User login
Assessing Outcomes Between Risperidone Microspheres and Paliperidone Palmitate Long-Acting Injectable Antipsychotics Among Veterans
Medication nonadherence is common with oral antipsychotic formulations, resulting in relapse, increased morbidity, and more frequent psychiatric hospitalization.1-7 Psychiatric hospitalization and illness decompensation is costly to health care systems and leads to reduced quality of life for veterans and families.6,7 Long-acting injectable antipsychotics (LAIAs) were developed to enhance antipsychotic adherence and improve patient outcomes, including reduced psychiatric hospitalization.8-12
Little outcomes data exist comparing LAIAs, including biweekly risperidone microspheres and monthly paliperidone palmitate.10-13 Risperidone microspheres require a 3-week oral crossover and are administered every 2 weeks, whereas paliperidone palmitate does not require an oral crossover and is administered every 4 weeks. The paliperidone palmitate loading regimen replaces an oral crossover.
The primary objective of this study was to compare the number of psychiatric hospitalizations between veterans administered risperidone microspheres and those on paliperidone palmitate pre- and post-LAIA initiation. Secondary objectives were to assess rehospitalization rates between patients taking risperidone microspheres and paliperidone palmitate, reduction in pre- and posthospitalization rates with LAIAs, and medication adherence.
Methods
This observational study with a retrospective cohort design was conducted at the Veterans Affairs Loma Linda Healthcare System (VALLHS) in California. We examined veterans who were initiated on LAIAs risperidone microspheres or paliperidone palmitate from January 01, 2016 through December 31, 2018. Veterans who were aged ≥ 18 years and received ≥ 2 injections of either risperidone microspheres or paliperidone palmitate during the study period were included. Veterans were excluded if they had received < 2 doses of either LAIA, received the LAIA outside of the review period, were nonadherent to risperidone crossover if they received risperidone microspheres, or transferred their care to another facility. At VALLHS, LAIA injections are administered by a nurse, and veterans must travel to the facility to receive the injections.
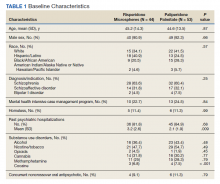
Extracted patient chart elements included participant demographics; diagnoses; comorbid alcohol, nicotine, opioid, or other substance use; duration on LAIA; psychiatric hospitalizations pre- and postinitiation of the LAIA; medication adherence; and medication discontinuation based on clinician documentation and clinic orders (Table 1).
Nonadherence to LAIA was defined as missing an injection by > 3 days for risperidone microspheres and > 7 days for paliperidone palmitate. This time frame was based on pharmacokinetic information listed in the products’ package inserts.14,15 Nonadherence to oral risperidone crossover with risperidone microspheres was defined as ≤ 80% of days covered.
Data Analysis
Patient demographics were analyzed using descriptive statistics and experimental comparisons between the risperidone microspheres and paliperidone palmitate groups to assess baseline differences between groups. Psychiatric hospitalizations pre- and post-LAIA were analyzed with parallel group (between veterans–independent groups) and pre-post (within veterans–dependent groups) designs. Index hospitalizations were examined for a period equivalent to the length of time veterans were on the LAIA. Psychiatric rehospitalization rates were analyzed for patients who had index hospitalizations and were rehospitalized for any period when they were receiving the LAIA. Incidences of pre- and post-LAIA hospitalizations were calculated in 100 person-years.
Parallel-group analysis was analyzed using the χ2 and Mann-Whitney U tests. Pre-post analyses were analyzed using the Wilcoxon rank sum test. P was set at < .05 for statistical significance.
Results
We screened 111 veterans, and 97 were included in this study (risperidone microspheres, 44; paliperidone palmitate, 53). Mean (SD) age was 46 (13.8) years, 92% were male, 38% were White, 94% were diagnosed with schizophrenia or schizoaffective disorder, and 11% were homeless. Substance use was documented as 52% for nicotine products, 40% for alcohol, 31% for cannabis, 27% for methamphetamine, 7% for cocaine, and 3% for opioids. Cannabis, methamphetamine, cocaine, and opioid use were based on clinician documentation and listed as active diagnoses at the time of LAIA initiation. Statistical significance was found in index hospitalizations P = .009) and history of cocaine use disorder (6.8% vs 7.5%, P < .001).
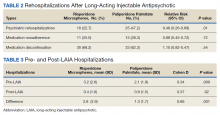
Veterans administered risperidone microspheres had fewer mean (SD) post-LAIA hospitalizations (0.4 [1.0] vs 0.9 [1.5]; P = .02) and were less likely to be rehospitalized (22.7% vs 47.2%, P = .01) compared with paliperidone palmitate. However, veterans taking risperidone microspheres had a shorter mean (SD) treatment duration (41.6 [40.2] vs 58.2 [45.7] weeks, P = .04) compared with paliperidone palmitate, mainly because patients switched to a different LAIA or oral antipsychotic. No differences were detected in nonadherence and discontinuation between risperidone microspheres and paliperidone palmitate. All veterans in the risperidone microspheres group adhered to oral risperidone crossover with an average 87.8% days covered (Table 2).
The average maintenance dose of risperidone microspheres was 42 mg every 2 weeks and 153 mg every 4 weeks for paliperidone palmitate.
Across the sample, 84% of veterans had a previous psychiatric hospitalization, although veterans initiated on risperidone microspheres had significantly higher mean (SD) index hospitalizations than those started on paliperidone palmitate (3.2 [2.6] risperidone microspheres vs 2.1 [1.9] paliperidone palmitate, P = .009). Both groups had significant decreases in mean (SD) hospitalizations (3.2 [2.6] to 0.4 [1.0], risperidone microspheres vs 2.1 [1.9] to 0.9 [1.5] paliperidone palmitate). The risperidone microspheres group had a larger decrease in mean (SD) hospitalizations post-LAIA (2.8 [2.9] risperidone microspheres vs 1.3 [1.7] paliperidone palmitate, P = .001) (Table 3).
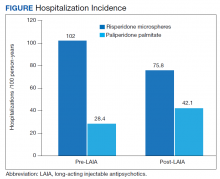
Differences in incidence per 100 person-years between pre- and post-LAIA hospitalizations were larger in risperidone microspheres users than in paliperidone palmitate (73.8 vs 33.7, P = .01) (Figure). No differences between risperidone microspheres and paliperidone palmitate were detected when looking at incidence pre-LAIA (102.2 vs 75.8, P = .22) and post-LAIA (28.4 vs 42.1, P = .38) separately.
Thirty veterans in the risperidone microspheres group discontinued LAIA: 11 were nonadherent, 5 experienced adverse effects (AEs), and 14 discontinued due to inconvenience. Among 33 veterans in the paliperidone palmitate group who discontinued the LAIA, 15 were nonadherent, 11 experienced AEs, 4 stopped due to of inconvenience, and 3 switched to a less frequently administered LAIA. The most common AEs reported were injection site reactions, cholinergic AEs (salivation, lacrimation, urination), orthostasis, and weight gain.
Discussion
The main finding of this study was that initiation of LAIAs significantly reduced hospitalizations. Veterans taking risperidone microspheres had higher index hospitalizations and lower posttreatment hospitalizations compared with paliperidone palmitate. We found that patients initiated on risperidone microspheres had more hospitalizations before use of a LAIA than those initiated on paliperidone palmitate. Risperidone microspheres reduced the number of hospitalization post-LAIA significantly more than paliperidone palmitate. We also found that veterans taking risperidone microspheres were on the medication for less mean (SD) time than those on paliperidone palmitate (41.6 [40.2] vs 58.2 [45.7] weeks; P = .04).
To our knowledge, this is one of the few studies that compared outcomes of psychiatric hospitalizations, medication adherence, and treatment discontinuation between risperidone microspheres and paliperidone palmitate, specifically in a veteran population.16-19 Limosin and colleagues aimed to compare length of stay during the initial hospitalization, rehospitalization risk, and treatment duration between risperidone microspheres and paliperidone palmitate in patients with schizophrenia.16 These researchers detected no differences in initial hospitalization duration and time to rehospitalization between risperidone microspheres and paliperidone palmitate.16 The study revealed a more favorable trend in time to discontinuation for paliperidone palmitate, but switching between LAIAs might have confounded the data.16 The authors note that their study lacked power, and patients on paliperidone palmitate had significantly more nonpsychiatric comorbidities.16 Joshi and colleagues looked at adherence, medication discontinuation, hospitalization rates, emergency department visits, and hospitalization costs between risperidone microspheres and paliperidone palmitate in patients identified in Truven MarketScan Commercial, Medicare Supplemental, and Medicaid Multi-State insurance databases.17 The authors found paliperidone palmitate to be superior in all objectives with better adherence, lower discontinuation rates, less likelihood of hospitalization, fewer emergency department visits, and lower hospitalization costs compared with risperidone microspheres.17 Korell and colleagues aimed to establish reference ranges for plasma concentrations of risperidone and paliperidone among adherent patients.18
The researchers established reference ranges for risperidone and paliperidone plasma concentrations that represented expected variability within a population and were derived from population pharmacokinetic models.18 Gopal and colleagues conducted a post hoc comparison between paliperidone palmitate and oral risperidone during initiation of long-acting injectable risperidone in patients with acute schizophrenia.19 The researchers found that during the first month after initiating long-acting injectable risperidone, paliperidone palmitate without oral supplementation had similar efficacy and safety to oral risperidone among these patients.19
LAIAs can create a steadier drug plasma concentration compared with oral antipsychotics and do not need to be taken daily. These agents improve adherence by reducing the frequency of medication administrations.20-24 Assessing nonadherence is easier with LAIAs by counting missed injections compared with oral antipsychotics that require calculation of percentage of days covered.25
The results in our study are somewhat unexpected in part because of the close relationship between risperidone and paliperidone. Risperidone is converted to paliperidone (9-OH-risperidone) via hepatic cytochrome P450 2D6. Although the molecules do not have identical pharmacologic profiles, it is accepted that they are similar enough that risperidone can establish oral tolerability when transitioning therapy to paliperidone palmitate and vice versa.24 Although the active moiety in risperidone microspheres and paliperidone palmitate is similar, the dosing interval for risperidone microspheres is 2 weeks compared with 4 weeks with paliperidone palmitate. One potential explanation as to why veterans started on risperidone microspheres experienced better outcomes is because they had twice as many office visits with the health care team. Facility procedures dictate veterans receive the LAIA at an on-site clinic. During the visits, a licensed vocational nurse administers the injection and monitors the patient for 15 to 30 minutes afterward.
Despite new LAIAs coming to market, high-quality data examining potential differences in treatment outcomes among agents are limited. This is problematic for clinicians who want to optimize care by understanding how administration schedules or other aspects of LAIA use could modify treatment outcomes. Our results suggest that an advantage might exist in selecting an agent with a more frequent administration schedule, at least initially. This could allow for close monitoring and regular therapeutic contact, which could improve short-term outcomes. This conclusion is supported by meta-analyses, randomized controlled trials, and conceptual articles conducted by Wehring and colleagues, Berwaerts and colleagues, and Parellada and colleagues, respectively, who examined patients on different LAIAs and contact with health care professionals as part of their research.26-28 These researchers concluded that patients who had regular contact with a health care professional had better outcomes when initiated on a LAIA.26-28
Limitations
There are several limitations in this study. Retrospective and observational methods introduce risks of bias and confounding variables. Sample size might have limited statistical power to detect differences. Veterans might have had undocumented pre- or posthospitalizations at other institutions, which was not accounted for and lack of rehospitalization is not conclusive of a positive outcome. Institutions could improve on our study and help to fill gaps in comparative data by conducting larger analyses over longer periods and including more LAIA agents.
Conclusions
Although veterans that were administered risperidone microspheres had a shorter treatment duration, they were less likely to be rehospitalized, had a fewer mean number of post-LAIA hospitalizations, and had a larger difference in incidence in 100 person-years compared with veterans on paliperidone palmitate. Nonadherence and discontinuation rates were comparable between risperidone microspheres and paliperidone palmitate. Future studies could aim to further clarify differences in outcomes among agents or administration schedules.
1. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
2. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223. doi:10.1056/NEJMoa051688
3. Swartz MS, Stroup TS, McEvoy JP, et al. What CATIE found: results from the schizophrenia trial. Psychiatr Serv. 2008;59(5):500-506. doi:10.1176/ps.2008.59.5.500
4. Haywood TW, Kravitz HM, Grossman LS, Cavanaugh JL Jr, Davis JM, Lewis DA. Predicting the “revolving door” phenomenon among patients with schizophrenic, schizoaffective, and affective disorders. Am J Psychiatry. 1995;152(6):856-561. doi:10.1176/ajp.152.6.856
5. Morken G, Widen JH, Grawe RW. Non-adherence to antipsychotic medication, relapse and rehospitalisation in recent-onset schizophrenia. BMC Psychiatry. 2008;8:32. doi:10.1186/1471-244X-8-32
6. Weiden PJ, Kozma C, Grogg A, Locklear J. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv. 2004;55(8):886-891. doi:10.1176/appi.ps.55.8.886
7. Gilmer TP, Dolder CR, Lacro JP, et al. Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. Am J Psychiatry. 2004;161(4):692-699. doi:10.1176/appi.ajp.161.4.692
8. Lafeuille MH, Dean J, Carter V, et al. Systematic review of long-acting injectables versus oral atypical antipsychotics on hospitalization in schizophrenia. Curr Med Res Opin. 2014;30(8):1643-1655. doi:10.1185/03007995.2014.915211
9. Yu W, Wagner TH, Chen S, Barnett PG. Average cost of VA rehabilitation, mental health, and long-term hospital stays. Med Care Res Rev. 2003;60(3 suppl):40S-53S. doi:10.1177/1077558703256724
10. Duncan EJ, Woolson SL, Hamer RM. Treatment compliance in veterans administration schizophrenia spectrum patients treated with risperidone long-acting injectable. Int Clin Psychopharmacol. 2012;27(5):283-290. doi:10.1097/YIC.0b013e328354b534
11. Romstadt N, Wonson E. Outcomes comparison of long-acting injectable antipsychotic initiation in treatment-naïve veterans in the inpatient versus outpatient setting. Ment Health Clin. 2018;8(1):24-27. doi:10.9740/mhc.2018.01.024
12. Dimitropoulos E, Drogemuller L, Wong K. Evaluation of concurrent oral and long-acting injectable antipsychotic prescribing at the Minneapolis Veterans Affairs Health Care System. J Clin Psychopharmacol. 2017;37(5):605-608. doi:10.1097/JCP.0000000000000755
13. Marcus SC, Zummo J, Pettit AR, Stoddard J, Doshi JA. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768. doi:10.18553/jmcp.2015.21.9.754
14. Risperdal Consta. Package insert. Janssen Pharmaceutical; 2007.
15. Invega Sustenna. Package insert. Janssen Pharmaceutical; 2009.
16. Limosin F, Belhadi D, Comet D, et al. Comparison of paliperidone palmitate and risperidone long-acting injection in schizophrenic patients: results from a multicenter retrospective cohort study in France. J Clin Psychopharmacol. 2018;38(1):19-26. doi:10.1097/JCP.0000000000000827
17. Joshi K, Pan X, Wang R, Yang E, Benson C. Healthcare resource utilization of second-generation long-acting injectable antipsychotics in schizophrenia: risperidone versus paliperidone palmitate. Curr Med Res Opin. 2016;32(11):1873-1881. doi: 10.1080/03007995.2016.1219706
18. Korell J, Green B, Remmerie B, Vermeulen A. Determination of plasma concentration reference ranges for risperidone and paliperidone. CPT Pharmacometrics Syst Pharmacol. 2017;6(9):589-595. doi:10.1002/psp4.12217
19. Gopal S, Pandina G, Lane R, et al. A post-hoc comparison of paliperidone palmitate to oral risperidone during initiation of long-acting risperidone injection in patients with acute schizophrenia. Innov Clin Neurosci. 2011;8(8):26-33.
20. Marcus SC, Zummo J, Pettit AR, Stoddard J, Doshi JA. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768. doi:10.18553/jmcp.2015.21.9.754
21. Romstadt N, Wonson E. Outcomes comparison of long-acting injectable antipsychotic initiation in treatment-naïve veterans in the inpatient versus outpatient setting. Ment Health Clin. 2018;8(1):24-27. doi:10.9740/mhc.2018.01.024
22. Green AI, Brunette MF, Dawson R, et al. Long-acting injectable vs oral risperidone for schizophrenia and co-occurring alcohol use disorder: a randomized trial. J Clin Psychiatry. 2015;76(10):1359-1365. doi:10.4088/JCP.13m08838
23. Rezansoff SN, Moniruzzaman A, Fazel S, Procyshyn R, Somers JM. Adherence to antipsychotic medication among homeless adults in Vancouver, Canada: a 15-year retrospective cohort study. Soc Psychiatry Psychiatr Epidemiol. 2016;51(12):1623-1632. doi:10.1007/s00127-016-1259-7
24. Castillo EG, Stroup TS. Effectiveness of long-acting injectable antipsychotics: a clinical perspective. Evid Based Ment Health. 2015;18(2):36-39. doi:10.1136/eb-2015-102086
25. Marder SR. Overview of partial compliance. J Clin Psychiatry. 2003;64 (suppl 16):3-9.
26. Wehring HJ, Thedford S, Koola M, Kelly DL. Patient and health care provider perspectives on long acting injectable antipsychotics in schizophrenia and the introduction of olanzapine long-acting injection. J Cent Nerv Syst Dis. 2011;2011(3):107-123. doi:10.4137/JCNSD.S4091
27. Berwaerts J, Liu Y, Gopal S, et al. Efficacy and safety of the 3-month formulation of paliperidone palmitate vs placebo for relapse prevention of schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2015;72(8):830-839. doi:10.1001/jamapsychiatry.2015.0241
28. Parellada E, Bioque M. Barriers to the use of long-acting injectable antipsychotics in the management of schizophrenia. CNS Drugs. 2016;30(8):689-701. doi:10.1007/s40263-016-0350-7
Medication nonadherence is common with oral antipsychotic formulations, resulting in relapse, increased morbidity, and more frequent psychiatric hospitalization.1-7 Psychiatric hospitalization and illness decompensation is costly to health care systems and leads to reduced quality of life for veterans and families.6,7 Long-acting injectable antipsychotics (LAIAs) were developed to enhance antipsychotic adherence and improve patient outcomes, including reduced psychiatric hospitalization.8-12
Little outcomes data exist comparing LAIAs, including biweekly risperidone microspheres and monthly paliperidone palmitate.10-13 Risperidone microspheres require a 3-week oral crossover and are administered every 2 weeks, whereas paliperidone palmitate does not require an oral crossover and is administered every 4 weeks. The paliperidone palmitate loading regimen replaces an oral crossover.
The primary objective of this study was to compare the number of psychiatric hospitalizations between veterans administered risperidone microspheres and those on paliperidone palmitate pre- and post-LAIA initiation. Secondary objectives were to assess rehospitalization rates between patients taking risperidone microspheres and paliperidone palmitate, reduction in pre- and posthospitalization rates with LAIAs, and medication adherence.
Methods
This observational study with a retrospective cohort design was conducted at the Veterans Affairs Loma Linda Healthcare System (VALLHS) in California. We examined veterans who were initiated on LAIAs risperidone microspheres or paliperidone palmitate from January 01, 2016 through December 31, 2018. Veterans who were aged ≥ 18 years and received ≥ 2 injections of either risperidone microspheres or paliperidone palmitate during the study period were included. Veterans were excluded if they had received < 2 doses of either LAIA, received the LAIA outside of the review period, were nonadherent to risperidone crossover if they received risperidone microspheres, or transferred their care to another facility. At VALLHS, LAIA injections are administered by a nurse, and veterans must travel to the facility to receive the injections.
Extracted patient chart elements included participant demographics; diagnoses; comorbid alcohol, nicotine, opioid, or other substance use; duration on LAIA; psychiatric hospitalizations pre- and postinitiation of the LAIA; medication adherence; and medication discontinuation based on clinician documentation and clinic orders (Table 1).
Nonadherence to LAIA was defined as missing an injection by > 3 days for risperidone microspheres and > 7 days for paliperidone palmitate. This time frame was based on pharmacokinetic information listed in the products’ package inserts.14,15 Nonadherence to oral risperidone crossover with risperidone microspheres was defined as ≤ 80% of days covered.
Data Analysis
Patient demographics were analyzed using descriptive statistics and experimental comparisons between the risperidone microspheres and paliperidone palmitate groups to assess baseline differences between groups. Psychiatric hospitalizations pre- and post-LAIA were analyzed with parallel group (between veterans–independent groups) and pre-post (within veterans–dependent groups) designs. Index hospitalizations were examined for a period equivalent to the length of time veterans were on the LAIA. Psychiatric rehospitalization rates were analyzed for patients who had index hospitalizations and were rehospitalized for any period when they were receiving the LAIA. Incidences of pre- and post-LAIA hospitalizations were calculated in 100 person-years.
Parallel-group analysis was analyzed using the χ2 and Mann-Whitney U tests. Pre-post analyses were analyzed using the Wilcoxon rank sum test. P was set at < .05 for statistical significance.
Results
We screened 111 veterans, and 97 were included in this study (risperidone microspheres, 44; paliperidone palmitate, 53). Mean (SD) age was 46 (13.8) years, 92% were male, 38% were White, 94% were diagnosed with schizophrenia or schizoaffective disorder, and 11% were homeless. Substance use was documented as 52% for nicotine products, 40% for alcohol, 31% for cannabis, 27% for methamphetamine, 7% for cocaine, and 3% for opioids. Cannabis, methamphetamine, cocaine, and opioid use were based on clinician documentation and listed as active diagnoses at the time of LAIA initiation. Statistical significance was found in index hospitalizations P = .009) and history of cocaine use disorder (6.8% vs 7.5%, P < .001).
Veterans administered risperidone microspheres had fewer mean (SD) post-LAIA hospitalizations (0.4 [1.0] vs 0.9 [1.5]; P = .02) and were less likely to be rehospitalized (22.7% vs 47.2%, P = .01) compared with paliperidone palmitate. However, veterans taking risperidone microspheres had a shorter mean (SD) treatment duration (41.6 [40.2] vs 58.2 [45.7] weeks, P = .04) compared with paliperidone palmitate, mainly because patients switched to a different LAIA or oral antipsychotic. No differences were detected in nonadherence and discontinuation between risperidone microspheres and paliperidone palmitate. All veterans in the risperidone microspheres group adhered to oral risperidone crossover with an average 87.8% days covered (Table 2).
The average maintenance dose of risperidone microspheres was 42 mg every 2 weeks and 153 mg every 4 weeks for paliperidone palmitate.
Across the sample, 84% of veterans had a previous psychiatric hospitalization, although veterans initiated on risperidone microspheres had significantly higher mean (SD) index hospitalizations than those started on paliperidone palmitate (3.2 [2.6] risperidone microspheres vs 2.1 [1.9] paliperidone palmitate, P = .009). Both groups had significant decreases in mean (SD) hospitalizations (3.2 [2.6] to 0.4 [1.0], risperidone microspheres vs 2.1 [1.9] to 0.9 [1.5] paliperidone palmitate). The risperidone microspheres group had a larger decrease in mean (SD) hospitalizations post-LAIA (2.8 [2.9] risperidone microspheres vs 1.3 [1.7] paliperidone palmitate, P = .001) (Table 3).
Differences in incidence per 100 person-years between pre- and post-LAIA hospitalizations were larger in risperidone microspheres users than in paliperidone palmitate (73.8 vs 33.7, P = .01) (Figure). No differences between risperidone microspheres and paliperidone palmitate were detected when looking at incidence pre-LAIA (102.2 vs 75.8, P = .22) and post-LAIA (28.4 vs 42.1, P = .38) separately.
Thirty veterans in the risperidone microspheres group discontinued LAIA: 11 were nonadherent, 5 experienced adverse effects (AEs), and 14 discontinued due to inconvenience. Among 33 veterans in the paliperidone palmitate group who discontinued the LAIA, 15 were nonadherent, 11 experienced AEs, 4 stopped due to of inconvenience, and 3 switched to a less frequently administered LAIA. The most common AEs reported were injection site reactions, cholinergic AEs (salivation, lacrimation, urination), orthostasis, and weight gain.
Discussion
The main finding of this study was that initiation of LAIAs significantly reduced hospitalizations. Veterans taking risperidone microspheres had higher index hospitalizations and lower posttreatment hospitalizations compared with paliperidone palmitate. We found that patients initiated on risperidone microspheres had more hospitalizations before use of a LAIA than those initiated on paliperidone palmitate. Risperidone microspheres reduced the number of hospitalization post-LAIA significantly more than paliperidone palmitate. We also found that veterans taking risperidone microspheres were on the medication for less mean (SD) time than those on paliperidone palmitate (41.6 [40.2] vs 58.2 [45.7] weeks; P = .04).
To our knowledge, this is one of the few studies that compared outcomes of psychiatric hospitalizations, medication adherence, and treatment discontinuation between risperidone microspheres and paliperidone palmitate, specifically in a veteran population.16-19 Limosin and colleagues aimed to compare length of stay during the initial hospitalization, rehospitalization risk, and treatment duration between risperidone microspheres and paliperidone palmitate in patients with schizophrenia.16 These researchers detected no differences in initial hospitalization duration and time to rehospitalization between risperidone microspheres and paliperidone palmitate.16 The study revealed a more favorable trend in time to discontinuation for paliperidone palmitate, but switching between LAIAs might have confounded the data.16 The authors note that their study lacked power, and patients on paliperidone palmitate had significantly more nonpsychiatric comorbidities.16 Joshi and colleagues looked at adherence, medication discontinuation, hospitalization rates, emergency department visits, and hospitalization costs between risperidone microspheres and paliperidone palmitate in patients identified in Truven MarketScan Commercial, Medicare Supplemental, and Medicaid Multi-State insurance databases.17 The authors found paliperidone palmitate to be superior in all objectives with better adherence, lower discontinuation rates, less likelihood of hospitalization, fewer emergency department visits, and lower hospitalization costs compared with risperidone microspheres.17 Korell and colleagues aimed to establish reference ranges for plasma concentrations of risperidone and paliperidone among adherent patients.18
The researchers established reference ranges for risperidone and paliperidone plasma concentrations that represented expected variability within a population and were derived from population pharmacokinetic models.18 Gopal and colleagues conducted a post hoc comparison between paliperidone palmitate and oral risperidone during initiation of long-acting injectable risperidone in patients with acute schizophrenia.19 The researchers found that during the first month after initiating long-acting injectable risperidone, paliperidone palmitate without oral supplementation had similar efficacy and safety to oral risperidone among these patients.19
LAIAs can create a steadier drug plasma concentration compared with oral antipsychotics and do not need to be taken daily. These agents improve adherence by reducing the frequency of medication administrations.20-24 Assessing nonadherence is easier with LAIAs by counting missed injections compared with oral antipsychotics that require calculation of percentage of days covered.25
The results in our study are somewhat unexpected in part because of the close relationship between risperidone and paliperidone. Risperidone is converted to paliperidone (9-OH-risperidone) via hepatic cytochrome P450 2D6. Although the molecules do not have identical pharmacologic profiles, it is accepted that they are similar enough that risperidone can establish oral tolerability when transitioning therapy to paliperidone palmitate and vice versa.24 Although the active moiety in risperidone microspheres and paliperidone palmitate is similar, the dosing interval for risperidone microspheres is 2 weeks compared with 4 weeks with paliperidone palmitate. One potential explanation as to why veterans started on risperidone microspheres experienced better outcomes is because they had twice as many office visits with the health care team. Facility procedures dictate veterans receive the LAIA at an on-site clinic. During the visits, a licensed vocational nurse administers the injection and monitors the patient for 15 to 30 minutes afterward.
Despite new LAIAs coming to market, high-quality data examining potential differences in treatment outcomes among agents are limited. This is problematic for clinicians who want to optimize care by understanding how administration schedules or other aspects of LAIA use could modify treatment outcomes. Our results suggest that an advantage might exist in selecting an agent with a more frequent administration schedule, at least initially. This could allow for close monitoring and regular therapeutic contact, which could improve short-term outcomes. This conclusion is supported by meta-analyses, randomized controlled trials, and conceptual articles conducted by Wehring and colleagues, Berwaerts and colleagues, and Parellada and colleagues, respectively, who examined patients on different LAIAs and contact with health care professionals as part of their research.26-28 These researchers concluded that patients who had regular contact with a health care professional had better outcomes when initiated on a LAIA.26-28
Limitations
There are several limitations in this study. Retrospective and observational methods introduce risks of bias and confounding variables. Sample size might have limited statistical power to detect differences. Veterans might have had undocumented pre- or posthospitalizations at other institutions, which was not accounted for and lack of rehospitalization is not conclusive of a positive outcome. Institutions could improve on our study and help to fill gaps in comparative data by conducting larger analyses over longer periods and including more LAIA agents.
Conclusions
Although veterans that were administered risperidone microspheres had a shorter treatment duration, they were less likely to be rehospitalized, had a fewer mean number of post-LAIA hospitalizations, and had a larger difference in incidence in 100 person-years compared with veterans on paliperidone palmitate. Nonadherence and discontinuation rates were comparable between risperidone microspheres and paliperidone palmitate. Future studies could aim to further clarify differences in outcomes among agents or administration schedules.
Medication nonadherence is common with oral antipsychotic formulations, resulting in relapse, increased morbidity, and more frequent psychiatric hospitalization.1-7 Psychiatric hospitalization and illness decompensation is costly to health care systems and leads to reduced quality of life for veterans and families.6,7 Long-acting injectable antipsychotics (LAIAs) were developed to enhance antipsychotic adherence and improve patient outcomes, including reduced psychiatric hospitalization.8-12
Little outcomes data exist comparing LAIAs, including biweekly risperidone microspheres and monthly paliperidone palmitate.10-13 Risperidone microspheres require a 3-week oral crossover and are administered every 2 weeks, whereas paliperidone palmitate does not require an oral crossover and is administered every 4 weeks. The paliperidone palmitate loading regimen replaces an oral crossover.
The primary objective of this study was to compare the number of psychiatric hospitalizations between veterans administered risperidone microspheres and those on paliperidone palmitate pre- and post-LAIA initiation. Secondary objectives were to assess rehospitalization rates between patients taking risperidone microspheres and paliperidone palmitate, reduction in pre- and posthospitalization rates with LAIAs, and medication adherence.
Methods
This observational study with a retrospective cohort design was conducted at the Veterans Affairs Loma Linda Healthcare System (VALLHS) in California. We examined veterans who were initiated on LAIAs risperidone microspheres or paliperidone palmitate from January 01, 2016 through December 31, 2018. Veterans who were aged ≥ 18 years and received ≥ 2 injections of either risperidone microspheres or paliperidone palmitate during the study period were included. Veterans were excluded if they had received < 2 doses of either LAIA, received the LAIA outside of the review period, were nonadherent to risperidone crossover if they received risperidone microspheres, or transferred their care to another facility. At VALLHS, LAIA injections are administered by a nurse, and veterans must travel to the facility to receive the injections.
Extracted patient chart elements included participant demographics; diagnoses; comorbid alcohol, nicotine, opioid, or other substance use; duration on LAIA; psychiatric hospitalizations pre- and postinitiation of the LAIA; medication adherence; and medication discontinuation based on clinician documentation and clinic orders (Table 1).
Nonadherence to LAIA was defined as missing an injection by > 3 days for risperidone microspheres and > 7 days for paliperidone palmitate. This time frame was based on pharmacokinetic information listed in the products’ package inserts.14,15 Nonadherence to oral risperidone crossover with risperidone microspheres was defined as ≤ 80% of days covered.
Data Analysis
Patient demographics were analyzed using descriptive statistics and experimental comparisons between the risperidone microspheres and paliperidone palmitate groups to assess baseline differences between groups. Psychiatric hospitalizations pre- and post-LAIA were analyzed with parallel group (between veterans–independent groups) and pre-post (within veterans–dependent groups) designs. Index hospitalizations were examined for a period equivalent to the length of time veterans were on the LAIA. Psychiatric rehospitalization rates were analyzed for patients who had index hospitalizations and were rehospitalized for any period when they were receiving the LAIA. Incidences of pre- and post-LAIA hospitalizations were calculated in 100 person-years.
Parallel-group analysis was analyzed using the χ2 and Mann-Whitney U tests. Pre-post analyses were analyzed using the Wilcoxon rank sum test. P was set at < .05 for statistical significance.
Results
We screened 111 veterans, and 97 were included in this study (risperidone microspheres, 44; paliperidone palmitate, 53). Mean (SD) age was 46 (13.8) years, 92% were male, 38% were White, 94% were diagnosed with schizophrenia or schizoaffective disorder, and 11% were homeless. Substance use was documented as 52% for nicotine products, 40% for alcohol, 31% for cannabis, 27% for methamphetamine, 7% for cocaine, and 3% for opioids. Cannabis, methamphetamine, cocaine, and opioid use were based on clinician documentation and listed as active diagnoses at the time of LAIA initiation. Statistical significance was found in index hospitalizations P = .009) and history of cocaine use disorder (6.8% vs 7.5%, P < .001).
Veterans administered risperidone microspheres had fewer mean (SD) post-LAIA hospitalizations (0.4 [1.0] vs 0.9 [1.5]; P = .02) and were less likely to be rehospitalized (22.7% vs 47.2%, P = .01) compared with paliperidone palmitate. However, veterans taking risperidone microspheres had a shorter mean (SD) treatment duration (41.6 [40.2] vs 58.2 [45.7] weeks, P = .04) compared with paliperidone palmitate, mainly because patients switched to a different LAIA or oral antipsychotic. No differences were detected in nonadherence and discontinuation between risperidone microspheres and paliperidone palmitate. All veterans in the risperidone microspheres group adhered to oral risperidone crossover with an average 87.8% days covered (Table 2).
The average maintenance dose of risperidone microspheres was 42 mg every 2 weeks and 153 mg every 4 weeks for paliperidone palmitate.
Across the sample, 84% of veterans had a previous psychiatric hospitalization, although veterans initiated on risperidone microspheres had significantly higher mean (SD) index hospitalizations than those started on paliperidone palmitate (3.2 [2.6] risperidone microspheres vs 2.1 [1.9] paliperidone palmitate, P = .009). Both groups had significant decreases in mean (SD) hospitalizations (3.2 [2.6] to 0.4 [1.0], risperidone microspheres vs 2.1 [1.9] to 0.9 [1.5] paliperidone palmitate). The risperidone microspheres group had a larger decrease in mean (SD) hospitalizations post-LAIA (2.8 [2.9] risperidone microspheres vs 1.3 [1.7] paliperidone palmitate, P = .001) (Table 3).
Differences in incidence per 100 person-years between pre- and post-LAIA hospitalizations were larger in risperidone microspheres users than in paliperidone palmitate (73.8 vs 33.7, P = .01) (Figure). No differences between risperidone microspheres and paliperidone palmitate were detected when looking at incidence pre-LAIA (102.2 vs 75.8, P = .22) and post-LAIA (28.4 vs 42.1, P = .38) separately.
Thirty veterans in the risperidone microspheres group discontinued LAIA: 11 were nonadherent, 5 experienced adverse effects (AEs), and 14 discontinued due to inconvenience. Among 33 veterans in the paliperidone palmitate group who discontinued the LAIA, 15 were nonadherent, 11 experienced AEs, 4 stopped due to of inconvenience, and 3 switched to a less frequently administered LAIA. The most common AEs reported were injection site reactions, cholinergic AEs (salivation, lacrimation, urination), orthostasis, and weight gain.
Discussion
The main finding of this study was that initiation of LAIAs significantly reduced hospitalizations. Veterans taking risperidone microspheres had higher index hospitalizations and lower posttreatment hospitalizations compared with paliperidone palmitate. We found that patients initiated on risperidone microspheres had more hospitalizations before use of a LAIA than those initiated on paliperidone palmitate. Risperidone microspheres reduced the number of hospitalization post-LAIA significantly more than paliperidone palmitate. We also found that veterans taking risperidone microspheres were on the medication for less mean (SD) time than those on paliperidone palmitate (41.6 [40.2] vs 58.2 [45.7] weeks; P = .04).
