User login
Things We Do for No Reason™: Prescribing Appetite Stimulants to Hospitalized Older Adults With Unintentional Weight Loss
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
An 87-year-old hospitalized man has lost 7% of his body weight in the past year. His family and the inpatient nutritionist ask about a prescription appetite stimulant.
Why You Might Think Prescribing Appetite Stimulants for Unintentional Weight Loss in Older Adults Is Helpful
Unintentional weight loss—the loss of more than 10 lb or 5% of usual body weight over 6 to 12 months—affects up to 27% of older adults in the community and 50% to 60% of older adults in nursing homes.1,2 Patients who report weight loss on hospital admission have an almost four times greater risk of death in the 12 months following discharge.3 To address unintentional weight loss, clinicians may prescribe appetite stimulants.
Megestrol acetate is approved by the US Food and Drug Administration (FDA) for the treatment of weight loss in patients with AIDS.4 Megestrol acetate promotes weight gain through inhibition of cytokines, interleukin-6, and tumor necrosis factor-alpha, which are increased in older adults. In a randomized, placebo-controlled trial of 69 nursing home residents with ≥6 months’ life expectancy and Karnofsky score of ≥40%, patients treated with megestrol acetate for 12 weeks reported increased appetite and well-being. They achieved significant weight gain (>1.82 kg), but not until 3 months after therapy ended.5 No significant adverse events were reported; however, adverse event monitoring continued only for the 12-week treatment period. This follow-up duration may have been insufficient to identify some adverse events, such as venous thromboembolism.
Mirtazapine, an antidepressant and serotonin receptor antagonist, reduces levels of serotonin, a neurotransmitter that promotes early satiety.6 In a meta-analysis of 11 trials comparing mirtazapine to selective serotonin reuptake inhibitors for depression, patients treated with mirtazapine demonstrated an increase in the composite secondary outcome of weight gain or increased appetite.7 The amount of weight gain was not specified. Weight gain is more common with low-dose mirtazapine, potentially due to increased antihistamine activity at lower doses.8 Overall, mirtazapine is well-tolerated and efficacious in the treatment of depression and may benefit older adults with concomitant weight loss.6
Cyproheptadine is a first-generation antihistamine with appetite-stimulating effects. It has been found to increase weight or appetite in various disease states, particularly in the pediatric population,9 including cystic fibrosis10 and malignancy.11 Given this evidence, there has been interest in its use in the geriatric population with unintentional weight loss.
Dronabinol is an orally active cannabinoid approved for anorexia-associated weight loss in patients with AIDS.12 In a randomized, placebo-controlled trial in patients with AIDS-related anorexia and weight loss, participants receiving dronabinol had a statistically significant increase in appetite but no change in weight. Participants receiving dronabinol also experienced more nervous system-related adverse events, including dizziness, thinking abnormalities, and somnolence.13
Why Prescribing Appetite Stimulants for Unintentional Weight Loss in Older Adults Is Not Helpful
Weight gain may not improve clinically meaningful outcomes. The absence of consistent evidence that prescription appetite stimulants improve patient-centered outcomes, such as quality of life or functional status, and the potential morbidity and mortality of these medications make prescribing appetite stimulants in older adults concerning.
Megestrol Acetate
A 2018 systematic review of randomized controlled trials studying megestrol acetate for treatment of anorexia-cachexia, primarily in adults with AIDS and cancer, found that treatment resulted in a 2.25-kg weight gain, with no improvement in quality of life and an increased risk of adverse events.14
Three prospective trials studied the effect of megestrol acetate in older adults (Appendix Table). One trial randomized 47 patients receiving skilled nursing services following an admission for acute illness to megestrol acetate vs placebo. While the investigators noted increases in appetite at higher doses of megestrol acetate, there was no change in weight or clinically relevant outcomes.15 In a second randomized controlled trial, 29 patients with illness-induced functional decline were enrolled in a strength training program in addition to being assigned to megestrol acetate or placebo. While patients receiving megestrol acetate with the exercise program had significant increases in weight and nutritional intake, they suffered a deterioration in physical function.16 In a pilot study, 17 nursing home residents who consistently ate less than 75% of their meals received megestrol acetate plus standard or optimal feeding assistance. The percentage of meals consumed increased only when patients received optimal feeding assistance in conjunction with megestrol acetate.17
The largest case-control study examining megestrol acetate for unintentional weight loss in older adults compared 709 residents in a multistate nursing home system treated with megestrol acetate to matched untreated controls. After 6 months of treatment, the median weight and change in weight did not differ significantly. Patients receiving megestrol acetate had a significant increase in mortality, surviving an average of 23.9 months, compared to 31.2 months for controls (P < .001).18
Additionally, two retrospective reviews of nursing home patients who were prescribed megestrol acetate showed incidences of venous thrombosis of 5% and 32%.19,20 Other potentially significant adverse effects include adrenal insufficiency and fluid retention.6 In 2019, the American Geriatrics Society’s Beers Criteria included megestrol acetate as a medication to avoid given its “minimal effect on weight; increases [in] risk of thrombotic events and possibly death in older adults.”21
Mirtazapine
No studies have evaluated mirtazapine for weight gain without concomitant depression. In older adults with depression, mirtazapine has minimal impact on promoting weight gain compared to other antidepressants. In two retrospective studies of older patients with depression and weight loss, researchers found no difference in weight gain in those treated with mirtazapine vs sertraline or other nontricyclic antidepressants, excluding fluoxetine.22,23
Cyproheptadine
There have been no controlled trials evaluating the use of cyproheptadine in older adults, in part due to anticholinergic side effects. In a trial of cancer patients, sedation and dizziness were common adverse effects.11 The 2019 American Geriatrics Society’s Beers Criteria include cyproheptadine as a medication to avoid based upon the “risk of confusion, dry mouth, constipation, and other anticholinergic effects or toxicity.”21
Dronabinol
In a retrospective cohort study of 28 long-term care residents with anorexia and weight loss, participants receiving dronabinol for 12 weeks had no statistically significant weight gain.24 The FDA cautions against prescribing dronabinol for older adults due to neurological side effects.12 A systematic review of randomized controlled trials found that cannabinoid-based medications in patients older than 50 years were associated with a significant increase in dizziness or lightheadedness and thinking or perception disorder.25
What You Should Do Instead
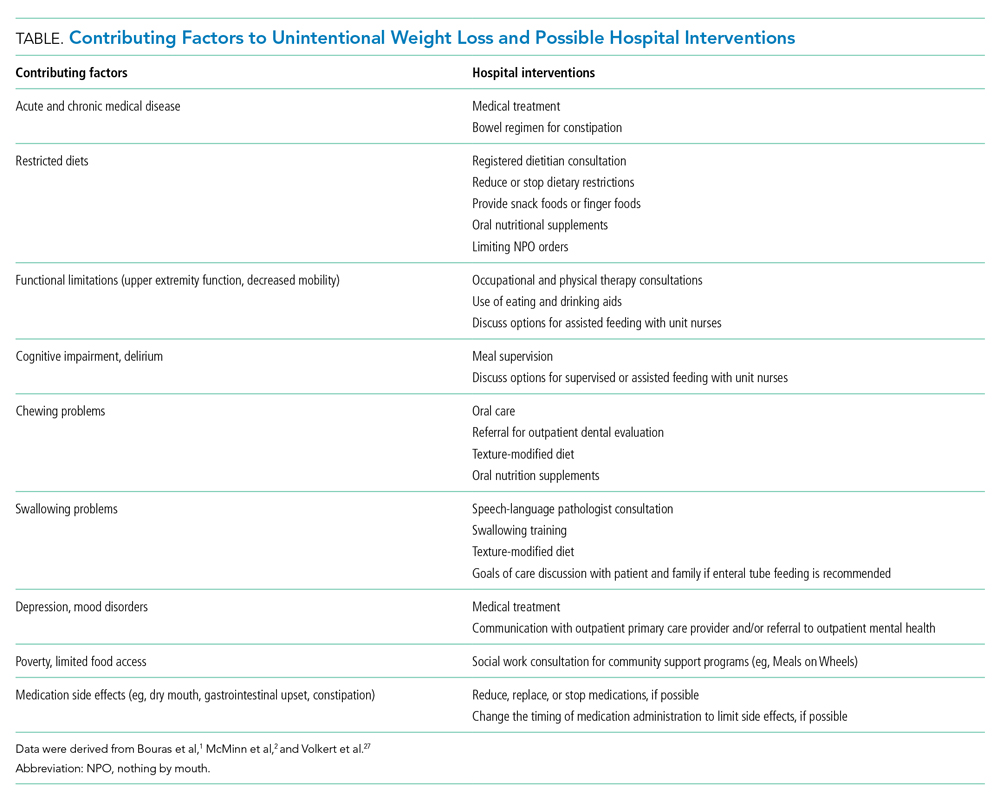
In the Choosing Wisely® initiative, the American Geriatrics Society recommends avoiding prescription appetite stimulants for patients with anorexia or cachexia.26 Instead, hospitalists should evaluate older patients for causes of unintentional weight loss, including malignancy, nonmalignant gastrointestinal disorders, depression, and dementia. Hospitalists can identify most causes based on the history, physical exam, and laboratory studies and initiate treatment for modifiable causes, such as constipation and depression.2
Hospitalists should work with an interprofessional team to develop an individualized plan to optimize caloric intake in the hospital (Table).27 One in five hospitalized older adults has insufficient caloric intake during admission, which is associated with increased risk for in-hospital and 90-day mortality.28 Removing dietary restrictions, increasing the variety of foods offered, and assisted eating may increase food intake.27,29 Hospitalists should also consider discontinuing or changing medications with gastrointestinal side effects, such as metformin, cholinesterase inhibitors, bisphosphonates, and oral iron supplements. Dietitians may recommend oral nutrition supplements; if started, patients should be offered supplements after discharge.27,29 For patients with limited access to food, social workers can help optimize social supports and identify community resources following discharge. Finally, hospitalists should coordinate with outpatient providers to monitor weight long-term.
Recommendations
- Recognize and address unintentional weight loss in older adults in the hospital.
- Do not prescribe appetite stimulants for unintentional weight loss in hospitalized older adults as they have no proven benefit for improving long-term outcomes and, in the case of megestrol acetate, may increase mortality.
- Work with an interprofessional team to address factors contributing to unintentional weight loss using nonpharmacologic options for improving food intake.
Conclusion
After discussing the lack of evidence supporting prescription appetite stimulants and the potential risks, we shifted the focus to optimizing oral intake. The team worked with the patient and the patient’s family to optimize nutrition following discharge and communicated the need for ongoing monitoring to the primary care provider.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected]
Acknowledgment
The authors thank Claire Campbell, MD, for her review of this manuscript.
1. Bouras EP, Lange SM, Scolapio JS. Rational approach to patients with unintentional weight loss. Mayo Clin Proc. 2001;76(9):923-929. https://doi.org/10.4065/76.9.923
2. McMinn J, Steel C, Bowman A. Investigation and management of unintentional weight loss in older adults. BMJ. 2011;342:d1732. https://doi.org/10.1136/bmj.d1732
3. Satish S, Winograd CH, Chavez C, Bloch DA. Geriatric targeting criteria as predictors of survival and health care utilization. J Am Geriatr Soc. 1996;44(8):914-921. https://doi.org/10.1111/j.1532-5415.1996.tb01860.x
4. Megace (megestrol acetate) [package insert]. Par Pharmaceutical Inc. Revised July 2005. Accessed January 27, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021778s000TOC.cfm
5. Yeh SS, Wu SY, Lee TP, et al. Improvement in quality-of-life measures and stimulation of weight gain after treatment with megestrol acetate oral suspension in geriatric cachexia: results of a double-blind, placebo-controlled study. J Am Geriatr Soc. 2000;48(5):485-492. https://doi.org/10.1111/j.1532-5415.2000.tb04993.x
6. Fox CB, Treadway AK, Blaszczyk AT, Sleeper RB. Reviews of therapeutics megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29(4):383-397. https://doi.org/10.1592/phco.29.4.383
7. Watanabe N, Omori IM, Nakagawa A, et al. Mirtazapine versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2011;(12):CD006528. https://doi.org/10.1002/14651858.CD006528.pub2
8. Fawcett J, Barkin RL. Review of the results from clinical studies on the efficacy, safety and tolerability of mirtazapine for the treatment of patients with major depression. J Affect Disord. 1998;51(3):267-285. https://doi.org/10.1016/S0165-0327(98)00224-9
9. Najib K, Moghtaderi M, Karamizadeh Z, Fallahzadeh E. Beneficial effect of cyproheptadine on body mass index in undernourished children: a randomized controlled trial. Iran J Pediatr. 2014;24(6):753-758.
10. Epifanio M, Marostica PC, Mattiello R, et al. A randomized, double-blind, placebo-controlled trial of cyproheptadine for appetite stimulation in cystic fibrosis. J Pediatr (Rio J). 2012;88(2):155-160. https://doi.org/10.2223/JPED.2174
11. Kardinal CG, Loprinzi CL, Schaid DJ, et al. A controlled trial of cyproheptadine in cancer patients with anorexia and/or cachexia. Cancer. 1990;65(12):2657-2662. https://doi.org/10.1002/1097-0142(19900615)65:12<2657::aid-cncr2820651210>3.0.co;2-s
12. MARINOL (dronabinol) [package insert]. Solvay Pharmaceuticals, Inc. Revised August 2017. Accessed April 27, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf.
13. Beal JE, Olson R, Laubenstein L, et al. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage. 1995;10(2):89-97. https://doi.org/10.1016/0885-3924(94)00117-4
14. Ruiz-García V, López-Briz E, Carbonell-Sanchis R, Bort-Martí S, Gonzálvez-Perales JL. Megestrol acetate for cachexia–anorexia syndrome. A systematic review. J Cachexia Sarcopenia Muscle. 2018;9(3):444-452. https://doi.org/10.1002/jcsm.12292
15. Reuben DB, Hirsch SH, Zhou K, Greendale GA. The effects of megestrol acetate suspension for elderly patients with reduced appetite after hospitalization: a phase II randomized clinical trial. J Am Geriatr Soc. 2005;53(6):970-975. https://doi.org/10.1111/j.1532-5415.2005.53307.x
16. Sullivan DH, Roberson PK, Smith ES, Price JA, Bopp MM. Effects of muscle strength training and megestrol acetate on strength, muscle mass, and function in frail older people. J Am Geriatr Soc. 2007;55(1):20-28. https://doi.org/10.1111/j.1532-5415.2006.01010.x
17. Simmons SF, Walker KA, Osterweil D. The effect of megestrol acetate on oral food and fluid intake in nursing home residents: a pilot study. J Am Med Dir Assoc. 2005;6(3):S5-S11. https://doi.org/10.1016/j.jamda.2005.03.014
18. Bodenner D, Spencer T, Riggs AT, Redman C, Strunk B, Hughes T. A retrospective study of the association between megestrol acetate administration and mortality among nursing home residents with clinically significant weight loss. Am J Geriatr Pharmacother. 2007;5(2):137-146. https://doi.org/10.1016/J.AMJOPHARM.2007.06.004
19. Kropsky B, Shi Y, Cherniack EP. Incidence of deep-venous thrombosis in nursing home residents using megestrol acetate. J Am Med Dir Assoc. 2003;4(5):255-256. https://doi.org/10.1097/01.JAM.0000083384.84558.75
20. Bolen JC, Andersen RE, Bennett RG. Deep vein thrombosis as a complication of megestrol acetate therapy among nursing home residents. J Am Med Dir Assoc. 2000;1(6):248-252.
21. Fick DM, Semla TP, Steinman M, et al. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674-694. https://doi.org/10.1111/jgs.15767
22. Mihara IQT, McCombs JS, Williams BR. The impact of mirtazapine compared with non-TCA antidepressants on weight change in nursing facility residents. Consult Pharm. 2005;20(3):217-223. https://doi.org/10.4140/tcp.n.2005.217
23. Goldberg RJ. Weight change in depressed nursing home patients on mirtazapine. J Am Geriatr Soc. 2002;50(8):1461. https://doi.org/10.1046/j.1532-5415.2002.50374.x
24. Wilson MMG, Philpot C, Morley JE. Anorexia of aging in long term care: is dronabinol an effective appetite stimulant?--a pilot study. J Nutr Health Aging. 2007;11(2):195-198.
25. Velayudhan L, McGoohan KL, Bhattacharyya S. Evaluation of THC-related neuropsychiatric symptoms among adults aged 50 years and older: a systematic review and metaregression analysis. JAMA Netw Open. 2021;4(2):e2035913. https://doi.org/10.1001/jamanetworkopen.2020.35913
26. AGS Choosing Wisely Workgroup. American Geriatrics Society identifies another five things that healthcare providers and patients should question. J Am Geriatr Soc. 2014;62(5):950-960. https://doi.org/10.1111/jgs.12770
27. Volkert D, Beck AM, Cederholm T, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. 2019;38(1):10-47. https://doi.org/10.1016/j.clnu.2018.05.024
28. Sullivan DH, Sun S, Walls RC. Protein-energy undernutrition among elderly hospitalized patients: a prospective study. JAMA. 1999;281(21):2013-2019. https://doi.org/10.1001/jama.281.21.2013
29. Feinberg J, Nielsen EE, Korang SK, et al. Nutrition support in hospitalised adults at nutritional risk. Cochrane Database Syst Rev. 2017;2017(5). https://doi.org/10.1002/14651858.CD011598.pub2
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
An 87-year-old hospitalized man has lost 7% of his body weight in the past year. His family and the inpatient nutritionist ask about a prescription appetite stimulant.
Why You Might Think Prescribing Appetite Stimulants for Unintentional Weight Loss in Older Adults Is Helpful
Unintentional weight loss—the loss of more than 10 lb or 5% of usual body weight over 6 to 12 months—affects up to 27% of older adults in the community and 50% to 60% of older adults in nursing homes.1,2 Patients who report weight loss on hospital admission have an almost four times greater risk of death in the 12 months following discharge.3 To address unintentional weight loss, clinicians may prescribe appetite stimulants.
Megestrol acetate is approved by the US Food and Drug Administration (FDA) for the treatment of weight loss in patients with AIDS.4 Megestrol acetate promotes weight gain through inhibition of cytokines, interleukin-6, and tumor necrosis factor-alpha, which are increased in older adults. In a randomized, placebo-controlled trial of 69 nursing home residents with ≥6 months’ life expectancy and Karnofsky score of ≥40%, patients treated with megestrol acetate for 12 weeks reported increased appetite and well-being. They achieved significant weight gain (>1.82 kg), but not until 3 months after therapy ended.5 No significant adverse events were reported; however, adverse event monitoring continued only for the 12-week treatment period. This follow-up duration may have been insufficient to identify some adverse events, such as venous thromboembolism.
Mirtazapine, an antidepressant and serotonin receptor antagonist, reduces levels of serotonin, a neurotransmitter that promotes early satiety.6 In a meta-analysis of 11 trials comparing mirtazapine to selective serotonin reuptake inhibitors for depression, patients treated with mirtazapine demonstrated an increase in the composite secondary outcome of weight gain or increased appetite.7 The amount of weight gain was not specified. Weight gain is more common with low-dose mirtazapine, potentially due to increased antihistamine activity at lower doses.8 Overall, mirtazapine is well-tolerated and efficacious in the treatment of depression and may benefit older adults with concomitant weight loss.6
Cyproheptadine is a first-generation antihistamine with appetite-stimulating effects. It has been found to increase weight or appetite in various disease states, particularly in the pediatric population,9 including cystic fibrosis10 and malignancy.11 Given this evidence, there has been interest in its use in the geriatric population with unintentional weight loss.
Dronabinol is an orally active cannabinoid approved for anorexia-associated weight loss in patients with AIDS.12 In a randomized, placebo-controlled trial in patients with AIDS-related anorexia and weight loss, participants receiving dronabinol had a statistically significant increase in appetite but no change in weight. Participants receiving dronabinol also experienced more nervous system-related adverse events, including dizziness, thinking abnormalities, and somnolence.13
Why Prescribing Appetite Stimulants for Unintentional Weight Loss in Older Adults Is Not Helpful
Weight gain may not improve clinically meaningful outcomes. The absence of consistent evidence that prescription appetite stimulants improve patient-centered outcomes, such as quality of life or functional status, and the potential morbidity and mortality of these medications make prescribing appetite stimulants in older adults concerning.
Megestrol Acetate
A 2018 systematic review of randomized controlled trials studying megestrol acetate for treatment of anorexia-cachexia, primarily in adults with AIDS and cancer, found that treatment resulted in a 2.25-kg weight gain, with no improvement in quality of life and an increased risk of adverse events.14
Three prospective trials studied the effect of megestrol acetate in older adults (Appendix Table). One trial randomized 47 patients receiving skilled nursing services following an admission for acute illness to megestrol acetate vs placebo. While the investigators noted increases in appetite at higher doses of megestrol acetate, there was no change in weight or clinically relevant outcomes.15 In a second randomized controlled trial, 29 patients with illness-induced functional decline were enrolled in a strength training program in addition to being assigned to megestrol acetate or placebo. While patients receiving megestrol acetate with the exercise program had significant increases in weight and nutritional intake, they suffered a deterioration in physical function.16 In a pilot study, 17 nursing home residents who consistently ate less than 75% of their meals received megestrol acetate plus standard or optimal feeding assistance. The percentage of meals consumed increased only when patients received optimal feeding assistance in conjunction with megestrol acetate.17
The largest case-control study examining megestrol acetate for unintentional weight loss in older adults compared 709 residents in a multistate nursing home system treated with megestrol acetate to matched untreated controls. After 6 months of treatment, the median weight and change in weight did not differ significantly. Patients receiving megestrol acetate had a significant increase in mortality, surviving an average of 23.9 months, compared to 31.2 months for controls (P < .001).18
Additionally, two retrospective reviews of nursing home patients who were prescribed megestrol acetate showed incidences of venous thrombosis of 5% and 32%.19,20 Other potentially significant adverse effects include adrenal insufficiency and fluid retention.6 In 2019, the American Geriatrics Society’s Beers Criteria included megestrol acetate as a medication to avoid given its “minimal effect on weight; increases [in] risk of thrombotic events and possibly death in older adults.”21
Mirtazapine
No studies have evaluated mirtazapine for weight gain without concomitant depression. In older adults with depression, mirtazapine has minimal impact on promoting weight gain compared to other antidepressants. In two retrospective studies of older patients with depression and weight loss, researchers found no difference in weight gain in those treated with mirtazapine vs sertraline or other nontricyclic antidepressants, excluding fluoxetine.22,23
Cyproheptadine
There have been no controlled trials evaluating the use of cyproheptadine in older adults, in part due to anticholinergic side effects. In a trial of cancer patients, sedation and dizziness were common adverse effects.11 The 2019 American Geriatrics Society’s Beers Criteria include cyproheptadine as a medication to avoid based upon the “risk of confusion, dry mouth, constipation, and other anticholinergic effects or toxicity.”21
Dronabinol
In a retrospective cohort study of 28 long-term care residents with anorexia and weight loss, participants receiving dronabinol for 12 weeks had no statistically significant weight gain.24 The FDA cautions against prescribing dronabinol for older adults due to neurological side effects.12 A systematic review of randomized controlled trials found that cannabinoid-based medications in patients older than 50 years were associated with a significant increase in dizziness or lightheadedness and thinking or perception disorder.25
What You Should Do Instead
In the Choosing Wisely® initiative, the American Geriatrics Society recommends avoiding prescription appetite stimulants for patients with anorexia or cachexia.26 Instead, hospitalists should evaluate older patients for causes of unintentional weight loss, including malignancy, nonmalignant gastrointestinal disorders, depression, and dementia. Hospitalists can identify most causes based on the history, physical exam, and laboratory studies and initiate treatment for modifiable causes, such as constipation and depression.2
Hospitalists should work with an interprofessional team to develop an individualized plan to optimize caloric intake in the hospital (Table).27 One in five hospitalized older adults has insufficient caloric intake during admission, which is associated with increased risk for in-hospital and 90-day mortality.28 Removing dietary restrictions, increasing the variety of foods offered, and assisted eating may increase food intake.27,29 Hospitalists should also consider discontinuing or changing medications with gastrointestinal side effects, such as metformin, cholinesterase inhibitors, bisphosphonates, and oral iron supplements. Dietitians may recommend oral nutrition supplements; if started, patients should be offered supplements after discharge.27,29 For patients with limited access to food, social workers can help optimize social supports and identify community resources following discharge. Finally, hospitalists should coordinate with outpatient providers to monitor weight long-term.

Recommendations
- Recognize and address unintentional weight loss in older adults in the hospital.
- Do not prescribe appetite stimulants for unintentional weight loss in hospitalized older adults as they have no proven benefit for improving long-term outcomes and, in the case of megestrol acetate, may increase mortality.
- Work with an interprofessional team to address factors contributing to unintentional weight loss using nonpharmacologic options for improving food intake.
Conclusion
After discussing the lack of evidence supporting prescription appetite stimulants and the potential risks, we shifted the focus to optimizing oral intake. The team worked with the patient and the patient’s family to optimize nutrition following discharge and communicated the need for ongoing monitoring to the primary care provider.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected]
Acknowledgment
The authors thank Claire Campbell, MD, for her review of this manuscript.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
An 87-year-old hospitalized man has lost 7% of his body weight in the past year. His family and the inpatient nutritionist ask about a prescription appetite stimulant.
Why You Might Think Prescribing Appetite Stimulants for Unintentional Weight Loss in Older Adults Is Helpful
Unintentional weight loss—the loss of more than 10 lb or 5% of usual body weight over 6 to 12 months—affects up to 27% of older adults in the community and 50% to 60% of older adults in nursing homes.1,2 Patients who report weight loss on hospital admission have an almost four times greater risk of death in the 12 months following discharge.3 To address unintentional weight loss, clinicians may prescribe appetite stimulants.
Megestrol acetate is approved by the US Food and Drug Administration (FDA) for the treatment of weight loss in patients with AIDS.4 Megestrol acetate promotes weight gain through inhibition of cytokines, interleukin-6, and tumor necrosis factor-alpha, which are increased in older adults. In a randomized, placebo-controlled trial of 69 nursing home residents with ≥6 months’ life expectancy and Karnofsky score of ≥40%, patients treated with megestrol acetate for 12 weeks reported increased appetite and well-being. They achieved significant weight gain (>1.82 kg), but not until 3 months after therapy ended.5 No significant adverse events were reported; however, adverse event monitoring continued only for the 12-week treatment period. This follow-up duration may have been insufficient to identify some adverse events, such as venous thromboembolism.
Mirtazapine, an antidepressant and serotonin receptor antagonist, reduces levels of serotonin, a neurotransmitter that promotes early satiety.6 In a meta-analysis of 11 trials comparing mirtazapine to selective serotonin reuptake inhibitors for depression, patients treated with mirtazapine demonstrated an increase in the composite secondary outcome of weight gain or increased appetite.7 The amount of weight gain was not specified. Weight gain is more common with low-dose mirtazapine, potentially due to increased antihistamine activity at lower doses.8 Overall, mirtazapine is well-tolerated and efficacious in the treatment of depression and may benefit older adults with concomitant weight loss.6
Cyproheptadine is a first-generation antihistamine with appetite-stimulating effects. It has been found to increase weight or appetite in various disease states, particularly in the pediatric population,9 including cystic fibrosis10 and malignancy.11 Given this evidence, there has been interest in its use in the geriatric population with unintentional weight loss.
Dronabinol is an orally active cannabinoid approved for anorexia-associated weight loss in patients with AIDS.12 In a randomized, placebo-controlled trial in patients with AIDS-related anorexia and weight loss, participants receiving dronabinol had a statistically significant increase in appetite but no change in weight. Participants receiving dronabinol also experienced more nervous system-related adverse events, including dizziness, thinking abnormalities, and somnolence.13
Why Prescribing Appetite Stimulants for Unintentional Weight Loss in Older Adults Is Not Helpful
Weight gain may not improve clinically meaningful outcomes. The absence of consistent evidence that prescription appetite stimulants improve patient-centered outcomes, such as quality of life or functional status, and the potential morbidity and mortality of these medications make prescribing appetite stimulants in older adults concerning.
Megestrol Acetate
A 2018 systematic review of randomized controlled trials studying megestrol acetate for treatment of anorexia-cachexia, primarily in adults with AIDS and cancer, found that treatment resulted in a 2.25-kg weight gain, with no improvement in quality of life and an increased risk of adverse events.14
Three prospective trials studied the effect of megestrol acetate in older adults (Appendix Table). One trial randomized 47 patients receiving skilled nursing services following an admission for acute illness to megestrol acetate vs placebo. While the investigators noted increases in appetite at higher doses of megestrol acetate, there was no change in weight or clinically relevant outcomes.15 In a second randomized controlled trial, 29 patients with illness-induced functional decline were enrolled in a strength training program in addition to being assigned to megestrol acetate or placebo. While patients receiving megestrol acetate with the exercise program had significant increases in weight and nutritional intake, they suffered a deterioration in physical function.16 In a pilot study, 17 nursing home residents who consistently ate less than 75% of their meals received megestrol acetate plus standard or optimal feeding assistance. The percentage of meals consumed increased only when patients received optimal feeding assistance in conjunction with megestrol acetate.17
The largest case-control study examining megestrol acetate for unintentional weight loss in older adults compared 709 residents in a multistate nursing home system treated with megestrol acetate to matched untreated controls. After 6 months of treatment, the median weight and change in weight did not differ significantly. Patients receiving megestrol acetate had a significant increase in mortality, surviving an average of 23.9 months, compared to 31.2 months for controls (P < .001).18
Additionally, two retrospective reviews of nursing home patients who were prescribed megestrol acetate showed incidences of venous thrombosis of 5% and 32%.19,20 Other potentially significant adverse effects include adrenal insufficiency and fluid retention.6 In 2019, the American Geriatrics Society’s Beers Criteria included megestrol acetate as a medication to avoid given its “minimal effect on weight; increases [in] risk of thrombotic events and possibly death in older adults.”21
Mirtazapine
No studies have evaluated mirtazapine for weight gain without concomitant depression. In older adults with depression, mirtazapine has minimal impact on promoting weight gain compared to other antidepressants. In two retrospective studies of older patients with depression and weight loss, researchers found no difference in weight gain in those treated with mirtazapine vs sertraline or other nontricyclic antidepressants, excluding fluoxetine.22,23
Cyproheptadine
There have been no controlled trials evaluating the use of cyproheptadine in older adults, in part due to anticholinergic side effects. In a trial of cancer patients, sedation and dizziness were common adverse effects.11 The 2019 American Geriatrics Society’s Beers Criteria include cyproheptadine as a medication to avoid based upon the “risk of confusion, dry mouth, constipation, and other anticholinergic effects or toxicity.”21
Dronabinol
In a retrospective cohort study of 28 long-term care residents with anorexia and weight loss, participants receiving dronabinol for 12 weeks had no statistically significant weight gain.24 The FDA cautions against prescribing dronabinol for older adults due to neurological side effects.12 A systematic review of randomized controlled trials found that cannabinoid-based medications in patients older than 50 years were associated with a significant increase in dizziness or lightheadedness and thinking or perception disorder.25
What You Should Do Instead
In the Choosing Wisely® initiative, the American Geriatrics Society recommends avoiding prescription appetite stimulants for patients with anorexia or cachexia.26 Instead, hospitalists should evaluate older patients for causes of unintentional weight loss, including malignancy, nonmalignant gastrointestinal disorders, depression, and dementia. Hospitalists can identify most causes based on the history, physical exam, and laboratory studies and initiate treatment for modifiable causes, such as constipation and depression.2
Hospitalists should work with an interprofessional team to develop an individualized plan to optimize caloric intake in the hospital (Table).27 One in five hospitalized older adults has insufficient caloric intake during admission, which is associated with increased risk for in-hospital and 90-day mortality.28 Removing dietary restrictions, increasing the variety of foods offered, and assisted eating may increase food intake.27,29 Hospitalists should also consider discontinuing or changing medications with gastrointestinal side effects, such as metformin, cholinesterase inhibitors, bisphosphonates, and oral iron supplements. Dietitians may recommend oral nutrition supplements; if started, patients should be offered supplements after discharge.27,29 For patients with limited access to food, social workers can help optimize social supports and identify community resources following discharge. Finally, hospitalists should coordinate with outpatient providers to monitor weight long-term.

Recommendations
- Recognize and address unintentional weight loss in older adults in the hospital.
- Do not prescribe appetite stimulants for unintentional weight loss in hospitalized older adults as they have no proven benefit for improving long-term outcomes and, in the case of megestrol acetate, may increase mortality.
- Work with an interprofessional team to address factors contributing to unintentional weight loss using nonpharmacologic options for improving food intake.
Conclusion
After discussing the lack of evidence supporting prescription appetite stimulants and the potential risks, we shifted the focus to optimizing oral intake. The team worked with the patient and the patient’s family to optimize nutrition following discharge and communicated the need for ongoing monitoring to the primary care provider.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected]
Acknowledgment
The authors thank Claire Campbell, MD, for her review of this manuscript.
1. Bouras EP, Lange SM, Scolapio JS. Rational approach to patients with unintentional weight loss. Mayo Clin Proc. 2001;76(9):923-929. https://doi.org/10.4065/76.9.923
2. McMinn J, Steel C, Bowman A. Investigation and management of unintentional weight loss in older adults. BMJ. 2011;342:d1732. https://doi.org/10.1136/bmj.d1732
3. Satish S, Winograd CH, Chavez C, Bloch DA. Geriatric targeting criteria as predictors of survival and health care utilization. J Am Geriatr Soc. 1996;44(8):914-921. https://doi.org/10.1111/j.1532-5415.1996.tb01860.x
4. Megace (megestrol acetate) [package insert]. Par Pharmaceutical Inc. Revised July 2005. Accessed January 27, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021778s000TOC.cfm
5. Yeh SS, Wu SY, Lee TP, et al. Improvement in quality-of-life measures and stimulation of weight gain after treatment with megestrol acetate oral suspension in geriatric cachexia: results of a double-blind, placebo-controlled study. J Am Geriatr Soc. 2000;48(5):485-492. https://doi.org/10.1111/j.1532-5415.2000.tb04993.x
6. Fox CB, Treadway AK, Blaszczyk AT, Sleeper RB. Reviews of therapeutics megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29(4):383-397. https://doi.org/10.1592/phco.29.4.383
7. Watanabe N, Omori IM, Nakagawa A, et al. Mirtazapine versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2011;(12):CD006528. https://doi.org/10.1002/14651858.CD006528.pub2
8. Fawcett J, Barkin RL. Review of the results from clinical studies on the efficacy, safety and tolerability of mirtazapine for the treatment of patients with major depression. J Affect Disord. 1998;51(3):267-285. https://doi.org/10.1016/S0165-0327(98)00224-9
9. Najib K, Moghtaderi M, Karamizadeh Z, Fallahzadeh E. Beneficial effect of cyproheptadine on body mass index in undernourished children: a randomized controlled trial. Iran J Pediatr. 2014;24(6):753-758.
