User login
Centers for Medicare & Medicaid Services Price Publication Requirement: If You Post It, Will They Come?
Patients in the United States continue to experience rising out-of-pocket medical costs, with little access to the price information they desire when making decisions regarding medical care.1 The Centers for Medicare & Medicaid Services (CMS) has taken steps toward transparency by requiring hospitals to publish price information.2 In this issue of the Journal of Hospital Medicine, White and Liao3 break down the new rule, and we further discuss how this policy affects patients, hospitals, and hospitalists.
The new CMS rule requires hospitals to publish the prices of 300 “shoppable” services, including those negotiated with different payors. The rule standardizes how this information is displayed and accessed, with a daily penalty for facilities that fail to comply. Clinics and ambulatory surgical centers are currently excluded, as are facility and ancillary fees, such as those billed by pathology or anesthesiology. As White and Liao point out, a limitation for hospitalists is that this rule will only affect orders for the outpatient setting at discharge. In addition, this rule separates cost from quality. Although quality data are publicly available via CMS, price data are posted directly by hospitals, making a true value assessment difficult. To strengthen the rule, White and Liao recommend the following: increasing the financial penalty for noncompliance; aggregating data centrally to allow for comparisons; adding quality data to cost; expanding included sites and types of services; and adding common additional fees to the service price.
The larger question is whether patients will use these data in the manner intended. Previous studies have found a paradoxical relationship between patients’ expressed desire to compare prices for medical services vs documented low levels of price-shopping behavior. Mehrotra et al1 found that lack of access to data as well as loyalty to providers were significant barriers to using price data effectively. The CMS rule increases access to the price information patients desire but cannot find. However, it is unclear whether available prices will be sufficient to change behaviors given that, aside from those with no insurance and those with high-deductible plans, most patients are fairly removed from the actual cost of service.
This rule may have a larger, unexpected impact on hospitals and access to care. Sharing price data could increase pressure on facilities to merge with larger systems in order to obtain more favorable rates via increased negotiating power. Hospitals that serve poorer communities may not be attractive merger candidates for large systems and could be left out of the push toward consolidation. Charging higher prices for the same services could lead to hospital closures or cuts in resources, potentially exacerbating health inequities for underserved populations.
On the provider end, it is unlikely that price transparency will influence resource utilization. Mummadi et al4 found that displaying price information in the electronic health record did not significantly influence physician ordering behavior. For hospitalists today, the emphasis on “high-value care” is already an important consideration when utilizing healthcare resources, considering the Accreditation Council for Graduate Medical Education (ACGME) requirements for residency, restrictive insurance protocols, and guidelines such as the ACR Appropriateness Criteria and the American Board of Internal Medicine’s Choosing Wisely® campaign. Outside of extremes, separate cost data likely will not make a difference in provider ordering practices.
Although the information from this rule may not cause dramatic practice change, it will allow us to help our patients by providing those interested in price-shopping with data. This policy represents a large step toward a more transparent healthcare system, though it may have limited impact on overall healthcare costs.
1. Mehrotra A, Dean KM, Sinaiko AD, Sood N. Americans support price shopping for health care, but few actually seek out price information. Health Aff (Millwood). 2017;36(8):1392-1400. https://doi.org/10.1377/hlthaff.2016.1471
2. Price Transparency Requirements for Hospitals to Make Standard Charges Public. 45 CFR § 180.20 (2019).
3. White AA, Liao JM. Policy in clinical practice: hospital price transparency. J Hosp Med. 2021;16(11):688-690. https://doi.org/10.12788/jhm.3698
4. Mummadi SR, Mishra R. Effectiveness of provider price display in computerized physician order entry (CPOE) on healthcare quality: a systematic review. J Am Med Inform Assoc. 2018;25(9):1228-1239. https://doi.org/10.1093/jamia/ocy076
Patients in the United States continue to experience rising out-of-pocket medical costs, with little access to the price information they desire when making decisions regarding medical care.1 The Centers for Medicare & Medicaid Services (CMS) has taken steps toward transparency by requiring hospitals to publish price information.2 In this issue of the Journal of Hospital Medicine, White and Liao3 break down the new rule, and we further discuss how this policy affects patients, hospitals, and hospitalists.
The new CMS rule requires hospitals to publish the prices of 300 “shoppable” services, including those negotiated with different payors. The rule standardizes how this information is displayed and accessed, with a daily penalty for facilities that fail to comply. Clinics and ambulatory surgical centers are currently excluded, as are facility and ancillary fees, such as those billed by pathology or anesthesiology. As White and Liao point out, a limitation for hospitalists is that this rule will only affect orders for the outpatient setting at discharge. In addition, this rule separates cost from quality. Although quality data are publicly available via CMS, price data are posted directly by hospitals, making a true value assessment difficult. To strengthen the rule, White and Liao recommend the following: increasing the financial penalty for noncompliance; aggregating data centrally to allow for comparisons; adding quality data to cost; expanding included sites and types of services; and adding common additional fees to the service price.
The larger question is whether patients will use these data in the manner intended. Previous studies have found a paradoxical relationship between patients’ expressed desire to compare prices for medical services vs documented low levels of price-shopping behavior. Mehrotra et al1 found that lack of access to data as well as loyalty to providers were significant barriers to using price data effectively. The CMS rule increases access to the price information patients desire but cannot find. However, it is unclear whether available prices will be sufficient to change behaviors given that, aside from those with no insurance and those with high-deductible plans, most patients are fairly removed from the actual cost of service.
This rule may have a larger, unexpected impact on hospitals and access to care. Sharing price data could increase pressure on facilities to merge with larger systems in order to obtain more favorable rates via increased negotiating power. Hospitals that serve poorer communities may not be attractive merger candidates for large systems and could be left out of the push toward consolidation. Charging higher prices for the same services could lead to hospital closures or cuts in resources, potentially exacerbating health inequities for underserved populations.
On the provider end, it is unlikely that price transparency will influence resource utilization. Mummadi et al4 found that displaying price information in the electronic health record did not significantly influence physician ordering behavior. For hospitalists today, the emphasis on “high-value care” is already an important consideration when utilizing healthcare resources, considering the Accreditation Council for Graduate Medical Education (ACGME) requirements for residency, restrictive insurance protocols, and guidelines such as the ACR Appropriateness Criteria and the American Board of Internal Medicine’s Choosing Wisely® campaign. Outside of extremes, separate cost data likely will not make a difference in provider ordering practices.
Although the information from this rule may not cause dramatic practice change, it will allow us to help our patients by providing those interested in price-shopping with data. This policy represents a large step toward a more transparent healthcare system, though it may have limited impact on overall healthcare costs.
Patients in the United States continue to experience rising out-of-pocket medical costs, with little access to the price information they desire when making decisions regarding medical care.1 The Centers for Medicare & Medicaid Services (CMS) has taken steps toward transparency by requiring hospitals to publish price information.2 In this issue of the Journal of Hospital Medicine, White and Liao3 break down the new rule, and we further discuss how this policy affects patients, hospitals, and hospitalists.
The new CMS rule requires hospitals to publish the prices of 300 “shoppable” services, including those negotiated with different payors. The rule standardizes how this information is displayed and accessed, with a daily penalty for facilities that fail to comply. Clinics and ambulatory surgical centers are currently excluded, as are facility and ancillary fees, such as those billed by pathology or anesthesiology. As White and Liao point out, a limitation for hospitalists is that this rule will only affect orders for the outpatient setting at discharge. In addition, this rule separates cost from quality. Although quality data are publicly available via CMS, price data are posted directly by hospitals, making a true value assessment difficult. To strengthen the rule, White and Liao recommend the following: increasing the financial penalty for noncompliance; aggregating data centrally to allow for comparisons; adding quality data to cost; expanding included sites and types of services; and adding common additional fees to the service price.
The larger question is whether patients will use these data in the manner intended. Previous studies have found a paradoxical relationship between patients’ expressed desire to compare prices for medical services vs documented low levels of price-shopping behavior. Mehrotra et al1 found that lack of access to data as well as loyalty to providers were significant barriers to using price data effectively. The CMS rule increases access to the price information patients desire but cannot find. However, it is unclear whether available prices will be sufficient to change behaviors given that, aside from those with no insurance and those with high-deductible plans, most patients are fairly removed from the actual cost of service.
This rule may have a larger, unexpected impact on hospitals and access to care. Sharing price data could increase pressure on facilities to merge with larger systems in order to obtain more favorable rates via increased negotiating power. Hospitals that serve poorer communities may not be attractive merger candidates for large systems and could be left out of the push toward consolidation. Charging higher prices for the same services could lead to hospital closures or cuts in resources, potentially exacerbating health inequities for underserved populations.
On the provider end, it is unlikely that price transparency will influence resource utilization. Mummadi et al4 found that displaying price information in the electronic health record did not significantly influence physician ordering behavior. For hospitalists today, the emphasis on “high-value care” is already an important consideration when utilizing healthcare resources, considering the Accreditation Council for Graduate Medical Education (ACGME) requirements for residency, restrictive insurance protocols, and guidelines such as the ACR Appropriateness Criteria and the American Board of Internal Medicine’s Choosing Wisely® campaign. Outside of extremes, separate cost data likely will not make a difference in provider ordering practices.
Although the information from this rule may not cause dramatic practice change, it will allow us to help our patients by providing those interested in price-shopping with data. This policy represents a large step toward a more transparent healthcare system, though it may have limited impact on overall healthcare costs.
1. Mehrotra A, Dean KM, Sinaiko AD, Sood N. Americans support price shopping for health care, but few actually seek out price information. Health Aff (Millwood). 2017;36(8):1392-1400. https://doi.org/10.1377/hlthaff.2016.1471
2. Price Transparency Requirements for Hospitals to Make Standard Charges Public. 45 CFR § 180.20 (2019).
3. White AA, Liao JM. Policy in clinical practice: hospital price transparency. J Hosp Med. 2021;16(11):688-690. https://doi.org/10.12788/jhm.3698
4. Mummadi SR, Mishra R. Effectiveness of provider price display in computerized physician order entry (CPOE) on healthcare quality: a systematic review. J Am Med Inform Assoc. 2018;25(9):1228-1239. https://doi.org/10.1093/jamia/ocy076
1. Mehrotra A, Dean KM, Sinaiko AD, Sood N. Americans support price shopping for health care, but few actually seek out price information. Health Aff (Millwood). 2017;36(8):1392-1400. https://doi.org/10.1377/hlthaff.2016.1471
2. Price Transparency Requirements for Hospitals to Make Standard Charges Public. 45 CFR § 180.20 (2019).
3. White AA, Liao JM. Policy in clinical practice: hospital price transparency. J Hosp Med. 2021;16(11):688-690. https://doi.org/10.12788/jhm.3698
4. Mummadi SR, Mishra R. Effectiveness of provider price display in computerized physician order entry (CPOE) on healthcare quality: a systematic review. J Am Med Inform Assoc. 2018;25(9):1228-1239. https://doi.org/10.1093/jamia/ocy076
© 2021 Society of Hospital Medicine
Goal-Concordant Care After Hospitalization for Serious Acute Illness: A Key Opportunity for Hospitalists in Patient-Centered Outcomes
Care concordant with patient goals of care (GOC) is a central component of quality. Communication about GOC is associated with improved quality of life, reduced resource utilization, and optimized end-of-life (EOL) care. Prior literature has focused on outpatient populations, with little knowledge based on preferences elicited from patients hospitalized for serious acute illness.1 The consequent knowledge gap relates to a dimension of practice through which hospitalists can improve patient-centered care by clarifying patient preferences for goal-directed treatments both during and following hospitalization.2 Implementing interventions that optimize shared decision-making through a personalized serious- illness care plan is a high-priority research area.2
In this issue, to estimate how frequently GOC are assessed during hospitalization for serious illness and the concordance between identified goals and postdischarge care, Taylor et al3 retrospectively evaluated a cohort of sepsis survivors through electronic health record (EHR) review. A standardized EHR care alignment tool and a comprehensive EHR assessment demonstrated that only 19% and 40% of patients, respectively, had identifiable GOC documented. Goal-concordant care was subsequently observed among 68% of patients with identified goals, consistent with prior work demonstrating goal-concordance in this range.1 Data on EOL care provided to decedents in an integrated health system notably showed that 89% received goal-concordant treatments.4 This difference may stem from clinicians’ emphasis on goal ascertainment at the EOL, a propensity reflected in the comparative characteristics of patients with goals documented in the current study’s Table.3 Investigators took advantage of unique inpatient and postdischarge clinical information from a sepsis patient sample to provide novel insights into the inadequacy of patient preference assessment and the substantial frequency of goal-discordant care resulting from insufficient attention to GOC.
This study suggests a critical need to improve practices related to identification of GOC in patients hospitalized with serious illness. After adjusting for relevant confounding characteristics, completion of a standardized EHR care alignment tool was strongly associated with receipt of goal-concordant care following discharge.3 Although this tool was only completed in 19% of patients, this finding suggests that elicitation of patient preferences is an under-addressed step in facilitating patient-centered transitions of care. In particular, the low 39% rate of goal-concordant care among patients prioritizing comfort over longevity is noteworthy, but consistent with prior literature.1 This degree of discordance highlights provision of goal-concordant care following hospitalization as a key, yet unfulfilled, patient-centered-care quality metric.
The identified shortcomings in communication and care represent an important opportunity for hospitalists to enhance the extent to which survivors of critical illness receive care respectful of their preferences and values. Given the importance of effective discharge handoff practices in hospital medicine,2 future work should address assertively incorporating GOC into transitions after serious acute illness. Enhancing communication of these goals at discharge may benefit patients at high risk of readmission and other postdischarge adverse events, particularly for patients with comfort-focused GOC.
The study is limited in its derivation from trial participants with a specific clinical syndrome in a single health system. Also, investigators’ classification of a single patient goal does not reflect the multifactorial objectives of health interventions. In addition, since patient-reported GOC discussions correlate more highly with goal-concordant care than those identified through EHRs,5 future work should ascertain the generalizability of the identified gaps in practice.
The findings of this study underscore the need for clinicians to promote GOC assessment and documentation during hospitalization for high-risk conditions, such as sepsis. Tracking rates of GOC elicitation and goal-concordant care following discharge should be incorporated into quality measurement systems as important patient-centered dimensions of care. Hospitalists can fill a critical void by helping to correct the deficiencies that exist in respecting the preferences of survivors of serious acute illness.
1. Modes ME, Heckbert SR, Engelberg RA, Nielsen EL, Curtis JR, Kross EK. Patient-reported receipt of goal-concordant care among seriously ill outpatients-prevalence and associated factors. J Pain Symptom Manage. 2020;60(4):765-773. https://doi.org/10.1016/j.jpainsymman.2020.04.026
2. Harrison JD, Archuleta M, Avitia E, et al. Developing a patient- and family-centered research agenda for hospital medicine: the Improving Hospital Outcomes through Patient Engagement (i-HOPE) Study. J Hosp Med. 2020;15(6):331-337. https://doi.org/10.12788/jhm.3386
3. Taylor SP, Kowalkowski MA, Courtright KR, et al. Deficits in identification of goals and goal-concordant care after sepsis hospitalization. J Hosp Med. 2021;16(11):645-651. https://doi.org/10.12788/jhm.3714
4. Glass DP, Wang SE, Minardi PM, Kanter MH. Concordance of end-of-life care with end-of-life wishes in an integrated health care system. JAMA Netw Open. 2021;4(4):e213053. https://doi.org/10.1001/jamanetworkopen.2021.3053
5. Modes ME, Engelberg RA, Downey L, Nielsen EL, Curtis JR, Kross EK. Did a goals-of-care discussion happen? Differences in the occurrence of goals-of-care discussions as reported by patients, clinicians, and in the electronic health record. J Pain Symptom Manage. 2019;57(2):251-259. https://doi.org/10.1016/j.jpainsymman.2018.10.507
Care concordant with patient goals of care (GOC) is a central component of quality. Communication about GOC is associated with improved quality of life, reduced resource utilization, and optimized end-of-life (EOL) care. Prior literature has focused on outpatient populations, with little knowledge based on preferences elicited from patients hospitalized for serious acute illness.1 The consequent knowledge gap relates to a dimension of practice through which hospitalists can improve patient-centered care by clarifying patient preferences for goal-directed treatments both during and following hospitalization.2 Implementing interventions that optimize shared decision-making through a personalized serious- illness care plan is a high-priority research area.2
In this issue, to estimate how frequently GOC are assessed during hospitalization for serious illness and the concordance between identified goals and postdischarge care, Taylor et al3 retrospectively evaluated a cohort of sepsis survivors through electronic health record (EHR) review. A standardized EHR care alignment tool and a comprehensive EHR assessment demonstrated that only 19% and 40% of patients, respectively, had identifiable GOC documented. Goal-concordant care was subsequently observed among 68% of patients with identified goals, consistent with prior work demonstrating goal-concordance in this range.1 Data on EOL care provided to decedents in an integrated health system notably showed that 89% received goal-concordant treatments.4 This difference may stem from clinicians’ emphasis on goal ascertainment at the EOL, a propensity reflected in the comparative characteristics of patients with goals documented in the current study’s Table.3 Investigators took advantage of unique inpatient and postdischarge clinical information from a sepsis patient sample to provide novel insights into the inadequacy of patient preference assessment and the substantial frequency of goal-discordant care resulting from insufficient attention to GOC.
This study suggests a critical need to improve practices related to identification of GOC in patients hospitalized with serious illness. After adjusting for relevant confounding characteristics, completion of a standardized EHR care alignment tool was strongly associated with receipt of goal-concordant care following discharge.3 Although this tool was only completed in 19% of patients, this finding suggests that elicitation of patient preferences is an under-addressed step in facilitating patient-centered transitions of care. In particular, the low 39% rate of goal-concordant care among patients prioritizing comfort over longevity is noteworthy, but consistent with prior literature.1 This degree of discordance highlights provision of goal-concordant care following hospitalization as a key, yet unfulfilled, patient-centered-care quality metric.
The identified shortcomings in communication and care represent an important opportunity for hospitalists to enhance the extent to which survivors of critical illness receive care respectful of their preferences and values. Given the importance of effective discharge handoff practices in hospital medicine,2 future work should address assertively incorporating GOC into transitions after serious acute illness. Enhancing communication of these goals at discharge may benefit patients at high risk of readmission and other postdischarge adverse events, particularly for patients with comfort-focused GOC.
The study is limited in its derivation from trial participants with a specific clinical syndrome in a single health system. Also, investigators’ classification of a single patient goal does not reflect the multifactorial objectives of health interventions. In addition, since patient-reported GOC discussions correlate more highly with goal-concordant care than those identified through EHRs,5 future work should ascertain the generalizability of the identified gaps in practice.
The findings of this study underscore the need for clinicians to promote GOC assessment and documentation during hospitalization for high-risk conditions, such as sepsis. Tracking rates of GOC elicitation and goal-concordant care following discharge should be incorporated into quality measurement systems as important patient-centered dimensions of care. Hospitalists can fill a critical void by helping to correct the deficiencies that exist in respecting the preferences of survivors of serious acute illness.
Care concordant with patient goals of care (GOC) is a central component of quality. Communication about GOC is associated with improved quality of life, reduced resource utilization, and optimized end-of-life (EOL) care. Prior literature has focused on outpatient populations, with little knowledge based on preferences elicited from patients hospitalized for serious acute illness.1 The consequent knowledge gap relates to a dimension of practice through which hospitalists can improve patient-centered care by clarifying patient preferences for goal-directed treatments both during and following hospitalization.2 Implementing interventions that optimize shared decision-making through a personalized serious- illness care plan is a high-priority research area.2
In this issue, to estimate how frequently GOC are assessed during hospitalization for serious illness and the concordance between identified goals and postdischarge care, Taylor et al3 retrospectively evaluated a cohort of sepsis survivors through electronic health record (EHR) review. A standardized EHR care alignment tool and a comprehensive EHR assessment demonstrated that only 19% and 40% of patients, respectively, had identifiable GOC documented. Goal-concordant care was subsequently observed among 68% of patients with identified goals, consistent with prior work demonstrating goal-concordance in this range.1 Data on EOL care provided to decedents in an integrated health system notably showed that 89% received goal-concordant treatments.4 This difference may stem from clinicians’ emphasis on goal ascertainment at the EOL, a propensity reflected in the comparative characteristics of patients with goals documented in the current study’s Table.3 Investigators took advantage of unique inpatient and postdischarge clinical information from a sepsis patient sample to provide novel insights into the inadequacy of patient preference assessment and the substantial frequency of goal-discordant care resulting from insufficient attention to GOC.
This study suggests a critical need to improve practices related to identification of GOC in patients hospitalized with serious illness. After adjusting for relevant confounding characteristics, completion of a standardized EHR care alignment tool was strongly associated with receipt of goal-concordant care following discharge.3 Although this tool was only completed in 19% of patients, this finding suggests that elicitation of patient preferences is an under-addressed step in facilitating patient-centered transitions of care. In particular, the low 39% rate of goal-concordant care among patients prioritizing comfort over longevity is noteworthy, but consistent with prior literature.1 This degree of discordance highlights provision of goal-concordant care following hospitalization as a key, yet unfulfilled, patient-centered-care quality metric.
The identified shortcomings in communication and care represent an important opportunity for hospitalists to enhance the extent to which survivors of critical illness receive care respectful of their preferences and values. Given the importance of effective discharge handoff practices in hospital medicine,2 future work should address assertively incorporating GOC into transitions after serious acute illness. Enhancing communication of these goals at discharge may benefit patients at high risk of readmission and other postdischarge adverse events, particularly for patients with comfort-focused GOC.
The study is limited in its derivation from trial participants with a specific clinical syndrome in a single health system. Also, investigators’ classification of a single patient goal does not reflect the multifactorial objectives of health interventions. In addition, since patient-reported GOC discussions correlate more highly with goal-concordant care than those identified through EHRs,5 future work should ascertain the generalizability of the identified gaps in practice.
The findings of this study underscore the need for clinicians to promote GOC assessment and documentation during hospitalization for high-risk conditions, such as sepsis. Tracking rates of GOC elicitation and goal-concordant care following discharge should be incorporated into quality measurement systems as important patient-centered dimensions of care. Hospitalists can fill a critical void by helping to correct the deficiencies that exist in respecting the preferences of survivors of serious acute illness.
1. Modes ME, Heckbert SR, Engelberg RA, Nielsen EL, Curtis JR, Kross EK. Patient-reported receipt of goal-concordant care among seriously ill outpatients-prevalence and associated factors. J Pain Symptom Manage. 2020;60(4):765-773. https://doi.org/10.1016/j.jpainsymman.2020.04.026
2. Harrison JD, Archuleta M, Avitia E, et al. Developing a patient- and family-centered research agenda for hospital medicine: the Improving Hospital Outcomes through Patient Engagement (i-HOPE) Study. J Hosp Med. 2020;15(6):331-337. https://doi.org/10.12788/jhm.3386
3. Taylor SP, Kowalkowski MA, Courtright KR, et al. Deficits in identification of goals and goal-concordant care after sepsis hospitalization. J Hosp Med. 2021;16(11):645-651. https://doi.org/10.12788/jhm.3714
4. Glass DP, Wang SE, Minardi PM, Kanter MH. Concordance of end-of-life care with end-of-life wishes in an integrated health care system. JAMA Netw Open. 2021;4(4):e213053. https://doi.org/10.1001/jamanetworkopen.2021.3053
5. Modes ME, Engelberg RA, Downey L, Nielsen EL, Curtis JR, Kross EK. Did a goals-of-care discussion happen? Differences in the occurrence of goals-of-care discussions as reported by patients, clinicians, and in the electronic health record. J Pain Symptom Manage. 2019;57(2):251-259. https://doi.org/10.1016/j.jpainsymman.2018.10.507
1. Modes ME, Heckbert SR, Engelberg RA, Nielsen EL, Curtis JR, Kross EK. Patient-reported receipt of goal-concordant care among seriously ill outpatients-prevalence and associated factors. J Pain Symptom Manage. 2020;60(4):765-773. https://doi.org/10.1016/j.jpainsymman.2020.04.026
2. Harrison JD, Archuleta M, Avitia E, et al. Developing a patient- and family-centered research agenda for hospital medicine: the Improving Hospital Outcomes through Patient Engagement (i-HOPE) Study. J Hosp Med. 2020;15(6):331-337. https://doi.org/10.12788/jhm.3386
3. Taylor SP, Kowalkowski MA, Courtright KR, et al. Deficits in identification of goals and goal-concordant care after sepsis hospitalization. J Hosp Med. 2021;16(11):645-651. https://doi.org/10.12788/jhm.3714
4. Glass DP, Wang SE, Minardi PM, Kanter MH. Concordance of end-of-life care with end-of-life wishes in an integrated health care system. JAMA Netw Open. 2021;4(4):e213053. https://doi.org/10.1001/jamanetworkopen.2021.3053
5. Modes ME, Engelberg RA, Downey L, Nielsen EL, Curtis JR, Kross EK. Did a goals-of-care discussion happen? Differences in the occurrence of goals-of-care discussions as reported by patients, clinicians, and in the electronic health record. J Pain Symptom Manage. 2019;57(2):251-259. https://doi.org/10.1016/j.jpainsymman.2018.10.507
© 2021 Society of Hospital Medicine
Where Have All the Medicare Inpatients Gone?
The advent of COVID-19 saw a precipitous decline in inpatient admissions. Even before the COVID-19 pandemic, hospitals were seeing a trend toward fewer inpatient admissions for Medicare beneficiaries, which has not been thoroughly examined or explained.1 In this issue, Keohane et al2 studied Medicare inpatient episode trends between 2009 and 2017 and found that, during this period, inpatient episodes per 1000 Medicare fee-for-service (FFS) beneficiaries declined by 18.2%, from 326 to 267 per 1000 beneficiaries.
This trend can be partly explained by changes in the way that care is delivered. First, observation stays have risen, and these are excluded in the authors’ analysis. From 2010 to 2017, observation visits per 1000 beneficiaries increased from 28 to 51.1 Second, due to improved outpatient management, margin constraints, and efficiency gains, hospitals are less likely to admit patients with less complex problems or keep patients overnight for uncomplicated procedural interventions. In cardiology, there has been an increase in the proportion of same-day percutaneous coronary interventions, from 4.5% in 2009 to 28.6% in 2017.3 The authors do not include a quantitative measure of complexity, but their data support this conclusion as they find larger declines in episodes that began with a planned admission and those that involved no use of post–acute care services, and thus were likely less complicated admissions. Finally, the increased use of alternative care sites such as home-based care settings and urgent care clinics, the proliferation of telemedicine, and the continual development of guideline-based therapy have resulted in better outpatient management of diseases.
The growth of value-based care has also contributed to the reduction in inpatient admission. The past decade has seen the growth of bundled-payment contracts, accountable care organizations (ACO), and advanced primary care models. In 2018, an estimated 20% of Medicare beneficiaries were part of an ACO.4 These changes have led healthcare systems to invest in care management and postdischarge interventions, such as postdischarge phone calls, transitional clinics, and transition guides to reduce admissions and readmissions. Johns Hopkins adopted all these strategies to drive performance on the Maryland Total Cost of Care Model, which like an ACO holds hospitals accountable for both inpatient and outpatient costs incurred by Medicare FFS beneficiaries. A consistent theme among successful ACOs has been a reduction in inpatient spending.5
The authors are likely undercounting the volume of admissions by Medicare beneficiaries. First, to define an episode, they leverage the Medicare definition of bundles and include traditional Medicare inpatient, outpatient, and Part D services 30 days prior to hospitalizations and up to 90 days after. Admissions for the same diagnosis related group that occur in the 90 days after the anchor hospitalization are included in the same episode. From a clinical perspective, it is not intuitively clear why an admission for heart failure or pneumonia that occurs 3 months after an anchor hospitalization would not be defined as a separate and distinct admission rather than a readmission. Second, their analysis focuses on Medicare FFS and does not include Medicare Advantage, which now accounts for 42% of total Medicare beneficiaries. In fact, Medicare Advantage experienced significant growth in enrollment during the study period, increasing from 10 million to 24 million beneficiaries.6
Despite the reduction in inpatient volumes, the authors find that inpatient spending has increased. Spending per episode increased by 11.4% over this period, when adjusted for Medicare payment increases. Actual spending per episode unadjusted for payment increases rose by 25%. Thus, they astutely point out that most of the increase has been driven by Medicare payment increases. It is likely that increases in the complexity of patients and more dedicated focus on appropriate coding have also contributed. The authors, however, do not provide information on changes to the total cost of care outside of their defined inpatient episodes, a relevant measure to those participating in value-based models.
It is likely that the trend toward fewer inpatient admissions and increased outpatient management of medical conditions will continue as value-based care models grow. Studies like these are important in documenting this trend, but it will be important in future studies to understand how these changes have impacted the quality of care delivered to patients. Prior studies have found that reductions in readmissions through the Hospital Readmission Reduction Program were associated with increases in mortality as a potential unintended consequence.7
1. The Medicare Payment Advisory Commission. A Data Book: Health Care Spending and the Medicare Program. June 2018. Accessed October 25, 2021. http://medpac.gov/docs/default-source/data-book/jun19_databook_entirereport_sec.pdf
2. Keohane LM, Kripalani S, Buntin MB, et al. Traditional Medicare spending on inpatient episodes as hospitalizations decline. J Hosp Med. 2021;16(11):652-658. https://doi.org/10.12788/jhm.3699
3. Bradley SM, Kaltenbach LA, Xiang K, et al. Trends in use and outcomes of same-day discharge following elective percutaneous coronary intervention. JACC Cardiovasc Interv. 2021;14(15):1655-1666. https://doi.org/10.1016/j.jcin.2021.05.043
4. National Association of ACOs. NAACOs overview of the 2018 Medicare ACO class. Accessed October 25, 2021. https://www.naacos.com/overview-of-the-2018-medicare-aco-class
5. McWilliams JM, Hatfield LA, Landon BE, Hamed P, Chernew ME. Medicare spending after 3 years of the Medicare shared savings program. N Engl J Med. 2018;379(12):1139-1149. https://doi.org/10.1056/NEJMsa1803388
6. Freed M, Fuglesten Biniek J, Damico A, Neuman T. Medicare Advantage in 2021: Enrollment update and key trends. KFF. June 21, 2021. Accessed October 25, 2021. https://www.kff.org/medicare/issue-brief/medicare-advantage-in-2021-enrollment-update-and-key-trends/
7. Gupta A, Allen LA, Bhatt DL, et al. Association of the hospital readmissions reduction program implementation with readmission and mortality outcomes in heart failure. JAMA Cardiol. 2018;3(1):44-53. https://doi.org/10.1001/jamacardio.2017.4265
The advent of COVID-19 saw a precipitous decline in inpatient admissions. Even before the COVID-19 pandemic, hospitals were seeing a trend toward fewer inpatient admissions for Medicare beneficiaries, which has not been thoroughly examined or explained.1 In this issue, Keohane et al2 studied Medicare inpatient episode trends between 2009 and 2017 and found that, during this period, inpatient episodes per 1000 Medicare fee-for-service (FFS) beneficiaries declined by 18.2%, from 326 to 267 per 1000 beneficiaries.
This trend can be partly explained by changes in the way that care is delivered. First, observation stays have risen, and these are excluded in the authors’ analysis. From 2010 to 2017, observation visits per 1000 beneficiaries increased from 28 to 51.1 Second, due to improved outpatient management, margin constraints, and efficiency gains, hospitals are less likely to admit patients with less complex problems or keep patients overnight for uncomplicated procedural interventions. In cardiology, there has been an increase in the proportion of same-day percutaneous coronary interventions, from 4.5% in 2009 to 28.6% in 2017.3 The authors do not include a quantitative measure of complexity, but their data support this conclusion as they find larger declines in episodes that began with a planned admission and those that involved no use of post–acute care services, and thus were likely less complicated admissions. Finally, the increased use of alternative care sites such as home-based care settings and urgent care clinics, the proliferation of telemedicine, and the continual development of guideline-based therapy have resulted in better outpatient management of diseases.
The growth of value-based care has also contributed to the reduction in inpatient admission. The past decade has seen the growth of bundled-payment contracts, accountable care organizations (ACO), and advanced primary care models. In 2018, an estimated 20% of Medicare beneficiaries were part of an ACO.4 These changes have led healthcare systems to invest in care management and postdischarge interventions, such as postdischarge phone calls, transitional clinics, and transition guides to reduce admissions and readmissions. Johns Hopkins adopted all these strategies to drive performance on the Maryland Total Cost of Care Model, which like an ACO holds hospitals accountable for both inpatient and outpatient costs incurred by Medicare FFS beneficiaries. A consistent theme among successful ACOs has been a reduction in inpatient spending.5
The authors are likely undercounting the volume of admissions by Medicare beneficiaries. First, to define an episode, they leverage the Medicare definition of bundles and include traditional Medicare inpatient, outpatient, and Part D services 30 days prior to hospitalizations and up to 90 days after. Admissions for the same diagnosis related group that occur in the 90 days after the anchor hospitalization are included in the same episode. From a clinical perspective, it is not intuitively clear why an admission for heart failure or pneumonia that occurs 3 months after an anchor hospitalization would not be defined as a separate and distinct admission rather than a readmission. Second, their analysis focuses on Medicare FFS and does not include Medicare Advantage, which now accounts for 42% of total Medicare beneficiaries. In fact, Medicare Advantage experienced significant growth in enrollment during the study period, increasing from 10 million to 24 million beneficiaries.6
Despite the reduction in inpatient volumes, the authors find that inpatient spending has increased. Spending per episode increased by 11.4% over this period, when adjusted for Medicare payment increases. Actual spending per episode unadjusted for payment increases rose by 25%. Thus, they astutely point out that most of the increase has been driven by Medicare payment increases. It is likely that increases in the complexity of patients and more dedicated focus on appropriate coding have also contributed. The authors, however, do not provide information on changes to the total cost of care outside of their defined inpatient episodes, a relevant measure to those participating in value-based models.
It is likely that the trend toward fewer inpatient admissions and increased outpatient management of medical conditions will continue as value-based care models grow. Studies like these are important in documenting this trend, but it will be important in future studies to understand how these changes have impacted the quality of care delivered to patients. Prior studies have found that reductions in readmissions through the Hospital Readmission Reduction Program were associated with increases in mortality as a potential unintended consequence.7
The advent of COVID-19 saw a precipitous decline in inpatient admissions. Even before the COVID-19 pandemic, hospitals were seeing a trend toward fewer inpatient admissions for Medicare beneficiaries, which has not been thoroughly examined or explained.1 In this issue, Keohane et al2 studied Medicare inpatient episode trends between 2009 and 2017 and found that, during this period, inpatient episodes per 1000 Medicare fee-for-service (FFS) beneficiaries declined by 18.2%, from 326 to 267 per 1000 beneficiaries.
This trend can be partly explained by changes in the way that care is delivered. First, observation stays have risen, and these are excluded in the authors’ analysis. From 2010 to 2017, observation visits per 1000 beneficiaries increased from 28 to 51.1 Second, due to improved outpatient management, margin constraints, and efficiency gains, hospitals are less likely to admit patients with less complex problems or keep patients overnight for uncomplicated procedural interventions. In cardiology, there has been an increase in the proportion of same-day percutaneous coronary interventions, from 4.5% in 2009 to 28.6% in 2017.3 The authors do not include a quantitative measure of complexity, but their data support this conclusion as they find larger declines in episodes that began with a planned admission and those that involved no use of post–acute care services, and thus were likely less complicated admissions. Finally, the increased use of alternative care sites such as home-based care settings and urgent care clinics, the proliferation of telemedicine, and the continual development of guideline-based therapy have resulted in better outpatient management of diseases.
The growth of value-based care has also contributed to the reduction in inpatient admission. The past decade has seen the growth of bundled-payment contracts, accountable care organizations (ACO), and advanced primary care models. In 2018, an estimated 20% of Medicare beneficiaries were part of an ACO.4 These changes have led healthcare systems to invest in care management and postdischarge interventions, such as postdischarge phone calls, transitional clinics, and transition guides to reduce admissions and readmissions. Johns Hopkins adopted all these strategies to drive performance on the Maryland Total Cost of Care Model, which like an ACO holds hospitals accountable for both inpatient and outpatient costs incurred by Medicare FFS beneficiaries. A consistent theme among successful ACOs has been a reduction in inpatient spending.5
The authors are likely undercounting the volume of admissions by Medicare beneficiaries. First, to define an episode, they leverage the Medicare definition of bundles and include traditional Medicare inpatient, outpatient, and Part D services 30 days prior to hospitalizations and up to 90 days after. Admissions for the same diagnosis related group that occur in the 90 days after the anchor hospitalization are included in the same episode. From a clinical perspective, it is not intuitively clear why an admission for heart failure or pneumonia that occurs 3 months after an anchor hospitalization would not be defined as a separate and distinct admission rather than a readmission. Second, their analysis focuses on Medicare FFS and does not include Medicare Advantage, which now accounts for 42% of total Medicare beneficiaries. In fact, Medicare Advantage experienced significant growth in enrollment during the study period, increasing from 10 million to 24 million beneficiaries.6
Despite the reduction in inpatient volumes, the authors find that inpatient spending has increased. Spending per episode increased by 11.4% over this period, when adjusted for Medicare payment increases. Actual spending per episode unadjusted for payment increases rose by 25%. Thus, they astutely point out that most of the increase has been driven by Medicare payment increases. It is likely that increases in the complexity of patients and more dedicated focus on appropriate coding have also contributed. The authors, however, do not provide information on changes to the total cost of care outside of their defined inpatient episodes, a relevant measure to those participating in value-based models.
It is likely that the trend toward fewer inpatient admissions and increased outpatient management of medical conditions will continue as value-based care models grow. Studies like these are important in documenting this trend, but it will be important in future studies to understand how these changes have impacted the quality of care delivered to patients. Prior studies have found that reductions in readmissions through the Hospital Readmission Reduction Program were associated with increases in mortality as a potential unintended consequence.7
1. The Medicare Payment Advisory Commission. A Data Book: Health Care Spending and the Medicare Program. June 2018. Accessed October 25, 2021. http://medpac.gov/docs/default-source/data-book/jun19_databook_entirereport_sec.pdf
2. Keohane LM, Kripalani S, Buntin MB, et al. Traditional Medicare spending on inpatient episodes as hospitalizations decline. J Hosp Med. 2021;16(11):652-658. https://doi.org/10.12788/jhm.3699
3. Bradley SM, Kaltenbach LA, Xiang K, et al. Trends in use and outcomes of same-day discharge following elective percutaneous coronary intervention. JACC Cardiovasc Interv. 2021;14(15):1655-1666. https://doi.org/10.1016/j.jcin.2021.05.043
4. National Association of ACOs. NAACOs overview of the 2018 Medicare ACO class. Accessed October 25, 2021. https://www.naacos.com/overview-of-the-2018-medicare-aco-class
5. McWilliams JM, Hatfield LA, Landon BE, Hamed P, Chernew ME. Medicare spending after 3 years of the Medicare shared savings program. N Engl J Med. 2018;379(12):1139-1149. https://doi.org/10.1056/NEJMsa1803388
6. Freed M, Fuglesten Biniek J, Damico A, Neuman T. Medicare Advantage in 2021: Enrollment update and key trends. KFF. June 21, 2021. Accessed October 25, 2021. https://www.kff.org/medicare/issue-brief/medicare-advantage-in-2021-enrollment-update-and-key-trends/
7. Gupta A, Allen LA, Bhatt DL, et al. Association of the hospital readmissions reduction program implementation with readmission and mortality outcomes in heart failure. JAMA Cardiol. 2018;3(1):44-53. https://doi.org/10.1001/jamacardio.2017.4265
1. The Medicare Payment Advisory Commission. A Data Book: Health Care Spending and the Medicare Program. June 2018. Accessed October 25, 2021. http://medpac.gov/docs/default-source/data-book/jun19_databook_entirereport_sec.pdf
2. Keohane LM, Kripalani S, Buntin MB, et al. Traditional Medicare spending on inpatient episodes as hospitalizations decline. J Hosp Med. 2021;16(11):652-658. https://doi.org/10.12788/jhm.3699
3. Bradley SM, Kaltenbach LA, Xiang K, et al. Trends in use and outcomes of same-day discharge following elective percutaneous coronary intervention. JACC Cardiovasc Interv. 2021;14(15):1655-1666. https://doi.org/10.1016/j.jcin.2021.05.043
4. National Association of ACOs. NAACOs overview of the 2018 Medicare ACO class. Accessed October 25, 2021. https://www.naacos.com/overview-of-the-2018-medicare-aco-class
5. McWilliams JM, Hatfield LA, Landon BE, Hamed P, Chernew ME. Medicare spending after 3 years of the Medicare shared savings program. N Engl J Med. 2018;379(12):1139-1149. https://doi.org/10.1056/NEJMsa1803388
6. Freed M, Fuglesten Biniek J, Damico A, Neuman T. Medicare Advantage in 2021: Enrollment update and key trends. KFF. June 21, 2021. Accessed October 25, 2021. https://www.kff.org/medicare/issue-brief/medicare-advantage-in-2021-enrollment-update-and-key-trends/
7. Gupta A, Allen LA, Bhatt DL, et al. Association of the hospital readmissions reduction program implementation with readmission and mortality outcomes in heart failure. JAMA Cardiol. 2018;3(1):44-53. https://doi.org/10.1001/jamacardio.2017.4265
© 2021 Society of Hospital Medicine
Problematic Trends in Observation Status for Children’s Hospitals
Two children who presented to emergency departments in different cities were diagnosed with diabetes mellitus and ketoacidosis. On presentation, both had significant anion gap metabolic acidosis. Because the patients were deemed unsafe for discharge, the admitting physician placed orders that dictated hospital care, including an order that designated the stay as observation (OBS) or inpatient (IP) status. During their stay, both patients received care including continuous infusion of insulin, intravenous fluids, and frequent lab monitoring. Each child recovered quickly and was discharged in less than 48 hours.
Despite both patients receiving comparable care, recovering well, and being discharged home after a similar length of stay, their encounter designation may be different. Although the patient outcome is of utmost importance, the consequences of labeling an encounter as OBS or IP status are complex and may impact the financial standing of patients, hospitals, and payors. Determining the trajectory of OBS status and its utilization in the pediatric population is vital to understanding the consequences of this designation.
In this issue of the Journal of Hospital Medicine, Tian et al1 describe the increase in OBS status hospitalizations between 2010 and 2019, with OBS stays accounting for approximately one-third of pediatric hospitalizations within children’s hospitals in 2019. The increase in OBS status use was described in 19 of 20 of the most common All Patient Refined Diagnosis Related Groups, with the highest growth noted for surgical conditions and diabetes mellitus.1 These frequently seen, high-stakes conditions, when expertly managed, may result in a safe discharge within 48 hours of admission, but the labor-intensive technical skills to ensure patient safety and high-value care often differ greatly from the idea of simply “observing” a patient.
The scope creep of OBS status in pediatrics is evident. OBS status was initially designed to acknowledge a prolonged outpatient period of monitoring with a goal of determining whether inpatient hospitalization was warranted. However, in most circumstances, the care of children under OBS status differs little from those under IP status; OBS status patients are usually cared for in the same wards and by the same providers as IP status patients. The similarities in care lead to nearly equivalent hospital costs for IP and OBS stays.2 Comparable hospital costs would be less concerning if reimbursement were proportional, but OBS status hospitalizations are reimbursed at lower outpatient rates.3 The combination of similar costs and lower reimbursement results in a financial liability for children’s hospitals.
Tian et al add to the growing body of literature that underscores concerns with OBS stays.1 Its increasing use over the past decade represents a troubling continuation of increased OBS status use described by Macy et al3 nearly a decade ago, and the variability with which it is applied suggests that the designation has little connection to the clinical status of patients. Instead, its use is more likely influenced by local payor contracts, individual state laws, and provider culture. For individual institutions, this differential application affects more than just reimbursement. OBS stays are often excluded from nationally representative administrative databases, which makes hospital benchmarking, research on outcomes, and accurate comparison of patient populations impossible.4,5
The trends described by Tian et al1 raise concerns about the potential impact that OBS stays have on patients and hospital systems across the country. OBS status was created to serve a clinical need, but its inconsistent use places hospitals and the children they treat at risk. This erratic application of OBS status and the serious results of its assignment to pediatric hospitalizations provide evidence that criteria for OBS need to be standardized or otherwise abandoned outright.
1. Tian Y, Hall M, Ingram M-CE, Hu A, Raval MV. Trends and variation in the use of observation stays at children’s hospitals. J Hosp Med. 2021;16(11):645-651. https://doi.org/10.12788/jhm.3622
2. Fieldston ES, Shah SS, Hall M, et al. Resource utilization for observation-status stays at children’s hospitals. Pediatrics. 2013;131(6):1050-1058. https://doi.org/10.1542/peds.2012-2494
3. Macy ML, Hall M, Shah SS, et al. Pediatric observation status: are we overlooking a growing population in children’s hospitals? J Hosp Med. 2012;7(7):530-536. https://doi.org/10.1002/jhm.1923
4. Synhorst DC, Hall M, Harris M, et al. Hospital observation status and readmission rates. Pediatrics. 2020;146(5):e2020003954. https://doi.org/10.1542/peds.2020-003954
5. Gay JC, Hall M, Morse R, Fieldston ES, Synhorst D, Macy ML. Observation encounters and length of stay benchmarking in children’s hospitals. Pediatrics. 2020;146(5):e20200120. https://doi.org/10.1542/peds.2020-0120
Two children who presented to emergency departments in different cities were diagnosed with diabetes mellitus and ketoacidosis. On presentation, both had significant anion gap metabolic acidosis. Because the patients were deemed unsafe for discharge, the admitting physician placed orders that dictated hospital care, including an order that designated the stay as observation (OBS) or inpatient (IP) status. During their stay, both patients received care including continuous infusion of insulin, intravenous fluids, and frequent lab monitoring. Each child recovered quickly and was discharged in less than 48 hours.
Despite both patients receiving comparable care, recovering well, and being discharged home after a similar length of stay, their encounter designation may be different. Although the patient outcome is of utmost importance, the consequences of labeling an encounter as OBS or IP status are complex and may impact the financial standing of patients, hospitals, and payors. Determining the trajectory of OBS status and its utilization in the pediatric population is vital to understanding the consequences of this designation.
In this issue of the Journal of Hospital Medicine, Tian et al1 describe the increase in OBS status hospitalizations between 2010 and 2019, with OBS stays accounting for approximately one-third of pediatric hospitalizations within children’s hospitals in 2019. The increase in OBS status use was described in 19 of 20 of the most common All Patient Refined Diagnosis Related Groups, with the highest growth noted for surgical conditions and diabetes mellitus.1 These frequently seen, high-stakes conditions, when expertly managed, may result in a safe discharge within 48 hours of admission, but the labor-intensive technical skills to ensure patient safety and high-value care often differ greatly from the idea of simply “observing” a patient.
The scope creep of OBS status in pediatrics is evident. OBS status was initially designed to acknowledge a prolonged outpatient period of monitoring with a goal of determining whether inpatient hospitalization was warranted. However, in most circumstances, the care of children under OBS status differs little from those under IP status; OBS status patients are usually cared for in the same wards and by the same providers as IP status patients. The similarities in care lead to nearly equivalent hospital costs for IP and OBS stays.2 Comparable hospital costs would be less concerning if reimbursement were proportional, but OBS status hospitalizations are reimbursed at lower outpatient rates.3 The combination of similar costs and lower reimbursement results in a financial liability for children’s hospitals.
Tian et al add to the growing body of literature that underscores concerns with OBS stays.1 Its increasing use over the past decade represents a troubling continuation of increased OBS status use described by Macy et al3 nearly a decade ago, and the variability with which it is applied suggests that the designation has little connection to the clinical status of patients. Instead, its use is more likely influenced by local payor contracts, individual state laws, and provider culture. For individual institutions, this differential application affects more than just reimbursement. OBS stays are often excluded from nationally representative administrative databases, which makes hospital benchmarking, research on outcomes, and accurate comparison of patient populations impossible.4,5
The trends described by Tian et al1 raise concerns about the potential impact that OBS stays have on patients and hospital systems across the country. OBS status was created to serve a clinical need, but its inconsistent use places hospitals and the children they treat at risk. This erratic application of OBS status and the serious results of its assignment to pediatric hospitalizations provide evidence that criteria for OBS need to be standardized or otherwise abandoned outright.
Two children who presented to emergency departments in different cities were diagnosed with diabetes mellitus and ketoacidosis. On presentation, both had significant anion gap metabolic acidosis. Because the patients were deemed unsafe for discharge, the admitting physician placed orders that dictated hospital care, including an order that designated the stay as observation (OBS) or inpatient (IP) status. During their stay, both patients received care including continuous infusion of insulin, intravenous fluids, and frequent lab monitoring. Each child recovered quickly and was discharged in less than 48 hours.
Despite both patients receiving comparable care, recovering well, and being discharged home after a similar length of stay, their encounter designation may be different. Although the patient outcome is of utmost importance, the consequences of labeling an encounter as OBS or IP status are complex and may impact the financial standing of patients, hospitals, and payors. Determining the trajectory of OBS status and its utilization in the pediatric population is vital to understanding the consequences of this designation.
In this issue of the Journal of Hospital Medicine, Tian et al1 describe the increase in OBS status hospitalizations between 2010 and 2019, with OBS stays accounting for approximately one-third of pediatric hospitalizations within children’s hospitals in 2019. The increase in OBS status use was described in 19 of 20 of the most common All Patient Refined Diagnosis Related Groups, with the highest growth noted for surgical conditions and diabetes mellitus.1 These frequently seen, high-stakes conditions, when expertly managed, may result in a safe discharge within 48 hours of admission, but the labor-intensive technical skills to ensure patient safety and high-value care often differ greatly from the idea of simply “observing” a patient.
The scope creep of OBS status in pediatrics is evident. OBS status was initially designed to acknowledge a prolonged outpatient period of monitoring with a goal of determining whether inpatient hospitalization was warranted. However, in most circumstances, the care of children under OBS status differs little from those under IP status; OBS status patients are usually cared for in the same wards and by the same providers as IP status patients. The similarities in care lead to nearly equivalent hospital costs for IP and OBS stays.2 Comparable hospital costs would be less concerning if reimbursement were proportional, but OBS status hospitalizations are reimbursed at lower outpatient rates.3 The combination of similar costs and lower reimbursement results in a financial liability for children’s hospitals.
Tian et al add to the growing body of literature that underscores concerns with OBS stays.1 Its increasing use over the past decade represents a troubling continuation of increased OBS status use described by Macy et al3 nearly a decade ago, and the variability with which it is applied suggests that the designation has little connection to the clinical status of patients. Instead, its use is more likely influenced by local payor contracts, individual state laws, and provider culture. For individual institutions, this differential application affects more than just reimbursement. OBS stays are often excluded from nationally representative administrative databases, which makes hospital benchmarking, research on outcomes, and accurate comparison of patient populations impossible.4,5
The trends described by Tian et al1 raise concerns about the potential impact that OBS stays have on patients and hospital systems across the country. OBS status was created to serve a clinical need, but its inconsistent use places hospitals and the children they treat at risk. This erratic application of OBS status and the serious results of its assignment to pediatric hospitalizations provide evidence that criteria for OBS need to be standardized or otherwise abandoned outright.
1. Tian Y, Hall M, Ingram M-CE, Hu A, Raval MV. Trends and variation in the use of observation stays at children’s hospitals. J Hosp Med. 2021;16(11):645-651. https://doi.org/10.12788/jhm.3622
2. Fieldston ES, Shah SS, Hall M, et al. Resource utilization for observation-status stays at children’s hospitals. Pediatrics. 2013;131(6):1050-1058. https://doi.org/10.1542/peds.2012-2494
3. Macy ML, Hall M, Shah SS, et al. Pediatric observation status: are we overlooking a growing population in children’s hospitals? J Hosp Med. 2012;7(7):530-536. https://doi.org/10.1002/jhm.1923
4. Synhorst DC, Hall M, Harris M, et al. Hospital observation status and readmission rates. Pediatrics. 2020;146(5):e2020003954. https://doi.org/10.1542/peds.2020-003954
5. Gay JC, Hall M, Morse R, Fieldston ES, Synhorst D, Macy ML. Observation encounters and length of stay benchmarking in children’s hospitals. Pediatrics. 2020;146(5):e20200120. https://doi.org/10.1542/peds.2020-0120
1. Tian Y, Hall M, Ingram M-CE, Hu A, Raval MV. Trends and variation in the use of observation stays at children’s hospitals. J Hosp Med. 2021;16(11):645-651. https://doi.org/10.12788/jhm.3622
2. Fieldston ES, Shah SS, Hall M, et al. Resource utilization for observation-status stays at children’s hospitals. Pediatrics. 2013;131(6):1050-1058. https://doi.org/10.1542/peds.2012-2494
3. Macy ML, Hall M, Shah SS, et al. Pediatric observation status: are we overlooking a growing population in children’s hospitals? J Hosp Med. 2012;7(7):530-536. https://doi.org/10.1002/jhm.1923
4. Synhorst DC, Hall M, Harris M, et al. Hospital observation status and readmission rates. Pediatrics. 2020;146(5):e2020003954. https://doi.org/10.1542/peds.2020-003954
5. Gay JC, Hall M, Morse R, Fieldston ES, Synhorst D, Macy ML. Observation encounters and length of stay benchmarking in children’s hospitals. Pediatrics. 2020;146(5):e20200120. https://doi.org/10.1542/peds.2020-0120
© 2021 Society of Hospital Medicine
Leadership & Professional Development: Everyone Resists Change
Nothing changes without personal transformation.
—W Edwards Deming, 1986
Failure is common among quality improvement projects, but also predictable. Health professionals have multiple competing priorities. Improvement projects rarely reduce an individual’s workload. In our experience coaching health professionals, we have found that improvement teams often overlook two important facts: improvement requires behavior change, and everyone resists change.
Quality improvement education focuses on the development of technical skills (eg, process mapping, measure development, data analysis). Technical skills are necessary, but insufficient, to lead change. Process maps and run charts guide improvement work but alone do not motivate frontline staff to change workflows. Rather, soft skills (eg, communication, negotiation, change management, influencing others) convince frontline staff and hospital leaders that change is worth their time and effort.1,2 Successful improvement teams combine technical skills and soft skills to inspire behavior change.
We propose three practical skills that all improvement teams can adopt to inspire change:
Understand your stakeholders’ needs. Early identification and engagement of stakeholders (individuals or groups who may affect or be affected by the project) is critical. Improvement teams must consider stakeholders at multiple levels in the organization, from frontline staff to executives. The easiest way to understand stakeholders is by talking to them. Often, stakeholders lack time for scheduled meetings, so teams must rely on informal conversations in hallways and elevators. The key is to understand what will motivate the stakeholder to change. Put yourself in the stakeholders’ shoes: What are their needs and priorities? How might their needs and priorities motivate them to change? What potential barriers exist that prevent the stakeholder from making a change?
Tailor your message to establish a rationale for change. Build upon what was learned from stakeholders and decide how the rationale for change will be communicated. What can you say that will influence others to see the problem as important? Recognize that the rationale is different for different stakeholders; a financial rationale may inspire hospital leaders but alienate staff who are driven by patient and staff satisfaction. Even carefully crafted messages may not resonate with stakeholders as intended. Improvement teams must monitor the impact of their message with different stakeholders. Developing a clear, concise, and compelling rationale for change is often challenging and iterative. Multiple communication channels (ie, email, newsletters, formal and informal conversations) must be employed to spread your message.
Share small and large wins. Talking with stakeholders is not a one-time event. Stakeholder interest may decrease over time. Frontline staff can become complacent, falling back into old behaviors. Priorities of hospital leadership can shift. Successful teams maintain lines of communication throughout the project to share successes and sustain stakeholder buy-in. Small and large wins matter. Project outcomes (large wins) may take months to achieve. Teams can maintain stakeholder interest by demonstrating that project processes are feasible and acceptable (small wins). Maintaining regular communication also affords teams the opportunity for early identification of organizational barriers and facilitators that may impact their project. Ongoing communication of project wins sets the stage for sustainment by embedding the change within the local culture.
The goal of any improvement project is to create sustainable change. To do this, improvement teams often need hundreds of people to change the way they work. Change is hard, but improvement teams can overcome resistance to it by strategically engaging stakeholders and thoughtfully communicating the rationale for change.
1. Myers JS, Lane-Fall MB, Perfetti AR, et al. Demonstrating the value of postgraduate fellowships for physicians in quality improvement and patient safety. BMJ Qual Saf. 2020;29(8):645-654. https://doi.org/10.1136/bmjqs-2019-010204
2. Rajashekara S, Naik AD, Campbell CM, et al. Using a logic model to design and evaluate a quality improvement leadership course. Acad Med. 2020;95(8):1201-1206. https://doi.org/10.1097/ACM.0000000000003191
Nothing changes without personal transformation.
—W Edwards Deming, 1986
Failure is common among quality improvement projects, but also predictable. Health professionals have multiple competing priorities. Improvement projects rarely reduce an individual’s workload. In our experience coaching health professionals, we have found that improvement teams often overlook two important facts: improvement requires behavior change, and everyone resists change.
Quality improvement education focuses on the development of technical skills (eg, process mapping, measure development, data analysis). Technical skills are necessary, but insufficient, to lead change. Process maps and run charts guide improvement work but alone do not motivate frontline staff to change workflows. Rather, soft skills (eg, communication, negotiation, change management, influencing others) convince frontline staff and hospital leaders that change is worth their time and effort.1,2 Successful improvement teams combine technical skills and soft skills to inspire behavior change.
We propose three practical skills that all improvement teams can adopt to inspire change:
Understand your stakeholders’ needs. Early identification and engagement of stakeholders (individuals or groups who may affect or be affected by the project) is critical. Improvement teams must consider stakeholders at multiple levels in the organization, from frontline staff to executives. The easiest way to understand stakeholders is by talking to them. Often, stakeholders lack time for scheduled meetings, so teams must rely on informal conversations in hallways and elevators. The key is to understand what will motivate the stakeholder to change. Put yourself in the stakeholders’ shoes: What are their needs and priorities? How might their needs and priorities motivate them to change? What potential barriers exist that prevent the stakeholder from making a change?
Tailor your message to establish a rationale for change. Build upon what was learned from stakeholders and decide how the rationale for change will be communicated. What can you say that will influence others to see the problem as important? Recognize that the rationale is different for different stakeholders; a financial rationale may inspire hospital leaders but alienate staff who are driven by patient and staff satisfaction. Even carefully crafted messages may not resonate with stakeholders as intended. Improvement teams must monitor the impact of their message with different stakeholders. Developing a clear, concise, and compelling rationale for change is often challenging and iterative. Multiple communication channels (ie, email, newsletters, formal and informal conversations) must be employed to spread your message.
Share small and large wins. Talking with stakeholders is not a one-time event. Stakeholder interest may decrease over time. Frontline staff can become complacent, falling back into old behaviors. Priorities of hospital leadership can shift. Successful teams maintain lines of communication throughout the project to share successes and sustain stakeholder buy-in. Small and large wins matter. Project outcomes (large wins) may take months to achieve. Teams can maintain stakeholder interest by demonstrating that project processes are feasible and acceptable (small wins). Maintaining regular communication also affords teams the opportunity for early identification of organizational barriers and facilitators that may impact their project. Ongoing communication of project wins sets the stage for sustainment by embedding the change within the local culture.
The goal of any improvement project is to create sustainable change. To do this, improvement teams often need hundreds of people to change the way they work. Change is hard, but improvement teams can overcome resistance to it by strategically engaging stakeholders and thoughtfully communicating the rationale for change.
Nothing changes without personal transformation.
—W Edwards Deming, 1986
Failure is common among quality improvement projects, but also predictable. Health professionals have multiple competing priorities. Improvement projects rarely reduce an individual’s workload. In our experience coaching health professionals, we have found that improvement teams often overlook two important facts: improvement requires behavior change, and everyone resists change.
Quality improvement education focuses on the development of technical skills (eg, process mapping, measure development, data analysis). Technical skills are necessary, but insufficient, to lead change. Process maps and run charts guide improvement work but alone do not motivate frontline staff to change workflows. Rather, soft skills (eg, communication, negotiation, change management, influencing others) convince frontline staff and hospital leaders that change is worth their time and effort.1,2 Successful improvement teams combine technical skills and soft skills to inspire behavior change.
We propose three practical skills that all improvement teams can adopt to inspire change:
Understand your stakeholders’ needs. Early identification and engagement of stakeholders (individuals or groups who may affect or be affected by the project) is critical. Improvement teams must consider stakeholders at multiple levels in the organization, from frontline staff to executives. The easiest way to understand stakeholders is by talking to them. Often, stakeholders lack time for scheduled meetings, so teams must rely on informal conversations in hallways and elevators. The key is to understand what will motivate the stakeholder to change. Put yourself in the stakeholders’ shoes: What are their needs and priorities? How might their needs and priorities motivate them to change? What potential barriers exist that prevent the stakeholder from making a change?
Tailor your message to establish a rationale for change. Build upon what was learned from stakeholders and decide how the rationale for change will be communicated. What can you say that will influence others to see the problem as important? Recognize that the rationale is different for different stakeholders; a financial rationale may inspire hospital leaders but alienate staff who are driven by patient and staff satisfaction. Even carefully crafted messages may not resonate with stakeholders as intended. Improvement teams must monitor the impact of their message with different stakeholders. Developing a clear, concise, and compelling rationale for change is often challenging and iterative. Multiple communication channels (ie, email, newsletters, formal and informal conversations) must be employed to spread your message.
Share small and large wins. Talking with stakeholders is not a one-time event. Stakeholder interest may decrease over time. Frontline staff can become complacent, falling back into old behaviors. Priorities of hospital leadership can shift. Successful teams maintain lines of communication throughout the project to share successes and sustain stakeholder buy-in. Small and large wins matter. Project outcomes (large wins) may take months to achieve. Teams can maintain stakeholder interest by demonstrating that project processes are feasible and acceptable (small wins). Maintaining regular communication also affords teams the opportunity for early identification of organizational barriers and facilitators that may impact their project. Ongoing communication of project wins sets the stage for sustainment by embedding the change within the local culture.
The goal of any improvement project is to create sustainable change. To do this, improvement teams often need hundreds of people to change the way they work. Change is hard, but improvement teams can overcome resistance to it by strategically engaging stakeholders and thoughtfully communicating the rationale for change.
1. Myers JS, Lane-Fall MB, Perfetti AR, et al. Demonstrating the value of postgraduate fellowships for physicians in quality improvement and patient safety. BMJ Qual Saf. 2020;29(8):645-654. https://doi.org/10.1136/bmjqs-2019-010204
2. Rajashekara S, Naik AD, Campbell CM, et al. Using a logic model to design and evaluate a quality improvement leadership course. Acad Med. 2020;95(8):1201-1206. https://doi.org/10.1097/ACM.0000000000003191
1. Myers JS, Lane-Fall MB, Perfetti AR, et al. Demonstrating the value of postgraduate fellowships for physicians in quality improvement and patient safety. BMJ Qual Saf. 2020;29(8):645-654. https://doi.org/10.1136/bmjqs-2019-010204
2. Rajashekara S, Naik AD, Campbell CM, et al. Using a logic model to design and evaluate a quality improvement leadership course. Acad Med. 2020;95(8):1201-1206. https://doi.org/10.1097/ACM.0000000000003191
© 2021 Society of Hospital Medicine
Association of Healthcare Access With Intensive Care Unit Utilization and Mortality in Patients of Hispanic Ethnicity Hospitalized With COVID-19
In the United States, health disparities in COVID-19 outcomes (including morbidity and mortality) based on race and ethnicity have been described in the scientific literature and mainstream media.1-7 According to the US Centers for Disease Control and Prevention (CDC), Hispanic people are 3.2 times more likely to be hospitalized with COVID-19 than non-Hispanic White people.8 Further, Hispanic people diagnosed with COVID-19 are 2.3 times more likely to die, adjusted for age, than non-Hispanic White people.9 As the epicenter of the COVID-19 pandemic shifted from the Northeast to the South, the CDC reported that, among people who died from COVID-19 in the United States from May to August 2020, the percentage of Hispanic people increased from 16.3% to 26.4%.10
Published studies on the effect of ethnicity on critical illness or mortality for hospitalized COVID-19 patients are limited and inconsistent. While some studies reported a higher mortality rate for Hispanic patients,11-15 others showed no difference.4,16,17 A recent meta-analysis found that intensive care unit (ICU) utilization and mortality were slightly higher among Hispanic COVID-19 inpatients, but this finding did not reach statistical significance.18 Past studies from different healthcare systems were limited by the small sample size of hospitalized Hispanic patients and the heterogeneity of patients. A comprehensive analysis from a large healthcare system with sufficient sample size is needed to understand the impact of ethnicity on clinical outcomes of hospitalized COVID-19 patients.
Texas Health Resources (THR) is a large integrated healthcare system serving the Dallas-Fort Worth-Arlington (DFW) metropolitan area. According to the 2019 US Census Bureau American Community Survey, Hispanic people comprise 18.4% of the population of this geographic area.19 Congruent with the CDC’s findings, Hispanic patients account for a disproportionate share (32.2%) of hospitalized COVID-19 patients at THR relative to the area’s demographic composition. Aware of the increased risk, we undertook an analysis of the clinical outcomes and the clinical, social, and demographic characteristics of Hispanic patients hospitalized at THR with COVID-19. Our primary goal was to investigate whether clinical outcomes differ by ethnicity among patients hospitalized with COVID-19 and, if so, whether inpatient care or preadmission factors contribute to this difference.
Methods
Study Setting and Overview
We collected data from the single electronic health record (EHR) used by 20 THR hospitals located across the DFW metropolitan area. THR is the largest faith-based, nonprofit health system in North Texas, operating 20 acute care hospitals. Including all access points, such as outpatient facilities and physician group practices, THR serves 7 million residents in 16 counties in North Texas, of whom 16.8% are Hispanic, 73.3% are non-Hispanic, and 9.9% are unclassified, congruent with demographics in the DFW area.
The institutional review boards at THR and UT Southwestern Medical Center approved the study under a waiver of informed consent (as a minimal-risk medical record review). After collection, all data were de-identified prior to statistical analysis.
Cohort, Outcomes, and Covariables
The study cohort included 6097 adult patients with laboratory-confirmed COVID-19 (age ≥18 years) who were admitted as inpatients from March 3 to November 5, 2020. The primary outcomes included ICU utilization and death during hospitalization. We described demographic characteristics using the following variables: age (18–49, 50–64, 65–79, ≥80 years), sex, self-reported ethnicity, and primary spoken language.
We defined a severe baseline condition as an elevated respiratory subscore parsed from the overall MSOFA (Modified Sequential Organ Failure Assessment),20 an elevated Epic Deterioration Index (EDI),21 or an elevated C-reactive protein level (CRP) at baseline (any elevated CRP). Baseline referred to the variable mean during the first available 12-hour window of measurement during the COVID-19 hospital admission, including variables obtained in the emergency department (ED). An elevated MSOFA referred to a score of 4, corresponding to an SpO2/FiO2 < 150. Elevated EDI referred to a baseline EDI > 45. An elevated CRP referred to a baseline CRP > 20 mg/dL.22
Variables reflecting access to healthcare included: THR EHR creation year (representing the first time patients accessed the THR health system), insurance payor type, and presence of a primary care provider (PCP). The federal government established the COVID-19 Claims Reimbursement to Health Care Providers and Facilities for Testing, Treatment, and Vaccine Administration for the Uninsured program. The insurance payor for patients covered by this program is designated as COVID-19 HRSA. Presence of a PCP reflects any documented PCP, regardless of affiliation with THR. We selected these access metrics opportunistically, as they were consistently documented in the EHR and readily available for analysis.
We used 12 variables to describe comorbidities or underlying conditions that, according to the CDC, increased patients’ risk of severe illness from COVID-1923: diagnoses of diabetes, hypertension, obesity, chronic obstructive pulmonary disease (COPD), asthma, smoking, other lung disease, heart failure, kidney disease without end-stage renal disease (ESRD), ESRD, liver disease, and cancer. We identified comorbidities by mining the structured diagnosis codes documented in the EHR prior to and during the COVID-19 admission. Sources for diagnoses included final billed diagnosis codes, working diagnosis codes, problem list, and reason for visit. The definition of diabetes included previously recorded diabetes or baseline hemoglobin A1c > 9%. We also recorded the presence of four major COVID-19 treatments: steroids, remdesivir, tocilizumab, and fresh frozen plasma (FFP) from convalescent patients.24-26 Each treatment variable was defined by receipt of one or more doses.
Statistical Analysis
To analyze patient outcomes based on ethnicity, we divided the study cohort into a Hispanic group and a non-Hispanic group based on self-reported ethnicity in the EHR. To study the potential impact of primary language among Hispanic patients, we divided them into English-speaking and non-English-speaking patients based on their self-reported primary language. As a result, we analyzed three groups of patients: (1) non-Hispanic, (2) Hispanic and English speaking, and (3) Hispanic and non-English speaking. We tested differences of a given categorical variable across the three groups using the chi-square test for each age subgroup (18–49, 50–64, 65–79, ≥80 years). The Cochran-Mantel-Haenszel test was used for the overall difference adjusted for age. To assess whether an observed disparity in treatment existed across the three groups, we tested the difference in the administration of four major therapeutics for COVID-19, including steroids, remdesivir, tocilizumab, and convalescent plasma. To determine whether any groups had elevated disease severity at hospital admission (baseline), we tested the difference in four disease-severity metrics across the ethnic-language groups: (1) elevated respiratory MSOFA score, (2) elevated EDI, (3) elevated CRP level, and (4) any of the three conditions.
To study the associations with ICU utilization and death, respectively, we performed a multivariable analysis using a generalized linear mixed model with binomial distribution and a logit link function. In each analysis model, the hospital of admission was included as a random-effect variable to account for the potential treatment variations among different hospitals, while other variables were regarded as fixed effects. In the first multivariable analysis (Model 1), all demographic variables, including age, sex, and ethnicity, and different types of comorbidities and underlying conditions, were included as fixed-effect variables in the initial model, and then backward stepwise variable selection was performed to establish the final model (Model 1). We performed the backward stepwise variable selection separately for the outcome of ICU use or mortality. Based on Akaike information criterion (AIC), during each iteration the fixed-effect variable that led to the largest decrease in the AIC value was removed, and the variable selection process was completed when the AIC value stopped decreasing. In Model 2, we added the disease-severity variable at baseline to the selected variable set derived from Model 1 to explore its effect on the associations between ethnicity and clinical outcomes. In Model 3, we added healthcare access–related variables, including first-time healthsystem access, payor type, and PCP availability to Model 2. We performed all statistical analyses using R, version 4.0.2 (R Foundation for Statistical Computing) in RStudio (version 1.3.1093).
Results
Distinct Demographic and Comorbidity Patterns for Three Ethnic-Language Groups
We identified 6097 adult patients (age ≥18 years) who had confirmed COVID-19 disease and were hospitalized between March 3 and November 5, 2020. Demographic characteristics and comorbidity for these patients are summarized in Table 1. Among these patients, 4139 (67.9%) were non-Hispanic and 1958 (32.1%) were Hispanic. Among the Hispanic patients, 1203 (61.4%) identified English as their primary language and 755 (38.6%) identified a non-English primary language. Age distribution was vastly different among the three ethnic-language groups (Table 1). Unlike the relatively balanced distribution across different age groups in the non-Hispanic group, more than half (55.8%) of the English-speaking Hispanic patients were in the youngest age group (18-49 years). A much lower fraction of Hispanic patients was among the oldest (≥80 years) age group (P < .001). Because COVID-19 clinical outcome is strongly associated with age,27 we used age-stratified analysis when comparing group-level differences in patient outcomes.