To our knowledge, this is one of the few studies that compared outcomes of psychiatric hospitalizations, medication adherence, and treatment discontinuation between risperidone microspheres and paliperidone palmitate, specifically in a veteran population.16-19 Limosin and colleagues aimed to compare length of stay during the initial hospitalization, rehospitalization risk, and treatment duration between risperidone microspheres and paliperidone palmitate in patients with schizophrenia.16 These researchers detected no differences in initial hospitalization duration and time to rehospitalization between risperidone microspheres and paliperidone palmitate.16 The study revealed a more favorable trend in time to discontinuation for paliperidone palmitate, but switching between LAIAs might have confounded the data.16 The authors note that their study lacked power, and patients on paliperidone palmitate had significantly more nonpsychiatric comorbidities.16 Joshi and colleagues looked at adherence, medication discontinuation, hospitalization rates, emergency department visits, and hospitalization costs between risperidone microspheres and paliperidone palmitate in patients identified in Truven MarketScan Commercial, Medicare Supplemental, and Medicaid Multi-State insurance databases.17 The authors found paliperidone palmitate to be superior in all objectives with better adherence, lower discontinuation rates, less likelihood of hospitalization, fewer emergency department visits, and lower hospitalization costs compared with risperidone microspheres.17 Korell and colleagues aimed to establish reference ranges for plasma concentrations of risperidone and paliperidone among adherent patients.18
The researchers established reference ranges for risperidone and paliperidone plasma concentrations that represented expected variability within a population and were derived from population pharmacokinetic models.18 Gopal and colleagues conducted a post hoc comparison between paliperidone palmitate and oral risperidone during initiation of long-acting injectable risperidone in patients with acute schizophrenia.19 The researchers found that during the first month after initiating long-acting injectable risperidone, paliperidone palmitate without oral supplementation had similar efficacy and safety to oral risperidone among these patients.19
LAIAs can create a steadier drug plasma concentration compared with oral antipsychotics and do not need to be taken daily. These agents improve adherence by reducing the frequency of medication administrations.20-24 Assessing nonadherence is easier with LAIAs by counting missed injections compared with oral antipsychotics that require calculation of percentage of days covered.25
The results in our study are somewhat unexpected in part because of the close relationship between risperidone and paliperidone. Risperidone is converted to paliperidone (9-OH-risperidone) via hepatic cytochrome P450 2D6. Although the molecules do not have identical pharmacologic profiles, it is accepted that they are similar enough that risperidone can establish oral tolerability when transitioning therapy to paliperidone palmitate and vice versa.24 Although the active moiety in risperidone microspheres and paliperidone palmitate is similar, the dosing interval for risperidone microspheres is 2 weeks compared with 4 weeks with paliperidone palmitate. One potential explanation as to why veterans started on risperidone microspheres experienced better outcomes is because they had twice as many office visits with the health care team. Facility procedures dictate veterans receive the LAIA at an on-site clinic. During the visits, a licensed vocational nurse administers the injection and monitors the patient for 15 to 30 minutes afterward.
Despite new LAIAs coming to market, high-quality data examining potential differences in treatment outcomes among agents are limited. This is problematic for clinicians who want to optimize care by understanding how administration schedules or other aspects of LAIA use could modify treatment outcomes. Our results suggest that an advantage might exist in selecting an agent with a more frequent administration schedule, at least initially. This could allow for close monitoring and regular therapeutic contact, which could improve short-term outcomes. This conclusion is supported by meta-analyses, randomized controlled trials, and conceptual articles conducted by Wehring and colleagues, Berwaerts and colleagues, and Parellada and colleagues, respectively, who examined patients on different LAIAs and contact with health care professionals as part of their research.26-28 These researchers concluded that patients who had regular contact with a health care professional had better outcomes when initiated on a LAIA.26-28
Limitations
There are several limitations in this study. Retrospective and observational methods introduce risks of bias and confounding variables. Sample size might have limited statistical power to detect differences. Veterans might have had undocumented pre- or posthospitalizations at other institutions, which was not accounted for and lack of rehospitalization is not conclusive of a positive outcome. Institutions could improve on our study and help to fill gaps in comparative data by conducting larger analyses over longer periods and including more LAIA agents.
Conclusions
Although veterans that were administered risperidone microspheres had a shorter treatment duration, they were less likely to be rehospitalized, had a fewer mean number of post-LAIA hospitalizations, and had a larger difference in incidence in 100 person-years compared with veterans on paliperidone palmitate. Nonadherence and discontinuation rates were comparable between risperidone microspheres and paliperidone palmitate. Future studies could aim to further clarify differences in outcomes among agents or administration schedules.
1. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
2. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223. doi:10.1056/NEJMoa051688
3. Swartz MS, Stroup TS, McEvoy JP, et al. What CATIE found: results from the schizophrenia trial. Psychiatr Serv. 2008;59(5):500-506. doi:10.1176/ps.2008.59.5.500
4. Haywood TW, Kravitz HM, Grossman LS, Cavanaugh JL Jr, Davis JM, Lewis DA. Predicting the “revolving door” phenomenon among patients with schizophrenic, schizoaffective, and affective disorders. Am J Psychiatry. 1995;152(6):856-561. doi:10.1176/ajp.152.6.856
5. Morken G, Widen JH, Grawe RW. Non-adherence to antipsychotic medication, relapse and rehospitalisation in recent-onset schizophrenia. BMC Psychiatry. 2008;8:32. doi:10.1186/1471-244X-8-32
6. Weiden PJ, Kozma C, Grogg A, Locklear J. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv. 2004;55(8):886-891. doi:10.1176/appi.ps.55.8.886
7. Gilmer TP, Dolder CR, Lacro JP, et al. Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. Am J Psychiatry. 2004;161(4):692-699. doi:10.1176/appi.ajp.161.4.692
8. Lafeuille MH, Dean J, Carter V, et al. Systematic review of long-acting injectables versus oral atypical antipsychotics on hospitalization in schizophrenia. Curr Med Res Opin. 2014;30(8):1643-1655. doi:10.1185/03007995.2014.915211
9. Yu W, Wagner TH, Chen S, Barnett PG. Average cost of VA rehabilitation, mental health, and long-term hospital stays. Med Care Res Rev. 2003;60(3 suppl):40S-53S. doi:10.1177/1077558703256724
10. Duncan EJ, Woolson SL, Hamer RM. Treatment compliance in veterans administration schizophrenia spectrum patients treated with risperidone long-acting injectable. Int Clin Psychopharmacol. 2012;27(5):283-290. doi:10.1097/YIC.0b013e328354b534
11. Romstadt N, Wonson E. Outcomes comparison of long-acting injectable antipsychotic initiation in treatment-naïve veterans in the inpatient versus outpatient setting. Ment Health Clin. 2018;8(1):24-27. doi:10.9740/mhc.2018.01.024
12. Dimitropoulos E, Drogemuller L, Wong K. Evaluation of concurrent oral and long-acting injectable antipsychotic prescribing at the Minneapolis Veterans Affairs Health Care System. J Clin Psychopharmacol. 2017;37(5):605-608. doi:10.1097/JCP.0000000000000755
13. Marcus SC, Zummo J, Pettit AR, Stoddard J, Doshi JA. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768. doi:10.18553/jmcp.2015.21.9.754
14. Risperdal Consta. Package insert. Janssen Pharmaceutical; 2007.
15. Invega Sustenna. Package insert. Janssen Pharmaceutical; 2009.
16. Limosin F, Belhadi D, Comet D, et al. Comparison of paliperidone palmitate and risperidone long-acting injection in schizophrenic patients: results from a multicenter retrospective cohort study in France. J Clin Psychopharmacol. 2018;38(1):19-26. doi:10.1097/JCP.0000000000000827
17. Joshi K, Pan X, Wang R, Yang E, Benson C. Healthcare resource utilization of second-generation long-acting injectable antipsychotics in schizophrenia: risperidone versus paliperidone palmitate. Curr Med Res Opin. 2016;32(11):1873-1881. doi: 10.1080/03007995.2016.1219706
18. Korell J, Green B, Remmerie B, Vermeulen A. Determination of plasma concentration reference ranges for risperidone and paliperidone. CPT Pharmacometrics Syst Pharmacol. 2017;6(9):589-595. doi:10.1002/psp4.12217
19. Gopal S, Pandina G, Lane R, et al. A post-hoc comparison of paliperidone palmitate to oral risperidone during initiation of long-acting risperidone injection in patients with acute schizophrenia. Innov Clin Neurosci. 2011;8(8):26-33.
20. Marcus SC, Zummo J, Pettit AR, Stoddard J, Doshi JA. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768. doi:10.18553/jmcp.2015.21.9.754
21. Romstadt N, Wonson E. Outcomes comparison of long-acting injectable antipsychotic initiation in treatment-naïve veterans in the inpatient versus outpatient setting. Ment Health Clin. 2018;8(1):24-27. doi:10.9740/mhc.2018.01.024
22. Green AI, Brunette MF, Dawson R, et al. Long-acting injectable vs oral risperidone for schizophrenia and co-occurring alcohol use disorder: a randomized trial. J Clin Psychiatry. 2015;76(10):1359-1365. doi:10.4088/JCP.13m08838
23. Rezansoff SN, Moniruzzaman A, Fazel S, Procyshyn R, Somers JM. Adherence to antipsychotic medication among homeless adults in Vancouver, Canada: a 15-year retrospective cohort study. Soc Psychiatry Psychiatr Epidemiol. 2016;51(12):1623-1632. doi:10.1007/s00127-016-1259-7
24. Castillo EG, Stroup TS. Effectiveness of long-acting injectable antipsychotics: a clinical perspective. Evid Based Ment Health. 2015;18(2):36-39. doi:10.1136/eb-2015-102086
25. Marder SR. Overview of partial compliance. J Clin Psychiatry. 2003;64 (suppl 16):3-9.
26. Wehring HJ, Thedford S, Koola M, Kelly DL. Patient and health care provider perspectives on long acting injectable antipsychotics in schizophrenia and the introduction of olanzapine long-acting injection. J Cent Nerv Syst Dis. 2011;2011(3):107-123. doi:10.4137/JCNSD.S4091
27. Berwaerts J, Liu Y, Gopal S, et al. Efficacy and safety of the 3-month formulation of paliperidone palmitate vs placebo for relapse prevention of schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2015;72(8):830-839. doi:10.1001/jamapsychiatry.2015.0241
28. Parellada E, Bioque M. Barriers to the use of long-acting injectable antipsychotics in the management of schizophrenia. CNS Drugs. 2016;30(8):689-701. doi:10.1007/s40263-016-0350-7
1. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
2. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223. doi:10.1056/NEJMoa051688
3. Swartz MS, Stroup TS, McEvoy JP, et al. What CATIE found: results from the schizophrenia trial. Psychiatr Serv. 2008;59(5):500-506. doi:10.1176/ps.2008.59.5.500
4. Haywood TW, Kravitz HM, Grossman LS, Cavanaugh JL Jr, Davis JM, Lewis DA. Predicting the “revolving door” phenomenon among patients with schizophrenic, schizoaffective, and affective disorders. Am J Psychiatry. 1995;152(6):856-561. doi:10.1176/ajp.152.6.856
5. Morken G, Widen JH, Grawe RW. Non-adherence to antipsychotic medication, relapse and rehospitalisation in recent-onset schizophrenia. BMC Psychiatry. 2008;8:32. doi:10.1186/1471-244X-8-32
6. Weiden PJ, Kozma C, Grogg A, Locklear J. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv. 2004;55(8):886-891. doi:10.1176/appi.ps.55.8.886
7. Gilmer TP, Dolder CR, Lacro JP, et al. Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. Am J Psychiatry. 2004;161(4):692-699. doi:10.1176/appi.ajp.161.4.692
8. Lafeuille MH, Dean J, Carter V, et al. Systematic review of long-acting injectables versus oral atypical antipsychotics on hospitalization in schizophrenia. Curr Med Res Opin. 2014;30(8):1643-1655. doi:10.1185/03007995.2014.915211
9. Yu W, Wagner TH, Chen S, Barnett PG. Average cost of VA rehabilitation, mental health, and long-term hospital stays. Med Care Res Rev. 2003;60(3 suppl):40S-53S. doi:10.1177/1077558703256724
10. Duncan EJ, Woolson SL, Hamer RM. Treatment compliance in veterans administration schizophrenia spectrum patients treated with risperidone long-acting injectable. Int Clin Psychopharmacol. 2012;27(5):283-290. doi:10.1097/YIC.0b013e328354b534
11. Romstadt N, Wonson E. Outcomes comparison of long-acting injectable antipsychotic initiation in treatment-naïve veterans in the inpatient versus outpatient setting. Ment Health Clin. 2018;8(1):24-27. doi:10.9740/mhc.2018.01.024
12. Dimitropoulos E, Drogemuller L, Wong K. Evaluation of concurrent oral and long-acting injectable antipsychotic prescribing at the Minneapolis Veterans Affairs Health Care System. J Clin Psychopharmacol. 2017;37(5):605-608. doi:10.1097/JCP.0000000000000755
13. Marcus SC, Zummo J, Pettit AR, Stoddard J, Doshi JA. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768. doi:10.18553/jmcp.2015.21.9.754
14. Risperdal Consta. Package insert. Janssen Pharmaceutical; 2007.
15. Invega Sustenna. Package insert. Janssen Pharmaceutical; 2009.
16. Limosin F, Belhadi D, Comet D, et al. Comparison of paliperidone palmitate and risperidone long-acting injection in schizophrenic patients: results from a multicenter retrospective cohort study in France. J Clin Psychopharmacol. 2018;38(1):19-26. doi:10.1097/JCP.0000000000000827
17. Joshi K, Pan X, Wang R, Yang E, Benson C. Healthcare resource utilization of second-generation long-acting injectable antipsychotics in schizophrenia: risperidone versus paliperidone palmitate. Curr Med Res Opin. 2016;32(11):1873-1881. doi: 10.1080/03007995.2016.1219706
18. Korell J, Green B, Remmerie B, Vermeulen A. Determination of plasma concentration reference ranges for risperidone and paliperidone. CPT Pharmacometrics Syst Pharmacol. 2017;6(9):589-595. doi:10.1002/psp4.12217
19. Gopal S, Pandina G, Lane R, et al. A post-hoc comparison of paliperidone palmitate to oral risperidone during initiation of long-acting risperidone injection in patients with acute schizophrenia. Innov Clin Neurosci. 2011;8(8):26-33.
20. Marcus SC, Zummo J, Pettit AR, Stoddard J, Doshi JA. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768. doi:10.18553/jmcp.2015.21.9.754
21. Romstadt N, Wonson E. Outcomes comparison of long-acting injectable antipsychotic initiation in treatment-naïve veterans in the inpatient versus outpatient setting. Ment Health Clin. 2018;8(1):24-27. doi:10.9740/mhc.2018.01.024
22. Green AI, Brunette MF, Dawson R, et al. Long-acting injectable vs oral risperidone for schizophrenia and co-occurring alcohol use disorder: a randomized trial. J Clin Psychiatry. 2015;76(10):1359-1365. doi:10.4088/JCP.13m08838
23. Rezansoff SN, Moniruzzaman A, Fazel S, Procyshyn R, Somers JM. Adherence to antipsychotic medication among homeless adults in Vancouver, Canada: a 15-year retrospective cohort study. Soc Psychiatry Psychiatr Epidemiol. 2016;51(12):1623-1632. doi:10.1007/s00127-016-1259-7
24. Castillo EG, Stroup TS. Effectiveness of long-acting injectable antipsychotics: a clinical perspective. Evid Based Ment Health. 2015;18(2):36-39. doi:10.1136/eb-2015-102086
25. Marder SR. Overview of partial compliance. J Clin Psychiatry. 2003;64 (suppl 16):3-9.
26. Wehring HJ, Thedford S, Koola M, Kelly DL. Patient and health care provider perspectives on long acting injectable antipsychotics in schizophrenia and the introduction of olanzapine long-acting injection. J Cent Nerv Syst Dis. 2011;2011(3):107-123. doi:10.4137/JCNSD.S4091
27. Berwaerts J, Liu Y, Gopal S, et al. Efficacy and safety of the 3-month formulation of paliperidone palmitate vs placebo for relapse prevention of schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2015;72(8):830-839. doi:10.1001/jamapsychiatry.2015.0241
28. Parellada E, Bioque M. Barriers to the use of long-acting injectable antipsychotics in the management of schizophrenia. CNS Drugs. 2016;30(8):689-701. doi:10.1007/s40263-016-0350-7
The Angel of Death in Clarksburg
Readers of this column may recall that since I have been the Editor-in-Chief of Federal Practitioner, my December editorial focuses on the best and worst of the year in federal medicine. In 2021, these evaluative terms fail to capture the sadness and global devastation that mark this grim epoch of the continuing pandemic, increasing climate disasters, rising political tensions, and racial violence. Thus, this year my editorial is framed in terms of the philosophical or theological categories of good and evil as the only concepts that can even begin to express the horrendous events that occurred in West Virginia.
On June 28, 2018, then US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) Executive-in-Charge, Carolyn Clancy, MD, contacted Inspector General Michael Missal to alert him that “there may be an ‘Angel of Death’ in Clarksburg [West Virginia].”1 Two years later Reta Mays, a 46-year-old VA nursing assistant, entered a guilty plea in federal court to the deaths of 7 vulnerable veterans. The legal charges were second-degree murder and 1 count of assault with intent to commit murder by injecting insulin. The victims were all patients on Ward 3 at the Louis A. Johnson VA, Medical Center in Clarksburg, where Mays worked the night shift from 2015 to 2018.2 Mays was sentenced in May of this year to 7 consecutive life terms for each of the veterans whose lives she cruelly ended and an additional 240 months for the eighth patient who survived her murder attempt.3
The term angel of death has religious roots in Judaism, although not strictly in the Hebrew scriptures. Neither the Jewish nor Christian Bible identifies a specific figure who is the angel of death. The idea first appears in Rabbinic literature and Jewish tradition.4 The angel God sends as a messenger of death is known as malakh ha-mavet in Hebrew. The revered Jewish physician and philosopher Moses Maimonides taught in his Guide for the Perplexed the angel of death is synonymous with the devil, and the evil inclination that dwells in the mind of all human beings.5In modern times, the concept of an angel of death has come to designate a serial killer who is a health care professional (HCP). A group of forensic scientists, HCPs, and attorneys, including former VA Under Secretary for Health Dr. Kenneth Kizer, published a study of HCPs who had been prosecuted or convicted of serial murder. Nurses constituted the largest group of offenders (60%) with nursing aides like Mays responsible for 18% of murders, and physicians 12%. The review found that though health care serial killers are rare, they operate in nations across the Western world, in many different states in this country, and in almost all health care settings, including previous VA angels of death.6Nursing aides who are not supposed to have access to medications—a major problem in Mays’ case—nor permitted to administer them more often resort to noncontrolled substances to kill their victims.1 Mays chose insulin as her murder weapon as did 13% of serial killers. Just as insulin may be difficult to detect in toxicology, so Mays and others like her committed their crimes on the night shift when they were less likely to be discovered.6
Many of us feel compelled to seek a rational motivation for why healers would mutate into killers: If we can find a reason for this heinous behavior it somehow helps us feel the world is more intelligible and controllable. Unfortunately, despite intensive forensic investigations of multiple angels of death, there is little definitive understanding of the motives of these murders.6 Mays disclosed more than most. As part of a plea bargain, she provided investigators with 2 rationales for her killing: She wanted to ease the patients’ suffering. Such claims of being an angel of mercy are common among HCP serial murders, which the patterns of the killings generally disprove. The patients Mays lethally injected, while mostly old and ill, were all expected to recover and leave the hospital. The Inspector General report uncovered a cautionary detail that has at least indirect bearing on the nursing assistant’s contention that she “wanted to let the patient’s die gently”: Contrary to VHA requirements, the facility had no functioning palliative care team. This finding in no way excuses or even explains Mays’ actions; it does, however, reinforce the essential value of palliative care expertise in an aging veteran population with many life-limiting conditions.7
Mays’ second motivation seems more plausible, based on her life narrative and the literature on HCP serial killers. Mays disclosed to investigators that she “had a lot of stress and chaos in [her] personal and professional life and these actions gave [her] a sense of control.”1 Her prior use of excessive force when employed at a prison as well as forensic science indicating that feelings of wielding power over life and death often drive health care murders, suggest this may have been a factor in Mays’ horrific conduct.8
It seems blasphemous to associate the word good in the same pages with this terrible evil. Nothing can compensate or justify the betrayal of the sacred oath of an HCP and the public trust of a VHA employee. Yet that very impossibility carries with it an obligation to ask, as did the author of an article about a recent Canadian nurse serial killer, “What can we learn from the [Mays] story?”9
Mays could never have taken the lives of 8 patients without clinical and administrative lapses and shortcuts at all levels of the health care system. Indeed, the 100-plus page Inspector General report makes 15 recommendations for the VHA, the Veterans Integrated Service Network, and the facility, encompassing areas of personnel hiring and performance evaluation, medication management and security, reporting and responding to unexplained events, quality and safety programs oversight, leaders’ responses, corrective actions, and even computer systems data analysis.
I want to suggest 2 ethical additions to this list addressed to all of us as VHA staff and especially to those of us who are HCPs. From the perspective of virtue ethics, Reta Mays is a tragedy about complacency and compromise in everyday work that the pandemic has made even more frequent and challenging to avoid and resist. This is what the Roman Virgil means in the epigraph that the road down to hell is easy and the road back very difficult.
I propose the need for discernment in trying to listen to our moral intuitions that tell us something is amiss and diligence in adhering to best practices even when we are fearful, exhausted, demoralized, or apathetic. These 2 habits of commitment to veterans, one of compassion and the other of competence, can help us follow the good inclinations of our hearts and together with system changes can bar the doors of our hospitals to the visits of future angels of death. This dedication is the least we owe to the families of the patients at Clarksburg whose loved ones never came home and whose questions likely can never be fully answered.
1. US Department of Veterans Affairs, Office of Inspector General. Veterans Health Administration: care and oversight deficiencies related to multiple homicides at the Louis A. Johnson VA Medical Center in Clarksburg, West Virginia. Healthcare Inspection Report #20-035993-140. Published May 11 2021. Accessed November 22, 2021. https://www.va.gov/oig/pubs/VAOIG-20-03593-140.pdf
2. Kennedy M, Schwartz M. Former VA medical worker pleads guilty to murdering 7 patients in West Virginia. Published July 14, 2020. Accessed November 22, 2021. https://www.npr.org/2020/07/14/890776010/former-va-medical-worker-charged-with-7-murders-in-west-virginia
3. US Department of Justice, US Attorney’s Office Northern District of West Virginia. Former VA hospital nursing assistant sentenced to seven consecutive life sentences for murdering seven veterans and assault with intent to commit murder of an eighth [press release]. Published May 11, 2021. Accessed November 22, 2021. https://www.justice.gov/usao-ndwv/pr/former-va-hospital-nursing-assistant-sentenced-seven-consecutive-life-sentences.
4. Jacobs L. The Jewish Religion: A Companion. 1st ed. Oxford University Press;1995:116.
5. Maimonides. Guide for the Perplexed. Frielander M, trans. Routledge and Kegan Paul Ltd; 1904:pt 3, chap 22.
6. Yorker BC, Kizer KW, Lampe P, Forrest AR, Lannan JM, Russell DA. Serial murder by healthcare professionals. J Forensic Sci. 2006;51(6):1362-1371. doi:10.1111/j.1556-4029.2006.00273.x
7. VHA Directive 1139. Palliative care consult teams (PCCT) and VISN leads. Published June 14, 2017.
8. Rourke S, Ward T. Healthcare serial killers: patterns and policies. Published August 14, 2017. Accessed November 22, 2021. https://www.medscape.com/viewarticle/884136
9. Frank C. Health care serial murder: what can we learn from the Wettlaufer story? Can Fam Physician. 2020;66(10):719-722.
Readers of this column may recall that since I have been the Editor-in-Chief of Federal Practitioner, my December editorial focuses on the best and worst of the year in federal medicine. In 2021, these evaluative terms fail to capture the sadness and global devastation that mark this grim epoch of the continuing pandemic, increasing climate disasters, rising political tensions, and racial violence. Thus, this year my editorial is framed in terms of the philosophical or theological categories of good and evil as the only concepts that can even begin to express the horrendous events that occurred in West Virginia.
On June 28, 2018, then US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) Executive-in-Charge, Carolyn Clancy, MD, contacted Inspector General Michael Missal to alert him that “there may be an ‘Angel of Death’ in Clarksburg [West Virginia].”1 Two years later Reta Mays, a 46-year-old VA nursing assistant, entered a guilty plea in federal court to the deaths of 7 vulnerable veterans. The legal charges were second-degree murder and 1 count of assault with intent to commit murder by injecting insulin. The victims were all patients on Ward 3 at the Louis A. Johnson VA, Medical Center in Clarksburg, where Mays worked the night shift from 2015 to 2018.2 Mays was sentenced in May of this year to 7 consecutive life terms for each of the veterans whose lives she cruelly ended and an additional 240 months for the eighth patient who survived her murder attempt.3
The term angel of death has religious roots in Judaism, although not strictly in the Hebrew scriptures. Neither the Jewish nor Christian Bible identifies a specific figure who is the angel of death. The idea first appears in Rabbinic literature and Jewish tradition.4 The angel God sends as a messenger of death is known as malakh ha-mavet in Hebrew. The revered Jewish physician and philosopher Moses Maimonides taught in his Guide for the Perplexed the angel of death is synonymous with the devil, and the evil inclination that dwells in the mind of all human beings.5In modern times, the concept of an angel of death has come to designate a serial killer who is a health care professional (HCP). A group of forensic scientists, HCPs, and attorneys, including former VA Under Secretary for Health Dr. Kenneth Kizer, published a study of HCPs who had been prosecuted or convicted of serial murder. Nurses constituted the largest group of offenders (60%) with nursing aides like Mays responsible for 18% of murders, and physicians 12%. The review found that though health care serial killers are rare, they operate in nations across the Western world, in many different states in this country, and in almost all health care settings, including previous VA angels of death.6Nursing aides who are not supposed to have access to medications—a major problem in Mays’ case—nor permitted to administer them more often resort to noncontrolled substances to kill their victims.1 Mays chose insulin as her murder weapon as did 13% of serial killers. Just as insulin may be difficult to detect in toxicology, so Mays and others like her committed their crimes on the night shift when they were less likely to be discovered.6
Many of us feel compelled to seek a rational motivation for why healers would mutate into killers: If we can find a reason for this heinous behavior it somehow helps us feel the world is more intelligible and controllable. Unfortunately, despite intensive forensic investigations of multiple angels of death, there is little definitive understanding of the motives of these murders.6 Mays disclosed more than most. As part of a plea bargain, she provided investigators with 2 rationales for her killing: She wanted to ease the patients’ suffering. Such claims of being an angel of mercy are common among HCP serial murders, which the patterns of the killings generally disprove. The patients Mays lethally injected, while mostly old and ill, were all expected to recover and leave the hospital. The Inspector General report uncovered a cautionary detail that has at least indirect bearing on the nursing assistant’s contention that she “wanted to let the patient’s die gently”: Contrary to VHA requirements, the facility had no functioning palliative care team. This finding in no way excuses or even explains Mays’ actions; it does, however, reinforce the essential value of palliative care expertise in an aging veteran population with many life-limiting conditions.7
Mays’ second motivation seems more plausible, based on her life narrative and the literature on HCP serial killers. Mays disclosed to investigators that she “had a lot of stress and chaos in [her] personal and professional life and these actions gave [her] a sense of control.”1 Her prior use of excessive force when employed at a prison as well as forensic science indicating that feelings of wielding power over life and death often drive health care murders, suggest this may have been a factor in Mays’ horrific conduct.8
It seems blasphemous to associate the word good in the same pages with this terrible evil. Nothing can compensate or justify the betrayal of the sacred oath of an HCP and the public trust of a VHA employee. Yet that very impossibility carries with it an obligation to ask, as did the author of an article about a recent Canadian nurse serial killer, “What can we learn from the [Mays] story?”9
Mays could never have taken the lives of 8 patients without clinical and administrative lapses and shortcuts at all levels of the health care system. Indeed, the 100-plus page Inspector General report makes 15 recommendations for the VHA, the Veterans Integrated Service Network, and the facility, encompassing areas of personnel hiring and performance evaluation, medication management and security, reporting and responding to unexplained events, quality and safety programs oversight, leaders’ responses, corrective actions, and even computer systems data analysis.
I want to suggest 2 ethical additions to this list addressed to all of us as VHA staff and especially to those of us who are HCPs. From the perspective of virtue ethics, Reta Mays is a tragedy about complacency and compromise in everyday work that the pandemic has made even more frequent and challenging to avoid and resist. This is what the Roman Virgil means in the epigraph that the road down to hell is easy and the road back very difficult.
I propose the need for discernment in trying to listen to our moral intuitions that tell us something is amiss and diligence in adhering to best practices even when we are fearful, exhausted, demoralized, or apathetic. These 2 habits of commitment to veterans, one of compassion and the other of competence, can help us follow the good inclinations of our hearts and together with system changes can bar the doors of our hospitals to the visits of future angels of death. This dedication is the least we owe to the families of the patients at Clarksburg whose loved ones never came home and whose questions likely can never be fully answered.
Readers of this column may recall that since I have been the Editor-in-Chief of Federal Practitioner, my December editorial focuses on the best and worst of the year in federal medicine. In 2021, these evaluative terms fail to capture the sadness and global devastation that mark this grim epoch of the continuing pandemic, increasing climate disasters, rising political tensions, and racial violence. Thus, this year my editorial is framed in terms of the philosophical or theological categories of good and evil as the only concepts that can even begin to express the horrendous events that occurred in West Virginia.
On June 28, 2018, then US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) Executive-in-Charge, Carolyn Clancy, MD, contacted Inspector General Michael Missal to alert him that “there may be an ‘Angel of Death’ in Clarksburg [West Virginia].”1 Two years later Reta Mays, a 46-year-old VA nursing assistant, entered a guilty plea in federal court to the deaths of 7 vulnerable veterans. The legal charges were second-degree murder and 1 count of assault with intent to commit murder by injecting insulin. The victims were all patients on Ward 3 at the Louis A. Johnson VA, Medical Center in Clarksburg, where Mays worked the night shift from 2015 to 2018.2 Mays was sentenced in May of this year to 7 consecutive life terms for each of the veterans whose lives she cruelly ended and an additional 240 months for the eighth patient who survived her murder attempt.3
The term angel of death has religious roots in Judaism, although not strictly in the Hebrew scriptures. Neither the Jewish nor Christian Bible identifies a specific figure who is the angel of death. The idea first appears in Rabbinic literature and Jewish tradition.4 The angel God sends as a messenger of death is known as malakh ha-mavet in Hebrew. The revered Jewish physician and philosopher Moses Maimonides taught in his Guide for the Perplexed the angel of death is synonymous with the devil, and the evil inclination that dwells in the mind of all human beings.5In modern times, the concept of an angel of death has come to designate a serial killer who is a health care professional (HCP). A group of forensic scientists, HCPs, and attorneys, including former VA Under Secretary for Health Dr. Kenneth Kizer, published a study of HCPs who had been prosecuted or convicted of serial murder. Nurses constituted the largest group of offenders (60%) with nursing aides like Mays responsible for 18% of murders, and physicians 12%. The review found that though health care serial killers are rare, they operate in nations across the Western world, in many different states in this country, and in almost all health care settings, including previous VA angels of death.6Nursing aides who are not supposed to have access to medications—a major problem in Mays’ case—nor permitted to administer them more often resort to noncontrolled substances to kill their victims.1 Mays chose insulin as her murder weapon as did 13% of serial killers. Just as insulin may be difficult to detect in toxicology, so Mays and others like her committed their crimes on the night shift when they were less likely to be discovered.6
Many of us feel compelled to seek a rational motivation for why healers would mutate into killers: If we can find a reason for this heinous behavior it somehow helps us feel the world is more intelligible and controllable. Unfortunately, despite intensive forensic investigations of multiple angels of death, there is little definitive understanding of the motives of these murders.6 Mays disclosed more than most. As part of a plea bargain, she provided investigators with 2 rationales for her killing: She wanted to ease the patients’ suffering. Such claims of being an angel of mercy are common among HCP serial murders, which the patterns of the killings generally disprove. The patients Mays lethally injected, while mostly old and ill, were all expected to recover and leave the hospital. The Inspector General report uncovered a cautionary detail that has at least indirect bearing on the nursing assistant’s contention that she “wanted to let the patient’s die gently”: Contrary to VHA requirements, the facility had no functioning palliative care team. This finding in no way excuses or even explains Mays’ actions; it does, however, reinforce the essential value of palliative care expertise in an aging veteran population with many life-limiting conditions.7
Mays’ second motivation seems more plausible, based on her life narrative and the literature on HCP serial killers. Mays disclosed to investigators that she “had a lot of stress and chaos in [her] personal and professional life and these actions gave [her] a sense of control.”1 Her prior use of excessive force when employed at a prison as well as forensic science indicating that feelings of wielding power over life and death often drive health care murders, suggest this may have been a factor in Mays’ horrific conduct.8
It seems blasphemous to associate the word good in the same pages with this terrible evil. Nothing can compensate or justify the betrayal of the sacred oath of an HCP and the public trust of a VHA employee. Yet that very impossibility carries with it an obligation to ask, as did the author of an article about a recent Canadian nurse serial killer, “What can we learn from the [Mays] story?”9
Mays could never have taken the lives of 8 patients without clinical and administrative lapses and shortcuts at all levels of the health care system. Indeed, the 100-plus page Inspector General report makes 15 recommendations for the VHA, the Veterans Integrated Service Network, and the facility, encompassing areas of personnel hiring and performance evaluation, medication management and security, reporting and responding to unexplained events, quality and safety programs oversight, leaders’ responses, corrective actions, and even computer systems data analysis.
I want to suggest 2 ethical additions to this list addressed to all of us as VHA staff and especially to those of us who are HCPs. From the perspective of virtue ethics, Reta Mays is a tragedy about complacency and compromise in everyday work that the pandemic has made even more frequent and challenging to avoid and resist. This is what the Roman Virgil means in the epigraph that the road down to hell is easy and the road back very difficult.
I propose the need for discernment in trying to listen to our moral intuitions that tell us something is amiss and diligence in adhering to best practices even when we are fearful, exhausted, demoralized, or apathetic. These 2 habits of commitment to veterans, one of compassion and the other of competence, can help us follow the good inclinations of our hearts and together with system changes can bar the doors of our hospitals to the visits of future angels of death. This dedication is the least we owe to the families of the patients at Clarksburg whose loved ones never came home and whose questions likely can never be fully answered.
1. US Department of Veterans Affairs, Office of Inspector General. Veterans Health Administration: care and oversight deficiencies related to multiple homicides at the Louis A. Johnson VA Medical Center in Clarksburg, West Virginia. Healthcare Inspection Report #20-035993-140. Published May 11 2021. Accessed November 22, 2021. https://www.va.gov/oig/pubs/VAOIG-20-03593-140.pdf
2. Kennedy M, Schwartz M. Former VA medical worker pleads guilty to murdering 7 patients in West Virginia. Published July 14, 2020. Accessed November 22, 2021. https://www.npr.org/2020/07/14/890776010/former-va-medical-worker-charged-with-7-murders-in-west-virginia
3. US Department of Justice, US Attorney’s Office Northern District of West Virginia. Former VA hospital nursing assistant sentenced to seven consecutive life sentences for murdering seven veterans and assault with intent to commit murder of an eighth [press release]. Published May 11, 2021. Accessed November 22, 2021. https://www.justice.gov/usao-ndwv/pr/former-va-hospital-nursing-assistant-sentenced-seven-consecutive-life-sentences.