10. Epifanio M, Marostica PC, Mattiello R, et al. A randomized, double-blind, placebo-controlled trial of cyproheptadine for appetite stimulation in cystic fibrosis. J Pediatr (Rio J). 2012;88(2):155-160. https://doi.org/10.2223/JPED.2174
11. Kardinal CG, Loprinzi CL, Schaid DJ, et al. A controlled trial of cyproheptadine in cancer patients with anorexia and/or cachexia. Cancer. 1990;65(12):2657-2662. https://doi.org/10.1002/1097-0142(19900615)65:12<2657::aid-cncr2820651210>3.0.co;2-s
12. MARINOL (dronabinol) [package insert]. Solvay Pharmaceuticals, Inc. Revised August 2017. Accessed April 27, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf.
13. Beal JE, Olson R, Laubenstein L, et al. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage. 1995;10(2):89-97. https://doi.org/10.1016/0885-3924(94)00117-4
14. Ruiz-García V, López-Briz E, Carbonell-Sanchis R, Bort-Martí S, Gonzálvez-Perales JL. Megestrol acetate for cachexia–anorexia syndrome. A systematic review. J Cachexia Sarcopenia Muscle. 2018;9(3):444-452. https://doi.org/10.1002/jcsm.12292
15. Reuben DB, Hirsch SH, Zhou K, Greendale GA. The effects of megestrol acetate suspension for elderly patients with reduced appetite after hospitalization: a phase II randomized clinical trial. J Am Geriatr Soc. 2005;53(6):970-975. https://doi.org/10.1111/j.1532-5415.2005.53307.x
16. Sullivan DH, Roberson PK, Smith ES, Price JA, Bopp MM. Effects of muscle strength training and megestrol acetate on strength, muscle mass, and function in frail older people. J Am Geriatr Soc. 2007;55(1):20-28. https://doi.org/10.1111/j.1532-5415.2006.01010.x
17. Simmons SF, Walker KA, Osterweil D. The effect of megestrol acetate on oral food and fluid intake in nursing home residents: a pilot study. J Am Med Dir Assoc. 2005;6(3):S5-S11. https://doi.org/10.1016/j.jamda.2005.03.014
18. Bodenner D, Spencer T, Riggs AT, Redman C, Strunk B, Hughes T. A retrospective study of the association between megestrol acetate administration and mortality among nursing home residents with clinically significant weight loss. Am J Geriatr Pharmacother. 2007;5(2):137-146. https://doi.org/10.1016/J.AMJOPHARM.2007.06.004
19. Kropsky B, Shi Y, Cherniack EP. Incidence of deep-venous thrombosis in nursing home residents using megestrol acetate. J Am Med Dir Assoc. 2003;4(5):255-256. https://doi.org/10.1097/01.JAM.0000083384.84558.75
20. Bolen JC, Andersen RE, Bennett RG. Deep vein thrombosis as a complication of megestrol acetate therapy among nursing home residents. J Am Med Dir Assoc. 2000;1(6):248-252.
21. Fick DM, Semla TP, Steinman M, et al. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674-694. https://doi.org/10.1111/jgs.15767
22. Mihara IQT, McCombs JS, Williams BR. The impact of mirtazapine compared with non-TCA antidepressants on weight change in nursing facility residents. Consult Pharm. 2005;20(3):217-223. https://doi.org/10.4140/tcp.n.2005.217
23. Goldberg RJ. Weight change in depressed nursing home patients on mirtazapine. J Am Geriatr Soc. 2002;50(8):1461. https://doi.org/10.1046/j.1532-5415.2002.50374.x
24. Wilson MMG, Philpot C, Morley JE. Anorexia of aging in long term care: is dronabinol an effective appetite stimulant?--a pilot study. J Nutr Health Aging. 2007;11(2):195-198.
25. Velayudhan L, McGoohan KL, Bhattacharyya S. Evaluation of THC-related neuropsychiatric symptoms among adults aged 50 years and older: a systematic review and metaregression analysis. JAMA Netw Open. 2021;4(2):e2035913. https://doi.org/10.1001/jamanetworkopen.2020.35913
26. AGS Choosing Wisely Workgroup. American Geriatrics Society identifies another five things that healthcare providers and patients should question. J Am Geriatr Soc. 2014;62(5):950-960. https://doi.org/10.1111/jgs.12770
27. Volkert D, Beck AM, Cederholm T, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. 2019;38(1):10-47. https://doi.org/10.1016/j.clnu.2018.05.024
28. Sullivan DH, Sun S, Walls RC. Protein-energy undernutrition among elderly hospitalized patients: a prospective study. JAMA. 1999;281(21):2013-2019. https://doi.org/10.1001/jama.281.21.2013
29. Feinberg J, Nielsen EE, Korang SK, et al. Nutrition support in hospitalised adults at nutritional risk. Cochrane Database Syst Rev. 2017;2017(5). https://doi.org/10.1002/14651858.CD011598.pub2
1. Bouras EP, Lange SM, Scolapio JS. Rational approach to patients with unintentional weight loss. Mayo Clin Proc. 2001;76(9):923-929. https://doi.org/10.4065/76.9.923
2. McMinn J, Steel C, Bowman A. Investigation and management of unintentional weight loss in older adults. BMJ. 2011;342:d1732. https://doi.org/10.1136/bmj.d1732
3. Satish S, Winograd CH, Chavez C, Bloch DA. Geriatric targeting criteria as predictors of survival and health care utilization. J Am Geriatr Soc. 1996;44(8):914-921. https://doi.org/10.1111/j.1532-5415.1996.tb01860.x
4. Megace (megestrol acetate) [package insert]. Par Pharmaceutical Inc. Revised July 2005. Accessed January 27, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021778s000TOC.cfm
5. Yeh SS, Wu SY, Lee TP, et al. Improvement in quality-of-life measures and stimulation of weight gain after treatment with megestrol acetate oral suspension in geriatric cachexia: results of a double-blind, placebo-controlled study. J Am Geriatr Soc. 2000;48(5):485-492. https://doi.org/10.1111/j.1532-5415.2000.tb04993.x
6. Fox CB, Treadway AK, Blaszczyk AT, Sleeper RB. Reviews of therapeutics megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29(4):383-397. https://doi.org/10.1592/phco.29.4.383
7. Watanabe N, Omori IM, Nakagawa A, et al. Mirtazapine versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2011;(12):CD006528. https://doi.org/10.1002/14651858.CD006528.pub2
8. Fawcett J, Barkin RL. Review of the results from clinical studies on the efficacy, safety and tolerability of mirtazapine for the treatment of patients with major depression. J Affect Disord. 1998;51(3):267-285. https://doi.org/10.1016/S0165-0327(98)00224-9
9. Najib K, Moghtaderi M, Karamizadeh Z, Fallahzadeh E. Beneficial effect of cyproheptadine on body mass index in undernourished children: a randomized controlled trial. Iran J Pediatr. 2014;24(6):753-758.
10. Epifanio M, Marostica PC, Mattiello R, et al. A randomized, double-blind, placebo-controlled trial of cyproheptadine for appetite stimulation in cystic fibrosis. J Pediatr (Rio J). 2012;88(2):155-160. https://doi.org/10.2223/JPED.2174
11. Kardinal CG, Loprinzi CL, Schaid DJ, et al. A controlled trial of cyproheptadine in cancer patients with anorexia and/or cachexia. Cancer. 1990;65(12):2657-2662. https://doi.org/10.1002/1097-0142(19900615)65:12<2657::aid-cncr2820651210>3.0.co;2-s
12. MARINOL (dronabinol) [package insert]. Solvay Pharmaceuticals, Inc. Revised August 2017. Accessed April 27, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf.
13. Beal JE, Olson R, Laubenstein L, et al. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage. 1995;10(2):89-97. https://doi.org/10.1016/0885-3924(94)00117-4
14. Ruiz-García V, López-Briz E, Carbonell-Sanchis R, Bort-Martí S, Gonzálvez-Perales JL. Megestrol acetate for cachexia–anorexia syndrome. A systematic review. J Cachexia Sarcopenia Muscle. 2018;9(3):444-452. https://doi.org/10.1002/jcsm.12292
15. Reuben DB, Hirsch SH, Zhou K, Greendale GA. The effects of megestrol acetate suspension for elderly patients with reduced appetite after hospitalization: a phase II randomized clinical trial. J Am Geriatr Soc. 2005;53(6):970-975. https://doi.org/10.1111/j.1532-5415.2005.53307.x
16. Sullivan DH, Roberson PK, Smith ES, Price JA, Bopp MM. Effects of muscle strength training and megestrol acetate on strength, muscle mass, and function in frail older people. J Am Geriatr Soc. 2007;55(1):20-28. https://doi.org/10.1111/j.1532-5415.2006.01010.x
17. Simmons SF, Walker KA, Osterweil D. The effect of megestrol acetate on oral food and fluid intake in nursing home residents: a pilot study. J Am Med Dir Assoc. 2005;6(3):S5-S11. https://doi.org/10.1016/j.jamda.2005.03.014
18. Bodenner D, Spencer T, Riggs AT, Redman C, Strunk B, Hughes T. A retrospective study of the association between megestrol acetate administration and mortality among nursing home residents with clinically significant weight loss. Am J Geriatr Pharmacother. 2007;5(2):137-146. https://doi.org/10.1016/J.AMJOPHARM.2007.06.004
19. Kropsky B, Shi Y, Cherniack EP. Incidence of deep-venous thrombosis in nursing home residents using megestrol acetate. J Am Med Dir Assoc. 2003;4(5):255-256. https://doi.org/10.1097/01.JAM.0000083384.84558.75
20. Bolen JC, Andersen RE, Bennett RG. Deep vein thrombosis as a complication of megestrol acetate therapy among nursing home residents. J Am Med Dir Assoc. 2000;1(6):248-252.
21. Fick DM, Semla TP, Steinman M, et al. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674-694. https://doi.org/10.1111/jgs.15767
22. Mihara IQT, McCombs JS, Williams BR. The impact of mirtazapine compared with non-TCA antidepressants on weight change in nursing facility residents. Consult Pharm. 2005;20(3):217-223. https://doi.org/10.4140/tcp.n.2005.217
23. Goldberg RJ. Weight change in depressed nursing home patients on mirtazapine. J Am Geriatr Soc. 2002;50(8):1461. https://doi.org/10.1046/j.1532-5415.2002.50374.x
24. Wilson MMG, Philpot C, Morley JE. Anorexia of aging in long term care: is dronabinol an effective appetite stimulant?--a pilot study. J Nutr Health Aging. 2007;11(2):195-198.
25. Velayudhan L, McGoohan KL, Bhattacharyya S. Evaluation of THC-related neuropsychiatric symptoms among adults aged 50 years and older: a systematic review and metaregression analysis. JAMA Netw Open. 2021;4(2):e2035913. https://doi.org/10.1001/jamanetworkopen.2020.35913
26. AGS Choosing Wisely Workgroup. American Geriatrics Society identifies another five things that healthcare providers and patients should question. J Am Geriatr Soc. 2014;62(5):950-960. https://doi.org/10.1111/jgs.12770
27. Volkert D, Beck AM, Cederholm T, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. 2019;38(1):10-47. https://doi.org/10.1016/j.clnu.2018.05.024
28. Sullivan DH, Sun S, Walls RC. Protein-energy undernutrition among elderly hospitalized patients: a prospective study. JAMA. 1999;281(21):2013-2019. https://doi.org/10.1001/jama.281.21.2013
29. Feinberg J, Nielsen EE, Korang SK, et al. Nutrition support in hospitalised adults at nutritional risk. Cochrane Database Syst Rev. 2017;2017(5). https://doi.org/10.1002/14651858.CD011598.pub2
© 2021 Society of Hospital Medicine
Clinical Guideline Highlights for the Hospitalist: Focused Updates to Pediatric Asthma Management
Asthma is a heterogeneous condition characterized by airway hyperresponsiveness and obstruction, with associated airway inflammation and remodeling.2 Asthma affects 25 million people in the United States and 334 million people worldwide, with significant healthcare disparities across race and ethnicity.2-6 Asthma is the third most common reason for hospitalizations in pediatrics, accounting for 180,000 annual hospitalizations for children and adults.3,7 In 2020, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel provided a focused update to the Asthma Management Guidelines, centered on six topics with sufficient new evidence. The management of status asthmaticus was not included in this update. We spotlight four of the recommendations applicable to the practice of pediatric hospital medicine.
Key Recommendations for the Hospitalist
Recommendation 1. Children 0 to 4 years old with recurrent wheezing triggered by a respiratory tract infection (RTI) and no wheezing between infections should receive a short course of daily inhaled corticosteroids (ICS) at the onset of a RTI, with an as-needed short-acting beta agonist (SABA) for quick-relief therapy compared to SABA alone (evidence quality: high; recommendation strength: conditional).
Recurrent wheezing is defined as clinically significant periods of wheezing that are reversible or consistent with bronchospasm and as ≥3 episodes in a lifetime or 2 episodes in the past year. It is important to adhere to this definition to prevent inappropriate use of ICS for bronchiolitis. This treatment is associated with a reduction of use of systemic steroids (relative risk [RR], 0.67; 95% CI, 0.46-0.98) without a statistical decrease in acute care visits (RR, 0.90; 95% CI, 0.77-1.05) or hospitalizations (RR, 0.77; 95% CI, 0.06-9.68). Improved transition of care is essential between the primary care provider, hospitalist, and family to ensure an understanding of how/when to initiate ICS at the onset of a RTI. Potential harms include effect on growth and overprescribing. Growth should be monitored because data are conflicting.
Recommendation 2. Individuals ages 12 years and older with mild persistent asthma should use as-needed SABA and may use either daily low-dose ICS or as-needed ICS when symptoms flare (evidence quality: moderate; recommendation strength: conditional).
In intermittent therapy, patients take a SABA followed by an ICS as needed for acute asthma symptoms. This recommendation is driven by asthma-control and quality-of-life outcomes, with caregivers reporting that intermittent dosing could “offer flexibility and potentially reduce side effects.” There were no differences between management regimens with respect to systemic steroid use (RR, 0.70; 95% CI, 0.30-1.64) or urgent care visits (RR, 0.25; 95% CI, 0.05-1.16). Differing perception of symptoms by individuals may lead to undertreating or overtreating, and intermittent administration makes it challenging for clinicians to assess the need to adjust therapy.
Recommendation 3. Children 4 years and older with moderate to severe persistent asthma should use ICS-formoterol in a single inhaler used as both daily controller and reliever therapy compared to either (a) higher-dose ICS as daily controller therapy and SABA for quick-relief therapy or (b) a same-dose ICS-long-acting beta agonist (LABA) as daily controller therapy and SABA for quick-relief therapy (evidence quality: high for ages ≥12 years, moderate for ages 4-11 years; recommendation strength: strong).
For children 4 years and older, it is recommended to use “single maintenance and reliever therapy” (SMART) with a single-inhaler containing either low- or medium-dose ICS and formoterol when stepping up from Step 2 (daily low-dose ICS and as-needed SABA) to Step 3 (daily and as-needed low-dose ICS-formoterol) and Step 4 (daily and as-needed medium-dose ICS-formoterol).
Recommendation 4. If individuals with asthma have symptoms related to indoor allergens, confirmed by history or allergy testing, they should use a multicomponent allergen-specific mitigation intervention. Allergen mitigation interventions should not be a part of routine asthma management for individuals with asthma who do not have symptoms related to exposure to specific indoor allergens (evidence quality: low; recommendation strength: conditional).
Providers often emphasize exposure to potential indoor allergens such as carpets and pets when taking an asthma history and counsel removal of these triggers. However, all recommendations related to allergies in the 2020 updates have low-moderate evidence quality and conditional recommendation strength. Hospitalists should instead focus their questions on allergy symptoms and triggers and recommend multicomponent mitigation intervention only if there is a confirmed allergy history. Families should continue routine good practices such as house cleaning and laundering, but other interventions are not evidence-based.
CRITIQUE
Methods
The Expert Panel included a diverse group of clinicians, a pharmacist, and health policy experts. In 2015, a needs assessment identified 6 out of 17 priority topics with sufficient new information for updates. Key questions were drafted, and systematic reviews were published through 2018. The Expert Panel made its recommendations using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach. The Expert Panel informed its recommendations with input from focus groups, including individuals with asthma and caregivers. The NHLBI posted the draft report for public review, and comments were considered. We believe these methods effectively developed evidence-based recommendations, and the diversity of stakeholders increases the value of this guideline. However, the infrequency of updates limits the utility of the NHLBI guidelines as compared with annual GINA (Global Initiative for Asthma) updates.
There are important considerations in assessing these guidelines. Specifically, the validity of systemic steroid courses as an outcome for children ages 0 to 4 years is controversial. Second, the studies cited in defense of intermittent ICS use in children >12 years of age excluded pediatric patients and did not include readmissions as a primary outcome, which is of particular interest to the hospitalist.
Potential Conflicts for Guideline Authors
The Expert Panel reported all potential conflicts of interest (COIs), which were rated by the Expert Panel Chair and Journal of Allergy and Clinical Immunology editors. Individuals with high COIs were excluded from the Expert Panel. Those with moderate COIs were recused for that topic. Low COIs were not related to the guideline.
Generalizability of the Guideline
These guidelines are based on systematic reviews with large sample sizes and patients of all ages. They are generalizable. However, the authors recognize that variations in asthma require individualized approaches. They identify this as a reason for the lack of strong recommendations for asthma standards of care.
AREAS OF FUTURE STUDY
Biologics have progressed considerably since revision of the guidelines. The 2020 guidelines did not address these to prevent delay of the guideline release, but recommendations should be included in future guidelines. Future studies should address healthcare disparities in asthma, barriers to equitable care, and how to eliminate them, as guided by the President’s Task Force.8 Status asthmaticus should be included in future updates.
1. Expert Panel Working Group of the National Heart, Lung, and Blood Institute (NHLBI) administered and coordinated National Asthma Education and Prevention Program Coordinating Committee (NAEPPCC), Cloutier MM, Baptist AP, Blake KV, et al. 2020 focused updates to the asthma management guidelines: a report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol. 2020;146(6):1217-1270. https://doi.org/10.1016/j.jaci.2020.10.003.
2. Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018;391(10122):783-800. https://doi.org/10.1016/S0140-6736(17)33311-1
3. Centers for Disease Control and Prevention. Most recent asthma data. Reviewed March 30 2021. Accessed October 5, 2021. www.cdc.gov/asthma/most_recent_data.htm
4. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163-2196. https://doi.org/10.1016/S0140-6736(12)61729-2
5. Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15(3):348-356. https://doi.org/10.1513/AnnalsATS.201703-259OC
6. Moorman JE, Akinbami LJ, Bailey CM, et al. National surveillance of asthma: United States, 2001-2010. Vital Health Stat 3. 2012;(35):1-58.
7. Witt WP, Weiss AJ, Elixhauser A. Overview of hospital stays for children in the United States, 2012: Statistical Brief #187. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Agency for Healthcare Research and Quality; February 2006.
8. U.S. Environmental Protection Agency. President’s Task Force on Environmental Health Risks and Safety Risks to Children: Coordinated Federal Action Plan to Reduce Racial and Ethnic Asthma Disparities. May 2012. https://19january2017snapshot.epa.gov/sites/production/files/2014-08/documents/federal_asthma_disparities_action_plan.pdf
Asthma is a heterogeneous condition characterized by airway hyperresponsiveness and obstruction, with associated airway inflammation and remodeling.2 Asthma affects 25 million people in the United States and 334 million people worldwide, with significant healthcare disparities across race and ethnicity.2-6 Asthma is the third most common reason for hospitalizations in pediatrics, accounting for 180,000 annual hospitalizations for children and adults.3,7 In 2020, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel provided a focused update to the Asthma Management Guidelines, centered on six topics with sufficient new evidence. The management of status asthmaticus was not included in this update. We spotlight four of the recommendations applicable to the practice of pediatric hospital medicine.
Key Recommendations for the Hospitalist
Recommendation 1. Children 0 to 4 years old with recurrent wheezing triggered by a respiratory tract infection (RTI) and no wheezing between infections should receive a short course of daily inhaled corticosteroids (ICS) at the onset of a RTI, with an as-needed short-acting beta agonist (SABA) for quick-relief therapy compared to SABA alone (evidence quality: high; recommendation strength: conditional).
Recurrent wheezing is defined as clinically significant periods of wheezing that are reversible or consistent with bronchospasm and as ≥3 episodes in a lifetime or 2 episodes in the past year. It is important to adhere to this definition to prevent inappropriate use of ICS for bronchiolitis. This treatment is associated with a reduction of use of systemic steroids (relative risk [RR], 0.67; 95% CI, 0.46-0.98) without a statistical decrease in acute care visits (RR, 0.90; 95% CI, 0.77-1.05) or hospitalizations (RR, 0.77; 95% CI, 0.06-9.68). Improved transition of care is essential between the primary care provider, hospitalist, and family to ensure an understanding of how/when to initiate ICS at the onset of a RTI. Potential harms include effect on growth and overprescribing. Growth should be monitored because data are conflicting.
Recommendation 2. Individuals ages 12 years and older with mild persistent asthma should use as-needed SABA and may use either daily low-dose ICS or as-needed ICS when symptoms flare (evidence quality: moderate; recommendation strength: conditional).
In intermittent therapy, patients take a SABA followed by an ICS as needed for acute asthma symptoms. This recommendation is driven by asthma-control and quality-of-life outcomes, with caregivers reporting that intermittent dosing could “offer flexibility and potentially reduce side effects.” There were no differences between management regimens with respect to systemic steroid use (RR, 0.70; 95% CI, 0.30-1.64) or urgent care visits (RR, 0.25; 95% CI, 0.05-1.16). Differing perception of symptoms by individuals may lead to undertreating or overtreating, and intermittent administration makes it challenging for clinicians to assess the need to adjust therapy.
Recommendation 3. Children 4 years and older with moderate to severe persistent asthma should use ICS-formoterol in a single inhaler used as both daily controller and reliever therapy compared to either (a) higher-dose ICS as daily controller therapy and SABA for quick-relief therapy or (b) a same-dose ICS-long-acting beta agonist (LABA) as daily controller therapy and SABA for quick-relief therapy (evidence quality: high for ages ≥12 years, moderate for ages 4-11 years; recommendation strength: strong).
For children 4 years and older, it is recommended to use “single maintenance and reliever therapy” (SMART) with a single-inhaler containing either low- or medium-dose ICS and formoterol when stepping up from Step 2 (daily low-dose ICS and as-needed SABA) to Step 3 (daily and as-needed low-dose ICS-formoterol) and Step 4 (daily and as-needed medium-dose ICS-formoterol).
Recommendation 4. If individuals with asthma have symptoms related to indoor allergens, confirmed by history or allergy testing, they should use a multicomponent allergen-specific mitigation intervention. Allergen mitigation interventions should not be a part of routine asthma management for individuals with asthma who do not have symptoms related to exposure to specific indoor allergens (evidence quality: low; recommendation strength: conditional).
Providers often emphasize exposure to potential indoor allergens such as carpets and pets when taking an asthma history and counsel removal of these triggers. However, all recommendations related to allergies in the 2020 updates have low-moderate evidence quality and conditional recommendation strength. Hospitalists should instead focus their questions on allergy symptoms and triggers and recommend multicomponent mitigation intervention only if there is a confirmed allergy history. Families should continue routine good practices such as house cleaning and laundering, but other interventions are not evidence-based.
CRITIQUE
Methods
The Expert Panel included a diverse group of clinicians, a pharmacist, and health policy experts. In 2015, a needs assessment identified 6 out of 17 priority topics with sufficient new information for updates. Key questions were drafted, and systematic reviews were published through 2018. The Expert Panel made its recommendations using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach. The Expert Panel informed its recommendations with input from focus groups, including individuals with asthma and caregivers. The NHLBI posted the draft report for public review, and comments were considered. We believe these methods effectively developed evidence-based recommendations, and the diversity of stakeholders increases the value of this guideline. However, the infrequency of updates limits the utility of the NHLBI guidelines as compared with annual GINA (Global Initiative for Asthma) updates.
There are important considerations in assessing these guidelines. Specifically, the validity of systemic steroid courses as an outcome for children ages 0 to 4 years is controversial. Second, the studies cited in defense of intermittent ICS use in children >12 years of age excluded pediatric patients and did not include readmissions as a primary outcome, which is of particular interest to the hospitalist.
Potential Conflicts for Guideline Authors
The Expert Panel reported all potential conflicts of interest (COIs), which were rated by the Expert Panel Chair and Journal of Allergy and Clinical Immunology editors. Individuals with high COIs were excluded from the Expert Panel. Those with moderate COIs were recused for that topic. Low COIs were not related to the guideline.
Generalizability of the Guideline
These guidelines are based on systematic reviews with large sample sizes and patients of all ages. They are generalizable. However, the authors recognize that variations in asthma require individualized approaches. They identify this as a reason for the lack of strong recommendations for asthma standards of care.
AREAS OF FUTURE STUDY
Biologics have progressed considerably since revision of the guidelines. The 2020 guidelines did not address these to prevent delay of the guideline release, but recommendations should be included in future guidelines. Future studies should address healthcare disparities in asthma, barriers to equitable care, and how to eliminate them, as guided by the President’s Task Force.8 Status asthmaticus should be included in future updates.
Asthma is a heterogeneous condition characterized by airway hyperresponsiveness and obstruction, with associated airway inflammation and remodeling.2 Asthma affects 25 million people in the United States and 334 million people worldwide, with significant healthcare disparities across race and ethnicity.2-6 Asthma is the third most common reason for hospitalizations in pediatrics, accounting for 180,000 annual hospitalizations for children and adults.3,7 In 2020, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel provided a focused update to the Asthma Management Guidelines, centered on six topics with sufficient new evidence. The management of status asthmaticus was not included in this update. We spotlight four of the recommendations applicable to the practice of pediatric hospital medicine.
Key Recommendations for the Hospitalist
Recommendation 1. Children 0 to 4 years old with recurrent wheezing triggered by a respiratory tract infection (RTI) and no wheezing between infections should receive a short course of daily inhaled corticosteroids (ICS) at the onset of a RTI, with an as-needed short-acting beta agonist (SABA) for quick-relief therapy compared to SABA alone (evidence quality: high; recommendation strength: conditional).
Recurrent wheezing is defined as clinically significant periods of wheezing that are reversible or consistent with bronchospasm and as ≥3 episodes in a lifetime or 2 episodes in the past year. It is important to adhere to this definition to prevent inappropriate use of ICS for bronchiolitis. This treatment is associated with a reduction of use of systemic steroids (relative risk [RR], 0.67; 95% CI, 0.46-0.98) without a statistical decrease in acute care visits (RR, 0.90; 95% CI, 0.77-1.05) or hospitalizations (RR, 0.77; 95% CI, 0.06-9.68). Improved transition of care is essential between the primary care provider, hospitalist, and family to ensure an understanding of how/when to initiate ICS at the onset of a RTI. Potential harms include effect on growth and overprescribing. Growth should be monitored because data are conflicting.
Recommendation 2. Individuals ages 12 years and older with mild persistent asthma should use as-needed SABA and may use either daily low-dose ICS or as-needed ICS when symptoms flare (evidence quality: moderate; recommendation strength: conditional).
In intermittent therapy, patients take a SABA followed by an ICS as needed for acute asthma symptoms. This recommendation is driven by asthma-control and quality-of-life outcomes, with caregivers reporting that intermittent dosing could “offer flexibility and potentially reduce side effects.” There were no differences between management regimens with respect to systemic steroid use (RR, 0.70; 95% CI, 0.30-1.64) or urgent care visits (RR, 0.25; 95% CI, 0.05-1.16). Differing perception of symptoms by individuals may lead to undertreating or overtreating, and intermittent administration makes it challenging for clinicians to assess the need to adjust therapy.
Recommendation 3. Children 4 years and older with moderate to severe persistent asthma should use ICS-formoterol in a single inhaler used as both daily controller and reliever therapy compared to either (a) higher-dose ICS as daily controller therapy and SABA for quick-relief therapy or (b) a same-dose ICS-long-acting beta agonist (LABA) as daily controller therapy and SABA for quick-relief therapy (evidence quality: high for ages ≥12 years, moderate for ages 4-11 years; recommendation strength: strong).
For children 4 years and older, it is recommended to use “single maintenance and reliever therapy” (SMART) with a single-inhaler containing either low- or medium-dose ICS and formoterol when stepping up from Step 2 (daily low-dose ICS and as-needed SABA) to Step 3 (daily and as-needed low-dose ICS-formoterol) and Step 4 (daily and as-needed medium-dose ICS-formoterol).
Recommendation 4. If individuals with asthma have symptoms related to indoor allergens, confirmed by history or allergy testing, they should use a multicomponent allergen-specific mitigation intervention. Allergen mitigation interventions should not be a part of routine asthma management for individuals with asthma who do not have symptoms related to exposure to specific indoor allergens (evidence quality: low; recommendation strength: conditional).
Providers often emphasize exposure to potential indoor allergens such as carpets and pets when taking an asthma history and counsel removal of these triggers. However, all recommendations related to allergies in the 2020 updates have low-moderate evidence quality and conditional recommendation strength. Hospitalists should instead focus their questions on allergy symptoms and triggers and recommend multicomponent mitigation intervention only if there is a confirmed allergy history. Families should continue routine good practices such as house cleaning and laundering, but other interventions are not evidence-based.
CRITIQUE
Methods
The Expert Panel included a diverse group of clinicians, a pharmacist, and health policy experts. In 2015, a needs assessment identified 6 out of 17 priority topics with sufficient new information for updates. Key questions were drafted, and systematic reviews were published through 2018. The Expert Panel made its recommendations using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach. The Expert Panel informed its recommendations with input from focus groups, including individuals with asthma and caregivers. The NHLBI posted the draft report for public review, and comments were considered. We believe these methods effectively developed evidence-based recommendations, and the diversity of stakeholders increases the value of this guideline. However, the infrequency of updates limits the utility of the NHLBI guidelines as compared with annual GINA (Global Initiative for Asthma) updates.
There are important considerations in assessing these guidelines. Specifically, the validity of systemic steroid courses as an outcome for children ages 0 to 4 years is controversial. Second, the studies cited in defense of intermittent ICS use in children >12 years of age excluded pediatric patients and did not include readmissions as a primary outcome, which is of particular interest to the hospitalist.
Potential Conflicts for Guideline Authors
The Expert Panel reported all potential conflicts of interest (COIs), which were rated by the Expert Panel Chair and Journal of Allergy and Clinical Immunology editors. Individuals with high COIs were excluded from the Expert Panel. Those with moderate COIs were recused for that topic. Low COIs were not related to the guideline.
Generalizability of the Guideline
These guidelines are based on systematic reviews with large sample sizes and patients of all ages. They are generalizable. However, the authors recognize that variations in asthma require individualized approaches. They identify this as a reason for the lack of strong recommendations for asthma standards of care.
AREAS OF FUTURE STUDY
Biologics have progressed considerably since revision of the guidelines. The 2020 guidelines did not address these to prevent delay of the guideline release, but recommendations should be included in future guidelines. Future studies should address healthcare disparities in asthma, barriers to equitable care, and how to eliminate them, as guided by the President’s Task Force.8 Status asthmaticus should be included in future updates.
1. Expert Panel Working Group of the National Heart, Lung, and Blood Institute (NHLBI) administered and coordinated National Asthma Education and Prevention Program Coordinating Committee (NAEPPCC), Cloutier MM, Baptist AP, Blake KV, et al. 2020 focused updates to the asthma management guidelines: a report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol. 2020;146(6):1217-1270. https://doi.org/10.1016/j.jaci.2020.10.003.
2. Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018;391(10122):783-800. https://doi.org/10.1016/S0140-6736(17)33311-1
3. Centers for Disease Control and Prevention. Most recent asthma data. Reviewed March 30 2021. Accessed October 5, 2021. www.cdc.gov/asthma/most_recent_data.htm
4. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163-2196. https://doi.org/10.1016/S0140-6736(12)61729-2
5. Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15(3):348-356. https://doi.org/10.1513/AnnalsATS.201703-259OC
6. Moorman JE, Akinbami LJ, Bailey CM, et al. National surveillance of asthma: United States, 2001-2010. Vital Health Stat 3. 2012;(35):1-58.
7. Witt WP, Weiss AJ, Elixhauser A. Overview of hospital stays for children in the United States, 2012: Statistical Brief #187. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Agency for Healthcare Research and Quality; February 2006.
8. U.S. Environmental Protection Agency. President’s Task Force on Environmental Health Risks and Safety Risks to Children: Coordinated Federal Action Plan to Reduce Racial and Ethnic Asthma Disparities. May 2012. https://19january2017snapshot.epa.gov/sites/production/files/2014-08/documents/federal_asthma_disparities_action_plan.pdf
1. Expert Panel Working Group of the National Heart, Lung, and Blood Institute (NHLBI) administered and coordinated National Asthma Education and Prevention Program Coordinating Committee (NAEPPCC), Cloutier MM, Baptist AP, Blake KV, et al. 2020 focused updates to the asthma management guidelines: a report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol. 2020;146(6):1217-1270. https://doi.org/10.1016/j.jaci.2020.10.003.
2. Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018;391(10122):783-800. https://doi.org/10.1016/S0140-6736(17)33311-1
3. Centers for Disease Control and Prevention. Most recent asthma data. Reviewed March 30 2021. Accessed October 5, 2021. www.cdc.gov/asthma/most_recent_data.htm
4. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163-2196. https://doi.org/10.1016/S0140-6736(12)61729-2
5. Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15(3):348-356. https://doi.org/10.1513/AnnalsATS.201703-259OC
6. Moorman JE, Akinbami LJ, Bailey CM, et al. National surveillance of asthma: United States, 2001-2010. Vital Health Stat 3. 2012;(35):1-58.
7. Witt WP, Weiss AJ, Elixhauser A. Overview of hospital stays for children in the United States, 2012: Statistical Brief #187. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Agency for Healthcare Research and Quality; February 2006.
