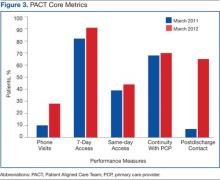
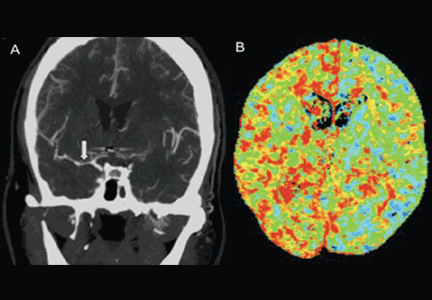
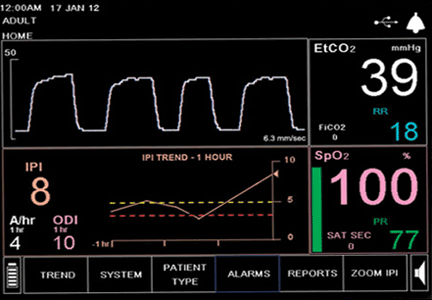
User login
Improving medication safety during hospital-based transitions of care
Any time patients enter or leave the hospital, they risk being harmed by errors in their medications.1 Adverse events from medication errors during transitions of care are common but often preventable. One key approach is to systematically review every patient’s medication list on admission and discharge and resolve any discrepancies. These transitions are also an opportunity to address other medication-related problems, such as adherence, drug interactions, and clinical appropriateness.
This article summarizes the types and prevalence of medication problems that occur during hospital-based transitions of care, and suggests strategies to decrease the risk of medication errors, focusing on medication reconciliation and related interventions that clinicians can use at the bedside to improve medication safety.
DEFINING TERMS
A medication discrepancy is any variance noted in a patient’s documented medication regimen across different medication lists or sites of care. While some differences reflect intentional and clinically appropriate changes to the regimen, others are unintentional and reflect inaccurate or incomplete information. These unintentional discrepancies are medication errors.
Depending on the clinical circumstances and medications involved, such errors may lead to an adverse drug event (ADE), defined as actual harm or injury resulting from a medication. Sometimes a medication error does not cause harm immediately but could if left uncorrected; this is called a potential ADE.
An important goal during transitions of care is to reduce unintentional medication discrepancies, thereby reducing potential and actual ADEs.
ERRORS ARISE AT DISCHARGE—AND EVEN MORE AT ADMISSION
Hospital discharge is a widely recognized transition in which patient harm occurs. As many as 70% of patients may have an unintentional medication discrepancy at hospital discharge, with many of those discrepancies having potential for harm.2 Indeed, during the first few weeks after discharge, 50% of patients have a clinically important medication error,3 and 20% experience an adverse event, most commonly an ADE.4 ADEs are associated with excess health care utilization,5–7 and many are preventable through strategies such as medication reconciliation.5,8
Importantly, more errors arise at hospital admission than at other times.9,10
Errors in medication histories are the most common source of discrepancies, affecting up to two-thirds of admitted patients.11,12 More than one-quarter of hospital prescribing errors can be attributed to incomplete medication histories at the time of admission,13 and nearly three times as many clinically important medication discrepancies are related to history-taking errors on admission rather than reconciliation errors at discharge.9
Most discrepancies occurring at the time of admission have the potential to cause harm, particularly if the errors persist beyond discharge.14 Therefore, taking a complete and accurate medication history on hospital admission is critical to ensuring safe care transitions.
MEDICATION RECONCILIATION: BARRIERS AND FACILITATORS
Medication reconciliation is a strategy for reducing medication discrepancies in patients moving across care settings. Simply put, it is the process by which a patient’s medication list is obtained, compared, and clarified across different sites of care.15 It has consistently been shown to decrease medication errors compared with usual care,16 and it is strongly supported by national and international organizations.17–21
In clinical practice, many physicians and institutions have found medication reconciliation difficult to implement, owing to barriers at the level of the patient, provider, and system (Table 1). In response to these challenges, two initiatives have synthesized best practices and offer toolkits that hospitals and clinicians can use: the Medications at Transitions and Clinical Handoffs (MATCH) program22 and the Multi-Center Medication Reconciliation Quality Improvement Study (MARQUIS).23
Lack of resources is a widely acknowledged challenge. Thus, the MARQUIS investigators23 suggested focusing on the admission history, where most errors occur, and applying the most resource-intensive interventions in patients at highest risk of ADEs, ie, those who are elderly, have multiple comorbid conditions, or take numerous medications.16
Although the risk of an ADE increases with the number of medications a patient takes,4 the exact number of drugs that defines high risk has not been well established. Targeting patients who take 10 or more maintenance medications is a reasonable initial approach,24 but institutions should tailor risk stratification to their patient populations and available resources. Patients taking high-risk medications such as anticoagulants and insulin could also be prioritized for review, since these medications are more likely to cause serious patient harm when used without appropriate clinical oversight.7
Using pharmacy staff to perform medication history-taking, reconciliation, and patient counseling has been shown to produce favorable patient outcomes, particularly for higher-risk patients.16,23
The MARQUIS investigators found they could boost the chances of success by sharing stories of patient harm to foster “buy-in” among frontline staff, providing formal training to clinicians on how to take a medication history, and obtaining the support of nursing leaders to champion improvement efforts.23
Additionally, patients should be empowered to maintain an accurate medication list. We address strategies for improving patient engagement and adherence in a later section.
BEST PRACTICES FOR IMPROVING MEDICATION SAFETY
Medication reconciliation is one of several measures necessary to optimize medication safety during transitions of care. It typically includes the following actions:
- Interview the patient or caregiver to determine the list of medications the patient is currently taking (or supposed to be taking); consult other sources if needed.
- List medications that are being ordered during the clinical encounter.
- Compare these two lists, making note of medications that are stopped, changed, or newly prescribed.
- Resolve any discrepancies.
- Communicate the reconciled list to the patient, appropriate caregivers, and providers of follow-up care.
At a rudimentary level, medication reconciliation encompasses medication list management along the continuum of care. However, we recommend leveraging medication reconciliation as an opportunity to further enhance medication safety by reviewing the appropriateness of each medication, seizing opportunities to streamline or simplify the regimen, assessing patient and caregiver understanding of medication instructions and potential ADEs, and delivering appropriate counseling to enhance medication use. Table 2 outlines our framework for medication management during hospital-based transitions.
STEP 1: OBTAIN A COMPLETE PREADMISSION MEDICATION LIST
The “best-possible medication history” is obtained in a systematic process of interviewing the patient or caregiver plus reviewing at least one other reliable source.23 The resulting list should include all medications the patient is taking (prescription and nonprescription), doses, directions for use, formulations if applicable, indications, start and stop dates, and medication allergies and reactions.
Review existing information. Before eliciting a history from the patient, review his or her recorded medical history and existing medication lists (eg, prior discharge summaries, records from other facilities, records from outpatient visits, pharmacy fill data). This will provide context about the regimen and help identify issues and questions that can be addressed during the history-taking.
Ask open-ended questions. Instead of just asking the patient to confirm the accuracy of the existing medication list, we recommend actively obtaining the full medication list from the patient or caregiver. The conversation should begin with an open-ended question such as, “What medications do you take at home?” This approach will also allow the clinician to gauge the patient’s level of understanding of each medication’s indication and dosing instructions. Using a series of prompts such as those recommended in Table 3 will elicit a best-possible medical history, while verifying all of the medications on the existing list.
Clarify discrepancies. Resolve differences between the existing medication lists and the patient’s or caregiver’s report during the preadmission interview. Examples include errors of omission (a medication is missing), errors of commission (an additional medication is present), and discrepancies in the strength, formulation, dosing instructions, and indications for the drugs. If necessary, other sources of information should be consulted, such as the patient’s medication bottles, pharmacy or pharmacies, primary care physician, and a family member or caregiver.
Assess adherence. The extent to which patients take their medications as directed is an important component of the history, but is often left out. Medication nonadherence rates in the United States are 40% to 70%,25 contributing to poor patient outcomes and imposing extraordinary costs on the health care system.26
Asking open-ended, nonjudgmental questions at the time of hospital admission will help to uncover medication-taking behaviors as well as barriers to adherence (Table 3). The patient’s responses should be taken into account when determining the treatment plan.
Document your findings. After completing the medication history and clarifying discrepancies, document the preadmission list in the medical record. All members of the health care team should have access to view and update the same list, as new information about the preadmission medications may be uncovered after the initial history.
Make clinical decisions. Complete the admission medication reconciliation by deciding whether each medication on the list should be continued, changed, held, or discontinued on the basis of the patient’s clinical condition. Well-designed information technology applications enable the provider to document each action and the rationale for it, as well as carry that information into the order-entry system. Medications marked as held or discontinued on admission should be revisited as the patient’s clinical condition changes and at discharge.
STEP 2: AVOID RECONCILIATION ERRORS
Reconciliation errors reflect discrepancies between the medication history and the medications that are ordered after admission.
Reconciliation errors are less common than medication history errors, accounting for approximately one-third of potentially harmful medication errors in hospitalized medical patients.9 These include errors of omission (a medication is omitted from the orders), errors of commission (a medication is prescribed with no indication for continuation), and therapeutic duplication.
Preventing errors of omission
Medications are often held at transition points for appropriate clinical reasons. Examples include holding anticoagulants and antiplatelet agents in patients who have gastrointestinal bleeding or an upcoming procedure, antihypertensives in patients with hemodynamic instability, and other chronic medications in patients with an acute illness.
Poor documentation and communication of these decisions can lead to a failure to resume the medications—an error of omission—at hospital discharge.
Hospitalized patients are at risk of unintentional discontinuation of their chronic medications, including antiplatelet drugs, anticoagulants, statins, and thyroid replacement, particularly if admitted to the intensive care unit.12 These errors can be minimized by a standardized medication reconciliation process at each transition and clear documentation of the medication plan.
Communication among providers can be improved if the admitting clinician documents clearly whether each preadmission medication is being continued, changed, or stopped, along with the reason for doing so, and makes this information available throughout the hospital stay. Upon transfer to another unit and at discharge, the physician should review each; preadmission medication that was held and the patient’s current clinical status and, based on that information, decide whether medications that were held should be resumed. If a medication will be restarted later, specific instructions should be documented and communicated to the patient and the physicians who are taking over his or her care.
Preventing errors of commission
Failure to perform a complete reconciliation at each transition of care and match each medication with an appropriate indication can lead to errors of commission.
One study showed that 44% of patients were prescribed at least one unnecessary drug at hospital discharge, one-fourth of which were started during the hospitalization.27 Commonly prescribed unnecessarily were gastrointestinal agents, central nervous system drugs, nutrients, and supplements.
It is critical to assess each medication’s ongoing need, appropriateness, and risk-benefit ratio at every transition. Medications no longer indicated should be discontinued in order to simplify the regimen, avoid unnecessary drug exposure, and prevent ADEs.
For example, proton pump inhibitors or histamine 2 receptor blockers are often started in the hospital for stress ulcer prophylaxis. One-third of patients are then discharged home on the medication, and 6 months later half of those patients are still taking the unnecessary drug.28 This situation can be avoided by limiting use of these medications to appropriate circumstances, clearly marking the indication as stress ulcer prophylaxis (as opposed to an ongoing condition that will require continuing it after discharge), and discontinuing the agent when appropriate.
All drugs, even common and seemingly benign ones, carry some risk and should be discontinued when no longer needed. Thus, medications added during the hospitalization to control acute symptoms should also be reviewed at each transition to prevent inappropriate continuation when symptoms have resolved.
One study, for example, found that many patients were discharged with inappropriate prescriptions for atypical antipsychotics after receiving them in the intensive care unit, likely for delirium.29 Documenting that an acute issue such as delirium has resolved should prompt the discontinuation of therapy.
Preventing therapeutic duplication
Therapeutic duplication occurs in about 8% of discharges.1 These errors often result from formulary substitutions or altering the dosage form in the acute setting. For example, patients who receive a prescription for the substituted agent at discharge and also resume their prehospitalization medications end up with duplicate therapy.
Therapeutic substitution is common at the time of admission to the hospital as a result of formulary restrictions. Drug classes that are frequently substituted include statins, antihypertensives, urinary antispasmodics, and proton pump inhibitors. Physicians should be familiar with the preferred agents on the hospital formulary and make careful note when a substitution occurs. Furthermore, hospital systems should be developed to remind the physician to switch back to the outpatient medication at discharge.
Similar problems occur when home medications are replaced with different dosage forms with different pharmacokinetic properties. For example, a long-acting medication may be temporarily replaced with an intravenous solution or immediate-release tablet for several reasons, including nothing-by-mouth status, unstable clinical condition, need for titration, and need to crush the tablet to give the drug per tube. The differing formulations must be reconciled throughout the patient’s hospital course and at discharge to avoid therapeutic duplication and serious medication errors. Deliberate changes to the dosage form should be clearly communicated in the discharge medication list so that patients and other clinicians are aware.
Hospital systems should also have the capability to identify duplications in the medication list and to warn prescribers of these errors. The ability to group medications by drug class or sort the medication list alphabetically by generic name can help uncover duplication errors.
STEP 3: REVIEW THE LIST IN VIEW OF THE CLINICAL PICTURE
Transitions of care should prompt providers to review the medication list for possible drug-disease interactions, confirm compliance with evidence-based guidelines, and evaluate the risks and benefits of each medication in the context of the patient’s age and acute and chronic medical issues. This is also an opportunity to screen the full list for potentially inappropriate medications and high-alert drugs such as insulin or anticoagulants, which are more likely than other drugs to cause severe harm when used in error.
Acute kidney injury. New drug-disease interactions can arise during a hospitalization and can affect dosing and the choice of drug. The onset of acute kidney injury, for example, often necessitates adjusting or discontinuing nephrotoxic and renally excreted medications. ADEs or potential ADEs have been reported in 43% of hospitalized patients with acute kidney injury.30
Because acute kidney injury is often transient, medications may need to be held or adjusted several times until renal function stabilizes. This can be challenging across the continuum of care and requires close monitoring of the serum creatinine level and associated drug doses and levels, if applicable. Well-designed clinical decision support tools can integrate laboratory data and alert the prescriber to a clinically important increase or decrease in serum creatinine that may warrant a change in therapy. Modifications to the regimen and a plan for timely follow-up of the serum creatinine level should be clearly documented in the discharge plan.
Liver disease. Similar attention should be given to drugs that are hepatically metabolized if a patient has acute or chronic liver impairment.
Geriatric patients, particularly those who present with altered mental status, falls, or urinary retention, should have their medication list reviewed for potentially inappropriate medications, which are drugs that pose increased risk of poor outcomes in older adults.31,32 Patients and providers may have been willing to accept the risk of medications such as anticholinergics or sedative-hypnotics when the drugs were initiated, but circumstances can change over time, especially in this patient population. Hospitalization is a prime opportunity to screen for medications that meet the Beers criteria31 for agents to avoid or use with caution in older adults.
As-needed medications. Medications prescribed on an as-needed basis in the hospital should be reviewed for continuation or discontinuation at discharge. How often the medication was given can inform this decision.
For example, if as-needed opioids were used frequently, failure to develop a plan of care for pain can lead to persistent symptoms and, possibly, to readmission.33,34 Similar scenarios occur with use of as-needed blood pressure medications, laxatives, and correction-dose insulin.
If an as-needed medication was used consistently during hospitalization, the physician should consider whether a regularly scheduled medication is needed. Conversely, if the medication was not used during the inpatient admission, it can likely be discontinued.
STEP 4: PREPARE THE PATIENT AND FOLLOW-UP PROVIDER
Once a clinician has performed medication reconciliation, including obtaining a best-possible medical history and carefully reviewing the medication list and orders for errors and clinical appropriateness, the next steps are to ensure the patient understands what he or she needs to do and to confirm that suitable follow-up plans are in place. These measures should be taken at all transitions of care but are critically important at hospital discharge.
Preparing the patient and caregiver
An accurate, reconciled medication list should be given to the patient, caregiver, or both, and should be reviewed before discharge.17
Approximately one-third of Americans have low health literacy skills, so medication lists and associated materials should be easy to understand.35 Medication lists should be written in plain language and formatted for optimal readability (Table 4), clearly stating which medications to continue, change, hold temporarily, and stop.
Patients recall and comprehend about half of the information provided during a medical encounter.36 Thus, medication teaching should focus on key points including changes or additions to the regimen, specific instructions for follow-up and monitoring, and how to handle common and serious side effects.
To confirm patient understanding, clinicians should use “teach-back,” ie, provide the patient with information and then ask him or her to repeat back key points.37,38 The patient and family should also be encouraged to ask questions before discharge.
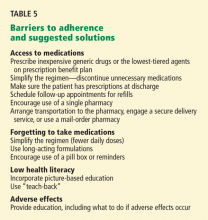
If not already addressed during the hospital stay, barriers to medication adherence and ability to obtain the medications should be attended to at this time (Table 5). Also, the plan to pick up the medications should be verified with the patient and caregiver. Verify that there is transportation to a particular pharmacy that is open at the time of discharge, and that the patient can afford the medications.
Ensuring appropriate follow-up
Studies have shown that timely in-home or telephone follow-up after discharge can decrease adverse events and postdischarge health care utilization.39,40 Telephone follow-up that includes thorough medication reconciliation can help detect and resolve medication issues early after discharge and can close gaps related to monitoring and follow-up.
Medication reconciliation by telephone can be time-consuming. Depending on the number of medications that need to be reviewed, calls can take between 10 and 60 minutes. Postdischarge phone calls should be performed by clinical personnel who are able to identify medication-related problems. A pharmacist should be an available resource to assist with complex regimens, to help resolve medication discrepancies, and to address patient concerns. Table 6 provides tips for conducting follow-up phone calls.
Resolving discrepancies identified during follow-up calls can be difficult, as changes to the medication regimen are often not communicated effectively to other members of the care team. Physicians should document the complete medication list and plan in the discharge summary, and there should be a method for the caller to record updates to the medication list in the medical record so that they are apparent at the outpatient follow-up visit.
An additional challenge is that it is frequently unclear which physician “owns” which medications. Therefore, designating a contact person for each medication until follow-up can be very valuable. At a minimum, a “physician owner” for high-alert medications such as insulin, anticoagulants, and diuretics should be identified to provide close follow-up, titration, and monitoring.
There should also be a plan for the patient to obtain refills of essential long-term medications, such as antiplatelet agents following stent placement.
SUMMARY AND RECOMMENDATIONS
Medication-related problems during hospital admission and discharge are common and range from minor discrepancies in the medication list to errors in history-taking, prescribing, and reconciliation that can lead to potential or actual patient harm. Putting systems in place to facilitate medication reconciliation can decrease the occurrence of medication discrepancies and ADEs, thereby improving patient safety during these critical transitions between care settings and providers. Institutional medication reconciliation programs should focus resources on the admission history-taking step, target the highest-risk patients for the most intensive interventions, and involve pharmacy personnel when possible.
On an individual level, clinicians can incorporate additional interventions into their workflows to optimize medication safety for hospitalized patients. Using a structured approach to obtain a complete and accurate medication list at the time of hospital admission will help providers identify medication-related problems and prevent the propagation of errors throughout the hospital stay and at discharge. Focusing additional time and effort on a comprehensive review of the medication list for errors of omission and commission, patient-specific needs, and high-alert drugs will further decrease the risk of medication errors. Finally, providing discharge counseling targeting patient barriers to adherence and ensuring a proper handover of medication information and rationale for medication changes to outpatient providers will improve the chances of a safe transition.
- Coleman EA, Smith JD, Raha D, Min SJ. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med 2005; 165:1842–1847.
- Wong JD, Bajcar JM, Wong GG, et al. Medication reconciliation at hospital discharge: evaluating discrepancies. Ann Pharmacother 2008; 42:1373–1379.
- Kripalani S, Roumie CL, Dalal AK, et al; PILL-CVD (Pharmacist Intervention for Low Literacy in Cardiovascular Disease) Study Group. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med 2012; 157:1-10.
- Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med 2003; 138:161–167.
- Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. Adverse drug events occurring following hospital discharge. J Gen Intern Med 2005; 20:317–323.
- Johnson JA, Bootman JL. Drug-related morbidity and mortality. A cost-of-illness model. Arch Intern Med 1995; 155:1949–1956.
- Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med 2011; 365:2002–2012.
- Bates DW, Boyle DL, Vander Vliet MB, Schneider J, Leape L. Relationship between medication errors and adverse drug events. J Gen Intern Med 1995;10:199–205.
- Pippins JR, Gandhi TK, Hamann C, et al. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med 2008; 23:1414–1422.
- Lau HS, Florax C, Porsius AJ, De Boer A.The completeness of medication histories in hospital medical records of patients admitted to general internal medicine wards. Br J Clin Pharmacol 2000; 49:597–603.
- Tam VC, Knowles SR, Cornish PL, Fine N, Marchesano R, Etchells EE. Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. CMAJ 2005; 173:510–515.
- Bell CM, Brener SS, Gunraj N, et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA 2011; 306:840–847.
- Dobranski S, Hammond I, Khan G, Holdsworth H. The nature of hospital prescribing errors. Br J Clin Governance 2002; 7:187–193.
- Gleason KM, Groszek JM, Sullivan C, Rooney D, Barnard C, Noskin GA. Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients. Am J Health Syst Pharm 2004; 61:1689–1695.
- Rozich JD, Resar KR. Medication safety: one organization’s approach to the challenge. J Clin Outcomes Manage 2001; 8:27–34.
- Mueller SK, Sponsler KC, Kripalani S, Schnipper JL. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med 2012; 172:1057–1069.
- Joint Commission. Using medication reconciliation to prevent errors. Sentinel Event Alert 2006, Issue 35. www.jointcommission.org/assets/1/18/SEA_35.pdf. Accessed March 31, 2015.
- Greenwald JL, Halasyamani L, Greene J, et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med 2010; 5:477–485.
- Berwick DM, Calkins DR, McCannon CJ, Hackbarth AD. The 100,000 lives campaign: setting a goal and a deadline for improving health care quality. JAMA 2006; 295:324–327.
- McCannon CJ, Hackbarth AD, Griffin FA. Miles to go: an introduction to the 5 Million Lives Campaign. Jt Comm J Qual Patient Saf 2007; 33:477–484.
- Leotsakos A, Caisley L, Karga M, Kelly E, O’Leary D, Timmons K. High 5s: addressing excellence in patient safety. World Hosp Health Serv 2009; 45:19–22.
- Gleason KM, Brake H, Agramonte V, Perfetti C. Medications at Transitions and Clinical Handoffs (MATCH) Toolkit for Medication Reconciliation. www.ahrq.gov/professionals/quality-patient-safety/patient-safety-resources/resources/match/match.pdf. Accessed March 31, 2015.
- Mueller SK, Kripalani S, Stein J, et al. A toolkit to disseminate best practices in inpatient medication reconciliation: multi-center medication reconciliation quality improvement study (MARQUIS). Jt Comm J Qual Patient Saf 2013; 39:371–382.
- Pal A, Babbott S, Wilkinson ST. Can the targeted use of a discharge pharmacist significantly decrease 30-day readmissions? Hosp Pharm 2013; 48:380–388.
- Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther 2001; 23:1296–1310.
- Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005; 353:487–497.
- Hajjar ER, Hanlon JT, Sloane RJ, et al. Unnecessary drug use in frail older people at hospital discharge. J Am Geriatr Soc 2005; 53:1518–1523.
- Zink DA, Pohlman M, Barnes M, Cannon ME. Long-term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther 2005; 21:1203–1209.
- Morandi A, Vasilevskis E, Pandharipande PP, et al. Inappropriate medication prescriptions in elderly adults surviving an intensive care unit hospitalization. J Am Geriatr Soc 2013; 61:1128–1134.
- Cox ZL, McCoy AB, Matheny ME, et al. Adverse drug events during AKI and its recovery. Clin J Am Soc Nephrol 2013; 8:1070–1078.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012; 60:616–631.
- Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers’ criteria. Age Ageing 2008; 37:673–679.
- Tjia J, Bonner A, Briesacher BA, McGee S, Terrill E, Miller K. Medication discrepancies upon hospital to skilled nursing facility transitions. J Gen Intern Med 2009; 24:630–635.
- Boockvar K, Fishman E, Kyriacou CK, Monias A, Gavi S, Cortes T. Adverse events due to discontinuations in drug use and dose changes in patients transferred between acute and long-term care facilities. Arch Intern Med 2004; 164:545–550.
- Kutner M, Greenberg E, Jin Y, Paulsen C. The Health Literacy of America’s Adults: Results From the 2003 National Assessment of Adult Literacy. http://nces.ed.gov/pubs2006/2006483_1.pdf. Accessed March 31, 2015.
- Crane JA. Patient comprehension of doctor-patient communication on discharge from the emergency department. J Emerg Med 1997; 15:1–7.
- DeWalt DA, Callahan LF, Hawk VH, et al. Health Literacy Universal Precautions Toolkit. www.ahrq.gov/qual/literacy/healthliteracytoolkit.pdf. Accessed March 31, 2015.
- Schillinger D, Piette J, Grumbach K, et al. Closing the loop: physician communication with diabetic patients who have low health literacy. Arch Intern Med 2003; 163:83–90.
- Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med 2006; 166:1822–1828.
- Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med 2009; 150:178–187.
Any time patients enter or leave the hospital, they risk being harmed by errors in their medications.1 Adverse events from medication errors during transitions of care are common but often preventable. One key approach is to systematically review every patient’s medication list on admission and discharge and resolve any discrepancies. These transitions are also an opportunity to address other medication-related problems, such as adherence, drug interactions, and clinical appropriateness.
This article summarizes the types and prevalence of medication problems that occur during hospital-based transitions of care, and suggests strategies to decrease the risk of medication errors, focusing on medication reconciliation and related interventions that clinicians can use at the bedside to improve medication safety.
DEFINING TERMS
A medication discrepancy is any variance noted in a patient’s documented medication regimen across different medication lists or sites of care. While some differences reflect intentional and clinically appropriate changes to the regimen, others are unintentional and reflect inaccurate or incomplete information. These unintentional discrepancies are medication errors.
Depending on the clinical circumstances and medications involved, such errors may lead to an adverse drug event (ADE), defined as actual harm or injury resulting from a medication. Sometimes a medication error does not cause harm immediately but could if left uncorrected; this is called a potential ADE.
An important goal during transitions of care is to reduce unintentional medication discrepancies, thereby reducing potential and actual ADEs.
ERRORS ARISE AT DISCHARGE—AND EVEN MORE AT ADMISSION
Hospital discharge is a widely recognized transition in which patient harm occurs. As many as 70% of patients may have an unintentional medication discrepancy at hospital discharge, with many of those discrepancies having potential for harm.2 Indeed, during the first few weeks after discharge, 50% of patients have a clinically important medication error,3 and 20% experience an adverse event, most commonly an ADE.4 ADEs are associated with excess health care utilization,5–7 and many are preventable through strategies such as medication reconciliation.5,8
Importantly, more errors arise at hospital admission than at other times.9,10
Errors in medication histories are the most common source of discrepancies, affecting up to two-thirds of admitted patients.11,12 More than one-quarter of hospital prescribing errors can be attributed to incomplete medication histories at the time of admission,13 and nearly three times as many clinically important medication discrepancies are related to history-taking errors on admission rather than reconciliation errors at discharge.9
Most discrepancies occurring at the time of admission have the potential to cause harm, particularly if the errors persist beyond discharge.14 Therefore, taking a complete and accurate medication history on hospital admission is critical to ensuring safe care transitions.
MEDICATION RECONCILIATION: BARRIERS AND FACILITATORS
Medication reconciliation is a strategy for reducing medication discrepancies in patients moving across care settings. Simply put, it is the process by which a patient’s medication list is obtained, compared, and clarified across different sites of care.15 It has consistently been shown to decrease medication errors compared with usual care,16 and it is strongly supported by national and international organizations.17–21
In clinical practice, many physicians and institutions have found medication reconciliation difficult to implement, owing to barriers at the level of the patient, provider, and system (Table 1). In response to these challenges, two initiatives have synthesized best practices and offer toolkits that hospitals and clinicians can use: the Medications at Transitions and Clinical Handoffs (MATCH) program22 and the Multi-Center Medication Reconciliation Quality Improvement Study (MARQUIS).23
Lack of resources is a widely acknowledged challenge. Thus, the MARQUIS investigators23 suggested focusing on the admission history, where most errors occur, and applying the most resource-intensive interventions in patients at highest risk of ADEs, ie, those who are elderly, have multiple comorbid conditions, or take numerous medications.16
Although the risk of an ADE increases with the number of medications a patient takes,4 the exact number of drugs that defines high risk has not been well established. Targeting patients who take 10 or more maintenance medications is a reasonable initial approach,24 but institutions should tailor risk stratification to their patient populations and available resources. Patients taking high-risk medications such as anticoagulants and insulin could also be prioritized for review, since these medications are more likely to cause serious patient harm when used without appropriate clinical oversight.7
Using pharmacy staff to perform medication history-taking, reconciliation, and patient counseling has been shown to produce favorable patient outcomes, particularly for higher-risk patients.16,23
The MARQUIS investigators found they could boost the chances of success by sharing stories of patient harm to foster “buy-in” among frontline staff, providing formal training to clinicians on how to take a medication history, and obtaining the support of nursing leaders to champion improvement efforts.23
Additionally, patients should be empowered to maintain an accurate medication list. We address strategies for improving patient engagement and adherence in a later section.
BEST PRACTICES FOR IMPROVING MEDICATION SAFETY
Medication reconciliation is one of several measures necessary to optimize medication safety during transitions of care. It typically includes the following actions:
- Interview the patient or caregiver to determine the list of medications the patient is currently taking (or supposed to be taking); consult other sources if needed.
- List medications that are being ordered during the clinical encounter.
- Compare these two lists, making note of medications that are stopped, changed, or newly prescribed.
- Resolve any discrepancies.
- Communicate the reconciled list to the patient, appropriate caregivers, and providers of follow-up care.
At a rudimentary level, medication reconciliation encompasses medication list management along the continuum of care. However, we recommend leveraging medication reconciliation as an opportunity to further enhance medication safety by reviewing the appropriateness of each medication, seizing opportunities to streamline or simplify the regimen, assessing patient and caregiver understanding of medication instructions and potential ADEs, and delivering appropriate counseling to enhance medication use. Table 2 outlines our framework for medication management during hospital-based transitions.
STEP 1: OBTAIN A COMPLETE PREADMISSION MEDICATION LIST
The “best-possible medication history” is obtained in a systematic process of interviewing the patient or caregiver plus reviewing at least one other reliable source.23 The resulting list should include all medications the patient is taking (prescription and nonprescription), doses, directions for use, formulations if applicable, indications, start and stop dates, and medication allergies and reactions.
Review existing information. Before eliciting a history from the patient, review his or her recorded medical history and existing medication lists (eg, prior discharge summaries, records from other facilities, records from outpatient visits, pharmacy fill data). This will provide context about the regimen and help identify issues and questions that can be addressed during the history-taking.
Ask open-ended questions. Instead of just asking the patient to confirm the accuracy of the existing medication list, we recommend actively obtaining the full medication list from the patient or caregiver. The conversation should begin with an open-ended question such as, “What medications do you take at home?” This approach will also allow the clinician to gauge the patient’s level of understanding of each medication’s indication and dosing instructions. Using a series of prompts such as those recommended in Table 3 will elicit a best-possible medical history, while verifying all of the medications on the existing list.
Clarify discrepancies. Resolve differences between the existing medication lists and the patient’s or caregiver’s report during the preadmission interview. Examples include errors of omission (a medication is missing), errors of commission (an additional medication is present), and discrepancies in the strength, formulation, dosing instructions, and indications for the drugs. If necessary, other sources of information should be consulted, such as the patient’s medication bottles, pharmacy or pharmacies, primary care physician, and a family member or caregiver.
Assess adherence. The extent to which patients take their medications as directed is an important component of the history, but is often left out. Medication nonadherence rates in the United States are 40% to 70%,25 contributing to poor patient outcomes and imposing extraordinary costs on the health care system.26
Asking open-ended, nonjudgmental questions at the time of hospital admission will help to uncover medication-taking behaviors as well as barriers to adherence (Table 3). The patient’s responses should be taken into account when determining the treatment plan.
Document your findings. After completing the medication history and clarifying discrepancies, document the preadmission list in the medical record. All members of the health care team should have access to view and update the same list, as new information about the preadmission medications may be uncovered after the initial history.
Make clinical decisions. Complete the admission medication reconciliation by deciding whether each medication on the list should be continued, changed, held, or discontinued on the basis of the patient’s clinical condition. Well-designed information technology applications enable the provider to document each action and the rationale for it, as well as carry that information into the order-entry system. Medications marked as held or discontinued on admission should be revisited as the patient’s clinical condition changes and at discharge.
STEP 2: AVOID RECONCILIATION ERRORS
Reconciliation errors reflect discrepancies between the medication history and the medications that are ordered after admission.
Reconciliation errors are less common than medication history errors, accounting for approximately one-third of potentially harmful medication errors in hospitalized medical patients.9 These include errors of omission (a medication is omitted from the orders), errors of commission (a medication is prescribed with no indication for continuation), and therapeutic duplication.
Preventing errors of omission
Medications are often held at transition points for appropriate clinical reasons. Examples include holding anticoagulants and antiplatelet agents in patients who have gastrointestinal bleeding or an upcoming procedure, antihypertensives in patients with hemodynamic instability, and other chronic medications in patients with an acute illness.
Poor documentation and communication of these decisions can lead to a failure to resume the medications—an error of omission—at hospital discharge.
Hospitalized patients are at risk of unintentional discontinuation of their chronic medications, including antiplatelet drugs, anticoagulants, statins, and thyroid replacement, particularly if admitted to the intensive care unit.12 These errors can be minimized by a standardized medication reconciliation process at each transition and clear documentation of the medication plan.
Communication among providers can be improved if the admitting clinician documents clearly whether each preadmission medication is being continued, changed, or stopped, along with the reason for doing so, and makes this information available throughout the hospital stay. Upon transfer to another unit and at discharge, the physician should review each; preadmission medication that was held and the patient’s current clinical status and, based on that information, decide whether medications that were held should be resumed. If a medication will be restarted later, specific instructions should be documented and communicated to the patient and the physicians who are taking over his or her care.
Preventing errors of commission
Failure to perform a complete reconciliation at each transition of care and match each medication with an appropriate indication can lead to errors of commission.
One study showed that 44% of patients were prescribed at least one unnecessary drug at hospital discharge, one-fourth of which were started during the hospitalization.27 Commonly prescribed unnecessarily were gastrointestinal agents, central nervous system drugs, nutrients, and supplements.
It is critical to assess each medication’s ongoing need, appropriateness, and risk-benefit ratio at every transition. Medications no longer indicated should be discontinued in order to simplify the regimen, avoid unnecessary drug exposure, and prevent ADEs.
For example, proton pump inhibitors or histamine 2 receptor blockers are often started in the hospital for stress ulcer prophylaxis. One-third of patients are then discharged home on the medication, and 6 months later half of those patients are still taking the unnecessary drug.28 This situation can be avoided by limiting use of these medications to appropriate circumstances, clearly marking the indication as stress ulcer prophylaxis (as opposed to an ongoing condition that will require continuing it after discharge), and discontinuing the agent when appropriate.
All drugs, even common and seemingly benign ones, carry some risk and should be discontinued when no longer needed. Thus, medications added during the hospitalization to control acute symptoms should also be reviewed at each transition to prevent inappropriate continuation when symptoms have resolved.
One study, for example, found that many patients were discharged with inappropriate prescriptions for atypical antipsychotics after receiving them in the intensive care unit, likely for delirium.29 Documenting that an acute issue such as delirium has resolved should prompt the discontinuation of therapy.
Preventing therapeutic duplication
Therapeutic duplication occurs in about 8% of discharges.1 These errors often result from formulary substitutions or altering the dosage form in the acute setting. For example, patients who receive a prescription for the substituted agent at discharge and also resume their prehospitalization medications end up with duplicate therapy.
Therapeutic substitution is common at the time of admission to the hospital as a result of formulary restrictions. Drug classes that are frequently substituted include statins, antihypertensives, urinary antispasmodics, and proton pump inhibitors. Physicians should be familiar with the preferred agents on the hospital formulary and make careful note when a substitution occurs. Furthermore, hospital systems should be developed to remind the physician to switch back to the outpatient medication at discharge.
Similar problems occur when home medications are replaced with different dosage forms with different pharmacokinetic properties. For example, a long-acting medication may be temporarily replaced with an intravenous solution or immediate-release tablet for several reasons, including nothing-by-mouth status, unstable clinical condition, need for titration, and need to crush the tablet to give the drug per tube. The differing formulations must be reconciled throughout the patient’s hospital course and at discharge to avoid therapeutic duplication and serious medication errors. Deliberate changes to the dosage form should be clearly communicated in the discharge medication list so that patients and other clinicians are aware.
Hospital systems should also have the capability to identify duplications in the medication list and to warn prescribers of these errors. The ability to group medications by drug class or sort the medication list alphabetically by generic name can help uncover duplication errors.
STEP 3: REVIEW THE LIST IN VIEW OF THE CLINICAL PICTURE
Transitions of care should prompt providers to review the medication list for possible drug-disease interactions, confirm compliance with evidence-based guidelines, and evaluate the risks and benefits of each medication in the context of the patient’s age and acute and chronic medical issues. This is also an opportunity to screen the full list for potentially inappropriate medications and high-alert drugs such as insulin or anticoagulants, which are more likely than other drugs to cause severe harm when used in error.
Acute kidney injury. New drug-disease interactions can arise during a hospitalization and can affect dosing and the choice of drug. The onset of acute kidney injury, for example, often necessitates adjusting or discontinuing nephrotoxic and renally excreted medications. ADEs or potential ADEs have been reported in 43% of hospitalized patients with acute kidney injury.30
Because acute kidney injury is often transient, medications may need to be held or adjusted several times until renal function stabilizes. This can be challenging across the continuum of care and requires close monitoring of the serum creatinine level and associated drug doses and levels, if applicable. Well-designed clinical decision support tools can integrate laboratory data and alert the prescriber to a clinically important increase or decrease in serum creatinine that may warrant a change in therapy. Modifications to the regimen and a plan for timely follow-up of the serum creatinine level should be clearly documented in the discharge plan.
Liver disease. Similar attention should be given to drugs that are hepatically metabolized if a patient has acute or chronic liver impairment.
Geriatric patients, particularly those who present with altered mental status, falls, or urinary retention, should have their medication list reviewed for potentially inappropriate medications, which are drugs that pose increased risk of poor outcomes in older adults.31,32 Patients and providers may have been willing to accept the risk of medications such as anticholinergics or sedative-hypnotics when the drugs were initiated, but circumstances can change over time, especially in this patient population. Hospitalization is a prime opportunity to screen for medications that meet the Beers criteria31 for agents to avoid or use with caution in older adults.
As-needed medications. Medications prescribed on an as-needed basis in the hospital should be reviewed for continuation or discontinuation at discharge. How often the medication was given can inform this decision.
For example, if as-needed opioids were used frequently, failure to develop a plan of care for pain can lead to persistent symptoms and, possibly, to readmission.33,34 Similar scenarios occur with use of as-needed blood pressure medications, laxatives, and correction-dose insulin.
If an as-needed medication was used consistently during hospitalization, the physician should consider whether a regularly scheduled medication is needed. Conversely, if the medication was not used during the inpatient admission, it can likely be discontinued.
STEP 4: PREPARE THE PATIENT AND FOLLOW-UP PROVIDER
Once a clinician has performed medication reconciliation, including obtaining a best-possible medical history and carefully reviewing the medication list and orders for errors and clinical appropriateness, the next steps are to ensure the patient understands what he or she needs to do and to confirm that suitable follow-up plans are in place. These measures should be taken at all transitions of care but are critically important at hospital discharge.
Preparing the patient and caregiver
An accurate, reconciled medication list should be given to the patient, caregiver, or both, and should be reviewed before discharge.17
Approximately one-third of Americans have low health literacy skills, so medication lists and associated materials should be easy to understand.35 Medication lists should be written in plain language and formatted for optimal readability (Table 4), clearly stating which medications to continue, change, hold temporarily, and stop.
Patients recall and comprehend about half of the information provided during a medical encounter.36 Thus, medication teaching should focus on key points including changes or additions to the regimen, specific instructions for follow-up and monitoring, and how to handle common and serious side effects.
To confirm patient understanding, clinicians should use “teach-back,” ie, provide the patient with information and then ask him or her to repeat back key points.37,38 The patient and family should also be encouraged to ask questions before discharge.
If not already addressed during the hospital stay, barriers to medication adherence and ability to obtain the medications should be attended to at this time (Table 5). Also, the plan to pick up the medications should be verified with the patient and caregiver. Verify that there is transportation to a particular pharmacy that is open at the time of discharge, and that the patient can afford the medications.
Ensuring appropriate follow-up
Studies have shown that timely in-home or telephone follow-up after discharge can decrease adverse events and postdischarge health care utilization.39,40 Telephone follow-up that includes thorough medication reconciliation can help detect and resolve medication issues early after discharge and can close gaps related to monitoring and follow-up.
Medication reconciliation by telephone can be time-consuming. Depending on the number of medications that need to be reviewed, calls can take between 10 and 60 minutes. Postdischarge phone calls should be performed by clinical personnel who are able to identify medication-related problems. A pharmacist should be an available resource to assist with complex regimens, to help resolve medication discrepancies, and to address patient concerns. Table 6 provides tips for conducting follow-up phone calls.
Resolving discrepancies identified during follow-up calls can be difficult, as changes to the medication regimen are often not communicated effectively to other members of the care team. Physicians should document the complete medication list and plan in the discharge summary, and there should be a method for the caller to record updates to the medication list in the medical record so that they are apparent at the outpatient follow-up visit.
An additional challenge is that it is frequently unclear which physician “owns” which medications. Therefore, designating a contact person for each medication until follow-up can be very valuable. At a minimum, a “physician owner” for high-alert medications such as insulin, anticoagulants, and diuretics should be identified to provide close follow-up, titration, and monitoring.
There should also be a plan for the patient to obtain refills of essential long-term medications, such as antiplatelet agents following stent placement.
SUMMARY AND RECOMMENDATIONS
Medication-related problems during hospital admission and discharge are common and range from minor discrepancies in the medication list to errors in history-taking, prescribing, and reconciliation that can lead to potential or actual patient harm. Putting systems in place to facilitate medication reconciliation can decrease the occurrence of medication discrepancies and ADEs, thereby improving patient safety during these critical transitions between care settings and providers. Institutional medication reconciliation programs should focus resources on the admission history-taking step, target the highest-risk patients for the most intensive interventions, and involve pharmacy personnel when possible.
On an individual level, clinicians can incorporate additional interventions into their workflows to optimize medication safety for hospitalized patients. Using a structured approach to obtain a complete and accurate medication list at the time of hospital admission will help providers identify medication-related problems and prevent the propagation of errors throughout the hospital stay and at discharge. Focusing additional time and effort on a comprehensive review of the medication list for errors of omission and commission, patient-specific needs, and high-alert drugs will further decrease the risk of medication errors. Finally, providing discharge counseling targeting patient barriers to adherence and ensuring a proper handover of medication information and rationale for medication changes to outpatient providers will improve the chances of a safe transition.
Any time patients enter or leave the hospital, they risk being harmed by errors in their medications.1 Adverse events from medication errors during transitions of care are common but often preventable. One key approach is to systematically review every patient’s medication list on admission and discharge and resolve any discrepancies. These transitions are also an opportunity to address other medication-related problems, such as adherence, drug interactions, and clinical appropriateness.
This article summarizes the types and prevalence of medication problems that occur during hospital-based transitions of care, and suggests strategies to decrease the risk of medication errors, focusing on medication reconciliation and related interventions that clinicians can use at the bedside to improve medication safety.
DEFINING TERMS
A medication discrepancy is any variance noted in a patient’s documented medication regimen across different medication lists or sites of care. While some differences reflect intentional and clinically appropriate changes to the regimen, others are unintentional and reflect inaccurate or incomplete information. These unintentional discrepancies are medication errors.
Depending on the clinical circumstances and medications involved, such errors may lead to an adverse drug event (ADE), defined as actual harm or injury resulting from a medication. Sometimes a medication error does not cause harm immediately but could if left uncorrected; this is called a potential ADE.
An important goal during transitions of care is to reduce unintentional medication discrepancies, thereby reducing potential and actual ADEs.
ERRORS ARISE AT DISCHARGE—AND EVEN MORE AT ADMISSION
Hospital discharge is a widely recognized transition in which patient harm occurs. As many as 70% of patients may have an unintentional medication discrepancy at hospital discharge, with many of those discrepancies having potential for harm.2 Indeed, during the first few weeks after discharge, 50% of patients have a clinically important medication error,3 and 20% experience an adverse event, most commonly an ADE.4 ADEs are associated with excess health care utilization,5–7 and many are preventable through strategies such as medication reconciliation.5,8
Importantly, more errors arise at hospital admission than at other times.9,10
Errors in medication histories are the most common source of discrepancies, affecting up to two-thirds of admitted patients.11,12 More than one-quarter of hospital prescribing errors can be attributed to incomplete medication histories at the time of admission,13 and nearly three times as many clinically important medication discrepancies are related to history-taking errors on admission rather than reconciliation errors at discharge.9
Most discrepancies occurring at the time of admission have the potential to cause harm, particularly if the errors persist beyond discharge.14 Therefore, taking a complete and accurate medication history on hospital admission is critical to ensuring safe care transitions.
MEDICATION RECONCILIATION: BARRIERS AND FACILITATORS
Medication reconciliation is a strategy for reducing medication discrepancies in patients moving across care settings. Simply put, it is the process by which a patient’s medication list is obtained, compared, and clarified across different sites of care.15 It has consistently been shown to decrease medication errors compared with usual care,16 and it is strongly supported by national and international organizations.17–21
In clinical practice, many physicians and institutions have found medication reconciliation difficult to implement, owing to barriers at the level of the patient, provider, and system (Table 1). In response to these challenges, two initiatives have synthesized best practices and offer toolkits that hospitals and clinicians can use: the Medications at Transitions and Clinical Handoffs (MATCH) program22 and the Multi-Center Medication Reconciliation Quality Improvement Study (MARQUIS).23
Lack of resources is a widely acknowledged challenge. Thus, the MARQUIS investigators23 suggested focusing on the admission history, where most errors occur, and applying the most resource-intensive interventions in patients at highest risk of ADEs, ie, those who are elderly, have multiple comorbid conditions, or take numerous medications.16
Although the risk of an ADE increases with the number of medications a patient takes,4 the exact number of drugs that defines high risk has not been well established. Targeting patients who take 10 or more maintenance medications is a reasonable initial approach,24 but institutions should tailor risk stratification to their patient populations and available resources. Patients taking high-risk medications such as anticoagulants and insulin could also be prioritized for review, since these medications are more likely to cause serious patient harm when used without appropriate clinical oversight.7
Using pharmacy staff to perform medication history-taking, reconciliation, and patient counseling has been shown to produce favorable patient outcomes, particularly for higher-risk patients.16,23
The MARQUIS investigators found they could boost the chances of success by sharing stories of patient harm to foster “buy-in” among frontline staff, providing formal training to clinicians on how to take a medication history, and obtaining the support of nursing leaders to champion improvement efforts.23
Additionally, patients should be empowered to maintain an accurate medication list. We address strategies for improving patient engagement and adherence in a later section.
BEST PRACTICES FOR IMPROVING MEDICATION SAFETY
Medication reconciliation is one of several measures necessary to optimize medication safety during transitions of care. It typically includes the following actions:
- Interview the patient or caregiver to determine the list of medications the patient is currently taking (or supposed to be taking); consult other sources if needed.
- List medications that are being ordered during the clinical encounter.
- Compare these two lists, making note of medications that are stopped, changed, or newly prescribed.
- Resolve any discrepancies.
- Communicate the reconciled list to the patient, appropriate caregivers, and providers of follow-up care.
At a rudimentary level, medication reconciliation encompasses medication list management along the continuum of care. However, we recommend leveraging medication reconciliation as an opportunity to further enhance medication safety by reviewing the appropriateness of each medication, seizing opportunities to streamline or simplify the regimen, assessing patient and caregiver understanding of medication instructions and potential ADEs, and delivering appropriate counseling to enhance medication use. Table 2 outlines our framework for medication management during hospital-based transitions.
STEP 1: OBTAIN A COMPLETE PREADMISSION MEDICATION LIST
The “best-possible medication history” is obtained in a systematic process of interviewing the patient or caregiver plus reviewing at least one other reliable source.23 The resulting list should include all medications the patient is taking (prescription and nonprescription), doses, directions for use, formulations if applicable, indications, start and stop dates, and medication allergies and reactions.
Review existing information. Before eliciting a history from the patient, review his or her recorded medical history and existing medication lists (eg, prior discharge summaries, records from other facilities, records from outpatient visits, pharmacy fill data). This will provide context about the regimen and help identify issues and questions that can be addressed during the history-taking.
Ask open-ended questions. Instead of just asking the patient to confirm the accuracy of the existing medication list, we recommend actively obtaining the full medication list from the patient or caregiver. The conversation should begin with an open-ended question such as, “What medications do you take at home?” This approach will also allow the clinician to gauge the patient’s level of understanding of each medication’s indication and dosing instructions. Using a series of prompts such as those recommended in Table 3 will elicit a best-possible medical history, while verifying all of the medications on the existing list.
Clarify discrepancies. Resolve differences between the existing medication lists and the patient’s or caregiver’s report during the preadmission interview. Examples include errors of omission (a medication is missing), errors of commission (an additional medication is present), and discrepancies in the strength, formulation, dosing instructions, and indications for the drugs. If necessary, other sources of information should be consulted, such as the patient’s medication bottles, pharmacy or pharmacies, primary care physician, and a family member or caregiver.
Assess adherence. The extent to which patients take their medications as directed is an important component of the history, but is often left out. Medication nonadherence rates in the United States are 40% to 70%,25 contributing to poor patient outcomes and imposing extraordinary costs on the health care system.26
Asking open-ended, nonjudgmental questions at the time of hospital admission will help to uncover medication-taking behaviors as well as barriers to adherence (Table 3). The patient’s responses should be taken into account when determining the treatment plan.
Document your findings. After completing the medication history and clarifying discrepancies, document the preadmission list in the medical record. All members of the health care team should have access to view and update the same list, as new information about the preadmission medications may be uncovered after the initial history.
Make clinical decisions. Complete the admission medication reconciliation by deciding whether each medication on the list should be continued, changed, held, or discontinued on the basis of the patient’s clinical condition. Well-designed information technology applications enable the provider to document each action and the rationale for it, as well as carry that information into the order-entry system. Medications marked as held or discontinued on admission should be revisited as the patient’s clinical condition changes and at discharge.
STEP 2: AVOID RECONCILIATION ERRORS
Reconciliation errors reflect discrepancies between the medication history and the medications that are ordered after admission.
Reconciliation errors are less common than medication history errors, accounting for approximately one-third of potentially harmful medication errors in hospitalized medical patients.9 These include errors of omission (a medication is omitted from the orders), errors of commission (a medication is prescribed with no indication for continuation), and therapeutic duplication.
Preventing errors of omission
Medications are often held at transition points for appropriate clinical reasons. Examples include holding anticoagulants and antiplatelet agents in patients who have gastrointestinal bleeding or an upcoming procedure, antihypertensives in patients with hemodynamic instability, and other chronic medications in patients with an acute illness.
Poor documentation and communication of these decisions can lead to a failure to resume the medications—an error of omission—at hospital discharge.
Hospitalized patients are at risk of unintentional discontinuation of their chronic medications, including antiplatelet drugs, anticoagulants, statins, and thyroid replacement, particularly if admitted to the intensive care unit.12 These errors can be minimized by a standardized medication reconciliation process at each transition and clear documentation of the medication plan.
Communication among providers can be improved if the admitting clinician documents clearly whether each preadmission medication is being continued, changed, or stopped, along with the reason for doing so, and makes this information available throughout the hospital stay. Upon transfer to another unit and at discharge, the physician should review each; preadmission medication that was held and the patient’s current clinical status and, based on that information, decide whether medications that were held should be resumed. If a medication will be restarted later, specific instructions should be documented and communicated to the patient and the physicians who are taking over his or her care.
Preventing errors of commission
Failure to perform a complete reconciliation at each transition of care and match each medication with an appropriate indication can lead to errors of commission.
One study showed that 44% of patients were prescribed at least one unnecessary drug at hospital discharge, one-fourth of which were started during the hospitalization.27 Commonly prescribed unnecessarily were gastrointestinal agents, central nervous system drugs, nutrients, and supplements.
It is critical to assess each medication’s ongoing need, appropriateness, and risk-benefit ratio at every transition. Medications no longer indicated should be discontinued in order to simplify the regimen, avoid unnecessary drug exposure, and prevent ADEs.
For example, proton pump inhibitors or histamine 2 receptor blockers are often started in the hospital for stress ulcer prophylaxis. One-third of patients are then discharged home on the medication, and 6 months later half of those patients are still taking the unnecessary drug.28 This situation can be avoided by limiting use of these medications to appropriate circumstances, clearly marking the indication as stress ulcer prophylaxis (as opposed to an ongoing condition that will require continuing it after discharge), and discontinuing the agent when appropriate.
All drugs, even common and seemingly benign ones, carry some risk and should be discontinued when no longer needed. Thus, medications added during the hospitalization to control acute symptoms should also be reviewed at each transition to prevent inappropriate continuation when symptoms have resolved.
One study, for example, found that many patients were discharged with inappropriate prescriptions for atypical antipsychotics after receiving them in the intensive care unit, likely for delirium.29 Documenting that an acute issue such as delirium has resolved should prompt the discontinuation of therapy.
Preventing therapeutic duplication
Therapeutic duplication occurs in about 8% of discharges.1 These errors often result from formulary substitutions or altering the dosage form in the acute setting. For example, patients who receive a prescription for the substituted agent at discharge and also resume their prehospitalization medications end up with duplicate therapy.
Therapeutic substitution is common at the time of admission to the hospital as a result of formulary restrictions. Drug classes that are frequently substituted include statins, antihypertensives, urinary antispasmodics, and proton pump inhibitors. Physicians should be familiar with the preferred agents on the hospital formulary and make careful note when a substitution occurs. Furthermore, hospital systems should be developed to remind the physician to switch back to the outpatient medication at discharge.
Similar problems occur when home medications are replaced with different dosage forms with different pharmacokinetic properties. For example, a long-acting medication may be temporarily replaced with an intravenous solution or immediate-release tablet for several reasons, including nothing-by-mouth status, unstable clinical condition, need for titration, and need to crush the tablet to give the drug per tube. The differing formulations must be reconciled throughout the patient’s hospital course and at discharge to avoid therapeutic duplication and serious medication errors. Deliberate changes to the dosage form should be clearly communicated in the discharge medication list so that patients and other clinicians are aware.
Hospital systems should also have the capability to identify duplications in the medication list and to warn prescribers of these errors. The ability to group medications by drug class or sort the medication list alphabetically by generic name can help uncover duplication errors.
STEP 3: REVIEW THE LIST IN VIEW OF THE CLINICAL PICTURE
Transitions of care should prompt providers to review the medication list for possible drug-disease interactions, confirm compliance with evidence-based guidelines, and evaluate the risks and benefits of each medication in the context of the patient’s age and acute and chronic medical issues. This is also an opportunity to screen the full list for potentially inappropriate medications and high-alert drugs such as insulin or anticoagulants, which are more likely than other drugs to cause severe harm when used in error.
Acute kidney injury. New drug-disease interactions can arise during a hospitalization and can affect dosing and the choice of drug. The onset of acute kidney injury, for example, often necessitates adjusting or discontinuing nephrotoxic and renally excreted medications. ADEs or potential ADEs have been reported in 43% of hospitalized patients with acute kidney injury.30
Because acute kidney injury is often transient, medications may need to be held or adjusted several times until renal function stabilizes. This can be challenging across the continuum of care and requires close monitoring of the serum creatinine level and associated drug doses and levels, if applicable. Well-designed clinical decision support tools can integrate laboratory data and alert the prescriber to a clinically important increase or decrease in serum creatinine that may warrant a change in therapy. Modifications to the regimen and a plan for timely follow-up of the serum creatinine level should be clearly documented in the discharge plan.
Liver disease. Similar attention should be given to drugs that are hepatically metabolized if a patient has acute or chronic liver impairment.
Geriatric patients, particularly those who present with altered mental status, falls, or urinary retention, should have their medication list reviewed for potentially inappropriate medications, which are drugs that pose increased risk of poor outcomes in older adults.31,32 Patients and providers may have been willing to accept the risk of medications such as anticholinergics or sedative-hypnotics when the drugs were initiated, but circumstances can change over time, especially in this patient population. Hospitalization is a prime opportunity to screen for medications that meet the Beers criteria31 for agents to avoid or use with caution in older adults.
As-needed medications. Medications prescribed on an as-needed basis in the hospital should be reviewed for continuation or discontinuation at discharge. How often the medication was given can inform this decision.
For example, if as-needed opioids were used frequently, failure to develop a plan of care for pain can lead to persistent symptoms and, possibly, to readmission.33,34 Similar scenarios occur with use of as-needed blood pressure medications, laxatives, and correction-dose insulin.
If an as-needed medication was used consistently during hospitalization, the physician should consider whether a regularly scheduled medication is needed. Conversely, if the medication was not used during the inpatient admission, it can likely be discontinued.
STEP 4: PREPARE THE PATIENT AND FOLLOW-UP PROVIDER
Once a clinician has performed medication reconciliation, including obtaining a best-possible medical history and carefully reviewing the medication list and orders for errors and clinical appropriateness, the next steps are to ensure the patient understands what he or she needs to do and to confirm that suitable follow-up plans are in place. These measures should be taken at all transitions of care but are critically important at hospital discharge.
Preparing the patient and caregiver
An accurate, reconciled medication list should be given to the patient, caregiver, or both, and should be reviewed before discharge.17
Approximately one-third of Americans have low health literacy skills, so medication lists and associated materials should be easy to understand.35 Medication lists should be written in plain language and formatted for optimal readability (Table 4), clearly stating which medications to continue, change, hold temporarily, and stop.
Patients recall and comprehend about half of the information provided during a medical encounter.36 Thus, medication teaching should focus on key points including changes or additions to the regimen, specific instructions for follow-up and monitoring, and how to handle common and serious side effects.
To confirm patient understanding, clinicians should use “teach-back,” ie, provide the patient with information and then ask him or her to repeat back key points.37,38 The patient and family should also be encouraged to ask questions before discharge.
If not already addressed during the hospital stay, barriers to medication adherence and ability to obtain the medications should be attended to at this time (Table 5). Also, the plan to pick up the medications should be verified with the patient and caregiver. Verify that there is transportation to a particular pharmacy that is open at the time of discharge, and that the patient can afford the medications.
Ensuring appropriate follow-up
Studies have shown that timely in-home or telephone follow-up after discharge can decrease adverse events and postdischarge health care utilization.39,40 Telephone follow-up that includes thorough medication reconciliation can help detect and resolve medication issues early after discharge and can close gaps related to monitoring and follow-up.
Medication reconciliation by telephone can be time-consuming. Depending on the number of medications that need to be reviewed, calls can take between 10 and 60 minutes. Postdischarge phone calls should be performed by clinical personnel who are able to identify medication-related problems. A pharmacist should be an available resource to assist with complex regimens, to help resolve medication discrepancies, and to address patient concerns. Table 6 provides tips for conducting follow-up phone calls.
Resolving discrepancies identified during follow-up calls can be difficult, as changes to the medication regimen are often not communicated effectively to other members of the care team. Physicians should document the complete medication list and plan in the discharge summary, and there should be a method for the caller to record updates to the medication list in the medical record so that they are apparent at the outpatient follow-up visit.
An additional challenge is that it is frequently unclear which physician “owns” which medications. Therefore, designating a contact person for each medication until follow-up can be very valuable. At a minimum, a “physician owner” for high-alert medications such as insulin, anticoagulants, and diuretics should be identified to provide close follow-up, titration, and monitoring.
There should also be a plan for the patient to obtain refills of essential long-term medications, such as antiplatelet agents following stent placement.
SUMMARY AND RECOMMENDATIONS
Medication-related problems during hospital admission and discharge are common and range from minor discrepancies in the medication list to errors in history-taking, prescribing, and reconciliation that can lead to potential or actual patient harm. Putting systems in place to facilitate medication reconciliation can decrease the occurrence of medication discrepancies and ADEs, thereby improving patient safety during these critical transitions between care settings and providers. Institutional medication reconciliation programs should focus resources on the admission history-taking step, target the highest-risk patients for the most intensive interventions, and involve pharmacy personnel when possible.
On an individual level, clinicians can incorporate additional interventions into their workflows to optimize medication safety for hospitalized patients. Using a structured approach to obtain a complete and accurate medication list at the time of hospital admission will help providers identify medication-related problems and prevent the propagation of errors throughout the hospital stay and at discharge. Focusing additional time and effort on a comprehensive review of the medication list for errors of omission and commission, patient-specific needs, and high-alert drugs will further decrease the risk of medication errors. Finally, providing discharge counseling targeting patient barriers to adherence and ensuring a proper handover of medication information and rationale for medication changes to outpatient providers will improve the chances of a safe transition.
- Coleman EA, Smith JD, Raha D, Min SJ. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med 2005; 165:1842–1847.
- Wong JD, Bajcar JM, Wong GG, et al. Medication reconciliation at hospital discharge: evaluating discrepancies. Ann Pharmacother 2008; 42:1373–1379.
- Kripalani S, Roumie CL, Dalal AK, et al; PILL-CVD (Pharmacist Intervention for Low Literacy in Cardiovascular Disease) Study Group. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med 2012; 157:1-10.
- Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med 2003; 138:161–167.
- Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. Adverse drug events occurring following hospital discharge. J Gen Intern Med 2005; 20:317–323.
- Johnson JA, Bootman JL. Drug-related morbidity and mortality. A cost-of-illness model. Arch Intern Med 1995; 155:1949–1956.
- Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med 2011; 365:2002–2012.
- Bates DW, Boyle DL, Vander Vliet MB, Schneider J, Leape L. Relationship between medication errors and adverse drug events. J Gen Intern Med 1995;10:199–205.
- Pippins JR, Gandhi TK, Hamann C, et al. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med 2008; 23:1414–1422.
- Lau HS, Florax C, Porsius AJ, De Boer A.The completeness of medication histories in hospital medical records of patients admitted to general internal medicine wards. Br J Clin Pharmacol 2000; 49:597–603.
- Tam VC, Knowles SR, Cornish PL, Fine N, Marchesano R, Etchells EE. Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. CMAJ 2005; 173:510–515.
- Bell CM, Brener SS, Gunraj N, et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA 2011; 306:840–847.
- Dobranski S, Hammond I, Khan G, Holdsworth H. The nature of hospital prescribing errors. Br J Clin Governance 2002; 7:187–193.
- Gleason KM, Groszek JM, Sullivan C, Rooney D, Barnard C, Noskin GA. Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients. Am J Health Syst Pharm 2004; 61:1689–1695.
- Rozich JD, Resar KR. Medication safety: one organization’s approach to the challenge. J Clin Outcomes Manage 2001; 8:27–34.
- Mueller SK, Sponsler KC, Kripalani S, Schnipper JL. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med 2012; 172:1057–1069.
- Joint Commission. Using medication reconciliation to prevent errors. Sentinel Event Alert 2006, Issue 35. www.jointcommission.org/assets/1/18/SEA_35.pdf. Accessed March 31, 2015.
- Greenwald JL, Halasyamani L, Greene J, et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med 2010; 5:477–485.
- Berwick DM, Calkins DR, McCannon CJ, Hackbarth AD. The 100,000 lives campaign: setting a goal and a deadline for improving health care quality. JAMA 2006; 295:324–327.
- McCannon CJ, Hackbarth AD, Griffin FA. Miles to go: an introduction to the 5 Million Lives Campaign. Jt Comm J Qual Patient Saf 2007; 33:477–484.
- Leotsakos A, Caisley L, Karga M, Kelly E, O’Leary D, Timmons K. High 5s: addressing excellence in patient safety. World Hosp Health Serv 2009; 45:19–22.
- Gleason KM, Brake H, Agramonte V, Perfetti C. Medications at Transitions and Clinical Handoffs (MATCH) Toolkit for Medication Reconciliation. www.ahrq.gov/professionals/quality-patient-safety/patient-safety-resources/resources/match/match.pdf. Accessed March 31, 2015.
- Mueller SK, Kripalani S, Stein J, et al. A toolkit to disseminate best practices in inpatient medication reconciliation: multi-center medication reconciliation quality improvement study (MARQUIS). Jt Comm J Qual Patient Saf 2013; 39:371–382.
- Pal A, Babbott S, Wilkinson ST. Can the targeted use of a discharge pharmacist significantly decrease 30-day readmissions? Hosp Pharm 2013; 48:380–388.
- Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther 2001; 23:1296–1310.
- Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005; 353:487–497.
- Hajjar ER, Hanlon JT, Sloane RJ, et al. Unnecessary drug use in frail older people at hospital discharge. J Am Geriatr Soc 2005; 53:1518–1523.
- Zink DA, Pohlman M, Barnes M, Cannon ME. Long-term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther 2005; 21:1203–1209.
- Morandi A, Vasilevskis E, Pandharipande PP, et al. Inappropriate medication prescriptions in elderly adults surviving an intensive care unit hospitalization. J Am Geriatr Soc 2013; 61:1128–1134.
- Cox ZL, McCoy AB, Matheny ME, et al. Adverse drug events during AKI and its recovery. Clin J Am Soc Nephrol 2013; 8:1070–1078.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012; 60:616–631.
- Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers’ criteria. Age Ageing 2008; 37:673–679.
- Tjia J, Bonner A, Briesacher BA, McGee S, Terrill E, Miller K. Medication discrepancies upon hospital to skilled nursing facility transitions. J Gen Intern Med 2009; 24:630–635.
- Boockvar K, Fishman E, Kyriacou CK, Monias A, Gavi S, Cortes T. Adverse events due to discontinuations in drug use and dose changes in patients transferred between acute and long-term care facilities. Arch Intern Med 2004; 164:545–550.
- Kutner M, Greenberg E, Jin Y, Paulsen C. The Health Literacy of America’s Adults: Results From the 2003 National Assessment of Adult Literacy. http://nces.ed.gov/pubs2006/2006483_1.pdf. Accessed March 31, 2015.
- Crane JA. Patient comprehension of doctor-patient communication on discharge from the emergency department. J Emerg Med 1997; 15:1–7.
- DeWalt DA, Callahan LF, Hawk VH, et al. Health Literacy Universal Precautions Toolkit. www.ahrq.gov/qual/literacy/healthliteracytoolkit.pdf. Accessed March 31, 2015.
- Schillinger D, Piette J, Grumbach K, et al. Closing the loop: physician communication with diabetic patients who have low health literacy. Arch Intern Med 2003; 163:83–90.
- Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med 2006; 166:1822–1828.
- Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med 2009; 150:178–187.
- Coleman EA, Smith JD, Raha D, Min SJ. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med 2005; 165:1842–1847.
- Wong JD, Bajcar JM, Wong GG, et al. Medication reconciliation at hospital discharge: evaluating discrepancies. Ann Pharmacother 2008; 42:1373–1379.
- Kripalani S, Roumie CL, Dalal AK, et al; PILL-CVD (Pharmacist Intervention for Low Literacy in Cardiovascular Disease) Study Group. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med 2012; 157:1-10.
- Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med 2003; 138:161–167.
- Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. Adverse drug events occurring following hospital discharge. J Gen Intern Med 2005; 20:317–323.
- Johnson JA, Bootman JL. Drug-related morbidity and mortality. A cost-of-illness model. Arch Intern Med 1995; 155:1949–1956.
- Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med 2011; 365:2002–2012.
- Bates DW, Boyle DL, Vander Vliet MB, Schneider J, Leape L. Relationship between medication errors and adverse drug events. J Gen Intern Med 1995;10:199–205.
- Pippins JR, Gandhi TK, Hamann C, et al. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med 2008; 23:1414–1422.
- Lau HS, Florax C, Porsius AJ, De Boer A.The completeness of medication histories in hospital medical records of patients admitted to general internal medicine wards. Br J Clin Pharmacol 2000; 49:597–603.
- Tam VC, Knowles SR, Cornish PL, Fine N, Marchesano R, Etchells EE. Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. CMAJ 2005; 173:510–515.
- Bell CM, Brener SS, Gunraj N, et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA 2011; 306:840–847.
- Dobranski S, Hammond I, Khan G, Holdsworth H. The nature of hospital prescribing errors. Br J Clin Governance 2002; 7:187–193.
- Gleason KM, Groszek JM, Sullivan C, Rooney D, Barnard C, Noskin GA. Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients. Am J Health Syst Pharm 2004; 61:1689–1695.
- Rozich JD, Resar KR. Medication safety: one organization’s approach to the challenge. J Clin Outcomes Manage 2001; 8:27–34.
- Mueller SK, Sponsler KC, Kripalani S, Schnipper JL. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med 2012; 172:1057–1069.
- Joint Commission. Using medication reconciliation to prevent errors. Sentinel Event Alert 2006, Issue 35. www.jointcommission.org/assets/1/18/SEA_35.pdf. Accessed March 31, 2015.
- Greenwald JL, Halasyamani L, Greene J, et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med 2010; 5:477–485.
- Berwick DM, Calkins DR, McCannon CJ, Hackbarth AD. The 100,000 lives campaign: setting a goal and a deadline for improving health care quality. JAMA 2006; 295:324–327.
- McCannon CJ, Hackbarth AD, Griffin FA. Miles to go: an introduction to the 5 Million Lives Campaign. Jt Comm J Qual Patient Saf 2007; 33:477–484.
- Leotsakos A, Caisley L, Karga M, Kelly E, O’Leary D, Timmons K. High 5s: addressing excellence in patient safety. World Hosp Health Serv 2009; 45:19–22.
- Gleason KM, Brake H, Agramonte V, Perfetti C. Medications at Transitions and Clinical Handoffs (MATCH) Toolkit for Medication Reconciliation. www.ahrq.gov/professionals/quality-patient-safety/patient-safety-resources/resources/match/match.pdf. Accessed March 31, 2015.
- Mueller SK, Kripalani S, Stein J, et al. A toolkit to disseminate best practices in inpatient medication reconciliation: multi-center medication reconciliation quality improvement study (MARQUIS). Jt Comm J Qual Patient Saf 2013; 39:371–382.
- Pal A, Babbott S, Wilkinson ST. Can the targeted use of a discharge pharmacist significantly decrease 30-day readmissions? Hosp Pharm 2013; 48:380–388.
- Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther 2001; 23:1296–1310.
- Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005; 353:487–497.
- Hajjar ER, Hanlon JT, Sloane RJ, et al. Unnecessary drug use in frail older people at hospital discharge. J Am Geriatr Soc 2005; 53:1518–1523.
- Zink DA, Pohlman M, Barnes M, Cannon ME. Long-term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther 2005; 21:1203–1209.
- Morandi A, Vasilevskis E, Pandharipande PP, et al. Inappropriate medication prescriptions in elderly adults surviving an intensive care unit hospitalization. J Am Geriatr Soc 2013; 61:1128–1134.
- Cox ZL, McCoy AB, Matheny ME, et al. Adverse drug events during AKI and its recovery. Clin J Am Soc Nephrol 2013; 8:1070–1078.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012; 60:616–631.
- Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers’ criteria. Age Ageing 2008; 37:673–679.
- Tjia J, Bonner A, Briesacher BA, McGee S, Terrill E, Miller K. Medication discrepancies upon hospital to skilled nursing facility transitions. J Gen Intern Med 2009; 24:630–635.
- Boockvar K, Fishman E, Kyriacou CK, Monias A, Gavi S, Cortes T. Adverse events due to discontinuations in drug use and dose changes in patients transferred between acute and long-term care facilities. Arch Intern Med 2004; 164:545–550.
- Kutner M, Greenberg E, Jin Y, Paulsen C. The Health Literacy of America’s Adults: Results From the 2003 National Assessment of Adult Literacy. http://nces.ed.gov/pubs2006/2006483_1.pdf. Accessed March 31, 2015.
- Crane JA. Patient comprehension of doctor-patient communication on discharge from the emergency department. J Emerg Med 1997; 15:1–7.
- DeWalt DA, Callahan LF, Hawk VH, et al. Health Literacy Universal Precautions Toolkit. www.ahrq.gov/qual/literacy/healthliteracytoolkit.pdf. Accessed March 31, 2015.
- Schillinger D, Piette J, Grumbach K, et al. Closing the loop: physician communication with diabetic patients who have low health literacy. Arch Intern Med 2003; 163:83–90.
- Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med 2006; 166:1822–1828.
- Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med 2009; 150:178–187.
KEY POINTS
- Institutional medication reconciliation programs should include taking a best-possible medication history at admission, intervening when patients are at high risk, and involving pharmacy staff when possible.
- Clinicians can incorporate additional interventions into their workflows to optimize medication safety for hospitalized patients.
- Reviewing the medication list for errors of omission and commission, patient-specific needs, and “high-alert” drugs further decreases the risk of medication errors.
- At discharge, patients should receive counseling to ensure understanding of medications and follow-up plans. Hospital physicians should communicate with outpatient providers about medications and rationales for medication changes.
The Surviving Sepsis Campaign: Where have we been and where are we going?
Sepsis is familiar to most physicians in clinical practice, but guidance from the medical literature on how best to manage it has traditionally been confusing.
Starting in 2002, the Surviving Sepsis Campaign has worked to reduce worldwide mortality from severe sepsis and septic shock by developing and publicizing guidelines of best practices based on evidence from the literature. The campaign published its first management guidelines in 2004.
In this article, I review the most recent guidelines1,2 (published in 2013) and discuss the campaign’s ongoing performance-improvement program.
DEFINING SEPSIS
Sepsis is a known or suspected infection plus systemic manifestations of infection. This includes the sepsis inflammatory response syndrome. Criteria include:
- Tachycardia (heart rate > 90 beats per minute)
- Tachypnea (> 20 breaths/minute or Paco2 < 32 mm Hg)
- Fever (temperature > 38.3°C [100.9°F]) or hypothermia (core temperature < 36°C [96.8°F])
- High or low white blood cell count (> 12.0 × 109/L or < 4.0 × 109/L), or a normal count with more than 10% immature cells.
The definition of sepsis was broadened in 2002 to include other systemic manifestations of infection, such as changes in blood glucose level and organ dysfunction.
Severe sepsis is defined as sepsis plus either acute organ dysfunction or tissue hypoperfusion due to infection, with tissue hypoperfusion defined as:
- Hypotension (systolic blood pressure < 90 mm Hg, or a drop in systolic blood pressure of > 40 mm Hg)
- Elevated lactate
- Low urine output
- Altered mental status.
In severe sepsis, organ dysfunction is caused by blood-borne toxins and involves acute lung and kidney injury, coagulopathy (thrombocytopenia and increased international normalized ratio), and liver dysfunction.
Septic shock is present when a patient requires vasopressors after adequate intravascular volume repletion.
SEPSIS IS DEADLY AND COSTLY
Severe sepsis is the leading cause of hospital death. Patients admitted with severe sepsis are eight times more likely to die than those admitted with other conditions.3 The economic burden is enormous: it is the most expensive condition treated in US hospitals, costing an estimated $20.3 billion in 2011, of which $12.7 billion came from Medicare.
THE SURVIVING SEPSIS CAMPAIGN
The Surviving Sepsis Campaign is a global effort to reduce the rate of death from severe sepsis. The campaign’s methods include:
- Educating physicians, the public, the media, and government about the high rates of morbidity and death in severe sepsis
- Creating evidence-based guidelines for managing sepsis and establishing global best-practice standards
- Facilitating the transfer of knowledge by developing performance-improvement programs to change bedside practice.
The campaign is funded with a grant from the Gordon and Betty Moore Foundation. The campaign’s guidelines are not associated with any direct or indirect industry support. The 2013 guidelines were backed by 30 international organizations.1,2
All recommendations are ranked with numerical and letter scores, according to the GRADE system: 1 indicates a strong recommendation and 2 a weak one. The letters A through D reflect the quality of evidence, ranging from high (A) to very low (D).
GIVING ANTIBIOTICS EARLY IMPROVES OUTCOMES
A number of studies have suggested that starting appropriate antibiotics early improves outcomes in severe sepsis and septic shock. The death rate increases with each hour of delay.4
Recommendation. Intravenous antibiotic therapy should be started as soon as possible, and within the first hour after recognition of septic shock (grade 1B) and severe sepsis without septic shock (grade 1C).
The feasibility of achieving this goal has not been scientifically validated, and the recommendation should not be misinterpreted as the current standard of care. Even hospitals that participate in performance-improvement programs often struggle to start antibiotics, even within 6 hours of recognition. Nevertheless, the goal is a good one.
Some have questioned the early antibiotic recommendation because of concerns about antibiotic overuse and resistance. For a patient with some manifestation of systemic inflammation, such as organ dysfunction or hypotension with no clear cause, the campaign’s position is to provide empiric antibiotics early and then, if a noninfectious cause is found, to stop the antibiotics. Moreover, as soon as a causative pathogen has been identified, the regimen should be switched to the most appropriate antimicrobial that covers the pathogen and is safe and cost-effective. Collaboration with an antimicrobial stewardship program, if available, is encouraged.
FIND THE INFECTION SOURCE PROMPTLY: SOURCE CONTROL MAY BE REQUIRED
Recommendation. A specific anatomic diagnosis of infection (eg, necrotizing soft-tissue infection, peritonitis complicated by intra-abdominal infection, cholangitis, intestinal infarction) requiring consideration of emergency source control should be confirmed or excluded as soon as possible. If needed, surgical drainage should be undertaken for source control within the first 12 hours after a diagnosis is made (grade 1C).
FLUID THERAPY: CRYSTALLOIDS FIRST
Recommendation. In fluid resuscitation of severe sepsis, use crystalloids first (grade 1B).
No head-to-head trial has shown albumin to be superior to crystalloids, and crystalloids are less expensive. However, normal saline has a higher chloride content than plasma, which leads to non-anion-gap metabolic acidosis. It is called an unbalanced crystalloid, having a high chloride content and no buffer. There is concern that this reduces renal blood flow and the glomerular filtration rate, creating the potential for acute kidney injury. Although no high-level evidence supports this concern, some animal studies and historical control studies suggest that a balanced crystalloid such as Ringer’s lactate, Ringer’s acetate, or PlasmaLyte (having a chloride content close to that of plasma and the buffers acetate or lactate) may be associated with better outcome in resuscitation of severe sepsis.
Use albumin solution if necessary
Recommendation. Albumin should be used in the fluid resuscitation of severe sepsis and septic shock for patients who require substantial amounts of crystalloids (grade 2C).
Finfer et al5 compared the effect of fluid resuscitation with either an albumin or saline solution in nearly 7,000 patients in intensive care and found that death rates over 28 days were nearly identical between the two groups. Although this study was not designed to measure an effect in subsets of patients, the subgroup with severe sepsis had a lower mortality rate with albumin (relative risk 0.87, 95% confidence interval 0.74–1.02). In a meta-analysis of 17 studies of albumin vs crystalloids or albumin vs saline, Delaney et al6 found a significant survival advantage with an albumin solution in patients with sepsis and severe septic shock.
Sometimes, in patients admitted to intensive care with septic shock and receiving two or three vasopressors and large amounts of a crystalloid solution, vasopressors can be reduced when fluid is being given, but as soon as the fluid infusion rate is decreased, the need for increasing vasopressors returns. This scenario is an indication for changing to an albumin solution.
Recommendation. Initial fluid challenge in sepsis-induced tissue hypoperfusion (as evidenced by hypotension or elevated lactate) with suspicion of hypovolemia should be a minimum of 30 mL/kg of crystalloids, a portion of which can be an albumin equivalent. Some patients require more rapid administration and greater amounts of fluid (grade 1B).
Other fluid resuscitation considerations
Recommendation. Hydroxyethyl starch (hetastarch) should not be used for fluid resuscitation of severe sepsis and septic shock (grade 1B).
Five large clinical trials7–11 compared hetastarch with crystalloids in the resuscitation of severe sepsis or septic shock. None found an advantage to using hetastarch, and three found it to be associated with higher rates of acute kidney injury and renal-replacement therapy.
Blood is not considered a resuscitation fluid.
Full fluid replacement is still needed in heart or kidney disease
Often, doctors hesitate to administer full fluid resuscitation to patients with septic shock or sepsis-induced hypotension who have baseline cardiomyopathy with a low ejection fraction or who have end-stage renal disease and are anuric. However, these patients’ baseline intravascular volume status has changed because of venodilation and capillary leak leading to reduced blood return to the heart. They require the same amount of fluids as other patients to return to their baseline state.
To avoid fluid overload in these patients, however, we recommend providing fluid in smaller boluses. For a young, previously healthy patient, 2 L of crystalloid should be provided as quickly as possible. Patients with heart or kidney disease should receive smaller (250- or 500-mL) boluses, with oxygen saturation checked after each dose, as hypoxemia is one of only two potential downsides of aggressive fluid resuscitation (the other being the further raising of intra-abdominal pressure in the intra-abdominal compartment syndrome).
WHAT DRIVES HYPOTENSION IN SEPTIC SHOCK?
In septic shock, mechanisms that can lower the blood pressure include capillary leakage (loss of intravascular volume), decreased arteriolar resistance, decreased cardiac contractility, increased ventricular compliance, and increased venous capacitance (loss of intra-arterial volume).
Capillary leakage ranges from moderate to severe, and it is difficult to know the severity early on during resuscitation. The extent of capillary leakage is often apparent only after 24 hours of fluid resuscitation, when the large amount of fluid needed to maintain intravascular volume produces significant tissue edema. Within the first 24 hours of resuscitation of a patient with septic shock or in the presence of ongoing inflammation, one cannot use intake and output to judge the adequacy of fluid resuscitation.
Reduced arteriolar resistance may be an advantage in the nonhypotensive severely septic patient, compensating for the decreased ejection fraction, but it becomes problematic in the presence of hypotension. In addition, venodilation increases venous capacitance, producing a “sink” for blood and inadequate return of blood volume to the heart.
Decreased contractility of the left and right ventricles leads to compensatory sinus tachycardia.12 Reduced heart contractility can be seen by radionuclide angiography: little difference in chamber size is apparent in systole (immediately before contraction) vs diastole (immediately after contraction) (Figure 1).
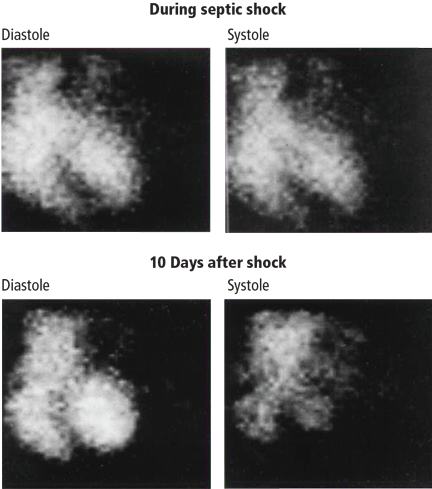
NOREPINEPHRINE IS THE FIRST-CHOICE VASOPRESSOR
If a patient remains hypotensive after replacement of intravascular volume, the hypotension is due to a combination of vasodilation and reduced contractility, and a combined inotrope-vasopressor is an appropriate drug to raise blood pressure. Therefore, the drug of first choice for raising blood pressure should be a combined inotrope-vasopressor.
There are three combined inotrope-vasopressors: dopamine, norepinephrine, and epinephrine. Head-to-head comparisons of norepinephrine and dopamine have supported a survival advantage with norepinephrine in patients with shock, including septic shock.13 A meta-analysis of six randomized trials totaling 2,768 patients also supports norepinephrine over dopamine in septic shock. Dopamine has been associated with a higher incidence of tachyarrhythmic events.14
Recommendations. Norepinephrine is the first choice for vasopressor therapy (grade 1B). If an additional agent is needed to maintain blood pressure, epinephrine should be added to norepinephrine (grade 2B). Alternatively, vasopressin (0.03 U/minute) can be added to norepinephrine to raise mean arterial pressure to target or to decrease the norepinephrine dose (ungraded recommendation).
Dopamine is not recommended as empiric or additive therapy for septic shock. It may be considered, however, in the presence of septic shock with sinus bradycardia.
Phenylephrine for special cases
Phenylephrine is a pure vasopressor: it decreases stroke volume and is particularly disadvantageous in patients with low cardiac output.
Recommendation. Phenylephrine is not recommended as empiric or additive therapy in the treatment of septic shock, with these exceptions (grade 1C):
- In unusual cases in which norepinephrine is associated with serious tachyarrhythmia, phenylephrine would be the least likely vasopressor to exacerbate arrhythmia
- If cardiac output is known to be high and blood pressure is persistently low
- If it is used as salvage therapy when combined inotrope-vasopressor drugs and low-dose vasopressin have failed to achieve the mean arterial pressure target.
RESUSCITATION OF SEPSIS-INDUCED TISSUE HYPOPERFUSION
A more severe form of sepsis-induced tissue hypoperfusion occurs in patients with severe sepsis, who require vasopressors after fluid challenge or have a lactate level of at least 4 mmol/L (36 mg/dL). Initial resuscitation is of utmost importance in these patients and often is done in the emergency department or regular hospital unit. These patients are targeted for “quantitative resuscitation,” ie, a protocol of fluid therapy and vasoactive agent support to achieve predefined end points.
Rivers et al15 published a landmark study of “early goal-directed therapy” targeting the early management of sepsis-induced tissue hypoperfusion (vasopressor requirement after fluid resuscitation or lactate > 4 mmol/L) and reported significant improvement in the survival rate when resuscitation was targeted to a superior vena cava oxygen saturation of 70%. Both control-group and active-treatment-group patients had central venous pressure targets of 8 mm Hg or greater. The Surviving Sepsis Campaign adopted these targets as recommendations in the original 2004 guidelines and continued through the 2013 guidelines, although the campaign’s sepsis management “bundles” that had originally included specific targets for central venous pressure and central venous oxygen saturation as above were changed in the 2013 guidelines to only measuring these variables (see discussion below).
Jones et al16 analyzed studies that involved early (within 24 hours of presentation) vs late (after 24 hours or unknown) quantitative resuscitation for sepsis-induced tissue hypoperfusion and found a significant reduction in the rate of death with early resuscitation but no difference with late resuscitation compared with standard therapy.
ALTERNATIVES TO MEASURING PRESSURE TO PREDICT RESPONSE TO FLUID
The campaign recognizes the limitation of pressure measurements to predict the response to fluid resuscitation. Some clinicians have objected to the guidelines, arguing that new bedside technology provides better information than central venous pressure or superior vena cava oxygen saturation.
It is useful to recall the Starling principle, which is based on the behavior of isolated myocardial fibrils that are put under the strain of graduated weights and then are stimulated to contract, modeling the contractility of the heart. The more the fibril is stretched, the more intense the contraction. Increased contractility explains why fluid resuscitation increases cardiac output; it is not simply a matter of increasing fluid volume in the veins. Increased volume in the left ventricle increases stretch, causing more intense contractility and higher stroke-volume cardiac output.
The guidelines are based on pressure measurements, but volume is the important measure that drives contractility. For this reason, the 2013 guidelines encourage the use of alternative measures if a hospital has the capability to assess and use them. These alternative measures include changes in pulse pressure, systolic pressure, and stroke volume during the respiratory cycle or with fluid bolus. The greater the variation in these measures, the more likely the patient will respond to additional fluid therapy.17 Normal values:
- Pulse pressure variation: < 13%
- Systolic pressure variation: < 10 mm Hg
- Stroke volume variation: < 10%.
The problem with the more sophisticated technologies is that they tend to be available only in academic centers and not at hospitals doing the critical early resuscitation of septic shock.
The serum lactate level
Measuring serum lactate levels is an alternative method for monitoring resuscitation of early septic shock. This method is widely available even with point-of-care testing. If the lactate level is elevated, quantitative resuscitation, fluids, inotropes, and oxygen delivery can be targeted to lactate clearance.
Recommendation. In patients in whom elevated lactate levels are used as a marker of tissue hypoperfusion, resuscitation should be targeted to normalize lactate as rapidly as possible (grade 2C).
STEROID THERAPY IS CONTROVERSIAL
Corticosteroid therapy for septic shock remains controversial. Although it has been deemphasized, it likely has a role in select patients.
Recommendation. Intravenous corticosteroids should not be used in adults with septic shock if adequate fluid resuscitation and vasopressor therapy restore hemodynamic stability (grade 2C). However, a patient on high doses of multiple vasopressors after adequate fluid resuscitation would likely benefit.
Recommendation. If corticosteroid therapy is used, hydrocortisone 200 mg should be given over 24 hours, preferentially by continuous intravenous infusion but alternatively 50 mg every 6 hours (grade 2D). This regimen can be continued for up to 7 days or tapered when shock resolves.
SURVIVING SEPSIS CAMPAIGN PERFORMANCE-IMPROVEMENT PROGRAM
By themselves, guidelines change bedside care very slowly. To effect change, protocols must be put in place and quality indicators must be measured. Beginning in 2005, the Surviving Sepsis Campaign converted its guidelines to selected sets of quality indicators, ie, severe sepsis bundles. The campaign published tools that hospitals could use to initiate performance improvement programs for diagnosis and management of severe sepsis and septic shock. The information was disseminated worldwide with a free software program. The program allowed data collection at the bedside to record performance with quality indicators.
In addition, the campaign requested user data so that performance could be tracked over time. In 2010, data on more than 10,000 patients in participating hospitals showed improved ability to achieve quality indicators. The longer a hospital continued the program, the better its compliance with management bundles; in addition, there was a concomitant reduction in hospital mortality rates.18
At this time, the database holds records for more than 30,000 patients. Mortality rates among campaign participants decreased from 37% in the first quarter to 26% in the 16th quarter worldwide, with a reduced relative risk of mortality of 28%.19 To assess whether background factors unrelated to campaign participation were contributing to the reduced rates, mortality rates of long-term participants were compared with those of new program participants; the finding supported the association with program participation.
Bundles revised
The campaign published updated performance bundles in the 2013 guidelines.
The 3-hour bundle remains the same. Within the first 3 hours of presentation with sepsis:
- Measure the serum lactate level.
- Obtain blood cultures before starting antibiotics.
- Start broad-spectrum antibiotics.
- Give a crystalloid (30 mL/kg) for hypotension or for lactate ≥ 4 mmol/L.
The 6-hour bundle has changed somewhat. Within 6 hours of presentation:
- If hypotension does not respond to initial fluid resuscitation, apply vasopressors to maintain mean arterial pressure ≥ 65 mm Hg.
- In the event of persistent arterial hypotension despite volume resuscitation (septic shock) or initial lactate ≥ 4 mmol/L, measure central venous pressure and central venous oxygen saturation.
- Remeasure lactate if the initial lactate level was elevated.
In light of the campaign’s recognition of alternatives to central venous pressure and central venous oxygen saturation for quantitative resuscitation targets, specific targets for these measures were not defined, allowing institutions the flexibility to base decisions on other technologies, such as inferior vena cava ultrasonography, systolic pressure variation, and changes in flow measures or estimates with fluid boluses if they have the capability.
Moreover, the second point in the 6-hour bundle is being further revised. The Protocolized Care for Early Septic Shock (ProCESS) trial20 and the Australasian Resuscitation in Sepsis Evaluation (ARISE) trial,21 both published in 2013, demonstrated that measuring central venous pressure and central venous oxygen saturation, although safe, is not necessary for successful resuscitation of patients with septic shock. Therefore, newer versions of the 6-hour bundle propose that physicians reassess intravascular volume status and tissue perfusion, after initial 30 mL/kg crystalloid administration, in the event of persistent hypotension (mean arterial pressure < 65 mm Hg, ie, vasopressor requirement) or an initial lactate level of 4 mmol/L or higher, and then document the findings. To meet the requirements, one must document either a repeat focused examination by a licensed independent practitioner (to include vital signs, cardiopulmonary, capillary refill, pulse, and skin findings) or two alternative items from the following options: central venous pressure, central venous oxygen saturation, bedside cardiovascular ultrasonography, and dynamic assessment of fluid responsiveness with passive leg-raising or fluid challenge.
