User login
Methodologic Progress Note: A Clinician’s Guide to Logistic Regression
The ability to read and correctly interpret research is an essential skill, but most hospitalists—and physicians in general—do not receive formal training in biostatistics during their medical education.1-3 In addition to straightforward statistical tests that compare a single exposure and outcome, researchers commonly use statistical models to identify and quantify complex relationships among many exposures (eg, demographics, clinical characteristics, interventions, or other variables) and an outcome. Understanding statistical models can be challenging. Still, it is important to recognize the advantages and limitations of statistical models, how to interpret their results, and the potential implications of findings on current clinical practice.
In the article “Rates and Characteristics of Medical Malpractice Claims Against Hospitalists” published in the July 2021 issue of the Journal of Hospital Medicine, Schaffer et al4 used the Comparative Benchmarking System database, which is maintained by a malpractice insurer, to characterize malpractice claims against hospitalists. The authors used multiple logistic regression models to understand the relationship among clinical factors and indemnity payments. In this Progress Note, we describe situations in which logistic regression is the proper statistical method to analyze a data set, explain results from logistic regression analyses, and equip readers with skills to critically appraise conclusions drawn from these models.
Choosing an Appropriate Statistical Model
Statistical models often are used to describe the relationship among one or more exposure variables (ie, independent variables) and an outcome (ie, dependent variable). These models allow researchers to evaluate the effects of multiple exposure variables simultaneously, which in turn allows them to “isolate” the effect of each variable; in other words, models facilitate an understanding of the relationship between each exposure variable and the outcome, adjusted for (ie, independent of) the other exposure variables in the model.
Several statistical models can be used to quantify relationships within the data, but each type of model has certain assumptions that must be satisfied. Two important assumptions include characteristics of the outcome (eg, the type and distribution) and the nature of the relationships among the outcome and independent variables (eg, linear vs nonlinear). Simple linear regression, one of the most basic statistical models used in research,5 assumes that (a) the outcome is continuous (ie, any numeric value is possible) and normally distributed (ie, its histogram is a bell-shaped curve) and (b) the relationship between the independent variable and the outcome is linear (ie, follows a straight line). If an investigator wanted to understand how weight is related to height, a simple linear regression could be used to develop a mathematical equation that tells us how the outcome (weight) generally increases as the independent variable (height) increases.
Often, the outcome in a study is not a continuous variable but a simple success/failure variable (ie, dichotomous variable that can be one of two possible values). Schaffer et al4 examined the binary outcome of whether a malpractice claim case would end in an indemnity payment or no payment. Linear regression models are not equipped to handle dichotomous outcomes. Instead, we need to use a different statistical model: logistic regression. In logistic regression, the probability (p) of a defined outcome event is estimated by creating a regression model.
The Logistic Model
A probability (p) is a measure of how likely an event (eg, a malpractice claim ends in an indemnity payment or not) is to occur. It is always between 0 (ie, the event will definitely not occur) and 1 (ie, the event will definitely occur). A p of 0.5 means there is a 50/50 chance that the event will occur (ie, equivalent to a coin flip). Because p is a probability, we need to make sure it is always between 0 and 1. If we were to try to model p with a linear regression, the model would assume that p could extend beyond 0 and 1. What can we do?
Applying a transformation is a commonly used tool in statistics to make data work better within statistical models.6 In this case, we will transform the variable p. In logistic regression, we model the probability of experiencing the outcome through a transformation called a logit. The logit represents the natural logarithm (ln) of the ratio of the probability of experiencing the outcome (p) vs the probability of not experiencing the outcome (1 – p), with the ratio being the odds of the event occurring.
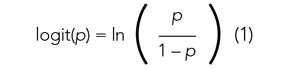
This transformation works well for dichotomous outcomes because the logit transformation approximates a straight line as long as p is not too large or too small (between 0.05 and 0.95).
If we are performing a logistic regression with only one independent variable (x) and want to understand the relationship between this variable (x) and the probability of an outcome event (p), then our model is the equation of a line. The equation for the base model of logistic regression with one independent variable (x) is
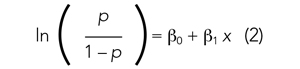
where β0 is the y-intercept and β1 is the slope of the line. Equation (2) is identical to the algebraic equation y = mx + b for a line, just rearranged slightly. In this algebraic equation, m is the slope (the same as β1) and b is the y-intercept (the same as β0). We will see that β0 and β1 are estimated (ie, assigned numeric values) from the data collected to help us understand how x and
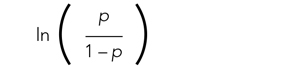
are related and are the basis for estimating odds ratios.
We can build more complex models using multivariable logistic regression by adding more independent variables to the right side of equation (2). Essentially, this is what S
There are two notable techniques used frequently with multivariable logistic regression models. The first involves choosing which independent variables to include in the model. One way to select variables for multivariable models is defining them a priori, that is deciding which variables are clinically or conceptually associated with the outcome before looking at the data. With this approach, we can test specific hypotheses about the relationships between the independent variables and the outcome. Another common approach is to look at the data and identify the variables that vary significantly between the two outcome groups. Schaffer et al4 used an a priori approach to define variables in their multivariable model (ie, “variables for inclusion into the multivariable model were determined a priori”).
A second technique is the evaluation of collinearity, which helps us understand whether the i
Understanding the Results of the Logistic Model
Fitting the model is the process by which statistical software (eg, SAS, Stata, R, SPSS) estimates the relationships among independent variables in the model and the outcome within a specific dataset. In equation (2), this essentially means that the software will evaluate the data and provide us with the best estimates for β0 (the y-intercept) and β1 (the slope) that describe the relationship between the variable x and
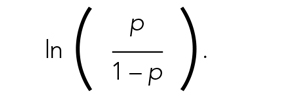
Modeling can be iterative, and part of the process may include removing variables from the model that are not significantly associated with the outcome to create a simpler solution, a process known as model reduction. The results from models describe the independent association between a specific characteristic and the outcome, meaning that the relationship has been adjusted for all the other characteristics in the model.
The relationships among the independent variables and outcome are most often represented as an odds ratio (OR), which quantifies the strength of the association between two variables and is directly calculated from the β values in the model. As the name suggests, an OR is a ratio of odds. But what are odds? Simply, the odds of an outcome (such as mortality) is the probability of experiencing the event divided by the probability of not experiencing that event; in other words, it is the ratio:

The concept of odds is often unfamiliar, so it can be helpful to consider the definition in the context of games of chance. For example, in horse race betting, the outcome of interest is that a horse will lose a race. Imagine that the probability of a horse losing a race is 0.8 and the probability of winning is 0.2. The odds of losing are
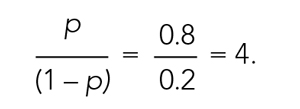
These odds usually are listed as 4-to-1, meaning that out of 5 races (ie, 4 + 1) the horse is expected to lose 4 times and win once. When odds are listed this way, we can easily calculate the associated probability by recognizing that the total number of expected races is the sum of two numbers (probability of losing: 4 races out of 5, or 0.80 vs probability of winning: 1 race out of 5, or 0.20).
In medical research, the OR typically represents the odds for one group of patients (A) compared with the odds for another group of patients (B) experiencing an outcome. If the odds of the outcome are the same for group A and group B, then OR = 1.0, meaning that the probability of the outcome is the same between the two groups. If the patients in group A have greater odds of experiencing the outcome compared with group B patients (and a greater probability of the outcome), then the OR will be >1. If the opposite is true, then the OR will be <1.
Schaffer et al4 estimated that the OR of an indemnity payment in malpractice cases involving errors in clinical judgment as a contributing factor was 5.01 (95% CI, 3.37-7.45). This means that malpractice cases involving errors in clinical judgement had a 5.01 times greater odds of indemnity payment compared with those without these errors after adjusting for all other variables in the model (eg, age, severity). Note that the 95% CI does not include 1.0. This indicates that the OR is statistically >1, and we can conclude that there is a significant relationship between errors in clinical judgment and payment that is unlikely to be attributed to chance alone.
In logistic regression for categorical independent variables, all categories are compared with a reference group within that variable, with the reference group serving as the denominator of the OR. The authors4 did not incorporate continuous independent variables in their multivariable logistic regression model. However, if the authors examined length of hospitalization as a contributing factor in indemnity payments, for example, the OR would represent a 1-unit increase in this variable (eg, 1-day increase in length of stay).
Conclusion
Logistic regression describes the relationships in data and is an important statistical model across many types of research. This Progress Note emphasizes the importance of weighing the advantages and limitations of logistic regression, provides a common approach to data transformation, and guides the correct interpretation of logistic regression model results.
1. Windish DM, Huot SJ, Green ML. Medicine residents’ understanding of the biostatistics and results in the medical literature. JAMA. 2007;298(9):1010. https://doi.org/10.1001/jama.298.9.1010
2. MacDougall M, Cameron HS, Maxwell SRJ. Medical graduate views on statistical learning needs for clinical practice: a comprehensive survey. BMC Med Educ. 2019;20(1):1. https://doi.org/10.1186/s12909-019-1842-1
3. Montori VM. Progress in evidence-based medicine. JAMA. 2008;300(15):1814-1816. https://doi.org/10.1001/jama.300.15.1814
4. Schaffer AC, Yu-Moe CW, Babayan A, Wachter RM, Einbinder JS. Rates and characteristics of medical malpractice claims against hospitalists. J Hosp Med. 2021;16(7):390-396. https://doi.org/10.12788/jhm.3557
5. Lane DM, Scott D, Hebl M, Guerra R, Osherson D, Zimmer H. Introducton to Statistics. Accessed April 13, 2021. https://onlinestatbook.com/Online_Statistics_Education.pdf
6. Marill KA. Advanced statistics: linear regression, part II: multiple linear regression. Acad Emerg Med Off J Soc Acad Emerg Med. 2004;11(1):94-102. https://doi.org/10.1197/j.aem.2003.09.006
The ability to read and correctly interpret research is an essential skill, but most hospitalists—and physicians in general—do not receive formal training in biostatistics during their medical education.1-3 In addition to straightforward statistical tests that compare a single exposure and outcome, researchers commonly use statistical models to identify and quantify complex relationships among many exposures (eg, demographics, clinical characteristics, interventions, or other variables) and an outcome. Understanding statistical models can be challenging. Still, it is important to recognize the advantages and limitations of statistical models, how to interpret their results, and the potential implications of findings on current clinical practice.
In the article “Rates and Characteristics of Medical Malpractice Claims Against Hospitalists” published in the July 2021 issue of the Journal of Hospital Medicine, Schaffer et al4 used the Comparative Benchmarking System database, which is maintained by a malpractice insurer, to characterize malpractice claims against hospitalists. The authors used multiple logistic regression models to understand the relationship among clinical factors and indemnity payments. In this Progress Note, we describe situations in which logistic regression is the proper statistical method to analyze a data set, explain results from logistic regression analyses, and equip readers with skills to critically appraise conclusions drawn from these models.
Choosing an Appropriate Statistical Model
Statistical models often are used to describe the relationship among one or more exposure variables (ie, independent variables) and an outcome (ie, dependent variable). These models allow researchers to evaluate the effects of multiple exposure variables simultaneously, which in turn allows them to “isolate” the effect of each variable; in other words, models facilitate an understanding of the relationship between each exposure variable and the outcome, adjusted for (ie, independent of) the other exposure variables in the model.
Several statistical models can be used to quantify relationships within the data, but each type of model has certain assumptions that must be satisfied. Two important assumptions include characteristics of the outcome (eg, the type and distribution) and the nature of the relationships among the outcome and independent variables (eg, linear vs nonlinear). Simple linear regression, one of the most basic statistical models used in research,5 assumes that (a) the outcome is continuous (ie, any numeric value is possible) and normally distributed (ie, its histogram is a bell-shaped curve) and (b) the relationship between the independent variable and the outcome is linear (ie, follows a straight line). If an investigator wanted to understand how weight is related to height, a simple linear regression could be used to develop a mathematical equation that tells us how the outcome (weight) generally increases as the independent variable (height) increases.
Often, the outcome in a study is not a continuous variable but a simple success/failure variable (ie, dichotomous variable that can be one of two possible values). Schaffer et al4 examined the binary outcome of whether a malpractice claim case would end in an indemnity payment or no payment. Linear regression models are not equipped to handle dichotomous outcomes. Instead, we need to use a different statistical model: logistic regression. In logistic regression, the probability (p) of a defined outcome event is estimated by creating a regression model.
The Logistic Model
A probability (p) is a measure of how likely an event (eg, a malpractice claim ends in an indemnity payment or not) is to occur. It is always between 0 (ie, the event will definitely not occur) and 1 (ie, the event will definitely occur). A p of 0.5 means there is a 50/50 chance that the event will occur (ie, equivalent to a coin flip). Because p is a probability, we need to make sure it is always between 0 and 1. If we were to try to model p with a linear regression, the model would assume that p could extend beyond 0 and 1. What can we do?
Applying a transformation is a commonly used tool in statistics to make data work better within statistical models.6 In this case, we will transform the variable p. In logistic regression, we model the probability of experiencing the outcome through a transformation called a logit. The logit represents the natural logarithm (ln) of the ratio of the probability of experiencing the outcome (p) vs the probability of not experiencing the outcome (1 – p), with the ratio being the odds of the event occurring.
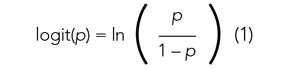
This transformation works well for dichotomous outcomes because the logit transformation approximates a straight line as long as p is not too large or too small (between 0.05 and 0.95).
If we are performing a logistic regression with only one independent variable (x) and want to understand the relationship between this variable (x) and the probability of an outcome event (p), then our model is the equation of a line. The equation for the base model of logistic regression with one independent variable (x) is
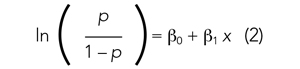
where β0 is the y-intercept and β1 is the slope of the line. Equation (2) is identical to the algebraic equation y = mx + b for a line, just rearranged slightly. In this algebraic equation, m is the slope (the same as β1) and b is the y-intercept (the same as β0). We will see that β0 and β1 are estimated (ie, assigned numeric values) from the data collected to help us understand how x and
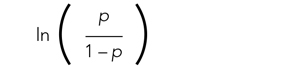
are related and are the basis for estimating odds ratios.
We can build more complex models using multivariable logistic regression by adding more independent variables to the right side of equation (2). Essentially, this is what S
There are two notable techniques used frequently with multivariable logistic regression models. The first involves choosing which independent variables to include in the model. One way to select variables for multivariable models is defining them a priori, that is deciding which variables are clinically or conceptually associated with the outcome before looking at the data. With this approach, we can test specific hypotheses about the relationships between the independent variables and the outcome. Another common approach is to look at the data and identify the variables that vary significantly between the two outcome groups. Schaffer et al4 used an a priori approach to define variables in their multivariable model (ie, “variables for inclusion into the multivariable model were determined a priori”).
A second technique is the evaluation of collinearity, which helps us understand whether the i
Understanding the Results of the Logistic Model
Fitting the model is the process by which statistical software (eg, SAS, Stata, R, SPSS) estimates the relationships among independent variables in the model and the outcome within a specific dataset. In equation (2), this essentially means that the software will evaluate the data and provide us with the best estimates for β0 (the y-intercept) and β1 (the slope) that describe the relationship between the variable x and
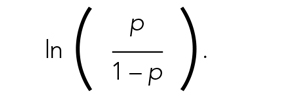
Modeling can be iterative, and part of the process may include removing variables from the model that are not significantly associated with the outcome to create a simpler solution, a process known as model reduction. The results from models describe the independent association between a specific characteristic and the outcome, meaning that the relationship has been adjusted for all the other characteristics in the model.
The relationships among the independent variables and outcome are most often represented as an odds ratio (OR), which quantifies the strength of the association between two variables and is directly calculated from the β values in the model. As the name suggests, an OR is a ratio of odds. But what are odds? Simply, the odds of an outcome (such as mortality) is the probability of experiencing the event divided by the probability of not experiencing that event; in other words, it is the ratio:

The concept of odds is often unfamiliar, so it can be helpful to consider the definition in the context of games of chance. For example, in horse race betting, the outcome of interest is that a horse will lose a race. Imagine that the probability of a horse losing a race is 0.8 and the probability of winning is 0.2. The odds of losing are
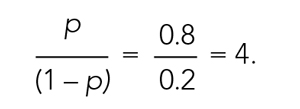
These odds usually are listed as 4-to-1, meaning that out of 5 races (ie, 4 + 1) the horse is expected to lose 4 times and win once. When odds are listed this way, we can easily calculate the associated probability by recognizing that the total number of expected races is the sum of two numbers (probability of losing: 4 races out of 5, or 0.80 vs probability of winning: 1 race out of 5, or 0.20).
In medical research, the OR typically represents the odds for one group of patients (A) compared with the odds for another group of patients (B) experiencing an outcome. If the odds of the outcome are the same for group A and group B, then OR = 1.0, meaning that the probability of the outcome is the same between the two groups. If the patients in group A have greater odds of experiencing the outcome compared with group B patients (and a greater probability of the outcome), then the OR will be >1. If the opposite is true, then the OR will be <1.
Schaffer et al4 estimated that the OR of an indemnity payment in malpractice cases involving errors in clinical judgment as a contributing factor was 5.01 (95% CI, 3.37-7.45). This means that malpractice cases involving errors in clinical judgement had a 5.01 times greater odds of indemnity payment compared with those without these errors after adjusting for all other variables in the model (eg, age, severity). Note that the 95% CI does not include 1.0. This indicates that the OR is statistically >1, and we can conclude that there is a significant relationship between errors in clinical judgment and payment that is unlikely to be attributed to chance alone.
In logistic regression for categorical independent variables, all categories are compared with a reference group within that variable, with the reference group serving as the denominator of the OR. The authors4 did not incorporate continuous independent variables in their multivariable logistic regression model. However, if the authors examined length of hospitalization as a contributing factor in indemnity payments, for example, the OR would represent a 1-unit increase in this variable (eg, 1-day increase in length of stay).
Conclusion
Logistic regression describes the relationships in data and is an important statistical model across many types of research. This Progress Note emphasizes the importance of weighing the advantages and limitations of logistic regression, provides a common approach to data transformation, and guides the correct interpretation of logistic regression model results.
The ability to read and correctly interpret research is an essential skill, but most hospitalists—and physicians in general—do not receive formal training in biostatistics during their medical education.1-3 In addition to straightforward statistical tests that compare a single exposure and outcome, researchers commonly use statistical models to identify and quantify complex relationships among many exposures (eg, demographics, clinical characteristics, interventions, or other variables) and an outcome. Understanding statistical models can be challenging. Still, it is important to recognize the advantages and limitations of statistical models, how to interpret their results, and the potential implications of findings on current clinical practice.
In the article “Rates and Characteristics of Medical Malpractice Claims Against Hospitalists” published in the July 2021 issue of the Journal of Hospital Medicine, Schaffer et al4 used the Comparative Benchmarking System database, which is maintained by a malpractice insurer, to characterize malpractice claims against hospitalists. The authors used multiple logistic regression models to understand the relationship among clinical factors and indemnity payments. In this Progress Note, we describe situations in which logistic regression is the proper statistical method to analyze a data set, explain results from logistic regression analyses, and equip readers with skills to critically appraise conclusions drawn from these models.
Choosing an Appropriate Statistical Model
Statistical models often are used to describe the relationship among one or more exposure variables (ie, independent variables) and an outcome (ie, dependent variable). These models allow researchers to evaluate the effects of multiple exposure variables simultaneously, which in turn allows them to “isolate” the effect of each variable; in other words, models facilitate an understanding of the relationship between each exposure variable and the outcome, adjusted for (ie, independent of) the other exposure variables in the model.
Several statistical models can be used to quantify relationships within the data, but each type of model has certain assumptions that must be satisfied. Two important assumptions include characteristics of the outcome (eg, the type and distribution) and the nature of the relationships among the outcome and independent variables (eg, linear vs nonlinear). Simple linear regression, one of the most basic statistical models used in research,5 assumes that (a) the outcome is continuous (ie, any numeric value is possible) and normally distributed (ie, its histogram is a bell-shaped curve) and (b) the relationship between the independent variable and the outcome is linear (ie, follows a straight line). If an investigator wanted to understand how weight is related to height, a simple linear regression could be used to develop a mathematical equation that tells us how the outcome (weight) generally increases as the independent variable (height) increases.
Often, the outcome in a study is not a continuous variable but a simple success/failure variable (ie, dichotomous variable that can be one of two possible values). Schaffer et al4 examined the binary outcome of whether a malpractice claim case would end in an indemnity payment or no payment. Linear regression models are not equipped to handle dichotomous outcomes. Instead, we need to use a different statistical model: logistic regression. In logistic regression, the probability (p) of a defined outcome event is estimated by creating a regression model.
The Logistic Model
A probability (p) is a measure of how likely an event (eg, a malpractice claim ends in an indemnity payment or not) is to occur. It is always between 0 (ie, the event will definitely not occur) and 1 (ie, the event will definitely occur). A p of 0.5 means there is a 50/50 chance that the event will occur (ie, equivalent to a coin flip). Because p is a probability, we need to make sure it is always between 0 and 1. If we were to try to model p with a linear regression, the model would assume that p could extend beyond 0 and 1. What can we do?
Applying a transformation is a commonly used tool in statistics to make data work better within statistical models.6 In this case, we will transform the variable p. In logistic regression, we model the probability of experiencing the outcome through a transformation called a logit. The logit represents the natural logarithm (ln) of the ratio of the probability of experiencing the outcome (p) vs the probability of not experiencing the outcome (1 – p), with the ratio being the odds of the event occurring.
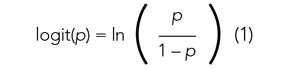
This transformation works well for dichotomous outcomes because the logit transformation approximates a straight line as long as p is not too large or too small (between 0.05 and 0.95).
If we are performing a logistic regression with only one independent variable (x) and want to understand the relationship between this variable (x) and the probability of an outcome event (p), then our model is the equation of a line. The equation for the base model of logistic regression with one independent variable (x) is
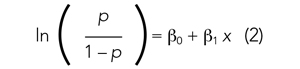
where β0 is the y-intercept and β1 is the slope of the line. Equation (2) is identical to the algebraic equation y = mx + b for a line, just rearranged slightly. In this algebraic equation, m is the slope (the same as β1) and b is the y-intercept (the same as β0). We will see that β0 and β1 are estimated (ie, assigned numeric values) from the data collected to help us understand how x and
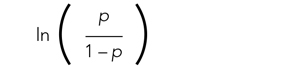
are related and are the basis for estimating odds ratios.
We can build more complex models using multivariable logistic regression by adding more independent variables to the right side of equation (2). Essentially, this is what S
There are two notable techniques used frequently with multivariable logistic regression models. The first involves choosing which independent variables to include in the model. One way to select variables for multivariable models is defining them a priori, that is deciding which variables are clinically or conceptually associated with the outcome before looking at the data. With this approach, we can test specific hypotheses about the relationships between the independent variables and the outcome. Another common approach is to look at the data and identify the variables that vary significantly between the two outcome groups. Schaffer et al4 used an a priori approach to define variables in their multivariable model (ie, “variables for inclusion into the multivariable model were determined a priori”).
A second technique is the evaluation of collinearity, which helps us understand whether the i
Understanding the Results of the Logistic Model
Fitting the model is the process by which statistical software (eg, SAS, Stata, R, SPSS) estimates the relationships among independent variables in the model and the outcome within a specific dataset. In equation (2), this essentially means that the software will evaluate the data and provide us with the best estimates for β0 (the y-intercept) and β1 (the slope) that describe the relationship between the variable x and
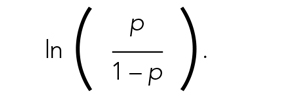
Modeling can be iterative, and part of the process may include removing variables from the model that are not significantly associated with the outcome to create a simpler solution, a process known as model reduction. The results from models describe the independent association between a specific characteristic and the outcome, meaning that the relationship has been adjusted for all the other characteristics in the model.
The relationships among the independent variables and outcome are most often represented as an odds ratio (OR), which quantifies the strength of the association between two variables and is directly calculated from the β values in the model. As the name suggests, an OR is a ratio of odds. But what are odds? Simply, the odds of an outcome (such as mortality) is the probability of experiencing the event divided by the probability of not experiencing that event; in other words, it is the ratio:

The concept of odds is often unfamiliar, so it can be helpful to consider the definition in the context of games of chance. For example, in horse race betting, the outcome of interest is that a horse will lose a race. Imagine that the probability of a horse losing a race is 0.8 and the probability of winning is 0.2. The odds of losing are
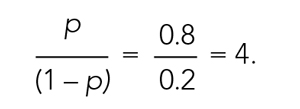
These odds usually are listed as 4-to-1, meaning that out of 5 races (ie, 4 + 1) the horse is expected to lose 4 times and win once. When odds are listed this way, we can easily calculate the associated probability by recognizing that the total number of expected races is the sum of two numbers (probability of losing: 4 races out of 5, or 0.80 vs probability of winning: 1 race out of 5, or 0.20).
In medical research, the OR typically represents the odds for one group of patients (A) compared with the odds for another group of patients (B) experiencing an outcome. If the odds of the outcome are the same for group A and group B, then OR = 1.0, meaning that the probability of the outcome is the same between the two groups. If the patients in group A have greater odds of experiencing the outcome compared with group B patients (and a greater probability of the outcome), then the OR will be >1. If the opposite is true, then the OR will be <1.
Schaffer et al4 estimated that the OR of an indemnity payment in malpractice cases involving errors in clinical judgment as a contributing factor was 5.01 (95% CI, 3.37-7.45). This means that malpractice cases involving errors in clinical judgement had a 5.01 times greater odds of indemnity payment compared with those without these errors after adjusting for all other variables in the model (eg, age, severity). Note that the 95% CI does not include 1.0. This indicates that the OR is statistically >1, and we can conclude that there is a significant relationship between errors in clinical judgment and payment that is unlikely to be attributed to chance alone.
In logistic regression for categorical independent variables, all categories are compared with a reference group within that variable, with the reference group serving as the denominator of the OR. The authors4 did not incorporate continuous independent variables in their multivariable logistic regression model. However, if the authors examined length of hospitalization as a contributing factor in indemnity payments, for example, the OR would represent a 1-unit increase in this variable (eg, 1-day increase in length of stay).
Conclusion
Logistic regression describes the relationships in data and is an important statistical model across many types of research. This Progress Note emphasizes the importance of weighing the advantages and limitations of logistic regression, provides a common approach to data transformation, and guides the correct interpretation of logistic regression model results.
1. Windish DM, Huot SJ, Green ML. Medicine residents’ understanding of the biostatistics and results in the medical literature. JAMA. 2007;298(9):1010. https://doi.org/10.1001/jama.298.9.1010
2. MacDougall M, Cameron HS, Maxwell SRJ. Medical graduate views on statistical learning needs for clinical practice: a comprehensive survey. BMC Med Educ. 2019;20(1):1. https://doi.org/10.1186/s12909-019-1842-1
3. Montori VM. Progress in evidence-based medicine. JAMA. 2008;300(15):1814-1816. https://doi.org/10.1001/jama.300.15.1814
4. Schaffer AC, Yu-Moe CW, Babayan A, Wachter RM, Einbinder JS. Rates and characteristics of medical malpractice claims against hospitalists. J Hosp Med. 2021;16(7):390-396. https://doi.org/10.12788/jhm.3557
5. Lane DM, Scott D, Hebl M, Guerra R, Osherson D, Zimmer H. Introducton to Statistics. Accessed April 13, 2021. https://onlinestatbook.com/Online_Statistics_Education.pdf
6. Marill KA. Advanced statistics: linear regression, part II: multiple linear regression. Acad Emerg Med Off J Soc Acad Emerg Med. 2004;11(1):94-102. https://doi.org/10.1197/j.aem.2003.09.006
1. Windish DM, Huot SJ, Green ML. Medicine residents’ understanding of the biostatistics and results in the medical literature. JAMA. 2007;298(9):1010. https://doi.org/10.1001/jama.298.9.1010
2. MacDougall M, Cameron HS, Maxwell SRJ. Medical graduate views on statistical learning needs for clinical practice: a comprehensive survey. BMC Med Educ. 2019;20(1):1. https://doi.org/10.1186/s12909-019-1842-1
3. Montori VM. Progress in evidence-based medicine. JAMA. 2008;300(15):1814-1816. https://doi.org/10.1001/jama.300.15.1814
4. Schaffer AC, Yu-Moe CW, Babayan A, Wachter RM, Einbinder JS. Rates and characteristics of medical malpractice claims against hospitalists. J Hosp Med. 2021;16(7):390-396. https://doi.org/10.12788/jhm.3557
5. Lane DM, Scott D, Hebl M, Guerra R, Osherson D, Zimmer H. Introducton to Statistics. Accessed April 13, 2021. https://onlinestatbook.com/Online_Statistics_Education.pdf
6. Marill KA. Advanced statistics: linear regression, part II: multiple linear regression. Acad Emerg Med Off J Soc Acad Emerg Med. 2004;11(1):94-102. https://doi.org/10.1197/j.aem.2003.09.006
© 2021 Society of Hospital Medicine
Clinical Progress Note: Intravenous Human Albumin in Patients With Cirrhosis
The burden of chronic liver disease (CLD) in the United States is growing, and it is currently the fourth leading cause of death in adults aged 45 to 64 years.1 From 2012 to 2016, there were 538,720 hospitalizations in the United States for patients with cirrhosis, with almost a quarter having at least one cirrhosis-related complication. Inpatient hospitalizations for cirrhosis contribute to healthcare resource utilization, with a mean cost per CLD-related hospitalization of $16,271, and the presence of cirrhosis results in higher mortality and cost burden.1
In hospitalized patients with decompensated cirrhosis with ascites, intravenous human albumin (HA) infusion has been utilized for decades for a variety of indications. Current guidance by the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL) recommends the use of albumin for the prevention of paracentesis-induced circulatory dysfunction (PICD) for the prevention of kidney injury in spontaneous bacterial peritonitis (SBP) and for the diagnosis and treatment of hepatorenal syndrome (HRS).2,3 There have been several major trials in recent years studying the use of HA for other indications in patients with cirrhosis, and the Society of Critical Care Medicine (SCCM) updated their guidelines in 2020 to recommend HA administration in resuscitation of critically ill patients with liver failure with hypoalbuminemia.4This Clinical Progress Note addresses the use of albumin in hospitalized patients with cirrhosis, focusing on current indications and discussing potential uses published after the 2018 EASL guidelines. We conducted a literature search via the PubMed database. The authors began by using the Medical Subject Heading (MeSH) terms albumins/administration AND dosage; organization AND administration; adverse effects; and therapeutic use combined with liver cirrhosis as a MeSH major topic, which yielded 107 English-language articles published in the previous 10 years, and MeSH major topics of albumins and liver cirrhosis, which yielded 461 English-language articles, with 178 published in the previous 10 years. The search results were reviewed for applicability to albumin strategies for patients with cirrhosis.
CURRENT EVIDENCE-BASED INDICATIONS FOR USE OF ALBUMIN IN PATIENTS WITH CIRRHOSIS
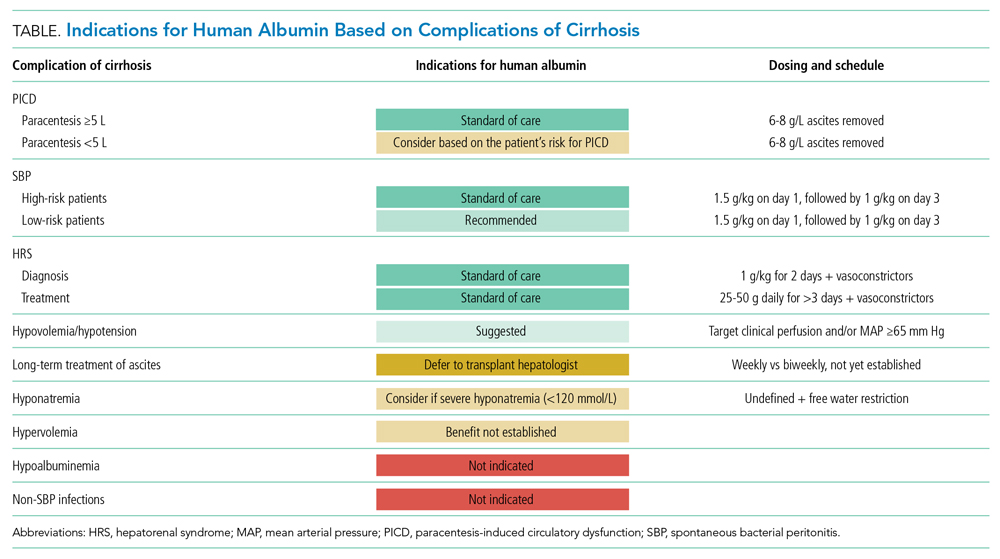
There are three widely accepted and evidence-based indications for HA infusion in patients with cirrhosis, considered standard of care (Table).
Prevention of PICD
Therapeutic large-volume paracentesis (LVP) leads to a rise in plasma renin activity (PRA) centrally through several mechanisms and is not impacted by the rate of ascites removal.5 LVP relieves abdominal pressure, increasing venous return to the heart and cardiac output, and the corresponding drop in systemic vascular resistance with splanchnic vasodilation decreases effective circulating volume and activates the renin-angiotensin system. This PRA activation and circulatory dysfunction are associated with reaccumulating ascites, renal impairment, hypervolemic hyponatremia, and increased mortality.6 A large meta-analysis of 17 trials with 1225 patients found that HA infusion improves outcomes and reduces mortality for patients undergoing LVP (odds ratio [OR], 0.64; 95% CI, 0.41-0.98), reduces the risk of PICD more than other volume expanders tested, and lowers the incidence of hyponatremia.6 More recently, in 2017, Kütting et al7 analyzed 21 trials with 1277 patients and did not observe a significant mortality benefit for HA after LVP (OR, 0.78; 95% CI, 0.55-1.11). However, negative outcomes such as rise in PRA (OR, 0.53; 95% CI, 0.29-0.97) and hyponatremia (OR, 0.62; 95% CI, 0.42-0.94) were prevented. Guidelines recommend HA after LVP ≥5 L to prevent PICD, with a replacement volume of 6 to 8 g of albumin per liter of ascitic fluid removed.2,3 Some patients may be at higher risk for PICD with less ascites removed, and the AASLD supports the use of HA to prevent PICD after smaller-volume paracentesis in patients who are already hypotensive (systolic blood pressure <90 mm Hg) or hyponatremic (<130 mmol/L), or have acute kidney injury.3
Spontaneous Bacterial Peritonitis
Spontaneous bacterial peritonitis is diagnosed by paracentesis, defined as ascitic neutrophil count ≥250 cells/µL with or without bacterascites (positive bacteriological culture). Bacterascites may be a precursor to the development of SBP, with the fluid neutrophil count of ≥250 determining the need for SBP treatment.2 SBP can lead to circulatory dysfunction, hepatic encephalopathy, and HRS. Treating SBP with HA in addition to antibiotics reduces the risk of kidney injury compared with antibiotics alone (OR for kidney injury with antibiotics alone, 4.6; 95% CI, 1.3-16.1) and also reduces the risk of death (OR for mortality with antibiotics alone, 4.5; 95% CI, 1.0-20.9).8 The AASLD recommends albumin in addition to antibiotics in SBP to prevent HRS and acute kidney injury, and high-risk patients who already have kidney dysfunction (creatinine >1 mg/dL) or jaundice (total bilirubin >5 mg/dL) are more likely to benefit from albumin. The treatment schedule is 25% HA at 1.5 g/kg on day 1 and 1 g/kg on day 3.3 The EASL recommends administering HA to all patients with cirrhosis with SBP regardless of renal or liver indices. They acknowledge, however, that the incidence of SBP-associated acute kidney injury will be low in patients without severe hepatic disease or baseline renal impairment.2
Hepatorenal Syndrome
Albumin combined with vasoconstrictors is effective in treating HRS with a response rate of 20% to 80% (average, 50%).3 Vasoactive medications can include combination midodrine and octreotide or norepinephrine (or terlipressin outside of the United States). In patients with suspected HRS, the recommended dosing of 25% HA is 1 g/kg (to a maximum of 100 g of albumin) on day 1 and then 40 to 50 g daily for at least 3 days after the diagnosis is confirmed.3 The optimal duration of therapy beyond 3 days of combined therapy with midodrine, albumin, and octreotide is not established. Terlipressin treatment is recommended for a maximum of 14 days in cases of partial response or nonresponse in renal recovery.2
INDICATIONS FOR ALBUMIN WITHOUT CLEAR EVIDENCE OF EFFICACY
Hypoalbuminemia
Albumin administration to raise serum albumin levels in hospitalized patients has been a common practice. However, new evidence suggests that treating hypoalbuminemia with infusion of HA in hospitalized patients with decompensated cirrhosis does not protect patients from risk and causes harm. The Albumin To prevenT Infection in chronic liveR (ATTIRE) trial, published in 2021, randomly assigned 777 patients across 35 centers in the United Kingdom to receive daily 20% HA to target a serum albumin level of 3.0 g/dL vs standard care, including HA for established indications.2,3 The primary end point was a composite of infection, kidney dysfunction, and death within 3 to 15 days of initiating treatment. There were no differences in the primary end point; secondary end points of death at 28 days, 3 months, or 6 months; or duration of hospitalization. The treatment group received 10 times more albumin than the control group and reported more adverse events, including pulmonary edema.9
Long-Term Treatment in Patients With Ascites
The human Albumin for the treatmeNt of aScites in patients With hEpatic ciRrhosis (ANSWER) trial, published in 2018, found improved 18-month survival in patients with cirrhosis and ascites treated with diuretics who received long-term albumin. This was an open-label trial of 431 patients at 33 sites in Italy, and the treatment arm received weekly infusions of 40 g of 20% HA. They observed a 38% reduction in mortality hazard ratio and half the number of hospital days annually.10 Based on these data and those from a 2006 Italian study with similar design and results, the Italian Association for the Study of the Liver (AISF) strongly recommends long-term albumin treatment in patients with cirrhosis with ascites.11 The lead author on the ANSWER trial also authored the AISF statement, although this recommendation has not been adopted by the EASL or the AASLD.
Conversely, the Midodrine and Albumin for CirrHoTic patients (MACHT) trial, also published in 2018, randomly assigned 173 patients with ascites awaiting liver transplant to receive 40 g of HA every 15 days and midodrine in addition to standard care vs placebo. MACHT found no difference in mortality or complications at 1 year.12
Long-term albumin therapy as a preventive measure may be a disease modifier, taking advantage of the pleiotropic effects of albumin, though the differing conclusions from ANSWER and MACHT necessitate additional trials. The ongoing PRECIOSA study in Spain is assessing dosage and schedule for this therapy.13
Augmenting Diuresis
Loop diuretics are highly protein-bound, and, with hypoalbuminemia, there is less effective drug delivered to the site of action. One clinical approach is to augment diuretics with concomitant HA infusion. This approach is not supported by strong evidence or guidelines.
Hyponatremia
In a retrospective cohort study of 2435 hospitalized patients with cirrhosis, 1126 of whom had hyponatremia, those patients with sodium <130 mmol/L who received HA were more likely to have resolution of hyponatremia to >135 mmol/L. This was associated with improved 30-day survival.14 From this observational data, the AASLD supports the use of albumin combined with extreme fluid restriction (<1000 mL/d) for patients with severe hyponatremia (<120 mmol/L).3
Non-SBP Infections
A 2019 meta-analysis found no evidence of a benefit of HA for bacterial infections other than SBP. However, only three trials encompassing 407 patients met the inclusion criteria.15
NEW GUIDELINE-SUGGESTED USE FOR ALBUMIN IN PATIENTS WITH CIRRHOSIS
SCCM Guideline Update: Hypoalbuminemia and Hypotension
The 2020 SCCM Guidelines for the Management of Adult Acute and Acute-on-Chronic Liver Failure in the ICU “suggest using albumin for resuscitation of patients [with liver failure] over other fluids, especially when serum albumin is low (<3 g/dL).” Acute-on-chronic liver failure is decompensation of cirrhosis combined with organ dysfunction (eg, coagulopathy, encephalopathy, kidney injury), a scenario that is frequently encountered by hospitalists outside of intensive care settings. In hypotensive patients with cirrhosis, the SCCM recommends administering albumin to a target mean arterial pressure of 65 mm Hg or otherwise adequate perfusion. This new recommendation is conditional, based on expert consensus, and derives from low-quality evidence, with acknowledgement that “costs may be prohibitive.”4
While the ATTIRE study demonstrated no benefit in treating hypoalbuminemia with infusion of HA in hospitalized patients with decompensated cirrhosis, the 2020 SCCM guidelines, released prior to the publication of the ATTIRE study, focused on more acutely ill patients. In the ATTIRE study, only 2% to 3% of the study population was in an intensive care unit.4,9 The use of albumin infusion in the critically ill, hypoalbuminemic, hypotensive patient is not well studied, and the SCCM acknowledges the lack of supportive evidence for this practice in their guideline statement.
CONCLUSION
The three cardinal clinical indications for human albumin in patients with cirrhosis—prevention of PICD after LVP, in SBP, and for HRS—remain supported by the literature and guidelines, with the most recent guidance adding more nuance in patient selection based on individual risk (Table). With the publication of several large-scale studies in the past few years and a 2021 update to the AASLD guidance statement, clinicians have more evidence to guide their use of HA in patients with cirrhosis. In particular, the practice of treating isolated hypoalbuminemia with HA is no longer supported by the best evidence and is potentially harmful. A professional society recommendation to preferentially use albumin as a resuscitation fluid in hypoalbuminemia was made without the benefit of the results of the 2021 ATTIRE trial. On the horizon, additional results from ongoing and upcoming studies exploring concepts of effective albumin concentration and the pleiotropic properties of HA will impact the use of this therapy in hospitalized patients with cirrhosis.
1. Hirode G, Saab S, Wong RJ. Trends in the burden of chronic liver disease among hospitalized US adults. JAMA Netw Open. 2020;3(4):e201997. https://doi.org/10.1001/jamanetworkopen.2020.1997
2. European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406-460. https://doi.org/10.1016/j.jhep.2018.03.024
3. Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74(2):1014-1048. https://doi.org/10.1002/hep.31884
4. Nanchal R, Subramanian R, Karvellas CJ, et al. Guidelines for the management of adult acute and acute-on-chronic liver failure in the ICU: cardiovascular, endocrine, hematologic, pulmonary, and renal considerations. Crit Care Med. 2020;48(3):e173-e191. https://doi.org/10.1097/CCM.0000000000004192
5. Elsabaawy MM, Abdelhamid SR, Alsebaey A, et al. The impact of paracentesis flow rate in patients with liver cirrhosis on the development of paracentesis induced circulatory dysfunction. Clin Mol Hepatol. 2015;21(4):365-371. https://doi.org/10.3350/cmh.2015.21.4.365
6. Bernardi M, Caraceni P, Navickis RJ, Wilkes MM. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55(4):1172-1181. https://doi.org/10.1002/hep.24786
7. Kütting F, Schubert J, Franklin J, et al. Insufficient evidence of benefit regarding mortality due to albumin substitution in HCC-free cirrhotic patients undergoing large volume paracentesis. J Gastroenterol Hepatol. 2017;32(2):327-338. https://doi.org/10.1111/jgh.13421
8. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341(6):403-409. https://doi.org/10.1056/NEJM199908053410603
9. China L, Freemantle N, Forrest E, et al. A randomized trial of albumin infusions in hospitalized patients with cirrhosis. N Engl J Med. 2021;384(9):808-817. https://doi.org/10.1056/NEJMoa2022166
10. Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391(10138):2417-2429. https://doi.org/10.1016/S0140-6736(18)30840-7
11. Caraceni P, Angeli P, Prati D, et al. AISF-SIMTI position paper on the appropriate use of albumin in patients with liver cirrhosis: a 2020 update. Blood Transfus. 2021;19(1):9-13. https://doi.org/10.2450/2020.0414-20
12. Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69(6):1250-1259. https://doi.org/10.1016/j.jhep.2018.08.006
13. Fernández J, Clària J, Amorós A, et al. Effects of albumin treatment on systemic and portal hemodynamics and systemic inflammation in patients with decompensated cirrhosis. Gastroenterology. 2019;157(1):149-162. https://doi.org/10.1053/j.gastro.2019.03.021
14. Bajaj JS, Tandon P, O’Leary JG, et al. The impact of albumin use on resolution of hyponatremia in hospitalized patients with cirrhosis. Am J Gastroenterol. 2018;113(9):1339. https://doi.org/10.1038/s41395-018-0119-3
15. Leão GS, Neto GJ, Jotz RdF, de Mattos AA, de Mattos ÂZ. Albumin for cirrhotic patients with extraperitoneal infections: a meta-analysis. J Gastroenterol Hepatol. 2019;34(12):2071-2076. https://doi.org/10.1111/jgh.14791
The burden of chronic liver disease (CLD) in the United States is growing, and it is currently the fourth leading cause of death in adults aged 45 to 64 years.1 From 2012 to 2016, there were 538,720 hospitalizations in the United States for patients with cirrhosis, with almost a quarter having at least one cirrhosis-related complication. Inpatient hospitalizations for cirrhosis contribute to healthcare resource utilization, with a mean cost per CLD-related hospitalization of $16,271, and the presence of cirrhosis results in higher mortality and cost burden.1
In hospitalized patients with decompensated cirrhosis with ascites, intravenous human albumin (HA) infusion has been utilized for decades for a variety of indications. Current guidance by the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL) recommends the use of albumin for the prevention of paracentesis-induced circulatory dysfunction (PICD) for the prevention of kidney injury in spontaneous bacterial peritonitis (SBP) and for the diagnosis and treatment of hepatorenal syndrome (HRS).2,3 There have been several major trials in recent years studying the use of HA for other indications in patients with cirrhosis, and the Society of Critical Care Medicine (SCCM) updated their guidelines in 2020 to recommend HA administration in resuscitation of critically ill patients with liver failure with hypoalbuminemia.4This Clinical Progress Note addresses the use of albumin in hospitalized patients with cirrhosis, focusing on current indications and discussing potential uses published after the 2018 EASL guidelines. We conducted a literature search via the PubMed database. The authors began by using the Medical Subject Heading (MeSH) terms albumins/administration AND dosage; organization AND administration; adverse effects; and therapeutic use combined with liver cirrhosis as a MeSH major topic, which yielded 107 English-language articles published in the previous 10 years, and MeSH major topics of albumins and liver cirrhosis, which yielded 461 English-language articles, with 178 published in the previous 10 years. The search results were reviewed for applicability to albumin strategies for patients with cirrhosis.
CURRENT EVIDENCE-BASED INDICATIONS FOR USE OF ALBUMIN IN PATIENTS WITH CIRRHOSIS
There are three widely accepted and evidence-based indications for HA infusion in patients with cirrhosis, considered standard of care (Table).

Prevention of PICD
Therapeutic large-volume paracentesis (LVP) leads to a rise in plasma renin activity (PRA) centrally through several mechanisms and is not impacted by the rate of ascites removal.5 LVP relieves abdominal pressure, increasing venous return to the heart and cardiac output, and the corresponding drop in systemic vascular resistance with splanchnic vasodilation decreases effective circulating volume and activates the renin-angiotensin system. This PRA activation and circulatory dysfunction are associated with reaccumulating ascites, renal impairment, hypervolemic hyponatremia, and increased mortality.6 A large meta-analysis of 17 trials with 1225 patients found that HA infusion improves outcomes and reduces mortality for patients undergoing LVP (odds ratio [OR], 0.64; 95% CI, 0.41-0.98), reduces the risk of PICD more than other volume expanders tested, and lowers the incidence of hyponatremia.6 More recently, in 2017, Kütting et al7 analyzed 21 trials with 1277 patients and did not observe a significant mortality benefit for HA after LVP (OR, 0.78; 95% CI, 0.55-1.11). However, negative outcomes such as rise in PRA (OR, 0.53; 95% CI, 0.29-0.97) and hyponatremia (OR, 0.62; 95% CI, 0.42-0.94) were prevented. Guidelines recommend HA after LVP ≥5 L to prevent PICD, with a replacement volume of 6 to 8 g of albumin per liter of ascitic fluid removed.2,3 Some patients may be at higher risk for PICD with less ascites removed, and the AASLD supports the use of HA to prevent PICD after smaller-volume paracentesis in patients who are already hypotensive (systolic blood pressure <90 mm Hg) or hyponatremic (<130 mmol/L), or have acute kidney injury.3
Spontaneous Bacterial Peritonitis
Spontaneous bacterial peritonitis is diagnosed by paracentesis, defined as ascitic neutrophil count ≥250 cells/µL with or without bacterascites (positive bacteriological culture). Bacterascites may be a precursor to the development of SBP, with the fluid neutrophil count of ≥250 determining the need for SBP treatment.2 SBP can lead to circulatory dysfunction, hepatic encephalopathy, and HRS. Treating SBP with HA in addition to antibiotics reduces the risk of kidney injury compared with antibiotics alone (OR for kidney injury with antibiotics alone, 4.6; 95% CI, 1.3-16.1) and also reduces the risk of death (OR for mortality with antibiotics alone, 4.5; 95% CI, 1.0-20.9).8 The AASLD recommends albumin in addition to antibiotics in SBP to prevent HRS and acute kidney injury, and high-risk patients who already have kidney dysfunction (creatinine >1 mg/dL) or jaundice (total bilirubin >5 mg/dL) are more likely to benefit from albumin. The treatment schedule is 25% HA at 1.5 g/kg on day 1 and 1 g/kg on day 3.3 The EASL recommends administering HA to all patients with cirrhosis with SBP regardless of renal or liver indices. They acknowledge, however, that the incidence of SBP-associated acute kidney injury will be low in patients without severe hepatic disease or baseline renal impairment.2
Hepatorenal Syndrome
Albumin combined with vasoconstrictors is effective in treating HRS with a response rate of 20% to 80% (average, 50%).3 Vasoactive medications can include combination midodrine and octreotide or norepinephrine (or terlipressin outside of the United States). In patients with suspected HRS, the recommended dosing of 25% HA is 1 g/kg (to a maximum of 100 g of albumin) on day 1 and then 40 to 50 g daily for at least 3 days after the diagnosis is confirmed.3 The optimal duration of therapy beyond 3 days of combined therapy with midodrine, albumin, and octreotide is not established. Terlipressin treatment is recommended for a maximum of 14 days in cases of partial response or nonresponse in renal recovery.2
INDICATIONS FOR ALBUMIN WITHOUT CLEAR EVIDENCE OF EFFICACY
Hypoalbuminemia
Albumin administration to raise serum albumin levels in hospitalized patients has been a common practice. However, new evidence suggests that treating hypoalbuminemia with infusion of HA in hospitalized patients with decompensated cirrhosis does not protect patients from risk and causes harm. The Albumin To prevenT Infection in chronic liveR (ATTIRE) trial, published in 2021, randomly assigned 777 patients across 35 centers in the United Kingdom to receive daily 20% HA to target a serum albumin level of 3.0 g/dL vs standard care, including HA for established indications.2,3 The primary end point was a composite of infection, kidney dysfunction, and death within 3 to 15 days of initiating treatment. There were no differences in the primary end point; secondary end points of death at 28 days, 3 months, or 6 months; or duration of hospitalization. The treatment group received 10 times more albumin than the control group and reported more adverse events, including pulmonary edema.9
Long-Term Treatment in Patients With Ascites
The human Albumin for the treatmeNt of aScites in patients With hEpatic ciRrhosis (ANSWER) trial, published in 2018, found improved 18-month survival in patients with cirrhosis and ascites treated with diuretics who received long-term albumin. This was an open-label trial of 431 patients at 33 sites in Italy, and the treatment arm received weekly infusions of 40 g of 20% HA. They observed a 38% reduction in mortality hazard ratio and half the number of hospital days annually.10 Based on these data and those from a 2006 Italian study with similar design and results, the Italian Association for the Study of the Liver (AISF) strongly recommends long-term albumin treatment in patients with cirrhosis with ascites.11 The lead author on the ANSWER trial also authored the AISF statement, although this recommendation has not been adopted by the EASL or the AASLD.
Conversely, the Midodrine and Albumin for CirrHoTic patients (MACHT) trial, also published in 2018, randomly assigned 173 patients with ascites awaiting liver transplant to receive 40 g of HA every 15 days and midodrine in addition to standard care vs placebo. MACHT found no difference in mortality or complications at 1 year.12
Long-term albumin therapy as a preventive measure may be a disease modifier, taking advantage of the pleiotropic effects of albumin, though the differing conclusions from ANSWER and MACHT necessitate additional trials. The ongoing PRECIOSA study in Spain is assessing dosage and schedule for this therapy.13
Augmenting Diuresis
Loop diuretics are highly protein-bound, and, with hypoalbuminemia, there is less effective drug delivered to the site of action. One clinical approach is to augment diuretics with concomitant HA infusion. This approach is not supported by strong evidence or guidelines.
Hyponatremia
In a retrospective cohort study of 2435 hospitalized patients with cirrhosis, 1126 of whom had hyponatremia, those patients with sodium <130 mmol/L who received HA were more likely to have resolution of hyponatremia to >135 mmol/L. This was associated with improved 30-day survival.14 From this observational data, the AASLD supports the use of albumin combined with extreme fluid restriction (<1000 mL/d) for patients with severe hyponatremia (<120 mmol/L).3
Non-SBP Infections
A 2019 meta-analysis found no evidence of a benefit of HA for bacterial infections other than SBP. However, only three trials encompassing 407 patients met the inclusion criteria.15
NEW GUIDELINE-SUGGESTED USE FOR ALBUMIN IN PATIENTS WITH CIRRHOSIS
SCCM Guideline Update: Hypoalbuminemia and Hypotension
The 2020 SCCM Guidelines for the Management of Adult Acute and Acute-on-Chronic Liver Failure in the ICU “suggest using albumin for resuscitation of patients [with liver failure] over other fluids, especially when serum albumin is low (<3 g/dL).” Acute-on-chronic liver failure is decompensation of cirrhosis combined with organ dysfunction (eg, coagulopathy, encephalopathy, kidney injury), a scenario that is frequently encountered by hospitalists outside of intensive care settings. In hypotensive patients with cirrhosis, the SCCM recommends administering albumin to a target mean arterial pressure of 65 mm Hg or otherwise adequate perfusion. This new recommendation is conditional, based on expert consensus, and derives from low-quality evidence, with acknowledgement that “costs may be prohibitive.”4
While the ATTIRE study demonstrated no benefit in treating hypoalbuminemia with infusion of HA in hospitalized patients with decompensated cirrhosis, the 2020 SCCM guidelines, released prior to the publication of the ATTIRE study, focused on more acutely ill patients. In the ATTIRE study, only 2% to 3% of the study population was in an intensive care unit.4,9 The use of albumin infusion in the critically ill, hypoalbuminemic, hypotensive patient is not well studied, and the SCCM acknowledges the lack of supportive evidence for this practice in their guideline statement.
CONCLUSION
The three cardinal clinical indications for human albumin in patients with cirrhosis—prevention of PICD after LVP, in SBP, and for HRS—remain supported by the literature and guidelines, with the most recent guidance adding more nuance in patient selection based on individual risk (Table). With the publication of several large-scale studies in the past few years and a 2021 update to the AASLD guidance statement, clinicians have more evidence to guide their use of HA in patients with cirrhosis. In particular, the practice of treating isolated hypoalbuminemia with HA is no longer supported by the best evidence and is potentially harmful. A professional society recommendation to preferentially use albumin as a resuscitation fluid in hypoalbuminemia was made without the benefit of the results of the 2021 ATTIRE trial. On the horizon, additional results from ongoing and upcoming studies exploring concepts of effective albumin concentration and the pleiotropic properties of HA will impact the use of this therapy in hospitalized patients with cirrhosis.
The burden of chronic liver disease (CLD) in the United States is growing, and it is currently the fourth leading cause of death in adults aged 45 to 64 years.1 From 2012 to 2016, there were 538,720 hospitalizations in the United States for patients with cirrhosis, with almost a quarter having at least one cirrhosis-related complication. Inpatient hospitalizations for cirrhosis contribute to healthcare resource utilization, with a mean cost per CLD-related hospitalization of $16,271, and the presence of cirrhosis results in higher mortality and cost burden.1
In hospitalized patients with decompensated cirrhosis with ascites, intravenous human albumin (HA) infusion has been utilized for decades for a variety of indications. Current guidance by the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL) recommends the use of albumin for the prevention of paracentesis-induced circulatory dysfunction (PICD) for the prevention of kidney injury in spontaneous bacterial peritonitis (SBP) and for the diagnosis and treatment of hepatorenal syndrome (HRS).2,3 There have been several major trials in recent years studying the use of HA for other indications in patients with cirrhosis, and the Society of Critical Care Medicine (SCCM) updated their guidelines in 2020 to recommend HA administration in resuscitation of critically ill patients with liver failure with hypoalbuminemia.4This Clinical Progress Note addresses the use of albumin in hospitalized patients with cirrhosis, focusing on current indications and discussing potential uses published after the 2018 EASL guidelines. We conducted a literature search via the PubMed database. The authors began by using the Medical Subject Heading (MeSH) terms albumins/administration AND dosage; organization AND administration; adverse effects; and therapeutic use combined with liver cirrhosis as a MeSH major topic, which yielded 107 English-language articles published in the previous 10 years, and MeSH major topics of albumins and liver cirrhosis, which yielded 461 English-language articles, with 178 published in the previous 10 years. The search results were reviewed for applicability to albumin strategies for patients with cirrhosis.
CURRENT EVIDENCE-BASED INDICATIONS FOR USE OF ALBUMIN IN PATIENTS WITH CIRRHOSIS
There are three widely accepted and evidence-based indications for HA infusion in patients with cirrhosis, considered standard of care (Table).