8. U.S. Environmental Protection Agency. President’s Task Force on Environmental Health Risks and Safety Risks to Children: Coordinated Federal Action Plan to Reduce Racial and Ethnic Asthma Disparities. May 2012. https://19january2017snapshot.epa.gov/sites/production/files/2014-08/documents/federal_asthma_disparities_action_plan.pdf
© 2021 Society of Hospital Medicine
Things We Do for No Reason™: Prescribing Thiamine, Folate and Multivitamins on Discharge for Patients With Alcohol Use Disorder
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old man with alcohol use disorder (AUD) is admitted with decompensated heart failure and experiences alcohol withdrawal during the hospitalization. He improves with guideline-directed heart failure therapy and benzodiazepines for alcohol withdrawal. Discharge medications are metoprolol succinate, lisinopril, furosemide, aspirin, atorvastatin, thiamine, folic acid, and a multivitamin. No medications are offered for AUD treatment. At follow-up a week later, he presents with dyspnea and reports poor medication adherence and a return to heavy drinking.
WHY YOU MIGHT THINK IT IS HELPFUL TO PRESCRIBE VITAMIN SUPPLEMENTATION TO PATIENTS WITH AUD AT HOSPITAL DISCHARGE
AUD is common among hospitalized patients.1 AUD increases the risk of vitamin deficiencies due to the toxic effects of alcohol on the gastrointestinal tract and liver, causing impaired digestion, reduced absorption, and increased degradation of key micronutrients.2,3 Other risk factors for AUD-associated vitamin deficiencies include food insecurity and the replacement of nutrient-rich food with alcohol. Since the body does not readily store water-soluble vitamins, including thiamine (vitamin B1) and folate (vitamin B9), people require regular dietary replenishment of these nutrients. Thus, if individuals with AUD eat less fortified food, they risk developing thiamine, folate, niacin, and other vitamin deficiencies. Since AUD puts patients at risk for vitamin deficiencies, hospitalized patients typically receive vitamin supplementation, including thiamine, folic acid, and a multivitamin (most formulations contain water-soluble vitamins B and C and micronutrients).1 Hospitalists often continue these medications at discharge.
Thiamine deficiency may manifest as Wernicke encephalopathy (WE), peripheral neuropathy, or a high-output heart failure state.
Hospitalists empirically treat with thiamine, folate, and other vitamins upon hospital admission with the intent of reducing morbidity associated with nutritional deficiencies.1 Repletion poses few risks to patients since the kidneys eliminate water-soluble vitamins. Multivitamins also have a low potential for direct harm and a low cost. Given the consequences of missing a deficiency, alcohol withdrawal–management order sets commonly embed vitamin repletion orders.6
WHY ROUTINELY PRESCRIBING VITAMIN SUPPLEMENTATION AT HOSPITAL DISCHARGE IN PATIENTS WITH AUD IS A TWDFNR
Hospitalists often reflexively continue vitamin supplementation on discharge. Unfortunately, there is no evidence that prescribing vitamin supplementation leads to clinically significant improvements for people with AUD, and patients can experience harms.
Literature and specialty guidelines lack consensus on rational vitamin supplementation in patients with AUD.2,7,8 Folate testing is not recommended due to inaccuracies.9 In fact, clinical data, such as body mass index, more accurately predict alcohol-related cognitive impairment than blood levels of vitamins.10 In one small study of vitamin deficiencies among patients with acute alcohol intoxication, none had low B12 or folate levels.11 A systematic review among people experiencing homelessness with unhealthy alcohol use showed no clear pattern of vitamin deficiencies across studies, although vitamin C and thiamine deficiencies predominated.12
In the absence of reliable thiamine and folate testing to confirm deficiencies, clinicians must use their clinical assessment skills. Clinicians rarely evaluate patients with AUD for vitamin deficiency risk factors and instead reflexively prescribe vitamin supplementation. An AUD diagnosis may serve as a sensitive, but not specific, risk factor for those in need of vitamin supplementation.
Other limitations make prescribing oral vitamins reflexively at discharge a low-value practice. Thiamine, often prescribed orally in the hospital and on discharge, has poor oral bioavailability.13 Unfortunately, people with AUD have decreased and variable thiamine absorption. To prevent WE, thiamine must cross the blood-brain barrier, and the literature provides insufficient evidence to guide clinicians on an appropriate oral thiamine dose, frequency, or duration of treatment.14 While early high-dose IV thiamine may treat or prevent WE during hospitalization, low-dose oral thiamine may not provide benefit to patients with AUD.5
The literature also provides sparse evidence for folate supplementation and its optimal dose. Since 1998, when the United States mandated fortifying grain products with folic acid, people rarely have low serum folate levels. Though patients with AUD have lower folate levels relative to the general population,15 this difference does not seem clinically significant. While limited data show an association between oral multivitamin supplementation and improved serum nutrient levels among people with AUD, we lack evidence on clinical outcomes.16
Most importantly, for a practice lacking strong evidence, prescribing multiple vitamins at discharge may result in harm from polypharmacy and unnecessary costs for the recently hospitalized patient. Alcohol use is associated with decreased adherence to medications for chronic conditions,17 including HIV, hypertension, hyperlipidemia, and psychiatric diseases. In addition, research shows an association between an increased number of discharge medications and higher risk for hospital readmission. The harm may actually correlate with the number of medications and complexity of the regimen rather than the risk profile of the medications themselves.18 Providers underestimate the impact of adding multiple vitamins at discharge, especially for patients who have several co-occurring medical conditions that require other medications. Furthermore, insurance rarely covers vitamins, leading hospitals or patients to incur the costs at discharge.
WHEN TO CONSIDER VITAMIN SUPPLEMENTATION AT DISCHARGE FOR PATIENTS WITH AUD
When treating patients with AUD, consider the potential benefit of vitamin supplementation for the individual. If a patient with regular, heavy alcohol use is at high risk of vitamin deficiencies due to ongoing risk factors (Table), hospitalists should discuss vitamin therapy via a patient-centered risk-benefit process.

When considering discharge vitamins, make concurrent efforts to enhance patient nutrition via decreased alcohol consumption and improved healthy food intake. While some patients do not have a goal of abstaining from alcohol, providing resources to food access may help decrease the harms of drinking. Education may help patients learn that vitamin deficiencies can result from heavy alcohol use.
Multivitamin formulations have variable doses of vitamins but can contain 100% or more of the daily value of thiamine and folic acid. For patients with AUD at lower risk of vitamin deficiencies (ie, mild alcohol use disorder with a healthy diet), discuss risks and benefits of supplementation. If they desire supplementation, a single thiamine-containing vitamin alone may be highest yield since it is the most morbid vitamin deficiency. Conversely, a patient with heavy alcohol intake and other risk factors for malnutrition may benefit from a higher dose of supplementation, achieved by prescribing a multivitamin alongside additional doses of thiamine and folate. However, the literature lacks evidence to guide clinicians on optimal vitamin dosing and formulations.
WHAT WE SHOULD DO INSTEAD
Instead of reflexively prescribing thiamine, folate, and multivitamin, clinicians can assess patients for AUD, provide motivational interviewing, and offer AUD treatment. Hospitalists should initiate and prescribe evidence-based medications for AUD for patients interested in reducing or stopping their alcohol intake. We can choose from Food and Drug Administration–approved AUD medications, including naltrexone and acamprosate. Unfortunately, less than 3% of patients with AUD receive medication therapy.19 Our healthcare systems can also refer individuals to community psychosocial treatment.
For patients with risk factors, prescribe empiric IV thiamine during hospitalization. Clinicians should then perform a risk-benefit assessment rather than reflexively prescribe vitamins to patients with AUD at discharge. We should also counsel patients to eat food when drinking to decrease alcohol-related harms.20 Patients experiencing food insecurity should be linked to food resources through inpatient nutritional and social work consultations.
Elicit patient preference around vitamin supplementation after discharge. For patients with AUD who desire supplementation without risk factors for malnutrition (Table), consider prescribing a single thiamine-containing vitamin for prevention of thiamine deficiency, which, unlike other vitamin deficiencies, has the potential to be irreversible and life-threatening. Though no evidence currently supports this practice, it stands to reason that prescribing a single tablet could decrease the number of pills for patients who struggle with pill burden.
RECOMMENDATIONS
- Offer evidence-based medication treatment for AUD.
- Connect patients experiencing food insecurity with appropriate resources.
- For patients initiated on a multivitamin, folate, and high-dose IV thiamine at admission, perform vitamin de-escalation during hospitalization.
- Risk-stratify hospitalized patients with AUD for additional risk factors for vitamin deficiencies (Table). In those with additional risk factors, offer supplementation if consistent with patient preference. Balance the benefits of vitamin supplementation with the risks of polypharmacy, particularly if the patient has conditions requiring multiple medications.
CONCLUSION
Returning to our case, the hospitalist initiates IV thiamine, folate, and a multivitamin at admission and assesses the patient’s nutritional status and food insecurity. The hospitalist deems the patient—who eats regular, balanced meals—to be at low risk for vitamin deficiencies. The medical team discontinues folate and multivitamins before discharge and continues IV thiamine throughout the 3-day hospitalization. The patient and clinician agree that unaddressed AUD played a key role in the patient’s heart failure exacerbation. The clinician elicits the patient’s goals around their alcohol use, discusses AUD treatment, and initiates naltrexone for AUD.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
1. Makdissi R, Stewart SH. Care for hospitalized patients with unhealthy alcohol use: a narrative review. Addict Sci Clin Pract. 2013;8(1):11. https://doi.org/10.1186/1940-0640-8-11
2. Lewis MJ. Alcoholism and nutrition: a review of vitamin supplementation and treatment. Curr Opin Clin Nutr Metab Care. 2020;23(2):138-144. https://doi.org/10.1097/mco.0000000000000622
3. Bergmans RS, Coughlin L, Wilson T, Malecki K. Cross-sectional associations of food insecurity with smoking cigarettes and heavy alcohol use in a population-based sample of adults. Drug Alcohol Depend. 2019;205:107646. https://doi.org/10.1016/j.drugalcdep.2019.107646
4. Latt N, Dore G. Thiamine in the treatment of Wernicke encephalopathy in patients with alcohol use disorders. Intern Med J. 2014;44(9):911-915. https://doi.org/10.1111/imj.12522
5. Flannery AH, Adkins DA, Cook AM. Unpeeling the evidence for the banana bag: evidence-based recommendations for the management of alcohol-associated vitamin and electrolyte deficiencies in the ICU. Crit Care Med. 2016;44(8):1545-1552. https://doi.org/10.1097/ccm.0000000000001659
6. Wai JM, Aloezos C, Mowrey WB, Baron SW, Cregin R, Forman HL. Using clinical decision support through the electronic medical record to increase prescribing of high-dose parenteral thiamine in hospitalized patients with alcohol use disorder. J Subst Abuse Treat. 2019;99:117-123. https://doi.org/10.1016/j.jsat.2019.01.017
7. American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. January 2020. https://www.asam.org/docs/default-source/quality-science/the_asam_clinical_practice_guideline_on_alcohol-1.pdf?sfvrsn=ba255c2_2
8. O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51(1):307-328. https://doi.org/10.1002/hep.23258
9. Breu AC, Theisen-Toupal J, Feldman LS. Serum and red blood cell folate testing on hospitalized patients. J Hosp Med. 2015;10(11):753-755. https://doi.org/10.1002/jhm.2385
10. Gautron M-A, Questel F, Lejoyeux M, Bellivier F, Vorspan F. Nutritional status during inpatient alcohol detoxification. Alcohol Alcohol. 2018;53(1):64-70. https://doi.org/10.1093/alcalc/agx086
11. Li SF, Jacob J, Feng J, Kulkarni M. Vitamin deficiencies in acutely intoxicated patients in the ED. Am J Emerg Med. 2008;26(7):792-795. https://doi.org/10.1016/j.ajem.2007.10.003
12. Ijaz S, Jackson J, Thorley H, et al. Nutritional deficiencies in homeless persons with problematic drinking: a systematic review. Int J Equity Health. 2017;16(1):71. https://doi.org/10.1186/s12939-017-0564-4
13. Day GS, Ladak S, Curley K, et al. Thiamine prescribing practices within university-affiliated hospitals: a multicenter retrospective review. J Hosp Med. 2015;10(4):246-253. https://doi.org/10.1002/jhm.2324
14. Day E, Bentham PW, Callaghan R, Kuruvilla T, George S. Thiamine for prevention and treatment of Wernicke-Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;2013(7):CD004033. https://doi.org/10.1002/14651858.CD004033.pub3
15. Medici V, Halsted CH. Folate, alcohol, and liver disease. Mol Nutr Food Res. 2013;57(4):596-606. https://doi.org/10.1002/mnfr.201200077
16. Ijaz S, Thorley H, Porter K, et al. Interventions for preventing or treating malnutrition in homeless problem-drinkers: a systematic review. Int J Equity Health. 2018;17(1):8. https://doi.org/10.1186/s12939-018-0722-3
17. Bryson CL, Au DH, Sun H, Williams EC, Kivlahan DR, Bradley KA. Alcohol screening scores and medication nonadherence. Ann Intern Med. 2008;149(11):795-803. https://doi.org/10.7326/0003-4819-149-11-200812020-00004
18. Picker D, Heard K, Bailey TC, Martin NR, LaRossa GN, Kollef MH. The number of discharge medications predicts thirty-day hospital readmission: a cohort study. BMC Health Serv Res. 2015;15:282. https://doi.org/10.1186/s12913-015-0950-9
19. Han B, Jones CM, Einstein EB, Powell PA, Compton WM. Use of medications for alcohol use disorder in the US: results From the 2019 National Survey on Drug Use and Health. JAMA Psychiatry. 2021;78(8):922–4. https://doi.org/10.1001/jamapsychiatry.2021.1271
20. Collins SE, Duncan MH, Saxon AJ, et al. Combining behavioral harm-reduction treatment and extended-release naltrexone for people experiencing homelessness and alcohol use disorder in the USA: a randomised clinical trial. Lancet Psychiatry. 2021;8(4):287-300. https://doi.org/10.1016/S2215-0366(20)30489-2
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old man with alcohol use disorder (AUD) is admitted with decompensated heart failure and experiences alcohol withdrawal during the hospitalization. He improves with guideline-directed heart failure therapy and benzodiazepines for alcohol withdrawal. Discharge medications are metoprolol succinate, lisinopril, furosemide, aspirin, atorvastatin, thiamine, folic acid, and a multivitamin. No medications are offered for AUD treatment. At follow-up a week later, he presents with dyspnea and reports poor medication adherence and a return to heavy drinking.
WHY YOU MIGHT THINK IT IS HELPFUL TO PRESCRIBE VITAMIN SUPPLEMENTATION TO PATIENTS WITH AUD AT HOSPITAL DISCHARGE
AUD is common among hospitalized patients.1 AUD increases the risk of vitamin deficiencies due to the toxic effects of alcohol on the gastrointestinal tract and liver, causing impaired digestion, reduced absorption, and increased degradation of key micronutrients.2,3 Other risk factors for AUD-associated vitamin deficiencies include food insecurity and the replacement of nutrient-rich food with alcohol. Since the body does not readily store water-soluble vitamins, including thiamine (vitamin B1) and folate (vitamin B9), people require regular dietary replenishment of these nutrients. Thus, if individuals with AUD eat less fortified food, they risk developing thiamine, folate, niacin, and other vitamin deficiencies. Since AUD puts patients at risk for vitamin deficiencies, hospitalized patients typically receive vitamin supplementation, including thiamine, folic acid, and a multivitamin (most formulations contain water-soluble vitamins B and C and micronutrients).1 Hospitalists often continue these medications at discharge.
Thiamine deficiency may manifest as Wernicke encephalopathy (WE), peripheral neuropathy, or a high-output heart failure state.
Hospitalists empirically treat with thiamine, folate, and other vitamins upon hospital admission with the intent of reducing morbidity associated with nutritional deficiencies.1 Repletion poses few risks to patients since the kidneys eliminate water-soluble vitamins. Multivitamins also have a low potential for direct harm and a low cost. Given the consequences of missing a deficiency, alcohol withdrawal–management order sets commonly embed vitamin repletion orders.6
WHY ROUTINELY PRESCRIBING VITAMIN SUPPLEMENTATION AT HOSPITAL DISCHARGE IN PATIENTS WITH AUD IS A TWDFNR
Hospitalists often reflexively continue vitamin supplementation on discharge. Unfortunately, there is no evidence that prescribing vitamin supplementation leads to clinically significant improvements for people with AUD, and patients can experience harms.
Literature and specialty guidelines lack consensus on rational vitamin supplementation in patients with AUD.2,7,8 Folate testing is not recommended due to inaccuracies.9 In fact, clinical data, such as body mass index, more accurately predict alcohol-related cognitive impairment than blood levels of vitamins.10 In one small study of vitamin deficiencies among patients with acute alcohol intoxication, none had low B12 or folate levels.11 A systematic review among people experiencing homelessness with unhealthy alcohol use showed no clear pattern of vitamin deficiencies across studies, although vitamin C and thiamine deficiencies predominated.12
In the absence of reliable thiamine and folate testing to confirm deficiencies, clinicians must use their clinical assessment skills. Clinicians rarely evaluate patients with AUD for vitamin deficiency risk factors and instead reflexively prescribe vitamin supplementation. An AUD diagnosis may serve as a sensitive, but not specific, risk factor for those in need of vitamin supplementation.
Other limitations make prescribing oral vitamins reflexively at discharge a low-value practice. Thiamine, often prescribed orally in the hospital and on discharge, has poor oral bioavailability.13 Unfortunately, people with AUD have decreased and variable thiamine absorption. To prevent WE, thiamine must cross the blood-brain barrier, and the literature provides insufficient evidence to guide clinicians on an appropriate oral thiamine dose, frequency, or duration of treatment.14 While early high-dose IV thiamine may treat or prevent WE during hospitalization, low-dose oral thiamine may not provide benefit to patients with AUD.5
The literature also provides sparse evidence for folate supplementation and its optimal dose. Since 1998, when the United States mandated fortifying grain products with folic acid, people rarely have low serum folate levels. Though patients with AUD have lower folate levels relative to the general population,15 this difference does not seem clinically significant. While limited data show an association between oral multivitamin supplementation and improved serum nutrient levels among people with AUD, we lack evidence on clinical outcomes.16
Most importantly, for a practice lacking strong evidence, prescribing multiple vitamins at discharge may result in harm from polypharmacy and unnecessary costs for the recently hospitalized patient. Alcohol use is associated with decreased adherence to medications for chronic conditions,17 including HIV, hypertension, hyperlipidemia, and psychiatric diseases. In addition, research shows an association between an increased number of discharge medications and higher risk for hospital readmission. The harm may actually correlate with the number of medications and complexity of the regimen rather than the risk profile of the medications themselves.18 Providers underestimate the impact of adding multiple vitamins at discharge, especially for patients who have several co-occurring medical conditions that require other medications. Furthermore, insurance rarely covers vitamins, leading hospitals or patients to incur the costs at discharge.
WHEN TO CONSIDER VITAMIN SUPPLEMENTATION AT DISCHARGE FOR PATIENTS WITH AUD
When treating patients with AUD, consider the potential benefit of vitamin supplementation for the individual. If a patient with regular, heavy alcohol use is at high risk of vitamin deficiencies due to ongoing risk factors (Table), hospitalists should discuss vitamin therapy via a patient-centered risk-benefit process.

When considering discharge vitamins, make concurrent efforts to enhance patient nutrition via decreased alcohol consumption and improved healthy food intake. While some patients do not have a goal of abstaining from alcohol, providing resources to food access may help decrease the harms of drinking. Education may help patients learn that vitamin deficiencies can result from heavy alcohol use.
Multivitamin formulations have variable doses of vitamins but can contain 100% or more of the daily value of thiamine and folic acid. For patients with AUD at lower risk of vitamin deficiencies (ie, mild alcohol use disorder with a healthy diet), discuss risks and benefits of supplementation. If they desire supplementation, a single thiamine-containing vitamin alone may be highest yield since it is the most morbid vitamin deficiency. Conversely, a patient with heavy alcohol intake and other risk factors for malnutrition may benefit from a higher dose of supplementation, achieved by prescribing a multivitamin alongside additional doses of thiamine and folate. However, the literature lacks evidence to guide clinicians on optimal vitamin dosing and formulations.
WHAT WE SHOULD DO INSTEAD
Instead of reflexively prescribing thiamine, folate, and multivitamin, clinicians can assess patients for AUD, provide motivational interviewing, and offer AUD treatment. Hospitalists should initiate and prescribe evidence-based medications for AUD for patients interested in reducing or stopping their alcohol intake. We can choose from Food and Drug Administration–approved AUD medications, including naltrexone and acamprosate. Unfortunately, less than 3% of patients with AUD receive medication therapy.19 Our healthcare systems can also refer individuals to community psychosocial treatment.
For patients with risk factors, prescribe empiric IV thiamine during hospitalization. Clinicians should then perform a risk-benefit assessment rather than reflexively prescribe vitamins to patients with AUD at discharge. We should also counsel patients to eat food when drinking to decrease alcohol-related harms.20 Patients experiencing food insecurity should be linked to food resources through inpatient nutritional and social work consultations.
Elicit patient preference around vitamin supplementation after discharge. For patients with AUD who desire supplementation without risk factors for malnutrition (Table), consider prescribing a single thiamine-containing vitamin for prevention of thiamine deficiency, which, unlike other vitamin deficiencies, has the potential to be irreversible and life-threatening. Though no evidence currently supports this practice, it stands to reason that prescribing a single tablet could decrease the number of pills for patients who struggle with pill burden.
RECOMMENDATIONS
- Offer evidence-based medication treatment for AUD.
- Connect patients experiencing food insecurity with appropriate resources.
- For patients initiated on a multivitamin, folate, and high-dose IV thiamine at admission, perform vitamin de-escalation during hospitalization.
- Risk-stratify hospitalized patients with AUD for additional risk factors for vitamin deficiencies (Table). In those with additional risk factors, offer supplementation if consistent with patient preference. Balance the benefits of vitamin supplementation with the risks of polypharmacy, particularly if the patient has conditions requiring multiple medications.
CONCLUSION
Returning to our case, the hospitalist initiates IV thiamine, folate, and a multivitamin at admission and assesses the patient’s nutritional status and food insecurity. The hospitalist deems the patient—who eats regular, balanced meals—to be at low risk for vitamin deficiencies. The medical team discontinues folate and multivitamins before discharge and continues IV thiamine throughout the 3-day hospitalization. The patient and clinician agree that unaddressed AUD played a key role in the patient’s heart failure exacerbation. The clinician elicits the patient’s goals around their alcohol use, discusses AUD treatment, and initiates naltrexone for AUD.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old man with alcohol use disorder (AUD) is admitted with decompensated heart failure and experiences alcohol withdrawal during the hospitalization. He improves with guideline-directed heart failure therapy and benzodiazepines for alcohol withdrawal. Discharge medications are metoprolol succinate, lisinopril, furosemide, aspirin, atorvastatin, thiamine, folic acid, and a multivitamin. No medications are offered for AUD treatment. At follow-up a week later, he presents with dyspnea and reports poor medication adherence and a return to heavy drinking.
WHY YOU MIGHT THINK IT IS HELPFUL TO PRESCRIBE VITAMIN SUPPLEMENTATION TO PATIENTS WITH AUD AT HOSPITAL DISCHARGE
AUD is common among hospitalized patients.1 AUD increases the risk of vitamin deficiencies due to the toxic effects of alcohol on the gastrointestinal tract and liver, causing impaired digestion, reduced absorption, and increased degradation of key micronutrients.2,3 Other risk factors for AUD-associated vitamin deficiencies include food insecurity and the replacement of nutrient-rich food with alcohol. Since the body does not readily store water-soluble vitamins, including thiamine (vitamin B1) and folate (vitamin B9), people require regular dietary replenishment of these nutrients. Thus, if individuals with AUD eat less fortified food, they risk developing thiamine, folate, niacin, and other vitamin deficiencies. Since AUD puts patients at risk for vitamin deficiencies, hospitalized patients typically receive vitamin supplementation, including thiamine, folic acid, and a multivitamin (most formulations contain water-soluble vitamins B and C and micronutrients).1 Hospitalists often continue these medications at discharge.
Thiamine deficiency may manifest as Wernicke encephalopathy (WE), peripheral neuropathy, or a high-output heart failure state.
Hospitalists empirically treat with thiamine, folate, and other vitamins upon hospital admission with the intent of reducing morbidity associated with nutritional deficiencies.1 Repletion poses few risks to patients since the kidneys eliminate water-soluble vitamins. Multivitamins also have a low potential for direct harm and a low cost. Given the consequences of missing a deficiency, alcohol withdrawal–management order sets commonly embed vitamin repletion orders.6
WHY ROUTINELY PRESCRIBING VITAMIN SUPPLEMENTATION AT HOSPITAL DISCHARGE IN PATIENTS WITH AUD IS A TWDFNR
Hospitalists often reflexively continue vitamin supplementation on discharge. Unfortunately, there is no evidence that prescribing vitamin supplementation leads to clinically significant improvements for people with AUD, and patients can experience harms.
Literature and specialty guidelines lack consensus on rational vitamin supplementation in patients with AUD.2,7,8 Folate testing is not recommended due to inaccuracies.9 In fact, clinical data, such as body mass index, more accurately predict alcohol-related cognitive impairment than blood levels of vitamins.10 In one small study of vitamin deficiencies among patients with acute alcohol intoxication, none had low B12 or folate levels.11 A systematic review among people experiencing homelessness with unhealthy alcohol use showed no clear pattern of vitamin deficiencies across studies, although vitamin C and thiamine deficiencies predominated.12
In the absence of reliable thiamine and folate testing to confirm deficiencies, clinicians must use their clinical assessment skills. Clinicians rarely evaluate patients with AUD for vitamin deficiency risk factors and instead reflexively prescribe vitamin supplementation. An AUD diagnosis may serve as a sensitive, but not specific, risk factor for those in need of vitamin supplementation.
Other limitations make prescribing oral vitamins reflexively at discharge a low-value practice. Thiamine, often prescribed orally in the hospital and on discharge, has poor oral bioavailability.13 Unfortunately, people with AUD have decreased and variable thiamine absorption. To prevent WE, thiamine must cross the blood-brain barrier, and the literature provides insufficient evidence to guide clinicians on an appropriate oral thiamine dose, frequency, or duration of treatment.14 While early high-dose IV thiamine may treat or prevent WE during hospitalization, low-dose oral thiamine may not provide benefit to patients with AUD.5
The literature also provides sparse evidence for folate supplementation and its optimal dose. Since 1998, when the United States mandated fortifying grain products with folic acid, people rarely have low serum folate levels. Though patients with AUD have lower folate levels relative to the general population,15 this difference does not seem clinically significant. While limited data show an association between oral multivitamin supplementation and improved serum nutrient levels among people with AUD, we lack evidence on clinical outcomes.16
Most importantly, for a practice lacking strong evidence, prescribing multiple vitamins at discharge may result in harm from polypharmacy and unnecessary costs for the recently hospitalized patient. Alcohol use is associated with decreased adherence to medications for chronic conditions,17 including HIV, hypertension, hyperlipidemia, and psychiatric diseases. In addition, research shows an association between an increased number of discharge medications and higher risk for hospital readmission. The harm may actually correlate with the number of medications and complexity of the regimen rather than the risk profile of the medications themselves.18 Providers underestimate the impact of adding multiple vitamins at discharge, especially for patients who have several co-occurring medical conditions that require other medications. Furthermore, insurance rarely covers vitamins, leading hospitals or patients to incur the costs at discharge.
WHEN TO CONSIDER VITAMIN SUPPLEMENTATION AT DISCHARGE FOR PATIENTS WITH AUD
When treating patients with AUD, consider the potential benefit of vitamin supplementation for the individual. If a patient with regular, heavy alcohol use is at high risk of vitamin deficiencies due to ongoing risk factors (Table), hospitalists should discuss vitamin therapy via a patient-centered risk-benefit process.

When considering discharge vitamins, make concurrent efforts to enhance patient nutrition via decreased alcohol consumption and improved healthy food intake. While some patients do not have a goal of abstaining from alcohol, providing resources to food access may help decrease the harms of drinking. Education may help patients learn that vitamin deficiencies can result from heavy alcohol use.
Multivitamin formulations have variable doses of vitamins but can contain 100% or more of the daily value of thiamine and folic acid. For patients with AUD at lower risk of vitamin deficiencies (ie, mild alcohol use disorder with a healthy diet), discuss risks and benefits of supplementation. If they desire supplementation, a single thiamine-containing vitamin alone may be highest yield since it is the most morbid vitamin deficiency. Conversely, a patient with heavy alcohol intake and other risk factors for malnutrition may benefit from a higher dose of supplementation, achieved by prescribing a multivitamin alongside additional doses of thiamine and folate. However, the literature lacks evidence to guide clinicians on optimal vitamin dosing and formulations.
WHAT WE SHOULD DO INSTEAD
Instead of reflexively prescribing thiamine, folate, and multivitamin, clinicians can assess patients for AUD, provide motivational interviewing, and offer AUD treatment. Hospitalists should initiate and prescribe evidence-based medications for AUD for patients interested in reducing or stopping their alcohol intake. We can choose from Food and Drug Administration–approved AUD medications, including naltrexone and acamprosate. Unfortunately, less than 3% of patients with AUD receive medication therapy.19 Our healthcare systems can also refer individuals to community psychosocial treatment.
For patients with risk factors, prescribe empiric IV thiamine during hospitalization. Clinicians should then perform a risk-benefit assessment rather than reflexively prescribe vitamins to patients with AUD at discharge. We should also counsel patients to eat food when drinking to decrease alcohol-related harms.20 Patients experiencing food insecurity should be linked to food resources through inpatient nutritional and social work consultations.
Elicit patient preference around vitamin supplementation after discharge. For patients with AUD who desire supplementation without risk factors for malnutrition (Table), consider prescribing a single thiamine-containing vitamin for prevention of thiamine deficiency, which, unlike other vitamin deficiencies, has the potential to be irreversible and life-threatening. Though no evidence currently supports this practice, it stands to reason that prescribing a single tablet could decrease the number of pills for patients who struggle with pill burden.
RECOMMENDATIONS
- Offer evidence-based medication treatment for AUD.
- Connect patients experiencing food insecurity with appropriate resources.
- For patients initiated on a multivitamin, folate, and high-dose IV thiamine at admission, perform vitamin de-escalation during hospitalization.
- Risk-stratify hospitalized patients with AUD for additional risk factors for vitamin deficiencies (Table). In those with additional risk factors, offer supplementation if consistent with patient preference. Balance the benefits of vitamin supplementation with the risks of polypharmacy, particularly if the patient has conditions requiring multiple medications.