Sex distribution also was different among the three groups, with the non-English-speaking Hispanic group having more male patients (53.0%). Diabetes and obesity, which are associated with clinical outcomes of COVID-19 patients, were more prevalent in Hispanic patients (Table 1). Non-English-speaking Hispanic patients had the highest diabetes rate (48.7% with documented diabetes; 15.8% with baseline HbA1c > 9%; P < .001). English-speaking Hispanic patients presented with the highest obesity rate (62.8%; P < .001). Appendix Table 1 provides detailed age-group-specific comorbidity distributions among ethnic-language groups.
Patients of Hispanic Ethnicity Experienced a Higher Rate of ICU Utilization and Mortality
Of the 6097 patients overall, 1365 (22.4%) were admitted to the ICU and 543 (8.9%) died in hospital. For non-Hispanic patients (n = 4139), 883 (21.3%) were admitted to the ICU and 373 (9.0%) died in hospital. For English-speaking Hispanic patients (n = 1203), 241 (20.0%) were admitted to the ICU and 91 (7.6%) died in hospital. For non-English-speaking Hispanic patients (n = 755), 241 (31.9%) were admitted to the ICU and 79 (10.5%) died in hospital. Figure 1 summarizes the age-stratified comparison of ICU utilization and mortality across the three ethnic-language patient groups. In all age groups, non-English-speaking Hispanic patients experienced a significantly higher ICU utilization rate compared to non-Hispanic patients (age-adjusted OR, 1.75; 95% CI, 1.47-2.08; P < .001). English-speaking and non-English-speaking Hispanic patients had a significantly higher mortality rate compared to non-Hispanic patients (age-adjusted OR, 1.53; 95% CI, 1.19-1.98; P = .001 for English-speaking Hispanic patients; age-adjusted OR, 1.43; 95% CI,: 1.10-1.86; P = .01 for non-English-speaking Hispanic patients).
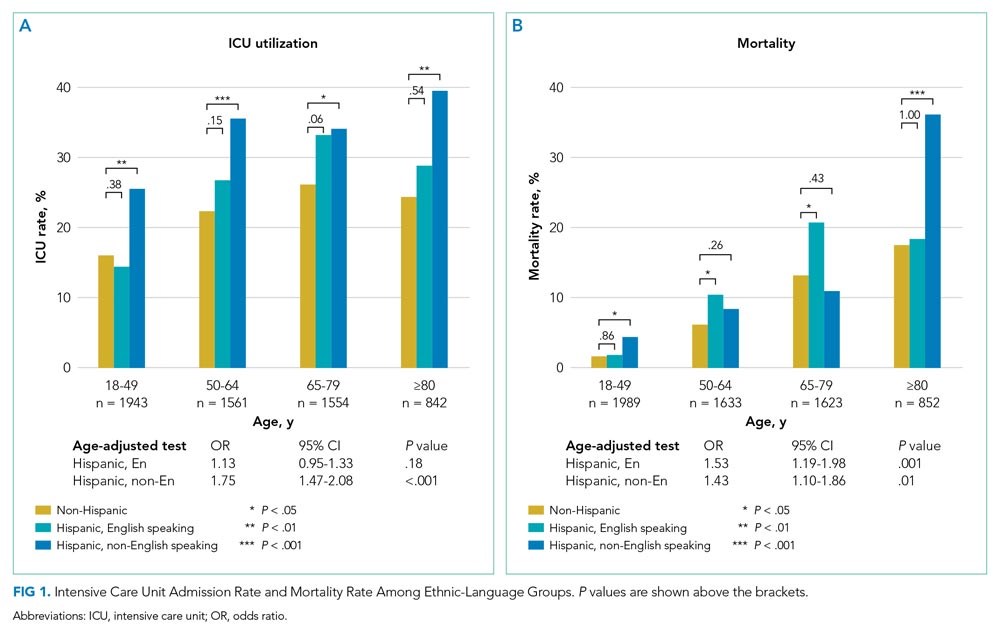
To delineate the risk factors associated with ICU utilization and death, we performed multivariable logistic regression with stepwise variable selection. After adjusting for age, sex, and comorbidity (Model 1), the factors ethnicity and primary language were still strongly associated with ICU utilization and mortality (Appendix Table 2). Non-English-speaking Hispanic patients had an OR of 1.74 (95% CI, 1.41-2.15; P < .001) for ICU utilization and an OR of 1.54 (95% CI, 1.12-2.12; P = .008) for mortality compared to non-Hispanic patients. Similarly, English-speaking Hispanic patients had higher ICU utilization (OR, 1.28; 95% CI, 1.05-1.55; P = .01) and a higher mortality rate (OR, 1.60; 95% CI, 1.19-2.14; P = .002).
No Disparity in COVID-19 Therapeutics Observed Across Three Ethnic-Language Groups
Appendix Figure 1 summarizes the comparison of the administration of four major treatments across the three ethnic-language groups. We did not observe any underuse of COVID-19 therapeutics for Hispanic patients. Usage rates for these therapies were significantly higher, after adjusting for age, in Hispanic groups when compared to non-Hispanic patients (OR ranged from 1.21 to 1.96). Steroids were the most common treatment in all patient groups. Tocilizumab was used almost twice as frequently (OR, 1.96; 95% CI, 1.64-2.33; P < .001) in non-English-speaking Hispanic patients compared to non-Hispanic patients.
Patients of Hispanic Ethnicity Had More Severe Disease at Hospital Admission
Figure 2 shows that non-English-speaking Hispanic patients had a higher rate of severe illness at admission based on each of these metrics: high respiratory MSOFA score (OR, 2.43; 95% CI, 1.77-3.33; P < .001), high EDI (OR, 1.85; 95% CI, 1.41-2.41; P < .001), and high CRP level (OR, 2.06; 95% CI, 1.64-2.58; P < .001). English-speaking Hispanic patients also had a greater rate of high CRP level (OR, 1.48; 95% CI, 1.17-1.86; P = .001) compared to non-Hispanic patients. When considering the presentation of any one of these clinical indicators, the English-speaking and non-English-speaking Hispanic patients had a higher rate of severe baseline condition (OR, 1.33; 95% CI, 1.10-1.61; P = .004 for English-speaking patients; OR, 2.27; 95% CI, 1.89-2.72; P < .001 for non-English-speaking patients).