Prevention of PICD
Therapeutic large-volume paracentesis (LVP) leads to a rise in plasma renin activity (PRA) centrally through several mechanisms and is not impacted by the rate of ascites removal.5 LVP relieves abdominal pressure, increasing venous return to the heart and cardiac output, and the corresponding drop in systemic vascular resistance with splanchnic vasodilation decreases effective circulating volume and activates the renin-angiotensin system. This PRA activation and circulatory dysfunction are associated with reaccumulating ascites, renal impairment, hypervolemic hyponatremia, and increased mortality.6 A large meta-analysis of 17 trials with 1225 patients found that HA infusion improves outcomes and reduces mortality for patients undergoing LVP (odds ratio [OR], 0.64; 95% CI, 0.41-0.98), reduces the risk of PICD more than other volume expanders tested, and lowers the incidence of hyponatremia.6 More recently, in 2017, Kütting et al7 analyzed 21 trials with 1277 patients and did not observe a significant mortality benefit for HA after LVP (OR, 0.78; 95% CI, 0.55-1.11). However, negative outcomes such as rise in PRA (OR, 0.53; 95% CI, 0.29-0.97) and hyponatremia (OR, 0.62; 95% CI, 0.42-0.94) were prevented. Guidelines recommend HA after LVP ≥5 L to prevent PICD, with a replacement volume of 6 to 8 g of albumin per liter of ascitic fluid removed.2,3 Some patients may be at higher risk for PICD with less ascites removed, and the AASLD supports the use of HA to prevent PICD after smaller-volume paracentesis in patients who are already hypotensive (systolic blood pressure <90 mm Hg) or hyponatremic (<130 mmol/L), or have acute kidney injury.3
Spontaneous Bacterial Peritonitis
Spontaneous bacterial peritonitis is diagnosed by paracentesis, defined as ascitic neutrophil count ≥250 cells/µL with or without bacterascites (positive bacteriological culture). Bacterascites may be a precursor to the development of SBP, with the fluid neutrophil count of ≥250 determining the need for SBP treatment.2 SBP can lead to circulatory dysfunction, hepatic encephalopathy, and HRS. Treating SBP with HA in addition to antibiotics reduces the risk of kidney injury compared with antibiotics alone (OR for kidney injury with antibiotics alone, 4.6; 95% CI, 1.3-16.1) and also reduces the risk of death (OR for mortality with antibiotics alone, 4.5; 95% CI, 1.0-20.9).8 The AASLD recommends albumin in addition to antibiotics in SBP to prevent HRS and acute kidney injury, and high-risk patients who already have kidney dysfunction (creatinine >1 mg/dL) or jaundice (total bilirubin >5 mg/dL) are more likely to benefit from albumin. The treatment schedule is 25% HA at 1.5 g/kg on day 1 and 1 g/kg on day 3.3 The EASL recommends administering HA to all patients with cirrhosis with SBP regardless of renal or liver indices. They acknowledge, however, that the incidence of SBP-associated acute kidney injury will be low in patients without severe hepatic disease or baseline renal impairment.2
Hepatorenal Syndrome
Albumin combined with vasoconstrictors is effective in treating HRS with a response rate of 20% to 80% (average, 50%).3 Vasoactive medications can include combination midodrine and octreotide or norepinephrine (or terlipressin outside of the United States). In patients with suspected HRS, the recommended dosing of 25% HA is 1 g/kg (to a maximum of 100 g of albumin) on day 1 and then 40 to 50 g daily for at least 3 days after the diagnosis is confirmed.3 The optimal duration of therapy beyond 3 days of combined therapy with midodrine, albumin, and octreotide is not established. Terlipressin treatment is recommended for a maximum of 14 days in cases of partial response or nonresponse in renal recovery.2
INDICATIONS FOR ALBUMIN WITHOUT CLEAR EVIDENCE OF EFFICACY
Hypoalbuminemia
Albumin administration to raise serum albumin levels in hospitalized patients has been a common practice. However, new evidence suggests that treating hypoalbuminemia with infusion of HA in hospitalized patients with decompensated cirrhosis does not protect patients from risk and causes harm. The Albumin To prevenT Infection in chronic liveR (ATTIRE) trial, published in 2021, randomly assigned 777 patients across 35 centers in the United Kingdom to receive daily 20% HA to target a serum albumin level of 3.0 g/dL vs standard care, including HA for established indications.2,3 The primary end point was a composite of infection, kidney dysfunction, and death within 3 to 15 days of initiating treatment. There were no differences in the primary end point; secondary end points of death at 28 days, 3 months, or 6 months; or duration of hospitalization. The treatment group received 10 times more albumin than the control group and reported more adverse events, including pulmonary edema.9
Long-Term Treatment in Patients With Ascites
The human Albumin for the treatmeNt of aScites in patients With hEpatic ciRrhosis (ANSWER) trial, published in 2018, found improved 18-month survival in patients with cirrhosis and ascites treated with diuretics who received long-term albumin. This was an open-label trial of 431 patients at 33 sites in Italy, and the treatment arm received weekly infusions of 40 g of 20% HA. They observed a 38% reduction in mortality hazard ratio and half the number of hospital days annually.10 Based on these data and those from a 2006 Italian study with similar design and results, the Italian Association for the Study of the Liver (AISF) strongly recommends long-term albumin treatment in patients with cirrhosis with ascites.11 The lead author on the ANSWER trial also authored the AISF statement, although this recommendation has not been adopted by the EASL or the AASLD.
Conversely, the Midodrine and Albumin for CirrHoTic patients (MACHT) trial, also published in 2018, randomly assigned 173 patients with ascites awaiting liver transplant to receive 40 g of HA every 15 days and midodrine in addition to standard care vs placebo. MACHT found no difference in mortality or complications at 1 year.12
Long-term albumin therapy as a preventive measure may be a disease modifier, taking advantage of the pleiotropic effects of albumin, though the differing conclusions from ANSWER and MACHT necessitate additional trials. The ongoing PRECIOSA study in Spain is assessing dosage and schedule for this therapy.13
Augmenting Diuresis
Loop diuretics are highly protein-bound, and, with hypoalbuminemia, there is less effective drug delivered to the site of action. One clinical approach is to augment diuretics with concomitant HA infusion. This approach is not supported by strong evidence or guidelines.
Hyponatremia
In a retrospective cohort study of 2435 hospitalized patients with cirrhosis, 1126 of whom had hyponatremia, those patients with sodium <130 mmol/L who received HA were more likely to have resolution of hyponatremia to >135 mmol/L. This was associated with improved 30-day survival.14 From this observational data, the AASLD supports the use of albumin combined with extreme fluid restriction (<1000 mL/d) for patients with severe hyponatremia (<120 mmol/L).3
Non-SBP Infections
A 2019 meta-analysis found no evidence of a benefit of HA for bacterial infections other than SBP. However, only three trials encompassing 407 patients met the inclusion criteria.15
NEW GUIDELINE-SUGGESTED USE FOR ALBUMIN IN PATIENTS WITH CIRRHOSIS
SCCM Guideline Update: Hypoalbuminemia and Hypotension
The 2020 SCCM Guidelines for the Management of Adult Acute and Acute-on-Chronic Liver Failure in the ICU “suggest using albumin for resuscitation of patients [with liver failure] over other fluids, especially when serum albumin is low (<3 g/dL).” Acute-on-chronic liver failure is decompensation of cirrhosis combined with organ dysfunction (eg, coagulopathy, encephalopathy, kidney injury), a scenario that is frequently encountered by hospitalists outside of intensive care settings. In hypotensive patients with cirrhosis, the SCCM recommends administering albumin to a target mean arterial pressure of 65 mm Hg or otherwise adequate perfusion. This new recommendation is conditional, based on expert consensus, and derives from low-quality evidence, with acknowledgement that “costs may be prohibitive.”4
While the ATTIRE study demonstrated no benefit in treating hypoalbuminemia with infusion of HA in hospitalized patients with decompensated cirrhosis, the 2020 SCCM guidelines, released prior to the publication of the ATTIRE study, focused on more acutely ill patients. In the ATTIRE study, only 2% to 3% of the study population was in an intensive care unit.4,9 The use of albumin infusion in the critically ill, hypoalbuminemic, hypotensive patient is not well studied, and the SCCM acknowledges the lack of supportive evidence for this practice in their guideline statement.
CONCLUSION
The three cardinal clinical indications for human albumin in patients with cirrhosis—prevention of PICD after LVP, in SBP, and for HRS—remain supported by the literature and guidelines, with the most recent guidance adding more nuance in patient selection based on individual risk (Table). With the publication of several large-scale studies in the past few years and a 2021 update to the AASLD guidance statement, clinicians have more evidence to guide their use of HA in patients with cirrhosis. In particular, the practice of treating isolated hypoalbuminemia with HA is no longer supported by the best evidence and is potentially harmful. A professional society recommendation to preferentially use albumin as a resuscitation fluid in hypoalbuminemia was made without the benefit of the results of the 2021 ATTIRE trial. On the horizon, additional results from ongoing and upcoming studies exploring concepts of effective albumin concentration and the pleiotropic properties of HA will impact the use of this therapy in hospitalized patients with cirrhosis.
1. Hirode G, Saab S, Wong RJ. Trends in the burden of chronic liver disease among hospitalized US adults. JAMA Netw Open. 2020;3(4):e201997. https://doi.org/10.1001/jamanetworkopen.2020.1997
2. European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406-460. https://doi.org/10.1016/j.jhep.2018.03.024
3. Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74(2):1014-1048. https://doi.org/10.1002/hep.31884
4. Nanchal R, Subramanian R, Karvellas CJ, et al. Guidelines for the management of adult acute and acute-on-chronic liver failure in the ICU: cardiovascular, endocrine, hematologic, pulmonary, and renal considerations. Crit Care Med. 2020;48(3):e173-e191. https://doi.org/10.1097/CCM.0000000000004192
5. Elsabaawy MM, Abdelhamid SR, Alsebaey A, et al. The impact of paracentesis flow rate in patients with liver cirrhosis on the development of paracentesis induced circulatory dysfunction. Clin Mol Hepatol. 2015;21(4):365-371. https://doi.org/10.3350/cmh.2015.21.4.365
6. Bernardi M, Caraceni P, Navickis RJ, Wilkes MM. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55(4):1172-1181. https://doi.org/10.1002/hep.24786
7. Kütting F, Schubert J, Franklin J, et al. Insufficient evidence of benefit regarding mortality due to albumin substitution in HCC-free cirrhotic patients undergoing large volume paracentesis. J Gastroenterol Hepatol. 2017;32(2):327-338. https://doi.org/10.1111/jgh.13421
8. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341(6):403-409. https://doi.org/10.1056/NEJM199908053410603
9. China L, Freemantle N, Forrest E, et al. A randomized trial of albumin infusions in hospitalized patients with cirrhosis. N Engl J Med. 2021;384(9):808-817. https://doi.org/10.1056/NEJMoa2022166
10. Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391(10138):2417-2429. https://doi.org/10.1016/S0140-6736(18)30840-7
11. Caraceni P, Angeli P, Prati D, et al. AISF-SIMTI position paper on the appropriate use of albumin in patients with liver cirrhosis: a 2020 update. Blood Transfus. 2021;19(1):9-13. https://doi.org/10.2450/2020.0414-20
12. Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69(6):1250-1259. https://doi.org/10.1016/j.jhep.2018.08.006
13. Fernández J, Clària J, Amorós A, et al. Effects of albumin treatment on systemic and portal hemodynamics and systemic inflammation in patients with decompensated cirrhosis. Gastroenterology. 2019;157(1):149-162. https://doi.org/10.1053/j.gastro.2019.03.021
14. Bajaj JS, Tandon P, O’Leary JG, et al. The impact of albumin use on resolution of hyponatremia in hospitalized patients with cirrhosis. Am J Gastroenterol. 2018;113(9):1339. https://doi.org/10.1038/s41395-018-0119-3
15. Leão GS, Neto GJ, Jotz RdF, de Mattos AA, de Mattos ÂZ. Albumin for cirrhotic patients with extraperitoneal infections: a meta-analysis. J Gastroenterol Hepatol. 2019;34(12):2071-2076. https://doi.org/10.1111/jgh.14791
1. Hirode G, Saab S, Wong RJ. Trends in the burden of chronic liver disease among hospitalized US adults. JAMA Netw Open. 2020;3(4):e201997. https://doi.org/10.1001/jamanetworkopen.2020.1997
2. European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406-460. https://doi.org/10.1016/j.jhep.2018.03.024
3. Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74(2):1014-1048. https://doi.org/10.1002/hep.31884
4. Nanchal R, Subramanian R, Karvellas CJ, et al. Guidelines for the management of adult acute and acute-on-chronic liver failure in the ICU: cardiovascular, endocrine, hematologic, pulmonary, and renal considerations. Crit Care Med. 2020;48(3):e173-e191. https://doi.org/10.1097/CCM.0000000000004192
5. Elsabaawy MM, Abdelhamid SR, Alsebaey A, et al. The impact of paracentesis flow rate in patients with liver cirrhosis on the development of paracentesis induced circulatory dysfunction. Clin Mol Hepatol. 2015;21(4):365-371. https://doi.org/10.3350/cmh.2015.21.4.365
6. Bernardi M, Caraceni P, Navickis RJ, Wilkes MM. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55(4):1172-1181. https://doi.org/10.1002/hep.24786
7. Kütting F, Schubert J, Franklin J, et al. Insufficient evidence of benefit regarding mortality due to albumin substitution in HCC-free cirrhotic patients undergoing large volume paracentesis. J Gastroenterol Hepatol. 2017;32(2):327-338. https://doi.org/10.1111/jgh.13421
8. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341(6):403-409. https://doi.org/10.1056/NEJM199908053410603
9. China L, Freemantle N, Forrest E, et al. A randomized trial of albumin infusions in hospitalized patients with cirrhosis. N Engl J Med. 2021;384(9):808-817. https://doi.org/10.1056/NEJMoa2022166
10. Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391(10138):2417-2429. https://doi.org/10.1016/S0140-6736(18)30840-7
11. Caraceni P, Angeli P, Prati D, et al. AISF-SIMTI position paper on the appropriate use of albumin in patients with liver cirrhosis: a 2020 update. Blood Transfus. 2021;19(1):9-13. https://doi.org/10.2450/2020.0414-20
12. Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69(6):1250-1259. https://doi.org/10.1016/j.jhep.2018.08.006
13. Fernández J, Clària J, Amorós A, et al. Effects of albumin treatment on systemic and portal hemodynamics and systemic inflammation in patients with decompensated cirrhosis. Gastroenterology. 2019;157(1):149-162. https://doi.org/10.1053/j.gastro.2019.03.021
14. Bajaj JS, Tandon P, O’Leary JG, et al. The impact of albumin use on resolution of hyponatremia in hospitalized patients with cirrhosis. Am J Gastroenterol. 2018;113(9):1339. https://doi.org/10.1038/s41395-018-0119-3
15. Leão GS, Neto GJ, Jotz RdF, de Mattos AA, de Mattos ÂZ. Albumin for cirrhotic patients with extraperitoneal infections: a meta-analysis. J Gastroenterol Hepatol. 2019;34(12):2071-2076. https://doi.org/10.1111/jgh.14791
© 2021 Society of Hospital Medicine
Deficits in Identification of Goals and Goal-Concordant Care After Sepsis Hospitalization
Identifying and supporting patients’ care goals through shared decision-making was named the highest priority in the Improving Hospital Outcomes through Patient Engagement (i-HOPE) study.1 Ensuring that seriously ill patients’ goals for their future care are understood and honored is particularly important for patients hospitalized with conditions known to be associated with high near-term mortality or functional disability, such as sepsis. It is increasingly recognized that a hospital admission for sepsis is associated with poor outcomes, including high rates of readmission and postdischarge mortality,2-5 yet little is known about the assessment, status, and stability of patient care goals after discharge for sepsis. Using a cohort of high-risk sepsis survivors enrolled in a clinical trial, we aimed to determine how frequently care goals were documented, describe patterns in care goals, and evaluate how frequently care goals changed over 90 days after sepsis discharge. We also used expert reviewers to assess care delivered in the 90 days after hospitalization and determine the proportion of patients who received goal-concordant care.6,7
METHODS
Design, Setting, Participants
We conducted a secondary analysis using data from the Improving Morbidity During Post-Acute Care Transitions for Sepsis (IMPACTS) study,8 a pragmatic randomized trial evaluating the effectiveness of a multicomponent transition program to reduce mortality and rehospitalization after sepsis among patients enrolled from three hospitals between January 2019 and March 2020 (NCT03865602). The study intervention emphasized preference-sensitive care for patients but did not specifically require documentation of care goals in the electronic health record (EHR).
Data Collection
Clinical and outcomes data were collected from the EHR and enterprise data warehouse. We included data collected as part of routine care at IMPACTS trial enrollment (ie, age at admission, gender, race, marital status, coexisting conditions) and during index hospitalization (ie, organ failures, hospital length of stay, discharge disposition). The Charlson Comorbidity Index score was calculated from diagnosis codes captured during both inpatient and outpatient healthcare encounters in the 12 months prior to trial enrollment. The Centers for Disease Control and Prevention Adult Sepsis Event definitions9 were applied to measure organ failures.
Two palliative care physicians, three internal medicine physicians, and one critical care clinician retrospectively reviewed the EHR of study patients to: (1) identify whether patient care goals were documented in a standardized care alignment tool at discharge or in the subsequent 90 days; (2) categorize each patient’s care goals as focused on longevity, function, or comfort6 using either standardized documentation or unstructured information from the EHR; and (3) determine whether care goals changed over the first 90 days after discharge. Reviewers also classified care received over the 90-day postdischarge period as focused on longevity, function, or comfort. A random sample of 75 cases was selected for double review by a palliative care reviewer to assess interrater agreement in these assessments. Reviewers indicated whether the goal changed and, if so, what the new goal was. The data collection form is provided in the Appendix. The study was approved by the Atrium Health Institutional Review Board.
Outcomes
The primary outcome was the proportion of cases with care goals documented in the standardized care alignment tool, an EHR-embedded tool prompting questions about goals for future health states, including choices among longevity-, function-, and comfort-focused goals. A secondary outcome was the proportion of cases for which a goal could be determined using all information available in the EHR, such as family meeting notes, discharge summaries, and inpatient or outpatient visit notes. We also measured the proportion of patients who received goal-concordant care, defined as agreement between reviewers’ categorizations of patients’ goals and the primary focus of the care delivered, using a well-defined approach.6 In this approach, reviewers first categorized the care delivered during the 90 days after hospital discharge as focused on longevity, function, or comfort using clinical documentation in each patient’s medical record. To enhance transparency of this decision process, reviewers indicated which specific treatments (eg, new medications, hospital admission, hospice enrollment) supported their categorization. Reviewers then separately categorized the patient’s primary goal over the same period. Reviewer training emphasized that classifications of goals and care delivered should be independent. Patients were considered to have received goal-concordant care if the category of care delivered matched the category of the primary care goal. For patients with changing goals, care delivered was compared with the most recent documented goal.
Analyses
We characterized distributions of care goals and care delivered and reported rates of goal-concordant care overall and by care goals. We calculated weighted kappa statistics to assess interrater reliability. We conducted a multivariable logistic regression analysis in the full cohort to evaluate the association of standardized care goal documentation in the EHR with the dependent outcome of goal-concordant care, adjusting for other risk factors (ie, gender, race, marital status, coexisting chronic conditions, organ failures, and hospital length of stay).
RESULTS
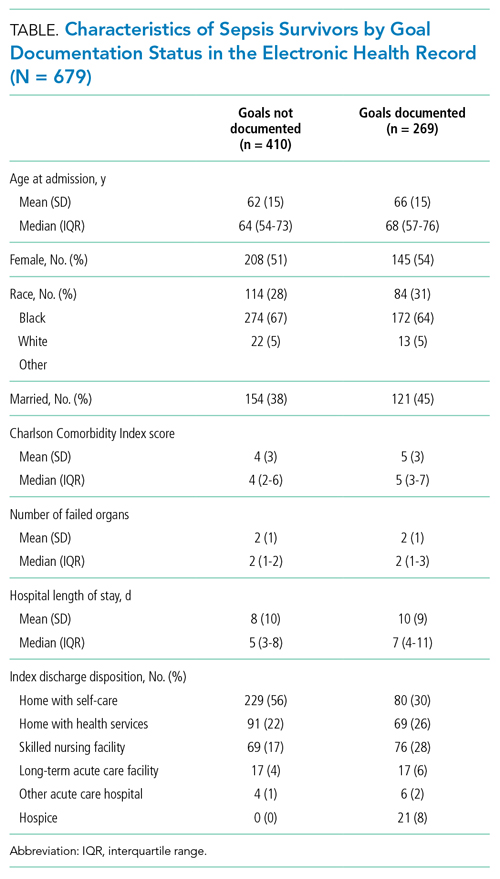
Characterization of Sepsis Survivors’ Goals
The Figure shows patterns of goal documentation and goal-concordant care in the study cohort. Care goals for sepsis survivors were documented in the standardized EHR care alignment tool at discharge for 130 (19%) patients. When reviewers used all information available in the EHR to categorize goals (73% interrater agreement; interrater reliability by weighted κ, 0.71; 95% CI, 0.58-0.83), reviewers were able to categorize patients’ goals in 269 (40%) cases. Among those categorized, goals were classified as prioritizing longevity in 95 (35%), function in 141 (52%), and comfort in 33 (12%) cases.
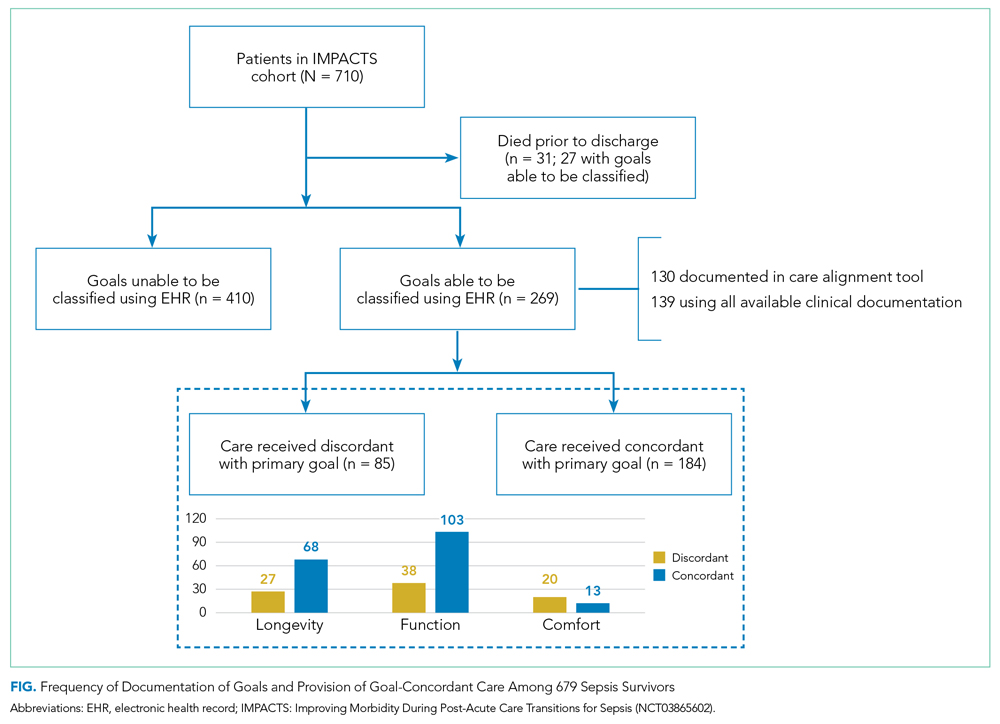
Goals changed over the 90-day observation period for 41 (6%) patients. Of patients whose goals changed, 15 (37%) initially had a goal focused on longevity, 24 (59%) had a goal focused on function, and 2 (5%) had a goal focused on comfort. Of goals that changed, the most frequent new goal was comfort, which was documented in 33 (80%) patients.
Characterization of Goal-Concordant Care
Interrater reliability was moderate for reviewer-based determination of care delivered (73% interrater agreement; weighted κ, 0.60; 95% CI, 0.43-0.78). Reviewers categorized care delivered as focused on longevity in 374 (55%), function in 290 (43%), and comfort in 13 (2%) patients, with <1% unable to be determined. Care elements most frequently cited for longevity-focused classification included intensive care unit (ICU) stay (39%) and new medications for nonsymptom benefit (29%). Care elements most frequently cited for function-focused classification included new medications for nonsymptom benefit (50%) and new medication for symptom benefit (41%). Care elements most frequently cited for comfort-focused classification included hospice enrollment (50%) and new medications for symptom benefit (48%). The rate of goal-concordant care was 68% among those with care goals determined and 27% when cases with unknown goals were classified as not concordant. Concordance was highest among those with longevity-focused (72%) and function-focused (73%) care goals compared with comfort-focused (39%) care goals (P < .01). Adjusting for other potential risk factors, completion of the standardized EHR care alignment tool was associated with higher odds of receiving goal-concordant care (OR, 3.6; 95% CI, 2.4-5.5).
DISCUSSION
Our study identified deficits in the current delivery of goal-concordant care in the first 90 days after sepsis hospitalization. First, goals were only documented in the standardized EHR care alignment tool in one-fifth of cases. Otherwise, information about goals, values, and treatment preferences of sepsis patients was documented idiosyncratically in progress notes, which may not be apparent to clinicians involved in patients’ future care. Lack of clinician attention to documenting the goals of sepsis patients post discharge may reflect suboptimal awareness of the lasting health consequences of sepsis, including persistently elevated risk of mortality up to 2 years following the index hospitalization.2-5 Second, even when goals could be classified by reviewers, the focus of care delivered did not match patients’ goals in nearly one-third of cases.
Our findings inspire several considerations for postsepsis care during hospitalization or in the peridischarge period. First, efforts should focus on increasing assessment and documentation of sepsis survivors’ goals—this might begin with enhanced education about the lasting health consequences after sepsis and communication skills training. Importantly, sepsis survivors’ goals were relatively stable over 90 days after discharge, suggesting that hospitalization for sepsis represents an important opportunity to assess and document patients’ goals. Improving documentation of care goals explicitly in a standardized EHR tool may be an important target for quality-improvement initiatives, as this practice was associated with higher odds of receiving goal-concordant care in our cohort. Second, our findings that one-third of patients received care that was not consistent with their goals is worrisome. Concordance was lowest among comfort-focused care goals, suggesting that some of the high rates of healthcare utilization after sepsis may be unwanted.10-12 For example, ICU stay and new medication for nonsymptom benefit were commonly cited as indications of longevity-focused care among patients with comfort-focused goals. Thus, improving the alignment between sepsis survivors’ goals and subsequent care received is an important target from both a patient-centered and value perspective. Consistent with the recommendations of the i-HOPE study,1 future interventions designed to improve posthospitalization care of sepsis patients should aim to capture goal-concordant care as a patient-centered outcome, if possible.
Our examination of goals and goal-concordant care after sepsis hospitalization advances the goal of enhancing understanding of survivorship in this population.4 Strengths of this study include the large, real-world sample and use of expert palliative care physicians conducting granular EHR review to assess goal-concordant care. Our utilization of this methodology to evaluate goal-concordant care provides information to refine efforts toward developing reliable measures of this important outcome—for example, interrater reliability was similar among reviewers in our study compared with studies assessing goal-concordant care using similar methodology.13
Limitations include potential generalizability challenges for goal and goal-concordant care assessments in other health systems with different EHR platforms or local documentation practices, although deficits in EHR documentation of care goals have been reported in other settings.14,15 We double-reviewed a sample of cases to evaluate interrater reliability, but double-review of all cases with a discussion and adjudication approach may have increased the number of goals that could ultimately be classified. However, this might overestimate the number of goals that are identifiable in real-world practice by a treating clinician. Finally, reviewers may have been challenged to select one goal when two or more competing goals existed. Future refinements of goal-concordant care measurement will need to define methods for handling tradeoffs and prioritization associated with competing goals.
CONCLUSION
The hospitalization and peridischarge periods represent an important opportunity to address deficits in the documentation of goals and provision of goal-concordant care for sepsis survivors. Doing so may improve patient-centered care and reduce the high rates of healthcare utilization after sepsis.
1. Harrison JD, Archuleta M, Avitia E, et al. Developing a patient- and family-centered research agenda for hospital medicine: the Improving Hospital Outcomes through Patient Engagement (i-HOPE) study. J Hosp Med. 2020;15(6):331-337. https://doi.org/10.12788/jhm.3386
2. Courtright KR, Jordan L, Murtaugh CM, et al. Risk factors for long-term mortality and patterns of end-of-life care among Medicare sepsis survivors discharged to home health care. JAMA Netw Open. 2020 ;3(2):e200038. https://doi.org/10.1001/jamanetworkopen.2020.0038
3. Prescott HC, Angus DC. Enhancing recovery from sepsis: a review. JAMA. 2018;319(1):62-75. https://doi.org/10.1001/jama.2017.17687
4. Prescott HC, Iwashyna TJ, Blackwood B, et al. Understanding and enhancing sepsis survivorship. Priorities for research and practice. Am J Respir Crit Care Med. 2019;200(8):972-981. https://doi.org/10.1164/rccm.201812-2383CP
5. Prescott HC, Osterholzer JJ, Langa KM, Angus DC, Iwashyna TJ. Late mortality after sepsis: propensity matched cohort study. BMJ. 2016;353:i2375. https://doi.org/10.1136/bmj.i2375
6. Halpern SD. Goal-concordant care - searching for the Holy Grail. N Engl J Med. 2019;381(17):1603-1606. https://doi.org/10.1056/NEJMp1908153
7. Ernecoff NC, Wessell KL, Bennett AV, Hanson LC. Measuring goal-concordant care in palliative care research. J Pain Symptom Manage. 2021;62(3):e305-e314. https://doi.org/10.1016/j.jpainsymman.2021.02.030
8. Kowalkowski M, Chou SH, McWilliams A, et al. Structured, proactive care coordination versus usual care for Improving Morbidity during Post-Acute Care Transitions for Sepsis (IMPACTS): a pragmatic, randomized controlled trial. Trials. 2019;20(1):660. https://doi.org/10.1186/s13063-019-3792-7
9. Centers for Disease Control and Prevention. Hospital Toolkit for Adult Sepsis Surveillance. March 2018. Accessed September 20, 2021. https://www.cdc.gov/sepsis/pdfs/Sepsis-Surveillance-Toolkit-Mar-2018_508.pdf
10. Liu V, Lei X, Prescott HC, Kipnis P, Iwashyna TJ, Escobar GJ. Hospital readmission and healthcare utilization following sepsis in community settings. J Hosp Med. 2014;9(8):502-507. https://doi.org/10.1002/jhm.2197
11. DeMerle KM, Vincent BM, Iwashyna TJ, Prescott HC. Increased healthcare facility use in veterans surviving sepsis hospitalization. J Crit Care. 2017;42:59-64. https://doi.org/10.1016/j.jcrc.2017.06.026
12. Shankar-Hari M, Saha R, Wilson J, et al. Rate and risk factors for rehospitalisation in sepsis survivors: systematic review and meta-analysis. Intensive Care Med. 2020;46(4):619-636. https://doi.org/10.1007/s00134-019-05908-3
13. Turnbull AE, Sahetya SK, Colantuoni E, Kweku J, Nikooie R, Curtis JR. Inter-rater agreement of intensivists evaluating the goal concordance of preference-sensitive ICU interventions. J Pain Symptom Manage. 2018;56(3):406-413.e3. https://doi.org/10.1016/j.jpainsymman.2018.06.003
14. Wilson CJ, Newman J, Tapper S, et al. Multiple locations of advance care planning documentation in an electronic health record: are they easy to find? J Palliat Med. 2013;16(9):1089-1094. https://doi.org/10.1089/jpm.2012.0472
15. Buck K, Detering KM, Pollard A, et al. Concordance between self-reported completion of advance care planning documentation and availability of documentation in Australian health and residential aged care services. J Pain Symptom Manage. 2019;58(2):264-274. https://.doi.org/10.1016/j.jpainsymman.2019.04.026
Identifying and supporting patients’ care goals through shared decision-making was named the highest priority in the Improving Hospital Outcomes through Patient Engagement (i-HOPE) study.1 Ensuring that seriously ill patients’ goals for their future care are understood and honored is particularly important for patients hospitalized with conditions known to be associated with high near-term mortality or functional disability, such as sepsis. It is increasingly recognized that a hospital admission for sepsis is associated with poor outcomes, including high rates of readmission and postdischarge mortality,2-5 yet little is known about the assessment, status, and stability of patient care goals after discharge for sepsis. Using a cohort of high-risk sepsis survivors enrolled in a clinical trial, we aimed to determine how frequently care goals were documented, describe patterns in care goals, and evaluate how frequently care goals changed over 90 days after sepsis discharge. We also used expert reviewers to assess care delivered in the 90 days after hospitalization and determine the proportion of patients who received goal-concordant care.6,7
METHODS
Design, Setting, Participants
We conducted a secondary analysis using data from the Improving Morbidity During Post-Acute Care Transitions for Sepsis (IMPACTS) study,8 a pragmatic randomized trial evaluating the effectiveness of a multicomponent transition program to reduce mortality and rehospitalization after sepsis among patients enrolled from three hospitals between January 2019 and March 2020 (NCT03865602). The study intervention emphasized preference-sensitive care for patients but did not specifically require documentation of care goals in the electronic health record (EHR).
Data Collection
Clinical and outcomes data were collected from the EHR and enterprise data warehouse. We included data collected as part of routine care at IMPACTS trial enrollment (ie, age at admission, gender, race, marital status, coexisting conditions) and during index hospitalization (ie, organ failures, hospital length of stay, discharge disposition). The Charlson Comorbidity Index score was calculated from diagnosis codes captured during both inpatient and outpatient healthcare encounters in the 12 months prior to trial enrollment. The Centers for Disease Control and Prevention Adult Sepsis Event definitions9 were applied to measure organ failures.
Two palliative care physicians, three internal medicine physicians, and one critical care clinician retrospectively reviewed the EHR of study patients to: (1) identify whether patient care goals were documented in a standardized care alignment tool at discharge or in the subsequent 90 days; (2) categorize each patient’s care goals as focused on longevity, function, or comfort6 using either standardized documentation or unstructured information from the EHR; and (3) determine whether care goals changed over the first 90 days after discharge. Reviewers also classified care received over the 90-day postdischarge period as focused on longevity, function, or comfort. A random sample of 75 cases was selected for double review by a palliative care reviewer to assess interrater agreement in these assessments. Reviewers indicated whether the goal changed and, if so, what the new goal was. The data collection form is provided in the Appendix. The study was approved by the Atrium Health Institutional Review Board.
Outcomes
The primary outcome was the proportion of cases with care goals documented in the standardized care alignment tool, an EHR-embedded tool prompting questions about goals for future health states, including choices among longevity-, function-, and comfort-focused goals. A secondary outcome was the proportion of cases for which a goal could be determined using all information available in the EHR, such as family meeting notes, discharge summaries, and inpatient or outpatient visit notes. We also measured the proportion of patients who received goal-concordant care, defined as agreement between reviewers’ categorizations of patients’ goals and the primary focus of the care delivered, using a well-defined approach.6 In this approach, reviewers first categorized the care delivered during the 90 days after hospital discharge as focused on longevity, function, or comfort using clinical documentation in each patient’s medical record. To enhance transparency of this decision process, reviewers indicated which specific treatments (eg, new medications, hospital admission, hospice enrollment) supported their categorization. Reviewers then separately categorized the patient’s primary goal over the same period. Reviewer training emphasized that classifications of goals and care delivered should be independent. Patients were considered to have received goal-concordant care if the category of care delivered matched the category of the primary care goal. For patients with changing goals, care delivered was compared with the most recent documented goal.
Analyses
We characterized distributions of care goals and care delivered and reported rates of goal-concordant care overall and by care goals. We calculated weighted kappa statistics to assess interrater reliability. We conducted a multivariable logistic regression analysis in the full cohort to evaluate the association of standardized care goal documentation in the EHR with the dependent outcome of goal-concordant care, adjusting for other risk factors (ie, gender, race, marital status, coexisting chronic conditions, organ failures, and hospital length of stay).
RESULTS

Characterization of Sepsis Survivors’ Goals
The Figure shows patterns of goal documentation and goal-concordant care in the study cohort. Care goals for sepsis survivors were documented in the standardized EHR care alignment tool at discharge for 130 (19%) patients. When reviewers used all information available in the EHR to categorize goals (73% interrater agreement; interrater reliability by weighted κ, 0.71; 95% CI, 0.58-0.83), reviewers were able to categorize patients’ goals in 269 (40%) cases. Among those categorized, goals were classified as prioritizing longevity in 95 (35%), function in 141 (52%), and comfort in 33 (12%) cases.

Goals changed over the 90-day observation period for 41 (6%) patients. Of patients whose goals changed, 15 (37%) initially had a goal focused on longevity, 24 (59%) had a goal focused on function, and 2 (5%) had a goal focused on comfort. Of goals that changed, the most frequent new goal was comfort, which was documented in 33 (80%) patients.
Characterization of Goal-Concordant Care
Interrater reliability was moderate for reviewer-based determination of care delivered (73% interrater agreement; weighted κ, 0.60; 95% CI, 0.43-0.78). Reviewers categorized care delivered as focused on longevity in 374 (55%), function in 290 (43%), and comfort in 13 (2%) patients, with <1% unable to be determined. Care elements most frequently cited for longevity-focused classification included intensive care unit (ICU) stay (39%) and new medications for nonsymptom benefit (29%). Care elements most frequently cited for function-focused classification included new medications for nonsymptom benefit (50%) and new medication for symptom benefit (41%). Care elements most frequently cited for comfort-focused classification included hospice enrollment (50%) and new medications for symptom benefit (48%). The rate of goal-concordant care was 68% among those with care goals determined and 27% when cases with unknown goals were classified as not concordant. Concordance was highest among those with longevity-focused (72%) and function-focused (73%) care goals compared with comfort-focused (39%) care goals (P < .01). Adjusting for other potential risk factors, completion of the standardized EHR care alignment tool was associated with higher odds of receiving goal-concordant care (OR, 3.6; 95% CI, 2.4-5.5).
DISCUSSION
Our study identified deficits in the current delivery of goal-concordant care in the first 90 days after sepsis hospitalization. First, goals were only documented in the standardized EHR care alignment tool in one-fifth of cases. Otherwise, information about goals, values, and treatment preferences of sepsis patients was documented idiosyncratically in progress notes, which may not be apparent to clinicians involved in patients’ future care. Lack of clinician attention to documenting the goals of sepsis patients post discharge may reflect suboptimal awareness of the lasting health consequences of sepsis, including persistently elevated risk of mortality up to 2 years following the index hospitalization.2-5 Second, even when goals could be classified by reviewers, the focus of care delivered did not match patients’ goals in nearly one-third of cases.
Our findings inspire several considerations for postsepsis care during hospitalization or in the peridischarge period. First, efforts should focus on increasing assessment and documentation of sepsis survivors’ goals—this might begin with enhanced education about the lasting health consequences after sepsis and communication skills training. Importantly, sepsis survivors’ goals were relatively stable over 90 days after discharge, suggesting that hospitalization for sepsis represents an important opportunity to assess and document patients’ goals. Improving documentation of care goals explicitly in a standardized EHR tool may be an important target for quality-improvement initiatives, as this practice was associated with higher odds of receiving goal-concordant care in our cohort. Second, our findings that one-third of patients received care that was not consistent with their goals is worrisome. Concordance was lowest among comfort-focused care goals, suggesting that some of the high rates of healthcare utilization after sepsis may be unwanted.10-12 For example, ICU stay and new medication for nonsymptom benefit were commonly cited as indications of longevity-focused care among patients with comfort-focused goals. Thus, improving the alignment between sepsis survivors’ goals and subsequent care received is an important target from both a patient-centered and value perspective. Consistent with the recommendations of the i-HOPE study,1 future interventions designed to improve posthospitalization care of sepsis patients should aim to capture goal-concordant care as a patient-centered outcome, if possible.
Our examination of goals and goal-concordant care after sepsis hospitalization advances the goal of enhancing understanding of survivorship in this population.4 Strengths of this study include the large, real-world sample and use of expert palliative care physicians conducting granular EHR review to assess goal-concordant care. Our utilization of this methodology to evaluate goal-concordant care provides information to refine efforts toward developing reliable measures of this important outcome—for example, interrater reliability was similar among reviewers in our study compared with studies assessing goal-concordant care using similar methodology.13
Limitations include potential generalizability challenges for goal and goal-concordant care assessments in other health systems with different EHR platforms or local documentation practices, although deficits in EHR documentation of care goals have been reported in other settings.14,15 We double-reviewed a sample of cases to evaluate interrater reliability, but double-review of all cases with a discussion and adjudication approach may have increased the number of goals that could ultimately be classified. However, this might overestimate the number of goals that are identifiable in real-world practice by a treating clinician. Finally, reviewers may have been challenged to select one goal when two or more competing goals existed. Future refinements of goal-concordant care measurement will need to define methods for handling tradeoffs and prioritization associated with competing goals.
CONCLUSION
The hospitalization and peridischarge periods represent an important opportunity to address deficits in the documentation of goals and provision of goal-concordant care for sepsis survivors. Doing so may improve patient-centered care and reduce the high rates of healthcare utilization after sepsis.
Identifying and supporting patients’ care goals through shared decision-making was named the highest priority in the Improving Hospital Outcomes through Patient Engagement (i-HOPE) study.1 Ensuring that seriously ill patients’ goals for their future care are understood and honored is particularly important for patients hospitalized with conditions known to be associated with high near-term mortality or functional disability, such as sepsis. It is increasingly recognized that a hospital admission for sepsis is associated with poor outcomes, including high rates of readmission and postdischarge mortality,2-5 yet little is known about the assessment, status, and stability of patient care goals after discharge for sepsis. Using a cohort of high-risk sepsis survivors enrolled in a clinical trial, we aimed to determine how frequently care goals were documented, describe patterns in care goals, and evaluate how frequently care goals changed over 90 days after sepsis discharge. We also used expert reviewers to assess care delivered in the 90 days after hospitalization and determine the proportion of patients who received goal-concordant care.6,7
METHODS
Design, Setting, Participants
We conducted a secondary analysis using data from the Improving Morbidity During Post-Acute Care Transitions for Sepsis (IMPACTS) study,8 a pragmatic randomized trial evaluating the effectiveness of a multicomponent transition program to reduce mortality and rehospitalization after sepsis among patients enrolled from three hospitals between January 2019 and March 2020 (NCT03865602). The study intervention emphasized preference-sensitive care for patients but did not specifically require documentation of care goals in the electronic health record (EHR).
Data Collection
Clinical and outcomes data were collected from the EHR and enterprise data warehouse. We included data collected as part of routine care at IMPACTS trial enrollment (ie, age at admission, gender, race, marital status, coexisting conditions) and during index hospitalization (ie, organ failures, hospital length of stay, discharge disposition). The Charlson Comorbidity Index score was calculated from diagnosis codes captured during both inpatient and outpatient healthcare encounters in the 12 months prior to trial enrollment. The Centers for Disease Control and Prevention Adult Sepsis Event definitions9 were applied to measure organ failures.
Two palliative care physicians, three internal medicine physicians, and one critical care clinician retrospectively reviewed the EHR of study patients to: (1) identify whether patient care goals were documented in a standardized care alignment tool at discharge or in the subsequent 90 days; (2) categorize each patient’s care goals as focused on longevity, function, or comfort6 using either standardized documentation or unstructured information from the EHR; and (3) determine whether care goals changed over the first 90 days after discharge. Reviewers also classified care received over the 90-day postdischarge period as focused on longevity, function, or comfort. A random sample of 75 cases was selected for double review by a palliative care reviewer to assess interrater agreement in these assessments. Reviewers indicated whether the goal changed and, if so, what the new goal was. The data collection form is provided in the Appendix. The study was approved by the Atrium Health Institutional Review Board.
Outcomes
The primary outcome was the proportion of cases with care goals documented in the standardized care alignment tool, an EHR-embedded tool prompting questions about goals for future health states, including choices among longevity-, function-, and comfort-focused goals. A secondary outcome was the proportion of cases for which a goal could be determined using all information available in the EHR, such as family meeting notes, discharge summaries, and inpatient or outpatient visit notes. We also measured the proportion of patients who received goal-concordant care, defined as agreement between reviewers’ categorizations of patients’ goals and the primary focus of the care delivered, using a well-defined approach.6 In this approach, reviewers first categorized the care delivered during the 90 days after hospital discharge as focused on longevity, function, or comfort using clinical documentation in each patient’s medical record. To enhance transparency of this decision process, reviewers indicated which specific treatments (eg, new medications, hospital admission, hospice enrollment) supported their categorization. Reviewers then separately categorized the patient’s primary goal over the same period. Reviewer training emphasized that classifications of goals and care delivered should be independent. Patients were considered to have received goal-concordant care if the category of care delivered matched the category of the primary care goal. For patients with changing goals, care delivered was compared with the most recent documented goal.
Analyses
We characterized distributions of care goals and care delivered and reported rates of goal-concordant care overall and by care goals. We calculated weighted kappa statistics to assess interrater reliability. We conducted a multivariable logistic regression analysis in the full cohort to evaluate the association of standardized care goal documentation in the EHR with the dependent outcome of goal-concordant care, adjusting for other risk factors (ie, gender, race, marital status, coexisting chronic conditions, organ failures, and hospital length of stay).
RESULTS

Characterization of Sepsis Survivors’ Goals
The Figure shows patterns of goal documentation and goal-concordant care in the study cohort. Care goals for sepsis survivors were documented in the standardized EHR care alignment tool at discharge for 130 (19%) patients. When reviewers used all information available in the EHR to categorize goals (73% interrater agreement; interrater reliability by weighted κ, 0.71; 95% CI, 0.58-0.83), reviewers were able to categorize patients’ goals in 269 (40%) cases. Among those categorized, goals were classified as prioritizing longevity in 95 (35%), function in 141 (52%), and comfort in 33 (12%) cases.