CONCLUSION
Returning to our case, the hospitalist initiates IV thiamine, folate, and a multivitamin at admission and assesses the patient’s nutritional status and food insecurity. The hospitalist deems the patient—who eats regular, balanced meals—to be at low risk for vitamin deficiencies. The medical team discontinues folate and multivitamins before discharge and continues IV thiamine throughout the 3-day hospitalization. The patient and clinician agree that unaddressed AUD played a key role in the patient’s heart failure exacerbation. The clinician elicits the patient’s goals around their alcohol use, discusses AUD treatment, and initiates naltrexone for AUD.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
1. Makdissi R, Stewart SH. Care for hospitalized patients with unhealthy alcohol use: a narrative review. Addict Sci Clin Pract. 2013;8(1):11. https://doi.org/10.1186/1940-0640-8-11
2. Lewis MJ. Alcoholism and nutrition: a review of vitamin supplementation and treatment. Curr Opin Clin Nutr Metab Care. 2020;23(2):138-144. https://doi.org/10.1097/mco.0000000000000622
3. Bergmans RS, Coughlin L, Wilson T, Malecki K. Cross-sectional associations of food insecurity with smoking cigarettes and heavy alcohol use in a population-based sample of adults. Drug Alcohol Depend. 2019;205:107646. https://doi.org/10.1016/j.drugalcdep.2019.107646
4. Latt N, Dore G. Thiamine in the treatment of Wernicke encephalopathy in patients with alcohol use disorders. Intern Med J. 2014;44(9):911-915. https://doi.org/10.1111/imj.12522
5. Flannery AH, Adkins DA, Cook AM. Unpeeling the evidence for the banana bag: evidence-based recommendations for the management of alcohol-associated vitamin and electrolyte deficiencies in the ICU. Crit Care Med. 2016;44(8):1545-1552. https://doi.org/10.1097/ccm.0000000000001659
6. Wai JM, Aloezos C, Mowrey WB, Baron SW, Cregin R, Forman HL. Using clinical decision support through the electronic medical record to increase prescribing of high-dose parenteral thiamine in hospitalized patients with alcohol use disorder. J Subst Abuse Treat. 2019;99:117-123. https://doi.org/10.1016/j.jsat.2019.01.017
7. American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. January 2020. https://www.asam.org/docs/default-source/quality-science/the_asam_clinical_practice_guideline_on_alcohol-1.pdf?sfvrsn=ba255c2_2
8. O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51(1):307-328. https://doi.org/10.1002/hep.23258
9. Breu AC, Theisen-Toupal J, Feldman LS. Serum and red blood cell folate testing on hospitalized patients. J Hosp Med. 2015;10(11):753-755. https://doi.org/10.1002/jhm.2385
10. Gautron M-A, Questel F, Lejoyeux M, Bellivier F, Vorspan F. Nutritional status during inpatient alcohol detoxification. Alcohol Alcohol. 2018;53(1):64-70. https://doi.org/10.1093/alcalc/agx086
11. Li SF, Jacob J, Feng J, Kulkarni M. Vitamin deficiencies in acutely intoxicated patients in the ED. Am J Emerg Med. 2008;26(7):792-795. https://doi.org/10.1016/j.ajem.2007.10.003
12. Ijaz S, Jackson J, Thorley H, et al. Nutritional deficiencies in homeless persons with problematic drinking: a systematic review. Int J Equity Health. 2017;16(1):71. https://doi.org/10.1186/s12939-017-0564-4
13. Day GS, Ladak S, Curley K, et al. Thiamine prescribing practices within university-affiliated hospitals: a multicenter retrospective review. J Hosp Med. 2015;10(4):246-253. https://doi.org/10.1002/jhm.2324
14. Day E, Bentham PW, Callaghan R, Kuruvilla T, George S. Thiamine for prevention and treatment of Wernicke-Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;2013(7):CD004033. https://doi.org/10.1002/14651858.CD004033.pub3
15. Medici V, Halsted CH. Folate, alcohol, and liver disease. Mol Nutr Food Res. 2013;57(4):596-606. https://doi.org/10.1002/mnfr.201200077
16. Ijaz S, Thorley H, Porter K, et al. Interventions for preventing or treating malnutrition in homeless problem-drinkers: a systematic review. Int J Equity Health. 2018;17(1):8. https://doi.org/10.1186/s12939-018-0722-3
17. Bryson CL, Au DH, Sun H, Williams EC, Kivlahan DR, Bradley KA. Alcohol screening scores and medication nonadherence. Ann Intern Med. 2008;149(11):795-803. https://doi.org/10.7326/0003-4819-149-11-200812020-00004
18. Picker D, Heard K, Bailey TC, Martin NR, LaRossa GN, Kollef MH. The number of discharge medications predicts thirty-day hospital readmission: a cohort study. BMC Health Serv Res. 2015;15:282. https://doi.org/10.1186/s12913-015-0950-9
19. Han B, Jones CM, Einstein EB, Powell PA, Compton WM. Use of medications for alcohol use disorder in the US: results From the 2019 National Survey on Drug Use and Health. JAMA Psychiatry. 2021;78(8):922–4. https://doi.org/10.1001/jamapsychiatry.2021.1271
20. Collins SE, Duncan MH, Saxon AJ, et al. Combining behavioral harm-reduction treatment and extended-release naltrexone for people experiencing homelessness and alcohol use disorder in the USA: a randomised clinical trial. Lancet Psychiatry. 2021;8(4):287-300. https://doi.org/10.1016/S2215-0366(20)30489-2
1. Makdissi R, Stewart SH. Care for hospitalized patients with unhealthy alcohol use: a narrative review. Addict Sci Clin Pract. 2013;8(1):11. https://doi.org/10.1186/1940-0640-8-11
2. Lewis MJ. Alcoholism and nutrition: a review of vitamin supplementation and treatment. Curr Opin Clin Nutr Metab Care. 2020;23(2):138-144. https://doi.org/10.1097/mco.0000000000000622
3. Bergmans RS, Coughlin L, Wilson T, Malecki K. Cross-sectional associations of food insecurity with smoking cigarettes and heavy alcohol use in a population-based sample of adults. Drug Alcohol Depend. 2019;205:107646. https://doi.org/10.1016/j.drugalcdep.2019.107646
4. Latt N, Dore G. Thiamine in the treatment of Wernicke encephalopathy in patients with alcohol use disorders. Intern Med J. 2014;44(9):911-915. https://doi.org/10.1111/imj.12522
5. Flannery AH, Adkins DA, Cook AM. Unpeeling the evidence for the banana bag: evidence-based recommendations for the management of alcohol-associated vitamin and electrolyte deficiencies in the ICU. Crit Care Med. 2016;44(8):1545-1552. https://doi.org/10.1097/ccm.0000000000001659
6. Wai JM, Aloezos C, Mowrey WB, Baron SW, Cregin R, Forman HL. Using clinical decision support through the electronic medical record to increase prescribing of high-dose parenteral thiamine in hospitalized patients with alcohol use disorder. J Subst Abuse Treat. 2019;99:117-123. https://doi.org/10.1016/j.jsat.2019.01.017
7. American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. January 2020. https://www.asam.org/docs/default-source/quality-science/the_asam_clinical_practice_guideline_on_alcohol-1.pdf?sfvrsn=ba255c2_2
8. O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51(1):307-328. https://doi.org/10.1002/hep.23258
9. Breu AC, Theisen-Toupal J, Feldman LS. Serum and red blood cell folate testing on hospitalized patients. J Hosp Med. 2015;10(11):753-755. https://doi.org/10.1002/jhm.2385
10. Gautron M-A, Questel F, Lejoyeux M, Bellivier F, Vorspan F. Nutritional status during inpatient alcohol detoxification. Alcohol Alcohol. 2018;53(1):64-70. https://doi.org/10.1093/alcalc/agx086
11. Li SF, Jacob J, Feng J, Kulkarni M. Vitamin deficiencies in acutely intoxicated patients in the ED. Am J Emerg Med. 2008;26(7):792-795. https://doi.org/10.1016/j.ajem.2007.10.003
12. Ijaz S, Jackson J, Thorley H, et al. Nutritional deficiencies in homeless persons with problematic drinking: a systematic review. Int J Equity Health. 2017;16(1):71. https://doi.org/10.1186/s12939-017-0564-4
13. Day GS, Ladak S, Curley K, et al. Thiamine prescribing practices within university-affiliated hospitals: a multicenter retrospective review. J Hosp Med. 2015;10(4):246-253. https://doi.org/10.1002/jhm.2324
14. Day E, Bentham PW, Callaghan R, Kuruvilla T, George S. Thiamine for prevention and treatment of Wernicke-Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;2013(7):CD004033. https://doi.org/10.1002/14651858.CD004033.pub3
15. Medici V, Halsted CH. Folate, alcohol, and liver disease. Mol Nutr Food Res. 2013;57(4):596-606. https://doi.org/10.1002/mnfr.201200077
16. Ijaz S, Thorley H, Porter K, et al. Interventions for preventing or treating malnutrition in homeless problem-drinkers: a systematic review. Int J Equity Health. 2018;17(1):8. https://doi.org/10.1186/s12939-018-0722-3
17. Bryson CL, Au DH, Sun H, Williams EC, Kivlahan DR, Bradley KA. Alcohol screening scores and medication nonadherence. Ann Intern Med. 2008;149(11):795-803. https://doi.org/10.7326/0003-4819-149-11-200812020-00004
18. Picker D, Heard K, Bailey TC, Martin NR, LaRossa GN, Kollef MH. The number of discharge medications predicts thirty-day hospital readmission: a cohort study. BMC Health Serv Res. 2015;15:282. https://doi.org/10.1186/s12913-015-0950-9
19. Han B, Jones CM, Einstein EB, Powell PA, Compton WM. Use of medications for alcohol use disorder in the US: results From the 2019 National Survey on Drug Use and Health. JAMA Psychiatry. 2021;78(8):922–4. https://doi.org/10.1001/jamapsychiatry.2021.1271
20. Collins SE, Duncan MH, Saxon AJ, et al. Combining behavioral harm-reduction treatment and extended-release naltrexone for people experiencing homelessness and alcohol use disorder in the USA: a randomised clinical trial. Lancet Psychiatry. 2021;8(4):287-300. https://doi.org/10.1016/S2215-0366(20)30489-2
© 2021 Society of Hospital Medicine
Things We Do for No Reason:™ Prescribing Tramadol for Inpatients in Pain
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits an 80-year-old man for a chronic obstructive pulmonary disease exacerbation. The patient’s history is significant for chronic right knee pain. While hospitalized, the patient reports worsening of his knee pain. Radiographs of the right knee show severe osteoarthritic changes. Since acetaminophen does not relieve the patient’s pain, the hospitalist orders tramadol as needed.
BACKGROUND
Hospitalists, who commonly evaluate and treat acute and chronic pain in the inpatient setting, have a wide selection of interventions from which to choose, including tramadol. Tramadol hydrochloride is a synthetic, central-acting analgesic with multiple mechanisms of action. It is a serotonin-norepinephrine reuptake inhibitor (SNRI) with a structure similar to venlafaxine and produces antineuropathic analgesic effects.1 Tramadol and its primary active metabolite O-desmethyltramadol (also known as the M1 metabolite) mediate its effects by binding at the mu-opioid receptor.2 Phase I metabolism in the liver by cytochrome P450 isoenzyme 2D6 (CYP2D6) facilitates conversion of tramadol to M1 (Figure). Importantly, genetic polymorphisms in CYP2D6 result in individual variations in gene expression, which impacts the metabolism of tramadol.2

Although tramadol is available over the counter in some countries, in the United States it is a Schedule IV controlled substance. Tramadol consistently ranks among the top 50 prescribed medications in the United States.3
WHY YOU MIGHT THINK PRESCRIBING TRAMADOL FOR PAIN MAY BE HELPFUL
Given the growing concerns regarding the use of opioids, the pharmaceutical industry has marketed tramadol as a safer opioid option for pain management. Tramadol binds at the mu-opioid receptor with an affinity that is less than 4000-fold that of morphine; the binding potency of M1, the metabolite of tramadol, is less than 5-fold that of morphine.4 Due to its lower binding affinity at the mu-opioid receptor, tramadol is considered a weak opioid, one believed to have minimal withdrawal symptoms and a lower potential for overdose or misuse compared to other opioids.1,5 Based on this characterization, many clinicians prescribe tramadol for elderly patients or patients otherwise at risk for medication misuse or adverse effects of opioids.6 In addition, hospitalized patients often have contraindications to nonopioid medications (eg, acetaminophen, nonsteroidal anti-inflammatory drugs [NSAIDs]), limiting their options for pain management.
WHY PRESCRIBING TRAMADOL FOR PAIN SHOULD BE AVOIDED
Despite being marketed as an effective and safe medication, tramadol has an unpredictable metabolism, complex pharmacology, and drug-drug interactions that can cause significant adverse effects. Similar to other opioids, tramadol is associated with a risk of misuse, physiologic dependency, and overdose. In addition, tramadol has a black box warning for addiction, misuse, respiratory depression, ultra-rapid metabolism, neonatal opioid withdrawal syndrome, CYP450 drug interactions, and interactions with other central nervous system depressants.
While tramadol has multiple mechanisms of action, the literature lacks high-quality evidence (eg, large randomized controlled trials) supporting its use, especially in hospitalized medical patients. A recent retrospective study of tramadol looked at the diagnoses of 250 hospitalized patients who received tramadol for pain management. While this study did not examine efficacy, it found mild-to-moderate acute noncancer pain to be the primary reason for prescribing tramadol.7 This study also showed the risk of severe drug-drug interactions increased the longer patients were on tramadol.7
As a result of the limited evidence in hospitalized patients, hospitalists must rely on outpatient studies.8-10 The size and quality of these studies, especially given the magnitude of tramadol prescribing in the United States, make them less useful. A series of Cochrane reviews examining the beneficial effects of tramadol for neuropathic pain, osteoarthritis, and cancer pain show insufficient evidence for tramadol when compared to placebo or active controls such as acetaminophen, NSAIDs, or other opioids.8-10
The side-effect profile of tramadol outweighs its mild analgesic effects. The 2019 American Geriatric Society Beers criteria for potentially inappropriate medication use in older adults strongly recommends clinicians use caution when prescribing tramadol to older adult patients, as tramadol may worsen or cause hyponatremia.11 In one large, population-based study, the use of tramadol doubled patients’ risk of hospitalization for hyponatremia when compared to codeine, though the incidence remains rather low at 4.6 per 10,000 person-months.12 Studies have also demonstrated an increased risk of hospitalization for hypoglycemia in nondiabetic patients receiving tramadol.13 A large propensity-score matched cohort study of patients with osteoarthritis found tramadol to have an associated higher all-cause mortality compared to NSAIDs; however, these differences may be due to confounding variables.1 In addition to hyponatremia and all-cause mortality, patients taking tramadol also have an associated increased risk of falls and hip fractures when compared to codeine or NSAIDs.14
The increased serotonergic activity associated with tramadol can lead to serotonin syndrome (serotonin toxicity), a rare but serious condition. Although serotonin syndrome can develop in patients taking tramadol as a monotherapy, the risk for this toxidrome increases when tramadol is taken in combination with other serotonergic agents or agents that inhibit metabolism of tramadol at CYP2D6.5 Seizures may also occur with tramadol at therapeutic and supratherapeutic doses. Population-based studies estimate seizures occur in 0.15% to 0.86% of patients receiving tramadol, which is two to six times the risk of those not on tramadol.5 Patients concurrently taking tramadol with a tricyclic antidepressant (TCA) or selective serotonin reuptake inhibitor (SSRI) are estimated to have seizures five to nine times more often than patients not taking a TCA or SSRI.5 Risk factors for tramadol-induced seizure include tramadol misuse or overdose, tramadol doses >1000 mg daily (maximum recommended dose is 400 mg/day), chronic tramadol use, concurrent use of a serotonergic agent or medications that inhibit CYP2D6, and history of epilepsy, renal disease, stroke, or traumatic brain injury.5
Differences in the genetic polymorphisms of CYP2D6 can produce a range of CYP2D6 activity from “poor metabolizers” (little-to-no analgesic effect) to “ultra-rapid metabolizers” (enhanced analgesia and increased risk of adverse effects), leading to unpredictable pharmacodynamic effects of tramadol.2 In North Africa and the Arabian peninsula, more than 25% of the population rapidly metabolizes tramadol; these pharmacogenomic effects result in higher rates of tramadol addiction and overdose in these regions.5 An estimated 7% to 10% of Caucasians slowly metabolize tramadol, which may place them at risk of adverse effects from tramadol in addition to inadequate analgesia.15 In contrast, Ethiopian populations have the highest rate of ultra-rapid tramadol metabolism at 29%.15
Drugs that induce CYP2D6 (eg, dexamethasone, rifampin) or inhibit CYP2D6 (eg, bupropion, fluoxetine) also impact tramadol efficacy, pharmacokinetics, and pharmacodynamics.16,17 Patients taking strong CYP2D6 inhibitors require significantly higher doses of tramadol to achieve analgesic effects.17 Tramadol undergoes extensive hepatic metabolism, producing several active metabolites, including M1 (Figure). Hepatic impairment increases the elimination half-life of tramadol and its metabolites.18 The majority of tramadol and its metabolites are eliminated through the kidneys. Accumulation of tramadol and its metabolites may occur in patients with renal impairment, placing them at increased risk of adverse effects.2
Finally, although some clinicians assume that tramadol has lower rates of misuse, diversion, or overdose compared to other opioids, rates of nonprescription use have increased with its proliferation.19,20 The US Substance Abuse and Mental Health Services Administration estimates that 1,287,000 persons misused tramadol in 2019.21 Patients may exhibit symptoms of physiologic opioid dependence and withdrawal from chronic tramadol use.2,22 In one study, patients prescribed tramadol monotherapy for acute pain from elective surgery had an increased risk for prolonged opioid use compared to patients prescribed other short-acting opioids.22
WHAT YOU SHOULD DO INSTEAD
Clinicians should determine the nature of the patient’s pain by obtaining a complete medical history, performing a thorough physical examination, and ordering diagnostic tests and imaging studies, as necessary. After consulting with the patient’s primary care physician, the clinician should employ a multimodal approach to pain that includes topical agents, psychotherapy, injections or interventions, and nonopioid medications. Patients with neuropathic pain may benefit from adjuvant analgesics such as gabapentinoids, TCAs, or SNRIs. In patients with evidence-based indications for opioid therapy (eg, pancreatitis, cancer pain, postsurgical pain), the hospitalist should assess the risk for opioid misuse and discuss risks and benefits with the patient before considering a time-limited trial of opioid therapy. If available and when indicated, clinicians should consult with specialists in pain management or palliative care. For cases wherein clinicians have already prescribed tramadol to the patient, they should discuss deprescribing strategies and alternative analgesic options with the patient and the patient’s primary care physician. Finally, before initiating tramadol therapy for hospitalized patients with pain, hospitalists should consider the risks, benefits, and alternative approaches to prescribing tramadol.
RECOMMENDATIONS
- For hospitalized patients reporting pain, complete a pain assessment by history, physical exam, chart review, and diagnostic studies to examine the etiology of the pain.
- Utilize multiple modalities for pain control when possible, including acetaminophen, NSAIDs, topical agents, ice or heat, neuropathic pain medications, and interventions such as injections, psychotherapy, or radiation, if indicated.
- Avoid prescribing tramadol due to unpredictable pharmacodynamics, adverse effects, and lack of quality evidence for efficacy in hospitalized medical patients.
CONCLUSION
Tramadol is a commonly used opioid medication associated with adverse effects and unpredictable analgesia. Regarding this case scenario, the use of tramadol in this patient places him at risk for drug-drug interactions, hyponatremia, hypoglycemia, serotonin syndrome, seizures, and pronounced side effects of opioid medications. Moderate quality evidence in the outpatient setting suggests that tramadol is unlikely to provide significant analgesia for his osteoarthritic pain.9 Instead of prescribing tramadol, the hospitalist should consider alternative treatments for this patient’s pain, such as intraarticular glucocorticoids, a short course of oral NSAIDs (unless contraindicated), topical treatments (eg, menthol, capsaicin, NSAIDs), physical therapy, and close follow-up with an orthopedist after hospital discharge. Further randomized controlled studies of tramadol vs active controls are needed.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
1. Zeng C, Dubreuil M, LaRochelle MR, et al. Association of tramadol with all-cause mortality among patients with osteoarthritis. JAMA. 2019;321(10):969-982. https://doi.org/10.1001/jama.2019.1347
2. Gong L, Stamer UM, Tzvetkov MV, Altman RB, Klein TE. PharmGKB summary: tramadol pathway. Pharmacogenet Genomics. 2014;24(7):374-380. https://doi.org/10.1097/FPC.0000000000000057
3. The top 200 drugs of 2019. ClinCalc DrugStats Database. Accessed June 10, 2021. https://clincalc.com/DrugStats
4. Gillen C, Haurand M, Kobelt DJ, Wnendt S. Affinity, potency and efficacy of tramadol and its metabolites at the cloned human µ-opioid receptor. Naunyn Schmiedebergs Arch Pharmacol. 2000;362(2):116-121. https://doi.org/10.1007/s002100000266
5. Hassamal S, Miotto K, Dale W, Danovitch I. Tramadol: understanding the risk of serotonin syndrome and seizures. Am J Med. 2018;131(11):1382.e1-1382.e6. https://doi.org/10.1016/j.amjmed.2018.04.025
6. Shipton EA. Tramadol—present and future. Anaesth Intensive Care. 2000;28(4):363-374. https://doi.org/10.1177/0310057X0002800403
7. Mohan N, Edmonds KP, Ajayi TA, Atayee RS, Clinical tolerability and safety of tramadol in hospitalized patients. J Pain & Palliat Care Pharmacother. 2020:34(4):211-218. https://doi.org/10.1080/15360288.2020.1817227
8. Duehmke RM, Derry S, Wiffen PJ, Bell RF, Aldington D, Moore RA. Tramadol for neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6(6):CD003726. https://doi.org/10.1002/14651858.cd003726.pub4
9. Toupin-April K, Bisaillon J, Welch V, et al. Tramadol for osteoarthritis. Cochrane Database Syst Rev. 2019;5(5):CD005522. https://doi.org/10.1002/14651858.cd005522.pub3
10. Wiffen PJ, Derry S, Moore RA. Tramadol with or without paracetamol (acetaminophen) for cancer pain. Cochrane Database Syst Rev. 2017;5(5):CD012508. https://doi.org/10.1002/14651858.cd012508.pub2
11. The American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. https://doi.org/10.1111/jgs.15767
12. Fournier JP, Yin H, Nessim SJ, Montastruc JL, Azoulay L. Tramadol for noncancer pain and the risk of hyponatremia. Am J Med. 2015;128(4):418-425.e5. https://doi.org/10.1016/j.amjmed.2014.10.046
13. Fournier JP, Azoulay L, Yin H, Montastruc JL, Suissa S. Tramadol use and the risk of hospitalization for hypoglycemia in patients with noncancer pain. JAMA Intern Med. 2015;175(2):186-193. https://doi.org/10.1001/jamainternmed.2014.6512
14. Wei J, Lane NE, Bolster MB, et al. Association of tramadol use with risk of hip fracture. J Bone Miner Res. 2020;35(4):631-640. https://doi.org/10.1002/jbmr.3935
15. Leppert W. CYP2D6 in the metabolism of opioids for mild to moderate pain. Pharmacology. 2011;87(5-6):274-285. https://doi.org/10.1159/000326085
16. Flockhart DA, Thacker D, McDonald C, Desta Z. The Flockhart cytochrome P450 drug-drug interaction table. Division of Clinical Pharmacology, Indiana University School of Medicine. Updated 2021. Accessed April 21, 2021. https://drug-interactions.medicine.iu.edu
17. Frost DA, Soric MM, Kaiser R, Neugebauer RE. Efficacy of tramadol for pain management in patients receiving strong cytochrome P450 2D6 inhibitors. Pharmacotherapy. 2019;39(6):724-729. https://doi.org/10.1002/phar.2269
18. Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43(13):879-923. https://doi.org/10.2165/00003088-200443130-00004
19. Bush DM. The CBHSQ report: emergency department visits for drug misuse or abuse involving the pain medication tramadol. Substance Abuse and Mental Health Service Administration. May 14, 2015. Accessed June 16, 2021. https://www.ncbi.nlm.nih.gov/books/NBK343535/
20. Bigal LM, Bibeau K, Dunbar S. Tramadol prescription over a 4-year period in the USA. Curr Pain Headache Rep. 2019;23(10):76. https://doi.org/10.1007/s11916-019-0777-x
21. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. National survey on drug use and health 2019 (NSDUH-2019). Accessed June 16, 2021. https://www.samhsa.gov/data/release/2019-national-survey-drug-use-and-health-nsduh-releases
22. Thiels CA, Habermann EB, Hooten WM, Jeffery MM. Chronic use of tramadol after acute pain episode: cohort study. BMJ. 2019;365:l1849. https://doi.org/10.1136/bmj.l1849
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits an 80-year-old man for a chronic obstructive pulmonary disease exacerbation. The patient’s history is significant for chronic right knee pain. While hospitalized, the patient reports worsening of his knee pain. Radiographs of the right knee show severe osteoarthritic changes. Since acetaminophen does not relieve the patient’s pain, the hospitalist orders tramadol as needed.
BACKGROUND
Hospitalists, who commonly evaluate and treat acute and chronic pain in the inpatient setting, have a wide selection of interventions from which to choose, including tramadol. Tramadol hydrochloride is a synthetic, central-acting analgesic with multiple mechanisms of action. It is a serotonin-norepinephrine reuptake inhibitor (SNRI) with a structure similar to venlafaxine and produces antineuropathic analgesic effects.1 Tramadol and its primary active metabolite O-desmethyltramadol (also known as the M1 metabolite) mediate its effects by binding at the mu-opioid receptor.2 Phase I metabolism in the liver by cytochrome P450 isoenzyme 2D6 (CYP2D6) facilitates conversion of tramadol to M1 (Figure). Importantly, genetic polymorphisms in CYP2D6 result in individual variations in gene expression, which impacts the metabolism of tramadol.2

Although tramadol is available over the counter in some countries, in the United States it is a Schedule IV controlled substance. Tramadol consistently ranks among the top 50 prescribed medications in the United States.3
WHY YOU MIGHT THINK PRESCRIBING TRAMADOL FOR PAIN MAY BE HELPFUL
Given the growing concerns regarding the use of opioids, the pharmaceutical industry has marketed tramadol as a safer opioid option for pain management. Tramadol binds at the mu-opioid receptor with an affinity that is less than 4000-fold that of morphine; the binding potency of M1, the metabolite of tramadol, is less than 5-fold that of morphine.4 Due to its lower binding affinity at the mu-opioid receptor, tramadol is considered a weak opioid, one believed to have minimal withdrawal symptoms and a lower potential for overdose or misuse compared to other opioids.1,5 Based on this characterization, many clinicians prescribe tramadol for elderly patients or patients otherwise at risk for medication misuse or adverse effects of opioids.6 In addition, hospitalized patients often have contraindications to nonopioid medications (eg, acetaminophen, nonsteroidal anti-inflammatory drugs [NSAIDs]), limiting their options for pain management.
WHY PRESCRIBING TRAMADOL FOR PAIN SHOULD BE AVOIDED
Despite being marketed as an effective and safe medication, tramadol has an unpredictable metabolism, complex pharmacology, and drug-drug interactions that can cause significant adverse effects. Similar to other opioids, tramadol is associated with a risk of misuse, physiologic dependency, and overdose. In addition, tramadol has a black box warning for addiction, misuse, respiratory depression, ultra-rapid metabolism, neonatal opioid withdrawal syndrome, CYP450 drug interactions, and interactions with other central nervous system depressants.
While tramadol has multiple mechanisms of action, the literature lacks high-quality evidence (eg, large randomized controlled trials) supporting its use, especially in hospitalized medical patients. A recent retrospective study of tramadol looked at the diagnoses of 250 hospitalized patients who received tramadol for pain management. While this study did not examine efficacy, it found mild-to-moderate acute noncancer pain to be the primary reason for prescribing tramadol.7 This study also showed the risk of severe drug-drug interactions increased the longer patients were on tramadol.7
As a result of the limited evidence in hospitalized patients, hospitalists must rely on outpatient studies.8-10 The size and quality of these studies, especially given the magnitude of tramadol prescribing in the United States, make them less useful. A series of Cochrane reviews examining the beneficial effects of tramadol for neuropathic pain, osteoarthritis, and cancer pain show insufficient evidence for tramadol when compared to placebo or active controls such as acetaminophen, NSAIDs, or other opioids.8-10
The side-effect profile of tramadol outweighs its mild analgesic effects. The 2019 American Geriatric Society Beers criteria for potentially inappropriate medication use in older adults strongly recommends clinicians use caution when prescribing tramadol to older adult patients, as tramadol may worsen or cause hyponatremia.11 In one large, population-based study, the use of tramadol doubled patients’ risk of hospitalization for hyponatremia when compared to codeine, though the incidence remains rather low at 4.6 per 10,000 person-months.12 Studies have also demonstrated an increased risk of hospitalization for hypoglycemia in nondiabetic patients receiving tramadol.13 A large propensity-score matched cohort study of patients with osteoarthritis found tramadol to have an associated higher all-cause mortality compared to NSAIDs; however, these differences may be due to confounding variables.1 In addition to hyponatremia and all-cause mortality, patients taking tramadol also have an associated increased risk of falls and hip fractures when compared to codeine or NSAIDs.14
The increased serotonergic activity associated with tramadol can lead to serotonin syndrome (serotonin toxicity), a rare but serious condition. Although serotonin syndrome can develop in patients taking tramadol as a monotherapy, the risk for this toxidrome increases when tramadol is taken in combination with other serotonergic agents or agents that inhibit metabolism of tramadol at CYP2D6.5 Seizures may also occur with tramadol at therapeutic and supratherapeutic doses. Population-based studies estimate seizures occur in 0.15% to 0.86% of patients receiving tramadol, which is two to six times the risk of those not on tramadol.5 Patients concurrently taking tramadol with a tricyclic antidepressant (TCA) or selective serotonin reuptake inhibitor (SSRI) are estimated to have seizures five to nine times more often than patients not taking a TCA or SSRI.5 Risk factors for tramadol-induced seizure include tramadol misuse or overdose, tramadol doses >1000 mg daily (maximum recommended dose is 400 mg/day), chronic tramadol use, concurrent use of a serotonergic agent or medications that inhibit CYP2D6, and history of epilepsy, renal disease, stroke, or traumatic brain injury.5
Differences in the genetic polymorphisms of CYP2D6 can produce a range of CYP2D6 activity from “poor metabolizers” (little-to-no analgesic effect) to “ultra-rapid metabolizers” (enhanced analgesia and increased risk of adverse effects), leading to unpredictable pharmacodynamic effects of tramadol.2 In North Africa and the Arabian peninsula, more than 25% of the population rapidly metabolizes tramadol; these pharmacogenomic effects result in higher rates of tramadol addiction and overdose in these regions.5 An estimated 7% to 10% of Caucasians slowly metabolize tramadol, which may place them at risk of adverse effects from tramadol in addition to inadequate analgesia.15 In contrast, Ethiopian populations have the highest rate of ultra-rapid tramadol metabolism at 29%.15
Drugs that induce CYP2D6 (eg, dexamethasone, rifampin) or inhibit CYP2D6 (eg, bupropion, fluoxetine) also impact tramadol efficacy, pharmacokinetics, and pharmacodynamics.16,17 Patients taking strong CYP2D6 inhibitors require significantly higher doses of tramadol to achieve analgesic effects.17 Tramadol undergoes extensive hepatic metabolism, producing several active metabolites, including M1 (Figure). Hepatic impairment increases the elimination half-life of tramadol and its metabolites.18 The majority of tramadol and its metabolites are eliminated through the kidneys. Accumulation of tramadol and its metabolites may occur in patients with renal impairment, placing them at increased risk of adverse effects.2
Finally, although some clinicians assume that tramadol has lower rates of misuse, diversion, or overdose compared to other opioids, rates of nonprescription use have increased with its proliferation.19,20 The US Substance Abuse and Mental Health Services Administration estimates that 1,287,000 persons misused tramadol in 2019.21 Patients may exhibit symptoms of physiologic opioid dependence and withdrawal from chronic tramadol use.2,22 In one study, patients prescribed tramadol monotherapy for acute pain from elective surgery had an increased risk for prolonged opioid use compared to patients prescribed other short-acting opioids.22
WHAT YOU SHOULD DO INSTEAD
Clinicians should determine the nature of the patient’s pain by obtaining a complete medical history, performing a thorough physical examination, and ordering diagnostic tests and imaging studies, as necessary. After consulting with the patient’s primary care physician, the clinician should employ a multimodal approach to pain that includes topical agents, psychotherapy, injections or interventions, and nonopioid medications. Patients with neuropathic pain may benefit from adjuvant analgesics such as gabapentinoids, TCAs, or SNRIs. In patients with evidence-based indications for opioid therapy (eg, pancreatitis, cancer pain, postsurgical pain), the hospitalist should assess the risk for opioid misuse and discuss risks and benefits with the patient before considering a time-limited trial of opioid therapy. If available and when indicated, clinicians should consult with specialists in pain management or palliative care. For cases wherein clinicians have already prescribed tramadol to the patient, they should discuss deprescribing strategies and alternative analgesic options with the patient and the patient’s primary care physician. Finally, before initiating tramadol therapy for hospitalized patients with pain, hospitalists should consider the risks, benefits, and alternative approaches to prescribing tramadol.
RECOMMENDATIONS
- For hospitalized patients reporting pain, complete a pain assessment by history, physical exam, chart review, and diagnostic studies to examine the etiology of the pain.
- Utilize multiple modalities for pain control when possible, including acetaminophen, NSAIDs, topical agents, ice or heat, neuropathic pain medications, and interventions such as injections, psychotherapy, or radiation, if indicated.
- Avoid prescribing tramadol due to unpredictable pharmacodynamics, adverse effects, and lack of quality evidence for efficacy in hospitalized medical patients.
CONCLUSION
Tramadol is a commonly used opioid medication associated with adverse effects and unpredictable analgesia. Regarding this case scenario, the use of tramadol in this patient places him at risk for drug-drug interactions, hyponatremia, hypoglycemia, serotonin syndrome, seizures, and pronounced side effects of opioid medications. Moderate quality evidence in the outpatient setting suggests that tramadol is unlikely to provide significant analgesia for his osteoarthritic pain.9 Instead of prescribing tramadol, the hospitalist should consider alternative treatments for this patient’s pain, such as intraarticular glucocorticoids, a short course of oral NSAIDs (unless contraindicated), topical treatments (eg, menthol, capsaicin, NSAIDs), physical therapy, and close follow-up with an orthopedist after hospital discharge. Further randomized controlled studies of tramadol vs active controls are needed.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits an 80-year-old man for a chronic obstructive pulmonary disease exacerbation. The patient’s history is significant for chronic right knee pain. While hospitalized, the patient reports worsening of his knee pain. Radiographs of the right knee show severe osteoarthritic changes. Since acetaminophen does not relieve the patient’s pain, the hospitalist orders tramadol as needed.
BACKGROUND
Hospitalists, who commonly evaluate and treat acute and chronic pain in the inpatient setting, have a wide selection of interventions from which to choose, including tramadol. Tramadol hydrochloride is a synthetic, central-acting analgesic with multiple mechanisms of action. It is a serotonin-norepinephrine reuptake inhibitor (SNRI) with a structure similar to venlafaxine and produces antineuropathic analgesic effects.1 Tramadol and its primary active metabolite O-desmethyltramadol (also known as the M1 metabolite) mediate its effects by binding at the mu-opioid receptor.2 Phase I metabolism in the liver by cytochrome P450 isoenzyme 2D6 (CYP2D6) facilitates conversion of tramadol to M1 (Figure). Importantly, genetic polymorphisms in CYP2D6 result in individual variations in gene expression, which impacts the metabolism of tramadol.2

Although tramadol is available over the counter in some countries, in the United States it is a Schedule IV controlled substance. Tramadol consistently ranks among the top 50 prescribed medications in the United States.3
WHY YOU MIGHT THINK PRESCRIBING TRAMADOL FOR PAIN MAY BE HELPFUL
Given the growing concerns regarding the use of opioids, the pharmaceutical industry has marketed tramadol as a safer opioid option for pain management. Tramadol binds at the mu-opioid receptor with an affinity that is less than 4000-fold that of morphine; the binding potency of M1, the metabolite of tramadol, is less than 5-fold that of morphine.4 Due to its lower binding affinity at the mu-opioid receptor, tramadol is considered a weak opioid, one believed to have minimal withdrawal symptoms and a lower potential for overdose or misuse compared to other opioids.1,5 Based on this characterization, many clinicians prescribe tramadol for elderly patients or patients otherwise at risk for medication misuse or adverse effects of opioids.6 In addition, hospitalized patients often have contraindications to nonopioid medications (eg, acetaminophen, nonsteroidal anti-inflammatory drugs [NSAIDs]), limiting their options for pain management.
WHY PRESCRIBING TRAMADOL FOR PAIN SHOULD BE AVOIDED
Despite being marketed as an effective and safe medication, tramadol has an unpredictable metabolism, complex pharmacology, and drug-drug interactions that can cause significant adverse effects. Similar to other opioids, tramadol is associated with a risk of misuse, physiologic dependency, and overdose. In addition, tramadol has a black box warning for addiction, misuse, respiratory depression, ultra-rapid metabolism, neonatal opioid withdrawal syndrome, CYP450 drug interactions, and interactions with other central nervous system depressants.