We then studied how the baseline disease condition affects the association between ethnicity and clinical outcomes. We performed a multivariable analysis including baseline disease severity as a covariable (Model 2, Table 2), which showed that baseline disease severity was strongly associated with ICU admission (OR, 4.52; 95% CI, 3.83-5.33; P < .001) and mortality (OR, 3.32; 95% CI, 2.67-4.13; P < .001). The associations between ethnicity and clinical outcomes were reduced after considering the baseline disease condition. The OR dropped to 1.47 (95% CI, 1.18-1.84; P < .001) and 1.34 (95% CI, 0.97-1.87; P = .08) for ICU utilization and mortality, respectively, when comparing non-English-speaking Hispanic patients to non-Hispanic patients. A similar reduction was observed for English-speaking Hispanic patients. Model comparison showed a significant improvement of Model 2 over Model 1 based on ANOVA test (P < .001) as well as AIC.
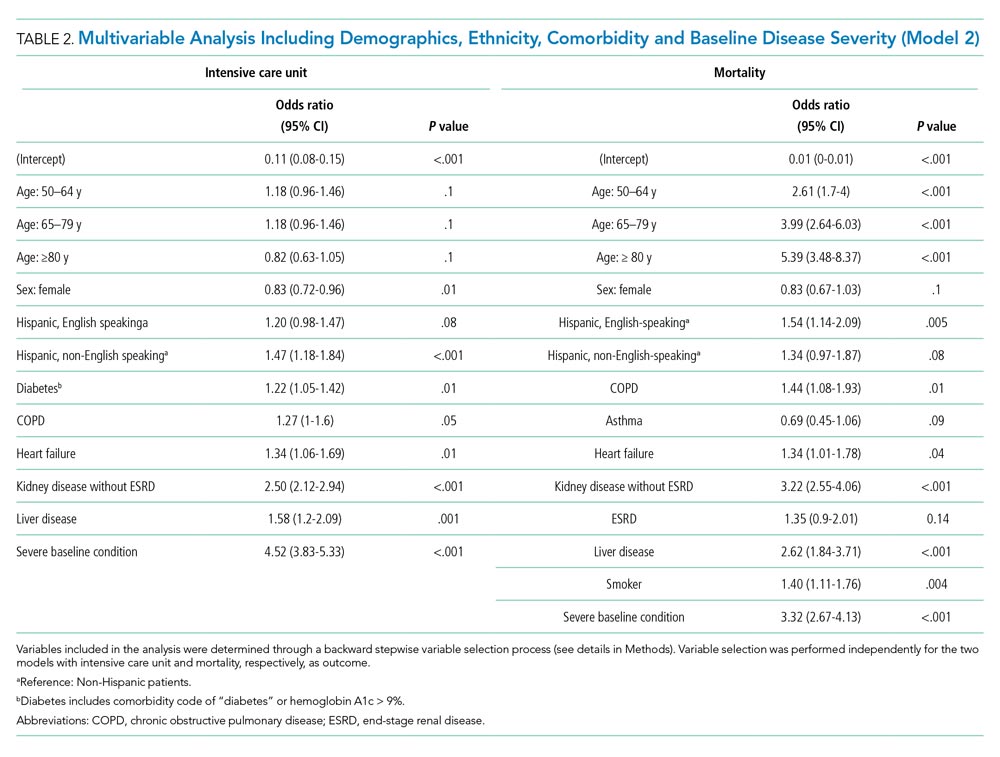
Hispanic Patients Had Worse Healthcare Access
To explore the etiology for the more severe disease conditions at hospital admission among Hispanic patients, we analyzed variables related to healthcare access. We found that Hispanic patients were likely to have reduced access to healthcare (Table 1; Appendix Figure 2). For a large proportion (16.9%) of the COVID-19 patients in this study, their medical records were first created at THR in 2020, corresponding to the initial time these patients accessed THR for their healthcare. This surge in 2020, compared to previous years with data (2005–2019), corresponds to the number of new patients seen because of COVID-19 (Appendix Figure 2A). Among this new patient population, the proportion of non-English-speaking Hispanic patients in 2020 was 28.3%, compared to 9.1% from 2005 to 2019 (P < .001). The proportion of new English-speaking Hispanic patients in 2020 was 22.1%, compared to an average of 19.2% from 2005 to 2019 (P < .001). In addition, a much smaller proportion of Hispanic patients had a PCP (P < .001) (Table 1; Appendix Figure 2B), with non-English-speaking Hispanic patients having the smallest proportion (58.5%).
Appendix Figure 2C illustrates the comparison of payor types across the three patient groups. A much higher proportion of Hispanic patients used COVID-19 HRSA (P < .001) compared to non-Hispanic patients. Breaking this down further by primary language, 29.1% of non-English-speaking Hispanic patients relied on COVID-19 HRSA due to otherwise uninsured status, compared to 12.7% of English-speaking Hispanic patients and only 5.1% of non-Hispanic patients. Similarly, non-English-speaking Hispanic patients have the highest self-pay rates (2.3%) compared to English-speaking Hispanic patients (1.4%) and non-Hispanic patients (0.7%). In summary, more Hispanic patients, and especially non-English-speaking Hispanic patients, lacked conventional health insurance and experienced limited access to healthcare.
Further evidence showed a trend of correlation between presentation of severe COVID-19 conditions when arriving at the hospital and each of the healthcare access factors analyzed (Appendix Figure 3).
Discussion
With a large sample size of hospitalized COVID-19 patients at an integrated health system in the DFW metropolitan area, we observed an increased rate of ICU utilization and mortality among Hispanic inpatients. After adjusting for age, we found that non-English-speaking Hispanic patients were 75% more likely to require critical care compared with non-Hispanic patients. English-speaking and non-English-speaking Hispanic patients had an increased mortality rate (age-adjusted) compared to non-Hispanic patients. The association between ethnicity and clinical outcomes remained significant after adjusting for age, sex, and comorbidities. We did not observe any underuse of major COVID-19 therapeutics in Hispanic patients, and excluded in-hospital treatments from the contributors to the outcome differences.
Hispanic patients, especially non-English-speaking Hispanic patients, had a higher rate of severe COVID-19 disease at the time of hospital admission (Figure 2). After including baseline disease severity into the multivariable analysis (Model 2), the overall model improved (P < .001) while the associations between ethnicity and outcomes decreased (Table 2). This suggests disease severity at admission was a main contributor to the observed associations between ethnicity and clinical outcomes. The higher rate of baseline COVID-19 severity in Hispanic patients might also explain their higher rate of receiving major COVID-19 therapeutics (Appendix Figure 1).
This study found that Hispanic patients were less likely to have a PCP and insurance coverage compared with non-Hispanic patients (P < .001). This disparity was more pronounced among non-English-speaking Hispanic patients (Appendix Figure 2). We also observed that a disproportionately larger proportion (50.4%) of patients who visited the healthcare system for the first time in 2020 (the year of the COVID-19 pandemic) was composed of Hispanic patients, compared to merely 28.4% prior to 2020. While there is a possibility that patients had primary care outside THR, the staggering number of Hispanic patients who were new to the health system in 2020, in conjunction with the fact that immigrants tend to be “healthier” compared to their native-born peers (the so-called immigrant paradox),28 led us to conclude that there were few other primary care options for these patients, making THR’s ED the primary care option of choice. The systemic, structural barriers to routine care might be a possible cause for delayed admission and, in turn, elevated baseline COVID-19 severity for Hispanic patients (Appendix Figure 3).
Recent studies have investigated the impact of socioeconomic factors on racial/ethnic disparities in the COVID-19 pandemic.7,16,17 To our knowledge, no study has directly analyzed the link between healthcare access metrics, COVID-19 severity at admission, and the Hispanic population stratified by primary language. Studies exist on this subject for other diseases, however. For example, healthcare access factors have been associated with sepsis-related mortality.29,30 In fact, a recent study that explored the potential effect of language barriers on healthcare access demonstrated an association between limited English proficiency and sepsis-related mortality.31 Our study found that Hispanic patients whose primary language is not English had the worst clinical outcomes, including more severe baseline COVID-19 conditions, and the least access to healthcare, highlighting the importance of addressing language barriers in COVID-19 care. Further research is needed to confirm the relationship between limited English proficiency and clinical outcomes, as well as potential factors that contribute to such a relationship in different types of diseases.
Our study has a number of limitations. First, it was limited to only one large healthcare system, which means the results may not be generalizable. Because THR is an open system, comorbidity data may be incomplete, and we cannot exclude the possibility that patients accessed care outside THR prior to or during the pandemic. We may overcome this limitation in the future with cross-system health information exchange data. Second, we did not have data for the time of symptom onset, so we were unable to analyze the direct evidence of the possible delayed care. As a result, we were unable to analyze whether treatments were administered in a timely manner or appropriately. Third, our analysis was not adjusted for other socioeconomic factors (eg, income, education) due to lack of data. We used self-identification for ethnicity, but unlike new approaches by the U.S. Census Bureau,32 our survey allowed only one choice to be selected.
Conclusion
Sociodemographic factors among Hispanic inpatients hospitalized for COVID-19 at a large integrated health system—including a primary non-English language, lack of a PCP, and insurance status—were associated with measures of reduced access to care and more severe illness at admission. Structural barriers to care, which may be associated with reduced health literacy and less access to health insurance, can result in delayed treatment and more severe illness at admission and underdiagnosis of medical conditions, contributing to worse outcomes in this population. Our findings suggest that interventions to promote early recognition of signs and symptoms of COVID-19 and to encourage prompt clinical care at the community level may reduce the burden of COVID-19 deaths in racial or ethnic minority communities with language and socioeconomic barriers.
1. Lopez L III, Hart LH III, Katz MH. Racial and ethnic health disparities related to COVID-19. JAMA. 2021;325(8):719-720. https://doi.org/10.1001/jama.2020.26443
2. Cooper LA, Williams DR. Excess deaths from COVID-19, community bereavement, and restorative justice for communities of color. JAMA. 2020;324(15):1491-1492. https://doi.org/10.1001/jama.2020.19567
3. Clay LA, Rogus S. Primary and secondary health impacts of COVID-19 among minority individuals in New York State. Int J Environ Res Public Health. 2021;18(2):683. https://doi.org/10.3390/ijerph18020683
4. Rodriguez F, Solomon N, de Lemos JA, et al. Racial and ethnic differences in presentation and outcomes for patients hospitalized with COVID-19: findings from the American Heart Association’s COVID-19 Cardiovascular Disease Registry. Circulation. 2021;143(24):2332-2342. https://doi.org/10.1161/CIRCULATIONAHA.120.052278
5. Moreira A, Chorath K, Rajasekaran K, Burmeister F, Ahmed M, Moreira A. Demographic predictors of hospitalization and mortality in US children with COVID-19. Eur J Pediatr. 2021;180(5):1659-1663. https://doi.org/10.1007/s00431-021-03955-x
6. Kolata G. Social inequities explain racial gaps in pandemic, studies find. The New York Times. December 9, 2020. https://www.nytimes.com/2020/12/09/health/coronavirus-black-hispanic.html
7. Liao TF, De Maio F. Association of social and economic inequality with coronavirus disease 2019 incidence and mortality across US counties. JAMA Netw Open. 2021;4(1):e2034578. https://doi.org/10.1001/jamanetworkopen.2020.34578
8. Centers for Disease Control and Prevention. A Weekly Surveillance Summary of U.S. COVID-19 Activity: Key Updates for Week 2. January 21, 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/pdf/covidview-01-22-2021.pdf
9. Centers for Disease Control and Prevention. Risk for COVID-19 infection, hospitalization, and death by race/ethnicity. Updated September 9, 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html
10. Gold JAW, Rossen LM, Ahmad FB, et al. Race, ethnicity, and age trends in persons who died from COVID-19 – United States, May-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(42):1517-1521. https://doi.org/10.15585/mmwr.mm6942e1
11. Pennington AF, Kompaniyets L, Summers AD, et al. Risk of clinical severity by age and race/ethnicity among adults hospitalized for COVID-19 – United States, March-September 2020. Open Forum Infect Dis. 2021;8(2):ofaa638. https://doi.org/10.1093/ofid/ofaa638.
12. Renelus BD, Khoury NC, Chandrasekaran K, et al. Racial disparities in COVID-19 hospitalization and in-hospital mortality at the height of the New York City pandemic. J Racial Ethn Health Disparities. 2021;8(5):1161-1167. https://doi.org/10.1007/s40615-020-00872-x
13. Wiley Z, Ross-Driscoll K, Wang Z, Smothers L, Mehta AK, Patzer RE. Racial and ethnic differences and clinical outcomes of COVID-19 patients presenting to the emergency department. Clin Infect Dis. 2021 Apr 2. [Epub ahead of print] https://doi.org/10.1093/cid/ciab290
14. Dai CL, Kornilov SA, Roper RT, et al. Characteristics and factors associated with COVID-19 infection, hospitalization, and mortality across race and ethnicity. Clin Infect Dis. 2021 Feb 20. [Epub ahead of print] https://doi.org/10.1093/cid/ciab154
15. Pan AP, Khan O, Meeks JR, et al. Disparities in COVID-19 hospitalizations and mortality among black and Hispanic patients: cross-sectional analysis from the greater Houston metropolitan area. BMC Public Health. 2021;21(1):1330. https://doi.org/10.1186/s12889-021-11431-2
16. Ogedegbe G, Ravenell J, Adhikari S, et al. Assessment of racial/ethnic disparities in hospitalization and mortality in patients with COVID-19 in New York City. JAMA Netw Open. 2020;3(12):e2026881. https://doi.org/10.1001/jamanetworkopen.2020.26881
17. Gershengorn HB, Patel S, Shukla B, et al. Association of race and ethnicity with COVID-19 test positivity and hospitalization is mediated by socioeconomic factors. Ann Am Thorac Soc. 2021;18(8):1326-1334. https://doi.org/10.1513/AnnalsATS.202011-1448OC
18. Sze S, Pan D, Nevill CR, et al. Ethnicity and clinical outcomes in COVID-19: a systematic review and meta-analysis. EClinicalMedicine. 2020;29:100630. https://doi.org/10.1016/j.eclinm.2020.100630
19. U.S. Census Bureau. 2019 U.S Census Bureau American Community Survey. https://www.census.gov/programs-surveys/acs
20. North Texas Mass Critical Care Task Force. North Texas Mass Critical Care Guidelines Document. Hospital and ICU Triage Guidelines for ADULTS. January 2014. https://www.dallas-cms.org/tmaimis/dcms/assets/files/communityhealth/MCC/GuidelinesAdult_JAN2014.pdf
21. Singh K, Valley TS, Tang S, et al. Evaluating a widely implemented proprietary deterioration index model among hospitalized COVID-19 patients. Ann Am Thorac Soc. 2021;18(7):1129-1137. https://doi.org/10.1513/AnnalsATS.202006-698OC
22. Keller MJ, Kitsis EA, Arora S, et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;15(8):489-493. https://doi.org/10.12788/jhm.3497
23. Centers for Disease Control and Prevention. Science Brief: Evidence used to update the list of underlying medical conditions that increase a person’s risk of severe illness from COVID-19. Updated May 12, 2021. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/underlying-evidence-table.html
24. Gupta S, Wang W, Hayek SS, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2021;181(1):41-51. https://doi.org/10.1001/jamainternmed.2020.6252
25. Baroutjian A, Sanchez C, Boneva D, McKenney M, Elkbuli A. SARS-CoV-2 pharmacologic therapies and their safety/effectiveness according to level of evidence. Am J Emerg Med. 2020;38(11):2405-2415. https://doi.org/10.1016/j.ajem.2020.08.091
26. Janiaud P, Axfors C, Schmitt AM, et al. Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. JAMA. 2021;325(12):1185-1195. https://doi.org/10.1001/jama.2021.2747
27. Panagiotou OA, Kosar CM, White EM, et al. Risk factors associated with all-cause 30-day mortality in nursing home residents with COVID-19. JAMA Intern Med. 2021;181(4):439-448. https://doi.org/10.1001/jamainternmed.2020.7968
28. Bacong AM, Menjívar C. Recasting the immigrant health paradox through intersections of legal status and race. J Immigr Minor Health. 2021;23(5):1092-1104. https://doi.org/10.1007/s10903-021-01162-2
29. Plopper GE, Sciarretta KL, Buchman TG. Disparities in sepsis outcomes may be attributable to access to care. Crit Care Med. 2021;49(8):1358-1360. https://doi.org/10.1097/CCM.0000000000005126
30. Jones JM, Fingar KR, Miller MA, et al. Racial disparities in sepsis-related in-hospital mortality: using a broad case capture method and multivariate controls for clinical and hospital variables, 2004-2013. Crit Care Med. 2017;45(12):e1209-e1217. https://doi.org/10.1097/CCM.0000000000002699
31. Jacobs ZG, Prasad PA, Fang MC, Abe-Jones Y, Kangelaris KN. The association between limited English proficiency and sepsis mortality. J Hosp Med. 2019;14:E1-E7. https://doi.org/10.12788/jhm.3334
32. Cohn D. Census considers new approach to asking about race – by not using the term at all. June 18, 2015. https://www.pewresearch.org/fact-tank/2015/06/18/census-considers-new-approach-to-asking-about-race-by-not-using-the-term-at-all/
In the United States, health disparities in COVID-19 outcomes (including morbidity and mortality) based on race and ethnicity have been described in the scientific literature and mainstream media.1-7 According to the US Centers for Disease Control and Prevention (CDC), Hispanic people are 3.2 times more likely to be hospitalized with COVID-19 than non-Hispanic White people.8 Further, Hispanic people diagnosed with COVID-19 are 2.3 times more likely to die, adjusted for age, than non-Hispanic White people.9 As the epicenter of the COVID-19 pandemic shifted from the Northeast to the South, the CDC reported that, among people who died from COVID-19 in the United States from May to August 2020, the percentage of Hispanic people increased from 16.3% to 26.4%.10
Published studies on the effect of ethnicity on critical illness or mortality for hospitalized COVID-19 patients are limited and inconsistent. While some studies reported a higher mortality rate for Hispanic patients,11-15 others showed no difference.4,16,17 A recent meta-analysis found that intensive care unit (ICU) utilization and mortality were slightly higher among Hispanic COVID-19 inpatients, but this finding did not reach statistical significance.18 Past studies from different healthcare systems were limited by the small sample size of hospitalized Hispanic patients and the heterogeneity of patients. A comprehensive analysis from a large healthcare system with sufficient sample size is needed to understand the impact of ethnicity on clinical outcomes of hospitalized COVID-19 patients.
Texas Health Resources (THR) is a large integrated healthcare system serving the Dallas-Fort Worth-Arlington (DFW) metropolitan area. According to the 2019 US Census Bureau American Community Survey, Hispanic people comprise 18.4% of the population of this geographic area.19 Congruent with the CDC’s findings, Hispanic patients account for a disproportionate share (32.2%) of hospitalized COVID-19 patients at THR relative to the area’s demographic composition. Aware of the increased risk, we undertook an analysis of the clinical outcomes and the clinical, social, and demographic characteristics of Hispanic patients hospitalized at THR with COVID-19. Our primary goal was to investigate whether clinical outcomes differ by ethnicity among patients hospitalized with COVID-19 and, if so, whether inpatient care or preadmission factors contribute to this difference.
Methods
Study Setting and Overview
We collected data from the single electronic health record (EHR) used by 20 THR hospitals located across the DFW metropolitan area. THR is the largest faith-based, nonprofit health system in North Texas, operating 20 acute care hospitals. Including all access points, such as outpatient facilities and physician group practices, THR serves 7 million residents in 16 counties in North Texas, of whom 16.8% are Hispanic, 73.3% are non-Hispanic, and 9.9% are unclassified, congruent with demographics in the DFW area.
The institutional review boards at THR and UT Southwestern Medical Center approved the study under a waiver of informed consent (as a minimal-risk medical record review). After collection, all data were de-identified prior to statistical analysis.
Cohort, Outcomes, and Covariables
The study cohort included 6097 adult patients with laboratory-confirmed COVID-19 (age ≥18 years) who were admitted as inpatients from March 3 to November 5, 2020. The primary outcomes included ICU utilization and death during hospitalization. We described demographic characteristics using the following variables: age (18–49, 50–64, 65–79, ≥80 years), sex, self-reported ethnicity, and primary spoken language.
We defined a severe baseline condition as an elevated respiratory subscore parsed from the overall MSOFA (Modified Sequential Organ Failure Assessment),20 an elevated Epic Deterioration Index (EDI),21 or an elevated C-reactive protein level (CRP) at baseline (any elevated CRP). Baseline referred to the variable mean during the first available 12-hour window of measurement during the COVID-19 hospital admission, including variables obtained in the emergency department (ED). An elevated MSOFA referred to a score of 4, corresponding to an SpO2/FiO2 < 150. Elevated EDI referred to a baseline EDI > 45. An elevated CRP referred to a baseline CRP > 20 mg/dL.22
Variables reflecting access to healthcare included: THR EHR creation year (representing the first time patients accessed the THR health system), insurance payor type, and presence of a primary care provider (PCP). The federal government established the COVID-19 Claims Reimbursement to Health Care Providers and Facilities for Testing, Treatment, and Vaccine Administration for the Uninsured program. The insurance payor for patients covered by this program is designated as COVID-19 HRSA. Presence of a PCP reflects any documented PCP, regardless of affiliation with THR. We selected these access metrics opportunistically, as they were consistently documented in the EHR and readily available for analysis.
We used 12 variables to describe comorbidities or underlying conditions that, according to the CDC, increased patients’ risk of severe illness from COVID-1923: diagnoses of diabetes, hypertension, obesity, chronic obstructive pulmonary disease (COPD), asthma, smoking, other lung disease, heart failure, kidney disease without end-stage renal disease (ESRD), ESRD, liver disease, and cancer. We identified comorbidities by mining the structured diagnosis codes documented in the EHR prior to and during the COVID-19 admission. Sources for diagnoses included final billed diagnosis codes, working diagnosis codes, problem list, and reason for visit. The definition of diabetes included previously recorded diabetes or baseline hemoglobin A1c > 9%. We also recorded the presence of four major COVID-19 treatments: steroids, remdesivir, tocilizumab, and fresh frozen plasma (FFP) from convalescent patients.24-26 Each treatment variable was defined by receipt of one or more doses.
Statistical Analysis
To analyze patient outcomes based on ethnicity, we divided the study cohort into a Hispanic group and a non-Hispanic group based on self-reported ethnicity in the EHR. To study the potential impact of primary language among Hispanic patients, we divided them into English-speaking and non-English-speaking patients based on their self-reported primary language. As a result, we analyzed three groups of patients: (1) non-Hispanic, (2) Hispanic and English speaking, and (3) Hispanic and non-English speaking. We tested differences of a given categorical variable across the three groups using the chi-square test for each age subgroup (18–49, 50–64, 65–79, ≥80 years). The Cochran-Mantel-Haenszel test was used for the overall difference adjusted for age. To assess whether an observed disparity in treatment existed across the three groups, we tested the difference in the administration of four major therapeutics for COVID-19, including steroids, remdesivir, tocilizumab, and convalescent plasma. To determine whether any groups had elevated disease severity at hospital admission (baseline), we tested the difference in four disease-severity metrics across the ethnic-language groups: (1) elevated respiratory MSOFA score, (2) elevated EDI, (3) elevated CRP level, and (4) any of the three conditions.
To study the associations with ICU utilization and death, respectively, we performed a multivariable analysis using a generalized linear mixed model with binomial distribution and a logit link function. In each analysis model, the hospital of admission was included as a random-effect variable to account for the potential treatment variations among different hospitals, while other variables were regarded as fixed effects. In the first multivariable analysis (Model 1), all demographic variables, including age, sex, and ethnicity, and different types of comorbidities and underlying conditions, were included as fixed-effect variables in the initial model, and then backward stepwise variable selection was performed to establish the final model (Model 1). We performed the backward stepwise variable selection separately for the outcome of ICU use or mortality. Based on Akaike information criterion (AIC), during each iteration the fixed-effect variable that led to the largest decrease in the AIC value was removed, and the variable selection process was completed when the AIC value stopped decreasing. In Model 2, we added the disease-severity variable at baseline to the selected variable set derived from Model 1 to explore its effect on the associations between ethnicity and clinical outcomes. In Model 3, we added healthcare access–related variables, including first-time healthsystem access, payor type, and PCP availability to Model 2. We performed all statistical analyses using R, version 4.0.2 (R Foundation for Statistical Computing) in RStudio (version 1.3.1093).
Results
Distinct Demographic and Comorbidity Patterns for Three Ethnic-Language Groups
We identified 6097 adult patients (age ≥18 years) who had confirmed COVID-19 disease and were hospitalized between March 3 and November 5, 2020. Demographic characteristics and comorbidity for these patients are summarized in Table 1. Among these patients, 4139 (67.9%) were non-Hispanic and 1958 (32.1%) were Hispanic. Among the Hispanic patients, 1203 (61.4%) identified English as their primary language and 755 (38.6%) identified a non-English primary language. Age distribution was vastly different among the three ethnic-language groups (Table 1). Unlike the relatively balanced distribution across different age groups in the non-Hispanic group, more than half (55.8%) of the English-speaking Hispanic patients were in the youngest age group (18-49 years). A much lower fraction of Hispanic patients was among the oldest (≥80 years) age group (P < .001). Because COVID-19 clinical outcome is strongly associated with age,27 we used age-stratified analysis when comparing group-level differences in patient outcomes.

Sex distribution also was different among the three groups, with the non-English-speaking Hispanic group having more male patients (53.0%). Diabetes and obesity, which are associated with clinical outcomes of COVID-19 patients, were more prevalent in Hispanic patients (Table 1). Non-English-speaking Hispanic patients had the highest diabetes rate (48.7% with documented diabetes; 15.8% with baseline HbA1c > 9%; P < .001). English-speaking Hispanic patients presented with the highest obesity rate (62.8%; P < .001). Appendix Table 1 provides detailed age-group-specific comorbidity distributions among ethnic-language groups.
Patients of Hispanic Ethnicity Experienced a Higher Rate of ICU Utilization and Mortality
Of the 6097 patients overall, 1365 (22.4%) were admitted to the ICU and 543 (8.9%) died in hospital. For non-Hispanic patients (n = 4139), 883 (21.3%) were admitted to the ICU and 373 (9.0%) died in hospital. For English-speaking Hispanic patients (n = 1203), 241 (20.0%) were admitted to the ICU and 91 (7.6%) died in hospital. For non-English-speaking Hispanic patients (n = 755), 241 (31.9%) were admitted to the ICU and 79 (10.5%) died in hospital. Figure 1 summarizes the age-stratified comparison of ICU utilization and mortality across the three ethnic-language patient groups. In all age groups, non-English-speaking Hispanic patients experienced a significantly higher ICU utilization rate compared to non-Hispanic patients (age-adjusted OR, 1.75; 95% CI, 1.47-2.08; P < .001). English-speaking and non-English-speaking Hispanic patients had a significantly higher mortality rate compared to non-Hispanic patients (age-adjusted OR, 1.53; 95% CI, 1.19-1.98; P = .001 for English-speaking Hispanic patients; age-adjusted OR, 1.43; 95% CI,: 1.10-1.86; P = .01 for non-English-speaking Hispanic patients).

To delineate the risk factors associated with ICU utilization and death, we performed multivariable logistic regression with stepwise variable selection. After adjusting for age, sex, and comorbidity (Model 1), the factors ethnicity and primary language were still strongly associated with ICU utilization and mortality (Appendix Table 2). Non-English-speaking Hispanic patients had an OR of 1.74 (95% CI, 1.41-2.15; P < .001) for ICU utilization and an OR of 1.54 (95% CI, 1.12-2.12; P = .008) for mortality compared to non-Hispanic patients. Similarly, English-speaking Hispanic patients had higher ICU utilization (OR, 1.28; 95% CI, 1.05-1.55; P = .01) and a higher mortality rate (OR, 1.60; 95% CI, 1.19-2.14; P = .002).
No Disparity in COVID-19 Therapeutics Observed Across Three Ethnic-Language Groups
Appendix Figure 1 summarizes the comparison of the administration of four major treatments across the three ethnic-language groups. We did not observe any underuse of COVID-19 therapeutics for Hispanic patients. Usage rates for these therapies were significantly higher, after adjusting for age, in Hispanic groups when compared to non-Hispanic patients (OR ranged from 1.21 to 1.96). Steroids were the most common treatment in all patient groups. Tocilizumab was used almost twice as frequently (OR, 1.96; 95% CI, 1.64-2.33; P < .001) in non-English-speaking Hispanic patients compared to non-Hispanic patients.
Patients of Hispanic Ethnicity Had More Severe Disease at Hospital Admission
Figure 2 shows that non-English-speaking Hispanic patients had a higher rate of severe illness at admission based on each of these metrics: high respiratory MSOFA score (OR, 2.43; 95% CI, 1.77-3.33; P < .001), high EDI (OR, 1.85; 95% CI, 1.41-2.41; P < .001), and high CRP level (OR, 2.06; 95% CI, 1.64-2.58; P < .001). English-speaking Hispanic patients also had a greater rate of high CRP level (OR, 1.48; 95% CI, 1.17-1.86; P = .001) compared to non-Hispanic patients. When considering the presentation of any one of these clinical indicators, the English-speaking and non-English-speaking Hispanic patients had a higher rate of severe baseline condition (OR, 1.33; 95% CI, 1.10-1.61; P = .004 for English-speaking patients; OR, 2.27; 95% CI, 1.89-2.72; P < .001 for non-English-speaking patients).

We then studied how the baseline disease condition affects the association between ethnicity and clinical outcomes. We performed a multivariable analysis including baseline disease severity as a covariable (Model 2, Table 2), which showed that baseline disease severity was strongly associated with ICU admission (OR, 4.52; 95% CI, 3.83-5.33; P < .001) and mortality (OR, 3.32; 95% CI, 2.67-4.13; P < .001). The associations between ethnicity and clinical outcomes were reduced after considering the baseline disease condition. The OR dropped to 1.47 (95% CI, 1.18-1.84; P < .001) and 1.34 (95% CI, 0.97-1.87; P = .08) for ICU utilization and mortality, respectively, when comparing non-English-speaking Hispanic patients to non-Hispanic patients. A similar reduction was observed for English-speaking Hispanic patients. Model comparison showed a significant improvement of Model 2 over Model 1 based on ANOVA test (P < .001) as well as AIC.

Hispanic Patients Had Worse Healthcare Access
To explore the etiology for the more severe disease conditions at hospital admission among Hispanic patients, we analyzed variables related to healthcare access. We found that Hispanic patients were likely to have reduced access to healthcare (Table 1; Appendix Figure 2). For a large proportion (16.9%) of the COVID-19 patients in this study, their medical records were first created at THR in 2020, corresponding to the initial time these patients accessed THR for their healthcare. This surge in 2020, compared to previous years with data (2005–2019), corresponds to the number of new patients seen because of COVID-19 (Appendix Figure 2A). Among this new patient population, the proportion of non-English-speaking Hispanic patients in 2020 was 28.3%, compared to 9.1% from 2005 to 2019 (P < .001). The proportion of new English-speaking Hispanic patients in 2020 was 22.1%, compared to an average of 19.2% from 2005 to 2019 (P < .001). In addition, a much smaller proportion of Hispanic patients had a PCP (P < .001) (Table 1; Appendix Figure 2B), with non-English-speaking Hispanic patients having the smallest proportion (58.5%).
Appendix Figure 2C illustrates the comparison of payor types across the three patient groups. A much higher proportion of Hispanic patients used COVID-19 HRSA (P < .001) compared to non-Hispanic patients. Breaking this down further by primary language, 29.1% of non-English-speaking Hispanic patients relied on COVID-19 HRSA due to otherwise uninsured status, compared to 12.7% of English-speaking Hispanic patients and only 5.1% of non-Hispanic patients. Similarly, non-English-speaking Hispanic patients have the highest self-pay rates (2.3%) compared to English-speaking Hispanic patients (1.4%) and non-Hispanic patients (0.7%). In summary, more Hispanic patients, and especially non-English-speaking Hispanic patients, lacked conventional health insurance and experienced limited access to healthcare.
Further evidence showed a trend of correlation between presentation of severe COVID-19 conditions when arriving at the hospital and each of the healthcare access factors analyzed (Appendix Figure 3).
Discussion
With a large sample size of hospitalized COVID-19 patients at an integrated health system in the DFW metropolitan area, we observed an increased rate of ICU utilization and mortality among Hispanic inpatients. After adjusting for age, we found that non-English-speaking Hispanic patients were 75% more likely to require critical care compared with non-Hispanic patients. English-speaking and non-English-speaking Hispanic patients had an increased mortality rate (age-adjusted) compared to non-Hispanic patients. The association between ethnicity and clinical outcomes remained significant after adjusting for age, sex, and comorbidities. We did not observe any underuse of major COVID-19 therapeutics in Hispanic patients, and excluded in-hospital treatments from the contributors to the outcome differences.
Hispanic patients, especially non-English-speaking Hispanic patients, had a higher rate of severe COVID-19 disease at the time of hospital admission (Figure 2). After including baseline disease severity into the multivariable analysis (Model 2), the overall model improved (P < .001) while the associations between ethnicity and outcomes decreased (Table 2). This suggests disease severity at admission was a main contributor to the observed associations between ethnicity and clinical outcomes. The higher rate of baseline COVID-19 severity in Hispanic patients might also explain their higher rate of receiving major COVID-19 therapeutics (Appendix Figure 1).
This study found that Hispanic patients were less likely to have a PCP and insurance coverage compared with non-Hispanic patients (P < .001). This disparity was more pronounced among non-English-speaking Hispanic patients (Appendix Figure 2). We also observed that a disproportionately larger proportion (50.4%) of patients who visited the healthcare system for the first time in 2020 (the year of the COVID-19 pandemic) was composed of Hispanic patients, compared to merely 28.4% prior to 2020. While there is a possibility that patients had primary care outside THR, the staggering number of Hispanic patients who were new to the health system in 2020, in conjunction with the fact that immigrants tend to be “healthier” compared to their native-born peers (the so-called immigrant paradox),28 led us to conclude that there were few other primary care options for these patients, making THR’s ED the primary care option of choice. The systemic, structural barriers to routine care might be a possible cause for delayed admission and, in turn, elevated baseline COVID-19 severity for Hispanic patients (Appendix Figure 3).
Recent studies have investigated the impact of socioeconomic factors on racial/ethnic disparities in the COVID-19 pandemic.7,16,17 To our knowledge, no study has directly analyzed the link between healthcare access metrics, COVID-19 severity at admission, and the Hispanic population stratified by primary language. Studies exist on this subject for other diseases, however. For example, healthcare access factors have been associated with sepsis-related mortality.29,30 In fact, a recent study that explored the potential effect of language barriers on healthcare access demonstrated an association between limited English proficiency and sepsis-related mortality.31 Our study found that Hispanic patients whose primary language is not English had the worst clinical outcomes, including more severe baseline COVID-19 conditions, and the least access to healthcare, highlighting the importance of addressing language barriers in COVID-19 care. Further research is needed to confirm the relationship between limited English proficiency and clinical outcomes, as well as potential factors that contribute to such a relationship in different types of diseases.
Our study has a number of limitations. First, it was limited to only one large healthcare system, which means the results may not be generalizable. Because THR is an open system, comorbidity data may be incomplete, and we cannot exclude the possibility that patients accessed care outside THR prior to or during the pandemic. We may overcome this limitation in the future with cross-system health information exchange data. Second, we did not have data for the time of symptom onset, so we were unable to analyze the direct evidence of the possible delayed care. As a result, we were unable to analyze whether treatments were administered in a timely manner or appropriately. Third, our analysis was not adjusted for other socioeconomic factors (eg, income, education) due to lack of data. We used self-identification for ethnicity, but unlike new approaches by the U.S. Census Bureau,32 our survey allowed only one choice to be selected.
Conclusion
Sociodemographic factors among Hispanic inpatients hospitalized for COVID-19 at a large integrated health system—including a primary non-English language, lack of a PCP, and insurance status—were associated with measures of reduced access to care and more severe illness at admission. Structural barriers to care, which may be associated with reduced health literacy and less access to health insurance, can result in delayed treatment and more severe illness at admission and underdiagnosis of medical conditions, contributing to worse outcomes in this population. Our findings suggest that interventions to promote early recognition of signs and symptoms of COVID-19 and to encourage prompt clinical care at the community level may reduce the burden of COVID-19 deaths in racial or ethnic minority communities with language and socioeconomic barriers.
In the United States, health disparities in COVID-19 outcomes (including morbidity and mortality) based on race and ethnicity have been described in the scientific literature and mainstream media.1-7 According to the US Centers for Disease Control and Prevention (CDC), Hispanic people are 3.2 times more likely to be hospitalized with COVID-19 than non-Hispanic White people.8 Further, Hispanic people diagnosed with COVID-19 are 2.3 times more likely to die, adjusted for age, than non-Hispanic White people.9 As the epicenter of the COVID-19 pandemic shifted from the Northeast to the South, the CDC reported that, among people who died from COVID-19 in the United States from May to August 2020, the percentage of Hispanic people increased from 16.3% to 26.4%.10
Published studies on the effect of ethnicity on critical illness or mortality for hospitalized COVID-19 patients are limited and inconsistent. While some studies reported a higher mortality rate for Hispanic patients,11-15 others showed no difference.4,16,17 A recent meta-analysis found that intensive care unit (ICU) utilization and mortality were slightly higher among Hispanic COVID-19 inpatients, but this finding did not reach statistical significance.18 Past studies from different healthcare systems were limited by the small sample size of hospitalized Hispanic patients and the heterogeneity of patients. A comprehensive analysis from a large healthcare system with sufficient sample size is needed to understand the impact of ethnicity on clinical outcomes of hospitalized COVID-19 patients.
Texas Health Resources (THR) is a large integrated healthcare system serving the Dallas-Fort Worth-Arlington (DFW) metropolitan area. According to the 2019 US Census Bureau American Community Survey, Hispanic people comprise 18.4% of the population of this geographic area.19 Congruent with the CDC’s findings, Hispanic patients account for a disproportionate share (32.2%) of hospitalized COVID-19 patients at THR relative to the area’s demographic composition. Aware of the increased risk, we undertook an analysis of the clinical outcomes and the clinical, social, and demographic characteristics of Hispanic patients hospitalized at THR with COVID-19. Our primary goal was to investigate whether clinical outcomes differ by ethnicity among patients hospitalized with COVID-19 and, if so, whether inpatient care or preadmission factors contribute to this difference.
Methods
Study Setting and Overview
We collected data from the single electronic health record (EHR) used by 20 THR hospitals located across the DFW metropolitan area. THR is the largest faith-based, nonprofit health system in North Texas, operating 20 acute care hospitals. Including all access points, such as outpatient facilities and physician group practices, THR serves 7 million residents in 16 counties in North Texas, of whom 16.8% are Hispanic, 73.3% are non-Hispanic, and 9.9% are unclassified, congruent with demographics in the DFW area.
The institutional review boards at THR and UT Southwestern Medical Center approved the study under a waiver of informed consent (as a minimal-risk medical record review). After collection, all data were de-identified prior to statistical analysis.
Cohort, Outcomes, and Covariables
The study cohort included 6097 adult patients with laboratory-confirmed COVID-19 (age ≥18 years) who were admitted as inpatients from March 3 to November 5, 2020. The primary outcomes included ICU utilization and death during hospitalization. We described demographic characteristics using the following variables: age (18–49, 50–64, 65–79, ≥80 years), sex, self-reported ethnicity, and primary spoken language.
We defined a severe baseline condition as an elevated respiratory subscore parsed from the overall MSOFA (Modified Sequential Organ Failure Assessment),20 an elevated Epic Deterioration Index (EDI),21 or an elevated C-reactive protein level (CRP) at baseline (any elevated CRP). Baseline referred to the variable mean during the first available 12-hour window of measurement during the COVID-19 hospital admission, including variables obtained in the emergency department (ED). An elevated MSOFA referred to a score of 4, corresponding to an SpO2/FiO2 < 150. Elevated EDI referred to a baseline EDI > 45. An elevated CRP referred to a baseline CRP > 20 mg/dL.22
Variables reflecting access to healthcare included: THR EHR creation year (representing the first time patients accessed the THR health system), insurance payor type, and presence of a primary care provider (PCP). The federal government established the COVID-19 Claims Reimbursement to Health Care Providers and Facilities for Testing, Treatment, and Vaccine Administration for the Uninsured program. The insurance payor for patients covered by this program is designated as COVID-19 HRSA. Presence of a PCP reflects any documented PCP, regardless of affiliation with THR. We selected these access metrics opportunistically, as they were consistently documented in the EHR and readily available for analysis.
We used 12 variables to describe comorbidities or underlying conditions that, according to the CDC, increased patients’ risk of severe illness from COVID-1923: diagnoses of diabetes, hypertension, obesity, chronic obstructive pulmonary disease (COPD), asthma, smoking, other lung disease, heart failure, kidney disease without end-stage renal disease (ESRD), ESRD, liver disease, and cancer. We identified comorbidities by mining the structured diagnosis codes documented in the EHR prior to and during the COVID-19 admission. Sources for diagnoses included final billed diagnosis codes, working diagnosis codes, problem list, and reason for visit. The definition of diabetes included previously recorded diabetes or baseline hemoglobin A1c > 9%. We also recorded the presence of four major COVID-19 treatments: steroids, remdesivir, tocilizumab, and fresh frozen plasma (FFP) from convalescent patients.24-26 Each treatment variable was defined by receipt of one or more doses.
Statistical Analysis
To analyze patient outcomes based on ethnicity, we divided the study cohort into a Hispanic group and a non-Hispanic group based on self-reported ethnicity in the EHR. To study the potential impact of primary language among Hispanic patients, we divided them into English-speaking and non-English-speaking patients based on their self-reported primary language. As a result, we analyzed three groups of patients: (1) non-Hispanic, (2) Hispanic and English speaking, and (3) Hispanic and non-English speaking. We tested differences of a given categorical variable across the three groups using the chi-square test for each age subgroup (18–49, 50–64, 65–79, ≥80 years). The Cochran-Mantel-Haenszel test was used for the overall difference adjusted for age. To assess whether an observed disparity in treatment existed across the three groups, we tested the difference in the administration of four major therapeutics for COVID-19, including steroids, remdesivir, tocilizumab, and convalescent plasma. To determine whether any groups had elevated disease severity at hospital admission (baseline), we tested the difference in four disease-severity metrics across the ethnic-language groups: (1) elevated respiratory MSOFA score, (2) elevated EDI, (3) elevated CRP level, and (4) any of the three conditions.
To study the associations with ICU utilization and death, respectively, we performed a multivariable analysis using a generalized linear mixed model with binomial distribution and a logit link function. In each analysis model, the hospital of admission was included as a random-effect variable to account for the potential treatment variations among different hospitals, while other variables were regarded as fixed effects. In the first multivariable analysis (Model 1), all demographic variables, including age, sex, and ethnicity, and different types of comorbidities and underlying conditions, were included as fixed-effect variables in the initial model, and then backward stepwise variable selection was performed to establish the final model (Model 1). We performed the backward stepwise variable selection separately for the outcome of ICU use or mortality. Based on Akaike information criterion (AIC), during each iteration the fixed-effect variable that led to the largest decrease in the AIC value was removed, and the variable selection process was completed when the AIC value stopped decreasing. In Model 2, we added the disease-severity variable at baseline to the selected variable set derived from Model 1 to explore its effect on the associations between ethnicity and clinical outcomes. In Model 3, we added healthcare access–related variables, including first-time healthsystem access, payor type, and PCP availability to Model 2. We performed all statistical analyses using R, version 4.0.2 (R Foundation for Statistical Computing) in RStudio (version 1.3.1093).
Results
Distinct Demographic and Comorbidity Patterns for Three Ethnic-Language Groups
We identified 6097 adult patients (age ≥18 years) who had confirmed COVID-19 disease and were hospitalized between March 3 and November 5, 2020. Demographic characteristics and comorbidity for these patients are summarized in Table 1. Among these patients, 4139 (67.9%) were non-Hispanic and 1958 (32.1%) were Hispanic. Among the Hispanic patients, 1203 (61.4%) identified English as their primary language and 755 (38.6%) identified a non-English primary language. Age distribution was vastly different among the three ethnic-language groups (Table 1). Unlike the relatively balanced distribution across different age groups in the non-Hispanic group, more than half (55.8%) of the English-speaking Hispanic patients were in the youngest age group (18-49 years). A much lower fraction of Hispanic patients was among the oldest (≥80 years) age group (P < .001). Because COVID-19 clinical outcome is strongly associated with age,27 we used age-stratified analysis when comparing group-level differences in patient outcomes.

Sex distribution also was different among the three groups, with the non-English-speaking Hispanic group having more male patients (53.0%). Diabetes and obesity, which are associated with clinical outcomes of COVID-19 patients, were more prevalent in Hispanic patients (Table 1). Non-English-speaking Hispanic patients had the highest diabetes rate (48.7% with documented diabetes; 15.8% with baseline HbA1c > 9%; P < .001). English-speaking Hispanic patients presented with the highest obesity rate (62.8%; P < .001). Appendix Table 1 provides detailed age-group-specific comorbidity distributions among ethnic-language groups.
Patients of Hispanic Ethnicity Experienced a Higher Rate of ICU Utilization and Mortality
Of the 6097 patients overall, 1365 (22.4%) were admitted to the ICU and 543 (8.9%) died in hospital. For non-Hispanic patients (n = 4139), 883 (21.3%) were admitted to the ICU and 373 (9.0%) died in hospital. For English-speaking Hispanic patients (n = 1203), 241 (20.0%) were admitted to the ICU and 91 (7.6%) died in hospital. For non-English-speaking Hispanic patients (n = 755), 241 (31.9%) were admitted to the ICU and 79 (10.5%) died in hospital. Figure 1 summarizes the age-stratified comparison of ICU utilization and mortality across the three ethnic-language patient groups. In all age groups, non-English-speaking Hispanic patients experienced a significantly higher ICU utilization rate compared to non-Hispanic patients (age-adjusted OR, 1.75; 95% CI, 1.47-2.08; P < .001). English-speaking and non-English-speaking Hispanic patients had a significantly higher mortality rate compared to non-Hispanic patients (age-adjusted OR, 1.53; 95% CI, 1.19-1.98; P = .001 for English-speaking Hispanic patients; age-adjusted OR, 1.43; 95% CI,: 1.10-1.86; P = .01 for non-English-speaking Hispanic patients).

To delineate the risk factors associated with ICU utilization and death, we performed multivariable logistic regression with stepwise variable selection. After adjusting for age, sex, and comorbidity (Model 1), the factors ethnicity and primary language were still strongly associated with ICU utilization and mortality (Appendix Table 2). Non-English-speaking Hispanic patients had an OR of 1.74 (95% CI, 1.41-2.15; P < .001) for ICU utilization and an OR of 1.54 (95% CI, 1.12-2.12; P = .008) for mortality compared to non-Hispanic patients. Similarly, English-speaking Hispanic patients had higher ICU utilization (OR, 1.28; 95% CI, 1.05-1.55; P = .01) and a higher mortality rate (OR, 1.60; 95% CI, 1.19-2.14; P = .002).
No Disparity in COVID-19 Therapeutics Observed Across Three Ethnic-Language Groups
Appendix Figure 1 summarizes the comparison of the administration of four major treatments across the three ethnic-language groups. We did not observe any underuse of COVID-19 therapeutics for Hispanic patients. Usage rates for these therapies were significantly higher, after adjusting for age, in Hispanic groups when compared to non-Hispanic patients (OR ranged from 1.21 to 1.96). Steroids were the most common treatment in all patient groups. Tocilizumab was used almost twice as frequently (OR, 1.96; 95% CI, 1.64-2.33; P < .001) in non-English-speaking Hispanic patients compared to non-Hispanic patients.
Patients of Hispanic Ethnicity Had More Severe Disease at Hospital Admission
Figure 2 shows that non-English-speaking Hispanic patients had a higher rate of severe illness at admission based on each of these metrics: high respiratory MSOFA score (OR, 2.43; 95% CI, 1.77-3.33; P < .001), high EDI (OR, 1.85; 95% CI, 1.41-2.41; P < .001), and high CRP level (OR, 2.06; 95% CI, 1.64-2.58; P < .001). English-speaking Hispanic patients also had a greater rate of high CRP level (OR, 1.48; 95% CI, 1.17-1.86; P = .001) compared to non-Hispanic patients. When considering the presentation of any one of these clinical indicators, the English-speaking and non-English-speaking Hispanic patients had a higher rate of severe baseline condition (OR, 1.33; 95% CI, 1.10-1.61; P = .004 for English-speaking patients; OR, 2.27; 95% CI, 1.89-2.72; P < .001 for non-English-speaking patients).

We then studied how the baseline disease condition affects the association between ethnicity and clinical outcomes. We performed a multivariable analysis including baseline disease severity as a covariable (Model 2, Table 2), which showed that baseline disease severity was strongly associated with ICU admission (OR, 4.52; 95% CI, 3.83-5.33; P < .001) and mortality (OR, 3.32; 95% CI, 2.67-4.13; P < .001). The associations between ethnicity and clinical outcomes were reduced after considering the baseline disease condition. The OR dropped to 1.47 (95% CI, 1.18-1.84; P < .001) and 1.34 (95% CI, 0.97-1.87; P = .08) for ICU utilization and mortality, respectively, when comparing non-English-speaking Hispanic patients to non-Hispanic patients. A similar reduction was observed for English-speaking Hispanic patients. Model comparison showed a significant improvement of Model 2 over Model 1 based on ANOVA test (P < .001) as well as AIC.