4. Jacobs L. The Jewish Religion: A Companion. 1st ed. Oxford University Press;1995:116.
5. Maimonides. Guide for the Perplexed. Frielander M, trans. Routledge and Kegan Paul Ltd; 1904:pt 3, chap 22.
6. Yorker BC, Kizer KW, Lampe P, Forrest AR, Lannan JM, Russell DA. Serial murder by healthcare professionals. J Forensic Sci. 2006;51(6):1362-1371. doi:10.1111/j.1556-4029.2006.00273.x
7. VHA Directive 1139. Palliative care consult teams (PCCT) and VISN leads. Published June 14, 2017.
8. Rourke S, Ward T. Healthcare serial killers: patterns and policies. Published August 14, 2017. Accessed November 22, 2021. https://www.medscape.com/viewarticle/884136
9. Frank C. Health care serial murder: what can we learn from the Wettlaufer story? Can Fam Physician. 2020;66(10):719-722.
1. US Department of Veterans Affairs, Office of Inspector General. Veterans Health Administration: care and oversight deficiencies related to multiple homicides at the Louis A. Johnson VA Medical Center in Clarksburg, West Virginia. Healthcare Inspection Report #20-035993-140. Published May 11 2021. Accessed November 22, 2021. https://www.va.gov/oig/pubs/VAOIG-20-03593-140.pdf
2. Kennedy M, Schwartz M. Former VA medical worker pleads guilty to murdering 7 patients in West Virginia. Published July 14, 2020. Accessed November 22, 2021. https://www.npr.org/2020/07/14/890776010/former-va-medical-worker-charged-with-7-murders-in-west-virginia
3. US Department of Justice, US Attorney’s Office Northern District of West Virginia. Former VA hospital nursing assistant sentenced to seven consecutive life sentences for murdering seven veterans and assault with intent to commit murder of an eighth [press release]. Published May 11, 2021. Accessed November 22, 2021. https://www.justice.gov/usao-ndwv/pr/former-va-hospital-nursing-assistant-sentenced-seven-consecutive-life-sentences.
4. Jacobs L. The Jewish Religion: A Companion. 1st ed. Oxford University Press;1995:116.
5. Maimonides. Guide for the Perplexed. Frielander M, trans. Routledge and Kegan Paul Ltd; 1904:pt 3, chap 22.
6. Yorker BC, Kizer KW, Lampe P, Forrest AR, Lannan JM, Russell DA. Serial murder by healthcare professionals. J Forensic Sci. 2006;51(6):1362-1371. doi:10.1111/j.1556-4029.2006.00273.x
7. VHA Directive 1139. Palliative care consult teams (PCCT) and VISN leads. Published June 14, 2017.
8. Rourke S, Ward T. Healthcare serial killers: patterns and policies. Published August 14, 2017. Accessed November 22, 2021. https://www.medscape.com/viewarticle/884136
9. Frank C. Health care serial murder: what can we learn from the Wettlaufer story? Can Fam Physician. 2020;66(10):719-722.
Evaluation of Intermittent Energy Restriction and Continuous Energy Restriction on Weight Loss and Blood Pressure Control in Overweight and Obese Patients With Hypertension
Study Overview
Objective. To compare the effects of intermittent energy restriction (IER) with those of continuous energy restriction (CER) on blood pressure control and weight loss in overweight and obese patients with hypertension during a 6-month period.
Design. Randomized controlled trial.
Settings and participants. The trial was conducted at the Affiliated Hospital of Jiaxing University from June 1, 2020, to April 30, 2021. Chinese adults were recruited using advertisements and flyers posted in the hospital and local communities. Prior to participation in study activities, all participants gave informed consent prior to recruitment and were provided compensation in the form of a $38 voucher at 3 and 6 months for their time for participating in the study.
The main inclusion criteria were patients between the ages of 18 and 70 years, hypertension, and body mass index (BMI) ranging from 24 to 40 kg/m2. The exclusion criteria were systolic blood pressure (SBP) ≥ 180 mmHg or diastolic blood pressure (DBP) ≥ 120 mmHg, type 1 or 2 diabetes with a history of severe hypoglycemic episodes, pregnancy or breastfeeding, usage of glucagon-like peptide 1 receptor agonists, weight loss > 5 kg within the past 3 months or previous weight loss surgery, and inability to adhere to the dietary protocol.
Of the 294 participants screened for eligibility, 205 were randomized in a 1:1 ratio to the IER group (n = 102) or the CER group (n = 103), stratified by sex and BMI (as overweight or obese). All participants were required to have a stable medication regimen and weight in the 3 months prior to enrollment and not to use weight-loss drugs or vitamin supplements for the duration of the study. Researchers and participants were not blinded to the study group assignment.
Interventions. Participants randomly assigned to the IER group followed a 5:2 eating pattern: a very-low-energy diet of 500-600 kcal for 2 days of the week along with their usual diet for the other 5 days. The 2 days of calorie restriction could be consecutive or nonconsecutive, with a minimum of 0.8 g supplemental protein per kg of body weight per day, in accordance with the 2016 Dietary Guidelines for Chinese Residents. The CER group was advised to consume 1000 kcal/day for women and 1200 kcal/day for men on a 7-day energy restriction. That is, they were prescribed a daily 25% restriction based on the general principles of a Mediterranean-type diet (30% fat, 45-50% carbohydrate, and 20-25% protein).
Both groups received dietary education from a qualified dietitian and were recommended to maintain their current daily activity levels throughout the trial. Written dietary information brochures with portion advice and sample meal plans were provided to improve compliance in each group. All participants received a digital cooking scale to weigh foods to ensure accuracy of intake and were required to keep a food diary while following the recommended recipe on 2 days/week during calorie restriction to help with adherence. No food was provided. All participants were followed up by regular outpatient visits to both cardiologists and dietitians once a month. Diet checklists, activity schedules, and weight were reviewed to assess compliance with dietary advice at each visit.
Of note, participants were encouraged to measure and record their BP twice daily, and if 2 consecutive BP readings were < 110/70 mmHg and/or accompanied by hypotensive episodes with symptoms (dizziness, nausea, headache, and fatigue), they were asked to contact the investigators directly. Antihypertensive medication changes were then made in consultation with cardiologists. In addition, a medication management protocol (ie, doses of antidiabetic medications, including insulin and sulfonylurea) was designed to avoid hypoglycemia. Medication could be reduced in the CER group based on the basal dose at the endocrinologist’s discretion. In the IER group, insulin and sulfonylureas were discontinued on calorie restriction days only, and long-acting insulin was discontinued the night before the IER day. Insulin was not to be resumed until a full day’s caloric intake was achieved.
Measures and analysis. The primary outcomes of this study were changes in BP and weight (measured using an automatic digital sphygmomanometer and an electronic scale), and the secondary outcomes were changes in body composition (assessed by dual-energy x-ray absorptiometry scanning), as well as glycosylated hemoglobin A1c (HbA1c) levels and blood lipids after 6 months. All outcome measures were recorded at baseline and at each monthly visit. Incidence rates of hypoglycemia were based on blood glucose (defined as blood glucose < 70 mg/dL) and/or symptomatic hypoglycemia (symptoms of sweating, paleness, dizziness, and confusion). Two cardiologists who were blind to the patients’ diet condition measured and recorded all pertinent clinical parameters and adjudicated serious adverse events.
Data were compared using independent-samples t-tests or the Mann–Whitney U test for continuous variables, and Pearson’s χ2 test or Fisher’s exact test for categorial variables as appropriate. Repeated-measures ANOVA via a linear mixed model was employed to test the effects of diet, time, and their interaction. In subgroup analyses, differential effects of the intervention on the primary outcomes were evaluated with respect to patients’ level of education, domicile, and sex based on the statistical significance of the interaction term for the subgroup of interest in the multivariate model. Analyses were performed based on completers and on an intention-to-treat principle.
Main results. Among the 205 randomized participants, 118 were women and 87 were men; mean (SD) age was 50.5 (8.8) years; mean (SD) BMI was 28.7 (2.6); mean (SD) SBP was 143 (10) mmHg; and mean (SD) DBP was 91 (9) mmHg. At the end of the 6-month intervention, 173 (84.4%) completed the study (IER group: n = 88; CER group: n = 85). Both groups had similar dropout rates at 6 months (IER group: 14 participants [13.7%]; CER group: 18 participants [17.5%]; P = .83) and were well matched for baseline characteristics except for triglyceride levels.
In the completers analysis, both groups experienced significant reductions in weight (mean [SEM]), but there was no difference between treatment groups (−7.2 [0.6] kg in the IER group vs −7.1 [0.6] kg in the CER group; diet by time P = .72). Similarly, the change in SBP and DBP achieved was statistically significant over time, but there was also no difference between the dietary interventions (−8 [0.7] mmHg in the IER group vs −8 [0.6] mmHg in the CER group, diet by time P = .68; −6 [0.6] mmHg in the IER group vs −6 [0.5] mmHg in the CER group, diet by time P = .53]. Subgroup analyses of the association of the intervention with weight, SBP and DBP by sex, education, and domicile showed no significant between-group differences.
All measures of body composition decreased significantly at 6 months with both groups experiencing comparable reductions in total fat mass (−5.5 [0.6] kg in the IER group vs −4.8 [0.5] kg in the CER group, diet by time P = .08) and android fat mass (−1.1 [0.2] kg in the IER group vs −0.8 [0.2] kg in the CER group, diet by time P = .16). Of note, participants in the CER group lost significantly more total fat-free mass than did participants in the IER group (mean [SEM], −2.3 [0.2] kg vs −1.7 [0.2] kg; P = .03], and there was a trend toward a greater change in total fat mass in the IER group (P = .08). The secondary outcome of mean (SEM) HbA1c (−0.2% [0.1%]) and blood lipid levels (triglyceride level, −1.0 [0.3] mmol/L; total cholesterol level, −0.9 [0.2] mmol/L; low-density lipoprotein cholesterol level, −0.9 [0.2 mmol/L; high-density lipoprotein cholesterol level, 0.7 [0.3] mmol/L] improved with weight loss (P < .05), with no differences between groups (diet by time P > .05).
The intention-to-treat analysis demonstrated that IER and CER are equally effective for weight loss and blood pressure control: both groups experienced significant reductions in weight, SBP, and DBP, but with no difference between treatment groups – mean (SEM) weight change with IER was −7.0 (0.6) kg vs −6.8 (0.6) kg with CER; the mean (SEM) SBP with IER was −7 (0.7) mmHg vs −7 (0.6) mmHg with CER; and the mean (SEM) DBP with IER was −6 (0.5) mmHg vs −5 (0.5) mmHg with CER, (diet by time P = .62, .39, and .41, respectively). There were favorable improvements in
Conclusion. A 2-day severe energy restriction with 5 days of habitual eating compared to 7 days of CER provides an acceptable alternative for BP control and weight loss in overweight and obese individuals with hypertension after 6 months. IER may offer a useful alternative strategy for this population, who find continuous weight-loss diets too difficult to maintain.
Commentary
Globally, obesity represents a major health challenge as it substantially increases the risk of diseases such as hypertension, type 2 diabetes, and coronary heart disease.1 Lifestyle modifications, including weight loss and increased physical activity, are recommended in major guidelines as a first-step intervention in the treatment of hypertensive patients.2 However, lifestyle and behavioral interventions aimed at reducing calorie intake through low-calorie dieting is challenging as it is dependent on individual motivation and adherence to a strict, continuous protocol. Further, CER strategies have limited effectiveness because complex and persistent hormonal, metabolic, and neurochemical adaptations defend against weight loss and promote weight regain.3-4 IER has drawn attention in the popular media as an alternative to CER due to its feasibility and even potential for higher rates of compliance.5
This study adds to the literature as it is the first randomized controlled trial (to the knowledge of the authors at the time of publication) to explore 2 forms of energy restriction – CER and IER – and their impact on weight loss, BP, body composition, HbA1c, and blood lipid levels in overweight and obese patients with high blood pressure. Results from this study showed that IER is as effective as, but not superior to, CER (in terms of the outcomes measures assessed). Specifically, findings highlighted that the 5:2 diet is an effective strategy and noninferior to that of daily calorie restriction for BP and weight control. In addition, both weight loss and BP reduction were greater in a subgroup of obese compared with overweight participants, which indicates that obese populations may benefit more from energy restriction. As the authors highlight, this study both aligns with and expands on current related literature.
This study has both strengths and limitations, especially with regard to the design and data analysis strategy. A key strength is the randomized controlled trial design which enables increased internal validity and decreases several sources of bias, including selection bias and confounding. In addition, it was also designed as a pragmatic trial, with the protocol reflecting efforts to replicate the real-world environment by not supplying meal replacements or food. Notably, only 9 patients could not comply with the protocol, indicating that acceptability of the diet protocol was high. However, as this was only a 6-month long study, further studies are needed to determine whether a 5:2 diet is sustainable (and effective) in the long-term compared with CER, which the authors highlight. The study was also adequately powered to detect clinically meaningful differences in weight loss and SBP, and appropriate analyses were performed on both the basis of completers and on an intention-to-treat principle. However, further studies are needed that are adequately powered to also detect clinically meaningful differences in the other measures, ie, body composition, HbA1c, and blood lipid levels. Importantly, generalizability of findings from this study is limited as the study population comprises only Chinese adults, predominately middle-aged, overweight, and had mildly to moderately elevated SBP and DBP, and excluded diabetic patients. Thus, findings are not necessarily applicable to individuals with highly elevated blood pressure or poorly controlled diabetes.
Applications for Clinical Practice
Results of this study demonstrated that IER is an effective alternative diet strategy for weight loss and blood pressure control in overweight and obese patients with hypertension and is comparable to CER. This is relevant for clinical practice as IER may be easier to maintain in this population compared to continuous weight-loss diets. Importantly, both types of calorie restriction require clinical oversight as medication changes and periodic monitoring of hypotensive and hypoglycemic episodes are needed. Clinicians should consider what is feasible and sustainable for their patients when recommending intermittent energy restriction.
Financial disclosures: None.
1. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288-298. doi:10.1038/s41574-019-0176-8
2. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension Global hypertension practice guidelines. J Hypertens. 2020;38(6):982-1004. doi:10.1097/HJH.0000000000002453
3. Müller MJ, Enderle J, Bosy-Westphal A. Changes in Energy Expenditure with Weight Gain and Weight Loss in Humans. Curr Obes Rep. 2016;5(4):413-423. doi:10.1007/s13679-016-0237-4
4. Sainsbury A, Wood RE, Seimon RV, et al. Rationale for novel intermittent dieting strategies to attenuate adaptive responses to energy restriction. Obes Rev. 2018;19 Suppl 1:47–60. doi:10.1111/obr.12787
5. Davis CS, Clarke RE, Coulter SN, et al. Intermittent energy restriction and weight loss: a systematic review. Eur J Clin Nutr. 2016;70(3):292-299. doi:10.1038/ejcn.2015.195
Study Overview
Objective. To compare the effects of intermittent energy restriction (IER) with those of continuous energy restriction (CER) on blood pressure control and weight loss in overweight and obese patients with hypertension during a 6-month period.
Design. Randomized controlled trial.
Settings and participants. The trial was conducted at the Affiliated Hospital of Jiaxing University from June 1, 2020, to April 30, 2021. Chinese adults were recruited using advertisements and flyers posted in the hospital and local communities. Prior to participation in study activities, all participants gave informed consent prior to recruitment and were provided compensation in the form of a $38 voucher at 3 and 6 months for their time for participating in the study.
The main inclusion criteria were patients between the ages of 18 and 70 years, hypertension, and body mass index (BMI) ranging from 24 to 40 kg/m2. The exclusion criteria were systolic blood pressure (SBP) ≥ 180 mmHg or diastolic blood pressure (DBP) ≥ 120 mmHg, type 1 or 2 diabetes with a history of severe hypoglycemic episodes, pregnancy or breastfeeding, usage of glucagon-like peptide 1 receptor agonists, weight loss > 5 kg within the past 3 months or previous weight loss surgery, and inability to adhere to the dietary protocol.
Of the 294 participants screened for eligibility, 205 were randomized in a 1:1 ratio to the IER group (n = 102) or the CER group (n = 103), stratified by sex and BMI (as overweight or obese). All participants were required to have a stable medication regimen and weight in the 3 months prior to enrollment and not to use weight-loss drugs or vitamin supplements for the duration of the study. Researchers and participants were not blinded to the study group assignment.
Interventions. Participants randomly assigned to the IER group followed a 5:2 eating pattern: a very-low-energy diet of 500-600 kcal for 2 days of the week along with their usual diet for the other 5 days. The 2 days of calorie restriction could be consecutive or nonconsecutive, with a minimum of 0.8 g supplemental protein per kg of body weight per day, in accordance with the 2016 Dietary Guidelines for Chinese Residents. The CER group was advised to consume 1000 kcal/day for women and 1200 kcal/day for men on a 7-day energy restriction. That is, they were prescribed a daily 25% restriction based on the general principles of a Mediterranean-type diet (30% fat, 45-50% carbohydrate, and 20-25% protein).
Both groups received dietary education from a qualified dietitian and were recommended to maintain their current daily activity levels throughout the trial. Written dietary information brochures with portion advice and sample meal plans were provided to improve compliance in each group. All participants received a digital cooking scale to weigh foods to ensure accuracy of intake and were required to keep a food diary while following the recommended recipe on 2 days/week during calorie restriction to help with adherence. No food was provided. All participants were followed up by regular outpatient visits to both cardiologists and dietitians once a month. Diet checklists, activity schedules, and weight were reviewed to assess compliance with dietary advice at each visit.
Of note, participants were encouraged to measure and record their BP twice daily, and if 2 consecutive BP readings were < 110/70 mmHg and/or accompanied by hypotensive episodes with symptoms (dizziness, nausea, headache, and fatigue), they were asked to contact the investigators directly. Antihypertensive medication changes were then made in consultation with cardiologists. In addition, a medication management protocol (ie, doses of antidiabetic medications, including insulin and sulfonylurea) was designed to avoid hypoglycemia. Medication could be reduced in the CER group based on the basal dose at the endocrinologist’s discretion. In the IER group, insulin and sulfonylureas were discontinued on calorie restriction days only, and long-acting insulin was discontinued the night before the IER day. Insulin was not to be resumed until a full day’s caloric intake was achieved.
Measures and analysis. The primary outcomes of this study were changes in BP and weight (measured using an automatic digital sphygmomanometer and an electronic scale), and the secondary outcomes were changes in body composition (assessed by dual-energy x-ray absorptiometry scanning), as well as glycosylated hemoglobin A1c (HbA1c) levels and blood lipids after 6 months. All outcome measures were recorded at baseline and at each monthly visit. Incidence rates of hypoglycemia were based on blood glucose (defined as blood glucose < 70 mg/dL) and/or symptomatic hypoglycemia (symptoms of sweating, paleness, dizziness, and confusion). Two cardiologists who were blind to the patients’ diet condition measured and recorded all pertinent clinical parameters and adjudicated serious adverse events.
Data were compared using independent-samples t-tests or the Mann–Whitney U test for continuous variables, and Pearson’s χ2 test or Fisher’s exact test for categorial variables as appropriate. Repeated-measures ANOVA via a linear mixed model was employed to test the effects of diet, time, and their interaction. In subgroup analyses, differential effects of the intervention on the primary outcomes were evaluated with respect to patients’ level of education, domicile, and sex based on the statistical significance of the interaction term for the subgroup of interest in the multivariate model. Analyses were performed based on completers and on an intention-to-treat principle.
Main results. Among the 205 randomized participants, 118 were women and 87 were men; mean (SD) age was 50.5 (8.8) years; mean (SD) BMI was 28.7 (2.6); mean (SD) SBP was 143 (10) mmHg; and mean (SD) DBP was 91 (9) mmHg. At the end of the 6-month intervention, 173 (84.4%) completed the study (IER group: n = 88; CER group: n = 85). Both groups had similar dropout rates at 6 months (IER group: 14 participants [13.7%]; CER group: 18 participants [17.5%]; P = .83) and were well matched for baseline characteristics except for triglyceride levels.
In the completers analysis, both groups experienced significant reductions in weight (mean [SEM]), but there was no difference between treatment groups (−7.2 [0.6] kg in the IER group vs −7.1 [0.6] kg in the CER group; diet by time P = .72). Similarly, the change in SBP and DBP achieved was statistically significant over time, but there was also no difference between the dietary interventions (−8 [0.7] mmHg in the IER group vs −8 [0.6] mmHg in the CER group, diet by time P = .68; −6 [0.6] mmHg in the IER group vs −6 [0.5] mmHg in the CER group, diet by time P = .53]. Subgroup analyses of the association of the intervention with weight, SBP and DBP by sex, education, and domicile showed no significant between-group differences.
All measures of body composition decreased significantly at 6 months with both groups experiencing comparable reductions in total fat mass (−5.5 [0.6] kg in the IER group vs −4.8 [0.5] kg in the CER group, diet by time P = .08) and android fat mass (−1.1 [0.2] kg in the IER group vs −0.8 [0.2] kg in the CER group, diet by time P = .16). Of note, participants in the CER group lost significantly more total fat-free mass than did participants in the IER group (mean [SEM], −2.3 [0.2] kg vs −1.7 [0.2] kg; P = .03], and there was a trend toward a greater change in total fat mass in the IER group (P = .08). The secondary outcome of mean (SEM) HbA1c (−0.2% [0.1%]) and blood lipid levels (triglyceride level, −1.0 [0.3] mmol/L; total cholesterol level, −0.9 [0.2] mmol/L; low-density lipoprotein cholesterol level, −0.9 [0.2 mmol/L; high-density lipoprotein cholesterol level, 0.7 [0.3] mmol/L] improved with weight loss (P < .05), with no differences between groups (diet by time P > .05).
The intention-to-treat analysis demonstrated that IER and CER are equally effective for weight loss and blood pressure control: both groups experienced significant reductions in weight, SBP, and DBP, but with no difference between treatment groups – mean (SEM) weight change with IER was −7.0 (0.6) kg vs −6.8 (0.6) kg with CER; the mean (SEM) SBP with IER was −7 (0.7) mmHg vs −7 (0.6) mmHg with CER; and the mean (SEM) DBP with IER was −6 (0.5) mmHg vs −5 (0.5) mmHg with CER, (diet by time P = .62, .39, and .41, respectively). There were favorable improvements in
Conclusion. A 2-day severe energy restriction with 5 days of habitual eating compared to 7 days of CER provides an acceptable alternative for BP control and weight loss in overweight and obese individuals with hypertension after 6 months. IER may offer a useful alternative strategy for this population, who find continuous weight-loss diets too difficult to maintain.
Commentary
Globally, obesity represents a major health challenge as it substantially increases the risk of diseases such as hypertension, type 2 diabetes, and coronary heart disease.1 Lifestyle modifications, including weight loss and increased physical activity, are recommended in major guidelines as a first-step intervention in the treatment of hypertensive patients.2 However, lifestyle and behavioral interventions aimed at reducing calorie intake through low-calorie dieting is challenging as it is dependent on individual motivation and adherence to a strict, continuous protocol. Further, CER strategies have limited effectiveness because complex and persistent hormonal, metabolic, and neurochemical adaptations defend against weight loss and promote weight regain.3-4 IER has drawn attention in the popular media as an alternative to CER due to its feasibility and even potential for higher rates of compliance.5
This study adds to the literature as it is the first randomized controlled trial (to the knowledge of the authors at the time of publication) to explore 2 forms of energy restriction – CER and IER – and their impact on weight loss, BP, body composition, HbA1c, and blood lipid levels in overweight and obese patients with high blood pressure. Results from this study showed that IER is as effective as, but not superior to, CER (in terms of the outcomes measures assessed). Specifically, findings highlighted that the 5:2 diet is an effective strategy and noninferior to that of daily calorie restriction for BP and weight control. In addition, both weight loss and BP reduction were greater in a subgroup of obese compared with overweight participants, which indicates that obese populations may benefit more from energy restriction. As the authors highlight, this study both aligns with and expands on current related literature.
This study has both strengths and limitations, especially with regard to the design and data analysis strategy. A key strength is the randomized controlled trial design which enables increased internal validity and decreases several sources of bias, including selection bias and confounding. In addition, it was also designed as a pragmatic trial, with the protocol reflecting efforts to replicate the real-world environment by not supplying meal replacements or food. Notably, only 9 patients could not comply with the protocol, indicating that acceptability of the diet protocol was high. However, as this was only a 6-month long study, further studies are needed to determine whether a 5:2 diet is sustainable (and effective) in the long-term compared with CER, which the authors highlight. The study was also adequately powered to detect clinically meaningful differences in weight loss and SBP, and appropriate analyses were performed on both the basis of completers and on an intention-to-treat principle. However, further studies are needed that are adequately powered to also detect clinically meaningful differences in the other measures, ie, body composition, HbA1c, and blood lipid levels. Importantly, generalizability of findings from this study is limited as the study population comprises only Chinese adults, predominately middle-aged, overweight, and had mildly to moderately elevated SBP and DBP, and excluded diabetic patients. Thus, findings are not necessarily applicable to individuals with highly elevated blood pressure or poorly controlled diabetes.
Applications for Clinical Practice
Results of this study demonstrated that IER is an effective alternative diet strategy for weight loss and blood pressure control in overweight and obese patients with hypertension and is comparable to CER. This is relevant for clinical practice as IER may be easier to maintain in this population compared to continuous weight-loss diets. Importantly, both types of calorie restriction require clinical oversight as medication changes and periodic monitoring of hypotensive and hypoglycemic episodes are needed. Clinicians should consider what is feasible and sustainable for their patients when recommending intermittent energy restriction.
Financial disclosures: None.
Study Overview
Objective. To compare the effects of intermittent energy restriction (IER) with those of continuous energy restriction (CER) on blood pressure control and weight loss in overweight and obese patients with hypertension during a 6-month period.
Design. Randomized controlled trial.
Settings and participants. The trial was conducted at the Affiliated Hospital of Jiaxing University from June 1, 2020, to April 30, 2021. Chinese adults were recruited using advertisements and flyers posted in the hospital and local communities. Prior to participation in study activities, all participants gave informed consent prior to recruitment and were provided compensation in the form of a $38 voucher at 3 and 6 months for their time for participating in the study.
The main inclusion criteria were patients between the ages of 18 and 70 years, hypertension, and body mass index (BMI) ranging from 24 to 40 kg/m2. The exclusion criteria were systolic blood pressure (SBP) ≥ 180 mmHg or diastolic blood pressure (DBP) ≥ 120 mmHg, type 1 or 2 diabetes with a history of severe hypoglycemic episodes, pregnancy or breastfeeding, usage of glucagon-like peptide 1 receptor agonists, weight loss > 5 kg within the past 3 months or previous weight loss surgery, and inability to adhere to the dietary protocol.
Of the 294 participants screened for eligibility, 205 were randomized in a 1:1 ratio to the IER group (n = 102) or the CER group (n = 103), stratified by sex and BMI (as overweight or obese). All participants were required to have a stable medication regimen and weight in the 3 months prior to enrollment and not to use weight-loss drugs or vitamin supplements for the duration of the study. Researchers and participants were not blinded to the study group assignment.
Interventions. Participants randomly assigned to the IER group followed a 5:2 eating pattern: a very-low-energy diet of 500-600 kcal for 2 days of the week along with their usual diet for the other 5 days. The 2 days of calorie restriction could be consecutive or nonconsecutive, with a minimum of 0.8 g supplemental protein per kg of body weight per day, in accordance with the 2016 Dietary Guidelines for Chinese Residents. The CER group was advised to consume 1000 kcal/day for women and 1200 kcal/day for men on a 7-day energy restriction. That is, they were prescribed a daily 25% restriction based on the general principles of a Mediterranean-type diet (30% fat, 45-50% carbohydrate, and 20-25% protein).
Both groups received dietary education from a qualified dietitian and were recommended to maintain their current daily activity levels throughout the trial. Written dietary information brochures with portion advice and sample meal plans were provided to improve compliance in each group. All participants received a digital cooking scale to weigh foods to ensure accuracy of intake and were required to keep a food diary while following the recommended recipe on 2 days/week during calorie restriction to help with adherence. No food was provided. All participants were followed up by regular outpatient visits to both cardiologists and dietitians once a month. Diet checklists, activity schedules, and weight were reviewed to assess compliance with dietary advice at each visit.
Of note, participants were encouraged to measure and record their BP twice daily, and if 2 consecutive BP readings were < 110/70 mmHg and/or accompanied by hypotensive episodes with symptoms (dizziness, nausea, headache, and fatigue), they were asked to contact the investigators directly. Antihypertensive medication changes were then made in consultation with cardiologists. In addition, a medication management protocol (ie, doses of antidiabetic medications, including insulin and sulfonylurea) was designed to avoid hypoglycemia. Medication could be reduced in the CER group based on the basal dose at the endocrinologist’s discretion. In the IER group, insulin and sulfonylureas were discontinued on calorie restriction days only, and long-acting insulin was discontinued the night before the IER day. Insulin was not to be resumed until a full day’s caloric intake was achieved.
Measures and analysis. The primary outcomes of this study were changes in BP and weight (measured using an automatic digital sphygmomanometer and an electronic scale), and the secondary outcomes were changes in body composition (assessed by dual-energy x-ray absorptiometry scanning), as well as glycosylated hemoglobin A1c (HbA1c) levels and blood lipids after 6 months. All outcome measures were recorded at baseline and at each monthly visit. Incidence rates of hypoglycemia were based on blood glucose (defined as blood glucose < 70 mg/dL) and/or symptomatic hypoglycemia (symptoms of sweating, paleness, dizziness, and confusion). Two cardiologists who were blind to the patients’ diet condition measured and recorded all pertinent clinical parameters and adjudicated serious adverse events.
Data were compared using independent-samples t-tests or the Mann–Whitney U test for continuous variables, and Pearson’s χ2 test or Fisher’s exact test for categorial variables as appropriate. Repeated-measures ANOVA via a linear mixed model was employed to test the effects of diet, time, and their interaction. In subgroup analyses, differential effects of the intervention on the primary outcomes were evaluated with respect to patients’ level of education, domicile, and sex based on the statistical significance of the interaction term for the subgroup of interest in the multivariate model. Analyses were performed based on completers and on an intention-to-treat principle.
Main results. Among the 205 randomized participants, 118 were women and 87 were men; mean (SD) age was 50.5 (8.8) years; mean (SD) BMI was 28.7 (2.6); mean (SD) SBP was 143 (10) mmHg; and mean (SD) DBP was 91 (9) mmHg. At the end of the 6-month intervention, 173 (84.4%) completed the study (IER group: n = 88; CER group: n = 85). Both groups had similar dropout rates at 6 months (IER group: 14 participants [13.7%]; CER group: 18 participants [17.5%]; P = .83) and were well matched for baseline characteristics except for triglyceride levels.
In the completers analysis, both groups experienced significant reductions in weight (mean [SEM]), but there was no difference between treatment groups (−7.2 [0.6] kg in the IER group vs −7.1 [0.6] kg in the CER group; diet by time P = .72). Similarly, the change in SBP and DBP achieved was statistically significant over time, but there was also no difference between the dietary interventions (−8 [0.7] mmHg in the IER group vs −8 [0.6] mmHg in the CER group, diet by time P = .68; −6 [0.6] mmHg in the IER group vs −6 [0.5] mmHg in the CER group, diet by time P = .53]. Subgroup analyses of the association of the intervention with weight, SBP and DBP by sex, education, and domicile showed no significant between-group differences.
All measures of body composition decreased significantly at 6 months with both groups experiencing comparable reductions in total fat mass (−5.5 [0.6] kg in the IER group vs −4.8 [0.5] kg in the CER group, diet by time P = .08) and android fat mass (−1.1 [0.2] kg in the IER group vs −0.8 [0.2] kg in the CER group, diet by time P = .16). Of note, participants in the CER group lost significantly more total fat-free mass than did participants in the IER group (mean [SEM], −2.3 [0.2] kg vs −1.7 [0.2] kg; P = .03], and there was a trend toward a greater change in total fat mass in the IER group (P = .08). The secondary outcome of mean (SEM) HbA1c (−0.2% [0.1%]) and blood lipid levels (triglyceride level, −1.0 [0.3] mmol/L; total cholesterol level, −0.9 [0.2] mmol/L; low-density lipoprotein cholesterol level, −0.9 [0.2 mmol/L; high-density lipoprotein cholesterol level, 0.7 [0.3] mmol/L] improved with weight loss (P < .05), with no differences between groups (diet by time P > .05).
The intention-to-treat analysis demonstrated that IER and CER are equally effective for weight loss and blood pressure control: both groups experienced significant reductions in weight, SBP, and DBP, but with no difference between treatment groups – mean (SEM) weight change with IER was −7.0 (0.6) kg vs −6.8 (0.6) kg with CER; the mean (SEM) SBP with IER was −7 (0.7) mmHg vs −7 (0.6) mmHg with CER; and the mean (SEM) DBP with IER was −6 (0.5) mmHg vs −5 (0.5) mmHg with CER, (diet by time P = .62, .39, and .41, respectively). There were favorable improvements in
Conclusion. A 2-day severe energy restriction with 5 days of habitual eating compared to 7 days of CER provides an acceptable alternative for BP control and weight loss in overweight and obese individuals with hypertension after 6 months. IER may offer a useful alternative strategy for this population, who find continuous weight-loss diets too difficult to maintain.
Commentary
Globally, obesity represents a major health challenge as it substantially increases the risk of diseases such as hypertension, type 2 diabetes, and coronary heart disease.1 Lifestyle modifications, including weight loss and increased physical activity, are recommended in major guidelines as a first-step intervention in the treatment of hypertensive patients.2 However, lifestyle and behavioral interventions aimed at reducing calorie intake through low-calorie dieting is challenging as it is dependent on individual motivation and adherence to a strict, continuous protocol. Further, CER strategies have limited effectiveness because complex and persistent hormonal, metabolic, and neurochemical adaptations defend against weight loss and promote weight regain.3-4 IER has drawn attention in the popular media as an alternative to CER due to its feasibility and even potential for higher rates of compliance.5
This study adds to the literature as it is the first randomized controlled trial (to the knowledge of the authors at the time of publication) to explore 2 forms of energy restriction – CER and IER – and their impact on weight loss, BP, body composition, HbA1c, and blood lipid levels in overweight and obese patients with high blood pressure. Results from this study showed that IER is as effective as, but not superior to, CER (in terms of the outcomes measures assessed). Specifically, findings highlighted that the 5:2 diet is an effective strategy and noninferior to that of daily calorie restriction for BP and weight control. In addition, both weight loss and BP reduction were greater in a subgroup of obese compared with overweight participants, which indicates that obese populations may benefit more from energy restriction. As the authors highlight, this study both aligns with and expands on current related literature.
This study has both strengths and limitations, especially with regard to the design and data analysis strategy. A key strength is the randomized controlled trial design which enables increased internal validity and decreases several sources of bias, including selection bias and confounding. In addition, it was also designed as a pragmatic trial, with the protocol reflecting efforts to replicate the real-world environment by not supplying meal replacements or food. Notably, only 9 patients could not comply with the protocol, indicating that acceptability of the diet protocol was high. However, as this was only a 6-month long study, further studies are needed to determine whether a 5:2 diet is sustainable (and effective) in the long-term compared with CER, which the authors highlight. The study was also adequately powered to detect clinically meaningful differences in weight loss and SBP, and appropriate analyses were performed on both the basis of completers and on an intention-to-treat principle. However, further studies are needed that are adequately powered to also detect clinically meaningful differences in the other measures, ie, body composition, HbA1c, and blood lipid levels. Importantly, generalizability of findings from this study is limited as the study population comprises only Chinese adults, predominately middle-aged, overweight, and had mildly to moderately elevated SBP and DBP, and excluded diabetic patients. Thus, findings are not necessarily applicable to individuals with highly elevated blood pressure or poorly controlled diabetes.
Applications for Clinical Practice
Results of this study demonstrated that IER is an effective alternative diet strategy for weight loss and blood pressure control in overweight and obese patients with hypertension and is comparable to CER. This is relevant for clinical practice as IER may be easier to maintain in this population compared to continuous weight-loss diets. Importantly, both types of calorie restriction require clinical oversight as medication changes and periodic monitoring of hypotensive and hypoglycemic episodes are needed. Clinicians should consider what is feasible and sustainable for their patients when recommending intermittent energy restriction.
Financial disclosures: None.
1. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288-298. doi:10.1038/s41574-019-0176-8
2. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension Global hypertension practice guidelines. J Hypertens. 2020;38(6):982-1004. doi:10.1097/HJH.0000000000002453
3. Müller MJ, Enderle J, Bosy-Westphal A. Changes in Energy Expenditure with Weight Gain and Weight Loss in Humans. Curr Obes Rep. 2016;5(4):413-423. doi:10.1007/s13679-016-0237-4
4. Sainsbury A, Wood RE, Seimon RV, et al. Rationale for novel intermittent dieting strategies to attenuate adaptive responses to energy restriction. Obes Rev. 2018;19 Suppl 1:47–60. doi:10.1111/obr.12787
5. Davis CS, Clarke RE, Coulter SN, et al. Intermittent energy restriction and weight loss: a systematic review. Eur J Clin Nutr. 2016;70(3):292-299. doi:10.1038/ejcn.2015.195
1. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288-298. doi:10.1038/s41574-019-0176-8
2. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension Global hypertension practice guidelines. J Hypertens. 2020;38(6):982-1004. doi:10.1097/HJH.0000000000002453
3. Müller MJ, Enderle J, Bosy-Westphal A. Changes in Energy Expenditure with Weight Gain and Weight Loss in Humans. Curr Obes Rep. 2016;5(4):413-423. doi:10.1007/s13679-016-0237-4
4. Sainsbury A, Wood RE, Seimon RV, et al. Rationale for novel intermittent dieting strategies to attenuate adaptive responses to energy restriction. Obes Rev. 2018;19 Suppl 1:47–60. doi:10.1111/obr.12787
5. Davis CS, Clarke RE, Coulter SN, et al. Intermittent energy restriction and weight loss: a systematic review. Eur J Clin Nutr. 2016;70(3):292-299. doi:10.1038/ejcn.2015.195
Preoperative Code Status Discussion in Older Adults: Are We Doing Enough?
Study Overview
Objective. The objective of this study was to evaluate orders and documentation describing perioperative management of code status in adults.
Design. A retrospective case series of all adult inpatients admitted to hospitals at 1 academic health system in the US.
Setting and participants. This retrospective case series was conducted at 5 hospitals within the University of Pennsylvania Health System. Cases included all adult inpatients admitted to hospitals between March 2017 and September 2018 who had a Do-Not-Resuscitate (DNR) order placed in their medical record during admission and subsequently underwent a surgical procedure that required anesthesia care.
Main outcome measures. Medical records of included cases were manually reviewed by the authors to verify whether a DNR order was in place at the time surgical intervention was discussed with a patient. Clinical notes and DNR orders of eligible cases were reviewed to identify documentation and outcome of goals of care discussions that were conducted within 48 hours prior to the surgical procedure. Collected data included patient demographics (age, sex, race); case characteristics (American Society of Anesthesiologists [ASA] physical status score, anesthesia type [general vs others such as regional], emergency status [emergent vs elective surgery], procedures by service [surgical including hip fracture repair, gastrostomy or jejunostomy, or exploratory laparotomy vs medical including endoscopy, bronchoscopy, or transesophageal echocardiogram]); and hospital policy for perioperative management of DNR orders (written policy encouraging discussion vs written policy plus additional initiatives, including procedure-specific DNR form). The primary outcome was the presence of a preoperative order or note documenting code status discussion or change. Data were analyzed using χ2 and Fisher exact tests and the threshold for statistical significance was P < .05.
Main results. Of the 27 665 inpatient procedures identified across 5 hospitals, 444 (1.6%) cases met the inclusion criteria. Patients from these cases aged 75 (SD 13) years (95% CI, 72-77 years); 247 (56%, 95% CI, 55%-57%) were women; and 300 (68%, 95% CI, 65%-71%) were White. A total of 426 patients (96%, 95% CI, 90%-100%) had an ASA physical status score of 3 or higher and 237 (53%, 95% CI, 51%-56%) received general anesthesia. The most common procedures performed were endoscopy (148 [33%]), hip fracture repair (43 [10%]), and gastrostomy or jejunostomy (28 [6%]). Reevaluation of code status was documented in 126 cases (28%, 95% CI, 25%-31%); code status orders were changed in 20 of 126 cases (16%, 95% CI, 7%-24%); and a note was filed without a corresponding order for 106 of 126 cases (84%, 95% CI, 75%-95%). In the majority of cases (109 of 126 [87%], 95% CI, 78%-95%) in which documented discussion occurred, DNR orders were suspended. Of 126 cases in which a discussion was documented, participants of these discussions included surgeons 10% of the time (13 cases, 95% CI, 8%-13%), members of the anesthesia team 51% of the time (64 cases, 95% CI, 49%-53%), and medicine or palliative care clinicians 39% of the time (49 cases, 95% CI, 37%-41%).
The rate of documented preoperative code status discussion was higher in patients with higher ASA physical status score (35% in patients with an ASA physical status score ≥ 4 [55 of 155] vs 25% in those with an ASA physical status score ≤ 3 [71 of 289]; P = .02). The rates of documented preoperative code status discussion were similar by anesthesia type (29% for general anesthesia [69 of 237 cases] vs 28% [57 of 207 cases] for other modalities; P = .70). The hospitals involved in this study all had a written policy encouraging rediscussion of code status before surgery. However, only 1 hospital reported added measures (eg, provision of a procedure-specific DNR form) to increase documentation of preoperative code status discussions. In this specific hospital, documentation of preoperative code status discussions was higher compared to other hospitals (67% [37 of 55 cases] vs 23% [89 of 389 cases]; P < .01).
Conclusion. In a retrospective case series conducted at 5 hospitals within 1 academic health system in the US, fewer than 1 in 5 patients with preexisting DNR orders had a documented discussion of code status prior to undergoing surgery. Additional strategies including the development of institutional protocols that facilitate perioperative management of advance directives, identification of local champions, and patient education, should be explored as means to improve preoperative code status reevaulation per guideline recommendations.
Commentary
It is not unusual that patients with a DNR order may require and undergo surgical interventions to treat reversible conditions, prevent progression of underlying disease, or mitigate distressing symptoms such as pain. For instance, intubation, mechanical ventilation, and administration of vasoactive drugs are resuscitative measures that may be needed to safely anesthetize and sedate a patient. As such, the American College of Surgeons1 has provided a statement on advance directives by patients with an existing DNR order to guide management. Specifically, the statement indicates that the best approach for these patients is a policy of “required reconsideration” of the existing DNR order. Required reconsideration means that “the patient or designated surrogate and the physicians who will be responsible for the patient’s care should, when possible, discuss the new intraoperative and perioperative risks associated with the surgical procedure, the patient’s treatment goals, and an approach for potentially life-threatening problems consistent with the patient’s values and preferences.” Moreover, the required reconsideration discussion needs to occur as early as it is practical once a decision is made to have surgery because the discussion “may result in the patient agreeing to suspend the DNR order during surgery and the perioperative period, retaining the original DNR order, or modifying the DNR order.” Given that surgical patients with DNR orders have significant comorbidities, many sustain postoperative complications, and nearly 1 in 4 die within 30 days of surgery, preoperative advance care planning (ACP) and code status discussions are particularly essential to delivering high quality surgical care.2
In the current study, Hadler et al3 conducted a retrospective analysis to evaluate orders and documentation describing perioperative management of code status in patients with existing DNR order at an academic health system in the US. The authors reported that fewer than 20% of patients with existing DNR orders had a documented discussion of code status prior to undergoing surgery. These findings add to the notion that compliance with such guidance on required reconsideration discussion is suboptimal in perioperative care in the US.4,5 A recently published study focused on patients aged more than 60 years undergoing high-risk oncologic or vascular surgeries similarly showed that the frequency of ACP discussions or advance directive documentations among older patients was low.6 This growing body of evidence is highly clinically relevant in that preoperative discussion on code status is highly relevant to the care of older adults, a population group that accounts for the majority of surgeries and is most vulnerable to poor surgical outcomes. Additionally, it highlights a disconnect between the shared recognition by surgeons and patients that ACP discussion is important in perioperative care and its low implementation rates.
Unsurprisingly, Hadler et al3 reported that added measures such as the provision of a procedure-specific DNR form led to an increase in the documentation of preoperative code status discussions in 1 of the hospitals studied. The authors suggested that strategies such as the development of institutional protocols aimed to facilitate perioperative advance directive discussions, identify local champions, and educate patients may be ways to improve preoperative code status reevaulation. The idea that institutional value and culture are key factors impacting surgeon behavior and may influence the practice of ACP discussion is not new. Thus, creative and adaptable strategies, resources, and trainings that are required by medical institutions and hospitals to support preoperative ACP discussions with patients undergoing surgeries need to be identified, validated, and implemented to optimize perioperative care in vulnerable patients.
Applications for Clinical Practice
The findings from the current study indicate that less than 20% of patients with preexisting DNR orders have a documented discussion of code status prior to undergoing surgery. Physicians and health care institutions need to identify barriers to, and implement strategies that, facilitate and optimize preoperative ACP discussions in order to provide patient-centered care in vulnerable surgical patients.
Financial disclosures: None.
1. American College of Surgeons Board of Regents. Statement on Advance Directives by Patients: “Do Not Resuscitate” in the Operating Room. American College of Surgeons. January 3, 2014. Accessed November 6, 2021. https://www.facs.org/about-acs/statements/19-advance-directives
2. Kazaure H, Roman S, Sosa JA. High mortality in surgical patients with do-not-resuscitate orders: analysis of 8256 patients. Arch Surg. 2011;146(8):922-928. doi:10.1001/archsurg.2011.69
3. Hadler RA, Fatuzzo M, Sahota G, Neuman MD. Perioperative Management of Do-Not-Resuscitate Orders at a Large Academic Health System. JAMA Surg. 2021;e214135. doi:10.1001/jamasurg.2021.4135
4. Coopmans VC, Gries CA. CRNA awareness and experience with perioperative DNR orders. AANA J. 2000;68(3):247-256.
5. Urman RD, Lilley EJ, Changala M, Lindvall C, Hepner DL, Bader AM. A Pilot Study to Evaluate Compliance with Guidelines for Preprocedural Reconsideration of Code Status Limitations. J Palliat Med. 2018;21(8):1152-1156. doi:10.1089/jpm.2017.0601
6. Kalbfell E, Kata A, Buffington AS, et al. Frequency of Preoperative Advance Care Planning for Older Adults Undergoing High-risk Surgery: A Secondary Analysis of a Randomized Clinical Trial. JAMA Surg. 2021;156(7):e211521. doi:10.1001/jamasurg.2021.1521
Study Overview
Objective. The objective of this study was to evaluate orders and documentation describing perioperative management of code status in adults.
Design. A retrospective case series of all adult inpatients admitted to hospitals at 1 academic health system in the US.
Setting and participants. This retrospective case series was conducted at 5 hospitals within the University of Pennsylvania Health System. Cases included all adult inpatients admitted to hospitals between March 2017 and September 2018 who had a Do-Not-Resuscitate (DNR) order placed in their medical record during admission and subsequently underwent a surgical procedure that required anesthesia care.
Main outcome measures. Medical records of included cases were manually reviewed by the authors to verify whether a DNR order was in place at the time surgical intervention was discussed with a patient. Clinical notes and DNR orders of eligible cases were reviewed to identify documentation and outcome of goals of care discussions that were conducted within 48 hours prior to the surgical procedure. Collected data included patient demographics (age, sex, race); case characteristics (American Society of Anesthesiologists [ASA] physical status score, anesthesia type [general vs others such as regional], emergency status [emergent vs elective surgery], procedures by service [surgical including hip fracture repair, gastrostomy or jejunostomy, or exploratory laparotomy vs medical including endoscopy, bronchoscopy, or transesophageal echocardiogram]); and hospital policy for perioperative management of DNR orders (written policy encouraging discussion vs written policy plus additional initiatives, including procedure-specific DNR form). The primary outcome was the presence of a preoperative order or note documenting code status discussion or change. Data were analyzed using χ2 and Fisher exact tests and the threshold for statistical significance was P < .05.
Main results. Of the 27 665 inpatient procedures identified across 5 hospitals, 444 (1.6%) cases met the inclusion criteria. Patients from these cases aged 75 (SD 13) years (95% CI, 72-77 years); 247 (56%, 95% CI, 55%-57%) were women; and 300 (68%, 95% CI, 65%-71%) were White. A total of 426 patients (96%, 95% CI, 90%-100%) had an ASA physical status score of 3 or higher and 237 (53%, 95% CI, 51%-56%) received general anesthesia. The most common procedures performed were endoscopy (148 [33%]), hip fracture repair (43 [10%]), and gastrostomy or jejunostomy (28 [6%]). Reevaluation of code status was documented in 126 cases (28%, 95% CI, 25%-31%); code status orders were changed in 20 of 126 cases (16%, 95% CI, 7%-24%); and a note was filed without a corresponding order for 106 of 126 cases (84%, 95% CI, 75%-95%). In the majority of cases (109 of 126 [87%], 95% CI, 78%-95%) in which documented discussion occurred, DNR orders were suspended. Of 126 cases in which a discussion was documented, participants of these discussions included surgeons 10% of the time (13 cases, 95% CI, 8%-13%), members of the anesthesia team 51% of the time (64 cases, 95% CI, 49%-53%), and medicine or palliative care clinicians 39% of the time (49 cases, 95% CI, 37%-41%).
The rate of documented preoperative code status discussion was higher in patients with higher ASA physical status score (35% in patients with an ASA physical status score ≥ 4 [55 of 155] vs 25% in those with an ASA physical status score ≤ 3 [71 of 289]; P = .02). The rates of documented preoperative code status discussion were similar by anesthesia type (29% for general anesthesia [69 of 237 cases] vs 28% [57 of 207 cases] for other modalities; P = .70). The hospitals involved in this study all had a written policy encouraging rediscussion of code status before surgery. However, only 1 hospital reported added measures (eg, provision of a procedure-specific DNR form) to increase documentation of preoperative code status discussions. In this specific hospital, documentation of preoperative code status discussions was higher compared to other hospitals (67% [37 of 55 cases] vs 23% [89 of 389 cases]; P < .01).
Conclusion. In a retrospective case series conducted at 5 hospitals within 1 academic health system in the US, fewer than 1 in 5 patients with preexisting DNR orders had a documented discussion of code status prior to undergoing surgery. Additional strategies including the development of institutional protocols that facilitate perioperative management of advance directives, identification of local champions, and patient education, should be explored as means to improve preoperative code status reevaulation per guideline recommendations.
Commentary
It is not unusual that patients with a DNR order may require and undergo surgical interventions to treat reversible conditions, prevent progression of underlying disease, or mitigate distressing symptoms such as pain. For instance, intubation, mechanical ventilation, and administration of vasoactive drugs are resuscitative measures that may be needed to safely anesthetize and sedate a patient. As such, the American College of Surgeons1 has provided a statement on advance directives by patients with an existing DNR order to guide management. Specifically, the statement indicates that the best approach for these patients is a policy of “required reconsideration” of the existing DNR order. Required reconsideration means that “the patient or designated surrogate and the physicians who will be responsible for the patient’s care should, when possible, discuss the new intraoperative and perioperative risks associated with the surgical procedure, the patient’s treatment goals, and an approach for potentially life-threatening problems consistent with the patient’s values and preferences.” Moreover, the required reconsideration discussion needs to occur as early as it is practical once a decision is made to have surgery because the discussion “may result in the patient agreeing to suspend the DNR order during surgery and the perioperative period, retaining the original DNR order, or modifying the DNR order.” Given that surgical patients with DNR orders have significant comorbidities, many sustain postoperative complications, and nearly 1 in 4 die within 30 days of surgery, preoperative advance care planning (ACP) and code status discussions are particularly essential to delivering high quality surgical care.2
In the current study, Hadler et al3 conducted a retrospective analysis to evaluate orders and documentation describing perioperative management of code status in patients with existing DNR order at an academic health system in the US. The authors reported that fewer than 20% of patients with existing DNR orders had a documented discussion of code status prior to undergoing surgery. These findings add to the notion that compliance with such guidance on required reconsideration discussion is suboptimal in perioperative care in the US.4,5 A recently published study focused on patients aged more than 60 years undergoing high-risk oncologic or vascular surgeries similarly showed that the frequency of ACP discussions or advance directive documentations among older patients was low.6 This growing body of evidence is highly clinically relevant in that preoperative discussion on code status is highly relevant to the care of older adults, a population group that accounts for the majority of surgeries and is most vulnerable to poor surgical outcomes. Additionally, it highlights a disconnect between the shared recognition by surgeons and patients that ACP discussion is important in perioperative care and its low implementation rates.
Unsurprisingly, Hadler et al3 reported that added measures such as the provision of a procedure-specific DNR form led to an increase in the documentation of preoperative code status discussions in 1 of the hospitals studied. The authors suggested that strategies such as the development of institutional protocols aimed to facilitate perioperative advance directive discussions, identify local champions, and educate patients may be ways to improve preoperative code status reevaulation. The idea that institutional value and culture are key factors impacting surgeon behavior and may influence the practice of ACP discussion is not new. Thus, creative and adaptable strategies, resources, and trainings that are required by medical institutions and hospitals to support preoperative ACP discussions with patients undergoing surgeries need to be identified, validated, and implemented to optimize perioperative care in vulnerable patients.
Applications for Clinical Practice
The findings from the current study indicate that less than 20% of patients with preexisting DNR orders have a documented discussion of code status prior to undergoing surgery. Physicians and health care institutions need to identify barriers to, and implement strategies that, facilitate and optimize preoperative ACP discussions in order to provide patient-centered care in vulnerable surgical patients.
Financial disclosures: None.
Study Overview
Objective. The objective of this study was to evaluate orders and documentation describing perioperative management of code status in adults.
Design. A retrospective case series of all adult inpatients admitted to hospitals at 1 academic health system in the US.
Setting and participants. This retrospective case series was conducted at 5 hospitals within the University of Pennsylvania Health System. Cases included all adult inpatients admitted to hospitals between March 2017 and September 2018 who had a Do-Not-Resuscitate (DNR) order placed in their medical record during admission and subsequently underwent a surgical procedure that required anesthesia care.
Main outcome measures. Medical records of included cases were manually reviewed by the authors to verify whether a DNR order was in place at the time surgical intervention was discussed with a patient. Clinical notes and DNR orders of eligible cases were reviewed to identify documentation and outcome of goals of care discussions that were conducted within 48 hours prior to the surgical procedure. Collected data included patient demographics (age, sex, race); case characteristics (American Society of Anesthesiologists [ASA] physical status score, anesthesia type [general vs others such as regional], emergency status [emergent vs elective surgery], procedures by service [surgical including hip fracture repair, gastrostomy or jejunostomy, or exploratory laparotomy vs medical including endoscopy, bronchoscopy, or transesophageal echocardiogram]); and hospital policy for perioperative management of DNR orders (written policy encouraging discussion vs written policy plus additional initiatives, including procedure-specific DNR form). The primary outcome was the presence of a preoperative order or note documenting code status discussion or change. Data were analyzed using χ2 and Fisher exact tests and the threshold for statistical significance was P < .05.
Main results. Of the 27 665 inpatient procedures identified across 5 hospitals, 444 (1.6%) cases met the inclusion criteria. Patients from these cases aged 75 (SD 13) years (95% CI, 72-77 years); 247 (56%, 95% CI, 55%-57%) were women; and 300 (68%, 95% CI, 65%-71%) were White. A total of 426 patients (96%, 95% CI, 90%-100%) had an ASA physical status score of 3 or higher and 237 (53%, 95% CI, 51%-56%) received general anesthesia. The most common procedures performed were endoscopy (148 [33%]), hip fracture repair (43 [10%]), and gastrostomy or jejunostomy (28 [6%]). Reevaluation of code status was documented in 126 cases (28%, 95% CI, 25%-31%); code status orders were changed in 20 of 126 cases (16%, 95% CI, 7%-24%); and a note was filed without a corresponding order for 106 of 126 cases (84%, 95% CI, 75%-95%). In the majority of cases (109 of 126 [87%], 95% CI, 78%-95%) in which documented discussion occurred, DNR orders were suspended. Of 126 cases in which a discussion was documented, participants of these discussions included surgeons 10% of the time (13 cases, 95% CI, 8%-13%), members of the anesthesia team 51% of the time (64 cases, 95% CI, 49%-53%), and medicine or palliative care clinicians 39% of the time (49 cases, 95% CI, 37%-41%).
The rate of documented preoperative code status discussion was higher in patients with higher ASA physical status score (35% in patients with an ASA physical status score ≥ 4 [55 of 155] vs 25% in those with an ASA physical status score ≤ 3 [71 of 289]; P = .02). The rates of documented preoperative code status discussion were similar by anesthesia type (29% for general anesthesia [69 of 237 cases] vs 28% [57 of 207 cases] for other modalities; P = .70). The hospitals involved in this study all had a written policy encouraging rediscussion of code status before surgery. However, only 1 hospital reported added measures (eg, provision of a procedure-specific DNR form) to increase documentation of preoperative code status discussions. In this specific hospital, documentation of preoperative code status discussions was higher compared to other hospitals (67% [37 of 55 cases] vs 23% [89 of 389 cases]; P < .01).
Conclusion. In a retrospective case series conducted at 5 hospitals within 1 academic health system in the US, fewer than 1 in 5 patients with preexisting DNR orders had a documented discussion of code status prior to undergoing surgery. Additional strategies including the development of institutional protocols that facilitate perioperative management of advance directives, identification of local champions, and patient education, should be explored as means to improve preoperative code status reevaulation per guideline recommendations.
Commentary
It is not unusual that patients with a DNR order may require and undergo surgical interventions to treat reversible conditions, prevent progression of underlying disease, or mitigate distressing symptoms such as pain. For instance, intubation, mechanical ventilation, and administration of vasoactive drugs are resuscitative measures that may be needed to safely anesthetize and sedate a patient. As such, the American College of Surgeons1 has provided a statement on advance directives by patients with an existing DNR order to guide management. Specifically, the statement indicates that the best approach for these patients is a policy of “required reconsideration” of the existing DNR order. Required reconsideration means that “the patient or designated surrogate and the physicians who will be responsible for the patient’s care should, when possible, discuss the new intraoperative and perioperative risks associated with the surgical procedure, the patient’s treatment goals, and an approach for potentially life-threatening problems consistent with the patient’s values and preferences.” Moreover, the required reconsideration discussion needs to occur as early as it is practical once a decision is made to have surgery because the discussion “may result in the patient agreeing to suspend the DNR order during surgery and the perioperative period, retaining the original DNR order, or modifying the DNR order.” Given that surgical patients with DNR orders have significant comorbidities, many sustain postoperative complications, and nearly 1 in 4 die within 30 days of surgery, preoperative advance care planning (ACP) and code status discussions are particularly essential to delivering high quality surgical care.2
In the current study, Hadler et al3 conducted a retrospective analysis to evaluate orders and documentation describing perioperative management of code status in patients with existing DNR order at an academic health system in the US. The authors reported that fewer than 20% of patients with existing DNR orders had a documented discussion of code status prior to undergoing surgery. These findings add to the notion that compliance with such guidance on required reconsideration discussion is suboptimal in perioperative care in the US.4,5 A recently published study focused on patients aged more than 60 years undergoing high-risk oncologic or vascular surgeries similarly showed that the frequency of ACP discussions or advance directive documentations among older patients was low.6 This growing body of evidence is highly clinically relevant in that preoperative discussion on code status is highly relevant to the care of older adults, a population group that accounts for the majority of surgeries and is most vulnerable to poor surgical outcomes. Additionally, it highlights a disconnect between the shared recognition by surgeons and patients that ACP discussion is important in perioperative care and its low implementation rates.
Unsurprisingly, Hadler et al3 reported that added measures such as the provision of a procedure-specific DNR form led to an increase in the documentation of preoperative code status discussions in 1 of the hospitals studied. The authors suggested that strategies such as the development of institutional protocols aimed to facilitate perioperative advance directive discussions, identify local champions, and educate patients may be ways to improve preoperative code status reevaulation. The idea that institutional value and culture are key factors impacting surgeon behavior and may influence the practice of ACP discussion is not new. Thus, creative and adaptable strategies, resources, and trainings that are required by medical institutions and hospitals to support preoperative ACP discussions with patients undergoing surgeries need to be identified, validated, and implemented to optimize perioperative care in vulnerable patients.
Applications for Clinical Practice
The findings from the current study indicate that less than 20% of patients with preexisting DNR orders have a documented discussion of code status prior to undergoing surgery. Physicians and health care institutions need to identify barriers to, and implement strategies that, facilitate and optimize preoperative ACP discussions in order to provide patient-centered care in vulnerable surgical patients.
Financial disclosures: None.
1. American College of Surgeons Board of Regents. Statement on Advance Directives by Patients: “Do Not Resuscitate” in the Operating Room. American College of Surgeons. January 3, 2014. Accessed November 6, 2021. https://www.facs.org/about-acs/statements/19-advance-directives
2. Kazaure H, Roman S, Sosa JA. High mortality in surgical patients with do-not-resuscitate orders: analysis of 8256 patients. Arch Surg. 2011;146(8):922-928. doi:10.1001/archsurg.2011.69
3. Hadler RA, Fatuzzo M, Sahota G, Neuman MD. Perioperative Management of Do-Not-Resuscitate Orders at a Large Academic Health System. JAMA Surg. 2021;e214135. doi:10.1001/jamasurg.2021.4135
4. Coopmans VC, Gries CA. CRNA awareness and experience with perioperative DNR orders. AANA J. 2000;68(3):247-256.
5. Urman RD, Lilley EJ, Changala M, Lindvall C, Hepner DL, Bader AM. A Pilot Study to Evaluate Compliance with Guidelines for Preprocedural Reconsideration of Code Status Limitations. J Palliat Med. 2018;21(8):1152-1156. doi:10.1089/jpm.2017.0601
6. Kalbfell E, Kata A, Buffington AS, et al. Frequency of Preoperative Advance Care Planning for Older Adults Undergoing High-risk Surgery: A Secondary Analysis of a Randomized Clinical Trial. JAMA Surg. 2021;156(7):e211521. doi:10.1001/jamasurg.2021.1521
1. American College of Surgeons Board of Regents. Statement on Advance Directives by Patients: “Do Not Resuscitate” in the Operating Room. American College of Surgeons. January 3, 2014. Accessed November 6, 2021. https://www.facs.org/about-acs/statements/19-advance-directives
2. Kazaure H, Roman S, Sosa JA. High mortality in surgical patients with do-not-resuscitate orders: analysis of 8256 patients. Arch Surg. 2011;146(8):922-928. doi:10.1001/archsurg.2011.69
3. Hadler RA, Fatuzzo M, Sahota G, Neuman MD. Perioperative Management of Do-Not-Resuscitate Orders at a Large Academic Health System. JAMA Surg. 2021;e214135. doi:10.1001/jamasurg.2021.4135
4. Coopmans VC, Gries CA. CRNA awareness and experience with perioperative DNR orders. AANA J. 2000;68(3):247-256.
5. Urman RD, Lilley EJ, Changala M, Lindvall C, Hepner DL, Bader AM. A Pilot Study to Evaluate Compliance with Guidelines for Preprocedural Reconsideration of Code Status Limitations. J Palliat Med. 2018;21(8):1152-1156. doi:10.1089/jpm.2017.0601
6. Kalbfell E, Kata A, Buffington AS, et al. Frequency of Preoperative Advance Care Planning for Older Adults Undergoing High-risk Surgery: A Secondary Analysis of a Randomized Clinical Trial. JAMA Surg. 2021;156(7):e211521. doi:10.1001/jamasurg.2021.1521
FFR-Guided or Angiography-Guided Nonculprit Lesion PCI in Patients With STEMI Without Cardiogenic Shock
Study Overview
Objective. To determine whether fractional flow reserve (FFR)-guided percutaneous coronary intervention (PCI) of nonculprit lesion in patients with ST-segment elevation myocardial infarction (STEMI) is superior to angiography-guided PCI.
Design. Multicenter randomized control trial blinded to outcome, conducted in 41 sites in France.
Setting and participants. A total of 1163 patients with STEMI and multivessel coronary disease, who had undergone successful PCI to the culprit lesion were randomized to either FFR-guided PCI or angiography-guided PCI for nonculprit lesions. Randomization was stratified according to the trial site and timing of the procedure (immediate or staged).
Main outcome measures. The primary outcome was a composite of death from any cause, nonfatal myocardial infarction (MI) or unplanned hospitalization leading to urgent revascularization at 1 year.
Main results. At 1 year, the primary outcome occurred in 32 of 586 patients (5.5%) in the FFR-guided group and in 24 of 577 (4.2%) in the angiography-guided group (hazard ratio [HR], 1.32; 95% CI, 0.78-2.23; P = .31). The rate of death (1.5% vs 1.7%), nonfatal MI (3.1% vs 1.7%), and unplanned hospitalization leading to urgent revascularization (3.1% vs 1.7%) were also similar between FFR-guided and angiography-guided groups.
Conclusion. Among patients with STEMI and multivessel disease who had undergone successful PCI of the culprit vessel, an FFR-guided strategy for complete revascularization was not superior to angiography-guided strategy for reducing death, MI, or urgent revascularization at 1 year.
Commentary
Patients presenting with STEMI often have multivessel disease.1 Recently, multiple studies have reported the benefit of nonculprit vessel revascularization in patients presenting with hemodynamically stable STEMI compared to culprit-only strategy including the most recent COMPLETE trial which showed reduction in death and MI.2-6 However, the previous studies have variable design in evaluating the nonculprit vessel, some utilized FFR guidance, while others used angiography guidance. Whether FFR-guided PCI of nonculprit vessel can improve outcome in patients presenting STEMI remains unknown.
In the FLOWER-MI study, Puymirat et al investigated the use of FFR compared to angiography-guided nonculprit vessel PCI. A total of 1163 patients presenting with STEMI and multivessel disease who had undergone successful PCI to the culprit vessel, were randomized to either FFR guidance or angiography guidance among 41 centers in France. The authors found that after 1 year, there was no difference in composite endpoint of death, nonfatal MI or unplanned hospitalization leading to urgent revascularization in the FFR-guided group compared to angiography-guided group (5.5% vs 4.2%, HR, 1.32; 95% CI, 0.678-2.23; P = .31). There was also no difference in individual components of primary outcomes or secondary outcomes such as rate of stent thrombosis, any revascularization, or hospitalization.
There are a few interesting points to consider in this study. Ever since the Fractional Flow Reserve vs Angiography for Multivessel Evaluation (FAME) trial reported the lower incidence of major adverse events in routine FFR measurement during PCI compared to angiography-guided PCI, physiological assessment has become the gold standard for treatment of stable ischemic heart disease.7 However, the results of the current FLOWER-MI trial were not consistent with the FAME trial and there are few possible reasons to consider.
First, the use of FFR in the setting of STEMI is less validated compared to stable ischemic heart disease.8 Microvascular dysfunction during the acute phase can affect the FFR reading and the lesion severity can be underestimated.8 Second, the rate of composite endpoint was much lower in this study compared to FAME despite using the same composite endpoint of death, nonfatal MI, and unplanned hospitalization leading to urgent revascularization. At 1 year, the incidence of primary outcome was 13.5% in the FFR-guided group compared to 18.6% in the angiography-guided group in the FAME study compared to 5.5% and 4.2% in the FLOWER-MI study, despite having a sicker population presenting with STEMI. This is likely due to improvement in the PCI techniques such as radial approach, imaging guidance, and advancement in medical therapy such as use of more potent antiplatelet therapy. With lower incidence of primary outcome, larger number of patients are needed to detect the difference in the composite outcome. Finally, the operators’ visual assessment may have been calibrated to the physiologic assessment as the operators are routinely using FFR assessment which may have diminished the benefit of FFR guidance seen in the early FAME study.
Another interesting finding from this study was that although the study protocol encouraged the operators to perform the nonculprit PCI in the same setting, only 4% had nonculprit PCI in the same setting and 96% of the patients underwent a staged PCI. The advantage of performing the nonculprit PCI on the same setting is to have 1 fewer procedure for the patient. On the other hand, the disadvantage of this approach includes prolongation of the index procedure, theoretically higher risk of complication during the acute phase and vasospasm leading to overestimation of the lesion severity. A recent analysis from the COMPLETE study did not show any difference when comparing staged PCI during the index hospitalization vs after discharge.9 The optimal timing of the staged PCI needs to be investigated in future studies.
A limitation of this study is the lower than expected incidence of clinical events decreasing the statistical power of the study. However, there was no signal that FFR-guided PCI is better compared to the angiography-guided group. In fact, the curve started to diverge at 6 months favoring the angiography-guided group. In addition, there was no core-lab analysis for completeness of revascularization.
Applications for Clinical Practice
In patients presenting with hemodynamically stable STEMI for undergoing nonculprit vessel PCI, both FFR-guided or angiography-guided strategies can be considered.
Financial disclosures: None.
1. Park DW, Clare RM, Schulte PJ, et al. Extent, location, and clinical significance of non-infarct-related coronary artery disease among patients with ST-elevation myocardial infarction. JAMA. 2014;312(19):2019-27. doi:10.1001/jama.2014.15095
2. Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369(12):1115-23. doi:10.1056/NEJMoa1305520
3. Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65(10):963-72. doi:10.1016/j.jacc.2014.12.038
4. Engstrøm T, Kelbæk H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386(9994):665-71. doi:10.1016/s0140-6736(15)60648-1
5. Smits PC, Abdel-Wahab M, Neumann FJ, , et al. Fractional Flow Reserve-Guided Multivessel Angioplasty in Myocardial Infarction. N Engl J Med. 2017;376(13):1234-44. doi:10.1056/NEJMoa1701067
6. Mehta SR, Wood DA, Storey RF, et al. Complete Revascularization with Multivessel PCI for Myocardial Infarction. N Engl J Med. 2019;381(15):1411-21. doi:10.1056/NEJMoa1907775
7. Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213-24. doi:10.1056/NEJMoa0807611
8. Thim T, van der Hoeven NW, Musto C, et al. Evaluation and Management of Nonculprit Lesions in STEMI. JACC Cardiovasc Interv. 2020;13(10):1145-54. doi:10.1016/j.jcin.2020.02.030
9. Wood DA, Cairns JA, Wang J, et al. Timing of Staged Nonculprit Artery Revascularization in Patients With ST-Segment Elevation Myocardial Infarction: COMPLETE Trial. J Am Coll Cardiol. 2019;74(22):2713-23. doi:10.1016/j.jacc.2019/09.051
Study Overview
Objective. To determine whether fractional flow reserve (FFR)-guided percutaneous coronary intervention (PCI) of nonculprit lesion in patients with ST-segment elevation myocardial infarction (STEMI) is superior to angiography-guided PCI.
Design. Multicenter randomized control trial blinded to outcome, conducted in 41 sites in France.
Setting and participants. A total of 1163 patients with STEMI and multivessel coronary disease, who had undergone successful PCI to the culprit lesion were randomized to either FFR-guided PCI or angiography-guided PCI for nonculprit lesions. Randomization was stratified according to the trial site and timing of the procedure (immediate or staged).
Main outcome measures. The primary outcome was a composite of death from any cause, nonfatal myocardial infarction (MI) or unplanned hospitalization leading to urgent revascularization at 1 year.
Main results. At 1 year, the primary outcome occurred in 32 of 586 patients (5.5%) in the FFR-guided group and in 24 of 577 (4.2%) in the angiography-guided group (hazard ratio [HR], 1.32; 95% CI, 0.78-2.23; P = .31). The rate of death (1.5% vs 1.7%), nonfatal MI (3.1% vs 1.7%), and unplanned hospitalization leading to urgent revascularization (3.1% vs 1.7%) were also similar between FFR-guided and angiography-guided groups.
Conclusion. Among patients with STEMI and multivessel disease who had undergone successful PCI of the culprit vessel, an FFR-guided strategy for complete revascularization was not superior to angiography-guided strategy for reducing death, MI, or urgent revascularization at 1 year.