While tramadol has multiple mechanisms of action, the literature lacks high-quality evidence (eg, large randomized controlled trials) supporting its use, especially in hospitalized medical patients. A recent retrospective study of tramadol looked at the diagnoses of 250 hospitalized patients who received tramadol for pain management. While this study did not examine efficacy, it found mild-to-moderate acute noncancer pain to be the primary reason for prescribing tramadol.7 This study also showed the risk of severe drug-drug interactions increased the longer patients were on tramadol.7
As a result of the limited evidence in hospitalized patients, hospitalists must rely on outpatient studies.8-10 The size and quality of these studies, especially given the magnitude of tramadol prescribing in the United States, make them less useful. A series of Cochrane reviews examining the beneficial effects of tramadol for neuropathic pain, osteoarthritis, and cancer pain show insufficient evidence for tramadol when compared to placebo or active controls such as acetaminophen, NSAIDs, or other opioids.8-10
The side-effect profile of tramadol outweighs its mild analgesic effects. The 2019 American Geriatric Society Beers criteria for potentially inappropriate medication use in older adults strongly recommends clinicians use caution when prescribing tramadol to older adult patients, as tramadol may worsen or cause hyponatremia.11 In one large, population-based study, the use of tramadol doubled patients’ risk of hospitalization for hyponatremia when compared to codeine, though the incidence remains rather low at 4.6 per 10,000 person-months.12 Studies have also demonstrated an increased risk of hospitalization for hypoglycemia in nondiabetic patients receiving tramadol.13 A large propensity-score matched cohort study of patients with osteoarthritis found tramadol to have an associated higher all-cause mortality compared to NSAIDs; however, these differences may be due to confounding variables.1 In addition to hyponatremia and all-cause mortality, patients taking tramadol also have an associated increased risk of falls and hip fractures when compared to codeine or NSAIDs.14
The increased serotonergic activity associated with tramadol can lead to serotonin syndrome (serotonin toxicity), a rare but serious condition. Although serotonin syndrome can develop in patients taking tramadol as a monotherapy, the risk for this toxidrome increases when tramadol is taken in combination with other serotonergic agents or agents that inhibit metabolism of tramadol at CYP2D6.5 Seizures may also occur with tramadol at therapeutic and supratherapeutic doses. Population-based studies estimate seizures occur in 0.15% to 0.86% of patients receiving tramadol, which is two to six times the risk of those not on tramadol.5 Patients concurrently taking tramadol with a tricyclic antidepressant (TCA) or selective serotonin reuptake inhibitor (SSRI) are estimated to have seizures five to nine times more often than patients not taking a TCA or SSRI.5 Risk factors for tramadol-induced seizure include tramadol misuse or overdose, tramadol doses >1000 mg daily (maximum recommended dose is 400 mg/day), chronic tramadol use, concurrent use of a serotonergic agent or medications that inhibit CYP2D6, and history of epilepsy, renal disease, stroke, or traumatic brain injury.5
Differences in the genetic polymorphisms of CYP2D6 can produce a range of CYP2D6 activity from “poor metabolizers” (little-to-no analgesic effect) to “ultra-rapid metabolizers” (enhanced analgesia and increased risk of adverse effects), leading to unpredictable pharmacodynamic effects of tramadol.2 In North Africa and the Arabian peninsula, more than 25% of the population rapidly metabolizes tramadol; these pharmacogenomic effects result in higher rates of tramadol addiction and overdose in these regions.5 An estimated 7% to 10% of Caucasians slowly metabolize tramadol, which may place them at risk of adverse effects from tramadol in addition to inadequate analgesia.15 In contrast, Ethiopian populations have the highest rate of ultra-rapid tramadol metabolism at 29%.15
Drugs that induce CYP2D6 (eg, dexamethasone, rifampin) or inhibit CYP2D6 (eg, bupropion, fluoxetine) also impact tramadol efficacy, pharmacokinetics, and pharmacodynamics.16,17 Patients taking strong CYP2D6 inhibitors require significantly higher doses of tramadol to achieve analgesic effects.17 Tramadol undergoes extensive hepatic metabolism, producing several active metabolites, including M1 (Figure). Hepatic impairment increases the elimination half-life of tramadol and its metabolites.18 The majority of tramadol and its metabolites are eliminated through the kidneys. Accumulation of tramadol and its metabolites may occur in patients with renal impairment, placing them at increased risk of adverse effects.2
Finally, although some clinicians assume that tramadol has lower rates of misuse, diversion, or overdose compared to other opioids, rates of nonprescription use have increased with its proliferation.19,20 The US Substance Abuse and Mental Health Services Administration estimates that 1,287,000 persons misused tramadol in 2019.21 Patients may exhibit symptoms of physiologic opioid dependence and withdrawal from chronic tramadol use.2,22 In one study, patients prescribed tramadol monotherapy for acute pain from elective surgery had an increased risk for prolonged opioid use compared to patients prescribed other short-acting opioids.22
WHAT YOU SHOULD DO INSTEAD
Clinicians should determine the nature of the patient’s pain by obtaining a complete medical history, performing a thorough physical examination, and ordering diagnostic tests and imaging studies, as necessary. After consulting with the patient’s primary care physician, the clinician should employ a multimodal approach to pain that includes topical agents, psychotherapy, injections or interventions, and nonopioid medications. Patients with neuropathic pain may benefit from adjuvant analgesics such as gabapentinoids, TCAs, or SNRIs. In patients with evidence-based indications for opioid therapy (eg, pancreatitis, cancer pain, postsurgical pain), the hospitalist should assess the risk for opioid misuse and discuss risks and benefits with the patient before considering a time-limited trial of opioid therapy. If available and when indicated, clinicians should consult with specialists in pain management or palliative care. For cases wherein clinicians have already prescribed tramadol to the patient, they should discuss deprescribing strategies and alternative analgesic options with the patient and the patient’s primary care physician. Finally, before initiating tramadol therapy for hospitalized patients with pain, hospitalists should consider the risks, benefits, and alternative approaches to prescribing tramadol.
RECOMMENDATIONS
- For hospitalized patients reporting pain, complete a pain assessment by history, physical exam, chart review, and diagnostic studies to examine the etiology of the pain.
- Utilize multiple modalities for pain control when possible, including acetaminophen, NSAIDs, topical agents, ice or heat, neuropathic pain medications, and interventions such as injections, psychotherapy, or radiation, if indicated.
- Avoid prescribing tramadol due to unpredictable pharmacodynamics, adverse effects, and lack of quality evidence for efficacy in hospitalized medical patients.
CONCLUSION
Tramadol is a commonly used opioid medication associated with adverse effects and unpredictable analgesia. Regarding this case scenario, the use of tramadol in this patient places him at risk for drug-drug interactions, hyponatremia, hypoglycemia, serotonin syndrome, seizures, and pronounced side effects of opioid medications. Moderate quality evidence in the outpatient setting suggests that tramadol is unlikely to provide significant analgesia for his osteoarthritic pain.9 Instead of prescribing tramadol, the hospitalist should consider alternative treatments for this patient’s pain, such as intraarticular glucocorticoids, a short course of oral NSAIDs (unless contraindicated), topical treatments (eg, menthol, capsaicin, NSAIDs), physical therapy, and close follow-up with an orthopedist after hospital discharge. Further randomized controlled studies of tramadol vs active controls are needed.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
1. Zeng C, Dubreuil M, LaRochelle MR, et al. Association of tramadol with all-cause mortality among patients with osteoarthritis. JAMA. 2019;321(10):969-982. https://doi.org/10.1001/jama.2019.1347
2. Gong L, Stamer UM, Tzvetkov MV, Altman RB, Klein TE. PharmGKB summary: tramadol pathway. Pharmacogenet Genomics. 2014;24(7):374-380. https://doi.org/10.1097/FPC.0000000000000057
3. The top 200 drugs of 2019. ClinCalc DrugStats Database. Accessed June 10, 2021. https://clincalc.com/DrugStats
4. Gillen C, Haurand M, Kobelt DJ, Wnendt S. Affinity, potency and efficacy of tramadol and its metabolites at the cloned human µ-opioid receptor. Naunyn Schmiedebergs Arch Pharmacol. 2000;362(2):116-121. https://doi.org/10.1007/s002100000266
5. Hassamal S, Miotto K, Dale W, Danovitch I. Tramadol: understanding the risk of serotonin syndrome and seizures. Am J Med. 2018;131(11):1382.e1-1382.e6. https://doi.org/10.1016/j.amjmed.2018.04.025
6. Shipton EA. Tramadol—present and future. Anaesth Intensive Care. 2000;28(4):363-374. https://doi.org/10.1177/0310057X0002800403
7. Mohan N, Edmonds KP, Ajayi TA, Atayee RS, Clinical tolerability and safety of tramadol in hospitalized patients. J Pain & Palliat Care Pharmacother. 2020:34(4):211-218. https://doi.org/10.1080/15360288.2020.1817227
8. Duehmke RM, Derry S, Wiffen PJ, Bell RF, Aldington D, Moore RA. Tramadol for neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6(6):CD003726. https://doi.org/10.1002/14651858.cd003726.pub4
9. Toupin-April K, Bisaillon J, Welch V, et al. Tramadol for osteoarthritis. Cochrane Database Syst Rev. 2019;5(5):CD005522. https://doi.org/10.1002/14651858.cd005522.pub3
10. Wiffen PJ, Derry S, Moore RA. Tramadol with or without paracetamol (acetaminophen) for cancer pain. Cochrane Database Syst Rev. 2017;5(5):CD012508. https://doi.org/10.1002/14651858.cd012508.pub2
11. The American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. https://doi.org/10.1111/jgs.15767
12. Fournier JP, Yin H, Nessim SJ, Montastruc JL, Azoulay L. Tramadol for noncancer pain and the risk of hyponatremia. Am J Med. 2015;128(4):418-425.e5. https://doi.org/10.1016/j.amjmed.2014.10.046
13. Fournier JP, Azoulay L, Yin H, Montastruc JL, Suissa S. Tramadol use and the risk of hospitalization for hypoglycemia in patients with noncancer pain. JAMA Intern Med. 2015;175(2):186-193. https://doi.org/10.1001/jamainternmed.2014.6512
14. Wei J, Lane NE, Bolster MB, et al. Association of tramadol use with risk of hip fracture. J Bone Miner Res. 2020;35(4):631-640. https://doi.org/10.1002/jbmr.3935
15. Leppert W. CYP2D6 in the metabolism of opioids for mild to moderate pain. Pharmacology. 2011;87(5-6):274-285. https://doi.org/10.1159/000326085
16. Flockhart DA, Thacker D, McDonald C, Desta Z. The Flockhart cytochrome P450 drug-drug interaction table. Division of Clinical Pharmacology, Indiana University School of Medicine. Updated 2021. Accessed April 21, 2021. https://drug-interactions.medicine.iu.edu
17. Frost DA, Soric MM, Kaiser R, Neugebauer RE. Efficacy of tramadol for pain management in patients receiving strong cytochrome P450 2D6 inhibitors. Pharmacotherapy. 2019;39(6):724-729. https://doi.org/10.1002/phar.2269
18. Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43(13):879-923. https://doi.org/10.2165/00003088-200443130-00004
19. Bush DM. The CBHSQ report: emergency department visits for drug misuse or abuse involving the pain medication tramadol. Substance Abuse and Mental Health Service Administration. May 14, 2015. Accessed June 16, 2021. https://www.ncbi.nlm.nih.gov/books/NBK343535/
20. Bigal LM, Bibeau K, Dunbar S. Tramadol prescription over a 4-year period in the USA. Curr Pain Headache Rep. 2019;23(10):76. https://doi.org/10.1007/s11916-019-0777-x
21. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. National survey on drug use and health 2019 (NSDUH-2019). Accessed June 16, 2021. https://www.samhsa.gov/data/release/2019-national-survey-drug-use-and-health-nsduh-releases
22. Thiels CA, Habermann EB, Hooten WM, Jeffery MM. Chronic use of tramadol after acute pain episode: cohort study. BMJ. 2019;365:l1849. https://doi.org/10.1136/bmj.l1849
1. Zeng C, Dubreuil M, LaRochelle MR, et al. Association of tramadol with all-cause mortality among patients with osteoarthritis. JAMA. 2019;321(10):969-982. https://doi.org/10.1001/jama.2019.1347
2. Gong L, Stamer UM, Tzvetkov MV, Altman RB, Klein TE. PharmGKB summary: tramadol pathway. Pharmacogenet Genomics. 2014;24(7):374-380. https://doi.org/10.1097/FPC.0000000000000057
3. The top 200 drugs of 2019. ClinCalc DrugStats Database. Accessed June 10, 2021. https://clincalc.com/DrugStats
4. Gillen C, Haurand M, Kobelt DJ, Wnendt S. Affinity, potency and efficacy of tramadol and its metabolites at the cloned human µ-opioid receptor. Naunyn Schmiedebergs Arch Pharmacol. 2000;362(2):116-121. https://doi.org/10.1007/s002100000266
5. Hassamal S, Miotto K, Dale W, Danovitch I. Tramadol: understanding the risk of serotonin syndrome and seizures. Am J Med. 2018;131(11):1382.e1-1382.e6. https://doi.org/10.1016/j.amjmed.2018.04.025
6. Shipton EA. Tramadol—present and future. Anaesth Intensive Care. 2000;28(4):363-374. https://doi.org/10.1177/0310057X0002800403
7. Mohan N, Edmonds KP, Ajayi TA, Atayee RS, Clinical tolerability and safety of tramadol in hospitalized patients. J Pain & Palliat Care Pharmacother. 2020:34(4):211-218. https://doi.org/10.1080/15360288.2020.1817227
8. Duehmke RM, Derry S, Wiffen PJ, Bell RF, Aldington D, Moore RA. Tramadol for neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6(6):CD003726. https://doi.org/10.1002/14651858.cd003726.pub4
9. Toupin-April K, Bisaillon J, Welch V, et al. Tramadol for osteoarthritis. Cochrane Database Syst Rev. 2019;5(5):CD005522. https://doi.org/10.1002/14651858.cd005522.pub3
10. Wiffen PJ, Derry S, Moore RA. Tramadol with or without paracetamol (acetaminophen) for cancer pain. Cochrane Database Syst Rev. 2017;5(5):CD012508. https://doi.org/10.1002/14651858.cd012508.pub2
11. The American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. https://doi.org/10.1111/jgs.15767
12. Fournier JP, Yin H, Nessim SJ, Montastruc JL, Azoulay L. Tramadol for noncancer pain and the risk of hyponatremia. Am J Med. 2015;128(4):418-425.e5. https://doi.org/10.1016/j.amjmed.2014.10.046
13. Fournier JP, Azoulay L, Yin H, Montastruc JL, Suissa S. Tramadol use and the risk of hospitalization for hypoglycemia in patients with noncancer pain. JAMA Intern Med. 2015;175(2):186-193. https://doi.org/10.1001/jamainternmed.2014.6512
14. Wei J, Lane NE, Bolster MB, et al. Association of tramadol use with risk of hip fracture. J Bone Miner Res. 2020;35(4):631-640. https://doi.org/10.1002/jbmr.3935
15. Leppert W. CYP2D6 in the metabolism of opioids for mild to moderate pain. Pharmacology. 2011;87(5-6):274-285. https://doi.org/10.1159/000326085
16. Flockhart DA, Thacker D, McDonald C, Desta Z. The Flockhart cytochrome P450 drug-drug interaction table. Division of Clinical Pharmacology, Indiana University School of Medicine. Updated 2021. Accessed April 21, 2021. https://drug-interactions.medicine.iu.edu
17. Frost DA, Soric MM, Kaiser R, Neugebauer RE. Efficacy of tramadol for pain management in patients receiving strong cytochrome P450 2D6 inhibitors. Pharmacotherapy. 2019;39(6):724-729. https://doi.org/10.1002/phar.2269
18. Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43(13):879-923. https://doi.org/10.2165/00003088-200443130-00004
19. Bush DM. The CBHSQ report: emergency department visits for drug misuse or abuse involving the pain medication tramadol. Substance Abuse and Mental Health Service Administration. May 14, 2015. Accessed June 16, 2021. https://www.ncbi.nlm.nih.gov/books/NBK343535/
20. Bigal LM, Bibeau K, Dunbar S. Tramadol prescription over a 4-year period in the USA. Curr Pain Headache Rep. 2019;23(10):76. https://doi.org/10.1007/s11916-019-0777-x
21. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. National survey on drug use and health 2019 (NSDUH-2019). Accessed June 16, 2021. https://www.samhsa.gov/data/release/2019-national-survey-drug-use-and-health-nsduh-releases
22. Thiels CA, Habermann EB, Hooten WM, Jeffery MM. Chronic use of tramadol after acute pain episode: cohort study. BMJ. 2019;365:l1849. https://doi.org/10.1136/bmj.l1849
© 2021 Society of Hospital Medicine
Operational Curriculum and Research Initiatives: Shaping the Future of Military Medicine
It is a time of significant change as the Military Health System (MHS) transitions to the purview of the Defense Health Agency (DHA). Additionally, the landscape of combat is ever changing, and military medicine needs to evolve to ensure that the lessons learned are utilized to optimize care of the war fighters. The purpose of this review is to evaluate the available literature on existing operational medicine curriculums and make recommendations to restructure current military medicine training to produce operationally prepared clinicians who are informed in operationally focused research principles.
Operational Medicine
Before diving into the importance of creating a curriculum and investing in training for scholarly activity proficiency, operational medicine needs to be defined. It can be defined as medical care provided in an austere environment with limited resources and possibly under hostile conditions. Another way to look at operational medicine is as the evaluation of normal human physiology and pathology under abnormal conditions. The mission set of each of the services is unique. The Marines and Army may operate forward past the wire vulnerable to the environment, gunfire, and improvised explosive devices, remote from fixed medical facilities. The Navy has divers exposed to the risks of decompression sickness. The Air Force has pilots exposed to altitude changes and strains of G-forces during flight. Locations vary from cold high-altitude mountainous regions to high-temperature desolate deserts. Many times, medical practitioners may be remotely stationed, far from specialty or immediate definitive care. Patient care may consist of low-acuity management of individual patients in sick call to mass casualty events where patient numbers and morbidity may outstrip available resources, making the difficult task of triage necessary.
Despite the challenges of being a uniformed physician, the benefits of being embedded is a better understanding of the roles and capability of the unit. Military physicians need to have the unique knowledge of the type of injuries sustained in that particular theater of war, such as differentiating between the trauma pattern and care required for blast injuries vs high-velocity missiles. There are also chemical, biologic, radiologic, and nuclear threats that military physicians need to recognize. Much of what disables a military fighting force is not a direct relationship to combat-related injuries; however, entire units have been taken down by infectious diarrhea or trench foot. There is also a need for familiarity of the infections and parasitology endemic to the particular theater with the aim of implementation of prevention whenever possible.
Military medicine does not fit in any box. Military physicians need to know the job requirements of various specialties, including elements of occupational medicine, such as aircrew piloting high-performance fighters or ground troops fully loaded with body armor and 80-lb backpacks. There are musculoskeletal injuries from the stressors of various military occupations. Working around weaponry and contact with hostile forces will create scenarios requiring emergent and critical care. In addition to physical injuries, there is the mental strain of combat with the risk of imminent personal injury, the guilt of survivorship, dealing with the scars and permanent physical damage of combat, and prolonged separation from family and other support systems.
The National Defense Authorization Act 2017 mandated the establishment of a standardized process to oversee all military graduate medical education (GME) programs with the goal of ensuring medical operational readiness.1 This is no small task with > 3000 residents in more than 70 specialties, comprising approximately 12% of US residents.1,2 Presently, 26 to 32% of the medical corps is enrolled in full-time training compared with 12% of the total force.2 With significant time and resources expended during this period, it is vital to maximize the potential of the training.
Literature Review
A literature review was performed, evaluating historical precedence of specialized military medical training and research as well as current operational curriculums. Literature search was conducted in the PubMed and Uniformed Services University (USU) Learning Resource databases using the terms “operational medicine curriculum,” “military medicine curriculum,” “operational medicine training,” “military medicine training,” “operational medicine research,” and “military medicine research,” and included all articles from 1997 to 2020. Inclusion criteria included studies that detailed military medicine training programs and/or outcomes. The source types used in this research project included peer-reviewed journal publications—both review articles and original research—from medical and military journals. The citations of these articles were also reviewed for additional usable publications. Secondary sources included official reports and studies by the RAND Corporation, the US Government Accountability Office, and the Institute for Defense Analysis (IDA). Due to lack of literature on the topic, other sources such as talking papers, letters, and formal presentations from subject matter experts were included to showcase the current state and gaps on this topic. Key findings from peer-reviewed publications are presented in Table 1.
Overall, the literature review showed that longitudinal deliberately mapped out curriculums can be well integrated into the existing medical curriculum.3 The military medicine course topics include environmental medicine, applied field medicine, combat casualty care, medical support planning, mass casualty incident preparation, and military-focused problem solving, decision making, and leadership.4
One 1997 study looked at the degree of implementation of military unique curriculum in 18 family medicine residencies. Only 30% of residents stated that their program had a specific operational medicine curriculum.5 Salerno and colleagues surveyed current residents and recently graduated internal medicine physicians at 14 facilities in the Army, Air Force, and Navy to determine confidence level with military medicine. More than half did not feel ready to practice deployment medicine; just 19% felt comfortable treating nuclear, biologic, and chemical warfare injuries; and 32% felt unfamiliar with the command and administrative duties. A subgroup analysis showed that USU graduates felt more prepared in these areas compared with civilian program graduates.6 Additional studies showed perceived smoother transition in the first active-duty tour after participation in an operational curriculum.7
Didactics can provide a foundation. However, just as the practice of medicine is learned in the clinic, the art of military medicine is learned in the field. Hands-on training in one study was accomplished through the Combat Casualty Care Course (C4), the USU Bushmaster exercise, and a field training exercise. The field exercise included components of mission planning, medical threat assessments, triage of a mass casualty situation, management of disease and nonbattle injuries, combat stress casualties, resource management, and patient evacuation.8
Another publication described a similar longitudinal curriculum with C4 after the first year of training and the Medical Management of Chemical and Biological Casualty Course during the second year. The operational curriculum 3-day capstone occurred at the end of medical training utilizing mannequins to realistically simulate combat casualty care, including emergency airways, chest tube, and tourniquets.9 Due to the current deployment tempo, just in time refresher courses like this could be valuable preparation.
While most of the operational curriculums evaluated assessed efficiency over a short time interval, one study looked at 1189 graduates from the military medical school from the past 20 years. Preparedness was perceived to be high for military-unique practice and leadership.10 The operational curriculum at USU had been purposefully structured to provide continuity. Didactics and casework were reinforced with hands-on training whether through realistic simulator training or field exercises. The authors note a weakness of many operational curriculums is inconsistency and fragmented training without deliberate longitudinal planning.
One of the more recent military GME curriculums include the creation of the operational medicine residency in 2013, which created a standardized longitudinal operational curriculum integrated along with the existing family medicine, emergency medicine, or internal medicine curriculum to create mission-ready military physicians upon graduation. Scheduled rotations include global medicine, aeromedical evacuation, occupational medicine, and tropical medicine. Completing military officer professional development and an operationally relevant research project is an expectation (Table 2).11
In addition to in-program training, other options include operational rotations offsite and military courses conducted outside the GME program.12 Some of these courses may include just-in-time training such as expeditionary medical support system training prior to scheduled deployments. Examples of experiential training are listed in Table 3.
Critical Analysis
Current gaps were identified in the military medicine training pipeline’s operational medicine curriculum and research programs. The analysis looked at specific components that make the operational medicine curriculum and research unique as well as current readiness goals, to determine how to best align both to meet the mission requirements. Some factors considered included efficiency, cost, program portability, duplication minimization, retention, and sustainability.
Efficiency
A well-created curriculum that meets objectives will require more than an assigned rotation and a few lectures. The most successful ones in the literature review were the ones that were deliberately planned and longitudinal, such as the ones at USU that combined a mixture of classroom and field exercises over the course of 4 years.4,8 In that way, the curriculum may not be considered time efficient, but if integrated well into the already existing medical training, the production of military physicians who are mission ready upon graduation—ready to serve as military medical leaders and deploy—will be invaluable.
Cost Comparison
Due to the associated overhead of running a training platform and the additional hours of operational training, military GME is more expensive initially compared with civilian outsourcing. In USU, for example, there is an additional 700 hours of operational curriculum alone. This cost difference more than doubles the cost of a USU education vs a Health Professional Scholarship Program (HPSP) scholarship at a civilian medical school. However, a causal analysis performed by the IDA to determine value basis noted that USU graduates deploy almost 3 times as much and serve 6 years longer on active duty.3
After graduating medical school through either accession source, physicians complete specialization training in a GME program. The IDA study noted an average $12,000 increased cost of military GME compared with civilian programs. The analysis included resident compensation and overhead costs of running the program as well as the net cost, which also accounted for resident productivity and workload by training in a military facility.3 Calculations due to mandated budget cuts estimated cost savings of closing the military medical school at < $100 million while significantly impacting the military physician pipeline and operational research output.3
Duplication of Effort
There are already established training programs such as Tactical Combat Casualty Care (TCCC) that could be incorporated into the curriculum to avoid expending additional resources to recreate the wheel. USU has a validated operational training curriculum and may be able to make opportunities available for outside trainees to participate in some of its military-unique training and leadership exercises. Other ways to decrease duplication of effort and improve cost efficiency include focusing on the creation of an academic health system (AHS) and consolidating similar programs to conserve resources. Increasing existing military program sizes will not only ensure the continuation of the military medicine pipeline, but will spread overhead costs over a larger cohort, decrease costs of civilian outsourcing, and ensure the less tangible benefits of military cultural exposure early in trainees’ careers. For example, increasing the class size of USU by 30 students actually reduces the cost per student to $239,000 per year from $253,000, while decreasing the need for HPSP accessions training in civilian programs, making the endeavor overall cost neutral.3
Program Portability
The operational medicine residency has proved that an operational curriculum can be remotely managed and reproduced at a variety of residency specialties.12 Remote education could be developed and distributed throughout the MHS, such as the proposed USU course Military Medicine and Leadership course.3 Centralized training programs like Global Medicine and C-STARS could be scheduled TDYs during the medical training calendar.
Retention
The military medical school, USU, is the largest military medicine accession source. An IDA report notes that retention of USU graduates is 15.2 years compared with 9.2 years served by civilian trainees. Due to the longevity in service, USU graduates also make up more than 25% of military medical leadership.4 The long-term outcome study that looked at the past 40 years of USU graduates observed that over 70% of graduates served until retirement eligibility and are overrepresented in special operations units.3,13 While some of this longevity may be attributed to the longer USU service contracts, military GME graduates were still noted to be 4 times more likely to commit to a multiyear service contract.14 A RAND study on the retention of military physicians in the Army, Air Force, and Navy noted that overall retention increased throughout all the services for physicians who went through the military GME pipeline.15 Conversely, civilian GME training was associated with a 45% chance in leaving active duty.16
It is theorized that early military acculturation during training increases the likelihood of instilling a sense of mission. Being involved in military GME on the teaching side also showed increased retention rates for 63% of survey respondents.17 Reduced burnout and increased work satisfaction for those involved in military GME was noted on another faculty satisfaction survey.17
Sustainability
Programs like USU, which have been around for decades, and the newer operational residency program evolving since 2013 have shown sustainability.4,11 Dissemination of proven curriculums as well as centralization of already validated training programs can help standardize operational medical training throughout the MHS. In order to flourish at individual programs, the faculty need to be well versed in a train the trainer model and have institutional support. The ability to engage with the line at individual locations may be a factor as well.18 In regard to research, once residents are taught the principles of scholarly activity, they will have the tools to continue operational medicine research advancements and mentoring students.
Discussion
The 2020 NDAA recommends the establishment of an AHS.3 This step will create a culture of military medical readiness from the top down as congressional mandates push reorganization of the MHS, including military GME programs. An overall restructuring of military medicine will require prioritization of resources toward operational requirements vs the historic significant division of attention to beneficiary care that has caused a lack of unity of effort and additional strain on an already heavily tasked medical force. The changes in military GME are just one aspect of that. It is vital to look at the restructuring with a comprehension of the unique challenges of combat health rather than only from an in-garrison, hospital-based aspect.19 Benefits of having a military medicine AHS include opportunities to share resources and successful business models as well as foster interdisciplinary teamwork and partnerships with civilian health care facilities and research institutions as a force multiplier.19
There has been recent discussion about budget cuts, including shutting down USU and military GME and transitioning all training to civilian programs to be cost-effective.4 If this were to happen, it would be a step backward from the goal of operational readiness. Maintaining US Department of Defense (DoD) control of the military medicine pipeline has innumerable benefits, including built-in mentorship from operationally-seasoned faculty, military leadership development, proficiency in MHS systems, open communication between GME programs and DoD, and curriculum control to ensure focus on readiness.20 Military GME programs are also a significant production source of military-related scholarly activity. Over fiscal year 2017/2018, 63% of the publications out of the San Antonio Uniformed Services Health Education Consortium—the largest Air Force GME platform and second largest multiservice GME platform—involved military relevant medical topics.17 Much of the volume of operational research as well as the relevant skills learned and future innovations secondary to conducting this research would be lost if military GME did not exist.17,21
Practically speaking, military GME provides the majority of the military medicine accessions. For example, a presentation by the Air Force Chief of Physician Education noted that the total military GME pipeline included 2875 students, but direct physician access averaged only 20 physicians a year.22 Even if the decision was made to defer to civilian education, capacity does not exist in civilian GME programs. This is worsened by the increased competitiveness of the GME match with the proliferation of medical schools without concurrent increase in residency spots. The 2018 National Resident Matching Program noted that there were more than 37,103 US and foreign applicants for only 33,000 residency positions, leaving many US applicants unmatched.17 It is doubtful that the civilian GME programs would be able to absorb the influx of military residents, affecting both the military and civilian medicine pipelines. As a secondary effect, the military treatment centers that house the military GME programs would have to close, with surrounding civilian medical facilities also likely unable to absorb the sudden influx of patients and residents losing the intangible benefits of caring for a military population.15 This was even recognized by the civilian president of the Accreditation Council for Graduate Medical Education:
Military physicians must be trained in the systems of care that are operative in military medicine, which is significantly unlike civilian medicine in many ways. It is often practiced in circumstances that are not seen in civilian medicine, within care structures that are not encountered in American medical practice… Military medicine has advanced research into the care of individuals suffering traumatic injury, critical care, rehabilitation medicine, prosthetics, psychiatric care of those traumatized, and closed head injury, to name a just a few. The sacrifices of our active military demand these advances, and the American Public benefit from these advances.21
Where deficiencies exist in military GME, it is possible to use the growing military-civilian training institution partnerships. Two prime examples are the just-in-time deployment training done with civilian trauma facilities by the Air Force Center for the Sustainment of Trauma Readiness Skills and the Air Force Special Operations Surgical Team-Special Operations Critical Care Evacuation Team being embedded in civilian facilities to maintain trauma, surgical, and emergency care skills. While military physicians can maintain competencies, at the same time, the civilian sector can benefit from the lessons learned in the military in regard to mass casualty and disaster responses. Fostering military and civilian training agreements can also enhance research opportunities.1
Just as the realities of operational medicine frequently require the military physician to think outside the box, the most successful methods of instruction of military medicine tend to be nontraditional. Classroom education should be involved beyond lectures and can include other methods, such as case-based, role-playing, small group discussion, and computer-based teaching. Maintaining flexibility in live vs distance learning as well as synchronous vs asynchronous learning can expand the capacity of available instructors and standardize material over several sites.23 Asking learners to consider operational concerns, such as whether certain medical conditions would be compatible with military duty in addition to the routine investigation is an easy way to incorporate military training in preexisting medical training.12 The advancement of technology has made simulation one of the best ways to engage in hands-on learning, whether through computer simulations, animal models, standardized or moulaged patients, or mannequins that can realistically mimic medical or trauma-related conditions.24 Many times, simulation can be combined with exercises in the field to create a realistic operational environment.23
There are 3 pillars of an operational curriculum that should be integrated into the existing residency curriculum—operational medicine, leadership, and research principles (Appendix).
Conclusions
Judging by the continuing operational tempo and evolution of warfare, maintaining enhanced military medical readiness will remain a priority. Operational medicine is a unique field that requires specialized preparation. Studies have shown that longitudinal deliberately mapped out curriculums are able to be integrated well into the existing medical curriculum. The recommendation moving forward is increasing the access of existing operational training structures that have well established programs and modeling individual GME program curriculums after those that have shown proven success with a focus on the 3 pillars of operational training, leadership, and research.
Acknowledgments
Previously submitted in April 2020 in expanded form as part of graduation requirements for the Masters of Military Arts and Science degree program at Air University, Maxwell Air Force Base in Alabama.
1. US Government Accountability Office. Defense Health Care: DoD’s proposed plan for oversight of graduate medical education program. Published March 2019. Accessed September 24, 2021. https://www.gao.gov/assets/700/698075.pdf
2. De Lorenzo RA. Accreditation status of U.S. military graduate medical education programs. Mil Med. 2008;173(7):635-640. doi:10.7205/milmed.173.7.635
3. John SK, Bishop JM, Hidreth LA, et al; Institute for Defense Analysis. Analysis of DoD accession alternatives for military physicians: readiness value and cost. Published October 2019. Accessed September 24, 2021. https://www.ida.org/-/media/feature/publications/a/an/analysis-of-dod-accession-alternatives-for-military-physicians-readiness-value-and-cost/p-10815.ashx.
4. O’Connor FG, Grunberg N, Kellermann AL, Schoomaker E. Leadership education and development at the Uniformed Services University. Mil Med. 2015;180(suppl 4):147-152. doi:10.7205/MILMED-D-14-00563
5. Suls H, Karnei K, Gardner JW, Fogarty JP, Llewellyn CH. The extent of military medicine topics taught in military family practice residency programs: Part II, a survey of residency graduates from 1987-1990. Mil Med. 1997;162(6):428-434. doi:10.1093/milmed/162.6.428
6. Salerno S, Cash B, Cranston M, Schoomaker E. Perceptions of current and recent military internal medicine residents on operational medicine, managed care, graduate medical education, and continued military service. Mil Med. 1998;163(6):392-397. doi:10.1093/milmed/163.6.392
7. Roop SA, Murray CK, Pugh AM, Phillips YY, Bolan CD. Operational medicine experience integrated into a military internal medicine residency curriculum. Mil Med. 2001;166(1):34-39. doi:10.1093/milmed/166.1.34
8. Perkins JG, Roy MJ, Bolan CD, Phillips YY. Operational experiences during medical residency: perspectives from the Walter Reed Army Medical Center Department of Medicine. Mil Med. 2001;166(12):1038-1045. doi:10.1093/milmed/166.12.1038
9. Murray CK, Reynolds JC, Boyer DA, et al. Development of a deployment course for graduating military internal medicine residents. Mil Med. 2006;171(10):933-936. doi:10.7205/milmed.171.10.933. doi:10.7205/milmed.171.10.933
10. Picho K, Gilliland WR, Artino AR Jr, et al. Assessing curriculum effectiveness: a survey of Uniformed Services University medical school graduates. Mil Med. 2015;180(suppl 4):113-128. doi:10.7205/MILMED-D-14-00570
11. Jacobson MD: Operational Aerospace medicine collaborative programs: past, present, and future. US Air Force School of Aerospace Medicine Presentation. November 1, 2018.