Goals changed over the 90-day observation period for 41 (6%) patients. Of patients whose goals changed, 15 (37%) initially had a goal focused on longevity, 24 (59%) had a goal focused on function, and 2 (5%) had a goal focused on comfort. Of goals that changed, the most frequent new goal was comfort, which was documented in 33 (80%) patients.
Characterization of Goal-Concordant Care
Interrater reliability was moderate for reviewer-based determination of care delivered (73% interrater agreement; weighted κ, 0.60; 95% CI, 0.43-0.78). Reviewers categorized care delivered as focused on longevity in 374 (55%), function in 290 (43%), and comfort in 13 (2%) patients, with <1% unable to be determined. Care elements most frequently cited for longevity-focused classification included intensive care unit (ICU) stay (39%) and new medications for nonsymptom benefit (29%). Care elements most frequently cited for function-focused classification included new medications for nonsymptom benefit (50%) and new medication for symptom benefit (41%). Care elements most frequently cited for comfort-focused classification included hospice enrollment (50%) and new medications for symptom benefit (48%). The rate of goal-concordant care was 68% among those with care goals determined and 27% when cases with unknown goals were classified as not concordant. Concordance was highest among those with longevity-focused (72%) and function-focused (73%) care goals compared with comfort-focused (39%) care goals (P < .01). Adjusting for other potential risk factors, completion of the standardized EHR care alignment tool was associated with higher odds of receiving goal-concordant care (OR, 3.6; 95% CI, 2.4-5.5).
DISCUSSION
Our study identified deficits in the current delivery of goal-concordant care in the first 90 days after sepsis hospitalization. First, goals were only documented in the standardized EHR care alignment tool in one-fifth of cases. Otherwise, information about goals, values, and treatment preferences of sepsis patients was documented idiosyncratically in progress notes, which may not be apparent to clinicians involved in patients’ future care. Lack of clinician attention to documenting the goals of sepsis patients post discharge may reflect suboptimal awareness of the lasting health consequences of sepsis, including persistently elevated risk of mortality up to 2 years following the index hospitalization.2-5 Second, even when goals could be classified by reviewers, the focus of care delivered did not match patients’ goals in nearly one-third of cases.
Our findings inspire several considerations for postsepsis care during hospitalization or in the peridischarge period. First, efforts should focus on increasing assessment and documentation of sepsis survivors’ goals—this might begin with enhanced education about the lasting health consequences after sepsis and communication skills training. Importantly, sepsis survivors’ goals were relatively stable over 90 days after discharge, suggesting that hospitalization for sepsis represents an important opportunity to assess and document patients’ goals. Improving documentation of care goals explicitly in a standardized EHR tool may be an important target for quality-improvement initiatives, as this practice was associated with higher odds of receiving goal-concordant care in our cohort. Second, our findings that one-third of patients received care that was not consistent with their goals is worrisome. Concordance was lowest among comfort-focused care goals, suggesting that some of the high rates of healthcare utilization after sepsis may be unwanted.10-12 For example, ICU stay and new medication for nonsymptom benefit were commonly cited as indications of longevity-focused care among patients with comfort-focused goals. Thus, improving the alignment between sepsis survivors’ goals and subsequent care received is an important target from both a patient-centered and value perspective. Consistent with the recommendations of the i-HOPE study,1 future interventions designed to improve posthospitalization care of sepsis patients should aim to capture goal-concordant care as a patient-centered outcome, if possible.
Our examination of goals and goal-concordant care after sepsis hospitalization advances the goal of enhancing understanding of survivorship in this population.4 Strengths of this study include the large, real-world sample and use of expert palliative care physicians conducting granular EHR review to assess goal-concordant care. Our utilization of this methodology to evaluate goal-concordant care provides information to refine efforts toward developing reliable measures of this important outcome—for example, interrater reliability was similar among reviewers in our study compared with studies assessing goal-concordant care using similar methodology.13
Limitations include potential generalizability challenges for goal and goal-concordant care assessments in other health systems with different EHR platforms or local documentation practices, although deficits in EHR documentation of care goals have been reported in other settings.14,15 We double-reviewed a sample of cases to evaluate interrater reliability, but double-review of all cases with a discussion and adjudication approach may have increased the number of goals that could ultimately be classified. However, this might overestimate the number of goals that are identifiable in real-world practice by a treating clinician. Finally, reviewers may have been challenged to select one goal when two or more competing goals existed. Future refinements of goal-concordant care measurement will need to define methods for handling tradeoffs and prioritization associated with competing goals.
CONCLUSION
The hospitalization and peridischarge periods represent an important opportunity to address deficits in the documentation of goals and provision of goal-concordant care for sepsis survivors. Doing so may improve patient-centered care and reduce the high rates of healthcare utilization after sepsis.
1. Harrison JD, Archuleta M, Avitia E, et al. Developing a patient- and family-centered research agenda for hospital medicine: the Improving Hospital Outcomes through Patient Engagement (i-HOPE) study. J Hosp Med. 2020;15(6):331-337. https://doi.org/10.12788/jhm.3386
2. Courtright KR, Jordan L, Murtaugh CM, et al. Risk factors for long-term mortality and patterns of end-of-life care among Medicare sepsis survivors discharged to home health care. JAMA Netw Open. 2020 ;3(2):e200038. https://doi.org/10.1001/jamanetworkopen.2020.0038
3. Prescott HC, Angus DC. Enhancing recovery from sepsis: a review. JAMA. 2018;319(1):62-75. https://doi.org/10.1001/jama.2017.17687
4. Prescott HC, Iwashyna TJ, Blackwood B, et al. Understanding and enhancing sepsis survivorship. Priorities for research and practice. Am J Respir Crit Care Med. 2019;200(8):972-981. https://doi.org/10.1164/rccm.201812-2383CP
5. Prescott HC, Osterholzer JJ, Langa KM, Angus DC, Iwashyna TJ. Late mortality after sepsis: propensity matched cohort study. BMJ. 2016;353:i2375. https://doi.org/10.1136/bmj.i2375
6. Halpern SD. Goal-concordant care - searching for the Holy Grail. N Engl J Med. 2019;381(17):1603-1606. https://doi.org/10.1056/NEJMp1908153
7. Ernecoff NC, Wessell KL, Bennett AV, Hanson LC. Measuring goal-concordant care in palliative care research. J Pain Symptom Manage. 2021;62(3):e305-e314. https://doi.org/10.1016/j.jpainsymman.2021.02.030
8. Kowalkowski M, Chou SH, McWilliams A, et al. Structured, proactive care coordination versus usual care for Improving Morbidity during Post-Acute Care Transitions for Sepsis (IMPACTS): a pragmatic, randomized controlled trial. Trials. 2019;20(1):660. https://doi.org/10.1186/s13063-019-3792-7
9. Centers for Disease Control and Prevention. Hospital Toolkit for Adult Sepsis Surveillance. March 2018. Accessed September 20, 2021. https://www.cdc.gov/sepsis/pdfs/Sepsis-Surveillance-Toolkit-Mar-2018_508.pdf
10. Liu V, Lei X, Prescott HC, Kipnis P, Iwashyna TJ, Escobar GJ. Hospital readmission and healthcare utilization following sepsis in community settings. J Hosp Med. 2014;9(8):502-507. https://doi.org/10.1002/jhm.2197
11. DeMerle KM, Vincent BM, Iwashyna TJ, Prescott HC. Increased healthcare facility use in veterans surviving sepsis hospitalization. J Crit Care. 2017;42:59-64. https://doi.org/10.1016/j.jcrc.2017.06.026
12. Shankar-Hari M, Saha R, Wilson J, et al. Rate and risk factors for rehospitalisation in sepsis survivors: systematic review and meta-analysis. Intensive Care Med. 2020;46(4):619-636. https://doi.org/10.1007/s00134-019-05908-3
13. Turnbull AE, Sahetya SK, Colantuoni E, Kweku J, Nikooie R, Curtis JR. Inter-rater agreement of intensivists evaluating the goal concordance of preference-sensitive ICU interventions. J Pain Symptom Manage. 2018;56(3):406-413.e3. https://doi.org/10.1016/j.jpainsymman.2018.06.003
14. Wilson CJ, Newman J, Tapper S, et al. Multiple locations of advance care planning documentation in an electronic health record: are they easy to find? J Palliat Med. 2013;16(9):1089-1094. https://doi.org/10.1089/jpm.2012.0472
15. Buck K, Detering KM, Pollard A, et al. Concordance between self-reported completion of advance care planning documentation and availability of documentation in Australian health and residential aged care services. J Pain Symptom Manage. 2019;58(2):264-274. https://.doi.org/10.1016/j.jpainsymman.2019.04.026
1. Harrison JD, Archuleta M, Avitia E, et al. Developing a patient- and family-centered research agenda for hospital medicine: the Improving Hospital Outcomes through Patient Engagement (i-HOPE) study. J Hosp Med. 2020;15(6):331-337. https://doi.org/10.12788/jhm.3386
2. Courtright KR, Jordan L, Murtaugh CM, et al. Risk factors for long-term mortality and patterns of end-of-life care among Medicare sepsis survivors discharged to home health care. JAMA Netw Open. 2020 ;3(2):e200038. https://doi.org/10.1001/jamanetworkopen.2020.0038
3. Prescott HC, Angus DC. Enhancing recovery from sepsis: a review. JAMA. 2018;319(1):62-75. https://doi.org/10.1001/jama.2017.17687
4. Prescott HC, Iwashyna TJ, Blackwood B, et al. Understanding and enhancing sepsis survivorship. Priorities for research and practice. Am J Respir Crit Care Med. 2019;200(8):972-981. https://doi.org/10.1164/rccm.201812-2383CP
5. Prescott HC, Osterholzer JJ, Langa KM, Angus DC, Iwashyna TJ. Late mortality after sepsis: propensity matched cohort study. BMJ. 2016;353:i2375. https://doi.org/10.1136/bmj.i2375
6. Halpern SD. Goal-concordant care - searching for the Holy Grail. N Engl J Med. 2019;381(17):1603-1606. https://doi.org/10.1056/NEJMp1908153
7. Ernecoff NC, Wessell KL, Bennett AV, Hanson LC. Measuring goal-concordant care in palliative care research. J Pain Symptom Manage. 2021;62(3):e305-e314. https://doi.org/10.1016/j.jpainsymman.2021.02.030
8. Kowalkowski M, Chou SH, McWilliams A, et al. Structured, proactive care coordination versus usual care for Improving Morbidity during Post-Acute Care Transitions for Sepsis (IMPACTS): a pragmatic, randomized controlled trial. Trials. 2019;20(1):660. https://doi.org/10.1186/s13063-019-3792-7
9. Centers for Disease Control and Prevention. Hospital Toolkit for Adult Sepsis Surveillance. March 2018. Accessed September 20, 2021. https://www.cdc.gov/sepsis/pdfs/Sepsis-Surveillance-Toolkit-Mar-2018_508.pdf
10. Liu V, Lei X, Prescott HC, Kipnis P, Iwashyna TJ, Escobar GJ. Hospital readmission and healthcare utilization following sepsis in community settings. J Hosp Med. 2014;9(8):502-507. https://doi.org/10.1002/jhm.2197
11. DeMerle KM, Vincent BM, Iwashyna TJ, Prescott HC. Increased healthcare facility use in veterans surviving sepsis hospitalization. J Crit Care. 2017;42:59-64. https://doi.org/10.1016/j.jcrc.2017.06.026
12. Shankar-Hari M, Saha R, Wilson J, et al. Rate and risk factors for rehospitalisation in sepsis survivors: systematic review and meta-analysis. Intensive Care Med. 2020;46(4):619-636. https://doi.org/10.1007/s00134-019-05908-3
13. Turnbull AE, Sahetya SK, Colantuoni E, Kweku J, Nikooie R, Curtis JR. Inter-rater agreement of intensivists evaluating the goal concordance of preference-sensitive ICU interventions. J Pain Symptom Manage. 2018;56(3):406-413.e3. https://doi.org/10.1016/j.jpainsymman.2018.06.003
14. Wilson CJ, Newman J, Tapper S, et al. Multiple locations of advance care planning documentation in an electronic health record: are they easy to find? J Palliat Med. 2013;16(9):1089-1094. https://doi.org/10.1089/jpm.2012.0472
15. Buck K, Detering KM, Pollard A, et al. Concordance between self-reported completion of advance care planning documentation and availability of documentation in Australian health and residential aged care services. J Pain Symptom Manage. 2019;58(2):264-274. https://.doi.org/10.1016/j.jpainsymman.2019.04.026
© 2021 Society of Hospital Medicine
Traditional Medicare Spending on Inpatient Episodes as Hospitalizations Decline
The rate of inpatient admissions among adults aged 65 years and older has decreased by approximately 25% since 2000.1,2 This long-term trend raises important questions about inpatient-related spending in the traditional Medicare program for hospitals and providers who treat beneficiaries after a hospitalization. As traditional Medicare’s most expensive sector (accounting for 21% of all Medicare spending3), reducing hospitalizations is often championed as an opportunity to moderate Medicare spending growth.
Medicare’s ability to achieve significant savings from declining inpatient use may be tempered by a shift toward more expensive hospitalizations. If marginal hospitalizations among healthier beneficiaries are avoided, then the remaining inpatient users may be sicker and have greater spending per hospitalization and greater need for follow-up services. This study examines trends in Medicare spending related to episodes initiated by an inpatient stay because of its importance to overall Medicare spending and the implications for several Medicare value-based payment initiatives. In care models seeking to contain spending at a population level, such as accountable care organizations and managed care plans, reducing inpatient use and associated services may have the largest impact in curbing overall spending growth per beneficiary. Other models focused on spending at an episode level, including bundled payment initiatives, may face challenges if inpatient episodes become more expensive over time.
As Medicare shifts toward value-based payments, hospitalists and other hospital leaders are often involved in redesigning care delivery models for the hospital or accountable care organization (eg, through readmission reduction initiatives, post–acute care coordination, and bundled-care delivery programs). Not all savings strategies rely on providers to change how services are delivered; Medicare can modify payment rates, such as Affordable Care Act provisions that slowed how quickly Medicare payment rates increased.4 For clinicians to navigate the shift toward new payment models, it is important to recognize how each of these elements—declining hospital admissions, spending per inpatient episode, and payment rates—affect spending trends for inpatient services and associated care. Previous articles on overall Medicare inpatient spending have examined inpatient stays alone5 or focused mainly on spending per episode6,7 without quantifying how these elements contributed to overall episode-related Medicare spending per beneficiary. This article addresses this gap by demonstrating how inpatient-related spending trends reflect each component.
This study examined trends in Medicare’s spending on inpatient episodes during the years 2009 to 2017. We described changes in the volume and spending on inpatient-initiated episodes across several dimensions, including beneficiary-level and hospitalization-level factors. We examined whether declines in spending associated with fewer inpatient-initiated episodes have been offset by increased spending per episode and how spending would have differed without changes in Medicare payment rates.
Episode Definition
We constructed an episode measure that captured traditional Medicare spending for 30 days prior to hospital admission, hospitalization duration, and 90 days following hospital discharge (additional details in the Appendix).
Any acute hospitalization triggered a new episode, with one exception: if a beneficiary was discharged and readmitted within 90 days for the same diagnosis-related group (DRG), then the readmission did not trigger a new episode. The spending for that readmission was attributed to the prior hospital stay. In effect, the annual number of episodes is equivalent to the annual number of hospital admissions minus subsequent rehospitalizations for the same DRG. Neither observation stays nor hospitalizations in inpatient rehabilitation, psychiatric, or long-term facilities were considered acute hospital admissions.
We assigned claims from noninpatient sectors to an episode based on whether the claim start date fell within the episode window. All traditional Medicare sectors were measured, including outpatient services, physician claims, post–acute care services, and Medicare Part D prescription drug events.
Our analysis aimed to measure all spending related to inpatient episodes without double-counting spending for overlapping episodes. If episodes overlapped, then spending for overlapping days was weighted to be evenly divided across episodes.
Outcome Measures
The study’s main outcomes summarized episode trends across the entire traditional Medicare population, including beneficiaries without an episode, in annual mean number of episodes per beneficiary and annual mean episode-related spending per beneficiary. The denominator of these measures is person-years, or total number of beneficiary months with Medicare Part A and B coverage divided by 12. The annual mean number of episodes per beneficiary is the total number of episodes initiated in a calendar year divided by person-years. The annual mean episode-related spending per beneficiary is the total amount of spending attributed to episodes divided by person-years. We also measured annual mean spending per episode, or total amount of spending attributed to episodes divided by the total number of episodes.
Medicare annually updates each sector’s payment rates for several factors, including inflation. We constructed an index for each sector to adjust for these annual payment rate changes. We also accounted for sequestration measures in effect since April 2013 that reduced Medicare payments to all sectors by 2%. We report our spending measures twice, with and without adjusting for changes in payment rates. Adjusted numbers reflect payment rates in effect in 2015.
Analysis Approach
We present annual trends on changes in the number of inpatient episodes per beneficiary, mean episode-related spending per beneficiary, and mean spending per episode. To quantify how changes in episode-related spending per beneficiary reflect changes in the number of episodes per beneficiary vs changes in spending per episode, we modified an approach implemented by Rosen and colleagues.8
To better understand which beneficiaries have declining inpatient use, we performed stratified analyses describing changes in the number of episodes per beneficiary between 2009 and 2017, spending per episode, and total episode-related spending per beneficiary. We report these measures for several subpopulations defined by age, sex, race, dual-eligible status, and whether the beneficiary used long-term nursing home services during the episode’s calendar year. Descriptive statistics also detail how these measures changed between 2009 and 2017 for episodes stratified by characteristics of the index hospital stay: planned vs unplanned, medical vs surgical, and any use of intensive care unit (ICU) or coronary care unit services. We also stratify study measures by whether an episode included any use of post–acute care services (skilled nursing facility, home health, or inpatient rehabilitation facility use). Finally, we aggregate the episodes into major diagnostic categories (MDCs) based on the index hospital stay’s DRG to report study outcomes by condition. Because of a shift in coding hospitalizations for pneumonia as sepsis,9,10 we exclude these two diseases from their respective MDCs and analyze them jointly as a unique category.
RESULTS
Changes in Number of Inpatient Episodes and Related Spending
From 2009 to 2017, the number of inpatient episodes per 1000 traditional Medicare beneficiaries declined from 326 to 267 (Table 1), or a relative decline of 18.2% (Figure 1). The total volume of inpatient episodes declined by only 13.4%, from 10.2 million to 8.8 million, reflecting that the size of the traditional Medicare population grew during these years. Over the same years, mean payment-rate–adjusted spending per episode increased 11.4% from $20,891 to $23,273.

When considering overall episode-related spending, the large decline in the volume of episodes outweighed increased spending per episode: the mean amount of episode-related Medicare spending per beneficiary decreased 8.9% from $6810 to $6206 (Table 1), or a net change of $604 (Figure 2). This net change reflects decreased spending due to fewer episodes per beneficiary ($1239 reduction in episode-related spending) offset by increased spending per episode (translating to a $776 increase in episode-related spending per beneficiary).
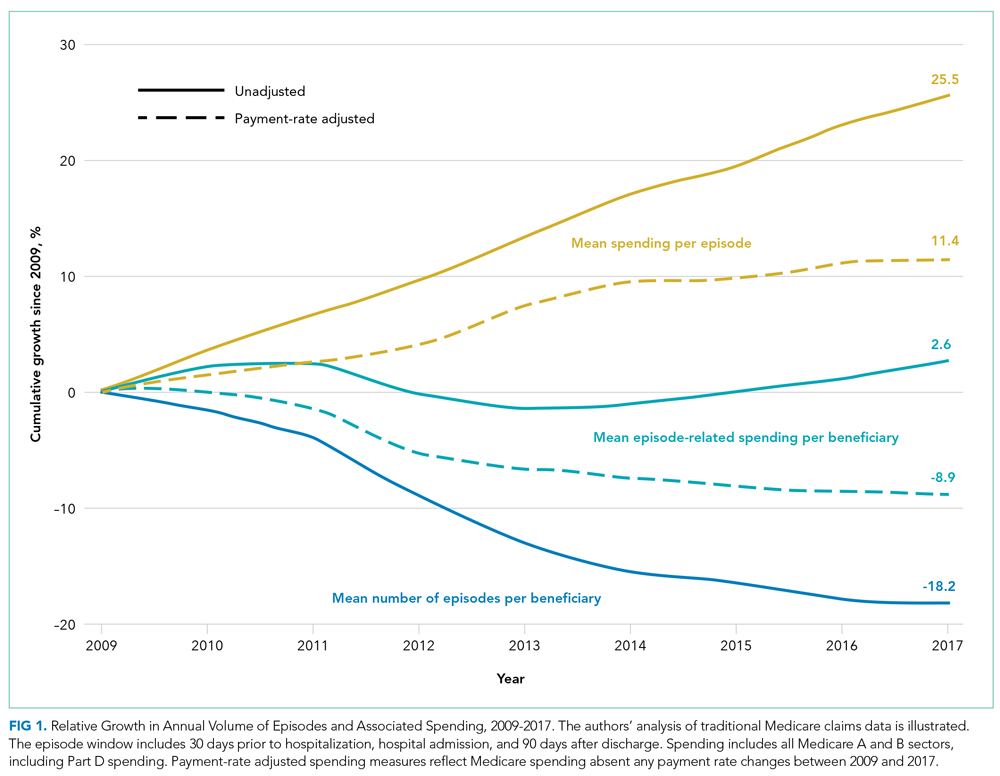
When these estimates are calculated separately for the inpatient sector and all other sectors, the inpatient sector experienced small increases in spending associated with greater spending per episode ($304) compared with noninpatient sectors ($472). Accordingly, the inpatient sector had a larger net decline in episode-related spending per beneficiary ($420) than noninpatient sectors ($184) after taking into account declining episode volume.
As expected, episode-related spending increased more when measures were not adjusted for annual payment rate increases. Without such adjustment, mean spending per episode increased 25.5%, and episode-related spending per beneficiary was nearly flat (2.6% between 2009 and 2017 [Figure 1]). The decline in unadjusted spending associated with fewer episodes ($1138) was offset by the spending increase associated with higher spending per episode ($1592) (Figure 2).
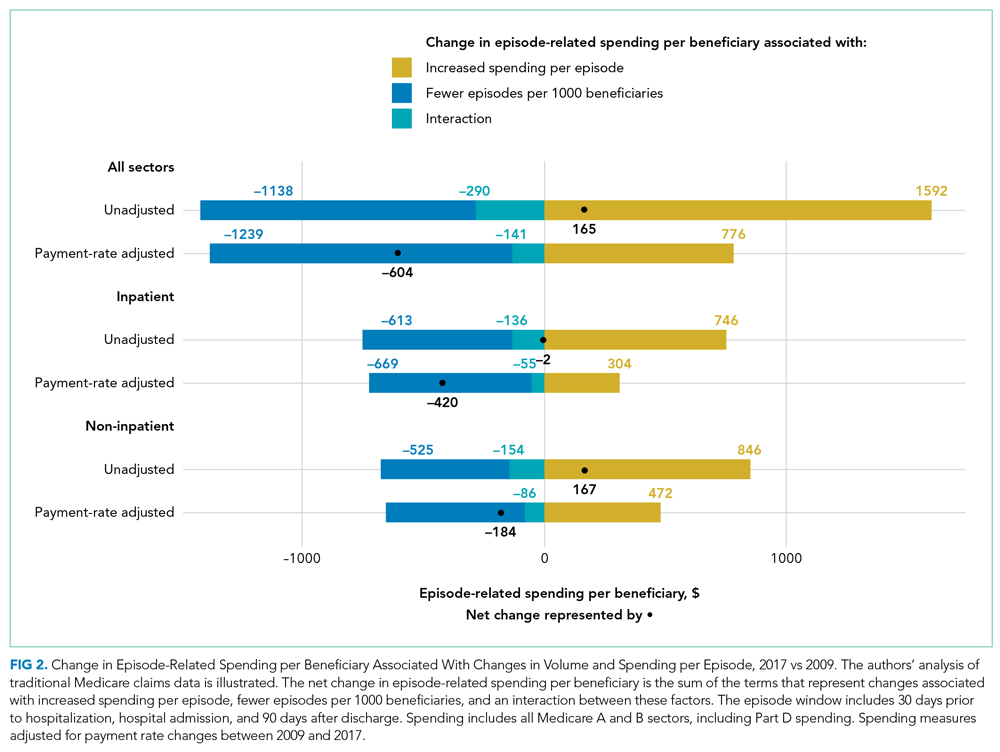
Analyses Stratified by Beneficiary Characteristics
Every population examined had declines in the number of inpatient episodes, even beneficiaries with more frequent inpatient use (Table 2). Among Medicare beneficiaries aged 85 years and older, the mean number of episodes per 1000 beneficiaries declined by 12.7%, from 524 to 457. Populations with less frequent inpatient use often experienced larger relative declines in number of episodes than populations with more frequent inpatient use. For example, the mean number of episodes per 1000 beneficiaries decreased by 17.7% for beneficiaries without nursing home use (306 to 252), as compared with an 8.1% decline for beneficiaries with nursing home use (from 888 to 816). In contrast, populations with less frequent inpatient use had larger relative increases in spending per episode with adjustment for payment rate changes. For example, spending per episode increased by 13.1% for beneficiaries aged 65 to 74 years ($20,904 to $23,644), but only by 8.6% for beneficiaries 85 years and older ($20,384 to $22,138).

Analyses Stratified by Service Use Characteristics
Some types of inpatient episodes had larger declines in the number of episodes, including episodes with planned admissions for the index hospital stay (28.8% decline from 68 to 48 episodes per 1000 beneficiaries) and episodes without post–acute care use (23.9% decline from 169 to 129 episodes per 1000 beneficiaries) (Appendix Table). In contrast, declines in the number of episodes were similar for index hospital admissions that did or did not involve ICU use (17.8% and 18.3% reduction in mean number of episodes per 1000 beneficiaries, respectively) or that included a surgical procedure or not (17.1% versus 18.6%, respectively). Several types of inpatient episodes had larger increases in spending per episode, such as a 15.1% increase for planned admissions and a 13.2% increase for hospitalizations without ICU use.
According to diagnosis information for an episode’s index hospital stay, inpatient episodes related to conditions affecting the circulatory system had the largest decline in mean number of episodes, decreasing by 31.8% from 78 to 53 episodes per 1000 beneficiaries (Appendix Table). Episodes for other diseases had much smaller declines in volume. Admissions for diagnoses of pneumonia or sepsis had notable increases in the volume of episodes, increasing by 20.7% from 25 to 30 admissions per 1000 beneficiaries.
DISCUSSION
Medicare spending per beneficiary on inpatient episodes, including services provided pre- and post hospitalization, declined by 8.9% from 2009 to 2017 after adjusting for payment rate changes. This decline reflects two components. First, the number of episodes per 1000 beneficiaries declined by 18.2%. Although the extent of this decrease varied across populations, every group examined had declines in inpatient use. In particular, hospitalizations for conditions affecting the circulatory system, such as heart attacks and cardiac procedures, decreased. Second, as inpatient volume declined, spending per episode increased by 11.4% to an average of $23,273 in 2017. This increase in spending per episode offset how much overall Medicare spending on episode-related care declined.
Medicare is increasingly challenging hospitals to demonstrate the value of inpatient services and associated treatment, which requires hospital leaders to recognize how their facilities’ spending trends relate to these national patterns. Understanding how much national episode-related spending has decreased over time with declining inpatient volume can help an accountable care organization evaluate whether it is feasible to achieve significant savings by reducing hospitalizations. Bundled payment providers focused on managing spending per episode can benefit from identifying which types of hospitalizations have increased spending per episode, especially for certain diagnoses.
These results also highlight the continued importance of a perennial factor in Medicare spending: payment rates. If Medicare payment rates had not increased over our study period, Medicare spending per inpatient episode would have increased by only 11%. Actual Medicare spending per episode increased by 25%, demonstrating that over half of the relative increase in spending per episode reflected increases in Medicare’s payment rates.
Increased spending per episode, even after adjustment for payment rate changes, suggests that services provided during an episode have increased in intensity or shifted toward higher-cost treatments.
When interpreting these trends, several points are notable. The underlying health of the Medicare population may contribute to declining inpatient use but is difficult to quantify. The observed decline in cardiac-related hospitalizations is consistent with evidence that the impact of ischemic heart disease, the leading source of disease or injury in the US population, has dramatically declined over recent decades15 and that the Medicare program has experienced large declines in overall spending and use related to cardiac conditions.16-18
Other potential factors include a shift toward hospitals treating Medicare beneficiaries as outpatients during an observation stay instead of admitting them as inpatients. Observation stays have increased as traditional Medicare implemented measures to penalize readmissions and limit payments for short inpatient stays.19-21 Even so, the increase in observation stays would have to be at least three times as large as described in other work to fully substitute for the decrease in inpatient stays: between the years 2007 and 2018, the number of observation stays per 1000 beneficiaries increased by only 26 stays, whereas the number of hospitalizations per 1000 beneficiaries decreased by 83 hospitalizations.20
Outpatient services may also broaden treatment availability in alternative settings or enable beneficiaries to avoid inpatient treatment with appropriate preventative care.22-27 These considerations are even more relevant as the COVID-19 pandemic spurred reduced admissions and shifted acute services outside of hospitals.28,29 Some services, such as elective surgeries, have probably shifted from an inpatient to an outpatient setting, which would be consistent with our finding that there are larger relative declines in planned hospitalizations. Although this analysis does not capture spending for outpatient services that are not linked to an inpatient admission, prior work demonstrates that annual growth in total Medicare spending per beneficiary (episode related or not) has recently declined for the inpatient sector but increased for outpatient and physician sectors.30 By offering other outpatient services, hospitals may be able to recoup some declining inpatient revenues. However, outpatient services are reimbursed at a lower rate than inpatient services, suggesting these trends may create financial pressure for hospitals.
There are several limitations to our analysis. First, our analysis is not designed to uncover the reason for the shift away from inpatient services nor to analyze how it has affected beneficiaries’ overall quality of care.
CONCLUSION
Over an 8-year period, Medicare spending per beneficiary on inpatient episodes, including all services immediately preceding and following hospitalizations, declined by 8.9% after taking into account payment rate increases. This broad shift away from inpatient services among all Medicare beneficiaries suggests policymakers should aim for payment policies that balance financial sustainability for hospitals and associated facilities with more efficient use of inpatient and related services.
Acknowledgments
The authors thank Sunita Thapa, Lucas Stewart, Christine Lai, and Liliana Podczerwinski for contributions in data analysis and manuscript preparation.
1. Sun R, Karaca Z, Wong HS. Trends in hospital inpatient stays by age and payer, 2000-2015: Statistical Brief #235. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality; 2006.
2. HCUP Fast Stats - trends in inpatient stays. Healthcare Cost and Utilization Project (HCUP). April 2021. Accessed August 29, 2021. www.hcup-us.ahrq.gov/faststats/national/inpatienttrends.jsp
3. The Medicare Payment Advisory Commission. Section 1: National health care and Medicare spending. In: A Data Book: Health Care Spending and the Medicare Program. June 2018. Accessed August 13, 2021. http://www.medpac.gov/docs/default-source/data-book/jun18_databooksec1_sec.pdf
4. Buntin MB, Graves JA. How the ACA dented the cost curve. Health Aff (Millwood). 2020;39(3):403-412. https://doi.org/10.1377/hlthaff.2019.01478
5. Krumholz HM, Nuti SV, Downing NS, Normand SLT, Wang Y. Mortality, hospitalizations, and expenditures for the Medicare population aged 65 years or older, 1999-2013. JAMA. 2015;314(4):355-365. https://doi.org/10.1001/jama.2015.8035
6. Chen LM, Norton EC, Banerjee M, Regenbogen SE, Cain-Nielsen AH, Birkmeyer JD. Spending on care after surgery driven by choice of care settings instead of intensity of services. Health Aff (Millwood). 2017;36(1):83-90. https://doi.org/10.1377/hlthaff.2016.0668
7. Ibrahim AM, Nuliyalu U, Lawton EJ, et al. Evaluation of US hospital episode spending for acute inpatient conditions after the Patient Protection and Affordable Care Act. JAMA Netw Open. 2020;3(11):e2023926. https://doi.org/10.1001/jamanetworkopen.2020.23926
8. Rosen A, Aizcorbe A, Ryu AJ, Nestoriak N, Cutler DM, Chernew ME. Policy makers will need a way to update bundled payments that reflects highly skewed spending growth of various care episodes. Health Aff (Millwood). 2013;32(5):944-951. https://doi.org/10.1377/hlthaff.2012.1246
9. Lindenauer PK, Lagu T, Shieh MS, Pekow PS, Rothberg MB. Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003-2009. JAMA. 2012;307(13):1405-1413. https://doi.org/10.1001/jama.2012.384
10. Buntin MB, Lai C, Podczerwinski L, Poon S, Wallis C. Changing diagnosis patterns are increasing Medicare spending for inpatient hospital services. The Commonwealth Fund. April 28, 2021. Accessed August 13, 2021. https://www.commonwealthfund.org/publications/2021/apr/changing-diagnosis-patterns-are-increasing-medicare-spending-inpatient
11. The Medicare Payment Advisory Commission. Hospital inpatient and outpatient services. In: Report to the Congress: Medicare Payment Policy. . March 2018. Accessed August 13, 2021. http://www.medpac.gov/docs/default-source/reports/mar18_medpac_ch3_sec.pdf?sfvrsn=0
12. Ody C, Msall L, Dafny LS, Grabowski DC, Cutler DM. Decreases In readmissions credited to Medicare’s program to reduce hospital readmissions have been overstated. Health Aff (Millwood). 2019;38(1):36-43. https://doi.org/10.1377/hlthaff.2018.05178
13. Dharmarajan K, Qin L, Lin Z, et al. Declining admission rates and thirty-day readmission rates positively associated even though patients grew sicker over time. Health Aff (Millwood). 2016;35(7):1294-1302. https://doi.org/10.1377/hlthaff.2015.1614
14. Sjoding MW, Prescott HC, Wunsch H, Iwashyna TJ, Cooke CR. Longitudinal changes in ICU admissions among elderly patients in the United States. Crit Care Med. 2016;44(7):1353-1360. https://doi.org/10.1097/CCM.0000000000001664
15. Murray CJ, Atkinson C, Bhalla K, et al. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591-608. https://doi.org/10.1001/jama.2013.13805
16. Cutler DM, Ghosh K, Messer KL, Raghunathan TE, Stewart ST, Rosen AB. Explaining the slowdown in medical spending growth among the elderly, 1999-2012. Health Aff (Millwood). 2019;38(2):222-229. https://doi.org/10.1377/hlthaff.2018.05372
17. Ward MJ, Kripalani S, Zhu Y, et al. Incidence of emergency department visits for ST-elevation myocardial infarction in a recent six-year period in the United States. Am J Cardiol. 2015;115(2):167-170. https://doi.org/10.1016/j.amjcard.2014.10.020
18. Keohane LM, Gambrel RJ, Freed SS, Stevenson D, Buntin MB. Understanding trends in Medicare spending, 2007-2014. Health Serv Res. 2018;53(5):3507-3527. https://doi.org/10.1111/1475-6773.12845
19. Nuckols TK, Fingar KR, Barrett M, Steiner CA, Stocks C, Owens PL. The shifting landscape in utilization of inpatient, observation, and emergency department services across payers. J Hosp Med. 2017;12(6):443-446. https://doi.org/10.12788/jhm.2751
20. Poon SJ, Wallis CJ, Lai P, Podczerwinski L, Buntin MB. Medicare two-midnight rule accelerated shift to observation stays. Health Affairs. In press.
21. Sheehy AM, Kaiksow F, Powell WR, et al. The Hospital Readmissions Reduction Program and observation hospitalizations. J Hosp Med. 2021;16(7):409-411. https://doi.org/10.12788/jhm.3634
22. Culler SD, Parchman ML, Przybylski M. Factors related to potentially preventable hospitalizations among the elderly. Med Care. 1998;36(6):804-817. https://doi.org/10.1097/00005650-199806000-00004
23. Kozak LJ, Hall MJ, Owings MF. Trends in avoidable hospitalizations, 1980-1998. Health Aff (Millwood). 2001;20(2):225-232. https://doi.org/10.1377/hlthaff.20.2.225
24. Ouslander JG, Lamb G, Perloe M, et al. Potentially avoidable hospitalizations of nursing home residents: frequency, causes, and costs. J Am Geriatr Soc. 2010;58(4):627-635. https://doi.org/10.1111/j.1532-5415.2010.02768.x
25. Konetzka RT, Karon SL, Potter DEB. Users of Medicaid home and community-based services are especially vulnerable to costly avoidable hospital admissions. Health Aff (Millwood). 2012;31(6):1167-1175. https://doi.org/10.1377/hlthaff.2011.0902
26. Nyweide DJ, Anthony DL, Bynum JPW, et al. Continuity of care and the risk of preventable hospitalization in older adults. JAMA Intern Med. 2013;173(20):1879-1885. https://doi.org/10.1001/jamainternmed.2013.10059
27. Auerbach AD, Kripalani S, Vasilevskis EE, et al. Preventability and causes of readmissions in a national cohort of general medicine patients. JAMA Intern Med. 2016;176(4):484-493. https://doi.org/10.1001/jamainternmed.2015.7863
28. Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood). 2020;39(11):2010-2017. https://doi.org/10.1377/hlthaff.2020.00980
29. Nundy S, Patel KK. Hospital-at-home to support COVID-19 surge—time to bring down the walls? JAMA Health Forum. 2020;1(5):e200504. https://doi.org/10.1001/jamahealthforum.2020.0504
30. Keohane LM, Stevenson DG, Freed S, Thapa S, Stewart L, Buntin MB. Trends in Medicare fee-for-service spending growth for dual-eligible beneficiaries, 2007–15. Health Aff (Millwood). 2018;37(8):1265-1273. https://doi.org/10.1377/hlthaff.2018.0143
31. Freed M, Biniek JF, Damico A, Neuman T. Medicare Advantage in 2021: enrollment update and key trends. June 21, 2021. Accessed August 13, 2021. https://www.kff.org/medicare/issue-brief/medicare-advantage-in-2021-enrollment-update-and-key-trends/
32. Li Q, Rahman M, Gozalo P, Keohane LM, Gold MR, Trivedi AN. Regional variations: the use of hospitals, home health, and skilled nursing in traditional Medicare and Medicare Advantage. Health Aff (Millwood). 2018;37(8):1274-1281. https://doi.org/10.1377/hlthaff.2018.0147
The rate of inpatient admissions among adults aged 65 years and older has decreased by approximately 25% since 2000.1,2 This long-term trend raises important questions about inpatient-related spending in the traditional Medicare program for hospitals and providers who treat beneficiaries after a hospitalization. As traditional Medicare’s most expensive sector (accounting for 21% of all Medicare spending3), reducing hospitalizations is often championed as an opportunity to moderate Medicare spending growth.
Medicare’s ability to achieve significant savings from declining inpatient use may be tempered by a shift toward more expensive hospitalizations. If marginal hospitalizations among healthier beneficiaries are avoided, then the remaining inpatient users may be sicker and have greater spending per hospitalization and greater need for follow-up services. This study examines trends in Medicare spending related to episodes initiated by an inpatient stay because of its importance to overall Medicare spending and the implications for several Medicare value-based payment initiatives. In care models seeking to contain spending at a population level, such as accountable care organizations and managed care plans, reducing inpatient use and associated services may have the largest impact in curbing overall spending growth per beneficiary. Other models focused on spending at an episode level, including bundled payment initiatives, may face challenges if inpatient episodes become more expensive over time.
As Medicare shifts toward value-based payments, hospitalists and other hospital leaders are often involved in redesigning care delivery models for the hospital or accountable care organization (eg, through readmission reduction initiatives, post–acute care coordination, and bundled-care delivery programs). Not all savings strategies rely on providers to change how services are delivered; Medicare can modify payment rates, such as Affordable Care Act provisions that slowed how quickly Medicare payment rates increased.4 For clinicians to navigate the shift toward new payment models, it is important to recognize how each of these elements—declining hospital admissions, spending per inpatient episode, and payment rates—affect spending trends for inpatient services and associated care. Previous articles on overall Medicare inpatient spending have examined inpatient stays alone5 or focused mainly on spending per episode6,7 without quantifying how these elements contributed to overall episode-related Medicare spending per beneficiary. This article addresses this gap by demonstrating how inpatient-related spending trends reflect each component.
This study examined trends in Medicare’s spending on inpatient episodes during the years 2009 to 2017. We described changes in the volume and spending on inpatient-initiated episodes across several dimensions, including beneficiary-level and hospitalization-level factors. We examined whether declines in spending associated with fewer inpatient-initiated episodes have been offset by increased spending per episode and how spending would have differed without changes in Medicare payment rates.
Episode Definition
We constructed an episode measure that captured traditional Medicare spending for 30 days prior to hospital admission, hospitalization duration, and 90 days following hospital discharge (additional details in the Appendix).
Any acute hospitalization triggered a new episode, with one exception: if a beneficiary was discharged and readmitted within 90 days for the same diagnosis-related group (DRG), then the readmission did not trigger a new episode. The spending for that readmission was attributed to the prior hospital stay. In effect, the annual number of episodes is equivalent to the annual number of hospital admissions minus subsequent rehospitalizations for the same DRG. Neither observation stays nor hospitalizations in inpatient rehabilitation, psychiatric, or long-term facilities were considered acute hospital admissions.
We assigned claims from noninpatient sectors to an episode based on whether the claim start date fell within the episode window. All traditional Medicare sectors were measured, including outpatient services, physician claims, post–acute care services, and Medicare Part D prescription drug events.
Our analysis aimed to measure all spending related to inpatient episodes without double-counting spending for overlapping episodes. If episodes overlapped, then spending for overlapping days was weighted to be evenly divided across episodes.
Outcome Measures
The study’s main outcomes summarized episode trends across the entire traditional Medicare population, including beneficiaries without an episode, in annual mean number of episodes per beneficiary and annual mean episode-related spending per beneficiary. The denominator of these measures is person-years, or total number of beneficiary months with Medicare Part A and B coverage divided by 12. The annual mean number of episodes per beneficiary is the total number of episodes initiated in a calendar year divided by person-years. The annual mean episode-related spending per beneficiary is the total amount of spending attributed to episodes divided by person-years. We also measured annual mean spending per episode, or total amount of spending attributed to episodes divided by the total number of episodes.
Medicare annually updates each sector’s payment rates for several factors, including inflation. We constructed an index for each sector to adjust for these annual payment rate changes. We also accounted for sequestration measures in effect since April 2013 that reduced Medicare payments to all sectors by 2%. We report our spending measures twice, with and without adjusting for changes in payment rates. Adjusted numbers reflect payment rates in effect in 2015.
Analysis Approach
We present annual trends on changes in the number of inpatient episodes per beneficiary, mean episode-related spending per beneficiary, and mean spending per episode. To quantify how changes in episode-related spending per beneficiary reflect changes in the number of episodes per beneficiary vs changes in spending per episode, we modified an approach implemented by Rosen and colleagues.8
To better understand which beneficiaries have declining inpatient use, we performed stratified analyses describing changes in the number of episodes per beneficiary between 2009 and 2017, spending per episode, and total episode-related spending per beneficiary. We report these measures for several subpopulations defined by age, sex, race, dual-eligible status, and whether the beneficiary used long-term nursing home services during the episode’s calendar year. Descriptive statistics also detail how these measures changed between 2009 and 2017 for episodes stratified by characteristics of the index hospital stay: planned vs unplanned, medical vs surgical, and any use of intensive care unit (ICU) or coronary care unit services. We also stratify study measures by whether an episode included any use of post–acute care services (skilled nursing facility, home health, or inpatient rehabilitation facility use). Finally, we aggregate the episodes into major diagnostic categories (MDCs) based on the index hospital stay’s DRG to report study outcomes by condition. Because of a shift in coding hospitalizations for pneumonia as sepsis,9,10 we exclude these two diseases from their respective MDCs and analyze them jointly as a unique category.
RESULTS
Changes in Number of Inpatient Episodes and Related Spending
From 2009 to 2017, the number of inpatient episodes per 1000 traditional Medicare beneficiaries declined from 326 to 267 (Table 1), or a relative decline of 18.2% (Figure 1). The total volume of inpatient episodes declined by only 13.4%, from 10.2 million to 8.8 million, reflecting that the size of the traditional Medicare population grew during these years. Over the same years, mean payment-rate–adjusted spending per episode increased 11.4% from $20,891 to $23,273.

When considering overall episode-related spending, the large decline in the volume of episodes outweighed increased spending per episode: the mean amount of episode-related Medicare spending per beneficiary decreased 8.9% from $6810 to $6206 (Table 1), or a net change of $604 (Figure 2). This net change reflects decreased spending due to fewer episodes per beneficiary ($1239 reduction in episode-related spending) offset by increased spending per episode (translating to a $776 increase in episode-related spending per beneficiary).

When these estimates are calculated separately for the inpatient sector and all other sectors, the inpatient sector experienced small increases in spending associated with greater spending per episode ($304) compared with noninpatient sectors ($472). Accordingly, the inpatient sector had a larger net decline in episode-related spending per beneficiary ($420) than noninpatient sectors ($184) after taking into account declining episode volume.
As expected, episode-related spending increased more when measures were not adjusted for annual payment rate increases. Without such adjustment, mean spending per episode increased 25.5%, and episode-related spending per beneficiary was nearly flat (2.6% between 2009 and 2017 [Figure 1]). The decline in unadjusted spending associated with fewer episodes ($1138) was offset by the spending increase associated with higher spending per episode ($1592) (Figure 2).

Analyses Stratified by Beneficiary Characteristics
Every population examined had declines in the number of inpatient episodes, even beneficiaries with more frequent inpatient use (Table 2). Among Medicare beneficiaries aged 85 years and older, the mean number of episodes per 1000 beneficiaries declined by 12.7%, from 524 to 457. Populations with less frequent inpatient use often experienced larger relative declines in number of episodes than populations with more frequent inpatient use. For example, the mean number of episodes per 1000 beneficiaries decreased by 17.7% for beneficiaries without nursing home use (306 to 252), as compared with an 8.1% decline for beneficiaries with nursing home use (from 888 to 816). In contrast, populations with less frequent inpatient use had larger relative increases in spending per episode with adjustment for payment rate changes. For example, spending per episode increased by 13.1% for beneficiaries aged 65 to 74 years ($20,904 to $23,644), but only by 8.6% for beneficiaries 85 years and older ($20,384 to $22,138).

Analyses Stratified by Service Use Characteristics
Some types of inpatient episodes had larger declines in the number of episodes, including episodes with planned admissions for the index hospital stay (28.8% decline from 68 to 48 episodes per 1000 beneficiaries) and episodes without post–acute care use (23.9% decline from 169 to 129 episodes per 1000 beneficiaries) (Appendix Table). In contrast, declines in the number of episodes were similar for index hospital admissions that did or did not involve ICU use (17.8% and 18.3% reduction in mean number of episodes per 1000 beneficiaries, respectively) or that included a surgical procedure or not (17.1% versus 18.6%, respectively). Several types of inpatient episodes had larger increases in spending per episode, such as a 15.1% increase for planned admissions and a 13.2% increase for hospitalizations without ICU use.
According to diagnosis information for an episode’s index hospital stay, inpatient episodes related to conditions affecting the circulatory system had the largest decline in mean number of episodes, decreasing by 31.8% from 78 to 53 episodes per 1000 beneficiaries (Appendix Table). Episodes for other diseases had much smaller declines in volume. Admissions for diagnoses of pneumonia or sepsis had notable increases in the volume of episodes, increasing by 20.7% from 25 to 30 admissions per 1000 beneficiaries.
DISCUSSION
Medicare spending per beneficiary on inpatient episodes, including services provided pre- and post hospitalization, declined by 8.9% from 2009 to 2017 after adjusting for payment rate changes. This decline reflects two components. First, the number of episodes per 1000 beneficiaries declined by 18.2%. Although the extent of this decrease varied across populations, every group examined had declines in inpatient use. In particular, hospitalizations for conditions affecting the circulatory system, such as heart attacks and cardiac procedures, decreased. Second, as inpatient volume declined, spending per episode increased by 11.4% to an average of $23,273 in 2017. This increase in spending per episode offset how much overall Medicare spending on episode-related care declined.
Medicare is increasingly challenging hospitals to demonstrate the value of inpatient services and associated treatment, which requires hospital leaders to recognize how their facilities’ spending trends relate to these national patterns. Understanding how much national episode-related spending has decreased over time with declining inpatient volume can help an accountable care organization evaluate whether it is feasible to achieve significant savings by reducing hospitalizations. Bundled payment providers focused on managing spending per episode can benefit from identifying which types of hospitalizations have increased spending per episode, especially for certain diagnoses.
These results also highlight the continued importance of a perennial factor in Medicare spending: payment rates. If Medicare payment rates had not increased over our study period, Medicare spending per inpatient episode would have increased by only 11%. Actual Medicare spending per episode increased by 25%, demonstrating that over half of the relative increase in spending per episode reflected increases in Medicare’s payment rates.
Increased spending per episode, even after adjustment for payment rate changes, suggests that services provided during an episode have increased in intensity or shifted toward higher-cost treatments.
When interpreting these trends, several points are notable. The underlying health of the Medicare population may contribute to declining inpatient use but is difficult to quantify. The observed decline in cardiac-related hospitalizations is consistent with evidence that the impact of ischemic heart disease, the leading source of disease or injury in the US population, has dramatically declined over recent decades15 and that the Medicare program has experienced large declines in overall spending and use related to cardiac conditions.16-18
Other potential factors include a shift toward hospitals treating Medicare beneficiaries as outpatients during an observation stay instead of admitting them as inpatients. Observation stays have increased as traditional Medicare implemented measures to penalize readmissions and limit payments for short inpatient stays.19-21 Even so, the increase in observation stays would have to be at least three times as large as described in other work to fully substitute for the decrease in inpatient stays: between the years 2007 and 2018, the number of observation stays per 1000 beneficiaries increased by only 26 stays, whereas the number of hospitalizations per 1000 beneficiaries decreased by 83 hospitalizations.20
Outpatient services may also broaden treatment availability in alternative settings or enable beneficiaries to avoid inpatient treatment with appropriate preventative care.22-27 These considerations are even more relevant as the COVID-19 pandemic spurred reduced admissions and shifted acute services outside of hospitals.28,29 Some services, such as elective surgeries, have probably shifted from an inpatient to an outpatient setting, which would be consistent with our finding that there are larger relative declines in planned hospitalizations. Although this analysis does not capture spending for outpatient services that are not linked to an inpatient admission, prior work demonstrates that annual growth in total Medicare spending per beneficiary (episode related or not) has recently declined for the inpatient sector but increased for outpatient and physician sectors.30 By offering other outpatient services, hospitals may be able to recoup some declining inpatient revenues. However, outpatient services are reimbursed at a lower rate than inpatient services, suggesting these trends may create financial pressure for hospitals.
There are several limitations to our analysis. First, our analysis is not designed to uncover the reason for the shift away from inpatient services nor to analyze how it has affected beneficiaries’ overall quality of care.
CONCLUSION
Over an 8-year period, Medicare spending per beneficiary on inpatient episodes, including all services immediately preceding and following hospitalizations, declined by 8.9% after taking into account payment rate increases. This broad shift away from inpatient services among all Medicare beneficiaries suggests policymakers should aim for payment policies that balance financial sustainability for hospitals and associated facilities with more efficient use of inpatient and related services.
Acknowledgments
The authors thank Sunita Thapa, Lucas Stewart, Christine Lai, and Liliana Podczerwinski for contributions in data analysis and manuscript preparation.
The rate of inpatient admissions among adults aged 65 years and older has decreased by approximately 25% since 2000.1,2 This long-term trend raises important questions about inpatient-related spending in the traditional Medicare program for hospitals and providers who treat beneficiaries after a hospitalization. As traditional Medicare’s most expensive sector (accounting for 21% of all Medicare spending3), reducing hospitalizations is often championed as an opportunity to moderate Medicare spending growth.
Medicare’s ability to achieve significant savings from declining inpatient use may be tempered by a shift toward more expensive hospitalizations. If marginal hospitalizations among healthier beneficiaries are avoided, then the remaining inpatient users may be sicker and have greater spending per hospitalization and greater need for follow-up services. This study examines trends in Medicare spending related to episodes initiated by an inpatient stay because of its importance to overall Medicare spending and the implications for several Medicare value-based payment initiatives. In care models seeking to contain spending at a population level, such as accountable care organizations and managed care plans, reducing inpatient use and associated services may have the largest impact in curbing overall spending growth per beneficiary. Other models focused on spending at an episode level, including bundled payment initiatives, may face challenges if inpatient episodes become more expensive over time.
As Medicare shifts toward value-based payments, hospitalists and other hospital leaders are often involved in redesigning care delivery models for the hospital or accountable care organization (eg, through readmission reduction initiatives, post–acute care coordination, and bundled-care delivery programs). Not all savings strategies rely on providers to change how services are delivered; Medicare can modify payment rates, such as Affordable Care Act provisions that slowed how quickly Medicare payment rates increased.4 For clinicians to navigate the shift toward new payment models, it is important to recognize how each of these elements—declining hospital admissions, spending per inpatient episode, and payment rates—affect spending trends for inpatient services and associated care. Previous articles on overall Medicare inpatient spending have examined inpatient stays alone5 or focused mainly on spending per episode6,7 without quantifying how these elements contributed to overall episode-related Medicare spending per beneficiary. This article addresses this gap by demonstrating how inpatient-related spending trends reflect each component.
This study examined trends in Medicare’s spending on inpatient episodes during the years 2009 to 2017. We described changes in the volume and spending on inpatient-initiated episodes across several dimensions, including beneficiary-level and hospitalization-level factors. We examined whether declines in spending associated with fewer inpatient-initiated episodes have been offset by increased spending per episode and how spending would have differed without changes in Medicare payment rates.
Episode Definition
We constructed an episode measure that captured traditional Medicare spending for 30 days prior to hospital admission, hospitalization duration, and 90 days following hospital discharge (additional details in the Appendix).
Any acute hospitalization triggered a new episode, with one exception: if a beneficiary was discharged and readmitted within 90 days for the same diagnosis-related group (DRG), then the readmission did not trigger a new episode. The spending for that readmission was attributed to the prior hospital stay. In effect, the annual number of episodes is equivalent to the annual number of hospital admissions minus subsequent rehospitalizations for the same DRG. Neither observation stays nor hospitalizations in inpatient rehabilitation, psychiatric, or long-term facilities were considered acute hospital admissions.
We assigned claims from noninpatient sectors to an episode based on whether the claim start date fell within the episode window. All traditional Medicare sectors were measured, including outpatient services, physician claims, post–acute care services, and Medicare Part D prescription drug events.
Our analysis aimed to measure all spending related to inpatient episodes without double-counting spending for overlapping episodes. If episodes overlapped, then spending for overlapping days was weighted to be evenly divided across episodes.
Outcome Measures
The study’s main outcomes summarized episode trends across the entire traditional Medicare population, including beneficiaries without an episode, in annual mean number of episodes per beneficiary and annual mean episode-related spending per beneficiary. The denominator of these measures is person-years, or total number of beneficiary months with Medicare Part A and B coverage divided by 12. The annual mean number of episodes per beneficiary is the total number of episodes initiated in a calendar year divided by person-years. The annual mean episode-related spending per beneficiary is the total amount of spending attributed to episodes divided by person-years. We also measured annual mean spending per episode, or total amount of spending attributed to episodes divided by the total number of episodes.
Medicare annually updates each sector’s payment rates for several factors, including inflation. We constructed an index for each sector to adjust for these annual payment rate changes. We also accounted for sequestration measures in effect since April 2013 that reduced Medicare payments to all sectors by 2%. We report our spending measures twice, with and without adjusting for changes in payment rates. Adjusted numbers reflect payment rates in effect in 2015.
Analysis Approach
We present annual trends on changes in the number of inpatient episodes per beneficiary, mean episode-related spending per beneficiary, and mean spending per episode. To quantify how changes in episode-related spending per beneficiary reflect changes in the number of episodes per beneficiary vs changes in spending per episode, we modified an approach implemented by Rosen and colleagues.8
To better understand which beneficiaries have declining inpatient use, we performed stratified analyses describing changes in the number of episodes per beneficiary between 2009 and 2017, spending per episode, and total episode-related spending per beneficiary. We report these measures for several subpopulations defined by age, sex, race, dual-eligible status, and whether the beneficiary used long-term nursing home services during the episode’s calendar year. Descriptive statistics also detail how these measures changed between 2009 and 2017 for episodes stratified by characteristics of the index hospital stay: planned vs unplanned, medical vs surgical, and any use of intensive care unit (ICU) or coronary care unit services. We also stratify study measures by whether an episode included any use of post–acute care services (skilled nursing facility, home health, or inpatient rehabilitation facility use). Finally, we aggregate the episodes into major diagnostic categories (MDCs) based on the index hospital stay’s DRG to report study outcomes by condition. Because of a shift in coding hospitalizations for pneumonia as sepsis,9,10 we exclude these two diseases from their respective MDCs and analyze them jointly as a unique category.
RESULTS
Changes in Number of Inpatient Episodes and Related Spending
From 2009 to 2017, the number of inpatient episodes per 1000 traditional Medicare beneficiaries declined from 326 to 267 (Table 1), or a relative decline of 18.2% (Figure 1). The total volume of inpatient episodes declined by only 13.4%, from 10.2 million to 8.8 million, reflecting that the size of the traditional Medicare population grew during these years. Over the same years, mean payment-rate–adjusted spending per episode increased 11.4% from $20,891 to $23,273.