Hispanic Patients Had Worse Healthcare Access
To explore the etiology for the more severe disease conditions at hospital admission among Hispanic patients, we analyzed variables related to healthcare access. We found that Hispanic patients were likely to have reduced access to healthcare (Table 1; Appendix Figure 2). For a large proportion (16.9%) of the COVID-19 patients in this study, their medical records were first created at THR in 2020, corresponding to the initial time these patients accessed THR for their healthcare. This surge in 2020, compared to previous years with data (2005–2019), corresponds to the number of new patients seen because of COVID-19 (Appendix Figure 2A). Among this new patient population, the proportion of non-English-speaking Hispanic patients in 2020 was 28.3%, compared to 9.1% from 2005 to 2019 (P < .001). The proportion of new English-speaking Hispanic patients in 2020 was 22.1%, compared to an average of 19.2% from 2005 to 2019 (P < .001). In addition, a much smaller proportion of Hispanic patients had a PCP (P < .001) (Table 1; Appendix Figure 2B), with non-English-speaking Hispanic patients having the smallest proportion (58.5%).
Appendix Figure 2C illustrates the comparison of payor types across the three patient groups. A much higher proportion of Hispanic patients used COVID-19 HRSA (P < .001) compared to non-Hispanic patients. Breaking this down further by primary language, 29.1% of non-English-speaking Hispanic patients relied on COVID-19 HRSA due to otherwise uninsured status, compared to 12.7% of English-speaking Hispanic patients and only 5.1% of non-Hispanic patients. Similarly, non-English-speaking Hispanic patients have the highest self-pay rates (2.3%) compared to English-speaking Hispanic patients (1.4%) and non-Hispanic patients (0.7%). In summary, more Hispanic patients, and especially non-English-speaking Hispanic patients, lacked conventional health insurance and experienced limited access to healthcare.
Further evidence showed a trend of correlation between presentation of severe COVID-19 conditions when arriving at the hospital and each of the healthcare access factors analyzed (Appendix Figure 3).
Discussion
With a large sample size of hospitalized COVID-19 patients at an integrated health system in the DFW metropolitan area, we observed an increased rate of ICU utilization and mortality among Hispanic inpatients. After adjusting for age, we found that non-English-speaking Hispanic patients were 75% more likely to require critical care compared with non-Hispanic patients. English-speaking and non-English-speaking Hispanic patients had an increased mortality rate (age-adjusted) compared to non-Hispanic patients. The association between ethnicity and clinical outcomes remained significant after adjusting for age, sex, and comorbidities. We did not observe any underuse of major COVID-19 therapeutics in Hispanic patients, and excluded in-hospital treatments from the contributors to the outcome differences.
Hispanic patients, especially non-English-speaking Hispanic patients, had a higher rate of severe COVID-19 disease at the time of hospital admission (Figure 2). After including baseline disease severity into the multivariable analysis (Model 2), the overall model improved (P < .001) while the associations between ethnicity and outcomes decreased (Table 2). This suggests disease severity at admission was a main contributor to the observed associations between ethnicity and clinical outcomes. The higher rate of baseline COVID-19 severity in Hispanic patients might also explain their higher rate of receiving major COVID-19 therapeutics (Appendix Figure 1).
This study found that Hispanic patients were less likely to have a PCP and insurance coverage compared with non-Hispanic patients (P < .001). This disparity was more pronounced among non-English-speaking Hispanic patients (Appendix Figure 2). We also observed that a disproportionately larger proportion (50.4%) of patients who visited the healthcare system for the first time in 2020 (the year of the COVID-19 pandemic) was composed of Hispanic patients, compared to merely 28.4% prior to 2020. While there is a possibility that patients had primary care outside THR, the staggering number of Hispanic patients who were new to the health system in 2020, in conjunction with the fact that immigrants tend to be “healthier” compared to their native-born peers (the so-called immigrant paradox),28 led us to conclude that there were few other primary care options for these patients, making THR’s ED the primary care option of choice. The systemic, structural barriers to routine care might be a possible cause for delayed admission and, in turn, elevated baseline COVID-19 severity for Hispanic patients (Appendix Figure 3).
Recent studies have investigated the impact of socioeconomic factors on racial/ethnic disparities in the COVID-19 pandemic.7,16,17 To our knowledge, no study has directly analyzed the link between healthcare access metrics, COVID-19 severity at admission, and the Hispanic population stratified by primary language. Studies exist on this subject for other diseases, however. For example, healthcare access factors have been associated with sepsis-related mortality.29,30 In fact, a recent study that explored the potential effect of language barriers on healthcare access demonstrated an association between limited English proficiency and sepsis-related mortality.31 Our study found that Hispanic patients whose primary language is not English had the worst clinical outcomes, including more severe baseline COVID-19 conditions, and the least access to healthcare, highlighting the importance of addressing language barriers in COVID-19 care. Further research is needed to confirm the relationship between limited English proficiency and clinical outcomes, as well as potential factors that contribute to such a relationship in different types of diseases.
Our study has a number of limitations. First, it was limited to only one large healthcare system, which means the results may not be generalizable. Because THR is an open system, comorbidity data may be incomplete, and we cannot exclude the possibility that patients accessed care outside THR prior to or during the pandemic. We may overcome this limitation in the future with cross-system health information exchange data. Second, we did not have data for the time of symptom onset, so we were unable to analyze the direct evidence of the possible delayed care. As a result, we were unable to analyze whether treatments were administered in a timely manner or appropriately. Third, our analysis was not adjusted for other socioeconomic factors (eg, income, education) due to lack of data. We used self-identification for ethnicity, but unlike new approaches by the U.S. Census Bureau,32 our survey allowed only one choice to be selected.
Conclusion
Sociodemographic factors among Hispanic inpatients hospitalized for COVID-19 at a large integrated health system—including a primary non-English language, lack of a PCP, and insurance status—were associated with measures of reduced access to care and more severe illness at admission. Structural barriers to care, which may be associated with reduced health literacy and less access to health insurance, can result in delayed treatment and more severe illness at admission and underdiagnosis of medical conditions, contributing to worse outcomes in this population. Our findings suggest that interventions to promote early recognition of signs and symptoms of COVID-19 and to encourage prompt clinical care at the community level may reduce the burden of COVID-19 deaths in racial or ethnic minority communities with language and socioeconomic barriers.
1. Lopez L III, Hart LH III, Katz MH. Racial and ethnic health disparities related to COVID-19. JAMA. 2021;325(8):719-720. https://doi.org/10.1001/jama.2020.26443
2. Cooper LA, Williams DR. Excess deaths from COVID-19, community bereavement, and restorative justice for communities of color. JAMA. 2020;324(15):1491-1492. https://doi.org/10.1001/jama.2020.19567
3. Clay LA, Rogus S. Primary and secondary health impacts of COVID-19 among minority individuals in New York State. Int J Environ Res Public Health. 2021;18(2):683. https://doi.org/10.3390/ijerph18020683
4. Rodriguez F, Solomon N, de Lemos JA, et al. Racial and ethnic differences in presentation and outcomes for patients hospitalized with COVID-19: findings from the American Heart Association’s COVID-19 Cardiovascular Disease Registry. Circulation. 2021;143(24):2332-2342. https://doi.org/10.1161/CIRCULATIONAHA.120.052278
5. Moreira A, Chorath K, Rajasekaran K, Burmeister F, Ahmed M, Moreira A. Demographic predictors of hospitalization and mortality in US children with COVID-19. Eur J Pediatr. 2021;180(5):1659-1663. https://doi.org/10.1007/s00431-021-03955-x
6. Kolata G. Social inequities explain racial gaps in pandemic, studies find. The New York Times. December 9, 2020. https://www.nytimes.com/2020/12/09/health/coronavirus-black-hispanic.html
7. Liao TF, De Maio F. Association of social and economic inequality with coronavirus disease 2019 incidence and mortality across US counties. JAMA Netw Open. 2021;4(1):e2034578. https://doi.org/10.1001/jamanetworkopen.2020.34578
8. Centers for Disease Control and Prevention. A Weekly Surveillance Summary of U.S. COVID-19 Activity: Key Updates for Week 2. January 21, 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/pdf/covidview-01-22-2021.pdf
9. Centers for Disease Control and Prevention. Risk for COVID-19 infection, hospitalization, and death by race/ethnicity. Updated September 9, 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html
10. Gold JAW, Rossen LM, Ahmad FB, et al. Race, ethnicity, and age trends in persons who died from COVID-19 – United States, May-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(42):1517-1521. https://doi.org/10.15585/mmwr.mm6942e1
11. Pennington AF, Kompaniyets L, Summers AD, et al. Risk of clinical severity by age and race/ethnicity among adults hospitalized for COVID-19 – United States, March-September 2020. Open Forum Infect Dis. 2021;8(2):ofaa638. https://doi.org/10.1093/ofid/ofaa638.
12. Renelus BD, Khoury NC, Chandrasekaran K, et al. Racial disparities in COVID-19 hospitalization and in-hospital mortality at the height of the New York City pandemic. J Racial Ethn Health Disparities. 2021;8(5):1161-1167. https://doi.org/10.1007/s40615-020-00872-x
13. Wiley Z, Ross-Driscoll K, Wang Z, Smothers L, Mehta AK, Patzer RE. Racial and ethnic differences and clinical outcomes of COVID-19 patients presenting to the emergency department. Clin Infect Dis. 2021 Apr 2. [Epub ahead of print] https://doi.org/10.1093/cid/ciab290
14. Dai CL, Kornilov SA, Roper RT, et al. Characteristics and factors associated with COVID-19 infection, hospitalization, and mortality across race and ethnicity. Clin Infect Dis. 2021 Feb 20. [Epub ahead of print] https://doi.org/10.1093/cid/ciab154
15. Pan AP, Khan O, Meeks JR, et al. Disparities in COVID-19 hospitalizations and mortality among black and Hispanic patients: cross-sectional analysis from the greater Houston metropolitan area. BMC Public Health. 2021;21(1):1330. https://doi.org/10.1186/s12889-021-11431-2
16. Ogedegbe G, Ravenell J, Adhikari S, et al. Assessment of racial/ethnic disparities in hospitalization and mortality in patients with COVID-19 in New York City. JAMA Netw Open. 2020;3(12):e2026881. https://doi.org/10.1001/jamanetworkopen.2020.26881
17. Gershengorn HB, Patel S, Shukla B, et al. Association of race and ethnicity with COVID-19 test positivity and hospitalization is mediated by socioeconomic factors. Ann Am Thorac Soc. 2021;18(8):1326-1334. https://doi.org/10.1513/AnnalsATS.202011-1448OC
18. Sze S, Pan D, Nevill CR, et al. Ethnicity and clinical outcomes in COVID-19: a systematic review and meta-analysis. EClinicalMedicine. 2020;29:100630. https://doi.org/10.1016/j.eclinm.2020.100630
19. U.S. Census Bureau. 2019 U.S Census Bureau American Community Survey. https://www.census.gov/programs-surveys/acs
20. North Texas Mass Critical Care Task Force. North Texas Mass Critical Care Guidelines Document. Hospital and ICU Triage Guidelines for ADULTS. January 2014. https://www.dallas-cms.org/tmaimis/dcms/assets/files/communityhealth/MCC/GuidelinesAdult_JAN2014.pdf
21. Singh K, Valley TS, Tang S, et al. Evaluating a widely implemented proprietary deterioration index model among hospitalized COVID-19 patients. Ann Am Thorac Soc. 2021;18(7):1129-1137. https://doi.org/10.1513/AnnalsATS.202006-698OC
22. Keller MJ, Kitsis EA, Arora S, et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;15(8):489-493. https://doi.org/10.12788/jhm.3497
23. Centers for Disease Control and Prevention. Science Brief: Evidence used to update the list of underlying medical conditions that increase a person’s risk of severe illness from COVID-19. Updated May 12, 2021. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/underlying-evidence-table.html
24. Gupta S, Wang W, Hayek SS, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2021;181(1):41-51. https://doi.org/10.1001/jamainternmed.2020.6252
25. Baroutjian A, Sanchez C, Boneva D, McKenney M, Elkbuli A. SARS-CoV-2 pharmacologic therapies and their safety/effectiveness according to level of evidence. Am J Emerg Med. 2020;38(11):2405-2415. https://doi.org/10.1016/j.ajem.2020.08.091
26. Janiaud P, Axfors C, Schmitt AM, et al. Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. JAMA. 2021;325(12):1185-1195. https://doi.org/10.1001/jama.2021.2747
27. Panagiotou OA, Kosar CM, White EM, et al. Risk factors associated with all-cause 30-day mortality in nursing home residents with COVID-19. JAMA Intern Med. 2021;181(4):439-448. https://doi.org/10.1001/jamainternmed.2020.7968
28. Bacong AM, Menjívar C. Recasting the immigrant health paradox through intersections of legal status and race. J Immigr Minor Health. 2021;23(5):1092-1104. https://doi.org/10.1007/s10903-021-01162-2
29. Plopper GE, Sciarretta KL, Buchman TG. Disparities in sepsis outcomes may be attributable to access to care. Crit Care Med. 2021;49(8):1358-1360. https://doi.org/10.1097/CCM.0000000000005126
30. Jones JM, Fingar KR, Miller MA, et al. Racial disparities in sepsis-related in-hospital mortality: using a broad case capture method and multivariate controls for clinical and hospital variables, 2004-2013. Crit Care Med. 2017;45(12):e1209-e1217. https://doi.org/10.1097/CCM.0000000000002699
31. Jacobs ZG, Prasad PA, Fang MC, Abe-Jones Y, Kangelaris KN. The association between limited English proficiency and sepsis mortality. J Hosp Med. 2019;14:E1-E7. https://doi.org/10.12788/jhm.3334
32. Cohn D. Census considers new approach to asking about race – by not using the term at all. June 18, 2015. https://www.pewresearch.org/fact-tank/2015/06/18/census-considers-new-approach-to-asking-about-race-by-not-using-the-term-at-all/
1. Lopez L III, Hart LH III, Katz MH. Racial and ethnic health disparities related to COVID-19. JAMA. 2021;325(8):719-720. https://doi.org/10.1001/jama.2020.26443
2. Cooper LA, Williams DR. Excess deaths from COVID-19, community bereavement, and restorative justice for communities of color. JAMA. 2020;324(15):1491-1492. https://doi.org/10.1001/jama.2020.19567
3. Clay LA, Rogus S. Primary and secondary health impacts of COVID-19 among minority individuals in New York State. Int J Environ Res Public Health. 2021;18(2):683. https://doi.org/10.3390/ijerph18020683
4. Rodriguez F, Solomon N, de Lemos JA, et al. Racial and ethnic differences in presentation and outcomes for patients hospitalized with COVID-19: findings from the American Heart Association’s COVID-19 Cardiovascular Disease Registry. Circulation. 2021;143(24):2332-2342. https://doi.org/10.1161/CIRCULATIONAHA.120.052278
5. Moreira A, Chorath K, Rajasekaran K, Burmeister F, Ahmed M, Moreira A. Demographic predictors of hospitalization and mortality in US children with COVID-19. Eur J Pediatr. 2021;180(5):1659-1663. https://doi.org/10.1007/s00431-021-03955-x
6. Kolata G. Social inequities explain racial gaps in pandemic, studies find. The New York Times. December 9, 2020. https://www.nytimes.com/2020/12/09/health/coronavirus-black-hispanic.html
7. Liao TF, De Maio F. Association of social and economic inequality with coronavirus disease 2019 incidence and mortality across US counties. JAMA Netw Open. 2021;4(1):e2034578. https://doi.org/10.1001/jamanetworkopen.2020.34578
8. Centers for Disease Control and Prevention. A Weekly Surveillance Summary of U.S. COVID-19 Activity: Key Updates for Week 2. January 21, 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/pdf/covidview-01-22-2021.pdf
9. Centers for Disease Control and Prevention. Risk for COVID-19 infection, hospitalization, and death by race/ethnicity. Updated September 9, 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html
10. Gold JAW, Rossen LM, Ahmad FB, et al. Race, ethnicity, and age trends in persons who died from COVID-19 – United States, May-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(42):1517-1521. https://doi.org/10.15585/mmwr.mm6942e1
11. Pennington AF, Kompaniyets L, Summers AD, et al. Risk of clinical severity by age and race/ethnicity among adults hospitalized for COVID-19 – United States, March-September 2020. Open Forum Infect Dis. 2021;8(2):ofaa638. https://doi.org/10.1093/ofid/ofaa638.
12. Renelus BD, Khoury NC, Chandrasekaran K, et al. Racial disparities in COVID-19 hospitalization and in-hospital mortality at the height of the New York City pandemic. J Racial Ethn Health Disparities. 2021;8(5):1161-1167. https://doi.org/10.1007/s40615-020-00872-x
13. Wiley Z, Ross-Driscoll K, Wang Z, Smothers L, Mehta AK, Patzer RE. Racial and ethnic differences and clinical outcomes of COVID-19 patients presenting to the emergency department. Clin Infect Dis. 2021 Apr 2. [Epub ahead of print] https://doi.org/10.1093/cid/ciab290
14. Dai CL, Kornilov SA, Roper RT, et al. Characteristics and factors associated with COVID-19 infection, hospitalization, and mortality across race and ethnicity. Clin Infect Dis. 2021 Feb 20. [Epub ahead of print] https://doi.org/10.1093/cid/ciab154
15. Pan AP, Khan O, Meeks JR, et al. Disparities in COVID-19 hospitalizations and mortality among black and Hispanic patients: cross-sectional analysis from the greater Houston metropolitan area. BMC Public Health. 2021;21(1):1330. https://doi.org/10.1186/s12889-021-11431-2
16. Ogedegbe G, Ravenell J, Adhikari S, et al. Assessment of racial/ethnic disparities in hospitalization and mortality in patients with COVID-19 in New York City. JAMA Netw Open. 2020;3(12):e2026881. https://doi.org/10.1001/jamanetworkopen.2020.26881
17. Gershengorn HB, Patel S, Shukla B, et al. Association of race and ethnicity with COVID-19 test positivity and hospitalization is mediated by socioeconomic factors. Ann Am Thorac Soc. 2021;18(8):1326-1334. https://doi.org/10.1513/AnnalsATS.202011-1448OC
18. Sze S, Pan D, Nevill CR, et al. Ethnicity and clinical outcomes in COVID-19: a systematic review and meta-analysis. EClinicalMedicine. 2020;29:100630. https://doi.org/10.1016/j.eclinm.2020.100630
19. U.S. Census Bureau. 2019 U.S Census Bureau American Community Survey. https://www.census.gov/programs-surveys/acs
20. North Texas Mass Critical Care Task Force. North Texas Mass Critical Care Guidelines Document. Hospital and ICU Triage Guidelines for ADULTS. January 2014. https://www.dallas-cms.org/tmaimis/dcms/assets/files/communityhealth/MCC/GuidelinesAdult_JAN2014.pdf
21. Singh K, Valley TS, Tang S, et al. Evaluating a widely implemented proprietary deterioration index model among hospitalized COVID-19 patients. Ann Am Thorac Soc. 2021;18(7):1129-1137. https://doi.org/10.1513/AnnalsATS.202006-698OC
22. Keller MJ, Kitsis EA, Arora S, et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;15(8):489-493. https://doi.org/10.12788/jhm.3497
23. Centers for Disease Control and Prevention. Science Brief: Evidence used to update the list of underlying medical conditions that increase a person’s risk of severe illness from COVID-19. Updated May 12, 2021. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/underlying-evidence-table.html
24. Gupta S, Wang W, Hayek SS, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2021;181(1):41-51. https://doi.org/10.1001/jamainternmed.2020.6252
25. Baroutjian A, Sanchez C, Boneva D, McKenney M, Elkbuli A. SARS-CoV-2 pharmacologic therapies and their safety/effectiveness according to level of evidence. Am J Emerg Med. 2020;38(11):2405-2415. https://doi.org/10.1016/j.ajem.2020.08.091
26. Janiaud P, Axfors C, Schmitt AM, et al. Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. JAMA. 2021;325(12):1185-1195. https://doi.org/10.1001/jama.2021.2747
27. Panagiotou OA, Kosar CM, White EM, et al. Risk factors associated with all-cause 30-day mortality in nursing home residents with COVID-19. JAMA Intern Med. 2021;181(4):439-448. https://doi.org/10.1001/jamainternmed.2020.7968
28. Bacong AM, Menjívar C. Recasting the immigrant health paradox through intersections of legal status and race. J Immigr Minor Health. 2021;23(5):1092-1104. https://doi.org/10.1007/s10903-021-01162-2
29. Plopper GE, Sciarretta KL, Buchman TG. Disparities in sepsis outcomes may be attributable to access to care. Crit Care Med. 2021;49(8):1358-1360. https://doi.org/10.1097/CCM.0000000000005126
30. Jones JM, Fingar KR, Miller MA, et al. Racial disparities in sepsis-related in-hospital mortality: using a broad case capture method and multivariate controls for clinical and hospital variables, 2004-2013. Crit Care Med. 2017;45(12):e1209-e1217. https://doi.org/10.1097/CCM.0000000000002699
31. Jacobs ZG, Prasad PA, Fang MC, Abe-Jones Y, Kangelaris KN. The association between limited English proficiency and sepsis mortality. J Hosp Med. 2019;14:E1-E7. https://doi.org/10.12788/jhm.3334
32. Cohn D. Census considers new approach to asking about race – by not using the term at all. June 18, 2015. https://www.pewresearch.org/fact-tank/2015/06/18/census-considers-new-approach-to-asking-about-race-by-not-using-the-term-at-all/
© 2021 Society of Hospital Medicine
Clinical Progress Note: Consolidated Guidelines on Management of Coagulopathy and Antithrombotic Agents for Common Bedside Procedures
The practice of internal medicine includes bedside procedures such as paracentesis, thoracentesis, and lumbar puncture (LP). The American Board of Internal Medicine requires graduates of internal medicine residency programs to be competent in the cognitive components of procedural training (eg, indications, contraindications, complications) and considers it essential that trainees have opportunities to perform procedures relevant to their intended career direction.1 Whether or not the performance of procedures is part of a given hospitalist’s practice, it is necessary that hospitalists understand each procedure’s risks and mitigation strategies to prevent a range of periprocedural complications, including clinically significant bleeding. Numerous recommendations and guidelines exist describing bleeding risk for common procedures. In this Progress Note, we summarize and consolidate this literature, covering a range of scenarios common to the hospital setting, including thrombocytopenia, elevated international normalized ratio (INR), and the use of medications such as antiplatelet and anticoagulant agents (Table 1 and Table 2). We performed electronic searches in PubMed, focusing on literature published since 2016. Key search terms included paracentesis, thoracentesis, lumbar puncture, anticoagulant, antiplatelet, coagulopathy, INR, thrombocytopenia, and guideline. In addition, we used the following MeSH terms: spinal puncture AND blood coagulation disorders, spinal puncture AND platelet aggregation inhibitors, spinal puncture AND anticoagulants, paracentesis AND blood coagulation disorders, paracentesis AND platelet aggregation inhibitors, paracentesis AND anticoagulants, thoracentesis AND blood coagulation disorders, thoracentesis AND platelet aggregation inhibitors, and thoracentesis AND anticoagulants.

GENERAL CONCEPTS
Weighing Risks and Benefits

Hepatic and Renal Dysfunction
In the setting of chronic liver disease, thrombocytopenia and elevated INR are generally not reliable indicators of bleeding risk.13 The included recommendations for INR and platelet count thresholds in the setting of chronic liver disease are derived from the referenced guidelines and supplemental personal communication with the guideline authors. Many antiplatelet and anticoagulant medications are partially cleared or metabolized by the liver, suggesting that hepatic dysfunction may impact drug clearance, but this has not been well studied. Impaired renal function should also be considered when determining appropriate hold times for antithrombotic drugs that are partially renally cleared. The periprocedural hold and restart times outlined in Table 2 are specific to patients without clinically significant hepatic or renal dysfunction. For patients with these conditions, further information on hold time adjustment can be found in the individual references.

Bridging Therapy
Resuming Therapy
Other Considerations
Some guidelines referenced in this article are based on data collected on procedures performed by interventional radiologists, which may or may not accurately reflect the bleeding risks of bedside procedures performed by hospitalists. In the case of LP, we included some regional anesthesia and pain procedure guidelines based on the assumption that certain procedures are analogous to LP and associated with similar bleeding risks.
PARACENTESIS
Paracentesis is a common procedure that can be performed safely at the bedside. The overall rate of serious complications is low (1%-2%), with severe hemorrhage accounting for the majority of those complications (0.97%).15 Bleeding usually occurs from puncture of an abdominal wall vein, a mesenteric varix, or an inferior epigastric artery. Certain techniques may help to mitigate serious bleeding, including the use of ultrasound to avoid overlying vessels. Paracentesis is frequently performed in patients with cirrhosis, a population at increased risk for coagulopathy, although INR and platelet counts may not reflect aggregate bleeding risk in patients with cirrhosis. The American Association for the Study of Liver Diseases released new guidelines in 2021, stating that elevated prothrombin time or thrombocytopenia is not a contraindication to paracentesis.6 The most liberal guidelines for patients without chronic liver disease suggest correcting to an INR of 2.0 to 3.0, with multiple societies suggesting that a platelet count as low as 20,000/µL is safe.2,3 As shown in Table 2, most guidelines recommend continuation of antiplatelet agents such as aspirin and thienopyridines (eg, clopidogrel, prasugrel), whereas recommendations vary regarding continuation of anticoagulant agents.
THORACENTESIS
Akin to paracentesis, thoracentesis is generally considered to be a safe bedside procedure, with an incidence of thoracentesis-associated bleeding of less than 1%.15 Certain techniques may help to mitigate serious bleeding, including the insertion of the needle over the superior aspect of the rib in an effort to avoid the intercostal neurovascular bundle, which runs along the inferior aspect of each rib. Various clinical societies have proposed INR and platelet thresholds at which the risk of bleeding from thoracentesis is thought to be acceptable. The most liberal guidelines include a target INR of
LUMBAR PUNCTURE
Compared to thoracentesis and paracentesis, LP is generally considered to be a higher-risk procedure owing to the rare possibility of spinal hematoma with associated neurologic compromise. In one retrospective review of more than 49,000 patients without coagulopathy who underwent LP, the risk for developing a spinal hematoma by 30 days post procedure was 0.20%.16 Certain techniques may help to mitigate serious bleeding, including the use of image guidance in patients with large body habitus or those with difficult anatomy. Compared with paracentesis and thoracentesis, guideline recommendations for safe INR and platelet thresholds in patients undergoing LP are based on a more limited body of evidence. Guidelines also suggest a target INR of anywhere from ≤1.5 to the most liberal suggestion of 2.0 to 3.0.2-4 The SIR guidelines categorize LP as a low–bleeding risk procedure, with a platelet threshold of 20,000/µL but note that most other societies and guidelines regard LP as a high–bleeding risk procedure with more conservative platelet thresholds.2 The Association of British Neurologists (ABN), however, allows platelets to be 40,000/µL or greater than 20,000/µL with an additional risk-benefit discussion.7 In contrast to paracentesis and thoracentesis, recommendations regarding hold times of antithrombotic medications prior to LP are more variable and sometimes more conservative. For example, some guidelines indicate that the thienopyridines can be continued, whereas others recommend holding them for up to 1 week prior to LP.2,4,7
GAPS IN KNOWLEDGE
A theme throughout the recent literature and recommendations from clinical societies is that it is uncommon for there to be one unifying recommendation for every situation, especially regarding LP. Recent guidelines remain largely based on studies that are decades old. With bedside ultrasound becoming more accessible and established in daily practice, the risk of bleeding has been decreasing, potentially making periprocedural coagulopathies and antithrombotic agents less of a concern. For example, in a retrospective study of 69,859 paracenteses, ultrasound guidance reduced the risk of bleeding complications by 68%, an odds ratio of 0.32 (95% CI, 0.25-0.41).17 More research is needed to assess procedural bleeding risks in the context of current practice standards. This article focuses on a subset of bedside procedures most commonly performed by hospitalists. Similar references for other common bedside procedures, such as arthrocentesis, central venous catheter, and arterial line placement, would be helpful. Finally, this article does not capture such nuances as needle gauge, operator experience, availability of (and comfort with) ultrasound, and variations in patient anatomy, all of which are factors that can contribute to the complexities and risks of these bedside procedures.
CONCLUSION
Although not every internal medicine physician performs bedside procedures in their practice, it is vital that all understand the cognitive aspects of common bedside procedures. This necessitates the understanding of periprocedural risks and possible complications and applying that to individual patients. Correcting coagulopathy and stopping or reversing antithrombotic agents are mitigation strategies that are associated with risk. It is therefore important to understand when coagulopathy should be corrected and when antithrombotic agents should be held and for how long. With multiple existing and sometimes conflicting guidelines regarding periprocedural management of coagulopathy and antithrombotic agents, we hope that providing consolidated tables with this information will increase efficiency, aid in risk-benefit discussions between patients and care teams, and enhance patient safety.
1. Nichani S, Fitterman N, Lukela M, Crocker J. The core competencies in hospital medicine 2017 Revision. Section 2: procedures. J Hosp Med. 2017;12(4 Suppl 1):S44-S54. https://doi.org/10.12788/jhm.2728
2. Patel IJ, Rahim S, Davidson JC, et al. Society of Interventional Radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions-part ii: recommendations: endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol. 2019;30(8):1168-1184.e1. https://doi.org/10.1016/j.jvir.2019.04.017
3. Hadi M, Walker C, Desborough M, et al. CIRSE standards of practice on peri-operative anticoagulation management during interventional radiology procedures. Cardiovasc Intervent Radiol. 2021;44(4):523-536. https://doi.org/10.1007/s00270-020-02763-4
4. Özütemiz C, Rykken JB. Lumbar puncture under fluoroscopy guidance: a technical review for radiologists. Diagn Interv Radiol. 2019;25(2):144-156. https://doi.org/10.5152/dir.2019.18291
5. Demirci NY, Koksal D, Bilaceroglu S, et al. Management of bleeding risk before pleural procedures: a consensus statement of Turkish Respiratory Society—Pleura study group. Consensus Report. Eurasian J Pulmonol. 2020;22(2):73-78. https://doi.org/10.4103/ejop.ejop_28_20
6. Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74(2):1014-1048. https://doi.org/10.1002/hep.31884
7. Dodd KC, Emsley HCA, Desborough MJR, Chhetri SK. Periprocedural antithrombotic management for lumbar puncture: Association of British Neurologists clinical guideline. Pract Neurol. 2018;18(6):436-446. https://doi.org/10.1136/practneurol-2017-001820
8. Horlocker TT, Vandermeuelen E, Kopp SL, Gogarten W, Leffert LR, Benzon HT. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Fourth Edition). Reg Anesth Pain Med. 2018;43(3):263-309. https://doi.org/10.1097/aap.0000000000000763
9. Narouze S, Benzon HT, Provenzano D, et al. Interventional spine and pain procedures in patients on antiplatelet and anticoagulant medications (Second Edition): guidelines from the American Society of Regional Anesthesia and Pain Medicine, the European Society of Regional Anaesthesia and Pain Therapy, the American Academy of Pain Medicine, the International Neuromodulation Society, the North American Neuromodulation Society, and the World Institute of Pain. Reg Anesth Pain Med. 2018;43(3):225-262. https://doi.org/:10.1097/aap.0000000000000700
10. Andrade JG, Aguilar M, Atzema C, et al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society Comprehensive Guidelines for the Management of Atrial Fibrillation. Can J Cardiol. 2020;36(12):1847-1948. https://doi.org/10.1016/j.cjca.2020.09.001
11. Doherty JU, Gluckman TJ, Hucker WJ, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation: a report of the American College of Cardiology Clinical Expert Consensus Document Task Force. J Am Coll Cardiol. 2017;69(7):871-898. https://doi.org/10.1016/j.jacc.2016.11.024
12. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e326S-e350S. https://doi.org/10.1378/chest.11-2298
13. Crowe B, Tahhan SG, Lacy C, Grzankowski J, Lessing JN. Things we do for no reason™: Routine correction of elevated INR and thrombocytopenia prior to paracentesis in patients with cirrhosis. J Hosp Med. 2021;16(2):102-104. https://doi.org/10.12788/jhm.3458
14. Kuo HC, Liu FL, Chen JT, Cherng YG, Tam KW, Tai YH. Thromboembolic and bleeding risk of periprocedural bridging anticoagulation: a systematic review and meta-analysis. Clin Cardiol. 2020;43(5):441-449. https://doi.org/10.1002/clc.23336
15. Wolfe KS, Kress JP. Risk of procedural hemorrhage. Chest. 2016;150(1):237-246. https://doi.org/10.1016/j.chest.2016.01.023
16. Bodilsen J, Mariager T, Vestergaard HH, et al. Association of lumbar puncture with spinal hematoma in patients with and without coagulopathy. JAMA. 2020;324(14):1419-1428. https://doi.org/10.1001/jama.2020.14895
17. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. https://doi.org/10.1378/chest.12-0447
The practice of internal medicine includes bedside procedures such as paracentesis, thoracentesis, and lumbar puncture (LP). The American Board of Internal Medicine requires graduates of internal medicine residency programs to be competent in the cognitive components of procedural training (eg, indications, contraindications, complications) and considers it essential that trainees have opportunities to perform procedures relevant to their intended career direction.1 Whether or not the performance of procedures is part of a given hospitalist’s practice, it is necessary that hospitalists understand each procedure’s risks and mitigation strategies to prevent a range of periprocedural complications, including clinically significant bleeding. Numerous recommendations and guidelines exist describing bleeding risk for common procedures. In this Progress Note, we summarize and consolidate this literature, covering a range of scenarios common to the hospital setting, including thrombocytopenia, elevated international normalized ratio (INR), and the use of medications such as antiplatelet and anticoagulant agents (Table 1 and Table 2). We performed electronic searches in PubMed, focusing on literature published since 2016. Key search terms included paracentesis, thoracentesis, lumbar puncture, anticoagulant, antiplatelet, coagulopathy, INR, thrombocytopenia, and guideline. In addition, we used the following MeSH terms: spinal puncture AND blood coagulation disorders, spinal puncture AND platelet aggregation inhibitors, spinal puncture AND anticoagulants, paracentesis AND blood coagulation disorders, paracentesis AND platelet aggregation inhibitors, paracentesis AND anticoagulants, thoracentesis AND blood coagulation disorders, thoracentesis AND platelet aggregation inhibitors, and thoracentesis AND anticoagulants.

GENERAL CONCEPTS
Weighing Risks and Benefits

Hepatic and Renal Dysfunction
In the setting of chronic liver disease, thrombocytopenia and elevated INR are generally not reliable indicators of bleeding risk.13 The included recommendations for INR and platelet count thresholds in the setting of chronic liver disease are derived from the referenced guidelines and supplemental personal communication with the guideline authors. Many antiplatelet and anticoagulant medications are partially cleared or metabolized by the liver, suggesting that hepatic dysfunction may impact drug clearance, but this has not been well studied. Impaired renal function should also be considered when determining appropriate hold times for antithrombotic drugs that are partially renally cleared. The periprocedural hold and restart times outlined in Table 2 are specific to patients without clinically significant hepatic or renal dysfunction. For patients with these conditions, further information on hold time adjustment can be found in the individual references.

Bridging Therapy
Resuming Therapy
Other Considerations
Some guidelines referenced in this article are based on data collected on procedures performed by interventional radiologists, which may or may not accurately reflect the bleeding risks of bedside procedures performed by hospitalists. In the case of LP, we included some regional anesthesia and pain procedure guidelines based on the assumption that certain procedures are analogous to LP and associated with similar bleeding risks.
PARACENTESIS
Paracentesis is a common procedure that can be performed safely at the bedside. The overall rate of serious complications is low (1%-2%), with severe hemorrhage accounting for the majority of those complications (0.97%).15 Bleeding usually occurs from puncture of an abdominal wall vein, a mesenteric varix, or an inferior epigastric artery. Certain techniques may help to mitigate serious bleeding, including the use of ultrasound to avoid overlying vessels. Paracentesis is frequently performed in patients with cirrhosis, a population at increased risk for coagulopathy, although INR and platelet counts may not reflect aggregate bleeding risk in patients with cirrhosis. The American Association for the Study of Liver Diseases released new guidelines in 2021, stating that elevated prothrombin time or thrombocytopenia is not a contraindication to paracentesis.6 The most liberal guidelines for patients without chronic liver disease suggest correcting to an INR of 2.0 to 3.0, with multiple societies suggesting that a platelet count as low as 20,000/µL is safe.2,3 As shown in Table 2, most guidelines recommend continuation of antiplatelet agents such as aspirin and thienopyridines (eg, clopidogrel, prasugrel), whereas recommendations vary regarding continuation of anticoagulant agents.
THORACENTESIS
Akin to paracentesis, thoracentesis is generally considered to be a safe bedside procedure, with an incidence of thoracentesis-associated bleeding of less than 1%.15 Certain techniques may help to mitigate serious bleeding, including the insertion of the needle over the superior aspect of the rib in an effort to avoid the intercostal neurovascular bundle, which runs along the inferior aspect of each rib. Various clinical societies have proposed INR and platelet thresholds at which the risk of bleeding from thoracentesis is thought to be acceptable. The most liberal guidelines include a target INR of
LUMBAR PUNCTURE
Compared to thoracentesis and paracentesis, LP is generally considered to be a higher-risk procedure owing to the rare possibility of spinal hematoma with associated neurologic compromise. In one retrospective review of more than 49,000 patients without coagulopathy who underwent LP, the risk for developing a spinal hematoma by 30 days post procedure was 0.20%.16 Certain techniques may help to mitigate serious bleeding, including the use of image guidance in patients with large body habitus or those with difficult anatomy. Compared with paracentesis and thoracentesis, guideline recommendations for safe INR and platelet thresholds in patients undergoing LP are based on a more limited body of evidence. Guidelines also suggest a target INR of anywhere from ≤1.5 to the most liberal suggestion of 2.0 to 3.0.2-4 The SIR guidelines categorize LP as a low–bleeding risk procedure, with a platelet threshold of 20,000/µL but note that most other societies and guidelines regard LP as a high–bleeding risk procedure with more conservative platelet thresholds.2 The Association of British Neurologists (ABN), however, allows platelets to be 40,000/µL or greater than 20,000/µL with an additional risk-benefit discussion.7 In contrast to paracentesis and thoracentesis, recommendations regarding hold times of antithrombotic medications prior to LP are more variable and sometimes more conservative. For example, some guidelines indicate that the thienopyridines can be continued, whereas others recommend holding them for up to 1 week prior to LP.2,4,7
GAPS IN KNOWLEDGE
A theme throughout the recent literature and recommendations from clinical societies is that it is uncommon for there to be one unifying recommendation for every situation, especially regarding LP. Recent guidelines remain largely based on studies that are decades old. With bedside ultrasound becoming more accessible and established in daily practice, the risk of bleeding has been decreasing, potentially making periprocedural coagulopathies and antithrombotic agents less of a concern. For example, in a retrospective study of 69,859 paracenteses, ultrasound guidance reduced the risk of bleeding complications by 68%, an odds ratio of 0.32 (95% CI, 0.25-0.41).17 More research is needed to assess procedural bleeding risks in the context of current practice standards. This article focuses on a subset of bedside procedures most commonly performed by hospitalists. Similar references for other common bedside procedures, such as arthrocentesis, central venous catheter, and arterial line placement, would be helpful. Finally, this article does not capture such nuances as needle gauge, operator experience, availability of (and comfort with) ultrasound, and variations in patient anatomy, all of which are factors that can contribute to the complexities and risks of these bedside procedures.
CONCLUSION
Although not every internal medicine physician performs bedside procedures in their practice, it is vital that all understand the cognitive aspects of common bedside procedures. This necessitates the understanding of periprocedural risks and possible complications and applying that to individual patients. Correcting coagulopathy and stopping or reversing antithrombotic agents are mitigation strategies that are associated with risk. It is therefore important to understand when coagulopathy should be corrected and when antithrombotic agents should be held and for how long. With multiple existing and sometimes conflicting guidelines regarding periprocedural management of coagulopathy and antithrombotic agents, we hope that providing consolidated tables with this information will increase efficiency, aid in risk-benefit discussions between patients and care teams, and enhance patient safety.
The practice of internal medicine includes bedside procedures such as paracentesis, thoracentesis, and lumbar puncture (LP). The American Board of Internal Medicine requires graduates of internal medicine residency programs to be competent in the cognitive components of procedural training (eg, indications, contraindications, complications) and considers it essential that trainees have opportunities to perform procedures relevant to their intended career direction.1 Whether or not the performance of procedures is part of a given hospitalist’s practice, it is necessary that hospitalists understand each procedure’s risks and mitigation strategies to prevent a range of periprocedural complications, including clinically significant bleeding. Numerous recommendations and guidelines exist describing bleeding risk for common procedures. In this Progress Note, we summarize and consolidate this literature, covering a range of scenarios common to the hospital setting, including thrombocytopenia, elevated international normalized ratio (INR), and the use of medications such as antiplatelet and anticoagulant agents (Table 1 and Table 2). We performed electronic searches in PubMed, focusing on literature published since 2016. Key search terms included paracentesis, thoracentesis, lumbar puncture, anticoagulant, antiplatelet, coagulopathy, INR, thrombocytopenia, and guideline. In addition, we used the following MeSH terms: spinal puncture AND blood coagulation disorders, spinal puncture AND platelet aggregation inhibitors, spinal puncture AND anticoagulants, paracentesis AND blood coagulation disorders, paracentesis AND platelet aggregation inhibitors, paracentesis AND anticoagulants, thoracentesis AND blood coagulation disorders, thoracentesis AND platelet aggregation inhibitors, and thoracentesis AND anticoagulants.

GENERAL CONCEPTS
Weighing Risks and Benefits

Hepatic and Renal Dysfunction
In the setting of chronic liver disease, thrombocytopenia and elevated INR are generally not reliable indicators of bleeding risk.13 The included recommendations for INR and platelet count thresholds in the setting of chronic liver disease are derived from the referenced guidelines and supplemental personal communication with the guideline authors. Many antiplatelet and anticoagulant medications are partially cleared or metabolized by the liver, suggesting that hepatic dysfunction may impact drug clearance, but this has not been well studied. Impaired renal function should also be considered when determining appropriate hold times for antithrombotic drugs that are partially renally cleared. The periprocedural hold and restart times outlined in Table 2 are specific to patients without clinically significant hepatic or renal dysfunction. For patients with these conditions, further information on hold time adjustment can be found in the individual references.

