User login
Simulation-Based Training in Medical Education: Immediate Growth or Cautious Optimism?
For years, professional athletes have used simulation-based training (SBT), a combination of virtual and experiential learning that aims to optimize technical skills, teamwork, and communication.1 In SBT, critical plays and skills are first watched on video or reviewed on a chalkboard, and then run in the presence of a coach who offers immediate feedback to the player. The hope is that the individual will then be able to perfectly execute that play or scenario when it is game time. While SBT is a developing tool in medical education—allowing learners to practice important clinical skills prior to practicing in the higher-stakes clinical environment—an important question remains: what training can go virtual and what needs to stay in person?
In this issue, Carter et al2 present a single-site, telesimulation curriculum that addresses consult request and handoff communication using SBT. Due to the COVID-19 pandemic, the authors converted an in-person intern bootcamp into a virtual, Zoom®-based workshop and compared assessments and evaluations to the previous year’s (2019) in-person bootcamp. Compared to the in-person class, the telesimulation-based cohort were equally or better trained in the consult request portion of the workshop. However, participants were significantly less likely to perform the assessed handoff skills optimally, with only a quarter (26%) appropriately prioritizing patients and less than half (49%) providing an appropriate amount of information in the patient summary. Additionally, postworkshop surveys found that SBT participants were more satisfied with their performance in both the consult request and handoff scenarios and felt more prepared (99% vs 91%) to perform handoffs in clinical practice compared to the previous year’s in-person cohort.
We focus on this work as it explores the role that SBT or virtual training could have in hospital communication and patient safety training. While previous work has highlighted that technical and procedural skills often lend themselves to in-person adaptation (eg, point-of-care ultrasound), this work suggests that nontechnical skills training could be adapted to the virtual environment. Hospitalists and internal medicine trainees perform a myriad of nontechnical activities, such as end-of-life discussions, obtaining informed consent, providing peer-to-peer feedback, and leading multidisciplinary teams. Activities like these, which require no hands-on interactions, may be well-suited for simulation or virtual-based training.3
However, we make this suggestion with some caution. In Carter et al’s study,2 while we assumed that telesimulation would work for the handoff portion of the workshop, interestingly, the telesimulation-based cohort performed worse than the interns who participated in the previous year’s in-person training while simultaneously and paradoxically reporting that they felt more prepared. The authors offer several possible explanations, including alterations in the assessment checklist and a shift in the facilitators from peer observers to faculty hospitalists. We suspect that differences in the participants’ experiences prior to the bootcamp may also be at play. Given the onset of the pandemic during their final year in undergraduate training, many in this intern cohort were likely removed from their fourth-year clinical clerkships,4 taking from them pivotal opportunities to hone and refine this skill set prior to starting their graduate medical education.
As telesimulation and other virtual care educational opportunities continue to evolve, we must ensure that such training does not sacrifice quality for ease and satisfaction. As the authors’ findings show, simply replicating an in-person curriculum in a virtual environment does not ensure equivalence for all skill sets. We remain cautiously optimistic that as we adjust to a postpandemic world, more SBT and virtual-based educational interventions will allow medical trainees to be ready to perform come game time.
1. McCaskill S. Sports tech comes of age with VR training, coaching apps and smart gear. Forbes. March 31, 2020. https://www.forbes.com/sites/stevemccaskill/2020/03/31/sports-tech-comes-of-age-with-vr-training-coaching-apps-and-smart-gear/?sh=309a8fa219c9
2. Carter K, Podczerwinski J, Love L, et al. Utilizing telesimulation for advanced skills training in consultation and handoff communication: a post-COVID-19 GME bootcamp experience. J Hosp Med. 2021;16(12)730-734. https://doi.org/10.12788/jhm.3733
3. Paige JT, Sonesh SC, Garbee DD, Bonanno LS. Comprensive Healthcare Simulation: Interprofessional Team Training and Simulation. 1st ed. Springer International Publishing; 2020. https://doi.org/10.1007/978-3-030-28845-7
4. Goldenberg MN, Hersh DC, Wilkins KM, Schwartz ML. Suspending medical student clerkships due to COVID-19. Med Sci Educ. 2020;30(3):1-4. https://doi.org/10.1007/s40670-020-00994-1
For years, professional athletes have used simulation-based training (SBT), a combination of virtual and experiential learning that aims to optimize technical skills, teamwork, and communication.1 In SBT, critical plays and skills are first watched on video or reviewed on a chalkboard, and then run in the presence of a coach who offers immediate feedback to the player. The hope is that the individual will then be able to perfectly execute that play or scenario when it is game time. While SBT is a developing tool in medical education—allowing learners to practice important clinical skills prior to practicing in the higher-stakes clinical environment—an important question remains: what training can go virtual and what needs to stay in person?
In this issue, Carter et al2 present a single-site, telesimulation curriculum that addresses consult request and handoff communication using SBT. Due to the COVID-19 pandemic, the authors converted an in-person intern bootcamp into a virtual, Zoom®-based workshop and compared assessments and evaluations to the previous year’s (2019) in-person bootcamp. Compared to the in-person class, the telesimulation-based cohort were equally or better trained in the consult request portion of the workshop. However, participants were significantly less likely to perform the assessed handoff skills optimally, with only a quarter (26%) appropriately prioritizing patients and less than half (49%) providing an appropriate amount of information in the patient summary. Additionally, postworkshop surveys found that SBT participants were more satisfied with their performance in both the consult request and handoff scenarios and felt more prepared (99% vs 91%) to perform handoffs in clinical practice compared to the previous year’s in-person cohort.
We focus on this work as it explores the role that SBT or virtual training could have in hospital communication and patient safety training. While previous work has highlighted that technical and procedural skills often lend themselves to in-person adaptation (eg, point-of-care ultrasound), this work suggests that nontechnical skills training could be adapted to the virtual environment. Hospitalists and internal medicine trainees perform a myriad of nontechnical activities, such as end-of-life discussions, obtaining informed consent, providing peer-to-peer feedback, and leading multidisciplinary teams. Activities like these, which require no hands-on interactions, may be well-suited for simulation or virtual-based training.3
However, we make this suggestion with some caution. In Carter et al’s study,2 while we assumed that telesimulation would work for the handoff portion of the workshop, interestingly, the telesimulation-based cohort performed worse than the interns who participated in the previous year’s in-person training while simultaneously and paradoxically reporting that they felt more prepared. The authors offer several possible explanations, including alterations in the assessment checklist and a shift in the facilitators from peer observers to faculty hospitalists. We suspect that differences in the participants’ experiences prior to the bootcamp may also be at play. Given the onset of the pandemic during their final year in undergraduate training, many in this intern cohort were likely removed from their fourth-year clinical clerkships,4 taking from them pivotal opportunities to hone and refine this skill set prior to starting their graduate medical education.
As telesimulation and other virtual care educational opportunities continue to evolve, we must ensure that such training does not sacrifice quality for ease and satisfaction. As the authors’ findings show, simply replicating an in-person curriculum in a virtual environment does not ensure equivalence for all skill sets. We remain cautiously optimistic that as we adjust to a postpandemic world, more SBT and virtual-based educational interventions will allow medical trainees to be ready to perform come game time.
For years, professional athletes have used simulation-based training (SBT), a combination of virtual and experiential learning that aims to optimize technical skills, teamwork, and communication.1 In SBT, critical plays and skills are first watched on video or reviewed on a chalkboard, and then run in the presence of a coach who offers immediate feedback to the player. The hope is that the individual will then be able to perfectly execute that play or scenario when it is game time. While SBT is a developing tool in medical education—allowing learners to practice important clinical skills prior to practicing in the higher-stakes clinical environment—an important question remains: what training can go virtual and what needs to stay in person?
In this issue, Carter et al2 present a single-site, telesimulation curriculum that addresses consult request and handoff communication using SBT. Due to the COVID-19 pandemic, the authors converted an in-person intern bootcamp into a virtual, Zoom®-based workshop and compared assessments and evaluations to the previous year’s (2019) in-person bootcamp. Compared to the in-person class, the telesimulation-based cohort were equally or better trained in the consult request portion of the workshop. However, participants were significantly less likely to perform the assessed handoff skills optimally, with only a quarter (26%) appropriately prioritizing patients and less than half (49%) providing an appropriate amount of information in the patient summary. Additionally, postworkshop surveys found that SBT participants were more satisfied with their performance in both the consult request and handoff scenarios and felt more prepared (99% vs 91%) to perform handoffs in clinical practice compared to the previous year’s in-person cohort.
We focus on this work as it explores the role that SBT or virtual training could have in hospital communication and patient safety training. While previous work has highlighted that technical and procedural skills often lend themselves to in-person adaptation (eg, point-of-care ultrasound), this work suggests that nontechnical skills training could be adapted to the virtual environment. Hospitalists and internal medicine trainees perform a myriad of nontechnical activities, such as end-of-life discussions, obtaining informed consent, providing peer-to-peer feedback, and leading multidisciplinary teams. Activities like these, which require no hands-on interactions, may be well-suited for simulation or virtual-based training.3
However, we make this suggestion with some caution. In Carter et al’s study,2 while we assumed that telesimulation would work for the handoff portion of the workshop, interestingly, the telesimulation-based cohort performed worse than the interns who participated in the previous year’s in-person training while simultaneously and paradoxically reporting that they felt more prepared. The authors offer several possible explanations, including alterations in the assessment checklist and a shift in the facilitators from peer observers to faculty hospitalists. We suspect that differences in the participants’ experiences prior to the bootcamp may also be at play. Given the onset of the pandemic during their final year in undergraduate training, many in this intern cohort were likely removed from their fourth-year clinical clerkships,4 taking from them pivotal opportunities to hone and refine this skill set prior to starting their graduate medical education.
As telesimulation and other virtual care educational opportunities continue to evolve, we must ensure that such training does not sacrifice quality for ease and satisfaction. As the authors’ findings show, simply replicating an in-person curriculum in a virtual environment does not ensure equivalence for all skill sets. We remain cautiously optimistic that as we adjust to a postpandemic world, more SBT and virtual-based educational interventions will allow medical trainees to be ready to perform come game time.
1. McCaskill S. Sports tech comes of age with VR training, coaching apps and smart gear. Forbes. March 31, 2020. https://www.forbes.com/sites/stevemccaskill/2020/03/31/sports-tech-comes-of-age-with-vr-training-coaching-apps-and-smart-gear/?sh=309a8fa219c9
2. Carter K, Podczerwinski J, Love L, et al. Utilizing telesimulation for advanced skills training in consultation and handoff communication: a post-COVID-19 GME bootcamp experience. J Hosp Med. 2021;16(12)730-734. https://doi.org/10.12788/jhm.3733
3. Paige JT, Sonesh SC, Garbee DD, Bonanno LS. Comprensive Healthcare Simulation: Interprofessional Team Training and Simulation. 1st ed. Springer International Publishing; 2020. https://doi.org/10.1007/978-3-030-28845-7
4. Goldenberg MN, Hersh DC, Wilkins KM, Schwartz ML. Suspending medical student clerkships due to COVID-19. Med Sci Educ. 2020;30(3):1-4. https://doi.org/10.1007/s40670-020-00994-1
1. McCaskill S. Sports tech comes of age with VR training, coaching apps and smart gear. Forbes. March 31, 2020. https://www.forbes.com/sites/stevemccaskill/2020/03/31/sports-tech-comes-of-age-with-vr-training-coaching-apps-and-smart-gear/?sh=309a8fa219c9
2. Carter K, Podczerwinski J, Love L, et al. Utilizing telesimulation for advanced skills training in consultation and handoff communication: a post-COVID-19 GME bootcamp experience. J Hosp Med. 2021;16(12)730-734. https://doi.org/10.12788/jhm.3733
3. Paige JT, Sonesh SC, Garbee DD, Bonanno LS. Comprensive Healthcare Simulation: Interprofessional Team Training and Simulation. 1st ed. Springer International Publishing; 2020. https://doi.org/10.1007/978-3-030-28845-7
4. Goldenberg MN, Hersh DC, Wilkins KM, Schwartz ML. Suspending medical student clerkships due to COVID-19. Med Sci Educ. 2020;30(3):1-4. https://doi.org/10.1007/s40670-020-00994-1
Techniques and Technologies to Improve Peripheral Intravenous Catheter Outcomes in Pediatric Patients: Systematic Review and Meta-Analysis
Peripheral intravenous catheters (PIVCs) are fundamental to the healthcare practitioners’ ability to provide vital intravenous fluids, medications, and blood products, and as a prophylactic measure prior to some procedures, making insertion of these devices the most common in-hospital invasive procedure in pediatrics.1,2 Despite the prevalence and ubiquity of PIVCs,1 successful insertion in pediatrics is problematic,3-5 and device dysfunction prior to completion of treatment is common.3,6 The inability to attain timely PIVC access and maintain postinsertion function has significant short- and long-term sequelae, including pain and anxiety for children and their parents,3,7 delays in treatment,3 prolonged hospitalization,8 and increased healthcare-associated costs.8-10
Approximately 50% of pediatric PIVC insertions are challenging, often requiring upwards of four insertion attempts, and a similar proportion fail prior to treatment completion.3,11 Exactly why PIVC insertion is difficult in children, and the mechanisms of failure, are unknown. It is likely to be multifaceted and related to factors concerning the patient (eg, comorbidities, age, gender, adiposity),11,12 provider (eg, insertion practice, care, and maintenance),3,13,14 device (eg, size, length, catheter-to-vein ratio),15,16 and therapy (eg, vessel irritation).11,13,17 Observational studies and randomized controlled trials (RCTs) in hospitalized pediatric patients report that the average PIVC dwell is approximately 48 hours, suggesting multiple PIVCs are required to complete a single admission.3,18
Conventionally, PIVC insertion involved physical assessment through palpation and visualization (landmark approach), and although postinsertion care varies among healthcare facilities, minimal requirements are a dressing over the insertion site and regular flushes to ensure device patency.1,3,19 Recently, clinicians have investigated insertion and management practices to improve PIVC outcomes. These can be grouped into techniques—the art of doing (the manner of performance, or the details, of any surgical operation, experiment, or mechanical act) and technologies—the application of scientific knowledge for practical purposes.20 Individual studies have examined the outcomes of new techniques and technologies; however, an overall estimation of their clinical significance or effect is unknown.11,18 Therefore, the aim of this review was to systematically search published studies, conduct a pooled analysis of findings, and report the success of various techniques and technologies to improve insertion success and reduce overall PIVC failure.
METHODS
Design
The protocol for this systematic review was prospectively registered with PROSPERO (CRD42020165288). This review followed Cochrane Collaboration systematic review methods21 and was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.22
Inclusion and Exclusion Criteria
Studies were eligible for inclusion if they met predefined criteria: (1) RCT design; (2) included standard-length PIVC; (3) participants aged 0 to 18 years, excluding preterm infants (less than 36 weeks’ gestation); (4) required PIVC insertion in an inpatient healthcare setting; and (5) reported PIVC insertion outcomes (described below). Studies were excluded if they were cluster or crossover RCTs, published before 2010, or not written in English.
Interventions
Interventions were PIVC insertion and management techniques, defined as “the manner of performance, or the details, of any surgical operation, experiment, or mechanical act” (eg, needle-tip positioning, vein selection [site of insertion], comfort measures, and flushing regimen), or technologies, defined as “the application of scientific knowledge for practical purpose” (eg, vessel visualization, catheter material, and catheter design), compared with current practice, defined as commonly known, practiced, or accepted (eg, landmark PIVC insertion).20
Primary and Secondary Outcomes
The primary outcome was first-time insertion success (one skin puncture to achieve PIVC insertion; can aspirate and flush PIVC without resistance).23 Secondary outcomes included: (1) overall PIVC insertion success23; (2) all-cause PIVC failure (cessation of PIVC function prior to treatment completion)6; (3) dwell time14; (4) PIVC insertion time; (5) insertion attempts23; (6) individual elements of failure (dislodgement, extravasation, infection, occlusion, pain, phlebitis, and thrombosis)6; and (7) patient/parent satisfaction. Some outcomes evaluated were author defined within each study (patient/parent satisfaction, pain score).
Systematic Search
A search of the Cochrane Library and Central Register of Controlled Trials (CENTRAL), Cumulative Index to Nursing and Allied Health (CINAHL), US National Institutes of Health National Library of Medicine (PubMed), and Embase databases between 2010 to 2020 was undertaken on June 23, 2020, and updated March 4, 2021. Medical Subject Heading (MeSH) terms and relevant keywords and their variants were used in collaboration with a healthcare librarian (Appendix Table 1). Additional studies were identified through hand searches of bibliographies.19 Studies were included if two authors (TMK and JS) independently agreed they met the inclusion criteria.
Data Extraction
Two authors (TMK/JS) independently abstracted study data using a standardized form managed in Microsoft Excel.
Quality Assessment
Included studies were assessed by two authors (TMK and JS) for quality using the Cochrane risk of bias (RoB2) tool.21,24 The overall quality of evidence for each outcome was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE)25 approach. Individual RCTs began at high quality, downgraded by one level for “serious” or two levels for “very serious” study limitations, including high risk of bias, serious inconsistency, publication bias, or indirectness of evidence.
Data Analysis and Synthesis
Where two or more trials with evidence of study homogeneity (trial interventions and population) were identified, meta-analysis using RevMan 5 (version 5.4.1)26 with random effects was conducted. Descriptive statistics summarized study population, interventions, and results. For dichotomous outcomes, we calculated risk ratio (RR) plus 95% CI. For continuous outcomes, we planned to calculate the mean difference (MD) plus 95% CI and the standardized mean difference (SMD) (difference between experimental and control groups across trials) reported as the summary statistic.
Subgroup analyses, where possible, included: difficult intravenous access (DIVA), defined by study authors; age (0-3 years; >3 years up to 18 years); hospital setting during PIVC insertion (awake clinical environment vs awake emergency department vs asleep operating room setting); and by operator (bedside nurse, anesthesiologist).
RESULTS
Search Strategy
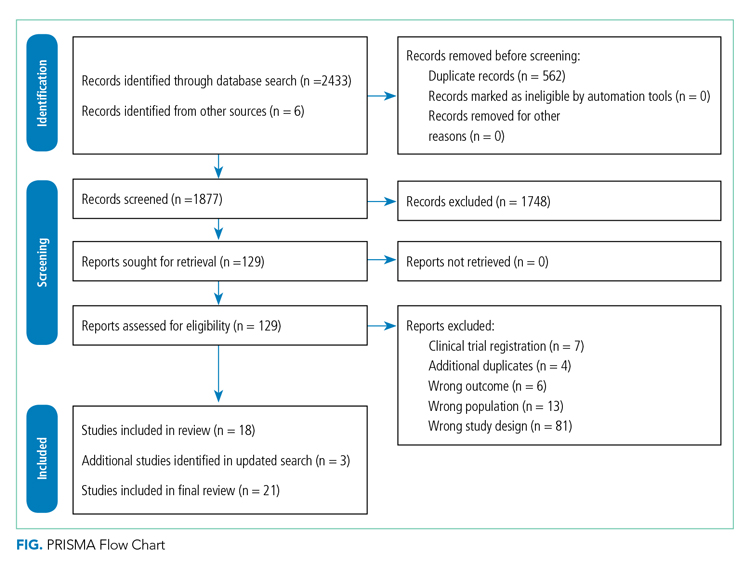
Figure 1 describes study selection in accordance with the PRISMA guidelines.22 We identified 1877 records, and 18 articles met the inclusion criteria. An additional 3 studies were identified in the updated search, totaling 21 studies included in the final review.
Study Characteristics
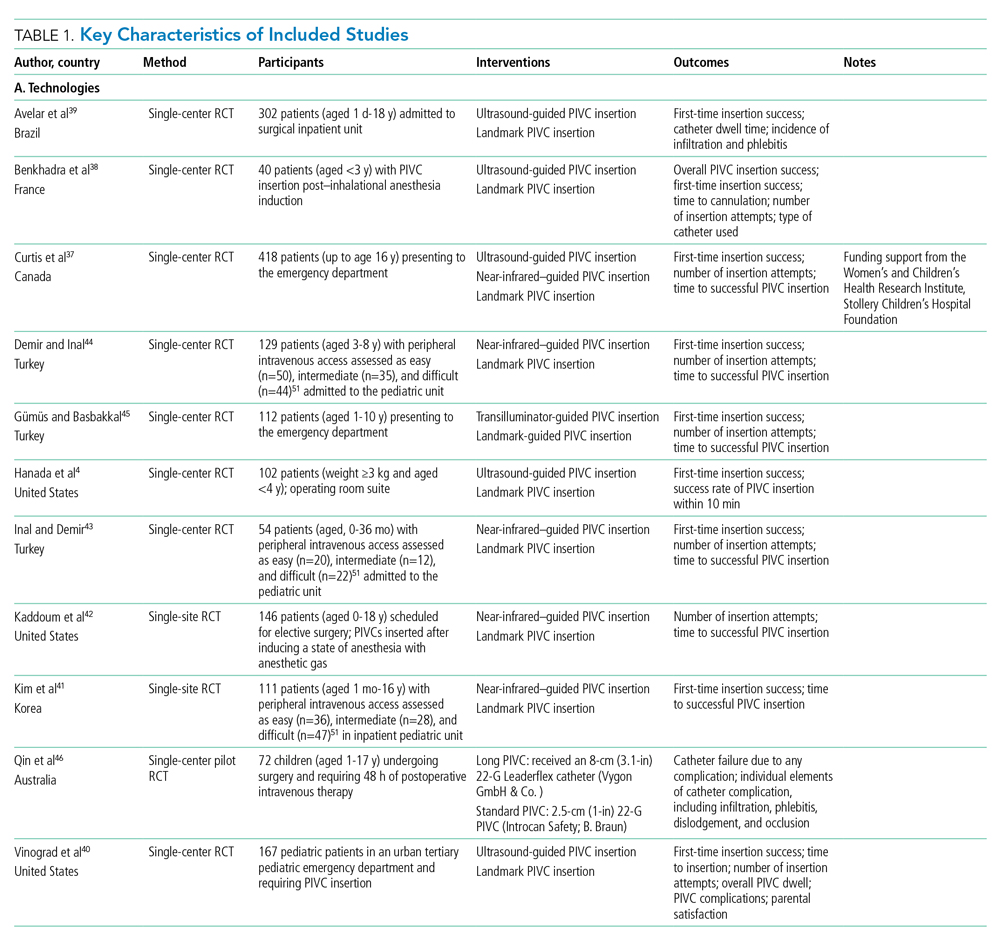
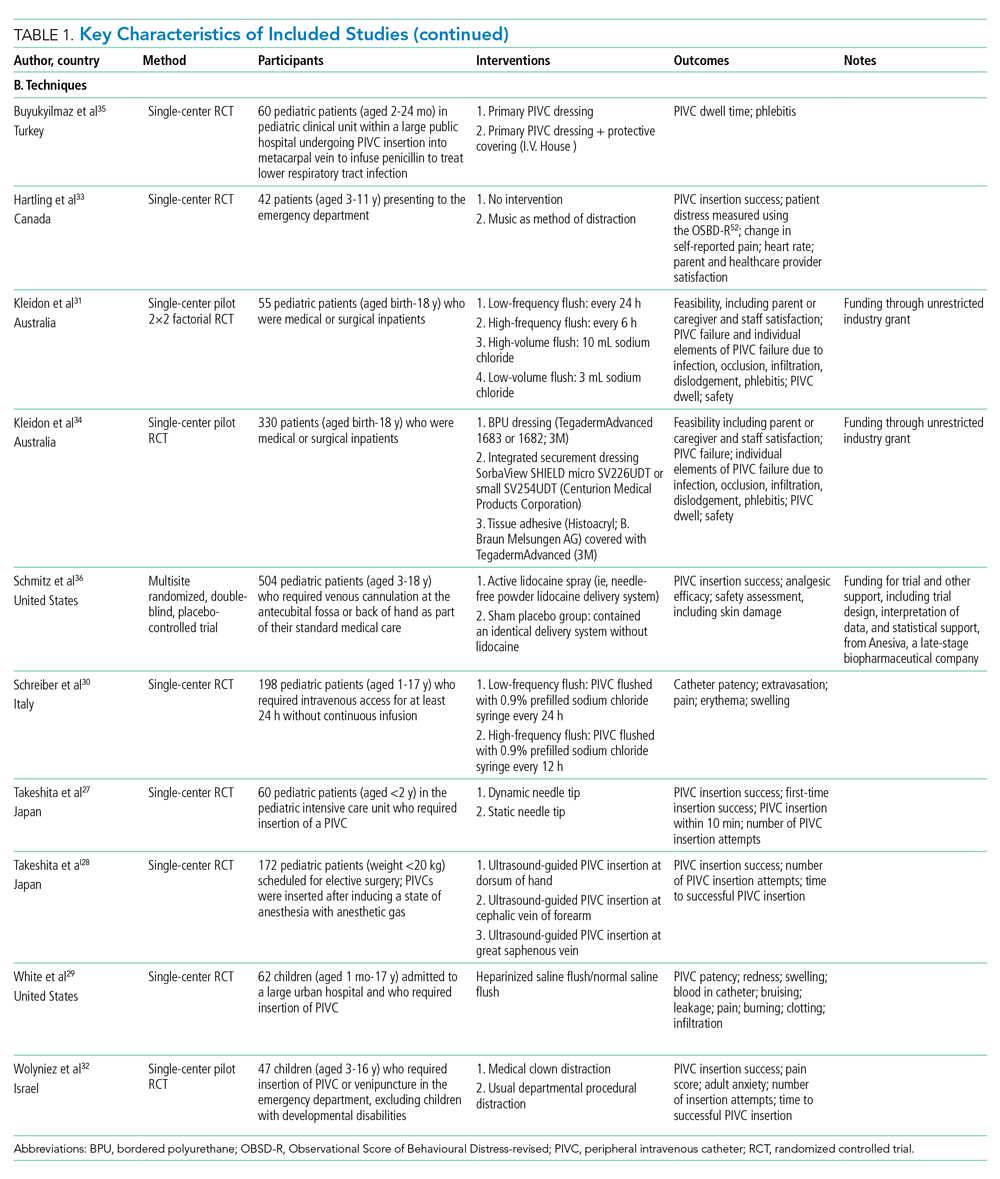
Collectively, 3237 patients and 3098 successful PIVC insertions were reported. In the included studies, 139 patients did not receive a PIVC owing to failed insertion. Ten studies examined techniques (needle-tip positioning,27 vein choice for PIVC insertion,28 flushing regimen,29-31 nonpharmacological32,33 dressing and securement,34,35 and pharmacological comfort measures36), and 11 studies examined technologies (vessel visualization including ultrasound,4,37-40 near-infrared [image of vein projected onto the skin],37,41-44 transillumination [transmission of light through the skin],45 and catheter design46). Table 1 outlines characteristics of included studies. Most trials were single center and conducted in an acute inpatient pediatric-specific setting4,27-34,36-41,44-46 or dedicated pediatric unit in a large public hospital35,43,44; one study was a multicenter trial.36 All trials described evidence of ethical review board approval and participant consent for trial participation.
Study Quality
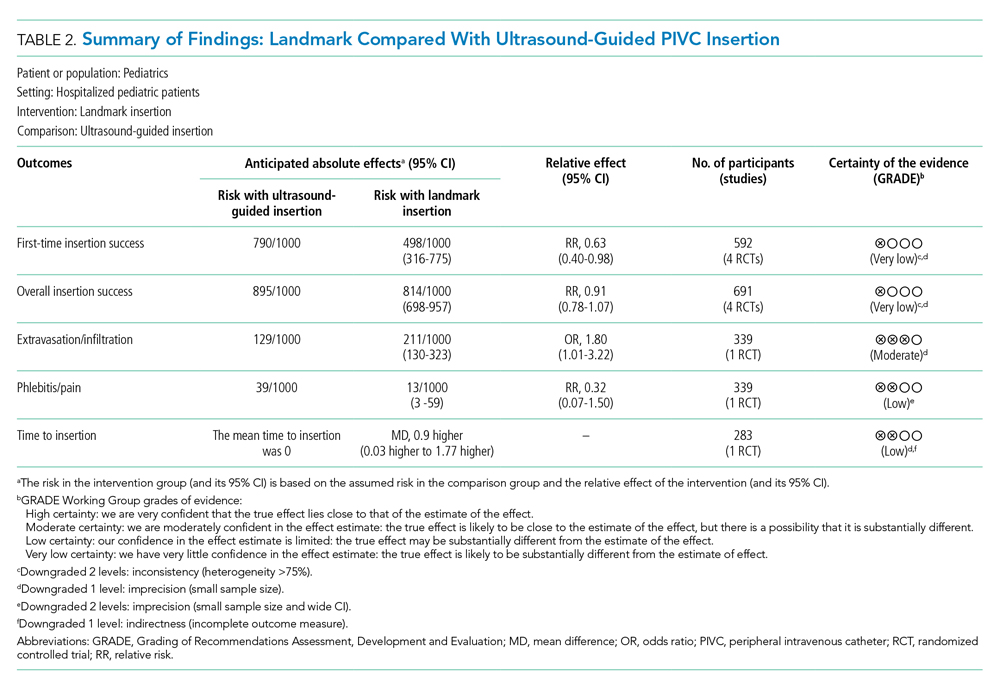
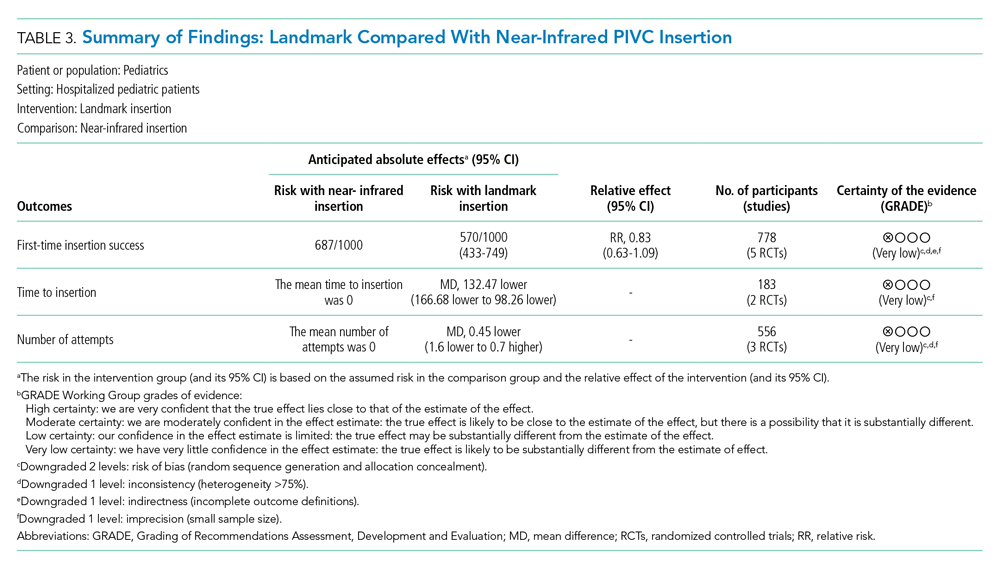
The certainty of evidence at the outcome level varied from moderate to very low. Table 2 and Table 3 outline the summary of findings for landmark insertion compared with ultrasound-guided and landmark insertion compared with near-infrared PIVC insertion, respectively. The remaining summary-of-findings comparisons that included more than one study or addressed clinically relevant questions can be found in Appendix Tables 2, 3, 4, 5, 6, 7, and 8. At the individual study level, most domains were assessed as low risk of bias (Appendix Figure 1).
Effectiveness of Interventions
Technology to Improve PIVC Outcomes
Landmark compared with ultrasound-guided PIVC insertion. Five studies compared PIVC insertion success outcomes when traditional landmark technique was used in comparison with ultrasound guidance (Appendix Figure 2). Four studies (592 patients)4,37,38,40 assessed the primary outcome of first-time insertion success. Appendix Figure 2.1 demonstrates PIVCs were 1.5 times more likely to be inserted on first attempt when ultrasound guidance was used compared with landmark insertion (RR, 1.60; 95% CI, 1.02-2.50). When examining only studies that included DIVA,4,38,40 the effect size increased and CIs tightened (RR, 1.87; 95% CI, 1.56-2.24). No evidence of effect was demonstrated when comparing this outcome in children aged 0 to 3 years (RR, 1.39; 95% CI, 0.88-2.18) or >3 years (RR, 0.72; 95% CI, 0.35-1.51. Two studies4,38 demonstrated that first-time insertion success with ultrasound (compared with landmark) was almost twice as likely (RR, 1.87; 95% CI, 1.44-2.42) after induction of anesthesia in contrast to no effect in studies undertaken in the emergency department37,40 (RR, 1.32; 95% CI, 0.68-2.56). One study39 (339 patients) reported the secondary outcomes of extravasation/infiltration and phlebitis. Extravasation/infiltration was nearly twice as likely with ultrasound compared with landmark insertion (RR, 1.80; 95% CI, 1.01-3.22); however, there was no evidence of effect related to phlebitis (RR, 0.32; 95% CI, 0.07-1.50).
Four studies4,38-40 compared the review’s secondary outcome of PIVC insertion success (Appendix Figure 2.2), with no evidence of an effect (RR, 1.10; 95% CI, 0.94-1.28). No improvement in overall insertion success was demonstrated in the following subgroup analyses: patients with DIVA (RR, 1.18; 95% CI, 0.95-1.47), children under 3 years of age (RR, 1.23; 95% CI, 0.90-1.68), and PIVCs inserted by anesthesiologists (RR, 1.25; 95% CI, 0.91-1.72). One study measured this outcome in children aged >3 years (RR, 1.13; 95% CI, 0.99-1.29) with no effect and in the emergency department (RR, 1.09; 95% CI, 1.00-1.20), where ultrasound guidance improved overall PIVC insertion success.
Landmark compared with near-infrared PIVC insertion. First-time insertion success (Appendix Figure 3.1) was reported in five studies37,41-44 and 778 patients with no evidence of effect (RR, 1.21; 95% CI, 0.91-1.59). Subgroup analysis by DIVA41-44 demonstrated first-time insertion success more than doubled with near-infrared technology compared with landmark (RR, 2.72; 95% CI, 1.02-7.24). Subgroup analysis by age did not demonstrate an effect in children younger than 3 years or children older than 3 years. Subgroup analysis by clinician inserting did not demonstrate an effect. Of the five studies reporting time to insertion,37,41-44 two41,42 reported median rather than mean, so could not be included in the analysis. Of the remaining three studies,37,43,44 near-infrared reduced PIVC time to insertion (Appendix Figure 3.2).
Four studies37,42-44 reported the number of attempts required for successful PIVC insertion where no difference was detected; however, subgroup analysis of patients with DIVA43,44 and insertion by bedside nurse43,44 demonstrated fewer PIVC insertion attempts and a reduction in insertion time, respectively, with the use of near-infrared technology (Appendix Figure 3.3).
Landmark compared with transillumination PIVC insertion. One study45 (112 participants) found a positive effect with the use of transillumination and first-time insertion success (RR, 1.29; 95% CI, 1.07-1.54), reduced time to insertion (MD, –9.70; 95% CI, –17.40 to –2.00), and fewer insertion attempts (MD, –0.24; 95% CI, –0.40 to –0.08) compared with landmark insertion.
Long PIVC compared with short PIVC. A single study46 demonstrated a 70% reduction in PIVC failure (RR, 0.29; 95% CI, 0.14-0.59) when long PIVCs were compared with standard PIVCs. Specifically, PIVC failure due to infiltration was reduced with the use of a long PIVC (RR, 0.08; 95% CI, 0.01-0.61). There was no difference in insertion success (RR, 1.00; 95% CI, 0.95-1.05) or phlebitis (RR, 1.00; 95% CI, 0.07-15.38).
Technique to Improve PIVC Outcomes
Static ultrasound-guided compared with dynamic needle-tip PIVC insertion. In a single study comparing variation in ultrasound-guided PIVC insertion technique27 (60 patients), dynamic needle-tip positioning improved first-time insertion success (RR, 1.44; 95% CI, 1.04-2.00) and overall PIVC insertion success (RR, 1.42; 95% CI, 1.06-1.91).
Variation in vein choice for successful PIVC insertion. Insertion of PIVC in the cephalic vein of the forearm improved insertion success in a single study28 of 172 patients compared with insertion in the dorsal vein of the hand (RR, 1.39; 95% CI, 1.15-1.69) and great saphenous vein (RR, 1.27; 95% CI, 1.08-1.49).
Variation in PIVC flush. Heparinized saline compared with 0.9% sodium chloride flush29 did not reduce infiltration (RR, 0.31; 95% CI, 0.03-2.84), occlusion (RR, 1.88; 95% CI, 0.18-19.63) during dwell, or hematoma (RR, 0.94; 95% CI, 0.06-14.33) at insertion.
Two studies30,31 (253 participants) compared PIVC flush frequency (daily compared with more frequent flush regimes). There was no reduction in overall PIVC failure, extravasation/infiltration, phlebitis, or occlusion during dwell (Appendix Figure 4.1-4.4). Additionally, no effect was demonstrated when a single study31 investigated volume of flush on extravasation/infiltration, dislodgement, phlebitis, or occlusion.
Variation in dressing and securement. One trial (330 participants)34 demonstrated that integrated securement and dressing (ISD) product reduced PIVC failure (RR, 0.65; 95% CI, 0.45-0.93) and occlusion (RR, 0.35; 95% CI, 0.13-0.94) compared with bordered polyurethane (BPU). There was no difference in the proportion of PIVC failure between BPU compared with tissue adhesive (TA) (RR, 0.74; 95% CI, 0.52-1.06). When comparing individual elements of PIVC failure, there was no evidence of effect between BPU and ISD in reducing infiltration (RR, 0.74; 95% CI, 0.43-1.27), dislodgement (RR, 0.49; 95% CI, 0.15-1.58), or phlebitis/pain (RR, 0.54; 95% CI, 0.21-1.39); similarly, the use of TA compared with BPU did not reduce failure due to infiltration (RR, 0.78; 95% CI, 0.45-1.33), dislodgement (RR, 0.37; 95% CI, 0.10-1.35), occlusion (RR, 0.91; 95% CI, 0.45-1.84), or phlebitis/pain (RR, 0.42; 95% CI, 0.17-1.05).
A comparison of protective covering35 (60 participants) did not demonstrate a significant improvement in PIVC dwell (RR, 0.83; 95% CI, 0.25-1.41).
Pharmacological and nonpharmacological interventions. A comparison of nonpharmacological comfort techniques, including music during insertion (one trial, 42 participants), did not improve first-time insertion success between the two groups (RR, 0.74; 95% CI, 0.53-1.03). Similarly, incorporation of a clown32 (47 patients) as method of distraction did not demonstrate an effect on PIVC insertion success (RR, 0.90; 95% CI, 0.77-1.06) or time to PIVC insertion (MD, –0.20; 95% CI, –1.74 to 1.34). In a double-blinded, placebo-controlled RCT36 of pharmacological techniques to reduce PIVC insertion-related pain (504 participants), no evidence of effect was established between the placebo control group and the active analgesia in overall PIVC insertion success (RR, 1.01; 95% CI, 0.97-1.04).
DISCUSSION
Despite their pervasiveness, PIVC insertion in children is problematic and premature device failure is common, yet effective strategies to overcome these challenges have not been systematically reviewed to date. This systematic review (including meta-analysis) examines techniques and technologies to improve PIVC insertion success and reduce overall failure. We demonstrated ultrasound-guided PIVC insertion significantly improved first-time insertion success in general pediatrics.
Analogous to a previous systematic review in adult patients (1660 patients, odds ratio, 2.49; 95% CI, 1.37-4.52; P = .003; I2, 69%),47 we confirm ultrasound improves first-time PIVC insertion success, most notably in pediatric patients with difficult intravenous access. However, widespread use of ultrasound-guided PIVC insertion is limited by operator skills, as it requires practice and dexterity, especially for DIVA patients.5,47 Healthcare facilities should prioritize teaching and training to support acquisition of this skill to reduce the deleterious effects of multiple insertion attempts, including vessel damage, delayed treatment, pain, and anxiety associated with needles.
Other vessel-visualization technologies (near-infrared and transillumination) did not improve PIVC insertion in generic pediatrics.5 However, they significantly improved first-time insertion, time to insertion, and number of insertion attempts in patients with DIVA and should be considered in the absence of ultrasound-proficient clinicians.
Although vessel-visualization technologies provide efficient PIVC insertion, complication-free PIVC dwell is equally important. Few studies examined both insertion outcomes and PIVC postinsertion outcomes (dwell time and complications during treatment). One study reported more postinsertion complications ( eg, infiltration) with ultrasound compared with landmark technique.39 Vessel-visualization tools should be used to assess the vein to guide PIVC choice. Pandurangadu et al15 reported increased PIVC failure when less than 65% of the catheter length resides within the vein; this was consistent with the single RCT46 included in this review that demonstrated reduced infiltration with long PIVCs compared with standard-length PIVCs. To reduce this knowledge practice gap, it is critical that clinicians continue to evaluate and publish findings of novel techniques to improve PIVC outcomes.
The review findings have important implications for future research, clinical practice, and policy. Unlike earlier reviews,48 vessel-visualization technologies, particularly ultrasound, improved PIVC insertion success; however, during-dwell outcomes were inconsistently reported, and future research should include these. In addition, while there is evidence to support these new technologies, adequate training and resources to ensure a sustained, skilled workforce to optimize PIVC insertion are necessary for successful implementation.
Our study had some limitations, including the methodological quality of included studies (small sample size and significant clinical and statistical heterogeneity). Subgroup analyses were undertaken to reduce the heterogeneity inherent in pediatric populations; however, future studies should stratify for patient (age, DIVA, indication for insertion) and setting (conscious/unconscious, emergent/nonemergent) factors. Incomplete or absent outcome definitions and varied reporting measures (eg, median vs mean) prevented calculation of the pooled incidence of catheter failure and dwell time.
Our review also has notable strengths. Two independent investigators performed a rigorous literature search. Only RCTs were included, ensuring the most robust methods to inform clinically important questions. The primary and secondary outcomes were derived from patient-centered outcomes.
CONCLUSION
This systematic review and meta-analysis describes the pooled incidence of PIVC insertion success and outcomes, including complication and failure in pediatric patients. PIVC insertion with ultrasound should be used to improve insertion success in generic pediatric patients, and any form of vessel-visualization technology (ultrasound, near-infrared, transillumination) should be considered for anticipated difficult insertions.
1. Ullman AJ, Takashima M, Kleidon T, Ray-Barruel G, Alexandrou E, Rickard CM. Global pediatric peripheral intravenous catheter practice and performance: a secondary analysis of 4206 catheters. J Pediatr Nurs. 2020;50:e18-e25. https://doi.org/10.1016/j.pedn.2019.09.023
2. Millington SJ, Hendin A, Shiloh AL, Koenig S. Better with ultrasound peripheral intravenous catheter insertion. Chest. 2020;157(2):369-375. https://doi.org/10.1016/j.chest.2019.04.139
3. Kleidon TM, Cattanach P, Mihala G, Ullman AJ. Implementation of a paediatric peripheral intravenous catheter care bundle: a quality improvement initiative. J Paediatr Child Health. 2019;55(10):1214-1223. https://doi.org/10.1111/jpc.14384
4. Hanada S, Van Winkle MT, Subramani S, Ueda K. Dynamic ultrasound-guided short-axis needle tip navigation technique vs. landmark technique for difficult saphenous vein access in children: a randomised study. Anaesthesia. 2017;72(12):1508-1515. https://doi.org/10.1111/anae.14082
5. Heinrichs J, Fritze Z, Klassen T, Curtis S. A systematic review and meta-analysis of new interventions for peripheral intravenous cannulation of children. Pediatr Emerg Care. 2013;29(7):858-866. https://doi.org/10.1097/PEC.0b013e3182999bcd
6. Indarwati F, Mathew S, Munday J, Keogh S. Incidence of peripheral intravenous catheter failure and complications in paediatric patients: systematic review and meta analysis. Int J Nurs Stud. 2020;102:103488. https://doi.org/10.1016/j.ijnurstu.2019.103488
7. Cooke M, Ullman AJ, Ray-Barruel G, Wallis M, Corley A, Rickard CM. Not “just” an intravenous line: consumer perspectives on peripheral intravenous cannulation (PIVC). An international cross-sectional survey of 25 countries. PLoS One. 2018;13(2):e0193436. https://doi.org/10.1371/journal.pone.0193436
8. Goff DA, Larsen P, Brinkley J, et al. Resource utilization and cost of inserting peripheral intravenous catheters in hospitalized children. Hosp Pediatr. 2013;3(3):185-191. https://doi.org/10.1542/hpeds.2012-0089
9. Tuffaha HW, Rickard CM, Webster J, et al. Cost-effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Heath Policy. 2014;12(1):51-58. https://doi.org/10.1007/s40258-013-0077-2
10. Suliman M, Saleh W, Al-Shiekh H, Taan W, AlBashtawy M. The incidence of peripheral intravenous catheter phlebitis and risk factors among pediatric patients. J Pediatr Nurs. 2020;50:89-93. https://doi.org/10.1016/j.pedn.2019.11.006
11. Ben Abdelaziz R, Hafsi H, Hajji H, et al. Peripheral venous catheter complications in children: predisposing factors in a multicenter prospective cohort study. BMC Pediatr. 2017;17(1):208. https://doi.org/10.1186/s12887-017-0965-y
12. Reigart JR, Camberlain KH, Eldridge D, et al. Peripheral intravenous access in pediatric inpatients. Clin Pediatr (Phila). 2012;51(1):468-472. https://doi.org/10.1177/0009922811435164
13. Holder MR, Stutzman SE, Olson DM. Impact of ultrasound on short peripheral intravenous catheter placement on vein thrombosis risk. J Infus Nurs. 2017;40(3):176-182. https://doi.org/10.1097/NAN.0000000000000214
14. Marsh N, Webster J, Larsen E, et al. Expert versus generalist inserters for peripheral intravenous catheter insertion: a pilot randomised controlled trial. Trials. 2018;19(1):564. https://doi.org/10.1186/s13063-018-2946-3
15. Pandurangadu AV, Tucker J, Brackney AR, Bahl A. Ultrasound-guided intravenous catheter survival impacted by amount of catheter residing in the vein. Emerg Med J. 2018;35(9):550-555. https://doi.org/10.1136/emermed-2017-206803
16. Bahl A, Hijazi M, Chen NW, Lachapelle-Clavette L, Price J. Ultralong versus standard long peripheral intravenous catheters: a randomized controlled trial of ultrasonographically guided catheter survival. Ann Emerg Med. 2020;76(2):134-142. https://doi.org/10.1016/j.annemergmed.2019.11.013
17. Takahashi T, Murayama R, Abe-Doi M, et al. Preventing peripheral intravenous catheter failure by reducing mechanical irritation. Sci Rep. 2020;10(1):1550. https://doi.org/10.1038/s41598-019-56873-2
18. Vinograd AM, Zorc JJ, Dean AJ, Abbadessa MKF, Chen AE. First-attempt success, longevity, and complication rates of ultrasound-guided peripheral intravenous catheters in children. Pediatr Emerg Care. 2018;34(6):376-380. https://doi.org/10.1097/PEC.0000000000001063
19. Gorski LA, Hadaway L, Hagle ME, et al. Infusion Therapy Standards of Practice, 8th edition. J Infus Nurs. 2021;44(1S Suppl 1):S1-S224. https://doi.org/10.1097/NAN.0000000000000396
20. Stedman’s Medical Dictionary for the Health Professions and Nursing. 7th ed.Lippincott Williams & Wilkins; 2012.
21. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.1. Cochrane; 2020. www.training.cochrane.org/handbook
22. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341. https://doi.org/10.1016/j.ijsu.2010.02.007
23. Stolz LA, Cappa AR, Minckler MR, et al. Prospective evaluation of the learning curve for ultrasound-guided peripheral intravenous catheter placement. J Vasc Access. 2016;17(4):366-370. https://doi.org/10.5301/jva.5000574
24. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. https://doi.org/10.1136/bmj.l4898
25. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. https://doi.org/10.1136/bmj.328.7454.1490
26. Diaz-Hennessey S, O’Shea ER, King K. Virtual reality: augmenting the acute pain experience in children. Pediatr Nurs. 2019;45(3):122-127.
27. Takeshita J, Yoshida T, Nakajima Y, et al. Superiority of dynamic needle tip positioning for ultrasound-guided peripheral venous catheterization in patients younger than 2 years old: a randomized controlled trial. Pediatr Crit Care Med. 2019;20(9):e410-e414. https://doi.org/10.1097/PCC.0000000000002034
28. Takeshita J, Nakayama Y, Nakajima Y, et al. Optimal site for ultrasound-guided venous catheterisation in paediatric patients: an observational study to investigate predictors for catheterisation success and a randomised controlled study to determine the most successful site. Crit Care. 2015;19(1):15. https://doi.org/10.1186/s13054-014-0733-4
29. White ML, Crawley J, Rennie EA, Lewandowski LA. Examining the effectiveness of 2 solutions used to flush capped pediatric peripheral intravenous catheters. J Infus Nurs. 2011;34(4):260-270. https://doi.org/10.1097/NAN.0b013e31821da29a
30. Schreiber S, Zanchi C, Ronfani L, et al. Normal saline flushes performed once daily maintain peripheral intravenous catheter patency: a randomised controlled trial. Arch Dis Child. 2015;100(7):700-703. https://doi.org/10.1136/archdischild-2014-307478
31. Kleidon TM, Keogh S, Flynn J, Schults J, Mihala G, Rickard CM. Flushing of peripheral intravenous catheters: a pilot, factorial, randomised controlled trial of high versus low frequency and volume in paediatrics. J Paediatr Child Health. 2019;56(1):22-29. https://doi.org/10.1111/jpc.14482
32. Wolyniez I, Rimon A, Scolnik D, et al. The effect of a medical clown on pain during intravenous access in the pediatric emergency department: a randomized prospective pilot study. Clin Pediatr (Phila). 2013;52(12):1168-1172. https://doi.org/10.1177/0009922813502257
33. Hartling L, Newton AS, Liang Y, et al. Music to reduce pain and distress in the pediatric emergency department: a randomized clinical trial. JAMA Pediatr. 2013;167(9):826‐835. https://doi.org/10.1001/jamapediatrics.2013.200
34. Kleidon TM, Rickard CM, Gibson V, et al. Smile - secure my intravenous line effectively: a pilot randomised controlled trial of peripheral intravenous catheter securement in paediatrics. J Tissue Viability. 2020;29(2):82-90. https://doi.org/10.1016/j.jtv.2020.03.006
35. Büyükyilmaz F, Sahiner NC, Caglar S, Eren H. Effectiveness of an intravenous protection device in pediatric patients on catheter dwell time and phlebitis score. Asian Nurs Res (Korean Soc Nurs Sci). 2019;13(4):236-241. https://doi.org/10.1016/j.anr.2019.09.001
36. Schmitz ML, Zempsky WT, Meyer JM. Safety and efficacy of a needle-free powder lidocaine delivery system in pediatric patients undergoing venipuncture or peripheral venous cannulation: randomized double-blind COMFORT-004 trial. Clin Ther. 2015;37(8):1761-1772. https://doi.org/10.1016/j.clinthera.2015.05.515
37. Curtis SJ, Craig WR, Logue E, Vandermeer B, Hanson A, Klassen T. Ultrasound or near-infrared vascular imaging to guide peripheral intravenous catheterization in children: a pragmatic randomized controlled trial. CMAJ. 2015;187(8):563-570. https://doi.org/10.1503/cmaj.141012
38. Benkhadra M, Collignon M, Fournel I, et al. Ultrasound guidance allows faster peripheral IV cannulation in children under 3 years of age with difficult venous access: a prospective randomized study. Paediatr Anaesth. 2012;22(5):449-454. https://doi.org/10.1111/j.1460-9592.2012.03830.x
39. Avelar AFM, Peterlini MAS, da Luz Gonçalves Pedreira M. Ultrasonography-guided peripheral intravenous access in children: a randomized controlled trial. J Infus Nurs. 2015;38(5):320‐327. https://doi.org/10.1097/NAN.0000000000000126
40. Vinograd AM, Chen AE, Woodford AL, et al. Ultrasonographic guidance to improve first-attempt success in children with predicted difficult intravenous access in the emergency department: a randomized controlled trial. Ann Emerg Med. 2019;74(1):19-27. https://doi.org/10.1016/j.annemergmed.2019.02.019
41. Kim MJ, Park JM, Rhee N, et al. Efficacy of VeinViewer in pediatric peripheral intravenous access: a randomized controlled trial. Eur J Pediatr. 2012;171(7):1121-1125. https://doi.org/10.1007/s00431-012-1713-9
42. Kaddoum RN, Anghelescu DL, et al. A randomized controlled trial comparing the AccuVein AV300 device to standard insertion technique for intravenous cannulation of anesthetized children. Paediatr Anaesth. 2012;22(9):884-889. https://doi.org/10.1111/j.1460-9592.2012.03896.x
43. Inal S, Demir D. Impact of peripheral venous catheter placement with vein visualization device support on success rate and pain levels in pediatric patients aged 0 to 3 years. Pediatr Emerg Care. 2021;37(3):138-144. https://doi.org/10.1097/PEC.0000000000001493
44. Demir D, Inal S. Does the use of a vein visualization device for peripheral venous catheter placement increase success rate in pediatric patients? Pediatr Emerg Care. 2019;35(7):474-479. https://doi.org/10.1097/PEC.0000000000001007
45. Gümüs M, Basbakkal Z. Efficacy of Veinlite PEDI in pediatric peripheral intravenous access: a randomized controlled trial. Pediatr Emerg Care. 2021;37(3):145-149. https://doi.org/10.1097/PEC.0000000000001515
46. Qin KR, Ensor N, Barnes R, Englin A, Nataraja RM, Pacilli M. Standard versus long peripheral catheters for multiday IV therapy: a randomized controlled trial. Pediatrics. 2021;147(2): e2020000877. https://doi.org/10.1542/peds.2020-000877
47. van Loon FHJ, Buise MP, Claassen JJF, Dierick-van Daele ATM, Bouwman ARA. Comparison of ultrasound guidance with palpation and direct visualisation for peripheral vein cannulation in adult patients: a systematic review and meta-analysis. Br J Anaesth. 2018;121(2):358-366. https://doi.org/10.1016/j.bja.2018.04.047
48. Parker SIA, Benzies KM, Hayden KA. A systematic review: effectiveness of pediatric peripheral intravenous catheterization strategies. J Adv Nurs. 2017;73(7):1570-1582. https://doi.org/10.1111/jan.13211
Peripheral intravenous catheters (PIVCs) are fundamental to the healthcare practitioners’ ability to provide vital intravenous fluids, medications, and blood products, and as a prophylactic measure prior to some procedures, making insertion of these devices the most common in-hospital invasive procedure in pediatrics.1,2 Despite the prevalence and ubiquity of PIVCs,1 successful insertion in pediatrics is problematic,3-5 and device dysfunction prior to completion of treatment is common.3,6 The inability to attain timely PIVC access and maintain postinsertion function has significant short- and long-term sequelae, including pain and anxiety for children and their parents,3,7 delays in treatment,3 prolonged hospitalization,8 and increased healthcare-associated costs.8-10
Approximately 50% of pediatric PIVC insertions are challenging, often requiring upwards of four insertion attempts, and a similar proportion fail prior to treatment completion.3,11 Exactly why PIVC insertion is difficult in children, and the mechanisms of failure, are unknown. It is likely to be multifaceted and related to factors concerning the patient (eg, comorbidities, age, gender, adiposity),11,12 provider (eg, insertion practice, care, and maintenance),3,13,14 device (eg, size, length, catheter-to-vein ratio),15,16 and therapy (eg, vessel irritation).11,13,17 Observational studies and randomized controlled trials (RCTs) in hospitalized pediatric patients report that the average PIVC dwell is approximately 48 hours, suggesting multiple PIVCs are required to complete a single admission.3,18
Conventionally, PIVC insertion involved physical assessment through palpation and visualization (landmark approach), and although postinsertion care varies among healthcare facilities, minimal requirements are a dressing over the insertion site and regular flushes to ensure device patency.1,3,19 Recently, clinicians have investigated insertion and management practices to improve PIVC outcomes. These can be grouped into techniques—the art of doing (the manner of performance, or the details, of any surgical operation, experiment, or mechanical act) and technologies—the application of scientific knowledge for practical purposes.20 Individual studies have examined the outcomes of new techniques and technologies; however, an overall estimation of their clinical significance or effect is unknown.11,18 Therefore, the aim of this review was to systematically search published studies, conduct a pooled analysis of findings, and report the success of various techniques and technologies to improve insertion success and reduce overall PIVC failure.
METHODS
Design
The protocol for this systematic review was prospectively registered with PROSPERO (CRD42020165288). This review followed Cochrane Collaboration systematic review methods21 and was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.22
Inclusion and Exclusion Criteria
Studies were eligible for inclusion if they met predefined criteria: (1) RCT design; (2) included standard-length PIVC; (3) participants aged 0 to 18 years, excluding preterm infants (less than 36 weeks’ gestation); (4) required PIVC insertion in an inpatient healthcare setting; and (5) reported PIVC insertion outcomes (described below). Studies were excluded if they were cluster or crossover RCTs, published before 2010, or not written in English.
Interventions
Interventions were PIVC insertion and management techniques, defined as “the manner of performance, or the details, of any surgical operation, experiment, or mechanical act” (eg, needle-tip positioning, vein selection [site of insertion], comfort measures, and flushing regimen), or technologies, defined as “the application of scientific knowledge for practical purpose” (eg, vessel visualization, catheter material, and catheter design), compared with current practice, defined as commonly known, practiced, or accepted (eg, landmark PIVC insertion).20
Primary and Secondary Outcomes
The primary outcome was first-time insertion success (one skin puncture to achieve PIVC insertion; can aspirate and flush PIVC without resistance).23 Secondary outcomes included: (1) overall PIVC insertion success23; (2) all-cause PIVC failure (cessation of PIVC function prior to treatment completion)6; (3) dwell time14; (4) PIVC insertion time; (5) insertion attempts23; (6) individual elements of failure (dislodgement, extravasation, infection, occlusion, pain, phlebitis, and thrombosis)6; and (7) patient/parent satisfaction. Some outcomes evaluated were author defined within each study (patient/parent satisfaction, pain score).
Systematic Search
A search of the Cochrane Library and Central Register of Controlled Trials (CENTRAL), Cumulative Index to Nursing and Allied Health (CINAHL), US National Institutes of Health National Library of Medicine (PubMed), and Embase databases between 2010 to 2020 was undertaken on June 23, 2020, and updated March 4, 2021. Medical Subject Heading (MeSH) terms and relevant keywords and their variants were used in collaboration with a healthcare librarian (Appendix Table 1). Additional studies were identified through hand searches of bibliographies.19 Studies were included if two authors (TMK and JS) independently agreed they met the inclusion criteria.
Data Extraction
Two authors (TMK/JS) independently abstracted study data using a standardized form managed in Microsoft Excel.
Quality Assessment
Included studies were assessed by two authors (TMK and JS) for quality using the Cochrane risk of bias (RoB2) tool.21,24 The overall quality of evidence for each outcome was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE)25 approach. Individual RCTs began at high quality, downgraded by one level for “serious” or two levels for “very serious” study limitations, including high risk of bias, serious inconsistency, publication bias, or indirectness of evidence.
Data Analysis and Synthesis
Where two or more trials with evidence of study homogeneity (trial interventions and population) were identified, meta-analysis using RevMan 5 (version 5.4.1)26 with random effects was conducted. Descriptive statistics summarized study population, interventions, and results. For dichotomous outcomes, we calculated risk ratio (RR) plus 95% CI. For continuous outcomes, we planned to calculate the mean difference (MD) plus 95% CI and the standardized mean difference (SMD) (difference between experimental and control groups across trials) reported as the summary statistic.
Subgroup analyses, where possible, included: difficult intravenous access (DIVA), defined by study authors; age (0-3 years; >3 years up to 18 years); hospital setting during PIVC insertion (awake clinical environment vs awake emergency department vs asleep operating room setting); and by operator (bedside nurse, anesthesiologist).
RESULTS
Search Strategy
Figure 1 describes study selection in accordance with the PRISMA guidelines.22 We identified 1877 records, and 18 articles met the inclusion criteria. An additional 3 studies were identified in the updated search, totaling 21 studies included in the final review.