Of interest, the ProCESS20 and ARISE21 trials supported early identification of septic shock, early use of antibiotics, and early aggressive fluid resuscitation as the likely reasons for the reduced mortality rates across all treatment groups in these studies.
REDUCING HOSPITAL MORTALITY RATES
Phase 3 of the campaign involves data from 30,000 patients with severe sepsis or septic shock in emergency departments (52%), medical and surgical units (35%), and critical care units (13%).
Hospital mortality rates were 28% for those who presented to the emergency department with sepsis vs 47% for those who developed it in the hospital.22 The reason for the substantial difference is unclear; possibly, diagnosis takes longer in medical and surgical units because of a lower nurse-to-patient ratio, leading to delay in diagnosis and treatment.
The finding of the greater risk of dying from sepsis in those who develop severe sepsis on medical and surgical floors has led to initiation of phase 4 of the campaign, conducted in four US-based collaborative groups in California, Illinois, New Jersey, and Florida, with 12 to 20 sites per collaborative. The collaborative is funded by the Moore Foundation and sponsored by the Society of Critical Care Medicine and the Society of Hospital Medicine. The purpose is to improve early recognition of severe sepsis through nurse screening of every patient during every shift of every day of hospitalization. The program empowers nurses to recognize and report sepsis, severe sepsis, and septic shock. The response differs depending on the hospital: some employ a rapid response or “sepsis alert,” others have a designated hospitalist on each shift who is informed, and hospitals that use private doctors may have a call-in system.
MUCH REMAINS TO BE DONE
The Surviving Sepsis Campaign has come far since the initial guidelines published in 2004. Thirty international organizations now sponsor and support the evidence-based guidelines. The sepsis performance improvement program deployed internationally has been associated with significant improvement in outcome in patients with severe sepsis.
How much of this is related to the campaign as opposed to other changes in health care cannot be clearly ascertained. In addition, how much of the Surviving Sepsis Campaign’s performance-improvement program effect is from attention to this patient group or from precise indicators is difficult to deduce. However, most experts in the field believe the Surviving Sepsis Campaign has significantly improved outcomes since its inception in 2002. Much still needs to be done as new evidence evolves.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41:580–637.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39:165–228.
- Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. HCHS Data Brief No. 62, June 2011. https://www.cdc.gov/nchs/products/databriefs/db62.htm.
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–1596.
- Finfer S, Bellomo R, Boyce N, Frency J, Myburgh J, Norton R; SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 2004; 350:2247–2256.
- Delaney AP, Dan A, McCaffrey J, et al. The role of albumin as a resuscitation fluid for patients with sepsis: a systematic review and meta-analysis. Crit Care Med 2011; 39:389–391.
- Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008; 358:125–139.
- Guidet B, Martinet O, Boulain T, et al. Assessment of haemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 vs. 0.9% NaCl fluid replacement in patients with severe sepsis: the CRYSTMAS study. Crit Care 2012; 16:R94.
- Perner A, Haase N, Guttormsen AB, et al; the 6S Trial Group and the Scandinavian Critical Care Trials Group. Hydroxyethyl starch 130.0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med 2012; 367:124–134.
- Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012; 367:1901–1911.
- Annane D, Siami S, Jaber S, et al; CRISTAL Investigators. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA 2013; 310:1809–1817.
- Dellinger RP. Cardiovascular management of septic shock. Crit Care Med 2003; 31:946–955.
- De Backer D, Biston P, Devriendt J, et al; SOAP II Investigators. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010; 362:779–789.
- De Backer D, Aldecoa C, Njimi H, Vincent JL. Dopamine versus norepinephrine in the treatment of septic shock: a meta-analysis. Crit Care Med 2012; 40:725–730.
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345:1368–1377.
- Jones AE, Brown MD, Trzeciak S, et al; Emergency Medicine Shock Research Network Investigators. The effect of a quantitative resuscitation strategy on mortality in patients with sepsis: a meta-analysis. Crit Care Med 2008; 36:2734–2739.
- Parry-Jones AJD, Pittman JAL. Arterial pressure and stroke volume variability as measurements for cardiovascular optimisation. Int J Intensive Care 2003; 2:67–72.
- Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med 2010; 38:367–374.
- Levy M, Artigas A, Phillips GS, et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis 2012; 12:919–924.
- ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014; 370:1683–1693.
- ARISE Investigators; ANZICS Clinical Trials Group, Peake SL, Delaney A, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014; 371:1496–1506.
- Levy MM, Dellinger RP, Townsend SA, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 2010; 36:222-231.
Sepsis is familiar to most physicians in clinical practice, but guidance from the medical literature on how best to manage it has traditionally been confusing.
Starting in 2002, the Surviving Sepsis Campaign has worked to reduce worldwide mortality from severe sepsis and septic shock by developing and publicizing guidelines of best practices based on evidence from the literature. The campaign published its first management guidelines in 2004.
In this article, I review the most recent guidelines1,2 (published in 2013) and discuss the campaign’s ongoing performance-improvement program.
DEFINING SEPSIS
Sepsis is a known or suspected infection plus systemic manifestations of infection. This includes the sepsis inflammatory response syndrome. Criteria include:
- Tachycardia (heart rate > 90 beats per minute)
- Tachypnea (> 20 breaths/minute or Paco2 < 32 mm Hg)
- Fever (temperature > 38.3°C [100.9°F]) or hypothermia (core temperature < 36°C [96.8°F])
- High or low white blood cell count (> 12.0 × 109/L or < 4.0 × 109/L), or a normal count with more than 10% immature cells.
The definition of sepsis was broadened in 2002 to include other systemic manifestations of infection, such as changes in blood glucose level and organ dysfunction.
Severe sepsis is defined as sepsis plus either acute organ dysfunction or tissue hypoperfusion due to infection, with tissue hypoperfusion defined as:
- Hypotension (systolic blood pressure < 90 mm Hg, or a drop in systolic blood pressure of > 40 mm Hg)
- Elevated lactate
- Low urine output
- Altered mental status.
In severe sepsis, organ dysfunction is caused by blood-borne toxins and involves acute lung and kidney injury, coagulopathy (thrombocytopenia and increased international normalized ratio), and liver dysfunction.
Septic shock is present when a patient requires vasopressors after adequate intravascular volume repletion.
SEPSIS IS DEADLY AND COSTLY
Severe sepsis is the leading cause of hospital death. Patients admitted with severe sepsis are eight times more likely to die than those admitted with other conditions.3 The economic burden is enormous: it is the most expensive condition treated in US hospitals, costing an estimated $20.3 billion in 2011, of which $12.7 billion came from Medicare.
THE SURVIVING SEPSIS CAMPAIGN
The Surviving Sepsis Campaign is a global effort to reduce the rate of death from severe sepsis. The campaign’s methods include:
- Educating physicians, the public, the media, and government about the high rates of morbidity and death in severe sepsis
- Creating evidence-based guidelines for managing sepsis and establishing global best-practice standards
- Facilitating the transfer of knowledge by developing performance-improvement programs to change bedside practice.
The campaign is funded with a grant from the Gordon and Betty Moore Foundation. The campaign’s guidelines are not associated with any direct or indirect industry support. The 2013 guidelines were backed by 30 international organizations.1,2
All recommendations are ranked with numerical and letter scores, according to the GRADE system: 1 indicates a strong recommendation and 2 a weak one. The letters A through D reflect the quality of evidence, ranging from high (A) to very low (D).
GIVING ANTIBIOTICS EARLY IMPROVES OUTCOMES
A number of studies have suggested that starting appropriate antibiotics early improves outcomes in severe sepsis and septic shock. The death rate increases with each hour of delay.4
Recommendation. Intravenous antibiotic therapy should be started as soon as possible, and within the first hour after recognition of septic shock (grade 1B) and severe sepsis without septic shock (grade 1C).
The feasibility of achieving this goal has not been scientifically validated, and the recommendation should not be misinterpreted as the current standard of care. Even hospitals that participate in performance-improvement programs often struggle to start antibiotics, even within 6 hours of recognition. Nevertheless, the goal is a good one.
Some have questioned the early antibiotic recommendation because of concerns about antibiotic overuse and resistance. For a patient with some manifestation of systemic inflammation, such as organ dysfunction or hypotension with no clear cause, the campaign’s position is to provide empiric antibiotics early and then, if a noninfectious cause is found, to stop the antibiotics. Moreover, as soon as a causative pathogen has been identified, the regimen should be switched to the most appropriate antimicrobial that covers the pathogen and is safe and cost-effective. Collaboration with an antimicrobial stewardship program, if available, is encouraged.
FIND THE INFECTION SOURCE PROMPTLY: SOURCE CONTROL MAY BE REQUIRED
Recommendation. A specific anatomic diagnosis of infection (eg, necrotizing soft-tissue infection, peritonitis complicated by intra-abdominal infection, cholangitis, intestinal infarction) requiring consideration of emergency source control should be confirmed or excluded as soon as possible. If needed, surgical drainage should be undertaken for source control within the first 12 hours after a diagnosis is made (grade 1C).
FLUID THERAPY: CRYSTALLOIDS FIRST
Recommendation. In fluid resuscitation of severe sepsis, use crystalloids first (grade 1B).
No head-to-head trial has shown albumin to be superior to crystalloids, and crystalloids are less expensive. However, normal saline has a higher chloride content than plasma, which leads to non-anion-gap metabolic acidosis. It is called an unbalanced crystalloid, having a high chloride content and no buffer. There is concern that this reduces renal blood flow and the glomerular filtration rate, creating the potential for acute kidney injury. Although no high-level evidence supports this concern, some animal studies and historical control studies suggest that a balanced crystalloid such as Ringer’s lactate, Ringer’s acetate, or PlasmaLyte (having a chloride content close to that of plasma and the buffers acetate or lactate) may be associated with better outcome in resuscitation of severe sepsis.
Use albumin solution if necessary
Recommendation. Albumin should be used in the fluid resuscitation of severe sepsis and septic shock for patients who require substantial amounts of crystalloids (grade 2C).
Finfer et al5 compared the effect of fluid resuscitation with either an albumin or saline solution in nearly 7,000 patients in intensive care and found that death rates over 28 days were nearly identical between the two groups. Although this study was not designed to measure an effect in subsets of patients, the subgroup with severe sepsis had a lower mortality rate with albumin (relative risk 0.87, 95% confidence interval 0.74–1.02). In a meta-analysis of 17 studies of albumin vs crystalloids or albumin vs saline, Delaney et al6 found a significant survival advantage with an albumin solution in patients with sepsis and severe septic shock.
Sometimes, in patients admitted to intensive care with septic shock and receiving two or three vasopressors and large amounts of a crystalloid solution, vasopressors can be reduced when fluid is being given, but as soon as the fluid infusion rate is decreased, the need for increasing vasopressors returns. This scenario is an indication for changing to an albumin solution.
Recommendation. Initial fluid challenge in sepsis-induced tissue hypoperfusion (as evidenced by hypotension or elevated lactate) with suspicion of hypovolemia should be a minimum of 30 mL/kg of crystalloids, a portion of which can be an albumin equivalent. Some patients require more rapid administration and greater amounts of fluid (grade 1B).
Other fluid resuscitation considerations
Recommendation. Hydroxyethyl starch (hetastarch) should not be used for fluid resuscitation of severe sepsis and septic shock (grade 1B).
Five large clinical trials7–11 compared hetastarch with crystalloids in the resuscitation of severe sepsis or septic shock. None found an advantage to using hetastarch, and three found it to be associated with higher rates of acute kidney injury and renal-replacement therapy.
Blood is not considered a resuscitation fluid.
Full fluid replacement is still needed in heart or kidney disease
Often, doctors hesitate to administer full fluid resuscitation to patients with septic shock or sepsis-induced hypotension who have baseline cardiomyopathy with a low ejection fraction or who have end-stage renal disease and are anuric. However, these patients’ baseline intravascular volume status has changed because of venodilation and capillary leak leading to reduced blood return to the heart. They require the same amount of fluids as other patients to return to their baseline state.
To avoid fluid overload in these patients, however, we recommend providing fluid in smaller boluses. For a young, previously healthy patient, 2 L of crystalloid should be provided as quickly as possible. Patients with heart or kidney disease should receive smaller (250- or 500-mL) boluses, with oxygen saturation checked after each dose, as hypoxemia is one of only two potential downsides of aggressive fluid resuscitation (the other being the further raising of intra-abdominal pressure in the intra-abdominal compartment syndrome).
WHAT DRIVES HYPOTENSION IN SEPTIC SHOCK?
In septic shock, mechanisms that can lower the blood pressure include capillary leakage (loss of intravascular volume), decreased arteriolar resistance, decreased cardiac contractility, increased ventricular compliance, and increased venous capacitance (loss of intra-arterial volume).
Capillary leakage ranges from moderate to severe, and it is difficult to know the severity early on during resuscitation. The extent of capillary leakage is often apparent only after 24 hours of fluid resuscitation, when the large amount of fluid needed to maintain intravascular volume produces significant tissue edema. Within the first 24 hours of resuscitation of a patient with septic shock or in the presence of ongoing inflammation, one cannot use intake and output to judge the adequacy of fluid resuscitation.
Reduced arteriolar resistance may be an advantage in the nonhypotensive severely septic patient, compensating for the decreased ejection fraction, but it becomes problematic in the presence of hypotension. In addition, venodilation increases venous capacitance, producing a “sink” for blood and inadequate return of blood volume to the heart.
Decreased contractility of the left and right ventricles leads to compensatory sinus tachycardia.12 Reduced heart contractility can be seen by radionuclide angiography: little difference in chamber size is apparent in systole (immediately before contraction) vs diastole (immediately after contraction) (Figure 1).

NOREPINEPHRINE IS THE FIRST-CHOICE VASOPRESSOR
If a patient remains hypotensive after replacement of intravascular volume, the hypotension is due to a combination of vasodilation and reduced contractility, and a combined inotrope-vasopressor is an appropriate drug to raise blood pressure. Therefore, the drug of first choice for raising blood pressure should be a combined inotrope-vasopressor.
There are three combined inotrope-vasopressors: dopamine, norepinephrine, and epinephrine. Head-to-head comparisons of norepinephrine and dopamine have supported a survival advantage with norepinephrine in patients with shock, including septic shock.13 A meta-analysis of six randomized trials totaling 2,768 patients also supports norepinephrine over dopamine in septic shock. Dopamine has been associated with a higher incidence of tachyarrhythmic events.14
Recommendations. Norepinephrine is the first choice for vasopressor therapy (grade 1B). If an additional agent is needed to maintain blood pressure, epinephrine should be added to norepinephrine (grade 2B). Alternatively, vasopressin (0.03 U/minute) can be added to norepinephrine to raise mean arterial pressure to target or to decrease the norepinephrine dose (ungraded recommendation).
Dopamine is not recommended as empiric or additive therapy for septic shock. It may be considered, however, in the presence of septic shock with sinus bradycardia.
Phenylephrine for special cases
Phenylephrine is a pure vasopressor: it decreases stroke volume and is particularly disadvantageous in patients with low cardiac output.
Recommendation. Phenylephrine is not recommended as empiric or additive therapy in the treatment of septic shock, with these exceptions (grade 1C):
- In unusual cases in which norepinephrine is associated with serious tachyarrhythmia, phenylephrine would be the least likely vasopressor to exacerbate arrhythmia
- If cardiac output is known to be high and blood pressure is persistently low
- If it is used as salvage therapy when combined inotrope-vasopressor drugs and low-dose vasopressin have failed to achieve the mean arterial pressure target.
RESUSCITATION OF SEPSIS-INDUCED TISSUE HYPOPERFUSION
A more severe form of sepsis-induced tissue hypoperfusion occurs in patients with severe sepsis, who require vasopressors after fluid challenge or have a lactate level of at least 4 mmol/L (36 mg/dL). Initial resuscitation is of utmost importance in these patients and often is done in the emergency department or regular hospital unit. These patients are targeted for “quantitative resuscitation,” ie, a protocol of fluid therapy and vasoactive agent support to achieve predefined end points.
Rivers et al15 published a landmark study of “early goal-directed therapy” targeting the early management of sepsis-induced tissue hypoperfusion (vasopressor requirement after fluid resuscitation or lactate > 4 mmol/L) and reported significant improvement in the survival rate when resuscitation was targeted to a superior vena cava oxygen saturation of 70%. Both control-group and active-treatment-group patients had central venous pressure targets of 8 mm Hg or greater. The Surviving Sepsis Campaign adopted these targets as recommendations in the original 2004 guidelines and continued through the 2013 guidelines, although the campaign’s sepsis management “bundles” that had originally included specific targets for central venous pressure and central venous oxygen saturation as above were changed in the 2013 guidelines to only measuring these variables (see discussion below).
Jones et al16 analyzed studies that involved early (within 24 hours of presentation) vs late (after 24 hours or unknown) quantitative resuscitation for sepsis-induced tissue hypoperfusion and found a significant reduction in the rate of death with early resuscitation but no difference with late resuscitation compared with standard therapy.
ALTERNATIVES TO MEASURING PRESSURE TO PREDICT RESPONSE TO FLUID
The campaign recognizes the limitation of pressure measurements to predict the response to fluid resuscitation. Some clinicians have objected to the guidelines, arguing that new bedside technology provides better information than central venous pressure or superior vena cava oxygen saturation.
It is useful to recall the Starling principle, which is based on the behavior of isolated myocardial fibrils that are put under the strain of graduated weights and then are stimulated to contract, modeling the contractility of the heart. The more the fibril is stretched, the more intense the contraction. Increased contractility explains why fluid resuscitation increases cardiac output; it is not simply a matter of increasing fluid volume in the veins. Increased volume in the left ventricle increases stretch, causing more intense contractility and higher stroke-volume cardiac output.
The guidelines are based on pressure measurements, but volume is the important measure that drives contractility. For this reason, the 2013 guidelines encourage the use of alternative measures if a hospital has the capability to assess and use them. These alternative measures include changes in pulse pressure, systolic pressure, and stroke volume during the respiratory cycle or with fluid bolus. The greater the variation in these measures, the more likely the patient will respond to additional fluid therapy.17 Normal values:
- Pulse pressure variation: < 13%
- Systolic pressure variation: < 10 mm Hg
- Stroke volume variation: < 10%.
The problem with the more sophisticated technologies is that they tend to be available only in academic centers and not at hospitals doing the critical early resuscitation of septic shock.
The serum lactate level
Measuring serum lactate levels is an alternative method for monitoring resuscitation of early septic shock. This method is widely available even with point-of-care testing. If the lactate level is elevated, quantitative resuscitation, fluids, inotropes, and oxygen delivery can be targeted to lactate clearance.
Recommendation. In patients in whom elevated lactate levels are used as a marker of tissue hypoperfusion, resuscitation should be targeted to normalize lactate as rapidly as possible (grade 2C).
STEROID THERAPY IS CONTROVERSIAL
Corticosteroid therapy for septic shock remains controversial. Although it has been deemphasized, it likely has a role in select patients.
Recommendation. Intravenous corticosteroids should not be used in adults with septic shock if adequate fluid resuscitation and vasopressor therapy restore hemodynamic stability (grade 2C). However, a patient on high doses of multiple vasopressors after adequate fluid resuscitation would likely benefit.
Recommendation. If corticosteroid therapy is used, hydrocortisone 200 mg should be given over 24 hours, preferentially by continuous intravenous infusion but alternatively 50 mg every 6 hours (grade 2D). This regimen can be continued for up to 7 days or tapered when shock resolves.
SURVIVING SEPSIS CAMPAIGN PERFORMANCE-IMPROVEMENT PROGRAM
By themselves, guidelines change bedside care very slowly. To effect change, protocols must be put in place and quality indicators must be measured. Beginning in 2005, the Surviving Sepsis Campaign converted its guidelines to selected sets of quality indicators, ie, severe sepsis bundles. The campaign published tools that hospitals could use to initiate performance improvement programs for diagnosis and management of severe sepsis and septic shock. The information was disseminated worldwide with a free software program. The program allowed data collection at the bedside to record performance with quality indicators.
In addition, the campaign requested user data so that performance could be tracked over time. In 2010, data on more than 10,000 patients in participating hospitals showed improved ability to achieve quality indicators. The longer a hospital continued the program, the better its compliance with management bundles; in addition, there was a concomitant reduction in hospital mortality rates.18
At this time, the database holds records for more than 30,000 patients. Mortality rates among campaign participants decreased from 37% in the first quarter to 26% in the 16th quarter worldwide, with a reduced relative risk of mortality of 28%.19 To assess whether background factors unrelated to campaign participation were contributing to the reduced rates, mortality rates of long-term participants were compared with those of new program participants; the finding supported the association with program participation.
Bundles revised
The campaign published updated performance bundles in the 2013 guidelines.
The 3-hour bundle remains the same. Within the first 3 hours of presentation with sepsis:
- Measure the serum lactate level.
- Obtain blood cultures before starting antibiotics.
- Start broad-spectrum antibiotics.
- Give a crystalloid (30 mL/kg) for hypotension or for lactate ≥ 4 mmol/L.
The 6-hour bundle has changed somewhat. Within 6 hours of presentation:
- If hypotension does not respond to initial fluid resuscitation, apply vasopressors to maintain mean arterial pressure ≥ 65 mm Hg.
- In the event of persistent arterial hypotension despite volume resuscitation (septic shock) or initial lactate ≥ 4 mmol/L, measure central venous pressure and central venous oxygen saturation.
- Remeasure lactate if the initial lactate level was elevated.
In light of the campaign’s recognition of alternatives to central venous pressure and central venous oxygen saturation for quantitative resuscitation targets, specific targets for these measures were not defined, allowing institutions the flexibility to base decisions on other technologies, such as inferior vena cava ultrasonography, systolic pressure variation, and changes in flow measures or estimates with fluid boluses if they have the capability.
Moreover, the second point in the 6-hour bundle is being further revised. The Protocolized Care for Early Septic Shock (ProCESS) trial20 and the Australasian Resuscitation in Sepsis Evaluation (ARISE) trial,21 both published in 2013, demonstrated that measuring central venous pressure and central venous oxygen saturation, although safe, is not necessary for successful resuscitation of patients with septic shock. Therefore, newer versions of the 6-hour bundle propose that physicians reassess intravascular volume status and tissue perfusion, after initial 30 mL/kg crystalloid administration, in the event of persistent hypotension (mean arterial pressure < 65 mm Hg, ie, vasopressor requirement) or an initial lactate level of 4 mmol/L or higher, and then document the findings. To meet the requirements, one must document either a repeat focused examination by a licensed independent practitioner (to include vital signs, cardiopulmonary, capillary refill, pulse, and skin findings) or two alternative items from the following options: central venous pressure, central venous oxygen saturation, bedside cardiovascular ultrasonography, and dynamic assessment of fluid responsiveness with passive leg-raising or fluid challenge.
Of interest, the ProCESS20 and ARISE21 trials supported early identification of septic shock, early use of antibiotics, and early aggressive fluid resuscitation as the likely reasons for the reduced mortality rates across all treatment groups in these studies.
REDUCING HOSPITAL MORTALITY RATES
Phase 3 of the campaign involves data from 30,000 patients with severe sepsis or septic shock in emergency departments (52%), medical and surgical units (35%), and critical care units (13%).
Hospital mortality rates were 28% for those who presented to the emergency department with sepsis vs 47% for those who developed it in the hospital.22 The reason for the substantial difference is unclear; possibly, diagnosis takes longer in medical and surgical units because of a lower nurse-to-patient ratio, leading to delay in diagnosis and treatment.
The finding of the greater risk of dying from sepsis in those who develop severe sepsis on medical and surgical floors has led to initiation of phase 4 of the campaign, conducted in four US-based collaborative groups in California, Illinois, New Jersey, and Florida, with 12 to 20 sites per collaborative. The collaborative is funded by the Moore Foundation and sponsored by the Society of Critical Care Medicine and the Society of Hospital Medicine. The purpose is to improve early recognition of severe sepsis through nurse screening of every patient during every shift of every day of hospitalization. The program empowers nurses to recognize and report sepsis, severe sepsis, and septic shock. The response differs depending on the hospital: some employ a rapid response or “sepsis alert,” others have a designated hospitalist on each shift who is informed, and hospitals that use private doctors may have a call-in system.
MUCH REMAINS TO BE DONE
The Surviving Sepsis Campaign has come far since the initial guidelines published in 2004. Thirty international organizations now sponsor and support the evidence-based guidelines. The sepsis performance improvement program deployed internationally has been associated with significant improvement in outcome in patients with severe sepsis.
How much of this is related to the campaign as opposed to other changes in health care cannot be clearly ascertained. In addition, how much of the Surviving Sepsis Campaign’s performance-improvement program effect is from attention to this patient group or from precise indicators is difficult to deduce. However, most experts in the field believe the Surviving Sepsis Campaign has significantly improved outcomes since its inception in 2002. Much still needs to be done as new evidence evolves.
Sepsis is familiar to most physicians in clinical practice, but guidance from the medical literature on how best to manage it has traditionally been confusing.
Starting in 2002, the Surviving Sepsis Campaign has worked to reduce worldwide mortality from severe sepsis and septic shock by developing and publicizing guidelines of best practices based on evidence from the literature. The campaign published its first management guidelines in 2004.
In this article, I review the most recent guidelines1,2 (published in 2013) and discuss the campaign’s ongoing performance-improvement program.
DEFINING SEPSIS
Sepsis is a known or suspected infection plus systemic manifestations of infection. This includes the sepsis inflammatory response syndrome. Criteria include:
- Tachycardia (heart rate > 90 beats per minute)
- Tachypnea (> 20 breaths/minute or Paco2 < 32 mm Hg)
- Fever (temperature > 38.3°C [100.9°F]) or hypothermia (core temperature < 36°C [96.8°F])
- High or low white blood cell count (> 12.0 × 109/L or < 4.0 × 109/L), or a normal count with more than 10% immature cells.
The definition of sepsis was broadened in 2002 to include other systemic manifestations of infection, such as changes in blood glucose level and organ dysfunction.
Severe sepsis is defined as sepsis plus either acute organ dysfunction or tissue hypoperfusion due to infection, with tissue hypoperfusion defined as:
- Hypotension (systolic blood pressure < 90 mm Hg, or a drop in systolic blood pressure of > 40 mm Hg)
- Elevated lactate
- Low urine output
- Altered mental status.
In severe sepsis, organ dysfunction is caused by blood-borne toxins and involves acute lung and kidney injury, coagulopathy (thrombocytopenia and increased international normalized ratio), and liver dysfunction.
Septic shock is present when a patient requires vasopressors after adequate intravascular volume repletion.
SEPSIS IS DEADLY AND COSTLY
Severe sepsis is the leading cause of hospital death. Patients admitted with severe sepsis are eight times more likely to die than those admitted with other conditions.3 The economic burden is enormous: it is the most expensive condition treated in US hospitals, costing an estimated $20.3 billion in 2011, of which $12.7 billion came from Medicare.
THE SURVIVING SEPSIS CAMPAIGN
The Surviving Sepsis Campaign is a global effort to reduce the rate of death from severe sepsis. The campaign’s methods include:
- Educating physicians, the public, the media, and government about the high rates of morbidity and death in severe sepsis
- Creating evidence-based guidelines for managing sepsis and establishing global best-practice standards
- Facilitating the transfer of knowledge by developing performance-improvement programs to change bedside practice.
The campaign is funded with a grant from the Gordon and Betty Moore Foundation. The campaign’s guidelines are not associated with any direct or indirect industry support. The 2013 guidelines were backed by 30 international organizations.1,2
All recommendations are ranked with numerical and letter scores, according to the GRADE system: 1 indicates a strong recommendation and 2 a weak one. The letters A through D reflect the quality of evidence, ranging from high (A) to very low (D).
GIVING ANTIBIOTICS EARLY IMPROVES OUTCOMES
A number of studies have suggested that starting appropriate antibiotics early improves outcomes in severe sepsis and septic shock. The death rate increases with each hour of delay.4
Recommendation. Intravenous antibiotic therapy should be started as soon as possible, and within the first hour after recognition of septic shock (grade 1B) and severe sepsis without septic shock (grade 1C).
The feasibility of achieving this goal has not been scientifically validated, and the recommendation should not be misinterpreted as the current standard of care. Even hospitals that participate in performance-improvement programs often struggle to start antibiotics, even within 6 hours of recognition. Nevertheless, the goal is a good one.
Some have questioned the early antibiotic recommendation because of concerns about antibiotic overuse and resistance. For a patient with some manifestation of systemic inflammation, such as organ dysfunction or hypotension with no clear cause, the campaign’s position is to provide empiric antibiotics early and then, if a noninfectious cause is found, to stop the antibiotics. Moreover, as soon as a causative pathogen has been identified, the regimen should be switched to the most appropriate antimicrobial that covers the pathogen and is safe and cost-effective. Collaboration with an antimicrobial stewardship program, if available, is encouraged.
FIND THE INFECTION SOURCE PROMPTLY: SOURCE CONTROL MAY BE REQUIRED
Recommendation. A specific anatomic diagnosis of infection (eg, necrotizing soft-tissue infection, peritonitis complicated by intra-abdominal infection, cholangitis, intestinal infarction) requiring consideration of emergency source control should be confirmed or excluded as soon as possible. If needed, surgical drainage should be undertaken for source control within the first 12 hours after a diagnosis is made (grade 1C).
FLUID THERAPY: CRYSTALLOIDS FIRST
Recommendation. In fluid resuscitation of severe sepsis, use crystalloids first (grade 1B).
No head-to-head trial has shown albumin to be superior to crystalloids, and crystalloids are less expensive. However, normal saline has a higher chloride content than plasma, which leads to non-anion-gap metabolic acidosis. It is called an unbalanced crystalloid, having a high chloride content and no buffer. There is concern that this reduces renal blood flow and the glomerular filtration rate, creating the potential for acute kidney injury. Although no high-level evidence supports this concern, some animal studies and historical control studies suggest that a balanced crystalloid such as Ringer’s lactate, Ringer’s acetate, or PlasmaLyte (having a chloride content close to that of plasma and the buffers acetate or lactate) may be associated with better outcome in resuscitation of severe sepsis.
Use albumin solution if necessary
Recommendation. Albumin should be used in the fluid resuscitation of severe sepsis and septic shock for patients who require substantial amounts of crystalloids (grade 2C).
Finfer et al5 compared the effect of fluid resuscitation with either an albumin or saline solution in nearly 7,000 patients in intensive care and found that death rates over 28 days were nearly identical between the two groups. Although this study was not designed to measure an effect in subsets of patients, the subgroup with severe sepsis had a lower mortality rate with albumin (relative risk 0.87, 95% confidence interval 0.74–1.02). In a meta-analysis of 17 studies of albumin vs crystalloids or albumin vs saline, Delaney et al6 found a significant survival advantage with an albumin solution in patients with sepsis and severe septic shock.
Sometimes, in patients admitted to intensive care with septic shock and receiving two or three vasopressors and large amounts of a crystalloid solution, vasopressors can be reduced when fluid is being given, but as soon as the fluid infusion rate is decreased, the need for increasing vasopressors returns. This scenario is an indication for changing to an albumin solution.
Recommendation. Initial fluid challenge in sepsis-induced tissue hypoperfusion (as evidenced by hypotension or elevated lactate) with suspicion of hypovolemia should be a minimum of 30 mL/kg of crystalloids, a portion of which can be an albumin equivalent. Some patients require more rapid administration and greater amounts of fluid (grade 1B).
Other fluid resuscitation considerations
Recommendation. Hydroxyethyl starch (hetastarch) should not be used for fluid resuscitation of severe sepsis and septic shock (grade 1B).
Five large clinical trials7–11 compared hetastarch with crystalloids in the resuscitation of severe sepsis or septic shock. None found an advantage to using hetastarch, and three found it to be associated with higher rates of acute kidney injury and renal-replacement therapy.
Blood is not considered a resuscitation fluid.
Full fluid replacement is still needed in heart or kidney disease
Often, doctors hesitate to administer full fluid resuscitation to patients with septic shock or sepsis-induced hypotension who have baseline cardiomyopathy with a low ejection fraction or who have end-stage renal disease and are anuric. However, these patients’ baseline intravascular volume status has changed because of venodilation and capillary leak leading to reduced blood return to the heart. They require the same amount of fluids as other patients to return to their baseline state.
To avoid fluid overload in these patients, however, we recommend providing fluid in smaller boluses. For a young, previously healthy patient, 2 L of crystalloid should be provided as quickly as possible. Patients with heart or kidney disease should receive smaller (250- or 500-mL) boluses, with oxygen saturation checked after each dose, as hypoxemia is one of only two potential downsides of aggressive fluid resuscitation (the other being the further raising of intra-abdominal pressure in the intra-abdominal compartment syndrome).
WHAT DRIVES HYPOTENSION IN SEPTIC SHOCK?
In septic shock, mechanisms that can lower the blood pressure include capillary leakage (loss of intravascular volume), decreased arteriolar resistance, decreased cardiac contractility, increased ventricular compliance, and increased venous capacitance (loss of intra-arterial volume).
Capillary leakage ranges from moderate to severe, and it is difficult to know the severity early on during resuscitation. The extent of capillary leakage is often apparent only after 24 hours of fluid resuscitation, when the large amount of fluid needed to maintain intravascular volume produces significant tissue edema. Within the first 24 hours of resuscitation of a patient with septic shock or in the presence of ongoing inflammation, one cannot use intake and output to judge the adequacy of fluid resuscitation.
Reduced arteriolar resistance may be an advantage in the nonhypotensive severely septic patient, compensating for the decreased ejection fraction, but it becomes problematic in the presence of hypotension. In addition, venodilation increases venous capacitance, producing a “sink” for blood and inadequate return of blood volume to the heart.
Decreased contractility of the left and right ventricles leads to compensatory sinus tachycardia.12 Reduced heart contractility can be seen by radionuclide angiography: little difference in chamber size is apparent in systole (immediately before contraction) vs diastole (immediately after contraction) (Figure 1).

NOREPINEPHRINE IS THE FIRST-CHOICE VASOPRESSOR
If a patient remains hypotensive after replacement of intravascular volume, the hypotension is due to a combination of vasodilation and reduced contractility, and a combined inotrope-vasopressor is an appropriate drug to raise blood pressure. Therefore, the drug of first choice for raising blood pressure should be a combined inotrope-vasopressor.
There are three combined inotrope-vasopressors: dopamine, norepinephrine, and epinephrine. Head-to-head comparisons of norepinephrine and dopamine have supported a survival advantage with norepinephrine in patients with shock, including septic shock.13 A meta-analysis of six randomized trials totaling 2,768 patients also supports norepinephrine over dopamine in septic shock. Dopamine has been associated with a higher incidence of tachyarrhythmic events.14
Recommendations. Norepinephrine is the first choice for vasopressor therapy (grade 1B). If an additional agent is needed to maintain blood pressure, epinephrine should be added to norepinephrine (grade 2B). Alternatively, vasopressin (0.03 U/minute) can be added to norepinephrine to raise mean arterial pressure to target or to decrease the norepinephrine dose (ungraded recommendation).
Dopamine is not recommended as empiric or additive therapy for septic shock. It may be considered, however, in the presence of septic shock with sinus bradycardia.
Phenylephrine for special cases
Phenylephrine is a pure vasopressor: it decreases stroke volume and is particularly disadvantageous in patients with low cardiac output.
Recommendation. Phenylephrine is not recommended as empiric or additive therapy in the treatment of septic shock, with these exceptions (grade 1C):
- In unusual cases in which norepinephrine is associated with serious tachyarrhythmia, phenylephrine would be the least likely vasopressor to exacerbate arrhythmia
- If cardiac output is known to be high and blood pressure is persistently low
- If it is used as salvage therapy when combined inotrope-vasopressor drugs and low-dose vasopressin have failed to achieve the mean arterial pressure target.
RESUSCITATION OF SEPSIS-INDUCED TISSUE HYPOPERFUSION
A more severe form of sepsis-induced tissue hypoperfusion occurs in patients with severe sepsis, who require vasopressors after fluid challenge or have a lactate level of at least 4 mmol/L (36 mg/dL). Initial resuscitation is of utmost importance in these patients and often is done in the emergency department or regular hospital unit. These patients are targeted for “quantitative resuscitation,” ie, a protocol of fluid therapy and vasoactive agent support to achieve predefined end points.
Rivers et al15 published a landmark study of “early goal-directed therapy” targeting the early management of sepsis-induced tissue hypoperfusion (vasopressor requirement after fluid resuscitation or lactate > 4 mmol/L) and reported significant improvement in the survival rate when resuscitation was targeted to a superior vena cava oxygen saturation of 70%. Both control-group and active-treatment-group patients had central venous pressure targets of 8 mm Hg or greater. The Surviving Sepsis Campaign adopted these targets as recommendations in the original 2004 guidelines and continued through the 2013 guidelines, although the campaign’s sepsis management “bundles” that had originally included specific targets for central venous pressure and central venous oxygen saturation as above were changed in the 2013 guidelines to only measuring these variables (see discussion below).
Jones et al16 analyzed studies that involved early (within 24 hours of presentation) vs late (after 24 hours or unknown) quantitative resuscitation for sepsis-induced tissue hypoperfusion and found a significant reduction in the rate of death with early resuscitation but no difference with late resuscitation compared with standard therapy.
ALTERNATIVES TO MEASURING PRESSURE TO PREDICT RESPONSE TO FLUID
The campaign recognizes the limitation of pressure measurements to predict the response to fluid resuscitation. Some clinicians have objected to the guidelines, arguing that new bedside technology provides better information than central venous pressure or superior vena cava oxygen saturation.
It is useful to recall the Starling principle, which is based on the behavior of isolated myocardial fibrils that are put under the strain of graduated weights and then are stimulated to contract, modeling the contractility of the heart. The more the fibril is stretched, the more intense the contraction. Increased contractility explains why fluid resuscitation increases cardiac output; it is not simply a matter of increasing fluid volume in the veins. Increased volume in the left ventricle increases stretch, causing more intense contractility and higher stroke-volume cardiac output.
The guidelines are based on pressure measurements, but volume is the important measure that drives contractility. For this reason, the 2013 guidelines encourage the use of alternative measures if a hospital has the capability to assess and use them. These alternative measures include changes in pulse pressure, systolic pressure, and stroke volume during the respiratory cycle or with fluid bolus. The greater the variation in these measures, the more likely the patient will respond to additional fluid therapy.17 Normal values:
- Pulse pressure variation: < 13%
- Systolic pressure variation: < 10 mm Hg
- Stroke volume variation: < 10%.
The problem with the more sophisticated technologies is that they tend to be available only in academic centers and not at hospitals doing the critical early resuscitation of septic shock.
The serum lactate level
Measuring serum lactate levels is an alternative method for monitoring resuscitation of early septic shock. This method is widely available even with point-of-care testing. If the lactate level is elevated, quantitative resuscitation, fluids, inotropes, and oxygen delivery can be targeted to lactate clearance.
Recommendation. In patients in whom elevated lactate levels are used as a marker of tissue hypoperfusion, resuscitation should be targeted to normalize lactate as rapidly as possible (grade 2C).
STEROID THERAPY IS CONTROVERSIAL
Corticosteroid therapy for septic shock remains controversial. Although it has been deemphasized, it likely has a role in select patients.
Recommendation. Intravenous corticosteroids should not be used in adults with septic shock if adequate fluid resuscitation and vasopressor therapy restore hemodynamic stability (grade 2C). However, a patient on high doses of multiple vasopressors after adequate fluid resuscitation would likely benefit.
Recommendation. If corticosteroid therapy is used, hydrocortisone 200 mg should be given over 24 hours, preferentially by continuous intravenous infusion but alternatively 50 mg every 6 hours (grade 2D). This regimen can be continued for up to 7 days or tapered when shock resolves.
SURVIVING SEPSIS CAMPAIGN PERFORMANCE-IMPROVEMENT PROGRAM
By themselves, guidelines change bedside care very slowly. To effect change, protocols must be put in place and quality indicators must be measured. Beginning in 2005, the Surviving Sepsis Campaign converted its guidelines to selected sets of quality indicators, ie, severe sepsis bundles. The campaign published tools that hospitals could use to initiate performance improvement programs for diagnosis and management of severe sepsis and septic shock. The information was disseminated worldwide with a free software program. The program allowed data collection at the bedside to record performance with quality indicators.
In addition, the campaign requested user data so that performance could be tracked over time. In 2010, data on more than 10,000 patients in participating hospitals showed improved ability to achieve quality indicators. The longer a hospital continued the program, the better its compliance with management bundles; in addition, there was a concomitant reduction in hospital mortality rates.18
At this time, the database holds records for more than 30,000 patients. Mortality rates among campaign participants decreased from 37% in the first quarter to 26% in the 16th quarter worldwide, with a reduced relative risk of mortality of 28%.19 To assess whether background factors unrelated to campaign participation were contributing to the reduced rates, mortality rates of long-term participants were compared with those of new program participants; the finding supported the association with program participation.
Bundles revised
The campaign published updated performance bundles in the 2013 guidelines.
The 3-hour bundle remains the same. Within the first 3 hours of presentation with sepsis:
- Measure the serum lactate level.
- Obtain blood cultures before starting antibiotics.
- Start broad-spectrum antibiotics.
- Give a crystalloid (30 mL/kg) for hypotension or for lactate ≥ 4 mmol/L.
The 6-hour bundle has changed somewhat. Within 6 hours of presentation:
- If hypotension does not respond to initial fluid resuscitation, apply vasopressors to maintain mean arterial pressure ≥ 65 mm Hg.
- In the event of persistent arterial hypotension despite volume resuscitation (septic shock) or initial lactate ≥ 4 mmol/L, measure central venous pressure and central venous oxygen saturation.
- Remeasure lactate if the initial lactate level was elevated.
In light of the campaign’s recognition of alternatives to central venous pressure and central venous oxygen saturation for quantitative resuscitation targets, specific targets for these measures were not defined, allowing institutions the flexibility to base decisions on other technologies, such as inferior vena cava ultrasonography, systolic pressure variation, and changes in flow measures or estimates with fluid boluses if they have the capability.
Moreover, the second point in the 6-hour bundle is being further revised. The Protocolized Care for Early Septic Shock (ProCESS) trial20 and the Australasian Resuscitation in Sepsis Evaluation (ARISE) trial,21 both published in 2013, demonstrated that measuring central venous pressure and central venous oxygen saturation, although safe, is not necessary for successful resuscitation of patients with septic shock. Therefore, newer versions of the 6-hour bundle propose that physicians reassess intravascular volume status and tissue perfusion, after initial 30 mL/kg crystalloid administration, in the event of persistent hypotension (mean arterial pressure < 65 mm Hg, ie, vasopressor requirement) or an initial lactate level of 4 mmol/L or higher, and then document the findings. To meet the requirements, one must document either a repeat focused examination by a licensed independent practitioner (to include vital signs, cardiopulmonary, capillary refill, pulse, and skin findings) or two alternative items from the following options: central venous pressure, central venous oxygen saturation, bedside cardiovascular ultrasonography, and dynamic assessment of fluid responsiveness with passive leg-raising or fluid challenge.
Of interest, the ProCESS20 and ARISE21 trials supported early identification of septic shock, early use of antibiotics, and early aggressive fluid resuscitation as the likely reasons for the reduced mortality rates across all treatment groups in these studies.
REDUCING HOSPITAL MORTALITY RATES
Phase 3 of the campaign involves data from 30,000 patients with severe sepsis or septic shock in emergency departments (52%), medical and surgical units (35%), and critical care units (13%).
Hospital mortality rates were 28% for those who presented to the emergency department with sepsis vs 47% for those who developed it in the hospital.22 The reason for the substantial difference is unclear; possibly, diagnosis takes longer in medical and surgical units because of a lower nurse-to-patient ratio, leading to delay in diagnosis and treatment.
The finding of the greater risk of dying from sepsis in those who develop severe sepsis on medical and surgical floors has led to initiation of phase 4 of the campaign, conducted in four US-based collaborative groups in California, Illinois, New Jersey, and Florida, with 12 to 20 sites per collaborative. The collaborative is funded by the Moore Foundation and sponsored by the Society of Critical Care Medicine and the Society of Hospital Medicine. The purpose is to improve early recognition of severe sepsis through nurse screening of every patient during every shift of every day of hospitalization. The program empowers nurses to recognize and report sepsis, severe sepsis, and septic shock. The response differs depending on the hospital: some employ a rapid response or “sepsis alert,” others have a designated hospitalist on each shift who is informed, and hospitals that use private doctors may have a call-in system.
MUCH REMAINS TO BE DONE
The Surviving Sepsis Campaign has come far since the initial guidelines published in 2004. Thirty international organizations now sponsor and support the evidence-based guidelines. The sepsis performance improvement program deployed internationally has been associated with significant improvement in outcome in patients with severe sepsis.
How much of this is related to the campaign as opposed to other changes in health care cannot be clearly ascertained. In addition, how much of the Surviving Sepsis Campaign’s performance-improvement program effect is from attention to this patient group or from precise indicators is difficult to deduce. However, most experts in the field believe the Surviving Sepsis Campaign has significantly improved outcomes since its inception in 2002. Much still needs to be done as new evidence evolves.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41:580–637.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39:165–228.
- Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. HCHS Data Brief No. 62, June 2011. https://www.cdc.gov/nchs/products/databriefs/db62.htm.
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–1596.
- Finfer S, Bellomo R, Boyce N, Frency J, Myburgh J, Norton R; SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 2004; 350:2247–2256.
- Delaney AP, Dan A, McCaffrey J, et al. The role of albumin as a resuscitation fluid for patients with sepsis: a systematic review and meta-analysis. Crit Care Med 2011; 39:389–391.
- Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008; 358:125–139.
- Guidet B, Martinet O, Boulain T, et al. Assessment of haemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 vs. 0.9% NaCl fluid replacement in patients with severe sepsis: the CRYSTMAS study. Crit Care 2012; 16:R94.
- Perner A, Haase N, Guttormsen AB, et al; the 6S Trial Group and the Scandinavian Critical Care Trials Group. Hydroxyethyl starch 130.0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med 2012; 367:124–134.
- Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012; 367:1901–1911.
- Annane D, Siami S, Jaber S, et al; CRISTAL Investigators. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA 2013; 310:1809–1817.
- Dellinger RP. Cardiovascular management of septic shock. Crit Care Med 2003; 31:946–955.
- De Backer D, Biston P, Devriendt J, et al; SOAP II Investigators. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010; 362:779–789.
- De Backer D, Aldecoa C, Njimi H, Vincent JL. Dopamine versus norepinephrine in the treatment of septic shock: a meta-analysis. Crit Care Med 2012; 40:725–730.
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345:1368–1377.
- Jones AE, Brown MD, Trzeciak S, et al; Emergency Medicine Shock Research Network Investigators. The effect of a quantitative resuscitation strategy on mortality in patients with sepsis: a meta-analysis. Crit Care Med 2008; 36:2734–2739.
- Parry-Jones AJD, Pittman JAL. Arterial pressure and stroke volume variability as measurements for cardiovascular optimisation. Int J Intensive Care 2003; 2:67–72.
- Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med 2010; 38:367–374.
- Levy M, Artigas A, Phillips GS, et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis 2012; 12:919–924.
- ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014; 370:1683–1693.
- ARISE Investigators; ANZICS Clinical Trials Group, Peake SL, Delaney A, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014; 371:1496–1506.
- Levy MM, Dellinger RP, Townsend SA, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 2010; 36:222-231.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41:580–637.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39:165–228.
- Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. HCHS Data Brief No. 62, June 2011. https://www.cdc.gov/nchs/products/databriefs/db62.htm.
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–1596.
- Finfer S, Bellomo R, Boyce N, Frency J, Myburgh J, Norton R; SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 2004; 350:2247–2256.
- Delaney AP, Dan A, McCaffrey J, et al. The role of albumin as a resuscitation fluid for patients with sepsis: a systematic review and meta-analysis. Crit Care Med 2011; 39:389–391.
- Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008; 358:125–139.
- Guidet B, Martinet O, Boulain T, et al. Assessment of haemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 vs. 0.9% NaCl fluid replacement in patients with severe sepsis: the CRYSTMAS study. Crit Care 2012; 16:R94.
- Perner A, Haase N, Guttormsen AB, et al; the 6S Trial Group and the Scandinavian Critical Care Trials Group. Hydroxyethyl starch 130.0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med 2012; 367:124–134.
- Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012; 367:1901–1911.
- Annane D, Siami S, Jaber S, et al; CRISTAL Investigators. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA 2013; 310:1809–1817.
- Dellinger RP. Cardiovascular management of septic shock. Crit Care Med 2003; 31:946–955.
- De Backer D, Biston P, Devriendt J, et al; SOAP II Investigators. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010; 362:779–789.
- De Backer D, Aldecoa C, Njimi H, Vincent JL. Dopamine versus norepinephrine in the treatment of septic shock: a meta-analysis. Crit Care Med 2012; 40:725–730.
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345:1368–1377.
- Jones AE, Brown MD, Trzeciak S, et al; Emergency Medicine Shock Research Network Investigators. The effect of a quantitative resuscitation strategy on mortality in patients with sepsis: a meta-analysis. Crit Care Med 2008; 36:2734–2739.
- Parry-Jones AJD, Pittman JAL. Arterial pressure and stroke volume variability as measurements for cardiovascular optimisation. Int J Intensive Care 2003; 2:67–72.
- Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med 2010; 38:367–374.
- Levy M, Artigas A, Phillips GS, et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis 2012; 12:919–924.
- ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014; 370:1683–1693.
- ARISE Investigators; ANZICS Clinical Trials Group, Peake SL, Delaney A, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014; 371:1496–1506.
- Levy MM, Dellinger RP, Townsend SA, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 2010; 36:222-231.
KEY POINTS
- Ideally, intravenous antibiotic therapy should start within the first hour after sepsis is recognized; performance improvement protocols set a target of within 3 hours.
- A specific source of infection that requires source control measures should be sought, diagnosed or excluded, and if located, treated as rapidly as possible.
- Crystalloids should be used for initial fluid resuscitation. Adding an albumin-based solution is suggested for patients who require substantial amounts of crystalloids.
- Vasopressors are indicated for those who remain hypotensive despite fluid resuscitation. Norepinephrine should be used initially, and if the target mean arterial pressure cannot be achieved, then epinephrine or low-dose vasopressin is added.
- Corticosteroids should be considered only for patients who remain unstable despite adequate fluid resuscitation and vasopressor therapy.
A 57-year-old woman with abdominal pain
A 57-year-old woman presented to the emergency department with left lower quadrant pain, which had started 1 week earlier and was constant, dull, aching, and nonradiating. There were no aggravating or alleviating factors. The pain was associated with low-grade fever and nausea. She reported no vomiting, no change in bowel habits, and no hematemesis, hematochezia, or melena. She did not have urinary urgency, frequency, or dysuria. She had no cardiac, respiratory, or neurologic symptoms.
Her medical history included hypothyroidism, type 2 diabetes mellitus, diverticulosis, and chronic obstructive pulmonary disease. Her medications included metformin, insulin, levothyroxine, and inhaled tiotropium. She had no allergies. She had never undergone surgery, including cesarean delivery. She was postmenopausal. She had two children, both of whom had been born vaginally at full term. She denied using alcohol, tobacco, and illicit drugs. Her family history was noncontributory.
On examination, she was not in acute distress. Her temperature was 36.7°C (98.1°F), blood pressure 130/90 mm Hg, heart rate 86 beats per minute and regular, respiratory rate 16 breaths per minute, and oxygen saturation 98% on ambient air. Examination of her head and neck was unremarkable. Cardiopulmonary examination was normal. Abdominal examination revealed normal bowel sounds, mild tenderness in the left lower quadrant with localized guarding, and rebound tenderness. Neurologic examination was unremarkable.
Initial laboratory data showed mild leukocytosis. Computed tomography with contrast of the abdomen and pelvis suggested acute diverticulitis.
ATRIAL FIBRILLATION, AND THEN A TURN FOR THE WORSE
The patient was admitted with an initial diagnosis of acute diverticulitis. She was started on antibiotics, hydration, and pain medications, and her abdominal pain gradually improved.
On the third hospital day, she suddenly experienced shortness of breath and palpitations. At the time of admission her electrocardiogram had been normal, but it now showed atrial fibrillation with a rapid ventricular response. She also developed elevated troponin levels, which were thought to represent type 2 non-ST-elevation myocardial infarction.
She was started on aspirin, clopidogrel, and anticoagulation with heparin bridged with warfarin for the new-onset atrial fibrillation. Her heart rate was controlled with metoprolol, and her shortness of breath improved. An echocardiogram was normal.
On the seventh hospital day, she developed severe right-sided lower abdominal pain and bruising. Her blood pressure was 90/60 mm Hg, heart rate 110 beats per minute and irregularly irregular, respiratory rate 22 breaths per minute, and oxygen saturation 97% on room air. Her abdomen was diffusely tender with a palpable mass in the right lower quadrant and hypoactive bowel sounds. Ecchymosis was noted (Figure 1).
DIFFERENTIAL DIAGNOSIS
1. What is the likely cause of her decompensation?
- Acute mesenteric ischemia
- Perforation of the gastrointestinal tract
- Rectus sheath hematoma
- Abdominal compartment syndrome due to acute pancreatitis
Acute mesenteric ischemia
Signs and symptoms of acute mesenteric ischemia can be vague. Moreover, when it leads to bowel necrosis the mortality rate is high, ranging from 30% to 65%.1 Hence, one should suspect it and try to diagnose it early.
Most patients with this condition have comorbidities; risk factors include atherosclerotic disease, cardiac conditions (congestive heart failure, recent myocardial infarction, and atrial fibrillation), systemic illness, and inherited or acquired hypercoagulable states.2
The four major causes are:
- Acute thromboembolic occlusion of the superior mesenteric artery (the most common site of occlusion because of the acute angle of origin from the aorta)
- Acute thrombosis of the mesenteric vein
- Acute thrombosis of the mesenteric artery
- Nonocclusive disease affecting the mesenteric vessels2
Nonocclusive disease is seen in conditions in which the mesenteric vessels are already compromised due to background stenosis owing to atherosclerosis. Also, conditions such as septic and cardiogenic shock can compromise these arteries, leading to ischemia, which, if it persists, can lead to bowel infarction. Ischemic colitis falls under this category. It commonly involves the descending and sigmoid colon.3
The initial symptom of ischemia may be abdominal pain that is brought on by eating large meals (“postprandial intestinal angina.”2 When the ischemia worsens to infarction, patients may have a diffusely tender abdomen and constant pain that does not vary with palpation. Surprisingly, patients do not exhibit peritoneal signs early on. This gives rise to the description of “pain out of proportion to the physical findings” traditionally associated with acute mesenteric ischemia.2
Diagnosis. Supportive laboratory data include marked leukocytosis, elevated hematocrit due to hemoconcentration, metabolic acidosis, and elevated lactate.4 Newer markers such as serum alpha-glutathione S-transferase (alpha-GST) and intestinal fatty acid-binding protein (I-FABP) may be used to support the diagnosis.
Elevated alpha-GST has 72% sensitivity and 77% specificity in the diagnosis of acute mesenteric ischemia.5 The caveat is that it cannot reliably differentiate ischemia from infarction. Its sensitivity can be improved to 97% to 100% by using the white blood cell count and lactate levels in combination.5
An I-FABP level higher than 100 ng/mL has 100% sensitivity for diagnosing mesenteric infarction but only 25% sensitivity for bowel strangulation.6
Early use of abdominal computed tomography with contrast can aid in recognizing this diagnosis.7 Thus, it should be ordered in suspected cases, even in patients who have elevated creatinine levels (which would normally preclude the use of contrast), since early diagnosis followed by endovascular therapy is associated with survival benefit, and the risk of contrast-induced nephropathy appears to be small.8 Computed tomography helps to determine the state of mesenteric vessels and bowel perfusion before ischemia progresses to infarction. It also helps to rule out other common diagnoses. Findings that suggest acute mesenteric ischemia include segmental bowel wall thickening, intestinal pneumatosis with gas in the portal vein, bowel dilation, mesenteric stranding, portomesenteric thrombosis, and solid-organ infarction.9
Treatment. If superior mesenteric artery occlusion is diagnosed on computed tomography, the next step is to determine if there is peritonitis.10 In patients who have evidence of peritonitis, exploratory laparotomy is performed. For emboli in such patients, open embolectomy followed by on-table angiography is carried out in combination with damage-control surgery. For patients with peritonitis and acute thrombosis, stenting along with damage-control surgery is preferred.10
On the other hand, if there is no peritonitis, the thrombosis may be amenable to endovascular intervention. For patients with acute embolic occlusion with no contraindications to thrombolysis, aspiration embolectomy in combination with local catheter-directed thrombolysis with recombinant tissue plasminogen activator can be performed. This can be combined with endovascular mechanical embolectomy for more complete management.10 Patients with contraindications to thrombolysis can be treated either with aspiration and mechanical embolectomy or with open embolectomy with angiography.10
During laparotomy, the surgeon carefully inspects the bowel for signs of necrosis. Signs that bowel is still viable include pink color, bleeding from cut surfaces, good peristalsis, and visible pulsations in the arterial arcade of the mesentery.
Acute mesenteric artery thrombosis arising from chronic atherosclerotic disease can be treated with stenting of the stenotic lesion.10 Patients with this condition would also benefit from aggressive management of atherosclerotic disease with statins along with antiplatelet agents.10
Mesenteric vein thrombosis requires prompt institution of anticoagulation. However, in advanced cases leading to bowel infarction, exploratory laparotomy with resection of the necrotic bowel may be required. Anticoagulation should be continued for at least 6 months, and further therapy should be determined by the underlying precipitating condition.10
Critically ill patients who develop mesenteric ischemia secondary to persistent hypotension usually respond to adequate volume resuscitation, cessation of vasopressors, and overall improvement in their hemodynamic status. These patients must be closely monitored for development of gangrene of the bowel because they may be intubated and not able to complain. Any sudden deterioration in their condition should prompt physicians to consider bowel necrosis developing in these patients. Elevation of lactate levels out of proportion to the degree of hypotension may be corroborative evidence.4
Our patient had risk factors for acute mesenteric ischemia that included atrial fibrillation and recent non-ST-elevation myocardial infarction. She could have had arterial emboli due to atrial fibrillation, in situ superior mesenteric arterial thrombosis, or splanchnic arterial vasoconstriction due to the myocardial infarction associated with transient hypotension.
Arguing against this diagnosis, although she had a grossly distended abdomen, abdominal bruising usually is not seen. Also, a palpable mass in the right lower quadrant is uncommon except when acute mesenteric ischemia occurs due to segmental intestinal strangulation, as with strangulated hernia or volvulus. She also had therapeutic international normalized ratio (INR) levels constantly while on anticoagulation. Nevertheless, acute mesenteric ischemia should be strongly considered in the initial differential diagnosis of this patient’s acute decompensation.
Perforation of the gastrointestinal tract
Diverticulitis is the acute inflammation of one or more diverticula, which are small pouches created by herniation of the mucosa into the wall of the colon. The condition is caused by microscopic or macroscopic perforation of the diverticula. Microscopic perforation is usually self-limited (uncomplicated diverticulitis) and responds to conservative treatment, whereas macroscopic perforation can be associated with fecal or purulent peritonitis, abscess, enteric fistula, bowel obstruction, and stricture (complicated diverticulitis), in which case surgery may be necessary.
Patients with peritonitis due to free perforation present with generalized tenderness with rebound tenderness and guarding on abdominal examination. The abdomen may be distended and tympanic to percussion, with diminished or absent bowel sounds. Patients may have hemodynamic compromise.
Plain upright abdominal radiographs may show free air under the diaphragm. Computed tomography may show oral contrast outside the lumen and detect even small amounts of free intraperitoneal air (more clearly seen on a lung window setting).
Our patient initially presented with acute diverticulitis. She later developed diffuse abdominal tenderness with hypoactive bowel sounds. Bowel perforation is certainly a possibility at this stage, though it is usually not associated with abdominal bruising. She would need additional imaging to rule out this complication.
Other differential diagnoses to be considered in this patient with right lower-quadrant pain include acute appendicitis, incarcerated inguinal hernia, volvulus (particularly cecal volvulus), small-bowel obstruction, pyelonephritis, and gynecologic causes such as adnexal torsion, ruptured ovarian cyst, and tubo-ovarian abscess. Computed tomography helps to differentiate most of these causes.
Rectus sheath hematoma
Rectus sheath hematoma is relatively uncommon and often not considered in the initial differential diagnosis of an acute abdomen. This gives it the rightful term “a great masquerader.” It usually results from bleeding into the rectus sheath from damage to the superior (more common) or inferior epigastric arteries and occasionally from a direct tear of the rectus abdominis muscle. Predisposing factors include anticoagulant therapy (most common), advanced age, hypertension, previous abdominal surgery, trauma, paroxysmal coughing, medication injections, pregnancy, blood dyscrasias, severe vomiting, violent physical activity, and leukemia.11
Clinical manifestations include acute abdominal pain, often associated with fever, nausea, and vomiting. Physical examination may reveal signs of hypovolemic shock, a palpable nonpulsatile abdominal mass, and signs of local peritoneal irritation. The Carnett sign11 (tenderness within the abdominal wall that persists and does not improve with raising the head) and the Fothergill sign11 (a tender abdominal mass that does not cross the midline and remains palpable with tensing of the rectus sheath) may be elicited.
Computed tomography is more sensitive than abdominal ultrasonography in differentiating rectus sheath hematoma from an intra-abdominal pathology.11 In addition, computed tomography also helps to determine if the bleeding is active or not, which has therapeutic implications.
In our patient, rectus sheath hematoma is a possibility because of her ongoing anticoagulation, findings of localized abdominal bruising, and palpable right lower-quadrant mass, and it is high on the list of differential diagnoses. Rectus sheath hematoma should be considered in the differential diagnosis of lower abdominal pain particularly in elderly women who are on anticoagulation and in whom the onset of pain coincides with a paroxysm of cough.12 Women are twice as likely as men to develop rectus sheath hematoma, owing to their different muscle mass.13 In addition, anterior abdominal wall muscles are stretched during pregnancy.13
Abdominal compartment syndrome
Abdominal compartment syndrome has been classically associated with surgical patients. However, it is being increasingly recognized in critically ill medical patients, in whom detecting and treating it early may result in significant reduction in rates of morbidity and death.14
Abdominal compartment syndrome is of three types: primary, secondary, and recurrent. Primary abdominal compartment syndrome refers to the classic surgical patients with evidence of direct injury to the abdominal or pelvic organs through major trauma or extensive abdominal surgeries. Secondary abdominal compartment syndrome refers to its development in critically ill intensive care patients in whom the pathology does not directly involve the abdominal or pelvic organs.
Various medical conditions can culminate in abdominal compartment syndrome and result in multiorgan failure. Recurrent abdominal compartment syndrome refers to its development after management of either primary or secondary intra-abdominal hypertension or abdominal compartment syndrome.15 Clinicians thus must be aware of secondary and recurrent abdominal compartment syndrome occurring in critically ill patients.
The normal intra-abdominal pressure is around 5 to 7 mm Hg, even in most critically ill patients. Persistent elevation, ie, higher than 12 mm Hg, is referred to as intra-abdominal hypertension.16–18 Intra-abdominal hypertension is subdivided into four grades:
- Grade I: 12–15 mm Hg
- Grade II: 16–20 mm Hg
- Grade III: 21–25 mm Hg
- Grade IV: > 25 mm Hg.
The World Society of the Abdominal Compartment Syndrome (WSACS) defines abdominal compartment syndrome as pressure higher than 20 mm Hg along with organ damage.18 It may or may not be associated with an abdominal perfusion pressure less than 60 mm Hg.18
Risk factors associated with abdominal compartment syndrome include conditions causing decreased gut motility (gastroparesis, ileus, and colonic pseudo-obstruction), intra-abdominal or retroperitoneal masses or abscesses, ascites, hemoperitoneum, acute pancreatitis, third-spacing due to massive fluid resuscitation with transfusions, peritoneal dialysis, and shock.18,19
Physical examination has a sensitivity of only 40% to 60% in detecting intra-abdominal hypertension.20 The gold-standard method of measuring the intra-abdominal pressure is the modified Kron technique,18 using a Foley catheter in the bladder connected to a pressure transducer. With the patient in the supine position, the transducer is zeroed at the mid-axillary line at the level of the iliac crest, and 25 mL of normal saline is instilled into the bladder and maintained for 30 to 60 seconds to let the detrusor muscle relax.15 Pressure tracings are then recorded at the end of expiration. Factors that are known to affect the transbladder pressure include patient position, respiratory movement, and body mass index, and should be taken into account when reading the pressure recordings.15,21 Other techniques that can be used include intragastric, intra-inferior vena cava, and intrarectal approaches.15
The WSACS recommends that any patient admitted to a critical care unit or in whom new organ failure develops should be screened for risk factors for intra-abdominal hypertension and abdominal compartment syndrome. If a patient has at least two of the risk factors suggested by WSACS, a baseline intra-abdominal pressure measurement should be obtained. Patients at risk for intra-abdominal hypertension should have the intra-abdominal pressure measured every 4 to 6 hours. However, in the face of hemodynamic instability and worsening multiorgan failure, the pressure may need to be measured hourly.18
Clinicians managing patients in the intensive care unit should think of intra-abdominal pressure alongside blood pressure, urine output, and mental status when evaluating hemodynamic status. Clinical manifestations of abdominal compartment syndrome reflect the underlying organ dysfunction and include hypotension, refractory shock, decreased urine output, intracranial hypertension, progressive hypoxemia and hypercarbia, elevated pulmonary peak pressures, and worsening of metabolic acidosis.22
Treatment. The standard treatment for primary abdominal compartment syndrome is surgical decompression. According to WSACS guidelines, insertion of a percutaneous drainage catheter should be advocated in patients with gross ascites and in whom decompressive surgery is not feasible. A damage-control resuscitation strategy used for patients undergoing damage-control laparotomy has been found to increase the 30-day survival rate.23 A damage-control resuscitation strategy consists of increasing the use of plasma and platelet transfusions over packed red cell transfusions, limiting the use of crystalloid solutions in early fluid resuscitation, and allowing for permissive hypotension.
Secondary abdominal compartment syndrome is treated conservatively in most cases, since patients with this condition are very poor surgical candidates owing to their comorbidities.18 However, in patients with progressive organ dysfunction in whom medical management has failed, surgical decompression should be considered.18 Medical management of secondary abdominal compartment syndrome depends on the underlying etiology. Strategies include nasogastric or colonic decompression, use of prokinetic agents, paracentesis in cases with gross ascites, and maintaining a cumulative negative fluid balance. The WSACS does not recommend routine use of diuretics, albumin infusion, or renal replacement strategies. Pain should be adequately controlled to improve abdominal wall compliance.18,24 Neuromuscular blockade agents may be used to aid this process. Neostigmine may be used to treat colonic pseudo-obstruction when other conservative methods fail. Use of enteral nutrition should be minimized.18
Our patient might have abdominal compartment syndrome, but a definitive diagnosis cannot be made at this point without measuring the intra-abdominal pressure.
WHICH IMAGING TEST WOULD BE BEST?
2. Which imaging test would be best for establishing the diagnosis in this patient?
- Plain abdominal radiography
- Abdominal ultrasonography
- Computed tomography of the abdomen and pelvis with contrast
- Magnetic resonance imaging of the abdomen and pelvis
Plain abdominal radiography
Plain abdominal radiography can help to determine if there is free gas under the diaphragm (due to bowel perforation), obstructed bowel, sentinel loop, volvulus, or fecoliths causing the abdominal pain. It cannot diagnose rectus sheath hematoma or acute mesenteric ischemia.
Abdominal ultrasonography
Abdominal ultrasonography can be used as the first diagnostic test, as it is widely available, safe, effective, and tolerable. It does not expose the patient to radiation or intravenous contrast agents. It helps to diagnose rectus sheath hematoma and helps to follow its maturation and resolution once a diagnosis is made. It can provide a rapid assessment of the size, location, extent, and physical characteristics of the mass.
Rectus sheath hematoma appears spindle-shaped on sagittal sections and ovoid on coronal sections. It often appears sonolucent in the early stages and sonodense in the late stage, but the appearance may be heterogeneous depending on the combined presence of clot and fresh blood. These findings are sufficient to make the diagnosis.
Abdominal ultrasonography has 85% to 96% sensitivity in diagnosing rectus sheath hematoma.25 It can help diagnose other causes of the abdominal pain, such as renal stones and cholecystitis. It is the preferred imaging test in pediatric patients, pregnant patients, and those with renal insufficiency.
Abdominal computed tomography
Abdominal computed tomography has a sensitivity and specificity of 100% for diagnosing acute rectus sheath hematoma with a duration of less than 5 days.25 It not only helps to determine the precise location and extent, but also helps to determine if there is active extravasation. Even in patients with renal insufficiency, noncontrast computed tomography will help to confirm the diagnosis, although it may not show extravasation or it may miss certain abdominal pathologies because of the lack of contrast.
Acute rectus sheath hematoma appears as a hyperdense mass posterior to the rectus abdominis muscle with ipsilateral anterolateral muscular enlargement. Chronic rectus sheath hematoma appears isodense or hypodense relative to the surrounding muscle. Above the arcuate line, rectus sheath hematoma has a spindle shape; below the arcuate line, it is typically spherical.
In 1996, Berná et al26 classified rectus sheath hematoma into three grades based on findings of computed tomography:
- Grade I is intramuscular and unilateral
- Grade II may involve bilateral rectus muscles without extension into the prevesicular space
- Grade III extends into the peritoneum and prevesicular space
Magnetic resonance imaging
Magnetic resonance imaging is useful to differentiate chronic rectus sheath hematoma (greater than 5-day duration) from an anterior abdominal wall mass. Chronic rectus sheath hematoma will have high signal intensity on both T1- and T2-weighted images up to 10 months after the onset of the hematoma.27
Back to our patient
Since our patient’s symptoms are acute and of less than 5 days’ duration, computed tomography of the abdomen and pelvis would be the best diagnostic test, with therapeutic implications if there is ongoing extravasation.
Computed tomography of the abdomen with contrast showed a new hematoma, measuring 25 by 14 by 13.5 cm, in the right rectus sheath (Figure 2), with no other findings. The hematoma was grade I, since it was intramuscular and unilateral without extension elsewhere.
Laboratory workup showed that the patient’s hematocrit was falling, from 34% to 24%, and her INR was elevated at 2.5. She was resuscitated with fluids, blood transfusion, and fresh-frozen plasma. Anticoagulation was withheld. In spite of resuscitation, her hematocrit kept falling, though she remained hemodynamically stable.
THE WAY FORWARD
3. At this point, what would be the best approach to management in this patient?
- Serial clinical examinations and frequent monitoring of the complete blood cell count
- Urgent surgical consult for exploratory laparotomy with evaluation of the hematoma and ligation of the bleeding vessel
- Repeat computed tomographic angiography to identify a possible bleeding vessel; consideration of radiographically guided arterial embolization
- Measuring the intra-abdominal pressure using the intrabladder pressure for abdominal compartment syndrome and consideration of surgical drainage
The key clinical concern in a patient with a rectus sheath hematoma who is hemodynamically stable is whether the hematoma is expanding. This patient responded to initial resuscitation, but her falling hematocrit was evidence of ongoing bleeding leading to an expanding rectus sheath hematoma. Thus, serial clinical examinations and frequent monitoring of the complete blood cell count would not be enough, as it could miss fatal ongoing bleeding.
Radiographically guided embolization with Gelfoam, thrombin, or coils should be attempted first, as this is less invasive than exploratory laparotomy.28 It can achieve hemostasis, decrease the size of the hematoma, limit the need for blood products, and prevent rupture into the abdomen. If this is unsuccessful, the next step is ligation of the bleeding vessel.29
Surgical treatment includes evacuation of the hematoma, repair of the rectus sheath, ligation of bleeding vessels, and abdominal wall closure. Surgical evacuation or guided drainage of a rectus sheath hematoma on its own is not normally indicated and may indeed cause persistent bleeding by diminishing a potential tamponade effect. However, it may become necessary if the hematoma is very large or infected, if it causes marked respiratory impairment, or if abdominal compartment syndrome is suspected.
Abdominal compartment syndrome is very rare but is associated with a 50% mortality rate.30 It should be suspected in patients with oliguria, low cardiac output, changes in minute ventilation, and altered splanchnic blood flow. The diagnosis is confirmed with indwelling catheter manometry of the bladder to measure intra-abdominal pressure. Intra-abominal pressure above 25 mm Hg should be treated with decompressive laparotomy.30 However, the clinical suspicion of abdominal compartment syndrome was low in this patient.
The best choice at this point would be urgent computed tomographic angiography to identify a bleeding vessel, along with consideration of radiographically guided arterial embolization.
TREATMENT IS USUALLY CONSERVATIVE
Treatment of rectus sheath hematoma is conservative in most hemodynamically stable patients, with embolization or surgical intervention reserved for unstable patients or those in whom the hematoma is expanding.
Knowledge of the grading system of Berná et al26 helps to assess the patient’s risk and to anticipate potential complications. Grade I hematomas are mild and do not necessitate admission. Patients with grade II hematoma can be admitted to the floor for 24 to 48 hours for observation. Grade III usually occurs in patients receiving anticoagulant therapy and frequently requires blood products. These patients have a prolonged hospital stay and more complications, including hypovolemic shock, myonecrosis, acute coronary syndrome, arrhythmias, acute renal failure, small-bowel infarction, and abdominal compartment syndrome—all of which increases the risk of morbidity and death. Thus, patients who are on anticoagulation who develop grade III rectus sheath hematoma should be admitted to the hospital, preferably to the intensive care unit, to ensure that the hematoma is not expanding and to plan reinstitution of anticoagulation as appropriate.
In most cases, rectus sheath hematomas resolve within 1 to 3 months. Resolution of large hematomas may be hastened with the use of pulsed ultrasound.31 However, this treatment should be used only after the acute phase is over, when there is evidence of an organized thrombus and coagulation measures have returned to the target range. This helps to reduce the risk of bleeding and to prevent symptoms from worsening.31
OUR PATIENT’S COURSE
Our patient underwent urgent computed tomographic angiography, which showed a modest increase in the size of the rectus sheath hematoma. However, no definitive blush of contrast was seen to suggest active arterial bleeding. Her hematocrit stabilized, and she remained hemodynamically stable without requiring additional intervention. Most likely her bleeding was self-contained. She had normal intra-abdominal pressure on serial monitoring. She was later transferred to acute inpatient rehabilitation in view of deconditioning and is currently doing well. The hematoma persisted, decreasing only slightly in size over the next 3 weeks.
- Kougias P, Lau D, El Sayed HF, Zhou W, Huynh TT, Lin PH. Determinants of mortality and treatment outcome following surgical interventions for acute mesenteric ischemia. J Vasc Surg 2007; 46:467–474.
- Sise MJ. Acute mesenteric ischemia. Surg Clin North Am 2014; 94:165–181.
- Scharff JR, Longo WE, Vartanian SM, Jacobs DL, Bahadursingh AN, Kaminski DL. Ischemic colitis: spectrum of disease and outcome. Surgery 2003; 134:624–629.
- Lange H, Jäckel R. Usefulness of plasma lactate concentration in the diagnosis of acute abdominal disease. Eur J Surg 1994; 160:381–384.
- Gearhart SL, Delaney CP, Senagore AJ, et al. Prospective assessment of the predictive value of alpha-glutathione S-transferase for intestinal ischemia. Am Surg 2003; 69:324–329.
- Kanda T, Fujii H, Tani T, et al. Intestinal fatty acid-binding protein is a useful diagnostic marker for mesenteric infarction in humans. Gastroenterology 1996; 110:339–343.
- Menke J. Diagnostic accuracy of multidetector CT in acute mesenteric ischemia: systematic review and meta-analysis. Radiology 2010; 256:93–101.
- Acosta S, Björnsson S, Ekberg O, Resch T. CT angiography followed by endovascular intervention for acute superior mesenteric artery occlusion does not increase risk of contrast-induced renal failure. Eur J Vasc Endovasc Surg 2010; 39:726–730.
- Clark RA. Computed tomography of bowel infarction. J Comput Assist Tomogr 1987; 11:757–762.
- Acosta S, Björck M. Modern treatment of acute mesenteric ischaemia. Br J Surg 2014; 101:e100–e108.
- Smithson A, Ruiz J, Perello R, Valverde M, Ramos J, Garzo L. Diagnostic and management of spontaneous rectus sheath hematoma. Eur J Intern Med 2013; 24:579–582.
- Moreno Gallego A, Aguayo JL, Flores B, et al. Ultrasonography and computed tomography reduce unnecessary surgery in abdominal rectus sheath haematoma. Br J Surg 1997; 84:1295–1297.
- Dubinsky IL. Hematoma of the rectus abdominis muscle: case report and review of the literature. J Emerg Med 1997; 15:165–167.
- Yi M, Yao G, Bai Y. The monitoring of intra-abdominal pressure in critically ill patients. (In Chinese.) Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2014; 26:175–178.
- Hunt L, Frost SA, Hillman K, Newton PJ, Davidson PM. Management of intra-abdominal hypertension and abdominal compartment syndrome: a review. J Trauma Manag Outcomes 2014; 8:2.
- Malbrain ML, Cheatham ML, Kirkpatrick A, et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med 2006; 32:1722–1732.
- Malbrain ML, Chiumello D, Cesana BM, et al; WAKE-Up! Investigators. A systematic review and individual patient data meta-analysis on intra-abdominal hypertension in critically ill patients: the wake-up project. World initiative on Abdominal Hypertension Epidemiology, a Unifying Project (WAKE-Up!). Minerva Anestesiol 2014; 80:293–306.
- Kirkpatrick AW, Roberts DJ, De Waele J, et al; Pediatric Guidelines Sub-Committee for the World Society of the Abdominal Compartment Syndrome. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med 2013; 39:1190–1206.
- Holodinsky JK, Roberts DJ, Ball CG, et al. Risk factors for intra-abdominal hypertension and abdominal compartment syndrome among adult intensive care unit patients: a systematic review and meta-analysis. Crit Care 2013; 17:R249.
- Sugrue M, Bauman A, Jones F, et al. Clinical examination is an inaccurate predictor of intraabdominal pressure. World J Surg 2002; 26:1428–1431.
- Cheatham ML, De Waele JJ, De Laet I, et al; World Society of the Abdominal Compartment Syndrome (WSACS) Clinical Trials Working Group. The impact of body position on intra-abdominal pressure measurement: a multicenter analysis. Crit Care Med 2009; 37:2187–2190.
- Ortiz-Diaz E, Lan CK. Intra-abdominal hypertension in medical critically ill patients: a narrative review. Shock 2014; 41:175–180.
- Cotton BA, Reddy N, Hatch QM, et al. Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg 2011; 254:598–605.
- An G, West MA. Abdominal compartment syndrome: a concise clinical review. Crit Care Med 2008; 36:1304–1310.
- Tolcher MC, Nitsche JF, Arendt KW, Rose CH. Spontaneous rectus sheath hematoma pregnancy: case report and review of the literature. Obstet Gynecol Surv 2010; 65:517–522.
- Berná JD, Garcia-Medina V, Guirao J, Garcia-Medina J. Rectus sheath hematoma: diagnostic classification by CT. Abdom Imaging 1996; 21:62–64.
- Unger EC, Glazer HS, Lee JK, Ling D. MRI of extracranial hematomas: preliminary observations. AJR Am J Roentgenol 1986; 146:403–407.
- Rimola J, Perendreu J, Falcó J, Fortuño JR, Massuet A, Branera J. Percutaneous arterial embolization in the management of rectus sheath hematoma. AJR Am J Roentgenol 2007; 188:W497–W502.
- Titone C, Lipsius M, Krakauer JS. “Spontaneous” hematoma of the rectus abdominis muscle: critical review of 50 cases with emphasis on early diagnosis and treatment. Surgery 1972; 72:568–572.
- Osinbowale O, Bartholomew JR. Rectus sheath hematoma. Vasc Med 2008; 13:275–279.
- Berná-Serna JD, Sánchez-Garre J, Madrigal M, Zuazu I, Berná-Mestre JD. Ultrasound therapy in rectus sheath hematoma. Phys Ther 2005; 85:352–357.
A 57-year-old woman presented to the emergency department with left lower quadrant pain, which had started 1 week earlier and was constant, dull, aching, and nonradiating. There were no aggravating or alleviating factors. The pain was associated with low-grade fever and nausea. She reported no vomiting, no change in bowel habits, and no hematemesis, hematochezia, or melena. She did not have urinary urgency, frequency, or dysuria. She had no cardiac, respiratory, or neurologic symptoms.
Her medical history included hypothyroidism, type 2 diabetes mellitus, diverticulosis, and chronic obstructive pulmonary disease. Her medications included metformin, insulin, levothyroxine, and inhaled tiotropium. She had no allergies. She had never undergone surgery, including cesarean delivery. She was postmenopausal. She had two children, both of whom had been born vaginally at full term. She denied using alcohol, tobacco, and illicit drugs. Her family history was noncontributory.
On examination, she was not in acute distress. Her temperature was 36.7°C (98.1°F), blood pressure 130/90 mm Hg, heart rate 86 beats per minute and regular, respiratory rate 16 breaths per minute, and oxygen saturation 98% on ambient air. Examination of her head and neck was unremarkable. Cardiopulmonary examination was normal. Abdominal examination revealed normal bowel sounds, mild tenderness in the left lower quadrant with localized guarding, and rebound tenderness. Neurologic examination was unremarkable.
Initial laboratory data showed mild leukocytosis. Computed tomography with contrast of the abdomen and pelvis suggested acute diverticulitis.
ATRIAL FIBRILLATION, AND THEN A TURN FOR THE WORSE
The patient was admitted with an initial diagnosis of acute diverticulitis. She was started on antibiotics, hydration, and pain medications, and her abdominal pain gradually improved.
On the third hospital day, she suddenly experienced shortness of breath and palpitations. At the time of admission her electrocardiogram had been normal, but it now showed atrial fibrillation with a rapid ventricular response. She also developed elevated troponin levels, which were thought to represent type 2 non-ST-elevation myocardial infarction.
She was started on aspirin, clopidogrel, and anticoagulation with heparin bridged with warfarin for the new-onset atrial fibrillation. Her heart rate was controlled with metoprolol, and her shortness of breath improved. An echocardiogram was normal.
On the seventh hospital day, she developed severe right-sided lower abdominal pain and bruising. Her blood pressure was 90/60 mm Hg, heart rate 110 beats per minute and irregularly irregular, respiratory rate 22 breaths per minute, and oxygen saturation 97% on room air. Her abdomen was diffusely tender with a palpable mass in the right lower quadrant and hypoactive bowel sounds. Ecchymosis was noted (Figure 1).
DIFFERENTIAL DIAGNOSIS
1. What is the likely cause of her decompensation?
- Acute mesenteric ischemia
- Perforation of the gastrointestinal tract
- Rectus sheath hematoma
- Abdominal compartment syndrome due to acute pancreatitis
Acute mesenteric ischemia
Signs and symptoms of acute mesenteric ischemia can be vague. Moreover, when it leads to bowel necrosis the mortality rate is high, ranging from 30% to 65%.1 Hence, one should suspect it and try to diagnose it early.
Most patients with this condition have comorbidities; risk factors include atherosclerotic disease, cardiac conditions (congestive heart failure, recent myocardial infarction, and atrial fibrillation), systemic illness, and inherited or acquired hypercoagulable states.2
The four major causes are:
- Acute thromboembolic occlusion of the superior mesenteric artery (the most common site of occlusion because of the acute angle of origin from the aorta)
- Acute thrombosis of the mesenteric vein
- Acute thrombosis of the mesenteric artery
- Nonocclusive disease affecting the mesenteric vessels2
Nonocclusive disease is seen in conditions in which the mesenteric vessels are already compromised due to background stenosis owing to atherosclerosis. Also, conditions such as septic and cardiogenic shock can compromise these arteries, leading to ischemia, which, if it persists, can lead to bowel infarction. Ischemic colitis falls under this category. It commonly involves the descending and sigmoid colon.3
The initial symptom of ischemia may be abdominal pain that is brought on by eating large meals (“postprandial intestinal angina.”2 When the ischemia worsens to infarction, patients may have a diffusely tender abdomen and constant pain that does not vary with palpation. Surprisingly, patients do not exhibit peritoneal signs early on. This gives rise to the description of “pain out of proportion to the physical findings” traditionally associated with acute mesenteric ischemia.2
Diagnosis. Supportive laboratory data include marked leukocytosis, elevated hematocrit due to hemoconcentration, metabolic acidosis, and elevated lactate.4 Newer markers such as serum alpha-glutathione S-transferase (alpha-GST) and intestinal fatty acid-binding protein (I-FABP) may be used to support the diagnosis.
Elevated alpha-GST has 72% sensitivity and 77% specificity in the diagnosis of acute mesenteric ischemia.5 The caveat is that it cannot reliably differentiate ischemia from infarction. Its sensitivity can be improved to 97% to 100% by using the white blood cell count and lactate levels in combination.5
An I-FABP level higher than 100 ng/mL has 100% sensitivity for diagnosing mesenteric infarction but only 25% sensitivity for bowel strangulation.6
Early use of abdominal computed tomography with contrast can aid in recognizing this diagnosis.7 Thus, it should be ordered in suspected cases, even in patients who have elevated creatinine levels (which would normally preclude the use of contrast), since early diagnosis followed by endovascular therapy is associated with survival benefit, and the risk of contrast-induced nephropathy appears to be small.8 Computed tomography helps to determine the state of mesenteric vessels and bowel perfusion before ischemia progresses to infarction. It also helps to rule out other common diagnoses. Findings that suggest acute mesenteric ischemia include segmental bowel wall thickening, intestinal pneumatosis with gas in the portal vein, bowel dilation, mesenteric stranding, portomesenteric thrombosis, and solid-organ infarction.9
Treatment. If superior mesenteric artery occlusion is diagnosed on computed tomography, the next step is to determine if there is peritonitis.10 In patients who have evidence of peritonitis, exploratory laparotomy is performed. For emboli in such patients, open embolectomy followed by on-table angiography is carried out in combination with damage-control surgery. For patients with peritonitis and acute thrombosis, stenting along with damage-control surgery is preferred.10
On the other hand, if there is no peritonitis, the thrombosis may be amenable to endovascular intervention. For patients with acute embolic occlusion with no contraindications to thrombolysis, aspiration embolectomy in combination with local catheter-directed thrombolysis with recombinant tissue plasminogen activator can be performed. This can be combined with endovascular mechanical embolectomy for more complete management.10 Patients with contraindications to thrombolysis can be treated either with aspiration and mechanical embolectomy or with open embolectomy with angiography.10
During laparotomy, the surgeon carefully inspects the bowel for signs of necrosis. Signs that bowel is still viable include pink color, bleeding from cut surfaces, good peristalsis, and visible pulsations in the arterial arcade of the mesentery.
Acute mesenteric artery thrombosis arising from chronic atherosclerotic disease can be treated with stenting of the stenotic lesion.10 Patients with this condition would also benefit from aggressive management of atherosclerotic disease with statins along with antiplatelet agents.10
Mesenteric vein thrombosis requires prompt institution of anticoagulation. However, in advanced cases leading to bowel infarction, exploratory laparotomy with resection of the necrotic bowel may be required. Anticoagulation should be continued for at least 6 months, and further therapy should be determined by the underlying precipitating condition.10
Critically ill patients who develop mesenteric ischemia secondary to persistent hypotension usually respond to adequate volume resuscitation, cessation of vasopressors, and overall improvement in their hemodynamic status. These patients must be closely monitored for development of gangrene of the bowel because they may be intubated and not able to complain. Any sudden deterioration in their condition should prompt physicians to consider bowel necrosis developing in these patients. Elevation of lactate levels out of proportion to the degree of hypotension may be corroborative evidence.4
Our patient had risk factors for acute mesenteric ischemia that included atrial fibrillation and recent non-ST-elevation myocardial infarction. She could have had arterial emboli due to atrial fibrillation, in situ superior mesenteric arterial thrombosis, or splanchnic arterial vasoconstriction due to the myocardial infarction associated with transient hypotension.
Arguing against this diagnosis, although she had a grossly distended abdomen, abdominal bruising usually is not seen. Also, a palpable mass in the right lower quadrant is uncommon except when acute mesenteric ischemia occurs due to segmental intestinal strangulation, as with strangulated hernia or volvulus. She also had therapeutic international normalized ratio (INR) levels constantly while on anticoagulation. Nevertheless, acute mesenteric ischemia should be strongly considered in the initial differential diagnosis of this patient’s acute decompensation.
Perforation of the gastrointestinal tract
Diverticulitis is the acute inflammation of one or more diverticula, which are small pouches created by herniation of the mucosa into the wall of the colon. The condition is caused by microscopic or macroscopic perforation of the diverticula. Microscopic perforation is usually self-limited (uncomplicated diverticulitis) and responds to conservative treatment, whereas macroscopic perforation can be associated with fecal or purulent peritonitis, abscess, enteric fistula, bowel obstruction, and stricture (complicated diverticulitis), in which case surgery may be necessary.
Patients with peritonitis due to free perforation present with generalized tenderness with rebound tenderness and guarding on abdominal examination. The abdomen may be distended and tympanic to percussion, with diminished or absent bowel sounds. Patients may have hemodynamic compromise.
Plain upright abdominal radiographs may show free air under the diaphragm. Computed tomography may show oral contrast outside the lumen and detect even small amounts of free intraperitoneal air (more clearly seen on a lung window setting).
Our patient initially presented with acute diverticulitis. She later developed diffuse abdominal tenderness with hypoactive bowel sounds. Bowel perforation is certainly a possibility at this stage, though it is usually not associated with abdominal bruising. She would need additional imaging to rule out this complication.
Other differential diagnoses to be considered in this patient with right lower-quadrant pain include acute appendicitis, incarcerated inguinal hernia, volvulus (particularly cecal volvulus), small-bowel obstruction, pyelonephritis, and gynecologic causes such as adnexal torsion, ruptured ovarian cyst, and tubo-ovarian abscess. Computed tomography helps to differentiate most of these causes.
Rectus sheath hematoma
Rectus sheath hematoma is relatively uncommon and often not considered in the initial differential diagnosis of an acute abdomen. This gives it the rightful term “a great masquerader.” It usually results from bleeding into the rectus sheath from damage to the superior (more common) or inferior epigastric arteries and occasionally from a direct tear of the rectus abdominis muscle. Predisposing factors include anticoagulant therapy (most common), advanced age, hypertension, previous abdominal surgery, trauma, paroxysmal coughing, medication injections, pregnancy, blood dyscrasias, severe vomiting, violent physical activity, and leukemia.11
Clinical manifestations include acute abdominal pain, often associated with fever, nausea, and vomiting. Physical examination may reveal signs of hypovolemic shock, a palpable nonpulsatile abdominal mass, and signs of local peritoneal irritation. The Carnett sign11 (tenderness within the abdominal wall that persists and does not improve with raising the head) and the Fothergill sign11 (a tender abdominal mass that does not cross the midline and remains palpable with tensing of the rectus sheath) may be elicited.
Computed tomography is more sensitive than abdominal ultrasonography in differentiating rectus sheath hematoma from an intra-abdominal pathology.11 In addition, computed tomography also helps to determine if the bleeding is active or not, which has therapeutic implications.
In our patient, rectus sheath hematoma is a possibility because of her ongoing anticoagulation, findings of localized abdominal bruising, and palpable right lower-quadrant mass, and it is high on the list of differential diagnoses. Rectus sheath hematoma should be considered in the differential diagnosis of lower abdominal pain particularly in elderly women who are on anticoagulation and in whom the onset of pain coincides with a paroxysm of cough.12 Women are twice as likely as men to develop rectus sheath hematoma, owing to their different muscle mass.13 In addition, anterior abdominal wall muscles are stretched during pregnancy.13
Abdominal compartment syndrome
Abdominal compartment syndrome has been classically associated with surgical patients. However, it is being increasingly recognized in critically ill medical patients, in whom detecting and treating it early may result in significant reduction in rates of morbidity and death.14
Abdominal compartment syndrome is of three types: primary, secondary, and recurrent. Primary abdominal compartment syndrome refers to the classic surgical patients with evidence of direct injury to the abdominal or pelvic organs through major trauma or extensive abdominal surgeries. Secondary abdominal compartment syndrome refers to its development in critically ill intensive care patients in whom the pathology does not directly involve the abdominal or pelvic organs.