When considering overall episode-related spending, the large decline in the volume of episodes outweighed increased spending per episode: the mean amount of episode-related Medicare spending per beneficiary decreased 8.9% from $6810 to $6206 (Table 1), or a net change of $604 (Figure 2). This net change reflects decreased spending due to fewer episodes per beneficiary ($1239 reduction in episode-related spending) offset by increased spending per episode (translating to a $776 increase in episode-related spending per beneficiary).

When these estimates are calculated separately for the inpatient sector and all other sectors, the inpatient sector experienced small increases in spending associated with greater spending per episode ($304) compared with noninpatient sectors ($472). Accordingly, the inpatient sector had a larger net decline in episode-related spending per beneficiary ($420) than noninpatient sectors ($184) after taking into account declining episode volume.
As expected, episode-related spending increased more when measures were not adjusted for annual payment rate increases. Without such adjustment, mean spending per episode increased 25.5%, and episode-related spending per beneficiary was nearly flat (2.6% between 2009 and 2017 [Figure 1]). The decline in unadjusted spending associated with fewer episodes ($1138) was offset by the spending increase associated with higher spending per episode ($1592) (Figure 2).

Analyses Stratified by Beneficiary Characteristics
Every population examined had declines in the number of inpatient episodes, even beneficiaries with more frequent inpatient use (Table 2). Among Medicare beneficiaries aged 85 years and older, the mean number of episodes per 1000 beneficiaries declined by 12.7%, from 524 to 457. Populations with less frequent inpatient use often experienced larger relative declines in number of episodes than populations with more frequent inpatient use. For example, the mean number of episodes per 1000 beneficiaries decreased by 17.7% for beneficiaries without nursing home use (306 to 252), as compared with an 8.1% decline for beneficiaries with nursing home use (from 888 to 816). In contrast, populations with less frequent inpatient use had larger relative increases in spending per episode with adjustment for payment rate changes. For example, spending per episode increased by 13.1% for beneficiaries aged 65 to 74 years ($20,904 to $23,644), but only by 8.6% for beneficiaries 85 years and older ($20,384 to $22,138).

Analyses Stratified by Service Use Characteristics
Some types of inpatient episodes had larger declines in the number of episodes, including episodes with planned admissions for the index hospital stay (28.8% decline from 68 to 48 episodes per 1000 beneficiaries) and episodes without post–acute care use (23.9% decline from 169 to 129 episodes per 1000 beneficiaries) (Appendix Table). In contrast, declines in the number of episodes were similar for index hospital admissions that did or did not involve ICU use (17.8% and 18.3% reduction in mean number of episodes per 1000 beneficiaries, respectively) or that included a surgical procedure or not (17.1% versus 18.6%, respectively). Several types of inpatient episodes had larger increases in spending per episode, such as a 15.1% increase for planned admissions and a 13.2% increase for hospitalizations without ICU use.
According to diagnosis information for an episode’s index hospital stay, inpatient episodes related to conditions affecting the circulatory system had the largest decline in mean number of episodes, decreasing by 31.8% from 78 to 53 episodes per 1000 beneficiaries (Appendix Table). Episodes for other diseases had much smaller declines in volume. Admissions for diagnoses of pneumonia or sepsis had notable increases in the volume of episodes, increasing by 20.7% from 25 to 30 admissions per 1000 beneficiaries.
DISCUSSION
Medicare spending per beneficiary on inpatient episodes, including services provided pre- and post hospitalization, declined by 8.9% from 2009 to 2017 after adjusting for payment rate changes. This decline reflects two components. First, the number of episodes per 1000 beneficiaries declined by 18.2%. Although the extent of this decrease varied across populations, every group examined had declines in inpatient use. In particular, hospitalizations for conditions affecting the circulatory system, such as heart attacks and cardiac procedures, decreased. Second, as inpatient volume declined, spending per episode increased by 11.4% to an average of $23,273 in 2017. This increase in spending per episode offset how much overall Medicare spending on episode-related care declined.
Medicare is increasingly challenging hospitals to demonstrate the value of inpatient services and associated treatment, which requires hospital leaders to recognize how their facilities’ spending trends relate to these national patterns. Understanding how much national episode-related spending has decreased over time with declining inpatient volume can help an accountable care organization evaluate whether it is feasible to achieve significant savings by reducing hospitalizations. Bundled payment providers focused on managing spending per episode can benefit from identifying which types of hospitalizations have increased spending per episode, especially for certain diagnoses.
These results also highlight the continued importance of a perennial factor in Medicare spending: payment rates. If Medicare payment rates had not increased over our study period, Medicare spending per inpatient episode would have increased by only 11%. Actual Medicare spending per episode increased by 25%, demonstrating that over half of the relative increase in spending per episode reflected increases in Medicare’s payment rates.
Increased spending per episode, even after adjustment for payment rate changes, suggests that services provided during an episode have increased in intensity or shifted toward higher-cost treatments.
When interpreting these trends, several points are notable. The underlying health of the Medicare population may contribute to declining inpatient use but is difficult to quantify. The observed decline in cardiac-related hospitalizations is consistent with evidence that the impact of ischemic heart disease, the leading source of disease or injury in the US population, has dramatically declined over recent decades15 and that the Medicare program has experienced large declines in overall spending and use related to cardiac conditions.16-18
Other potential factors include a shift toward hospitals treating Medicare beneficiaries as outpatients during an observation stay instead of admitting them as inpatients. Observation stays have increased as traditional Medicare implemented measures to penalize readmissions and limit payments for short inpatient stays.19-21 Even so, the increase in observation stays would have to be at least three times as large as described in other work to fully substitute for the decrease in inpatient stays: between the years 2007 and 2018, the number of observation stays per 1000 beneficiaries increased by only 26 stays, whereas the number of hospitalizations per 1000 beneficiaries decreased by 83 hospitalizations.20
Outpatient services may also broaden treatment availability in alternative settings or enable beneficiaries to avoid inpatient treatment with appropriate preventative care.22-27 These considerations are even more relevant as the COVID-19 pandemic spurred reduced admissions and shifted acute services outside of hospitals.28,29 Some services, such as elective surgeries, have probably shifted from an inpatient to an outpatient setting, which would be consistent with our finding that there are larger relative declines in planned hospitalizations. Although this analysis does not capture spending for outpatient services that are not linked to an inpatient admission, prior work demonstrates that annual growth in total Medicare spending per beneficiary (episode related or not) has recently declined for the inpatient sector but increased for outpatient and physician sectors.30 By offering other outpatient services, hospitals may be able to recoup some declining inpatient revenues. However, outpatient services are reimbursed at a lower rate than inpatient services, suggesting these trends may create financial pressure for hospitals.
There are several limitations to our analysis. First, our analysis is not designed to uncover the reason for the shift away from inpatient services nor to analyze how it has affected beneficiaries’ overall quality of care.
CONCLUSION
Over an 8-year period, Medicare spending per beneficiary on inpatient episodes, including all services immediately preceding and following hospitalizations, declined by 8.9% after taking into account payment rate increases. This broad shift away from inpatient services among all Medicare beneficiaries suggests policymakers should aim for payment policies that balance financial sustainability for hospitals and associated facilities with more efficient use of inpatient and related services.
Acknowledgments
The authors thank Sunita Thapa, Lucas Stewart, Christine Lai, and Liliana Podczerwinski for contributions in data analysis and manuscript preparation.
1. Sun R, Karaca Z, Wong HS. Trends in hospital inpatient stays by age and payer, 2000-2015: Statistical Brief #235. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality; 2006.
2. HCUP Fast Stats - trends in inpatient stays. Healthcare Cost and Utilization Project (HCUP). April 2021. Accessed August 29, 2021. www.hcup-us.ahrq.gov/faststats/national/inpatienttrends.jsp
3. The Medicare Payment Advisory Commission. Section 1: National health care and Medicare spending. In: A Data Book: Health Care Spending and the Medicare Program. June 2018. Accessed August 13, 2021. http://www.medpac.gov/docs/default-source/data-book/jun18_databooksec1_sec.pdf
4. Buntin MB, Graves JA. How the ACA dented the cost curve. Health Aff (Millwood). 2020;39(3):403-412. https://doi.org/10.1377/hlthaff.2019.01478
5. Krumholz HM, Nuti SV, Downing NS, Normand SLT, Wang Y. Mortality, hospitalizations, and expenditures for the Medicare population aged 65 years or older, 1999-2013. JAMA. 2015;314(4):355-365. https://doi.org/10.1001/jama.2015.8035
6. Chen LM, Norton EC, Banerjee M, Regenbogen SE, Cain-Nielsen AH, Birkmeyer JD. Spending on care after surgery driven by choice of care settings instead of intensity of services. Health Aff (Millwood). 2017;36(1):83-90. https://doi.org/10.1377/hlthaff.2016.0668
7. Ibrahim AM, Nuliyalu U, Lawton EJ, et al. Evaluation of US hospital episode spending for acute inpatient conditions after the Patient Protection and Affordable Care Act. JAMA Netw Open. 2020;3(11):e2023926. https://doi.org/10.1001/jamanetworkopen.2020.23926
8. Rosen A, Aizcorbe A, Ryu AJ, Nestoriak N, Cutler DM, Chernew ME. Policy makers will need a way to update bundled payments that reflects highly skewed spending growth of various care episodes. Health Aff (Millwood). 2013;32(5):944-951. https://doi.org/10.1377/hlthaff.2012.1246
9. Lindenauer PK, Lagu T, Shieh MS, Pekow PS, Rothberg MB. Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003-2009. JAMA. 2012;307(13):1405-1413. https://doi.org/10.1001/jama.2012.384
10. Buntin MB, Lai C, Podczerwinski L, Poon S, Wallis C. Changing diagnosis patterns are increasing Medicare spending for inpatient hospital services. The Commonwealth Fund. April 28, 2021. Accessed August 13, 2021. https://www.commonwealthfund.org/publications/2021/apr/changing-diagnosis-patterns-are-increasing-medicare-spending-inpatient
11. The Medicare Payment Advisory Commission. Hospital inpatient and outpatient services. In: Report to the Congress: Medicare Payment Policy. . March 2018. Accessed August 13, 2021. http://www.medpac.gov/docs/default-source/reports/mar18_medpac_ch3_sec.pdf?sfvrsn=0
12. Ody C, Msall L, Dafny LS, Grabowski DC, Cutler DM. Decreases In readmissions credited to Medicare’s program to reduce hospital readmissions have been overstated. Health Aff (Millwood). 2019;38(1):36-43. https://doi.org/10.1377/hlthaff.2018.05178
13. Dharmarajan K, Qin L, Lin Z, et al. Declining admission rates and thirty-day readmission rates positively associated even though patients grew sicker over time. Health Aff (Millwood). 2016;35(7):1294-1302. https://doi.org/10.1377/hlthaff.2015.1614
14. Sjoding MW, Prescott HC, Wunsch H, Iwashyna TJ, Cooke CR. Longitudinal changes in ICU admissions among elderly patients in the United States. Crit Care Med. 2016;44(7):1353-1360. https://doi.org/10.1097/CCM.0000000000001664
15. Murray CJ, Atkinson C, Bhalla K, et al. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591-608. https://doi.org/10.1001/jama.2013.13805
16. Cutler DM, Ghosh K, Messer KL, Raghunathan TE, Stewart ST, Rosen AB. Explaining the slowdown in medical spending growth among the elderly, 1999-2012. Health Aff (Millwood). 2019;38(2):222-229. https://doi.org/10.1377/hlthaff.2018.05372
17. Ward MJ, Kripalani S, Zhu Y, et al. Incidence of emergency department visits for ST-elevation myocardial infarction in a recent six-year period in the United States. Am J Cardiol. 2015;115(2):167-170. https://doi.org/10.1016/j.amjcard.2014.10.020
18. Keohane LM, Gambrel RJ, Freed SS, Stevenson D, Buntin MB. Understanding trends in Medicare spending, 2007-2014. Health Serv Res. 2018;53(5):3507-3527. https://doi.org/10.1111/1475-6773.12845
19. Nuckols TK, Fingar KR, Barrett M, Steiner CA, Stocks C, Owens PL. The shifting landscape in utilization of inpatient, observation, and emergency department services across payers. J Hosp Med. 2017;12(6):443-446. https://doi.org/10.12788/jhm.2751
20. Poon SJ, Wallis CJ, Lai P, Podczerwinski L, Buntin MB. Medicare two-midnight rule accelerated shift to observation stays. Health Affairs. In press.
21. Sheehy AM, Kaiksow F, Powell WR, et al. The Hospital Readmissions Reduction Program and observation hospitalizations. J Hosp Med. 2021;16(7):409-411. https://doi.org/10.12788/jhm.3634
22. Culler SD, Parchman ML, Przybylski M. Factors related to potentially preventable hospitalizations among the elderly. Med Care. 1998;36(6):804-817. https://doi.org/10.1097/00005650-199806000-00004
23. Kozak LJ, Hall MJ, Owings MF. Trends in avoidable hospitalizations, 1980-1998. Health Aff (Millwood). 2001;20(2):225-232. https://doi.org/10.1377/hlthaff.20.2.225
24. Ouslander JG, Lamb G, Perloe M, et al. Potentially avoidable hospitalizations of nursing home residents: frequency, causes, and costs. J Am Geriatr Soc. 2010;58(4):627-635. https://doi.org/10.1111/j.1532-5415.2010.02768.x
25. Konetzka RT, Karon SL, Potter DEB. Users of Medicaid home and community-based services are especially vulnerable to costly avoidable hospital admissions. Health Aff (Millwood). 2012;31(6):1167-1175. https://doi.org/10.1377/hlthaff.2011.0902
26. Nyweide DJ, Anthony DL, Bynum JPW, et al. Continuity of care and the risk of preventable hospitalization in older adults. JAMA Intern Med. 2013;173(20):1879-1885. https://doi.org/10.1001/jamainternmed.2013.10059
27. Auerbach AD, Kripalani S, Vasilevskis EE, et al. Preventability and causes of readmissions in a national cohort of general medicine patients. JAMA Intern Med. 2016;176(4):484-493. https://doi.org/10.1001/jamainternmed.2015.7863
28. Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood). 2020;39(11):2010-2017. https://doi.org/10.1377/hlthaff.2020.00980
29. Nundy S, Patel KK. Hospital-at-home to support COVID-19 surge—time to bring down the walls? JAMA Health Forum. 2020;1(5):e200504. https://doi.org/10.1001/jamahealthforum.2020.0504
30. Keohane LM, Stevenson DG, Freed S, Thapa S, Stewart L, Buntin MB. Trends in Medicare fee-for-service spending growth for dual-eligible beneficiaries, 2007–15. Health Aff (Millwood). 2018;37(8):1265-1273. https://doi.org/10.1377/hlthaff.2018.0143
31. Freed M, Biniek JF, Damico A, Neuman T. Medicare Advantage in 2021: enrollment update and key trends. June 21, 2021. Accessed August 13, 2021. https://www.kff.org/medicare/issue-brief/medicare-advantage-in-2021-enrollment-update-and-key-trends/
32. Li Q, Rahman M, Gozalo P, Keohane LM, Gold MR, Trivedi AN. Regional variations: the use of hospitals, home health, and skilled nursing in traditional Medicare and Medicare Advantage. Health Aff (Millwood). 2018;37(8):1274-1281. https://doi.org/10.1377/hlthaff.2018.0147
1. Sun R, Karaca Z, Wong HS. Trends in hospital inpatient stays by age and payer, 2000-2015: Statistical Brief #235. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality; 2006.
2. HCUP Fast Stats - trends in inpatient stays. Healthcare Cost and Utilization Project (HCUP). April 2021. Accessed August 29, 2021. www.hcup-us.ahrq.gov/faststats/national/inpatienttrends.jsp
3. The Medicare Payment Advisory Commission. Section 1: National health care and Medicare spending. In: A Data Book: Health Care Spending and the Medicare Program. June 2018. Accessed August 13, 2021. http://www.medpac.gov/docs/default-source/data-book/jun18_databooksec1_sec.pdf
4. Buntin MB, Graves JA. How the ACA dented the cost curve. Health Aff (Millwood). 2020;39(3):403-412. https://doi.org/10.1377/hlthaff.2019.01478
5. Krumholz HM, Nuti SV, Downing NS, Normand SLT, Wang Y. Mortality, hospitalizations, and expenditures for the Medicare population aged 65 years or older, 1999-2013. JAMA. 2015;314(4):355-365. https://doi.org/10.1001/jama.2015.8035
6. Chen LM, Norton EC, Banerjee M, Regenbogen SE, Cain-Nielsen AH, Birkmeyer JD. Spending on care after surgery driven by choice of care settings instead of intensity of services. Health Aff (Millwood). 2017;36(1):83-90. https://doi.org/10.1377/hlthaff.2016.0668
7. Ibrahim AM, Nuliyalu U, Lawton EJ, et al. Evaluation of US hospital episode spending for acute inpatient conditions after the Patient Protection and Affordable Care Act. JAMA Netw Open. 2020;3(11):e2023926. https://doi.org/10.1001/jamanetworkopen.2020.23926
8. Rosen A, Aizcorbe A, Ryu AJ, Nestoriak N, Cutler DM, Chernew ME. Policy makers will need a way to update bundled payments that reflects highly skewed spending growth of various care episodes. Health Aff (Millwood). 2013;32(5):944-951. https://doi.org/10.1377/hlthaff.2012.1246
9. Lindenauer PK, Lagu T, Shieh MS, Pekow PS, Rothberg MB. Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003-2009. JAMA. 2012;307(13):1405-1413. https://doi.org/10.1001/jama.2012.384
10. Buntin MB, Lai C, Podczerwinski L, Poon S, Wallis C. Changing diagnosis patterns are increasing Medicare spending for inpatient hospital services. The Commonwealth Fund. April 28, 2021. Accessed August 13, 2021. https://www.commonwealthfund.org/publications/2021/apr/changing-diagnosis-patterns-are-increasing-medicare-spending-inpatient
11. The Medicare Payment Advisory Commission. Hospital inpatient and outpatient services. In: Report to the Congress: Medicare Payment Policy. . March 2018. Accessed August 13, 2021. http://www.medpac.gov/docs/default-source/reports/mar18_medpac_ch3_sec.pdf?sfvrsn=0
12. Ody C, Msall L, Dafny LS, Grabowski DC, Cutler DM. Decreases In readmissions credited to Medicare’s program to reduce hospital readmissions have been overstated. Health Aff (Millwood). 2019;38(1):36-43. https://doi.org/10.1377/hlthaff.2018.05178
13. Dharmarajan K, Qin L, Lin Z, et al. Declining admission rates and thirty-day readmission rates positively associated even though patients grew sicker over time. Health Aff (Millwood). 2016;35(7):1294-1302. https://doi.org/10.1377/hlthaff.2015.1614
14. Sjoding MW, Prescott HC, Wunsch H, Iwashyna TJ, Cooke CR. Longitudinal changes in ICU admissions among elderly patients in the United States. Crit Care Med. 2016;44(7):1353-1360. https://doi.org/10.1097/CCM.0000000000001664
15. Murray CJ, Atkinson C, Bhalla K, et al. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591-608. https://doi.org/10.1001/jama.2013.13805
16. Cutler DM, Ghosh K, Messer KL, Raghunathan TE, Stewart ST, Rosen AB. Explaining the slowdown in medical spending growth among the elderly, 1999-2012. Health Aff (Millwood). 2019;38(2):222-229. https://doi.org/10.1377/hlthaff.2018.05372
17. Ward MJ, Kripalani S, Zhu Y, et al. Incidence of emergency department visits for ST-elevation myocardial infarction in a recent six-year period in the United States. Am J Cardiol. 2015;115(2):167-170. https://doi.org/10.1016/j.amjcard.2014.10.020
18. Keohane LM, Gambrel RJ, Freed SS, Stevenson D, Buntin MB. Understanding trends in Medicare spending, 2007-2014. Health Serv Res. 2018;53(5):3507-3527. https://doi.org/10.1111/1475-6773.12845
19. Nuckols TK, Fingar KR, Barrett M, Steiner CA, Stocks C, Owens PL. The shifting landscape in utilization of inpatient, observation, and emergency department services across payers. J Hosp Med. 2017;12(6):443-446. https://doi.org/10.12788/jhm.2751
20. Poon SJ, Wallis CJ, Lai P, Podczerwinski L, Buntin MB. Medicare two-midnight rule accelerated shift to observation stays. Health Affairs. In press.
21. Sheehy AM, Kaiksow F, Powell WR, et al. The Hospital Readmissions Reduction Program and observation hospitalizations. J Hosp Med. 2021;16(7):409-411. https://doi.org/10.12788/jhm.3634
22. Culler SD, Parchman ML, Przybylski M. Factors related to potentially preventable hospitalizations among the elderly. Med Care. 1998;36(6):804-817. https://doi.org/10.1097/00005650-199806000-00004
23. Kozak LJ, Hall MJ, Owings MF. Trends in avoidable hospitalizations, 1980-1998. Health Aff (Millwood). 2001;20(2):225-232. https://doi.org/10.1377/hlthaff.20.2.225
24. Ouslander JG, Lamb G, Perloe M, et al. Potentially avoidable hospitalizations of nursing home residents: frequency, causes, and costs. J Am Geriatr Soc. 2010;58(4):627-635. https://doi.org/10.1111/j.1532-5415.2010.02768.x
25. Konetzka RT, Karon SL, Potter DEB. Users of Medicaid home and community-based services are especially vulnerable to costly avoidable hospital admissions. Health Aff (Millwood). 2012;31(6):1167-1175. https://doi.org/10.1377/hlthaff.2011.0902
26. Nyweide DJ, Anthony DL, Bynum JPW, et al. Continuity of care and the risk of preventable hospitalization in older adults. JAMA Intern Med. 2013;173(20):1879-1885. https://doi.org/10.1001/jamainternmed.2013.10059
27. Auerbach AD, Kripalani S, Vasilevskis EE, et al. Preventability and causes of readmissions in a national cohort of general medicine patients. JAMA Intern Med. 2016;176(4):484-493. https://doi.org/10.1001/jamainternmed.2015.7863
28. Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood). 2020;39(11):2010-2017. https://doi.org/10.1377/hlthaff.2020.00980
29. Nundy S, Patel KK. Hospital-at-home to support COVID-19 surge—time to bring down the walls? JAMA Health Forum. 2020;1(5):e200504. https://doi.org/10.1001/jamahealthforum.2020.0504
30. Keohane LM, Stevenson DG, Freed S, Thapa S, Stewart L, Buntin MB. Trends in Medicare fee-for-service spending growth for dual-eligible beneficiaries, 2007–15. Health Aff (Millwood). 2018;37(8):1265-1273. https://doi.org/10.1377/hlthaff.2018.0143
31. Freed M, Biniek JF, Damico A, Neuman T. Medicare Advantage in 2021: enrollment update and key trends. June 21, 2021. Accessed August 13, 2021. https://www.kff.org/medicare/issue-brief/medicare-advantage-in-2021-enrollment-update-and-key-trends/
32. Li Q, Rahman M, Gozalo P, Keohane LM, Gold MR, Trivedi AN. Regional variations: the use of hospitals, home health, and skilled nursing in traditional Medicare and Medicare Advantage. Health Aff (Millwood). 2018;37(8):1274-1281. https://doi.org/10.1377/hlthaff.2018.0147
© 2021 Society of Hospital Medicine
Things We Do for No Reason™: Routine Use of Corticosteroids for the Treatment of Anaphylaxis
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old man with coronary artery disease (CAD) undergoes hospital treatment for diverticulitis. He receives ketorolac for abdominal pain upon arrival to the medical ward despite his known allergy to nonsteroidal anti-inflammatory drugs. Fifteen minutes after administration, he develops lightheadedness and experiences swelling of his lips. On exam, he has tachycardia and a diffuse urticarial rash across his torso. The admitting physician prescribes methylprednisolone, diphenhydramine, and a liter bolus of normal saline for suspected anaphylaxis. Epinephrine is not administered for fear of precipitating an adverse cardiovascular event given the patient’s history of CAD.
BACKGROUND
Anaphylaxis, a rapid-onset generalized immunoglobulin E (IgE)–mediated hypersensitivity reaction, can lead to significant morbidity and mortality when not managed properly. Patients can present with anaphylaxis in heterogeneous ways. Fulfilling any one of three criteria establishes the diagnosis of anaphylaxis: (1) rapid onset of skin or mucosal symptoms complicated by either respiratory compromise or hypotension; (2) two or more symptoms involving the respiratory, mucosal, cardiovascular, or gastrointestinal systems following exposure to a likely allergen; and (3) reduced blood pressure in response to a known allergen.1 Up to 5% of the population experiences anaphylaxis in a lifetime. Medication and stinging insects account for the majority of anaphylactic reactions in adults, while food and insect stings commonly trigger it in children and adolescents.2
The majority of anaphylactic reactions, known as uniphasic or monophasic, occur rapidly as single episodes following exposure to a specific trigger and resolve within minutes to hours after treatment. Meanwhile, biphasic, or delayed-phase, anaphylaxis occurs when symptoms recur after an apparent resolution and in the absence of reexposure to the trigger. Symptoms restart within 1 to 72 hours after resolution of an initial anaphylaxis episode, with a median time to onset of 11 hours. Biphasic reactions occur in roughly 5% of patients with anaphylaxis.3
Epinephrine is the only recommended first-line medication for the treatment of anaphylaxis in all age groups.4 Epinephrine counteracts the cardiovascular and respiratory compromise induced by anaphylaxis through its α- and β-adrenergic activity and stabilizes mast cells.4 Early administration of intramuscular epinephrine decreases the need for additional interventions, reduces the likelihood of hospitalization, and is associated with reduced biphasic reactions.5-7 Paradoxically, patients receive corticosteroids more often than epinephrine for suspected anaphylaxis, despite no robust evidence for their efficacy.4,8,9
WHY YOU MIGHT THINK STEROIDS aRE HELPFUL FOR ANAPHYLAXIS
Corticosteroids act as potent anti-inflammatory medications that modulate mast-cell maturation, activation, and degranulation. Known to work primarily through downregulation of gene transcription responsible for cytokine, chemokine, and arachidonic acid production, their maximal anti-inflammatory effects manifest 2 to 6 hours after administration. Demonstrated efficacy in treating and preventing relapse of other inflammatory conditions, such as asthma and croup, may, in part, explain the widespread glucocorticoid use in anaphylaxis. Some believe that administration of corticosteroids may also help reduce the risk of biphasic or delayed-phase anaphylaxis.10
WHY THERE IS NO REASON TO PRESCRIBE CORTICOSTEROIDS FOR ANAPHYLAXIS
Based on their mechanism of action, corticosteroids do not exert any anti-inflammatory effects for several hours, regardless of their route of administration.10 In contrast, epinephrine exerts an almost immediate effect to increase cardiac output and vascular resistance, to reverse edema and bronchoconstriction, and to stabilize mast cells, preventing release of harmful chemokines and cytokines.4
The American Academy of Allergy, Asthma & Immunology (AAAAI) recommends early administration of epinephrine as the first-line treatment of anaphylaxis and emphasizes that evidence does not support routine corticosteroid use in the management of acute anaphylaxis or for prevention of biphasic reactions.9 To date, no randomized controlled trials have explored the role of corticosteroids in the treatment of acute anaphylaxis, although one is currently under way looking at whether dexamethasone has an impact on preventing biphasic reactions (Table).11
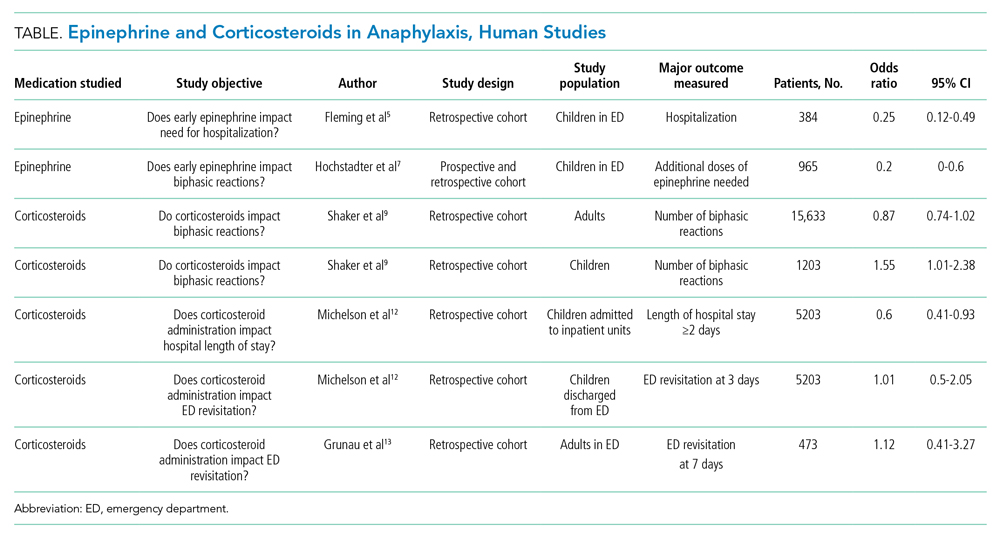
The AAAAI Joint Task Force on Practice Parameters (JTFPP) conducted a pooled analysis of observational studies that did not find a reduction in biphasic reactions in adult patients receiving corticosteroids (odds ratio [OR], 0.87; 95% CI, 0.74-1.02).9 Further, their analysis suggests an association with administration of corticosteroids and an increased likelihood of biphasic reactions in children (OR, 1.55; 95% CI, 1.01-2.38).9
An observational study in children across 35 hospitals demonstrated an association with corticosteroid administration and a reduced length of hospital stay for anaphylaxis, but the same study found no reduction in repeat emergency department (ED) visits within 72 hours.12 Similarly, a retrospective cohort study in adults did not find that corticosteroid administration reduced the 7-day risk of returning to the hospital.13 These studies highlight the importance of anticipatory guidance in both ED and hospital discharges for anaphylaxis since the literature does not provide data that corticosteroid administration reduces the likelihood of a biphasic course.
Long-term corticosteroids have well-known deleterious health effects. Recent evidence highlights the possible adverse events associated with even short courses of corticosteroids. A large case series from Taiwan containing 2,623,327 adults administered brief courses (<14 days) of corticosteroids demonstrated increased incidence of gastrointestinal bleeding, sepsis, and heart failure beginning 5 to 30 days after starting corticosteroid treatments for common medical conditions, with respective absolute risk increases of 10.3, 0.1, and 1 per 1000 patient-years for each condition.14 The same group of researchers found a nearly two-fold increased risk of sepsis, gastrointestinal bleeding, and pneumonia in a nearly 1 million children who had received corticosteroids within the previous year.15 Other common side effects of short-term corticosteroids include insomnia, agitation, mood disturbances, and hyperglycemia.
A growing body of evidence demonstrates that corticosteroids likely do not alter the natural disease course of anaphylaxis and carry increased risks of significant adverse events. The AAAAI recommends against the use of glucocorticoids as a first-line agent for anaphylaxis and suggests against the use of glucocorticoids to prevent biphasic reactions.9
WHEN TREATING WITH CORTICOSTEROIDS MAY BE INDICATED
The recent JTFPP analysis of observational studies demonstrated reduced hypersensitivity reactions to chemotherapeutics with corticosteroid premedication (OR, 0.49; 95% CI, 0.37-0.66). The AAAAI favors administration of corticosteroids to reduce the risk of anaphylactoid reactions—non–IgE-mediated mast cell activation—for some chemotherapeutic protocols.9
There is robust evidence regarding the benefits of corticosteroids in the treatment of asthma and upper-airway edema.16,17 Allergen exposures can precipitate significant bronchospasm in individuals with asthma and trigger an exacerbation. Although routine corticosteroid use for anaphylaxis in these populations has not been directly studied, their use as an adjunctive therapy may be beneficial if there is clinical evidence of bronchospasm or significant upper-airway edema.
WHAT YOU SHOULD DO INSTEAD
Rapid administration of epinephrine saves lives, reduces need for adjuvant treatments and hospitalization, and is associated with decreased risk of developing biphasic anaphylactic reactions (OR, 0.2; 95% CI, 0-0.6).5-7 Some clinicians are apprehensive about using epinephrine owing to fears related to negative side effects, particularly adverse cardiovascular events. Kawano et al18 performed a retrospective evaluation of 492 ED visits for anaphylaxis and found that epinephrine is administered less often in older patients (age >50 years); however, when administered intramuscularly, there was no significant difference in adverse cardiovascular events in this population compared with younger individuals. The study did demonstrate an increased rate of adverse cardiac events in older patients receiving intravenous epinephrine, an observation that the authors attributed partly to dosing errors that were reported more often with intravenous use.18
RECOMMENDATIONS
- Always promptly administer intramuscular epinephrine when treating anaphylaxis.
- Routine administration of corticosteroids in the treatment of anaphylaxis is not advised owing to insufficient data supporting their efficacy and potential for adverse events. Some patient populations may derive benefit from corticosteroids, including individuals with history of asthma exhibiting bronchospastic symptoms, individuals with significant upper-airway edema, and those undergoing certain chemotherapy regimens.
CONCLUSIONS
In the clinical vignette, the hospitalist withheld the first-line treatment for anaphylaxis, epinephrine. Without the support of evidence in the literature, patients receive corticosteroids and antihistamines more often than epinephrine for suspected anaphylaxis. No evidence supports the routine use of corticosteroids in the management of anaphylaxis or in the prevention of biphasic reactions. Further, recent research demonstrates significant adverse events are associated with even short courses of corticosteroids.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
1. Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391-397. https://doi.org/10.1016/j.jaci.2005.12.1303
2. Wood RA, Camargo CA Jr, Lieberman P, et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133(2):461-467. https://doi.org/10.1016/j.jaci.2013.08.016
3. Lee S, Bellolio MF, Hess EP, Erwin P, Murad MH, Campbell RL. Time of onset and predictors of biphasic anaphylactic reactions: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2015;3(3):408-16.e162. https://doi.org/10.1016/j.jaip.2014.12.010
4. Simons KJ, Simons FE. Epinephrine and its use in anaphylaxis: current issues. Curr Opin Allergy Clin Immunol. 2010;10(4):354-361. https://doi.org/10.1097/ACI.0b013e32833bc670
5. Fleming JT, Clark S, Camargo CA Jr, Rudders SA. Early treatment of food-induced anaphylaxis with epinephrine is associated with a lower risk of hospitalization. J Allergy Clin Immunol Pract. 2015;3(1):57-62. https://doi.org/10.1016/j.jaip.2014.07.004
6. Sundquist BK, Jose J, Pauze D, Pauze D, Wang H, Järvinen KM. Anaphylaxis risk factors for hospitalization and intensive care: a comparison between adults and children in an upstate New York emergency department. Allergy Asthma Proc. 2019;40(1):41-47. https://doi.org/10.2500/aap.2019.40.4189
7. Hochstadter E, Clarke A, De Schryver S, et al. Increasing visits for anaphylaxis and the benefits of early epinephrine administration: a 4-year study at a pediatric emergency department in Montreal, Canada. J Allergy Clin Immunol. 2016;137(6):1888-1890.e4. https://doi.org/10.1016/j.jaci.2016.02.016
8. Worm M, Moneret-Vautrin A, Scherer K, et al. First European data from the network of severe allergic reactions (NORA). Allergy. 2014;69(10):1397-1404. https://doi.org/10.1111/all.12475
9. Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis—a 2020 practice parameter update, systemic review, and Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020;145(4):1082-1123. https://doi.org/10.1016/j.jaci.2020.01.017
10. Liyanage CK, Galappatthy P, Seneviratne SL. Corticosteroids in management of anaphylaxis; a systematic review of evidence. Eur Ann Allergy Clin Immunol. 2017;49(5):196-207. https://doi.org/10.23822/EurAnnACI.1764-1489.15
11. Use of dexamethasone in prevention of the second phase of a biphasic reaction of anaphylaxis. ClinicalTrials.gov identifier: NCT03523221. Updated July 29, 2020. Accessed July 16, 2021. https://clinicaltrials.gov/ct2/show/NCT03523221
12. Michelson KA, Monuteaux MC, Neuman MI. Glucocorticoids and hospital length of stay for children with anaphylaxis: a retrospective study. J Pediatr. 2015;167(3):719-24.e243. https://doi.org/10.1016/j.jpeds.2015.05.033
13. Grunau BE, Wiens MO, Rowe BH, et al. Emergency department corticosteroid use for allergy or anaphylaxis is not associated with decreased relapses. Ann Emerg Med. 2015;66(4):381-389. https://doi.org/10.1016/j.annemergmed.2015.03.003
14. Yao TC, Huang YW, Chang SM, Tsai SY, Wu AC, Tsai HJ. Association between oral corticosteroid bursts and severe adverse events: a nationwide population-based cohort study. Ann Intern Med. 2020;173(5):325-330. https://doi.org/10.7326/M20-0432
15. Yao TC, Wang JY, Chang SM, et al. Association of oral corticosteroid bursts with severe adverse events in children. JAMA Pediatr. 2021;175(7):723-729. https://doi.org/10.1001/jamapediatrics.2021.0433
16. Rowe BH, Spooner CH, Ducharme FM, Bretzlaff JA, Bota GW. Corticosteroids for preventing relapse following acute exacerbations of asthma. Cochrane Database Syst Rev. 2007 Jul 18;(3):CD000195. https://doi.org/10.1002/14651858.CD000195.pub2
17. Gates A, Gates M, Vandermeer B, et al. Glucocorticoids for croup in children. Cochrane Database Syst Rev. 2018;8(8):CD001955. https://doi.org/10.1002/14651858.CD001955.pub4
18. Kawano T, Scheuermeyer FX, Stenstrom R, Rowe BH, Grafstein E, Grunau B. Epinephrine use in older patients with anaphylaxis: clinical outcomes and cardiovascular complications. Resuscitation. 2017;112:53-58. https://doi.org/10.1016/j.resuscitation.2016.12.020
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old man with coronary artery disease (CAD) undergoes hospital treatment for diverticulitis. He receives ketorolac for abdominal pain upon arrival to the medical ward despite his known allergy to nonsteroidal anti-inflammatory drugs. Fifteen minutes after administration, he develops lightheadedness and experiences swelling of his lips. On exam, he has tachycardia and a diffuse urticarial rash across his torso. The admitting physician prescribes methylprednisolone, diphenhydramine, and a liter bolus of normal saline for suspected anaphylaxis. Epinephrine is not administered for fear of precipitating an adverse cardiovascular event given the patient’s history of CAD.
BACKGROUND
Anaphylaxis, a rapid-onset generalized immunoglobulin E (IgE)–mediated hypersensitivity reaction, can lead to significant morbidity and mortality when not managed properly. Patients can present with anaphylaxis in heterogeneous ways. Fulfilling any one of three criteria establishes the diagnosis of anaphylaxis: (1) rapid onset of skin or mucosal symptoms complicated by either respiratory compromise or hypotension; (2) two or more symptoms involving the respiratory, mucosal, cardiovascular, or gastrointestinal systems following exposure to a likely allergen; and (3) reduced blood pressure in response to a known allergen.1 Up to 5% of the population experiences anaphylaxis in a lifetime. Medication and stinging insects account for the majority of anaphylactic reactions in adults, while food and insect stings commonly trigger it in children and adolescents.2
The majority of anaphylactic reactions, known as uniphasic or monophasic, occur rapidly as single episodes following exposure to a specific trigger and resolve within minutes to hours after treatment. Meanwhile, biphasic, or delayed-phase, anaphylaxis occurs when symptoms recur after an apparent resolution and in the absence of reexposure to the trigger. Symptoms restart within 1 to 72 hours after resolution of an initial anaphylaxis episode, with a median time to onset of 11 hours. Biphasic reactions occur in roughly 5% of patients with anaphylaxis.3
Epinephrine is the only recommended first-line medication for the treatment of anaphylaxis in all age groups.4 Epinephrine counteracts the cardiovascular and respiratory compromise induced by anaphylaxis through its α- and β-adrenergic activity and stabilizes mast cells.4 Early administration of intramuscular epinephrine decreases the need for additional interventions, reduces the likelihood of hospitalization, and is associated with reduced biphasic reactions.5-7 Paradoxically, patients receive corticosteroids more often than epinephrine for suspected anaphylaxis, despite no robust evidence for their efficacy.4,8,9
WHY YOU MIGHT THINK STEROIDS aRE HELPFUL FOR ANAPHYLAXIS
Corticosteroids act as potent anti-inflammatory medications that modulate mast-cell maturation, activation, and degranulation. Known to work primarily through downregulation of gene transcription responsible for cytokine, chemokine, and arachidonic acid production, their maximal anti-inflammatory effects manifest 2 to 6 hours after administration. Demonstrated efficacy in treating and preventing relapse of other inflammatory conditions, such as asthma and croup, may, in part, explain the widespread glucocorticoid use in anaphylaxis. Some believe that administration of corticosteroids may also help reduce the risk of biphasic or delayed-phase anaphylaxis.10
WHY THERE IS NO REASON TO PRESCRIBE CORTICOSTEROIDS FOR ANAPHYLAXIS
Based on their mechanism of action, corticosteroids do not exert any anti-inflammatory effects for several hours, regardless of their route of administration.10 In contrast, epinephrine exerts an almost immediate effect to increase cardiac output and vascular resistance, to reverse edema and bronchoconstriction, and to stabilize mast cells, preventing release of harmful chemokines and cytokines.4
The American Academy of Allergy, Asthma & Immunology (AAAAI) recommends early administration of epinephrine as the first-line treatment of anaphylaxis and emphasizes that evidence does not support routine corticosteroid use in the management of acute anaphylaxis or for prevention of biphasic reactions.9 To date, no randomized controlled trials have explored the role of corticosteroids in the treatment of acute anaphylaxis, although one is currently under way looking at whether dexamethasone has an impact on preventing biphasic reactions (Table).11

The AAAAI Joint Task Force on Practice Parameters (JTFPP) conducted a pooled analysis of observational studies that did not find a reduction in biphasic reactions in adult patients receiving corticosteroids (odds ratio [OR], 0.87; 95% CI, 0.74-1.02).9 Further, their analysis suggests an association with administration of corticosteroids and an increased likelihood of biphasic reactions in children (OR, 1.55; 95% CI, 1.01-2.38).9
An observational study in children across 35 hospitals demonstrated an association with corticosteroid administration and a reduced length of hospital stay for anaphylaxis, but the same study found no reduction in repeat emergency department (ED) visits within 72 hours.12 Similarly, a retrospective cohort study in adults did not find that corticosteroid administration reduced the 7-day risk of returning to the hospital.13 These studies highlight the importance of anticipatory guidance in both ED and hospital discharges for anaphylaxis since the literature does not provide data that corticosteroid administration reduces the likelihood of a biphasic course.
Long-term corticosteroids have well-known deleterious health effects. Recent evidence highlights the possible adverse events associated with even short courses of corticosteroids. A large case series from Taiwan containing 2,623,327 adults administered brief courses (<14 days) of corticosteroids demonstrated increased incidence of gastrointestinal bleeding, sepsis, and heart failure beginning 5 to 30 days after starting corticosteroid treatments for common medical conditions, with respective absolute risk increases of 10.3, 0.1, and 1 per 1000 patient-years for each condition.14 The same group of researchers found a nearly two-fold increased risk of sepsis, gastrointestinal bleeding, and pneumonia in a nearly 1 million children who had received corticosteroids within the previous year.15 Other common side effects of short-term corticosteroids include insomnia, agitation, mood disturbances, and hyperglycemia.
A growing body of evidence demonstrates that corticosteroids likely do not alter the natural disease course of anaphylaxis and carry increased risks of significant adverse events. The AAAAI recommends against the use of glucocorticoids as a first-line agent for anaphylaxis and suggests against the use of glucocorticoids to prevent biphasic reactions.9
WHEN TREATING WITH CORTICOSTEROIDS MAY BE INDICATED
The recent JTFPP analysis of observational studies demonstrated reduced hypersensitivity reactions to chemotherapeutics with corticosteroid premedication (OR, 0.49; 95% CI, 0.37-0.66). The AAAAI favors administration of corticosteroids to reduce the risk of anaphylactoid reactions—non–IgE-mediated mast cell activation—for some chemotherapeutic protocols.9
There is robust evidence regarding the benefits of corticosteroids in the treatment of asthma and upper-airway edema.16,17 Allergen exposures can precipitate significant bronchospasm in individuals with asthma and trigger an exacerbation. Although routine corticosteroid use for anaphylaxis in these populations has not been directly studied, their use as an adjunctive therapy may be beneficial if there is clinical evidence of bronchospasm or significant upper-airway edema.
WHAT YOU SHOULD DO INSTEAD
Rapid administration of epinephrine saves lives, reduces need for adjuvant treatments and hospitalization, and is associated with decreased risk of developing biphasic anaphylactic reactions (OR, 0.2; 95% CI, 0-0.6).5-7 Some clinicians are apprehensive about using epinephrine owing to fears related to negative side effects, particularly adverse cardiovascular events. Kawano et al18 performed a retrospective evaluation of 492 ED visits for anaphylaxis and found that epinephrine is administered less often in older patients (age >50 years); however, when administered intramuscularly, there was no significant difference in adverse cardiovascular events in this population compared with younger individuals. The study did demonstrate an increased rate of adverse cardiac events in older patients receiving intravenous epinephrine, an observation that the authors attributed partly to dosing errors that were reported more often with intravenous use.18
RECOMMENDATIONS
- Always promptly administer intramuscular epinephrine when treating anaphylaxis.
- Routine administration of corticosteroids in the treatment of anaphylaxis is not advised owing to insufficient data supporting their efficacy and potential for adverse events. Some patient populations may derive benefit from corticosteroids, including individuals with history of asthma exhibiting bronchospastic symptoms, individuals with significant upper-airway edema, and those undergoing certain chemotherapy regimens.
CONCLUSIONS
In the clinical vignette, the hospitalist withheld the first-line treatment for anaphylaxis, epinephrine. Without the support of evidence in the literature, patients receive corticosteroids and antihistamines more often than epinephrine for suspected anaphylaxis. No evidence supports the routine use of corticosteroids in the management of anaphylaxis or in the prevention of biphasic reactions. Further, recent research demonstrates significant adverse events are associated with even short courses of corticosteroids.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old man with coronary artery disease (CAD) undergoes hospital treatment for diverticulitis. He receives ketorolac for abdominal pain upon arrival to the medical ward despite his known allergy to nonsteroidal anti-inflammatory drugs. Fifteen minutes after administration, he develops lightheadedness and experiences swelling of his lips. On exam, he has tachycardia and a diffuse urticarial rash across his torso. The admitting physician prescribes methylprednisolone, diphenhydramine, and a liter bolus of normal saline for suspected anaphylaxis. Epinephrine is not administered for fear of precipitating an adverse cardiovascular event given the patient’s history of CAD.
BACKGROUND
Anaphylaxis, a rapid-onset generalized immunoglobulin E (IgE)–mediated hypersensitivity reaction, can lead to significant morbidity and mortality when not managed properly. Patients can present with anaphylaxis in heterogeneous ways. Fulfilling any one of three criteria establishes the diagnosis of anaphylaxis: (1) rapid onset of skin or mucosal symptoms complicated by either respiratory compromise or hypotension; (2) two or more symptoms involving the respiratory, mucosal, cardiovascular, or gastrointestinal systems following exposure to a likely allergen; and (3) reduced blood pressure in response to a known allergen.1 Up to 5% of the population experiences anaphylaxis in a lifetime. Medication and stinging insects account for the majority of anaphylactic reactions in adults, while food and insect stings commonly trigger it in children and adolescents.2
The majority of anaphylactic reactions, known as uniphasic or monophasic, occur rapidly as single episodes following exposure to a specific trigger and resolve within minutes to hours after treatment. Meanwhile, biphasic, or delayed-phase, anaphylaxis occurs when symptoms recur after an apparent resolution and in the absence of reexposure to the trigger. Symptoms restart within 1 to 72 hours after resolution of an initial anaphylaxis episode, with a median time to onset of 11 hours. Biphasic reactions occur in roughly 5% of patients with anaphylaxis.3
Epinephrine is the only recommended first-line medication for the treatment of anaphylaxis in all age groups.4 Epinephrine counteracts the cardiovascular and respiratory compromise induced by anaphylaxis through its α- and β-adrenergic activity and stabilizes mast cells.4 Early administration of intramuscular epinephrine decreases the need for additional interventions, reduces the likelihood of hospitalization, and is associated with reduced biphasic reactions.5-7 Paradoxically, patients receive corticosteroids more often than epinephrine for suspected anaphylaxis, despite no robust evidence for their efficacy.4,8,9
WHY YOU MIGHT THINK STEROIDS aRE HELPFUL FOR ANAPHYLAXIS
Corticosteroids act as potent anti-inflammatory medications that modulate mast-cell maturation, activation, and degranulation. Known to work primarily through downregulation of gene transcription responsible for cytokine, chemokine, and arachidonic acid production, their maximal anti-inflammatory effects manifest 2 to 6 hours after administration. Demonstrated efficacy in treating and preventing relapse of other inflammatory conditions, such as asthma and croup, may, in part, explain the widespread glucocorticoid use in anaphylaxis. Some believe that administration of corticosteroids may also help reduce the risk of biphasic or delayed-phase anaphylaxis.10
WHY THERE IS NO REASON TO PRESCRIBE CORTICOSTEROIDS FOR ANAPHYLAXIS
Based on their mechanism of action, corticosteroids do not exert any anti-inflammatory effects for several hours, regardless of their route of administration.10 In contrast, epinephrine exerts an almost immediate effect to increase cardiac output and vascular resistance, to reverse edema and bronchoconstriction, and to stabilize mast cells, preventing release of harmful chemokines and cytokines.4
The American Academy of Allergy, Asthma & Immunology (AAAAI) recommends early administration of epinephrine as the first-line treatment of anaphylaxis and emphasizes that evidence does not support routine corticosteroid use in the management of acute anaphylaxis or for prevention of biphasic reactions.9 To date, no randomized controlled trials have explored the role of corticosteroids in the treatment of acute anaphylaxis, although one is currently under way looking at whether dexamethasone has an impact on preventing biphasic reactions (Table).11