Study Characteristics
Collectively, 3237 patients and 3098 successful PIVC insertions were reported. In the included studies, 139 patients did not receive a PIVC owing to failed insertion. Ten studies examined techniques (needle-tip positioning,27 vein choice for PIVC insertion,28 flushing regimen,29-31 nonpharmacological32,33 dressing and securement,34,35 and pharmacological comfort measures36), and 11 studies examined technologies (vessel visualization including ultrasound,4,37-40 near-infrared [image of vein projected onto the skin],37,41-44 transillumination [transmission of light through the skin],45 and catheter design46). Table 1 outlines characteristics of included studies. Most trials were single center and conducted in an acute inpatient pediatric-specific setting4,27-34,36-41,44-46 or dedicated pediatric unit in a large public hospital35,43,44; one study was a multicenter trial.36 All trials described evidence of ethical review board approval and participant consent for trial participation.


Study Quality
The certainty of evidence at the outcome level varied from moderate to very low. Table 2 and Table 3 outline the summary of findings for landmark insertion compared with ultrasound-guided and landmark insertion compared with near-infrared PIVC insertion, respectively. The remaining summary-of-findings comparisons that included more than one study or addressed clinically relevant questions can be found in Appendix Tables 2, 3, 4, 5, 6, 7, and 8. At the individual study level, most domains were assessed as low risk of bias (Appendix Figure 1).

Effectiveness of Interventions
Technology to Improve PIVC Outcomes
Landmark compared with ultrasound-guided PIVC insertion. Five studies compared PIVC insertion success outcomes when traditional landmark technique was used in comparison with ultrasound guidance (Appendix Figure 2). Four studies (592 patients)4,37,38,40 assessed the primary outcome of first-time insertion success. Appendix Figure 2.1 demonstrates PIVCs were 1.5 times more likely to be inserted on first attempt when ultrasound guidance was used compared with landmark insertion (RR, 1.60; 95% CI, 1.02-2.50). When examining only studies that included DIVA,4,38,40 the effect size increased and CIs tightened (RR, 1.87; 95% CI, 1.56-2.24). No evidence of effect was demonstrated when comparing this outcome in children aged 0 to 3 years (RR, 1.39; 95% CI, 0.88-2.18) or >3 years (RR, 0.72; 95% CI, 0.35-1.51. Two studies4,38 demonstrated that first-time insertion success with ultrasound (compared with landmark) was almost twice as likely (RR, 1.87; 95% CI, 1.44-2.42) after induction of anesthesia in contrast to no effect in studies undertaken in the emergency department37,40 (RR, 1.32; 95% CI, 0.68-2.56). One study39 (339 patients) reported the secondary outcomes of extravasation/infiltration and phlebitis. Extravasation/infiltration was nearly twice as likely with ultrasound compared with landmark insertion (RR, 1.80; 95% CI, 1.01-3.22); however, there was no evidence of effect related to phlebitis (RR, 0.32; 95% CI, 0.07-1.50).

Four studies4,38-40 compared the review’s secondary outcome of PIVC insertion success (Appendix Figure 2.2), with no evidence of an effect (RR, 1.10; 95% CI, 0.94-1.28). No improvement in overall insertion success was demonstrated in the following subgroup analyses: patients with DIVA (RR, 1.18; 95% CI, 0.95-1.47), children under 3 years of age (RR, 1.23; 95% CI, 0.90-1.68), and PIVCs inserted by anesthesiologists (RR, 1.25; 95% CI, 0.91-1.72). One study measured this outcome in children aged >3 years (RR, 1.13; 95% CI, 0.99-1.29) with no effect and in the emergency department (RR, 1.09; 95% CI, 1.00-1.20), where ultrasound guidance improved overall PIVC insertion success.
Landmark compared with near-infrared PIVC insertion. First-time insertion success (Appendix Figure 3.1) was reported in five studies37,41-44 and 778 patients with no evidence of effect (RR, 1.21; 95% CI, 0.91-1.59). Subgroup analysis by DIVA41-44 demonstrated first-time insertion success more than doubled with near-infrared technology compared with landmark (RR, 2.72; 95% CI, 1.02-7.24). Subgroup analysis by age did not demonstrate an effect in children younger than 3 years or children older than 3 years. Subgroup analysis by clinician inserting did not demonstrate an effect. Of the five studies reporting time to insertion,37,41-44 two41,42 reported median rather than mean, so could not be included in the analysis. Of the remaining three studies,37,43,44 near-infrared reduced PIVC time to insertion (Appendix Figure 3.2).
Four studies37,42-44 reported the number of attempts required for successful PIVC insertion where no difference was detected; however, subgroup analysis of patients with DIVA43,44 and insertion by bedside nurse43,44 demonstrated fewer PIVC insertion attempts and a reduction in insertion time, respectively, with the use of near-infrared technology (Appendix Figure 3.3).
Landmark compared with transillumination PIVC insertion. One study45 (112 participants) found a positive effect with the use of transillumination and first-time insertion success (RR, 1.29; 95% CI, 1.07-1.54), reduced time to insertion (MD, –9.70; 95% CI, –17.40 to –2.00), and fewer insertion attempts (MD, –0.24; 95% CI, –0.40 to –0.08) compared with landmark insertion.
Long PIVC compared with short PIVC. A single study46 demonstrated a 70% reduction in PIVC failure (RR, 0.29; 95% CI, 0.14-0.59) when long PIVCs were compared with standard PIVCs. Specifically, PIVC failure due to infiltration was reduced with the use of a long PIVC (RR, 0.08; 95% CI, 0.01-0.61). There was no difference in insertion success (RR, 1.00; 95% CI, 0.95-1.05) or phlebitis (RR, 1.00; 95% CI, 0.07-15.38).
Technique to Improve PIVC Outcomes
Static ultrasound-guided compared with dynamic needle-tip PIVC insertion. In a single study comparing variation in ultrasound-guided PIVC insertion technique27 (60 patients), dynamic needle-tip positioning improved first-time insertion success (RR, 1.44; 95% CI, 1.04-2.00) and overall PIVC insertion success (RR, 1.42; 95% CI, 1.06-1.91).
Variation in vein choice for successful PIVC insertion. Insertion of PIVC in the cephalic vein of the forearm improved insertion success in a single study28 of 172 patients compared with insertion in the dorsal vein of the hand (RR, 1.39; 95% CI, 1.15-1.69) and great saphenous vein (RR, 1.27; 95% CI, 1.08-1.49).
Variation in PIVC flush. Heparinized saline compared with 0.9% sodium chloride flush29 did not reduce infiltration (RR, 0.31; 95% CI, 0.03-2.84), occlusion (RR, 1.88; 95% CI, 0.18-19.63) during dwell, or hematoma (RR, 0.94; 95% CI, 0.06-14.33) at insertion.
Two studies30,31 (253 participants) compared PIVC flush frequency (daily compared with more frequent flush regimes). There was no reduction in overall PIVC failure, extravasation/infiltration, phlebitis, or occlusion during dwell (Appendix Figure 4.1-4.4). Additionally, no effect was demonstrated when a single study31 investigated volume of flush on extravasation/infiltration, dislodgement, phlebitis, or occlusion.
Variation in dressing and securement. One trial (330 participants)34 demonstrated that integrated securement and dressing (ISD) product reduced PIVC failure (RR, 0.65; 95% CI, 0.45-0.93) and occlusion (RR, 0.35; 95% CI, 0.13-0.94) compared with bordered polyurethane (BPU). There was no difference in the proportion of PIVC failure between BPU compared with tissue adhesive (TA) (RR, 0.74; 95% CI, 0.52-1.06). When comparing individual elements of PIVC failure, there was no evidence of effect between BPU and ISD in reducing infiltration (RR, 0.74; 95% CI, 0.43-1.27), dislodgement (RR, 0.49; 95% CI, 0.15-1.58), or phlebitis/pain (RR, 0.54; 95% CI, 0.21-1.39); similarly, the use of TA compared with BPU did not reduce failure due to infiltration (RR, 0.78; 95% CI, 0.45-1.33), dislodgement (RR, 0.37; 95% CI, 0.10-1.35), occlusion (RR, 0.91; 95% CI, 0.45-1.84), or phlebitis/pain (RR, 0.42; 95% CI, 0.17-1.05).
A comparison of protective covering35 (60 participants) did not demonstrate a significant improvement in PIVC dwell (RR, 0.83; 95% CI, 0.25-1.41).
Pharmacological and nonpharmacological interventions. A comparison of nonpharmacological comfort techniques, including music during insertion (one trial, 42 participants), did not improve first-time insertion success between the two groups (RR, 0.74; 95% CI, 0.53-1.03). Similarly, incorporation of a clown32 (47 patients) as method of distraction did not demonstrate an effect on PIVC insertion success (RR, 0.90; 95% CI, 0.77-1.06) or time to PIVC insertion (MD, –0.20; 95% CI, –1.74 to 1.34). In a double-blinded, placebo-controlled RCT36 of pharmacological techniques to reduce PIVC insertion-related pain (504 participants), no evidence of effect was established between the placebo control group and the active analgesia in overall PIVC insertion success (RR, 1.01; 95% CI, 0.97-1.04).
DISCUSSION
Despite their pervasiveness, PIVC insertion in children is problematic and premature device failure is common, yet effective strategies to overcome these challenges have not been systematically reviewed to date. This systematic review (including meta-analysis) examines techniques and technologies to improve PIVC insertion success and reduce overall failure. We demonstrated ultrasound-guided PIVC insertion significantly improved first-time insertion success in general pediatrics.
Analogous to a previous systematic review in adult patients (1660 patients, odds ratio, 2.49; 95% CI, 1.37-4.52; P = .003; I2, 69%),47 we confirm ultrasound improves first-time PIVC insertion success, most notably in pediatric patients with difficult intravenous access. However, widespread use of ultrasound-guided PIVC insertion is limited by operator skills, as it requires practice and dexterity, especially for DIVA patients.5,47 Healthcare facilities should prioritize teaching and training to support acquisition of this skill to reduce the deleterious effects of multiple insertion attempts, including vessel damage, delayed treatment, pain, and anxiety associated with needles.
Other vessel-visualization technologies (near-infrared and transillumination) did not improve PIVC insertion in generic pediatrics.5 However, they significantly improved first-time insertion, time to insertion, and number of insertion attempts in patients with DIVA and should be considered in the absence of ultrasound-proficient clinicians.
Although vessel-visualization technologies provide efficient PIVC insertion, complication-free PIVC dwell is equally important. Few studies examined both insertion outcomes and PIVC postinsertion outcomes (dwell time and complications during treatment). One study reported more postinsertion complications ( eg, infiltration) with ultrasound compared with landmark technique.39 Vessel-visualization tools should be used to assess the vein to guide PIVC choice. Pandurangadu et al15 reported increased PIVC failure when less than 65% of the catheter length resides within the vein; this was consistent with the single RCT46 included in this review that demonstrated reduced infiltration with long PIVCs compared with standard-length PIVCs. To reduce this knowledge practice gap, it is critical that clinicians continue to evaluate and publish findings of novel techniques to improve PIVC outcomes.
The review findings have important implications for future research, clinical practice, and policy. Unlike earlier reviews,48 vessel-visualization technologies, particularly ultrasound, improved PIVC insertion success; however, during-dwell outcomes were inconsistently reported, and future research should include these. In addition, while there is evidence to support these new technologies, adequate training and resources to ensure a sustained, skilled workforce to optimize PIVC insertion are necessary for successful implementation.
Our study had some limitations, including the methodological quality of included studies (small sample size and significant clinical and statistical heterogeneity). Subgroup analyses were undertaken to reduce the heterogeneity inherent in pediatric populations; however, future studies should stratify for patient (age, DIVA, indication for insertion) and setting (conscious/unconscious, emergent/nonemergent) factors. Incomplete or absent outcome definitions and varied reporting measures (eg, median vs mean) prevented calculation of the pooled incidence of catheter failure and dwell time.
Our review also has notable strengths. Two independent investigators performed a rigorous literature search. Only RCTs were included, ensuring the most robust methods to inform clinically important questions. The primary and secondary outcomes were derived from patient-centered outcomes.
CONCLUSION
This systematic review and meta-analysis describes the pooled incidence of PIVC insertion success and outcomes, including complication and failure in pediatric patients. PIVC insertion with ultrasound should be used to improve insertion success in generic pediatric patients, and any form of vessel-visualization technology (ultrasound, near-infrared, transillumination) should be considered for anticipated difficult insertions.
Peripheral intravenous catheters (PIVCs) are fundamental to the healthcare practitioners’ ability to provide vital intravenous fluids, medications, and blood products, and as a prophylactic measure prior to some procedures, making insertion of these devices the most common in-hospital invasive procedure in pediatrics.1,2 Despite the prevalence and ubiquity of PIVCs,1 successful insertion in pediatrics is problematic,3-5 and device dysfunction prior to completion of treatment is common.3,6 The inability to attain timely PIVC access and maintain postinsertion function has significant short- and long-term sequelae, including pain and anxiety for children and their parents,3,7 delays in treatment,3 prolonged hospitalization,8 and increased healthcare-associated costs.8-10
Approximately 50% of pediatric PIVC insertions are challenging, often requiring upwards of four insertion attempts, and a similar proportion fail prior to treatment completion.3,11 Exactly why PIVC insertion is difficult in children, and the mechanisms of failure, are unknown. It is likely to be multifaceted and related to factors concerning the patient (eg, comorbidities, age, gender, adiposity),11,12 provider (eg, insertion practice, care, and maintenance),3,13,14 device (eg, size, length, catheter-to-vein ratio),15,16 and therapy (eg, vessel irritation).11,13,17 Observational studies and randomized controlled trials (RCTs) in hospitalized pediatric patients report that the average PIVC dwell is approximately 48 hours, suggesting multiple PIVCs are required to complete a single admission.3,18
Conventionally, PIVC insertion involved physical assessment through palpation and visualization (landmark approach), and although postinsertion care varies among healthcare facilities, minimal requirements are a dressing over the insertion site and regular flushes to ensure device patency.1,3,19 Recently, clinicians have investigated insertion and management practices to improve PIVC outcomes. These can be grouped into techniques—the art of doing (the manner of performance, or the details, of any surgical operation, experiment, or mechanical act) and technologies—the application of scientific knowledge for practical purposes.20 Individual studies have examined the outcomes of new techniques and technologies; however, an overall estimation of their clinical significance or effect is unknown.11,18 Therefore, the aim of this review was to systematically search published studies, conduct a pooled analysis of findings, and report the success of various techniques and technologies to improve insertion success and reduce overall PIVC failure.
METHODS
Design
The protocol for this systematic review was prospectively registered with PROSPERO (CRD42020165288). This review followed Cochrane Collaboration systematic review methods21 and was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.22
Inclusion and Exclusion Criteria
Studies were eligible for inclusion if they met predefined criteria: (1) RCT design; (2) included standard-length PIVC; (3) participants aged 0 to 18 years, excluding preterm infants (less than 36 weeks’ gestation); (4) required PIVC insertion in an inpatient healthcare setting; and (5) reported PIVC insertion outcomes (described below). Studies were excluded if they were cluster or crossover RCTs, published before 2010, or not written in English.
Interventions
Interventions were PIVC insertion and management techniques, defined as “the manner of performance, or the details, of any surgical operation, experiment, or mechanical act” (eg, needle-tip positioning, vein selection [site of insertion], comfort measures, and flushing regimen), or technologies, defined as “the application of scientific knowledge for practical purpose” (eg, vessel visualization, catheter material, and catheter design), compared with current practice, defined as commonly known, practiced, or accepted (eg, landmark PIVC insertion).20
Primary and Secondary Outcomes
The primary outcome was first-time insertion success (one skin puncture to achieve PIVC insertion; can aspirate and flush PIVC without resistance).23 Secondary outcomes included: (1) overall PIVC insertion success23; (2) all-cause PIVC failure (cessation of PIVC function prior to treatment completion)6; (3) dwell time14; (4) PIVC insertion time; (5) insertion attempts23; (6) individual elements of failure (dislodgement, extravasation, infection, occlusion, pain, phlebitis, and thrombosis)6; and (7) patient/parent satisfaction. Some outcomes evaluated were author defined within each study (patient/parent satisfaction, pain score).
Systematic Search
A search of the Cochrane Library and Central Register of Controlled Trials (CENTRAL), Cumulative Index to Nursing and Allied Health (CINAHL), US National Institutes of Health National Library of Medicine (PubMed), and Embase databases between 2010 to 2020 was undertaken on June 23, 2020, and updated March 4, 2021. Medical Subject Heading (MeSH) terms and relevant keywords and their variants were used in collaboration with a healthcare librarian (Appendix Table 1). Additional studies were identified through hand searches of bibliographies.19 Studies were included if two authors (TMK and JS) independently agreed they met the inclusion criteria.
Data Extraction
Two authors (TMK/JS) independently abstracted study data using a standardized form managed in Microsoft Excel.
Quality Assessment
Included studies were assessed by two authors (TMK and JS) for quality using the Cochrane risk of bias (RoB2) tool.21,24 The overall quality of evidence for each outcome was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE)25 approach. Individual RCTs began at high quality, downgraded by one level for “serious” or two levels for “very serious” study limitations, including high risk of bias, serious inconsistency, publication bias, or indirectness of evidence.
Data Analysis and Synthesis
Where two or more trials with evidence of study homogeneity (trial interventions and population) were identified, meta-analysis using RevMan 5 (version 5.4.1)26 with random effects was conducted. Descriptive statistics summarized study population, interventions, and results. For dichotomous outcomes, we calculated risk ratio (RR) plus 95% CI. For continuous outcomes, we planned to calculate the mean difference (MD) plus 95% CI and the standardized mean difference (SMD) (difference between experimental and control groups across trials) reported as the summary statistic.
Subgroup analyses, where possible, included: difficult intravenous access (DIVA), defined by study authors; age (0-3 years; >3 years up to 18 years); hospital setting during PIVC insertion (awake clinical environment vs awake emergency department vs asleep operating room setting); and by operator (bedside nurse, anesthesiologist).
RESULTS
Search Strategy
Figure 1 describes study selection in accordance with the PRISMA guidelines.22 We identified 1877 records, and 18 articles met the inclusion criteria. An additional 3 studies were identified in the updated search, totaling 21 studies included in the final review.

Study Characteristics
Collectively, 3237 patients and 3098 successful PIVC insertions were reported. In the included studies, 139 patients did not receive a PIVC owing to failed insertion. Ten studies examined techniques (needle-tip positioning,27 vein choice for PIVC insertion,28 flushing regimen,29-31 nonpharmacological32,33 dressing and securement,34,35 and pharmacological comfort measures36), and 11 studies examined technologies (vessel visualization including ultrasound,4,37-40 near-infrared [image of vein projected onto the skin],37,41-44 transillumination [transmission of light through the skin],45 and catheter design46). Table 1 outlines characteristics of included studies. Most trials were single center and conducted in an acute inpatient pediatric-specific setting4,27-34,36-41,44-46 or dedicated pediatric unit in a large public hospital35,43,44; one study was a multicenter trial.36 All trials described evidence of ethical review board approval and participant consent for trial participation.


Study Quality
The certainty of evidence at the outcome level varied from moderate to very low. Table 2 and Table 3 outline the summary of findings for landmark insertion compared with ultrasound-guided and landmark insertion compared with near-infrared PIVC insertion, respectively. The remaining summary-of-findings comparisons that included more than one study or addressed clinically relevant questions can be found in Appendix Tables 2, 3, 4, 5, 6, 7, and 8. At the individual study level, most domains were assessed as low risk of bias (Appendix Figure 1).

Effectiveness of Interventions
Technology to Improve PIVC Outcomes
Landmark compared with ultrasound-guided PIVC insertion. Five studies compared PIVC insertion success outcomes when traditional landmark technique was used in comparison with ultrasound guidance (Appendix Figure 2). Four studies (592 patients)4,37,38,40 assessed the primary outcome of first-time insertion success. Appendix Figure 2.1 demonstrates PIVCs were 1.5 times more likely to be inserted on first attempt when ultrasound guidance was used compared with landmark insertion (RR, 1.60; 95% CI, 1.02-2.50). When examining only studies that included DIVA,4,38,40 the effect size increased and CIs tightened (RR, 1.87; 95% CI, 1.56-2.24). No evidence of effect was demonstrated when comparing this outcome in children aged 0 to 3 years (RR, 1.39; 95% CI, 0.88-2.18) or >3 years (RR, 0.72; 95% CI, 0.35-1.51. Two studies4,38 demonstrated that first-time insertion success with ultrasound (compared with landmark) was almost twice as likely (RR, 1.87; 95% CI, 1.44-2.42) after induction of anesthesia in contrast to no effect in studies undertaken in the emergency department37,40 (RR, 1.32; 95% CI, 0.68-2.56). One study39 (339 patients) reported the secondary outcomes of extravasation/infiltration and phlebitis. Extravasation/infiltration was nearly twice as likely with ultrasound compared with landmark insertion (RR, 1.80; 95% CI, 1.01-3.22); however, there was no evidence of effect related to phlebitis (RR, 0.32; 95% CI, 0.07-1.50).

Four studies4,38-40 compared the review’s secondary outcome of PIVC insertion success (Appendix Figure 2.2), with no evidence of an effect (RR, 1.10; 95% CI, 0.94-1.28). No improvement in overall insertion success was demonstrated in the following subgroup analyses: patients with DIVA (RR, 1.18; 95% CI, 0.95-1.47), children under 3 years of age (RR, 1.23; 95% CI, 0.90-1.68), and PIVCs inserted by anesthesiologists (RR, 1.25; 95% CI, 0.91-1.72). One study measured this outcome in children aged >3 years (RR, 1.13; 95% CI, 0.99-1.29) with no effect and in the emergency department (RR, 1.09; 95% CI, 1.00-1.20), where ultrasound guidance improved overall PIVC insertion success.
Landmark compared with near-infrared PIVC insertion. First-time insertion success (Appendix Figure 3.1) was reported in five studies37,41-44 and 778 patients with no evidence of effect (RR, 1.21; 95% CI, 0.91-1.59). Subgroup analysis by DIVA41-44 demonstrated first-time insertion success more than doubled with near-infrared technology compared with landmark (RR, 2.72; 95% CI, 1.02-7.24). Subgroup analysis by age did not demonstrate an effect in children younger than 3 years or children older than 3 years. Subgroup analysis by clinician inserting did not demonstrate an effect. Of the five studies reporting time to insertion,37,41-44 two41,42 reported median rather than mean, so could not be included in the analysis. Of the remaining three studies,37,43,44 near-infrared reduced PIVC time to insertion (Appendix Figure 3.2).
Four studies37,42-44 reported the number of attempts required for successful PIVC insertion where no difference was detected; however, subgroup analysis of patients with DIVA43,44 and insertion by bedside nurse43,44 demonstrated fewer PIVC insertion attempts and a reduction in insertion time, respectively, with the use of near-infrared technology (Appendix Figure 3.3).
Landmark compared with transillumination PIVC insertion. One study45 (112 participants) found a positive effect with the use of transillumination and first-time insertion success (RR, 1.29; 95% CI, 1.07-1.54), reduced time to insertion (MD, –9.70; 95% CI, –17.40 to –2.00), and fewer insertion attempts (MD, –0.24; 95% CI, –0.40 to –0.08) compared with landmark insertion.
Long PIVC compared with short PIVC. A single study46 demonstrated a 70% reduction in PIVC failure (RR, 0.29; 95% CI, 0.14-0.59) when long PIVCs were compared with standard PIVCs. Specifically, PIVC failure due to infiltration was reduced with the use of a long PIVC (RR, 0.08; 95% CI, 0.01-0.61). There was no difference in insertion success (RR, 1.00; 95% CI, 0.95-1.05) or phlebitis (RR, 1.00; 95% CI, 0.07-15.38).
Technique to Improve PIVC Outcomes
Static ultrasound-guided compared with dynamic needle-tip PIVC insertion. In a single study comparing variation in ultrasound-guided PIVC insertion technique27 (60 patients), dynamic needle-tip positioning improved first-time insertion success (RR, 1.44; 95% CI, 1.04-2.00) and overall PIVC insertion success (RR, 1.42; 95% CI, 1.06-1.91).
Variation in vein choice for successful PIVC insertion. Insertion of PIVC in the cephalic vein of the forearm improved insertion success in a single study28 of 172 patients compared with insertion in the dorsal vein of the hand (RR, 1.39; 95% CI, 1.15-1.69) and great saphenous vein (RR, 1.27; 95% CI, 1.08-1.49).
Variation in PIVC flush. Heparinized saline compared with 0.9% sodium chloride flush29 did not reduce infiltration (RR, 0.31; 95% CI, 0.03-2.84), occlusion (RR, 1.88; 95% CI, 0.18-19.63) during dwell, or hematoma (RR, 0.94; 95% CI, 0.06-14.33) at insertion.
Two studies30,31 (253 participants) compared PIVC flush frequency (daily compared with more frequent flush regimes). There was no reduction in overall PIVC failure, extravasation/infiltration, phlebitis, or occlusion during dwell (Appendix Figure 4.1-4.4). Additionally, no effect was demonstrated when a single study31 investigated volume of flush on extravasation/infiltration, dislodgement, phlebitis, or occlusion.
Variation in dressing and securement. One trial (330 participants)34 demonstrated that integrated securement and dressing (ISD) product reduced PIVC failure (RR, 0.65; 95% CI, 0.45-0.93) and occlusion (RR, 0.35; 95% CI, 0.13-0.94) compared with bordered polyurethane (BPU). There was no difference in the proportion of PIVC failure between BPU compared with tissue adhesive (TA) (RR, 0.74; 95% CI, 0.52-1.06). When comparing individual elements of PIVC failure, there was no evidence of effect between BPU and ISD in reducing infiltration (RR, 0.74; 95% CI, 0.43-1.27), dislodgement (RR, 0.49; 95% CI, 0.15-1.58), or phlebitis/pain (RR, 0.54; 95% CI, 0.21-1.39); similarly, the use of TA compared with BPU did not reduce failure due to infiltration (RR, 0.78; 95% CI, 0.45-1.33), dislodgement (RR, 0.37; 95% CI, 0.10-1.35), occlusion (RR, 0.91; 95% CI, 0.45-1.84), or phlebitis/pain (RR, 0.42; 95% CI, 0.17-1.05).
A comparison of protective covering35 (60 participants) did not demonstrate a significant improvement in PIVC dwell (RR, 0.83; 95% CI, 0.25-1.41).
Pharmacological and nonpharmacological interventions. A comparison of nonpharmacological comfort techniques, including music during insertion (one trial, 42 participants), did not improve first-time insertion success between the two groups (RR, 0.74; 95% CI, 0.53-1.03). Similarly, incorporation of a clown32 (47 patients) as method of distraction did not demonstrate an effect on PIVC insertion success (RR, 0.90; 95% CI, 0.77-1.06) or time to PIVC insertion (MD, –0.20; 95% CI, –1.74 to 1.34). In a double-blinded, placebo-controlled RCT36 of pharmacological techniques to reduce PIVC insertion-related pain (504 participants), no evidence of effect was established between the placebo control group and the active analgesia in overall PIVC insertion success (RR, 1.01; 95% CI, 0.97-1.04).
DISCUSSION
Despite their pervasiveness, PIVC insertion in children is problematic and premature device failure is common, yet effective strategies to overcome these challenges have not been systematically reviewed to date. This systematic review (including meta-analysis) examines techniques and technologies to improve PIVC insertion success and reduce overall failure. We demonstrated ultrasound-guided PIVC insertion significantly improved first-time insertion success in general pediatrics.
Analogous to a previous systematic review in adult patients (1660 patients, odds ratio, 2.49; 95% CI, 1.37-4.52; P = .003; I2, 69%),47 we confirm ultrasound improves first-time PIVC insertion success, most notably in pediatric patients with difficult intravenous access. However, widespread use of ultrasound-guided PIVC insertion is limited by operator skills, as it requires practice and dexterity, especially for DIVA patients.5,47 Healthcare facilities should prioritize teaching and training to support acquisition of this skill to reduce the deleterious effects of multiple insertion attempts, including vessel damage, delayed treatment, pain, and anxiety associated with needles.
Other vessel-visualization technologies (near-infrared and transillumination) did not improve PIVC insertion in generic pediatrics.5 However, they significantly improved first-time insertion, time to insertion, and number of insertion attempts in patients with DIVA and should be considered in the absence of ultrasound-proficient clinicians.
Although vessel-visualization technologies provide efficient PIVC insertion, complication-free PIVC dwell is equally important. Few studies examined both insertion outcomes and PIVC postinsertion outcomes (dwell time and complications during treatment). One study reported more postinsertion complications ( eg, infiltration) with ultrasound compared with landmark technique.39 Vessel-visualization tools should be used to assess the vein to guide PIVC choice. Pandurangadu et al15 reported increased PIVC failure when less than 65% of the catheter length resides within the vein; this was consistent with the single RCT46 included in this review that demonstrated reduced infiltration with long PIVCs compared with standard-length PIVCs. To reduce this knowledge practice gap, it is critical that clinicians continue to evaluate and publish findings of novel techniques to improve PIVC outcomes.
The review findings have important implications for future research, clinical practice, and policy. Unlike earlier reviews,48 vessel-visualization technologies, particularly ultrasound, improved PIVC insertion success; however, during-dwell outcomes were inconsistently reported, and future research should include these. In addition, while there is evidence to support these new technologies, adequate training and resources to ensure a sustained, skilled workforce to optimize PIVC insertion are necessary for successful implementation.
Our study had some limitations, including the methodological quality of included studies (small sample size and significant clinical and statistical heterogeneity). Subgroup analyses were undertaken to reduce the heterogeneity inherent in pediatric populations; however, future studies should stratify for patient (age, DIVA, indication for insertion) and setting (conscious/unconscious, emergent/nonemergent) factors. Incomplete or absent outcome definitions and varied reporting measures (eg, median vs mean) prevented calculation of the pooled incidence of catheter failure and dwell time.
Our review also has notable strengths. Two independent investigators performed a rigorous literature search. Only RCTs were included, ensuring the most robust methods to inform clinically important questions. The primary and secondary outcomes were derived from patient-centered outcomes.
CONCLUSION
This systematic review and meta-analysis describes the pooled incidence of PIVC insertion success and outcomes, including complication and failure in pediatric patients. PIVC insertion with ultrasound should be used to improve insertion success in generic pediatric patients, and any form of vessel-visualization technology (ultrasound, near-infrared, transillumination) should be considered for anticipated difficult insertions.
1. Ullman AJ, Takashima M, Kleidon T, Ray-Barruel G, Alexandrou E, Rickard CM. Global pediatric peripheral intravenous catheter practice and performance: a secondary analysis of 4206 catheters. J Pediatr Nurs. 2020;50:e18-e25. https://doi.org/10.1016/j.pedn.2019.09.023
2. Millington SJ, Hendin A, Shiloh AL, Koenig S. Better with ultrasound peripheral intravenous catheter insertion. Chest. 2020;157(2):369-375. https://doi.org/10.1016/j.chest.2019.04.139
3. Kleidon TM, Cattanach P, Mihala G, Ullman AJ. Implementation of a paediatric peripheral intravenous catheter care bundle: a quality improvement initiative. J Paediatr Child Health. 2019;55(10):1214-1223. https://doi.org/10.1111/jpc.14384
4. Hanada S, Van Winkle MT, Subramani S, Ueda K. Dynamic ultrasound-guided short-axis needle tip navigation technique vs. landmark technique for difficult saphenous vein access in children: a randomised study. Anaesthesia. 2017;72(12):1508-1515. https://doi.org/10.1111/anae.14082
5. Heinrichs J, Fritze Z, Klassen T, Curtis S. A systematic review and meta-analysis of new interventions for peripheral intravenous cannulation of children. Pediatr Emerg Care. 2013;29(7):858-866. https://doi.org/10.1097/PEC.0b013e3182999bcd
6. Indarwati F, Mathew S, Munday J, Keogh S. Incidence of peripheral intravenous catheter failure and complications in paediatric patients: systematic review and meta analysis. Int J Nurs Stud. 2020;102:103488. https://doi.org/10.1016/j.ijnurstu.2019.103488
7. Cooke M, Ullman AJ, Ray-Barruel G, Wallis M, Corley A, Rickard CM. Not “just” an intravenous line: consumer perspectives on peripheral intravenous cannulation (PIVC). An international cross-sectional survey of 25 countries. PLoS One. 2018;13(2):e0193436. https://doi.org/10.1371/journal.pone.0193436
8. Goff DA, Larsen P, Brinkley J, et al. Resource utilization and cost of inserting peripheral intravenous catheters in hospitalized children. Hosp Pediatr. 2013;3(3):185-191. https://doi.org/10.1542/hpeds.2012-0089
9. Tuffaha HW, Rickard CM, Webster J, et al. Cost-effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Heath Policy. 2014;12(1):51-58. https://doi.org/10.1007/s40258-013-0077-2
10. Suliman M, Saleh W, Al-Shiekh H, Taan W, AlBashtawy M. The incidence of peripheral intravenous catheter phlebitis and risk factors among pediatric patients. J Pediatr Nurs. 2020;50:89-93. https://doi.org/10.1016/j.pedn.2019.11.006
11. Ben Abdelaziz R, Hafsi H, Hajji H, et al. Peripheral venous catheter complications in children: predisposing factors in a multicenter prospective cohort study. BMC Pediatr. 2017;17(1):208. https://doi.org/10.1186/s12887-017-0965-y
12. Reigart JR, Camberlain KH, Eldridge D, et al. Peripheral intravenous access in pediatric inpatients. Clin Pediatr (Phila). 2012;51(1):468-472. https://doi.org/10.1177/0009922811435164
13. Holder MR, Stutzman SE, Olson DM. Impact of ultrasound on short peripheral intravenous catheter placement on vein thrombosis risk. J Infus Nurs. 2017;40(3):176-182. https://doi.org/10.1097/NAN.0000000000000214
14. Marsh N, Webster J, Larsen E, et al. Expert versus generalist inserters for peripheral intravenous catheter insertion: a pilot randomised controlled trial. Trials. 2018;19(1):564. https://doi.org/10.1186/s13063-018-2946-3
15. Pandurangadu AV, Tucker J, Brackney AR, Bahl A. Ultrasound-guided intravenous catheter survival impacted by amount of catheter residing in the vein. Emerg Med J. 2018;35(9):550-555. https://doi.org/10.1136/emermed-2017-206803
16. Bahl A, Hijazi M, Chen NW, Lachapelle-Clavette L, Price J. Ultralong versus standard long peripheral intravenous catheters: a randomized controlled trial of ultrasonographically guided catheter survival. Ann Emerg Med. 2020;76(2):134-142. https://doi.org/10.1016/j.annemergmed.2019.11.013
17. Takahashi T, Murayama R, Abe-Doi M, et al. Preventing peripheral intravenous catheter failure by reducing mechanical irritation. Sci Rep. 2020;10(1):1550. https://doi.org/10.1038/s41598-019-56873-2
18. Vinograd AM, Zorc JJ, Dean AJ, Abbadessa MKF, Chen AE. First-attempt success, longevity, and complication rates of ultrasound-guided peripheral intravenous catheters in children. Pediatr Emerg Care. 2018;34(6):376-380. https://doi.org/10.1097/PEC.0000000000001063
19. Gorski LA, Hadaway L, Hagle ME, et al. Infusion Therapy Standards of Practice, 8th edition. J Infus Nurs. 2021;44(1S Suppl 1):S1-S224. https://doi.org/10.1097/NAN.0000000000000396
20. Stedman’s Medical Dictionary for the Health Professions and Nursing. 7th ed.Lippincott Williams & Wilkins; 2012.
21. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.1. Cochrane; 2020. www.training.cochrane.org/handbook
22. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341. https://doi.org/10.1016/j.ijsu.2010.02.007
23. Stolz LA, Cappa AR, Minckler MR, et al. Prospective evaluation of the learning curve for ultrasound-guided peripheral intravenous catheter placement. J Vasc Access. 2016;17(4):366-370. https://doi.org/10.5301/jva.5000574
24. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. https://doi.org/10.1136/bmj.l4898
25. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. https://doi.org/10.1136/bmj.328.7454.1490
26. Diaz-Hennessey S, O’Shea ER, King K. Virtual reality: augmenting the acute pain experience in children. Pediatr Nurs. 2019;45(3):122-127.
27. Takeshita J, Yoshida T, Nakajima Y, et al. Superiority of dynamic needle tip positioning for ultrasound-guided peripheral venous catheterization in patients younger than 2 years old: a randomized controlled trial. Pediatr Crit Care Med. 2019;20(9):e410-e414. https://doi.org/10.1097/PCC.0000000000002034
28. Takeshita J, Nakayama Y, Nakajima Y, et al. Optimal site for ultrasound-guided venous catheterisation in paediatric patients: an observational study to investigate predictors for catheterisation success and a randomised controlled study to determine the most successful site. Crit Care. 2015;19(1):15. https://doi.org/10.1186/s13054-014-0733-4
29. White ML, Crawley J, Rennie EA, Lewandowski LA. Examining the effectiveness of 2 solutions used to flush capped pediatric peripheral intravenous catheters. J Infus Nurs. 2011;34(4):260-270. https://doi.org/10.1097/NAN.0b013e31821da29a
30. Schreiber S, Zanchi C, Ronfani L, et al. Normal saline flushes performed once daily maintain peripheral intravenous catheter patency: a randomised controlled trial. Arch Dis Child. 2015;100(7):700-703. https://doi.org/10.1136/archdischild-2014-307478
31. Kleidon TM, Keogh S, Flynn J, Schults J, Mihala G, Rickard CM. Flushing of peripheral intravenous catheters: a pilot, factorial, randomised controlled trial of high versus low frequency and volume in paediatrics. J Paediatr Child Health. 2019;56(1):22-29. https://doi.org/10.1111/jpc.14482
32. Wolyniez I, Rimon A, Scolnik D, et al. The effect of a medical clown on pain during intravenous access in the pediatric emergency department: a randomized prospective pilot study. Clin Pediatr (Phila). 2013;52(12):1168-1172. https://doi.org/10.1177/0009922813502257
33. Hartling L, Newton AS, Liang Y, et al. Music to reduce pain and distress in the pediatric emergency department: a randomized clinical trial. JAMA Pediatr. 2013;167(9):826‐835. https://doi.org/10.1001/jamapediatrics.2013.200
34. Kleidon TM, Rickard CM, Gibson V, et al. Smile - secure my intravenous line effectively: a pilot randomised controlled trial of peripheral intravenous catheter securement in paediatrics. J Tissue Viability. 2020;29(2):82-90. https://doi.org/10.1016/j.jtv.2020.03.006
35. Büyükyilmaz F, Sahiner NC, Caglar S, Eren H. Effectiveness of an intravenous protection device in pediatric patients on catheter dwell time and phlebitis score. Asian Nurs Res (Korean Soc Nurs Sci). 2019;13(4):236-241. https://doi.org/10.1016/j.anr.2019.09.001
36. Schmitz ML, Zempsky WT, Meyer JM. Safety and efficacy of a needle-free powder lidocaine delivery system in pediatric patients undergoing venipuncture or peripheral venous cannulation: randomized double-blind COMFORT-004 trial. Clin Ther. 2015;37(8):1761-1772. https://doi.org/10.1016/j.clinthera.2015.05.515
37. Curtis SJ, Craig WR, Logue E, Vandermeer B, Hanson A, Klassen T. Ultrasound or near-infrared vascular imaging to guide peripheral intravenous catheterization in children: a pragmatic randomized controlled trial. CMAJ. 2015;187(8):563-570. https://doi.org/10.1503/cmaj.141012
38. Benkhadra M, Collignon M, Fournel I, et al. Ultrasound guidance allows faster peripheral IV cannulation in children under 3 years of age with difficult venous access: a prospective randomized study. Paediatr Anaesth. 2012;22(5):449-454. https://doi.org/10.1111/j.1460-9592.2012.03830.x
39. Avelar AFM, Peterlini MAS, da Luz Gonçalves Pedreira M. Ultrasonography-guided peripheral intravenous access in children: a randomized controlled trial. J Infus Nurs. 2015;38(5):320‐327. https://doi.org/10.1097/NAN.0000000000000126
40. Vinograd AM, Chen AE, Woodford AL, et al. Ultrasonographic guidance to improve first-attempt success in children with predicted difficult intravenous access in the emergency department: a randomized controlled trial. Ann Emerg Med. 2019;74(1):19-27. https://doi.org/10.1016/j.annemergmed.2019.02.019
41. Kim MJ, Park JM, Rhee N, et al. Efficacy of VeinViewer in pediatric peripheral intravenous access: a randomized controlled trial. Eur J Pediatr. 2012;171(7):1121-1125. https://doi.org/10.1007/s00431-012-1713-9
42. Kaddoum RN, Anghelescu DL, et al. A randomized controlled trial comparing the AccuVein AV300 device to standard insertion technique for intravenous cannulation of anesthetized children. Paediatr Anaesth. 2012;22(9):884-889. https://doi.org/10.1111/j.1460-9592.2012.03896.x
43. Inal S, Demir D. Impact of peripheral venous catheter placement with vein visualization device support on success rate and pain levels in pediatric patients aged 0 to 3 years. Pediatr Emerg Care. 2021;37(3):138-144. https://doi.org/10.1097/PEC.0000000000001493
44. Demir D, Inal S. Does the use of a vein visualization device for peripheral venous catheter placement increase success rate in pediatric patients? Pediatr Emerg Care. 2019;35(7):474-479. https://doi.org/10.1097/PEC.0000000000001007
45. Gümüs M, Basbakkal Z. Efficacy of Veinlite PEDI in pediatric peripheral intravenous access: a randomized controlled trial. Pediatr Emerg Care. 2021;37(3):145-149. https://doi.org/10.1097/PEC.0000000000001515
46. Qin KR, Ensor N, Barnes R, Englin A, Nataraja RM, Pacilli M. Standard versus long peripheral catheters for multiday IV therapy: a randomized controlled trial. Pediatrics. 2021;147(2): e2020000877. https://doi.org/10.1542/peds.2020-000877
47. van Loon FHJ, Buise MP, Claassen JJF, Dierick-van Daele ATM, Bouwman ARA. Comparison of ultrasound guidance with palpation and direct visualisation for peripheral vein cannulation in adult patients: a systematic review and meta-analysis. Br J Anaesth. 2018;121(2):358-366. https://doi.org/10.1016/j.bja.2018.04.047
48. Parker SIA, Benzies KM, Hayden KA. A systematic review: effectiveness of pediatric peripheral intravenous catheterization strategies. J Adv Nurs. 2017;73(7):1570-1582. https://doi.org/10.1111/jan.13211
1. Ullman AJ, Takashima M, Kleidon T, Ray-Barruel G, Alexandrou E, Rickard CM. Global pediatric peripheral intravenous catheter practice and performance: a secondary analysis of 4206 catheters. J Pediatr Nurs. 2020;50:e18-e25. https://doi.org/10.1016/j.pedn.2019.09.023
2. Millington SJ, Hendin A, Shiloh AL, Koenig S. Better with ultrasound peripheral intravenous catheter insertion. Chest. 2020;157(2):369-375. https://doi.org/10.1016/j.chest.2019.04.139
3. Kleidon TM, Cattanach P, Mihala G, Ullman AJ. Implementation of a paediatric peripheral intravenous catheter care bundle: a quality improvement initiative. J Paediatr Child Health. 2019;55(10):1214-1223. https://doi.org/10.1111/jpc.14384
4. Hanada S, Van Winkle MT, Subramani S, Ueda K. Dynamic ultrasound-guided short-axis needle tip navigation technique vs. landmark technique for difficult saphenous vein access in children: a randomised study. Anaesthesia. 2017;72(12):1508-1515. https://doi.org/10.1111/anae.14082
5. Heinrichs J, Fritze Z, Klassen T, Curtis S. A systematic review and meta-analysis of new interventions for peripheral intravenous cannulation of children. Pediatr Emerg Care. 2013;29(7):858-866. https://doi.org/10.1097/PEC.0b013e3182999bcd
6. Indarwati F, Mathew S, Munday J, Keogh S. Incidence of peripheral intravenous catheter failure and complications in paediatric patients: systematic review and meta analysis. Int J Nurs Stud. 2020;102:103488. https://doi.org/10.1016/j.ijnurstu.2019.103488
7. Cooke M, Ullman AJ, Ray-Barruel G, Wallis M, Corley A, Rickard CM. Not “just” an intravenous line: consumer perspectives on peripheral intravenous cannulation (PIVC). An international cross-sectional survey of 25 countries. PLoS One. 2018;13(2):e0193436. https://doi.org/10.1371/journal.pone.0193436
8. Goff DA, Larsen P, Brinkley J, et al. Resource utilization and cost of inserting peripheral intravenous catheters in hospitalized children. Hosp Pediatr. 2013;3(3):185-191. https://doi.org/10.1542/hpeds.2012-0089
9. Tuffaha HW, Rickard CM, Webster J, et al. Cost-effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Heath Policy. 2014;12(1):51-58. https://doi.org/10.1007/s40258-013-0077-2
10. Suliman M, Saleh W, Al-Shiekh H, Taan W, AlBashtawy M. The incidence of peripheral intravenous catheter phlebitis and risk factors among pediatric patients. J Pediatr Nurs. 2020;50:89-93. https://doi.org/10.1016/j.pedn.2019.11.006
11. Ben Abdelaziz R, Hafsi H, Hajji H, et al. Peripheral venous catheter complications in children: predisposing factors in a multicenter prospective cohort study. BMC Pediatr. 2017;17(1):208. https://doi.org/10.1186/s12887-017-0965-y
12. Reigart JR, Camberlain KH, Eldridge D, et al. Peripheral intravenous access in pediatric inpatients. Clin Pediatr (Phila). 2012;51(1):468-472. https://doi.org/10.1177/0009922811435164
13. Holder MR, Stutzman SE, Olson DM. Impact of ultrasound on short peripheral intravenous catheter placement on vein thrombosis risk. J Infus Nurs. 2017;40(3):176-182. https://doi.org/10.1097/NAN.0000000000000214
14. Marsh N, Webster J, Larsen E, et al. Expert versus generalist inserters for peripheral intravenous catheter insertion: a pilot randomised controlled trial. Trials. 2018;19(1):564. https://doi.org/10.1186/s13063-018-2946-3
15. Pandurangadu AV, Tucker J, Brackney AR, Bahl A. Ultrasound-guided intravenous catheter survival impacted by amount of catheter residing in the vein. Emerg Med J. 2018;35(9):550-555. https://doi.org/10.1136/emermed-2017-206803
16. Bahl A, Hijazi M, Chen NW, Lachapelle-Clavette L, Price J. Ultralong versus standard long peripheral intravenous catheters: a randomized controlled trial of ultrasonographically guided catheter survival. Ann Emerg Med. 2020;76(2):134-142. https://doi.org/10.1016/j.annemergmed.2019.11.013
17. Takahashi T, Murayama R, Abe-Doi M, et al. Preventing peripheral intravenous catheter failure by reducing mechanical irritation. Sci Rep. 2020;10(1):1550. https://doi.org/10.1038/s41598-019-56873-2
18. Vinograd AM, Zorc JJ, Dean AJ, Abbadessa MKF, Chen AE. First-attempt success, longevity, and complication rates of ultrasound-guided peripheral intravenous catheters in children. Pediatr Emerg Care. 2018;34(6):376-380. https://doi.org/10.1097/PEC.0000000000001063
19. Gorski LA, Hadaway L, Hagle ME, et al. Infusion Therapy Standards of Practice, 8th edition. J Infus Nurs. 2021;44(1S Suppl 1):S1-S224. https://doi.org/10.1097/NAN.0000000000000396
20. Stedman’s Medical Dictionary for the Health Professions and Nursing. 7th ed.Lippincott Williams & Wilkins; 2012.
21. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.1. Cochrane; 2020. www.training.cochrane.org/handbook
22. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341. https://doi.org/10.1016/j.ijsu.2010.02.007
23. Stolz LA, Cappa AR, Minckler MR, et al. Prospective evaluation of the learning curve for ultrasound-guided peripheral intravenous catheter placement. J Vasc Access. 2016;17(4):366-370. https://doi.org/10.5301/jva.5000574
24. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. https://doi.org/10.1136/bmj.l4898
25. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. https://doi.org/10.1136/bmj.328.7454.1490
26. Diaz-Hennessey S, O’Shea ER, King K. Virtual reality: augmenting the acute pain experience in children. Pediatr Nurs. 2019;45(3):122-127.
27. Takeshita J, Yoshida T, Nakajima Y, et al. Superiority of dynamic needle tip positioning for ultrasound-guided peripheral venous catheterization in patients younger than 2 years old: a randomized controlled trial. Pediatr Crit Care Med. 2019;20(9):e410-e414. https://doi.org/10.1097/PCC.0000000000002034
28. Takeshita J, Nakayama Y, Nakajima Y, et al. Optimal site for ultrasound-guided venous catheterisation in paediatric patients: an observational study to investigate predictors for catheterisation success and a randomised controlled study to determine the most successful site. Crit Care. 2015;19(1):15. https://doi.org/10.1186/s13054-014-0733-4
29. White ML, Crawley J, Rennie EA, Lewandowski LA. Examining the effectiveness of 2 solutions used to flush capped pediatric peripheral intravenous catheters. J Infus Nurs. 2011;34(4):260-270. https://doi.org/10.1097/NAN.0b013e31821da29a
30. Schreiber S, Zanchi C, Ronfani L, et al. Normal saline flushes performed once daily maintain peripheral intravenous catheter patency: a randomised controlled trial. Arch Dis Child. 2015;100(7):700-703. https://doi.org/10.1136/archdischild-2014-307478
31. Kleidon TM, Keogh S, Flynn J, Schults J, Mihala G, Rickard CM. Flushing of peripheral intravenous catheters: a pilot, factorial, randomised controlled trial of high versus low frequency and volume in paediatrics. J Paediatr Child Health. 2019;56(1):22-29. https://doi.org/10.1111/jpc.14482
32. Wolyniez I, Rimon A, Scolnik D, et al. The effect of a medical clown on pain during intravenous access in the pediatric emergency department: a randomized prospective pilot study. Clin Pediatr (Phila). 2013;52(12):1168-1172. https://doi.org/10.1177/0009922813502257
33. Hartling L, Newton AS, Liang Y, et al. Music to reduce pain and distress in the pediatric emergency department: a randomized clinical trial. JAMA Pediatr. 2013;167(9):826‐835. https://doi.org/10.1001/jamapediatrics.2013.200
34. Kleidon TM, Rickard CM, Gibson V, et al. Smile - secure my intravenous line effectively: a pilot randomised controlled trial of peripheral intravenous catheter securement in paediatrics. J Tissue Viability. 2020;29(2):82-90. https://doi.org/10.1016/j.jtv.2020.03.006
35. Büyükyilmaz F, Sahiner NC, Caglar S, Eren H. Effectiveness of an intravenous protection device in pediatric patients on catheter dwell time and phlebitis score. Asian Nurs Res (Korean Soc Nurs Sci). 2019;13(4):236-241. https://doi.org/10.1016/j.anr.2019.09.001
36. Schmitz ML, Zempsky WT, Meyer JM. Safety and efficacy of a needle-free powder lidocaine delivery system in pediatric patients undergoing venipuncture or peripheral venous cannulation: randomized double-blind COMFORT-004 trial. Clin Ther. 2015;37(8):1761-1772. https://doi.org/10.1016/j.clinthera.2015.05.515
37. Curtis SJ, Craig WR, Logue E, Vandermeer B, Hanson A, Klassen T. Ultrasound or near-infrared vascular imaging to guide peripheral intravenous catheterization in children: a pragmatic randomized controlled trial. CMAJ. 2015;187(8):563-570. https://doi.org/10.1503/cmaj.141012
38. Benkhadra M, Collignon M, Fournel I, et al. Ultrasound guidance allows faster peripheral IV cannulation in children under 3 years of age with difficult venous access: a prospective randomized study. Paediatr Anaesth. 2012;22(5):449-454. https://doi.org/10.1111/j.1460-9592.2012.03830.x
39. Avelar AFM, Peterlini MAS, da Luz Gonçalves Pedreira M. Ultrasonography-guided peripheral intravenous access in children: a randomized controlled trial. J Infus Nurs. 2015;38(5):320‐327. https://doi.org/10.1097/NAN.0000000000000126
40. Vinograd AM, Chen AE, Woodford AL, et al. Ultrasonographic guidance to improve first-attempt success in children with predicted difficult intravenous access in the emergency department: a randomized controlled trial. Ann Emerg Med. 2019;74(1):19-27. https://doi.org/10.1016/j.annemergmed.2019.02.019
41. Kim MJ, Park JM, Rhee N, et al. Efficacy of VeinViewer in pediatric peripheral intravenous access: a randomized controlled trial. Eur J Pediatr. 2012;171(7):1121-1125. https://doi.org/10.1007/s00431-012-1713-9
42. Kaddoum RN, Anghelescu DL, et al. A randomized controlled trial comparing the AccuVein AV300 device to standard insertion technique for intravenous cannulation of anesthetized children. Paediatr Anaesth. 2012;22(9):884-889. https://doi.org/10.1111/j.1460-9592.2012.03896.x
43. Inal S, Demir D. Impact of peripheral venous catheter placement with vein visualization device support on success rate and pain levels in pediatric patients aged 0 to 3 years. Pediatr Emerg Care. 2021;37(3):138-144. https://doi.org/10.1097/PEC.0000000000001493
44. Demir D, Inal S. Does the use of a vein visualization device for peripheral venous catheter placement increase success rate in pediatric patients? Pediatr Emerg Care. 2019;35(7):474-479. https://doi.org/10.1097/PEC.0000000000001007
45. Gümüs M, Basbakkal Z. Efficacy of Veinlite PEDI in pediatric peripheral intravenous access: a randomized controlled trial. Pediatr Emerg Care. 2021;37(3):145-149. https://doi.org/10.1097/PEC.0000000000001515
46. Qin KR, Ensor N, Barnes R, Englin A, Nataraja RM, Pacilli M. Standard versus long peripheral catheters for multiday IV therapy: a randomized controlled trial. Pediatrics. 2021;147(2): e2020000877. https://doi.org/10.1542/peds.2020-000877
47. van Loon FHJ, Buise MP, Claassen JJF, Dierick-van Daele ATM, Bouwman ARA. Comparison of ultrasound guidance with palpation and direct visualisation for peripheral vein cannulation in adult patients: a systematic review and meta-analysis. Br J Anaesth. 2018;121(2):358-366. https://doi.org/10.1016/j.bja.2018.04.047
48. Parker SIA, Benzies KM, Hayden KA. A systematic review: effectiveness of pediatric peripheral intravenous catheterization strategies. J Adv Nurs. 2017;73(7):1570-1582. https://doi.org/10.1111/jan.13211
© 2021 Society of Hospital Medicine
Responsibilities and Interests of Pediatricians Practicing Hospital Medicine in the United States
As one of the youngest fields of pediatric practice in the United States, pediatric hospital medicine (PHM) has grown rapidly over the past 2 decades. Approximately 10% of recent graduates from pediatric residency programs in the United States have entered PHM, with two-thirds reporting an intention to remain as hospitalists long term.1,2
In October 2016, the American Board of Medical Specialties (ABMS) approved a petition for PHM to become the newest pediatric subspecialty.3 The application for subspeciality status, led by the Joint Council of Pediatric Hospital Medicine, articulated that subspecialty certification would more clearly define subspecialty hospitalists’ scope of practice, create a “new and larger cadre” of quality improvement (QI) experts, and strengthen opportunities for professional development related to child health safety within healthcare systems.4 Approximately 1500 pediatric hospitalists sat for the first PHM board-certification exam in November 2019, illustrating broad interest and commitment to this subspecialty.5
Characterizing the current responsibilities, practice settings, and professional interests of pediatric hospitalists is critical to understanding the continued development of the field. However, the most recent national survey of pediatric hospitalists’ roles and responsibilities was conducted more than a decade ago, and shared definitions of what constitutes PHM across institutions are lacking.6 Furthermore, studies suggest wide variability in PHM workload.7-9 We therefore aimed to describe the characteristics, responsibilities, and practice settings of pediatricians who reported practicing PHM in the United States and determine how exclusive PHM practice, compared with PHM practice in combination with primary or subspecialty care, was associated with professional responsibilities and interests. We hypothesized that those reporting exclusive PHM practice would be more likely to report interest in QI leadership and intention to take the PHM certifying exam than those practicing PHM in combination with primary or subspecialty care.
METHODS
Participants and Survey
Pediatricians enrolling in the American Board of Pediatrics (ABP) Maintenance of Certification (MOC) program in 2017 and 2018 were asked to complete a voluntary survey about their professional roles and scope of practice (Appendix Methods). The survey, offered to all MOC enrollees, included a hospital medicine module administered to those reporting PHM practice, given the ABP’s interest in characterizing PHM roles, responsibilities, practice settings, and interests in QI. Respondents were excluded if they were practicing outside of the United States, if they were unemployed or in a volunteer position, or if they were in fellowship training.
To ascertain areas of clinical practice, respondents were provided with a list of clinical practice areas and asked, “In which of the following areas are you practicing?” Those selecting “hospital medicine” were classified as self-identified hospitalists (hereafter, “hospitalists”). Given variation across institutions in physician roles and responsibilities, we stratified hospitalists into three groups: (1) exclusive PHM practice, representing those who reported PHM as their only area of practice; (2) PHM in combination with general pediatrics, representing those who reported practicing PHM and general pediatrics; and (3) PHM in combination with other subspecialties, representing those who reported practicing PHM in addition to one or more subspecialties. Respondents who reported practicing hospital medicine, general pediatrics, and another subspecialty were classified in the subspecialty group. The ABP’s institutional review board of record deemed the survey exempt from human subjects review.
Hospitalist Characteristics and Clinical Roles
To characterize respondents, we examined their age, gender, medical school location (American medical school or international medical school), and survey year (2017 or 2018). We also examined the following practice characteristics: US Census region, part-time versus full-time employment, academic appointment (yes or no), proportion of time spent providing direct and/or consultative patient care and fulfilling nonclinical responsibilities (research, administration, medical education, and QI), hospital setting (children’s hospital, community hospital, or mix of these hospital types), and work schedule type (shift schedule, on-service work in blocks, or a combination of shift and block schedules).
To examine variation in clinical roles, we determined the proportion of total direct and/or consultative clinical care that was spent in each of the following areas: (1) inpatient pediatric care, defined as inpatient general or subspecialty care in patients up to 21 years of age; (2) neonatal care, defined as labor and delivery, inpatient normal newborn care, and/or neonatal intensive care; (3) outpatient practice, defined as outpatient general or subspecialty care in patients up to 21 years of age; (4) emergency department care; and (5) other, which included pediatric intensive care as well inpatient adult care. Recognizing that scope of practice may differ at community hospitals and children’s hospitals, we stratified this analysis by practice setting (children’s hospital, community hospital).
Dependent Variables
We examined four dependent variables, two that were hypothesis driven and two that were exploratory. To test our hypothesis that respondents practicing PHM exclusively would be more likely to report interest in QI leadership or consultation (given the emphasis on QI in the ABMS application for subspecialty status), we examined the frequency with which respondents endorsed being “somewhat interested” or “very interested” in “serving as a leader or consultant for QI activities.” To test our hypothesis that respondents practicing PHM exclusively would be more likely to report plans to take the PHM certifying exam, we noted the frequency with which respondents reported “yes” to the question, “Do you plan to take a certifying exam in hospitalist medicine when it becomes available?” As an exploratory outcome, we examined satisfaction with allocation of professional time, available on the 2017 survey only; satisfaction was defined as an affirmative response to the question, “Is the allocation of your total professional time approximately what you wanted in your current position?” Finally, intention to maintain more than one ABP certification, also reported only in 2017 and examined as an exploratory outcome, was defined as a reported intention to maintain more than one ABP certification, including general pediatrics, PHM, or any other subspecialty.
Statistical Analysis
We used chi-square tests and analysis of variance as appropriate to examine differences in sociodemographic and professional characteristics among respondents who reported exclusive PHM practice, PHM in combination with general pediatrics, and PHM in combination with another subspecialty. To examine differences across the three PHM groups in their allocation of time to various clinical responsibilities (eg, inpatient care, newborn care), we used Kruskal-Wallis equality-of-population rank tests, stratifying by hospital type. We used multivariable logistic regression to identify associations between exclusive PHM practice and our four dependent variables, adjusting for the sociodemographic and professional characteristics described above. All analyses were conducted using Stata 15 (StataCorp LLC), using two-sided tests, and defining P < .05 as statistically significant.
RESULTS
Study Sample
Of the 19,763 pediatricians enrolling in MOC in 2017 and 2018, 13,839 responded the survey, representing a response rate of 70.0%. There were no significant differences between survey respondents and nonrespondents with respect to gender; differences between respondents and nonrespondents in age, medical school location, and initial year of ABP certification year were small (mean age, 48.1 years and 47.1 years, respectively [P < .01]; 77.0% of respondents were graduates of US medical schools compared with 73.7% of nonrespondents [P < .01]; mean certification year for respondents was 2003 compared with 2004 for nonrespondents [P < .01]). After applying the described exclusion criteria, 1662 of 12,665 respondents self-identified as hospitalists, reflecting 13.1% of the sample and the focus of this analysis (Appendix Figure).
Participant Characteristics and Areas of Practice
Of 1662 self-identified hospitalists, 881 (53.0%) also reported practicing general pediatrics, and 653 (39.3%) also reported practicing at least one subspecialty in addition to PHM. The most frequently reported additional subspecialty practice areas included: (1) neonatology (n = 155, 9.3%); (2) adolescent medicine (n = 138, 8.3%); (3) pediatric critical care (n = 89, 5.4%); (4) pediatric emergency medicine (n = 80, 4.8%); and (5) medicine-pediatrics (n = 30, 4.7%, asked only on the 2018 survey). When stratified into mutually exclusive groups, 491 respondents (29.5%) identified as practicing PHM exclusively, 518 (31.2%) identified as practicing PHM in combination with general pediatrics, and 653 (39.3%) identified as practicing PHM in combination with one or more other subspecialties.
Table 1 summarizes the characteristics of respondents in these three groups. Respondents reporting exclusive PHM practice were, on average, younger, more likely to be female, and more likely to be graduates of US medical schools than those reporting PHM in combination with general or subspecialty pediatrics. In total, approximately two-thirds of the sample (n = 1068, 64.3%) reported holding an academic appointment, including 72.9% (n = 358) of those reporting exclusive PHM practice compared with 56.9% (n = 295) of those also reporting general pediatrics and 63.6% (n = 415) of those also reporting subspecialty care (P < .001). Respondents who reported practicing PHM exclusively most frequently worked at children’s hospitals (64.6%, n = 317), compared with 40.0% (n = 207) and 42.1% (n = 275) of those practicing PHM in combination with general and subspecialty pediatrics, respectively (P < .001).
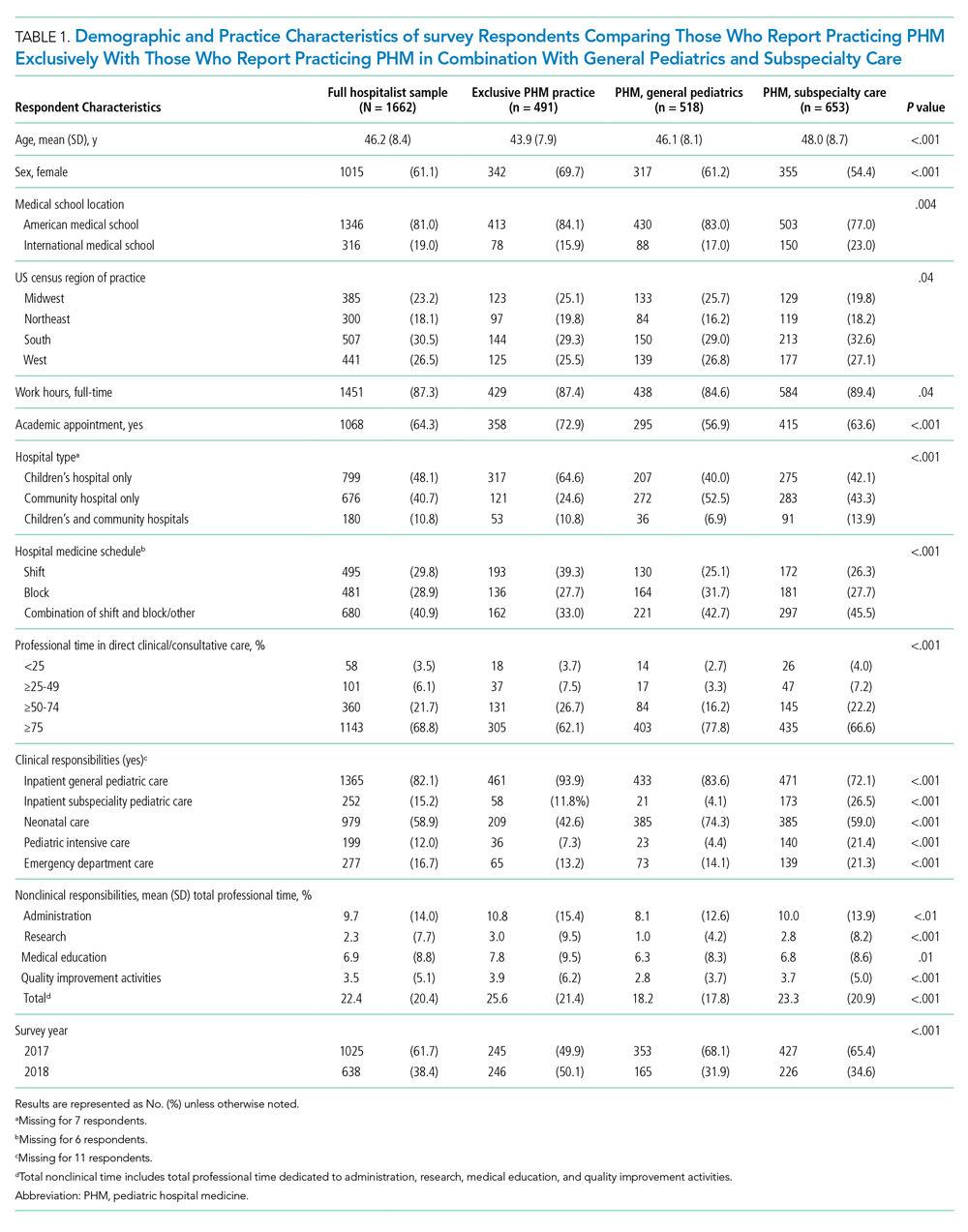
Clinical and Nonclinical Roles and Responsibilities
The majority of respondents reported that they spent >75% of their professional time in direct clinical or consultative care, including 62.1% (n = 305) of those reporting PHM exclusively and 77.8% (n = 403) and 66.6% (n = 435) of those reporting PHM with general and subspecialty pediatrics, respectively (P < .001). Overall, <10% reported spending less than 50% of their time proving direct patient care, including 11.2% (n = 55) of those reporting exclusive PHM practice, 11.2% (n = 73) reporting PHM in combination with a subspecialty, and 6% (n = 31) in combination with general pediatrics. The mean proportion of time spent in nonclinical roles was 22.4% (SD, 20.4%), and the mean proportions of time spent in any one area (administration, research, education, or QI) were all <10%.
The proportion of time allocated to inpatient pediatric care, neonatal care, emergency care, and outpatient pediatric care varied substantially across PHM practice groups and settings. Among respondents who practiced at children’s hospitals, the median percentage of clinical time dedicated to inpatient pediatric care was 66.5% (interquartile range [IQR], 15%-100%), with neonatal care being the second most common clinical practice area (Figure, part A; Appendix Table). At community hospitals, the percentage of clinical time dedicated to inpatient pediatric care was lower, with a median of 10% (IQR, 3%-40%) (Figure, part B). Among those reporting exclusive PHM practice, the median proportion of clinical time spent delivering inpatient pediatric care was 100% (IQR, 80%-100%) at children’s hospitals and 40% (IQR, 20%-85%) at community hospitals. At community hospitals, neonatal care accounted for a similar proportion of clinical time as inpatient pediatric care for these respondents (median, 40% [IQR, 0%-70%]). With the exception of emergency room care, we observed significant differences in how clinical time was allocated by respondents reporting exclusive PHM practice compared with those reporting PHM in combination with general or specialty care (all P values < .001, Appendix Table).