Bridging Therapy
Resuming Therapy
Other Considerations
Some guidelines referenced in this article are based on data collected on procedures performed by interventional radiologists, which may or may not accurately reflect the bleeding risks of bedside procedures performed by hospitalists. In the case of LP, we included some regional anesthesia and pain procedure guidelines based on the assumption that certain procedures are analogous to LP and associated with similar bleeding risks.
PARACENTESIS
Paracentesis is a common procedure that can be performed safely at the bedside. The overall rate of serious complications is low (1%-2%), with severe hemorrhage accounting for the majority of those complications (0.97%).15 Bleeding usually occurs from puncture of an abdominal wall vein, a mesenteric varix, or an inferior epigastric artery. Certain techniques may help to mitigate serious bleeding, including the use of ultrasound to avoid overlying vessels. Paracentesis is frequently performed in patients with cirrhosis, a population at increased risk for coagulopathy, although INR and platelet counts may not reflect aggregate bleeding risk in patients with cirrhosis. The American Association for the Study of Liver Diseases released new guidelines in 2021, stating that elevated prothrombin time or thrombocytopenia is not a contraindication to paracentesis.6 The most liberal guidelines for patients without chronic liver disease suggest correcting to an INR of 2.0 to 3.0, with multiple societies suggesting that a platelet count as low as 20,000/µL is safe.2,3 As shown in Table 2, most guidelines recommend continuation of antiplatelet agents such as aspirin and thienopyridines (eg, clopidogrel, prasugrel), whereas recommendations vary regarding continuation of anticoagulant agents.
THORACENTESIS
Akin to paracentesis, thoracentesis is generally considered to be a safe bedside procedure, with an incidence of thoracentesis-associated bleeding of less than 1%.15 Certain techniques may help to mitigate serious bleeding, including the insertion of the needle over the superior aspect of the rib in an effort to avoid the intercostal neurovascular bundle, which runs along the inferior aspect of each rib. Various clinical societies have proposed INR and platelet thresholds at which the risk of bleeding from thoracentesis is thought to be acceptable. The most liberal guidelines include a target INR of
LUMBAR PUNCTURE
Compared to thoracentesis and paracentesis, LP is generally considered to be a higher-risk procedure owing to the rare possibility of spinal hematoma with associated neurologic compromise. In one retrospective review of more than 49,000 patients without coagulopathy who underwent LP, the risk for developing a spinal hematoma by 30 days post procedure was 0.20%.16 Certain techniques may help to mitigate serious bleeding, including the use of image guidance in patients with large body habitus or those with difficult anatomy. Compared with paracentesis and thoracentesis, guideline recommendations for safe INR and platelet thresholds in patients undergoing LP are based on a more limited body of evidence. Guidelines also suggest a target INR of anywhere from ≤1.5 to the most liberal suggestion of 2.0 to 3.0.2-4 The SIR guidelines categorize LP as a low–bleeding risk procedure, with a platelet threshold of 20,000/µL but note that most other societies and guidelines regard LP as a high–bleeding risk procedure with more conservative platelet thresholds.2 The Association of British Neurologists (ABN), however, allows platelets to be 40,000/µL or greater than 20,000/µL with an additional risk-benefit discussion.7 In contrast to paracentesis and thoracentesis, recommendations regarding hold times of antithrombotic medications prior to LP are more variable and sometimes more conservative. For example, some guidelines indicate that the thienopyridines can be continued, whereas others recommend holding them for up to 1 week prior to LP.2,4,7
GAPS IN KNOWLEDGE
A theme throughout the recent literature and recommendations from clinical societies is that it is uncommon for there to be one unifying recommendation for every situation, especially regarding LP. Recent guidelines remain largely based on studies that are decades old. With bedside ultrasound becoming more accessible and established in daily practice, the risk of bleeding has been decreasing, potentially making periprocedural coagulopathies and antithrombotic agents less of a concern. For example, in a retrospective study of 69,859 paracenteses, ultrasound guidance reduced the risk of bleeding complications by 68%, an odds ratio of 0.32 (95% CI, 0.25-0.41).17 More research is needed to assess procedural bleeding risks in the context of current practice standards. This article focuses on a subset of bedside procedures most commonly performed by hospitalists. Similar references for other common bedside procedures, such as arthrocentesis, central venous catheter, and arterial line placement, would be helpful. Finally, this article does not capture such nuances as needle gauge, operator experience, availability of (and comfort with) ultrasound, and variations in patient anatomy, all of which are factors that can contribute to the complexities and risks of these bedside procedures.
CONCLUSION
Although not every internal medicine physician performs bedside procedures in their practice, it is vital that all understand the cognitive aspects of common bedside procedures. This necessitates the understanding of periprocedural risks and possible complications and applying that to individual patients. Correcting coagulopathy and stopping or reversing antithrombotic agents are mitigation strategies that are associated with risk. It is therefore important to understand when coagulopathy should be corrected and when antithrombotic agents should be held and for how long. With multiple existing and sometimes conflicting guidelines regarding periprocedural management of coagulopathy and antithrombotic agents, we hope that providing consolidated tables with this information will increase efficiency, aid in risk-benefit discussions between patients and care teams, and enhance patient safety.
1. Nichani S, Fitterman N, Lukela M, Crocker J. The core competencies in hospital medicine 2017 Revision. Section 2: procedures. J Hosp Med. 2017;12(4 Suppl 1):S44-S54. https://doi.org/10.12788/jhm.2728
2. Patel IJ, Rahim S, Davidson JC, et al. Society of Interventional Radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions-part ii: recommendations: endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol. 2019;30(8):1168-1184.e1. https://doi.org/10.1016/j.jvir.2019.04.017
3. Hadi M, Walker C, Desborough M, et al. CIRSE standards of practice on peri-operative anticoagulation management during interventional radiology procedures. Cardiovasc Intervent Radiol. 2021;44(4):523-536. https://doi.org/10.1007/s00270-020-02763-4
4. Özütemiz C, Rykken JB. Lumbar puncture under fluoroscopy guidance: a technical review for radiologists. Diagn Interv Radiol. 2019;25(2):144-156. https://doi.org/10.5152/dir.2019.18291
5. Demirci NY, Koksal D, Bilaceroglu S, et al. Management of bleeding risk before pleural procedures: a consensus statement of Turkish Respiratory Society—Pleura study group. Consensus Report. Eurasian J Pulmonol. 2020;22(2):73-78. https://doi.org/10.4103/ejop.ejop_28_20
6. Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74(2):1014-1048. https://doi.org/10.1002/hep.31884
7. Dodd KC, Emsley HCA, Desborough MJR, Chhetri SK. Periprocedural antithrombotic management for lumbar puncture: Association of British Neurologists clinical guideline. Pract Neurol. 2018;18(6):436-446. https://doi.org/10.1136/practneurol-2017-001820
8. Horlocker TT, Vandermeuelen E, Kopp SL, Gogarten W, Leffert LR, Benzon HT. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Fourth Edition). Reg Anesth Pain Med. 2018;43(3):263-309. https://doi.org/10.1097/aap.0000000000000763
9. Narouze S, Benzon HT, Provenzano D, et al. Interventional spine and pain procedures in patients on antiplatelet and anticoagulant medications (Second Edition): guidelines from the American Society of Regional Anesthesia and Pain Medicine, the European Society of Regional Anaesthesia and Pain Therapy, the American Academy of Pain Medicine, the International Neuromodulation Society, the North American Neuromodulation Society, and the World Institute of Pain. Reg Anesth Pain Med. 2018;43(3):225-262. https://doi.org/:10.1097/aap.0000000000000700
10. Andrade JG, Aguilar M, Atzema C, et al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society Comprehensive Guidelines for the Management of Atrial Fibrillation. Can J Cardiol. 2020;36(12):1847-1948. https://doi.org/10.1016/j.cjca.2020.09.001
11. Doherty JU, Gluckman TJ, Hucker WJ, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation: a report of the American College of Cardiology Clinical Expert Consensus Document Task Force. J Am Coll Cardiol. 2017;69(7):871-898. https://doi.org/10.1016/j.jacc.2016.11.024
12. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e326S-e350S. https://doi.org/10.1378/chest.11-2298
13. Crowe B, Tahhan SG, Lacy C, Grzankowski J, Lessing JN. Things we do for no reason™: Routine correction of elevated INR and thrombocytopenia prior to paracentesis in patients with cirrhosis. J Hosp Med. 2021;16(2):102-104. https://doi.org/10.12788/jhm.3458
14. Kuo HC, Liu FL, Chen JT, Cherng YG, Tam KW, Tai YH. Thromboembolic and bleeding risk of periprocedural bridging anticoagulation: a systematic review and meta-analysis. Clin Cardiol. 2020;43(5):441-449. https://doi.org/10.1002/clc.23336
15. Wolfe KS, Kress JP. Risk of procedural hemorrhage. Chest. 2016;150(1):237-246. https://doi.org/10.1016/j.chest.2016.01.023
16. Bodilsen J, Mariager T, Vestergaard HH, et al. Association of lumbar puncture with spinal hematoma in patients with and without coagulopathy. JAMA. 2020;324(14):1419-1428. https://doi.org/10.1001/jama.2020.14895
17. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. https://doi.org/10.1378/chest.12-0447
1. Nichani S, Fitterman N, Lukela M, Crocker J. The core competencies in hospital medicine 2017 Revision. Section 2: procedures. J Hosp Med. 2017;12(4 Suppl 1):S44-S54. https://doi.org/10.12788/jhm.2728
2. Patel IJ, Rahim S, Davidson JC, et al. Society of Interventional Radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions-part ii: recommendations: endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol. 2019;30(8):1168-1184.e1. https://doi.org/10.1016/j.jvir.2019.04.017
3. Hadi M, Walker C, Desborough M, et al. CIRSE standards of practice on peri-operative anticoagulation management during interventional radiology procedures. Cardiovasc Intervent Radiol. 2021;44(4):523-536. https://doi.org/10.1007/s00270-020-02763-4
4. Özütemiz C, Rykken JB. Lumbar puncture under fluoroscopy guidance: a technical review for radiologists. Diagn Interv Radiol. 2019;25(2):144-156. https://doi.org/10.5152/dir.2019.18291
5. Demirci NY, Koksal D, Bilaceroglu S, et al. Management of bleeding risk before pleural procedures: a consensus statement of Turkish Respiratory Society—Pleura study group. Consensus Report. Eurasian J Pulmonol. 2020;22(2):73-78. https://doi.org/10.4103/ejop.ejop_28_20
6. Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74(2):1014-1048. https://doi.org/10.1002/hep.31884
7. Dodd KC, Emsley HCA, Desborough MJR, Chhetri SK. Periprocedural antithrombotic management for lumbar puncture: Association of British Neurologists clinical guideline. Pract Neurol. 2018;18(6):436-446. https://doi.org/10.1136/practneurol-2017-001820
8. Horlocker TT, Vandermeuelen E, Kopp SL, Gogarten W, Leffert LR, Benzon HT. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Fourth Edition). Reg Anesth Pain Med. 2018;43(3):263-309. https://doi.org/10.1097/aap.0000000000000763
9. Narouze S, Benzon HT, Provenzano D, et al. Interventional spine and pain procedures in patients on antiplatelet and anticoagulant medications (Second Edition): guidelines from the American Society of Regional Anesthesia and Pain Medicine, the European Society of Regional Anaesthesia and Pain Therapy, the American Academy of Pain Medicine, the International Neuromodulation Society, the North American Neuromodulation Society, and the World Institute of Pain. Reg Anesth Pain Med. 2018;43(3):225-262. https://doi.org/:10.1097/aap.0000000000000700
10. Andrade JG, Aguilar M, Atzema C, et al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society Comprehensive Guidelines for the Management of Atrial Fibrillation. Can J Cardiol. 2020;36(12):1847-1948. https://doi.org/10.1016/j.cjca.2020.09.001
11. Doherty JU, Gluckman TJ, Hucker WJ, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation: a report of the American College of Cardiology Clinical Expert Consensus Document Task Force. J Am Coll Cardiol. 2017;69(7):871-898. https://doi.org/10.1016/j.jacc.2016.11.024
12. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e326S-e350S. https://doi.org/10.1378/chest.11-2298
13. Crowe B, Tahhan SG, Lacy C, Grzankowski J, Lessing JN. Things we do for no reason™: Routine correction of elevated INR and thrombocytopenia prior to paracentesis in patients with cirrhosis. J Hosp Med. 2021;16(2):102-104. https://doi.org/10.12788/jhm.3458
14. Kuo HC, Liu FL, Chen JT, Cherng YG, Tam KW, Tai YH. Thromboembolic and bleeding risk of periprocedural bridging anticoagulation: a systematic review and meta-analysis. Clin Cardiol. 2020;43(5):441-449. https://doi.org/10.1002/clc.23336
15. Wolfe KS, Kress JP. Risk of procedural hemorrhage. Chest. 2016;150(1):237-246. https://doi.org/10.1016/j.chest.2016.01.023
16. Bodilsen J, Mariager T, Vestergaard HH, et al. Association of lumbar puncture with spinal hematoma in patients with and without coagulopathy. JAMA. 2020;324(14):1419-1428. https://doi.org/10.1001/jama.2020.14895
17. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. https://doi.org/10.1378/chest.12-0447
© 2021 Society of Hospital Medicine
Improving Healthcare Value: Reducing Overuse in Hospital Pediatrics
Most hospital pediatricians can recall cases where an abnormal result in one unnecessary test led to a cascade of multiple further unnecessary treatments, procedures, and tests. These cases are well described in the literature and written off as a side effect of delivering high-quality, comprehensive pediatric care.1 Unfortunately, however, these frequent events are not without consequence and can cause significant harm to patients, as well as stress and fear for parents and families, and indirectly waste valuable resources.
As we look forward to recovering from the COVID-19 pandemic, there are calls to prioritize high-value and more equitable care in the postpandemic world.2 Choosing Wisely is a global movement comprised of clinician-led campaigns that partner with national specialty societies to develop lists of evidence-based recommendations of tests, treatments, and procedures that offer no added clinical value and may cause harm.3
In pediatrics, there is a growing recognition and published literature on the harms of overdiagnosis and unnecessary care in children.4-6 Choosing Wisely recommendations are being used as a resource to drive healthcare prioritization and ensure low-value care is avoided so that greater focus can be placed on areas of need exacerbated by the pandemic. Using a Choosing Wisely perspective can drive quality and help inform a shift in practice, creating a roadmap for reducing testing or treatment cascades that harm patients and waste resources as we move toward the goal of high-value pediatric care. However, adoption of Choosing Wisely recommendations in pediatrics has been slow. For example, the pediatric working group of the Society of Hospital Medicine released a Choosing Wisely® recommendation in 2013 against the use of continuous pulse oximetry monitoring in children with acute respiratory illness who are not on supplementary oxygen.7 Data from a cross-sectional study across 56 hospitals 6 years later found significant variation in this practice for infants hospitalized with bronchiolitis and not receiving supplemental oxygen; 46% were continuously monitored with pulse oximetry (range, 2%-92%).8
WHY HAS CHOOSING WISELY LAGGED IN PEDIATRICS?
Traditionally, attention in children’s healthcare has focused on underuse (eg, immunizations or mental health) rather than overuse. Further, the weakness of the evidence base, with very few randomized controlled trials in children, limits our ability to provide sufficient confidence in the evidence supporting some of our recommendations.9
Second, there is also tremendous anxiety for both parents and frontline clinicians around diagnostic uncertainty of any kind when it comes to children. We endeavour to reassure ourselves and patients’ families by leaving no stone unturned. This approach can lead to unnecessary care, including false-positive test results, “incidentalomas,” and adverse effects from unnecessary medications. Despite the best intentions of assuaging caregivers’ anxiety, overuse of invasive and uncomfortable tests can have the opposite effect of increasing stress and trauma for both children and parents.
Third, there is compelling evidence that practice habits, once established, are difficult to break.10 Particularly in the high-stakes practice of hospital pediatric medicine, where we are conditioned to expect the worst and anticipate the unexpected. This “do everything to everyone” approach, however, can lead to significant harms for pediatric patients. For example, the exposure to ionizing radiation through unnecessary computed tomography (CT) scans can increase a child’s lifetime cancer risk.11
The perpetuation of unnecessary care needs to change in pediatrics, especially for the most vulnerable young patients seeking hospital care. Implementation is a necessary next step to introduce recommendations into practice, and the Choosing Wisely efforts of the Hospital for Sick Children in Toronto, Canada, can offer insights into opportunities to embed this approach across similar quaternary care teaching hospitals, as well as general hospitals and the systems they support.
STEPS TO IMPLEMENTING CHOOSING WISELY HOSPITAL-WIDE
Creating Lists of Recommendations Aligned With Quality Metrics
The Hospital for Sick Children developed a hospital-specific Choosing Wisely list in 2016 to address a gap in existing Choosing Wisely Canada campaign recommendations related to pediatric hospitals.12 Choosing Wisely Canada was initially focused on adult medicine, and a list of recommendations developed by the Canadian Paediatric Society relates mostly to overuse in pediatric outpatient settings and is not applicable to hospital-based practice.13 The Society of Hospital Medicine-Pediatric Hospital Medicine Choosing Wisely® list predominantly pertained to unnecessary care of infants with bronchiolitis (eg, not to order chest radiographs in uncomplicated asthma and bronchiolitis). We had measured our compliance with this recommendation and found it was already well below the achievable benchmark of care in the United States,14 so we preferred to create a list that would resonate with our clinicians. Since the original list was created at the Hospital for Sick Children,12 we have developed two subsequent lists of recommendations, which were released in 2018 and 2021 (Table).
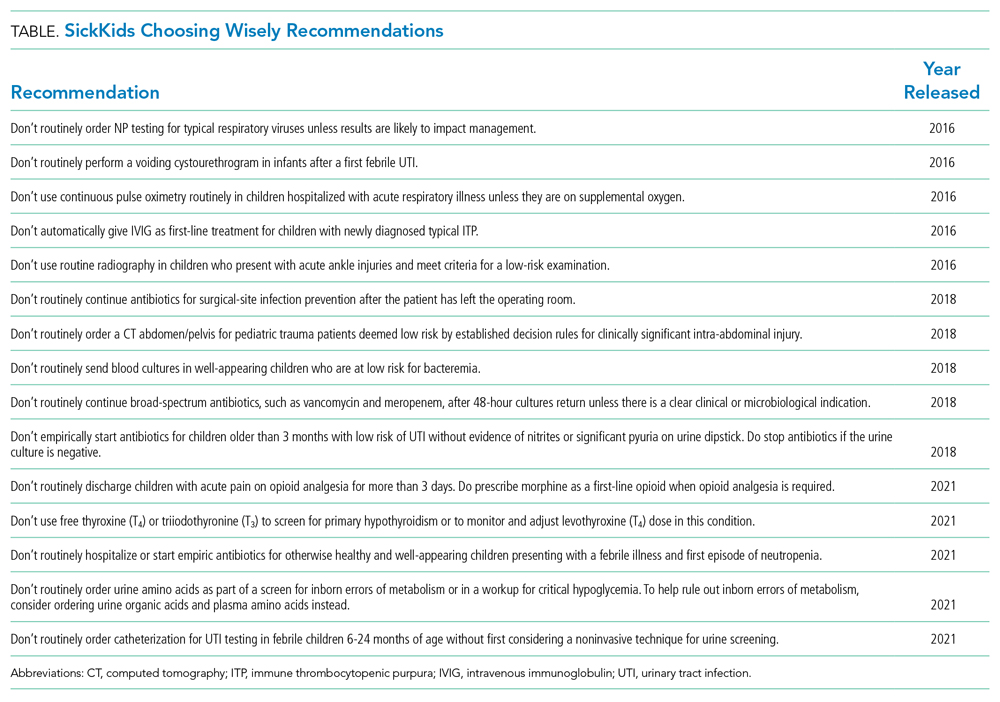
The approach to list development used by staff pediatricians and trainees, with input from hospital staff and family advisors, has been described elsewhere.12 The goal was to self-identify five local practices that we felt would help us reduce unnecessary care. This list served as the foundation of an organization-wide quality initiative driven by a steering committee that consisted of the clinician champions as well as representation from various groups at the hospital, including decision support, information services, the family advisory committee, and public affairs.
Each recommendation needed to be evidence-based and measurable, have a clinician champion to implement the recommendation, and have the potential to improve the quality and safety of the care we provided. “Balancing” measures needed to be carefully monitored to ensure that no diagnoses were being missed or negative effects resulted from decreasing these interventions. In order for a recommendation to be considered, a subgroup of the pediatric department’s clinical advisory committee reviewed the references provided to ensure that what was being suggested was based on published evidence and part of current national guidelines. The clinician champion needed to agree to lead the implementation project, and specific outcomes, including appropriate balancing measures, needed to be identified a priori, in addition to an appropriate mechanism to collect the data. Hospital executive leaders were supportive of the initiative and facilitated access to “in-kind” hospital resources as required, although no financial budget was provided. After some early success, the Department of Paediatrics provided part-time project management support to help coordinate the growth and administration of the initiative.
Measuring and Supporting Practice Change
The main implementation principles included targeted education/awareness, transparent measurement with audit/feedback, and, most importantly, embedding changes in the ordering process, essentially making it easy for frontline clinicians to do the right thing (and trickier to do the “wrong” thing). Audit and feedback have been used at both the individual provider level (eg, respiratory viral testing–ordering practices) and the divisional level (eg, ordering of postoperative antibiotics). These quality improvement initiatives have had a compelling impact. Scorecards have been developed and results shared internally using local divisional as well as hospital-wide tools, varying from staff meetings to screensavers across hospital computers and television screens and the hospital intranet. Evaluation is ongoing, but many of the initial results have been encouraging.15-17
For example, the 2016 list includes recommendations related to emergency department (ED) test ordering. Implementation efforts to address unnecessary nasopharyngeal swabs for viral testing in bronchiolitis reduced this practice by 80%,15 and there has been a 50% reduction in ankle X-rays in children with acute ankle injuries who meet criteria for a low-risk examination.16 The 2016 list also included a recommendation related to inappropriate intravenous immunoglobulin (IVIG) use in children with typical acute immune thrombocytopenic purpura (ITP), and a targeted quality improvement initiative reduced inappropriate IVIG use by 50%, with no detectable increase in bleeding complications or readmission to hospital.17 These results have been sustained over a period of 3 or more years. Examples from the 2018 list include a 40% decrease in inappropriate urinary tract infection diagnosis and treatment in the ED and a four-fold decrease in the CT abdomen/pelvis imaging rate for low-risk trauma.18
The steering committee meets every 2 months and includes all of the clinician champions as well as representatives from strategic hospital resources and two family advisors (NGS). These meetings are chaired by the Associate Pediatrician-in-Chief (JNF) and the project manager. The progress of the active projects is discussed, and the experience of the group is used to problem-solve, plan ahead, and encourage academic presentation and publication of the various projects. Patient partnership and participation in committees has ensured that improvements to patient experience, satisfaction, and education are considered in the outcomes of implementation. Moreover, it has safeguarded that this effort is not misperceived as limiting care and remains focused on advancing quality, safety, and the patient experience.
SOME LESSONS LEARNED
While most projects have surpassed expectations, not all have proceeded as anticipated. The biggest challenge is finding a reliable and practical source for data collection. For example, at the time of initiation of the voiding cystourethrography (VCUG) recommendation, practice had presumably changed over the recent years, and compliance already exceeded the goal, illustrating the importance of current accurate data. The oxygen saturation–monitoring recommendation highlighted the challenge presented by data collection that requires manual audits; the inability to find staff to do this regularly significantly hampered this project. The critical role of the clinician champion was highlighted in a few projects when a lead was absent for a prolonged period of time (eg, due to a parental leave or change in job), with no willing replacement. There does seem to be a strong correlation between the commitment and passion of the clinician lead and the success of the project. We have incorporated the lessons learned into the development and rollout of the 2018 and 2021 lists.
SPREAD AND SCALE
The challenge is to scale up these successes to impact and change practice across the hospital pediatrics community. After 5 years, awareness of and engagement with this process are still not uniform across our hospital campus. Nevertheless, anecdotally, at the Hospital for Sick Children, there is a shift in culture where clinicians have processed the imperative to reduce overuse and unnecessary tests and treatments, with phrases such as “this is not very Choosing Wisely” entering the vernacular. It is becoming part of the culture. Second, the new generation of medical school trainees and residents has displayed a tremendous appetite and passion for stewardship and a sense that practice can change from the ground up. The SickKids Choosing Wisely efforts have been a hub for resident-led quality improvement projects and leadership for implementation of recommendations.19 As we continue to engage all providers at our hospital, we are also reaching out to the other community hospitals in our region, and all children’s hospitals in Canada, to share the principles and lessons learned from our program through a national community of practice.
CONCLUSION
Practicing pediatric medicine in a well-resourced hospital setting should not drive us to overuse in practice “just because we can.” The harms of this approach to our patients and health systems, coupled with the pressures of the pandemic, are compelling reasons to be responsible stewards. There are opportunities to reshape and rethink practice patterns and habits.20 Overuse and overdiagnosis harm our patients and families physically and emotionally and indirectly waste resources urgently needed for investment upstream. Providing safe, quality, high-value care to our young patients requires constant critical thinking. The time is here to advance Choosing Wisely into pediatric hospital practice.
1. Elliott DK, Rose SR, Ronan JC. Changing the culture around cultures. Hosp Pediatr. 2014;4(6):405-407. https://doi.org/10.1542/hpeds.2014-0064
2. Gupta R, Simpson LA, Morgan DJ. Prioritizing high-value, equitable care after the COVID-19 shutdown: an opportunity for a healthcare renaissance. J Hosp Med. 2021;16(2):114-116. https://doi.org/10.12788/jhm.3526
3. Born K, Kool T, Levinson W. Reducing overuse in healthcare: advancing Choosing Wisely. BMJ. 2019;367:l6317. https://doi.org/10.1136/bmj.l6317
4. Coon ER, Young PC, Quinonez RA, Morgan DJ, Dhruva SS, Schroeder AR. Update on pediatric overuse. Pediatrics. 2017;139(2):e20162797. https://doi.org/10.1542/peds.2016-2797
5. Coon ER, Quinonez RA, Moyer VA, Schroeder AR. Overdiagnosis: how our compulsion for diagnosis may be harming children. Pediatrics. 2014;134(5):1013-1023. https://doi.org/10.1542/peds.2014-1778
6. Wolf ER, Krist AH, Schroeder AR. Deimplementation in pediatrics: past, present, and future. JAMA Pediatr. 2021;175(3):230-232. https://doi.org/10.1001/jamapediatrics.2020.4681
7. Quinonez RA, Garber MD, Schroeder AR, et al. Choosing Wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):479-485. https://doi.org/10.1002/jhm.2064
8. Bonafide CP, Xiao R, Brady PW, et al. Prevalence of continuous pulse oximetry monitoring in hospitalized children with bronchiolitis not requiring supplemental oxygen. JAMA. 2020;323(15):1467-1477. https://doi.org/10.1001/jama.2020.2998
9. Ralston SL, Schroeder AR. Why is it so hard to talk about overuse in pediatrics and why it matters. JAMA Pediatr. 2017;17(10):931-932. https://doi.org/10.1001/jamapediatrics.2017.2239
10. Stammen LA, Stalmeijer RE, Paternotte E, et al. Training physicians to provide high-value, cost-conscious care: a systematic review. JAMA. 2015;314(22):2384-2400. https://doi.org/10.1001/jama.2015.16353
11. Mathews JD, Forsythe AV, Brady Z, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ. 2013;346:f2360. https://doi.org/10.1136/bmj.f2360
12. Friedman JN. Saying yes to the less: making it easier to choose wisely [editorial]. J Pediatr. 2017;145:4-5. https://doi.org/10.1016/j.jpeds.2017.01.062
13. Canadian Paediatric Society. Five things physicians and patients should question. Choosing Wisely Canada. Updated July 2019. Accessed June 17, 2021. https://choosingwiselycanada.org/wp-content/uploads/2020/07/Paediatrics_EN.pdf
14. Parikh K, Hall M, Montalbano A, et al. Establishing benchmarks for the hospitalized care of children with asthma, bronciolitis, and pneumonia. Pediatrics. 2014;134(3):555-562. https://doi.org/10.1542/peds.2014-1052
15. Ostrow O, Richardson S, Savlov D, Friedman JN. Reducing unnecessary respiratory viral testing to promote high value care. Pediatrics. In press.
16. Al-Sani F, Ben-Yakov M, Harvey G, et al. P016: Low risk ankle rule, high reward—a quality improvement initiative to reduce ankle x-rays in the pediatric emergency department [poster]. CJEM. 2017;19(S1):S83. https://doi.org/10.1017/cem.2017.218
17. Beck CE, Carcao M, Cada M, Porter S, Blanchette VS, Parkin PC. A quality improvement bundle to improve informed choice for children with typical, newly diagnosed immune thrombocytopenia. J Pediatr Hematol Oncol. 2018;40(8):e537-e543. https://doi.org/10.1097/MPH.0000000000001247
18. Beno S, Lenton-Brym T, Rosenfield D, McDowall D, Wales P, Principi T. Safe reduction of abdominal CT imaging in pediatric trauma patients: a quality-improvement initiative [abstract]. Can J Surg. 2019;62(3 Suppl 2):S29-S30.
19. Bal C, Tesch M, Blair G, Ostrow O, Premji L. Engaging medical trainees in resource stewardship through resident-led teaching sessions: a choosing wisely educational initiative. Can Med Educ J. 2021;12(1):e98-e100. https://doi.org/10.36834/cmej.70563
20. Berwick DM. Choices for the “new normal.” JAMA. 2020;323(21):2125-2126. https://doi.org/10.1001/jama.2020.6949
Most hospital pediatricians can recall cases where an abnormal result in one unnecessary test led to a cascade of multiple further unnecessary treatments, procedures, and tests. These cases are well described in the literature and written off as a side effect of delivering high-quality, comprehensive pediatric care.1 Unfortunately, however, these frequent events are not without consequence and can cause significant harm to patients, as well as stress and fear for parents and families, and indirectly waste valuable resources.
As we look forward to recovering from the COVID-19 pandemic, there are calls to prioritize high-value and more equitable care in the postpandemic world.2 Choosing Wisely is a global movement comprised of clinician-led campaigns that partner with national specialty societies to develop lists of evidence-based recommendations of tests, treatments, and procedures that offer no added clinical value and may cause harm.3
In pediatrics, there is a growing recognition and published literature on the harms of overdiagnosis and unnecessary care in children.4-6 Choosing Wisely recommendations are being used as a resource to drive healthcare prioritization and ensure low-value care is avoided so that greater focus can be placed on areas of need exacerbated by the pandemic. Using a Choosing Wisely perspective can drive quality and help inform a shift in practice, creating a roadmap for reducing testing or treatment cascades that harm patients and waste resources as we move toward the goal of high-value pediatric care. However, adoption of Choosing Wisely recommendations in pediatrics has been slow. For example, the pediatric working group of the Society of Hospital Medicine released a Choosing Wisely® recommendation in 2013 against the use of continuous pulse oximetry monitoring in children with acute respiratory illness who are not on supplementary oxygen.7 Data from a cross-sectional study across 56 hospitals 6 years later found significant variation in this practice for infants hospitalized with bronchiolitis and not receiving supplemental oxygen; 46% were continuously monitored with pulse oximetry (range, 2%-92%).8
WHY HAS CHOOSING WISELY LAGGED IN PEDIATRICS?
Traditionally, attention in children’s healthcare has focused on underuse (eg, immunizations or mental health) rather than overuse. Further, the weakness of the evidence base, with very few randomized controlled trials in children, limits our ability to provide sufficient confidence in the evidence supporting some of our recommendations.9
Second, there is also tremendous anxiety for both parents and frontline clinicians around diagnostic uncertainty of any kind when it comes to children. We endeavour to reassure ourselves and patients’ families by leaving no stone unturned. This approach can lead to unnecessary care, including false-positive test results, “incidentalomas,” and adverse effects from unnecessary medications. Despite the best intentions of assuaging caregivers’ anxiety, overuse of invasive and uncomfortable tests can have the opposite effect of increasing stress and trauma for both children and parents.
Third, there is compelling evidence that practice habits, once established, are difficult to break.10 Particularly in the high-stakes practice of hospital pediatric medicine, where we are conditioned to expect the worst and anticipate the unexpected. This “do everything to everyone” approach, however, can lead to significant harms for pediatric patients. For example, the exposure to ionizing radiation through unnecessary computed tomography (CT) scans can increase a child’s lifetime cancer risk.11
The perpetuation of unnecessary care needs to change in pediatrics, especially for the most vulnerable young patients seeking hospital care. Implementation is a necessary next step to introduce recommendations into practice, and the Choosing Wisely efforts of the Hospital for Sick Children in Toronto, Canada, can offer insights into opportunities to embed this approach across similar quaternary care teaching hospitals, as well as general hospitals and the systems they support.
STEPS TO IMPLEMENTING CHOOSING WISELY HOSPITAL-WIDE
Creating Lists of Recommendations Aligned With Quality Metrics
The Hospital for Sick Children developed a hospital-specific Choosing Wisely list in 2016 to address a gap in existing Choosing Wisely Canada campaign recommendations related to pediatric hospitals.12 Choosing Wisely Canada was initially focused on adult medicine, and a list of recommendations developed by the Canadian Paediatric Society relates mostly to overuse in pediatric outpatient settings and is not applicable to hospital-based practice.13 The Society of Hospital Medicine-Pediatric Hospital Medicine Choosing Wisely® list predominantly pertained to unnecessary care of infants with bronchiolitis (eg, not to order chest radiographs in uncomplicated asthma and bronchiolitis). We had measured our compliance with this recommendation and found it was already well below the achievable benchmark of care in the United States,14 so we preferred to create a list that would resonate with our clinicians. Since the original list was created at the Hospital for Sick Children,12 we have developed two subsequent lists of recommendations, which were released in 2018 and 2021 (Table).

The approach to list development used by staff pediatricians and trainees, with input from hospital staff and family advisors, has been described elsewhere.12 The goal was to self-identify five local practices that we felt would help us reduce unnecessary care. This list served as the foundation of an organization-wide quality initiative driven by a steering committee that consisted of the clinician champions as well as representation from various groups at the hospital, including decision support, information services, the family advisory committee, and public affairs.
Each recommendation needed to be evidence-based and measurable, have a clinician champion to implement the recommendation, and have the potential to improve the quality and safety of the care we provided. “Balancing” measures needed to be carefully monitored to ensure that no diagnoses were being missed or negative effects resulted from decreasing these interventions. In order for a recommendation to be considered, a subgroup of the pediatric department’s clinical advisory committee reviewed the references provided to ensure that what was being suggested was based on published evidence and part of current national guidelines. The clinician champion needed to agree to lead the implementation project, and specific outcomes, including appropriate balancing measures, needed to be identified a priori, in addition to an appropriate mechanism to collect the data. Hospital executive leaders were supportive of the initiative and facilitated access to “in-kind” hospital resources as required, although no financial budget was provided. After some early success, the Department of Paediatrics provided part-time project management support to help coordinate the growth and administration of the initiative.
Measuring and Supporting Practice Change
The main implementation principles included targeted education/awareness, transparent measurement with audit/feedback, and, most importantly, embedding changes in the ordering process, essentially making it easy for frontline clinicians to do the right thing (and trickier to do the “wrong” thing). Audit and feedback have been used at both the individual provider level (eg, respiratory viral testing–ordering practices) and the divisional level (eg, ordering of postoperative antibiotics). These quality improvement initiatives have had a compelling impact. Scorecards have been developed and results shared internally using local divisional as well as hospital-wide tools, varying from staff meetings to screensavers across hospital computers and television screens and the hospital intranet. Evaluation is ongoing, but many of the initial results have been encouraging.15-17
For example, the 2016 list includes recommendations related to emergency department (ED) test ordering. Implementation efforts to address unnecessary nasopharyngeal swabs for viral testing in bronchiolitis reduced this practice by 80%,15 and there has been a 50% reduction in ankle X-rays in children with acute ankle injuries who meet criteria for a low-risk examination.16 The 2016 list also included a recommendation related to inappropriate intravenous immunoglobulin (IVIG) use in children with typical acute immune thrombocytopenic purpura (ITP), and a targeted quality improvement initiative reduced inappropriate IVIG use by 50%, with no detectable increase in bleeding complications or readmission to hospital.17 These results have been sustained over a period of 3 or more years. Examples from the 2018 list include a 40% decrease in inappropriate urinary tract infection diagnosis and treatment in the ED and a four-fold decrease in the CT abdomen/pelvis imaging rate for low-risk trauma.18
The steering committee meets every 2 months and includes all of the clinician champions as well as representatives from strategic hospital resources and two family advisors (NGS). These meetings are chaired by the Associate Pediatrician-in-Chief (JNF) and the project manager. The progress of the active projects is discussed, and the experience of the group is used to problem-solve, plan ahead, and encourage academic presentation and publication of the various projects. Patient partnership and participation in committees has ensured that improvements to patient experience, satisfaction, and education are considered in the outcomes of implementation. Moreover, it has safeguarded that this effort is not misperceived as limiting care and remains focused on advancing quality, safety, and the patient experience.
SOME LESSONS LEARNED
While most projects have surpassed expectations, not all have proceeded as anticipated. The biggest challenge is finding a reliable and practical source for data collection. For example, at the time of initiation of the voiding cystourethrography (VCUG) recommendation, practice had presumably changed over the recent years, and compliance already exceeded the goal, illustrating the importance of current accurate data. The oxygen saturation–monitoring recommendation highlighted the challenge presented by data collection that requires manual audits; the inability to find staff to do this regularly significantly hampered this project. The critical role of the clinician champion was highlighted in a few projects when a lead was absent for a prolonged period of time (eg, due to a parental leave or change in job), with no willing replacement. There does seem to be a strong correlation between the commitment and passion of the clinician lead and the success of the project. We have incorporated the lessons learned into the development and rollout of the 2018 and 2021 lists.
SPREAD AND SCALE
The challenge is to scale up these successes to impact and change practice across the hospital pediatrics community. After 5 years, awareness of and engagement with this process are still not uniform across our hospital campus. Nevertheless, anecdotally, at the Hospital for Sick Children, there is a shift in culture where clinicians have processed the imperative to reduce overuse and unnecessary tests and treatments, with phrases such as “this is not very Choosing Wisely” entering the vernacular. It is becoming part of the culture. Second, the new generation of medical school trainees and residents has displayed a tremendous appetite and passion for stewardship and a sense that practice can change from the ground up. The SickKids Choosing Wisely efforts have been a hub for resident-led quality improvement projects and leadership for implementation of recommendations.19 As we continue to engage all providers at our hospital, we are also reaching out to the other community hospitals in our region, and all children’s hospitals in Canada, to share the principles and lessons learned from our program through a national community of practice.
CONCLUSION
Practicing pediatric medicine in a well-resourced hospital setting should not drive us to overuse in practice “just because we can.” The harms of this approach to our patients and health systems, coupled with the pressures of the pandemic, are compelling reasons to be responsible stewards. There are opportunities to reshape and rethink practice patterns and habits.20 Overuse and overdiagnosis harm our patients and families physically and emotionally and indirectly waste resources urgently needed for investment upstream. Providing safe, quality, high-value care to our young patients requires constant critical thinking. The time is here to advance Choosing Wisely into pediatric hospital practice.
Most hospital pediatricians can recall cases where an abnormal result in one unnecessary test led to a cascade of multiple further unnecessary treatments, procedures, and tests. These cases are well described in the literature and written off as a side effect of delivering high-quality, comprehensive pediatric care.1 Unfortunately, however, these frequent events are not without consequence and can cause significant harm to patients, as well as stress and fear for parents and families, and indirectly waste valuable resources.
As we look forward to recovering from the COVID-19 pandemic, there are calls to prioritize high-value and more equitable care in the postpandemic world.2 Choosing Wisely is a global movement comprised of clinician-led campaigns that partner with national specialty societies to develop lists of evidence-based recommendations of tests, treatments, and procedures that offer no added clinical value and may cause harm.3
In pediatrics, there is a growing recognition and published literature on the harms of overdiagnosis and unnecessary care in children.4-6 Choosing Wisely recommendations are being used as a resource to drive healthcare prioritization and ensure low-value care is avoided so that greater focus can be placed on areas of need exacerbated by the pandemic. Using a Choosing Wisely perspective can drive quality and help inform a shift in practice, creating a roadmap for reducing testing or treatment cascades that harm patients and waste resources as we move toward the goal of high-value pediatric care. However, adoption of Choosing Wisely recommendations in pediatrics has been slow. For example, the pediatric working group of the Society of Hospital Medicine released a Choosing Wisely® recommendation in 2013 against the use of continuous pulse oximetry monitoring in children with acute respiratory illness who are not on supplementary oxygen.7 Data from a cross-sectional study across 56 hospitals 6 years later found significant variation in this practice for infants hospitalized with bronchiolitis and not receiving supplemental oxygen; 46% were continuously monitored with pulse oximetry (range, 2%-92%).8
WHY HAS CHOOSING WISELY LAGGED IN PEDIATRICS?
Traditionally, attention in children’s healthcare has focused on underuse (eg, immunizations or mental health) rather than overuse. Further, the weakness of the evidence base, with very few randomized controlled trials in children, limits our ability to provide sufficient confidence in the evidence supporting some of our recommendations.9
Second, there is also tremendous anxiety for both parents and frontline clinicians around diagnostic uncertainty of any kind when it comes to children. We endeavour to reassure ourselves and patients’ families by leaving no stone unturned. This approach can lead to unnecessary care, including false-positive test results, “incidentalomas,” and adverse effects from unnecessary medications. Despite the best intentions of assuaging caregivers’ anxiety, overuse of invasive and uncomfortable tests can have the opposite effect of increasing stress and trauma for both children and parents.
Third, there is compelling evidence that practice habits, once established, are difficult to break.10 Particularly in the high-stakes practice of hospital pediatric medicine, where we are conditioned to expect the worst and anticipate the unexpected. This “do everything to everyone” approach, however, can lead to significant harms for pediatric patients. For example, the exposure to ionizing radiation through unnecessary computed tomography (CT) scans can increase a child’s lifetime cancer risk.11
The perpetuation of unnecessary care needs to change in pediatrics, especially for the most vulnerable young patients seeking hospital care. Implementation is a necessary next step to introduce recommendations into practice, and the Choosing Wisely efforts of the Hospital for Sick Children in Toronto, Canada, can offer insights into opportunities to embed this approach across similar quaternary care teaching hospitals, as well as general hospitals and the systems they support.
STEPS TO IMPLEMENTING CHOOSING WISELY HOSPITAL-WIDE
Creating Lists of Recommendations Aligned With Quality Metrics
The Hospital for Sick Children developed a hospital-specific Choosing Wisely list in 2016 to address a gap in existing Choosing Wisely Canada campaign recommendations related to pediatric hospitals.12 Choosing Wisely Canada was initially focused on adult medicine, and a list of recommendations developed by the Canadian Paediatric Society relates mostly to overuse in pediatric outpatient settings and is not applicable to hospital-based practice.13 The Society of Hospital Medicine-Pediatric Hospital Medicine Choosing Wisely® list predominantly pertained to unnecessary care of infants with bronchiolitis (eg, not to order chest radiographs in uncomplicated asthma and bronchiolitis). We had measured our compliance with this recommendation and found it was already well below the achievable benchmark of care in the United States,14 so we preferred to create a list that would resonate with our clinicians. Since the original list was created at the Hospital for Sick Children,12 we have developed two subsequent lists of recommendations, which were released in 2018 and 2021 (Table).