Various medical conditions can culminate in abdominal compartment syndrome and result in multiorgan failure. Recurrent abdominal compartment syndrome refers to its development after management of either primary or secondary intra-abdominal hypertension or abdominal compartment syndrome.15 Clinicians thus must be aware of secondary and recurrent abdominal compartment syndrome occurring in critically ill patients.
The normal intra-abdominal pressure is around 5 to 7 mm Hg, even in most critically ill patients. Persistent elevation, ie, higher than 12 mm Hg, is referred to as intra-abdominal hypertension.16–18 Intra-abdominal hypertension is subdivided into four grades:
- Grade I: 12–15 mm Hg
- Grade II: 16–20 mm Hg
- Grade III: 21–25 mm Hg
- Grade IV: > 25 mm Hg.
The World Society of the Abdominal Compartment Syndrome (WSACS) defines abdominal compartment syndrome as pressure higher than 20 mm Hg along with organ damage.18 It may or may not be associated with an abdominal perfusion pressure less than 60 mm Hg.18
Risk factors associated with abdominal compartment syndrome include conditions causing decreased gut motility (gastroparesis, ileus, and colonic pseudo-obstruction), intra-abdominal or retroperitoneal masses or abscesses, ascites, hemoperitoneum, acute pancreatitis, third-spacing due to massive fluid resuscitation with transfusions, peritoneal dialysis, and shock.18,19
Physical examination has a sensitivity of only 40% to 60% in detecting intra-abdominal hypertension.20 The gold-standard method of measuring the intra-abdominal pressure is the modified Kron technique,18 using a Foley catheter in the bladder connected to a pressure transducer. With the patient in the supine position, the transducer is zeroed at the mid-axillary line at the level of the iliac crest, and 25 mL of normal saline is instilled into the bladder and maintained for 30 to 60 seconds to let the detrusor muscle relax.15 Pressure tracings are then recorded at the end of expiration. Factors that are known to affect the transbladder pressure include patient position, respiratory movement, and body mass index, and should be taken into account when reading the pressure recordings.15,21 Other techniques that can be used include intragastric, intra-inferior vena cava, and intrarectal approaches.15
The WSACS recommends that any patient admitted to a critical care unit or in whom new organ failure develops should be screened for risk factors for intra-abdominal hypertension and abdominal compartment syndrome. If a patient has at least two of the risk factors suggested by WSACS, a baseline intra-abdominal pressure measurement should be obtained. Patients at risk for intra-abdominal hypertension should have the intra-abdominal pressure measured every 4 to 6 hours. However, in the face of hemodynamic instability and worsening multiorgan failure, the pressure may need to be measured hourly.18
Clinicians managing patients in the intensive care unit should think of intra-abdominal pressure alongside blood pressure, urine output, and mental status when evaluating hemodynamic status. Clinical manifestations of abdominal compartment syndrome reflect the underlying organ dysfunction and include hypotension, refractory shock, decreased urine output, intracranial hypertension, progressive hypoxemia and hypercarbia, elevated pulmonary peak pressures, and worsening of metabolic acidosis.22
Treatment. The standard treatment for primary abdominal compartment syndrome is surgical decompression. According to WSACS guidelines, insertion of a percutaneous drainage catheter should be advocated in patients with gross ascites and in whom decompressive surgery is not feasible. A damage-control resuscitation strategy used for patients undergoing damage-control laparotomy has been found to increase the 30-day survival rate.23 A damage-control resuscitation strategy consists of increasing the use of plasma and platelet transfusions over packed red cell transfusions, limiting the use of crystalloid solutions in early fluid resuscitation, and allowing for permissive hypotension.
Secondary abdominal compartment syndrome is treated conservatively in most cases, since patients with this condition are very poor surgical candidates owing to their comorbidities.18 However, in patients with progressive organ dysfunction in whom medical management has failed, surgical decompression should be considered.18 Medical management of secondary abdominal compartment syndrome depends on the underlying etiology. Strategies include nasogastric or colonic decompression, use of prokinetic agents, paracentesis in cases with gross ascites, and maintaining a cumulative negative fluid balance. The WSACS does not recommend routine use of diuretics, albumin infusion, or renal replacement strategies. Pain should be adequately controlled to improve abdominal wall compliance.18,24 Neuromuscular blockade agents may be used to aid this process. Neostigmine may be used to treat colonic pseudo-obstruction when other conservative methods fail. Use of enteral nutrition should be minimized.18
Our patient might have abdominal compartment syndrome, but a definitive diagnosis cannot be made at this point without measuring the intra-abdominal pressure.
WHICH IMAGING TEST WOULD BE BEST?
2. Which imaging test would be best for establishing the diagnosis in this patient?
- Plain abdominal radiography
- Abdominal ultrasonography
- Computed tomography of the abdomen and pelvis with contrast
- Magnetic resonance imaging of the abdomen and pelvis
Plain abdominal radiography
Plain abdominal radiography can help to determine if there is free gas under the diaphragm (due to bowel perforation), obstructed bowel, sentinel loop, volvulus, or fecoliths causing the abdominal pain. It cannot diagnose rectus sheath hematoma or acute mesenteric ischemia.
Abdominal ultrasonography
Abdominal ultrasonography can be used as the first diagnostic test, as it is widely available, safe, effective, and tolerable. It does not expose the patient to radiation or intravenous contrast agents. It helps to diagnose rectus sheath hematoma and helps to follow its maturation and resolution once a diagnosis is made. It can provide a rapid assessment of the size, location, extent, and physical characteristics of the mass.
Rectus sheath hematoma appears spindle-shaped on sagittal sections and ovoid on coronal sections. It often appears sonolucent in the early stages and sonodense in the late stage, but the appearance may be heterogeneous depending on the combined presence of clot and fresh blood. These findings are sufficient to make the diagnosis.
Abdominal ultrasonography has 85% to 96% sensitivity in diagnosing rectus sheath hematoma.25 It can help diagnose other causes of the abdominal pain, such as renal stones and cholecystitis. It is the preferred imaging test in pediatric patients, pregnant patients, and those with renal insufficiency.
Abdominal computed tomography
Abdominal computed tomography has a sensitivity and specificity of 100% for diagnosing acute rectus sheath hematoma with a duration of less than 5 days.25 It not only helps to determine the precise location and extent, but also helps to determine if there is active extravasation. Even in patients with renal insufficiency, noncontrast computed tomography will help to confirm the diagnosis, although it may not show extravasation or it may miss certain abdominal pathologies because of the lack of contrast.
Acute rectus sheath hematoma appears as a hyperdense mass posterior to the rectus abdominis muscle with ipsilateral anterolateral muscular enlargement. Chronic rectus sheath hematoma appears isodense or hypodense relative to the surrounding muscle. Above the arcuate line, rectus sheath hematoma has a spindle shape; below the arcuate line, it is typically spherical.
In 1996, Berná et al26 classified rectus sheath hematoma into three grades based on findings of computed tomography:
- Grade I is intramuscular and unilateral
- Grade II may involve bilateral rectus muscles without extension into the prevesicular space
- Grade III extends into the peritoneum and prevesicular space
Magnetic resonance imaging
Magnetic resonance imaging is useful to differentiate chronic rectus sheath hematoma (greater than 5-day duration) from an anterior abdominal wall mass. Chronic rectus sheath hematoma will have high signal intensity on both T1- and T2-weighted images up to 10 months after the onset of the hematoma.27
Back to our patient
Since our patient’s symptoms are acute and of less than 5 days’ duration, computed tomography of the abdomen and pelvis would be the best diagnostic test, with therapeutic implications if there is ongoing extravasation.
Computed tomography of the abdomen with contrast showed a new hematoma, measuring 25 by 14 by 13.5 cm, in the right rectus sheath (Figure 2), with no other findings. The hematoma was grade I, since it was intramuscular and unilateral without extension elsewhere.
Laboratory workup showed that the patient’s hematocrit was falling, from 34% to 24%, and her INR was elevated at 2.5. She was resuscitated with fluids, blood transfusion, and fresh-frozen plasma. Anticoagulation was withheld. In spite of resuscitation, her hematocrit kept falling, though she remained hemodynamically stable.
THE WAY FORWARD
3. At this point, what would be the best approach to management in this patient?
- Serial clinical examinations and frequent monitoring of the complete blood cell count
- Urgent surgical consult for exploratory laparotomy with evaluation of the hematoma and ligation of the bleeding vessel
- Repeat computed tomographic angiography to identify a possible bleeding vessel; consideration of radiographically guided arterial embolization
- Measuring the intra-abdominal pressure using the intrabladder pressure for abdominal compartment syndrome and consideration of surgical drainage
The key clinical concern in a patient with a rectus sheath hematoma who is hemodynamically stable is whether the hematoma is expanding. This patient responded to initial resuscitation, but her falling hematocrit was evidence of ongoing bleeding leading to an expanding rectus sheath hematoma. Thus, serial clinical examinations and frequent monitoring of the complete blood cell count would not be enough, as it could miss fatal ongoing bleeding.
Radiographically guided embolization with Gelfoam, thrombin, or coils should be attempted first, as this is less invasive than exploratory laparotomy.28 It can achieve hemostasis, decrease the size of the hematoma, limit the need for blood products, and prevent rupture into the abdomen. If this is unsuccessful, the next step is ligation of the bleeding vessel.29
Surgical treatment includes evacuation of the hematoma, repair of the rectus sheath, ligation of bleeding vessels, and abdominal wall closure. Surgical evacuation or guided drainage of a rectus sheath hematoma on its own is not normally indicated and may indeed cause persistent bleeding by diminishing a potential tamponade effect. However, it may become necessary if the hematoma is very large or infected, if it causes marked respiratory impairment, or if abdominal compartment syndrome is suspected.
Abdominal compartment syndrome is very rare but is associated with a 50% mortality rate.30 It should be suspected in patients with oliguria, low cardiac output, changes in minute ventilation, and altered splanchnic blood flow. The diagnosis is confirmed with indwelling catheter manometry of the bladder to measure intra-abdominal pressure. Intra-abominal pressure above 25 mm Hg should be treated with decompressive laparotomy.30 However, the clinical suspicion of abdominal compartment syndrome was low in this patient.
The best choice at this point would be urgent computed tomographic angiography to identify a bleeding vessel, along with consideration of radiographically guided arterial embolization.
TREATMENT IS USUALLY CONSERVATIVE
Treatment of rectus sheath hematoma is conservative in most hemodynamically stable patients, with embolization or surgical intervention reserved for unstable patients or those in whom the hematoma is expanding.
Knowledge of the grading system of Berná et al26 helps to assess the patient’s risk and to anticipate potential complications. Grade I hematomas are mild and do not necessitate admission. Patients with grade II hematoma can be admitted to the floor for 24 to 48 hours for observation. Grade III usually occurs in patients receiving anticoagulant therapy and frequently requires blood products. These patients have a prolonged hospital stay and more complications, including hypovolemic shock, myonecrosis, acute coronary syndrome, arrhythmias, acute renal failure, small-bowel infarction, and abdominal compartment syndrome—all of which increases the risk of morbidity and death. Thus, patients who are on anticoagulation who develop grade III rectus sheath hematoma should be admitted to the hospital, preferably to the intensive care unit, to ensure that the hematoma is not expanding and to plan reinstitution of anticoagulation as appropriate.
In most cases, rectus sheath hematomas resolve within 1 to 3 months. Resolution of large hematomas may be hastened with the use of pulsed ultrasound.31 However, this treatment should be used only after the acute phase is over, when there is evidence of an organized thrombus and coagulation measures have returned to the target range. This helps to reduce the risk of bleeding and to prevent symptoms from worsening.31
OUR PATIENT’S COURSE
Our patient underwent urgent computed tomographic angiography, which showed a modest increase in the size of the rectus sheath hematoma. However, no definitive blush of contrast was seen to suggest active arterial bleeding. Her hematocrit stabilized, and she remained hemodynamically stable without requiring additional intervention. Most likely her bleeding was self-contained. She had normal intra-abdominal pressure on serial monitoring. She was later transferred to acute inpatient rehabilitation in view of deconditioning and is currently doing well. The hematoma persisted, decreasing only slightly in size over the next 3 weeks.
A 57-year-old woman presented to the emergency department with left lower quadrant pain, which had started 1 week earlier and was constant, dull, aching, and nonradiating. There were no aggravating or alleviating factors. The pain was associated with low-grade fever and nausea. She reported no vomiting, no change in bowel habits, and no hematemesis, hematochezia, or melena. She did not have urinary urgency, frequency, or dysuria. She had no cardiac, respiratory, or neurologic symptoms.
Her medical history included hypothyroidism, type 2 diabetes mellitus, diverticulosis, and chronic obstructive pulmonary disease. Her medications included metformin, insulin, levothyroxine, and inhaled tiotropium. She had no allergies. She had never undergone surgery, including cesarean delivery. She was postmenopausal. She had two children, both of whom had been born vaginally at full term. She denied using alcohol, tobacco, and illicit drugs. Her family history was noncontributory.
On examination, she was not in acute distress. Her temperature was 36.7°C (98.1°F), blood pressure 130/90 mm Hg, heart rate 86 beats per minute and regular, respiratory rate 16 breaths per minute, and oxygen saturation 98% on ambient air. Examination of her head and neck was unremarkable. Cardiopulmonary examination was normal. Abdominal examination revealed normal bowel sounds, mild tenderness in the left lower quadrant with localized guarding, and rebound tenderness. Neurologic examination was unremarkable.
Initial laboratory data showed mild leukocytosis. Computed tomography with contrast of the abdomen and pelvis suggested acute diverticulitis.
ATRIAL FIBRILLATION, AND THEN A TURN FOR THE WORSE
The patient was admitted with an initial diagnosis of acute diverticulitis. She was started on antibiotics, hydration, and pain medications, and her abdominal pain gradually improved.
On the third hospital day, she suddenly experienced shortness of breath and palpitations. At the time of admission her electrocardiogram had been normal, but it now showed atrial fibrillation with a rapid ventricular response. She also developed elevated troponin levels, which were thought to represent type 2 non-ST-elevation myocardial infarction.
She was started on aspirin, clopidogrel, and anticoagulation with heparin bridged with warfarin for the new-onset atrial fibrillation. Her heart rate was controlled with metoprolol, and her shortness of breath improved. An echocardiogram was normal.
On the seventh hospital day, she developed severe right-sided lower abdominal pain and bruising. Her blood pressure was 90/60 mm Hg, heart rate 110 beats per minute and irregularly irregular, respiratory rate 22 breaths per minute, and oxygen saturation 97% on room air. Her abdomen was diffusely tender with a palpable mass in the right lower quadrant and hypoactive bowel sounds. Ecchymosis was noted (Figure 1).
DIFFERENTIAL DIAGNOSIS
1. What is the likely cause of her decompensation?
- Acute mesenteric ischemia
- Perforation of the gastrointestinal tract
- Rectus sheath hematoma
- Abdominal compartment syndrome due to acute pancreatitis
Acute mesenteric ischemia
Signs and symptoms of acute mesenteric ischemia can be vague. Moreover, when it leads to bowel necrosis the mortality rate is high, ranging from 30% to 65%.1 Hence, one should suspect it and try to diagnose it early.
Most patients with this condition have comorbidities; risk factors include atherosclerotic disease, cardiac conditions (congestive heart failure, recent myocardial infarction, and atrial fibrillation), systemic illness, and inherited or acquired hypercoagulable states.2
The four major causes are:
- Acute thromboembolic occlusion of the superior mesenteric artery (the most common site of occlusion because of the acute angle of origin from the aorta)
- Acute thrombosis of the mesenteric vein
- Acute thrombosis of the mesenteric artery
- Nonocclusive disease affecting the mesenteric vessels2
Nonocclusive disease is seen in conditions in which the mesenteric vessels are already compromised due to background stenosis owing to atherosclerosis. Also, conditions such as septic and cardiogenic shock can compromise these arteries, leading to ischemia, which, if it persists, can lead to bowel infarction. Ischemic colitis falls under this category. It commonly involves the descending and sigmoid colon.3
The initial symptom of ischemia may be abdominal pain that is brought on by eating large meals (“postprandial intestinal angina.”2 When the ischemia worsens to infarction, patients may have a diffusely tender abdomen and constant pain that does not vary with palpation. Surprisingly, patients do not exhibit peritoneal signs early on. This gives rise to the description of “pain out of proportion to the physical findings” traditionally associated with acute mesenteric ischemia.2
Diagnosis. Supportive laboratory data include marked leukocytosis, elevated hematocrit due to hemoconcentration, metabolic acidosis, and elevated lactate.4 Newer markers such as serum alpha-glutathione S-transferase (alpha-GST) and intestinal fatty acid-binding protein (I-FABP) may be used to support the diagnosis.
Elevated alpha-GST has 72% sensitivity and 77% specificity in the diagnosis of acute mesenteric ischemia.5 The caveat is that it cannot reliably differentiate ischemia from infarction. Its sensitivity can be improved to 97% to 100% by using the white blood cell count and lactate levels in combination.5
An I-FABP level higher than 100 ng/mL has 100% sensitivity for diagnosing mesenteric infarction but only 25% sensitivity for bowel strangulation.6
Early use of abdominal computed tomography with contrast can aid in recognizing this diagnosis.7 Thus, it should be ordered in suspected cases, even in patients who have elevated creatinine levels (which would normally preclude the use of contrast), since early diagnosis followed by endovascular therapy is associated with survival benefit, and the risk of contrast-induced nephropathy appears to be small.8 Computed tomography helps to determine the state of mesenteric vessels and bowel perfusion before ischemia progresses to infarction. It also helps to rule out other common diagnoses. Findings that suggest acute mesenteric ischemia include segmental bowel wall thickening, intestinal pneumatosis with gas in the portal vein, bowel dilation, mesenteric stranding, portomesenteric thrombosis, and solid-organ infarction.9
Treatment. If superior mesenteric artery occlusion is diagnosed on computed tomography, the next step is to determine if there is peritonitis.10 In patients who have evidence of peritonitis, exploratory laparotomy is performed. For emboli in such patients, open embolectomy followed by on-table angiography is carried out in combination with damage-control surgery. For patients with peritonitis and acute thrombosis, stenting along with damage-control surgery is preferred.10
On the other hand, if there is no peritonitis, the thrombosis may be amenable to endovascular intervention. For patients with acute embolic occlusion with no contraindications to thrombolysis, aspiration embolectomy in combination with local catheter-directed thrombolysis with recombinant tissue plasminogen activator can be performed. This can be combined with endovascular mechanical embolectomy for more complete management.10 Patients with contraindications to thrombolysis can be treated either with aspiration and mechanical embolectomy or with open embolectomy with angiography.10
During laparotomy, the surgeon carefully inspects the bowel for signs of necrosis. Signs that bowel is still viable include pink color, bleeding from cut surfaces, good peristalsis, and visible pulsations in the arterial arcade of the mesentery.
Acute mesenteric artery thrombosis arising from chronic atherosclerotic disease can be treated with stenting of the stenotic lesion.10 Patients with this condition would also benefit from aggressive management of atherosclerotic disease with statins along with antiplatelet agents.10
Mesenteric vein thrombosis requires prompt institution of anticoagulation. However, in advanced cases leading to bowel infarction, exploratory laparotomy with resection of the necrotic bowel may be required. Anticoagulation should be continued for at least 6 months, and further therapy should be determined by the underlying precipitating condition.10
Critically ill patients who develop mesenteric ischemia secondary to persistent hypotension usually respond to adequate volume resuscitation, cessation of vasopressors, and overall improvement in their hemodynamic status. These patients must be closely monitored for development of gangrene of the bowel because they may be intubated and not able to complain. Any sudden deterioration in their condition should prompt physicians to consider bowel necrosis developing in these patients. Elevation of lactate levels out of proportion to the degree of hypotension may be corroborative evidence.4
Our patient had risk factors for acute mesenteric ischemia that included atrial fibrillation and recent non-ST-elevation myocardial infarction. She could have had arterial emboli due to atrial fibrillation, in situ superior mesenteric arterial thrombosis, or splanchnic arterial vasoconstriction due to the myocardial infarction associated with transient hypotension.
Arguing against this diagnosis, although she had a grossly distended abdomen, abdominal bruising usually is not seen. Also, a palpable mass in the right lower quadrant is uncommon except when acute mesenteric ischemia occurs due to segmental intestinal strangulation, as with strangulated hernia or volvulus. She also had therapeutic international normalized ratio (INR) levels constantly while on anticoagulation. Nevertheless, acute mesenteric ischemia should be strongly considered in the initial differential diagnosis of this patient’s acute decompensation.
Perforation of the gastrointestinal tract
Diverticulitis is the acute inflammation of one or more diverticula, which are small pouches created by herniation of the mucosa into the wall of the colon. The condition is caused by microscopic or macroscopic perforation of the diverticula. Microscopic perforation is usually self-limited (uncomplicated diverticulitis) and responds to conservative treatment, whereas macroscopic perforation can be associated with fecal or purulent peritonitis, abscess, enteric fistula, bowel obstruction, and stricture (complicated diverticulitis), in which case surgery may be necessary.
Patients with peritonitis due to free perforation present with generalized tenderness with rebound tenderness and guarding on abdominal examination. The abdomen may be distended and tympanic to percussion, with diminished or absent bowel sounds. Patients may have hemodynamic compromise.
Plain upright abdominal radiographs may show free air under the diaphragm. Computed tomography may show oral contrast outside the lumen and detect even small amounts of free intraperitoneal air (more clearly seen on a lung window setting).
Our patient initially presented with acute diverticulitis. She later developed diffuse abdominal tenderness with hypoactive bowel sounds. Bowel perforation is certainly a possibility at this stage, though it is usually not associated with abdominal bruising. She would need additional imaging to rule out this complication.
Other differential diagnoses to be considered in this patient with right lower-quadrant pain include acute appendicitis, incarcerated inguinal hernia, volvulus (particularly cecal volvulus), small-bowel obstruction, pyelonephritis, and gynecologic causes such as adnexal torsion, ruptured ovarian cyst, and tubo-ovarian abscess. Computed tomography helps to differentiate most of these causes.
Rectus sheath hematoma
Rectus sheath hematoma is relatively uncommon and often not considered in the initial differential diagnosis of an acute abdomen. This gives it the rightful term “a great masquerader.” It usually results from bleeding into the rectus sheath from damage to the superior (more common) or inferior epigastric arteries and occasionally from a direct tear of the rectus abdominis muscle. Predisposing factors include anticoagulant therapy (most common), advanced age, hypertension, previous abdominal surgery, trauma, paroxysmal coughing, medication injections, pregnancy, blood dyscrasias, severe vomiting, violent physical activity, and leukemia.11
Clinical manifestations include acute abdominal pain, often associated with fever, nausea, and vomiting. Physical examination may reveal signs of hypovolemic shock, a palpable nonpulsatile abdominal mass, and signs of local peritoneal irritation. The Carnett sign11 (tenderness within the abdominal wall that persists and does not improve with raising the head) and the Fothergill sign11 (a tender abdominal mass that does not cross the midline and remains palpable with tensing of the rectus sheath) may be elicited.
Computed tomography is more sensitive than abdominal ultrasonography in differentiating rectus sheath hematoma from an intra-abdominal pathology.11 In addition, computed tomography also helps to determine if the bleeding is active or not, which has therapeutic implications.
In our patient, rectus sheath hematoma is a possibility because of her ongoing anticoagulation, findings of localized abdominal bruising, and palpable right lower-quadrant mass, and it is high on the list of differential diagnoses. Rectus sheath hematoma should be considered in the differential diagnosis of lower abdominal pain particularly in elderly women who are on anticoagulation and in whom the onset of pain coincides with a paroxysm of cough.12 Women are twice as likely as men to develop rectus sheath hematoma, owing to their different muscle mass.13 In addition, anterior abdominal wall muscles are stretched during pregnancy.13
Abdominal compartment syndrome
Abdominal compartment syndrome has been classically associated with surgical patients. However, it is being increasingly recognized in critically ill medical patients, in whom detecting and treating it early may result in significant reduction in rates of morbidity and death.14
Abdominal compartment syndrome is of three types: primary, secondary, and recurrent. Primary abdominal compartment syndrome refers to the classic surgical patients with evidence of direct injury to the abdominal or pelvic organs through major trauma or extensive abdominal surgeries. Secondary abdominal compartment syndrome refers to its development in critically ill intensive care patients in whom the pathology does not directly involve the abdominal or pelvic organs.
Various medical conditions can culminate in abdominal compartment syndrome and result in multiorgan failure. Recurrent abdominal compartment syndrome refers to its development after management of either primary or secondary intra-abdominal hypertension or abdominal compartment syndrome.15 Clinicians thus must be aware of secondary and recurrent abdominal compartment syndrome occurring in critically ill patients.
The normal intra-abdominal pressure is around 5 to 7 mm Hg, even in most critically ill patients. Persistent elevation, ie, higher than 12 mm Hg, is referred to as intra-abdominal hypertension.16–18 Intra-abdominal hypertension is subdivided into four grades:
- Grade I: 12–15 mm Hg
- Grade II: 16–20 mm Hg
- Grade III: 21–25 mm Hg
- Grade IV: > 25 mm Hg.
The World Society of the Abdominal Compartment Syndrome (WSACS) defines abdominal compartment syndrome as pressure higher than 20 mm Hg along with organ damage.18 It may or may not be associated with an abdominal perfusion pressure less than 60 mm Hg.18
Risk factors associated with abdominal compartment syndrome include conditions causing decreased gut motility (gastroparesis, ileus, and colonic pseudo-obstruction), intra-abdominal or retroperitoneal masses or abscesses, ascites, hemoperitoneum, acute pancreatitis, third-spacing due to massive fluid resuscitation with transfusions, peritoneal dialysis, and shock.18,19
Physical examination has a sensitivity of only 40% to 60% in detecting intra-abdominal hypertension.20 The gold-standard method of measuring the intra-abdominal pressure is the modified Kron technique,18 using a Foley catheter in the bladder connected to a pressure transducer. With the patient in the supine position, the transducer is zeroed at the mid-axillary line at the level of the iliac crest, and 25 mL of normal saline is instilled into the bladder and maintained for 30 to 60 seconds to let the detrusor muscle relax.15 Pressure tracings are then recorded at the end of expiration. Factors that are known to affect the transbladder pressure include patient position, respiratory movement, and body mass index, and should be taken into account when reading the pressure recordings.15,21 Other techniques that can be used include intragastric, intra-inferior vena cava, and intrarectal approaches.15
The WSACS recommends that any patient admitted to a critical care unit or in whom new organ failure develops should be screened for risk factors for intra-abdominal hypertension and abdominal compartment syndrome. If a patient has at least two of the risk factors suggested by WSACS, a baseline intra-abdominal pressure measurement should be obtained. Patients at risk for intra-abdominal hypertension should have the intra-abdominal pressure measured every 4 to 6 hours. However, in the face of hemodynamic instability and worsening multiorgan failure, the pressure may need to be measured hourly.18
Clinicians managing patients in the intensive care unit should think of intra-abdominal pressure alongside blood pressure, urine output, and mental status when evaluating hemodynamic status. Clinical manifestations of abdominal compartment syndrome reflect the underlying organ dysfunction and include hypotension, refractory shock, decreased urine output, intracranial hypertension, progressive hypoxemia and hypercarbia, elevated pulmonary peak pressures, and worsening of metabolic acidosis.22
Treatment. The standard treatment for primary abdominal compartment syndrome is surgical decompression. According to WSACS guidelines, insertion of a percutaneous drainage catheter should be advocated in patients with gross ascites and in whom decompressive surgery is not feasible. A damage-control resuscitation strategy used for patients undergoing damage-control laparotomy has been found to increase the 30-day survival rate.23 A damage-control resuscitation strategy consists of increasing the use of plasma and platelet transfusions over packed red cell transfusions, limiting the use of crystalloid solutions in early fluid resuscitation, and allowing for permissive hypotension.
Secondary abdominal compartment syndrome is treated conservatively in most cases, since patients with this condition are very poor surgical candidates owing to their comorbidities.18 However, in patients with progressive organ dysfunction in whom medical management has failed, surgical decompression should be considered.18 Medical management of secondary abdominal compartment syndrome depends on the underlying etiology. Strategies include nasogastric or colonic decompression, use of prokinetic agents, paracentesis in cases with gross ascites, and maintaining a cumulative negative fluid balance. The WSACS does not recommend routine use of diuretics, albumin infusion, or renal replacement strategies. Pain should be adequately controlled to improve abdominal wall compliance.18,24 Neuromuscular blockade agents may be used to aid this process. Neostigmine may be used to treat colonic pseudo-obstruction when other conservative methods fail. Use of enteral nutrition should be minimized.18
Our patient might have abdominal compartment syndrome, but a definitive diagnosis cannot be made at this point without measuring the intra-abdominal pressure.
WHICH IMAGING TEST WOULD BE BEST?
2. Which imaging test would be best for establishing the diagnosis in this patient?
- Plain abdominal radiography
- Abdominal ultrasonography
- Computed tomography of the abdomen and pelvis with contrast
- Magnetic resonance imaging of the abdomen and pelvis
Plain abdominal radiography
Plain abdominal radiography can help to determine if there is free gas under the diaphragm (due to bowel perforation), obstructed bowel, sentinel loop, volvulus, or fecoliths causing the abdominal pain. It cannot diagnose rectus sheath hematoma or acute mesenteric ischemia.
Abdominal ultrasonography
Abdominal ultrasonography can be used as the first diagnostic test, as it is widely available, safe, effective, and tolerable. It does not expose the patient to radiation or intravenous contrast agents. It helps to diagnose rectus sheath hematoma and helps to follow its maturation and resolution once a diagnosis is made. It can provide a rapid assessment of the size, location, extent, and physical characteristics of the mass.
Rectus sheath hematoma appears spindle-shaped on sagittal sections and ovoid on coronal sections. It often appears sonolucent in the early stages and sonodense in the late stage, but the appearance may be heterogeneous depending on the combined presence of clot and fresh blood. These findings are sufficient to make the diagnosis.
Abdominal ultrasonography has 85% to 96% sensitivity in diagnosing rectus sheath hematoma.25 It can help diagnose other causes of the abdominal pain, such as renal stones and cholecystitis. It is the preferred imaging test in pediatric patients, pregnant patients, and those with renal insufficiency.
Abdominal computed tomography
Abdominal computed tomography has a sensitivity and specificity of 100% for diagnosing acute rectus sheath hematoma with a duration of less than 5 days.25 It not only helps to determine the precise location and extent, but also helps to determine if there is active extravasation. Even in patients with renal insufficiency, noncontrast computed tomography will help to confirm the diagnosis, although it may not show extravasation or it may miss certain abdominal pathologies because of the lack of contrast.
Acute rectus sheath hematoma appears as a hyperdense mass posterior to the rectus abdominis muscle with ipsilateral anterolateral muscular enlargement. Chronic rectus sheath hematoma appears isodense or hypodense relative to the surrounding muscle. Above the arcuate line, rectus sheath hematoma has a spindle shape; below the arcuate line, it is typically spherical.
In 1996, Berná et al26 classified rectus sheath hematoma into three grades based on findings of computed tomography:
- Grade I is intramuscular and unilateral
- Grade II may involve bilateral rectus muscles without extension into the prevesicular space
- Grade III extends into the peritoneum and prevesicular space
Magnetic resonance imaging
Magnetic resonance imaging is useful to differentiate chronic rectus sheath hematoma (greater than 5-day duration) from an anterior abdominal wall mass. Chronic rectus sheath hematoma will have high signal intensity on both T1- and T2-weighted images up to 10 months after the onset of the hematoma.27
Back to our patient
Since our patient’s symptoms are acute and of less than 5 days’ duration, computed tomography of the abdomen and pelvis would be the best diagnostic test, with therapeutic implications if there is ongoing extravasation.
Computed tomography of the abdomen with contrast showed a new hematoma, measuring 25 by 14 by 13.5 cm, in the right rectus sheath (Figure 2), with no other findings. The hematoma was grade I, since it was intramuscular and unilateral without extension elsewhere.
Laboratory workup showed that the patient’s hematocrit was falling, from 34% to 24%, and her INR was elevated at 2.5. She was resuscitated with fluids, blood transfusion, and fresh-frozen plasma. Anticoagulation was withheld. In spite of resuscitation, her hematocrit kept falling, though she remained hemodynamically stable.
THE WAY FORWARD
3. At this point, what would be the best approach to management in this patient?
- Serial clinical examinations and frequent monitoring of the complete blood cell count
- Urgent surgical consult for exploratory laparotomy with evaluation of the hematoma and ligation of the bleeding vessel
- Repeat computed tomographic angiography to identify a possible bleeding vessel; consideration of radiographically guided arterial embolization
- Measuring the intra-abdominal pressure using the intrabladder pressure for abdominal compartment syndrome and consideration of surgical drainage
The key clinical concern in a patient with a rectus sheath hematoma who is hemodynamically stable is whether the hematoma is expanding. This patient responded to initial resuscitation, but her falling hematocrit was evidence of ongoing bleeding leading to an expanding rectus sheath hematoma. Thus, serial clinical examinations and frequent monitoring of the complete blood cell count would not be enough, as it could miss fatal ongoing bleeding.
Radiographically guided embolization with Gelfoam, thrombin, or coils should be attempted first, as this is less invasive than exploratory laparotomy.28 It can achieve hemostasis, decrease the size of the hematoma, limit the need for blood products, and prevent rupture into the abdomen. If this is unsuccessful, the next step is ligation of the bleeding vessel.29
Surgical treatment includes evacuation of the hematoma, repair of the rectus sheath, ligation of bleeding vessels, and abdominal wall closure. Surgical evacuation or guided drainage of a rectus sheath hematoma on its own is not normally indicated and may indeed cause persistent bleeding by diminishing a potential tamponade effect. However, it may become necessary if the hematoma is very large or infected, if it causes marked respiratory impairment, or if abdominal compartment syndrome is suspected.
Abdominal compartment syndrome is very rare but is associated with a 50% mortality rate.30 It should be suspected in patients with oliguria, low cardiac output, changes in minute ventilation, and altered splanchnic blood flow. The diagnosis is confirmed with indwelling catheter manometry of the bladder to measure intra-abdominal pressure. Intra-abominal pressure above 25 mm Hg should be treated with decompressive laparotomy.30 However, the clinical suspicion of abdominal compartment syndrome was low in this patient.
The best choice at this point would be urgent computed tomographic angiography to identify a bleeding vessel, along with consideration of radiographically guided arterial embolization.
TREATMENT IS USUALLY CONSERVATIVE
Treatment of rectus sheath hematoma is conservative in most hemodynamically stable patients, with embolization or surgical intervention reserved for unstable patients or those in whom the hematoma is expanding.
Knowledge of the grading system of Berná et al26 helps to assess the patient’s risk and to anticipate potential complications. Grade I hematomas are mild and do not necessitate admission. Patients with grade II hematoma can be admitted to the floor for 24 to 48 hours for observation. Grade III usually occurs in patients receiving anticoagulant therapy and frequently requires blood products. These patients have a prolonged hospital stay and more complications, including hypovolemic shock, myonecrosis, acute coronary syndrome, arrhythmias, acute renal failure, small-bowel infarction, and abdominal compartment syndrome—all of which increases the risk of morbidity and death. Thus, patients who are on anticoagulation who develop grade III rectus sheath hematoma should be admitted to the hospital, preferably to the intensive care unit, to ensure that the hematoma is not expanding and to plan reinstitution of anticoagulation as appropriate.
In most cases, rectus sheath hematomas resolve within 1 to 3 months. Resolution of large hematomas may be hastened with the use of pulsed ultrasound.31 However, this treatment should be used only after the acute phase is over, when there is evidence of an organized thrombus and coagulation measures have returned to the target range. This helps to reduce the risk of bleeding and to prevent symptoms from worsening.31
OUR PATIENT’S COURSE
Our patient underwent urgent computed tomographic angiography, which showed a modest increase in the size of the rectus sheath hematoma. However, no definitive blush of contrast was seen to suggest active arterial bleeding. Her hematocrit stabilized, and she remained hemodynamically stable without requiring additional intervention. Most likely her bleeding was self-contained. She had normal intra-abdominal pressure on serial monitoring. She was later transferred to acute inpatient rehabilitation in view of deconditioning and is currently doing well. The hematoma persisted, decreasing only slightly in size over the next 3 weeks.
- Kougias P, Lau D, El Sayed HF, Zhou W, Huynh TT, Lin PH. Determinants of mortality and treatment outcome following surgical interventions for acute mesenteric ischemia. J Vasc Surg 2007; 46:467–474.
- Sise MJ. Acute mesenteric ischemia. Surg Clin North Am 2014; 94:165–181.
- Scharff JR, Longo WE, Vartanian SM, Jacobs DL, Bahadursingh AN, Kaminski DL. Ischemic colitis: spectrum of disease and outcome. Surgery 2003; 134:624–629.
- Lange H, Jäckel R. Usefulness of plasma lactate concentration in the diagnosis of acute abdominal disease. Eur J Surg 1994; 160:381–384.
- Gearhart SL, Delaney CP, Senagore AJ, et al. Prospective assessment of the predictive value of alpha-glutathione S-transferase for intestinal ischemia. Am Surg 2003; 69:324–329.
- Kanda T, Fujii H, Tani T, et al. Intestinal fatty acid-binding protein is a useful diagnostic marker for mesenteric infarction in humans. Gastroenterology 1996; 110:339–343.
- Menke J. Diagnostic accuracy of multidetector CT in acute mesenteric ischemia: systematic review and meta-analysis. Radiology 2010; 256:93–101.
- Acosta S, Björnsson S, Ekberg O, Resch T. CT angiography followed by endovascular intervention for acute superior mesenteric artery occlusion does not increase risk of contrast-induced renal failure. Eur J Vasc Endovasc Surg 2010; 39:726–730.
- Clark RA. Computed tomography of bowel infarction. J Comput Assist Tomogr 1987; 11:757–762.
- Acosta S, Björck M. Modern treatment of acute mesenteric ischaemia. Br J Surg 2014; 101:e100–e108.
- Smithson A, Ruiz J, Perello R, Valverde M, Ramos J, Garzo L. Diagnostic and management of spontaneous rectus sheath hematoma. Eur J Intern Med 2013; 24:579–582.
- Moreno Gallego A, Aguayo JL, Flores B, et al. Ultrasonography and computed tomography reduce unnecessary surgery in abdominal rectus sheath haematoma. Br J Surg 1997; 84:1295–1297.
- Dubinsky IL. Hematoma of the rectus abdominis muscle: case report and review of the literature. J Emerg Med 1997; 15:165–167.
- Yi M, Yao G, Bai Y. The monitoring of intra-abdominal pressure in critically ill patients. (In Chinese.) Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2014; 26:175–178.
- Hunt L, Frost SA, Hillman K, Newton PJ, Davidson PM. Management of intra-abdominal hypertension and abdominal compartment syndrome: a review. J Trauma Manag Outcomes 2014; 8:2.
- Malbrain ML, Cheatham ML, Kirkpatrick A, et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med 2006; 32:1722–1732.
- Malbrain ML, Chiumello D, Cesana BM, et al; WAKE-Up! Investigators. A systematic review and individual patient data meta-analysis on intra-abdominal hypertension in critically ill patients: the wake-up project. World initiative on Abdominal Hypertension Epidemiology, a Unifying Project (WAKE-Up!). Minerva Anestesiol 2014; 80:293–306.
- Kirkpatrick AW, Roberts DJ, De Waele J, et al; Pediatric Guidelines Sub-Committee for the World Society of the Abdominal Compartment Syndrome. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med 2013; 39:1190–1206.
- Holodinsky JK, Roberts DJ, Ball CG, et al. Risk factors for intra-abdominal hypertension and abdominal compartment syndrome among adult intensive care unit patients: a systematic review and meta-analysis. Crit Care 2013; 17:R249.
- Sugrue M, Bauman A, Jones F, et al. Clinical examination is an inaccurate predictor of intraabdominal pressure. World J Surg 2002; 26:1428–1431.
- Cheatham ML, De Waele JJ, De Laet I, et al; World Society of the Abdominal Compartment Syndrome (WSACS) Clinical Trials Working Group. The impact of body position on intra-abdominal pressure measurement: a multicenter analysis. Crit Care Med 2009; 37:2187–2190.
- Ortiz-Diaz E, Lan CK. Intra-abdominal hypertension in medical critically ill patients: a narrative review. Shock 2014; 41:175–180.
- Cotton BA, Reddy N, Hatch QM, et al. Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg 2011; 254:598–605.
- An G, West MA. Abdominal compartment syndrome: a concise clinical review. Crit Care Med 2008; 36:1304–1310.
- Tolcher MC, Nitsche JF, Arendt KW, Rose CH. Spontaneous rectus sheath hematoma pregnancy: case report and review of the literature. Obstet Gynecol Surv 2010; 65:517–522.
- Berná JD, Garcia-Medina V, Guirao J, Garcia-Medina J. Rectus sheath hematoma: diagnostic classification by CT. Abdom Imaging 1996; 21:62–64.
- Unger EC, Glazer HS, Lee JK, Ling D. MRI of extracranial hematomas: preliminary observations. AJR Am J Roentgenol 1986; 146:403–407.
- Rimola J, Perendreu J, Falcó J, Fortuño JR, Massuet A, Branera J. Percutaneous arterial embolization in the management of rectus sheath hematoma. AJR Am J Roentgenol 2007; 188:W497–W502.
- Titone C, Lipsius M, Krakauer JS. “Spontaneous” hematoma of the rectus abdominis muscle: critical review of 50 cases with emphasis on early diagnosis and treatment. Surgery 1972; 72:568–572.
- Osinbowale O, Bartholomew JR. Rectus sheath hematoma. Vasc Med 2008; 13:275–279.
- Berná-Serna JD, Sánchez-Garre J, Madrigal M, Zuazu I, Berná-Mestre JD. Ultrasound therapy in rectus sheath hematoma. Phys Ther 2005; 85:352–357.
- Kougias P, Lau D, El Sayed HF, Zhou W, Huynh TT, Lin PH. Determinants of mortality and treatment outcome following surgical interventions for acute mesenteric ischemia. J Vasc Surg 2007; 46:467–474.
- Sise MJ. Acute mesenteric ischemia. Surg Clin North Am 2014; 94:165–181.
- Scharff JR, Longo WE, Vartanian SM, Jacobs DL, Bahadursingh AN, Kaminski DL. Ischemic colitis: spectrum of disease and outcome. Surgery 2003; 134:624–629.
- Lange H, Jäckel R. Usefulness of plasma lactate concentration in the diagnosis of acute abdominal disease. Eur J Surg 1994; 160:381–384.
- Gearhart SL, Delaney CP, Senagore AJ, et al. Prospective assessment of the predictive value of alpha-glutathione S-transferase for intestinal ischemia. Am Surg 2003; 69:324–329.
- Kanda T, Fujii H, Tani T, et al. Intestinal fatty acid-binding protein is a useful diagnostic marker for mesenteric infarction in humans. Gastroenterology 1996; 110:339–343.
- Menke J. Diagnostic accuracy of multidetector CT in acute mesenteric ischemia: systematic review and meta-analysis. Radiology 2010; 256:93–101.
- Acosta S, Björnsson S, Ekberg O, Resch T. CT angiography followed by endovascular intervention for acute superior mesenteric artery occlusion does not increase risk of contrast-induced renal failure. Eur J Vasc Endovasc Surg 2010; 39:726–730.
- Clark RA. Computed tomography of bowel infarction. J Comput Assist Tomogr 1987; 11:757–762.
- Acosta S, Björck M. Modern treatment of acute mesenteric ischaemia. Br J Surg 2014; 101:e100–e108.
- Smithson A, Ruiz J, Perello R, Valverde M, Ramos J, Garzo L. Diagnostic and management of spontaneous rectus sheath hematoma. Eur J Intern Med 2013; 24:579–582.
- Moreno Gallego A, Aguayo JL, Flores B, et al. Ultrasonography and computed tomography reduce unnecessary surgery in abdominal rectus sheath haematoma. Br J Surg 1997; 84:1295–1297.
- Dubinsky IL. Hematoma of the rectus abdominis muscle: case report and review of the literature. J Emerg Med 1997; 15:165–167.
- Yi M, Yao G, Bai Y. The monitoring of intra-abdominal pressure in critically ill patients. (In Chinese.) Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2014; 26:175–178.
- Hunt L, Frost SA, Hillman K, Newton PJ, Davidson PM. Management of intra-abdominal hypertension and abdominal compartment syndrome: a review. J Trauma Manag Outcomes 2014; 8:2.
- Malbrain ML, Cheatham ML, Kirkpatrick A, et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med 2006; 32:1722–1732.
- Malbrain ML, Chiumello D, Cesana BM, et al; WAKE-Up! Investigators. A systematic review and individual patient data meta-analysis on intra-abdominal hypertension in critically ill patients: the wake-up project. World initiative on Abdominal Hypertension Epidemiology, a Unifying Project (WAKE-Up!). Minerva Anestesiol 2014; 80:293–306.
- Kirkpatrick AW, Roberts DJ, De Waele J, et al; Pediatric Guidelines Sub-Committee for the World Society of the Abdominal Compartment Syndrome. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med 2013; 39:1190–1206.
- Holodinsky JK, Roberts DJ, Ball CG, et al. Risk factors for intra-abdominal hypertension and abdominal compartment syndrome among adult intensive care unit patients: a systematic review and meta-analysis. Crit Care 2013; 17:R249.
- Sugrue M, Bauman A, Jones F, et al. Clinical examination is an inaccurate predictor of intraabdominal pressure. World J Surg 2002; 26:1428–1431.
- Cheatham ML, De Waele JJ, De Laet I, et al; World Society of the Abdominal Compartment Syndrome (WSACS) Clinical Trials Working Group. The impact of body position on intra-abdominal pressure measurement: a multicenter analysis. Crit Care Med 2009; 37:2187–2190.
- Ortiz-Diaz E, Lan CK. Intra-abdominal hypertension in medical critically ill patients: a narrative review. Shock 2014; 41:175–180.
- Cotton BA, Reddy N, Hatch QM, et al. Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg 2011; 254:598–605.
- An G, West MA. Abdominal compartment syndrome: a concise clinical review. Crit Care Med 2008; 36:1304–1310.
- Tolcher MC, Nitsche JF, Arendt KW, Rose CH. Spontaneous rectus sheath hematoma pregnancy: case report and review of the literature. Obstet Gynecol Surv 2010; 65:517–522.
- Berná JD, Garcia-Medina V, Guirao J, Garcia-Medina J. Rectus sheath hematoma: diagnostic classification by CT. Abdom Imaging 1996; 21:62–64.
- Unger EC, Glazer HS, Lee JK, Ling D. MRI of extracranial hematomas: preliminary observations. AJR Am J Roentgenol 1986; 146:403–407.
- Rimola J, Perendreu J, Falcó J, Fortuño JR, Massuet A, Branera J. Percutaneous arterial embolization in the management of rectus sheath hematoma. AJR Am J Roentgenol 2007; 188:W497–W502.
- Titone C, Lipsius M, Krakauer JS. “Spontaneous” hematoma of the rectus abdominis muscle: critical review of 50 cases with emphasis on early diagnosis and treatment. Surgery 1972; 72:568–572.
- Osinbowale O, Bartholomew JR. Rectus sheath hematoma. Vasc Med 2008; 13:275–279.
- Berná-Serna JD, Sánchez-Garre J, Madrigal M, Zuazu I, Berná-Mestre JD. Ultrasound therapy in rectus sheath hematoma. Phys Ther 2005; 85:352–357.
Alcoholic hepatitis: Challenges in diagnosis and management
Alcoholic hepatitis, a severe manifestation of alcoholic liver disease, is rising in incidence. Complete abstinence from alcohol remains the cornerstone of treatment, while other specific interventions aim to decrease short-term mortality rates.
Despite current treatments, about 25% of patients with severe alcoholic hepatitis eventually die of it. For those who survive hospitalization, measures need to be taken to prevent recidivism. Although liver transplantation seems to hold promise, early transplantation is still largely experimental in alcoholic hepatitis and will likely be available to only a small subset of patients, especially in view of ethical issues and the possible wider implications for transplant centers.
New treatments will largely depend on a better understanding of the disease’s pathophysiology, and future clinical trials should evaluate therapies that improve short-term as well as long-term outcomes.
ACUTE HEPATIC DECOMPENSATION IN A HEAVY DRINKER
Excessive alcohol consumption is very common worldwide, is a major risk factor for liver disease, and is a leading cause of preventable death. Alcoholic cirrhosis is the eighth most common cause of death in the United States and in 2010 was responsible for nearly half of cirrhosis-related deaths worldwide.1
Alcoholic liver disease is a spectrum. Nearly all heavy drinkers (ie, those consuming 40 g or more of alcohol per day, Table 1) have fatty liver changes, 20% to 40% develop fibrosis, 10% to 20% progress to cirrhosis, and of those with cirrhosis, 1% to 2% are diagnosed with hepatocellular carcinoma every year.2
Within this spectrum, alcoholic hepatitis is a well-defined clinical syndrome characterized by acute hepatic decompensation that typically results from long-standing alcohol abuse. Binge drinkers may also be at risk for alcoholic hepatitis, but good data on the association between drinking patterns and the risk of alcoholic hepatitis are limited.
Alcoholic hepatitis varies in severity from mild to life-threatening.3 Although its exact incidence is unknown, its prevalence in alcoholics has been estimated at 20%.4 Nearly half of patients with alcoholic hepatitis have cirrhosis at the time of their acute presentation, and these patients generally have a poor prognosis, with a 28-day death rate as high as 50% in severe cases.5,6 Moreover, although alcoholic hepatitis develops in only a subset of patients with alcoholic liver disease, hospitalizations for it are increasing in the United States.7
Women are at higher risk of developing alcoholic hepatitis, an observation attributed to the effect of estrogens on oxidative stress and inflammation, lower gastric alcohol dehydrogenase levels resulting in slower first-pass metabolism of alcohol, and higher body fat content causing a lower volume of distribution for alcohol than in men.8 The incidence of alcoholic hepatitis is also influenced by a number of demographic and genetic factors as well as nutritional status and coexistence of other liver diseases.9 Most patients diagnosed with alcoholic hepatitis are active drinkers, but it can develop even after significantly reducing or stopping alcohol consumption.
FATTY ACIDS, ENZYMES, CYTOKINES, INFLAMMATION
Alcohol consumption induces fatty acid synthesis and inhibits fatty acid oxidation, thereby promoting fat deposition in the liver.
The major enzymes involved in alcohol metabolism are cytochrome P450 2E1 (CYP2E1) and alcohol dehydrogenase. CYP2E1 is inducible and is up-regulated when excess alcohol is ingested, while alcohol dehydrogen-
ase function is relatively stable. Oxidative degradation of alcohol by these enzymes generates reactive oxygen species and acetaldehyde, inducing liver injury.10 Interestingly, it has been proposed that variations in the genes for these enzymes influence alcohol consumption and dependency as well as alcohol-driven tissue damage.
In addition, alcohol disrupts the intestinal mucosal barrier, allowing lipopolysaccharides from gram-negative bacteria to travel to the liver via the portal vein. These lipopolysaccharides then bind to and activate sinusoidal Kupffer cells, leading to production of several cytokines such as tumor necrosis factor alpha, interleukin 1, and transforming growth factor beta. These cytokines promote hepatocyte inflammation, apoptosis, and necrosis (Figure 1).11
Besides activating the innate immune system, the reactive oxygen species resulting from alcohol metabolism interact with cellular components, leading to production of protein adducts. These act as antigens that activate the adaptive immune response, followed by B- and T-lymphocyte infiltration, which in turn contribute to liver injury and inflammation.12
THE DIAGNOSIS IS MAINLY CLINICAL
The diagnosis of alcoholic hepatitis is mainly clinical. In its usual presentation, jaundice develops rapidly in a person with a known history of heavy alcohol use. Other symptoms and signs may include ascites, encephalopathy, and fever. On examination, the liver may be enlarged and tender, and a hepatic bruit has been reported.13
Other classic signs of liver disease such as parotid enlargement, Dupuytren contracture, dilated abdominal wall veins, and spider nevi can be present, but none is highly specific or sensitive for alcoholic hepatitis.
Elevated liver enzymes and other clues
Laboratory tests are important in evaluating potential alcoholic hepatitis, although no single laboratory marker can definitively establish alcohol as the cause of liver disease. To detect alcohol consumption, biochemical markers such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), mean corpuscular volume, carbohydrate-deficient transferrin, and, more commonly, gamma-glutamyl transpeptidase are used.
In the acute setting, typical biochemical derangements in alcoholic hepatitis include elevated AST (up to 2 to 6 times the upper limit of normal; usually less than 300 IU/L) and elevated ALT to a lesser extent,14 with an AST-to-ALT ratio greater than 2. Neutrophilia, anemia, hyperbilirubinemia, and coagulopathy with an elevated international normalized ratio are common.
Patients with alcoholic hepatitis are also prone to develop bacterial infections, and about 7% develop hepatorenal syndrome, itself an ominous sign.15
Imaging studies are valuable in excluding other causes of abnormal liver test results in patients who abuse alcohol, such as biliary obstruction, infiltrative liver diseases, and hepatocellular carcinoma.
Screen for alcohol intake
During the initial evaluation of suspected alcoholic hepatitis, one should screen for excessive drinking. In a US Centers for Disease Control and Prevention study, only one of six US adults, including binge drinkers, said they had ever discussed alcohol consumption with a health professional.16 Many patients with alcoholic liver disease in general and alcoholic hepatitis in particular deny alcohol abuse or underreport their intake.17
Screening tests such as the CAGE questionnaire and the Alcohol Use Disorders Identification Test can be used to assess alcohol dependence or abuse.18,19 The CAGE questionnaire consists of four questions:
- Have you ever felt you should cut down on your drinking?
- Have people annoyed you by criticizing your drinking?
- Have you ever felt guilty about your drinking?
- Have you ever had a drink first thing in the morning (an eye-opener) to steady your nerves or to get rid of a hangover?
A yes answer to two or more questions is considered clinically significant.
Is liver biopsy always needed?
Although alcoholic hepatitis can be suspected on the basis of clinical and biochemical clues, liver biopsy remains the gold standard diagnostic tool. It confirms the clinical diagnosis of alcoholic hepatitis in about 85% of all patients and in up to 95% when significant hyperbilirubinemia is present.20
However, whether a particular patient needs a biopsy is not always clear. The American Association for the Study of Liver Diseases (AASLD) recommends biopsy in patients who have a clinical diagnosis of severe alcoholic hepatitis for whom medical treatment is being considered and in those with an uncertain underlying diagnosis.
Findings on liver biopsy in alcoholic hepatitis include steatosis, hepatocyte ballooning, neutrophilic infiltration, Mallory bodies (which represent aggregated cytokeratin intermediate filaments and other proteins), and scarring with a typical perivenular distribution as opposed to the periportal fibrosis seen in chronic viral hepatitis. Some histologic findings, such as centrilobular necrosis, may overlap alcoholic hepatitis and nonalcoholic steatohepatitis.
In addition to confirming the diagnosis and staging the disease, liver biopsy has prognostic value. The severity of inflammation and cholestatic changes correlates with poor prognosis and may also predict response to corticosteroid treatment in severe cases of alcoholic hepatitis.21
However, the utility of liver biopsy in confirming the diagnosis and assessing the prognosis of alcoholic hepatitis is controversial for several reasons. Coagulopathy, thrombocytopenia, and ascites are all common in patients with alcoholic hepatitis, often making percutaneous liver biopsy contraindicated. Trans-
jugular liver biopsy is not universally available outside tertiary care centers.
Needed is a minimally invasive test for assessing this disease. Breath analysis might be such a test, offering a noninvasive means to study the composition of volatile organic compounds and elemental gases and an attractive method to evaluate health and disease in a patient-friendly manner. Our group devised a model based on breath levels of trimethylamine and pentane. When we tested it, we found that it distinguishes patients with alcoholic hepatitis from those with acute liver decompensation from causes other than alcohol and controls without liver disease with up to 90% sensitivity and 80% specificity.22
ASSESSING THE SEVERITY OF ALCOHOLIC HEPATITIS
Several models have been developed to assess the severity of alcoholic hepatitis and guide treatment decisions (Table 2).
The MDF (Maddrey Discriminant Function)6 system was the first scoring system developed and is still the most widely used. A score of 32 or higher indicates severe alcoholic hepatitis and has been used as the threshold for starting treatment with corticosteroids.6
The MDF has limitations. Patients with a score lower than 32 are considered not to have severe alcoholic hepatitis, but up to 17% of them still die. Also, since it uses the prothrombin time, its results can vary considerably among laboratories, depending on the sensitivity of the thromboplastin reagent used.
The MELD (Model for End-stage Liver Disease) score. Sheth et al23 compared the MELD and the MDF scores in assessing the severity of alcoholic hepatitis. They found that the MELD performed as well as the MDF in predicting 30-day mortality. A MELD score of greater than 11 had a sensitivity in predicting 30-day mortality of 86% and a specificity of 81%, compared with 86% and 48%, respectively, for MDF scores greater than 32.
Another study found a MELD score of 21 to have the highest sensitivity and specificity in predicting mortality (an estimated 90-day death rate of 20%). Thus, a MELD score of 21 is an appropriate threshold for prompt consideration of specific therapies such as corticosteroids.24
The MELD score has become increasingly important in patients with alcoholic hepatitis, as some of them may become candidates for liver transplantation (see below). Also, serial MELD scores in hospitalized patients have prognostic implications, since an increase of 2 or more points in the first week has been shown to predict in-hospital mortality.25
The GAHS (Glasgow Alcoholic Hepatitis Score)26 was shown to identify patients with alcoholic hepatitis who have an especially poor prognosis and need corticosteroid therapy. In those with a GAHS of 9 or higher, the 28-day survival rate was 78% with corticosteroid treatment and 52% without corticosteroid treatment; survival rates at 84 days were 59% and 38%, respectively.26
The ABIC scoring system (Age, Serum Bilirubin, INR, and Serum Creatinine) stratifies patients by risk of death at 90 days27:
- Score less than 6.71: low risk (100% survival)
- A score 6.71–8.99: intermediate risk (70% survival)
- A score 9.0 or higher: high risk (25% survival).
Both the GAHS and ABIC score are limited by lack of external validation.
The Lille score.28 While the above scores are used to identify patients at risk of death from alcoholic hepatitis and to decide on starting corticosteroids, the Lille score is designed to assess response to corticosteroids after 1 week of treatment. It is calculated based on five pretreatment variables and the change in serum bilirubin level at day 7 of corticosteroid therapy. Lille scores range from 0 to 1; a score higher than 0.45 is associated with a 75% mortality rate at 6 months and indicates a lack of response to corticosteroids and that these drugs should be discontinued.28
MANAGEMENT
Supportive treatment
Abstinence from alcohol is the cornerstone of treatment of alcoholic hepatitis. Early management of alcohol abuse or dependence is, therefore, warranted in all patients with alcoholic hepatitis. Referral to addiction specialists, motivational therapies, and anticraving drugs such as baclofen can be utilized.
Treat alcohol withdrawal. Alcoholics who suddenly decrease or discontinue their alcohol use are at high risk of alcohol withdrawal syndrome. Within 24 hours after the last drink, patients can experience increases in their heart rate and blood pressure, along with irritability and hyperreflexia. Within the next few days, more dangerous complications including seizures and delirium tremens can arise.
Alcohol withdrawal symptoms should be treated with short-acting benzodiazepines or clomethiazole, keeping the risk of worsening encephalopathy in mind.29 If present, complications of cirrhosis such as encephalopathy, ascites, and variceal bleeding should be managed.
Nutritional support is important. Protein-calorie malnutrition is common in alcoholics, as are deficiencies of vitamin A, vitamin D, thiamine, folate, pyridoxine, and zinc.30 Although a randomized controlled trial comparing enteral nutrition (2,000 kcal/day) vs corticosteroids (prednisolone 40 mg/day) in patients with alcoholic hepatitis did not show any difference in the 28-day mortality rate, those who received nutritional support and survived the first month had a lower mortality rate than those treated with corticosteroids (8% vs 37%).31 A daily protein intake of 1.5 g per kilogram of body weight is therefore recommended, even in patients with hepatic encephalopathy.15
Combining enteral nutrition and corticosteroid treatment may have a synergistic effect but is yet to be investigated.
Screen for infection. Patients with alcoholic hepatitis should be screened for infection, as about 25% of those with severe alcoholic hepatitis have an infection at admission.32 Since many of these patients meet the criteria for systemic inflammatory response syndrome, infections can be particularly difficult to diagnose. Patients require close clinical monitoring as well as regular pancultures for early detection. Antibiotics are frequently started empirically even though we lack specific evidence-based guidelines on this practice.33
Corticosteroids
Various studies have evaluated the role of corticosteroids in treating alcoholic hepatitis, differing considerably in sample populations, methods, and end points. Although the results of individual trials differ, meta-analyses indicate that corticosteroids have a moderate beneficial effect in patients with severe alcoholic hepatitis.
For example, Rambaldi et al34 performed a meta-analysis that concluded the mortality rate was lower in alcoholic hepatitis patients with MDF scores of at least 32 or hepatic encephalopathy who were treated with corticosteroids than in controls (relative risk 0.37, 95% confidence interval 0.16–0.86).
Therefore, in the absence of contraindications, the AASLD recommends starting corticosteroids in patients with severe alcoholic hepatitis, defined as an MDF score of 32 or higher.21 The preferred agent is oral prednisolone 40 mg daily or parenteral methylprednisolone 32 mg daily for 4 weeks and then tapered over the next 2 to 4 weeks or abruptly discontinued. Because activation of prednisone is decreased in patients with liver disease, prednisolone (the active form) is preferred over prednisone (the inactive precursor).35 In alcoholic hepatitis, the number needed to treat with corticosteroids to prevent one death has been calculated36 at 5.
As mentioned, response to corticosteroids is commonly assessed at 1 week of treatment using the Lille score. A score higher than 0.45 predicts a poor response and should trigger discontinuation of corticosteroids, particularly in those classified as null responders (Lille score > 0.56).
Adverse effects of steroids include sepsis, gastrointestinal bleeding, and steroid psychosis. Of note, patients who have evidence of hepatorenal syndrome or gastrointestinal bleeding tend to have a less favorable response to corticosteroids. Also, while infections were once considered a contraindication to steroid therapy, recent evidence suggests that steroid use might not be precluded in infected patients after appropriate antibiotic therapy. Infections occur in about a quarter of all alcoholic hepatitis patients treated with steroids, more frequently in null responders (42.5%) than in responders (11.1%), which supports corticosteroid discontinuance at 1 week in null responders.32
Pentoxifylline
An oral phosphodiesterase inhibitor, pentoxifylline, also inhibits production of several cytokines, including tumor necrosis factor alpha. At a dose of 400 mg orally three times daily for 4 weeks, pentoxifylline has been used in treating severe alcoholic hepatitis (MDF score ≥ 32) and is recommended especially if corticosteroids are contraindicated, as with sepsis.21
An early double-blind clinical trial randomized patients with severe alcoholic hepatitis to receive either pentoxifylline 400 mg orally three times daily or placebo. Of the patients who received pentoxifylline, 24.5% died during the index hospitalization, compared with 46.1% of patients who received placebo. This survival benefit was mainly related to a markedly lower incidence of hepatorenal syndrome as the cause of death in the pentoxifylline group than in the placebo group (50% vs 91.7% of deaths).37
In a small clinical trial in patients with severe alcoholic hepatitis, pentoxifylline recipients had a higher 3-month survival rate than prednisolone recipients (35.29% vs 14.71%, P = .04).38 However, a larger trial showed no improvement in 6-month survival with the combination of prednisolone and pentoxifylline compared with prednisolone alone (69.9% vs 69.2%, P = .91).39 Also, a meta-analysis of five randomized clinical trials found no survival benefit with pentoxifylline therapy.40
Of note, in the unfortunate subgroup of patients who have a poor response to corticosteroids, no alternative treatment, including pentoxifylline, has been shown to be effective.41
Prednisone or pentoxifylline? Very recently, results of the Steroids or Pentoxifylline for Alcoholic Hepatitis (STOPAH) trial have been released.42 This is a large, multicenter, double-blinded clinical trial that aimed to provide a definitive answer to whether corticosteroids or pentoxifylline (or both) are beneficial in patients with alcoholic hepatitis. The study included 1,103 adult patients with severe alcoholic hepatitis (MDF score ≥ 32) who were randomized to monotherapy with prednisolone or pentoxifylline, combination therapy, or placebo. The primary end point was mortality at 28 days, and secondary end points included mortality at 90 days and at 1 year. Prednisolone reduced 28-day mortality by about 39%. In contrast, the 28-day mortality rate was similar in patients who received pentoxifylline and those who did not. Also, neither drug was significantly associated with a survival benefit beyond 28 days. The investigators concluded that pentoxifylline has no impact on disease progression and should not be used for the treatment of severe alcoholic hepatitis.42
Other tumor necrosis factor alpha inhibitors not recommended
Two other tumor necrosis factor alpha inhibitors, infliximab and etanercept, have been tested in clinical trials in alcoholic hepatitis. Unfortunately, the results were not encouraging, with no major reduction in mortality.43–45 In fact, these trials demonstrated a significantly increased risk of infections in the treatment groups. Therefore, these drugs are not recommended for treating alcoholic hepatitis.
A possible explanation is that tumor necrosis factor alpha plays an important role in liver regeneration, aiding in recovery from alcohol-induced liver injury, and inhibiting it can have deleterious consequences.
Other agents
A number of other agents have undergone clinical trials in alcoholic hepatitis.
N-acetylcysteine, an antioxidant that replenishes glutathione stores in hepatocytes, was evaluated in a randomized clinical trial in combination with prednisolone.46 Although the 1-month mortality rate was significantly lower in the combination group than in the prednisolone-only group (8% vs 24%, P = .006), 3-month and 6-month mortality rates were not. Nonetheless, the rates of infection and hepatorenal syndrome were lower in the combination group. Therefore, corticosteroids and N-acetylcysteine may have synergistic effects, but the optimum duration of N-acetylcysteine therapy needs to be determined in further studies.
Vitamin E, silymarin, propylthiouracil, colchicine, and oxandrolone (an anabolic steroid) have also been studied, but with no convincing benefit.21
Role of liver transplantation
Liver transplantation for alcoholic liver disease has been a topic of great medical and social controversy. The view that alcoholic patients are responsible for their own illness led to caution when contemplating liver transplantation. Many countries require 6 months of abstinence from alcohol before placing a patient on the liver transplant list, posing a major obstacle to patients with alcoholic hepatitis, as almost all are active drinkers at the time of presentation and many will die within 6 months. Reasons for this 6-month rule include donor shortage and risk of recidivism.47
With regard to survival following alcoholic hepatitis, a study utilizing the United Network for Organ Sharing database matched patients with alcoholic hepatitis and alcoholic cirrhosis who underwent liver transplantation. Rates of 5-year graft survival were 75% in those with alcoholic hepatitis and 73% in those with alcoholic cirrhosis (P = .97), and rates of patient survival were 80% and 78% (P = .90), respectively. Proportional regression analysis adjusting for other variables showed no impact of the etiology of liver disease on graft or patient survival. The investigators concluded that liver transplantation could be considered in a select group of patients with alcoholic hepatitis who do not improve with medical therapy.48
In a pivotal case-control prospective study,49 26 patients with Lille scores greater than 0.45 were listed for liver transplantation within a median of 13 days after nonresponse to medical therapy. The cumulative 6-month survival rate was higher in patients who received a liver transplant early than in those who did not (77% vs 23%, P < .001). This benefit was maintained through 2 years of follow-up (hazard ratio 6.08, P = .004). Of note, all these patients had supportive family members, no severe coexisting conditions, and a commitment to alcohol abstinence (although 3 patients resumed drinking after liver transplantation).49
Although these studies support early liver transplantation in carefully selected patients with severe alcoholic hepatitis, the criteria for transplantation in this group need to be refined. Views on alcoholism also need to be reconciled, as strong evidence is emerging that implicates genetic and environmental influences on alcohol dependence.
Management algorithm
Figure 2 shows a suggested management algorithm for alcoholic hepatitis, adapted from the guidelines of the AASLD and European Association for the Study of the Liver.
NEW THERAPIES NEEDED
Novel therapies for severe alcoholic hepatitis are urgently needed to help combat this devastating condition. Advances in understanding its pathophysiology have uncovered several new therapeutic targets, and new agents are already being evaluated in clinical trials.
IMM 124-E, a hyperimmune bovine colostrum enriched with immunoglobulin G anti-lipopolysaccharide, is going to be evaluated in combination with prednisolone in patients with severe alcoholic hepatitis.
Anakinra, an interleukin 1 receptor antagonist, has significant anti-inflammatory activity and is used to treat rheumatoid arthritis. A clinical trial to evaluate its role in alcoholic hepatitis has been designed in which patients with severe alcoholic hepatitis (defined as a MELD score ≥ 21) will be randomized to receive either methylprednisolone or a combination of anakinra, pentoxifylline, and zinc (a mineral that improves gut integrity).
Emricasan, an orally active caspase protease inhibitor, is another agent currently being tested in a phase 2 clinical trial in patients with severe alcoholic hepatitis. Since caspases induce apoptosis, inhibiting them should theoretically dampen alcohol-induced hepatocyte injury.
Interleukin 22, a hepatoprotective cytokine, shows promise as a treatment and will soon be evaluated in alcoholic hepatitis.
- Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol 2013; 59:160–168.
- Teli MR, Day CP, Burt AD, Bennett MK, James OF. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet 1995; 346:987–990.
- Alcoholic liver disease: morphological manifestations. Review by an international group. Lancet 1981; 1:707–711.
- Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput JC. Excess weight risk factor for alcoholic liver disease. Hepatology 1997; 25:108–111.
- Basra S, Anand BS. Definition, epidemiology and magnitude of alcoholic hepatitis. World J Hepatol 2011; 3:108–113.
- Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978; 75:193–199.
- Jinjuvadia R, Liangpunsakul S, for the Translational Research and Evolving Alcoholic Hepatitis Treatment Consortium. Trends in alcoholic hepatitis-related hospitalizations, financial burden, and mortality in the United States. J Clin Gastroenterol 2014 Jun 25 (Epub ahead of print).
- Sato N, Lindros KO, Baraona E, et al. Sex difference in alcohol-related organ injury. Alcohol Clin Exp Res 2001; 25(suppl s1):40S–45S.
- Singal AK, Kamath PS, Gores GJ, Shah VH. Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol 2014; 12:555–564.
- Seitz HK, Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol Chem 2006; 387:349–360.
- Thurman RG. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol 1998; 275:G605–G611.
- Duddempudi AT. Immunology in alcoholic liver disease. Clin Liver Dis 2012; 16:687–698.
- Lischner MW, Alexander JF, Galambos JT. Natural history of alcoholic hepatitis. I. The acute disease. Am J Dig Dis 1971; 16:481–494.
- Cohen JA, Kaplan MM. The SGOT/SGPT ratio—an indicator of alcoholic liver disease. Dig Dis Sci 1979; 24:835–838.
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 2009; 360:2758–2769.
- McKnight-Eily LR, Liu Y, Brewer RD, et al; Centers for Disease Control and Prevention (CDC). Vital signs: communication between health professionals and their patients about alcohol use—44 states and the District of Columbia, 2011. MMWR Morb Mortal Wkly Rep 2014; 63:16–22.
- Grant BF. Barriers to alcoholism treatment: reasons for not seeking treatment in a general population sample. J Stud Alcohol 1997; 58:365–371.
- Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol 2004; 57:30–39.
- The Alcohol Use Disorders Identification Test Guidelines for Use in Primary Care. Second Edition. World Health Organization. Department of Mental Health and Substance Dependence. http://whqlibdoc.who.int/hq/2001/who_msd_msb_01.6a.pdf. Accessed February 3, 2015.
- Hamid R, Forrest EH. Is histology required for the diagnosis of alcoholic hepatitis? A review of published randomised controlled trials. Gut 2011; 60(suppl 1):A233.
- O’Shea RS, Dasarathy S, McCullough AJ; Practice Guideline Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Alcoholic liver disease. Hepatology 2010; 51:307–328.
- Hanouneh IA, Zein NN, Cikach F, et al. The breathprints in patients with liver disease identify novel breath biomarkers in alcoholic hepatitis. Clin Gastroenterol Hepatol 2014; 12:516–523.
- Sheth M, Riggs M, Patel T. Utility of the Mayo End-Stage Liver Disease (MELD) score in assessing prognosis of patients with alcoholic hepatitis. BMC Gastroenterol 2002; 2:2.
- Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology 2005; 41:353–358.
- Srikureja W, Kyulo NL, Runyon BA, Hu KQ. MELD score is a better prognostic model than Child-Turcotte-Pugh score or Discriminant Function score in patients with alcoholic hepatitis. J Hepatol 2005; 42:700–706.
- Forrest EH, Morris AJ, Stewart S, et al. The Glasgow alcoholic hepatitis score identifies patients who may benefit from corticosteroids. Gut 2007; 56:1743–1746.
- Dominguez M, Rincón D, Abraldes JG, et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol 2008; 103:2747–2756.
- Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007; 45:1348–1354.
- Mayo-Smith MF, Beecher LH, Fischer TL, et al; Working Group on the Management of Alcohol Withdrawal Delirium, Practice Guidelines Committee, American Society of Addiction Medicine. Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med 2004; 164:1405–1412.
- Mezey E. Interaction between alcohol and nutrition in the pathogenesis of alcoholic liver disease. Semin Liver Dis 1991; 11:340–348.
- Cabré E, Rodríguez-Iglesias P, Caballería J, et al. Short- and long-term outcome of severe alcohol-induced hepatitis treated with steroids or enteral nutrition: a multicenter randomized trial. Hepatology 2000; 32:36–42.
- Louvet A, Wartel F, Castel H, et al. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology 2009; 137:541–548.
- European Association for the Study of Liver. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol 2012; 57:399–420.
- Rambaldi A, Saconato HH, Christensen E, Thorlund K, Wetterslev J, Gluud C. Systematic review: glucocorticosteroids for alcoholic hepatitis—a Cochrane Hepato-Biliary Group systematic review with meta-analyses and trial sequential analyses of randomized clinical trials. Aliment Pharmacol Ther 2008; 27:1167–1178.
- Powell LW, Axelsen E. Corticosteroids in liver disease: studies on the biological conversion of prednisone to prednisolone and plasma protein binding. Gut 1972; 13:690–696.
- Mathurin P, O’Grady J, Carithers RL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut 2011; 60:255–260.
- Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology 2000; 119:1637–1648.
- De BK, Gangopadhyay S, Dutta D, Baksi SD, Pani A, Ghosh P. Pentoxifylline versus prednisolone for severe alcoholic hepatitis: a randomized controlled trial. World J Gastroenterol 2009; 15:1613–1619.
- Mathurin P, Louvet A, Dao T, et al. Addition of pentoxifylline to prednisolone for severe alcoholic hepatitis does not improve 6-month survival: results of the CORPENTOX trial (abstract). Hepatology 2011; 54(suppl 1):81A.
- Whitfield K, Rambaldi A, Wetterslev J, Gluud C. Pentoxifylline for alcoholic hepatitis. Cochrane Database Syst Rev 2009; CD007339.
- Louvet A, Diaz E, Dharancy S, et al. Early switch to pentoxifylline in patients with severe alcoholic hepatitis is inefficient in non-responders to corticosteroids. J Hepatol 2008; 48:465–470.
- Thursz MR, Richardson P, Allison ME, et al. Steroids or pentoxifylline for alcoholic hepatitis: results of the STOPAH trial [abstract LB-1]. 65th Annual Meeting of the American Association for the Study of Liver Diseases; November 7–11, 2014; Boston, MA.
- Naveau S, Chollet-Martin S, Dharancy S, et al; Foie-Alcool group of the Association Française pour l’Etude du Foie. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology 2004; 39:1390–1397.
- Menon KV, Stadheim L, Kamath PS, et al. A pilot study of the safety and tolerability of etanercept in patients with alcoholic hepatitis. Am J Gastroenterol 2004; 99:255–260.
- Boetticher NC, Peine CJ, Kwo P, et al. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology 2008; 135:1953–1960.
- Nguyen-Khac E, Thevenot T, Piquet MA, et al; AAH-NAC Study Group. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med 2011; 365:1781–1789.
- Singal AK, Duchini A. Liver transplantation in acute alcoholic hepatitis: current status and future development. World J Hepatol 2011; 3:215–218.
- Singal AK, Bashar H, Anand BS, Jampana SC, Singal V, Kuo YF. Outcomes after liver transplantation for alcoholic hepatitis are similar to alcoholic cirrhosis: exploratory analysis from the UNOS database. Hepatology 2012; 55:1398–1405.
- Mathurin P, Moreno C, Samuel D, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med 2011; 365:1790–1800.
Alcoholic hepatitis, a severe manifestation of alcoholic liver disease, is rising in incidence. Complete abstinence from alcohol remains the cornerstone of treatment, while other specific interventions aim to decrease short-term mortality rates.
Despite current treatments, about 25% of patients with severe alcoholic hepatitis eventually die of it. For those who survive hospitalization, measures need to be taken to prevent recidivism. Although liver transplantation seems to hold promise, early transplantation is still largely experimental in alcoholic hepatitis and will likely be available to only a small subset of patients, especially in view of ethical issues and the possible wider implications for transplant centers.
New treatments will largely depend on a better understanding of the disease’s pathophysiology, and future clinical trials should evaluate therapies that improve short-term as well as long-term outcomes.
ACUTE HEPATIC DECOMPENSATION IN A HEAVY DRINKER
Excessive alcohol consumption is very common worldwide, is a major risk factor for liver disease, and is a leading cause of preventable death. Alcoholic cirrhosis is the eighth most common cause of death in the United States and in 2010 was responsible for nearly half of cirrhosis-related deaths worldwide.1
Alcoholic liver disease is a spectrum. Nearly all heavy drinkers (ie, those consuming 40 g or more of alcohol per day, Table 1) have fatty liver changes, 20% to 40% develop fibrosis, 10% to 20% progress to cirrhosis, and of those with cirrhosis, 1% to 2% are diagnosed with hepatocellular carcinoma every year.2
Within this spectrum, alcoholic hepatitis is a well-defined clinical syndrome characterized by acute hepatic decompensation that typically results from long-standing alcohol abuse. Binge drinkers may also be at risk for alcoholic hepatitis, but good data on the association between drinking patterns and the risk of alcoholic hepatitis are limited.
Alcoholic hepatitis varies in severity from mild to life-threatening.3 Although its exact incidence is unknown, its prevalence in alcoholics has been estimated at 20%.4 Nearly half of patients with alcoholic hepatitis have cirrhosis at the time of their acute presentation, and these patients generally have a poor prognosis, with a 28-day death rate as high as 50% in severe cases.5,6 Moreover, although alcoholic hepatitis develops in only a subset of patients with alcoholic liver disease, hospitalizations for it are increasing in the United States.7
Women are at higher risk of developing alcoholic hepatitis, an observation attributed to the effect of estrogens on oxidative stress and inflammation, lower gastric alcohol dehydrogenase levels resulting in slower first-pass metabolism of alcohol, and higher body fat content causing a lower volume of distribution for alcohol than in men.8 The incidence of alcoholic hepatitis is also influenced by a number of demographic and genetic factors as well as nutritional status and coexistence of other liver diseases.9 Most patients diagnosed with alcoholic hepatitis are active drinkers, but it can develop even after significantly reducing or stopping alcohol consumption.
FATTY ACIDS, ENZYMES, CYTOKINES, INFLAMMATION
Alcohol consumption induces fatty acid synthesis and inhibits fatty acid oxidation, thereby promoting fat deposition in the liver.
The major enzymes involved in alcohol metabolism are cytochrome P450 2E1 (CYP2E1) and alcohol dehydrogenase. CYP2E1 is inducible and is up-regulated when excess alcohol is ingested, while alcohol dehydrogen-
ase function is relatively stable. Oxidative degradation of alcohol by these enzymes generates reactive oxygen species and acetaldehyde, inducing liver injury.10 Interestingly, it has been proposed that variations in the genes for these enzymes influence alcohol consumption and dependency as well as alcohol-driven tissue damage.
In addition, alcohol disrupts the intestinal mucosal barrier, allowing lipopolysaccharides from gram-negative bacteria to travel to the liver via the portal vein. These lipopolysaccharides then bind to and activate sinusoidal Kupffer cells, leading to production of several cytokines such as tumor necrosis factor alpha, interleukin 1, and transforming growth factor beta. These cytokines promote hepatocyte inflammation, apoptosis, and necrosis (Figure 1).11
Besides activating the innate immune system, the reactive oxygen species resulting from alcohol metabolism interact with cellular components, leading to production of protein adducts. These act as antigens that activate the adaptive immune response, followed by B- and T-lymphocyte infiltration, which in turn contribute to liver injury and inflammation.12
THE DIAGNOSIS IS MAINLY CLINICAL
The diagnosis of alcoholic hepatitis is mainly clinical. In its usual presentation, jaundice develops rapidly in a person with a known history of heavy alcohol use. Other symptoms and signs may include ascites, encephalopathy, and fever. On examination, the liver may be enlarged and tender, and a hepatic bruit has been reported.13
Other classic signs of liver disease such as parotid enlargement, Dupuytren contracture, dilated abdominal wall veins, and spider nevi can be present, but none is highly specific or sensitive for alcoholic hepatitis.
Elevated liver enzymes and other clues
Laboratory tests are important in evaluating potential alcoholic hepatitis, although no single laboratory marker can definitively establish alcohol as the cause of liver disease. To detect alcohol consumption, biochemical markers such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), mean corpuscular volume, carbohydrate-deficient transferrin, and, more commonly, gamma-glutamyl transpeptidase are used.
In the acute setting, typical biochemical derangements in alcoholic hepatitis include elevated AST (up to 2 to 6 times the upper limit of normal; usually less than 300 IU/L) and elevated ALT to a lesser extent,14 with an AST-to-ALT ratio greater than 2. Neutrophilia, anemia, hyperbilirubinemia, and coagulopathy with an elevated international normalized ratio are common.
Patients with alcoholic hepatitis are also prone to develop bacterial infections, and about 7% develop hepatorenal syndrome, itself an ominous sign.15
Imaging studies are valuable in excluding other causes of abnormal liver test results in patients who abuse alcohol, such as biliary obstruction, infiltrative liver diseases, and hepatocellular carcinoma.
Screen for alcohol intake
During the initial evaluation of suspected alcoholic hepatitis, one should screen for excessive drinking. In a US Centers for Disease Control and Prevention study, only one of six US adults, including binge drinkers, said they had ever discussed alcohol consumption with a health professional.16 Many patients with alcoholic liver disease in general and alcoholic hepatitis in particular deny alcohol abuse or underreport their intake.17
Screening tests such as the CAGE questionnaire and the Alcohol Use Disorders Identification Test can be used to assess alcohol dependence or abuse.18,19 The CAGE questionnaire consists of four questions:
- Have you ever felt you should cut down on your drinking?
- Have people annoyed you by criticizing your drinking?
- Have you ever felt guilty about your drinking?
- Have you ever had a drink first thing in the morning (an eye-opener) to steady your nerves or to get rid of a hangover?
A yes answer to two or more questions is considered clinically significant.
Is liver biopsy always needed?
Although alcoholic hepatitis can be suspected on the basis of clinical and biochemical clues, liver biopsy remains the gold standard diagnostic tool. It confirms the clinical diagnosis of alcoholic hepatitis in about 85% of all patients and in up to 95% when significant hyperbilirubinemia is present.20
However, whether a particular patient needs a biopsy is not always clear. The American Association for the Study of Liver Diseases (AASLD) recommends biopsy in patients who have a clinical diagnosis of severe alcoholic hepatitis for whom medical treatment is being considered and in those with an uncertain underlying diagnosis.
Findings on liver biopsy in alcoholic hepatitis include steatosis, hepatocyte ballooning, neutrophilic infiltration, Mallory bodies (which represent aggregated cytokeratin intermediate filaments and other proteins), and scarring with a typical perivenular distribution as opposed to the periportal fibrosis seen in chronic viral hepatitis. Some histologic findings, such as centrilobular necrosis, may overlap alcoholic hepatitis and nonalcoholic steatohepatitis.
In addition to confirming the diagnosis and staging the disease, liver biopsy has prognostic value. The severity of inflammation and cholestatic changes correlates with poor prognosis and may also predict response to corticosteroid treatment in severe cases of alcoholic hepatitis.21
However, the utility of liver biopsy in confirming the diagnosis and assessing the prognosis of alcoholic hepatitis is controversial for several reasons. Coagulopathy, thrombocytopenia, and ascites are all common in patients with alcoholic hepatitis, often making percutaneous liver biopsy contraindicated. Trans-
jugular liver biopsy is not universally available outside tertiary care centers.
Needed is a minimally invasive test for assessing this disease. Breath analysis might be such a test, offering a noninvasive means to study the composition of volatile organic compounds and elemental gases and an attractive method to evaluate health and disease in a patient-friendly manner. Our group devised a model based on breath levels of trimethylamine and pentane. When we tested it, we found that it distinguishes patients with alcoholic hepatitis from those with acute liver decompensation from causes other than alcohol and controls without liver disease with up to 90% sensitivity and 80% specificity.22
ASSESSING THE SEVERITY OF ALCOHOLIC HEPATITIS
Several models have been developed to assess the severity of alcoholic hepatitis and guide treatment decisions (Table 2).
The MDF (Maddrey Discriminant Function)6 system was the first scoring system developed and is still the most widely used. A score of 32 or higher indicates severe alcoholic hepatitis and has been used as the threshold for starting treatment with corticosteroids.6
The MDF has limitations. Patients with a score lower than 32 are considered not to have severe alcoholic hepatitis, but up to 17% of them still die. Also, since it uses the prothrombin time, its results can vary considerably among laboratories, depending on the sensitivity of the thromboplastin reagent used.
The MELD (Model for End-stage Liver Disease) score. Sheth et al23 compared the MELD and the MDF scores in assessing the severity of alcoholic hepatitis. They found that the MELD performed as well as the MDF in predicting 30-day mortality. A MELD score of greater than 11 had a sensitivity in predicting 30-day mortality of 86% and a specificity of 81%, compared with 86% and 48%, respectively, for MDF scores greater than 32.
Another study found a MELD score of 21 to have the highest sensitivity and specificity in predicting mortality (an estimated 90-day death rate of 20%). Thus, a MELD score of 21 is an appropriate threshold for prompt consideration of specific therapies such as corticosteroids.24
The MELD score has become increasingly important in patients with alcoholic hepatitis, as some of them may become candidates for liver transplantation (see below). Also, serial MELD scores in hospitalized patients have prognostic implications, since an increase of 2 or more points in the first week has been shown to predict in-hospital mortality.25
The GAHS (Glasgow Alcoholic Hepatitis Score)26 was shown to identify patients with alcoholic hepatitis who have an especially poor prognosis and need corticosteroid therapy. In those with a GAHS of 9 or higher, the 28-day survival rate was 78% with corticosteroid treatment and 52% without corticosteroid treatment; survival rates at 84 days were 59% and 38%, respectively.26
The ABIC scoring system (Age, Serum Bilirubin, INR, and Serum Creatinine) stratifies patients by risk of death at 90 days27:
- Score less than 6.71: low risk (100% survival)
- A score 6.71–8.99: intermediate risk (70% survival)
- A score 9.0 or higher: high risk (25% survival).
Both the GAHS and ABIC score are limited by lack of external validation.
The Lille score.28 While the above scores are used to identify patients at risk of death from alcoholic hepatitis and to decide on starting corticosteroids, the Lille score is designed to assess response to corticosteroids after 1 week of treatment. It is calculated based on five pretreatment variables and the change in serum bilirubin level at day 7 of corticosteroid therapy. Lille scores range from 0 to 1; a score higher than 0.45 is associated with a 75% mortality rate at 6 months and indicates a lack of response to corticosteroids and that these drugs should be discontinued.28
MANAGEMENT
Supportive treatment
Abstinence from alcohol is the cornerstone of treatment of alcoholic hepatitis. Early management of alcohol abuse or dependence is, therefore, warranted in all patients with alcoholic hepatitis. Referral to addiction specialists, motivational therapies, and anticraving drugs such as baclofen can be utilized.
Treat alcohol withdrawal. Alcoholics who suddenly decrease or discontinue their alcohol use are at high risk of alcohol withdrawal syndrome. Within 24 hours after the last drink, patients can experience increases in their heart rate and blood pressure, along with irritability and hyperreflexia. Within the next few days, more dangerous complications including seizures and delirium tremens can arise.
Alcohol withdrawal symptoms should be treated with short-acting benzodiazepines or clomethiazole, keeping the risk of worsening encephalopathy in mind.29 If present, complications of cirrhosis such as encephalopathy, ascites, and variceal bleeding should be managed.
Nutritional support is important. Protein-calorie malnutrition is common in alcoholics, as are deficiencies of vitamin A, vitamin D, thiamine, folate, pyridoxine, and zinc.30 Although a randomized controlled trial comparing enteral nutrition (2,000 kcal/day) vs corticosteroids (prednisolone 40 mg/day) in patients with alcoholic hepatitis did not show any difference in the 28-day mortality rate, those who received nutritional support and survived the first month had a lower mortality rate than those treated with corticosteroids (8% vs 37%).31 A daily protein intake of 1.5 g per kilogram of body weight is therefore recommended, even in patients with hepatic encephalopathy.15
Combining enteral nutrition and corticosteroid treatment may have a synergistic effect but is yet to be investigated.
Screen for infection. Patients with alcoholic hepatitis should be screened for infection, as about 25% of those with severe alcoholic hepatitis have an infection at admission.32 Since many of these patients meet the criteria for systemic inflammatory response syndrome, infections can be particularly difficult to diagnose. Patients require close clinical monitoring as well as regular pancultures for early detection. Antibiotics are frequently started empirically even though we lack specific evidence-based guidelines on this practice.33
Corticosteroids
Various studies have evaluated the role of corticosteroids in treating alcoholic hepatitis, differing considerably in sample populations, methods, and end points. Although the results of individual trials differ, meta-analyses indicate that corticosteroids have a moderate beneficial effect in patients with severe alcoholic hepatitis.
For example, Rambaldi et al34 performed a meta-analysis that concluded the mortality rate was lower in alcoholic hepatitis patients with MDF scores of at least 32 or hepatic encephalopathy who were treated with corticosteroids than in controls (relative risk 0.37, 95% confidence interval 0.16–0.86).
Therefore, in the absence of contraindications, the AASLD recommends starting corticosteroids in patients with severe alcoholic hepatitis, defined as an MDF score of 32 or higher.21 The preferred agent is oral prednisolone 40 mg daily or parenteral methylprednisolone 32 mg daily for 4 weeks and then tapered over the next 2 to 4 weeks or abruptly discontinued. Because activation of prednisone is decreased in patients with liver disease, prednisolone (the active form) is preferred over prednisone (the inactive precursor).35 In alcoholic hepatitis, the number needed to treat with corticosteroids to prevent one death has been calculated36 at 5.
As mentioned, response to corticosteroids is commonly assessed at 1 week of treatment using the Lille score. A score higher than 0.45 predicts a poor response and should trigger discontinuation of corticosteroids, particularly in those classified as null responders (Lille score > 0.56).