The AAAAI Joint Task Force on Practice Parameters (JTFPP) conducted a pooled analysis of observational studies that did not find a reduction in biphasic reactions in adult patients receiving corticosteroids (odds ratio [OR], 0.87; 95% CI, 0.74-1.02).9 Further, their analysis suggests an association with administration of corticosteroids and an increased likelihood of biphasic reactions in children (OR, 1.55; 95% CI, 1.01-2.38).9
An observational study in children across 35 hospitals demonstrated an association with corticosteroid administration and a reduced length of hospital stay for anaphylaxis, but the same study found no reduction in repeat emergency department (ED) visits within 72 hours.12 Similarly, a retrospective cohort study in adults did not find that corticosteroid administration reduced the 7-day risk of returning to the hospital.13 These studies highlight the importance of anticipatory guidance in both ED and hospital discharges for anaphylaxis since the literature does not provide data that corticosteroid administration reduces the likelihood of a biphasic course.
Long-term corticosteroids have well-known deleterious health effects. Recent evidence highlights the possible adverse events associated with even short courses of corticosteroids. A large case series from Taiwan containing 2,623,327 adults administered brief courses (<14 days) of corticosteroids demonstrated increased incidence of gastrointestinal bleeding, sepsis, and heart failure beginning 5 to 30 days after starting corticosteroid treatments for common medical conditions, with respective absolute risk increases of 10.3, 0.1, and 1 per 1000 patient-years for each condition.14 The same group of researchers found a nearly two-fold increased risk of sepsis, gastrointestinal bleeding, and pneumonia in a nearly 1 million children who had received corticosteroids within the previous year.15 Other common side effects of short-term corticosteroids include insomnia, agitation, mood disturbances, and hyperglycemia.
A growing body of evidence demonstrates that corticosteroids likely do not alter the natural disease course of anaphylaxis and carry increased risks of significant adverse events. The AAAAI recommends against the use of glucocorticoids as a first-line agent for anaphylaxis and suggests against the use of glucocorticoids to prevent biphasic reactions.9
WHEN TREATING WITH CORTICOSTEROIDS MAY BE INDICATED
The recent JTFPP analysis of observational studies demonstrated reduced hypersensitivity reactions to chemotherapeutics with corticosteroid premedication (OR, 0.49; 95% CI, 0.37-0.66). The AAAAI favors administration of corticosteroids to reduce the risk of anaphylactoid reactions—non–IgE-mediated mast cell activation—for some chemotherapeutic protocols.9
There is robust evidence regarding the benefits of corticosteroids in the treatment of asthma and upper-airway edema.16,17 Allergen exposures can precipitate significant bronchospasm in individuals with asthma and trigger an exacerbation. Although routine corticosteroid use for anaphylaxis in these populations has not been directly studied, their use as an adjunctive therapy may be beneficial if there is clinical evidence of bronchospasm or significant upper-airway edema.
WHAT YOU SHOULD DO INSTEAD
Rapid administration of epinephrine saves lives, reduces need for adjuvant treatments and hospitalization, and is associated with decreased risk of developing biphasic anaphylactic reactions (OR, 0.2; 95% CI, 0-0.6).5-7 Some clinicians are apprehensive about using epinephrine owing to fears related to negative side effects, particularly adverse cardiovascular events. Kawano et al18 performed a retrospective evaluation of 492 ED visits for anaphylaxis and found that epinephrine is administered less often in older patients (age >50 years); however, when administered intramuscularly, there was no significant difference in adverse cardiovascular events in this population compared with younger individuals. The study did demonstrate an increased rate of adverse cardiac events in older patients receiving intravenous epinephrine, an observation that the authors attributed partly to dosing errors that were reported more often with intravenous use.18
RECOMMENDATIONS
- Always promptly administer intramuscular epinephrine when treating anaphylaxis.
- Routine administration of corticosteroids in the treatment of anaphylaxis is not advised owing to insufficient data supporting their efficacy and potential for adverse events. Some patient populations may derive benefit from corticosteroids, including individuals with history of asthma exhibiting bronchospastic symptoms, individuals with significant upper-airway edema, and those undergoing certain chemotherapy regimens.
CONCLUSIONS
In the clinical vignette, the hospitalist withheld the first-line treatment for anaphylaxis, epinephrine. Without the support of evidence in the literature, patients receive corticosteroids and antihistamines more often than epinephrine for suspected anaphylaxis. No evidence supports the routine use of corticosteroids in the management of anaphylaxis or in the prevention of biphasic reactions. Further, recent research demonstrates significant adverse events are associated with even short courses of corticosteroids.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
1. Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391-397. https://doi.org/10.1016/j.jaci.2005.12.1303
2. Wood RA, Camargo CA Jr, Lieberman P, et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133(2):461-467. https://doi.org/10.1016/j.jaci.2013.08.016
3. Lee S, Bellolio MF, Hess EP, Erwin P, Murad MH, Campbell RL. Time of onset and predictors of biphasic anaphylactic reactions: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2015;3(3):408-16.e162. https://doi.org/10.1016/j.jaip.2014.12.010
4. Simons KJ, Simons FE. Epinephrine and its use in anaphylaxis: current issues. Curr Opin Allergy Clin Immunol. 2010;10(4):354-361. https://doi.org/10.1097/ACI.0b013e32833bc670
5. Fleming JT, Clark S, Camargo CA Jr, Rudders SA. Early treatment of food-induced anaphylaxis with epinephrine is associated with a lower risk of hospitalization. J Allergy Clin Immunol Pract. 2015;3(1):57-62. https://doi.org/10.1016/j.jaip.2014.07.004
6. Sundquist BK, Jose J, Pauze D, Pauze D, Wang H, Järvinen KM. Anaphylaxis risk factors for hospitalization and intensive care: a comparison between adults and children in an upstate New York emergency department. Allergy Asthma Proc. 2019;40(1):41-47. https://doi.org/10.2500/aap.2019.40.4189
7. Hochstadter E, Clarke A, De Schryver S, et al. Increasing visits for anaphylaxis and the benefits of early epinephrine administration: a 4-year study at a pediatric emergency department in Montreal, Canada. J Allergy Clin Immunol. 2016;137(6):1888-1890.e4. https://doi.org/10.1016/j.jaci.2016.02.016
8. Worm M, Moneret-Vautrin A, Scherer K, et al. First European data from the network of severe allergic reactions (NORA). Allergy. 2014;69(10):1397-1404. https://doi.org/10.1111/all.12475
9. Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis—a 2020 practice parameter update, systemic review, and Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020;145(4):1082-1123. https://doi.org/10.1016/j.jaci.2020.01.017
10. Liyanage CK, Galappatthy P, Seneviratne SL. Corticosteroids in management of anaphylaxis; a systematic review of evidence. Eur Ann Allergy Clin Immunol. 2017;49(5):196-207. https://doi.org/10.23822/EurAnnACI.1764-1489.15
11. Use of dexamethasone in prevention of the second phase of a biphasic reaction of anaphylaxis. ClinicalTrials.gov identifier: NCT03523221. Updated July 29, 2020. Accessed July 16, 2021. https://clinicaltrials.gov/ct2/show/NCT03523221
12. Michelson KA, Monuteaux MC, Neuman MI. Glucocorticoids and hospital length of stay for children with anaphylaxis: a retrospective study. J Pediatr. 2015;167(3):719-24.e243. https://doi.org/10.1016/j.jpeds.2015.05.033
13. Grunau BE, Wiens MO, Rowe BH, et al. Emergency department corticosteroid use for allergy or anaphylaxis is not associated with decreased relapses. Ann Emerg Med. 2015;66(4):381-389. https://doi.org/10.1016/j.annemergmed.2015.03.003
14. Yao TC, Huang YW, Chang SM, Tsai SY, Wu AC, Tsai HJ. Association between oral corticosteroid bursts and severe adverse events: a nationwide population-based cohort study. Ann Intern Med. 2020;173(5):325-330. https://doi.org/10.7326/M20-0432
15. Yao TC, Wang JY, Chang SM, et al. Association of oral corticosteroid bursts with severe adverse events in children. JAMA Pediatr. 2021;175(7):723-729. https://doi.org/10.1001/jamapediatrics.2021.0433
16. Rowe BH, Spooner CH, Ducharme FM, Bretzlaff JA, Bota GW. Corticosteroids for preventing relapse following acute exacerbations of asthma. Cochrane Database Syst Rev. 2007 Jul 18;(3):CD000195. https://doi.org/10.1002/14651858.CD000195.pub2
17. Gates A, Gates M, Vandermeer B, et al. Glucocorticoids for croup in children. Cochrane Database Syst Rev. 2018;8(8):CD001955. https://doi.org/10.1002/14651858.CD001955.pub4
18. Kawano T, Scheuermeyer FX, Stenstrom R, Rowe BH, Grafstein E, Grunau B. Epinephrine use in older patients with anaphylaxis: clinical outcomes and cardiovascular complications. Resuscitation. 2017;112:53-58. https://doi.org/10.1016/j.resuscitation.2016.12.020
1. Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391-397. https://doi.org/10.1016/j.jaci.2005.12.1303
2. Wood RA, Camargo CA Jr, Lieberman P, et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133(2):461-467. https://doi.org/10.1016/j.jaci.2013.08.016
3. Lee S, Bellolio MF, Hess EP, Erwin P, Murad MH, Campbell RL. Time of onset and predictors of biphasic anaphylactic reactions: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2015;3(3):408-16.e162. https://doi.org/10.1016/j.jaip.2014.12.010
4. Simons KJ, Simons FE. Epinephrine and its use in anaphylaxis: current issues. Curr Opin Allergy Clin Immunol. 2010;10(4):354-361. https://doi.org/10.1097/ACI.0b013e32833bc670
5. Fleming JT, Clark S, Camargo CA Jr, Rudders SA. Early treatment of food-induced anaphylaxis with epinephrine is associated with a lower risk of hospitalization. J Allergy Clin Immunol Pract. 2015;3(1):57-62. https://doi.org/10.1016/j.jaip.2014.07.004
6. Sundquist BK, Jose J, Pauze D, Pauze D, Wang H, Järvinen KM. Anaphylaxis risk factors for hospitalization and intensive care: a comparison between adults and children in an upstate New York emergency department. Allergy Asthma Proc. 2019;40(1):41-47. https://doi.org/10.2500/aap.2019.40.4189
7. Hochstadter E, Clarke A, De Schryver S, et al. Increasing visits for anaphylaxis and the benefits of early epinephrine administration: a 4-year study at a pediatric emergency department in Montreal, Canada. J Allergy Clin Immunol. 2016;137(6):1888-1890.e4. https://doi.org/10.1016/j.jaci.2016.02.016
8. Worm M, Moneret-Vautrin A, Scherer K, et al. First European data from the network of severe allergic reactions (NORA). Allergy. 2014;69(10):1397-1404. https://doi.org/10.1111/all.12475
9. Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis—a 2020 practice parameter update, systemic review, and Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020;145(4):1082-1123. https://doi.org/10.1016/j.jaci.2020.01.017
10. Liyanage CK, Galappatthy P, Seneviratne SL. Corticosteroids in management of anaphylaxis; a systematic review of evidence. Eur Ann Allergy Clin Immunol. 2017;49(5):196-207. https://doi.org/10.23822/EurAnnACI.1764-1489.15
11. Use of dexamethasone in prevention of the second phase of a biphasic reaction of anaphylaxis. ClinicalTrials.gov identifier: NCT03523221. Updated July 29, 2020. Accessed July 16, 2021. https://clinicaltrials.gov/ct2/show/NCT03523221
12. Michelson KA, Monuteaux MC, Neuman MI. Glucocorticoids and hospital length of stay for children with anaphylaxis: a retrospective study. J Pediatr. 2015;167(3):719-24.e243. https://doi.org/10.1016/j.jpeds.2015.05.033
13. Grunau BE, Wiens MO, Rowe BH, et al. Emergency department corticosteroid use for allergy or anaphylaxis is not associated with decreased relapses. Ann Emerg Med. 2015;66(4):381-389. https://doi.org/10.1016/j.annemergmed.2015.03.003
14. Yao TC, Huang YW, Chang SM, Tsai SY, Wu AC, Tsai HJ. Association between oral corticosteroid bursts and severe adverse events: a nationwide population-based cohort study. Ann Intern Med. 2020;173(5):325-330. https://doi.org/10.7326/M20-0432
15. Yao TC, Wang JY, Chang SM, et al. Association of oral corticosteroid bursts with severe adverse events in children. JAMA Pediatr. 2021;175(7):723-729. https://doi.org/10.1001/jamapediatrics.2021.0433
16. Rowe BH, Spooner CH, Ducharme FM, Bretzlaff JA, Bota GW. Corticosteroids for preventing relapse following acute exacerbations of asthma. Cochrane Database Syst Rev. 2007 Jul 18;(3):CD000195. https://doi.org/10.1002/14651858.CD000195.pub2
17. Gates A, Gates M, Vandermeer B, et al. Glucocorticoids for croup in children. Cochrane Database Syst Rev. 2018;8(8):CD001955. https://doi.org/10.1002/14651858.CD001955.pub4
18. Kawano T, Scheuermeyer FX, Stenstrom R, Rowe BH, Grafstein E, Grunau B. Epinephrine use in older patients with anaphylaxis: clinical outcomes and cardiovascular complications. Resuscitation. 2017;112:53-58. https://doi.org/10.1016/j.resuscitation.2016.12.020
© 2021 Society of Hospital Medicine
Policy in Clinical Practice: Emergency Medicaid and Access to Allogeneic Stem Cell Transplant for Undocumented Immigrants
Clinical Scenario
Juan, a 50-year-old man with acute myeloid leukemia (AML), sat on the edge of his bed, dejected. Juan’s leukemia had relapsed for a third time, and he was low on options and optimism. Originally from Mexico, he had made the journey to Colorado to work as a mechanic and care for his disabled son. Like millions of other individuals in the United States, he did not obtain a visa and had no affordable options for health insurance. For nearly a decade, that had seemed not to matter, until he became ill. Initially presenting to the emergency department with fatigue and night sweats, Juan was diagnosed with poor-risk AML and underwent emergent induction chemotherapy reimbursed under Emergency Medicaid (Table). Just when his bone marrow biopsy showed remission, however, Juan was told there was no chance to cure him, as his documentation status precluded him from receiving the next recommended therapy: stem cell transplant (SCT). Without transplant, Juan’s leukemia relapsed within a few months. He decided to undergo all the salvage chemotherapy that was offered, worrying about how his son would survive without his father.
Background and History
For the patient with a new cancer diagnosis, a difference in immigration status may be the difference between life and death. Undocumented immigrants are excluded from federally funded benefits, including those offered under Medicare, most Medicaid programs, and the Patient Protection and Affordable Care Act (Table).1 The nearly 11 million undocumented immigrants residing in the United States are integral to the workforce and economy. Although they pay taxes that fund Medicaid, contributing approximately $11.7 billion nationally in 2017, undocumented immigrants are ineligible to benefit from such programs.2 The inequity of this policy is highlighted by Juan, an undocumented immigrant presenting with a new diagnosis of AML.
The Emergency Medical Treatment and Active Labor Act (EMTALA) is a 1986 federal law which mandates that patients who present to the hospital with an emergency medical condition receive appropriate evaluation and stabilizing treatment. An emergency condition is defined as “manifesting itself by acute symptoms of sufficient severity … such that the absence of immediate medical attention could reasonably be expected to result in (A) placing the patient’s health in serious jeopardy; (B) serious impairment to bodily functions; or (C) serious dysfunction of any bodily organ or part” (Table).3,4 The Centers for Medicare & Medicaid manual restates the EMTALA definition and notes that services for an emergency medical condition cannot include care related to organ transplantation. Most state Emergency Medicaid programs have adopted the federal definition of what constitutes a medical emergency.5 As a result, undocumented individuals who qualify for Medicaid benefits but who do not meet citizenship requirements are eligible to “receive Medical Assistance benefits for emergency medical care only.”3
Similar to our patient Juan, individuals who initially present with an acute leukemia would be eligible for induction chemotherapy, as blast crisis is imminently fatal. Once in remission, however, standard-of-care therapy for patients without disqualifying comorbidities, depending on cytogenetic disease phenotypes, recommends the only current potential cure: allogeneic SCT, a treatment that was far from routine practice at the time EMTALA was enacted.6 When preparing for transplant, a patient is stable and no longer fits EMTALA’s “emergency” criteria, even though their health is still in “serious jeopardy,” as their cancer has been incompletely treated. Because most state Emergency Medicaid programs adopt the federal definition of an emergency medical condition, the cure is out of reach.
Policy in Clinical Practice
This policy requires clinicians to deviate from the usual standard of care and results in inferior outcomes. For AML patients in the poor-risk category, allogeneic SCT is recommended following induction chemotherapy.7 The risk of relapse is 30% to 40% if consolidation therapy includes SCT, vs 70% to 80% if treated with chemotherapeutic consolidation alone.6 AML patients in the intermediate-, and sometimes even favorable- risk categories, have been shown to benefit from allogeneic SCT as well, with risk of relapse half that of a patient who undergoes consolidation without transplant. Undocumented individuals with AML are therefore resigned to inadequate cancer treatment, including lifelong salvage chemotherapy, and have a substantially decreased chance of achieving sustained remission.6 Furthermore, providing inequitable care for undocumented patients with other medical conditions, such as end-stage kidney disease (ESKD), has been associated with inferior patient-reported outcomes, higher mortality and hospital costs, and clinician burnout. In many states, undocumented immigrants with ESKD rely on emergency dialysis (dialysis made available only after presenting critically ill to an emergency department). In 2019, Colorado’s Medicaid agency opted to include ESKD as a qualifying condition for Emergency Medicaid, thereby expanding access to scheduled dialysis. This led to improved patient quality of life, a decreased emotional toll on patients and clinicians, and reduced costs.8,9
Economic Considerations
Policy discussions must consider cost. The average cost of allogeneic SCT in the United States was approximately $226,000 in 2018, which is often compared to the cost of managing a patient with refractory disease who does not receive transplant.10 This study reported a cost of active disease without transplant, including chemotherapy and hospitalizations, of approximately $69,000, plus terminal care costs of nearly $89,000; at a total of $158,000, this comes out to $68,000 less than SCT.10 This cost savings, however, results in a patient’s death rather than an up to 85% chance of long-term, relapse-free survival.6
To more completely capture the relationship between the healthcare value and cost-effectiveness of SCT, a second study calculated the incremental cost-effectiveness ratio (ICER) of transplantation in acute leukemias in the first 100 days post transplant, including management of complications, such as hospitalization, acute graft-versus-host disease (GVHD), infection, and blood product transfusions. ICER represents the economic value of an intervention compared to an alternative, calculated as cost per quality-adjusted life years. The ICER of SCT compared to no transplant is $16,346 to $34,360, depending on type of transplant and conditioning regimen.11 An ICER of less than $50,000 is considered an acceptable expense for the value achieved—in this case, a significant opportunity for cure. This finding supports SCT, including management of complications, as an economically valuable intervention. Furthermore, if a sustained remission is achieved with SCT, this difference in expense buys the individual patient potentially decades of productivity to contribute back into society and the economy. According to the National Bureau of Economic Research, undocumented workers as a whole contribute $5 trillion to the US Gross Domestic Product over a 10-year period, or about $45,000 per worker per year.12 According to the costs cited, curing a single undocumented worker with acute leukemia via SCT and allowing them to return to work would lead to a return on investment in less than 2 years. If the goal is high-quality, high-value, equitable care, it is logical to spend the money upfront and allow all patients the best chance for recovery.
One might suggest that patients instead receive treatment in their country of origin. This proposition, however, is often unrealistic. Latin American countries, for example, lack access to many standard-of-care cancer treatments available domestically. In Mexico, SCT is only available at a single facility in Mexico City, which is unable to track outcomes.13 The mortality-to-incidence ratio for cancer, a marker of availability of effective treatment, for Latin America is 0.48, substantially inferior to that of the United States (0.29).14 Importantly, almost two thirds of undocumented immigrants in the United States have lived in the country for 10 or more years, and 43% are parents of minor children, an increasing proportion of whom are American citizens.15 This highlights the impracticality of these individuals returning to their country of origin for treatment.
Commentary and Recommendations
Medicaid laws in several states have made it possible for undocumented immigrants to receive access to standard-of-care therapies. Washington and California have included provisions that enable undocumented immigrants to receive allogeneic SCT if they are otherwise medically eligible. In the course of this policy change, legal arguments from the California Court of Appeals expressed that the language of the law was not intended to deny lifesaving treatment to an individual.16 California’s Emergency Medicaid policy is comparable to that of other states, but because the courts considered SCT a “continuation of medically necessary inpatient hospital services … directly related to the emergency” for which the patient initially presented, they concluded that it could be covered under California Medicaid. Despite covering SCT for undocumented immigrants, California maintains lower costs for those patients compared to US citizens on Medicaid while providing evidence-based cancer care.17 This exemplifies sustainable and equitable healthcare policy for the rest of the nation.
A proposed change in policy could occur at either the federal or state level. One option would be to follow the example set by the State of Washington. Under Emergency Medicaid, Washington modified qualifying conditions to include “emergency room care, inpatient admission, or outpatient surgery; a cancer treatment plan; dialysis treatment; anti-rejection medication for an organ transplant” and long-term care services.18 Federal policy reform for undocumented immigrants would also improve access to care. The US Citizenship Act of 2021, introduced to the House of Representatives in February 2021, offers a path to citizenship for undocumented immigrants, ultimately allowing for undocumented individuals to be eligible for the same programs as citizens, though after a period of up to 8 years.19 More immediate revisions of qualifying conditions under state Emergency Medicaid programs, coupled with a path to citizenship, would make significant progress towards reducing structural health inequities. Such policy change would also have broader implications. Three quarters of undocumented immigrants in the United States originate from Mexico, Central America, and South America, and the incidence rate of AML for Latinx individuals is 3.6 per 100,000, a figure which can be extrapolated to an estimated 380 cases per year in the US undocumented population.20-22 In addition to benefiting patients with acute leukemias, the proposed policy change would also benefit numerous others who are frequently hospitalized for acute decompensations of chronic conditions, including congestive heart failure, liver disease, ESKD, and chronic lung conditions. Enabling follow-up care for these diseases under Emergency Medicaid would likewise be expected to reduce costs and improve both quality of care and patient-centered and clinical outcomes.
What Should I Tell My Patient?
Hospitalists frequently care for undocumented immigrants with acute leukemias because the hospital can only be reimbursed by Emergency Medicaid when a patient is admitted to the hospital. Patients may ask about what they can expect in the course of their illness and, while details may be left to the oncologist, hospitalists will be faced with responding to many of these questions. Clinicians at our institution hold honest conversations with patients like Juan. We are compelled to provide the care that hospital and state policies allow, and can only offer the best care available to them because of the restrictions of an insurance system to which they contribute financially, yet cannot benefit from, in their time of need. We can tell our undocumented immigrant patients that we find this unacceptable and are actively advocating to change this policy.
Conclusion
The State of Colorado and the nation must amend its healthcare policy to include comprehensive cancer care for everyone. Offering standard-of-care therapy to all patients is not only ethical, but also an economically sound policy benefiting patients, clinicians, and the workforce.
1. Skopec L, Holahan J, Elmendorf C. Changes in Health Insurance Coverage in 2013-2016: Medicaid Expansion States Lead the Way. Urban Institute. September 11, 2018. Accessed July 12, 2021. https://www.urban.org/research/publication/changes-health-insurance-coverage-2013-2016-medicaid-expansion-states-lead-way
2. Christensen Gee L, Gardner M, Hill ME, Wiehe M. Undocumented Immigrants’ State & Local Tax Contributions. Institute on Taxation & Economic Policy. Updated March 2017. Accessed July 12, 2021. https://www.immigrationresearch.org/system/files/immigration_taxes_2017.pdf
3. Emergency Medical Treatment and Labor Act (EMTALA), Public Law 42 U.S.C. 1395dd. 2010.
4. Social Security Act. Sec. 1903 [42 U.S.C. 1396b]. Accessed July 12, 2021. https://www.ssa.gov/OP_Home/ssact/title19/1903.htm.
5. Cervantes L, Mundo W, Powe NR. The status of provision of standard outpatient dialysis for US undocumented immigrants with ESKD. Clin J Am Soc Nephrol. 2019;14(8):1258-1260. https://doi.org/10.2215/CJN.03460319
6. Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood. 2016;127(1):62-70. https://doi.org/10.1182/blood-2015-07-604546
7. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Acute Myeloid Leukemia. 2021.
8. Cervantes L, Richardson S, Raghavan R, et al. Clinicians’ perspectives on providing emergency-only hemodialysis to undocumented immigrants: a qualitative study. Ann Intern Med. 2018;169(2):78-86. https://doi.org/10.7326/M18-0400
9. Cervantes L, Tong A, Camacho C, Collings A, Powe NR. Patient-reported outcomes and experiences in the transition of undocumented patients from emergency to scheduled hemodialysis. Kidney Int. 2021;99(1):198-207. https://doi.org/10.1016/j.kint.2020.07.024
10. Stein E, Xie J, Duchesneau E, et al. Cost effectiveness of midostaurin in the treatment of newly diagnosed FLT3-mutated acute myeloid leukemia in the United States. Pharmacoeconomics. 2019;37(2):239-253. https://doi.org/10.1007/s40273-018-0732-4
11. Preussler JM, Denzen EM, Majhail NS. Costs and cost-effectiveness of hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(11):1620-1628. https://doi.org/10.1016/j.bbmt.2012.04.001
12. Edwards R, Ortega F. The Economic Contribution of Unauthorized Workers: An Industry Analysis. National Bureau of Economic Research. November 2016. Accessed July 12, 2021. https://www.nber.org/system/files/working_papers/w22834/w22834.pdf
13. Nunnery SE, Fintel AE, Jackson WC, Chandler JC, Ugwueke MO, Martin MG. Treatment disparities faced by undocumented workers from low- and middle-income countries in the United States with hematologic malignancies. J Natl Compr Canc Netw. 2016;14(4):483-486. https://doi.org/10.6004/jnccn.2016.0053
14. World Cancer Initiative. Cancer Preparedness in Latin America: The Need to Build on Recent Progress. 2019. Accessed July 7, 2021. https://worldcancerinitiative.economist.com/cancer-preparedness-latin-america
15. Taylor P, Lopez MH, Passel JS, Motel S; Pew Research Center. Unauthorized Immigrants: Length of Residency, Patterns of Parenthood. December 1, 2011. Accessed July 12, 2021. https://www.pewresearch.org/hispanic/2011/12/01/unauthorized-immigrants-length-of-residency-patterns-of-parenthood/
16. California Supreme Court, Records and Briefs: S019427, Dominguez vs. Superior Court of Alameda County. 1990.
17. Wallace SP, Torres J, Sadegh-Nobari T, Pourat N, Brown ER. Undocumented Immigrants and Health Care Reform. UCLA Center for Health Policy Research. August 31, 2012. Accessed July 7, 2021. https://healthpolicy.ucla.edu/publications/Documents/PDF/undocumentedreport-aug2013.pdf
18. Washington State Health Care Authority. Health care services and supports. Noncitizens. Accessed July 12, 2021. https://www.hca.wa.gov/health-care-services-supports/apple-health-medicaid-coverage/non-citizens
19. 117th Congress of the United States. H.R.1177, U.S. Citizenship Act of 2021.
20. National Institutes of Health. Surveillance, Epidemiology, and End Results (SEER) Program. Accessed July 7, 2021. https://seer.cancer.gov/
21. Migration Policy Institute. Profile of the unauthorized population: United States. Accessed July 12, 2021. https://www.migrationpolicy.org/data/unauthorized-immigrant-population/state/US. 2021.
22. Torres L. Latinx? Lat Stud. 2018;16:283-285. https://doi.org/10.1057/s41276-018-0142-y
Clinical Scenario
Juan, a 50-year-old man with acute myeloid leukemia (AML), sat on the edge of his bed, dejected. Juan’s leukemia had relapsed for a third time, and he was low on options and optimism. Originally from Mexico, he had made the journey to Colorado to work as a mechanic and care for his disabled son. Like millions of other individuals in the United States, he did not obtain a visa and had no affordable options for health insurance. For nearly a decade, that had seemed not to matter, until he became ill. Initially presenting to the emergency department with fatigue and night sweats, Juan was diagnosed with poor-risk AML and underwent emergent induction chemotherapy reimbursed under Emergency Medicaid (Table). Just when his bone marrow biopsy showed remission, however, Juan was told there was no chance to cure him, as his documentation status precluded him from receiving the next recommended therapy: stem cell transplant (SCT). Without transplant, Juan’s leukemia relapsed within a few months. He decided to undergo all the salvage chemotherapy that was offered, worrying about how his son would survive without his father.
Background and History
For the patient with a new cancer diagnosis, a difference in immigration status may be the difference between life and death. Undocumented immigrants are excluded from federally funded benefits, including those offered under Medicare, most Medicaid programs, and the Patient Protection and Affordable Care Act (Table).1 The nearly 11 million undocumented immigrants residing in the United States are integral to the workforce and economy. Although they pay taxes that fund Medicaid, contributing approximately $11.7 billion nationally in 2017, undocumented immigrants are ineligible to benefit from such programs.2 The inequity of this policy is highlighted by Juan, an undocumented immigrant presenting with a new diagnosis of AML.
The Emergency Medical Treatment and Active Labor Act (EMTALA) is a 1986 federal law which mandates that patients who present to the hospital with an emergency medical condition receive appropriate evaluation and stabilizing treatment. An emergency condition is defined as “manifesting itself by acute symptoms of sufficient severity … such that the absence of immediate medical attention could reasonably be expected to result in (A) placing the patient’s health in serious jeopardy; (B) serious impairment to bodily functions; or (C) serious dysfunction of any bodily organ or part” (Table).3,4 The Centers for Medicare & Medicaid manual restates the EMTALA definition and notes that services for an emergency medical condition cannot include care related to organ transplantation. Most state Emergency Medicaid programs have adopted the federal definition of what constitutes a medical emergency.5 As a result, undocumented individuals who qualify for Medicaid benefits but who do not meet citizenship requirements are eligible to “receive Medical Assistance benefits for emergency medical care only.”3
Similar to our patient Juan, individuals who initially present with an acute leukemia would be eligible for induction chemotherapy, as blast crisis is imminently fatal. Once in remission, however, standard-of-care therapy for patients without disqualifying comorbidities, depending on cytogenetic disease phenotypes, recommends the only current potential cure: allogeneic SCT, a treatment that was far from routine practice at the time EMTALA was enacted.6 When preparing for transplant, a patient is stable and no longer fits EMTALA’s “emergency” criteria, even though their health is still in “serious jeopardy,” as their cancer has been incompletely treated. Because most state Emergency Medicaid programs adopt the federal definition of an emergency medical condition, the cure is out of reach.
Policy in Clinical Practice
This policy requires clinicians to deviate from the usual standard of care and results in inferior outcomes. For AML patients in the poor-risk category, allogeneic SCT is recommended following induction chemotherapy.7 The risk of relapse is 30% to 40% if consolidation therapy includes SCT, vs 70% to 80% if treated with chemotherapeutic consolidation alone.6 AML patients in the intermediate-, and sometimes even favorable- risk categories, have been shown to benefit from allogeneic SCT as well, with risk of relapse half that of a patient who undergoes consolidation without transplant. Undocumented individuals with AML are therefore resigned to inadequate cancer treatment, including lifelong salvage chemotherapy, and have a substantially decreased chance of achieving sustained remission.6 Furthermore, providing inequitable care for undocumented patients with other medical conditions, such as end-stage kidney disease (ESKD), has been associated with inferior patient-reported outcomes, higher mortality and hospital costs, and clinician burnout. In many states, undocumented immigrants with ESKD rely on emergency dialysis (dialysis made available only after presenting critically ill to an emergency department). In 2019, Colorado’s Medicaid agency opted to include ESKD as a qualifying condition for Emergency Medicaid, thereby expanding access to scheduled dialysis. This led to improved patient quality of life, a decreased emotional toll on patients and clinicians, and reduced costs.8,9
Economic Considerations
Policy discussions must consider cost. The average cost of allogeneic SCT in the United States was approximately $226,000 in 2018, which is often compared to the cost of managing a patient with refractory disease who does not receive transplant.10 This study reported a cost of active disease without transplant, including chemotherapy and hospitalizations, of approximately $69,000, plus terminal care costs of nearly $89,000; at a total of $158,000, this comes out to $68,000 less than SCT.10 This cost savings, however, results in a patient’s death rather than an up to 85% chance of long-term, relapse-free survival.6
To more completely capture the relationship between the healthcare value and cost-effectiveness of SCT, a second study calculated the incremental cost-effectiveness ratio (ICER) of transplantation in acute leukemias in the first 100 days post transplant, including management of complications, such as hospitalization, acute graft-versus-host disease (GVHD), infection, and blood product transfusions. ICER represents the economic value of an intervention compared to an alternative, calculated as cost per quality-adjusted life years. The ICER of SCT compared to no transplant is $16,346 to $34,360, depending on type of transplant and conditioning regimen.11 An ICER of less than $50,000 is considered an acceptable expense for the value achieved—in this case, a significant opportunity for cure. This finding supports SCT, including management of complications, as an economically valuable intervention. Furthermore, if a sustained remission is achieved with SCT, this difference in expense buys the individual patient potentially decades of productivity to contribute back into society and the economy. According to the National Bureau of Economic Research, undocumented workers as a whole contribute $5 trillion to the US Gross Domestic Product over a 10-year period, or about $45,000 per worker per year.12 According to the costs cited, curing a single undocumented worker with acute leukemia via SCT and allowing them to return to work would lead to a return on investment in less than 2 years. If the goal is high-quality, high-value, equitable care, it is logical to spend the money upfront and allow all patients the best chance for recovery.
One might suggest that patients instead receive treatment in their country of origin. This proposition, however, is often unrealistic. Latin American countries, for example, lack access to many standard-of-care cancer treatments available domestically. In Mexico, SCT is only available at a single facility in Mexico City, which is unable to track outcomes.13 The mortality-to-incidence ratio for cancer, a marker of availability of effective treatment, for Latin America is 0.48, substantially inferior to that of the United States (0.29).14 Importantly, almost two thirds of undocumented immigrants in the United States have lived in the country for 10 or more years, and 43% are parents of minor children, an increasing proportion of whom are American citizens.15 This highlights the impracticality of these individuals returning to their country of origin for treatment.
Commentary and Recommendations
Medicaid laws in several states have made it possible for undocumented immigrants to receive access to standard-of-care therapies. Washington and California have included provisions that enable undocumented immigrants to receive allogeneic SCT if they are otherwise medically eligible. In the course of this policy change, legal arguments from the California Court of Appeals expressed that the language of the law was not intended to deny lifesaving treatment to an individual.16 California’s Emergency Medicaid policy is comparable to that of other states, but because the courts considered SCT a “continuation of medically necessary inpatient hospital services … directly related to the emergency” for which the patient initially presented, they concluded that it could be covered under California Medicaid. Despite covering SCT for undocumented immigrants, California maintains lower costs for those patients compared to US citizens on Medicaid while providing evidence-based cancer care.17 This exemplifies sustainable and equitable healthcare policy for the rest of the nation.
A proposed change in policy could occur at either the federal or state level. One option would be to follow the example set by the State of Washington. Under Emergency Medicaid, Washington modified qualifying conditions to include “emergency room care, inpatient admission, or outpatient surgery; a cancer treatment plan; dialysis treatment; anti-rejection medication for an organ transplant” and long-term care services.18 Federal policy reform for undocumented immigrants would also improve access to care. The US Citizenship Act of 2021, introduced to the House of Representatives in February 2021, offers a path to citizenship for undocumented immigrants, ultimately allowing for undocumented individuals to be eligible for the same programs as citizens, though after a period of up to 8 years.19 More immediate revisions of qualifying conditions under state Emergency Medicaid programs, coupled with a path to citizenship, would make significant progress towards reducing structural health inequities. Such policy change would also have broader implications. Three quarters of undocumented immigrants in the United States originate from Mexico, Central America, and South America, and the incidence rate of AML for Latinx individuals is 3.6 per 100,000, a figure which can be extrapolated to an estimated 380 cases per year in the US undocumented population.20-22 In addition to benefiting patients with acute leukemias, the proposed policy change would also benefit numerous others who are frequently hospitalized for acute decompensations of chronic conditions, including congestive heart failure, liver disease, ESKD, and chronic lung conditions. Enabling follow-up care for these diseases under Emergency Medicaid would likewise be expected to reduce costs and improve both quality of care and patient-centered and clinical outcomes.
What Should I Tell My Patient?
Hospitalists frequently care for undocumented immigrants with acute leukemias because the hospital can only be reimbursed by Emergency Medicaid when a patient is admitted to the hospital. Patients may ask about what they can expect in the course of their illness and, while details may be left to the oncologist, hospitalists will be faced with responding to many of these questions. Clinicians at our institution hold honest conversations with patients like Juan. We are compelled to provide the care that hospital and state policies allow, and can only offer the best care available to them because of the restrictions of an insurance system to which they contribute financially, yet cannot benefit from, in their time of need. We can tell our undocumented immigrant patients that we find this unacceptable and are actively advocating to change this policy.
Conclusion
The State of Colorado and the nation must amend its healthcare policy to include comprehensive cancer care for everyone. Offering standard-of-care therapy to all patients is not only ethical, but also an economically sound policy benefiting patients, clinicians, and the workforce.
Clinical Scenario
Juan, a 50-year-old man with acute myeloid leukemia (AML), sat on the edge of his bed, dejected. Juan’s leukemia had relapsed for a third time, and he was low on options and optimism. Originally from Mexico, he had made the journey to Colorado to work as a mechanic and care for his disabled son. Like millions of other individuals in the United States, he did not obtain a visa and had no affordable options for health insurance. For nearly a decade, that had seemed not to matter, until he became ill. Initially presenting to the emergency department with fatigue and night sweats, Juan was diagnosed with poor-risk AML and underwent emergent induction chemotherapy reimbursed under Emergency Medicaid (Table). Just when his bone marrow biopsy showed remission, however, Juan was told there was no chance to cure him, as his documentation status precluded him from receiving the next recommended therapy: stem cell transplant (SCT). Without transplant, Juan’s leukemia relapsed within a few months. He decided to undergo all the salvage chemotherapy that was offered, worrying about how his son would survive without his father.
Background and History
For the patient with a new cancer diagnosis, a difference in immigration status may be the difference between life and death. Undocumented immigrants are excluded from federally funded benefits, including those offered under Medicare, most Medicaid programs, and the Patient Protection and Affordable Care Act (Table).1 The nearly 11 million undocumented immigrants residing in the United States are integral to the workforce and economy. Although they pay taxes that fund Medicaid, contributing approximately $11.7 billion nationally in 2017, undocumented immigrants are ineligible to benefit from such programs.2 The inequity of this policy is highlighted by Juan, an undocumented immigrant presenting with a new diagnosis of AML.
The Emergency Medical Treatment and Active Labor Act (EMTALA) is a 1986 federal law which mandates that patients who present to the hospital with an emergency medical condition receive appropriate evaluation and stabilizing treatment. An emergency condition is defined as “manifesting itself by acute symptoms of sufficient severity … such that the absence of immediate medical attention could reasonably be expected to result in (A) placing the patient’s health in serious jeopardy; (B) serious impairment to bodily functions; or (C) serious dysfunction of any bodily organ or part” (Table).3,4 The Centers for Medicare & Medicaid manual restates the EMTALA definition and notes that services for an emergency medical condition cannot include care related to organ transplantation. Most state Emergency Medicaid programs have adopted the federal definition of what constitutes a medical emergency.5 As a result, undocumented individuals who qualify for Medicaid benefits but who do not meet citizenship requirements are eligible to “receive Medical Assistance benefits for emergency medical care only.”3
Similar to our patient Juan, individuals who initially present with an acute leukemia would be eligible for induction chemotherapy, as blast crisis is imminently fatal. Once in remission, however, standard-of-care therapy for patients without disqualifying comorbidities, depending on cytogenetic disease phenotypes, recommends the only current potential cure: allogeneic SCT, a treatment that was far from routine practice at the time EMTALA was enacted.6 When preparing for transplant, a patient is stable and no longer fits EMTALA’s “emergency” criteria, even though their health is still in “serious jeopardy,” as their cancer has been incompletely treated. Because most state Emergency Medicaid programs adopt the federal definition of an emergency medical condition, the cure is out of reach.
Policy in Clinical Practice
This policy requires clinicians to deviate from the usual standard of care and results in inferior outcomes. For AML patients in the poor-risk category, allogeneic SCT is recommended following induction chemotherapy.7 The risk of relapse is 30% to 40% if consolidation therapy includes SCT, vs 70% to 80% if treated with chemotherapeutic consolidation alone.6 AML patients in the intermediate-, and sometimes even favorable- risk categories, have been shown to benefit from allogeneic SCT as well, with risk of relapse half that of a patient who undergoes consolidation without transplant. Undocumented individuals with AML are therefore resigned to inadequate cancer treatment, including lifelong salvage chemotherapy, and have a substantially decreased chance of achieving sustained remission.6 Furthermore, providing inequitable care for undocumented patients with other medical conditions, such as end-stage kidney disease (ESKD), has been associated with inferior patient-reported outcomes, higher mortality and hospital costs, and clinician burnout. In many states, undocumented immigrants with ESKD rely on emergency dialysis (dialysis made available only after presenting critically ill to an emergency department). In 2019, Colorado’s Medicaid agency opted to include ESKD as a qualifying condition for Emergency Medicaid, thereby expanding access to scheduled dialysis. This led to improved patient quality of life, a decreased emotional toll on patients and clinicians, and reduced costs.8,9
Economic Considerations
Policy discussions must consider cost. The average cost of allogeneic SCT in the United States was approximately $226,000 in 2018, which is often compared to the cost of managing a patient with refractory disease who does not receive transplant.10 This study reported a cost of active disease without transplant, including chemotherapy and hospitalizations, of approximately $69,000, plus terminal care costs of nearly $89,000; at a total of $158,000, this comes out to $68,000 less than SCT.10 This cost savings, however, results in a patient’s death rather than an up to 85% chance of long-term, relapse-free survival.6
To more completely capture the relationship between the healthcare value and cost-effectiveness of SCT, a second study calculated the incremental cost-effectiveness ratio (ICER) of transplantation in acute leukemias in the first 100 days post transplant, including management of complications, such as hospitalization, acute graft-versus-host disease (GVHD), infection, and blood product transfusions. ICER represents the economic value of an intervention compared to an alternative, calculated as cost per quality-adjusted life years. The ICER of SCT compared to no transplant is $16,346 to $34,360, depending on type of transplant and conditioning regimen.11 An ICER of less than $50,000 is considered an acceptable expense for the value achieved—in this case, a significant opportunity for cure. This finding supports SCT, including management of complications, as an economically valuable intervention. Furthermore, if a sustained remission is achieved with SCT, this difference in expense buys the individual patient potentially decades of productivity to contribute back into society and the economy. According to the National Bureau of Economic Research, undocumented workers as a whole contribute $5 trillion to the US Gross Domestic Product over a 10-year period, or about $45,000 per worker per year.12 According to the costs cited, curing a single undocumented worker with acute leukemia via SCT and allowing them to return to work would lead to a return on investment in less than 2 years. If the goal is high-quality, high-value, equitable care, it is logical to spend the money upfront and allow all patients the best chance for recovery.
One might suggest that patients instead receive treatment in their country of origin. This proposition, however, is often unrealistic. Latin American countries, for example, lack access to many standard-of-care cancer treatments available domestically. In Mexico, SCT is only available at a single facility in Mexico City, which is unable to track outcomes.13 The mortality-to-incidence ratio for cancer, a marker of availability of effective treatment, for Latin America is 0.48, substantially inferior to that of the United States (0.29).14 Importantly, almost two thirds of undocumented immigrants in the United States have lived in the country for 10 or more years, and 43% are parents of minor children, an increasing proportion of whom are American citizens.15 This highlights the impracticality of these individuals returning to their country of origin for treatment.
Commentary and Recommendations
Medicaid laws in several states have made it possible for undocumented immigrants to receive access to standard-of-care therapies. Washington and California have included provisions that enable undocumented immigrants to receive allogeneic SCT if they are otherwise medically eligible. In the course of this policy change, legal arguments from the California Court of Appeals expressed that the language of the law was not intended to deny lifesaving treatment to an individual.16 California’s Emergency Medicaid policy is comparable to that of other states, but because the courts considered SCT a “continuation of medically necessary inpatient hospital services … directly related to the emergency” for which the patient initially presented, they concluded that it could be covered under California Medicaid. Despite covering SCT for undocumented immigrants, California maintains lower costs for those patients compared to US citizens on Medicaid while providing evidence-based cancer care.17 This exemplifies sustainable and equitable healthcare policy for the rest of the nation.
A proposed change in policy could occur at either the federal or state level. One option would be to follow the example set by the State of Washington. Under Emergency Medicaid, Washington modified qualifying conditions to include “emergency room care, inpatient admission, or outpatient surgery; a cancer treatment plan; dialysis treatment; anti-rejection medication for an organ transplant” and long-term care services.18 Federal policy reform for undocumented immigrants would also improve access to care. The US Citizenship Act of 2021, introduced to the House of Representatives in February 2021, offers a path to citizenship for undocumented immigrants, ultimately allowing for undocumented individuals to be eligible for the same programs as citizens, though after a period of up to 8 years.19 More immediate revisions of qualifying conditions under state Emergency Medicaid programs, coupled with a path to citizenship, would make significant progress towards reducing structural health inequities. Such policy change would also have broader implications. Three quarters of undocumented immigrants in the United States originate from Mexico, Central America, and South America, and the incidence rate of AML for Latinx individuals is 3.6 per 100,000, a figure which can be extrapolated to an estimated 380 cases per year in the US undocumented population.20-22 In addition to benefiting patients with acute leukemias, the proposed policy change would also benefit numerous others who are frequently hospitalized for acute decompensations of chronic conditions, including congestive heart failure, liver disease, ESKD, and chronic lung conditions. Enabling follow-up care for these diseases under Emergency Medicaid would likewise be expected to reduce costs and improve both quality of care and patient-centered and clinical outcomes.
What Should I Tell My Patient?
Hospitalists frequently care for undocumented immigrants with acute leukemias because the hospital can only be reimbursed by Emergency Medicaid when a patient is admitted to the hospital. Patients may ask about what they can expect in the course of their illness and, while details may be left to the oncologist, hospitalists will be faced with responding to many of these questions. Clinicians at our institution hold honest conversations with patients like Juan. We are compelled to provide the care that hospital and state policies allow, and can only offer the best care available to them because of the restrictions of an insurance system to which they contribute financially, yet cannot benefit from, in their time of need. We can tell our undocumented immigrant patients that we find this unacceptable and are actively advocating to change this policy.
Conclusion
The State of Colorado and the nation must amend its healthcare policy to include comprehensive cancer care for everyone. Offering standard-of-care therapy to all patients is not only ethical, but also an economically sound policy benefiting patients, clinicians, and the workforce.
1. Skopec L, Holahan J, Elmendorf C. Changes in Health Insurance Coverage in 2013-2016: Medicaid Expansion States Lead the Way. Urban Institute. September 11, 2018. Accessed July 12, 2021. https://www.urban.org/research/publication/changes-health-insurance-coverage-2013-2016-medicaid-expansion-states-lead-way
2. Christensen Gee L, Gardner M, Hill ME, Wiehe M. Undocumented Immigrants’ State & Local Tax Contributions. Institute on Taxation & Economic Policy. Updated March 2017. Accessed July 12, 2021. https://www.immigrationresearch.org/system/files/immigration_taxes_2017.pdf
3. Emergency Medical Treatment and Labor Act (EMTALA), Public Law 42 U.S.C. 1395dd. 2010.
4. Social Security Act. Sec. 1903 [42 U.S.C. 1396b]. Accessed July 12, 2021. https://www.ssa.gov/OP_Home/ssact/title19/1903.htm.
5. Cervantes L, Mundo W, Powe NR. The status of provision of standard outpatient dialysis for US undocumented immigrants with ESKD. Clin J Am Soc Nephrol. 2019;14(8):1258-1260. https://doi.org/10.2215/CJN.03460319
6. Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood. 2016;127(1):62-70. https://doi.org/10.1182/blood-2015-07-604546
7. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Acute Myeloid Leukemia. 2021.
8. Cervantes L, Richardson S, Raghavan R, et al. Clinicians’ perspectives on providing emergency-only hemodialysis to undocumented immigrants: a qualitative study. Ann Intern Med. 2018;169(2):78-86. https://doi.org/10.7326/M18-0400
9. Cervantes L, Tong A, Camacho C, Collings A, Powe NR. Patient-reported outcomes and experiences in the transition of undocumented patients from emergency to scheduled hemodialysis. Kidney Int. 2021;99(1):198-207. https://doi.org/10.1016/j.kint.2020.07.024
10. Stein E, Xie J, Duchesneau E, et al. Cost effectiveness of midostaurin in the treatment of newly diagnosed FLT3-mutated acute myeloid leukemia in the United States. Pharmacoeconomics. 2019;37(2):239-253. https://doi.org/10.1007/s40273-018-0732-4
11. Preussler JM, Denzen EM, Majhail NS. Costs and cost-effectiveness of hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(11):1620-1628. https://doi.org/10.1016/j.bbmt.2012.04.001
12. Edwards R, Ortega F. The Economic Contribution of Unauthorized Workers: An Industry Analysis. National Bureau of Economic Research. November 2016. Accessed July 12, 2021. https://www.nber.org/system/files/working_papers/w22834/w22834.pdf
13. Nunnery SE, Fintel AE, Jackson WC, Chandler JC, Ugwueke MO, Martin MG. Treatment disparities faced by undocumented workers from low- and middle-income countries in the United States with hematologic malignancies. J Natl Compr Canc Netw. 2016;14(4):483-486. https://doi.org/10.6004/jnccn.2016.0053
14. World Cancer Initiative. Cancer Preparedness in Latin America: The Need to Build on Recent Progress. 2019. Accessed July 7, 2021. https://worldcancerinitiative.economist.com/cancer-preparedness-latin-america
15. Taylor P, Lopez MH, Passel JS, Motel S; Pew Research Center. Unauthorized Immigrants: Length of Residency, Patterns of Parenthood. December 1, 2011. Accessed July 12, 2021. https://www.pewresearch.org/hispanic/2011/12/01/unauthorized-immigrants-length-of-residency-patterns-of-parenthood/
16. California Supreme Court, Records and Briefs: S019427, Dominguez vs. Superior Court of Alameda County. 1990.
17. Wallace SP, Torres J, Sadegh-Nobari T, Pourat N, Brown ER. Undocumented Immigrants and Health Care Reform. UCLA Center for Health Policy Research. August 31, 2012. Accessed July 7, 2021. https://healthpolicy.ucla.edu/publications/Documents/PDF/undocumentedreport-aug2013.pdf
18. Washington State Health Care Authority. Health care services and supports. Noncitizens. Accessed July 12, 2021. https://www.hca.wa.gov/health-care-services-supports/apple-health-medicaid-coverage/non-citizens
19. 117th Congress of the United States. H.R.1177, U.S. Citizenship Act of 2021.
20. National Institutes of Health. Surveillance, Epidemiology, and End Results (SEER) Program. Accessed July 7, 2021. https://seer.cancer.gov/
21. Migration Policy Institute. Profile of the unauthorized population: United States. Accessed July 12, 2021. https://www.migrationpolicy.org/data/unauthorized-immigrant-population/state/US. 2021.
22. Torres L. Latinx? Lat Stud. 2018;16:283-285. https://doi.org/10.1057/s41276-018-0142-y
1. Skopec L, Holahan J, Elmendorf C. Changes in Health Insurance Coverage in 2013-2016: Medicaid Expansion States Lead the Way. Urban Institute. September 11, 2018. Accessed July 12, 2021. https://www.urban.org/research/publication/changes-health-insurance-coverage-2013-2016-medicaid-expansion-states-lead-way
2. Christensen Gee L, Gardner M, Hill ME, Wiehe M. Undocumented Immigrants’ State & Local Tax Contributions. Institute on Taxation & Economic Policy. Updated March 2017. Accessed July 12, 2021. https://www.immigrationresearch.org/system/files/immigration_taxes_2017.pdf
3. Emergency Medical Treatment and Labor Act (EMTALA), Public Law 42 U.S.C. 1395dd. 2010.
4. Social Security Act. Sec. 1903 [42 U.S.C. 1396b]. Accessed July 12, 2021. https://www.ssa.gov/OP_Home/ssact/title19/1903.htm.
5. Cervantes L, Mundo W, Powe NR. The status of provision of standard outpatient dialysis for US undocumented immigrants with ESKD. Clin J Am Soc Nephrol. 2019;14(8):1258-1260. https://doi.org/10.2215/CJN.03460319
6. Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood. 2016;127(1):62-70. https://doi.org/10.1182/blood-2015-07-604546
7. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Acute Myeloid Leukemia. 2021.
8. Cervantes L, Richardson S, Raghavan R, et al. Clinicians’ perspectives on providing emergency-only hemodialysis to undocumented immigrants: a qualitative study. Ann Intern Med. 2018;169(2):78-86. https://doi.org/10.7326/M18-0400
9. Cervantes L, Tong A, Camacho C, Collings A, Powe NR. Patient-reported outcomes and experiences in the transition of undocumented patients from emergency to scheduled hemodialysis. Kidney Int. 2021;99(1):198-207. https://doi.org/10.1016/j.kint.2020.07.024
10. Stein E, Xie J, Duchesneau E, et al. Cost effectiveness of midostaurin in the treatment of newly diagnosed FLT3-mutated acute myeloid leukemia in the United States. Pharmacoeconomics. 2019;37(2):239-253. https://doi.org/10.1007/s40273-018-0732-4
11. Preussler JM, Denzen EM, Majhail NS. Costs and cost-effectiveness of hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(11):1620-1628. https://doi.org/10.1016/j.bbmt.2012.04.001
12. Edwards R, Ortega F. The Economic Contribution of Unauthorized Workers: An Industry Analysis. National Bureau of Economic Research. November 2016. Accessed July 12, 2021. https://www.nber.org/system/files/working_papers/w22834/w22834.pdf
13. Nunnery SE, Fintel AE, Jackson WC, Chandler JC, Ugwueke MO, Martin MG. Treatment disparities faced by undocumented workers from low- and middle-income countries in the United States with hematologic malignancies. J Natl Compr Canc Netw. 2016;14(4):483-486. https://doi.org/10.6004/jnccn.2016.0053
14. World Cancer Initiative. Cancer Preparedness in Latin America: The Need to Build on Recent Progress. 2019. Accessed July 7, 2021. https://worldcancerinitiative.economist.com/cancer-preparedness-latin-america
15. Taylor P, Lopez MH, Passel JS, Motel S; Pew Research Center. Unauthorized Immigrants: Length of Residency, Patterns of Parenthood. December 1, 2011. Accessed July 12, 2021. https://www.pewresearch.org/hispanic/2011/12/01/unauthorized-immigrants-length-of-residency-patterns-of-parenthood/
16. California Supreme Court, Records and Briefs: S019427, Dominguez vs. Superior Court of Alameda County. 1990.
17. Wallace SP, Torres J, Sadegh-Nobari T, Pourat N, Brown ER. Undocumented Immigrants and Health Care Reform. UCLA Center for Health Policy Research. August 31, 2012. Accessed July 7, 2021. https://healthpolicy.ucla.edu/publications/Documents/PDF/undocumentedreport-aug2013.pdf
18. Washington State Health Care Authority. Health care services and supports. Noncitizens. Accessed July 12, 2021. https://www.hca.wa.gov/health-care-services-supports/apple-health-medicaid-coverage/non-citizens
19. 117th Congress of the United States. H.R.1177, U.S. Citizenship Act of 2021.
20. National Institutes of Health. Surveillance, Epidemiology, and End Results (SEER) Program. Accessed July 7, 2021. https://seer.cancer.gov/
21. Migration Policy Institute. Profile of the unauthorized population: United States. Accessed July 12, 2021. https://www.migrationpolicy.org/data/unauthorized-immigrant-population/state/US. 2021.
22. Torres L. Latinx? Lat Stud. 2018;16:283-285. https://doi.org/10.1057/s41276-018-0142-y
© 2021 Society of Hospital Medicine
Things We Do for No Reason™: Prescribing Appetite Stimulants to Hospitalized Older Adults With Unintentional Weight Loss
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
An 87-year-old hospitalized man has lost 7% of his body weight in the past year. His family and the inpatient nutritionist ask about a prescription appetite stimulant.
Why You Might Think Prescribing Appetite Stimulants for Unintentional Weight Loss in Older Adults Is Helpful
Unintentional weight loss—the loss of more than 10 lb or 5% of usual body weight over 6 to 12 months—affects up to 27% of older adults in the community and 50% to 60% of older adults in nursing homes.1,2 Patients who report weight loss on hospital admission have an almost four times greater risk of death in the 12 months following discharge.3 To address unintentional weight loss, clinicians may prescribe appetite stimulants.
Megestrol acetate is approved by the US Food and Drug Administration (FDA) for the treatment of weight loss in patients with AIDS.4 Megestrol acetate promotes weight gain through inhibition of cytokines, interleukin-6, and tumor necrosis factor-alpha, which are increased in older adults. In a randomized, placebo-controlled trial of 69 nursing home residents with ≥6 months’ life expectancy and Karnofsky score of ≥40%, patients treated with megestrol acetate for 12 weeks reported increased appetite and well-being. They achieved significant weight gain (>1.82 kg), but not until 3 months after therapy ended.5 No significant adverse events were reported; however, adverse event monitoring continued only for the 12-week treatment period. This follow-up duration may have been insufficient to identify some adverse events, such as venous thromboembolism.
Mirtazapine, an antidepressant and serotonin receptor antagonist, reduces levels of serotonin, a neurotransmitter that promotes early satiety.6 In a meta-analysis of 11 trials comparing mirtazapine to selective serotonin reuptake inhibitors for depression, patients treated with mirtazapine demonstrated an increase in the composite secondary outcome of weight gain or increased appetite.7 The amount of weight gain was not specified. Weight gain is more common with low-dose mirtazapine, potentially due to increased antihistamine activity at lower doses.8 Overall, mirtazapine is well-tolerated and efficacious in the treatment of depression and may benefit older adults with concomitant weight loss.6
Cyproheptadine is a first-generation antihistamine with appetite-stimulating effects. It has been found to increase weight or appetite in various disease states, particularly in the pediatric population,9 including cystic fibrosis10 and malignancy.11 Given this evidence, there has been interest in its use in the geriatric population with unintentional weight loss.
Dronabinol is an orally active cannabinoid approved for anorexia-associated weight loss in patients with AIDS.12 In a randomized, placebo-controlled trial in patients with AIDS-related anorexia and weight loss, participants receiving dronabinol had a statistically significant increase in appetite but no change in weight. Participants receiving dronabinol also experienced more nervous system-related adverse events, including dizziness, thinking abnormalities, and somnolence.13
Why Prescribing Appetite Stimulants for Unintentional Weight Loss in Older Adults Is Not Helpful
Weight gain may not improve clinically meaningful outcomes. The absence of consistent evidence that prescription appetite stimulants improve patient-centered outcomes, such as quality of life or functional status, and the potential morbidity and mortality of these medications make prescribing appetite stimulants in older adults concerning.
Megestrol Acetate
A 2018 systematic review of randomized controlled trials studying megestrol acetate for treatment of anorexia-cachexia, primarily in adults with AIDS and cancer, found that treatment resulted in a 2.25-kg weight gain, with no improvement in quality of life and an increased risk of adverse events.14
Three prospective trials studied the effect of megestrol acetate in older adults (Appendix Table). One trial randomized 47 patients receiving skilled nursing services following an admission for acute illness to megestrol acetate vs placebo. While the investigators noted increases in appetite at higher doses of megestrol acetate, there was no change in weight or clinically relevant outcomes.15 In a second randomized controlled trial, 29 patients with illness-induced functional decline were enrolled in a strength training program in addition to being assigned to megestrol acetate or placebo. While patients receiving megestrol acetate with the exercise program had significant increases in weight and nutritional intake, they suffered a deterioration in physical function.16 In a pilot study, 17 nursing home residents who consistently ate less than 75% of their meals received megestrol acetate plus standard or optimal feeding assistance. The percentage of meals consumed increased only when patients received optimal feeding assistance in conjunction with megestrol acetate.17
The largest case-control study examining megestrol acetate for unintentional weight loss in older adults compared 709 residents in a multistate nursing home system treated with megestrol acetate to matched untreated controls. After 6 months of treatment, the median weight and change in weight did not differ significantly. Patients receiving megestrol acetate had a significant increase in mortality, surviving an average of 23.9 months, compared to 31.2 months for controls (P < .001).18
Additionally, two retrospective reviews of nursing home patients who were prescribed megestrol acetate showed incidences of venous thrombosis of 5% and 32%.19,20 Other potentially significant adverse effects include adrenal insufficiency and fluid retention.6 In 2019, the American Geriatrics Society’s Beers Criteria included megestrol acetate as a medication to avoid given its “minimal effect on weight; increases [in] risk of thrombotic events and possibly death in older adults.”21
Mirtazapine
No studies have evaluated mirtazapine for weight gain without concomitant depression. In older adults with depression, mirtazapine has minimal impact on promoting weight gain compared to other antidepressants. In two retrospective studies of older patients with depression and weight loss, researchers found no difference in weight gain in those treated with mirtazapine vs sertraline or other nontricyclic antidepressants, excluding fluoxetine.22,23
Cyproheptadine
There have been no controlled trials evaluating the use of cyproheptadine in older adults, in part due to anticholinergic side effects. In a trial of cancer patients, sedation and dizziness were common adverse effects.11 The 2019 American Geriatrics Society’s Beers Criteria include cyproheptadine as a medication to avoid based upon the “risk of confusion, dry mouth, constipation, and other anticholinergic effects or toxicity.”21
Dronabinol
In a retrospective cohort study of 28 long-term care residents with anorexia and weight loss, participants receiving dronabinol for 12 weeks had no statistically significant weight gain.24 The FDA cautions against prescribing dronabinol for older adults due to neurological side effects.12 A systematic review of randomized controlled trials found that cannabinoid-based medications in patients older than 50 years were associated with a significant increase in dizziness or lightheadedness and thinking or perception disorder.25
What You Should Do Instead
In the Choosing Wisely® initiative, the American Geriatrics Society recommends avoiding prescription appetite stimulants for patients with anorexia or cachexia.26 Instead, hospitalists should evaluate older patients for causes of unintentional weight loss, including malignancy, nonmalignant gastrointestinal disorders, depression, and dementia. Hospitalists can identify most causes based on the history, physical exam, and laboratory studies and initiate treatment for modifiable causes, such as constipation and depression.2
Hospitalists should work with an interprofessional team to develop an individualized plan to optimize caloric intake in the hospital (Table).27 One in five hospitalized older adults has insufficient caloric intake during admission, which is associated with increased risk for in-hospital and 90-day mortality.28 Removing dietary restrictions, increasing the variety of foods offered, and assisted eating may increase food intake.27,29 Hospitalists should also consider discontinuing or changing medications with gastrointestinal side effects, such as metformin, cholinesterase inhibitors, bisphosphonates, and oral iron supplements. Dietitians may recommend oral nutrition supplements; if started, patients should be offered supplements after discharge.27,29 For patients with limited access to food, social workers can help optimize social supports and identify community resources following discharge. Finally, hospitalists should coordinate with outpatient providers to monitor weight long-term.
Recommendations
- Recognize and address unintentional weight loss in older adults in the hospital.
- Do not prescribe appetite stimulants for unintentional weight loss in hospitalized older adults as they have no proven benefit for improving long-term outcomes and, in the case of megestrol acetate, may increase mortality.
- Work with an interprofessional team to address factors contributing to unintentional weight loss using nonpharmacologic options for improving food intake.
Conclusion
After discussing the lack of evidence supporting prescription appetite stimulants and the potential risks, we shifted the focus to optimizing oral intake. The team worked with the patient and the patient’s family to optimize nutrition following discharge and communicated the need for ongoing monitoring to the primary care provider.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected]
Acknowledgment
The authors thank Claire Campbell, MD, for her review of this manuscript.
1. Bouras EP, Lange SM, Scolapio JS. Rational approach to patients with unintentional weight loss. Mayo Clin Proc. 2001;76(9):923-929. https://doi.org/10.4065/76.9.923
2. McMinn J, Steel C, Bowman A. Investigation and management of unintentional weight loss in older adults. BMJ. 2011;342:d1732. https://doi.org/10.1136/bmj.d1732
3. Satish S, Winograd CH, Chavez C, Bloch DA. Geriatric targeting criteria as predictors of survival and health care utilization. J Am Geriatr Soc. 1996;44(8):914-921. https://doi.org/10.1111/j.1532-5415.1996.tb01860.x
4. Megace (megestrol acetate) [package insert]. Par Pharmaceutical Inc. Revised July 2005. Accessed January 27, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021778s000TOC.cfm
5. Yeh SS, Wu SY, Lee TP, et al. Improvement in quality-of-life measures and stimulation of weight gain after treatment with megestrol acetate oral suspension in geriatric cachexia: results of a double-blind, placebo-controlled study. J Am Geriatr Soc. 2000;48(5):485-492. https://doi.org/10.1111/j.1532-5415.2000.tb04993.x
6. Fox CB, Treadway AK, Blaszczyk AT, Sleeper RB. Reviews of therapeutics megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29(4):383-397. https://doi.org/10.1592/phco.29.4.383
7. Watanabe N, Omori IM, Nakagawa A, et al. Mirtazapine versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2011;(12):CD006528. https://doi.org/10.1002/14651858.CD006528.pub2
8. Fawcett J, Barkin RL. Review of the results from clinical studies on the efficacy, safety and tolerability of mirtazapine for the treatment of patients with major depression. J Affect Disord. 1998;51(3):267-285. https://doi.org/10.1016/S0165-0327(98)00224-9
9. Najib K, Moghtaderi M, Karamizadeh Z, Fallahzadeh E. Beneficial effect of cyproheptadine on body mass index in undernourished children: a randomized controlled trial. Iran J Pediatr. 2014;24(6):753-758.
10. Epifanio M, Marostica PC, Mattiello R, et al. A randomized, double-blind, placebo-controlled trial of cyproheptadine for appetite stimulation in cystic fibrosis. J Pediatr (Rio J). 2012;88(2):155-160. https://doi.org/10.2223/JPED.2174
11. Kardinal CG, Loprinzi CL, Schaid DJ, et al. A controlled trial of cyproheptadine in cancer patients with anorexia and/or cachexia. Cancer. 1990;65(12):2657-2662. https://doi.org/10.1002/1097-0142(19900615)65:12<2657::aid-cncr2820651210>3.0.co;2-s
12. MARINOL (dronabinol) [package insert]. Solvay Pharmaceuticals, Inc. Revised August 2017. Accessed April 27, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf.
13. Beal JE, Olson R, Laubenstein L, et al. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage. 1995;10(2):89-97. https://doi.org/10.1016/0885-3924(94)00117-4
14. Ruiz-García V, López-Briz E, Carbonell-Sanchis R, Bort-Martí S, Gonzálvez-Perales JL. Megestrol acetate for cachexia–anorexia syndrome. A systematic review. J Cachexia Sarcopenia Muscle. 2018;9(3):444-452. https://doi.org/10.1002/jcsm.12292
15. Reuben DB, Hirsch SH, Zhou K, Greendale GA. The effects of megestrol acetate suspension for elderly patients with reduced appetite after hospitalization: a phase II randomized clinical trial. J Am Geriatr Soc. 2005;53(6):970-975. https://doi.org/10.1111/j.1532-5415.2005.53307.x
16. Sullivan DH, Roberson PK, Smith ES, Price JA, Bopp MM. Effects of muscle strength training and megestrol acetate on strength, muscle mass, and function in frail older people. J Am Geriatr Soc. 2007;55(1):20-28. https://doi.org/10.1111/j.1532-5415.2006.01010.x
17. Simmons SF, Walker KA, Osterweil D. The effect of megestrol acetate on oral food and fluid intake in nursing home residents: a pilot study. J Am Med Dir Assoc. 2005;6(3):S5-S11. https://doi.org/10.1016/j.jamda.2005.03.014
18. Bodenner D, Spencer T, Riggs AT, Redman C, Strunk B, Hughes T. A retrospective study of the association between megestrol acetate administration and mortality among nursing home residents with clinically significant weight loss. Am J Geriatr Pharmacother. 2007;5(2):137-146. https://doi.org/10.1016/J.AMJOPHARM.2007.06.004
19. Kropsky B, Shi Y, Cherniack EP. Incidence of deep-venous thrombosis in nursing home residents using megestrol acetate. J Am Med Dir Assoc. 2003;4(5):255-256. https://doi.org/10.1097/01.JAM.0000083384.84558.75
20. Bolen JC, Andersen RE, Bennett RG. Deep vein thrombosis as a complication of megestrol acetate therapy among nursing home residents. J Am Med Dir Assoc. 2000;1(6):248-252.
21. Fick DM, Semla TP, Steinman M, et al. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674-694. https://doi.org/10.1111/jgs.15767
22. Mihara IQT, McCombs JS, Williams BR. The impact of mirtazapine compared with non-TCA antidepressants on weight change in nursing facility residents. Consult Pharm. 2005;20(3):217-223. https://doi.org/10.4140/tcp.n.2005.217
23. Goldberg RJ. Weight change in depressed nursing home patients on mirtazapine. J Am Geriatr Soc. 2002;50(8):1461. https://doi.org/10.1046/j.1532-5415.2002.50374.x
24. Wilson MMG, Philpot C, Morley JE. Anorexia of aging in long term care: is dronabinol an effective appetite stimulant?--a pilot study. J Nutr Health Aging. 2007;11(2):195-198.
25. Velayudhan L, McGoohan KL, Bhattacharyya S. Evaluation of THC-related neuropsychiatric symptoms among adults aged 50 years and older: a systematic review and metaregression analysis. JAMA Netw Open. 2021;4(2):e2035913. https://doi.org/10.1001/jamanetworkopen.2020.35913
26. AGS Choosing Wisely Workgroup. American Geriatrics Society identifies another five things that healthcare providers and patients should question. J Am Geriatr Soc. 2014;62(5):950-960. https://doi.org/10.1111/jgs.12770
27. Volkert D, Beck AM, Cederholm T, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. 2019;38(1):10-47. https://doi.org/10.1016/j.clnu.2018.05.024
28. Sullivan DH, Sun S, Walls RC. Protein-energy undernutrition among elderly hospitalized patients: a prospective study. JAMA. 1999;281(21):2013-2019. https://doi.org/10.1001/jama.281.21.2013
29. Feinberg J, Nielsen EE, Korang SK, et al. Nutrition support in hospitalised adults at nutritional risk. Cochrane Database Syst Rev. 2017;2017(5). https://doi.org/10.1002/14651858.CD011598.pub2
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
An 87-year-old hospitalized man has lost 7% of his body weight in the past year. His family and the inpatient nutritionist ask about a prescription appetite stimulant.
Why You Might Think Prescribing Appetite Stimulants for Unintentional Weight Loss in Older Adults Is Helpful
Unintentional weight loss—the loss of more than 10 lb or 5% of usual body weight over 6 to 12 months—affects up to 27% of older adults in the community and 50% to 60% of older adults in nursing homes.1,2 Patients who report weight loss on hospital admission have an almost four times greater risk of death in the 12 months following discharge.3 To address unintentional weight loss, clinicians may prescribe appetite stimulants.
Megestrol acetate is approved by the US Food and Drug Administration (FDA) for the treatment of weight loss in patients with AIDS.4 Megestrol acetate promotes weight gain through inhibition of cytokines, interleukin-6, and tumor necrosis factor-alpha, which are increased in older adults. In a randomized, placebo-controlled trial of 69 nursing home residents with ≥6 months’ life expectancy and Karnofsky score of ≥40%, patients treated with megestrol acetate for 12 weeks reported increased appetite and well-being. They achieved significant weight gain (>1.82 kg), but not until 3 months after therapy ended.5 No significant adverse events were reported; however, adverse event monitoring continued only for the 12-week treatment period. This follow-up duration may have been insufficient to identify some adverse events, such as venous thromboembolism.
Mirtazapine, an antidepressant and serotonin receptor antagonist, reduces levels of serotonin, a neurotransmitter that promotes early satiety.6 In a meta-analysis of 11 trials comparing mirtazapine to selective serotonin reuptake inhibitors for depression, patients treated with mirtazapine demonstrated an increase in the composite secondary outcome of weight gain or increased appetite.7 The amount of weight gain was not specified. Weight gain is more common with low-dose mirtazapine, potentially due to increased antihistamine activity at lower doses.8 Overall, mirtazapine is well-tolerated and efficacious in the treatment of depression and may benefit older adults with concomitant weight loss.6
Cyproheptadine is a first-generation antihistamine with appetite-stimulating effects. It has been found to increase weight or appetite in various disease states, particularly in the pediatric population,9 including cystic fibrosis10 and malignancy.11 Given this evidence, there has been interest in its use in the geriatric population with unintentional weight loss.
Dronabinol is an orally active cannabinoid approved for anorexia-associated weight loss in patients with AIDS.12 In a randomized, placebo-controlled trial in patients with AIDS-related anorexia and weight loss, participants receiving dronabinol had a statistically significant increase in appetite but no change in weight. Participants receiving dronabinol also experienced more nervous system-related adverse events, including dizziness, thinking abnormalities, and somnolence.13
Why Prescribing Appetite Stimulants for Unintentional Weight Loss in Older Adults Is Not Helpful
Weight gain may not improve clinically meaningful outcomes. The absence of consistent evidence that prescription appetite stimulants improve patient-centered outcomes, such as quality of life or functional status, and the potential morbidity and mortality of these medications make prescribing appetite stimulants in older adults concerning.
Megestrol Acetate
A 2018 systematic review of randomized controlled trials studying megestrol acetate for treatment of anorexia-cachexia, primarily in adults with AIDS and cancer, found that treatment resulted in a 2.25-kg weight gain, with no improvement in quality of life and an increased risk of adverse events.14
Three prospective trials studied the effect of megestrol acetate in older adults (Appendix Table). One trial randomized 47 patients receiving skilled nursing services following an admission for acute illness to megestrol acetate vs placebo. While the investigators noted increases in appetite at higher doses of megestrol acetate, there was no change in weight or clinically relevant outcomes.15 In a second randomized controlled trial, 29 patients with illness-induced functional decline were enrolled in a strength training program in addition to being assigned to megestrol acetate or placebo. While patients receiving megestrol acetate with the exercise program had significant increases in weight and nutritional intake, they suffered a deterioration in physical function.16 In a pilot study, 17 nursing home residents who consistently ate less than 75% of their meals received megestrol acetate plus standard or optimal feeding assistance. The percentage of meals consumed increased only when patients received optimal feeding assistance in conjunction with megestrol acetate.17
The largest case-control study examining megestrol acetate for unintentional weight loss in older adults compared 709 residents in a multistate nursing home system treated with megestrol acetate to matched untreated controls. After 6 months of treatment, the median weight and change in weight did not differ significantly. Patients receiving megestrol acetate had a significant increase in mortality, surviving an average of 23.9 months, compared to 31.2 months for controls (P < .001).18
Additionally, two retrospective reviews of nursing home patients who were prescribed megestrol acetate showed incidences of venous thrombosis of 5% and 32%.19,20 Other potentially significant adverse effects include adrenal insufficiency and fluid retention.6 In 2019, the American Geriatrics Society’s Beers Criteria included megestrol acetate as a medication to avoid given its “minimal effect on weight; increases [in] risk of thrombotic events and possibly death in older adults.”21
Mirtazapine
No studies have evaluated mirtazapine for weight gain without concomitant depression. In older adults with depression, mirtazapine has minimal impact on promoting weight gain compared to other antidepressants. In two retrospective studies of older patients with depression and weight loss, researchers found no difference in weight gain in those treated with mirtazapine vs sertraline or other nontricyclic antidepressants, excluding fluoxetine.22,23
Cyproheptadine
There have been no controlled trials evaluating the use of cyproheptadine in older adults, in part due to anticholinergic side effects. In a trial of cancer patients, sedation and dizziness were common adverse effects.11 The 2019 American Geriatrics Society’s Beers Criteria include cyproheptadine as a medication to avoid based upon the “risk of confusion, dry mouth, constipation, and other anticholinergic effects or toxicity.”21
Dronabinol
In a retrospective cohort study of 28 long-term care residents with anorexia and weight loss, participants receiving dronabinol for 12 weeks had no statistically significant weight gain.24 The FDA cautions against prescribing dronabinol for older adults due to neurological side effects.12 A systematic review of randomized controlled trials found that cannabinoid-based medications in patients older than 50 years were associated with a significant increase in dizziness or lightheadedness and thinking or perception disorder.25
What You Should Do Instead
In the Choosing Wisely® initiative, the American Geriatrics Society recommends avoiding prescription appetite stimulants for patients with anorexia or cachexia.26 Instead, hospitalists should evaluate older patients for causes of unintentional weight loss, including malignancy, nonmalignant gastrointestinal disorders, depression, and dementia. Hospitalists can identify most causes based on the history, physical exam, and laboratory studies and initiate treatment for modifiable causes, such as constipation and depression.2
Hospitalists should work with an interprofessional team to develop an individualized plan to optimize caloric intake in the hospital (Table).27 One in five hospitalized older adults has insufficient caloric intake during admission, which is associated with increased risk for in-hospital and 90-day mortality.28 Removing dietary restrictions, increasing the variety of foods offered, and assisted eating may increase food intake.27,29 Hospitalists should also consider discontinuing or changing medications with gastrointestinal side effects, such as metformin, cholinesterase inhibitors, bisphosphonates, and oral iron supplements. Dietitians may recommend oral nutrition supplements; if started, patients should be offered supplements after discharge.27,29 For patients with limited access to food, social workers can help optimize social supports and identify community resources following discharge. Finally, hospitalists should coordinate with outpatient providers to monitor weight long-term.
Recommendations
- Recognize and address unintentional weight loss in older adults in the hospital.
- Do not prescribe appetite stimulants for unintentional weight loss in hospitalized older adults as they have no proven benefit for improving long-term outcomes and, in the case of megestrol acetate, may increase mortality.
- Work with an interprofessional team to address factors contributing to unintentional weight loss using nonpharmacologic options for improving food intake.
Conclusion
After discussing the lack of evidence supporting prescription appetite stimulants and the potential risks, we shifted the focus to optimizing oral intake. The team worked with the patient and the patient’s family to optimize nutrition following discharge and communicated the need for ongoing monitoring to the primary care provider.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected]
Acknowledgment
The authors thank Claire Campbell, MD, for her review of this manuscript.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
An 87-year-old hospitalized man has lost 7% of his body weight in the past year. His family and the inpatient nutritionist ask about a prescription appetite stimulant.
Why You Might Think Prescribing Appetite Stimulants for Unintentional Weight Loss in Older Adults Is Helpful
Unintentional weight loss—the loss of more than 10 lb or 5% of usual body weight over 6 to 12 months—affects up to 27% of older adults in the community and 50% to 60% of older adults in nursing homes.1,2 Patients who report weight loss on hospital admission have an almost four times greater risk of death in the 12 months following discharge.3 To address unintentional weight loss, clinicians may prescribe appetite stimulants.
Megestrol acetate is approved by the US Food and Drug Administration (FDA) for the treatment of weight loss in patients with AIDS.4 Megestrol acetate promotes weight gain through inhibition of cytokines, interleukin-6, and tumor necrosis factor-alpha, which are increased in older adults. In a randomized, placebo-controlled trial of 69 nursing home residents with ≥6 months’ life expectancy and Karnofsky score of ≥40%, patients treated with megestrol acetate for 12 weeks reported increased appetite and well-being. They achieved significant weight gain (>1.82 kg), but not until 3 months after therapy ended.5 No significant adverse events were reported; however, adverse event monitoring continued only for the 12-week treatment period. This follow-up duration may have been insufficient to identify some adverse events, such as venous thromboembolism.
Mirtazapine, an antidepressant and serotonin receptor antagonist, reduces levels of serotonin, a neurotransmitter that promotes early satiety.6 In a meta-analysis of 11 trials comparing mirtazapine to selective serotonin reuptake inhibitors for depression, patients treated with mirtazapine demonstrated an increase in the composite secondary outcome of weight gain or increased appetite.7 The amount of weight gain was not specified. Weight gain is more common with low-dose mirtazapine, potentially due to increased antihistamine activity at lower doses.8 Overall, mirtazapine is well-tolerated and efficacious in the treatment of depression and may benefit older adults with concomitant weight loss.6
Cyproheptadine is a first-generation antihistamine with appetite-stimulating effects. It has been found to increase weight or appetite in various disease states, particularly in the pediatric population,9 including cystic fibrosis10 and malignancy.11 Given this evidence, there has been interest in its use in the geriatric population with unintentional weight loss.
Dronabinol is an orally active cannabinoid approved for anorexia-associated weight loss in patients with AIDS.12 In a randomized, placebo-controlled trial in patients with AIDS-related anorexia and weight loss, participants receiving dronabinol had a statistically significant increase in appetite but no change in weight. Participants receiving dronabinol also experienced more nervous system-related adverse events, including dizziness, thinking abnormalities, and somnolence.13
Why Prescribing Appetite Stimulants for Unintentional Weight Loss in Older Adults Is Not Helpful
Weight gain may not improve clinically meaningful outcomes. The absence of consistent evidence that prescription appetite stimulants improve patient-centered outcomes, such as quality of life or functional status, and the potential morbidity and mortality of these medications make prescribing appetite stimulants in older adults concerning.
Megestrol Acetate
A 2018 systematic review of randomized controlled trials studying megestrol acetate for treatment of anorexia-cachexia, primarily in adults with AIDS and cancer, found that treatment resulted in a 2.25-kg weight gain, with no improvement in quality of life and an increased risk of adverse events.14
Three prospective trials studied the effect of megestrol acetate in older adults (Appendix Table). One trial randomized 47 patients receiving skilled nursing services following an admission for acute illness to megestrol acetate vs placebo. While the investigators noted increases in appetite at higher doses of megestrol acetate, there was no change in weight or clinically relevant outcomes.15 In a second randomized controlled trial, 29 patients with illness-induced functional decline were enrolled in a strength training program in addition to being assigned to megestrol acetate or placebo. While patients receiving megestrol acetate with the exercise program had significant increases in weight and nutritional intake, they suffered a deterioration in physical function.16 In a pilot study, 17 nursing home residents who consistently ate less than 75% of their meals received megestrol acetate plus standard or optimal feeding assistance. The percentage of meals consumed increased only when patients received optimal feeding assistance in conjunction with megestrol acetate.17
The largest case-control study examining megestrol acetate for unintentional weight loss in older adults compared 709 residents in a multistate nursing home system treated with megestrol acetate to matched untreated controls. After 6 months of treatment, the median weight and change in weight did not differ significantly. Patients receiving megestrol acetate had a significant increase in mortality, surviving an average of 23.9 months, compared to 31.2 months for controls (P < .001).18
Additionally, two retrospective reviews of nursing home patients who were prescribed megestrol acetate showed incidences of venous thrombosis of 5% and 32%.19,20 Other potentially significant adverse effects include adrenal insufficiency and fluid retention.6 In 2019, the American Geriatrics Society’s Beers Criteria included megestrol acetate as a medication to avoid given its “minimal effect on weight; increases [in] risk of thrombotic events and possibly death in older adults.”21
Mirtazapine
No studies have evaluated mirtazapine for weight gain without concomitant depression. In older adults with depression, mirtazapine has minimal impact on promoting weight gain compared to other antidepressants. In two retrospective studies of older patients with depression and weight loss, researchers found no difference in weight gain in those treated with mirtazapine vs sertraline or other nontricyclic antidepressants, excluding fluoxetine.22,23
Cyproheptadine
There have been no controlled trials evaluating the use of cyproheptadine in older adults, in part due to anticholinergic side effects. In a trial of cancer patients, sedation and dizziness were common adverse effects.11 The 2019 American Geriatrics Society’s Beers Criteria include cyproheptadine as a medication to avoid based upon the “risk of confusion, dry mouth, constipation, and other anticholinergic effects or toxicity.”21
Dronabinol
In a retrospective cohort study of 28 long-term care residents with anorexia and weight loss, participants receiving dronabinol for 12 weeks had no statistically significant weight gain.24 The FDA cautions against prescribing dronabinol for older adults due to neurological side effects.12 A systematic review of randomized controlled trials found that cannabinoid-based medications in patients older than 50 years were associated with a significant increase in dizziness or lightheadedness and thinking or perception disorder.25
What You Should Do Instead
In the Choosing Wisely® initiative, the American Geriatrics Society recommends avoiding prescription appetite stimulants for patients with anorexia or cachexia.26 Instead, hospitalists should evaluate older patients for causes of unintentional weight loss, including malignancy, nonmalignant gastrointestinal disorders, depression, and dementia. Hospitalists can identify most causes based on the history, physical exam, and laboratory studies and initiate treatment for modifiable causes, such as constipation and depression.2
Hospitalists should work with an interprofessional team to develop an individualized plan to optimize caloric intake in the hospital (Table).27 One in five hospitalized older adults has insufficient caloric intake during admission, which is associated with increased risk for in-hospital and 90-day mortality.28 Removing dietary restrictions, increasing the variety of foods offered, and assisted eating may increase food intake.27,29 Hospitalists should also consider discontinuing or changing medications with gastrointestinal side effects, such as metformin, cholinesterase inhibitors, bisphosphonates, and oral iron supplements. Dietitians may recommend oral nutrition supplements; if started, patients should be offered supplements after discharge.27,29 For patients with limited access to food, social workers can help optimize social supports and identify community resources following discharge. Finally, hospitalists should coordinate with outpatient providers to monitor weight long-term.
Recommendations
- Recognize and address unintentional weight loss in older adults in the hospital.
- Do not prescribe appetite stimulants for unintentional weight loss in hospitalized older adults as they have no proven benefit for improving long-term outcomes and, in the case of megestrol acetate, may increase mortality.
- Work with an interprofessional team to address factors contributing to unintentional weight loss using nonpharmacologic options for improving food intake.
Conclusion
After discussing the lack of evidence supporting prescription appetite stimulants and the potential risks, we shifted the focus to optimizing oral intake. The team worked with the patient and the patient’s family to optimize nutrition following discharge and communicated the need for ongoing monitoring to the primary care provider.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected]
Acknowledgment
The authors thank Claire Campbell, MD, for her review of this manuscript.
1. Bouras EP, Lange SM, Scolapio JS. Rational approach to patients with unintentional weight loss. Mayo Clin Proc. 2001;76(9):923-929. https://doi.org/10.4065/76.9.923
2. McMinn J, Steel C, Bowman A. Investigation and management of unintentional weight loss in older adults. BMJ. 2011;342:d1732. https://doi.org/10.1136/bmj.d1732
3. Satish S, Winograd CH, Chavez C, Bloch DA. Geriatric targeting criteria as predictors of survival and health care utilization. J Am Geriatr Soc. 1996;44(8):914-921. https://doi.org/10.1111/j.1532-5415.1996.tb01860.x
4. Megace (megestrol acetate) [package insert]. Par Pharmaceutical Inc. Revised July 2005. Accessed January 27, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021778s000TOC.cfm
5. Yeh SS, Wu SY, Lee TP, et al. Improvement in quality-of-life measures and stimulation of weight gain after treatment with megestrol acetate oral suspension in geriatric cachexia: results of a double-blind, placebo-controlled study. J Am Geriatr Soc. 2000;48(5):485-492. https://doi.org/10.1111/j.1532-5415.2000.tb04993.x
6. Fox CB, Treadway AK, Blaszczyk AT, Sleeper RB. Reviews of therapeutics megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29(4):383-397. https://doi.org/10.1592/phco.29.4.383
7. Watanabe N, Omori IM, Nakagawa A, et al. Mirtazapine versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2011;(12):CD006528. https://doi.org/10.1002/14651858.CD006528.pub2
8. Fawcett J, Barkin RL. Review of the results from clinical studies on the efficacy, safety and tolerability of mirtazapine for the treatment of patients with major depression. J Affect Disord. 1998;51(3):267-285. https://doi.org/10.1016/S0165-0327(98)00224-9
9. Najib K, Moghtaderi M, Karamizadeh Z, Fallahzadeh E. Beneficial effect of cyproheptadine on body mass index in undernourished children: a randomized controlled trial. Iran J Pediatr. 2014;24(6):753-758.
10. Epifanio M, Marostica PC, Mattiello R, et al. A randomized, double-blind, placebo-controlled trial of cyproheptadine for appetite stimulation in cystic fibrosis. J Pediatr (Rio J). 2012;88(2):155-160. https://doi.org/10.2223/JPED.2174
11. Kardinal CG, Loprinzi CL, Schaid DJ, et al. A controlled trial of cyproheptadine in cancer patients with anorexia and/or cachexia. Cancer. 1990;65(12):2657-2662. https://doi.org/10.1002/1097-0142(19900615)65:12<2657::aid-cncr2820651210>3.0.co;2-s
12. MARINOL (dronabinol) [package insert]. Solvay Pharmaceuticals, Inc. Revised August 2017. Accessed April 27, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf.
13. Beal JE, Olson R, Laubenstein L, et al. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage. 1995;10(2):89-97. https://doi.org/10.1016/0885-3924(94)00117-4
14. Ruiz-García V, López-Briz E, Carbonell-Sanchis R, Bort-Martí S, Gonzálvez-Perales JL. Megestrol acetate for cachexia–anorexia syndrome. A systematic review. J Cachexia Sarcopenia Muscle. 2018;9(3):444-452. https://doi.org/10.1002/jcsm.12292
15. Reuben DB, Hirsch SH, Zhou K, Greendale GA. The effects of megestrol acetate suspension for elderly patients with reduced appetite after hospitalization: a phase II randomized clinical trial. J Am Geriatr Soc. 2005;53(6):970-975. https://doi.org/10.1111/j.1532-5415.2005.53307.x
16. Sullivan DH, Roberson PK, Smith ES, Price JA, Bopp MM. Effects of muscle strength training and megestrol acetate on strength, muscle mass, and function in frail older people. J Am Geriatr Soc. 2007;55(1):20-28. https://doi.org/10.1111/j.1532-5415.2006.01010.x
17. Simmons SF, Walker KA, Osterweil D. The effect of megestrol acetate on oral food and fluid intake in nursing home residents: a pilot study. J Am Med Dir Assoc. 2005;6(3):S5-S11. https://doi.org/10.1016/j.jamda.2005.03.014
18. Bodenner D, Spencer T, Riggs AT, Redman C, Strunk B, Hughes T. A retrospective study of the association between megestrol acetate administration and mortality among nursing home residents with clinically significant weight loss. Am J Geriatr Pharmacother. 2007;5(2):137-146. https://doi.org/10.1016/J.AMJOPHARM.2007.06.004
19. Kropsky B, Shi Y, Cherniack EP. Incidence of deep-venous thrombosis in nursing home residents using megestrol acetate. J Am Med Dir Assoc. 2003;4(5):255-256. https://doi.org/10.1097/01.JAM.0000083384.84558.75
20. Bolen JC, Andersen RE, Bennett RG. Deep vein thrombosis as a complication of megestrol acetate therapy among nursing home residents. J Am Med Dir Assoc. 2000;1(6):248-252.
21. Fick DM, Semla TP, Steinman M, et al. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674-694. https://doi.org/10.1111/jgs.15767
22. Mihara IQT, McCombs JS, Williams BR. The impact of mirtazapine compared with non-TCA antidepressants on weight change in nursing facility residents. Consult Pharm. 2005;20(3):217-223. https://doi.org/10.4140/tcp.n.2005.217
23. Goldberg RJ. Weight change in depressed nursing home patients on mirtazapine. J Am Geriatr Soc. 2002;50(8):1461. https://doi.org/10.1046/j.1532-5415.2002.50374.x
24. Wilson MMG, Philpot C, Morley JE. Anorexia of aging in long term care: is dronabinol an effective appetite stimulant?--a pilot study. J Nutr Health Aging. 2007;11(2):195-198.
25. Velayudhan L, McGoohan KL, Bhattacharyya S. Evaluation of THC-related neuropsychiatric symptoms among adults aged 50 years and older: a systematic review and metaregression analysis. JAMA Netw Open. 2021;4(2):e2035913. https://doi.org/10.1001/jamanetworkopen.2020.35913
26. AGS Choosing Wisely Workgroup. American Geriatrics Society identifies another five things that healthcare providers and patients should question. J Am Geriatr Soc. 2014;62(5):950-960. https://doi.org/10.1111/jgs.12770
27. Volkert D, Beck AM, Cederholm T, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. 2019;38(1):10-47. https://doi.org/10.1016/j.clnu.2018.05.024
28. Sullivan DH, Sun S, Walls RC. Protein-energy undernutrition among elderly hospitalized patients: a prospective study. JAMA. 1999;281(21):2013-2019. https://doi.org/10.1001/jama.281.21.2013
29. Feinberg J, Nielsen EE, Korang SK, et al. Nutrition support in hospitalised adults at nutritional risk. Cochrane Database Syst Rev. 2017;2017(5). https://doi.org/10.1002/14651858.CD011598.pub2
1. Bouras EP, Lange SM, Scolapio JS. Rational approach to patients with unintentional weight loss. Mayo Clin Proc. 2001;76(9):923-929. https://doi.org/10.4065/76.9.923
2. McMinn J, Steel C, Bowman A. Investigation and management of unintentional weight loss in older adults. BMJ. 2011;342:d1732. https://doi.org/10.1136/bmj.d1732
3. Satish S, Winograd CH, Chavez C, Bloch DA. Geriatric targeting criteria as predictors of survival and health care utilization. J Am Geriatr Soc. 1996;44(8):914-921. https://doi.org/10.1111/j.1532-5415.1996.tb01860.x
4. Megace (megestrol acetate) [package insert]. Par Pharmaceutical Inc. Revised July 2005. Accessed January 27, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021778s000TOC.cfm
5. Yeh SS, Wu SY, Lee TP, et al. Improvement in quality-of-life measures and stimulation of weight gain after treatment with megestrol acetate oral suspension in geriatric cachexia: results of a double-blind, placebo-controlled study. J Am Geriatr Soc. 2000;48(5):485-492. https://doi.org/10.1111/j.1532-5415.2000.tb04993.x
6. Fox CB, Treadway AK, Blaszczyk AT, Sleeper RB. Reviews of therapeutics megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29(4):383-397. https://doi.org/10.1592/phco.29.4.383
7. Watanabe N, Omori IM, Nakagawa A, et al. Mirtazapine versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2011;(12):CD006528. https://doi.org/10.1002/14651858.CD006528.pub2
8. Fawcett J, Barkin RL. Review of the results from clinical studies on the efficacy, safety and tolerability of mirtazapine for the treatment of patients with major depression. J Affect Disord. 1998;51(3):267-285. https://doi.org/10.1016/S0165-0327(98)00224-9
9. Najib K, Moghtaderi M, Karamizadeh Z, Fallahzadeh E. Beneficial effect of cyproheptadine on body mass index in undernourished children: a randomized controlled trial. Iran J Pediatr. 2014;24(6):753-758.
10. Epifanio M, Marostica PC, Mattiello R, et al. A randomized, double-blind, placebo-controlled trial of cyproheptadine for appetite stimulation in cystic fibrosis. J Pediatr (Rio J). 2012;88(2):155-160. https://doi.org/10.2223/JPED.2174
11. Kardinal CG, Loprinzi CL, Schaid DJ, et al. A controlled trial of cyproheptadine in cancer patients with anorexia and/or cachexia. Cancer. 1990;65(12):2657-2662. https://doi.org/10.1002/1097-0142(19900615)65:12<2657::aid-cncr2820651210>3.0.co;2-s
12. MARINOL (dronabinol) [package insert]. Solvay Pharmaceuticals, Inc. Revised August 2017. Accessed April 27, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf.
13. Beal JE, Olson R, Laubenstein L, et al. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage. 1995;10(2):89-97. https://doi.org/10.1016/0885-3924(94)00117-4
14. Ruiz-García V, López-Briz E, Carbonell-Sanchis R, Bort-Martí S, Gonzálvez-Perales JL. Megestrol acetate for cachexia–anorexia syndrome. A systematic review. J Cachexia Sarcopenia Muscle. 2018;9(3):444-452. https://doi.org/10.1002/jcsm.12292
15. Reuben DB, Hirsch SH, Zhou K, Greendale GA. The effects of megestrol acetate suspension for elderly patients with reduced appetite after hospitalization: a phase II randomized clinical trial. J Am Geriatr Soc. 2005;53(6):970-975. https://doi.org/10.1111/j.1532-5415.2005.53307.x
16. Sullivan DH, Roberson PK, Smith ES, Price JA, Bopp MM. Effects of muscle strength training and megestrol acetate on strength, muscle mass, and function in frail older people. J Am Geriatr Soc. 2007;55(1):20-28. https://doi.org/10.1111/j.1532-5415.2006.01010.x
17. Simmons SF, Walker KA, Osterweil D. The effect of megestrol acetate on oral food and fluid intake in nursing home residents: a pilot study. J Am Med Dir Assoc. 2005;6(3):S5-S11. https://doi.org/10.1016/j.jamda.2005.03.014
18. Bodenner D, Spencer T, Riggs AT, Redman C, Strunk B, Hughes T. A retrospective study of the association between megestrol acetate administration and mortality among nursing home residents with clinically significant weight loss. Am J Geriatr Pharmacother. 2007;5(2):137-146. https://doi.org/10.1016/J.AMJOPHARM.2007.06.004
19. Kropsky B, Shi Y, Cherniack EP. Incidence of deep-venous thrombosis in nursing home residents using megestrol acetate. J Am Med Dir Assoc. 2003;4(5):255-256. https://doi.org/10.1097/01.JAM.0000083384.84558.75
20. Bolen JC, Andersen RE, Bennett RG. Deep vein thrombosis as a complication of megestrol acetate therapy among nursing home residents. J Am Med Dir Assoc. 2000;1(6):248-252.
21. Fick DM, Semla TP, Steinman M, et al. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674-694. https://doi.org/10.1111/jgs.15767
22. Mihara IQT, McCombs JS, Williams BR. The impact of mirtazapine compared with non-TCA antidepressants on weight change in nursing facility residents. Consult Pharm. 2005;20(3):217-223. https://doi.org/10.4140/tcp.n.2005.217
23. Goldberg RJ. Weight change in depressed nursing home patients on mirtazapine. J Am Geriatr Soc. 2002;50(8):1461. https://doi.org/10.1046/j.1532-5415.2002.50374.x
24. Wilson MMG, Philpot C, Morley JE. Anorexia of aging in long term care: is dronabinol an effective appetite stimulant?--a pilot study. J Nutr Health Aging. 2007;11(2):195-198.
25. Velayudhan L, McGoohan KL, Bhattacharyya S. Evaluation of THC-related neuropsychiatric symptoms among adults aged 50 years and older: a systematic review and metaregression analysis. JAMA Netw Open. 2021;4(2):e2035913. https://doi.org/10.1001/jamanetworkopen.2020.35913
26. AGS Choosing Wisely Workgroup. American Geriatrics Society identifies another five things that healthcare providers and patients should question. J Am Geriatr Soc. 2014;62(5):950-960. https://doi.org/10.1111/jgs.12770
27. Volkert D, Beck AM, Cederholm T, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. 2019;38(1):10-47. https://doi.org/10.1016/j.clnu.2018.05.024
28. Sullivan DH, Sun S, Walls RC. Protein-energy undernutrition among elderly hospitalized patients: a prospective study. JAMA. 1999;281(21):2013-2019. https://doi.org/10.1001/jama.281.21.2013
29. Feinberg J, Nielsen EE, Korang SK, et al. Nutrition support in hospitalised adults at nutritional risk. Cochrane Database Syst Rev. 2017;2017(5). https://doi.org/10.1002/14651858.CD011598.pub2
© 2021 Society of Hospital Medicine
Clinical Guideline Highlights for the Hospitalist: Focused Updates to Pediatric Asthma Management
Asthma is a heterogeneous condition characterized by airway hyperresponsiveness and obstruction, with associated airway inflammation and remodeling.2 Asthma affects 25 million people in the United States and 334 million people worldwide, with significant healthcare disparities across race and ethnicity.2-6 Asthma is the third most common reason for hospitalizations in pediatrics, accounting for 180,000 annual hospitalizations for children and adults.3,7 In 2020, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel provided a focused update to the Asthma Management Guidelines, centered on six topics with sufficient new evidence. The management of status asthmaticus was not included in this update. We spotlight four of the recommendations applicable to the practice of pediatric hospital medicine.
Key Recommendations for the Hospitalist
Recommendation 1. Children 0 to 4 years old with recurrent wheezing triggered by a respiratory tract infection (RTI) and no wheezing between infections should receive a short course of daily inhaled corticosteroids (ICS) at the onset of a RTI, with an as-needed short-acting beta agonist (SABA) for quick-relief therapy compared to SABA alone (evidence quality: high; recommendation strength: conditional).
Recurrent wheezing is defined as clinically significant periods of wheezing that are reversible or consistent with bronchospasm and as ≥3 episodes in a lifetime or 2 episodes in the past year. It is important to adhere to this definition to prevent inappropriate use of ICS for bronchiolitis. This treatment is associated with a reduction of use of systemic steroids (relative risk [RR], 0.67; 95% CI, 0.46-0.98) without a statistical decrease in acute care visits (RR, 0.90; 95% CI, 0.77-1.05) or hospitalizations (RR, 0.77; 95% CI, 0.06-9.68). Improved transition of care is essential between the primary care provider, hospitalist, and family to ensure an understanding of how/when to initiate ICS at the onset of a RTI. Potential harms include effect on growth and overprescribing. Growth should be monitored because data are conflicting.
Recommendation 2. Individuals ages 12 years and older with mild persistent asthma should use as-needed SABA and may use either daily low-dose ICS or as-needed ICS when symptoms flare (evidence quality: moderate; recommendation strength: conditional).
In intermittent therapy, patients take a SABA followed by an ICS as needed for acute asthma symptoms. This recommendation is driven by asthma-control and quality-of-life outcomes, with caregivers reporting that intermittent dosing could “offer flexibility and potentially reduce side effects.” There were no differences between management regimens with respect to systemic steroid use (RR, 0.70; 95% CI, 0.30-1.64) or urgent care visits (RR, 0.25; 95% CI, 0.05-1.16). Differing perception of symptoms by individuals may lead to undertreating or overtreating, and intermittent administration makes it challenging for clinicians to assess the need to adjust therapy.
Recommendation 3. Children 4 years and older with moderate to severe persistent asthma should use ICS-formoterol in a single inhaler used as both daily controller and reliever therapy compared to either (a) higher-dose ICS as daily controller therapy and SABA for quick-relief therapy or (b) a same-dose ICS-long-acting beta agonist (LABA) as daily controller therapy and SABA for quick-relief therapy (evidence quality: high for ages ≥12 years, moderate for ages 4-11 years; recommendation strength: strong).
For children 4 years and older, it is recommended to use “single maintenance and reliever therapy” (SMART) with a single-inhaler containing either low- or medium-dose ICS and formoterol when stepping up from Step 2 (daily low-dose ICS and as-needed SABA) to Step 3 (daily and as-needed low-dose ICS-formoterol) and Step 4 (daily and as-needed medium-dose ICS-formoterol).
Recommendation 4. If individuals with asthma have symptoms related to indoor allergens, confirmed by history or allergy testing, they should use a multicomponent allergen-specific mitigation intervention. Allergen mitigation interventions should not be a part of routine asthma management for individuals with asthma who do not have symptoms related to exposure to specific indoor allergens (evidence quality: low; recommendation strength: conditional).
Providers often emphasize exposure to potential indoor allergens such as carpets and pets when taking an asthma history and counsel removal of these triggers. However, all recommendations related to allergies in the 2020 updates have low-moderate evidence quality and conditional recommendation strength. Hospitalists should instead focus their questions on allergy symptoms and triggers and recommend multicomponent mitigation intervention only if there is a confirmed allergy history. Families should continue routine good practices such as house cleaning and laundering, but other interventions are not evidence-based.
CRITIQUE
Methods
The Expert Panel included a diverse group of clinicians, a pharmacist, and health policy experts. In 2015, a needs assessment identified 6 out of 17 priority topics with sufficient new information for updates. Key questions were drafted, and systematic reviews were published through 2018. The Expert Panel made its recommendations using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach. The Expert Panel informed its recommendations with input from focus groups, including individuals with asthma and caregivers. The NHLBI posted the draft report for public review, and comments were considered. We believe these methods effectively developed evidence-based recommendations, and the diversity of stakeholders increases the value of this guideline. However, the infrequency of updates limits the utility of the NHLBI guidelines as compared with annual GINA (Global Initiative for Asthma) updates.
There are important considerations in assessing these guidelines. Specifically, the validity of systemic steroid courses as an outcome for children ages 0 to 4 years is controversial. Second, the studies cited in defense of intermittent ICS use in children >12 years of age excluded pediatric patients and did not include readmissions as a primary outcome, which is of particular interest to the hospitalist.
Potential Conflicts for Guideline Authors
The Expert Panel reported all potential conflicts of interest (COIs), which were rated by the Expert Panel Chair and Journal of Allergy and Clinical Immunology editors. Individuals with high COIs were excluded from the Expert Panel. Those with moderate COIs were recused for that topic. Low COIs were not related to the guideline.
Generalizability of the Guideline
These guidelines are based on systematic reviews with large sample sizes and patients of all ages. They are generalizable. However, the authors recognize that variations in asthma require individualized approaches. They identify this as a reason for the lack of strong recommendations for asthma standards of care.
AREAS OF FUTURE STUDY
Biologics have progressed considerably since revision of the guidelines. The 2020 guidelines did not address these to prevent delay of the guideline release, but recommendations should be included in future guidelines. Future studies should address healthcare disparities in asthma, barriers to equitable care, and how to eliminate them, as guided by the President’s Task Force.8 Status asthmaticus should be included in future updates.
1. Expert Panel Working Group of the National Heart, Lung, and Blood Institute (NHLBI) administered and coordinated National Asthma Education and Prevention Program Coordinating Committee (NAEPPCC), Cloutier MM, Baptist AP, Blake KV, et al. 2020 focused updates to the asthma management guidelines: a report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol. 2020;146(6):1217-1270. https://doi.org/10.1016/j.jaci.2020.10.003.
2. Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018;391(10122):783-800. https://doi.org/10.1016/S0140-6736(17)33311-1
3. Centers for Disease Control and Prevention. Most recent asthma data. Reviewed March 30 2021. Accessed October 5, 2021. www.cdc.gov/asthma/most_recent_data.htm
4. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163-2196. https://doi.org/10.1016/S0140-6736(12)61729-2
5. Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15(3):348-356. https://doi.org/10.1513/AnnalsATS.201703-259OC
6. Moorman JE, Akinbami LJ, Bailey CM, et al. National surveillance of asthma: United States, 2001-2010. Vital Health Stat 3. 2012;(35):1-58.
7. Witt WP, Weiss AJ, Elixhauser A. Overview of hospital stays for children in the United States, 2012: Statistical Brief #187. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Agency for Healthcare Research and Quality; February 2006.
8. U.S. Environmental Protection Agency. President’s Task Force on Environmental Health Risks and Safety Risks to Children: Coordinated Federal Action Plan to Reduce Racial and Ethnic Asthma Disparities. May 2012. https://19january2017snapshot.epa.gov/sites/production/files/2014-08/documents/federal_asthma_disparities_action_plan.pdf
Asthma is a heterogeneous condition characterized by airway hyperresponsiveness and obstruction, with associated airway inflammation and remodeling.2 Asthma affects 25 million people in the United States and 334 million people worldwide, with significant healthcare disparities across race and ethnicity.2-6 Asthma is the third most common reason for hospitalizations in pediatrics, accounting for 180,000 annual hospitalizations for children and adults.3,7 In 2020, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel provided a focused update to the Asthma Management Guidelines, centered on six topics with sufficient new evidence. The management of status asthmaticus was not included in this update. We spotlight four of the recommendations applicable to the practice of pediatric hospital medicine.
Key Recommendations for the Hospitalist
Recommendation 1. Children 0 to 4 years old with recurrent wheezing triggered by a respiratory tract infection (RTI) and no wheezing between infections should receive a short course of daily inhaled corticosteroids (ICS) at the onset of a RTI, with an as-needed short-acting beta agonist (SABA) for quick-relief therapy compared to SABA alone (evidence quality: high; recommendation strength: conditional).
Recurrent wheezing is defined as clinically significant periods of wheezing that are reversible or consistent with bronchospasm and as ≥3 episodes in a lifetime or 2 episodes in the past year. It is important to adhere to this definition to prevent inappropriate use of ICS for bronchiolitis. This treatment is associated with a reduction of use of systemic steroids (relative risk [RR], 0.67; 95% CI, 0.46-0.98) without a statistical decrease in acute care visits (RR, 0.90; 95% CI, 0.77-1.05) or hospitalizations (RR, 0.77; 95% CI, 0.06-9.68). Improved transition of care is essential between the primary care provider, hospitalist, and family to ensure an understanding of how/when to initiate ICS at the onset of a RTI. Potential harms include effect on growth and overprescribing. Growth should be monitored because data are conflicting.
Recommendation 2. Individuals ages 12 years and older with mild persistent asthma should use as-needed SABA and may use either daily low-dose ICS or as-needed ICS when symptoms flare (evidence quality: moderate; recommendation strength: conditional).
In intermittent therapy, patients take a SABA followed by an ICS as needed for acute asthma symptoms. This recommendation is driven by asthma-control and quality-of-life outcomes, with caregivers reporting that intermittent dosing could “offer flexibility and potentially reduce side effects.” There were no differences between management regimens with respect to systemic steroid use (RR, 0.70; 95% CI, 0.30-1.64) or urgent care visits (RR, 0.25; 95% CI, 0.05-1.16). Differing perception of symptoms by individuals may lead to undertreating or overtreating, and intermittent administration makes it challenging for clinicians to assess the need to adjust therapy.
Recommendation 3. Children 4 years and older with moderate to severe persistent asthma should use ICS-formoterol in a single inhaler used as both daily controller and reliever therapy compared to either (a) higher-dose ICS as daily controller therapy and SABA for quick-relief therapy or (b) a same-dose ICS-long-acting beta agonist (LABA) as daily controller therapy and SABA for quick-relief therapy (evidence quality: high for ages ≥12 years, moderate for ages 4-11 years; recommendation strength: strong).
For children 4 years and older, it is recommended to use “single maintenance and reliever therapy” (SMART) with a single-inhaler containing either low- or medium-dose ICS and formoterol when stepping up from Step 2 (daily low-dose ICS and as-needed SABA) to Step 3 (daily and as-needed low-dose ICS-formoterol) and Step 4 (daily and as-needed medium-dose ICS-formoterol).
Recommendation 4. If individuals with asthma have symptoms related to indoor allergens, confirmed by history or allergy testing, they should use a multicomponent allergen-specific mitigation intervention. Allergen mitigation interventions should not be a part of routine asthma management for individuals with asthma who do not have symptoms related to exposure to specific indoor allergens (evidence quality: low; recommendation strength: conditional).
Providers often emphasize exposure to potential indoor allergens such as carpets and pets when taking an asthma history and counsel removal of these triggers. However, all recommendations related to allergies in the 2020 updates have low-moderate evidence quality and conditional recommendation strength. Hospitalists should instead focus their questions on allergy symptoms and triggers and recommend multicomponent mitigation intervention only if there is a confirmed allergy history. Families should continue routine good practices such as house cleaning and laundering, but other interventions are not evidence-based.
CRITIQUE
Methods
The Expert Panel included a diverse group of clinicians, a pharmacist, and health policy experts. In 2015, a needs assessment identified 6 out of 17 priority topics with sufficient new information for updates. Key questions were drafted, and systematic reviews were published through 2018. The Expert Panel made its recommendations using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach. The Expert Panel informed its recommendations with input from focus groups, including individuals with asthma and caregivers. The NHLBI posted the draft report for public review, and comments were considered. We believe these methods effectively developed evidence-based recommendations, and the diversity of stakeholders increases the value of this guideline. However, the infrequency of updates limits the utility of the NHLBI guidelines as compared with annual GINA (Global Initiative for Asthma) updates.
There are important considerations in assessing these guidelines. Specifically, the validity of systemic steroid courses as an outcome for children ages 0 to 4 years is controversial. Second, the studies cited in defense of intermittent ICS use in children >12 years of age excluded pediatric patients and did not include readmissions as a primary outcome, which is of particular interest to the hospitalist.
Potential Conflicts for Guideline Authors
The Expert Panel reported all potential conflicts of interest (COIs), which were rated by the Expert Panel Chair and Journal of Allergy and Clinical Immunology editors. Individuals with high COIs were excluded from the Expert Panel. Those with moderate COIs were recused for that topic. Low COIs were not related to the guideline.
Generalizability of the Guideline
These guidelines are based on systematic reviews with large sample sizes and patients of all ages. They are generalizable. However, the authors recognize that variations in asthma require individualized approaches. They identify this as a reason for the lack of strong recommendations for asthma standards of care.
AREAS OF FUTURE STUDY
Biologics have progressed considerably since revision of the guidelines. The 2020 guidelines did not address these to prevent delay of the guideline release, but recommendations should be included in future guidelines. Future studies should address healthcare disparities in asthma, barriers to equitable care, and how to eliminate them, as guided by the President’s Task Force.8 Status asthmaticus should be included in future updates.
Asthma is a heterogeneous condition characterized by airway hyperresponsiveness and obstruction, with associated airway inflammation and remodeling.2 Asthma affects 25 million people in the United States and 334 million people worldwide, with significant healthcare disparities across race and ethnicity.2-6 Asthma is the third most common reason for hospitalizations in pediatrics, accounting for 180,000 annual hospitalizations for children and adults.3,7 In 2020, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel provided a focused update to the Asthma Management Guidelines, centered on six topics with sufficient new evidence. The management of status asthmaticus was not included in this update. We spotlight four of the recommendations applicable to the practice of pediatric hospital medicine.
Key Recommendations for the Hospitalist
Recommendation 1. Children 0 to 4 years old with recurrent wheezing triggered by a respiratory tract infection (RTI) and no wheezing between infections should receive a short course of daily inhaled corticosteroids (ICS) at the onset of a RTI, with an as-needed short-acting beta agonist (SABA) for quick-relief therapy compared to SABA alone (evidence quality: high; recommendation strength: conditional).
Recurrent wheezing is defined as clinically significant periods of wheezing that are reversible or consistent with bronchospasm and as ≥3 episodes in a lifetime or 2 episodes in the past year. It is important to adhere to this definition to prevent inappropriate use of ICS for bronchiolitis. This treatment is associated with a reduction of use of systemic steroids (relative risk [RR], 0.67; 95% CI, 0.46-0.98) without a statistical decrease in acute care visits (RR, 0.90; 95% CI, 0.77-1.05) or hospitalizations (RR, 0.77; 95% CI, 0.06-9.68). Improved transition of care is essential between the primary care provider, hospitalist, and family to ensure an understanding of how/when to initiate ICS at the onset of a RTI. Potential harms include effect on growth and overprescribing. Growth should be monitored because data are conflicting.
Recommendation 2. Individuals ages 12 years and older with mild persistent asthma should use as-needed SABA and may use either daily low-dose ICS or as-needed ICS when symptoms flare (evidence quality: moderate; recommendation strength: conditional).
In intermittent therapy, patients take a SABA followed by an ICS as needed for acute asthma symptoms. This recommendation is driven by asthma-control and quality-of-life outcomes, with caregivers reporting that intermittent dosing could “offer flexibility and potentially reduce side effects.” There were no differences between management regimens with respect to systemic steroid use (RR, 0.70; 95% CI, 0.30-1.64) or urgent care visits (RR, 0.25; 95% CI, 0.05-1.16). Differing perception of symptoms by individuals may lead to undertreating or overtreating, and intermittent administration makes it challenging for clinicians to assess the need to adjust therapy.
Recommendation 3. Children 4 years and older with moderate to severe persistent asthma should use ICS-formoterol in a single inhaler used as both daily controller and reliever therapy compared to either (a) higher-dose ICS as daily controller therapy and SABA for quick-relief therapy or (b) a same-dose ICS-long-acting beta agonist (LABA) as daily controller therapy and SABA for quick-relief therapy (evidence quality: high for ages ≥12 years, moderate for ages 4-11 years; recommendation strength: strong).
For children 4 years and older, it is recommended to use “single maintenance and reliever therapy” (SMART) with a single-inhaler containing either low- or medium-dose ICS and formoterol when stepping up from Step 2 (daily low-dose ICS and as-needed SABA) to Step 3 (daily and as-needed low-dose ICS-formoterol) and Step 4 (daily and as-needed medium-dose ICS-formoterol).
Recommendation 4. If individuals with asthma have symptoms related to indoor allergens, confirmed by history or allergy testing, they should use a multicomponent allergen-specific mitigation intervention. Allergen mitigation interventions should not be a part of routine asthma management for individuals with asthma who do not have symptoms related to exposure to specific indoor allergens (evidence quality: low; recommendation strength: conditional).
Providers often emphasize exposure to potential indoor allergens such as carpets and pets when taking an asthma history and counsel removal of these triggers. However, all recommendations related to allergies in the 2020 updates have low-moderate evidence quality and conditional recommendation strength. Hospitalists should instead focus their questions on allergy symptoms and triggers and recommend multicomponent mitigation intervention only if there is a confirmed allergy history. Families should continue routine good practices such as house cleaning and laundering, but other interventions are not evidence-based.
CRITIQUE
Methods
The Expert Panel included a diverse group of clinicians, a pharmacist, and health policy experts. In 2015, a needs assessment identified 6 out of 17 priority topics with sufficient new information for updates. Key questions were drafted, and systematic reviews were published through 2018. The Expert Panel made its recommendations using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach. The Expert Panel informed its recommendations with input from focus groups, including individuals with asthma and caregivers. The NHLBI posted the draft report for public review, and comments were considered. We believe these methods effectively developed evidence-based recommendations, and the diversity of stakeholders increases the value of this guideline. However, the infrequency of updates limits the utility of the NHLBI guidelines as compared with annual GINA (Global Initiative for Asthma) updates.
There are important considerations in assessing these guidelines. Specifically, the validity of systemic steroid courses as an outcome for children ages 0 to 4 years is controversial. Second, the studies cited in defense of intermittent ICS use in children >12 years of age excluded pediatric patients and did not include readmissions as a primary outcome, which is of particular interest to the hospitalist.
Potential Conflicts for Guideline Authors
The Expert Panel reported all potential conflicts of interest (COIs), which were rated by the Expert Panel Chair and Journal of Allergy and Clinical Immunology editors. Individuals with high COIs were excluded from the Expert Panel. Those with moderate COIs were recused for that topic. Low COIs were not related to the guideline.
Generalizability of the Guideline
These guidelines are based on systematic reviews with large sample sizes and patients of all ages. They are generalizable. However, the authors recognize that variations in asthma require individualized approaches. They identify this as a reason for the lack of strong recommendations for asthma standards of care.
AREAS OF FUTURE STUDY
Biologics have progressed considerably since revision of the guidelines. The 2020 guidelines did not address these to prevent delay of the guideline release, but recommendations should be included in future guidelines. Future studies should address healthcare disparities in asthma, barriers to equitable care, and how to eliminate them, as guided by the President’s Task Force.8 Status asthmaticus should be included in future updates.
1. Expert Panel Working Group of the National Heart, Lung, and Blood Institute (NHLBI) administered and coordinated National Asthma Education and Prevention Program Coordinating Committee (NAEPPCC), Cloutier MM, Baptist AP, Blake KV, et al. 2020 focused updates to the asthma management guidelines: a report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol. 2020;146(6):1217-1270. https://doi.org/10.1016/j.jaci.2020.10.003.
2. Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018;391(10122):783-800. https://doi.org/10.1016/S0140-6736(17)33311-1
3. Centers for Disease Control and Prevention. Most recent asthma data. Reviewed March 30 2021. Accessed October 5, 2021. www.cdc.gov/asthma/most_recent_data.htm
4. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163-2196. https://doi.org/10.1016/S0140-6736(12)61729-2
5. Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15(3):348-356. https://doi.org/10.1513/AnnalsATS.201703-259OC
6. Moorman JE, Akinbami LJ, Bailey CM, et al. National surveillance of asthma: United States, 2001-2010. Vital Health Stat 3. 2012;(35):1-58.
7. Witt WP, Weiss AJ, Elixhauser A. Overview of hospital stays for children in the United States, 2012: Statistical Brief #187. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Agency for Healthcare Research and Quality; February 2006.
8. U.S. Environmental Protection Agency. President’s Task Force on Environmental Health Risks and Safety Risks to Children: Coordinated Federal Action Plan to Reduce Racial and Ethnic Asthma Disparities. May 2012. https://19january2017snapshot.epa.gov/sites/production/files/2014-08/documents/federal_asthma_disparities_action_plan.pdf
1. Expert Panel Working Group of the National Heart, Lung, and Blood Institute (NHLBI) administered and coordinated National Asthma Education and Prevention Program Coordinating Committee (NAEPPCC), Cloutier MM, Baptist AP, Blake KV, et al. 2020 focused updates to the asthma management guidelines: a report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol. 2020;146(6):1217-1270. https://doi.org/10.1016/j.jaci.2020.10.003.
2. Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018;391(10122):783-800. https://doi.org/10.1016/S0140-6736(17)33311-1
3. Centers for Disease Control and Prevention. Most recent asthma data. Reviewed March 30 2021. Accessed October 5, 2021. www.cdc.gov/asthma/most_recent_data.htm
4. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163-2196. https://doi.org/10.1016/S0140-6736(12)61729-2
5. Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15(3):348-356. https://doi.org/10.1513/AnnalsATS.201703-259OC
6. Moorman JE, Akinbami LJ, Bailey CM, et al. National surveillance of asthma: United States, 2001-2010. Vital Health Stat 3. 2012;(35):1-58.
7. Witt WP, Weiss AJ, Elixhauser A. Overview of hospital stays for children in the United States, 2012: Statistical Brief #187. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Agency for Healthcare Research and Quality; February 2006.
8. U.S. Environmental Protection Agency. President’s Task Force on Environmental Health Risks and Safety Risks to Children: Coordinated Federal Action Plan to Reduce Racial and Ethnic Asthma Disparities. May 2012. https://19january2017snapshot.epa.gov/sites/production/files/2014-08/documents/federal_asthma_disparities_action_plan.pdf
© 2021 Society of Hospital Medicine
Things We Do for No Reason™: Prescribing Thiamine, Folate and Multivitamins on Discharge for Patients With Alcohol Use Disorder
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old man with alcohol use disorder (AUD) is admitted with decompensated heart failure and experiences alcohol withdrawal during the hospitalization. He improves with guideline-directed heart failure therapy and benzodiazepines for alcohol withdrawal. Discharge medications are metoprolol succinate, lisinopril, furosemide, aspirin, atorvastatin, thiamine, folic acid, and a multivitamin. No medications are offered for AUD treatment. At follow-up a week later, he presents with dyspnea and reports poor medication adherence and a return to heavy drinking.
WHY YOU MIGHT THINK IT IS HELPFUL TO PRESCRIBE VITAMIN SUPPLEMENTATION TO PATIENTS WITH AUD AT HOSPITAL DISCHARGE
AUD is common among hospitalized patients.1 AUD increases the risk of vitamin deficiencies due to the toxic effects of alcohol on the gastrointestinal tract and liver, causing impaired digestion, reduced absorption, and increased degradation of key micronutrients.2,3 Other risk factors for AUD-associated vitamin deficiencies include food insecurity and the replacement of nutrient-rich food with alcohol. Since the body does not readily store water-soluble vitamins, including thiamine (vitamin B1) and folate (vitamin B9), people require regular dietary replenishment of these nutrients. Thus, if individuals with AUD eat less fortified food, they risk developing thiamine, folate, niacin, and other vitamin deficiencies. Since AUD puts patients at risk for vitamin deficiencies, hospitalized patients typically receive vitamin supplementation, including thiamine, folic acid, and a multivitamin (most formulations contain water-soluble vitamins B and C and micronutrients).1 Hospitalists often continue these medications at discharge.
Thiamine deficiency may manifest as Wernicke encephalopathy (WE), peripheral neuropathy, or a high-output heart failure state.
Hospitalists empirically treat with thiamine, folate, and other vitamins upon hospital admission with the intent of reducing morbidity associated with nutritional deficiencies.1 Repletion poses few risks to patients since the kidneys eliminate water-soluble vitamins. Multivitamins also have a low potential for direct harm and a low cost. Given the consequences of missing a deficiency, alcohol withdrawal–management order sets commonly embed vitamin repletion orders.6
WHY ROUTINELY PRESCRIBING VITAMIN SUPPLEMENTATION AT HOSPITAL DISCHARGE IN PATIENTS WITH AUD IS A TWDFNR
Hospitalists often reflexively continue vitamin supplementation on discharge. Unfortunately, there is no evidence that prescribing vitamin supplementation leads to clinically significant improvements for people with AUD, and patients can experience harms.
Literature and specialty guidelines lack consensus on rational vitamin supplementation in patients with AUD.2,7,8 Folate testing is not recommended due to inaccuracies.9 In fact, clinical data, such as body mass index, more accurately predict alcohol-related cognitive impairment than blood levels of vitamins.10 In one small study of vitamin deficiencies among patients with acute alcohol intoxication, none had low B12 or folate levels.11 A systematic review among people experiencing homelessness with unhealthy alcohol use showed no clear pattern of vitamin deficiencies across studies, although vitamin C and thiamine deficiencies predominated.12
In the absence of reliable thiamine and folate testing to confirm deficiencies, clinicians must use their clinical assessment skills. Clinicians rarely evaluate patients with AUD for vitamin deficiency risk factors and instead reflexively prescribe vitamin supplementation. An AUD diagnosis may serve as a sensitive, but not specific, risk factor for those in need of vitamin supplementation.
Other limitations make prescribing oral vitamins reflexively at discharge a low-value practice. Thiamine, often prescribed orally in the hospital and on discharge, has poor oral bioavailability.13 Unfortunately, people with AUD have decreased and variable thiamine absorption. To prevent WE, thiamine must cross the blood-brain barrier, and the literature provides insufficient evidence to guide clinicians on an appropriate oral thiamine dose, frequency, or duration of treatment.14 While early high-dose IV thiamine may treat or prevent WE during hospitalization, low-dose oral thiamine may not provide benefit to patients with AUD.5
The literature also provides sparse evidence for folate supplementation and its optimal dose. Since 1998, when the United States mandated fortifying grain products with folic acid, people rarely have low serum folate levels. Though patients with AUD have lower folate levels relative to the general population,15 this difference does not seem clinically significant. While limited data show an association between oral multivitamin supplementation and improved serum nutrient levels among people with AUD, we lack evidence on clinical outcomes.16
Most importantly, for a practice lacking strong evidence, prescribing multiple vitamins at discharge may result in harm from polypharmacy and unnecessary costs for the recently hospitalized patient. Alcohol use is associated with decreased adherence to medications for chronic conditions,17 including HIV, hypertension, hyperlipidemia, and psychiatric diseases. In addition, research shows an association between an increased number of discharge medications and higher risk for hospital readmission. The harm may actually correlate with the number of medications and complexity of the regimen rather than the risk profile of the medications themselves.18 Providers underestimate the impact of adding multiple vitamins at discharge, especially for patients who have several co-occurring medical conditions that require other medications. Furthermore, insurance rarely covers vitamins, leading hospitals or patients to incur the costs at discharge.
WHEN TO CONSIDER VITAMIN SUPPLEMENTATION AT DISCHARGE FOR PATIENTS WITH AUD
When treating patients with AUD, consider the potential benefit of vitamin supplementation for the individual. If a patient with regular, heavy alcohol use is at high risk of vitamin deficiencies due to ongoing risk factors (Table), hospitalists should discuss vitamin therapy via a patient-centered risk-benefit process.