Professional Development Interests
Approximately two-thirds of respondents reported interest in QI leadership or consultation (Table 2), with those reporting exclusive PHM practice significantly more likely to report this (70.3% [n = 345] compared with 57.7% [n = 297] of those practicing PHM with general pediatrics and 66.3% [n = 431] of those practicing PHM with another subspecialty, P < .001). Similarly, 69% (n = 339) of respondents who reported exclusive PHM practice described an intention to take the PHM certifying examination, compared with 20.4% (n = 105) of those practicing PHM and general pediatrics and 17.7% (n = 115) of those practicing PHM and subspeciality pediatrics (P < .001). A total of 82.5% (n = 846) of respondents reported that they were satisfied with the allocation of their professional time; there were no significant differences between those reporting exclusive PHM practice and those reporting PHM in combination with general or subspecialty pediatrics. Of hospitalists reporting exclusive PHM practice, 67.8% (n = 166) reported an intention to maintain more than one ABP certification, compared with 22.1% (n = 78) of those practicing PHM and general pediatrics and 53.9% (n = 230) of those practicing PHM and subspecialty pediatrics (P < .001).
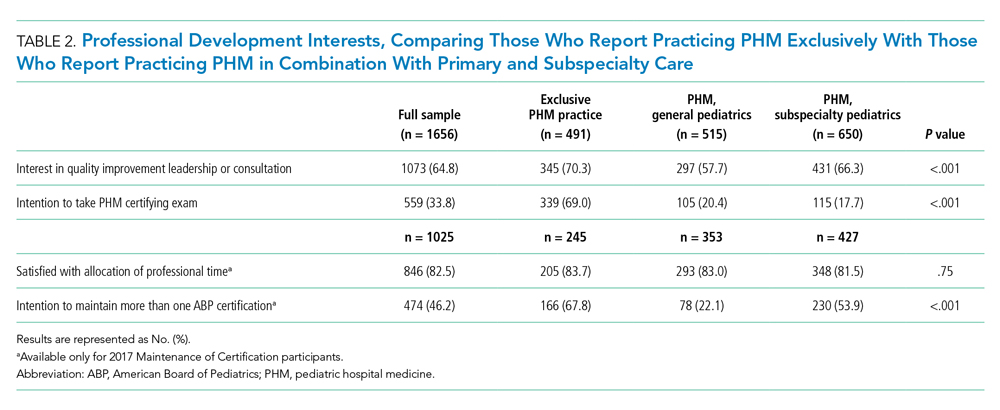
In multivariate regression analyses, hospitalists reporting exclusive PHM practice had significantly greater odds of reported interest in QI leadership or consultation (adjusted odds ratio [OR], 1.39; 95% CI, 1.09-1.79), intention to take the PHM certifying exam (adjusted OR, 7.10; 95% CI, 5.45-9.25), and intention to maintain more than one ABP certification (adjusted OR, 2.64; 95% CI, 1.89-3.68) than those practicing PHM in combination with general or subspecialty pediatrics (Table 3). There was no significant difference across the three groups in the satisfaction with the allocation of professional time.
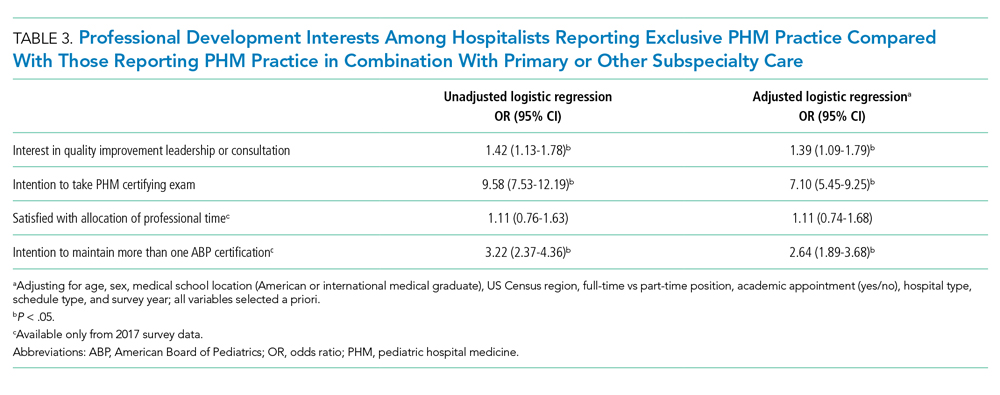
DISCUSSION
In this national survey of pediatricians seeking MOC from the ABP, 13.1% reported that they practiced hospital medicine, with approximately one-third of these individuals reporting that they practiced PHM exclusively. The distribution of clinical and nonclinical responsibilities differed across those reporting exclusive PHM practice relative to those practicing PHM in combination with general or subspecialty pediatrics. Relative to hospitalists who reported practicing PHM in addition to general or subspecialty care, those reporting exclusive PHM practice were significantly more likely to report an interest in QI leadership or consultation, intention to sit for the PHM board-certification exam, and intention to maintain more than one ABP certification.
These findings offer insight into the evolution of PHM and have important implications for workforce planning. The last nationally representative analysis of the PHM workforce was conducted in 2006, at which time 73% of hospitalists reported working at children’s hospitals.6 In the current analysis, less than 50% of hospitalists reported practicing PHM at children’s hospitals only; 10% reported working at both children’s hospitals and community hospitals and 40% at community hospitals alone. This diffusion of PHM from children’s hospitals into community hospitals represents an important development in the field and aligns with the epidemiology of pediatric hospitalization.10 Pediatric hospitalists who practice at community hospitals experience unique challenges, including a relative paucity of pediatric-specific clinical resources, limited mentorship opportunities and resources for scholarly work, and limited access to data from which to prioritize QI interventions.11,12 Our findings also illustrate that the scope of practice for hospitalists differs at community hospitals relative to children’s hospitals. Although the PHM fellowship curriculum requires training at a community hospital, the requirement is limited to one 4-week block, which may not provide sufficient preparation for the unique clinical responsibilities in this setting.13,14
Relative to past analyses of PHM workforce roles and responsibilities, a substantially greater proportion of respondents in the current study reported clinical responsibility for neonatal care, including more than 40% of those self-reporting practicing PHM exclusively and almost three-quarters of those self-reporting PHM in conjunction with general pediatrics.6,15 Given that more than half of the six million US pediatric hospitalizations that occur each year represent birth hospitalizations,16 pediatric hospitalists’ responsibilities for newborn care are consistent with these patterns of hospital-based care. Expanding hospitalists’ responsibilities to provide newborn care has also been shown to improve the financial performance of PHM programs with relatively low pediatric volumes, which may further explain this finding, particularly at community hospitals.17,18 Interestingly, although emergency department care has also been demonstrated as a model to improve the financial stability of PHM programs, relatively few hospitalists reported this as an area of clinical responsibility.19,20 This finding contrasts with past analyses and may reflect how the scope of PHM clinical responsibilities has changed since these prior studies were conducted.6,15
Because PHM had not been recognized as a subspecialty prior to 2016, a national count of pediatric hospitalists is lacking. In this study, approximately one in eight pediatricians reported that they practiced PHM, but less than 4% of the survey sample reported practicing PHM exclusively. Based on these results, we estimate that of the 76,214 to 89,608 ABP-certified pediatricians currently practicing in the United States, between 9984 and 11,738 would self-identify as practicing PHM, with between 2945 and 3462 reporting exclusive PHM practice.
Hospitalists who reported practicing PHM exclusively were significantly more likely to report an interest in QI leadership or consultation and plans to take the PHM certifying exam. These findings are consistent with PHM’s focus on QI, as articulated in the application to the ABMS for subspecialty status as well as the PHM Core Competencies and fellowship curriculum.4,13,21,22 Despite past research questioning the sustainability of some community- and university-based PHM programs and wide variability in workload,7-9 more than 80% of hospitalists reported satisfaction with the allocation of their professional time, with no significant differences between respondents practicing PHM exclusively or in combination with general or subspecialty care.
This analysis should be interpreted in light of its strengths and limitations. Strengths of this work include its national focus, large sample size, and comprehensive characterization of respondents’ professional roles and characteristics. Study limitations include the fact that respondents were classified as hospitalists based on self-report; we were unable to ascertain if they were classified as hospitalists at their place of employment or if they met the ABP’s eligibility criteria to sit for the PHM subspecialty certifying exam.19 Additionally, respondents self-reported their allocations of clinical and nonclinical time, and we are unable to correlate this with actual work hours. Respondents’ reported interest in QI leadership or consultation may not be correlated with QI effort in practice; the mean time reportedly dedicated to QI activities was quite low. Additionally, two of our outcomes were available only for respondents who enrolled in MOC in 2017, and the proportion practicing medicine-pediatrics was available only in 2018. Although this analysis represents approximately 40% of all pediatricians enrolling in MOC (2 years of the 5-year MOC cycle), it may not be representative of pediatricians who are not certified by the ABP. Finally, our outcomes related to board certification examined interest and intentions; future study will be needed to determine how many pediatricians take the PHM exam and maintain certification.
In conclusion, the field of PHM has evolved considerably since its inception, with pediatric hospitalists reporting diverse clinical and nonclinical responsibilities. Hospitalists practicing PHM exclusively were more likely to report an interest in QI leadership and intent to sit for the PHM certifying exam than those practicing PHM in combination with general pediatrics or another specialty. Continuing to monitor the evolution of PHM roles and responsibilities over time and across settings will be important to support the professional development needs of the PHM workforce.
1. House S, Frintner MP, Leyenaar JK. Factors influencing career longevity in pediatric hospital medicine. Hosp Pediatr. 2019;9(12):983-988. https://doi.org/10.1542/hpeds.2019-0151
2. Leyenaar JK, Frintner MP. Graduating pediatric residents entering the hospital medicine workforce, 2006-2015. Acad Pediatr. 2018;18(2):200-207. https://doi.org/10.1016/j.acap.2017.05.001
3. The American Board of Pediatrics. ABMS approves pediatric hospital medicine certification. November 8, 2016. Accessed October 12, 2021. https://www.abp.org/news/abms-approves-pediatric-hospital-medicine-certification
4. American Board of Medical Specialities. Application for a new subspecialty certificate: pediatric hospital medicine.
5. American Board of Pediatrics. 2019 Annual Report. Accessed October 12, 2021. https://www.abp.org/sites/abp/files/pdf/annual-report-2019.pdf
6. Freed GL, Dunham KM, Research Advisory Committee of the American Board of Pediatrics. Pediatric hospitalists: training, current practice, and career goals. J Hosp Med. 2009;4(3):179-186. https://doi.org/10.1002/jhm.458
7. Alvarez F, McDaniel CE, Birnie K, et al. Community pediatric hospitalist workload: results from a national survey. J Hosp Med. 2019;14(11):682-685. https://doi.org/10.12788/jhm.3263
8. Fromme HB, Chen CO, Fine BR, Gosdin C, Shaughnessy EE. Pediatric hospitalist workload and sustainability in university-based programs: results from a national interview-based survey. J Hosp Med. 2018;13(10):702-705. https://doi.org/10.12788/jhm.2977
9. Gosdin C, Simmons J, Yau C, Sucharew H, Carlson D, Paciorkowski N. Survey of academic pediatric hospitalist programs in the US: organizational, administrative, and financial factors. J Hosp Med. 2013;8(6):285-291. https://doi.org/10.1002/jhm.2020
10. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624
11. Leary JC, Walsh KE, Morin RA, Schainker EG, Leyenaar JK. Quality and safety of pediatric inpatient care in community hospitals: a scoping review. J Hosp Med. 2019;14:694-703. https://doi.org/10.12788/jhm.3268
12. Leyenaar JK, Capra LA, O’Brien ER, Leslie LK, Mackie TI. Determinants of career satisfaction among pediatric hospitalists: a qualitative exploration. Acad Pediatr. 2014;14(4):361-368. https://doi.org/10.1016/j.acap.2014.03.015
13. Jerardi KE, Fisher E, Rassbach C, et al. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2017;140(1):e20170698. https://doi.org/10.1542/peds.2017-0698
14. ACGME Program Requirements for Graduate Medical Education in Pediatric Hospital Medicine. July 1, 2021. Accessed October 4, 2021.https://www.acgme.org/globalassets/PFAssets/ProgramRequirements/334_PediatricHospitalMedicine_2020.pdf?ver=2020-06-29-163350-910&ver=2020-06-29-163350-910
15. Freed GL, Brzoznowski K, Neighbors K, Lakhani I, American Board of Pediatrics, Research Advisory Committee. Characteristics of the pediatric hospitalist workforce: its roles and work environment. Pediatrics. 2007;120(1):33-39. https://doi.org/10.1542/peds.2007-0304
16. Moore B, Freeman W, Jiang H. Costs of Pediatric Hospital Stays, 2016. Healthcare Cost and Utilization Project Statistical Brief #250. Accessed October 25, 2021. https://www.ncbi.nlm.nih.gov/books/NBK547762/
17. Carlson DW, Fentzke KM, Dawson JG. Pediatric hospitalists: fill varied roles in the care of newborns. Pediatr Ann. 2003;32(12):802-810. https://doi.org/10.3928/0090-4481-20031201-09
18. Tieder JS, Migita DS, Cowan CA, Melzer SM. Newborn care by pediatric hospitalists in a community hospital: effect on physician productivity and financial performance. Arch Pediatr Adolesc Med. 2008;162(1):74-78. https://doi.org/10.1001/archpediatrics.2007.15
19. Krugman SD, Suggs A, Photowala HY, Beck A. Redefining the community pediatric hospitalist: the combined pediatric ED/inpatient unit. Pediatr Emerg Care. 2007;23(1):33-37. https://doi.org/10.1097/01.pec.0000248685.94647.01
20. Dudas RA, Monroe D, McColligan Borger M. Community pediatric hospitalists providing care in the emergency department: an analysis of physician productivity and financial performance. Pediatr Emerg Care. 2011;27(11):1099-1103. https://doi.org/10.1097/PEC.0b013e31823606f5
21. Stucky ER, Ottolini MC, Maniscalco J. Pediatric hospital medicine core competencies: development and methodology. J Hosp Med. 2010;5(6):339-343. https://doi.org/10.1002/jhm.843
22. Maniscalco J, Gage S, Teferi S, Fisher ES. The Pediatric Hospital Medicine Core Competencies: 2020 revision. J Hosp Med. 2020;15(7):389-394. https://doi.org/10.12788/jhm.3391
As one of the youngest fields of pediatric practice in the United States, pediatric hospital medicine (PHM) has grown rapidly over the past 2 decades. Approximately 10% of recent graduates from pediatric residency programs in the United States have entered PHM, with two-thirds reporting an intention to remain as hospitalists long term.1,2
In October 2016, the American Board of Medical Specialties (ABMS) approved a petition for PHM to become the newest pediatric subspecialty.3 The application for subspeciality status, led by the Joint Council of Pediatric Hospital Medicine, articulated that subspecialty certification would more clearly define subspecialty hospitalists’ scope of practice, create a “new and larger cadre” of quality improvement (QI) experts, and strengthen opportunities for professional development related to child health safety within healthcare systems.4 Approximately 1500 pediatric hospitalists sat for the first PHM board-certification exam in November 2019, illustrating broad interest and commitment to this subspecialty.5
Characterizing the current responsibilities, practice settings, and professional interests of pediatric hospitalists is critical to understanding the continued development of the field. However, the most recent national survey of pediatric hospitalists’ roles and responsibilities was conducted more than a decade ago, and shared definitions of what constitutes PHM across institutions are lacking.6 Furthermore, studies suggest wide variability in PHM workload.7-9 We therefore aimed to describe the characteristics, responsibilities, and practice settings of pediatricians who reported practicing PHM in the United States and determine how exclusive PHM practice, compared with PHM practice in combination with primary or subspecialty care, was associated with professional responsibilities and interests. We hypothesized that those reporting exclusive PHM practice would be more likely to report interest in QI leadership and intention to take the PHM certifying exam than those practicing PHM in combination with primary or subspecialty care.
METHODS
Participants and Survey
Pediatricians enrolling in the American Board of Pediatrics (ABP) Maintenance of Certification (MOC) program in 2017 and 2018 were asked to complete a voluntary survey about their professional roles and scope of practice (Appendix Methods). The survey, offered to all MOC enrollees, included a hospital medicine module administered to those reporting PHM practice, given the ABP’s interest in characterizing PHM roles, responsibilities, practice settings, and interests in QI. Respondents were excluded if they were practicing outside of the United States, if they were unemployed or in a volunteer position, or if they were in fellowship training.
To ascertain areas of clinical practice, respondents were provided with a list of clinical practice areas and asked, “In which of the following areas are you practicing?” Those selecting “hospital medicine” were classified as self-identified hospitalists (hereafter, “hospitalists”). Given variation across institutions in physician roles and responsibilities, we stratified hospitalists into three groups: (1) exclusive PHM practice, representing those who reported PHM as their only area of practice; (2) PHM in combination with general pediatrics, representing those who reported practicing PHM and general pediatrics; and (3) PHM in combination with other subspecialties, representing those who reported practicing PHM in addition to one or more subspecialties. Respondents who reported practicing hospital medicine, general pediatrics, and another subspecialty were classified in the subspecialty group. The ABP’s institutional review board of record deemed the survey exempt from human subjects review.
Hospitalist Characteristics and Clinical Roles
To characterize respondents, we examined their age, gender, medical school location (American medical school or international medical school), and survey year (2017 or 2018). We also examined the following practice characteristics: US Census region, part-time versus full-time employment, academic appointment (yes or no), proportion of time spent providing direct and/or consultative patient care and fulfilling nonclinical responsibilities (research, administration, medical education, and QI), hospital setting (children’s hospital, community hospital, or mix of these hospital types), and work schedule type (shift schedule, on-service work in blocks, or a combination of shift and block schedules).
To examine variation in clinical roles, we determined the proportion of total direct and/or consultative clinical care that was spent in each of the following areas: (1) inpatient pediatric care, defined as inpatient general or subspecialty care in patients up to 21 years of age; (2) neonatal care, defined as labor and delivery, inpatient normal newborn care, and/or neonatal intensive care; (3) outpatient practice, defined as outpatient general or subspecialty care in patients up to 21 years of age; (4) emergency department care; and (5) other, which included pediatric intensive care as well inpatient adult care. Recognizing that scope of practice may differ at community hospitals and children’s hospitals, we stratified this analysis by practice setting (children’s hospital, community hospital).
Dependent Variables
We examined four dependent variables, two that were hypothesis driven and two that were exploratory. To test our hypothesis that respondents practicing PHM exclusively would be more likely to report interest in QI leadership or consultation (given the emphasis on QI in the ABMS application for subspecialty status), we examined the frequency with which respondents endorsed being “somewhat interested” or “very interested” in “serving as a leader or consultant for QI activities.” To test our hypothesis that respondents practicing PHM exclusively would be more likely to report plans to take the PHM certifying exam, we noted the frequency with which respondents reported “yes” to the question, “Do you plan to take a certifying exam in hospitalist medicine when it becomes available?” As an exploratory outcome, we examined satisfaction with allocation of professional time, available on the 2017 survey only; satisfaction was defined as an affirmative response to the question, “Is the allocation of your total professional time approximately what you wanted in your current position?” Finally, intention to maintain more than one ABP certification, also reported only in 2017 and examined as an exploratory outcome, was defined as a reported intention to maintain more than one ABP certification, including general pediatrics, PHM, or any other subspecialty.
Statistical Analysis
We used chi-square tests and analysis of variance as appropriate to examine differences in sociodemographic and professional characteristics among respondents who reported exclusive PHM practice, PHM in combination with general pediatrics, and PHM in combination with another subspecialty. To examine differences across the three PHM groups in their allocation of time to various clinical responsibilities (eg, inpatient care, newborn care), we used Kruskal-Wallis equality-of-population rank tests, stratifying by hospital type. We used multivariable logistic regression to identify associations between exclusive PHM practice and our four dependent variables, adjusting for the sociodemographic and professional characteristics described above. All analyses were conducted using Stata 15 (StataCorp LLC), using two-sided tests, and defining P < .05 as statistically significant.
RESULTS
Study Sample
Of the 19,763 pediatricians enrolling in MOC in 2017 and 2018, 13,839 responded the survey, representing a response rate of 70.0%. There were no significant differences between survey respondents and nonrespondents with respect to gender; differences between respondents and nonrespondents in age, medical school location, and initial year of ABP certification year were small (mean age, 48.1 years and 47.1 years, respectively [P < .01]; 77.0% of respondents were graduates of US medical schools compared with 73.7% of nonrespondents [P < .01]; mean certification year for respondents was 2003 compared with 2004 for nonrespondents [P < .01]). After applying the described exclusion criteria, 1662 of 12,665 respondents self-identified as hospitalists, reflecting 13.1% of the sample and the focus of this analysis (Appendix Figure).
Participant Characteristics and Areas of Practice
Of 1662 self-identified hospitalists, 881 (53.0%) also reported practicing general pediatrics, and 653 (39.3%) also reported practicing at least one subspecialty in addition to PHM. The most frequently reported additional subspecialty practice areas included: (1) neonatology (n = 155, 9.3%); (2) adolescent medicine (n = 138, 8.3%); (3) pediatric critical care (n = 89, 5.4%); (4) pediatric emergency medicine (n = 80, 4.8%); and (5) medicine-pediatrics (n = 30, 4.7%, asked only on the 2018 survey). When stratified into mutually exclusive groups, 491 respondents (29.5%) identified as practicing PHM exclusively, 518 (31.2%) identified as practicing PHM in combination with general pediatrics, and 653 (39.3%) identified as practicing PHM in combination with one or more other subspecialties.
Table 1 summarizes the characteristics of respondents in these three groups. Respondents reporting exclusive PHM practice were, on average, younger, more likely to be female, and more likely to be graduates of US medical schools than those reporting PHM in combination with general or subspecialty pediatrics. In total, approximately two-thirds of the sample (n = 1068, 64.3%) reported holding an academic appointment, including 72.9% (n = 358) of those reporting exclusive PHM practice compared with 56.9% (n = 295) of those also reporting general pediatrics and 63.6% (n = 415) of those also reporting subspecialty care (P < .001). Respondents who reported practicing PHM exclusively most frequently worked at children’s hospitals (64.6%, n = 317), compared with 40.0% (n = 207) and 42.1% (n = 275) of those practicing PHM in combination with general and subspecialty pediatrics, respectively (P < .001).

Clinical and Nonclinical Roles and Responsibilities
The majority of respondents reported that they spent >75% of their professional time in direct clinical or consultative care, including 62.1% (n = 305) of those reporting PHM exclusively and 77.8% (n = 403) and 66.6% (n = 435) of those reporting PHM with general and subspecialty pediatrics, respectively (P < .001). Overall, <10% reported spending less than 50% of their time proving direct patient care, including 11.2% (n = 55) of those reporting exclusive PHM practice, 11.2% (n = 73) reporting PHM in combination with a subspecialty, and 6% (n = 31) in combination with general pediatrics. The mean proportion of time spent in nonclinical roles was 22.4% (SD, 20.4%), and the mean proportions of time spent in any one area (administration, research, education, or QI) were all <10%.
The proportion of time allocated to inpatient pediatric care, neonatal care, emergency care, and outpatient pediatric care varied substantially across PHM practice groups and settings. Among respondents who practiced at children’s hospitals, the median percentage of clinical time dedicated to inpatient pediatric care was 66.5% (interquartile range [IQR], 15%-100%), with neonatal care being the second most common clinical practice area (Figure, part A; Appendix Table). At community hospitals, the percentage of clinical time dedicated to inpatient pediatric care was lower, with a median of 10% (IQR, 3%-40%) (Figure, part B). Among those reporting exclusive PHM practice, the median proportion of clinical time spent delivering inpatient pediatric care was 100% (IQR, 80%-100%) at children’s hospitals and 40% (IQR, 20%-85%) at community hospitals. At community hospitals, neonatal care accounted for a similar proportion of clinical time as inpatient pediatric care for these respondents (median, 40% [IQR, 0%-70%]). With the exception of emergency room care, we observed significant differences in how clinical time was allocated by respondents reporting exclusive PHM practice compared with those reporting PHM in combination with general or specialty care (all P values < .001, Appendix Table).

Professional Development Interests
Approximately two-thirds of respondents reported interest in QI leadership or consultation (Table 2), with those reporting exclusive PHM practice significantly more likely to report this (70.3% [n = 345] compared with 57.7% [n = 297] of those practicing PHM with general pediatrics and 66.3% [n = 431] of those practicing PHM with another subspecialty, P < .001). Similarly, 69% (n = 339) of respondents who reported exclusive PHM practice described an intention to take the PHM certifying examination, compared with 20.4% (n = 105) of those practicing PHM and general pediatrics and 17.7% (n = 115) of those practicing PHM and subspeciality pediatrics (P < .001). A total of 82.5% (n = 846) of respondents reported that they were satisfied with the allocation of their professional time; there were no significant differences between those reporting exclusive PHM practice and those reporting PHM in combination with general or subspecialty pediatrics. Of hospitalists reporting exclusive PHM practice, 67.8% (n = 166) reported an intention to maintain more than one ABP certification, compared with 22.1% (n = 78) of those practicing PHM and general pediatrics and 53.9% (n = 230) of those practicing PHM and subspecialty pediatrics (P < .001).

In multivariate regression analyses, hospitalists reporting exclusive PHM practice had significantly greater odds of reported interest in QI leadership or consultation (adjusted odds ratio [OR], 1.39; 95% CI, 1.09-1.79), intention to take the PHM certifying exam (adjusted OR, 7.10; 95% CI, 5.45-9.25), and intention to maintain more than one ABP certification (adjusted OR, 2.64; 95% CI, 1.89-3.68) than those practicing PHM in combination with general or subspecialty pediatrics (Table 3). There was no significant difference across the three groups in the satisfaction with the allocation of professional time.