The approach to list development used by staff pediatricians and trainees, with input from hospital staff and family advisors, has been described elsewhere.12 The goal was to self-identify five local practices that we felt would help us reduce unnecessary care. This list served as the foundation of an organization-wide quality initiative driven by a steering committee that consisted of the clinician champions as well as representation from various groups at the hospital, including decision support, information services, the family advisory committee, and public affairs.
Each recommendation needed to be evidence-based and measurable, have a clinician champion to implement the recommendation, and have the potential to improve the quality and safety of the care we provided. “Balancing” measures needed to be carefully monitored to ensure that no diagnoses were being missed or negative effects resulted from decreasing these interventions. In order for a recommendation to be considered, a subgroup of the pediatric department’s clinical advisory committee reviewed the references provided to ensure that what was being suggested was based on published evidence and part of current national guidelines. The clinician champion needed to agree to lead the implementation project, and specific outcomes, including appropriate balancing measures, needed to be identified a priori, in addition to an appropriate mechanism to collect the data. Hospital executive leaders were supportive of the initiative and facilitated access to “in-kind” hospital resources as required, although no financial budget was provided. After some early success, the Department of Paediatrics provided part-time project management support to help coordinate the growth and administration of the initiative.
Measuring and Supporting Practice Change
The main implementation principles included targeted education/awareness, transparent measurement with audit/feedback, and, most importantly, embedding changes in the ordering process, essentially making it easy for frontline clinicians to do the right thing (and trickier to do the “wrong” thing). Audit and feedback have been used at both the individual provider level (eg, respiratory viral testing–ordering practices) and the divisional level (eg, ordering of postoperative antibiotics). These quality improvement initiatives have had a compelling impact. Scorecards have been developed and results shared internally using local divisional as well as hospital-wide tools, varying from staff meetings to screensavers across hospital computers and television screens and the hospital intranet. Evaluation is ongoing, but many of the initial results have been encouraging.15-17
For example, the 2016 list includes recommendations related to emergency department (ED) test ordering. Implementation efforts to address unnecessary nasopharyngeal swabs for viral testing in bronchiolitis reduced this practice by 80%,15 and there has been a 50% reduction in ankle X-rays in children with acute ankle injuries who meet criteria for a low-risk examination.16 The 2016 list also included a recommendation related to inappropriate intravenous immunoglobulin (IVIG) use in children with typical acute immune thrombocytopenic purpura (ITP), and a targeted quality improvement initiative reduced inappropriate IVIG use by 50%, with no detectable increase in bleeding complications or readmission to hospital.17 These results have been sustained over a period of 3 or more years. Examples from the 2018 list include a 40% decrease in inappropriate urinary tract infection diagnosis and treatment in the ED and a four-fold decrease in the CT abdomen/pelvis imaging rate for low-risk trauma.18
The steering committee meets every 2 months and includes all of the clinician champions as well as representatives from strategic hospital resources and two family advisors (NGS). These meetings are chaired by the Associate Pediatrician-in-Chief (JNF) and the project manager. The progress of the active projects is discussed, and the experience of the group is used to problem-solve, plan ahead, and encourage academic presentation and publication of the various projects. Patient partnership and participation in committees has ensured that improvements to patient experience, satisfaction, and education are considered in the outcomes of implementation. Moreover, it has safeguarded that this effort is not misperceived as limiting care and remains focused on advancing quality, safety, and the patient experience.
SOME LESSONS LEARNED
While most projects have surpassed expectations, not all have proceeded as anticipated. The biggest challenge is finding a reliable and practical source for data collection. For example, at the time of initiation of the voiding cystourethrography (VCUG) recommendation, practice had presumably changed over the recent years, and compliance already exceeded the goal, illustrating the importance of current accurate data. The oxygen saturation–monitoring recommendation highlighted the challenge presented by data collection that requires manual audits; the inability to find staff to do this regularly significantly hampered this project. The critical role of the clinician champion was highlighted in a few projects when a lead was absent for a prolonged period of time (eg, due to a parental leave or change in job), with no willing replacement. There does seem to be a strong correlation between the commitment and passion of the clinician lead and the success of the project. We have incorporated the lessons learned into the development and rollout of the 2018 and 2021 lists.
SPREAD AND SCALE
The challenge is to scale up these successes to impact and change practice across the hospital pediatrics community. After 5 years, awareness of and engagement with this process are still not uniform across our hospital campus. Nevertheless, anecdotally, at the Hospital for Sick Children, there is a shift in culture where clinicians have processed the imperative to reduce overuse and unnecessary tests and treatments, with phrases such as “this is not very Choosing Wisely” entering the vernacular. It is becoming part of the culture. Second, the new generation of medical school trainees and residents has displayed a tremendous appetite and passion for stewardship and a sense that practice can change from the ground up. The SickKids Choosing Wisely efforts have been a hub for resident-led quality improvement projects and leadership for implementation of recommendations.19 As we continue to engage all providers at our hospital, we are also reaching out to the other community hospitals in our region, and all children’s hospitals in Canada, to share the principles and lessons learned from our program through a national community of practice.
CONCLUSION
Practicing pediatric medicine in a well-resourced hospital setting should not drive us to overuse in practice “just because we can.” The harms of this approach to our patients and health systems, coupled with the pressures of the pandemic, are compelling reasons to be responsible stewards. There are opportunities to reshape and rethink practice patterns and habits.20 Overuse and overdiagnosis harm our patients and families physically and emotionally and indirectly waste resources urgently needed for investment upstream. Providing safe, quality, high-value care to our young patients requires constant critical thinking. The time is here to advance Choosing Wisely into pediatric hospital practice.
1. Elliott DK, Rose SR, Ronan JC. Changing the culture around cultures. Hosp Pediatr. 2014;4(6):405-407. https://doi.org/10.1542/hpeds.2014-0064
2. Gupta R, Simpson LA, Morgan DJ. Prioritizing high-value, equitable care after the COVID-19 shutdown: an opportunity for a healthcare renaissance. J Hosp Med. 2021;16(2):114-116. https://doi.org/10.12788/jhm.3526
3. Born K, Kool T, Levinson W. Reducing overuse in healthcare: advancing Choosing Wisely. BMJ. 2019;367:l6317. https://doi.org/10.1136/bmj.l6317
4. Coon ER, Young PC, Quinonez RA, Morgan DJ, Dhruva SS, Schroeder AR. Update on pediatric overuse. Pediatrics. 2017;139(2):e20162797. https://doi.org/10.1542/peds.2016-2797
5. Coon ER, Quinonez RA, Moyer VA, Schroeder AR. Overdiagnosis: how our compulsion for diagnosis may be harming children. Pediatrics. 2014;134(5):1013-1023. https://doi.org/10.1542/peds.2014-1778
6. Wolf ER, Krist AH, Schroeder AR. Deimplementation in pediatrics: past, present, and future. JAMA Pediatr. 2021;175(3):230-232. https://doi.org/10.1001/jamapediatrics.2020.4681
7. Quinonez RA, Garber MD, Schroeder AR, et al. Choosing Wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):479-485. https://doi.org/10.1002/jhm.2064
8. Bonafide CP, Xiao R, Brady PW, et al. Prevalence of continuous pulse oximetry monitoring in hospitalized children with bronchiolitis not requiring supplemental oxygen. JAMA. 2020;323(15):1467-1477. https://doi.org/10.1001/jama.2020.2998
9. Ralston SL, Schroeder AR. Why is it so hard to talk about overuse in pediatrics and why it matters. JAMA Pediatr. 2017;17(10):931-932. https://doi.org/10.1001/jamapediatrics.2017.2239
10. Stammen LA, Stalmeijer RE, Paternotte E, et al. Training physicians to provide high-value, cost-conscious care: a systematic review. JAMA. 2015;314(22):2384-2400. https://doi.org/10.1001/jama.2015.16353
11. Mathews JD, Forsythe AV, Brady Z, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ. 2013;346:f2360. https://doi.org/10.1136/bmj.f2360
12. Friedman JN. Saying yes to the less: making it easier to choose wisely [editorial]. J Pediatr. 2017;145:4-5. https://doi.org/10.1016/j.jpeds.2017.01.062
13. Canadian Paediatric Society. Five things physicians and patients should question. Choosing Wisely Canada. Updated July 2019. Accessed June 17, 2021. https://choosingwiselycanada.org/wp-content/uploads/2020/07/Paediatrics_EN.pdf
14. Parikh K, Hall M, Montalbano A, et al. Establishing benchmarks for the hospitalized care of children with asthma, bronciolitis, and pneumonia. Pediatrics. 2014;134(3):555-562. https://doi.org/10.1542/peds.2014-1052
15. Ostrow O, Richardson S, Savlov D, Friedman JN. Reducing unnecessary respiratory viral testing to promote high value care. Pediatrics. In press.
16. Al-Sani F, Ben-Yakov M, Harvey G, et al. P016: Low risk ankle rule, high reward—a quality improvement initiative to reduce ankle x-rays in the pediatric emergency department [poster]. CJEM. 2017;19(S1):S83. https://doi.org/10.1017/cem.2017.218
17. Beck CE, Carcao M, Cada M, Porter S, Blanchette VS, Parkin PC. A quality improvement bundle to improve informed choice for children with typical, newly diagnosed immune thrombocytopenia. J Pediatr Hematol Oncol. 2018;40(8):e537-e543. https://doi.org/10.1097/MPH.0000000000001247
18. Beno S, Lenton-Brym T, Rosenfield D, McDowall D, Wales P, Principi T. Safe reduction of abdominal CT imaging in pediatric trauma patients: a quality-improvement initiative [abstract]. Can J Surg. 2019;62(3 Suppl 2):S29-S30.
19. Bal C, Tesch M, Blair G, Ostrow O, Premji L. Engaging medical trainees in resource stewardship through resident-led teaching sessions: a choosing wisely educational initiative. Can Med Educ J. 2021;12(1):e98-e100. https://doi.org/10.36834/cmej.70563
20. Berwick DM. Choices for the “new normal.” JAMA. 2020;323(21):2125-2126. https://doi.org/10.1001/jama.2020.6949
1. Elliott DK, Rose SR, Ronan JC. Changing the culture around cultures. Hosp Pediatr. 2014;4(6):405-407. https://doi.org/10.1542/hpeds.2014-0064
2. Gupta R, Simpson LA, Morgan DJ. Prioritizing high-value, equitable care after the COVID-19 shutdown: an opportunity for a healthcare renaissance. J Hosp Med. 2021;16(2):114-116. https://doi.org/10.12788/jhm.3526
3. Born K, Kool T, Levinson W. Reducing overuse in healthcare: advancing Choosing Wisely. BMJ. 2019;367:l6317. https://doi.org/10.1136/bmj.l6317
4. Coon ER, Young PC, Quinonez RA, Morgan DJ, Dhruva SS, Schroeder AR. Update on pediatric overuse. Pediatrics. 2017;139(2):e20162797. https://doi.org/10.1542/peds.2016-2797
5. Coon ER, Quinonez RA, Moyer VA, Schroeder AR. Overdiagnosis: how our compulsion for diagnosis may be harming children. Pediatrics. 2014;134(5):1013-1023. https://doi.org/10.1542/peds.2014-1778
6. Wolf ER, Krist AH, Schroeder AR. Deimplementation in pediatrics: past, present, and future. JAMA Pediatr. 2021;175(3):230-232. https://doi.org/10.1001/jamapediatrics.2020.4681
7. Quinonez RA, Garber MD, Schroeder AR, et al. Choosing Wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):479-485. https://doi.org/10.1002/jhm.2064
8. Bonafide CP, Xiao R, Brady PW, et al. Prevalence of continuous pulse oximetry monitoring in hospitalized children with bronchiolitis not requiring supplemental oxygen. JAMA. 2020;323(15):1467-1477. https://doi.org/10.1001/jama.2020.2998
9. Ralston SL, Schroeder AR. Why is it so hard to talk about overuse in pediatrics and why it matters. JAMA Pediatr. 2017;17(10):931-932. https://doi.org/10.1001/jamapediatrics.2017.2239
10. Stammen LA, Stalmeijer RE, Paternotte E, et al. Training physicians to provide high-value, cost-conscious care: a systematic review. JAMA. 2015;314(22):2384-2400. https://doi.org/10.1001/jama.2015.16353
11. Mathews JD, Forsythe AV, Brady Z, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ. 2013;346:f2360. https://doi.org/10.1136/bmj.f2360
12. Friedman JN. Saying yes to the less: making it easier to choose wisely [editorial]. J Pediatr. 2017;145:4-5. https://doi.org/10.1016/j.jpeds.2017.01.062
13. Canadian Paediatric Society. Five things physicians and patients should question. Choosing Wisely Canada. Updated July 2019. Accessed June 17, 2021. https://choosingwiselycanada.org/wp-content/uploads/2020/07/Paediatrics_EN.pdf
14. Parikh K, Hall M, Montalbano A, et al. Establishing benchmarks for the hospitalized care of children with asthma, bronciolitis, and pneumonia. Pediatrics. 2014;134(3):555-562. https://doi.org/10.1542/peds.2014-1052
15. Ostrow O, Richardson S, Savlov D, Friedman JN. Reducing unnecessary respiratory viral testing to promote high value care. Pediatrics. In press.
16. Al-Sani F, Ben-Yakov M, Harvey G, et al. P016: Low risk ankle rule, high reward—a quality improvement initiative to reduce ankle x-rays in the pediatric emergency department [poster]. CJEM. 2017;19(S1):S83. https://doi.org/10.1017/cem.2017.218
17. Beck CE, Carcao M, Cada M, Porter S, Blanchette VS, Parkin PC. A quality improvement bundle to improve informed choice for children with typical, newly diagnosed immune thrombocytopenia. J Pediatr Hematol Oncol. 2018;40(8):e537-e543. https://doi.org/10.1097/MPH.0000000000001247
18. Beno S, Lenton-Brym T, Rosenfield D, McDowall D, Wales P, Principi T. Safe reduction of abdominal CT imaging in pediatric trauma patients: a quality-improvement initiative [abstract]. Can J Surg. 2019;62(3 Suppl 2):S29-S30.
19. Bal C, Tesch M, Blair G, Ostrow O, Premji L. Engaging medical trainees in resource stewardship through resident-led teaching sessions: a choosing wisely educational initiative. Can Med Educ J. 2021;12(1):e98-e100. https://doi.org/10.36834/cmej.70563
20. Berwick DM. Choices for the “new normal.” JAMA. 2020;323(21):2125-2126. https://doi.org/10.1001/jama.2020.6949
© 2021 Society of Hospital Medicine
Policy in Clinical Practice: Hospital Price Transparency
CLINICAL SCENARIO
A 59-year-old man is observed in the hospital for substernal chest pain initially concerning for angina. Serial troponin testing is negative, and based on additional history of intermittent dysphagia, an elective upper endoscopy is recommended after discharge. The patient does not have health insurance and expresses anxiety about the cost of endoscopy. He asks how he could compare the costs at different hospitals. How do federal price transparency rules assist the hospitalist in addressing this patient’s question?
BACKGROUND AND HISTORY
Healthcare costs continue to rise in the United States despite mounting concerns about wasteful spending and unaffordability.1 One contributor is a lack of price transparency.2 In theory, price transparency allows individuals to shop for services, spurring competition and lower prices. However, healthcare prices have historically been opaque to both physicians and patients; unlike other licensed professionals who provide clients estimates for their work (eg, lawyers, electricians), physicians are rarely able to offer patients real-time insight or guidance about costs, which most patients discover only when the bill arrives. The situation is particularly problematic for patients who bear higher out-of-pocket costs, such as the uninsured or those with high-deductible health plans.3
Decades of work to improve healthcare price transparency have unfortunately borne little fruit. Multiple states and organizations have attempted to disseminate price information on comparison websites.4 These efforts only modestly reduced some prices, with benefits confined to elective, single-episode, commodifiable services such as magnetic resonance imaging scans.5 The Affordable Care Act required hospitals to publish standard charges, also called a chargemaster (Table).6 However, chargemaster fees are notoriously inflated and inaccessible at the point of service, undercutting transparency.

POLICY IN CLINICAL PRACTICE
Beginning January 2021, the Centers for Medicare & Medicaid Services (CMS) required all hospitals to publish negotiated prices—including payor-specific negotiated charges—for 300 “shoppable services” (Table).6 The list must include 70 common CMS-specified services, such as a basic metabolic panel, upper endoscopy, and prostate biopsy, as well as another 230 services that each hospital determines relevant to its patient population.
In circumstances where hospitals have negotiated different prices for a service, they must list each third-party payor and their payor-specific charge. The information must be prominently displayed, accessible without requiring the patient to enter personal information, and provided in a machine-readable file. CMS may impose a $300 daily penalty on hospitals failing to comply with the policy. Of note, the policy does not apply to clinics or ambulatory surgery centers.
As more hospitals share data, this policy will directly benefit both patients and physicians. It can benefit patients with the time, foresight, and ability to search for the lowest price for shoppable services. Other patients may also benefit indirectly, to the extent that insurers and other purchasers apply this information to negotiate lower and more uniform prices. Decreased price variation may also encourage hospitals to compete on quality to distinguish the value of their services. Hospitalists could benefit through the ability to directly help patients locate price information.
Despite these potential benefits, the policy has limitations. Price information about shoppable services is most useful for discharge planning, and other solutions are needed to address transparency before and during unplanned admissions. Patients who prioritize continuity with a hospital or physician may be less price sensitive, particularly for more complex services. Patients with commercial insurance may be shielded from cost considerations and personal incentives to comparison shop. Interpreting hospitals’ estimates remains difficult, as it can be unclear if professional fees are included or if certain prices are offered to outpatients.7 Price information is not accompanied by corresponding quality data. Additionally, price transparency may also fail to lower prices in heavily concentrated payor or provider markets, and it remains unknown whether some providers may actually raise prices after learning about higher rates negotiated by competitors.8,9
Another issue is hospital participation. Early evidence suggests that most hospitals have not complied with the letter or spirit of the regulation.
Despite its limitations, this policy represents a meaningful advance for healthcare competition and patient empowerment. Additionally, it signals federal willingness to address the lack of price transparency as a source of widespread patient and clinician frustration—a commitment that will be needed to sustain this policy and implement additional measures in the future.
COMMENTARY AND RECOMMENDATIONS
CMS could consider five steps to augment the policy and maximize transparency and value for patients.
First, CMS could consider increasing daily nonparticipation penalties. Hospitals, particularly those in areas with less competition, have less incentive to participate given meager current penalties. Because the magnitude needed to compel action remains unknown, CMS could gradually escalate penalties over time until there is broader participation across hospitals.
Second, policymakers could aggregate price information centrally, organize the data around patients’ clinical scenarios, and advertise its availability. Currently, this information is scattered and time-consuming for hospitalists and patients to gather for decision-making. Additionally, CMS could encourage the development of third-party tools that aggregate and analyze machine-readable price data or require that prices be posted at the point of service.
Third, CMS could revise the policy to include quality as well as price information. Price alone does not offer a full enough picture of what consumers can expect from hospitals for shoppable services. Pairing price and quality information is better aligned to addressing costs in the context of value, rather than cost-cutting for its own purposes.
Fourth, over time, CMS could expand the list of services and sites required to report (eg, clinics and ambulatory surgical centers as well as hospitals).
Fifth, CMS rule-makers could set reporting standards and contextualize price information in common clinical scenarios. Patients may have difficulty shopping for complex healthcare services without understanding how they apply in different clinical situations. Decision-making would also be aided by reporting standards—for instance, for how prices are displayed and whether they include certain fees (eg, professional fees, pathology studies).
WHAT SHOULD I TELL MY PATIENT?
Hospitalists planning follow-up care should inform patients that price information is increasingly available and encourage them to search on the internet or contact hospital billing offices to request information (eg, discounted cash prices and minimum negotiated charges) before obtaining elective services after discharge. Hospitalists can also encourage patients to discuss shoppable services with their primary care physicians to understand the clinical context and make high-value decisions. Hospitalists who wish to build communication skills discussing costs with patients can increasingly find resources for these conversations and request that prices be displayed in the electronic health record for this purpose.13,14 As conversations occur, hospitalists should seek to understand other factors, such as convenience and continuity relationships, that might influence choices.
CONCLUSIONS
Starting in 2021, CMS policy requires that hospitals report prices for services such as the endoscopy recommended for the patient in the scenario. Though the policy gives patients new hope for greater transparency and better prices, additional steps are needed to help patients and hospitalists achieve these benefits.
1. Shrank WH, Rogstad TL, Parekh N. Waste in the US health care system: estimated costs and potential for savings. JAMA. 2019;322(15):1501-1509. https://doi.org/10.1001/jama.2019.13978
2. Wetzell S. Transparency: a needed step towards health care affordability. American Health Policy Institute. March 2014. Accessed August 26, 2021. https://www.americanhealthpolicy.org/Content/documents/resources/Transparency%20Study%201%20-%20The%20Need%20for%20Health%20Care%20Transparency.pdf
3. Mehrotra A, Dean KM, Sinaiko AD, Sood N. Americans support price shopping for health care, but few actually seek out price information. Health Aff (Millwood). 2017;36(8):1392-1400. https://doi.org/10.1377/hlthaff.2016.1471
4. Kullgren JT, Duey KA, Werner RM. A census of state health care price transparency websites. JAMA. 2013;309(23):2437-2438. https://doi.org/10.1001/jama.2013.6557
5. Brown ZY. Equilibrium effects of health care price information. Rev Econ Stat. 2019;101(4):699-712. https://doi.org/10.1162/rest_a_00765
6. Medicare and Medicaid Programs: CY 2020 hospital outpatient PPS policy changes and payment rates and ambulatory surgical center payment system policy changes and payment rates. Price transparency requirements for hospitals to make standard charges public. 45 CFR §180.20 (2019).
7. Kurani N, Ramirez G, Hudman J, Cox C, Kamal R. Early results from federal price transparency rule show difficulty in estimating the cost of care. Peterson-Kaiser Family Foundation. April 9, 2021. Accessed August 26, 2021. https://www.healthsystemtracker.org/brief/early-results-from-federal-price-transparency-rule-show-difficultly-in-estimating-the-cost-of-care/
8. Miller BJ, Mandelberg MC, Griffith NC, Ehrenfeld JM. Price transparency: empowering patient choice and promoting provider competition. J Med Syst. 2020;44(4):80. https://doi.org/10.1007/s10916-020-01553-2
9. Glied S. Price transparency–promise and peril. JAMA. 2021;325(15):1496-1497. https://doi.org/10.1001/jama.2021.4640
10. Haque W, Ahmadzada M, Allahrakha H, Haque E, Hsiehchen D. Transparency, accessibility, and variability of US hospital price data. JAMA Netw Open. 2021;4(5):e2110109. https://doi.org/10.1001/jamanetworkopen.2021.10109
11. Henderson M, Mouslim MC. Low compliance from big hospitals on CMS’s hospital price transparency rule. Health Affairs Blog. March 16, 2021. Accessed August 26, 2021. https://doi.org/10.1377/hblog20210311.899634
12. McGinty T, Wilde Mathews A, Evans M. Hospitals hide pricing data from search results. The Wall Street Journal. March 22, 2021. Accessed August 26, 2021. https://www.wsj.com/articles/hospitals-hide-pricing-data-from-search-results-11616405402
13. Dine CJ, Masi D, Smith CD. Tools to help overcome barriers to cost-of-care conversations. Ann Intern Med. 2019;170(9 suppl):S36-S38. https://doi.org/10.7326/M19-0778
14. Miller BJ, Slota JM, Ehrenfeld JM. Redefining the physician’s role in cost-conscious care: the potential role of the electronic health record. JAMA. 2019;322(8):721-722. https://doi.org/10.1001/jama.2019.9114
CLINICAL SCENARIO
A 59-year-old man is observed in the hospital for substernal chest pain initially concerning for angina. Serial troponin testing is negative, and based on additional history of intermittent dysphagia, an elective upper endoscopy is recommended after discharge. The patient does not have health insurance and expresses anxiety about the cost of endoscopy. He asks how he could compare the costs at different hospitals. How do federal price transparency rules assist the hospitalist in addressing this patient’s question?
BACKGROUND AND HISTORY
Healthcare costs continue to rise in the United States despite mounting concerns about wasteful spending and unaffordability.1 One contributor is a lack of price transparency.2 In theory, price transparency allows individuals to shop for services, spurring competition and lower prices. However, healthcare prices have historically been opaque to both physicians and patients; unlike other licensed professionals who provide clients estimates for their work (eg, lawyers, electricians), physicians are rarely able to offer patients real-time insight or guidance about costs, which most patients discover only when the bill arrives. The situation is particularly problematic for patients who bear higher out-of-pocket costs, such as the uninsured or those with high-deductible health plans.3
Decades of work to improve healthcare price transparency have unfortunately borne little fruit. Multiple states and organizations have attempted to disseminate price information on comparison websites.4 These efforts only modestly reduced some prices, with benefits confined to elective, single-episode, commodifiable services such as magnetic resonance imaging scans.5 The Affordable Care Act required hospitals to publish standard charges, also called a chargemaster (Table).6 However, chargemaster fees are notoriously inflated and inaccessible at the point of service, undercutting transparency.

POLICY IN CLINICAL PRACTICE
Beginning January 2021, the Centers for Medicare & Medicaid Services (CMS) required all hospitals to publish negotiated prices—including payor-specific negotiated charges—for 300 “shoppable services” (Table).6 The list must include 70 common CMS-specified services, such as a basic metabolic panel, upper endoscopy, and prostate biopsy, as well as another 230 services that each hospital determines relevant to its patient population.
In circumstances where hospitals have negotiated different prices for a service, they must list each third-party payor and their payor-specific charge. The information must be prominently displayed, accessible without requiring the patient to enter personal information, and provided in a machine-readable file. CMS may impose a $300 daily penalty on hospitals failing to comply with the policy. Of note, the policy does not apply to clinics or ambulatory surgery centers.
As more hospitals share data, this policy will directly benefit both patients and physicians. It can benefit patients with the time, foresight, and ability to search for the lowest price for shoppable services. Other patients may also benefit indirectly, to the extent that insurers and other purchasers apply this information to negotiate lower and more uniform prices. Decreased price variation may also encourage hospitals to compete on quality to distinguish the value of their services. Hospitalists could benefit through the ability to directly help patients locate price information.
Despite these potential benefits, the policy has limitations. Price information about shoppable services is most useful for discharge planning, and other solutions are needed to address transparency before and during unplanned admissions. Patients who prioritize continuity with a hospital or physician may be less price sensitive, particularly for more complex services. Patients with commercial insurance may be shielded from cost considerations and personal incentives to comparison shop. Interpreting hospitals’ estimates remains difficult, as it can be unclear if professional fees are included or if certain prices are offered to outpatients.7 Price information is not accompanied by corresponding quality data. Additionally, price transparency may also fail to lower prices in heavily concentrated payor or provider markets, and it remains unknown whether some providers may actually raise prices after learning about higher rates negotiated by competitors.8,9
Another issue is hospital participation. Early evidence suggests that most hospitals have not complied with the letter or spirit of the regulation.
Despite its limitations, this policy represents a meaningful advance for healthcare competition and patient empowerment. Additionally, it signals federal willingness to address the lack of price transparency as a source of widespread patient and clinician frustration—a commitment that will be needed to sustain this policy and implement additional measures in the future.
COMMENTARY AND RECOMMENDATIONS
CMS could consider five steps to augment the policy and maximize transparency and value for patients.
First, CMS could consider increasing daily nonparticipation penalties. Hospitals, particularly those in areas with less competition, have less incentive to participate given meager current penalties. Because the magnitude needed to compel action remains unknown, CMS could gradually escalate penalties over time until there is broader participation across hospitals.
Second, policymakers could aggregate price information centrally, organize the data around patients’ clinical scenarios, and advertise its availability. Currently, this information is scattered and time-consuming for hospitalists and patients to gather for decision-making. Additionally, CMS could encourage the development of third-party tools that aggregate and analyze machine-readable price data or require that prices be posted at the point of service.
Third, CMS could revise the policy to include quality as well as price information. Price alone does not offer a full enough picture of what consumers can expect from hospitals for shoppable services. Pairing price and quality information is better aligned to addressing costs in the context of value, rather than cost-cutting for its own purposes.
Fourth, over time, CMS could expand the list of services and sites required to report (eg, clinics and ambulatory surgical centers as well as hospitals).
Fifth, CMS rule-makers could set reporting standards and contextualize price information in common clinical scenarios. Patients may have difficulty shopping for complex healthcare services without understanding how they apply in different clinical situations. Decision-making would also be aided by reporting standards—for instance, for how prices are displayed and whether they include certain fees (eg, professional fees, pathology studies).
WHAT SHOULD I TELL MY PATIENT?
Hospitalists planning follow-up care should inform patients that price information is increasingly available and encourage them to search on the internet or contact hospital billing offices to request information (eg, discounted cash prices and minimum negotiated charges) before obtaining elective services after discharge. Hospitalists can also encourage patients to discuss shoppable services with their primary care physicians to understand the clinical context and make high-value decisions. Hospitalists who wish to build communication skills discussing costs with patients can increasingly find resources for these conversations and request that prices be displayed in the electronic health record for this purpose.13,14 As conversations occur, hospitalists should seek to understand other factors, such as convenience and continuity relationships, that might influence choices.
CONCLUSIONS
Starting in 2021, CMS policy requires that hospitals report prices for services such as the endoscopy recommended for the patient in the scenario. Though the policy gives patients new hope for greater transparency and better prices, additional steps are needed to help patients and hospitalists achieve these benefits.
CLINICAL SCENARIO
A 59-year-old man is observed in the hospital for substernal chest pain initially concerning for angina. Serial troponin testing is negative, and based on additional history of intermittent dysphagia, an elective upper endoscopy is recommended after discharge. The patient does not have health insurance and expresses anxiety about the cost of endoscopy. He asks how he could compare the costs at different hospitals. How do federal price transparency rules assist the hospitalist in addressing this patient’s question?
BACKGROUND AND HISTORY
Healthcare costs continue to rise in the United States despite mounting concerns about wasteful spending and unaffordability.1 One contributor is a lack of price transparency.2 In theory, price transparency allows individuals to shop for services, spurring competition and lower prices. However, healthcare prices have historically been opaque to both physicians and patients; unlike other licensed professionals who provide clients estimates for their work (eg, lawyers, electricians), physicians are rarely able to offer patients real-time insight or guidance about costs, which most patients discover only when the bill arrives. The situation is particularly problematic for patients who bear higher out-of-pocket costs, such as the uninsured or those with high-deductible health plans.3
Decades of work to improve healthcare price transparency have unfortunately borne little fruit. Multiple states and organizations have attempted to disseminate price information on comparison websites.4 These efforts only modestly reduced some prices, with benefits confined to elective, single-episode, commodifiable services such as magnetic resonance imaging scans.5 The Affordable Care Act required hospitals to publish standard charges, also called a chargemaster (Table).6 However, chargemaster fees are notoriously inflated and inaccessible at the point of service, undercutting transparency.

POLICY IN CLINICAL PRACTICE
Beginning January 2021, the Centers for Medicare & Medicaid Services (CMS) required all hospitals to publish negotiated prices—including payor-specific negotiated charges—for 300 “shoppable services” (Table).6 The list must include 70 common CMS-specified services, such as a basic metabolic panel, upper endoscopy, and prostate biopsy, as well as another 230 services that each hospital determines relevant to its patient population.
In circumstances where hospitals have negotiated different prices for a service, they must list each third-party payor and their payor-specific charge. The information must be prominently displayed, accessible without requiring the patient to enter personal information, and provided in a machine-readable file. CMS may impose a $300 daily penalty on hospitals failing to comply with the policy. Of note, the policy does not apply to clinics or ambulatory surgery centers.
As more hospitals share data, this policy will directly benefit both patients and physicians. It can benefit patients with the time, foresight, and ability to search for the lowest price for shoppable services. Other patients may also benefit indirectly, to the extent that insurers and other purchasers apply this information to negotiate lower and more uniform prices. Decreased price variation may also encourage hospitals to compete on quality to distinguish the value of their services. Hospitalists could benefit through the ability to directly help patients locate price information.
Despite these potential benefits, the policy has limitations. Price information about shoppable services is most useful for discharge planning, and other solutions are needed to address transparency before and during unplanned admissions. Patients who prioritize continuity with a hospital or physician may be less price sensitive, particularly for more complex services. Patients with commercial insurance may be shielded from cost considerations and personal incentives to comparison shop. Interpreting hospitals’ estimates remains difficult, as it can be unclear if professional fees are included or if certain prices are offered to outpatients.7 Price information is not accompanied by corresponding quality data. Additionally, price transparency may also fail to lower prices in heavily concentrated payor or provider markets, and it remains unknown whether some providers may actually raise prices after learning about higher rates negotiated by competitors.8,9
Another issue is hospital participation. Early evidence suggests that most hospitals have not complied with the letter or spirit of the regulation.
Despite its limitations, this policy represents a meaningful advance for healthcare competition and patient empowerment. Additionally, it signals federal willingness to address the lack of price transparency as a source of widespread patient and clinician frustration—a commitment that will be needed to sustain this policy and implement additional measures in the future.
COMMENTARY AND RECOMMENDATIONS
CMS could consider five steps to augment the policy and maximize transparency and value for patients.
First, CMS could consider increasing daily nonparticipation penalties. Hospitals, particularly those in areas with less competition, have less incentive to participate given meager current penalties. Because the magnitude needed to compel action remains unknown, CMS could gradually escalate penalties over time until there is broader participation across hospitals.
Second, policymakers could aggregate price information centrally, organize the data around patients’ clinical scenarios, and advertise its availability. Currently, this information is scattered and time-consuming for hospitalists and patients to gather for decision-making. Additionally, CMS could encourage the development of third-party tools that aggregate and analyze machine-readable price data or require that prices be posted at the point of service.
Third, CMS could revise the policy to include quality as well as price information. Price alone does not offer a full enough picture of what consumers can expect from hospitals for shoppable services. Pairing price and quality information is better aligned to addressing costs in the context of value, rather than cost-cutting for its own purposes.
Fourth, over time, CMS could expand the list of services and sites required to report (eg, clinics and ambulatory surgical centers as well as hospitals).
Fifth, CMS rule-makers could set reporting standards and contextualize price information in common clinical scenarios. Patients may have difficulty shopping for complex healthcare services without understanding how they apply in different clinical situations. Decision-making would also be aided by reporting standards—for instance, for how prices are displayed and whether they include certain fees (eg, professional fees, pathology studies).
WHAT SHOULD I TELL MY PATIENT?
Hospitalists planning follow-up care should inform patients that price information is increasingly available and encourage them to search on the internet or contact hospital billing offices to request information (eg, discounted cash prices and minimum negotiated charges) before obtaining elective services after discharge. Hospitalists can also encourage patients to discuss shoppable services with their primary care physicians to understand the clinical context and make high-value decisions. Hospitalists who wish to build communication skills discussing costs with patients can increasingly find resources for these conversations and request that prices be displayed in the electronic health record for this purpose.13,14 As conversations occur, hospitalists should seek to understand other factors, such as convenience and continuity relationships, that might influence choices.
CONCLUSIONS
Starting in 2021, CMS policy requires that hospitals report prices for services such as the endoscopy recommended for the patient in the scenario. Though the policy gives patients new hope for greater transparency and better prices, additional steps are needed to help patients and hospitalists achieve these benefits.
1. Shrank WH, Rogstad TL, Parekh N. Waste in the US health care system: estimated costs and potential for savings. JAMA. 2019;322(15):1501-1509. https://doi.org/10.1001/jama.2019.13978
2. Wetzell S. Transparency: a needed step towards health care affordability. American Health Policy Institute. March 2014. Accessed August 26, 2021. https://www.americanhealthpolicy.org/Content/documents/resources/Transparency%20Study%201%20-%20The%20Need%20for%20Health%20Care%20Transparency.pdf
3. Mehrotra A, Dean KM, Sinaiko AD, Sood N. Americans support price shopping for health care, but few actually seek out price information. Health Aff (Millwood). 2017;36(8):1392-1400. https://doi.org/10.1377/hlthaff.2016.1471
4. Kullgren JT, Duey KA, Werner RM. A census of state health care price transparency websites. JAMA. 2013;309(23):2437-2438. https://doi.org/10.1001/jama.2013.6557
5. Brown ZY. Equilibrium effects of health care price information. Rev Econ Stat. 2019;101(4):699-712. https://doi.org/10.1162/rest_a_00765
6. Medicare and Medicaid Programs: CY 2020 hospital outpatient PPS policy changes and payment rates and ambulatory surgical center payment system policy changes and payment rates. Price transparency requirements for hospitals to make standard charges public. 45 CFR §180.20 (2019).
7. Kurani N, Ramirez G, Hudman J, Cox C, Kamal R. Early results from federal price transparency rule show difficulty in estimating the cost of care. Peterson-Kaiser Family Foundation. April 9, 2021. Accessed August 26, 2021. https://www.healthsystemtracker.org/brief/early-results-from-federal-price-transparency-rule-show-difficultly-in-estimating-the-cost-of-care/
8. Miller BJ, Mandelberg MC, Griffith NC, Ehrenfeld JM. Price transparency: empowering patient choice and promoting provider competition. J Med Syst. 2020;44(4):80. https://doi.org/10.1007/s10916-020-01553-2
9. Glied S. Price transparency–promise and peril. JAMA. 2021;325(15):1496-1497. https://doi.org/10.1001/jama.2021.4640
10. Haque W, Ahmadzada M, Allahrakha H, Haque E, Hsiehchen D. Transparency, accessibility, and variability of US hospital price data. JAMA Netw Open. 2021;4(5):e2110109. https://doi.org/10.1001/jamanetworkopen.2021.10109
11. Henderson M, Mouslim MC. Low compliance from big hospitals on CMS’s hospital price transparency rule. Health Affairs Blog. March 16, 2021. Accessed August 26, 2021. https://doi.org/10.1377/hblog20210311.899634
12. McGinty T, Wilde Mathews A, Evans M. Hospitals hide pricing data from search results. The Wall Street Journal. March 22, 2021. Accessed August 26, 2021. https://www.wsj.com/articles/hospitals-hide-pricing-data-from-search-results-11616405402
13. Dine CJ, Masi D, Smith CD. Tools to help overcome barriers to cost-of-care conversations. Ann Intern Med. 2019;170(9 suppl):S36-S38. https://doi.org/10.7326/M19-0778
14. Miller BJ, Slota JM, Ehrenfeld JM. Redefining the physician’s role in cost-conscious care: the potential role of the electronic health record. JAMA. 2019;322(8):721-722. https://doi.org/10.1001/jama.2019.9114
1. Shrank WH, Rogstad TL, Parekh N. Waste in the US health care system: estimated costs and potential for savings. JAMA. 2019;322(15):1501-1509. https://doi.org/10.1001/jama.2019.13978
2. Wetzell S. Transparency: a needed step towards health care affordability. American Health Policy Institute. March 2014. Accessed August 26, 2021. https://www.americanhealthpolicy.org/Content/documents/resources/Transparency%20Study%201%20-%20The%20Need%20for%20Health%20Care%20Transparency.pdf
3. Mehrotra A, Dean KM, Sinaiko AD, Sood N. Americans support price shopping for health care, but few actually seek out price information. Health Aff (Millwood). 2017;36(8):1392-1400. https://doi.org/10.1377/hlthaff.2016.1471
4. Kullgren JT, Duey KA, Werner RM. A census of state health care price transparency websites. JAMA. 2013;309(23):2437-2438. https://doi.org/10.1001/jama.2013.6557
5. Brown ZY. Equilibrium effects of health care price information. Rev Econ Stat. 2019;101(4):699-712. https://doi.org/10.1162/rest_a_00765
6. Medicare and Medicaid Programs: CY 2020 hospital outpatient PPS policy changes and payment rates and ambulatory surgical center payment system policy changes and payment rates. Price transparency requirements for hospitals to make standard charges public. 45 CFR §180.20 (2019).
7. Kurani N, Ramirez G, Hudman J, Cox C, Kamal R. Early results from federal price transparency rule show difficulty in estimating the cost of care. Peterson-Kaiser Family Foundation. April 9, 2021. Accessed August 26, 2021. https://www.healthsystemtracker.org/brief/early-results-from-federal-price-transparency-rule-show-difficultly-in-estimating-the-cost-of-care/
8. Miller BJ, Mandelberg MC, Griffith NC, Ehrenfeld JM. Price transparency: empowering patient choice and promoting provider competition. J Med Syst. 2020;44(4):80. https://doi.org/10.1007/s10916-020-01553-2
9. Glied S. Price transparency–promise and peril. JAMA. 2021;325(15):1496-1497. https://doi.org/10.1001/jama.2021.4640
10. Haque W, Ahmadzada M, Allahrakha H, Haque E, Hsiehchen D. Transparency, accessibility, and variability of US hospital price data. JAMA Netw Open. 2021;4(5):e2110109. https://doi.org/10.1001/jamanetworkopen.2021.10109
11. Henderson M, Mouslim MC. Low compliance from big hospitals on CMS’s hospital price transparency rule. Health Affairs Blog. March 16, 2021. Accessed August 26, 2021. https://doi.org/10.1377/hblog20210311.899634
12. McGinty T, Wilde Mathews A, Evans M. Hospitals hide pricing data from search results. The Wall Street Journal. March 22, 2021. Accessed August 26, 2021. https://www.wsj.com/articles/hospitals-hide-pricing-data-from-search-results-11616405402
13. Dine CJ, Masi D, Smith CD. Tools to help overcome barriers to cost-of-care conversations. Ann Intern Med. 2019;170(9 suppl):S36-S38. https://doi.org/10.7326/M19-0778
14. Miller BJ, Slota JM, Ehrenfeld JM. Redefining the physician’s role in cost-conscious care: the potential role of the electronic health record. JAMA. 2019;322(8):721-722. https://doi.org/10.1001/jama.2019.9114
© 2021 Society of Hospital Medicine
Evaluation and Medical Management of the Pediatric Patient With Orbital Cellulitis/Abscess: A Systematic Review
Orbital cellulitis/abscess (OCA) is a potential complication of sinusitis. If not treated promptly, it can result in vision loss, intracranial infection, or cavernous sinus thrombosis.1,2 In 1970, Chandler et al3 classified orbital complications of acute sinusitis into five groups: inflammatory edema (group 1); orbital cellulitis (group 2); subperiosteal abscess (SPA) (group 3); orbital abscess (group 4); and cavernous sinus thrombosis (group 5). Group 1, or preseptal cellulitis, is significantly different from groups 2, 3, and 4, collectively referred to as OCA, which affect the actual orbital content.
Children with OCA are generally hospitalized so they can be treated with intravenous antibiotics. While orbital abscesses (group 4) are typically treated surgically, successful medical management has been reported for cases of orbital cellulitis and SPA (groups 2 and 3).4,5 No widely accepted guidelines exist for the evaluation and medical management of OCA, resulting in significant variation in care.6 The purpose of this systematic review is to summarize existing evidence guiding the medical management of OCA regarding laboratory testing, imaging, and microbiology. This review does not address surgical considerations.
METHODS
The review protocol has been registered in the PROSPERO International Prospective Register of Systematic Reviews (crd.york.ac.uk/prospero/index.asp; identifier: CRD42020158463), and the review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.7
Search Strategy
A systematic search of the literature was designed and conducted by a medical librarian (ES), with input from the research team (AB, SM). The search strategy included Medical Subject Headings (MeSH) terms and keywords related to orbital or subperiosteal cellulitis/abscess and children; see Appendix Table 1 for the complete search strategy. Searches were conducted in MEDLINE (Ovid), Web of Science Core Collection, Scopus, CINAHL (EBSCO), and Cochrane Central Register of Controlled Trials (CENTRAL) using advanced search techniques relative to each database. Searches were last conducted on February 9, 2021.
Eligibility Criteria
The study designs (retrospective and prospective) included in the search were limited to randomized clinical trials, cohort studies, case-control studies, and case series with participants <18 years of age. Case reports describing fewer than 5 patients and literature reviews were excluded. Studies including a combination of adult and pediatric patients were included if pediatric outcomes were reported separately. Only studies available in English were included.
Outcome Measures
The outcome measures were determined a priori based on three clinical questions:
- Q1. What is the role of inflammatory markers—white blood cell (WBC) count, C-reactive protein (CRP), and fever—in distinguishing between the following: preseptal cellulitis (group 1) and OCA (groups 2, 3, and 4); orbital cellulitis (group 2) and abscess (groups 3 and 4); and patients who do and do not require surgery?
- Q2. What is the role of imaging in the evaluation of OCA?
- Q3. What is the microbiology of OCA over the past 2 decades? What is the prevalence of methicillin-resistant Staphylococcus aureus (MRSA)?
Screening
Two review authors (AB, SM) performed both the title/abstract and full-text screen, independently applying the eligibility criteria. Disagreements were discussed, and conflicts were resolved with input from a third reviewer author (ES). Duplications were removed. When two studies had overlapping patient data, the study with fewer data points was excluded.
Data Extraction and Synthesis
All studies included after the full-text screen were divided based on the clinical question they answered (Q1, Q2, Q3 above). Some studies reported outcomes pertinent to more than one question. Two review authors were assigned to each clinical question. They independently reviewed each article and extracted the pertinent data into question-specific extraction sheets. Articles assigned to Q2 were reviewed by two pediatric neuroradiologists. For each study, the following details were extracted: authors, location, year, study type, study period, population, and number and ages of participants. Details that were question-specific included: (Q1) values and/or percentages for inflammatory markers; (Q2) reasons for imaging or type of imaging; and (Q3) participants managed surgically and culture results. The data were then synthesized in table and/or narrative format. For Q3, the organisms identified from intraoperative and blood cultures in each study were mathematically combined. When possible, prevalence was calculated using the number of patients with at least one pathogen recovered as the denominator. If this number was not available, the number of patients who underwent surgery was used as the denominator.
Quality Assessment
No randomized controlled trials were identified. More than 90% of the studies identified and included were retrospective descriptive studies. By the nature of the case series design, the study quality was felt to be poor, with high risk of bias. The Joanna Briggs Institute Critical Appraisal tools for systematic reviews were used to appraise each individual study included (Appendix Table 2).8 The Grading of Recommendations, Assessment, Development and Evaluations (GRADE) criteria were used in rating the quality of evidence for each question.9
RESULTS
A summary of the search strategy and study selection is provided in the Figure (PRISMA flow diagram). The initial search identified 3007 studies. After duplicates were removed and general eligibility criteria applied, 94 articles remained. Question-specific eligibility criteria, discussed in the following sections, were then applied, resulting in 63 articles included in the review.
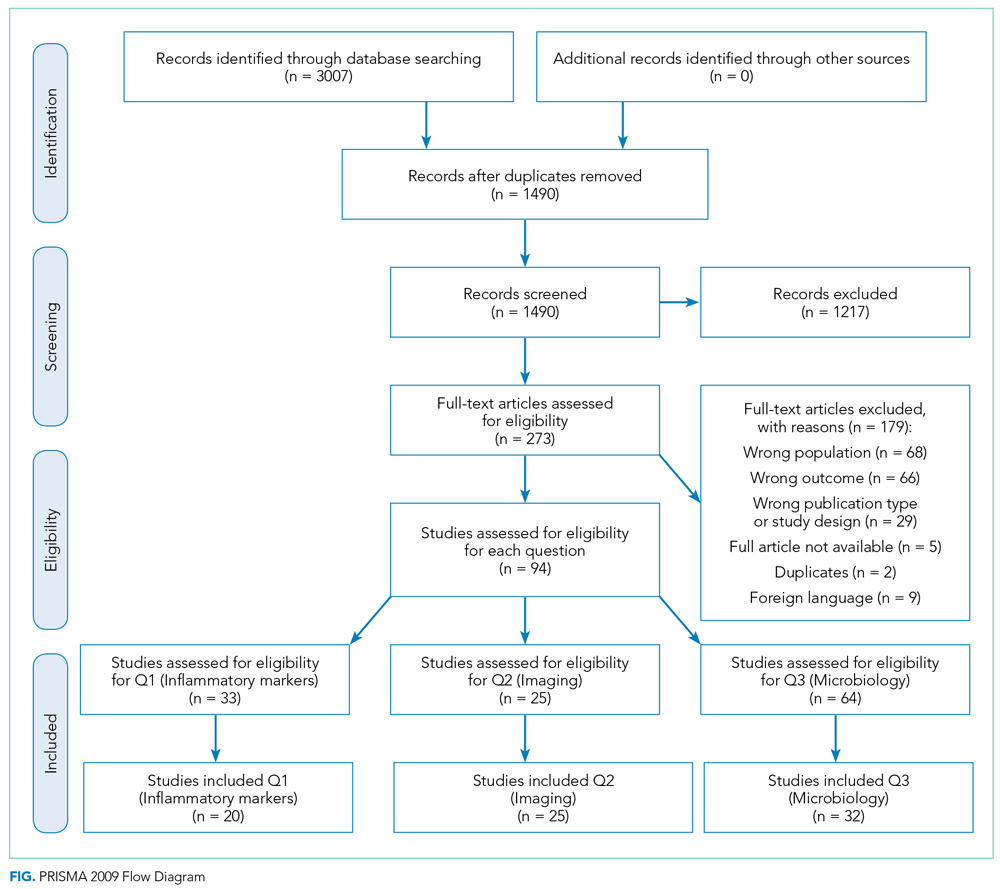
Q1: Are Inflammatory Markers, Including Fever, WBC, and CRP, Useful in Distinguishing Preseptal Cellulitis (group 1) From OCA (Groups 2, 3, and 4); Orbital Cellulitis (group 2) From Abscess (Groups 3 and 4); or Identifying Patients Who Require Surgical Intervention?
Fever and elevation of the WBC count and CRP have been used to assess the severity of certain pediatric infections10,11 and therefore may be helpful in distinguishing severity of illness in OCA. Studies included in this section provided numerical values for at least one of the following: WBC count, CRP, or percentage of patients with fever for at least one type of orbital infection. Included studies had at least five patients per group.
Thirty-three articles were screened for the inflammatory marker section. Thirteen were excluded for the following reasons: no numbers reported for inflammatory markers (n = 6); group 1 and groups 2, 3, and 4 results combined (n = 6); fewer than five patients with orbital cellulitis included (n = 1). Twenty studies were included: 18 case series and 2 retrospective cohorts. Appendix Table 3 summarizes the data from studies included. Based on GRADE criteria, the body of evidence included in this section is of low quality.9
Distinguishing Between Preseptal and OCA
Eleven studies were included in this section (Table 1). WBC count was significantly higher in patients with groups 2, 3, and 4 than group 1 in two studies (Devrim et al,12P < .01; Santos et al,13P = .025). CRP was significantly higher in patients with groups 2, 3, and 4 than group 1 in four studies (Öcal Demir et al,14P = .02; Devrim et al,12P < .01; Ohana-Sarna-Cahan et al,18P < .001; Santos et al,13P < .001). Patients with groups 2, 3, and 4 had a significantly higher fever rate in three studies (Botting et al,21P < .001; Ohana-Sarna-Cahan et al,18P = .0001; Santos et al,13 P = .029).
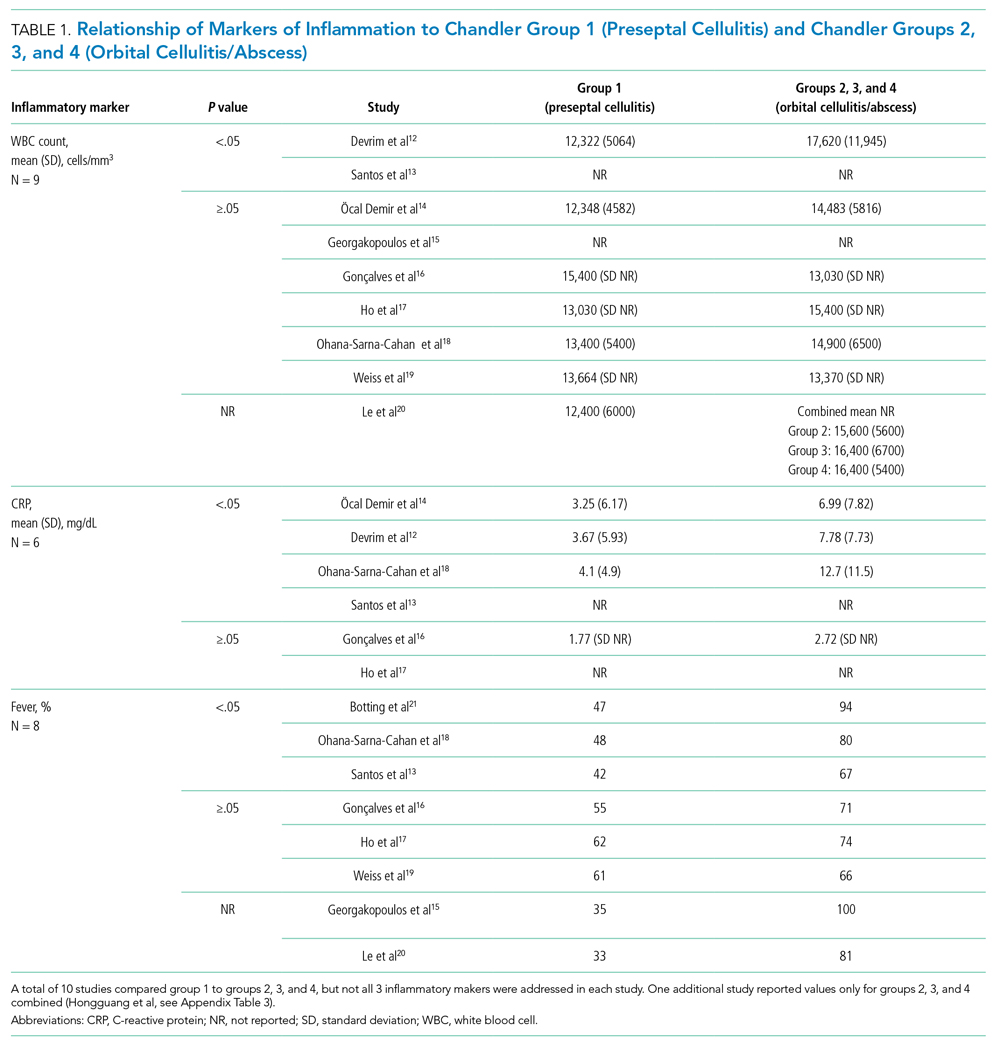
Distinguishing Between Orbital Cellulitis and Abscess
Seven studies were included in this section (Appendix Table 3). One study showed significantly higher WBC count in group 3 than group 2 (P = .004), although results were reported as percentage of patients above a cutoff number calculated to distinguish between cellulitis and abscess (Appendix Table 3).22 CRP was not significantly different between group 2 and groups 3 and 4. One study found a significantly higher fever rate in patients with group 3 compared to patients with group 2 (P < .001).22
Identifying Patients Requiring Surgery
Six studies were included in this section (Appendix Table 3). One study found a significantly higher WBC count in patients treated surgically (Tabarino et al,24P < .05). Patients treated surgically had a significantly higher CRP in two studies (Cohen et al,25P = .02; Friling et al,26 P = .04). Fever was inconsistently reported in the studies, with some using mean presenting temperatures and some using rates of fever. One study found a significantly higher mean presenting temperature in patients treated surgically (P = .027), but the difference between the two groups was 0.7 °C.23
Summary
Most studies found no significant difference in WBC count, CRP, or fever between preseptal and OCA, cellulitis and abscess, or patients receiving medical and surgical interventions.
Q2: What Is the Role of Imaging in Evaluation of OCA?
Twenty-five articles were selected for the imaging section review. All the included studies were retrospective descriptive studies. Quantitative data extraction and analysis of these studies could not be performed because of their heterogeneous methodologies and lack of objective data. Therefore, the information gleaned from these studies is summarized in narrative format. Per GRADE criteria, the body of evidence included in this section is of low quality.
Who Needs Imaging?
Proptosis, ophthalmoplegia, decreased vision, and pain with eye movements are widely agreed-upon indications for imaging evaluation.21,27,28 Because of concern for radiation exposure in pediatric patients, some authors suggested that computed tomography (CT) should only be obtained if patients fail to respond to medical therapy or if surgery is being considered.17,29,30 However, Rudloe et al31 found that half of the patients with group 3 or higher disease on CT did not have proptosis, ophthalmoplegia, or pain with extraocular movement. In addition, evaluation of young children with acute periorbital swelling can be difficult, so a lower threshold for imaging is likely warranted in these younger patients.
What Type of Imaging Should Be Obtained?
The American College of Radiology 2018 Appropriateness Criteria (ACR criteria) for orbital imaging state that orbital CT is usually indicated for patients with suspected Chandler groups 2, 3, and 4 infections.32 CT with contrast is useful for evaluating the extent of orbital infection and size of the abscess and for delineating the adjacent osseous anatomy, which is essential for cases in which surgical intervention is planned.20,21,26,27,30,31,33,34 Distinguishing abscess from cellulitis on CT sometimes can be challenging; therefore, serial clinical examinations and, occasionally, surgical exploration may be required.35,36
Magnetic resonance imaging (MRI) is helpful for evaluating intracranial complications (eg, epidural abscess),27,37 but it is limited for evaluating the osseous components of the paranasal sinuses. Although one study suggested that rapid MRI is comparable to contrast CT for differentiating group 1 infections from groups 2, 3, and 4 infections, it provided limited assessment of other complications.38 With no definitive studies comparing CT with MRI for orbital infections, adherence to the ACR criteria is recommended.
Orbital ultrasound is limited by its small field of view and artifact produced by the surrounding bony interface, both of which can obscure posterior intraorbital pathologies.29,39,40 Plain radiographs are not helpful for evaluating OCA due to limited soft-tissue contrast.41
When Should Repeat Imaging Be Obtained?
Children with group 3 OCA have been successfully managed medically in a carefully monitored setting.42 Repeat CT imaging is sometimes useful in these patients, particularly if the clinical examination is difficult.42-44 However, improvement in CT findings may lag behind clinical improvement.39
Summary
Per ACR criteria, orbital CT with contrast is recommended to evaluate patients with suspected Chandler groups 2, 3, and 4 OCA. MRI is reserved for evaluating intracranial complications.
Q3: What Is the Microbiology of OCA? What Is the MRSA Prevalence?
Knowledge of the microbiology of OCA is essential for the appropriate selection of empiric antibiotics. Because fewer children with groups 2 and 3 OCA undergo surgery, intraoperative cultures often are not available to guide antibiotic selection.45 As a result, significant variation exists in antibiotic prescribing.6
Studies discussing the microbiology of OCA were included only if they were published in the past 2 decades (2000-2020) and were excluded if the study period was before 1990, as microbiology changes over time and new vaccines are introduced. To be included, the majority of cultures reported had to be intraoperative (orbital or sinus) specimens. Studies reporting only nasal, conjunctival, or other surface cultures were excluded. When studies included patients with group 1 OCA, only microbiology data for groups 2, 3, and 4 OCA were extracted. The pattern of resistance for S aureus was not always explicitly reported; however, when non-MRSA active antibiotics were used, methicillin-susceptible S aureus was assumed.
A total of 63 studies were screened for the microbiology section; 32 were excluded for the following reasons: published before 2000 or study period before 1990 (n = 18), reported surface cultures or culture site not clearly stated (n = 4), microbiology mixed between preseptal and orbital (n = 6), wrong study type (n = 2), and study group overlaps with a different article included (n = 2). Of the 32 studies included, 3 were prospective observational, 4 were retrospective cohort, and 25 were case series. Based on GRADE criteria, the body of evidence included in this section is of low quality.42
Appendix Table 4 summarizes the microbiologic data from the studies included. In the group of children that had a positive culture (orbital, sinus, or blood), the most commonly recovered organisms reported were S aureus (median, 22%; range, 0%-100%), Streptococcus anginosus group (median, 16%; range, 0%-100%), group A Streptococcus (median, 12%; range, 0%-80%), and Streptococcus pneumoniae (median, 8%; range, 0%-100%). Streptococcus as a group had a median prevalence of 57%, ranging from 0% to 100%. MRSA prevalence had a median of 3% (interquartile range [IQR], 0%-13%). Median prevalence of polymicrobial cultures was 20%, and median prevalence of anaerobic organisms was 14% (Table 2). Orbital and sinus cultures had the highest yield, with an average return of an organism of 72% (median, 75%; IQR, 64%-84%).