When considering discharge vitamins, make concurrent efforts to enhance patient nutrition via decreased alcohol consumption and improved healthy food intake. While some patients do not have a goal of abstaining from alcohol, providing resources to food access may help decrease the harms of drinking. Education may help patients learn that vitamin deficiencies can result from heavy alcohol use.
Multivitamin formulations have variable doses of vitamins but can contain 100% or more of the daily value of thiamine and folic acid. For patients with AUD at lower risk of vitamin deficiencies (ie, mild alcohol use disorder with a healthy diet), discuss risks and benefits of supplementation. If they desire supplementation, a single thiamine-containing vitamin alone may be highest yield since it is the most morbid vitamin deficiency. Conversely, a patient with heavy alcohol intake and other risk factors for malnutrition may benefit from a higher dose of supplementation, achieved by prescribing a multivitamin alongside additional doses of thiamine and folate. However, the literature lacks evidence to guide clinicians on optimal vitamin dosing and formulations.
WHAT WE SHOULD DO INSTEAD
Instead of reflexively prescribing thiamine, folate, and multivitamin, clinicians can assess patients for AUD, provide motivational interviewing, and offer AUD treatment. Hospitalists should initiate and prescribe evidence-based medications for AUD for patients interested in reducing or stopping their alcohol intake. We can choose from Food and Drug Administration–approved AUD medications, including naltrexone and acamprosate. Unfortunately, less than 3% of patients with AUD receive medication therapy.19 Our healthcare systems can also refer individuals to community psychosocial treatment.
For patients with risk factors, prescribe empiric IV thiamine during hospitalization. Clinicians should then perform a risk-benefit assessment rather than reflexively prescribe vitamins to patients with AUD at discharge. We should also counsel patients to eat food when drinking to decrease alcohol-related harms.20 Patients experiencing food insecurity should be linked to food resources through inpatient nutritional and social work consultations.
Elicit patient preference around vitamin supplementation after discharge. For patients with AUD who desire supplementation without risk factors for malnutrition (Table), consider prescribing a single thiamine-containing vitamin for prevention of thiamine deficiency, which, unlike other vitamin deficiencies, has the potential to be irreversible and life-threatening. Though no evidence currently supports this practice, it stands to reason that prescribing a single tablet could decrease the number of pills for patients who struggle with pill burden.
RECOMMENDATIONS
- Offer evidence-based medication treatment for AUD.
- Connect patients experiencing food insecurity with appropriate resources.
- For patients initiated on a multivitamin, folate, and high-dose IV thiamine at admission, perform vitamin de-escalation during hospitalization.
- Risk-stratify hospitalized patients with AUD for additional risk factors for vitamin deficiencies (Table). In those with additional risk factors, offer supplementation if consistent with patient preference. Balance the benefits of vitamin supplementation with the risks of polypharmacy, particularly if the patient has conditions requiring multiple medications.
CONCLUSION
Returning to our case, the hospitalist initiates IV thiamine, folate, and a multivitamin at admission and assesses the patient’s nutritional status and food insecurity. The hospitalist deems the patient—who eats regular, balanced meals—to be at low risk for vitamin deficiencies. The medical team discontinues folate and multivitamins before discharge and continues IV thiamine throughout the 3-day hospitalization. The patient and clinician agree that unaddressed AUD played a key role in the patient’s heart failure exacerbation. The clinician elicits the patient’s goals around their alcohol use, discusses AUD treatment, and initiates naltrexone for AUD.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
1. Makdissi R, Stewart SH. Care for hospitalized patients with unhealthy alcohol use: a narrative review. Addict Sci Clin Pract. 2013;8(1):11. https://doi.org/10.1186/1940-0640-8-11
2. Lewis MJ. Alcoholism and nutrition: a review of vitamin supplementation and treatment. Curr Opin Clin Nutr Metab Care. 2020;23(2):138-144. https://doi.org/10.1097/mco.0000000000000622
3. Bergmans RS, Coughlin L, Wilson T, Malecki K. Cross-sectional associations of food insecurity with smoking cigarettes and heavy alcohol use in a population-based sample of adults. Drug Alcohol Depend. 2019;205:107646. https://doi.org/10.1016/j.drugalcdep.2019.107646
4. Latt N, Dore G. Thiamine in the treatment of Wernicke encephalopathy in patients with alcohol use disorders. Intern Med J. 2014;44(9):911-915. https://doi.org/10.1111/imj.12522
5. Flannery AH, Adkins DA, Cook AM. Unpeeling the evidence for the banana bag: evidence-based recommendations for the management of alcohol-associated vitamin and electrolyte deficiencies in the ICU. Crit Care Med. 2016;44(8):1545-1552. https://doi.org/10.1097/ccm.0000000000001659
6. Wai JM, Aloezos C, Mowrey WB, Baron SW, Cregin R, Forman HL. Using clinical decision support through the electronic medical record to increase prescribing of high-dose parenteral thiamine in hospitalized patients with alcohol use disorder. J Subst Abuse Treat. 2019;99:117-123. https://doi.org/10.1016/j.jsat.2019.01.017
7. American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. January 2020. https://www.asam.org/docs/default-source/quality-science/the_asam_clinical_practice_guideline_on_alcohol-1.pdf?sfvrsn=ba255c2_2
8. O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51(1):307-328. https://doi.org/10.1002/hep.23258
9. Breu AC, Theisen-Toupal J, Feldman LS. Serum and red blood cell folate testing on hospitalized patients. J Hosp Med. 2015;10(11):753-755. https://doi.org/10.1002/jhm.2385
10. Gautron M-A, Questel F, Lejoyeux M, Bellivier F, Vorspan F. Nutritional status during inpatient alcohol detoxification. Alcohol Alcohol. 2018;53(1):64-70. https://doi.org/10.1093/alcalc/agx086
11. Li SF, Jacob J, Feng J, Kulkarni M. Vitamin deficiencies in acutely intoxicated patients in the ED. Am J Emerg Med. 2008;26(7):792-795. https://doi.org/10.1016/j.ajem.2007.10.003
12. Ijaz S, Jackson J, Thorley H, et al. Nutritional deficiencies in homeless persons with problematic drinking: a systematic review. Int J Equity Health. 2017;16(1):71. https://doi.org/10.1186/s12939-017-0564-4
13. Day GS, Ladak S, Curley K, et al. Thiamine prescribing practices within university-affiliated hospitals: a multicenter retrospective review. J Hosp Med. 2015;10(4):246-253. https://doi.org/10.1002/jhm.2324
14. Day E, Bentham PW, Callaghan R, Kuruvilla T, George S. Thiamine for prevention and treatment of Wernicke-Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;2013(7):CD004033. https://doi.org/10.1002/14651858.CD004033.pub3
15. Medici V, Halsted CH. Folate, alcohol, and liver disease. Mol Nutr Food Res. 2013;57(4):596-606. https://doi.org/10.1002/mnfr.201200077
16. Ijaz S, Thorley H, Porter K, et al. Interventions for preventing or treating malnutrition in homeless problem-drinkers: a systematic review. Int J Equity Health. 2018;17(1):8. https://doi.org/10.1186/s12939-018-0722-3
17. Bryson CL, Au DH, Sun H, Williams EC, Kivlahan DR, Bradley KA. Alcohol screening scores and medication nonadherence. Ann Intern Med. 2008;149(11):795-803. https://doi.org/10.7326/0003-4819-149-11-200812020-00004
18. Picker D, Heard K, Bailey TC, Martin NR, LaRossa GN, Kollef MH. The number of discharge medications predicts thirty-day hospital readmission: a cohort study. BMC Health Serv Res. 2015;15:282. https://doi.org/10.1186/s12913-015-0950-9
19. Han B, Jones CM, Einstein EB, Powell PA, Compton WM. Use of medications for alcohol use disorder in the US: results From the 2019 National Survey on Drug Use and Health. JAMA Psychiatry. 2021;78(8):922–4. https://doi.org/10.1001/jamapsychiatry.2021.1271
20. Collins SE, Duncan MH, Saxon AJ, et al. Combining behavioral harm-reduction treatment and extended-release naltrexone for people experiencing homelessness and alcohol use disorder in the USA: a randomised clinical trial. Lancet Psychiatry. 2021;8(4):287-300. https://doi.org/10.1016/S2215-0366(20)30489-2
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old man with alcohol use disorder (AUD) is admitted with decompensated heart failure and experiences alcohol withdrawal during the hospitalization. He improves with guideline-directed heart failure therapy and benzodiazepines for alcohol withdrawal. Discharge medications are metoprolol succinate, lisinopril, furosemide, aspirin, atorvastatin, thiamine, folic acid, and a multivitamin. No medications are offered for AUD treatment. At follow-up a week later, he presents with dyspnea and reports poor medication adherence and a return to heavy drinking.
WHY YOU MIGHT THINK IT IS HELPFUL TO PRESCRIBE VITAMIN SUPPLEMENTATION TO PATIENTS WITH AUD AT HOSPITAL DISCHARGE
AUD is common among hospitalized patients.1 AUD increases the risk of vitamin deficiencies due to the toxic effects of alcohol on the gastrointestinal tract and liver, causing impaired digestion, reduced absorption, and increased degradation of key micronutrients.2,3 Other risk factors for AUD-associated vitamin deficiencies include food insecurity and the replacement of nutrient-rich food with alcohol. Since the body does not readily store water-soluble vitamins, including thiamine (vitamin B1) and folate (vitamin B9), people require regular dietary replenishment of these nutrients. Thus, if individuals with AUD eat less fortified food, they risk developing thiamine, folate, niacin, and other vitamin deficiencies. Since AUD puts patients at risk for vitamin deficiencies, hospitalized patients typically receive vitamin supplementation, including thiamine, folic acid, and a multivitamin (most formulations contain water-soluble vitamins B and C and micronutrients).1 Hospitalists often continue these medications at discharge.
Thiamine deficiency may manifest as Wernicke encephalopathy (WE), peripheral neuropathy, or a high-output heart failure state.
Hospitalists empirically treat with thiamine, folate, and other vitamins upon hospital admission with the intent of reducing morbidity associated with nutritional deficiencies.1 Repletion poses few risks to patients since the kidneys eliminate water-soluble vitamins. Multivitamins also have a low potential for direct harm and a low cost. Given the consequences of missing a deficiency, alcohol withdrawal–management order sets commonly embed vitamin repletion orders.6
WHY ROUTINELY PRESCRIBING VITAMIN SUPPLEMENTATION AT HOSPITAL DISCHARGE IN PATIENTS WITH AUD IS A TWDFNR
Hospitalists often reflexively continue vitamin supplementation on discharge. Unfortunately, there is no evidence that prescribing vitamin supplementation leads to clinically significant improvements for people with AUD, and patients can experience harms.
Literature and specialty guidelines lack consensus on rational vitamin supplementation in patients with AUD.2,7,8 Folate testing is not recommended due to inaccuracies.9 In fact, clinical data, such as body mass index, more accurately predict alcohol-related cognitive impairment than blood levels of vitamins.10 In one small study of vitamin deficiencies among patients with acute alcohol intoxication, none had low B12 or folate levels.11 A systematic review among people experiencing homelessness with unhealthy alcohol use showed no clear pattern of vitamin deficiencies across studies, although vitamin C and thiamine deficiencies predominated.12
In the absence of reliable thiamine and folate testing to confirm deficiencies, clinicians must use their clinical assessment skills. Clinicians rarely evaluate patients with AUD for vitamin deficiency risk factors and instead reflexively prescribe vitamin supplementation. An AUD diagnosis may serve as a sensitive, but not specific, risk factor for those in need of vitamin supplementation.
Other limitations make prescribing oral vitamins reflexively at discharge a low-value practice. Thiamine, often prescribed orally in the hospital and on discharge, has poor oral bioavailability.13 Unfortunately, people with AUD have decreased and variable thiamine absorption. To prevent WE, thiamine must cross the blood-brain barrier, and the literature provides insufficient evidence to guide clinicians on an appropriate oral thiamine dose, frequency, or duration of treatment.14 While early high-dose IV thiamine may treat or prevent WE during hospitalization, low-dose oral thiamine may not provide benefit to patients with AUD.5
The literature also provides sparse evidence for folate supplementation and its optimal dose. Since 1998, when the United States mandated fortifying grain products with folic acid, people rarely have low serum folate levels. Though patients with AUD have lower folate levels relative to the general population,15 this difference does not seem clinically significant. While limited data show an association between oral multivitamin supplementation and improved serum nutrient levels among people with AUD, we lack evidence on clinical outcomes.16
Most importantly, for a practice lacking strong evidence, prescribing multiple vitamins at discharge may result in harm from polypharmacy and unnecessary costs for the recently hospitalized patient. Alcohol use is associated with decreased adherence to medications for chronic conditions,17 including HIV, hypertension, hyperlipidemia, and psychiatric diseases. In addition, research shows an association between an increased number of discharge medications and higher risk for hospital readmission. The harm may actually correlate with the number of medications and complexity of the regimen rather than the risk profile of the medications themselves.18 Providers underestimate the impact of adding multiple vitamins at discharge, especially for patients who have several co-occurring medical conditions that require other medications. Furthermore, insurance rarely covers vitamins, leading hospitals or patients to incur the costs at discharge.
WHEN TO CONSIDER VITAMIN SUPPLEMENTATION AT DISCHARGE FOR PATIENTS WITH AUD
When treating patients with AUD, consider the potential benefit of vitamin supplementation for the individual. If a patient with regular, heavy alcohol use is at high risk of vitamin deficiencies due to ongoing risk factors (Table), hospitalists should discuss vitamin therapy via a patient-centered risk-benefit process.