DISCUSSION
In this national survey of pediatricians seeking MOC from the ABP, 13.1% reported that they practiced hospital medicine, with approximately one-third of these individuals reporting that they practiced PHM exclusively. The distribution of clinical and nonclinical responsibilities differed across those reporting exclusive PHM practice relative to those practicing PHM in combination with general or subspecialty pediatrics. Relative to hospitalists who reported practicing PHM in addition to general or subspecialty care, those reporting exclusive PHM practice were significantly more likely to report an interest in QI leadership or consultation, intention to sit for the PHM board-certification exam, and intention to maintain more than one ABP certification.
These findings offer insight into the evolution of PHM and have important implications for workforce planning. The last nationally representative analysis of the PHM workforce was conducted in 2006, at which time 73% of hospitalists reported working at children’s hospitals.6 In the current analysis, less than 50% of hospitalists reported practicing PHM at children’s hospitals only; 10% reported working at both children’s hospitals and community hospitals and 40% at community hospitals alone. This diffusion of PHM from children’s hospitals into community hospitals represents an important development in the field and aligns with the epidemiology of pediatric hospitalization.10 Pediatric hospitalists who practice at community hospitals experience unique challenges, including a relative paucity of pediatric-specific clinical resources, limited mentorship opportunities and resources for scholarly work, and limited access to data from which to prioritize QI interventions.11,12 Our findings also illustrate that the scope of practice for hospitalists differs at community hospitals relative to children’s hospitals. Although the PHM fellowship curriculum requires training at a community hospital, the requirement is limited to one 4-week block, which may not provide sufficient preparation for the unique clinical responsibilities in this setting.13,14
Relative to past analyses of PHM workforce roles and responsibilities, a substantially greater proportion of respondents in the current study reported clinical responsibility for neonatal care, including more than 40% of those self-reporting practicing PHM exclusively and almost three-quarters of those self-reporting PHM in conjunction with general pediatrics.6,15 Given that more than half of the six million US pediatric hospitalizations that occur each year represent birth hospitalizations,16 pediatric hospitalists’ responsibilities for newborn care are consistent with these patterns of hospital-based care. Expanding hospitalists’ responsibilities to provide newborn care has also been shown to improve the financial performance of PHM programs with relatively low pediatric volumes, which may further explain this finding, particularly at community hospitals.17,18 Interestingly, although emergency department care has also been demonstrated as a model to improve the financial stability of PHM programs, relatively few hospitalists reported this as an area of clinical responsibility.19,20 This finding contrasts with past analyses and may reflect how the scope of PHM clinical responsibilities has changed since these prior studies were conducted.6,15
Because PHM had not been recognized as a subspecialty prior to 2016, a national count of pediatric hospitalists is lacking. In this study, approximately one in eight pediatricians reported that they practiced PHM, but less than 4% of the survey sample reported practicing PHM exclusively. Based on these results, we estimate that of the 76,214 to 89,608 ABP-certified pediatricians currently practicing in the United States, between 9984 and 11,738 would self-identify as practicing PHM, with between 2945 and 3462 reporting exclusive PHM practice.
Hospitalists who reported practicing PHM exclusively were significantly more likely to report an interest in QI leadership or consultation and plans to take the PHM certifying exam. These findings are consistent with PHM’s focus on QI, as articulated in the application to the ABMS for subspecialty status as well as the PHM Core Competencies and fellowship curriculum.4,13,21,22 Despite past research questioning the sustainability of some community- and university-based PHM programs and wide variability in workload,7-9 more than 80% of hospitalists reported satisfaction with the allocation of their professional time, with no significant differences between respondents practicing PHM exclusively or in combination with general or subspecialty care.
This analysis should be interpreted in light of its strengths and limitations. Strengths of this work include its national focus, large sample size, and comprehensive characterization of respondents’ professional roles and characteristics. Study limitations include the fact that respondents were classified as hospitalists based on self-report; we were unable to ascertain if they were classified as hospitalists at their place of employment or if they met the ABP’s eligibility criteria to sit for the PHM subspecialty certifying exam.19 Additionally, respondents self-reported their allocations of clinical and nonclinical time, and we are unable to correlate this with actual work hours. Respondents’ reported interest in QI leadership or consultation may not be correlated with QI effort in practice; the mean time reportedly dedicated to QI activities was quite low. Additionally, two of our outcomes were available only for respondents who enrolled in MOC in 2017, and the proportion practicing medicine-pediatrics was available only in 2018. Although this analysis represents approximately 40% of all pediatricians enrolling in MOC (2 years of the 5-year MOC cycle), it may not be representative of pediatricians who are not certified by the ABP. Finally, our outcomes related to board certification examined interest and intentions; future study will be needed to determine how many pediatricians take the PHM exam and maintain certification.
In conclusion, the field of PHM has evolved considerably since its inception, with pediatric hospitalists reporting diverse clinical and nonclinical responsibilities. Hospitalists practicing PHM exclusively were more likely to report an interest in QI leadership and intent to sit for the PHM certifying exam than those practicing PHM in combination with general pediatrics or another specialty. Continuing to monitor the evolution of PHM roles and responsibilities over time and across settings will be important to support the professional development needs of the PHM workforce.
As one of the youngest fields of pediatric practice in the United States, pediatric hospital medicine (PHM) has grown rapidly over the past 2 decades. Approximately 10% of recent graduates from pediatric residency programs in the United States have entered PHM, with two-thirds reporting an intention to remain as hospitalists long term.1,2
In October 2016, the American Board of Medical Specialties (ABMS) approved a petition for PHM to become the newest pediatric subspecialty.3 The application for subspeciality status, led by the Joint Council of Pediatric Hospital Medicine, articulated that subspecialty certification would more clearly define subspecialty hospitalists’ scope of practice, create a “new and larger cadre” of quality improvement (QI) experts, and strengthen opportunities for professional development related to child health safety within healthcare systems.4 Approximately 1500 pediatric hospitalists sat for the first PHM board-certification exam in November 2019, illustrating broad interest and commitment to this subspecialty.5
Characterizing the current responsibilities, practice settings, and professional interests of pediatric hospitalists is critical to understanding the continued development of the field. However, the most recent national survey of pediatric hospitalists’ roles and responsibilities was conducted more than a decade ago, and shared definitions of what constitutes PHM across institutions are lacking.6 Furthermore, studies suggest wide variability in PHM workload.7-9 We therefore aimed to describe the characteristics, responsibilities, and practice settings of pediatricians who reported practicing PHM in the United States and determine how exclusive PHM practice, compared with PHM practice in combination with primary or subspecialty care, was associated with professional responsibilities and interests. We hypothesized that those reporting exclusive PHM practice would be more likely to report interest in QI leadership and intention to take the PHM certifying exam than those practicing PHM in combination with primary or subspecialty care.
METHODS
Participants and Survey
Pediatricians enrolling in the American Board of Pediatrics (ABP) Maintenance of Certification (MOC) program in 2017 and 2018 were asked to complete a voluntary survey about their professional roles and scope of practice (Appendix Methods). The survey, offered to all MOC enrollees, included a hospital medicine module administered to those reporting PHM practice, given the ABP’s interest in characterizing PHM roles, responsibilities, practice settings, and interests in QI. Respondents were excluded if they were practicing outside of the United States, if they were unemployed or in a volunteer position, or if they were in fellowship training.
To ascertain areas of clinical practice, respondents were provided with a list of clinical practice areas and asked, “In which of the following areas are you practicing?” Those selecting “hospital medicine” were classified as self-identified hospitalists (hereafter, “hospitalists”). Given variation across institutions in physician roles and responsibilities, we stratified hospitalists into three groups: (1) exclusive PHM practice, representing those who reported PHM as their only area of practice; (2) PHM in combination with general pediatrics, representing those who reported practicing PHM and general pediatrics; and (3) PHM in combination with other subspecialties, representing those who reported practicing PHM in addition to one or more subspecialties. Respondents who reported practicing hospital medicine, general pediatrics, and another subspecialty were classified in the subspecialty group. The ABP’s institutional review board of record deemed the survey exempt from human subjects review.
Hospitalist Characteristics and Clinical Roles
To characterize respondents, we examined their age, gender, medical school location (American medical school or international medical school), and survey year (2017 or 2018). We also examined the following practice characteristics: US Census region, part-time versus full-time employment, academic appointment (yes or no), proportion of time spent providing direct and/or consultative patient care and fulfilling nonclinical responsibilities (research, administration, medical education, and QI), hospital setting (children’s hospital, community hospital, or mix of these hospital types), and work schedule type (shift schedule, on-service work in blocks, or a combination of shift and block schedules).
To examine variation in clinical roles, we determined the proportion of total direct and/or consultative clinical care that was spent in each of the following areas: (1) inpatient pediatric care, defined as inpatient general or subspecialty care in patients up to 21 years of age; (2) neonatal care, defined as labor and delivery, inpatient normal newborn care, and/or neonatal intensive care; (3) outpatient practice, defined as outpatient general or subspecialty care in patients up to 21 years of age; (4) emergency department care; and (5) other, which included pediatric intensive care as well inpatient adult care. Recognizing that scope of practice may differ at community hospitals and children’s hospitals, we stratified this analysis by practice setting (children’s hospital, community hospital).
Dependent Variables
We examined four dependent variables, two that were hypothesis driven and two that were exploratory. To test our hypothesis that respondents practicing PHM exclusively would be more likely to report interest in QI leadership or consultation (given the emphasis on QI in the ABMS application for subspecialty status), we examined the frequency with which respondents endorsed being “somewhat interested” or “very interested” in “serving as a leader or consultant for QI activities.” To test our hypothesis that respondents practicing PHM exclusively would be more likely to report plans to take the PHM certifying exam, we noted the frequency with which respondents reported “yes” to the question, “Do you plan to take a certifying exam in hospitalist medicine when it becomes available?” As an exploratory outcome, we examined satisfaction with allocation of professional time, available on the 2017 survey only; satisfaction was defined as an affirmative response to the question, “Is the allocation of your total professional time approximately what you wanted in your current position?” Finally, intention to maintain more than one ABP certification, also reported only in 2017 and examined as an exploratory outcome, was defined as a reported intention to maintain more than one ABP certification, including general pediatrics, PHM, or any other subspecialty.
Statistical Analysis
We used chi-square tests and analysis of variance as appropriate to examine differences in sociodemographic and professional characteristics among respondents who reported exclusive PHM practice, PHM in combination with general pediatrics, and PHM in combination with another subspecialty. To examine differences across the three PHM groups in their allocation of time to various clinical responsibilities (eg, inpatient care, newborn care), we used Kruskal-Wallis equality-of-population rank tests, stratifying by hospital type. We used multivariable logistic regression to identify associations between exclusive PHM practice and our four dependent variables, adjusting for the sociodemographic and professional characteristics described above. All analyses were conducted using Stata 15 (StataCorp LLC), using two-sided tests, and defining P < .05 as statistically significant.
RESULTS
Study Sample
Of the 19,763 pediatricians enrolling in MOC in 2017 and 2018, 13,839 responded the survey, representing a response rate of 70.0%. There were no significant differences between survey respondents and nonrespondents with respect to gender; differences between respondents and nonrespondents in age, medical school location, and initial year of ABP certification year were small (mean age, 48.1 years and 47.1 years, respectively [P < .01]; 77.0% of respondents were graduates of US medical schools compared with 73.7% of nonrespondents [P < .01]; mean certification year for respondents was 2003 compared with 2004 for nonrespondents [P < .01]). After applying the described exclusion criteria, 1662 of 12,665 respondents self-identified as hospitalists, reflecting 13.1% of the sample and the focus of this analysis (Appendix Figure).
Participant Characteristics and Areas of Practice
Of 1662 self-identified hospitalists, 881 (53.0%) also reported practicing general pediatrics, and 653 (39.3%) also reported practicing at least one subspecialty in addition to PHM. The most frequently reported additional subspecialty practice areas included: (1) neonatology (n = 155, 9.3%); (2) adolescent medicine (n = 138, 8.3%); (3) pediatric critical care (n = 89, 5.4%); (4) pediatric emergency medicine (n = 80, 4.8%); and (5) medicine-pediatrics (n = 30, 4.7%, asked only on the 2018 survey). When stratified into mutually exclusive groups, 491 respondents (29.5%) identified as practicing PHM exclusively, 518 (31.2%) identified as practicing PHM in combination with general pediatrics, and 653 (39.3%) identified as practicing PHM in combination with one or more other subspecialties.
Table 1 summarizes the characteristics of respondents in these three groups. Respondents reporting exclusive PHM practice were, on average, younger, more likely to be female, and more likely to be graduates of US medical schools than those reporting PHM in combination with general or subspecialty pediatrics. In total, approximately two-thirds of the sample (n = 1068, 64.3%) reported holding an academic appointment, including 72.9% (n = 358) of those reporting exclusive PHM practice compared with 56.9% (n = 295) of those also reporting general pediatrics and 63.6% (n = 415) of those also reporting subspecialty care (P < .001). Respondents who reported practicing PHM exclusively most frequently worked at children’s hospitals (64.6%, n = 317), compared with 40.0% (n = 207) and 42.1% (n = 275) of those practicing PHM in combination with general and subspecialty pediatrics, respectively (P < .001).

Clinical and Nonclinical Roles and Responsibilities
The majority of respondents reported that they spent >75% of their professional time in direct clinical or consultative care, including 62.1% (n = 305) of those reporting PHM exclusively and 77.8% (n = 403) and 66.6% (n = 435) of those reporting PHM with general and subspecialty pediatrics, respectively (P < .001). Overall, <10% reported spending less than 50% of their time proving direct patient care, including 11.2% (n = 55) of those reporting exclusive PHM practice, 11.2% (n = 73) reporting PHM in combination with a subspecialty, and 6% (n = 31) in combination with general pediatrics. The mean proportion of time spent in nonclinical roles was 22.4% (SD, 20.4%), and the mean proportions of time spent in any one area (administration, research, education, or QI) were all <10%.
The proportion of time allocated to inpatient pediatric care, neonatal care, emergency care, and outpatient pediatric care varied substantially across PHM practice groups and settings. Among respondents who practiced at children’s hospitals, the median percentage of clinical time dedicated to inpatient pediatric care was 66.5% (interquartile range [IQR], 15%-100%), with neonatal care being the second most common clinical practice area (Figure, part A; Appendix Table). At community hospitals, the percentage of clinical time dedicated to inpatient pediatric care was lower, with a median of 10% (IQR, 3%-40%) (Figure, part B). Among those reporting exclusive PHM practice, the median proportion of clinical time spent delivering inpatient pediatric care was 100% (IQR, 80%-100%) at children’s hospitals and 40% (IQR, 20%-85%) at community hospitals. At community hospitals, neonatal care accounted for a similar proportion of clinical time as inpatient pediatric care for these respondents (median, 40% [IQR, 0%-70%]). With the exception of emergency room care, we observed significant differences in how clinical time was allocated by respondents reporting exclusive PHM practice compared with those reporting PHM in combination with general or specialty care (all P values < .001, Appendix Table).

Professional Development Interests
Approximately two-thirds of respondents reported interest in QI leadership or consultation (Table 2), with those reporting exclusive PHM practice significantly more likely to report this (70.3% [n = 345] compared with 57.7% [n = 297] of those practicing PHM with general pediatrics and 66.3% [n = 431] of those practicing PHM with another subspecialty, P < .001). Similarly, 69% (n = 339) of respondents who reported exclusive PHM practice described an intention to take the PHM certifying examination, compared with 20.4% (n = 105) of those practicing PHM and general pediatrics and 17.7% (n = 115) of those practicing PHM and subspeciality pediatrics (P < .001). A total of 82.5% (n = 846) of respondents reported that they were satisfied with the allocation of their professional time; there were no significant differences between those reporting exclusive PHM practice and those reporting PHM in combination with general or subspecialty pediatrics. Of hospitalists reporting exclusive PHM practice, 67.8% (n = 166) reported an intention to maintain more than one ABP certification, compared with 22.1% (n = 78) of those practicing PHM and general pediatrics and 53.9% (n = 230) of those practicing PHM and subspecialty pediatrics (P < .001).

In multivariate regression analyses, hospitalists reporting exclusive PHM practice had significantly greater odds of reported interest in QI leadership or consultation (adjusted odds ratio [OR], 1.39; 95% CI, 1.09-1.79), intention to take the PHM certifying exam (adjusted OR, 7.10; 95% CI, 5.45-9.25), and intention to maintain more than one ABP certification (adjusted OR, 2.64; 95% CI, 1.89-3.68) than those practicing PHM in combination with general or subspecialty pediatrics (Table 3). There was no significant difference across the three groups in the satisfaction with the allocation of professional time.

DISCUSSION
In this national survey of pediatricians seeking MOC from the ABP, 13.1% reported that they practiced hospital medicine, with approximately one-third of these individuals reporting that they practiced PHM exclusively. The distribution of clinical and nonclinical responsibilities differed across those reporting exclusive PHM practice relative to those practicing PHM in combination with general or subspecialty pediatrics. Relative to hospitalists who reported practicing PHM in addition to general or subspecialty care, those reporting exclusive PHM practice were significantly more likely to report an interest in QI leadership or consultation, intention to sit for the PHM board-certification exam, and intention to maintain more than one ABP certification.
These findings offer insight into the evolution of PHM and have important implications for workforce planning. The last nationally representative analysis of the PHM workforce was conducted in 2006, at which time 73% of hospitalists reported working at children’s hospitals.6 In the current analysis, less than 50% of hospitalists reported practicing PHM at children’s hospitals only; 10% reported working at both children’s hospitals and community hospitals and 40% at community hospitals alone. This diffusion of PHM from children’s hospitals into community hospitals represents an important development in the field and aligns with the epidemiology of pediatric hospitalization.10 Pediatric hospitalists who practice at community hospitals experience unique challenges, including a relative paucity of pediatric-specific clinical resources, limited mentorship opportunities and resources for scholarly work, and limited access to data from which to prioritize QI interventions.11,12 Our findings also illustrate that the scope of practice for hospitalists differs at community hospitals relative to children’s hospitals. Although the PHM fellowship curriculum requires training at a community hospital, the requirement is limited to one 4-week block, which may not provide sufficient preparation for the unique clinical responsibilities in this setting.13,14
Relative to past analyses of PHM workforce roles and responsibilities, a substantially greater proportion of respondents in the current study reported clinical responsibility for neonatal care, including more than 40% of those self-reporting practicing PHM exclusively and almost three-quarters of those self-reporting PHM in conjunction with general pediatrics.6,15 Given that more than half of the six million US pediatric hospitalizations that occur each year represent birth hospitalizations,16 pediatric hospitalists’ responsibilities for newborn care are consistent with these patterns of hospital-based care. Expanding hospitalists’ responsibilities to provide newborn care has also been shown to improve the financial performance of PHM programs with relatively low pediatric volumes, which may further explain this finding, particularly at community hospitals.17,18 Interestingly, although emergency department care has also been demonstrated as a model to improve the financial stability of PHM programs, relatively few hospitalists reported this as an area of clinical responsibility.19,20 This finding contrasts with past analyses and may reflect how the scope of PHM clinical responsibilities has changed since these prior studies were conducted.6,15
Because PHM had not been recognized as a subspecialty prior to 2016, a national count of pediatric hospitalists is lacking. In this study, approximately one in eight pediatricians reported that they practiced PHM, but less than 4% of the survey sample reported practicing PHM exclusively. Based on these results, we estimate that of the 76,214 to 89,608 ABP-certified pediatricians currently practicing in the United States, between 9984 and 11,738 would self-identify as practicing PHM, with between 2945 and 3462 reporting exclusive PHM practice.
Hospitalists who reported practicing PHM exclusively were significantly more likely to report an interest in QI leadership or consultation and plans to take the PHM certifying exam. These findings are consistent with PHM’s focus on QI, as articulated in the application to the ABMS for subspecialty status as well as the PHM Core Competencies and fellowship curriculum.4,13,21,22 Despite past research questioning the sustainability of some community- and university-based PHM programs and wide variability in workload,7-9 more than 80% of hospitalists reported satisfaction with the allocation of their professional time, with no significant differences between respondents practicing PHM exclusively or in combination with general or subspecialty care.
This analysis should be interpreted in light of its strengths and limitations. Strengths of this work include its national focus, large sample size, and comprehensive characterization of respondents’ professional roles and characteristics. Study limitations include the fact that respondents were classified as hospitalists based on self-report; we were unable to ascertain if they were classified as hospitalists at their place of employment or if they met the ABP’s eligibility criteria to sit for the PHM subspecialty certifying exam.19 Additionally, respondents self-reported their allocations of clinical and nonclinical time, and we are unable to correlate this with actual work hours. Respondents’ reported interest in QI leadership or consultation may not be correlated with QI effort in practice; the mean time reportedly dedicated to QI activities was quite low. Additionally, two of our outcomes were available only for respondents who enrolled in MOC in 2017, and the proportion practicing medicine-pediatrics was available only in 2018. Although this analysis represents approximately 40% of all pediatricians enrolling in MOC (2 years of the 5-year MOC cycle), it may not be representative of pediatricians who are not certified by the ABP. Finally, our outcomes related to board certification examined interest and intentions; future study will be needed to determine how many pediatricians take the PHM exam and maintain certification.
In conclusion, the field of PHM has evolved considerably since its inception, with pediatric hospitalists reporting diverse clinical and nonclinical responsibilities. Hospitalists practicing PHM exclusively were more likely to report an interest in QI leadership and intent to sit for the PHM certifying exam than those practicing PHM in combination with general pediatrics or another specialty. Continuing to monitor the evolution of PHM roles and responsibilities over time and across settings will be important to support the professional development needs of the PHM workforce.
1. House S, Frintner MP, Leyenaar JK. Factors influencing career longevity in pediatric hospital medicine. Hosp Pediatr. 2019;9(12):983-988. https://doi.org/10.1542/hpeds.2019-0151
2. Leyenaar JK, Frintner MP. Graduating pediatric residents entering the hospital medicine workforce, 2006-2015. Acad Pediatr. 2018;18(2):200-207. https://doi.org/10.1016/j.acap.2017.05.001
3. The American Board of Pediatrics. ABMS approves pediatric hospital medicine certification. November 8, 2016. Accessed October 12, 2021. https://www.abp.org/news/abms-approves-pediatric-hospital-medicine-certification
4. American Board of Medical Specialities. Application for a new subspecialty certificate: pediatric hospital medicine.
5. American Board of Pediatrics. 2019 Annual Report. Accessed October 12, 2021. https://www.abp.org/sites/abp/files/pdf/annual-report-2019.pdf
6. Freed GL, Dunham KM, Research Advisory Committee of the American Board of Pediatrics. Pediatric hospitalists: training, current practice, and career goals. J Hosp Med. 2009;4(3):179-186. https://doi.org/10.1002/jhm.458
7. Alvarez F, McDaniel CE, Birnie K, et al. Community pediatric hospitalist workload: results from a national survey. J Hosp Med. 2019;14(11):682-685. https://doi.org/10.12788/jhm.3263
8. Fromme HB, Chen CO, Fine BR, Gosdin C, Shaughnessy EE. Pediatric hospitalist workload and sustainability in university-based programs: results from a national interview-based survey. J Hosp Med. 2018;13(10):702-705. https://doi.org/10.12788/jhm.2977
9. Gosdin C, Simmons J, Yau C, Sucharew H, Carlson D, Paciorkowski N. Survey of academic pediatric hospitalist programs in the US: organizational, administrative, and financial factors. J Hosp Med. 2013;8(6):285-291. https://doi.org/10.1002/jhm.2020
10. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624
11. Leary JC, Walsh KE, Morin RA, Schainker EG, Leyenaar JK. Quality and safety of pediatric inpatient care in community hospitals: a scoping review. J Hosp Med. 2019;14:694-703. https://doi.org/10.12788/jhm.3268
12. Leyenaar JK, Capra LA, O’Brien ER, Leslie LK, Mackie TI. Determinants of career satisfaction among pediatric hospitalists: a qualitative exploration. Acad Pediatr. 2014;14(4):361-368. https://doi.org/10.1016/j.acap.2014.03.015
13. Jerardi KE, Fisher E, Rassbach C, et al. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2017;140(1):e20170698. https://doi.org/10.1542/peds.2017-0698
14. ACGME Program Requirements for Graduate Medical Education in Pediatric Hospital Medicine. July 1, 2021. Accessed October 4, 2021.https://www.acgme.org/globalassets/PFAssets/ProgramRequirements/334_PediatricHospitalMedicine_2020.pdf?ver=2020-06-29-163350-910&ver=2020-06-29-163350-910
15. Freed GL, Brzoznowski K, Neighbors K, Lakhani I, American Board of Pediatrics, Research Advisory Committee. Characteristics of the pediatric hospitalist workforce: its roles and work environment. Pediatrics. 2007;120(1):33-39. https://doi.org/10.1542/peds.2007-0304
16. Moore B, Freeman W, Jiang H. Costs of Pediatric Hospital Stays, 2016. Healthcare Cost and Utilization Project Statistical Brief #250. Accessed October 25, 2021. https://www.ncbi.nlm.nih.gov/books/NBK547762/
17. Carlson DW, Fentzke KM, Dawson JG. Pediatric hospitalists: fill varied roles in the care of newborns. Pediatr Ann. 2003;32(12):802-810. https://doi.org/10.3928/0090-4481-20031201-09
18. Tieder JS, Migita DS, Cowan CA, Melzer SM. Newborn care by pediatric hospitalists in a community hospital: effect on physician productivity and financial performance. Arch Pediatr Adolesc Med. 2008;162(1):74-78. https://doi.org/10.1001/archpediatrics.2007.15
19. Krugman SD, Suggs A, Photowala HY, Beck A. Redefining the community pediatric hospitalist: the combined pediatric ED/inpatient unit. Pediatr Emerg Care. 2007;23(1):33-37. https://doi.org/10.1097/01.pec.0000248685.94647.01
20. Dudas RA, Monroe D, McColligan Borger M. Community pediatric hospitalists providing care in the emergency department: an analysis of physician productivity and financial performance. Pediatr Emerg Care. 2011;27(11):1099-1103. https://doi.org/10.1097/PEC.0b013e31823606f5
21. Stucky ER, Ottolini MC, Maniscalco J. Pediatric hospital medicine core competencies: development and methodology. J Hosp Med. 2010;5(6):339-343. https://doi.org/10.1002/jhm.843
22. Maniscalco J, Gage S, Teferi S, Fisher ES. The Pediatric Hospital Medicine Core Competencies: 2020 revision. J Hosp Med. 2020;15(7):389-394. https://doi.org/10.12788/jhm.3391
1. House S, Frintner MP, Leyenaar JK. Factors influencing career longevity in pediatric hospital medicine. Hosp Pediatr. 2019;9(12):983-988. https://doi.org/10.1542/hpeds.2019-0151
2. Leyenaar JK, Frintner MP. Graduating pediatric residents entering the hospital medicine workforce, 2006-2015. Acad Pediatr. 2018;18(2):200-207. https://doi.org/10.1016/j.acap.2017.05.001
3. The American Board of Pediatrics. ABMS approves pediatric hospital medicine certification. November 8, 2016. Accessed October 12, 2021. https://www.abp.org/news/abms-approves-pediatric-hospital-medicine-certification
4. American Board of Medical Specialities. Application for a new subspecialty certificate: pediatric hospital medicine.
5. American Board of Pediatrics. 2019 Annual Report. Accessed October 12, 2021. https://www.abp.org/sites/abp/files/pdf/annual-report-2019.pdf
6. Freed GL, Dunham KM, Research Advisory Committee of the American Board of Pediatrics. Pediatric hospitalists: training, current practice, and career goals. J Hosp Med. 2009;4(3):179-186. https://doi.org/10.1002/jhm.458
7. Alvarez F, McDaniel CE, Birnie K, et al. Community pediatric hospitalist workload: results from a national survey. J Hosp Med. 2019;14(11):682-685. https://doi.org/10.12788/jhm.3263
8. Fromme HB, Chen CO, Fine BR, Gosdin C, Shaughnessy EE. Pediatric hospitalist workload and sustainability in university-based programs: results from a national interview-based survey. J Hosp Med. 2018;13(10):702-705. https://doi.org/10.12788/jhm.2977
9. Gosdin C, Simmons J, Yau C, Sucharew H, Carlson D, Paciorkowski N. Survey of academic pediatric hospitalist programs in the US: organizational, administrative, and financial factors. J Hosp Med. 2013;8(6):285-291. https://doi.org/10.1002/jhm.2020
10. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624
11. Leary JC, Walsh KE, Morin RA, Schainker EG, Leyenaar JK. Quality and safety of pediatric inpatient care in community hospitals: a scoping review. J Hosp Med. 2019;14:694-703. https://doi.org/10.12788/jhm.3268
12. Leyenaar JK, Capra LA, O’Brien ER, Leslie LK, Mackie TI. Determinants of career satisfaction among pediatric hospitalists: a qualitative exploration. Acad Pediatr. 2014;14(4):361-368. https://doi.org/10.1016/j.acap.2014.03.015
13. Jerardi KE, Fisher E, Rassbach C, et al. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2017;140(1):e20170698. https://doi.org/10.1542/peds.2017-0698
14. ACGME Program Requirements for Graduate Medical Education in Pediatric Hospital Medicine. July 1, 2021. Accessed October 4, 2021.https://www.acgme.org/globalassets/PFAssets/ProgramRequirements/334_PediatricHospitalMedicine_2020.pdf?ver=2020-06-29-163350-910&ver=2020-06-29-163350-910
15. Freed GL, Brzoznowski K, Neighbors K, Lakhani I, American Board of Pediatrics, Research Advisory Committee. Characteristics of the pediatric hospitalist workforce: its roles and work environment. Pediatrics. 2007;120(1):33-39. https://doi.org/10.1542/peds.2007-0304
16. Moore B, Freeman W, Jiang H. Costs of Pediatric Hospital Stays, 2016. Healthcare Cost and Utilization Project Statistical Brief #250. Accessed October 25, 2021. https://www.ncbi.nlm.nih.gov/books/NBK547762/
17. Carlson DW, Fentzke KM, Dawson JG. Pediatric hospitalists: fill varied roles in the care of newborns. Pediatr Ann. 2003;32(12):802-810. https://doi.org/10.3928/0090-4481-20031201-09
18. Tieder JS, Migita DS, Cowan CA, Melzer SM. Newborn care by pediatric hospitalists in a community hospital: effect on physician productivity and financial performance. Arch Pediatr Adolesc Med. 2008;162(1):74-78. https://doi.org/10.1001/archpediatrics.2007.15
19. Krugman SD, Suggs A, Photowala HY, Beck A. Redefining the community pediatric hospitalist: the combined pediatric ED/inpatient unit. Pediatr Emerg Care. 2007;23(1):33-37. https://doi.org/10.1097/01.pec.0000248685.94647.01
20. Dudas RA, Monroe D, McColligan Borger M. Community pediatric hospitalists providing care in the emergency department: an analysis of physician productivity and financial performance. Pediatr Emerg Care. 2011;27(11):1099-1103. https://doi.org/10.1097/PEC.0b013e31823606f5
21. Stucky ER, Ottolini MC, Maniscalco J. Pediatric hospital medicine core competencies: development and methodology. J Hosp Med. 2010;5(6):339-343. https://doi.org/10.1002/jhm.843
22. Maniscalco J, Gage S, Teferi S, Fisher ES. The Pediatric Hospital Medicine Core Competencies: 2020 revision. J Hosp Med. 2020;15(7):389-394. https://doi.org/10.12788/jhm.3391
© 2021 Society of Hospital Medicine
Pooled Testing for SARS-CoV-2 for Resource Conservation in the Hospital: A Dynamic Process
Pooled testing for SARS-CoV-2 has been proposed as a strategy to facilitate testing and conserve scarce laboratory resources in a variety of settings. Previously in the Journal of Hospital Medicine, we reported our initial experience with pooled testing in low-risk admitted patients from April 17, 2020, to May 11, 2020, at Saratoga Hospital, Saratoga Springs, New York.1 Early in the pandemic, when testing resources were critically short, pooling allowed us to meet our clinical goal of testing all admitted inpatients. We now present our subsequent experience to emphasize the dynamic nature of this strategy when used to offer testing while conserving resources within a hospital system.
From April 17, 2020, to December 10, 2020, pooled testing using the GeneXpert system (Cepheid) was performed as previously described on all patients admitted from the emergency department (ED) of Saratoga Hospital who met criteria for being at low risk for SARS-CoV-2 infection.1 During this period, we had a low community prevalence (<1%-2%). In our low-risk admitted patients, an overall positive rate of 0.5% allowed us to expand the pool size from our initial reported size of three samples to a maximum of five samples. As ED volumes changed, pool sizes could be adjusted by clinical leaders as supplies allowed the demands of throughput to be met. These adjustments were facilitated by regular discussion of aggregate testing results, pool size, patient-flow issues, and supply levels among our staff. In December 2020, we experienced a marked increase in community prevalence and hospital admissions. This surge ended our use of pooling and required us to test each admitted patient with a single cartridge, which fortunately had become available.
During our period of pooling, we tested 7755 low-risk patients using 1738 cartridges (1177 pools of five samples; 211 pools of four samples; 326 pools of three samples; and 24 pools of two samples). We had 39 positive pooled cartridges, which required the use of 174 additional single cartridges. The instructions for use of this system with single cartridges report a negative percent agreement (sensitivity) of 95.6% and a positive percent agreement (specificity) of 97.8% in the lab.2 We did not have any patients who tested negative in a pool subsequently turn positive during admission unless they had a known in-hospital exposure; however, our public health service alerted us to several patients with high-risk exposures who were excluded from pooling. Our pooling strategy resulted in use of 5843 fewer cartridges than if each test had been performed on a single patient. The total savings on cartridges was $225,000. Pooling did not directly increase staff costs, but required significant individual and organizational energy and commitment. At times, pooling could delay throughput of admitted patients from the ED to inpatient beds. The testing process often added 60 to 90 minutes to throughput time. During the night, waiting for admissions to create a pool could also cause delay. Close and ongoing communication among our ED, inpatient teams, nursing, and laboratory was required to minimize these negative effects.
Pooling can be an effective method of resource conservation in low-risk populations. The theoretical benefits of pooling have been calculated in various scenarios3 and recently comprehensively reviewed with emphasis on selecting the pooling method.4 Practically, pooling has been aptly described as a complex undertaking that should be one part of a broad approach to achieving various COVID-19 control goals.5 Our experience is that, in the hospital setting, it is a dynamic process that requires repeatedly balancing clinical goals, organizational realities, laboratory and mathematical parameters, and competing staff duties. The potential costs and benefits may change over time. We found success was highly dependent on our staff, who were highly motivated by strongly agreeing with our commitment to test all inpatients and our desire to maintain adequate supplies to accomplish this goal.
1. Mastrianni D, Falivena R, Brooks T, et al. Pooled testing for SARS-CoV-2 in hospitalized patients. J Hosp Med. 2020;15:538-539. https://doi.org/10.12788/jhm.3501
2. Xpert Xpress SARS-CoV-2. Instructions for use. Cepheid; 2020. Accessed October 7, 2021. https://www.cepheid.com/Package%20Insert%20Files/Xpert%20Xpress%20SARS-CoV-2%20Assay%20ENGLISH%20Package%20Insert%20302-3787%20Rev.%20B.pdf
3. Abdalhamid B, Bilder CR, McCutchen EL, Hinrichs SH, Koepsell SA, Iwen PC. Assessment of specimen pooling to conserve SARS CoV-2 testing resources. Am J Clin Pathol. 2020;153(6):715-718. https://doi.org/10.1093/ajcp/aqaa064
4. Daniel EA, Esakialraj L BH, Anbalagan S, et al. Pooled testing strategies for SARS-CoV-2 diagnosis: a comprehensive review. Diagn Microbiol Infect Dis. 2021;101(2):115432. https://doi.org/10.1016/j.diagmicrobio.2021.115432
5. Schulte PA, Weissman DN, Luckhaupt SE, et al. Considerations for pooled testing of employees for SARS-CoV-2. J Occup Environ Med. 2021;63(1):1-9. https://doi.org/10.1097/JOM.0000000000002049
Pooled testing for SARS-CoV-2 has been proposed as a strategy to facilitate testing and conserve scarce laboratory resources in a variety of settings. Previously in the Journal of Hospital Medicine, we reported our initial experience with pooled testing in low-risk admitted patients from April 17, 2020, to May 11, 2020, at Saratoga Hospital, Saratoga Springs, New York.1 Early in the pandemic, when testing resources were critically short, pooling allowed us to meet our clinical goal of testing all admitted inpatients. We now present our subsequent experience to emphasize the dynamic nature of this strategy when used to offer testing while conserving resources within a hospital system.
From April 17, 2020, to December 10, 2020, pooled testing using the GeneXpert system (Cepheid) was performed as previously described on all patients admitted from the emergency department (ED) of Saratoga Hospital who met criteria for being at low risk for SARS-CoV-2 infection.1 During this period, we had a low community prevalence (<1%-2%). In our low-risk admitted patients, an overall positive rate of 0.5% allowed us to expand the pool size from our initial reported size of three samples to a maximum of five samples. As ED volumes changed, pool sizes could be adjusted by clinical leaders as supplies allowed the demands of throughput to be met. These adjustments were facilitated by regular discussion of aggregate testing results, pool size, patient-flow issues, and supply levels among our staff. In December 2020, we experienced a marked increase in community prevalence and hospital admissions. This surge ended our use of pooling and required us to test each admitted patient with a single cartridge, which fortunately had become available.
During our period of pooling, we tested 7755 low-risk patients using 1738 cartridges (1177 pools of five samples; 211 pools of four samples; 326 pools of three samples; and 24 pools of two samples). We had 39 positive pooled cartridges, which required the use of 174 additional single cartridges. The instructions for use of this system with single cartridges report a negative percent agreement (sensitivity) of 95.6% and a positive percent agreement (specificity) of 97.8% in the lab.2 We did not have any patients who tested negative in a pool subsequently turn positive during admission unless they had a known in-hospital exposure; however, our public health service alerted us to several patients with high-risk exposures who were excluded from pooling. Our pooling strategy resulted in use of 5843 fewer cartridges than if each test had been performed on a single patient. The total savings on cartridges was $225,000. Pooling did not directly increase staff costs, but required significant individual and organizational energy and commitment. At times, pooling could delay throughput of admitted patients from the ED to inpatient beds. The testing process often added 60 to 90 minutes to throughput time. During the night, waiting for admissions to create a pool could also cause delay. Close and ongoing communication among our ED, inpatient teams, nursing, and laboratory was required to minimize these negative effects.
Pooling can be an effective method of resource conservation in low-risk populations. The theoretical benefits of pooling have been calculated in various scenarios3 and recently comprehensively reviewed with emphasis on selecting the pooling method.4 Practically, pooling has been aptly described as a complex undertaking that should be one part of a broad approach to achieving various COVID-19 control goals.5 Our experience is that, in the hospital setting, it is a dynamic process that requires repeatedly balancing clinical goals, organizational realities, laboratory and mathematical parameters, and competing staff duties. The potential costs and benefits may change over time. We found success was highly dependent on our staff, who were highly motivated by strongly agreeing with our commitment to test all inpatients and our desire to maintain adequate supplies to accomplish this goal.
Pooled testing for SARS-CoV-2 has been proposed as a strategy to facilitate testing and conserve scarce laboratory resources in a variety of settings. Previously in the Journal of Hospital Medicine, we reported our initial experience with pooled testing in low-risk admitted patients from April 17, 2020, to May 11, 2020, at Saratoga Hospital, Saratoga Springs, New York.1 Early in the pandemic, when testing resources were critically short, pooling allowed us to meet our clinical goal of testing all admitted inpatients. We now present our subsequent experience to emphasize the dynamic nature of this strategy when used to offer testing while conserving resources within a hospital system.
From April 17, 2020, to December 10, 2020, pooled testing using the GeneXpert system (Cepheid) was performed as previously described on all patients admitted from the emergency department (ED) of Saratoga Hospital who met criteria for being at low risk for SARS-CoV-2 infection.1 During this period, we had a low community prevalence (<1%-2%). In our low-risk admitted patients, an overall positive rate of 0.5% allowed us to expand the pool size from our initial reported size of three samples to a maximum of five samples. As ED volumes changed, pool sizes could be adjusted by clinical leaders as supplies allowed the demands of throughput to be met. These adjustments were facilitated by regular discussion of aggregate testing results, pool size, patient-flow issues, and supply levels among our staff. In December 2020, we experienced a marked increase in community prevalence and hospital admissions. This surge ended our use of pooling and required us to test each admitted patient with a single cartridge, which fortunately had become available.
During our period of pooling, we tested 7755 low-risk patients using 1738 cartridges (1177 pools of five samples; 211 pools of four samples; 326 pools of three samples; and 24 pools of two samples). We had 39 positive pooled cartridges, which required the use of 174 additional single cartridges. The instructions for use of this system with single cartridges report a negative percent agreement (sensitivity) of 95.6% and a positive percent agreement (specificity) of 97.8% in the lab.2 We did not have any patients who tested negative in a pool subsequently turn positive during admission unless they had a known in-hospital exposure; however, our public health service alerted us to several patients with high-risk exposures who were excluded from pooling. Our pooling strategy resulted in use of 5843 fewer cartridges than if each test had been performed on a single patient. The total savings on cartridges was $225,000. Pooling did not directly increase staff costs, but required significant individual and organizational energy and commitment. At times, pooling could delay throughput of admitted patients from the ED to inpatient beds. The testing process often added 60 to 90 minutes to throughput time. During the night, waiting for admissions to create a pool could also cause delay. Close and ongoing communication among our ED, inpatient teams, nursing, and laboratory was required to minimize these negative effects.
Pooling can be an effective method of resource conservation in low-risk populations. The theoretical benefits of pooling have been calculated in various scenarios3 and recently comprehensively reviewed with emphasis on selecting the pooling method.4 Practically, pooling has been aptly described as a complex undertaking that should be one part of a broad approach to achieving various COVID-19 control goals.5 Our experience is that, in the hospital setting, it is a dynamic process that requires repeatedly balancing clinical goals, organizational realities, laboratory and mathematical parameters, and competing staff duties. The potential costs and benefits may change over time. We found success was highly dependent on our staff, who were highly motivated by strongly agreeing with our commitment to test all inpatients and our desire to maintain adequate supplies to accomplish this goal.
1. Mastrianni D, Falivena R, Brooks T, et al. Pooled testing for SARS-CoV-2 in hospitalized patients. J Hosp Med. 2020;15:538-539. https://doi.org/10.12788/jhm.3501
2. Xpert Xpress SARS-CoV-2. Instructions for use. Cepheid; 2020. Accessed October 7, 2021. https://www.cepheid.com/Package%20Insert%20Files/Xpert%20Xpress%20SARS-CoV-2%20Assay%20ENGLISH%20Package%20Insert%20302-3787%20Rev.%20B.pdf
3. Abdalhamid B, Bilder CR, McCutchen EL, Hinrichs SH, Koepsell SA, Iwen PC. Assessment of specimen pooling to conserve SARS CoV-2 testing resources. Am J Clin Pathol. 2020;153(6):715-718. https://doi.org/10.1093/ajcp/aqaa064
4. Daniel EA, Esakialraj L BH, Anbalagan S, et al. Pooled testing strategies for SARS-CoV-2 diagnosis: a comprehensive review. Diagn Microbiol Infect Dis. 2021;101(2):115432. https://doi.org/10.1016/j.diagmicrobio.2021.115432
5. Schulte PA, Weissman DN, Luckhaupt SE, et al. Considerations for pooled testing of employees for SARS-CoV-2. J Occup Environ Med. 2021;63(1):1-9. https://doi.org/10.1097/JOM.0000000000002049
1. Mastrianni D, Falivena R, Brooks T, et al. Pooled testing for SARS-CoV-2 in hospitalized patients. J Hosp Med. 2020;15:538-539. https://doi.org/10.12788/jhm.3501
2. Xpert Xpress SARS-CoV-2. Instructions for use. Cepheid; 2020. Accessed October 7, 2021. https://www.cepheid.com/Package%20Insert%20Files/Xpert%20Xpress%20SARS-CoV-2%20Assay%20ENGLISH%20Package%20Insert%20302-3787%20Rev.%20B.pdf
3. Abdalhamid B, Bilder CR, McCutchen EL, Hinrichs SH, Koepsell SA, Iwen PC. Assessment of specimen pooling to conserve SARS CoV-2 testing resources. Am J Clin Pathol. 2020;153(6):715-718. https://doi.org/10.1093/ajcp/aqaa064
4. Daniel EA, Esakialraj L BH, Anbalagan S, et al. Pooled testing strategies for SARS-CoV-2 diagnosis: a comprehensive review. Diagn Microbiol Infect Dis. 2021;101(2):115432. https://doi.org/10.1016/j.diagmicrobio.2021.115432
5. Schulte PA, Weissman DN, Luckhaupt SE, et al. Considerations for pooled testing of employees for SARS-CoV-2. J Occup Environ Med. 2021;63(1):1-9. https://doi.org/10.1097/JOM.0000000000002049
© 2021 Society of Hospital Medicine
Principles and Practice of Gossiping About Colleagues in Medicine
CLINICAL SCENARIO PROLOGUE
You are signing over to a colleague on the COVID-19 inpatient hospital ward. You are stressed after having failed to reach the chief medical resident who did not respond despite repeated texts. You think about mentioning this apparent professional lapse to your colleague. You pause, however, because you are uncertain about the appropriate norm, hesitant around finding the right words, and unsure about a mutual feeling of camaraderie.
OVERVIEW
Lay and scientific perspectives about gossip diverge widely. Lay definitions of gossip generally include malicious, salacious, immoral, trivial, or unfair comments that attack someone else’s reputation. Scientific definitions of gossip, in contrast, also include neutral or positive social information intended to align group dynamics.1 The common feature of both is that the named individual is not present to hear about themselves.2 A further commonality is that gossip involves informal assessments loaded with subjective judgments, unlike professional comments about patients from clinicians providing care. In contrast to stereotype remarks, gossip focuses on a specific person and not a group.
Gossip is widespread. A recent study in nonhospital settings suggests nearly all adults engage in gossip during normal interactions, averaging 52 minutes on a typical day.3 Most gossip is neutral (74%) rather than negative or positive. The content usually (92%) concerns relationships, and the typical person identified (82%) is an acquaintance. Some of the potential benefits include conveying information for social learning, defining what is socially acceptable, or promoting personal connections. Men and women gossip to nearly the same degree.4 Indeed, evolutionary theory suggests gossip is not deviant behavior and arises even in small hunter-gatherer communities.5Social psychology science provides some insights on fundamental principles of gossip that may be relevant to clinicians in medicine.6 In this article, we review three important findings from social psychology science relevant to team cooperation, the specific transmitter, and the individual receiver (Table 1). Clinicians working in groups may benefit from recognizing the prosocial function of healthy gossip and avoiding the antisocial adverse effects of harmful gossip.7 At a time when work-related conversations have radically shifted online,8 hospitalists need to be aware of positives and pitfalls of gossip to help provide effective medical care and avoid adverse events.

GOSSIP AS TEAM COMMUNICATION
Large team endeavors often require social signals to coordinate people.9 Gossip helps groups establish reputations, monitor their members, deter antisocial behavior, and protect newcomers from exploitation.10 Sharing social information can also indirectly promote cooperation because individuals place a high value on their own reputations and want to avoid embarrassment.11,12 The absence of gossip, in contrast, may lead individuals to be oblivious to team expectations and fail to do their fair share. A lack of gossip, in particular, may add to inefficiencies during the COVID-19 pandemic since exchanging gossip seems to feel awkward over email or other digital channels (albeit a chat function for side conversations in virtual meetings is a partial substitute).13,14
A paradigm for testing the positive effects of gossip involves a trust game where participants consider making small contributions for later rewards in recurrent rounds of cooperation.15-17 In one online study of volunteers, for example, individuals contributed to a group account and gained rewards equal to a doubling of total contributions shared over everyone equally (even those contributing nothing).18 Half the experiments allowed participants to send notes about other participants, whereas the other experiments allowed no such “gossip.” As predicted, gossip increased the proportion contributed (40% vs 32%, P = .020) and average total reward (64 vs 56, P = .002). In this and other studies of healthy volunteers, gossip builds trust and increases gains for the entire group.19-22
Effective medical practice inside hospitals often involves constructive gossip for pointers on how to behave (eg, how quickly to reply to a text message from the ward pharmacist). The blend of objective facts with subjective opinion provides a compelling message otherwise lacking from institutional guidelines or directives on how not to behave (eg, how quickly to complete an annual report with an arbitrary deadline). Gossip is the antithesis of a cursory interaction between strangers and is also less awkward than open flattery or public ridicule that may occur when the third person is in earshot.23 Even negative social comparisons can be constructive to listeners since people want to know how to avoid bad gossip about themselves in a world with changing morality.24
GOSSIP AND THE TRANSMITTER
Gossip can provide a distinct emotional benefit for the gossiper as a form of self-expression, an exercise of justice, and a validation of one’s perspective.25 Consider, for example, witnessing an antisocial act that leads to subsequent feelings of unfairness yet having no way to communicate personal dissatisfaction. Similarly, expressing prosocial gossip may help relieve some of the annoyance after a hassle (eg, talking with a friend after encountering a new onerous hospital protocol). The sharing of gossip might also help bolster solidarity after an offense (eg, talking with a friend on how to deal with another warning from health records).26 In contrast, lost opportunities to gossip about unfairness could be exacerbating the social isolation and emotional distress of the COVID-19 pandemic.27,28
A rigorous example of the emotional benefits of expressing gossip involves undergraduates witnessing staged behavior under laboratory conditions where one actor appeared to exploit the generosity of another actor.29 By random assignment, half the participants had an opportunity to gossip, and the other half had no such opportunity. All participants reacted to the antisocial behavior by feeling frustrated (self-report survey scale of 0-100, where higher scores indicate worse frustration). Importantly, almost all chose to engage in gossip when feasible, and those who had the opportunity to gossip experienced more relief than those who had no opportunity (absolute improvement in frustration scores, 9.69 vs 0.16; P < .01). Evidentially, engaging in prosocial gossip can sometimes provide solace.
Sharing gossip might strengthen social bonds, bolster self-esteem, promote personal power, elicit reciprocal favors, or telegraph the presence of a larger network of personal connections. Gossip is cheap and efficient compared with peer-sanctioning or formal sanctioning to control behavior.30 Airing grievances through gossip may also solve some social dilemmas more easily than channeling messages through institutional reporting structures or formal performance reviews. Gossip has another advantage of raising delicate comparative judgments without the discomfort of direct confrontation (eg, defining the appropriate level of detail for a case presentation is perhaps best done by identifying those who are judged too verbose).31
GOSSIP AND THE RECEIVER
People tend to enjoy listening to gossip despite the uneven quality where some comments are more valuable than others. The receiver, therefore, faces an irregular payoff similar to random intermittent reinforcement. Ironically, random intermittent reinforcement can be particularly addictive when compared with steady rewards with predictable payoffs. This includes cases where gossip conveys good news that helps elevate, inspire, or motivate the receiver. The thirst for more gossip may partially explain why receivers keep seeking gossip despite knowing the material may be unimportant. The shortfall of enticing gossip might also be another factor adding to a feeling of loneliness that prevails widely during the COVID-19 pandemic.32,33
Classic research on reinforcement includes experiments examining operant conditioning for creating addiction.34,35 An important distinction is the contrast between random reinforcement (eg, variable reward akin to gambling on a roulette wheel) and consistent reinforcement (eg, regular pay akin to a steady salary each week). In a study of pigeons trained to peck a lever for food, for example, random reinforcement resulted in twice the response compared with consistent reinforcement (despite an equalized total amount of food received).36 Moreover, random reinforcement was hard to extinguish, and the behavior continued long after all food ended. In general, random compared with consistent reinforcement tended to cause a more intense and persistent change of behavior.
The inconsistent quality makes the prospect of new, exciting gossip seem nearly impossible to resist; indeed, gossip from any source is surprisingly tantalizing. Moreover, the validity of gossip is rarely challenged, unlike the typical norm of lively thoughtful debate that surrounds new ideas (eg, whether to prescribe a novel medication).2 Gossip, of course, can also lead to a positive thrill where, for example, a recipient subsequently feels emboldened with passionate enthusiasm to relay the point to others. This means that spreading inaccurate characterizations may be particularly destructive for a listener who is gullible or easily provoked.37 Conversely, gossip can also lead to anxiety about future uncertainties.38
DISCUSSION
This perspective summarizes positive and negative features of gossip drawn from social psychology science on a normally hidden activity. The main benefits in medical care are to support team communication, the specific transmitter, and the individual receiver. Some specific gains are to enhance team cooperation, deter exploitation, signal trust, and convey codes of conduct. Sharing gossip might also promote honest dialogue, foster friendships, facilitate reciprocity, and curtail excessive use of force by a dominant individual. Listening to gossip possibly also reduces loneliness, affirms an innate desire for inclusion, and provides a way to share insights. Of course, gossip has downsides from direct or indirect adverse effects that merit attention and mitigation (Table 2).
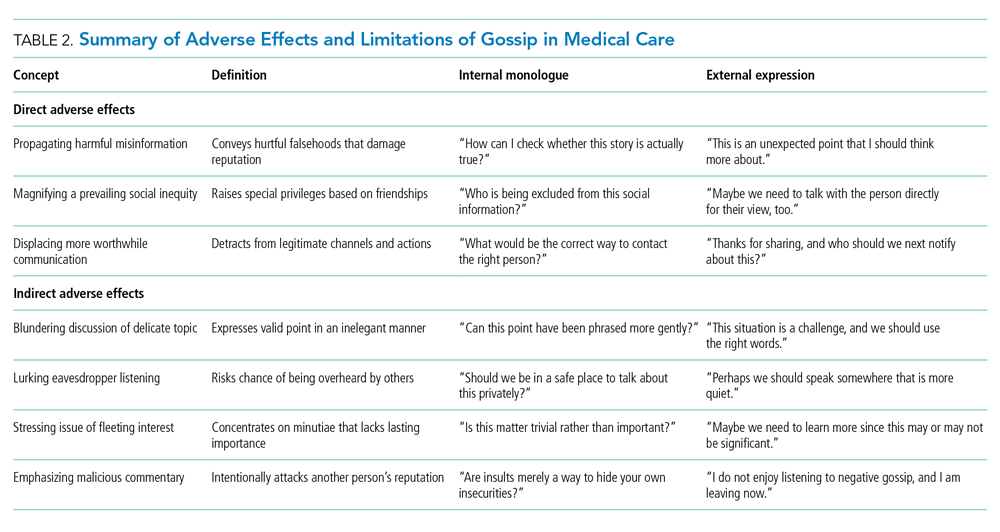
A large direct downside of gossip is in propagating damaging misinformation that harms individuals.24 Toxic gossip can wreck relationships, hurt feelings, violate privacy, and manipulate others. Malicious gossip may become further accentuated because of groupthink, polarization, or selfish biases.39 Presumably, these downsides of gossip are sufficiently infrequent because regular people spend substantial time, attention, and effort engaging in gossip.3 In society, healthy gossip that propagates positive information goes by synonyms having a less negative connotation, including socializing, networking, chatting, schmoozing, friendly banter, small talk, and scuttlebutt. The net benefits must be real since one person is often both a transmitter and a receiver of gossip over time.
Another large direct limitation of gossip is that it can magnify social inequities by allowing some people but not others to access hidden information. In essence, receiving gossip is a privilege that is not universally available within a community and depends on social capital.40 Gossip helps strengthen personal bonds, so marginalized individuals can become further disempowered by not receiving gossip. Social exclusion is painful when different individuals realize they are left out of gossip circles. In summary, gossip can provide an unfair advantage because it allows only some people to learn what is going on behind their backs (eg, different hospitalists within the same institution may have differing circles of friendships for different professional advantages).
Gossip is a way to communicate priorities and regulate behavior. Without interpersonal comparisons, clinicians might find themselves adrift in a complex, difficult, and mysterious medical world. Listening to intelligent gossip can also be an effective way to learn lessons that are otherwise difficult to grasp (eg, an impolite comment may be more easily recognized in someone else than in yourself).41 Perhaps this explains why hospital executives gossip about physicians and vice versa.42 Healthy gossip tends to be positive or neutral (not malicious or negative), propagates accurate information (not hurtful falsehoods), and corrects social inequities (not worsening unearned privileges).43 We suggest that a careful practice of healthy gossip may help regulate trust, enhance social bonding, shape how people feel working together, and promote collective benefit.
CLINICAL SCENARIO EPILOGUE
Your colleague spontaneously comments that the chief medical resident is away because of a death in the family. In turn, you realize you were unaware of this personal nuance because the point was unmentioned in the (virtual) staff meeting last week. You thank your colleague for tactfully relaying the point. You also secretly wonder what other interpersonal details you might be missing during the COVID-19 pandemic.
Acknowledgments
The authors thank Cindy Kao, Fizza Manzoor, Sheharyar Raza, Lee Ross, Miriam Shuchman, and William Silverstein for helpful suggestions on specific points.
1. Foster EK. Research on gossip: taxonomy, methods, and future directions. Rev Gen Psychol. 2004;8(2):78-99. https://doi.org/10.1037/1089-2680.8.2.78
2. Eder D, Enke JL. The structure of gossip: opportunities and constraints on collective expression among adolescents. Am Sociol Rev. 1991;56(4):494-508. https://doi.org/10.2307/2096270
3. Robbins ML, Karan A. Who gossips and how in everyday life. Soc Psychol Pers Sci. 2020;11(2):185-195. https://doi.org/10.1177/1948550619837000
4. Nevo O, Nevo B, Derech-Zehavi A. The development of the Tendency to Gossip Questionnaire: construct and concurrent validation for a sample of Israeli college students. Educ Psychol Meas. 1993;53(4):973-981. https://doi.org/10.1177/0013164493053004010
5. Nishi A. Evolution and social epidemiology. Soc Sci Med. 2015;145:132-137. https://doi.org/10.1016/j.socscimed.2015.08.015
6. Redelmeier DA, Ross LD. Practicing medicine with colleagues: pitfalls from social psychology science. J Gen Intern Med. 2019;34(4):624-626. https://doi.org/10.1007/s11606-019-04839-5
7. Baumeister RF, Zhang L, Vohs KD. Gossip as cultural learning. Rev Gen Psychol. 2004;8(2):111-121. https://doi.org/10.1037/1089-2680.8.2.111
8. Kulkarni A. Navigating loneliness in the era of virtual care. N Engl J Med. 2019;380(4):307-309. https://doi.org/10.1056/NEJMp1813713
9. Nowak MA, Sigmund K. Evolution of indirect reciprocity. Nature. 2005;437(7063):1291-1298. https://doi.org/10.1038/nature04131
10. Dunbar RIM. Gossip in evolutionary perspective. Rev Gen Psychol. 2004;8(2):100-110. https://doi.org/10.1037/1089-2680.8.2.100
11. Emler N. A social psychology of reputation. Eur Rev Social Psychol. 2011;1(1):171-193. https://doi.org/10.1080/14792779108401861
12. Arendt F, Forrai M, Findl O. Dealing with negative reviews on physician-rating websites: an experimental test of how physicians can prevent reputational damage via effective response strategies. Soc Sci Med. 2020;266:113422. https://doi.org/10.1016/j.socscimed.2020.113422
13. Seo H. Blah blah blah: the lack of small talk is breaking our brains. The Walrus. April 22, 2021. Updated April 22, 2021. Accessed September 6, 2021. https://thewalrus.ca/blah-blah-blah-the-lack-of-small-talk-is-breaking-our-brains/
14. Houchens N, Tipirneni R. Compassionate communication amid the COVID-19 pandemic. J Hosp Med. 2020;15(7):437-439. https://doi.org/10.12788/jhm.3472
15. Camerer CE. Behavioral Game Theory: Experiments in Strategic Interaction. Princeton University Press; 2003.
16. Sommerfeld RD, Krambeck HJ, Semmann D, Milinski M. Gossip as an alternative for direct observation in games of indirect reciprocity. Proc Natl Acad Sci U S A. 2007;104(44):17435-17440. https://doi.org/10.1073/pnas.0704598104
17. Hendriks A. SoPHIE - Software Platform for Human Interaction Experiments. Working Paper. 2012.
18. Wu J, Balliet D, Van Lange PAM. Gossip versus punishment: the efficiency of reputation to promote and maintain cooperation. Sci Rep. 2016;6:23919. https://doi.org/10.1038/srep23919
19. Milinski M, Semmann D, Krambeck HJ. Reputation helps solve the “tragedy of the commons.” Nature. 2002;415(6870):424-426. https://doi.org/10.1038/415424a
20. Bolton GE, Katok E, Ockenfels A. Cooperation among strangers with limited information about reputation. J Publ Econ. 2005;89(8):1457-1468. https://doi.org/10.1016/j.jpubeco.2004.03.008
21. Seinen I, Schram A. Social status and group norms: indirect reciprocity in a repeated helping experiment. Eur Econ Rev. 2006;50(3):581-602. https://doi.org/10.1016/j.euroecorev.2004.10.005
22. Feinberg M, Willer R, Schultz M. Gossip and ostracism promote cooperation in groups. Psychol Sci. 2014;25(3):656-664. https://doi.org/10.1177/0956797613510184
23. Farley SD. Is gossip power? The inverse relationships between gossip, power, and likability. Eur J Soc Psychol. 2011;41(5):574-579. https://doi.org/10.1002/ejsp.821
24. Wert SR, Salovey P. A social comparison account of gossip. Rev Gen Psychol. 2004;8(2):122-137. https://doi.org/10.1037/1089-2680.8.2.122
25. Peters K, Kashima Y. From social talk to social action: shaping the social triad with emotion sharing. J Pers Soc Psychol. 2007;93(5):780-797. https://doi.org/10.1037/0022-3514.93.5.780
26. Cruz TDD, Beersma B, Dijkstra MTM, Bechtoldt MN. The bright and dark side of gossip for cooperation in groups. Front Psychol. 2019;10:1374. https://doi.org/10.3389/fpsyg.2019.01374
27. Connolly R. The year in gossip. Hazlitt. December 4, 2020. Accessed September 6, 2021. https://hazlitt.net/feature/year-gossip
28. Rosenbluth G, Good BP, Litterer KP, et al. Communicating effectively with hospitalized patients and families during the COVID-19 pandemic. J Hosp Med. 2020;15(7):440-442. https://doi.org/10.12788/jhm.3466
29. Feinberg M, Willer R, Stellar J, Keltner D. The virtues of gossip: reputational information sharing as prosocial behavior. J Pers Soc Psychol. 2012;102(5):1015-1030. https://doi.org/10.1037/a0026650
30. Panchanathan K, Boyd R. Indirect reciprocity can stabilize cooperation without the second-order free rider problem. Nature. 2004;432(7016):499-502. https://doi.org/10.1038/nature02978
31. Suls JM. Gossip as social comparison. J Commun. 1977;27(1):164-168. https://doi.org/10.1111/j.1460-2466.1977.tb01812.x
32. Gottfriend S. The science behind why people gossip—and when it can be a good thing. Time. September 25, 2019. Accessed September 6, 2021. https://time.com/5680457/why-do-people-gossip/
33. Auerbach A, O’Leary KJ, Greysen SR, et al. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15(8):483-488. https://doi.org/10.12788/jhm.3476
34. Skinner BF. Science and Human Behavior. The Macmillan Company; 1953.
35. Andrzejewski ME, Cardinal CD, Field DP, et al. Pigeons’ choices between fixed-interval and random-interval schedules: utility of variability? J Exp Anal Behav. 2005;83(2):129-145. https://doi.org/10.1901/jeab.2005.30-04
36. Kendall SB. Preference for intermittent reinforcement. J Exp Anal Behav. 1974;21(3):463-473. https://doi.org/10.1901/jeab.1974.21-463
37. Redelmeier DA, Ross LD. Pitfalls from psychology science that worsen with practice. J Gen Intern Med. 2020;35(10):3050-3052. https://doi.org/10.1007/s11606-020-05864-5
38. Rosnow RL. Inside rumor: a personal journey. Am Psychol. 1991;46(5):484-496. https://doi.org/10.1037/0003-066X.46.5.484
39. Cinelli M, De Francisci Moreales G, Galeazzi A, Quattrociocchi W, Starnini M. The echo chamber effect on social media. Proc Natl Acad Sci U S A. 2021;118(9):e2023301118. https://doi.org/10.1073/pnas.2023301118
40. Chaikof M, Tannenbaum E, Mathur S, Bodley J, Farrugia M. Approaching gossip and rumor in medical education. J Grad Med Educ. 2019;11(2):239-240. https://doi.org/10.4300/JGME-D-19-00119.1
41. Redelmeier DA, Najeeb U, Etchells EE. Understanding patient personality in medical care: five-factor model. J Gen Intern Med. 2021;36(7):2111-2114. https://doi.org/10.1007/s11606-021-06598-8
42. Ribeiro VE, Blakeley JA. The proactive management of rumor and gossip. J Nurs Adm. 1995;25(6):43-50. https://doi.org/10.1097/00005110-199506000-00010
43. Beersma B, van Kleef GA. Why people gossip: an empirical analysis of social motives, antecedents, and consequences. J Appl Soc Psychol. 2012;42(11):2640-2670. https://doi.org/10.1111/j.1559-1816.2012.00956.x
CLINICAL SCENARIO PROLOGUE
You are signing over to a colleague on the COVID-19 inpatient hospital ward. You are stressed after having failed to reach the chief medical resident who did not respond despite repeated texts. You think about mentioning this apparent professional lapse to your colleague. You pause, however, because you are uncertain about the appropriate norm, hesitant around finding the right words, and unsure about a mutual feeling of camaraderie.
OVERVIEW
Lay and scientific perspectives about gossip diverge widely. Lay definitions of gossip generally include malicious, salacious, immoral, trivial, or unfair comments that attack someone else’s reputation. Scientific definitions of gossip, in contrast, also include neutral or positive social information intended to align group dynamics.1 The common feature of both is that the named individual is not present to hear about themselves.2 A further commonality is that gossip involves informal assessments loaded with subjective judgments, unlike professional comments about patients from clinicians providing care. In contrast to stereotype remarks, gossip focuses on a specific person and not a group.
Gossip is widespread. A recent study in nonhospital settings suggests nearly all adults engage in gossip during normal interactions, averaging 52 minutes on a typical day.3 Most gossip is neutral (74%) rather than negative or positive. The content usually (92%) concerns relationships, and the typical person identified (82%) is an acquaintance. Some of the potential benefits include conveying information for social learning, defining what is socially acceptable, or promoting personal connections. Men and women gossip to nearly the same degree.4 Indeed, evolutionary theory suggests gossip is not deviant behavior and arises even in small hunter-gatherer communities.5Social psychology science provides some insights on fundamental principles of gossip that may be relevant to clinicians in medicine.6 In this article, we review three important findings from social psychology science relevant to team cooperation, the specific transmitter, and the individual receiver (Table 1). Clinicians working in groups may benefit from recognizing the prosocial function of healthy gossip and avoiding the antisocial adverse effects of harmful gossip.7 At a time when work-related conversations have radically shifted online,8 hospitalists need to be aware of positives and pitfalls of gossip to help provide effective medical care and avoid adverse events.

