User login
Perioperative medicine: Combining the science and the art
In this issue of the Cleveland Clinic Journal of Medicine,1 Dr. Steven L. Cohn provides a succinct review of the recently published guidelines by the American College of Cardiology and American Heart Association (ACC/AHA) on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery.2 Although no drastic changes have been made in these guidelines, several significant modifications have been implemented and are highlighted in his review.
A BREACH OF SCIENTIFIC INTEGRITY
First, I am pleased Dr. Cohn described how the writing committee of the new guidelines handled the well-publicized breaches of scientific integrity by Dr. Don Poldermans, a prolific perioperative-medicine researcher at Erasmus University in the Netherlands who has contributed an abundance of literature that influenced clinical practice. Although some of his key publications were excluded by the ACC/AHA committee in its overall analysis, it remains unclear to me if simply ignoring some of his work is truly possible. For better or for worse, his publications have significantly shaped clinical practice in addition to guiding subsequent research in this field.
ASSESSING RISK
Along with continuing to endorse the Revised Cardiac Risk Index (RCRI),3 the guidelines now include another option for objective preoperative cardiovascular risk assessment. Dr. Cohn nicely outlines the pros and cons of the surgical risk calculator (often referred to as the “Gupta calculator”) derived from the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database.4
Although the RCRI is not perfect, I agree with Dr. Cohn that the ACS NSQIP tool has limitations, including a cumbersome calculation (requiring a smartphone application or online calculator), lack of external validation, and use of the American Society of Anesthesiologists Physical Status Classification System, which has been notoriously confusing for generalists and has demonstrated poor inter-rater reliability among anesthesiologists.5,6
Of note, a patient may have very different risk-prediction scores depending on which tool is used. For example, a 66-year-old man with a history of ischemic heart disease, diabetes on insulin therapy, hypertension, and chronic kidney disease with a serum creatinine level greater than 2.0 mg/dL who is scheduled to undergo total hip arthroplasty would have a risk of a perioperative cardiovascular event of about 10% according to the RCRI, but only 1.1% according to the ACS NSQIP calculator. How widely this newer risk-stratification tool will be adopted in clinical practice will be interesting to observe.
In what appears to be an effort to simplify the guidelines, the ACC/AHA now recommends combining the patient’s clinical and surgical risks into estimating an overall perioperative risk for developing major adverse cardiac events. This estimate is now whittled down to only two categories: “low risk” and “elevated risk.” I am concerned that the new guidelines may have become too streamlined and lack the direction to assist providers in making important clinical decisions. Most notably, and as Dr. Cohn appropriately suggests, many patients will be in a gray zone with respect to whether cardiac stress testing should be obtained before surgery.
STRESS TESTING
Significant background knowledge is required to answer the important question in the ACC/AHA algorithm, ie, whether further testing will have an impact on decision-making or perioperative care.2 Dr. Cohn provides some of this information by noting the abysmal positive predictive value of preoperative noninvasive cardiac testing (with studies ranging from 0% to 37%) and by correctly stating that no benefit has been observed with preoperative cardiac revascularization.
If this is not widely known, I share Dr. Cohn’s fear that the new guidelines may stimulate increased ordering of preoperative stress tests. We observed this trend with the highly scripted 2002 ACC/AHA perioperative guidelines7 and subsequently learned that stress testing before surgery very seldom changes patient management.
A preoperative stress test should be reserved for patients with symptoms suggestive of ischemic heart disease. As a diagnostic study, the value of stress testing is excellent. This is not true when it is used as a screening test for asymptomatic patients, where its ability to predict perioperative cardiovascular events is extremely poor. The only other indication for preoperative stress testing is the rare occasion when further risk stratification is desired for exceptionally high-risk patients. In this scenario, test results may influence the decision to proceed with surgery vs seeking nonoperative approaches or palliative care.
MANAGING MEDICATIONS
Dr. Cohn discusses pertinent issues in the perioperative management of patients’ medications, an important component of the preoperative evaluation.
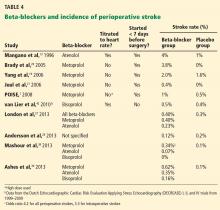
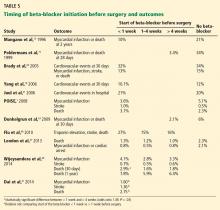
Despite the inconsistent clinical trial results on perioperative beta-blockers, his assessment of their risks and benefits is clinically accurate and practical. Furthermore, I fully agree with Dr. Cohn’s thoughtful approach regarding perioperative statins, despite the limited data available from randomized controlled trials.
With respect to perioperative aspirin use, I have concerns with Dr. Cohn’s statement that it may be reasonable to continue aspirin perioperatively if the risk of potential cardiac events outweighs the risk of bleeding. Given the result of the recently published second Perioperative Ischemic Evaluation (POISE-2) trial8 that showed a significantly higher risk of major perioperative bleeding in patients randomized to low-dose aspirin, it is difficult to advocate continuing aspirin when no cardiovascular protection was found in this very large trial. I agree with Dr. Cohn that this applies only to patients with no history of coronary artery stent placement, as patients with a stent should remain on low-dose aspirin throughout the entire perioperative period.
Controversy also surrounds angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Dr. Cohn agrees with the ACC/AHA guidelines to continue these agents before surgery; however, I favor holding them on the day of surgery. Although the risk of hypotension-induced cardiac events has not been clearly demonstrated, a recent retrospective study involving more than 1,100 patients showed significantly more acute kidney injury (even after adjusting for hypotension) as well as an increased length of hospital stay in the patients exposed to these agents before surgery.9 Given these findings, in addition to the postinduction hypotension (which can be profound) commonly observed by our anesthesiology colleagues, I recommend holding angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on the day of surgery, with very few exceptions.
THE SCIENCE AND ART OF MEDICINE
Dr. Cohn acknowledges that we lack scientific data to answer many questions that arise when caring for the perioperative patient and thus we rely on the ACC/AHA guidelines to provide a framework. These scientific knowledge gaps emphasize the importance of the art of medicine in the perioperative arena. Although we may desire “cookbook” guidelines, the significant gaps in the perioperative medicine evidence base reinforce the necessity to provide individual patient-level care in a multidisciplinary environment with our surgery and anesthesiology colleagues. Without the proper balance of science and art in perioperative medicine, we sacrifice our ability to deliver optimal care for this high-risk patient population.
- Cohn SL. Updated guidelines on cardiovascular evaluation before noncardiac surgery: a view from the trenches. Cleve Clin J Med 2014; 81:742–751.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05536-3. doi: 10.1016/j.jacc.2014.07.944. [Epub ahead of print].
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124:381–387.
- Aronson WL, McAuliffe MS, Miller K. Variability in the American Society of Anesthesiologists Physical Status Classification Scale. AANA J 2003; 71:265–274.
- Mak PH, Campbell RC, Irwin MG; American Society of Anesthesiologists. The ASA Physical Status Classification: inter-observer consistency. American Society of Anesthesiologists. Anaesth Intensive Care 2002; 30:633–640.
- Eagle KA, Berger PB, Calkins H, et al; American College of Cardiology; American Heart Association. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2002; 39:542–553.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370:1494–1503.
- Nielson E, Hennrikus E, Lehman E, Mets B. Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med 2014; 9:283–288.
In this issue of the Cleveland Clinic Journal of Medicine,1 Dr. Steven L. Cohn provides a succinct review of the recently published guidelines by the American College of Cardiology and American Heart Association (ACC/AHA) on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery.2 Although no drastic changes have been made in these guidelines, several significant modifications have been implemented and are highlighted in his review.
A BREACH OF SCIENTIFIC INTEGRITY
First, I am pleased Dr. Cohn described how the writing committee of the new guidelines handled the well-publicized breaches of scientific integrity by Dr. Don Poldermans, a prolific perioperative-medicine researcher at Erasmus University in the Netherlands who has contributed an abundance of literature that influenced clinical practice. Although some of his key publications were excluded by the ACC/AHA committee in its overall analysis, it remains unclear to me if simply ignoring some of his work is truly possible. For better or for worse, his publications have significantly shaped clinical practice in addition to guiding subsequent research in this field.
ASSESSING RISK
Along with continuing to endorse the Revised Cardiac Risk Index (RCRI),3 the guidelines now include another option for objective preoperative cardiovascular risk assessment. Dr. Cohn nicely outlines the pros and cons of the surgical risk calculator (often referred to as the “Gupta calculator”) derived from the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database.4
Although the RCRI is not perfect, I agree with Dr. Cohn that the ACS NSQIP tool has limitations, including a cumbersome calculation (requiring a smartphone application or online calculator), lack of external validation, and use of the American Society of Anesthesiologists Physical Status Classification System, which has been notoriously confusing for generalists and has demonstrated poor inter-rater reliability among anesthesiologists.5,6
Of note, a patient may have very different risk-prediction scores depending on which tool is used. For example, a 66-year-old man with a history of ischemic heart disease, diabetes on insulin therapy, hypertension, and chronic kidney disease with a serum creatinine level greater than 2.0 mg/dL who is scheduled to undergo total hip arthroplasty would have a risk of a perioperative cardiovascular event of about 10% according to the RCRI, but only 1.1% according to the ACS NSQIP calculator. How widely this newer risk-stratification tool will be adopted in clinical practice will be interesting to observe.
In what appears to be an effort to simplify the guidelines, the ACC/AHA now recommends combining the patient’s clinical and surgical risks into estimating an overall perioperative risk for developing major adverse cardiac events. This estimate is now whittled down to only two categories: “low risk” and “elevated risk.” I am concerned that the new guidelines may have become too streamlined and lack the direction to assist providers in making important clinical decisions. Most notably, and as Dr. Cohn appropriately suggests, many patients will be in a gray zone with respect to whether cardiac stress testing should be obtained before surgery.
STRESS TESTING
Significant background knowledge is required to answer the important question in the ACC/AHA algorithm, ie, whether further testing will have an impact on decision-making or perioperative care.2 Dr. Cohn provides some of this information by noting the abysmal positive predictive value of preoperative noninvasive cardiac testing (with studies ranging from 0% to 37%) and by correctly stating that no benefit has been observed with preoperative cardiac revascularization.
If this is not widely known, I share Dr. Cohn’s fear that the new guidelines may stimulate increased ordering of preoperative stress tests. We observed this trend with the highly scripted 2002 ACC/AHA perioperative guidelines7 and subsequently learned that stress testing before surgery very seldom changes patient management.
A preoperative stress test should be reserved for patients with symptoms suggestive of ischemic heart disease. As a diagnostic study, the value of stress testing is excellent. This is not true when it is used as a screening test for asymptomatic patients, where its ability to predict perioperative cardiovascular events is extremely poor. The only other indication for preoperative stress testing is the rare occasion when further risk stratification is desired for exceptionally high-risk patients. In this scenario, test results may influence the decision to proceed with surgery vs seeking nonoperative approaches or palliative care.
MANAGING MEDICATIONS
Dr. Cohn discusses pertinent issues in the perioperative management of patients’ medications, an important component of the preoperative evaluation.
Despite the inconsistent clinical trial results on perioperative beta-blockers, his assessment of their risks and benefits is clinically accurate and practical. Furthermore, I fully agree with Dr. Cohn’s thoughtful approach regarding perioperative statins, despite the limited data available from randomized controlled trials.
With respect to perioperative aspirin use, I have concerns with Dr. Cohn’s statement that it may be reasonable to continue aspirin perioperatively if the risk of potential cardiac events outweighs the risk of bleeding. Given the result of the recently published second Perioperative Ischemic Evaluation (POISE-2) trial8 that showed a significantly higher risk of major perioperative bleeding in patients randomized to low-dose aspirin, it is difficult to advocate continuing aspirin when no cardiovascular protection was found in this very large trial. I agree with Dr. Cohn that this applies only to patients with no history of coronary artery stent placement, as patients with a stent should remain on low-dose aspirin throughout the entire perioperative period.
Controversy also surrounds angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Dr. Cohn agrees with the ACC/AHA guidelines to continue these agents before surgery; however, I favor holding them on the day of surgery. Although the risk of hypotension-induced cardiac events has not been clearly demonstrated, a recent retrospective study involving more than 1,100 patients showed significantly more acute kidney injury (even after adjusting for hypotension) as well as an increased length of hospital stay in the patients exposed to these agents before surgery.9 Given these findings, in addition to the postinduction hypotension (which can be profound) commonly observed by our anesthesiology colleagues, I recommend holding angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on the day of surgery, with very few exceptions.
THE SCIENCE AND ART OF MEDICINE
Dr. Cohn acknowledges that we lack scientific data to answer many questions that arise when caring for the perioperative patient and thus we rely on the ACC/AHA guidelines to provide a framework. These scientific knowledge gaps emphasize the importance of the art of medicine in the perioperative arena. Although we may desire “cookbook” guidelines, the significant gaps in the perioperative medicine evidence base reinforce the necessity to provide individual patient-level care in a multidisciplinary environment with our surgery and anesthesiology colleagues. Without the proper balance of science and art in perioperative medicine, we sacrifice our ability to deliver optimal care for this high-risk patient population.
In this issue of the Cleveland Clinic Journal of Medicine,1 Dr. Steven L. Cohn provides a succinct review of the recently published guidelines by the American College of Cardiology and American Heart Association (ACC/AHA) on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery.2 Although no drastic changes have been made in these guidelines, several significant modifications have been implemented and are highlighted in his review.
A BREACH OF SCIENTIFIC INTEGRITY
First, I am pleased Dr. Cohn described how the writing committee of the new guidelines handled the well-publicized breaches of scientific integrity by Dr. Don Poldermans, a prolific perioperative-medicine researcher at Erasmus University in the Netherlands who has contributed an abundance of literature that influenced clinical practice. Although some of his key publications were excluded by the ACC/AHA committee in its overall analysis, it remains unclear to me if simply ignoring some of his work is truly possible. For better or for worse, his publications have significantly shaped clinical practice in addition to guiding subsequent research in this field.
ASSESSING RISK
Along with continuing to endorse the Revised Cardiac Risk Index (RCRI),3 the guidelines now include another option for objective preoperative cardiovascular risk assessment. Dr. Cohn nicely outlines the pros and cons of the surgical risk calculator (often referred to as the “Gupta calculator”) derived from the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database.4
Although the RCRI is not perfect, I agree with Dr. Cohn that the ACS NSQIP tool has limitations, including a cumbersome calculation (requiring a smartphone application or online calculator), lack of external validation, and use of the American Society of Anesthesiologists Physical Status Classification System, which has been notoriously confusing for generalists and has demonstrated poor inter-rater reliability among anesthesiologists.5,6
Of note, a patient may have very different risk-prediction scores depending on which tool is used. For example, a 66-year-old man with a history of ischemic heart disease, diabetes on insulin therapy, hypertension, and chronic kidney disease with a serum creatinine level greater than 2.0 mg/dL who is scheduled to undergo total hip arthroplasty would have a risk of a perioperative cardiovascular event of about 10% according to the RCRI, but only 1.1% according to the ACS NSQIP calculator. How widely this newer risk-stratification tool will be adopted in clinical practice will be interesting to observe.
In what appears to be an effort to simplify the guidelines, the ACC/AHA now recommends combining the patient’s clinical and surgical risks into estimating an overall perioperative risk for developing major adverse cardiac events. This estimate is now whittled down to only two categories: “low risk” and “elevated risk.” I am concerned that the new guidelines may have become too streamlined and lack the direction to assist providers in making important clinical decisions. Most notably, and as Dr. Cohn appropriately suggests, many patients will be in a gray zone with respect to whether cardiac stress testing should be obtained before surgery.
STRESS TESTING
Significant background knowledge is required to answer the important question in the ACC/AHA algorithm, ie, whether further testing will have an impact on decision-making or perioperative care.2 Dr. Cohn provides some of this information by noting the abysmal positive predictive value of preoperative noninvasive cardiac testing (with studies ranging from 0% to 37%) and by correctly stating that no benefit has been observed with preoperative cardiac revascularization.
If this is not widely known, I share Dr. Cohn’s fear that the new guidelines may stimulate increased ordering of preoperative stress tests. We observed this trend with the highly scripted 2002 ACC/AHA perioperative guidelines7 and subsequently learned that stress testing before surgery very seldom changes patient management.
A preoperative stress test should be reserved for patients with symptoms suggestive of ischemic heart disease. As a diagnostic study, the value of stress testing is excellent. This is not true when it is used as a screening test for asymptomatic patients, where its ability to predict perioperative cardiovascular events is extremely poor. The only other indication for preoperative stress testing is the rare occasion when further risk stratification is desired for exceptionally high-risk patients. In this scenario, test results may influence the decision to proceed with surgery vs seeking nonoperative approaches or palliative care.
MANAGING MEDICATIONS
Dr. Cohn discusses pertinent issues in the perioperative management of patients’ medications, an important component of the preoperative evaluation.
Despite the inconsistent clinical trial results on perioperative beta-blockers, his assessment of their risks and benefits is clinically accurate and practical. Furthermore, I fully agree with Dr. Cohn’s thoughtful approach regarding perioperative statins, despite the limited data available from randomized controlled trials.
With respect to perioperative aspirin use, I have concerns with Dr. Cohn’s statement that it may be reasonable to continue aspirin perioperatively if the risk of potential cardiac events outweighs the risk of bleeding. Given the result of the recently published second Perioperative Ischemic Evaluation (POISE-2) trial8 that showed a significantly higher risk of major perioperative bleeding in patients randomized to low-dose aspirin, it is difficult to advocate continuing aspirin when no cardiovascular protection was found in this very large trial. I agree with Dr. Cohn that this applies only to patients with no history of coronary artery stent placement, as patients with a stent should remain on low-dose aspirin throughout the entire perioperative period.
Controversy also surrounds angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Dr. Cohn agrees with the ACC/AHA guidelines to continue these agents before surgery; however, I favor holding them on the day of surgery. Although the risk of hypotension-induced cardiac events has not been clearly demonstrated, a recent retrospective study involving more than 1,100 patients showed significantly more acute kidney injury (even after adjusting for hypotension) as well as an increased length of hospital stay in the patients exposed to these agents before surgery.9 Given these findings, in addition to the postinduction hypotension (which can be profound) commonly observed by our anesthesiology colleagues, I recommend holding angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on the day of surgery, with very few exceptions.
THE SCIENCE AND ART OF MEDICINE
Dr. Cohn acknowledges that we lack scientific data to answer many questions that arise when caring for the perioperative patient and thus we rely on the ACC/AHA guidelines to provide a framework. These scientific knowledge gaps emphasize the importance of the art of medicine in the perioperative arena. Although we may desire “cookbook” guidelines, the significant gaps in the perioperative medicine evidence base reinforce the necessity to provide individual patient-level care in a multidisciplinary environment with our surgery and anesthesiology colleagues. Without the proper balance of science and art in perioperative medicine, we sacrifice our ability to deliver optimal care for this high-risk patient population.
- Cohn SL. Updated guidelines on cardiovascular evaluation before noncardiac surgery: a view from the trenches. Cleve Clin J Med 2014; 81:742–751.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05536-3. doi: 10.1016/j.jacc.2014.07.944. [Epub ahead of print].
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124:381–387.
- Aronson WL, McAuliffe MS, Miller K. Variability in the American Society of Anesthesiologists Physical Status Classification Scale. AANA J 2003; 71:265–274.
- Mak PH, Campbell RC, Irwin MG; American Society of Anesthesiologists. The ASA Physical Status Classification: inter-observer consistency. American Society of Anesthesiologists. Anaesth Intensive Care 2002; 30:633–640.
- Eagle KA, Berger PB, Calkins H, et al; American College of Cardiology; American Heart Association. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2002; 39:542–553.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370:1494–1503.
- Nielson E, Hennrikus E, Lehman E, Mets B. Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med 2014; 9:283–288.
- Cohn SL. Updated guidelines on cardiovascular evaluation before noncardiac surgery: a view from the trenches. Cleve Clin J Med 2014; 81:742–751.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05536-3. doi: 10.1016/j.jacc.2014.07.944. [Epub ahead of print].
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124:381–387.
- Aronson WL, McAuliffe MS, Miller K. Variability in the American Society of Anesthesiologists Physical Status Classification Scale. AANA J 2003; 71:265–274.
- Mak PH, Campbell RC, Irwin MG; American Society of Anesthesiologists. The ASA Physical Status Classification: inter-observer consistency. American Society of Anesthesiologists. Anaesth Intensive Care 2002; 30:633–640.
- Eagle KA, Berger PB, Calkins H, et al; American College of Cardiology; American Heart Association. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2002; 39:542–553.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370:1494–1503.
- Nielson E, Hennrikus E, Lehman E, Mets B. Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med 2014; 9:283–288.
Ebola—lessons still to be learned
In this issue of the Journal, Dr. Kyle Brizendine reviews the basics of the Ebola virus and its natural history, diagnosis, and management.
Like many of you, I have followed the Ebola story with disquietude. So far, the disease has barely touched our country, with fewer than 10 confirmed cases on US soil, but it has had a big impact on our health care system and our national psyche.
The creation of specialized containment and management units may deplete some hospitals and their communities of intensive care beds. Specially trained caregivers will need to be diverted to staff these units, and the public’s fear may dissuade patients from undergoing elective procedures at hospitals caring for patients with Ebola. All of these pose a financial challenge to the hospitals most capable of dealing with these patients.
We have yet to hear about management guidelines dealing with renal replacement therapy and ventilator support, which may extend life but also pose extra risks to caregivers. Do we understand the disease well enough to know when advanced supportive therapies might be futile? Many lessons were learned from the Liberian patient who died of Ebola in Dallas, but many more clinical questions remain. I had hoped that in our sophisticated ICUs patients treated relatively early with aggressive supportive care would likely survive. We do not yet know if that is true. One death does not make it false, but it does give one pause.
About a half dozen other Ebola patients have survived with treatment here, but they were not African. Does genetic background play a role in disease severity and survival? Were the survivors treated sooner or differently in ways that matter? How much of the end-organ damage from the virus is from direct organ infection that cannot be reversed or prevented by even the best supportive treatment? Does the ability of the virus to suppress the immune system doom patients to opportunistic infections during prolonged supportive therapy? Is the viral-associated immunosuppression enough to prevent some patients from mounting an effective innate (interferon-based) or acquired (viral-specific T-cell or humoral) antiviral response? And is transfusing blood from survivors, presumably conferring passive immunity, actually efficacious?
I was relieved there were no new Ebola cases among the staff caring for Mr. Duncan at his second emergency room visit in Dallas, since at that time he was clearly quite ill, viremic, and contagious. Universal safety precautions must have helped. But how did the other nurses become infected, even though they presumably wore better protection? Hopefully, we will gain further understanding of transmissibility and resistance. We need this knowledge to inform safe and manageable protocols of care, particularly if successful vaccine development is delayed.
In this issue of the Journal, Dr. Kyle Brizendine reviews the basics of the Ebola virus and its natural history, diagnosis, and management.
Like many of you, I have followed the Ebola story with disquietude. So far, the disease has barely touched our country, with fewer than 10 confirmed cases on US soil, but it has had a big impact on our health care system and our national psyche.
The creation of specialized containment and management units may deplete some hospitals and their communities of intensive care beds. Specially trained caregivers will need to be diverted to staff these units, and the public’s fear may dissuade patients from undergoing elective procedures at hospitals caring for patients with Ebola. All of these pose a financial challenge to the hospitals most capable of dealing with these patients.
We have yet to hear about management guidelines dealing with renal replacement therapy and ventilator support, which may extend life but also pose extra risks to caregivers. Do we understand the disease well enough to know when advanced supportive therapies might be futile? Many lessons were learned from the Liberian patient who died of Ebola in Dallas, but many more clinical questions remain. I had hoped that in our sophisticated ICUs patients treated relatively early with aggressive supportive care would likely survive. We do not yet know if that is true. One death does not make it false, but it does give one pause.
About a half dozen other Ebola patients have survived with treatment here, but they were not African. Does genetic background play a role in disease severity and survival? Were the survivors treated sooner or differently in ways that matter? How much of the end-organ damage from the virus is from direct organ infection that cannot be reversed or prevented by even the best supportive treatment? Does the ability of the virus to suppress the immune system doom patients to opportunistic infections during prolonged supportive therapy? Is the viral-associated immunosuppression enough to prevent some patients from mounting an effective innate (interferon-based) or acquired (viral-specific T-cell or humoral) antiviral response? And is transfusing blood from survivors, presumably conferring passive immunity, actually efficacious?
I was relieved there were no new Ebola cases among the staff caring for Mr. Duncan at his second emergency room visit in Dallas, since at that time he was clearly quite ill, viremic, and contagious. Universal safety precautions must have helped. But how did the other nurses become infected, even though they presumably wore better protection? Hopefully, we will gain further understanding of transmissibility and resistance. We need this knowledge to inform safe and manageable protocols of care, particularly if successful vaccine development is delayed.
In this issue of the Journal, Dr. Kyle Brizendine reviews the basics of the Ebola virus and its natural history, diagnosis, and management.
Like many of you, I have followed the Ebola story with disquietude. So far, the disease has barely touched our country, with fewer than 10 confirmed cases on US soil, but it has had a big impact on our health care system and our national psyche.
The creation of specialized containment and management units may deplete some hospitals and their communities of intensive care beds. Specially trained caregivers will need to be diverted to staff these units, and the public’s fear may dissuade patients from undergoing elective procedures at hospitals caring for patients with Ebola. All of these pose a financial challenge to the hospitals most capable of dealing with these patients.
We have yet to hear about management guidelines dealing with renal replacement therapy and ventilator support, which may extend life but also pose extra risks to caregivers. Do we understand the disease well enough to know when advanced supportive therapies might be futile? Many lessons were learned from the Liberian patient who died of Ebola in Dallas, but many more clinical questions remain. I had hoped that in our sophisticated ICUs patients treated relatively early with aggressive supportive care would likely survive. We do not yet know if that is true. One death does not make it false, but it does give one pause.
About a half dozen other Ebola patients have survived with treatment here, but they were not African. Does genetic background play a role in disease severity and survival? Were the survivors treated sooner or differently in ways that matter? How much of the end-organ damage from the virus is from direct organ infection that cannot be reversed or prevented by even the best supportive treatment? Does the ability of the virus to suppress the immune system doom patients to opportunistic infections during prolonged supportive therapy? Is the viral-associated immunosuppression enough to prevent some patients from mounting an effective innate (interferon-based) or acquired (viral-specific T-cell or humoral) antiviral response? And is transfusing blood from survivors, presumably conferring passive immunity, actually efficacious?
I was relieved there were no new Ebola cases among the staff caring for Mr. Duncan at his second emergency room visit in Dallas, since at that time he was clearly quite ill, viremic, and contagious. Universal safety precautions must have helped. But how did the other nurses become infected, even though they presumably wore better protection? Hopefully, we will gain further understanding of transmissibility and resistance. We need this knowledge to inform safe and manageable protocols of care, particularly if successful vaccine development is delayed.
Ebola virus: Questions, answers, and more questions
A 50-year-old man who returned from a business trip to Nigeria 24 days ago presents with complaints of the sudden onset of fever, diarrhea, myalgia, and headache. He reports 10 bowel movements per day and has seen bloody stools.
During his trip he flew in to Murtala Muhammed International Airport in Lagos, ate meals only in his hotel, and attended meetings in Lagos central business district. He had no exposure to animals, mosquitoes, ticks, or sick people, and no sexual activity. After returning home, he felt well for the first 3 weeks.
The patient has a history of hypertension. He does not smoke, drink alcohol, or use injection drugs. He is married, works with commercial banks and financial institutions, and lives in Cleveland, OH.
On physical examination his temperature is 100.0˚F (37.8˚C), pulse 98, respirations 15, blood pressure 105/70 mm Hg, and weight 78 kg (172 lb). He appears comfortable but is a little diaphoretic. His abdomen is tender to palpation in the epigastrium and slightly to the right; he has no signs of peritonitis. His skin is without rash, bleeding, or bruising. The remainder of the examination is normal.
His white blood cell count is 17 × 109/L, hemoglobin 15 g/dL, hematocrit 41%, and platelet count 172 × 109/L. His sodium level is 126 mmol/L, potassium 3.8 mmol/L, chloride 95 mmol/L, carbon dioxide 20 mmol/L, blood urea nitrogen 11 mg/dL, creatinine 0.7 mg/dL, and glucose 130 mg/dL. His aminotransferase and alkaline phosphatase levels are normal.
Could this patient have Ebola virus disease?
With Ebola virus disease on the rise in West Africa, physicians who encounter patients like this one need to include it in the differential diagnosis. Because the disease is new, many questions are raised for which we as yet have no answers. Here, I will review what we know and do not know in an effort to remove some of the fear and uncertainty.
A NEW DISEASE
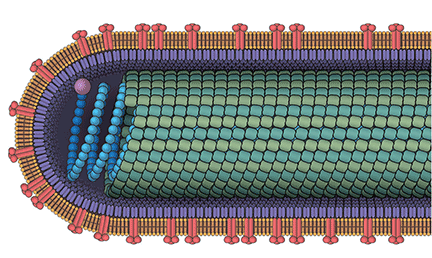
Ebola virus disease is a severe hemorrhagic fever caused by negative-sense single-stranded RNA viruses classified by the International Committee on Taxonomy of Viruses as belonging to the genus Ebolavirus in the family Filoviridae. Filoviruses get their name from the Latin filum, or thread-like structure.
The family Filoviridae was discovered in 1967 after inadvertent importation of infected monkeys from Uganda into Yugoslavia and Marburg, Germany. Outbreaks of severe illness occurred in workers at a vaccine plant who came into direct contact with the animals by killing them, removing their kidneys, or preparing primary cell cultures for polio vaccine production.
Ebola virus was discovered in 1976 by Peter Piot, who was working at the Institute of Tropical Medicine in Antwerp, Belgium. The blood of a Belgian woman who had been working in what is now the Democratic Republic of the Congo (formerly Zaire) had been sent to the institute; she and Mabalo Lokela, a school headmaster and the first recorded victim of Ebola virus, had been working near Yambuku, about 96 km from the Ebola River.
Before the 2014 outbreak, all known outbreaks had caused fewer than 2,400 cases across a dozen African countries over 3 decades.
Five species of Ebola virus
The genus Ebolavirus contains five species, each associated with a consistent case-fatality rate and a more or less well-identified endemic area.1
Zaire ebolavirus was recognized in 1976; it has caused multiple outbreaks, with high case-fatality rates.
Sudan ebolavirus was seen first in the 1970s; it has a 50% case-fatality rate.
Tai Forest ebolavirus has been found in only one person, an ethologist working with deceased chimpanzees.
Bundibugyo ebolavirus emerged in 2007 and has a 30% case-fatality rate.
Reston ebolavirus is maintained in an animal reservoir in the Philippines and is not found in Africa. It caused an outbreak of lethal infection in macaques imported into the United States in 1989. There is evidence that Reston ebolavirus can cause asymptomatic infection in humans. None of the caretakers of the macaques became ill, nor did farmers working with infected pigs, although both groups seroconverted.
A reservoir in bats?
A reservoir in nonhuman primates was initially suspected. However, studies subsequently showed that monkeys are susceptible to rapidly lethal filoviral disease, precluding any role as a host for persistent viral infection. It is likely that Ebola virus is maintained in small animals that serve as a source of infection for both humans and wild primates. A prominent suspect is fruit bats, which are consumed in soup in West Africa.
Transmission is person-to-person or nosocomial
Ebola virus is transmitted by direct contact with body fluids such as blood, urine, sweat, vomitus, semen, and breast milk. Filoviruses can initiate infection via ingestion, inhalation (although probably not Ebola), or passage through breaks in the skin. Droplet inoculation into the mouth or eyes has been shown to result from inadvertent transfer of virus from contaminated hands. Patients transmit the virus while febrile and through later stages of disease, as well as postmortem through contact with the body during funeral preparations. The virus has been isolated in semen for as many as 61 days after illness onset.
Ebola virus can also be spread nosocomially. In 1976, a 44-year-old teacher sought care for fever at the Yambuku Mission Hospital. He was given parenteral chloroquine as empiric treatment for presumed malaria, which was routine for all febrile patients. However, he had unrecognized Ebola virus infection. Moreover, syringes were rinsed in the same pan of water and reused, which spread the infection to nearly 100 people, all of whom developed fulminant Ebola virus disease and died. Infection then spread to family caregivers, the hospital staff, and those who prepared the bodies for burial.
Nosocomial transmission was also responsible for an outbreak of Lake Victoria Marburg virus in Uige Province in northern Angola in 2005, with 374 putative cases and 329 deaths. When teams from Médecins Sans Frontières started setting up the Marburg ward, there were five patients with hemorrhagic fever in a makeshift isolation room in the hospital, together with corpses that the hospital staff had been too afraid to remove. Healers found in many rural African communities were administering injections in homes or in makeshift clinics with reused needles or syringes.2
There is no evidence that filoviruses are carried by mosquitoes or other biting arthropods. Also, the risk of transmission via fomites appears to be low when currently recommended infection-control guidelines for the viral hemorrhagic fevers are followed.3 One primary human case generates only one to three secondary cases on average.
EBOLA IS AN IMMUNODEFICIENCY VIRUS
The main targets of infection are endothelial cells, mononuclear phagocytes, and hepatocytes. Ebola virus replicates at an unusually high rate. Macrophages infected with Zaire ebolavirus produce tumor necrosis factor alpha, interleukin (IL) 1 beta, IL-6, macrophage chemotactic protein 1, and nitric oxide. Virus-infected macrophages synthesize cell-surface tissue factor, triggering the extrinsic coagulation pathway.
Ebola is an immunodeficiency virus. Dendritic cells, which initiate adaptive immune responses, are a major site of filoviral replication. Infected cells cannot present antigens to naïve lymphocytes. Patients who die of Ebola virus disease do not develop antibodies to the virus. Lymphocytes remain uninfected, but undergo “bystander” apoptosis induced by inflammatory mediators.
CLINICAL MANIFESTATIONS
The incubation period is generally 5 to 7 days (range 2 to 28 days), during which the patient is not infectious. Symptoms begin abruptly, with fever, chills, general malaise, weakness, severe headache, and myalgia. By the time of case detection in West Africa, most patients also had nausea, vomiting, diarrhea, and abdominal pain. Once symptoms arise, patients have high levels of the virus in their blood and fluids and are infectious. Hemorrhagic symptoms have apparently been uncommon in West Africa, occurring in 1.0% to 5.7%, but “unexplained bleeding” has been documented in 18% of cases.4 Among those in whom the disease enters its hemorrhagic terminal phase, there is characteristic internal and subcutaneous bleeding, vomiting of blood, and subconjunctival hemorrhage.4
Laboratory findings include lymphocytopenia (often with counts as low as 1.0 × 109/L), thrombocytopenia (with counts in the range of 50 to 100 × 109/L), elevated aminotransferase levels (including aspartate aminotransferase levels 7 to 12 times higher than alanine aminotransferase in fatal cases), low total protein (due to capillary leak), and disseminated intravascular coagulation. Those who survive begin to improve in the second week, during which viremia resolves in association with the appearance of virus-specific antibodies.4
DIAGNOSIS
In symptomatic patients, Ebola virus infection is diagnosed by detection in blood or body fluids of viral antigens by enzyme-linked immunosorbent assay, or RNA sequences by reverse transcriptase polymerase chain reaction. The diagnosis is confirmed with cell culture (in a BSL-4 containment laboratory) showing characteristic viral particles by electron microscopy.
CARING FOR PATIENTS
The most detailed descriptions of the care of patients with Ebola virus disease have come from Dr. Bruce Ribner, of Emory University Hospital, in an October 2014 report of his experience caring for Ebola-infected patients at Emory University Hospital in Atlanta, GA.5 He described fluid losses of 5 to 10 L/day, profound hyponatremia, hypokalemia, and hypocalcemia, which were associated with cardiac arrhythmias and the need for intravenous and oral electrolyte repletion and hemodialysis. Intensive one-to-one nursing was critical, as was the coordination of many medical subspecialties. The Emory team arranged point-of-care testing near the unit and generally kept laboratory testing to a minimum. The team was surprised to learn that commercial carriers refused to transport specimens even when they were licensed for category A agents. Difficulties with the local water authority and waste disposal contractor required the hospital to dedicate an autoclave to process all materials used in clinical care.
TREATMENT: SUPPORTIVE AND EXPERIMENTAL
Treatment is supportive to maintain circulatory function and blood pressure and to correct coagulopathy. However, a variety of vaccines, antibodies, small-molecule agents, and antiviral agents are undergoing testing, mostly in animals at this point.
Vaccines. A therapeutic vaccine that worked only slightly was a live-attenuated recombinant vesicular stomatitis virus expressing Ebola virus transmembrane glycoproteins, which was tested in mice, guinea pigs, and rhesus macaques who had been exposed to Ebola virus.6
A preventive vaccine worked better. Stanley et al7 evaluated a replication-defective chimpanzee adenovirus 3-vectored vaccine that also contained Ebola virus glycoprotein. They gave macaques a single injection of this vaccine, and then 5 weeks later gave them a lethal dose of Ebola virus. All the vaccinated animals survived the infection, and half (2 of 4) survived when challenged 10 months later. With a prime-boost strategy (modified vaccinia virus Ankara, a poxvirus), all survived when challenged 10 months later.
KZ52, a neutralizing antibody, did not work. Oswald et al8 gave a human IgG monoclonal antibody against Zaire Ebola virus, designated KZ52, to four rhesus macaques, challenged them with the virus 24 hours later, and administered a second shot of KZ52 on day 4. All of them died.
ZMAb is a combination of three murine monoclonal antibodies, designated 1H3, 2G4, and 4G7. Ad-IFN is a human adenovirus, serotype 5, that expresses human interferon alpha. Qui et al9 gave ZMAb and Ad-IFN to macaques in several experiments. In experiment 1, eight macaques were infected and then were given ZMAb and Ad-IFN 3 days later, and ZMAb again on days 6 and 9. Seven of the eight survived. In a second experiment, Ad-IFN was given first, when the viral load was still less than the limit of detection of known assays, and then ZMAb was given upon detection of viremia and fever. Two of four macaques survived. Control animals had undetectable levels of IgG, whereas Ebola virus GP–specific IgG levels were detected in all survivors. IFN-gamma ELISpots showed high EBOV-GP–specific T-cell response in all survivors.
ZMapp is another cocktail of monoclonal antibodies, containing two from ZMab (2G4 and 4G7), plus a third, c13C6. In experiments in rhesus macaques, three groups of six animals each received three doses of ZMapp at varying times after being infected with Ebola virus: at 3, 6, and 9 days; at 4, 7, and 10 days, and at 5, 8, and 11 days. All 18 macaques treated with ZMapp survived. Thus, Zmapp extended the treatment window to 5 days postexposure.10 One of the American health care workers who contracted Ebola virus in Liberia received this medication.
HSPA5-PMO. Endoplasmic reticulum chaperone heat shock 70 kDa protein 5 (HSPA5) is instrumental in the maturation of envelope proteins in hepatitis C and influenza A virus. It plays a role in viral entry for coxsackievirus A9 and dengue virus serotype 2, and it may be involved in Ebola viral budding. Phosphorodiamidate morpholino oligomers (PMOs) are a class of antisense DNA nucleotide analogs.
Reid et al11 reported that mice treated with HSPA5–PMO were completely protected from lethal Ebola challenge. Therefore, HSPA5 appears to be a promising target for the development of antifilovirus countermeasures.
Favipiravir, an antiviral agent also known as T-705, is a pyrazinecarboxamide derivative. Invented in 2002 by Toyama Chemicals as an inhibitor of influenza virus replication, it acts as a nucleotide analog, selectively inhibiting the viral RNA-dependent RNA polymerase, or causes lethal mutagenesis upon incorporation into the virus RNA. Favipiravir suppresses Ebola virus replication by 4 log10 units in cell culture.12
Mice were challenged with intranasal inoculation of 1,000 focus-forming units of Ebola virus diluted in phosphate-buffered saline. Until the first day of treatment (postinfection day 6), all mice in the T-705 group lost weight similarly to control mice, developed viremia, and showed elevated serum levels of aspartate aminotransferase and alanine aminotransferase. Within 4 days of T-705 treatment (post-infection day 10), the animals had cleared the virus from blood. Surviving mice developed Ebola virus-specific antibodies and CD8+ T cells specific for the viral nucleoprotein.12
The authors hypothesized that suppression of virus replication by T-705 allowed the host to mount a virus-specific adaptive immune response, and concluded that T-705 was 100% effective in the treatment of Zaire Ebola virus infection up to postinfection day 6 but was hardly beneficial at the terminal stage of disease.12 Of note, favipiravir is undergoing phase 2 and phase 3 trials as an anti-influenza agent in Japan.
THE CURRENT OUTBREAK
The current outbreak is with Zaire ebolavirus. It seems to have started in a 2-year-old child who died in Meliandou in Guéckédou Prefecture, Guinea, on December 6, 2013. On March 21, 2014, the Guinea Ministry of Health reported the outbreak of an illness characterized by fever, severe diarrhea, vomiting, and a high case-fatality rate (59%) in 49 persons. On May 25, 2014, Kenema Government Hospital confirmed the first case of Ebola virus disease in Sierra Leone, probably brought there by a traditional healer who had treated Ebola patients from Guinea. Tracing led to 13 additional cases—all women who attended the burial.13
The Center for Systems Biology at Harvard University and the Broad Institute of Massachusetts Institute of Technology generated 99 Ebola virus genome sequences from 78 patients with confirmed disease, representing more than 70% of the patients diagnosed with the disease in Sierra Leone from May to mid-June 2014. They found genetic similarity across the sequenced 2014 samples, suggesting a single transmission from the natural reservoir, followed by human-to-human transmission during the outbreak. Continued human-reservoir exposure is unlikely to have contributed to the growth of this epidemic.14
As of October 14, 2014, there were 8,914 suspected and confirmed cases of Ebola virus infection, and 4,477 deaths.15
But how did Zaire Ebola virus make the 2,000-mile trek from Central Africa to Guinea in West Africa? There are two possibilities: it has always been present in the region but we just never noticed, or it was recently introduced. Bayesian phylogenetic analyses and sequence divergence studies suggest the virus has been present in bat populations in Guinea without previously infecting humans.
Why Guinea and why Guéckédou? Guinea is one of the poorest countries in the world, ranking 178th of 187 countries on the Human Development Index of the United Nations Development Programme, just behind Liberia (174th) and Sierra Leone (177th). In Guinea, the life expectancy is 56 years and the gross national income per capita is $440. The region has been systematically plundered and the forest decimated by clear-cut logging, leaving the Guinea Forest Region largely deforested, resulting in increased contact between humans and the small animals that serve as the source of infection.1
LIMITED CAPACITY, EVEN IN THE UNITED STATES
A few hospitals in the United States have dedicated units to handle serious infectious diseases such as Ebola: Emory University Hospital; Nebraska Medicine in Omaha; Providence St. Patrick Hospital in Missoula, MT; and the National Institutes of Health in Bethesda, MD. However, in total they have only 19 beds.
QUESTIONS, ANSWERS—AND MORE QUESTIONS
(The following is from a question-and-answer discussion that followed Dr. Brizendine’s Grand Rounds presentation.)
Q: Are there any differences between survivors and those who die of the disease? A: We do not know. Patient survival depends on early recognition and supportive care. There are disparities in the care of patients. Schieffelin et al16 analyzed the characteristics of patients who died or who survived in Sierra Leone and found that the mortality rate was higher in older patients and those with a higher viral load on presentation.
Q: Does the virus block production or release of interferon early in infection? A: Yes, it has been shown17 that Ebola virus protein VP24 inhibits signaling downstream of both interferon alpha/beta and interferon gamma by indirectly impairing the transport of a transcription factor termed STAT1. VP24 is also able to bind STAT1 directly. The resulting suppression of host interferon very early on in the incubation phase is key to the virulence of the virus.
Q: Does infection with one of the viral species confer immunity from other species? A: No, there is no cross-immunity.
Q: How soon do patients test positive? A: About 5 days after exposure, when they develop a fever. At this time patients are highly viremic, which PCR can detect.
Q: Before the virus is detectable in the blood, where is it? A: The liver, endothelial cells, antigen-presenting cells, and adrenal glands.
Q: Do we really need to quarantine ill patients and health care workers returning from Africa, per CDC recommendations? A: We don’t know everything, and some people do make bad decisions, such as traveling while symptomatic. I support a period of observation, although confinement is not reasonable, as it may pose a disincentive to cooperation.
Q: What is the role of giving plasma from survivors? A: Dr. Kent Brantly (see American citizens infected with Ebola) received the blood of a 14-year-old who survived. We don’t know. It is not proved. It did not result in improvement in animal models.
Q: Is the bleeding caused by a mechanism similar to that in enterohemorrhagic Escherichia coli infection? A: No. That is a bacterial toxin, whereas this is more like disseminated intravascular coagulation, with an intrinsic pathway anticoagulation cascade.
Q: How long does the virus remain viable outside the body? A: In one study,18 Ebola virus could not be recovered from experimentally contaminated surfaces (plastic, metal or glass) at room temperature. In another in which it was dried onto a surface,19 Ebola virus survived in the dark for several hours between 20 and 25°C. When dried in tissue culture media onto glass and stored at 4°C, it has survived for over 50 days.
Q: How long does the virus remain in breast milk? A: We know it has been detected 15 days after disease onset and think possibly as late as 28 days from symptom onset.3
Q: How are people actually infected? A: I believe people get the virus on their hands and then touch their face, eyes, or mouth. If you are wearing personal protective equipment, it must occur while doffing the equipment.
Q: Could we increase the sensitivity of the test so that we could detect the virus before the onset of symptoms? A: In theory it may be possible. The virus is somewhere in the body during the incubation period. Perhaps we could sample the right compartment in an enriched mononuclear cell line.
Q: When can patients who recover resume their normal activities? A: After their viral load returns to 0, I would still advise abstaining from unprotected sex and from breastfeeding for a few months. but as for other activities, no special precautions are needed.
Q: Does the virus appear to be mutating at a high rate? A: Looking back to 2004, mutations are occurring, but there is no sign that any of these mutations has contributed to the size of the outbreak by changing the characteristics of the Ebola virus. Can it become aerosolized? It has been suggested that the virus that caused the outbreak separated from those that caused past Ebola outbreaks but does not seem to be affecting the spread or efficacy of experimental drugs and vaccines. So, even though it is an RNA virus and mutations are occurring, no serious changes have emerged.14
BACK TO OUR PATIENT
The differential diagnosis for the patient described at the beginning of this paper includes travelers’ diarrhea, malaria, typhoid fever, yellow fever, meningococcal disease … and Ebola virus disease, although this is much less likely in view of the epidemiology and incubation period of this disease. When his stool was tested by enzyme immunoassay and culture, it was found to be positive for Campylobacter. He recovered with oral rehydration.
- Bausch DG, Schwarz L. Outbreak of ebola virus disease in Guinea: where ecology meets economy. PLoS Negl Trop Dis 2014; 8:e3056.
- Roddy P, Thomas SL, Jeffs B, et al. Factors associated with Marburg hemorrhagic fever: analysis of patient data from Uige, Angola. J Infect Dis 2010; 201:1909–1918.
- Bausch DG, Towner JS, Dowell SF, et al. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis 2007; 196(suppl 2):S142–S147.
- WHO Ebola Response Team. Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N Engl J Med 2014; 371:1481–1495.
- Ribner BS. Treating patients with Ebola virus infections in the US: lessons learned. Presented at IDWeek, October 8, 2014. Philadelphia PA.
- Feldman H, Jones SM, Daddario-DiCaprio KM, et al. Effective post-exposure treatment of Ebola infection. PLoS Pathog 2007; 3:e2.
- Stanley DA, Honko AN, Asiedu C, et al. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nat Med 2014; 20:1126–1129.
- Oswald WB, Geisbert TW, Davis KJ, et al. Neutralizing antibody fails to impact the course of Ebola virus infection in monkeys. PLos Pathog 2007; 3:e9.
- Qui X, Wong G, Fernando L, et al. mAbs and Ad-vectored IFN-a therapy rescue Ebola-infected nonhuman primates when administered after the detection of viremia and symptoms. Sci Transl Med 2013; 5:207ra143.
- Qui X, Wong G, Audet J, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 2014; 514:47–53.
- Reid SP, Shurtleff AC, Costantino JA, et al. HSPA5 is an essential host factor for Ebola virus infection. Antiviral Res 2014; 109:171–174.
- Oestereich L, Lüdtke A, Wurr S, Rieger T, Muñoz-Fontela C, Günther S. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antiviral Res 2014; 105:17–21.
- Baize S, Pannetier D, Oestereich L, et al. Emergence of Zaire Ebola virus dsease in Guinea. N Engl J Med 2014; 371:1418–1425.
- Gire SK, Goba A, Andersen KG, et al. Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science 2014; 345:1369–1372.
- Chamary JV. 4000 deaths and counting: the Ebola epidemic in 4 charts. Forbes. http://www.forbes.com/sites/jvchamary/2014/10/13/ebola-trends. Accessed November 5, 2014.
- Schieffelin JS, Shaffer JG, Goba A, et al, for the KGH Lassa Fever Program, the Viral Hemorrhagic Fever Consortium, and the WHO Clinical Response Team. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med 2014 Oct 29 [Epub ahead of print]. DOI: 10.1056/NEJMoa1411680.
- Zhang AP, Bornholdt ZA, Liu T, et al. The ebola virus interferon antagonist VP24 directly binds STAT1 and has a novel, pyramidal fold. PLoS Pathog 2012; 8:e1002550.
- Piercy TJ, Smither SJ, Steward JA, Eastaugh L, Lever MS. The survival of filoviruses in liquids, on solid substrates and in a dynamic aerosol. J Appl Microbiol 2010; 109:1531–1539.
- Sagripanti JL, Rom AM, Holland LE. Persistence in darkness of virulent alphaviruses, Ebola virus, and Lassa virus deposited on solid surfaces. Arch Virol 2010; 155:2035–2039.
A 50-year-old man who returned from a business trip to Nigeria 24 days ago presents with complaints of the sudden onset of fever, diarrhea, myalgia, and headache. He reports 10 bowel movements per day and has seen bloody stools.
During his trip he flew in to Murtala Muhammed International Airport in Lagos, ate meals only in his hotel, and attended meetings in Lagos central business district. He had no exposure to animals, mosquitoes, ticks, or sick people, and no sexual activity. After returning home, he felt well for the first 3 weeks.
The patient has a history of hypertension. He does not smoke, drink alcohol, or use injection drugs. He is married, works with commercial banks and financial institutions, and lives in Cleveland, OH.
On physical examination his temperature is 100.0˚F (37.8˚C), pulse 98, respirations 15, blood pressure 105/70 mm Hg, and weight 78 kg (172 lb). He appears comfortable but is a little diaphoretic. His abdomen is tender to palpation in the epigastrium and slightly to the right; he has no signs of peritonitis. His skin is without rash, bleeding, or bruising. The remainder of the examination is normal.
His white blood cell count is 17 × 109/L, hemoglobin 15 g/dL, hematocrit 41%, and platelet count 172 × 109/L. His sodium level is 126 mmol/L, potassium 3.8 mmol/L, chloride 95 mmol/L, carbon dioxide 20 mmol/L, blood urea nitrogen 11 mg/dL, creatinine 0.7 mg/dL, and glucose 130 mg/dL. His aminotransferase and alkaline phosphatase levels are normal.
Could this patient have Ebola virus disease?
With Ebola virus disease on the rise in West Africa, physicians who encounter patients like this one need to include it in the differential diagnosis. Because the disease is new, many questions are raised for which we as yet have no answers. Here, I will review what we know and do not know in an effort to remove some of the fear and uncertainty.
A NEW DISEASE
Ebola virus disease is a severe hemorrhagic fever caused by negative-sense single-stranded RNA viruses classified by the International Committee on Taxonomy of Viruses as belonging to the genus Ebolavirus in the family Filoviridae. Filoviruses get their name from the Latin filum, or thread-like structure.
The family Filoviridae was discovered in 1967 after inadvertent importation of infected monkeys from Uganda into Yugoslavia and Marburg, Germany. Outbreaks of severe illness occurred in workers at a vaccine plant who came into direct contact with the animals by killing them, removing their kidneys, or preparing primary cell cultures for polio vaccine production.
Ebola virus was discovered in 1976 by Peter Piot, who was working at the Institute of Tropical Medicine in Antwerp, Belgium. The blood of a Belgian woman who had been working in what is now the Democratic Republic of the Congo (formerly Zaire) had been sent to the institute; she and Mabalo Lokela, a school headmaster and the first recorded victim of Ebola virus, had been working near Yambuku, about 96 km from the Ebola River.
Before the 2014 outbreak, all known outbreaks had caused fewer than 2,400 cases across a dozen African countries over 3 decades.
Five species of Ebola virus
The genus Ebolavirus contains five species, each associated with a consistent case-fatality rate and a more or less well-identified endemic area.1
Zaire ebolavirus was recognized in 1976; it has caused multiple outbreaks, with high case-fatality rates.
Sudan ebolavirus was seen first in the 1970s; it has a 50% case-fatality rate.
Tai Forest ebolavirus has been found in only one person, an ethologist working with deceased chimpanzees.
Bundibugyo ebolavirus emerged in 2007 and has a 30% case-fatality rate.
Reston ebolavirus is maintained in an animal reservoir in the Philippines and is not found in Africa. It caused an outbreak of lethal infection in macaques imported into the United States in 1989. There is evidence that Reston ebolavirus can cause asymptomatic infection in humans. None of the caretakers of the macaques became ill, nor did farmers working with infected pigs, although both groups seroconverted.
A reservoir in bats?
A reservoir in nonhuman primates was initially suspected. However, studies subsequently showed that monkeys are susceptible to rapidly lethal filoviral disease, precluding any role as a host for persistent viral infection. It is likely that Ebola virus is maintained in small animals that serve as a source of infection for both humans and wild primates. A prominent suspect is fruit bats, which are consumed in soup in West Africa.
Transmission is person-to-person or nosocomial
Ebola virus is transmitted by direct contact with body fluids such as blood, urine, sweat, vomitus, semen, and breast milk. Filoviruses can initiate infection via ingestion, inhalation (although probably not Ebola), or passage through breaks in the skin. Droplet inoculation into the mouth or eyes has been shown to result from inadvertent transfer of virus from contaminated hands. Patients transmit the virus while febrile and through later stages of disease, as well as postmortem through contact with the body during funeral preparations. The virus has been isolated in semen for as many as 61 days after illness onset.
Ebola virus can also be spread nosocomially. In 1976, a 44-year-old teacher sought care for fever at the Yambuku Mission Hospital. He was given parenteral chloroquine as empiric treatment for presumed malaria, which was routine for all febrile patients. However, he had unrecognized Ebola virus infection. Moreover, syringes were rinsed in the same pan of water and reused, which spread the infection to nearly 100 people, all of whom developed fulminant Ebola virus disease and died. Infection then spread to family caregivers, the hospital staff, and those who prepared the bodies for burial.
Nosocomial transmission was also responsible for an outbreak of Lake Victoria Marburg virus in Uige Province in northern Angola in 2005, with 374 putative cases and 329 deaths. When teams from Médecins Sans Frontières started setting up the Marburg ward, there were five patients with hemorrhagic fever in a makeshift isolation room in the hospital, together with corpses that the hospital staff had been too afraid to remove. Healers found in many rural African communities were administering injections in homes or in makeshift clinics with reused needles or syringes.2
There is no evidence that filoviruses are carried by mosquitoes or other biting arthropods. Also, the risk of transmission via fomites appears to be low when currently recommended infection-control guidelines for the viral hemorrhagic fevers are followed.3 One primary human case generates only one to three secondary cases on average.
EBOLA IS AN IMMUNODEFICIENCY VIRUS
The main targets of infection are endothelial cells, mononuclear phagocytes, and hepatocytes. Ebola virus replicates at an unusually high rate. Macrophages infected with Zaire ebolavirus produce tumor necrosis factor alpha, interleukin (IL) 1 beta, IL-6, macrophage chemotactic protein 1, and nitric oxide. Virus-infected macrophages synthesize cell-surface tissue factor, triggering the extrinsic coagulation pathway.
Ebola is an immunodeficiency virus. Dendritic cells, which initiate adaptive immune responses, are a major site of filoviral replication. Infected cells cannot present antigens to naïve lymphocytes. Patients who die of Ebola virus disease do not develop antibodies to the virus. Lymphocytes remain uninfected, but undergo “bystander” apoptosis induced by inflammatory mediators.
CLINICAL MANIFESTATIONS
The incubation period is generally 5 to 7 days (range 2 to 28 days), during which the patient is not infectious. Symptoms begin abruptly, with fever, chills, general malaise, weakness, severe headache, and myalgia. By the time of case detection in West Africa, most patients also had nausea, vomiting, diarrhea, and abdominal pain. Once symptoms arise, patients have high levels of the virus in their blood and fluids and are infectious. Hemorrhagic symptoms have apparently been uncommon in West Africa, occurring in 1.0% to 5.7%, but “unexplained bleeding” has been documented in 18% of cases.4 Among those in whom the disease enters its hemorrhagic terminal phase, there is characteristic internal and subcutaneous bleeding, vomiting of blood, and subconjunctival hemorrhage.4
Laboratory findings include lymphocytopenia (often with counts as low as 1.0 × 109/L), thrombocytopenia (with counts in the range of 50 to 100 × 109/L), elevated aminotransferase levels (including aspartate aminotransferase levels 7 to 12 times higher than alanine aminotransferase in fatal cases), low total protein (due to capillary leak), and disseminated intravascular coagulation. Those who survive begin to improve in the second week, during which viremia resolves in association with the appearance of virus-specific antibodies.4
DIAGNOSIS
In symptomatic patients, Ebola virus infection is diagnosed by detection in blood or body fluids of viral antigens by enzyme-linked immunosorbent assay, or RNA sequences by reverse transcriptase polymerase chain reaction. The diagnosis is confirmed with cell culture (in a BSL-4 containment laboratory) showing characteristic viral particles by electron microscopy.
CARING FOR PATIENTS
The most detailed descriptions of the care of patients with Ebola virus disease have come from Dr. Bruce Ribner, of Emory University Hospital, in an October 2014 report of his experience caring for Ebola-infected patients at Emory University Hospital in Atlanta, GA.5 He described fluid losses of 5 to 10 L/day, profound hyponatremia, hypokalemia, and hypocalcemia, which were associated with cardiac arrhythmias and the need for intravenous and oral electrolyte repletion and hemodialysis. Intensive one-to-one nursing was critical, as was the coordination of many medical subspecialties. The Emory team arranged point-of-care testing near the unit and generally kept laboratory testing to a minimum. The team was surprised to learn that commercial carriers refused to transport specimens even when they were licensed for category A agents. Difficulties with the local water authority and waste disposal contractor required the hospital to dedicate an autoclave to process all materials used in clinical care.
TREATMENT: SUPPORTIVE AND EXPERIMENTAL
Treatment is supportive to maintain circulatory function and blood pressure and to correct coagulopathy. However, a variety of vaccines, antibodies, small-molecule agents, and antiviral agents are undergoing testing, mostly in animals at this point.
Vaccines. A therapeutic vaccine that worked only slightly was a live-attenuated recombinant vesicular stomatitis virus expressing Ebola virus transmembrane glycoproteins, which was tested in mice, guinea pigs, and rhesus macaques who had been exposed to Ebola virus.6
A preventive vaccine worked better. Stanley et al7 evaluated a replication-defective chimpanzee adenovirus 3-vectored vaccine that also contained Ebola virus glycoprotein. They gave macaques a single injection of this vaccine, and then 5 weeks later gave them a lethal dose of Ebola virus. All the vaccinated animals survived the infection, and half (2 of 4) survived when challenged 10 months later. With a prime-boost strategy (modified vaccinia virus Ankara, a poxvirus), all survived when challenged 10 months later.
KZ52, a neutralizing antibody, did not work. Oswald et al8 gave a human IgG monoclonal antibody against Zaire Ebola virus, designated KZ52, to four rhesus macaques, challenged them with the virus 24 hours later, and administered a second shot of KZ52 on day 4. All of them died.
ZMAb is a combination of three murine monoclonal antibodies, designated 1H3, 2G4, and 4G7. Ad-IFN is a human adenovirus, serotype 5, that expresses human interferon alpha. Qui et al9 gave ZMAb and Ad-IFN to macaques in several experiments. In experiment 1, eight macaques were infected and then were given ZMAb and Ad-IFN 3 days later, and ZMAb again on days 6 and 9. Seven of the eight survived. In a second experiment, Ad-IFN was given first, when the viral load was still less than the limit of detection of known assays, and then ZMAb was given upon detection of viremia and fever. Two of four macaques survived. Control animals had undetectable levels of IgG, whereas Ebola virus GP–specific IgG levels were detected in all survivors. IFN-gamma ELISpots showed high EBOV-GP–specific T-cell response in all survivors.
ZMapp is another cocktail of monoclonal antibodies, containing two from ZMab (2G4 and 4G7), plus a third, c13C6. In experiments in rhesus macaques, three groups of six animals each received three doses of ZMapp at varying times after being infected with Ebola virus: at 3, 6, and 9 days; at 4, 7, and 10 days, and at 5, 8, and 11 days. All 18 macaques treated with ZMapp survived. Thus, Zmapp extended the treatment window to 5 days postexposure.10 One of the American health care workers who contracted Ebola virus in Liberia received this medication.
HSPA5-PMO. Endoplasmic reticulum chaperone heat shock 70 kDa protein 5 (HSPA5) is instrumental in the maturation of envelope proteins in hepatitis C and influenza A virus. It plays a role in viral entry for coxsackievirus A9 and dengue virus serotype 2, and it may be involved in Ebola viral budding. Phosphorodiamidate morpholino oligomers (PMOs) are a class of antisense DNA nucleotide analogs.
Reid et al11 reported that mice treated with HSPA5–PMO were completely protected from lethal Ebola challenge. Therefore, HSPA5 appears to be a promising target for the development of antifilovirus countermeasures.