When considering discharge vitamins, make concurrent efforts to enhance patient nutrition via decreased alcohol consumption and improved healthy food intake. While some patients do not have a goal of abstaining from alcohol, providing resources to food access may help decrease the harms of drinking. Education may help patients learn that vitamin deficiencies can result from heavy alcohol use.
Multivitamin formulations have variable doses of vitamins but can contain 100% or more of the daily value of thiamine and folic acid. For patients with AUD at lower risk of vitamin deficiencies (ie, mild alcohol use disorder with a healthy diet), discuss risks and benefits of supplementation. If they desire supplementation, a single thiamine-containing vitamin alone may be highest yield since it is the most morbid vitamin deficiency. Conversely, a patient with heavy alcohol intake and other risk factors for malnutrition may benefit from a higher dose of supplementation, achieved by prescribing a multivitamin alongside additional doses of thiamine and folate. However, the literature lacks evidence to guide clinicians on optimal vitamin dosing and formulations.
WHAT WE SHOULD DO INSTEAD
Instead of reflexively prescribing thiamine, folate, and multivitamin, clinicians can assess patients for AUD, provide motivational interviewing, and offer AUD treatment. Hospitalists should initiate and prescribe evidence-based medications for AUD for patients interested in reducing or stopping their alcohol intake. We can choose from Food and Drug Administration–approved AUD medications, including naltrexone and acamprosate. Unfortunately, less than 3% of patients with AUD receive medication therapy.19 Our healthcare systems can also refer individuals to community psychosocial treatment.
For patients with risk factors, prescribe empiric IV thiamine during hospitalization. Clinicians should then perform a risk-benefit assessment rather than reflexively prescribe vitamins to patients with AUD at discharge. We should also counsel patients to eat food when drinking to decrease alcohol-related harms.20 Patients experiencing food insecurity should be linked to food resources through inpatient nutritional and social work consultations.
Elicit patient preference around vitamin supplementation after discharge. For patients with AUD who desire supplementation without risk factors for malnutrition (Table), consider prescribing a single thiamine-containing vitamin for prevention of thiamine deficiency, which, unlike other vitamin deficiencies, has the potential to be irreversible and life-threatening. Though no evidence currently supports this practice, it stands to reason that prescribing a single tablet could decrease the number of pills for patients who struggle with pill burden.
RECOMMENDATIONS
- Offer evidence-based medication treatment for AUD.
- Connect patients experiencing food insecurity with appropriate resources.
- For patients initiated on a multivitamin, folate, and high-dose IV thiamine at admission, perform vitamin de-escalation during hospitalization.
- Risk-stratify hospitalized patients with AUD for additional risk factors for vitamin deficiencies (Table). In those with additional risk factors, offer supplementation if consistent with patient preference. Balance the benefits of vitamin supplementation with the risks of polypharmacy, particularly if the patient has conditions requiring multiple medications.
CONCLUSION
Returning to our case, the hospitalist initiates IV thiamine, folate, and a multivitamin at admission and assesses the patient’s nutritional status and food insecurity. The hospitalist deems the patient—who eats regular, balanced meals—to be at low risk for vitamin deficiencies. The medical team discontinues folate and multivitamins before discharge and continues IV thiamine throughout the 3-day hospitalization. The patient and clinician agree that unaddressed AUD played a key role in the patient’s heart failure exacerbation. The clinician elicits the patient’s goals around their alcohol use, discusses AUD treatment, and initiates naltrexone for AUD.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old man with alcohol use disorder (AUD) is admitted with decompensated heart failure and experiences alcohol withdrawal during the hospitalization. He improves with guideline-directed heart failure therapy and benzodiazepines for alcohol withdrawal. Discharge medications are metoprolol succinate, lisinopril, furosemide, aspirin, atorvastatin, thiamine, folic acid, and a multivitamin. No medications are offered for AUD treatment. At follow-up a week later, he presents with dyspnea and reports poor medication adherence and a return to heavy drinking.
WHY YOU MIGHT THINK IT IS HELPFUL TO PRESCRIBE VITAMIN SUPPLEMENTATION TO PATIENTS WITH AUD AT HOSPITAL DISCHARGE
AUD is common among hospitalized patients.1 AUD increases the risk of vitamin deficiencies due to the toxic effects of alcohol on the gastrointestinal tract and liver, causing impaired digestion, reduced absorption, and increased degradation of key micronutrients.2,3 Other risk factors for AUD-associated vitamin deficiencies include food insecurity and the replacement of nutrient-rich food with alcohol. Since the body does not readily store water-soluble vitamins, including thiamine (vitamin B1) and folate (vitamin B9), people require regular dietary replenishment of these nutrients. Thus, if individuals with AUD eat less fortified food, they risk developing thiamine, folate, niacin, and other vitamin deficiencies. Since AUD puts patients at risk for vitamin deficiencies, hospitalized patients typically receive vitamin supplementation, including thiamine, folic acid, and a multivitamin (most formulations contain water-soluble vitamins B and C and micronutrients).1 Hospitalists often continue these medications at discharge.
Thiamine deficiency may manifest as Wernicke encephalopathy (WE), peripheral neuropathy, or a high-output heart failure state.
Hospitalists empirically treat with thiamine, folate, and other vitamins upon hospital admission with the intent of reducing morbidity associated with nutritional deficiencies.1 Repletion poses few risks to patients since the kidneys eliminate water-soluble vitamins. Multivitamins also have a low potential for direct harm and a low cost. Given the consequences of missing a deficiency, alcohol withdrawal–management order sets commonly embed vitamin repletion orders.6
WHY ROUTINELY PRESCRIBING VITAMIN SUPPLEMENTATION AT HOSPITAL DISCHARGE IN PATIENTS WITH AUD IS A TWDFNR
Hospitalists often reflexively continue vitamin supplementation on discharge. Unfortunately, there is no evidence that prescribing vitamin supplementation leads to clinically significant improvements for people with AUD, and patients can experience harms.
Literature and specialty guidelines lack consensus on rational vitamin supplementation in patients with AUD.2,7,8 Folate testing is not recommended due to inaccuracies.9 In fact, clinical data, such as body mass index, more accurately predict alcohol-related cognitive impairment than blood levels of vitamins.10 In one small study of vitamin deficiencies among patients with acute alcohol intoxication, none had low B12 or folate levels.11 A systematic review among people experiencing homelessness with unhealthy alcohol use showed no clear pattern of vitamin deficiencies across studies, although vitamin C and thiamine deficiencies predominated.12
In the absence of reliable thiamine and folate testing to confirm deficiencies, clinicians must use their clinical assessment skills. Clinicians rarely evaluate patients with AUD for vitamin deficiency risk factors and instead reflexively prescribe vitamin supplementation. An AUD diagnosis may serve as a sensitive, but not specific, risk factor for those in need of vitamin supplementation.
Other limitations make prescribing oral vitamins reflexively at discharge a low-value practice. Thiamine, often prescribed orally in the hospital and on discharge, has poor oral bioavailability.13 Unfortunately, people with AUD have decreased and variable thiamine absorption. To prevent WE, thiamine must cross the blood-brain barrier, and the literature provides insufficient evidence to guide clinicians on an appropriate oral thiamine dose, frequency, or duration of treatment.14 While early high-dose IV thiamine may treat or prevent WE during hospitalization, low-dose oral thiamine may not provide benefit to patients with AUD.5
The literature also provides sparse evidence for folate supplementation and its optimal dose. Since 1998, when the United States mandated fortifying grain products with folic acid, people rarely have low serum folate levels. Though patients with AUD have lower folate levels relative to the general population,15 this difference does not seem clinically significant. While limited data show an association between oral multivitamin supplementation and improved serum nutrient levels among people with AUD, we lack evidence on clinical outcomes.16
Most importantly, for a practice lacking strong evidence, prescribing multiple vitamins at discharge may result in harm from polypharmacy and unnecessary costs for the recently hospitalized patient. Alcohol use is associated with decreased adherence to medications for chronic conditions,17 including HIV, hypertension, hyperlipidemia, and psychiatric diseases. In addition, research shows an association between an increased number of discharge medications and higher risk for hospital readmission. The harm may actually correlate with the number of medications and complexity of the regimen rather than the risk profile of the medications themselves.18 Providers underestimate the impact of adding multiple vitamins at discharge, especially for patients who have several co-occurring medical conditions that require other medications. Furthermore, insurance rarely covers vitamins, leading hospitals or patients to incur the costs at discharge.
WHEN TO CONSIDER VITAMIN SUPPLEMENTATION AT DISCHARGE FOR PATIENTS WITH AUD
When treating patients with AUD, consider the potential benefit of vitamin supplementation for the individual. If a patient with regular, heavy alcohol use is at high risk of vitamin deficiencies due to ongoing risk factors (Table), hospitalists should discuss vitamin therapy via a patient-centered risk-benefit process.