GOSSIP AS TEAM COMMUNICATION
Large team endeavors often require social signals to coordinate people.9 Gossip helps groups establish reputations, monitor their members, deter antisocial behavior, and protect newcomers from exploitation.10 Sharing social information can also indirectly promote cooperation because individuals place a high value on their own reputations and want to avoid embarrassment.11,12 The absence of gossip, in contrast, may lead individuals to be oblivious to team expectations and fail to do their fair share. A lack of gossip, in particular, may add to inefficiencies during the COVID-19 pandemic since exchanging gossip seems to feel awkward over email or other digital channels (albeit a chat function for side conversations in virtual meetings is a partial substitute).13,14
A paradigm for testing the positive effects of gossip involves a trust game where participants consider making small contributions for later rewards in recurrent rounds of cooperation.15-17 In one online study of volunteers, for example, individuals contributed to a group account and gained rewards equal to a doubling of total contributions shared over everyone equally (even those contributing nothing).18 Half the experiments allowed participants to send notes about other participants, whereas the other experiments allowed no such “gossip.” As predicted, gossip increased the proportion contributed (40% vs 32%, P = .020) and average total reward (64 vs 56, P = .002). In this and other studies of healthy volunteers, gossip builds trust and increases gains for the entire group.19-22
Effective medical practice inside hospitals often involves constructive gossip for pointers on how to behave (eg, how quickly to reply to a text message from the ward pharmacist). The blend of objective facts with subjective opinion provides a compelling message otherwise lacking from institutional guidelines or directives on how not to behave (eg, how quickly to complete an annual report with an arbitrary deadline). Gossip is the antithesis of a cursory interaction between strangers and is also less awkward than open flattery or public ridicule that may occur when the third person is in earshot.23 Even negative social comparisons can be constructive to listeners since people want to know how to avoid bad gossip about themselves in a world with changing morality.24
GOSSIP AND THE TRANSMITTER
Gossip can provide a distinct emotional benefit for the gossiper as a form of self-expression, an exercise of justice, and a validation of one’s perspective.25 Consider, for example, witnessing an antisocial act that leads to subsequent feelings of unfairness yet having no way to communicate personal dissatisfaction. Similarly, expressing prosocial gossip may help relieve some of the annoyance after a hassle (eg, talking with a friend after encountering a new onerous hospital protocol). The sharing of gossip might also help bolster solidarity after an offense (eg, talking with a friend on how to deal with another warning from health records).26 In contrast, lost opportunities to gossip about unfairness could be exacerbating the social isolation and emotional distress of the COVID-19 pandemic.27,28
A rigorous example of the emotional benefits of expressing gossip involves undergraduates witnessing staged behavior under laboratory conditions where one actor appeared to exploit the generosity of another actor.29 By random assignment, half the participants had an opportunity to gossip, and the other half had no such opportunity. All participants reacted to the antisocial behavior by feeling frustrated (self-report survey scale of 0-100, where higher scores indicate worse frustration). Importantly, almost all chose to engage in gossip when feasible, and those who had the opportunity to gossip experienced more relief than those who had no opportunity (absolute improvement in frustration scores, 9.69 vs 0.16; P < .01). Evidentially, engaging in prosocial gossip can sometimes provide solace.
Sharing gossip might strengthen social bonds, bolster self-esteem, promote personal power, elicit reciprocal favors, or telegraph the presence of a larger network of personal connections. Gossip is cheap and efficient compared with peer-sanctioning or formal sanctioning to control behavior.30 Airing grievances through gossip may also solve some social dilemmas more easily than channeling messages through institutional reporting structures or formal performance reviews. Gossip has another advantage of raising delicate comparative judgments without the discomfort of direct confrontation (eg, defining the appropriate level of detail for a case presentation is perhaps best done by identifying those who are judged too verbose).31
GOSSIP AND THE RECEIVER
People tend to enjoy listening to gossip despite the uneven quality where some comments are more valuable than others. The receiver, therefore, faces an irregular payoff similar to random intermittent reinforcement. Ironically, random intermittent reinforcement can be particularly addictive when compared with steady rewards with predictable payoffs. This includes cases where gossip conveys good news that helps elevate, inspire, or motivate the receiver. The thirst for more gossip may partially explain why receivers keep seeking gossip despite knowing the material may be unimportant. The shortfall of enticing gossip might also be another factor adding to a feeling of loneliness that prevails widely during the COVID-19 pandemic.32,33
Classic research on reinforcement includes experiments examining operant conditioning for creating addiction.34,35 An important distinction is the contrast between random reinforcement (eg, variable reward akin to gambling on a roulette wheel) and consistent reinforcement (eg, regular pay akin to a steady salary each week). In a study of pigeons trained to peck a lever for food, for example, random reinforcement resulted in twice the response compared with consistent reinforcement (despite an equalized total amount of food received).36 Moreover, random reinforcement was hard to extinguish, and the behavior continued long after all food ended. In general, random compared with consistent reinforcement tended to cause a more intense and persistent change of behavior.
The inconsistent quality makes the prospect of new, exciting gossip seem nearly impossible to resist; indeed, gossip from any source is surprisingly tantalizing. Moreover, the validity of gossip is rarely challenged, unlike the typical norm of lively thoughtful debate that surrounds new ideas (eg, whether to prescribe a novel medication).2 Gossip, of course, can also lead to a positive thrill where, for example, a recipient subsequently feels emboldened with passionate enthusiasm to relay the point to others. This means that spreading inaccurate characterizations may be particularly destructive for a listener who is gullible or easily provoked.37 Conversely, gossip can also lead to anxiety about future uncertainties.38
DISCUSSION
This perspective summarizes positive and negative features of gossip drawn from social psychology science on a normally hidden activity. The main benefits in medical care are to support team communication, the specific transmitter, and the individual receiver. Some specific gains are to enhance team cooperation, deter exploitation, signal trust, and convey codes of conduct. Sharing gossip might also promote honest dialogue, foster friendships, facilitate reciprocity, and curtail excessive use of force by a dominant individual. Listening to gossip possibly also reduces loneliness, affirms an innate desire for inclusion, and provides a way to share insights. Of course, gossip has downsides from direct or indirect adverse effects that merit attention and mitigation (Table 2).

A large direct downside of gossip is in propagating damaging misinformation that harms individuals.24 Toxic gossip can wreck relationships, hurt feelings, violate privacy, and manipulate others. Malicious gossip may become further accentuated because of groupthink, polarization, or selfish biases.39 Presumably, these downsides of gossip are sufficiently infrequent because regular people spend substantial time, attention, and effort engaging in gossip.3 In society, healthy gossip that propagates positive information goes by synonyms having a less negative connotation, including socializing, networking, chatting, schmoozing, friendly banter, small talk, and scuttlebutt. The net benefits must be real since one person is often both a transmitter and a receiver of gossip over time.
Another large direct limitation of gossip is that it can magnify social inequities by allowing some people but not others to access hidden information. In essence, receiving gossip is a privilege that is not universally available within a community and depends on social capital.40 Gossip helps strengthen personal bonds, so marginalized individuals can become further disempowered by not receiving gossip. Social exclusion is painful when different individuals realize they are left out of gossip circles. In summary, gossip can provide an unfair advantage because it allows only some people to learn what is going on behind their backs (eg, different hospitalists within the same institution may have differing circles of friendships for different professional advantages).
Gossip is a way to communicate priorities and regulate behavior. Without interpersonal comparisons, clinicians might find themselves adrift in a complex, difficult, and mysterious medical world. Listening to intelligent gossip can also be an effective way to learn lessons that are otherwise difficult to grasp (eg, an impolite comment may be more easily recognized in someone else than in yourself).41 Perhaps this explains why hospital executives gossip about physicians and vice versa.42 Healthy gossip tends to be positive or neutral (not malicious or negative), propagates accurate information (not hurtful falsehoods), and corrects social inequities (not worsening unearned privileges).43 We suggest that a careful practice of healthy gossip may help regulate trust, enhance social bonding, shape how people feel working together, and promote collective benefit.
CLINICAL SCENARIO EPILOGUE
Your colleague spontaneously comments that the chief medical resident is away because of a death in the family. In turn, you realize you were unaware of this personal nuance because the point was unmentioned in the (virtual) staff meeting last week. You thank your colleague for tactfully relaying the point. You also secretly wonder what other interpersonal details you might be missing during the COVID-19 pandemic.
Acknowledgments
The authors thank Cindy Kao, Fizza Manzoor, Sheharyar Raza, Lee Ross, Miriam Shuchman, and William Silverstein for helpful suggestions on specific points.
CLINICAL SCENARIO PROLOGUE
You are signing over to a colleague on the COVID-19 inpatient hospital ward. You are stressed after having failed to reach the chief medical resident who did not respond despite repeated texts. You think about mentioning this apparent professional lapse to your colleague. You pause, however, because you are uncertain about the appropriate norm, hesitant around finding the right words, and unsure about a mutual feeling of camaraderie.
OVERVIEW
Lay and scientific perspectives about gossip diverge widely. Lay definitions of gossip generally include malicious, salacious, immoral, trivial, or unfair comments that attack someone else’s reputation. Scientific definitions of gossip, in contrast, also include neutral or positive social information intended to align group dynamics.1 The common feature of both is that the named individual is not present to hear about themselves.2 A further commonality is that gossip involves informal assessments loaded with subjective judgments, unlike professional comments about patients from clinicians providing care. In contrast to stereotype remarks, gossip focuses on a specific person and not a group.
Gossip is widespread. A recent study in nonhospital settings suggests nearly all adults engage in gossip during normal interactions, averaging 52 minutes on a typical day.3 Most gossip is neutral (74%) rather than negative or positive. The content usually (92%) concerns relationships, and the typical person identified (82%) is an acquaintance. Some of the potential benefits include conveying information for social learning, defining what is socially acceptable, or promoting personal connections. Men and women gossip to nearly the same degree.4 Indeed, evolutionary theory suggests gossip is not deviant behavior and arises even in small hunter-gatherer communities.5Social psychology science provides some insights on fundamental principles of gossip that may be relevant to clinicians in medicine.6 In this article, we review three important findings from social psychology science relevant to team cooperation, the specific transmitter, and the individual receiver (Table 1). Clinicians working in groups may benefit from recognizing the prosocial function of healthy gossip and avoiding the antisocial adverse effects of harmful gossip.7 At a time when work-related conversations have radically shifted online,8 hospitalists need to be aware of positives and pitfalls of gossip to help provide effective medical care and avoid adverse events.

GOSSIP AS TEAM COMMUNICATION
Large team endeavors often require social signals to coordinate people.9 Gossip helps groups establish reputations, monitor their members, deter antisocial behavior, and protect newcomers from exploitation.10 Sharing social information can also indirectly promote cooperation because individuals place a high value on their own reputations and want to avoid embarrassment.11,12 The absence of gossip, in contrast, may lead individuals to be oblivious to team expectations and fail to do their fair share. A lack of gossip, in particular, may add to inefficiencies during the COVID-19 pandemic since exchanging gossip seems to feel awkward over email or other digital channels (albeit a chat function for side conversations in virtual meetings is a partial substitute).13,14
A paradigm for testing the positive effects of gossip involves a trust game where participants consider making small contributions for later rewards in recurrent rounds of cooperation.15-17 In one online study of volunteers, for example, individuals contributed to a group account and gained rewards equal to a doubling of total contributions shared over everyone equally (even those contributing nothing).18 Half the experiments allowed participants to send notes about other participants, whereas the other experiments allowed no such “gossip.” As predicted, gossip increased the proportion contributed (40% vs 32%, P = .020) and average total reward (64 vs 56, P = .002). In this and other studies of healthy volunteers, gossip builds trust and increases gains for the entire group.19-22
Effective medical practice inside hospitals often involves constructive gossip for pointers on how to behave (eg, how quickly to reply to a text message from the ward pharmacist). The blend of objective facts with subjective opinion provides a compelling message otherwise lacking from institutional guidelines or directives on how not to behave (eg, how quickly to complete an annual report with an arbitrary deadline). Gossip is the antithesis of a cursory interaction between strangers and is also less awkward than open flattery or public ridicule that may occur when the third person is in earshot.23 Even negative social comparisons can be constructive to listeners since people want to know how to avoid bad gossip about themselves in a world with changing morality.24
GOSSIP AND THE TRANSMITTER
Gossip can provide a distinct emotional benefit for the gossiper as a form of self-expression, an exercise of justice, and a validation of one’s perspective.25 Consider, for example, witnessing an antisocial act that leads to subsequent feelings of unfairness yet having no way to communicate personal dissatisfaction. Similarly, expressing prosocial gossip may help relieve some of the annoyance after a hassle (eg, talking with a friend after encountering a new onerous hospital protocol). The sharing of gossip might also help bolster solidarity after an offense (eg, talking with a friend on how to deal with another warning from health records).26 In contrast, lost opportunities to gossip about unfairness could be exacerbating the social isolation and emotional distress of the COVID-19 pandemic.27,28
A rigorous example of the emotional benefits of expressing gossip involves undergraduates witnessing staged behavior under laboratory conditions where one actor appeared to exploit the generosity of another actor.29 By random assignment, half the participants had an opportunity to gossip, and the other half had no such opportunity. All participants reacted to the antisocial behavior by feeling frustrated (self-report survey scale of 0-100, where higher scores indicate worse frustration). Importantly, almost all chose to engage in gossip when feasible, and those who had the opportunity to gossip experienced more relief than those who had no opportunity (absolute improvement in frustration scores, 9.69 vs 0.16; P < .01). Evidentially, engaging in prosocial gossip can sometimes provide solace.
Sharing gossip might strengthen social bonds, bolster self-esteem, promote personal power, elicit reciprocal favors, or telegraph the presence of a larger network of personal connections. Gossip is cheap and efficient compared with peer-sanctioning or formal sanctioning to control behavior.30 Airing grievances through gossip may also solve some social dilemmas more easily than channeling messages through institutional reporting structures or formal performance reviews. Gossip has another advantage of raising delicate comparative judgments without the discomfort of direct confrontation (eg, defining the appropriate level of detail for a case presentation is perhaps best done by identifying those who are judged too verbose).31
GOSSIP AND THE RECEIVER
People tend to enjoy listening to gossip despite the uneven quality where some comments are more valuable than others. The receiver, therefore, faces an irregular payoff similar to random intermittent reinforcement. Ironically, random intermittent reinforcement can be particularly addictive when compared with steady rewards with predictable payoffs. This includes cases where gossip conveys good news that helps elevate, inspire, or motivate the receiver. The thirst for more gossip may partially explain why receivers keep seeking gossip despite knowing the material may be unimportant. The shortfall of enticing gossip might also be another factor adding to a feeling of loneliness that prevails widely during the COVID-19 pandemic.32,33
Classic research on reinforcement includes experiments examining operant conditioning for creating addiction.34,35 An important distinction is the contrast between random reinforcement (eg, variable reward akin to gambling on a roulette wheel) and consistent reinforcement (eg, regular pay akin to a steady salary each week). In a study of pigeons trained to peck a lever for food, for example, random reinforcement resulted in twice the response compared with consistent reinforcement (despite an equalized total amount of food received).36 Moreover, random reinforcement was hard to extinguish, and the behavior continued long after all food ended. In general, random compared with consistent reinforcement tended to cause a more intense and persistent change of behavior.
The inconsistent quality makes the prospect of new, exciting gossip seem nearly impossible to resist; indeed, gossip from any source is surprisingly tantalizing. Moreover, the validity of gossip is rarely challenged, unlike the typical norm of lively thoughtful debate that surrounds new ideas (eg, whether to prescribe a novel medication).2 Gossip, of course, can also lead to a positive thrill where, for example, a recipient subsequently feels emboldened with passionate enthusiasm to relay the point to others. This means that spreading inaccurate characterizations may be particularly destructive for a listener who is gullible or easily provoked.37 Conversely, gossip can also lead to anxiety about future uncertainties.38
DISCUSSION
This perspective summarizes positive and negative features of gossip drawn from social psychology science on a normally hidden activity. The main benefits in medical care are to support team communication, the specific transmitter, and the individual receiver. Some specific gains are to enhance team cooperation, deter exploitation, signal trust, and convey codes of conduct. Sharing gossip might also promote honest dialogue, foster friendships, facilitate reciprocity, and curtail excessive use of force by a dominant individual. Listening to gossip possibly also reduces loneliness, affirms an innate desire for inclusion, and provides a way to share insights. Of course, gossip has downsides from direct or indirect adverse effects that merit attention and mitigation (Table 2).