Commentary
Patients presenting with STEMI often have multivessel disease.1 Recently, multiple studies have reported the benefit of nonculprit vessel revascularization in patients presenting with hemodynamically stable STEMI compared to culprit-only strategy including the most recent COMPLETE trial which showed reduction in death and MI.2-6 However, the previous studies have variable design in evaluating the nonculprit vessel, some utilized FFR guidance, while others used angiography guidance. Whether FFR-guided PCI of nonculprit vessel can improve outcome in patients presenting STEMI remains unknown.
In the FLOWER-MI study, Puymirat et al investigated the use of FFR compared to angiography-guided nonculprit vessel PCI. A total of 1163 patients presenting with STEMI and multivessel disease who had undergone successful PCI to the culprit vessel, were randomized to either FFR guidance or angiography guidance among 41 centers in France. The authors found that after 1 year, there was no difference in composite endpoint of death, nonfatal MI or unplanned hospitalization leading to urgent revascularization in the FFR-guided group compared to angiography-guided group (5.5% vs 4.2%, HR, 1.32; 95% CI, 0.678-2.23; P = .31). There was also no difference in individual components of primary outcomes or secondary outcomes such as rate of stent thrombosis, any revascularization, or hospitalization.
There are a few interesting points to consider in this study. Ever since the Fractional Flow Reserve vs Angiography for Multivessel Evaluation (FAME) trial reported the lower incidence of major adverse events in routine FFR measurement during PCI compared to angiography-guided PCI, physiological assessment has become the gold standard for treatment of stable ischemic heart disease.7 However, the results of the current FLOWER-MI trial were not consistent with the FAME trial and there are few possible reasons to consider.
First, the use of FFR in the setting of STEMI is less validated compared to stable ischemic heart disease.8 Microvascular dysfunction during the acute phase can affect the FFR reading and the lesion severity can be underestimated.8 Second, the rate of composite endpoint was much lower in this study compared to FAME despite using the same composite endpoint of death, nonfatal MI, and unplanned hospitalization leading to urgent revascularization. At 1 year, the incidence of primary outcome was 13.5% in the FFR-guided group compared to 18.6% in the angiography-guided group in the FAME study compared to 5.5% and 4.2% in the FLOWER-MI study, despite having a sicker population presenting with STEMI. This is likely due to improvement in the PCI techniques such as radial approach, imaging guidance, and advancement in medical therapy such as use of more potent antiplatelet therapy. With lower incidence of primary outcome, larger number of patients are needed to detect the difference in the composite outcome. Finally, the operators’ visual assessment may have been calibrated to the physiologic assessment as the operators are routinely using FFR assessment which may have diminished the benefit of FFR guidance seen in the early FAME study.
Another interesting finding from this study was that although the study protocol encouraged the operators to perform the nonculprit PCI in the same setting, only 4% had nonculprit PCI in the same setting and 96% of the patients underwent a staged PCI. The advantage of performing the nonculprit PCI on the same setting is to have 1 fewer procedure for the patient. On the other hand, the disadvantage of this approach includes prolongation of the index procedure, theoretically higher risk of complication during the acute phase and vasospasm leading to overestimation of the lesion severity. A recent analysis from the COMPLETE study did not show any difference when comparing staged PCI during the index hospitalization vs after discharge.9 The optimal timing of the staged PCI needs to be investigated in future studies.
A limitation of this study is the lower than expected incidence of clinical events decreasing the statistical power of the study. However, there was no signal that FFR-guided PCI is better compared to the angiography-guided group. In fact, the curve started to diverge at 6 months favoring the angiography-guided group. In addition, there was no core-lab analysis for completeness of revascularization.
Applications for Clinical Practice
In patients presenting with hemodynamically stable STEMI for undergoing nonculprit vessel PCI, both FFR-guided or angiography-guided strategies can be considered.
Financial disclosures: None.
Study Overview
Objective. To determine whether fractional flow reserve (FFR)-guided percutaneous coronary intervention (PCI) of nonculprit lesion in patients with ST-segment elevation myocardial infarction (STEMI) is superior to angiography-guided PCI.
Design. Multicenter randomized control trial blinded to outcome, conducted in 41 sites in France.
Setting and participants. A total of 1163 patients with STEMI and multivessel coronary disease, who had undergone successful PCI to the culprit lesion were randomized to either FFR-guided PCI or angiography-guided PCI for nonculprit lesions. Randomization was stratified according to the trial site and timing of the procedure (immediate or staged).
Main outcome measures. The primary outcome was a composite of death from any cause, nonfatal myocardial infarction (MI) or unplanned hospitalization leading to urgent revascularization at 1 year.
Main results. At 1 year, the primary outcome occurred in 32 of 586 patients (5.5%) in the FFR-guided group and in 24 of 577 (4.2%) in the angiography-guided group (hazard ratio [HR], 1.32; 95% CI, 0.78-2.23; P = .31). The rate of death (1.5% vs 1.7%), nonfatal MI (3.1% vs 1.7%), and unplanned hospitalization leading to urgent revascularization (3.1% vs 1.7%) were also similar between FFR-guided and angiography-guided groups.
Conclusion. Among patients with STEMI and multivessel disease who had undergone successful PCI of the culprit vessel, an FFR-guided strategy for complete revascularization was not superior to angiography-guided strategy for reducing death, MI, or urgent revascularization at 1 year.
Commentary
Patients presenting with STEMI often have multivessel disease.1 Recently, multiple studies have reported the benefit of nonculprit vessel revascularization in patients presenting with hemodynamically stable STEMI compared to culprit-only strategy including the most recent COMPLETE trial which showed reduction in death and MI.2-6 However, the previous studies have variable design in evaluating the nonculprit vessel, some utilized FFR guidance, while others used angiography guidance. Whether FFR-guided PCI of nonculprit vessel can improve outcome in patients presenting STEMI remains unknown.
In the FLOWER-MI study, Puymirat et al investigated the use of FFR compared to angiography-guided nonculprit vessel PCI. A total of 1163 patients presenting with STEMI and multivessel disease who had undergone successful PCI to the culprit vessel, were randomized to either FFR guidance or angiography guidance among 41 centers in France. The authors found that after 1 year, there was no difference in composite endpoint of death, nonfatal MI or unplanned hospitalization leading to urgent revascularization in the FFR-guided group compared to angiography-guided group (5.5% vs 4.2%, HR, 1.32; 95% CI, 0.678-2.23; P = .31). There was also no difference in individual components of primary outcomes or secondary outcomes such as rate of stent thrombosis, any revascularization, or hospitalization.
There are a few interesting points to consider in this study. Ever since the Fractional Flow Reserve vs Angiography for Multivessel Evaluation (FAME) trial reported the lower incidence of major adverse events in routine FFR measurement during PCI compared to angiography-guided PCI, physiological assessment has become the gold standard for treatment of stable ischemic heart disease.7 However, the results of the current FLOWER-MI trial were not consistent with the FAME trial and there are few possible reasons to consider.
First, the use of FFR in the setting of STEMI is less validated compared to stable ischemic heart disease.8 Microvascular dysfunction during the acute phase can affect the FFR reading and the lesion severity can be underestimated.8 Second, the rate of composite endpoint was much lower in this study compared to FAME despite using the same composite endpoint of death, nonfatal MI, and unplanned hospitalization leading to urgent revascularization. At 1 year, the incidence of primary outcome was 13.5% in the FFR-guided group compared to 18.6% in the angiography-guided group in the FAME study compared to 5.5% and 4.2% in the FLOWER-MI study, despite having a sicker population presenting with STEMI. This is likely due to improvement in the PCI techniques such as radial approach, imaging guidance, and advancement in medical therapy such as use of more potent antiplatelet therapy. With lower incidence of primary outcome, larger number of patients are needed to detect the difference in the composite outcome. Finally, the operators’ visual assessment may have been calibrated to the physiologic assessment as the operators are routinely using FFR assessment which may have diminished the benefit of FFR guidance seen in the early FAME study.
Another interesting finding from this study was that although the study protocol encouraged the operators to perform the nonculprit PCI in the same setting, only 4% had nonculprit PCI in the same setting and 96% of the patients underwent a staged PCI. The advantage of performing the nonculprit PCI on the same setting is to have 1 fewer procedure for the patient. On the other hand, the disadvantage of this approach includes prolongation of the index procedure, theoretically higher risk of complication during the acute phase and vasospasm leading to overestimation of the lesion severity. A recent analysis from the COMPLETE study did not show any difference when comparing staged PCI during the index hospitalization vs after discharge.9 The optimal timing of the staged PCI needs to be investigated in future studies.
A limitation of this study is the lower than expected incidence of clinical events decreasing the statistical power of the study. However, there was no signal that FFR-guided PCI is better compared to the angiography-guided group. In fact, the curve started to diverge at 6 months favoring the angiography-guided group. In addition, there was no core-lab analysis for completeness of revascularization.
Applications for Clinical Practice
In patients presenting with hemodynamically stable STEMI for undergoing nonculprit vessel PCI, both FFR-guided or angiography-guided strategies can be considered.
Financial disclosures: None.
1. Park DW, Clare RM, Schulte PJ, et al. Extent, location, and clinical significance of non-infarct-related coronary artery disease among patients with ST-elevation myocardial infarction. JAMA. 2014;312(19):2019-27. doi:10.1001/jama.2014.15095
2. Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369(12):1115-23. doi:10.1056/NEJMoa1305520
3. Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65(10):963-72. doi:10.1016/j.jacc.2014.12.038
4. Engstrøm T, Kelbæk H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386(9994):665-71. doi:10.1016/s0140-6736(15)60648-1
5. Smits PC, Abdel-Wahab M, Neumann FJ, , et al. Fractional Flow Reserve-Guided Multivessel Angioplasty in Myocardial Infarction. N Engl J Med. 2017;376(13):1234-44. doi:10.1056/NEJMoa1701067
6. Mehta SR, Wood DA, Storey RF, et al. Complete Revascularization with Multivessel PCI for Myocardial Infarction. N Engl J Med. 2019;381(15):1411-21. doi:10.1056/NEJMoa1907775
7. Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213-24. doi:10.1056/NEJMoa0807611
8. Thim T, van der Hoeven NW, Musto C, et al. Evaluation and Management of Nonculprit Lesions in STEMI. JACC Cardiovasc Interv. 2020;13(10):1145-54. doi:10.1016/j.jcin.2020.02.030
9. Wood DA, Cairns JA, Wang J, et al. Timing of Staged Nonculprit Artery Revascularization in Patients With ST-Segment Elevation Myocardial Infarction: COMPLETE Trial. J Am Coll Cardiol. 2019;74(22):2713-23. doi:10.1016/j.jacc.2019/09.051
1. Park DW, Clare RM, Schulte PJ, et al. Extent, location, and clinical significance of non-infarct-related coronary artery disease among patients with ST-elevation myocardial infarction. JAMA. 2014;312(19):2019-27. doi:10.1001/jama.2014.15095
2. Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369(12):1115-23. doi:10.1056/NEJMoa1305520
3. Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65(10):963-72. doi:10.1016/j.jacc.2014.12.038
4. Engstrøm T, Kelbæk H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386(9994):665-71. doi:10.1016/s0140-6736(15)60648-1
5. Smits PC, Abdel-Wahab M, Neumann FJ, , et al. Fractional Flow Reserve-Guided Multivessel Angioplasty in Myocardial Infarction. N Engl J Med. 2017;376(13):1234-44. doi:10.1056/NEJMoa1701067
6. Mehta SR, Wood DA, Storey RF, et al. Complete Revascularization with Multivessel PCI for Myocardial Infarction. N Engl J Med. 2019;381(15):1411-21. doi:10.1056/NEJMoa1907775
7. Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213-24. doi:10.1056/NEJMoa0807611
8. Thim T, van der Hoeven NW, Musto C, et al. Evaluation and Management of Nonculprit Lesions in STEMI. JACC Cardiovasc Interv. 2020;13(10):1145-54. doi:10.1016/j.jcin.2020.02.030
9. Wood DA, Cairns JA, Wang J, et al. Timing of Staged Nonculprit Artery Revascularization in Patients With ST-Segment Elevation Myocardial Infarction: COMPLETE Trial. J Am Coll Cardiol. 2019;74(22):2713-23. doi:10.1016/j.jacc.2019/09.051
Association Between Physiotherapy Outcome Measures and the Functional Independence Measure: A Retrospective Analysis
From Illawarra Shoalhaven Local Health District, New South Wales, Australia (Maren Jones, Dr. Hewitt, Philippa King, Rhiannon Thorn, Edward Davidson, and Tiana-Lee Elphick), and Illawarra Health and Medical Research Institute, University of Wollongong, Wollongong, New South Wales, Australia (Dr. Hewitt)
Objective: To assess the association between change scores in the Functional Independence Measure (FIM) with evaluative measures used in physiotherapy to objectively show that use of the FIM in isolation is limited.
Design: Retrospective observational study.
Setting: Five rehabilitation inpatient wards from 1 public local health district in NSW Australia.
Participants: Patient data over a 5-year time frame (2015 to 2019) were reviewed (N = 2378). The patient data from the 3 most prevalent impairment groups (Australasian Rehabilitation Outcome Centre classification) were identified for inclusion in this study: Reconditioning (n = 742, mean age 76.88 years); Orthopedic Fracture (n = 585, mean age 77.46 years); and Orthopedic Replacement (n = 377, mean age 73.84 years).
Measurements: The difference between the admission and discharge scores were calculated for each measure. Kruskal-Wallis and χ2 tests were used to analyze the data.
Results: Pearson correlation (r) coefficients between FIM Motor change to the de Morton’s Mobility Index (DEMMI) change was r = 0.396, FIM Motor change to the Timed Up and Go (TUG) change was r = -0.217, and the FIM Motor change to the Ten Meter Walk Test (10MWT) change was .194.
Conclusion: The FIM Motor change scores showed a weak positive association to the DEMMI change and no association to the TUG and 10MWT change, demonstrating that the outcome measures do not measure the same attributes. To review rehabilitation effectiveness from a management perspective, it is recommended that all measures are reviewed to assess the burden of care, functional mobility, and dynamic balance.
Keywords: physiotherapy; rehabilitation; clinical outcome measures.
Patients receive interdisciplinary inpatient rehabilitation treatment after they have sustained a lower limb fracture, a lower limb joint replacement, or have generalized deconditioning (muscle wasting and disuse atrophy) following hospitalization for surgery or illness. The degree of a patient’s impairment or loss of functional capacity, as well as their ability to manage at home safely, is assessed using standardized outcome measures during their recovery and rehabilitation.1,2
Physiotherapists routinely use validated outcome measures to assess patient progress and to measure goal attainment through assessment of functional independence, dynamic balance performance, and ambulatory ability. These objective assessments provide clinicians with information about the effectiveness of the rehabilitation program, as well as the patient’s ability to manage in their home environment, to determine the need for assistive devices, level of caregiver support, future level of autonomy, and strategies for falls prevention.3-7
There is a view among service providers that rehabilitation decisions can be based on a singular measure of function known as the Functional Independence Measure (FIM). This is an understandable position because not only is the FIM an internationally recognized, valid, and reliable tool, but, as a singular measure, it also means measurement consistency across rehabilitation sites is more likely. However, rehabilitation is complex, and it is risky to base decisions on a single measure, which might not capture the results of rehabilitation treatment ingredients on individual patient targets.8,9
The patient’s progress is objectively assessed using functional outcome measures such as the FIM. Other measures used typically in our service include the de Morton’s Mobility Index (DEMMI), Timed Up and Go (TUG), and the Ten Meter Walk Test (10MWT), which measure patient mobility, balance during directional changes, and walking ability, respectively. Additional measures include patient progression to a less supportive level of assistance (ie, number of persons required to assist or level of supervision) or the selection of a walking aid (eg, forearm support frame, crutches). This progression—or lack thereof—assists in decision-making regarding the individual’s future once they are discharged from rehabilitation. Such considerations would include the need to modify the home environment, selection of assistive devices, community access (walking indoors, outdoors, and shopping), personal care needs, and age-appropriate care facility recommendations (ie, level of care). The use of outcome measures also indicates the need for further referrals to other care providers upon discharge from the rehabilitation facility.
There is widespread support in the literature for the use of the FIM, DEMMI, TUG, and 10MWT in rehabilitation population groups. For example, DEMMI has been validated in hip fracture patients during rehabilitation,10 as well as among older people hospitalized for medical illness.11-13 It has also been shown to be a predictor of discharge destination for patients living with frailty in geriatric rehabilitation settings,14 and to have moderate predictive validity for functional independence after 4 weeks of rehabilitation.15 Similarly, TUG has been validated for use among hospitalized and community-dwelling individuals,16-18 and for patients after joint arthroplasty19,20 or hip fracture.21 It has also been shown to be an indicator of fall risk,22-24 as well as a predictor of fracture incidence.25 Furthermore, TUG has been identified as an indicator of a patient’s ability to walk in the community without the need for a walking device.26 It has also been shown to be an early identifier of patients in need of rehabilitation.27 Normative values for TUG have been reported, and the association with gait time established.28
Gait speed has been shown to predict adverse outcomes in community-dwelling older people.29 In fact, the 10MWT has been established as a powerful tool to benchmark rehabilitation recovery after a medical event.30 Results of the test relate to overall quality of walking, health status, morbidity, and the rate of mortality.31-33 Meaningful improvement, minimum detectable change (0.19-0.34 m/s), and responsiveness in common physical performance in older adults has been reported.26,34,36
Structural and functional impairment has been used to define rehabilitation classes by the Australasian Rehabilitation Outcome Centre (AROC) in the Australian National Sub-Acute and Non-Acute Patient Classification (AN-SNAP) Version 4.37-43 Variables used for grouping are age, care type, function, and impairment for rehabilitation. FIM was developed in order to assess patients’ outcomes after inpatient multidisciplinary care, and is an internationally accepted measure of functioning.44 It is a holistic outcome measure, which can be used to determine the patient’s level of disability and burden of care, and is widely used in both public and private inpatient rehabilitation settings. Each patient classification is reported separately within the case mix structure.45 Inpatient rehabilitation centers are evaluated and compared by the AROC,46 with an emphasis on length of stay and the FIM change. The most successful centers demonstrate shorter length of stay and greater FIM improvement. Although the FIM is a valuable measure, it does not provide a complete picture of the individual patient’s rehabilitation gain: ie, the specific attributes of patients’ mobility, walking ability, or balance during directional changes.
A large-scale analysis of the association between the holistic disability measure of the FIM and the more mobility- and ambulation-focused physiotherapy outcomes has not been documented.
The well-documented DEMMI accumulates points for the patient’s mobility in a similar fashion to the FIM, but with more mobility detail. These 2 outcome measures allow for the full range of patients, from the very dependent up to and including the independently ambulant patients. The DEMMI may show a positive relationship to the FIM, yet the association is unknown. The association of the TUG to the 10MWT has been established28; however, their relationship to the FIM is unknown.
Current practice in the participating public health inpatient rehabilitation wards is to use the DEMMI, TUG, 10MWT, and FIM to ensure physiotherapy and allow the wider multidisciplinary team to more effectively evaluate patient mobility outcomes. The 3 most frequent patient groups identified within the current patient population are expected to present clinical differences and will be analyzed for comparison. If an association is found between the outcome measures in question, clinical efficiency could be improved.
The aim of the current study is to assess the association between change scores in the FIM with evaluative measures of outcomes typically used in physiotherapy to objectively show that use of the FIM in isolation is limited in our population of patients.
Methods
Study design and setting
This retrospective descriptive observational study complied with the STROBE-RECORD guidance and checklist (available at mdedge.com/jcomjournal) and analyzed the routinely collected data from rehabilitation patients who were admitted to 5 different rehabilitation wards in 4 different public hospitals from 1 regional local health district (20-24 beds per ward) from 2015 to 2019. As this study conducted secondary analyses using existing de-identified data from a public health facility and did not involve interaction with any human subjects, ethical approval was not required.46 Approval to conduct this study was granted by the health district’s institutional review committee, as per the National Statement on Ethical Conduct in Human Research 2015.
Participants
Patient data over a 5-year time frame were reviewed (N = 2378). The patient data from the 3 most prevalent impairment groups were identified for inclusion in this study: reconditioning, orthopedic fracture, and orthopedic replacement. (See Table 1 for the specific AN-SNAP impairment groups used in this study.)
Patient data from the less-frequent impairment groups were excluded (n = 673, 28.19%), including stroke (n = 343), brain dysfunction (n = 45), amputation of limb (n = 45), spinal cord dysfunction (n = 36), neurological dysfunction (n = 34), cardiac (n = 24), and others (n = 25) who may have benefitted from other outcome measures due to their medical condition. Ten patient data sets were excluded for missing discharge outcome measure data, from when the patient became ill and returned to acute services or was discharged at short notice. To be included in the study, both the admission and discharge scores from the FIM and the admission and discharge scores from at least 1 of the physiotherapy outcome measures were required for each patient (n = 1704, 71.39%): Reconditioning (n = 742), Orthopedic Fracture (n = 585), and Orthopedic Replacement (n = 377). Information regarding the type of walking aid and the amount of assistance required for safe ambulation was also recorded. These items were included in the study’s descriptive analysis. Only 1.7% of these descriptors were missing.
Outcome measures
DEMMI tasks of bed mobility, sitting balance, transfers, walking, and balance were scored with an assigned value according to the patient’s performance. This was then tallied and the results scaled, to provide an overall score out of 100 available points. The total score from admission and discharge was then compared. Improvement (change) was identified by the increase in scores.
The TUG assesses a patient’s dynamic balance performance.47 The number of seconds it took the patient to complete the procedure was recorded at admission and discharge. Improvement (change) was identified by the reduction in time taken at discharge from the admission score.
The 10MWT measures the unidirectional walking speed of a person over 10 meters and is recorded in seconds and reported in meters per second. Improvement (change) was identified by the reduction in the time taken to increase walking speed.
Concurrent to the physiotherapy measures were the FIM scores, recorded by the accredited nursing staff from each rehabilitation ward. Improvement is demonstrated by the accumulation of points on the ordinal scale of the FIM Total, including mobility, dressing, bladder and bowel care, cognition, and social interaction, and is represented as a score between 18 and 126. The FIM Motor category is reported as a score between 13 and 91.
The 2 data sets were matched by unique identifier and admission dates, then de-identified for analysis.
Statistical analysis
Patient demographic information was analyzed using descriptive statistics (mean, SD, frequencies, percentages) for each impairment group (orthopedic fracture, orthopedic replacement, reconditioning). Differences in continuous demographic variables for each impairment group were assessed using Kruskal-Wallis tests and χ2 tests for categorical variables. Functional outcome scores were compared at admission, discharge, and change between the impairment groups. Association of the functional outcome change scores was determined with the Pearson correlation coefficient (r) between the FIM and the DEMMI, TUG, and 10MWT. Graphs were plotted for each of these (Figure available online at mdedge.com/jcomjournal). A strong, moderate, and weak association was described as > 0.6, > 0.4, and > 0.2, respectively.46 Statistical significance was set at P < .05. Analyses were conducted using Stata (StataCorp LLC, USA).
Results
The patient descriptive data (site from which data were collected, admission length of stay, age at admission, discharge destination, walk aid improvement, and walk assistance improvement) from the 3 impairment groups are reported in Table 2. The functional outcomes for DEMMI, TUG, 10MWT, FIM Motor, FIM Total at admission, discharge, and the change scores are presented in Table 3.
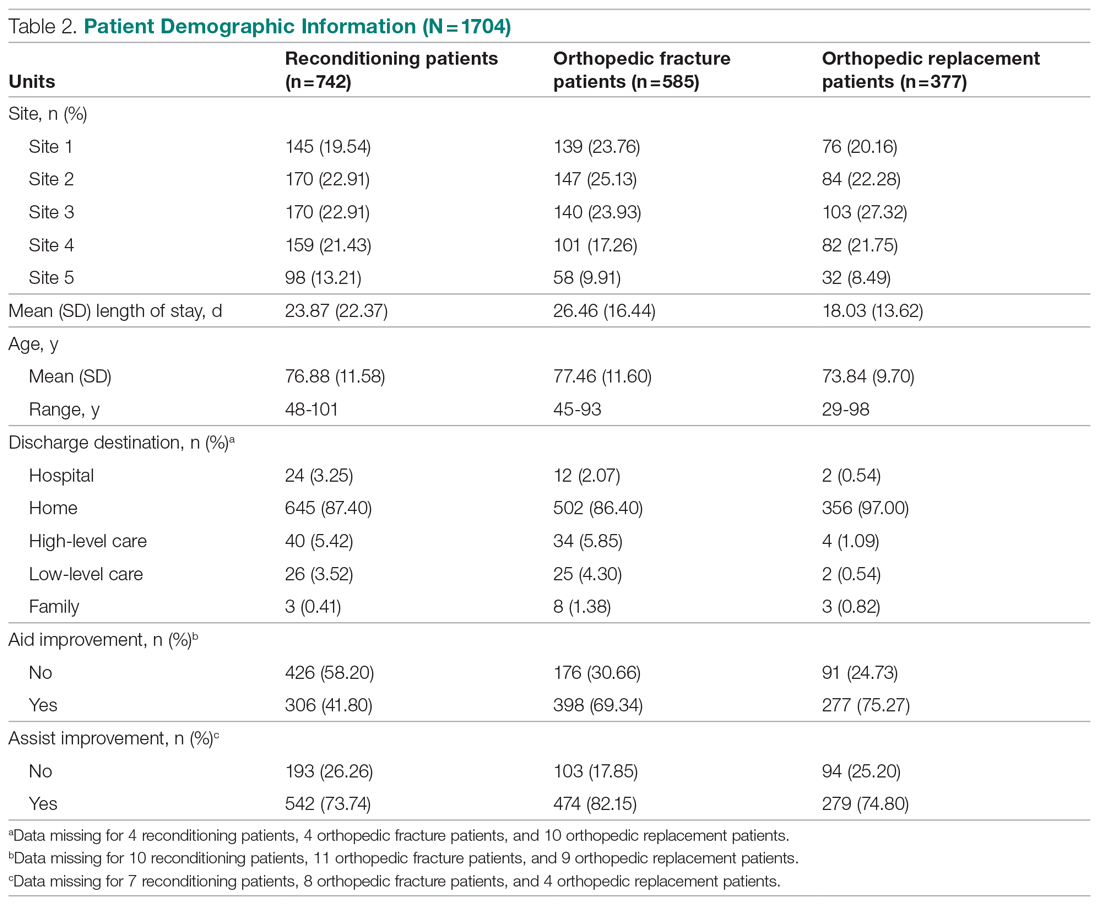
Orthopedic fracture patients had the greatest improvement in their functional outcomes, with a DEMMI improvement of 18 points, TUG score change of 23.49 seconds (s), 10MWT change of 0.30 meters/second (m/s), FIM Motor change of 20.62, and a FIM Total change of 21.9 points. The outcome measures exceeded the minimum detectable change as reported in the literature for DEMMI (8.8 points48), TUG (2.08 s26), walking speed 0.19 m/s26, and FIM Motor (14.6 points49).
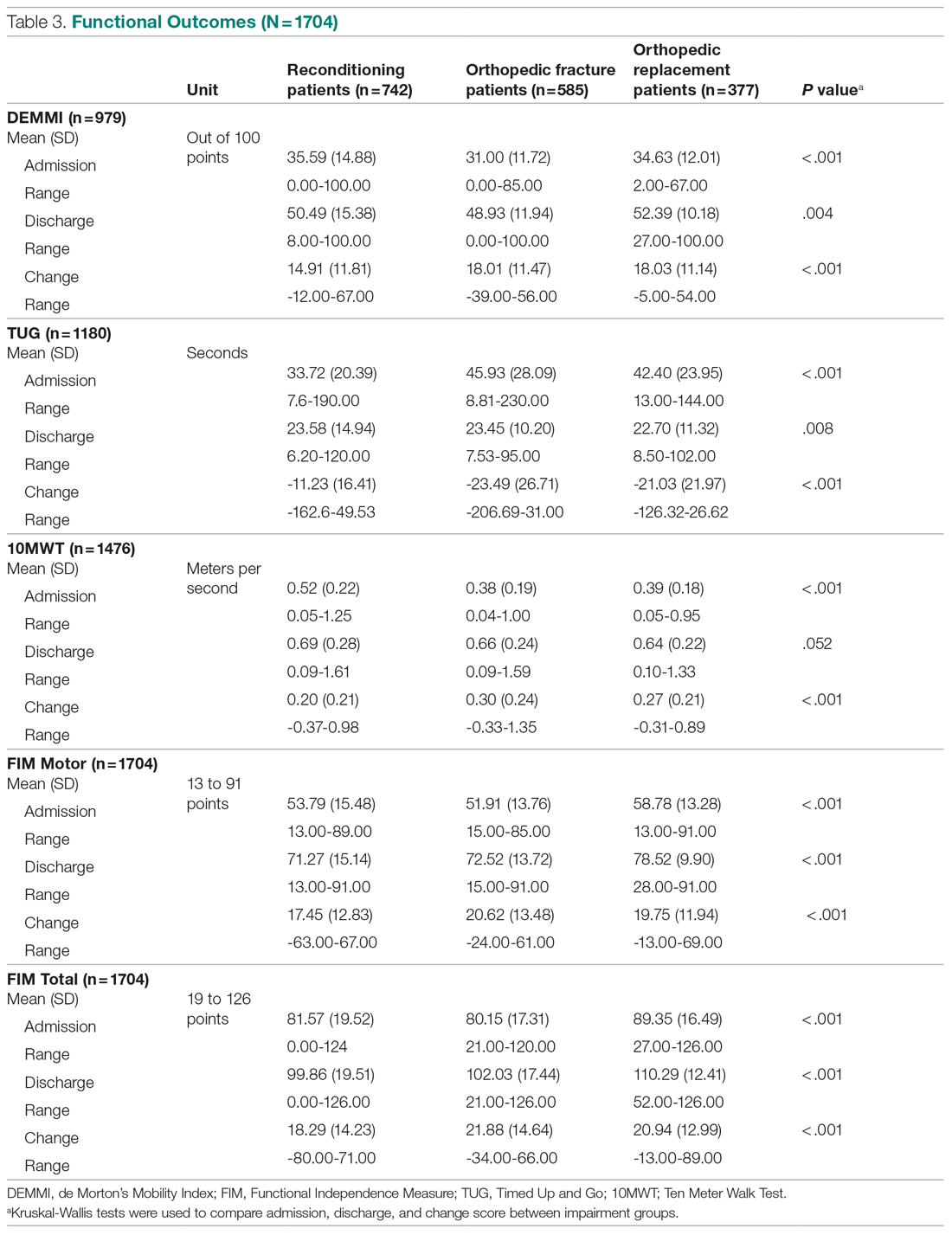
Association of functional outcomes (change scores)
There was a significant weak positive correlation between DEMMI change score and both the FIM Motor (r = 0.396) and FIM Total change scores (r = 0.373). When viewing the specific items within the FIM Motor labelled FIM Walk change, FIM MobilityBedChair change, and FIM stairs change, r values were 0.100, 0.379, and 0.126, respectively. In addition, there was a weak negative correlation between TUG change scores and both FIM Motor (r = -0.217) and FIM Total change scores (r = -0.207). There was a very weak positive correlation between 10MWT (m/s) change scores and both FIM Motor (r = 0.194) and FIM Total change scores (r = 0.187) (Table 4, Figure). There was a moderate correlation between 10MWT change (s) and TUG change (s) (r = 0.72, P < .001).
Discussion
The purpose of this study was to ascertain the association between the DEMMI, TUG, 10MWT, and FIM measures using retrospective data collected from 5 public hospital inpatient rehabilitation wards. The results of this retrospective analysis demonstrate that a variety of objective outcome measures are required for the multidisciplinary team to accurately measure a patient’s functional improvement during their inpatient rehabilitation stay. No single outcome measure in this study fully reported all mobility attributes, and we note the risk of basing decisions on a single measure evaluating rehabilitation outcomes. Although the internationally used FIM has a strong place in rehabilitation reporting and benchmarking, it does not predict change nor provide a proxy for the patient’s whole-body motor control as they extend their mobility, dynamic balance, and ambulatory ability. Multiple objective outcome measures should therefore be required to evaluate the patient’s progress and functional performance toward discharge planning.
The FIM is a measure of disability or care needs, incorporating cognitive, social, and physical components of disability. It is a valid, holistic measure of an individual’s functional ability at a given time. Rehabilitation sites internationally utilize this assessment tool to evaluate a patient’s progress and the efficacy of intervention. The strength of this measure is its widespread use and the inclusion of the personal activities of daily living to provide an overall evaluation encompassing all aspects of a person’s ability to function independently. However, as our study results suggest, patient improvement measured by the FIM Motor components were not correlated to other widely used physiotherapy measures of ambulation and balance, such as the 10MWT or TUG. This is perhaps largely because the FIM Motor components only consider the level of assistance (eg, physical assistance, assistive device, independence) and do not consider assessment of balance and gait ability as assessed in the 10MWT and TUG. The 10MWT and TUG provide assessment of velocity and dynamic balance during walking, which have been shown to predict an individual’s risk of falling.22,23 This is a pertinent issue in the rehabilitation and geriatric population.29 Furthermore, the use of the FIM as a benchmarking tool to compare facility efficiency may not provide a complete assessment of all outcomes achieved on the inpatient rehabilitation ward, such as reduced falls risk or improved ambulatory ability and balance.
Of the objective measures evaluated in our paper, the DEMMI assessment has the most similar components to those of the FIM Motor. It includes evaluating independence with bed mobility, standing up, and ambulation. In addition, the DEMMI includes assessment of both static and dynamic balance. As a result of these commonalities, there was only a weak positive correlation between the change in DEMMI and the change in FIM Motor and FIM Total. However, this correlation is not statistically significant. Therefore, the FIM is not recommended as a replacement of the DEMMI, nor can one be used to predict the other.
It has previously been confirmed that there is a significant positive correlation between the 10MWT and the TUG.27 This retrospective analysis has also supported these findings. This is possibly due to the similarity in the assessments, as they both incorporate ambulation ability and dynamic movement.
Each of the 4 outcome measures assess different yet vital aspects of an individual’s functional mobility and ambulation ability during their subacute rehabilitation journey. The diversity of patient age, functional impairment, and mobility level needs a range of outcomes to provide baselines, targets, and goal attainment for discharge home.
Consistent with the AROC AN-SNAP reporting of Length of Stay and FIM change separated into the weighted impairment groups, the data analysis of this study demonstrated significant differences between the Reconditioning, Orthopedic Fracture, and Orthopedic Replacement patient data. Tables 2 and 3 describe the differences between the groups. The fracture population in this study improved the most across each outcome measure. In contrast, the reconditioning population showed the least improvement. This may be expected due to the pathophysiological differences between the groups. Furthermore, for the elderly who sustain fractures because of a fall, rehabilitation will be required to address not only the presenting injury but also the premorbid falls risk factors which may include polypharmacy or impaired balance.
Any conclusions drawn from the findings of this study need to take into consideration that it has focused on patients from 1 local health district and therefore may not be generalizable to a wider national or international context. As this study was a retrospective study, controlling for data collection quality, measurement bias due to nonblinding and missing data is a limitation. However, clinicians regularly completed these outcome assessments and recorded this information as part of their standard care practices within this health district. There may have been slight differences in definitions of practice between the 5 rehabilitation sites. To ensure reliability, each individual site’s protocols for the FIM, DEMMI, TUG, and 10MWT were reviewed and confirmed to be consistent.
It is important, too, to consider the ceiling effect for the FIM scores. For patients requiring a walking aid well after discharge, the highest level of independence from the walking aid will not be achieved. It is acknowledged that the floor effect of the 10MWT and TUG may also influence the outcomes of this study. In addition, data were not collected on preadmission functional measures to enable further evaluation of the population groups. The proportion of variance in change from admission to discharge for TUG and 10MWT to FIM was less than 5%, so the correlation interpretation from this type of scaling is limited. Further research into outcome measures for inpatient rehabilitation in respect to variables such as patient age, length of stay, discharge destination, and efficacy of intervention is warranted.
Conclusion
The FIM Motor change scores showed a weak positive association to the DEMMI change, and no association to the TUG and 10MWT change, demonstrating that the outcome measures do not measure the same attributes. Thorough reporting of clinical outcomes is much more meaningful to assess and guide the physiotherapy component of rehabilitation. To review rehabilitation effectiveness from a management perspective, it is recommended that all measures are reviewed to assess the burden of care, mobility, functional capacity, and dynamic balance.