Adverse effects of steroids include sepsis, gastrointestinal bleeding, and steroid psychosis. Of note, patients who have evidence of hepatorenal syndrome or gastrointestinal bleeding tend to have a less favorable response to corticosteroids. Also, while infections were once considered a contraindication to steroid therapy, recent evidence suggests that steroid use might not be precluded in infected patients after appropriate antibiotic therapy. Infections occur in about a quarter of all alcoholic hepatitis patients treated with steroids, more frequently in null responders (42.5%) than in responders (11.1%), which supports corticosteroid discontinuance at 1 week in null responders.32
Pentoxifylline
An oral phosphodiesterase inhibitor, pentoxifylline, also inhibits production of several cytokines, including tumor necrosis factor alpha. At a dose of 400 mg orally three times daily for 4 weeks, pentoxifylline has been used in treating severe alcoholic hepatitis (MDF score ≥ 32) and is recommended especially if corticosteroids are contraindicated, as with sepsis.21
An early double-blind clinical trial randomized patients with severe alcoholic hepatitis to receive either pentoxifylline 400 mg orally three times daily or placebo. Of the patients who received pentoxifylline, 24.5% died during the index hospitalization, compared with 46.1% of patients who received placebo. This survival benefit was mainly related to a markedly lower incidence of hepatorenal syndrome as the cause of death in the pentoxifylline group than in the placebo group (50% vs 91.7% of deaths).37
In a small clinical trial in patients with severe alcoholic hepatitis, pentoxifylline recipients had a higher 3-month survival rate than prednisolone recipients (35.29% vs 14.71%, P = .04).38 However, a larger trial showed no improvement in 6-month survival with the combination of prednisolone and pentoxifylline compared with prednisolone alone (69.9% vs 69.2%, P = .91).39 Also, a meta-analysis of five randomized clinical trials found no survival benefit with pentoxifylline therapy.40
Of note, in the unfortunate subgroup of patients who have a poor response to corticosteroids, no alternative treatment, including pentoxifylline, has been shown to be effective.41
Prednisone or pentoxifylline? Very recently, results of the Steroids or Pentoxifylline for Alcoholic Hepatitis (STOPAH) trial have been released.42 This is a large, multicenter, double-blinded clinical trial that aimed to provide a definitive answer to whether corticosteroids or pentoxifylline (or both) are beneficial in patients with alcoholic hepatitis. The study included 1,103 adult patients with severe alcoholic hepatitis (MDF score ≥ 32) who were randomized to monotherapy with prednisolone or pentoxifylline, combination therapy, or placebo. The primary end point was mortality at 28 days, and secondary end points included mortality at 90 days and at 1 year. Prednisolone reduced 28-day mortality by about 39%. In contrast, the 28-day mortality rate was similar in patients who received pentoxifylline and those who did not. Also, neither drug was significantly associated with a survival benefit beyond 28 days. The investigators concluded that pentoxifylline has no impact on disease progression and should not be used for the treatment of severe alcoholic hepatitis.42
Other tumor necrosis factor alpha inhibitors not recommended
Two other tumor necrosis factor alpha inhibitors, infliximab and etanercept, have been tested in clinical trials in alcoholic hepatitis. Unfortunately, the results were not encouraging, with no major reduction in mortality.43–45 In fact, these trials demonstrated a significantly increased risk of infections in the treatment groups. Therefore, these drugs are not recommended for treating alcoholic hepatitis.
A possible explanation is that tumor necrosis factor alpha plays an important role in liver regeneration, aiding in recovery from alcohol-induced liver injury, and inhibiting it can have deleterious consequences.
Other agents
A number of other agents have undergone clinical trials in alcoholic hepatitis.
N-acetylcysteine, an antioxidant that replenishes glutathione stores in hepatocytes, was evaluated in a randomized clinical trial in combination with prednisolone.46 Although the 1-month mortality rate was significantly lower in the combination group than in the prednisolone-only group (8% vs 24%, P = .006), 3-month and 6-month mortality rates were not. Nonetheless, the rates of infection and hepatorenal syndrome were lower in the combination group. Therefore, corticosteroids and N-acetylcysteine may have synergistic effects, but the optimum duration of N-acetylcysteine therapy needs to be determined in further studies.
Vitamin E, silymarin, propylthiouracil, colchicine, and oxandrolone (an anabolic steroid) have also been studied, but with no convincing benefit.21
Role of liver transplantation
Liver transplantation for alcoholic liver disease has been a topic of great medical and social controversy. The view that alcoholic patients are responsible for their own illness led to caution when contemplating liver transplantation. Many countries require 6 months of abstinence from alcohol before placing a patient on the liver transplant list, posing a major obstacle to patients with alcoholic hepatitis, as almost all are active drinkers at the time of presentation and many will die within 6 months. Reasons for this 6-month rule include donor shortage and risk of recidivism.47
With regard to survival following alcoholic hepatitis, a study utilizing the United Network for Organ Sharing database matched patients with alcoholic hepatitis and alcoholic cirrhosis who underwent liver transplantation. Rates of 5-year graft survival were 75% in those with alcoholic hepatitis and 73% in those with alcoholic cirrhosis (P = .97), and rates of patient survival were 80% and 78% (P = .90), respectively. Proportional regression analysis adjusting for other variables showed no impact of the etiology of liver disease on graft or patient survival. The investigators concluded that liver transplantation could be considered in a select group of patients with alcoholic hepatitis who do not improve with medical therapy.48
In a pivotal case-control prospective study,49 26 patients with Lille scores greater than 0.45 were listed for liver transplantation within a median of 13 days after nonresponse to medical therapy. The cumulative 6-month survival rate was higher in patients who received a liver transplant early than in those who did not (77% vs 23%, P < .001). This benefit was maintained through 2 years of follow-up (hazard ratio 6.08, P = .004). Of note, all these patients had supportive family members, no severe coexisting conditions, and a commitment to alcohol abstinence (although 3 patients resumed drinking after liver transplantation).49
Although these studies support early liver transplantation in carefully selected patients with severe alcoholic hepatitis, the criteria for transplantation in this group need to be refined. Views on alcoholism also need to be reconciled, as strong evidence is emerging that implicates genetic and environmental influences on alcohol dependence.
Management algorithm
Figure 2 shows a suggested management algorithm for alcoholic hepatitis, adapted from the guidelines of the AASLD and European Association for the Study of the Liver.
NEW THERAPIES NEEDED
Novel therapies for severe alcoholic hepatitis are urgently needed to help combat this devastating condition. Advances in understanding its pathophysiology have uncovered several new therapeutic targets, and new agents are already being evaluated in clinical trials.
IMM 124-E, a hyperimmune bovine colostrum enriched with immunoglobulin G anti-lipopolysaccharide, is going to be evaluated in combination with prednisolone in patients with severe alcoholic hepatitis.
Anakinra, an interleukin 1 receptor antagonist, has significant anti-inflammatory activity and is used to treat rheumatoid arthritis. A clinical trial to evaluate its role in alcoholic hepatitis has been designed in which patients with severe alcoholic hepatitis (defined as a MELD score ≥ 21) will be randomized to receive either methylprednisolone or a combination of anakinra, pentoxifylline, and zinc (a mineral that improves gut integrity).
Emricasan, an orally active caspase protease inhibitor, is another agent currently being tested in a phase 2 clinical trial in patients with severe alcoholic hepatitis. Since caspases induce apoptosis, inhibiting them should theoretically dampen alcohol-induced hepatocyte injury.
Interleukin 22, a hepatoprotective cytokine, shows promise as a treatment and will soon be evaluated in alcoholic hepatitis.
Alcoholic hepatitis, a severe manifestation of alcoholic liver disease, is rising in incidence. Complete abstinence from alcohol remains the cornerstone of treatment, while other specific interventions aim to decrease short-term mortality rates.
Despite current treatments, about 25% of patients with severe alcoholic hepatitis eventually die of it. For those who survive hospitalization, measures need to be taken to prevent recidivism. Although liver transplantation seems to hold promise, early transplantation is still largely experimental in alcoholic hepatitis and will likely be available to only a small subset of patients, especially in view of ethical issues and the possible wider implications for transplant centers.
New treatments will largely depend on a better understanding of the disease’s pathophysiology, and future clinical trials should evaluate therapies that improve short-term as well as long-term outcomes.
ACUTE HEPATIC DECOMPENSATION IN A HEAVY DRINKER
Excessive alcohol consumption is very common worldwide, is a major risk factor for liver disease, and is a leading cause of preventable death. Alcoholic cirrhosis is the eighth most common cause of death in the United States and in 2010 was responsible for nearly half of cirrhosis-related deaths worldwide.1
Alcoholic liver disease is a spectrum. Nearly all heavy drinkers (ie, those consuming 40 g or more of alcohol per day, Table 1) have fatty liver changes, 20% to 40% develop fibrosis, 10% to 20% progress to cirrhosis, and of those with cirrhosis, 1% to 2% are diagnosed with hepatocellular carcinoma every year.2
Within this spectrum, alcoholic hepatitis is a well-defined clinical syndrome characterized by acute hepatic decompensation that typically results from long-standing alcohol abuse. Binge drinkers may also be at risk for alcoholic hepatitis, but good data on the association between drinking patterns and the risk of alcoholic hepatitis are limited.
Alcoholic hepatitis varies in severity from mild to life-threatening.3 Although its exact incidence is unknown, its prevalence in alcoholics has been estimated at 20%.4 Nearly half of patients with alcoholic hepatitis have cirrhosis at the time of their acute presentation, and these patients generally have a poor prognosis, with a 28-day death rate as high as 50% in severe cases.5,6 Moreover, although alcoholic hepatitis develops in only a subset of patients with alcoholic liver disease, hospitalizations for it are increasing in the United States.7
Women are at higher risk of developing alcoholic hepatitis, an observation attributed to the effect of estrogens on oxidative stress and inflammation, lower gastric alcohol dehydrogenase levels resulting in slower first-pass metabolism of alcohol, and higher body fat content causing a lower volume of distribution for alcohol than in men.8 The incidence of alcoholic hepatitis is also influenced by a number of demographic and genetic factors as well as nutritional status and coexistence of other liver diseases.9 Most patients diagnosed with alcoholic hepatitis are active drinkers, but it can develop even after significantly reducing or stopping alcohol consumption.
FATTY ACIDS, ENZYMES, CYTOKINES, INFLAMMATION
Alcohol consumption induces fatty acid synthesis and inhibits fatty acid oxidation, thereby promoting fat deposition in the liver.
The major enzymes involved in alcohol metabolism are cytochrome P450 2E1 (CYP2E1) and alcohol dehydrogenase. CYP2E1 is inducible and is up-regulated when excess alcohol is ingested, while alcohol dehydrogen-
ase function is relatively stable. Oxidative degradation of alcohol by these enzymes generates reactive oxygen species and acetaldehyde, inducing liver injury.10 Interestingly, it has been proposed that variations in the genes for these enzymes influence alcohol consumption and dependency as well as alcohol-driven tissue damage.
In addition, alcohol disrupts the intestinal mucosal barrier, allowing lipopolysaccharides from gram-negative bacteria to travel to the liver via the portal vein. These lipopolysaccharides then bind to and activate sinusoidal Kupffer cells, leading to production of several cytokines such as tumor necrosis factor alpha, interleukin 1, and transforming growth factor beta. These cytokines promote hepatocyte inflammation, apoptosis, and necrosis (Figure 1).11
Besides activating the innate immune system, the reactive oxygen species resulting from alcohol metabolism interact with cellular components, leading to production of protein adducts. These act as antigens that activate the adaptive immune response, followed by B- and T-lymphocyte infiltration, which in turn contribute to liver injury and inflammation.12
THE DIAGNOSIS IS MAINLY CLINICAL
The diagnosis of alcoholic hepatitis is mainly clinical. In its usual presentation, jaundice develops rapidly in a person with a known history of heavy alcohol use. Other symptoms and signs may include ascites, encephalopathy, and fever. On examination, the liver may be enlarged and tender, and a hepatic bruit has been reported.13
Other classic signs of liver disease such as parotid enlargement, Dupuytren contracture, dilated abdominal wall veins, and spider nevi can be present, but none is highly specific or sensitive for alcoholic hepatitis.
Elevated liver enzymes and other clues
Laboratory tests are important in evaluating potential alcoholic hepatitis, although no single laboratory marker can definitively establish alcohol as the cause of liver disease. To detect alcohol consumption, biochemical markers such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), mean corpuscular volume, carbohydrate-deficient transferrin, and, more commonly, gamma-glutamyl transpeptidase are used.
In the acute setting, typical biochemical derangements in alcoholic hepatitis include elevated AST (up to 2 to 6 times the upper limit of normal; usually less than 300 IU/L) and elevated ALT to a lesser extent,14 with an AST-to-ALT ratio greater than 2. Neutrophilia, anemia, hyperbilirubinemia, and coagulopathy with an elevated international normalized ratio are common.
Patients with alcoholic hepatitis are also prone to develop bacterial infections, and about 7% develop hepatorenal syndrome, itself an ominous sign.15
Imaging studies are valuable in excluding other causes of abnormal liver test results in patients who abuse alcohol, such as biliary obstruction, infiltrative liver diseases, and hepatocellular carcinoma.
Screen for alcohol intake
During the initial evaluation of suspected alcoholic hepatitis, one should screen for excessive drinking. In a US Centers for Disease Control and Prevention study, only one of six US adults, including binge drinkers, said they had ever discussed alcohol consumption with a health professional.16 Many patients with alcoholic liver disease in general and alcoholic hepatitis in particular deny alcohol abuse or underreport their intake.17
Screening tests such as the CAGE questionnaire and the Alcohol Use Disorders Identification Test can be used to assess alcohol dependence or abuse.18,19 The CAGE questionnaire consists of four questions:
- Have you ever felt you should cut down on your drinking?
- Have people annoyed you by criticizing your drinking?
- Have you ever felt guilty about your drinking?
- Have you ever had a drink first thing in the morning (an eye-opener) to steady your nerves or to get rid of a hangover?
A yes answer to two or more questions is considered clinically significant.
Is liver biopsy always needed?
Although alcoholic hepatitis can be suspected on the basis of clinical and biochemical clues, liver biopsy remains the gold standard diagnostic tool. It confirms the clinical diagnosis of alcoholic hepatitis in about 85% of all patients and in up to 95% when significant hyperbilirubinemia is present.20
However, whether a particular patient needs a biopsy is not always clear. The American Association for the Study of Liver Diseases (AASLD) recommends biopsy in patients who have a clinical diagnosis of severe alcoholic hepatitis for whom medical treatment is being considered and in those with an uncertain underlying diagnosis.
Findings on liver biopsy in alcoholic hepatitis include steatosis, hepatocyte ballooning, neutrophilic infiltration, Mallory bodies (which represent aggregated cytokeratin intermediate filaments and other proteins), and scarring with a typical perivenular distribution as opposed to the periportal fibrosis seen in chronic viral hepatitis. Some histologic findings, such as centrilobular necrosis, may overlap alcoholic hepatitis and nonalcoholic steatohepatitis.
In addition to confirming the diagnosis and staging the disease, liver biopsy has prognostic value. The severity of inflammation and cholestatic changes correlates with poor prognosis and may also predict response to corticosteroid treatment in severe cases of alcoholic hepatitis.21
However, the utility of liver biopsy in confirming the diagnosis and assessing the prognosis of alcoholic hepatitis is controversial for several reasons. Coagulopathy, thrombocytopenia, and ascites are all common in patients with alcoholic hepatitis, often making percutaneous liver biopsy contraindicated. Trans-
jugular liver biopsy is not universally available outside tertiary care centers.
Needed is a minimally invasive test for assessing this disease. Breath analysis might be such a test, offering a noninvasive means to study the composition of volatile organic compounds and elemental gases and an attractive method to evaluate health and disease in a patient-friendly manner. Our group devised a model based on breath levels of trimethylamine and pentane. When we tested it, we found that it distinguishes patients with alcoholic hepatitis from those with acute liver decompensation from causes other than alcohol and controls without liver disease with up to 90% sensitivity and 80% specificity.22
ASSESSING THE SEVERITY OF ALCOHOLIC HEPATITIS
Several models have been developed to assess the severity of alcoholic hepatitis and guide treatment decisions (Table 2).
The MDF (Maddrey Discriminant Function)6 system was the first scoring system developed and is still the most widely used. A score of 32 or higher indicates severe alcoholic hepatitis and has been used as the threshold for starting treatment with corticosteroids.6
The MDF has limitations. Patients with a score lower than 32 are considered not to have severe alcoholic hepatitis, but up to 17% of them still die. Also, since it uses the prothrombin time, its results can vary considerably among laboratories, depending on the sensitivity of the thromboplastin reagent used.
The MELD (Model for End-stage Liver Disease) score. Sheth et al23 compared the MELD and the MDF scores in assessing the severity of alcoholic hepatitis. They found that the MELD performed as well as the MDF in predicting 30-day mortality. A MELD score of greater than 11 had a sensitivity in predicting 30-day mortality of 86% and a specificity of 81%, compared with 86% and 48%, respectively, for MDF scores greater than 32.
Another study found a MELD score of 21 to have the highest sensitivity and specificity in predicting mortality (an estimated 90-day death rate of 20%). Thus, a MELD score of 21 is an appropriate threshold for prompt consideration of specific therapies such as corticosteroids.24
The MELD score has become increasingly important in patients with alcoholic hepatitis, as some of them may become candidates for liver transplantation (see below). Also, serial MELD scores in hospitalized patients have prognostic implications, since an increase of 2 or more points in the first week has been shown to predict in-hospital mortality.25
The GAHS (Glasgow Alcoholic Hepatitis Score)26 was shown to identify patients with alcoholic hepatitis who have an especially poor prognosis and need corticosteroid therapy. In those with a GAHS of 9 or higher, the 28-day survival rate was 78% with corticosteroid treatment and 52% without corticosteroid treatment; survival rates at 84 days were 59% and 38%, respectively.26
The ABIC scoring system (Age, Serum Bilirubin, INR, and Serum Creatinine) stratifies patients by risk of death at 90 days27:
- Score less than 6.71: low risk (100% survival)
- A score 6.71–8.99: intermediate risk (70% survival)
- A score 9.0 or higher: high risk (25% survival).
Both the GAHS and ABIC score are limited by lack of external validation.
The Lille score.28 While the above scores are used to identify patients at risk of death from alcoholic hepatitis and to decide on starting corticosteroids, the Lille score is designed to assess response to corticosteroids after 1 week of treatment. It is calculated based on five pretreatment variables and the change in serum bilirubin level at day 7 of corticosteroid therapy. Lille scores range from 0 to 1; a score higher than 0.45 is associated with a 75% mortality rate at 6 months and indicates a lack of response to corticosteroids and that these drugs should be discontinued.28
MANAGEMENT
Supportive treatment
Abstinence from alcohol is the cornerstone of treatment of alcoholic hepatitis. Early management of alcohol abuse or dependence is, therefore, warranted in all patients with alcoholic hepatitis. Referral to addiction specialists, motivational therapies, and anticraving drugs such as baclofen can be utilized.
Treat alcohol withdrawal. Alcoholics who suddenly decrease or discontinue their alcohol use are at high risk of alcohol withdrawal syndrome. Within 24 hours after the last drink, patients can experience increases in their heart rate and blood pressure, along with irritability and hyperreflexia. Within the next few days, more dangerous complications including seizures and delirium tremens can arise.
Alcohol withdrawal symptoms should be treated with short-acting benzodiazepines or clomethiazole, keeping the risk of worsening encephalopathy in mind.29 If present, complications of cirrhosis such as encephalopathy, ascites, and variceal bleeding should be managed.
Nutritional support is important. Protein-calorie malnutrition is common in alcoholics, as are deficiencies of vitamin A, vitamin D, thiamine, folate, pyridoxine, and zinc.30 Although a randomized controlled trial comparing enteral nutrition (2,000 kcal/day) vs corticosteroids (prednisolone 40 mg/day) in patients with alcoholic hepatitis did not show any difference in the 28-day mortality rate, those who received nutritional support and survived the first month had a lower mortality rate than those treated with corticosteroids (8% vs 37%).31 A daily protein intake of 1.5 g per kilogram of body weight is therefore recommended, even in patients with hepatic encephalopathy.15
Combining enteral nutrition and corticosteroid treatment may have a synergistic effect but is yet to be investigated.
Screen for infection. Patients with alcoholic hepatitis should be screened for infection, as about 25% of those with severe alcoholic hepatitis have an infection at admission.32 Since many of these patients meet the criteria for systemic inflammatory response syndrome, infections can be particularly difficult to diagnose. Patients require close clinical monitoring as well as regular pancultures for early detection. Antibiotics are frequently started empirically even though we lack specific evidence-based guidelines on this practice.33
Corticosteroids
Various studies have evaluated the role of corticosteroids in treating alcoholic hepatitis, differing considerably in sample populations, methods, and end points. Although the results of individual trials differ, meta-analyses indicate that corticosteroids have a moderate beneficial effect in patients with severe alcoholic hepatitis.
For example, Rambaldi et al34 performed a meta-analysis that concluded the mortality rate was lower in alcoholic hepatitis patients with MDF scores of at least 32 or hepatic encephalopathy who were treated with corticosteroids than in controls (relative risk 0.37, 95% confidence interval 0.16–0.86).
Therefore, in the absence of contraindications, the AASLD recommends starting corticosteroids in patients with severe alcoholic hepatitis, defined as an MDF score of 32 or higher.21 The preferred agent is oral prednisolone 40 mg daily or parenteral methylprednisolone 32 mg daily for 4 weeks and then tapered over the next 2 to 4 weeks or abruptly discontinued. Because activation of prednisone is decreased in patients with liver disease, prednisolone (the active form) is preferred over prednisone (the inactive precursor).35 In alcoholic hepatitis, the number needed to treat with corticosteroids to prevent one death has been calculated36 at 5.
As mentioned, response to corticosteroids is commonly assessed at 1 week of treatment using the Lille score. A score higher than 0.45 predicts a poor response and should trigger discontinuation of corticosteroids, particularly in those classified as null responders (Lille score > 0.56).
Adverse effects of steroids include sepsis, gastrointestinal bleeding, and steroid psychosis. Of note, patients who have evidence of hepatorenal syndrome or gastrointestinal bleeding tend to have a less favorable response to corticosteroids. Also, while infections were once considered a contraindication to steroid therapy, recent evidence suggests that steroid use might not be precluded in infected patients after appropriate antibiotic therapy. Infections occur in about a quarter of all alcoholic hepatitis patients treated with steroids, more frequently in null responders (42.5%) than in responders (11.1%), which supports corticosteroid discontinuance at 1 week in null responders.32
Pentoxifylline
An oral phosphodiesterase inhibitor, pentoxifylline, also inhibits production of several cytokines, including tumor necrosis factor alpha. At a dose of 400 mg orally three times daily for 4 weeks, pentoxifylline has been used in treating severe alcoholic hepatitis (MDF score ≥ 32) and is recommended especially if corticosteroids are contraindicated, as with sepsis.21
An early double-blind clinical trial randomized patients with severe alcoholic hepatitis to receive either pentoxifylline 400 mg orally three times daily or placebo. Of the patients who received pentoxifylline, 24.5% died during the index hospitalization, compared with 46.1% of patients who received placebo. This survival benefit was mainly related to a markedly lower incidence of hepatorenal syndrome as the cause of death in the pentoxifylline group than in the placebo group (50% vs 91.7% of deaths).37
In a small clinical trial in patients with severe alcoholic hepatitis, pentoxifylline recipients had a higher 3-month survival rate than prednisolone recipients (35.29% vs 14.71%, P = .04).38 However, a larger trial showed no improvement in 6-month survival with the combination of prednisolone and pentoxifylline compared with prednisolone alone (69.9% vs 69.2%, P = .91).39 Also, a meta-analysis of five randomized clinical trials found no survival benefit with pentoxifylline therapy.40
Of note, in the unfortunate subgroup of patients who have a poor response to corticosteroids, no alternative treatment, including pentoxifylline, has been shown to be effective.41
Prednisone or pentoxifylline? Very recently, results of the Steroids or Pentoxifylline for Alcoholic Hepatitis (STOPAH) trial have been released.42 This is a large, multicenter, double-blinded clinical trial that aimed to provide a definitive answer to whether corticosteroids or pentoxifylline (or both) are beneficial in patients with alcoholic hepatitis. The study included 1,103 adult patients with severe alcoholic hepatitis (MDF score ≥ 32) who were randomized to monotherapy with prednisolone or pentoxifylline, combination therapy, or placebo. The primary end point was mortality at 28 days, and secondary end points included mortality at 90 days and at 1 year. Prednisolone reduced 28-day mortality by about 39%. In contrast, the 28-day mortality rate was similar in patients who received pentoxifylline and those who did not. Also, neither drug was significantly associated with a survival benefit beyond 28 days. The investigators concluded that pentoxifylline has no impact on disease progression and should not be used for the treatment of severe alcoholic hepatitis.42
Other tumor necrosis factor alpha inhibitors not recommended
Two other tumor necrosis factor alpha inhibitors, infliximab and etanercept, have been tested in clinical trials in alcoholic hepatitis. Unfortunately, the results were not encouraging, with no major reduction in mortality.43–45 In fact, these trials demonstrated a significantly increased risk of infections in the treatment groups. Therefore, these drugs are not recommended for treating alcoholic hepatitis.
A possible explanation is that tumor necrosis factor alpha plays an important role in liver regeneration, aiding in recovery from alcohol-induced liver injury, and inhibiting it can have deleterious consequences.
Other agents
A number of other agents have undergone clinical trials in alcoholic hepatitis.
N-acetylcysteine, an antioxidant that replenishes glutathione stores in hepatocytes, was evaluated in a randomized clinical trial in combination with prednisolone.46 Although the 1-month mortality rate was significantly lower in the combination group than in the prednisolone-only group (8% vs 24%, P = .006), 3-month and 6-month mortality rates were not. Nonetheless, the rates of infection and hepatorenal syndrome were lower in the combination group. Therefore, corticosteroids and N-acetylcysteine may have synergistic effects, but the optimum duration of N-acetylcysteine therapy needs to be determined in further studies.
Vitamin E, silymarin, propylthiouracil, colchicine, and oxandrolone (an anabolic steroid) have also been studied, but with no convincing benefit.21
Role of liver transplantation
Liver transplantation for alcoholic liver disease has been a topic of great medical and social controversy. The view that alcoholic patients are responsible for their own illness led to caution when contemplating liver transplantation. Many countries require 6 months of abstinence from alcohol before placing a patient on the liver transplant list, posing a major obstacle to patients with alcoholic hepatitis, as almost all are active drinkers at the time of presentation and many will die within 6 months. Reasons for this 6-month rule include donor shortage and risk of recidivism.47
With regard to survival following alcoholic hepatitis, a study utilizing the United Network for Organ Sharing database matched patients with alcoholic hepatitis and alcoholic cirrhosis who underwent liver transplantation. Rates of 5-year graft survival were 75% in those with alcoholic hepatitis and 73% in those with alcoholic cirrhosis (P = .97), and rates of patient survival were 80% and 78% (P = .90), respectively. Proportional regression analysis adjusting for other variables showed no impact of the etiology of liver disease on graft or patient survival. The investigators concluded that liver transplantation could be considered in a select group of patients with alcoholic hepatitis who do not improve with medical therapy.48
In a pivotal case-control prospective study,49 26 patients with Lille scores greater than 0.45 were listed for liver transplantation within a median of 13 days after nonresponse to medical therapy. The cumulative 6-month survival rate was higher in patients who received a liver transplant early than in those who did not (77% vs 23%, P < .001). This benefit was maintained through 2 years of follow-up (hazard ratio 6.08, P = .004). Of note, all these patients had supportive family members, no severe coexisting conditions, and a commitment to alcohol abstinence (although 3 patients resumed drinking after liver transplantation).49
Although these studies support early liver transplantation in carefully selected patients with severe alcoholic hepatitis, the criteria for transplantation in this group need to be refined. Views on alcoholism also need to be reconciled, as strong evidence is emerging that implicates genetic and environmental influences on alcohol dependence.
Management algorithm
Figure 2 shows a suggested management algorithm for alcoholic hepatitis, adapted from the guidelines of the AASLD and European Association for the Study of the Liver.
NEW THERAPIES NEEDED
Novel therapies for severe alcoholic hepatitis are urgently needed to help combat this devastating condition. Advances in understanding its pathophysiology have uncovered several new therapeutic targets, and new agents are already being evaluated in clinical trials.
IMM 124-E, a hyperimmune bovine colostrum enriched with immunoglobulin G anti-lipopolysaccharide, is going to be evaluated in combination with prednisolone in patients with severe alcoholic hepatitis.
Anakinra, an interleukin 1 receptor antagonist, has significant anti-inflammatory activity and is used to treat rheumatoid arthritis. A clinical trial to evaluate its role in alcoholic hepatitis has been designed in which patients with severe alcoholic hepatitis (defined as a MELD score ≥ 21) will be randomized to receive either methylprednisolone or a combination of anakinra, pentoxifylline, and zinc (a mineral that improves gut integrity).
Emricasan, an orally active caspase protease inhibitor, is another agent currently being tested in a phase 2 clinical trial in patients with severe alcoholic hepatitis. Since caspases induce apoptosis, inhibiting them should theoretically dampen alcohol-induced hepatocyte injury.
Interleukin 22, a hepatoprotective cytokine, shows promise as a treatment and will soon be evaluated in alcoholic hepatitis.
- Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol 2013; 59:160–168.
- Teli MR, Day CP, Burt AD, Bennett MK, James OF. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet 1995; 346:987–990.
- Alcoholic liver disease: morphological manifestations. Review by an international group. Lancet 1981; 1:707–711.
- Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput JC. Excess weight risk factor for alcoholic liver disease. Hepatology 1997; 25:108–111.
- Basra S, Anand BS. Definition, epidemiology and magnitude of alcoholic hepatitis. World J Hepatol 2011; 3:108–113.
- Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978; 75:193–199.
- Jinjuvadia R, Liangpunsakul S, for the Translational Research and Evolving Alcoholic Hepatitis Treatment Consortium. Trends in alcoholic hepatitis-related hospitalizations, financial burden, and mortality in the United States. J Clin Gastroenterol 2014 Jun 25 (Epub ahead of print).
- Sato N, Lindros KO, Baraona E, et al. Sex difference in alcohol-related organ injury. Alcohol Clin Exp Res 2001; 25(suppl s1):40S–45S.
- Singal AK, Kamath PS, Gores GJ, Shah VH. Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol 2014; 12:555–564.
- Seitz HK, Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol Chem 2006; 387:349–360.
- Thurman RG. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol 1998; 275:G605–G611.
- Duddempudi AT. Immunology in alcoholic liver disease. Clin Liver Dis 2012; 16:687–698.
- Lischner MW, Alexander JF, Galambos JT. Natural history of alcoholic hepatitis. I. The acute disease. Am J Dig Dis 1971; 16:481–494.
- Cohen JA, Kaplan MM. The SGOT/SGPT ratio—an indicator of alcoholic liver disease. Dig Dis Sci 1979; 24:835–838.
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 2009; 360:2758–2769.
- McKnight-Eily LR, Liu Y, Brewer RD, et al; Centers for Disease Control and Prevention (CDC). Vital signs: communication between health professionals and their patients about alcohol use—44 states and the District of Columbia, 2011. MMWR Morb Mortal Wkly Rep 2014; 63:16–22.
- Grant BF. Barriers to alcoholism treatment: reasons for not seeking treatment in a general population sample. J Stud Alcohol 1997; 58:365–371.
- Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol 2004; 57:30–39.
- The Alcohol Use Disorders Identification Test Guidelines for Use in Primary Care. Second Edition. World Health Organization. Department of Mental Health and Substance Dependence. http://whqlibdoc.who.int/hq/2001/who_msd_msb_01.6a.pdf. Accessed February 3, 2015.
- Hamid R, Forrest EH. Is histology required for the diagnosis of alcoholic hepatitis? A review of published randomised controlled trials. Gut 2011; 60(suppl 1):A233.
- O’Shea RS, Dasarathy S, McCullough AJ; Practice Guideline Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Alcoholic liver disease. Hepatology 2010; 51:307–328.
- Hanouneh IA, Zein NN, Cikach F, et al. The breathprints in patients with liver disease identify novel breath biomarkers in alcoholic hepatitis. Clin Gastroenterol Hepatol 2014; 12:516–523.
- Sheth M, Riggs M, Patel T. Utility of the Mayo End-Stage Liver Disease (MELD) score in assessing prognosis of patients with alcoholic hepatitis. BMC Gastroenterol 2002; 2:2.
- Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology 2005; 41:353–358.
- Srikureja W, Kyulo NL, Runyon BA, Hu KQ. MELD score is a better prognostic model than Child-Turcotte-Pugh score or Discriminant Function score in patients with alcoholic hepatitis. J Hepatol 2005; 42:700–706.
- Forrest EH, Morris AJ, Stewart S, et al. The Glasgow alcoholic hepatitis score identifies patients who may benefit from corticosteroids. Gut 2007; 56:1743–1746.
- Dominguez M, Rincón D, Abraldes JG, et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol 2008; 103:2747–2756.
- Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007; 45:1348–1354.
- Mayo-Smith MF, Beecher LH, Fischer TL, et al; Working Group on the Management of Alcohol Withdrawal Delirium, Practice Guidelines Committee, American Society of Addiction Medicine. Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med 2004; 164:1405–1412.
- Mezey E. Interaction between alcohol and nutrition in the pathogenesis of alcoholic liver disease. Semin Liver Dis 1991; 11:340–348.
- Cabré E, Rodríguez-Iglesias P, Caballería J, et al. Short- and long-term outcome of severe alcohol-induced hepatitis treated with steroids or enteral nutrition: a multicenter randomized trial. Hepatology 2000; 32:36–42.
- Louvet A, Wartel F, Castel H, et al. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology 2009; 137:541–548.
- European Association for the Study of Liver. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol 2012; 57:399–420.
- Rambaldi A, Saconato HH, Christensen E, Thorlund K, Wetterslev J, Gluud C. Systematic review: glucocorticosteroids for alcoholic hepatitis—a Cochrane Hepato-Biliary Group systematic review with meta-analyses and trial sequential analyses of randomized clinical trials. Aliment Pharmacol Ther 2008; 27:1167–1178.
- Powell LW, Axelsen E. Corticosteroids in liver disease: studies on the biological conversion of prednisone to prednisolone and plasma protein binding. Gut 1972; 13:690–696.
- Mathurin P, O’Grady J, Carithers RL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut 2011; 60:255–260.
- Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology 2000; 119:1637–1648.
- De BK, Gangopadhyay S, Dutta D, Baksi SD, Pani A, Ghosh P. Pentoxifylline versus prednisolone for severe alcoholic hepatitis: a randomized controlled trial. World J Gastroenterol 2009; 15:1613–1619.
- Mathurin P, Louvet A, Dao T, et al. Addition of pentoxifylline to prednisolone for severe alcoholic hepatitis does not improve 6-month survival: results of the CORPENTOX trial (abstract). Hepatology 2011; 54(suppl 1):81A.
- Whitfield K, Rambaldi A, Wetterslev J, Gluud C. Pentoxifylline for alcoholic hepatitis. Cochrane Database Syst Rev 2009; CD007339.
- Louvet A, Diaz E, Dharancy S, et al. Early switch to pentoxifylline in patients with severe alcoholic hepatitis is inefficient in non-responders to corticosteroids. J Hepatol 2008; 48:465–470.
- Thursz MR, Richardson P, Allison ME, et al. Steroids or pentoxifylline for alcoholic hepatitis: results of the STOPAH trial [abstract LB-1]. 65th Annual Meeting of the American Association for the Study of Liver Diseases; November 7–11, 2014; Boston, MA.
- Naveau S, Chollet-Martin S, Dharancy S, et al; Foie-Alcool group of the Association Française pour l’Etude du Foie. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology 2004; 39:1390–1397.
- Menon KV, Stadheim L, Kamath PS, et al. A pilot study of the safety and tolerability of etanercept in patients with alcoholic hepatitis. Am J Gastroenterol 2004; 99:255–260.
- Boetticher NC, Peine CJ, Kwo P, et al. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology 2008; 135:1953–1960.
- Nguyen-Khac E, Thevenot T, Piquet MA, et al; AAH-NAC Study Group. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med 2011; 365:1781–1789.
- Singal AK, Duchini A. Liver transplantation in acute alcoholic hepatitis: current status and future development. World J Hepatol 2011; 3:215–218.
- Singal AK, Bashar H, Anand BS, Jampana SC, Singal V, Kuo YF. Outcomes after liver transplantation for alcoholic hepatitis are similar to alcoholic cirrhosis: exploratory analysis from the UNOS database. Hepatology 2012; 55:1398–1405.
- Mathurin P, Moreno C, Samuel D, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med 2011; 365:1790–1800.
- Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol 2013; 59:160–168.
- Teli MR, Day CP, Burt AD, Bennett MK, James OF. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet 1995; 346:987–990.
- Alcoholic liver disease: morphological manifestations. Review by an international group. Lancet 1981; 1:707–711.
- Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput JC. Excess weight risk factor for alcoholic liver disease. Hepatology 1997; 25:108–111.
- Basra S, Anand BS. Definition, epidemiology and magnitude of alcoholic hepatitis. World J Hepatol 2011; 3:108–113.
- Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978; 75:193–199.
- Jinjuvadia R, Liangpunsakul S, for the Translational Research and Evolving Alcoholic Hepatitis Treatment Consortium. Trends in alcoholic hepatitis-related hospitalizations, financial burden, and mortality in the United States. J Clin Gastroenterol 2014 Jun 25 (Epub ahead of print).
- Sato N, Lindros KO, Baraona E, et al. Sex difference in alcohol-related organ injury. Alcohol Clin Exp Res 2001; 25(suppl s1):40S–45S.
- Singal AK, Kamath PS, Gores GJ, Shah VH. Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol 2014; 12:555–564.
- Seitz HK, Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol Chem 2006; 387:349–360.
- Thurman RG. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol 1998; 275:G605–G611.
- Duddempudi AT. Immunology in alcoholic liver disease. Clin Liver Dis 2012; 16:687–698.
- Lischner MW, Alexander JF, Galambos JT. Natural history of alcoholic hepatitis. I. The acute disease. Am J Dig Dis 1971; 16:481–494.
- Cohen JA, Kaplan MM. The SGOT/SGPT ratio—an indicator of alcoholic liver disease. Dig Dis Sci 1979; 24:835–838.
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 2009; 360:2758–2769.
- McKnight-Eily LR, Liu Y, Brewer RD, et al; Centers for Disease Control and Prevention (CDC). Vital signs: communication between health professionals and their patients about alcohol use—44 states and the District of Columbia, 2011. MMWR Morb Mortal Wkly Rep 2014; 63:16–22.
- Grant BF. Barriers to alcoholism treatment: reasons for not seeking treatment in a general population sample. J Stud Alcohol 1997; 58:365–371.
- Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol 2004; 57:30–39.
- The Alcohol Use Disorders Identification Test Guidelines for Use in Primary Care. Second Edition. World Health Organization. Department of Mental Health and Substance Dependence. http://whqlibdoc.who.int/hq/2001/who_msd_msb_01.6a.pdf. Accessed February 3, 2015.
- Hamid R, Forrest EH. Is histology required for the diagnosis of alcoholic hepatitis? A review of published randomised controlled trials. Gut 2011; 60(suppl 1):A233.
- O’Shea RS, Dasarathy S, McCullough AJ; Practice Guideline Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Alcoholic liver disease. Hepatology 2010; 51:307–328.
- Hanouneh IA, Zein NN, Cikach F, et al. The breathprints in patients with liver disease identify novel breath biomarkers in alcoholic hepatitis. Clin Gastroenterol Hepatol 2014; 12:516–523.
- Sheth M, Riggs M, Patel T. Utility of the Mayo End-Stage Liver Disease (MELD) score in assessing prognosis of patients with alcoholic hepatitis. BMC Gastroenterol 2002; 2:2.
- Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology 2005; 41:353–358.
- Srikureja W, Kyulo NL, Runyon BA, Hu KQ. MELD score is a better prognostic model than Child-Turcotte-Pugh score or Discriminant Function score in patients with alcoholic hepatitis. J Hepatol 2005; 42:700–706.
- Forrest EH, Morris AJ, Stewart S, et al. The Glasgow alcoholic hepatitis score identifies patients who may benefit from corticosteroids. Gut 2007; 56:1743–1746.
- Dominguez M, Rincón D, Abraldes JG, et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol 2008; 103:2747–2756.
- Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007; 45:1348–1354.
- Mayo-Smith MF, Beecher LH, Fischer TL, et al; Working Group on the Management of Alcohol Withdrawal Delirium, Practice Guidelines Committee, American Society of Addiction Medicine. Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med 2004; 164:1405–1412.
- Mezey E. Interaction between alcohol and nutrition in the pathogenesis of alcoholic liver disease. Semin Liver Dis 1991; 11:340–348.
- Cabré E, Rodríguez-Iglesias P, Caballería J, et al. Short- and long-term outcome of severe alcohol-induced hepatitis treated with steroids or enteral nutrition: a multicenter randomized trial. Hepatology 2000; 32:36–42.
- Louvet A, Wartel F, Castel H, et al. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology 2009; 137:541–548.
- European Association for the Study of Liver. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol 2012; 57:399–420.
- Rambaldi A, Saconato HH, Christensen E, Thorlund K, Wetterslev J, Gluud C. Systematic review: glucocorticosteroids for alcoholic hepatitis—a Cochrane Hepato-Biliary Group systematic review with meta-analyses and trial sequential analyses of randomized clinical trials. Aliment Pharmacol Ther 2008; 27:1167–1178.
- Powell LW, Axelsen E. Corticosteroids in liver disease: studies on the biological conversion of prednisone to prednisolone and plasma protein binding. Gut 1972; 13:690–696.
- Mathurin P, O’Grady J, Carithers RL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut 2011; 60:255–260.
- Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology 2000; 119:1637–1648.
- De BK, Gangopadhyay S, Dutta D, Baksi SD, Pani A, Ghosh P. Pentoxifylline versus prednisolone for severe alcoholic hepatitis: a randomized controlled trial. World J Gastroenterol 2009; 15:1613–1619.
- Mathurin P, Louvet A, Dao T, et al. Addition of pentoxifylline to prednisolone for severe alcoholic hepatitis does not improve 6-month survival: results of the CORPENTOX trial (abstract). Hepatology 2011; 54(suppl 1):81A.
- Whitfield K, Rambaldi A, Wetterslev J, Gluud C. Pentoxifylline for alcoholic hepatitis. Cochrane Database Syst Rev 2009; CD007339.
- Louvet A, Diaz E, Dharancy S, et al. Early switch to pentoxifylline in patients with severe alcoholic hepatitis is inefficient in non-responders to corticosteroids. J Hepatol 2008; 48:465–470.
- Thursz MR, Richardson P, Allison ME, et al. Steroids or pentoxifylline for alcoholic hepatitis: results of the STOPAH trial [abstract LB-1]. 65th Annual Meeting of the American Association for the Study of Liver Diseases; November 7–11, 2014; Boston, MA.
- Naveau S, Chollet-Martin S, Dharancy S, et al; Foie-Alcool group of the Association Française pour l’Etude du Foie. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology 2004; 39:1390–1397.
- Menon KV, Stadheim L, Kamath PS, et al. A pilot study of the safety and tolerability of etanercept in patients with alcoholic hepatitis. Am J Gastroenterol 2004; 99:255–260.
- Boetticher NC, Peine CJ, Kwo P, et al. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology 2008; 135:1953–1960.
- Nguyen-Khac E, Thevenot T, Piquet MA, et al; AAH-NAC Study Group. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med 2011; 365:1781–1789.
- Singal AK, Duchini A. Liver transplantation in acute alcoholic hepatitis: current status and future development. World J Hepatol 2011; 3:215–218.
- Singal AK, Bashar H, Anand BS, Jampana SC, Singal V, Kuo YF. Outcomes after liver transplantation for alcoholic hepatitis are similar to alcoholic cirrhosis: exploratory analysis from the UNOS database. Hepatology 2012; 55:1398–1405.
- Mathurin P, Moreno C, Samuel D, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med 2011; 365:1790–1800.
KEY POINTS
- One should assess the severity of alcoholic hepatitis, using defined scoring systems, to allocate resources and initiate appropriate therapy.
- Supportive care should focus on alcohol withdrawal and enteral nutrition while managing the complications of liver failure.
- Corticosteroids or pentoxifylline are commonly used, but increase the survival rate only by about 50%.
- Opinion is shifting toward allowing some patients with alcoholic hepatitis to receive liver transplants early in the course of their disease.
- Many new therapies are undergoing clinical trials.
Coaching Supports Patient Aligned Care Teams
In 2010, the VHA implemented the patient-centered medical home model of primary care health care as part of its transformational T-21 Initiatives.1 Now known as Patient Aligned Care Teams (PACTs), the key pillars of the model include the expanded roles and responsibilities of multidisciplinary care teams who provide enhanced access and coordinated care. This model is based on a foundation of adequate resources, patient centeredness, and process improvement (Figure 1).
The national implementation strategy consisted of an initial educational conference with 3,600 attendees. The conference included a series of PACT learning collaboratives that engaged > 300 primary care teams, 5 demonstration laboratories, and educational outreach through learning centers and on-site consultations. Despite an aggressive national implementation plan, many frontline primary care teams struggled to translate the medical home theory into process.
Background
The Richard L. Roudebush VAMC (RLRVAMC) is a large tertiary medical center providing care to > 44,000 primary care patients. This care is delivered by 58 primary care physicians (PCPs) in 5 hospital-based outpatient clinics, including 1 large teaching clinic, 3 community-based outpatient clinics (CBOCs), and a clinic that serves recently returned active-duty veterans. Administrative nursing and clerical associates report to the Office of Ambulatory Care, and physicians and nurse practitioners report to the Medicine Service. Before the implementation of the PACT model, the functional unit of primary care was an entire clinic, typically consisting of 4 to 10 PCPs, nurses, and clerical associates.
Discussions about process change had previously occurred through monthly service or clinic meetings in which administrative leaders provided direction to frontline staff. This culture of top-down leadership drove process change but was not always effective and empowering for practice change. With the implementation of PACT, the functional unit of primary care shifted from the larger clinic to a team composed of a PCP, a nurse, a licensed nurse practitioner or health technician, and a clerical associate.
The care delivery system fundamentally changed from the traditional model to a medical home model (Figure 2). This group now represented the fundamental clinical microsystem for the delivery of primary care within the VA medical home model.2 The experience of Batalden and colleagues at the Dartmouth Hitchcock Medical Center suggests that such microsystems are very effective units of change.3 The key challenge presented to the primary care leadership was how to link these clinical PACT microsystems with an effective process that would guide practice redesign.
The concept of practice coaching or facilitation as a mechanism for physician offices to adopt evidence-based medicine and quality improvement dates to the early 1980s in England. This model spread to the U.S. in the 1990s and has continued to be used as a mechanism for leading clinical practice redesign.4 In traditional practice facilitation, a trained individual is brought in from outside the practice to help adopt evidence-based medicine guidelines.5 This individual works with the practice to implement changes that translate into patient outcome improvements.
Unlike consultation, this facilitator maintains a long-term relationship with the team as they work together to achieve goals. More important, the facilitator assists the team in developing improvement processes that are sustainable as they become incorporated within the fabric of the team culture and remain after the coach is gone. There are several reviews of clinical practice coaching that support its effectiveness in implementing evidence-based primary care guidelines.6,7 The Affordable Care Act contains provisions for the use of this model in promoting best practices and quality care.8 Manuals developed by the Agency for Healthcare Research and Quality outline how to develop a practice facilitation program.9,10
Related: Infusing Gerontologic Practice Into PACT
Essential to all practice facilitation models is the effective use of quality improvement tools. The RLRVAMC adopted the VHA Lean Healthcare Improvement Framework, which includes an approach for rapid cycle change.
The RLRVAMC adopted the facilitative coaching model in November 2011, using internal coaches who were assigned to the fundamental microsystem of its medical home.
Coach Selection
Many facilitative coaching models described in the literature use external coaches. Frequently cited advantages of external coaches include having dedicated time, receiving standardized training in facilitation, and being regarded as neutral to internal conflicts. The RLRVAMC staff elected to identify internal coaches. Advantages of this approach include the use of existing resources, the ability to develop long-term continuous relationships with PACTs, and the ability to access key internal resources to assist the team. Also, using internal individuals holding primary care leadership positions was critical to the coaching model.
Thirty-eight PACTs were initially created, and 15 internal coaches were identified. These individuals included the associate chief of staff of Ambulatory Care, chief nurse for Clinic Operations, business administrators in primary care, and all frontline unit managers and supervisors. This level of management involvement provided content expertise about primary care operations and equally important, carried the authority to implement change.
Related: Using H-PACT to Overcome Treatment Obstacles for Homeless Veterans
In addition, this approach provided considerable leadership credibility among frontline PACT staff. Given the large number of PACTs requiring coaching, coach recruitment was expanded to include other primary care administrative staff, such as the leads for the CBOCs, Prevention and Behavioral Health programs, System Redesign, and Telehealth Services. The most recent phase has included registered nurses, licensed practical nurses, health technicians, and physicians from high- functioning PACT teams who have experienced the process and who can now devote time to being coaches.
Qulifications and Training
Although the literature suggests multiple qualifications for practice coaches, there is a general agreement regarding core skills for strong facilitation, which includes interpersonal skills, knowledge of process improvement techniques, and an understanding of data acquisition and analysis.10 Strong interpersonal skills are often inherent but are a critical factor in motivating team members and managing conflicts that arise. Potential coaches were not selected if these skills were poorly developed.
The authors’ experiences have shown that although content knowledge about primary care operations is very helpful, it is not essential to being an effective coach. The facilitation model that was adopted for the program, as described by Bens, focuses predominately on process and not content expertise.11 The facilitator’s role is to apply a structural framework; ie, methods and tools that capitalize on content knowledge of frontline staff in identifying changes needed to implement the medical home.
Related: Updates in Specialty Care
Also, although knowledge of primary care operations was not required, formal training in understanding the goals of the medical home and the metrics related to PACT was essential for successful coaching. All coaches were required to attend PACT training sessions. Coaches were also expected to have basic training or experience in system redesign with the majority of the coaches completing Yellow Belt training, which is an introduction to the methods of process improvement through the lean thinking business model. A coaching manual was developed that contained information related to meeting structures, data definitions, extraction, and interpretation. A coaching website was developed that provided links to data sources and definitions. PACT-related tools, such as instructions on conducting group visits, phone visits, and use of population management were disseminated.
Coach-Team Meeting Structure
Coaches were assigned to teams by matching the skills of the coach with the team needs. Initially, sessions were held weekly for 1 hour, though this typically evolved into biweekly meetings. Clinic schedules were blocked, allowing PACTs the time to meet with their coaches. A ratio of 1 coach to 2 PACTs was considered optimal for individualized team meetings. The exceptions were the CBOCs, where several teams met together due to the need for coaches to travel. Meetings were held away from clinical areas to avoid distractions. Plan-Do-Study-Act (PDSA) cycles were used to plan and implement process improvements. The average time commitment for a coach assigned to 2 teams was between 2 to 4 hours a week.
The initial coaching sessions tended to be more structured, clearly defining the coach role, developing team building, identifying the goals, and outlining process improvement tools. Common challenges for the coaches were keeping teams focused and optimally managing time by preventing prolonged conversations unrelated to process improvement. Many frontline staff had never been empowered to change their practices, so their initial reaction was to focus on problems and not solutions. Once team relationships were established, the strong influence of nursing or clerical associates exerted on the PCP’ s willingness to change became evident and a key factor for success. Often the leaders of change are not the physicians, highlighting the influence of team building and the willingness of individuals to change practice due to team relationships and not by authority.12
Data Use
Before the implementation of this model, PACT data and metrics were posted in the clinics and briefly discussed at service-level meetings. However, this data-sharing approach rarely generated team members’ interest. By using coaching, personalized data reports that displayed team-specific information in comparison to the overall service and national VA goals were found to be a more effective technique for sharing data and performance metrics. National VA PACT core metrics tracked the following: (1) percentage of same-day appointments with PCP ratio—target 70%; (2) ratio of nontraditional encounters—target 20%; (3) percentage of continuity with PCP—target 77%; and (4) percentage of 2-day contact postdischarge ratio—target 75%. Figure 3 shows the improvements made as a facility from March 2011 (pre-PACT implementation) to March 2012 (post-PACT implementation).
A graphic display of the team’s data, including metrics related to access, continuity, and postdischarge follow-up was reviewed monthly, and the coach provided detailed explanations (Figure 4). Of particular importance to the teams was the ability to individually identify those patients who failed the metric. Review of these “fallouts” at a coaching session often resulted in reliable, consistent process improvements that addressed the failed process.
Coach-to-Coach Meetings
Critical to the RLRVAMC coaching model were the weekly 1-hour coach-to-coach meetings. Most of the coach training occurred during these sessions, either formally or via feedback and discussion. Coaches discussed their teams’ progress, brought back questions from the teams, and sought guidance from one another. Executive leaders, who were also coaches, were present at these meetings and provided the opportunity to implement broader operational changes quickly. Coaches also served as a communication venue for frontline staff to express their concerns to primary care leaders during these meetings.
Limitations
Practice facilitation that uses internal coaches for a clinical PACT microsystem may present several potential challenges. Large primary care practices require a pool of coaches who are willing to commit the necessary time required for successful implementation of this model. Although the coaches dedicate this time as collateral duty, many express that the time spent with their teams is a rewarding experience outside of their administrative roles. The coaches express satisfaction when teams meet their goals and PDSA cycles are successful.
Coaches require significant amounts of training to reach the level of effectiveness required. Teams must realize and appreciate the importance of dedicating time away from the competing priority of patient care.
Implementation of the coaching model for physician trainees in the teaching clinic has not been successful due to the teaching clinic schedule and other issues. Also related to the complexity of the teaching clinic schedule, the coaching model did not significantly improve continuity. Coaches have recently been assigned to the teaching clinic, and each team will be identifying PDSA cycles to approach the implementation of PACT principles.
Conclusion
Despite the aforementioned challenges, the outcomes are clear. The implementation of the coaching model, using internal coaches, resulted in a significant improvement of the ability of the staff to achieve the national PACT metrics (Figure 3). More important, the model created a new structural organization for change within primary care that reversed a culture of top-down leadership to that of team empowerment.
Teams that experienced practice facilitation developed ownership in their processes, data, and performance improvement and now have a more direct mechanism of communicating with primary care leadership. The coaching model moved the teams forward from having received PACT education to having the confidence and tools to implement PACTs. Staff progressed from looking at the data given to them to collecting and interpreting the data themselves. The teams are able to articulate how they fit in to the PACT model and enthusiastically monitor their progress. As primary care moves forward with the medical home, the facilitative coaching model offers a promising option for successful implementation.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects— before administering pharmacologic therapy to patients.
1. Klein S. The Veterans Health Administration: Implementing patient-centered medical homes in the nation’s largest integrated delivery system. Issue Brief (Commonw Fund). 2011;16:1537.
2. Godfrey MM, ed. Clinical Microsystem Action Guide: Improving Health Care by Improving Your Microsystem. Version 2.1. The Dartmouth Institute Website. http://clinicalmicrosys.dartmouth.edu/wp -content/uploads/2014/07/CMAG040104.pdf. Published 2004. Accessed January 23, 2015.
3. Batalden PB, Nelson EC, Edwards WH, Godfrey MM, Mohr JJ. Microsystems in health care: Part 9. Developing a small clinical unit to attain peak performance. Jt Comm J Qual Saf. 2003;29(11):575-585.
4. Nutting PA, Crabtree BF, Stewart EE, et al. Effect of facilitation on practice outcomes in the National Demonstration Project model of the patient-centered medical home. Ann Fam Med. 2010;8(suppl 1):S33-S34, S92.
5. Nagykaldi Z, Mold JW, Robinson A, Niebauer L, Ford A. Practice facilitators and practice-based research networks. J Am Board Fam Med. 2006;19(5):506-510.
6. Baskerville NB, Liddy C, Hogg W. Systematic review and meta-analysis of practice facilitation within primary care settings. Ann Fam Med. 2012;10(1):63-74.
7. Nagykaldi Z, Mold JW, Aspy CB. Practice facilitators: A review of the literature. Fam Med. 2005;37(8):581-588.
8. Grumbach K, Bainbridge E, Bodenheimer T. Facilitating improvement in primary care: The promise of practice coaching. Issue Brief (Commonw Fund). 2012;15:1-14.
9. Knox L, Taylor EF, Geonnotti K, et al. Developing and Running a Primary Care Practice Facilitation Program: A How-To Guide. Rockville, MD: Agency for Healthcare Research and Quality. U.S. Department of Health and Human Services; 2011. AHRQ Publication No. 12-0011.
10. Agency for Healthcare Research and Quality. Integrating Chronic Care and Business Strategies in the Safety Net: A Practice Coaching Manual. Agency for Healthcare Research and Quality Website. http://www.ahrq.gov/professionals/systems/primary-care /coachmnl/index.html. Published December 2012. Accessed February 26, 2015.
11. Bens I. Facilitation With Ease! Core Skills for Facilitators, Team Leaders and Members, Managers, Consultants and Trainers. 2nd ed. San Francisco, CA: Jossey-Bass; 2005.
12. Bodenheimer T. Building Teams in Primary Care: Lessons Learned: Part 1 and 2. Oakland, CA: California Health Care Foundation; July 2007.
In 2010, the VHA implemented the patient-centered medical home model of primary care health care as part of its transformational T-21 Initiatives.1 Now known as Patient Aligned Care Teams (PACTs), the key pillars of the model include the expanded roles and responsibilities of multidisciplinary care teams who provide enhanced access and coordinated care. This model is based on a foundation of adequate resources, patient centeredness, and process improvement (Figure 1).
The national implementation strategy consisted of an initial educational conference with 3,600 attendees. The conference included a series of PACT learning collaboratives that engaged > 300 primary care teams, 5 demonstration laboratories, and educational outreach through learning centers and on-site consultations. Despite an aggressive national implementation plan, many frontline primary care teams struggled to translate the medical home theory into process.
Background
The Richard L. Roudebush VAMC (RLRVAMC) is a large tertiary medical center providing care to > 44,000 primary care patients. This care is delivered by 58 primary care physicians (PCPs) in 5 hospital-based outpatient clinics, including 1 large teaching clinic, 3 community-based outpatient clinics (CBOCs), and a clinic that serves recently returned active-duty veterans. Administrative nursing and clerical associates report to the Office of Ambulatory Care, and physicians and nurse practitioners report to the Medicine Service. Before the implementation of the PACT model, the functional unit of primary care was an entire clinic, typically consisting of 4 to 10 PCPs, nurses, and clerical associates.
Discussions about process change had previously occurred through monthly service or clinic meetings in which administrative leaders provided direction to frontline staff. This culture of top-down leadership drove process change but was not always effective and empowering for practice change. With the implementation of PACT, the functional unit of primary care shifted from the larger clinic to a team composed of a PCP, a nurse, a licensed nurse practitioner or health technician, and a clerical associate.
The care delivery system fundamentally changed from the traditional model to a medical home model (Figure 2). This group now represented the fundamental clinical microsystem for the delivery of primary care within the VA medical home model.2 The experience of Batalden and colleagues at the Dartmouth Hitchcock Medical Center suggests that such microsystems are very effective units of change.3 The key challenge presented to the primary care leadership was how to link these clinical PACT microsystems with an effective process that would guide practice redesign.
The concept of practice coaching or facilitation as a mechanism for physician offices to adopt evidence-based medicine and quality improvement dates to the early 1980s in England. This model spread to the U.S. in the 1990s and has continued to be used as a mechanism for leading clinical practice redesign.4 In traditional practice facilitation, a trained individual is brought in from outside the practice to help adopt evidence-based medicine guidelines.5 This individual works with the practice to implement changes that translate into patient outcome improvements.
Unlike consultation, this facilitator maintains a long-term relationship with the team as they work together to achieve goals. More important, the facilitator assists the team in developing improvement processes that are sustainable as they become incorporated within the fabric of the team culture and remain after the coach is gone. There are several reviews of clinical practice coaching that support its effectiveness in implementing evidence-based primary care guidelines.6,7 The Affordable Care Act contains provisions for the use of this model in promoting best practices and quality care.8 Manuals developed by the Agency for Healthcare Research and Quality outline how to develop a practice facilitation program.9,10
Related: Infusing Gerontologic Practice Into PACT
Essential to all practice facilitation models is the effective use of quality improvement tools. The RLRVAMC adopted the VHA Lean Healthcare Improvement Framework, which includes an approach for rapid cycle change.
The RLRVAMC adopted the facilitative coaching model in November 2011, using internal coaches who were assigned to the fundamental microsystem of its medical home.
Coach Selection
Many facilitative coaching models described in the literature use external coaches. Frequently cited advantages of external coaches include having dedicated time, receiving standardized training in facilitation, and being regarded as neutral to internal conflicts. The RLRVAMC staff elected to identify internal coaches. Advantages of this approach include the use of existing resources, the ability to develop long-term continuous relationships with PACTs, and the ability to access key internal resources to assist the team. Also, using internal individuals holding primary care leadership positions was critical to the coaching model.
Thirty-eight PACTs were initially created, and 15 internal coaches were identified. These individuals included the associate chief of staff of Ambulatory Care, chief nurse for Clinic Operations, business administrators in primary care, and all frontline unit managers and supervisors. This level of management involvement provided content expertise about primary care operations and equally important, carried the authority to implement change.
Related: Using H-PACT to Overcome Treatment Obstacles for Homeless Veterans
In addition, this approach provided considerable leadership credibility among frontline PACT staff. Given the large number of PACTs requiring coaching, coach recruitment was expanded to include other primary care administrative staff, such as the leads for the CBOCs, Prevention and Behavioral Health programs, System Redesign, and Telehealth Services. The most recent phase has included registered nurses, licensed practical nurses, health technicians, and physicians from high- functioning PACT teams who have experienced the process and who can now devote time to being coaches.
Qulifications and Training
Although the literature suggests multiple qualifications for practice coaches, there is a general agreement regarding core skills for strong facilitation, which includes interpersonal skills, knowledge of process improvement techniques, and an understanding of data acquisition and analysis.10 Strong interpersonal skills are often inherent but are a critical factor in motivating team members and managing conflicts that arise. Potential coaches were not selected if these skills were poorly developed.
The authors’ experiences have shown that although content knowledge about primary care operations is very helpful, it is not essential to being an effective coach. The facilitation model that was adopted for the program, as described by Bens, focuses predominately on process and not content expertise.11 The facilitator’s role is to apply a structural framework; ie, methods and tools that capitalize on content knowledge of frontline staff in identifying changes needed to implement the medical home.
Related: Updates in Specialty Care
Also, although knowledge of primary care operations was not required, formal training in understanding the goals of the medical home and the metrics related to PACT was essential for successful coaching. All coaches were required to attend PACT training sessions. Coaches were also expected to have basic training or experience in system redesign with the majority of the coaches completing Yellow Belt training, which is an introduction to the methods of process improvement through the lean thinking business model. A coaching manual was developed that contained information related to meeting structures, data definitions, extraction, and interpretation. A coaching website was developed that provided links to data sources and definitions. PACT-related tools, such as instructions on conducting group visits, phone visits, and use of population management were disseminated.
Coach-Team Meeting Structure
Coaches were assigned to teams by matching the skills of the coach with the team needs. Initially, sessions were held weekly for 1 hour, though this typically evolved into biweekly meetings. Clinic schedules were blocked, allowing PACTs the time to meet with their coaches. A ratio of 1 coach to 2 PACTs was considered optimal for individualized team meetings. The exceptions were the CBOCs, where several teams met together due to the need for coaches to travel. Meetings were held away from clinical areas to avoid distractions. Plan-Do-Study-Act (PDSA) cycles were used to plan and implement process improvements. The average time commitment for a coach assigned to 2 teams was between 2 to 4 hours a week.
The initial coaching sessions tended to be more structured, clearly defining the coach role, developing team building, identifying the goals, and outlining process improvement tools. Common challenges for the coaches were keeping teams focused and optimally managing time by preventing prolonged conversations unrelated to process improvement. Many frontline staff had never been empowered to change their practices, so their initial reaction was to focus on problems and not solutions. Once team relationships were established, the strong influence of nursing or clerical associates exerted on the PCP’ s willingness to change became evident and a key factor for success. Often the leaders of change are not the physicians, highlighting the influence of team building and the willingness of individuals to change practice due to team relationships and not by authority.12
Data Use
Before the implementation of this model, PACT data and metrics were posted in the clinics and briefly discussed at service-level meetings. However, this data-sharing approach rarely generated team members’ interest. By using coaching, personalized data reports that displayed team-specific information in comparison to the overall service and national VA goals were found to be a more effective technique for sharing data and performance metrics. National VA PACT core metrics tracked the following: (1) percentage of same-day appointments with PCP ratio—target 70%; (2) ratio of nontraditional encounters—target 20%; (3) percentage of continuity with PCP—target 77%; and (4) percentage of 2-day contact postdischarge ratio—target 75%. Figure 3 shows the improvements made as a facility from March 2011 (pre-PACT implementation) to March 2012 (post-PACT implementation).
A graphic display of the team’s data, including metrics related to access, continuity, and postdischarge follow-up was reviewed monthly, and the coach provided detailed explanations (Figure 4). Of particular importance to the teams was the ability to individually identify those patients who failed the metric. Review of these “fallouts” at a coaching session often resulted in reliable, consistent process improvements that addressed the failed process.
Coach-to-Coach Meetings
Critical to the RLRVAMC coaching model were the weekly 1-hour coach-to-coach meetings. Most of the coach training occurred during these sessions, either formally or via feedback and discussion. Coaches discussed their teams’ progress, brought back questions from the teams, and sought guidance from one another. Executive leaders, who were also coaches, were present at these meetings and provided the opportunity to implement broader operational changes quickly. Coaches also served as a communication venue for frontline staff to express their concerns to primary care leaders during these meetings.
Limitations
Practice facilitation that uses internal coaches for a clinical PACT microsystem may present several potential challenges. Large primary care practices require a pool of coaches who are willing to commit the necessary time required for successful implementation of this model. Although the coaches dedicate this time as collateral duty, many express that the time spent with their teams is a rewarding experience outside of their administrative roles. The coaches express satisfaction when teams meet their goals and PDSA cycles are successful.
Coaches require significant amounts of training to reach the level of effectiveness required. Teams must realize and appreciate the importance of dedicating time away from the competing priority of patient care.
Implementation of the coaching model for physician trainees in the teaching clinic has not been successful due to the teaching clinic schedule and other issues. Also related to the complexity of the teaching clinic schedule, the coaching model did not significantly improve continuity. Coaches have recently been assigned to the teaching clinic, and each team will be identifying PDSA cycles to approach the implementation of PACT principles.
Conclusion
Despite the aforementioned challenges, the outcomes are clear. The implementation of the coaching model, using internal coaches, resulted in a significant improvement of the ability of the staff to achieve the national PACT metrics (Figure 3). More important, the model created a new structural organization for change within primary care that reversed a culture of top-down leadership to that of team empowerment.
Teams that experienced practice facilitation developed ownership in their processes, data, and performance improvement and now have a more direct mechanism of communicating with primary care leadership. The coaching model moved the teams forward from having received PACT education to having the confidence and tools to implement PACTs. Staff progressed from looking at the data given to them to collecting and interpreting the data themselves. The teams are able to articulate how they fit in to the PACT model and enthusiastically monitor their progress. As primary care moves forward with the medical home, the facilitative coaching model offers a promising option for successful implementation.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects— before administering pharmacologic therapy to patients.
In 2010, the VHA implemented the patient-centered medical home model of primary care health care as part of its transformational T-21 Initiatives.1 Now known as Patient Aligned Care Teams (PACTs), the key pillars of the model include the expanded roles and responsibilities of multidisciplinary care teams who provide enhanced access and coordinated care. This model is based on a foundation of adequate resources, patient centeredness, and process improvement (Figure 1).
The national implementation strategy consisted of an initial educational conference with 3,600 attendees. The conference included a series of PACT learning collaboratives that engaged > 300 primary care teams, 5 demonstration laboratories, and educational outreach through learning centers and on-site consultations. Despite an aggressive national implementation plan, many frontline primary care teams struggled to translate the medical home theory into process.
Background
The Richard L. Roudebush VAMC (RLRVAMC) is a large tertiary medical center providing care to > 44,000 primary care patients. This care is delivered by 58 primary care physicians (PCPs) in 5 hospital-based outpatient clinics, including 1 large teaching clinic, 3 community-based outpatient clinics (CBOCs), and a clinic that serves recently returned active-duty veterans. Administrative nursing and clerical associates report to the Office of Ambulatory Care, and physicians and nurse practitioners report to the Medicine Service. Before the implementation of the PACT model, the functional unit of primary care was an entire clinic, typically consisting of 4 to 10 PCPs, nurses, and clerical associates.
Discussions about process change had previously occurred through monthly service or clinic meetings in which administrative leaders provided direction to frontline staff. This culture of top-down leadership drove process change but was not always effective and empowering for practice change. With the implementation of PACT, the functional unit of primary care shifted from the larger clinic to a team composed of a PCP, a nurse, a licensed nurse practitioner or health technician, and a clerical associate.
The care delivery system fundamentally changed from the traditional model to a medical home model (Figure 2). This group now represented the fundamental clinical microsystem for the delivery of primary care within the VA medical home model.2 The experience of Batalden and colleagues at the Dartmouth Hitchcock Medical Center suggests that such microsystems are very effective units of change.3 The key challenge presented to the primary care leadership was how to link these clinical PACT microsystems with an effective process that would guide practice redesign.
The concept of practice coaching or facilitation as a mechanism for physician offices to adopt evidence-based medicine and quality improvement dates to the early 1980s in England. This model spread to the U.S. in the 1990s and has continued to be used as a mechanism for leading clinical practice redesign.4 In traditional practice facilitation, a trained individual is brought in from outside the practice to help adopt evidence-based medicine guidelines.5 This individual works with the practice to implement changes that translate into patient outcome improvements.
Unlike consultation, this facilitator maintains a long-term relationship with the team as they work together to achieve goals. More important, the facilitator assists the team in developing improvement processes that are sustainable as they become incorporated within the fabric of the team culture and remain after the coach is gone. There are several reviews of clinical practice coaching that support its effectiveness in implementing evidence-based primary care guidelines.6,7 The Affordable Care Act contains provisions for the use of this model in promoting best practices and quality care.8 Manuals developed by the Agency for Healthcare Research and Quality outline how to develop a practice facilitation program.9,10
Related: Infusing Gerontologic Practice Into PACT
Essential to all practice facilitation models is the effective use of quality improvement tools. The RLRVAMC adopted the VHA Lean Healthcare Improvement Framework, which includes an approach for rapid cycle change.
The RLRVAMC adopted the facilitative coaching model in November 2011, using internal coaches who were assigned to the fundamental microsystem of its medical home.
Coach Selection
Many facilitative coaching models described in the literature use external coaches. Frequently cited advantages of external coaches include having dedicated time, receiving standardized training in facilitation, and being regarded as neutral to internal conflicts. The RLRVAMC staff elected to identify internal coaches. Advantages of this approach include the use of existing resources, the ability to develop long-term continuous relationships with PACTs, and the ability to access key internal resources to assist the team. Also, using internal individuals holding primary care leadership positions was critical to the coaching model.
Thirty-eight PACTs were initially created, and 15 internal coaches were identified. These individuals included the associate chief of staff of Ambulatory Care, chief nurse for Clinic Operations, business administrators in primary care, and all frontline unit managers and supervisors. This level of management involvement provided content expertise about primary care operations and equally important, carried the authority to implement change.
Related: Using H-PACT to Overcome Treatment Obstacles for Homeless Veterans
In addition, this approach provided considerable leadership credibility among frontline PACT staff. Given the large number of PACTs requiring coaching, coach recruitment was expanded to include other primary care administrative staff, such as the leads for the CBOCs, Prevention and Behavioral Health programs, System Redesign, and Telehealth Services. The most recent phase has included registered nurses, licensed practical nurses, health technicians, and physicians from high- functioning PACT teams who have experienced the process and who can now devote time to being coaches.
Qulifications and Training
Although the literature suggests multiple qualifications for practice coaches, there is a general agreement regarding core skills for strong facilitation, which includes interpersonal skills, knowledge of process improvement techniques, and an understanding of data acquisition and analysis.10 Strong interpersonal skills are often inherent but are a critical factor in motivating team members and managing conflicts that arise. Potential coaches were not selected if these skills were poorly developed.
The authors’ experiences have shown that although content knowledge about primary care operations is very helpful, it is not essential to being an effective coach. The facilitation model that was adopted for the program, as described by Bens, focuses predominately on process and not content expertise.11 The facilitator’s role is to apply a structural framework; ie, methods and tools that capitalize on content knowledge of frontline staff in identifying changes needed to implement the medical home.
Related: Updates in Specialty Care
Also, although knowledge of primary care operations was not required, formal training in understanding the goals of the medical home and the metrics related to PACT was essential for successful coaching. All coaches were required to attend PACT training sessions. Coaches were also expected to have basic training or experience in system redesign with the majority of the coaches completing Yellow Belt training, which is an introduction to the methods of process improvement through the lean thinking business model. A coaching manual was developed that contained information related to meeting structures, data definitions, extraction, and interpretation. A coaching website was developed that provided links to data sources and definitions. PACT-related tools, such as instructions on conducting group visits, phone visits, and use of population management were disseminated.
Coach-Team Meeting Structure
Coaches were assigned to teams by matching the skills of the coach with the team needs. Initially, sessions were held weekly for 1 hour, though this typically evolved into biweekly meetings. Clinic schedules were blocked, allowing PACTs the time to meet with their coaches. A ratio of 1 coach to 2 PACTs was considered optimal for individualized team meetings. The exceptions were the CBOCs, where several teams met together due to the need for coaches to travel. Meetings were held away from clinical areas to avoid distractions. Plan-Do-Study-Act (PDSA) cycles were used to plan and implement process improvements. The average time commitment for a coach assigned to 2 teams was between 2 to 4 hours a week.
The initial coaching sessions tended to be more structured, clearly defining the coach role, developing team building, identifying the goals, and outlining process improvement tools. Common challenges for the coaches were keeping teams focused and optimally managing time by preventing prolonged conversations unrelated to process improvement. Many frontline staff had never been empowered to change their practices, so their initial reaction was to focus on problems and not solutions. Once team relationships were established, the strong influence of nursing or clerical associates exerted on the PCP’ s willingness to change became evident and a key factor for success. Often the leaders of change are not the physicians, highlighting the influence of team building and the willingness of individuals to change practice due to team relationships and not by authority.12
Data Use
Before the implementation of this model, PACT data and metrics were posted in the clinics and briefly discussed at service-level meetings. However, this data-sharing approach rarely generated team members’ interest. By using coaching, personalized data reports that displayed team-specific information in comparison to the overall service and national VA goals were found to be a more effective technique for sharing data and performance metrics. National VA PACT core metrics tracked the following: (1) percentage of same-day appointments with PCP ratio—target 70%; (2) ratio of nontraditional encounters—target 20%; (3) percentage of continuity with PCP—target 77%; and (4) percentage of 2-day contact postdischarge ratio—target 75%. Figure 3 shows the improvements made as a facility from March 2011 (pre-PACT implementation) to March 2012 (post-PACT implementation).
A graphic display of the team’s data, including metrics related to access, continuity, and postdischarge follow-up was reviewed monthly, and the coach provided detailed explanations (Figure 4). Of particular importance to the teams was the ability to individually identify those patients who failed the metric. Review of these “fallouts” at a coaching session often resulted in reliable, consistent process improvements that addressed the failed process.
Coach-to-Coach Meetings
Critical to the RLRVAMC coaching model were the weekly 1-hour coach-to-coach meetings. Most of the coach training occurred during these sessions, either formally or via feedback and discussion. Coaches discussed their teams’ progress, brought back questions from the teams, and sought guidance from one another. Executive leaders, who were also coaches, were present at these meetings and provided the opportunity to implement broader operational changes quickly. Coaches also served as a communication venue for frontline staff to express their concerns to primary care leaders during these meetings.
Limitations
Practice facilitation that uses internal coaches for a clinical PACT microsystem may present several potential challenges. Large primary care practices require a pool of coaches who are willing to commit the necessary time required for successful implementation of this model. Although the coaches dedicate this time as collateral duty, many express that the time spent with their teams is a rewarding experience outside of their administrative roles. The coaches express satisfaction when teams meet their goals and PDSA cycles are successful.
Coaches require significant amounts of training to reach the level of effectiveness required. Teams must realize and appreciate the importance of dedicating time away from the competing priority of patient care.
Implementation of the coaching model for physician trainees in the teaching clinic has not been successful due to the teaching clinic schedule and other issues. Also related to the complexity of the teaching clinic schedule, the coaching model did not significantly improve continuity. Coaches have recently been assigned to the teaching clinic, and each team will be identifying PDSA cycles to approach the implementation of PACT principles.
Conclusion
Despite the aforementioned challenges, the outcomes are clear. The implementation of the coaching model, using internal coaches, resulted in a significant improvement of the ability of the staff to achieve the national PACT metrics (Figure 3). More important, the model created a new structural organization for change within primary care that reversed a culture of top-down leadership to that of team empowerment.
Teams that experienced practice facilitation developed ownership in their processes, data, and performance improvement and now have a more direct mechanism of communicating with primary care leadership. The coaching model moved the teams forward from having received PACT education to having the confidence and tools to implement PACTs. Staff progressed from looking at the data given to them to collecting and interpreting the data themselves. The teams are able to articulate how they fit in to the PACT model and enthusiastically monitor their progress. As primary care moves forward with the medical home, the facilitative coaching model offers a promising option for successful implementation.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects— before administering pharmacologic therapy to patients.
1. Klein S. The Veterans Health Administration: Implementing patient-centered medical homes in the nation’s largest integrated delivery system. Issue Brief (Commonw Fund). 2011;16:1537.
2. Godfrey MM, ed. Clinical Microsystem Action Guide: Improving Health Care by Improving Your Microsystem. Version 2.1. The Dartmouth Institute Website. http://clinicalmicrosys.dartmouth.edu/wp -content/uploads/2014/07/CMAG040104.pdf. Published 2004. Accessed January 23, 2015.
3. Batalden PB, Nelson EC, Edwards WH, Godfrey MM, Mohr JJ. Microsystems in health care: Part 9. Developing a small clinical unit to attain peak performance. Jt Comm J Qual Saf. 2003;29(11):575-585.
4. Nutting PA, Crabtree BF, Stewart EE, et al. Effect of facilitation on practice outcomes in the National Demonstration Project model of the patient-centered medical home. Ann Fam Med. 2010;8(suppl 1):S33-S34, S92.
5. Nagykaldi Z, Mold JW, Robinson A, Niebauer L, Ford A. Practice facilitators and practice-based research networks. J Am Board Fam Med. 2006;19(5):506-510.
6. Baskerville NB, Liddy C, Hogg W. Systematic review and meta-analysis of practice facilitation within primary care settings. Ann Fam Med. 2012;10(1):63-74.
7. Nagykaldi Z, Mold JW, Aspy CB. Practice facilitators: A review of the literature. Fam Med. 2005;37(8):581-588.
8. Grumbach K, Bainbridge E, Bodenheimer T. Facilitating improvement in primary care: The promise of practice coaching. Issue Brief (Commonw Fund). 2012;15:1-14.
9. Knox L, Taylor EF, Geonnotti K, et al. Developing and Running a Primary Care Practice Facilitation Program: A How-To Guide. Rockville, MD: Agency for Healthcare Research and Quality. U.S. Department of Health and Human Services; 2011. AHRQ Publication No. 12-0011.
10. Agency for Healthcare Research and Quality. Integrating Chronic Care and Business Strategies in the Safety Net: A Practice Coaching Manual. Agency for Healthcare Research and Quality Website. http://www.ahrq.gov/professionals/systems/primary-care /coachmnl/index.html. Published December 2012. Accessed February 26, 2015.
11. Bens I. Facilitation With Ease! Core Skills for Facilitators, Team Leaders and Members, Managers, Consultants and Trainers. 2nd ed. San Francisco, CA: Jossey-Bass; 2005.
12. Bodenheimer T. Building Teams in Primary Care: Lessons Learned: Part 1 and 2. Oakland, CA: California Health Care Foundation; July 2007.
1. Klein S. The Veterans Health Administration: Implementing patient-centered medical homes in the nation’s largest integrated delivery system. Issue Brief (Commonw Fund). 2011;16:1537.
2. Godfrey MM, ed. Clinical Microsystem Action Guide: Improving Health Care by Improving Your Microsystem. Version 2.1. The Dartmouth Institute Website. http://clinicalmicrosys.dartmouth.edu/wp -content/uploads/2014/07/CMAG040104.pdf. Published 2004. Accessed January 23, 2015.
3. Batalden PB, Nelson EC, Edwards WH, Godfrey MM, Mohr JJ. Microsystems in health care: Part 9. Developing a small clinical unit to attain peak performance. Jt Comm J Qual Saf. 2003;29(11):575-585.
4. Nutting PA, Crabtree BF, Stewart EE, et al. Effect of facilitation on practice outcomes in the National Demonstration Project model of the patient-centered medical home. Ann Fam Med. 2010;8(suppl 1):S33-S34, S92.
5. Nagykaldi Z, Mold JW, Robinson A, Niebauer L, Ford A. Practice facilitators and practice-based research networks. J Am Board Fam Med. 2006;19(5):506-510.
6. Baskerville NB, Liddy C, Hogg W. Systematic review and meta-analysis of practice facilitation within primary care settings. Ann Fam Med. 2012;10(1):63-74.
7. Nagykaldi Z, Mold JW, Aspy CB. Practice facilitators: A review of the literature. Fam Med. 2005;37(8):581-588.
8. Grumbach K, Bainbridge E, Bodenheimer T. Facilitating improvement in primary care: The promise of practice coaching. Issue Brief (Commonw Fund). 2012;15:1-14.
9. Knox L, Taylor EF, Geonnotti K, et al. Developing and Running a Primary Care Practice Facilitation Program: A How-To Guide. Rockville, MD: Agency for Healthcare Research and Quality. U.S. Department of Health and Human Services; 2011. AHRQ Publication No. 12-0011.
10. Agency for Healthcare Research and Quality. Integrating Chronic Care and Business Strategies in the Safety Net: A Practice Coaching Manual. Agency for Healthcare Research and Quality Website. http://www.ahrq.gov/professionals/systems/primary-care /coachmnl/index.html. Published December 2012. Accessed February 26, 2015.
11. Bens I. Facilitation With Ease! Core Skills for Facilitators, Team Leaders and Members, Managers, Consultants and Trainers. 2nd ed. San Francisco, CA: Jossey-Bass; 2005.
12. Bodenheimer T. Building Teams in Primary Care: Lessons Learned: Part 1 and 2. Oakland, CA: California Health Care Foundation; July 2007.
Importance of Early Initiation of Advance Care Planning
Every day, health care providers (HCPs) care for patients with advanced chronic illnesses. At times, HCPs make critical treatment decisions without input from the patient. As a result, patients are often confused about their disease trajectory, prognosis, benefits and burdens of treatments, and outcome preferences.1 Unfortunately, limited research has been conducted on patients who have chronic illnesses, such as congestive heart failure (CHF) and chronic obstructive pulmonary disease (COPD), regarding early discussion of advance care planning (ACP) and advance directives (ADs). This gap in the knowledge base has contributed to a delay in the initiation of ACP for patients with COPD or CHF, especially for those with end-stage illness.
Background
The Patient Self-Determination Act (PSDA) was passed in 1990 to inform patients of their rights about health care choices while in the hospital, but the completion rate for ADs remains poor.2 The 3 key elements include the right of patients to facilitate their own health care decisions, the right to refuse or accept treatment, and the right to make an AD.
One of the reasons for the poor AD completion rate may be increased confusion about the difference between ACP and an AD.3 Advance care planning is a discussion about overall goals of care related to health care and progression through the life cycle. Advance directives focus on more specific information, including who will be designated as health care proxy, which health care interventions would be requested and which would be declined, and decisions regarding code status and organ donation.
A case study was conducted at a long-term care facility to test beliefs that residents who made their wishes known through ACP would have a positive experience at the end of life (EOL).3 The study allowed the residents to provide direction on what are and are not acceptable treatments at EOL. Before this study, most residents did not have a health care proxy and had not discussed the topic of EOL care with their HCP. Treatment choices were also not designated.3 Results demonstrated that ACP had positive outcomes for residents and family members, including documentation of an AD, autonomy in decision making, person-centered approaches to care, and dying with dignity.
Related: Fiduciary Services for Veterans With Psychiatric Disabilities
Much of the research regarding ADs has been conducted with seniors, hospitalized patients, and those with critical or terminal illnesses. A study by Jackson and colleagues examined attitudes, experiences, and preferences about ADs among adults of all ages. The study used an age-stratified random sample of patients from a large managed care organization.4 Findings revealed that older subjects were likely to be comfortable with and complete an AD. The most valuable outcome was the discussion of personal wishes with family and loved ones. Overall, the findings of this study concurred with the findings of other studies demonstrating that patients wanted control over EOL care decisions or wanted family members or loved ones to make those decisions. Consequently, patients not only believe the decisions are their responsibility, but also feel comfortable if their HCP initiates this conversation.4
Open and direct discussion regarding care planning can ease many of the fears related to EOL care. Discussion of an AD is a way to prepare for death and dying, rather than just a preparation for being incapacitated in the future. The process allows improved communication between patients, surrogates, and HCPs.3
The importance of communication with the patient’s primary care provider (PCP) regarding discussion of ACP or an AD is demonstrated in a longitudinal study completed by Ramsaroop and colleagues from January 1991 through July 2005. This systematic review of studies was designed to increase the completion of an AD in primary care settings.5 The study reviewed interventions that were most successful in improving the AD completion rate. The investigators extracted physician and patient barriers to completion of an AD. Findings suggested that the most successful intervention for completion of an AD were conversations that took place between patients and HCPs about ACP and occurred over multiple visits. By contrast, passive education using written materials without any direct counseling was relatively ineffective.5 The study also demonstrated the importance of completion of an AD in the primary care setting, gauging patient readiness to complete an AD, and having the PCP initiate the AD conversation.5
Related: Personal Counseling Helps Prevent Cancer-Related Malnutrition
If communication does not occur between patients and HCPs, care preferences are often not documented. Without this documentation many patients do not receive appropriate palliative care services when needed. Palliative care is not available to and therefore often not used with patients with nononcologic diseases.6 A study by Mahtani-Chugani and colleagues evaluated barriers to providing palliative care to nononcologic patients and proposed strategies to overcome them. Findings suggested 4 barriers: (1) lack of clarity about illness and prognosis; (2) discussion limited exclusively to the curative approach; (3) avoiding terms such as “terminal illness”; and (4) cheating death, including linking nononcologic disease and death.6 A strategy to overcoming these barriers highlighted improved communication between HCP and patient and understanding that the communication process is as important as the content of the message. Therefore, equitable palliative care services should be offered to both nononcologic and oncologic patients.6
One life-limiting nononcologic disease is COPD. Chronic obstructive pulmonary disease remains a major public health problem. It is the fourth leading cause of chronic morbidity and mortality in the U.S. and is projected to rank fifth in 2020 in disease burden worldwide.7 Given its prevalence, COPD is found in all adult health care settings.
Among hospitalized veterans in the VHA in 2005, COPD was the fourth most common discharge diagnosis.8 In the veteran population, a high prevalence for developing COPD also exists due to high-risk factors including tobacco use in the military. According to a study conducted at the Cincinnati VAMC in Ohio, a 40% greater prevalence of COPD existed in this veteran population than in the general U.S. population.8
Related: Lifestyle Intervention for Veterans With Chronic Diseases
Another nononcologic, life-limiting disease is CHF. Both the prevalence and hospitalization rates for CHF show an upward trend since the 1970s, resulting in a continued increase in CHF death rates.9 According to 2008 estimates from the National Institutes of Health, there are 5 million CHF patients in the U.S. and hospitalization rates approach 1 million per year.9 Congestive heart failure affects 2.4% of the adult population and > 11% of the expanding population aged > 80 years. Existing care may slow the progression of the disease but can rarely reverse it, which usually results in a prolonged period of advanced illness. As a result of the increasing prevalence, there remains a high symptom burden for patients living with advanced CHF.10
In managing the high symptom burden of CHF and COPD, patient-centered care must be acknowledged and used. Patient-centered care mandates that beneficial therapies and recommended guidelines be offered and discussed with the patient, giving attention to patient preferences.10
Study Design
The theoretical framework for the development and implementation of this project is based on Ruland and Moore’s Peaceful End of Life Theory.11 This theory is based on 2 assumptions. The first is that each person’s approach to EOL is personal. The second is that nursing care plays a major role in making EOL a peaceful experience. The 5 outcome measures include: (1) not experiencing pain; (2) the experience of comfort; (3) the experience of dignity and respect; (4) being at peace; and (5) closeness to significant others or other caring persons.
The outcome indicator of the Peaceful End of Life Theory— experience of dignity/respect with its related criteria and prescriptors—provided structure for the development and implementation of this project. The prescriptors related to the experience of dignity/respect include involving the patient and significant others in decision making; treating the patient with dignity, empathy, and respect; and being attentive to the patient’s expressed needs, wishes, and preferences.11
Due to the increased prevalence of chronic illnesses in the VA system, veterans need encouragement to complete ADs. The VA instituted a national directive guiding education and implementation of an AD.12 These discussions occur at the first contact a veteran has with the system and at other times when appropriate. The purpose of the directive is to allow veterans to guide the course of their treatment and to assure that they are aware of the ability to refuse treatment at any time.12
Inconsistencies in Advance Directive Completion
Inconsistencies were noted with how ADs were completed at the VA Northern Indiana Health Care System in Muncie. For outpatients, the clinic nursing staff received an electronic medical record (EMR) reminder if the veteran did not have an AD. This reminder prompted the nurse to ask the veteran about completing an AD. If the veteran agreed, a social work consult was initiated by nursing. Of concern, the social worker is usually responsible for several clinics so it is unlikely the process of completing the AD would be accomplished on the day the veteran was already in the clinic.
Discussions in the inpatient setting included a physician, a nurse practitioner, or a social worker and were often disease specific and patient oriented. However, in an acute hospitalization, it was less likely that patients initiated ADs due to acute illness and rapid nature of treatment.
Another concern was related to the amount of clinical knowledge the social worker had about the specifics of each patient’s case. Without specifics, a social worker can make the AD discussion very broad. Patients want information regarding disease progression and prognosis specific to their own condition to be able to make an informed choice regarding ADs.13
A study population with the diagnoses of CHF and COPD was selected due to the prevalence in the facility and at the request of facility leadership.
Methods
The primary aim of this quality improvement (QI) project was to educate the PCP about the importance of allowing veterans to express their care goals in the form of ADs and to understand that veterans would prefer to discuss these goals with their PCP. A secondary aim was to improve goal-directed care for veterans with COPD or CHF by increasing the number of completed ADs.
By using a systems approach, ACP can be addressed in a uniform manner. This approach allows veterans to discuss their goals of care prior to the need for emergent interventions, avoiding burdensome and unwanted treatments. Through the completion of ADs, veterans are able to designate a surrogate decision maker and identify specific desired treatments and interventions as their illness advances.
Two physicians and 3 nurse practitioners volunteered to contribute to this study. In the participating clinics, veterans with a diagnosis of CHF or COPD were identified. Each veteran had 20 to 30 minutes per appointment to discuss concerns, be examined, have HCPs address concerns, and complete all clinical reminders.
The study design was a QI project focused on evaluating the following process: An EMR reminder alerted the clinic nurse who asked the veteran if he or she was interested in completing an AD. If the patient agreed, a consult was placed to the social worker for completion of the VA national form for AD. The completion rate for a sample of primary care clinics at the facility was 10% to 12%, with no participation from the PCP in the process.
The providers were educated in 5 areas: (1) the prevalence of CHF and COPD in the U.S.; (2) the difference between ACP and AD; (3) the percentage of ADs completed in the U.S. adult population and in the facility; (4) the importance of addressing ACP early in the disease trajectory of this population; and (5) the use of the EMR reminder and the template to guide discussion of ACP ( eAppendix A
The template was developed following the literature review and addressed the reoccurring themes that patients wanted to discuss concerning their specific diagnosis and treatment. The template was formatted to include 3 components: (1) health care surrogate; (2) code status; and (3) organ donation preference ( eAppendix B
The natural progression from discussion of goals of care led to the discussion regarding the initiation of an AD. When completed, the note automatically appeared in the Postings section of the EMR, making it easily accessible to all other providers in different care settings.
The project time was 3 months (December 2012 through February 2013). The education was completed and the EMR reminder was turned on at the beginning of the project for veterans with a diagnosis of CHF or COPD who had not completed an AD. At the conclusion of the project, the PCPs completed a post project survey to provide information regarding their opinions on facilitators and barriers in the AD completion process ( eAppendix C
Results
Five different primary care clinics in 4 different locations throughout the health care system provided a total of 294 veterans with diagnoses of CHF or COPD. On completion of the project, 35 veterans had completed ADs. These 35 veterans represent an additional 12% of patients who previously did not complete an AD despite being approached multiple times. The veterans completed an AD following PCP-initiated discussion due to this QI project.
All 5 providers completed the post project survey and agreed that it would be beneficial to have the information regarding ADs easily accessible in the EMR. Four out of 5 providers admitted to cutting corners by not opening a new note every time to complete the AD template. They reported completing the EMR reminder within the clinic note, making it difficult to locate the information. Providers also reported on the various facilitators and barriers to AD discussion with patients (Table).