Microbiology was compared between studies completed in the United States and in other countries (Table 2). Based on median prevalence across studies, both S anginosus group and MRSA were more prevalent in the United States than internationally (28% vs 0% and 11% vs 0%, respectively). No clear trend in MRSA prevalence was evident over the 2 decades; however, the studies included were heterogeneous and did not have the power to detect such a trend.
Two reports suggest a difference of MRSA prevalence by patient age. Hsu et al46 found that three of eight MRSA infections were in infants age <1 year, which accounted for 50% (3/6) of infants included in the study. Miller et al47 reported MRSA in 4 of 9 (44%) infants with OCA. Age <1 year may be associated with increased frequency of MRSA infection in OCA.
Summary
Blood cultures have low yield. The most common organisms recovered from OCA are Streptococcus species (most commonly S anginosus group, group A Streptococcus, and pneumococcus) and S aureus. Polymicrobial infections including anaerobes are common. MRSA prevalence is low globally but varies significantly among geographic areas.
DISCUSSION
Our systematic review of the literature for the medical management of OCA revealed predominantly descriptive studies and only a limited number of comparison-based studies, likely reflecting the rarity of advanced forms of OCA. Given the lack of high-quality evidence and the level of heterogeneity among studies, the conclusions that can be drawn are limited.
Distinguishing between disease severity and OCA requiring surgical intervention remains challenging. Although studies in our review suggest a trend toward markers of inflammation (fever, elevated WBC count and CRP) being more common in more severe presentations, the results were mixed, and studies were low quality and underpowered to detect meaningful differences. For example, most studies do not define what constitutes a fever in their cohort. Our review suggests that markers of inflammation cannot be used to distinguish between Chandler groups or to identify patients requiring surgery. Of note, the presence of fever and elevated inflammatory markers may have influenced the decision to obtain imaging or to proceed to surgery, thereby also potentially biasing these clinical indicators toward predictors for more severe disease. Decisions regarding surgery should therefore be based on the entire clinical picture, including response to appropriate antibiotics.
We found a lack of high-quality evidence regarding the role of imaging in OCA, and the studies reviewed were heterogeneous. Recommendations for imaging therefore remain at the level of expert opinion (ACR criteria). CT imaging is the first-line modality for imaging in suspected OCA given the limitations of alternative imaging modalities, but the sensitivity and specificity of CT imaging remain unknown for diagnosis of orbital abscesses.
Our review of the published microbiology confirmed that Staphylococcus and Streptococcus species are the most common pathogens identified in OCA. Prevalence across the different studies varied greatly. Owing to the significant heterogeneity in studies, calculation of pooled prevalence was not possible. By using the number of positive cultures as our denominator (or total surgeries if number of positive cultures was unavailable), we likely overestimated the prevalence of S aureus. S aureus is generally recognized as a pyogenic pathogen, more likely to be associated with abscess formation.48 Therefore, culture results obtained predominantly from abscesses likely result in an overestimate of S aureus in OCA (groups 2, 3, and 4). Regardless, MRSA prevalence was generally low, both nationally and internationally. The MRSA results from the study by McKinley at el49 (Texas) was a notable outlier in the United States, with MRSA prevalence as high as 44% compared with the median prevalence of 3% (IQR, 0-13), highlighting the importance of local resistance patterns when choosing empiric antibiotics.
Limitations to the microbiology review included significant heterogeneity in both the types of cultures included and the reporting of results. Although we excluded studies that reported only surface culture results or did not specify culture type, we did include studies that had surface culture results combined with intraoperative culture results, making it impossible to separate the two. Since most of the cultures included in combined results reported organisms based on intraoperative cultures, we felt they provided valuable information that should be included. In most studies, blood cultures were not obtained in all participants, so the yield of blood cultures is likely an overestimate, as blood cultures are more likely to be obtained in higher-acuity patients.
CONCLUSION
Although the available evidence regarding the medical management of OCA remains low quality, certain limited conclusions can be drawn, as presented in this review. Further high-quality studies are needed to better inform the medical management of OCA.
Acknowledgment
The authors thank Dr Kyle Pronko for his help with data extraction for the imaging section.
1. Reynolds D.J, Kodsi SR, Rubin SE, Rodgers IR. Intracranial infection associated with preseptal and orbital cellulitis in the pediatric patient. J AAPOS. 2003;7(6):413-417. https://doi.org/10.1016/j.jaapos.2003.09.013
2. Chaudhry IA, Shamsi FA, Elzaridi E, et al. Outcome of treated orbital cellulitis in a tertiary eye care center in the Middle East. Ophthalmology. 2007;114(2):345-354. https://doi.org/10.1016/j.ophtha.2006.07.059
3. Chandler JR, Langenbrunner DJ, Stevens ER. Pathogenesis of orbital complications in acute sinusitis. Laryngoscope. 1970;1414-1428. https://doi.org/10.1288/00005537-197009000-00007
4. Wong SJ, Levi J. Management of pediatric orbital cellulitis: a systematic review. Int J Pediatr Otorhinolaryngol. 2018;110:123-129. https://doi.org/10.1016/j.ijporl.2018.05.006
5. Liao JC, Harris GJ. Subperiosteal abscess of the orbit: evolving pathogens and the therapeutic protocol. Ophthalmology. 2015;122(3):639-647. https://doi.org/10.1016/j.ophtha.2014.09.009
6. Markham JL, Hall M, Bettenhausen JL, Myers AL, Puls HT, McColloh RJ. Variation in care and clinical outcomes in children hospitalized with orbital cellulitis. Hosp Pediatr. 2018;8(1):28-35. https://doi.org/10.1542/hpeds.2017-0040
7. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
8. Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18(10):2127-2133. https://doi.org/10.11124/JBISRIR-D-19-00099
9. Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401-406. https://doi.org/10.1016/j.jclinepi.2010.07.015
10. Dean P, Florin TA. Factors associated with pneumonia severity in children: a systematic review. J Pediatric Infect Dis Soc. 2018;7(4):323-334. https://doi.org/10.1093/jpids/piy046
11. Hofer N, Zacharias E, Müller W, Resch B. An update on the use of C-reactive protein in early-onset neonatal sepsis: current insights and new tasks. Neonatology. 2012;102(1):25-36. https://doi.org/10.1159/000336629
12. Devrim I, Kanra G, Kara A, et al. Preseptal and orbital cellulitis: 15-year experience with sulbactam ampicillin treatment. Turk J Pediatr. 2008;50(3):214-218.
13. Santos JC, Pinto S, Ferreira S, Maia C, Alves S, da Silva V. Pediatric preseptal and orbital cellulitis: a 10-year experience. Int J Pediatr Otorhinolaryngol. 2019;120:82-88. https://doi.org/10.1016/j.ijporl.2019.02.003
14. Öcal Demir S , Çagan E, Kepenekli Kadayifci E, et al. Clinical features and outcome of preseptal and orbital cellulitis in hospitalized children: four years experience. Medeni Med J. 2017;32(1):7-13. https://doi.org/10.5222/MMJ.2017.007
15. Georgakopoulos CD, Eliopoulou MI, Stasinos S, Exarchou A, Pharmakakis N, Varvarigou A. Periorbital and orbitaln cellulitis: a 10-year review of hospitalized children. Eur J Ophthalmol. 2010;20(6):1066-1072. https://doi.org/10.1177/112067211002000607
16. Gonçalves R, Menezes C, Machado R, Ribeiro I, Lemos JA. Periorbital cellulitis in children: analysis of outcome of intravenous antibiotic therapy. Orbit. 2016;34(4):175-180. https://doi.org/10.1080/01676830.2016.1176205
17. Ho CF, Huang YC, Wang CJ, Chiu CH, Lin TY. Clinical analysis of computed tomography-staged orbital cellulitis in children. J Microbiol Immunol Infect. 2017;40(6):518-524.
18. Ohana-Sarna-Cahan L, Hurvitz N, Gross I, Cohen A, Hashavya S. Factors associated with increased risk of pediatric orbital cellulitis—who should be scanned? Pediatr Emerg Care. Published online ahead of print March 19, 2020. https://doi.org/10.1097/PEC.0000000000002083
19. Weiss A, Friendly D, Eglin K, Chang M, Gold B. Bacterial periorbital and orbital cellulitis in childhood. Ophthalmology. 1983;90(3):195-203. https://doi.org/10.1016/s0161-6420(83)34573-5
20. Le TD, Liu ES, Adatia FA, Buncic JR Blaser S. The effect of adding orbital computed tomography findings to the Chandler criteria for classifying pediatric orbital cellulitis in predicting which patients will require surgical intervention. J AAPOS. 2014;18(3):271-277. https://doi.org/10.1016/j.jaapos.2014.01.015
21. Botting AM, McIntosh D, Mahadevan M. Paediatric pre- and post-septal peri-orbital infections are different diseases. A retrospective review of 262 cases. Int J Pediatr Otorhinolaryngol. 2008;72(3):377-383. https://doi.org/10.1016/j.ijporl.2007.11.013
22. Huang SF, Lee TJ, Lee YS, Chen CC, Chin SC, Wang NC. Acute rhinosinusitis-related orbital infection in pediatric patients: a retrospective analysis. Ann Otol Rhinol Laryngol. 2011;120(3):185-190. https://doi.org/10.1177/000348941112000307
23. Ryan JT, Preciado A, Bauman N, et al. Management of pediatric orbital cellulitis in patients with radiographic findings of subperiosteal abscess. Otolaryngol Head Neck Surg. 2009;140(6):907-911. https://doi.org/10.1016/j.otohns.2009.02.014
24. Tabarino F, Elmaleh-Bergès M, Quesnel S, Lorrot M, Van Den Abbeele T, Teissier N. Subperiosteal orbital abscess: volumetric criteria for surgical drainage. Int J Pediatr Otorhinolaryngol. 2015;79(2):131-135. https://doi.org/10.1016/j.ijporl.2014.11.021
25. Cohen N, Erisson S, Anafy A, et al. Clinicians need to consider surgery when presented with some markers for severe paediatric orbital cellulitis. Acta Paediatr. 2020;109(6):1269-1270. https://doi.org/10.1111/apa.15125
26. Friling R, Garty BZ, Kornreich L, et al. Medical and surgical management of orbital cellulitis in children. Folia Med (Plovdiv). 2014;56(4):253-258. https://doi.org/10.1515/folmed-2015-0004
27. Gavriel H, Yeheskeli E, Aviram E, Yehoshua L, Eviatar E. Dimension of subperiosteal orbital abscess as an indication for surgical management in children. Otolaryngol Head Neck Surg. 2011;145(5):823-827. https://doi.org/10.1177/0194599811416559
28. Mathew AV, Craig E, Al-Mahmoud R, et al. Paediatric post-septal and pre-septal cellulitis: 10 years’ experience at a tertiary-level children’s hospital. Br J Radiol. 2014;87(1033):20130503. https://doi.org/10.1259/bjr.20130503
29. Goodwin WJ Jr, Weinshall M, Chandler JR. The role of high resolution computerized tomography and standardized ultrasound in the evaluation of orbital cellulitis. Laryngoscope. 1982;92(7 pt 1):729-731.
30. Bilaniuk LT, Zimmerman RA. Computer‐assisted tomography: sinus lesions with orbital involvement. Head Neck Surg. 1980;2(4):293-301. https://doi.org/10.1002/hed.2890020407
31. Rudloe TF, Harper MB, Prabhu SP, Rahbar R, Vanderveen D, Kimia AA. Acute periorbital infections: who needs emergent imaging? Pediatrics. 2010;125(4):e719-e726. https://doi.org/10.1542/peds.2009-1709
32. Kennedy TA, Corey AS, Policeni B, et al. ACR Appropriateness Criteria® orbits vision and visual loss. J Am Coll Radiol. 2018;15(5S):S116-S131. https://doi.org/10.1016/j.jacr.2018.03.023
33. De Silva M, Lam V, Broadfoot J. C.T. findings of orbital inflammation in children. Australas Radiol. 1987;31(3):241-245. https://doi.org/10.1111/j.1440-1673.1987.tb01822.x
34. Hirsch M, Lifshitz T. Computerized tomography in the diagnosis and treatment of orbital cellulitis. Pediatr Radiol. 1988;18(4):302-305. https://doi.org/10.1007/BF02388996
35. Andrews TM, Myer CM 3rd. The role of computed tomography in the diagnosis of subperiosteal abscess of the orbit. Clin Pediatr (Phila). 1992;31(1):37-43. https://doi.org/10.1177/000992289203100108
36. Clary RA, Cunningham MJ, Eavey RD. Orbital complications of acute sinusitis: comparison of computed tomography scan and surgical findings. Ann Otol Rhinol Laryngol. 1992;101(7):598-600. https://doi.org/10.1177/000348949210100710
37. Arjmand EM, LuskRP, Muntz HR. Pediatric sinusitis and subperiosteal orbital abscess formation: diagnosis and treatment. Otolaryngol Neck Surg. 1993;109(5):886.894. https://doi.org/10.1177/019459989310900518
38. Jain SF, Ishihara R, Wheelock L, et al. Feasibility of rapid magnetic resonance imaging (rMRI) for the emergency evaluation of suspected pediatric orbital cellulitis. J AAPOS. 2020;24(5):289.e1-289.e4. https://doi.org/10.1016/j.jaapos.2020.05.018
39. Harris GJ. Subperiosteal abscess of the orbit: computed tomography and the clinical course. Ophthal Plast Reconstr Surg. 1996;12:1-8. https://doi.org/10.1097/00002341-199603000-00001
40. Kaplan DM, Briscoe D, Gatot A, Niv A, Leiberman A, Fliss DM. The use of standardized orbital ultrasound in the diagnosis of sinus induced infections of the orbit in children: a preliminary report. Int J Pediatr Otorhinolaryngol. 1999;48(2):155-162. https://doi.org/10.1016/s0165-5876(99)00023-3
41. Towbin R, Han BK, Kaufman RA, Burke M. Postseptal cellulitis: CT in diagnosis and management. Radiology. 1986;158(3):735-737. https://doi.org/10.1148/radiology.158.3.3945747
42. Starkey CR, Steele RW. Medical management of orbital cellulitis. Pediatr Infect Dis J. 2001;20(10):1002-1005. https://doi.org/10.1097/00006454-200110000-00017
43. Brown CL, Graham SM, Griffin MC, et al. Pediatric medial subperiosteal orbital abscess: medical management where possible. Am J Rhinol. 2004;18(5):321-327.
44. Cossack MT, Herretes SP, Cham A, Sniegowski MC, Lyon DB. Radiographic course of medically managed pediatric orbital subperiosteal abscesses. J Pediatr Ophthalmol Strabismus. 2018;55(6):387-392. https://doi.org/10.3928/01913913-20180802-02
45. Zhao EE, Koochakzadeh S, Nguyen SA, et al. Orbital complications of acute bacterial rhinosinusitis in the pediatric population: a systematic review and meta-analysis. Int J Pediatr Otorhinolaryngol. 2020;135:110078. https://doi.org/10.1016/j.ijporl.2020.110078
46. Hsu J, Treister AD, Ralay Ranaivo H, Rowley AH, Rahmani B. Microbiology of pediatric orbital cellulitis and trends in methicillin-resistant Staphylococcus aureus cases. Clin Pediatr (Phila). 2019;58(10):1056-1062. https://doi.org/10.1177/0009922819864587
47. Miller A, Castanes M, Yen M, Coats D, Yen K. Infantile orbital cellulitis. Ophthalmology. 2008;115(3):594. https://doi.org/10.1016/j.ophtha.2007.10.011
48. Dajani AS, Garcia RE, Wolinsky E. Etiology of cervical lymphadenitis in children. N Engl J Med. 1963;268:1329-1333. https://doi.org/10.1056/NEJM196306132682403
49. McKinley SH, Yen MT, Miller AM, Yen KG. Microbiology of pediatric orbital cellulitis. Am J Ophthalmol. 2007;144(4):497-501. https://doi.org/10.1016/j.ajo.2007.04.049
Orbital cellulitis/abscess (OCA) is a potential complication of sinusitis. If not treated promptly, it can result in vision loss, intracranial infection, or cavernous sinus thrombosis.1,2 In 1970, Chandler et al3 classified orbital complications of acute sinusitis into five groups: inflammatory edema (group 1); orbital cellulitis (group 2); subperiosteal abscess (SPA) (group 3); orbital abscess (group 4); and cavernous sinus thrombosis (group 5). Group 1, or preseptal cellulitis, is significantly different from groups 2, 3, and 4, collectively referred to as OCA, which affect the actual orbital content.
Children with OCA are generally hospitalized so they can be treated with intravenous antibiotics. While orbital abscesses (group 4) are typically treated surgically, successful medical management has been reported for cases of orbital cellulitis and SPA (groups 2 and 3).4,5 No widely accepted guidelines exist for the evaluation and medical management of OCA, resulting in significant variation in care.6 The purpose of this systematic review is to summarize existing evidence guiding the medical management of OCA regarding laboratory testing, imaging, and microbiology. This review does not address surgical considerations.
METHODS
The review protocol has been registered in the PROSPERO International Prospective Register of Systematic Reviews (crd.york.ac.uk/prospero/index.asp; identifier: CRD42020158463), and the review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.7
Search Strategy
A systematic search of the literature was designed and conducted by a medical librarian (ES), with input from the research team (AB, SM). The search strategy included Medical Subject Headings (MeSH) terms and keywords related to orbital or subperiosteal cellulitis/abscess and children; see Appendix Table 1 for the complete search strategy. Searches were conducted in MEDLINE (Ovid), Web of Science Core Collection, Scopus, CINAHL (EBSCO), and Cochrane Central Register of Controlled Trials (CENTRAL) using advanced search techniques relative to each database. Searches were last conducted on February 9, 2021.
Eligibility Criteria
The study designs (retrospective and prospective) included in the search were limited to randomized clinical trials, cohort studies, case-control studies, and case series with participants <18 years of age. Case reports describing fewer than 5 patients and literature reviews were excluded. Studies including a combination of adult and pediatric patients were included if pediatric outcomes were reported separately. Only studies available in English were included.
Outcome Measures
The outcome measures were determined a priori based on three clinical questions:
- Q1. What is the role of inflammatory markers—white blood cell (WBC) count, C-reactive protein (CRP), and fever—in distinguishing between the following: preseptal cellulitis (group 1) and OCA (groups 2, 3, and 4); orbital cellulitis (group 2) and abscess (groups 3 and 4); and patients who do and do not require surgery?
- Q2. What is the role of imaging in the evaluation of OCA?
- Q3. What is the microbiology of OCA over the past 2 decades? What is the prevalence of methicillin-resistant Staphylococcus aureus (MRSA)?
Screening
Two review authors (AB, SM) performed both the title/abstract and full-text screen, independently applying the eligibility criteria. Disagreements were discussed, and conflicts were resolved with input from a third reviewer author (ES). Duplications were removed. When two studies had overlapping patient data, the study with fewer data points was excluded.
Data Extraction and Synthesis
All studies included after the full-text screen were divided based on the clinical question they answered (Q1, Q2, Q3 above). Some studies reported outcomes pertinent to more than one question. Two review authors were assigned to each clinical question. They independently reviewed each article and extracted the pertinent data into question-specific extraction sheets. Articles assigned to Q2 were reviewed by two pediatric neuroradiologists. For each study, the following details were extracted: authors, location, year, study type, study period, population, and number and ages of participants. Details that were question-specific included: (Q1) values and/or percentages for inflammatory markers; (Q2) reasons for imaging or type of imaging; and (Q3) participants managed surgically and culture results. The data were then synthesized in table and/or narrative format. For Q3, the organisms identified from intraoperative and blood cultures in each study were mathematically combined. When possible, prevalence was calculated using the number of patients with at least one pathogen recovered as the denominator. If this number was not available, the number of patients who underwent surgery was used as the denominator.
Quality Assessment
No randomized controlled trials were identified. More than 90% of the studies identified and included were retrospective descriptive studies. By the nature of the case series design, the study quality was felt to be poor, with high risk of bias. The Joanna Briggs Institute Critical Appraisal tools for systematic reviews were used to appraise each individual study included (Appendix Table 2).8 The Grading of Recommendations, Assessment, Development and Evaluations (GRADE) criteria were used in rating the quality of evidence for each question.9
RESULTS
A summary of the search strategy and study selection is provided in the Figure (PRISMA flow diagram). The initial search identified 3007 studies. After duplicates were removed and general eligibility criteria applied, 94 articles remained. Question-specific eligibility criteria, discussed in the following sections, were then applied, resulting in 63 articles included in the review.

Q1: Are Inflammatory Markers, Including Fever, WBC, and CRP, Useful in Distinguishing Preseptal Cellulitis (group 1) From OCA (Groups 2, 3, and 4); Orbital Cellulitis (group 2) From Abscess (Groups 3 and 4); or Identifying Patients Who Require Surgical Intervention?
Fever and elevation of the WBC count and CRP have been used to assess the severity of certain pediatric infections10,11 and therefore may be helpful in distinguishing severity of illness in OCA. Studies included in this section provided numerical values for at least one of the following: WBC count, CRP, or percentage of patients with fever for at least one type of orbital infection. Included studies had at least five patients per group.
Thirty-three articles were screened for the inflammatory marker section. Thirteen were excluded for the following reasons: no numbers reported for inflammatory markers (n = 6); group 1 and groups 2, 3, and 4 results combined (n = 6); fewer than five patients with orbital cellulitis included (n = 1). Twenty studies were included: 18 case series and 2 retrospective cohorts. Appendix Table 3 summarizes the data from studies included. Based on GRADE criteria, the body of evidence included in this section is of low quality.9
Distinguishing Between Preseptal and OCA
Eleven studies were included in this section (Table 1). WBC count was significantly higher in patients with groups 2, 3, and 4 than group 1 in two studies (Devrim et al,12P < .01; Santos et al,13P = .025). CRP was significantly higher in patients with groups 2, 3, and 4 than group 1 in four studies (Öcal Demir et al,14P = .02; Devrim et al,12P < .01; Ohana-Sarna-Cahan et al,18P < .001; Santos et al,13P < .001). Patients with groups 2, 3, and 4 had a significantly higher fever rate in three studies (Botting et al,21P < .001; Ohana-Sarna-Cahan et al,18P = .0001; Santos et al,13 P = .029).

Distinguishing Between Orbital Cellulitis and Abscess
Seven studies were included in this section (Appendix Table 3). One study showed significantly higher WBC count in group 3 than group 2 (P = .004), although results were reported as percentage of patients above a cutoff number calculated to distinguish between cellulitis and abscess (Appendix Table 3).22 CRP was not significantly different between group 2 and groups 3 and 4. One study found a significantly higher fever rate in patients with group 3 compared to patients with group 2 (P < .001).22
Identifying Patients Requiring Surgery
Six studies were included in this section (Appendix Table 3). One study found a significantly higher WBC count in patients treated surgically (Tabarino et al,24P < .05). Patients treated surgically had a significantly higher CRP in two studies (Cohen et al,25P = .02; Friling et al,26 P = .04). Fever was inconsistently reported in the studies, with some using mean presenting temperatures and some using rates of fever. One study found a significantly higher mean presenting temperature in patients treated surgically (P = .027), but the difference between the two groups was 0.7 °C.23
Summary
Most studies found no significant difference in WBC count, CRP, or fever between preseptal and OCA, cellulitis and abscess, or patients receiving medical and surgical interventions.
Q2: What Is the Role of Imaging in Evaluation of OCA?
Twenty-five articles were selected for the imaging section review. All the included studies were retrospective descriptive studies. Quantitative data extraction and analysis of these studies could not be performed because of their heterogeneous methodologies and lack of objective data. Therefore, the information gleaned from these studies is summarized in narrative format. Per GRADE criteria, the body of evidence included in this section is of low quality.
Who Needs Imaging?
Proptosis, ophthalmoplegia, decreased vision, and pain with eye movements are widely agreed-upon indications for imaging evaluation.21,27,28 Because of concern for radiation exposure in pediatric patients, some authors suggested that computed tomography (CT) should only be obtained if patients fail to respond to medical therapy or if surgery is being considered.17,29,30 However, Rudloe et al31 found that half of the patients with group 3 or higher disease on CT did not have proptosis, ophthalmoplegia, or pain with extraocular movement. In addition, evaluation of young children with acute periorbital swelling can be difficult, so a lower threshold for imaging is likely warranted in these younger patients.
What Type of Imaging Should Be Obtained?
The American College of Radiology 2018 Appropriateness Criteria (ACR criteria) for orbital imaging state that orbital CT is usually indicated for patients with suspected Chandler groups 2, 3, and 4 infections.32 CT with contrast is useful for evaluating the extent of orbital infection and size of the abscess and for delineating the adjacent osseous anatomy, which is essential for cases in which surgical intervention is planned.20,21,26,27,30,31,33,34 Distinguishing abscess from cellulitis on CT sometimes can be challenging; therefore, serial clinical examinations and, occasionally, surgical exploration may be required.35,36
Magnetic resonance imaging (MRI) is helpful for evaluating intracranial complications (eg, epidural abscess),27,37 but it is limited for evaluating the osseous components of the paranasal sinuses. Although one study suggested that rapid MRI is comparable to contrast CT for differentiating group 1 infections from groups 2, 3, and 4 infections, it provided limited assessment of other complications.38 With no definitive studies comparing CT with MRI for orbital infections, adherence to the ACR criteria is recommended.
Orbital ultrasound is limited by its small field of view and artifact produced by the surrounding bony interface, both of which can obscure posterior intraorbital pathologies.29,39,40 Plain radiographs are not helpful for evaluating OCA due to limited soft-tissue contrast.41
When Should Repeat Imaging Be Obtained?
Children with group 3 OCA have been successfully managed medically in a carefully monitored setting.42 Repeat CT imaging is sometimes useful in these patients, particularly if the clinical examination is difficult.42-44 However, improvement in CT findings may lag behind clinical improvement.39
Summary
Per ACR criteria, orbital CT with contrast is recommended to evaluate patients with suspected Chandler groups 2, 3, and 4 OCA. MRI is reserved for evaluating intracranial complications.
Q3: What Is the Microbiology of OCA? What Is the MRSA Prevalence?
Knowledge of the microbiology of OCA is essential for the appropriate selection of empiric antibiotics. Because fewer children with groups 2 and 3 OCA undergo surgery, intraoperative cultures often are not available to guide antibiotic selection.45 As a result, significant variation exists in antibiotic prescribing.6
Studies discussing the microbiology of OCA were included only if they were published in the past 2 decades (2000-2020) and were excluded if the study period was before 1990, as microbiology changes over time and new vaccines are introduced. To be included, the majority of cultures reported had to be intraoperative (orbital or sinus) specimens. Studies reporting only nasal, conjunctival, or other surface cultures were excluded. When studies included patients with group 1 OCA, only microbiology data for groups 2, 3, and 4 OCA were extracted. The pattern of resistance for S aureus was not always explicitly reported; however, when non-MRSA active antibiotics were used, methicillin-susceptible S aureus was assumed.
A total of 63 studies were screened for the microbiology section; 32 were excluded for the following reasons: published before 2000 or study period before 1990 (n = 18), reported surface cultures or culture site not clearly stated (n = 4), microbiology mixed between preseptal and orbital (n = 6), wrong study type (n = 2), and study group overlaps with a different article included (n = 2). Of the 32 studies included, 3 were prospective observational, 4 were retrospective cohort, and 25 were case series. Based on GRADE criteria, the body of evidence included in this section is of low quality.42
Appendix Table 4 summarizes the microbiologic data from the studies included. In the group of children that had a positive culture (orbital, sinus, or blood), the most commonly recovered organisms reported were S aureus (median, 22%; range, 0%-100%), Streptococcus anginosus group (median, 16%; range, 0%-100%), group A Streptococcus (median, 12%; range, 0%-80%), and Streptococcus pneumoniae (median, 8%; range, 0%-100%). Streptococcus as a group had a median prevalence of 57%, ranging from 0% to 100%. MRSA prevalence had a median of 3% (interquartile range [IQR], 0%-13%). Median prevalence of polymicrobial cultures was 20%, and median prevalence of anaerobic organisms was 14% (Table 2). Orbital and sinus cultures had the highest yield, with an average return of an organism of 72% (median, 75%; IQR, 64%-84%).