Acknowledgements: The authors thank Anne Smith, MSHLM, BAppSc, Head of the Physiotherapy Department, and the physiotherapists and allied health assistants who have contributed to the collection of this valuable data over several years. They also thank Lina Baytieh, MS, BS, from Research Central, Illawarra Shoalhaven Local Health District, for her assistance with the analysis.
Corresponding author: Maren Jones, MPH, BS, Physiotherapy Department, Port Kembla Hospital, Illawarra Shoalhaven Local Health District, Warrawong, New South Wales, 2505 Australia; [email protected].
Financial disclosures: None.
1. Centers for Disease Control and Prevention. Disability and health overview. Impairments, activity limitations and participation restrictions. September 16, 2020. https://www.cdc.gov/ncbddd/disabilityandhealth/disability.html
2. The Royal Australasian College of Physicians. Australasian Faculty of Rehabilitation Medicine. Standards for the Provision of Inpatient Adult Rehabilitation Medicine Services in Public and Private Hospitals. February 2019:7-9. https://www.racp.edu.au/docs/default-source/advocacy-library/afrm-standards-for-the-provision-of-inpatient-adult-rehabilitation-medicine-services-in-public-and-private-hospitals.pdf?sfvrsn=4690171a_4
3. NSW Agency for Clinical Innovation. NSW rehabilitation model of care. June 1, 2015. https://aci.health.nsw.gov.au/resources/rehabilitation/rehabilitation-model-of-care/rehabilitation-moc
4. The State of Queensland (Queensland Health). Clinical task instructions. June 22, 2021. https://www.health.qld.gov.au/ahwac/html/clintaskinstructions
5. Panel on Prevention of Falls in Older Persons, American Geriatrics Society and British Geriatrics Society. Summary of the updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc. 2011;59(1):148-157. doi:10.1111/j.1532-5415.2010.03234.x
6. Suwannarat P, Kaewsanmung S, Thaweewannakij T, Amatachaya S. The use of functional performance tests by primary health-care providers to determine walking ability with and without a walking device in community-dwelling elderly. Physiother Theory Pract. 2021;37(1):64-72. doi:10.1080/09593985.2019.1606372
7. Lee K-J, Um S-H, Kim Y-H. Postoperative rehabilitation after hip fracture: a literature review. Hip Pelvis. 2020;32(3):125-131. doi:10.5371/hp.2020.32.3.125
8. Wade DT, Smeets RJEM, Verbunt JA. Research in rehabilitation medicine: methodological challenges. J Clin Epidemiol. 2010;63(7):699-704. doi:10.1016/j.clinepi.2009.07.010
9. Wade DT. Outcome measures for clinical rehabilitation trials: impairment, function, quality of life, or value? Am J Phys Med Rehabil. 2003;82(suppl 10):S26-S31. doi:10.1097/01.PHM.0000086996.89383.A1
10. de Morton NA, Harding KE, Taylor NF, Harrison G. Validity of the de Morton NA Mobility Index (DEMMI) for measuring the mobility of patients with hip fracture during rehabilitation. Disabil Rehabil. 2013;35(4):325-333. doi:10.3109/09638288.2012.705220
11. Trøstrup J, Andersen H, Kam CAM, et al. Assessment of mobility in older people hospitalized for medical illness using the de Morton Mobility Index and cumulated ambulation score—validity and minimal clinical important difference. J Geriatr Phys Ther. 2019;42(3):153-160. doi:10.1519/JPT.0000000000000170
12. Gazzoti A, Meyer U, Freystaetter G, et al. Physical performance among patients aged 70+ in acute care: a preliminary comparison between the Short Physical Performance Battery and the De Morton Mobility Index with regard to sensitivity to change and prediction of discharge destination. Aging Clin Exp Res. 2020;32(4):579-586. doi:10.1007/s40520-019-1249-9
13. Tavares LS, Moreno NA, de Aquino BG, et al. Reliability, validity, interpretability and responsiveness of the DEMMI mobility index for Brazilian older hospitalized patients. PLoS One. 2020;15(3):e0230047. doi:10.1371/journal.pone.0230047
14. Braun T, Schulz R-J, Reinke J. Reliability and validity of the German translation of the de Morton Mobility Index performed by physiotherapists in patients admitted to a sub-acute inpatient geriatric rehabilitation hospital. BMC Geriatr. 2015;15:38. doi:10.1186/s12877-015-0035-y
15. Søndergaard K, Petersen LE, Pedersen MK, et al. The responsiveness and predictive validity of the de Morton Mobility Index in geriatric rehabilitation. Disabil Rehabil. 2020 Jun 12. [Epub ahead of print] doi:10.1080/09638288.2020.1771438
16. de Morton NA, Brusco NK, Wood L, et al. The de Morton Mobility Index (DEMMI) provides a valid method for measuring and monitoring the mobility of patients making the transition from hospital to the community: an observational study. J Physiother. 2011;57(2):109-116. doi:10.1016/S1836-9553(11)70021-2
17. Caronni A, Sterpi I, Antoniotti P, et al. Criterion validity of the instrumented Timed Up and Go test: a partial least square regression study. Gait Posture. 2018;61(3):287-293. doi:10.1016/j.gaitpost.2018.01.015
18. Kristensen MT, Bloch ML, Jonsson LR, Jakobsen TL. Interrater reliability of the standardized Timed Up and Go Test when used in hospitalized and community-dwelling individuals. Physiother Res Int. 2019;24(2):e1769. doi:10.1002/pri.1769
19. Yuksel E, Kalkan S, Cekmece S, et al. Assessing minimal detectable changes and test-retest reliability of the timed up and go test and 2-minute walk test in patients with total knee arthroplasty. J Arthroplasty. 2017;32(2):426-430. doi:10.1016/j.arth.2016.07.031
20. Yuksel E, Unver B, Kalkan S, Karatosun V. Reliability and minimal detectable change of the 2-minute walk test and Timed Up and Go test in patients with total hip arthroplasty. Hip Int. 2021;31(1):43-49. doi:10.1177/1120700019888614
21. Faleide AGH, Bogen BE, Magnussen LH. Intra-session test-retest reliability of the Timed “Up & Go” Test when performed by patients with hip fractures. Eur J Physiother. 2015;17(2):89-97. doi:10.3109/21679169.2015.1043579
22. Barry E, Galvin R, Keogh C, et al. Is the timed up and go test a useful predictor of risk of falls in community dwelling older adults: a systematic review and meta- analysis. BMC Geriatr. 2014;14:14. doi:10.1186/1471-2318-14-14
23. Kojima G, Masud T, Kendrick D, et al. Does the timed up and go test predict future falls among British community-dwelling older people? Prospective cohort study nested within a randomised controlled trial. BMC Geriatr. 2015;15:38. doi:10.1186/s12877-015-0039-7
24. Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the timed up & go test. Phys Ther. 2000;80(9):896-903.
25. Jeong SM, Shin DW, Han K, et al. Timed Up-and-Go test is a useful predictor of fracture incidence. Bone. 2019;127:474-481. doi:10.1016/j.bone.2019.07.018
26. Donaghue OA, Savva GM, Börsch-Supan A, Kenny RA. Reliability, measurement error and minimum detectable change in reliability measurement error and minimum detectable change in mobility measures: a cohort study of community dwelling adults aged 50 years and over in Ireland. BMJ Open. 2019;9(11):e030475. doi:10/1136.bmjopen-2019-030475
27. Freter SH, Fruchter N. Relationship between timed ‘up and go’ and gait time in an elderly orthopaedic rehabilitation population. Clin Rehabil. 2000;14(1):96-101. doi:10.1191/026921500675545616
28. Kear BM, Guck TP, McGaha AL. Timed up and go (TUG) test: normative reference values for ages 20 to 59 years and relationships with physical and mental health risk factors. J Prim Care Community Health. 2017;8(1):9-13. doi:10.1177/2150131916659282
29. Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people: an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10)881-889. doi:10.1007/s12603-009-0246-z
30. Unver B, Baris RH, Yusel E, et al. Reliability of 4-meter and 10-meter walk tests after lower extremity surgery. Disabil Rehabil. 2017;39(25):2572-2576. doi:10.1080/09638288.2016.1236153
31. Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign.” J Geriatr Phys Ther. 2009;32(2):46-49.
32. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50-58. doi:10.1001/jama.2010.1923
33. Bohannon R. Comfortable and maximum walking speed of adults aged 20-79 years: reference values and determinants. Age Ageing. 1997;26(1):15-19. doi:10.1093/ageing/26.1.15
34. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance in older adults. J Am Geriatr Soc. 2006;54(5):743-749. doi:10.1111/j.1532-5415.2006.00701.x
35. Hollman J, Beckman B, Brandt R, et al. Minimum detectable change in gait velocity during acute rehabilitation following hip fracture. J Geriatr Phys Ther. 2008;31(2):53-56. doi:10.1519/00139143-200831020-00003
36. Bohannon RW, Andrews AW. Normal walking speed: a descriptive meta-analysis. Physiotherapy. 2011;97(3):182-189. doi:10.1016/j.physio.2010.12.004
37. Granger CV, Hamilton BB, Keith RA, et al. Advances in functional assessment for medical rehabilitation. Top Geriatr Rehabil. 1986;1:59-74.
38. Keith RA, Granger CV, Hamilton BB, Sherwin FS. The Functional Independence Measure: a new tool for rehabilitation. In: Eisenberg MG, Grzesiak RC, eds. Advances in Clinical Rehabilitation. Springer-Verlag; 1987:6-18.
39. Linacre JM, Heinemann AW, Wright BD, et al. The structure and stability of the Functional Independence Measure. Arch Phys Med Rehabil. 1994;75(2):127-132.
40. Coster WJ, Haley SM, Jette AM. Measuring patient-reported outcomes after discharge from inpatient rehabilitation settings. J Rehabil Med. 2006;38(4):237-242. doi:10.1080/16501970600609774
41. Street L. Frequently asked questions about FIM. Journal of the Australasian Rehabilitation Nurses Association. 2014;17(1):21-22. https://ro.uow.edu.au/ahsri/296/
42. Green JP, Gordon R, Blanchard MB, et al. Development of the Australian National Subacute and Non-acute Patient (AN-SNAP) Classification. Version 4 Final Report. Australian Health Services Research Institute, University of Wollongong, 2015. https://ro.uow.edu.au/ahsri/760
43. Australasian Rehabilitation Outcomes Centre. University of Wollongong, Australia. https://www.uow.edu.au/ahsri/aroc/
44. Green J, Gordon R, Kobel C, et al; Centre for Health Service Development. The Australian National Subacute and Non-acute Patient Classification. AN-SNAP V4 User Manual. May 2015. https://documents.uow.edu.au/content/groups/public/@web/@chsd/@aroc/documents/doc/uow194637.pdf
45. Alexander TL, Simmonds FD, Capelle JT, Green LJ. Anywhere Hospital AROC Impairment Specific Report on Reconditioning (Inpatient–Pathway 3), July 2018–June 2019. Australasian Rehabilitation Outcomes Centre, Australian Health Services Research Institute, University of Wollongong; 2019. ro.uow.edu.au/ahsri/1110
46. Evans JD. Straightforward Statistics for the Behavioural Sciences. Brooks/Cole Publishing; 1996.
47. Lee SP, Dufek J, Hickman R, Schuerman S. Influence of procedural factors on the reliability and performance of the timed up-and-go test in older adults. Int J Gerontol. 2016;10(1):37-42. doi:10.1016/j.ijge.2015
48. New PW, Scroggie GD, Williams CM. The validity, reliability, responsiveness and minimal clinically important difference of the de Morton Mobility Index in rehabilitation. Disabil Rehabil. 2017;39(10):1039-1043. doi:10.10801/09638288.2016.1179800
49. Nakaguchi T, Ishimoto Y, Akazawa N. Functional Independence Measure for patients with locomotor disorders in convalescent rehabilitation wards. Clinically significant minimum difference in exercise score gain. Physiotherapy Science. 2018;33(2):235-240.
From Illawarra Shoalhaven Local Health District, New South Wales, Australia (Maren Jones, Dr. Hewitt, Philippa King, Rhiannon Thorn, Edward Davidson, and Tiana-Lee Elphick), and Illawarra Health and Medical Research Institute, University of Wollongong, Wollongong, New South Wales, Australia (Dr. Hewitt)
Objective: To assess the association between change scores in the Functional Independence Measure (FIM) with evaluative measures used in physiotherapy to objectively show that use of the FIM in isolation is limited.
Design: Retrospective observational study.
Setting: Five rehabilitation inpatient wards from 1 public local health district in NSW Australia.
Participants: Patient data over a 5-year time frame (2015 to 2019) were reviewed (N = 2378). The patient data from the 3 most prevalent impairment groups (Australasian Rehabilitation Outcome Centre classification) were identified for inclusion in this study: Reconditioning (n = 742, mean age 76.88 years); Orthopedic Fracture (n = 585, mean age 77.46 years); and Orthopedic Replacement (n = 377, mean age 73.84 years).
Measurements: The difference between the admission and discharge scores were calculated for each measure. Kruskal-Wallis and χ2 tests were used to analyze the data.
Results: Pearson correlation (r) coefficients between FIM Motor change to the de Morton’s Mobility Index (DEMMI) change was r = 0.396, FIM Motor change to the Timed Up and Go (TUG) change was r = -0.217, and the FIM Motor change to the Ten Meter Walk Test (10MWT) change was .194.
Conclusion: The FIM Motor change scores showed a weak positive association to the DEMMI change and no association to the TUG and 10MWT change, demonstrating that the outcome measures do not measure the same attributes. To review rehabilitation effectiveness from a management perspective, it is recommended that all measures are reviewed to assess the burden of care, functional mobility, and dynamic balance.
Keywords: physiotherapy; rehabilitation; clinical outcome measures.
Patients receive interdisciplinary inpatient rehabilitation treatment after they have sustained a lower limb fracture, a lower limb joint replacement, or have generalized deconditioning (muscle wasting and disuse atrophy) following hospitalization for surgery or illness. The degree of a patient’s impairment or loss of functional capacity, as well as their ability to manage at home safely, is assessed using standardized outcome measures during their recovery and rehabilitation.1,2
Physiotherapists routinely use validated outcome measures to assess patient progress and to measure goal attainment through assessment of functional independence, dynamic balance performance, and ambulatory ability. These objective assessments provide clinicians with information about the effectiveness of the rehabilitation program, as well as the patient’s ability to manage in their home environment, to determine the need for assistive devices, level of caregiver support, future level of autonomy, and strategies for falls prevention.3-7
There is a view among service providers that rehabilitation decisions can be based on a singular measure of function known as the Functional Independence Measure (FIM). This is an understandable position because not only is the FIM an internationally recognized, valid, and reliable tool, but, as a singular measure, it also means measurement consistency across rehabilitation sites is more likely. However, rehabilitation is complex, and it is risky to base decisions on a single measure, which might not capture the results of rehabilitation treatment ingredients on individual patient targets.8,9
The patient’s progress is objectively assessed using functional outcome measures such as the FIM. Other measures used typically in our service include the de Morton’s Mobility Index (DEMMI), Timed Up and Go (TUG), and the Ten Meter Walk Test (10MWT), which measure patient mobility, balance during directional changes, and walking ability, respectively. Additional measures include patient progression to a less supportive level of assistance (ie, number of persons required to assist or level of supervision) or the selection of a walking aid (eg, forearm support frame, crutches). This progression—or lack thereof—assists in decision-making regarding the individual’s future once they are discharged from rehabilitation. Such considerations would include the need to modify the home environment, selection of assistive devices, community access (walking indoors, outdoors, and shopping), personal care needs, and age-appropriate care facility recommendations (ie, level of care). The use of outcome measures also indicates the need for further referrals to other care providers upon discharge from the rehabilitation facility.
There is widespread support in the literature for the use of the FIM, DEMMI, TUG, and 10MWT in rehabilitation population groups. For example, DEMMI has been validated in hip fracture patients during rehabilitation,10 as well as among older people hospitalized for medical illness.11-13 It has also been shown to be a predictor of discharge destination for patients living with frailty in geriatric rehabilitation settings,14 and to have moderate predictive validity for functional independence after 4 weeks of rehabilitation.15 Similarly, TUG has been validated for use among hospitalized and community-dwelling individuals,16-18 and for patients after joint arthroplasty19,20 or hip fracture.21 It has also been shown to be an indicator of fall risk,22-24 as well as a predictor of fracture incidence.25 Furthermore, TUG has been identified as an indicator of a patient’s ability to walk in the community without the need for a walking device.26 It has also been shown to be an early identifier of patients in need of rehabilitation.27 Normative values for TUG have been reported, and the association with gait time established.28
Gait speed has been shown to predict adverse outcomes in community-dwelling older people.29 In fact, the 10MWT has been established as a powerful tool to benchmark rehabilitation recovery after a medical event.30 Results of the test relate to overall quality of walking, health status, morbidity, and the rate of mortality.31-33 Meaningful improvement, minimum detectable change (0.19-0.34 m/s), and responsiveness in common physical performance in older adults has been reported.26,34,36
Structural and functional impairment has been used to define rehabilitation classes by the Australasian Rehabilitation Outcome Centre (AROC) in the Australian National Sub-Acute and Non-Acute Patient Classification (AN-SNAP) Version 4.37-43 Variables used for grouping are age, care type, function, and impairment for rehabilitation. FIM was developed in order to assess patients’ outcomes after inpatient multidisciplinary care, and is an internationally accepted measure of functioning.44 It is a holistic outcome measure, which can be used to determine the patient’s level of disability and burden of care, and is widely used in both public and private inpatient rehabilitation settings. Each patient classification is reported separately within the case mix structure.45 Inpatient rehabilitation centers are evaluated and compared by the AROC,46 with an emphasis on length of stay and the FIM change. The most successful centers demonstrate shorter length of stay and greater FIM improvement. Although the FIM is a valuable measure, it does not provide a complete picture of the individual patient’s rehabilitation gain: ie, the specific attributes of patients’ mobility, walking ability, or balance during directional changes.
A large-scale analysis of the association between the holistic disability measure of the FIM and the more mobility- and ambulation-focused physiotherapy outcomes has not been documented.
The well-documented DEMMI accumulates points for the patient’s mobility in a similar fashion to the FIM, but with more mobility detail. These 2 outcome measures allow for the full range of patients, from the very dependent up to and including the independently ambulant patients. The DEMMI may show a positive relationship to the FIM, yet the association is unknown. The association of the TUG to the 10MWT has been established28; however, their relationship to the FIM is unknown.
Current practice in the participating public health inpatient rehabilitation wards is to use the DEMMI, TUG, 10MWT, and FIM to ensure physiotherapy and allow the wider multidisciplinary team to more effectively evaluate patient mobility outcomes. The 3 most frequent patient groups identified within the current patient population are expected to present clinical differences and will be analyzed for comparison. If an association is found between the outcome measures in question, clinical efficiency could be improved.
The aim of the current study is to assess the association between change scores in the FIM with evaluative measures of outcomes typically used in physiotherapy to objectively show that use of the FIM in isolation is limited in our population of patients.
Methods
Study design and setting
This retrospective descriptive observational study complied with the STROBE-RECORD guidance and checklist (available at mdedge.com/jcomjournal) and analyzed the routinely collected data from rehabilitation patients who were admitted to 5 different rehabilitation wards in 4 different public hospitals from 1 regional local health district (20-24 beds per ward) from 2015 to 2019. As this study conducted secondary analyses using existing de-identified data from a public health facility and did not involve interaction with any human subjects, ethical approval was not required.46 Approval to conduct this study was granted by the health district’s institutional review committee, as per the National Statement on Ethical Conduct in Human Research 2015.
Participants
Patient data over a 5-year time frame were reviewed (N = 2378). The patient data from the 3 most prevalent impairment groups were identified for inclusion in this study: reconditioning, orthopedic fracture, and orthopedic replacement. (See Table 1 for the specific AN-SNAP impairment groups used in this study.)

Patient data from the less-frequent impairment groups were excluded (n = 673, 28.19%), including stroke (n = 343), brain dysfunction (n = 45), amputation of limb (n = 45), spinal cord dysfunction (n = 36), neurological dysfunction (n = 34), cardiac (n = 24), and others (n = 25) who may have benefitted from other outcome measures due to their medical condition. Ten patient data sets were excluded for missing discharge outcome measure data, from when the patient became ill and returned to acute services or was discharged at short notice. To be included in the study, both the admission and discharge scores from the FIM and the admission and discharge scores from at least 1 of the physiotherapy outcome measures were required for each patient (n = 1704, 71.39%): Reconditioning (n = 742), Orthopedic Fracture (n = 585), and Orthopedic Replacement (n = 377). Information regarding the type of walking aid and the amount of assistance required for safe ambulation was also recorded. These items were included in the study’s descriptive analysis. Only 1.7% of these descriptors were missing.
Outcome measures
DEMMI tasks of bed mobility, sitting balance, transfers, walking, and balance were scored with an assigned value according to the patient’s performance. This was then tallied and the results scaled, to provide an overall score out of 100 available points. The total score from admission and discharge was then compared. Improvement (change) was identified by the increase in scores.
The TUG assesses a patient’s dynamic balance performance.47 The number of seconds it took the patient to complete the procedure was recorded at admission and discharge. Improvement (change) was identified by the reduction in time taken at discharge from the admission score.
The 10MWT measures the unidirectional walking speed of a person over 10 meters and is recorded in seconds and reported in meters per second. Improvement (change) was identified by the reduction in the time taken to increase walking speed.
Concurrent to the physiotherapy measures were the FIM scores, recorded by the accredited nursing staff from each rehabilitation ward. Improvement is demonstrated by the accumulation of points on the ordinal scale of the FIM Total, including mobility, dressing, bladder and bowel care, cognition, and social interaction, and is represented as a score between 18 and 126. The FIM Motor category is reported as a score between 13 and 91.
The 2 data sets were matched by unique identifier and admission dates, then de-identified for analysis.
Statistical analysis
Patient demographic information was analyzed using descriptive statistics (mean, SD, frequencies, percentages) for each impairment group (orthopedic fracture, orthopedic replacement, reconditioning). Differences in continuous demographic variables for each impairment group were assessed using Kruskal-Wallis tests and χ2 tests for categorical variables. Functional outcome scores were compared at admission, discharge, and change between the impairment groups. Association of the functional outcome change scores was determined with the Pearson correlation coefficient (r) between the FIM and the DEMMI, TUG, and 10MWT. Graphs were plotted for each of these (Figure available online at mdedge.com/jcomjournal). A strong, moderate, and weak association was described as > 0.6, > 0.4, and > 0.2, respectively.46 Statistical significance was set at P < .05. Analyses were conducted using Stata (StataCorp LLC, USA).
Results
The patient descriptive data (site from which data were collected, admission length of stay, age at admission, discharge destination, walk aid improvement, and walk assistance improvement) from the 3 impairment groups are reported in Table 2. The functional outcomes for DEMMI, TUG, 10MWT, FIM Motor, FIM Total at admission, discharge, and the change scores are presented in Table 3.

Orthopedic fracture patients had the greatest improvement in their functional outcomes, with a DEMMI improvement of 18 points, TUG score change of 23.49 seconds (s), 10MWT change of 0.30 meters/second (m/s), FIM Motor change of 20.62, and a FIM Total change of 21.9 points. The outcome measures exceeded the minimum detectable change as reported in the literature for DEMMI (8.8 points48), TUG (2.08 s26), walking speed 0.19 m/s26, and FIM Motor (14.6 points49).

Association of functional outcomes (change scores)
There was a significant weak positive correlation between DEMMI change score and both the FIM Motor (r = 0.396) and FIM Total change scores (r = 0.373). When viewing the specific items within the FIM Motor labelled FIM Walk change, FIM MobilityBedChair change, and FIM stairs change, r values were 0.100, 0.379, and 0.126, respectively. In addition, there was a weak negative correlation between TUG change scores and both FIM Motor (r = -0.217) and FIM Total change scores (r = -0.207). There was a very weak positive correlation between 10MWT (m/s) change scores and both FIM Motor (r = 0.194) and FIM Total change scores (r = 0.187) (Table 4, Figure). There was a moderate correlation between 10MWT change (s) and TUG change (s) (r = 0.72, P < .001).

Discussion
The purpose of this study was to ascertain the association between the DEMMI, TUG, 10MWT, and FIM measures using retrospective data collected from 5 public hospital inpatient rehabilitation wards. The results of this retrospective analysis demonstrate that a variety of objective outcome measures are required for the multidisciplinary team to accurately measure a patient’s functional improvement during their inpatient rehabilitation stay. No single outcome measure in this study fully reported all mobility attributes, and we note the risk of basing decisions on a single measure evaluating rehabilitation outcomes. Although the internationally used FIM has a strong place in rehabilitation reporting and benchmarking, it does not predict change nor provide a proxy for the patient’s whole-body motor control as they extend their mobility, dynamic balance, and ambulatory ability. Multiple objective outcome measures should therefore be required to evaluate the patient’s progress and functional performance toward discharge planning.
The FIM is a measure of disability or care needs, incorporating cognitive, social, and physical components of disability. It is a valid, holistic measure of an individual’s functional ability at a given time. Rehabilitation sites internationally utilize this assessment tool to evaluate a patient’s progress and the efficacy of intervention. The strength of this measure is its widespread use and the inclusion of the personal activities of daily living to provide an overall evaluation encompassing all aspects of a person’s ability to function independently. However, as our study results suggest, patient improvement measured by the FIM Motor components were not correlated to other widely used physiotherapy measures of ambulation and balance, such as the 10MWT or TUG. This is perhaps largely because the FIM Motor components only consider the level of assistance (eg, physical assistance, assistive device, independence) and do not consider assessment of balance and gait ability as assessed in the 10MWT and TUG. The 10MWT and TUG provide assessment of velocity and dynamic balance during walking, which have been shown to predict an individual’s risk of falling.22,23 This is a pertinent issue in the rehabilitation and geriatric population.29 Furthermore, the use of the FIM as a benchmarking tool to compare facility efficiency may not provide a complete assessment of all outcomes achieved on the inpatient rehabilitation ward, such as reduced falls risk or improved ambulatory ability and balance.
Of the objective measures evaluated in our paper, the DEMMI assessment has the most similar components to those of the FIM Motor. It includes evaluating independence with bed mobility, standing up, and ambulation. In addition, the DEMMI includes assessment of both static and dynamic balance. As a result of these commonalities, there was only a weak positive correlation between the change in DEMMI and the change in FIM Motor and FIM Total. However, this correlation is not statistically significant. Therefore, the FIM is not recommended as a replacement of the DEMMI, nor can one be used to predict the other.
It has previously been confirmed that there is a significant positive correlation between the 10MWT and the TUG.27 This retrospective analysis has also supported these findings. This is possibly due to the similarity in the assessments, as they both incorporate ambulation ability and dynamic movement.
Each of the 4 outcome measures assess different yet vital aspects of an individual’s functional mobility and ambulation ability during their subacute rehabilitation journey. The diversity of patient age, functional impairment, and mobility level needs a range of outcomes to provide baselines, targets, and goal attainment for discharge home.
Consistent with the AROC AN-SNAP reporting of Length of Stay and FIM change separated into the weighted impairment groups, the data analysis of this study demonstrated significant differences between the Reconditioning, Orthopedic Fracture, and Orthopedic Replacement patient data. Tables 2 and 3 describe the differences between the groups. The fracture population in this study improved the most across each outcome measure. In contrast, the reconditioning population showed the least improvement. This may be expected due to the pathophysiological differences between the groups. Furthermore, for the elderly who sustain fractures because of a fall, rehabilitation will be required to address not only the presenting injury but also the premorbid falls risk factors which may include polypharmacy or impaired balance.
Any conclusions drawn from the findings of this study need to take into consideration that it has focused on patients from 1 local health district and therefore may not be generalizable to a wider national or international context. As this study was a retrospective study, controlling for data collection quality, measurement bias due to nonblinding and missing data is a limitation. However, clinicians regularly completed these outcome assessments and recorded this information as part of their standard care practices within this health district. There may have been slight differences in definitions of practice between the 5 rehabilitation sites. To ensure reliability, each individual site’s protocols for the FIM, DEMMI, TUG, and 10MWT were reviewed and confirmed to be consistent.
It is important, too, to consider the ceiling effect for the FIM scores. For patients requiring a walking aid well after discharge, the highest level of independence from the walking aid will not be achieved. It is acknowledged that the floor effect of the 10MWT and TUG may also influence the outcomes of this study. In addition, data were not collected on preadmission functional measures to enable further evaluation of the population groups. The proportion of variance in change from admission to discharge for TUG and 10MWT to FIM was less than 5%, so the correlation interpretation from this type of scaling is limited. Further research into outcome measures for inpatient rehabilitation in respect to variables such as patient age, length of stay, discharge destination, and efficacy of intervention is warranted.
Conclusion
The FIM Motor change scores showed a weak positive association to the DEMMI change, and no association to the TUG and 10MWT change, demonstrating that the outcome measures do not measure the same attributes. Thorough reporting of clinical outcomes is much more meaningful to assess and guide the physiotherapy component of rehabilitation. To review rehabilitation effectiveness from a management perspective, it is recommended that all measures are reviewed to assess the burden of care, mobility, functional capacity, and dynamic balance.
Acknowledgements: The authors thank Anne Smith, MSHLM, BAppSc, Head of the Physiotherapy Department, and the physiotherapists and allied health assistants who have contributed to the collection of this valuable data over several years. They also thank Lina Baytieh, MS, BS, from Research Central, Illawarra Shoalhaven Local Health District, for her assistance with the analysis.
Corresponding author: Maren Jones, MPH, BS, Physiotherapy Department, Port Kembla Hospital, Illawarra Shoalhaven Local Health District, Warrawong, New South Wales, 2505 Australia; [email protected].
Financial disclosures: None.
From Illawarra Shoalhaven Local Health District, New South Wales, Australia (Maren Jones, Dr. Hewitt, Philippa King, Rhiannon Thorn, Edward Davidson, and Tiana-Lee Elphick), and Illawarra Health and Medical Research Institute, University of Wollongong, Wollongong, New South Wales, Australia (Dr. Hewitt)
Objective: To assess the association between change scores in the Functional Independence Measure (FIM) with evaluative measures used in physiotherapy to objectively show that use of the FIM in isolation is limited.
Design: Retrospective observational study.
Setting: Five rehabilitation inpatient wards from 1 public local health district in NSW Australia.
Participants: Patient data over a 5-year time frame (2015 to 2019) were reviewed (N = 2378). The patient data from the 3 most prevalent impairment groups (Australasian Rehabilitation Outcome Centre classification) were identified for inclusion in this study: Reconditioning (n = 742, mean age 76.88 years); Orthopedic Fracture (n = 585, mean age 77.46 years); and Orthopedic Replacement (n = 377, mean age 73.84 years).
Measurements: The difference between the admission and discharge scores were calculated for each measure. Kruskal-Wallis and χ2 tests were used to analyze the data.
Results: Pearson correlation (r) coefficients between FIM Motor change to the de Morton’s Mobility Index (DEMMI) change was r = 0.396, FIM Motor change to the Timed Up and Go (TUG) change was r = -0.217, and the FIM Motor change to the Ten Meter Walk Test (10MWT) change was .194.
Conclusion: The FIM Motor change scores showed a weak positive association to the DEMMI change and no association to the TUG and 10MWT change, demonstrating that the outcome measures do not measure the same attributes. To review rehabilitation effectiveness from a management perspective, it is recommended that all measures are reviewed to assess the burden of care, functional mobility, and dynamic balance.
Keywords: physiotherapy; rehabilitation; clinical outcome measures.
Patients receive interdisciplinary inpatient rehabilitation treatment after they have sustained a lower limb fracture, a lower limb joint replacement, or have generalized deconditioning (muscle wasting and disuse atrophy) following hospitalization for surgery or illness. The degree of a patient’s impairment or loss of functional capacity, as well as their ability to manage at home safely, is assessed using standardized outcome measures during their recovery and rehabilitation.1,2
Physiotherapists routinely use validated outcome measures to assess patient progress and to measure goal attainment through assessment of functional independence, dynamic balance performance, and ambulatory ability. These objective assessments provide clinicians with information about the effectiveness of the rehabilitation program, as well as the patient’s ability to manage in their home environment, to determine the need for assistive devices, level of caregiver support, future level of autonomy, and strategies for falls prevention.3-7
There is a view among service providers that rehabilitation decisions can be based on a singular measure of function known as the Functional Independence Measure (FIM). This is an understandable position because not only is the FIM an internationally recognized, valid, and reliable tool, but, as a singular measure, it also means measurement consistency across rehabilitation sites is more likely. However, rehabilitation is complex, and it is risky to base decisions on a single measure, which might not capture the results of rehabilitation treatment ingredients on individual patient targets.8,9
The patient’s progress is objectively assessed using functional outcome measures such as the FIM. Other measures used typically in our service include the de Morton’s Mobility Index (DEMMI), Timed Up and Go (TUG), and the Ten Meter Walk Test (10MWT), which measure patient mobility, balance during directional changes, and walking ability, respectively. Additional measures include patient progression to a less supportive level of assistance (ie, number of persons required to assist or level of supervision) or the selection of a walking aid (eg, forearm support frame, crutches). This progression—or lack thereof—assists in decision-making regarding the individual’s future once they are discharged from rehabilitation. Such considerations would include the need to modify the home environment, selection of assistive devices, community access (walking indoors, outdoors, and shopping), personal care needs, and age-appropriate care facility recommendations (ie, level of care). The use of outcome measures also indicates the need for further referrals to other care providers upon discharge from the rehabilitation facility.
There is widespread support in the literature for the use of the FIM, DEMMI, TUG, and 10MWT in rehabilitation population groups. For example, DEMMI has been validated in hip fracture patients during rehabilitation,10 as well as among older people hospitalized for medical illness.11-13 It has also been shown to be a predictor of discharge destination for patients living with frailty in geriatric rehabilitation settings,14 and to have moderate predictive validity for functional independence after 4 weeks of rehabilitation.15 Similarly, TUG has been validated for use among hospitalized and community-dwelling individuals,16-18 and for patients after joint arthroplasty19,20 or hip fracture.21 It has also been shown to be an indicator of fall risk,22-24 as well as a predictor of fracture incidence.25 Furthermore, TUG has been identified as an indicator of a patient’s ability to walk in the community without the need for a walking device.26 It has also been shown to be an early identifier of patients in need of rehabilitation.27 Normative values for TUG have been reported, and the association with gait time established.28
Gait speed has been shown to predict adverse outcomes in community-dwelling older people.29 In fact, the 10MWT has been established as a powerful tool to benchmark rehabilitation recovery after a medical event.30 Results of the test relate to overall quality of walking, health status, morbidity, and the rate of mortality.31-33 Meaningful improvement, minimum detectable change (0.19-0.34 m/s), and responsiveness in common physical performance in older adults has been reported.26,34,36
Structural and functional impairment has been used to define rehabilitation classes by the Australasian Rehabilitation Outcome Centre (AROC) in the Australian National Sub-Acute and Non-Acute Patient Classification (AN-SNAP) Version 4.37-43 Variables used for grouping are age, care type, function, and impairment for rehabilitation. FIM was developed in order to assess patients’ outcomes after inpatient multidisciplinary care, and is an internationally accepted measure of functioning.44 It is a holistic outcome measure, which can be used to determine the patient’s level of disability and burden of care, and is widely used in both public and private inpatient rehabilitation settings. Each patient classification is reported separately within the case mix structure.45 Inpatient rehabilitation centers are evaluated and compared by the AROC,46 with an emphasis on length of stay and the FIM change. The most successful centers demonstrate shorter length of stay and greater FIM improvement. Although the FIM is a valuable measure, it does not provide a complete picture of the individual patient’s rehabilitation gain: ie, the specific attributes of patients’ mobility, walking ability, or balance during directional changes.
A large-scale analysis of the association between the holistic disability measure of the FIM and the more mobility- and ambulation-focused physiotherapy outcomes has not been documented.