A large direct downside of gossip is in propagating damaging misinformation that harms individuals.24 Toxic gossip can wreck relationships, hurt feelings, violate privacy, and manipulate others. Malicious gossip may become further accentuated because of groupthink, polarization, or selfish biases.39 Presumably, these downsides of gossip are sufficiently infrequent because regular people spend substantial time, attention, and effort engaging in gossip.3 In society, healthy gossip that propagates positive information goes by synonyms having a less negative connotation, including socializing, networking, chatting, schmoozing, friendly banter, small talk, and scuttlebutt. The net benefits must be real since one person is often both a transmitter and a receiver of gossip over time.
Another large direct limitation of gossip is that it can magnify social inequities by allowing some people but not others to access hidden information. In essence, receiving gossip is a privilege that is not universally available within a community and depends on social capital.40 Gossip helps strengthen personal bonds, so marginalized individuals can become further disempowered by not receiving gossip. Social exclusion is painful when different individuals realize they are left out of gossip circles. In summary, gossip can provide an unfair advantage because it allows only some people to learn what is going on behind their backs (eg, different hospitalists within the same institution may have differing circles of friendships for different professional advantages).
Gossip is a way to communicate priorities and regulate behavior. Without interpersonal comparisons, clinicians might find themselves adrift in a complex, difficult, and mysterious medical world. Listening to intelligent gossip can also be an effective way to learn lessons that are otherwise difficult to grasp (eg, an impolite comment may be more easily recognized in someone else than in yourself).41 Perhaps this explains why hospital executives gossip about physicians and vice versa.42 Healthy gossip tends to be positive or neutral (not malicious or negative), propagates accurate information (not hurtful falsehoods), and corrects social inequities (not worsening unearned privileges).43 We suggest that a careful practice of healthy gossip may help regulate trust, enhance social bonding, shape how people feel working together, and promote collective benefit.
CLINICAL SCENARIO EPILOGUE
Your colleague spontaneously comments that the chief medical resident is away because of a death in the family. In turn, you realize you were unaware of this personal nuance because the point was unmentioned in the (virtual) staff meeting last week. You thank your colleague for tactfully relaying the point. You also secretly wonder what other interpersonal details you might be missing during the COVID-19 pandemic.
Acknowledgments
The authors thank Cindy Kao, Fizza Manzoor, Sheharyar Raza, Lee Ross, Miriam Shuchman, and William Silverstein for helpful suggestions on specific points.
1. Foster EK. Research on gossip: taxonomy, methods, and future directions. Rev Gen Psychol. 2004;8(2):78-99. https://doi.org/10.1037/1089-2680.8.2.78
2. Eder D, Enke JL. The structure of gossip: opportunities and constraints on collective expression among adolescents. Am Sociol Rev. 1991;56(4):494-508. https://doi.org/10.2307/2096270
3. Robbins ML, Karan A. Who gossips and how in everyday life. Soc Psychol Pers Sci. 2020;11(2):185-195. https://doi.org/10.1177/1948550619837000
4. Nevo O, Nevo B, Derech-Zehavi A. The development of the Tendency to Gossip Questionnaire: construct and concurrent validation for a sample of Israeli college students. Educ Psychol Meas. 1993;53(4):973-981. https://doi.org/10.1177/0013164493053004010
5. Nishi A. Evolution and social epidemiology. Soc Sci Med. 2015;145:132-137. https://doi.org/10.1016/j.socscimed.2015.08.015
6. Redelmeier DA, Ross LD. Practicing medicine with colleagues: pitfalls from social psychology science. J Gen Intern Med. 2019;34(4):624-626. https://doi.org/10.1007/s11606-019-04839-5
7. Baumeister RF, Zhang L, Vohs KD. Gossip as cultural learning. Rev Gen Psychol. 2004;8(2):111-121. https://doi.org/10.1037/1089-2680.8.2.111
8. Kulkarni A. Navigating loneliness in the era of virtual care. N Engl J Med. 2019;380(4):307-309. https://doi.org/10.1056/NEJMp1813713
9. Nowak MA, Sigmund K. Evolution of indirect reciprocity. Nature. 2005;437(7063):1291-1298. https://doi.org/10.1038/nature04131
10. Dunbar RIM. Gossip in evolutionary perspective. Rev Gen Psychol. 2004;8(2):100-110. https://doi.org/10.1037/1089-2680.8.2.100
11. Emler N. A social psychology of reputation. Eur Rev Social Psychol. 2011;1(1):171-193. https://doi.org/10.1080/14792779108401861
12. Arendt F, Forrai M, Findl O. Dealing with negative reviews on physician-rating websites: an experimental test of how physicians can prevent reputational damage via effective response strategies. Soc Sci Med. 2020;266:113422. https://doi.org/10.1016/j.socscimed.2020.113422
13. Seo H. Blah blah blah: the lack of small talk is breaking our brains. The Walrus. April 22, 2021. Updated April 22, 2021. Accessed September 6, 2021. https://thewalrus.ca/blah-blah-blah-the-lack-of-small-talk-is-breaking-our-brains/
14. Houchens N, Tipirneni R. Compassionate communication amid the COVID-19 pandemic. J Hosp Med. 2020;15(7):437-439. https://doi.org/10.12788/jhm.3472
15. Camerer CE. Behavioral Game Theory: Experiments in Strategic Interaction. Princeton University Press; 2003.
16. Sommerfeld RD, Krambeck HJ, Semmann D, Milinski M. Gossip as an alternative for direct observation in games of indirect reciprocity. Proc Natl Acad Sci U S A. 2007;104(44):17435-17440. https://doi.org/10.1073/pnas.0704598104
17. Hendriks A. SoPHIE - Software Platform for Human Interaction Experiments. Working Paper. 2012.
18. Wu J, Balliet D, Van Lange PAM. Gossip versus punishment: the efficiency of reputation to promote and maintain cooperation. Sci Rep. 2016;6:23919. https://doi.org/10.1038/srep23919
19. Milinski M, Semmann D, Krambeck HJ. Reputation helps solve the “tragedy of the commons.” Nature. 2002;415(6870):424-426. https://doi.org/10.1038/415424a
20. Bolton GE, Katok E, Ockenfels A. Cooperation among strangers with limited information about reputation. J Publ Econ. 2005;89(8):1457-1468. https://doi.org/10.1016/j.jpubeco.2004.03.008
21. Seinen I, Schram A. Social status and group norms: indirect reciprocity in a repeated helping experiment. Eur Econ Rev. 2006;50(3):581-602. https://doi.org/10.1016/j.euroecorev.2004.10.005
22. Feinberg M, Willer R, Schultz M. Gossip and ostracism promote cooperation in groups. Psychol Sci. 2014;25(3):656-664. https://doi.org/10.1177/0956797613510184
23. Farley SD. Is gossip power? The inverse relationships between gossip, power, and likability. Eur J Soc Psychol. 2011;41(5):574-579. https://doi.org/10.1002/ejsp.821
24. Wert SR, Salovey P. A social comparison account of gossip. Rev Gen Psychol. 2004;8(2):122-137. https://doi.org/10.1037/1089-2680.8.2.122
25. Peters K, Kashima Y. From social talk to social action: shaping the social triad with emotion sharing. J Pers Soc Psychol. 2007;93(5):780-797. https://doi.org/10.1037/0022-3514.93.5.780
26. Cruz TDD, Beersma B, Dijkstra MTM, Bechtoldt MN. The bright and dark side of gossip for cooperation in groups. Front Psychol. 2019;10:1374. https://doi.org/10.3389/fpsyg.2019.01374
27. Connolly R. The year in gossip. Hazlitt. December 4, 2020. Accessed September 6, 2021. https://hazlitt.net/feature/year-gossip
28. Rosenbluth G, Good BP, Litterer KP, et al. Communicating effectively with hospitalized patients and families during the COVID-19 pandemic. J Hosp Med. 2020;15(7):440-442. https://doi.org/10.12788/jhm.3466
29. Feinberg M, Willer R, Stellar J, Keltner D. The virtues of gossip: reputational information sharing as prosocial behavior. J Pers Soc Psychol. 2012;102(5):1015-1030. https://doi.org/10.1037/a0026650
30. Panchanathan K, Boyd R. Indirect reciprocity can stabilize cooperation without the second-order free rider problem. Nature. 2004;432(7016):499-502. https://doi.org/10.1038/nature02978
31. Suls JM. Gossip as social comparison. J Commun. 1977;27(1):164-168. https://doi.org/10.1111/j.1460-2466.1977.tb01812.x
32. Gottfriend S. The science behind why people gossip—and when it can be a good thing. Time. September 25, 2019. Accessed September 6, 2021. https://time.com/5680457/why-do-people-gossip/
33. Auerbach A, O’Leary KJ, Greysen SR, et al. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15(8):483-488. https://doi.org/10.12788/jhm.3476
34. Skinner BF. Science and Human Behavior. The Macmillan Company; 1953.
35. Andrzejewski ME, Cardinal CD, Field DP, et al. Pigeons’ choices between fixed-interval and random-interval schedules: utility of variability? J Exp Anal Behav. 2005;83(2):129-145. https://doi.org/10.1901/jeab.2005.30-04
36. Kendall SB. Preference for intermittent reinforcement. J Exp Anal Behav. 1974;21(3):463-473. https://doi.org/10.1901/jeab.1974.21-463
37. Redelmeier DA, Ross LD. Pitfalls from psychology science that worsen with practice. J Gen Intern Med. 2020;35(10):3050-3052. https://doi.org/10.1007/s11606-020-05864-5
38. Rosnow RL. Inside rumor: a personal journey. Am Psychol. 1991;46(5):484-496. https://doi.org/10.1037/0003-066X.46.5.484
39. Cinelli M, De Francisci Moreales G, Galeazzi A, Quattrociocchi W, Starnini M. The echo chamber effect on social media. Proc Natl Acad Sci U S A. 2021;118(9):e2023301118. https://doi.org/10.1073/pnas.2023301118
40. Chaikof M, Tannenbaum E, Mathur S, Bodley J, Farrugia M. Approaching gossip and rumor in medical education. J Grad Med Educ. 2019;11(2):239-240. https://doi.org/10.4300/JGME-D-19-00119.1
41. Redelmeier DA, Najeeb U, Etchells EE. Understanding patient personality in medical care: five-factor model. J Gen Intern Med. 2021;36(7):2111-2114. https://doi.org/10.1007/s11606-021-06598-8
42. Ribeiro VE, Blakeley JA. The proactive management of rumor and gossip. J Nurs Adm. 1995;25(6):43-50. https://doi.org/10.1097/00005110-199506000-00010
43. Beersma B, van Kleef GA. Why people gossip: an empirical analysis of social motives, antecedents, and consequences. J Appl Soc Psychol. 2012;42(11):2640-2670. https://doi.org/10.1111/j.1559-1816.2012.00956.x
1. Foster EK. Research on gossip: taxonomy, methods, and future directions. Rev Gen Psychol. 2004;8(2):78-99. https://doi.org/10.1037/1089-2680.8.2.78
2. Eder D, Enke JL. The structure of gossip: opportunities and constraints on collective expression among adolescents. Am Sociol Rev. 1991;56(4):494-508. https://doi.org/10.2307/2096270
3. Robbins ML, Karan A. Who gossips and how in everyday life. Soc Psychol Pers Sci. 2020;11(2):185-195. https://doi.org/10.1177/1948550619837000
4. Nevo O, Nevo B, Derech-Zehavi A. The development of the Tendency to Gossip Questionnaire: construct and concurrent validation for a sample of Israeli college students. Educ Psychol Meas. 1993;53(4):973-981. https://doi.org/10.1177/0013164493053004010
5. Nishi A. Evolution and social epidemiology. Soc Sci Med. 2015;145:132-137. https://doi.org/10.1016/j.socscimed.2015.08.015
6. Redelmeier DA, Ross LD. Practicing medicine with colleagues: pitfalls from social psychology science. J Gen Intern Med. 2019;34(4):624-626. https://doi.org/10.1007/s11606-019-04839-5
7. Baumeister RF, Zhang L, Vohs KD. Gossip as cultural learning. Rev Gen Psychol. 2004;8(2):111-121. https://doi.org/10.1037/1089-2680.8.2.111
8. Kulkarni A. Navigating loneliness in the era of virtual care. N Engl J Med. 2019;380(4):307-309. https://doi.org/10.1056/NEJMp1813713
9. Nowak MA, Sigmund K. Evolution of indirect reciprocity. Nature. 2005;437(7063):1291-1298. https://doi.org/10.1038/nature04131
10. Dunbar RIM. Gossip in evolutionary perspective. Rev Gen Psychol. 2004;8(2):100-110. https://doi.org/10.1037/1089-2680.8.2.100
11. Emler N. A social psychology of reputation. Eur Rev Social Psychol. 2011;1(1):171-193. https://doi.org/10.1080/14792779108401861
12. Arendt F, Forrai M, Findl O. Dealing with negative reviews on physician-rating websites: an experimental test of how physicians can prevent reputational damage via effective response strategies. Soc Sci Med. 2020;266:113422. https://doi.org/10.1016/j.socscimed.2020.113422
13. Seo H. Blah blah blah: the lack of small talk is breaking our brains. The Walrus. April 22, 2021. Updated April 22, 2021. Accessed September 6, 2021. https://thewalrus.ca/blah-blah-blah-the-lack-of-small-talk-is-breaking-our-brains/
14. Houchens N, Tipirneni R. Compassionate communication amid the COVID-19 pandemic. J Hosp Med. 2020;15(7):437-439. https://doi.org/10.12788/jhm.3472
15. Camerer CE. Behavioral Game Theory: Experiments in Strategic Interaction. Princeton University Press; 2003.
16. Sommerfeld RD, Krambeck HJ, Semmann D, Milinski M. Gossip as an alternative for direct observation in games of indirect reciprocity. Proc Natl Acad Sci U S A. 2007;104(44):17435-17440. https://doi.org/10.1073/pnas.0704598104
17. Hendriks A. SoPHIE - Software Platform for Human Interaction Experiments. Working Paper. 2012.
18. Wu J, Balliet D, Van Lange PAM. Gossip versus punishment: the efficiency of reputation to promote and maintain cooperation. Sci Rep. 2016;6:23919. https://doi.org/10.1038/srep23919
19. Milinski M, Semmann D, Krambeck HJ. Reputation helps solve the “tragedy of the commons.” Nature. 2002;415(6870):424-426. https://doi.org/10.1038/415424a
20. Bolton GE, Katok E, Ockenfels A. Cooperation among strangers with limited information about reputation. J Publ Econ. 2005;89(8):1457-1468. https://doi.org/10.1016/j.jpubeco.2004.03.008
21. Seinen I, Schram A. Social status and group norms: indirect reciprocity in a repeated helping experiment. Eur Econ Rev. 2006;50(3):581-602. https://doi.org/10.1016/j.euroecorev.2004.10.005
22. Feinberg M, Willer R, Schultz M. Gossip and ostracism promote cooperation in groups. Psychol Sci. 2014;25(3):656-664. https://doi.org/10.1177/0956797613510184
23. Farley SD. Is gossip power? The inverse relationships between gossip, power, and likability. Eur J Soc Psychol. 2011;41(5):574-579. https://doi.org/10.1002/ejsp.821
24. Wert SR, Salovey P. A social comparison account of gossip. Rev Gen Psychol. 2004;8(2):122-137. https://doi.org/10.1037/1089-2680.8.2.122
25. Peters K, Kashima Y. From social talk to social action: shaping the social triad with emotion sharing. J Pers Soc Psychol. 2007;93(5):780-797. https://doi.org/10.1037/0022-3514.93.5.780
26. Cruz TDD, Beersma B, Dijkstra MTM, Bechtoldt MN. The bright and dark side of gossip for cooperation in groups. Front Psychol. 2019;10:1374. https://doi.org/10.3389/fpsyg.2019.01374
27. Connolly R. The year in gossip. Hazlitt. December 4, 2020. Accessed September 6, 2021. https://hazlitt.net/feature/year-gossip
28. Rosenbluth G, Good BP, Litterer KP, et al. Communicating effectively with hospitalized patients and families during the COVID-19 pandemic. J Hosp Med. 2020;15(7):440-442. https://doi.org/10.12788/jhm.3466
29. Feinberg M, Willer R, Stellar J, Keltner D. The virtues of gossip: reputational information sharing as prosocial behavior. J Pers Soc Psychol. 2012;102(5):1015-1030. https://doi.org/10.1037/a0026650
30. Panchanathan K, Boyd R. Indirect reciprocity can stabilize cooperation without the second-order free rider problem. Nature. 2004;432(7016):499-502. https://doi.org/10.1038/nature02978
31. Suls JM. Gossip as social comparison. J Commun. 1977;27(1):164-168. https://doi.org/10.1111/j.1460-2466.1977.tb01812.x
32. Gottfriend S. The science behind why people gossip—and when it can be a good thing. Time. September 25, 2019. Accessed September 6, 2021. https://time.com/5680457/why-do-people-gossip/
33. Auerbach A, O’Leary KJ, Greysen SR, et al. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15(8):483-488. https://doi.org/10.12788/jhm.3476
34. Skinner BF. Science and Human Behavior. The Macmillan Company; 1953.
35. Andrzejewski ME, Cardinal CD, Field DP, et al. Pigeons’ choices between fixed-interval and random-interval schedules: utility of variability? J Exp Anal Behav. 2005;83(2):129-145. https://doi.org/10.1901/jeab.2005.30-04
36. Kendall SB. Preference for intermittent reinforcement. J Exp Anal Behav. 1974;21(3):463-473. https://doi.org/10.1901/jeab.1974.21-463
37. Redelmeier DA, Ross LD. Pitfalls from psychology science that worsen with practice. J Gen Intern Med. 2020;35(10):3050-3052. https://doi.org/10.1007/s11606-020-05864-5
38. Rosnow RL. Inside rumor: a personal journey. Am Psychol. 1991;46(5):484-496. https://doi.org/10.1037/0003-066X.46.5.484
39. Cinelli M, De Francisci Moreales G, Galeazzi A, Quattrociocchi W, Starnini M. The echo chamber effect on social media. Proc Natl Acad Sci U S A. 2021;118(9):e2023301118. https://doi.org/10.1073/pnas.2023301118
40. Chaikof M, Tannenbaum E, Mathur S, Bodley J, Farrugia M. Approaching gossip and rumor in medical education. J Grad Med Educ. 2019;11(2):239-240. https://doi.org/10.4300/JGME-D-19-00119.1
41. Redelmeier DA, Najeeb U, Etchells EE. Understanding patient personality in medical care: five-factor model. J Gen Intern Med. 2021;36(7):2111-2114. https://doi.org/10.1007/s11606-021-06598-8
42. Ribeiro VE, Blakeley JA. The proactive management of rumor and gossip. J Nurs Adm. 1995;25(6):43-50. https://doi.org/10.1097/00005110-199506000-00010
43. Beersma B, van Kleef GA. Why people gossip: an empirical analysis of social motives, antecedents, and consequences. J Appl Soc Psychol. 2012;42(11):2640-2670. https://doi.org/10.1111/j.1559-1816.2012.00956.x
© 2021 Society of Hospital Medicine
Things We Do For No Reason™: Ultrasonography After an Initial Negative CT in Patients Presenting With Acute Abdominal or Pelvic Pain
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
A 70-year-old woman presented to the emergency department (ED) with diffuse abdominal pain, nausea, and vomiting with normal liver function tests and lipase. Computed tomography (CT) of the abdomen and pelvis with intravenous contrast revealed no acute intraabdominal pathology except for an incidentally noted, mildly enlarged but nondistended gallbladder without evident cholelithiasis, pericholecystic fluid, or gallbladder wall edema. The hospitalist orders an abdominal ultrasound to evaluate for acute biliary pathology potentially missed by CT.
Why You Might Consider Ordering an Abdominal Ultrasound After a Negative CT
Guidelines and expert opinion recommend an “ultrasound-first” approach when patients present with right upper quadrant (RUQ) abdominal pain or pelvic pain of suspected gynecologic origin.1-3 When evaluating suspected biliary disease, experts recommend beginning with ultrasonography based on the speed of obtaining results, absence of radiation exposure, reduced cost, and good diagnostic accuracy.1 Ultrasound has superior sensitivity, of 98%,4 in identifying radiolucent gallstones, compared to CT’s 79% sensitivity.5 Ultrasonography also differentiates gallbladder sludge from cholelithiasis, evaluates the extrahepatic and intrahepatic bile ducts, and can identify alternate causes of RUQ pain.1,3 Since ultrasound has important advantages, a negative initial CT may lead the clinician to consider an ultrasound to evaluate for gallbladder diseases.
Additionally, ultrasound provides improved anatomic detail of pelvic structures when diagnosing endometrial or ovarian pathology2 and improves diagnostic accuracy when the initial CT reveals an abnormal pelvic finding (eg, defining an enlarged ovary on CT as ovarian torsion, a cyst, or an adnexal mass).6 While CT excludes emergent surgical diagnoses, ultrasound may add value in elucidating a cause of the pain, even when urgent surgical management is not necessary.7
Many providers believe that a CT lacks sensitivity for acute biliary or pelvic pathology and will order an ultrasound to avoid missing an important diagnosis.7 Within 6 months at a single center, clinicians ordered 614 abdominal ultrasounds within 72 hours of an abdominal CT; 227 of these orders were to evaluate the gallbladder. Clinicians documented a discussion with a radiologist in only 19% of cases.8
Why Ordering an Ultrasound After a Negative CT Is Unnecessary
While ultrasound is more sensitive for detecting gallstones, the data do not indicate that it is more sensitive than CT for detecting acute cholecystitis. Abdominal ultrasound has a sensitivity for the diagnosis of acute cholecystitis of 81%, with a specificity of 83%,9 while CT has a comparable 85% to 94%9,10 sensitivity and specificity ranging from 59% to 99%.9,11 A recent study using more stringent radiographic criteria (two or more abnormal features) for diagnosing acute cholecystitis found ultrasound and CT had near equivalent sensitivities of 61% and 55%, respectively.12 Even with these stringent criteria, CT had a negative predictive value of 90% and approached 95% when applying a less strict (one feature) criterion.12 As a result, an abdominal ultrasound will rarely diagnose cholecystitis after a normal CT.
A 2020 study evaluated the diagnostic yield and clinical impact of ordering an abdominal or pelvic ultrasound within 24 hours of a negative abdominal CT.7
As with abdominal CT and ultrasound, the recommendation for an initial pelvic ultrasound when evaluating female pelvic pain also stems from the reduced cost, absence of radiation exposure, and superior anatomic visualization of the pelvic organs when compared with pelvic CT.2,13 However, as with the results of studies investigating the use of abdominal ultrasound after negative CT, a study of pelvic ultrasound after a negative CT revealed that only 4/126 (3.2%) follow-up ultrasounds had an abnormal finding not identified on CT.13 Pelvic ultrasound found four endometrial abnormalities that did not alter acute management.13 Notably, in 58% of the cases, the indication for ordering the subsequent ultrasound was “rule out ovarian torsion.” However, CT almost always finds a morphologically abnormal ovary in the case of torsion.6 One study and literature review found that all 28 patients studied and all 85 patients from previous studies with proven ovarian torsion had either an adnexal mass or an enlarged ovary on pelvic CT.6 Harfouch et al found that 0 out of 199 pelvic ultrasounds ordered after a negative CT revealed acute surgical pathology, but pelvic ultrasound did identify nonsurgical uterine and ovarian abnormalities.7 In conclusion, when clinicians order CT as the first study to diagnose acute, surgical biliary or gynecologic causes of pain, follow-up ultrasound has a low probability of affecting diagnosis or management if the CT is normal.
When You Should Consider Ultrasound After CT
The previous discussion only applies if hospitalists order an ultrasound within 24 to 48 hours of the initial CT. Time and clinical course are critical diagnostic tools during an admission for abdominal pain. Consider pelvic or abdominal ultrasound based on guideline recommendations if a patient develops new or evolving RUQ or pelvic pain.1,2 The rationale for obtaining the initial negative CT may no longer apply, and the clinician must consider the changing characteristics of the patient’s symptoms. For example, initial CT imaging may miss cholelithiasis in a patient presenting for biliary colic. Under observation, the patient may develop acute cholecystitis, potentially requiring an abdominal ultrasound. Also, the data for pelvic ultrasound apply to a normal CT of the abdomen and pelvis. Ultrasound may help to further evaluate indeterminate findings present on initial CT or if recommended by radiology.
What You Should Do Instead
When the hospitalist assumes care for a patient with abdominal pain and a negative CT, appropriate next steps include taking time to reexamine the differential diagnosis, repeating the history and physical, and communicating directly with a radiologist. These steps ensure the highest diagnostic yield and the lowest cost and help prevent diagnostic error arising from anchoring on the initial negative ED evaluation. Prior research demonstrates that the initial history alone can lead to the correct diagnosis in up to 76% of cases of abdominal pain.14 If repeat evaluation determines that additional imaging is necessary, the American College of Radiology provides evidence-based guidelines to help clinicians determine the correct imaging test based on the clinical situation (Appendix Table).1,2 For example, an equivocal ultrasound or CT exam with continued suspicion for acute cholecystitis or an alternate diagnosis, such as acalculous cholecystitis or choledocholithiasis, merits alternative tests with improved sensitivity and specificity profiles (Tc 99 m hepatobiliary iminodiacetic acid scan, also known as cholescintigraphy, for cholecystitis and acalculous cholecystitis, or magnetic resonance cholangiopancreatography for choledocholithiasis).1
Remember to communicate with the radiologist to rule out “can’t miss” diagnoses, increase mutual understanding of the radiographic test characteristics for specific disease processes, and improve the radiologist’s understanding of the patient’s history and clinical question.15 Collaboration with the radiologist can also determine the need for follow-up imaging and its timing. One single-center study found that surgeons’ diagnostic impression and management changed in 35/100 (35%) cases after an in-person review with the radiologist.15 Observing patients in the hospital with a nondiagnostic initial evaluation but concerning clinical features often allows for either a trial of cure or for the disease process to “declare itself.”14 This allows clinicians to target additional testing to a specific diagnosis and avoid reflexive ordering of additional radiographic studies.
Recommendations
- Order an ultrasound for initial imaging of RUQ and female pelvic pain.
- Do not reflexively order an ultrasound within 24 to 48 hours of a negative CT scan to pursue biliary or pelvic pathology.
- Only order repeat abdominal imaging if clinical circumstances evolve or discussions with a radiologist conclude it will answer a more specific diagnostic question.
Conclusion
In our clinical scenario involving a patient with diffuse abdominal pain and a negative CT, the hospitalist should reevaluate the history, exam, and differential diagnosis before pursuing further diagnostic imaging. Based on the evidence presented, CT has similar diagnostic accuracy to ultrasound for biliary and gynecologic pathologies necessitating urgent surgical management (eg, acute cholecystitis, ovarian torsion), and a follow-up ultrasound adds little. If the utility of imaging remains in question, hospitalist consultation with a radiologist can clarify whether prior imaging answered the clinical question and the diagnostic utility of repeat abdominal imaging. With thoughtful reevaluation of the history and physical, and communication with radiology, hospitalists can reduce unnecessary, low-yield imaging and reduce healthcare costs when evaluating patients with abdominal pain.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected]
1. Expert Panel on Gastrointestinal Imaging; Peterson CM, McNamara MM, Kamel IR, et al. ACR Appropriateness Criteria® Right Upper Quadrant Pain. J Am Coll Radiol. 2019;16(5S):S235-S243. https://doi.org/10.1016/j.jacr.2019.02.013
2. Bhosale PR, Javitt MC, Atri M, et al. ACR Appropriateness Criteria® Acute Pelvic Pain in the Reproductive Age Group. Ultrasound Q. 2016;32(2):108-115. https://doi.org/10.1097/RUQ.0000000000000200
3. Revzin MV, Scoutt LM, Garner JG, Moore CL. Right upper quadrant pain: ultrasound first! J Ultrasound Med. 2017;36(10):1975-1985. https://doi.org/10.1002/jum.14274
4. Cooperberg PL, Burhenne HJ. Real-time ultrasonography. Diagnostic technique of choice in calculous gallbladder disease. N Engl J Med. 1980;302(23):1277-1279. https://doi.org/10.1056/NEJM198006053022303
5. Barakos JA, Ralls PW, Lapin SA, et al. Cholelithiasis: evaluation with CT. Radiology. 1987;162(2):415-418. https://doi.org/10.1148/radiology.162.2.3797654
6. Moore C, Meyers AB, Capotasto J, Bokhari J. Prevalence of abnormal CT findings in patients with proven ovarian torsion and a proposed triage schema. Emerg Radiol. 2009;16(2):115-120. https://doi.org/10.1007/s10140-008-0754-x
7. Harfouch N, Stern J, Chowdhary V, et al. Utility of ultrasound after a negative CT abdomen and pelvis in the emergency department. Clin Imaging. 2020;68:29-35. https://doi.org/10.1016/j.clinimag.2020.06.007
8. Adenaw N, Wen J, Pahwa AK, Sheth S, Johnson PT. Decreasing duplicative imaging: inpatient and emergency medicine abdominal ultrasound within 72 hours of abdominal CT. J Am Coll Radiol. 2020;17(5):590-596. https://doi.org/10.1016/j.jacr.2020.03.010
9. Kiewiet JJ, Leeuwenburgh MM, Bipat S, Bossuyt PM, Stoker J, Boermeester MA. A systematic review and meta-analysis of diagnostic performance of imaging in acute cholecystitis. Radiology. 2012;264(3):708-720. https://doi.org/10.1148/radiol.12111561
10. Wertz JR, Lopez JM, Olson D, Thompson WM. Comparing the diagnostic accuracy of ultrasound and CT in evaluating acute cholecystitis. AJR Am J Roentgenol. 2018;211(2):W92-W97. https://doi.org/10.2214/AJR.17.18884
11. Bennett GL, Rusinek H, Lisi V, et al. CT findings in acute gangrenous cholecystitis. AJR Am J Roentgenol. 2002;178(2):275-281. https://doi.org/10.2214/ajr.178.2.1780275
12. Hiatt KD, Ou JJ, Childs DD. Role of ultrasound and CT in the workup of right upper quadrant pain in adults in the emergency department: a retrospective review of more than 2800 cases. AJR Am J Roentgenol. 2020;214(6):1305-1310. https://doi.org/10.2214/AJR.19.22188
13. Gao Y, Lee K, Camacho M. Utility of pelvic ultrasound following negative abdominal and pelvic CT in the emergency room. Clin Radiol. 2013;68(11):e586-e592. https://doi.org/10.1016/j.crad.2013.05.101
14. Natesan S, Lee J, Volkamer H, Thoureen T. Evidence-based medicine approach to abdominal pain. Emerg Med Clin North Am. 2016;34(2):165-190. https://doi.org/10.1016/j.emc.2015.12.008.
15. Dickerson EC, Alam HB, Brown RK, Stojanovska J, Davenport MS; Michigan Radiology Quality Collaborative. In-person communication between radiologists and acute care surgeons leads to significant alterations in surgical decision making. J Am Coll Radiol. 2016;13(8):943-949. https://doi.org/10.1016/j.jacr.2016.02.005
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
A 70-year-old woman presented to the emergency department (ED) with diffuse abdominal pain, nausea, and vomiting with normal liver function tests and lipase. Computed tomography (CT) of the abdomen and pelvis with intravenous contrast revealed no acute intraabdominal pathology except for an incidentally noted, mildly enlarged but nondistended gallbladder without evident cholelithiasis, pericholecystic fluid, or gallbladder wall edema. The hospitalist orders an abdominal ultrasound to evaluate for acute biliary pathology potentially missed by CT.
Why You Might Consider Ordering an Abdominal Ultrasound After a Negative CT
Guidelines and expert opinion recommend an “ultrasound-first” approach when patients present with right upper quadrant (RUQ) abdominal pain or pelvic pain of suspected gynecologic origin.1-3 When evaluating suspected biliary disease, experts recommend beginning with ultrasonography based on the speed of obtaining results, absence of radiation exposure, reduced cost, and good diagnostic accuracy.1 Ultrasound has superior sensitivity, of 98%,4 in identifying radiolucent gallstones, compared to CT’s 79% sensitivity.5 Ultrasonography also differentiates gallbladder sludge from cholelithiasis, evaluates the extrahepatic and intrahepatic bile ducts, and can identify alternate causes of RUQ pain.1,3 Since ultrasound has important advantages, a negative initial CT may lead the clinician to consider an ultrasound to evaluate for gallbladder diseases.
Additionally, ultrasound provides improved anatomic detail of pelvic structures when diagnosing endometrial or ovarian pathology2 and improves diagnostic accuracy when the initial CT reveals an abnormal pelvic finding (eg, defining an enlarged ovary on CT as ovarian torsion, a cyst, or an adnexal mass).6 While CT excludes emergent surgical diagnoses, ultrasound may add value in elucidating a cause of the pain, even when urgent surgical management is not necessary.7
Many providers believe that a CT lacks sensitivity for acute biliary or pelvic pathology and will order an ultrasound to avoid missing an important diagnosis.7 Within 6 months at a single center, clinicians ordered 614 abdominal ultrasounds within 72 hours of an abdominal CT; 227 of these orders were to evaluate the gallbladder. Clinicians documented a discussion with a radiologist in only 19% of cases.8
Why Ordering an Ultrasound After a Negative CT Is Unnecessary
While ultrasound is more sensitive for detecting gallstones, the data do not indicate that it is more sensitive than CT for detecting acute cholecystitis. Abdominal ultrasound has a sensitivity for the diagnosis of acute cholecystitis of 81%, with a specificity of 83%,9 while CT has a comparable 85% to 94%9,10 sensitivity and specificity ranging from 59% to 99%.9,11 A recent study using more stringent radiographic criteria (two or more abnormal features) for diagnosing acute cholecystitis found ultrasound and CT had near equivalent sensitivities of 61% and 55%, respectively.12 Even with these stringent criteria, CT had a negative predictive value of 90% and approached 95% when applying a less strict (one feature) criterion.12 As a result, an abdominal ultrasound will rarely diagnose cholecystitis after a normal CT.
A 2020 study evaluated the diagnostic yield and clinical impact of ordering an abdominal or pelvic ultrasound within 24 hours of a negative abdominal CT.7
As with abdominal CT and ultrasound, the recommendation for an initial pelvic ultrasound when evaluating female pelvic pain also stems from the reduced cost, absence of radiation exposure, and superior anatomic visualization of the pelvic organs when compared with pelvic CT.2,13 However, as with the results of studies investigating the use of abdominal ultrasound after negative CT, a study of pelvic ultrasound after a negative CT revealed that only 4/126 (3.2%) follow-up ultrasounds had an abnormal finding not identified on CT.13 Pelvic ultrasound found four endometrial abnormalities that did not alter acute management.13 Notably, in 58% of the cases, the indication for ordering the subsequent ultrasound was “rule out ovarian torsion.” However, CT almost always finds a morphologically abnormal ovary in the case of torsion.6 One study and literature review found that all 28 patients studied and all 85 patients from previous studies with proven ovarian torsion had either an adnexal mass or an enlarged ovary on pelvic CT.6 Harfouch et al found that 0 out of 199 pelvic ultrasounds ordered after a negative CT revealed acute surgical pathology, but pelvic ultrasound did identify nonsurgical uterine and ovarian abnormalities.7 In conclusion, when clinicians order CT as the first study to diagnose acute, surgical biliary or gynecologic causes of pain, follow-up ultrasound has a low probability of affecting diagnosis or management if the CT is normal.
When You Should Consider Ultrasound After CT
The previous discussion only applies if hospitalists order an ultrasound within 24 to 48 hours of the initial CT. Time and clinical course are critical diagnostic tools during an admission for abdominal pain. Consider pelvic or abdominal ultrasound based on guideline recommendations if a patient develops new or evolving RUQ or pelvic pain.1,2 The rationale for obtaining the initial negative CT may no longer apply, and the clinician must consider the changing characteristics of the patient’s symptoms. For example, initial CT imaging may miss cholelithiasis in a patient presenting for biliary colic. Under observation, the patient may develop acute cholecystitis, potentially requiring an abdominal ultrasound. Also, the data for pelvic ultrasound apply to a normal CT of the abdomen and pelvis. Ultrasound may help to further evaluate indeterminate findings present on initial CT or if recommended by radiology.
What You Should Do Instead
When the hospitalist assumes care for a patient with abdominal pain and a negative CT, appropriate next steps include taking time to reexamine the differential diagnosis, repeating the history and physical, and communicating directly with a radiologist. These steps ensure the highest diagnostic yield and the lowest cost and help prevent diagnostic error arising from anchoring on the initial negative ED evaluation. Prior research demonstrates that the initial history alone can lead to the correct diagnosis in up to 76% of cases of abdominal pain.14 If repeat evaluation determines that additional imaging is necessary, the American College of Radiology provides evidence-based guidelines to help clinicians determine the correct imaging test based on the clinical situation (Appendix Table).1,2 For example, an equivocal ultrasound or CT exam with continued suspicion for acute cholecystitis or an alternate diagnosis, such as acalculous cholecystitis or choledocholithiasis, merits alternative tests with improved sensitivity and specificity profiles (Tc 99 m hepatobiliary iminodiacetic acid scan, also known as cholescintigraphy, for cholecystitis and acalculous cholecystitis, or magnetic resonance cholangiopancreatography for choledocholithiasis).1
Remember to communicate with the radiologist to rule out “can’t miss” diagnoses, increase mutual understanding of the radiographic test characteristics for specific disease processes, and improve the radiologist’s understanding of the patient’s history and clinical question.15 Collaboration with the radiologist can also determine the need for follow-up imaging and its timing. One single-center study found that surgeons’ diagnostic impression and management changed in 35/100 (35%) cases after an in-person review with the radiologist.15 Observing patients in the hospital with a nondiagnostic initial evaluation but concerning clinical features often allows for either a trial of cure or for the disease process to “declare itself.”14 This allows clinicians to target additional testing to a specific diagnosis and avoid reflexive ordering of additional radiographic studies.
Recommendations
- Order an ultrasound for initial imaging of RUQ and female pelvic pain.
- Do not reflexively order an ultrasound within 24 to 48 hours of a negative CT scan to pursue biliary or pelvic pathology.
- Only order repeat abdominal imaging if clinical circumstances evolve or discussions with a radiologist conclude it will answer a more specific diagnostic question.
Conclusion
In our clinical scenario involving a patient with diffuse abdominal pain and a negative CT, the hospitalist should reevaluate the history, exam, and differential diagnosis before pursuing further diagnostic imaging. Based on the evidence presented, CT has similar diagnostic accuracy to ultrasound for biliary and gynecologic pathologies necessitating urgent surgical management (eg, acute cholecystitis, ovarian torsion), and a follow-up ultrasound adds little. If the utility of imaging remains in question, hospitalist consultation with a radiologist can clarify whether prior imaging answered the clinical question and the diagnostic utility of repeat abdominal imaging. With thoughtful reevaluation of the history and physical, and communication with radiology, hospitalists can reduce unnecessary, low-yield imaging and reduce healthcare costs when evaluating patients with abdominal pain.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected]
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
A 70-year-old woman presented to the emergency department (ED) with diffuse abdominal pain, nausea, and vomiting with normal liver function tests and lipase. Computed tomography (CT) of the abdomen and pelvis with intravenous contrast revealed no acute intraabdominal pathology except for an incidentally noted, mildly enlarged but nondistended gallbladder without evident cholelithiasis, pericholecystic fluid, or gallbladder wall edema. The hospitalist orders an abdominal ultrasound to evaluate for acute biliary pathology potentially missed by CT.
Why You Might Consider Ordering an Abdominal Ultrasound After a Negative CT
Guidelines and expert opinion recommend an “ultrasound-first” approach when patients present with right upper quadrant (RUQ) abdominal pain or pelvic pain of suspected gynecologic origin.1-3 When evaluating suspected biliary disease, experts recommend beginning with ultrasonography based on the speed of obtaining results, absence of radiation exposure, reduced cost, and good diagnostic accuracy.1 Ultrasound has superior sensitivity, of 98%,4 in identifying radiolucent gallstones, compared to CT’s 79% sensitivity.5 Ultrasonography also differentiates gallbladder sludge from cholelithiasis, evaluates the extrahepatic and intrahepatic bile ducts, and can identify alternate causes of RUQ pain.1,3 Since ultrasound has important advantages, a negative initial CT may lead the clinician to consider an ultrasound to evaluate for gallbladder diseases.
Additionally, ultrasound provides improved anatomic detail of pelvic structures when diagnosing endometrial or ovarian pathology2 and improves diagnostic accuracy when the initial CT reveals an abnormal pelvic finding (eg, defining an enlarged ovary on CT as ovarian torsion, a cyst, or an adnexal mass).6 While CT excludes emergent surgical diagnoses, ultrasound may add value in elucidating a cause of the pain, even when urgent surgical management is not necessary.7
Many providers believe that a CT lacks sensitivity for acute biliary or pelvic pathology and will order an ultrasound to avoid missing an important diagnosis.7 Within 6 months at a single center, clinicians ordered 614 abdominal ultrasounds within 72 hours of an abdominal CT; 227 of these orders were to evaluate the gallbladder. Clinicians documented a discussion with a radiologist in only 19% of cases.8
Why Ordering an Ultrasound After a Negative CT Is Unnecessary
While ultrasound is more sensitive for detecting gallstones, the data do not indicate that it is more sensitive than CT for detecting acute cholecystitis. Abdominal ultrasound has a sensitivity for the diagnosis of acute cholecystitis of 81%, with a specificity of 83%,9 while CT has a comparable 85% to 94%9,10 sensitivity and specificity ranging from 59% to 99%.9,11 A recent study using more stringent radiographic criteria (two or more abnormal features) for diagnosing acute cholecystitis found ultrasound and CT had near equivalent sensitivities of 61% and 55%, respectively.12 Even with these stringent criteria, CT had a negative predictive value of 90% and approached 95% when applying a less strict (one feature) criterion.12 As a result, an abdominal ultrasound will rarely diagnose cholecystitis after a normal CT.
A 2020 study evaluated the diagnostic yield and clinical impact of ordering an abdominal or pelvic ultrasound within 24 hours of a negative abdominal CT.7
As with abdominal CT and ultrasound, the recommendation for an initial pelvic ultrasound when evaluating female pelvic pain also stems from the reduced cost, absence of radiation exposure, and superior anatomic visualization of the pelvic organs when compared with pelvic CT.2,13 However, as with the results of studies investigating the use of abdominal ultrasound after negative CT, a study of pelvic ultrasound after a negative CT revealed that only 4/126 (3.2%) follow-up ultrasounds had an abnormal finding not identified on CT.13 Pelvic ultrasound found four endometrial abnormalities that did not alter acute management.13 Notably, in 58% of the cases, the indication for ordering the subsequent ultrasound was “rule out ovarian torsion.” However, CT almost always finds a morphologically abnormal ovary in the case of torsion.6 One study and literature review found that all 28 patients studied and all 85 patients from previous studies with proven ovarian torsion had either an adnexal mass or an enlarged ovary on pelvic CT.6 Harfouch et al found that 0 out of 199 pelvic ultrasounds ordered after a negative CT revealed acute surgical pathology, but pelvic ultrasound did identify nonsurgical uterine and ovarian abnormalities.7 In conclusion, when clinicians order CT as the first study to diagnose acute, surgical biliary or gynecologic causes of pain, follow-up ultrasound has a low probability of affecting diagnosis or management if the CT is normal.
When You Should Consider Ultrasound After CT
The previous discussion only applies if hospitalists order an ultrasound within 24 to 48 hours of the initial CT. Time and clinical course are critical diagnostic tools during an admission for abdominal pain. Consider pelvic or abdominal ultrasound based on guideline recommendations if a patient develops new or evolving RUQ or pelvic pain.1,2 The rationale for obtaining the initial negative CT may no longer apply, and the clinician must consider the changing characteristics of the patient’s symptoms. For example, initial CT imaging may miss cholelithiasis in a patient presenting for biliary colic. Under observation, the patient may develop acute cholecystitis, potentially requiring an abdominal ultrasound. Also, the data for pelvic ultrasound apply to a normal CT of the abdomen and pelvis. Ultrasound may help to further evaluate indeterminate findings present on initial CT or if recommended by radiology.
What You Should Do Instead
When the hospitalist assumes care for a patient with abdominal pain and a negative CT, appropriate next steps include taking time to reexamine the differential diagnosis, repeating the history and physical, and communicating directly with a radiologist. These steps ensure the highest diagnostic yield and the lowest cost and help prevent diagnostic error arising from anchoring on the initial negative ED evaluation. Prior research demonstrates that the initial history alone can lead to the correct diagnosis in up to 76% of cases of abdominal pain.14 If repeat evaluation determines that additional imaging is necessary, the American College of Radiology provides evidence-based guidelines to help clinicians determine the correct imaging test based on the clinical situation (Appendix Table).1,2 For example, an equivocal ultrasound or CT exam with continued suspicion for acute cholecystitis or an alternate diagnosis, such as acalculous cholecystitis or choledocholithiasis, merits alternative tests with improved sensitivity and specificity profiles (Tc 99 m hepatobiliary iminodiacetic acid scan, also known as cholescintigraphy, for cholecystitis and acalculous cholecystitis, or magnetic resonance cholangiopancreatography for choledocholithiasis).1
Remember to communicate with the radiologist to rule out “can’t miss” diagnoses, increase mutual understanding of the radiographic test characteristics for specific disease processes, and improve the radiologist’s understanding of the patient’s history and clinical question.15 Collaboration with the radiologist can also determine the need for follow-up imaging and its timing. One single-center study found that surgeons’ diagnostic impression and management changed in 35/100 (35%) cases after an in-person review with the radiologist.15 Observing patients in the hospital with a nondiagnostic initial evaluation but concerning clinical features often allows for either a trial of cure or for the disease process to “declare itself.”14 This allows clinicians to target additional testing to a specific diagnosis and avoid reflexive ordering of additional radiographic studies.
Recommendations
- Order an ultrasound for initial imaging of RUQ and female pelvic pain.
- Do not reflexively order an ultrasound within 24 to 48 hours of a negative CT scan to pursue biliary or pelvic pathology.
- Only order repeat abdominal imaging if clinical circumstances evolve or discussions with a radiologist conclude it will answer a more specific diagnostic question.
Conclusion
In our clinical scenario involving a patient with diffuse abdominal pain and a negative CT, the hospitalist should reevaluate the history, exam, and differential diagnosis before pursuing further diagnostic imaging. Based on the evidence presented, CT has similar diagnostic accuracy to ultrasound for biliary and gynecologic pathologies necessitating urgent surgical management (eg, acute cholecystitis, ovarian torsion), and a follow-up ultrasound adds little. If the utility of imaging remains in question, hospitalist consultation with a radiologist can clarify whether prior imaging answered the clinical question and the diagnostic utility of repeat abdominal imaging. With thoughtful reevaluation of the history and physical, and communication with radiology, hospitalists can reduce unnecessary, low-yield imaging and reduce healthcare costs when evaluating patients with abdominal pain.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected]
1. Expert Panel on Gastrointestinal Imaging; Peterson CM, McNamara MM, Kamel IR, et al. ACR Appropriateness Criteria® Right Upper Quadrant Pain. J Am Coll Radiol. 2019;16(5S):S235-S243. https://doi.org/10.1016/j.jacr.2019.02.013
2. Bhosale PR, Javitt MC, Atri M, et al. ACR Appropriateness Criteria® Acute Pelvic Pain in the Reproductive Age Group. Ultrasound Q. 2016;32(2):108-115. https://doi.org/10.1097/RUQ.0000000000000200
3. Revzin MV, Scoutt LM, Garner JG, Moore CL. Right upper quadrant pain: ultrasound first! J Ultrasound Med. 2017;36(10):1975-1985. https://doi.org/10.1002/jum.14274
4. Cooperberg PL, Burhenne HJ. Real-time ultrasonography. Diagnostic technique of choice in calculous gallbladder disease. N Engl J Med. 1980;302(23):1277-1279. https://doi.org/10.1056/NEJM198006053022303
5. Barakos JA, Ralls PW, Lapin SA, et al. Cholelithiasis: evaluation with CT. Radiology. 1987;162(2):415-418. https://doi.org/10.1148/radiology.162.2.3797654
6. Moore C, Meyers AB, Capotasto J, Bokhari J. Prevalence of abnormal CT findings in patients with proven ovarian torsion and a proposed triage schema. Emerg Radiol. 2009;16(2):115-120. https://doi.org/10.1007/s10140-008-0754-x
7. Harfouch N, Stern J, Chowdhary V, et al. Utility of ultrasound after a negative CT abdomen and pelvis in the emergency department. Clin Imaging. 2020;68:29-35. https://doi.org/10.1016/j.clinimag.2020.06.007
8. Adenaw N, Wen J, Pahwa AK, Sheth S, Johnson PT. Decreasing duplicative imaging: inpatient and emergency medicine abdominal ultrasound within 72 hours of abdominal CT. J Am Coll Radiol. 2020;17(5):590-596. https://doi.org/10.1016/j.jacr.2020.03.010
9. Kiewiet JJ, Leeuwenburgh MM, Bipat S, Bossuyt PM, Stoker J, Boermeester MA. A systematic review and meta-analysis of diagnostic performance of imaging in acute cholecystitis. Radiology. 2012;264(3):708-720. https://doi.org/10.1148/radiol.12111561
10. Wertz JR, Lopez JM, Olson D, Thompson WM. Comparing the diagnostic accuracy of ultrasound and CT in evaluating acute cholecystitis. AJR Am J Roentgenol. 2018;211(2):W92-W97. https://doi.org/10.2214/AJR.17.18884
11. Bennett GL, Rusinek H, Lisi V, et al. CT findings in acute gangrenous cholecystitis. AJR Am J Roentgenol. 2002;178(2):275-281. https://doi.org/10.2214/ajr.178.2.1780275
12. Hiatt KD, Ou JJ, Childs DD. Role of ultrasound and CT in the workup of right upper quadrant pain in adults in the emergency department: a retrospective review of more than 2800 cases. AJR Am J Roentgenol. 2020;214(6):1305-1310. https://doi.org/10.2214/AJR.19.22188
13. Gao Y, Lee K, Camacho M. Utility of pelvic ultrasound following negative abdominal and pelvic CT in the emergency room. Clin Radiol. 2013;68(11):e586-e592. https://doi.org/10.1016/j.crad.2013.05.101
14. Natesan S, Lee J, Volkamer H, Thoureen T. Evidence-based medicine approach to abdominal pain. Emerg Med Clin North Am. 2016;34(2):165-190. https://doi.org/10.1016/j.emc.2015.12.008.
15. Dickerson EC, Alam HB, Brown RK, Stojanovska J, Davenport MS; Michigan Radiology Quality Collaborative. In-person communication between radiologists and acute care surgeons leads to significant alterations in surgical decision making. J Am Coll Radiol. 2016;13(8):943-949. https://doi.org/10.1016/j.jacr.2016.02.005
1. Expert Panel on Gastrointestinal Imaging; Peterson CM, McNamara MM, Kamel IR, et al. ACR Appropriateness Criteria® Right Upper Quadrant Pain. J Am Coll Radiol. 2019;16(5S):S235-S243. https://doi.org/10.1016/j.jacr.2019.02.013
2. Bhosale PR, Javitt MC, Atri M, et al. ACR Appropriateness Criteria® Acute Pelvic Pain in the Reproductive Age Group. Ultrasound Q. 2016;32(2):108-115. https://doi.org/10.1097/RUQ.0000000000000200
3. Revzin MV, Scoutt LM, Garner JG, Moore CL. Right upper quadrant pain: ultrasound first! J Ultrasound Med. 2017;36(10):1975-1985. https://doi.org/10.1002/jum.14274
4. Cooperberg PL, Burhenne HJ. Real-time ultrasonography. Diagnostic technique of choice in calculous gallbladder disease. N Engl J Med. 1980;302(23):1277-1279. https://doi.org/10.1056/NEJM198006053022303
5. Barakos JA, Ralls PW, Lapin SA, et al. Cholelithiasis: evaluation with CT. Radiology. 1987;162(2):415-418. https://doi.org/10.1148/radiology.162.2.3797654
6. Moore C, Meyers AB, Capotasto J, Bokhari J. Prevalence of abnormal CT findings in patients with proven ovarian torsion and a proposed triage schema. Emerg Radiol. 2009;16(2):115-120. https://doi.org/10.1007/s10140-008-0754-x
7. Harfouch N, Stern J, Chowdhary V, et al. Utility of ultrasound after a negative CT abdomen and pelvis in the emergency department. Clin Imaging. 2020;68:29-35. https://doi.org/10.1016/j.clinimag.2020.06.007
8. Adenaw N, Wen J, Pahwa AK, Sheth S, Johnson PT. Decreasing duplicative imaging: inpatient and emergency medicine abdominal ultrasound within 72 hours of abdominal CT. J Am Coll Radiol. 2020;17(5):590-596. https://doi.org/10.1016/j.jacr.2020.03.010
9. Kiewiet JJ, Leeuwenburgh MM, Bipat S, Bossuyt PM, Stoker J, Boermeester MA. A systematic review and meta-analysis of diagnostic performance of imaging in acute cholecystitis. Radiology. 2012;264(3):708-720. https://doi.org/10.1148/radiol.12111561
10. Wertz JR, Lopez JM, Olson D, Thompson WM. Comparing the diagnostic accuracy of ultrasound and CT in evaluating acute cholecystitis. AJR Am J Roentgenol. 2018;211(2):W92-W97. https://doi.org/10.2214/AJR.17.18884
11. Bennett GL, Rusinek H, Lisi V, et al. CT findings in acute gangrenous cholecystitis. AJR Am J Roentgenol. 2002;178(2):275-281. https://doi.org/10.2214/ajr.178.2.1780275
12. Hiatt KD, Ou JJ, Childs DD. Role of ultrasound and CT in the workup of right upper quadrant pain in adults in the emergency department: a retrospective review of more than 2800 cases. AJR Am J Roentgenol. 2020;214(6):1305-1310. https://doi.org/10.2214/AJR.19.22188
13. Gao Y, Lee K, Camacho M. Utility of pelvic ultrasound following negative abdominal and pelvic CT in the emergency room. Clin Radiol. 2013;68(11):e586-e592. https://doi.org/10.1016/j.crad.2013.05.101
14. Natesan S, Lee J, Volkamer H, Thoureen T. Evidence-based medicine approach to abdominal pain. Emerg Med Clin North Am. 2016;34(2):165-190. https://doi.org/10.1016/j.emc.2015.12.008.
15. Dickerson EC, Alam HB, Brown RK, Stojanovska J, Davenport MS; Michigan Radiology Quality Collaborative. In-person communication between radiologists and acute care surgeons leads to significant alterations in surgical decision making. J Am Coll Radiol. 2016;13(8):943-949. https://doi.org/10.1016/j.jacr.2016.02.005
© 2021 Society of Hospital Medicine
Clinical Guideline Highlights for the Hospitalist: Management of Upper Gastrointestinal and Ulcer Bleeding
Upper gastrointestinal bleeding (UGIB) is defined as a bleed originating from the esophagus, stomach, or duodenum. Approximately 80% of patients with UGIB presenting to the emergency department are admitted to the hospital, accounting for more than 200,000 hospital admissions and 4000 in-hospital deaths per year.1 In this article, we highlight 9 of the 16 recommendations from the 2021 American College of Gastroenterology (ACG) guidelines that are most pertinent to the hospitalist, presented in sections corresponding to the stages of inpatient clinical management.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Initial Triage
Recommendation 1. Patients with UGIB presenting to the emergency department who are classified as very low risk, defined as a risk assessment score with ≤1% false-negative rate for the outcome of hospital-based intervention or death (ie, Glasgow-Blatchford score of 0-1), should be discharged with outpatient follow-up rather than admitted to the hospital (conditional recommendation, very-low-quality evidence). The Glasgow-Blatchford score is an effective risk-assessment tool that can classify patients at high risk for death or needing a hospital-based intervention (eg, endoscopy or blood transfusion) with a sensitivity of 99%.2 Triage decisions should incorporate other patient factors, such as age, comorbidities, and reliability of close follow-up after discharge.
Pre-endoscopy Management
Recommendation 2. A restrictive threshold for red blood cell transfusion of 7 g/dL is recommended for patients with UGIB (conditional recommendation, low-quality evidence) as it appears to reduce death and further bleeding.3 It is reasonable to transfuse patients with preexisting cardiovascular disease whose hemoglobin is below 8 g/dL. For patients who are exsanguinating with hemodynamic instability, it is reasonable to transfuse before the hemoglobin reaches 7 g/dL.
Recommendation 3. An infusion of erythromycin is recommended before endoscopy in patients with UGIB (conditional recommendation, very-low-quality evidence). Erythromycin (250 mg intravenously [IV]) improves endoscopic visualization and diagnostic accuracy by moving the blood and clot out of the upper GI tract. A meta-analysis showed a reduction of need for repeat endoscopy (odds ratio [OR], 0.51; 95% CI, 0.34-0.77) and length of hospitalization (mean difference, –1.75 d).4
Recommendation 4. There is no consensus for or against pre-endoscopic proton pump inhibitor (PPI) therapy for patients with UGIB, owing to overall limited available data.
Recommendation 5. Patients hospitalized for UGIB should undergo endoscopy within 24 hours of presentation (conditional recommendation, very-low-quality evidence). Performing endoscopy within 24 hours, rather than 12 hours, of presentation demonstrated a potential trend toward decreased length of stay, mortality, and need for surgery. The potential harm in performing earlier endoscopy was attributed to inadequate resuscitation and insufficient optimization of active comorbidities.
Post-endoscopy Management
Recommendation 6. High-dose PPI therapy should be given for 3 days after successful endoscopic hemostatic therapy of a bleeding ulcer (strong recommendation, moderate- to high-quality evidence). When compared with placebo, there is an absolute risk reduction of 3% in mortality and 10% in further bleeding when administering continuous (80 mg bolus with 8 mg/h infusion) or intermittent high-dose PPI therapy (80 mg bolus with 40 mg 2-4 times daily thereafter) for 3 days after endoscopic therapy.5,6 Cost and ease of administration should be considered when choosing between intermittent or continuous PPI therapy. Oral PPI therapy may be appropriate for patients who are able to tolerate oral intake (no nausea, vomiting, dysphagia, or somnolence).
Recommendation 7. High-risk patients (defined as a Rockall score of ≥6 ) with UGIB due to ulcers who received endoscopic hemostatic therapy followed by short-term high-dose PPI therapy in hospital should be continued on twice-daily PPI therapy until 2 weeks after index endoscopy (conditional recommendation, low-quality evidence). A randomized controlled trial of high-risk patients showed significantly lower recurrence of bleeding with twice-daily vs once daily PPI.7 It remains uncertain whether patients benefit from PPI therapy beyond 4 weeks.
Rebleeding Management
Recommendation 8. Patients with recurrent bleeding after endoscopic therapy for a bleeding ulcer should undergo repeat endoscopic therapy rather than surgery or transcatheter arterial embolization (TAE) (conditional recommendation, low-quality evidence for comparison with surgery, very-low-quality evidence for comparison with TAE). In a small randomized controlled trial of repeat endoscopy vs surgery in patients with rebleeding after initial successful endoscopic treatment, there were more subsequent bleeding episodes in the repeat endoscopy group, but no significant difference in mortality and length of stay.8 The repeat endoscopy group had fewer complications, though, and a successful treatment rate of 75%. Because of the lack of high-quality studies in support of TAE and the known safety and efficacy of repeat endoscopy, repeat endoscopy is preferred over TAE for recurrent UGIB.
Recommendation 9. Patients with bleeding ulcers who have failed repeat endoscopic therapy should be treated with TAE (conditional recommendation, very-low-quality evidence). Based on a meta-analysis, when comparing TAE with surgery in patients with UGIB who fail endoscopic therapy, overall mortality was the same, and TAE patients had fewer complications and shorter hospital stays despite having a higher risk of further bleeding.9
CRITIQUE
The guidelines were formulated by panel members with input from the ACG Practice Parameters Committee using the population, intervention, comparator, and outcome (PICO) format to frame each question. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was used to assess the strength of the recommendation and the quality of evidence.
Most of the recommendations are conditional and/or based on low-quality or very-low-quality evidence. Although randomized control trials were sought, observational studies were sometimes included when randomized controlled trials were lacking. The literature review process appeared to focus on the primary outcome of further bleeding, which, although critical in patients with UGIB, could have limited the scope of evidence used in making the recommendations. It was stated that studies identified as relevant to the panel members or authors were considered for review without mentioning any standardized approach. The composition of the panel members was not discussed, and it is uncertain whether the guidelines underwent any formal peer-review process. Furthermore, although competing interests were declared, the panel did not discuss how conflicts were managed and what potential impact they had in the guideline recommendations. Finally, some of the recommendations (eg, TAE) will depend on local expertise and may not be available at all medical centers.
AREAS IN NEED OF FUTURE STUDY
Further study is needed to address the integration of risk-assessment tools into electronic health records to assist with timely decisions on managing patients with acute UGIB, to clarify the role for pre-endoscopic PPI therapy, and to specify fluid resuscitation and blood pressure goals in patients with more severe bleeding episodes and determine whether a subset of patients might benefit from very-early endoscopy (the 2012 ACG guidelines suggested that endoscopy within 12 hours may be considered in patients with high-risk clinical features such as hemodynamic instability or cirrhosis).
Other Resources
Glasgow-Blatchford Score (https://www.mdcalc.com/glasgow-blatchford-bleeding-score-gbs)
Rockall Score (https://www.mdcalc.com/rockall-score-upper-gi-bleeding-pre-endoscopy)
1. Peery AF, Crockett SD, Murphy CC, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology. 2019;156(1):254-272.e11. https://doi.org/10.1053/j.gastro.2018.08.063
2. Stanley AJ, Laine L, Dalton HR, et al. Comparison of risk scoring systems for patients presenting with upper gastrointestinal bleeding: international multicentre prospective study. BMJ. 2017;356:i6432. https://doi.org/10.1136/bmj.i6432
3. Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368(1):11-21. https://doi.org/10.1056/NEJMoa1211801
4. Rahman R, Nguyen DL, Sohail U, et al. Pre-endoscopic erythromycin administration in upper gastrointestinal bleeding: an updated meta analysis and systematic review. Ann Gastroenterol. 2016;29(3):312-317. https://doi.org/10.20524/aog.2016.0045
5. Hung WK, Li VKM, Chung CK, et al. Randomized trial comparing pantoprazole infusion, bolus and no treatment on gastric pH and recurrent bleeding in peptic ulcers. ANZ J Surg. 2007;77(8):677-681. https://doi.org/10.1111/j.1445-2197.2007.04185.x
6. Lau JY, Sung JJ, Lee KK, et al. Effect of intravenous omeprazole on recurrent bleeding after endoscopic treatment of bleeding peptic ulcers. N Engl J Med. 2000;343(5):310-316. https://doi.org/10.1056/NEJM200008033430501
7. Cheng HC, Wu CT, Chang WL, Cheng WC, Chen WY, Sheu BS. Double oral esomeprazole after a 3-day intravenous esomeprazole infusion reduces recurrent peptic ulcer bleeding in high-risk patients: a randomised controlled study. Gut. 2014;63(12):1864-1872. https://doi.org/10.1136/gutjnl-2013-306531
8. Lau JY, Sung JJ, Lam YH, et al. Endoscopic retreatment compared with surgery in patients with recurrent bleeding after initial endoscopic control of bleeding ulcers. N Engl J Med. 1999;340(10):751-756. https://doi.org/10.1056/NEJM199903113401002
9. Tarasconi A, Baiocchi GL, Pattonieri V, et al. Transcatheter arterial embolization versus surgery for refractory non-variceal upper gastrointestinal bleeding: a meta-analysis. World J Emerg Surg. 2019;14:3. https://doi.org/10.1186/s13017-019-0223-8
Upper gastrointestinal bleeding (UGIB) is defined as a bleed originating from the esophagus, stomach, or duodenum. Approximately 80% of patients with UGIB presenting to the emergency department are admitted to the hospital, accounting for more than 200,000 hospital admissions and 4000 in-hospital deaths per year.1 In this article, we highlight 9 of the 16 recommendations from the 2021 American College of Gastroenterology (ACG) guidelines that are most pertinent to the hospitalist, presented in sections corresponding to the stages of inpatient clinical management.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Initial Triage
Recommendation 1. Patients with UGIB presenting to the emergency department who are classified as very low risk, defined as a risk assessment score with ≤1% false-negative rate for the outcome of hospital-based intervention or death (ie, Glasgow-Blatchford score of 0-1), should be discharged with outpatient follow-up rather than admitted to the hospital (conditional recommendation, very-low-quality evidence). The Glasgow-Blatchford score is an effective risk-assessment tool that can classify patients at high risk for death or needing a hospital-based intervention (eg, endoscopy or blood transfusion) with a sensitivity of 99%.2 Triage decisions should incorporate other patient factors, such as age, comorbidities, and reliability of close follow-up after discharge.
Pre-endoscopy Management
Recommendation 2. A restrictive threshold for red blood cell transfusion of 7 g/dL is recommended for patients with UGIB (conditional recommendation, low-quality evidence) as it appears to reduce death and further bleeding.3 It is reasonable to transfuse patients with preexisting cardiovascular disease whose hemoglobin is below 8 g/dL. For patients who are exsanguinating with hemodynamic instability, it is reasonable to transfuse before the hemoglobin reaches 7 g/dL.
Recommendation 3. An infusion of erythromycin is recommended before endoscopy in patients with UGIB (conditional recommendation, very-low-quality evidence). Erythromycin (250 mg intravenously [IV]) improves endoscopic visualization and diagnostic accuracy by moving the blood and clot out of the upper GI tract. A meta-analysis showed a reduction of need for repeat endoscopy (odds ratio [OR], 0.51; 95% CI, 0.34-0.77) and length of hospitalization (mean difference, –1.75 d).4
Recommendation 4. There is no consensus for or against pre-endoscopic proton pump inhibitor (PPI) therapy for patients with UGIB, owing to overall limited available data.
Recommendation 5. Patients hospitalized for UGIB should undergo endoscopy within 24 hours of presentation (conditional recommendation, very-low-quality evidence). Performing endoscopy within 24 hours, rather than 12 hours, of presentation demonstrated a potential trend toward decreased length of stay, mortality, and need for surgery. The potential harm in performing earlier endoscopy was attributed to inadequate resuscitation and insufficient optimization of active comorbidities.
Post-endoscopy Management
Recommendation 6. High-dose PPI therapy should be given for 3 days after successful endoscopic hemostatic therapy of a bleeding ulcer (strong recommendation, moderate- to high-quality evidence). When compared with placebo, there is an absolute risk reduction of 3% in mortality and 10% in further bleeding when administering continuous (80 mg bolus with 8 mg/h infusion) or intermittent high-dose PPI therapy (80 mg bolus with 40 mg 2-4 times daily thereafter) for 3 days after endoscopic therapy.5,6 Cost and ease of administration should be considered when choosing between intermittent or continuous PPI therapy. Oral PPI therapy may be appropriate for patients who are able to tolerate oral intake (no nausea, vomiting, dysphagia, or somnolence).
Recommendation 7. High-risk patients (defined as a Rockall score of ≥6 ) with UGIB due to ulcers who received endoscopic hemostatic therapy followed by short-term high-dose PPI therapy in hospital should be continued on twice-daily PPI therapy until 2 weeks after index endoscopy (conditional recommendation, low-quality evidence). A randomized controlled trial of high-risk patients showed significantly lower recurrence of bleeding with twice-daily vs once daily PPI.7 It remains uncertain whether patients benefit from PPI therapy beyond 4 weeks.
Rebleeding Management
Recommendation 8. Patients with recurrent bleeding after endoscopic therapy for a bleeding ulcer should undergo repeat endoscopic therapy rather than surgery or transcatheter arterial embolization (TAE) (conditional recommendation, low-quality evidence for comparison with surgery, very-low-quality evidence for comparison with TAE). In a small randomized controlled trial of repeat endoscopy vs surgery in patients with rebleeding after initial successful endoscopic treatment, there were more subsequent bleeding episodes in the repeat endoscopy group, but no significant difference in mortality and length of stay.8 The repeat endoscopy group had fewer complications, though, and a successful treatment rate of 75%. Because of the lack of high-quality studies in support of TAE and the known safety and efficacy of repeat endoscopy, repeat endoscopy is preferred over TAE for recurrent UGIB.
Recommendation 9. Patients with bleeding ulcers who have failed repeat endoscopic therapy should be treated with TAE (conditional recommendation, very-low-quality evidence). Based on a meta-analysis, when comparing TAE with surgery in patients with UGIB who fail endoscopic therapy, overall mortality was the same, and TAE patients had fewer complications and shorter hospital stays despite having a higher risk of further bleeding.9
CRITIQUE
The guidelines were formulated by panel members with input from the ACG Practice Parameters Committee using the population, intervention, comparator, and outcome (PICO) format to frame each question. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was used to assess the strength of the recommendation and the quality of evidence.
Most of the recommendations are conditional and/or based on low-quality or very-low-quality evidence. Although randomized control trials were sought, observational studies were sometimes included when randomized controlled trials were lacking. The literature review process appeared to focus on the primary outcome of further bleeding, which, although critical in patients with UGIB, could have limited the scope of evidence used in making the recommendations. It was stated that studies identified as relevant to the panel members or authors were considered for review without mentioning any standardized approach. The composition of the panel members was not discussed, and it is uncertain whether the guidelines underwent any formal peer-review process. Furthermore, although competing interests were declared, the panel did not discuss how conflicts were managed and what potential impact they had in the guideline recommendations. Finally, some of the recommendations (eg, TAE) will depend on local expertise and may not be available at all medical centers.
AREAS IN NEED OF FUTURE STUDY
Further study is needed to address the integration of risk-assessment tools into electronic health records to assist with timely decisions on managing patients with acute UGIB, to clarify the role for pre-endoscopic PPI therapy, and to specify fluid resuscitation and blood pressure goals in patients with more severe bleeding episodes and determine whether a subset of patients might benefit from very-early endoscopy (the 2012 ACG guidelines suggested that endoscopy within 12 hours may be considered in patients with high-risk clinical features such as hemodynamic instability or cirrhosis).
Other Resources
Glasgow-Blatchford Score (https://www.mdcalc.com/glasgow-blatchford-bleeding-score-gbs)
Rockall Score (https://www.mdcalc.com/rockall-score-upper-gi-bleeding-pre-endoscopy)
Upper gastrointestinal bleeding (UGIB) is defined as a bleed originating from the esophagus, stomach, or duodenum. Approximately 80% of patients with UGIB presenting to the emergency department are admitted to the hospital, accounting for more than 200,000 hospital admissions and 4000 in-hospital deaths per year.1 In this article, we highlight 9 of the 16 recommendations from the 2021 American College of Gastroenterology (ACG) guidelines that are most pertinent to the hospitalist, presented in sections corresponding to the stages of inpatient clinical management.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Initial Triage
Recommendation 1. Patients with UGIB presenting to the emergency department who are classified as very low risk, defined as a risk assessment score with ≤1% false-negative rate for the outcome of hospital-based intervention or death (ie, Glasgow-Blatchford score of 0-1), should be discharged with outpatient follow-up rather than admitted to the hospital (conditional recommendation, very-low-quality evidence). The Glasgow-Blatchford score is an effective risk-assessment tool that can classify patients at high risk for death or needing a hospital-based intervention (eg, endoscopy or blood transfusion) with a sensitivity of 99%.2 Triage decisions should incorporate other patient factors, such as age, comorbidities, and reliability of close follow-up after discharge.
Pre-endoscopy Management
Recommendation 2. A restrictive threshold for red blood cell transfusion of 7 g/dL is recommended for patients with UGIB (conditional recommendation, low-quality evidence) as it appears to reduce death and further bleeding.3 It is reasonable to transfuse patients with preexisting cardiovascular disease whose hemoglobin is below 8 g/dL. For patients who are exsanguinating with hemodynamic instability, it is reasonable to transfuse before the hemoglobin reaches 7 g/dL.
Recommendation 3. An infusion of erythromycin is recommended before endoscopy in patients with UGIB (conditional recommendation, very-low-quality evidence). Erythromycin (250 mg intravenously [IV]) improves endoscopic visualization and diagnostic accuracy by moving the blood and clot out of the upper GI tract. A meta-analysis showed a reduction of need for repeat endoscopy (odds ratio [OR], 0.51; 95% CI, 0.34-0.77) and length of hospitalization (mean difference, –1.75 d).4
Recommendation 4. There is no consensus for or against pre-endoscopic proton pump inhibitor (PPI) therapy for patients with UGIB, owing to overall limited available data.
Recommendation 5. Patients hospitalized for UGIB should undergo endoscopy within 24 hours of presentation (conditional recommendation, very-low-quality evidence). Performing endoscopy within 24 hours, rather than 12 hours, of presentation demonstrated a potential trend toward decreased length of stay, mortality, and need for surgery. The potential harm in performing earlier endoscopy was attributed to inadequate resuscitation and insufficient optimization of active comorbidities.
Post-endoscopy Management
Recommendation 6. High-dose PPI therapy should be given for 3 days after successful endoscopic hemostatic therapy of a bleeding ulcer (strong recommendation, moderate- to high-quality evidence). When compared with placebo, there is an absolute risk reduction of 3% in mortality and 10% in further bleeding when administering continuous (80 mg bolus with 8 mg/h infusion) or intermittent high-dose PPI therapy (80 mg bolus with 40 mg 2-4 times daily thereafter) for 3 days after endoscopic therapy.5,6 Cost and ease of administration should be considered when choosing between intermittent or continuous PPI therapy. Oral PPI therapy may be appropriate for patients who are able to tolerate oral intake (no nausea, vomiting, dysphagia, or somnolence).
Recommendation 7. High-risk patients (defined as a Rockall score of ≥6 ) with UGIB due to ulcers who received endoscopic hemostatic therapy followed by short-term high-dose PPI therapy in hospital should be continued on twice-daily PPI therapy until 2 weeks after index endoscopy (conditional recommendation, low-quality evidence). A randomized controlled trial of high-risk patients showed significantly lower recurrence of bleeding with twice-daily vs once daily PPI.7 It remains uncertain whether patients benefit from PPI therapy beyond 4 weeks.
Rebleeding Management
Recommendation 8. Patients with recurrent bleeding after endoscopic therapy for a bleeding ulcer should undergo repeat endoscopic therapy rather than surgery or transcatheter arterial embolization (TAE) (conditional recommendation, low-quality evidence for comparison with surgery, very-low-quality evidence for comparison with TAE). In a small randomized controlled trial of repeat endoscopy vs surgery in patients with rebleeding after initial successful endoscopic treatment, there were more subsequent bleeding episodes in the repeat endoscopy group, but no significant difference in mortality and length of stay.8 The repeat endoscopy group had fewer complications, though, and a successful treatment rate of 75%. Because of the lack of high-quality studies in support of TAE and the known safety and efficacy of repeat endoscopy, repeat endoscopy is preferred over TAE for recurrent UGIB.
Recommendation 9. Patients with bleeding ulcers who have failed repeat endoscopic therapy should be treated with TAE (conditional recommendation, very-low-quality evidence). Based on a meta-analysis, when comparing TAE with surgery in patients with UGIB who fail endoscopic therapy, overall mortality was the same, and TAE patients had fewer complications and shorter hospital stays despite having a higher risk of further bleeding.9
CRITIQUE
The guidelines were formulated by panel members with input from the ACG Practice Parameters Committee using the population, intervention, comparator, and outcome (PICO) format to frame each question. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was used to assess the strength of the recommendation and the quality of evidence.
Most of the recommendations are conditional and/or based on low-quality or very-low-quality evidence. Although randomized control trials were sought, observational studies were sometimes included when randomized controlled trials were lacking. The literature review process appeared to focus on the primary outcome of further bleeding, which, although critical in patients with UGIB, could have limited the scope of evidence used in making the recommendations. It was stated that studies identified as relevant to the panel members or authors were considered for review without mentioning any standardized approach. The composition of the panel members was not discussed, and it is uncertain whether the guidelines underwent any formal peer-review process. Furthermore, although competing interests were declared, the panel did not discuss how conflicts were managed and what potential impact they had in the guideline recommendations. Finally, some of the recommendations (eg, TAE) will depend on local expertise and may not be available at all medical centers.
AREAS IN NEED OF FUTURE STUDY
Further study is needed to address the integration of risk-assessment tools into electronic health records to assist with timely decisions on managing patients with acute UGIB, to clarify the role for pre-endoscopic PPI therapy, and to specify fluid resuscitation and blood pressure goals in patients with more severe bleeding episodes and determine whether a subset of patients might benefit from very-early endoscopy (the 2012 ACG guidelines suggested that endoscopy within 12 hours may be considered in patients with high-risk clinical features such as hemodynamic instability or cirrhosis).
Other Resources
Glasgow-Blatchford Score (https://www.mdcalc.com/glasgow-blatchford-bleeding-score-gbs)
Rockall Score (https://www.mdcalc.com/rockall-score-upper-gi-bleeding-pre-endoscopy)
1. Peery AF, Crockett SD, Murphy CC, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology. 2019;156(1):254-272.e11. https://doi.org/10.1053/j.gastro.2018.08.063
2. Stanley AJ, Laine L, Dalton HR, et al. Comparison of risk scoring systems for patients presenting with upper gastrointestinal bleeding: international multicentre prospective study. BMJ. 2017;356:i6432. https://doi.org/10.1136/bmj.i6432
3. Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368(1):11-21. https://doi.org/10.1056/NEJMoa1211801
4. Rahman R, Nguyen DL, Sohail U, et al. Pre-endoscopic erythromycin administration in upper gastrointestinal bleeding: an updated meta analysis and systematic review. Ann Gastroenterol. 2016;29(3):312-317. https://doi.org/10.20524/aog.2016.0045
5. Hung WK, Li VKM, Chung CK, et al. Randomized trial comparing pantoprazole infusion, bolus and no treatment on gastric pH and recurrent bleeding in peptic ulcers. ANZ J Surg. 2007;77(8):677-681. https://doi.org/10.1111/j.1445-2197.2007.04185.x
6. Lau JY, Sung JJ, Lee KK, et al. Effect of intravenous omeprazole on recurrent bleeding after endoscopic treatment of bleeding peptic ulcers. N Engl J Med. 2000;343(5):310-316. https://doi.org/10.1056/NEJM200008033430501
7. Cheng HC, Wu CT, Chang WL, Cheng WC, Chen WY, Sheu BS. Double oral esomeprazole after a 3-day intravenous esomeprazole infusion reduces recurrent peptic ulcer bleeding in high-risk patients: a randomised controlled study. Gut. 2014;63(12):1864-1872. https://doi.org/10.1136/gutjnl-2013-306531
8. Lau JY, Sung JJ, Lam YH, et al. Endoscopic retreatment compared with surgery in patients with recurrent bleeding after initial endoscopic control of bleeding ulcers. N Engl J Med. 1999;340(10):751-756. https://doi.org/10.1056/NEJM199903113401002
9. Tarasconi A, Baiocchi GL, Pattonieri V, et al. Transcatheter arterial embolization versus surgery for refractory non-variceal upper gastrointestinal bleeding: a meta-analysis. World J Emerg Surg. 2019;14:3. https://doi.org/10.1186/s13017-019-0223-8
1. Peery AF, Crockett SD, Murphy CC, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology. 2019;156(1):254-272.e11. https://doi.org/10.1053/j.gastro.2018.08.063
2. Stanley AJ, Laine L, Dalton HR, et al. Comparison of risk scoring systems for patients presenting with upper gastrointestinal bleeding: international multicentre prospective study. BMJ. 2017;356:i6432. https://doi.org/10.1136/bmj.i6432
3. Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368(1):11-21. https://doi.org/10.1056/NEJMoa1211801
4. Rahman R, Nguyen DL, Sohail U, et al. Pre-endoscopic erythromycin administration in upper gastrointestinal bleeding: an updated meta analysis and systematic review. Ann Gastroenterol. 2016;29(3):312-317. https://doi.org/10.20524/aog.2016.0045
5. Hung WK, Li VKM, Chung CK, et al. Randomized trial comparing pantoprazole infusion, bolus and no treatment on gastric pH and recurrent bleeding in peptic ulcers. ANZ J Surg. 2007;77(8):677-681. https://doi.org/10.1111/j.1445-2197.2007.04185.x
6. Lau JY, Sung JJ, Lee KK, et al. Effect of intravenous omeprazole on recurrent bleeding after endoscopic treatment of bleeding peptic ulcers. N Engl J Med. 2000;343(5):310-316. https://doi.org/10.1056/NEJM200008033430501
7. Cheng HC, Wu CT, Chang WL, Cheng WC, Chen WY, Sheu BS. Double oral esomeprazole after a 3-day intravenous esomeprazole infusion reduces recurrent peptic ulcer bleeding in high-risk patients: a randomised controlled study. Gut. 2014;63(12):1864-1872. https://doi.org/10.1136/gutjnl-2013-306531
8. Lau JY, Sung JJ, Lam YH, et al. Endoscopic retreatment compared with surgery in patients with recurrent bleeding after initial endoscopic control of bleeding ulcers. N Engl J Med. 1999;340(10):751-756. https://doi.org/10.1056/NEJM199903113401002
9. Tarasconi A, Baiocchi GL, Pattonieri V, et al. Transcatheter arterial embolization versus surgery for refractory non-variceal upper gastrointestinal bleeding: a meta-analysis. World J Emerg Surg. 2019;14:3. https://doi.org/10.1186/s13017-019-0223-8
© 2021 Society of Hospital Medicine
Utilizing Telesimulation for Advanced Skills Training in Consultation and Handoff Communication: A Post-COVID-19 GME Bootcamp Experience
Events requiring communication among and within teams are vulnerable points in patient care in hospital medicine, with communication failures representing important contributors to adverse events.1-4 Consultations and handoffs are exceptionally common inpatient practices, yet training in these practices is variable across educational and practice domains.5,6 Advanced inpatient communication-skills training requires an effective, feasible, and scalable format. Simulation-based bootcamps can effectively support clinical skills training, often in procedural domains, and have been increasingly utilized for communication skills.7,8 We previously described the development and implementation of an in-person bootcamp for training and feedback in consultation and handoff communication.5,8
As hospitalist leaders grapple with how to systematically support and assess essential clinical skills, the COVID-19 pandemic has presented another impetus to rethink current processes. The rapid shift to virtual activities met immediate needs of the pandemic, but also inspired creativity in applying new methodologies to improve teaching strategies and implementation long-term.9,10 One such strategy, telesimulation, offers a way to continue simulation-based training limited by the need for physical distancing.10 Furthermore, recent calls to study the efficacy of virtual bootcamp structures have acknowledged potential benefits, even outside of the pandemic.11
The primary objective of this feasibility study was to convert our previously described consultation and handoff bootcamp to a telesimulation bootcamp (TBC), preserving rigorous performance evaluation and opportunities for skills-based feedback. We additionally compared evaluation between virtual and in-person formats to understand the utility of telesimulation for bootcamp-based clinical education moving forward.
METHODS
Setting and Participants
The TBC occurred in June 2020 during the University of Chicago institution-wide graduate medical education (GME) orientation; 130 interns entering 13 residency programs participated. The comparison group was 128 interns who underwent the traditional University of Chicago GME orientation “Advanced Communication Skills Bootcamp” (ACSBC) in 2019.5,8
Program Description
To develop TBC, we adapted observed structured clinical experiences (OSCEs) created for ACSBC. Until 2020, ACSBC included three in-person OSCEs: (1) requesting a consultation; (2) conducting handoffs; and (3) acquiring informed consent. COVID-19 necessitated conversion of ACSBC to virtual in June 2020. For this, we selected the consultation and handoff OSCEs, as these skills require near-universal and immediate application in clinical practice. Additionally, they required only trained facilitators (TFs), whereas informed consent required standardized patients. Hospitalist and emergency medicine faculty were recruited as TFs; 7 of 12 TFs were hospitalists. Each OSCE had two parts: an asynchronous, mandatory training module and a clinical simulation. For TBC, we adapted the simulations, previously separate experiences, into a 20-minute combined handoff/consultation telesimulation using the Zoom® video platform. Interns were paired with one TF who served as both standardized consultant (for one mock case) and handoff receiver (for three mock cases, including the consultation case). TFs rated intern performance and provided feedback.
TBC occurred on June 17 and 18, 2020. Interns were emailed asynchronous modules on June 1, and mock cases and instructions on June 12. When TBC began, GME staff proctors oriented interns in the Zoom® platform. Proctors placed TFs into private breakout rooms into which interns rotated through 20-minute timeslots. Faculty received copies of all TBC materials for review (Appendix 1) and underwent Zoom®-based training 1 to 2 weeks prior.
We evaluated TBC using several methods: (1) consultation and handoff skills performance measured by two validated checklists5,8; (2) survey of intern self-reported preparedness to practice consultations and handoffs; and (3) survey of intern satisfaction. Surveys were administered both immediately post bootcamp (Appendix 2) and 8 weeks into internship (Appendix 3). Skills performance checklists were a 12-item consultation checklist5 and 6-item handoff checklist.8 The handoff checklist was modified to remove activities impossible to assess virtually (ie, orienting sign-outs in a shared space) and to add a three-level rating scale of “outstanding,” “satisfactory,” and “needs improvement.” This was done based on feedback from ACSBC to allow more nuanced feedback for interns. A rating of “outstanding” was used to define successful completion of the item (Appendix 1). Interns rated preparedness and satisfaction on 5-point Likert-type items. All measures were compared to the 2019 in-person ACSBC cohort.
Data Analysis
Stata 16.1 (StataCorp LP) was used for analysis. We dichotomized preparedness and satisfaction scores, defining ratings of “4” or “5” as “prepared” or “satisfied.” As previously described,5 we created a composite score averaging both checklist scores for each intern. We normalized this score by rater to a z score (mean, 0; SD, 1) to account for rater differences. “Poor” and “outstanding” performances were defined as z scores below and above 1 SD, respectively. Fisher’s exact test was used to compare proportions, and Pearson correlation test to correlate z scores. The University of Chicago Institutional Review Board granted exemption.
RESULTS
All 130 entering interns participated in TBC. Internal medicine (IM) was the largest specialty (n = 37), followed by pediatrics (n = 22), emergency medicine (EM) (n = 16), and anesthesiology (n = 12). The remaining 9 programs ranged from 2 to 10 interns per program. The 128 interns in ACSBC were similar, including 40 IM, 23 pediatrics, 14 EM, and 12 anesthesia interns, with 2 to 10 interns in remaining programs.
TBC skills performance evaluations were compared to ACSBC (Table 1). The TBC intern cohort’s consultation performance was the same or better than the ACSBC intern cohort’s. For handoffs, TBC interns completed significantly fewer checklist items compared to ACSBC. Performance in each exercise was moderately correlated (r = 0.39, P < .05). For z scores, 14 TBC interns (10.8%) had “outstanding” and 15 (11.6%) had “poor” performances, compared to ACSBC interns with 7 (5.5%) “outstanding” and 10 (7.81%) “poor” performances (P = .15).