Favipiravir, an antiviral agent also known as T-705, is a pyrazinecarboxamide derivative. Invented in 2002 by Toyama Chemicals as an inhibitor of influenza virus replication, it acts as a nucleotide analog, selectively inhibiting the viral RNA-dependent RNA polymerase, or causes lethal mutagenesis upon incorporation into the virus RNA. Favipiravir suppresses Ebola virus replication by 4 log10 units in cell culture.12
Mice were challenged with intranasal inoculation of 1,000 focus-forming units of Ebola virus diluted in phosphate-buffered saline. Until the first day of treatment (postinfection day 6), all mice in the T-705 group lost weight similarly to control mice, developed viremia, and showed elevated serum levels of aspartate aminotransferase and alanine aminotransferase. Within 4 days of T-705 treatment (post-infection day 10), the animals had cleared the virus from blood. Surviving mice developed Ebola virus-specific antibodies and CD8+ T cells specific for the viral nucleoprotein.12
The authors hypothesized that suppression of virus replication by T-705 allowed the host to mount a virus-specific adaptive immune response, and concluded that T-705 was 100% effective in the treatment of Zaire Ebola virus infection up to postinfection day 6 but was hardly beneficial at the terminal stage of disease.12 Of note, favipiravir is undergoing phase 2 and phase 3 trials as an anti-influenza agent in Japan.
THE CURRENT OUTBREAK
The current outbreak is with Zaire ebolavirus. It seems to have started in a 2-year-old child who died in Meliandou in Guéckédou Prefecture, Guinea, on December 6, 2013. On March 21, 2014, the Guinea Ministry of Health reported the outbreak of an illness characterized by fever, severe diarrhea, vomiting, and a high case-fatality rate (59%) in 49 persons. On May 25, 2014, Kenema Government Hospital confirmed the first case of Ebola virus disease in Sierra Leone, probably brought there by a traditional healer who had treated Ebola patients from Guinea. Tracing led to 13 additional cases—all women who attended the burial.13
The Center for Systems Biology at Harvard University and the Broad Institute of Massachusetts Institute of Technology generated 99 Ebola virus genome sequences from 78 patients with confirmed disease, representing more than 70% of the patients diagnosed with the disease in Sierra Leone from May to mid-June 2014. They found genetic similarity across the sequenced 2014 samples, suggesting a single transmission from the natural reservoir, followed by human-to-human transmission during the outbreak. Continued human-reservoir exposure is unlikely to have contributed to the growth of this epidemic.14
As of October 14, 2014, there were 8,914 suspected and confirmed cases of Ebola virus infection, and 4,477 deaths.15
But how did Zaire Ebola virus make the 2,000-mile trek from Central Africa to Guinea in West Africa? There are two possibilities: it has always been present in the region but we just never noticed, or it was recently introduced. Bayesian phylogenetic analyses and sequence divergence studies suggest the virus has been present in bat populations in Guinea without previously infecting humans.
Why Guinea and why Guéckédou? Guinea is one of the poorest countries in the world, ranking 178th of 187 countries on the Human Development Index of the United Nations Development Programme, just behind Liberia (174th) and Sierra Leone (177th). In Guinea, the life expectancy is 56 years and the gross national income per capita is $440. The region has been systematically plundered and the forest decimated by clear-cut logging, leaving the Guinea Forest Region largely deforested, resulting in increased contact between humans and the small animals that serve as the source of infection.1
LIMITED CAPACITY, EVEN IN THE UNITED STATES
A few hospitals in the United States have dedicated units to handle serious infectious diseases such as Ebola: Emory University Hospital; Nebraska Medicine in Omaha; Providence St. Patrick Hospital in Missoula, MT; and the National Institutes of Health in Bethesda, MD. However, in total they have only 19 beds.
QUESTIONS, ANSWERS—AND MORE QUESTIONS
(The following is from a question-and-answer discussion that followed Dr. Brizendine’s Grand Rounds presentation.)
Q: Are there any differences between survivors and those who die of the disease? A: We do not know. Patient survival depends on early recognition and supportive care. There are disparities in the care of patients. Schieffelin et al16 analyzed the characteristics of patients who died or who survived in Sierra Leone and found that the mortality rate was higher in older patients and those with a higher viral load on presentation.
Q: Does the virus block production or release of interferon early in infection? A: Yes, it has been shown17 that Ebola virus protein VP24 inhibits signaling downstream of both interferon alpha/beta and interferon gamma by indirectly impairing the transport of a transcription factor termed STAT1. VP24 is also able to bind STAT1 directly. The resulting suppression of host interferon very early on in the incubation phase is key to the virulence of the virus.
Q: Does infection with one of the viral species confer immunity from other species? A: No, there is no cross-immunity.
Q: How soon do patients test positive? A: About 5 days after exposure, when they develop a fever. At this time patients are highly viremic, which PCR can detect.
Q: Before the virus is detectable in the blood, where is it? A: The liver, endothelial cells, antigen-presenting cells, and adrenal glands.
Q: Do we really need to quarantine ill patients and health care workers returning from Africa, per CDC recommendations? A: We don’t know everything, and some people do make bad decisions, such as traveling while symptomatic. I support a period of observation, although confinement is not reasonable, as it may pose a disincentive to cooperation.
Q: What is the role of giving plasma from survivors? A: Dr. Kent Brantly (see American citizens infected with Ebola) received the blood of a 14-year-old who survived. We don’t know. It is not proved. It did not result in improvement in animal models.
Q: Is the bleeding caused by a mechanism similar to that in enterohemorrhagic Escherichia coli infection? A: No. That is a bacterial toxin, whereas this is more like disseminated intravascular coagulation, with an intrinsic pathway anticoagulation cascade.
Q: How long does the virus remain viable outside the body? A: In one study,18 Ebola virus could not be recovered from experimentally contaminated surfaces (plastic, metal or glass) at room temperature. In another in which it was dried onto a surface,19 Ebola virus survived in the dark for several hours between 20 and 25°C. When dried in tissue culture media onto glass and stored at 4°C, it has survived for over 50 days.
Q: How long does the virus remain in breast milk? A: We know it has been detected 15 days after disease onset and think possibly as late as 28 days from symptom onset.3
Q: How are people actually infected? A: I believe people get the virus on their hands and then touch their face, eyes, or mouth. If you are wearing personal protective equipment, it must occur while doffing the equipment.
Q: Could we increase the sensitivity of the test so that we could detect the virus before the onset of symptoms? A: In theory it may be possible. The virus is somewhere in the body during the incubation period. Perhaps we could sample the right compartment in an enriched mononuclear cell line.
Q: When can patients who recover resume their normal activities? A: After their viral load returns to 0, I would still advise abstaining from unprotected sex and from breastfeeding for a few months. but as for other activities, no special precautions are needed.
Q: Does the virus appear to be mutating at a high rate? A: Looking back to 2004, mutations are occurring, but there is no sign that any of these mutations has contributed to the size of the outbreak by changing the characteristics of the Ebola virus. Can it become aerosolized? It has been suggested that the virus that caused the outbreak separated from those that caused past Ebola outbreaks but does not seem to be affecting the spread or efficacy of experimental drugs and vaccines. So, even though it is an RNA virus and mutations are occurring, no serious changes have emerged.14
BACK TO OUR PATIENT
The differential diagnosis for the patient described at the beginning of this paper includes travelers’ diarrhea, malaria, typhoid fever, yellow fever, meningococcal disease … and Ebola virus disease, although this is much less likely in view of the epidemiology and incubation period of this disease. When his stool was tested by enzyme immunoassay and culture, it was found to be positive for Campylobacter. He recovered with oral rehydration.
A 50-year-old man who returned from a business trip to Nigeria 24 days ago presents with complaints of the sudden onset of fever, diarrhea, myalgia, and headache. He reports 10 bowel movements per day and has seen bloody stools.
During his trip he flew in to Murtala Muhammed International Airport in Lagos, ate meals only in his hotel, and attended meetings in Lagos central business district. He had no exposure to animals, mosquitoes, ticks, or sick people, and no sexual activity. After returning home, he felt well for the first 3 weeks.
The patient has a history of hypertension. He does not smoke, drink alcohol, or use injection drugs. He is married, works with commercial banks and financial institutions, and lives in Cleveland, OH.
On physical examination his temperature is 100.0˚F (37.8˚C), pulse 98, respirations 15, blood pressure 105/70 mm Hg, and weight 78 kg (172 lb). He appears comfortable but is a little diaphoretic. His abdomen is tender to palpation in the epigastrium and slightly to the right; he has no signs of peritonitis. His skin is without rash, bleeding, or bruising. The remainder of the examination is normal.
His white blood cell count is 17 × 109/L, hemoglobin 15 g/dL, hematocrit 41%, and platelet count 172 × 109/L. His sodium level is 126 mmol/L, potassium 3.8 mmol/L, chloride 95 mmol/L, carbon dioxide 20 mmol/L, blood urea nitrogen 11 mg/dL, creatinine 0.7 mg/dL, and glucose 130 mg/dL. His aminotransferase and alkaline phosphatase levels are normal.
Could this patient have Ebola virus disease?
With Ebola virus disease on the rise in West Africa, physicians who encounter patients like this one need to include it in the differential diagnosis. Because the disease is new, many questions are raised for which we as yet have no answers. Here, I will review what we know and do not know in an effort to remove some of the fear and uncertainty.
A NEW DISEASE
Ebola virus disease is a severe hemorrhagic fever caused by negative-sense single-stranded RNA viruses classified by the International Committee on Taxonomy of Viruses as belonging to the genus Ebolavirus in the family Filoviridae. Filoviruses get their name from the Latin filum, or thread-like structure.
The family Filoviridae was discovered in 1967 after inadvertent importation of infected monkeys from Uganda into Yugoslavia and Marburg, Germany. Outbreaks of severe illness occurred in workers at a vaccine plant who came into direct contact with the animals by killing them, removing their kidneys, or preparing primary cell cultures for polio vaccine production.
Ebola virus was discovered in 1976 by Peter Piot, who was working at the Institute of Tropical Medicine in Antwerp, Belgium. The blood of a Belgian woman who had been working in what is now the Democratic Republic of the Congo (formerly Zaire) had been sent to the institute; she and Mabalo Lokela, a school headmaster and the first recorded victim of Ebola virus, had been working near Yambuku, about 96 km from the Ebola River.
Before the 2014 outbreak, all known outbreaks had caused fewer than 2,400 cases across a dozen African countries over 3 decades.
Five species of Ebola virus
The genus Ebolavirus contains five species, each associated with a consistent case-fatality rate and a more or less well-identified endemic area.1
Zaire ebolavirus was recognized in 1976; it has caused multiple outbreaks, with high case-fatality rates.
Sudan ebolavirus was seen first in the 1970s; it has a 50% case-fatality rate.
Tai Forest ebolavirus has been found in only one person, an ethologist working with deceased chimpanzees.
Bundibugyo ebolavirus emerged in 2007 and has a 30% case-fatality rate.
Reston ebolavirus is maintained in an animal reservoir in the Philippines and is not found in Africa. It caused an outbreak of lethal infection in macaques imported into the United States in 1989. There is evidence that Reston ebolavirus can cause asymptomatic infection in humans. None of the caretakers of the macaques became ill, nor did farmers working with infected pigs, although both groups seroconverted.
A reservoir in bats?
A reservoir in nonhuman primates was initially suspected. However, studies subsequently showed that monkeys are susceptible to rapidly lethal filoviral disease, precluding any role as a host for persistent viral infection. It is likely that Ebola virus is maintained in small animals that serve as a source of infection for both humans and wild primates. A prominent suspect is fruit bats, which are consumed in soup in West Africa.
Transmission is person-to-person or nosocomial
Ebola virus is transmitted by direct contact with body fluids such as blood, urine, sweat, vomitus, semen, and breast milk. Filoviruses can initiate infection via ingestion, inhalation (although probably not Ebola), or passage through breaks in the skin. Droplet inoculation into the mouth or eyes has been shown to result from inadvertent transfer of virus from contaminated hands. Patients transmit the virus while febrile and through later stages of disease, as well as postmortem through contact with the body during funeral preparations. The virus has been isolated in semen for as many as 61 days after illness onset.
Ebola virus can also be spread nosocomially. In 1976, a 44-year-old teacher sought care for fever at the Yambuku Mission Hospital. He was given parenteral chloroquine as empiric treatment for presumed malaria, which was routine for all febrile patients. However, he had unrecognized Ebola virus infection. Moreover, syringes were rinsed in the same pan of water and reused, which spread the infection to nearly 100 people, all of whom developed fulminant Ebola virus disease and died. Infection then spread to family caregivers, the hospital staff, and those who prepared the bodies for burial.
Nosocomial transmission was also responsible for an outbreak of Lake Victoria Marburg virus in Uige Province in northern Angola in 2005, with 374 putative cases and 329 deaths. When teams from Médecins Sans Frontières started setting up the Marburg ward, there were five patients with hemorrhagic fever in a makeshift isolation room in the hospital, together with corpses that the hospital staff had been too afraid to remove. Healers found in many rural African communities were administering injections in homes or in makeshift clinics with reused needles or syringes.2
There is no evidence that filoviruses are carried by mosquitoes or other biting arthropods. Also, the risk of transmission via fomites appears to be low when currently recommended infection-control guidelines for the viral hemorrhagic fevers are followed.3 One primary human case generates only one to three secondary cases on average.
EBOLA IS AN IMMUNODEFICIENCY VIRUS
The main targets of infection are endothelial cells, mononuclear phagocytes, and hepatocytes. Ebola virus replicates at an unusually high rate. Macrophages infected with Zaire ebolavirus produce tumor necrosis factor alpha, interleukin (IL) 1 beta, IL-6, macrophage chemotactic protein 1, and nitric oxide. Virus-infected macrophages synthesize cell-surface tissue factor, triggering the extrinsic coagulation pathway.
Ebola is an immunodeficiency virus. Dendritic cells, which initiate adaptive immune responses, are a major site of filoviral replication. Infected cells cannot present antigens to naïve lymphocytes. Patients who die of Ebola virus disease do not develop antibodies to the virus. Lymphocytes remain uninfected, but undergo “bystander” apoptosis induced by inflammatory mediators.
CLINICAL MANIFESTATIONS
The incubation period is generally 5 to 7 days (range 2 to 28 days), during which the patient is not infectious. Symptoms begin abruptly, with fever, chills, general malaise, weakness, severe headache, and myalgia. By the time of case detection in West Africa, most patients also had nausea, vomiting, diarrhea, and abdominal pain. Once symptoms arise, patients have high levels of the virus in their blood and fluids and are infectious. Hemorrhagic symptoms have apparently been uncommon in West Africa, occurring in 1.0% to 5.7%, but “unexplained bleeding” has been documented in 18% of cases.4 Among those in whom the disease enters its hemorrhagic terminal phase, there is characteristic internal and subcutaneous bleeding, vomiting of blood, and subconjunctival hemorrhage.4
Laboratory findings include lymphocytopenia (often with counts as low as 1.0 × 109/L), thrombocytopenia (with counts in the range of 50 to 100 × 109/L), elevated aminotransferase levels (including aspartate aminotransferase levels 7 to 12 times higher than alanine aminotransferase in fatal cases), low total protein (due to capillary leak), and disseminated intravascular coagulation. Those who survive begin to improve in the second week, during which viremia resolves in association with the appearance of virus-specific antibodies.4
DIAGNOSIS
In symptomatic patients, Ebola virus infection is diagnosed by detection in blood or body fluids of viral antigens by enzyme-linked immunosorbent assay, or RNA sequences by reverse transcriptase polymerase chain reaction. The diagnosis is confirmed with cell culture (in a BSL-4 containment laboratory) showing characteristic viral particles by electron microscopy.
CARING FOR PATIENTS
The most detailed descriptions of the care of patients with Ebola virus disease have come from Dr. Bruce Ribner, of Emory University Hospital, in an October 2014 report of his experience caring for Ebola-infected patients at Emory University Hospital in Atlanta, GA.5 He described fluid losses of 5 to 10 L/day, profound hyponatremia, hypokalemia, and hypocalcemia, which were associated with cardiac arrhythmias and the need for intravenous and oral electrolyte repletion and hemodialysis. Intensive one-to-one nursing was critical, as was the coordination of many medical subspecialties. The Emory team arranged point-of-care testing near the unit and generally kept laboratory testing to a minimum. The team was surprised to learn that commercial carriers refused to transport specimens even when they were licensed for category A agents. Difficulties with the local water authority and waste disposal contractor required the hospital to dedicate an autoclave to process all materials used in clinical care.
TREATMENT: SUPPORTIVE AND EXPERIMENTAL
Treatment is supportive to maintain circulatory function and blood pressure and to correct coagulopathy. However, a variety of vaccines, antibodies, small-molecule agents, and antiviral agents are undergoing testing, mostly in animals at this point.
Vaccines. A therapeutic vaccine that worked only slightly was a live-attenuated recombinant vesicular stomatitis virus expressing Ebola virus transmembrane glycoproteins, which was tested in mice, guinea pigs, and rhesus macaques who had been exposed to Ebola virus.6
A preventive vaccine worked better. Stanley et al7 evaluated a replication-defective chimpanzee adenovirus 3-vectored vaccine that also contained Ebola virus glycoprotein. They gave macaques a single injection of this vaccine, and then 5 weeks later gave them a lethal dose of Ebola virus. All the vaccinated animals survived the infection, and half (2 of 4) survived when challenged 10 months later. With a prime-boost strategy (modified vaccinia virus Ankara, a poxvirus), all survived when challenged 10 months later.
KZ52, a neutralizing antibody, did not work. Oswald et al8 gave a human IgG monoclonal antibody against Zaire Ebola virus, designated KZ52, to four rhesus macaques, challenged them with the virus 24 hours later, and administered a second shot of KZ52 on day 4. All of them died.
ZMAb is a combination of three murine monoclonal antibodies, designated 1H3, 2G4, and 4G7. Ad-IFN is a human adenovirus, serotype 5, that expresses human interferon alpha. Qui et al9 gave ZMAb and Ad-IFN to macaques in several experiments. In experiment 1, eight macaques were infected and then were given ZMAb and Ad-IFN 3 days later, and ZMAb again on days 6 and 9. Seven of the eight survived. In a second experiment, Ad-IFN was given first, when the viral load was still less than the limit of detection of known assays, and then ZMAb was given upon detection of viremia and fever. Two of four macaques survived. Control animals had undetectable levels of IgG, whereas Ebola virus GP–specific IgG levels were detected in all survivors. IFN-gamma ELISpots showed high EBOV-GP–specific T-cell response in all survivors.
ZMapp is another cocktail of monoclonal antibodies, containing two from ZMab (2G4 and 4G7), plus a third, c13C6. In experiments in rhesus macaques, three groups of six animals each received three doses of ZMapp at varying times after being infected with Ebola virus: at 3, 6, and 9 days; at 4, 7, and 10 days, and at 5, 8, and 11 days. All 18 macaques treated with ZMapp survived. Thus, Zmapp extended the treatment window to 5 days postexposure.10 One of the American health care workers who contracted Ebola virus in Liberia received this medication.
HSPA5-PMO. Endoplasmic reticulum chaperone heat shock 70 kDa protein 5 (HSPA5) is instrumental in the maturation of envelope proteins in hepatitis C and influenza A virus. It plays a role in viral entry for coxsackievirus A9 and dengue virus serotype 2, and it may be involved in Ebola viral budding. Phosphorodiamidate morpholino oligomers (PMOs) are a class of antisense DNA nucleotide analogs.
Reid et al11 reported that mice treated with HSPA5–PMO were completely protected from lethal Ebola challenge. Therefore, HSPA5 appears to be a promising target for the development of antifilovirus countermeasures.
Favipiravir, an antiviral agent also known as T-705, is a pyrazinecarboxamide derivative. Invented in 2002 by Toyama Chemicals as an inhibitor of influenza virus replication, it acts as a nucleotide analog, selectively inhibiting the viral RNA-dependent RNA polymerase, or causes lethal mutagenesis upon incorporation into the virus RNA. Favipiravir suppresses Ebola virus replication by 4 log10 units in cell culture.12
Mice were challenged with intranasal inoculation of 1,000 focus-forming units of Ebola virus diluted in phosphate-buffered saline. Until the first day of treatment (postinfection day 6), all mice in the T-705 group lost weight similarly to control mice, developed viremia, and showed elevated serum levels of aspartate aminotransferase and alanine aminotransferase. Within 4 days of T-705 treatment (post-infection day 10), the animals had cleared the virus from blood. Surviving mice developed Ebola virus-specific antibodies and CD8+ T cells specific for the viral nucleoprotein.12
The authors hypothesized that suppression of virus replication by T-705 allowed the host to mount a virus-specific adaptive immune response, and concluded that T-705 was 100% effective in the treatment of Zaire Ebola virus infection up to postinfection day 6 but was hardly beneficial at the terminal stage of disease.12 Of note, favipiravir is undergoing phase 2 and phase 3 trials as an anti-influenza agent in Japan.
THE CURRENT OUTBREAK
The current outbreak is with Zaire ebolavirus. It seems to have started in a 2-year-old child who died in Meliandou in Guéckédou Prefecture, Guinea, on December 6, 2013. On March 21, 2014, the Guinea Ministry of Health reported the outbreak of an illness characterized by fever, severe diarrhea, vomiting, and a high case-fatality rate (59%) in 49 persons. On May 25, 2014, Kenema Government Hospital confirmed the first case of Ebola virus disease in Sierra Leone, probably brought there by a traditional healer who had treated Ebola patients from Guinea. Tracing led to 13 additional cases—all women who attended the burial.13
The Center for Systems Biology at Harvard University and the Broad Institute of Massachusetts Institute of Technology generated 99 Ebola virus genome sequences from 78 patients with confirmed disease, representing more than 70% of the patients diagnosed with the disease in Sierra Leone from May to mid-June 2014. They found genetic similarity across the sequenced 2014 samples, suggesting a single transmission from the natural reservoir, followed by human-to-human transmission during the outbreak. Continued human-reservoir exposure is unlikely to have contributed to the growth of this epidemic.14
As of October 14, 2014, there were 8,914 suspected and confirmed cases of Ebola virus infection, and 4,477 deaths.15
But how did Zaire Ebola virus make the 2,000-mile trek from Central Africa to Guinea in West Africa? There are two possibilities: it has always been present in the region but we just never noticed, or it was recently introduced. Bayesian phylogenetic analyses and sequence divergence studies suggest the virus has been present in bat populations in Guinea without previously infecting humans.
Why Guinea and why Guéckédou? Guinea is one of the poorest countries in the world, ranking 178th of 187 countries on the Human Development Index of the United Nations Development Programme, just behind Liberia (174th) and Sierra Leone (177th). In Guinea, the life expectancy is 56 years and the gross national income per capita is $440. The region has been systematically plundered and the forest decimated by clear-cut logging, leaving the Guinea Forest Region largely deforested, resulting in increased contact between humans and the small animals that serve as the source of infection.1
LIMITED CAPACITY, EVEN IN THE UNITED STATES
A few hospitals in the United States have dedicated units to handle serious infectious diseases such as Ebola: Emory University Hospital; Nebraska Medicine in Omaha; Providence St. Patrick Hospital in Missoula, MT; and the National Institutes of Health in Bethesda, MD. However, in total they have only 19 beds.
QUESTIONS, ANSWERS—AND MORE QUESTIONS
(The following is from a question-and-answer discussion that followed Dr. Brizendine’s Grand Rounds presentation.)
Q: Are there any differences between survivors and those who die of the disease? A: We do not know. Patient survival depends on early recognition and supportive care. There are disparities in the care of patients. Schieffelin et al16 analyzed the characteristics of patients who died or who survived in Sierra Leone and found that the mortality rate was higher in older patients and those with a higher viral load on presentation.
Q: Does the virus block production or release of interferon early in infection? A: Yes, it has been shown17 that Ebola virus protein VP24 inhibits signaling downstream of both interferon alpha/beta and interferon gamma by indirectly impairing the transport of a transcription factor termed STAT1. VP24 is also able to bind STAT1 directly. The resulting suppression of host interferon very early on in the incubation phase is key to the virulence of the virus.
Q: Does infection with one of the viral species confer immunity from other species? A: No, there is no cross-immunity.
Q: How soon do patients test positive? A: About 5 days after exposure, when they develop a fever. At this time patients are highly viremic, which PCR can detect.
Q: Before the virus is detectable in the blood, where is it? A: The liver, endothelial cells, antigen-presenting cells, and adrenal glands.
Q: Do we really need to quarantine ill patients and health care workers returning from Africa, per CDC recommendations? A: We don’t know everything, and some people do make bad decisions, such as traveling while symptomatic. I support a period of observation, although confinement is not reasonable, as it may pose a disincentive to cooperation.
Q: What is the role of giving plasma from survivors? A: Dr. Kent Brantly (see American citizens infected with Ebola) received the blood of a 14-year-old who survived. We don’t know. It is not proved. It did not result in improvement in animal models.
Q: Is the bleeding caused by a mechanism similar to that in enterohemorrhagic Escherichia coli infection? A: No. That is a bacterial toxin, whereas this is more like disseminated intravascular coagulation, with an intrinsic pathway anticoagulation cascade.
Q: How long does the virus remain viable outside the body? A: In one study,18 Ebola virus could not be recovered from experimentally contaminated surfaces (plastic, metal or glass) at room temperature. In another in which it was dried onto a surface,19 Ebola virus survived in the dark for several hours between 20 and 25°C. When dried in tissue culture media onto glass and stored at 4°C, it has survived for over 50 days.
Q: How long does the virus remain in breast milk? A: We know it has been detected 15 days after disease onset and think possibly as late as 28 days from symptom onset.3
Q: How are people actually infected? A: I believe people get the virus on their hands and then touch their face, eyes, or mouth. If you are wearing personal protective equipment, it must occur while doffing the equipment.
Q: Could we increase the sensitivity of the test so that we could detect the virus before the onset of symptoms? A: In theory it may be possible. The virus is somewhere in the body during the incubation period. Perhaps we could sample the right compartment in an enriched mononuclear cell line.
Q: When can patients who recover resume their normal activities? A: After their viral load returns to 0, I would still advise abstaining from unprotected sex and from breastfeeding for a few months. but as for other activities, no special precautions are needed.
Q: Does the virus appear to be mutating at a high rate? A: Looking back to 2004, mutations are occurring, but there is no sign that any of these mutations has contributed to the size of the outbreak by changing the characteristics of the Ebola virus. Can it become aerosolized? It has been suggested that the virus that caused the outbreak separated from those that caused past Ebola outbreaks but does not seem to be affecting the spread or efficacy of experimental drugs and vaccines. So, even though it is an RNA virus and mutations are occurring, no serious changes have emerged.14
BACK TO OUR PATIENT
The differential diagnosis for the patient described at the beginning of this paper includes travelers’ diarrhea, malaria, typhoid fever, yellow fever, meningococcal disease … and Ebola virus disease, although this is much less likely in view of the epidemiology and incubation period of this disease. When his stool was tested by enzyme immunoassay and culture, it was found to be positive for Campylobacter. He recovered with oral rehydration.
- Bausch DG, Schwarz L. Outbreak of ebola virus disease in Guinea: where ecology meets economy. PLoS Negl Trop Dis 2014; 8:e3056.
- Roddy P, Thomas SL, Jeffs B, et al. Factors associated with Marburg hemorrhagic fever: analysis of patient data from Uige, Angola. J Infect Dis 2010; 201:1909–1918.
- Bausch DG, Towner JS, Dowell SF, et al. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis 2007; 196(suppl 2):S142–S147.
- WHO Ebola Response Team. Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N Engl J Med 2014; 371:1481–1495.
- Ribner BS. Treating patients with Ebola virus infections in the US: lessons learned. Presented at IDWeek, October 8, 2014. Philadelphia PA.
- Feldman H, Jones SM, Daddario-DiCaprio KM, et al. Effective post-exposure treatment of Ebola infection. PLoS Pathog 2007; 3:e2.
- Stanley DA, Honko AN, Asiedu C, et al. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nat Med 2014; 20:1126–1129.
- Oswald WB, Geisbert TW, Davis KJ, et al. Neutralizing antibody fails to impact the course of Ebola virus infection in monkeys. PLos Pathog 2007; 3:e9.
- Qui X, Wong G, Fernando L, et al. mAbs and Ad-vectored IFN-a therapy rescue Ebola-infected nonhuman primates when administered after the detection of viremia and symptoms. Sci Transl Med 2013; 5:207ra143.
- Qui X, Wong G, Audet J, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 2014; 514:47–53.
- Reid SP, Shurtleff AC, Costantino JA, et al. HSPA5 is an essential host factor for Ebola virus infection. Antiviral Res 2014; 109:171–174.
- Oestereich L, Lüdtke A, Wurr S, Rieger T, Muñoz-Fontela C, Günther S. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antiviral Res 2014; 105:17–21.
- Baize S, Pannetier D, Oestereich L, et al. Emergence of Zaire Ebola virus dsease in Guinea. N Engl J Med 2014; 371:1418–1425.
- Gire SK, Goba A, Andersen KG, et al. Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science 2014; 345:1369–1372.
- Chamary JV. 4000 deaths and counting: the Ebola epidemic in 4 charts. Forbes. http://www.forbes.com/sites/jvchamary/2014/10/13/ebola-trends. Accessed November 5, 2014.
- Schieffelin JS, Shaffer JG, Goba A, et al, for the KGH Lassa Fever Program, the Viral Hemorrhagic Fever Consortium, and the WHO Clinical Response Team. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med 2014 Oct 29 [Epub ahead of print]. DOI: 10.1056/NEJMoa1411680.
- Zhang AP, Bornholdt ZA, Liu T, et al. The ebola virus interferon antagonist VP24 directly binds STAT1 and has a novel, pyramidal fold. PLoS Pathog 2012; 8:e1002550.
- Piercy TJ, Smither SJ, Steward JA, Eastaugh L, Lever MS. The survival of filoviruses in liquids, on solid substrates and in a dynamic aerosol. J Appl Microbiol 2010; 109:1531–1539.
- Sagripanti JL, Rom AM, Holland LE. Persistence in darkness of virulent alphaviruses, Ebola virus, and Lassa virus deposited on solid surfaces. Arch Virol 2010; 155:2035–2039.
- Bausch DG, Schwarz L. Outbreak of ebola virus disease in Guinea: where ecology meets economy. PLoS Negl Trop Dis 2014; 8:e3056.
- Roddy P, Thomas SL, Jeffs B, et al. Factors associated with Marburg hemorrhagic fever: analysis of patient data from Uige, Angola. J Infect Dis 2010; 201:1909–1918.
- Bausch DG, Towner JS, Dowell SF, et al. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis 2007; 196(suppl 2):S142–S147.
- WHO Ebola Response Team. Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N Engl J Med 2014; 371:1481–1495.
- Ribner BS. Treating patients with Ebola virus infections in the US: lessons learned. Presented at IDWeek, October 8, 2014. Philadelphia PA.
- Feldman H, Jones SM, Daddario-DiCaprio KM, et al. Effective post-exposure treatment of Ebola infection. PLoS Pathog 2007; 3:e2.
- Stanley DA, Honko AN, Asiedu C, et al. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nat Med 2014; 20:1126–1129.
- Oswald WB, Geisbert TW, Davis KJ, et al. Neutralizing antibody fails to impact the course of Ebola virus infection in monkeys. PLos Pathog 2007; 3:e9.
- Qui X, Wong G, Fernando L, et al. mAbs and Ad-vectored IFN-a therapy rescue Ebola-infected nonhuman primates when administered after the detection of viremia and symptoms. Sci Transl Med 2013; 5:207ra143.
- Qui X, Wong G, Audet J, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 2014; 514:47–53.
- Reid SP, Shurtleff AC, Costantino JA, et al. HSPA5 is an essential host factor for Ebola virus infection. Antiviral Res 2014; 109:171–174.
- Oestereich L, Lüdtke A, Wurr S, Rieger T, Muñoz-Fontela C, Günther S. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antiviral Res 2014; 105:17–21.
- Baize S, Pannetier D, Oestereich L, et al. Emergence of Zaire Ebola virus dsease in Guinea. N Engl J Med 2014; 371:1418–1425.
- Gire SK, Goba A, Andersen KG, et al. Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science 2014; 345:1369–1372.
- Chamary JV. 4000 deaths and counting: the Ebola epidemic in 4 charts. Forbes. http://www.forbes.com/sites/jvchamary/2014/10/13/ebola-trends. Accessed November 5, 2014.
- Schieffelin JS, Shaffer JG, Goba A, et al, for the KGH Lassa Fever Program, the Viral Hemorrhagic Fever Consortium, and the WHO Clinical Response Team. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med 2014 Oct 29 [Epub ahead of print]. DOI: 10.1056/NEJMoa1411680.
- Zhang AP, Bornholdt ZA, Liu T, et al. The ebola virus interferon antagonist VP24 directly binds STAT1 and has a novel, pyramidal fold. PLoS Pathog 2012; 8:e1002550.
- Piercy TJ, Smither SJ, Steward JA, Eastaugh L, Lever MS. The survival of filoviruses in liquids, on solid substrates and in a dynamic aerosol. J Appl Microbiol 2010; 109:1531–1539.
- Sagripanti JL, Rom AM, Holland LE. Persistence in darkness of virulent alphaviruses, Ebola virus, and Lassa virus deposited on solid surfaces. Arch Virol 2010; 155:2035–2039.
KEY POINTS
- Ebola virus is spread by contact with body fluids, with no evidence to date that it is airborne.
- Ebola virus is likely maintained in a reservoir of small animals, possibly bats.
- The incubation period is about 5 to 7 days, during which the patient is not infectious.
- Symptoms begin abruptly, with fever, chills, and general malaise, which in some patients leads to weakness, severe headache, myalgia, nausea, vomiting, diarrhea, and abdominal pain.
- Once the disease is symptomatic, patients have high levels of virus in the blood and other body fluids and are therefore infectious.
- Survivors show improvement in the second week of illness, during which viremia resolves and virus-specific antibodies appear.
Acute respiratory distress syndrome: Implications of recent studies
Continued progress in understanding the pathophysiology of acute respiratory distress syndrome (ARDS) is translating into changes in the way we diagnose and manage it. Over the past 20 years, low tidal volume,1 positive end-expiratory pressure (PEEP),2 and fluid restriction3 have become the standard of care. A multidisciplinary approach, including targeted use of sedatives, early mobilization, and protocols for weaning from the ventilator, has also brought about substantial changes in ARDS management and its outcomes.4–6
In this article, we review the most relevant articles about ARDS in the last 5 years. We include the new definition of ARDS and studies of ventilatory and nonventilatory therapies that have implications in managing patients with ARDS.
A STANDARDIZED APPROACH
ARDS is characterized by damage to the alveolar architecture, severe hypoxemia, and bilateral parenchymal opacities.
The working definition of ARDS developed in 1994 by the American-European Consensus Conference (AECC) was the basis for enrollment in most of the landmark trials and observational studies over the past 20 years.7,8 However, it was limited in its reliability and validity.
An updated definition
In 2011, the ARDS Definition Task Force, using a novel consensus process, updated the ARDS definition,9 focusing on its feasibility, reliability, and validity in predicting response to therapies and outcomes in ARDS. This new “Berlin” definition is not substantially different from the old, but defines the criteria more specifically:
- Bilateral opacities, unexplained by nodules, atelectasis, or effusion, on chest radiography or computed tomography
- New or worsening respiratory symptoms, or a clinical insult associated with ARDS within 7 days of diagnosis
- Objective assessment of cardiac function (eg, with echocardiography) to exclude cardiogenic pulmonary edema
- Hypoxemia, with a partial pressure of arterial oxygen divided by the percentage of inspired oxygen (PaO2/FiO2 ratio) of 300 mm Hg or less despite noninvasive or invasive mechanical ventilation with PEEP or continuous positive airway pressure (CPAP) of at least 5 cm H2O.
In addition, the new definition classifies the severity of disease on the basis of the degree of hypoxemia, ie, the PaO2/FiO2 ratio:
- Mild: PaO2/FiO2 ratio > 200 and ≤ 300 mm Hg
- Moderate: PaO2/FiO2 ratio > 100 and ≤ 200 mm Hg
- Severe: PaO2/FiO2 ratio ≤ 100 mm Hg.
The term “acute lung injury” has been eliminated, as has the previous criterion of a pulmonary artery wedge pressure of 18 mm Hg or less.
The panel also evaluated four ancillary variables for predicting outcomes in severe ARDS:
- Compliance of the respiratory system less than or equal to 40 mL/cm H2O
- Radiographic severity (involvement of three or four quadrants on chest radiography)
- PEEP of 10 cm H2O or greater
- Corrected expired volume 10 L/min or greater.
The task force evaluated the reliability and validity of this definition in a meta-analysis of 4,400 patients previously enrolled in randomized controlled trials or observational studies.
Findings. The Berlin definition predicted the risk of death better than the AECC definition. The mortality rate increased with the severity of ARDS, from 27% with mild disease to 32% with moderate disease to 45% with severe disease. The four ancillary variables did not contribute to the predictive validity of severe ARDS for mortality and were removed from the definition.
Thille et al10 retrospectively reviewed autopsy findings from 712 patients and found that the new definition identified a homogeneous group who had severe ARDS.10
Conclusions. The new definition may overcome some of the limitations of the old one, but it needs to be validated in clinical practice, especially its ability to predict death.
VENTILATORY SUPPORT
Prompt recognition, lung-protective ventilation, and a conservative fluid strategy remain the cornerstones of ARDS management. However, other strategies are being tested.
Prone-position ventilation in severe ARDS: The right therapy in a specific population
Prone-position ventilation was first described almost 30 years ago, but it has been used inconsistently in clinical practice.
Physiologic and observational studies indicated that prone positioning might improve survival in patients with ARDS, but several randomized trials failed to demonstrate any positive effect on outcomes.11,12 Some trials also reported a higher rate of complications with this intervention.13 However, meta-analyses suggested that prone-position ventilation might have a beneficial effect in patients with severe ARDS (defined as a PaO2/FiO2 ratio ≤ 100 mm Hg).14
In view of these findings, investigators conducted a trial of prone-position ventilation exclusively in patients with severe ARDS.
The PROSEVA study
The Proning Severe ARDS Patients (PROSEVA) study was a randomized controlled trial designed to determine whether prone-position ventilation, applied early, would improve outcomes in patients with severe ARDS.15
In PROSEVA, 466 patients with severe ARDS (defined as a PaO2/FiO2 ratio < 150 mm Hg, FiO2 ≥ 60%, and PEEP ≥ 5 cm H2O) underwent either at least 16 hours of prone positioning or were left in the supine position after 12 to 24 hours of initial conventional mechanical ventilation. The patients were recruited from centers in France and Spain where prone-position ventilation had been used in daily practice for more than 5 years.
The primary outcome studied was the rate of death at 28 days. The secondary end points were the death rate at day 90, rates of successful extubation, the length of stay in the intensive care unit, and complications.
Findings. At study entry, the patients in the supine group were sicker, more of them required a vasopressor, and fewer of them were receiving neuromuscular blocking agents than those in the prone group. These baseline differences may have influenced the outcomes; the unadjusted 28-day mortality rate was 16.0% in the prone group compared with 32.8% in the supine group (P < .001). However, the hazard ratio for death with prone positioning was 0.39 (95% confidence interval [CI] 0.25–0.63) even after adjusting for severity and the use of vasopressors and neuromuscular blocking agents. Prone-position ventilation was not associated with a higher incidence of complications, and the rate of successful extubation was higher.
Conclusions. In patients with severe ARDS, early use of prolonged prone positioning significantly decreased the 28-day and 90-day mortality rates. This trial has made prone positioning one of the strategies in managing patients with early severe ARDS. To minimize complications such as pressure ulcers and line or tube dislodgement, personnel caring for these patients must follow a protocol and undergo specific training.
These results were corroborated by a meta-analysis by Beitler et al16 that found a significant decrease in mortality rate with prone-position ventilation even in older studies when lung-protective ventilation strategies were separated from high-tidal-volume ventilation.
High-frequency oscillatory ventilation: No benefit in two trials
Observational data and experimental studies suggested that high-frequency oscillatory ventilation (HFOV) is superior to conventional mechanical ventilation in ARDS patients.17,18 However, outdated and cumbersome equipment, lack of protocols, and a lack of high-quality evidence led to limited and inconsistent use of HFOV, mainly as a rescue therapy in ARDS.19
Over the last few years, HFOV has been gaining acceptance, especially earlier in the course of ARDS.20 After preliminary clinical trials reported promising results, two trials conducted in Canada and the United Kingdom compared HFOV vs conventional mechanical ventilation in patients with ARDS.
The OSCAR study
The Oscillation in ARDS (OSCAR) study21 was a “pragmatic” trial22 (ie, it had minimal exclusion criteria) of the safety and effectiveness of HFOV as a primary ventilatory strategy for ARDS. It included 795 patients randomized to receive conventional ventilation (n = 397) or HFOV (n = 398). Research centers followed detailed algorithms for HFOV management and adopted their usual practice for conventional ventilation. Medical care was given according to the clinician’s judgment.
The primary outcome studied was survival at 30 days. The secondary outcomes were all-cause mortality in the intensive care unit and the hospital, duration of mechanical ventilation, and use of antimicrobial, sedative, vasoactive, and neuromuscular-blocking drugs.
Findings. The patient baseline characteristics were similar in both groups.
There was no significant difference in intensive care unit mortality rates, hospital mortality rates, or mortality rates at 30 days (41.7% in the HFOV group vs 41.1% in the conventional ventilation group; P = .85, 95% CI 6.1–7.5) even after adjustments for center or severity of illness.
The duration of mechanical ventilation was similar in both groups (14.9 ± 13.3 days in the HFOV group vs 14.1 ± 13.4 days in the conventional ventilation group, P = .41). However, sedatives and neuromuscular-blocking drugs were used more often and longer in the HFOV group than in the conventional ventilation group. There was no difference in the use of vasoactive or antimicrobial medications.
Conclusions. This multicenter randomized control trial did not demonstrate any benefit from using HFOV for routine management of ARDS. Its pragmatic design made it less likely to reach a firm conclusion,22 but it at least made a case against routinely using HFOV in patients with ARDS.
The OSCILLATE study
The Oscillation for Acute Respiratory Distress Syndrome Treated Early (OSCILLATE) study23 assessed the safety and efficacy of HFOV as a treatment for early-onset moderate-to-severe ARDS.
The inclusion criteria were similar to those in the OSCAR trial except that pulmonary symptoms had to be present less than 2 weeks and ARDS assessment was done under standard ventilator settings. As this was an efficacy trial, it had more exclusion criteria than the OSCAR trial. A total of 548 patients were randomized to receive conventional ventilation (n = 273) or HFOV (n = 275). The baseline characteristics were similar between groups.
Conventional ventilation was given according to a protocol used in an earlier trial2 and included recruitment maneuvers. HFOV was given in centers that had experience in this treatment, and there were protocols for ventilation management, hemodynamic optimization, and weaning. All other care was left to the clinician’s choice.
The primary outcome studied was in-hospital mortality. The investigators also evaluated whether there were interactions between the treatment and baseline severity of lung injury and center experience with HFOV.
Findings. The trial was stopped after an interim analysis found that HFOV might be harmful, although the statistical threshold for stopping was not reached. The in-hospital mortality rate was 47% in the HFOV group and 35% in the control group (relative risk of death with HFOV 1.33, 95% CI 1.09–1.64, P = .005). HFOV was worse than conventional ventilation regardless of the severity of disease or center experience. The HFOV group had higher mean airway pressures but similar FiO2 compared with the conventional ventilation group.
The HFOV group received significantly more vasopressors, sedatives, and neuromuscular blockers. This group’s fluid balance was higher as well, but not significantly so. Refractory hypoxemia (defined as PaO2 < 60 mm Hg for 1 hour with an FiO2 of 1.0 and neuromuscular blockade) was more frequent in the conventional ventilation group, but the number of deaths in the subgroup with refractory hypoxemia was similar with either treatment.
Conclusions. This multicenter randomized controlled trial demonstrated that HFOV was harmful when used routinely to manage ARDS. The trial’s protocol was based on the results of a pilot study carried out by the same investigators, which provided the best evidence available regarding the safety of HFOV at that time.
The results of the OSCAR and OSCILLATE trials have quelled enthusiasm for early, routine use of HFOV in ARDS. Although there are concerns that the protocol (ie, the way HFOV was implemented) rather than HFOV itself may have led to worse outcomes, there is no signal to support its routine use. We need further studies to define if it remains a viable rescue therapy.
Extracorporeal membrane oxygenation: Is it a viable option in severe ARDS?
Extracorporeal membrane oxygenation (ECMO) uses cardiopulmonary bypass technology to provide gas exchange. In patients with severe hypoxemia, ECMO can ensure adequate oxygenation and ventilation while ensuring the optimization of lung-protective ventilation. But ECMO was never as successful in adults with ARDS as it was in children and neonates.24
The first two trials of ECMO in ARDS24,25 reported equal or worse survival rates compared with conventional ventilation, and the overall mortality rate in these studies was staggeringly high. However, these studies were carried out before the era of lung-protective ventilation and at a time when ECMO technology was relatively primitive.
With new technology such as venovenous circuits and smaller cannulas, ECMO has gained more acceptance. It was used in patients with severe or refractory hypoxemia associated with ARDS during the H1N1 pandemic.26,27
The CESAR trial
The Conventional Ventilatory Support Versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure (CESAR) trial28 assessed the safety, clinical efficacy, and cost-effectiveness of ECMO in managing severe ARDS. It compared best standard practice vs a protocol that included ECMO. The trial was conducted from 2001 to 2006.
Patients with severe ARDS, as defined by a Murray score29 greater than 3 or uncompensated hypercapnea, were prospectively randomized and recruited from an ECMO center and 148 tertiary intensive care units and referral hospitals in England. This was a pragmatic trial, with minimal exclusion criteria (essentially, mechanical ventilation with high pressures and high FiO2 for more than 7 days, intracranial bleeding, or contraindication to heparinization).
A total of 180 patients were randomized in a one-to-one ratio to receive ECMO or conventional management. The ventilator management in the conventional treatment group was not done according to a protocol but in general was low-volume and low-pressure. All patients randomized to ECMO were transferred to the ECMO center and treated according to a standardized ventilation protocol. After 12 hours, if predefined goals were not reached, venovenous ECMO was started. Patients assigned to conventional management could not cross over to ECMO.
The primary outcomes were death or severe disability at 6 months after randomization, and cost-effectiveness. The secondary outcomes were hospital resource use (eg, rescue techniques, length of stay, duration of ECMO) and health status after 6 months.
Findings. The groups were similar at baseline. Sixty-eight (75%) of the 90 patients randomized to receive ECMO actually received it. Of the 22 patients who did not receive ECMO, 16 (18% of the 90) improved on conventional therapy, 5 (6%) died during or before transfer, and 1 could not receive heparin.
Two patients had severe complications in the ECMO group: one had an arterial puncture, and one had an oxygen delivery failure during transport. In each case, these events contributed to the death of the patient.
More patients in the ECMO group received lung-protective ventilation, 84 (93%) vs 63 (70%).
The primary outcome, ie, death or severe disability at 6 months, occurred in 33 (37%) of the 90 patients in the ECMO group and in 46 (53%) of the patients in the conventional management group (relative risk 0.69, 95% CI 0.05–0.97, P = .03). More patients in the ECMO group survived, but the difference was not statistically significant (relative risk of death 0.73, 95% CI 0.52–1.03, P = .07). The most common cause of death in the ECMO group was multiorgan failure (42%), whereas in the conventional management group, the most common cause of death was respiratory failure (60%).
Length of stay in the hospital and in the critical care unit and health care costs were double for patients in the ECMO group. There was no difference in quality-of-life markers at 6 months in the survivors.
Conclusions. This pragmatic trial demonstrated that a protocol that includes ECMO could improve survival rates in ARDS.
Of note, the ECMO group got care in regional centers that used protocols. Therefore, in interpreting the results of this trial, we have to consider that being in a center with protocol-specified care for ARDS could drive some of the difference in mortality rates.
Regardless, this trial demonstrated that ECMO is feasible and led to better outcomes than expected. The findings were encouraging, and spurred the use of ECMO in severe ARDS during the 2009 H1N1 pandemic. Two propensity-matched studies and a number of case series reported a survival benefit associated with the use of ECMO in patients with severe ARDS.27,30
A recent meta-analysis also reported that ECMO might lower the mortality rate in ARDS; however, the patients in the H1N1 pandemic were younger and usually had isolated respiratory failure.31
The success of ECMO has opened new possibilities in the management of ARDS. As the technology improves and our experience increases, ECMO will likely gain more acceptance as a treatment for severe ARDS.
Airway pressure release ventilation
The use of airway pressure release ventilation and other ventilator modalities in ARDS is not supported by current evidence, though results of clinical trials may influence our practice in the future.
PHARMACOTHERAPY IN ARDS
The pathogenesis of ARDS includes damage to the alveolar-capillary membrane, with leakage of protein-rich edema fluid into alveoli. This damage is propagated by a complex inflammatory response including but not limited to neutrophil activation, free-radical formation, dysregulation of the coagulation system, and extensive release of inflammatory mediators.32,33 As a consequence, there are multiple potential targets for pharmacologic therapy in ARDS.
A variety of drugs, including corticosteroids, anti-inflammatory agents, immune-modulating agents, pulmonary vasodilators, antioxidants, and surfactants, have been studied in patients with ARDS.34 But effective pharmacotherapy for ARDS remains extremely limited.
Neuromuscular blockade in early severe ARDS
Mechanical ventilation can result in injurious stretching of the lung parenchyma, either from alveolar overdistention (volutrauma) or from continual recruitment and derecruitment of unstable lung units during the ventilator cycle (atelectrauma).35 Ventilator-induced lung injury can be exacerbated by asynchronous breathing.
In theory, neuromuscular blockers could minimize patient-ventilator asynchrony and provide much better control of tidal volume and pressure in patients with ARDS. This may result in less volutrauma and atelectrauma associated with asynchronous breathing. Data also suggest that cisatracurium (Nimbex), a neuromuscular blocking agent, may have a direct effect on the amount of inflammation in lungs with ARDS.36
The ACURASYS study
The ARDS et Curarisation Systématique (ACURASYS) study37 was a randomized trial in 340 patients undergoing mechanical ventilation for severe ARDS to evaluate the impact of neuromuscular blockade within the first 48 hours in this population.
The primary outcome was the mortality rate before hospital discharge or within 90 days of study entry. Secondary outcomes included the 28-day mortality rate, the rate of intensive care unit-acquired paresis, and the number of ventilator-free days. To be included, patients had to have been mechanically ventilated for less than 48 hours and to meet the AECC criteria for severe ARDS, with a PaO2/FiO2 ratio less than 150 mm Hg.
The intervention group received a continuous infusion of cisatracurium for 48 hours, while the control patients received placebo. Muscle strength was evaluated by clinical scoring of strength in different muscle groups.
Findings. The study groups were similar at baseline.
The crude 90-day mortality rate was lower in the cisatracurium group (31.6% vs 40.7%, P = .08). Regression analysis showed an improved 90-day survival rate with the use of this neuromuscular blocker after adjustment for severity of illness and the severity of ARDS (based on degree of hypoxemia and plateau pressures) (hazard ratio for death at 90 days 0.68; 95% CI 0.48–0.98; P = .04). The rate of paresis acquired in the intensive care unit did not differ significantly between the two groups.
Conclusion. In patients with severe ARDS, giving a neuromuscular blocking agent early improved the survival rate and increased the time off the ventilator without increasing muscle weakness.
These data are in line with similar findings from two other studies published by the same group.38,39 A meta-analysis of 432 patients showed that the use of neuromuscular blockade in early severe ARDS is associated with a statistically significant effect on early mortality (relative risk 0.66, 95% CI 0.50–0.87).40 The pooled analysis of these trials did not show any statistically significant critical-illness polyneuropathy.
These results need to be interpreted carefully, as we have inadequate data to see if they generalize to different intensive care units, and the evaluation and categorization of critical-illness polyneuropathy remains to be defined.
Cisatracurium is a promising treatment for moderate to severe ARDS and merits investigation in a large confirmatory randomized controlled trial.
Other pharmacologic agents
A number of other drugs have been studied in ARDS patients, including both inhaled and intravenous beta agonists,41,42 statins,43 and nutritional supplements.44 But as with other drugs previously studied in ARDS such as corticosteroids, N-acetylcysteine, and surfactant,34 these agents showed no effect on outcomes. In fact, a recent trial of intravenous salbutamol in ARDS patients was stopped after an interim analysis because of a higher incidence of arrhythmias and lactic acidosis with this agent.42
These findings reaffirm that pharmacologic therapy needs to be carefully considered, and potential harms associated with these therapies need to be addressed before they are introduced in the care of critically ill patients.
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. Acute Respiratory Distress Syndrome Network. N Engl J Med 2000; 342:1301–1308.
- Meade MO, Cook DJ, Guyatt GH, et al; Lung Open Ventilation Study Investigators. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008; 299:637–645.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575.
- Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008; 371:126–134.
- Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009; 373:1874–1882.
- Ely EW, Baker AM, Dunagan DP, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med 1996; 335:1864–1869.
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994; 149:818–824.
- Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med 2012; 38:1573–1582.
- ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307:2526–2533.
- Thille AW, Esteban A, Fernández-Segoviano P, et al. Comparison of the Berlin definition for acute respiratory distress syndrome with autopsy. Am J Respir Crit Care Med 2013; 187:761–767.
- Gattinoni L, Tognoni G, Pesenti A, et al; Prone-Supine Study Group. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med 2001; 345:568–573.
- Taccone P, Pesenti A, Latini R, et al; Prone-Supine II Study Group. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA 2009; 302:1977–1984.
- Mancebo J, Fernández R, Blanch L, et al. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med 2006; 173:1233–1239.
- Sud S, Friedrich JO, Taccone P, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med 2010; 36:585–599.
- Guérin C, Reignier J, Richard JC, et al; PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013; 368:2159–2168.
- Beitler JR, Shaefi S, Montesi SB, et al. Prone positioning reduces mortality from acute respiratory distress syndrome in the low tidal volume era: a meta-analysis. Intensive Care Med 2014; 40:332–341.
- Chan KP, Stewart TE, Mehta S. High-frequency oscillatory ventilation for adult patients with ARDS. Chest 2007; 131:1907–1916.
- Fessler HE, Hager DN, Brower RG. Feasibility of very high-frequency ventilation in adults with acute respiratory distress syndrome. Crit Care Med 2008; 36:1043–1048.
- Mehta S, Granton J, MacDonald RJ, et al. High-frequency oscillatory ventilation in adults: the Toronto experience. Chest 2004; 126:518–527.
- Ferguson ND, Chiche JD, Kacmarek RM, et al. Combining high-frequency oscillatory ventilation and recruitment maneuvers in adults with early acute respiratory distress syndrome: the Treatment with Oscillation and an Open Lung Strategy (TOOLS) Trial pilot study. Crit Care Med 2005; 33:479–486.
- Young D, Lamb SE, Shah S, et al; OSCAR Study Group. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med 2013; 368:806–813.
- Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol 2009; 62:464–475.
- Ferguson ND, Cook DJ, Guyatt GH, et al; OSCILLATE Trial Investigators; Canadian Critical Care Trials Group. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med 2013; 368:795–805.
- Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 1994; 149:295–305.
- Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 1979; 242:2193–2196.
- Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators; Davies A, Jones D, Bailey M, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA 2009; 302:1888–1895.
- Pham T, Combes A, Rozé H, et al; REVA Research Network. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med 2013; 187:276–285.
- Peek GJ, Mugford M, Tiruvoipati R, et al; CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009; 374:1351–1363.
- Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 1988; 138:720–723.
- Noah MA, Peek GJ, Finney SJ, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA 2011; 306:1659–1668.
- Zampieri FG, Mendes PV, Ranzani OT, et al. Extracorporeal membrane oxygenation for severe respiratory failure in adult patients: a systematic review and meta-analysis of current evidence. J Crit Care 2013; 28:998–1005.
- Raghavendran K, Pryhuber GS, Chess PR, Davidson BA, Knight PR, Notter RH. Pharmacotherapy of acute lung injury and acute respiratory distress syndrome. Curr Med Chem 2008; 15:1911–1924.
- Adhikari N, Burns KE, Meade MO. Pharmacologic treatments for acute respiratory distress syndrome and acute lung injury: systematic review and meta-analysis. Treat Respir Med 2004; 3:307–328.
- Adhikari N, Burns KE, Meade MO. Pharmacologic therapies for adults with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev 2004; 4:CD004477.
- Terragni PP, Rosboch GL, Lisi A, Viale AG, Ranieri VM. How respiratory system mechanics may help in minimising ventilator-induced lung injury in ARDS patients. Eur Respir J Suppl 2003; 42:15s–21s.
- Forel JM, Roch A, Papazian L. Paralytics in critical care: not always the bad guy. Curr Opin Crit Care 2009; 15:59–66.
- Papazian L, Forel JM, Gacouin A, et al; ACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010; 363:1107–1116.
- Gainnier M, Roch A, Forel JM, et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med 2004; 32:113–19.
- Forel JM, Roch A, Marin V, et al. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med 2006; 34:2749–2757.
- Alhazzani W, Alshahrani M, Jaeschke R, et al. Neuromuscular blocking agents in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. Crit Care 2013; 17:R43.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Matthay MA, Brower RG, Carson S, et al. Randomized, placebo-controlled clinical trial of an aerosolized beta-2-agonist for treatment of acute lung injury. Am J Respir Crit Care Med 2011; 184:561–568.
- Gao Smith F, Perkins GD, Gates S, et al; BALTI-2 study investigators. Effect of intravenous beta-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet 2012; 379:229–235.
- Craig TR, Duffy MJ, Shyamsundar M, et al. A randomized clinical trial of hydroxymethylglutaryl-coenzyme a reductase inhibition for acute lung injury (The HARP Study). Am J Respir Crit Care Med 2011; 183:620–626.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Rice TW, Wheeler AP, Thompson BT, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA 2012; 307:795–803.
Continued progress in understanding the pathophysiology of acute respiratory distress syndrome (ARDS) is translating into changes in the way we diagnose and manage it. Over the past 20 years, low tidal volume,1 positive end-expiratory pressure (PEEP),2 and fluid restriction3 have become the standard of care. A multidisciplinary approach, including targeted use of sedatives, early mobilization, and protocols for weaning from the ventilator, has also brought about substantial changes in ARDS management and its outcomes.4–6
In this article, we review the most relevant articles about ARDS in the last 5 years. We include the new definition of ARDS and studies of ventilatory and nonventilatory therapies that have implications in managing patients with ARDS.
A STANDARDIZED APPROACH
ARDS is characterized by damage to the alveolar architecture, severe hypoxemia, and bilateral parenchymal opacities.
The working definition of ARDS developed in 1994 by the American-European Consensus Conference (AECC) was the basis for enrollment in most of the landmark trials and observational studies over the past 20 years.7,8 However, it was limited in its reliability and validity.
An updated definition
In 2011, the ARDS Definition Task Force, using a novel consensus process, updated the ARDS definition,9 focusing on its feasibility, reliability, and validity in predicting response to therapies and outcomes in ARDS. This new “Berlin” definition is not substantially different from the old, but defines the criteria more specifically:
- Bilateral opacities, unexplained by nodules, atelectasis, or effusion, on chest radiography or computed tomography
- New or worsening respiratory symptoms, or a clinical insult associated with ARDS within 7 days of diagnosis
- Objective assessment of cardiac function (eg, with echocardiography) to exclude cardiogenic pulmonary edema
- Hypoxemia, with a partial pressure of arterial oxygen divided by the percentage of inspired oxygen (PaO2/FiO2 ratio) of 300 mm Hg or less despite noninvasive or invasive mechanical ventilation with PEEP or continuous positive airway pressure (CPAP) of at least 5 cm H2O.
In addition, the new definition classifies the severity of disease on the basis of the degree of hypoxemia, ie, the PaO2/FiO2 ratio:
- Mild: PaO2/FiO2 ratio > 200 and ≤ 300 mm Hg
- Moderate: PaO2/FiO2 ratio > 100 and ≤ 200 mm Hg
- Severe: PaO2/FiO2 ratio ≤ 100 mm Hg.
The term “acute lung injury” has been eliminated, as has the previous criterion of a pulmonary artery wedge pressure of 18 mm Hg or less.
The panel also evaluated four ancillary variables for predicting outcomes in severe ARDS:
- Compliance of the respiratory system less than or equal to 40 mL/cm H2O
- Radiographic severity (involvement of three or four quadrants on chest radiography)
- PEEP of 10 cm H2O or greater
- Corrected expired volume 10 L/min or greater.
The task force evaluated the reliability and validity of this definition in a meta-analysis of 4,400 patients previously enrolled in randomized controlled trials or observational studies.
Findings. The Berlin definition predicted the risk of death better than the AECC definition. The mortality rate increased with the severity of ARDS, from 27% with mild disease to 32% with moderate disease to 45% with severe disease. The four ancillary variables did not contribute to the predictive validity of severe ARDS for mortality and were removed from the definition.
Thille et al10 retrospectively reviewed autopsy findings from 712 patients and found that the new definition identified a homogeneous group who had severe ARDS.10
Conclusions. The new definition may overcome some of the limitations of the old one, but it needs to be validated in clinical practice, especially its ability to predict death.
VENTILATORY SUPPORT
Prompt recognition, lung-protective ventilation, and a conservative fluid strategy remain the cornerstones of ARDS management. However, other strategies are being tested.
Prone-position ventilation in severe ARDS: The right therapy in a specific population
Prone-position ventilation was first described almost 30 years ago, but it has been used inconsistently in clinical practice.
Physiologic and observational studies indicated that prone positioning might improve survival in patients with ARDS, but several randomized trials failed to demonstrate any positive effect on outcomes.11,12 Some trials also reported a higher rate of complications with this intervention.13 However, meta-analyses suggested that prone-position ventilation might have a beneficial effect in patients with severe ARDS (defined as a PaO2/FiO2 ratio ≤ 100 mm Hg).14
In view of these findings, investigators conducted a trial of prone-position ventilation exclusively in patients with severe ARDS.