12. Roy MJ, Brietzke S, Hemmer P, Pangaro L, Goldstein R. Teaching military medicine: enhancing military relevance within the fabric of current medical training. Mil Med. 2002;167(4):277-280. doi:10.1093/miled.milmed.167.4.277
13. Durning SJ, Dong T, LaRochelle JL, et al. The long-term career outcome study: lessons learned and implications for educational practice. Mil Med. 2015;180(suppl 4):164-170. doi:10.7205/MILMED-D-14-00574
14. Keating EG, Brauner MK, Galway LA, Mele JD, Burks JJ, Saloner B. The Air Force Medical Corps’ status and how its physicians respond to multiyear special pay. Mil Med. 2009;174(11):1155-1162. doi:10.7205/milmed-d-01-4309
15. Mundell BF. Retention of military physicians: the differential effects of practice opportunities across the three services. RAND Corporation; 2010:74-77. Accessed September 24, 2021. https://www.rand.org/pubs/rgs_dissertations/RGSD275.html
16. Nagy CJ. The importance of a military-unique curriculum in active duty graduate medical education. Mil Med. 2012;177(3):243-244. doi:10.7205/milmed-d-11-00280
17. True M: The value of military graduate medical education. SAUSHEC interim dean talking paper. November 2, 2018.
18. Hatzfeld JJ, Khalili RA, Hendrickson TL, Reilly PA. Publishing military medical research: appreciating the process. Mil Med. 2016;181(suppl 5):5-6. doi:10.7205/MILMED-D-15-00517
19. Sauer SW, Robinson JB, Smith MP, et al. Lessons learned: saving lives on the battlefield. J Spec Oper Med. 2016;15(2). 25-41.
20. Tankersley MS: Air Force Physician Education Branch response to GME questions. Talking Paper. Feb 23, 2015.
21. Nasca TJ. [Letter] Published October 26, 2019. Accessed September 24, 2021. https://www.moaa.org/uploadedfiles/nasca-to-kellerman-a--cordts-p-2019-10-26.pdf
22. Forgione MA: USAF-SAM GME Brief. Air Force Personnel Center. October 2018.
23. Turner M, Wilson C, Gausman K, Roy MJ. Optimal methods of learning for military medical education. Mil Med. 2003;168(suppl 9):46-50. doi:10.1093/milmed/168.suppl_1.46
24. Goolsby C, Deering S. Hybrid simulation during military medical student field training--a novel curriculum. Mil Med. 2013;178(7):742-745. doi:10.7205/MILMED-D-12-00541
25. Hartzell JD, Yu CE, Cohee BM, Nelson MR, Wilson RL. Moving beyond accidental leadership: a graduate medical education leadership curriculum needs assessment. Mil Med. 2017;182(7):e1815-e1822. doi:10.7205/MILMED-D-16-00365
26. Barry ES, Dong T, Durning SJ, Schreiber-Gregory D, Torre D, Grunberg NE. Medical Student Leader Performance in an Applied Medical Field Practicum. Mil Med. 2019;184(11-12):653-660. doi:10.1093/milmed/usz121
27. Air Force Medical Corps Development Team: Medical corps integrated OPS career path. MC Pyramids 2019 Presentation. January 18, 2019. https://kx.health.mil [Nonpublic source, not verified]
28. Polski MM: Back to basics—research design for the operational level of war. Naval War College Rev. 2019;72(3):1-23. https://digital-commons.usnwc.edu/nwc-review/vol72/iss3/6.
It is a time of significant change as the Military Health System (MHS) transitions to the purview of the Defense Health Agency (DHA). Additionally, the landscape of combat is ever changing, and military medicine needs to evolve to ensure that the lessons learned are utilized to optimize care of the war fighters. The purpose of this review is to evaluate the available literature on existing operational medicine curriculums and make recommendations to restructure current military medicine training to produce operationally prepared clinicians who are informed in operationally focused research principles.
Operational Medicine
Before diving into the importance of creating a curriculum and investing in training for scholarly activity proficiency, operational medicine needs to be defined. It can be defined as medical care provided in an austere environment with limited resources and possibly under hostile conditions. Another way to look at operational medicine is as the evaluation of normal human physiology and pathology under abnormal conditions. The mission set of each of the services is unique. The Marines and Army may operate forward past the wire vulnerable to the environment, gunfire, and improvised explosive devices, remote from fixed medical facilities. The Navy has divers exposed to the risks of decompression sickness. The Air Force has pilots exposed to altitude changes and strains of G-forces during flight. Locations vary from cold high-altitude mountainous regions to high-temperature desolate deserts. Many times, medical practitioners may be remotely stationed, far from specialty or immediate definitive care. Patient care may consist of low-acuity management of individual patients in sick call to mass casualty events where patient numbers and morbidity may outstrip available resources, making the difficult task of triage necessary.
Despite the challenges of being a uniformed physician, the benefits of being embedded is a better understanding of the roles and capability of the unit. Military physicians need to have the unique knowledge of the type of injuries sustained in that particular theater of war, such as differentiating between the trauma pattern and care required for blast injuries vs high-velocity missiles. There are also chemical, biologic, radiologic, and nuclear threats that military physicians need to recognize. Much of what disables a military fighting force is not a direct relationship to combat-related injuries; however, entire units have been taken down by infectious diarrhea or trench foot. There is also a need for familiarity of the infections and parasitology endemic to the particular theater with the aim of implementation of prevention whenever possible.
Military medicine does not fit in any box. Military physicians need to know the job requirements of various specialties, including elements of occupational medicine, such as aircrew piloting high-performance fighters or ground troops fully loaded with body armor and 80-lb backpacks. There are musculoskeletal injuries from the stressors of various military occupations. Working around weaponry and contact with hostile forces will create scenarios requiring emergent and critical care. In addition to physical injuries, there is the mental strain of combat with the risk of imminent personal injury, the guilt of survivorship, dealing with the scars and permanent physical damage of combat, and prolonged separation from family and other support systems.
The National Defense Authorization Act 2017 mandated the establishment of a standardized process to oversee all military graduate medical education (GME) programs with the goal of ensuring medical operational readiness.1 This is no small task with > 3000 residents in more than 70 specialties, comprising approximately 12% of US residents.1,2 Presently, 26 to 32% of the medical corps is enrolled in full-time training compared with 12% of the total force.2 With significant time and resources expended during this period, it is vital to maximize the potential of the training.
Literature Review
A literature review was performed, evaluating historical precedence of specialized military medical training and research as well as current operational curriculums. Literature search was conducted in the PubMed and Uniformed Services University (USU) Learning Resource databases using the terms “operational medicine curriculum,” “military medicine curriculum,” “operational medicine training,” “military medicine training,” “operational medicine research,” and “military medicine research,” and included all articles from 1997 to 2020. Inclusion criteria included studies that detailed military medicine training programs and/or outcomes. The source types used in this research project included peer-reviewed journal publications—both review articles and original research—from medical and military journals. The citations of these articles were also reviewed for additional usable publications. Secondary sources included official reports and studies by the RAND Corporation, the US Government Accountability Office, and the Institute for Defense Analysis (IDA). Due to lack of literature on the topic, other sources such as talking papers, letters, and formal presentations from subject matter experts were included to showcase the current state and gaps on this topic. Key findings from peer-reviewed publications are presented in Table 1.
Overall, the literature review showed that longitudinal deliberately mapped out curriculums can be well integrated into the existing medical curriculum.3 The military medicine course topics include environmental medicine, applied field medicine, combat casualty care, medical support planning, mass casualty incident preparation, and military-focused problem solving, decision making, and leadership.4
One 1997 study looked at the degree of implementation of military unique curriculum in 18 family medicine residencies. Only 30% of residents stated that their program had a specific operational medicine curriculum.5 Salerno and colleagues surveyed current residents and recently graduated internal medicine physicians at 14 facilities in the Army, Air Force, and Navy to determine confidence level with military medicine. More than half did not feel ready to practice deployment medicine; just 19% felt comfortable treating nuclear, biologic, and chemical warfare injuries; and 32% felt unfamiliar with the command and administrative duties. A subgroup analysis showed that USU graduates felt more prepared in these areas compared with civilian program graduates.6 Additional studies showed perceived smoother transition in the first active-duty tour after participation in an operational curriculum.7
Didactics can provide a foundation. However, just as the practice of medicine is learned in the clinic, the art of military medicine is learned in the field. Hands-on training in one study was accomplished through the Combat Casualty Care Course (C4), the USU Bushmaster exercise, and a field training exercise. The field exercise included components of mission planning, medical threat assessments, triage of a mass casualty situation, management of disease and nonbattle injuries, combat stress casualties, resource management, and patient evacuation.8
Another publication described a similar longitudinal curriculum with C4 after the first year of training and the Medical Management of Chemical and Biological Casualty Course during the second year. The operational curriculum 3-day capstone occurred at the end of medical training utilizing mannequins to realistically simulate combat casualty care, including emergency airways, chest tube, and tourniquets.9 Due to the current deployment tempo, just in time refresher courses like this could be valuable preparation.
While most of the operational curriculums evaluated assessed efficiency over a short time interval, one study looked at 1189 graduates from the military medical school from the past 20 years. Preparedness was perceived to be high for military-unique practice and leadership.10 The operational curriculum at USU had been purposefully structured to provide continuity. Didactics and casework were reinforced with hands-on training whether through realistic simulator training or field exercises. The authors note a weakness of many operational curriculums is inconsistency and fragmented training without deliberate longitudinal planning.
One of the more recent military GME curriculums include the creation of the operational medicine residency in 2013, which created a standardized longitudinal operational curriculum integrated along with the existing family medicine, emergency medicine, or internal medicine curriculum to create mission-ready military physicians upon graduation. Scheduled rotations include global medicine, aeromedical evacuation, occupational medicine, and tropical medicine. Completing military officer professional development and an operationally relevant research project is an expectation (Table 2).11
In addition to in-program training, other options include operational rotations offsite and military courses conducted outside the GME program.12 Some of these courses may include just-in-time training such as expeditionary medical support system training prior to scheduled deployments. Examples of experiential training are listed in Table 3.
Critical Analysis
Current gaps were identified in the military medicine training pipeline’s operational medicine curriculum and research programs. The analysis looked at specific components that make the operational medicine curriculum and research unique as well as current readiness goals, to determine how to best align both to meet the mission requirements. Some factors considered included efficiency, cost, program portability, duplication minimization, retention, and sustainability.
Efficiency
A well-created curriculum that meets objectives will require more than an assigned rotation and a few lectures. The most successful ones in the literature review were the ones that were deliberately planned and longitudinal, such as the ones at USU that combined a mixture of classroom and field exercises over the course of 4 years.4,8 In that way, the curriculum may not be considered time efficient, but if integrated well into the already existing medical training, the production of military physicians who are mission ready upon graduation—ready to serve as military medical leaders and deploy—will be invaluable.
Cost Comparison
Due to the associated overhead of running a training platform and the additional hours of operational training, military GME is more expensive initially compared with civilian outsourcing. In USU, for example, there is an additional 700 hours of operational curriculum alone. This cost difference more than doubles the cost of a USU education vs a Health Professional Scholarship Program (HPSP) scholarship at a civilian medical school. However, a causal analysis performed by the IDA to determine value basis noted that USU graduates deploy almost 3 times as much and serve 6 years longer on active duty.3
After graduating medical school through either accession source, physicians complete specialization training in a GME program. The IDA study noted an average $12,000 increased cost of military GME compared with civilian programs. The analysis included resident compensation and overhead costs of running the program as well as the net cost, which also accounted for resident productivity and workload by training in a military facility.3 Calculations due to mandated budget cuts estimated cost savings of closing the military medical school at < $100 million while significantly impacting the military physician pipeline and operational research output.3
Duplication of Effort
There are already established training programs such as Tactical Combat Casualty Care (TCCC) that could be incorporated into the curriculum to avoid expending additional resources to recreate the wheel. USU has a validated operational training curriculum and may be able to make opportunities available for outside trainees to participate in some of its military-unique training and leadership exercises. Other ways to decrease duplication of effort and improve cost efficiency include focusing on the creation of an academic health system (AHS) and consolidating similar programs to conserve resources. Increasing existing military program sizes will not only ensure the continuation of the military medicine pipeline, but will spread overhead costs over a larger cohort, decrease costs of civilian outsourcing, and ensure the less tangible benefits of military cultural exposure early in trainees’ careers. For example, increasing the class size of USU by 30 students actually reduces the cost per student to $239,000 per year from $253,000, while decreasing the need for HPSP accessions training in civilian programs, making the endeavor overall cost neutral.3
Program Portability
The operational medicine residency has proved that an operational curriculum can be remotely managed and reproduced at a variety of residency specialties.12 Remote education could be developed and distributed throughout the MHS, such as the proposed USU course Military Medicine and Leadership course.3 Centralized training programs like Global Medicine and C-STARS could be scheduled TDYs during the medical training calendar.
Retention
The military medical school, USU, is the largest military medicine accession source. An IDA report notes that retention of USU graduates is 15.2 years compared with 9.2 years served by civilian trainees. Due to the longevity in service, USU graduates also make up more than 25% of military medical leadership.4 The long-term outcome study that looked at the past 40 years of USU graduates observed that over 70% of graduates served until retirement eligibility and are overrepresented in special operations units.3,13 While some of this longevity may be attributed to the longer USU service contracts, military GME graduates were still noted to be 4 times more likely to commit to a multiyear service contract.14 A RAND study on the retention of military physicians in the Army, Air Force, and Navy noted that overall retention increased throughout all the services for physicians who went through the military GME pipeline.15 Conversely, civilian GME training was associated with a 45% chance in leaving active duty.16
It is theorized that early military acculturation during training increases the likelihood of instilling a sense of mission. Being involved in military GME on the teaching side also showed increased retention rates for 63% of survey respondents.17 Reduced burnout and increased work satisfaction for those involved in military GME was noted on another faculty satisfaction survey.17
Sustainability
Programs like USU, which have been around for decades, and the newer operational residency program evolving since 2013 have shown sustainability.4,11 Dissemination of proven curriculums as well as centralization of already validated training programs can help standardize operational medical training throughout the MHS. In order to flourish at individual programs, the faculty need to be well versed in a train the trainer model and have institutional support. The ability to engage with the line at individual locations may be a factor as well.18 In regard to research, once residents are taught the principles of scholarly activity, they will have the tools to continue operational medicine research advancements and mentoring students.
Discussion
The 2020 NDAA recommends the establishment of an AHS.3 This step will create a culture of military medical readiness from the top down as congressional mandates push reorganization of the MHS, including military GME programs. An overall restructuring of military medicine will require prioritization of resources toward operational requirements vs the historic significant division of attention to beneficiary care that has caused a lack of unity of effort and additional strain on an already heavily tasked medical force. The changes in military GME are just one aspect of that. It is vital to look at the restructuring with a comprehension of the unique challenges of combat health rather than only from an in-garrison, hospital-based aspect.19 Benefits of having a military medicine AHS include opportunities to share resources and successful business models as well as foster interdisciplinary teamwork and partnerships with civilian health care facilities and research institutions as a force multiplier.19
There has been recent discussion about budget cuts, including shutting down USU and military GME and transitioning all training to civilian programs to be cost-effective.4 If this were to happen, it would be a step backward from the goal of operational readiness. Maintaining US Department of Defense (DoD) control of the military medicine pipeline has innumerable benefits, including built-in mentorship from operationally-seasoned faculty, military leadership development, proficiency in MHS systems, open communication between GME programs and DoD, and curriculum control to ensure focus on readiness.20 Military GME programs are also a significant production source of military-related scholarly activity. Over fiscal year 2017/2018, 63% of the publications out of the San Antonio Uniformed Services Health Education Consortium—the largest Air Force GME platform and second largest multiservice GME platform—involved military relevant medical topics.17 Much of the volume of operational research as well as the relevant skills learned and future innovations secondary to conducting this research would be lost if military GME did not exist.17,21
Practically speaking, military GME provides the majority of the military medicine accessions. For example, a presentation by the Air Force Chief of Physician Education noted that the total military GME pipeline included 2875 students, but direct physician access averaged only 20 physicians a year.22 Even if the decision was made to defer to civilian education, capacity does not exist in civilian GME programs. This is worsened by the increased competitiveness of the GME match with the proliferation of medical schools without concurrent increase in residency spots. The 2018 National Resident Matching Program noted that there were more than 37,103 US and foreign applicants for only 33,000 residency positions, leaving many US applicants unmatched.17 It is doubtful that the civilian GME programs would be able to absorb the influx of military residents, affecting both the military and civilian medicine pipelines. As a secondary effect, the military treatment centers that house the military GME programs would have to close, with surrounding civilian medical facilities also likely unable to absorb the sudden influx of patients and residents losing the intangible benefits of caring for a military population.15 This was even recognized by the civilian president of the Accreditation Council for Graduate Medical Education:
Military physicians must be trained in the systems of care that are operative in military medicine, which is significantly unlike civilian medicine in many ways. It is often practiced in circumstances that are not seen in civilian medicine, within care structures that are not encountered in American medical practice… Military medicine has advanced research into the care of individuals suffering traumatic injury, critical care, rehabilitation medicine, prosthetics, psychiatric care of those traumatized, and closed head injury, to name a just a few. The sacrifices of our active military demand these advances, and the American Public benefit from these advances.21
Where deficiencies exist in military GME, it is possible to use the growing military-civilian training institution partnerships. Two prime examples are the just-in-time deployment training done with civilian trauma facilities by the Air Force Center for the Sustainment of Trauma Readiness Skills and the Air Force Special Operations Surgical Team-Special Operations Critical Care Evacuation Team being embedded in civilian facilities to maintain trauma, surgical, and emergency care skills. While military physicians can maintain competencies, at the same time, the civilian sector can benefit from the lessons learned in the military in regard to mass casualty and disaster responses. Fostering military and civilian training agreements can also enhance research opportunities.1
Just as the realities of operational medicine frequently require the military physician to think outside the box, the most successful methods of instruction of military medicine tend to be nontraditional. Classroom education should be involved beyond lectures and can include other methods, such as case-based, role-playing, small group discussion, and computer-based teaching. Maintaining flexibility in live vs distance learning as well as synchronous vs asynchronous learning can expand the capacity of available instructors and standardize material over several sites.23 Asking learners to consider operational concerns, such as whether certain medical conditions would be compatible with military duty in addition to the routine investigation is an easy way to incorporate military training in preexisting medical training.12 The advancement of technology has made simulation one of the best ways to engage in hands-on learning, whether through computer simulations, animal models, standardized or moulaged patients, or mannequins that can realistically mimic medical or trauma-related conditions.24 Many times, simulation can be combined with exercises in the field to create a realistic operational environment.23
There are 3 pillars of an operational curriculum that should be integrated into the existing residency curriculum—operational medicine, leadership, and research principles (Appendix).
Conclusions
Judging by the continuing operational tempo and evolution of warfare, maintaining enhanced military medical readiness will remain a priority. Operational medicine is a unique field that requires specialized preparation. Studies have shown that longitudinal deliberately mapped out curriculums are able to be integrated well into the existing medical curriculum. The recommendation moving forward is increasing the access of existing operational training structures that have well established programs and modeling individual GME program curriculums after those that have shown proven success with a focus on the 3 pillars of operational training, leadership, and research.
Acknowledgments
Previously submitted in April 2020 in expanded form as part of graduation requirements for the Masters of Military Arts and Science degree program at Air University, Maxwell Air Force Base in Alabama.
It is a time of significant change as the Military Health System (MHS) transitions to the purview of the Defense Health Agency (DHA). Additionally, the landscape of combat is ever changing, and military medicine needs to evolve to ensure that the lessons learned are utilized to optimize care of the war fighters. The purpose of this review is to evaluate the available literature on existing operational medicine curriculums and make recommendations to restructure current military medicine training to produce operationally prepared clinicians who are informed in operationally focused research principles.
Operational Medicine
Before diving into the importance of creating a curriculum and investing in training for scholarly activity proficiency, operational medicine needs to be defined. It can be defined as medical care provided in an austere environment with limited resources and possibly under hostile conditions. Another way to look at operational medicine is as the evaluation of normal human physiology and pathology under abnormal conditions. The mission set of each of the services is unique. The Marines and Army may operate forward past the wire vulnerable to the environment, gunfire, and improvised explosive devices, remote from fixed medical facilities. The Navy has divers exposed to the risks of decompression sickness. The Air Force has pilots exposed to altitude changes and strains of G-forces during flight. Locations vary from cold high-altitude mountainous regions to high-temperature desolate deserts. Many times, medical practitioners may be remotely stationed, far from specialty or immediate definitive care. Patient care may consist of low-acuity management of individual patients in sick call to mass casualty events where patient numbers and morbidity may outstrip available resources, making the difficult task of triage necessary.
Despite the challenges of being a uniformed physician, the benefits of being embedded is a better understanding of the roles and capability of the unit. Military physicians need to have the unique knowledge of the type of injuries sustained in that particular theater of war, such as differentiating between the trauma pattern and care required for blast injuries vs high-velocity missiles. There are also chemical, biologic, radiologic, and nuclear threats that military physicians need to recognize. Much of what disables a military fighting force is not a direct relationship to combat-related injuries; however, entire units have been taken down by infectious diarrhea or trench foot. There is also a need for familiarity of the infections and parasitology endemic to the particular theater with the aim of implementation of prevention whenever possible.
Military medicine does not fit in any box. Military physicians need to know the job requirements of various specialties, including elements of occupational medicine, such as aircrew piloting high-performance fighters or ground troops fully loaded with body armor and 80-lb backpacks. There are musculoskeletal injuries from the stressors of various military occupations. Working around weaponry and contact with hostile forces will create scenarios requiring emergent and critical care. In addition to physical injuries, there is the mental strain of combat with the risk of imminent personal injury, the guilt of survivorship, dealing with the scars and permanent physical damage of combat, and prolonged separation from family and other support systems.
The National Defense Authorization Act 2017 mandated the establishment of a standardized process to oversee all military graduate medical education (GME) programs with the goal of ensuring medical operational readiness.1 This is no small task with > 3000 residents in more than 70 specialties, comprising approximately 12% of US residents.1,2 Presently, 26 to 32% of the medical corps is enrolled in full-time training compared with 12% of the total force.2 With significant time and resources expended during this period, it is vital to maximize the potential of the training.
Literature Review
A literature review was performed, evaluating historical precedence of specialized military medical training and research as well as current operational curriculums. Literature search was conducted in the PubMed and Uniformed Services University (USU) Learning Resource databases using the terms “operational medicine curriculum,” “military medicine curriculum,” “operational medicine training,” “military medicine training,” “operational medicine research,” and “military medicine research,” and included all articles from 1997 to 2020. Inclusion criteria included studies that detailed military medicine training programs and/or outcomes. The source types used in this research project included peer-reviewed journal publications—both review articles and original research—from medical and military journals. The citations of these articles were also reviewed for additional usable publications. Secondary sources included official reports and studies by the RAND Corporation, the US Government Accountability Office, and the Institute for Defense Analysis (IDA). Due to lack of literature on the topic, other sources such as talking papers, letters, and formal presentations from subject matter experts were included to showcase the current state and gaps on this topic. Key findings from peer-reviewed publications are presented in Table 1.
Overall, the literature review showed that longitudinal deliberately mapped out curriculums can be well integrated into the existing medical curriculum.3 The military medicine course topics include environmental medicine, applied field medicine, combat casualty care, medical support planning, mass casualty incident preparation, and military-focused problem solving, decision making, and leadership.4
One 1997 study looked at the degree of implementation of military unique curriculum in 18 family medicine residencies. Only 30% of residents stated that their program had a specific operational medicine curriculum.5 Salerno and colleagues surveyed current residents and recently graduated internal medicine physicians at 14 facilities in the Army, Air Force, and Navy to determine confidence level with military medicine. More than half did not feel ready to practice deployment medicine; just 19% felt comfortable treating nuclear, biologic, and chemical warfare injuries; and 32% felt unfamiliar with the command and administrative duties. A subgroup analysis showed that USU graduates felt more prepared in these areas compared with civilian program graduates.6 Additional studies showed perceived smoother transition in the first active-duty tour after participation in an operational curriculum.7
Didactics can provide a foundation. However, just as the practice of medicine is learned in the clinic, the art of military medicine is learned in the field. Hands-on training in one study was accomplished through the Combat Casualty Care Course (C4), the USU Bushmaster exercise, and a field training exercise. The field exercise included components of mission planning, medical threat assessments, triage of a mass casualty situation, management of disease and nonbattle injuries, combat stress casualties, resource management, and patient evacuation.8
Another publication described a similar longitudinal curriculum with C4 after the first year of training and the Medical Management of Chemical and Biological Casualty Course during the second year. The operational curriculum 3-day capstone occurred at the end of medical training utilizing mannequins to realistically simulate combat casualty care, including emergency airways, chest tube, and tourniquets.9 Due to the current deployment tempo, just in time refresher courses like this could be valuable preparation.
While most of the operational curriculums evaluated assessed efficiency over a short time interval, one study looked at 1189 graduates from the military medical school from the past 20 years. Preparedness was perceived to be high for military-unique practice and leadership.10 The operational curriculum at USU had been purposefully structured to provide continuity. Didactics and casework were reinforced with hands-on training whether through realistic simulator training or field exercises. The authors note a weakness of many operational curriculums is inconsistency and fragmented training without deliberate longitudinal planning.
One of the more recent military GME curriculums include the creation of the operational medicine residency in 2013, which created a standardized longitudinal operational curriculum integrated along with the existing family medicine, emergency medicine, or internal medicine curriculum to create mission-ready military physicians upon graduation. Scheduled rotations include global medicine, aeromedical evacuation, occupational medicine, and tropical medicine. Completing military officer professional development and an operationally relevant research project is an expectation (Table 2).11
In addition to in-program training, other options include operational rotations offsite and military courses conducted outside the GME program.12 Some of these courses may include just-in-time training such as expeditionary medical support system training prior to scheduled deployments. Examples of experiential training are listed in Table 3.
Critical Analysis
Current gaps were identified in the military medicine training pipeline’s operational medicine curriculum and research programs. The analysis looked at specific components that make the operational medicine curriculum and research unique as well as current readiness goals, to determine how to best align both to meet the mission requirements. Some factors considered included efficiency, cost, program portability, duplication minimization, retention, and sustainability.
Efficiency
A well-created curriculum that meets objectives will require more than an assigned rotation and a few lectures. The most successful ones in the literature review were the ones that were deliberately planned and longitudinal, such as the ones at USU that combined a mixture of classroom and field exercises over the course of 4 years.4,8 In that way, the curriculum may not be considered time efficient, but if integrated well into the already existing medical training, the production of military physicians who are mission ready upon graduation—ready to serve as military medical leaders and deploy—will be invaluable.
Cost Comparison
Due to the associated overhead of running a training platform and the additional hours of operational training, military GME is more expensive initially compared with civilian outsourcing. In USU, for example, there is an additional 700 hours of operational curriculum alone. This cost difference more than doubles the cost of a USU education vs a Health Professional Scholarship Program (HPSP) scholarship at a civilian medical school. However, a causal analysis performed by the IDA to determine value basis noted that USU graduates deploy almost 3 times as much and serve 6 years longer on active duty.3
After graduating medical school through either accession source, physicians complete specialization training in a GME program. The IDA study noted an average $12,000 increased cost of military GME compared with civilian programs. The analysis included resident compensation and overhead costs of running the program as well as the net cost, which also accounted for resident productivity and workload by training in a military facility.3 Calculations due to mandated budget cuts estimated cost savings of closing the military medical school at < $100 million while significantly impacting the military physician pipeline and operational research output.3
Duplication of Effort
There are already established training programs such as Tactical Combat Casualty Care (TCCC) that could be incorporated into the curriculum to avoid expending additional resources to recreate the wheel. USU has a validated operational training curriculum and may be able to make opportunities available for outside trainees to participate in some of its military-unique training and leadership exercises. Other ways to decrease duplication of effort and improve cost efficiency include focusing on the creation of an academic health system (AHS) and consolidating similar programs to conserve resources. Increasing existing military program sizes will not only ensure the continuation of the military medicine pipeline, but will spread overhead costs over a larger cohort, decrease costs of civilian outsourcing, and ensure the less tangible benefits of military cultural exposure early in trainees’ careers. For example, increasing the class size of USU by 30 students actually reduces the cost per student to $239,000 per year from $253,000, while decreasing the need for HPSP accessions training in civilian programs, making the endeavor overall cost neutral.3
Program Portability
The operational medicine residency has proved that an operational curriculum can be remotely managed and reproduced at a variety of residency specialties.12 Remote education could be developed and distributed throughout the MHS, such as the proposed USU course Military Medicine and Leadership course.3 Centralized training programs like Global Medicine and C-STARS could be scheduled TDYs during the medical training calendar.
Retention
The military medical school, USU, is the largest military medicine accession source. An IDA report notes that retention of USU graduates is 15.2 years compared with 9.2 years served by civilian trainees. Due to the longevity in service, USU graduates also make up more than 25% of military medical leadership.4 The long-term outcome study that looked at the past 40 years of USU graduates observed that over 70% of graduates served until retirement eligibility and are overrepresented in special operations units.3,13 While some of this longevity may be attributed to the longer USU service contracts, military GME graduates were still noted to be 4 times more likely to commit to a multiyear service contract.14 A RAND study on the retention of military physicians in the Army, Air Force, and Navy noted that overall retention increased throughout all the services for physicians who went through the military GME pipeline.15 Conversely, civilian GME training was associated with a 45% chance in leaving active duty.16
It is theorized that early military acculturation during training increases the likelihood of instilling a sense of mission. Being involved in military GME on the teaching side also showed increased retention rates for 63% of survey respondents.17 Reduced burnout and increased work satisfaction for those involved in military GME was noted on another faculty satisfaction survey.17
Sustainability
Programs like USU, which have been around for decades, and the newer operational residency program evolving since 2013 have shown sustainability.4,11 Dissemination of proven curriculums as well as centralization of already validated training programs can help standardize operational medical training throughout the MHS. In order to flourish at individual programs, the faculty need to be well versed in a train the trainer model and have institutional support. The ability to engage with the line at individual locations may be a factor as well.18 In regard to research, once residents are taught the principles of scholarly activity, they will have the tools to continue operational medicine research advancements and mentoring students.
Discussion
The 2020 NDAA recommends the establishment of an AHS.3 This step will create a culture of military medical readiness from the top down as congressional mandates push reorganization of the MHS, including military GME programs. An overall restructuring of military medicine will require prioritization of resources toward operational requirements vs the historic significant division of attention to beneficiary care that has caused a lack of unity of effort and additional strain on an already heavily tasked medical force. The changes in military GME are just one aspect of that. It is vital to look at the restructuring with a comprehension of the unique challenges of combat health rather than only from an in-garrison, hospital-based aspect.19 Benefits of having a military medicine AHS include opportunities to share resources and successful business models as well as foster interdisciplinary teamwork and partnerships with civilian health care facilities and research institutions as a force multiplier.19
There has been recent discussion about budget cuts, including shutting down USU and military GME and transitioning all training to civilian programs to be cost-effective.4 If this were to happen, it would be a step backward from the goal of operational readiness. Maintaining US Department of Defense (DoD) control of the military medicine pipeline has innumerable benefits, including built-in mentorship from operationally-seasoned faculty, military leadership development, proficiency in MHS systems, open communication between GME programs and DoD, and curriculum control to ensure focus on readiness.20 Military GME programs are also a significant production source of military-related scholarly activity. Over fiscal year 2017/2018, 63% of the publications out of the San Antonio Uniformed Services Health Education Consortium—the largest Air Force GME platform and second largest multiservice GME platform—involved military relevant medical topics.17 Much of the volume of operational research as well as the relevant skills learned and future innovations secondary to conducting this research would be lost if military GME did not exist.17,21
Practically speaking, military GME provides the majority of the military medicine accessions. For example, a presentation by the Air Force Chief of Physician Education noted that the total military GME pipeline included 2875 students, but direct physician access averaged only 20 physicians a year.22 Even if the decision was made to defer to civilian education, capacity does not exist in civilian GME programs. This is worsened by the increased competitiveness of the GME match with the proliferation of medical schools without concurrent increase in residency spots. The 2018 National Resident Matching Program noted that there were more than 37,103 US and foreign applicants for only 33,000 residency positions, leaving many US applicants unmatched.17 It is doubtful that the civilian GME programs would be able to absorb the influx of military residents, affecting both the military and civilian medicine pipelines. As a secondary effect, the military treatment centers that house the military GME programs would have to close, with surrounding civilian medical facilities also likely unable to absorb the sudden influx of patients and residents losing the intangible benefits of caring for a military population.15 This was even recognized by the civilian president of the Accreditation Council for Graduate Medical Education:
Military physicians must be trained in the systems of care that are operative in military medicine, which is significantly unlike civilian medicine in many ways. It is often practiced in circumstances that are not seen in civilian medicine, within care structures that are not encountered in American medical practice… Military medicine has advanced research into the care of individuals suffering traumatic injury, critical care, rehabilitation medicine, prosthetics, psychiatric care of those traumatized, and closed head injury, to name a just a few. The sacrifices of our active military demand these advances, and the American Public benefit from these advances.21
Where deficiencies exist in military GME, it is possible to use the growing military-civilian training institution partnerships. Two prime examples are the just-in-time deployment training done with civilian trauma facilities by the Air Force Center for the Sustainment of Trauma Readiness Skills and the Air Force Special Operations Surgical Team-Special Operations Critical Care Evacuation Team being embedded in civilian facilities to maintain trauma, surgical, and emergency care skills. While military physicians can maintain competencies, at the same time, the civilian sector can benefit from the lessons learned in the military in regard to mass casualty and disaster responses. Fostering military and civilian training agreements can also enhance research opportunities.1
Just as the realities of operational medicine frequently require the military physician to think outside the box, the most successful methods of instruction of military medicine tend to be nontraditional. Classroom education should be involved beyond lectures and can include other methods, such as case-based, role-playing, small group discussion, and computer-based teaching. Maintaining flexibility in live vs distance learning as well as synchronous vs asynchronous learning can expand the capacity of available instructors and standardize material over several sites.23 Asking learners to consider operational concerns, such as whether certain medical conditions would be compatible with military duty in addition to the routine investigation is an easy way to incorporate military training in preexisting medical training.12 The advancement of technology has made simulation one of the best ways to engage in hands-on learning, whether through computer simulations, animal models, standardized or moulaged patients, or mannequins that can realistically mimic medical or trauma-related conditions.24 Many times, simulation can be combined with exercises in the field to create a realistic operational environment.23
There are 3 pillars of an operational curriculum that should be integrated into the existing residency curriculum—operational medicine, leadership, and research principles (Appendix).