All 130 interns (100%) completed the immediate post-TBC survey. Overall, TBC satisfaction was comparable to ACSBC, and significantly improved for satisfaction with performance (Table 2). Compared to ACSBC, TBC interns felt more prepared for simulation and handoff clinical practice. Nearly all interns would recommend TBC (99% vs 96% of ACSBC interns, P = 0.28), and 99% felt the software used for the simulation ran smoothly.
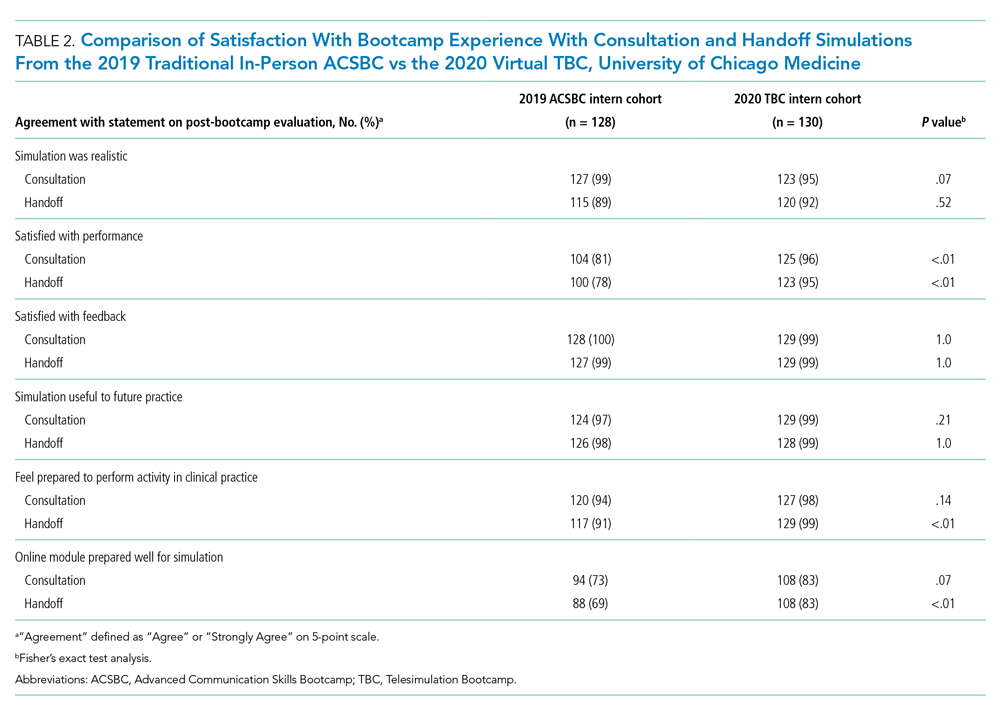
The 8-week post-TBC survey had a response rate of 88% (115/130); 69% of interns reported conducting more effective handoffs due to TBC, and 79% felt confident in handoff skills. Similarly, 73% felt more effective at calling consultations, and 75% reported retained knowledge of consultation frameworks taught during TBC. Additionally, 71% of interns reported that TBC helped identify areas for self-directed improvement. There were no significant differences in 8-week postsurvey ratings between ACSBC and TBC.
DISCUSSION
In converting the advanced communication skills bootcamp from an in-person to a virtual format, telesimulation was well-received by interns and rated similarly to in-person bootcamp in most respects. Nearly all interns agreed the experience was realistic, provided useful feedback, and prepared them for clinical practice. Although we shifted to virtual out of necessity, our results demonstrate a high-quality, streamlined bootcamp experience that was less labor-intensive for interns, staff, and faculty. Telesimulation may represent an effective strategy beyond the COVID-19 pandemic to increase ease of administration and scale the use of bootcamps in supporting advanced clinical skill training for hospital-based practice.
TBC interns felt better prepared for simulation and more satisfied with their performance than ACSBC interns, potentially due to the revised format. The mock cases were adapted and consolidated for TBC, such that the handoff and consultation simulations shared a common case, whereas previously they were separate. Thus, intern preparation for TBC required familiarity with fewer overall cases. Ultimately, TBC maintained the quality of training but required review of less information.
In comparing performance, TBC interns were rated as well or better during consultation simulation compared to ASCBC, but handoffs were rated lower. This was likely due to the change in the handoff checklist from a dichotomous to a three-level rating scale. This change was made after receiving feedback from ACSBC TFs that a rating scale allowing for more nuance was needed to provide adequate feedback to interns. Although we defined handoff item completion for TBC interns as being rated “outstanding,” if the top two rankings, “outstanding” and “satisfactory,” are dichotomized to reflect completion, TBC handoff performance is equivalent or better than ACSBC. TF recruitment additionally differed between TBC and ACSBC cohorts. In ACSBC, resident physicians served as handoff TFs, whereas only faculty were recruited for TBC. Faculty were primarily clinically active hospitalists, whose expertise in handoffs may resulted in more stringent performance ratings, contributing to differences seen.
Hospitalist groups require clinicians to be immediately proficient in essential communication skills like consultation and handoffs, potentially requiring just-in-time training and feedback for large cohorts.12 Bootcamps can meet this need but require participation and time investment by many faculty members, staff, and administrators.5,8 Combining TBC into one virtual handoff/consultation simulation required recruitment and training of 50% fewer TFs and reduced administrative burden. ACSBC consultation simulations were high-fidelity but resource-heavy, requiring reliable two-way telephones with reliable connections and separate spaces for simulation and feedback.5 Conversely, TBC only required consultations to be “called” via audio-only Zoom® discussion, then both individuals turned on cameras for feedback. The slight decrease in perceived fidelity was certainly outweighed by ease of administration. TBC’s more efficient and less labor-intensive format is an appealing strategy for hospitalist groups looking to train up clinicians, including those operating across multiple or geographically distant sites.
Our study has limitations. It occurred with one group of learners at a single site with consistent consultation and handoff communication practices, which may not be the case elsewhere. Our comparison group was a separate cohort, and groups were not randomized; thus, differences seen may reflect inherent dissimilarities in these groups. Changes to the handoff checklist rating scale between 2019 and 2020 additionally may limit the direct comparison of handoff performance between cohorts. While overall fewer resources were required, TBC implementation did require time and institutional support, along with full virtual platform capability without user or time limitations. Our preparedness outcomes were self-reported without direct measurement of clinical performance, which is an area for future work.
We describe a feasible implementation of an adapted telesimulation communication bootcamp, with comparison to a previous in-person cohort’s skills performance and satisfaction. While COVID-19 has made the future of in-person training activities uncertain, it also served as a catalyst for educational innovation that may be sustained beyond the pandemic. Although developed out of necessity, the telesimulation communication bootcamp was effective and well-received. Telesimulation represents an opportunity for hospital medicine groups to implement advanced communication skills training and assessment in a more efficient, flexible, and potentially preferable way, even after the pandemic ends.
Acknowledgments
The authors thank the staff at the University of Chicago Office of Graduate Medical Education and the UChicago Medicine Simulation Center.
1. Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186-194. https://doi.org/ 10.1097/00001888-200402000-00019
2. Inadequate hand-off communication. Sentinel Event Alert. 2017;(58):1-6.
3. Horwitz LI, Meredith T, Schuur JD, Shah NR, Kulkarni RG, Jenq JY. Dropping the baton: a qualitative analysis of failures during the transition from emergency department to inpatient care. Ann Emerg Med. 2009;53(6):701-710. https://doi.org/ 10.1016/j.annemergmed.2008.05.007
4. Jagsi R, Kitch BT, Weinstein DF, Campbell EG, Hutter M, Weissman JS. Residents report on adverse events and their causes. Arch Intern Med. 2005;165(22):2607-2613. https://doi.org/10.1001/archinte.165.22.2607
5. Martin SK, Carter K, Hellerman N, et al. The consultation observed simulated clinical experience: training, assessment, and feedback for incoming interns on requesting consultations. Acad Med. 2018; 93(12):1814-1820. https://doi.org/10.1097/ACM.0000000000002337
6. Lopez MA, Campbell J. Developing a communication curriculum for primary and consulting services. Med Educ Online. 2020;25(1):1794341. https://doi.org/10.1080/10872981.2020
7. Cohen, ER, Barsuk JH, Moazed F, et al. Making July safer: simulation-based mastery learning during intern bootcamp. Acad Med. 2013;88(2):233-239. https://doi.org/10.1097/ACM.0b013e31827bfc0a
8. Gaffney S, Farnan JM, Hirsch K, McGinty M, Arora VM. The Modified, Multi-patient Observed Simulated Handoff Experience (M-OSHE): assessment and feedback for entering residents on handoff performance. J Gen Intern Med. 2016;31(4):438-441. https://doi.org/10.1007/s11606-016-3591-8.
9. Woolliscroft, J. Innovation in response to the COVID-19 pandemic crisis. Acad Med. 2020;95(8):1140-1142. https://doi.org/10.1097/ACM.0000000000003402.
10. Anderson ML, Turbow S, Willgerodt MA, Ruhnke G. Education in a crisis: the opportunity of our lives. J Hosp. Med 2020;5;287-291. https://doi.org/10.12788/jhm.3431
11. Farr DE, Zeh HJ, Abdelfattah KR. Virtual bootcamps—an emerging solution to the undergraduate medical education-graduate medical education transition. JAMA Surg. 2021;156(3):282-283. https://doi.org/10.1001/jamasurg.2020.6162
12. Hepps JH, Yu CE, Calaman S. Simulation in medical education for the hospitalist: moving beyond the mock code. Pediatr Clin North Am. 2019;66(4):855-866. https://doi.org/10.1016/j.pcl.2019.03.014
Events requiring communication among and within teams are vulnerable points in patient care in hospital medicine, with communication failures representing important contributors to adverse events.1-4 Consultations and handoffs are exceptionally common inpatient practices, yet training in these practices is variable across educational and practice domains.5,6 Advanced inpatient communication-skills training requires an effective, feasible, and scalable format. Simulation-based bootcamps can effectively support clinical skills training, often in procedural domains, and have been increasingly utilized for communication skills.7,8 We previously described the development and implementation of an in-person bootcamp for training and feedback in consultation and handoff communication.5,8
As hospitalist leaders grapple with how to systematically support and assess essential clinical skills, the COVID-19 pandemic has presented another impetus to rethink current processes. The rapid shift to virtual activities met immediate needs of the pandemic, but also inspired creativity in applying new methodologies to improve teaching strategies and implementation long-term.9,10 One such strategy, telesimulation, offers a way to continue simulation-based training limited by the need for physical distancing.10 Furthermore, recent calls to study the efficacy of virtual bootcamp structures have acknowledged potential benefits, even outside of the pandemic.11
The primary objective of this feasibility study was to convert our previously described consultation and handoff bootcamp to a telesimulation bootcamp (TBC), preserving rigorous performance evaluation and opportunities for skills-based feedback. We additionally compared evaluation between virtual and in-person formats to understand the utility of telesimulation for bootcamp-based clinical education moving forward.
METHODS
Setting and Participants
The TBC occurred in June 2020 during the University of Chicago institution-wide graduate medical education (GME) orientation; 130 interns entering 13 residency programs participated. The comparison group was 128 interns who underwent the traditional University of Chicago GME orientation “Advanced Communication Skills Bootcamp” (ACSBC) in 2019.5,8
Program Description
To develop TBC, we adapted observed structured clinical experiences (OSCEs) created for ACSBC. Until 2020, ACSBC included three in-person OSCEs: (1) requesting a consultation; (2) conducting handoffs; and (3) acquiring informed consent. COVID-19 necessitated conversion of ACSBC to virtual in June 2020. For this, we selected the consultation and handoff OSCEs, as these skills require near-universal and immediate application in clinical practice. Additionally, they required only trained facilitators (TFs), whereas informed consent required standardized patients. Hospitalist and emergency medicine faculty were recruited as TFs; 7 of 12 TFs were hospitalists. Each OSCE had two parts: an asynchronous, mandatory training module and a clinical simulation. For TBC, we adapted the simulations, previously separate experiences, into a 20-minute combined handoff/consultation telesimulation using the Zoom® video platform. Interns were paired with one TF who served as both standardized consultant (for one mock case) and handoff receiver (for three mock cases, including the consultation case). TFs rated intern performance and provided feedback.
TBC occurred on June 17 and 18, 2020. Interns were emailed asynchronous modules on June 1, and mock cases and instructions on June 12. When TBC began, GME staff proctors oriented interns in the Zoom® platform. Proctors placed TFs into private breakout rooms into which interns rotated through 20-minute timeslots. Faculty received copies of all TBC materials for review (Appendix 1) and underwent Zoom®-based training 1 to 2 weeks prior.
We evaluated TBC using several methods: (1) consultation and handoff skills performance measured by two validated checklists5,8; (2) survey of intern self-reported preparedness to practice consultations and handoffs; and (3) survey of intern satisfaction. Surveys were administered both immediately post bootcamp (Appendix 2) and 8 weeks into internship (Appendix 3). Skills performance checklists were a 12-item consultation checklist5 and 6-item handoff checklist.8 The handoff checklist was modified to remove activities impossible to assess virtually (ie, orienting sign-outs in a shared space) and to add a three-level rating scale of “outstanding,” “satisfactory,” and “needs improvement.” This was done based on feedback from ACSBC to allow more nuanced feedback for interns. A rating of “outstanding” was used to define successful completion of the item (Appendix 1). Interns rated preparedness and satisfaction on 5-point Likert-type items. All measures were compared to the 2019 in-person ACSBC cohort.
Data Analysis
Stata 16.1 (StataCorp LP) was used for analysis. We dichotomized preparedness and satisfaction scores, defining ratings of “4” or “5” as “prepared” or “satisfied.” As previously described,5 we created a composite score averaging both checklist scores for each intern. We normalized this score by rater to a z score (mean, 0; SD, 1) to account for rater differences. “Poor” and “outstanding” performances were defined as z scores below and above 1 SD, respectively. Fisher’s exact test was used to compare proportions, and Pearson correlation test to correlate z scores. The University of Chicago Institutional Review Board granted exemption.
RESULTS
All 130 entering interns participated in TBC. Internal medicine (IM) was the largest specialty (n = 37), followed by pediatrics (n = 22), emergency medicine (EM) (n = 16), and anesthesiology (n = 12). The remaining 9 programs ranged from 2 to 10 interns per program. The 128 interns in ACSBC were similar, including 40 IM, 23 pediatrics, 14 EM, and 12 anesthesia interns, with 2 to 10 interns in remaining programs.
TBC skills performance evaluations were compared to ACSBC (Table 1). The TBC intern cohort’s consultation performance was the same or better than the ACSBC intern cohort’s. For handoffs, TBC interns completed significantly fewer checklist items compared to ACSBC. Performance in each exercise was moderately correlated (r = 0.39, P < .05). For z scores, 14 TBC interns (10.8%) had “outstanding” and 15 (11.6%) had “poor” performances, compared to ACSBC interns with 7 (5.5%) “outstanding” and 10 (7.81%) “poor” performances (P = .15).

All 130 interns (100%) completed the immediate post-TBC survey. Overall, TBC satisfaction was comparable to ACSBC, and significantly improved for satisfaction with performance (Table 2). Compared to ACSBC, TBC interns felt more prepared for simulation and handoff clinical practice. Nearly all interns would recommend TBC (99% vs 96% of ACSBC interns, P = 0.28), and 99% felt the software used for the simulation ran smoothly.

The 8-week post-TBC survey had a response rate of 88% (115/130); 69% of interns reported conducting more effective handoffs due to TBC, and 79% felt confident in handoff skills. Similarly, 73% felt more effective at calling consultations, and 75% reported retained knowledge of consultation frameworks taught during TBC. Additionally, 71% of interns reported that TBC helped identify areas for self-directed improvement. There were no significant differences in 8-week postsurvey ratings between ACSBC and TBC.
DISCUSSION
In converting the advanced communication skills bootcamp from an in-person to a virtual format, telesimulation was well-received by interns and rated similarly to in-person bootcamp in most respects. Nearly all interns agreed the experience was realistic, provided useful feedback, and prepared them for clinical practice. Although we shifted to virtual out of necessity, our results demonstrate a high-quality, streamlined bootcamp experience that was less labor-intensive for interns, staff, and faculty. Telesimulation may represent an effective strategy beyond the COVID-19 pandemic to increase ease of administration and scale the use of bootcamps in supporting advanced clinical skill training for hospital-based practice.
TBC interns felt better prepared for simulation and more satisfied with their performance than ACSBC interns, potentially due to the revised format. The mock cases were adapted and consolidated for TBC, such that the handoff and consultation simulations shared a common case, whereas previously they were separate. Thus, intern preparation for TBC required familiarity with fewer overall cases. Ultimately, TBC maintained the quality of training but required review of less information.
In comparing performance, TBC interns were rated as well or better during consultation simulation compared to ASCBC, but handoffs were rated lower. This was likely due to the change in the handoff checklist from a dichotomous to a three-level rating scale. This change was made after receiving feedback from ACSBC TFs that a rating scale allowing for more nuance was needed to provide adequate feedback to interns. Although we defined handoff item completion for TBC interns as being rated “outstanding,” if the top two rankings, “outstanding” and “satisfactory,” are dichotomized to reflect completion, TBC handoff performance is equivalent or better than ACSBC. TF recruitment additionally differed between TBC and ACSBC cohorts. In ACSBC, resident physicians served as handoff TFs, whereas only faculty were recruited for TBC. Faculty were primarily clinically active hospitalists, whose expertise in handoffs may resulted in more stringent performance ratings, contributing to differences seen.
Hospitalist groups require clinicians to be immediately proficient in essential communication skills like consultation and handoffs, potentially requiring just-in-time training and feedback for large cohorts.12 Bootcamps can meet this need but require participation and time investment by many faculty members, staff, and administrators.5,8 Combining TBC into one virtual handoff/consultation simulation required recruitment and training of 50% fewer TFs and reduced administrative burden. ACSBC consultation simulations were high-fidelity but resource-heavy, requiring reliable two-way telephones with reliable connections and separate spaces for simulation and feedback.5 Conversely, TBC only required consultations to be “called” via audio-only Zoom® discussion, then both individuals turned on cameras for feedback. The slight decrease in perceived fidelity was certainly outweighed by ease of administration. TBC’s more efficient and less labor-intensive format is an appealing strategy for hospitalist groups looking to train up clinicians, including those operating across multiple or geographically distant sites.
Our study has limitations. It occurred with one group of learners at a single site with consistent consultation and handoff communication practices, which may not be the case elsewhere. Our comparison group was a separate cohort, and groups were not randomized; thus, differences seen may reflect inherent dissimilarities in these groups. Changes to the handoff checklist rating scale between 2019 and 2020 additionally may limit the direct comparison of handoff performance between cohorts. While overall fewer resources were required, TBC implementation did require time and institutional support, along with full virtual platform capability without user or time limitations. Our preparedness outcomes were self-reported without direct measurement of clinical performance, which is an area for future work.
We describe a feasible implementation of an adapted telesimulation communication bootcamp, with comparison to a previous in-person cohort’s skills performance and satisfaction. While COVID-19 has made the future of in-person training activities uncertain, it also served as a catalyst for educational innovation that may be sustained beyond the pandemic. Although developed out of necessity, the telesimulation communication bootcamp was effective and well-received. Telesimulation represents an opportunity for hospital medicine groups to implement advanced communication skills training and assessment in a more efficient, flexible, and potentially preferable way, even after the pandemic ends.
Acknowledgments
The authors thank the staff at the University of Chicago Office of Graduate Medical Education and the UChicago Medicine Simulation Center.
Events requiring communication among and within teams are vulnerable points in patient care in hospital medicine, with communication failures representing important contributors to adverse events.1-4 Consultations and handoffs are exceptionally common inpatient practices, yet training in these practices is variable across educational and practice domains.5,6 Advanced inpatient communication-skills training requires an effective, feasible, and scalable format. Simulation-based bootcamps can effectively support clinical skills training, often in procedural domains, and have been increasingly utilized for communication skills.7,8 We previously described the development and implementation of an in-person bootcamp for training and feedback in consultation and handoff communication.5,8
As hospitalist leaders grapple with how to systematically support and assess essential clinical skills, the COVID-19 pandemic has presented another impetus to rethink current processes. The rapid shift to virtual activities met immediate needs of the pandemic, but also inspired creativity in applying new methodologies to improve teaching strategies and implementation long-term.9,10 One such strategy, telesimulation, offers a way to continue simulation-based training limited by the need for physical distancing.10 Furthermore, recent calls to study the efficacy of virtual bootcamp structures have acknowledged potential benefits, even outside of the pandemic.11
The primary objective of this feasibility study was to convert our previously described consultation and handoff bootcamp to a telesimulation bootcamp (TBC), preserving rigorous performance evaluation and opportunities for skills-based feedback. We additionally compared evaluation between virtual and in-person formats to understand the utility of telesimulation for bootcamp-based clinical education moving forward.
METHODS
Setting and Participants
The TBC occurred in June 2020 during the University of Chicago institution-wide graduate medical education (GME) orientation; 130 interns entering 13 residency programs participated. The comparison group was 128 interns who underwent the traditional University of Chicago GME orientation “Advanced Communication Skills Bootcamp” (ACSBC) in 2019.5,8
Program Description
To develop TBC, we adapted observed structured clinical experiences (OSCEs) created for ACSBC. Until 2020, ACSBC included three in-person OSCEs: (1) requesting a consultation; (2) conducting handoffs; and (3) acquiring informed consent. COVID-19 necessitated conversion of ACSBC to virtual in June 2020. For this, we selected the consultation and handoff OSCEs, as these skills require near-universal and immediate application in clinical practice. Additionally, they required only trained facilitators (TFs), whereas informed consent required standardized patients. Hospitalist and emergency medicine faculty were recruited as TFs; 7 of 12 TFs were hospitalists. Each OSCE had two parts: an asynchronous, mandatory training module and a clinical simulation. For TBC, we adapted the simulations, previously separate experiences, into a 20-minute combined handoff/consultation telesimulation using the Zoom® video platform. Interns were paired with one TF who served as both standardized consultant (for one mock case) and handoff receiver (for three mock cases, including the consultation case). TFs rated intern performance and provided feedback.
TBC occurred on June 17 and 18, 2020. Interns were emailed asynchronous modules on June 1, and mock cases and instructions on June 12. When TBC began, GME staff proctors oriented interns in the Zoom® platform. Proctors placed TFs into private breakout rooms into which interns rotated through 20-minute timeslots. Faculty received copies of all TBC materials for review (Appendix 1) and underwent Zoom®-based training 1 to 2 weeks prior.
We evaluated TBC using several methods: (1) consultation and handoff skills performance measured by two validated checklists5,8; (2) survey of intern self-reported preparedness to practice consultations and handoffs; and (3) survey of intern satisfaction. Surveys were administered both immediately post bootcamp (Appendix 2) and 8 weeks into internship (Appendix 3). Skills performance checklists were a 12-item consultation checklist5 and 6-item handoff checklist.8 The handoff checklist was modified to remove activities impossible to assess virtually (ie, orienting sign-outs in a shared space) and to add a three-level rating scale of “outstanding,” “satisfactory,” and “needs improvement.” This was done based on feedback from ACSBC to allow more nuanced feedback for interns. A rating of “outstanding” was used to define successful completion of the item (Appendix 1). Interns rated preparedness and satisfaction on 5-point Likert-type items. All measures were compared to the 2019 in-person ACSBC cohort.
Data Analysis
Stata 16.1 (StataCorp LP) was used for analysis. We dichotomized preparedness and satisfaction scores, defining ratings of “4” or “5” as “prepared” or “satisfied.” As previously described,5 we created a composite score averaging both checklist scores for each intern. We normalized this score by rater to a z score (mean, 0; SD, 1) to account for rater differences. “Poor” and “outstanding” performances were defined as z scores below and above 1 SD, respectively. Fisher’s exact test was used to compare proportions, and Pearson correlation test to correlate z scores. The University of Chicago Institutional Review Board granted exemption.
RESULTS
All 130 entering interns participated in TBC. Internal medicine (IM) was the largest specialty (n = 37), followed by pediatrics (n = 22), emergency medicine (EM) (n = 16), and anesthesiology (n = 12). The remaining 9 programs ranged from 2 to 10 interns per program. The 128 interns in ACSBC were similar, including 40 IM, 23 pediatrics, 14 EM, and 12 anesthesia interns, with 2 to 10 interns in remaining programs.
TBC skills performance evaluations were compared to ACSBC (Table 1). The TBC intern cohort’s consultation performance was the same or better than the ACSBC intern cohort’s. For handoffs, TBC interns completed significantly fewer checklist items compared to ACSBC. Performance in each exercise was moderately correlated (r = 0.39, P < .05). For z scores, 14 TBC interns (10.8%) had “outstanding” and 15 (11.6%) had “poor” performances, compared to ACSBC interns with 7 (5.5%) “outstanding” and 10 (7.81%) “poor” performances (P = .15).

All 130 interns (100%) completed the immediate post-TBC survey. Overall, TBC satisfaction was comparable to ACSBC, and significantly improved for satisfaction with performance (Table 2). Compared to ACSBC, TBC interns felt more prepared for simulation and handoff clinical practice. Nearly all interns would recommend TBC (99% vs 96% of ACSBC interns, P = 0.28), and 99% felt the software used for the simulation ran smoothly.

The 8-week post-TBC survey had a response rate of 88% (115/130); 69% of interns reported conducting more effective handoffs due to TBC, and 79% felt confident in handoff skills. Similarly, 73% felt more effective at calling consultations, and 75% reported retained knowledge of consultation frameworks taught during TBC. Additionally, 71% of interns reported that TBC helped identify areas for self-directed improvement. There were no significant differences in 8-week postsurvey ratings between ACSBC and TBC.
DISCUSSION
In converting the advanced communication skills bootcamp from an in-person to a virtual format, telesimulation was well-received by interns and rated similarly to in-person bootcamp in most respects. Nearly all interns agreed the experience was realistic, provided useful feedback, and prepared them for clinical practice. Although we shifted to virtual out of necessity, our results demonstrate a high-quality, streamlined bootcamp experience that was less labor-intensive for interns, staff, and faculty. Telesimulation may represent an effective strategy beyond the COVID-19 pandemic to increase ease of administration and scale the use of bootcamps in supporting advanced clinical skill training for hospital-based practice.
TBC interns felt better prepared for simulation and more satisfied with their performance than ACSBC interns, potentially due to the revised format. The mock cases were adapted and consolidated for TBC, such that the handoff and consultation simulations shared a common case, whereas previously they were separate. Thus, intern preparation for TBC required familiarity with fewer overall cases. Ultimately, TBC maintained the quality of training but required review of less information.
In comparing performance, TBC interns were rated as well or better during consultation simulation compared to ASCBC, but handoffs were rated lower. This was likely due to the change in the handoff checklist from a dichotomous to a three-level rating scale. This change was made after receiving feedback from ACSBC TFs that a rating scale allowing for more nuance was needed to provide adequate feedback to interns. Although we defined handoff item completion for TBC interns as being rated “outstanding,” if the top two rankings, “outstanding” and “satisfactory,” are dichotomized to reflect completion, TBC handoff performance is equivalent or better than ACSBC. TF recruitment additionally differed between TBC and ACSBC cohorts. In ACSBC, resident physicians served as handoff TFs, whereas only faculty were recruited for TBC. Faculty were primarily clinically active hospitalists, whose expertise in handoffs may resulted in more stringent performance ratings, contributing to differences seen.
Hospitalist groups require clinicians to be immediately proficient in essential communication skills like consultation and handoffs, potentially requiring just-in-time training and feedback for large cohorts.12 Bootcamps can meet this need but require participation and time investment by many faculty members, staff, and administrators.5,8 Combining TBC into one virtual handoff/consultation simulation required recruitment and training of 50% fewer TFs and reduced administrative burden. ACSBC consultation simulations were high-fidelity but resource-heavy, requiring reliable two-way telephones with reliable connections and separate spaces for simulation and feedback.5 Conversely, TBC only required consultations to be “called” via audio-only Zoom® discussion, then both individuals turned on cameras for feedback. The slight decrease in perceived fidelity was certainly outweighed by ease of administration. TBC’s more efficient and less labor-intensive format is an appealing strategy for hospitalist groups looking to train up clinicians, including those operating across multiple or geographically distant sites.
Our study has limitations. It occurred with one group of learners at a single site with consistent consultation and handoff communication practices, which may not be the case elsewhere. Our comparison group was a separate cohort, and groups were not randomized; thus, differences seen may reflect inherent dissimilarities in these groups. Changes to the handoff checklist rating scale between 2019 and 2020 additionally may limit the direct comparison of handoff performance between cohorts. While overall fewer resources were required, TBC implementation did require time and institutional support, along with full virtual platform capability without user or time limitations. Our preparedness outcomes were self-reported without direct measurement of clinical performance, which is an area for future work.
We describe a feasible implementation of an adapted telesimulation communication bootcamp, with comparison to a previous in-person cohort’s skills performance and satisfaction. While COVID-19 has made the future of in-person training activities uncertain, it also served as a catalyst for educational innovation that may be sustained beyond the pandemic. Although developed out of necessity, the telesimulation communication bootcamp was effective and well-received. Telesimulation represents an opportunity for hospital medicine groups to implement advanced communication skills training and assessment in a more efficient, flexible, and potentially preferable way, even after the pandemic ends.
Acknowledgments
The authors thank the staff at the University of Chicago Office of Graduate Medical Education and the UChicago Medicine Simulation Center.
1. Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186-194. https://doi.org/ 10.1097/00001888-200402000-00019
2. Inadequate hand-off communication. Sentinel Event Alert. 2017;(58):1-6.
3. Horwitz LI, Meredith T, Schuur JD, Shah NR, Kulkarni RG, Jenq JY. Dropping the baton: a qualitative analysis of failures during the transition from emergency department to inpatient care. Ann Emerg Med. 2009;53(6):701-710. https://doi.org/ 10.1016/j.annemergmed.2008.05.007
4. Jagsi R, Kitch BT, Weinstein DF, Campbell EG, Hutter M, Weissman JS. Residents report on adverse events and their causes. Arch Intern Med. 2005;165(22):2607-2613. https://doi.org/10.1001/archinte.165.22.2607
5. Martin SK, Carter K, Hellerman N, et al. The consultation observed simulated clinical experience: training, assessment, and feedback for incoming interns on requesting consultations. Acad Med. 2018; 93(12):1814-1820. https://doi.org/10.1097/ACM.0000000000002337
6. Lopez MA, Campbell J. Developing a communication curriculum for primary and consulting services. Med Educ Online. 2020;25(1):1794341. https://doi.org/10.1080/10872981.2020
7. Cohen, ER, Barsuk JH, Moazed F, et al. Making July safer: simulation-based mastery learning during intern bootcamp. Acad Med. 2013;88(2):233-239. https://doi.org/10.1097/ACM.0b013e31827bfc0a
8. Gaffney S, Farnan JM, Hirsch K, McGinty M, Arora VM. The Modified, Multi-patient Observed Simulated Handoff Experience (M-OSHE): assessment and feedback for entering residents on handoff performance. J Gen Intern Med. 2016;31(4):438-441. https://doi.org/10.1007/s11606-016-3591-8.
9. Woolliscroft, J. Innovation in response to the COVID-19 pandemic crisis. Acad Med. 2020;95(8):1140-1142. https://doi.org/10.1097/ACM.0000000000003402.
10. Anderson ML, Turbow S, Willgerodt MA, Ruhnke G. Education in a crisis: the opportunity of our lives. J Hosp. Med 2020;5;287-291. https://doi.org/10.12788/jhm.3431
11. Farr DE, Zeh HJ, Abdelfattah KR. Virtual bootcamps—an emerging solution to the undergraduate medical education-graduate medical education transition. JAMA Surg. 2021;156(3):282-283. https://doi.org/10.1001/jamasurg.2020.6162
12. Hepps JH, Yu CE, Calaman S. Simulation in medical education for the hospitalist: moving beyond the mock code. Pediatr Clin North Am. 2019;66(4):855-866. https://doi.org/10.1016/j.pcl.2019.03.014
1. Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186-194. https://doi.org/ 10.1097/00001888-200402000-00019
2. Inadequate hand-off communication. Sentinel Event Alert. 2017;(58):1-6.
3. Horwitz LI, Meredith T, Schuur JD, Shah NR, Kulkarni RG, Jenq JY. Dropping the baton: a qualitative analysis of failures during the transition from emergency department to inpatient care. Ann Emerg Med. 2009;53(6):701-710. https://doi.org/ 10.1016/j.annemergmed.2008.05.007
4. Jagsi R, Kitch BT, Weinstein DF, Campbell EG, Hutter M, Weissman JS. Residents report on adverse events and their causes. Arch Intern Med. 2005;165(22):2607-2613. https://doi.org/10.1001/archinte.165.22.2607
5. Martin SK, Carter K, Hellerman N, et al. The consultation observed simulated clinical experience: training, assessment, and feedback for incoming interns on requesting consultations. Acad Med. 2018; 93(12):1814-1820. https://doi.org/10.1097/ACM.0000000000002337
6. Lopez MA, Campbell J. Developing a communication curriculum for primary and consulting services. Med Educ Online. 2020;25(1):1794341. https://doi.org/10.1080/10872981.2020
7. Cohen, ER, Barsuk JH, Moazed F, et al. Making July safer: simulation-based mastery learning during intern bootcamp. Acad Med. 2013;88(2):233-239. https://doi.org/10.1097/ACM.0b013e31827bfc0a
8. Gaffney S, Farnan JM, Hirsch K, McGinty M, Arora VM. The Modified, Multi-patient Observed Simulated Handoff Experience (M-OSHE): assessment and feedback for entering residents on handoff performance. J Gen Intern Med. 2016;31(4):438-441. https://doi.org/10.1007/s11606-016-3591-8.
9. Woolliscroft, J. Innovation in response to the COVID-19 pandemic crisis. Acad Med. 2020;95(8):1140-1142. https://doi.org/10.1097/ACM.0000000000003402.
10. Anderson ML, Turbow S, Willgerodt MA, Ruhnke G. Education in a crisis: the opportunity of our lives. J Hosp. Med 2020;5;287-291. https://doi.org/10.12788/jhm.3431
11. Farr DE, Zeh HJ, Abdelfattah KR. Virtual bootcamps—an emerging solution to the undergraduate medical education-graduate medical education transition. JAMA Surg. 2021;156(3):282-283. https://doi.org/10.1001/jamasurg.2020.6162
12. Hepps JH, Yu CE, Calaman S. Simulation in medical education for the hospitalist: moving beyond the mock code. Pediatr Clin North Am. 2019;66(4):855-866. https://doi.org/10.1016/j.pcl.2019.03.014
© 2021 Society of Hospital Medicine
The Alarm Burden of Excess Continuous Pulse Oximetry Monitoring Among Patients With Bronchiolitis
Practice guidelines discourage continuous pulse oximetry (SpO2) monitoring of patients with bronchiolitis who are not receiving supplemental oxygen.1,2 Overuse of SpO2 monitoring in this patient population has been associated with increased length of stay, unnecessary oxygen therapy, and excess hospital costs, without measurable patient benefit.3-5 In spite of this evidence base and expert guidance, nearly half of the more than 100,000 infants admitted for bronchiolitis each year receive excess SpO2 monitoring.6,7
Bronchiolitis guidelines suggest that guideline-discordant SpO2 monitoring may result in excess alarms, which disrupt families’ sleep and engender alarm fatigue among staff.1 Pediatric nurses receive up to 155 alarms per monitored patient per day.8,9 Frequent alarms are associated with slower nurse response times10,11 and increased nurse subjective workload.12
Methods
Cohort
We retrospectively evaluated SpO2 monitoring patterns and alarm rates of children 0 to 24 months old admitted to a general pediatrics service at a tertiary care children’s hospital. We included patients who had a discharge diagnosis of bronchiolitis (International Classification of Diseases, Tenth Revision codes J45x, T17.2x, T17.3x, T17.4x, T17.5x, T17.8x, T17.9x, A37xx, J04x, J05x, J05.1x, J69.0x, J69.1x, J69.8x) between November 24, 2019, and January 21, 2020, the period of time during which alarm data and monitor data were concurrently available for analysis. In order to conservatively assure applicability of clinical practice guidelines, we excluded patients with discharge diagnoses that included other respiratory conditions (eg, reactive airway disease), patients with complex chronic conditions (CCC) as defined by the CCC version 2 classification system,13 and patients with intensive care unit (ICU) stays during the admission.
Time
Flowsheet data detailing nursing respiratory assessments were extracted from the electronic health record (EHR) database (Clarity, Epic Systems). Using previously validated methodology,14 we identified minutes during which patients received supplemental oxygen or high-flow nasal cannula (supplemental oxygen) based on the documented fraction of inspired oxygen (FiO2), flow rate, and support devices. We then identified the final discontinuation of respiratory support during the hospital admission, and censored the 60 minutes after final discontinuation of supplemental oxygen based upon recent monitoring guidelines.2 Minutes up to an hour after supplemental oxygen discontinuation were classified as receiving supplemental oxygen and not included in our analysis. Only minutes between the end of the censored period and hospital discharge were used in the analysis. For patients who never received respiratory support during the admission, we censored the first 60 minutes of the admission and analyzed the remainder of their stay.
SpO2 Monitoring
We used device-integrated, physiologic-monitor, vital sign data sent each minute from the General Electric monitor network to the EHR to identify minutes during which patients were connected to physiologic monitors and transmitting signals from SpO2 sensors. We extracted minute-level SpO2 data from the hospital clinical data warehouse (CDW). Minutes in which SpO2 data were present were classified as “monitored,” an approach previously validated using in-person observation.14
To categorize time as “not receiving supplemental oxygen and continuously monitored (guideline-discordant monitoring),” or “not receiving supplemental oxygen and not continuously monitored (guideline-concordant intermittent measurement),” we evaluated the percent of minutes within an hour during which the patient received SpO2 monitoring and applied an a priori conservative rule. Hours during which ≥90% of minutes had SpO2 monitoring data were classified as “continuously monitored.” Hours during which ≤10% of minutes had SpO2 monitoring data were classified as “intermittently measured.” Hours during which 11% to 89% of minutes included monitor data were excluded from further analysis. The number of continuously monitored hours was tabulated for each patient. The median number of continuously monitored hours was computed; results were stratified by prior receipt of respiratory support.
Alarm Counts
Minute-level monitor alarm counts (the absolute number of abnormal vital signs that triggered a monitor to alarm) were extracted from the CDW. Alarm counts were tabulated for each patient hour. For each patient, the alarm rate (total number of alarms divided by time) was computed for continuously monitored and intermittently measured time. Results were stratified by prior receipt of respiratory support.
The study was reviewed by the institutional review board and determined to meet exemption criteria.
Results
Our cohort included 201 admissions by 197 unique patients (Table). We evaluated 4402 hours that occurred ≥60 minutes following final discontinuation of supplemental oxygen, the time period during which guidelines discourage routine use of continuous SpO2 monitoring. This represented a median of 19 hours (interquartile range [IQR], 14-25) per admission. We excluded 474 hours (11%) that could not be classified as either continuously or intermittently measured.
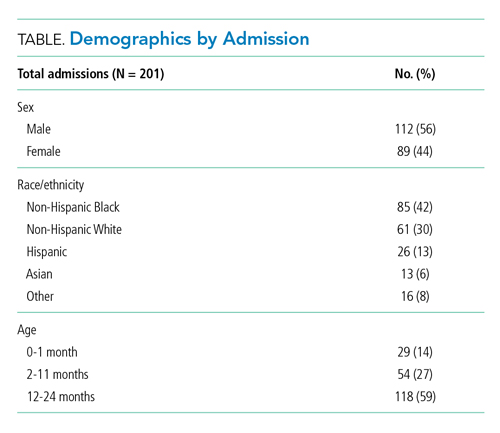
During time ≥60 minutes following discontinuation of supplemental oxygen, our cohort experienced 1537 hours of guideline-discordant continuous monitoring, a median of 6 hours (IQR, 3-12) per admission. Patients experienced a median of 12 hours (IQR, 5-17) of intermittent measurement. Among patients who received supplemental oxygen, 91% experienced guideline-discordant continuous SpO2 monitoring, as compared to 68% of patients who did not receive supplemental oxygen. Among those who received guideline-discordant continuous SpO2 monitoring, the duration of this monitoring did not differ significantly between those who had received supplemental oxygen during the admission and those who had not.
During classifiable time ≥60 minutes following discontinuation of supplemental oxygen, our cohort experienced 14,371 alarms; 77% (11,101) of these alarms were generated during periods of guideline-discordant continuous monitoring. The median hourly alarm rate during these periods of guideline-discordant continuous monitoring was 6.7 alarms per hour (IQR, 2.1-12.3), representing a median of 35 (IQR, 10-81) additional alarms per patient. During periods of guideline-concordant intermittent measurement, the median hourly alarm rate was 0.5 (IQR, 0.1-0.8), with a median of 5 (IQR, 1-13) alarms per patient.
Those who received supplemental oxygen earlier in the admission had higher alarm rates during continuously monitored time (7.3 per hour [IQR, 2.7-12.7]) than patients who had not received supplemental oxygen (3.3 per hour [IQR, 0.6-11.8]), likely reflecting clinical differences between these patient populations. The most frequent alarm type among continuously monitored patients who had previously received supplemental oxygen was “SpO2 low.”
Discussion
Across 4402 patient hours, guideline-discordant continuous SpO2 monitoring of patients with bronchiolitis resulted in 11,101 alarms, at a rate of approximately 1 additional alarm every 9 minutes. Patients in our cohort received a median of 6 hours of guideline-discordant monitoring, which imposes a significant alarm burden that is potentially modifiable using targeted reduction strategies.15
Transient, self-resolved hypoxemia is a common feature of bronchiolitis and likely of little clinical consequence.16 Therefore, this rate of hypoxemia alarms is not unexpected. Though we evaluated only the period of time following final discontinuation of respiratory support, this finding is in keeping with the literature associating excess physiologic monitoring of patients with bronchiolitis with unnecessary oxygen therapy and increased length of stay,3-5 largely because clinicians may feel compelled to respond to hypoxemia alarms with either supplemental oxygen or longer monitoring.
Our findings must be contextualized in light of the limitations of our approach. We did not evaluate nurse workload associated with guideline-discordant continuous SpO2 monitoring. Prior work conducted by our lab has demonstrated that when nurses experience more than 40 alarms within a 2-hour period, their measured subjective workload increases to a degree associated with missing important tasks, threatening the quality and safety of the care they deliver.12,17 Given that nurses care for multiple patients, it is likely that the excess alarms introduced by guideline-discordant continuous monitoring contribute to increased nurse workload and alarm fatigue.
Similarly, we could not evaluate whether the alarms nurses experienced were actionable. Although our lab has previously reported that ≥99% of alarms occurring on non-ICU pediatric wards are nonactionable,10,11 it is possible that some of the alarms during guideline-discordant monitoring periods required action. However, it is unlikely that any life-sustaining actions were taken because (1) we only evaluated time >60 minutes after final discontinuation of supplemental oxygen, so by definition none of these alarms required treatment with supplemental oxygen, and (2) none of the patients we included received ICU care during their admission.
The avoidable alarm burden identified in our analysis suggests that eliminating continuous SpO2 monitoring overuse in bronchiolitis will likely reduce nurses’ workload and alarm fatigue in hospital settings that care for children with bronchiolitis.
1. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742
2. Schondelmeyer AC, Dewan ML, Brady PW, et al. Cardiorespiratory and pulse oximetry monitoring in hospitalized children: a Delphi process. Pediatrics. 2020;146(2):e20193336. https://doi.org/10.1542/peds.2019-3336
3. Cunningham S, Rodriguez A, Boyd KA, McIntosh E, Lewis SC, BIDS Collaborators Group. Bronchiolitis of Infancy Discharge Study (BIDS): A multicentre, parallel-group, double-blind, randomised controlled, equivalence trial with economic evaluation. Health Technol Assess. 2015;19(71):i-xxiii, 1-172. https://doi.org/10.3310/hta19710
4. McCulloh R, Koster M, Ralston S, et al. Use of intermittent vs continuous pulse oximetry for nonhypoxemic infants and young children hospitalized for bronchiolitis: a randomized clinical trial. JAMA Pediatr. 2015;169(10):898-904. https://doi.org/10.1001/jamapediatrics.2015.1746
5. Schuh S, Freedman S, Coates A, et al. Effect of oximetry on hospitalization in bronchiolitis: a randomized clinical trial. JAMA. 2014;312(7):712-718. https://doi.org/10.1001/jama.2014.8637
6. Fujiogi M, Goto T, Yasunaga H, et al. Trends in bronchiolitis hospitalizations in the United States: 2000–2016. Pediatrics. 2019;144(6):e20192614. https://doi.org/10.1542/peds.2019-2614
7. Bonafide CP, Xiao R, Brady PW, et al. Prevalence of continuous pulse oximetry monitoring in hospitalized children with bronchiolitis not requiring supplemental oxygen. JAMA. 2020;323(15):1467-1477. https://doi.org/10.1001/jama.2020.2998
8. Schondelmeyer AC, Brady PW, Goel VV, et al. Physiologic monitor alarm rates at 5 children’s hospitals. J Hosp Med. 2018;13(6):396-398. https://doi.org/10.12788/jhm.2918
9. Schondelmeyer AC, Bonafide CP, Goel VV, et al. The frequency of physiologic monitor alarms in a children’s hospital. J Hosp Med. 2016;11(11):796-798. https://doi.org/10.1002/jhm.2612
10. Bonafide CP, Lin R, Zander M, et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children’s hospital. J Hosp Med. 2015;10(6):345-351. https://doi.org/10.1002/jhm.2331
11. Bonafide CP, Localio AR, Holmes JH, et al. Video analysis of factors associated with response time to physiologic monitor alarms in a children’s hospital. JAMA Pediatr. 2017;171(6):524-531. https://doi.org/10.1001/jamapediatrics.2016.5123
12. Rasooly IR, Kern-Goldberger AS, Xiao R, et al. Physiologic monitor alarm burden and nurses’ subjective workload in a children’s hospital. Hosp Pediatr. 2021;11(7):703-710. https://doi.org/10.1542/hpeds.2020-003509
13. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199
14. Kern-Goldberger AS, Rasooly IR, Luo B, et al. EHR-integrated monitor data to measure pulse oximetry use in bronchiolitis. Hosp Pediatr. 2021;11(10):1073-1082. https://doi.org/10.1542/hpeds.2021-005894
15. Schondelmeyer AC, Bettencourt AP, Xiao R, et al. Evaluation of an educational outreach and audit and feedback program to reduce continuous pulse oximetry use in hospitalized infants with stable bronchiolitis. JAMA Netw Open. 2021;4(9):e2122826. https://doi.org/10.1001/jamanetworkopen.2021.22826
16. Principi T, Coates AL, Parkin PC, Stephens D, DaSilva Z, Schuh S. Effect of oxygen desaturations on subsequent medical visits in infants discharged from the emergency department with bronchiolitis. JAMA Pediatr. 2016;170(6):602-608. https://doi.org/10.1001/jamapediatrics.2016.0114
17. Tubbs-Cooley HL, Mara CA, Carle AC, Mark BA, Pickler RH. Association of nurse workload with missed nursing care in the neonatal intensive care unit. JAMA Pediatr. 2019;173(1):44-51. https://doi.org/10.1001/jamapediatrics.2018.3619
Practice guidelines discourage continuous pulse oximetry (SpO2) monitoring of patients with bronchiolitis who are not receiving supplemental oxygen.1,2 Overuse of SpO2 monitoring in this patient population has been associated with increased length of stay, unnecessary oxygen therapy, and excess hospital costs, without measurable patient benefit.3-5 In spite of this evidence base and expert guidance, nearly half of the more than 100,000 infants admitted for bronchiolitis each year receive excess SpO2 monitoring.6,7
Bronchiolitis guidelines suggest that guideline-discordant SpO2 monitoring may result in excess alarms, which disrupt families’ sleep and engender alarm fatigue among staff.1 Pediatric nurses receive up to 155 alarms per monitored patient per day.8,9 Frequent alarms are associated with slower nurse response times10,11 and increased nurse subjective workload.12
Methods
Cohort
We retrospectively evaluated SpO2 monitoring patterns and alarm rates of children 0 to 24 months old admitted to a general pediatrics service at a tertiary care children’s hospital. We included patients who had a discharge diagnosis of bronchiolitis (International Classification of Diseases, Tenth Revision codes J45x, T17.2x, T17.3x, T17.4x, T17.5x, T17.8x, T17.9x, A37xx, J04x, J05x, J05.1x, J69.0x, J69.1x, J69.8x) between November 24, 2019, and January 21, 2020, the period of time during which alarm data and monitor data were concurrently available for analysis. In order to conservatively assure applicability of clinical practice guidelines, we excluded patients with discharge diagnoses that included other respiratory conditions (eg, reactive airway disease), patients with complex chronic conditions (CCC) as defined by the CCC version 2 classification system,13 and patients with intensive care unit (ICU) stays during the admission.
Time
Flowsheet data detailing nursing respiratory assessments were extracted from the electronic health record (EHR) database (Clarity, Epic Systems). Using previously validated methodology,14 we identified minutes during which patients received supplemental oxygen or high-flow nasal cannula (supplemental oxygen) based on the documented fraction of inspired oxygen (FiO2), flow rate, and support devices. We then identified the final discontinuation of respiratory support during the hospital admission, and censored the 60 minutes after final discontinuation of supplemental oxygen based upon recent monitoring guidelines.2 Minutes up to an hour after supplemental oxygen discontinuation were classified as receiving supplemental oxygen and not included in our analysis. Only minutes between the end of the censored period and hospital discharge were used in the analysis. For patients who never received respiratory support during the admission, we censored the first 60 minutes of the admission and analyzed the remainder of their stay.
SpO2 Monitoring
We used device-integrated, physiologic-monitor, vital sign data sent each minute from the General Electric monitor network to the EHR to identify minutes during which patients were connected to physiologic monitors and transmitting signals from SpO2 sensors. We extracted minute-level SpO2 data from the hospital clinical data warehouse (CDW). Minutes in which SpO2 data were present were classified as “monitored,” an approach previously validated using in-person observation.14
To categorize time as “not receiving supplemental oxygen and continuously monitored (guideline-discordant monitoring),” or “not receiving supplemental oxygen and not continuously monitored (guideline-concordant intermittent measurement),” we evaluated the percent of minutes within an hour during which the patient received SpO2 monitoring and applied an a priori conservative rule. Hours during which ≥90% of minutes had SpO2 monitoring data were classified as “continuously monitored.” Hours during which ≤10% of minutes had SpO2 monitoring data were classified as “intermittently measured.” Hours during which 11% to 89% of minutes included monitor data were excluded from further analysis. The number of continuously monitored hours was tabulated for each patient. The median number of continuously monitored hours was computed; results were stratified by prior receipt of respiratory support.
Alarm Counts
Minute-level monitor alarm counts (the absolute number of abnormal vital signs that triggered a monitor to alarm) were extracted from the CDW. Alarm counts were tabulated for each patient hour. For each patient, the alarm rate (total number of alarms divided by time) was computed for continuously monitored and intermittently measured time. Results were stratified by prior receipt of respiratory support.
The study was reviewed by the institutional review board and determined to meet exemption criteria.
Results
Our cohort included 201 admissions by 197 unique patients (Table). We evaluated 4402 hours that occurred ≥60 minutes following final discontinuation of supplemental oxygen, the time period during which guidelines discourage routine use of continuous SpO2 monitoring. This represented a median of 19 hours (interquartile range [IQR], 14-25) per admission. We excluded 474 hours (11%) that could not be classified as either continuously or intermittently measured.