The PROSEVA study
The Proning Severe ARDS Patients (PROSEVA) study was a randomized controlled trial designed to determine whether prone-position ventilation, applied early, would improve outcomes in patients with severe ARDS.15
In PROSEVA, 466 patients with severe ARDS (defined as a PaO2/FiO2 ratio < 150 mm Hg, FiO2 ≥ 60%, and PEEP ≥ 5 cm H2O) underwent either at least 16 hours of prone positioning or were left in the supine position after 12 to 24 hours of initial conventional mechanical ventilation. The patients were recruited from centers in France and Spain where prone-position ventilation had been used in daily practice for more than 5 years.
The primary outcome studied was the rate of death at 28 days. The secondary end points were the death rate at day 90, rates of successful extubation, the length of stay in the intensive care unit, and complications.
Findings. At study entry, the patients in the supine group were sicker, more of them required a vasopressor, and fewer of them were receiving neuromuscular blocking agents than those in the prone group. These baseline differences may have influenced the outcomes; the unadjusted 28-day mortality rate was 16.0% in the prone group compared with 32.8% in the supine group (P < .001). However, the hazard ratio for death with prone positioning was 0.39 (95% confidence interval [CI] 0.25–0.63) even after adjusting for severity and the use of vasopressors and neuromuscular blocking agents. Prone-position ventilation was not associated with a higher incidence of complications, and the rate of successful extubation was higher.
Conclusions. In patients with severe ARDS, early use of prolonged prone positioning significantly decreased the 28-day and 90-day mortality rates. This trial has made prone positioning one of the strategies in managing patients with early severe ARDS. To minimize complications such as pressure ulcers and line or tube dislodgement, personnel caring for these patients must follow a protocol and undergo specific training.
These results were corroborated by a meta-analysis by Beitler et al16 that found a significant decrease in mortality rate with prone-position ventilation even in older studies when lung-protective ventilation strategies were separated from high-tidal-volume ventilation.
High-frequency oscillatory ventilation: No benefit in two trials
Observational data and experimental studies suggested that high-frequency oscillatory ventilation (HFOV) is superior to conventional mechanical ventilation in ARDS patients.17,18 However, outdated and cumbersome equipment, lack of protocols, and a lack of high-quality evidence led to limited and inconsistent use of HFOV, mainly as a rescue therapy in ARDS.19
Over the last few years, HFOV has been gaining acceptance, especially earlier in the course of ARDS.20 After preliminary clinical trials reported promising results, two trials conducted in Canada and the United Kingdom compared HFOV vs conventional mechanical ventilation in patients with ARDS.
The OSCAR study
The Oscillation in ARDS (OSCAR) study21 was a “pragmatic” trial22 (ie, it had minimal exclusion criteria) of the safety and effectiveness of HFOV as a primary ventilatory strategy for ARDS. It included 795 patients randomized to receive conventional ventilation (n = 397) or HFOV (n = 398). Research centers followed detailed algorithms for HFOV management and adopted their usual practice for conventional ventilation. Medical care was given according to the clinician’s judgment.
The primary outcome studied was survival at 30 days. The secondary outcomes were all-cause mortality in the intensive care unit and the hospital, duration of mechanical ventilation, and use of antimicrobial, sedative, vasoactive, and neuromuscular-blocking drugs.
Findings. The patient baseline characteristics were similar in both groups.
There was no significant difference in intensive care unit mortality rates, hospital mortality rates, or mortality rates at 30 days (41.7% in the HFOV group vs 41.1% in the conventional ventilation group; P = .85, 95% CI 6.1–7.5) even after adjustments for center or severity of illness.
The duration of mechanical ventilation was similar in both groups (14.9 ± 13.3 days in the HFOV group vs 14.1 ± 13.4 days in the conventional ventilation group, P = .41). However, sedatives and neuromuscular-blocking drugs were used more often and longer in the HFOV group than in the conventional ventilation group. There was no difference in the use of vasoactive or antimicrobial medications.
Conclusions. This multicenter randomized control trial did not demonstrate any benefit from using HFOV for routine management of ARDS. Its pragmatic design made it less likely to reach a firm conclusion,22 but it at least made a case against routinely using HFOV in patients with ARDS.
The OSCILLATE study
The Oscillation for Acute Respiratory Distress Syndrome Treated Early (OSCILLATE) study23 assessed the safety and efficacy of HFOV as a treatment for early-onset moderate-to-severe ARDS.
The inclusion criteria were similar to those in the OSCAR trial except that pulmonary symptoms had to be present less than 2 weeks and ARDS assessment was done under standard ventilator settings. As this was an efficacy trial, it had more exclusion criteria than the OSCAR trial. A total of 548 patients were randomized to receive conventional ventilation (n = 273) or HFOV (n = 275). The baseline characteristics were similar between groups.
Conventional ventilation was given according to a protocol used in an earlier trial2 and included recruitment maneuvers. HFOV was given in centers that had experience in this treatment, and there were protocols for ventilation management, hemodynamic optimization, and weaning. All other care was left to the clinician’s choice.
The primary outcome studied was in-hospital mortality. The investigators also evaluated whether there were interactions between the treatment and baseline severity of lung injury and center experience with HFOV.
Findings. The trial was stopped after an interim analysis found that HFOV might be harmful, although the statistical threshold for stopping was not reached. The in-hospital mortality rate was 47% in the HFOV group and 35% in the control group (relative risk of death with HFOV 1.33, 95% CI 1.09–1.64, P = .005). HFOV was worse than conventional ventilation regardless of the severity of disease or center experience. The HFOV group had higher mean airway pressures but similar FiO2 compared with the conventional ventilation group.
The HFOV group received significantly more vasopressors, sedatives, and neuromuscular blockers. This group’s fluid balance was higher as well, but not significantly so. Refractory hypoxemia (defined as PaO2 < 60 mm Hg for 1 hour with an FiO2 of 1.0 and neuromuscular blockade) was more frequent in the conventional ventilation group, but the number of deaths in the subgroup with refractory hypoxemia was similar with either treatment.
Conclusions. This multicenter randomized controlled trial demonstrated that HFOV was harmful when used routinely to manage ARDS. The trial’s protocol was based on the results of a pilot study carried out by the same investigators, which provided the best evidence available regarding the safety of HFOV at that time.
The results of the OSCAR and OSCILLATE trials have quelled enthusiasm for early, routine use of HFOV in ARDS. Although there are concerns that the protocol (ie, the way HFOV was implemented) rather than HFOV itself may have led to worse outcomes, there is no signal to support its routine use. We need further studies to define if it remains a viable rescue therapy.
Extracorporeal membrane oxygenation: Is it a viable option in severe ARDS?
Extracorporeal membrane oxygenation (ECMO) uses cardiopulmonary bypass technology to provide gas exchange. In patients with severe hypoxemia, ECMO can ensure adequate oxygenation and ventilation while ensuring the optimization of lung-protective ventilation. But ECMO was never as successful in adults with ARDS as it was in children and neonates.24
The first two trials of ECMO in ARDS24,25 reported equal or worse survival rates compared with conventional ventilation, and the overall mortality rate in these studies was staggeringly high. However, these studies were carried out before the era of lung-protective ventilation and at a time when ECMO technology was relatively primitive.
With new technology such as venovenous circuits and smaller cannulas, ECMO has gained more acceptance. It was used in patients with severe or refractory hypoxemia associated with ARDS during the H1N1 pandemic.26,27
The CESAR trial
The Conventional Ventilatory Support Versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure (CESAR) trial28 assessed the safety, clinical efficacy, and cost-effectiveness of ECMO in managing severe ARDS. It compared best standard practice vs a protocol that included ECMO. The trial was conducted from 2001 to 2006.
Patients with severe ARDS, as defined by a Murray score29 greater than 3 or uncompensated hypercapnea, were prospectively randomized and recruited from an ECMO center and 148 tertiary intensive care units and referral hospitals in England. This was a pragmatic trial, with minimal exclusion criteria (essentially, mechanical ventilation with high pressures and high FiO2 for more than 7 days, intracranial bleeding, or contraindication to heparinization).
A total of 180 patients were randomized in a one-to-one ratio to receive ECMO or conventional management. The ventilator management in the conventional treatment group was not done according to a protocol but in general was low-volume and low-pressure. All patients randomized to ECMO were transferred to the ECMO center and treated according to a standardized ventilation protocol. After 12 hours, if predefined goals were not reached, venovenous ECMO was started. Patients assigned to conventional management could not cross over to ECMO.
The primary outcomes were death or severe disability at 6 months after randomization, and cost-effectiveness. The secondary outcomes were hospital resource use (eg, rescue techniques, length of stay, duration of ECMO) and health status after 6 months.
Findings. The groups were similar at baseline. Sixty-eight (75%) of the 90 patients randomized to receive ECMO actually received it. Of the 22 patients who did not receive ECMO, 16 (18% of the 90) improved on conventional therapy, 5 (6%) died during or before transfer, and 1 could not receive heparin.
Two patients had severe complications in the ECMO group: one had an arterial puncture, and one had an oxygen delivery failure during transport. In each case, these events contributed to the death of the patient.
More patients in the ECMO group received lung-protective ventilation, 84 (93%) vs 63 (70%).
The primary outcome, ie, death or severe disability at 6 months, occurred in 33 (37%) of the 90 patients in the ECMO group and in 46 (53%) of the patients in the conventional management group (relative risk 0.69, 95% CI 0.05–0.97, P = .03). More patients in the ECMO group survived, but the difference was not statistically significant (relative risk of death 0.73, 95% CI 0.52–1.03, P = .07). The most common cause of death in the ECMO group was multiorgan failure (42%), whereas in the conventional management group, the most common cause of death was respiratory failure (60%).
Length of stay in the hospital and in the critical care unit and health care costs were double for patients in the ECMO group. There was no difference in quality-of-life markers at 6 months in the survivors.
Conclusions. This pragmatic trial demonstrated that a protocol that includes ECMO could improve survival rates in ARDS.
Of note, the ECMO group got care in regional centers that used protocols. Therefore, in interpreting the results of this trial, we have to consider that being in a center with protocol-specified care for ARDS could drive some of the difference in mortality rates.
Regardless, this trial demonstrated that ECMO is feasible and led to better outcomes than expected. The findings were encouraging, and spurred the use of ECMO in severe ARDS during the 2009 H1N1 pandemic. Two propensity-matched studies and a number of case series reported a survival benefit associated with the use of ECMO in patients with severe ARDS.27,30
A recent meta-analysis also reported that ECMO might lower the mortality rate in ARDS; however, the patients in the H1N1 pandemic were younger and usually had isolated respiratory failure.31
The success of ECMO has opened new possibilities in the management of ARDS. As the technology improves and our experience increases, ECMO will likely gain more acceptance as a treatment for severe ARDS.
Airway pressure release ventilation
The use of airway pressure release ventilation and other ventilator modalities in ARDS is not supported by current evidence, though results of clinical trials may influence our practice in the future.
PHARMACOTHERAPY IN ARDS
The pathogenesis of ARDS includes damage to the alveolar-capillary membrane, with leakage of protein-rich edema fluid into alveoli. This damage is propagated by a complex inflammatory response including but not limited to neutrophil activation, free-radical formation, dysregulation of the coagulation system, and extensive release of inflammatory mediators.32,33 As a consequence, there are multiple potential targets for pharmacologic therapy in ARDS.
A variety of drugs, including corticosteroids, anti-inflammatory agents, immune-modulating agents, pulmonary vasodilators, antioxidants, and surfactants, have been studied in patients with ARDS.34 But effective pharmacotherapy for ARDS remains extremely limited.
Neuromuscular blockade in early severe ARDS
Mechanical ventilation can result in injurious stretching of the lung parenchyma, either from alveolar overdistention (volutrauma) or from continual recruitment and derecruitment of unstable lung units during the ventilator cycle (atelectrauma).35 Ventilator-induced lung injury can be exacerbated by asynchronous breathing.
In theory, neuromuscular blockers could minimize patient-ventilator asynchrony and provide much better control of tidal volume and pressure in patients with ARDS. This may result in less volutrauma and atelectrauma associated with asynchronous breathing. Data also suggest that cisatracurium (Nimbex), a neuromuscular blocking agent, may have a direct effect on the amount of inflammation in lungs with ARDS.36
The ACURASYS study
The ARDS et Curarisation Systématique (ACURASYS) study37 was a randomized trial in 340 patients undergoing mechanical ventilation for severe ARDS to evaluate the impact of neuromuscular blockade within the first 48 hours in this population.
The primary outcome was the mortality rate before hospital discharge or within 90 days of study entry. Secondary outcomes included the 28-day mortality rate, the rate of intensive care unit-acquired paresis, and the number of ventilator-free days. To be included, patients had to have been mechanically ventilated for less than 48 hours and to meet the AECC criteria for severe ARDS, with a PaO2/FiO2 ratio less than 150 mm Hg.
The intervention group received a continuous infusion of cisatracurium for 48 hours, while the control patients received placebo. Muscle strength was evaluated by clinical scoring of strength in different muscle groups.
Findings. The study groups were similar at baseline.
The crude 90-day mortality rate was lower in the cisatracurium group (31.6% vs 40.7%, P = .08). Regression analysis showed an improved 90-day survival rate with the use of this neuromuscular blocker after adjustment for severity of illness and the severity of ARDS (based on degree of hypoxemia and plateau pressures) (hazard ratio for death at 90 days 0.68; 95% CI 0.48–0.98; P = .04). The rate of paresis acquired in the intensive care unit did not differ significantly between the two groups.
Conclusion. In patients with severe ARDS, giving a neuromuscular blocking agent early improved the survival rate and increased the time off the ventilator without increasing muscle weakness.
These data are in line with similar findings from two other studies published by the same group.38,39 A meta-analysis of 432 patients showed that the use of neuromuscular blockade in early severe ARDS is associated with a statistically significant effect on early mortality (relative risk 0.66, 95% CI 0.50–0.87).40 The pooled analysis of these trials did not show any statistically significant critical-illness polyneuropathy.
These results need to be interpreted carefully, as we have inadequate data to see if they generalize to different intensive care units, and the evaluation and categorization of critical-illness polyneuropathy remains to be defined.
Cisatracurium is a promising treatment for moderate to severe ARDS and merits investigation in a large confirmatory randomized controlled trial.
Other pharmacologic agents
A number of other drugs have been studied in ARDS patients, including both inhaled and intravenous beta agonists,41,42 statins,43 and nutritional supplements.44 But as with other drugs previously studied in ARDS such as corticosteroids, N-acetylcysteine, and surfactant,34 these agents showed no effect on outcomes. In fact, a recent trial of intravenous salbutamol in ARDS patients was stopped after an interim analysis because of a higher incidence of arrhythmias and lactic acidosis with this agent.42
These findings reaffirm that pharmacologic therapy needs to be carefully considered, and potential harms associated with these therapies need to be addressed before they are introduced in the care of critically ill patients.
Continued progress in understanding the pathophysiology of acute respiratory distress syndrome (ARDS) is translating into changes in the way we diagnose and manage it. Over the past 20 years, low tidal volume,1 positive end-expiratory pressure (PEEP),2 and fluid restriction3 have become the standard of care. A multidisciplinary approach, including targeted use of sedatives, early mobilization, and protocols for weaning from the ventilator, has also brought about substantial changes in ARDS management and its outcomes.4–6
In this article, we review the most relevant articles about ARDS in the last 5 years. We include the new definition of ARDS and studies of ventilatory and nonventilatory therapies that have implications in managing patients with ARDS.
A STANDARDIZED APPROACH
ARDS is characterized by damage to the alveolar architecture, severe hypoxemia, and bilateral parenchymal opacities.
The working definition of ARDS developed in 1994 by the American-European Consensus Conference (AECC) was the basis for enrollment in most of the landmark trials and observational studies over the past 20 years.7,8 However, it was limited in its reliability and validity.
An updated definition
In 2011, the ARDS Definition Task Force, using a novel consensus process, updated the ARDS definition,9 focusing on its feasibility, reliability, and validity in predicting response to therapies and outcomes in ARDS. This new “Berlin” definition is not substantially different from the old, but defines the criteria more specifically:
- Bilateral opacities, unexplained by nodules, atelectasis, or effusion, on chest radiography or computed tomography
- New or worsening respiratory symptoms, or a clinical insult associated with ARDS within 7 days of diagnosis
- Objective assessment of cardiac function (eg, with echocardiography) to exclude cardiogenic pulmonary edema
- Hypoxemia, with a partial pressure of arterial oxygen divided by the percentage of inspired oxygen (PaO2/FiO2 ratio) of 300 mm Hg or less despite noninvasive or invasive mechanical ventilation with PEEP or continuous positive airway pressure (CPAP) of at least 5 cm H2O.
In addition, the new definition classifies the severity of disease on the basis of the degree of hypoxemia, ie, the PaO2/FiO2 ratio:
- Mild: PaO2/FiO2 ratio > 200 and ≤ 300 mm Hg
- Moderate: PaO2/FiO2 ratio > 100 and ≤ 200 mm Hg
- Severe: PaO2/FiO2 ratio ≤ 100 mm Hg.
The term “acute lung injury” has been eliminated, as has the previous criterion of a pulmonary artery wedge pressure of 18 mm Hg or less.
The panel also evaluated four ancillary variables for predicting outcomes in severe ARDS:
- Compliance of the respiratory system less than or equal to 40 mL/cm H2O
- Radiographic severity (involvement of three or four quadrants on chest radiography)
- PEEP of 10 cm H2O or greater
- Corrected expired volume 10 L/min or greater.
The task force evaluated the reliability and validity of this definition in a meta-analysis of 4,400 patients previously enrolled in randomized controlled trials or observational studies.
Findings. The Berlin definition predicted the risk of death better than the AECC definition. The mortality rate increased with the severity of ARDS, from 27% with mild disease to 32% with moderate disease to 45% with severe disease. The four ancillary variables did not contribute to the predictive validity of severe ARDS for mortality and were removed from the definition.
Thille et al10 retrospectively reviewed autopsy findings from 712 patients and found that the new definition identified a homogeneous group who had severe ARDS.10
Conclusions. The new definition may overcome some of the limitations of the old one, but it needs to be validated in clinical practice, especially its ability to predict death.
VENTILATORY SUPPORT
Prompt recognition, lung-protective ventilation, and a conservative fluid strategy remain the cornerstones of ARDS management. However, other strategies are being tested.
Prone-position ventilation in severe ARDS: The right therapy in a specific population
Prone-position ventilation was first described almost 30 years ago, but it has been used inconsistently in clinical practice.
Physiologic and observational studies indicated that prone positioning might improve survival in patients with ARDS, but several randomized trials failed to demonstrate any positive effect on outcomes.11,12 Some trials also reported a higher rate of complications with this intervention.13 However, meta-analyses suggested that prone-position ventilation might have a beneficial effect in patients with severe ARDS (defined as a PaO2/FiO2 ratio ≤ 100 mm Hg).14
In view of these findings, investigators conducted a trial of prone-position ventilation exclusively in patients with severe ARDS.
The PROSEVA study
The Proning Severe ARDS Patients (PROSEVA) study was a randomized controlled trial designed to determine whether prone-position ventilation, applied early, would improve outcomes in patients with severe ARDS.15
In PROSEVA, 466 patients with severe ARDS (defined as a PaO2/FiO2 ratio < 150 mm Hg, FiO2 ≥ 60%, and PEEP ≥ 5 cm H2O) underwent either at least 16 hours of prone positioning or were left in the supine position after 12 to 24 hours of initial conventional mechanical ventilation. The patients were recruited from centers in France and Spain where prone-position ventilation had been used in daily practice for more than 5 years.
The primary outcome studied was the rate of death at 28 days. The secondary end points were the death rate at day 90, rates of successful extubation, the length of stay in the intensive care unit, and complications.
Findings. At study entry, the patients in the supine group were sicker, more of them required a vasopressor, and fewer of them were receiving neuromuscular blocking agents than those in the prone group. These baseline differences may have influenced the outcomes; the unadjusted 28-day mortality rate was 16.0% in the prone group compared with 32.8% in the supine group (P < .001). However, the hazard ratio for death with prone positioning was 0.39 (95% confidence interval [CI] 0.25–0.63) even after adjusting for severity and the use of vasopressors and neuromuscular blocking agents. Prone-position ventilation was not associated with a higher incidence of complications, and the rate of successful extubation was higher.
Conclusions. In patients with severe ARDS, early use of prolonged prone positioning significantly decreased the 28-day and 90-day mortality rates. This trial has made prone positioning one of the strategies in managing patients with early severe ARDS. To minimize complications such as pressure ulcers and line or tube dislodgement, personnel caring for these patients must follow a protocol and undergo specific training.
These results were corroborated by a meta-analysis by Beitler et al16 that found a significant decrease in mortality rate with prone-position ventilation even in older studies when lung-protective ventilation strategies were separated from high-tidal-volume ventilation.
High-frequency oscillatory ventilation: No benefit in two trials
Observational data and experimental studies suggested that high-frequency oscillatory ventilation (HFOV) is superior to conventional mechanical ventilation in ARDS patients.17,18 However, outdated and cumbersome equipment, lack of protocols, and a lack of high-quality evidence led to limited and inconsistent use of HFOV, mainly as a rescue therapy in ARDS.19
Over the last few years, HFOV has been gaining acceptance, especially earlier in the course of ARDS.20 After preliminary clinical trials reported promising results, two trials conducted in Canada and the United Kingdom compared HFOV vs conventional mechanical ventilation in patients with ARDS.
The OSCAR study
The Oscillation in ARDS (OSCAR) study21 was a “pragmatic” trial22 (ie, it had minimal exclusion criteria) of the safety and effectiveness of HFOV as a primary ventilatory strategy for ARDS. It included 795 patients randomized to receive conventional ventilation (n = 397) or HFOV (n = 398). Research centers followed detailed algorithms for HFOV management and adopted their usual practice for conventional ventilation. Medical care was given according to the clinician’s judgment.
The primary outcome studied was survival at 30 days. The secondary outcomes were all-cause mortality in the intensive care unit and the hospital, duration of mechanical ventilation, and use of antimicrobial, sedative, vasoactive, and neuromuscular-blocking drugs.
Findings. The patient baseline characteristics were similar in both groups.
There was no significant difference in intensive care unit mortality rates, hospital mortality rates, or mortality rates at 30 days (41.7% in the HFOV group vs 41.1% in the conventional ventilation group; P = .85, 95% CI 6.1–7.5) even after adjustments for center or severity of illness.
The duration of mechanical ventilation was similar in both groups (14.9 ± 13.3 days in the HFOV group vs 14.1 ± 13.4 days in the conventional ventilation group, P = .41). However, sedatives and neuromuscular-blocking drugs were used more often and longer in the HFOV group than in the conventional ventilation group. There was no difference in the use of vasoactive or antimicrobial medications.
Conclusions. This multicenter randomized control trial did not demonstrate any benefit from using HFOV for routine management of ARDS. Its pragmatic design made it less likely to reach a firm conclusion,22 but it at least made a case against routinely using HFOV in patients with ARDS.
The OSCILLATE study
The Oscillation for Acute Respiratory Distress Syndrome Treated Early (OSCILLATE) study23 assessed the safety and efficacy of HFOV as a treatment for early-onset moderate-to-severe ARDS.
The inclusion criteria were similar to those in the OSCAR trial except that pulmonary symptoms had to be present less than 2 weeks and ARDS assessment was done under standard ventilator settings. As this was an efficacy trial, it had more exclusion criteria than the OSCAR trial. A total of 548 patients were randomized to receive conventional ventilation (n = 273) or HFOV (n = 275). The baseline characteristics were similar between groups.
Conventional ventilation was given according to a protocol used in an earlier trial2 and included recruitment maneuvers. HFOV was given in centers that had experience in this treatment, and there were protocols for ventilation management, hemodynamic optimization, and weaning. All other care was left to the clinician’s choice.
The primary outcome studied was in-hospital mortality. The investigators also evaluated whether there were interactions between the treatment and baseline severity of lung injury and center experience with HFOV.
Findings. The trial was stopped after an interim analysis found that HFOV might be harmful, although the statistical threshold for stopping was not reached. The in-hospital mortality rate was 47% in the HFOV group and 35% in the control group (relative risk of death with HFOV 1.33, 95% CI 1.09–1.64, P = .005). HFOV was worse than conventional ventilation regardless of the severity of disease or center experience. The HFOV group had higher mean airway pressures but similar FiO2 compared with the conventional ventilation group.
The HFOV group received significantly more vasopressors, sedatives, and neuromuscular blockers. This group’s fluid balance was higher as well, but not significantly so. Refractory hypoxemia (defined as PaO2 < 60 mm Hg for 1 hour with an FiO2 of 1.0 and neuromuscular blockade) was more frequent in the conventional ventilation group, but the number of deaths in the subgroup with refractory hypoxemia was similar with either treatment.
Conclusions. This multicenter randomized controlled trial demonstrated that HFOV was harmful when used routinely to manage ARDS. The trial’s protocol was based on the results of a pilot study carried out by the same investigators, which provided the best evidence available regarding the safety of HFOV at that time.
The results of the OSCAR and OSCILLATE trials have quelled enthusiasm for early, routine use of HFOV in ARDS. Although there are concerns that the protocol (ie, the way HFOV was implemented) rather than HFOV itself may have led to worse outcomes, there is no signal to support its routine use. We need further studies to define if it remains a viable rescue therapy.
Extracorporeal membrane oxygenation: Is it a viable option in severe ARDS?
Extracorporeal membrane oxygenation (ECMO) uses cardiopulmonary bypass technology to provide gas exchange. In patients with severe hypoxemia, ECMO can ensure adequate oxygenation and ventilation while ensuring the optimization of lung-protective ventilation. But ECMO was never as successful in adults with ARDS as it was in children and neonates.24
The first two trials of ECMO in ARDS24,25 reported equal or worse survival rates compared with conventional ventilation, and the overall mortality rate in these studies was staggeringly high. However, these studies were carried out before the era of lung-protective ventilation and at a time when ECMO technology was relatively primitive.
With new technology such as venovenous circuits and smaller cannulas, ECMO has gained more acceptance. It was used in patients with severe or refractory hypoxemia associated with ARDS during the H1N1 pandemic.26,27
The CESAR trial
The Conventional Ventilatory Support Versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure (CESAR) trial28 assessed the safety, clinical efficacy, and cost-effectiveness of ECMO in managing severe ARDS. It compared best standard practice vs a protocol that included ECMO. The trial was conducted from 2001 to 2006.
Patients with severe ARDS, as defined by a Murray score29 greater than 3 or uncompensated hypercapnea, were prospectively randomized and recruited from an ECMO center and 148 tertiary intensive care units and referral hospitals in England. This was a pragmatic trial, with minimal exclusion criteria (essentially, mechanical ventilation with high pressures and high FiO2 for more than 7 days, intracranial bleeding, or contraindication to heparinization).
A total of 180 patients were randomized in a one-to-one ratio to receive ECMO or conventional management. The ventilator management in the conventional treatment group was not done according to a protocol but in general was low-volume and low-pressure. All patients randomized to ECMO were transferred to the ECMO center and treated according to a standardized ventilation protocol. After 12 hours, if predefined goals were not reached, venovenous ECMO was started. Patients assigned to conventional management could not cross over to ECMO.
The primary outcomes were death or severe disability at 6 months after randomization, and cost-effectiveness. The secondary outcomes were hospital resource use (eg, rescue techniques, length of stay, duration of ECMO) and health status after 6 months.
Findings. The groups were similar at baseline. Sixty-eight (75%) of the 90 patients randomized to receive ECMO actually received it. Of the 22 patients who did not receive ECMO, 16 (18% of the 90) improved on conventional therapy, 5 (6%) died during or before transfer, and 1 could not receive heparin.
Two patients had severe complications in the ECMO group: one had an arterial puncture, and one had an oxygen delivery failure during transport. In each case, these events contributed to the death of the patient.
More patients in the ECMO group received lung-protective ventilation, 84 (93%) vs 63 (70%).
The primary outcome, ie, death or severe disability at 6 months, occurred in 33 (37%) of the 90 patients in the ECMO group and in 46 (53%) of the patients in the conventional management group (relative risk 0.69, 95% CI 0.05–0.97, P = .03). More patients in the ECMO group survived, but the difference was not statistically significant (relative risk of death 0.73, 95% CI 0.52–1.03, P = .07). The most common cause of death in the ECMO group was multiorgan failure (42%), whereas in the conventional management group, the most common cause of death was respiratory failure (60%).
Length of stay in the hospital and in the critical care unit and health care costs were double for patients in the ECMO group. There was no difference in quality-of-life markers at 6 months in the survivors.
Conclusions. This pragmatic trial demonstrated that a protocol that includes ECMO could improve survival rates in ARDS.
Of note, the ECMO group got care in regional centers that used protocols. Therefore, in interpreting the results of this trial, we have to consider that being in a center with protocol-specified care for ARDS could drive some of the difference in mortality rates.
Regardless, this trial demonstrated that ECMO is feasible and led to better outcomes than expected. The findings were encouraging, and spurred the use of ECMO in severe ARDS during the 2009 H1N1 pandemic. Two propensity-matched studies and a number of case series reported a survival benefit associated with the use of ECMO in patients with severe ARDS.27,30
A recent meta-analysis also reported that ECMO might lower the mortality rate in ARDS; however, the patients in the H1N1 pandemic were younger and usually had isolated respiratory failure.31
The success of ECMO has opened new possibilities in the management of ARDS. As the technology improves and our experience increases, ECMO will likely gain more acceptance as a treatment for severe ARDS.
Airway pressure release ventilation
The use of airway pressure release ventilation and other ventilator modalities in ARDS is not supported by current evidence, though results of clinical trials may influence our practice in the future.
PHARMACOTHERAPY IN ARDS
The pathogenesis of ARDS includes damage to the alveolar-capillary membrane, with leakage of protein-rich edema fluid into alveoli. This damage is propagated by a complex inflammatory response including but not limited to neutrophil activation, free-radical formation, dysregulation of the coagulation system, and extensive release of inflammatory mediators.32,33 As a consequence, there are multiple potential targets for pharmacologic therapy in ARDS.
A variety of drugs, including corticosteroids, anti-inflammatory agents, immune-modulating agents, pulmonary vasodilators, antioxidants, and surfactants, have been studied in patients with ARDS.34 But effective pharmacotherapy for ARDS remains extremely limited.
Neuromuscular blockade in early severe ARDS
Mechanical ventilation can result in injurious stretching of the lung parenchyma, either from alveolar overdistention (volutrauma) or from continual recruitment and derecruitment of unstable lung units during the ventilator cycle (atelectrauma).35 Ventilator-induced lung injury can be exacerbated by asynchronous breathing.
In theory, neuromuscular blockers could minimize patient-ventilator asynchrony and provide much better control of tidal volume and pressure in patients with ARDS. This may result in less volutrauma and atelectrauma associated with asynchronous breathing. Data also suggest that cisatracurium (Nimbex), a neuromuscular blocking agent, may have a direct effect on the amount of inflammation in lungs with ARDS.36
The ACURASYS study
The ARDS et Curarisation Systématique (ACURASYS) study37 was a randomized trial in 340 patients undergoing mechanical ventilation for severe ARDS to evaluate the impact of neuromuscular blockade within the first 48 hours in this population.
The primary outcome was the mortality rate before hospital discharge or within 90 days of study entry. Secondary outcomes included the 28-day mortality rate, the rate of intensive care unit-acquired paresis, and the number of ventilator-free days. To be included, patients had to have been mechanically ventilated for less than 48 hours and to meet the AECC criteria for severe ARDS, with a PaO2/FiO2 ratio less than 150 mm Hg.
The intervention group received a continuous infusion of cisatracurium for 48 hours, while the control patients received placebo. Muscle strength was evaluated by clinical scoring of strength in different muscle groups.
Findings. The study groups were similar at baseline.
The crude 90-day mortality rate was lower in the cisatracurium group (31.6% vs 40.7%, P = .08). Regression analysis showed an improved 90-day survival rate with the use of this neuromuscular blocker after adjustment for severity of illness and the severity of ARDS (based on degree of hypoxemia and plateau pressures) (hazard ratio for death at 90 days 0.68; 95% CI 0.48–0.98; P = .04). The rate of paresis acquired in the intensive care unit did not differ significantly between the two groups.
Conclusion. In patients with severe ARDS, giving a neuromuscular blocking agent early improved the survival rate and increased the time off the ventilator without increasing muscle weakness.
These data are in line with similar findings from two other studies published by the same group.38,39 A meta-analysis of 432 patients showed that the use of neuromuscular blockade in early severe ARDS is associated with a statistically significant effect on early mortality (relative risk 0.66, 95% CI 0.50–0.87).40 The pooled analysis of these trials did not show any statistically significant critical-illness polyneuropathy.
These results need to be interpreted carefully, as we have inadequate data to see if they generalize to different intensive care units, and the evaluation and categorization of critical-illness polyneuropathy remains to be defined.
Cisatracurium is a promising treatment for moderate to severe ARDS and merits investigation in a large confirmatory randomized controlled trial.
Other pharmacologic agents
A number of other drugs have been studied in ARDS patients, including both inhaled and intravenous beta agonists,41,42 statins,43 and nutritional supplements.44 But as with other drugs previously studied in ARDS such as corticosteroids, N-acetylcysteine, and surfactant,34 these agents showed no effect on outcomes. In fact, a recent trial of intravenous salbutamol in ARDS patients was stopped after an interim analysis because of a higher incidence of arrhythmias and lactic acidosis with this agent.42
These findings reaffirm that pharmacologic therapy needs to be carefully considered, and potential harms associated with these therapies need to be addressed before they are introduced in the care of critically ill patients.
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. Acute Respiratory Distress Syndrome Network. N Engl J Med 2000; 342:1301–1308.
- Meade MO, Cook DJ, Guyatt GH, et al; Lung Open Ventilation Study Investigators. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008; 299:637–645.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575.
- Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008; 371:126–134.
- Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009; 373:1874–1882.
- Ely EW, Baker AM, Dunagan DP, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med 1996; 335:1864–1869.
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994; 149:818–824.
- Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med 2012; 38:1573–1582.
- ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307:2526–2533.
- Thille AW, Esteban A, Fernández-Segoviano P, et al. Comparison of the Berlin definition for acute respiratory distress syndrome with autopsy. Am J Respir Crit Care Med 2013; 187:761–767.
- Gattinoni L, Tognoni G, Pesenti A, et al; Prone-Supine Study Group. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med 2001; 345:568–573.
- Taccone P, Pesenti A, Latini R, et al; Prone-Supine II Study Group. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA 2009; 302:1977–1984.
- Mancebo J, Fernández R, Blanch L, et al. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med 2006; 173:1233–1239.
- Sud S, Friedrich JO, Taccone P, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med 2010; 36:585–599.
- Guérin C, Reignier J, Richard JC, et al; PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013; 368:2159–2168.
- Beitler JR, Shaefi S, Montesi SB, et al. Prone positioning reduces mortality from acute respiratory distress syndrome in the low tidal volume era: a meta-analysis. Intensive Care Med 2014; 40:332–341.
- Chan KP, Stewart TE, Mehta S. High-frequency oscillatory ventilation for adult patients with ARDS. Chest 2007; 131:1907–1916.
- Fessler HE, Hager DN, Brower RG. Feasibility of very high-frequency ventilation in adults with acute respiratory distress syndrome. Crit Care Med 2008; 36:1043–1048.
- Mehta S, Granton J, MacDonald RJ, et al. High-frequency oscillatory ventilation in adults: the Toronto experience. Chest 2004; 126:518–527.
- Ferguson ND, Chiche JD, Kacmarek RM, et al. Combining high-frequency oscillatory ventilation and recruitment maneuvers in adults with early acute respiratory distress syndrome: the Treatment with Oscillation and an Open Lung Strategy (TOOLS) Trial pilot study. Crit Care Med 2005; 33:479–486.
- Young D, Lamb SE, Shah S, et al; OSCAR Study Group. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med 2013; 368:806–813.
- Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol 2009; 62:464–475.
- Ferguson ND, Cook DJ, Guyatt GH, et al; OSCILLATE Trial Investigators; Canadian Critical Care Trials Group. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med 2013; 368:795–805.
- Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 1994; 149:295–305.
- Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 1979; 242:2193–2196.
- Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators; Davies A, Jones D, Bailey M, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA 2009; 302:1888–1895.
- Pham T, Combes A, Rozé H, et al; REVA Research Network. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med 2013; 187:276–285.
- Peek GJ, Mugford M, Tiruvoipati R, et al; CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009; 374:1351–1363.
- Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 1988; 138:720–723.
- Noah MA, Peek GJ, Finney SJ, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA 2011; 306:1659–1668.
- Zampieri FG, Mendes PV, Ranzani OT, et al. Extracorporeal membrane oxygenation for severe respiratory failure in adult patients: a systematic review and meta-analysis of current evidence. J Crit Care 2013; 28:998–1005.
- Raghavendran K, Pryhuber GS, Chess PR, Davidson BA, Knight PR, Notter RH. Pharmacotherapy of acute lung injury and acute respiratory distress syndrome. Curr Med Chem 2008; 15:1911–1924.
- Adhikari N, Burns KE, Meade MO. Pharmacologic treatments for acute respiratory distress syndrome and acute lung injury: systematic review and meta-analysis. Treat Respir Med 2004; 3:307–328.
- Adhikari N, Burns KE, Meade MO. Pharmacologic therapies for adults with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev 2004; 4:CD004477.
- Terragni PP, Rosboch GL, Lisi A, Viale AG, Ranieri VM. How respiratory system mechanics may help in minimising ventilator-induced lung injury in ARDS patients. Eur Respir J Suppl 2003; 42:15s–21s.
- Forel JM, Roch A, Papazian L. Paralytics in critical care: not always the bad guy. Curr Opin Crit Care 2009; 15:59–66.
- Papazian L, Forel JM, Gacouin A, et al; ACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010; 363:1107–1116.
- Gainnier M, Roch A, Forel JM, et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med 2004; 32:113–19.
- Forel JM, Roch A, Marin V, et al. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med 2006; 34:2749–2757.
- Alhazzani W, Alshahrani M, Jaeschke R, et al. Neuromuscular blocking agents in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. Crit Care 2013; 17:R43.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Matthay MA, Brower RG, Carson S, et al. Randomized, placebo-controlled clinical trial of an aerosolized beta-2-agonist for treatment of acute lung injury. Am J Respir Crit Care Med 2011; 184:561–568.
- Gao Smith F, Perkins GD, Gates S, et al; BALTI-2 study investigators. Effect of intravenous beta-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet 2012; 379:229–235.
- Craig TR, Duffy MJ, Shyamsundar M, et al. A randomized clinical trial of hydroxymethylglutaryl-coenzyme a reductase inhibition for acute lung injury (The HARP Study). Am J Respir Crit Care Med 2011; 183:620–626.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Rice TW, Wheeler AP, Thompson BT, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA 2012; 307:795–803.
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. Acute Respiratory Distress Syndrome Network. N Engl J Med 2000; 342:1301–1308.
- Meade MO, Cook DJ, Guyatt GH, et al; Lung Open Ventilation Study Investigators. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008; 299:637–645.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575.
- Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008; 371:126–134.
- Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009; 373:1874–1882.
- Ely EW, Baker AM, Dunagan DP, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med 1996; 335:1864–1869.
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994; 149:818–824.
- Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med 2012; 38:1573–1582.
- ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307:2526–2533.
- Thille AW, Esteban A, Fernández-Segoviano P, et al. Comparison of the Berlin definition for acute respiratory distress syndrome with autopsy. Am J Respir Crit Care Med 2013; 187:761–767.
- Gattinoni L, Tognoni G, Pesenti A, et al; Prone-Supine Study Group. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med 2001; 345:568–573.
- Taccone P, Pesenti A, Latini R, et al; Prone-Supine II Study Group. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA 2009; 302:1977–1984.
- Mancebo J, Fernández R, Blanch L, et al. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med 2006; 173:1233–1239.
- Sud S, Friedrich JO, Taccone P, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med 2010; 36:585–599.
- Guérin C, Reignier J, Richard JC, et al; PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013; 368:2159–2168.
- Beitler JR, Shaefi S, Montesi SB, et al. Prone positioning reduces mortality from acute respiratory distress syndrome in the low tidal volume era: a meta-analysis. Intensive Care Med 2014; 40:332–341.
- Chan KP, Stewart TE, Mehta S. High-frequency oscillatory ventilation for adult patients with ARDS. Chest 2007; 131:1907–1916.
- Fessler HE, Hager DN, Brower RG. Feasibility of very high-frequency ventilation in adults with acute respiratory distress syndrome. Crit Care Med 2008; 36:1043–1048.
- Mehta S, Granton J, MacDonald RJ, et al. High-frequency oscillatory ventilation in adults: the Toronto experience. Chest 2004; 126:518–527.
- Ferguson ND, Chiche JD, Kacmarek RM, et al. Combining high-frequency oscillatory ventilation and recruitment maneuvers in adults with early acute respiratory distress syndrome: the Treatment with Oscillation and an Open Lung Strategy (TOOLS) Trial pilot study. Crit Care Med 2005; 33:479–486.
- Young D, Lamb SE, Shah S, et al; OSCAR Study Group. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med 2013; 368:806–813.
- Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol 2009; 62:464–475.
- Ferguson ND, Cook DJ, Guyatt GH, et al; OSCILLATE Trial Investigators; Canadian Critical Care Trials Group. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med 2013; 368:795–805.
- Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 1994; 149:295–305.
- Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 1979; 242:2193–2196.
- Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators; Davies A, Jones D, Bailey M, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA 2009; 302:1888–1895.
- Pham T, Combes A, Rozé H, et al; REVA Research Network. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med 2013; 187:276–285.
- Peek GJ, Mugford M, Tiruvoipati R, et al; CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009; 374:1351–1363.
- Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 1988; 138:720–723.
- Noah MA, Peek GJ, Finney SJ, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA 2011; 306:1659–1668.
- Zampieri FG, Mendes PV, Ranzani OT, et al. Extracorporeal membrane oxygenation for severe respiratory failure in adult patients: a systematic review and meta-analysis of current evidence. J Crit Care 2013; 28:998–1005.
- Raghavendran K, Pryhuber GS, Chess PR, Davidson BA, Knight PR, Notter RH. Pharmacotherapy of acute lung injury and acute respiratory distress syndrome. Curr Med Chem 2008; 15:1911–1924.
- Adhikari N, Burns KE, Meade MO. Pharmacologic treatments for acute respiratory distress syndrome and acute lung injury: systematic review and meta-analysis. Treat Respir Med 2004; 3:307–328.
- Adhikari N, Burns KE, Meade MO. Pharmacologic therapies for adults with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev 2004; 4:CD004477.
- Terragni PP, Rosboch GL, Lisi A, Viale AG, Ranieri VM. How respiratory system mechanics may help in minimising ventilator-induced lung injury in ARDS patients. Eur Respir J Suppl 2003; 42:15s–21s.
- Forel JM, Roch A, Papazian L. Paralytics in critical care: not always the bad guy. Curr Opin Crit Care 2009; 15:59–66.
- Papazian L, Forel JM, Gacouin A, et al; ACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010; 363:1107–1116.
- Gainnier M, Roch A, Forel JM, et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med 2004; 32:113–19.
- Forel JM, Roch A, Marin V, et al. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med 2006; 34:2749–2757.
- Alhazzani W, Alshahrani M, Jaeschke R, et al. Neuromuscular blocking agents in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. Crit Care 2013; 17:R43.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Matthay MA, Brower RG, Carson S, et al. Randomized, placebo-controlled clinical trial of an aerosolized beta-2-agonist for treatment of acute lung injury. Am J Respir Crit Care Med 2011; 184:561–568.
- Gao Smith F, Perkins GD, Gates S, et al; BALTI-2 study investigators. Effect of intravenous beta-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet 2012; 379:229–235.
- Craig TR, Duffy MJ, Shyamsundar M, et al. A randomized clinical trial of hydroxymethylglutaryl-coenzyme a reductase inhibition for acute lung injury (The HARP Study). Am J Respir Crit Care Med 2011; 183:620–626.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Rice TW, Wheeler AP, Thompson BT, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA 2012; 307:795–803.
KEY POINTS
- The new definition of ARDS categorizes it as mild, moderate, or severe on the basis of oxygenation, specifically, the PaO2/FiO2 ratio.
- Neuromuscular blockade and prone positioning, used early in moderate or severe cases of ARDS, have shown some promise in trials, but questions remain about their application in critically ill patients.
- Based on two large trials, HFOV is no longer recommended as a primary therapy for ARDS, but it may still be considered as a rescue therapy in patients with refractory hypoxemia.
- In light of observational studies and randomized trials, ECMO should be considered an option in cases of refractory hypoxemia.
A 61-year-old man with fluctuating hypertension
A 61-year-old man with type 2 diabetes mellitus on glimepiride therapy presented with somnolence and slurred speech. His capillary glucose level was 17 mg/dL and his serum glucose level was 28 mg/dL. He was treated with intravenous dextrose, and his glucose level promptly returned to normal.
He had been adherent to his medication regimen and denied overmedicating or accidental overdosing. Over the past 7 months, he had noted redness on his palms, a rash on his legs, intermittent moderate to severe headaches, weight loss, and decreased appetite. In addition, his blood pressure had been labile, which his physicians had attributed to autonomic instability. He had continued on the same dose of glimepiride despite losing weight.
His history included multivessel coronary artery disease treated with angioplasty and placement of multiple coronary stents; ischemic cardiomyopathy with a left ventricular ejection fraction of 28%; implantation of a cardioverter-defibrillator for secondary prevention of ventricular arrhythmia; an ischemic stroke; and multiple sclerosis complicated by bilateral blindness, with optic nerve involvement and autonomic instability, present for over a year and manifested by labile blood pressure. He was a long-time tobacco user. His daily medications included ticagrelor 90 mg, aspirin 81 mg, metoprolol 50 mg, ramipril 10 mg, simvastatin 20 mg, glimepiride 2 mg, and esomeprazole 40 mg. He needed help taking his medications.
At the time of hospital admission, his heart rate was 69 beats per minute with a regular rhythm, blood pressure 115/73 mm Hg, respiratory rate 11 breaths per minute with an oxygen saturation of 99% on room air, and oral temperature 34.7°C (94.5°F). He appeared to be in no distress.
Cardiovascular examination revealed no murmurs or gallops; there was mild nonpitting edema of the lower extremities. Pulmonary, abdominal, and neurologic examinations were unrevealing except for bilateral blindness. Vascular examination revealed no bruits. Results of a complete blood cell count and metabolic panel were normal except for a hemoglobin level of 9.9 g/dL (reference range 13.5–17.5) and a platelet count of 477 × 109/L (150–450).
Although he continued to receive the same medications he had been taking at home, his blood pressure fluctuated. On the second hospital day, it reached 186/135 mm Hg, at which time he also had palpitations, dyspnea, and crackles in the lower lobes of both lungs. Volume resuscitation on admission was suspected to have played a role, and he received furosemide, which improved his symptoms. But several hours later, his blood pressure rose again, and he became diaphoretic. Despite aggressive treatment with different antihypertensive agents, his blood pressure remained high and his symptoms persisted. Chest radiography showed no evidence of pulmonary edema. Because of his progressive dyspnea, the diagnosis of pulmonary embolism was entertained.
CAUSES OF RESISTANT HYPERTENSION
1. What could explain this patient’s high blood pressure?
- A drug effect
- Renovascular disease
- Excess circulating catecholamines
- Obstructive sleep apnea
- Primary aldosteronism
Sympathomimetic drugs such as epinephrine, norepinephrine, dopamine, and vasopressin, which are used when hemodynamic support is required, can raise both systolic and diastolic blood pressure. Nonsteroidal anti-inflammatory drugs and nasal decongestants are common culprits in the community. However, our patient was using none of these drugs.
Renovascular disease is one of many causes of resistant hypertension, accounting for 8% of all cases.1,2 Despite fluctuations, the blood pressure often remains chronically elevated, its changes are less paroxysmal than in our patient, and a precipitating factor such as a dietary indiscretion is sometimes identified.1
Excess circulating catecholamines can be a result of stress, exogenous administration, or endogenous oversecretion. Our patient’s clinical presentation is highly suspicious for a high-catecholamine state, and this should be further evaluated.
Obstructive sleep apnea is common in patients with resistant hypertension, with an estimated prevalence as high as 60% in this group.3,4
Primary aldosteronism has an estimated prevalence of about 20% in patients evaluated for resistant hypertension.5
AN ADRENAL MASS IS INCIDENTALLY DISCOVERED
Computed tomographic angiography of the chest revealed no evidence of pulmonary emboli. There was mild dilation of the central pulmonary arteries and an incidental, incompletely imaged 4.7-by-3.4-cm mass of mixed attenuation in the right adrenal gland, with macroscopic fat within the lesion.
Computed tomography (CT) of the abdomen with dedicated cuts through the adrenal glands revealed a 4.7-cm heterogeneous right adrenal mass with a density of 34 Hounsfield units (HU). The left adrenal gland appeared diffusely enlarged without a discretely seen mass, consistent with hyperplasticity (Figure 1).
2. Based on the patient’s clinical presentation and findings on CT, what would be the most likely diagnosis for this incidentally found adrenal mass?
- Adrenocortical adenoma
- Adrenocortical carcinoma
- Metastatic mass
- Pheochromocytoma
Adrenocortical adenoma can present as a small homogeneous mass of variable size, with smooth margins, and rarely containing hemorrhagic tissue or calcifications. The typical density on nonenhanced CT is less than 10 HU. On enhanced CT, it is nonvascular. T2-weighted magnetic resonance imaging (MRI) shows a lesion of the same intensity as liver tissue.6
Adrenocortical adenoma is not classically associated with autologous activity and thus is less likely to explain our patient’s symptoms.
Adrenocortical carcinoma can present as a large heterogeneous mass, usually greater than 4 cm in diameter, with irregular margins and areas of necrosis, hemorrhage, or calcification. The typical density on nonenhanced CT is greater than 10 HU. On enhanced CT, the mass is usually vascular, and T2-weighted MRI will show a lesion more intense than liver tissue.6
Adrenocortical carcinoma is also not classically associated with autologous activity, and so is not likely to explain our patient’s symptoms.6
Metastatic disease can present with masses of variable size, often bilaterally, and occasionally with cysts or areas of hemorrhage. The typical density of metastatic lesions on nonenhanced CT is greater than 10 HU. On enhanced CT, they are usually vascular, and on T2-weighted MRI they are hyperintense.6 The characteristics of the mass and the absence of a primary malignancy on CT of the chest and abdomen do not support the diagnosis of metastatic disease.
Pheochromocytoma is a neuroendocrine tumor of the adrenal medulla that can present as a large heterogeneous mass, greater than 3 cm in diameter, with clear margins and cysts or areas of hemorrhage. Extra-adrenal neuroendocrine tumors are typically called paragangliomas and have features similar to those of pheochromocytoma. The typical density of pheochromocytoma on nonenhanced CT is greater than 10 HU. On enhanced CT, it is usually vascular, and T2-weighted MRI shows a hyperintense lesion. Pheochromocytoma can be biochemically active and thus can cause signs and symptoms that will lead to the diagnosis.6
Other imaging tests may play a role in the evaluation of adrenal masses but are not required for the diagnosis of pheochromocytoma. Functional positron emission tomography using metaiodobenzylguanidine labeled with iodine 123 or-iodine 131 or using the glucose analogue F-18 fluorodeoxyglucose has been used in the initial assessment of pheochromocytoma, with good sensitivity and specificity.7,8
Our patient’s pacemaker-defibrillator precluded him from undergoing MRI.
DIAGNOSIS: PHEOCHROMOCYTOMA
Pheochromocytoma was highly suspected on the basis of the patient’s clinical presentation, and metoprolol was immediately discontinued. He was started on the calcium channel blocker verapamil and the alpha-blocker phenoxybenzamine.
Serum samples were obtained to measure metanephrines, dehydroepiandrosterone, aldosterone, and cortisol, and a 24-hour urine collection was obtained to measure creatinine, dopamine, epinephrine, norepinephrine, cortisol, and metanephrines. Based on the results (Table 1) and on the findings on imaging, the patient was diagnosed with pheochromocytoma. A surgical consultation was obtained, and surgery was recommended.
WHEN DOES PHEOCHROMOCYTOMA CALL FOR SURGERY?
3. Which criterion is most important when determining the need for surgery for pheochromocytoma?
- Findings on fine-needle aspiration biopsy
- Biochemical activity
- Size of the mass
- Bilateral masses
Fine-needle aspiration biopsy can be done when a mass is found incidentally and no evidence of biochemical activity is detected, although it is not an essential part of the diagnostic workup.9 In most cases, the sampling from fine-needle aspiration is not sufficient to achieve a diagnosis.
Biochemical activity is the most important factor when determining the need for prompt surgical intervention. The excess circulating catecholamines have been associated with increased risk of cardiovascular morbidity and death independent of the morbidity associated with hypertension alone.10 Biochemical activity can be independent of the size of the mass, but larger masses typically present with symptoms.
Bilateral masses have been associated with metastatic disease.11 In retrospect, the patient’s history of hypertension and cerebrovascular accident could be associated with the development of a catecholamine-releasing tumor.
A GOOD OUTCOME FROM SURGERY
The patient was continued on phenoxybenzamine for 7 days and responded well to this therapy.
After this preoperative preparation, he underwent laparoscopic right adrenalectomy with excision of a retroperitoneal adrenal mass. His postoperative course was complicated by transient hypotension requiring low-dose vasopressin support for less than 24 hours. He was then restarted on his previous dosage of metoprolol and was discharged home on postoperative day 5 with stable blood pressure. Follow-up 24-hour urine collection 1 month after he was discharged showed normalization of metanephrine, normetanephrine, epinephrine, and norepinephrine levels.
Despite low suspicion for an underlying genetic syndrome, he was referred for genetic testing and was scheduled to have a repeat 24-hour urine collection and imaging in 6 months to follow his enlarged left adrenal gland, which did not appear to be metabolically hyperactive.
4. What is the most common perioperative complication of pheochromocytoma excision?
- Hypoglycemia
- Hypotension
- Hypocortisolism
- Hypertension
- Tachycardia
Hypoglycemia has been observed after removal of pheochromocytoma, as levels of catecholamines (which normally inhibit pancreatic beta cells) decrease and insulin secretion consequently increases.12 Our patient developed hypoglycemia before surgery, not after, and it was likely due to the combination of his antidiabetic therapy, weight loss, and decreased oral intake.
Hypotension is the most common complication in the perioperative period. It is associated with excessive loss of catecholamine secretion. It is usually short-lived but may require aggressive administration of intravenous fluids and use of sympathomimetic agents.
Hypocortisolism is unlikely in patients with pheochromocytoma, but it is likely after removal of adrenocortical adenoma.
Hypertension and tachycardia affect up to 40% of pheochromocytoma patients in some case series.12
PHEOCHROMOCYTOMA: A CATECHOLAMINE-SECRETING TUMOR
The pathophysiology of pheochromocytoma is complex. It is characterized by accelerated growth of cells producing catecholamines, which may produce symptoms when secreted into the bloodstream. The classic triad of symptoms is headache, hypertension, and hyperglycemia, although our patient had very low blood sugar levels. Other common symptoms are nausea, orthostasis, and tremor, although not all symptoms are invariably seen.
Genetic testing recommended
Genetic associations have been described and are thought to be responsible for 20% to 30% of cases of pheochromocytoma. All associated germline mutations are autosomal dominant, some with variable penetrance. These include:
- Succinate dehydrogenase subunit B, C, and D mutations
- von Hippel-Lindau syndrome
- Multiple endocrine neoplasia type 1 and type 2 syndromes
- Neurofibromatosis type 1.13,14
The succinate dehydrogenase subunit mutations have been associated with, but not limited to, extra-adrenal adenomas (paragangliomas) and carry a worse prognosis.
Some experts recommend genetic testing in all cases of pheochromocytoma, sporadic or familial, and this testing should be followed by counseling if a mutation is found.15 Others recommend genetic testing based on the patient’s age (under age 50), history, imaging, and biochemical features of the tumor (metanephrines predominate in multiple endocrine neoplasia syndromes, and normetanephrines in von Hippel-Lindau syndrome).13
Serious consequences
A thorough evaluation is recommended, since pheochromocytoma has been associated with increased cardiovascular morbidity. In a retrospective series, Stolk et al10 reported that patients with pheochromocytoma had a higher incidence of myocardial infarction, angina, and stroke in the years preceding the diagnosis than did patients with essential hypertension (13.8% vs 1.1%, P < .001).10
Catecholamine cardiomyopathy has been described and shares clinical features with Takotsubo or stress cardiomyopathy, with global left ventricular systolic and diastolic dysfunction that improve or resolve after the adrenergic insult is removed.16
Conditions that warrant further evaluation or that may suggest pheochromocytoma are malignant hypertension, hypertensive encephalopathy, ischemic stroke, subarachnoid hemorrhage, acute pulmonary edema, angina pectoris, myocardial infarction, aortic dissection, and kidney injury.
When to suspect pheochromocytoma
Pheochromocytoma should be suspected in a patient with resistant hypertension, family history, or imaging findings that suggest an adrenal mass with a heterogeneous appearance. The diagnostic algorithm follows the same pathway as for the evaluation of an incidentally found adrenal mass, with determination of its dimension and characteristics by CT or MRI, and with biochemical testing of urine catecholamines, plasma free metanephrines, renin, aldosterone, and cortisol.
The diagnosis of pheochromocytoma is established by obtaining fractionated metanephrines and catecholamines in a 24-hour urine collection (sensitivity 90%, specificity 98%). Analysis of plasma metanephrines has a higher sensitivity (97%) but lower specificity (85%).17 The combination of typical signs, symptoms, and laboratory findings makes the diagnosis likely, especially in combination with a unilateral adrenal mass.
Laparoscopic surgery after medical preparation for active tumors
If the mass appears benign and not biochemically hyperactive, then follow-up at 1 year is recommended, with repeat testing. Surgical evaluation and intervention is recommended for lesions that appear malignant or that are biochemically active and clinically symptomatic.9
Preoperative hemodynamic control is essential in the management of pheochromocytoma to prevent or minimize hemodynamic changes that can be driven by increased catecholamines. Control is typically achieved with initial alpha-blockade and then beta-blockade to avoid worsening hypertension and to prevent an acute hypertensive crisis during surgical intervention. Phenoxybenzamine, the mainstay of therapy, is a nonselective alpha-blocker with a long duration of action that requires titration over several days up to 3 weeks.
A selective alpha-1-blocker such as doxazosin can be used to control postoperative hypotension, as it has a shorter half-life than phenoxybenzamine. Alternative strategies include calcium channel blockers, centrally acting sympathetic blockers, and magnesium.18
Laparoscopic adrenalectomy by an experienced surgeon after excellent medical preparation is often considered the treatment of choice, but for larger or malignant masses, an open procedure is recommended. The risk of perioperative morbidity and death can be reduced by adequate medical management. With successful surgical resection, the long-term prognosis is favorable.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2008; 51:1403–1419.
- Kumar N, Calhoun DA, Dudenbostel T. Management of patients with resistant hypertension: current treatment options. Integr Blood Press Control 2013; 6:139–151.
- Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension 2011; 58:811–817.
- Marcus JA, Pothineni A, Marcus CZ, Bisognano JD. The role of obesity and obstructive sleep apnea in the pathogenesis and treatment of resistant hypertension. Curr Hypertens Rep 2014; 16:411.
- Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB, Weissmann P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension 2002; 40:892–896.
- Young WF Clinical practice. The incidentally discovered adrenal mass. N Engl J Med 2007; 356:601–610.
- Lin M, Wong V, Yap J, Jin R, Leong P, Campbell P. FDG PET in the evaluation of phaeochromocytoma: a correlative study with MIBG scintigraphy and Ki-67 proliferative index. Clin Imaging 2013; 37:1084–1088.
- Raja A, Leung K, Stamm M, Girgis S, Low G. Multimodality imaging findings of pheochromocytoma with associated clinical and biochemical features in 53 patients with histologically confirmed tumors. AJR Am J Roentgenol 2013; 201:825–833.
- Nieman LK. Approach to the patient with an adrenal incidentaloma. J Clin Endocrinol Metab 2010; 95:4106–4113.
- Stolk RF, Bakx C, Mulder J, Timmers HJ, Lenders JW. Is the excess cardiovascular morbidity in pheochromocytoma related to blood pressure or to catecholamines? J Clin Endocrinol Metab 2013; 98:1100–1106.
- Grumbach MM, Biller BM, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (‘incidentaloma’). Ann Intern Med 2003; 138:424–429.
- Lentschener C, Gaujoux S, Tesniere A, Dousset B. Point of controversy: perioperative care of patients undergoing pheochromocytoma removal—time for a reappraisal? Eur J Endocrinol 2011; 165:365–373.
- Bryant J, Farmer J, Kessler LJ, Townsend RR, Nathanson KL. Pheochromocytoma: the expanding genetic differential diagnosis. J Natl Cancer Inst 2003; 95:1196–1204.
- Lee P, Leonard J. Textbook on endocrinology. BMJ 1994; 308:1512.
- Fishbein L, Merrill S, Fraker DL, Cohen DL, Nathanson KL. Inherited mutations in pheochromocytoma and paraganglioma: why all patients should be offered genetic testing. Ann Surg Oncol 2013; 20:1444–1450.
- Agarwal G, Sadacharan D, Kapoor A, et al. Cardiovascular dysfunction and catecholamine cardiomyopathy in pheochromocytoma patients and their reversal following surgical cure: results of a prospective case-control study. Surgery 2011; 150:1202–1211.
- Sawka AM, Jaeschke R, Singh RJ, Young WF A comparison of biochemical tests for pheochromocytoma: measurement of fractionated plasma metanephrines compared with the combination of 24-hour urinary metanephrines and catecholamines. J Clin Endocrinol Metab 2003; 88:553–558.
- Domi R, Laho H. Management of pheochromocytoma: old ideas and new drugs. Niger J Clin Pract 2012; 15:253–257.
A 61-year-old man with type 2 diabetes mellitus on glimepiride therapy presented with somnolence and slurred speech. His capillary glucose level was 17 mg/dL and his serum glucose level was 28 mg/dL. He was treated with intravenous dextrose, and his glucose level promptly returned to normal.