When considering discharge vitamins, make concurrent efforts to enhance patient nutrition via decreased alcohol consumption and improved healthy food intake. While some patients do not have a goal of abstaining from alcohol, providing resources to food access may help decrease the harms of drinking. Education may help patients learn that vitamin deficiencies can result from heavy alcohol use.
Multivitamin formulations have variable doses of vitamins but can contain 100% or more of the daily value of thiamine and folic acid. For patients with AUD at lower risk of vitamin deficiencies (ie, mild alcohol use disorder with a healthy diet), discuss risks and benefits of supplementation. If they desire supplementation, a single thiamine-containing vitamin alone may be highest yield since it is the most morbid vitamin deficiency. Conversely, a patient with heavy alcohol intake and other risk factors for malnutrition may benefit from a higher dose of supplementation, achieved by prescribing a multivitamin alongside additional doses of thiamine and folate. However, the literature lacks evidence to guide clinicians on optimal vitamin dosing and formulations.
WHAT WE SHOULD DO INSTEAD
Instead of reflexively prescribing thiamine, folate, and multivitamin, clinicians can assess patients for AUD, provide motivational interviewing, and offer AUD treatment. Hospitalists should initiate and prescribe evidence-based medications for AUD for patients interested in reducing or stopping their alcohol intake. We can choose from Food and Drug Administration–approved AUD medications, including naltrexone and acamprosate. Unfortunately, less than 3% of patients with AUD receive medication therapy.19 Our healthcare systems can also refer individuals to community psychosocial treatment.
For patients with risk factors, prescribe empiric IV thiamine during hospitalization. Clinicians should then perform a risk-benefit assessment rather than reflexively prescribe vitamins to patients with AUD at discharge. We should also counsel patients to eat food when drinking to decrease alcohol-related harms.20 Patients experiencing food insecurity should be linked to food resources through inpatient nutritional and social work consultations.
Elicit patient preference around vitamin supplementation after discharge. For patients with AUD who desire supplementation without risk factors for malnutrition (Table), consider prescribing a single thiamine-containing vitamin for prevention of thiamine deficiency, which, unlike other vitamin deficiencies, has the potential to be irreversible and life-threatening. Though no evidence currently supports this practice, it stands to reason that prescribing a single tablet could decrease the number of pills for patients who struggle with pill burden.
RECOMMENDATIONS
- Offer evidence-based medication treatment for AUD.
- Connect patients experiencing food insecurity with appropriate resources.
- For patients initiated on a multivitamin, folate, and high-dose IV thiamine at admission, perform vitamin de-escalation during hospitalization.
- Risk-stratify hospitalized patients with AUD for additional risk factors for vitamin deficiencies (Table). In those with additional risk factors, offer supplementation if consistent with patient preference. Balance the benefits of vitamin supplementation with the risks of polypharmacy, particularly if the patient has conditions requiring multiple medications.
CONCLUSION
Returning to our case, the hospitalist initiates IV thiamine, folate, and a multivitamin at admission and assesses the patient’s nutritional status and food insecurity. The hospitalist deems the patient—who eats regular, balanced meals—to be at low risk for vitamin deficiencies. The medical team discontinues folate and multivitamins before discharge and continues IV thiamine throughout the 3-day hospitalization. The patient and clinician agree that unaddressed AUD played a key role in the patient’s heart failure exacerbation. The clinician elicits the patient’s goals around their alcohol use, discusses AUD treatment, and initiates naltrexone for AUD.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
1. Makdissi R, Stewart SH. Care for hospitalized patients with unhealthy alcohol use: a narrative review. Addict Sci Clin Pract. 2013;8(1):11. https://doi.org/10.1186/1940-0640-8-11
2. Lewis MJ. Alcoholism and nutrition: a review of vitamin supplementation and treatment. Curr Opin Clin Nutr Metab Care. 2020;23(2):138-144. https://doi.org/10.1097/mco.0000000000000622
3. Bergmans RS, Coughlin L, Wilson T, Malecki K. Cross-sectional associations of food insecurity with smoking cigarettes and heavy alcohol use in a population-based sample of adults. Drug Alcohol Depend. 2019;205:107646. https://doi.org/10.1016/j.drugalcdep.2019.107646
4. Latt N, Dore G. Thiamine in the treatment of Wernicke encephalopathy in patients with alcohol use disorders. Intern Med J. 2014;44(9):911-915. https://doi.org/10.1111/imj.12522
5. Flannery AH, Adkins DA, Cook AM. Unpeeling the evidence for the banana bag: evidence-based recommendations for the management of alcohol-associated vitamin and electrolyte deficiencies in the ICU. Crit Care Med. 2016;44(8):1545-1552. https://doi.org/10.1097/ccm.0000000000001659
6. Wai JM, Aloezos C, Mowrey WB, Baron SW, Cregin R, Forman HL. Using clinical decision support through the electronic medical record to increase prescribing of high-dose parenteral thiamine in hospitalized patients with alcohol use disorder. J Subst Abuse Treat. 2019;99:117-123. https://doi.org/10.1016/j.jsat.2019.01.017
7. American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. January 2020. https://www.asam.org/docs/default-source/quality-science/the_asam_clinical_practice_guideline_on_alcohol-1.pdf?sfvrsn=ba255c2_2
8. O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51(1):307-328. https://doi.org/10.1002/hep.23258
9. Breu AC, Theisen-Toupal J, Feldman LS. Serum and red blood cell folate testing on hospitalized patients. J Hosp Med. 2015;10(11):753-755. https://doi.org/10.1002/jhm.2385
10. Gautron M-A, Questel F, Lejoyeux M, Bellivier F, Vorspan F. Nutritional status during inpatient alcohol detoxification. Alcohol Alcohol. 2018;53(1):64-70. https://doi.org/10.1093/alcalc/agx086
11. Li SF, Jacob J, Feng J, Kulkarni M. Vitamin deficiencies in acutely intoxicated patients in the ED. Am J Emerg Med. 2008;26(7):792-795. https://doi.org/10.1016/j.ajem.2007.10.003
12. Ijaz S, Jackson J, Thorley H, et al. Nutritional deficiencies in homeless persons with problematic drinking: a systematic review. Int J Equity Health. 2017;16(1):71. https://doi.org/10.1186/s12939-017-0564-4
13. Day GS, Ladak S, Curley K, et al. Thiamine prescribing practices within university-affiliated hospitals: a multicenter retrospective review. J Hosp Med. 2015;10(4):246-253. https://doi.org/10.1002/jhm.2324
14. Day E, Bentham PW, Callaghan R, Kuruvilla T, George S. Thiamine for prevention and treatment of Wernicke-Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;2013(7):CD004033. https://doi.org/10.1002/14651858.CD004033.pub3
15. Medici V, Halsted CH. Folate, alcohol, and liver disease. Mol Nutr Food Res. 2013;57(4):596-606. https://doi.org/10.1002/mnfr.201200077
16. Ijaz S, Thorley H, Porter K, et al. Interventions for preventing or treating malnutrition in homeless problem-drinkers: a systematic review. Int J Equity Health. 2018;17(1):8. https://doi.org/10.1186/s12939-018-0722-3
17. Bryson CL, Au DH, Sun H, Williams EC, Kivlahan DR, Bradley KA. Alcohol screening scores and medication nonadherence. Ann Intern Med. 2008;149(11):795-803. https://doi.org/10.7326/0003-4819-149-11-200812020-00004
18. Picker D, Heard K, Bailey TC, Martin NR, LaRossa GN, Kollef MH. The number of discharge medications predicts thirty-day hospital readmission: a cohort study. BMC Health Serv Res. 2015;15:282. https://doi.org/10.1186/s12913-015-0950-9
19. Han B, Jones CM, Einstein EB, Powell PA, Compton WM. Use of medications for alcohol use disorder in the US: results From the 2019 National Survey on Drug Use and Health. JAMA Psychiatry. 2021;78(8):922–4. https://doi.org/10.1001/jamapsychiatry.2021.1271
20. Collins SE, Duncan MH, Saxon AJ, et al. Combining behavioral harm-reduction treatment and extended-release naltrexone for people experiencing homelessness and alcohol use disorder in the USA: a randomised clinical trial. Lancet Psychiatry. 2021;8(4):287-300. https://doi.org/10.1016/S2215-0366(20)30489-2
1. Makdissi R, Stewart SH. Care for hospitalized patients with unhealthy alcohol use: a narrative review. Addict Sci Clin Pract. 2013;8(1):11. https://doi.org/10.1186/1940-0640-8-11
2. Lewis MJ. Alcoholism and nutrition: a review of vitamin supplementation and treatment. Curr Opin Clin Nutr Metab Care. 2020;23(2):138-144. https://doi.org/10.1097/mco.0000000000000622
3. Bergmans RS, Coughlin L, Wilson T, Malecki K. Cross-sectional associations of food insecurity with smoking cigarettes and heavy alcohol use in a population-based sample of adults. Drug Alcohol Depend. 2019;205:107646. https://doi.org/10.1016/j.drugalcdep.2019.107646
4. Latt N, Dore G. Thiamine in the treatment of Wernicke encephalopathy in patients with alcohol use disorders. Intern Med J. 2014;44(9):911-915. https://doi.org/10.1111/imj.12522
5. Flannery AH, Adkins DA, Cook AM. Unpeeling the evidence for the banana bag: evidence-based recommendations for the management of alcohol-associated vitamin and electrolyte deficiencies in the ICU. Crit Care Med. 2016;44(8):1545-1552. https://doi.org/10.1097/ccm.0000000000001659
6. Wai JM, Aloezos C, Mowrey WB, Baron SW, Cregin R, Forman HL. Using clinical decision support through the electronic medical record to increase prescribing of high-dose parenteral thiamine in hospitalized patients with alcohol use disorder. J Subst Abuse Treat. 2019;99:117-123. https://doi.org/10.1016/j.jsat.2019.01.017
7. American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. January 2020. https://www.asam.org/docs/default-source/quality-science/the_asam_clinical_practice_guideline_on_alcohol-1.pdf?sfvrsn=ba255c2_2
8. O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51(1):307-328. https://doi.org/10.1002/hep.23258
9. Breu AC, Theisen-Toupal J, Feldman LS. Serum and red blood cell folate testing on hospitalized patients. J Hosp Med. 2015;10(11):753-755. https://doi.org/10.1002/jhm.2385
10. Gautron M-A, Questel F, Lejoyeux M, Bellivier F, Vorspan F. Nutritional status during inpatient alcohol detoxification. Alcohol Alcohol. 2018;53(1):64-70. https://doi.org/10.1093/alcalc/agx086
11. Li SF, Jacob J, Feng J, Kulkarni M. Vitamin deficiencies in acutely intoxicated patients in the ED. Am J Emerg Med. 2008;26(7):792-795. https://doi.org/10.1016/j.ajem.2007.10.003
12. Ijaz S, Jackson J, Thorley H, et al. Nutritional deficiencies in homeless persons with problematic drinking: a systematic review. Int J Equity Health. 2017;16(1):71. https://doi.org/10.1186/s12939-017-0564-4
13. Day GS, Ladak S, Curley K, et al. Thiamine prescribing practices within university-affiliated hospitals: a multicenter retrospective review. J Hosp Med. 2015;10(4):246-253. https://doi.org/10.1002/jhm.2324
14. Day E, Bentham PW, Callaghan R, Kuruvilla T, George S. Thiamine for prevention and treatment of Wernicke-Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;2013(7):CD004033. https://doi.org/10.1002/14651858.CD004033.pub3
15. Medici V, Halsted CH. Folate, alcohol, and liver disease. Mol Nutr Food Res. 2013;57(4):596-606. https://doi.org/10.1002/mnfr.201200077
16. Ijaz S, Thorley H, Porter K, et al. Interventions for preventing or treating malnutrition in homeless problem-drinkers: a systematic review. Int J Equity Health. 2018;17(1):8. https://doi.org/10.1186/s12939-018-0722-3
17. Bryson CL, Au DH, Sun H, Williams EC, Kivlahan DR, Bradley KA. Alcohol screening scores and medication nonadherence. Ann Intern Med. 2008;149(11):795-803. https://doi.org/10.7326/0003-4819-149-11-200812020-00004
18. Picker D, Heard K, Bailey TC, Martin NR, LaRossa GN, Kollef MH. The number of discharge medications predicts thirty-day hospital readmission: a cohort study. BMC Health Serv Res. 2015;15:282. https://doi.org/10.1186/s12913-015-0950-9
19. Han B, Jones CM, Einstein EB, Powell PA, Compton WM. Use of medications for alcohol use disorder in the US: results From the 2019 National Survey on Drug Use and Health. JAMA Psychiatry. 2021;78(8):922–4. https://doi.org/10.1001/jamapsychiatry.2021.1271
20. Collins SE, Duncan MH, Saxon AJ, et al. Combining behavioral harm-reduction treatment and extended-release naltrexone for people experiencing homelessness and alcohol use disorder in the USA: a randomised clinical trial. Lancet Psychiatry. 2021;8(4):287-300. https://doi.org/10.1016/S2215-0366(20)30489-2
© 2021 Society of Hospital Medicine
Things We Do for No Reason:™ Prescribing Tramadol for Inpatients in Pain
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits an 80-year-old man for a chronic obstructive pulmonary disease exacerbation. The patient’s history is significant for chronic right knee pain. While hospitalized, the patient reports worsening of his knee pain. Radiographs of the right knee show severe osteoarthritic changes. Since acetaminophen does not relieve the patient’s pain, the hospitalist orders tramadol as needed.
BACKGROUND
Hospitalists, who commonly evaluate and treat acute and chronic pain in the inpatient setting, have a wide selection of interventions from which to choose, including tramadol. Tramadol hydrochloride is a synthetic, central-acting analgesic with multiple mechanisms of action. It is a serotonin-norepinephrine reuptake inhibitor (SNRI) with a structure similar to venlafaxine and produces antineuropathic analgesic effects.1 Tramadol and its primary active metabolite O-desmethyltramadol (also known as the M1 metabolite) mediate its effects by binding at the mu-opioid receptor.2 Phase I metabolism in the liver by cytochrome P450 isoenzyme 2D6 (CYP2D6) facilitates conversion of tramadol to M1 (Figure). Importantly, genetic polymorphisms in CYP2D6 result in individual variations in gene expression, which impacts the metabolism of tramadol.2
Although tramadol is available over the counter in some countries, in the United States it is a Schedule IV controlled substance. Tramadol consistently ranks among the top 50 prescribed medications in the United States.3
WHY YOU MIGHT THINK PRESCRIBING TRAMADOL FOR PAIN MAY BE HELPFUL
Given the growing concerns regarding the use of opioids, the pharmaceutical industry has marketed tramadol as a safer opioid option for pain management. Tramadol binds at the mu-opioid receptor with an affinity that is less than 4000-fold that of morphine; the binding potency of M1, the metabolite of tramadol, is less than 5-fold that of morphine.4 Due to its lower binding affinity at the mu-opioid receptor, tramadol is considered a weak opioid, one believed to have minimal withdrawal symptoms and a lower potential for overdose or misuse compared to other opioids.1,5 Based on this characterization, many clinicians prescribe tramadol for elderly patients or patients otherwise at risk for medication misuse or adverse effects of opioids.6 In addition, hospitalized patients often have contraindications to nonopioid medications (eg, acetaminophen, nonsteroidal anti-inflammatory drugs [NSAIDs]), limiting their options for pain management.
WHY PRESCRIBING TRAMADOL FOR PAIN SHOULD BE AVOIDED
Despite being marketed as an effective and safe medication, tramadol has an unpredictable metabolism, complex pharmacology, and drug-drug interactions that can cause significant adverse effects. Similar to other opioids, tramadol is associated with a risk of misuse, physiologic dependency, and overdose. In addition, tramadol has a black box warning for addiction, misuse, respiratory depression, ultra-rapid metabolism, neonatal opioid withdrawal syndrome, CYP450 drug interactions, and interactions with other central nervous system depressants.
While tramadol has multiple mechanisms of action, the literature lacks high-quality evidence (eg, large randomized controlled trials) supporting its use, especially in hospitalized medical patients. A recent retrospective study of tramadol looked at the diagnoses of 250 hospitalized patients who received tramadol for pain management. While this study did not examine efficacy, it found mild-to-moderate acute noncancer pain to be the primary reason for prescribing tramadol.7 This study also showed the risk of severe drug-drug interactions increased the longer patients were on tramadol.7
As a result of the limited evidence in hospitalized patients, hospitalists must rely on outpatient studies.8-10 The size and quality of these studies, especially given the magnitude of tramadol prescribing in the United States, make them less useful. A series of Cochrane reviews examining the beneficial effects of tramadol for neuropathic pain, osteoarthritis, and cancer pain show insufficient evidence for tramadol when compared to placebo or active controls such as acetaminophen, NSAIDs, or other opioids.8-10
The side-effect profile of tramadol outweighs its mild analgesic effects. The 2019 American Geriatric Society Beers criteria for potentially inappropriate medication use in older adults strongly recommends clinicians use caution when prescribing tramadol to older adult patients, as tramadol may worsen or cause hyponatremia.11 In one large, population-based study, the use of tramadol doubled patients’ risk of hospitalization for hyponatremia when compared to codeine, though the incidence remains rather low at 4.6 per 10,000 person-months.12 Studies have also demonstrated an increased risk of hospitalization for hypoglycemia in nondiabetic patients receiving tramadol.13 A large propensity-score matched cohort study of patients with osteoarthritis found tramadol to have an associated higher all-cause mortality compared to NSAIDs; however, these differences may be due to confounding variables.1 In addition to hyponatremia and all-cause mortality, patients taking tramadol also have an associated increased risk of falls and hip fractures when compared to codeine or NSAIDs.14
The increased serotonergic activity associated with tramadol can lead to serotonin syndrome (serotonin toxicity), a rare but serious condition. Although serotonin syndrome can develop in patients taking tramadol as a monotherapy, the risk for this toxidrome increases when tramadol is taken in combination with other serotonergic agents or agents that inhibit metabolism of tramadol at CYP2D6.5 Seizures may also occur with tramadol at therapeutic and supratherapeutic doses. Population-based studies estimate seizures occur in 0.15% to 0.86% of patients receiving tramadol, which is two to six times the risk of those not on tramadol.5 Patients concurrently taking tramadol with a tricyclic antidepressant (TCA) or selective serotonin reuptake inhibitor (SSRI) are estimated to have seizures five to nine times more often than patients not taking a TCA or SSRI.5 Risk factors for tramadol-induced seizure include tramadol misuse or overdose, tramadol doses >1000 mg daily (maximum recommended dose is 400 mg/day), chronic tramadol use, concurrent use of a serotonergic agent or medications that inhibit CYP2D6, and history of epilepsy, renal disease, stroke, or traumatic brain injury.5
Differences in the genetic polymorphisms of CYP2D6 can produce a range of CYP2D6 activity from “poor metabolizers” (little-to-no analgesic effect) to “ultra-rapid metabolizers” (enhanced analgesia and increased risk of adverse effects), leading to unpredictable pharmacodynamic effects of tramadol.2 In North Africa and the Arabian peninsula, more than 25% of the population rapidly metabolizes tramadol; these pharmacogenomic effects result in higher rates of tramadol addiction and overdose in these regions.5 An estimated 7% to 10% of Caucasians slowly metabolize tramadol, which may place them at risk of adverse effects from tramadol in addition to inadequate analgesia.15 In contrast, Ethiopian populations have the highest rate of ultra-rapid tramadol metabolism at 29%.15
Drugs that induce CYP2D6 (eg, dexamethasone, rifampin) or inhibit CYP2D6 (eg, bupropion, fluoxetine) also impact tramadol efficacy, pharmacokinetics, and pharmacodynamics.16,17 Patients taking strong CYP2D6 inhibitors require significantly higher doses of tramadol to achieve analgesic effects.17 Tramadol undergoes extensive hepatic metabolism, producing several active metabolites, including M1 (Figure). Hepatic impairment increases the elimination half-life of tramadol and its metabolites.18 The majority of tramadol and its metabolites are eliminated through the kidneys. Accumulation of tramadol and its metabolites may occur in patients with renal impairment, placing them at increased risk of adverse effects.2
Finally, although some clinicians assume that tramadol has lower rates of misuse, diversion, or overdose compared to other opioids, rates of nonprescription use have increased with its proliferation.19,20 The US Substance Abuse and Mental Health Services Administration estimates that 1,287,000 persons misused tramadol in 2019.21 Patients may exhibit symptoms of physiologic opioid dependence and withdrawal from chronic tramadol use.2,22 In one study, patients prescribed tramadol monotherapy for acute pain from elective surgery had an increased risk for prolonged opioid use compared to patients prescribed other short-acting opioids.22
WHAT YOU SHOULD DO INSTEAD
Clinicians should determine the nature of the patient’s pain by obtaining a complete medical history, performing a thorough physical examination, and ordering diagnostic tests and imaging studies, as necessary. After consulting with the patient’s primary care physician, the clinician should employ a multimodal approach to pain that includes topical agents, psychotherapy, injections or interventions, and nonopioid medications. Patients with neuropathic pain may benefit from adjuvant analgesics such as gabapentinoids, TCAs, or SNRIs. In patients with evidence-based indications for opioid therapy (eg, pancreatitis, cancer pain, postsurgical pain), the hospitalist should assess the risk for opioid misuse and discuss risks and benefits with the patient before considering a time-limited trial of opioid therapy. If available and when indicated, clinicians should consult with specialists in pain management or palliative care. For cases wherein clinicians have already prescribed tramadol to the patient, they should discuss deprescribing strategies and alternative analgesic options with the patient and the patient’s primary care physician. Finally, before initiating tramadol therapy for hospitalized patients with pain, hospitalists should consider the risks, benefits, and alternative approaches to prescribing tramadol.
RECOMMENDATIONS
- For hospitalized patients reporting pain, complete a pain assessment by history, physical exam, chart review, and diagnostic studies to examine the etiology of the pain.
- Utilize multiple modalities for pain control when possible, including acetaminophen, NSAIDs, topical agents, ice or heat, neuropathic pain medications, and interventions such as injections, psychotherapy, or radiation, if indicated.
- Avoid prescribing tramadol due to unpredictable pharmacodynamics, adverse effects, and lack of quality evidence for efficacy in hospitalized medical patients.
CONCLUSION
Tramadol is a commonly used opioid medication associated with adverse effects and unpredictable analgesia. Regarding this case scenario, the use of tramadol in this patient places him at risk for drug-drug interactions, hyponatremia, hypoglycemia, serotonin syndrome, seizures, and pronounced side effects of opioid medications. Moderate quality evidence in the outpatient setting suggests that tramadol is unlikely to provide significant analgesia for his osteoarthritic pain.9 Instead of prescribing tramadol, the hospitalist should consider alternative treatments for this patient’s pain, such as intraarticular glucocorticoids, a short course of oral NSAIDs (unless contraindicated), topical treatments (eg, menthol, capsaicin, NSAIDs), physical therapy, and close follow-up with an orthopedist after hospital discharge. Further randomized controlled studies of tramadol vs active controls are needed.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
1. Zeng C, Dubreuil M, LaRochelle MR, et al. Association of tramadol with all-cause mortality among patients with osteoarthritis. JAMA. 2019;321(10):969-982. https://doi.org/10.1001/jama.2019.1347
2. Gong L, Stamer UM, Tzvetkov MV, Altman RB, Klein TE. PharmGKB summary: tramadol pathway. Pharmacogenet Genomics. 2014;24(7):374-380. https://doi.org/10.1097/FPC.0000000000000057
3. The top 200 drugs of 2019. ClinCalc DrugStats Database. Accessed June 10, 2021. https://clincalc.com/DrugStats
4. Gillen C, Haurand M, Kobelt DJ, Wnendt S. Affinity, potency and efficacy of tramadol and its metabolites at the cloned human µ-opioid receptor. Naunyn Schmiedebergs Arch Pharmacol. 2000;362(2):116-121. https://doi.org/10.1007/s002100000266
5. Hassamal S, Miotto K, Dale W, Danovitch I. Tramadol: understanding the risk of serotonin syndrome and seizures. Am J Med. 2018;131(11):1382.e1-1382.e6. https://doi.org/10.1016/j.amjmed.2018.04.025
6. Shipton EA. Tramadol—present and future. Anaesth Intensive Care. 2000;28(4):363-374. https://doi.org/10.1177/0310057X0002800403
7. Mohan N, Edmonds KP, Ajayi TA, Atayee RS, Clinical tolerability and safety of tramadol in hospitalized patients. J Pain & Palliat Care Pharmacother. 2020:34(4):211-218. https://doi.org/10.1080/15360288.2020.1817227
8. Duehmke RM, Derry S, Wiffen PJ, Bell RF, Aldington D, Moore RA. Tramadol for neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6(6):CD003726. https://doi.org/10.1002/14651858.cd003726.pub4
9. Toupin-April K, Bisaillon J, Welch V, et al. Tramadol for osteoarthritis. Cochrane Database Syst Rev. 2019;5(5):CD005522. https://doi.org/10.1002/14651858.cd005522.pub3
10. Wiffen PJ, Derry S, Moore RA. Tramadol with or without paracetamol (acetaminophen) for cancer pain. Cochrane Database Syst Rev. 2017;5(5):CD012508. https://doi.org/10.1002/14651858.cd012508.pub2
11. The American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. https://doi.org/10.1111/jgs.15767
12. Fournier JP, Yin H, Nessim SJ, Montastruc JL, Azoulay L. Tramadol for noncancer pain and the risk of hyponatremia. Am J Med. 2015;128(4):418-425.e5. https://doi.org/10.1016/j.amjmed.2014.10.046
13. Fournier JP, Azoulay L, Yin H, Montastruc JL, Suissa S. Tramadol use and the risk of hospitalization for hypoglycemia in patients with noncancer pain. JAMA Intern Med. 2015;175(2):186-193. https://doi.org/10.1001/jamainternmed.2014.6512
14. Wei J, Lane NE, Bolster MB, et al. Association of tramadol use with risk of hip fracture. J Bone Miner Res. 2020;35(4):631-640. https://doi.org/10.1002/jbmr.3935
15. Leppert W. CYP2D6 in the metabolism of opioids for mild to moderate pain. Pharmacology. 2011;87(5-6):274-285. https://doi.org/10.1159/000326085
16. Flockhart DA, Thacker D, McDonald C, Desta Z. The Flockhart cytochrome P450 drug-drug interaction table. Division of Clinical Pharmacology, Indiana University School of Medicine. Updated 2021. Accessed April 21, 2021. https://drug-interactions.medicine.iu.edu
17. Frost DA, Soric MM, Kaiser R, Neugebauer RE. Efficacy of tramadol for pain management in patients receiving strong cytochrome P450 2D6 inhibitors. Pharmacotherapy. 2019;39(6):724-729. https://doi.org/10.1002/phar.2269
18. Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43(13):879-923. https://doi.org/10.2165/00003088-200443130-00004
19. Bush DM. The CBHSQ report: emergency department visits for drug misuse or abuse involving the pain medication tramadol. Substance Abuse and Mental Health Service Administration. May 14, 2015. Accessed June 16, 2021. https://www.ncbi.nlm.nih.gov/books/NBK343535/
20. Bigal LM, Bibeau K, Dunbar S. Tramadol prescription over a 4-year period in the USA. Curr Pain Headache Rep. 2019;23(10):76. https://doi.org/10.1007/s11916-019-0777-x
21. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. National survey on drug use and health 2019 (NSDUH-2019). Accessed June 16, 2021. https://www.samhsa.gov/data/release/2019-national-survey-drug-use-and-health-nsduh-releases
22. Thiels CA, Habermann EB, Hooten WM, Jeffery MM. Chronic use of tramadol after acute pain episode: cohort study. BMJ. 2019;365:l1849. https://doi.org/10.1136/bmj.l1849
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits an 80-year-old man for a chronic obstructive pulmonary disease exacerbation. The patient’s history is significant for chronic right knee pain. While hospitalized, the patient reports worsening of his knee pain. Radiographs of the right knee show severe osteoarthritic changes. Since acetaminophen does not relieve the patient’s pain, the hospitalist orders tramadol as needed.
BACKGROUND
Hospitalists, who commonly evaluate and treat acute and chronic pain in the inpatient setting, have a wide selection of interventions from which to choose, including tramadol. Tramadol hydrochloride is a synthetic, central-acting analgesic with multiple mechanisms of action. It is a serotonin-norepinephrine reuptake inhibitor (SNRI) with a structure similar to venlafaxine and produces antineuropathic analgesic effects.1 Tramadol and its primary active metabolite O-desmethyltramadol (also known as the M1 metabolite) mediate its effects by binding at the mu-opioid receptor.2 Phase I metabolism in the liver by cytochrome P450 isoenzyme 2D6 (CYP2D6) facilitates conversion of tramadol to M1 (Figure). Importantly, genetic polymorphisms in CYP2D6 result in individual variations in gene expression, which impacts the metabolism of tramadol.2
Although tramadol is available over the counter in some countries, in the United States it is a Schedule IV controlled substance. Tramadol consistently ranks among the top 50 prescribed medications in the United States.3
WHY YOU MIGHT THINK PRESCRIBING TRAMADOL FOR PAIN MAY BE HELPFUL
Given the growing concerns regarding the use of opioids, the pharmaceutical industry has marketed tramadol as a safer opioid option for pain management. Tramadol binds at the mu-opioid receptor with an affinity that is less than 4000-fold that of morphine; the binding potency of M1, the metabolite of tramadol, is less than 5-fold that of morphine.4 Due to its lower binding affinity at the mu-opioid receptor, tramadol is considered a weak opioid, one believed to have minimal withdrawal symptoms and a lower potential for overdose or misuse compared to other opioids.1,5 Based on this characterization, many clinicians prescribe tramadol for elderly patients or patients otherwise at risk for medication misuse or adverse effects of opioids.6 In addition, hospitalized patients often have contraindications to nonopioid medications (eg, acetaminophen, nonsteroidal anti-inflammatory drugs [NSAIDs]), limiting their options for pain management.
WHY PRESCRIBING TRAMADOL FOR PAIN SHOULD BE AVOIDED
Despite being marketed as an effective and safe medication, tramadol has an unpredictable metabolism, complex pharmacology, and drug-drug interactions that can cause significant adverse effects. Similar to other opioids, tramadol is associated with a risk of misuse, physiologic dependency, and overdose. In addition, tramadol has a black box warning for addiction, misuse, respiratory depression, ultra-rapid metabolism, neonatal opioid withdrawal syndrome, CYP450 drug interactions, and interactions with other central nervous system depressants.
While tramadol has multiple mechanisms of action, the literature lacks high-quality evidence (eg, large randomized controlled trials) supporting its use, especially in hospitalized medical patients. A recent retrospective study of tramadol looked at the diagnoses of 250 hospitalized patients who received tramadol for pain management. While this study did not examine efficacy, it found mild-to-moderate acute noncancer pain to be the primary reason for prescribing tramadol.7 This study also showed the risk of severe drug-drug interactions increased the longer patients were on tramadol.7
As a result of the limited evidence in hospitalized patients, hospitalists must rely on outpatient studies.8-10 The size and quality of these studies, especially given the magnitude of tramadol prescribing in the United States, make them less useful. A series of Cochrane reviews examining the beneficial effects of tramadol for neuropathic pain, osteoarthritis, and cancer pain show insufficient evidence for tramadol when compared to placebo or active controls such as acetaminophen, NSAIDs, or other opioids.8-10
The side-effect profile of tramadol outweighs its mild analgesic effects. The 2019 American Geriatric Society Beers criteria for potentially inappropriate medication use in older adults strongly recommends clinicians use caution when prescribing tramadol to older adult patients, as tramadol may worsen or cause hyponatremia.11 In one large, population-based study, the use of tramadol doubled patients’ risk of hospitalization for hyponatremia when compared to codeine, though the incidence remains rather low at 4.6 per 10,000 person-months.12 Studies have also demonstrated an increased risk of hospitalization for hypoglycemia in nondiabetic patients receiving tramadol.13 A large propensity-score matched cohort study of patients with osteoarthritis found tramadol to have an associated higher all-cause mortality compared to NSAIDs; however, these differences may be due to confounding variables.1 In addition to hyponatremia and all-cause mortality, patients taking tramadol also have an associated increased risk of falls and hip fractures when compared to codeine or NSAIDs.14
The increased serotonergic activity associated with tramadol can lead to serotonin syndrome (serotonin toxicity), a rare but serious condition. Although serotonin syndrome can develop in patients taking tramadol as a monotherapy, the risk for this toxidrome increases when tramadol is taken in combination with other serotonergic agents or agents that inhibit metabolism of tramadol at CYP2D6.5 Seizures may also occur with tramadol at therapeutic and supratherapeutic doses. Population-based studies estimate seizures occur in 0.15% to 0.86% of patients receiving tramadol, which is two to six times the risk of those not on tramadol.5 Patients concurrently taking tramadol with a tricyclic antidepressant (TCA) or selective serotonin reuptake inhibitor (SSRI) are estimated to have seizures five to nine times more often than patients not taking a TCA or SSRI.5 Risk factors for tramadol-induced seizure include tramadol misuse or overdose, tramadol doses >1000 mg daily (maximum recommended dose is 400 mg/day), chronic tramadol use, concurrent use of a serotonergic agent or medications that inhibit CYP2D6, and history of epilepsy, renal disease, stroke, or traumatic brain injury.5
Differences in the genetic polymorphisms of CYP2D6 can produce a range of CYP2D6 activity from “poor metabolizers” (little-to-no analgesic effect) to “ultra-rapid metabolizers” (enhanced analgesia and increased risk of adverse effects), leading to unpredictable pharmacodynamic effects of tramadol.2 In North Africa and the Arabian peninsula, more than 25% of the population rapidly metabolizes tramadol; these pharmacogenomic effects result in higher rates of tramadol addiction and overdose in these regions.5 An estimated 7% to 10% of Caucasians slowly metabolize tramadol, which may place them at risk of adverse effects from tramadol in addition to inadequate analgesia.15 In contrast, Ethiopian populations have the highest rate of ultra-rapid tramadol metabolism at 29%.15
Drugs that induce CYP2D6 (eg, dexamethasone, rifampin) or inhibit CYP2D6 (eg, bupropion, fluoxetine) also impact tramadol efficacy, pharmacokinetics, and pharmacodynamics.16,17 Patients taking strong CYP2D6 inhibitors require significantly higher doses of tramadol to achieve analgesic effects.17 Tramadol undergoes extensive hepatic metabolism, producing several active metabolites, including M1 (Figure). Hepatic impairment increases the elimination half-life of tramadol and its metabolites.18 The majority of tramadol and its metabolites are eliminated through the kidneys. Accumulation of tramadol and its metabolites may occur in patients with renal impairment, placing them at increased risk of adverse effects.2
Finally, although some clinicians assume that tramadol has lower rates of misuse, diversion, or overdose compared to other opioids, rates of nonprescription use have increased with its proliferation.19,20 The US Substance Abuse and Mental Health Services Administration estimates that 1,287,000 persons misused tramadol in 2019.21 Patients may exhibit symptoms of physiologic opioid dependence and withdrawal from chronic tramadol use.2,22 In one study, patients prescribed tramadol monotherapy for acute pain from elective surgery had an increased risk for prolonged opioid use compared to patients prescribed other short-acting opioids.22
WHAT YOU SHOULD DO INSTEAD
Clinicians should determine the nature of the patient’s pain by obtaining a complete medical history, performing a thorough physical examination, and ordering diagnostic tests and imaging studies, as necessary. After consulting with the patient’s primary care physician, the clinician should employ a multimodal approach to pain that includes topical agents, psychotherapy, injections or interventions, and nonopioid medications. Patients with neuropathic pain may benefit from adjuvant analgesics such as gabapentinoids, TCAs, or SNRIs. In patients with evidence-based indications for opioid therapy (eg, pancreatitis, cancer pain, postsurgical pain), the hospitalist should assess the risk for opioid misuse and discuss risks and benefits with the patient before considering a time-limited trial of opioid therapy. If available and when indicated, clinicians should consult with specialists in pain management or palliative care. For cases wherein clinicians have already prescribed tramadol to the patient, they should discuss deprescribing strategies and alternative analgesic options with the patient and the patient’s primary care physician. Finally, before initiating tramadol therapy for hospitalized patients with pain, hospitalists should consider the risks, benefits, and alternative approaches to prescribing tramadol.
RECOMMENDATIONS
- For hospitalized patients reporting pain, complete a pain assessment by history, physical exam, chart review, and diagnostic studies to examine the etiology of the pain.
- Utilize multiple modalities for pain control when possible, including acetaminophen, NSAIDs, topical agents, ice or heat, neuropathic pain medications, and interventions such as injections, psychotherapy, or radiation, if indicated.
- Avoid prescribing tramadol due to unpredictable pharmacodynamics, adverse effects, and lack of quality evidence for efficacy in hospitalized medical patients.
CONCLUSION
Tramadol is a commonly used opioid medication associated with adverse effects and unpredictable analgesia. Regarding this case scenario, the use of tramadol in this patient places him at risk for drug-drug interactions, hyponatremia, hypoglycemia, serotonin syndrome, seizures, and pronounced side effects of opioid medications. Moderate quality evidence in the outpatient setting suggests that tramadol is unlikely to provide significant analgesia for his osteoarthritic pain.9 Instead of prescribing tramadol, the hospitalist should consider alternative treatments for this patient’s pain, such as intraarticular glucocorticoids, a short course of oral NSAIDs (unless contraindicated), topical treatments (eg, menthol, capsaicin, NSAIDs), physical therapy, and close follow-up with an orthopedist after hospital discharge. Further randomized controlled studies of tramadol vs active controls are needed.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits an 80-year-old man for a chronic obstructive pulmonary disease exacerbation. The patient’s history is significant for chronic right knee pain. While hospitalized, the patient reports worsening of his knee pain. Radiographs of the right knee show severe osteoarthritic changes. Since acetaminophen does not relieve the patient’s pain, the hospitalist orders tramadol as needed.
BACKGROUND
Hospitalists, who commonly evaluate and treat acute and chronic pain in the inpatient setting, have a wide selection of interventions from which to choose, including tramadol. Tramadol hydrochloride is a synthetic, central-acting analgesic with multiple mechanisms of action. It is a serotonin-norepinephrine reuptake inhibitor (SNRI) with a structure similar to venlafaxine and produces antineuropathic analgesic effects.1 Tramadol and its primary active metabolite O-desmethyltramadol (also known as the M1 metabolite) mediate its effects by binding at the mu-opioid receptor.2 Phase I metabolism in the liver by cytochrome P450 isoenzyme 2D6 (CYP2D6) facilitates conversion of tramadol to M1 (Figure). Importantly, genetic polymorphisms in CYP2D6 result in individual variations in gene expression, which impacts the metabolism of tramadol.2
Although tramadol is available over the counter in some countries, in the United States it is a Schedule IV controlled substance. Tramadol consistently ranks among the top 50 prescribed medications in the United States.3
WHY YOU MIGHT THINK PRESCRIBING TRAMADOL FOR PAIN MAY BE HELPFUL
Given the growing concerns regarding the use of opioids, the pharmaceutical industry has marketed tramadol as a safer opioid option for pain management. Tramadol binds at the mu-opioid receptor with an affinity that is less than 4000-fold that of morphine; the binding potency of M1, the metabolite of tramadol, is less than 5-fold that of morphine.4 Due to its lower binding affinity at the mu-opioid receptor, tramadol is considered a weak opioid, one believed to have minimal withdrawal symptoms and a lower potential for overdose or misuse compared to other opioids.1,5 Based on this characterization, many clinicians prescribe tramadol for elderly patients or patients otherwise at risk for medication misuse or adverse effects of opioids.6 In addition, hospitalized patients often have contraindications to nonopioid medications (eg, acetaminophen, nonsteroidal anti-inflammatory drugs [NSAIDs]), limiting their options for pain management.
WHY PRESCRIBING TRAMADOL FOR PAIN SHOULD BE AVOIDED
Despite being marketed as an effective and safe medication, tramadol has an unpredictable metabolism, complex pharmacology, and drug-drug interactions that can cause significant adverse effects. Similar to other opioids, tramadol is associated with a risk of misuse, physiologic dependency, and overdose. In addition, tramadol has a black box warning for addiction, misuse, respiratory depression, ultra-rapid metabolism, neonatal opioid withdrawal syndrome, CYP450 drug interactions, and interactions with other central nervous system depressants.
While tramadol has multiple mechanisms of action, the literature lacks high-quality evidence (eg, large randomized controlled trials) supporting its use, especially in hospitalized medical patients. A recent retrospective study of tramadol looked at the diagnoses of 250 hospitalized patients who received tramadol for pain management. While this study did not examine efficacy, it found mild-to-moderate acute noncancer pain to be the primary reason for prescribing tramadol.7 This study also showed the risk of severe drug-drug interactions increased the longer patients were on tramadol.7
As a result of the limited evidence in hospitalized patients, hospitalists must rely on outpatient studies.8-10 The size and quality of these studies, especially given the magnitude of tramadol prescribing in the United States, make them less useful. A series of Cochrane reviews examining the beneficial effects of tramadol for neuropathic pain, osteoarthritis, and cancer pain show insufficient evidence for tramadol when compared to placebo or active controls such as acetaminophen, NSAIDs, or other opioids.8-10
The side-effect profile of tramadol outweighs its mild analgesic effects. The 2019 American Geriatric Society Beers criteria for potentially inappropriate medication use in older adults strongly recommends clinicians use caution when prescribing tramadol to older adult patients, as tramadol may worsen or cause hyponatremia.11 In one large, population-based study, the use of tramadol doubled patients’ risk of hospitalization for hyponatremia when compared to codeine, though the incidence remains rather low at 4.6 per 10,000 person-months.12 Studies have also demonstrated an increased risk of hospitalization for hypoglycemia in nondiabetic patients receiving tramadol.13 A large propensity-score matched cohort study of patients with osteoarthritis found tramadol to have an associated higher all-cause mortality compared to NSAIDs; however, these differences may be due to confounding variables.1 In addition to hyponatremia and all-cause mortality, patients taking tramadol also have an associated increased risk of falls and hip fractures when compared to codeine or NSAIDs.14
The increased serotonergic activity associated with tramadol can lead to serotonin syndrome (serotonin toxicity), a rare but serious condition. Although serotonin syndrome can develop in patients taking tramadol as a monotherapy, the risk for this toxidrome increases when tramadol is taken in combination with other serotonergic agents or agents that inhibit metabolism of tramadol at CYP2D6.5 Seizures may also occur with tramadol at therapeutic and supratherapeutic doses. Population-based studies estimate seizures occur in 0.15% to 0.86% of patients receiving tramadol, which is two to six times the risk of those not on tramadol.5 Patients concurrently taking tramadol with a tricyclic antidepressant (TCA) or selective serotonin reuptake inhibitor (SSRI) are estimated to have seizures five to nine times more often than patients not taking a TCA or SSRI.5 Risk factors for tramadol-induced seizure include tramadol misuse or overdose, tramadol doses >1000 mg daily (maximum recommended dose is 400 mg/day), chronic tramadol use, concurrent use of a serotonergic agent or medications that inhibit CYP2D6, and history of epilepsy, renal disease, stroke, or traumatic brain injury.5
Differences in the genetic polymorphisms of CYP2D6 can produce a range of CYP2D6 activity from “poor metabolizers” (little-to-no analgesic effect) to “ultra-rapid metabolizers” (enhanced analgesia and increased risk of adverse effects), leading to unpredictable pharmacodynamic effects of tramadol.2 In North Africa and the Arabian peninsula, more than 25% of the population rapidly metabolizes tramadol; these pharmacogenomic effects result in higher rates of tramadol addiction and overdose in these regions.5 An estimated 7% to 10% of Caucasians slowly metabolize tramadol, which may place them at risk of adverse effects from tramadol in addition to inadequate analgesia.15 In contrast, Ethiopian populations have the highest rate of ultra-rapid tramadol metabolism at 29%.15
Drugs that induce CYP2D6 (eg, dexamethasone, rifampin) or inhibit CYP2D6 (eg, bupropion, fluoxetine) also impact tramadol efficacy, pharmacokinetics, and pharmacodynamics.16,17 Patients taking strong CYP2D6 inhibitors require significantly higher doses of tramadol to achieve analgesic effects.17 Tramadol undergoes extensive hepatic metabolism, producing several active metabolites, including M1 (Figure). Hepatic impairment increases the elimination half-life of tramadol and its metabolites.18 The majority of tramadol and its metabolites are eliminated through the kidneys. Accumulation of tramadol and its metabolites may occur in patients with renal impairment, placing them at increased risk of adverse effects.2
Finally, although some clinicians assume that tramadol has lower rates of misuse, diversion, or overdose compared to other opioids, rates of nonprescription use have increased with its proliferation.19,20 The US Substance Abuse and Mental Health Services Administration estimates that 1,287,000 persons misused tramadol in 2019.21 Patients may exhibit symptoms of physiologic opioid dependence and withdrawal from chronic tramadol use.2,22 In one study, patients prescribed tramadol monotherapy for acute pain from elective surgery had an increased risk for prolonged opioid use compared to patients prescribed other short-acting opioids.22
WHAT YOU SHOULD DO INSTEAD
Clinicians should determine the nature of the patient’s pain by obtaining a complete medical history, performing a thorough physical examination, and ordering diagnostic tests and imaging studies, as necessary. After consulting with the patient’s primary care physician, the clinician should employ a multimodal approach to pain that includes topical agents, psychotherapy, injections or interventions, and nonopioid medications. Patients with neuropathic pain may benefit from adjuvant analgesics such as gabapentinoids, TCAs, or SNRIs. In patients with evidence-based indications for opioid therapy (eg, pancreatitis, cancer pain, postsurgical pain), the hospitalist should assess the risk for opioid misuse and discuss risks and benefits with the patient before considering a time-limited trial of opioid therapy. If available and when indicated, clinicians should consult with specialists in pain management or palliative care. For cases wherein clinicians have already prescribed tramadol to the patient, they should discuss deprescribing strategies and alternative analgesic options with the patient and the patient’s primary care physician. Finally, before initiating tramadol therapy for hospitalized patients with pain, hospitalists should consider the risks, benefits, and alternative approaches to prescribing tramadol.
RECOMMENDATIONS
- For hospitalized patients reporting pain, complete a pain assessment by history, physical exam, chart review, and diagnostic studies to examine the etiology of the pain.
- Utilize multiple modalities for pain control when possible, including acetaminophen, NSAIDs, topical agents, ice or heat, neuropathic pain medications, and interventions such as injections, psychotherapy, or radiation, if indicated.
- Avoid prescribing tramadol due to unpredictable pharmacodynamics, adverse effects, and lack of quality evidence for efficacy in hospitalized medical patients.
CONCLUSION
Tramadol is a commonly used opioid medication associated with adverse effects and unpredictable analgesia. Regarding this case scenario, the use of tramadol in this patient places him at risk for drug-drug interactions, hyponatremia, hypoglycemia, serotonin syndrome, seizures, and pronounced side effects of opioid medications. Moderate quality evidence in the outpatient setting suggests that tramadol is unlikely to provide significant analgesia for his osteoarthritic pain.9 Instead of prescribing tramadol, the hospitalist should consider alternative treatments for this patient’s pain, such as intraarticular glucocorticoids, a short course of oral NSAIDs (unless contraindicated), topical treatments (eg, menthol, capsaicin, NSAIDs), physical therapy, and close follow-up with an orthopedist after hospital discharge. Further randomized controlled studies of tramadol vs active controls are needed.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
1. Zeng C, Dubreuil M, LaRochelle MR, et al. Association of tramadol with all-cause mortality among patients with osteoarthritis. JAMA. 2019;321(10):969-982. https://doi.org/10.1001/jama.2019.1347
2. Gong L, Stamer UM, Tzvetkov MV, Altman RB, Klein TE. PharmGKB summary: tramadol pathway. Pharmacogenet Genomics. 2014;24(7):374-380. https://doi.org/10.1097/FPC.0000000000000057
3. The top 200 drugs of 2019. ClinCalc DrugStats Database. Accessed June 10, 2021. https://clincalc.com/DrugStats
4. Gillen C, Haurand M, Kobelt DJ, Wnendt S. Affinity, potency and efficacy of tramadol and its metabolites at the cloned human µ-opioid receptor. Naunyn Schmiedebergs Arch Pharmacol. 2000;362(2):116-121. https://doi.org/10.1007/s002100000266
5. Hassamal S, Miotto K, Dale W, Danovitch I. Tramadol: understanding the risk of serotonin syndrome and seizures. Am J Med. 2018;131(11):1382.e1-1382.e6. https://doi.org/10.1016/j.amjmed.2018.04.025
6. Shipton EA. Tramadol—present and future. Anaesth Intensive Care. 2000;28(4):363-374. https://doi.org/10.1177/0310057X0002800403
7. Mohan N, Edmonds KP, Ajayi TA, Atayee RS, Clinical tolerability and safety of tramadol in hospitalized patients. J Pain & Palliat Care Pharmacother. 2020:34(4):211-218. https://doi.org/10.1080/15360288.2020.1817227
8. Duehmke RM, Derry S, Wiffen PJ, Bell RF, Aldington D, Moore RA. Tramadol for neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6(6):CD003726. https://doi.org/10.1002/14651858.cd003726.pub4
9. Toupin-April K, Bisaillon J, Welch V, et al. Tramadol for osteoarthritis. Cochrane Database Syst Rev. 2019;5(5):CD005522. https://doi.org/10.1002/14651858.cd005522.pub3
10. Wiffen PJ, Derry S, Moore RA. Tramadol with or without paracetamol (acetaminophen) for cancer pain. Cochrane Database Syst Rev. 2017;5(5):CD012508. https://doi.org/10.1002/14651858.cd012508.pub2
11. The American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. https://doi.org/10.1111/jgs.15767
12. Fournier JP, Yin H, Nessim SJ, Montastruc JL, Azoulay L. Tramadol for noncancer pain and the risk of hyponatremia. Am J Med. 2015;128(4):418-425.e5. https://doi.org/10.1016/j.amjmed.2014.10.046
13. Fournier JP, Azoulay L, Yin H, Montastruc JL, Suissa S. Tramadol use and the risk of hospitalization for hypoglycemia in patients with noncancer pain. JAMA Intern Med. 2015;175(2):186-193. https://doi.org/10.1001/jamainternmed.2014.6512
14. Wei J, Lane NE, Bolster MB, et al. Association of tramadol use with risk of hip fracture. J Bone Miner Res. 2020;35(4):631-640. https://doi.org/10.1002/jbmr.3935
15. Leppert W. CYP2D6 in the metabolism of opioids for mild to moderate pain. Pharmacology. 2011;87(5-6):274-285. https://doi.org/10.1159/000326085
16. Flockhart DA, Thacker D, McDonald C, Desta Z. The Flockhart cytochrome P450 drug-drug interaction table. Division of Clinical Pharmacology, Indiana University School of Medicine. Updated 2021. Accessed April 21, 2021. https://drug-interactions.medicine.iu.edu
17. Frost DA, Soric MM, Kaiser R, Neugebauer RE. Efficacy of tramadol for pain management in patients receiving strong cytochrome P450 2D6 inhibitors. Pharmacotherapy. 2019;39(6):724-729. https://doi.org/10.1002/phar.2269
18. Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43(13):879-923. https://doi.org/10.2165/00003088-200443130-00004
19. Bush DM. The CBHSQ report: emergency department visits for drug misuse or abuse involving the pain medication tramadol. Substance Abuse and Mental Health Service Administration. May 14, 2015. Accessed June 16, 2021. https://www.ncbi.nlm.nih.gov/books/NBK343535/
20. Bigal LM, Bibeau K, Dunbar S. Tramadol prescription over a 4-year period in the USA. Curr Pain Headache Rep. 2019;23(10):76. https://doi.org/10.1007/s11916-019-0777-x
21. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. National survey on drug use and health 2019 (NSDUH-2019). Accessed June 16, 2021. https://www.samhsa.gov/data/release/2019-national-survey-drug-use-and-health-nsduh-releases
22. Thiels CA, Habermann EB, Hooten WM, Jeffery MM. Chronic use of tramadol after acute pain episode: cohort study. BMJ. 2019;365:l1849. https://doi.org/10.1136/bmj.l1849
1. Zeng C, Dubreuil M, LaRochelle MR, et al. Association of tramadol with all-cause mortality among patients with osteoarthritis. JAMA. 2019;321(10):969-982. https://doi.org/10.1001/jama.2019.1347
2. Gong L, Stamer UM, Tzvetkov MV, Altman RB, Klein TE. PharmGKB summary: tramadol pathway. Pharmacogenet Genomics. 2014;24(7):374-380. https://doi.org/10.1097/FPC.0000000000000057
3. The top 200 drugs of 2019. ClinCalc DrugStats Database. Accessed June 10, 2021. https://clincalc.com/DrugStats
4. Gillen C, Haurand M, Kobelt DJ, Wnendt S. Affinity, potency and efficacy of tramadol and its metabolites at the cloned human µ-opioid receptor. Naunyn Schmiedebergs Arch Pharmacol. 2000;362(2):116-121. https://doi.org/10.1007/s002100000266
5. Hassamal S, Miotto K, Dale W, Danovitch I. Tramadol: understanding the risk of serotonin syndrome and seizures. Am J Med. 2018;131(11):1382.e1-1382.e6. https://doi.org/10.1016/j.amjmed.2018.04.025
6. Shipton EA. Tramadol—present and future. Anaesth Intensive Care. 2000;28(4):363-374. https://doi.org/10.1177/0310057X0002800403
7. Mohan N, Edmonds KP, Ajayi TA, Atayee RS, Clinical tolerability and safety of tramadol in hospitalized patients. J Pain & Palliat Care Pharmacother. 2020:34(4):211-218. https://doi.org/10.1080/15360288.2020.1817227
8. Duehmke RM, Derry S, Wiffen PJ, Bell RF, Aldington D, Moore RA. Tramadol for neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6(6):CD003726. https://doi.org/10.1002/14651858.cd003726.pub4
9. Toupin-April K, Bisaillon J, Welch V, et al. Tramadol for osteoarthritis. Cochrane Database Syst Rev. 2019;5(5):CD005522. https://doi.org/10.1002/14651858.cd005522.pub3
10. Wiffen PJ, Derry S, Moore RA. Tramadol with or without paracetamol (acetaminophen) for cancer pain. Cochrane Database Syst Rev. 2017;5(5):CD012508. https://doi.org/10.1002/14651858.cd012508.pub2
11. The American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. https://doi.org/10.1111/jgs.15767
12. Fournier JP, Yin H, Nessim SJ, Montastruc JL, Azoulay L. Tramadol for noncancer pain and the risk of hyponatremia. Am J Med. 2015;128(4):418-425.e5. https://doi.org/10.1016/j.amjmed.2014.10.046
13. Fournier JP, Azoulay L, Yin H, Montastruc JL, Suissa S. Tramadol use and the risk of hospitalization for hypoglycemia in patients with noncancer pain. JAMA Intern Med. 2015;175(2):186-193. https://doi.org/10.1001/jamainternmed.2014.6512
14. Wei J, Lane NE, Bolster MB, et al. Association of tramadol use with risk of hip fracture. J Bone Miner Res. 2020;35(4):631-640. https://doi.org/10.1002/jbmr.3935
15. Leppert W. CYP2D6 in the metabolism of opioids for mild to moderate pain. Pharmacology. 2011;87(5-6):274-285. https://doi.org/10.1159/000326085
16. Flockhart DA, Thacker D, McDonald C, Desta Z. The Flockhart cytochrome P450 drug-drug interaction table. Division of Clinical Pharmacology, Indiana University School of Medicine. Updated 2021. Accessed April 21, 2021. https://drug-interactions.medicine.iu.edu
17. Frost DA, Soric MM, Kaiser R, Neugebauer RE. Efficacy of tramadol for pain management in patients receiving strong cytochrome P450 2D6 inhibitors. Pharmacotherapy. 2019;39(6):724-729. https://doi.org/10.1002/phar.2269
18. Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43(13):879-923. https://doi.org/10.2165/00003088-200443130-00004
19. Bush DM. The CBHSQ report: emergency department visits for drug misuse or abuse involving the pain medication tramadol. Substance Abuse and Mental Health Service Administration. May 14, 2015. Accessed June 16, 2021. https://www.ncbi.nlm.nih.gov/books/NBK343535/
20. Bigal LM, Bibeau K, Dunbar S. Tramadol prescription over a 4-year period in the USA. Curr Pain Headache Rep. 2019;23(10):76. https://doi.org/10.1007/s11916-019-0777-x
21. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. National survey on drug use and health 2019 (NSDUH-2019). Accessed June 16, 2021. https://www.samhsa.gov/data/release/2019-national-survey-drug-use-and-health-nsduh-releases
22. Thiels CA, Habermann EB, Hooten WM, Jeffery MM. Chronic use of tramadol after acute pain episode: cohort study. BMJ. 2019;365:l1849. https://doi.org/10.1136/bmj.l1849
© 2021 Society of Hospital Medicine