Discussion
The completion of an AD remains an important part of health care that is often neglected. When patients receive care and treatments, they often do not desire an AD, because the goals of care have not been clearly communicated and clearly documented. This can lead to poor quality of care with increased dissatisfaction and burden on the patient and health care system.14 However, if goals of care are discussed and documented, the veteran may avoid these burdensome treatments, and health care will be congruent with patient wishes. Better communication and documentation promotes increased patient satisfaction and improved quality of health care.1
This project endorses findings from a previous case study that demonstrated better patient-centered, goal-directed care results when patients have the opportunity to complete an AD, thereby improving health care quality and patient/family satisfaction.3 Previous studies suggested one way to increase the AD completion rate involves the PCP initiating a discussion with the patient.5 This project supports that conclusion.
Limitations
All the project providers expressed support regarding the importance of discussing ACPs with their patients. The major limitation identified by the project providers was time constraints in a busy primary care clinic. One provider suggested initiation of an EMR reminder once per year to prompt discussion. The same provider also recommended rescheduling an additional clinic visit to have an in-depth discussion regarding ACP.
Another limitation to this project involved the EMR. Currently, there is no way to have information in the postings section without a separate note. The project providers all agreed that it was not always possible to open a new note to use the template due to limited clinic time. This allowed information regarding health care surrogates and discussions regarding code status and organ donation to be embedded in a clinic note, which can make it difficult for other providers at different levels of care to effectively locate. Incorporating a method to allow information from an EMR reminder to be automatically placed in the postings section would alleviate this limitation.
A further limitation involved the setting. The VA provides care only to veterans. The project can be generalized to other VA primary care clinics, but generalizability beyond the VA may be limited.
This QI project took place over 3 months, another potential limitation due to the limited study period. Also, due to the short time frame of the project, a small sample size was used. Further investigation of this topic by expanding the time frame and sample size would further develop this body of knowledge.
The VA uses an EMR that is accessible to all VA providers locally and nationwide. Due to the nationwide network, expansion of the project would be possible with the support of facility leadership and the EMR reminder staff. By using the education and the template for discussion, the project could be replicated throughout the system.
Conclusion
Advance care planning and ADs should be a regular part of the health care process, especially for veterans with noncancer diagnoses, such as CHF and COPD. Clear communication about disease trajectory and prognosis are an important part of this discussion. Primary care providers are in the optimal setting to initiate this discussion.
This project supports previous findings that a PCP initiating or participating in the ACP discussion would result in an improved completion rate for ADs.5 Theoretically, improved AD completions result in patient-centered care, leading to higher patient satisfaction.
Acknowledgements
The authors would like to acknowledge the VA Northern Indiana Health Care System for its support of this project.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Pautex S, Herrmann FR, Zulian GB. Role of advance directives in palliative care units: A prospective study. Palliat Med. 2008;22(7):835-841.
2. Cohen MJ, McCannon JB, Edgman-Levitan S, Kormos WA. Exploring attitudes toward advance care directives in two diverse settings. J Palliat Med. 2010;13(12):1427-1432.
3. Jeong SY-S, Higgins I, McMillan M. The essentials of Advance Care Planning for end-of-life care for older people. J Clin Nurs. 2010;19(3-4):389-397.
4. Jackson JM, Rolnick SJ, Asche SE, Heinrich RL. Knowledge, attitudes, and p regarding advance directives among patients of a managed care organization. Am J Manag Care. 2009;15(3):177-186.
5. Ramsaroop SD, Reid MC, Adelman RD. Completing an advance directive in the primary care setting: What do we need for success? J Am Geriatr Soc. 2007;55(2):277-283.
6. Mahtani-Chugani V, González-Castro I, de Ormijana-Hernández AS, Martín-Fernández R, de la Vega EF. How to provide care for patients suffering from terminal non-oncological diseases: Barriers to a palliative care approach. Palliat Med. 2010;24(8):787-795.
7. Rabe KF, Hurd S, Anzueto A, et al; Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532-555.
8. Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560.
9. National Institutes of Health. Division of cardiovascular disease strategic plan. National Heart, Lung, and Blood Institute Website. http://www .nhlbi.nih.gov/about/org/dcvs/sp/goal-2.4b. 2012. Accessed January 29, 2015.
10. Allen LA, Stevenson LW, Grady KL; American Heart Association; Council on Quality of Care and Outcomes Research; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia. Decision making in advanced heart failure: A scientific statement from the American Heart Association. Circulation. 2012;125(15):1928-1952.
11. Ruland CM, Moore SM. Theory construction based on standards of care: A proposed theory of the peaceful end of life. Nurs Outlook. 1998;46(4):169-175.
12. US Department of Veterans Affairs, Veterans Health Administration . VHA Handbook 1004.02 transmittal sheet. Published July 2, 2009. http://www.ethics.va.gov/docs/policy/ADTraining /vha_handbk_1004_02.pdf. Accessed January 29, 2015.
13. Gott M, Gardiner C, Small N, et al. Barriers to advance care planning in chronic obstructive pulmonary disease. Palliat Med. 2009;23(7): 642-648.
14. Teno JM, Gruneir A, Schwartz Z, Nanda A, Wetle T. Association between advance directives and quality end-of-life care: A national study. J Am Geriatr Soc. 2007;55(2):189-194.
Every day, health care providers (HCPs) care for patients with advanced chronic illnesses. At times, HCPs make critical treatment decisions without input from the patient. As a result, patients are often confused about their disease trajectory, prognosis, benefits and burdens of treatments, and outcome preferences.1 Unfortunately, limited research has been conducted on patients who have chronic illnesses, such as congestive heart failure (CHF) and chronic obstructive pulmonary disease (COPD), regarding early discussion of advance care planning (ACP) and advance directives (ADs). This gap in the knowledge base has contributed to a delay in the initiation of ACP for patients with COPD or CHF, especially for those with end-stage illness.
Background
The Patient Self-Determination Act (PSDA) was passed in 1990 to inform patients of their rights about health care choices while in the hospital, but the completion rate for ADs remains poor.2 The 3 key elements include the right of patients to facilitate their own health care decisions, the right to refuse or accept treatment, and the right to make an AD.
One of the reasons for the poor AD completion rate may be increased confusion about the difference between ACP and an AD.3 Advance care planning is a discussion about overall goals of care related to health care and progression through the life cycle. Advance directives focus on more specific information, including who will be designated as health care proxy, which health care interventions would be requested and which would be declined, and decisions regarding code status and organ donation.
A case study was conducted at a long-term care facility to test beliefs that residents who made their wishes known through ACP would have a positive experience at the end of life (EOL).3 The study allowed the residents to provide direction on what are and are not acceptable treatments at EOL. Before this study, most residents did not have a health care proxy and had not discussed the topic of EOL care with their HCP. Treatment choices were also not designated.3 Results demonstrated that ACP had positive outcomes for residents and family members, including documentation of an AD, autonomy in decision making, person-centered approaches to care, and dying with dignity.
Related: Fiduciary Services for Veterans With Psychiatric Disabilities
Much of the research regarding ADs has been conducted with seniors, hospitalized patients, and those with critical or terminal illnesses. A study by Jackson and colleagues examined attitudes, experiences, and preferences about ADs among adults of all ages. The study used an age-stratified random sample of patients from a large managed care organization.4 Findings revealed that older subjects were likely to be comfortable with and complete an AD. The most valuable outcome was the discussion of personal wishes with family and loved ones. Overall, the findings of this study concurred with the findings of other studies demonstrating that patients wanted control over EOL care decisions or wanted family members or loved ones to make those decisions. Consequently, patients not only believe the decisions are their responsibility, but also feel comfortable if their HCP initiates this conversation.4
Open and direct discussion regarding care planning can ease many of the fears related to EOL care. Discussion of an AD is a way to prepare for death and dying, rather than just a preparation for being incapacitated in the future. The process allows improved communication between patients, surrogates, and HCPs.3
The importance of communication with the patient’s primary care provider (PCP) regarding discussion of ACP or an AD is demonstrated in a longitudinal study completed by Ramsaroop and colleagues from January 1991 through July 2005. This systematic review of studies was designed to increase the completion of an AD in primary care settings.5 The study reviewed interventions that were most successful in improving the AD completion rate. The investigators extracted physician and patient barriers to completion of an AD. Findings suggested that the most successful intervention for completion of an AD were conversations that took place between patients and HCPs about ACP and occurred over multiple visits. By contrast, passive education using written materials without any direct counseling was relatively ineffective.5 The study also demonstrated the importance of completion of an AD in the primary care setting, gauging patient readiness to complete an AD, and having the PCP initiate the AD conversation.5
Related: Personal Counseling Helps Prevent Cancer-Related Malnutrition
If communication does not occur between patients and HCPs, care preferences are often not documented. Without this documentation many patients do not receive appropriate palliative care services when needed. Palliative care is not available to and therefore often not used with patients with nononcologic diseases.6 A study by Mahtani-Chugani and colleagues evaluated barriers to providing palliative care to nononcologic patients and proposed strategies to overcome them. Findings suggested 4 barriers: (1) lack of clarity about illness and prognosis; (2) discussion limited exclusively to the curative approach; (3) avoiding terms such as “terminal illness”; and (4) cheating death, including linking nononcologic disease and death.6 A strategy to overcoming these barriers highlighted improved communication between HCP and patient and understanding that the communication process is as important as the content of the message. Therefore, equitable palliative care services should be offered to both nononcologic and oncologic patients.6
One life-limiting nononcologic disease is COPD. Chronic obstructive pulmonary disease remains a major public health problem. It is the fourth leading cause of chronic morbidity and mortality in the U.S. and is projected to rank fifth in 2020 in disease burden worldwide.7 Given its prevalence, COPD is found in all adult health care settings.
Among hospitalized veterans in the VHA in 2005, COPD was the fourth most common discharge diagnosis.8 In the veteran population, a high prevalence for developing COPD also exists due to high-risk factors including tobacco use in the military. According to a study conducted at the Cincinnati VAMC in Ohio, a 40% greater prevalence of COPD existed in this veteran population than in the general U.S. population.8
Related: Lifestyle Intervention for Veterans With Chronic Diseases
Another nononcologic, life-limiting disease is CHF. Both the prevalence and hospitalization rates for CHF show an upward trend since the 1970s, resulting in a continued increase in CHF death rates.9 According to 2008 estimates from the National Institutes of Health, there are 5 million CHF patients in the U.S. and hospitalization rates approach 1 million per year.9 Congestive heart failure affects 2.4% of the adult population and > 11% of the expanding population aged > 80 years. Existing care may slow the progression of the disease but can rarely reverse it, which usually results in a prolonged period of advanced illness. As a result of the increasing prevalence, there remains a high symptom burden for patients living with advanced CHF.10
In managing the high symptom burden of CHF and COPD, patient-centered care must be acknowledged and used. Patient-centered care mandates that beneficial therapies and recommended guidelines be offered and discussed with the patient, giving attention to patient preferences.10
Study Design
The theoretical framework for the development and implementation of this project is based on Ruland and Moore’s Peaceful End of Life Theory.11 This theory is based on 2 assumptions. The first is that each person’s approach to EOL is personal. The second is that nursing care plays a major role in making EOL a peaceful experience. The 5 outcome measures include: (1) not experiencing pain; (2) the experience of comfort; (3) the experience of dignity and respect; (4) being at peace; and (5) closeness to significant others or other caring persons.
The outcome indicator of the Peaceful End of Life Theory— experience of dignity/respect with its related criteria and prescriptors—provided structure for the development and implementation of this project. The prescriptors related to the experience of dignity/respect include involving the patient and significant others in decision making; treating the patient with dignity, empathy, and respect; and being attentive to the patient’s expressed needs, wishes, and preferences.11
Due to the increased prevalence of chronic illnesses in the VA system, veterans need encouragement to complete ADs. The VA instituted a national directive guiding education and implementation of an AD.12 These discussions occur at the first contact a veteran has with the system and at other times when appropriate. The purpose of the directive is to allow veterans to guide the course of their treatment and to assure that they are aware of the ability to refuse treatment at any time.12
Inconsistencies in Advance Directive Completion
Inconsistencies were noted with how ADs were completed at the VA Northern Indiana Health Care System in Muncie. For outpatients, the clinic nursing staff received an electronic medical record (EMR) reminder if the veteran did not have an AD. This reminder prompted the nurse to ask the veteran about completing an AD. If the veteran agreed, a social work consult was initiated by nursing. Of concern, the social worker is usually responsible for several clinics so it is unlikely the process of completing the AD would be accomplished on the day the veteran was already in the clinic.
Discussions in the inpatient setting included a physician, a nurse practitioner, or a social worker and were often disease specific and patient oriented. However, in an acute hospitalization, it was less likely that patients initiated ADs due to acute illness and rapid nature of treatment.
Another concern was related to the amount of clinical knowledge the social worker had about the specifics of each patient’s case. Without specifics, a social worker can make the AD discussion very broad. Patients want information regarding disease progression and prognosis specific to their own condition to be able to make an informed choice regarding ADs.13
A study population with the diagnoses of CHF and COPD was selected due to the prevalence in the facility and at the request of facility leadership.
Methods
The primary aim of this quality improvement (QI) project was to educate the PCP about the importance of allowing veterans to express their care goals in the form of ADs and to understand that veterans would prefer to discuss these goals with their PCP. A secondary aim was to improve goal-directed care for veterans with COPD or CHF by increasing the number of completed ADs.
By using a systems approach, ACP can be addressed in a uniform manner. This approach allows veterans to discuss their goals of care prior to the need for emergent interventions, avoiding burdensome and unwanted treatments. Through the completion of ADs, veterans are able to designate a surrogate decision maker and identify specific desired treatments and interventions as their illness advances.
Two physicians and 3 nurse practitioners volunteered to contribute to this study. In the participating clinics, veterans with a diagnosis of CHF or COPD were identified. Each veteran had 20 to 30 minutes per appointment to discuss concerns, be examined, have HCPs address concerns, and complete all clinical reminders.
The study design was a QI project focused on evaluating the following process: An EMR reminder alerted the clinic nurse who asked the veteran if he or she was interested in completing an AD. If the patient agreed, a consult was placed to the social worker for completion of the VA national form for AD. The completion rate for a sample of primary care clinics at the facility was 10% to 12%, with no participation from the PCP in the process.
The providers were educated in 5 areas: (1) the prevalence of CHF and COPD in the U.S.; (2) the difference between ACP and AD; (3) the percentage of ADs completed in the U.S. adult population and in the facility; (4) the importance of addressing ACP early in the disease trajectory of this population; and (5) the use of the EMR reminder and the template to guide discussion of ACP ( eAppendix A
The template was developed following the literature review and addressed the reoccurring themes that patients wanted to discuss concerning their specific diagnosis and treatment. The template was formatted to include 3 components: (1) health care surrogate; (2) code status; and (3) organ donation preference ( eAppendix B
The natural progression from discussion of goals of care led to the discussion regarding the initiation of an AD. When completed, the note automatically appeared in the Postings section of the EMR, making it easily accessible to all other providers in different care settings.
The project time was 3 months (December 2012 through February 2013). The education was completed and the EMR reminder was turned on at the beginning of the project for veterans with a diagnosis of CHF or COPD who had not completed an AD. At the conclusion of the project, the PCPs completed a post project survey to provide information regarding their opinions on facilitators and barriers in the AD completion process ( eAppendix C
Results
Five different primary care clinics in 4 different locations throughout the health care system provided a total of 294 veterans with diagnoses of CHF or COPD. On completion of the project, 35 veterans had completed ADs. These 35 veterans represent an additional 12% of patients who previously did not complete an AD despite being approached multiple times. The veterans completed an AD following PCP-initiated discussion due to this QI project.
All 5 providers completed the post project survey and agreed that it would be beneficial to have the information regarding ADs easily accessible in the EMR. Four out of 5 providers admitted to cutting corners by not opening a new note every time to complete the AD template. They reported completing the EMR reminder within the clinic note, making it difficult to locate the information. Providers also reported on the various facilitators and barriers to AD discussion with patients (Table).
Discussion
The completion of an AD remains an important part of health care that is often neglected. When patients receive care and treatments, they often do not desire an AD, because the goals of care have not been clearly communicated and clearly documented. This can lead to poor quality of care with increased dissatisfaction and burden on the patient and health care system.14 However, if goals of care are discussed and documented, the veteran may avoid these burdensome treatments, and health care will be congruent with patient wishes. Better communication and documentation promotes increased patient satisfaction and improved quality of health care.1
This project endorses findings from a previous case study that demonstrated better patient-centered, goal-directed care results when patients have the opportunity to complete an AD, thereby improving health care quality and patient/family satisfaction.3 Previous studies suggested one way to increase the AD completion rate involves the PCP initiating a discussion with the patient.5 This project supports that conclusion.
Limitations
All the project providers expressed support regarding the importance of discussing ACPs with their patients. The major limitation identified by the project providers was time constraints in a busy primary care clinic. One provider suggested initiation of an EMR reminder once per year to prompt discussion. The same provider also recommended rescheduling an additional clinic visit to have an in-depth discussion regarding ACP.
Another limitation to this project involved the EMR. Currently, there is no way to have information in the postings section without a separate note. The project providers all agreed that it was not always possible to open a new note to use the template due to limited clinic time. This allowed information regarding health care surrogates and discussions regarding code status and organ donation to be embedded in a clinic note, which can make it difficult for other providers at different levels of care to effectively locate. Incorporating a method to allow information from an EMR reminder to be automatically placed in the postings section would alleviate this limitation.
A further limitation involved the setting. The VA provides care only to veterans. The project can be generalized to other VA primary care clinics, but generalizability beyond the VA may be limited.
This QI project took place over 3 months, another potential limitation due to the limited study period. Also, due to the short time frame of the project, a small sample size was used. Further investigation of this topic by expanding the time frame and sample size would further develop this body of knowledge.
The VA uses an EMR that is accessible to all VA providers locally and nationwide. Due to the nationwide network, expansion of the project would be possible with the support of facility leadership and the EMR reminder staff. By using the education and the template for discussion, the project could be replicated throughout the system.
Conclusion
Advance care planning and ADs should be a regular part of the health care process, especially for veterans with noncancer diagnoses, such as CHF and COPD. Clear communication about disease trajectory and prognosis are an important part of this discussion. Primary care providers are in the optimal setting to initiate this discussion.
This project supports previous findings that a PCP initiating or participating in the ACP discussion would result in an improved completion rate for ADs.5 Theoretically, improved AD completions result in patient-centered care, leading to higher patient satisfaction.
Acknowledgements
The authors would like to acknowledge the VA Northern Indiana Health Care System for its support of this project.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Every day, health care providers (HCPs) care for patients with advanced chronic illnesses. At times, HCPs make critical treatment decisions without input from the patient. As a result, patients are often confused about their disease trajectory, prognosis, benefits and burdens of treatments, and outcome preferences.1 Unfortunately, limited research has been conducted on patients who have chronic illnesses, such as congestive heart failure (CHF) and chronic obstructive pulmonary disease (COPD), regarding early discussion of advance care planning (ACP) and advance directives (ADs). This gap in the knowledge base has contributed to a delay in the initiation of ACP for patients with COPD or CHF, especially for those with end-stage illness.
Background
The Patient Self-Determination Act (PSDA) was passed in 1990 to inform patients of their rights about health care choices while in the hospital, but the completion rate for ADs remains poor.2 The 3 key elements include the right of patients to facilitate their own health care decisions, the right to refuse or accept treatment, and the right to make an AD.
One of the reasons for the poor AD completion rate may be increased confusion about the difference between ACP and an AD.3 Advance care planning is a discussion about overall goals of care related to health care and progression through the life cycle. Advance directives focus on more specific information, including who will be designated as health care proxy, which health care interventions would be requested and which would be declined, and decisions regarding code status and organ donation.
A case study was conducted at a long-term care facility to test beliefs that residents who made their wishes known through ACP would have a positive experience at the end of life (EOL).3 The study allowed the residents to provide direction on what are and are not acceptable treatments at EOL. Before this study, most residents did not have a health care proxy and had not discussed the topic of EOL care with their HCP. Treatment choices were also not designated.3 Results demonstrated that ACP had positive outcomes for residents and family members, including documentation of an AD, autonomy in decision making, person-centered approaches to care, and dying with dignity.
Related: Fiduciary Services for Veterans With Psychiatric Disabilities
Much of the research regarding ADs has been conducted with seniors, hospitalized patients, and those with critical or terminal illnesses. A study by Jackson and colleagues examined attitudes, experiences, and preferences about ADs among adults of all ages. The study used an age-stratified random sample of patients from a large managed care organization.4 Findings revealed that older subjects were likely to be comfortable with and complete an AD. The most valuable outcome was the discussion of personal wishes with family and loved ones. Overall, the findings of this study concurred with the findings of other studies demonstrating that patients wanted control over EOL care decisions or wanted family members or loved ones to make those decisions. Consequently, patients not only believe the decisions are their responsibility, but also feel comfortable if their HCP initiates this conversation.4
Open and direct discussion regarding care planning can ease many of the fears related to EOL care. Discussion of an AD is a way to prepare for death and dying, rather than just a preparation for being incapacitated in the future. The process allows improved communication between patients, surrogates, and HCPs.3
The importance of communication with the patient’s primary care provider (PCP) regarding discussion of ACP or an AD is demonstrated in a longitudinal study completed by Ramsaroop and colleagues from January 1991 through July 2005. This systematic review of studies was designed to increase the completion of an AD in primary care settings.5 The study reviewed interventions that were most successful in improving the AD completion rate. The investigators extracted physician and patient barriers to completion of an AD. Findings suggested that the most successful intervention for completion of an AD were conversations that took place between patients and HCPs about ACP and occurred over multiple visits. By contrast, passive education using written materials without any direct counseling was relatively ineffective.5 The study also demonstrated the importance of completion of an AD in the primary care setting, gauging patient readiness to complete an AD, and having the PCP initiate the AD conversation.5
Related: Personal Counseling Helps Prevent Cancer-Related Malnutrition
If communication does not occur between patients and HCPs, care preferences are often not documented. Without this documentation many patients do not receive appropriate palliative care services when needed. Palliative care is not available to and therefore often not used with patients with nononcologic diseases.6 A study by Mahtani-Chugani and colleagues evaluated barriers to providing palliative care to nononcologic patients and proposed strategies to overcome them. Findings suggested 4 barriers: (1) lack of clarity about illness and prognosis; (2) discussion limited exclusively to the curative approach; (3) avoiding terms such as “terminal illness”; and (4) cheating death, including linking nononcologic disease and death.6 A strategy to overcoming these barriers highlighted improved communication between HCP and patient and understanding that the communication process is as important as the content of the message. Therefore, equitable palliative care services should be offered to both nononcologic and oncologic patients.6
One life-limiting nononcologic disease is COPD. Chronic obstructive pulmonary disease remains a major public health problem. It is the fourth leading cause of chronic morbidity and mortality in the U.S. and is projected to rank fifth in 2020 in disease burden worldwide.7 Given its prevalence, COPD is found in all adult health care settings.
Among hospitalized veterans in the VHA in 2005, COPD was the fourth most common discharge diagnosis.8 In the veteran population, a high prevalence for developing COPD also exists due to high-risk factors including tobacco use in the military. According to a study conducted at the Cincinnati VAMC in Ohio, a 40% greater prevalence of COPD existed in this veteran population than in the general U.S. population.8
Related: Lifestyle Intervention for Veterans With Chronic Diseases
Another nononcologic, life-limiting disease is CHF. Both the prevalence and hospitalization rates for CHF show an upward trend since the 1970s, resulting in a continued increase in CHF death rates.9 According to 2008 estimates from the National Institutes of Health, there are 5 million CHF patients in the U.S. and hospitalization rates approach 1 million per year.9 Congestive heart failure affects 2.4% of the adult population and > 11% of the expanding population aged > 80 years. Existing care may slow the progression of the disease but can rarely reverse it, which usually results in a prolonged period of advanced illness. As a result of the increasing prevalence, there remains a high symptom burden for patients living with advanced CHF.10
In managing the high symptom burden of CHF and COPD, patient-centered care must be acknowledged and used. Patient-centered care mandates that beneficial therapies and recommended guidelines be offered and discussed with the patient, giving attention to patient preferences.10
Study Design
The theoretical framework for the development and implementation of this project is based on Ruland and Moore’s Peaceful End of Life Theory.11 This theory is based on 2 assumptions. The first is that each person’s approach to EOL is personal. The second is that nursing care plays a major role in making EOL a peaceful experience. The 5 outcome measures include: (1) not experiencing pain; (2) the experience of comfort; (3) the experience of dignity and respect; (4) being at peace; and (5) closeness to significant others or other caring persons.
The outcome indicator of the Peaceful End of Life Theory— experience of dignity/respect with its related criteria and prescriptors—provided structure for the development and implementation of this project. The prescriptors related to the experience of dignity/respect include involving the patient and significant others in decision making; treating the patient with dignity, empathy, and respect; and being attentive to the patient’s expressed needs, wishes, and preferences.11
Due to the increased prevalence of chronic illnesses in the VA system, veterans need encouragement to complete ADs. The VA instituted a national directive guiding education and implementation of an AD.12 These discussions occur at the first contact a veteran has with the system and at other times when appropriate. The purpose of the directive is to allow veterans to guide the course of their treatment and to assure that they are aware of the ability to refuse treatment at any time.12
Inconsistencies in Advance Directive Completion
Inconsistencies were noted with how ADs were completed at the VA Northern Indiana Health Care System in Muncie. For outpatients, the clinic nursing staff received an electronic medical record (EMR) reminder if the veteran did not have an AD. This reminder prompted the nurse to ask the veteran about completing an AD. If the veteran agreed, a social work consult was initiated by nursing. Of concern, the social worker is usually responsible for several clinics so it is unlikely the process of completing the AD would be accomplished on the day the veteran was already in the clinic.
Discussions in the inpatient setting included a physician, a nurse practitioner, or a social worker and were often disease specific and patient oriented. However, in an acute hospitalization, it was less likely that patients initiated ADs due to acute illness and rapid nature of treatment.
Another concern was related to the amount of clinical knowledge the social worker had about the specifics of each patient’s case. Without specifics, a social worker can make the AD discussion very broad. Patients want information regarding disease progression and prognosis specific to their own condition to be able to make an informed choice regarding ADs.13
A study population with the diagnoses of CHF and COPD was selected due to the prevalence in the facility and at the request of facility leadership.
Methods
The primary aim of this quality improvement (QI) project was to educate the PCP about the importance of allowing veterans to express their care goals in the form of ADs and to understand that veterans would prefer to discuss these goals with their PCP. A secondary aim was to improve goal-directed care for veterans with COPD or CHF by increasing the number of completed ADs.
By using a systems approach, ACP can be addressed in a uniform manner. This approach allows veterans to discuss their goals of care prior to the need for emergent interventions, avoiding burdensome and unwanted treatments. Through the completion of ADs, veterans are able to designate a surrogate decision maker and identify specific desired treatments and interventions as their illness advances.
Two physicians and 3 nurse practitioners volunteered to contribute to this study. In the participating clinics, veterans with a diagnosis of CHF or COPD were identified. Each veteran had 20 to 30 minutes per appointment to discuss concerns, be examined, have HCPs address concerns, and complete all clinical reminders.
The study design was a QI project focused on evaluating the following process: An EMR reminder alerted the clinic nurse who asked the veteran if he or she was interested in completing an AD. If the patient agreed, a consult was placed to the social worker for completion of the VA national form for AD. The completion rate for a sample of primary care clinics at the facility was 10% to 12%, with no participation from the PCP in the process.
The providers were educated in 5 areas: (1) the prevalence of CHF and COPD in the U.S.; (2) the difference between ACP and AD; (3) the percentage of ADs completed in the U.S. adult population and in the facility; (4) the importance of addressing ACP early in the disease trajectory of this population; and (5) the use of the EMR reminder and the template to guide discussion of ACP ( eAppendix A
The template was developed following the literature review and addressed the reoccurring themes that patients wanted to discuss concerning their specific diagnosis and treatment. The template was formatted to include 3 components: (1) health care surrogate; (2) code status; and (3) organ donation preference ( eAppendix B
The natural progression from discussion of goals of care led to the discussion regarding the initiation of an AD. When completed, the note automatically appeared in the Postings section of the EMR, making it easily accessible to all other providers in different care settings.
The project time was 3 months (December 2012 through February 2013). The education was completed and the EMR reminder was turned on at the beginning of the project for veterans with a diagnosis of CHF or COPD who had not completed an AD. At the conclusion of the project, the PCPs completed a post project survey to provide information regarding their opinions on facilitators and barriers in the AD completion process ( eAppendix C
Results
Five different primary care clinics in 4 different locations throughout the health care system provided a total of 294 veterans with diagnoses of CHF or COPD. On completion of the project, 35 veterans had completed ADs. These 35 veterans represent an additional 12% of patients who previously did not complete an AD despite being approached multiple times. The veterans completed an AD following PCP-initiated discussion due to this QI project.
All 5 providers completed the post project survey and agreed that it would be beneficial to have the information regarding ADs easily accessible in the EMR. Four out of 5 providers admitted to cutting corners by not opening a new note every time to complete the AD template. They reported completing the EMR reminder within the clinic note, making it difficult to locate the information. Providers also reported on the various facilitators and barriers to AD discussion with patients (Table).
Discussion
The completion of an AD remains an important part of health care that is often neglected. When patients receive care and treatments, they often do not desire an AD, because the goals of care have not been clearly communicated and clearly documented. This can lead to poor quality of care with increased dissatisfaction and burden on the patient and health care system.14 However, if goals of care are discussed and documented, the veteran may avoid these burdensome treatments, and health care will be congruent with patient wishes. Better communication and documentation promotes increased patient satisfaction and improved quality of health care.1
This project endorses findings from a previous case study that demonstrated better patient-centered, goal-directed care results when patients have the opportunity to complete an AD, thereby improving health care quality and patient/family satisfaction.3 Previous studies suggested one way to increase the AD completion rate involves the PCP initiating a discussion with the patient.5 This project supports that conclusion.
Limitations
All the project providers expressed support regarding the importance of discussing ACPs with their patients. The major limitation identified by the project providers was time constraints in a busy primary care clinic. One provider suggested initiation of an EMR reminder once per year to prompt discussion. The same provider also recommended rescheduling an additional clinic visit to have an in-depth discussion regarding ACP.
Another limitation to this project involved the EMR. Currently, there is no way to have information in the postings section without a separate note. The project providers all agreed that it was not always possible to open a new note to use the template due to limited clinic time. This allowed information regarding health care surrogates and discussions regarding code status and organ donation to be embedded in a clinic note, which can make it difficult for other providers at different levels of care to effectively locate. Incorporating a method to allow information from an EMR reminder to be automatically placed in the postings section would alleviate this limitation.
A further limitation involved the setting. The VA provides care only to veterans. The project can be generalized to other VA primary care clinics, but generalizability beyond the VA may be limited.
This QI project took place over 3 months, another potential limitation due to the limited study period. Also, due to the short time frame of the project, a small sample size was used. Further investigation of this topic by expanding the time frame and sample size would further develop this body of knowledge.
The VA uses an EMR that is accessible to all VA providers locally and nationwide. Due to the nationwide network, expansion of the project would be possible with the support of facility leadership and the EMR reminder staff. By using the education and the template for discussion, the project could be replicated throughout the system.
Conclusion
Advance care planning and ADs should be a regular part of the health care process, especially for veterans with noncancer diagnoses, such as CHF and COPD. Clear communication about disease trajectory and prognosis are an important part of this discussion. Primary care providers are in the optimal setting to initiate this discussion.
This project supports previous findings that a PCP initiating or participating in the ACP discussion would result in an improved completion rate for ADs.5 Theoretically, improved AD completions result in patient-centered care, leading to higher patient satisfaction.
Acknowledgements
The authors would like to acknowledge the VA Northern Indiana Health Care System for its support of this project.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Pautex S, Herrmann FR, Zulian GB. Role of advance directives in palliative care units: A prospective study. Palliat Med. 2008;22(7):835-841.
2. Cohen MJ, McCannon JB, Edgman-Levitan S, Kormos WA. Exploring attitudes toward advance care directives in two diverse settings. J Palliat Med. 2010;13(12):1427-1432.
3. Jeong SY-S, Higgins I, McMillan M. The essentials of Advance Care Planning for end-of-life care for older people. J Clin Nurs. 2010;19(3-4):389-397.
4. Jackson JM, Rolnick SJ, Asche SE, Heinrich RL. Knowledge, attitudes, and p regarding advance directives among patients of a managed care organization. Am J Manag Care. 2009;15(3):177-186.
5. Ramsaroop SD, Reid MC, Adelman RD. Completing an advance directive in the primary care setting: What do we need for success? J Am Geriatr Soc. 2007;55(2):277-283.
6. Mahtani-Chugani V, González-Castro I, de Ormijana-Hernández AS, Martín-Fernández R, de la Vega EF. How to provide care for patients suffering from terminal non-oncological diseases: Barriers to a palliative care approach. Palliat Med. 2010;24(8):787-795.
7. Rabe KF, Hurd S, Anzueto A, et al; Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532-555.
8. Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560.
9. National Institutes of Health. Division of cardiovascular disease strategic plan. National Heart, Lung, and Blood Institute Website. http://www .nhlbi.nih.gov/about/org/dcvs/sp/goal-2.4b. 2012. Accessed January 29, 2015.
10. Allen LA, Stevenson LW, Grady KL; American Heart Association; Council on Quality of Care and Outcomes Research; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia. Decision making in advanced heart failure: A scientific statement from the American Heart Association. Circulation. 2012;125(15):1928-1952.
11. Ruland CM, Moore SM. Theory construction based on standards of care: A proposed theory of the peaceful end of life. Nurs Outlook. 1998;46(4):169-175.
12. US Department of Veterans Affairs, Veterans Health Administration . VHA Handbook 1004.02 transmittal sheet. Published July 2, 2009. http://www.ethics.va.gov/docs/policy/ADTraining /vha_handbk_1004_02.pdf. Accessed January 29, 2015.
13. Gott M, Gardiner C, Small N, et al. Barriers to advance care planning in chronic obstructive pulmonary disease. Palliat Med. 2009;23(7): 642-648.
14. Teno JM, Gruneir A, Schwartz Z, Nanda A, Wetle T. Association between advance directives and quality end-of-life care: A national study. J Am Geriatr Soc. 2007;55(2):189-194.
1. Pautex S, Herrmann FR, Zulian GB. Role of advance directives in palliative care units: A prospective study. Palliat Med. 2008;22(7):835-841.
2. Cohen MJ, McCannon JB, Edgman-Levitan S, Kormos WA. Exploring attitudes toward advance care directives in two diverse settings. J Palliat Med. 2010;13(12):1427-1432.
3. Jeong SY-S, Higgins I, McMillan M. The essentials of Advance Care Planning for end-of-life care for older people. J Clin Nurs. 2010;19(3-4):389-397.
4. Jackson JM, Rolnick SJ, Asche SE, Heinrich RL. Knowledge, attitudes, and p regarding advance directives among patients of a managed care organization. Am J Manag Care. 2009;15(3):177-186.
5. Ramsaroop SD, Reid MC, Adelman RD. Completing an advance directive in the primary care setting: What do we need for success? J Am Geriatr Soc. 2007;55(2):277-283.
6. Mahtani-Chugani V, González-Castro I, de Ormijana-Hernández AS, Martín-Fernández R, de la Vega EF. How to provide care for patients suffering from terminal non-oncological diseases: Barriers to a palliative care approach. Palliat Med. 2010;24(8):787-795.
7. Rabe KF, Hurd S, Anzueto A, et al; Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532-555.
8. Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560.
9. National Institutes of Health. Division of cardiovascular disease strategic plan. National Heart, Lung, and Blood Institute Website. http://www .nhlbi.nih.gov/about/org/dcvs/sp/goal-2.4b. 2012. Accessed January 29, 2015.
10. Allen LA, Stevenson LW, Grady KL; American Heart Association; Council on Quality of Care and Outcomes Research; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia. Decision making in advanced heart failure: A scientific statement from the American Heart Association. Circulation. 2012;125(15):1928-1952.
11. Ruland CM, Moore SM. Theory construction based on standards of care: A proposed theory of the peaceful end of life. Nurs Outlook. 1998;46(4):169-175.
12. US Department of Veterans Affairs, Veterans Health Administration . VHA Handbook 1004.02 transmittal sheet. Published July 2, 2009. http://www.ethics.va.gov/docs/policy/ADTraining /vha_handbk_1004_02.pdf. Accessed January 29, 2015.
13. Gott M, Gardiner C, Small N, et al. Barriers to advance care planning in chronic obstructive pulmonary disease. Palliat Med. 2009;23(7): 642-648.
14. Teno JM, Gruneir A, Schwartz Z, Nanda A, Wetle T. Association between advance directives and quality end-of-life care: A national study. J Am Geriatr Soc. 2007;55(2):189-194.
Managing aneurysmal subarachnoid hemorrhage: It takes a team
Aneurysmal subarachnoid hemorrhage is a devastating condition, with an estimated death rate of 30% during the initial episode.1,2 Approximately the same number of patients survive but leave the hospital with disabling neurologic deficits.3
However, better outcomes can be achieved by systems that are able to work as a team on the collective goal of quick intervention to secure the ruptured aneurysm, followed by the implementation of measures to minimize secondary brain injury. Although the search for new diagnostic, prognostic, and therapeutic modalities continues, it is clear that there exists no “silver bullet” that will help all patients. Instead, it is the systematic integration and application of small advances that will ultimately maximize the patient’s chances of survival and neurologic recovery.
This review focuses on the management of aneurysmal subarachnoid hemorrhage and its systemic and neurologic complications.
ANEURYSM IS THE MOST COMMON CAUSE OF SUBARACHNOID BLEEDING
Aneurysmal subarachnoid hemorrhage, ie, rupture of an intracranial aneurysm, flooding the subarachnoid space with blood, affects about 24,000 Americans each year.1,2 A ruptured aneurysm is the most common cause of subarachnoid hemorrhage, accounting for about 85% of cases. Less common causes include idiopathic benign perimesencephalic hemorrhage, arteriovenous malformation, dural arteriovenous fistula, and hemorrhagic mycotic aneurysm. These have their own natural history, pathophysiology, and specific treatment, and will not be addressed in this article.
Risk factors for aneurysmal subarachnoid hemorrhage include having a first-degree relative who had the disease, hypertension, smoking, and consuming more than 150 g of alcohol per week.4
CLINICAL PRESENTATION AND DIAGNOSIS
The key symptom of aneurysmal subarachnoid hemorrhage is the abrupt onset of severe headache that peaks in intensity over 1 hour,5 often described as “the worst headache of my life.” Headache is accompanied by brief loss of consciousness in 53% of cases (conversely, nearly half of patients maintain normal mental status), by nausea or vomiting in 77%, and by meningismus (neck pain or stiffness) in 35%.6
These clinical manifestations and risk factors have been incorporated into a decision rule:
Obtain brain imaging if the patient has acute headache reaching maximal intensity within 1 hour, associated with any of the following factors:
- Age 40 or older
- Neck pain or stiffness
- Witnessed loss of consciousness
- Onset during exertion
- “Thunderclap” headache (ie, instantly peaking pain)
- Limited neck flexion on examination.5
This decision rule has nearly 100% sensitivity for aneurysmal subarachnoid hemorrhage in clinical practice.5 All patients require brain imaging if they have a severe headache plus either abnormal neurologic findings (eg, a focal neurologic deficit) or a history of cerebral aneurysm.
Emergency physicians should have a low threshold for ordering noncontrast computed tomography (CT) of the head in patients with even mild symptoms suggesting aneurysmal subarachnoid hemorrhage. Failure to order CT is the most common diagnostic error in this situation.6 CT performed within 6 hours of headache onset is nearly 100% sensitive for this condition,7 but the sensitivity falls to 93% after the first 24 hours and to less than 60% after 5 days.8 In patients who have symptoms highly suggestive of aneurysmal subarachnoid hemorrhage but a normal CT, lumbar puncture is the next diagnostic step.
There are two alternatives to CT followed by lumbar puncture: ie, noncontrast CT followed by CT angiography,9,10 and magnetic resonance imaging followed by magnetic resonance angiography. In patients with suspicious clinical symptoms but negative CT results, CT followed by CT angiography can rule out aneurysmal subarachnoid hemorrhage with a 99% probability.9,10 However, CT followed by lumbar puncture remains the standard of care and carries a class I recommendation in the American Heart Association guidelines for ruling out subarachnoid hemorrhage.5
GRADING THE SEVERITY OF SUBARACHNOID HEMORRHAGE
Age, the thickness of the blood layer in the subarachnoid space, intraventricular hemorrhage and the findings of the neurologic examination at presentation are predictors of long-term outcomes in aneurysmal subarachnoid hemorrhage (Figure 1).
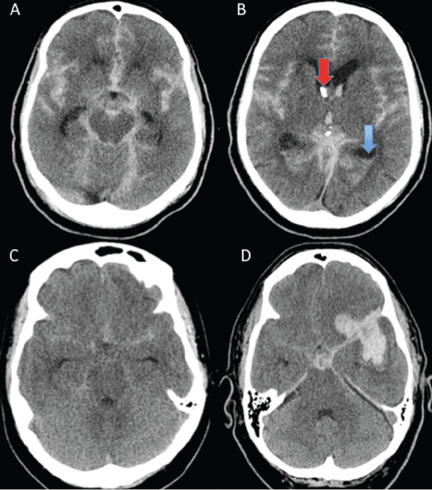
Different grading systems used in clinical practice are based on the findings on the initial neurologic examination and on the initial noncontrast CT (ie, the thickness of the blood, and whether intraventricular hemorrhage is present). Among the most widely used are those developed by Hunt and Hess12 and by the World Federation of Neurological Surgeons11 (WFNS), and the CT grading scales (Fisher13 or its modified version14) (Tables 1 and 2). With either the Hunt and Hess scale or the WFNS scale, the higher the score, the worse the patient’s probable outcome. Scores on both Fisher scales correlate with the risk of angiographic vasospasm. The higher the grade, the higher the risk of angiographic vasospasm.
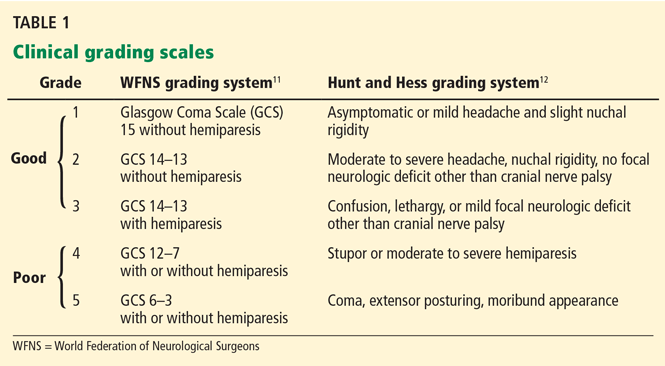
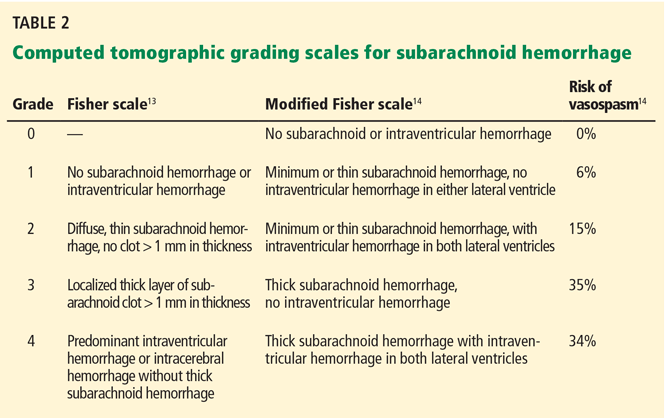
The VASOGRADE score—a combination of the WFNS score and the modified Fisher scale—stratifies patients at risk of delayed cerebral ischemia, allowing for a tailored monitoring strategy.15 There are three variations:
- VASOGRADE green—Modified Fisher 1 or 2 and WFNS 1 or 2
- VASOGRADE yellow—Modified Fisher 3 or 4 and WFNS 1, 2, or 3
- VASOGRADE red—WFNS 4 or 5.
After the initial bleeding event, patients with aneurysmal subarachnoid hemorrhage are at high risk of delayed systemic and neurologic complications, with poor functional outcomes. Delayed cerebral ischemia holds the greatest risk of an unfavorable outcome and ultimately can lead to cerebral infarction, disability, and death.6,7
INITIAL MANAGEMENT
After aneurysmal subarachnoid hemorrhage is diagnosed, the initial management (Figure 2) includes appropriate medical prevention of rebleeding (which includes supportive care, blood pressure management, and, perhaps, the early use of a short course of an antifibrinolytic drug) and early transfer to a high-volume center for securing the aneurysm. The reported incidence of rebleeding varies from 5% to 22% in the first 72 hours. “Ultra-early” rebleeding (within 24 hours of hemorrhage) has been reported, with an incidence as high as 15% and a fatality rate around 70%. Patients with poor-grade aneurysmal subarachnoid hemorrhage, larger aneurysms, and “sentinel bleeds” are at higher risk of rebleeding.16
Outcomes are much better when patients are managed in a high-volume center, with a specialized neurointensive care unit17 and access to an interdisciplinary team.18 Regardless of the initial grade, patients with aneurysmal subarachnoid hemorrhage should be quickly transferred to a high-volume center, defined as one treating at least 35 cases per year, and the benefit is greater in centers treating more than 60 cases per year.19 The higher the caseload in any given hospital, the better the clinical outcomes in this population.20
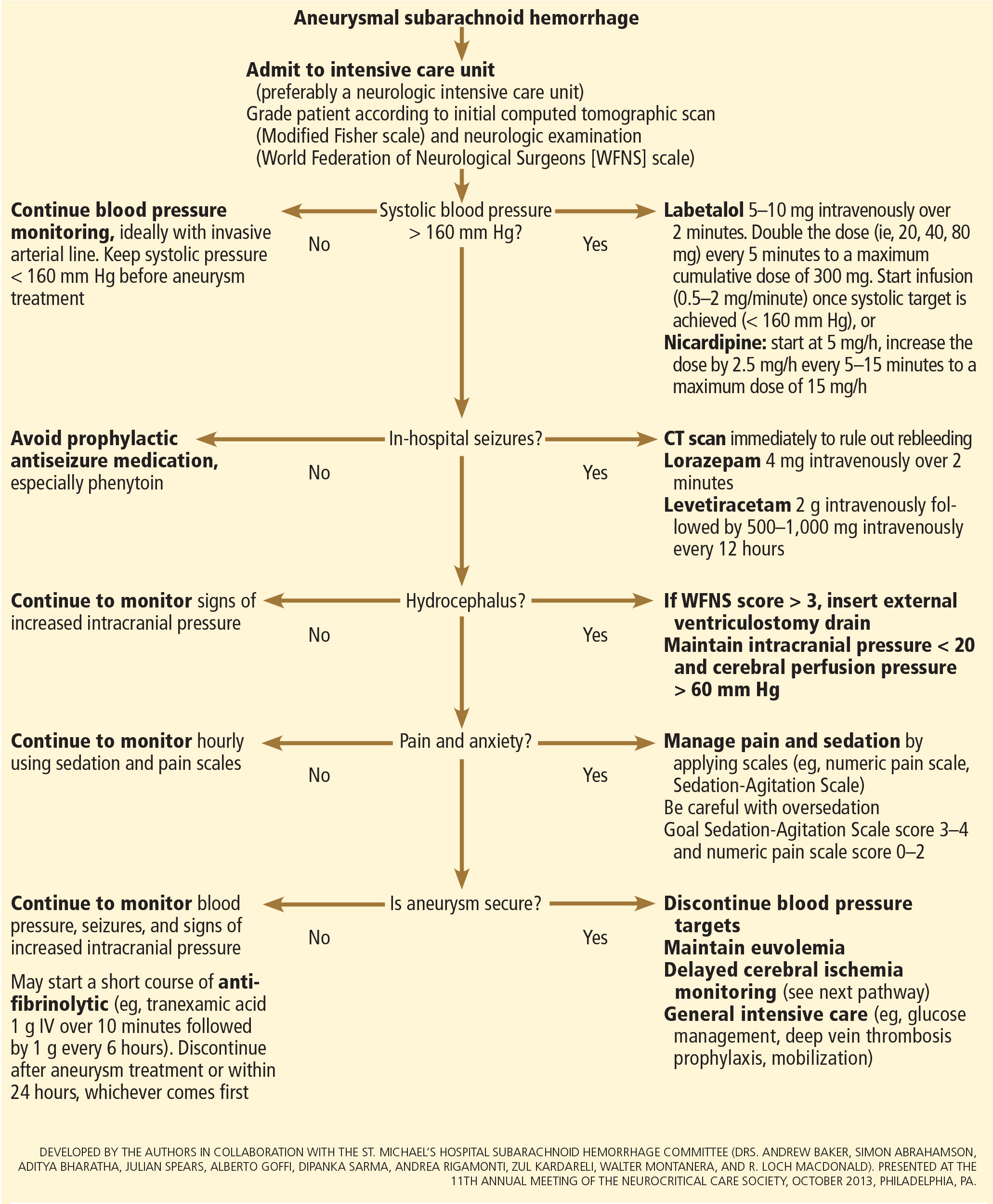
Treating cerebral aneurysm: Clipping or coiling
Early aneurysm repair is generally considered the standard of care and the best strategy to reduce the risk of rebleeding. Further, early treatment may be associated with a lower risk of delayed cerebral ischemia21 and better outcomes.22
Three randomized clinical trials have compared surgical clipping and endovascular repair (placement of small metal coils within the aneurysm to promote clotting).
The International Subarachnoid Aneurysm Trial23 showed a reduction of 23% in relative risk and of 7% in absolute risk in patients who underwent endovascular treatment compared with surgery. The survival benefit persisted at a mean of 9 years (range 6–14 years), but with a higher annual rate of aneurysm recurrence in the coiling group (2.9% vs 0.9%).24 Of note, this trial included only patients with aneurysms deemed suitable for both coiling and clipping, so that the exclusion rate was high. Most of the patients presented with good-grade (WFNS score 1–3), small aneurysms (< 5 mm) in the anterior circulation.
A single-center Finnish study25 found no differences in rates of recovery, disability, and death at 1 year, comparing surgery and endovascular treatment. Additionally, survival rates at a mean follow-up of 39 months were similar, with no late recurrences or aneurysmal bleeding.
Lastly, the Barrow Ruptured Aneurysm Trial26,27 found that patients assigned to endovascular treatment had better 1-year neurologic outcomes, defined as a modified Rankin score of 2 or less. Importantly, 37.7% of patients originally assigned to endovascular treatment crossed over to surgical treatment. The authors then performed intention-to-treat and as-treated analyses. Either way, patients treated by endovascular means had better neurologic outcomes at 1 year. However, no difference in the relative risk reduction in worse outcome was found on 3-year follow-up, and patients treated surgically had higher rates of aneurysm obliteration and required less aneurysm retreatment, both of which were statistically significant.
The question that remains is not whether to clip or whether to coil, but whom to clip and whom to coil.28 That question must be answered on a patient-to-patient basis and requires the expertise of an interventional neuroradiologist and a vascular neurosurgeon—one of the reasons these patients are best cared for in high-volume centers providing such expertise.
MEDICAL PREVENTION OF REBLEEDING
Blood pressure management
There are no systematic data on the optimal blood pressure before securing an aneurysm. Early studies of hemodynamic augmentation in cases of ruptured untreated aneurysm reported rebleeding when the systolic blood pressure was allowed to rise above 160 mm Hg.29,30 A recent study evaluating hypertensive intracerebral hemorrhage revealed better functional outcomes with intensive lowering of blood pressure (defined as systolic blood pressure < 140 mm Hg) but no significant reduction in the combined rate of death or severe disability.31 It is difficult to know if these results can be extrapolated to patients with aneurysmal subarachnoid hemorrhage. Current guidelines3,32 say that before the aneurysm is treated, the systolic pressure should be lower than 160 mm Hg.
There is no specific drug of choice, but a short-acting, titratable medication is preferable. Nicardipine is a very good option, and labetalol might be an appropriate alternative.33 Once the aneurysm is secured, all antihypertensive drugs should be held. Hypertension should not be treated unless the patient has clinical signs of a hypertensive crisis, such as flash pulmonary edema, myocardial infarction, or hypertensive encephalopathy.
Antifibrinolytic therapy
The role of antifibrinolytic therapy in aneurysmal subarachnoid hemorrhage is controversial and has been studied in 10 clinical trials. In a Swedish study,34 early use of tranexamic acid (1 g intravenously over 10 minutes followed by 1 g every 6 hours for a maximum of 24 hours) reduced the rebleeding rate substantially, from 10.8% to 2.4%, with an 80% reduction in the mortality rate from ultra-early rebleeding. However, a recent Cochrane review that included this study found no overall benefit.35
An ongoing multicenter randomized trial in the Netherlands will, we hope, answer this question in the near future.36 At present, some centers would consider a short course of tranexamic acid before aneurysm treatment.
DIAGNOSIS AND TREATMENT OF COMPLICATIONS
Medical complications are extremely common after aneurysmal subarachnoid hemorrhage. Between 75% and 100% of patients develop some type of systemic or further neurologic derangement, which in turn has a negative impact on the long-term outcome.37,38 In the first 72 hours, rebleeding is the most feared complication, and as mentioned previously, appropriate blood pressure management and early securing of the aneurysm minimize its risk.
NEUROLOGIC COMPLICATIONS
Hydrocephalus
Hydrocephalus is the most common early neurologic complication after aneurysmal subarachnoid hemorrhage, with an overall incidence of 50%.39 Many patients with poor-grade aneurysmal subarachnoid hemorrhage and patients whose condition deteriorates due to worsening of hydrocephalus require the insertion of an external ventricular drain (Figure 1).
Up to 30% of patients who have a poor-grade aneurysmal subarachnoid hemorrhage improve neurologically with cerebrospinal fluid drainage.40 An external ventricular drain can be safely placed, even before aneurysm treatment, and placement does not appear to increase the risk of rebleeding.39,41 After placement, rapid weaning from the drain (clamping within 24 hours of insertion) is safe, decreases length of stay in the intensive care unit and hospital, and may be more cost-effective than gradual weaning over 96 hours.42
Increased intracranial pressure
Intracranial hypertension is another potential early complication, and is frequently due to the development of hydrocephalus, cerebral edema, or rebleeding. The treatment of increased intracranial pressure does not differ from the approach used in managing severe traumatic brain injury, which includes elevating the head of the bed, sedation, analgesia, normoventilation, and cerebrospinal fluid drainage.
Hypertonic saline has been tested in several studies that were very small but nevertheless consistently showed control of intracranial pressure levels and improvement in cerebral blood flow measured by xenon CT.43–47 Two of these studies even showed better outcomes at discharge.43,44 However, the small number of patients prevents any meaningful conclusion regarding the use of hypertonic saline and functional outcomes.
Barbiturates, hypothermia, and decompressive craniectomy could be tried in refractory cases.48 Seule et al49 evaluated the role of therapeutic hypothermia with or without barbiturate coma in 100 patients with refractory intracranial hypertension. Only 13 patients received hypothermia by itself. At 1 year, 32 patients had achieved a good functional outcome (Glasgow Outcome Scale score 4 or 5). The remaining patients were severely disabled or had died. Of interest, the median duration of hypothermia was 7 days, and 93% of patients developed some medical complication such as electrolyte disorders (77%), pneumonia (52%), thrombocytopenia (47%), or septic shock syndrome (40%). Six patients died as a consequence of one of these complications.
Decompressive craniectomy can be life-saving in patients with refractory intracranial hypertension. However, most of these patients will die or remain severely disabled or comatose.50
Seizure prophylaxis is controversial
Seizures can occur at the onset of intracranial hemorrhage, perioperatively, or later (ie, after the first week). The incidence varied considerably in different reports, ranging from 4% to 26%.51 Seizures occurring perioperatively, ie, after hospital admission, are less frequent and are usually the manifestation of aneurysm rebleeding.24
Seizure prophylaxis remains controversial, especially because the use of phenytoin is associated with increased incidence of cerebral vasospasm, infarction, and worse cognitive outcomes after aneurysmal subarachnoid hemorrhage.52,53 Therefore, routine prophylactic use of phenytoin is not recommended in these patients,3 although the effect of other antiepileptic drugs is less studied and less clear. Patients may be considered for this therapy if they have multiple risk factors for seizures, such as intraparenchymal hematoma, advanced age (> 65), middle cerebral artery aneurysm, craniotomy for aneurysm clipping, and a short course (≤ 72 hours) of an antiepileptic drug other than phenytoin, especially while the aneurysm is unsecured.3
Levetiracetam may be an alternative to phenytoin, having better pharmacodynamic and kinetic profiles, minimal protein binding, and absence of hepatic metabolism, resulting in a very low risk of drug interaction and better tolerability.54,55 Because of these advantages, levetiracetam has become the drug of choice in several centers treating aneurysmal subarachnoid hemorrhage in the United States.
Addressing this question, a survey was sent to 25 high-volume aneurysmal subarachnoid hemorrhage academic centers in the United States. All 25 institutions answered the survey, and interestingly, levetiracetam was the first-line agent for 16 (94%) of the 17 responders that used prophylaxis, while only 1 used phenytoin as the agent of choice.56
A retrospective cohort study by Murphy-Human et al57 showed that a short course of levetiracetam (≤ 72 hours) was associated with higher rates of in-hospital seizures compared with an extended course of phenytoin (eg, entire hospital stay). However, the study did not address functional outcomes.57
Continuous electroencephalographic monitoring may be considered in comatose patients, in patients requiring controlled ventilation and sedation, or in patients with unexplained alteration in consciousness. In one series of patients with aneurysmal subarachnoid hemorrhage who received continuous monitoring, the incidence of nonconvulsive status epilepticus was 19%, with an associated mortality rate of 100%.58
Continuous quantitative electroencephalography is useful to monitor and to detect angiographic vasospasm and delayed cerebral ischemia. Relative alpha variability and the alpha-delta ratio decrease with ischemia, and this effect can precede angiographic vasospasm by 3 days.59,60
Delayed cerebral ischemia
Delayed cerebral ischemia is defined as the occurrence of focal neurologic impairment, or a decrease of at least 2 points on the Glasgow Coma Scale that lasts for at least 1 hour, is not apparent immediately after aneurysm occlusion, and not attributable to other causes (eg, hyponatremia, fever).61
Classically, neurologic deficits that occurred within 2 weeks of aneurysm rupture were ascribed to reduced cerebral blood flow caused by delayed large-vessel vasospasm causing cerebral ischemia.62 However, perfusion abnormalities have also been observed with either mild or no demonstrable vasospasm.63 Almost 70% of patients who survive the initial hemorrhage develop some degree of angiographic vasospasm. However, only 30% of those patients will experience symptoms.
In addition to vasospasm of large cerebral arteries, impaired autoregulation and early brain injury within the first 72 hours following subarachnoid hemorrhage may play important roles in the development of delayed cerebral ischemia.64 Therefore, the modern concept of delayed cerebral ischemia monitoring should focus on cerebral perfusion rather than vessel diameter measurements. This underscores the importance of comprehensive, standardized monitoring techniques that provide information not only on microvasculature, but also at the level of the microcirculation, with information on perfusion, oxygen utilization and extraction, and autoregulation.
Although transcranial Doppler has been the most commonly applied tool to monitor for angiographic vasospasm, it has a low sensitivity and negative predictive value.37 It is nevertheless a useful technique to monitor good-grade aneurysmal subarachnoid hemorrhage patients (WFNS score 1–3) combined with frequent neurologic examinations (Figure 3).
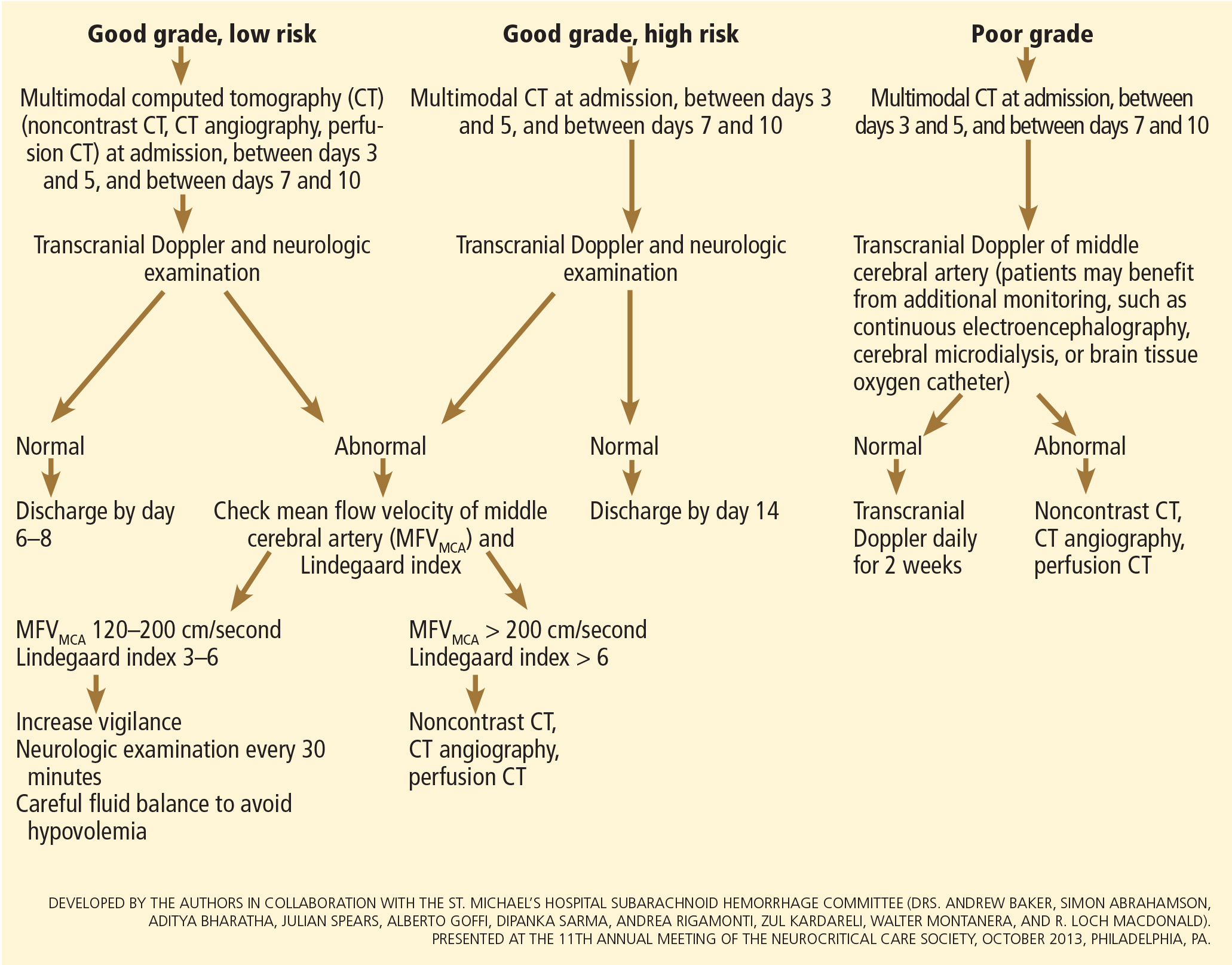
CT angiography is a good noninvasive alternative to digital subtraction angiography. However, it tends to overestimate the degree of vasoconstriction and does not provide information about perfusion and autoregulation.65 Nevertheless, CT angiography combined with a CT perfusion scan can add information about autoregulation and cerebral perfusion and has been shown to be more sensitive for the diagnosis of angiographic vasospasm than transcranial Doppler and digital subtraction angiography (Figure 4).
Patients with a poor clinical condition (WFNS score 4 or 5) or receiving continuous sedation constitute a challenge in monitoring for delayed neurologic deterioration. Neurologic examination is not sensitive enough in this setting to detect subtle changes. In these specific and challenging circumstances, multimodality neuromonitoring may be useful in the early detection of delayed cerebral ischemia and may help guide therapy.67
Several noninvasive and invasive techniques have been studied to monitor patients at risk of delayed cerebral ischemia after subarachnoid hemorrhage.66 These include continuous electroencephalography, brain tissue oxygenation monitoring (Ptio2), cerebral microdialysis, thermal diffusion flowmetry, and near-infrared spectroscopy. Of these techniques, Ptio2, cerebral microdialysis, and continuous electroencephalography (see discussion of seizure prophylaxis above) have been more extensively studied. However, most of the studies were observational and very small, limiting any recommendations for using these techniques in routine clinical practice.68
Ptio2 is measured by inserting an intraparenchymal oxygen-sensitive microelectrode, and microdialysis requires a microcatheter with a semipermeable membrane that allows small soluble substances to cross it into the dialysate. These substances, which include markers of ischemia (ie, glucose, lactate, and pyruvate), excitotoxins (ie, glutamate and aspartate), and membrane cell damage products (ie, glycerol), can be measured. Low Ptio2 values (< 15 mm Hg) and abnormal mycrodialysate findings (eg, glucose < 0.8 mmol/L, lactate-to-pyruvate ratio > 40) have both been associated with cerebral ischemic events and poor outcome.68
Preventing delayed cerebral ischemia
Oral nimodipine 60 mg every 4 hours for 21 days, started on admission, carries a class I, level of evidence A recommendation in the management of aneurysmal subarachnoid hemorrhage.3,32,69 It improves clinical outcome despite having no effect on the risk of angiographic vasospasm. The mechanism of improved outcome is unclear, but the effect may be a neuroprotective phenomenon limiting the extension of delayed cerebral ischemia.70
If hypotension occurs, the dose can be lowered to 30 mg every 2 hours. Whether to discontinue nimodipine in this situation is controversial. Of note, the clinical benefits of nimodipine have not been replicated with other calcium channel blockers (eg, nicardipine).71
Prophylactic hyperdynamic fluid therapy, known as “triple-H” (hypervolemia, hemodilution, and hypertension) was for years the mainstay of treatment in preventing delayed cerebral ischemia due to vasospasm. However, the clinical data supporting this intervention have been called into question, as analysis of two trials found that hypervolemia did not improve outcomes or reduce the incidence of delayed cerebral ischemia, and in fact increased the rate of complications.72,73 Based on these findings, current guidelines recommend maintaining euvolemia rather than prophylactic hypervolemia in patients with aneurysmal subarachnoid hemorrhage.3,32,69
TREATING DELAYED CEREBRAL ISCHEMIA
Hemodynamic augmentation
In patients with neurologic deterioration due to delayed cerebral ischemia, hemodynamic augmentation is the cornerstone of treatment. This is done according to a protocol, started early, involving specific physiologic goals, clinical improvement, and escalation to invasive therapies in a timely fashion in patients at high risk of further neurologic insult (Figure 5).
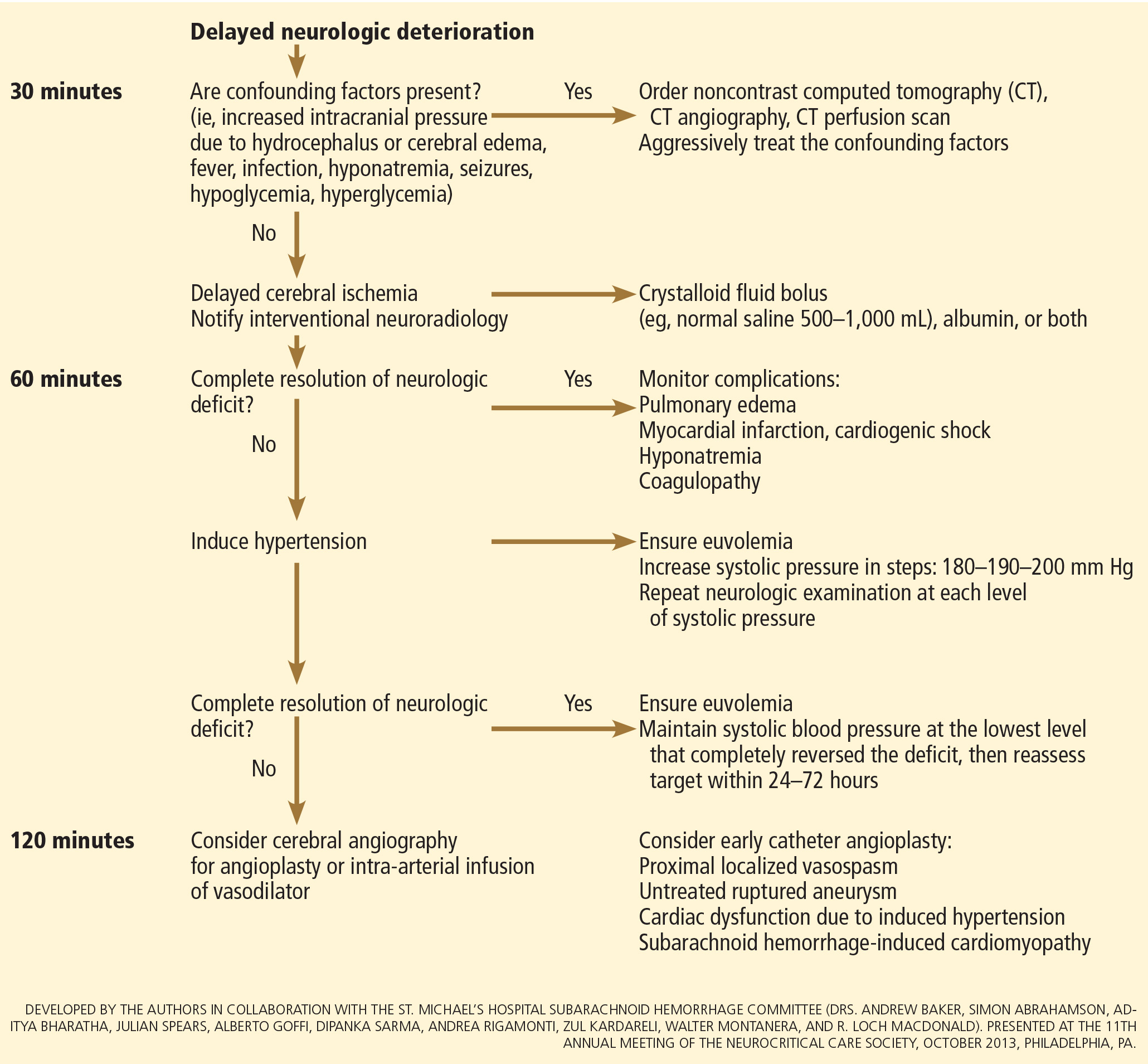
The physiologic goal is to increase the delivery of oxygen and glucose to the ischemic brain. Hypertension seems to be the most effective component of hemodynamic augmentation regardless of volume status, increasing cerebral blood flow and brain tissue oxygenation, with reversal of delayed cerebral ischemic symptoms in up to two-thirds of treated patients.74,75 However, this information comes from very small studies, with no randomized trials of induced hypertension available.
The effect of a normal saline fluid bolus in patients suspected of having delayed cerebral ischemia has been shown to increase cerebral blood flow in areas of cerebral ischemia.74 If volume augmentation fails to improve the neurologic status, the next step is to artificially induce hypertension using vasopressors. The blood pressure target should be based on clinical improvement. A stepwise approach is reasonable in this situation, and the lowest level of blood pressure at which there is a complete reversal of the new focal neurologic deficit should be maintained.3,29
Inotropic agents such as dobutamine or milrinone can be considered as alternatives in patients who have new neurologic deficits that are refractory to fluid boluses and vasopressors, or in a setting of subarachnoid hemorrhage-induced cardiomyopathy.76,77
Once the neurologic deficit is reversed by hemodynamic augmentation, the blood pressure should be maintained for 48 to 72 hours at the level that reversed the deficit completely, carefully reassessed thereafter, and the patient weaned slowly. Unruptured unsecured aneurysms should not prevent blood pressure augmentation in a setting of delayed cerebral ischemia if the culprit aneurysm is treated.3,32 If the ruptured aneurysm has not been secured, careful blood pressure augmentation can be attempted, keeping in mind that hypertension (> 160/95 mm Hg) is a risk factor for fatal aneurysm rupture.
Endovascular management of delayed cerebral ischemia
When medical augmentation fails to completely reverse the neurologic deficits, endovascular treatment can be considered. Although patients treated early in the course of delayed cerebral ischemia have better neurologic recovery, prophylactic endovascular treatment in asymptomatic patients, even if angiographic signs of spasm are present, does not improve clinical outcomes and carries the risk of fatal arterial rupture.78
SYSTEMIC COMPLICATIONS
Hyponatremia and hypovolemia
Aneurysmal subarachnoid hemorrhage is commonly associated with abnormalities of fluid balance and electrolyte derangements. Hyponatremia (serum sodium < 135 mmol/L) occurs in 30% to 50% of patients, while the rate of hypovolemia (decreased circulating blood volume) ranges from 17% to 30%.79 Both can negatively affect long-term outcomes.80,81
Decreased circulating blood volume is a well-described contributor to delayed cerebral ischemia and cerebral infarction after aneurysmal subarachnoid hemorrhage.80–82 Clinical variables such as heart rate, blood pressure, fluid balance, and serum sodium concentration are usually the cornerstones of intravascular volume status assessment. However, these variables correlate poorly with measured circulating blood volumes in those with aneurysmal subarachnoid hemorrhage.83,84
The mechanisms responsible for the development of hyponatremia and hypovolemia after aneurysmal subarachnoid hemorrhage are not completely understood. Several factors have been described and may contribute to the increased natriuresis and, hence, to a reduction in circulating blood volume: increased circulating natriuretic peptide concentrations,85–87 sympathetic nervous system hyperactivation,88 and hyperreninemic hypo-
aldosteronism syndrome.89,90
Lastly, the cerebral salt wasting syndrome, described in the 1950s,91 was thought to be a key mechanism in the development of hyponatremia and hypovolemia after aneurysmal subarachnoid hemorrhage. In contrast to the syndrome of inappropriate antidiuretic hormone, which is characterized by hyponatremia with a normal or slightly elevated intravascular volume, the characteristic feature of cerebral salt wasting syndrome is the development of hyponatremia in a setting of intravascular volume depletion.92 In critically ill neurologic and neurosurgical patients, this differential diagnosis is very difficult, especially in those with aneurysmal subarachnoid hemorrhage in whom the clinical assessment of fluid status is not reliable. These two syndromes might coexist and contribute to the development of hyponatremia after aneurysmal subarachnoid hemorrhage.92,93
Hoff et al83,84 prospectively compared the clinical assessment of fluid status by critical and intermediate care nurses and direct measurements of blood volume using pulse dye densitometry. The clinical assessment failed to accurately assess patients’ volume status. Using the same technique to measure circulating blood volume, this group showed that calculation of fluid balance does not provide adequate assessment of fluid status.83,84
Hemodynamic monitoring tools can help guide fluid replacement in this population. Mutoh et al94 randomized 160 patients within 24 hours of hemorrhage to receive early goal-directed fluid therapy (ie, preload volume and cardiac output monitored by transpulmonary thermodilution) vs standard therapy (ie, fluid balance or central venous pressure). Overall, no difference was found in the rates of delayed cerebral ischemia (33% vs 42%; P = .33) or favorable outcome (67% vs 57%; P = .22). However, in the subgroup of poor-grade patients (WFNS score 4 or 5), early goal-directed therapy was associated with a lower rate of delayed cerebral ischemia (5% vs 14%; P = .036) and with better functional outcomes at 3 months (52% vs 36%; P = .026).
Fluid restriction to treat hyponatremia in aneurysmal subarachnoid hemorrhage is no longer recommended because of the increased risk of cerebral infarction due to hypovolemic hypoperfusion.82
Prophylactic use of mineralocorticoids (eg, fludrocortisone, hydrocortisone) has been shown to limit natriuresis, hyponatremia, and the amount of fluid required to maintain euvolemia.95,96 Higher rates of hypokalemia and hyperglycemia, which can be easily treated, are the most common complications associated with this approach. Additionally, hypertonic saline (eg, 3% saline) can be used to correct hyponatremia in a setting of aneurysmal subarachnoid hemorrhage.79
Cardiac complications
Cardiac complications after subarachnoid hemorrhage are most likely related to sympathetic hyperactivity and catecholamine-induced myocyte dysfunction. The pathophysiology is complex, but cardiac complications have a significant negative impact on long-term outcome in these patients.97
Electrocardiographic changes and positive cardiac enzymes associated with aneurysmal subarachnoid hemorrhage have been extensively reported. More recently, data from studies of two-dimensional echocardiography have shown that subarachnoid hemorrhage can also be associated with significant wall-motion abnormalities and even overt cardiogenic shock.98–100
There is no specific curative therapy; the treatment is mainly supportive. Vasopressors and inotropes may be used for hemodynamic augmentation.
Pulmonary complications
Pulmonary complications occur in 20% to 30% of all aneurysmal subarachnoid hemorrhage patients and are associated with a higher risk of delayed cerebral ischemia and death. Common pulmonary complications in this population are mild acute respiratory distress syndrome (27%), hospital-acquired pneumonia (9%), cardiogenic pulmonary edema (8%), aspiration pneumonia (6%), neurogenic pulmonary edema (2%), and pulmonary embolism (1%).101–103
SUPPORTIVE CARE
Hyperthermia, hyperglycemia, and liberal use of transfusions have all been associated with longer stays in the intensive care unit and hospital, poorer neurologic outcomes, and higher mortality rates in patients with acute brain injury.104 Noninfectious fever is the most common systemic complication after subarachnoid hemorrhage.
Antipyretic drugs such as acetaminophen and ibuprofen are not very effective in reducing fever in the subarachnoid hemorrhage population, but should still be used as first-line therapy. The use of surface and intravascular devices can be considered when fevers do not respond to nonsteroidal anti-inflammatory drugs.
Although no prospective randomized trial has addressed the impact of induced normothermia on long-term outcome and mortality in aneurysmal subarachnoid hemorrhage patients, fever control has been shown to reduce cerebral metabolic distress, irrespective of intracranial pressure.105 Maintenance of normothermia (< 37.5°C) seems reasonable, especially in aneurysmal subarachnoid hemorrhage patients at risk of or with active delayed cerebral ischemia.106
Current guidelines3,32,69 strongly recommend avoiding hypoglycemia, defined as a serum glucose level less than 80 mg/dL, but suggest keeping the blood sugar level below 180 or 200 mg/dL.
At the moment, there is no clear threshold for transfusion in patients with aneurysmal subarachnoid hemorrhage. Current guidelines suggest keeping hemoglobin levels between 8 and 10 g/dL.3
Preventing venous thromboembolism
The incidence of venous thromboembolism after aneurysmal subarachnoid hemorrhage varies widely, from 1.5% to 18%.107 Active surveillance with venous Doppler ultrasonography has found asymptomatic deep vein thrombosis in up to 3.4% of poor-grade aneurysmal subarachnoid hemorrhage patients receiving pharmacologic thromboprophylaxis.108
In a retrospective study of 170 patients, our group showed that giving drugs to prevent venous thromboembolism (unfractionated heparin 5,000 IU subcutaneously every 12 hours or dalteparin 5,000 IU subcutaneously daily), starting within 24 hours of aneurysm treatment, could be safe.109 Fifty-eight percent of these patients had an external ventricular drain in place. One patient developed a major cerebral hemorrhagic complication and died while on unfractionated heparin; however, the patient was also on dual antiplatelet therapy with aspirin and clopidogrel.109
Current guidelines suggest that intermittent compression devices be applied in all patients before aneurysm treatment. Pharmacologic thromboprophylaxis with a heparinoid can be started 12 to 24 hours after aneurysm treatment.3,109
A TEAM APPROACH
Patients with subarachnoid hemorrhage need integrated care from different medical and nursing specialties. The best outcomes are achieved by systems that can focus as a team on the collective goal of quick intervention to secure the aneurysm, followed by measures to minimize secondary brain injury.
The modern concept of cerebral monitoring in a setting of subarachnoid hemorrhage should focus on brain perfusion rather than vascular diameter. Although the search continues for new diagnostic, prognostic, and therapeutic tools, there is no “silver bullet” that will help all patients. Instead, it is the systematic integration and application of many small advances that will ultimately lead to better outcomes.
ACKNOWLEDGMENT
This work was supported by research funding provided by the Bitove Foundation, which has been supportive of our clinical and research work for several years.
- van Gijn J, Rinkel GJ. Subarachnoid haemorrhage: diagnosis, causes and management. Brain 2001; 124:249–278.
- Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 2014; 129:e28–e292.
- Diringer MN, Bleck TP, Claude Hemphill J 3rd, et al; Neurocritical Care Society. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society’s Multidisciplinary Consensus Conference. Neurocrit Care 2011; 15:211–240.
- Feigin VL, Rinkel GJ, Lawes CM, et al. Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies. Stroke 2005; 36:2773–2780.
- Perry JJ, Stiell IG, Sivilotti ML, et al. Clinical decision rules to rule out subarachnoid hemorrhage for acute headache. JAMA 2013; 310:1248–1255.
- Kowalski RG, Claassen J, Kreiter KT, et al. Initial misdiagnosis and outcome after subarachnoid hemorrhage. JAMA 2004; 291:866–869.
- Perry JJ, Stiell IG, Sivilotti ML, et al. Sensitivity of computed tomography performed within six hours of onset of headache for diagnosis of subarachnoid haemorrhage: prospective cohort study. BMJ 2011; 343:d4277.
- van Gijn J, van Dongen KJ. The time course of aneurysmal haemorrhage on computed tomograms. Neuroradiology 1982; 23:153–156.
- McCormack RF, Hutson A. Can computed tomography angiography of the brain replace lumbar puncture in the evaluation of acute-onset headache after a negative noncontrast cranial computed tomography scan? Acad Emerg Med 2010; 17:444–451.
- Agid R, Andersson T, Almqvist H, et al. Negative CT angiography findings in patients with spontaneous subarachnoid hemorrhage: when is digital subtraction angiography still needed? AJNR Am J Neuroradiol 2010; 31:696–705.
- Teasdale GM, Drake CG, Hunt W, et al. A universal subarachnoid hemorrhage scale: report of a committee of the World Federation of Neurosurgical Societies. J Neurol Neurosurg Psychiatry 1988; 51:1457.
- Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 1968; 28:14–20.
- Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 1980; 6:1–9.
- Frontera JA, Claassen J, Schmidt JM, et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified Fisher scale. Neurosurgery 2006; 59:21–27.
- de Oliveira Manoel AL, Turkel-Parrella D, Kouzmina E, et al. The VASOGRADE—a simple, reliable grading scale for aneurysmal subarachnoid hemorrhage. Neurology 2014; 82(suppl 10): P5.123.
- Naidech AM, Janjua N, Kreiter KT, et al. Predictors and impact of aneurysm rebleeding after subarachnoid hemorrhage. Arch Neurol 2005; 62:410–416.
- Rincon F, Mayer SA. Neurocritical care: a distinct discipline? Curr Opin Crit Care 2007; 13:115–121.
- Rabinstein AA, Lanzino G, Wijdicks EF. Multidisciplinary management and emerging therapeutic strategies in aneurysmal subarachnoid haemorrhage. Lancet Neurol 2010; 9:504–519.
- Vespa P, Diringer MN; Participants in the International Multi-Disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage. High-volume centers. Neurocrit Care 2011; 15:369–372.
- McNeill L, English SW, Borg N, Matta BF, Menon DK. Effects of institutional caseload of subarachnoid hemorrhage on mortality: a secondary analysis of administrative data. Stroke 2013; 44:647–652.
- Dorhout Mees SM, Molyneux AJ, Kerr RS, Algra A, Rinkel GJ. Timing of aneurysm treatment after subarachnoid hemorrhage: relationship with delayed cerebral ischemia and poor outcome. Stroke 2012; 43:2126–2129.
- Laidlaw JD, Siu KH. Ultra-early surgery for aneurysmal subarachnoid hemorrhage: outcomes for a consecutive series of 391 patients not selected by grade or age. J Neurosurg 2002; 97:250–259.
- Molyneux A, Kerr R, Stratton I, et al; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2,143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002; 360:1267–1274.
- Molyneux AJ, Kerr RS, Yu LM, et al; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2,143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005; 366:809–817.
- Koivisto T, Vanninen R, Hurskainen H, Saari T, Hernesniemi J, Vapalahti M. Outcomes of early endovascular versus surgical treatment of ruptured cerebral aneurysms. A prospective randomized study. Stroke 2000; 31:2369–2377.
- McDougall CG, Spetzler RF, Zabramski JM, et al. The Barrow Ruptured Aneurysm Trial. J Neurosurg 2012; 116:135–144.
- Spetzler RF, McDougall CG, Albuquerque FC, et al. The Barrow Ruptured Aneurysm Trial: 3-year results. J Neurosurg 2013; 119:146–157.
- Connolly ES Jr, Meyers PM. Cerebral aneurysms: to clip or to coil? That is no longer the question. Nat Rev Neurol 2009; 5:412–413.
- Kassell NF, Peerless SJ, Durward QJ, Beck DW, Drake CG, Adams HP. Treatment of ischemic deficits from vasospasm with intravascular volume expansion and induced arterial hypertension. Neurosurgery 1982; 11:337–343.
- Otsubo H, Takemae T, Inoue T, Kobayashi S, Sugita K. Normovolaemic induced hypertension therapy for cerebral vasospasm after subarachnoid haemorrhage. Acta Neurochir (Wien) 1990; 103:18–26.
- Anderson CS, Heeley E, Huang Y, et al; INTERACT2 Investigators. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med 2013; 368:2355–2365.
- Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al; American Heart Association Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2012; 43:1711–1737.
- Ortega-Gutierrez S, Thomas J, Reccius A, et al. Effectiveness and safety of nicardipine and labetalol infusion for blood pressure management in patients with intracerebral and subarachnoid hemorrhage. Neurocrit Care 2013; 18:13–19.
- Hillman J, Fridriksson S, Nilsson O, Yu Z, Saveland H, Jakobsson KE. Immediate administration of tranexamic acid and reduced incidence of early rebleeding after aneurysmal subarachnoid hemorrhage: a prospective randomized study. J Neurosurg 2002; 97:771–778.
- Baharoglu MI, Germans MR, Rinkel GJ, et al. Antifibrinolytic therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 2013; 8:CD001245.
- Germans MR, Post R, Coert BA, Rinkel GJ, Vandertop WP, Verbaan D. Ultra-early tranexamic acid after subarachnoid hemorrhage (ULTRA): study protocol for a randomized controlled trial. Trials 2013; 14:143.
- Sloan MA, Alexandrov AV, Tegeler CH, et al; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Assessment: transcranial Doppler ultrasonography: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2004; 62:1468–1481.
- Wartenberg KE, Schmidt JM, Claassen J, et al. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med 2006; 34:617–623;
- Hellingman CA, van den Bergh WM, Beijer IS, et al. Risk of rebleeding after treatment of acute hydrocephalus in patients with aneurysmal subarachnoid hemorrhage. Stroke 2007; 38:96–99.
- Ransom ER, Mocco J, Komotar RJ, et al. External ventricular drainage response in poor grade aneurysmal subarachnoid hemorrhage: effect on preoperative grading and prognosis. Neurocrit Care 2007; 6:174–180.
- McIver JI, Friedman JA, Wijdicks EF, et al. Preoperative ventriculostomy and rebleeding after aneurysmal subarachnoid hemorrhage. J Neurosurg 2002; 97:1042–1044.
- Klopfenstein JD, Kim LJ, Feiz-Erfan I, et al. Comparison of rapid and gradual weaning from external ventricular drainage in patients with aneurysmal subarachnoid hemorrhage: a prospective randomized trial. J Neurosurg 2004; 100:225–229.
- Tseng MY, Al-Rawi PG, Czosnyka M, et al. Enhancement of cerebral blood flow using systemic hypertonic saline therapy improves outcome in patients with poor-grade spontaneous subarachnoid hemorrhage. J Neurosurg 2007; 107:274–282.
- Al-Rawi PG, Tseng MY, Richards HK, et al. Hypertonic saline in patients with poor-grade subarachnoid hemorrhage improves cerebral blood flow, brain tissue oxygen, and pH. Stroke 2010; 41:122–128.
- Tseng MY, Al-Rawi PG, Pickard JD, Rasulo FA, Kirkpatrick PJ. Effect of hypertonic saline on cerebral blood flow in poor-grade patients with subarachnoid hemorrhage. Stroke 2003; 34:1389–1396.
- Bentsen G, Breivik H, Lundar T, Stubhaug A. Hypertonic saline (7.2%) in 6% hydroxyethyl starch reduces intracranial pressure and improves hemodynamics in a placebo-controlled study involving stable patients with subarachnoid hemorrhage. Crit Care Med 2006; 34:2912–2917.
- Suarez JI, Qureshi AI, Parekh PD, et al. Administration of hypertonic (3%) sodium chloride/acetate in hyponatremic patients with symptomatic vasospasm following subarachnoid hemorrhage. J Neurosurg Anesthesiol 1999; 11:178–184.
- Stevens RD, Huff JS, Duckworth J, Papangelou A, Weingart SD, Smith WS. Emergency neurological life support: intracranial hypertension and herniation. Neurocrit Care 2012;17(suppl 1):S60–S65.
- Seule MA, Muroi C, Mink S, Yonekawa Y, Keller E. Therapeutic hypothermia in patients with aneurysmal subarachnoid hemorrhage, refractory intracranial hypertension, or cerebral vasospasm. Neurosurgery 2009; 64:86–93.
- Otani N, Takasato Y, Masaoka H, et al. Surgical outcome following decompressive craniectomy for poor-grade aneurysmal subarachnoid hemorrhage in patients with associated massive intracerebral or Sylvian hematomas. Cerebrovasc Dis 2008; 26:612–617.
- Lanzino G, D’Urso PI, Suarez J; Participants in the International Multi-Disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage. Seizures and anticonvulsants after aneurysmal subarachnoid hemorrhage. Neurocrit Care 2011; 15:247–256.
- Naidech AM, Kreiter KT, Janjua N, et al. Phenytoin exposure is associated with functional and cognitive disability after subarachnoid hemorrhage. Stroke 2005; 36:583–587.
- Rosengart AJ, Huo JD, Tolentino J, et al. Outcome in patients with subarachnoid hemorrhage treated with antiepileptic drugs. J Neurosurg 2007; 107:253–260.
- Shah D, Husain AM. Utility of levetiracetam in patients with subarachnoid hemorrhage. Seizure 2009; 18:676–679.
- Patsalos PN. Pharmacokinetic profile of levetiracetam: toward ideal characteristics. Pharmacol Ther 2000; 85:77–85.
- Dewan MC, Mocco J. Current practice regarding seizure prophylaxis in aneurysmal subarachnoid hemorrhage across academic centers. J Neurointerv Surg 2014 Jan 28. doi: 10.1136/neurintsurg-2013-011075 [Epub ahead of print]
- Murphy-Human T, Welch E, Zipfel G, Diringer MN, Dhar R. Comparison of short-duration levetiracetam with extended-course phenytoin for seizure prophylaxis after subarachnoid hemorrhage. World Neurosurg 2011; 75:269–274.
- Dennis LJ, Claassen J, Hirsch LJ, Emerson RG, Connolly ES, Mayer SA. Nonconvulsive status epilepticus after subarachnoid hemorrhage. Neurosurgery 2002; 51:1136–1144.
- Vespa PM, Nuwer MR, Juhász C, et al. Early detection of vasospasm after acute subarachnoid hemorrhage using continuous EEG ICU monitoring. Electroencephalogr Clin Neurophysiol 1997; 103:607–615.
- Claassen J, Hirsch LJ, Kreiter KT, et al. Quantitative continuous EEG for detecting delayed cerebral ischemia in patients with poor-grade subarachnoid hemorrhage. Clin Neurophysiol 2004; 115:2699–2710.
- Vergouwen MD, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 2010; 41:2391–2395.
- Kelly PJ, Gorten RJ, Grossman RG, Eisenberg HM. Cerebral perfusion, vascular spasm, and outcome in patients with ruptured intracranial aneurysms. J Neurosurg 1977; 47:44–49.
- Aralasmak A, Akyuz M, Ozkaynak C, Sindel T, Tuncer R. CT angiography and perfusion imaging in patients with subarachnoid hemorrhage: correlation of vasospasm to perfusion abnormality. Neuroradiology 2009; 51:85–93.