He had been adherent to his medication regimen and denied overmedicating or accidental overdosing. Over the past 7 months, he had noted redness on his palms, a rash on his legs, intermittent moderate to severe headaches, weight loss, and decreased appetite. In addition, his blood pressure had been labile, which his physicians had attributed to autonomic instability. He had continued on the same dose of glimepiride despite losing weight.
His history included multivessel coronary artery disease treated with angioplasty and placement of multiple coronary stents; ischemic cardiomyopathy with a left ventricular ejection fraction of 28%; implantation of a cardioverter-defibrillator for secondary prevention of ventricular arrhythmia; an ischemic stroke; and multiple sclerosis complicated by bilateral blindness, with optic nerve involvement and autonomic instability, present for over a year and manifested by labile blood pressure. He was a long-time tobacco user. His daily medications included ticagrelor 90 mg, aspirin 81 mg, metoprolol 50 mg, ramipril 10 mg, simvastatin 20 mg, glimepiride 2 mg, and esomeprazole 40 mg. He needed help taking his medications.
At the time of hospital admission, his heart rate was 69 beats per minute with a regular rhythm, blood pressure 115/73 mm Hg, respiratory rate 11 breaths per minute with an oxygen saturation of 99% on room air, and oral temperature 34.7°C (94.5°F). He appeared to be in no distress.
Cardiovascular examination revealed no murmurs or gallops; there was mild nonpitting edema of the lower extremities. Pulmonary, abdominal, and neurologic examinations were unrevealing except for bilateral blindness. Vascular examination revealed no bruits. Results of a complete blood cell count and metabolic panel were normal except for a hemoglobin level of 9.9 g/dL (reference range 13.5–17.5) and a platelet count of 477 × 109/L (150–450).
Although he continued to receive the same medications he had been taking at home, his blood pressure fluctuated. On the second hospital day, it reached 186/135 mm Hg, at which time he also had palpitations, dyspnea, and crackles in the lower lobes of both lungs. Volume resuscitation on admission was suspected to have played a role, and he received furosemide, which improved his symptoms. But several hours later, his blood pressure rose again, and he became diaphoretic. Despite aggressive treatment with different antihypertensive agents, his blood pressure remained high and his symptoms persisted. Chest radiography showed no evidence of pulmonary edema. Because of his progressive dyspnea, the diagnosis of pulmonary embolism was entertained.
CAUSES OF RESISTANT HYPERTENSION
1. What could explain this patient’s high blood pressure?
- A drug effect
- Renovascular disease
- Excess circulating catecholamines
- Obstructive sleep apnea
- Primary aldosteronism
Sympathomimetic drugs such as epinephrine, norepinephrine, dopamine, and vasopressin, which are used when hemodynamic support is required, can raise both systolic and diastolic blood pressure. Nonsteroidal anti-inflammatory drugs and nasal decongestants are common culprits in the community. However, our patient was using none of these drugs.
Renovascular disease is one of many causes of resistant hypertension, accounting for 8% of all cases.1,2 Despite fluctuations, the blood pressure often remains chronically elevated, its changes are less paroxysmal than in our patient, and a precipitating factor such as a dietary indiscretion is sometimes identified.1
Excess circulating catecholamines can be a result of stress, exogenous administration, or endogenous oversecretion. Our patient’s clinical presentation is highly suspicious for a high-catecholamine state, and this should be further evaluated.
Obstructive sleep apnea is common in patients with resistant hypertension, with an estimated prevalence as high as 60% in this group.3,4
Primary aldosteronism has an estimated prevalence of about 20% in patients evaluated for resistant hypertension.5
AN ADRENAL MASS IS INCIDENTALLY DISCOVERED
Computed tomographic angiography of the chest revealed no evidence of pulmonary emboli. There was mild dilation of the central pulmonary arteries and an incidental, incompletely imaged 4.7-by-3.4-cm mass of mixed attenuation in the right adrenal gland, with macroscopic fat within the lesion.
Computed tomography (CT) of the abdomen with dedicated cuts through the adrenal glands revealed a 4.7-cm heterogeneous right adrenal mass with a density of 34 Hounsfield units (HU). The left adrenal gland appeared diffusely enlarged without a discretely seen mass, consistent with hyperplasticity (Figure 1).
2. Based on the patient’s clinical presentation and findings on CT, what would be the most likely diagnosis for this incidentally found adrenal mass?
- Adrenocortical adenoma
- Adrenocortical carcinoma
- Metastatic mass
- Pheochromocytoma
Adrenocortical adenoma can present as a small homogeneous mass of variable size, with smooth margins, and rarely containing hemorrhagic tissue or calcifications. The typical density on nonenhanced CT is less than 10 HU. On enhanced CT, it is nonvascular. T2-weighted magnetic resonance imaging (MRI) shows a lesion of the same intensity as liver tissue.6
Adrenocortical adenoma is not classically associated with autologous activity and thus is less likely to explain our patient’s symptoms.
Adrenocortical carcinoma can present as a large heterogeneous mass, usually greater than 4 cm in diameter, with irregular margins and areas of necrosis, hemorrhage, or calcification. The typical density on nonenhanced CT is greater than 10 HU. On enhanced CT, the mass is usually vascular, and T2-weighted MRI will show a lesion more intense than liver tissue.6
Adrenocortical carcinoma is also not classically associated with autologous activity, and so is not likely to explain our patient’s symptoms.6
Metastatic disease can present with masses of variable size, often bilaterally, and occasionally with cysts or areas of hemorrhage. The typical density of metastatic lesions on nonenhanced CT is greater than 10 HU. On enhanced CT, they are usually vascular, and on T2-weighted MRI they are hyperintense.6 The characteristics of the mass and the absence of a primary malignancy on CT of the chest and abdomen do not support the diagnosis of metastatic disease.
Pheochromocytoma is a neuroendocrine tumor of the adrenal medulla that can present as a large heterogeneous mass, greater than 3 cm in diameter, with clear margins and cysts or areas of hemorrhage. Extra-adrenal neuroendocrine tumors are typically called paragangliomas and have features similar to those of pheochromocytoma. The typical density of pheochromocytoma on nonenhanced CT is greater than 10 HU. On enhanced CT, it is usually vascular, and T2-weighted MRI shows a hyperintense lesion. Pheochromocytoma can be biochemically active and thus can cause signs and symptoms that will lead to the diagnosis.6
Other imaging tests may play a role in the evaluation of adrenal masses but are not required for the diagnosis of pheochromocytoma. Functional positron emission tomography using metaiodobenzylguanidine labeled with iodine 123 or-iodine 131 or using the glucose analogue F-18 fluorodeoxyglucose has been used in the initial assessment of pheochromocytoma, with good sensitivity and specificity.7,8
Our patient’s pacemaker-defibrillator precluded him from undergoing MRI.
DIAGNOSIS: PHEOCHROMOCYTOMA
Pheochromocytoma was highly suspected on the basis of the patient’s clinical presentation, and metoprolol was immediately discontinued. He was started on the calcium channel blocker verapamil and the alpha-blocker phenoxybenzamine.
Serum samples were obtained to measure metanephrines, dehydroepiandrosterone, aldosterone, and cortisol, and a 24-hour urine collection was obtained to measure creatinine, dopamine, epinephrine, norepinephrine, cortisol, and metanephrines. Based on the results (Table 1) and on the findings on imaging, the patient was diagnosed with pheochromocytoma. A surgical consultation was obtained, and surgery was recommended.
WHEN DOES PHEOCHROMOCYTOMA CALL FOR SURGERY?
3. Which criterion is most important when determining the need for surgery for pheochromocytoma?
- Findings on fine-needle aspiration biopsy
- Biochemical activity
- Size of the mass
- Bilateral masses
Fine-needle aspiration biopsy can be done when a mass is found incidentally and no evidence of biochemical activity is detected, although it is not an essential part of the diagnostic workup.9 In most cases, the sampling from fine-needle aspiration is not sufficient to achieve a diagnosis.
Biochemical activity is the most important factor when determining the need for prompt surgical intervention. The excess circulating catecholamines have been associated with increased risk of cardiovascular morbidity and death independent of the morbidity associated with hypertension alone.10 Biochemical activity can be independent of the size of the mass, but larger masses typically present with symptoms.
Bilateral masses have been associated with metastatic disease.11 In retrospect, the patient’s history of hypertension and cerebrovascular accident could be associated with the development of a catecholamine-releasing tumor.
A GOOD OUTCOME FROM SURGERY
The patient was continued on phenoxybenzamine for 7 days and responded well to this therapy.
After this preoperative preparation, he underwent laparoscopic right adrenalectomy with excision of a retroperitoneal adrenal mass. His postoperative course was complicated by transient hypotension requiring low-dose vasopressin support for less than 24 hours. He was then restarted on his previous dosage of metoprolol and was discharged home on postoperative day 5 with stable blood pressure. Follow-up 24-hour urine collection 1 month after he was discharged showed normalization of metanephrine, normetanephrine, epinephrine, and norepinephrine levels.
Despite low suspicion for an underlying genetic syndrome, he was referred for genetic testing and was scheduled to have a repeat 24-hour urine collection and imaging in 6 months to follow his enlarged left adrenal gland, which did not appear to be metabolically hyperactive.
4. What is the most common perioperative complication of pheochromocytoma excision?
- Hypoglycemia
- Hypotension
- Hypocortisolism
- Hypertension
- Tachycardia
Hypoglycemia has been observed after removal of pheochromocytoma, as levels of catecholamines (which normally inhibit pancreatic beta cells) decrease and insulin secretion consequently increases.12 Our patient developed hypoglycemia before surgery, not after, and it was likely due to the combination of his antidiabetic therapy, weight loss, and decreased oral intake.
Hypotension is the most common complication in the perioperative period. It is associated with excessive loss of catecholamine secretion. It is usually short-lived but may require aggressive administration of intravenous fluids and use of sympathomimetic agents.
Hypocortisolism is unlikely in patients with pheochromocytoma, but it is likely after removal of adrenocortical adenoma.
Hypertension and tachycardia affect up to 40% of pheochromocytoma patients in some case series.12
PHEOCHROMOCYTOMA: A CATECHOLAMINE-SECRETING TUMOR
The pathophysiology of pheochromocytoma is complex. It is characterized by accelerated growth of cells producing catecholamines, which may produce symptoms when secreted into the bloodstream. The classic triad of symptoms is headache, hypertension, and hyperglycemia, although our patient had very low blood sugar levels. Other common symptoms are nausea, orthostasis, and tremor, although not all symptoms are invariably seen.
Genetic testing recommended
Genetic associations have been described and are thought to be responsible for 20% to 30% of cases of pheochromocytoma. All associated germline mutations are autosomal dominant, some with variable penetrance. These include:
- Succinate dehydrogenase subunit B, C, and D mutations
- von Hippel-Lindau syndrome
- Multiple endocrine neoplasia type 1 and type 2 syndromes
- Neurofibromatosis type 1.13,14
The succinate dehydrogenase subunit mutations have been associated with, but not limited to, extra-adrenal adenomas (paragangliomas) and carry a worse prognosis.
Some experts recommend genetic testing in all cases of pheochromocytoma, sporadic or familial, and this testing should be followed by counseling if a mutation is found.15 Others recommend genetic testing based on the patient’s age (under age 50), history, imaging, and biochemical features of the tumor (metanephrines predominate in multiple endocrine neoplasia syndromes, and normetanephrines in von Hippel-Lindau syndrome).13
Serious consequences
A thorough evaluation is recommended, since pheochromocytoma has been associated with increased cardiovascular morbidity. In a retrospective series, Stolk et al10 reported that patients with pheochromocytoma had a higher incidence of myocardial infarction, angina, and stroke in the years preceding the diagnosis than did patients with essential hypertension (13.8% vs 1.1%, P < .001).10
Catecholamine cardiomyopathy has been described and shares clinical features with Takotsubo or stress cardiomyopathy, with global left ventricular systolic and diastolic dysfunction that improve or resolve after the adrenergic insult is removed.16
Conditions that warrant further evaluation or that may suggest pheochromocytoma are malignant hypertension, hypertensive encephalopathy, ischemic stroke, subarachnoid hemorrhage, acute pulmonary edema, angina pectoris, myocardial infarction, aortic dissection, and kidney injury.
When to suspect pheochromocytoma
Pheochromocytoma should be suspected in a patient with resistant hypertension, family history, or imaging findings that suggest an adrenal mass with a heterogeneous appearance. The diagnostic algorithm follows the same pathway as for the evaluation of an incidentally found adrenal mass, with determination of its dimension and characteristics by CT or MRI, and with biochemical testing of urine catecholamines, plasma free metanephrines, renin, aldosterone, and cortisol.
The diagnosis of pheochromocytoma is established by obtaining fractionated metanephrines and catecholamines in a 24-hour urine collection (sensitivity 90%, specificity 98%). Analysis of plasma metanephrines has a higher sensitivity (97%) but lower specificity (85%).17 The combination of typical signs, symptoms, and laboratory findings makes the diagnosis likely, especially in combination with a unilateral adrenal mass.
Laparoscopic surgery after medical preparation for active tumors
If the mass appears benign and not biochemically hyperactive, then follow-up at 1 year is recommended, with repeat testing. Surgical evaluation and intervention is recommended for lesions that appear malignant or that are biochemically active and clinically symptomatic.9
Preoperative hemodynamic control is essential in the management of pheochromocytoma to prevent or minimize hemodynamic changes that can be driven by increased catecholamines. Control is typically achieved with initial alpha-blockade and then beta-blockade to avoid worsening hypertension and to prevent an acute hypertensive crisis during surgical intervention. Phenoxybenzamine, the mainstay of therapy, is a nonselective alpha-blocker with a long duration of action that requires titration over several days up to 3 weeks.
A selective alpha-1-blocker such as doxazosin can be used to control postoperative hypotension, as it has a shorter half-life than phenoxybenzamine. Alternative strategies include calcium channel blockers, centrally acting sympathetic blockers, and magnesium.18
Laparoscopic adrenalectomy by an experienced surgeon after excellent medical preparation is often considered the treatment of choice, but for larger or malignant masses, an open procedure is recommended. The risk of perioperative morbidity and death can be reduced by adequate medical management. With successful surgical resection, the long-term prognosis is favorable.
A 61-year-old man with type 2 diabetes mellitus on glimepiride therapy presented with somnolence and slurred speech. His capillary glucose level was 17 mg/dL and his serum glucose level was 28 mg/dL. He was treated with intravenous dextrose, and his glucose level promptly returned to normal.
He had been adherent to his medication regimen and denied overmedicating or accidental overdosing. Over the past 7 months, he had noted redness on his palms, a rash on his legs, intermittent moderate to severe headaches, weight loss, and decreased appetite. In addition, his blood pressure had been labile, which his physicians had attributed to autonomic instability. He had continued on the same dose of glimepiride despite losing weight.
His history included multivessel coronary artery disease treated with angioplasty and placement of multiple coronary stents; ischemic cardiomyopathy with a left ventricular ejection fraction of 28%; implantation of a cardioverter-defibrillator for secondary prevention of ventricular arrhythmia; an ischemic stroke; and multiple sclerosis complicated by bilateral blindness, with optic nerve involvement and autonomic instability, present for over a year and manifested by labile blood pressure. He was a long-time tobacco user. His daily medications included ticagrelor 90 mg, aspirin 81 mg, metoprolol 50 mg, ramipril 10 mg, simvastatin 20 mg, glimepiride 2 mg, and esomeprazole 40 mg. He needed help taking his medications.
At the time of hospital admission, his heart rate was 69 beats per minute with a regular rhythm, blood pressure 115/73 mm Hg, respiratory rate 11 breaths per minute with an oxygen saturation of 99% on room air, and oral temperature 34.7°C (94.5°F). He appeared to be in no distress.
Cardiovascular examination revealed no murmurs or gallops; there was mild nonpitting edema of the lower extremities. Pulmonary, abdominal, and neurologic examinations were unrevealing except for bilateral blindness. Vascular examination revealed no bruits. Results of a complete blood cell count and metabolic panel were normal except for a hemoglobin level of 9.9 g/dL (reference range 13.5–17.5) and a platelet count of 477 × 109/L (150–450).
Although he continued to receive the same medications he had been taking at home, his blood pressure fluctuated. On the second hospital day, it reached 186/135 mm Hg, at which time he also had palpitations, dyspnea, and crackles in the lower lobes of both lungs. Volume resuscitation on admission was suspected to have played a role, and he received furosemide, which improved his symptoms. But several hours later, his blood pressure rose again, and he became diaphoretic. Despite aggressive treatment with different antihypertensive agents, his blood pressure remained high and his symptoms persisted. Chest radiography showed no evidence of pulmonary edema. Because of his progressive dyspnea, the diagnosis of pulmonary embolism was entertained.
CAUSES OF RESISTANT HYPERTENSION
1. What could explain this patient’s high blood pressure?
- A drug effect
- Renovascular disease
- Excess circulating catecholamines
- Obstructive sleep apnea
- Primary aldosteronism
Sympathomimetic drugs such as epinephrine, norepinephrine, dopamine, and vasopressin, which are used when hemodynamic support is required, can raise both systolic and diastolic blood pressure. Nonsteroidal anti-inflammatory drugs and nasal decongestants are common culprits in the community. However, our patient was using none of these drugs.
Renovascular disease is one of many causes of resistant hypertension, accounting for 8% of all cases.1,2 Despite fluctuations, the blood pressure often remains chronically elevated, its changes are less paroxysmal than in our patient, and a precipitating factor such as a dietary indiscretion is sometimes identified.1
Excess circulating catecholamines can be a result of stress, exogenous administration, or endogenous oversecretion. Our patient’s clinical presentation is highly suspicious for a high-catecholamine state, and this should be further evaluated.
Obstructive sleep apnea is common in patients with resistant hypertension, with an estimated prevalence as high as 60% in this group.3,4
Primary aldosteronism has an estimated prevalence of about 20% in patients evaluated for resistant hypertension.5
AN ADRENAL MASS IS INCIDENTALLY DISCOVERED
Computed tomographic angiography of the chest revealed no evidence of pulmonary emboli. There was mild dilation of the central pulmonary arteries and an incidental, incompletely imaged 4.7-by-3.4-cm mass of mixed attenuation in the right adrenal gland, with macroscopic fat within the lesion.
Computed tomography (CT) of the abdomen with dedicated cuts through the adrenal glands revealed a 4.7-cm heterogeneous right adrenal mass with a density of 34 Hounsfield units (HU). The left adrenal gland appeared diffusely enlarged without a discretely seen mass, consistent with hyperplasticity (Figure 1).
2. Based on the patient’s clinical presentation and findings on CT, what would be the most likely diagnosis for this incidentally found adrenal mass?
- Adrenocortical adenoma
- Adrenocortical carcinoma
- Metastatic mass
- Pheochromocytoma
Adrenocortical adenoma can present as a small homogeneous mass of variable size, with smooth margins, and rarely containing hemorrhagic tissue or calcifications. The typical density on nonenhanced CT is less than 10 HU. On enhanced CT, it is nonvascular. T2-weighted magnetic resonance imaging (MRI) shows a lesion of the same intensity as liver tissue.6
Adrenocortical adenoma is not classically associated with autologous activity and thus is less likely to explain our patient’s symptoms.
Adrenocortical carcinoma can present as a large heterogeneous mass, usually greater than 4 cm in diameter, with irregular margins and areas of necrosis, hemorrhage, or calcification. The typical density on nonenhanced CT is greater than 10 HU. On enhanced CT, the mass is usually vascular, and T2-weighted MRI will show a lesion more intense than liver tissue.6
Adrenocortical carcinoma is also not classically associated with autologous activity, and so is not likely to explain our patient’s symptoms.6
Metastatic disease can present with masses of variable size, often bilaterally, and occasionally with cysts or areas of hemorrhage. The typical density of metastatic lesions on nonenhanced CT is greater than 10 HU. On enhanced CT, they are usually vascular, and on T2-weighted MRI they are hyperintense.6 The characteristics of the mass and the absence of a primary malignancy on CT of the chest and abdomen do not support the diagnosis of metastatic disease.
Pheochromocytoma is a neuroendocrine tumor of the adrenal medulla that can present as a large heterogeneous mass, greater than 3 cm in diameter, with clear margins and cysts or areas of hemorrhage. Extra-adrenal neuroendocrine tumors are typically called paragangliomas and have features similar to those of pheochromocytoma. The typical density of pheochromocytoma on nonenhanced CT is greater than 10 HU. On enhanced CT, it is usually vascular, and T2-weighted MRI shows a hyperintense lesion. Pheochromocytoma can be biochemically active and thus can cause signs and symptoms that will lead to the diagnosis.6
Other imaging tests may play a role in the evaluation of adrenal masses but are not required for the diagnosis of pheochromocytoma. Functional positron emission tomography using metaiodobenzylguanidine labeled with iodine 123 or-iodine 131 or using the glucose analogue F-18 fluorodeoxyglucose has been used in the initial assessment of pheochromocytoma, with good sensitivity and specificity.7,8
Our patient’s pacemaker-defibrillator precluded him from undergoing MRI.
DIAGNOSIS: PHEOCHROMOCYTOMA
Pheochromocytoma was highly suspected on the basis of the patient’s clinical presentation, and metoprolol was immediately discontinued. He was started on the calcium channel blocker verapamil and the alpha-blocker phenoxybenzamine.
Serum samples were obtained to measure metanephrines, dehydroepiandrosterone, aldosterone, and cortisol, and a 24-hour urine collection was obtained to measure creatinine, dopamine, epinephrine, norepinephrine, cortisol, and metanephrines. Based on the results (Table 1) and on the findings on imaging, the patient was diagnosed with pheochromocytoma. A surgical consultation was obtained, and surgery was recommended.
WHEN DOES PHEOCHROMOCYTOMA CALL FOR SURGERY?
3. Which criterion is most important when determining the need for surgery for pheochromocytoma?
- Findings on fine-needle aspiration biopsy
- Biochemical activity
- Size of the mass
- Bilateral masses
Fine-needle aspiration biopsy can be done when a mass is found incidentally and no evidence of biochemical activity is detected, although it is not an essential part of the diagnostic workup.9 In most cases, the sampling from fine-needle aspiration is not sufficient to achieve a diagnosis.
Biochemical activity is the most important factor when determining the need for prompt surgical intervention. The excess circulating catecholamines have been associated with increased risk of cardiovascular morbidity and death independent of the morbidity associated with hypertension alone.10 Biochemical activity can be independent of the size of the mass, but larger masses typically present with symptoms.
Bilateral masses have been associated with metastatic disease.11 In retrospect, the patient’s history of hypertension and cerebrovascular accident could be associated with the development of a catecholamine-releasing tumor.
A GOOD OUTCOME FROM SURGERY
The patient was continued on phenoxybenzamine for 7 days and responded well to this therapy.
After this preoperative preparation, he underwent laparoscopic right adrenalectomy with excision of a retroperitoneal adrenal mass. His postoperative course was complicated by transient hypotension requiring low-dose vasopressin support for less than 24 hours. He was then restarted on his previous dosage of metoprolol and was discharged home on postoperative day 5 with stable blood pressure. Follow-up 24-hour urine collection 1 month after he was discharged showed normalization of metanephrine, normetanephrine, epinephrine, and norepinephrine levels.
Despite low suspicion for an underlying genetic syndrome, he was referred for genetic testing and was scheduled to have a repeat 24-hour urine collection and imaging in 6 months to follow his enlarged left adrenal gland, which did not appear to be metabolically hyperactive.
4. What is the most common perioperative complication of pheochromocytoma excision?
- Hypoglycemia
- Hypotension
- Hypocortisolism
- Hypertension
- Tachycardia
Hypoglycemia has been observed after removal of pheochromocytoma, as levels of catecholamines (which normally inhibit pancreatic beta cells) decrease and insulin secretion consequently increases.12 Our patient developed hypoglycemia before surgery, not after, and it was likely due to the combination of his antidiabetic therapy, weight loss, and decreased oral intake.
Hypotension is the most common complication in the perioperative period. It is associated with excessive loss of catecholamine secretion. It is usually short-lived but may require aggressive administration of intravenous fluids and use of sympathomimetic agents.
Hypocortisolism is unlikely in patients with pheochromocytoma, but it is likely after removal of adrenocortical adenoma.
Hypertension and tachycardia affect up to 40% of pheochromocytoma patients in some case series.12
PHEOCHROMOCYTOMA: A CATECHOLAMINE-SECRETING TUMOR
The pathophysiology of pheochromocytoma is complex. It is characterized by accelerated growth of cells producing catecholamines, which may produce symptoms when secreted into the bloodstream. The classic triad of symptoms is headache, hypertension, and hyperglycemia, although our patient had very low blood sugar levels. Other common symptoms are nausea, orthostasis, and tremor, although not all symptoms are invariably seen.
Genetic testing recommended
Genetic associations have been described and are thought to be responsible for 20% to 30% of cases of pheochromocytoma. All associated germline mutations are autosomal dominant, some with variable penetrance. These include:
- Succinate dehydrogenase subunit B, C, and D mutations
- von Hippel-Lindau syndrome
- Multiple endocrine neoplasia type 1 and type 2 syndromes
- Neurofibromatosis type 1.13,14
The succinate dehydrogenase subunit mutations have been associated with, but not limited to, extra-adrenal adenomas (paragangliomas) and carry a worse prognosis.
Some experts recommend genetic testing in all cases of pheochromocytoma, sporadic or familial, and this testing should be followed by counseling if a mutation is found.15 Others recommend genetic testing based on the patient’s age (under age 50), history, imaging, and biochemical features of the tumor (metanephrines predominate in multiple endocrine neoplasia syndromes, and normetanephrines in von Hippel-Lindau syndrome).13
Serious consequences
A thorough evaluation is recommended, since pheochromocytoma has been associated with increased cardiovascular morbidity. In a retrospective series, Stolk et al10 reported that patients with pheochromocytoma had a higher incidence of myocardial infarction, angina, and stroke in the years preceding the diagnosis than did patients with essential hypertension (13.8% vs 1.1%, P < .001).10
Catecholamine cardiomyopathy has been described and shares clinical features with Takotsubo or stress cardiomyopathy, with global left ventricular systolic and diastolic dysfunction that improve or resolve after the adrenergic insult is removed.16
Conditions that warrant further evaluation or that may suggest pheochromocytoma are malignant hypertension, hypertensive encephalopathy, ischemic stroke, subarachnoid hemorrhage, acute pulmonary edema, angina pectoris, myocardial infarction, aortic dissection, and kidney injury.
When to suspect pheochromocytoma
Pheochromocytoma should be suspected in a patient with resistant hypertension, family history, or imaging findings that suggest an adrenal mass with a heterogeneous appearance. The diagnostic algorithm follows the same pathway as for the evaluation of an incidentally found adrenal mass, with determination of its dimension and characteristics by CT or MRI, and with biochemical testing of urine catecholamines, plasma free metanephrines, renin, aldosterone, and cortisol.
The diagnosis of pheochromocytoma is established by obtaining fractionated metanephrines and catecholamines in a 24-hour urine collection (sensitivity 90%, specificity 98%). Analysis of plasma metanephrines has a higher sensitivity (97%) but lower specificity (85%).17 The combination of typical signs, symptoms, and laboratory findings makes the diagnosis likely, especially in combination with a unilateral adrenal mass.
Laparoscopic surgery after medical preparation for active tumors
If the mass appears benign and not biochemically hyperactive, then follow-up at 1 year is recommended, with repeat testing. Surgical evaluation and intervention is recommended for lesions that appear malignant or that are biochemically active and clinically symptomatic.9
Preoperative hemodynamic control is essential in the management of pheochromocytoma to prevent or minimize hemodynamic changes that can be driven by increased catecholamines. Control is typically achieved with initial alpha-blockade and then beta-blockade to avoid worsening hypertension and to prevent an acute hypertensive crisis during surgical intervention. Phenoxybenzamine, the mainstay of therapy, is a nonselective alpha-blocker with a long duration of action that requires titration over several days up to 3 weeks.
A selective alpha-1-blocker such as doxazosin can be used to control postoperative hypotension, as it has a shorter half-life than phenoxybenzamine. Alternative strategies include calcium channel blockers, centrally acting sympathetic blockers, and magnesium.18
Laparoscopic adrenalectomy by an experienced surgeon after excellent medical preparation is often considered the treatment of choice, but for larger or malignant masses, an open procedure is recommended. The risk of perioperative morbidity and death can be reduced by adequate medical management. With successful surgical resection, the long-term prognosis is favorable.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2008; 51:1403–1419.
- Kumar N, Calhoun DA, Dudenbostel T. Management of patients with resistant hypertension: current treatment options. Integr Blood Press Control 2013; 6:139–151.
- Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension 2011; 58:811–817.
- Marcus JA, Pothineni A, Marcus CZ, Bisognano JD. The role of obesity and obstructive sleep apnea in the pathogenesis and treatment of resistant hypertension. Curr Hypertens Rep 2014; 16:411.
- Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB, Weissmann P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension 2002; 40:892–896.
- Young WF Clinical practice. The incidentally discovered adrenal mass. N Engl J Med 2007; 356:601–610.
- Lin M, Wong V, Yap J, Jin R, Leong P, Campbell P. FDG PET in the evaluation of phaeochromocytoma: a correlative study with MIBG scintigraphy and Ki-67 proliferative index. Clin Imaging 2013; 37:1084–1088.
- Raja A, Leung K, Stamm M, Girgis S, Low G. Multimodality imaging findings of pheochromocytoma with associated clinical and biochemical features in 53 patients with histologically confirmed tumors. AJR Am J Roentgenol 2013; 201:825–833.
- Nieman LK. Approach to the patient with an adrenal incidentaloma. J Clin Endocrinol Metab 2010; 95:4106–4113.
- Stolk RF, Bakx C, Mulder J, Timmers HJ, Lenders JW. Is the excess cardiovascular morbidity in pheochromocytoma related to blood pressure or to catecholamines? J Clin Endocrinol Metab 2013; 98:1100–1106.
- Grumbach MM, Biller BM, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (‘incidentaloma’). Ann Intern Med 2003; 138:424–429.
- Lentschener C, Gaujoux S, Tesniere A, Dousset B. Point of controversy: perioperative care of patients undergoing pheochromocytoma removal—time for a reappraisal? Eur J Endocrinol 2011; 165:365–373.
- Bryant J, Farmer J, Kessler LJ, Townsend RR, Nathanson KL. Pheochromocytoma: the expanding genetic differential diagnosis. J Natl Cancer Inst 2003; 95:1196–1204.
- Lee P, Leonard J. Textbook on endocrinology. BMJ 1994; 308:1512.
- Fishbein L, Merrill S, Fraker DL, Cohen DL, Nathanson KL. Inherited mutations in pheochromocytoma and paraganglioma: why all patients should be offered genetic testing. Ann Surg Oncol 2013; 20:1444–1450.
- Agarwal G, Sadacharan D, Kapoor A, et al. Cardiovascular dysfunction and catecholamine cardiomyopathy in pheochromocytoma patients and their reversal following surgical cure: results of a prospective case-control study. Surgery 2011; 150:1202–1211.
- Sawka AM, Jaeschke R, Singh RJ, Young WF A comparison of biochemical tests for pheochromocytoma: measurement of fractionated plasma metanephrines compared with the combination of 24-hour urinary metanephrines and catecholamines. J Clin Endocrinol Metab 2003; 88:553–558.
- Domi R, Laho H. Management of pheochromocytoma: old ideas and new drugs. Niger J Clin Pract 2012; 15:253–257.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2008; 51:1403–1419.
- Kumar N, Calhoun DA, Dudenbostel T. Management of patients with resistant hypertension: current treatment options. Integr Blood Press Control 2013; 6:139–151.
- Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension 2011; 58:811–817.
- Marcus JA, Pothineni A, Marcus CZ, Bisognano JD. The role of obesity and obstructive sleep apnea in the pathogenesis and treatment of resistant hypertension. Curr Hypertens Rep 2014; 16:411.
- Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB, Weissmann P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension 2002; 40:892–896.
- Young WF Clinical practice. The incidentally discovered adrenal mass. N Engl J Med 2007; 356:601–610.
- Lin M, Wong V, Yap J, Jin R, Leong P, Campbell P. FDG PET in the evaluation of phaeochromocytoma: a correlative study with MIBG scintigraphy and Ki-67 proliferative index. Clin Imaging 2013; 37:1084–1088.
- Raja A, Leung K, Stamm M, Girgis S, Low G. Multimodality imaging findings of pheochromocytoma with associated clinical and biochemical features in 53 patients with histologically confirmed tumors. AJR Am J Roentgenol 2013; 201:825–833.
- Nieman LK. Approach to the patient with an adrenal incidentaloma. J Clin Endocrinol Metab 2010; 95:4106–4113.
- Stolk RF, Bakx C, Mulder J, Timmers HJ, Lenders JW. Is the excess cardiovascular morbidity in pheochromocytoma related to blood pressure or to catecholamines? J Clin Endocrinol Metab 2013; 98:1100–1106.
- Grumbach MM, Biller BM, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (‘incidentaloma’). Ann Intern Med 2003; 138:424–429.
- Lentschener C, Gaujoux S, Tesniere A, Dousset B. Point of controversy: perioperative care of patients undergoing pheochromocytoma removal—time for a reappraisal? Eur J Endocrinol 2011; 165:365–373.
- Bryant J, Farmer J, Kessler LJ, Townsend RR, Nathanson KL. Pheochromocytoma: the expanding genetic differential diagnosis. J Natl Cancer Inst 2003; 95:1196–1204.
- Lee P, Leonard J. Textbook on endocrinology. BMJ 1994; 308:1512.
- Fishbein L, Merrill S, Fraker DL, Cohen DL, Nathanson KL. Inherited mutations in pheochromocytoma and paraganglioma: why all patients should be offered genetic testing. Ann Surg Oncol 2013; 20:1444–1450.
- Agarwal G, Sadacharan D, Kapoor A, et al. Cardiovascular dysfunction and catecholamine cardiomyopathy in pheochromocytoma patients and their reversal following surgical cure: results of a prospective case-control study. Surgery 2011; 150:1202–1211.
- Sawka AM, Jaeschke R, Singh RJ, Young WF A comparison of biochemical tests for pheochromocytoma: measurement of fractionated plasma metanephrines compared with the combination of 24-hour urinary metanephrines and catecholamines. J Clin Endocrinol Metab 2003; 88:553–558.
- Domi R, Laho H. Management of pheochromocytoma: old ideas and new drugs. Niger J Clin Pract 2012; 15:253–257.
Should all patients have a resting 12-lead ECG before elective noncardiac surgery?
A 55-year-old lawyer with hypertension well controlled on lisinopril and amlodipine is scheduled for elective hernia repair under general anesthesia. His surgeon has referred him for a preoperative evaluation. He has never smoked, runs 4 miles on days off from work, and enjoys long hiking trips. On examination, his body mass index is 26 kg/m2 and his blood pressure is 130/78 mm Hg; his cardiac examination and the rest of the clinical examination are unremarkable. He asks if he should have an electrocardiogram (ECG) as a part of his workup.
A preoperative ECG is not routinely recommended in all asymptomatic patients undergoing noncardiac surgery.
Consider obtaining an ECG in patients planning to undergo a high-risk surgical procedure, especially if they have one or more clinical risk factors for coronary artery disease, and in patients undergoing elevated-cardiac-risk surgery who are known to have coronary artery disease, chronic heart failure, peripheral arterial disease, or cerebrovascular disease. However, a preoperative ECG is not routinely recommended for patients perceived to be at low cardiac risk who are planning to undergo low-risk surgery. In those patients it could delay surgery unnecessarily, cause further unnecessary testing, drive up costs, and increase patient anxiety.
Here we discuss the perioperative cardiac risk based on type of surgery and patient characteristics and summarize the current guidelines and recommendations on obtaining a preoperative 12-lead ECG in patients undergoing noncardiac surgery.
RISK DEPENDS ON TYPE OF SURGERY AND PATIENT FACTORS
Physicians, including primary care physicians, hospitalists, cardiologists, and anesthesiologists, are routinely asked to evaluate patients before surgical procedures. The purpose of the preoperative evaluation is to optimize existing medical conditions, to identify undiagnosed conditions that can increase risk of perioperative morbidity and death, and to suggest strategies to mitigate risk.1,2
Cardiac risk is multifactorial, and risk factors for postoperative adverse cardiac events include the type of surgery and patient factors.1,3
Cardiac risk based on type of surgery
Low-risk procedures are those in which the risk of a perioperative major adverse cardiac event is less than 1%.1,4 Examples:
- Ambulatory surgery
- Breast or plastic surgery
- Cataract surgery
- Endoscopic procedures.
Elevated-risk procedures are those in which the risk is 1% or higher. Examples:
- Intraperitoneal surgery
- Intrathoracic surgery
- Carotid endarterectomy
- Head and neck surgery
- Orthopedic surgery
- Prostate surgery
- Aortic surgery
- Major vascular surgery
- Peripheral arterial surgery.
Cardiac risk based on patient factors
The 2014 American College of Cardiology and American Heart Association (ACC/AHA) perioperative guidelines list a number of clinical risk factors for perioperative cardiac morbidity and death.1 These include coronary artery disease, chronic heart failure, clinically suspected moderate or greater degrees of valvular heart disease, arrhythmias, conduction disorders, pulmonary vascular disease, and adult congenital heart disease.
Patients with these conditions and patients with unstable coronary syndromes warrant preoperative ECGs and sometimes even urgent interventions before any nonemergency surgery, provided such interventions would affect decision-making and perioperative care.1
The risk of perioperative cardiac morbidity and death can be calculated using either the Revised Cardiac Risk Index scoring system or the American College of Surgeons National Surgical Quality Improvement Program calculator.157 The former is fairly simple, validated, and accepted, while the latter requires use of online calculators (eg, www.surgicalriskcalculator.com/miorcardiacarrest, www.riskcalculator.facs.org).
The Revised Cardiac Risk Index has six clinical predictors of major perioperative cardiac events:
- History of cerebrovascular disease
- Prior or current compensated congestive heart failure
- History of coronary artery disease
- Insulin-dependent diabetes mellitus
- Renal insufficiency, defined as a serum creatinine level of 2 mg/dL or higher
- Patient undergoing suprainguinal vascular, intraperitoneal, or intrathoracic surgery.
A patient who has 0 or 1 of these predictors would have a low risk of a major adverse cardiac event, whereas a patient with 2 or more would have elevated risk. These risk factors must be taken into consideration to determine the need, if any, for a preoperative ECG.
What an ECG can tell us
Abnormalities such as left ventricular hypertrophy, ST-segment depression, and pathologic Q waves on a preoperative ECG in a patient undergoing an elevated-risk surgical procedure may predict adverse perioperative cardiac events.3,6
In a retrospective study of 23,036 patients, Noordzij et al7 found that in patients undergoing elevated-risk surgery, those with an abnormal preoperative ECG had a higher incidence of cardiovascular death than those with a normal ECG. However, a preoperative ECG was obtained only in patients with established coronary artery disease or risk factors for cardiovascular disease. Hence, although an abnormal ECG in such patients undergoing elevated-risk surgery was predictive of adverse postoperative cardiac outcomes, we cannot say that the same would apply to patients without these characteristics undergoing elevated-risk surgery.
In a prospective observational study of patients with known coronary artery disease undergoing major noncardiac surgery, a preoperative ECG was found to contain prognostic information and was predictive of long-term outcome independent of clinical findings and perioperative ischemia.8
CURRENT GUIDELINES AND RECOMMENDATIONS
Several guidelines address whether to order a preoperative ECG but are mostly based on low-level evidence and expert opinion.1,2,6,9
Current guidelines recommend obtaining a preoperative ECG in patients with known coronary, peripheral arterial, or cerebrovascular disease.1,6,9
Obesity and associated comorbidities such as coronary artery disease, heart failure, systemic hypertension, and sleep apnea can predispose to increased perioperative complications. A preoperative 12-lead ECG is reasonable in morbidly obese patients (body mass index ≥ 40 kg/m2) and in obese patients (body mass index ≥ 30 kg/m2) with at least one risk factor for coronary artery disease or poor exercise tolerance, or both.10
Liu et al11 looked at the predictive value of a preoperative 12-lead ECG in 513 elderly patients (age ≥ 70) undergoing noncardiac surgery and found that electrocardiographic abnormalities were not predictive of adverse cardiac outcomes. In this study, although electrocardiographic abnormalities were common (noted in 75% of the patients), they were nonspecific and less useful in predicting postoperative cardiac complications than was the presence of comorbidities.11 Age alone as a cutoff for obtaining a preoperative ECG is not predictive of postoperative outcomes and a preoperative ECG is not warranted in all elderly patients. This is also reflected in current ACC/AHA guidelines on perioperative cardiovascular evaluation1 and is a change from prior ACC/AHA guidelines when age was used as a criterion for preoperative ECGs.12
Current guidelines do not recommend getting a preoperative ECG in asymptomatic patients undergoing low-cardiac-risk surgery.1,4,9
Although the ideal time for ordering an ECG before a planned surgery is unknown, obtaining one within 90 days before the surgery is considered adequate in stable patients in whom an ECG is indicated.1
BACK TO OUR PATIENT
On the basis of current evidence, our patient does not need a preoperative ECG, as it is unlikely to alter his perioperative management and instead may delay his surgery unnecessarily if any nonspecific changes prompt further cardiac workup.
CLINICAL BOTTOM LINE
Although frequently ordered in clinical practice, preoperative electrocardiography has a limited role in predicting postoperative outcome and should be ordered only in the appropriate clinical setting.1 Moreover, there is little evidence that outcomes are better if we obtain an ECG before surgery. The clinician should consider patient factors and the type of surgery before ordering diagnostic tests, including electrocardiography.
In asymptomatic patients undergoing nonemergent surgery:
- It is reasonable to obtain a preoperative ECG in patients with known coronary artery disease, significant arrhythmia, peripheral arterial disease, cerebrovascular disease, chronic heart failure, or other significant structural heart disease undergoing elevated-cardiac-risk surgery.
- Do not order a preoperative ECG in asymptomatic patients undergoing low-risk surgery.
- Obtaining a preoperative ECG is reasonable in morbidly obese patients and in obese patients with one or more risk factors for coronary artery disease, or poor exercise tolerance, undergoing high-risk surgery.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. J Am Coll Cardiol 2014; Jul 29. 10.1016/j.jacc.2014.07.944. [Epub ahead of print]
- Feely MA, Collins CS, Daniels PR, Kebede EB, Jatoi A, Mauck KF. Preoperative testing before noncardiac surgery: guidelines and recommendations. Am Fam Physician 2013; 87:414–418.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Task Force for Preoperative Cardiac Risk Assessment and Perioperative Cardiac Management in Non-cardiac Surgery; European Society of Cardiology (ESC); Poldermans D, Bax JJ, Boersma E, et al. Guidelines for pre-operative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery. Eur Heart J 2009; 30:2769–2812.
- Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217:833–842.
- Landesberg G, Einav S, Christopherson R, et al. Perioperative ischemia and cardiac complications in major vascular surgery: importance of the preoperative twelve-lead electrocardiogram. J Vasc Surg 1997; 26:570–578.
- Noordzij PG, Boersma E, Bax JJ, et al. Prognostic value of routine preoperative electrocardiography in patients undergoing noncardiac surgery. Am J Cardiol 2006; 97:1103–1106.
- Jeger RV, Probst C, Arsenic R, et al. Long-term prognostic value of the preoperative 12-lead electrocardiogram before major noncardiac surgery in coronary artery disease. Am Heart J 2006; 151:508–513.
- Committee on Standards and Practice Parameters; Apfelbaum JL, Connis RT, Nickinovich DG, Pasternak LR, Arens JF, Caplan RA, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology 2012; 116:522–538.
- Poirier P, Alpert MA, Fleisher LA, et al. Cardiovascular evaluation and management of severely obese patients undergoing surgery: a science advisory from the American Heart Association. Circulation 2009; 120:86–95.
- Liu LL, Dzankic S, Leung JM. Preoperative electrocardiogram abnormalities do not predict postoperative cardiac complications in geriatric surgical patients. J Am Geriatr Soc 2002; 50:1186–1191.
- Eagle KA, Berger PB, Calkins H, et al; American College of Cardiology; American Heart Association. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary. J Am Coll Cardiol 2002; 39:542–553.
A 55-year-old lawyer with hypertension well controlled on lisinopril and amlodipine is scheduled for elective hernia repair under general anesthesia. His surgeon has referred him for a preoperative evaluation. He has never smoked, runs 4 miles on days off from work, and enjoys long hiking trips. On examination, his body mass index is 26 kg/m2 and his blood pressure is 130/78 mm Hg; his cardiac examination and the rest of the clinical examination are unremarkable. He asks if he should have an electrocardiogram (ECG) as a part of his workup.
A preoperative ECG is not routinely recommended in all asymptomatic patients undergoing noncardiac surgery.
Consider obtaining an ECG in patients planning to undergo a high-risk surgical procedure, especially if they have one or more clinical risk factors for coronary artery disease, and in patients undergoing elevated-cardiac-risk surgery who are known to have coronary artery disease, chronic heart failure, peripheral arterial disease, or cerebrovascular disease. However, a preoperative ECG is not routinely recommended for patients perceived to be at low cardiac risk who are planning to undergo low-risk surgery. In those patients it could delay surgery unnecessarily, cause further unnecessary testing, drive up costs, and increase patient anxiety.
Here we discuss the perioperative cardiac risk based on type of surgery and patient characteristics and summarize the current guidelines and recommendations on obtaining a preoperative 12-lead ECG in patients undergoing noncardiac surgery.
RISK DEPENDS ON TYPE OF SURGERY AND PATIENT FACTORS
Physicians, including primary care physicians, hospitalists, cardiologists, and anesthesiologists, are routinely asked to evaluate patients before surgical procedures. The purpose of the preoperative evaluation is to optimize existing medical conditions, to identify undiagnosed conditions that can increase risk of perioperative morbidity and death, and to suggest strategies to mitigate risk.1,2
Cardiac risk is multifactorial, and risk factors for postoperative adverse cardiac events include the type of surgery and patient factors.1,3
Cardiac risk based on type of surgery
Low-risk procedures are those in which the risk of a perioperative major adverse cardiac event is less than 1%.1,4 Examples:
- Ambulatory surgery
- Breast or plastic surgery
- Cataract surgery
- Endoscopic procedures.
Elevated-risk procedures are those in which the risk is 1% or higher. Examples:
- Intraperitoneal surgery
- Intrathoracic surgery
- Carotid endarterectomy
- Head and neck surgery
- Orthopedic surgery
- Prostate surgery
- Aortic surgery
- Major vascular surgery
- Peripheral arterial surgery.
Cardiac risk based on patient factors
The 2014 American College of Cardiology and American Heart Association (ACC/AHA) perioperative guidelines list a number of clinical risk factors for perioperative cardiac morbidity and death.1 These include coronary artery disease, chronic heart failure, clinically suspected moderate or greater degrees of valvular heart disease, arrhythmias, conduction disorders, pulmonary vascular disease, and adult congenital heart disease.
Patients with these conditions and patients with unstable coronary syndromes warrant preoperative ECGs and sometimes even urgent interventions before any nonemergency surgery, provided such interventions would affect decision-making and perioperative care.1
The risk of perioperative cardiac morbidity and death can be calculated using either the Revised Cardiac Risk Index scoring system or the American College of Surgeons National Surgical Quality Improvement Program calculator.157 The former is fairly simple, validated, and accepted, while the latter requires use of online calculators (eg, www.surgicalriskcalculator.com/miorcardiacarrest, www.riskcalculator.facs.org).
The Revised Cardiac Risk Index has six clinical predictors of major perioperative cardiac events:
- History of cerebrovascular disease
- Prior or current compensated congestive heart failure
- History of coronary artery disease
- Insulin-dependent diabetes mellitus
- Renal insufficiency, defined as a serum creatinine level of 2 mg/dL or higher
- Patient undergoing suprainguinal vascular, intraperitoneal, or intrathoracic surgery.
A patient who has 0 or 1 of these predictors would have a low risk of a major adverse cardiac event, whereas a patient with 2 or more would have elevated risk. These risk factors must be taken into consideration to determine the need, if any, for a preoperative ECG.
What an ECG can tell us
Abnormalities such as left ventricular hypertrophy, ST-segment depression, and pathologic Q waves on a preoperative ECG in a patient undergoing an elevated-risk surgical procedure may predict adverse perioperative cardiac events.3,6
In a retrospective study of 23,036 patients, Noordzij et al7 found that in patients undergoing elevated-risk surgery, those with an abnormal preoperative ECG had a higher incidence of cardiovascular death than those with a normal ECG. However, a preoperative ECG was obtained only in patients with established coronary artery disease or risk factors for cardiovascular disease. Hence, although an abnormal ECG in such patients undergoing elevated-risk surgery was predictive of adverse postoperative cardiac outcomes, we cannot say that the same would apply to patients without these characteristics undergoing elevated-risk surgery.
In a prospective observational study of patients with known coronary artery disease undergoing major noncardiac surgery, a preoperative ECG was found to contain prognostic information and was predictive of long-term outcome independent of clinical findings and perioperative ischemia.8
CURRENT GUIDELINES AND RECOMMENDATIONS
Several guidelines address whether to order a preoperative ECG but are mostly based on low-level evidence and expert opinion.1,2,6,9
Current guidelines recommend obtaining a preoperative ECG in patients with known coronary, peripheral arterial, or cerebrovascular disease.1,6,9
Obesity and associated comorbidities such as coronary artery disease, heart failure, systemic hypertension, and sleep apnea can predispose to increased perioperative complications. A preoperative 12-lead ECG is reasonable in morbidly obese patients (body mass index ≥ 40 kg/m2) and in obese patients (body mass index ≥ 30 kg/m2) with at least one risk factor for coronary artery disease or poor exercise tolerance, or both.10
Liu et al11 looked at the predictive value of a preoperative 12-lead ECG in 513 elderly patients (age ≥ 70) undergoing noncardiac surgery and found that electrocardiographic abnormalities were not predictive of adverse cardiac outcomes. In this study, although electrocardiographic abnormalities were common (noted in 75% of the patients), they were nonspecific and less useful in predicting postoperative cardiac complications than was the presence of comorbidities.11 Age alone as a cutoff for obtaining a preoperative ECG is not predictive of postoperative outcomes and a preoperative ECG is not warranted in all elderly patients. This is also reflected in current ACC/AHA guidelines on perioperative cardiovascular evaluation1 and is a change from prior ACC/AHA guidelines when age was used as a criterion for preoperative ECGs.12
Current guidelines do not recommend getting a preoperative ECG in asymptomatic patients undergoing low-cardiac-risk surgery.1,4,9
Although the ideal time for ordering an ECG before a planned surgery is unknown, obtaining one within 90 days before the surgery is considered adequate in stable patients in whom an ECG is indicated.1
BACK TO OUR PATIENT
On the basis of current evidence, our patient does not need a preoperative ECG, as it is unlikely to alter his perioperative management and instead may delay his surgery unnecessarily if any nonspecific changes prompt further cardiac workup.
CLINICAL BOTTOM LINE
Although frequently ordered in clinical practice, preoperative electrocardiography has a limited role in predicting postoperative outcome and should be ordered only in the appropriate clinical setting.1 Moreover, there is little evidence that outcomes are better if we obtain an ECG before surgery. The clinician should consider patient factors and the type of surgery before ordering diagnostic tests, including electrocardiography.
In asymptomatic patients undergoing nonemergent surgery:
- It is reasonable to obtain a preoperative ECG in patients with known coronary artery disease, significant arrhythmia, peripheral arterial disease, cerebrovascular disease, chronic heart failure, or other significant structural heart disease undergoing elevated-cardiac-risk surgery.
- Do not order a preoperative ECG in asymptomatic patients undergoing low-risk surgery.
- Obtaining a preoperative ECG is reasonable in morbidly obese patients and in obese patients with one or more risk factors for coronary artery disease, or poor exercise tolerance, undergoing high-risk surgery.
A 55-year-old lawyer with hypertension well controlled on lisinopril and amlodipine is scheduled for elective hernia repair under general anesthesia. His surgeon has referred him for a preoperative evaluation. He has never smoked, runs 4 miles on days off from work, and enjoys long hiking trips. On examination, his body mass index is 26 kg/m2 and his blood pressure is 130/78 mm Hg; his cardiac examination and the rest of the clinical examination are unremarkable. He asks if he should have an electrocardiogram (ECG) as a part of his workup.
A preoperative ECG is not routinely recommended in all asymptomatic patients undergoing noncardiac surgery.
Consider obtaining an ECG in patients planning to undergo a high-risk surgical procedure, especially if they have one or more clinical risk factors for coronary artery disease, and in patients undergoing elevated-cardiac-risk surgery who are known to have coronary artery disease, chronic heart failure, peripheral arterial disease, or cerebrovascular disease. However, a preoperative ECG is not routinely recommended for patients perceived to be at low cardiac risk who are planning to undergo low-risk surgery. In those patients it could delay surgery unnecessarily, cause further unnecessary testing, drive up costs, and increase patient anxiety.
Here we discuss the perioperative cardiac risk based on type of surgery and patient characteristics and summarize the current guidelines and recommendations on obtaining a preoperative 12-lead ECG in patients undergoing noncardiac surgery.
RISK DEPENDS ON TYPE OF SURGERY AND PATIENT FACTORS
Physicians, including primary care physicians, hospitalists, cardiologists, and anesthesiologists, are routinely asked to evaluate patients before surgical procedures. The purpose of the preoperative evaluation is to optimize existing medical conditions, to identify undiagnosed conditions that can increase risk of perioperative morbidity and death, and to suggest strategies to mitigate risk.1,2
Cardiac risk is multifactorial, and risk factors for postoperative adverse cardiac events include the type of surgery and patient factors.1,3
Cardiac risk based on type of surgery
Low-risk procedures are those in which the risk of a perioperative major adverse cardiac event is less than 1%.1,4 Examples:
- Ambulatory surgery
- Breast or plastic surgery
- Cataract surgery
- Endoscopic procedures.
Elevated-risk procedures are those in which the risk is 1% or higher. Examples:
- Intraperitoneal surgery
- Intrathoracic surgery
- Carotid endarterectomy
- Head and neck surgery
- Orthopedic surgery
- Prostate surgery
- Aortic surgery
- Major vascular surgery
- Peripheral arterial surgery.
Cardiac risk based on patient factors
The 2014 American College of Cardiology and American Heart Association (ACC/AHA) perioperative guidelines list a number of clinical risk factors for perioperative cardiac morbidity and death.1 These include coronary artery disease, chronic heart failure, clinically suspected moderate or greater degrees of valvular heart disease, arrhythmias, conduction disorders, pulmonary vascular disease, and adult congenital heart disease.
Patients with these conditions and patients with unstable coronary syndromes warrant preoperative ECGs and sometimes even urgent interventions before any nonemergency surgery, provided such interventions would affect decision-making and perioperative care.1
The risk of perioperative cardiac morbidity and death can be calculated using either the Revised Cardiac Risk Index scoring system or the American College of Surgeons National Surgical Quality Improvement Program calculator.157 The former is fairly simple, validated, and accepted, while the latter requires use of online calculators (eg, www.surgicalriskcalculator.com/miorcardiacarrest, www.riskcalculator.facs.org).
The Revised Cardiac Risk Index has six clinical predictors of major perioperative cardiac events:
- History of cerebrovascular disease
- Prior or current compensated congestive heart failure
- History of coronary artery disease
- Insulin-dependent diabetes mellitus
- Renal insufficiency, defined as a serum creatinine level of 2 mg/dL or higher
- Patient undergoing suprainguinal vascular, intraperitoneal, or intrathoracic surgery.
A patient who has 0 or 1 of these predictors would have a low risk of a major adverse cardiac event, whereas a patient with 2 or more would have elevated risk. These risk factors must be taken into consideration to determine the need, if any, for a preoperative ECG.
What an ECG can tell us
Abnormalities such as left ventricular hypertrophy, ST-segment depression, and pathologic Q waves on a preoperative ECG in a patient undergoing an elevated-risk surgical procedure may predict adverse perioperative cardiac events.3,6
In a retrospective study of 23,036 patients, Noordzij et al7 found that in patients undergoing elevated-risk surgery, those with an abnormal preoperative ECG had a higher incidence of cardiovascular death than those with a normal ECG. However, a preoperative ECG was obtained only in patients with established coronary artery disease or risk factors for cardiovascular disease. Hence, although an abnormal ECG in such patients undergoing elevated-risk surgery was predictive of adverse postoperative cardiac outcomes, we cannot say that the same would apply to patients without these characteristics undergoing elevated-risk surgery.
In a prospective observational study of patients with known coronary artery disease undergoing major noncardiac surgery, a preoperative ECG was found to contain prognostic information and was predictive of long-term outcome independent of clinical findings and perioperative ischemia.8
CURRENT GUIDELINES AND RECOMMENDATIONS
Several guidelines address whether to order a preoperative ECG but are mostly based on low-level evidence and expert opinion.1,2,6,9
Current guidelines recommend obtaining a preoperative ECG in patients with known coronary, peripheral arterial, or cerebrovascular disease.1,6,9
Obesity and associated comorbidities such as coronary artery disease, heart failure, systemic hypertension, and sleep apnea can predispose to increased perioperative complications. A preoperative 12-lead ECG is reasonable in morbidly obese patients (body mass index ≥ 40 kg/m2) and in obese patients (body mass index ≥ 30 kg/m2) with at least one risk factor for coronary artery disease or poor exercise tolerance, or both.10
Liu et al11 looked at the predictive value of a preoperative 12-lead ECG in 513 elderly patients (age ≥ 70) undergoing noncardiac surgery and found that electrocardiographic abnormalities were not predictive of adverse cardiac outcomes. In this study, although electrocardiographic abnormalities were common (noted in 75% of the patients), they were nonspecific and less useful in predicting postoperative cardiac complications than was the presence of comorbidities.11 Age alone as a cutoff for obtaining a preoperative ECG is not predictive of postoperative outcomes and a preoperative ECG is not warranted in all elderly patients. This is also reflected in current ACC/AHA guidelines on perioperative cardiovascular evaluation1 and is a change from prior ACC/AHA guidelines when age was used as a criterion for preoperative ECGs.12
Current guidelines do not recommend getting a preoperative ECG in asymptomatic patients undergoing low-cardiac-risk surgery.1,4,9
Although the ideal time for ordering an ECG before a planned surgery is unknown, obtaining one within 90 days before the surgery is considered adequate in stable patients in whom an ECG is indicated.1
BACK TO OUR PATIENT
On the basis of current evidence, our patient does not need a preoperative ECG, as it is unlikely to alter his perioperative management and instead may delay his surgery unnecessarily if any nonspecific changes prompt further cardiac workup.
CLINICAL BOTTOM LINE
Although frequently ordered in clinical practice, preoperative electrocardiography has a limited role in predicting postoperative outcome and should be ordered only in the appropriate clinical setting.1 Moreover, there is little evidence that outcomes are better if we obtain an ECG before surgery. The clinician should consider patient factors and the type of surgery before ordering diagnostic tests, including electrocardiography.
In asymptomatic patients undergoing nonemergent surgery:
- It is reasonable to obtain a preoperative ECG in patients with known coronary artery disease, significant arrhythmia, peripheral arterial disease, cerebrovascular disease, chronic heart failure, or other significant structural heart disease undergoing elevated-cardiac-risk surgery.
- Do not order a preoperative ECG in asymptomatic patients undergoing low-risk surgery.
- Obtaining a preoperative ECG is reasonable in morbidly obese patients and in obese patients with one or more risk factors for coronary artery disease, or poor exercise tolerance, undergoing high-risk surgery.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. J Am Coll Cardiol 2014; Jul 29. 10.1016/j.jacc.2014.07.944. [Epub ahead of print]
- Feely MA, Collins CS, Daniels PR, Kebede EB, Jatoi A, Mauck KF. Preoperative testing before noncardiac surgery: guidelines and recommendations. Am Fam Physician 2013; 87:414–418.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Task Force for Preoperative Cardiac Risk Assessment and Perioperative Cardiac Management in Non-cardiac Surgery; European Society of Cardiology (ESC); Poldermans D, Bax JJ, Boersma E, et al. Guidelines for pre-operative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery. Eur Heart J 2009; 30:2769–2812.
- Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217:833–842.
- Landesberg G, Einav S, Christopherson R, et al. Perioperative ischemia and cardiac complications in major vascular surgery: importance of the preoperative twelve-lead electrocardiogram. J Vasc Surg 1997; 26:570–578.
- Noordzij PG, Boersma E, Bax JJ, et al. Prognostic value of routine preoperative electrocardiography in patients undergoing noncardiac surgery. Am J Cardiol 2006; 97:1103–1106.
- Jeger RV, Probst C, Arsenic R, et al. Long-term prognostic value of the preoperative 12-lead electrocardiogram before major noncardiac surgery in coronary artery disease. Am Heart J 2006; 151:508–513.
- Committee on Standards and Practice Parameters; Apfelbaum JL, Connis RT, Nickinovich DG, Pasternak LR, Arens JF, Caplan RA, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology 2012; 116:522–538.
- Poirier P, Alpert MA, Fleisher LA, et al. Cardiovascular evaluation and management of severely obese patients undergoing surgery: a science advisory from the American Heart Association. Circulation 2009; 120:86–95.
- Liu LL, Dzankic S, Leung JM. Preoperative electrocardiogram abnormalities do not predict postoperative cardiac complications in geriatric surgical patients. J Am Geriatr Soc 2002; 50:1186–1191.
- Eagle KA, Berger PB, Calkins H, et al; American College of Cardiology; American Heart Association. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary. J Am Coll Cardiol 2002; 39:542–553.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. J Am Coll Cardiol 2014; Jul 29. 10.1016/j.jacc.2014.07.944. [Epub ahead of print]
- Feely MA, Collins CS, Daniels PR, Kebede EB, Jatoi A, Mauck KF. Preoperative testing before noncardiac surgery: guidelines and recommendations. Am Fam Physician 2013; 87:414–418.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Task Force for Preoperative Cardiac Risk Assessment and Perioperative Cardiac Management in Non-cardiac Surgery; European Society of Cardiology (ESC); Poldermans D, Bax JJ, Boersma E, et al. Guidelines for pre-operative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery. Eur Heart J 2009; 30:2769–2812.
- Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217:833–842.
- Landesberg G, Einav S, Christopherson R, et al. Perioperative ischemia and cardiac complications in major vascular surgery: importance of the preoperative twelve-lead electrocardiogram. J Vasc Surg 1997; 26:570–578.
- Noordzij PG, Boersma E, Bax JJ, et al. Prognostic value of routine preoperative electrocardiography in patients undergoing noncardiac surgery. Am J Cardiol 2006; 97:1103–1106.
- Jeger RV, Probst C, Arsenic R, et al. Long-term prognostic value of the preoperative 12-lead electrocardiogram before major noncardiac surgery in coronary artery disease. Am Heart J 2006; 151:508–513.
- Committee on Standards and Practice Parameters; Apfelbaum JL, Connis RT, Nickinovich DG, Pasternak LR, Arens JF, Caplan RA, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology 2012; 116:522–538.
- Poirier P, Alpert MA, Fleisher LA, et al. Cardiovascular evaluation and management of severely obese patients undergoing surgery: a science advisory from the American Heart Association. Circulation 2009; 120:86–95.
- Liu LL, Dzankic S, Leung JM. Preoperative electrocardiogram abnormalities do not predict postoperative cardiac complications in geriatric surgical patients. J Am Geriatr Soc 2002; 50:1186–1191.
- Eagle KA, Berger PB, Calkins H, et al; American College of Cardiology; American Heart Association. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary. J Am Coll Cardiol 2002; 39:542–553.
When patients on target-specific oral anticoagulants need surgery
More then 2.5 million patients in the United States are on long-term anticoagulation therapy for atrial fibrillation, venous thromboembolic disease, or mechanical heart valves,1 and the number is expected to rise as the population ages. Each year, about 10% of these patients undergo an invasive procedure or surgery that requires temporary interruption of anticoagulation.2
Most physicians are familiar with the perioperative management of warfarin, a vitamin K antagonist, since for decades it has been the sole oral anticoagulant available. However, many physicians lack experience with the three target-specific oral anticoagulants (TSOACs; also known as “novel” oral anticoagulants) approved so far: the direct thrombin inhibitor dabigatran (Pradaxa) and the direct factor Xa inhibitors rivaroxaban (Xarelto) and apixaban (Eliquis).
With their rapid onset of action, predictable pharmacokinetics, relatively short half-lives, and fewer drug-drug interactions than warfarin, TSOACs overcome many of the limitations of the older oral anticoagulant warfarin. In many ways, these qualities simplify the perioperative management of anticoagulation. At the same time, these new drugs also bring new challenges: caution is needed in patients with renal impairment; the level of anticoagulation is difficult to assess; and there is no specific antidote or standardized procedure to reverse their anticoagulant effect. While various periprocedural protocols for TSOAC therapy have been proposed, evidence-based guidelines are still to come.
This article first discusses the pharmacology of dabigatran, rivaroxaban, and apixaban that is pertinent to the perioperative period. It then briefly reviews the general principles of perioperative management of anticoagulation. The final section provides specific recommendations for the perioperative management of TSOACs.
PHARMACOLOGY OF TARGET-SPECIFIC ORAL ANTICOAGULANTS
Dabigatran, a factor IIa inhibitor
Dabigatran is an oral direct thrombin (factor IIa) inhibitor. It exerts its anticoagulant effect by blocking the generation of fibrin, inhibiting platelet aggregation, and dampening the activity of factors V, VIII, and XI (Figure 1).3,4 From its introduction in October 2010 through August 2012, nearly 3.7 million prescriptions were dispensed to 725,000 patients in the United States.5
Indications for dabigatran. Dabigatran is approved in the United States and Canada for preventing stroke in nonvalvular atrial fibrillation (Table 1).6 More recently, it received US approval for treating deep vein thrombosis or pulmonary embolism after 5 to 10 days of a parenteral anticoagulant.7,8 It is also approved in Europe and Canada for preventing venous thromboembolism (VTE) after total hip replacement and knee arthroplasty.9,10
Dabigatran is contraindicated in patients with a mechanical heart valve, based on a phase 2 study in which it conferred a higher risk of thromboembolism and bleeding than warfarin.3,11
Pharmacokinetics of dabigatran. Dabigatran is formulated as a prodrug, dabigatran etexilate, in a capsule containing multiple small pellets.12 The capsules should not be crushed, as this significantly increases oral bioavailability. The prodrug is absorbed across the gastric mucosa and is then rapidly converted to the active form (Table 2).
Plasma concentrations peak within 2 hours of ingestion, which means that therapeutic anticoagulation is achieved shortly after taking the drug.
Only 35% of dabigatran is protein-bound, which allows it to be removed by hemodialysis. Nearly 85% of the drug is eliminated in the urine. It has a half-life of 13 to 15 hours in patients with normal renal function.3 However, its half-life increases to about 27 hours in patients whose creatinine clearance is less than 30 mL/min. As a result, the dose must be reduced in patients with renal impairment (Table 1).
Dabigatran is not metabolized by the cytochrome P450 enzymes, but it is a substrate for P-glycoprotein, so it still has the potential for drug-drug interactions.3 Practitioners should be familiar with these potential interactions (Table 3), as they can result in higher- or lower-than-expected plasma concentrations of dabigatran in the perioperative period.13
Rivaroxaban, a factor Xa inhibitor
Rivaroxaban is an oral direct factor Xa inhibitor. It has been approved by the US Food and Drug Administration (FDA) for the prevention of stroke in nonvalvular atrial fibrillation, for VTE treatment, and for VTE prophylaxis after hip replacement or knee replacement (Table 1).14–20 It has not yet been studied in patients with hip fracture.
Pharmacokinetics of rivaroxaban. Rivaroxaban is manufactured as a tablet that is best absorbed in the stomach (Table 2).14 In contrast to dabigatran, it can be crushed and, for example, mixed with applesauce for patients who have trouble swallowing. It can also be mixed with water and given via nasogastric tube; however, postpyloric administration should be avoided.
Plasma concentrations peak within a few hours after ingestion. Rivaroxaban is highly protein-bound, so it cannot be eliminated by hemodialysis.
The drug relies on renal elimination to a smaller degree than dabigatran, with one-third of the dose eliminated unchanged in the urine, one-third eliminated in the urine as inactive metabolite, and the remaining one-third eliminated in the feces. However, enough parent compound is cleared through the kidneys that the half-life of rivaroxaban increases from 8.3 hours in healthy individuals to 9.5 hours in patients whose creatinine clearance is less than 30 mL/min.21 As with dabigatran, the dose must be adjusted for renal impairment (Table 1).
Rivaroxaban has significant liver metabolism, specifically through the cytochrome P450 3A4 enzyme, and it is also a substrate of P-glycoprotein. Therefore, potential drug-drug interactions must be taken into account, as they may lead to important alterations in plasma concentrations (Table 3).
Apixaban, a factor Xa inhibitor
Apixaban is also an oral direct factor Xa inhibitor. It is the newest of the oral anticoagulants to be approved in the United States, specifically for preventing stroke in nonvalvular atrial fibrillation (Table 1).22
Pharmacokinetics of apixaban. Apixaban is produced as a tablet that is absorbed slowly through the gastrointestinal tract, mainly the distal small bowel and ascending colon (Table 2).23
Peak plasma concentrations are reached a few hours after ingestion. Like rivaroxaban, apixaban is highly protein-bound, so it cannot be removed by hemodialysis.
Apixaban is similar to rivaroxaban in that 27% of the parent compound is cleared through the kidneys, it undergoes significant hepatic metabolism through cytochrome P450 3A4, and it is a substrate for P-glycoprotein.
Drug-drug interactions must be considered as a potential source of altered drug exposure and clearance (Table 3).
Unlike dabigatran and rivaroxaban, dose reduction is not based on the calculated creatinine clearance. Instead, a reduced dose is required if the patient meets two of the following three criteria:
- Serum creatinine level ≥ 1.5 mg/dL
- Age ≥ 80
- Weight ≤ 60 kg (Table 1).
The American Heart Association/American Stroke Association guidelines further recommend against using apixaban in patients with a creatinine clearance less than 25 mL/min.24
Edoxaban, a factor Xa inhibitor in development
Edoxaban (Savaysa), another factor Xa inhibitor, is available in Japan and has been submitted for approval in the United States for treating VTE and for preventing stroke in patients with
PERIOPERATIVE CONSIDERATIONS IN ANTICOAGULATION
Before addressing the perioperative management of TSOACs, let us review the evidence guiding the perioperative management of any chronic anticoagulant.
In fact, no large prospective randomized trial has clearly defined the risks and benefits of using or withholding a bridging anticoagulation strategy around surgery and other procedures, though the PERIOP 2 and BRIDGE trials are currently ongoing.25,26 There are some data regarding continuing anticoagulation without interruption, but they have mainly been derived from specific groups (eg, patients on warfarin undergoing cardiac pacemaker or defibrillator placement) and in procedures that pose a very low risk of bleeding complications (eg, minor dental extractions, cataract surgery, dermatologic procedures).2,27 Recommendations are, therefore, necessarily based on small perioperative trials and data gleaned from cohort review and from studies that did not involve surgical patients.
Ultimately, the decisions whether to discontinue oral anticoagulants and whether to employ bridging anticoagulation are based on assumptions about the risks of bleeding and the risk of thrombotic events, with similar assumptions regarding the effects of anticoagulants on both outcomes. In addition, the relative acceptance of bleeding vs thrombotic risks implicitly guides these complex decisions.
Perioperative bleeding risk
Many risk factors specific to the patient and to the type of surgery affect the rates and severity of perioperative bleeding.28
As for patient-specific risk factors, a small retrospective cohort analysis revealed that a HAS-BLED score of 3 or higher was highly discriminating in predicting perioperative bleeding in atrial fibrillation patients receiving anticoagulation.29 (The HAS-BLED score is based on hypertension, abnormal renal or liver function, stroke, bleeding, labile international normalized ratio [INR], elderly [age > 65] and drug therapy.30) However, there are no widely validated tools that incorporate patient-specific factors to accurately predict bleeding risk in an individual patient.
Therefore, the American College of Chest Physicians (ACCP) guidelines suggest coarsely categorizing bleeding risk as either low or high solely on the basis of the type of procedure.2 Procedures considered “high-risk” have a risk greater than 1.5% to 2% and include urologic surgery involving the prostate or kidney, colonic polyp resections, surgeries involving highly vascular organs such as the liver or spleen, joint replacements, cancer surgeries, and cardiac or neurosurgical procedures.
Perioperative thrombotic risk
The ACCP guidelines2 place patients with atrial fibrillation, VTE, or mechanical heart valves in three risk groups for perioperative thromboembolism without anticoagulation, based on their annual risk of a thrombotic event:
- High risk—annual risk of a thrombotic event > 10%
- Moderate risk—5% to 10%
- Low risk—< 5%.
Comparing the risks calculated by these methods with the real-world risk of perioperative thrombosis highlights the problem of applying nonperioperative risk calculations: the perioperative period exposes patients to a higher risk than these models would predict.31 Nonetheless, these risk categorizations likely have some validity in stratifying patients into risk groups, even if the absolute risks are inaccurate.
Perioperative bridging for patients taking warfarin
Many patients with atrial fibrillation, VTE, or a mechanical heart valve need to interrupt their warfarin therapy because of the bleeding risk of an upcoming procedure.
The perioperative management of warfarin and other vitamin K antagonists is challenging because of the pharmacokinetics and pharmacodynamics of these drugs. Because it has a long half-life, warfarin usually must be stopped 4 to 5 days before a procedure in order to allow not only adequate clearance of the drug itself, but also restoration of functional clotting factors to normal or near-normal levels.12 Warfarin can generally be resumed 12 to 24 hours after surgery, assuming adequate hemostasis has been achieved, and it will again take several days for the INR to reach the therapeutic range.
The ACCP guidelines recommend using the perioperative risk of thromboembolism to make decisions about the need for bridging anticoagulation during warfarin interruption.2 They suggest that patients at high risk of thrombosis receive bridging with an alternative anticoagulant such as low-molecular-weight heparin or unfractionated heparin, because of the prolonged duration of subtherapeutic anticoagulation.
There has been clinical interest in using a TSOAC instead of low-molecular-weight or unfractionated heparin for bridging in the perioperative setting. Although this approach may be attractive from a cost and convenience perspective, it cannot be endorsed as yet because of the lack of information on the pros and cons of such an approach.
Patients at low thrombotic risk do not require bridging. In patients at moderate risk, the decision to bridge or not to bridge is based on careful consideration of patient-specific and surgery-specific factors.
PERIOPERATIVE MANAGEMENT OF TARGET-SPECIFIC ORAL ANTICOAGULANTS
As summarized above, the perioperative management strategy for chronic anticoagulation is based on limited evidence, even for drugs as well established as warfarin.
The most recent ACCP guidelines on the perioperative management of antithrombotic therapy do not mention TSOACs.2 For now, the management strategy must be based on the pharmacokinetics of the drugs, package inserts from the manufacturers, and expert recommendations.3,14,23,32–34 Fortunately, because TSOACs have a more favorable pharmacokinetic profile than that of warfarin, their perioperative uses should be more streamlined. As always, the goal is to minimize the risk of both periprocedural bleeding and thromboembolism.
Timing of cessation of anticoagulation
The timing of cessation of TSOACs before an elective procedure depends primarily on two factors: the bleeding risk of the procedure and the patient’s renal function. Complete clearance of the medication is not necessary in all circumstances.
TSOACs should be stopped four to five half-lives before a procedure with a high bleeding risk, so that there is no or only minimal residual anticoagulant effect. The drug can be stopped two to three half-lives before a procedure with a low bleeding risk. Remember: the half-life increases as creatinine clearance decreases.
Specific recommendations may vary across institutions, but a suggested strategy is shown in Table 4.3,4,21,23,32–35 For the small subset of patients on P-glycoprotein or cytochrome P450 inhibitors or inducers, further adjustment in the time of discontinuation may be required.
Therapy does not need to be interrupted for procedures with a very low bleeding risk, as defined above.33,34 There is also preliminary evidence that TSOACs, similar to warfarin, may be continued during cardiac pacemaker or defibrillator placement.36
Evidence from clinical trials of perioperative TSOAC management
While the above recommendations are logical, studies are needed to prospectively evaluate perioperative management strategies.
The RE-LY trial (Randomized Evaluation of Long-Term Anticoagulation Therapy), which compared the effects of dabigatran and warfarin in preventing stroke in patients with atrial fibrillation, is one of the few clinical trials that also looked at periprocedural bleeding.37 About a quarter of the RE-LY participants required interruption of anticoagulation for a procedure.
Warfarin was managed according to local practices. For most of the study, the protocol required that dabigatran be discontinued 24 hours before a procedure, regardless of renal function or procedure type. The protocol was later amended and closely mirrored the management plan outlined in Table 4.
With either protocol, there was no statistically significant difference between dabigatran and warfarin in the rates of bleeding and thrombotic complications in the 7 days before or 30 days after the procedure.
A major limitation of the study was that most patients underwent a procedure with a low bleeding risk, so the analysis was likely underpowered to evaluate rates of bleeding in higher-risk procedures.
The ROCKET-AF trial (Rivaroxaban Once-daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) also shed light on periprocedural bleeding.15 About 15% of the participants required temporary interruption of anticoagulation for a surgical or invasive procedure.38
The study protocol called for discontinuing rivaroxaban 2 days before any procedure. Warfarin was to be held for 4 days to achieve a goal INR of 1.5 or less.15
Rates of major and nonmajor clinically significant bleeding at 30 days were similar with rivaroxaban and with warfarin.38 As with the RE-LY trial, the retrospective analysis was probably underpowered for assessing rates of bleeding in procedures with higher risk.
Perioperative bridging
While stopping a TSOAC in the perioperative period decreases the risk of bleeding, it naturally increases the risk of thromboembolism. However, patients on TSOACs should not routinely require perioperative bridging with an alternative anticoagulant, regardless of thrombotic risk.
Of note, dabigatran, rivaroxaban, and apixaban carry black-box warnings that discontinuation places patients at higher risk of thrombotic events.3,14,23 These warnings further state that coverage with an alternative anticoagulant should be strongly considered during interruption of therapy for reasons other than pathologic bleeding.
However, it does not necessarily follow that perioperative bridging is required. For example, the warning for rivaroxaban is based on the finding in the ROCKET-AF trial that patients in the rivaroxaban group had higher rates of stroke than those in the warfarin group after the study drugs were stopped at the end of the trial.39 While there was initial concern that this could represent a prothrombotic rebound effect, the authors subsequently showed that patients in the rivaroxaban group were more likely to have had a subtherapeutic INR when transitioning to open-label vitamin-K-antagonist therapy.39,40 There was no difference in the rate of stroke or systemic embolism between the rivaroxaban and warfarin groups when anticoagulation was temporarily interrupted for a procedure.38
The risks and benefits of perioperative bridging with TSOACs are difficult to evaluate, given the dearth of trial data. In the RE-LY trial, only 17% of patients on dabigatran and 28% of patients on warfarin underwent periprocedural bridging.37 The selection criteria and protocol for bridging were not reported. In the ROCKET-AF trial, only 9% of patients received bridging therapy despite a mean CHADS2 score of 3.4.38 (The CHADS2 score is calculated as 1 point each for congestive heart failure, hypertension, age ≥ 75, and diabetes; 2 points for stroke or transient ischemic attack.) The decision to bridge or not was left to the individual investigator. As a result, the literature offers diverse opinions about the appropriateness of transitioning to an alternative anticoagulant.41–43
Bridging does not make sense in most instances, since anticoagulants such as low-molecular-weight heparin have pharmacokinetics similar to those of the available TSOACs and also depend on renal clearance.41 However, there may be situations in which patients must be switched to a parenteral anticoagulant such as unfractionated or low-molecular-weight heparin. For example, if a TSOAC has to be held, the patient has acute renal failure, and a needed procedure is still several days away, it would be reasonable to start a heparin drip for an inpatient at increased thrombotic risk.
In patients with normal renal function, these alternative anticoagulants should be started at the time the next TSOAC dose would have been due.3,14,23 In patients with reduced renal function, initiation of an alternative anticoagulant may need to be delayed 12 to 48 hours depending on which TSOAC is being used, as well as on the degree of renal dysfunction. This delay would help ensure that the onset of anticoagulation with the alternative anticoagulant is timed with the offset of therapeutic anticoagulation with the TSOAC.
Although limited, information from available coagulation assays may assist with the timing of initiation of an alternative anticoagulant (see the following section on laboratory monitoring). Serial testing with appropriate coagulation assays may help identify when most of a TSOAC has been cleared from a patient.
Laboratory monitoring
Inevitably, some patients on TSOACs require urgent or emergency surgery. In certain situations, such as before an orthopedic spine procedure, in which the complications of bleeding could be devastating, it may be necessary to know if any residual anticoagulant effect is present.
Monitoring dabigatran. As one might expect, direct thrombin inhibitors such as dabigatran can prolong the prothrombin time and activated partial thromboplastin time (aPTT).44–47 However, the prothrombin time is not recommended for assessing the level of anticoagulation from dabigatran. Many institutions may be using a normal aPTT to rule out therapeutic concentrations of dabigatran, based on results from early in vitro and ex vivo studies.46 While appealing from a practical standpoint, practitioners should exercise caution when relying on the aPTT to assess the risk of perioperative bleeding. A more recent investigation in patients treated with dabigatran found that up to 35% of patients with a normal aPTT still had a plasma concentration in the therapeutic range.48
The thrombin time and ecarin clotting time are more sensitive tests for dabigatran. A normal thrombin time or ecarin clotting time indicates that no or only minimal dabigatran is present.48 Unfortunately, these two tests often are either unavailable or are associated with long turnaround times, which limits their usefulness in the perioperative setting.
Monitoring rivaroxaban and apixaban. Factor Xa inhibitors such as rivaroxaban and apixaban can also influence the prothrombin time and aPTT (Figure 1).44–47,49,50 The aPTT is relatively insensitive to these drugs at low concentrations. It has been suggested that a normal prothrombin time can reasonably exclude therapeutic concentrations of rivaroxaban.45,46 However, the effects on the prothrombin time are highly variable, changing with the reagent used.49,50 In addition, apixaban appears to have less impact on the prothrombin time overall. The INR is not recommended for monitoring the effect of factor Xa inhibitors.
Anti-factor Xa assays likely represent the best option to provide true quantitative information on the level of anticoagulation with either rivaroxaban or apixaban. However, the assays must be specifically calibrated for each drug for results to be useful. (Anti-factor Xa assays cannot be used for heparin or low-molecular-weight heparin.) Further, most institutions do not yet have this capability. When appropriately calibrated, normal anti-factor Xa levels would exclude any effect of rivaroxaban or apixaban.
Reversal of anticoagulation
If patients on TSOACs require emergency surgery or present with significant bleeding in the setting of persistent anticoagulation, it may be necessary to try to reverse the anticoagulation.
Unlike warfarin or heparin, TSOACS do not have specific reversal agents, though specific antidotes are being developed. For example, researchers are evaluating antibodies capable of neutralizing dabigatran, as well as recombinant thrombin and factor Xa molecules that could antagonize dabigatran and rivaroxaban, respectively.51–53
Reversal can be attempted by neutralizing or removing the offending drug. Activated charcoal may be able to reduce absorption of TSOACs that were recently ingested,44 and dabigatran can be removed by hemodialysis.
However, certain practical considerations may limit the use of dialysis in the perioperative period. Insertion of a temporary dialysis line in an anticoagulated patient poses additional bleeding risks. A standard 4-hour hemodialysis session may remove only about 70% of dabigatran from the plasma, which may not be enough to prevent perioperative bleeding.54 Dabigatran also tends to redistribute from adipose tissue back into plasma after each dialysis session.55 Serial sessions of high-flux intermittent hemodialysis or continuous renal replacement therapy may therefore be needed to counteract rebound elevations in the dabigatran concentration.
Reversal can also be attempted through activation of the coagulation cascade via other mechanisms. Fresh-frozen plasma is unlikely to be a practical solution for reversal.44 Although it can readily replace the clotting factors depleted by vitamin K antagonists, large volumes of fresh-frozen plasma would be needed to overwhelm thrombin or factor Xa inhibition by TSOACs.
There are limited data on the use of prothrombin complex concentrates or recombinant activated factor VIIa in patients on TSOACs, though their use can be considered.56 In a trial in 12 healthy participants, a nonactivated four-factor prothrombin complex concentrate containing factors II, VII, IX, and X immediately and completely reversed the anticoagulant effect of rivaroxaban but had no effect on dabigatran.57 Before 2013, there were no nonactivated four-factor prothrombin complex concentrates available in the United States. The FDA has since approved Kcentra for the urgent reversal of vitamin K antagonists, meaning that the reversal of TSOACs in major bleeding events would still be off-label.58 Giving any of the clotting factors carries a risk of thromboembolism.
Resumption of anticoagulation
TSOACs have a rapid onset of action, and therapeutic levels are reached within a few hours of administration.
Extrapolating from the ACCP guidelines, TSOACs can generally be restarted at therapeutic doses 24 hours after low-bleeding-risk procedures.2 Therapeutic dosing should be delayed 48 to 72 hours after a procedure with a high bleeding risk, assuming adequate hemostasis has been achieved. Prophylactic unfractionated heparin or low-molecular-weight heparin therapy can be given in the interim if deemed safe. Alternatively, for orthopedic patients ultimately transitioning back to therapeutic rivaroxaban after hip or knee arthroplasty, prophylactic rivaroxaban doses can be started 6 to 10 hours after surgery.14
There are numerous reasons why the resumption of TSOACs may have to be delayed after surgery, including nothing-by-mouth status, postoperative nausea and vomiting, ileus, gastric or bowel resection, and the anticipated need for future procedures. Since dabigatran capsules cannot be crushed, they cannot be given via nasogastric tube in patients with postoperative dysphagia. Parenteral anticoagulants should be used until these issues resolve.
Unfractionated heparin is still the preferred anticoagulant in unstable or potentially unstable patients, given its ease of monitoring, quick offset of action, and reversibility. When patients have stabilized, TSOACs can be resumed when the next dose of low-molecular-weight heparin would have been due or when the unfractionated heparin drip is discontinued.3,14,23
UNTIL EVIDENCE-BASED GUIDELINES ARE DEVELOPED
The development of TSOACs has ushered in an exciting new era for anticoagulant therapy. Providers involved in perioperative medicine will increasingly encounter patients on dabigatran, rivaroxaban, and apixaban. However, until evidence-based guidelines are developed for these new anticoagulants, clinicians will have to apply their knowledge of pharmacology and critically evaluate expert recommendations in order to manage patients safely throughout the perioperative period.
- Douketis JD, Berger PB, Dunn AS, et al; American College of Chest Physicians. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):299S–339S.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e326S–e350S.
- Boehringer Ingelheim Pharmaceuticals, Inc. PRADAXA (dabigatran) package insert. http://bidocs.boehringer-ingelheim.com/BIWebAc-cess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf. Accessed August 6, 2014.
- Levy JH, Faraoni D, Spring JL, Douketis JD, Samama CM. Managing new oral anticoagulants in the perioperative and intensive care unit setting. Anesthesiology 2013; 118:1466–1474.
- US Food and Drug Administration (FDA). FDA drug safety communication: update on the risk for serious bleeding events with the anticoagulant Pradaxa (dabigatran). www.fda.gov/drugs/drugsafety/ucm326580.htm. Accessed August 6, 2014.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- Schulman S, Kearon C, Kakkar AK, et al; RE-MEDY Trial Investigators. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013; 368:709–718.
- Eriksson BI, Dahl OE, Rosencher N, Büller HR, et al; RE-NOVATE Study Group. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 2007; 370:949–956.
- Eriksson BI, Dahl OE, Rosencher N, et al; RE-MODEL Study Group. Oral dabigatran etexilate vs subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007; 5:2178–2185.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013; 369:1206–1214.
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G; American College of Chest Physicians. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e44S–e88S.
- Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos 2008; 36:386–399.
- Janssen Pharmaceuticals, Inc. XARELTO (rivaroxaban) package insert. www.xareltohcp.com/about-xarelto/about-xarelto.html?utm_source=google&utm_medium=cpc&utm_campaign=Branded+-+Broad&utm_term=xarelto%20rivaroxaban&utm_content=Xarelto+Rivaroxaban|mkwid|sxSDxPb4m_dc|pcrid|34667840494. Accessed August 6, 2014.
- Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–391.
- EINSTEIN Investigators; Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363:2499–2510.
- EINSTEIN–PE Investigators; Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012; 366:1287–1297.
- Eriksson BI, Borris LC, Friedman RJ, et al; RECORD1 Study Group. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008; 358:2765–2775.
- Kakkar AK, Brenner B, Dahl OE, et al; RECORD2 Investigators. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet 2008; 372:31–39.
- Lassen MR, Ageno W, Borris LC, et al; RECORD3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008; 358:2776–2786.
- Kubitza D, Becka M, Mueck W, et al. Effects of renal impairment on the pharmacokinetics, pharmacodynamics and safety of rivaroxaban, an oral, direct factor Xa inhibitor. Br J Clin Pharmacol 2010; 70:703–712.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Bristol-Myers Squibb Company. ELIQUIS (apixaban) package insert. www.eliquis.com/index.aspx. Accessed August 6, 2014.
- Furie KL, Goldstein LB, Albers GW, et al; American Heart Association Stroke Council. Oral antithrombotic agents for the prevention of stroke in nonvalvular atrial fibrillation: a science advisory for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2012; 43:3442–3453.
- ClinicalTrials.gov, US National Institutes of Health. PERIOP 2 - A Safety and Effectiveness Study of LMWH Bridging Therapy Versus Placebo Bridging Therapy for Patients on Long Term Warfarin and Require Temporary Interruption of Their Warfarin. http://clinicaltri-als.gov/show/NCT00432796. Accessed August 6, 2014.
- ClinicalTrials.gov, US National Institutes of Health. Effectiveness of Bridging Anticoagulation for Surgery (The BRIDGE Study). http://clinicaltrials.gov/ct2/show/NCT00786474. Accessed August 6, 2014.
- Birnie DH, Healey JS, Wells GA, et al; BRUISE CONTROL Investigators. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med 2013; 368:2084–2093.
- Oberweis BS, Nukala S, Rosenberg A, et al. Thrombotic and bleeding complications after orthopedic surgery. Am Heart J 2013; 165:427.e1–433.e1.
- Omran H, Bauersachs R, Rübenacker S, Goss F, Hammerstingl C. The HAS-BLED score predicts bleedings during bridging of chronic oral anticoagulation. Results from the national multicentre BNK Online bRiDging REgistRy (BORDER). Thromb Haemost 2012; 108:65–73.
- Pisters R, Lane DA, Nieuwlaaat R, de Vos CB, Crijns HJGM, Lip GYH. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation. The Euro Heart Survey. Chest 2010; 138:1093–1100.
- Kaatz S, Douketis JD, Zhou H, Gage BF, White RH. Risk of stroke after surgery in patients with and without chronic atrial fibrillation. J Thromb Haemost 2010; 8:884–890.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Connolly G, Spyropoulos AC. Practical issues, limitations, and periprocedural management of the NOAC’s. J Thromb Thrombolysis 2013; 36:212–222.
- Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood 2012; 120:2954–2962.
- Kaatz S, Kouides PA, Garcia DA, et al. Guidance on the emergent reversal of oral thrombin and factor Xa inhibitors. Am J Hematol 2012; 87(suppl 1):S141–S145.
- Rowley CP, Bernard ML, Brabham WW, et al. Safety of continuous anticoagulation with dabigatran during implantation of cardiac rhythm devices. Am J Cardiol 2013; 111:1165–1168.
- Healey JS, Eikelboom J, Douketis J, et al; RE-LY Investigators. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) randomized trial. Circulation 2012; 126:343–348.
- Sherwood MW, Douketis JD, Patel MR, et al; on behalf of the ROCKET AF Investigators. Outcomes of temporary interruption of rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: results from the Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Circulation 2014; 129:1850–1859.
- Patel MR, Hellkamp AS, Lokhnygina Y, et al. Outcomes of discontinuing rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: analysis from the ROCKET AF trial (Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation). J Am Coll Cardiol 2013; 61:651–658.
- Reynolds MR. Discontinuation of rivaroxaban: filling in the gaps. J Am Coll Cardiol 2013; 61:659–660.
- Turpie AG, Kreutz R, Llau J, Norrving B, Haas S. Management consensus guidance for the use of rivaroxaban—an oral, direct factor Xa inhibitor. Thromb Haemost 2012; 108:876–886.
- Gallego P, Apostolakis S, Lip GY. Bridging evidence-based practice and practice-based evidence in periprocedural anticoagulation. Circulation 2012; 126:1573–1576.
- Sié P, Samama CM, Godier A, et al; Working Group on Perioperative Haemostasis. Surgery and invasive procedures in patients on long-term treatment with direct oral anticoagulants: thrombin or factor-Xa inhibitors. Recommendations of the Working Group on Perioperative Haemostasis and the French Study Group on Thrombosis and Haemostasis. Arch Cardiovasc Dis 2011; 104:669–676.
- King CS, Holley AB, Moores LK. Moving toward a more ideal anticoagulant: the oral direct thrombin and factor Xa inhibitors. Chest 2013; 143:1106–1116.
- Baglin T, Hillarp A, Tripodi A, Elalamy I, Buller H, Ageno W. Measuring oral direct inhibitors (ODIs) of thrombin and factor Xa: a recommendation from the Subcommittee on Control of Anticoagulation of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2013; 11:756–760.
- Baglin T, Keeling D, Kitchen S; British Committee for Standards in Haematology. Effects on routine coagulation screens and assessment of anticoagulant intensity in patients taking oral dabigatran or rivaroxaban: guidance from the British Committee for Standards in Haematology. Br J Haematol 2012; 159:427–429.
- Mani H, Kasper A, Lindhoff-Last E. Measuring the anticoagulant effects of target specific oral anticoagulants—reasons, methods and current limitations. J Thromb Thrombolysis 2013; 36:187–194.
- Hawes EM, Deal AM, Funk-Adcock D, et al. Performance of coagulation tests in patients on therapeutic doses of dabigatran: a cross-sectional pharmacodynamic study based on peak and trough plasma levels. J Thromb Haemost 2013; 11:1493–1502.
- Barrett YC, Wang Z, Frost C, Shenker A. Clinical laboratory measurement of direct factor Xa inhibitors: anti-Xa assay is preferable to prothrombin time assay. Thromb Haemost 2010; 104:1263–1271.
- Smythe MA, Fanikos J, Gulseth MP, et al. Rivaroxaban: practical considerations for ensuring safety and efficacy. Pharmacotherapy 2013; 33:1223–1245.
- Van Ryn J, Litzenburger T, Waterman A, et al. Dabigatran anticoagulant activity is neutralized by an antibody selective to dabigatran in in vitro and in vivo models. J Am Coll Cardiol 2011; 57:E1130.
- Sheffield W, Lambourne M, Bhakta V, Eltringham-Smith L, Arnold D, Crowther M. Active site-mutated thrombin S195A but not active site-blocked thrombin counteracts the anticoagulant activity of dabigatran in plasma. Abstract presented at the International Society of Thrombosis and Haemostasis 2013 Congress. http://onlinelibrary.wiley.com/doi/10.1111/jth.2013.11.issue-s2/issuetoc. Accessed August 6, 2014.
- Lu G, Luan P, Hollenbach SJ, et al. Reconstructed recombinant factor Xa as an antidote to reverse anticoagulation by factor Xa inhibitors (abstract). J Thromb Haemost 2009; 7(suppl 2):abstract OC-TH-107.
- Stangier J, Rathgen K, Stähle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet 2010; 49:259–268.
- Singh T, Maw TT, Henry BL, et al. Extracorporeal therapy for dabigatran removal in the treatment of acute bleeding: a single center experience. Clin J Am Soc Nephrol 2013; 8:1533–1539.
- Kaatz S, Crowther M. Reversal of target-specific oral anticoagulants. J Thromb Thrombolysis 2013; 36:195–202.
- Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011; 124:1573–1579.
- Sarode R, Milling TJ, Refaai MA, et al. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation 2013; 128:1234–1243.
More then 2.5 million patients in the United States are on long-term anticoagulation therapy for atrial fibrillation, venous thromboembolic disease, or mechanical heart valves,1 and the number is expected to rise as the population ages. Each year, about 10% of these patients undergo an invasive procedure or surgery that requires temporary interruption of anticoagulation.2
Most physicians are familiar with the perioperative management of warfarin, a vitamin K antagonist, since for decades it has been the sole oral anticoagulant available. However, many physicians lack experience with the three target-specific oral anticoagulants (TSOACs; also known as “novel” oral anticoagulants) approved so far: the direct thrombin inhibitor dabigatran (Pradaxa) and the direct factor Xa inhibitors rivaroxaban (Xarelto) and apixaban (Eliquis).
With their rapid onset of action, predictable pharmacokinetics, relatively short half-lives, and fewer drug-drug interactions than warfarin, TSOACs overcome many of the limitations of the older oral anticoagulant warfarin. In many ways, these qualities simplify the perioperative management of anticoagulation. At the same time, these new drugs also bring new challenges: caution is needed in patients with renal impairment; the level of anticoagulation is difficult to assess; and there is no specific antidote or standardized procedure to reverse their anticoagulant effect. While various periprocedural protocols for TSOAC therapy have been proposed, evidence-based guidelines are still to come.
This article first discusses the pharmacology of dabigatran, rivaroxaban, and apixaban that is pertinent to the perioperative period. It then briefly reviews the general principles of perioperative management of anticoagulation. The final section provides specific recommendations for the perioperative management of TSOACs.
PHARMACOLOGY OF TARGET-SPECIFIC ORAL ANTICOAGULANTS
Dabigatran, a factor IIa inhibitor
Dabigatran is an oral direct thrombin (factor IIa) inhibitor. It exerts its anticoagulant effect by blocking the generation of fibrin, inhibiting platelet aggregation, and dampening the activity of factors V, VIII, and XI (Figure 1).3,4 From its introduction in October 2010 through August 2012, nearly 3.7 million prescriptions were dispensed to 725,000 patients in the United States.5
Indications for dabigatran. Dabigatran is approved in the United States and Canada for preventing stroke in nonvalvular atrial fibrillation (Table 1).6 More recently, it received US approval for treating deep vein thrombosis or pulmonary embolism after 5 to 10 days of a parenteral anticoagulant.7,8 It is also approved in Europe and Canada for preventing venous thromboembolism (VTE) after total hip replacement and knee arthroplasty.9,10
Dabigatran is contraindicated in patients with a mechanical heart valve, based on a phase 2 study in which it conferred a higher risk of thromboembolism and bleeding than warfarin.3,11
Pharmacokinetics of dabigatran. Dabigatran is formulated as a prodrug, dabigatran etexilate, in a capsule containing multiple small pellets.12 The capsules should not be crushed, as this significantly increases oral bioavailability. The prodrug is absorbed across the gastric mucosa and is then rapidly converted to the active form (Table 2).
Plasma concentrations peak within 2 hours of ingestion, which means that therapeutic anticoagulation is achieved shortly after taking the drug.
Only 35% of dabigatran is protein-bound, which allows it to be removed by hemodialysis. Nearly 85% of the drug is eliminated in the urine. It has a half-life of 13 to 15 hours in patients with normal renal function.3 However, its half-life increases to about 27 hours in patients whose creatinine clearance is less than 30 mL/min. As a result, the dose must be reduced in patients with renal impairment (Table 1).
Dabigatran is not metabolized by the cytochrome P450 enzymes, but it is a substrate for P-glycoprotein, so it still has the potential for drug-drug interactions.3 Practitioners should be familiar with these potential interactions (Table 3), as they can result in higher- or lower-than-expected plasma concentrations of dabigatran in the perioperative period.13
Rivaroxaban, a factor Xa inhibitor
Rivaroxaban is an oral direct factor Xa inhibitor. It has been approved by the US Food and Drug Administration (FDA) for the prevention of stroke in nonvalvular atrial fibrillation, for VTE treatment, and for VTE prophylaxis after hip replacement or knee replacement (Table 1).14–20 It has not yet been studied in patients with hip fracture.
Pharmacokinetics of rivaroxaban. Rivaroxaban is manufactured as a tablet that is best absorbed in the stomach (Table 2).14 In contrast to dabigatran, it can be crushed and, for example, mixed with applesauce for patients who have trouble swallowing. It can also be mixed with water and given via nasogastric tube; however, postpyloric administration should be avoided.
Plasma concentrations peak within a few hours after ingestion. Rivaroxaban is highly protein-bound, so it cannot be eliminated by hemodialysis.
The drug relies on renal elimination to a smaller degree than dabigatran, with one-third of the dose eliminated unchanged in the urine, one-third eliminated in the urine as inactive metabolite, and the remaining one-third eliminated in the feces. However, enough parent compound is cleared through the kidneys that the half-life of rivaroxaban increases from 8.3 hours in healthy individuals to 9.5 hours in patients whose creatinine clearance is less than 30 mL/min.21 As with dabigatran, the dose must be adjusted for renal impairment (Table 1).
Rivaroxaban has significant liver metabolism, specifically through the cytochrome P450 3A4 enzyme, and it is also a substrate of P-glycoprotein. Therefore, potential drug-drug interactions must be taken into account, as they may lead to important alterations in plasma concentrations (Table 3).
Apixaban, a factor Xa inhibitor
Apixaban is also an oral direct factor Xa inhibitor. It is the newest of the oral anticoagulants to be approved in the United States, specifically for preventing stroke in nonvalvular atrial fibrillation (Table 1).22
Pharmacokinetics of apixaban. Apixaban is produced as a tablet that is absorbed slowly through the gastrointestinal tract, mainly the distal small bowel and ascending colon (Table 2).23
Peak plasma concentrations are reached a few hours after ingestion. Like rivaroxaban, apixaban is highly protein-bound, so it cannot be removed by hemodialysis.
Apixaban is similar to rivaroxaban in that 27% of the parent compound is cleared through the kidneys, it undergoes significant hepatic metabolism through cytochrome P450 3A4, and it is a substrate for P-glycoprotein.
Drug-drug interactions must be considered as a potential source of altered drug exposure and clearance (Table 3).
Unlike dabigatran and rivaroxaban, dose reduction is not based on the calculated creatinine clearance. Instead, a reduced dose is required if the patient meets two of the following three criteria:
- Serum creatinine level ≥ 1.5 mg/dL
- Age ≥ 80
- Weight ≤ 60 kg (Table 1).
The American Heart Association/American Stroke Association guidelines further recommend against using apixaban in patients with a creatinine clearance less than 25 mL/min.24
Edoxaban, a factor Xa inhibitor in development
Edoxaban (Savaysa), another factor Xa inhibitor, is available in Japan and has been submitted for approval in the United States for treating VTE and for preventing stroke in patients with
PERIOPERATIVE CONSIDERATIONS IN ANTICOAGULATION
Before addressing the perioperative management of TSOACs, let us review the evidence guiding the perioperative management of any chronic anticoagulant.
In fact, no large prospective randomized trial has clearly defined the risks and benefits of using or withholding a bridging anticoagulation strategy around surgery and other procedures, though the PERIOP 2 and BRIDGE trials are currently ongoing.25,26 There are some data regarding continuing anticoagulation without interruption, but they have mainly been derived from specific groups (eg, patients on warfarin undergoing cardiac pacemaker or defibrillator placement) and in procedures that pose a very low risk of bleeding complications (eg, minor dental extractions, cataract surgery, dermatologic procedures).2,27 Recommendations are, therefore, necessarily based on small perioperative trials and data gleaned from cohort review and from studies that did not involve surgical patients.
Ultimately, the decisions whether to discontinue oral anticoagulants and whether to employ bridging anticoagulation are based on assumptions about the risks of bleeding and the risk of thrombotic events, with similar assumptions regarding the effects of anticoagulants on both outcomes. In addition, the relative acceptance of bleeding vs thrombotic risks implicitly guides these complex decisions.
Perioperative bleeding risk
Many risk factors specific to the patient and to the type of surgery affect the rates and severity of perioperative bleeding.28
As for patient-specific risk factors, a small retrospective cohort analysis revealed that a HAS-BLED score of 3 or higher was highly discriminating in predicting perioperative bleeding in atrial fibrillation patients receiving anticoagulation.29 (The HAS-BLED score is based on hypertension, abnormal renal or liver function, stroke, bleeding, labile international normalized ratio [INR], elderly [age > 65] and drug therapy.30) However, there are no widely validated tools that incorporate patient-specific factors to accurately predict bleeding risk in an individual patient.
Therefore, the American College of Chest Physicians (ACCP) guidelines suggest coarsely categorizing bleeding risk as either low or high solely on the basis of the type of procedure.2 Procedures considered “high-risk” have a risk greater than 1.5% to 2% and include urologic surgery involving the prostate or kidney, colonic polyp resections, surgeries involving highly vascular organs such as the liver or spleen, joint replacements, cancer surgeries, and cardiac or neurosurgical procedures.
Perioperative thrombotic risk
The ACCP guidelines2 place patients with atrial fibrillation, VTE, or mechanical heart valves in three risk groups for perioperative thromboembolism without anticoagulation, based on their annual risk of a thrombotic event:
- High risk—annual risk of a thrombotic event > 10%
- Moderate risk—5% to 10%
- Low risk—< 5%.
Comparing the risks calculated by these methods with the real-world risk of perioperative thrombosis highlights the problem of applying nonperioperative risk calculations: the perioperative period exposes patients to a higher risk than these models would predict.31 Nonetheless, these risk categorizations likely have some validity in stratifying patients into risk groups, even if the absolute risks are inaccurate.
Perioperative bridging for patients taking warfarin
Many patients with atrial fibrillation, VTE, or a mechanical heart valve need to interrupt their warfarin therapy because of the bleeding risk of an upcoming procedure.
The perioperative management of warfarin and other vitamin K antagonists is challenging because of the pharmacokinetics and pharmacodynamics of these drugs. Because it has a long half-life, warfarin usually must be stopped 4 to 5 days before a procedure in order to allow not only adequate clearance of the drug itself, but also restoration of functional clotting factors to normal or near-normal levels.12 Warfarin can generally be resumed 12 to 24 hours after surgery, assuming adequate hemostasis has been achieved, and it will again take several days for the INR to reach the therapeutic range.
The ACCP guidelines recommend using the perioperative risk of thromboembolism to make decisions about the need for bridging anticoagulation during warfarin interruption.2 They suggest that patients at high risk of thrombosis receive bridging with an alternative anticoagulant such as low-molecular-weight heparin or unfractionated heparin, because of the prolonged duration of subtherapeutic anticoagulation.
There has been clinical interest in using a TSOAC instead of low-molecular-weight or unfractionated heparin for bridging in the perioperative setting. Although this approach may be attractive from a cost and convenience perspective, it cannot be endorsed as yet because of the lack of information on the pros and cons of such an approach.
Patients at low thrombotic risk do not require bridging. In patients at moderate risk, the decision to bridge or not to bridge is based on careful consideration of patient-specific and surgery-specific factors.
PERIOPERATIVE MANAGEMENT OF TARGET-SPECIFIC ORAL ANTICOAGULANTS
As summarized above, the perioperative management strategy for chronic anticoagulation is based on limited evidence, even for drugs as well established as warfarin.
The most recent ACCP guidelines on the perioperative management of antithrombotic therapy do not mention TSOACs.2 For now, the management strategy must be based on the pharmacokinetics of the drugs, package inserts from the manufacturers, and expert recommendations.3,14,23,32–34 Fortunately, because TSOACs have a more favorable pharmacokinetic profile than that of warfarin, their perioperative uses should be more streamlined. As always, the goal is to minimize the risk of both periprocedural bleeding and thromboembolism.
Timing of cessation of anticoagulation
The timing of cessation of TSOACs before an elective procedure depends primarily on two factors: the bleeding risk of the procedure and the patient’s renal function. Complete clearance of the medication is not necessary in all circumstances.
TSOACs should be stopped four to five half-lives before a procedure with a high bleeding risk, so that there is no or only minimal residual anticoagulant effect. The drug can be stopped two to three half-lives before a procedure with a low bleeding risk. Remember: the half-life increases as creatinine clearance decreases.
Specific recommendations may vary across institutions, but a suggested strategy is shown in Table 4.3,4,21,23,32–35 For the small subset of patients on P-glycoprotein or cytochrome P450 inhibitors or inducers, further adjustment in the time of discontinuation may be required.
Therapy does not need to be interrupted for procedures with a very low bleeding risk, as defined above.33,34 There is also preliminary evidence that TSOACs, similar to warfarin, may be continued during cardiac pacemaker or defibrillator placement.36
Evidence from clinical trials of perioperative TSOAC management
While the above recommendations are logical, studies are needed to prospectively evaluate perioperative management strategies.
The RE-LY trial (Randomized Evaluation of Long-Term Anticoagulation Therapy), which compared the effects of dabigatran and warfarin in preventing stroke in patients with atrial fibrillation, is one of the few clinical trials that also looked at periprocedural bleeding.37 About a quarter of the RE-LY participants required interruption of anticoagulation for a procedure.
Warfarin was managed according to local practices. For most of the study, the protocol required that dabigatran be discontinued 24 hours before a procedure, regardless of renal function or procedure type. The protocol was later amended and closely mirrored the management plan outlined in Table 4.
With either protocol, there was no statistically significant difference between dabigatran and warfarin in the rates of bleeding and thrombotic complications in the 7 days before or 30 days after the procedure.
A major limitation of the study was that most patients underwent a procedure with a low bleeding risk, so the analysis was likely underpowered to evaluate rates of bleeding in higher-risk procedures.
The ROCKET-AF trial (Rivaroxaban Once-daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) also shed light on periprocedural bleeding.15 About 15% of the participants required temporary interruption of anticoagulation for a surgical or invasive procedure.38
The study protocol called for discontinuing rivaroxaban 2 days before any procedure. Warfarin was to be held for 4 days to achieve a goal INR of 1.5 or less.15
Rates of major and nonmajor clinically significant bleeding at 30 days were similar with rivaroxaban and with warfarin.38 As with the RE-LY trial, the retrospective analysis was probably underpowered for assessing rates of bleeding in procedures with higher risk.
Perioperative bridging
While stopping a TSOAC in the perioperative period decreases the risk of bleeding, it naturally increases the risk of thromboembolism. However, patients on TSOACs should not routinely require perioperative bridging with an alternative anticoagulant, regardless of thrombotic risk.
Of note, dabigatran, rivaroxaban, and apixaban carry black-box warnings that discontinuation places patients at higher risk of thrombotic events.3,14,23 These warnings further state that coverage with an alternative anticoagulant should be strongly considered during interruption of therapy for reasons other than pathologic bleeding.
However, it does not necessarily follow that perioperative bridging is required. For example, the warning for rivaroxaban is based on the finding in the ROCKET-AF trial that patients in the rivaroxaban group had higher rates of stroke than those in the warfarin group after the study drugs were stopped at the end of the trial.39 While there was initial concern that this could represent a prothrombotic rebound effect, the authors subsequently showed that patients in the rivaroxaban group were more likely to have had a subtherapeutic INR when transitioning to open-label vitamin-K-antagonist therapy.39,40 There was no difference in the rate of stroke or systemic embolism between the rivaroxaban and warfarin groups when anticoagulation was temporarily interrupted for a procedure.38
The risks and benefits of perioperative bridging with TSOACs are difficult to evaluate, given the dearth of trial data. In the RE-LY trial, only 17% of patients on dabigatran and 28% of patients on warfarin underwent periprocedural bridging.37 The selection criteria and protocol for bridging were not reported. In the ROCKET-AF trial, only 9% of patients received bridging therapy despite a mean CHADS2 score of 3.4.38 (The CHADS2 score is calculated as 1 point each for congestive heart failure, hypertension, age ≥ 75, and diabetes; 2 points for stroke or transient ischemic attack.) The decision to bridge or not was left to the individual investigator. As a result, the literature offers diverse opinions about the appropriateness of transitioning to an alternative anticoagulant.41–43
Bridging does not make sense in most instances, since anticoagulants such as low-molecular-weight heparin have pharmacokinetics similar to those of the available TSOACs and also depend on renal clearance.41 However, there may be situations in which patients must be switched to a parenteral anticoagulant such as unfractionated or low-molecular-weight heparin. For example, if a TSOAC has to be held, the patient has acute renal failure, and a needed procedure is still several days away, it would be reasonable to start a heparin drip for an inpatient at increased thrombotic risk.
In patients with normal renal function, these alternative anticoagulants should be started at the time the next TSOAC dose would have been due.3,14,23 In patients with reduced renal function, initiation of an alternative anticoagulant may need to be delayed 12 to 48 hours depending on which TSOAC is being used, as well as on the degree of renal dysfunction. This delay would help ensure that the onset of anticoagulation with the alternative anticoagulant is timed with the offset of therapeutic anticoagulation with the TSOAC.
Although limited, information from available coagulation assays may assist with the timing of initiation of an alternative anticoagulant (see the following section on laboratory monitoring). Serial testing with appropriate coagulation assays may help identify when most of a TSOAC has been cleared from a patient.
Laboratory monitoring
Inevitably, some patients on TSOACs require urgent or emergency surgery. In certain situations, such as before an orthopedic spine procedure, in which the complications of bleeding could be devastating, it may be necessary to know if any residual anticoagulant effect is present.
Monitoring dabigatran. As one might expect, direct thrombin inhibitors such as dabigatran can prolong the prothrombin time and activated partial thromboplastin time (aPTT).44–47 However, the prothrombin time is not recommended for assessing the level of anticoagulation from dabigatran. Many institutions may be using a normal aPTT to rule out therapeutic concentrations of dabigatran, based on results from early in vitro and ex vivo studies.46 While appealing from a practical standpoint, practitioners should exercise caution when relying on the aPTT to assess the risk of perioperative bleeding. A more recent investigation in patients treated with dabigatran found that up to 35% of patients with a normal aPTT still had a plasma concentration in the therapeutic range.48
The thrombin time and ecarin clotting time are more sensitive tests for dabigatran. A normal thrombin time or ecarin clotting time indicates that no or only minimal dabigatran is present.48 Unfortunately, these two tests often are either unavailable or are associated with long turnaround times, which limits their usefulness in the perioperative setting.
Monitoring rivaroxaban and apixaban. Factor Xa inhibitors such as rivaroxaban and apixaban can also influence the prothrombin time and aPTT (Figure 1).44–47,49,50 The aPTT is relatively insensitive to these drugs at low concentrations. It has been suggested that a normal prothrombin time can reasonably exclude therapeutic concentrations of rivaroxaban.45,46 However, the effects on the prothrombin time are highly variable, changing with the reagent used.49,50 In addition, apixaban appears to have less impact on the prothrombin time overall. The INR is not recommended for monitoring the effect of factor Xa inhibitors.
Anti-factor Xa assays likely represent the best option to provide true quantitative information on the level of anticoagulation with either rivaroxaban or apixaban. However, the assays must be specifically calibrated for each drug for results to be useful. (Anti-factor Xa assays cannot be used for heparin or low-molecular-weight heparin.) Further, most institutions do not yet have this capability. When appropriately calibrated, normal anti-factor Xa levels would exclude any effect of rivaroxaban or apixaban.
Reversal of anticoagulation
If patients on TSOACs require emergency surgery or present with significant bleeding in the setting of persistent anticoagulation, it may be necessary to try to reverse the anticoagulation.
Unlike warfarin or heparin, TSOACS do not have specific reversal agents, though specific antidotes are being developed. For example, researchers are evaluating antibodies capable of neutralizing dabigatran, as well as recombinant thrombin and factor Xa molecules that could antagonize dabigatran and rivaroxaban, respectively.51–53
Reversal can be attempted by neutralizing or removing the offending drug. Activated charcoal may be able to reduce absorption of TSOACs that were recently ingested,44 and dabigatran can be removed by hemodialysis.
However, certain practical considerations may limit the use of dialysis in the perioperative period. Insertion of a temporary dialysis line in an anticoagulated patient poses additional bleeding risks. A standard 4-hour hemodialysis session may remove only about 70% of dabigatran from the plasma, which may not be enough to prevent perioperative bleeding.54 Dabigatran also tends to redistribute from adipose tissue back into plasma after each dialysis session.55 Serial sessions of high-flux intermittent hemodialysis or continuous renal replacement therapy may therefore be needed to counteract rebound elevations in the dabigatran concentration.
Reversal can also be attempted through activation of the coagulation cascade via other mechanisms. Fresh-frozen plasma is unlikely to be a practical solution for reversal.44 Although it can readily replace the clotting factors depleted by vitamin K antagonists, large volumes of fresh-frozen plasma would be needed to overwhelm thrombin or factor Xa inhibition by TSOACs.
There are limited data on the use of prothrombin complex concentrates or recombinant activated factor VIIa in patients on TSOACs, though their use can be considered.56 In a trial in 12 healthy participants, a nonactivated four-factor prothrombin complex concentrate containing factors II, VII, IX, and X immediately and completely reversed the anticoagulant effect of rivaroxaban but had no effect on dabigatran.57 Before 2013, there were no nonactivated four-factor prothrombin complex concentrates available in the United States. The FDA has since approved Kcentra for the urgent reversal of vitamin K antagonists, meaning that the reversal of TSOACs in major bleeding events would still be off-label.58 Giving any of the clotting factors carries a risk of thromboembolism.
Resumption of anticoagulation
TSOACs have a rapid onset of action, and therapeutic levels are reached within a few hours of administration.
Extrapolating from the ACCP guidelines, TSOACs can generally be restarted at therapeutic doses 24 hours after low-bleeding-risk procedures.2 Therapeutic dosing should be delayed 48 to 72 hours after a procedure with a high bleeding risk, assuming adequate hemostasis has been achieved. Prophylactic unfractionated heparin or low-molecular-weight heparin therapy can be given in the interim if deemed safe. Alternatively, for orthopedic patients ultimately transitioning back to therapeutic rivaroxaban after hip or knee arthroplasty, prophylactic rivaroxaban doses can be started 6 to 10 hours after surgery.14
There are numerous reasons why the resumption of TSOACs may have to be delayed after surgery, including nothing-by-mouth status, postoperative nausea and vomiting, ileus, gastric or bowel resection, and the anticipated need for future procedures. Since dabigatran capsules cannot be crushed, they cannot be given via nasogastric tube in patients with postoperative dysphagia. Parenteral anticoagulants should be used until these issues resolve.
Unfractionated heparin is still the preferred anticoagulant in unstable or potentially unstable patients, given its ease of monitoring, quick offset of action, and reversibility. When patients have stabilized, TSOACs can be resumed when the next dose of low-molecular-weight heparin would have been due or when the unfractionated heparin drip is discontinued.3,14,23
UNTIL EVIDENCE-BASED GUIDELINES ARE DEVELOPED
The development of TSOACs has ushered in an exciting new era for anticoagulant therapy. Providers involved in perioperative medicine will increasingly encounter patients on dabigatran, rivaroxaban, and apixaban. However, until evidence-based guidelines are developed for these new anticoagulants, clinicians will have to apply their knowledge of pharmacology and critically evaluate expert recommendations in order to manage patients safely throughout the perioperative period.
More then 2.5 million patients in the United States are on long-term anticoagulation therapy for atrial fibrillation, venous thromboembolic disease, or mechanical heart valves,1 and the number is expected to rise as the population ages. Each year, about 10% of these patients undergo an invasive procedure or surgery that requires temporary interruption of anticoagulation.2
Most physicians are familiar with the perioperative management of warfarin, a vitamin K antagonist, since for decades it has been the sole oral anticoagulant available. However, many physicians lack experience with the three target-specific oral anticoagulants (TSOACs; also known as “novel” oral anticoagulants) approved so far: the direct thrombin inhibitor dabigatran (Pradaxa) and the direct factor Xa inhibitors rivaroxaban (Xarelto) and apixaban (Eliquis).
With their rapid onset of action, predictable pharmacokinetics, relatively short half-lives, and fewer drug-drug interactions than warfarin, TSOACs overcome many of the limitations of the older oral anticoagulant warfarin. In many ways, these qualities simplify the perioperative management of anticoagulation. At the same time, these new drugs also bring new challenges: caution is needed in patients with renal impairment; the level of anticoagulation is difficult to assess; and there is no specific antidote or standardized procedure to reverse their anticoagulant effect. While various periprocedural protocols for TSOAC therapy have been proposed, evidence-based guidelines are still to come.
This article first discusses the pharmacology of dabigatran, rivaroxaban, and apixaban that is pertinent to the perioperative period. It then briefly reviews the general principles of perioperative management of anticoagulation. The final section provides specific recommendations for the perioperative management of TSOACs.
PHARMACOLOGY OF TARGET-SPECIFIC ORAL ANTICOAGULANTS
Dabigatran, a factor IIa inhibitor
Dabigatran is an oral direct thrombin (factor IIa) inhibitor. It exerts its anticoagulant effect by blocking the generation of fibrin, inhibiting platelet aggregation, and dampening the activity of factors V, VIII, and XI (Figure 1).3,4 From its introduction in October 2010 through August 2012, nearly 3.7 million prescriptions were dispensed to 725,000 patients in the United States.5
Indications for dabigatran. Dabigatran is approved in the United States and Canada for preventing stroke in nonvalvular atrial fibrillation (Table 1).6 More recently, it received US approval for treating deep vein thrombosis or pulmonary embolism after 5 to 10 days of a parenteral anticoagulant.7,8 It is also approved in Europe and Canada for preventing venous thromboembolism (VTE) after total hip replacement and knee arthroplasty.9,10
Dabigatran is contraindicated in patients with a mechanical heart valve, based on a phase 2 study in which it conferred a higher risk of thromboembolism and bleeding than warfarin.3,11
Pharmacokinetics of dabigatran. Dabigatran is formulated as a prodrug, dabigatran etexilate, in a capsule containing multiple small pellets.12 The capsules should not be crushed, as this significantly increases oral bioavailability. The prodrug is absorbed across the gastric mucosa and is then rapidly converted to the active form (Table 2).
Plasma concentrations peak within 2 hours of ingestion, which means that therapeutic anticoagulation is achieved shortly after taking the drug.
Only 35% of dabigatran is protein-bound, which allows it to be removed by hemodialysis. Nearly 85% of the drug is eliminated in the urine. It has a half-life of 13 to 15 hours in patients with normal renal function.3 However, its half-life increases to about 27 hours in patients whose creatinine clearance is less than 30 mL/min. As a result, the dose must be reduced in patients with renal impairment (Table 1).
Dabigatran is not metabolized by the cytochrome P450 enzymes, but it is a substrate for P-glycoprotein, so it still has the potential for drug-drug interactions.3 Practitioners should be familiar with these potential interactions (Table 3), as they can result in higher- or lower-than-expected plasma concentrations of dabigatran in the perioperative period.13
Rivaroxaban, a factor Xa inhibitor
Rivaroxaban is an oral direct factor Xa inhibitor. It has been approved by the US Food and Drug Administration (FDA) for the prevention of stroke in nonvalvular atrial fibrillation, for VTE treatment, and for VTE prophylaxis after hip replacement or knee replacement (Table 1).14–20 It has not yet been studied in patients with hip fracture.
Pharmacokinetics of rivaroxaban. Rivaroxaban is manufactured as a tablet that is best absorbed in the stomach (Table 2).14 In contrast to dabigatran, it can be crushed and, for example, mixed with applesauce for patients who have trouble swallowing. It can also be mixed with water and given via nasogastric tube; however, postpyloric administration should be avoided.
Plasma concentrations peak within a few hours after ingestion. Rivaroxaban is highly protein-bound, so it cannot be eliminated by hemodialysis.
The drug relies on renal elimination to a smaller degree than dabigatran, with one-third of the dose eliminated unchanged in the urine, one-third eliminated in the urine as inactive metabolite, and the remaining one-third eliminated in the feces. However, enough parent compound is cleared through the kidneys that the half-life of rivaroxaban increases from 8.3 hours in healthy individuals to 9.5 hours in patients whose creatinine clearance is less than 30 mL/min.21 As with dabigatran, the dose must be adjusted for renal impairment (Table 1).