Conclusions
Judging by the continuing operational tempo and evolution of warfare, maintaining enhanced military medical readiness will remain a priority. Operational medicine is a unique field that requires specialized preparation. Studies have shown that longitudinal deliberately mapped out curriculums are able to be integrated well into the existing medical curriculum. The recommendation moving forward is increasing the access of existing operational training structures that have well established programs and modeling individual GME program curriculums after those that have shown proven success with a focus on the 3 pillars of operational training, leadership, and research.
Acknowledgments
Previously submitted in April 2020 in expanded form as part of graduation requirements for the Masters of Military Arts and Science degree program at Air University, Maxwell Air Force Base in Alabama.
1. US Government Accountability Office. Defense Health Care: DoD’s proposed plan for oversight of graduate medical education program. Published March 2019. Accessed September 24, 2021. https://www.gao.gov/assets/700/698075.pdf
2. De Lorenzo RA. Accreditation status of U.S. military graduate medical education programs. Mil Med. 2008;173(7):635-640. doi:10.7205/milmed.173.7.635
3. John SK, Bishop JM, Hidreth LA, et al; Institute for Defense Analysis. Analysis of DoD accession alternatives for military physicians: readiness value and cost. Published October 2019. Accessed September 24, 2021. https://www.ida.org/-/media/feature/publications/a/an/analysis-of-dod-accession-alternatives-for-military-physicians-readiness-value-and-cost/p-10815.ashx.
4. O’Connor FG, Grunberg N, Kellermann AL, Schoomaker E. Leadership education and development at the Uniformed Services University. Mil Med. 2015;180(suppl 4):147-152. doi:10.7205/MILMED-D-14-00563
5. Suls H, Karnei K, Gardner JW, Fogarty JP, Llewellyn CH. The extent of military medicine topics taught in military family practice residency programs: Part II, a survey of residency graduates from 1987-1990. Mil Med. 1997;162(6):428-434. doi:10.1093/milmed/162.6.428
6. Salerno S, Cash B, Cranston M, Schoomaker E. Perceptions of current and recent military internal medicine residents on operational medicine, managed care, graduate medical education, and continued military service. Mil Med. 1998;163(6):392-397. doi:10.1093/milmed/163.6.392
7. Roop SA, Murray CK, Pugh AM, Phillips YY, Bolan CD. Operational medicine experience integrated into a military internal medicine residency curriculum. Mil Med. 2001;166(1):34-39. doi:10.1093/milmed/166.1.34
8. Perkins JG, Roy MJ, Bolan CD, Phillips YY. Operational experiences during medical residency: perspectives from the Walter Reed Army Medical Center Department of Medicine. Mil Med. 2001;166(12):1038-1045. doi:10.1093/milmed/166.12.1038
9. Murray CK, Reynolds JC, Boyer DA, et al. Development of a deployment course for graduating military internal medicine residents. Mil Med. 2006;171(10):933-936. doi:10.7205/milmed.171.10.933. doi:10.7205/milmed.171.10.933
10. Picho K, Gilliland WR, Artino AR Jr, et al. Assessing curriculum effectiveness: a survey of Uniformed Services University medical school graduates. Mil Med. 2015;180(suppl 4):113-128. doi:10.7205/MILMED-D-14-00570
11. Jacobson MD: Operational Aerospace medicine collaborative programs: past, present, and future. US Air Force School of Aerospace Medicine Presentation. November 1, 2018.
12. Roy MJ, Brietzke S, Hemmer P, Pangaro L, Goldstein R. Teaching military medicine: enhancing military relevance within the fabric of current medical training. Mil Med. 2002;167(4):277-280. doi:10.1093/miled.milmed.167.4.277
13. Durning SJ, Dong T, LaRochelle JL, et al. The long-term career outcome study: lessons learned and implications for educational practice. Mil Med. 2015;180(suppl 4):164-170. doi:10.7205/MILMED-D-14-00574
14. Keating EG, Brauner MK, Galway LA, Mele JD, Burks JJ, Saloner B. The Air Force Medical Corps’ status and how its physicians respond to multiyear special pay. Mil Med. 2009;174(11):1155-1162. doi:10.7205/milmed-d-01-4309
15. Mundell BF. Retention of military physicians: the differential effects of practice opportunities across the three services. RAND Corporation; 2010:74-77. Accessed September 24, 2021. https://www.rand.org/pubs/rgs_dissertations/RGSD275.html
16. Nagy CJ. The importance of a military-unique curriculum in active duty graduate medical education. Mil Med. 2012;177(3):243-244. doi:10.7205/milmed-d-11-00280
17. True M: The value of military graduate medical education. SAUSHEC interim dean talking paper. November 2, 2018.
18. Hatzfeld JJ, Khalili RA, Hendrickson TL, Reilly PA. Publishing military medical research: appreciating the process. Mil Med. 2016;181(suppl 5):5-6. doi:10.7205/MILMED-D-15-00517
19. Sauer SW, Robinson JB, Smith MP, et al. Lessons learned: saving lives on the battlefield. J Spec Oper Med. 2016;15(2). 25-41.
20. Tankersley MS: Air Force Physician Education Branch response to GME questions. Talking Paper. Feb 23, 2015.
21. Nasca TJ. [Letter] Published October 26, 2019. Accessed September 24, 2021. https://www.moaa.org/uploadedfiles/nasca-to-kellerman-a--cordts-p-2019-10-26.pdf
22. Forgione MA: USAF-SAM GME Brief. Air Force Personnel Center. October 2018.
23. Turner M, Wilson C, Gausman K, Roy MJ. Optimal methods of learning for military medical education. Mil Med. 2003;168(suppl 9):46-50. doi:10.1093/milmed/168.suppl_1.46
24. Goolsby C, Deering S. Hybrid simulation during military medical student field training--a novel curriculum. Mil Med. 2013;178(7):742-745. doi:10.7205/MILMED-D-12-00541
25. Hartzell JD, Yu CE, Cohee BM, Nelson MR, Wilson RL. Moving beyond accidental leadership: a graduate medical education leadership curriculum needs assessment. Mil Med. 2017;182(7):e1815-e1822. doi:10.7205/MILMED-D-16-00365
26. Barry ES, Dong T, Durning SJ, Schreiber-Gregory D, Torre D, Grunberg NE. Medical Student Leader Performance in an Applied Medical Field Practicum. Mil Med. 2019;184(11-12):653-660. doi:10.1093/milmed/usz121
27. Air Force Medical Corps Development Team: Medical corps integrated OPS career path. MC Pyramids 2019 Presentation. January 18, 2019. https://kx.health.mil [Nonpublic source, not verified]
28. Polski MM: Back to basics—research design for the operational level of war. Naval War College Rev. 2019;72(3):1-23. https://digital-commons.usnwc.edu/nwc-review/vol72/iss3/6.
1. US Government Accountability Office. Defense Health Care: DoD’s proposed plan for oversight of graduate medical education program. Published March 2019. Accessed September 24, 2021. https://www.gao.gov/assets/700/698075.pdf
2. De Lorenzo RA. Accreditation status of U.S. military graduate medical education programs. Mil Med. 2008;173(7):635-640. doi:10.7205/milmed.173.7.635
3. John SK, Bishop JM, Hidreth LA, et al; Institute for Defense Analysis. Analysis of DoD accession alternatives for military physicians: readiness value and cost. Published October 2019. Accessed September 24, 2021. https://www.ida.org/-/media/feature/publications/a/an/analysis-of-dod-accession-alternatives-for-military-physicians-readiness-value-and-cost/p-10815.ashx.
4. O’Connor FG, Grunberg N, Kellermann AL, Schoomaker E. Leadership education and development at the Uniformed Services University. Mil Med. 2015;180(suppl 4):147-152. doi:10.7205/MILMED-D-14-00563
5. Suls H, Karnei K, Gardner JW, Fogarty JP, Llewellyn CH. The extent of military medicine topics taught in military family practice residency programs: Part II, a survey of residency graduates from 1987-1990. Mil Med. 1997;162(6):428-434. doi:10.1093/milmed/162.6.428
6. Salerno S, Cash B, Cranston M, Schoomaker E. Perceptions of current and recent military internal medicine residents on operational medicine, managed care, graduate medical education, and continued military service. Mil Med. 1998;163(6):392-397. doi:10.1093/milmed/163.6.392
7. Roop SA, Murray CK, Pugh AM, Phillips YY, Bolan CD. Operational medicine experience integrated into a military internal medicine residency curriculum. Mil Med. 2001;166(1):34-39. doi:10.1093/milmed/166.1.34
8. Perkins JG, Roy MJ, Bolan CD, Phillips YY. Operational experiences during medical residency: perspectives from the Walter Reed Army Medical Center Department of Medicine. Mil Med. 2001;166(12):1038-1045. doi:10.1093/milmed/166.12.1038
9. Murray CK, Reynolds JC, Boyer DA, et al. Development of a deployment course for graduating military internal medicine residents. Mil Med. 2006;171(10):933-936. doi:10.7205/milmed.171.10.933. doi:10.7205/milmed.171.10.933
10. Picho K, Gilliland WR, Artino AR Jr, et al. Assessing curriculum effectiveness: a survey of Uniformed Services University medical school graduates. Mil Med. 2015;180(suppl 4):113-128. doi:10.7205/MILMED-D-14-00570
11. Jacobson MD: Operational Aerospace medicine collaborative programs: past, present, and future. US Air Force School of Aerospace Medicine Presentation. November 1, 2018.
12. Roy MJ, Brietzke S, Hemmer P, Pangaro L, Goldstein R. Teaching military medicine: enhancing military relevance within the fabric of current medical training. Mil Med. 2002;167(4):277-280. doi:10.1093/miled.milmed.167.4.277
13. Durning SJ, Dong T, LaRochelle JL, et al. The long-term career outcome study: lessons learned and implications for educational practice. Mil Med. 2015;180(suppl 4):164-170. doi:10.7205/MILMED-D-14-00574
14. Keating EG, Brauner MK, Galway LA, Mele JD, Burks JJ, Saloner B. The Air Force Medical Corps’ status and how its physicians respond to multiyear special pay. Mil Med. 2009;174(11):1155-1162. doi:10.7205/milmed-d-01-4309
15. Mundell BF. Retention of military physicians: the differential effects of practice opportunities across the three services. RAND Corporation; 2010:74-77. Accessed September 24, 2021. https://www.rand.org/pubs/rgs_dissertations/RGSD275.html
16. Nagy CJ. The importance of a military-unique curriculum in active duty graduate medical education. Mil Med. 2012;177(3):243-244. doi:10.7205/milmed-d-11-00280
17. True M: The value of military graduate medical education. SAUSHEC interim dean talking paper. November 2, 2018.
18. Hatzfeld JJ, Khalili RA, Hendrickson TL, Reilly PA. Publishing military medical research: appreciating the process. Mil Med. 2016;181(suppl 5):5-6. doi:10.7205/MILMED-D-15-00517
19. Sauer SW, Robinson JB, Smith MP, et al. Lessons learned: saving lives on the battlefield. J Spec Oper Med. 2016;15(2). 25-41.
20. Tankersley MS: Air Force Physician Education Branch response to GME questions. Talking Paper. Feb 23, 2015.
21. Nasca TJ. [Letter] Published October 26, 2019. Accessed September 24, 2021. https://www.moaa.org/uploadedfiles/nasca-to-kellerman-a--cordts-p-2019-10-26.pdf
22. Forgione MA: USAF-SAM GME Brief. Air Force Personnel Center. October 2018.
23. Turner M, Wilson C, Gausman K, Roy MJ. Optimal methods of learning for military medical education. Mil Med. 2003;168(suppl 9):46-50. doi:10.1093/milmed/168.suppl_1.46
24. Goolsby C, Deering S. Hybrid simulation during military medical student field training--a novel curriculum. Mil Med. 2013;178(7):742-745. doi:10.7205/MILMED-D-12-00541
25. Hartzell JD, Yu CE, Cohee BM, Nelson MR, Wilson RL. Moving beyond accidental leadership: a graduate medical education leadership curriculum needs assessment. Mil Med. 2017;182(7):e1815-e1822. doi:10.7205/MILMED-D-16-00365
26. Barry ES, Dong T, Durning SJ, Schreiber-Gregory D, Torre D, Grunberg NE. Medical Student Leader Performance in an Applied Medical Field Practicum. Mil Med. 2019;184(11-12):653-660. doi:10.1093/milmed/usz121
27. Air Force Medical Corps Development Team: Medical corps integrated OPS career path. MC Pyramids 2019 Presentation. January 18, 2019. https://kx.health.mil [Nonpublic source, not verified]
28. Polski MM: Back to basics—research design for the operational level of war. Naval War College Rev. 2019;72(3):1-23. https://digital-commons.usnwc.edu/nwc-review/vol72/iss3/6.
VA Firearm Policy Got It Half Right
To the Editor: September is National Suicide Prevention and Awareness month. In 2021, the US Department of Veterans Affairs (VA) Office of Mental Health and Suicide Prevention marked the month by demonstrating why it is the national visionary when it comes to preventing suicide. The office rolled out several public service announcements (PSAs) about creating “space between thought and trigger.”1 These incredibly sensitive spots, the first of their kind, encourage safer storage and reduced access to firearms at points of heightened crises. The PSAs are timely, especially given the just released annual report showing that 69.2% of veteran suicide deaths are by firearm.2 Wide PSA dissemination is vital.
But concerningly, the PSAs completely missed the importance of critical partnerships. As described in Federal Practitioner 2 years ago, VA forged a groundbreaking collaboration with the National Shooting Sports Foundation (NSSF), the firearms industry trade association, and the American Foundation for Suicide Prevention (AFSP).3 Having NSSF as a partner advanced VA’s effort to ensure that lethal means safety counseling is culturally relevant, comes from a trusted source, and contains no antifirearm bias. Since then, VA and NSSF cobranded billboards in 8 states, encouraging storing firearms responsibly to prevent suicide. They collectively developed an educational, training, and resource toolkit that guides communities through the process of building coalitions to raise awareness about securely storing firearms when not in use.4 VA and NSSF have cross-listed safe storage websites. In May 2020, the VA cosponsored a COVID-19 suicide prevention video with the NSSF, AFSP, and the US Concealed Carry Association, including ways that the firearm industry, gun owners, and their families can help.5
Yet when the VA launched its PSA campaign last month, NSSF’s name was conspicuously absent. That must be corrected going forward. Reaching vulnerable veterans who own firearms requires partnerships with individuals and groups who own firearms. Going it alone undercuts the essence of what VA has worked so hard to achieve in the past few years.
Russell B. Lemle, PhD
Veterans Healthcare
Policy Institute
1. US Department of Veterans Affairs. Firearm suicide and lethal means safety, space between thought and trigger. Updated September 22, 2021. Accessed October 1, 2021. https://www.va.gov/reach/lethal-means
2. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. 2021 National veteran suicide prevention annual report. Published September 8, 2021. Accessed October 1, 2021. https://www.mentalhealth.va.gov/docs/data-sheets/2021/2021-National-Veteran-Suicide-Prevention-Annual-Report-FINAL-9-8-21.pdf
3. Lemle, RB. VA forges a historic partnership with the national shooting sports foundation and the American foundation for suicide prevention to prevent veteran suicide. Published February 15, 2019. Accessed October 1, 2021. https://www.mdedge.com/fedprac/article/194610/mental-health/va-forges-historic-partnership-national-shooting-sports
4. US Department of Veterans Affairs, National Shooting Sports Foundation, American Foundation for Suicide Prevention. Suicide prevention is everyone’s business: a toolkit for safe firearm storage in your community. Published February 24, 2020. Accessed October 1, 2021. https://www.mentalhealth.va.gov/suicide_prevention/docs/Toolkit_Safe_Firearm_Storage_CLEARED_508_2-24-20.pdf
5. US Concealed Carry Association. Protecting mental health and preventing suicide during COVID 19. Published May 14, 2020. Accessed October 1, 2021. https://www.youtube.com/watch?app=desktop&v=Rp48Pnl5fUA&feature=youtube
To the Editor: September is National Suicide Prevention and Awareness month. In 2021, the US Department of Veterans Affairs (VA) Office of Mental Health and Suicide Prevention marked the month by demonstrating why it is the national visionary when it comes to preventing suicide. The office rolled out several public service announcements (PSAs) about creating “space between thought and trigger.”1 These incredibly sensitive spots, the first of their kind, encourage safer storage and reduced access to firearms at points of heightened crises. The PSAs are timely, especially given the just released annual report showing that 69.2% of veteran suicide deaths are by firearm.2 Wide PSA dissemination is vital.
But concerningly, the PSAs completely missed the importance of critical partnerships. As described in Federal Practitioner 2 years ago, VA forged a groundbreaking collaboration with the National Shooting Sports Foundation (NSSF), the firearms industry trade association, and the American Foundation for Suicide Prevention (AFSP).3 Having NSSF as a partner advanced VA’s effort to ensure that lethal means safety counseling is culturally relevant, comes from a trusted source, and contains no antifirearm bias. Since then, VA and NSSF cobranded billboards in 8 states, encouraging storing firearms responsibly to prevent suicide. They collectively developed an educational, training, and resource toolkit that guides communities through the process of building coalitions to raise awareness about securely storing firearms when not in use.4 VA and NSSF have cross-listed safe storage websites. In May 2020, the VA cosponsored a COVID-19 suicide prevention video with the NSSF, AFSP, and the US Concealed Carry Association, including ways that the firearm industry, gun owners, and their families can help.5
Yet when the VA launched its PSA campaign last month, NSSF’s name was conspicuously absent. That must be corrected going forward. Reaching vulnerable veterans who own firearms requires partnerships with individuals and groups who own firearms. Going it alone undercuts the essence of what VA has worked so hard to achieve in the past few years.
Russell B. Lemle, PhD
Veterans Healthcare
Policy Institute
To the Editor: September is National Suicide Prevention and Awareness month. In 2021, the US Department of Veterans Affairs (VA) Office of Mental Health and Suicide Prevention marked the month by demonstrating why it is the national visionary when it comes to preventing suicide. The office rolled out several public service announcements (PSAs) about creating “space between thought and trigger.”1 These incredibly sensitive spots, the first of their kind, encourage safer storage and reduced access to firearms at points of heightened crises. The PSAs are timely, especially given the just released annual report showing that 69.2% of veteran suicide deaths are by firearm.2 Wide PSA dissemination is vital.
But concerningly, the PSAs completely missed the importance of critical partnerships. As described in Federal Practitioner 2 years ago, VA forged a groundbreaking collaboration with the National Shooting Sports Foundation (NSSF), the firearms industry trade association, and the American Foundation for Suicide Prevention (AFSP).3 Having NSSF as a partner advanced VA’s effort to ensure that lethal means safety counseling is culturally relevant, comes from a trusted source, and contains no antifirearm bias. Since then, VA and NSSF cobranded billboards in 8 states, encouraging storing firearms responsibly to prevent suicide. They collectively developed an educational, training, and resource toolkit that guides communities through the process of building coalitions to raise awareness about securely storing firearms when not in use.4 VA and NSSF have cross-listed safe storage websites. In May 2020, the VA cosponsored a COVID-19 suicide prevention video with the NSSF, AFSP, and the US Concealed Carry Association, including ways that the firearm industry, gun owners, and their families can help.5
Yet when the VA launched its PSA campaign last month, NSSF’s name was conspicuously absent. That must be corrected going forward. Reaching vulnerable veterans who own firearms requires partnerships with individuals and groups who own firearms. Going it alone undercuts the essence of what VA has worked so hard to achieve in the past few years.
Russell B. Lemle, PhD
Veterans Healthcare
Policy Institute
1. US Department of Veterans Affairs. Firearm suicide and lethal means safety, space between thought and trigger. Updated September 22, 2021. Accessed October 1, 2021. https://www.va.gov/reach/lethal-means
2. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. 2021 National veteran suicide prevention annual report. Published September 8, 2021. Accessed October 1, 2021. https://www.mentalhealth.va.gov/docs/data-sheets/2021/2021-National-Veteran-Suicide-Prevention-Annual-Report-FINAL-9-8-21.pdf
3. Lemle, RB. VA forges a historic partnership with the national shooting sports foundation and the American foundation for suicide prevention to prevent veteran suicide. Published February 15, 2019. Accessed October 1, 2021. https://www.mdedge.com/fedprac/article/194610/mental-health/va-forges-historic-partnership-national-shooting-sports
4. US Department of Veterans Affairs, National Shooting Sports Foundation, American Foundation for Suicide Prevention. Suicide prevention is everyone’s business: a toolkit for safe firearm storage in your community. Published February 24, 2020. Accessed October 1, 2021. https://www.mentalhealth.va.gov/suicide_prevention/docs/Toolkit_Safe_Firearm_Storage_CLEARED_508_2-24-20.pdf
5. US Concealed Carry Association. Protecting mental health and preventing suicide during COVID 19. Published May 14, 2020. Accessed October 1, 2021. https://www.youtube.com/watch?app=desktop&v=Rp48Pnl5fUA&feature=youtube
1. US Department of Veterans Affairs. Firearm suicide and lethal means safety, space between thought and trigger. Updated September 22, 2021. Accessed October 1, 2021. https://www.va.gov/reach/lethal-means
2. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. 2021 National veteran suicide prevention annual report. Published September 8, 2021. Accessed October 1, 2021. https://www.mentalhealth.va.gov/docs/data-sheets/2021/2021-National-Veteran-Suicide-Prevention-Annual-Report-FINAL-9-8-21.pdf
3. Lemle, RB. VA forges a historic partnership with the national shooting sports foundation and the American foundation for suicide prevention to prevent veteran suicide. Published February 15, 2019. Accessed October 1, 2021. https://www.mdedge.com/fedprac/article/194610/mental-health/va-forges-historic-partnership-national-shooting-sports
4. US Department of Veterans Affairs, National Shooting Sports Foundation, American Foundation for Suicide Prevention. Suicide prevention is everyone’s business: a toolkit for safe firearm storage in your community. Published February 24, 2020. Accessed October 1, 2021. https://www.mentalhealth.va.gov/suicide_prevention/docs/Toolkit_Safe_Firearm_Storage_CLEARED_508_2-24-20.pdf
5. US Concealed Carry Association. Protecting mental health and preventing suicide during COVID 19. Published May 14, 2020. Accessed October 1, 2021. https://www.youtube.com/watch?app=desktop&v=Rp48Pnl5fUA&feature=youtube
Evaluating the Impact of a Simulated Hypersensitivity Reaction Case Study for New Fellows and Chemotherapy Nurses in an Outpatient Infusion Clinic
Background
All chemotherapeutic agents have potential to cause infusion reactions. Our primary objective was to develop a project to assist in appropriate training of nursing staff and incoming fellows for clinic efficiency and patient safety.
Methods
A multi-disciplinary team, including physicians, nurses, and a pharmacist met and following a pre-assessment, a pareto chart was created to determine where to focus our efforts. The results revealed the following areas of concern from most important to least important: utilization of an infusion reaction “kit,” team discussion with staff, infusion reaction simulation, a competency checklist for reactions and “other.” Other responses included: reaction orders in the chart, hands on scenarios, and continued reinforcements. The team resolved to conduct an infusion reaction simulation program to provide an environment to meet many needs of the team, new and experienced. Set in the outpatient infusion center, the program included: a patient/actor, a facilitator, infusion nursing staff, and physicians/fellows. Physicians were invited to participate in the training, but infusion staff were unaware of the program to provide another real life aspect to the simulation; however, both were blinded to the scenario. The pharmacist facilitated the event where the patient actor proceeded to start with a minor infusion reaction that progressed to full anaphylaxis.
Results
Using a Likert scale, a post simulation assessment included 6 questions: 90% of participants felt strongly the exercise increased awareness of the infusion reaction e-kit, 80% felt strongly the exercise was meaningful to their practice, 90% strongly agreed or agreed the scenario simulated a real life situation, also 90% strongly agreed or agreed the program helped them think critically. Finally, 100% of participants strongly agreed or agreed they felt confident in their ability to intervene in the event of a hypersensitivity reaction. Our objectives were achieved: identify the signs and symptoms of a hypersensitivity reaction, utilize the proper intervention in the event of a hypersensitivity reaction. Other outcomes include an updated chemotherapy order consult complete with standing reaction orders in the medical record.
Conclusion
Ultimately, our interdisciplinary simulation concluded with increased awareness, improved confidence, and strengthened collaboration, communication and accountability among our infusion staff and oncology providers
Background
All chemotherapeutic agents have potential to cause infusion reactions. Our primary objective was to develop a project to assist in appropriate training of nursing staff and incoming fellows for clinic efficiency and patient safety.
Methods
A multi-disciplinary team, including physicians, nurses, and a pharmacist met and following a pre-assessment, a pareto chart was created to determine where to focus our efforts. The results revealed the following areas of concern from most important to least important: utilization of an infusion reaction “kit,” team discussion with staff, infusion reaction simulation, a competency checklist for reactions and “other.” Other responses included: reaction orders in the chart, hands on scenarios, and continued reinforcements. The team resolved to conduct an infusion reaction simulation program to provide an environment to meet many needs of the team, new and experienced. Set in the outpatient infusion center, the program included: a patient/actor, a facilitator, infusion nursing staff, and physicians/fellows. Physicians were invited to participate in the training, but infusion staff were unaware of the program to provide another real life aspect to the simulation; however, both were blinded to the scenario. The pharmacist facilitated the event where the patient actor proceeded to start with a minor infusion reaction that progressed to full anaphylaxis.
Results
Using a Likert scale, a post simulation assessment included 6 questions: 90% of participants felt strongly the exercise increased awareness of the infusion reaction e-kit, 80% felt strongly the exercise was meaningful to their practice, 90% strongly agreed or agreed the scenario simulated a real life situation, also 90% strongly agreed or agreed the program helped them think critically. Finally, 100% of participants strongly agreed or agreed they felt confident in their ability to intervene in the event of a hypersensitivity reaction. Our objectives were achieved: identify the signs and symptoms of a hypersensitivity reaction, utilize the proper intervention in the event of a hypersensitivity reaction. Other outcomes include an updated chemotherapy order consult complete with standing reaction orders in the medical record.
Conclusion
Ultimately, our interdisciplinary simulation concluded with increased awareness, improved confidence, and strengthened collaboration, communication and accountability among our infusion staff and oncology providers
Background
All chemotherapeutic agents have potential to cause infusion reactions. Our primary objective was to develop a project to assist in appropriate training of nursing staff and incoming fellows for clinic efficiency and patient safety.
Methods
A multi-disciplinary team, including physicians, nurses, and a pharmacist met and following a pre-assessment, a pareto chart was created to determine where to focus our efforts. The results revealed the following areas of concern from most important to least important: utilization of an infusion reaction “kit,” team discussion with staff, infusion reaction simulation, a competency checklist for reactions and “other.” Other responses included: reaction orders in the chart, hands on scenarios, and continued reinforcements. The team resolved to conduct an infusion reaction simulation program to provide an environment to meet many needs of the team, new and experienced. Set in the outpatient infusion center, the program included: a patient/actor, a facilitator, infusion nursing staff, and physicians/fellows. Physicians were invited to participate in the training, but infusion staff were unaware of the program to provide another real life aspect to the simulation; however, both were blinded to the scenario. The pharmacist facilitated the event where the patient actor proceeded to start with a minor infusion reaction that progressed to full anaphylaxis.
Results
Using a Likert scale, a post simulation assessment included 6 questions: 90% of participants felt strongly the exercise increased awareness of the infusion reaction e-kit, 80% felt strongly the exercise was meaningful to their practice, 90% strongly agreed or agreed the scenario simulated a real life situation, also 90% strongly agreed or agreed the program helped them think critically. Finally, 100% of participants strongly agreed or agreed they felt confident in their ability to intervene in the event of a hypersensitivity reaction. Our objectives were achieved: identify the signs and symptoms of a hypersensitivity reaction, utilize the proper intervention in the event of a hypersensitivity reaction. Other outcomes include an updated chemotherapy order consult complete with standing reaction orders in the medical record.
Conclusion
Ultimately, our interdisciplinary simulation concluded with increased awareness, improved confidence, and strengthened collaboration, communication and accountability among our infusion staff and oncology providers
The Transitions of Care Clinic: Demonstrating the Utility of the Single-Site Quality Improvement Study
A significant literature describes efforts to reduce hospital readmissions through improving care transitions. Many approaches have been tried, alone or in combination, targeting different points across the spectrum of discharge activities. These approaches encompass interventions initiated prior to discharge, such as patient education and enhanced discharge planning; bridging interventions, such as transition coaches; and postdischarge interventions, such as home visits or early follow-up appointments. Transitions of care clinics (TOCC) attempt to improve posthospital care by providing dedicated, rapid follow-up for patients after discharge.1
The impact of care transitions interventions is mixed, with inconsistent results across interventions and contexts. More complex, multipronged, context- and patient-sensitive interventions, however, are more likely to be associated with lower readmission rates.2,3
In this issue of the journal, Griffin and colleagues4 report on their TOCC implementation. Their focus on a high-risk, rural veteran population is different from prior studies, as is their use of in-person or virtual follow-up options. While the authors describe their intervention as a TOCC, their model serves as an organizer for an interprofessional team, including hospitalists, that coordinates multiple activities that complement the postdischarge appointments: identification of high-risk patients, pharmacist-led medication reconciliation, dietary counseling, contingency planning for potential changes, follow-up on pending tests and studies, and coordination of primary care and specialty care appointments. The multipronged, patient-sensitive nature of their intervention makes their positive findings consistent with other care transition literature.
Griffin and colleagues’ reporting of their TOCC experience is worth highlighting, as they present their experience and results in a way that maximizes our ability to learn from their implementation. Unfortunately, reports of improvement initiatives often lack sufficient detail regarding the context or intervention to potentially apply their findings. Griffin and colleagues applied the Revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines, a standardized framework for describing improvement initiatives that captures critical contextual and intervention elements.5Griffin and colleagues describe their baseline readmission performance and how the TOCC model was relevant to this issue. They describe the context, including their patient population, and their intervention with sufficient detail for us to understand what they actually did. Importantly, Griffin and colleagues clearly delineate the dynamic phases of the implementation, their use of Plan-Do-Study-Act cycles to assess and improve their implementation, and the specific changes they made. The Figure clearly puts their results in the context of their program evolution, and their secondary outcomes support our understanding of program growth. Their use of a committee for ongoing monitoring could be important for ongoing adaptation and sustainability.
There are several limitations worth noting. There may have been subjectivity in teams’ decisions to refer specific patients with lower
In this era of multisite collaborative studies and analyses of large administrative datasets, Griffin et al4 demonstrate that there is still much to learn from a well-done, single-site improvement study.
Funding: Drs Leykum and Penney reported funding from the Department of Veterans Affairs.
1. Nall RW, Herndon BB, Mramba LK, Vogel-Anderson K, Hagen MG. An interprofessional primary care-based transition of care clinic to reduce hospital readmission. J Am Med. 2019; 133(6):E260-E268. https://doi.org/10.1016/j.amjmed.2019.10.040
2. Leppin AL, Gionfriddo MR, Kessler M, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095-1107. https://doi.org/10.1001/jamainternmed.2014.1608
3. Pugh J, Penney LS, Noel PH, Neller S, Mader M, Finley EP, Lanham HJ, Leykum LK. Evidence-based processes to prevent readmissions: more is better, a ten-site observational study. BMC Health Serv Res. 2021; 21:189. https://doi.org/10.1186/s12913-021-06193-x
4. Griffin BR, Agarwal N, Amberker R, et al. An initiative to improve 30-day readmission rates using a transitions-of-care clinic among a mixed urban and rural Veteran population. J Hosp Med. 2021;16(10):583-588. https://doi.org/10.12788/jhm.3659
5. Squire 2.0 guidelines. Accessed September 17, 2021. http://squire-statement.org
A significant literature describes efforts to reduce hospital readmissions through improving care transitions. Many approaches have been tried, alone or in combination, targeting different points across the spectrum of discharge activities. These approaches encompass interventions initiated prior to discharge, such as patient education and enhanced discharge planning; bridging interventions, such as transition coaches; and postdischarge interventions, such as home visits or early follow-up appointments. Transitions of care clinics (TOCC) attempt to improve posthospital care by providing dedicated, rapid follow-up for patients after discharge.1
The impact of care transitions interventions is mixed, with inconsistent results across interventions and contexts. More complex, multipronged, context- and patient-sensitive interventions, however, are more likely to be associated with lower readmission rates.2,3
In this issue of the journal, Griffin and colleagues4 report on their TOCC implementation. Their focus on a high-risk, rural veteran population is different from prior studies, as is their use of in-person or virtual follow-up options. While the authors describe their intervention as a TOCC, their model serves as an organizer for an interprofessional team, including hospitalists, that coordinates multiple activities that complement the postdischarge appointments: identification of high-risk patients, pharmacist-led medication reconciliation, dietary counseling, contingency planning for potential changes, follow-up on pending tests and studies, and coordination of primary care and specialty care appointments. The multipronged, patient-sensitive nature of their intervention makes their positive findings consistent with other care transition literature.
Griffin and colleagues’ reporting of their TOCC experience is worth highlighting, as they present their experience and results in a way that maximizes our ability to learn from their implementation. Unfortunately, reports of improvement initiatives often lack sufficient detail regarding the context or intervention to potentially apply their findings. Griffin and colleagues applied the Revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines, a standardized framework for describing improvement initiatives that captures critical contextual and intervention elements.5Griffin and colleagues describe their baseline readmission performance and how the TOCC model was relevant to this issue. They describe the context, including their patient population, and their intervention with sufficient detail for us to understand what they actually did. Importantly, Griffin and colleagues clearly delineate the dynamic phases of the implementation, their use of Plan-Do-Study-Act cycles to assess and improve their implementation, and the specific changes they made. The Figure clearly puts their results in the context of their program evolution, and their secondary outcomes support our understanding of program growth. Their use of a committee for ongoing monitoring could be important for ongoing adaptation and sustainability.
There are several limitations worth noting. There may have been subjectivity in teams’ decisions to refer specific patients with lower
In this era of multisite collaborative studies and analyses of large administrative datasets, Griffin et al4 demonstrate that there is still much to learn from a well-done, single-site improvement study.
Funding: Drs Leykum and Penney reported funding from the Department of Veterans Affairs.