- Sabri M, Lass E, Macdonald RL. Early brain injury: a common mechanism in subarachnoid hemorrhage and global cerebral ischemia. Stroke Res Treat 2013 Feb 28. doi: 10.1155/2013/394036 [Epub 2013 ahead of print]
- Yoon DY, Choi CS, Kim KH, Cho BM. Multidetector-row CT angiography of cerebral vasospasm after aneurysmal subarachnoid hemorrhage: comparison of volume-rendered images and digital subtraction angiography. AJNR Am J Neuroradiol 2006; 27:370–377.
- Wintermark M1, Ko NU, Smith WS, Liu S, Higashida RT, Dillon WP. Vasospasm after subarachnoid hemorrhage: utility of perfusion CT and CT angiography on diagnosis and management. AJNR Am J Neuroradiol 2006; 27:26–34.
- Helbok R, Madineni RC, Schmidt MJ, et al. Intracerebral monitoring of silent infarcts after subarachnoid hemorrhage. Neurocrit Care 2011; 14:162–167.
- Hänggi D; Participants in the International Multi-Disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage. Monitoring and detection of vasospasm II: EEG and invasive monitoring. Neurocrit Care 2011; 15:318–323.
- Steiner T, Juvela S, Unterberg A, Jung C, Forsting M, Rinkel G; European Stroke Organization. European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis 2013; 35:93–112.
- Pickard JD, Murray GD, Illingworth R, et al. Effect of oral nimodipine on cerebral infarction and outcome after subarachnoid haemorrhage: British aneurysm nimodipine trial. BMJ 1989; 298:636–642.
- Dorhout Mees SM, Rinkel GJ, Feigin VL, et al. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 2007; 3:CD000277.
- Lennihan L, Mayer SA, Fink ME, et al. Effect of hypervolemic therapy on cerebral blood flow after subarachnoid hemorrhage: a randomized controlled trial. Stroke 2000; 31:383–391.
- Egge A, Waterloo K, Sjøholm H, Solberg T, Ingebrigtsen T, Romner B. Prophylactic hyperdynamic postoperative fluid therapy after aneurysmal subarachnoid hemorrhage: a clinical, prospective, randomized, controlled study. Neurosurgery 2001; 49:593–606.
- Jost SC, Diringer MN, Zazulia AR, et al. Effect of normal saline bolus on cerebral blood flow in regions with low baseline flow in patients with vasospasm following subarachnoid hemorrhage. J Neurosurg 2005; 103:25–30.
- Muizelaar JP, Becker DP. Induced hypertension for the treatment of cerebral ischemia after subarachnoid hemorrhage. Direct effect on cerebral blood flow. Surg Neurol 1986; 25:317–325.
- Levy ML, Rabb CH, Zelman V, Giannotta SL. Cardiac performance enhancement from dobutamine in patients refractory to hypervolemic therapy for cerebral vasospasm. J Neurosurg 1993; 79:494–499.
- Lannes M, Teitelbaum J, del Pilar Cortés M, Cardoso M, Angle M. Milrinone and homeostasis to treat cerebral vasospasm associated with subarachnoid hemorrhage: the Montreal Neurological Hospital protocol. Neurocrit Care 2012; 16:354–362.
- Zwienenberg-Lee M, Hartman J, Rudisill N, et al; Balloon Prophylaxis for Aneurysmal Vasospasm (BPAV) Study Group. Effect of prophylactic transluminal balloon angioplasty on cerebral vasospasm and outcome in patients with Fisher grade III subarachnoid hemorrhage: results of a phase II multicenter, randomized, clinical trial. Stroke 2008; 39:1759–1765.
- Rabinstein AA, Bruder N. Management of hyponatremia and volume contraction. Neurocrit Care 2011; 15:354–360.
- Wijdicks EF, Vermeulen M, Hijdra A, van Gijn J. Hyponatremia and cerebral infarction in patients with ruptured intracranial aneurysms: is fluid restriction harmful? Ann Neurol 1985; 17:137–140.
- Hasan D, Wijdicks EF, Vermeulen M. Hyponatremia is associated with cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage. Ann Neurol 1990; 27:106–108.
- Wijdicks EF, Vermeulen M, ten Haaf JA, Hijdra A, Bakker WH, van Gijn J. Volume depletion and natriuresis in patients with a ruptured intracranial aneurysm. Ann Neurol 1985; 18:211–216.
- Hoff RG, Rinkel GJ, Verweij BH, Algra A, Kalkman CJ. Nurses’ prediction of volume status after aneurysmal subarachnoid haemorrhage: a prospective cohort study. Crit Care 2008; 12:R153.
- Hoff RG, van Dijk GW, Algra A, Kalkman CJ, Rinkel GJ. Fluid balance and blood volume measurement after aneurysmal subarachnoid hemorrhage. Neurocrit Care 2008; 8:391–397.
- Berendes E, Walter M, Cullen P, et al. Secretion of brain natriuretic peptide in patients with aneurysmal subarachnoid haemorrhage. Lancet 1997; 349:245–249.
- Espiner EA, Leikis R, Ferch RD, et al. The neuro-cardio-endocrine response to acute subarachnoid haemorrhage. Clin Endocrinol (Oxf) 2002; 56:629–635.
- Isotani E, Suzuki R, Tomita K, et al. Alterations in plasma concentrations of natriuretic peptides and antidiuretic hormone after subarachnoid hemorrhage. Stroke 1994; 25:2198–2203.
- Benedict CR, Loach AB. Sympathetic nervous system activity in patients with subarachnoid hemorrhage. Stroke 1978; 9:237–244.
- Findling JW, Waters VO, Raff H. The dissociation of renin and aldosterone during critical illness. J Clin Endocrinol Metab 1987; 64:592–595.
- Solomon RA, Post KD, McMurtry JG 3rd. Depression of circulating blood volume in patients after subarachnoid hemorrhage: implications for the management of symptomatic vasospasm. Neurosurgery 1984; 15:354–361.
- Peters JP, Welt LG, Sims EA, Orloff J, Needham J. A salt-wasting syndrome associated with cerebral disease. Trans Assoc Am Physicians 1950; 63:57–64.
- Brimioulle S, Orellana-Jimenez C, Aminian A, Vincent JL. Hyponatremia in neurological patients: cerebral salt wasting versus inappropriate antidiuretic hormone secretion. Intensive Care Med 2008; 34:125–131.
- Singh S, Bohn D, Carlotti AP, Cusimano M, Rutka JT, Halperin ML. Cerebral salt wasting: truths, fallacies, theories, and challenges. Crit Care Med 2002; 30:2575–2579.
- Mutoh T, Kazumata K, Terasaka S, Taki Y, Suzuki A, Ishikawa T. Early intensive versus minimally invasive approach to postoperative hemodynamic management after subarachnoid hemorrhage. Stroke 2014; 45:1280–1284.
- Hasan D, Lindsay KW, Wijdicks EF, et al. Effect of fludrocortisone acetate in patients with subarachnoid hemorrhage. Stroke 1989; 20:1156–1161.
- Moro N, Katayama Y, Kojima J, Mori T, Kawamata T. Prophylactic management of excessive natriuresis with hydrocortisone for efficient hypervolemic therapy after subarachnoid hemorrhage. Stroke 2003; 34:2807–2811.
- Kilbourn KJ, Levy S, Staff I, Kureshi I, McCullough L. Clinical characteristics and outcomes of neurogenic stress cadiomyopathy in aneurysmal subarachnoid hemorrhage. Clin Neurol Neurosurg 2013; 115:909–914.
- Mayer SA, LiMandri G, Sherman D, et al. Electrocardiographic markers of abnormal left ventricular wall motion in acute subarachnoid hemorrhage. J Neurosurg 1995; 83:889–896.
- Deibert E, Barzilai B, Braverman AC, et al. Clinical significance of elevated troponin I levels in patients with nontraumatic subarachnoid hemorrhage. J Neurosurg 2003; 98:741–746.
- Banki N, Kopelnik A, Tung P, et al. Prospective analysis of prevalence, distribution, and rate of recovery of left ventricular systolic dysfunction in patients with subarachnoid hemorrhage. J Neurosurg 2006; 105:15–20.
- Kahn JM, Caldwell EC, Deem S, Newell DW, Heckbert SR, Rubenfeld GD. Acute lung injury in patients with subarachnoid hemorrhage: incidence, risk factors, and outcome. Crit Care Med 2006; 34:196–202.
- Kitamura Y, Nomura M, Shima H, et al. Acute lung injury associated with systemic inflammatory response syndrome following subarachnoid hemorrhage: a survey by the Shonan Neurosurgical Association. Neurol Med Chir (Tokyo) 2010; 50:456–460.
- Friedman JA, Pichelmann MA, Piepgras DG, et al. Pulmonary complications of aneurysmal subarachnoid hemorrhage. Neurosurgery 2003; 52:1025–1032.
- Oh HS, Jeong HS, Seo WS. Non-infectious hyperthermia in acute brain injury patients: relationships to mortality, blood pressure, intracranial pressure and cerebral perfusion pressure. Int J Nurs Pract 2012; 18:295–302.
- Oddo M, Frangos S, Milby A, et al. Induced normothermia attenuates cerebral metabolic distress in patients with aneurysmal subarachnoid hemorrhage and refractory fever. Stroke 2009; 40:1913–1916.
- Badjatia N, Fernandez L, Schmidt JM, et al. Impact of induced normothermia on outcome after subarachnoid hemorrhage: a case-control study. Neurosurgery 2010; 66:696-701.
- Serrone JC1, Wash EM, Hartings JA, Andaluz N, Zuccarello M. Venous thromboembolism in subarachnoid hemorrhage. World Neurosurg 2013; 80:859–863.
- Mack WJ, Ducruet AF, Hickman ZL, et al. Doppler ultrasonography screening of poor-grade subarachnoid hemorrhage patients increases the diagnosis of deep venous thrombosis. Neurol Res 2008; 30:889–892.
- de Oliveira Manoel AL, Turkel-Parrella D, Germans M, et al. Safety of early pharmacological thromboprophylaxis after subarachnoid hemorrhage. Can J Neurol Sci 2014; 41:554–561.
Aneurysmal subarachnoid hemorrhage is a devastating condition, with an estimated death rate of 30% during the initial episode.1,2 Approximately the same number of patients survive but leave the hospital with disabling neurologic deficits.3
However, better outcomes can be achieved by systems that are able to work as a team on the collective goal of quick intervention to secure the ruptured aneurysm, followed by the implementation of measures to minimize secondary brain injury. Although the search for new diagnostic, prognostic, and therapeutic modalities continues, it is clear that there exists no “silver bullet” that will help all patients. Instead, it is the systematic integration and application of small advances that will ultimately maximize the patient’s chances of survival and neurologic recovery.
This review focuses on the management of aneurysmal subarachnoid hemorrhage and its systemic and neurologic complications.
ANEURYSM IS THE MOST COMMON CAUSE OF SUBARACHNOID BLEEDING
Aneurysmal subarachnoid hemorrhage, ie, rupture of an intracranial aneurysm, flooding the subarachnoid space with blood, affects about 24,000 Americans each year.1,2 A ruptured aneurysm is the most common cause of subarachnoid hemorrhage, accounting for about 85% of cases. Less common causes include idiopathic benign perimesencephalic hemorrhage, arteriovenous malformation, dural arteriovenous fistula, and hemorrhagic mycotic aneurysm. These have their own natural history, pathophysiology, and specific treatment, and will not be addressed in this article.
Risk factors for aneurysmal subarachnoid hemorrhage include having a first-degree relative who had the disease, hypertension, smoking, and consuming more than 150 g of alcohol per week.4
CLINICAL PRESENTATION AND DIAGNOSIS
The key symptom of aneurysmal subarachnoid hemorrhage is the abrupt onset of severe headache that peaks in intensity over 1 hour,5 often described as “the worst headache of my life.” Headache is accompanied by brief loss of consciousness in 53% of cases (conversely, nearly half of patients maintain normal mental status), by nausea or vomiting in 77%, and by meningismus (neck pain or stiffness) in 35%.6
These clinical manifestations and risk factors have been incorporated into a decision rule:
Obtain brain imaging if the patient has acute headache reaching maximal intensity within 1 hour, associated with any of the following factors:
- Age 40 or older
- Neck pain or stiffness
- Witnessed loss of consciousness
- Onset during exertion
- “Thunderclap” headache (ie, instantly peaking pain)
- Limited neck flexion on examination.5
This decision rule has nearly 100% sensitivity for aneurysmal subarachnoid hemorrhage in clinical practice.5 All patients require brain imaging if they have a severe headache plus either abnormal neurologic findings (eg, a focal neurologic deficit) or a history of cerebral aneurysm.
Emergency physicians should have a low threshold for ordering noncontrast computed tomography (CT) of the head in patients with even mild symptoms suggesting aneurysmal subarachnoid hemorrhage. Failure to order CT is the most common diagnostic error in this situation.6 CT performed within 6 hours of headache onset is nearly 100% sensitive for this condition,7 but the sensitivity falls to 93% after the first 24 hours and to less than 60% after 5 days.8 In patients who have symptoms highly suggestive of aneurysmal subarachnoid hemorrhage but a normal CT, lumbar puncture is the next diagnostic step.
There are two alternatives to CT followed by lumbar puncture: ie, noncontrast CT followed by CT angiography,9,10 and magnetic resonance imaging followed by magnetic resonance angiography. In patients with suspicious clinical symptoms but negative CT results, CT followed by CT angiography can rule out aneurysmal subarachnoid hemorrhage with a 99% probability.9,10 However, CT followed by lumbar puncture remains the standard of care and carries a class I recommendation in the American Heart Association guidelines for ruling out subarachnoid hemorrhage.5
GRADING THE SEVERITY OF SUBARACHNOID HEMORRHAGE
Age, the thickness of the blood layer in the subarachnoid space, intraventricular hemorrhage and the findings of the neurologic examination at presentation are predictors of long-term outcomes in aneurysmal subarachnoid hemorrhage (Figure 1).

Different grading systems used in clinical practice are based on the findings on the initial neurologic examination and on the initial noncontrast CT (ie, the thickness of the blood, and whether intraventricular hemorrhage is present). Among the most widely used are those developed by Hunt and Hess12 and by the World Federation of Neurological Surgeons11 (WFNS), and the CT grading scales (Fisher13 or its modified version14) (Tables 1 and 2). With either the Hunt and Hess scale or the WFNS scale, the higher the score, the worse the patient’s probable outcome. Scores on both Fisher scales correlate with the risk of angiographic vasospasm. The higher the grade, the higher the risk of angiographic vasospasm.


The VASOGRADE score—a combination of the WFNS score and the modified Fisher scale—stratifies patients at risk of delayed cerebral ischemia, allowing for a tailored monitoring strategy.15 There are three variations:
- VASOGRADE green—Modified Fisher 1 or 2 and WFNS 1 or 2
- VASOGRADE yellow—Modified Fisher 3 or 4 and WFNS 1, 2, or 3
- VASOGRADE red—WFNS 4 or 5.
After the initial bleeding event, patients with aneurysmal subarachnoid hemorrhage are at high risk of delayed systemic and neurologic complications, with poor functional outcomes. Delayed cerebral ischemia holds the greatest risk of an unfavorable outcome and ultimately can lead to cerebral infarction, disability, and death.6,7
INITIAL MANAGEMENT
After aneurysmal subarachnoid hemorrhage is diagnosed, the initial management (Figure 2) includes appropriate medical prevention of rebleeding (which includes supportive care, blood pressure management, and, perhaps, the early use of a short course of an antifibrinolytic drug) and early transfer to a high-volume center for securing the aneurysm. The reported incidence of rebleeding varies from 5% to 22% in the first 72 hours. “Ultra-early” rebleeding (within 24 hours of hemorrhage) has been reported, with an incidence as high as 15% and a fatality rate around 70%. Patients with poor-grade aneurysmal subarachnoid hemorrhage, larger aneurysms, and “sentinel bleeds” are at higher risk of rebleeding.16
Outcomes are much better when patients are managed in a high-volume center, with a specialized neurointensive care unit17 and access to an interdisciplinary team.18 Regardless of the initial grade, patients with aneurysmal subarachnoid hemorrhage should be quickly transferred to a high-volume center, defined as one treating at least 35 cases per year, and the benefit is greater in centers treating more than 60 cases per year.19 The higher the caseload in any given hospital, the better the clinical outcomes in this population.20

Treating cerebral aneurysm: Clipping or coiling
Early aneurysm repair is generally considered the standard of care and the best strategy to reduce the risk of rebleeding. Further, early treatment may be associated with a lower risk of delayed cerebral ischemia21 and better outcomes.22
Three randomized clinical trials have compared surgical clipping and endovascular repair (placement of small metal coils within the aneurysm to promote clotting).
The International Subarachnoid Aneurysm Trial23 showed a reduction of 23% in relative risk and of 7% in absolute risk in patients who underwent endovascular treatment compared with surgery. The survival benefit persisted at a mean of 9 years (range 6–14 years), but with a higher annual rate of aneurysm recurrence in the coiling group (2.9% vs 0.9%).24 Of note, this trial included only patients with aneurysms deemed suitable for both coiling and clipping, so that the exclusion rate was high. Most of the patients presented with good-grade (WFNS score 1–3), small aneurysms (< 5 mm) in the anterior circulation.
A single-center Finnish study25 found no differences in rates of recovery, disability, and death at 1 year, comparing surgery and endovascular treatment. Additionally, survival rates at a mean follow-up of 39 months were similar, with no late recurrences or aneurysmal bleeding.
Lastly, the Barrow Ruptured Aneurysm Trial26,27 found that patients assigned to endovascular treatment had better 1-year neurologic outcomes, defined as a modified Rankin score of 2 or less. Importantly, 37.7% of patients originally assigned to endovascular treatment crossed over to surgical treatment. The authors then performed intention-to-treat and as-treated analyses. Either way, patients treated by endovascular means had better neurologic outcomes at 1 year. However, no difference in the relative risk reduction in worse outcome was found on 3-year follow-up, and patients treated surgically had higher rates of aneurysm obliteration and required less aneurysm retreatment, both of which were statistically significant.
The question that remains is not whether to clip or whether to coil, but whom to clip and whom to coil.28 That question must be answered on a patient-to-patient basis and requires the expertise of an interventional neuroradiologist and a vascular neurosurgeon—one of the reasons these patients are best cared for in high-volume centers providing such expertise.
MEDICAL PREVENTION OF REBLEEDING
Blood pressure management
There are no systematic data on the optimal blood pressure before securing an aneurysm. Early studies of hemodynamic augmentation in cases of ruptured untreated aneurysm reported rebleeding when the systolic blood pressure was allowed to rise above 160 mm Hg.29,30 A recent study evaluating hypertensive intracerebral hemorrhage revealed better functional outcomes with intensive lowering of blood pressure (defined as systolic blood pressure < 140 mm Hg) but no significant reduction in the combined rate of death or severe disability.31 It is difficult to know if these results can be extrapolated to patients with aneurysmal subarachnoid hemorrhage. Current guidelines3,32 say that before the aneurysm is treated, the systolic pressure should be lower than 160 mm Hg.
There is no specific drug of choice, but a short-acting, titratable medication is preferable. Nicardipine is a very good option, and labetalol might be an appropriate alternative.33 Once the aneurysm is secured, all antihypertensive drugs should be held. Hypertension should not be treated unless the patient has clinical signs of a hypertensive crisis, such as flash pulmonary edema, myocardial infarction, or hypertensive encephalopathy.
Antifibrinolytic therapy
The role of antifibrinolytic therapy in aneurysmal subarachnoid hemorrhage is controversial and has been studied in 10 clinical trials. In a Swedish study,34 early use of tranexamic acid (1 g intravenously over 10 minutes followed by 1 g every 6 hours for a maximum of 24 hours) reduced the rebleeding rate substantially, from 10.8% to 2.4%, with an 80% reduction in the mortality rate from ultra-early rebleeding. However, a recent Cochrane review that included this study found no overall benefit.35
An ongoing multicenter randomized trial in the Netherlands will, we hope, answer this question in the near future.36 At present, some centers would consider a short course of tranexamic acid before aneurysm treatment.
DIAGNOSIS AND TREATMENT OF COMPLICATIONS
Medical complications are extremely common after aneurysmal subarachnoid hemorrhage. Between 75% and 100% of patients develop some type of systemic or further neurologic derangement, which in turn has a negative impact on the long-term outcome.37,38 In the first 72 hours, rebleeding is the most feared complication, and as mentioned previously, appropriate blood pressure management and early securing of the aneurysm minimize its risk.
NEUROLOGIC COMPLICATIONS
Hydrocephalus
Hydrocephalus is the most common early neurologic complication after aneurysmal subarachnoid hemorrhage, with an overall incidence of 50%.39 Many patients with poor-grade aneurysmal subarachnoid hemorrhage and patients whose condition deteriorates due to worsening of hydrocephalus require the insertion of an external ventricular drain (Figure 1).
Up to 30% of patients who have a poor-grade aneurysmal subarachnoid hemorrhage improve neurologically with cerebrospinal fluid drainage.40 An external ventricular drain can be safely placed, even before aneurysm treatment, and placement does not appear to increase the risk of rebleeding.39,41 After placement, rapid weaning from the drain (clamping within 24 hours of insertion) is safe, decreases length of stay in the intensive care unit and hospital, and may be more cost-effective than gradual weaning over 96 hours.42
Increased intracranial pressure
Intracranial hypertension is another potential early complication, and is frequently due to the development of hydrocephalus, cerebral edema, or rebleeding. The treatment of increased intracranial pressure does not differ from the approach used in managing severe traumatic brain injury, which includes elevating the head of the bed, sedation, analgesia, normoventilation, and cerebrospinal fluid drainage.
Hypertonic saline has been tested in several studies that were very small but nevertheless consistently showed control of intracranial pressure levels and improvement in cerebral blood flow measured by xenon CT.43–47 Two of these studies even showed better outcomes at discharge.43,44 However, the small number of patients prevents any meaningful conclusion regarding the use of hypertonic saline and functional outcomes.
Barbiturates, hypothermia, and decompressive craniectomy could be tried in refractory cases.48 Seule et al49 evaluated the role of therapeutic hypothermia with or without barbiturate coma in 100 patients with refractory intracranial hypertension. Only 13 patients received hypothermia by itself. At 1 year, 32 patients had achieved a good functional outcome (Glasgow Outcome Scale score 4 or 5). The remaining patients were severely disabled or had died. Of interest, the median duration of hypothermia was 7 days, and 93% of patients developed some medical complication such as electrolyte disorders (77%), pneumonia (52%), thrombocytopenia (47%), or septic shock syndrome (40%). Six patients died as a consequence of one of these complications.
Decompressive craniectomy can be life-saving in patients with refractory intracranial hypertension. However, most of these patients will die or remain severely disabled or comatose.50
Seizure prophylaxis is controversial
Seizures can occur at the onset of intracranial hemorrhage, perioperatively, or later (ie, after the first week). The incidence varied considerably in different reports, ranging from 4% to 26%.51 Seizures occurring perioperatively, ie, after hospital admission, are less frequent and are usually the manifestation of aneurysm rebleeding.24
Seizure prophylaxis remains controversial, especially because the use of phenytoin is associated with increased incidence of cerebral vasospasm, infarction, and worse cognitive outcomes after aneurysmal subarachnoid hemorrhage.52,53 Therefore, routine prophylactic use of phenytoin is not recommended in these patients,3 although the effect of other antiepileptic drugs is less studied and less clear. Patients may be considered for this therapy if they have multiple risk factors for seizures, such as intraparenchymal hematoma, advanced age (> 65), middle cerebral artery aneurysm, craniotomy for aneurysm clipping, and a short course (≤ 72 hours) of an antiepileptic drug other than phenytoin, especially while the aneurysm is unsecured.3
Levetiracetam may be an alternative to phenytoin, having better pharmacodynamic and kinetic profiles, minimal protein binding, and absence of hepatic metabolism, resulting in a very low risk of drug interaction and better tolerability.54,55 Because of these advantages, levetiracetam has become the drug of choice in several centers treating aneurysmal subarachnoid hemorrhage in the United States.
Addressing this question, a survey was sent to 25 high-volume aneurysmal subarachnoid hemorrhage academic centers in the United States. All 25 institutions answered the survey, and interestingly, levetiracetam was the first-line agent for 16 (94%) of the 17 responders that used prophylaxis, while only 1 used phenytoin as the agent of choice.56
A retrospective cohort study by Murphy-Human et al57 showed that a short course of levetiracetam (≤ 72 hours) was associated with higher rates of in-hospital seizures compared with an extended course of phenytoin (eg, entire hospital stay). However, the study did not address functional outcomes.57
Continuous electroencephalographic monitoring may be considered in comatose patients, in patients requiring controlled ventilation and sedation, or in patients with unexplained alteration in consciousness. In one series of patients with aneurysmal subarachnoid hemorrhage who received continuous monitoring, the incidence of nonconvulsive status epilepticus was 19%, with an associated mortality rate of 100%.58
Continuous quantitative electroencephalography is useful to monitor and to detect angiographic vasospasm and delayed cerebral ischemia. Relative alpha variability and the alpha-delta ratio decrease with ischemia, and this effect can precede angiographic vasospasm by 3 days.59,60
Delayed cerebral ischemia
Delayed cerebral ischemia is defined as the occurrence of focal neurologic impairment, or a decrease of at least 2 points on the Glasgow Coma Scale that lasts for at least 1 hour, is not apparent immediately after aneurysm occlusion, and not attributable to other causes (eg, hyponatremia, fever).61
Classically, neurologic deficits that occurred within 2 weeks of aneurysm rupture were ascribed to reduced cerebral blood flow caused by delayed large-vessel vasospasm causing cerebral ischemia.62 However, perfusion abnormalities have also been observed with either mild or no demonstrable vasospasm.63 Almost 70% of patients who survive the initial hemorrhage develop some degree of angiographic vasospasm. However, only 30% of those patients will experience symptoms.
In addition to vasospasm of large cerebral arteries, impaired autoregulation and early brain injury within the first 72 hours following subarachnoid hemorrhage may play important roles in the development of delayed cerebral ischemia.64 Therefore, the modern concept of delayed cerebral ischemia monitoring should focus on cerebral perfusion rather than vessel diameter measurements. This underscores the importance of comprehensive, standardized monitoring techniques that provide information not only on microvasculature, but also at the level of the microcirculation, with information on perfusion, oxygen utilization and extraction, and autoregulation.
Although transcranial Doppler has been the most commonly applied tool to monitor for angiographic vasospasm, it has a low sensitivity and negative predictive value.37 It is nevertheless a useful technique to monitor good-grade aneurysmal subarachnoid hemorrhage patients (WFNS score 1–3) combined with frequent neurologic examinations (Figure 3).

CT angiography is a good noninvasive alternative to digital subtraction angiography. However, it tends to overestimate the degree of vasoconstriction and does not provide information about perfusion and autoregulation.65 Nevertheless, CT angiography combined with a CT perfusion scan can add information about autoregulation and cerebral perfusion and has been shown to be more sensitive for the diagnosis of angiographic vasospasm than transcranial Doppler and digital subtraction angiography (Figure 4).

Patients with a poor clinical condition (WFNS score 4 or 5) or receiving continuous sedation constitute a challenge in monitoring for delayed neurologic deterioration. Neurologic examination is not sensitive enough in this setting to detect subtle changes. In these specific and challenging circumstances, multimodality neuromonitoring may be useful in the early detection of delayed cerebral ischemia and may help guide therapy.67
Several noninvasive and invasive techniques have been studied to monitor patients at risk of delayed cerebral ischemia after subarachnoid hemorrhage.66 These include continuous electroencephalography, brain tissue oxygenation monitoring (Ptio2), cerebral microdialysis, thermal diffusion flowmetry, and near-infrared spectroscopy. Of these techniques, Ptio2, cerebral microdialysis, and continuous electroencephalography (see discussion of seizure prophylaxis above) have been more extensively studied. However, most of the studies were observational and very small, limiting any recommendations for using these techniques in routine clinical practice.68
Ptio2 is measured by inserting an intraparenchymal oxygen-sensitive microelectrode, and microdialysis requires a microcatheter with a semipermeable membrane that allows small soluble substances to cross it into the dialysate. These substances, which include markers of ischemia (ie, glucose, lactate, and pyruvate), excitotoxins (ie, glutamate and aspartate), and membrane cell damage products (ie, glycerol), can be measured. Low Ptio2 values (< 15 mm Hg) and abnormal mycrodialysate findings (eg, glucose < 0.8 mmol/L, lactate-to-pyruvate ratio > 40) have both been associated with cerebral ischemic events and poor outcome.68
Preventing delayed cerebral ischemia
Oral nimodipine 60 mg every 4 hours for 21 days, started on admission, carries a class I, level of evidence A recommendation in the management of aneurysmal subarachnoid hemorrhage.3,32,69 It improves clinical outcome despite having no effect on the risk of angiographic vasospasm. The mechanism of improved outcome is unclear, but the effect may be a neuroprotective phenomenon limiting the extension of delayed cerebral ischemia.70
If hypotension occurs, the dose can be lowered to 30 mg every 2 hours. Whether to discontinue nimodipine in this situation is controversial. Of note, the clinical benefits of nimodipine have not been replicated with other calcium channel blockers (eg, nicardipine).71
Prophylactic hyperdynamic fluid therapy, known as “triple-H” (hypervolemia, hemodilution, and hypertension) was for years the mainstay of treatment in preventing delayed cerebral ischemia due to vasospasm. However, the clinical data supporting this intervention have been called into question, as analysis of two trials found that hypervolemia did not improve outcomes or reduce the incidence of delayed cerebral ischemia, and in fact increased the rate of complications.72,73 Based on these findings, current guidelines recommend maintaining euvolemia rather than prophylactic hypervolemia in patients with aneurysmal subarachnoid hemorrhage.3,32,69
TREATING DELAYED CEREBRAL ISCHEMIA
Hemodynamic augmentation
In patients with neurologic deterioration due to delayed cerebral ischemia, hemodynamic augmentation is the cornerstone of treatment. This is done according to a protocol, started early, involving specific physiologic goals, clinical improvement, and escalation to invasive therapies in a timely fashion in patients at high risk of further neurologic insult (Figure 5).

The physiologic goal is to increase the delivery of oxygen and glucose to the ischemic brain. Hypertension seems to be the most effective component of hemodynamic augmentation regardless of volume status, increasing cerebral blood flow and brain tissue oxygenation, with reversal of delayed cerebral ischemic symptoms in up to two-thirds of treated patients.74,75 However, this information comes from very small studies, with no randomized trials of induced hypertension available.
The effect of a normal saline fluid bolus in patients suspected of having delayed cerebral ischemia has been shown to increase cerebral blood flow in areas of cerebral ischemia.74 If volume augmentation fails to improve the neurologic status, the next step is to artificially induce hypertension using vasopressors. The blood pressure target should be based on clinical improvement. A stepwise approach is reasonable in this situation, and the lowest level of blood pressure at which there is a complete reversal of the new focal neurologic deficit should be maintained.3,29
Inotropic agents such as dobutamine or milrinone can be considered as alternatives in patients who have new neurologic deficits that are refractory to fluid boluses and vasopressors, or in a setting of subarachnoid hemorrhage-induced cardiomyopathy.76,77
Once the neurologic deficit is reversed by hemodynamic augmentation, the blood pressure should be maintained for 48 to 72 hours at the level that reversed the deficit completely, carefully reassessed thereafter, and the patient weaned slowly. Unruptured unsecured aneurysms should not prevent blood pressure augmentation in a setting of delayed cerebral ischemia if the culprit aneurysm is treated.3,32 If the ruptured aneurysm has not been secured, careful blood pressure augmentation can be attempted, keeping in mind that hypertension (> 160/95 mm Hg) is a risk factor for fatal aneurysm rupture.
Endovascular management of delayed cerebral ischemia
When medical augmentation fails to completely reverse the neurologic deficits, endovascular treatment can be considered. Although patients treated early in the course of delayed cerebral ischemia have better neurologic recovery, prophylactic endovascular treatment in asymptomatic patients, even if angiographic signs of spasm are present, does not improve clinical outcomes and carries the risk of fatal arterial rupture.78
SYSTEMIC COMPLICATIONS
Hyponatremia and hypovolemia
Aneurysmal subarachnoid hemorrhage is commonly associated with abnormalities of fluid balance and electrolyte derangements. Hyponatremia (serum sodium < 135 mmol/L) occurs in 30% to 50% of patients, while the rate of hypovolemia (decreased circulating blood volume) ranges from 17% to 30%.79 Both can negatively affect long-term outcomes.80,81
Decreased circulating blood volume is a well-described contributor to delayed cerebral ischemia and cerebral infarction after aneurysmal subarachnoid hemorrhage.80–82 Clinical variables such as heart rate, blood pressure, fluid balance, and serum sodium concentration are usually the cornerstones of intravascular volume status assessment. However, these variables correlate poorly with measured circulating blood volumes in those with aneurysmal subarachnoid hemorrhage.83,84
The mechanisms responsible for the development of hyponatremia and hypovolemia after aneurysmal subarachnoid hemorrhage are not completely understood. Several factors have been described and may contribute to the increased natriuresis and, hence, to a reduction in circulating blood volume: increased circulating natriuretic peptide concentrations,85–87 sympathetic nervous system hyperactivation,88 and hyperreninemic hypo-
aldosteronism syndrome.89,90
Lastly, the cerebral salt wasting syndrome, described in the 1950s,91 was thought to be a key mechanism in the development of hyponatremia and hypovolemia after aneurysmal subarachnoid hemorrhage. In contrast to the syndrome of inappropriate antidiuretic hormone, which is characterized by hyponatremia with a normal or slightly elevated intravascular volume, the characteristic feature of cerebral salt wasting syndrome is the development of hyponatremia in a setting of intravascular volume depletion.92 In critically ill neurologic and neurosurgical patients, this differential diagnosis is very difficult, especially in those with aneurysmal subarachnoid hemorrhage in whom the clinical assessment of fluid status is not reliable. These two syndromes might coexist and contribute to the development of hyponatremia after aneurysmal subarachnoid hemorrhage.92,93
Hoff et al83,84 prospectively compared the clinical assessment of fluid status by critical and intermediate care nurses and direct measurements of blood volume using pulse dye densitometry. The clinical assessment failed to accurately assess patients’ volume status. Using the same technique to measure circulating blood volume, this group showed that calculation of fluid balance does not provide adequate assessment of fluid status.83,84
Hemodynamic monitoring tools can help guide fluid replacement in this population. Mutoh et al94 randomized 160 patients within 24 hours of hemorrhage to receive early goal-directed fluid therapy (ie, preload volume and cardiac output monitored by transpulmonary thermodilution) vs standard therapy (ie, fluid balance or central venous pressure). Overall, no difference was found in the rates of delayed cerebral ischemia (33% vs 42%; P = .33) or favorable outcome (67% vs 57%; P = .22). However, in the subgroup of poor-grade patients (WFNS score 4 or 5), early goal-directed therapy was associated with a lower rate of delayed cerebral ischemia (5% vs 14%; P = .036) and with better functional outcomes at 3 months (52% vs 36%; P = .026).
Fluid restriction to treat hyponatremia in aneurysmal subarachnoid hemorrhage is no longer recommended because of the increased risk of cerebral infarction due to hypovolemic hypoperfusion.82
Prophylactic use of mineralocorticoids (eg, fludrocortisone, hydrocortisone) has been shown to limit natriuresis, hyponatremia, and the amount of fluid required to maintain euvolemia.95,96 Higher rates of hypokalemia and hyperglycemia, which can be easily treated, are the most common complications associated with this approach. Additionally, hypertonic saline (eg, 3% saline) can be used to correct hyponatremia in a setting of aneurysmal subarachnoid hemorrhage.79
Cardiac complications
Cardiac complications after subarachnoid hemorrhage are most likely related to sympathetic hyperactivity and catecholamine-induced myocyte dysfunction. The pathophysiology is complex, but cardiac complications have a significant negative impact on long-term outcome in these patients.97
Electrocardiographic changes and positive cardiac enzymes associated with aneurysmal subarachnoid hemorrhage have been extensively reported. More recently, data from studies of two-dimensional echocardiography have shown that subarachnoid hemorrhage can also be associated with significant wall-motion abnormalities and even overt cardiogenic shock.98–100
There is no specific curative therapy; the treatment is mainly supportive. Vasopressors and inotropes may be used for hemodynamic augmentation.
Pulmonary complications
Pulmonary complications occur in 20% to 30% of all aneurysmal subarachnoid hemorrhage patients and are associated with a higher risk of delayed cerebral ischemia and death. Common pulmonary complications in this population are mild acute respiratory distress syndrome (27%), hospital-acquired pneumonia (9%), cardiogenic pulmonary edema (8%), aspiration pneumonia (6%), neurogenic pulmonary edema (2%), and pulmonary embolism (1%).101–103
SUPPORTIVE CARE
Hyperthermia, hyperglycemia, and liberal use of transfusions have all been associated with longer stays in the intensive care unit and hospital, poorer neurologic outcomes, and higher mortality rates in patients with acute brain injury.104 Noninfectious fever is the most common systemic complication after subarachnoid hemorrhage.
Antipyretic drugs such as acetaminophen and ibuprofen are not very effective in reducing fever in the subarachnoid hemorrhage population, but should still be used as first-line therapy. The use of surface and intravascular devices can be considered when fevers do not respond to nonsteroidal anti-inflammatory drugs.
Although no prospective randomized trial has addressed the impact of induced normothermia on long-term outcome and mortality in aneurysmal subarachnoid hemorrhage patients, fever control has been shown to reduce cerebral metabolic distress, irrespective of intracranial pressure.105 Maintenance of normothermia (< 37.5°C) seems reasonable, especially in aneurysmal subarachnoid hemorrhage patients at risk of or with active delayed cerebral ischemia.106
Current guidelines3,32,69 strongly recommend avoiding hypoglycemia, defined as a serum glucose level less than 80 mg/dL, but suggest keeping the blood sugar level below 180 or 200 mg/dL.
At the moment, there is no clear threshold for transfusion in patients with aneurysmal subarachnoid hemorrhage. Current guidelines suggest keeping hemoglobin levels between 8 and 10 g/dL.3
Preventing venous thromboembolism
The incidence of venous thromboembolism after aneurysmal subarachnoid hemorrhage varies widely, from 1.5% to 18%.107 Active surveillance with venous Doppler ultrasonography has found asymptomatic deep vein thrombosis in up to 3.4% of poor-grade aneurysmal subarachnoid hemorrhage patients receiving pharmacologic thromboprophylaxis.108
In a retrospective study of 170 patients, our group showed that giving drugs to prevent venous thromboembolism (unfractionated heparin 5,000 IU subcutaneously every 12 hours or dalteparin 5,000 IU subcutaneously daily), starting within 24 hours of aneurysm treatment, could be safe.109 Fifty-eight percent of these patients had an external ventricular drain in place. One patient developed a major cerebral hemorrhagic complication and died while on unfractionated heparin; however, the patient was also on dual antiplatelet therapy with aspirin and clopidogrel.109
Current guidelines suggest that intermittent compression devices be applied in all patients before aneurysm treatment. Pharmacologic thromboprophylaxis with a heparinoid can be started 12 to 24 hours after aneurysm treatment.3,109
A TEAM APPROACH
Patients with subarachnoid hemorrhage need integrated care from different medical and nursing specialties. The best outcomes are achieved by systems that can focus as a team on the collective goal of quick intervention to secure the aneurysm, followed by measures to minimize secondary brain injury.
The modern concept of cerebral monitoring in a setting of subarachnoid hemorrhage should focus on brain perfusion rather than vascular diameter. Although the search continues for new diagnostic, prognostic, and therapeutic tools, there is no “silver bullet” that will help all patients. Instead, it is the systematic integration and application of many small advances that will ultimately lead to better outcomes.
ACKNOWLEDGMENT
This work was supported by research funding provided by the Bitove Foundation, which has been supportive of our clinical and research work for several years.
Aneurysmal subarachnoid hemorrhage is a devastating condition, with an estimated death rate of 30% during the initial episode.1,2 Approximately the same number of patients survive but leave the hospital with disabling neurologic deficits.3
However, better outcomes can be achieved by systems that are able to work as a team on the collective goal of quick intervention to secure the ruptured aneurysm, followed by the implementation of measures to minimize secondary brain injury. Although the search for new diagnostic, prognostic, and therapeutic modalities continues, it is clear that there exists no “silver bullet” that will help all patients. Instead, it is the systematic integration and application of small advances that will ultimately maximize the patient’s chances of survival and neurologic recovery.
This review focuses on the management of aneurysmal subarachnoid hemorrhage and its systemic and neurologic complications.
ANEURYSM IS THE MOST COMMON CAUSE OF SUBARACHNOID BLEEDING
Aneurysmal subarachnoid hemorrhage, ie, rupture of an intracranial aneurysm, flooding the subarachnoid space with blood, affects about 24,000 Americans each year.1,2 A ruptured aneurysm is the most common cause of subarachnoid hemorrhage, accounting for about 85% of cases. Less common causes include idiopathic benign perimesencephalic hemorrhage, arteriovenous malformation, dural arteriovenous fistula, and hemorrhagic mycotic aneurysm. These have their own natural history, pathophysiology, and specific treatment, and will not be addressed in this article.
Risk factors for aneurysmal subarachnoid hemorrhage include having a first-degree relative who had the disease, hypertension, smoking, and consuming more than 150 g of alcohol per week.4
CLINICAL PRESENTATION AND DIAGNOSIS
The key symptom of aneurysmal subarachnoid hemorrhage is the abrupt onset of severe headache that peaks in intensity over 1 hour,5 often described as “the worst headache of my life.” Headache is accompanied by brief loss of consciousness in 53% of cases (conversely, nearly half of patients maintain normal mental status), by nausea or vomiting in 77%, and by meningismus (neck pain or stiffness) in 35%.6
These clinical manifestations and risk factors have been incorporated into a decision rule:
Obtain brain imaging if the patient has acute headache reaching maximal intensity within 1 hour, associated with any of the following factors:
- Age 40 or older
- Neck pain or stiffness
- Witnessed loss of consciousness
- Onset during exertion
- “Thunderclap” headache (ie, instantly peaking pain)
- Limited neck flexion on examination.5
This decision rule has nearly 100% sensitivity for aneurysmal subarachnoid hemorrhage in clinical practice.5 All patients require brain imaging if they have a severe headache plus either abnormal neurologic findings (eg, a focal neurologic deficit) or a history of cerebral aneurysm.
Emergency physicians should have a low threshold for ordering noncontrast computed tomography (CT) of the head in patients with even mild symptoms suggesting aneurysmal subarachnoid hemorrhage. Failure to order CT is the most common diagnostic error in this situation.6 CT performed within 6 hours of headache onset is nearly 100% sensitive for this condition,7 but the sensitivity falls to 93% after the first 24 hours and to less than 60% after 5 days.8 In patients who have symptoms highly suggestive of aneurysmal subarachnoid hemorrhage but a normal CT, lumbar puncture is the next diagnostic step.
There are two alternatives to CT followed by lumbar puncture: ie, noncontrast CT followed by CT angiography,9,10 and magnetic resonance imaging followed by magnetic resonance angiography. In patients with suspicious clinical symptoms but negative CT results, CT followed by CT angiography can rule out aneurysmal subarachnoid hemorrhage with a 99% probability.9,10 However, CT followed by lumbar puncture remains the standard of care and carries a class I recommendation in the American Heart Association guidelines for ruling out subarachnoid hemorrhage.5
GRADING THE SEVERITY OF SUBARACHNOID HEMORRHAGE
Age, the thickness of the blood layer in the subarachnoid space, intraventricular hemorrhage and the findings of the neurologic examination at presentation are predictors of long-term outcomes in aneurysmal subarachnoid hemorrhage (Figure 1).

Different grading systems used in clinical practice are based on the findings on the initial neurologic examination and on the initial noncontrast CT (ie, the thickness of the blood, and whether intraventricular hemorrhage is present). Among the most widely used are those developed by Hunt and Hess12 and by the World Federation of Neurological Surgeons11 (WFNS), and the CT grading scales (Fisher13 or its modified version14) (Tables 1 and 2). With either the Hunt and Hess scale or the WFNS scale, the higher the score, the worse the patient’s probable outcome. Scores on both Fisher scales correlate with the risk of angiographic vasospasm. The higher the grade, the higher the risk of angiographic vasospasm.


The VASOGRADE score—a combination of the WFNS score and the modified Fisher scale—stratifies patients at risk of delayed cerebral ischemia, allowing for a tailored monitoring strategy.15 There are three variations:
- VASOGRADE green—Modified Fisher 1 or 2 and WFNS 1 or 2
- VASOGRADE yellow—Modified Fisher 3 or 4 and WFNS 1, 2, or 3
- VASOGRADE red—WFNS 4 or 5.
After the initial bleeding event, patients with aneurysmal subarachnoid hemorrhage are at high risk of delayed systemic and neurologic complications, with poor functional outcomes. Delayed cerebral ischemia holds the greatest risk of an unfavorable outcome and ultimately can lead to cerebral infarction, disability, and death.6,7
INITIAL MANAGEMENT
After aneurysmal subarachnoid hemorrhage is diagnosed, the initial management (Figure 2) includes appropriate medical prevention of rebleeding (which includes supportive care, blood pressure management, and, perhaps, the early use of a short course of an antifibrinolytic drug) and early transfer to a high-volume center for securing the aneurysm. The reported incidence of rebleeding varies from 5% to 22% in the first 72 hours. “Ultra-early” rebleeding (within 24 hours of hemorrhage) has been reported, with an incidence as high as 15% and a fatality rate around 70%. Patients with poor-grade aneurysmal subarachnoid hemorrhage, larger aneurysms, and “sentinel bleeds” are at higher risk of rebleeding.16
Outcomes are much better when patients are managed in a high-volume center, with a specialized neurointensive care unit17 and access to an interdisciplinary team.18 Regardless of the initial grade, patients with aneurysmal subarachnoid hemorrhage should be quickly transferred to a high-volume center, defined as one treating at least 35 cases per year, and the benefit is greater in centers treating more than 60 cases per year.19 The higher the caseload in any given hospital, the better the clinical outcomes in this population.20

Treating cerebral aneurysm: Clipping or coiling
Early aneurysm repair is generally considered the standard of care and the best strategy to reduce the risk of rebleeding. Further, early treatment may be associated with a lower risk of delayed cerebral ischemia21 and better outcomes.22
Three randomized clinical trials have compared surgical clipping and endovascular repair (placement of small metal coils within the aneurysm to promote clotting).
The International Subarachnoid Aneurysm Trial23 showed a reduction of 23% in relative risk and of 7% in absolute risk in patients who underwent endovascular treatment compared with surgery. The survival benefit persisted at a mean of 9 years (range 6–14 years), but with a higher annual rate of aneurysm recurrence in the coiling group (2.9% vs 0.9%).24 Of note, this trial included only patients with aneurysms deemed suitable for both coiling and clipping, so that the exclusion rate was high. Most of the patients presented with good-grade (WFNS score 1–3), small aneurysms (< 5 mm) in the anterior circulation.
A single-center Finnish study25 found no differences in rates of recovery, disability, and death at 1 year, comparing surgery and endovascular treatment. Additionally, survival rates at a mean follow-up of 39 months were similar, with no late recurrences or aneurysmal bleeding.
Lastly, the Barrow Ruptured Aneurysm Trial26,27 found that patients assigned to endovascular treatment had better 1-year neurologic outcomes, defined as a modified Rankin score of 2 or less. Importantly, 37.7% of patients originally assigned to endovascular treatment crossed over to surgical treatment. The authors then performed intention-to-treat and as-treated analyses. Either way, patients treated by endovascular means had better neurologic outcomes at 1 year. However, no difference in the relative risk reduction in worse outcome was found on 3-year follow-up, and patients treated surgically had higher rates of aneurysm obliteration and required less aneurysm retreatment, both of which were statistically significant.
The question that remains is not whether to clip or whether to coil, but whom to clip and whom to coil.28 That question must be answered on a patient-to-patient basis and requires the expertise of an interventional neuroradiologist and a vascular neurosurgeon—one of the reasons these patients are best cared for in high-volume centers providing such expertise.
MEDICAL PREVENTION OF REBLEEDING
Blood pressure management
There are no systematic data on the optimal blood pressure before securing an aneurysm. Early studies of hemodynamic augmentation in cases of ruptured untreated aneurysm reported rebleeding when the systolic blood pressure was allowed to rise above 160 mm Hg.29,30 A recent study evaluating hypertensive intracerebral hemorrhage revealed better functional outcomes with intensive lowering of blood pressure (defined as systolic blood pressure < 140 mm Hg) but no significant reduction in the combined rate of death or severe disability.31 It is difficult to know if these results can be extrapolated to patients with aneurysmal subarachnoid hemorrhage. Current guidelines3,32 say that before the aneurysm is treated, the systolic pressure should be lower than 160 mm Hg.
There is no specific drug of choice, but a short-acting, titratable medication is preferable. Nicardipine is a very good option, and labetalol might be an appropriate alternative.33 Once the aneurysm is secured, all antihypertensive drugs should be held. Hypertension should not be treated unless the patient has clinical signs of a hypertensive crisis, such as flash pulmonary edema, myocardial infarction, or hypertensive encephalopathy.
Antifibrinolytic therapy
The role of antifibrinolytic therapy in aneurysmal subarachnoid hemorrhage is controversial and has been studied in 10 clinical trials. In a Swedish study,34 early use of tranexamic acid (1 g intravenously over 10 minutes followed by 1 g every 6 hours for a maximum of 24 hours) reduced the rebleeding rate substantially, from 10.8% to 2.4%, with an 80% reduction in the mortality rate from ultra-early rebleeding. However, a recent Cochrane review that included this study found no overall benefit.35
An ongoing multicenter randomized trial in the Netherlands will, we hope, answer this question in the near future.36 At present, some centers would consider a short course of tranexamic acid before aneurysm treatment.
DIAGNOSIS AND TREATMENT OF COMPLICATIONS
Medical complications are extremely common after aneurysmal subarachnoid hemorrhage. Between 75% and 100% of patients develop some type of systemic or further neurologic derangement, which in turn has a negative impact on the long-term outcome.37,38 In the first 72 hours, rebleeding is the most feared complication, and as mentioned previously, appropriate blood pressure management and early securing of the aneurysm minimize its risk.
NEUROLOGIC COMPLICATIONS
Hydrocephalus
Hydrocephalus is the most common early neurologic complication after aneurysmal subarachnoid hemorrhage, with an overall incidence of 50%.39 Many patients with poor-grade aneurysmal subarachnoid hemorrhage and patients whose condition deteriorates due to worsening of hydrocephalus require the insertion of an external ventricular drain (Figure 1).
Up to 30% of patients who have a poor-grade aneurysmal subarachnoid hemorrhage improve neurologically with cerebrospinal fluid drainage.40 An external ventricular drain can be safely placed, even before aneurysm treatment, and placement does not appear to increase the risk of rebleeding.39,41 After placement, rapid weaning from the drain (clamping within 24 hours of insertion) is safe, decreases length of stay in the intensive care unit and hospital, and may be more cost-effective than gradual weaning over 96 hours.42
Increased intracranial pressure
Intracranial hypertension is another potential early complication, and is frequently due to the development of hydrocephalus, cerebral edema, or rebleeding. The treatment of increased intracranial pressure does not differ from the approach used in managing severe traumatic brain injury, which includes elevating the head of the bed, sedation, analgesia, normoventilation, and cerebrospinal fluid drainage.
Hypertonic saline has been tested in several studies that were very small but nevertheless consistently showed control of intracranial pressure levels and improvement in cerebral blood flow measured by xenon CT.43–47 Two of these studies even showed better outcomes at discharge.43,44 However, the small number of patients prevents any meaningful conclusion regarding the use of hypertonic saline and functional outcomes.
Barbiturates, hypothermia, and decompressive craniectomy could be tried in refractory cases.48 Seule et al49 evaluated the role of therapeutic hypothermia with or without barbiturate coma in 100 patients with refractory intracranial hypertension. Only 13 patients received hypothermia by itself. At 1 year, 32 patients had achieved a good functional outcome (Glasgow Outcome Scale score 4 or 5). The remaining patients were severely disabled or had died. Of interest, the median duration of hypothermia was 7 days, and 93% of patients developed some medical complication such as electrolyte disorders (77%), pneumonia (52%), thrombocytopenia (47%), or septic shock syndrome (40%). Six patients died as a consequence of one of these complications.
Decompressive craniectomy can be life-saving in patients with refractory intracranial hypertension. However, most of these patients will die or remain severely disabled or comatose.50
Seizure prophylaxis is controversial
Seizures can occur at the onset of intracranial hemorrhage, perioperatively, or later (ie, after the first week). The incidence varied considerably in different reports, ranging from 4% to 26%.51 Seizures occurring perioperatively, ie, after hospital admission, are less frequent and are usually the manifestation of aneurysm rebleeding.24
Seizure prophylaxis remains controversial, especially because the use of phenytoin is associated with increased incidence of cerebral vasospasm, infarction, and worse cognitive outcomes after aneurysmal subarachnoid hemorrhage.52,53 Therefore, routine prophylactic use of phenytoin is not recommended in these patients,3 although the effect of other antiepileptic drugs is less studied and less clear. Patients may be considered for this therapy if they have multiple risk factors for seizures, such as intraparenchymal hematoma, advanced age (> 65), middle cerebral artery aneurysm, craniotomy for aneurysm clipping, and a short course (≤ 72 hours) of an antiepileptic drug other than phenytoin, especially while the aneurysm is unsecured.3
Levetiracetam may be an alternative to phenytoin, having better pharmacodynamic and kinetic profiles, minimal protein binding, and absence of hepatic metabolism, resulting in a very low risk of drug interaction and better tolerability.54,55 Because of these advantages, levetiracetam has become the drug of choice in several centers treating aneurysmal subarachnoid hemorrhage in the United States.
Addressing this question, a survey was sent to 25 high-volume aneurysmal subarachnoid hemorrhage academic centers in the United States. All 25 institutions answered the survey, and interestingly, levetiracetam was the first-line agent for 16 (94%) of the 17 responders that used prophylaxis, while only 1 used phenytoin as the agent of choice.56
A retrospective cohort study by Murphy-Human et al57 showed that a short course of levetiracetam (≤ 72 hours) was associated with higher rates of in-hospital seizures compared with an extended course of phenytoin (eg, entire hospital stay). However, the study did not address functional outcomes.57
Continuous electroencephalographic monitoring may be considered in comatose patients, in patients requiring controlled ventilation and sedation, or in patients with unexplained alteration in consciousness. In one series of patients with aneurysmal subarachnoid hemorrhage who received continuous monitoring, the incidence of nonconvulsive status epilepticus was 19%, with an associated mortality rate of 100%.58
Continuous quantitative electroencephalography is useful to monitor and to detect angiographic vasospasm and delayed cerebral ischemia. Relative alpha variability and the alpha-delta ratio decrease with ischemia, and this effect can precede angiographic vasospasm by 3 days.59,60
Delayed cerebral ischemia
Delayed cerebral ischemia is defined as the occurrence of focal neurologic impairment, or a decrease of at least 2 points on the Glasgow Coma Scale that lasts for at least 1 hour, is not apparent immediately after aneurysm occlusion, and not attributable to other causes (eg, hyponatremia, fever).61
Classically, neurologic deficits that occurred within 2 weeks of aneurysm rupture were ascribed to reduced cerebral blood flow caused by delayed large-vessel vasospasm causing cerebral ischemia.62 However, perfusion abnormalities have also been observed with either mild or no demonstrable vasospasm.63 Almost 70% of patients who survive the initial hemorrhage develop some degree of angiographic vasospasm. However, only 30% of those patients will experience symptoms.
In addition to vasospasm of large cerebral arteries, impaired autoregulation and early brain injury within the first 72 hours following subarachnoid hemorrhage may play important roles in the development of delayed cerebral ischemia.64 Therefore, the modern concept of delayed cerebral ischemia monitoring should focus on cerebral perfusion rather than vessel diameter measurements. This underscores the importance of comprehensive, standardized monitoring techniques that provide information not only on microvasculature, but also at the level of the microcirculation, with information on perfusion, oxygen utilization and extraction, and autoregulation.
Although transcranial Doppler has been the most commonly applied tool to monitor for angiographic vasospasm, it has a low sensitivity and negative predictive value.37 It is nevertheless a useful technique to monitor good-grade aneurysmal subarachnoid hemorrhage patients (WFNS score 1–3) combined with frequent neurologic examinations (Figure 3).

CT angiography is a good noninvasive alternative to digital subtraction angiography. However, it tends to overestimate the degree of vasoconstriction and does not provide information about perfusion and autoregulation.65 Nevertheless, CT angiography combined with a CT perfusion scan can add information about autoregulation and cerebral perfusion and has been shown to be more sensitive for the diagnosis of angiographic vasospasm than transcranial Doppler and digital subtraction angiography (Figure 4).

Patients with a poor clinical condition (WFNS score 4 or 5) or receiving continuous sedation constitute a challenge in monitoring for delayed neurologic deterioration. Neurologic examination is not sensitive enough in this setting to detect subtle changes. In these specific and challenging circumstances, multimodality neuromonitoring may be useful in the early detection of delayed cerebral ischemia and may help guide therapy.67
Several noninvasive and invasive techniques have been studied to monitor patients at risk of delayed cerebral ischemia after subarachnoid hemorrhage.66 These include continuous electroencephalography, brain tissue oxygenation monitoring (Ptio2), cerebral microdialysis, thermal diffusion flowmetry, and near-infrared spectroscopy. Of these techniques, Ptio2, cerebral microdialysis, and continuous electroencephalography (see discussion of seizure prophylaxis above) have been more extensively studied. However, most of the studies were observational and very small, limiting any recommendations for using these techniques in routine clinical practice.68
Ptio2 is measured by inserting an intraparenchymal oxygen-sensitive microelectrode, and microdialysis requires a microcatheter with a semipermeable membrane that allows small soluble substances to cross it into the dialysate. These substances, which include markers of ischemia (ie, glucose, lactate, and pyruvate), excitotoxins (ie, glutamate and aspartate), and membrane cell damage products (ie, glycerol), can be measured. Low Ptio2 values (< 15 mm Hg) and abnormal mycrodialysate findings (eg, glucose < 0.8 mmol/L, lactate-to-pyruvate ratio > 40) have both been associated with cerebral ischemic events and poor outcome.68
Preventing delayed cerebral ischemia
Oral nimodipine 60 mg every 4 hours for 21 days, started on admission, carries a class I, level of evidence A recommendation in the management of aneurysmal subarachnoid hemorrhage.3,32,69 It improves clinical outcome despite having no effect on the risk of angiographic vasospasm. The mechanism of improved outcome is unclear, but the effect may be a neuroprotective phenomenon limiting the extension of delayed cerebral ischemia.70
If hypotension occurs, the dose can be lowered to 30 mg every 2 hours. Whether to discontinue nimodipine in this situation is controversial. Of note, the clinical benefits of nimodipine have not been replicated with other calcium channel blockers (eg, nicardipine).71
Prophylactic hyperdynamic fluid therapy, known as “triple-H” (hypervolemia, hemodilution, and hypertension) was for years the mainstay of treatment in preventing delayed cerebral ischemia due to vasospasm. However, the clinical data supporting this intervention have been called into question, as analysis of two trials found that hypervolemia did not improve outcomes or reduce the incidence of delayed cerebral ischemia, and in fact increased the rate of complications.72,73 Based on these findings, current guidelines recommend maintaining euvolemia rather than prophylactic hypervolemia in patients with aneurysmal subarachnoid hemorrhage.3,32,69
TREATING DELAYED CEREBRAL ISCHEMIA
Hemodynamic augmentation
In patients with neurologic deterioration due to delayed cerebral ischemia, hemodynamic augmentation is the cornerstone of treatment. This is done according to a protocol, started early, involving specific physiologic goals, clinical improvement, and escalation to invasive therapies in a timely fashion in patients at high risk of further neurologic insult (Figure 5).

The physiologic goal is to increase the delivery of oxygen and glucose to the ischemic brain. Hypertension seems to be the most effective component of hemodynamic augmentation regardless of volume status, increasing cerebral blood flow and brain tissue oxygenation, with reversal of delayed cerebral ischemic symptoms in up to two-thirds of treated patients.74,75 However, this information comes from very small studies, with no randomized trials of induced hypertension available.
The effect of a normal saline fluid bolus in patients suspected of having delayed cerebral ischemia has been shown to increase cerebral blood flow in areas of cerebral ischemia.74 If volume augmentation fails to improve the neurologic status, the next step is to artificially induce hypertension using vasopressors. The blood pressure target should be based on clinical improvement. A stepwise approach is reasonable in this situation, and the lowest level of blood pressure at which there is a complete reversal of the new focal neurologic deficit should be maintained.3,29
Inotropic agents such as dobutamine or milrinone can be considered as alternatives in patients who have new neurologic deficits that are refractory to fluid boluses and vasopressors, or in a setting of subarachnoid hemorrhage-induced cardiomyopathy.76,77
Once the neurologic deficit is reversed by hemodynamic augmentation, the blood pressure should be maintained for 48 to 72 hours at the level that reversed the deficit completely, carefully reassessed thereafter, and the patient weaned slowly. Unruptured unsecured aneurysms should not prevent blood pressure augmentation in a setting of delayed cerebral ischemia if the culprit aneurysm is treated.3,32 If the ruptured aneurysm has not been secured, careful blood pressure augmentation can be attempted, keeping in mind that hypertension (> 160/95 mm Hg) is a risk factor for fatal aneurysm rupture.
Endovascular management of delayed cerebral ischemia
When medical augmentation fails to completely reverse the neurologic deficits, endovascular treatment can be considered. Although patients treated early in the course of delayed cerebral ischemia have better neurologic recovery, prophylactic endovascular treatment in asymptomatic patients, even if angiographic signs of spasm are present, does not improve clinical outcomes and carries the risk of fatal arterial rupture.78
SYSTEMIC COMPLICATIONS
Hyponatremia and hypovolemia
Aneurysmal subarachnoid hemorrhage is commonly associated with abnormalities of fluid balance and electrolyte derangements. Hyponatremia (serum sodium < 135 mmol/L) occurs in 30% to 50% of patients, while the rate of hypovolemia (decreased circulating blood volume) ranges from 17% to 30%.79 Both can negatively affect long-term outcomes.80,81
Decreased circulating blood volume is a well-described contributor to delayed cerebral ischemia and cerebral infarction after aneurysmal subarachnoid hemorrhage.80–82 Clinical variables such as heart rate, blood pressure, fluid balance, and serum sodium concentration are usually the cornerstones of intravascular volume status assessment. However, these variables correlate poorly with measured circulating blood volumes in those with aneurysmal subarachnoid hemorrhage.83,84
The mechanisms responsible for the development of hyponatremia and hypovolemia after aneurysmal subarachnoid hemorrhage are not completely understood. Several factors have been described and may contribute to the increased natriuresis and, hence, to a reduction in circulating blood volume: increased circulating natriuretic peptide concentrations,85–87 sympathetic nervous system hyperactivation,88 and hyperreninemic hypo-
aldosteronism syndrome.89,90
Lastly, the cerebral salt wasting syndrome, described in the 1950s,91 was thought to be a key mechanism in the development of hyponatremia and hypovolemia after aneurysmal subarachnoid hemorrhage. In contrast to the syndrome of inappropriate antidiuretic hormone, which is characterized by hyponatremia with a normal or slightly elevated intravascular volume, the characteristic feature of cerebral salt wasting syndrome is the development of hyponatremia in a setting of intravascular volume depletion.92 In critically ill neurologic and neurosurgical patients, this differential diagnosis is very difficult, especially in those with aneurysmal subarachnoid hemorrhage in whom the clinical assessment of fluid status is not reliable. These two syndromes might coexist and contribute to the development of hyponatremia after aneurysmal subarachnoid hemorrhage.92,93
Hoff et al83,84 prospectively compared the clinical assessment of fluid status by critical and intermediate care nurses and direct measurements of blood volume using pulse dye densitometry. The clinical assessment failed to accurately assess patients’ volume status. Using the same technique to measure circulating blood volume, this group showed that calculation of fluid balance does not provide adequate assessment of fluid status.83,84
Hemodynamic monitoring tools can help guide fluid replacement in this population. Mutoh et al94 randomized 160 patients within 24 hours of hemorrhage to receive early goal-directed fluid therapy (ie, preload volume and cardiac output monitored by transpulmonary thermodilution) vs standard therapy (ie, fluid balance or central venous pressure). Overall, no difference was found in the rates of delayed cerebral ischemia (33% vs 42%; P = .33) or favorable outcome (67% vs 57%; P = .22). However, in the subgroup of poor-grade patients (WFNS score 4 or 5), early goal-directed therapy was associated with a lower rate of delayed cerebral ischemia (5% vs 14%; P = .036) and with better functional outcomes at 3 months (52% vs 36%; P = .026).
Fluid restriction to treat hyponatremia in aneurysmal subarachnoid hemorrhage is no longer recommended because of the increased risk of cerebral infarction due to hypovolemic hypoperfusion.82
Prophylactic use of mineralocorticoids (eg, fludrocortisone, hydrocortisone) has been shown to limit natriuresis, hyponatremia, and the amount of fluid required to maintain euvolemia.95,96 Higher rates of hypokalemia and hyperglycemia, which can be easily treated, are the most common complications associated with this approach. Additionally, hypertonic saline (eg, 3% saline) can be used to correct hyponatremia in a setting of aneurysmal subarachnoid hemorrhage.79
Cardiac complications
Cardiac complications after subarachnoid hemorrhage are most likely related to sympathetic hyperactivity and catecholamine-induced myocyte dysfunction. The pathophysiology is complex, but cardiac complications have a significant negative impact on long-term outcome in these patients.97
Electrocardiographic changes and positive cardiac enzymes associated with aneurysmal subarachnoid hemorrhage have been extensively reported. More recently, data from studies of two-dimensional echocardiography have shown that subarachnoid hemorrhage can also be associated with significant wall-motion abnormalities and even overt cardiogenic shock.98–100
There is no specific curative therapy; the treatment is mainly supportive. Vasopressors and inotropes may be used for hemodynamic augmentation.
Pulmonary complications
Pulmonary complications occur in 20% to 30% of all aneurysmal subarachnoid hemorrhage patients and are associated with a higher risk of delayed cerebral ischemia and death. Common pulmonary complications in this population are mild acute respiratory distress syndrome (27%), hospital-acquired pneumonia (9%), cardiogenic pulmonary edema (8%), aspiration pneumonia (6%), neurogenic pulmonary edema (2%), and pulmonary embolism (1%).101–103
SUPPORTIVE CARE
Hyperthermia, hyperglycemia, and liberal use of transfusions have all been associated with longer stays in the intensive care unit and hospital, poorer neurologic outcomes, and higher mortality rates in patients with acute brain injury.104 Noninfectious fever is the most common systemic complication after subarachnoid hemorrhage.
Antipyretic drugs such as acetaminophen and ibuprofen are not very effective in reducing fever in the subarachnoid hemorrhage population, but should still be used as first-line therapy. The use of surface and intravascular devices can be considered when fevers do not respond to nonsteroidal anti-inflammatory drugs.
Although no prospective randomized trial has addressed the impact of induced normothermia on long-term outcome and mortality in aneurysmal subarachnoid hemorrhage patients, fever control has been shown to reduce cerebral metabolic distress, irrespective of intracranial pressure.105 Maintenance of normothermia (< 37.5°C) seems reasonable, especially in aneurysmal subarachnoid hemorrhage patients at risk of or with active delayed cerebral ischemia.106
Current guidelines3,32,69 strongly recommend avoiding hypoglycemia, defined as a serum glucose level less than 80 mg/dL, but suggest keeping the blood sugar level below 180 or 200 mg/dL.
At the moment, there is no clear threshold for transfusion in patients with aneurysmal subarachnoid hemorrhage. Current guidelines suggest keeping hemoglobin levels between 8 and 10 g/dL.3
Preventing venous thromboembolism
The incidence of venous thromboembolism after aneurysmal subarachnoid hemorrhage varies widely, from 1.5% to 18%.107 Active surveillance with venous Doppler ultrasonography has found asymptomatic deep vein thrombosis in up to 3.4% of poor-grade aneurysmal subarachnoid hemorrhage patients receiving pharmacologic thromboprophylaxis.108
In a retrospective study of 170 patients, our group showed that giving drugs to prevent venous thromboembolism (unfractionated heparin 5,000 IU subcutaneously every 12 hours or dalteparin 5,000 IU subcutaneously daily), starting within 24 hours of aneurysm treatment, could be safe.109 Fifty-eight percent of these patients had an external ventricular drain in place. One patient developed a major cerebral hemorrhagic complication and died while on unfractionated heparin; however, the patient was also on dual antiplatelet therapy with aspirin and clopidogrel.109
Current guidelines suggest that intermittent compression devices be applied in all patients before aneurysm treatment. Pharmacologic thromboprophylaxis with a heparinoid can be started 12 to 24 hours after aneurysm treatment.3,109
A TEAM APPROACH
Patients with subarachnoid hemorrhage need integrated care from different medical and nursing specialties. The best outcomes are achieved by systems that can focus as a team on the collective goal of quick intervention to secure the aneurysm, followed by measures to minimize secondary brain injury.
The modern concept of cerebral monitoring in a setting of subarachnoid hemorrhage should focus on brain perfusion rather than vascular diameter. Although the search continues for new diagnostic, prognostic, and therapeutic tools, there is no “silver bullet” that will help all patients. Instead, it is the systematic integration and application of many small advances that will ultimately lead to better outcomes.
ACKNOWLEDGMENT
This work was supported by research funding provided by the Bitove Foundation, which has been supportive of our clinical and research work for several years.
- van Gijn J, Rinkel GJ. Subarachnoid haemorrhage: diagnosis, causes and management. Brain 2001; 124:249–278.
- Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 2014; 129:e28–e292.
- Diringer MN, Bleck TP, Claude Hemphill J 3rd, et al; Neurocritical Care Society. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society’s Multidisciplinary Consensus Conference. Neurocrit Care 2011; 15:211–240.
- Feigin VL, Rinkel GJ, Lawes CM, et al. Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies. Stroke 2005; 36:2773–2780.
- Perry JJ, Stiell IG, Sivilotti ML, et al. Clinical decision rules to rule out subarachnoid hemorrhage for acute headache. JAMA 2013; 310:1248–1255.
- Kowalski RG, Claassen J, Kreiter KT, et al. Initial misdiagnosis and outcome after subarachnoid hemorrhage. JAMA 2004; 291:866–869.
- Perry JJ, Stiell IG, Sivilotti ML, et al. Sensitivity of computed tomography performed within six hours of onset of headache for diagnosis of subarachnoid haemorrhage: prospective cohort study. BMJ 2011; 343:d4277.
- van Gijn J, van Dongen KJ. The time course of aneurysmal haemorrhage on computed tomograms. Neuroradiology 1982; 23:153–156.
- McCormack RF, Hutson A. Can computed tomography angiography of the brain replace lumbar puncture in the evaluation of acute-onset headache after a negative noncontrast cranial computed tomography scan? Acad Emerg Med 2010; 17:444–451.
- Agid R, Andersson T, Almqvist H, et al. Negative CT angiography findings in patients with spontaneous subarachnoid hemorrhage: when is digital subtraction angiography still needed? AJNR Am J Neuroradiol 2010; 31:696–705.
- Teasdale GM, Drake CG, Hunt W, et al. A universal subarachnoid hemorrhage scale: report of a committee of the World Federation of Neurosurgical Societies. J Neurol Neurosurg Psychiatry 1988; 51:1457.
- Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 1968; 28:14–20.
- Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 1980; 6:1–9.
- Frontera JA, Claassen J, Schmidt JM, et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified Fisher scale. Neurosurgery 2006; 59:21–27.
- de Oliveira Manoel AL, Turkel-Parrella D, Kouzmina E, et al. The VASOGRADE—a simple, reliable grading scale for aneurysmal subarachnoid hemorrhage. Neurology 2014; 82(suppl 10): P5.123.
- Naidech AM, Janjua N, Kreiter KT, et al. Predictors and impact of aneurysm rebleeding after subarachnoid hemorrhage. Arch Neurol 2005; 62:410–416.
- Rincon F, Mayer SA. Neurocritical care: a distinct discipline? Curr Opin Crit Care 2007; 13:115–121.
- Rabinstein AA, Lanzino G, Wijdicks EF. Multidisciplinary management and emerging therapeutic strategies in aneurysmal subarachnoid haemorrhage. Lancet Neurol 2010; 9:504–519.
- Vespa P, Diringer MN; Participants in the International Multi-Disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage. High-volume centers. Neurocrit Care 2011; 15:369–372.
- McNeill L, English SW, Borg N, Matta BF, Menon DK. Effects of institutional caseload of subarachnoid hemorrhage on mortality: a secondary analysis of administrative data. Stroke 2013; 44:647–652.
- Dorhout Mees SM, Molyneux AJ, Kerr RS, Algra A, Rinkel GJ. Timing of aneurysm treatment after subarachnoid hemorrhage: relationship with delayed cerebral ischemia and poor outcome. Stroke 2012; 43:2126–2129.
- Laidlaw JD, Siu KH. Ultra-early surgery for aneurysmal subarachnoid hemorrhage: outcomes for a consecutive series of 391 patients not selected by grade or age. J Neurosurg 2002; 97:250–259.
- Molyneux A, Kerr R, Stratton I, et al; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2,143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002; 360:1267–1274.
- Molyneux AJ, Kerr RS, Yu LM, et al; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2,143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005; 366:809–817.
- Koivisto T, Vanninen R, Hurskainen H, Saari T, Hernesniemi J, Vapalahti M. Outcomes of early endovascular versus surgical treatment of ruptured cerebral aneurysms. A prospective randomized study. Stroke 2000; 31:2369–2377.
- McDougall CG, Spetzler RF, Zabramski JM, et al. The Barrow Ruptured Aneurysm Trial. J Neurosurg 2012; 116:135–144.
- Spetzler RF, McDougall CG, Albuquerque FC, et al. The Barrow Ruptured Aneurysm Trial: 3-year results. J Neurosurg 2013; 119:146–157.
- Connolly ES Jr, Meyers PM. Cerebral aneurysms: to clip or to coil? That is no longer the question. Nat Rev Neurol 2009; 5:412–413.
- Kassell NF, Peerless SJ, Durward QJ, Beck DW, Drake CG, Adams HP. Treatment of ischemic deficits from vasospasm with intravascular volume expansion and induced arterial hypertension. Neurosurgery 1982; 11:337–343.
- Otsubo H, Takemae T, Inoue T, Kobayashi S, Sugita K. Normovolaemic induced hypertension therapy for cerebral vasospasm after subarachnoid haemorrhage. Acta Neurochir (Wien) 1990; 103:18–26.
- Anderson CS, Heeley E, Huang Y, et al; INTERACT2 Investigators. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med 2013; 368:2355–2365.
- Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al; American Heart Association Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2012; 43:1711–1737.
- Ortega-Gutierrez S, Thomas J, Reccius A, et al. Effectiveness and safety of nicardipine and labetalol infusion for blood pressure management in patients with intracerebral and subarachnoid hemorrhage. Neurocrit Care 2013; 18:13–19.
- Hillman J, Fridriksson S, Nilsson O, Yu Z, Saveland H, Jakobsson KE. Immediate administration of tranexamic acid and reduced incidence of early rebleeding after aneurysmal subarachnoid hemorrhage: a prospective randomized study. J Neurosurg 2002; 97:771–778.
- Baharoglu MI, Germans MR, Rinkel GJ, et al. Antifibrinolytic therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 2013; 8:CD001245.
- Germans MR, Post R, Coert BA, Rinkel GJ, Vandertop WP, Verbaan D. Ultra-early tranexamic acid after subarachnoid hemorrhage (ULTRA): study protocol for a randomized controlled trial. Trials 2013; 14:143.
- Sloan MA, Alexandrov AV, Tegeler CH, et al; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Assessment: transcranial Doppler ultrasonography: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2004; 62:1468–1481.
- Wartenberg KE, Schmidt JM, Claassen J, et al. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med 2006; 34:617–623;
- Hellingman CA, van den Bergh WM, Beijer IS, et al. Risk of rebleeding after treatment of acute hydrocephalus in patients with aneurysmal subarachnoid hemorrhage. Stroke 2007; 38:96–99.
- Ransom ER, Mocco J, Komotar RJ, et al. External ventricular drainage response in poor grade aneurysmal subarachnoid hemorrhage: effect on preoperative grading and prognosis. Neurocrit Care 2007; 6:174–180.
- McIver JI, Friedman JA, Wijdicks EF, et al. Preoperative ventriculostomy and rebleeding after aneurysmal subarachnoid hemorrhage. J Neurosurg 2002; 97:1042–1044.
- Klopfenstein JD, Kim LJ, Feiz-Erfan I, et al. Comparison of rapid and gradual weaning from external ventricular drainage in patients with aneurysmal subarachnoid hemorrhage: a prospective randomized trial. J Neurosurg 2004; 100:225–229.
- Tseng MY, Al-Rawi PG, Czosnyka M, et al. Enhancement of cerebral blood flow using systemic hypertonic saline therapy improves outcome in patients with poor-grade spontaneous subarachnoid hemorrhage. J Neurosurg 2007; 107:274–282.
- Al-Rawi PG, Tseng MY, Richards HK, et al. Hypertonic saline in patients with poor-grade subarachnoid hemorrhage improves cerebral blood flow, brain tissue oxygen, and pH. Stroke 2010; 41:122–128.
- Tseng MY, Al-Rawi PG, Pickard JD, Rasulo FA, Kirkpatrick PJ. Effect of hypertonic saline on cerebral blood flow in poor-grade patients with subarachnoid hemorrhage. Stroke 2003; 34:1389–1396.
- Bentsen G, Breivik H, Lundar T, Stubhaug A. Hypertonic saline (7.2%) in 6% hydroxyethyl starch reduces intracranial pressure and improves hemodynamics in a placebo-controlled study involving stable patients with subarachnoid hemorrhage. Crit Care Med 2006; 34:2912–2917.
- Suarez JI, Qureshi AI, Parekh PD, et al. Administration of hypertonic (3%) sodium chloride/acetate in hyponatremic patients with symptomatic vasospasm following subarachnoid hemorrhage. J Neurosurg Anesthesiol 1999; 11:178–184.
- Stevens RD, Huff JS, Duckworth J, Papangelou A, Weingart SD, Smith WS. Emergency neurological life support: intracranial hypertension and herniation. Neurocrit Care 2012;17(suppl 1):S60–S65.
- Seule MA, Muroi C, Mink S, Yonekawa Y, Keller E. Therapeutic hypothermia in patients with aneurysmal subarachnoid hemorrhage, refractory intracranial hypertension, or cerebral vasospasm. Neurosurgery 2009; 64:86–93.
- Otani N, Takasato Y, Masaoka H, et al. Surgical outcome following decompressive craniectomy for poor-grade aneurysmal subarachnoid hemorrhage in patients with associated massive intracerebral or Sylvian hematomas. Cerebrovasc Dis 2008; 26:612–617.
- Lanzino G, D’Urso PI, Suarez J; Participants in the International Multi-Disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage. Seizures and anticonvulsants after aneurysmal subarachnoid hemorrhage. Neurocrit Care 2011; 15:247–256.
- Naidech AM, Kreiter KT, Janjua N, et al. Phenytoin exposure is associated with functional and cognitive disability after subarachnoid hemorrhage. Stroke 2005; 36:583–587.
- Rosengart AJ, Huo JD, Tolentino J, et al. Outcome in patients with subarachnoid hemorrhage treated with antiepileptic drugs. J Neurosurg 2007; 107:253–260.
- Shah D, Husain AM. Utility of levetiracetam in patients with subarachnoid hemorrhage. Seizure 2009; 18:676–679.
- Patsalos PN. Pharmacokinetic profile of levetiracetam: toward ideal characteristics. Pharmacol Ther 2000; 85:77–85.
- Dewan MC, Mocco J. Current practice regarding seizure prophylaxis in aneurysmal subarachnoid hemorrhage across academic centers. J Neurointerv Surg 2014 Jan 28. doi: 10.1136/neurintsurg-2013-011075 [Epub ahead of print]
- Murphy-Human T, Welch E, Zipfel G, Diringer MN, Dhar R. Comparison of short-duration levetiracetam with extended-course phenytoin for seizure prophylaxis after subarachnoid hemorrhage. World Neurosurg 2011; 75:269–274.
- Dennis LJ, Claassen J, Hirsch LJ, Emerson RG, Connolly ES, Mayer SA. Nonconvulsive status epilepticus after subarachnoid hemorrhage. Neurosurgery 2002; 51:1136–1144.
- Vespa PM, Nuwer MR, Juhász C, et al. Early detection of vasospasm after acute subarachnoid hemorrhage using continuous EEG ICU monitoring. Electroencephalogr Clin Neurophysiol 1997; 103:607–615.
- Claassen J, Hirsch LJ, Kreiter KT, et al. Quantitative continuous EEG for detecting delayed cerebral ischemia in patients with poor-grade subarachnoid hemorrhage. Clin Neurophysiol 2004; 115:2699–2710.
- Vergouwen MD, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 2010; 41:2391–2395.
- Kelly PJ, Gorten RJ, Grossman RG, Eisenberg HM. Cerebral perfusion, vascular spasm, and outcome in patients with ruptured intracranial aneurysms. J Neurosurg 1977; 47:44–49.
- Aralasmak A, Akyuz M, Ozkaynak C, Sindel T, Tuncer R. CT angiography and perfusion imaging in patients with subarachnoid hemorrhage: correlation of vasospasm to perfusion abnormality. Neuroradiology 2009; 51:85–93.
- Sabri M, Lass E, Macdonald RL. Early brain injury: a common mechanism in subarachnoid hemorrhage and global cerebral ischemia. Stroke Res Treat 2013 Feb 28. doi: 10.1155/2013/394036 [Epub 2013 ahead of print]
- Yoon DY, Choi CS, Kim KH, Cho BM. Multidetector-row CT angiography of cerebral vasospasm after aneurysmal subarachnoid hemorrhage: comparison of volume-rendered images and digital subtraction angiography. AJNR Am J Neuroradiol 2006; 27:370–377.
- Wintermark M1, Ko NU, Smith WS, Liu S, Higashida RT, Dillon WP. Vasospasm after subarachnoid hemorrhage: utility of perfusion CT and CT angiography on diagnosis and management. AJNR Am J Neuroradiol 2006; 27:26–34.
- Helbok R, Madineni RC, Schmidt MJ, et al. Intracerebral monitoring of silent infarcts after subarachnoid hemorrhage. Neurocrit Care 2011; 14:162–167.
- Hänggi D; Participants in the International Multi-Disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage. Monitoring and detection of vasospasm II: EEG and invasive monitoring. Neurocrit Care 2011; 15:318–323.
- Steiner T, Juvela S, Unterberg A, Jung C, Forsting M, Rinkel G; European Stroke Organization. European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis 2013; 35:93–112.
- Pickard JD, Murray GD, Illingworth R, et al. Effect of oral nimodipine on cerebral infarction and outcome after subarachnoid haemorrhage: British aneurysm nimodipine trial. BMJ 1989; 298:636–642.
- Dorhout Mees SM, Rinkel GJ, Feigin VL, et al. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 2007; 3:CD000277.
- Lennihan L, Mayer SA, Fink ME, et al. Effect of hypervolemic therapy on cerebral blood flow after subarachnoid hemorrhage: a randomized controlled trial. Stroke 2000; 31:383–391.
- Egge A, Waterloo K, Sjøholm H, Solberg T, Ingebrigtsen T, Romner B. Prophylactic hyperdynamic postoperative fluid therapy after aneurysmal subarachnoid hemorrhage: a clinical, prospective, randomized, controlled study. Neurosurgery 2001; 49:593–606.
- Jost SC, Diringer MN, Zazulia AR, et al. Effect of normal saline bolus on cerebral blood flow in regions with low baseline flow in patients with vasospasm following subarachnoid hemorrhage. J Neurosurg 2005; 103:25–30.
- Muizelaar JP, Becker DP. Induced hypertension for the treatment of cerebral ischemia after subarachnoid hemorrhage. Direct effect on cerebral blood flow. Surg Neurol 1986; 25:317–325.
- Levy ML, Rabb CH, Zelman V, Giannotta SL. Cardiac performance enhancement from dobutamine in patients refractory to hypervolemic therapy for cerebral vasospasm. J Neurosurg 1993; 79:494–499.
- Lannes M, Teitelbaum J, del Pilar Cortés M, Cardoso M, Angle M. Milrinone and homeostasis to treat cerebral vasospasm associated with subarachnoid hemorrhage: the Montreal Neurological Hospital protocol. Neurocrit Care 2012; 16:354–362.
- Zwienenberg-Lee M, Hartman J, Rudisill N, et al; Balloon Prophylaxis for Aneurysmal Vasospasm (BPAV) Study Group. Effect of prophylactic transluminal balloon angioplasty on cerebral vasospasm and outcome in patients with Fisher grade III subarachnoid hemorrhage: results of a phase II multicenter, randomized, clinical trial. Stroke 2008; 39:1759–1765.
- Rabinstein AA, Bruder N. Management of hyponatremia and volume contraction. Neurocrit Care 2011; 15:354–360.
- Wijdicks EF, Vermeulen M, Hijdra A, van Gijn J. Hyponatremia and cerebral infarction in patients with ruptured intracranial aneurysms: is fluid restriction harmful? Ann Neurol 1985; 17:137–140.
- Hasan D, Wijdicks EF, Vermeulen M. Hyponatremia is associated with cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage. Ann Neurol 1990; 27:106–108.
- Wijdicks EF, Vermeulen M, ten Haaf JA, Hijdra A, Bakker WH, van Gijn J. Volume depletion and natriuresis in patients with a ruptured intracranial aneurysm. Ann Neurol 1985; 18:211–216.
- Hoff RG, Rinkel GJ, Verweij BH, Algra A, Kalkman CJ. Nurses’ prediction of volume status after aneurysmal subarachnoid haemorrhage: a prospective cohort study. Crit Care 2008; 12:R153.
- Hoff RG, van Dijk GW, Algra A, Kalkman CJ, Rinkel GJ. Fluid balance and blood volume measurement after aneurysmal subarachnoid hemorrhage. Neurocrit Care 2008; 8:391–397.
- Berendes E, Walter M, Cullen P, et al. Secretion of brain natriuretic peptide in patients with aneurysmal subarachnoid haemorrhage. Lancet 1997; 349:245–249.
- Espiner EA, Leikis R, Ferch RD, et al. The neuro-cardio-endocrine response to acute subarachnoid haemorrhage. Clin Endocrinol (Oxf) 2002; 56:629–635.
- Isotani E, Suzuki R, Tomita K, et al. Alterations in plasma concentrations of natriuretic peptides and antidiuretic hormone after subarachnoid hemorrhage. Stroke 1994; 25:2198–2203.
- Benedict CR, Loach AB. Sympathetic nervous system activity in patients with subarachnoid hemorrhage. Stroke 1978; 9:237–244.
- Findling JW, Waters VO, Raff H. The dissociation of renin and aldosterone during critical illness. J Clin Endocrinol Metab 1987; 64:592–595.
- Solomon RA, Post KD, McMurtry JG 3rd. Depression of circulating blood volume in patients after subarachnoid hemorrhage: implications for the management of symptomatic vasospasm. Neurosurgery 1984; 15:354–361.
- Peters JP, Welt LG, Sims EA, Orloff J, Needham J. A salt-wasting syndrome associated with cerebral disease. Trans Assoc Am Physicians 1950; 63:57–64.
- Brimioulle S, Orellana-Jimenez C, Aminian A, Vincent JL. Hyponatremia in neurological patients: cerebral salt wasting versus inappropriate antidiuretic hormone secretion. Intensive Care Med 2008; 34:125–131.
- Singh S, Bohn D, Carlotti AP, Cusimano M, Rutka JT, Halperin ML. Cerebral salt wasting: truths, fallacies, theories, and challenges. Crit Care Med 2002; 30:2575–2579.
- Mutoh T, Kazumata K, Terasaka S, Taki Y, Suzuki A, Ishikawa T. Early intensive versus minimally invasive approach to postoperative hemodynamic management after subarachnoid hemorrhage. Stroke 2014; 45:1280–1284.
- Hasan D, Lindsay KW, Wijdicks EF, et al. Effect of fludrocortisone acetate in patients with subarachnoid hemorrhage. Stroke 1989; 20:1156–1161.
- Moro N, Katayama Y, Kojima J, Mori T, Kawamata T. Prophylactic management of excessive natriuresis with hydrocortisone for efficient hypervolemic therapy after subarachnoid hemorrhage. Stroke 2003; 34:2807–2811.
- Kilbourn KJ, Levy S, Staff I, Kureshi I, McCullough L. Clinical characteristics and outcomes of neurogenic stress cadiomyopathy in aneurysmal subarachnoid hemorrhage. Clin Neurol Neurosurg 2013; 115:909–914.
- Mayer SA, LiMandri G, Sherman D, et al. Electrocardiographic markers of abnormal left ventricular wall motion in acute subarachnoid hemorrhage. J Neurosurg 1995; 83:889–896.
- Deibert E, Barzilai B, Braverman AC, et al. Clinical significance of elevated troponin I levels in patients with nontraumatic subarachnoid hemorrhage. J Neurosurg 2003; 98:741–746.
- Banki N, Kopelnik A, Tung P, et al. Prospective analysis of prevalence, distribution, and rate of recovery of left ventricular systolic dysfunction in patients with subarachnoid hemorrhage. J Neurosurg 2006; 105:15–20.
- Kahn JM, Caldwell EC, Deem S, Newell DW, Heckbert SR, Rubenfeld GD. Acute lung injury in patients with subarachnoid hemorrhage: incidence, risk factors, and outcome. Crit Care Med 2006; 34:196–202.
- Kitamura Y, Nomura M, Shima H, et al. Acute lung injury associated with systemic inflammatory response syndrome following subarachnoid hemorrhage: a survey by the Shonan Neurosurgical Association. Neurol Med Chir (Tokyo) 2010; 50:456–460.
- Friedman JA, Pichelmann MA, Piepgras DG, et al. Pulmonary complications of aneurysmal subarachnoid hemorrhage. Neurosurgery 2003; 52:1025–1032.
- Oh HS, Jeong HS, Seo WS. Non-infectious hyperthermia in acute brain injury patients: relationships to mortality, blood pressure, intracranial pressure and cerebral perfusion pressure. Int J Nurs Pract 2012; 18:295–302.
- Oddo M, Frangos S, Milby A, et al. Induced normothermia attenuates cerebral metabolic distress in patients with aneurysmal subarachnoid hemorrhage and refractory fever. Stroke 2009; 40:1913–1916.
- Badjatia N, Fernandez L, Schmidt JM, et al. Impact of induced normothermia on outcome after subarachnoid hemorrhage: a case-control study. Neurosurgery 2010; 66:696-701.
- Serrone JC1, Wash EM, Hartings JA, Andaluz N, Zuccarello M. Venous thromboembolism in subarachnoid hemorrhage. World Neurosurg 2013; 80:859–863.
- Mack WJ, Ducruet AF, Hickman ZL, et al. Doppler ultrasonography screening of poor-grade subarachnoid hemorrhage patients increases the diagnosis of deep venous thrombosis. Neurol Res 2008; 30:889–892.
- de Oliveira Manoel AL, Turkel-Parrella D, Germans M, et al. Safety of early pharmacological thromboprophylaxis after subarachnoid hemorrhage. Can J Neurol Sci 2014; 41:554–561.
- van Gijn J, Rinkel GJ. Subarachnoid haemorrhage: diagnosis, causes and management. Brain 2001; 124:249–278.
- Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 2014; 129:e28–e292.
- Diringer MN, Bleck TP, Claude Hemphill J 3rd, et al; Neurocritical Care Society. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society’s Multidisciplinary Consensus Conference. Neurocrit Care 2011; 15:211–240.
- Feigin VL, Rinkel GJ, Lawes CM, et al. Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies. Stroke 2005; 36:2773–2780.
- Perry JJ, Stiell IG, Sivilotti ML, et al. Clinical decision rules to rule out subarachnoid hemorrhage for acute headache. JAMA 2013; 310:1248–1255.