Rivaroxaban has significant liver metabolism, specifically through the cytochrome P450 3A4 enzyme, and it is also a substrate of P-glycoprotein. Therefore, potential drug-drug interactions must be taken into account, as they may lead to important alterations in plasma concentrations (Table 3).
Apixaban, a factor Xa inhibitor
Apixaban is also an oral direct factor Xa inhibitor. It is the newest of the oral anticoagulants to be approved in the United States, specifically for preventing stroke in nonvalvular atrial fibrillation (Table 1).22
Pharmacokinetics of apixaban. Apixaban is produced as a tablet that is absorbed slowly through the gastrointestinal tract, mainly the distal small bowel and ascending colon (Table 2).23
Peak plasma concentrations are reached a few hours after ingestion. Like rivaroxaban, apixaban is highly protein-bound, so it cannot be removed by hemodialysis.
Apixaban is similar to rivaroxaban in that 27% of the parent compound is cleared through the kidneys, it undergoes significant hepatic metabolism through cytochrome P450 3A4, and it is a substrate for P-glycoprotein.
Drug-drug interactions must be considered as a potential source of altered drug exposure and clearance (Table 3).
Unlike dabigatran and rivaroxaban, dose reduction is not based on the calculated creatinine clearance. Instead, a reduced dose is required if the patient meets two of the following three criteria:
- Serum creatinine level ≥ 1.5 mg/dL
- Age ≥ 80
- Weight ≤ 60 kg (Table 1).
The American Heart Association/American Stroke Association guidelines further recommend against using apixaban in patients with a creatinine clearance less than 25 mL/min.24
Edoxaban, a factor Xa inhibitor in development
Edoxaban (Savaysa), another factor Xa inhibitor, is available in Japan and has been submitted for approval in the United States for treating VTE and for preventing stroke in patients with
PERIOPERATIVE CONSIDERATIONS IN ANTICOAGULATION
Before addressing the perioperative management of TSOACs, let us review the evidence guiding the perioperative management of any chronic anticoagulant.
In fact, no large prospective randomized trial has clearly defined the risks and benefits of using or withholding a bridging anticoagulation strategy around surgery and other procedures, though the PERIOP 2 and BRIDGE trials are currently ongoing.25,26 There are some data regarding continuing anticoagulation without interruption, but they have mainly been derived from specific groups (eg, patients on warfarin undergoing cardiac pacemaker or defibrillator placement) and in procedures that pose a very low risk of bleeding complications (eg, minor dental extractions, cataract surgery, dermatologic procedures).2,27 Recommendations are, therefore, necessarily based on small perioperative trials and data gleaned from cohort review and from studies that did not involve surgical patients.
Ultimately, the decisions whether to discontinue oral anticoagulants and whether to employ bridging anticoagulation are based on assumptions about the risks of bleeding and the risk of thrombotic events, with similar assumptions regarding the effects of anticoagulants on both outcomes. In addition, the relative acceptance of bleeding vs thrombotic risks implicitly guides these complex decisions.
Perioperative bleeding risk
Many risk factors specific to the patient and to the type of surgery affect the rates and severity of perioperative bleeding.28
As for patient-specific risk factors, a small retrospective cohort analysis revealed that a HAS-BLED score of 3 or higher was highly discriminating in predicting perioperative bleeding in atrial fibrillation patients receiving anticoagulation.29 (The HAS-BLED score is based on hypertension, abnormal renal or liver function, stroke, bleeding, labile international normalized ratio [INR], elderly [age > 65] and drug therapy.30) However, there are no widely validated tools that incorporate patient-specific factors to accurately predict bleeding risk in an individual patient.
Therefore, the American College of Chest Physicians (ACCP) guidelines suggest coarsely categorizing bleeding risk as either low or high solely on the basis of the type of procedure.2 Procedures considered “high-risk” have a risk greater than 1.5% to 2% and include urologic surgery involving the prostate or kidney, colonic polyp resections, surgeries involving highly vascular organs such as the liver or spleen, joint replacements, cancer surgeries, and cardiac or neurosurgical procedures.
Perioperative thrombotic risk
The ACCP guidelines2 place patients with atrial fibrillation, VTE, or mechanical heart valves in three risk groups for perioperative thromboembolism without anticoagulation, based on their annual risk of a thrombotic event:
- High risk—annual risk of a thrombotic event > 10%
- Moderate risk—5% to 10%
- Low risk—< 5%.
Comparing the risks calculated by these methods with the real-world risk of perioperative thrombosis highlights the problem of applying nonperioperative risk calculations: the perioperative period exposes patients to a higher risk than these models would predict.31 Nonetheless, these risk categorizations likely have some validity in stratifying patients into risk groups, even if the absolute risks are inaccurate.
Perioperative bridging for patients taking warfarin
Many patients with atrial fibrillation, VTE, or a mechanical heart valve need to interrupt their warfarin therapy because of the bleeding risk of an upcoming procedure.
The perioperative management of warfarin and other vitamin K antagonists is challenging because of the pharmacokinetics and pharmacodynamics of these drugs. Because it has a long half-life, warfarin usually must be stopped 4 to 5 days before a procedure in order to allow not only adequate clearance of the drug itself, but also restoration of functional clotting factors to normal or near-normal levels.12 Warfarin can generally be resumed 12 to 24 hours after surgery, assuming adequate hemostasis has been achieved, and it will again take several days for the INR to reach the therapeutic range.
The ACCP guidelines recommend using the perioperative risk of thromboembolism to make decisions about the need for bridging anticoagulation during warfarin interruption.2 They suggest that patients at high risk of thrombosis receive bridging with an alternative anticoagulant such as low-molecular-weight heparin or unfractionated heparin, because of the prolonged duration of subtherapeutic anticoagulation.
There has been clinical interest in using a TSOAC instead of low-molecular-weight or unfractionated heparin for bridging in the perioperative setting. Although this approach may be attractive from a cost and convenience perspective, it cannot be endorsed as yet because of the lack of information on the pros and cons of such an approach.
Patients at low thrombotic risk do not require bridging. In patients at moderate risk, the decision to bridge or not to bridge is based on careful consideration of patient-specific and surgery-specific factors.
PERIOPERATIVE MANAGEMENT OF TARGET-SPECIFIC ORAL ANTICOAGULANTS
As summarized above, the perioperative management strategy for chronic anticoagulation is based on limited evidence, even for drugs as well established as warfarin.
The most recent ACCP guidelines on the perioperative management of antithrombotic therapy do not mention TSOACs.2 For now, the management strategy must be based on the pharmacokinetics of the drugs, package inserts from the manufacturers, and expert recommendations.3,14,23,32–34 Fortunately, because TSOACs have a more favorable pharmacokinetic profile than that of warfarin, their perioperative uses should be more streamlined. As always, the goal is to minimize the risk of both periprocedural bleeding and thromboembolism.
Timing of cessation of anticoagulation
The timing of cessation of TSOACs before an elective procedure depends primarily on two factors: the bleeding risk of the procedure and the patient’s renal function. Complete clearance of the medication is not necessary in all circumstances.
TSOACs should be stopped four to five half-lives before a procedure with a high bleeding risk, so that there is no or only minimal residual anticoagulant effect. The drug can be stopped two to three half-lives before a procedure with a low bleeding risk. Remember: the half-life increases as creatinine clearance decreases.
Specific recommendations may vary across institutions, but a suggested strategy is shown in Table 4.3,4,21,23,32–35 For the small subset of patients on P-glycoprotein or cytochrome P450 inhibitors or inducers, further adjustment in the time of discontinuation may be required.
Therapy does not need to be interrupted for procedures with a very low bleeding risk, as defined above.33,34 There is also preliminary evidence that TSOACs, similar to warfarin, may be continued during cardiac pacemaker or defibrillator placement.36
Evidence from clinical trials of perioperative TSOAC management
While the above recommendations are logical, studies are needed to prospectively evaluate perioperative management strategies.
The RE-LY trial (Randomized Evaluation of Long-Term Anticoagulation Therapy), which compared the effects of dabigatran and warfarin in preventing stroke in patients with atrial fibrillation, is one of the few clinical trials that also looked at periprocedural bleeding.37 About a quarter of the RE-LY participants required interruption of anticoagulation for a procedure.
Warfarin was managed according to local practices. For most of the study, the protocol required that dabigatran be discontinued 24 hours before a procedure, regardless of renal function or procedure type. The protocol was later amended and closely mirrored the management plan outlined in Table 4.
With either protocol, there was no statistically significant difference between dabigatran and warfarin in the rates of bleeding and thrombotic complications in the 7 days before or 30 days after the procedure.
A major limitation of the study was that most patients underwent a procedure with a low bleeding risk, so the analysis was likely underpowered to evaluate rates of bleeding in higher-risk procedures.
The ROCKET-AF trial (Rivaroxaban Once-daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) also shed light on periprocedural bleeding.15 About 15% of the participants required temporary interruption of anticoagulation for a surgical or invasive procedure.38
The study protocol called for discontinuing rivaroxaban 2 days before any procedure. Warfarin was to be held for 4 days to achieve a goal INR of 1.5 or less.15
Rates of major and nonmajor clinically significant bleeding at 30 days were similar with rivaroxaban and with warfarin.38 As with the RE-LY trial, the retrospective analysis was probably underpowered for assessing rates of bleeding in procedures with higher risk.
Perioperative bridging
While stopping a TSOAC in the perioperative period decreases the risk of bleeding, it naturally increases the risk of thromboembolism. However, patients on TSOACs should not routinely require perioperative bridging with an alternative anticoagulant, regardless of thrombotic risk.
Of note, dabigatran, rivaroxaban, and apixaban carry black-box warnings that discontinuation places patients at higher risk of thrombotic events.3,14,23 These warnings further state that coverage with an alternative anticoagulant should be strongly considered during interruption of therapy for reasons other than pathologic bleeding.
However, it does not necessarily follow that perioperative bridging is required. For example, the warning for rivaroxaban is based on the finding in the ROCKET-AF trial that patients in the rivaroxaban group had higher rates of stroke than those in the warfarin group after the study drugs were stopped at the end of the trial.39 While there was initial concern that this could represent a prothrombotic rebound effect, the authors subsequently showed that patients in the rivaroxaban group were more likely to have had a subtherapeutic INR when transitioning to open-label vitamin-K-antagonist therapy.39,40 There was no difference in the rate of stroke or systemic embolism between the rivaroxaban and warfarin groups when anticoagulation was temporarily interrupted for a procedure.38
The risks and benefits of perioperative bridging with TSOACs are difficult to evaluate, given the dearth of trial data. In the RE-LY trial, only 17% of patients on dabigatran and 28% of patients on warfarin underwent periprocedural bridging.37 The selection criteria and protocol for bridging were not reported. In the ROCKET-AF trial, only 9% of patients received bridging therapy despite a mean CHADS2 score of 3.4.38 (The CHADS2 score is calculated as 1 point each for congestive heart failure, hypertension, age ≥ 75, and diabetes; 2 points for stroke or transient ischemic attack.) The decision to bridge or not was left to the individual investigator. As a result, the literature offers diverse opinions about the appropriateness of transitioning to an alternative anticoagulant.41–43
Bridging does not make sense in most instances, since anticoagulants such as low-molecular-weight heparin have pharmacokinetics similar to those of the available TSOACs and also depend on renal clearance.41 However, there may be situations in which patients must be switched to a parenteral anticoagulant such as unfractionated or low-molecular-weight heparin. For example, if a TSOAC has to be held, the patient has acute renal failure, and a needed procedure is still several days away, it would be reasonable to start a heparin drip for an inpatient at increased thrombotic risk.
In patients with normal renal function, these alternative anticoagulants should be started at the time the next TSOAC dose would have been due.3,14,23 In patients with reduced renal function, initiation of an alternative anticoagulant may need to be delayed 12 to 48 hours depending on which TSOAC is being used, as well as on the degree of renal dysfunction. This delay would help ensure that the onset of anticoagulation with the alternative anticoagulant is timed with the offset of therapeutic anticoagulation with the TSOAC.
Although limited, information from available coagulation assays may assist with the timing of initiation of an alternative anticoagulant (see the following section on laboratory monitoring). Serial testing with appropriate coagulation assays may help identify when most of a TSOAC has been cleared from a patient.
Laboratory monitoring
Inevitably, some patients on TSOACs require urgent or emergency surgery. In certain situations, such as before an orthopedic spine procedure, in which the complications of bleeding could be devastating, it may be necessary to know if any residual anticoagulant effect is present.
Monitoring dabigatran. As one might expect, direct thrombin inhibitors such as dabigatran can prolong the prothrombin time and activated partial thromboplastin time (aPTT).44–47 However, the prothrombin time is not recommended for assessing the level of anticoagulation from dabigatran. Many institutions may be using a normal aPTT to rule out therapeutic concentrations of dabigatran, based on results from early in vitro and ex vivo studies.46 While appealing from a practical standpoint, practitioners should exercise caution when relying on the aPTT to assess the risk of perioperative bleeding. A more recent investigation in patients treated with dabigatran found that up to 35% of patients with a normal aPTT still had a plasma concentration in the therapeutic range.48
The thrombin time and ecarin clotting time are more sensitive tests for dabigatran. A normal thrombin time or ecarin clotting time indicates that no or only minimal dabigatran is present.48 Unfortunately, these two tests often are either unavailable or are associated with long turnaround times, which limits their usefulness in the perioperative setting.
Monitoring rivaroxaban and apixaban. Factor Xa inhibitors such as rivaroxaban and apixaban can also influence the prothrombin time and aPTT (Figure 1).44–47,49,50 The aPTT is relatively insensitive to these drugs at low concentrations. It has been suggested that a normal prothrombin time can reasonably exclude therapeutic concentrations of rivaroxaban.45,46 However, the effects on the prothrombin time are highly variable, changing with the reagent used.49,50 In addition, apixaban appears to have less impact on the prothrombin time overall. The INR is not recommended for monitoring the effect of factor Xa inhibitors.
Anti-factor Xa assays likely represent the best option to provide true quantitative information on the level of anticoagulation with either rivaroxaban or apixaban. However, the assays must be specifically calibrated for each drug for results to be useful. (Anti-factor Xa assays cannot be used for heparin or low-molecular-weight heparin.) Further, most institutions do not yet have this capability. When appropriately calibrated, normal anti-factor Xa levels would exclude any effect of rivaroxaban or apixaban.
Reversal of anticoagulation
If patients on TSOACs require emergency surgery or present with significant bleeding in the setting of persistent anticoagulation, it may be necessary to try to reverse the anticoagulation.
Unlike warfarin or heparin, TSOACS do not have specific reversal agents, though specific antidotes are being developed. For example, researchers are evaluating antibodies capable of neutralizing dabigatran, as well as recombinant thrombin and factor Xa molecules that could antagonize dabigatran and rivaroxaban, respectively.51–53
Reversal can be attempted by neutralizing or removing the offending drug. Activated charcoal may be able to reduce absorption of TSOACs that were recently ingested,44 and dabigatran can be removed by hemodialysis.
However, certain practical considerations may limit the use of dialysis in the perioperative period. Insertion of a temporary dialysis line in an anticoagulated patient poses additional bleeding risks. A standard 4-hour hemodialysis session may remove only about 70% of dabigatran from the plasma, which may not be enough to prevent perioperative bleeding.54 Dabigatran also tends to redistribute from adipose tissue back into plasma after each dialysis session.55 Serial sessions of high-flux intermittent hemodialysis or continuous renal replacement therapy may therefore be needed to counteract rebound elevations in the dabigatran concentration.
Reversal can also be attempted through activation of the coagulation cascade via other mechanisms. Fresh-frozen plasma is unlikely to be a practical solution for reversal.44 Although it can readily replace the clotting factors depleted by vitamin K antagonists, large volumes of fresh-frozen plasma would be needed to overwhelm thrombin or factor Xa inhibition by TSOACs.
There are limited data on the use of prothrombin complex concentrates or recombinant activated factor VIIa in patients on TSOACs, though their use can be considered.56 In a trial in 12 healthy participants, a nonactivated four-factor prothrombin complex concentrate containing factors II, VII, IX, and X immediately and completely reversed the anticoagulant effect of rivaroxaban but had no effect on dabigatran.57 Before 2013, there were no nonactivated four-factor prothrombin complex concentrates available in the United States. The FDA has since approved Kcentra for the urgent reversal of vitamin K antagonists, meaning that the reversal of TSOACs in major bleeding events would still be off-label.58 Giving any of the clotting factors carries a risk of thromboembolism.
Resumption of anticoagulation
TSOACs have a rapid onset of action, and therapeutic levels are reached within a few hours of administration.
Extrapolating from the ACCP guidelines, TSOACs can generally be restarted at therapeutic doses 24 hours after low-bleeding-risk procedures.2 Therapeutic dosing should be delayed 48 to 72 hours after a procedure with a high bleeding risk, assuming adequate hemostasis has been achieved. Prophylactic unfractionated heparin or low-molecular-weight heparin therapy can be given in the interim if deemed safe. Alternatively, for orthopedic patients ultimately transitioning back to therapeutic rivaroxaban after hip or knee arthroplasty, prophylactic rivaroxaban doses can be started 6 to 10 hours after surgery.14
There are numerous reasons why the resumption of TSOACs may have to be delayed after surgery, including nothing-by-mouth status, postoperative nausea and vomiting, ileus, gastric or bowel resection, and the anticipated need for future procedures. Since dabigatran capsules cannot be crushed, they cannot be given via nasogastric tube in patients with postoperative dysphagia. Parenteral anticoagulants should be used until these issues resolve.
Unfractionated heparin is still the preferred anticoagulant in unstable or potentially unstable patients, given its ease of monitoring, quick offset of action, and reversibility. When patients have stabilized, TSOACs can be resumed when the next dose of low-molecular-weight heparin would have been due or when the unfractionated heparin drip is discontinued.3,14,23
UNTIL EVIDENCE-BASED GUIDELINES ARE DEVELOPED
The development of TSOACs has ushered in an exciting new era for anticoagulant therapy. Providers involved in perioperative medicine will increasingly encounter patients on dabigatran, rivaroxaban, and apixaban. However, until evidence-based guidelines are developed for these new anticoagulants, clinicians will have to apply their knowledge of pharmacology and critically evaluate expert recommendations in order to manage patients safely throughout the perioperative period.
- Douketis JD, Berger PB, Dunn AS, et al; American College of Chest Physicians. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):299S–339S.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e326S–e350S.
- Boehringer Ingelheim Pharmaceuticals, Inc. PRADAXA (dabigatran) package insert. http://bidocs.boehringer-ingelheim.com/BIWebAc-cess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf. Accessed August 6, 2014.
- Levy JH, Faraoni D, Spring JL, Douketis JD, Samama CM. Managing new oral anticoagulants in the perioperative and intensive care unit setting. Anesthesiology 2013; 118:1466–1474.
- US Food and Drug Administration (FDA). FDA drug safety communication: update on the risk for serious bleeding events with the anticoagulant Pradaxa (dabigatran). www.fda.gov/drugs/drugsafety/ucm326580.htm. Accessed August 6, 2014.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- Schulman S, Kearon C, Kakkar AK, et al; RE-MEDY Trial Investigators. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013; 368:709–718.
- Eriksson BI, Dahl OE, Rosencher N, Büller HR, et al; RE-NOVATE Study Group. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 2007; 370:949–956.
- Eriksson BI, Dahl OE, Rosencher N, et al; RE-MODEL Study Group. Oral dabigatran etexilate vs subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007; 5:2178–2185.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013; 369:1206–1214.
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G; American College of Chest Physicians. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e44S–e88S.
- Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos 2008; 36:386–399.
- Janssen Pharmaceuticals, Inc. XARELTO (rivaroxaban) package insert. www.xareltohcp.com/about-xarelto/about-xarelto.html?utm_source=google&utm_medium=cpc&utm_campaign=Branded+-+Broad&utm_term=xarelto%20rivaroxaban&utm_content=Xarelto+Rivaroxaban|mkwid|sxSDxPb4m_dc|pcrid|34667840494. Accessed August 6, 2014.
- Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–391.
- EINSTEIN Investigators; Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363:2499–2510.
- EINSTEIN–PE Investigators; Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012; 366:1287–1297.
- Eriksson BI, Borris LC, Friedman RJ, et al; RECORD1 Study Group. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008; 358:2765–2775.
- Kakkar AK, Brenner B, Dahl OE, et al; RECORD2 Investigators. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet 2008; 372:31–39.
- Lassen MR, Ageno W, Borris LC, et al; RECORD3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008; 358:2776–2786.
- Kubitza D, Becka M, Mueck W, et al. Effects of renal impairment on the pharmacokinetics, pharmacodynamics and safety of rivaroxaban, an oral, direct factor Xa inhibitor. Br J Clin Pharmacol 2010; 70:703–712.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Bristol-Myers Squibb Company. ELIQUIS (apixaban) package insert. www.eliquis.com/index.aspx. Accessed August 6, 2014.
- Furie KL, Goldstein LB, Albers GW, et al; American Heart Association Stroke Council. Oral antithrombotic agents for the prevention of stroke in nonvalvular atrial fibrillation: a science advisory for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2012; 43:3442–3453.
- ClinicalTrials.gov, US National Institutes of Health. PERIOP 2 - A Safety and Effectiveness Study of LMWH Bridging Therapy Versus Placebo Bridging Therapy for Patients on Long Term Warfarin and Require Temporary Interruption of Their Warfarin. http://clinicaltri-als.gov/show/NCT00432796. Accessed August 6, 2014.
- ClinicalTrials.gov, US National Institutes of Health. Effectiveness of Bridging Anticoagulation for Surgery (The BRIDGE Study). http://clinicaltrials.gov/ct2/show/NCT00786474. Accessed August 6, 2014.
- Birnie DH, Healey JS, Wells GA, et al; BRUISE CONTROL Investigators. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med 2013; 368:2084–2093.
- Oberweis BS, Nukala S, Rosenberg A, et al. Thrombotic and bleeding complications after orthopedic surgery. Am Heart J 2013; 165:427.e1–433.e1.
- Omran H, Bauersachs R, Rübenacker S, Goss F, Hammerstingl C. The HAS-BLED score predicts bleedings during bridging of chronic oral anticoagulation. Results from the national multicentre BNK Online bRiDging REgistRy (BORDER). Thromb Haemost 2012; 108:65–73.
- Pisters R, Lane DA, Nieuwlaaat R, de Vos CB, Crijns HJGM, Lip GYH. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation. The Euro Heart Survey. Chest 2010; 138:1093–1100.
- Kaatz S, Douketis JD, Zhou H, Gage BF, White RH. Risk of stroke after surgery in patients with and without chronic atrial fibrillation. J Thromb Haemost 2010; 8:884–890.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Connolly G, Spyropoulos AC. Practical issues, limitations, and periprocedural management of the NOAC’s. J Thromb Thrombolysis 2013; 36:212–222.
- Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood 2012; 120:2954–2962.
- Kaatz S, Kouides PA, Garcia DA, et al. Guidance on the emergent reversal of oral thrombin and factor Xa inhibitors. Am J Hematol 2012; 87(suppl 1):S141–S145.
- Rowley CP, Bernard ML, Brabham WW, et al. Safety of continuous anticoagulation with dabigatran during implantation of cardiac rhythm devices. Am J Cardiol 2013; 111:1165–1168.
- Healey JS, Eikelboom J, Douketis J, et al; RE-LY Investigators. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) randomized trial. Circulation 2012; 126:343–348.
- Sherwood MW, Douketis JD, Patel MR, et al; on behalf of the ROCKET AF Investigators. Outcomes of temporary interruption of rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: results from the Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Circulation 2014; 129:1850–1859.
- Patel MR, Hellkamp AS, Lokhnygina Y, et al. Outcomes of discontinuing rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: analysis from the ROCKET AF trial (Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation). J Am Coll Cardiol 2013; 61:651–658.
- Reynolds MR. Discontinuation of rivaroxaban: filling in the gaps. J Am Coll Cardiol 2013; 61:659–660.
- Turpie AG, Kreutz R, Llau J, Norrving B, Haas S. Management consensus guidance for the use of rivaroxaban—an oral, direct factor Xa inhibitor. Thromb Haemost 2012; 108:876–886.
- Gallego P, Apostolakis S, Lip GY. Bridging evidence-based practice and practice-based evidence in periprocedural anticoagulation. Circulation 2012; 126:1573–1576.
- Sié P, Samama CM, Godier A, et al; Working Group on Perioperative Haemostasis. Surgery and invasive procedures in patients on long-term treatment with direct oral anticoagulants: thrombin or factor-Xa inhibitors. Recommendations of the Working Group on Perioperative Haemostasis and the French Study Group on Thrombosis and Haemostasis. Arch Cardiovasc Dis 2011; 104:669–676.
- King CS, Holley AB, Moores LK. Moving toward a more ideal anticoagulant: the oral direct thrombin and factor Xa inhibitors. Chest 2013; 143:1106–1116.
- Baglin T, Hillarp A, Tripodi A, Elalamy I, Buller H, Ageno W. Measuring oral direct inhibitors (ODIs) of thrombin and factor Xa: a recommendation from the Subcommittee on Control of Anticoagulation of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2013; 11:756–760.
- Baglin T, Keeling D, Kitchen S; British Committee for Standards in Haematology. Effects on routine coagulation screens and assessment of anticoagulant intensity in patients taking oral dabigatran or rivaroxaban: guidance from the British Committee for Standards in Haematology. Br J Haematol 2012; 159:427–429.
- Mani H, Kasper A, Lindhoff-Last E. Measuring the anticoagulant effects of target specific oral anticoagulants—reasons, methods and current limitations. J Thromb Thrombolysis 2013; 36:187–194.
- Hawes EM, Deal AM, Funk-Adcock D, et al. Performance of coagulation tests in patients on therapeutic doses of dabigatran: a cross-sectional pharmacodynamic study based on peak and trough plasma levels. J Thromb Haemost 2013; 11:1493–1502.
- Barrett YC, Wang Z, Frost C, Shenker A. Clinical laboratory measurement of direct factor Xa inhibitors: anti-Xa assay is preferable to prothrombin time assay. Thromb Haemost 2010; 104:1263–1271.
- Smythe MA, Fanikos J, Gulseth MP, et al. Rivaroxaban: practical considerations for ensuring safety and efficacy. Pharmacotherapy 2013; 33:1223–1245.
- Van Ryn J, Litzenburger T, Waterman A, et al. Dabigatran anticoagulant activity is neutralized by an antibody selective to dabigatran in in vitro and in vivo models. J Am Coll Cardiol 2011; 57:E1130.
- Sheffield W, Lambourne M, Bhakta V, Eltringham-Smith L, Arnold D, Crowther M. Active site-mutated thrombin S195A but not active site-blocked thrombin counteracts the anticoagulant activity of dabigatran in plasma. Abstract presented at the International Society of Thrombosis and Haemostasis 2013 Congress. http://onlinelibrary.wiley.com/doi/10.1111/jth.2013.11.issue-s2/issuetoc. Accessed August 6, 2014.
- Lu G, Luan P, Hollenbach SJ, et al. Reconstructed recombinant factor Xa as an antidote to reverse anticoagulation by factor Xa inhibitors (abstract). J Thromb Haemost 2009; 7(suppl 2):abstract OC-TH-107.
- Stangier J, Rathgen K, Stähle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet 2010; 49:259–268.
- Singh T, Maw TT, Henry BL, et al. Extracorporeal therapy for dabigatran removal in the treatment of acute bleeding: a single center experience. Clin J Am Soc Nephrol 2013; 8:1533–1539.
- Kaatz S, Crowther M. Reversal of target-specific oral anticoagulants. J Thromb Thrombolysis 2013; 36:195–202.
- Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011; 124:1573–1579.
- Sarode R, Milling TJ, Refaai MA, et al. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation 2013; 128:1234–1243.
- Douketis JD, Berger PB, Dunn AS, et al; American College of Chest Physicians. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):299S–339S.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e326S–e350S.
- Boehringer Ingelheim Pharmaceuticals, Inc. PRADAXA (dabigatran) package insert. http://bidocs.boehringer-ingelheim.com/BIWebAc-cess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf. Accessed August 6, 2014.
- Levy JH, Faraoni D, Spring JL, Douketis JD, Samama CM. Managing new oral anticoagulants in the perioperative and intensive care unit setting. Anesthesiology 2013; 118:1466–1474.
- US Food and Drug Administration (FDA). FDA drug safety communication: update on the risk for serious bleeding events with the anticoagulant Pradaxa (dabigatran). www.fda.gov/drugs/drugsafety/ucm326580.htm. Accessed August 6, 2014.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- Schulman S, Kearon C, Kakkar AK, et al; RE-MEDY Trial Investigators. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013; 368:709–718.
- Eriksson BI, Dahl OE, Rosencher N, Büller HR, et al; RE-NOVATE Study Group. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 2007; 370:949–956.
- Eriksson BI, Dahl OE, Rosencher N, et al; RE-MODEL Study Group. Oral dabigatran etexilate vs subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007; 5:2178–2185.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013; 369:1206–1214.
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G; American College of Chest Physicians. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e44S–e88S.
- Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos 2008; 36:386–399.
- Janssen Pharmaceuticals, Inc. XARELTO (rivaroxaban) package insert. www.xareltohcp.com/about-xarelto/about-xarelto.html?utm_source=google&utm_medium=cpc&utm_campaign=Branded+-+Broad&utm_term=xarelto%20rivaroxaban&utm_content=Xarelto+Rivaroxaban|mkwid|sxSDxPb4m_dc|pcrid|34667840494. Accessed August 6, 2014.
- Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–391.
- EINSTEIN Investigators; Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363:2499–2510.
- EINSTEIN–PE Investigators; Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012; 366:1287–1297.
- Eriksson BI, Borris LC, Friedman RJ, et al; RECORD1 Study Group. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008; 358:2765–2775.
- Kakkar AK, Brenner B, Dahl OE, et al; RECORD2 Investigators. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet 2008; 372:31–39.
- Lassen MR, Ageno W, Borris LC, et al; RECORD3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008; 358:2776–2786.
- Kubitza D, Becka M, Mueck W, et al. Effects of renal impairment on the pharmacokinetics, pharmacodynamics and safety of rivaroxaban, an oral, direct factor Xa inhibitor. Br J Clin Pharmacol 2010; 70:703–712.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Bristol-Myers Squibb Company. ELIQUIS (apixaban) package insert. www.eliquis.com/index.aspx. Accessed August 6, 2014.
- Furie KL, Goldstein LB, Albers GW, et al; American Heart Association Stroke Council. Oral antithrombotic agents for the prevention of stroke in nonvalvular atrial fibrillation: a science advisory for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2012; 43:3442–3453.
- ClinicalTrials.gov, US National Institutes of Health. PERIOP 2 - A Safety and Effectiveness Study of LMWH Bridging Therapy Versus Placebo Bridging Therapy for Patients on Long Term Warfarin and Require Temporary Interruption of Their Warfarin. http://clinicaltri-als.gov/show/NCT00432796. Accessed August 6, 2014.
- ClinicalTrials.gov, US National Institutes of Health. Effectiveness of Bridging Anticoagulation for Surgery (The BRIDGE Study). http://clinicaltrials.gov/ct2/show/NCT00786474. Accessed August 6, 2014.
- Birnie DH, Healey JS, Wells GA, et al; BRUISE CONTROL Investigators. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med 2013; 368:2084–2093.
- Oberweis BS, Nukala S, Rosenberg A, et al. Thrombotic and bleeding complications after orthopedic surgery. Am Heart J 2013; 165:427.e1–433.e1.
- Omran H, Bauersachs R, Rübenacker S, Goss F, Hammerstingl C. The HAS-BLED score predicts bleedings during bridging of chronic oral anticoagulation. Results from the national multicentre BNK Online bRiDging REgistRy (BORDER). Thromb Haemost 2012; 108:65–73.
- Pisters R, Lane DA, Nieuwlaaat R, de Vos CB, Crijns HJGM, Lip GYH. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation. The Euro Heart Survey. Chest 2010; 138:1093–1100.
- Kaatz S, Douketis JD, Zhou H, Gage BF, White RH. Risk of stroke after surgery in patients with and without chronic atrial fibrillation. J Thromb Haemost 2010; 8:884–890.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Connolly G, Spyropoulos AC. Practical issues, limitations, and periprocedural management of the NOAC’s. J Thromb Thrombolysis 2013; 36:212–222.
- Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood 2012; 120:2954–2962.
- Kaatz S, Kouides PA, Garcia DA, et al. Guidance on the emergent reversal of oral thrombin and factor Xa inhibitors. Am J Hematol 2012; 87(suppl 1):S141–S145.
- Rowley CP, Bernard ML, Brabham WW, et al. Safety of continuous anticoagulation with dabigatran during implantation of cardiac rhythm devices. Am J Cardiol 2013; 111:1165–1168.
- Healey JS, Eikelboom J, Douketis J, et al; RE-LY Investigators. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) randomized trial. Circulation 2012; 126:343–348.
- Sherwood MW, Douketis JD, Patel MR, et al; on behalf of the ROCKET AF Investigators. Outcomes of temporary interruption of rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: results from the Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Circulation 2014; 129:1850–1859.
- Patel MR, Hellkamp AS, Lokhnygina Y, et al. Outcomes of discontinuing rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: analysis from the ROCKET AF trial (Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation). J Am Coll Cardiol 2013; 61:651–658.
- Reynolds MR. Discontinuation of rivaroxaban: filling in the gaps. J Am Coll Cardiol 2013; 61:659–660.
- Turpie AG, Kreutz R, Llau J, Norrving B, Haas S. Management consensus guidance for the use of rivaroxaban—an oral, direct factor Xa inhibitor. Thromb Haemost 2012; 108:876–886.
- Gallego P, Apostolakis S, Lip GY. Bridging evidence-based practice and practice-based evidence in periprocedural anticoagulation. Circulation 2012; 126:1573–1576.
- Sié P, Samama CM, Godier A, et al; Working Group on Perioperative Haemostasis. Surgery and invasive procedures in patients on long-term treatment with direct oral anticoagulants: thrombin or factor-Xa inhibitors. Recommendations of the Working Group on Perioperative Haemostasis and the French Study Group on Thrombosis and Haemostasis. Arch Cardiovasc Dis 2011; 104:669–676.
- King CS, Holley AB, Moores LK. Moving toward a more ideal anticoagulant: the oral direct thrombin and factor Xa inhibitors. Chest 2013; 143:1106–1116.
- Baglin T, Hillarp A, Tripodi A, Elalamy I, Buller H, Ageno W. Measuring oral direct inhibitors (ODIs) of thrombin and factor Xa: a recommendation from the Subcommittee on Control of Anticoagulation of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2013; 11:756–760.
- Baglin T, Keeling D, Kitchen S; British Committee for Standards in Haematology. Effects on routine coagulation screens and assessment of anticoagulant intensity in patients taking oral dabigatran or rivaroxaban: guidance from the British Committee for Standards in Haematology. Br J Haematol 2012; 159:427–429.
- Mani H, Kasper A, Lindhoff-Last E. Measuring the anticoagulant effects of target specific oral anticoagulants—reasons, methods and current limitations. J Thromb Thrombolysis 2013; 36:187–194.
- Hawes EM, Deal AM, Funk-Adcock D, et al. Performance of coagulation tests in patients on therapeutic doses of dabigatran: a cross-sectional pharmacodynamic study based on peak and trough plasma levels. J Thromb Haemost 2013; 11:1493–1502.
- Barrett YC, Wang Z, Frost C, Shenker A. Clinical laboratory measurement of direct factor Xa inhibitors: anti-Xa assay is preferable to prothrombin time assay. Thromb Haemost 2010; 104:1263–1271.
- Smythe MA, Fanikos J, Gulseth MP, et al. Rivaroxaban: practical considerations for ensuring safety and efficacy. Pharmacotherapy 2013; 33:1223–1245.
- Van Ryn J, Litzenburger T, Waterman A, et al. Dabigatran anticoagulant activity is neutralized by an antibody selective to dabigatran in in vitro and in vivo models. J Am Coll Cardiol 2011; 57:E1130.
- Sheffield W, Lambourne M, Bhakta V, Eltringham-Smith L, Arnold D, Crowther M. Active site-mutated thrombin S195A but not active site-blocked thrombin counteracts the anticoagulant activity of dabigatran in plasma. Abstract presented at the International Society of Thrombosis and Haemostasis 2013 Congress. http://onlinelibrary.wiley.com/doi/10.1111/jth.2013.11.issue-s2/issuetoc. Accessed August 6, 2014.
- Lu G, Luan P, Hollenbach SJ, et al. Reconstructed recombinant factor Xa as an antidote to reverse anticoagulation by factor Xa inhibitors (abstract). J Thromb Haemost 2009; 7(suppl 2):abstract OC-TH-107.
- Stangier J, Rathgen K, Stähle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet 2010; 49:259–268.
- Singh T, Maw TT, Henry BL, et al. Extracorporeal therapy for dabigatran removal in the treatment of acute bleeding: a single center experience. Clin J Am Soc Nephrol 2013; 8:1533–1539.
- Kaatz S, Crowther M. Reversal of target-specific oral anticoagulants. J Thromb Thrombolysis 2013; 36:195–202.
- Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011; 124:1573–1579.
- Sarode R, Milling TJ, Refaai MA, et al. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation 2013; 128:1234–1243.
KEY POINTS
- How long before surgery to stop a TSOAC depends on the bleeding risk of the procedure and the patient’s renal function.
- Perioperative bridging is generally unnecessary for patients on TSOACs.
- Routine coagulation assays such as the prothrombin time and activated partial thromboplastin time do not reliably reflect the degree of anticoagulation with TSOACs.
- There are no specific antidotes or standardized reversal strategies for TSOACs.
- TSOACs have a rapid onset of action and should only be restarted postoperatively once hemostasis has been confirmed.
Can we reduce the risk of readmission for a patient with an exacerbation of COPD?
We think so. Some strategies to reduce readmission rates, such as coordinating care and managing comorbidities, apply to chronic diseases in general, while others are disease-specific. To reduce the need for hospital readmission for chronic obstructive pulmonary disease (COPD), coordinated efforts involving both inpatient and outpatient care are necessary. This can be achieved by using a checklist before discharge (Table 1) and by implementing outpatient COPD programs that continue patient education and provide rapid access to medical support if needed.
There is room for improvement. COPD is common and expensive, with high rates of hospital readmission,1 and up to 70% of the money we spend on it goes for hospital care.2 No wonder then that the Centers for Medicare and Medicaid Services has now expanded its Readmissions Reduction Program to include acute COPD exacerbations.3 Yet in a retrospective study, Yip et al4 found that fewer than half of patients hospitalized with acute exacerbation of COPD received appropriate vaccinations, counseling on smoking cessation, and long-acting inhalers—all of which are on our checklist.4
The following interventions have been demonstrated to be useful in reducing COPD hospital admissions and the risk of death.
SMOKING CESSATION
Cigarette smoking is the most common and easily identifiable risk factor for COPD exacerbation.5
Au et al5 found that quitting smoking reduces the risk of COPD exacerbation (adjusted hazard ratio 0.78, 95% confidence interval [CI] 0.75–0.87), and the risk keeps decreasing the longer the patient stays off tobacco.5
Whether counseling hospitalized patients on smoking cessation reduces the COPD readmission rate has not been well studied. However, a meta-analysis of nine randomized controlled trials, two of which were done in the hospital, revealed higher abstinence rates in COPD patients who received extensive counseling on smoking cessation.7 For these reasons, hospitalized COPD patients who smoke should be strongly encouraged to quit.6
PNEUMOCOCCAL AND INFLUENZA VACCINATIONS
In a large retrospective study,8 pneumococcal vaccination was associated with a significantly lower risk of hospitalization for pneumonia in patients with chronic lung disease, including those with COPD (relative risk [RR] 0.57, 95% CI 0.38–0.84). The benefit was even greater with pneumococcal and influenza vaccinations during the influenza season (RR 0.28, 95% CI 0.14–0.58).
Randomized controlled trials indicate that influenza vaccination may reduce the rate of COPD exacerbations, especially in epidemic years when the proportion of exacerbations due to influenza is higher.9
Wongsurakiat et al10 found a significant reduction in the incidence of influenza-related acute respiratory illness in COPD patients in a well-designed randomized, placebo-controlled trial (RR 0.24, P = .005).10
Similarly, in another randomized controlled trial, pneumococcal vaccination was effective in preventing community-acquired pneumonia in COPD patients under age 65 and in those with severe airflow obstruction, although no statistically significant differences were found among other groups of patients with COPD.11
Therefore, influenza and pneumococcal vaccinations are recommended by major COPD guidelines, such as GOLD (Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease).6
INHALERS
Inhaler therapy is recommended based on COPD severity according to GOLD classification, and appropriate inhaler therapy with proper inhaler technique reduces the number of COPD exacerbations and hospitalizations.6
Long-acting beta-agonists and anticholinergics reduce the risk of COPD exacerbation and hospitalization and so are preferred over short-acting formulations except for patients in GOLD group A, ie, those who have few symptoms and are at low risk of exacerbations.6
Long-term treatment with inhaled corticosteroids with long-acting bronchodilators is recommended for patients at high risk of exacerbations (ie, those with two or more exacerbations in the previous year or a forced expiratory volume in 1 second [FEV1] less than 50% of predicted).6
OXYGEN THERAPY
Two older randomized controlled trials, the Nocturnal Oxygen Therapy Trial and the Medical Research Council study, reviewed by Stoller et al,12 provided clear evidence that oxygen therapy reduces the death rate and improves quality of life in COPD patients who have chronic resting hypoxemia (room air Pao2 ≤ 55 mm Hg, or ≤ 59 mm Hg with signs of right-sided heart strain or polycythemia).
PULMONARY REHABILITATION
Pulmonary rehabilitation likely reduces hospital admissions by improving exercise capacity.13 A systematic review of six trials in 230 patients found that respiratory rehabilitation after an acute COPD exacerbation reduced the risk of COPD hospital admission (RR 0.26, 95% CI 0.12–0.54) and the risk of death (RR 0.45, 95% CI 0.22–0.91).13
OTHER INTERVENTIONS
Home noninvasive ventilator support reduced hospital and intensive care unit readmissions in select patients recurrently hospitalized for acidotic exacerbations of COPD in one small study.14
Long-term antibiotic therapy. Although there is evidence that azithromycin, taken daily for 1 year, decreases the frequency of COPD exacerbations,15 concern persists that this approach promotes antibiotic resistance, and the GOLD guidelines do not recommend routinely using antibiotics in patients with clinically stable COPD.6
Roflumilast. According to the GOLD guidelines, the phosphodiesterase-4 inhibitor roflumilast (Daliresp) may be useful in reducing exacerbations in patients who have an FEV1 less than 50% of predicted, chronic bronchitis, and frequent exacerbations.6
Referral. Patients who have severe recurrent COPD exacerbations despite appropriate therapy will likely benefit from referral to a pulmonary specialist for other options such as theophylline, lung-reduction surgery, and lung transplantation.
PATIENT EDUCATION AND OUTPATIENT COPD PROGRAMS
There is growing evidence that outpatient programs that provide education and medical support significantly reduce the rate of hospitalizations for COPD.16–18 Patient education includes symptom monitoring, early recognition of an exacerbation, appropriate use of inhalers and nebulizers, and advice on smoking cessation.16
On the other hand, a Veterans Administration randomized controlled trial was stopped early because of a higher rate of death in the group that underwent a comprehensive care-management program of COPD education, an action plan for identification and treatment of exacerbations, and scheduled proactive telephone calls for case management.19
Further study is needed to investigate the cost-effectiveness and safety of COPD management programs and whether to adopt such programs on a systematic level.
In conclusion, COPD patients require a comprehensive approach based on studied interventions. This may be achieved through systematic methods that allow each patient to benefit from all possible interventions appropriate for him or her. Hospitalization of COPD patients provides an excellent opportunity to implement this comprehensive approach.
- Westert GP, Lagoe RJ, Keskimäki I, Leyland A, Murphy M. An international study of hospital readmissions and related utilization in Europe and the USA. Health Policy 2002; 61:269–278.
- Halpern MT, Stanford RH, Borker R. The burden of COPD in the USA: results from the Confronting COPD survey. Respir Med 2003; 97(suppl C):S81–S89.
- Centers for Medicare and Medicaid Services. Readmissions reduction program. www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. Accessed August 9, 2014.
- Yip NH, Yuen G, Lazar EJ, et al. Analysis of hospitalizations for COPD exacerbation: opportunities for improving care. COPD 2010; 7:85–92.
- Au DH, Bryson CL, Chien JW, et al. The effects of smoking cessation on the risk of chronic obstructive pulmonary disease exacerbations. J Gen Intern Med 2009; 24:457–463.
- Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187:347–365.
- Thabane MCOPD Working Group. Smoking cessation for patients with chronic obstructive pulmonary disease (COPD): an evidence-based analysis. Ont Health Technol Assess Ser 2012; 12:1–50.
- Nichol KL, Baken L, Wuorenma J, Nelson A. The health and economic benefits associated with pneumococcal vaccination of elderly persons with chronic lung disease. Arch Intern Med 1999; 159:2437–2442.
- Poole PJ, Chacko E, Wood-Baker RW, Cates CJ. Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006; 1:CD002733.
- Wongsurakiat P, Maranetra KN, Wasi C, Kositanont U, Dejsomritrutai W, Charoenratanakul S. Acute respiratory illness in patients with COPD and the effectiveness of influenza vaccination: a randomized controlled study. Chest 2004; 125:2011–2020.
- Alfageme I, Vazquez R, Reyes N, et al. Clinical efficacy of anti-pneumococcal vaccination in patients with COPD. Thorax 2006; 61:189–195.
- Stoller JK, Panos RJ, Krachman S, Doherty DE, Make B; Long-term Oxygen Treatment Trial Research Group. Oxygen therapy for patients with COPD: current evidence and the long-term oxygen treatment trial. Chest 2010; 138:179–187.
- Puhan MA, Scharplatz M, Troosters T, Steurer J. Respiratory rehabilitation after acute exacerbation of COPD may reduce risk for readmission and mortality—a systematic review. Respir Res 2005; 6:54.
- Tuggey JM, Plant PK, Elliott MW. Domiciliary non-invasive ventilation for recurrent acidotic exacerbations of COPD: an economic analysis. Thorax 2003; 58:867–871.
- Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011; 365:689–698.
- Lawlor M, Kealy S, Agnew M, et al. Early discharge care with ongoing follow-up support may reduce hospital readmissions in COPD. Int J Chron Obstruct Pulmon Dis 2009; 4:55–60.
- Gadoury MA, Schwartzman K, Rouleau M, et al; Chronic Obstructive Pulmonary Disease axis of the Respiratory Health Network, Fonds de la Recherche en Santé du Québec (FRSQ). Self-management reduces both short- and long-term hospitalisation in COPD. Eur Respir J 2005; 26:853–857.
- Rice KL, Dewan N, Bloomfield HE, et al. Disease management program for chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med 2010; 182:890–896.
- Fan VS, Gaziano JM, Lew R, et al. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations: a randomized, controlled trial. Ann Intern Med 2012; 156:673–683.
- COPD Working Group. Noninvasive positive pressure ventilation for chronic respiratory failure patients with stable chronic obstructive pulmonary disease (COPD): an evidence-based analysis. Ont Health Technol Assess Ser 2012; 12( 9):1–51.
We think so. Some strategies to reduce readmission rates, such as coordinating care and managing comorbidities, apply to chronic diseases in general, while others are disease-specific. To reduce the need for hospital readmission for chronic obstructive pulmonary disease (COPD), coordinated efforts involving both inpatient and outpatient care are necessary. This can be achieved by using a checklist before discharge (Table 1) and by implementing outpatient COPD programs that continue patient education and provide rapid access to medical support if needed.
There is room for improvement. COPD is common and expensive, with high rates of hospital readmission,1 and up to 70% of the money we spend on it goes for hospital care.2 No wonder then that the Centers for Medicare and Medicaid Services has now expanded its Readmissions Reduction Program to include acute COPD exacerbations.3 Yet in a retrospective study, Yip et al4 found that fewer than half of patients hospitalized with acute exacerbation of COPD received appropriate vaccinations, counseling on smoking cessation, and long-acting inhalers—all of which are on our checklist.4
The following interventions have been demonstrated to be useful in reducing COPD hospital admissions and the risk of death.
SMOKING CESSATION
Cigarette smoking is the most common and easily identifiable risk factor for COPD exacerbation.5
Au et al5 found that quitting smoking reduces the risk of COPD exacerbation (adjusted hazard ratio 0.78, 95% confidence interval [CI] 0.75–0.87), and the risk keeps decreasing the longer the patient stays off tobacco.5
Whether counseling hospitalized patients on smoking cessation reduces the COPD readmission rate has not been well studied. However, a meta-analysis of nine randomized controlled trials, two of which were done in the hospital, revealed higher abstinence rates in COPD patients who received extensive counseling on smoking cessation.7 For these reasons, hospitalized COPD patients who smoke should be strongly encouraged to quit.6
PNEUMOCOCCAL AND INFLUENZA VACCINATIONS
In a large retrospective study,8 pneumococcal vaccination was associated with a significantly lower risk of hospitalization for pneumonia in patients with chronic lung disease, including those with COPD (relative risk [RR] 0.57, 95% CI 0.38–0.84). The benefit was even greater with pneumococcal and influenza vaccinations during the influenza season (RR 0.28, 95% CI 0.14–0.58).
Randomized controlled trials indicate that influenza vaccination may reduce the rate of COPD exacerbations, especially in epidemic years when the proportion of exacerbations due to influenza is higher.9
Wongsurakiat et al10 found a significant reduction in the incidence of influenza-related acute respiratory illness in COPD patients in a well-designed randomized, placebo-controlled trial (RR 0.24, P = .005).10
Similarly, in another randomized controlled trial, pneumococcal vaccination was effective in preventing community-acquired pneumonia in COPD patients under age 65 and in those with severe airflow obstruction, although no statistically significant differences were found among other groups of patients with COPD.11
Therefore, influenza and pneumococcal vaccinations are recommended by major COPD guidelines, such as GOLD (Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease).6
INHALERS
Inhaler therapy is recommended based on COPD severity according to GOLD classification, and appropriate inhaler therapy with proper inhaler technique reduces the number of COPD exacerbations and hospitalizations.6
Long-acting beta-agonists and anticholinergics reduce the risk of COPD exacerbation and hospitalization and so are preferred over short-acting formulations except for patients in GOLD group A, ie, those who have few symptoms and are at low risk of exacerbations.6
Long-term treatment with inhaled corticosteroids with long-acting bronchodilators is recommended for patients at high risk of exacerbations (ie, those with two or more exacerbations in the previous year or a forced expiratory volume in 1 second [FEV1] less than 50% of predicted).6
OXYGEN THERAPY
Two older randomized controlled trials, the Nocturnal Oxygen Therapy Trial and the Medical Research Council study, reviewed by Stoller et al,12 provided clear evidence that oxygen therapy reduces the death rate and improves quality of life in COPD patients who have chronic resting hypoxemia (room air Pao2 ≤ 55 mm Hg, or ≤ 59 mm Hg with signs of right-sided heart strain or polycythemia).
PULMONARY REHABILITATION
Pulmonary rehabilitation likely reduces hospital admissions by improving exercise capacity.13 A systematic review of six trials in 230 patients found that respiratory rehabilitation after an acute COPD exacerbation reduced the risk of COPD hospital admission (RR 0.26, 95% CI 0.12–0.54) and the risk of death (RR 0.45, 95% CI 0.22–0.91).13
OTHER INTERVENTIONS
Home noninvasive ventilator support reduced hospital and intensive care unit readmissions in select patients recurrently hospitalized for acidotic exacerbations of COPD in one small study.14
Long-term antibiotic therapy. Although there is evidence that azithromycin, taken daily for 1 year, decreases the frequency of COPD exacerbations,15 concern persists that this approach promotes antibiotic resistance, and the GOLD guidelines do not recommend routinely using antibiotics in patients with clinically stable COPD.6
Roflumilast. According to the GOLD guidelines, the phosphodiesterase-4 inhibitor roflumilast (Daliresp) may be useful in reducing exacerbations in patients who have an FEV1 less than 50% of predicted, chronic bronchitis, and frequent exacerbations.6
Referral. Patients who have severe recurrent COPD exacerbations despite appropriate therapy will likely benefit from referral to a pulmonary specialist for other options such as theophylline, lung-reduction surgery, and lung transplantation.
PATIENT EDUCATION AND OUTPATIENT COPD PROGRAMS
There is growing evidence that outpatient programs that provide education and medical support significantly reduce the rate of hospitalizations for COPD.16–18 Patient education includes symptom monitoring, early recognition of an exacerbation, appropriate use of inhalers and nebulizers, and advice on smoking cessation.16
On the other hand, a Veterans Administration randomized controlled trial was stopped early because of a higher rate of death in the group that underwent a comprehensive care-management program of COPD education, an action plan for identification and treatment of exacerbations, and scheduled proactive telephone calls for case management.19
Further study is needed to investigate the cost-effectiveness and safety of COPD management programs and whether to adopt such programs on a systematic level.
In conclusion, COPD patients require a comprehensive approach based on studied interventions. This may be achieved through systematic methods that allow each patient to benefit from all possible interventions appropriate for him or her. Hospitalization of COPD patients provides an excellent opportunity to implement this comprehensive approach.
We think so. Some strategies to reduce readmission rates, such as coordinating care and managing comorbidities, apply to chronic diseases in general, while others are disease-specific. To reduce the need for hospital readmission for chronic obstructive pulmonary disease (COPD), coordinated efforts involving both inpatient and outpatient care are necessary. This can be achieved by using a checklist before discharge (Table 1) and by implementing outpatient COPD programs that continue patient education and provide rapid access to medical support if needed.
There is room for improvement. COPD is common and expensive, with high rates of hospital readmission,1 and up to 70% of the money we spend on it goes for hospital care.2 No wonder then that the Centers for Medicare and Medicaid Services has now expanded its Readmissions Reduction Program to include acute COPD exacerbations.3 Yet in a retrospective study, Yip et al4 found that fewer than half of patients hospitalized with acute exacerbation of COPD received appropriate vaccinations, counseling on smoking cessation, and long-acting inhalers—all of which are on our checklist.4
The following interventions have been demonstrated to be useful in reducing COPD hospital admissions and the risk of death.
SMOKING CESSATION
Cigarette smoking is the most common and easily identifiable risk factor for COPD exacerbation.5
Au et al5 found that quitting smoking reduces the risk of COPD exacerbation (adjusted hazard ratio 0.78, 95% confidence interval [CI] 0.75–0.87), and the risk keeps decreasing the longer the patient stays off tobacco.5
Whether counseling hospitalized patients on smoking cessation reduces the COPD readmission rate has not been well studied. However, a meta-analysis of nine randomized controlled trials, two of which were done in the hospital, revealed higher abstinence rates in COPD patients who received extensive counseling on smoking cessation.7 For these reasons, hospitalized COPD patients who smoke should be strongly encouraged to quit.6
PNEUMOCOCCAL AND INFLUENZA VACCINATIONS
In a large retrospective study,8 pneumococcal vaccination was associated with a significantly lower risk of hospitalization for pneumonia in patients with chronic lung disease, including those with COPD (relative risk [RR] 0.57, 95% CI 0.38–0.84). The benefit was even greater with pneumococcal and influenza vaccinations during the influenza season (RR 0.28, 95% CI 0.14–0.58).
Randomized controlled trials indicate that influenza vaccination may reduce the rate of COPD exacerbations, especially in epidemic years when the proportion of exacerbations due to influenza is higher.9
Wongsurakiat et al10 found a significant reduction in the incidence of influenza-related acute respiratory illness in COPD patients in a well-designed randomized, placebo-controlled trial (RR 0.24, P = .005).10
Similarly, in another randomized controlled trial, pneumococcal vaccination was effective in preventing community-acquired pneumonia in COPD patients under age 65 and in those with severe airflow obstruction, although no statistically significant differences were found among other groups of patients with COPD.11
Therefore, influenza and pneumococcal vaccinations are recommended by major COPD guidelines, such as GOLD (Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease).6
INHALERS
Inhaler therapy is recommended based on COPD severity according to GOLD classification, and appropriate inhaler therapy with proper inhaler technique reduces the number of COPD exacerbations and hospitalizations.6
Long-acting beta-agonists and anticholinergics reduce the risk of COPD exacerbation and hospitalization and so are preferred over short-acting formulations except for patients in GOLD group A, ie, those who have few symptoms and are at low risk of exacerbations.6
Long-term treatment with inhaled corticosteroids with long-acting bronchodilators is recommended for patients at high risk of exacerbations (ie, those with two or more exacerbations in the previous year or a forced expiratory volume in 1 second [FEV1] less than 50% of predicted).6
OXYGEN THERAPY
Two older randomized controlled trials, the Nocturnal Oxygen Therapy Trial and the Medical Research Council study, reviewed by Stoller et al,12 provided clear evidence that oxygen therapy reduces the death rate and improves quality of life in COPD patients who have chronic resting hypoxemia (room air Pao2 ≤ 55 mm Hg, or ≤ 59 mm Hg with signs of right-sided heart strain or polycythemia).
PULMONARY REHABILITATION
Pulmonary rehabilitation likely reduces hospital admissions by improving exercise capacity.13 A systematic review of six trials in 230 patients found that respiratory rehabilitation after an acute COPD exacerbation reduced the risk of COPD hospital admission (RR 0.26, 95% CI 0.12–0.54) and the risk of death (RR 0.45, 95% CI 0.22–0.91).13
OTHER INTERVENTIONS
Home noninvasive ventilator support reduced hospital and intensive care unit readmissions in select patients recurrently hospitalized for acidotic exacerbations of COPD in one small study.14
Long-term antibiotic therapy. Although there is evidence that azithromycin, taken daily for 1 year, decreases the frequency of COPD exacerbations,15 concern persists that this approach promotes antibiotic resistance, and the GOLD guidelines do not recommend routinely using antibiotics in patients with clinically stable COPD.6
Roflumilast. According to the GOLD guidelines, the phosphodiesterase-4 inhibitor roflumilast (Daliresp) may be useful in reducing exacerbations in patients who have an FEV1 less than 50% of predicted, chronic bronchitis, and frequent exacerbations.6
Referral. Patients who have severe recurrent COPD exacerbations despite appropriate therapy will likely benefit from referral to a pulmonary specialist for other options such as theophylline, lung-reduction surgery, and lung transplantation.
PATIENT EDUCATION AND OUTPATIENT COPD PROGRAMS
There is growing evidence that outpatient programs that provide education and medical support significantly reduce the rate of hospitalizations for COPD.16–18 Patient education includes symptom monitoring, early recognition of an exacerbation, appropriate use of inhalers and nebulizers, and advice on smoking cessation.16
On the other hand, a Veterans Administration randomized controlled trial was stopped early because of a higher rate of death in the group that underwent a comprehensive care-management program of COPD education, an action plan for identification and treatment of exacerbations, and scheduled proactive telephone calls for case management.19
Further study is needed to investigate the cost-effectiveness and safety of COPD management programs and whether to adopt such programs on a systematic level.
In conclusion, COPD patients require a comprehensive approach based on studied interventions. This may be achieved through systematic methods that allow each patient to benefit from all possible interventions appropriate for him or her. Hospitalization of COPD patients provides an excellent opportunity to implement this comprehensive approach.
- Westert GP, Lagoe RJ, Keskimäki I, Leyland A, Murphy M. An international study of hospital readmissions and related utilization in Europe and the USA. Health Policy 2002; 61:269–278.
- Halpern MT, Stanford RH, Borker R. The burden of COPD in the USA: results from the Confronting COPD survey. Respir Med 2003; 97(suppl C):S81–S89.
- Centers for Medicare and Medicaid Services. Readmissions reduction program. www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. Accessed August 9, 2014.
- Yip NH, Yuen G, Lazar EJ, et al. Analysis of hospitalizations for COPD exacerbation: opportunities for improving care. COPD 2010; 7:85–92.
- Au DH, Bryson CL, Chien JW, et al. The effects of smoking cessation on the risk of chronic obstructive pulmonary disease exacerbations. J Gen Intern Med 2009; 24:457–463.
- Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187:347–365.
- Thabane MCOPD Working Group. Smoking cessation for patients with chronic obstructive pulmonary disease (COPD): an evidence-based analysis. Ont Health Technol Assess Ser 2012; 12:1–50.
- Nichol KL, Baken L, Wuorenma J, Nelson A. The health and economic benefits associated with pneumococcal vaccination of elderly persons with chronic lung disease. Arch Intern Med 1999; 159:2437–2442.
- Poole PJ, Chacko E, Wood-Baker RW, Cates CJ. Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006; 1:CD002733.
- Wongsurakiat P, Maranetra KN, Wasi C, Kositanont U, Dejsomritrutai W, Charoenratanakul S. Acute respiratory illness in patients with COPD and the effectiveness of influenza vaccination: a randomized controlled study. Chest 2004; 125:2011–2020.
- Alfageme I, Vazquez R, Reyes N, et al. Clinical efficacy of anti-pneumococcal vaccination in patients with COPD. Thorax 2006; 61:189–195.
- Stoller JK, Panos RJ, Krachman S, Doherty DE, Make B; Long-term Oxygen Treatment Trial Research Group. Oxygen therapy for patients with COPD: current evidence and the long-term oxygen treatment trial. Chest 2010; 138:179–187.
- Puhan MA, Scharplatz M, Troosters T, Steurer J. Respiratory rehabilitation after acute exacerbation of COPD may reduce risk for readmission and mortality—a systematic review. Respir Res 2005; 6:54.
- Tuggey JM, Plant PK, Elliott MW. Domiciliary non-invasive ventilation for recurrent acidotic exacerbations of COPD: an economic analysis. Thorax 2003; 58:867–871.
- Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011; 365:689–698.
- Lawlor M, Kealy S, Agnew M, et al. Early discharge care with ongoing follow-up support may reduce hospital readmissions in COPD. Int J Chron Obstruct Pulmon Dis 2009; 4:55–60.