A significant literature describes efforts to reduce hospital readmissions through improving care transitions. Many approaches have been tried, alone or in combination, targeting different points across the spectrum of discharge activities. These approaches encompass interventions initiated prior to discharge, such as patient education and enhanced discharge planning; bridging interventions, such as transition coaches; and postdischarge interventions, such as home visits or early follow-up appointments. Transitions of care clinics (TOCC) attempt to improve posthospital care by providing dedicated, rapid follow-up for patients after discharge.1
The impact of care transitions interventions is mixed, with inconsistent results across interventions and contexts. More complex, multipronged, context- and patient-sensitive interventions, however, are more likely to be associated with lower readmission rates.2,3
In this issue of the journal, Griffin and colleagues4 report on their TOCC implementation. Their focus on a high-risk, rural veteran population is different from prior studies, as is their use of in-person or virtual follow-up options. While the authors describe their intervention as a TOCC, their model serves as an organizer for an interprofessional team, including hospitalists, that coordinates multiple activities that complement the postdischarge appointments: identification of high-risk patients, pharmacist-led medication reconciliation, dietary counseling, contingency planning for potential changes, follow-up on pending tests and studies, and coordination of primary care and specialty care appointments. The multipronged, patient-sensitive nature of their intervention makes their positive findings consistent with other care transition literature.
Griffin and colleagues’ reporting of their TOCC experience is worth highlighting, as they present their experience and results in a way that maximizes our ability to learn from their implementation. Unfortunately, reports of improvement initiatives often lack sufficient detail regarding the context or intervention to potentially apply their findings. Griffin and colleagues applied the Revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines, a standardized framework for describing improvement initiatives that captures critical contextual and intervention elements.5Griffin and colleagues describe their baseline readmission performance and how the TOCC model was relevant to this issue. They describe the context, including their patient population, and their intervention with sufficient detail for us to understand what they actually did. Importantly, Griffin and colleagues clearly delineate the dynamic phases of the implementation, their use of Plan-Do-Study-Act cycles to assess and improve their implementation, and the specific changes they made. The Figure clearly puts their results in the context of their program evolution, and their secondary outcomes support our understanding of program growth. Their use of a committee for ongoing monitoring could be important for ongoing adaptation and sustainability.
There are several limitations worth noting. There may have been subjectivity in teams’ decisions to refer specific patients with lower
In this era of multisite collaborative studies and analyses of large administrative datasets, Griffin et al4 demonstrate that there is still much to learn from a well-done, single-site improvement study.
Funding: Drs Leykum and Penney reported funding from the Department of Veterans Affairs.
1. Nall RW, Herndon BB, Mramba LK, Vogel-Anderson K, Hagen MG. An interprofessional primary care-based transition of care clinic to reduce hospital readmission. J Am Med. 2019; 133(6):E260-E268. https://doi.org/10.1016/j.amjmed.2019.10.040
2. Leppin AL, Gionfriddo MR, Kessler M, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095-1107. https://doi.org/10.1001/jamainternmed.2014.1608
3. Pugh J, Penney LS, Noel PH, Neller S, Mader M, Finley EP, Lanham HJ, Leykum LK. Evidence-based processes to prevent readmissions: more is better, a ten-site observational study. BMC Health Serv Res. 2021; 21:189. https://doi.org/10.1186/s12913-021-06193-x
4. Griffin BR, Agarwal N, Amberker R, et al. An initiative to improve 30-day readmission rates using a transitions-of-care clinic among a mixed urban and rural Veteran population. J Hosp Med. 2021;16(10):583-588. https://doi.org/10.12788/jhm.3659
5. Squire 2.0 guidelines. Accessed September 17, 2021. http://squire-statement.org
1. Nall RW, Herndon BB, Mramba LK, Vogel-Anderson K, Hagen MG. An interprofessional primary care-based transition of care clinic to reduce hospital readmission. J Am Med. 2019; 133(6):E260-E268. https://doi.org/10.1016/j.amjmed.2019.10.040
2. Leppin AL, Gionfriddo MR, Kessler M, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095-1107. https://doi.org/10.1001/jamainternmed.2014.1608
3. Pugh J, Penney LS, Noel PH, Neller S, Mader M, Finley EP, Lanham HJ, Leykum LK. Evidence-based processes to prevent readmissions: more is better, a ten-site observational study. BMC Health Serv Res. 2021; 21:189. https://doi.org/10.1186/s12913-021-06193-x
4. Griffin BR, Agarwal N, Amberker R, et al. An initiative to improve 30-day readmission rates using a transitions-of-care clinic among a mixed urban and rural Veteran population. J Hosp Med. 2021;16(10):583-588. https://doi.org/10.12788/jhm.3659
5. Squire 2.0 guidelines. Accessed September 17, 2021. http://squire-statement.org
© 2021 Society of Hospital Medicine
Predictive Models for In-Hospital Deterioration in Ward Patients
Adults admitted to general medical-surgical wards who experience in-hospital deterioration have a disproportionate effect on hospital mortality and length of stay.1 Not long ago, systematic electronic capture of vital signs—arguably the most important predictors of impending deterioration—was restricted to intensive care units (ICUs). Deployment of comprehensive electronic health records (EHRs) and handheld charting tools have made vital signs data more accessible, expanding the possibilities of early detection.
In this issue, Peelen et al2 report their scoping review of contemporary EHR-based predictive models for identifying ward patients at risk for deterioration. They identified 22 publications suitable for review. Impressively, some studies report extraordinary statistical performance, with positive predictive values (PPVs) exceeding 50% and with 12- to 24-hour lead times to prepare a clinician response. However, only five algorithms were implemented in an EHR and only three were used clinically. Peelen et al also quantified 48 barriers to and 54 facilitators of the implementation and use of these models. Improved statistical performance (higher PPVs) compared to manually assigned scores were the most important facilitators, while implementation in the context of daily practice (alarm fatigue, integration with existing workflows) were the most important barriers.
These reports invite an obvious question: If the models are this good, why have we not seen more reports of improved patient outcomes? Based on our own recent experience successfully deploying and evaluating the Advance Alert Monitor Program for early detection in a 21-hospital system,3 we suspect that there are several factors at play. Despite the relative computational ease of developing high-performing predictive models, it can be very challenging to create the right dataset (extracting and formatting data, standardizing variable definitions across different EHR builds). Investigators may also underestimate the difficulty of what can be implemented—and sustained—in real-world clinical practice. We encountered substantial difficulty, for example, around alarm fatigue mitigation and the relationship of alerts to end-of-life decisions. Greater attention to implementation is necessary to advance the field.
We suggest that four critical questions be considered when creating in-hospital predictive models. First, what are the statistical characteristics of a model around the likely clinical decision point? Simply having a high C-statistic is insufficient—what matters is the alert’s PPV at a clinically actionable threshold.4 Second, workflow burden—how many alerts per day at my hospital—must be measured, including other processes potentially affected by the new system. Third, will the extra work identify a meaningful proportion of the avoidable bad outcomes? Finally, how will model use affect care of patients near the end of life? Alerts for these patients may not make clinical sense and might even interfere with overall care (eg, by triggering an unwanted ICU transfer).
Implementation requires more than data scientists. Consideration must be given to system governance, predictive model maintenance (models can actually decalibrate over time!), and financing (not just the computation side—someone needs to pay for training clinicians and ensuring proper staffing of the clinical response).
Last, rigorous model evaluation must be undertaken. Given the increasing capabilities of comprehensive EHRs, patient-level randomization is becoming more feasible. But even randomized deployments present challenges. Since ward patients are a heterogeneous population, quantifying process-outcome relationships may be difficult. Alternative approaches to quantification of the impact of bundled interventions may need to be considered—not just for initial deployment, but on an ongoing basis. Peelen et al2 have effectively summarized the state of published predictive models, which hold the tantalizing possibility of meaningful improvement: saved lives, decreased morbidity. Now, we must work together to address the identified gaps so that, one day, implementation of real-time models is routine, and the promise of in-hospital predictive analytics is fulfilled.
1. Escobar GJ, Greene JD, Gardner MN, Marelich GP, Quick B, Kipnis P. Intra-hospital transfers to a higher level of care: contribution to total hospital and intensive care unit (ICU) mortality and length of stay (LOS). J Hosp Med. 2011;6(2):74-80. https://doi.org/10.1002/jhm.817
2. Peelen REY, Koeneman M, van de Belt T, van Goor H, Bredie S. Predicting algorithms for clinical deterioration on the general ward. J Hosp Med. 2021;16(9):612-619. https://doi.org/10.12788/jhm.3675
3. Escobar GJ, Liu VX, Schuler A, Lawson B, Greene JD, Kipnis P. Automated identification of adults at risk for in-hospital clinical deterioration. N Engl J Med. 2020;383(20):1951-1960. https://doi.org/10.1056/NEJMsa2001090
4. Romero-Brufau S, Huddleston JM, Escobar GJ, Liebow M. Why the C-statistic is not informative to evaluate early warning scores and what metrics to use. Crit Care. 2015;19(1):285. https://doi.org/10.1186/s13054-015-0999-1
Adults admitted to general medical-surgical wards who experience in-hospital deterioration have a disproportionate effect on hospital mortality and length of stay.1 Not long ago, systematic electronic capture of vital signs—arguably the most important predictors of impending deterioration—was restricted to intensive care units (ICUs). Deployment of comprehensive electronic health records (EHRs) and handheld charting tools have made vital signs data more accessible, expanding the possibilities of early detection.
In this issue, Peelen et al2 report their scoping review of contemporary EHR-based predictive models for identifying ward patients at risk for deterioration. They identified 22 publications suitable for review. Impressively, some studies report extraordinary statistical performance, with positive predictive values (PPVs) exceeding 50% and with 12- to 24-hour lead times to prepare a clinician response. However, only five algorithms were implemented in an EHR and only three were used clinically. Peelen et al also quantified 48 barriers to and 54 facilitators of the implementation and use of these models. Improved statistical performance (higher PPVs) compared to manually assigned scores were the most important facilitators, while implementation in the context of daily practice (alarm fatigue, integration with existing workflows) were the most important barriers.
These reports invite an obvious question: If the models are this good, why have we not seen more reports of improved patient outcomes? Based on our own recent experience successfully deploying and evaluating the Advance Alert Monitor Program for early detection in a 21-hospital system,3 we suspect that there are several factors at play. Despite the relative computational ease of developing high-performing predictive models, it can be very challenging to create the right dataset (extracting and formatting data, standardizing variable definitions across different EHR builds). Investigators may also underestimate the difficulty of what can be implemented—and sustained—in real-world clinical practice. We encountered substantial difficulty, for example, around alarm fatigue mitigation and the relationship of alerts to end-of-life decisions. Greater attention to implementation is necessary to advance the field.
We suggest that four critical questions be considered when creating in-hospital predictive models. First, what are the statistical characteristics of a model around the likely clinical decision point? Simply having a high C-statistic is insufficient—what matters is the alert’s PPV at a clinically actionable threshold.4 Second, workflow burden—how many alerts per day at my hospital—must be measured, including other processes potentially affected by the new system. Third, will the extra work identify a meaningful proportion of the avoidable bad outcomes? Finally, how will model use affect care of patients near the end of life? Alerts for these patients may not make clinical sense and might even interfere with overall care (eg, by triggering an unwanted ICU transfer).
Implementation requires more than data scientists. Consideration must be given to system governance, predictive model maintenance (models can actually decalibrate over time!), and financing (not just the computation side—someone needs to pay for training clinicians and ensuring proper staffing of the clinical response).
Last, rigorous model evaluation must be undertaken. Given the increasing capabilities of comprehensive EHRs, patient-level randomization is becoming more feasible. But even randomized deployments present challenges. Since ward patients are a heterogeneous population, quantifying process-outcome relationships may be difficult. Alternative approaches to quantification of the impact of bundled interventions may need to be considered—not just for initial deployment, but on an ongoing basis. Peelen et al2 have effectively summarized the state of published predictive models, which hold the tantalizing possibility of meaningful improvement: saved lives, decreased morbidity. Now, we must work together to address the identified gaps so that, one day, implementation of real-time models is routine, and the promise of in-hospital predictive analytics is fulfilled.
Adults admitted to general medical-surgical wards who experience in-hospital deterioration have a disproportionate effect on hospital mortality and length of stay.1 Not long ago, systematic electronic capture of vital signs—arguably the most important predictors of impending deterioration—was restricted to intensive care units (ICUs). Deployment of comprehensive electronic health records (EHRs) and handheld charting tools have made vital signs data more accessible, expanding the possibilities of early detection.
In this issue, Peelen et al2 report their scoping review of contemporary EHR-based predictive models for identifying ward patients at risk for deterioration. They identified 22 publications suitable for review. Impressively, some studies report extraordinary statistical performance, with positive predictive values (PPVs) exceeding 50% and with 12- to 24-hour lead times to prepare a clinician response. However, only five algorithms were implemented in an EHR and only three were used clinically. Peelen et al also quantified 48 barriers to and 54 facilitators of the implementation and use of these models. Improved statistical performance (higher PPVs) compared to manually assigned scores were the most important facilitators, while implementation in the context of daily practice (alarm fatigue, integration with existing workflows) were the most important barriers.
These reports invite an obvious question: If the models are this good, why have we not seen more reports of improved patient outcomes? Based on our own recent experience successfully deploying and evaluating the Advance Alert Monitor Program for early detection in a 21-hospital system,3 we suspect that there are several factors at play. Despite the relative computational ease of developing high-performing predictive models, it can be very challenging to create the right dataset (extracting and formatting data, standardizing variable definitions across different EHR builds). Investigators may also underestimate the difficulty of what can be implemented—and sustained—in real-world clinical practice. We encountered substantial difficulty, for example, around alarm fatigue mitigation and the relationship of alerts to end-of-life decisions. Greater attention to implementation is necessary to advance the field.
We suggest that four critical questions be considered when creating in-hospital predictive models. First, what are the statistical characteristics of a model around the likely clinical decision point? Simply having a high C-statistic is insufficient—what matters is the alert’s PPV at a clinically actionable threshold.4 Second, workflow burden—how many alerts per day at my hospital—must be measured, including other processes potentially affected by the new system. Third, will the extra work identify a meaningful proportion of the avoidable bad outcomes? Finally, how will model use affect care of patients near the end of life? Alerts for these patients may not make clinical sense and might even interfere with overall care (eg, by triggering an unwanted ICU transfer).
Implementation requires more than data scientists. Consideration must be given to system governance, predictive model maintenance (models can actually decalibrate over time!), and financing (not just the computation side—someone needs to pay for training clinicians and ensuring proper staffing of the clinical response).
Last, rigorous model evaluation must be undertaken. Given the increasing capabilities of comprehensive EHRs, patient-level randomization is becoming more feasible. But even randomized deployments present challenges. Since ward patients are a heterogeneous population, quantifying process-outcome relationships may be difficult. Alternative approaches to quantification of the impact of bundled interventions may need to be considered—not just for initial deployment, but on an ongoing basis. Peelen et al2 have effectively summarized the state of published predictive models, which hold the tantalizing possibility of meaningful improvement: saved lives, decreased morbidity. Now, we must work together to address the identified gaps so that, one day, implementation of real-time models is routine, and the promise of in-hospital predictive analytics is fulfilled.
1. Escobar GJ, Greene JD, Gardner MN, Marelich GP, Quick B, Kipnis P. Intra-hospital transfers to a higher level of care: contribution to total hospital and intensive care unit (ICU) mortality and length of stay (LOS). J Hosp Med. 2011;6(2):74-80. https://doi.org/10.1002/jhm.817
2. Peelen REY, Koeneman M, van de Belt T, van Goor H, Bredie S. Predicting algorithms for clinical deterioration on the general ward. J Hosp Med. 2021;16(9):612-619. https://doi.org/10.12788/jhm.3675
3. Escobar GJ, Liu VX, Schuler A, Lawson B, Greene JD, Kipnis P. Automated identification of adults at risk for in-hospital clinical deterioration. N Engl J Med. 2020;383(20):1951-1960. https://doi.org/10.1056/NEJMsa2001090
4. Romero-Brufau S, Huddleston JM, Escobar GJ, Liebow M. Why the C-statistic is not informative to evaluate early warning scores and what metrics to use. Crit Care. 2015;19(1):285. https://doi.org/10.1186/s13054-015-0999-1
1. Escobar GJ, Greene JD, Gardner MN, Marelich GP, Quick B, Kipnis P. Intra-hospital transfers to a higher level of care: contribution to total hospital and intensive care unit (ICU) mortality and length of stay (LOS). J Hosp Med. 2011;6(2):74-80. https://doi.org/10.1002/jhm.817
2. Peelen REY, Koeneman M, van de Belt T, van Goor H, Bredie S. Predicting algorithms for clinical deterioration on the general ward. J Hosp Med. 2021;16(9):612-619. https://doi.org/10.12788/jhm.3675
3. Escobar GJ, Liu VX, Schuler A, Lawson B, Greene JD, Kipnis P. Automated identification of adults at risk for in-hospital clinical deterioration. N Engl J Med. 2020;383(20):1951-1960. https://doi.org/10.1056/NEJMsa2001090
4. Romero-Brufau S, Huddleston JM, Escobar GJ, Liebow M. Why the C-statistic is not informative to evaluate early warning scores and what metrics to use. Crit Care. 2015;19(1):285. https://doi.org/10.1186/s13054-015-0999-1
© 2021 Society of Hospital Medicine
Black Pain Matters: Prioritizing Antiracism and Equity in the Opioid Epidemic
In 2016, a study was published that continues to shock observers today.1 Examining 200 medical trainees, researchers reported that an alarming percentage of these individuals held false beliefs about Black bodies, including 22% believing that nerve endings in Black persons are less sensitive than nerve endings in White persons and 63% believing that Black skin is thicker than White skin. Furthermore, the study found that those who held these false beliefs about biological differences between Black and White individuals were also less likely to recommend pain treatment to Black patients in a follow-up case vignette. Two years later, in an evaluation of racial differences in opioid prescribing in the United States published in Epidemiology, one of the authors suggested, “It’s an extremely rare case where racial biases actually protected the population [Black individuals] being discriminated against.”2
These studies provide the background for the analysis by Rambachan et al3 published in this issue of the Journal of Hospital Medicine. The authors examined a diverse cohort of more than 10,000 patients hospitalized on a general medicine service at an academic medical center in San Francisco from 2012 to 2018. Black patients were significantly less likely to receive an opioid prescription at discharge, and when they did, were discharged on opioids for fewer days than White patients. No other racial group experienced such a disparity, with Asian patients more likely to receive opioids at discharge. Whereas these findings align with myriad studies demonstrating racial disparities in opioid prescribing,4 the authors focus on patients admitted to a general medicine service, where most hospitalized patients receive medical care daily.
The authors concede that determining the etiology of these disparities was beyond the scope of their study, yet this is the exact question we must answer today. Why should the color of a patient’s skin continue to determine the type, and duration, of care they receive, especially when treating pain? The authors hypothesize that individual factors such as provider bias and systemic factors, including limited guidelines on pain management, may drive the observed racial inequities. This progression from individual- and institutional- to community- and policy-level determinants offers a useful framework for understanding the drivers of disparities in opioid prescribing. It also provides an agenda for future research that can guide us from simply detecting disparities to understanding and eliminating them. Furthermore, it is important to examine care team provider characteristics, including race/ethnicity, years in practice, education level (eg, resident vs attending),5 experience with implicit bias training, and differential referral to specialists, such as pain, palliative care, and addiction providers. Factors associated with the facility where a patient is hospitalized also warrant further exploration, including the diversity of medical and nonmedical staff as well as patients.6 Examining these factors will allow us to move closer toward implementing effective interventions that eliminate disparities in pain treatment.
The authors begin to provide us with possible levers to pull to address the inequities in opioid prescribing. They suggest provider-level bias training, improved institutional tracking of disparities, and policy-level solutions to address the persistent dearth of diversity in the healthcare workforce. While these broad solutions may address health disparities across the medical field, targeted solutions are needed to directly address inequities in pain treatment. First, we must explore the reasons for disparities in the prevalence, presentation, and management of pain in Black populations. These reasons may include occupational exposures or injuries, psychological stress (often associated with racism), and a disproportionate presence of chronic medical comorbidities. Second, health systems can implement a standardized system for opioid prescribing, supported by pharmacy expertise and considering clinical diagnoses, to reduce subjectivity associated with determining the appropriateness of an opioid prescription. Third, health systems must improve access to addiction, harm reduction, and pain specialty services to effectively manage comorbid conditions in at-risk patients.7 Furthermore, we must look beyond traditional measures of healthcare access, such as insurance coverage, to address social determinants of health, such as distance to pharmacy, housing security, employment status, and experience with the criminal justice system, which may influence a patient’s receipt of a prescription. Finally, as a society, we must prioritize early training of healthcare providers, long before the undergraduate and graduate medical education level, to practice medicine without stigmatizing biases and stereotypes related to drug use in communities of color.8
The pattern of racial and ethnic disparities in healthcare has been documented for decades, with an ever-increasing depth of the different ways in which minoritized patients are undertreated. Despite this breadth of research, our understanding of the etiology of these inequities and development and implementation of interventions to reduce them remain limited. Rambachan et al3 do a commendable job highlighting further racial disparities in opioid prescribing in hospitalized patients and provide another opportunity to answer the important questions plaguing health care today: Why do these disparities exist and what can be done to address them? The urgency we take towards answering these questions will confirm our commitment to achieving antiracism in medicine and prioritizing health equity. Black lives are depending on it.
1. Hoffman KM, Trawalter S, Axt JR, Oliver MN. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc Natl Acad Sci U S A. 2016;113(16):4296-4301. https://doi.org/10.1073/pnas.1516047113
2. Alexander MJ, Kiang MV, Barbieri M. Trends in Black and White opioid mortality in the United States, 1979-2015. Epidemiology. 2018;29(5):707-715. https://doi.org/10.1097/EDE.0000000000000858
3. Rambachan A, Fang MA, Prasad P, Iverson N. Racial and ethnic disparities in discharge opioid prescribing from a hospital medicine service. J Hosp Med. 2021;16(10):589-595. https://doi.org/10.12788/jhm.3667
4. Essien UR, Sileanu FE, Zhao X, et al. Racial/ethnic differences in the medical treatment of opioid use disorders within the VA healthcare system following non-fatal opioid overdose. J Gen Intern Med. 2020;35(5):1537-1544. https://doi.org/10.1007/s11606-020-05645-0
5. Essien UR, He W, Ray A, et al. Disparities in quality of primary care by resident and staff physicians: is there a conflict between training and equity? J Gen Intern Med. 2019;34(7):1184-1191. https://doi.org/10.1007/s11606-019-04960-5
6. Hollingsworth JM, Yu X, Yan PL, et al. Provider care team segregation and operative mortality following coronary artery bypass grafting. Circ Cardiovasc Qual Outcomes. 2021;14(5):e007778. https://doi.org/10.1161/CIRCOUTCOMES.120.007778
7. Sue KL, Fiellin DA. Bringing harm reduction into health policy - combating the overdose crisis. N Engl J Med. 2021;384(19):1781-1783. https://doi.org/10.1056/NEJMp2103274
8. James K, Jordan A. The opioid crisis in Black communities. J Law Med Ethics. 2018;46(2):404-421. https://doi.org/10.1038/jes.2015.55
In 2016, a study was published that continues to shock observers today.1 Examining 200 medical trainees, researchers reported that an alarming percentage of these individuals held false beliefs about Black bodies, including 22% believing that nerve endings in Black persons are less sensitive than nerve endings in White persons and 63% believing that Black skin is thicker than White skin. Furthermore, the study found that those who held these false beliefs about biological differences between Black and White individuals were also less likely to recommend pain treatment to Black patients in a follow-up case vignette. Two years later, in an evaluation of racial differences in opioid prescribing in the United States published in Epidemiology, one of the authors suggested, “It’s an extremely rare case where racial biases actually protected the population [Black individuals] being discriminated against.”2
These studies provide the background for the analysis by Rambachan et al3 published in this issue of the Journal of Hospital Medicine. The authors examined a diverse cohort of more than 10,000 patients hospitalized on a general medicine service at an academic medical center in San Francisco from 2012 to 2018. Black patients were significantly less likely to receive an opioid prescription at discharge, and when they did, were discharged on opioids for fewer days than White patients. No other racial group experienced such a disparity, with Asian patients more likely to receive opioids at discharge. Whereas these findings align with myriad studies demonstrating racial disparities in opioid prescribing,4 the authors focus on patients admitted to a general medicine service, where most hospitalized patients receive medical care daily.
The authors concede that determining the etiology of these disparities was beyond the scope of their study, yet this is the exact question we must answer today. Why should the color of a patient’s skin continue to determine the type, and duration, of care they receive, especially when treating pain? The authors hypothesize that individual factors such as provider bias and systemic factors, including limited guidelines on pain management, may drive the observed racial inequities. This progression from individual- and institutional- to community- and policy-level determinants offers a useful framework for understanding the drivers of disparities in opioid prescribing. It also provides an agenda for future research that can guide us from simply detecting disparities to understanding and eliminating them. Furthermore, it is important to examine care team provider characteristics, including race/ethnicity, years in practice, education level (eg, resident vs attending),5 experience with implicit bias training, and differential referral to specialists, such as pain, palliative care, and addiction providers. Factors associated with the facility where a patient is hospitalized also warrant further exploration, including the diversity of medical and nonmedical staff as well as patients.6 Examining these factors will allow us to move closer toward implementing effective interventions that eliminate disparities in pain treatment.
The authors begin to provide us with possible levers to pull to address the inequities in opioid prescribing. They suggest provider-level bias training, improved institutional tracking of disparities, and policy-level solutions to address the persistent dearth of diversity in the healthcare workforce. While these broad solutions may address health disparities across the medical field, targeted solutions are needed to directly address inequities in pain treatment. First, we must explore the reasons for disparities in the prevalence, presentation, and management of pain in Black populations. These reasons may include occupational exposures or injuries, psychological stress (often associated with racism), and a disproportionate presence of chronic medical comorbidities. Second, health systems can implement a standardized system for opioid prescribing, supported by pharmacy expertise and considering clinical diagnoses, to reduce subjectivity associated with determining the appropriateness of an opioid prescription. Third, health systems must improve access to addiction, harm reduction, and pain specialty services to effectively manage comorbid conditions in at-risk patients.7 Furthermore, we must look beyond traditional measures of healthcare access, such as insurance coverage, to address social determinants of health, such as distance to pharmacy, housing security, employment status, and experience with the criminal justice system, which may influence a patient’s receipt of a prescription. Finally, as a society, we must prioritize early training of healthcare providers, long before the undergraduate and graduate medical education level, to practice medicine without stigmatizing biases and stereotypes related to drug use in communities of color.8
The pattern of racial and ethnic disparities in healthcare has been documented for decades, with an ever-increasing depth of the different ways in which minoritized patients are undertreated. Despite this breadth of research, our understanding of the etiology of these inequities and development and implementation of interventions to reduce them remain limited. Rambachan et al3 do a commendable job highlighting further racial disparities in opioid prescribing in hospitalized patients and provide another opportunity to answer the important questions plaguing health care today: Why do these disparities exist and what can be done to address them? The urgency we take towards answering these questions will confirm our commitment to achieving antiracism in medicine and prioritizing health equity. Black lives are depending on it.
In 2016, a study was published that continues to shock observers today.1 Examining 200 medical trainees, researchers reported that an alarming percentage of these individuals held false beliefs about Black bodies, including 22% believing that nerve endings in Black persons are less sensitive than nerve endings in White persons and 63% believing that Black skin is thicker than White skin. Furthermore, the study found that those who held these false beliefs about biological differences between Black and White individuals were also less likely to recommend pain treatment to Black patients in a follow-up case vignette. Two years later, in an evaluation of racial differences in opioid prescribing in the United States published in Epidemiology, one of the authors suggested, “It’s an extremely rare case where racial biases actually protected the population [Black individuals] being discriminated against.”2
These studies provide the background for the analysis by Rambachan et al3 published in this issue of the Journal of Hospital Medicine. The authors examined a diverse cohort of more than 10,000 patients hospitalized on a general medicine service at an academic medical center in San Francisco from 2012 to 2018. Black patients were significantly less likely to receive an opioid prescription at discharge, and when they did, were discharged on opioids for fewer days than White patients. No other racial group experienced such a disparity, with Asian patients more likely to receive opioids at discharge. Whereas these findings align with myriad studies demonstrating racial disparities in opioid prescribing,4 the authors focus on patients admitted to a general medicine service, where most hospitalized patients receive medical care daily.
The authors concede that determining the etiology of these disparities was beyond the scope of their study, yet this is the exact question we must answer today. Why should the color of a patient’s skin continue to determine the type, and duration, of care they receive, especially when treating pain? The authors hypothesize that individual factors such as provider bias and systemic factors, including limited guidelines on pain management, may drive the observed racial inequities. This progression from individual- and institutional- to community- and policy-level determinants offers a useful framework for understanding the drivers of disparities in opioid prescribing. It also provides an agenda for future research that can guide us from simply detecting disparities to understanding and eliminating them. Furthermore, it is important to examine care team provider characteristics, including race/ethnicity, years in practice, education level (eg, resident vs attending),5 experience with implicit bias training, and differential referral to specialists, such as pain, palliative care, and addiction providers. Factors associated with the facility where a patient is hospitalized also warrant further exploration, including the diversity of medical and nonmedical staff as well as patients.6 Examining these factors will allow us to move closer toward implementing effective interventions that eliminate disparities in pain treatment.
The authors begin to provide us with possible levers to pull to address the inequities in opioid prescribing. They suggest provider-level bias training, improved institutional tracking of disparities, and policy-level solutions to address the persistent dearth of diversity in the healthcare workforce. While these broad solutions may address health disparities across the medical field, targeted solutions are needed to directly address inequities in pain treatment. First, we must explore the reasons for disparities in the prevalence, presentation, and management of pain in Black populations. These reasons may include occupational exposures or injuries, psychological stress (often associated with racism), and a disproportionate presence of chronic medical comorbidities. Second, health systems can implement a standardized system for opioid prescribing, supported by pharmacy expertise and considering clinical diagnoses, to reduce subjectivity associated with determining the appropriateness of an opioid prescription. Third, health systems must improve access to addiction, harm reduction, and pain specialty services to effectively manage comorbid conditions in at-risk patients.7 Furthermore, we must look beyond traditional measures of healthcare access, such as insurance coverage, to address social determinants of health, such as distance to pharmacy, housing security, employment status, and experience with the criminal justice system, which may influence a patient’s receipt of a prescription. Finally, as a society, we must prioritize early training of healthcare providers, long before the undergraduate and graduate medical education level, to practice medicine without stigmatizing biases and stereotypes related to drug use in communities of color.8
The pattern of racial and ethnic disparities in healthcare has been documented for decades, with an ever-increasing depth of the different ways in which minoritized patients are undertreated. Despite this breadth of research, our understanding of the etiology of these inequities and development and implementation of interventions to reduce them remain limited. Rambachan et al3 do a commendable job highlighting further racial disparities in opioid prescribing in hospitalized patients and provide another opportunity to answer the important questions plaguing health care today: Why do these disparities exist and what can be done to address them? The urgency we take towards answering these questions will confirm our commitment to achieving antiracism in medicine and prioritizing health equity. Black lives are depending on it.
1. Hoffman KM, Trawalter S, Axt JR, Oliver MN. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc Natl Acad Sci U S A. 2016;113(16):4296-4301. https://doi.org/10.1073/pnas.1516047113
2. Alexander MJ, Kiang MV, Barbieri M. Trends in Black and White opioid mortality in the United States, 1979-2015. Epidemiology. 2018;29(5):707-715. https://doi.org/10.1097/EDE.0000000000000858
3. Rambachan A, Fang MA, Prasad P, Iverson N. Racial and ethnic disparities in discharge opioid prescribing from a hospital medicine service. J Hosp Med. 2021;16(10):589-595. https://doi.org/10.12788/jhm.3667
4. Essien UR, Sileanu FE, Zhao X, et al. Racial/ethnic differences in the medical treatment of opioid use disorders within the VA healthcare system following non-fatal opioid overdose. J Gen Intern Med. 2020;35(5):1537-1544. https://doi.org/10.1007/s11606-020-05645-0
5. Essien UR, He W, Ray A, et al. Disparities in quality of primary care by resident and staff physicians: is there a conflict between training and equity? J Gen Intern Med. 2019;34(7):1184-1191. https://doi.org/10.1007/s11606-019-04960-5
6. Hollingsworth JM, Yu X, Yan PL, et al. Provider care team segregation and operative mortality following coronary artery bypass grafting. Circ Cardiovasc Qual Outcomes. 2021;14(5):e007778. https://doi.org/10.1161/CIRCOUTCOMES.120.007778
7. Sue KL, Fiellin DA. Bringing harm reduction into health policy - combating the overdose crisis. N Engl J Med. 2021;384(19):1781-1783. https://doi.org/10.1056/NEJMp2103274
8. James K, Jordan A. The opioid crisis in Black communities. J Law Med Ethics. 2018;46(2):404-421. https://doi.org/10.1038/jes.2015.55
1. Hoffman KM, Trawalter S, Axt JR, Oliver MN. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc Natl Acad Sci U S A. 2016;113(16):4296-4301. https://doi.org/10.1073/pnas.1516047113
2. Alexander MJ, Kiang MV, Barbieri M. Trends in Black and White opioid mortality in the United States, 1979-2015. Epidemiology. 2018;29(5):707-715. https://doi.org/10.1097/EDE.0000000000000858
3. Rambachan A, Fang MA, Prasad P, Iverson N. Racial and ethnic disparities in discharge opioid prescribing from a hospital medicine service. J Hosp Med. 2021;16(10):589-595. https://doi.org/10.12788/jhm.3667
4. Essien UR, Sileanu FE, Zhao X, et al. Racial/ethnic differences in the medical treatment of opioid use disorders within the VA healthcare system following non-fatal opioid overdose. J Gen Intern Med. 2020;35(5):1537-1544. https://doi.org/10.1007/s11606-020-05645-0
5. Essien UR, He W, Ray A, et al. Disparities in quality of primary care by resident and staff physicians: is there a conflict between training and equity? J Gen Intern Med. 2019;34(7):1184-1191. https://doi.org/10.1007/s11606-019-04960-5
6. Hollingsworth JM, Yu X, Yan PL, et al. Provider care team segregation and operative mortality following coronary artery bypass grafting. Circ Cardiovasc Qual Outcomes. 2021;14(5):e007778. https://doi.org/10.1161/CIRCOUTCOMES.120.007778
7. Sue KL, Fiellin DA. Bringing harm reduction into health policy - combating the overdose crisis. N Engl J Med. 2021;384(19):1781-1783. https://doi.org/10.1056/NEJMp2103274
8. James K, Jordan A. The opioid crisis in Black communities. J Law Med Ethics. 2018;46(2):404-421. https://doi.org/10.1038/jes.2015.55
© 2021 Society of Hospital Medicine