- Kowalski RG, Claassen J, Kreiter KT, et al. Initial misdiagnosis and outcome after subarachnoid hemorrhage. JAMA 2004; 291:866–869.
- Perry JJ, Stiell IG, Sivilotti ML, et al. Sensitivity of computed tomography performed within six hours of onset of headache for diagnosis of subarachnoid haemorrhage: prospective cohort study. BMJ 2011; 343:d4277.
- van Gijn J, van Dongen KJ. The time course of aneurysmal haemorrhage on computed tomograms. Neuroradiology 1982; 23:153–156.
- McCormack RF, Hutson A. Can computed tomography angiography of the brain replace lumbar puncture in the evaluation of acute-onset headache after a negative noncontrast cranial computed tomography scan? Acad Emerg Med 2010; 17:444–451.
- Agid R, Andersson T, Almqvist H, et al. Negative CT angiography findings in patients with spontaneous subarachnoid hemorrhage: when is digital subtraction angiography still needed? AJNR Am J Neuroradiol 2010; 31:696–705.
- Teasdale GM, Drake CG, Hunt W, et al. A universal subarachnoid hemorrhage scale: report of a committee of the World Federation of Neurosurgical Societies. J Neurol Neurosurg Psychiatry 1988; 51:1457.
- Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 1968; 28:14–20.
- Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 1980; 6:1–9.
- Frontera JA, Claassen J, Schmidt JM, et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified Fisher scale. Neurosurgery 2006; 59:21–27.
- de Oliveira Manoel AL, Turkel-Parrella D, Kouzmina E, et al. The VASOGRADE—a simple, reliable grading scale for aneurysmal subarachnoid hemorrhage. Neurology 2014; 82(suppl 10): P5.123.
- Naidech AM, Janjua N, Kreiter KT, et al. Predictors and impact of aneurysm rebleeding after subarachnoid hemorrhage. Arch Neurol 2005; 62:410–416.
- Rincon F, Mayer SA. Neurocritical care: a distinct discipline? Curr Opin Crit Care 2007; 13:115–121.
- Rabinstein AA, Lanzino G, Wijdicks EF. Multidisciplinary management and emerging therapeutic strategies in aneurysmal subarachnoid haemorrhage. Lancet Neurol 2010; 9:504–519.
- Vespa P, Diringer MN; Participants in the International Multi-Disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage. High-volume centers. Neurocrit Care 2011; 15:369–372.
- McNeill L, English SW, Borg N, Matta BF, Menon DK. Effects of institutional caseload of subarachnoid hemorrhage on mortality: a secondary analysis of administrative data. Stroke 2013; 44:647–652.
- Dorhout Mees SM, Molyneux AJ, Kerr RS, Algra A, Rinkel GJ. Timing of aneurysm treatment after subarachnoid hemorrhage: relationship with delayed cerebral ischemia and poor outcome. Stroke 2012; 43:2126–2129.
- Laidlaw JD, Siu KH. Ultra-early surgery for aneurysmal subarachnoid hemorrhage: outcomes for a consecutive series of 391 patients not selected by grade or age. J Neurosurg 2002; 97:250–259.
- Molyneux A, Kerr R, Stratton I, et al; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2,143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002; 360:1267–1274.
- Molyneux AJ, Kerr RS, Yu LM, et al; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2,143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005; 366:809–817.
- Koivisto T, Vanninen R, Hurskainen H, Saari T, Hernesniemi J, Vapalahti M. Outcomes of early endovascular versus surgical treatment of ruptured cerebral aneurysms. A prospective randomized study. Stroke 2000; 31:2369–2377.
- McDougall CG, Spetzler RF, Zabramski JM, et al. The Barrow Ruptured Aneurysm Trial. J Neurosurg 2012; 116:135–144.
- Spetzler RF, McDougall CG, Albuquerque FC, et al. The Barrow Ruptured Aneurysm Trial: 3-year results. J Neurosurg 2013; 119:146–157.
- Connolly ES Jr, Meyers PM. Cerebral aneurysms: to clip or to coil? That is no longer the question. Nat Rev Neurol 2009; 5:412–413.
- Kassell NF, Peerless SJ, Durward QJ, Beck DW, Drake CG, Adams HP. Treatment of ischemic deficits from vasospasm with intravascular volume expansion and induced arterial hypertension. Neurosurgery 1982; 11:337–343.
- Otsubo H, Takemae T, Inoue T, Kobayashi S, Sugita K. Normovolaemic induced hypertension therapy for cerebral vasospasm after subarachnoid haemorrhage. Acta Neurochir (Wien) 1990; 103:18–26.
- Anderson CS, Heeley E, Huang Y, et al; INTERACT2 Investigators. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med 2013; 368:2355–2365.
- Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al; American Heart Association Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2012; 43:1711–1737.
- Ortega-Gutierrez S, Thomas J, Reccius A, et al. Effectiveness and safety of nicardipine and labetalol infusion for blood pressure management in patients with intracerebral and subarachnoid hemorrhage. Neurocrit Care 2013; 18:13–19.
- Hillman J, Fridriksson S, Nilsson O, Yu Z, Saveland H, Jakobsson KE. Immediate administration of tranexamic acid and reduced incidence of early rebleeding after aneurysmal subarachnoid hemorrhage: a prospective randomized study. J Neurosurg 2002; 97:771–778.
- Baharoglu MI, Germans MR, Rinkel GJ, et al. Antifibrinolytic therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 2013; 8:CD001245.
- Germans MR, Post R, Coert BA, Rinkel GJ, Vandertop WP, Verbaan D. Ultra-early tranexamic acid after subarachnoid hemorrhage (ULTRA): study protocol for a randomized controlled trial. Trials 2013; 14:143.
- Sloan MA, Alexandrov AV, Tegeler CH, et al; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Assessment: transcranial Doppler ultrasonography: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2004; 62:1468–1481.
- Wartenberg KE, Schmidt JM, Claassen J, et al. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med 2006; 34:617–623;
- Hellingman CA, van den Bergh WM, Beijer IS, et al. Risk of rebleeding after treatment of acute hydrocephalus in patients with aneurysmal subarachnoid hemorrhage. Stroke 2007; 38:96–99.
- Ransom ER, Mocco J, Komotar RJ, et al. External ventricular drainage response in poor grade aneurysmal subarachnoid hemorrhage: effect on preoperative grading and prognosis. Neurocrit Care 2007; 6:174–180.
- McIver JI, Friedman JA, Wijdicks EF, et al. Preoperative ventriculostomy and rebleeding after aneurysmal subarachnoid hemorrhage. J Neurosurg 2002; 97:1042–1044.
- Klopfenstein JD, Kim LJ, Feiz-Erfan I, et al. Comparison of rapid and gradual weaning from external ventricular drainage in patients with aneurysmal subarachnoid hemorrhage: a prospective randomized trial. J Neurosurg 2004; 100:225–229.
- Tseng MY, Al-Rawi PG, Czosnyka M, et al. Enhancement of cerebral blood flow using systemic hypertonic saline therapy improves outcome in patients with poor-grade spontaneous subarachnoid hemorrhage. J Neurosurg 2007; 107:274–282.
- Al-Rawi PG, Tseng MY, Richards HK, et al. Hypertonic saline in patients with poor-grade subarachnoid hemorrhage improves cerebral blood flow, brain tissue oxygen, and pH. Stroke 2010; 41:122–128.
- Tseng MY, Al-Rawi PG, Pickard JD, Rasulo FA, Kirkpatrick PJ. Effect of hypertonic saline on cerebral blood flow in poor-grade patients with subarachnoid hemorrhage. Stroke 2003; 34:1389–1396.
- Bentsen G, Breivik H, Lundar T, Stubhaug A. Hypertonic saline (7.2%) in 6% hydroxyethyl starch reduces intracranial pressure and improves hemodynamics in a placebo-controlled study involving stable patients with subarachnoid hemorrhage. Crit Care Med 2006; 34:2912–2917.
- Suarez JI, Qureshi AI, Parekh PD, et al. Administration of hypertonic (3%) sodium chloride/acetate in hyponatremic patients with symptomatic vasospasm following subarachnoid hemorrhage. J Neurosurg Anesthesiol 1999; 11:178–184.
- Stevens RD, Huff JS, Duckworth J, Papangelou A, Weingart SD, Smith WS. Emergency neurological life support: intracranial hypertension and herniation. Neurocrit Care 2012;17(suppl 1):S60–S65.
- Seule MA, Muroi C, Mink S, Yonekawa Y, Keller E. Therapeutic hypothermia in patients with aneurysmal subarachnoid hemorrhage, refractory intracranial hypertension, or cerebral vasospasm. Neurosurgery 2009; 64:86–93.
- Otani N, Takasato Y, Masaoka H, et al. Surgical outcome following decompressive craniectomy for poor-grade aneurysmal subarachnoid hemorrhage in patients with associated massive intracerebral or Sylvian hematomas. Cerebrovasc Dis 2008; 26:612–617.
- Lanzino G, D’Urso PI, Suarez J; Participants in the International Multi-Disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage. Seizures and anticonvulsants after aneurysmal subarachnoid hemorrhage. Neurocrit Care 2011; 15:247–256.
- Naidech AM, Kreiter KT, Janjua N, et al. Phenytoin exposure is associated with functional and cognitive disability after subarachnoid hemorrhage. Stroke 2005; 36:583–587.
- Rosengart AJ, Huo JD, Tolentino J, et al. Outcome in patients with subarachnoid hemorrhage treated with antiepileptic drugs. J Neurosurg 2007; 107:253–260.
- Shah D, Husain AM. Utility of levetiracetam in patients with subarachnoid hemorrhage. Seizure 2009; 18:676–679.
- Patsalos PN. Pharmacokinetic profile of levetiracetam: toward ideal characteristics. Pharmacol Ther 2000; 85:77–85.
- Dewan MC, Mocco J. Current practice regarding seizure prophylaxis in aneurysmal subarachnoid hemorrhage across academic centers. J Neurointerv Surg 2014 Jan 28. doi: 10.1136/neurintsurg-2013-011075 [Epub ahead of print]
- Murphy-Human T, Welch E, Zipfel G, Diringer MN, Dhar R. Comparison of short-duration levetiracetam with extended-course phenytoin for seizure prophylaxis after subarachnoid hemorrhage. World Neurosurg 2011; 75:269–274.
- Dennis LJ, Claassen J, Hirsch LJ, Emerson RG, Connolly ES, Mayer SA. Nonconvulsive status epilepticus after subarachnoid hemorrhage. Neurosurgery 2002; 51:1136–1144.
- Vespa PM, Nuwer MR, Juhász C, et al. Early detection of vasospasm after acute subarachnoid hemorrhage using continuous EEG ICU monitoring. Electroencephalogr Clin Neurophysiol 1997; 103:607–615.
- Claassen J, Hirsch LJ, Kreiter KT, et al. Quantitative continuous EEG for detecting delayed cerebral ischemia in patients with poor-grade subarachnoid hemorrhage. Clin Neurophysiol 2004; 115:2699–2710.
- Vergouwen MD, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 2010; 41:2391–2395.
- Kelly PJ, Gorten RJ, Grossman RG, Eisenberg HM. Cerebral perfusion, vascular spasm, and outcome in patients with ruptured intracranial aneurysms. J Neurosurg 1977; 47:44–49.
- Aralasmak A, Akyuz M, Ozkaynak C, Sindel T, Tuncer R. CT angiography and perfusion imaging in patients with subarachnoid hemorrhage: correlation of vasospasm to perfusion abnormality. Neuroradiology 2009; 51:85–93.
- Sabri M, Lass E, Macdonald RL. Early brain injury: a common mechanism in subarachnoid hemorrhage and global cerebral ischemia. Stroke Res Treat 2013 Feb 28. doi: 10.1155/2013/394036 [Epub 2013 ahead of print]
- Yoon DY, Choi CS, Kim KH, Cho BM. Multidetector-row CT angiography of cerebral vasospasm after aneurysmal subarachnoid hemorrhage: comparison of volume-rendered images and digital subtraction angiography. AJNR Am J Neuroradiol 2006; 27:370–377.
- Wintermark M1, Ko NU, Smith WS, Liu S, Higashida RT, Dillon WP. Vasospasm after subarachnoid hemorrhage: utility of perfusion CT and CT angiography on diagnosis and management. AJNR Am J Neuroradiol 2006; 27:26–34.
- Helbok R, Madineni RC, Schmidt MJ, et al. Intracerebral monitoring of silent infarcts after subarachnoid hemorrhage. Neurocrit Care 2011; 14:162–167.
- Hänggi D; Participants in the International Multi-Disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage. Monitoring and detection of vasospasm II: EEG and invasive monitoring. Neurocrit Care 2011; 15:318–323.
- Steiner T, Juvela S, Unterberg A, Jung C, Forsting M, Rinkel G; European Stroke Organization. European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis 2013; 35:93–112.
- Pickard JD, Murray GD, Illingworth R, et al. Effect of oral nimodipine on cerebral infarction and outcome after subarachnoid haemorrhage: British aneurysm nimodipine trial. BMJ 1989; 298:636–642.
- Dorhout Mees SM, Rinkel GJ, Feigin VL, et al. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 2007; 3:CD000277.
- Lennihan L, Mayer SA, Fink ME, et al. Effect of hypervolemic therapy on cerebral blood flow after subarachnoid hemorrhage: a randomized controlled trial. Stroke 2000; 31:383–391.
- Egge A, Waterloo K, Sjøholm H, Solberg T, Ingebrigtsen T, Romner B. Prophylactic hyperdynamic postoperative fluid therapy after aneurysmal subarachnoid hemorrhage: a clinical, prospective, randomized, controlled study. Neurosurgery 2001; 49:593–606.
- Jost SC, Diringer MN, Zazulia AR, et al. Effect of normal saline bolus on cerebral blood flow in regions with low baseline flow in patients with vasospasm following subarachnoid hemorrhage. J Neurosurg 2005; 103:25–30.
- Muizelaar JP, Becker DP. Induced hypertension for the treatment of cerebral ischemia after subarachnoid hemorrhage. Direct effect on cerebral blood flow. Surg Neurol 1986; 25:317–325.
- Levy ML, Rabb CH, Zelman V, Giannotta SL. Cardiac performance enhancement from dobutamine in patients refractory to hypervolemic therapy for cerebral vasospasm. J Neurosurg 1993; 79:494–499.
- Lannes M, Teitelbaum J, del Pilar Cortés M, Cardoso M, Angle M. Milrinone and homeostasis to treat cerebral vasospasm associated with subarachnoid hemorrhage: the Montreal Neurological Hospital protocol. Neurocrit Care 2012; 16:354–362.
- Zwienenberg-Lee M, Hartman J, Rudisill N, et al; Balloon Prophylaxis for Aneurysmal Vasospasm (BPAV) Study Group. Effect of prophylactic transluminal balloon angioplasty on cerebral vasospasm and outcome in patients with Fisher grade III subarachnoid hemorrhage: results of a phase II multicenter, randomized, clinical trial. Stroke 2008; 39:1759–1765.
- Rabinstein AA, Bruder N. Management of hyponatremia and volume contraction. Neurocrit Care 2011; 15:354–360.
- Wijdicks EF, Vermeulen M, Hijdra A, van Gijn J. Hyponatremia and cerebral infarction in patients with ruptured intracranial aneurysms: is fluid restriction harmful? Ann Neurol 1985; 17:137–140.
- Hasan D, Wijdicks EF, Vermeulen M. Hyponatremia is associated with cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage. Ann Neurol 1990; 27:106–108.
- Wijdicks EF, Vermeulen M, ten Haaf JA, Hijdra A, Bakker WH, van Gijn J. Volume depletion and natriuresis in patients with a ruptured intracranial aneurysm. Ann Neurol 1985; 18:211–216.
- Hoff RG, Rinkel GJ, Verweij BH, Algra A, Kalkman CJ. Nurses’ prediction of volume status after aneurysmal subarachnoid haemorrhage: a prospective cohort study. Crit Care 2008; 12:R153.
- Hoff RG, van Dijk GW, Algra A, Kalkman CJ, Rinkel GJ. Fluid balance and blood volume measurement after aneurysmal subarachnoid hemorrhage. Neurocrit Care 2008; 8:391–397.
- Berendes E, Walter M, Cullen P, et al. Secretion of brain natriuretic peptide in patients with aneurysmal subarachnoid haemorrhage. Lancet 1997; 349:245–249.
- Espiner EA, Leikis R, Ferch RD, et al. The neuro-cardio-endocrine response to acute subarachnoid haemorrhage. Clin Endocrinol (Oxf) 2002; 56:629–635.
- Isotani E, Suzuki R, Tomita K, et al. Alterations in plasma concentrations of natriuretic peptides and antidiuretic hormone after subarachnoid hemorrhage. Stroke 1994; 25:2198–2203.
- Benedict CR, Loach AB. Sympathetic nervous system activity in patients with subarachnoid hemorrhage. Stroke 1978; 9:237–244.
- Findling JW, Waters VO, Raff H. The dissociation of renin and aldosterone during critical illness. J Clin Endocrinol Metab 1987; 64:592–595.
- Solomon RA, Post KD, McMurtry JG 3rd. Depression of circulating blood volume in patients after subarachnoid hemorrhage: implications for the management of symptomatic vasospasm. Neurosurgery 1984; 15:354–361.
- Peters JP, Welt LG, Sims EA, Orloff J, Needham J. A salt-wasting syndrome associated with cerebral disease. Trans Assoc Am Physicians 1950; 63:57–64.
- Brimioulle S, Orellana-Jimenez C, Aminian A, Vincent JL. Hyponatremia in neurological patients: cerebral salt wasting versus inappropriate antidiuretic hormone secretion. Intensive Care Med 2008; 34:125–131.
- Singh S, Bohn D, Carlotti AP, Cusimano M, Rutka JT, Halperin ML. Cerebral salt wasting: truths, fallacies, theories, and challenges. Crit Care Med 2002; 30:2575–2579.
- Mutoh T, Kazumata K, Terasaka S, Taki Y, Suzuki A, Ishikawa T. Early intensive versus minimally invasive approach to postoperative hemodynamic management after subarachnoid hemorrhage. Stroke 2014; 45:1280–1284.
- Hasan D, Lindsay KW, Wijdicks EF, et al. Effect of fludrocortisone acetate in patients with subarachnoid hemorrhage. Stroke 1989; 20:1156–1161.
- Moro N, Katayama Y, Kojima J, Mori T, Kawamata T. Prophylactic management of excessive natriuresis with hydrocortisone for efficient hypervolemic therapy after subarachnoid hemorrhage. Stroke 2003; 34:2807–2811.
- Kilbourn KJ, Levy S, Staff I, Kureshi I, McCullough L. Clinical characteristics and outcomes of neurogenic stress cadiomyopathy in aneurysmal subarachnoid hemorrhage. Clin Neurol Neurosurg 2013; 115:909–914.
- Mayer SA, LiMandri G, Sherman D, et al. Electrocardiographic markers of abnormal left ventricular wall motion in acute subarachnoid hemorrhage. J Neurosurg 1995; 83:889–896.
- Deibert E, Barzilai B, Braverman AC, et al. Clinical significance of elevated troponin I levels in patients with nontraumatic subarachnoid hemorrhage. J Neurosurg 2003; 98:741–746.
- Banki N, Kopelnik A, Tung P, et al. Prospective analysis of prevalence, distribution, and rate of recovery of left ventricular systolic dysfunction in patients with subarachnoid hemorrhage. J Neurosurg 2006; 105:15–20.
- Kahn JM, Caldwell EC, Deem S, Newell DW, Heckbert SR, Rubenfeld GD. Acute lung injury in patients with subarachnoid hemorrhage: incidence, risk factors, and outcome. Crit Care Med 2006; 34:196–202.
- Kitamura Y, Nomura M, Shima H, et al. Acute lung injury associated with systemic inflammatory response syndrome following subarachnoid hemorrhage: a survey by the Shonan Neurosurgical Association. Neurol Med Chir (Tokyo) 2010; 50:456–460.
- Friedman JA, Pichelmann MA, Piepgras DG, et al. Pulmonary complications of aneurysmal subarachnoid hemorrhage. Neurosurgery 2003; 52:1025–1032.
- Oh HS, Jeong HS, Seo WS. Non-infectious hyperthermia in acute brain injury patients: relationships to mortality, blood pressure, intracranial pressure and cerebral perfusion pressure. Int J Nurs Pract 2012; 18:295–302.
- Oddo M, Frangos S, Milby A, et al. Induced normothermia attenuates cerebral metabolic distress in patients with aneurysmal subarachnoid hemorrhage and refractory fever. Stroke 2009; 40:1913–1916.
- Badjatia N, Fernandez L, Schmidt JM, et al. Impact of induced normothermia on outcome after subarachnoid hemorrhage: a case-control study. Neurosurgery 2010; 66:696-701.
- Serrone JC1, Wash EM, Hartings JA, Andaluz N, Zuccarello M. Venous thromboembolism in subarachnoid hemorrhage. World Neurosurg 2013; 80:859–863.
- Mack WJ, Ducruet AF, Hickman ZL, et al. Doppler ultrasonography screening of poor-grade subarachnoid hemorrhage patients increases the diagnosis of deep venous thrombosis. Neurol Res 2008; 30:889–892.
- de Oliveira Manoel AL, Turkel-Parrella D, Germans M, et al. Safety of early pharmacological thromboprophylaxis after subarachnoid hemorrhage. Can J Neurol Sci 2014; 41:554–561.
KEY POINTS
- The key symptom is the abrupt onset of severe headache, commonly described as “the worst headache of my life.
- Computed tomography without contrast should be done promptly when this condition is suspected.
- Outcomes are improved when patients are managed in a high-volume center with a specialized neurointensive care unit and access to an interdisciplinary team.
- Early aneurysm repair by surgical clipping or endovascular coiling is considered the standard of care and is the best strategy to reduce the risk of rebleeding.
- Medical and neurologic complications are extremely common and include hydrocephalus, increased intracranial pressure, seizures, delayed cerebral ischemia, hyponatremia, hypovolemia, and cardiac and pulmonary abnormalities.
When the dissociation curve shifts to the left
A 48-year-old woman presented to the emergency department after 2 days of nonproductive cough, chest discomfort, worsening shortness of breath, and subjective fever. She had a history of systemic sclerosis. She was currently taking prednisone 20 mg daily and aspirin 81 mg daily.
Physical examination revealed tachypnea (28 breaths per minute), and bronchial breath sounds in the left lower chest posteriorly.
The initial laboratory workup revealed:
- Hemoglobin 106 g/L (reference range 115–155)
- Mean corpuscular volume 84 fL (80–100)
- White blood cell count 29.4 × 109/L (3.70–11.0), with 85% neutrophils
- Platelet count 180 × 109/L (150–350)
- Lactate dehydrogenase 312 U/L (100–220).
Chest radiography showed opacification of the lower lobe of the left lung.
She was admitted to the hospital and started treatment with intravenous azithromycin and ceftriaxone for presumed community-acquired pneumonia, based on the clinical presentation and findings on chest radiography. Because of her immunosuppression (due to chronic prednisone therapy) and her high lactate dehydrogenase level, Pneumocystis jirovecii pneumonia was suspected, and because she had a history of allergy to trimethoprim-sulfamethoxazole and pentamidine, she was started on dapsone.
During the next 24 hours, she developed worsening dyspnea, hypoxia, and cyanosis. She was placed on an air-entrainment mask, with a fraction of inspired oxygen of 0.5. Pulse oximetry showed an oxygen saturation of 85%, but arterial blood gas analysis indicated an oxyhemoglobin concentration of 95%.
THE ‘SATURATION GAP’
1. Which is most likely to have caused the discrepancy between the oxyhemoglobin concentration and the oxygen saturation by pulse oximetry in this patient?
- Methemoglobinemia
- Carbon monoxide poisoning
- Inappropriate placement of the pulse oximeter probe
- Pulmonary embolism
Methemoglobinemia is the most likely cause of the discrepancy between the oxyhemoglobin levels and the oxygen saturation by pulse oximetry, a phenomenon also known as the “saturation gap.” Other common causes are cyanide poisoning and carbon monoxide poisoning.
Carbon monoxide poisoning, however, does not explain our patient’s cyanosis. On the contrary, carbon monoxide poisoning can actually cause the patient’s lips and mucous membranes to appear unnaturally bright pink. Also, carbon monoxide poisoning raises the blood concentration of carboxyhemoglobin (which has a high affinity for oxygen), and this usually causes pulse oximetry to read inappropriately high, whereas in our patient it read low.
Incorrect placement of the pulse oximeter probe can result in an inaccurate measurement of oxygen saturation. Visualization of the waveform on the plethysmograph or the signal quality index can be used to assess adequate placement of the pulse oximeter probe. However, inadequate probe placement does not explain our patient’s dyspnea and cyanosis.
Pulmonary embolism can lead to hypoxia as a result of ventilation-perfusion mismatch. However, pulmonary embolism leading to low oxygen saturation on pulse oximetry will also lead to concomitantly low oxyhemoglobin levels as measured by arterial blood gas analysis, and this was not seen in our patient.
BACK TO OUR PATIENT
Because there was a discrepancy between our patient’s pulse oximetry reading and oxyhemoglobin concentration by arterial blood gas measurement, her methemoglobin level was checked and was found to be 30%, thus confirming the diagnosis of methemoglobinemia.
WHAT IS METHEMOGLOBINEMIA, AND WHAT CAUSES IT?
Oxygen is normally bound to iron in its ferrous (Fe2+) form in hemoglobin to form oxyhemoglobin. Oxidative stress in the body can cause iron to change from the ferrous to the ferric (Fe3+) state, forming methemoglobin. Methemoglobin is normally present in the blood in low levels (< 1% of the total hemoglobin), and ferric iron is reduced and recycled back to the ferrous form by NADH-cytochrome b5 reductase, an enzyme present in red blood cells. This protective mechanism maintains methemoglobin levels within safe limits. But increased production can lead to accumulation of methemoglobin, resulting in dyspnea and hypoxia and the condition referred to as methemoglobinemia.1
Increased levels of methemoglobin relative to normal hemoglobin cause tissue hypoxia by several mechanisms. Methemoglobin cannot efficiently carry oxygen; instead, it binds to water or to a hydroxide ion depending on the pH of the environment.2 Therefore, the hemoglobin molecule does not carry its usual load of oxygen, and hypoxia results from the reduced delivery of oxygen to tissues. In addition, an increased concentration of methemoglobin causes a leftward shift in the oxygen-hemoglobin dissociation curve, representing an increased affinity to bound oxygen in the remaining heme groups. The tightly bound oxygen is not adequately released at the tissue level, thus causing cellular hypoxia.
Methemoglobinemia is most often caused by exposure to an oxidizing chemical or drug that increases production of methemoglobin. In rare cases, it is caused by a congenital deficiency of NADH-cytochrome b5 reductase.3
2. Which of the following drugs can cause methemoglobinemia?
- Acetaminophen
- Dapsone
- Benzocaine
- Primaquine
All four of these drugs are common culprits for causing acquired methemoglobinemia; others include chloroquine, nitroglycerin, and sulfonamides.4–6
The increased production of methemoglobin caused by these drugs overwhelms the protective effect of reducing enzymes and can lead to an accumulation of methemoglobin. However, because of variability in cellular metabolism, not every person who takes these drugs develops dangerous levels of methemoglobin.
Dapsone and benzocaine are the most commonly encountered drugs known to cause methemoglobinemia (Table 1). Dapsone is an anti-inflammatory and antimicrobial agent most commonly used for treating lepromatous leprosy and dermatitis herpetiformis. It is also often prescribed for prophylaxis and treatment of P jirovecii pneumonia in immunosuppressed individuals.7 Benzocaine is a local anesthetic and was commonly used before procedures such as oral or dental surgery, transesophageal echocardiography, and endoscopy.8–10 Even low doses of benzocaine can lead to high levels of methemoglobinemia. However, the availability of other, safer anesthetics now limits the use of benzocaine in major US centers. In addition, the topical anesthetic Emla (lidocaine plus prilocaine) has been recently reported as a cause of methemoglobinemia in infants and children.11,12
Also, potentially fatal methemoglobinemia has been reported in patients with a deficiency of G-6-phosphate dehydrogenase (G6PD) who received rasburicase, a recombinant version of urate oxidase enzyme used to prevent and treat tumor lysis syndrome.13,14
Lastly, methemoglobinemia has been reported in patients with inflammatory bowel disease treated with mesalamine.
Although this adverse reaction is rare, clinicians should be aware of it, since these agents are commonly used in everyday medical practice.15
RECOGNIZING THE DANGER SIGNS
The clinical manifestations of methemoglobinemia are directly proportional to the percentage of methemoglobin in red blood cells. Cyanosis generally becomes apparent at concentrations around 15%, at which point the patient may still have no symptoms. Anxiety, lightheadedness, tachycardia, and dizziness manifest at levels of 20% to 30%. Fatigue, confusion, dizziness, tachypnea, and worsening tachycardia occur at levels of 30% to 50%. Levels of 50% to 70% cause coma, seizures, arrhythmias, and acidosis, and levels over 70% are considered lethal.16
While these levels provide a general guideline of symptomatology in an otherwise healthy person, it is important to remember that patients with underlying conditions such as anemia, lung disease (both of which our patient had), sepsis, thalassemia, G6PD deficiency, and sickle cell disease can manifest symptoms at lower concentrations of methemoglobin.1,17
Most patients who develop clinically significant levels of methemoglobin do so within the first few hours of starting one of the culprit drugs.
DIAGNOSIS: METHEMOGLOBINEMIA AND THE SATURATION GAP
In patients with methemoglobinemia, pulse oximetry gives lower values than arterial blood gas oxygen measurements. Regular pulse oximetry works by measuring light absorbance at two distinct wavelengths (660 and 940 nm) to calculate the ratio of oxyhemoglobin to deoxyhemoglobin. Methemoglobin absorbs light at both these wavelengths, thus lowering the pulse oximetry values.1
In contrast, oxygen saturation of arterial blood gas (oxyhemoglobin) is calculated indirectly from the concentration of dissolved oxygen in the blood and does not include oxygen bound to hemoglobin. Therefore, the measured arterial oxygen saturation is often normal in patients with methemoglobinemia since it relies only on inspired oxygen content and is independent of the methemoglobin concentration.18
Oxygen supplementation can raise the level of oxyhemoglobin, which is a measure of dissolved oxygen, but the oxygen saturation as measured by pulse oximetry remains largely unchanged—ie, the saturation gap. A difference of more than 5% between the oxygen saturation by pulse oximetry and blood gas analysis is abnormal. Patients with clinically significant methemoglobinemia usually have a saturation gap greater than 10%.
Several other unique features should raise suspicion of methemoglobinemia. It should be considered in a patient presenting with cyanosis out of proportion to the oxygen saturation and in a patient with low oxygen saturation and a normal chest radiograph. Other clues include blood that is chocolate-colored on gross examination, rather than the dark red of deoxygenated blood.
Co-oximetry measures oxygen saturation using different wavelengths of light to distinguish between fractions of oxyhemoglobin, deoxyhemoglobin, and methemoglobin, but it is not widely available.
THE NEXT STEP
3. What is the next step in the management of our patient?
- Discontinue the dapsone
- Start methylene blue
- Start hyperbaric oxygen
- Give sodium thiosulfate
- Discontinue dapsone and start methylene blue
The next step in her management should be to stop the dapsone and start an infusion of methylene blue. Hyperbaric oxygen is used in treating carbon monoxide poisoning, and sodium thiosulfate is used in treating cyanide toxicity. They would not be appropriate in this patient’s care.
MANAGEMENT OF ACQUIRED METHEMOGLOBINEMIA
The first, most critical step in managing acquired methemoglobinemia is to immediately discontinue the suspected offending agent. In most patients without a concomitant condition such as anemia or lung disease and with a methemoglobin level below 20%, discontinuing the offending agent may suffice. Patients with a level of 20% or greater and patients with cardiac and pulmonary disease, who develop symptoms at lower concentrations of methemoglobin, require infusion of methylene blue.
Methylene blue is converted to its reduced form, leukomethylene blue, by NADPH-methemoglobin reductase. As it is oxidized, leukomethylene blue reduces methemoglobin to hemoglobin. A dose of 1 mg/kg intravenously is given at first. The response is usually dramatic, with a reduction in methemoglobin levels and improvement in symptoms often within 30 to 60 minutes. If levels remain high, the dose can be repeated 1 hour later.19
A caveat: methylene blue therapy should be avoided in patients with complete G6PD deficiency. Methylene blue works through the enzyme NADPH-methemoglobin reductase, and since patients with G6PD deficiency lack this enzyme, methylene blue is ineffective. In fact, since it cannot be reduced, excessive methylene blue can oxidize hemoglobin to methemoglobin, further exacerbating the condition. In patients with partial G6PD deficiency, methylene blue is still recommended as a first-line treatment, but at a lower initial dose (0.3–0.5 mg/kg). However, in patients with significant hemolysis, an exchange transfusion is the only treatment option.
CASE CONCLUDED
Since dapsone was identified as the likely cause of methemoglobinemia in our patient, it was immediately discontinued. Because she was symptomatic, 70 mg of methylene blue was given intravenously. Over the next 60 minutes, her clinical condition improved significantly. A repeat methemoglobin measurement was 3%.
She was discharged home the next day on oral antibiotics to complete treatment for community-acquired pneumonia.
TAKE-HOME POINTS
- Consider methemoglobinemia in a patient with unexplained cyanosis.
- Pulse oximetry gives lower values than arterial blood gas oxygen measurements in patients with methemoglobinemia, and pulse oximetry readings do not improve with supplemental oxygen.
- A saturation gap greater than 5% strongly suggests methemoglobinemia.
- The diagnosis of methemoglobinemia is confirmed by measuring the methemoglobin concentration.
- Most healthy patients develop symptoms at methemoglobin levels of 20%, but patients with comorbidities can develop symptoms at lower levels.
- A number of drugs can cause methemoglobinemia, even at therapeutic dosages.
- Treatment is generally indicated in patients who have symptoms or in healthy patients who have a methemoglobin level of 20% or greater.
- Identifying and promptly discontinuing the causative agent and initiating methylene blue infusion (1 mg/kg over 5 minutes) is the preferred treatment.
- Cortazzo JA, Lichtman AD. Methemoglobinemia: a review and recommendations for management. J Cardiothorac Vasc Anesth 2014; 28:1055–1059.
- Margulies DR, Manookian CM. Methemoglobinemia as a cause of respiratory failure. J Trauma 2002; 52:796–797.
- Skold A, Cosco DL, Klein R. Methemoglobinemia: pathogenesis, diagnosis, and management. South Med J 2011; 104:757–761.
- Ash-Bernal R, Wise R, Wright SM. Acquired methemoglobinemia: a retrospective series of 138 cases at 2 teaching hospitals. Medicine (Baltimore) 2004; 83:265–273.
- Kanji HD, Mithani S, Boucher P, Dias VC, Yarema MC. Coma, metabolic acidosis, and methemoglobinemia in a patient with acetaminophen toxicity. J Popul Ther Clin Pharmacol 2013; 20:e207–e211.
- Kawasumi H, Tanaka E, Hoshi D, Kawaguchi Y, Yamanaka H. Methemoglobinemia induced by trimethoprim-sulfamethoxazole in a patient with systemic lupus erythematosus. Intern Med 2013; 52:1741–1743.
- Wieringa A, Bethlehem C, Hoogendoorn M, van der Maten J, van Roon EN. Very late recovery of dapsone-induced methemoglobinemia. Clin Toxicol (Phila) 2014; 52:80–81.
- Barclay JA, Ziemba SE, Ibrahim RB. Dapsone-induced methemoglobinemia: a primer for clinicians. Ann Pharmacother 2011; 45:1103–1115.
- Taleb M, Ashraf Z, Valavoor S, Tinkel J. Evaluation and management of acquired methemoglobinemia associated with topical benzocaine use. Am J Cardiovasc Drugs 2013; 13:325–330.
- Chowdhary S, Bukoye B, Bhansali AM, et al. Risk of topical anesthetic-induced methemoglobinemia: a 10-year retrospective case-control study. JAMA Intern Med 2013; 173:771–776.
- Larson A, Stidham T, Banerji S, Kaufman J. Seizures and methemoglobinemia in an infant after excessive EMLA application. Pediatr Emerg Care 2013; 29:377–379.
- Schmitt C, Matulic M, Kervégant M, et al. Methaemoglobinaemia in a child treated with Emla cream: circumstances and consequences of overdose [in French]. Ann Dermatol Venereol 2012; 139:824–827.
- Bucklin MH, Groth CM. Mortality following rasburicase-induced methemoglobinemia. Ann Pharmacother 2013; 47:1353–1358.
- Cheah CY, Lew TE, Seymour JF, Burbury K. Rasburicase causing severe oxidative hemolysis and methemoglobinemia in a patient with previously unrecognized glucose-6-phosphate dehydrogenase deficiency. Acta Haematol 2013; 130:254–259.
- Druez A, Rahier JF, Hébuterne X. Methaemoglobinaemia and renal failure following mesalazine for treatment of inflammatory bowel disease. J Crohns Colitis 2014; 8:900–901.
- Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med 1999; 34:646–656.
- Groeper K, Katcher K, Tobias JD. Anesthetic management of a patient with methemoglobinemia. South Med J 2003; 96:504–509.
- Haymond S, Cariappa R, Eby CS, Scott MG. Laboratory assessment of oxygenation in methemoglobinemia. Clin Chem 2005; 51:434–444.
- Jang DH, Nelson LS, Hoffman RS. Methylene blue for distributive shock: a potential new use of an old antidote. J Med Toxicol 2013; 9:242–249.
A 48-year-old woman presented to the emergency department after 2 days of nonproductive cough, chest discomfort, worsening shortness of breath, and subjective fever. She had a history of systemic sclerosis. She was currently taking prednisone 20 mg daily and aspirin 81 mg daily.
Physical examination revealed tachypnea (28 breaths per minute), and bronchial breath sounds in the left lower chest posteriorly.
The initial laboratory workup revealed:
- Hemoglobin 106 g/L (reference range 115–155)
- Mean corpuscular volume 84 fL (80–100)
- White blood cell count 29.4 × 109/L (3.70–11.0), with 85% neutrophils
- Platelet count 180 × 109/L (150–350)
- Lactate dehydrogenase 312 U/L (100–220).
Chest radiography showed opacification of the lower lobe of the left lung.
She was admitted to the hospital and started treatment with intravenous azithromycin and ceftriaxone for presumed community-acquired pneumonia, based on the clinical presentation and findings on chest radiography. Because of her immunosuppression (due to chronic prednisone therapy) and her high lactate dehydrogenase level, Pneumocystis jirovecii pneumonia was suspected, and because she had a history of allergy to trimethoprim-sulfamethoxazole and pentamidine, she was started on dapsone.
During the next 24 hours, she developed worsening dyspnea, hypoxia, and cyanosis. She was placed on an air-entrainment mask, with a fraction of inspired oxygen of 0.5. Pulse oximetry showed an oxygen saturation of 85%, but arterial blood gas analysis indicated an oxyhemoglobin concentration of 95%.
THE ‘SATURATION GAP’
1. Which is most likely to have caused the discrepancy between the oxyhemoglobin concentration and the oxygen saturation by pulse oximetry in this patient?
- Methemoglobinemia
- Carbon monoxide poisoning
- Inappropriate placement of the pulse oximeter probe
- Pulmonary embolism
Methemoglobinemia is the most likely cause of the discrepancy between the oxyhemoglobin levels and the oxygen saturation by pulse oximetry, a phenomenon also known as the “saturation gap.” Other common causes are cyanide poisoning and carbon monoxide poisoning.
Carbon monoxide poisoning, however, does not explain our patient’s cyanosis. On the contrary, carbon monoxide poisoning can actually cause the patient’s lips and mucous membranes to appear unnaturally bright pink. Also, carbon monoxide poisoning raises the blood concentration of carboxyhemoglobin (which has a high affinity for oxygen), and this usually causes pulse oximetry to read inappropriately high, whereas in our patient it read low.
Incorrect placement of the pulse oximeter probe can result in an inaccurate measurement of oxygen saturation. Visualization of the waveform on the plethysmograph or the signal quality index can be used to assess adequate placement of the pulse oximeter probe. However, inadequate probe placement does not explain our patient’s dyspnea and cyanosis.
Pulmonary embolism can lead to hypoxia as a result of ventilation-perfusion mismatch. However, pulmonary embolism leading to low oxygen saturation on pulse oximetry will also lead to concomitantly low oxyhemoglobin levels as measured by arterial blood gas analysis, and this was not seen in our patient.
BACK TO OUR PATIENT
Because there was a discrepancy between our patient’s pulse oximetry reading and oxyhemoglobin concentration by arterial blood gas measurement, her methemoglobin level was checked and was found to be 30%, thus confirming the diagnosis of methemoglobinemia.
WHAT IS METHEMOGLOBINEMIA, AND WHAT CAUSES IT?
Oxygen is normally bound to iron in its ferrous (Fe2+) form in hemoglobin to form oxyhemoglobin. Oxidative stress in the body can cause iron to change from the ferrous to the ferric (Fe3+) state, forming methemoglobin. Methemoglobin is normally present in the blood in low levels (< 1% of the total hemoglobin), and ferric iron is reduced and recycled back to the ferrous form by NADH-cytochrome b5 reductase, an enzyme present in red blood cells. This protective mechanism maintains methemoglobin levels within safe limits. But increased production can lead to accumulation of methemoglobin, resulting in dyspnea and hypoxia and the condition referred to as methemoglobinemia.1
Increased levels of methemoglobin relative to normal hemoglobin cause tissue hypoxia by several mechanisms. Methemoglobin cannot efficiently carry oxygen; instead, it binds to water or to a hydroxide ion depending on the pH of the environment.2 Therefore, the hemoglobin molecule does not carry its usual load of oxygen, and hypoxia results from the reduced delivery of oxygen to tissues. In addition, an increased concentration of methemoglobin causes a leftward shift in the oxygen-hemoglobin dissociation curve, representing an increased affinity to bound oxygen in the remaining heme groups. The tightly bound oxygen is not adequately released at the tissue level, thus causing cellular hypoxia.
Methemoglobinemia is most often caused by exposure to an oxidizing chemical or drug that increases production of methemoglobin. In rare cases, it is caused by a congenital deficiency of NADH-cytochrome b5 reductase.3
2. Which of the following drugs can cause methemoglobinemia?
- Acetaminophen
- Dapsone
- Benzocaine
- Primaquine
All four of these drugs are common culprits for causing acquired methemoglobinemia; others include chloroquine, nitroglycerin, and sulfonamides.4–6
The increased production of methemoglobin caused by these drugs overwhelms the protective effect of reducing enzymes and can lead to an accumulation of methemoglobin. However, because of variability in cellular metabolism, not every person who takes these drugs develops dangerous levels of methemoglobin.
Dapsone and benzocaine are the most commonly encountered drugs known to cause methemoglobinemia (Table 1). Dapsone is an anti-inflammatory and antimicrobial agent most commonly used for treating lepromatous leprosy and dermatitis herpetiformis. It is also often prescribed for prophylaxis and treatment of P jirovecii pneumonia in immunosuppressed individuals.7 Benzocaine is a local anesthetic and was commonly used before procedures such as oral or dental surgery, transesophageal echocardiography, and endoscopy.8–10 Even low doses of benzocaine can lead to high levels of methemoglobinemia. However, the availability of other, safer anesthetics now limits the use of benzocaine in major US centers. In addition, the topical anesthetic Emla (lidocaine plus prilocaine) has been recently reported as a cause of methemoglobinemia in infants and children.11,12
Also, potentially fatal methemoglobinemia has been reported in patients with a deficiency of G-6-phosphate dehydrogenase (G6PD) who received rasburicase, a recombinant version of urate oxidase enzyme used to prevent and treat tumor lysis syndrome.13,14
Lastly, methemoglobinemia has been reported in patients with inflammatory bowel disease treated with mesalamine.
Although this adverse reaction is rare, clinicians should be aware of it, since these agents are commonly used in everyday medical practice.15
RECOGNIZING THE DANGER SIGNS
The clinical manifestations of methemoglobinemia are directly proportional to the percentage of methemoglobin in red blood cells. Cyanosis generally becomes apparent at concentrations around 15%, at which point the patient may still have no symptoms. Anxiety, lightheadedness, tachycardia, and dizziness manifest at levels of 20% to 30%. Fatigue, confusion, dizziness, tachypnea, and worsening tachycardia occur at levels of 30% to 50%. Levels of 50% to 70% cause coma, seizures, arrhythmias, and acidosis, and levels over 70% are considered lethal.16
While these levels provide a general guideline of symptomatology in an otherwise healthy person, it is important to remember that patients with underlying conditions such as anemia, lung disease (both of which our patient had), sepsis, thalassemia, G6PD deficiency, and sickle cell disease can manifest symptoms at lower concentrations of methemoglobin.1,17
Most patients who develop clinically significant levels of methemoglobin do so within the first few hours of starting one of the culprit drugs.
DIAGNOSIS: METHEMOGLOBINEMIA AND THE SATURATION GAP
In patients with methemoglobinemia, pulse oximetry gives lower values than arterial blood gas oxygen measurements. Regular pulse oximetry works by measuring light absorbance at two distinct wavelengths (660 and 940 nm) to calculate the ratio of oxyhemoglobin to deoxyhemoglobin. Methemoglobin absorbs light at both these wavelengths, thus lowering the pulse oximetry values.1
In contrast, oxygen saturation of arterial blood gas (oxyhemoglobin) is calculated indirectly from the concentration of dissolved oxygen in the blood and does not include oxygen bound to hemoglobin. Therefore, the measured arterial oxygen saturation is often normal in patients with methemoglobinemia since it relies only on inspired oxygen content and is independent of the methemoglobin concentration.18
Oxygen supplementation can raise the level of oxyhemoglobin, which is a measure of dissolved oxygen, but the oxygen saturation as measured by pulse oximetry remains largely unchanged—ie, the saturation gap. A difference of more than 5% between the oxygen saturation by pulse oximetry and blood gas analysis is abnormal. Patients with clinically significant methemoglobinemia usually have a saturation gap greater than 10%.
Several other unique features should raise suspicion of methemoglobinemia. It should be considered in a patient presenting with cyanosis out of proportion to the oxygen saturation and in a patient with low oxygen saturation and a normal chest radiograph. Other clues include blood that is chocolate-colored on gross examination, rather than the dark red of deoxygenated blood.
Co-oximetry measures oxygen saturation using different wavelengths of light to distinguish between fractions of oxyhemoglobin, deoxyhemoglobin, and methemoglobin, but it is not widely available.
THE NEXT STEP
3. What is the next step in the management of our patient?
- Discontinue the dapsone
- Start methylene blue
- Start hyperbaric oxygen
- Give sodium thiosulfate
- Discontinue dapsone and start methylene blue
The next step in her management should be to stop the dapsone and start an infusion of methylene blue. Hyperbaric oxygen is used in treating carbon monoxide poisoning, and sodium thiosulfate is used in treating cyanide toxicity. They would not be appropriate in this patient’s care.
MANAGEMENT OF ACQUIRED METHEMOGLOBINEMIA
The first, most critical step in managing acquired methemoglobinemia is to immediately discontinue the suspected offending agent. In most patients without a concomitant condition such as anemia or lung disease and with a methemoglobin level below 20%, discontinuing the offending agent may suffice. Patients with a level of 20% or greater and patients with cardiac and pulmonary disease, who develop symptoms at lower concentrations of methemoglobin, require infusion of methylene blue.
Methylene blue is converted to its reduced form, leukomethylene blue, by NADPH-methemoglobin reductase. As it is oxidized, leukomethylene blue reduces methemoglobin to hemoglobin. A dose of 1 mg/kg intravenously is given at first. The response is usually dramatic, with a reduction in methemoglobin levels and improvement in symptoms often within 30 to 60 minutes. If levels remain high, the dose can be repeated 1 hour later.19
A caveat: methylene blue therapy should be avoided in patients with complete G6PD deficiency. Methylene blue works through the enzyme NADPH-methemoglobin reductase, and since patients with G6PD deficiency lack this enzyme, methylene blue is ineffective. In fact, since it cannot be reduced, excessive methylene blue can oxidize hemoglobin to methemoglobin, further exacerbating the condition. In patients with partial G6PD deficiency, methylene blue is still recommended as a first-line treatment, but at a lower initial dose (0.3–0.5 mg/kg). However, in patients with significant hemolysis, an exchange transfusion is the only treatment option.
CASE CONCLUDED
Since dapsone was identified as the likely cause of methemoglobinemia in our patient, it was immediately discontinued. Because she was symptomatic, 70 mg of methylene blue was given intravenously. Over the next 60 minutes, her clinical condition improved significantly. A repeat methemoglobin measurement was 3%.
She was discharged home the next day on oral antibiotics to complete treatment for community-acquired pneumonia.
TAKE-HOME POINTS
- Consider methemoglobinemia in a patient with unexplained cyanosis.
- Pulse oximetry gives lower values than arterial blood gas oxygen measurements in patients with methemoglobinemia, and pulse oximetry readings do not improve with supplemental oxygen.
- A saturation gap greater than 5% strongly suggests methemoglobinemia.
- The diagnosis of methemoglobinemia is confirmed by measuring the methemoglobin concentration.
- Most healthy patients develop symptoms at methemoglobin levels of 20%, but patients with comorbidities can develop symptoms at lower levels.
- A number of drugs can cause methemoglobinemia, even at therapeutic dosages.
- Treatment is generally indicated in patients who have symptoms or in healthy patients who have a methemoglobin level of 20% or greater.
- Identifying and promptly discontinuing the causative agent and initiating methylene blue infusion (1 mg/kg over 5 minutes) is the preferred treatment.
A 48-year-old woman presented to the emergency department after 2 days of nonproductive cough, chest discomfort, worsening shortness of breath, and subjective fever. She had a history of systemic sclerosis. She was currently taking prednisone 20 mg daily and aspirin 81 mg daily.
Physical examination revealed tachypnea (28 breaths per minute), and bronchial breath sounds in the left lower chest posteriorly.
The initial laboratory workup revealed:
- Hemoglobin 106 g/L (reference range 115–155)
- Mean corpuscular volume 84 fL (80–100)
- White blood cell count 29.4 × 109/L (3.70–11.0), with 85% neutrophils
- Platelet count 180 × 109/L (150–350)
- Lactate dehydrogenase 312 U/L (100–220).
Chest radiography showed opacification of the lower lobe of the left lung.
She was admitted to the hospital and started treatment with intravenous azithromycin and ceftriaxone for presumed community-acquired pneumonia, based on the clinical presentation and findings on chest radiography. Because of her immunosuppression (due to chronic prednisone therapy) and her high lactate dehydrogenase level, Pneumocystis jirovecii pneumonia was suspected, and because she had a history of allergy to trimethoprim-sulfamethoxazole and pentamidine, she was started on dapsone.
During the next 24 hours, she developed worsening dyspnea, hypoxia, and cyanosis. She was placed on an air-entrainment mask, with a fraction of inspired oxygen of 0.5. Pulse oximetry showed an oxygen saturation of 85%, but arterial blood gas analysis indicated an oxyhemoglobin concentration of 95%.
THE ‘SATURATION GAP’
1. Which is most likely to have caused the discrepancy between the oxyhemoglobin concentration and the oxygen saturation by pulse oximetry in this patient?
- Methemoglobinemia
- Carbon monoxide poisoning
- Inappropriate placement of the pulse oximeter probe
- Pulmonary embolism
Methemoglobinemia is the most likely cause of the discrepancy between the oxyhemoglobin levels and the oxygen saturation by pulse oximetry, a phenomenon also known as the “saturation gap.” Other common causes are cyanide poisoning and carbon monoxide poisoning.
Carbon monoxide poisoning, however, does not explain our patient’s cyanosis. On the contrary, carbon monoxide poisoning can actually cause the patient’s lips and mucous membranes to appear unnaturally bright pink. Also, carbon monoxide poisoning raises the blood concentration of carboxyhemoglobin (which has a high affinity for oxygen), and this usually causes pulse oximetry to read inappropriately high, whereas in our patient it read low.
Incorrect placement of the pulse oximeter probe can result in an inaccurate measurement of oxygen saturation. Visualization of the waveform on the plethysmograph or the signal quality index can be used to assess adequate placement of the pulse oximeter probe. However, inadequate probe placement does not explain our patient’s dyspnea and cyanosis.
Pulmonary embolism can lead to hypoxia as a result of ventilation-perfusion mismatch. However, pulmonary embolism leading to low oxygen saturation on pulse oximetry will also lead to concomitantly low oxyhemoglobin levels as measured by arterial blood gas analysis, and this was not seen in our patient.
BACK TO OUR PATIENT
Because there was a discrepancy between our patient’s pulse oximetry reading and oxyhemoglobin concentration by arterial blood gas measurement, her methemoglobin level was checked and was found to be 30%, thus confirming the diagnosis of methemoglobinemia.
WHAT IS METHEMOGLOBINEMIA, AND WHAT CAUSES IT?
Oxygen is normally bound to iron in its ferrous (Fe2+) form in hemoglobin to form oxyhemoglobin. Oxidative stress in the body can cause iron to change from the ferrous to the ferric (Fe3+) state, forming methemoglobin. Methemoglobin is normally present in the blood in low levels (< 1% of the total hemoglobin), and ferric iron is reduced and recycled back to the ferrous form by NADH-cytochrome b5 reductase, an enzyme present in red blood cells. This protective mechanism maintains methemoglobin levels within safe limits. But increased production can lead to accumulation of methemoglobin, resulting in dyspnea and hypoxia and the condition referred to as methemoglobinemia.1
Increased levels of methemoglobin relative to normal hemoglobin cause tissue hypoxia by several mechanisms. Methemoglobin cannot efficiently carry oxygen; instead, it binds to water or to a hydroxide ion depending on the pH of the environment.2 Therefore, the hemoglobin molecule does not carry its usual load of oxygen, and hypoxia results from the reduced delivery of oxygen to tissues. In addition, an increased concentration of methemoglobin causes a leftward shift in the oxygen-hemoglobin dissociation curve, representing an increased affinity to bound oxygen in the remaining heme groups. The tightly bound oxygen is not adequately released at the tissue level, thus causing cellular hypoxia.
Methemoglobinemia is most often caused by exposure to an oxidizing chemical or drug that increases production of methemoglobin. In rare cases, it is caused by a congenital deficiency of NADH-cytochrome b5 reductase.3
2. Which of the following drugs can cause methemoglobinemia?
- Acetaminophen
- Dapsone
- Benzocaine
- Primaquine
All four of these drugs are common culprits for causing acquired methemoglobinemia; others include chloroquine, nitroglycerin, and sulfonamides.4–6
The increased production of methemoglobin caused by these drugs overwhelms the protective effect of reducing enzymes and can lead to an accumulation of methemoglobin. However, because of variability in cellular metabolism, not every person who takes these drugs develops dangerous levels of methemoglobin.
Dapsone and benzocaine are the most commonly encountered drugs known to cause methemoglobinemia (Table 1). Dapsone is an anti-inflammatory and antimicrobial agent most commonly used for treating lepromatous leprosy and dermatitis herpetiformis. It is also often prescribed for prophylaxis and treatment of P jirovecii pneumonia in immunosuppressed individuals.7 Benzocaine is a local anesthetic and was commonly used before procedures such as oral or dental surgery, transesophageal echocardiography, and endoscopy.8–10 Even low doses of benzocaine can lead to high levels of methemoglobinemia. However, the availability of other, safer anesthetics now limits the use of benzocaine in major US centers. In addition, the topical anesthetic Emla (lidocaine plus prilocaine) has been recently reported as a cause of methemoglobinemia in infants and children.11,12
Also, potentially fatal methemoglobinemia has been reported in patients with a deficiency of G-6-phosphate dehydrogenase (G6PD) who received rasburicase, a recombinant version of urate oxidase enzyme used to prevent and treat tumor lysis syndrome.13,14
Lastly, methemoglobinemia has been reported in patients with inflammatory bowel disease treated with mesalamine.
Although this adverse reaction is rare, clinicians should be aware of it, since these agents are commonly used in everyday medical practice.15
RECOGNIZING THE DANGER SIGNS
The clinical manifestations of methemoglobinemia are directly proportional to the percentage of methemoglobin in red blood cells. Cyanosis generally becomes apparent at concentrations around 15%, at which point the patient may still have no symptoms. Anxiety, lightheadedness, tachycardia, and dizziness manifest at levels of 20% to 30%. Fatigue, confusion, dizziness, tachypnea, and worsening tachycardia occur at levels of 30% to 50%. Levels of 50% to 70% cause coma, seizures, arrhythmias, and acidosis, and levels over 70% are considered lethal.16
While these levels provide a general guideline of symptomatology in an otherwise healthy person, it is important to remember that patients with underlying conditions such as anemia, lung disease (both of which our patient had), sepsis, thalassemia, G6PD deficiency, and sickle cell disease can manifest symptoms at lower concentrations of methemoglobin.1,17
Most patients who develop clinically significant levels of methemoglobin do so within the first few hours of starting one of the culprit drugs.
DIAGNOSIS: METHEMOGLOBINEMIA AND THE SATURATION GAP
In patients with methemoglobinemia, pulse oximetry gives lower values than arterial blood gas oxygen measurements. Regular pulse oximetry works by measuring light absorbance at two distinct wavelengths (660 and 940 nm) to calculate the ratio of oxyhemoglobin to deoxyhemoglobin. Methemoglobin absorbs light at both these wavelengths, thus lowering the pulse oximetry values.1
In contrast, oxygen saturation of arterial blood gas (oxyhemoglobin) is calculated indirectly from the concentration of dissolved oxygen in the blood and does not include oxygen bound to hemoglobin. Therefore, the measured arterial oxygen saturation is often normal in patients with methemoglobinemia since it relies only on inspired oxygen content and is independent of the methemoglobin concentration.18
Oxygen supplementation can raise the level of oxyhemoglobin, which is a measure of dissolved oxygen, but the oxygen saturation as measured by pulse oximetry remains largely unchanged—ie, the saturation gap. A difference of more than 5% between the oxygen saturation by pulse oximetry and blood gas analysis is abnormal. Patients with clinically significant methemoglobinemia usually have a saturation gap greater than 10%.
Several other unique features should raise suspicion of methemoglobinemia. It should be considered in a patient presenting with cyanosis out of proportion to the oxygen saturation and in a patient with low oxygen saturation and a normal chest radiograph. Other clues include blood that is chocolate-colored on gross examination, rather than the dark red of deoxygenated blood.
Co-oximetry measures oxygen saturation using different wavelengths of light to distinguish between fractions of oxyhemoglobin, deoxyhemoglobin, and methemoglobin, but it is not widely available.
THE NEXT STEP
3. What is the next step in the management of our patient?
- Discontinue the dapsone
- Start methylene blue
- Start hyperbaric oxygen
- Give sodium thiosulfate
- Discontinue dapsone and start methylene blue
The next step in her management should be to stop the dapsone and start an infusion of methylene blue. Hyperbaric oxygen is used in treating carbon monoxide poisoning, and sodium thiosulfate is used in treating cyanide toxicity. They would not be appropriate in this patient’s care.
MANAGEMENT OF ACQUIRED METHEMOGLOBINEMIA
The first, most critical step in managing acquired methemoglobinemia is to immediately discontinue the suspected offending agent. In most patients without a concomitant condition such as anemia or lung disease and with a methemoglobin level below 20%, discontinuing the offending agent may suffice. Patients with a level of 20% or greater and patients with cardiac and pulmonary disease, who develop symptoms at lower concentrations of methemoglobin, require infusion of methylene blue.
Methylene blue is converted to its reduced form, leukomethylene blue, by NADPH-methemoglobin reductase. As it is oxidized, leukomethylene blue reduces methemoglobin to hemoglobin. A dose of 1 mg/kg intravenously is given at first. The response is usually dramatic, with a reduction in methemoglobin levels and improvement in symptoms often within 30 to 60 minutes. If levels remain high, the dose can be repeated 1 hour later.19
A caveat: methylene blue therapy should be avoided in patients with complete G6PD deficiency. Methylene blue works through the enzyme NADPH-methemoglobin reductase, and since patients with G6PD deficiency lack this enzyme, methylene blue is ineffective. In fact, since it cannot be reduced, excessive methylene blue can oxidize hemoglobin to methemoglobin, further exacerbating the condition. In patients with partial G6PD deficiency, methylene blue is still recommended as a first-line treatment, but at a lower initial dose (0.3–0.5 mg/kg). However, in patients with significant hemolysis, an exchange transfusion is the only treatment option.
CASE CONCLUDED
Since dapsone was identified as the likely cause of methemoglobinemia in our patient, it was immediately discontinued. Because she was symptomatic, 70 mg of methylene blue was given intravenously. Over the next 60 minutes, her clinical condition improved significantly. A repeat methemoglobin measurement was 3%.
She was discharged home the next day on oral antibiotics to complete treatment for community-acquired pneumonia.
TAKE-HOME POINTS
- Consider methemoglobinemia in a patient with unexplained cyanosis.
- Pulse oximetry gives lower values than arterial blood gas oxygen measurements in patients with methemoglobinemia, and pulse oximetry readings do not improve with supplemental oxygen.
- A saturation gap greater than 5% strongly suggests methemoglobinemia.
- The diagnosis of methemoglobinemia is confirmed by measuring the methemoglobin concentration.
- Most healthy patients develop symptoms at methemoglobin levels of 20%, but patients with comorbidities can develop symptoms at lower levels.
- A number of drugs can cause methemoglobinemia, even at therapeutic dosages.
- Treatment is generally indicated in patients who have symptoms or in healthy patients who have a methemoglobin level of 20% or greater.
- Identifying and promptly discontinuing the causative agent and initiating methylene blue infusion (1 mg/kg over 5 minutes) is the preferred treatment.
- Cortazzo JA, Lichtman AD. Methemoglobinemia: a review and recommendations for management. J Cardiothorac Vasc Anesth 2014; 28:1055–1059.
- Margulies DR, Manookian CM. Methemoglobinemia as a cause of respiratory failure. J Trauma 2002; 52:796–797.
- Skold A, Cosco DL, Klein R. Methemoglobinemia: pathogenesis, diagnosis, and management. South Med J 2011; 104:757–761.
- Ash-Bernal R, Wise R, Wright SM. Acquired methemoglobinemia: a retrospective series of 138 cases at 2 teaching hospitals. Medicine (Baltimore) 2004; 83:265–273.
- Kanji HD, Mithani S, Boucher P, Dias VC, Yarema MC. Coma, metabolic acidosis, and methemoglobinemia in a patient with acetaminophen toxicity. J Popul Ther Clin Pharmacol 2013; 20:e207–e211.
- Kawasumi H, Tanaka E, Hoshi D, Kawaguchi Y, Yamanaka H. Methemoglobinemia induced by trimethoprim-sulfamethoxazole in a patient with systemic lupus erythematosus. Intern Med 2013; 52:1741–1743.
- Wieringa A, Bethlehem C, Hoogendoorn M, van der Maten J, van Roon EN. Very late recovery of dapsone-induced methemoglobinemia. Clin Toxicol (Phila) 2014; 52:80–81.
- Barclay JA, Ziemba SE, Ibrahim RB. Dapsone-induced methemoglobinemia: a primer for clinicians. Ann Pharmacother 2011; 45:1103–1115.
- Taleb M, Ashraf Z, Valavoor S, Tinkel J. Evaluation and management of acquired methemoglobinemia associated with topical benzocaine use. Am J Cardiovasc Drugs 2013; 13:325–330.
- Chowdhary S, Bukoye B, Bhansali AM, et al. Risk of topical anesthetic-induced methemoglobinemia: a 10-year retrospective case-control study. JAMA Intern Med 2013; 173:771–776.
- Larson A, Stidham T, Banerji S, Kaufman J. Seizures and methemoglobinemia in an infant after excessive EMLA application. Pediatr Emerg Care 2013; 29:377–379.
- Schmitt C, Matulic M, Kervégant M, et al. Methaemoglobinaemia in a child treated with Emla cream: circumstances and consequences of overdose [in French]. Ann Dermatol Venereol 2012; 139:824–827.
- Bucklin MH, Groth CM. Mortality following rasburicase-induced methemoglobinemia. Ann Pharmacother 2013; 47:1353–1358.
- Cheah CY, Lew TE, Seymour JF, Burbury K. Rasburicase causing severe oxidative hemolysis and methemoglobinemia in a patient with previously unrecognized glucose-6-phosphate dehydrogenase deficiency. Acta Haematol 2013; 130:254–259.
- Druez A, Rahier JF, Hébuterne X. Methaemoglobinaemia and renal failure following mesalazine for treatment of inflammatory bowel disease. J Crohns Colitis 2014; 8:900–901.
- Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med 1999; 34:646–656.
- Groeper K, Katcher K, Tobias JD. Anesthetic management of a patient with methemoglobinemia. South Med J 2003; 96:504–509.
- Haymond S, Cariappa R, Eby CS, Scott MG. Laboratory assessment of oxygenation in methemoglobinemia. Clin Chem 2005; 51:434–444.
- Jang DH, Nelson LS, Hoffman RS. Methylene blue for distributive shock: a potential new use of an old antidote. J Med Toxicol 2013; 9:242–249.
- Cortazzo JA, Lichtman AD. Methemoglobinemia: a review and recommendations for management. J Cardiothorac Vasc Anesth 2014; 28:1055–1059.
- Margulies DR, Manookian CM. Methemoglobinemia as a cause of respiratory failure. J Trauma 2002; 52:796–797.
- Skold A, Cosco DL, Klein R. Methemoglobinemia: pathogenesis, diagnosis, and management. South Med J 2011; 104:757–761.
- Ash-Bernal R, Wise R, Wright SM. Acquired methemoglobinemia: a retrospective series of 138 cases at 2 teaching hospitals. Medicine (Baltimore) 2004; 83:265–273.
- Kanji HD, Mithani S, Boucher P, Dias VC, Yarema MC. Coma, metabolic acidosis, and methemoglobinemia in a patient with acetaminophen toxicity. J Popul Ther Clin Pharmacol 2013; 20:e207–e211.
- Kawasumi H, Tanaka E, Hoshi D, Kawaguchi Y, Yamanaka H. Methemoglobinemia induced by trimethoprim-sulfamethoxazole in a patient with systemic lupus erythematosus. Intern Med 2013; 52:1741–1743.
- Wieringa A, Bethlehem C, Hoogendoorn M, van der Maten J, van Roon EN. Very late recovery of dapsone-induced methemoglobinemia. Clin Toxicol (Phila) 2014; 52:80–81.
- Barclay JA, Ziemba SE, Ibrahim RB. Dapsone-induced methemoglobinemia: a primer for clinicians. Ann Pharmacother 2011; 45:1103–1115.
- Taleb M, Ashraf Z, Valavoor S, Tinkel J. Evaluation and management of acquired methemoglobinemia associated with topical benzocaine use. Am J Cardiovasc Drugs 2013; 13:325–330.
- Chowdhary S, Bukoye B, Bhansali AM, et al. Risk of topical anesthetic-induced methemoglobinemia: a 10-year retrospective case-control study. JAMA Intern Med 2013; 173:771–776.
- Larson A, Stidham T, Banerji S, Kaufman J. Seizures and methemoglobinemia in an infant after excessive EMLA application. Pediatr Emerg Care 2013; 29:377–379.
- Schmitt C, Matulic M, Kervégant M, et al. Methaemoglobinaemia in a child treated with Emla cream: circumstances and consequences of overdose [in French]. Ann Dermatol Venereol 2012; 139:824–827.
- Bucklin MH, Groth CM. Mortality following rasburicase-induced methemoglobinemia. Ann Pharmacother 2013; 47:1353–1358.
- Cheah CY, Lew TE, Seymour JF, Burbury K. Rasburicase causing severe oxidative hemolysis and methemoglobinemia in a patient with previously unrecognized glucose-6-phosphate dehydrogenase deficiency. Acta Haematol 2013; 130:254–259.
- Druez A, Rahier JF, Hébuterne X. Methaemoglobinaemia and renal failure following mesalazine for treatment of inflammatory bowel disease. J Crohns Colitis 2014; 8:900–901.
- Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med 1999; 34:646–656.
- Groeper K, Katcher K, Tobias JD. Anesthetic management of a patient with methemoglobinemia. South Med J 2003; 96:504–509.
- Haymond S, Cariappa R, Eby CS, Scott MG. Laboratory assessment of oxygenation in methemoglobinemia. Clin Chem 2005; 51:434–444.
- Jang DH, Nelson LS, Hoffman RS. Methylene blue for distributive shock: a potential new use of an old antidote. J Med Toxicol 2013; 9:242–249.
Short and sweet: Writing better consult notes in the era of the electronic medical record
After 4 decades of clinical practice in a teaching hospital, I believe that the notes we write to document medical consultations are too long. When I review them for my own patients, the only part I read is the consultant’s assessment and diagnostic and therapeutic recommendations. Many of my colleagues and trainees do the same.
In the old days, when medical records were handwritten, the first three pages of my hospital’s four-page consultation form were for the history, review of systems, physical examination, and test results. The top two-thirds of the last page was for diagnostic impressions and recommendations for additional testing and treatment, to be completed by the trainee performing the consultation.
This left only the bottom third of this page for attestation and additional remarks from the senior consultant. Often, this last (but most used) page was just a bullet list of diagnostic possibilities and suggested tests and treatments, with nothing about the critical reasoning underlying the differential diagnosis and recommendations. This was probably the result of fatigue from having to fill in the first three pages by hand, and then having only limited space on the final page.
Even though the written record has been replaced by the electronic medical record in my hospital, consult notes continue to be at least as long as before, without any change in the length of the assessment and recommendations section. I would guess this is true in most institutions and practices that have switched to an electronic record system.
WHY ARE CONSULT NOTES SO LONG?
The main factor contributing to the lengthy consultation document is that the Center for Medicare and Medicaid Services, with other third-party payers following suit, ties the level of reimbursement to detailed documentation of the history (present, past medical, past surgical, medications, allergies, social, and family), review of systems, and physical examination in the consultation.1 Physicians are under constant pressure from professional fee-coders to meet these requirements.
Since most of this information is already in the medical record, to require that it be documented again in the consultation note is unnecessary duplication. I believe that consultants comply with this requirement mainly to ensure adequate reimbursement, even though they know that the referring medical team will probably not read the repeated information.
Electronic medical record systems, which focus disproportionately on meeting insurers’ requirements governing reimbursement,2–5 have made it easier to create a lengthy consult note by checking boxes in templates and copying and pasting from other parts of the electronic record.2,6–12 Although verbatim copying and pasting may result in punitive audits by insurers, this practice remains common,13 including, in my experience, in consultations.
WHAT ARE THE NEGATIVE EFFECTS OF A NEEDLESSLY LONG CONSULT NOTE?
Time spent on repeating information—even if less time is required when using an electronic system—is clearly time wasted, since this part of the consult note is hardly ever read. Equally bad, the assessment and recommendations section in consult notes continues to be very short, probably because long-standing physician practices change slowly.
An ideal consult note has been described as one that, in addition to addressing the patient care issues, is as brief as possible, avoids duplication of already documented information, and has educational value to the person requesting it.14,15 The educational value of the consultation is especially important in teaching hospitals.
If the only part of the consultation perused in depth consists merely of lists of diagnoses, recommended tests, and therapy and does not include the consultant’s critical reasoning underlying them, the educational value of the consultation is lost.
HOW CAN THE FORMAT BE MADE SHORTER, YET MORE USEFUL?
The note should begin by briefly documenting the reason the consultation was requested. Ideally, institutions should train their staff to state this very specifically. For example, instead of “clearance for surgery,” it is better to ask, “Please identify risks involved in proposed surgery and suggest ways to reduce them.” The former steers the consultant to merely say “cleared for surgery, but with increased risk,” whereas the latter ensures a more specific and detailed response.
The consulting team must review in detail and verify the accuracy of all available information in the patient’s record. Once this is done, instead of repeating it, a statement that all existing information has been thoroughly reviewed should suffice, with mention in a separate paragraph of only the additional relevant positive or negative points in the history related to the issue the consultant has been asked to address.
The consultant shares with all users of the medical record the responsibility of pointing out and correcting any errors in the previously recorded information, thereby decreasing perpetuation of erroneous “chart lore,” an undesirable consequence of copying and pasting. If only previously unrecorded data and corrections to existing information are documented, the referring team is more likely to read the note because it points out relevant information that has been overlooked.
The main part of the document should consist of a detailed assessment and recommendations section, which should include not only a list of diagnoses and recommendations for testing and treatment, but also the consultant’s reasoning behind them, the results of tests already obtained that support the consultant’s conclusions, and information of value for teaching and cost-effective practice. A critically reasoned assessment and recommendation section not only will prove very educational, but by challenging the consultant to justify his or her choices, may discourage unnecessary testing and questionable therapy4,14 and thereby contribute to cost-saving.
My suggestions would not shorten the time spent by the consulting team in evaluating the patient, but only eliminate redundant documentation. I believe the consultation document will be shorter but adequate for patient care, the referring team will read and use the entire document, its educational value will be enhanced, and the time spent on redundant documentation will be saved.
A CASE VIGNETTE
The following vignette (from my own subspecialty) of a patient with acute kidney injury illustrates how a consult note can be made shorter but more useful and educational.
A 78-year-old man had a history of long-standing insulin-requiring diabetes mellitus, hypertension (treated with lisinopril and amlodipine), and benign prostatic hypertrophy. One month earlier, his blood urea nitrogen level had been 15 mg/dL and his serum creatinine had been 1.2 mg/dL.
He presented with a 3-day history of vomiting, diarrhea, and fever, presumed to be viral gastroenteritis. His blood urea nitrogen level was 100 mg, serum creatinine 2.5 mg, and blood glucose 450 mg/dL. Urinalysis revealed 2+ albuminuria, 3+ glucosuria, and 6 red blood cells per high-power field.
In the emergency department he received 2 L of normal saline and regular insulin intravenously, and an indwelling bladder catheter was inserted. He was admitted after 6 hours.
Tests obtained on arrival on the inpatient floor revealed a urinary fractional excretion of sodium of 2.5% and a blood glucose level of 295 mg/dL. His admission history and physical listed his home medications as insulin glargine, amlodipine, lisinopril, and tamsulosin. It also listed the differential diagnosis for acute kidney injury as:
- Prerenal azotemia due to volume depletion
- Rapidly progressive glomerulonephritis to be ruled out in view of proteinuria and microhematuria
- Obstructive uropathy to be ruled out.
Ultrasonography the morning after admission showed normal kidneys and no hydronephrosis. The absence of hydronephrosis was interpreted by the primary team as ruling out obstruction secondary to benign prostatic hypertrophy. The nephrology team saw the patient in consultation the day after admission and discovered the following additional information: urinalysis done 6 months earlier had also shown albuminuria and microhematuria, and the patient had been taking over-the-counter ibuprofen 400 mg three times daily for several days prior to admission.
Table 1 compares consultation documentation in the usual format and in the format I am suggesting. The revised format has much more information of educational value (eg, the importance of reviewing past urinalysis results, asking about over-the-counter medications, factors affecting fractional excretion of sodium, effect of bladder catheterization on hydronephrosis due to benign prostatic hypertrophy, and measuring urine protein only after acute kidney injury resolves). It also encourages cost-effective care (ultrasonography could have been delayed or avoided, and the patient could have been cautioned about ibuprofen-like drugs to decrease the risk of recurrent acute kidney injury).
FINAL THOUGHTS
The modifications I have suggested in consult notes will be accepted only if they are reimbursement-neutral. I hope insurers will not equate a shorter note with an opportunity to lower reimbursement and will see the value in not paying for things almost never read. I hope they will recognize and pay for the effort that went into creating a shorter document that contributes adequately to patient care, provides greater educational value, and may promote cost-effective medical practice. Also, not requiring redundant documentation may reduce or even eliminate undesirable copying and pasting.
Accountable-care organizations are an important part of the Affordable Care Act,16 which went into effect in 2014. Many organizations had already come into existence in the United States before the act became effective, and their numbers and the number of patients covered by them are projected to grow enormously over the next few years.17
Since the accountable-care organization model will rely heavily on capitated reimbursement to contain costs, these organizations are likely to scrutinize and curtail the use of consultations. I believe that a shorter consultation note—yet one that is more useful for patient care, education, and cost-containment—is more likely to pass such scrutiny, especially if it decreases time spent on documentation. Furthermore, unlike the fee-for-service model, in a capitated-payment system it may not be necessary to lengthen consultation documentation just to ensure adequate reimbursement.
- Department of Health and Human Services; Office of Inspector General. Consultations in Medicare: coding and reimbursement. http://oig.hhs.gov/oei/reports/oei-09-02-00030.pdf. Accessed November 24, 2014.
- Hartzband P, Groopman J. Off the record—avoiding the pitfalls of going electronic. N Engl J Med 2008; 358:1656–1658.
- O’Malley AS, Grossman JM, Cohen GR, Kemper NM, Pham HH. Are electronic medical records helpful for care coordination? Experiences of physician practices. J Gen Intern Med 2010; 25:177–185.
- The Center for Public Integrity; Schulte F. Electronic medical records probed for over-billing. Critics question credibility of federal panel charged with investigating. www.publicintegrity.org/2013/02/14/12208/electronic-medical-records-probed-over-billing. Accessed November 24, 2014.
- Li B. Cracking the codes: do electronic medical records facilitate hospital revenue enhancement? www.kellogg.northwestern.edu/faculty/b-li/JMP.pdf. Accessed November 24, 2014.
- Hirschtick RE. A piece of my mind. Copy-and-paste. JAMA 2006; 295:2335–2336.
- Thielke S, Hammond K, Helbig S. Copying and pasting of examinations within the electronic medical record. Int J Med Inform 2007; 76(suppl 1):S122–S128.
- Hanlon JT. The electronic medical record: diving into a shallow pool? Cleve Clin J Med 2010; 77:408–411.
- Fitzgerald FT. The emperor’s new clothes. Ann Intern Med 2012; 156:396–397.
- Bernat JL. Ethical and quality pitfalls in electronic health records. Neurology 2013; 80:1057–1061.
- Thornton JD, Schold JD, Venkateshaiah L, Lander B. Prevalence of copied information by attendings and residents in critical care progress notes. Crit Care Med 2013; 41:382–388.
- Foote RS. The challenge to the medical record. JAMA Intern Med 2013; 173:1171–1172.
- Tamburello LM. The road to EMR noncompliance and fraud is paved with cut and paste. MD Advis 2013; 6:24–30.
- Goldman L, Lee T, Rudd P. Ten commandments for effective consultations. Arch Intern Med 1983; 143:1753–1755.
- Salerno SM, Hurst FP, Halvorson S, Mercado DL. Principles of effective consultation: an update for the 21st-century consultant. Arch Intern Med 2007; 167:271–275.
- Longworth DL. Accountable care organizations, the patient-centered medical home, and health care reform: what does it all mean? Cleve Clin J Med 2011; 78:571–582.
- Meyer H. Many accountable care organizations are now up and running, if not off to the races. Health Aff (Millwood) 2012; 31:2363–2367.
After 4 decades of clinical practice in a teaching hospital, I believe that the notes we write to document medical consultations are too long. When I review them for my own patients, the only part I read is the consultant’s assessment and diagnostic and therapeutic recommendations. Many of my colleagues and trainees do the same.
In the old days, when medical records were handwritten, the first three pages of my hospital’s four-page consultation form were for the history, review of systems, physical examination, and test results. The top two-thirds of the last page was for diagnostic impressions and recommendations for additional testing and treatment, to be completed by the trainee performing the consultation.
This left only the bottom third of this page for attestation and additional remarks from the senior consultant. Often, this last (but most used) page was just a bullet list of diagnostic possibilities and suggested tests and treatments, with nothing about the critical reasoning underlying the differential diagnosis and recommendations. This was probably the result of fatigue from having to fill in the first three pages by hand, and then having only limited space on the final page.
Even though the written record has been replaced by the electronic medical record in my hospital, consult notes continue to be at least as long as before, without any change in the length of the assessment and recommendations section. I would guess this is true in most institutions and practices that have switched to an electronic record system.
WHY ARE CONSULT NOTES SO LONG?
The main factor contributing to the lengthy consultation document is that the Center for Medicare and Medicaid Services, with other third-party payers following suit, ties the level of reimbursement to detailed documentation of the history (present, past medical, past surgical, medications, allergies, social, and family), review of systems, and physical examination in the consultation.1 Physicians are under constant pressure from professional fee-coders to meet these requirements.
Since most of this information is already in the medical record, to require that it be documented again in the consultation note is unnecessary duplication. I believe that consultants comply with this requirement mainly to ensure adequate reimbursement, even though they know that the referring medical team will probably not read the repeated information.
Electronic medical record systems, which focus disproportionately on meeting insurers’ requirements governing reimbursement,2–5 have made it easier to create a lengthy consult note by checking boxes in templates and copying and pasting from other parts of the electronic record.2,6–12 Although verbatim copying and pasting may result in punitive audits by insurers, this practice remains common,13 including, in my experience, in consultations.
WHAT ARE THE NEGATIVE EFFECTS OF A NEEDLESSLY LONG CONSULT NOTE?
Time spent on repeating information—even if less time is required when using an electronic system—is clearly time wasted, since this part of the consult note is hardly ever read. Equally bad, the assessment and recommendations section in consult notes continues to be very short, probably because long-standing physician practices change slowly.
An ideal consult note has been described as one that, in addition to addressing the patient care issues, is as brief as possible, avoids duplication of already documented information, and has educational value to the person requesting it.14,15 The educational value of the consultation is especially important in teaching hospitals.
If the only part of the consultation perused in depth consists merely of lists of diagnoses, recommended tests, and therapy and does not include the consultant’s critical reasoning underlying them, the educational value of the consultation is lost.
HOW CAN THE FORMAT BE MADE SHORTER, YET MORE USEFUL?
The note should begin by briefly documenting the reason the consultation was requested. Ideally, institutions should train their staff to state this very specifically. For example, instead of “clearance for surgery,” it is better to ask, “Please identify risks involved in proposed surgery and suggest ways to reduce them.” The former steers the consultant to merely say “cleared for surgery, but with increased risk,” whereas the latter ensures a more specific and detailed response.
The consulting team must review in detail and verify the accuracy of all available information in the patient’s record. Once this is done, instead of repeating it, a statement that all existing information has been thoroughly reviewed should suffice, with mention in a separate paragraph of only the additional relevant positive or negative points in the history related to the issue the consultant has been asked to address.
The consultant shares with all users of the medical record the responsibility of pointing out and correcting any errors in the previously recorded information, thereby decreasing perpetuation of erroneous “chart lore,” an undesirable consequence of copying and pasting. If only previously unrecorded data and corrections to existing information are documented, the referring team is more likely to read the note because it points out relevant information that has been overlooked.
The main part of the document should consist of a detailed assessment and recommendations section, which should include not only a list of diagnoses and recommendations for testing and treatment, but also the consultant’s reasoning behind them, the results of tests already obtained that support the consultant’s conclusions, and information of value for teaching and cost-effective practice. A critically reasoned assessment and recommendation section not only will prove very educational, but by challenging the consultant to justify his or her choices, may discourage unnecessary testing and questionable therapy4,14 and thereby contribute to cost-saving.
My suggestions would not shorten the time spent by the consulting team in evaluating the patient, but only eliminate redundant documentation. I believe the consultation document will be shorter but adequate for patient care, the referring team will read and use the entire document, its educational value will be enhanced, and the time spent on redundant documentation will be saved.
A CASE VIGNETTE
The following vignette (from my own subspecialty) of a patient with acute kidney injury illustrates how a consult note can be made shorter but more useful and educational.
A 78-year-old man had a history of long-standing insulin-requiring diabetes mellitus, hypertension (treated with lisinopril and amlodipine), and benign prostatic hypertrophy. One month earlier, his blood urea nitrogen level had been 15 mg/dL and his serum creatinine had been 1.2 mg/dL.
He presented with a 3-day history of vomiting, diarrhea, and fever, presumed to be viral gastroenteritis. His blood urea nitrogen level was 100 mg, serum creatinine 2.5 mg, and blood glucose 450 mg/dL. Urinalysis revealed 2+ albuminuria, 3+ glucosuria, and 6 red blood cells per high-power field.
In the emergency department he received 2 L of normal saline and regular insulin intravenously, and an indwelling bladder catheter was inserted. He was admitted after 6 hours.
Tests obtained on arrival on the inpatient floor revealed a urinary fractional excretion of sodium of 2.5% and a blood glucose level of 295 mg/dL. His admission history and physical listed his home medications as insulin glargine, amlodipine, lisinopril, and tamsulosin. It also listed the differential diagnosis for acute kidney injury as:
- Prerenal azotemia due to volume depletion
- Rapidly progressive glomerulonephritis to be ruled out in view of proteinuria and microhematuria
- Obstructive uropathy to be ruled out.
Ultrasonography the morning after admission showed normal kidneys and no hydronephrosis. The absence of hydronephrosis was interpreted by the primary team as ruling out obstruction secondary to benign prostatic hypertrophy. The nephrology team saw the patient in consultation the day after admission and discovered the following additional information: urinalysis done 6 months earlier had also shown albuminuria and microhematuria, and the patient had been taking over-the-counter ibuprofen 400 mg three times daily for several days prior to admission.
Table 1 compares consultation documentation in the usual format and in the format I am suggesting. The revised format has much more information of educational value (eg, the importance of reviewing past urinalysis results, asking about over-the-counter medications, factors affecting fractional excretion of sodium, effect of bladder catheterization on hydronephrosis due to benign prostatic hypertrophy, and measuring urine protein only after acute kidney injury resolves). It also encourages cost-effective care (ultrasonography could have been delayed or avoided, and the patient could have been cautioned about ibuprofen-like drugs to decrease the risk of recurrent acute kidney injury).
FINAL THOUGHTS
The modifications I have suggested in consult notes will be accepted only if they are reimbursement-neutral. I hope insurers will not equate a shorter note with an opportunity to lower reimbursement and will see the value in not paying for things almost never read. I hope they will recognize and pay for the effort that went into creating a shorter document that contributes adequately to patient care, provides greater educational value, and may promote cost-effective medical practice. Also, not requiring redundant documentation may reduce or even eliminate undesirable copying and pasting.
Accountable-care organizations are an important part of the Affordable Care Act,16 which went into effect in 2014. Many organizations had already come into existence in the United States before the act became effective, and their numbers and the number of patients covered by them are projected to grow enormously over the next few years.17
Since the accountable-care organization model will rely heavily on capitated reimbursement to contain costs, these organizations are likely to scrutinize and curtail the use of consultations. I believe that a shorter consultation note—yet one that is more useful for patient care, education, and cost-containment—is more likely to pass such scrutiny, especially if it decreases time spent on documentation. Furthermore, unlike the fee-for-service model, in a capitated-payment system it may not be necessary to lengthen consultation documentation just to ensure adequate reimbursement.
After 4 decades of clinical practice in a teaching hospital, I believe that the notes we write to document medical consultations are too long. When I review them for my own patients, the only part I read is the consultant’s assessment and diagnostic and therapeutic recommendations. Many of my colleagues and trainees do the same.
In the old days, when medical records were handwritten, the first three pages of my hospital’s four-page consultation form were for the history, review of systems, physical examination, and test results. The top two-thirds of the last page was for diagnostic impressions and recommendations for additional testing and treatment, to be completed by the trainee performing the consultation.
This left only the bottom third of this page for attestation and additional remarks from the senior consultant. Often, this last (but most used) page was just a bullet list of diagnostic possibilities and suggested tests and treatments, with nothing about the critical reasoning underlying the differential diagnosis and recommendations. This was probably the result of fatigue from having to fill in the first three pages by hand, and then having only limited space on the final page.
Even though the written record has been replaced by the electronic medical record in my hospital, consult notes continue to be at least as long as before, without any change in the length of the assessment and recommendations section. I would guess this is true in most institutions and practices that have switched to an electronic record system.
WHY ARE CONSULT NOTES SO LONG?
The main factor contributing to the lengthy consultation document is that the Center for Medicare and Medicaid Services, with other third-party payers following suit, ties the level of reimbursement to detailed documentation of the history (present, past medical, past surgical, medications, allergies, social, and family), review of systems, and physical examination in the consultation.1 Physicians are under constant pressure from professional fee-coders to meet these requirements.
Since most of this information is already in the medical record, to require that it be documented again in the consultation note is unnecessary duplication. I believe that consultants comply with this requirement mainly to ensure adequate reimbursement, even though they know that the referring medical team will probably not read the repeated information.
Electronic medical record systems, which focus disproportionately on meeting insurers’ requirements governing reimbursement,2–5 have made it easier to create a lengthy consult note by checking boxes in templates and copying and pasting from other parts of the electronic record.2,6–12 Although verbatim copying and pasting may result in punitive audits by insurers, this practice remains common,13 including, in my experience, in consultations.
WHAT ARE THE NEGATIVE EFFECTS OF A NEEDLESSLY LONG CONSULT NOTE?
Time spent on repeating information—even if less time is required when using an electronic system—is clearly time wasted, since this part of the consult note is hardly ever read. Equally bad, the assessment and recommendations section in consult notes continues to be very short, probably because long-standing physician practices change slowly.
An ideal consult note has been described as one that, in addition to addressing the patient care issues, is as brief as possible, avoids duplication of already documented information, and has educational value to the person requesting it.14,15 The educational value of the consultation is especially important in teaching hospitals.
If the only part of the consultation perused in depth consists merely of lists of diagnoses, recommended tests, and therapy and does not include the consultant’s critical reasoning underlying them, the educational value of the consultation is lost.
HOW CAN THE FORMAT BE MADE SHORTER, YET MORE USEFUL?
The note should begin by briefly documenting the reason the consultation was requested. Ideally, institutions should train their staff to state this very specifically. For example, instead of “clearance for surgery,” it is better to ask, “Please identify risks involved in proposed surgery and suggest ways to reduce them.” The former steers the consultant to merely say “cleared for surgery, but with increased risk,” whereas the latter ensures a more specific and detailed response.
The consulting team must review in detail and verify the accuracy of all available information in the patient’s record. Once this is done, instead of repeating it, a statement that all existing information has been thoroughly reviewed should suffice, with mention in a separate paragraph of only the additional relevant positive or negative points in the history related to the issue the consultant has been asked to address.
The consultant shares with all users of the medical record the responsibility of pointing out and correcting any errors in the previously recorded information, thereby decreasing perpetuation of erroneous “chart lore,” an undesirable consequence of copying and pasting. If only previously unrecorded data and corrections to existing information are documented, the referring team is more likely to read the note because it points out relevant information that has been overlooked.
The main part of the document should consist of a detailed assessment and recommendations section, which should include not only a list of diagnoses and recommendations for testing and treatment, but also the consultant’s reasoning behind them, the results of tests already obtained that support the consultant’s conclusions, and information of value for teaching and cost-effective practice. A critically reasoned assessment and recommendation section not only will prove very educational, but by challenging the consultant to justify his or her choices, may discourage unnecessary testing and questionable therapy4,14 and thereby contribute to cost-saving.
My suggestions would not shorten the time spent by the consulting team in evaluating the patient, but only eliminate redundant documentation. I believe the consultation document will be shorter but adequate for patient care, the referring team will read and use the entire document, its educational value will be enhanced, and the time spent on redundant documentation will be saved.
A CASE VIGNETTE
The following vignette (from my own subspecialty) of a patient with acute kidney injury illustrates how a consult note can be made shorter but more useful and educational.
A 78-year-old man had a history of long-standing insulin-requiring diabetes mellitus, hypertension (treated with lisinopril and amlodipine), and benign prostatic hypertrophy. One month earlier, his blood urea nitrogen level had been 15 mg/dL and his serum creatinine had been 1.2 mg/dL.
He presented with a 3-day history of vomiting, diarrhea, and fever, presumed to be viral gastroenteritis. His blood urea nitrogen level was 100 mg, serum creatinine 2.5 mg, and blood glucose 450 mg/dL. Urinalysis revealed 2+ albuminuria, 3+ glucosuria, and 6 red blood cells per high-power field.
In the emergency department he received 2 L of normal saline and regular insulin intravenously, and an indwelling bladder catheter was inserted. He was admitted after 6 hours.