The well-documented DEMMI accumulates points for the patient’s mobility in a similar fashion to the FIM, but with more mobility detail. These 2 outcome measures allow for the full range of patients, from the very dependent up to and including the independently ambulant patients. The DEMMI may show a positive relationship to the FIM, yet the association is unknown. The association of the TUG to the 10MWT has been established28; however, their relationship to the FIM is unknown.
Current practice in the participating public health inpatient rehabilitation wards is to use the DEMMI, TUG, 10MWT, and FIM to ensure physiotherapy and allow the wider multidisciplinary team to more effectively evaluate patient mobility outcomes. The 3 most frequent patient groups identified within the current patient population are expected to present clinical differences and will be analyzed for comparison. If an association is found between the outcome measures in question, clinical efficiency could be improved.
The aim of the current study is to assess the association between change scores in the FIM with evaluative measures of outcomes typically used in physiotherapy to objectively show that use of the FIM in isolation is limited in our population of patients.
Methods
Study design and setting
This retrospective descriptive observational study complied with the STROBE-RECORD guidance and checklist (available at mdedge.com/jcomjournal) and analyzed the routinely collected data from rehabilitation patients who were admitted to 5 different rehabilitation wards in 4 different public hospitals from 1 regional local health district (20-24 beds per ward) from 2015 to 2019. As this study conducted secondary analyses using existing de-identified data from a public health facility and did not involve interaction with any human subjects, ethical approval was not required.46 Approval to conduct this study was granted by the health district’s institutional review committee, as per the National Statement on Ethical Conduct in Human Research 2015.
Participants
Patient data over a 5-year time frame were reviewed (N = 2378). The patient data from the 3 most prevalent impairment groups were identified for inclusion in this study: reconditioning, orthopedic fracture, and orthopedic replacement. (See Table 1 for the specific AN-SNAP impairment groups used in this study.)

Patient data from the less-frequent impairment groups were excluded (n = 673, 28.19%), including stroke (n = 343), brain dysfunction (n = 45), amputation of limb (n = 45), spinal cord dysfunction (n = 36), neurological dysfunction (n = 34), cardiac (n = 24), and others (n = 25) who may have benefitted from other outcome measures due to their medical condition. Ten patient data sets were excluded for missing discharge outcome measure data, from when the patient became ill and returned to acute services or was discharged at short notice. To be included in the study, both the admission and discharge scores from the FIM and the admission and discharge scores from at least 1 of the physiotherapy outcome measures were required for each patient (n = 1704, 71.39%): Reconditioning (n = 742), Orthopedic Fracture (n = 585), and Orthopedic Replacement (n = 377). Information regarding the type of walking aid and the amount of assistance required for safe ambulation was also recorded. These items were included in the study’s descriptive analysis. Only 1.7% of these descriptors were missing.
Outcome measures
DEMMI tasks of bed mobility, sitting balance, transfers, walking, and balance were scored with an assigned value according to the patient’s performance. This was then tallied and the results scaled, to provide an overall score out of 100 available points. The total score from admission and discharge was then compared. Improvement (change) was identified by the increase in scores.
The TUG assesses a patient’s dynamic balance performance.47 The number of seconds it took the patient to complete the procedure was recorded at admission and discharge. Improvement (change) was identified by the reduction in time taken at discharge from the admission score.
The 10MWT measures the unidirectional walking speed of a person over 10 meters and is recorded in seconds and reported in meters per second. Improvement (change) was identified by the reduction in the time taken to increase walking speed.
Concurrent to the physiotherapy measures were the FIM scores, recorded by the accredited nursing staff from each rehabilitation ward. Improvement is demonstrated by the accumulation of points on the ordinal scale of the FIM Total, including mobility, dressing, bladder and bowel care, cognition, and social interaction, and is represented as a score between 18 and 126. The FIM Motor category is reported as a score between 13 and 91.
The 2 data sets were matched by unique identifier and admission dates, then de-identified for analysis.
Statistical analysis
Patient demographic information was analyzed using descriptive statistics (mean, SD, frequencies, percentages) for each impairment group (orthopedic fracture, orthopedic replacement, reconditioning). Differences in continuous demographic variables for each impairment group were assessed using Kruskal-Wallis tests and χ2 tests for categorical variables. Functional outcome scores were compared at admission, discharge, and change between the impairment groups. Association of the functional outcome change scores was determined with the Pearson correlation coefficient (r) between the FIM and the DEMMI, TUG, and 10MWT. Graphs were plotted for each of these (Figure available online at mdedge.com/jcomjournal). A strong, moderate, and weak association was described as > 0.6, > 0.4, and > 0.2, respectively.46 Statistical significance was set at P < .05. Analyses were conducted using Stata (StataCorp LLC, USA).
Results
The patient descriptive data (site from which data were collected, admission length of stay, age at admission, discharge destination, walk aid improvement, and walk assistance improvement) from the 3 impairment groups are reported in Table 2. The functional outcomes for DEMMI, TUG, 10MWT, FIM Motor, FIM Total at admission, discharge, and the change scores are presented in Table 3.

Orthopedic fracture patients had the greatest improvement in their functional outcomes, with a DEMMI improvement of 18 points, TUG score change of 23.49 seconds (s), 10MWT change of 0.30 meters/second (m/s), FIM Motor change of 20.62, and a FIM Total change of 21.9 points. The outcome measures exceeded the minimum detectable change as reported in the literature for DEMMI (8.8 points48), TUG (2.08 s26), walking speed 0.19 m/s26, and FIM Motor (14.6 points49).

Association of functional outcomes (change scores)
There was a significant weak positive correlation between DEMMI change score and both the FIM Motor (r = 0.396) and FIM Total change scores (r = 0.373). When viewing the specific items within the FIM Motor labelled FIM Walk change, FIM MobilityBedChair change, and FIM stairs change, r values were 0.100, 0.379, and 0.126, respectively. In addition, there was a weak negative correlation between TUG change scores and both FIM Motor (r = -0.217) and FIM Total change scores (r = -0.207). There was a very weak positive correlation between 10MWT (m/s) change scores and both FIM Motor (r = 0.194) and FIM Total change scores (r = 0.187) (Table 4, Figure). There was a moderate correlation between 10MWT change (s) and TUG change (s) (r = 0.72, P < .001).

Discussion
The purpose of this study was to ascertain the association between the DEMMI, TUG, 10MWT, and FIM measures using retrospective data collected from 5 public hospital inpatient rehabilitation wards. The results of this retrospective analysis demonstrate that a variety of objective outcome measures are required for the multidisciplinary team to accurately measure a patient’s functional improvement during their inpatient rehabilitation stay. No single outcome measure in this study fully reported all mobility attributes, and we note the risk of basing decisions on a single measure evaluating rehabilitation outcomes. Although the internationally used FIM has a strong place in rehabilitation reporting and benchmarking, it does not predict change nor provide a proxy for the patient’s whole-body motor control as they extend their mobility, dynamic balance, and ambulatory ability. Multiple objective outcome measures should therefore be required to evaluate the patient’s progress and functional performance toward discharge planning.
The FIM is a measure of disability or care needs, incorporating cognitive, social, and physical components of disability. It is a valid, holistic measure of an individual’s functional ability at a given time. Rehabilitation sites internationally utilize this assessment tool to evaluate a patient’s progress and the efficacy of intervention. The strength of this measure is its widespread use and the inclusion of the personal activities of daily living to provide an overall evaluation encompassing all aspects of a person’s ability to function independently. However, as our study results suggest, patient improvement measured by the FIM Motor components were not correlated to other widely used physiotherapy measures of ambulation and balance, such as the 10MWT or TUG. This is perhaps largely because the FIM Motor components only consider the level of assistance (eg, physical assistance, assistive device, independence) and do not consider assessment of balance and gait ability as assessed in the 10MWT and TUG. The 10MWT and TUG provide assessment of velocity and dynamic balance during walking, which have been shown to predict an individual’s risk of falling.22,23 This is a pertinent issue in the rehabilitation and geriatric population.29 Furthermore, the use of the FIM as a benchmarking tool to compare facility efficiency may not provide a complete assessment of all outcomes achieved on the inpatient rehabilitation ward, such as reduced falls risk or improved ambulatory ability and balance.
Of the objective measures evaluated in our paper, the DEMMI assessment has the most similar components to those of the FIM Motor. It includes evaluating independence with bed mobility, standing up, and ambulation. In addition, the DEMMI includes assessment of both static and dynamic balance. As a result of these commonalities, there was only a weak positive correlation between the change in DEMMI and the change in FIM Motor and FIM Total. However, this correlation is not statistically significant. Therefore, the FIM is not recommended as a replacement of the DEMMI, nor can one be used to predict the other.
It has previously been confirmed that there is a significant positive correlation between the 10MWT and the TUG.27 This retrospective analysis has also supported these findings. This is possibly due to the similarity in the assessments, as they both incorporate ambulation ability and dynamic movement.
Each of the 4 outcome measures assess different yet vital aspects of an individual’s functional mobility and ambulation ability during their subacute rehabilitation journey. The diversity of patient age, functional impairment, and mobility level needs a range of outcomes to provide baselines, targets, and goal attainment for discharge home.
Consistent with the AROC AN-SNAP reporting of Length of Stay and FIM change separated into the weighted impairment groups, the data analysis of this study demonstrated significant differences between the Reconditioning, Orthopedic Fracture, and Orthopedic Replacement patient data. Tables 2 and 3 describe the differences between the groups. The fracture population in this study improved the most across each outcome measure. In contrast, the reconditioning population showed the least improvement. This may be expected due to the pathophysiological differences between the groups. Furthermore, for the elderly who sustain fractures because of a fall, rehabilitation will be required to address not only the presenting injury but also the premorbid falls risk factors which may include polypharmacy or impaired balance.
Any conclusions drawn from the findings of this study need to take into consideration that it has focused on patients from 1 local health district and therefore may not be generalizable to a wider national or international context. As this study was a retrospective study, controlling for data collection quality, measurement bias due to nonblinding and missing data is a limitation. However, clinicians regularly completed these outcome assessments and recorded this information as part of their standard care practices within this health district. There may have been slight differences in definitions of practice between the 5 rehabilitation sites. To ensure reliability, each individual site’s protocols for the FIM, DEMMI, TUG, and 10MWT were reviewed and confirmed to be consistent.
It is important, too, to consider the ceiling effect for the FIM scores. For patients requiring a walking aid well after discharge, the highest level of independence from the walking aid will not be achieved. It is acknowledged that the floor effect of the 10MWT and TUG may also influence the outcomes of this study. In addition, data were not collected on preadmission functional measures to enable further evaluation of the population groups. The proportion of variance in change from admission to discharge for TUG and 10MWT to FIM was less than 5%, so the correlation interpretation from this type of scaling is limited. Further research into outcome measures for inpatient rehabilitation in respect to variables such as patient age, length of stay, discharge destination, and efficacy of intervention is warranted.
Conclusion
The FIM Motor change scores showed a weak positive association to the DEMMI change, and no association to the TUG and 10MWT change, demonstrating that the outcome measures do not measure the same attributes. Thorough reporting of clinical outcomes is much more meaningful to assess and guide the physiotherapy component of rehabilitation. To review rehabilitation effectiveness from a management perspective, it is recommended that all measures are reviewed to assess the burden of care, mobility, functional capacity, and dynamic balance.
Acknowledgements: The authors thank Anne Smith, MSHLM, BAppSc, Head of the Physiotherapy Department, and the physiotherapists and allied health assistants who have contributed to the collection of this valuable data over several years. They also thank Lina Baytieh, MS, BS, from Research Central, Illawarra Shoalhaven Local Health District, for her assistance with the analysis.
Corresponding author: Maren Jones, MPH, BS, Physiotherapy Department, Port Kembla Hospital, Illawarra Shoalhaven Local Health District, Warrawong, New South Wales, 2505 Australia; [email protected].
Financial disclosures: None.
1. Centers for Disease Control and Prevention. Disability and health overview. Impairments, activity limitations and participation restrictions. September 16, 2020. https://www.cdc.gov/ncbddd/disabilityandhealth/disability.html
2. The Royal Australasian College of Physicians. Australasian Faculty of Rehabilitation Medicine. Standards for the Provision of Inpatient Adult Rehabilitation Medicine Services in Public and Private Hospitals. February 2019:7-9. https://www.racp.edu.au/docs/default-source/advocacy-library/afrm-standards-for-the-provision-of-inpatient-adult-rehabilitation-medicine-services-in-public-and-private-hospitals.pdf?sfvrsn=4690171a_4
3. NSW Agency for Clinical Innovation. NSW rehabilitation model of care. June 1, 2015. https://aci.health.nsw.gov.au/resources/rehabilitation/rehabilitation-model-of-care/rehabilitation-moc
4. The State of Queensland (Queensland Health). Clinical task instructions. June 22, 2021. https://www.health.qld.gov.au/ahwac/html/clintaskinstructions
5. Panel on Prevention of Falls in Older Persons, American Geriatrics Society and British Geriatrics Society. Summary of the updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc. 2011;59(1):148-157. doi:10.1111/j.1532-5415.2010.03234.x
6. Suwannarat P, Kaewsanmung S, Thaweewannakij T, Amatachaya S. The use of functional performance tests by primary health-care providers to determine walking ability with and without a walking device in community-dwelling elderly. Physiother Theory Pract. 2021;37(1):64-72. doi:10.1080/09593985.2019.1606372
7. Lee K-J, Um S-H, Kim Y-H. Postoperative rehabilitation after hip fracture: a literature review. Hip Pelvis. 2020;32(3):125-131. doi:10.5371/hp.2020.32.3.125
8. Wade DT, Smeets RJEM, Verbunt JA. Research in rehabilitation medicine: methodological challenges. J Clin Epidemiol. 2010;63(7):699-704. doi:10.1016/j.clinepi.2009.07.010
9. Wade DT. Outcome measures for clinical rehabilitation trials: impairment, function, quality of life, or value? Am J Phys Med Rehabil. 2003;82(suppl 10):S26-S31. doi:10.1097/01.PHM.0000086996.89383.A1
10. de Morton NA, Harding KE, Taylor NF, Harrison G. Validity of the de Morton NA Mobility Index (DEMMI) for measuring the mobility of patients with hip fracture during rehabilitation. Disabil Rehabil. 2013;35(4):325-333. doi:10.3109/09638288.2012.705220
11. Trøstrup J, Andersen H, Kam CAM, et al. Assessment of mobility in older people hospitalized for medical illness using the de Morton Mobility Index and cumulated ambulation score—validity and minimal clinical important difference. J Geriatr Phys Ther. 2019;42(3):153-160. doi:10.1519/JPT.0000000000000170
12. Gazzoti A, Meyer U, Freystaetter G, et al. Physical performance among patients aged 70+ in acute care: a preliminary comparison between the Short Physical Performance Battery and the De Morton Mobility Index with regard to sensitivity to change and prediction of discharge destination. Aging Clin Exp Res. 2020;32(4):579-586. doi:10.1007/s40520-019-1249-9
13. Tavares LS, Moreno NA, de Aquino BG, et al. Reliability, validity, interpretability and responsiveness of the DEMMI mobility index for Brazilian older hospitalized patients. PLoS One. 2020;15(3):e0230047. doi:10.1371/journal.pone.0230047
14. Braun T, Schulz R-J, Reinke J. Reliability and validity of the German translation of the de Morton Mobility Index performed by physiotherapists in patients admitted to a sub-acute inpatient geriatric rehabilitation hospital. BMC Geriatr. 2015;15:38. doi:10.1186/s12877-015-0035-y
15. Søndergaard K, Petersen LE, Pedersen MK, et al. The responsiveness and predictive validity of the de Morton Mobility Index in geriatric rehabilitation. Disabil Rehabil. 2020 Jun 12. [Epub ahead of print] doi:10.1080/09638288.2020.1771438
16. de Morton NA, Brusco NK, Wood L, et al. The de Morton Mobility Index (DEMMI) provides a valid method for measuring and monitoring the mobility of patients making the transition from hospital to the community: an observational study. J Physiother. 2011;57(2):109-116. doi:10.1016/S1836-9553(11)70021-2
17. Caronni A, Sterpi I, Antoniotti P, et al. Criterion validity of the instrumented Timed Up and Go test: a partial least square regression study. Gait Posture. 2018;61(3):287-293. doi:10.1016/j.gaitpost.2018.01.015
18. Kristensen MT, Bloch ML, Jonsson LR, Jakobsen TL. Interrater reliability of the standardized Timed Up and Go Test when used in hospitalized and community-dwelling individuals. Physiother Res Int. 2019;24(2):e1769. doi:10.1002/pri.1769
19. Yuksel E, Kalkan S, Cekmece S, et al. Assessing minimal detectable changes and test-retest reliability of the timed up and go test and 2-minute walk test in patients with total knee arthroplasty. J Arthroplasty. 2017;32(2):426-430. doi:10.1016/j.arth.2016.07.031
20. Yuksel E, Unver B, Kalkan S, Karatosun V. Reliability and minimal detectable change of the 2-minute walk test and Timed Up and Go test in patients with total hip arthroplasty. Hip Int. 2021;31(1):43-49. doi:10.1177/1120700019888614
21. Faleide AGH, Bogen BE, Magnussen LH. Intra-session test-retest reliability of the Timed “Up & Go” Test when performed by patients with hip fractures. Eur J Physiother. 2015;17(2):89-97. doi:10.3109/21679169.2015.1043579
22. Barry E, Galvin R, Keogh C, et al. Is the timed up and go test a useful predictor of risk of falls in community dwelling older adults: a systematic review and meta- analysis. BMC Geriatr. 2014;14:14. doi:10.1186/1471-2318-14-14
23. Kojima G, Masud T, Kendrick D, et al. Does the timed up and go test predict future falls among British community-dwelling older people? Prospective cohort study nested within a randomised controlled trial. BMC Geriatr. 2015;15:38. doi:10.1186/s12877-015-0039-7
24. Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the timed up & go test. Phys Ther. 2000;80(9):896-903.
25. Jeong SM, Shin DW, Han K, et al. Timed Up-and-Go test is a useful predictor of fracture incidence. Bone. 2019;127:474-481. doi:10.1016/j.bone.2019.07.018
26. Donaghue OA, Savva GM, Börsch-Supan A, Kenny RA. Reliability, measurement error and minimum detectable change in reliability measurement error and minimum detectable change in mobility measures: a cohort study of community dwelling adults aged 50 years and over in Ireland. BMJ Open. 2019;9(11):e030475. doi:10/1136.bmjopen-2019-030475
27. Freter SH, Fruchter N. Relationship between timed ‘up and go’ and gait time in an elderly orthopaedic rehabilitation population. Clin Rehabil. 2000;14(1):96-101. doi:10.1191/026921500675545616
28. Kear BM, Guck TP, McGaha AL. Timed up and go (TUG) test: normative reference values for ages 20 to 59 years and relationships with physical and mental health risk factors. J Prim Care Community Health. 2017;8(1):9-13. doi:10.1177/2150131916659282
29. Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people: an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10)881-889. doi:10.1007/s12603-009-0246-z
30. Unver B, Baris RH, Yusel E, et al. Reliability of 4-meter and 10-meter walk tests after lower extremity surgery. Disabil Rehabil. 2017;39(25):2572-2576. doi:10.1080/09638288.2016.1236153
31. Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign.” J Geriatr Phys Ther. 2009;32(2):46-49.
32. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50-58. doi:10.1001/jama.2010.1923
33. Bohannon R. Comfortable and maximum walking speed of adults aged 20-79 years: reference values and determinants. Age Ageing. 1997;26(1):15-19. doi:10.1093/ageing/26.1.15
34. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance in older adults. J Am Geriatr Soc. 2006;54(5):743-749. doi:10.1111/j.1532-5415.2006.00701.x
35. Hollman J, Beckman B, Brandt R, et al. Minimum detectable change in gait velocity during acute rehabilitation following hip fracture. J Geriatr Phys Ther. 2008;31(2):53-56. doi:10.1519/00139143-200831020-00003
36. Bohannon RW, Andrews AW. Normal walking speed: a descriptive meta-analysis. Physiotherapy. 2011;97(3):182-189. doi:10.1016/j.physio.2010.12.004
37. Granger CV, Hamilton BB, Keith RA, et al. Advances in functional assessment for medical rehabilitation. Top Geriatr Rehabil. 1986;1:59-74.
38. Keith RA, Granger CV, Hamilton BB, Sherwin FS. The Functional Independence Measure: a new tool for rehabilitation. In: Eisenberg MG, Grzesiak RC, eds. Advances in Clinical Rehabilitation. Springer-Verlag; 1987:6-18.
39. Linacre JM, Heinemann AW, Wright BD, et al. The structure and stability of the Functional Independence Measure. Arch Phys Med Rehabil. 1994;75(2):127-132.
40. Coster WJ, Haley SM, Jette AM. Measuring patient-reported outcomes after discharge from inpatient rehabilitation settings. J Rehabil Med. 2006;38(4):237-242. doi:10.1080/16501970600609774
41. Street L. Frequently asked questions about FIM. Journal of the Australasian Rehabilitation Nurses Association. 2014;17(1):21-22. https://ro.uow.edu.au/ahsri/296/
42. Green JP, Gordon R, Blanchard MB, et al. Development of the Australian National Subacute and Non-acute Patient (AN-SNAP) Classification. Version 4 Final Report. Australian Health Services Research Institute, University of Wollongong, 2015. https://ro.uow.edu.au/ahsri/760
43. Australasian Rehabilitation Outcomes Centre. University of Wollongong, Australia. https://www.uow.edu.au/ahsri/aroc/
44. Green J, Gordon R, Kobel C, et al; Centre for Health Service Development. The Australian National Subacute and Non-acute Patient Classification. AN-SNAP V4 User Manual. May 2015. https://documents.uow.edu.au/content/groups/public/@web/@chsd/@aroc/documents/doc/uow194637.pdf
45. Alexander TL, Simmonds FD, Capelle JT, Green LJ. Anywhere Hospital AROC Impairment Specific Report on Reconditioning (Inpatient–Pathway 3), July 2018–June 2019. Australasian Rehabilitation Outcomes Centre, Australian Health Services Research Institute, University of Wollongong; 2019. ro.uow.edu.au/ahsri/1110
46. Evans JD. Straightforward Statistics for the Behavioural Sciences. Brooks/Cole Publishing; 1996.
47. Lee SP, Dufek J, Hickman R, Schuerman S. Influence of procedural factors on the reliability and performance of the timed up-and-go test in older adults. Int J Gerontol. 2016;10(1):37-42. doi:10.1016/j.ijge.2015
48. New PW, Scroggie GD, Williams CM. The validity, reliability, responsiveness and minimal clinically important difference of the de Morton Mobility Index in rehabilitation. Disabil Rehabil. 2017;39(10):1039-1043. doi:10.10801/09638288.2016.1179800
49. Nakaguchi T, Ishimoto Y, Akazawa N. Functional Independence Measure for patients with locomotor disorders in convalescent rehabilitation wards. Clinically significant minimum difference in exercise score gain. Physiotherapy Science. 2018;33(2):235-240.
1. Centers for Disease Control and Prevention. Disability and health overview. Impairments, activity limitations and participation restrictions. September 16, 2020. https://www.cdc.gov/ncbddd/disabilityandhealth/disability.html
2. The Royal Australasian College of Physicians. Australasian Faculty of Rehabilitation Medicine. Standards for the Provision of Inpatient Adult Rehabilitation Medicine Services in Public and Private Hospitals. February 2019:7-9. https://www.racp.edu.au/docs/default-source/advocacy-library/afrm-standards-for-the-provision-of-inpatient-adult-rehabilitation-medicine-services-in-public-and-private-hospitals.pdf?sfvrsn=4690171a_4
3. NSW Agency for Clinical Innovation. NSW rehabilitation model of care. June 1, 2015. https://aci.health.nsw.gov.au/resources/rehabilitation/rehabilitation-model-of-care/rehabilitation-moc
4. The State of Queensland (Queensland Health). Clinical task instructions. June 22, 2021. https://www.health.qld.gov.au/ahwac/html/clintaskinstructions
5. Panel on Prevention of Falls in Older Persons, American Geriatrics Society and British Geriatrics Society. Summary of the updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc. 2011;59(1):148-157. doi:10.1111/j.1532-5415.2010.03234.x
6. Suwannarat P, Kaewsanmung S, Thaweewannakij T, Amatachaya S. The use of functional performance tests by primary health-care providers to determine walking ability with and without a walking device in community-dwelling elderly. Physiother Theory Pract. 2021;37(1):64-72. doi:10.1080/09593985.2019.1606372
7. Lee K-J, Um S-H, Kim Y-H. Postoperative rehabilitation after hip fracture: a literature review. Hip Pelvis. 2020;32(3):125-131. doi:10.5371/hp.2020.32.3.125
8. Wade DT, Smeets RJEM, Verbunt JA. Research in rehabilitation medicine: methodological challenges. J Clin Epidemiol. 2010;63(7):699-704. doi:10.1016/j.clinepi.2009.07.010
9. Wade DT. Outcome measures for clinical rehabilitation trials: impairment, function, quality of life, or value? Am J Phys Med Rehabil. 2003;82(suppl 10):S26-S31. doi:10.1097/01.PHM.0000086996.89383.A1
10. de Morton NA, Harding KE, Taylor NF, Harrison G. Validity of the de Morton NA Mobility Index (DEMMI) for measuring the mobility of patients with hip fracture during rehabilitation. Disabil Rehabil. 2013;35(4):325-333. doi:10.3109/09638288.2012.705220
11. Trøstrup J, Andersen H, Kam CAM, et al. Assessment of mobility in older people hospitalized for medical illness using the de Morton Mobility Index and cumulated ambulation score—validity and minimal clinical important difference. J Geriatr Phys Ther. 2019;42(3):153-160. doi:10.1519/JPT.0000000000000170
12. Gazzoti A, Meyer U, Freystaetter G, et al. Physical performance among patients aged 70+ in acute care: a preliminary comparison between the Short Physical Performance Battery and the De Morton Mobility Index with regard to sensitivity to change and prediction of discharge destination. Aging Clin Exp Res. 2020;32(4):579-586. doi:10.1007/s40520-019-1249-9
13. Tavares LS, Moreno NA, de Aquino BG, et al. Reliability, validity, interpretability and responsiveness of the DEMMI mobility index for Brazilian older hospitalized patients. PLoS One. 2020;15(3):e0230047. doi:10.1371/journal.pone.0230047
14. Braun T, Schulz R-J, Reinke J. Reliability and validity of the German translation of the de Morton Mobility Index performed by physiotherapists in patients admitted to a sub-acute inpatient geriatric rehabilitation hospital. BMC Geriatr. 2015;15:38. doi:10.1186/s12877-015-0035-y
15. Søndergaard K, Petersen LE, Pedersen MK, et al. The responsiveness and predictive validity of the de Morton Mobility Index in geriatric rehabilitation. Disabil Rehabil. 2020 Jun 12. [Epub ahead of print] doi:10.1080/09638288.2020.1771438
16. de Morton NA, Brusco NK, Wood L, et al. The de Morton Mobility Index (DEMMI) provides a valid method for measuring and monitoring the mobility of patients making the transition from hospital to the community: an observational study. J Physiother. 2011;57(2):109-116. doi:10.1016/S1836-9553(11)70021-2
17. Caronni A, Sterpi I, Antoniotti P, et al. Criterion validity of the instrumented Timed Up and Go test: a partial least square regression study. Gait Posture. 2018;61(3):287-293. doi:10.1016/j.gaitpost.2018.01.015
18. Kristensen MT, Bloch ML, Jonsson LR, Jakobsen TL. Interrater reliability of the standardized Timed Up and Go Test when used in hospitalized and community-dwelling individuals. Physiother Res Int. 2019;24(2):e1769. doi:10.1002/pri.1769
19. Yuksel E, Kalkan S, Cekmece S, et al. Assessing minimal detectable changes and test-retest reliability of the timed up and go test and 2-minute walk test in patients with total knee arthroplasty. J Arthroplasty. 2017;32(2):426-430. doi:10.1016/j.arth.2016.07.031
20. Yuksel E, Unver B, Kalkan S, Karatosun V. Reliability and minimal detectable change of the 2-minute walk test and Timed Up and Go test in patients with total hip arthroplasty. Hip Int. 2021;31(1):43-49. doi:10.1177/1120700019888614
21. Faleide AGH, Bogen BE, Magnussen LH. Intra-session test-retest reliability of the Timed “Up & Go” Test when performed by patients with hip fractures. Eur J Physiother. 2015;17(2):89-97. doi:10.3109/21679169.2015.1043579
22. Barry E, Galvin R, Keogh C, et al. Is the timed up and go test a useful predictor of risk of falls in community dwelling older adults: a systematic review and meta- analysis. BMC Geriatr. 2014;14:14. doi:10.1186/1471-2318-14-14
23. Kojima G, Masud T, Kendrick D, et al. Does the timed up and go test predict future falls among British community-dwelling older people? Prospective cohort study nested within a randomised controlled trial. BMC Geriatr. 2015;15:38. doi:10.1186/s12877-015-0039-7
24. Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the timed up & go test. Phys Ther. 2000;80(9):896-903.
25. Jeong SM, Shin DW, Han K, et al. Timed Up-and-Go test is a useful predictor of fracture incidence. Bone. 2019;127:474-481. doi:10.1016/j.bone.2019.07.018
26. Donaghue OA, Savva GM, Börsch-Supan A, Kenny RA. Reliability, measurement error and minimum detectable change in reliability measurement error and minimum detectable change in mobility measures: a cohort study of community dwelling adults aged 50 years and over in Ireland. BMJ Open. 2019;9(11):e030475. doi:10/1136.bmjopen-2019-030475
27. Freter SH, Fruchter N. Relationship between timed ‘up and go’ and gait time in an elderly orthopaedic rehabilitation population. Clin Rehabil. 2000;14(1):96-101. doi:10.1191/026921500675545616
28. Kear BM, Guck TP, McGaha AL. Timed up and go (TUG) test: normative reference values for ages 20 to 59 years and relationships with physical and mental health risk factors. J Prim Care Community Health. 2017;8(1):9-13. doi:10.1177/2150131916659282
29. Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people: an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10)881-889. doi:10.1007/s12603-009-0246-z
30. Unver B, Baris RH, Yusel E, et al. Reliability of 4-meter and 10-meter walk tests after lower extremity surgery. Disabil Rehabil. 2017;39(25):2572-2576. doi:10.1080/09638288.2016.1236153
31. Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign.” J Geriatr Phys Ther. 2009;32(2):46-49.
32. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50-58. doi:10.1001/jama.2010.1923
33. Bohannon R. Comfortable and maximum walking speed of adults aged 20-79 years: reference values and determinants. Age Ageing. 1997;26(1):15-19. doi:10.1093/ageing/26.1.15
34. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance in older adults. J Am Geriatr Soc. 2006;54(5):743-749. doi:10.1111/j.1532-5415.2006.00701.x
35. Hollman J, Beckman B, Brandt R, et al. Minimum detectable change in gait velocity during acute rehabilitation following hip fracture. J Geriatr Phys Ther. 2008;31(2):53-56. doi:10.1519/00139143-200831020-00003
36. Bohannon RW, Andrews AW. Normal walking speed: a descriptive meta-analysis. Physiotherapy. 2011;97(3):182-189. doi:10.1016/j.physio.2010.12.004
37. Granger CV, Hamilton BB, Keith RA, et al. Advances in functional assessment for medical rehabilitation. Top Geriatr Rehabil. 1986;1:59-74.
38. Keith RA, Granger CV, Hamilton BB, Sherwin FS. The Functional Independence Measure: a new tool for rehabilitation. In: Eisenberg MG, Grzesiak RC, eds. Advances in Clinical Rehabilitation. Springer-Verlag; 1987:6-18.
39. Linacre JM, Heinemann AW, Wright BD, et al. The structure and stability of the Functional Independence Measure. Arch Phys Med Rehabil. 1994;75(2):127-132.
40. Coster WJ, Haley SM, Jette AM. Measuring patient-reported outcomes after discharge from inpatient rehabilitation settings. J Rehabil Med. 2006;38(4):237-242. doi:10.1080/16501970600609774
41. Street L. Frequently asked questions about FIM. Journal of the Australasian Rehabilitation Nurses Association. 2014;17(1):21-22. https://ro.uow.edu.au/ahsri/296/
42. Green JP, Gordon R, Blanchard MB, et al. Development of the Australian National Subacute and Non-acute Patient (AN-SNAP) Classification. Version 4 Final Report. Australian Health Services Research Institute, University of Wollongong, 2015. https://ro.uow.edu.au/ahsri/760
43. Australasian Rehabilitation Outcomes Centre. University of Wollongong, Australia. https://www.uow.edu.au/ahsri/aroc/
44. Green J, Gordon R, Kobel C, et al; Centre for Health Service Development. The Australian National Subacute and Non-acute Patient Classification. AN-SNAP V4 User Manual. May 2015. https://documents.uow.edu.au/content/groups/public/@web/@chsd/@aroc/documents/doc/uow194637.pdf
45. Alexander TL, Simmonds FD, Capelle JT, Green LJ. Anywhere Hospital AROC Impairment Specific Report on Reconditioning (Inpatient–Pathway 3), July 2018–June 2019. Australasian Rehabilitation Outcomes Centre, Australian Health Services Research Institute, University of Wollongong; 2019. ro.uow.edu.au/ahsri/1110
46. Evans JD. Straightforward Statistics for the Behavioural Sciences. Brooks/Cole Publishing; 1996.
47. Lee SP, Dufek J, Hickman R, Schuerman S. Influence of procedural factors on the reliability and performance of the timed up-and-go test in older adults. Int J Gerontol. 2016;10(1):37-42. doi:10.1016/j.ijge.2015
48. New PW, Scroggie GD, Williams CM. The validity, reliability, responsiveness and minimal clinically important difference of the de Morton Mobility Index in rehabilitation. Disabil Rehabil. 2017;39(10):1039-1043. doi:10.10801/09638288.2016.1179800
49. Nakaguchi T, Ishimoto Y, Akazawa N. Functional Independence Measure for patients with locomotor disorders in convalescent rehabilitation wards. Clinically significant minimum difference in exercise score gain. Physiotherapy Science. 2018;33(2):235-240.
Positive Outcomes Following a Multidisciplinary Approach in the Diagnosis and Prevention of Hospital Delirium
From the Department of Neurology, Cedars-Sinai Medical Center, Los Angeles, CA (Drs. Ching, Darwish, Li, Wong, Simpson, and Funk), the Department of Anesthesia, Cedars-Sinai Medical Center, Los Angeles, CA (Keith Siegel), and the Department of Psychiatry, Cedars-Sinai Medical Center, Los Angeles, CA (Dr. Bamgbose).
Objectives: To reduce the incidence and duration of delirium among patients in a hospital ward through standardized delirium screening tools and nonpharmacologic interventions. To advance nursing-focused education on delirium-prevention strategies. To measure the efficacy of the interventions with the aim of reproducing best practices.
Background: Delirium is associated with poor patient outcomes but may be preventable in a significant percentage of hospitalized patients.
Methods: Following nursing-focused education to prevent delirium, we prospectively evaluated patient care outcomes in a consecutive series of patients who were admitted to a hospital medical-surgical ward within a 25-week period. All patients who had at least 1 Confusion Assessment Method (CAM) documented by a nurse during hospitalization met our inclusion criteria (N = 353). Standards for Quality Improvement Reporting Excellence guidelines were adhered to.
Results: There were 187 patients in the control group, and 166 in the postintervention group. Compared to the control group, the postintervention group had a significant decrease in the incidence of delirium during hospitalization (14.4% vs 4.2%) and a significant decrease in the mean percentage of tested nursing shifts with 1 or more positive CAM (4.9% vs 1.1%). Significant differences in secondary outcomes between the control and postintervention groups included median length of stay (6 days vs 4 days), mean length of stay (8.5 days vs 5.9 days), and use of an indwelling urinary catheter (9.1% vs 2.4%).
Conclusion: A multimodal strategy involving nursing-focused training and nonpharmacologic interventions to address hospital delirium is associated with improved patient care outcomes and nursing confidence. Nurses play an integral role in the early recognition and prevention of hospital delirium, which directly translates to reducing burdens in both patient functionality and health care costs.