Microbiology was compared between studies completed in the United States and in other countries (Table 2). Based on median prevalence across studies, both S anginosus group and MRSA were more prevalent in the United States than internationally (28% vs 0% and 11% vs 0%, respectively). No clear trend in MRSA prevalence was evident over the 2 decades; however, the studies included were heterogeneous and did not have the power to detect such a trend.
Two reports suggest a difference of MRSA prevalence by patient age. Hsu et al46 found that three of eight MRSA infections were in infants age <1 year, which accounted for 50% (3/6) of infants included in the study. Miller et al47 reported MRSA in 4 of 9 (44%) infants with OCA. Age <1 year may be associated with increased frequency of MRSA infection in OCA.
Summary
Blood cultures have low yield. The most common organisms recovered from OCA are Streptococcus species (most commonly S anginosus group, group A Streptococcus, and pneumococcus) and S aureus. Polymicrobial infections including anaerobes are common. MRSA prevalence is low globally but varies significantly among geographic areas.
DISCUSSION
Our systematic review of the literature for the medical management of OCA revealed predominantly descriptive studies and only a limited number of comparison-based studies, likely reflecting the rarity of advanced forms of OCA. Given the lack of high-quality evidence and the level of heterogeneity among studies, the conclusions that can be drawn are limited.
Distinguishing between disease severity and OCA requiring surgical intervention remains challenging. Although studies in our review suggest a trend toward markers of inflammation (fever, elevated WBC count and CRP) being more common in more severe presentations, the results were mixed, and studies were low quality and underpowered to detect meaningful differences. For example, most studies do not define what constitutes a fever in their cohort. Our review suggests that markers of inflammation cannot be used to distinguish between Chandler groups or to identify patients requiring surgery. Of note, the presence of fever and elevated inflammatory markers may have influenced the decision to obtain imaging or to proceed to surgery, thereby also potentially biasing these clinical indicators toward predictors for more severe disease. Decisions regarding surgery should therefore be based on the entire clinical picture, including response to appropriate antibiotics.
We found a lack of high-quality evidence regarding the role of imaging in OCA, and the studies reviewed were heterogeneous. Recommendations for imaging therefore remain at the level of expert opinion (ACR criteria). CT imaging is the first-line modality for imaging in suspected OCA given the limitations of alternative imaging modalities, but the sensitivity and specificity of CT imaging remain unknown for diagnosis of orbital abscesses.
Our review of the published microbiology confirmed that Staphylococcus and Streptococcus species are the most common pathogens identified in OCA. Prevalence across the different studies varied greatly. Owing to the significant heterogeneity in studies, calculation of pooled prevalence was not possible. By using the number of positive cultures as our denominator (or total surgeries if number of positive cultures was unavailable), we likely overestimated the prevalence of S aureus. S aureus is generally recognized as a pyogenic pathogen, more likely to be associated with abscess formation.48 Therefore, culture results obtained predominantly from abscesses likely result in an overestimate of S aureus in OCA (groups 2, 3, and 4). Regardless, MRSA prevalence was generally low, both nationally and internationally. The MRSA results from the study by McKinley at el49 (Texas) was a notable outlier in the United States, with MRSA prevalence as high as 44% compared with the median prevalence of 3% (IQR, 0-13), highlighting the importance of local resistance patterns when choosing empiric antibiotics.
Limitations to the microbiology review included significant heterogeneity in both the types of cultures included and the reporting of results. Although we excluded studies that reported only surface culture results or did not specify culture type, we did include studies that had surface culture results combined with intraoperative culture results, making it impossible to separate the two. Since most of the cultures included in combined results reported organisms based on intraoperative cultures, we felt they provided valuable information that should be included. In most studies, blood cultures were not obtained in all participants, so the yield of blood cultures is likely an overestimate, as blood cultures are more likely to be obtained in higher-acuity patients.
CONCLUSION
Although the available evidence regarding the medical management of OCA remains low quality, certain limited conclusions can be drawn, as presented in this review. Further high-quality studies are needed to better inform the medical management of OCA.
Acknowledgment
The authors thank Dr Kyle Pronko for his help with data extraction for the imaging section.
Orbital cellulitis/abscess (OCA) is a potential complication of sinusitis. If not treated promptly, it can result in vision loss, intracranial infection, or cavernous sinus thrombosis.1,2 In 1970, Chandler et al3 classified orbital complications of acute sinusitis into five groups: inflammatory edema (group 1); orbital cellulitis (group 2); subperiosteal abscess (SPA) (group 3); orbital abscess (group 4); and cavernous sinus thrombosis (group 5). Group 1, or preseptal cellulitis, is significantly different from groups 2, 3, and 4, collectively referred to as OCA, which affect the actual orbital content.
Children with OCA are generally hospitalized so they can be treated with intravenous antibiotics. While orbital abscesses (group 4) are typically treated surgically, successful medical management has been reported for cases of orbital cellulitis and SPA (groups 2 and 3).4,5 No widely accepted guidelines exist for the evaluation and medical management of OCA, resulting in significant variation in care.6 The purpose of this systematic review is to summarize existing evidence guiding the medical management of OCA regarding laboratory testing, imaging, and microbiology. This review does not address surgical considerations.
METHODS
The review protocol has been registered in the PROSPERO International Prospective Register of Systematic Reviews (crd.york.ac.uk/prospero/index.asp; identifier: CRD42020158463), and the review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.7
Search Strategy
A systematic search of the literature was designed and conducted by a medical librarian (ES), with input from the research team (AB, SM). The search strategy included Medical Subject Headings (MeSH) terms and keywords related to orbital or subperiosteal cellulitis/abscess and children; see Appendix Table 1 for the complete search strategy. Searches were conducted in MEDLINE (Ovid), Web of Science Core Collection, Scopus, CINAHL (EBSCO), and Cochrane Central Register of Controlled Trials (CENTRAL) using advanced search techniques relative to each database. Searches were last conducted on February 9, 2021.
Eligibility Criteria
The study designs (retrospective and prospective) included in the search were limited to randomized clinical trials, cohort studies, case-control studies, and case series with participants <18 years of age. Case reports describing fewer than 5 patients and literature reviews were excluded. Studies including a combination of adult and pediatric patients were included if pediatric outcomes were reported separately. Only studies available in English were included.
Outcome Measures
The outcome measures were determined a priori based on three clinical questions:
- Q1. What is the role of inflammatory markers—white blood cell (WBC) count, C-reactive protein (CRP), and fever—in distinguishing between the following: preseptal cellulitis (group 1) and OCA (groups 2, 3, and 4); orbital cellulitis (group 2) and abscess (groups 3 and 4); and patients who do and do not require surgery?
- Q2. What is the role of imaging in the evaluation of OCA?
- Q3. What is the microbiology of OCA over the past 2 decades? What is the prevalence of methicillin-resistant Staphylococcus aureus (MRSA)?
Screening
Two review authors (AB, SM) performed both the title/abstract and full-text screen, independently applying the eligibility criteria. Disagreements were discussed, and conflicts were resolved with input from a third reviewer author (ES). Duplications were removed. When two studies had overlapping patient data, the study with fewer data points was excluded.
Data Extraction and Synthesis
All studies included after the full-text screen were divided based on the clinical question they answered (Q1, Q2, Q3 above). Some studies reported outcomes pertinent to more than one question. Two review authors were assigned to each clinical question. They independently reviewed each article and extracted the pertinent data into question-specific extraction sheets. Articles assigned to Q2 were reviewed by two pediatric neuroradiologists. For each study, the following details were extracted: authors, location, year, study type, study period, population, and number and ages of participants. Details that were question-specific included: (Q1) values and/or percentages for inflammatory markers; (Q2) reasons for imaging or type of imaging; and (Q3) participants managed surgically and culture results. The data were then synthesized in table and/or narrative format. For Q3, the organisms identified from intraoperative and blood cultures in each study were mathematically combined. When possible, prevalence was calculated using the number of patients with at least one pathogen recovered as the denominator. If this number was not available, the number of patients who underwent surgery was used as the denominator.
Quality Assessment
No randomized controlled trials were identified. More than 90% of the studies identified and included were retrospective descriptive studies. By the nature of the case series design, the study quality was felt to be poor, with high risk of bias. The Joanna Briggs Institute Critical Appraisal tools for systematic reviews were used to appraise each individual study included (Appendix Table 2).8 The Grading of Recommendations, Assessment, Development and Evaluations (GRADE) criteria were used in rating the quality of evidence for each question.9
RESULTS
A summary of the search strategy and study selection is provided in the Figure (PRISMA flow diagram). The initial search identified 3007 studies. After duplicates were removed and general eligibility criteria applied, 94 articles remained. Question-specific eligibility criteria, discussed in the following sections, were then applied, resulting in 63 articles included in the review.

Q1: Are Inflammatory Markers, Including Fever, WBC, and CRP, Useful in Distinguishing Preseptal Cellulitis (group 1) From OCA (Groups 2, 3, and 4); Orbital Cellulitis (group 2) From Abscess (Groups 3 and 4); or Identifying Patients Who Require Surgical Intervention?
Fever and elevation of the WBC count and CRP have been used to assess the severity of certain pediatric infections10,11 and therefore may be helpful in distinguishing severity of illness in OCA. Studies included in this section provided numerical values for at least one of the following: WBC count, CRP, or percentage of patients with fever for at least one type of orbital infection. Included studies had at least five patients per group.
Thirty-three articles were screened for the inflammatory marker section. Thirteen were excluded for the following reasons: no numbers reported for inflammatory markers (n = 6); group 1 and groups 2, 3, and 4 results combined (n = 6); fewer than five patients with orbital cellulitis included (n = 1). Twenty studies were included: 18 case series and 2 retrospective cohorts. Appendix Table 3 summarizes the data from studies included. Based on GRADE criteria, the body of evidence included in this section is of low quality.9
Distinguishing Between Preseptal and OCA
Eleven studies were included in this section (Table 1). WBC count was significantly higher in patients with groups 2, 3, and 4 than group 1 in two studies (Devrim et al,12P < .01; Santos et al,13P = .025). CRP was significantly higher in patients with groups 2, 3, and 4 than group 1 in four studies (Öcal Demir et al,14P = .02; Devrim et al,12P < .01; Ohana-Sarna-Cahan et al,18P < .001; Santos et al,13P < .001). Patients with groups 2, 3, and 4 had a significantly higher fever rate in three studies (Botting et al,21P < .001; Ohana-Sarna-Cahan et al,18P = .0001; Santos et al,13 P = .029).

Distinguishing Between Orbital Cellulitis and Abscess
Seven studies were included in this section (Appendix Table 3). One study showed significantly higher WBC count in group 3 than group 2 (P = .004), although results were reported as percentage of patients above a cutoff number calculated to distinguish between cellulitis and abscess (Appendix Table 3).22 CRP was not significantly different between group 2 and groups 3 and 4. One study found a significantly higher fever rate in patients with group 3 compared to patients with group 2 (P < .001).22
Identifying Patients Requiring Surgery
Six studies were included in this section (Appendix Table 3). One study found a significantly higher WBC count in patients treated surgically (Tabarino et al,24P < .05). Patients treated surgically had a significantly higher CRP in two studies (Cohen et al,25P = .02; Friling et al,26 P = .04). Fever was inconsistently reported in the studies, with some using mean presenting temperatures and some using rates of fever. One study found a significantly higher mean presenting temperature in patients treated surgically (P = .027), but the difference between the two groups was 0.7 °C.23
Summary
Most studies found no significant difference in WBC count, CRP, or fever between preseptal and OCA, cellulitis and abscess, or patients receiving medical and surgical interventions.
Q2: What Is the Role of Imaging in Evaluation of OCA?
Twenty-five articles were selected for the imaging section review. All the included studies were retrospective descriptive studies. Quantitative data extraction and analysis of these studies could not be performed because of their heterogeneous methodologies and lack of objective data. Therefore, the information gleaned from these studies is summarized in narrative format. Per GRADE criteria, the body of evidence included in this section is of low quality.
Who Needs Imaging?
Proptosis, ophthalmoplegia, decreased vision, and pain with eye movements are widely agreed-upon indications for imaging evaluation.21,27,28 Because of concern for radiation exposure in pediatric patients, some authors suggested that computed tomography (CT) should only be obtained if patients fail to respond to medical therapy or if surgery is being considered.17,29,30 However, Rudloe et al31 found that half of the patients with group 3 or higher disease on CT did not have proptosis, ophthalmoplegia, or pain with extraocular movement. In addition, evaluation of young children with acute periorbital swelling can be difficult, so a lower threshold for imaging is likely warranted in these younger patients.
What Type of Imaging Should Be Obtained?
The American College of Radiology 2018 Appropriateness Criteria (ACR criteria) for orbital imaging state that orbital CT is usually indicated for patients with suspected Chandler groups 2, 3, and 4 infections.32 CT with contrast is useful for evaluating the extent of orbital infection and size of the abscess and for delineating the adjacent osseous anatomy, which is essential for cases in which surgical intervention is planned.20,21,26,27,30,31,33,34 Distinguishing abscess from cellulitis on CT sometimes can be challenging; therefore, serial clinical examinations and, occasionally, surgical exploration may be required.35,36
Magnetic resonance imaging (MRI) is helpful for evaluating intracranial complications (eg, epidural abscess),27,37 but it is limited for evaluating the osseous components of the paranasal sinuses. Although one study suggested that rapid MRI is comparable to contrast CT for differentiating group 1 infections from groups 2, 3, and 4 infections, it provided limited assessment of other complications.38 With no definitive studies comparing CT with MRI for orbital infections, adherence to the ACR criteria is recommended.
Orbital ultrasound is limited by its small field of view and artifact produced by the surrounding bony interface, both of which can obscure posterior intraorbital pathologies.29,39,40 Plain radiographs are not helpful for evaluating OCA due to limited soft-tissue contrast.41
When Should Repeat Imaging Be Obtained?
Children with group 3 OCA have been successfully managed medically in a carefully monitored setting.42 Repeat CT imaging is sometimes useful in these patients, particularly if the clinical examination is difficult.42-44 However, improvement in CT findings may lag behind clinical improvement.39
Summary
Per ACR criteria, orbital CT with contrast is recommended to evaluate patients with suspected Chandler groups 2, 3, and 4 OCA. MRI is reserved for evaluating intracranial complications.
Q3: What Is the Microbiology of OCA? What Is the MRSA Prevalence?
Knowledge of the microbiology of OCA is essential for the appropriate selection of empiric antibiotics. Because fewer children with groups 2 and 3 OCA undergo surgery, intraoperative cultures often are not available to guide antibiotic selection.45 As a result, significant variation exists in antibiotic prescribing.6
Studies discussing the microbiology of OCA were included only if they were published in the past 2 decades (2000-2020) and were excluded if the study period was before 1990, as microbiology changes over time and new vaccines are introduced. To be included, the majority of cultures reported had to be intraoperative (orbital or sinus) specimens. Studies reporting only nasal, conjunctival, or other surface cultures were excluded. When studies included patients with group 1 OCA, only microbiology data for groups 2, 3, and 4 OCA were extracted. The pattern of resistance for S aureus was not always explicitly reported; however, when non-MRSA active antibiotics were used, methicillin-susceptible S aureus was assumed.
A total of 63 studies were screened for the microbiology section; 32 were excluded for the following reasons: published before 2000 or study period before 1990 (n = 18), reported surface cultures or culture site not clearly stated (n = 4), microbiology mixed between preseptal and orbital (n = 6), wrong study type (n = 2), and study group overlaps with a different article included (n = 2). Of the 32 studies included, 3 were prospective observational, 4 were retrospective cohort, and 25 were case series. Based on GRADE criteria, the body of evidence included in this section is of low quality.42
Appendix Table 4 summarizes the microbiologic data from the studies included. In the group of children that had a positive culture (orbital, sinus, or blood), the most commonly recovered organisms reported were S aureus (median, 22%; range, 0%-100%), Streptococcus anginosus group (median, 16%; range, 0%-100%), group A Streptococcus (median, 12%; range, 0%-80%), and Streptococcus pneumoniae (median, 8%; range, 0%-100%). Streptococcus as a group had a median prevalence of 57%, ranging from 0% to 100%. MRSA prevalence had a median of 3% (interquartile range [IQR], 0%-13%). Median prevalence of polymicrobial cultures was 20%, and median prevalence of anaerobic organisms was 14% (Table 2). Orbital and sinus cultures had the highest yield, with an average return of an organism of 72% (median, 75%; IQR, 64%-84%).

Microbiology was compared between studies completed in the United States and in other countries (Table 2). Based on median prevalence across studies, both S anginosus group and MRSA were more prevalent in the United States than internationally (28% vs 0% and 11% vs 0%, respectively). No clear trend in MRSA prevalence was evident over the 2 decades; however, the studies included were heterogeneous and did not have the power to detect such a trend.
Two reports suggest a difference of MRSA prevalence by patient age. Hsu et al46 found that three of eight MRSA infections were in infants age <1 year, which accounted for 50% (3/6) of infants included in the study. Miller et al47 reported MRSA in 4 of 9 (44%) infants with OCA. Age <1 year may be associated with increased frequency of MRSA infection in OCA.
Summary
Blood cultures have low yield. The most common organisms recovered from OCA are Streptococcus species (most commonly S anginosus group, group A Streptococcus, and pneumococcus) and S aureus. Polymicrobial infections including anaerobes are common. MRSA prevalence is low globally but varies significantly among geographic areas.
DISCUSSION
Our systematic review of the literature for the medical management of OCA revealed predominantly descriptive studies and only a limited number of comparison-based studies, likely reflecting the rarity of advanced forms of OCA. Given the lack of high-quality evidence and the level of heterogeneity among studies, the conclusions that can be drawn are limited.
Distinguishing between disease severity and OCA requiring surgical intervention remains challenging. Although studies in our review suggest a trend toward markers of inflammation (fever, elevated WBC count and CRP) being more common in more severe presentations, the results were mixed, and studies were low quality and underpowered to detect meaningful differences. For example, most studies do not define what constitutes a fever in their cohort. Our review suggests that markers of inflammation cannot be used to distinguish between Chandler groups or to identify patients requiring surgery. Of note, the presence of fever and elevated inflammatory markers may have influenced the decision to obtain imaging or to proceed to surgery, thereby also potentially biasing these clinical indicators toward predictors for more severe disease. Decisions regarding surgery should therefore be based on the entire clinical picture, including response to appropriate antibiotics.
We found a lack of high-quality evidence regarding the role of imaging in OCA, and the studies reviewed were heterogeneous. Recommendations for imaging therefore remain at the level of expert opinion (ACR criteria). CT imaging is the first-line modality for imaging in suspected OCA given the limitations of alternative imaging modalities, but the sensitivity and specificity of CT imaging remain unknown for diagnosis of orbital abscesses.
Our review of the published microbiology confirmed that Staphylococcus and Streptococcus species are the most common pathogens identified in OCA. Prevalence across the different studies varied greatly. Owing to the significant heterogeneity in studies, calculation of pooled prevalence was not possible. By using the number of positive cultures as our denominator (or total surgeries if number of positive cultures was unavailable), we likely overestimated the prevalence of S aureus. S aureus is generally recognized as a pyogenic pathogen, more likely to be associated with abscess formation.48 Therefore, culture results obtained predominantly from abscesses likely result in an overestimate of S aureus in OCA (groups 2, 3, and 4). Regardless, MRSA prevalence was generally low, both nationally and internationally. The MRSA results from the study by McKinley at el49 (Texas) was a notable outlier in the United States, with MRSA prevalence as high as 44% compared with the median prevalence of 3% (IQR, 0-13), highlighting the importance of local resistance patterns when choosing empiric antibiotics.
Limitations to the microbiology review included significant heterogeneity in both the types of cultures included and the reporting of results. Although we excluded studies that reported only surface culture results or did not specify culture type, we did include studies that had surface culture results combined with intraoperative culture results, making it impossible to separate the two. Since most of the cultures included in combined results reported organisms based on intraoperative cultures, we felt they provided valuable information that should be included. In most studies, blood cultures were not obtained in all participants, so the yield of blood cultures is likely an overestimate, as blood cultures are more likely to be obtained in higher-acuity patients.
CONCLUSION
Although the available evidence regarding the medical management of OCA remains low quality, certain limited conclusions can be drawn, as presented in this review. Further high-quality studies are needed to better inform the medical management of OCA.
Acknowledgment
The authors thank Dr Kyle Pronko for his help with data extraction for the imaging section.
1. Reynolds D.J, Kodsi SR, Rubin SE, Rodgers IR. Intracranial infection associated with preseptal and orbital cellulitis in the pediatric patient. J AAPOS. 2003;7(6):413-417. https://doi.org/10.1016/j.jaapos.2003.09.013
2. Chaudhry IA, Shamsi FA, Elzaridi E, et al. Outcome of treated orbital cellulitis in a tertiary eye care center in the Middle East. Ophthalmology. 2007;114(2):345-354. https://doi.org/10.1016/j.ophtha.2006.07.059
3. Chandler JR, Langenbrunner DJ, Stevens ER. Pathogenesis of orbital complications in acute sinusitis. Laryngoscope. 1970;1414-1428. https://doi.org/10.1288/00005537-197009000-00007
4. Wong SJ, Levi J. Management of pediatric orbital cellulitis: a systematic review. Int J Pediatr Otorhinolaryngol. 2018;110:123-129. https://doi.org/10.1016/j.ijporl.2018.05.006
5. Liao JC, Harris GJ. Subperiosteal abscess of the orbit: evolving pathogens and the therapeutic protocol. Ophthalmology. 2015;122(3):639-647. https://doi.org/10.1016/j.ophtha.2014.09.009
6. Markham JL, Hall M, Bettenhausen JL, Myers AL, Puls HT, McColloh RJ. Variation in care and clinical outcomes in children hospitalized with orbital cellulitis. Hosp Pediatr. 2018;8(1):28-35. https://doi.org/10.1542/hpeds.2017-0040
7. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
8. Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18(10):2127-2133. https://doi.org/10.11124/JBISRIR-D-19-00099
9. Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401-406. https://doi.org/10.1016/j.jclinepi.2010.07.015
10. Dean P, Florin TA. Factors associated with pneumonia severity in children: a systematic review. J Pediatric Infect Dis Soc. 2018;7(4):323-334. https://doi.org/10.1093/jpids/piy046
11. Hofer N, Zacharias E, Müller W, Resch B. An update on the use of C-reactive protein in early-onset neonatal sepsis: current insights and new tasks. Neonatology. 2012;102(1):25-36. https://doi.org/10.1159/000336629
12. Devrim I, Kanra G, Kara A, et al. Preseptal and orbital cellulitis: 15-year experience with sulbactam ampicillin treatment. Turk J Pediatr. 2008;50(3):214-218.
13. Santos JC, Pinto S, Ferreira S, Maia C, Alves S, da Silva V. Pediatric preseptal and orbital cellulitis: a 10-year experience. Int J Pediatr Otorhinolaryngol. 2019;120:82-88. https://doi.org/10.1016/j.ijporl.2019.02.003
14. Öcal Demir S , Çagan E, Kepenekli Kadayifci E, et al. Clinical features and outcome of preseptal and orbital cellulitis in hospitalized children: four years experience. Medeni Med J. 2017;32(1):7-13. https://doi.org/10.5222/MMJ.2017.007
15. Georgakopoulos CD, Eliopoulou MI, Stasinos S, Exarchou A, Pharmakakis N, Varvarigou A. Periorbital and orbitaln cellulitis: a 10-year review of hospitalized children. Eur J Ophthalmol. 2010;20(6):1066-1072. https://doi.org/10.1177/112067211002000607
16. Gonçalves R, Menezes C, Machado R, Ribeiro I, Lemos JA. Periorbital cellulitis in children: analysis of outcome of intravenous antibiotic therapy. Orbit. 2016;34(4):175-180. https://doi.org/10.1080/01676830.2016.1176205
17. Ho CF, Huang YC, Wang CJ, Chiu CH, Lin TY. Clinical analysis of computed tomography-staged orbital cellulitis in children. J Microbiol Immunol Infect. 2017;40(6):518-524.
18. Ohana-Sarna-Cahan L, Hurvitz N, Gross I, Cohen A, Hashavya S. Factors associated with increased risk of pediatric orbital cellulitis—who should be scanned? Pediatr Emerg Care. Published online ahead of print March 19, 2020. https://doi.org/10.1097/PEC.0000000000002083
19. Weiss A, Friendly D, Eglin K, Chang M, Gold B. Bacterial periorbital and orbital cellulitis in childhood. Ophthalmology. 1983;90(3):195-203. https://doi.org/10.1016/s0161-6420(83)34573-5
20. Le TD, Liu ES, Adatia FA, Buncic JR Blaser S. The effect of adding orbital computed tomography findings to the Chandler criteria for classifying pediatric orbital cellulitis in predicting which patients will require surgical intervention. J AAPOS. 2014;18(3):271-277. https://doi.org/10.1016/j.jaapos.2014.01.015
21. Botting AM, McIntosh D, Mahadevan M. Paediatric pre- and post-septal peri-orbital infections are different diseases. A retrospective review of 262 cases. Int J Pediatr Otorhinolaryngol. 2008;72(3):377-383. https://doi.org/10.1016/j.ijporl.2007.11.013
22. Huang SF, Lee TJ, Lee YS, Chen CC, Chin SC, Wang NC. Acute rhinosinusitis-related orbital infection in pediatric patients: a retrospective analysis. Ann Otol Rhinol Laryngol. 2011;120(3):185-190. https://doi.org/10.1177/000348941112000307
23. Ryan JT, Preciado A, Bauman N, et al. Management of pediatric orbital cellulitis in patients with radiographic findings of subperiosteal abscess. Otolaryngol Head Neck Surg. 2009;140(6):907-911. https://doi.org/10.1016/j.otohns.2009.02.014
24. Tabarino F, Elmaleh-Bergès M, Quesnel S, Lorrot M, Van Den Abbeele T, Teissier N. Subperiosteal orbital abscess: volumetric criteria for surgical drainage. Int J Pediatr Otorhinolaryngol. 2015;79(2):131-135. https://doi.org/10.1016/j.ijporl.2014.11.021
25. Cohen N, Erisson S, Anafy A, et al. Clinicians need to consider surgery when presented with some markers for severe paediatric orbital cellulitis. Acta Paediatr. 2020;109(6):1269-1270. https://doi.org/10.1111/apa.15125
26. Friling R, Garty BZ, Kornreich L, et al. Medical and surgical management of orbital cellulitis in children. Folia Med (Plovdiv). 2014;56(4):253-258. https://doi.org/10.1515/folmed-2015-0004
27. Gavriel H, Yeheskeli E, Aviram E, Yehoshua L, Eviatar E. Dimension of subperiosteal orbital abscess as an indication for surgical management in children. Otolaryngol Head Neck Surg. 2011;145(5):823-827. https://doi.org/10.1177/0194599811416559
28. Mathew AV, Craig E, Al-Mahmoud R, et al. Paediatric post-septal and pre-septal cellulitis: 10 years’ experience at a tertiary-level children’s hospital. Br J Radiol. 2014;87(1033):20130503. https://doi.org/10.1259/bjr.20130503
29. Goodwin WJ Jr, Weinshall M, Chandler JR. The role of high resolution computerized tomography and standardized ultrasound in the evaluation of orbital cellulitis. Laryngoscope. 1982;92(7 pt 1):729-731.
30. Bilaniuk LT, Zimmerman RA. Computer‐assisted tomography: sinus lesions with orbital involvement. Head Neck Surg. 1980;2(4):293-301. https://doi.org/10.1002/hed.2890020407
31. Rudloe TF, Harper MB, Prabhu SP, Rahbar R, Vanderveen D, Kimia AA. Acute periorbital infections: who needs emergent imaging? Pediatrics. 2010;125(4):e719-e726. https://doi.org/10.1542/peds.2009-1709
32. Kennedy TA, Corey AS, Policeni B, et al. ACR Appropriateness Criteria® orbits vision and visual loss. J Am Coll Radiol. 2018;15(5S):S116-S131. https://doi.org/10.1016/j.jacr.2018.03.023
33. De Silva M, Lam V, Broadfoot J. C.T. findings of orbital inflammation in children. Australas Radiol. 1987;31(3):241-245. https://doi.org/10.1111/j.1440-1673.1987.tb01822.x
34. Hirsch M, Lifshitz T. Computerized tomography in the diagnosis and treatment of orbital cellulitis. Pediatr Radiol. 1988;18(4):302-305. https://doi.org/10.1007/BF02388996
35. Andrews TM, Myer CM 3rd. The role of computed tomography in the diagnosis of subperiosteal abscess of the orbit. Clin Pediatr (Phila). 1992;31(1):37-43. https://doi.org/10.1177/000992289203100108
36. Clary RA, Cunningham MJ, Eavey RD. Orbital complications of acute sinusitis: comparison of computed tomography scan and surgical findings. Ann Otol Rhinol Laryngol. 1992;101(7):598-600. https://doi.org/10.1177/000348949210100710
37. Arjmand EM, LuskRP, Muntz HR. Pediatric sinusitis and subperiosteal orbital abscess formation: diagnosis and treatment. Otolaryngol Neck Surg. 1993;109(5):886.894. https://doi.org/10.1177/019459989310900518
38. Jain SF, Ishihara R, Wheelock L, et al. Feasibility of rapid magnetic resonance imaging (rMRI) for the emergency evaluation of suspected pediatric orbital cellulitis. J AAPOS. 2020;24(5):289.e1-289.e4. https://doi.org/10.1016/j.jaapos.2020.05.018
39. Harris GJ. Subperiosteal abscess of the orbit: computed tomography and the clinical course. Ophthal Plast Reconstr Surg. 1996;12:1-8. https://doi.org/10.1097/00002341-199603000-00001
40. Kaplan DM, Briscoe D, Gatot A, Niv A, Leiberman A, Fliss DM. The use of standardized orbital ultrasound in the diagnosis of sinus induced infections of the orbit in children: a preliminary report. Int J Pediatr Otorhinolaryngol. 1999;48(2):155-162. https://doi.org/10.1016/s0165-5876(99)00023-3
41. Towbin R, Han BK, Kaufman RA, Burke M. Postseptal cellulitis: CT in diagnosis and management. Radiology. 1986;158(3):735-737. https://doi.org/10.1148/radiology.158.3.3945747
42. Starkey CR, Steele RW. Medical management of orbital cellulitis. Pediatr Infect Dis J. 2001;20(10):1002-1005. https://doi.org/10.1097/00006454-200110000-00017
43. Brown CL, Graham SM, Griffin MC, et al. Pediatric medial subperiosteal orbital abscess: medical management where possible. Am J Rhinol. 2004;18(5):321-327.
44. Cossack MT, Herretes SP, Cham A, Sniegowski MC, Lyon DB. Radiographic course of medically managed pediatric orbital subperiosteal abscesses. J Pediatr Ophthalmol Strabismus. 2018;55(6):387-392. https://doi.org/10.3928/01913913-20180802-02
45. Zhao EE, Koochakzadeh S, Nguyen SA, et al. Orbital complications of acute bacterial rhinosinusitis in the pediatric population: a systematic review and meta-analysis. Int J Pediatr Otorhinolaryngol. 2020;135:110078. https://doi.org/10.1016/j.ijporl.2020.110078
46. Hsu J, Treister AD, Ralay Ranaivo H, Rowley AH, Rahmani B. Microbiology of pediatric orbital cellulitis and trends in methicillin-resistant Staphylococcus aureus cases. Clin Pediatr (Phila). 2019;58(10):1056-1062. https://doi.org/10.1177/0009922819864587
47. Miller A, Castanes M, Yen M, Coats D, Yen K. Infantile orbital cellulitis. Ophthalmology. 2008;115(3):594. https://doi.org/10.1016/j.ophtha.2007.10.011
48. Dajani AS, Garcia RE, Wolinsky E. Etiology of cervical lymphadenitis in children. N Engl J Med. 1963;268:1329-1333. https://doi.org/10.1056/NEJM196306132682403
49. McKinley SH, Yen MT, Miller AM, Yen KG. Microbiology of pediatric orbital cellulitis. Am J Ophthalmol. 2007;144(4):497-501. https://doi.org/10.1016/j.ajo.2007.04.049
1. Reynolds D.J, Kodsi SR, Rubin SE, Rodgers IR. Intracranial infection associated with preseptal and orbital cellulitis in the pediatric patient. J AAPOS. 2003;7(6):413-417. https://doi.org/10.1016/j.jaapos.2003.09.013
2. Chaudhry IA, Shamsi FA, Elzaridi E, et al. Outcome of treated orbital cellulitis in a tertiary eye care center in the Middle East. Ophthalmology. 2007;114(2):345-354. https://doi.org/10.1016/j.ophtha.2006.07.059
3. Chandler JR, Langenbrunner DJ, Stevens ER. Pathogenesis of orbital complications in acute sinusitis. Laryngoscope. 1970;1414-1428. https://doi.org/10.1288/00005537-197009000-00007
4. Wong SJ, Levi J. Management of pediatric orbital cellulitis: a systematic review. Int J Pediatr Otorhinolaryngol. 2018;110:123-129. https://doi.org/10.1016/j.ijporl.2018.05.006
5. Liao JC, Harris GJ. Subperiosteal abscess of the orbit: evolving pathogens and the therapeutic protocol. Ophthalmology. 2015;122(3):639-647. https://doi.org/10.1016/j.ophtha.2014.09.009
6. Markham JL, Hall M, Bettenhausen JL, Myers AL, Puls HT, McColloh RJ. Variation in care and clinical outcomes in children hospitalized with orbital cellulitis. Hosp Pediatr. 2018;8(1):28-35. https://doi.org/10.1542/hpeds.2017-0040
7. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
8. Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18(10):2127-2133. https://doi.org/10.11124/JBISRIR-D-19-00099
9. Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401-406. https://doi.org/10.1016/j.jclinepi.2010.07.015
10. Dean P, Florin TA. Factors associated with pneumonia severity in children: a systematic review. J Pediatric Infect Dis Soc. 2018;7(4):323-334. https://doi.org/10.1093/jpids/piy046
11. Hofer N, Zacharias E, Müller W, Resch B. An update on the use of C-reactive protein in early-onset neonatal sepsis: current insights and new tasks. Neonatology. 2012;102(1):25-36. https://doi.org/10.1159/000336629
12. Devrim I, Kanra G, Kara A, et al. Preseptal and orbital cellulitis: 15-year experience with sulbactam ampicillin treatment. Turk J Pediatr. 2008;50(3):214-218.
13. Santos JC, Pinto S, Ferreira S, Maia C, Alves S, da Silva V. Pediatric preseptal and orbital cellulitis: a 10-year experience. Int J Pediatr Otorhinolaryngol. 2019;120:82-88. https://doi.org/10.1016/j.ijporl.2019.02.003
14. Öcal Demir S , Çagan E, Kepenekli Kadayifci E, et al. Clinical features and outcome of preseptal and orbital cellulitis in hospitalized children: four years experience. Medeni Med J. 2017;32(1):7-13. https://doi.org/10.5222/MMJ.2017.007
15. Georgakopoulos CD, Eliopoulou MI, Stasinos S, Exarchou A, Pharmakakis N, Varvarigou A. Periorbital and orbitaln cellulitis: a 10-year review of hospitalized children. Eur J Ophthalmol. 2010;20(6):1066-1072. https://doi.org/10.1177/112067211002000607
16. Gonçalves R, Menezes C, Machado R, Ribeiro I, Lemos JA. Periorbital cellulitis in children: analysis of outcome of intravenous antibiotic therapy. Orbit. 2016;34(4):175-180. https://doi.org/10.1080/01676830.2016.1176205
17. Ho CF, Huang YC, Wang CJ, Chiu CH, Lin TY. Clinical analysis of computed tomography-staged orbital cellulitis in children. J Microbiol Immunol Infect. 2017;40(6):518-524.
18. Ohana-Sarna-Cahan L, Hurvitz N, Gross I, Cohen A, Hashavya S. Factors associated with increased risk of pediatric orbital cellulitis—who should be scanned? Pediatr Emerg Care. Published online ahead of print March 19, 2020. https://doi.org/10.1097/PEC.0000000000002083
19. Weiss A, Friendly D, Eglin K, Chang M, Gold B. Bacterial periorbital and orbital cellulitis in childhood. Ophthalmology. 1983;90(3):195-203. https://doi.org/10.1016/s0161-6420(83)34573-5
20. Le TD, Liu ES, Adatia FA, Buncic JR Blaser S. The effect of adding orbital computed tomography findings to the Chandler criteria for classifying pediatric orbital cellulitis in predicting which patients will require surgical intervention. J AAPOS. 2014;18(3):271-277. https://doi.org/10.1016/j.jaapos.2014.01.015
21. Botting AM, McIntosh D, Mahadevan M. Paediatric pre- and post-septal peri-orbital infections are different diseases. A retrospective review of 262 cases. Int J Pediatr Otorhinolaryngol. 2008;72(3):377-383. https://doi.org/10.1016/j.ijporl.2007.11.013
22. Huang SF, Lee TJ, Lee YS, Chen CC, Chin SC, Wang NC. Acute rhinosinusitis-related orbital infection in pediatric patients: a retrospective analysis. Ann Otol Rhinol Laryngol. 2011;120(3):185-190. https://doi.org/10.1177/000348941112000307
23. Ryan JT, Preciado A, Bauman N, et al. Management of pediatric orbital cellulitis in patients with radiographic findings of subperiosteal abscess. Otolaryngol Head Neck Surg. 2009;140(6):907-911. https://doi.org/10.1016/j.otohns.2009.02.014
24. Tabarino F, Elmaleh-Bergès M, Quesnel S, Lorrot M, Van Den Abbeele T, Teissier N. Subperiosteal orbital abscess: volumetric criteria for surgical drainage. Int J Pediatr Otorhinolaryngol. 2015;79(2):131-135. https://doi.org/10.1016/j.ijporl.2014.11.021
25. Cohen N, Erisson S, Anafy A, et al. Clinicians need to consider surgery when presented with some markers for severe paediatric orbital cellulitis. Acta Paediatr. 2020;109(6):1269-1270. https://doi.org/10.1111/apa.15125
26. Friling R, Garty BZ, Kornreich L, et al. Medical and surgical management of orbital cellulitis in children. Folia Med (Plovdiv). 2014;56(4):253-258. https://doi.org/10.1515/folmed-2015-0004
27. Gavriel H, Yeheskeli E, Aviram E, Yehoshua L, Eviatar E. Dimension of subperiosteal orbital abscess as an indication for surgical management in children. Otolaryngol Head Neck Surg. 2011;145(5):823-827. https://doi.org/10.1177/0194599811416559
28. Mathew AV, Craig E, Al-Mahmoud R, et al. Paediatric post-septal and pre-septal cellulitis: 10 years’ experience at a tertiary-level children’s hospital. Br J Radiol. 2014;87(1033):20130503. https://doi.org/10.1259/bjr.20130503
29. Goodwin WJ Jr, Weinshall M, Chandler JR. The role of high resolution computerized tomography and standardized ultrasound in the evaluation of orbital cellulitis. Laryngoscope. 1982;92(7 pt 1):729-731.
30. Bilaniuk LT, Zimmerman RA. Computer‐assisted tomography: sinus lesions with orbital involvement. Head Neck Surg. 1980;2(4):293-301. https://doi.org/10.1002/hed.2890020407
31. Rudloe TF, Harper MB, Prabhu SP, Rahbar R, Vanderveen D, Kimia AA. Acute periorbital infections: who needs emergent imaging? Pediatrics. 2010;125(4):e719-e726. https://doi.org/10.1542/peds.2009-1709
32. Kennedy TA, Corey AS, Policeni B, et al. ACR Appropriateness Criteria® orbits vision and visual loss. J Am Coll Radiol. 2018;15(5S):S116-S131. https://doi.org/10.1016/j.jacr.2018.03.023
33. De Silva M, Lam V, Broadfoot J. C.T. findings of orbital inflammation in children. Australas Radiol. 1987;31(3):241-245. https://doi.org/10.1111/j.1440-1673.1987.tb01822.x
34. Hirsch M, Lifshitz T. Computerized tomography in the diagnosis and treatment of orbital cellulitis. Pediatr Radiol. 1988;18(4):302-305. https://doi.org/10.1007/BF02388996
35. Andrews TM, Myer CM 3rd. The role of computed tomography in the diagnosis of subperiosteal abscess of the orbit. Clin Pediatr (Phila). 1992;31(1):37-43. https://doi.org/10.1177/000992289203100108
36. Clary RA, Cunningham MJ, Eavey RD. Orbital complications of acute sinusitis: comparison of computed tomography scan and surgical findings. Ann Otol Rhinol Laryngol. 1992;101(7):598-600. https://doi.org/10.1177/000348949210100710
37. Arjmand EM, LuskRP, Muntz HR. Pediatric sinusitis and subperiosteal orbital abscess formation: diagnosis and treatment. Otolaryngol Neck Surg. 1993;109(5):886.894. https://doi.org/10.1177/019459989310900518
38. Jain SF, Ishihara R, Wheelock L, et al. Feasibility of rapid magnetic resonance imaging (rMRI) for the emergency evaluation of suspected pediatric orbital cellulitis. J AAPOS. 2020;24(5):289.e1-289.e4. https://doi.org/10.1016/j.jaapos.2020.05.018
39. Harris GJ. Subperiosteal abscess of the orbit: computed tomography and the clinical course. Ophthal Plast Reconstr Surg. 1996;12:1-8. https://doi.org/10.1097/00002341-199603000-00001
40. Kaplan DM, Briscoe D, Gatot A, Niv A, Leiberman A, Fliss DM. The use of standardized orbital ultrasound in the diagnosis of sinus induced infections of the orbit in children: a preliminary report. Int J Pediatr Otorhinolaryngol. 1999;48(2):155-162. https://doi.org/10.1016/s0165-5876(99)00023-3
41. Towbin R, Han BK, Kaufman RA, Burke M. Postseptal cellulitis: CT in diagnosis and management. Radiology. 1986;158(3):735-737. https://doi.org/10.1148/radiology.158.3.3945747
42. Starkey CR, Steele RW. Medical management of orbital cellulitis. Pediatr Infect Dis J. 2001;20(10):1002-1005. https://doi.org/10.1097/00006454-200110000-00017
43. Brown CL, Graham SM, Griffin MC, et al. Pediatric medial subperiosteal orbital abscess: medical management where possible. Am J Rhinol. 2004;18(5):321-327.
44. Cossack MT, Herretes SP, Cham A, Sniegowski MC, Lyon DB. Radiographic course of medically managed pediatric orbital subperiosteal abscesses. J Pediatr Ophthalmol Strabismus. 2018;55(6):387-392. https://doi.org/10.3928/01913913-20180802-02
45. Zhao EE, Koochakzadeh S, Nguyen SA, et al. Orbital complications of acute bacterial rhinosinusitis in the pediatric population: a systematic review and meta-analysis. Int J Pediatr Otorhinolaryngol. 2020;135:110078. https://doi.org/10.1016/j.ijporl.2020.110078
46. Hsu J, Treister AD, Ralay Ranaivo H, Rowley AH, Rahmani B. Microbiology of pediatric orbital cellulitis and trends in methicillin-resistant Staphylococcus aureus cases. Clin Pediatr (Phila). 2019;58(10):1056-1062. https://doi.org/10.1177/0009922819864587
47. Miller A, Castanes M, Yen M, Coats D, Yen K. Infantile orbital cellulitis. Ophthalmology. 2008;115(3):594. https://doi.org/10.1016/j.ophtha.2007.10.011
48. Dajani AS, Garcia RE, Wolinsky E. Etiology of cervical lymphadenitis in children. N Engl J Med. 1963;268:1329-1333. https://doi.org/10.1056/NEJM196306132682403
49. McKinley SH, Yen MT, Miller AM, Yen KG. Microbiology of pediatric orbital cellulitis. Am J Ophthalmol. 2007;144(4):497-501. https://doi.org/10.1016/j.ajo.2007.04.049
© 2021 Society of Hospital Medicine