Tests obtained on arrival on the inpatient floor revealed a urinary fractional excretion of sodium of 2.5% and a blood glucose level of 295 mg/dL. His admission history and physical listed his home medications as insulin glargine, amlodipine, lisinopril, and tamsulosin. It also listed the differential diagnosis for acute kidney injury as:
- Prerenal azotemia due to volume depletion
- Rapidly progressive glomerulonephritis to be ruled out in view of proteinuria and microhematuria
- Obstructive uropathy to be ruled out.
Ultrasonography the morning after admission showed normal kidneys and no hydronephrosis. The absence of hydronephrosis was interpreted by the primary team as ruling out obstruction secondary to benign prostatic hypertrophy. The nephrology team saw the patient in consultation the day after admission and discovered the following additional information: urinalysis done 6 months earlier had also shown albuminuria and microhematuria, and the patient had been taking over-the-counter ibuprofen 400 mg three times daily for several days prior to admission.
Table 1 compares consultation documentation in the usual format and in the format I am suggesting. The revised format has much more information of educational value (eg, the importance of reviewing past urinalysis results, asking about over-the-counter medications, factors affecting fractional excretion of sodium, effect of bladder catheterization on hydronephrosis due to benign prostatic hypertrophy, and measuring urine protein only after acute kidney injury resolves). It also encourages cost-effective care (ultrasonography could have been delayed or avoided, and the patient could have been cautioned about ibuprofen-like drugs to decrease the risk of recurrent acute kidney injury).
FINAL THOUGHTS
The modifications I have suggested in consult notes will be accepted only if they are reimbursement-neutral. I hope insurers will not equate a shorter note with an opportunity to lower reimbursement and will see the value in not paying for things almost never read. I hope they will recognize and pay for the effort that went into creating a shorter document that contributes adequately to patient care, provides greater educational value, and may promote cost-effective medical practice. Also, not requiring redundant documentation may reduce or even eliminate undesirable copying and pasting.
Accountable-care organizations are an important part of the Affordable Care Act,16 which went into effect in 2014. Many organizations had already come into existence in the United States before the act became effective, and their numbers and the number of patients covered by them are projected to grow enormously over the next few years.17
Since the accountable-care organization model will rely heavily on capitated reimbursement to contain costs, these organizations are likely to scrutinize and curtail the use of consultations. I believe that a shorter consultation note—yet one that is more useful for patient care, education, and cost-containment—is more likely to pass such scrutiny, especially if it decreases time spent on documentation. Furthermore, unlike the fee-for-service model, in a capitated-payment system it may not be necessary to lengthen consultation documentation just to ensure adequate reimbursement.
- Department of Health and Human Services; Office of Inspector General. Consultations in Medicare: coding and reimbursement. http://oig.hhs.gov/oei/reports/oei-09-02-00030.pdf. Accessed November 24, 2014.
- Hartzband P, Groopman J. Off the record—avoiding the pitfalls of going electronic. N Engl J Med 2008; 358:1656–1658.
- O’Malley AS, Grossman JM, Cohen GR, Kemper NM, Pham HH. Are electronic medical records helpful for care coordination? Experiences of physician practices. J Gen Intern Med 2010; 25:177–185.
- The Center for Public Integrity; Schulte F. Electronic medical records probed for over-billing. Critics question credibility of federal panel charged with investigating. www.publicintegrity.org/2013/02/14/12208/electronic-medical-records-probed-over-billing. Accessed November 24, 2014.
- Li B. Cracking the codes: do electronic medical records facilitate hospital revenue enhancement? www.kellogg.northwestern.edu/faculty/b-li/JMP.pdf. Accessed November 24, 2014.
- Hirschtick RE. A piece of my mind. Copy-and-paste. JAMA 2006; 295:2335–2336.
- Thielke S, Hammond K, Helbig S. Copying and pasting of examinations within the electronic medical record. Int J Med Inform 2007; 76(suppl 1):S122–S128.
- Hanlon JT. The electronic medical record: diving into a shallow pool? Cleve Clin J Med 2010; 77:408–411.
- Fitzgerald FT. The emperor’s new clothes. Ann Intern Med 2012; 156:396–397.
- Bernat JL. Ethical and quality pitfalls in electronic health records. Neurology 2013; 80:1057–1061.
- Thornton JD, Schold JD, Venkateshaiah L, Lander B. Prevalence of copied information by attendings and residents in critical care progress notes. Crit Care Med 2013; 41:382–388.
- Foote RS. The challenge to the medical record. JAMA Intern Med 2013; 173:1171–1172.
- Tamburello LM. The road to EMR noncompliance and fraud is paved with cut and paste. MD Advis 2013; 6:24–30.
- Goldman L, Lee T, Rudd P. Ten commandments for effective consultations. Arch Intern Med 1983; 143:1753–1755.
- Salerno SM, Hurst FP, Halvorson S, Mercado DL. Principles of effective consultation: an update for the 21st-century consultant. Arch Intern Med 2007; 167:271–275.
- Longworth DL. Accountable care organizations, the patient-centered medical home, and health care reform: what does it all mean? Cleve Clin J Med 2011; 78:571–582.
- Meyer H. Many accountable care organizations are now up and running, if not off to the races. Health Aff (Millwood) 2012; 31:2363–2367.
- Department of Health and Human Services; Office of Inspector General. Consultations in Medicare: coding and reimbursement. http://oig.hhs.gov/oei/reports/oei-09-02-00030.pdf. Accessed November 24, 2014.
- Hartzband P, Groopman J. Off the record—avoiding the pitfalls of going electronic. N Engl J Med 2008; 358:1656–1658.
- O’Malley AS, Grossman JM, Cohen GR, Kemper NM, Pham HH. Are electronic medical records helpful for care coordination? Experiences of physician practices. J Gen Intern Med 2010; 25:177–185.
- The Center for Public Integrity; Schulte F. Electronic medical records probed for over-billing. Critics question credibility of federal panel charged with investigating. www.publicintegrity.org/2013/02/14/12208/electronic-medical-records-probed-over-billing. Accessed November 24, 2014.
- Li B. Cracking the codes: do electronic medical records facilitate hospital revenue enhancement? www.kellogg.northwestern.edu/faculty/b-li/JMP.pdf. Accessed November 24, 2014.
- Hirschtick RE. A piece of my mind. Copy-and-paste. JAMA 2006; 295:2335–2336.
- Thielke S, Hammond K, Helbig S. Copying and pasting of examinations within the electronic medical record. Int J Med Inform 2007; 76(suppl 1):S122–S128.
- Hanlon JT. The electronic medical record: diving into a shallow pool? Cleve Clin J Med 2010; 77:408–411.
- Fitzgerald FT. The emperor’s new clothes. Ann Intern Med 2012; 156:396–397.
- Bernat JL. Ethical and quality pitfalls in electronic health records. Neurology 2013; 80:1057–1061.
- Thornton JD, Schold JD, Venkateshaiah L, Lander B. Prevalence of copied information by attendings and residents in critical care progress notes. Crit Care Med 2013; 41:382–388.
- Foote RS. The challenge to the medical record. JAMA Intern Med 2013; 173:1171–1172.
- Tamburello LM. The road to EMR noncompliance and fraud is paved with cut and paste. MD Advis 2013; 6:24–30.
- Goldman L, Lee T, Rudd P. Ten commandments for effective consultations. Arch Intern Med 1983; 143:1753–1755.
- Salerno SM, Hurst FP, Halvorson S, Mercado DL. Principles of effective consultation: an update for the 21st-century consultant. Arch Intern Med 2007; 167:271–275.
- Longworth DL. Accountable care organizations, the patient-centered medical home, and health care reform: what does it all mean? Cleve Clin J Med 2011; 78:571–582.
- Meyer H. Many accountable care organizations are now up and running, if not off to the races. Health Aff (Millwood) 2012; 31:2363–2367.
Updated guidelines on cardiovascular evaluation before noncardiac surgery: A view from the trenches
Guidelines jointly issued by the American College of Cardiology and American Heart Association (ACC/AHA)1 provide a framework for evaluating and managing perioperative cardiac risk in noncardiac surgery. An overriding theme in successive documents from these organizations through the years has been that preoperative intervention, coronary artery bypass grafting, or percutaneous coronary intervention is rarely necessary just to get the patient through surgery, unless it is otherwise indicated independent of the need for surgery.
This article highlights some of the key recommendations in the 2014 updates to these guidelines,1–3 how they differ from previous guidelines,4 and the ongoing challenges and unresolved issues facing physicians involved in perioperative care.
Of note, while these guidelines were being updated, Erasmus University5 expressed concern about the scientific integrity of some of the Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE) trials. As a result, the evidence review committee included these trials in its analysis but not in a systematic review of beta-blockers.2 These trials were not included in the clinical practice guideline supplements and tables but were cited in the text if relevant.
The European Society of Cardiology and European Society of Anesthesiology6 revised their guidelines concurrently with but independently of the ACC/AHA, and although they discussed and aligned some recommendations, many differences remain between the two sets of guidelines. Readers should consult the full guidelines for more detailed information.1
THE ROLE OF THE PREOPERATIVE CARDIAC EVALUATION
The purpose of preoperative medical evaluation is not to "get medical clearance" but rather to evaluate the patient’s medical status and risk of complications. The process includes:
- Identifying risk factors and assessing their severity and stability
- Establishing a clinical risk profile for informed and shared decision-making
- Recommending needed changes in management, further testing, or specialty consultation.
The updated guidelines emphasize the importance of communication among the perioperative team and with the patient. They reiterate the focus on appropriateness of care and cost containment—one should order a test only if the result may change the patient’s management.
HOW URGENT IS SURGERY? HOW RISKY?
The new guidelines classify the urgency of surgery as follows:
- Emergency (necessary within 6 hours)
- Urgent (necessary within 6–24 hours)
- Time-sensitive (can delay 1–6 weeks)
- Elective (can delay up to 1 year).
Surgical risk is now classified as either low (< 1% risk of major adverse cardiac events) or elevated (≥ 1%) on the basis of surgical and patient characteristics. Previous schemas included an intermediate-risk category. Low-risk procedures include endoscopic procedures, superficial procedures, cataract surgery, breast surgery, and ambulatory surgery. Elevated-risk procedures include vascular surgery, intraperitoneal and intrathoracic surgery, head and neck surgery, orthopedic surgery, and prostate surgery.
Risk calculators and biomarkers
To estimate the perioperative risk of major adverse cardiac events, the guidelines suggest incorporating the Revised Cardiac Risk Index (RCRI)7 with an estimation of surgical risk or using a newer surgical risk calculator derived from a database of the American College of Surgeons’ National Surgical Quality Improvement Project (ACS NSQIP).
The RCRI is based on six risk factors, each worth 1 point:
- High-risk surgery
- Ischemic heart disease
- Heart failure
- Stroke or transient ischemic attack
- Diabetes requiring insulin
- Renal insufficiency (serum creatinine > 2.0 mg/dL).7
MICA. The Myocardial Infarction or Cardiac Arrest (MICA) calculator8 has a narrower focus and was validated in only one center.
ACS NSQIP. The recommended newer ACS NSQIP surgical risk calculator9 provides an estimate of procedure-specific risk based on Current Procedural Terminology code and includes 21 patient-specific variables to predict death, major adverse cardiac events, and eight other outcomes. While more comprehensive, this risk calculator has yet to be validated outside of the ACS NSQIP database.
Reconstructed RCRI. The RCRI has been externally validated, but it underestimates risk in major vascular surgery and was outperformed by the MICA calculator. Although not discussed in the new guidelines, a recently published "reconstructed RCRI,"10 in which a serum creatinine level greater than 2 mg/dL in the original RCRI is replaced by a glomerular filtration rate less than 30 mL/min and diabetes is eliminated, may outperform the standard RCRI. A patient with either an RCRI score or a reconstructed RCRI score of 0 or 1 would be considered to be at low risk, whereas patients with two or more risk factors would have an elevated risk.
Cardiac biomarkers, primarily B-type natriuretic peptide (BNP) and N-terminal (NT) proBNP, are independent predictors of cardiac risk, and their addition to preoperative risk indices may provide incremental predictive value. However, how to use these biomarkers and whether any treatment aimed at them will reduce risk is unclear, and the new guidelines did not recommend their routine use.
CLINICAL RISK FACTORS
Coronary artery disease
Ischemic symptoms, a history of myocardial infarction, and elevated cardiac biomarkers are individually associated with perioperative risk of morbidity and death. The risk is modified by how long ago the infarction occurred, whether the patient underwent coronary revascularization, and if so, what type (bypass grafting or percutaneous coronary intervention). A patient with acute coronary syndrome (currently or in the recent past) is at higher risk, and should have elective surgery delayed and be referred for cardiac evaluation and management according to guidelines.
Heart failure
In terms of posing a risk for major adverse cardiac events, heart failure is at least equal to coronary artery disease, and is possibly worse. Its impact depends on its stability, its symptoms, and the patient’s left ventricular function. Symptomatic decompensated heart failure and depressed left ventricular function (ejection fraction < 30% or 40%) confer higher risk than asymptomatic heart failure and preserved left ventricular function. However, evidence is limited with respect to asymptomatic left ventricular dysfunction and diastolic dysfunction. Patients with stable heart failure treated according to guidelines may have better perioperative outcomes.
Valvular heart disease
Significant valvular heart disease is associated with increased risk of postoperative cardiac complications. This risk depends on the type and severity of the valvular lesion and type of noncardiac surgery, but can be minimized by clinical and echocardiographic assessment, choosing appropriate anesthesia, and closer perioperative monitoring. Aortic and mitral stenosis are associated with greater risk of perioperative adverse cardiac events than regurgitant valvular disease.
Echocardiography is recommended in patients suspected of having moderate to severe stenotic or regurgitant lesions if it has not been done within the past year or if the patient’s clinical condition has worsened.
If indicated, valvular intervention can reduce perioperative risk in these patients. Even if the planned noncardiac surgery is high-risk, it may be reasonable to proceed with it (using appropriate perioperative hemodynamic monitoring, which is not specified but typically would be with an arterial line, central line, and possibly a pulmonary arterial catheter) in patients who have asymptomatic severe aortic or mitral regurgitation or aortic stenosis. Surgery may also be reasonable in patients with asymptomatic severe mitral stenosis who are not candidates for repair.
Arrhythmias
Cardiac arrhythmias and conduction defects are often seen in the perioperative period, but there is only limited evidence as to how they affect surgical risk. In addition to their hemodynamic effects, certain arrhythmias (atrial fibrillation, ventricular tachycardia) often indicate underlying structural heart disease, which requires further evaluation before surgery.
The new guidelines refer the reader to previously published clinical practice guidelines for atrial fibrillation,11 supraventricular arrhythmias,12 and device-based therapy.13
ALGORITHM FOR PREOPERATIVE CARDIAC ASSESSMENT
The new algorithm for evaluating a patient who is known to have coronary artery disease or risk factors for it has seven steps (Figure 1).1,11,12,14–17 It differs from the previous algorithm in several details:
- Instead of listing the four active cardiac conditions for which elective surgery should be delayed while the patient is being evaluated and treated (unstable coronary syndrome, decompensated heart failure, significant arrhythmias, severe valvular heart disease), the new version specifically asks about acute coronary syndrome and recommends cardiac evaluation and treatment according to guidelines. A footnote directs readers to other clinical practice guidelines for symptomatic heart failure,14 valvular heart disease,15 and arrhythmias.11,12
- Instead of asking if the procedure is low-risk, the guidelines recommend estimating risk of major adverse cardiac events on the basis of combined clinical and surgical risk and define only two categories: low or elevated. Patients at low risk proceed to surgery with no further testing, as in the earlier algorithm.
- "Excellent" exercise capacity (> 10 metabolic equivalents of task [METs]) is separated from "moderate/good" (4–10 METs), presumably to indicate a stronger recommendation, but patients in both categories proceed to surgery as before.
- If the patient cannot exercise to at least 4 METs, the new algorithm asks whether further testing will affect decision-making or perioperative care (an addition to the previous algorithm). This entails discussing with the patient and perioperative team whether the original surgery will be performed and whether the patient is willing to undergo revascularization if indicated. If so, pharmacologic stress testing is recommended. Previously, this decision also included the number of RCRI factors as well as the type of surgery (vascular or nonvascular).
- If testing will not affect the decision or if the stress test is normal, in addition to recommending proceeding to surgery according to guidelines the new algorithm also lists an option for alternative strategies, including palliation.
- If the stress test is abnormal, especially with left main disease, it recommends coronary revascularization according to the 2011 clinical practice guidelines.18,19
TESTING FOR LEFT VENTRICULAR DYSFUNCTION OR ISCHEMIA
In patients with dyspnea of unexplained cause or worsening dyspnea, assessment of left ventricular function is reasonable, but this is not part of a routine preoperative evaluation.
Pharmacologic stress testing is reasonable for patients at elevated risk with poor functional capacity if the results will change their management, but it is not useful for patients undergoing low-risk surgery. Although dobutamine stress echocardiography may be slightly superior to pharmacologic myocardial perfusion imaging, there are no head-to-head randomized controlled trials, and the guidelines suggest considering local expertise in deciding which test to use.
The presence of moderate to large areas of ischemia (reversible perfusion defects or new wall-motion abnormalities) is associated with risk of perioperative myocardial infarction or death, whereas evidence of an old infarction is associated with long-term but not short-term risk. The negative predictive value of these tests in predicting postoperative cardiac events is high (> 90%), but the positive predictive value is low.
CORONARY REVASCULARIZATION
Coronary artery bypass grafting and percutaneous coronary intervention
The guidelines recommend coronary revascularization before noncardiac surgery only when it is indicated anyway, on the basis of existing clinical practice guidelines.
Whether performing percutaneous coronary intervention before surgery will reduce perioperative cardiac complications is uncertain, and coronary revascularization should not be routinely performed solely to reduce perioperative cardiac events. The only two randomized controlled trials, Coronary Artery Revascularization Prophylaxis (CARP)20 and DECREASE V21 evaluating prophylactic coronary revascularization before noncardiac surgery found no difference in either short-term or long-term outcomes, although subgroup analysis found a survival benefit in patients with left main disease who underwent bypass grafting. Preoperative percutaneous coronary intervention should be limited to patients with left main disease in whom comorbidities preclude bypass surgery and those with unstable coronary disease who may benefit from early invasive management.
The urgency and timing of the noncardiac surgery needs to be taken into account if percutaneous coronary intervention is being considered because of the need for antiplatelet therapy after the procedure, and the potential risks of bleeding and stent thrombosis. If the planned surgery is deemed time-sensitive, then balloon angioplasty or bare-metal stenting is preferred over placement of a drug-eluting stent.
The new guidelines continue to recommend that elective noncardiac surgery be delayed at least 14 days after balloon angioplasty, 30 days after bare-metal stent implantation, and ideally 365 days after drug-eluting stent placement, and reiterate that it is potentially harmful to perform elective surgery within these time frames without any antiplatelet therapy. However, a new class IIb recommendation (benefit ≥ risk) states that "elective noncardiac surgery after [drug-eluting stent] implantation may be considered after 180 days if the risk of further delay is greater than the expected risks of ischemia and stent thrombosis."
This is an important addition to the guidelines because we are often faced with patients needing to undergo surgery in the 6 to 12 months after placement of a drug-eluting stent. Based on previous guidelines, whether it was safe to proceed in this setting created controversy among the perioperative team caring for the patient, and surgery was often delayed unnecessarily. Recent studies22,23 suggest that the newer drug-eluting stents may require a shorter duration of dual antiplatelet therapy, at least in the nonsurgical setting.
MEDICAL THERAPY
Antiplatelet therapy: Stop or continue?
The risk of perioperative bleeding if antiplatelet drugs are continued must be weighed against the risk of stent thrombosis and ischemia if they are stopped before the recommended duration of therapy. Ideally, some antiplatelet therapy should be continued perioperatively in these situations, but the guidelines recommend that a consensus decision among the treating physicians should be made regarding the relative risks of surgery and discontinuation or continuation of antiplatelet therapy. Whenever possible, aspirin should be continued in these patients.
Although the Perioperative Ischemic Evaluation (POISE)-2 trial24 found that perioperative aspirin use was not associated with lower rates of postoperative myocardial infarction or death, it increased bleeding. Patients with stents who had not completed the recommended duration of antiplatelet therapy were excluded from the trial. Additionally, only 5% of the study patients had undergone percutaneous coronary intervention.
According to the guidelines and package inserts, if antiplatelet agents need to be discontinued before surgery, aspirin can be stopped 3 to 7 days before, clopidogrel and ticagrelor 5 days before, and prasugrel 7 days before. In patients without stents, it may be reasonable to continue aspirin perioperatively if the risk of cardiac events outweighs the risk of bleeding, but starting aspirin is not beneficial for patients undergoing elective noncardiac noncarotid surgery unless the risk of ischemic events outweighs the risk of bleeding.
Beta-blockers
In view of the issue of scientific integrity of the DECREASE trials, a separately commissioned systematic review2 of perioperative beta-blocker therapy was performed. This review suggested that giving beta-blockers before surgery was associated with fewer postoperative cardiac events, primarily ischemia and nonfatal myocardial infarction, but few data supported their use to reduce postoperative mortality. Beta-blocker use was associated with adverse outcomes that included bradycardia and stroke. These findings were similar with the inclusion or exclusion of the DECREASE trials in question or of the POISE trial.25
In addition to recommending continuing beta-blockers in patients already on them (class I—the highest recommendation), the guidelines say that it may be reasonable to start them in patients with intermediate- or high-risk ischemia on stress tests as well as in patients with three or more RCRI risk factors (class IIb). In the absence of these indications, initiating beta-blockers preoperatively to reduce risk even in patients with long-term indications is of uncertain benefit. They also recommended starting beta-blockers more than 1 day preoperatively, preferably at least 2 to 7 days before, and note that it was harmful to start them on the day of surgery, particularly at high doses, and with long-acting formulations.
Additionally, there is evidence of differences in outcome within the class of beta-blockers, with the more cardioselective drugs bisoprolol and atenolol being associated with more favorable outcomes than metoprolol in observational studies.
Statins
Multiple observational trials have reported that statins are associated with decreased perioperative morbidity and mortality. Limited evidence from three randomized controlled trials (including two from the discredited DECREASE group) suggests that there is a benefit in patients undergoing vascular surgery, but it is unclear for nonvascular surgery.26–30
The ACC/AHA guidelines again give a class I recommendation to continue statin therapy perioperatively in patients already taking statins and undergoing noncardiac surgery, as there is some evidence that statin withdrawal is associated with increased risk. The guidelines comment that starting statin therapy perioperatively is reasonable for patients undergoing vascular surgery (class IIa) and may be considered in patients with other clinical guideline indications who are undergoing elevated-risk surgery (class IIb).
The mechanism of this benefit is unclear and may relate to the pleotropic as well as the lipid-lowering effects of the statins. Statins may also have beneficial effects in reducing the incidence of acute kidney injury and postoperative atrial fibrillation.
Whether a particular statin, dose, or time of initiation before surgery affects risk is also unknown at this time. The European guidelines6 recommend starting a longer-acting statin ideally at least 2 weeks before surgery for maximal plaque-stabilizing effects.
The risk of statin-induced myopathy, rhabdomyolysis, and hepatic injury appears to be minimal.
Other medications
Of note, the new guidelines do not recommend starting alpha-2 agonists for preventing cardiac events in patients undergoing noncardiac surgery. Despite previous evidence from smaller studies suggesting a benefit, the POISE-2 trial31 demonstrated that perioperative use of clonidine did not reduce cardiac events and was associated with a significant increase in hypotension and nonfatal cardiac arrest. However, clonidine should be continued in patients already taking it.
A somewhat surprising recommendation is that it is reasonable to continue angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), and if they are held before surgery, to restart them as soon as possible postoperatively (class IIa). The guidelines note reports of increased hypotension associated with induction of anesthesia in patients taking these drugs but also note that there was no change in important postoperative cardiac and other outcomes. Although evidence of harm if these drugs are temporarily discontinued before surgery is sparse, the guidelines advocate continuing them in patients with heart failure or hypertension.
ANESTHESIA AND INTRAOPERATIVE MANAGEMENT
The classes of anesthesia include local, regional (nerve block or neuraxial), monitored anesthesia care (ie, intravenous sedation), and general (volatile agent, total intravenous, or a combination). The guideline committee found no evidence to support the use of neuraxial over general anesthesia, volatile over total intravenous anesthesia, or monitored anesthesia care over general anesthesia. Neuraxial anesthesia for postoperative pain relief in patients undergoing abdominal aortic surgery did reduce the incidence of myocardial infarction.
The guidelines do not recommend routinely using intraoperative transesophageal echocardiography during noncardiac surgery to screen for cardiac abnormalities or to monitor for myocardial ischemia in patients without risk factors or procedural risks for significant hemodynamic, pulmonary, or neurologic compromise. Only in emergency settings do they deem perioperative transesophageal echocardiography reasonable to determine the cause of hemodynamic instability when it persists despite attempted corrective therapy.
Maintenance of normothermia is reasonable, as studies evaluating hypothermia or use of warmed air did not find a lower rate of cardiac events.32,33
POSTOPERATIVE SURVEILLANCE
In observational studies, elevated troponin levels, and even detectable levels within the normal range, have been associated with adverse outcomes and predict mortality after noncardiac surgery—the higher the level, the higher the mortality rate.34 Elevated troponins have many potential causes, both cardiac and noncardiac.
An entity termed myocardial injury after noncardiac surgery (MINS)35 was described as prognostically relevant myocardial injury with a troponin T level higher than 0.03 ng/mL in the absence of a nonischemic etiology but not requiring the presence of ischemic features. Patients who had MINS had a higher 30-day mortality rate (9.8% vs 1.1%) and were also at higher risk of nonfatal cardiac arrest, heart failure, and stroke compared with patients who did not.
The guidelines recommend obtaining an electrocardiogram and troponin levels if there are signs or symptoms suggesting myocardial ischemia or infarction. However, despite the association between troponin and mortality, the guidelines state that "the usefulness of postoperative screening with troponin levels (and electrocardiograms) in patients at high risk for perioperative myocardial infarction, but without signs or symptoms suggestive of myocardial ischemia or infarction, is uncertain in the absence of established risks and benefits of a defined management strategy." They also recommend against routinely measuring postoperative troponins in unselected patients without signs or symptoms suggestive of myocardial ischemia or infarction, stating it is not useful for guiding perioperative management.
Although there was a suggestion that patients in the POISE trial36 who suffered postoperative myocardial infarction had better outcomes if they had received aspirin and statins, and another study37 showed that intensification of cardiac therapy in patients with elevated postoperative troponin levels after vascular surgery led to better 1-year outcomes, there are no randomized controlled trials at this time to support any specific plan or intervention.
IMPACT ON CLINICAL PRACTICE: A PERIOPERATIVE HOSPITALIST'S VIEW
Regarding testing
Although the updated guidelines provide some novel concepts in risk stratification, the new algorithm still leaves many patients in a gray zone with respect to noninvasive testing. Patients with heart failure, valvular heart disease, and arrhythmias appear to be somewhat disconnected from the algorithm in this version, and management according to clinical practice guidelines is recommended.
Patients with acute coronary syndrome remain embedded in the algorithm, with recommendations for cardiology evaluation and management according to standard guidelines before proceeding to elective surgery.
The concept of a combined risk based on clinical factors along with the surgical procedure is important, and an alternative to the RCRI factors is offered. However, while this new NSQIP surgical risk calculator is more comprehensive, it may be too time-consuming for routine clinical use and still needs to be externally validated.
The concept of shared decision-making and team communication is stressed, but the physician may still have difficulty deciding when further testing may influence management. The guidelines remain somewhat vague, and many physicians may be uncomfortable and will continue to look for further guidance in this area.
Without more specific recommendations, this uncertainty may result in more stress tests being ordered—often inappropriately, as they rarely change management. Future prospective studies using biomarkers in conjunction with risk calculators may shed some light on this decision.
The new perioperative guidelines incorporate other ACC/AHA guidelines for valvular heart disease15 and heart failure.14 Some of their recommendations, in my opinion, may lead to excessive testing (eg, repeat echocardiograms) that will not change perioperative management.
Regarding revascularization
The ACC/AHA guidelines continue to emphasize the important concept that coronary revascularization is rarely indicated just to get the patient through surgery.
The new guidelines give physicians some leeway in allowing patients with drug-eluting stents to undergo surgery after 6 rather than 12 months of dual antiplatelet therapy if they believe that delaying surgery would place the patient at more risk than that of stent thrombosis. There is evidence in the nonsurgical setting that the newer stents currently being used may require no more than 6 months of therapy. In my opinion it was never clear that there was a statistically significant benefit in delaying surgery more than 6 months after placement of a drug-eluting stent, so this is a welcome addition.
Regarding beta-blockers
The systematic review of beta-blockers reinforces the importance of continuing them preoperatively while downgrading recommendations for their prophylactic use in patients who are not at increased risk.
Although the debate continues, there is no doubt that beta-blockers are associated with a decrease in myocardial ischemia and infarction but an increase in bradycardia and hypotension. They probably are associated with some increased risk of stroke, although this may be related to the specific beta-blocker used as well as the time of initiation before surgery. Evidence of a possible effect on mortality depends on whether the DECREASE and POISE trials are included or excluded in the analysis.
In the absence of new large-scale randomized controlled trials, we are forced to rely on observational trials and expert opinion in the meantime. I think that if a beta-blocker is to be started preoperatively, it should be done at least 1 week before surgery, and a more cardioselective beta-blocker should be used.
Regarding other drugs and tests
I agree with the recommendation to continue ACE inhibitors and ARBs preoperatively in patients with heart failure and poorly controlled hypertension. Although somewhat contrary to current practice, continuance of these drugs has not been associated with an increase in myocardial infarction or death despite concern about intraoperative hypotension.
Data from randomized controlled trials of perioperative statins are limited, but the information from observational studies is favorable, and I see little downside to initiating statins preoperatively in patients who otherwise have indications for their use, particularly if undergoing vascular or other high-risk noncardiac surgery. It is not known whether the specific drug, dose, or timing of initiation of statins influences outcome.
Although multiple studies of biomarkers suggest that there is an association with outcome, there are no randomized controlled trials or specific interventions shown to improve outcome.
Some of the recommended interventions have included various cardiac medications, stress testing, possible coronary angiography, and revascularization, which are not without risk. In the absence of data and following the directive to "first do no harm," the ACC/AHA has been appropriately cautious in not recommending them for routine use at this time.
The updated guidelines have summarized the new evidence in perioperative cardiac evaluation and management. Many of their recommendations were reinforced by this information and remain essentially unchanged. Several new recommendations will lead to changes in management going forward. Unfortunately, we lack the evidence to answer many questions that arise in routine practice and are therefore forced to rely on expert opinion and our clinical judgment in these cases. The ACC/AHA guidelines do provide a framework for our evaluation and management and help keep clinicians up-to-date with the latest evidence.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05536-3. doi: 10.1016/j.jacc.2014.07.944. [Epub ahead of print].
- Wijeysundera DN, Duncan D, Nkonde-Price C, et al. Perioperative beta blockade in noncardiac surgery: a systematic review for the 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05528-4. doi: 10.1016/j.jacc.2014.07.939. [Epub ahead of print].
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05537-5. doi: 10.1016/j.jacc.2014.07.945. [Epub ahead of print].
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol 2007; 50:e159–e242.
- Erasmus MC Follow-up Investigation Committee. Report on the 2012 follow-up investigation of possible breaches of academic integrity. September 30, 2012. http://cardiobrief.files.wordpress.com/2012/10/integrity-report-2012-10-english-translation.pdf. Accessed October 30, 2014.
- Anderson JL, Antman EM, Harold JG, et al. Clinical practice guidelines on perioperative cardiovascular evaluation: collaborative efforts among the ACC, AHA, and ESC. J Am Coll Cardiol 2014 Jul 29. pii: S0735-1097(14)05527-2. doi: 10.1016/j.jacc.2014.07.938. [Epub ahead of print].
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124:381–387.
- Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217:833–842. e1-3.
- Davis C, Tait G, Carroll J, Wijeysundera DN, Beattie WS. The Revised Cardiac Risk Index in the new millennium: a single-centre prospective cohort re-evaluation of the original variables in 9,519 consecutive elective surgical patients. Can J Anaesth 2013; 60:855–863.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; e-pub before print. doi:10.1016/j.jacc.2014.03.022.
- Aliot EM, Alpert JS, Calkins H, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias. http://www.escardio.org/guidelines-surveys/esc-guidelines/GuidelinesDocuments/guidelines-SVA-FT.pdf. Accessed October 30,2014.
- Crossley GH, Poole JE, Rozner MA, et al. The Heart Rhythm Society (HRS)/American Society of Anesthesiologists (ASA) Expert Consensus Statement on the perioperative management of patients with implantable defibrillators, pacemakers and arrhythmia monitors: facilities and patient management. Developed as a joint project with the American Society of Anesthesiologists (ASA), and in collaboration with the American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Heart Rhythm 2011; 8:1114–1154.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62:e147–e239.
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:e57–e185.
- Jneid H, Anderson JL, Wright RS, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2012; 60:645-681.
- O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:e78–e140.
- Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol 2011; 58:e123–e210.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011; 58:e44–e122.
- McFalls EO, Ward HB, Krupski WC, et al. Prophylactic coronary artery revascularization for elective vascular surgery: study design. Veterans Affairs Cooperative Study Group on Coronary Artery Revascularization Prophylaxis for Elective Vascular Surgery. Control Clin Trials 1999; 20:297–308.
- Schouten O, van Kuijk JP, Flu WJ, et al. Long-term outcome of prophylactic coronary revascularization in cardiac high-risk patients undergoing major vascular surgery (from the randomized DECREASE-V Pilot Study). Am J Cardiol 2009; 103:897–901.
- Wijeysundera DN, Wijeysundera HC, Yun L, et al. risk of elective major noncardiac surgery after coronary stent insertion: a population-based study. Circulation 2012; 126:1355-1362.
- Hawn MT, Graham LA, Richman JS, et al. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA 2013; 310:1462–1472.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370:1494–1503.
- Group PS, Devereaux PJ, Yang H, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; 371:1839–1847.
- Lindenauer PK, Pekow P, Wang K, et al. Lipid-lowering therapy and in-hospital mortality following major noncardiac surgery. JAMA 2004; 291:2092–2099.
- Kennedy J, Quan H, Buchan AM, et al. Statins are associated with better outcomes after carotid endarterectomy in symptomatic patients. Stroke 2005; 36:2072–2076.
- Raju MG, Pachika A, Punnam SR, et al. Statin therapy in the reduction of cardiovascular events in patients undergoing intermediate-risk noncardiac, nonvascular surgery. Clin Cardiol 2013; 36:456–461.
- Desai H, Aronow WS, Ahn C, et al. Incidence of perioperative myocardial infarction and of 2-year mortality in 577 elderly patients undergoing noncardiac vascular surgery treated with and without statins. Arch Gerontol Geriatr 2010; 51:149–151.
- Durazzo AES, Machado FS, Ikeoka DT, et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg 2004; 39:967–975.
- Devereaux PJ, Sessler DI, Leslie K, et al. Clonidine in patients undergoing noncardiac surgery. N Engl J Med 2014; 370:1504–1513.
- Nguyen HP, Zaroff JG, Bayman EO, et al. Perioperative hypothermia (33 degrees C) does not increase the occurrence of cardiovascular events in patients undergoing cerebral aneurysm surgery: findings from the Intraoperative Hypothermia for Aneurysm Surgery Trial. Anesthesiology 2010; 113:327–342.
- Frank SM, Fleisher LA, Breslow MJ, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA 1997; 277:1127–1134.
- Vascular Events In Noncardiac Surgery Patients Cohort Evaluation Study I, Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307:2295–2304.
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120:564–578.
- Devereaux PJ, Xavier D, Pogue J, et al. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med 2011; 154:523–528.
- Foucrier A, Rodseth R, Aissaoui M, Ibanes C, et al. The long-term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg 2014; 119:1053–1063.
Guidelines jointly issued by the American College of Cardiology and American Heart Association (ACC/AHA)1 provide a framework for evaluating and managing perioperative cardiac risk in noncardiac surgery. An overriding theme in successive documents from these organizations through the years has been that preoperative intervention, coronary artery bypass grafting, or percutaneous coronary intervention is rarely necessary just to get the patient through surgery, unless it is otherwise indicated independent of the need for surgery.
This article highlights some of the key recommendations in the 2014 updates to these guidelines,1–3 how they differ from previous guidelines,4 and the ongoing challenges and unresolved issues facing physicians involved in perioperative care.
Of note, while these guidelines were being updated, Erasmus University5 expressed concern about the scientific integrity of some of the Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE) trials. As a result, the evidence review committee included these trials in its analysis but not in a systematic review of beta-blockers.2 These trials were not included in the clinical practice guideline supplements and tables but were cited in the text if relevant.
The European Society of Cardiology and European Society of Anesthesiology6 revised their guidelines concurrently with but independently of the ACC/AHA, and although they discussed and aligned some recommendations, many differences remain between the two sets of guidelines. Readers should consult the full guidelines for more detailed information.1
THE ROLE OF THE PREOPERATIVE CARDIAC EVALUATION
The purpose of preoperative medical evaluation is not to "get medical clearance" but rather to evaluate the patient’s medical status and risk of complications. The process includes:
- Identifying risk factors and assessing their severity and stability
- Establishing a clinical risk profile for informed and shared decision-making
- Recommending needed changes in management, further testing, or specialty consultation.
The updated guidelines emphasize the importance of communication among the perioperative team and with the patient. They reiterate the focus on appropriateness of care and cost containment—one should order a test only if the result may change the patient’s management.
HOW URGENT IS SURGERY? HOW RISKY?
The new guidelines classify the urgency of surgery as follows:
- Emergency (necessary within 6 hours)
- Urgent (necessary within 6–24 hours)
- Time-sensitive (can delay 1–6 weeks)
- Elective (can delay up to 1 year).
Surgical risk is now classified as either low (< 1% risk of major adverse cardiac events) or elevated (≥ 1%) on the basis of surgical and patient characteristics. Previous schemas included an intermediate-risk category. Low-risk procedures include endoscopic procedures, superficial procedures, cataract surgery, breast surgery, and ambulatory surgery. Elevated-risk procedures include vascular surgery, intraperitoneal and intrathoracic surgery, head and neck surgery, orthopedic surgery, and prostate surgery.
Risk calculators and biomarkers
To estimate the perioperative risk of major adverse cardiac events, the guidelines suggest incorporating the Revised Cardiac Risk Index (RCRI)7 with an estimation of surgical risk or using a newer surgical risk calculator derived from a database of the American College of Surgeons’ National Surgical Quality Improvement Project (ACS NSQIP).
The RCRI is based on six risk factors, each worth 1 point:
- High-risk surgery
- Ischemic heart disease
- Heart failure
- Stroke or transient ischemic attack
- Diabetes requiring insulin
- Renal insufficiency (serum creatinine > 2.0 mg/dL).7
MICA. The Myocardial Infarction or Cardiac Arrest (MICA) calculator8 has a narrower focus and was validated in only one center.
ACS NSQIP. The recommended newer ACS NSQIP surgical risk calculator9 provides an estimate of procedure-specific risk based on Current Procedural Terminology code and includes 21 patient-specific variables to predict death, major adverse cardiac events, and eight other outcomes. While more comprehensive, this risk calculator has yet to be validated outside of the ACS NSQIP database.
Reconstructed RCRI. The RCRI has been externally validated, but it underestimates risk in major vascular surgery and was outperformed by the MICA calculator. Although not discussed in the new guidelines, a recently published "reconstructed RCRI,"10 in which a serum creatinine level greater than 2 mg/dL in the original RCRI is replaced by a glomerular filtration rate less than 30 mL/min and diabetes is eliminated, may outperform the standard RCRI. A patient with either an RCRI score or a reconstructed RCRI score of 0 or 1 would be considered to be at low risk, whereas patients with two or more risk factors would have an elevated risk.
Cardiac biomarkers, primarily B-type natriuretic peptide (BNP) and N-terminal (NT) proBNP, are independent predictors of cardiac risk, and their addition to preoperative risk indices may provide incremental predictive value. However, how to use these biomarkers and whether any treatment aimed at them will reduce risk is unclear, and the new guidelines did not recommend their routine use.
CLINICAL RISK FACTORS
Coronary artery disease
Ischemic symptoms, a history of myocardial infarction, and elevated cardiac biomarkers are individually associated with perioperative risk of morbidity and death. The risk is modified by how long ago the infarction occurred, whether the patient underwent coronary revascularization, and if so, what type (bypass grafting or percutaneous coronary intervention). A patient with acute coronary syndrome (currently or in the recent past) is at higher risk, and should have elective surgery delayed and be referred for cardiac evaluation and management according to guidelines.
Heart failure
In terms of posing a risk for major adverse cardiac events, heart failure is at least equal to coronary artery disease, and is possibly worse. Its impact depends on its stability, its symptoms, and the patient’s left ventricular function. Symptomatic decompensated heart failure and depressed left ventricular function (ejection fraction < 30% or 40%) confer higher risk than asymptomatic heart failure and preserved left ventricular function. However, evidence is limited with respect to asymptomatic left ventricular dysfunction and diastolic dysfunction. Patients with stable heart failure treated according to guidelines may have better perioperative outcomes.
Valvular heart disease
Significant valvular heart disease is associated with increased risk of postoperative cardiac complications. This risk depends on the type and severity of the valvular lesion and type of noncardiac surgery, but can be minimized by clinical and echocardiographic assessment, choosing appropriate anesthesia, and closer perioperative monitoring. Aortic and mitral stenosis are associated with greater risk of perioperative adverse cardiac events than regurgitant valvular disease.
Echocardiography is recommended in patients suspected of having moderate to severe stenotic or regurgitant lesions if it has not been done within the past year or if the patient’s clinical condition has worsened.
If indicated, valvular intervention can reduce perioperative risk in these patients. Even if the planned noncardiac surgery is high-risk, it may be reasonable to proceed with it (using appropriate perioperative hemodynamic monitoring, which is not specified but typically would be with an arterial line, central line, and possibly a pulmonary arterial catheter) in patients who have asymptomatic severe aortic or mitral regurgitation or aortic stenosis. Surgery may also be reasonable in patients with asymptomatic severe mitral stenosis who are not candidates for repair.
Arrhythmias
Cardiac arrhythmias and conduction defects are often seen in the perioperative period, but there is only limited evidence as to how they affect surgical risk. In addition to their hemodynamic effects, certain arrhythmias (atrial fibrillation, ventricular tachycardia) often indicate underlying structural heart disease, which requires further evaluation before surgery.
The new guidelines refer the reader to previously published clinical practice guidelines for atrial fibrillation,11 supraventricular arrhythmias,12 and device-based therapy.13
ALGORITHM FOR PREOPERATIVE CARDIAC ASSESSMENT
The new algorithm for evaluating a patient who is known to have coronary artery disease or risk factors for it has seven steps (Figure 1).1,11,12,14–17 It differs from the previous algorithm in several details:
- Instead of listing the four active cardiac conditions for which elective surgery should be delayed while the patient is being evaluated and treated (unstable coronary syndrome, decompensated heart failure, significant arrhythmias, severe valvular heart disease), the new version specifically asks about acute coronary syndrome and recommends cardiac evaluation and treatment according to guidelines. A footnote directs readers to other clinical practice guidelines for symptomatic heart failure,14 valvular heart disease,15 and arrhythmias.11,12
- Instead of asking if the procedure is low-risk, the guidelines recommend estimating risk of major adverse cardiac events on the basis of combined clinical and surgical risk and define only two categories: low or elevated. Patients at low risk proceed to surgery with no further testing, as in the earlier algorithm.
- "Excellent" exercise capacity (> 10 metabolic equivalents of task [METs]) is separated from "moderate/good" (4–10 METs), presumably to indicate a stronger recommendation, but patients in both categories proceed to surgery as before.
- If the patient cannot exercise to at least 4 METs, the new algorithm asks whether further testing will affect decision-making or perioperative care (an addition to the previous algorithm). This entails discussing with the patient and perioperative team whether the original surgery will be performed and whether the patient is willing to undergo revascularization if indicated. If so, pharmacologic stress testing is recommended. Previously, this decision also included the number of RCRI factors as well as the type of surgery (vascular or nonvascular).
- If testing will not affect the decision or if the stress test is normal, in addition to recommending proceeding to surgery according to guidelines the new algorithm also lists an option for alternative strategies, including palliation.
- If the stress test is abnormal, especially with left main disease, it recommends coronary revascularization according to the 2011 clinical practice guidelines.18,19
TESTING FOR LEFT VENTRICULAR DYSFUNCTION OR ISCHEMIA
In patients with dyspnea of unexplained cause or worsening dyspnea, assessment of left ventricular function is reasonable, but this is not part of a routine preoperative evaluation.
Pharmacologic stress testing is reasonable for patients at elevated risk with poor functional capacity if the results will change their management, but it is not useful for patients undergoing low-risk surgery. Although dobutamine stress echocardiography may be slightly superior to pharmacologic myocardial perfusion imaging, there are no head-to-head randomized controlled trials, and the guidelines suggest considering local expertise in deciding which test to use.
The presence of moderate to large areas of ischemia (reversible perfusion defects or new wall-motion abnormalities) is associated with risk of perioperative myocardial infarction or death, whereas evidence of an old infarction is associated with long-term but not short-term risk. The negative predictive value of these tests in predicting postoperative cardiac events is high (> 90%), but the positive predictive value is low.
CORONARY REVASCULARIZATION
Coronary artery bypass grafting and percutaneous coronary intervention
The guidelines recommend coronary revascularization before noncardiac surgery only when it is indicated anyway, on the basis of existing clinical practice guidelines.
Whether performing percutaneous coronary intervention before surgery will reduce perioperative cardiac complications is uncertain, and coronary revascularization should not be routinely performed solely to reduce perioperative cardiac events. The only two randomized controlled trials, Coronary Artery Revascularization Prophylaxis (CARP)20 and DECREASE V21 evaluating prophylactic coronary revascularization before noncardiac surgery found no difference in either short-term or long-term outcomes, although subgroup analysis found a survival benefit in patients with left main disease who underwent bypass grafting. Preoperative percutaneous coronary intervention should be limited to patients with left main disease in whom comorbidities preclude bypass surgery and those with unstable coronary disease who may benefit from early invasive management.
The urgency and timing of the noncardiac surgery needs to be taken into account if percutaneous coronary intervention is being considered because of the need for antiplatelet therapy after the procedure, and the potential risks of bleeding and stent thrombosis. If the planned surgery is deemed time-sensitive, then balloon angioplasty or bare-metal stenting is preferred over placement of a drug-eluting stent.
The new guidelines continue to recommend that elective noncardiac surgery be delayed at least 14 days after balloon angioplasty, 30 days after bare-metal stent implantation, and ideally 365 days after drug-eluting stent placement, and reiterate that it is potentially harmful to perform elective surgery within these time frames without any antiplatelet therapy. However, a new class IIb recommendation (benefit ≥ risk) states that "elective noncardiac surgery after [drug-eluting stent] implantation may be considered after 180 days if the risk of further delay is greater than the expected risks of ischemia and stent thrombosis."
This is an important addition to the guidelines because we are often faced with patients needing to undergo surgery in the 6 to 12 months after placement of a drug-eluting stent. Based on previous guidelines, whether it was safe to proceed in this setting created controversy among the perioperative team caring for the patient, and surgery was often delayed unnecessarily. Recent studies22,23 suggest that the newer drug-eluting stents may require a shorter duration of dual antiplatelet therapy, at least in the nonsurgical setting.
MEDICAL THERAPY
Antiplatelet therapy: Stop or continue?
The risk of perioperative bleeding if antiplatelet drugs are continued must be weighed against the risk of stent thrombosis and ischemia if they are stopped before the recommended duration of therapy. Ideally, some antiplatelet therapy should be continued perioperatively in these situations, but the guidelines recommend that a consensus decision among the treating physicians should be made regarding the relative risks of surgery and discontinuation or continuation of antiplatelet therapy. Whenever possible, aspirin should be continued in these patients.
Although the Perioperative Ischemic Evaluation (POISE)-2 trial24 found that perioperative aspirin use was not associated with lower rates of postoperative myocardial infarction or death, it increased bleeding. Patients with stents who had not completed the recommended duration of antiplatelet therapy were excluded from the trial. Additionally, only 5% of the study patients had undergone percutaneous coronary intervention.
According to the guidelines and package inserts, if antiplatelet agents need to be discontinued before surgery, aspirin can be stopped 3 to 7 days before, clopidogrel and ticagrelor 5 days before, and prasugrel 7 days before. In patients without stents, it may be reasonable to continue aspirin perioperatively if the risk of cardiac events outweighs the risk of bleeding, but starting aspirin is not beneficial for patients undergoing elective noncardiac noncarotid surgery unless the risk of ischemic events outweighs the risk of bleeding.
Beta-blockers
In view of the issue of scientific integrity of the DECREASE trials, a separately commissioned systematic review2 of perioperative beta-blocker therapy was performed. This review suggested that giving beta-blockers before surgery was associated with fewer postoperative cardiac events, primarily ischemia and nonfatal myocardial infarction, but few data supported their use to reduce postoperative mortality. Beta-blocker use was associated with adverse outcomes that included bradycardia and stroke. These findings were similar with the inclusion or exclusion of the DECREASE trials in question or of the POISE trial.25
In addition to recommending continuing beta-blockers in patients already on them (class I—the highest recommendation), the guidelines say that it may be reasonable to start them in patients with intermediate- or high-risk ischemia on stress tests as well as in patients with three or more RCRI risk factors (class IIb). In the absence of these indications, initiating beta-blockers preoperatively to reduce risk even in patients with long-term indications is of uncertain benefit. They also recommended starting beta-blockers more than 1 day preoperatively, preferably at least 2 to 7 days before, and note that it was harmful to start them on the day of surgery, particularly at high doses, and with long-acting formulations.
Additionally, there is evidence of differences in outcome within the class of beta-blockers, with the more cardioselective drugs bisoprolol and atenolol being associated with more favorable outcomes than metoprolol in observational studies.
Statins
Multiple observational trials have reported that statins are associated with decreased perioperative morbidity and mortality. Limited evidence from three randomized controlled trials (including two from the discredited DECREASE group) suggests that there is a benefit in patients undergoing vascular surgery, but it is unclear for nonvascular surgery.26–30
The ACC/AHA guidelines again give a class I recommendation to continue statin therapy perioperatively in patients already taking statins and undergoing noncardiac surgery, as there is some evidence that statin withdrawal is associated with increased risk. The guidelines comment that starting statin therapy perioperatively is reasonable for patients undergoing vascular surgery (class IIa) and may be considered in patients with other clinical guideline indications who are undergoing elevated-risk surgery (class IIb).
The mechanism of this benefit is unclear and may relate to the pleotropic as well as the lipid-lowering effects of the statins. Statins may also have beneficial effects in reducing the incidence of acute kidney injury and postoperative atrial fibrillation.
Whether a particular statin, dose, or time of initiation before surgery affects risk is also unknown at this time. The European guidelines6 recommend starting a longer-acting statin ideally at least 2 weeks before surgery for maximal plaque-stabilizing effects.
The risk of statin-induced myopathy, rhabdomyolysis, and hepatic injury appears to be minimal.
Other medications
Of note, the new guidelines do not recommend starting alpha-2 agonists for preventing cardiac events in patients undergoing noncardiac surgery. Despite previous evidence from smaller studies suggesting a benefit, the POISE-2 trial31 demonstrated that perioperative use of clonidine did not reduce cardiac events and was associated with a significant increase in hypotension and nonfatal cardiac arrest. However, clonidine should be continued in patients already taking it.
A somewhat surprising recommendation is that it is reasonable to continue angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), and if they are held before surgery, to restart them as soon as possible postoperatively (class IIa). The guidelines note reports of increased hypotension associated with induction of anesthesia in patients taking these drugs but also note that there was no change in important postoperative cardiac and other outcomes. Although evidence of harm if these drugs are temporarily discontinued before surgery is sparse, the guidelines advocate continuing them in patients with heart failure or hypertension.
ANESTHESIA AND INTRAOPERATIVE MANAGEMENT
The classes of anesthesia include local, regional (nerve block or neuraxial), monitored anesthesia care (ie, intravenous sedation), and general (volatile agent, total intravenous, or a combination). The guideline committee found no evidence to support the use of neuraxial over general anesthesia, volatile over total intravenous anesthesia, or monitored anesthesia care over general anesthesia. Neuraxial anesthesia for postoperative pain relief in patients undergoing abdominal aortic surgery did reduce the incidence of myocardial infarction.
The guidelines do not recommend routinely using intraoperative transesophageal echocardiography during noncardiac surgery to screen for cardiac abnormalities or to monitor for myocardial ischemia in patients without risk factors or procedural risks for significant hemodynamic, pulmonary, or neurologic compromise. Only in emergency settings do they deem perioperative transesophageal echocardiography reasonable to determine the cause of hemodynamic instability when it persists despite attempted corrective therapy.
Maintenance of normothermia is reasonable, as studies evaluating hypothermia or use of warmed air did not find a lower rate of cardiac events.32,33
POSTOPERATIVE SURVEILLANCE
In observational studies, elevated troponin levels, and even detectable levels within the normal range, have been associated with adverse outcomes and predict mortality after noncardiac surgery—the higher the level, the higher the mortality rate.34 Elevated troponins have many potential causes, both cardiac and noncardiac.
An entity termed myocardial injury after noncardiac surgery (MINS)35 was described as prognostically relevant myocardial injury with a troponin T level higher than 0.03 ng/mL in the absence of a nonischemic etiology but not requiring the presence of ischemic features. Patients who had MINS had a higher 30-day mortality rate (9.8% vs 1.1%) and were also at higher risk of nonfatal cardiac arrest, heart failure, and stroke compared with patients who did not.
The guidelines recommend obtaining an electrocardiogram and troponin levels if there are signs or symptoms suggesting myocardial ischemia or infarction. However, despite the association between troponin and mortality, the guidelines state that "the usefulness of postoperative screening with troponin levels (and electrocardiograms) in patients at high risk for perioperative myocardial infarction, but without signs or symptoms suggestive of myocardial ischemia or infarction, is uncertain in the absence of established risks and benefits of a defined management strategy." They also recommend against routinely measuring postoperative troponins in unselected patients without signs or symptoms suggestive of myocardial ischemia or infarction, stating it is not useful for guiding perioperative management.
Although there was a suggestion that patients in the POISE trial36 who suffered postoperative myocardial infarction had better outcomes if they had received aspirin and statins, and another study37 showed that intensification of cardiac therapy in patients with elevated postoperative troponin levels after vascular surgery led to better 1-year outcomes, there are no randomized controlled trials at this time to support any specific plan or intervention.
IMPACT ON CLINICAL PRACTICE: A PERIOPERATIVE HOSPITALIST'S VIEW
Regarding testing
Although the updated guidelines provide some novel concepts in risk stratification, the new algorithm still leaves many patients in a gray zone with respect to noninvasive testing. Patients with heart failure, valvular heart disease, and arrhythmias appear to be somewhat disconnected from the algorithm in this version, and management according to clinical practice guidelines is recommended.
Patients with acute coronary syndrome remain embedded in the algorithm, with recommendations for cardiology evaluation and management according to standard guidelines before proceeding to elective surgery.
The concept of a combined risk based on clinical factors along with the surgical procedure is important, and an alternative to the RCRI factors is offered. However, while this new NSQIP surgical risk calculator is more comprehensive, it may be too time-consuming for routine clinical use and still needs to be externally validated.
The concept of shared decision-making and team communication is stressed, but the physician may still have difficulty deciding when further testing may influence management. The guidelines remain somewhat vague, and many physicians may be uncomfortable and will continue to look for further guidance in this area.
Without more specific recommendations, this uncertainty may result in more stress tests being ordered—often inappropriately, as they rarely change management. Future prospective studies using biomarkers in conjunction with risk calculators may shed some light on this decision.
The new perioperative guidelines incorporate other ACC/AHA guidelines for valvular heart disease15 and heart failure.14 Some of their recommendations, in my opinion, may lead to excessive testing (eg, repeat echocardiograms) that will not change perioperative management.
Regarding revascularization
The ACC/AHA guidelines continue to emphasize the important concept that coronary revascularization is rarely indicated just to get the patient through surgery.
The new guidelines give physicians some leeway in allowing patients with drug-eluting stents to undergo surgery after 6 rather than 12 months of dual antiplatelet therapy if they believe that delaying surgery would place the patient at more risk than that of stent thrombosis. There is evidence in the nonsurgical setting that the newer stents currently being used may require no more than 6 months of therapy. In my opinion it was never clear that there was a statistically significant benefit in delaying surgery more than 6 months after placement of a drug-eluting stent, so this is a welcome addition.
Regarding beta-blockers
The systematic review of beta-blockers reinforces the importance of continuing them preoperatively while downgrading recommendations for their prophylactic use in patients who are not at increased risk.
Although the debate continues, there is no doubt that beta-blockers are associated with a decrease in myocardial ischemia and infarction but an increase in bradycardia and hypotension. They probably are associated with some increased risk of stroke, although this may be related to the specific beta-blocker used as well as the time of initiation before surgery. Evidence of a possible effect on mortality depends on whether the DECREASE and POISE trials are included or excluded in the analysis.
In the absence of new large-scale randomized controlled trials, we are forced to rely on observational trials and expert opinion in the meantime. I think that if a beta-blocker is to be started preoperatively, it should be done at least 1 week before surgery, and a more cardioselective beta-blocker should be used.
Regarding other drugs and tests
I agree with the recommendation to continue ACE inhibitors and ARBs preoperatively in patients with heart failure and poorly controlled hypertension. Although somewhat contrary to current practice, continuance of these drugs has not been associated with an increase in myocardial infarction or death despite concern about intraoperative hypotension.
Data from randomized controlled trials of perioperative statins are limited, but the information from observational studies is favorable, and I see little downside to initiating statins preoperatively in patients who otherwise have indications for their use, particularly if undergoing vascular or other high-risk noncardiac surgery. It is not known whether the specific drug, dose, or timing of initiation of statins influences outcome.
Although multiple studies of biomarkers suggest that there is an association with outcome, there are no randomized controlled trials or specific interventions shown to improve outcome.
Some of the recommended interventions have included various cardiac medications, stress testing, possible coronary angiography, and revascularization, which are not without risk. In the absence of data and following the directive to "first do no harm," the ACC/AHA has been appropriately cautious in not recommending them for routine use at this time.
The updated guidelines have summarized the new evidence in perioperative cardiac evaluation and management. Many of their recommendations were reinforced by this information and remain essentially unchanged. Several new recommendations will lead to changes in management going forward. Unfortunately, we lack the evidence to answer many questions that arise in routine practice and are therefore forced to rely on expert opinion and our clinical judgment in these cases. The ACC/AHA guidelines do provide a framework for our evaluation and management and help keep clinicians up-to-date with the latest evidence.
Guidelines jointly issued by the American College of Cardiology and American Heart Association (ACC/AHA)1 provide a framework for evaluating and managing perioperative cardiac risk in noncardiac surgery. An overriding theme in successive documents from these organizations through the years has been that preoperative intervention, coronary artery bypass grafting, or percutaneous coronary intervention is rarely necessary just to get the patient through surgery, unless it is otherwise indicated independent of the need for surgery.
This article highlights some of the key recommendations in the 2014 updates to these guidelines,1–3 how they differ from previous guidelines,4 and the ongoing challenges and unresolved issues facing physicians involved in perioperative care.
Of note, while these guidelines were being updated, Erasmus University5 expressed concern about the scientific integrity of some of the Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE) trials. As a result, the evidence review committee included these trials in its analysis but not in a systematic review of beta-blockers.2 These trials were not included in the clinical practice guideline supplements and tables but were cited in the text if relevant.
The European Society of Cardiology and European Society of Anesthesiology6 revised their guidelines concurrently with but independently of the ACC/AHA, and although they discussed and aligned some recommendations, many differences remain between the two sets of guidelines. Readers should consult the full guidelines for more detailed information.1
THE ROLE OF THE PREOPERATIVE CARDIAC EVALUATION
The purpose of preoperative medical evaluation is not to "get medical clearance" but rather to evaluate the patient’s medical status and risk of complications. The process includes:
- Identifying risk factors and assessing their severity and stability
- Establishing a clinical risk profile for informed and shared decision-making
- Recommending needed changes in management, further testing, or specialty consultation.
The updated guidelines emphasize the importance of communication among the perioperative team and with the patient. They reiterate the focus on appropriateness of care and cost containment—one should order a test only if the result may change the patient’s management.
HOW URGENT IS SURGERY? HOW RISKY?
The new guidelines classify the urgency of surgery as follows:
- Emergency (necessary within 6 hours)
- Urgent (necessary within 6–24 hours)
- Time-sensitive (can delay 1–6 weeks)
- Elective (can delay up to 1 year).
Surgical risk is now classified as either low (< 1% risk of major adverse cardiac events) or elevated (≥ 1%) on the basis of surgical and patient characteristics. Previous schemas included an intermediate-risk category. Low-risk procedures include endoscopic procedures, superficial procedures, cataract surgery, breast surgery, and ambulatory surgery. Elevated-risk procedures include vascular surgery, intraperitoneal and intrathoracic surgery, head and neck surgery, orthopedic surgery, and prostate surgery.
Risk calculators and biomarkers
To estimate the perioperative risk of major adverse cardiac events, the guidelines suggest incorporating the Revised Cardiac Risk Index (RCRI)7 with an estimation of surgical risk or using a newer surgical risk calculator derived from a database of the American College of Surgeons’ National Surgical Quality Improvement Project (ACS NSQIP).
The RCRI is based on six risk factors, each worth 1 point:
- High-risk surgery
- Ischemic heart disease
- Heart failure
- Stroke or transient ischemic attack
- Diabetes requiring insulin
- Renal insufficiency (serum creatinine > 2.0 mg/dL).7
MICA. The Myocardial Infarction or Cardiac Arrest (MICA) calculator8 has a narrower focus and was validated in only one center.
ACS NSQIP. The recommended newer ACS NSQIP surgical risk calculator9 provides an estimate of procedure-specific risk based on Current Procedural Terminology code and includes 21 patient-specific variables to predict death, major adverse cardiac events, and eight other outcomes. While more comprehensive, this risk calculator has yet to be validated outside of the ACS NSQIP database.
Reconstructed RCRI. The RCRI has been externally validated, but it underestimates risk in major vascular surgery and was outperformed by the MICA calculator. Although not discussed in the new guidelines, a recently published "reconstructed RCRI,"10 in which a serum creatinine level greater than 2 mg/dL in the original RCRI is replaced by a glomerular filtration rate less than 30 mL/min and diabetes is eliminated, may outperform the standard RCRI. A patient with either an RCRI score or a reconstructed RCRI score of 0 or 1 would be considered to be at low risk, whereas patients with two or more risk factors would have an elevated risk.
Cardiac biomarkers, primarily B-type natriuretic peptide (BNP) and N-terminal (NT) proBNP, are independent predictors of cardiac risk, and their addition to preoperative risk indices may provide incremental predictive value. However, how to use these biomarkers and whether any treatment aimed at them will reduce risk is unclear, and the new guidelines did not recommend their routine use.
CLINICAL RISK FACTORS
Coronary artery disease
Ischemic symptoms, a history of myocardial infarction, and elevated cardiac biomarkers are individually associated with perioperative risk of morbidity and death. The risk is modified by how long ago the infarction occurred, whether the patient underwent coronary revascularization, and if so, what type (bypass grafting or percutaneous coronary intervention). A patient with acute coronary syndrome (currently or in the recent past) is at higher risk, and should have elective surgery delayed and be referred for cardiac evaluation and management according to guidelines.
Heart failure
In terms of posing a risk for major adverse cardiac events, heart failure is at least equal to coronary artery disease, and is possibly worse. Its impact depends on its stability, its symptoms, and the patient’s left ventricular function. Symptomatic decompensated heart failure and depressed left ventricular function (ejection fraction < 30% or 40%) confer higher risk than asymptomatic heart failure and preserved left ventricular function. However, evidence is limited with respect to asymptomatic left ventricular dysfunction and diastolic dysfunction. Patients with stable heart failure treated according to guidelines may have better perioperative outcomes.
Valvular heart disease
Significant valvular heart disease is associated with increased risk of postoperative cardiac complications. This risk depends on the type and severity of the valvular lesion and type of noncardiac surgery, but can be minimized by clinical and echocardiographic assessment, choosing appropriate anesthesia, and closer perioperative monitoring. Aortic and mitral stenosis are associated with greater risk of perioperative adverse cardiac events than regurgitant valvular disease.
Echocardiography is recommended in patients suspected of having moderate to severe stenotic or regurgitant lesions if it has not been done within the past year or if the patient’s clinical condition has worsened.
If indicated, valvular intervention can reduce perioperative risk in these patients. Even if the planned noncardiac surgery is high-risk, it may be reasonable to proceed with it (using appropriate perioperative hemodynamic monitoring, which is not specified but typically would be with an arterial line, central line, and possibly a pulmonary arterial catheter) in patients who have asymptomatic severe aortic or mitral regurgitation or aortic stenosis. Surgery may also be reasonable in patients with asymptomatic severe mitral stenosis who are not candidates for repair.
Arrhythmias
Cardiac arrhythmias and conduction defects are often seen in the perioperative period, but there is only limited evidence as to how they affect surgical risk. In addition to their hemodynamic effects, certain arrhythmias (atrial fibrillation, ventricular tachycardia) often indicate underlying structural heart disease, which requires further evaluation before surgery.
The new guidelines refer the reader to previously published clinical practice guidelines for atrial fibrillation,11 supraventricular arrhythmias,12 and device-based therapy.13
ALGORITHM FOR PREOPERATIVE CARDIAC ASSESSMENT
The new algorithm for evaluating a patient who is known to have coronary artery disease or risk factors for it has seven steps (Figure 1).1,11,12,14–17 It differs from the previous algorithm in several details:
- Instead of listing the four active cardiac conditions for which elective surgery should be delayed while the patient is being evaluated and treated (unstable coronary syndrome, decompensated heart failure, significant arrhythmias, severe valvular heart disease), the new version specifically asks about acute coronary syndrome and recommends cardiac evaluation and treatment according to guidelines. A footnote directs readers to other clinical practice guidelines for symptomatic heart failure,14 valvular heart disease,15 and arrhythmias.11,12
- Instead of asking if the procedure is low-risk, the guidelines recommend estimating risk of major adverse cardiac events on the basis of combined clinical and surgical risk and define only two categories: low or elevated. Patients at low risk proceed to surgery with no further testing, as in the earlier algorithm.
- "Excellent" exercise capacity (> 10 metabolic equivalents of task [METs]) is separated from "moderate/good" (4–10 METs), presumably to indicate a stronger recommendation, but patients in both categories proceed to surgery as before.
- If the patient cannot exercise to at least 4 METs, the new algorithm asks whether further testing will affect decision-making or perioperative care (an addition to the previous algorithm). This entails discussing with the patient and perioperative team whether the original surgery will be performed and whether the patient is willing to undergo revascularization if indicated. If so, pharmacologic stress testing is recommended. Previously, this decision also included the number of RCRI factors as well as the type of surgery (vascular or nonvascular).
- If testing will not affect the decision or if the stress test is normal, in addition to recommending proceeding to surgery according to guidelines the new algorithm also lists an option for alternative strategies, including palliation.
- If the stress test is abnormal, especially with left main disease, it recommends coronary revascularization according to the 2011 clinical practice guidelines.18,19
TESTING FOR LEFT VENTRICULAR DYSFUNCTION OR ISCHEMIA
In patients with dyspnea of unexplained cause or worsening dyspnea, assessment of left ventricular function is reasonable, but this is not part of a routine preoperative evaluation.
Pharmacologic stress testing is reasonable for patients at elevated risk with poor functional capacity if the results will change their management, but it is not useful for patients undergoing low-risk surgery. Although dobutamine stress echocardiography may be slightly superior to pharmacologic myocardial perfusion imaging, there are no head-to-head randomized controlled trials, and the guidelines suggest considering local expertise in deciding which test to use.
The presence of moderate to large areas of ischemia (reversible perfusion defects or new wall-motion abnormalities) is associated with risk of perioperative myocardial infarction or death, whereas evidence of an old infarction is associated with long-term but not short-term risk. The negative predictive value of these tests in predicting postoperative cardiac events is high (> 90%), but the positive predictive value is low.
CORONARY REVASCULARIZATION
Coronary artery bypass grafting and percutaneous coronary intervention
The guidelines recommend coronary revascularization before noncardiac surgery only when it is indicated anyway, on the basis of existing clinical practice guidelines.
Whether performing percutaneous coronary intervention before surgery will reduce perioperative cardiac complications is uncertain, and coronary revascularization should not be routinely performed solely to reduce perioperative cardiac events. The only two randomized controlled trials, Coronary Artery Revascularization Prophylaxis (CARP)20 and DECREASE V21 evaluating prophylactic coronary revascularization before noncardiac surgery found no difference in either short-term or long-term outcomes, although subgroup analysis found a survival benefit in patients with left main disease who underwent bypass grafting. Preoperative percutaneous coronary intervention should be limited to patients with left main disease in whom comorbidities preclude bypass surgery and those with unstable coronary disease who may benefit from early invasive management.
The urgency and timing of the noncardiac surgery needs to be taken into account if percutaneous coronary intervention is being considered because of the need for antiplatelet therapy after the procedure, and the potential risks of bleeding and stent thrombosis. If the planned surgery is deemed time-sensitive, then balloon angioplasty or bare-metal stenting is preferred over placement of a drug-eluting stent.
The new guidelines continue to recommend that elective noncardiac surgery be delayed at least 14 days after balloon angioplasty, 30 days after bare-metal stent implantation, and ideally 365 days after drug-eluting stent placement, and reiterate that it is potentially harmful to perform elective surgery within these time frames without any antiplatelet therapy. However, a new class IIb recommendation (benefit ≥ risk) states that "elective noncardiac surgery after [drug-eluting stent] implantation may be considered after 180 days if the risk of further delay is greater than the expected risks of ischemia and stent thrombosis."
This is an important addition to the guidelines because we are often faced with patients needing to undergo surgery in the 6 to 12 months after placement of a drug-eluting stent. Based on previous guidelines, whether it was safe to proceed in this setting created controversy among the perioperative team caring for the patient, and surgery was often delayed unnecessarily. Recent studies22,23 suggest that the newer drug-eluting stents may require a shorter duration of dual antiplatelet therapy, at least in the nonsurgical setting.
MEDICAL THERAPY
Antiplatelet therapy: Stop or continue?
The risk of perioperative bleeding if antiplatelet drugs are continued must be weighed against the risk of stent thrombosis and ischemia if they are stopped before the recommended duration of therapy. Ideally, some antiplatelet therapy should be continued perioperatively in these situations, but the guidelines recommend that a consensus decision among the treating physicians should be made regarding the relative risks of surgery and discontinuation or continuation of antiplatelet therapy. Whenever possible, aspirin should be continued in these patients.
Although the Perioperative Ischemic Evaluation (POISE)-2 trial24 found that perioperative aspirin use was not associated with lower rates of postoperative myocardial infarction or death, it increased bleeding. Patients with stents who had not completed the recommended duration of antiplatelet therapy were excluded from the trial. Additionally, only 5% of the study patients had undergone percutaneous coronary intervention.
According to the guidelines and package inserts, if antiplatelet agents need to be discontinued before surgery, aspirin can be stopped 3 to 7 days before, clopidogrel and ticagrelor 5 days before, and prasugrel 7 days before. In patients without stents, it may be reasonable to continue aspirin perioperatively if the risk of cardiac events outweighs the risk of bleeding, but starting aspirin is not beneficial for patients undergoing elective noncardiac noncarotid surgery unless the risk of ischemic events outweighs the risk of bleeding.
Beta-blockers
In view of the issue of scientific integrity of the DECREASE trials, a separately commissioned systematic review2 of perioperative beta-blocker therapy was performed. This review suggested that giving beta-blockers before surgery was associated with fewer postoperative cardiac events, primarily ischemia and nonfatal myocardial infarction, but few data supported their use to reduce postoperative mortality. Beta-blocker use was associated with adverse outcomes that included bradycardia and stroke. These findings were similar with the inclusion or exclusion of the DECREASE trials in question or of the POISE trial.25
In addition to recommending continuing beta-blockers in patients already on them (class I—the highest recommendation), the guidelines say that it may be reasonable to start them in patients with intermediate- or high-risk ischemia on stress tests as well as in patients with three or more RCRI risk factors (class IIb). In the absence of these indications, initiating beta-blockers preoperatively to reduce risk even in patients with long-term indications is of uncertain benefit. They also recommended starting beta-blockers more than 1 day preoperatively, preferably at least 2 to 7 days before, and note that it was harmful to start them on the day of surgery, particularly at high doses, and with long-acting formulations.
Additionally, there is evidence of differences in outcome within the class of beta-blockers, with the more cardioselective drugs bisoprolol and atenolol being associated with more favorable outcomes than metoprolol in observational studies.
Statins
Multiple observational trials have reported that statins are associated with decreased perioperative morbidity and mortality. Limited evidence from three randomized controlled trials (including two from the discredited DECREASE group) suggests that there is a benefit in patients undergoing vascular surgery, but it is unclear for nonvascular surgery.26–30
The ACC/AHA guidelines again give a class I recommendation to continue statin therapy perioperatively in patients already taking statins and undergoing noncardiac surgery, as there is some evidence that statin withdrawal is associated with increased risk. The guidelines comment that starting statin therapy perioperatively is reasonable for patients undergoing vascular surgery (class IIa) and may be considered in patients with other clinical guideline indications who are undergoing elevated-risk surgery (class IIb).
The mechanism of this benefit is unclear and may relate to the pleotropic as well as the lipid-lowering effects of the statins. Statins may also have beneficial effects in reducing the incidence of acute kidney injury and postoperative atrial fibrillation.
Whether a particular statin, dose, or time of initiation before surgery affects risk is also unknown at this time. The European guidelines6 recommend starting a longer-acting statin ideally at least 2 weeks before surgery for maximal plaque-stabilizing effects.
The risk of statin-induced myopathy, rhabdomyolysis, and hepatic injury appears to be minimal.
Other medications
Of note, the new guidelines do not recommend starting alpha-2 agonists for preventing cardiac events in patients undergoing noncardiac surgery. Despite previous evidence from smaller studies suggesting a benefit, the POISE-2 trial31 demonstrated that perioperative use of clonidine did not reduce cardiac events and was associated with a significant increase in hypotension and nonfatal cardiac arrest. However, clonidine should be continued in patients already taking it.
A somewhat surprising recommendation is that it is reasonable to continue angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), and if they are held before surgery, to restart them as soon as possible postoperatively (class IIa). The guidelines note reports of increased hypotension associated with induction of anesthesia in patients taking these drugs but also note that there was no change in important postoperative cardiac and other outcomes. Although evidence of harm if these drugs are temporarily discontinued before surgery is sparse, the guidelines advocate continuing them in patients with heart failure or hypertension.
ANESTHESIA AND INTRAOPERATIVE MANAGEMENT
The classes of anesthesia include local, regional (nerve block or neuraxial), monitored anesthesia care (ie, intravenous sedation), and general (volatile agent, total intravenous, or a combination). The guideline committee found no evidence to support the use of neuraxial over general anesthesia, volatile over total intravenous anesthesia, or monitored anesthesia care over general anesthesia. Neuraxial anesthesia for postoperative pain relief in patients undergoing abdominal aortic surgery did reduce the incidence of myocardial infarction.
The guidelines do not recommend routinely using intraoperative transesophageal echocardiography during noncardiac surgery to screen for cardiac abnormalities or to monitor for myocardial ischemia in patients without risk factors or procedural risks for significant hemodynamic, pulmonary, or neurologic compromise. Only in emergency settings do they deem perioperative transesophageal echocardiography reasonable to determine the cause of hemodynamic instability when it persists despite attempted corrective therapy.
Maintenance of normothermia is reasonable, as studies evaluating hypothermia or use of warmed air did not find a lower rate of cardiac events.32,33
POSTOPERATIVE SURVEILLANCE
In observational studies, elevated troponin levels, and even detectable levels within the normal range, have been associated with adverse outcomes and predict mortality after noncardiac surgery—the higher the level, the higher the mortality rate.34 Elevated troponins have many potential causes, both cardiac and noncardiac.
An entity termed myocardial injury after noncardiac surgery (MINS)35 was described as prognostically relevant myocardial injury with a troponin T level higher than 0.03 ng/mL in the absence of a nonischemic etiology but not requiring the presence of ischemic features. Patients who had MINS had a higher 30-day mortality rate (9.8% vs 1.1%) and were also at higher risk of nonfatal cardiac arrest, heart failure, and stroke compared with patients who did not.
The guidelines recommend obtaining an electrocardiogram and troponin levels if there are signs or symptoms suggesting myocardial ischemia or infarction. However, despite the association between troponin and mortality, the guidelines state that "the usefulness of postoperative screening with troponin levels (and electrocardiograms) in patients at high risk for perioperative myocardial infarction, but without signs or symptoms suggestive of myocardial ischemia or infarction, is uncertain in the absence of established risks and benefits of a defined management strategy." They also recommend against routinely measuring postoperative troponins in unselected patients without signs or symptoms suggestive of myocardial ischemia or infarction, stating it is not useful for guiding perioperative management.
Although there was a suggestion that patients in the POISE trial36 who suffered postoperative myocardial infarction had better outcomes if they had received aspirin and statins, and another study37 showed that intensification of cardiac therapy in patients with elevated postoperative troponin levels after vascular surgery led to better 1-year outcomes, there are no randomized controlled trials at this time to support any specific plan or intervention.
IMPACT ON CLINICAL PRACTICE: A PERIOPERATIVE HOSPITALIST'S VIEW
Regarding testing
Although the updated guidelines provide some novel concepts in risk stratification, the new algorithm still leaves many patients in a gray zone with respect to noninvasive testing. Patients with heart failure, valvular heart disease, and arrhythmias appear to be somewhat disconnected from the algorithm in this version, and management according to clinical practice guidelines is recommended.
Patients with acute coronary syndrome remain embedded in the algorithm, with recommendations for cardiology evaluation and management according to standard guidelines before proceeding to elective surgery.
The concept of a combined risk based on clinical factors along with the surgical procedure is important, and an alternative to the RCRI factors is offered. However, while this new NSQIP surgical risk calculator is more comprehensive, it may be too time-consuming for routine clinical use and still needs to be externally validated.
The concept of shared decision-making and team communication is stressed, but the physician may still have difficulty deciding when further testing may influence management. The guidelines remain somewhat vague, and many physicians may be uncomfortable and will continue to look for further guidance in this area.
Without more specific recommendations, this uncertainty may result in more stress tests being ordered—often inappropriately, as they rarely change management. Future prospective studies using biomarkers in conjunction with risk calculators may shed some light on this decision.
The new perioperative guidelines incorporate other ACC/AHA guidelines for valvular heart disease15 and heart failure.14 Some of their recommendations, in my opinion, may lead to excessive testing (eg, repeat echocardiograms) that will not change perioperative management.
Regarding revascularization
The ACC/AHA guidelines continue to emphasize the important concept that coronary revascularization is rarely indicated just to get the patient through surgery.
The new guidelines give physicians some leeway in allowing patients with drug-eluting stents to undergo surgery after 6 rather than 12 months of dual antiplatelet therapy if they believe that delaying surgery would place the patient at more risk than that of stent thrombosis. There is evidence in the nonsurgical setting that the newer stents currently being used may require no more than 6 months of therapy. In my opinion it was never clear that there was a statistically significant benefit in delaying surgery more than 6 months after placement of a drug-eluting stent, so this is a welcome addition.
Regarding beta-blockers
The systematic review of beta-blockers reinforces the importance of continuing them preoperatively while downgrading recommendations for their prophylactic use in patients who are not at increased risk.
Although the debate continues, there is no doubt that beta-blockers are associated with a decrease in myocardial ischemia and infarction but an increase in bradycardia and hypotension. They probably are associated with some increased risk of stroke, although this may be related to the specific beta-blocker used as well as the time of initiation before surgery. Evidence of a possible effect on mortality depends on whether the DECREASE and POISE trials are included or excluded in the analysis.
In the absence of new large-scale randomized controlled trials, we are forced to rely on observational trials and expert opinion in the meantime. I think that if a beta-blocker is to be started preoperatively, it should be done at least 1 week before surgery, and a more cardioselective beta-blocker should be used.
Regarding other drugs and tests
I agree with the recommendation to continue ACE inhibitors and ARBs preoperatively in patients with heart failure and poorly controlled hypertension. Although somewhat contrary to current practice, continuance of these drugs has not been associated with an increase in myocardial infarction or death despite concern about intraoperative hypotension.
Data from randomized controlled trials of perioperative statins are limited, but the information from observational studies is favorable, and I see little downside to initiating statins preoperatively in patients who otherwise have indications for their use, particularly if undergoing vascular or other high-risk noncardiac surgery. It is not known whether the specific drug, dose, or timing of initiation of statins influences outcome.
Although multiple studies of biomarkers suggest that there is an association with outcome, there are no randomized controlled trials or specific interventions shown to improve outcome.
Some of the recommended interventions have included various cardiac medications, stress testing, possible coronary angiography, and revascularization, which are not without risk. In the absence of data and following the directive to "first do no harm," the ACC/AHA has been appropriately cautious in not recommending them for routine use at this time.
The updated guidelines have summarized the new evidence in perioperative cardiac evaluation and management. Many of their recommendations were reinforced by this information and remain essentially unchanged. Several new recommendations will lead to changes in management going forward. Unfortunately, we lack the evidence to answer many questions that arise in routine practice and are therefore forced to rely on expert opinion and our clinical judgment in these cases. The ACC/AHA guidelines do provide a framework for our evaluation and management and help keep clinicians up-to-date with the latest evidence.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05536-3. doi: 10.1016/j.jacc.2014.07.944. [Epub ahead of print].
- Wijeysundera DN, Duncan D, Nkonde-Price C, et al. Perioperative beta blockade in noncardiac surgery: a systematic review for the 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05528-4. doi: 10.1016/j.jacc.2014.07.939. [Epub ahead of print].
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05537-5. doi: 10.1016/j.jacc.2014.07.945. [Epub ahead of print].
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol 2007; 50:e159–e242.
- Erasmus MC Follow-up Investigation Committee. Report on the 2012 follow-up investigation of possible breaches of academic integrity. September 30, 2012. http://cardiobrief.files.wordpress.com/2012/10/integrity-report-2012-10-english-translation.pdf. Accessed October 30, 2014.
- Anderson JL, Antman EM, Harold JG, et al. Clinical practice guidelines on perioperative cardiovascular evaluation: collaborative efforts among the ACC, AHA, and ESC. J Am Coll Cardiol 2014 Jul 29. pii: S0735-1097(14)05527-2. doi: 10.1016/j.jacc.2014.07.938. [Epub ahead of print].
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124:381–387.
- Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217:833–842. e1-3.
- Davis C, Tait G, Carroll J, Wijeysundera DN, Beattie WS. The Revised Cardiac Risk Index in the new millennium: a single-centre prospective cohort re-evaluation of the original variables in 9,519 consecutive elective surgical patients. Can J Anaesth 2013; 60:855–863.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; e-pub before print. doi:10.1016/j.jacc.2014.03.022.
- Aliot EM, Alpert JS, Calkins H, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias. http://www.escardio.org/guidelines-surveys/esc-guidelines/GuidelinesDocuments/guidelines-SVA-FT.pdf. Accessed October 30,2014.
- Crossley GH, Poole JE, Rozner MA, et al. The Heart Rhythm Society (HRS)/American Society of Anesthesiologists (ASA) Expert Consensus Statement on the perioperative management of patients with implantable defibrillators, pacemakers and arrhythmia monitors: facilities and patient management. Developed as a joint project with the American Society of Anesthesiologists (ASA), and in collaboration with the American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Heart Rhythm 2011; 8:1114–1154.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62:e147–e239.
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:e57–e185.
- Jneid H, Anderson JL, Wright RS, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2012; 60:645-681.
- O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:e78–e140.
- Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol 2011; 58:e123–e210.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011; 58:e44–e122.
- McFalls EO, Ward HB, Krupski WC, et al. Prophylactic coronary artery revascularization for elective vascular surgery: study design. Veterans Affairs Cooperative Study Group on Coronary Artery Revascularization Prophylaxis for Elective Vascular Surgery. Control Clin Trials 1999; 20:297–308.
- Schouten O, van Kuijk JP, Flu WJ, et al. Long-term outcome of prophylactic coronary revascularization in cardiac high-risk patients undergoing major vascular surgery (from the randomized DECREASE-V Pilot Study). Am J Cardiol 2009; 103:897–901.
- Wijeysundera DN, Wijeysundera HC, Yun L, et al. risk of elective major noncardiac surgery after coronary stent insertion: a population-based study. Circulation 2012; 126:1355-1362.
- Hawn MT, Graham LA, Richman JS, et al. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA 2013; 310:1462–1472.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370:1494–1503.
- Group PS, Devereaux PJ, Yang H, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; 371:1839–1847.
- Lindenauer PK, Pekow P, Wang K, et al. Lipid-lowering therapy and in-hospital mortality following major noncardiac surgery. JAMA 2004; 291:2092–2099.
- Kennedy J, Quan H, Buchan AM, et al. Statins are associated with better outcomes after carotid endarterectomy in symptomatic patients. Stroke 2005; 36:2072–2076.
- Raju MG, Pachika A, Punnam SR, et al. Statin therapy in the reduction of cardiovascular events in patients undergoing intermediate-risk noncardiac, nonvascular surgery. Clin Cardiol 2013; 36:456–461.
- Desai H, Aronow WS, Ahn C, et al. Incidence of perioperative myocardial infarction and of 2-year mortality in 577 elderly patients undergoing noncardiac vascular surgery treated with and without statins. Arch Gerontol Geriatr 2010; 51:149–151.
- Durazzo AES, Machado FS, Ikeoka DT, et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg 2004; 39:967–975.
- Devereaux PJ, Sessler DI, Leslie K, et al. Clonidine in patients undergoing noncardiac surgery. N Engl J Med 2014; 370:1504–1513.
- Nguyen HP, Zaroff JG, Bayman EO, et al. Perioperative hypothermia (33 degrees C) does not increase the occurrence of cardiovascular events in patients undergoing cerebral aneurysm surgery: findings from the Intraoperative Hypothermia for Aneurysm Surgery Trial. Anesthesiology 2010; 113:327–342.
- Frank SM, Fleisher LA, Breslow MJ, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA 1997; 277:1127–1134.
- Vascular Events In Noncardiac Surgery Patients Cohort Evaluation Study I, Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307:2295–2304.
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120:564–578.
- Devereaux PJ, Xavier D, Pogue J, et al. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med 2011; 154:523–528.
- Foucrier A, Rodseth R, Aissaoui M, Ibanes C, et al. The long-term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg 2014; 119:1053–1063.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05536-3. doi: 10.1016/j.jacc.2014.07.944. [Epub ahead of print].
- Wijeysundera DN, Duncan D, Nkonde-Price C, et al. Perioperative beta blockade in noncardiac surgery: a systematic review for the 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05528-4. doi: 10.1016/j.jacc.2014.07.939. [Epub ahead of print].
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05537-5. doi: 10.1016/j.jacc.2014.07.945. [Epub ahead of print].
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol 2007; 50:e159–e242.
- Erasmus MC Follow-up Investigation Committee. Report on the 2012 follow-up investigation of possible breaches of academic integrity. September 30, 2012. http://cardiobrief.files.wordpress.com/2012/10/integrity-report-2012-10-english-translation.pdf. Accessed October 30, 2014.
- Anderson JL, Antman EM, Harold JG, et al. Clinical practice guidelines on perioperative cardiovascular evaluation: collaborative efforts among the ACC, AHA, and ESC. J Am Coll Cardiol 2014 Jul 29. pii: S0735-1097(14)05527-2. doi: 10.1016/j.jacc.2014.07.938. [Epub ahead of print].
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124:381–387.
- Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217:833–842. e1-3.
- Davis C, Tait G, Carroll J, Wijeysundera DN, Beattie WS. The Revised Cardiac Risk Index in the new millennium: a single-centre prospective cohort re-evaluation of the original variables in 9,519 consecutive elective surgical patients. Can J Anaesth 2013; 60:855–863.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; e-pub before print. doi:10.1016/j.jacc.2014.03.022.
- Aliot EM, Alpert JS, Calkins H, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias. http://www.escardio.org/guidelines-surveys/esc-guidelines/GuidelinesDocuments/guidelines-SVA-FT.pdf. Accessed October 30,2014.
- Crossley GH, Poole JE, Rozner MA, et al. The Heart Rhythm Society (HRS)/American Society of Anesthesiologists (ASA) Expert Consensus Statement on the perioperative management of patients with implantable defibrillators, pacemakers and arrhythmia monitors: facilities and patient management. Developed as a joint project with the American Society of Anesthesiologists (ASA), and in collaboration with the American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Heart Rhythm 2011; 8:1114–1154.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62:e147–e239.
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:e57–e185.
- Jneid H, Anderson JL, Wright RS, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2012; 60:645-681.
- O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:e78–e140.
- Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol 2011; 58:e123–e210.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011; 58:e44–e122.
- McFalls EO, Ward HB, Krupski WC, et al. Prophylactic coronary artery revascularization for elective vascular surgery: study design. Veterans Affairs Cooperative Study Group on Coronary Artery Revascularization Prophylaxis for Elective Vascular Surgery. Control Clin Trials 1999; 20:297–308.
- Schouten O, van Kuijk JP, Flu WJ, et al. Long-term outcome of prophylactic coronary revascularization in cardiac high-risk patients undergoing major vascular surgery (from the randomized DECREASE-V Pilot Study). Am J Cardiol 2009; 103:897–901.
- Wijeysundera DN, Wijeysundera HC, Yun L, et al. risk of elective major noncardiac surgery after coronary stent insertion: a population-based study. Circulation 2012; 126:1355-1362.
- Hawn MT, Graham LA, Richman JS, et al. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA 2013; 310:1462–1472.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370:1494–1503.
- Group PS, Devereaux PJ, Yang H, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; 371:1839–1847.
- Lindenauer PK, Pekow P, Wang K, et al. Lipid-lowering therapy and in-hospital mortality following major noncardiac surgery. JAMA 2004; 291:2092–2099.
- Kennedy J, Quan H, Buchan AM, et al. Statins are associated with better outcomes after carotid endarterectomy in symptomatic patients. Stroke 2005; 36:2072–2076.
- Raju MG, Pachika A, Punnam SR, et al. Statin therapy in the reduction of cardiovascular events in patients undergoing intermediate-risk noncardiac, nonvascular surgery. Clin Cardiol 2013; 36:456–461.
- Desai H, Aronow WS, Ahn C, et al. Incidence of perioperative myocardial infarction and of 2-year mortality in 577 elderly patients undergoing noncardiac vascular surgery treated with and without statins. Arch Gerontol Geriatr 2010; 51:149–151.
- Durazzo AES, Machado FS, Ikeoka DT, et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg 2004; 39:967–975.
- Devereaux PJ, Sessler DI, Leslie K, et al. Clonidine in patients undergoing noncardiac surgery. N Engl J Med 2014; 370:1504–1513.
- Nguyen HP, Zaroff JG, Bayman EO, et al. Perioperative hypothermia (33 degrees C) does not increase the occurrence of cardiovascular events in patients undergoing cerebral aneurysm surgery: findings from the Intraoperative Hypothermia for Aneurysm Surgery Trial. Anesthesiology 2010; 113:327–342.
- Frank SM, Fleisher LA, Breslow MJ, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA 1997; 277:1127–1134.
- Vascular Events In Noncardiac Surgery Patients Cohort Evaluation Study I, Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307:2295–2304.
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120:564–578.
- Devereaux PJ, Xavier D, Pogue J, et al. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med 2011; 154:523–528.
- Foucrier A, Rodseth R, Aissaoui M, Ibanes C, et al. The long-term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg 2014; 119:1053–1063.
KEY POINTS
- Like earlier guidelines, the update recommends preoperative cardiac testing only when the results may influence the patient’s management.
- Preoperative intervention is rarely necessary just to get the patient through surgery, unless it is otherwise indicated independent of the need for surgery.
- The update proposes a modified algorithm for preoperative risk assessment and management and suggests using a new calculator of surgical risk.
- The report also updates information on the timing of surgery after percutaneous coronary intervention, as well as on antiplatelet therapy, other medical therapy, and biomarkers.