During time ≥60 minutes following discontinuation of supplemental oxygen, our cohort experienced 1537 hours of guideline-discordant continuous monitoring, a median of 6 hours (IQR, 3-12) per admission. Patients experienced a median of 12 hours (IQR, 5-17) of intermittent measurement. Among patients who received supplemental oxygen, 91% experienced guideline-discordant continuous SpO2 monitoring, as compared to 68% of patients who did not receive supplemental oxygen. Among those who received guideline-discordant continuous SpO2 monitoring, the duration of this monitoring did not differ significantly between those who had received supplemental oxygen during the admission and those who had not.
During classifiable time ≥60 minutes following discontinuation of supplemental oxygen, our cohort experienced 14,371 alarms; 77% (11,101) of these alarms were generated during periods of guideline-discordant continuous monitoring. The median hourly alarm rate during these periods of guideline-discordant continuous monitoring was 6.7 alarms per hour (IQR, 2.1-12.3), representing a median of 35 (IQR, 10-81) additional alarms per patient. During periods of guideline-concordant intermittent measurement, the median hourly alarm rate was 0.5 (IQR, 0.1-0.8), with a median of 5 (IQR, 1-13) alarms per patient.
Those who received supplemental oxygen earlier in the admission had higher alarm rates during continuously monitored time (7.3 per hour [IQR, 2.7-12.7]) than patients who had not received supplemental oxygen (3.3 per hour [IQR, 0.6-11.8]), likely reflecting clinical differences between these patient populations. The most frequent alarm type among continuously monitored patients who had previously received supplemental oxygen was “SpO2 low.”
Discussion
Across 4402 patient hours, guideline-discordant continuous SpO2 monitoring of patients with bronchiolitis resulted in 11,101 alarms, at a rate of approximately 1 additional alarm every 9 minutes. Patients in our cohort received a median of 6 hours of guideline-discordant monitoring, which imposes a significant alarm burden that is potentially modifiable using targeted reduction strategies.15
Transient, self-resolved hypoxemia is a common feature of bronchiolitis and likely of little clinical consequence.16 Therefore, this rate of hypoxemia alarms is not unexpected. Though we evaluated only the period of time following final discontinuation of respiratory support, this finding is in keeping with the literature associating excess physiologic monitoring of patients with bronchiolitis with unnecessary oxygen therapy and increased length of stay,3-5 largely because clinicians may feel compelled to respond to hypoxemia alarms with either supplemental oxygen or longer monitoring.
Our findings must be contextualized in light of the limitations of our approach. We did not evaluate nurse workload associated with guideline-discordant continuous SpO2 monitoring. Prior work conducted by our lab has demonstrated that when nurses experience more than 40 alarms within a 2-hour period, their measured subjective workload increases to a degree associated with missing important tasks, threatening the quality and safety of the care they deliver.12,17 Given that nurses care for multiple patients, it is likely that the excess alarms introduced by guideline-discordant continuous monitoring contribute to increased nurse workload and alarm fatigue.
Similarly, we could not evaluate whether the alarms nurses experienced were actionable. Although our lab has previously reported that ≥99% of alarms occurring on non-ICU pediatric wards are nonactionable,10,11 it is possible that some of the alarms during guideline-discordant monitoring periods required action. However, it is unlikely that any life-sustaining actions were taken because (1) we only evaluated time >60 minutes after final discontinuation of supplemental oxygen, so by definition none of these alarms required treatment with supplemental oxygen, and (2) none of the patients we included received ICU care during their admission.
The avoidable alarm burden identified in our analysis suggests that eliminating continuous SpO2 monitoring overuse in bronchiolitis will likely reduce nurses’ workload and alarm fatigue in hospital settings that care for children with bronchiolitis.
Practice guidelines discourage continuous pulse oximetry (SpO2) monitoring of patients with bronchiolitis who are not receiving supplemental oxygen.1,2 Overuse of SpO2 monitoring in this patient population has been associated with increased length of stay, unnecessary oxygen therapy, and excess hospital costs, without measurable patient benefit.3-5 In spite of this evidence base and expert guidance, nearly half of the more than 100,000 infants admitted for bronchiolitis each year receive excess SpO2 monitoring.6,7
Bronchiolitis guidelines suggest that guideline-discordant SpO2 monitoring may result in excess alarms, which disrupt families’ sleep and engender alarm fatigue among staff.1 Pediatric nurses receive up to 155 alarms per monitored patient per day.8,9 Frequent alarms are associated with slower nurse response times10,11 and increased nurse subjective workload.12
Methods
Cohort
We retrospectively evaluated SpO2 monitoring patterns and alarm rates of children 0 to 24 months old admitted to a general pediatrics service at a tertiary care children’s hospital. We included patients who had a discharge diagnosis of bronchiolitis (International Classification of Diseases, Tenth Revision codes J45x, T17.2x, T17.3x, T17.4x, T17.5x, T17.8x, T17.9x, A37xx, J04x, J05x, J05.1x, J69.0x, J69.1x, J69.8x) between November 24, 2019, and January 21, 2020, the period of time during which alarm data and monitor data were concurrently available for analysis. In order to conservatively assure applicability of clinical practice guidelines, we excluded patients with discharge diagnoses that included other respiratory conditions (eg, reactive airway disease), patients with complex chronic conditions (CCC) as defined by the CCC version 2 classification system,13 and patients with intensive care unit (ICU) stays during the admission.
Time
Flowsheet data detailing nursing respiratory assessments were extracted from the electronic health record (EHR) database (Clarity, Epic Systems). Using previously validated methodology,14 we identified minutes during which patients received supplemental oxygen or high-flow nasal cannula (supplemental oxygen) based on the documented fraction of inspired oxygen (FiO2), flow rate, and support devices. We then identified the final discontinuation of respiratory support during the hospital admission, and censored the 60 minutes after final discontinuation of supplemental oxygen based upon recent monitoring guidelines.2 Minutes up to an hour after supplemental oxygen discontinuation were classified as receiving supplemental oxygen and not included in our analysis. Only minutes between the end of the censored period and hospital discharge were used in the analysis. For patients who never received respiratory support during the admission, we censored the first 60 minutes of the admission and analyzed the remainder of their stay.
SpO2 Monitoring
We used device-integrated, physiologic-monitor, vital sign data sent each minute from the General Electric monitor network to the EHR to identify minutes during which patients were connected to physiologic monitors and transmitting signals from SpO2 sensors. We extracted minute-level SpO2 data from the hospital clinical data warehouse (CDW). Minutes in which SpO2 data were present were classified as “monitored,” an approach previously validated using in-person observation.14
To categorize time as “not receiving supplemental oxygen and continuously monitored (guideline-discordant monitoring),” or “not receiving supplemental oxygen and not continuously monitored (guideline-concordant intermittent measurement),” we evaluated the percent of minutes within an hour during which the patient received SpO2 monitoring and applied an a priori conservative rule. Hours during which ≥90% of minutes had SpO2 monitoring data were classified as “continuously monitored.” Hours during which ≤10% of minutes had SpO2 monitoring data were classified as “intermittently measured.” Hours during which 11% to 89% of minutes included monitor data were excluded from further analysis. The number of continuously monitored hours was tabulated for each patient. The median number of continuously monitored hours was computed; results were stratified by prior receipt of respiratory support.
Alarm Counts
Minute-level monitor alarm counts (the absolute number of abnormal vital signs that triggered a monitor to alarm) were extracted from the CDW. Alarm counts were tabulated for each patient hour. For each patient, the alarm rate (total number of alarms divided by time) was computed for continuously monitored and intermittently measured time. Results were stratified by prior receipt of respiratory support.
The study was reviewed by the institutional review board and determined to meet exemption criteria.
Results
Our cohort included 201 admissions by 197 unique patients (Table). We evaluated 4402 hours that occurred ≥60 minutes following final discontinuation of supplemental oxygen, the time period during which guidelines discourage routine use of continuous SpO2 monitoring. This represented a median of 19 hours (interquartile range [IQR], 14-25) per admission. We excluded 474 hours (11%) that could not be classified as either continuously or intermittently measured.

During time ≥60 minutes following discontinuation of supplemental oxygen, our cohort experienced 1537 hours of guideline-discordant continuous monitoring, a median of 6 hours (IQR, 3-12) per admission. Patients experienced a median of 12 hours (IQR, 5-17) of intermittent measurement. Among patients who received supplemental oxygen, 91% experienced guideline-discordant continuous SpO2 monitoring, as compared to 68% of patients who did not receive supplemental oxygen. Among those who received guideline-discordant continuous SpO2 monitoring, the duration of this monitoring did not differ significantly between those who had received supplemental oxygen during the admission and those who had not.
During classifiable time ≥60 minutes following discontinuation of supplemental oxygen, our cohort experienced 14,371 alarms; 77% (11,101) of these alarms were generated during periods of guideline-discordant continuous monitoring. The median hourly alarm rate during these periods of guideline-discordant continuous monitoring was 6.7 alarms per hour (IQR, 2.1-12.3), representing a median of 35 (IQR, 10-81) additional alarms per patient. During periods of guideline-concordant intermittent measurement, the median hourly alarm rate was 0.5 (IQR, 0.1-0.8), with a median of 5 (IQR, 1-13) alarms per patient.
Those who received supplemental oxygen earlier in the admission had higher alarm rates during continuously monitored time (7.3 per hour [IQR, 2.7-12.7]) than patients who had not received supplemental oxygen (3.3 per hour [IQR, 0.6-11.8]), likely reflecting clinical differences between these patient populations. The most frequent alarm type among continuously monitored patients who had previously received supplemental oxygen was “SpO2 low.”
Discussion
Across 4402 patient hours, guideline-discordant continuous SpO2 monitoring of patients with bronchiolitis resulted in 11,101 alarms, at a rate of approximately 1 additional alarm every 9 minutes. Patients in our cohort received a median of 6 hours of guideline-discordant monitoring, which imposes a significant alarm burden that is potentially modifiable using targeted reduction strategies.15
Transient, self-resolved hypoxemia is a common feature of bronchiolitis and likely of little clinical consequence.16 Therefore, this rate of hypoxemia alarms is not unexpected. Though we evaluated only the period of time following final discontinuation of respiratory support, this finding is in keeping with the literature associating excess physiologic monitoring of patients with bronchiolitis with unnecessary oxygen therapy and increased length of stay,3-5 largely because clinicians may feel compelled to respond to hypoxemia alarms with either supplemental oxygen or longer monitoring.
Our findings must be contextualized in light of the limitations of our approach. We did not evaluate nurse workload associated with guideline-discordant continuous SpO2 monitoring. Prior work conducted by our lab has demonstrated that when nurses experience more than 40 alarms within a 2-hour period, their measured subjective workload increases to a degree associated with missing important tasks, threatening the quality and safety of the care they deliver.12,17 Given that nurses care for multiple patients, it is likely that the excess alarms introduced by guideline-discordant continuous monitoring contribute to increased nurse workload and alarm fatigue.
Similarly, we could not evaluate whether the alarms nurses experienced were actionable. Although our lab has previously reported that ≥99% of alarms occurring on non-ICU pediatric wards are nonactionable,10,11 it is possible that some of the alarms during guideline-discordant monitoring periods required action. However, it is unlikely that any life-sustaining actions were taken because (1) we only evaluated time >60 minutes after final discontinuation of supplemental oxygen, so by definition none of these alarms required treatment with supplemental oxygen, and (2) none of the patients we included received ICU care during their admission.
The avoidable alarm burden identified in our analysis suggests that eliminating continuous SpO2 monitoring overuse in bronchiolitis will likely reduce nurses’ workload and alarm fatigue in hospital settings that care for children with bronchiolitis.
1. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742
2. Schondelmeyer AC, Dewan ML, Brady PW, et al. Cardiorespiratory and pulse oximetry monitoring in hospitalized children: a Delphi process. Pediatrics. 2020;146(2):e20193336. https://doi.org/10.1542/peds.2019-3336
3. Cunningham S, Rodriguez A, Boyd KA, McIntosh E, Lewis SC, BIDS Collaborators Group. Bronchiolitis of Infancy Discharge Study (BIDS): A multicentre, parallel-group, double-blind, randomised controlled, equivalence trial with economic evaluation. Health Technol Assess. 2015;19(71):i-xxiii, 1-172. https://doi.org/10.3310/hta19710
4. McCulloh R, Koster M, Ralston S, et al. Use of intermittent vs continuous pulse oximetry for nonhypoxemic infants and young children hospitalized for bronchiolitis: a randomized clinical trial. JAMA Pediatr. 2015;169(10):898-904. https://doi.org/10.1001/jamapediatrics.2015.1746
5. Schuh S, Freedman S, Coates A, et al. Effect of oximetry on hospitalization in bronchiolitis: a randomized clinical trial. JAMA. 2014;312(7):712-718. https://doi.org/10.1001/jama.2014.8637
6. Fujiogi M, Goto T, Yasunaga H, et al. Trends in bronchiolitis hospitalizations in the United States: 2000–2016. Pediatrics. 2019;144(6):e20192614. https://doi.org/10.1542/peds.2019-2614
7. Bonafide CP, Xiao R, Brady PW, et al. Prevalence of continuous pulse oximetry monitoring in hospitalized children with bronchiolitis not requiring supplemental oxygen. JAMA. 2020;323(15):1467-1477. https://doi.org/10.1001/jama.2020.2998
8. Schondelmeyer AC, Brady PW, Goel VV, et al. Physiologic monitor alarm rates at 5 children’s hospitals. J Hosp Med. 2018;13(6):396-398. https://doi.org/10.12788/jhm.2918
9. Schondelmeyer AC, Bonafide CP, Goel VV, et al. The frequency of physiologic monitor alarms in a children’s hospital. J Hosp Med. 2016;11(11):796-798. https://doi.org/10.1002/jhm.2612
10. Bonafide CP, Lin R, Zander M, et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children’s hospital. J Hosp Med. 2015;10(6):345-351. https://doi.org/10.1002/jhm.2331
11. Bonafide CP, Localio AR, Holmes JH, et al. Video analysis of factors associated with response time to physiologic monitor alarms in a children’s hospital. JAMA Pediatr. 2017;171(6):524-531. https://doi.org/10.1001/jamapediatrics.2016.5123
12. Rasooly IR, Kern-Goldberger AS, Xiao R, et al. Physiologic monitor alarm burden and nurses’ subjective workload in a children’s hospital. Hosp Pediatr. 2021;11(7):703-710. https://doi.org/10.1542/hpeds.2020-003509
13. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199
14. Kern-Goldberger AS, Rasooly IR, Luo B, et al. EHR-integrated monitor data to measure pulse oximetry use in bronchiolitis. Hosp Pediatr. 2021;11(10):1073-1082. https://doi.org/10.1542/hpeds.2021-005894
15. Schondelmeyer AC, Bettencourt AP, Xiao R, et al. Evaluation of an educational outreach and audit and feedback program to reduce continuous pulse oximetry use in hospitalized infants with stable bronchiolitis. JAMA Netw Open. 2021;4(9):e2122826. https://doi.org/10.1001/jamanetworkopen.2021.22826
16. Principi T, Coates AL, Parkin PC, Stephens D, DaSilva Z, Schuh S. Effect of oxygen desaturations on subsequent medical visits in infants discharged from the emergency department with bronchiolitis. JAMA Pediatr. 2016;170(6):602-608. https://doi.org/10.1001/jamapediatrics.2016.0114
17. Tubbs-Cooley HL, Mara CA, Carle AC, Mark BA, Pickler RH. Association of nurse workload with missed nursing care in the neonatal intensive care unit. JAMA Pediatr. 2019;173(1):44-51. https://doi.org/10.1001/jamapediatrics.2018.3619
1. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742
2. Schondelmeyer AC, Dewan ML, Brady PW, et al. Cardiorespiratory and pulse oximetry monitoring in hospitalized children: a Delphi process. Pediatrics. 2020;146(2):e20193336. https://doi.org/10.1542/peds.2019-3336
3. Cunningham S, Rodriguez A, Boyd KA, McIntosh E, Lewis SC, BIDS Collaborators Group. Bronchiolitis of Infancy Discharge Study (BIDS): A multicentre, parallel-group, double-blind, randomised controlled, equivalence trial with economic evaluation. Health Technol Assess. 2015;19(71):i-xxiii, 1-172. https://doi.org/10.3310/hta19710
4. McCulloh R, Koster M, Ralston S, et al. Use of intermittent vs continuous pulse oximetry for nonhypoxemic infants and young children hospitalized for bronchiolitis: a randomized clinical trial. JAMA Pediatr. 2015;169(10):898-904. https://doi.org/10.1001/jamapediatrics.2015.1746
5. Schuh S, Freedman S, Coates A, et al. Effect of oximetry on hospitalization in bronchiolitis: a randomized clinical trial. JAMA. 2014;312(7):712-718. https://doi.org/10.1001/jama.2014.8637
6. Fujiogi M, Goto T, Yasunaga H, et al. Trends in bronchiolitis hospitalizations in the United States: 2000–2016. Pediatrics. 2019;144(6):e20192614. https://doi.org/10.1542/peds.2019-2614
7. Bonafide CP, Xiao R, Brady PW, et al. Prevalence of continuous pulse oximetry monitoring in hospitalized children with bronchiolitis not requiring supplemental oxygen. JAMA. 2020;323(15):1467-1477. https://doi.org/10.1001/jama.2020.2998
8. Schondelmeyer AC, Brady PW, Goel VV, et al. Physiologic monitor alarm rates at 5 children’s hospitals. J Hosp Med. 2018;13(6):396-398. https://doi.org/10.12788/jhm.2918
9. Schondelmeyer AC, Bonafide CP, Goel VV, et al. The frequency of physiologic monitor alarms in a children’s hospital. J Hosp Med. 2016;11(11):796-798. https://doi.org/10.1002/jhm.2612
10. Bonafide CP, Lin R, Zander M, et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children’s hospital. J Hosp Med. 2015;10(6):345-351. https://doi.org/10.1002/jhm.2331
11. Bonafide CP, Localio AR, Holmes JH, et al. Video analysis of factors associated with response time to physiologic monitor alarms in a children’s hospital. JAMA Pediatr. 2017;171(6):524-531. https://doi.org/10.1001/jamapediatrics.2016.5123
12. Rasooly IR, Kern-Goldberger AS, Xiao R, et al. Physiologic monitor alarm burden and nurses’ subjective workload in a children’s hospital. Hosp Pediatr. 2021;11(7):703-710. https://doi.org/10.1542/hpeds.2020-003509
13. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199
14. Kern-Goldberger AS, Rasooly IR, Luo B, et al. EHR-integrated monitor data to measure pulse oximetry use in bronchiolitis. Hosp Pediatr. 2021;11(10):1073-1082. https://doi.org/10.1542/hpeds.2021-005894
15. Schondelmeyer AC, Bettencourt AP, Xiao R, et al. Evaluation of an educational outreach and audit and feedback program to reduce continuous pulse oximetry use in hospitalized infants with stable bronchiolitis. JAMA Netw Open. 2021;4(9):e2122826. https://doi.org/10.1001/jamanetworkopen.2021.22826
16. Principi T, Coates AL, Parkin PC, Stephens D, DaSilva Z, Schuh S. Effect of oxygen desaturations on subsequent medical visits in infants discharged from the emergency department with bronchiolitis. JAMA Pediatr. 2016;170(6):602-608. https://doi.org/10.1001/jamapediatrics.2016.0114
17. Tubbs-Cooley HL, Mara CA, Carle AC, Mark BA, Pickler RH. Association of nurse workload with missed nursing care in the neonatal intensive care unit. JAMA Pediatr. 2019;173(1):44-51. https://doi.org/10.1001/jamapediatrics.2018.3619
© 2021 Society of Hospital Medicine
Initiation of Long-Acting Opioids Following Hospital Discharge Among Medicare Beneficiaries
Transition out of the hospital is a vulnerable time for older adults. Medications, particularly opioids, are a common cause of adverse events during this transitionary period.1,2 For hospitalized patients with acute noncancer pain that necessitates opioid treatment, guidelines recommend using short-acting, rather than long-acting, opioids.3,4 Long-acting opioids have a longer duration of action but also have a significantly elevated risk of unintentional overdose and morbidity compared to short-acting opioids, even when total daily dosing is identical.5,6 This risk is highest in the first 2 weeks following initial prescription.7,8
Despite the recent decrease in overall prescription of opioids,9 a small but significant proportion continue to be prescribed as long-acting formulations.10-12 We sought to understand the incidence of, and patient characteristics associated with, long-acting opioid initiation following hospital discharge among opioid-naïve older adults.
METHODS
We examined the 20% random sample of US Medicare beneficiaries ≥65 years old who were hospitalized in 2016 and continuously enrolled in Parts A, B, and D for 1 year prior and 1 month following discharge, excluding beneficiaries with cancer or hospice care, those transferred from or discharged to a care facility, and those who had filled a prescription for an opioid within 90 days prior to hospitalization. We identified beneficiaries with a Part D claim for an opioid, excluding methadone and buprenorphine, within 7 days of discharge. We compared beneficiaries with at least one claim for a long-acting opioid (including extended-release formulations) within 7 days of hospital discharge to those with short-acting opioid claims only.
We used a multivariable, generalized estimating equation to determine patient-level factors independently associated with prescription of any long-acting opioids. We selected characteristics that we hypothesized to be associated with new opioid prescription, based on clinical experience and previous literature, including sociodemographics, patient clinical characteristics such as a modified Elixhauser index (a composite index of nearly 30 comorbidities, excluding cancer),13 substance use-related factors, co-prescribed medications, and hospitalization-related factors. The latter included being hospitalized for a medical vs surgical reason, defined based on diagnosis-related group (DRG), primary diagnosis, and primary procedure, grouped using the Agency for Healthcare Research and Quality Clinical Classification System14 (Table 1).
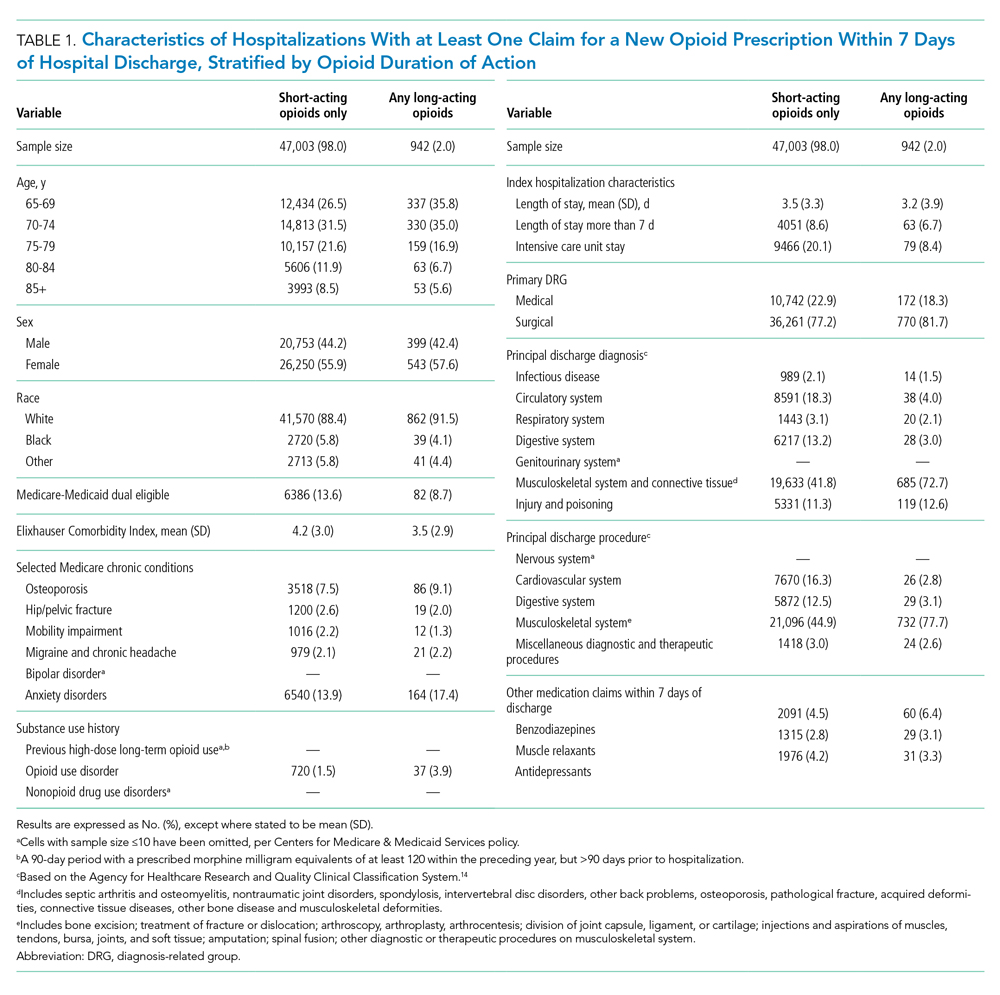
We conducted a sensitivity analysis, excluding beneficiaries with high-dose long-term opioid use in the year before hospitalization.
RESULTS
Of 258,193 hospitalizations meeting eligibility criteria, 47,945 (18.6%) had an opioid claim within 7 days of discharge and comprised our analytic cohort (see the Appendix Figure for the study consort diagram), including 47,003 (18.2%) with short-acting opioids only and 942 (0.4%) with at least one claim for long-acting opioids, of whom 817 received both short- and long-acting opioids (Table 1).
Beneficiaries with long-acting opioid claims were more likely to be younger (ages 65-69 and 70-74 years) and White than those with claims for short-acting opioids only. They had a lower mean number of Elixhauser comorbidities but a higher prevalence of mental health conditions, including anxiety disorders and opioid use disorder, as well as a higher prevalence of previous high-dose long-term opioid use (occurring more than 90 days prior to hospitalization). They were more likely to have been hospitalized for a procedural rather than a medical reason, with 770 of the 942 (81.7%) beneficiaries receiving long-acting opioids having been hospitalized for a procedural reason (based on DRG). They were also more likely to have benzodiazepine co-prescription.
Factors independently associated with receipt of long-acting opioids compared to short-acting opioids only included younger age, having been admitted for a musculoskeletal problem, and presence of known risk factors for opioid-related adverse events, including anxiety disorders, opioid use disorder, prior long-term high-dose opioid use, and benzodiazepine co-prescription (Table 2). After excluding 33 beneficiaries with previous high-dose long-term opioid use in the year before hospitalization, associations were unchanged (Appendix Table).
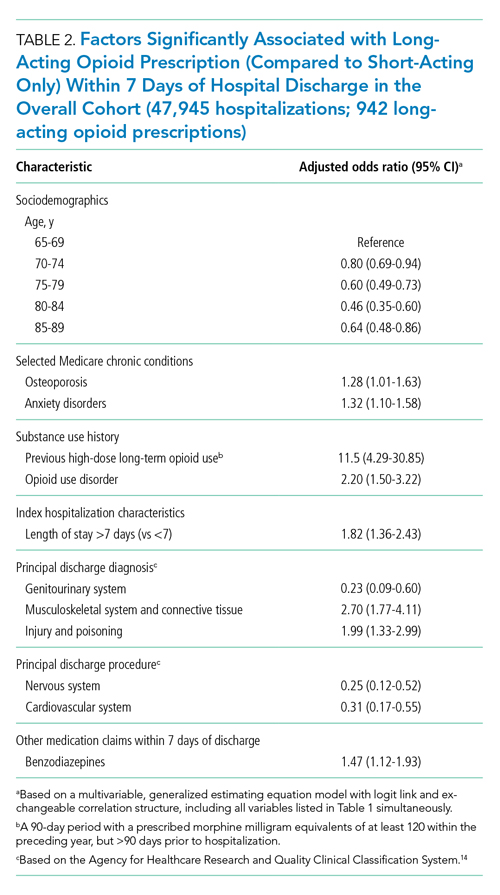
DISCUSSION
Among a nationally representative sample of opioid-naïve Medicare beneficiaries without cancer, almost 20% filled a new opioid prescription within 7 days of hospital discharge. While prescription of long-acting opioids was uncommon, 81.7% who were prescribed a long-acting opioid had a procedural reason for hospitalization, raising concern since postoperative pain is typically acute and limited. Beneficiaries started on long-acting opioids more frequently had risk factors for opioid-related adverse events, including history of opioid use disorder and benzodiazepine co-prescription. With nearly three-quarters of patients with a long-acting opioid claim having been hospitalized for musculoskeletal disorders or orthopedic procedures, this population represents a key target for quality improvement interventions.
This is the first analysis describing the incidence and factors associated with long-acting opioid receipt shortly after hospital discharge among Medicare beneficiaries. Given that our data predate the publication of the Society of Hospital Medicine’s consensus statement on safe opioid prescribing in hospitalized patients,3 it is possible that there have been changes to prescribing patterns since 2016 that we are unable to characterize with our data. We are also limited by an inability to determine the appropriateness of any individual long-acting opioid prescription, though previous research has shown that long-acting opioids are frequently inappropriately initiated in older adults.15 Finally, our findings may not be generalizable to non-Medicare populations.
While long-acting opioid initiation following hospitalization is uncommon, these medications are most often prescribed to individuals with pain that is typically of limited duration and those at high risk for harm. Our findings highlight potential targets for systems-based solutions to improve guideline-concordant prescribing of long-acting opioids.
1. Tsilimingras D, Schnipper J, Duke A, et al. Post-discharge adverse events among urban and rural patients of an urban community hospital: a prospective cohort study. J Gen Intern Med. 2015;30(8):1164-1171. https://doi.org/10.1007/s11606-015-3260-3
2. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. https://doi.org/10.7326/0003-4819-138-3-200302040-00007
3. Herzig SJ, Mosher HJ, Calcaterra SL, Jena AB, Nuckols TK. Improving the safety of opioid use for acute noncancer pain in hospitalized adults: a consensus statement from the Society of Hospital Medicine. J Hosp Med. 2018;13(4):263-271. https://doi.org/10.12788/jhm.2980
4. Herzig SJ, Calcaterra SL, Mosher HJ, et al. Safe opioid prescribing for acute noncancer pain in hospitalized adults: a systematic review of existing guidelines. J Hosp Med. 2018;13(4):256-262. https://doi.org/10.12788/jhm.2979
5. Barnett ML, Olenski AR, Thygeson NM, et al. A health plan’s formulary led to reduced use of extended-release opioids but did not lower overall opioid use. Health Aff (Millwood). 2018;37(9):1509-1516. https://doi.org/10.1377/hlthaff.2018.0391
6. Carey CM, Jena AB, Barnett ML. Patterns of potential opioid misuse and subsequent adverse outcomes in Medicare, 2008 to 2012. Ann Intern Med. 2018;168(12):837-845. https://doi.org/10.7326/M17-3065
7. Miller M, Barber CW, Leatherman S, et al. Prescription opioid duration of action and the risk of unintentional overdose among patients receiving opioid therapy. JAMA Intern Med. 2015;175(4):608-615. https://doi.org/10.1001/jamainternmed.2014.8071
8. Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Prescription of long-acting opioids and mortality in patients with chronic noncancer pain. JAMA. 2016;315(22):2415-2423. https://doi.org/10.1001/jama.2016.7789
9. Zhu W, Chernew ME, Sherry TB, Maestas N. Initial opioid prescriptions among U.S. commercially insured patients, 2012-2017. N Engl J Med. 2019;380(11):1043-1052. https://doi.org/10.1056/NEJMsa1807069
10. Starner I, Gleason P. Short-acting, long-acting, and abuse-deterrent opioid utilization patterns among 15 million commercially insured members. Presented at: Academy of Managed Care Pharmacy (AMCP) Nexus; October 3-6, 2016; National Harbor, MD.
11. Young JC, Lund JL, Dasgupta N, Jonsson Funk M. Opioid tolerance and clinically recognized opioid poisoning among patients prescribed extended-release long-acting opioids. Pharmacoepidemiol Drug Saf. 2019;28(1):39-47. https://doi.org/10.1002/pds.4572
12. Hwang CS, Kang EM, Ding Y, et al. Patterns of immediate-release and extended-release opioid analgesic use in the management of chronic pain, 2003-2014. JAMA Netw Open. 2018;1(2):e180216. https://doi.org/10.1001/jamanetworkopen.2018.0216
13. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. https://doi.org/10.1097/00005650-199801000-00004
14. Agency for Healthcare Research and Quality. Clinical Classifications Software (CCS) for ICD-10-CM/PCS. Healthcare Cost and Utilization Project (HCUP). October 2018. www.hcup-us.ahrq.gov/toolssoftware/ccs10/ccs10.jsp
15. Willy ME, Graham DJ, Racoosin JA, et al. Candidate metrics for evaluating the impact of prescriber education on the safe use of extended-release/long-acting (ER/LA) opioid analgesics. Pain Med. 2014;15(9):1558-1568. https://doi.org/10.1111/pme.12459
Transition out of the hospital is a vulnerable time for older adults. Medications, particularly opioids, are a common cause of adverse events during this transitionary period.1,2 For hospitalized patients with acute noncancer pain that necessitates opioid treatment, guidelines recommend using short-acting, rather than long-acting, opioids.3,4 Long-acting opioids have a longer duration of action but also have a significantly elevated risk of unintentional overdose and morbidity compared to short-acting opioids, even when total daily dosing is identical.5,6 This risk is highest in the first 2 weeks following initial prescription.7,8
Despite the recent decrease in overall prescription of opioids,9 a small but significant proportion continue to be prescribed as long-acting formulations.10-12 We sought to understand the incidence of, and patient characteristics associated with, long-acting opioid initiation following hospital discharge among opioid-naïve older adults.
METHODS
We examined the 20% random sample of US Medicare beneficiaries ≥65 years old who were hospitalized in 2016 and continuously enrolled in Parts A, B, and D for 1 year prior and 1 month following discharge, excluding beneficiaries with cancer or hospice care, those transferred from or discharged to a care facility, and those who had filled a prescription for an opioid within 90 days prior to hospitalization. We identified beneficiaries with a Part D claim for an opioid, excluding methadone and buprenorphine, within 7 days of discharge. We compared beneficiaries with at least one claim for a long-acting opioid (including extended-release formulations) within 7 days of hospital discharge to those with short-acting opioid claims only.
We used a multivariable, generalized estimating equation to determine patient-level factors independently associated with prescription of any long-acting opioids. We selected characteristics that we hypothesized to be associated with new opioid prescription, based on clinical experience and previous literature, including sociodemographics, patient clinical characteristics such as a modified Elixhauser index (a composite index of nearly 30 comorbidities, excluding cancer),13 substance use-related factors, co-prescribed medications, and hospitalization-related factors. The latter included being hospitalized for a medical vs surgical reason, defined based on diagnosis-related group (DRG), primary diagnosis, and primary procedure, grouped using the Agency for Healthcare Research and Quality Clinical Classification System14 (Table 1).

We conducted a sensitivity analysis, excluding beneficiaries with high-dose long-term opioid use in the year before hospitalization.
RESULTS
Of 258,193 hospitalizations meeting eligibility criteria, 47,945 (18.6%) had an opioid claim within 7 days of discharge and comprised our analytic cohort (see the Appendix Figure for the study consort diagram), including 47,003 (18.2%) with short-acting opioids only and 942 (0.4%) with at least one claim for long-acting opioids, of whom 817 received both short- and long-acting opioids (Table 1).
Beneficiaries with long-acting opioid claims were more likely to be younger (ages 65-69 and 70-74 years) and White than those with claims for short-acting opioids only. They had a lower mean number of Elixhauser comorbidities but a higher prevalence of mental health conditions, including anxiety disorders and opioid use disorder, as well as a higher prevalence of previous high-dose long-term opioid use (occurring more than 90 days prior to hospitalization). They were more likely to have been hospitalized for a procedural rather than a medical reason, with 770 of the 942 (81.7%) beneficiaries receiving long-acting opioids having been hospitalized for a procedural reason (based on DRG). They were also more likely to have benzodiazepine co-prescription.
Factors independently associated with receipt of long-acting opioids compared to short-acting opioids only included younger age, having been admitted for a musculoskeletal problem, and presence of known risk factors for opioid-related adverse events, including anxiety disorders, opioid use disorder, prior long-term high-dose opioid use, and benzodiazepine co-prescription (Table 2). After excluding 33 beneficiaries with previous high-dose long-term opioid use in the year before hospitalization, associations were unchanged (Appendix Table).

DISCUSSION
Among a nationally representative sample of opioid-naïve Medicare beneficiaries without cancer, almost 20% filled a new opioid prescription within 7 days of hospital discharge. While prescription of long-acting opioids was uncommon, 81.7% who were prescribed a long-acting opioid had a procedural reason for hospitalization, raising concern since postoperative pain is typically acute and limited. Beneficiaries started on long-acting opioids more frequently had risk factors for opioid-related adverse events, including history of opioid use disorder and benzodiazepine co-prescription. With nearly three-quarters of patients with a long-acting opioid claim having been hospitalized for musculoskeletal disorders or orthopedic procedures, this population represents a key target for quality improvement interventions.
This is the first analysis describing the incidence and factors associated with long-acting opioid receipt shortly after hospital discharge among Medicare beneficiaries. Given that our data predate the publication of the Society of Hospital Medicine’s consensus statement on safe opioid prescribing in hospitalized patients,3 it is possible that there have been changes to prescribing patterns since 2016 that we are unable to characterize with our data. We are also limited by an inability to determine the appropriateness of any individual long-acting opioid prescription, though previous research has shown that long-acting opioids are frequently inappropriately initiated in older adults.15 Finally, our findings may not be generalizable to non-Medicare populations.
While long-acting opioid initiation following hospitalization is uncommon, these medications are most often prescribed to individuals with pain that is typically of limited duration and those at high risk for harm. Our findings highlight potential targets for systems-based solutions to improve guideline-concordant prescribing of long-acting opioids.
Transition out of the hospital is a vulnerable time for older adults. Medications, particularly opioids, are a common cause of adverse events during this transitionary period.1,2 For hospitalized patients with acute noncancer pain that necessitates opioid treatment, guidelines recommend using short-acting, rather than long-acting, opioids.3,4 Long-acting opioids have a longer duration of action but also have a significantly elevated risk of unintentional overdose and morbidity compared to short-acting opioids, even when total daily dosing is identical.5,6 This risk is highest in the first 2 weeks following initial prescription.7,8
Despite the recent decrease in overall prescription of opioids,9 a small but significant proportion continue to be prescribed as long-acting formulations.10-12 We sought to understand the incidence of, and patient characteristics associated with, long-acting opioid initiation following hospital discharge among opioid-naïve older adults.
METHODS
We examined the 20% random sample of US Medicare beneficiaries ≥65 years old who were hospitalized in 2016 and continuously enrolled in Parts A, B, and D for 1 year prior and 1 month following discharge, excluding beneficiaries with cancer or hospice care, those transferred from or discharged to a care facility, and those who had filled a prescription for an opioid within 90 days prior to hospitalization. We identified beneficiaries with a Part D claim for an opioid, excluding methadone and buprenorphine, within 7 days of discharge. We compared beneficiaries with at least one claim for a long-acting opioid (including extended-release formulations) within 7 days of hospital discharge to those with short-acting opioid claims only.
We used a multivariable, generalized estimating equation to determine patient-level factors independently associated with prescription of any long-acting opioids. We selected characteristics that we hypothesized to be associated with new opioid prescription, based on clinical experience and previous literature, including sociodemographics, patient clinical characteristics such as a modified Elixhauser index (a composite index of nearly 30 comorbidities, excluding cancer),13 substance use-related factors, co-prescribed medications, and hospitalization-related factors. The latter included being hospitalized for a medical vs surgical reason, defined based on diagnosis-related group (DRG), primary diagnosis, and primary procedure, grouped using the Agency for Healthcare Research and Quality Clinical Classification System14 (Table 1).

We conducted a sensitivity analysis, excluding beneficiaries with high-dose long-term opioid use in the year before hospitalization.
RESULTS
Of 258,193 hospitalizations meeting eligibility criteria, 47,945 (18.6%) had an opioid claim within 7 days of discharge and comprised our analytic cohort (see the Appendix Figure for the study consort diagram), including 47,003 (18.2%) with short-acting opioids only and 942 (0.4%) with at least one claim for long-acting opioids, of whom 817 received both short- and long-acting opioids (Table 1).
Beneficiaries with long-acting opioid claims were more likely to be younger (ages 65-69 and 70-74 years) and White than those with claims for short-acting opioids only. They had a lower mean number of Elixhauser comorbidities but a higher prevalence of mental health conditions, including anxiety disorders and opioid use disorder, as well as a higher prevalence of previous high-dose long-term opioid use (occurring more than 90 days prior to hospitalization). They were more likely to have been hospitalized for a procedural rather than a medical reason, with 770 of the 942 (81.7%) beneficiaries receiving long-acting opioids having been hospitalized for a procedural reason (based on DRG). They were also more likely to have benzodiazepine co-prescription.
Factors independently associated with receipt of long-acting opioids compared to short-acting opioids only included younger age, having been admitted for a musculoskeletal problem, and presence of known risk factors for opioid-related adverse events, including anxiety disorders, opioid use disorder, prior long-term high-dose opioid use, and benzodiazepine co-prescription (Table 2). After excluding 33 beneficiaries with previous high-dose long-term opioid use in the year before hospitalization, associations were unchanged (Appendix Table).

DISCUSSION
Among a nationally representative sample of opioid-naïve Medicare beneficiaries without cancer, almost 20% filled a new opioid prescription within 7 days of hospital discharge. While prescription of long-acting opioids was uncommon, 81.7% who were prescribed a long-acting opioid had a procedural reason for hospitalization, raising concern since postoperative pain is typically acute and limited. Beneficiaries started on long-acting opioids more frequently had risk factors for opioid-related adverse events, including history of opioid use disorder and benzodiazepine co-prescription. With nearly three-quarters of patients with a long-acting opioid claim having been hospitalized for musculoskeletal disorders or orthopedic procedures, this population represents a key target for quality improvement interventions.
This is the first analysis describing the incidence and factors associated with long-acting opioid receipt shortly after hospital discharge among Medicare beneficiaries. Given that our data predate the publication of the Society of Hospital Medicine’s consensus statement on safe opioid prescribing in hospitalized patients,3 it is possible that there have been changes to prescribing patterns since 2016 that we are unable to characterize with our data. We are also limited by an inability to determine the appropriateness of any individual long-acting opioid prescription, though previous research has shown that long-acting opioids are frequently inappropriately initiated in older adults.15 Finally, our findings may not be generalizable to non-Medicare populations.
While long-acting opioid initiation following hospitalization is uncommon, these medications are most often prescribed to individuals with pain that is typically of limited duration and those at high risk for harm. Our findings highlight potential targets for systems-based solutions to improve guideline-concordant prescribing of long-acting opioids.
1. Tsilimingras D, Schnipper J, Duke A, et al. Post-discharge adverse events among urban and rural patients of an urban community hospital: a prospective cohort study. J Gen Intern Med. 2015;30(8):1164-1171. https://doi.org/10.1007/s11606-015-3260-3
2. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. https://doi.org/10.7326/0003-4819-138-3-200302040-00007
3. Herzig SJ, Mosher HJ, Calcaterra SL, Jena AB, Nuckols TK. Improving the safety of opioid use for acute noncancer pain in hospitalized adults: a consensus statement from the Society of Hospital Medicine. J Hosp Med. 2018;13(4):263-271. https://doi.org/10.12788/jhm.2980
4. Herzig SJ, Calcaterra SL, Mosher HJ, et al. Safe opioid prescribing for acute noncancer pain in hospitalized adults: a systematic review of existing guidelines. J Hosp Med. 2018;13(4):256-262. https://doi.org/10.12788/jhm.2979
5. Barnett ML, Olenski AR, Thygeson NM, et al. A health plan’s formulary led to reduced use of extended-release opioids but did not lower overall opioid use. Health Aff (Millwood). 2018;37(9):1509-1516. https://doi.org/10.1377/hlthaff.2018.0391
6. Carey CM, Jena AB, Barnett ML. Patterns of potential opioid misuse and subsequent adverse outcomes in Medicare, 2008 to 2012. Ann Intern Med. 2018;168(12):837-845. https://doi.org/10.7326/M17-3065
7. Miller M, Barber CW, Leatherman S, et al. Prescription opioid duration of action and the risk of unintentional overdose among patients receiving opioid therapy. JAMA Intern Med. 2015;175(4):608-615. https://doi.org/10.1001/jamainternmed.2014.8071
8. Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Prescription of long-acting opioids and mortality in patients with chronic noncancer pain. JAMA. 2016;315(22):2415-2423. https://doi.org/10.1001/jama.2016.7789
9. Zhu W, Chernew ME, Sherry TB, Maestas N. Initial opioid prescriptions among U.S. commercially insured patients, 2012-2017. N Engl J Med. 2019;380(11):1043-1052. https://doi.org/10.1056/NEJMsa1807069
10. Starner I, Gleason P. Short-acting, long-acting, and abuse-deterrent opioid utilization patterns among 15 million commercially insured members. Presented at: Academy of Managed Care Pharmacy (AMCP) Nexus; October 3-6, 2016; National Harbor, MD.
11. Young JC, Lund JL, Dasgupta N, Jonsson Funk M. Opioid tolerance and clinically recognized opioid poisoning among patients prescribed extended-release long-acting opioids. Pharmacoepidemiol Drug Saf. 2019;28(1):39-47. https://doi.org/10.1002/pds.4572
12. Hwang CS, Kang EM, Ding Y, et al. Patterns of immediate-release and extended-release opioid analgesic use in the management of chronic pain, 2003-2014. JAMA Netw Open. 2018;1(2):e180216. https://doi.org/10.1001/jamanetworkopen.2018.0216
13. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. https://doi.org/10.1097/00005650-199801000-00004
14. Agency for Healthcare Research and Quality. Clinical Classifications Software (CCS) for ICD-10-CM/PCS. Healthcare Cost and Utilization Project (HCUP). October 2018. www.hcup-us.ahrq.gov/toolssoftware/ccs10/ccs10.jsp
15. Willy ME, Graham DJ, Racoosin JA, et al. Candidate metrics for evaluating the impact of prescriber education on the safe use of extended-release/long-acting (ER/LA) opioid analgesics. Pain Med. 2014;15(9):1558-1568. https://doi.org/10.1111/pme.12459
1. Tsilimingras D, Schnipper J, Duke A, et al. Post-discharge adverse events among urban and rural patients of an urban community hospital: a prospective cohort study. J Gen Intern Med. 2015;30(8):1164-1171. https://doi.org/10.1007/s11606-015-3260-3
2. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. https://doi.org/10.7326/0003-4819-138-3-200302040-00007
3. Herzig SJ, Mosher HJ, Calcaterra SL, Jena AB, Nuckols TK. Improving the safety of opioid use for acute noncancer pain in hospitalized adults: a consensus statement from the Society of Hospital Medicine. J Hosp Med. 2018;13(4):263-271. https://doi.org/10.12788/jhm.2980
4. Herzig SJ, Calcaterra SL, Mosher HJ, et al. Safe opioid prescribing for acute noncancer pain in hospitalized adults: a systematic review of existing guidelines. J Hosp Med. 2018;13(4):256-262. https://doi.org/10.12788/jhm.2979
5. Barnett ML, Olenski AR, Thygeson NM, et al. A health plan’s formulary led to reduced use of extended-release opioids but did not lower overall opioid use. Health Aff (Millwood). 2018;37(9):1509-1516. https://doi.org/10.1377/hlthaff.2018.0391
6. Carey CM, Jena AB, Barnett ML. Patterns of potential opioid misuse and subsequent adverse outcomes in Medicare, 2008 to 2012. Ann Intern Med. 2018;168(12):837-845. https://doi.org/10.7326/M17-3065
7. Miller M, Barber CW, Leatherman S, et al. Prescription opioid duration of action and the risk of unintentional overdose among patients receiving opioid therapy. JAMA Intern Med. 2015;175(4):608-615. https://doi.org/10.1001/jamainternmed.2014.8071
8. Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Prescription of long-acting opioids and mortality in patients with chronic noncancer pain. JAMA. 2016;315(22):2415-2423. https://doi.org/10.1001/jama.2016.7789
9. Zhu W, Chernew ME, Sherry TB, Maestas N. Initial opioid prescriptions among U.S. commercially insured patients, 2012-2017. N Engl J Med. 2019;380(11):1043-1052. https://doi.org/10.1056/NEJMsa1807069
10. Starner I, Gleason P. Short-acting, long-acting, and abuse-deterrent opioid utilization patterns among 15 million commercially insured members. Presented at: Academy of Managed Care Pharmacy (AMCP) Nexus; October 3-6, 2016; National Harbor, MD.
11. Young JC, Lund JL, Dasgupta N, Jonsson Funk M. Opioid tolerance and clinically recognized opioid poisoning among patients prescribed extended-release long-acting opioids. Pharmacoepidemiol Drug Saf. 2019;28(1):39-47. https://doi.org/10.1002/pds.4572
12. Hwang CS, Kang EM, Ding Y, et al. Patterns of immediate-release and extended-release opioid analgesic use in the management of chronic pain, 2003-2014. JAMA Netw Open. 2018;1(2):e180216. https://doi.org/10.1001/jamanetworkopen.2018.0216
13. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. https://doi.org/10.1097/00005650-199801000-00004
14. Agency for Healthcare Research and Quality. Clinical Classifications Software (CCS) for ICD-10-CM/PCS. Healthcare Cost and Utilization Project (HCUP). October 2018. www.hcup-us.ahrq.gov/toolssoftware/ccs10/ccs10.jsp
15. Willy ME, Graham DJ, Racoosin JA, et al. Candidate metrics for evaluating the impact of prescriber education on the safe use of extended-release/long-acting (ER/LA) opioid analgesics. Pain Med. 2014;15(9):1558-1568. https://doi.org/10.1111/pme.12459
© 2021 Society of Hospital Medicine