- Gadoury MA, Schwartzman K, Rouleau M, et al; Chronic Obstructive Pulmonary Disease axis of the Respiratory Health Network, Fonds de la Recherche en Santé du Québec (FRSQ). Self-management reduces both short- and long-term hospitalisation in COPD. Eur Respir J 2005; 26:853–857.
- Rice KL, Dewan N, Bloomfield HE, et al. Disease management program for chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med 2010; 182:890–896.
- Fan VS, Gaziano JM, Lew R, et al. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations: a randomized, controlled trial. Ann Intern Med 2012; 156:673–683.
- COPD Working Group. Noninvasive positive pressure ventilation for chronic respiratory failure patients with stable chronic obstructive pulmonary disease (COPD): an evidence-based analysis. Ont Health Technol Assess Ser 2012; 12( 9):1–51.
- Westert GP, Lagoe RJ, Keskimäki I, Leyland A, Murphy M. An international study of hospital readmissions and related utilization in Europe and the USA. Health Policy 2002; 61:269–278.
- Halpern MT, Stanford RH, Borker R. The burden of COPD in the USA: results from the Confronting COPD survey. Respir Med 2003; 97(suppl C):S81–S89.
- Centers for Medicare and Medicaid Services. Readmissions reduction program. www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. Accessed August 9, 2014.
- Yip NH, Yuen G, Lazar EJ, et al. Analysis of hospitalizations for COPD exacerbation: opportunities for improving care. COPD 2010; 7:85–92.
- Au DH, Bryson CL, Chien JW, et al. The effects of smoking cessation on the risk of chronic obstructive pulmonary disease exacerbations. J Gen Intern Med 2009; 24:457–463.
- Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187:347–365.
- Thabane MCOPD Working Group. Smoking cessation for patients with chronic obstructive pulmonary disease (COPD): an evidence-based analysis. Ont Health Technol Assess Ser 2012; 12:1–50.
- Nichol KL, Baken L, Wuorenma J, Nelson A. The health and economic benefits associated with pneumococcal vaccination of elderly persons with chronic lung disease. Arch Intern Med 1999; 159:2437–2442.
- Poole PJ, Chacko E, Wood-Baker RW, Cates CJ. Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006; 1:CD002733.
- Wongsurakiat P, Maranetra KN, Wasi C, Kositanont U, Dejsomritrutai W, Charoenratanakul S. Acute respiratory illness in patients with COPD and the effectiveness of influenza vaccination: a randomized controlled study. Chest 2004; 125:2011–2020.
- Alfageme I, Vazquez R, Reyes N, et al. Clinical efficacy of anti-pneumococcal vaccination in patients with COPD. Thorax 2006; 61:189–195.
- Stoller JK, Panos RJ, Krachman S, Doherty DE, Make B; Long-term Oxygen Treatment Trial Research Group. Oxygen therapy for patients with COPD: current evidence and the long-term oxygen treatment trial. Chest 2010; 138:179–187.
- Puhan MA, Scharplatz M, Troosters T, Steurer J. Respiratory rehabilitation after acute exacerbation of COPD may reduce risk for readmission and mortality—a systematic review. Respir Res 2005; 6:54.
- Tuggey JM, Plant PK, Elliott MW. Domiciliary non-invasive ventilation for recurrent acidotic exacerbations of COPD: an economic analysis. Thorax 2003; 58:867–871.
- Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011; 365:689–698.
- Lawlor M, Kealy S, Agnew M, et al. Early discharge care with ongoing follow-up support may reduce hospital readmissions in COPD. Int J Chron Obstruct Pulmon Dis 2009; 4:55–60.
- Gadoury MA, Schwartzman K, Rouleau M, et al; Chronic Obstructive Pulmonary Disease axis of the Respiratory Health Network, Fonds de la Recherche en Santé du Québec (FRSQ). Self-management reduces both short- and long-term hospitalisation in COPD. Eur Respir J 2005; 26:853–857.
- Rice KL, Dewan N, Bloomfield HE, et al. Disease management program for chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med 2010; 182:890–896.
- Fan VS, Gaziano JM, Lew R, et al. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations: a randomized, controlled trial. Ann Intern Med 2012; 156:673–683.
- COPD Working Group. Noninvasive positive pressure ventilation for chronic respiratory failure patients with stable chronic obstructive pulmonary disease (COPD): an evidence-based analysis. Ont Health Technol Assess Ser 2012; 12( 9):1–51.
To improve our patients’ health, look beyond reducing readmissions
In this issue of the Cleveland Clinic Journal of Medicine, Drs. Ayache, Boyaji, and Pile share evidence-based strategies for reducing the risk of readmission for patients with acute exacerbations of chronic obstructive pulmonary disease (COPD).1 They emphasize standardizing practice by combining effective clinical management with appropriate patient education, communication, and postdischarge follow-up.
Reducing the rate of preventable hospital readmissions (as well as avoiding admissions in the first place) is the right thing to do for the patient. Moreover, broader adoption of the strategies that they outline in their article will be critical to the success of health care organizations in improving patient outcomes and navigating a rapidly evolving landscape of reimbursement and reporting changes associated with the Centers for Medicare and Medicaid Services (CMS) Readmissions Reduction Program. Hospital readmission rates, while imperfect measures of the quality of care, demonstrate opportunities to optimize transitions of care. Success in our efforts to improve the health of our patients will likely be aligned with reductions in preventable admissions and improved attention to care coordination.
HOSPITALS ARE PENALIZED FOR EXCESSIVE READMISSION RATES
With nearly 20% of Medicare beneficiaries being rehospitalized within 30 days of discharge, at a cost of $17 billion annually,2 Congress enacted the Hospital Readmissions Reduction Program3 as part of the Affordable Care Act (ACA) in 2012. The Centers for Medicare and Medicaid Services (CMS) had already been reporting the readmission rates for heart failure, acute myocardial infarction, and pneumonia since 2009 (www.medicare.gov/hospitalcompare). Building on this work, the Affordable Care Act implemented financial penalties against hospitals that had excessive rates of readmissions for these conditions.
The Affordable Care Act put 1% of a hospital’s Medicare base payment at risk for all inpatient diagnoses in 2013—not just the three listed here. The risk is 2% in 2014 and will rise to 3% in 2015. In its first year, more than 2,200 United States hospitals were penalized a total of approximately $280 million because of readmission rates above the national mean. Nearly 10% of hospitals incurred the maximum 1% penalty, and about 30% paid no penalty.
The Secretary of the Department of Health and Human Services has the authority to extend the Readmissions Reduction Program to additional high-volume or high-expenditure conditions, and the department has announced it will expand the program in October 2014 (fiscal year 2015) to include two additional conditions: elective hip or knee replacement and COPD.4 In both cases, CMS began by publicly reporting these rates before including them in the program. Additional readmission measures, including those for stroke and hospital-wide all-cause readmissions, are also publicly reported and receive increased attention but are not yet included in the Readmissions Reduction Program.
UNFAIRLY PENALIZING THOSE THAT SERVE THE POOR
Avoidable causes of readmissions include hospital-acquired infections and complications, inadequate medication reconciliation and management, poor communication and coordination of care among the members of the health care team, and suboptimal care transitions.5 But other important drivers of readmissions are outside of a hospital’s direct control. These include mental illness, lack of social support, and poverty.6
A criticism of the Readmissions Reduction Program is that it disproportionately penalizes hospitals that serve the poorest patients.7 Currently, CMS readmission risk models do not adjust for socioeconomic factors. Further, CMS responds to these concerns by noting that it does not want different outcome standards for poor patients, and that adjusting for these factors may conceal potential health care disparities in disadvantaged populations.
NEW MISSION FOR HOSPITALS: MITIGATE SOCIOECONOMIC BARRIERS
Effective programs to reduce hospital readmissions must address the clinical interventions and patient education needs in the COPD discharge checklist discussed by Ayache et al, but must also attempt to mitigate social disadvantages that drive up readmissions for patients at highest risk.
Are hospitals in a position to do this? Too often, it is assumed that patients have access to medications, transportation to follow-up appointments, and social support. Early identification of patients at highest risk of being affected by lack of these factors and innovative solutions for mitigating these risks are important considerations in our efforts to reduce hospital readmissions.
HOW MANY READMISSIONS ARE TRULY PREVENTABLE?
Experts disagree on how many readmissions are truly preventable. Readmission rates for the sickest patients treated at tertiary or academic medical centers may reflect high-quality care in well-managed patients who otherwise would have died during the index admission.8
In early studies, the Medicare Payment Advisory Commission estimated that up to three-quarters of readmissions are preventable.9 In contrast, studies that used clinical instead of administrative data suggest preventable readmissions make up as little as 12% of total readmissions.10
Regardless of the actual percentage, Medicare’s risk-adjustment model relies exclusively on administrative data that do not fully account for nonpreventable factors and do not completely address unrelated or planned rehospitalizations. CMS is attempting to address these issues with an expanded readmission algorithm that excludes more planned and unrelated readmissions from the penalty calculation.
Ironically, the current structure of the Readmissions Reduction Program does little to address its intended goal of eliminating the perverse financial incentives for hospitals and physicians to readmit patients. Payments are still episode-based and reward readmissions. The $280 million that CMS expects to receive from the program this year covers less than 5% of the nearly $12 billion attributed to preventable rehospitalizations.11
WHAT PATIENTS NEED, NOT WHAT SUITS PROVIDERS
Hospital readmission rates are publicly reported, but it is shortsighted to think about readmissions outside of the broader context of the “medical home.” One must consider the role of primary care providers before and after an index admission in addition to the role of postacute care providers for some patients after discharge. Neither is directly affected by the current penalty program, but both are critical to effective solutions and optimizing value-oriented care.
Readmission rates are suboptimal measures, as they address presumed failures of hospital transitions rather than measuring care coordination and providing meaningful incentives to coordinate care. Yes, there is much to do to ensure effective transitions from the hospital to home or postacute settings. But to truly transform health care and deliver value, shouldn’t we strive to redesign the work flow around what patients need rather than what suits providers?
This effort should focus on managing the conditions that bring patients to the hospital. Medical homes and optimizing chronic disease care can play pivotal roles in improving quality and reducing costs. Coordination of care and disease-management programs have led to cost reductions of 30% or more12 and have reduced admission rates by more than 10%.13 While the nation waits for health care reimbursement models to better reward patient quality outcomes and population health while reducing costs, we can use measures such as the Agency for Healthcare Research and Quality’s Prevention Quality Indicators to identify early interventions in the ambulatory care setting that can prevent admissions, complications, and exacerbation of disease.
Payers should also experiment with and promote innovative bundled-payment models such as Geisinger Health System’s ProvenCare program, which sets a fixed price for surgical procedures and up to 90 days of posthospital care, including readmission. These warranty-like programs overcome financial incentives to readmit patients in Medicare’s volume-based diagnosis-related group payment system.5
Re-engineering the delivery of care requires realigning resources to improve efficiency and effectiveness. In the short term, hospitals that successfully reduce readmission rates can expect reduced net reimbursements, as the penalties currently do not exceed the lost revenue of readmissions.
Reducing preventable readmissions is the right thing to do, but not all hospitalizations and rehospitalizations are avoidable. Many readmissions reflect appropriate and necessary care. The relentless focus on the readmission rate diverts attention and resources from more proactive solutions and innovative approaches for increasing health care safety, quality outcomes, and value.
Hospitals are caught between the volume and value paradigms. Payment programs that reward proactive disease management and care coordination will do the most to reduce health care costs and improve the quality of care. Hospitals have a responsibility to efficiently and effectively manage acute care and optimize handoffs to the next provider. Medicare’s payment policies do not do enough today to align the financial and quality-of-care incentives.
- Ayache MB, Boyaji S, Pile J. Can we reduce the risk of readmission for a patient with an exacerbation of COPD? Cleve Clin J Med 2014; 81:525–527.
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med 2009; 360:1418–1428.
- Department of Health and Human Services. Medicare Program; Hospital Inpatient Prospective Payment Systems for Acute Care Hospitals and the Long-Term Care Hospital Prospective Payment System and FY 2012 Rates; Hospitals’ FTE Resident Caps for Graduate Medical Education Payment; Final Rule. Federal Register 2011; 76:51475–51846. www.gpo.gov/fdsys/pkg/FR-2011-08-18/pdf/2011-19719.pdf. Accessed August 5, 2014.
- Department of Health and Human Services. Medicare program; hospital inpatient prospective payment systems for acute care hospitals and the long term care; hospital prospective payment system and fiscal year 2014 rates; quality reporting requirements for specific providers; hospital conditions of participation; payment policies related to patient status; final rule. Federal Register 2013; 78:50495–51040. www.gpo.gov/fdsys/pkg/FR-2013-08-19/pdf/2013-18956.pdf. Accessed August 5, 2014.
- Berenson RA, Paulus RA, Kalman NS. Medicare’s readmissions-reduction program—a positive alternative. N Engl J Med 2012; 366:1364–1366.
- Joynt KE, Jha AK. Thirty-day readmissions—truth and consequences. N Engl J Med 2012; 366:1366–1369.
- Rau J. Medicare to penalize 2,217 hospitals for excess readmissions. Kaiser Health News 2012. www.kaiserhealthnews.org/stories/2012/august/13/medicare-hospitals-readmissions-penalties.aspx. Accessed August 5, 2014.
- Gorodeski EZ, Starling RC, Blackstone EH. Are all readmissions bad readmissions? N Engl J Med 2010; 363:297–298.
- Medicare Payment Advisory Commission. Report to the Congress: Promoting Greater Efficiency in Medicare, June 2007. www.medpac.gov/documents/jun07_entirereport.pdf. Accessed August 5, 2014.
- van Walraven C, Bennett C, Jennings A, Austin PC, Forster AJ. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ 2011; 183:E391–E402.
- CMS Fee For Service IPPS Payment File, Fiscal Year 2014. cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Downloads/FY_14_FR_Impact_File.zip. Accessed August 5, 2014.
- Dartmouth Medical School Center for the Evaluative Clinical Sciences. The Dartmouth Atlas of Health Care, 2006. www.dartmouthat-las.org/downloads/atlases/2006_Chronic_Care_Atlas.pdf. Accessed August 5, 2014.
- Gold W, Kongstvedt P. How broadening DM’s focus helped shrink one plan’s costs. Managed Care 2003. www.managedcaremag.com/archives/0311/0311.minnesota.html. Accessed August 5, 2014.
In this issue of the Cleveland Clinic Journal of Medicine, Drs. Ayache, Boyaji, and Pile share evidence-based strategies for reducing the risk of readmission for patients with acute exacerbations of chronic obstructive pulmonary disease (COPD).1 They emphasize standardizing practice by combining effective clinical management with appropriate patient education, communication, and postdischarge follow-up.
Reducing the rate of preventable hospital readmissions (as well as avoiding admissions in the first place) is the right thing to do for the patient. Moreover, broader adoption of the strategies that they outline in their article will be critical to the success of health care organizations in improving patient outcomes and navigating a rapidly evolving landscape of reimbursement and reporting changes associated with the Centers for Medicare and Medicaid Services (CMS) Readmissions Reduction Program. Hospital readmission rates, while imperfect measures of the quality of care, demonstrate opportunities to optimize transitions of care. Success in our efforts to improve the health of our patients will likely be aligned with reductions in preventable admissions and improved attention to care coordination.
HOSPITALS ARE PENALIZED FOR EXCESSIVE READMISSION RATES
With nearly 20% of Medicare beneficiaries being rehospitalized within 30 days of discharge, at a cost of $17 billion annually,2 Congress enacted the Hospital Readmissions Reduction Program3 as part of the Affordable Care Act (ACA) in 2012. The Centers for Medicare and Medicaid Services (CMS) had already been reporting the readmission rates for heart failure, acute myocardial infarction, and pneumonia since 2009 (www.medicare.gov/hospitalcompare). Building on this work, the Affordable Care Act implemented financial penalties against hospitals that had excessive rates of readmissions for these conditions.
The Affordable Care Act put 1% of a hospital’s Medicare base payment at risk for all inpatient diagnoses in 2013—not just the three listed here. The risk is 2% in 2014 and will rise to 3% in 2015. In its first year, more than 2,200 United States hospitals were penalized a total of approximately $280 million because of readmission rates above the national mean. Nearly 10% of hospitals incurred the maximum 1% penalty, and about 30% paid no penalty.
The Secretary of the Department of Health and Human Services has the authority to extend the Readmissions Reduction Program to additional high-volume or high-expenditure conditions, and the department has announced it will expand the program in October 2014 (fiscal year 2015) to include two additional conditions: elective hip or knee replacement and COPD.4 In both cases, CMS began by publicly reporting these rates before including them in the program. Additional readmission measures, including those for stroke and hospital-wide all-cause readmissions, are also publicly reported and receive increased attention but are not yet included in the Readmissions Reduction Program.
UNFAIRLY PENALIZING THOSE THAT SERVE THE POOR
Avoidable causes of readmissions include hospital-acquired infections and complications, inadequate medication reconciliation and management, poor communication and coordination of care among the members of the health care team, and suboptimal care transitions.5 But other important drivers of readmissions are outside of a hospital’s direct control. These include mental illness, lack of social support, and poverty.6
A criticism of the Readmissions Reduction Program is that it disproportionately penalizes hospitals that serve the poorest patients.7 Currently, CMS readmission risk models do not adjust for socioeconomic factors. Further, CMS responds to these concerns by noting that it does not want different outcome standards for poor patients, and that adjusting for these factors may conceal potential health care disparities in disadvantaged populations.
NEW MISSION FOR HOSPITALS: MITIGATE SOCIOECONOMIC BARRIERS
Effective programs to reduce hospital readmissions must address the clinical interventions and patient education needs in the COPD discharge checklist discussed by Ayache et al, but must also attempt to mitigate social disadvantages that drive up readmissions for patients at highest risk.
Are hospitals in a position to do this? Too often, it is assumed that patients have access to medications, transportation to follow-up appointments, and social support. Early identification of patients at highest risk of being affected by lack of these factors and innovative solutions for mitigating these risks are important considerations in our efforts to reduce hospital readmissions.
HOW MANY READMISSIONS ARE TRULY PREVENTABLE?
Experts disagree on how many readmissions are truly preventable. Readmission rates for the sickest patients treated at tertiary or academic medical centers may reflect high-quality care in well-managed patients who otherwise would have died during the index admission.8
In early studies, the Medicare Payment Advisory Commission estimated that up to three-quarters of readmissions are preventable.9 In contrast, studies that used clinical instead of administrative data suggest preventable readmissions make up as little as 12% of total readmissions.10
Regardless of the actual percentage, Medicare’s risk-adjustment model relies exclusively on administrative data that do not fully account for nonpreventable factors and do not completely address unrelated or planned rehospitalizations. CMS is attempting to address these issues with an expanded readmission algorithm that excludes more planned and unrelated readmissions from the penalty calculation.
Ironically, the current structure of the Readmissions Reduction Program does little to address its intended goal of eliminating the perverse financial incentives for hospitals and physicians to readmit patients. Payments are still episode-based and reward readmissions. The $280 million that CMS expects to receive from the program this year covers less than 5% of the nearly $12 billion attributed to preventable rehospitalizations.11
WHAT PATIENTS NEED, NOT WHAT SUITS PROVIDERS
Hospital readmission rates are publicly reported, but it is shortsighted to think about readmissions outside of the broader context of the “medical home.” One must consider the role of primary care providers before and after an index admission in addition to the role of postacute care providers for some patients after discharge. Neither is directly affected by the current penalty program, but both are critical to effective solutions and optimizing value-oriented care.
Readmission rates are suboptimal measures, as they address presumed failures of hospital transitions rather than measuring care coordination and providing meaningful incentives to coordinate care. Yes, there is much to do to ensure effective transitions from the hospital to home or postacute settings. But to truly transform health care and deliver value, shouldn’t we strive to redesign the work flow around what patients need rather than what suits providers?
This effort should focus on managing the conditions that bring patients to the hospital. Medical homes and optimizing chronic disease care can play pivotal roles in improving quality and reducing costs. Coordination of care and disease-management programs have led to cost reductions of 30% or more12 and have reduced admission rates by more than 10%.13 While the nation waits for health care reimbursement models to better reward patient quality outcomes and population health while reducing costs, we can use measures such as the Agency for Healthcare Research and Quality’s Prevention Quality Indicators to identify early interventions in the ambulatory care setting that can prevent admissions, complications, and exacerbation of disease.
Payers should also experiment with and promote innovative bundled-payment models such as Geisinger Health System’s ProvenCare program, which sets a fixed price for surgical procedures and up to 90 days of posthospital care, including readmission. These warranty-like programs overcome financial incentives to readmit patients in Medicare’s volume-based diagnosis-related group payment system.5
Re-engineering the delivery of care requires realigning resources to improve efficiency and effectiveness. In the short term, hospitals that successfully reduce readmission rates can expect reduced net reimbursements, as the penalties currently do not exceed the lost revenue of readmissions.
Reducing preventable readmissions is the right thing to do, but not all hospitalizations and rehospitalizations are avoidable. Many readmissions reflect appropriate and necessary care. The relentless focus on the readmission rate diverts attention and resources from more proactive solutions and innovative approaches for increasing health care safety, quality outcomes, and value.
Hospitals are caught between the volume and value paradigms. Payment programs that reward proactive disease management and care coordination will do the most to reduce health care costs and improve the quality of care. Hospitals have a responsibility to efficiently and effectively manage acute care and optimize handoffs to the next provider. Medicare’s payment policies do not do enough today to align the financial and quality-of-care incentives.
In this issue of the Cleveland Clinic Journal of Medicine, Drs. Ayache, Boyaji, and Pile share evidence-based strategies for reducing the risk of readmission for patients with acute exacerbations of chronic obstructive pulmonary disease (COPD).1 They emphasize standardizing practice by combining effective clinical management with appropriate patient education, communication, and postdischarge follow-up.
Reducing the rate of preventable hospital readmissions (as well as avoiding admissions in the first place) is the right thing to do for the patient. Moreover, broader adoption of the strategies that they outline in their article will be critical to the success of health care organizations in improving patient outcomes and navigating a rapidly evolving landscape of reimbursement and reporting changes associated with the Centers for Medicare and Medicaid Services (CMS) Readmissions Reduction Program. Hospital readmission rates, while imperfect measures of the quality of care, demonstrate opportunities to optimize transitions of care. Success in our efforts to improve the health of our patients will likely be aligned with reductions in preventable admissions and improved attention to care coordination.
HOSPITALS ARE PENALIZED FOR EXCESSIVE READMISSION RATES
With nearly 20% of Medicare beneficiaries being rehospitalized within 30 days of discharge, at a cost of $17 billion annually,2 Congress enacted the Hospital Readmissions Reduction Program3 as part of the Affordable Care Act (ACA) in 2012. The Centers for Medicare and Medicaid Services (CMS) had already been reporting the readmission rates for heart failure, acute myocardial infarction, and pneumonia since 2009 (www.medicare.gov/hospitalcompare). Building on this work, the Affordable Care Act implemented financial penalties against hospitals that had excessive rates of readmissions for these conditions.
The Affordable Care Act put 1% of a hospital’s Medicare base payment at risk for all inpatient diagnoses in 2013—not just the three listed here. The risk is 2% in 2014 and will rise to 3% in 2015. In its first year, more than 2,200 United States hospitals were penalized a total of approximately $280 million because of readmission rates above the national mean. Nearly 10% of hospitals incurred the maximum 1% penalty, and about 30% paid no penalty.
The Secretary of the Department of Health and Human Services has the authority to extend the Readmissions Reduction Program to additional high-volume or high-expenditure conditions, and the department has announced it will expand the program in October 2014 (fiscal year 2015) to include two additional conditions: elective hip or knee replacement and COPD.4 In both cases, CMS began by publicly reporting these rates before including them in the program. Additional readmission measures, including those for stroke and hospital-wide all-cause readmissions, are also publicly reported and receive increased attention but are not yet included in the Readmissions Reduction Program.
UNFAIRLY PENALIZING THOSE THAT SERVE THE POOR
Avoidable causes of readmissions include hospital-acquired infections and complications, inadequate medication reconciliation and management, poor communication and coordination of care among the members of the health care team, and suboptimal care transitions.5 But other important drivers of readmissions are outside of a hospital’s direct control. These include mental illness, lack of social support, and poverty.6
A criticism of the Readmissions Reduction Program is that it disproportionately penalizes hospitals that serve the poorest patients.7 Currently, CMS readmission risk models do not adjust for socioeconomic factors. Further, CMS responds to these concerns by noting that it does not want different outcome standards for poor patients, and that adjusting for these factors may conceal potential health care disparities in disadvantaged populations.
NEW MISSION FOR HOSPITALS: MITIGATE SOCIOECONOMIC BARRIERS
Effective programs to reduce hospital readmissions must address the clinical interventions and patient education needs in the COPD discharge checklist discussed by Ayache et al, but must also attempt to mitigate social disadvantages that drive up readmissions for patients at highest risk.
Are hospitals in a position to do this? Too often, it is assumed that patients have access to medications, transportation to follow-up appointments, and social support. Early identification of patients at highest risk of being affected by lack of these factors and innovative solutions for mitigating these risks are important considerations in our efforts to reduce hospital readmissions.
HOW MANY READMISSIONS ARE TRULY PREVENTABLE?
Experts disagree on how many readmissions are truly preventable. Readmission rates for the sickest patients treated at tertiary or academic medical centers may reflect high-quality care in well-managed patients who otherwise would have died during the index admission.8
In early studies, the Medicare Payment Advisory Commission estimated that up to three-quarters of readmissions are preventable.9 In contrast, studies that used clinical instead of administrative data suggest preventable readmissions make up as little as 12% of total readmissions.10
Regardless of the actual percentage, Medicare’s risk-adjustment model relies exclusively on administrative data that do not fully account for nonpreventable factors and do not completely address unrelated or planned rehospitalizations. CMS is attempting to address these issues with an expanded readmission algorithm that excludes more planned and unrelated readmissions from the penalty calculation.
Ironically, the current structure of the Readmissions Reduction Program does little to address its intended goal of eliminating the perverse financial incentives for hospitals and physicians to readmit patients. Payments are still episode-based and reward readmissions. The $280 million that CMS expects to receive from the program this year covers less than 5% of the nearly $12 billion attributed to preventable rehospitalizations.11
WHAT PATIENTS NEED, NOT WHAT SUITS PROVIDERS
Hospital readmission rates are publicly reported, but it is shortsighted to think about readmissions outside of the broader context of the “medical home.” One must consider the role of primary care providers before and after an index admission in addition to the role of postacute care providers for some patients after discharge. Neither is directly affected by the current penalty program, but both are critical to effective solutions and optimizing value-oriented care.
Readmission rates are suboptimal measures, as they address presumed failures of hospital transitions rather than measuring care coordination and providing meaningful incentives to coordinate care. Yes, there is much to do to ensure effective transitions from the hospital to home or postacute settings. But to truly transform health care and deliver value, shouldn’t we strive to redesign the work flow around what patients need rather than what suits providers?
This effort should focus on managing the conditions that bring patients to the hospital. Medical homes and optimizing chronic disease care can play pivotal roles in improving quality and reducing costs. Coordination of care and disease-management programs have led to cost reductions of 30% or more12 and have reduced admission rates by more than 10%.13 While the nation waits for health care reimbursement models to better reward patient quality outcomes and population health while reducing costs, we can use measures such as the Agency for Healthcare Research and Quality’s Prevention Quality Indicators to identify early interventions in the ambulatory care setting that can prevent admissions, complications, and exacerbation of disease.
Payers should also experiment with and promote innovative bundled-payment models such as Geisinger Health System’s ProvenCare program, which sets a fixed price for surgical procedures and up to 90 days of posthospital care, including readmission. These warranty-like programs overcome financial incentives to readmit patients in Medicare’s volume-based diagnosis-related group payment system.5
Re-engineering the delivery of care requires realigning resources to improve efficiency and effectiveness. In the short term, hospitals that successfully reduce readmission rates can expect reduced net reimbursements, as the penalties currently do not exceed the lost revenue of readmissions.
Reducing preventable readmissions is the right thing to do, but not all hospitalizations and rehospitalizations are avoidable. Many readmissions reflect appropriate and necessary care. The relentless focus on the readmission rate diverts attention and resources from more proactive solutions and innovative approaches for increasing health care safety, quality outcomes, and value.
Hospitals are caught between the volume and value paradigms. Payment programs that reward proactive disease management and care coordination will do the most to reduce health care costs and improve the quality of care. Hospitals have a responsibility to efficiently and effectively manage acute care and optimize handoffs to the next provider. Medicare’s payment policies do not do enough today to align the financial and quality-of-care incentives.
- Ayache MB, Boyaji S, Pile J. Can we reduce the risk of readmission for a patient with an exacerbation of COPD? Cleve Clin J Med 2014; 81:525–527.
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med 2009; 360:1418–1428.
- Department of Health and Human Services. Medicare Program; Hospital Inpatient Prospective Payment Systems for Acute Care Hospitals and the Long-Term Care Hospital Prospective Payment System and FY 2012 Rates; Hospitals’ FTE Resident Caps for Graduate Medical Education Payment; Final Rule. Federal Register 2011; 76:51475–51846. www.gpo.gov/fdsys/pkg/FR-2011-08-18/pdf/2011-19719.pdf. Accessed August 5, 2014.
- Department of Health and Human Services. Medicare program; hospital inpatient prospective payment systems for acute care hospitals and the long term care; hospital prospective payment system and fiscal year 2014 rates; quality reporting requirements for specific providers; hospital conditions of participation; payment policies related to patient status; final rule. Federal Register 2013; 78:50495–51040. www.gpo.gov/fdsys/pkg/FR-2013-08-19/pdf/2013-18956.pdf. Accessed August 5, 2014.
- Berenson RA, Paulus RA, Kalman NS. Medicare’s readmissions-reduction program—a positive alternative. N Engl J Med 2012; 366:1364–1366.
- Joynt KE, Jha AK. Thirty-day readmissions—truth and consequences. N Engl J Med 2012; 366:1366–1369.
- Rau J. Medicare to penalize 2,217 hospitals for excess readmissions. Kaiser Health News 2012. www.kaiserhealthnews.org/stories/2012/august/13/medicare-hospitals-readmissions-penalties.aspx. Accessed August 5, 2014.
- Gorodeski EZ, Starling RC, Blackstone EH. Are all readmissions bad readmissions? N Engl J Med 2010; 363:297–298.
- Medicare Payment Advisory Commission. Report to the Congress: Promoting Greater Efficiency in Medicare, June 2007. www.medpac.gov/documents/jun07_entirereport.pdf. Accessed August 5, 2014.
- van Walraven C, Bennett C, Jennings A, Austin PC, Forster AJ. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ 2011; 183:E391–E402.
- CMS Fee For Service IPPS Payment File, Fiscal Year 2014. cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Downloads/FY_14_FR_Impact_File.zip. Accessed August 5, 2014.
- Dartmouth Medical School Center for the Evaluative Clinical Sciences. The Dartmouth Atlas of Health Care, 2006. www.dartmouthat-las.org/downloads/atlases/2006_Chronic_Care_Atlas.pdf. Accessed August 5, 2014.
- Gold W, Kongstvedt P. How broadening DM’s focus helped shrink one plan’s costs. Managed Care 2003. www.managedcaremag.com/archives/0311/0311.minnesota.html. Accessed August 5, 2014.
- Ayache MB, Boyaji S, Pile J. Can we reduce the risk of readmission for a patient with an exacerbation of COPD? Cleve Clin J Med 2014; 81:525–527.
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med 2009; 360:1418–1428.
- Department of Health and Human Services. Medicare Program; Hospital Inpatient Prospective Payment Systems for Acute Care Hospitals and the Long-Term Care Hospital Prospective Payment System and FY 2012 Rates; Hospitals’ FTE Resident Caps for Graduate Medical Education Payment; Final Rule. Federal Register 2011; 76:51475–51846. www.gpo.gov/fdsys/pkg/FR-2011-08-18/pdf/2011-19719.pdf. Accessed August 5, 2014.
- Department of Health and Human Services. Medicare program; hospital inpatient prospective payment systems for acute care hospitals and the long term care; hospital prospective payment system and fiscal year 2014 rates; quality reporting requirements for specific providers; hospital conditions of participation; payment policies related to patient status; final rule. Federal Register 2013; 78:50495–51040. www.gpo.gov/fdsys/pkg/FR-2013-08-19/pdf/2013-18956.pdf. Accessed August 5, 2014.
- Berenson RA, Paulus RA, Kalman NS. Medicare’s readmissions-reduction program—a positive alternative. N Engl J Med 2012; 366:1364–1366.
- Joynt KE, Jha AK. Thirty-day readmissions—truth and consequences. N Engl J Med 2012; 366:1366–1369.
- Rau J. Medicare to penalize 2,217 hospitals for excess readmissions. Kaiser Health News 2012. www.kaiserhealthnews.org/stories/2012/august/13/medicare-hospitals-readmissions-penalties.aspx. Accessed August 5, 2014.
- Gorodeski EZ, Starling RC, Blackstone EH. Are all readmissions bad readmissions? N Engl J Med 2010; 363:297–298.
- Medicare Payment Advisory Commission. Report to the Congress: Promoting Greater Efficiency in Medicare, June 2007. www.medpac.gov/documents/jun07_entirereport.pdf. Accessed August 5, 2014.
- van Walraven C, Bennett C, Jennings A, Austin PC, Forster AJ. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ 2011; 183:E391–E402.
- CMS Fee For Service IPPS Payment File, Fiscal Year 2014. cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Downloads/FY_14_FR_Impact_File.zip. Accessed August 5, 2014.
- Dartmouth Medical School Center for the Evaluative Clinical Sciences. The Dartmouth Atlas of Health Care, 2006. www.dartmouthat-las.org/downloads/atlases/2006_Chronic_Care_Atlas.pdf. Accessed August 5, 2014.
- Gold W, Kongstvedt P. How broadening DM’s focus helped shrink one plan’s costs. Managed Care 2003. www.managedcaremag.com/archives/0311/0311.minnesota.html. Accessed August 5, 2014.
Perioperative beta-blockers in noncardiac surgery: The evidence continues to evolve
Prophylactic use of beta-blockers in the perioperative period is highly controversial. Initial studies in the 1990s were favorable, but evidence has been conflicting since then.
The pendulum swung away from routinely recommending beta-blockers after the publication of negative results from several studies, including the Perioperative Ischemic Evaluation (POISE) trial in 2008.1 Highlighting this change in practice, a Canadian study2 found that the use of perioperative beta-blockade increased between 1999 and 2005 but subsequently declined from 2005 to 2010. However, there was no appreciable change in this pattern after the POISE trial or after changes in the American College of Cardiology guidelines in 2002 and 2006.3
In 2008, Harte and Jaffer reviewed the perioperative use of beta-blockers in noncardiac surgery in this journal.4 Since then, a number of meta-analyses and retrospective observational studies have reported variable findings related to specific beta-blockers and specific complications.
In this paper, we review the rationale and recent evidence for and against the perioperative use of beta-blockers as guidance for internists and hospitalists.
POTENTIAL CARDIOPROTECTIVE EFFECTS OF BETA-BLOCKERS
Myocardial infarction and unstable angina are the leading cardiovascular causes of death after surgery.5 These events are multifactorial. Some are caused by the stress of surgery, which precipitates physiologic changes related to inflammatory mediators, sympathetic tone, and oxygen supply and demand; others are caused by acute plaque rupture, thrombosis, and occlusion.6 Most perioperative infarcts are non-Q-wave events7 and occur within the first 2 days after the procedure, when the effects of anesthetics, pain, fluid shifts, and physiologic changes are greatest. Because multiple causes may contribute to perioperative myocardial infarction, a single preventive strategy may not be sufficient.8,9
Beta-blockers do several things that may be beneficial in the perioperative setting. They reduce myocardial oxygen demand by decreasing the force of contraction and by slowing the heart rate, and slowing the heart rate increases diastolic perfusion time.10 They suppress arrhythmias; they limit leukocyte recruitment, the production of free radicals, metalloproteinase activity, monocyte activation, release of growth factors, and inflammatory cytokine response; and they stabilize plaque.11 Their long-term use may also alter intracellular signaling processes, thus improving cell survival by decreasing the expression of receptors for substances that induce apoptosis.12
INITIAL POSITIVE TRIALS
Mangano et al13 began the beta-blocker trend in 1996 with a study in 200 patients known to have coronary artery disease or risk factors for it who were undergoing noncardiac surgery. Patients were randomized to receive either atenolol orally and intravenously, titrated to control the heart rate, or placebo in the immediate perioperative period.
The atenolol group had less perioperative ischemia but no difference in short-term rates of myocardial infarction and death. However, the death rate was lower in the atenolol group at 6 months after discharge and at 2 years, although patients who died in the immediate postoperative period were excluded from the analysis.
Although this finding did not appear to make sense physiologically, we now know that patients may experience myocardial injury without infarction after noncardiac surgery, a phenomenon associated with an increased risk of death in the short term and the long term.14 Preventing these episodes may be the explanation for the improved outcome.
The DECREASE trial15 (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography) provided additional support for beta-blocker use. The patients were at high risk, had abnormal dobutamine stress echocardiograms, and were undergoing vascular surgery; 112 patients were randomized to receive either oral bisoprolol (started 1 month before surgery, titrated to control the heart rate, and continued for 1 month after surgery) or placebo.
The study was stopped early because the bisoprolol group reportedly had a 90% lower rate of myocardial infarction and cardiac death 1 month after surgery. However, the study was criticized because the total number of patients enrolled was small and the benefit was much greater than usual for any pharmacologic intervention, thus calling the results into question.
In a follow-up study,16 survivors continued to be followed while receiving bisoprolol or usual care. The incidence of myocardial infarction or cardiac death at 2 years was significantly lower in the group receiving bisoprolol (12% vs 32%, odds ratio [OR] 0.30, P = .025).
Boersma et al,17 in an observational study, analyzed data from all 1,351 patients scheduled for major vascular surgery being considered for enrollment in the DECREASE trial. The DECREASE protocol required patients to undergo dobutamine stress echocardiography if they had one or more risk factors (age 70 or older, angina, prior myocardial infarction, congestive heart failure, treatment for ventricular arrhythmia, treatment for diabetes mellitus, or limited exercise capacity) or if their physician requested it. Twenty-seven percent received beta-blockers.
In multivariate analysis, clinical predictors of adverse outcome were age 70 or older; current or prior history of angina; and prior myocardial infarction, heart failure, or cerebrovascular accident.
In patients who had fewer than three clinical risk factors, beta-blocker use was associated with a lower rate of complications (0.8% vs 2.3%). Dobutamine stress echocardiography had minimal predictive value in this lower-risk group, suggesting that stress testing may not be necessary in this group if beta-blockers are used appropriately. However, in patients who had three or more risk factors, this test did provide additional prognostic information; those without stress-induced ischemia had lower event rates than those with ischemia, and beta-blocker use further reduced those rates, except in patients with extensive ischemia (more than five left ventricular segments involved).
The Revised Cardiac Risk Index. Lee et al18 devised an index to assist in preoperative cardiac risk stratification that was subsequently incorporated into the 2007 American College of Cardiology/American Heart Association preoperative risk guidelines. (It does not, however, address the beta-blocker issue.) It consists of six independent risk-predictors of major cardiac complications derived from 4,315 patients over age 50 undergoing non-cardiac surgery. The risk factors, each of which is given 1 point, are:
- Congestive heart failure based on history or examination
- Renal insufficiency (serum creatinine level > 2 mg/dL)
- Myocardial infarction, symptomatic ischemic heart disease, or a positive stress test
- History of transient ischemic attack or stroke
- Diabetes requiring insulin
- High-risk surgery (defined as intrathoracic, intra-abdominal, or suprainguinal vascular surgery).
Patients with 3 or more points are considered to be at high risk, and those with 1 or 2 points are considered to be at intermediate risk. The American College of Cardiology/American Heart Association preoperative cardiac risk algorithm subsequently included five of these six risk factors (the type of surgery was considered separately) and made recommendations concerning noninvasive stress testing and heart rate control.
On the basis of these studies, specialty societies, guideline committees, and hospitals enthusiastically recommended the prophylactic use of beta-blockers to decrease postoperative cardiac complications.
THREE NEGATIVE TRIALS OF METOPROLOL
In 2005 and 2006, two studies in vascular surgery patients and another in patients with diabetes cast doubt on the role of beta-blockers when the results failed to show a benefit. The trials used metoprolol, started shortly before surgery, and with no titration to control the heart rate.
The MaVS study19 (Metoprolol After Vascular Surgery) randomized 496 patients to receive metoprolol or placebo 2 hours before surgery and until hospital discharge or a maximum of 5 days after surgery. The metoprolol dose varied by weight: patients weighing 75 kg or more got 100 mg, those weighing between 40 and 75 kg got 50 mg, and those weighing less than 40 kg got 25 mg. Overall effects at 6 months were not significantly different, but intraoperative bradycardia and hypotension requiring intervention were more frequent in the metoprolol group.
The POBBLE study20 (Perioperative Beta Blockade) randomized 103 patients who had no history of myocardial infarction to receive either metoprolol 50 mg twice daily or placebo from admission to 7 days after surgery. Myocardial ischemia was present in one-third of the patients after surgery. Metoprolol did not reduce the 30-day cardiac mortality rate, but it was associated with a shorter length of stay.
The DIPOM trial21 (Diabetic Postoperative Mortality and Morbidity) randomized 921 diabetic patients to receive long-acting metoprolol succinate controlled-release/extended release (CR/XL) or placebo. Patients in the metoprolol group received a test dose of 50 mg the evening before surgery, another dose 2 hours before surgery (100 mg if the heart rate was more than 65 bpm, or 50 mg if between 55 and 65 bpm), and daily thereafter until discharge or a maximum of 8 days. The dose was not titrated to heart-rate control.
Metoprolol had no statistically significant effect on the composite primary outcome measures of time to death from any cause, acute myocardial infarction, unstable angina, or congestive heart failure or on the secondary outcome measures of time to death from any cause, death from a cardiac cause, and nonfatal cardiac morbidity.
ADDITIONAL POSITIVE STUDIES
Lindenauer et al22 retrospectively evaluated the use of beta-blockers in the first 2 days after surgery in 782,969 patients undergoing non-cardiac surgery. Using propensity score matching and Revised Cardiac Risk Index scores, they found a lower rate of postoperative mortality in patients with three or more risk factors who received a beta-blocker. There was no significant difference in the group with two risk factors, but in the lowest-risk group (with a score of 0 to 1), beta-blockers were not beneficial and may have been associated with harm as evidenced by a higher odds ratio for death, although this was probably artifactual and reflecting database limitations.
Feringa et al,23 in an observational cohort study of 272 patients undergoing vascular surgery, reported that higher doses of beta-blockers and tight heart-rate control were associated with less perioperative myocardial ischemia, lower troponin T levels, and better long-term outcome.
THE POISE TRIAL: MIXED RESULTS
The randomized POISE trial,1 published in 2008, compared the effects of extended-release metoprolol succinate vs placebo on the 30-day risk of major cardiovascular events in 8,351 patients with or at risk of atherosclerotic disease who were undergoing noncardiac surgery. The metoprolol regimen was 100 mg 2 to 4 hours before surgery, another 100 mg by 6 hours after surgery, and then 200 mg 12 hours later and once daily for 30 days.
The incidence of the composite primary end point of cardiovascular death, nonfatal myocardial infarction, and nonfatal cardiac arrest at 30 days was lower in the metoprolol group than in the placebo group (5.8% vs 6.9%; P = .04), primarily because of fewer nonfatal myocardial infarctions. However, more patients in the metoprolol group died of any cause (3.1% vs 2.3% P = .03) or had a stroke (1.0% vs 0.5% P = .005) than in the placebo group.
The metoprolol group had a higher incidence of clinically significant hypotension, bradycardia, and stroke, which could account for much of the increase in the mortality rate. Sepsis was the major cause of death in this group; hypotension may have increased the risk of infection, and beta-blockers may have potentiated hypotension in patients who were already septic. Also, the bradycardic and negative inotropic effects of the beta-blocker could have masked the physiologic response to systemic infection, thereby delaying recognition and treatment or impeding the normal immune response.
One of the major criticisms of the POISE trial was its aggressive dosing regimen (200 to 400 mg within a 36-hour period) in patients who had not been on beta-blockers before then. Also, the drug was started only a few hours before surgery. In addition, these patients were at higher risk of death and stroke than those in other trials based on a high baseline rate of cerebrovascular disease, and inclusion of urgent and emergency surgical procedures.
STUDIES SINCE POISE
The POISE trial results1 prompted further questioning of the prophylactic perioperative use of beta-blockers. However, proponents of beta-blockers voiced serious criticisms of the trial, particularly the dosing regimen, and continued to believe that these drugs were beneficial if used appropriately.
The DECREASE IV trial. Dunkelgrun et al,24 in a study using bisoprolol started approximately 1 month before surgery and titrated to control the heart rate, reported beneficial results in intermediate-risk patients. In their randomized open-label study with a 2 × 2 factorial design, 1,066 patients at intermediate cardiac risk were assigned to receive bisoprolol, fluvastatin, combination treatment, or control therapy at least 34 days before surgery. Bisoprolol was started at 2.5 mg orally daily and slowly titrated up to a maximum dose of 10 mg to keep the heart rate between 50 and 70 beats per minute. The group of 533 patients randomized to receive bisoprolol had a lower incidence rate of cardiac death and nonfatal myocardial infarction than the control group (2.1% vs 6.0%, HR 0.34, P = .002). A potential limitation of this study was its open-label design, which might have led to treatment bias.
Updated guidelines. Based on the results from POISE and DECREASE IV, the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines25 published a focused update on beta-blockers in 2009 as an amendment to their 2007 guidelines on perioperative evaluation and care for noncardiac surgery. The European Society of Cardiology26 released similar but somewhat more liberal guidelines (Table 1).
London et al,27 in an observational study published in 2013, found a lower 30-day overall mortality rate with beta-blockers (relative risk [RR] 0.73, 95% confidence interval [CI] 0.65–0.83, P < .001, number needed to treat [NNT] 241), as well as a lower rate of cardiac morbidity (nonfatal myocardial infarction and cardiac death), but only in nonvascular surgery patients who were on beta-blockers within 7 days of scheduled surgery. Moreover, similar to the findings of Lindenauer et al,22 only patients with a Revised Cardiac Risk Index score of 2 or more benefited from beta-blocker use in terms of a lower risk of death, whereas the lower-risk patients did not:
- Risk score of 0 or 1—no association
- Score of 2—RR 0.63, 95% CI 0.50–0.80, P < .001, NNT 105
- Score of 3—RR 0.54, 95% CI 0.39–0.73, P < .001, NNT 41
- Score of 4 or more—RR 0.40, 95% CI 0.24–0.73, P < .001, NNT 18).
Beta-blocker exposure was associated with a significantly lower rate of cardiac complications (RR 0.67, 95% CI 0.57–0.79, P < .001, NNT 339), also limited to nonvascular surgery patients with a risk score of 2 or 3.
The Danish Nationwide Cohort Study28 examined the effect of beta-blockers on major adverse cardiac events (MACE, ie, myocardial infarction, cerebrovascular accident, and death) in 28,263 patients with ischemic heart disease undergoing noncardiac surgery; 7,990 with heart failure and 20,273 without. Beta-blockers were used in 53% of patients with heart failure and 36% of those without heart failure. Outcomes for all of the beta-blocker recipients:
- MACE—HR 0.90, 95% CI 0.79–1.02
- All-cause mortality—HR 0.95, 95% CI 0.85–1.06.
Outcomes for patients with heart failure if they received beta-blockers:
- MACE—HR 0.75, 95% CI 0.70–0.87
- All-cause mortality—HR 0.80, 95% CI 0.70–0.92.
There was no significant benefit from beta-blockers in patients without heart failure. Outcomes for those patients if they received beta-blockers:
- MACE—HR 1.11, 95% CI 0.92–1.33
- All-cause mortality—HR 1.15, 95% CI 0.98–1.35.
However, in patients without heart failure but with a history of myocardial infarction within the past 2 years, beta-blockers were associated with a lower risk of MACE and all-cause mortality. In patients with neither heart failure nor a recent myocardial infarction, beta-blockers were associated with an increased risk of MACE and all-cause mortality.
This difference in efficacy depending on the presence and timing of a prior myocardial infarction is consistent with the 2012 American College of Cardiology/American Heart Association guidelines for secondary prevention, in which beta-blockers are given a class I recommendation only for patients with a myocardial infarction within the past 3 years.
Meta-analyses and outcomes
A number of meta-analyses have been published over the past 10 years, with conflicting results (Table 2). The divergent findings are primarily due to the different studies included in the analyses as well as the strong influence of the POISE trial.1 The studies varied in terms of the specific beta-blocker used, dose titration and heart rate control, time of initiation of beta-blocker use before surgery, type of surgery, patient characteristics, comorbidities, biomarkers and diagnosis of myocardial infarction, and clinical end points.
In general, these meta-analyses have found that prophylactic perioperative use of beta-blockers decreases ischemia and tends to reduce the risk of nonfatal myocardial infarction. They vary on whether the overall mortality risk is decreased. The meta-analyses that included POISE1 found an increased incidence of stroke, whereas those that excluded POISE found no significant difference, although there appeared to be slightly more strokes in the beta-blocker groups.
The beta-blocker controversy increased even further when Dr. Don Poldermans was fired by Erasmus Medical Center in November 2011 for violations of academic integrity involving his research, including the DECREASE trials. The most recent meta-analysis, by Bouri et al,29 included nine “secure trials” and excluded the DECREASE trials in view of the controversy about their authenticity. The analysis showed an increase in overall mortality as well as stroke, primarily because it was heavily influenced by POISE.1 In contrast, the DECREASE trials had reported a decreased risk of myocardial infarction and death, with no significant increase in stroke. The authors concluded that guideline bodies should “retract their recommendations based on the fictitious data without further delay.”29
Although the design of the DECREASE trials (in which beta-blockers were started well in advance of surgery and doses were titrated to achieve heart rate control) is physiologically more compelling than those of the negative trials, the results have been questioned in light of the integrity issue. However, to date, none of the published DECREASE trials have been retracted.
Two other meta-analyses,30,31 published in 2013, also found a decreased risk of myocardial infarction and increased risk of stroke but no significant difference in short-term all-cause mortality.
ARE ALL BETA-BLOCKERS EQUIVALENT?
In various studies evaluating specific beta-blockers, the more cardioselective agents bisoprolol and atenolol were associated with better outcomes than metoprolol. The affinity ratios for beta-1/beta-2 receptors range from 13.5 for bisoprolol to 4.7 for atenolol and 2.3 for metoprolol.32 Blocking beta-1 receptors blunts tachycardia, whereas blocking beta-2 receptors may block systemic or cerebral vasodilation.
In patients with anemia, beta-blockade in general may be harmful, but beta-2 blockade may be even worse. Beta-blockers were associated with an increased risk of MACE (6.5% vs 3.0%)33 in patients with acute surgical anemia if the hemoglobin concentration decreased to less than 35% of baseline, and increased risks of hospital death (OR 6.65) and multiorgan dysfunction syndrome (OR 4.18) with severe bleeding during aortic surgery.34
In addition, the pathway by which the beta-blocker is metabolized may also affect outcome, with less benefit from beta-blockers metabolized by the CYP2D6 isoenzyme of the cytochrome P450 system. Individual variations in CYP2D6 activity related to genetics or drug interactions may result in insufficient or excessive beta-blockade. Because metoprolol is the most dependent on this system, patients using it may be more susceptible to bradycardia.35
Studies comparing atenolol and metoprolol found that the atenolol groups had fewer myocardial infarctions and deaths36 and lower 30-day and 1-year mortality rates37 than the groups on metoprolol. Studies comparing the three beta-blockers found better outcomes with atenolol and bisoprolol than with metoprolol—fewer strokes,38,39 a lower mortality rate,31 and a better composite outcome39 (Table 3 and Table 4).
START THE BETA-BLOCKER EARLY, TITRATE TO CONTROL THE HEART RATE
A number of studies suggest that how long the beta-blocker is given before surgery may influence the outcome (Table 5). The best results were achieved when beta-blockers were started approximately 1 month before surgery and titrated to control the heart rate.
Because this long lead-in time is not always practical, it is important to determine the shortest time before surgery in which starting beta-blockers may be beneficial and yet safe. Some evidence suggests that results are better when the beta-blocker is started more than 1 week preoperatively compared with less than 1 week, but it is unknown what the minimum or optimal time period should be.
If a beta-blocker is started well in advance of the scheduled surgery, there is adequate time for dose titration and tighter heart rate control. Most of the studies demonstrating beneficial effects of perioperative beta-blockers used dose titration and achieved lower heart rates in the treatment group than in the control group. A criticism of the MaVs,19 POBBLE,20 and DIPOM21 trials was that the patients did not receive adequate beta-blockade. The POISE trial1 used a much higher dose of metoprolol in an attempt to assure beta-blockade without dose titration, and although the regimen decreased nonfatal myocardial infarctions, it increased strokes and the overall mortality rate, probably related to excess bradycardia and hypotension. The target heart rate should probably be between 55 and 70 beats per minute.
RISK OF STROKE
POISE1 was the first trial to note a clinically and statistically significant increase in strokes with perioperative beta-blocker use. Although no other study has shown a similar increased risk, almost all reported a higher number of strokes in the beta-blocker groups, although the absolute numbers and differences were small and not statistically significant. This risk may also vary from one beta-blocker to another (Table 4).
The usual incidence rate of postoperative stroke after noncardiac, noncarotid surgery is well under 1% in patients with no prior history of stroke but increases to approximately 3% in patients with a previous stroke.40 An observational study from the Dutch group reported a very low incidence of stroke overall (0.02%) in 186,779 patients undergoing noncardiac surgery with no significant difference in those on chronic beta-blocker therapy.41 The DECREASE trials, with a total of 3,884 patients, also found no statistically significant increase in stroke with beta-blocker use (0.46% overall vs 0.5% with a beta-blocker),42 which in this case was bisoprolol started well in advance of surgery and titrated to control the heart rate. Although the DECREASE data are under suspicion, they seem reasonable and consistent with those of observational studies.
Proposed mechanisms by which beta-blockers may increase stroke risk include the side effects of hypotension and bradycardia, particularly in the setting of anemia. They may also cause cerebral ischemia by blocking cerebral vasodilation. This effect on cerebral blood flow may be more pronounced with the less cardioselective beta-blockers, which may explain the apparent increased stroke risk associated with metoprolol.
WHAT SHOULD WE DO NOW?
The evidence for the safety and efficacy of beta-blockers in the perioperative setting continues to evolve, and new clinical trials are needed to clarify the ongoing controversy, particularly regarding the risk of stroke.
If patients have other indications for beta-blocker therapy, such as history of heart failure, myocardial infarction in the past 3 years, or atrial fibrillation for rate control, they should be receiving them if time permits.
If prophylactic beta-blockers are to be effective in minimizing perioperative complications, it appears that they may need to be more cardioselective, started at least 1 week before surgery, titrated to control heart rate, and used in high-risk patients (Revised Cardiac Risk Index score > 2 or 3) undergoing high-risk surgery.
Ideally, a large randomized controlled trial using a cardioselective beta-blocker started in advance of surgery in patients with a Revised Cardiac Risk Index score greater than 2, undergoing intermediate or high-risk procedures, is needed to fully answer the questions raised by the current data.
- POISE Study Group; Devereaux PJ, Yang H, Yusuf S, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; 371:1839–1847.
- Wijeysundera DN, Mamdani M, Laupacis A, et al. Clinical evidence, practice guidelines, and ß-blocker utilization before major noncardiac surgery. Circ Cardiovasc Qual Outcomes 2012; 5:558–565.
- American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery); American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Rhythm Society; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery: focused update on perioperative beta-blocker therapy: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society for Vascular Medicine and Biology. J Am Coll Cardiol 2006; 47:2343–2355.
- Harte B, Jaffer AK. Perioperative beta-blockers in noncardiac surgery: evolution of the evidence. Cleve Clin J Med 2008; 75:513–519.
- Mangano DT. Perioperative cardiac morbidity. Anesthesiology 1990; 72:153–184.
- London MJ, Zaugg M, Schaub MC, Spahn DR. Perioperative beta-adrenergic receptor blockade: physiologic foundations and clinical controversies. Anesthesiology 2004; 100:170–175.
- Badner NH, Knill RL, Brown JE, Novick TV, Gelb AW. Myocardial infarction after noncardiac surgery. Anesthesiology 1998; 88:572–578.
- Priebe HJ. Triggers of perioperative myocardial ischaemia and infarction. Br J Anaesth 2004; 93:9–20.
- Zaugg M, Schaub MC, Foëx P. Myocardial injury and its prevention in the perioperative setting. Br J Anaesth 2004; 93:21–33.
- Zaugg M, Schaub MC, Pasch T, Spahn DR. Modulation of beta-adrenergic receptor subtype activities in perioperative medicine: mechanisms and sites of action. Br J Anaesth 2002; 88:101–123.
- Landesberg G. The pathophysiology of perioperative myocardial infarction: facts and perspectives. J Cardiothorac Vasc Anesth 2003; 17:90–100.
- Yeager MP, Fillinger MP, Hettleman BD, Hartman GS. Perioperative beta-blockade and late cardiac outcomes: a complementary hypothesis. J Cardiothorac Vasc Anesth 2005; 19:237–241.
- Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 1996; 335:1713–1720.
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120:564–578.
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med 1999; 341:1789–1794.
- Poldermans D, Boersma E, Bax JJ, et al; Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. Bisoprolol reduces cardiac death and myocardial infarction in high-risk patients as long as 2 years after successful major vascular surgery. Eur Heart J 2001; 22:1353–1358.
- Boersma E, Poldermans D, Bax JJ, et al; DECREASE Study Group (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiogrpahy). Predictors of cardiac events after major vascular surgery: role of clinical characteristics, dobutamine echocardiography, and beta-blocker therapy. JAMA 2001; 285:1865–1873.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Yang H, Raymer K, Butler R, Parlow J, Roberts R. The effects of perioperative beta-blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J 2006; 152:983–990.
- Brady AR, Gibbs JS, Greenhalgh RM, Powell JT, Sydes MR; POBBLE trial investigators. Perioperative beta-blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double-blind controlled trial. J Vasc Surg 2005; 41:602–609.
- Juul AB, Wetterslev J, Gluud C, et al; DIPOM Trial Group. Effect of perioperative beta blockade in patients with diabetes undergoing major non-cardiac surgery: randomised placebo controlled, blinded multicentre trial. BMJ 2006; 332:1482.
- Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major non-cardiac surgery. N Engl J Med 2005; 353:349–361.
- Feringa HH, Bax JJ, Boersma E, et al. High-dose beta-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation 2006; 114(suppl 1):1344–1349.
- Dunkelgrun M, Boersma E, Schouten O, et al; Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate-risk patients undergoing noncardiovascular surgery: a randomized controlled trial (DECREASE-IV). Ann Surg 2009; 249:921–926.
- American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Rhythm Society; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine; Society for Vascular Surgery; Fleisher LA, Beckman JA, Brown KA, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol 2009; 54:e13–e118.
- Task Force for Preoperative Cardiac Risk Assessment and Perioperative Cardiac Management in Non-cardiac Surgery; European Society of Cardiology (ESC); Poldermans D, Bax JJ, Boersma E, et al. Guidelines for preoperative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery. Eur Heart J 2009; 30:2769–2812.
- London MJ, Hur K, Schwartz GG, Henderson WG. Association of perioperative beta-blockade with mortality and cardiovascular morbidity following major noncardiac surgery. JAMA 2013; 309:1704–1713.
- Andersson C, Mérie C, Jørgensen M, et al. Association of beta-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing noncardiac surgery: a Danish nationwide cohort study. JAMA Intern Med 2014; 174:336–344.
- Bouri S, Shun-Shin MJ, Cole GD, Mayet J, Francis DP. Meta-analysis of secure randomised controlled trials of beta-blockade to prevent perioperative death in non-cardiac surgery. Heart 2014; 100:456–464.
- Guay J, Ochroch EA. Beta-blocking agents for surgery: influence on mortality and major outcomes. A meta-analysis. J Cardiothorac Vasc Anesth 2013; 27:834–844.
- Dai N, Xu D, Zhang J, et al. Different beta-blockers and initiation time in patients undergoing noncardiac surgery: a meta-analysis. Am J Med Sci 2014; 347:235–244.
- Baker JG. The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br J Pharmacol 2005; 144:317–322.
- Beattie WS, Wijeysundera DN, Karkouti K, et al. Acute surgical anemia influences the cardioprotective effects of beta-blockade: a single-center, propensity-matched cohort study. Anesthesiology 2010; 112:25–33.
- Le Manach Y, Collins GS, Ibanez C, et al. Impact of perioperative bleeding on the protective effect of beta-blockers during infrarenal aortic reconstruction. Anesthesiology 2012; 117:1203–1211.
- Badgett RG, Lawrence VA, Cohn SL. Variations in pharmacology of beta-blockers may contribute to heterogeneous results in trials of perioperative beta-blockade. Anesthesiology 2010; 113:585–592.
- Redelmeier D, Scales D, Kopp A. Beta blockers for elective surgery in elderly patients: population based, retrospective cohort study. BMJ 2005; 331:932.
- Wallace AW, Au S, Cason BA. Perioperative beta-blockade: atenolol is associated with reduced mortality when compared to metoprolol. Anesthesiology 2011; 114:824–836.
- Mashour GA, Sharifpour M, Freundlich RE, et al. Perioperative metoprolol and risk of stroke after noncardiac surgery. Anesthesiology 2013; 119:1340–1346.
- Ashes C, Judelman S, Wijeysundera DN, et al. Selective beta1-antagonism with bisoprolol is associated with fewer postoperative strokes than atenolol or metoprolol: a single-center cohort study of 44,092 consecutive patients. Anesthesiology 2013; 119:777–787.
- Selim M. Perioperative stroke. N Engl J Med 2007; 356:706–713.
- van Lier F, Schouten O, van Domburg RT, et al. Effect of chronic beta-blocker use on stroke after noncardiac surgery. Am J Cardiol 2009; 104:429–433.
- van Lier F, Schouten O, Hoeks SE, et al. Impact of prophylactic beta-blocker therapy to prevent stroke after noncardiac surgery. Am J Cardiol 2010; 105:43–47.
- Devereaux PJ, Beattie WS, Choi PT, et al. How strong is the evidence for the use of perioperative beta blockers in non-cardiac surgery? Systematic review and meta-analysis of randomised controlled trials. BMJ 2005; 331:313–321.
- McGory ML, Maggard MA, Ko CY. A meta-analysis of perioperative beta blockade: what is the actual risk reduction? Surgery 2005; 138:171–179.
- Schouten O, Shaw LJ, Boersma E, et al. A meta-analysis of safety and effectiveness of perioperative beta-blocker use for the prevention of cardiac events in different types of noncardiac surgery. Coron Artery Dis 2006; 17:173–179.
- Wiesbauer F, Schlager O, Domanovits H, et al. Perioperative beta-blockers for preventing surgery-related mortality and morbidity: a systematic review and meta-analysis. Anesth Analg 2007; 104:27–41.
- Bangalore S, Wetterslev J, Pranesh S, Sawhney S, Gluud C, Messerli FH. Perioperative beta blockers in patients having non-cardiac surgery: a meta-analysis. Lancet 2008; 372:1962–1976.
- Flu WJ, van Kuijk JP, Chonchol M, et al. Timing of preoperative beta-blocker treatment in vascular surgery patients: influence on postoperative outcome. J Am Coll Cardiol 2010; 56:1922–1929.
- Wijeysundera DN, Beattie WS, Wijeysundera HC, Yun L, Austin PC, Ko DT. Duration of preoperative beta-blockade and outcomes after major elective noncardiac surgery. Can J Cardiol 2014; 30:217–223.
Prophylactic use of beta-blockers in the perioperative period is highly controversial. Initial studies in the 1990s were favorable, but evidence has been conflicting since then.
The pendulum swung away from routinely recommending beta-blockers after the publication of negative results from several studies, including the Perioperative Ischemic Evaluation (POISE) trial in 2008.1 Highlighting this change in practice, a Canadian study2 found that the use of perioperative beta-blockade increased between 1999 and 2005 but subsequently declined from 2005 to 2010. However, there was no appreciable change in this pattern after the POISE trial or after changes in the American College of Cardiology guidelines in 2002 and 2006.3
In 2008, Harte and Jaffer reviewed the perioperative use of beta-blockers in noncardiac surgery in this journal.4 Since then, a number of meta-analyses and retrospective observational studies have reported variable findings related to specific beta-blockers and specific complications.
In this paper, we review the rationale and recent evidence for and against the perioperative use of beta-blockers as guidance for internists and hospitalists.
POTENTIAL CARDIOPROTECTIVE EFFECTS OF BETA-BLOCKERS
Myocardial infarction and unstable angina are the leading cardiovascular causes of death after surgery.5 These events are multifactorial. Some are caused by the stress of surgery, which precipitates physiologic changes related to inflammatory mediators, sympathetic tone, and oxygen supply and demand; others are caused by acute plaque rupture, thrombosis, and occlusion.6 Most perioperative infarcts are non-Q-wave events7 and occur within the first 2 days after the procedure, when the effects of anesthetics, pain, fluid shifts, and physiologic changes are greatest. Because multiple causes may contribute to perioperative myocardial infarction, a single preventive strategy may not be sufficient.8,9
Beta-blockers do several things that may be beneficial in the perioperative setting. They reduce myocardial oxygen demand by decreasing the force of contraction and by slowing the heart rate, and slowing the heart rate increases diastolic perfusion time.10 They suppress arrhythmias; they limit leukocyte recruitment, the production of free radicals, metalloproteinase activity, monocyte activation, release of growth factors, and inflammatory cytokine response; and they stabilize plaque.11 Their long-term use may also alter intracellular signaling processes, thus improving cell survival by decreasing the expression of receptors for substances that induce apoptosis.12
INITIAL POSITIVE TRIALS
Mangano et al13 began the beta-blocker trend in 1996 with a study in 200 patients known to have coronary artery disease or risk factors for it who were undergoing noncardiac surgery. Patients were randomized to receive either atenolol orally and intravenously, titrated to control the heart rate, or placebo in the immediate perioperative period.
The atenolol group had less perioperative ischemia but no difference in short-term rates of myocardial infarction and death. However, the death rate was lower in the atenolol group at 6 months after discharge and at 2 years, although patients who died in the immediate postoperative period were excluded from the analysis.
Although this finding did not appear to make sense physiologically, we now know that patients may experience myocardial injury without infarction after noncardiac surgery, a phenomenon associated with an increased risk of death in the short term and the long term.14 Preventing these episodes may be the explanation for the improved outcome.
The DECREASE trial15 (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography) provided additional support for beta-blocker use. The patients were at high risk, had abnormal dobutamine stress echocardiograms, and were undergoing vascular surgery; 112 patients were randomized to receive either oral bisoprolol (started 1 month before surgery, titrated to control the heart rate, and continued for 1 month after surgery) or placebo.
The study was stopped early because the bisoprolol group reportedly had a 90% lower rate of myocardial infarction and cardiac death 1 month after surgery. However, the study was criticized because the total number of patients enrolled was small and the benefit was much greater than usual for any pharmacologic intervention, thus calling the results into question.
In a follow-up study,16 survivors continued to be followed while receiving bisoprolol or usual care. The incidence of myocardial infarction or cardiac death at 2 years was significantly lower in the group receiving bisoprolol (12% vs 32%, odds ratio [OR] 0.30, P = .025).
Boersma et al,17 in an observational study, analyzed data from all 1,351 patients scheduled for major vascular surgery being considered for enrollment in the DECREASE trial. The DECREASE protocol required patients to undergo dobutamine stress echocardiography if they had one or more risk factors (age 70 or older, angina, prior myocardial infarction, congestive heart failure, treatment for ventricular arrhythmia, treatment for diabetes mellitus, or limited exercise capacity) or if their physician requested it. Twenty-seven percent received beta-blockers.
In multivariate analysis, clinical predictors of adverse outcome were age 70 or older; current or prior history of angina; and prior myocardial infarction, heart failure, or cerebrovascular accident.
In patients who had fewer than three clinical risk factors, beta-blocker use was associated with a lower rate of complications (0.8% vs 2.3%). Dobutamine stress echocardiography had minimal predictive value in this lower-risk group, suggesting that stress testing may not be necessary in this group if beta-blockers are used appropriately. However, in patients who had three or more risk factors, this test did provide additional prognostic information; those without stress-induced ischemia had lower event rates than those with ischemia, and beta-blocker use further reduced those rates, except in patients with extensive ischemia (more than five left ventricular segments involved).
The Revised Cardiac Risk Index. Lee et al18 devised an index to assist in preoperative cardiac risk stratification that was subsequently incorporated into the 2007 American College of Cardiology/American Heart Association preoperative risk guidelines. (It does not, however, address the beta-blocker issue.) It consists of six independent risk-predictors of major cardiac complications derived from 4,315 patients over age 50 undergoing non-cardiac surgery. The risk factors, each of which is given 1 point, are:
- Congestive heart failure based on history or examination
- Renal insufficiency (serum creatinine level > 2 mg/dL)
- Myocardial infarction, symptomatic ischemic heart disease, or a positive stress test
- History of transient ischemic attack or stroke
- Diabetes requiring insulin
- High-risk surgery (defined as intrathoracic, intra-abdominal, or suprainguinal vascular surgery).
Patients with 3 or more points are considered to be at high risk, and those with 1 or 2 points are considered to be at intermediate risk. The American College of Cardiology/American Heart Association preoperative cardiac risk algorithm subsequently included five of these six risk factors (the type of surgery was considered separately) and made recommendations concerning noninvasive stress testing and heart rate control.
On the basis of these studies, specialty societies, guideline committees, and hospitals enthusiastically recommended the prophylactic use of beta-blockers to decrease postoperative cardiac complications.
THREE NEGATIVE TRIALS OF METOPROLOL
In 2005 and 2006, two studies in vascular surgery patients and another in patients with diabetes cast doubt on the role of beta-blockers when the results failed to show a benefit. The trials used metoprolol, started shortly before surgery, and with no titration to control the heart rate.
The MaVS study19 (Metoprolol After Vascular Surgery) randomized 496 patients to receive metoprolol or placebo 2 hours before surgery and until hospital discharge or a maximum of 5 days after surgery. The metoprolol dose varied by weight: patients weighing 75 kg or more got 100 mg, those weighing between 40 and 75 kg got 50 mg, and those weighing less than 40 kg got 25 mg. Overall effects at 6 months were not significantly different, but intraoperative bradycardia and hypotension requiring intervention were more frequent in the metoprolol group.
The POBBLE study20 (Perioperative Beta Blockade) randomized 103 patients who had no history of myocardial infarction to receive either metoprolol 50 mg twice daily or placebo from admission to 7 days after surgery. Myocardial ischemia was present in one-third of the patients after surgery. Metoprolol did not reduce the 30-day cardiac mortality rate, but it was associated with a shorter length of stay.
The DIPOM trial21 (Diabetic Postoperative Mortality and Morbidity) randomized 921 diabetic patients to receive long-acting metoprolol succinate controlled-release/extended release (CR/XL) or placebo. Patients in the metoprolol group received a test dose of 50 mg the evening before surgery, another dose 2 hours before surgery (100 mg if the heart rate was more than 65 bpm, or 50 mg if between 55 and 65 bpm), and daily thereafter until discharge or a maximum of 8 days. The dose was not titrated to heart-rate control.
Metoprolol had no statistically significant effect on the composite primary outcome measures of time to death from any cause, acute myocardial infarction, unstable angina, or congestive heart failure or on the secondary outcome measures of time to death from any cause, death from a cardiac cause, and nonfatal cardiac morbidity.
ADDITIONAL POSITIVE STUDIES
Lindenauer et al22 retrospectively evaluated the use of beta-blockers in the first 2 days after surgery in 782,969 patients undergoing non-cardiac surgery. Using propensity score matching and Revised Cardiac Risk Index scores, they found a lower rate of postoperative mortality in patients with three or more risk factors who received a beta-blocker. There was no significant difference in the group with two risk factors, but in the lowest-risk group (with a score of 0 to 1), beta-blockers were not beneficial and may have been associated with harm as evidenced by a higher odds ratio for death, although this was probably artifactual and reflecting database limitations.
Feringa et al,23 in an observational cohort study of 272 patients undergoing vascular surgery, reported that higher doses of beta-blockers and tight heart-rate control were associated with less perioperative myocardial ischemia, lower troponin T levels, and better long-term outcome.
THE POISE TRIAL: MIXED RESULTS
The randomized POISE trial,1 published in 2008, compared the effects of extended-release metoprolol succinate vs placebo on the 30-day risk of major cardiovascular events in 8,351 patients with or at risk of atherosclerotic disease who were undergoing noncardiac surgery. The metoprolol regimen was 100 mg 2 to 4 hours before surgery, another 100 mg by 6 hours after surgery, and then 200 mg 12 hours later and once daily for 30 days.
The incidence of the composite primary end point of cardiovascular death, nonfatal myocardial infarction, and nonfatal cardiac arrest at 30 days was lower in the metoprolol group than in the placebo group (5.8% vs 6.9%; P = .04), primarily because of fewer nonfatal myocardial infarctions. However, more patients in the metoprolol group died of any cause (3.1% vs 2.3% P = .03) or had a stroke (1.0% vs 0.5% P = .005) than in the placebo group.
The metoprolol group had a higher incidence of clinically significant hypotension, bradycardia, and stroke, which could account for much of the increase in the mortality rate. Sepsis was the major cause of death in this group; hypotension may have increased the risk of infection, and beta-blockers may have potentiated hypotension in patients who were already septic. Also, the bradycardic and negative inotropic effects of the beta-blocker could have masked the physiologic response to systemic infection, thereby delaying recognition and treatment or impeding the normal immune response.
One of the major criticisms of the POISE trial was its aggressive dosing regimen (200 to 400 mg within a 36-hour period) in patients who had not been on beta-blockers before then. Also, the drug was started only a few hours before surgery. In addition, these patients were at higher risk of death and stroke than those in other trials based on a high baseline rate of cerebrovascular disease, and inclusion of urgent and emergency surgical procedures.
STUDIES SINCE POISE
The POISE trial results1 prompted further questioning of the prophylactic perioperative use of beta-blockers. However, proponents of beta-blockers voiced serious criticisms of the trial, particularly the dosing regimen, and continued to believe that these drugs were beneficial if used appropriately.
The DECREASE IV trial. Dunkelgrun et al,24 in a study using bisoprolol started approximately 1 month before surgery and titrated to control the heart rate, reported beneficial results in intermediate-risk patients. In their randomized open-label study with a 2 × 2 factorial design, 1,066 patients at intermediate cardiac risk were assigned to receive bisoprolol, fluvastatin, combination treatment, or control therapy at least 34 days before surgery. Bisoprolol was started at 2.5 mg orally daily and slowly titrated up to a maximum dose of 10 mg to keep the heart rate between 50 and 70 beats per minute. The group of 533 patients randomized to receive bisoprolol had a lower incidence rate of cardiac death and nonfatal myocardial infarction than the control group (2.1% vs 6.0%, HR 0.34, P = .002). A potential limitation of this study was its open-label design, which might have led to treatment bias.
Updated guidelines. Based on the results from POISE and DECREASE IV, the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines25 published a focused update on beta-blockers in 2009 as an amendment to their 2007 guidelines on perioperative evaluation and care for noncardiac surgery. The European Society of Cardiology26 released similar but somewhat more liberal guidelines (Table 1).
London et al,27 in an observational study published in 2013, found a lower 30-day overall mortality rate with beta-blockers (relative risk [RR] 0.73, 95% confidence interval [CI] 0.65–0.83, P < .001, number needed to treat [NNT] 241), as well as a lower rate of cardiac morbidity (nonfatal myocardial infarction and cardiac death), but only in nonvascular surgery patients who were on beta-blockers within 7 days of scheduled surgery. Moreover, similar to the findings of Lindenauer et al,22 only patients with a Revised Cardiac Risk Index score of 2 or more benefited from beta-blocker use in terms of a lower risk of death, whereas the lower-risk patients did not:
- Risk score of 0 or 1—no association
- Score of 2—RR 0.63, 95% CI 0.50–0.80, P < .001, NNT 105
- Score of 3—RR 0.54, 95% CI 0.39–0.73, P < .001, NNT 41
- Score of 4 or more—RR 0.40, 95% CI 0.24–0.73, P < .001, NNT 18).
Beta-blocker exposure was associated with a significantly lower rate of cardiac complications (RR 0.67, 95% CI 0.57–0.79, P < .001, NNT 339), also limited to nonvascular surgery patients with a risk score of 2 or 3.
The Danish Nationwide Cohort Study28 examined the effect of beta-blockers on major adverse cardiac events (MACE, ie, myocardial infarction, cerebrovascular accident, and death) in 28,263 patients with ischemic heart disease undergoing noncardiac surgery; 7,990 with heart failure and 20,273 without. Beta-blockers were used in 53% of patients with heart failure and 36% of those without heart failure. Outcomes for all of the beta-blocker recipients:
- MACE—HR 0.90, 95% CI 0.79–1.02
- All-cause mortality—HR 0.95, 95% CI 0.85–1.06.
Outcomes for patients with heart failure if they received beta-blockers:
- MACE—HR 0.75, 95% CI 0.70–0.87
- All-cause mortality—HR 0.80, 95% CI 0.70–0.92.
There was no significant benefit from beta-blockers in patients without heart failure. Outcomes for those patients if they received beta-blockers:
- MACE—HR 1.11, 95% CI 0.92–1.33
- All-cause mortality—HR 1.15, 95% CI 0.98–1.35.
However, in patients without heart failure but with a history of myocardial infarction within the past 2 years, beta-blockers were associated with a lower risk of MACE and all-cause mortality. In patients with neither heart failure nor a recent myocardial infarction, beta-blockers were associated with an increased risk of MACE and all-cause mortality.
This difference in efficacy depending on the presence and timing of a prior myocardial infarction is consistent with the 2012 American College of Cardiology/American Heart Association guidelines for secondary prevention, in which beta-blockers are given a class I recommendation only for patients with a myocardial infarction within the past 3 years.
Meta-analyses and outcomes
A number of meta-analyses have been published over the past 10 years, with conflicting results (Table 2). The divergent findings are primarily due to the different studies included in the analyses as well as the strong influence of the POISE trial.1 The studies varied in terms of the specific beta-blocker used, dose titration and heart rate control, time of initiation of beta-blocker use before surgery, type of surgery, patient characteristics, comorbidities, biomarkers and diagnosis of myocardial infarction, and clinical end points.
In general, these meta-analyses have found that prophylactic perioperative use of beta-blockers decreases ischemia and tends to reduce the risk of nonfatal myocardial infarction. They vary on whether the overall mortality risk is decreased. The meta-analyses that included POISE1 found an increased incidence of stroke, whereas those that excluded POISE found no significant difference, although there appeared to be slightly more strokes in the beta-blocker groups.
The beta-blocker controversy increased even further when Dr. Don Poldermans was fired by Erasmus Medical Center in November 2011 for violations of academic integrity involving his research, including the DECREASE trials. The most recent meta-analysis, by Bouri et al,29 included nine “secure trials” and excluded the DECREASE trials in view of the controversy about their authenticity. The analysis showed an increase in overall mortality as well as stroke, primarily because it was heavily influenced by POISE.1 In contrast, the DECREASE trials had reported a decreased risk of myocardial infarction and death, with no significant increase in stroke. The authors concluded that guideline bodies should “retract their recommendations based on the fictitious data without further delay.”29
Although the design of the DECREASE trials (in which beta-blockers were started well in advance of surgery and doses were titrated to achieve heart rate control) is physiologically more compelling than those of the negative trials, the results have been questioned in light of the integrity issue. However, to date, none of the published DECREASE trials have been retracted.
Two other meta-analyses,30,31 published in 2013, also found a decreased risk of myocardial infarction and increased risk of stroke but no significant difference in short-term all-cause mortality.
ARE ALL BETA-BLOCKERS EQUIVALENT?
In various studies evaluating specific beta-blockers, the more cardioselective agents bisoprolol and atenolol were associated with better outcomes than metoprolol. The affinity ratios for beta-1/beta-2 receptors range from 13.5 for bisoprolol to 4.7 for atenolol and 2.3 for metoprolol.32 Blocking beta-1 receptors blunts tachycardia, whereas blocking beta-2 receptors may block systemic or cerebral vasodilation.
In patients with anemia, beta-blockade in general may be harmful, but beta-2 blockade may be even worse. Beta-blockers were associated with an increased risk of MACE (6.5% vs 3.0%)33 in patients with acute surgical anemia if the hemoglobin concentration decreased to less than 35% of baseline, and increased risks of hospital death (OR 6.65) and multiorgan dysfunction syndrome (OR 4.18) with severe bleeding during aortic surgery.34
In addition, the pathway by which the beta-blocker is metabolized may also affect outcome, with less benefit from beta-blockers metabolized by the CYP2D6 isoenzyme of the cytochrome P450 system. Individual variations in CYP2D6 activity related to genetics or drug interactions may result in insufficient or excessive beta-blockade. Because metoprolol is the most dependent on this system, patients using it may be more susceptible to bradycardia.35
Studies comparing atenolol and metoprolol found that the atenolol groups had fewer myocardial infarctions and deaths36 and lower 30-day and 1-year mortality rates37 than the groups on metoprolol. Studies comparing the three beta-blockers found better outcomes with atenolol and bisoprolol than with metoprolol—fewer strokes,38,39 a lower mortality rate,31 and a better composite outcome39 (Table 3 and Table 4).
START THE BETA-BLOCKER EARLY, TITRATE TO CONTROL THE HEART RATE
A number of studies suggest that how long the beta-blocker is given before surgery may influence the outcome (Table 5). The best results were achieved when beta-blockers were started approximately 1 month before surgery and titrated to control the heart rate.
Because this long lead-in time is not always practical, it is important to determine the shortest time before surgery in which starting beta-blockers may be beneficial and yet safe. Some evidence suggests that results are better when the beta-blocker is started more than 1 week preoperatively compared with less than 1 week, but it is unknown what the minimum or optimal time period should be.
If a beta-blocker is started well in advance of the scheduled surgery, there is adequate time for dose titration and tighter heart rate control. Most of the studies demonstrating beneficial effects of perioperative beta-blockers used dose titration and achieved lower heart rates in the treatment group than in the control group. A criticism of the MaVs,19 POBBLE,20 and DIPOM21 trials was that the patients did not receive adequate beta-blockade. The POISE trial1 used a much higher dose of metoprolol in an attempt to assure beta-blockade without dose titration, and although the regimen decreased nonfatal myocardial infarctions, it increased strokes and the overall mortality rate, probably related to excess bradycardia and hypotension. The target heart rate should probably be between 55 and 70 beats per minute.
RISK OF STROKE
POISE1 was the first trial to note a clinically and statistically significant increase in strokes with perioperative beta-blocker use. Although no other study has shown a similar increased risk, almost all reported a higher number of strokes in the beta-blocker groups, although the absolute numbers and differences were small and not statistically significant. This risk may also vary from one beta-blocker to another (Table 4).
The usual incidence rate of postoperative stroke after noncardiac, noncarotid surgery is well under 1% in patients with no prior history of stroke but increases to approximately 3% in patients with a previous stroke.40 An observational study from the Dutch group reported a very low incidence of stroke overall (0.02%) in 186,779 patients undergoing noncardiac surgery with no significant difference in those on chronic beta-blocker therapy.41 The DECREASE trials, with a total of 3,884 patients, also found no statistically significant increase in stroke with beta-blocker use (0.46% overall vs 0.5% with a beta-blocker),42 which in this case was bisoprolol started well in advance of surgery and titrated to control the heart rate. Although the DECREASE data are under suspicion, they seem reasonable and consistent with those of observational studies.
Proposed mechanisms by which beta-blockers may increase stroke risk include the side effects of hypotension and bradycardia, particularly in the setting of anemia. They may also cause cerebral ischemia by blocking cerebral vasodilation. This effect on cerebral blood flow may be more pronounced with the less cardioselective beta-blockers, which may explain the apparent increased stroke risk associated with metoprolol.
WHAT SHOULD WE DO NOW?
The evidence for the safety and efficacy of beta-blockers in the perioperative setting continues to evolve, and new clinical trials are needed to clarify the ongoing controversy, particularly regarding the risk of stroke.
If patients have other indications for beta-blocker therapy, such as history of heart failure, myocardial infarction in the past 3 years, or atrial fibrillation for rate control, they should be receiving them if time permits.
If prophylactic beta-blockers are to be effective in minimizing perioperative complications, it appears that they may need to be more cardioselective, started at least 1 week before surgery, titrated to control heart rate, and used in high-risk patients (Revised Cardiac Risk Index score > 2 or 3) undergoing high-risk surgery.
Ideally, a large randomized controlled trial using a cardioselective beta-blocker started in advance of surgery in patients with a Revised Cardiac Risk Index score greater than 2, undergoing intermediate or high-risk procedures, is needed to fully answer the questions raised by the current data.
Prophylactic use of beta-blockers in the perioperative period is highly controversial. Initial studies in the 1990s were favorable, but evidence has been conflicting since then.
The pendulum swung away from routinely recommending beta-blockers after the publication of negative results from several studies, including the Perioperative Ischemic Evaluation (POISE) trial in 2008.1 Highlighting this change in practice, a Canadian study2 found that the use of perioperative beta-blockade increased between 1999 and 2005 but subsequently declined from 2005 to 2010. However, there was no appreciable change in this pattern after the POISE trial or after changes in the American College of Cardiology guidelines in 2002 and 2006.3
In 2008, Harte and Jaffer reviewed the perioperative use of beta-blockers in noncardiac surgery in this journal.4 Since then, a number of meta-analyses and retrospective observational studies have reported variable findings related to specific beta-blockers and specific complications.
In this paper, we review the rationale and recent evidence for and against the perioperative use of beta-blockers as guidance for internists and hospitalists.
POTENTIAL CARDIOPROTECTIVE EFFECTS OF BETA-BLOCKERS
Myocardial infarction and unstable angina are the leading cardiovascular causes of death after surgery.5 These events are multifactorial. Some are caused by the stress of surgery, which precipitates physiologic changes related to inflammatory mediators, sympathetic tone, and oxygen supply and demand; others are caused by acute plaque rupture, thrombosis, and occlusion.6 Most perioperative infarcts are non-Q-wave events7 and occur within the first 2 days after the procedure, when the effects of anesthetics, pain, fluid shifts, and physiologic changes are greatest. Because multiple causes may contribute to perioperative myocardial infarction, a single preventive strategy may not be sufficient.8,9
Beta-blockers do several things that may be beneficial in the perioperative setting. They reduce myocardial oxygen demand by decreasing the force of contraction and by slowing the heart rate, and slowing the heart rate increases diastolic perfusion time.10 They suppress arrhythmias; they limit leukocyte recruitment, the production of free radicals, metalloproteinase activity, monocyte activation, release of growth factors, and inflammatory cytokine response; and they stabilize plaque.11 Their long-term use may also alter intracellular signaling processes, thus improving cell survival by decreasing the expression of receptors for substances that induce apoptosis.12
INITIAL POSITIVE TRIALS
Mangano et al13 began the beta-blocker trend in 1996 with a study in 200 patients known to have coronary artery disease or risk factors for it who were undergoing noncardiac surgery. Patients were randomized to receive either atenolol orally and intravenously, titrated to control the heart rate, or placebo in the immediate perioperative period.
The atenolol group had less perioperative ischemia but no difference in short-term rates of myocardial infarction and death. However, the death rate was lower in the atenolol group at 6 months after discharge and at 2 years, although patients who died in the immediate postoperative period were excluded from the analysis.
Although this finding did not appear to make sense physiologically, we now know that patients may experience myocardial injury without infarction after noncardiac surgery, a phenomenon associated with an increased risk of death in the short term and the long term.14 Preventing these episodes may be the explanation for the improved outcome.
The DECREASE trial15 (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography) provided additional support for beta-blocker use. The patients were at high risk, had abnormal dobutamine stress echocardiograms, and were undergoing vascular surgery; 112 patients were randomized to receive either oral bisoprolol (started 1 month before surgery, titrated to control the heart rate, and continued for 1 month after surgery) or placebo.
The study was stopped early because the bisoprolol group reportedly had a 90% lower rate of myocardial infarction and cardiac death 1 month after surgery. However, the study was criticized because the total number of patients enrolled was small and the benefit was much greater than usual for any pharmacologic intervention, thus calling the results into question.
In a follow-up study,16 survivors continued to be followed while receiving bisoprolol or usual care. The incidence of myocardial infarction or cardiac death at 2 years was significantly lower in the group receiving bisoprolol (12% vs 32%, odds ratio [OR] 0.30, P = .025).
Boersma et al,17 in an observational study, analyzed data from all 1,351 patients scheduled for major vascular surgery being considered for enrollment in the DECREASE trial. The DECREASE protocol required patients to undergo dobutamine stress echocardiography if they had one or more risk factors (age 70 or older, angina, prior myocardial infarction, congestive heart failure, treatment for ventricular arrhythmia, treatment for diabetes mellitus, or limited exercise capacity) or if their physician requested it. Twenty-seven percent received beta-blockers.
In multivariate analysis, clinical predictors of adverse outcome were age 70 or older; current or prior history of angina; and prior myocardial infarction, heart failure, or cerebrovascular accident.
In patients who had fewer than three clinical risk factors, beta-blocker use was associated with a lower rate of complications (0.8% vs 2.3%). Dobutamine stress echocardiography had minimal predictive value in this lower-risk group, suggesting that stress testing may not be necessary in this group if beta-blockers are used appropriately. However, in patients who had three or more risk factors, this test did provide additional prognostic information; those without stress-induced ischemia had lower event rates than those with ischemia, and beta-blocker use further reduced those rates, except in patients with extensive ischemia (more than five left ventricular segments involved).
The Revised Cardiac Risk Index. Lee et al18 devised an index to assist in preoperative cardiac risk stratification that was subsequently incorporated into the 2007 American College of Cardiology/American Heart Association preoperative risk guidelines. (It does not, however, address the beta-blocker issue.) It consists of six independent risk-predictors of major cardiac complications derived from 4,315 patients over age 50 undergoing non-cardiac surgery. The risk factors, each of which is given 1 point, are:
- Congestive heart failure based on history or examination
- Renal insufficiency (serum creatinine level > 2 mg/dL)
- Myocardial infarction, symptomatic ischemic heart disease, or a positive stress test
- History of transient ischemic attack or stroke
- Diabetes requiring insulin
- High-risk surgery (defined as intrathoracic, intra-abdominal, or suprainguinal vascular surgery).
Patients with 3 or more points are considered to be at high risk, and those with 1 or 2 points are considered to be at intermediate risk. The American College of Cardiology/American Heart Association preoperative cardiac risk algorithm subsequently included five of these six risk factors (the type of surgery was considered separately) and made recommendations concerning noninvasive stress testing and heart rate control.
On the basis of these studies, specialty societies, guideline committees, and hospitals enthusiastically recommended the prophylactic use of beta-blockers to decrease postoperative cardiac complications.
THREE NEGATIVE TRIALS OF METOPROLOL
In 2005 and 2006, two studies in vascular surgery patients and another in patients with diabetes cast doubt on the role of beta-blockers when the results failed to show a benefit. The trials used metoprolol, started shortly before surgery, and with no titration to control the heart rate.
The MaVS study19 (Metoprolol After Vascular Surgery) randomized 496 patients to receive metoprolol or placebo 2 hours before surgery and until hospital discharge or a maximum of 5 days after surgery. The metoprolol dose varied by weight: patients weighing 75 kg or more got 100 mg, those weighing between 40 and 75 kg got 50 mg, and those weighing less than 40 kg got 25 mg. Overall effects at 6 months were not significantly different, but intraoperative bradycardia and hypotension requiring intervention were more frequent in the metoprolol group.
The POBBLE study20 (Perioperative Beta Blockade) randomized 103 patients who had no history of myocardial infarction to receive either metoprolol 50 mg twice daily or placebo from admission to 7 days after surgery. Myocardial ischemia was present in one-third of the patients after surgery. Metoprolol did not reduce the 30-day cardiac mortality rate, but it was associated with a shorter length of stay.
The DIPOM trial21 (Diabetic Postoperative Mortality and Morbidity) randomized 921 diabetic patients to receive long-acting metoprolol succinate controlled-release/extended release (CR/XL) or placebo. Patients in the metoprolol group received a test dose of 50 mg the evening before surgery, another dose 2 hours before surgery (100 mg if the heart rate was more than 65 bpm, or 50 mg if between 55 and 65 bpm), and daily thereafter until discharge or a maximum of 8 days. The dose was not titrated to heart-rate control.
Metoprolol had no statistically significant effect on the composite primary outcome measures of time to death from any cause, acute myocardial infarction, unstable angina, or congestive heart failure or on the secondary outcome measures of time to death from any cause, death from a cardiac cause, and nonfatal cardiac morbidity.
ADDITIONAL POSITIVE STUDIES
Lindenauer et al22 retrospectively evaluated the use of beta-blockers in the first 2 days after surgery in 782,969 patients undergoing non-cardiac surgery. Using propensity score matching and Revised Cardiac Risk Index scores, they found a lower rate of postoperative mortality in patients with three or more risk factors who received a beta-blocker. There was no significant difference in the group with two risk factors, but in the lowest-risk group (with a score of 0 to 1), beta-blockers were not beneficial and may have been associated with harm as evidenced by a higher odds ratio for death, although this was probably artifactual and reflecting database limitations.
Feringa et al,23 in an observational cohort study of 272 patients undergoing vascular surgery, reported that higher doses of beta-blockers and tight heart-rate control were associated with less perioperative myocardial ischemia, lower troponin T levels, and better long-term outcome.
THE POISE TRIAL: MIXED RESULTS
The randomized POISE trial,1 published in 2008, compared the effects of extended-release metoprolol succinate vs placebo on the 30-day risk of major cardiovascular events in 8,351 patients with or at risk of atherosclerotic disease who were undergoing noncardiac surgery. The metoprolol regimen was 100 mg 2 to 4 hours before surgery, another 100 mg by 6 hours after surgery, and then 200 mg 12 hours later and once daily for 30 days.
The incidence of the composite primary end point of cardiovascular death, nonfatal myocardial infarction, and nonfatal cardiac arrest at 30 days was lower in the metoprolol group than in the placebo group (5.8% vs 6.9%; P = .04), primarily because of fewer nonfatal myocardial infarctions. However, more patients in the metoprolol group died of any cause (3.1% vs 2.3% P = .03) or had a stroke (1.0% vs 0.5% P = .005) than in the placebo group.
The metoprolol group had a higher incidence of clinically significant hypotension, bradycardia, and stroke, which could account for much of the increase in the mortality rate. Sepsis was the major cause of death in this group; hypotension may have increased the risk of infection, and beta-blockers may have potentiated hypotension in patients who were already septic. Also, the bradycardic and negative inotropic effects of the beta-blocker could have masked the physiologic response to systemic infection, thereby delaying recognition and treatment or impeding the normal immune response.
One of the major criticisms of the POISE trial was its aggressive dosing regimen (200 to 400 mg within a 36-hour period) in patients who had not been on beta-blockers before then. Also, the drug was started only a few hours before surgery. In addition, these patients were at higher risk of death and stroke than those in other trials based on a high baseline rate of cerebrovascular disease, and inclusion of urgent and emergency surgical procedures.
STUDIES SINCE POISE
The POISE trial results1 prompted further questioning of the prophylactic perioperative use of beta-blockers. However, proponents of beta-blockers voiced serious criticisms of the trial, particularly the dosing regimen, and continued to believe that these drugs were beneficial if used appropriately.
The DECREASE IV trial. Dunkelgrun et al,24 in a study using bisoprolol started approximately 1 month before surgery and titrated to control the heart rate, reported beneficial results in intermediate-risk patients. In their randomized open-label study with a 2 × 2 factorial design, 1,066 patients at intermediate cardiac risk were assigned to receive bisoprolol, fluvastatin, combination treatment, or control therapy at least 34 days before surgery. Bisoprolol was started at 2.5 mg orally daily and slowly titrated up to a maximum dose of 10 mg to keep the heart rate between 50 and 70 beats per minute. The group of 533 patients randomized to receive bisoprolol had a lower incidence rate of cardiac death and nonfatal myocardial infarction than the control group (2.1% vs 6.0%, HR 0.34, P = .002). A potential limitation of this study was its open-label design, which might have led to treatment bias.
Updated guidelines. Based on the results from POISE and DECREASE IV, the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines25 published a focused update on beta-blockers in 2009 as an amendment to their 2007 guidelines on perioperative evaluation and care for noncardiac surgery. The European Society of Cardiology26 released similar but somewhat more liberal guidelines (Table 1).
London et al,27 in an observational study published in 2013, found a lower 30-day overall mortality rate with beta-blockers (relative risk [RR] 0.73, 95% confidence interval [CI] 0.65–0.83, P < .001, number needed to treat [NNT] 241), as well as a lower rate of cardiac morbidity (nonfatal myocardial infarction and cardiac death), but only in nonvascular surgery patients who were on beta-blockers within 7 days of scheduled surgery. Moreover, similar to the findings of Lindenauer et al,22 only patients with a Revised Cardiac Risk Index score of 2 or more benefited from beta-blocker use in terms of a lower risk of death, whereas the lower-risk patients did not:
- Risk score of 0 or 1—no association
- Score of 2—RR 0.63, 95% CI 0.50–0.80, P < .001, NNT 105
- Score of 3—RR 0.54, 95% CI 0.39–0.73, P < .001, NNT 41
- Score of 4 or more—RR 0.40, 95% CI 0.24–0.73, P < .001, NNT 18).
Beta-blocker exposure was associated with a significantly lower rate of cardiac complications (RR 0.67, 95% CI 0.57–0.79, P < .001, NNT 339), also limited to nonvascular surgery patients with a risk score of 2 or 3.
The Danish Nationwide Cohort Study28 examined the effect of beta-blockers on major adverse cardiac events (MACE, ie, myocardial infarction, cerebrovascular accident, and death) in 28,263 patients with ischemic heart disease undergoing noncardiac surgery; 7,990 with heart failure and 20,273 without. Beta-blockers were used in 53% of patients with heart failure and 36% of those without heart failure. Outcomes for all of the beta-blocker recipients:
- MACE—HR 0.90, 95% CI 0.79–1.02
- All-cause mortality—HR 0.95, 95% CI 0.85–1.06.
Outcomes for patients with heart failure if they received beta-blockers:
- MACE—HR 0.75, 95% CI 0.70–0.87
- All-cause mortality—HR 0.80, 95% CI 0.70–0.92.
There was no significant benefit from beta-blockers in patients without heart failure. Outcomes for those patients if they received beta-blockers:
- MACE—HR 1.11, 95% CI 0.92–1.33
- All-cause mortality—HR 1.15, 95% CI 0.98–1.35.
However, in patients without heart failure but with a history of myocardial infarction within the past 2 years, beta-blockers were associated with a lower risk of MACE and all-cause mortality. In patients with neither heart failure nor a recent myocardial infarction, beta-blockers were associated with an increased risk of MACE and all-cause mortality.
This difference in efficacy depending on the presence and timing of a prior myocardial infarction is consistent with the 2012 American College of Cardiology/American Heart Association guidelines for secondary prevention, in which beta-blockers are given a class I recommendation only for patients with a myocardial infarction within the past 3 years.
Meta-analyses and outcomes
A number of meta-analyses have been published over the past 10 years, with conflicting results (Table 2). The divergent findings are primarily due to the different studies included in the analyses as well as the strong influence of the POISE trial.1 The studies varied in terms of the specific beta-blocker used, dose titration and heart rate control, time of initiation of beta-blocker use before surgery, type of surgery, patient characteristics, comorbidities, biomarkers and diagnosis of myocardial infarction, and clinical end points.
In general, these meta-analyses have found that prophylactic perioperative use of beta-blockers decreases ischemia and tends to reduce the risk of nonfatal myocardial infarction. They vary on whether the overall mortality risk is decreased. The meta-analyses that included POISE1 found an increased incidence of stroke, whereas those that excluded POISE found no significant difference, although there appeared to be slightly more strokes in the beta-blocker groups.
The beta-blocker controversy increased even further when Dr. Don Poldermans was fired by Erasmus Medical Center in November 2011 for violations of academic integrity involving his research, including the DECREASE trials. The most recent meta-analysis, by Bouri et al,29 included nine “secure trials” and excluded the DECREASE trials in view of the controversy about their authenticity. The analysis showed an increase in overall mortality as well as stroke, primarily because it was heavily influenced by POISE.1 In contrast, the DECREASE trials had reported a decreased risk of myocardial infarction and death, with no significant increase in stroke. The authors concluded that guideline bodies should “retract their recommendations based on the fictitious data without further delay.”29
Although the design of the DECREASE trials (in which beta-blockers were started well in advance of surgery and doses were titrated to achieve heart rate control) is physiologically more compelling than those of the negative trials, the results have been questioned in light of the integrity issue. However, to date, none of the published DECREASE trials have been retracted.
Two other meta-analyses,30,31 published in 2013, also found a decreased risk of myocardial infarction and increased risk of stroke but no significant difference in short-term all-cause mortality.
ARE ALL BETA-BLOCKERS EQUIVALENT?
In various studies evaluating specific beta-blockers, the more cardioselective agents bisoprolol and atenolol were associated with better outcomes than metoprolol. The affinity ratios for beta-1/beta-2 receptors range from 13.5 for bisoprolol to 4.7 for atenolol and 2.3 for metoprolol.32 Blocking beta-1 receptors blunts tachycardia, whereas blocking beta-2 receptors may block systemic or cerebral vasodilation.
In patients with anemia, beta-blockade in general may be harmful, but beta-2 blockade may be even worse. Beta-blockers were associated with an increased risk of MACE (6.5% vs 3.0%)33 in patients with acute surgical anemia if the hemoglobin concentration decreased to less than 35% of baseline, and increased risks of hospital death (OR 6.65) and multiorgan dysfunction syndrome (OR 4.18) with severe bleeding during aortic surgery.34
In addition, the pathway by which the beta-blocker is metabolized may also affect outcome, with less benefit from beta-blockers metabolized by the CYP2D6 isoenzyme of the cytochrome P450 system. Individual variations in CYP2D6 activity related to genetics or drug interactions may result in insufficient or excessive beta-blockade. Because metoprolol is the most dependent on this system, patients using it may be more susceptible to bradycardia.35
Studies comparing atenolol and metoprolol found that the atenolol groups had fewer myocardial infarctions and deaths36 and lower 30-day and 1-year mortality rates37 than the groups on metoprolol. Studies comparing the three beta-blockers found better outcomes with atenolol and bisoprolol than with metoprolol—fewer strokes,38,39 a lower mortality rate,31 and a better composite outcome39 (Table 3 and Table 4).
START THE BETA-BLOCKER EARLY, TITRATE TO CONTROL THE HEART RATE
A number of studies suggest that how long the beta-blocker is given before surgery may influence the outcome (Table 5). The best results were achieved when beta-blockers were started approximately 1 month before surgery and titrated to control the heart rate.
Because this long lead-in time is not always practical, it is important to determine the shortest time before surgery in which starting beta-blockers may be beneficial and yet safe. Some evidence suggests that results are better when the beta-blocker is started more than 1 week preoperatively compared with less than 1 week, but it is unknown what the minimum or optimal time period should be.
If a beta-blocker is started well in advance of the scheduled surgery, there is adequate time for dose titration and tighter heart rate control. Most of the studies demonstrating beneficial effects of perioperative beta-blockers used dose titration and achieved lower heart rates in the treatment group than in the control group. A criticism of the MaVs,19 POBBLE,20 and DIPOM21 trials was that the patients did not receive adequate beta-blockade. The POISE trial1 used a much higher dose of metoprolol in an attempt to assure beta-blockade without dose titration, and although the regimen decreased nonfatal myocardial infarctions, it increased strokes and the overall mortality rate, probably related to excess bradycardia and hypotension. The target heart rate should probably be between 55 and 70 beats per minute.
RISK OF STROKE
POISE1 was the first trial to note a clinically and statistically significant increase in strokes with perioperative beta-blocker use. Although no other study has shown a similar increased risk, almost all reported a higher number of strokes in the beta-blocker groups, although the absolute numbers and differences were small and not statistically significant. This risk may also vary from one beta-blocker to another (Table 4).
The usual incidence rate of postoperative stroke after noncardiac, noncarotid surgery is well under 1% in patients with no prior history of stroke but increases to approximately 3% in patients with a previous stroke.40 An observational study from the Dutch group reported a very low incidence of stroke overall (0.02%) in 186,779 patients undergoing noncardiac surgery with no significant difference in those on chronic beta-blocker therapy.41 The DECREASE trials, with a total of 3,884 patients, also found no statistically significant increase in stroke with beta-blocker use (0.46% overall vs 0.5% with a beta-blocker),42 which in this case was bisoprolol started well in advance of surgery and titrated to control the heart rate. Although the DECREASE data are under suspicion, they seem reasonable and consistent with those of observational studies.
Proposed mechanisms by which beta-blockers may increase stroke risk include the side effects of hypotension and bradycardia, particularly in the setting of anemia. They may also cause cerebral ischemia by blocking cerebral vasodilation. This effect on cerebral blood flow may be more pronounced with the less cardioselective beta-blockers, which may explain the apparent increased stroke risk associated with metoprolol.
WHAT SHOULD WE DO NOW?
The evidence for the safety and efficacy of beta-blockers in the perioperative setting continues to evolve, and new clinical trials are needed to clarify the ongoing controversy, particularly regarding the risk of stroke.
If patients have other indications for beta-blocker therapy, such as history of heart failure, myocardial infarction in the past 3 years, or atrial fibrillation for rate control, they should be receiving them if time permits.
If prophylactic beta-blockers are to be effective in minimizing perioperative complications, it appears that they may need to be more cardioselective, started at least 1 week before surgery, titrated to control heart rate, and used in high-risk patients (Revised Cardiac Risk Index score > 2 or 3) undergoing high-risk surgery.
Ideally, a large randomized controlled trial using a cardioselective beta-blocker started in advance of surgery in patients with a Revised Cardiac Risk Index score greater than 2, undergoing intermediate or high-risk procedures, is needed to fully answer the questions raised by the current data.
- POISE Study Group; Devereaux PJ, Yang H, Yusuf S, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; 371:1839–1847.
- Wijeysundera DN, Mamdani M, Laupacis A, et al. Clinical evidence, practice guidelines, and ß-blocker utilization before major noncardiac surgery. Circ Cardiovasc Qual Outcomes 2012; 5:558–565.
- American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery); American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Rhythm Society; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery: focused update on perioperative beta-blocker therapy: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society for Vascular Medicine and Biology. J Am Coll Cardiol 2006; 47:2343–2355.
- Harte B, Jaffer AK. Perioperative beta-blockers in noncardiac surgery: evolution of the evidence. Cleve Clin J Med 2008; 75:513–519.
- Mangano DT. Perioperative cardiac morbidity. Anesthesiology 1990; 72:153–184.
- London MJ, Zaugg M, Schaub MC, Spahn DR. Perioperative beta-adrenergic receptor blockade: physiologic foundations and clinical controversies. Anesthesiology 2004; 100:170–175.
- Badner NH, Knill RL, Brown JE, Novick TV, Gelb AW. Myocardial infarction after noncardiac surgery. Anesthesiology 1998; 88:572–578.
- Priebe HJ. Triggers of perioperative myocardial ischaemia and infarction. Br J Anaesth 2004; 93:9–20.
- Zaugg M, Schaub MC, Foëx P. Myocardial injury and its prevention in the perioperative setting. Br J Anaesth 2004; 93:21–33.
- Zaugg M, Schaub MC, Pasch T, Spahn DR. Modulation of beta-adrenergic receptor subtype activities in perioperative medicine: mechanisms and sites of action. Br J Anaesth 2002; 88:101–123.
- Landesberg G. The pathophysiology of perioperative myocardial infarction: facts and perspectives. J Cardiothorac Vasc Anesth 2003; 17:90–100.
- Yeager MP, Fillinger MP, Hettleman BD, Hartman GS. Perioperative beta-blockade and late cardiac outcomes: a complementary hypothesis. J Cardiothorac Vasc Anesth 2005; 19:237–241.
- Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 1996; 335:1713–1720.
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120:564–578.
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med 1999; 341:1789–1794.
- Poldermans D, Boersma E, Bax JJ, et al; Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. Bisoprolol reduces cardiac death and myocardial infarction in high-risk patients as long as 2 years after successful major vascular surgery. Eur Heart J 2001; 22:1353–1358.
- Boersma E, Poldermans D, Bax JJ, et al; DECREASE Study Group (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiogrpahy). Predictors of cardiac events after major vascular surgery: role of clinical characteristics, dobutamine echocardiography, and beta-blocker therapy. JAMA 2001; 285:1865–1873.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Yang H, Raymer K, Butler R, Parlow J, Roberts R. The effects of perioperative beta-blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J 2006; 152:983–990.
- Brady AR, Gibbs JS, Greenhalgh RM, Powell JT, Sydes MR; POBBLE trial investigators. Perioperative beta-blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double-blind controlled trial. J Vasc Surg 2005; 41:602–609.
- Juul AB, Wetterslev J, Gluud C, et al; DIPOM Trial Group. Effect of perioperative beta blockade in patients with diabetes undergoing major non-cardiac surgery: randomised placebo controlled, blinded multicentre trial. BMJ 2006; 332:1482.
- Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major non-cardiac surgery. N Engl J Med 2005; 353:349–361.
- Feringa HH, Bax JJ, Boersma E, et al. High-dose beta-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation 2006; 114(suppl 1):1344–1349.
- Dunkelgrun M, Boersma E, Schouten O, et al; Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate-risk patients undergoing noncardiovascular surgery: a randomized controlled trial (DECREASE-IV). Ann Surg 2009; 249:921–926.
- American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Rhythm Society; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine; Society for Vascular Surgery; Fleisher LA, Beckman JA, Brown KA, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol 2009; 54:e13–e118.
- Task Force for Preoperative Cardiac Risk Assessment and Perioperative Cardiac Management in Non-cardiac Surgery; European Society of Cardiology (ESC); Poldermans D, Bax JJ, Boersma E, et al. Guidelines for preoperative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery. Eur Heart J 2009; 30:2769–2812.
- London MJ, Hur K, Schwartz GG, Henderson WG. Association of perioperative beta-blockade with mortality and cardiovascular morbidity following major noncardiac surgery. JAMA 2013; 309:1704–1713.
- Andersson C, Mérie C, Jørgensen M, et al. Association of beta-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing noncardiac surgery: a Danish nationwide cohort study. JAMA Intern Med 2014; 174:336–344.
- Bouri S, Shun-Shin MJ, Cole GD, Mayet J, Francis DP. Meta-analysis of secure randomised controlled trials of beta-blockade to prevent perioperative death in non-cardiac surgery. Heart 2014; 100:456–464.
- Guay J, Ochroch EA. Beta-blocking agents for surgery: influence on mortality and major outcomes. A meta-analysis. J Cardiothorac Vasc Anesth 2013; 27:834–844.
- Dai N, Xu D, Zhang J, et al. Different beta-blockers and initiation time in patients undergoing noncardiac surgery: a meta-analysis. Am J Med Sci 2014; 347:235–244.
- Baker JG. The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br J Pharmacol 2005; 144:317–322.
- Beattie WS, Wijeysundera DN, Karkouti K, et al. Acute surgical anemia influences the cardioprotective effects of beta-blockade: a single-center, propensity-matched cohort study. Anesthesiology 2010; 112:25–33.
- Le Manach Y, Collins GS, Ibanez C, et al. Impact of perioperative bleeding on the protective effect of beta-blockers during infrarenal aortic reconstruction. Anesthesiology 2012; 117:1203–1211.
- Badgett RG, Lawrence VA, Cohn SL. Variations in pharmacology of beta-blockers may contribute to heterogeneous results in trials of perioperative beta-blockade. Anesthesiology 2010; 113:585–592.
- Redelmeier D, Scales D, Kopp A. Beta blockers for elective surgery in elderly patients: population based, retrospective cohort study. BMJ 2005; 331:932.
- Wallace AW, Au S, Cason BA. Perioperative beta-blockade: atenolol is associated with reduced mortality when compared to metoprolol. Anesthesiology 2011; 114:824–836.
- Mashour GA, Sharifpour M, Freundlich RE, et al. Perioperative metoprolol and risk of stroke after noncardiac surgery. Anesthesiology 2013; 119:1340–1346.
- Ashes C, Judelman S, Wijeysundera DN, et al. Selective beta1-antagonism with bisoprolol is associated with fewer postoperative strokes than atenolol or metoprolol: a single-center cohort study of 44,092 consecutive patients. Anesthesiology 2013; 119:777–787.
- Selim M. Perioperative stroke. N Engl J Med 2007; 356:706–713.
- van Lier F, Schouten O, van Domburg RT, et al. Effect of chronic beta-blocker use on stroke after noncardiac surgery. Am J Cardiol 2009; 104:429–433.
- van Lier F, Schouten O, Hoeks SE, et al. Impact of prophylactic beta-blocker therapy to prevent stroke after noncardiac surgery. Am J Cardiol 2010; 105:43–47.
- Devereaux PJ, Beattie WS, Choi PT, et al. How strong is the evidence for the use of perioperative beta blockers in non-cardiac surgery? Systematic review and meta-analysis of randomised controlled trials. BMJ 2005; 331:313–321.
- McGory ML, Maggard MA, Ko CY. A meta-analysis of perioperative beta blockade: what is the actual risk reduction? Surgery 2005; 138:171–179.
- Schouten O, Shaw LJ, Boersma E, et al. A meta-analysis of safety and effectiveness of perioperative beta-blocker use for the prevention of cardiac events in different types of noncardiac surgery. Coron Artery Dis 2006; 17:173–179.
- Wiesbauer F, Schlager O, Domanovits H, et al. Perioperative beta-blockers for preventing surgery-related mortality and morbidity: a systematic review and meta-analysis. Anesth Analg 2007; 104:27–41.
- Bangalore S, Wetterslev J, Pranesh S, Sawhney S, Gluud C, Messerli FH. Perioperative beta blockers in patients having non-cardiac surgery: a meta-analysis. Lancet 2008; 372:1962–1976.
- Flu WJ, van Kuijk JP, Chonchol M, et al. Timing of preoperative beta-blocker treatment in vascular surgery patients: influence on postoperative outcome. J Am Coll Cardiol 2010; 56:1922–1929.
- Wijeysundera DN, Beattie WS, Wijeysundera HC, Yun L, Austin PC, Ko DT. Duration of preoperative beta-blockade and outcomes after major elective noncardiac surgery. Can J Cardiol 2014; 30:217–223.
- POISE Study Group; Devereaux PJ, Yang H, Yusuf S, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; 371:1839–1847.
- Wijeysundera DN, Mamdani M, Laupacis A, et al. Clinical evidence, practice guidelines, and ß-blocker utilization before major noncardiac surgery. Circ Cardiovasc Qual Outcomes 2012; 5:558–565.
- American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery); American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Rhythm Society; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery: focused update on perioperative beta-blocker therapy: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society for Vascular Medicine and Biology. J Am Coll Cardiol 2006; 47:2343–2355.
- Harte B, Jaffer AK. Perioperative beta-blockers in noncardiac surgery: evolution of the evidence. Cleve Clin J Med 2008; 75:513–519.
- Mangano DT. Perioperative cardiac morbidity. Anesthesiology 1990; 72:153–184.
- London MJ, Zaugg M, Schaub MC, Spahn DR. Perioperative beta-adrenergic receptor blockade: physiologic foundations and clinical controversies. Anesthesiology 2004; 100:170–175.
- Badner NH, Knill RL, Brown JE, Novick TV, Gelb AW. Myocardial infarction after noncardiac surgery. Anesthesiology 1998; 88:572–578.
- Priebe HJ. Triggers of perioperative myocardial ischaemia and infarction. Br J Anaesth 2004; 93:9–20.
- Zaugg M, Schaub MC, Foëx P. Myocardial injury and its prevention in the perioperative setting. Br J Anaesth 2004; 93:21–33.
- Zaugg M, Schaub MC, Pasch T, Spahn DR. Modulation of beta-adrenergic receptor subtype activities in perioperative medicine: mechanisms and sites of action. Br J Anaesth 2002; 88:101–123.
- Landesberg G. The pathophysiology of perioperative myocardial infarction: facts and perspectives. J Cardiothorac Vasc Anesth 2003; 17:90–100.
- Yeager MP, Fillinger MP, Hettleman BD, Hartman GS. Perioperative beta-blockade and late cardiac outcomes: a complementary hypothesis. J Cardiothorac Vasc Anesth 2005; 19:237–241.
- Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 1996; 335:1713–1720.
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120:564–578.
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med 1999; 341:1789–1794.
- Poldermans D, Boersma E, Bax JJ, et al; Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. Bisoprolol reduces cardiac death and myocardial infarction in high-risk patients as long as 2 years after successful major vascular surgery. Eur Heart J 2001; 22:1353–1358.
- Boersma E, Poldermans D, Bax JJ, et al; DECREASE Study Group (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiogrpahy). Predictors of cardiac events after major vascular surgery: role of clinical characteristics, dobutamine echocardiography, and beta-blocker therapy. JAMA 2001; 285:1865–1873.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Yang H, Raymer K, Butler R, Parlow J, Roberts R. The effects of perioperative beta-blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J 2006; 152:983–990.
- Brady AR, Gibbs JS, Greenhalgh RM, Powell JT, Sydes MR; POBBLE trial investigators. Perioperative beta-blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double-blind controlled trial. J Vasc Surg 2005; 41:602–609.
- Juul AB, Wetterslev J, Gluud C, et al; DIPOM Trial Group. Effect of perioperative beta blockade in patients with diabetes undergoing major non-cardiac surgery: randomised placebo controlled, blinded multicentre trial. BMJ 2006; 332:1482.
- Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major non-cardiac surgery. N Engl J Med 2005; 353:349–361.
- Feringa HH, Bax JJ, Boersma E, et al. High-dose beta-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation 2006; 114(suppl 1):1344–1349.
- Dunkelgrun M, Boersma E, Schouten O, et al; Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate-risk patients undergoing noncardiovascular surgery: a randomized controlled trial (DECREASE-IV). Ann Surg 2009; 249:921–926.
- American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Rhythm Society; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine; Society for Vascular Surgery; Fleisher LA, Beckman JA, Brown KA, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol 2009; 54:e13–e118.
- Task Force for Preoperative Cardiac Risk Assessment and Perioperative Cardiac Management in Non-cardiac Surgery; European Society of Cardiology (ESC); Poldermans D, Bax JJ, Boersma E, et al. Guidelines for preoperative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery. Eur Heart J 2009; 30:2769–2812.
- London MJ, Hur K, Schwartz GG, Henderson WG. Association of perioperative beta-blockade with mortality and cardiovascular morbidity following major noncardiac surgery. JAMA 2013; 309:1704–1713.
- Andersson C, Mérie C, Jørgensen M, et al. Association of beta-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing noncardiac surgery: a Danish nationwide cohort study. JAMA Intern Med 2014; 174:336–344.
- Bouri S, Shun-Shin MJ, Cole GD, Mayet J, Francis DP. Meta-analysis of secure randomised controlled trials of beta-blockade to prevent perioperative death in non-cardiac surgery. Heart 2014; 100:456–464.
- Guay J, Ochroch EA. Beta-blocking agents for surgery: influence on mortality and major outcomes. A meta-analysis. J Cardiothorac Vasc Anesth 2013; 27:834–844.
- Dai N, Xu D, Zhang J, et al. Different beta-blockers and initiation time in patients undergoing noncardiac surgery: a meta-analysis. Am J Med Sci 2014; 347:235–244.
- Baker JG. The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br J Pharmacol 2005; 144:317–322.
- Beattie WS, Wijeysundera DN, Karkouti K, et al. Acute surgical anemia influences the cardioprotective effects of beta-blockade: a single-center, propensity-matched cohort study. Anesthesiology 2010; 112:25–33.
- Le Manach Y, Collins GS, Ibanez C, et al. Impact of perioperative bleeding on the protective effect of beta-blockers during infrarenal aortic reconstruction. Anesthesiology 2012; 117:1203–1211.
- Badgett RG, Lawrence VA, Cohn SL. Variations in pharmacology of beta-blockers may contribute to heterogeneous results in trials of perioperative beta-blockade. Anesthesiology 2010; 113:585–592.
- Redelmeier D, Scales D, Kopp A. Beta blockers for elective surgery in elderly patients: population based, retrospective cohort study. BMJ 2005; 331:932.
- Wallace AW, Au S, Cason BA. Perioperative beta-blockade: atenolol is associated with reduced mortality when compared to metoprolol. Anesthesiology 2011; 114:824–836.
- Mashour GA, Sharifpour M, Freundlich RE, et al. Perioperative metoprolol and risk of stroke after noncardiac surgery. Anesthesiology 2013; 119:1340–1346.
- Ashes C, Judelman S, Wijeysundera DN, et al. Selective beta1-antagonism with bisoprolol is associated with fewer postoperative strokes than atenolol or metoprolol: a single-center cohort study of 44,092 consecutive patients. Anesthesiology 2013; 119:777–787.
- Selim M. Perioperative stroke. N Engl J Med 2007; 356:706–713.
- van Lier F, Schouten O, van Domburg RT, et al. Effect of chronic beta-blocker use on stroke after noncardiac surgery. Am J Cardiol 2009; 104:429–433.
- van Lier F, Schouten O, Hoeks SE, et al. Impact of prophylactic beta-blocker therapy to prevent stroke after noncardiac surgery. Am J Cardiol 2010; 105:43–47.
- Devereaux PJ, Beattie WS, Choi PT, et al. How strong is the evidence for the use of perioperative beta blockers in non-cardiac surgery? Systematic review and meta-analysis of randomised controlled trials. BMJ 2005; 331:313–321.
- McGory ML, Maggard MA, Ko CY. A meta-analysis of perioperative beta blockade: what is the actual risk reduction? Surgery 2005; 138:171–179.
- Schouten O, Shaw LJ, Boersma E, et al. A meta-analysis of safety and effectiveness of perioperative beta-blocker use for the prevention of cardiac events in different types of noncardiac surgery. Coron Artery Dis 2006; 17:173–179.
- Wiesbauer F, Schlager O, Domanovits H, et al. Perioperative beta-blockers for preventing surgery-related mortality and morbidity: a systematic review and meta-analysis. Anesth Analg 2007; 104:27–41.
- Bangalore S, Wetterslev J, Pranesh S, Sawhney S, Gluud C, Messerli FH. Perioperative beta blockers in patients having non-cardiac surgery: a meta-analysis. Lancet 2008; 372:1962–1976.
- Flu WJ, van Kuijk JP, Chonchol M, et al. Timing of preoperative beta-blocker treatment in vascular surgery patients: influence on postoperative outcome. J Am Coll Cardiol 2010; 56:1922–1929.
- Wijeysundera DN, Beattie WS, Wijeysundera HC, Yun L, Austin PC, Ko DT. Duration of preoperative beta-blockade and outcomes after major elective noncardiac surgery. Can J Cardiol 2014; 30:217–223.
KEY POINTS
- If patients have other indications for beta-blocker therapy, such as a history of heart failure, myocardial infarction in the past 3 years, or atrial fibrillation, they should be started on a beta-blocker before surgery if time permits.
- Of the various beta-blockers, the cardioselective ones appear to be preferable in the perioperative setting.
- Beta-blockers may need to be started at least 1 week before surgery, titrated to control the heart rate, and used only in patients at high risk (Revised Cardiac Risk Index score > 2 or 3) undergoing high-risk surgery.
- Further clinical trials are necessary to clarify the ongoing controversy, particularly regarding the risk of stroke, which was increased in the large Perioperative Ischemic Evaluation (POISE) trial.