Delirium is a disorder characterized by inattention and acute changes in cognition. It is defined by the American Psychiatric Association’s fifth edition of the Diagnostic and Statistical Manual of Mental Disorders as a disturbance in attention, awareness, and cognition over hours to a few days that is not better explained by a preexisting, established, or other evolving neurocognitive disorder.1 Delirium is common yet often under-recognized among hospitalized patients, particularly in the elderly. The incidence of delirium in elderly patients on admission is estimated to be 11% to 25%, and an additional 29% to 31% of elderly patients will develop delirium during the hospitalization.2 Delirium costs the health care system an estimated $38 billion to $152 billion per year.3 It is associated with negative outcomes, such as increased new placements to nursing homes, increased mortality, increased risk of dementia, and further cognitive deterioration among patients with dementia.4-6
Despite its prevalence, delirium may be preventable in a significant percentage of hospitalized patients. Targeted intervention strategies, such as frequent reorientation, maximizing sleep, early mobilization, restricting use of psychoactive medications, and addressing hearing or vision impairment, have been demonstrated to significantly reduce the incidence of hospital delirium.7,8 To achieve these goals, we explored the use of a multimodal strategy centered on nursing education. We integrated consistent, standardized delirium screening and nonpharmacologic interventions as part of a preventative protocol to reduce the incidence of delirium in the hospital ward.
Methods
We evaluated a consecutive series of patients who were admitted to a designated hospital medical-surgical ward within a 25-week period between October 2019 and April 2020. All patients during this period who had at least 1 Confusion Assessment Method (CAM) documented by a nurse during hospitalization met our inclusion criteria. Patients who did not have a CAM documented were excluded from the analysis. Delirium was defined according to the CAM diagnostic algorithm.9
Core nursing staff regularly assigned to the ward completed a multimodal training program designed to improve recognition, documentation, and prevention of hospital delirium. Prior to the training, the nurses completed a 5-point Likert scale survey assessing their level of confidence with recognizing delirium risk factors, preventing delirium, addressing delirium, utilizing the CAM tool, and educating others about delirium. Nurses completed the same survey after the study period ended.
The training curriculum for nurses began with an online module reviewing the epidemiology and risk factors for delirium. Nurses then participated in a series of in-service training sessions led by a team of physicians, during which the CAM and nonpharmacologic delirium prevention measures were reviewed then practiced first-hand. Nursing staff attended an in-person lecture reviewing the current body of literature on delirium risk factors and effective nursing interventions. After formal training was completed, nurses were instructed to document CAM screens
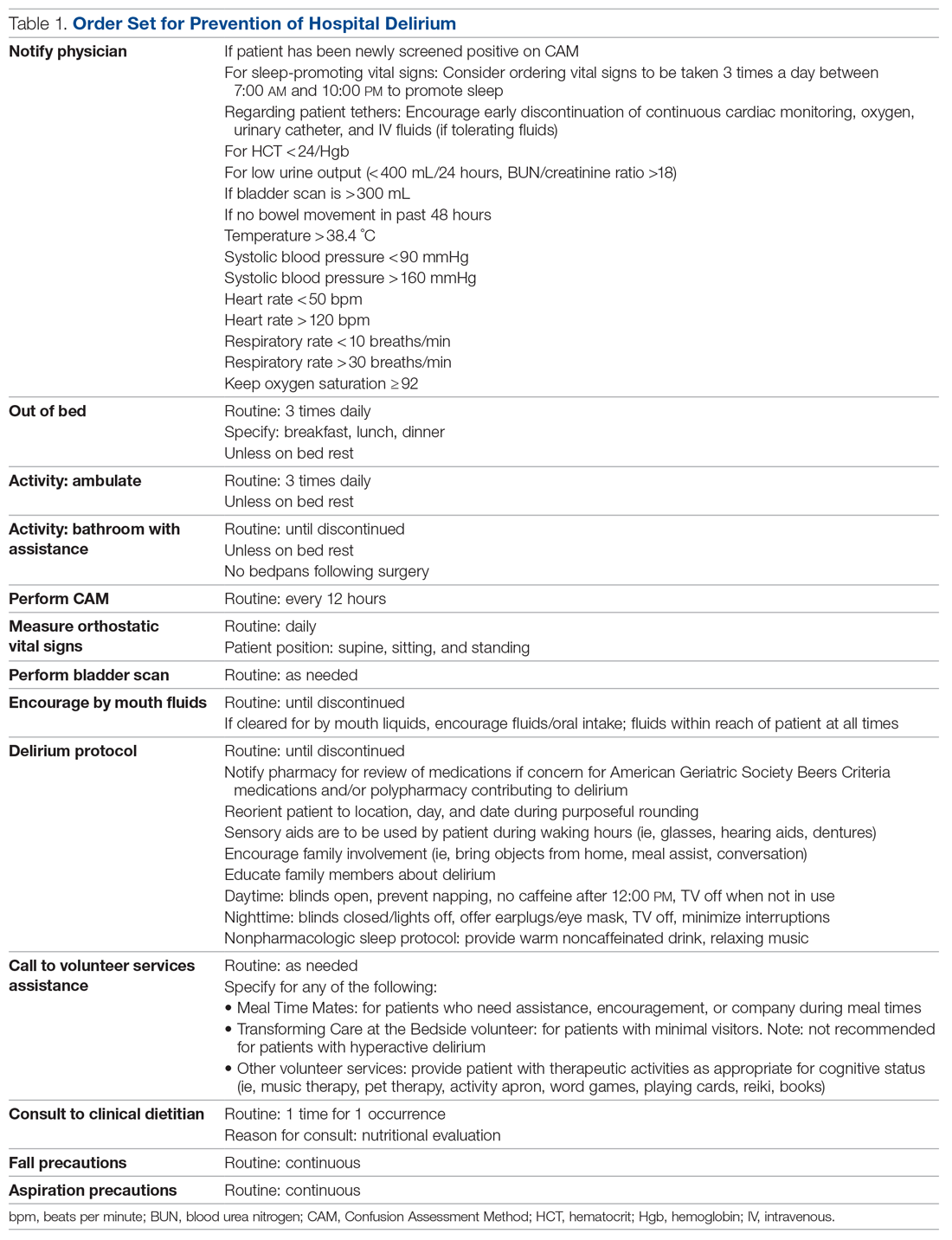
Patients admitted to the hospital unit from the start of the training program (week 1) until the order set was made available (week 15) constituted our control group. The postintervention study group consisted of patients admitted for 10 weeks after the completion of the interventions (weeks 16-25). A timeline of the study events is shown in Figure 1.
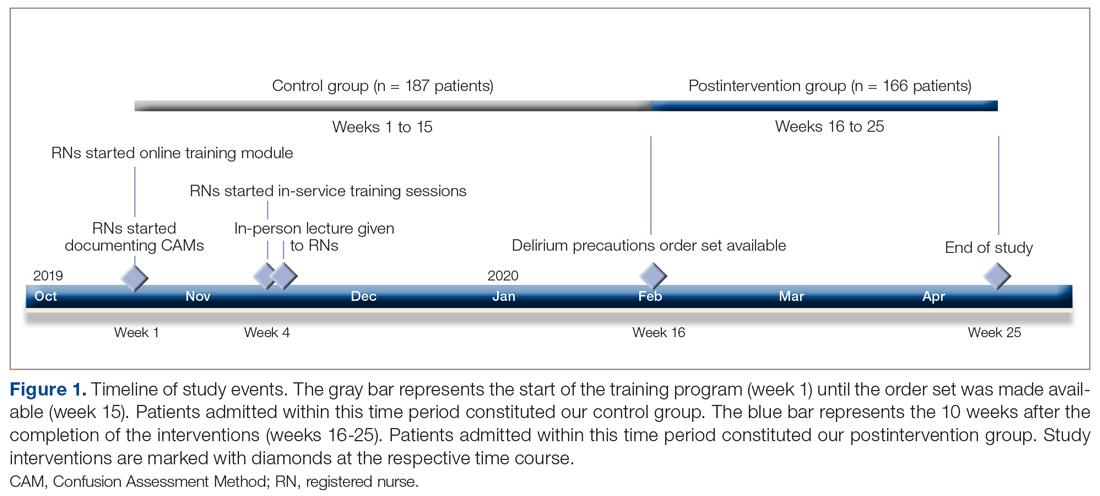
Patient demographics and hospital-stay metrics determined a priori were attained via the Cedars-Sinai Enterprise Information Services core. Age, sex, medical history, and incidence of surgery with anesthesia during hospitalization were recorded. The Charlson Comorbidity Index was calculated from patients’ listed diagnoses following discharge. Primary outcomes included incidence of patients with delirium during hospitalization, percentage of tested shifts with positive CAM screens, length of hospital stay, and survival. Secondary outcomes included measures associated with delirium, including the use of chemical restraints, physical restraints, sitters, indwelling urinary catheters, and new psychiatry and neurology consults. Chemical restraints were defined as administration of a new antipsychotic medication or benzodiazepine for the specific indication of hyperactive delirium or agitation.
Statistical analysis was conducted by a statistician, using R version 3.6.3.10P values of < .05 were considered significant. Categorical variables were analyzed using Fisher’s exact test. Continuous variables were analyzed with Welch’s t-test or, for highly skewed continuous variables, with Wilcoxon rank-sum test or Mood’s median test. All patient data were anonymized and stored securely in accordance with institutional guidelines.
Our project was deemed to represent nonhuman subject research and therefore did not require Institutional Review Board (IRB) approval upon review by our institution’s IRB committee and Office of Research Compliance and Quality Improvement. Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines were adhered to (Supplementary File can be found at mdedge.com/jcomjournal).
Results
We evaluated 353 patients who met our inclusion criteria: 187 in the control group, and 166 in the postintervention group. Ten patients were readmitted to the ward after their initial discharge; only the initial admission encounters were included in our analysis. Median age, sex, median Charlson Comorbidity Index, and incidence of surgery with anesthesia during hospitalization were comparable between the control and postintervention groups and are summarized in Table 2.
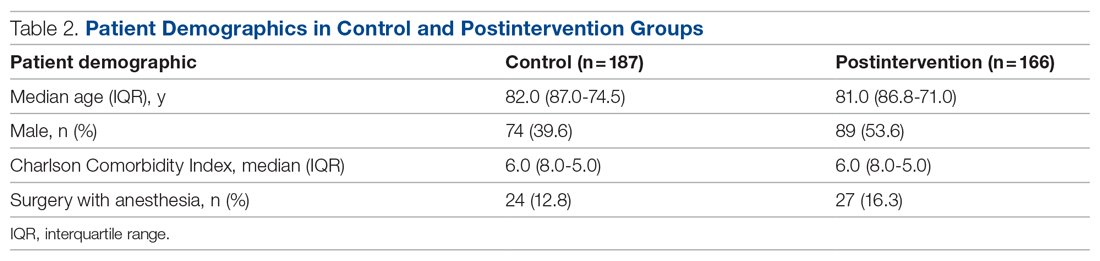
In the control group, 1572 CAMs were performed, with 74 positive CAMs recorded among 27 patients with delirium. In the postintervention group, 1298 CAMs were performed, with 12 positive CAMs recorded among 7 patients with delirium (Figure 2). Primary and secondary outcomes, as well as CAM compliance measures, are summarized in Table 3.
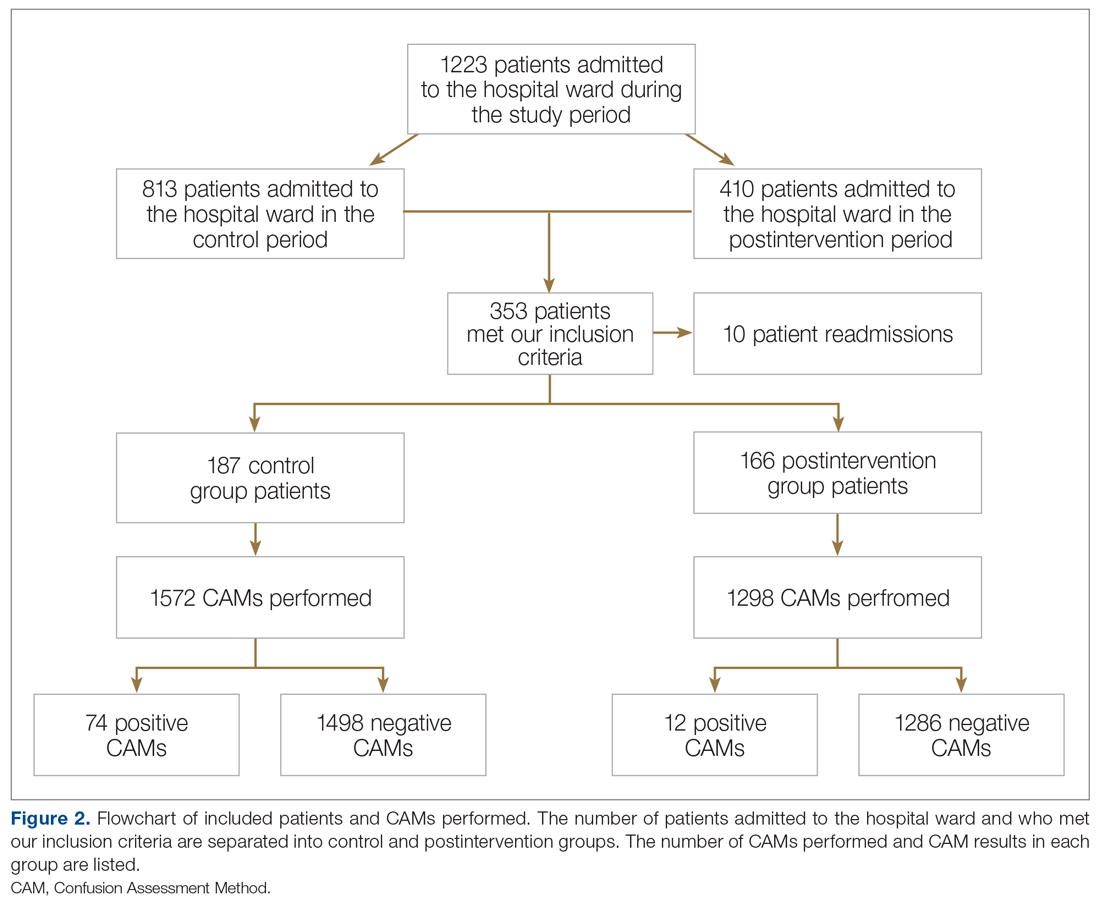
Compared to the control group, the postintervention group had a significant decrease in the incidence of delirium during hospitalization (14.4% vs 4.2%, P = .002) and a significant decrease in the mean percentage of tested nursing shifts with 1 or more positive CAM (4.9% vs 1.1%, P = .002). Significant differences in secondary outcomes between the control and postintervention groups included median length of stay (6 days vs 4 days, P = .004), mean length of stay (8.5 days vs 5.9 days, P = .003), and use of an indwelling urinary catheter (9.1% vs 2.4%, P = .012). There was a trend towards decreased incidence of chemical restraints and psychiatry consults, which did not reach statistical significance. Differences in mortality during hospitalization, physical restraint use, and sitter use could not be assessed due to low incidence.
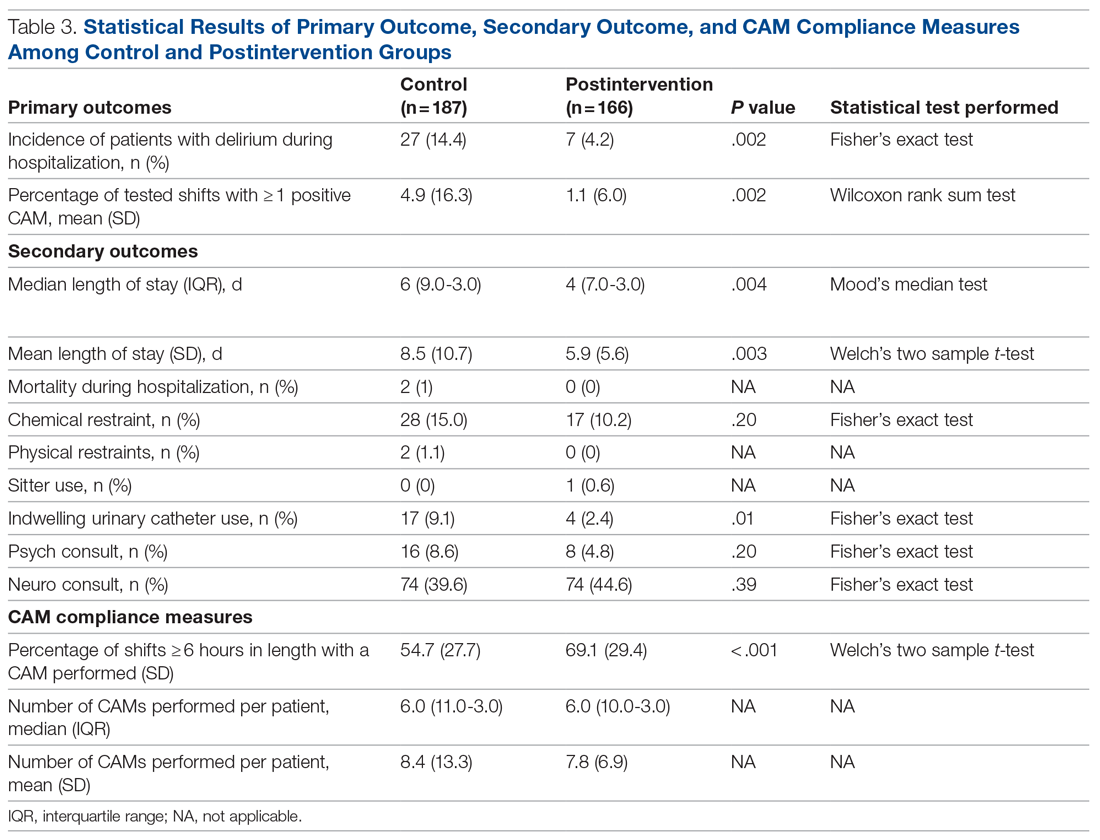
Compliance with nursing CAM assessments was evaluated. Compared to the control group, the postintervention group saw a significant increase in the percentage of shifts with a CAM performed (54.7% vs 69.1%, P < .001). The median and mean number of CAMs performed per patient were similar between the control and postintervention groups.
Results of nursing surveys completed before and after the training program are listed in Table 4. After training, nurses had a greater level of confidence with recognizing delirium risk factors, preventing delirium, addressing delirium, utilizing the CAM tool, and educating others about delirium.
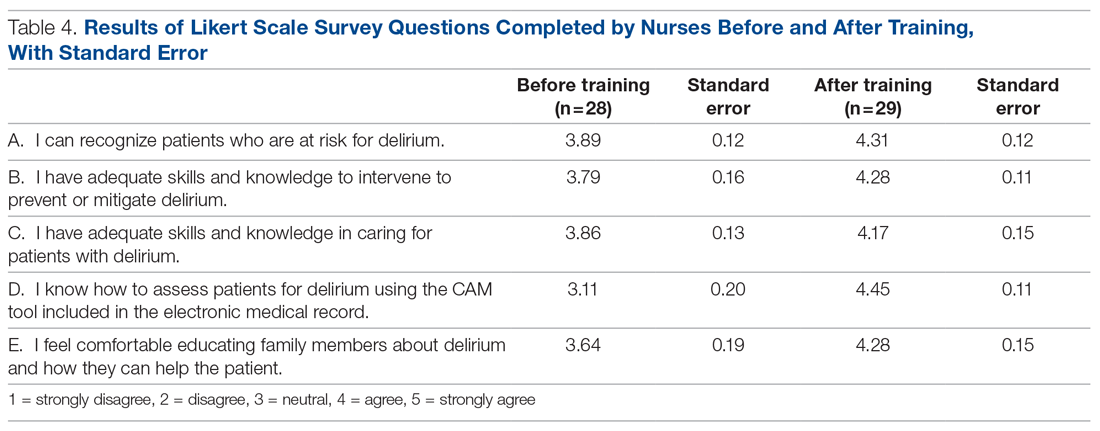
Discussion
Our study utilized a standardized delirium assessment tool to compare patient cohorts before and after nurse-targeted training interventions on delirium recognition and prevention. Our interventions emphasized nonpharmacologic intervention strategies, which are recommended as first-line in the management of patients with delirium.11 Patients were not excluded from the analysis based on preexisting medical conditions or recent surgery with anesthesia, to allow for conditions that are representative of community hospitals. We also did not use an inclusion criterion based on age; however, the majority of our patients were greater than 70 years old, representing those at highest risk for delirium.2 Significant outcomes among patients in the postintervention group include decreased incidence of delirium, lower average length of stay, decreased indwelling urinary catheter use, and increased compliance with delirium screening by nursing staff.
While the study’s focus was primarily on delirium prevention rather than treatment, these strategies may also have conferred the benefit of reversing delirium symptoms. In addition to measuring incidence of delirium, our primary outcome of percentage of tested shifts with 1 or more positive CAM was intended to assess the overall duration in which patients had delirium during their hospitalization. The reduction in shifts with positive CAMs observed in the postintervention group is notable, given that a significant percentage of patients with hospital delirium have the potential for symptom reversibility.12
Multiple studies have shown that admitted patients who develop delirium experience prolonged hospital stays, often up to 5 to 10 days longer.12-14 The decreased incidence and duration of delirium in our postintervention group is a reasonable explanation for the observed decrease in average length of stay. Our study is in line with previously documented initiatives that show that nonpharmacologic interventions can effectively address downstream health and fiscal sequelae of hospital delirium. For example, a volunteer-based initiative named the Hospital Elder Life Program, from which elements in our order set were modeled after, demonstrated significant reductions in delirium incidence, length of stay, and health care costs.14-16 Other initiatives that focused on educational training for nurses to assess and prevent delirium have also demonstrated similar positive results.17-19 Our study provides a model for effective nursing-focused education that can be reproduced in the hospital setting.
Unlike some other studies, which identified delirium based only on physician assessments, our initiative utilized the CAM performed by floor nurses to identify delirium. While this method
Our study demonstrated an increase in the overall compliance with the CAM screening during the postintervention period, which is significant given the under-recognition of delirium by health care professionals.20 We attribute this increase to greater realized importance and a higher level of confidence from nursing staff in recognizing and addressing delirium, as supported by survey data. While the increased screening of patients should be considered a positive outcome, it also poses the possibility that the observed decrease in delirium incidence in the postintervention group was in fact due to more CAMs performed on patients without delirium. Likewise, nurses may have become more adept at recognizing true delirium, as opposed to delirium mimics, in the latter period of the study.
Perhaps the greatest limitation of our study is the variability in performing and recording CAMs, as some patients had multiple CAMs recorded while others did not have any CAMs recorded. This may have been affected in part by the increase in COVID-19 cases in our hospital towards the latter half of the study, which resulted in changes in nursing assignments as well as patient comorbidities in ways that cannot be easily quantified. Given the limited size of our patient cohorts, certain outcomes, such as the use of sitters, physical restraints, and in-hospital mortality, were unable to be assessed for changes statistically. Causative relationships between our interventions and associated outcome measures are necessarily limited in a binary comparison between control and postintervention groups.
Within these limitations, our study demonstrates promising results in core dimensions of patient care. We anticipate further quality improvement initiatives involving greater numbers of nursing staff and patients to better quantify the impact of nonpharmacologic nursing-centered interventions for preventing hospital delirium.
Conclusion
A multimodal strategy involving nursing-focused training and nonpharmacologic interventions to address hospital delirium is associated with improved patient care outcomes and nursing confidence. Nurses play an integral role in the early recognition and prevention of hospital delirium, which directly translates to reducing burdens in both patient functionality and health care costs. Education and tools to equip nurses to perform standardized delirium screening and interventions should be prioritized.
Acknowledgment: The authors thanks Olena Svetlov, NP, Oscar Abarca, Jose Chavez, and Jenita Gutierrez.
Corresponding author: Jason Ching, MD, Department of Neurology, Cedars-Sinai Medical Center, 8700 Beverly Blvd, Los Angeles, CA 90048; [email protected].
Financial disclosures: None.
Funding: This research was supported by NIH National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition. American Psychiatric Association; 2013.
2. Vasilevskis EE, Han JH, Hughes CG, et al. Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol. 2012;26(3):277-287. doi:10.1016/j.bpa.2012.07003
3. Leslie DL, Marcantonio ER, Zhang Y, et al. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27-32. doi:10.1001/archinternmed.2007.4
4. McCusker J, Cole M, Abrahamowicz M, et al. Delirium predicts 12-month mortality. Arch Intern Med. 2002;162(4):457-463. doi:10.1001/archinte.162.4.457
5. Witlox J, Eurelings LS, de Jonghe JF, et al. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443-451. doi:10.1001/jama.2010.1013
6. Gross AL, Jones RN, Habtemariam DA, et al. Delirium and long-term cognitive trajectory among persons with dementia. Arch Intern Med. 2012;172(17):1324-1331. doi:10.1001/archinternmed.2012.3203
7. Inouye SK. Prevention of delirium in hospitalized older patients: risk factors and targeted intervention strategies. Ann Med. 2000;32(4):257-263. doi:10.3109/07853890009011770
8. Siddiqi N, Harrison JK, Clegg A, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2016;3:CD005563. doi:10.1002/14651858.CD005563.pub3
9. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. doi:10.7326/0003-4819-113-12-941
10. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2017.
11. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220. doi:10.1038/nrneurol.2009.24
12. Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35(4):350-364. doi:10.1093/ageing/afl005
13. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762. doi:10.1001/jama.291.14.1753
14. Chen CC, Lin MT, Tien YW, et al. Modified Hospital Elder Life Program: effects on abdominal surgery patients. J Am Coll Surg. 2011;213(2):245-252. doi:10.1016/j.jamcollsurg.2011.05.004
15. Zaubler TS, Murphy K, Rizzuto L, et al. Quality improvement and cost savings with multicomponent delirium interventions: replication of the Hospital Elder Life Program in a community hospital. Psychosomatics. 2013;54(3):219-226. doi:10.1016/j.psym.2013.01.010
16. Rubin FH, Neal K, Fenlon K, et al. Sustainability and scalability of the Hospital Elder Life Program at a community hospital. J Am Geriatr Soc. 2011;59(2):359-365. doi:10.1111/j.1532-5415.2010.03243.x
17. Milisen K, Foreman MD, Abraham IL, et al. A nurse-led interdisciplinary intervention program for delirium in elderly hip-fracture patients. J Am Geriatr Soc. 2001;49(5):523-532. doi:10.1046/j.1532-5415.2001.49109.x
18. Lundström M, Edlund A, Karlsson S, et al. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53(4):622-628. doi:10.1111/j.1532-5415.2005.53210.x
19. Tabet N, Hudson S, Sweeney V, et al. An educational intervention can prevent delirium on acute medical wards. Age Ageing. 2005;34(2):152-156. doi:10.1093/ageing/afi0320. Han JH, Zimmerman EE, Cutler N, et al. Delirium in older emergency department patients: recognition, risk factors, and psychomotor subtypes. Acad Emerg Med. 2009;16(3):193-200. doi:10.1111/j.1553-2712.2008.00339.x
From the Department of Neurology, Cedars-Sinai Medical Center, Los Angeles, CA (Drs. Ching, Darwish, Li, Wong, Simpson, and Funk), the Department of Anesthesia, Cedars-Sinai Medical Center, Los Angeles, CA (Keith Siegel), and the Department of Psychiatry, Cedars-Sinai Medical Center, Los Angeles, CA (Dr. Bamgbose).
Objectives: To reduce the incidence and duration of delirium among patients in a hospital ward through standardized delirium screening tools and nonpharmacologic interventions. To advance nursing-focused education on delirium-prevention strategies. To measure the efficacy of the interventions with the aim of reproducing best practices.
Background: Delirium is associated with poor patient outcomes but may be preventable in a significant percentage of hospitalized patients.
Methods: Following nursing-focused education to prevent delirium, we prospectively evaluated patient care outcomes in a consecutive series of patients who were admitted to a hospital medical-surgical ward within a 25-week period. All patients who had at least 1 Confusion Assessment Method (CAM) documented by a nurse during hospitalization met our inclusion criteria (N = 353). Standards for Quality Improvement Reporting Excellence guidelines were adhered to.
Results: There were 187 patients in the control group, and 166 in the postintervention group. Compared to the control group, the postintervention group had a significant decrease in the incidence of delirium during hospitalization (14.4% vs 4.2%) and a significant decrease in the mean percentage of tested nursing shifts with 1 or more positive CAM (4.9% vs 1.1%). Significant differences in secondary outcomes between the control and postintervention groups included median length of stay (6 days vs 4 days), mean length of stay (8.5 days vs 5.9 days), and use of an indwelling urinary catheter (9.1% vs 2.4%).
Conclusion: A multimodal strategy involving nursing-focused training and nonpharmacologic interventions to address hospital delirium is associated with improved patient care outcomes and nursing confidence. Nurses play an integral role in the early recognition and prevention of hospital delirium, which directly translates to reducing burdens in both patient functionality and health care costs.
Delirium is a disorder characterized by inattention and acute changes in cognition. It is defined by the American Psychiatric Association’s fifth edition of the Diagnostic and Statistical Manual of Mental Disorders as a disturbance in attention, awareness, and cognition over hours to a few days that is not better explained by a preexisting, established, or other evolving neurocognitive disorder.1 Delirium is common yet often under-recognized among hospitalized patients, particularly in the elderly. The incidence of delirium in elderly patients on admission is estimated to be 11% to 25%, and an additional 29% to 31% of elderly patients will develop delirium during the hospitalization.2 Delirium costs the health care system an estimated $38 billion to $152 billion per year.3 It is associated with negative outcomes, such as increased new placements to nursing homes, increased mortality, increased risk of dementia, and further cognitive deterioration among patients with dementia.4-6
Despite its prevalence, delirium may be preventable in a significant percentage of hospitalized patients. Targeted intervention strategies, such as frequent reorientation, maximizing sleep, early mobilization, restricting use of psychoactive medications, and addressing hearing or vision impairment, have been demonstrated to significantly reduce the incidence of hospital delirium.7,8 To achieve these goals, we explored the use of a multimodal strategy centered on nursing education. We integrated consistent, standardized delirium screening and nonpharmacologic interventions as part of a preventative protocol to reduce the incidence of delirium in the hospital ward.
Methods
We evaluated a consecutive series of patients who were admitted to a designated hospital medical-surgical ward within a 25-week period between October 2019 and April 2020. All patients during this period who had at least 1 Confusion Assessment Method (CAM) documented by a nurse during hospitalization met our inclusion criteria. Patients who did not have a CAM documented were excluded from the analysis. Delirium was defined according to the CAM diagnostic algorithm.9
Core nursing staff regularly assigned to the ward completed a multimodal training program designed to improve recognition, documentation, and prevention of hospital delirium. Prior to the training, the nurses completed a 5-point Likert scale survey assessing their level of confidence with recognizing delirium risk factors, preventing delirium, addressing delirium, utilizing the CAM tool, and educating others about delirium. Nurses completed the same survey after the study period ended.
The training curriculum for nurses began with an online module reviewing the epidemiology and risk factors for delirium. Nurses then participated in a series of in-service training sessions led by a team of physicians, during which the CAM and nonpharmacologic delirium prevention measures were reviewed then practiced first-hand. Nursing staff attended an in-person lecture reviewing the current body of literature on delirium risk factors and effective nursing interventions. After formal training was completed, nurses were instructed to document CAM screens

Patients admitted to the hospital unit from the start of the training program (week 1) until the order set was made available (week 15) constituted our control group. The postintervention study group consisted of patients admitted for 10 weeks after the completion of the interventions (weeks 16-25). A timeline of the study events is shown in Figure 1.

Patient demographics and hospital-stay metrics determined a priori were attained via the Cedars-Sinai Enterprise Information Services core. Age, sex, medical history, and incidence of surgery with anesthesia during hospitalization were recorded. The Charlson Comorbidity Index was calculated from patients’ listed diagnoses following discharge. Primary outcomes included incidence of patients with delirium during hospitalization, percentage of tested shifts with positive CAM screens, length of hospital stay, and survival. Secondary outcomes included measures associated with delirium, including the use of chemical restraints, physical restraints, sitters, indwelling urinary catheters, and new psychiatry and neurology consults. Chemical restraints were defined as administration of a new antipsychotic medication or benzodiazepine for the specific indication of hyperactive delirium or agitation.
Statistical analysis was conducted by a statistician, using R version 3.6.3.10P values of < .05 were considered significant. Categorical variables were analyzed using Fisher’s exact test. Continuous variables were analyzed with Welch’s t-test or, for highly skewed continuous variables, with Wilcoxon rank-sum test or Mood’s median test. All patient data were anonymized and stored securely in accordance with institutional guidelines.
Our project was deemed to represent nonhuman subject research and therefore did not require Institutional Review Board (IRB) approval upon review by our institution’s IRB committee and Office of Research Compliance and Quality Improvement. Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines were adhered to (Supplementary File can be found at mdedge.com/jcomjournal).
Results
We evaluated 353 patients who met our inclusion criteria: 187 in the control group, and 166 in the postintervention group. Ten patients were readmitted to the ward after their initial discharge; only the initial admission encounters were included in our analysis. Median age, sex, median Charlson Comorbidity Index, and incidence of surgery with anesthesia during hospitalization were comparable between the control and postintervention groups and are summarized in Table 2.

In the control group, 1572 CAMs were performed, with 74 positive CAMs recorded among 27 patients with delirium. In the postintervention group, 1298 CAMs were performed, with 12 positive CAMs recorded among 7 patients with delirium (Figure 2). Primary and secondary outcomes, as well as CAM compliance measures, are summarized in Table 3.

Compared to the control group, the postintervention group had a significant decrease in the incidence of delirium during hospitalization (14.4% vs 4.2%, P = .002) and a significant decrease in the mean percentage of tested nursing shifts with 1 or more positive CAM (4.9% vs 1.1%, P = .002). Significant differences in secondary outcomes between the control and postintervention groups included median length of stay (6 days vs 4 days, P = .004), mean length of stay (8.5 days vs 5.9 days, P = .003), and use of an indwelling urinary catheter (9.1% vs 2.4%, P = .012). There was a trend towards decreased incidence of chemical restraints and psychiatry consults, which did not reach statistical significance. Differences in mortality during hospitalization, physical restraint use, and sitter use could not be assessed due to low incidence.

Compliance with nursing CAM assessments was evaluated. Compared to the control group, the postintervention group saw a significant increase in the percentage of shifts with a CAM performed (54.7% vs 69.1%, P < .001). The median and mean number of CAMs performed per patient were similar between the control and postintervention groups.
Results of nursing surveys completed before and after the training program are listed in Table 4. After training, nurses had a greater level of confidence with recognizing delirium risk factors, preventing delirium, addressing delirium, utilizing the CAM tool, and educating others about delirium.

Discussion
Our study utilized a standardized delirium assessment tool to compare patient cohorts before and after nurse-targeted training interventions on delirium recognition and prevention. Our interventions emphasized nonpharmacologic intervention strategies, which are recommended as first-line in the management of patients with delirium.11 Patients were not excluded from the analysis based on preexisting medical conditions or recent surgery with anesthesia, to allow for conditions that are representative of community hospitals. We also did not use an inclusion criterion based on age; however, the majority of our patients were greater than 70 years old, representing those at highest risk for delirium.2 Significant outcomes among patients in the postintervention group include decreased incidence of delirium, lower average length of stay, decreased indwelling urinary catheter use, and increased compliance with delirium screening by nursing staff.
While the study’s focus was primarily on delirium prevention rather than treatment, these strategies may also have conferred the benefit of reversing delirium symptoms. In addition to measuring incidence of delirium, our primary outcome of percentage of tested shifts with 1 or more positive CAM was intended to assess the overall duration in which patients had delirium during their hospitalization. The reduction in shifts with positive CAMs observed in the postintervention group is notable, given that a significant percentage of patients with hospital delirium have the potential for symptom reversibility.12
Multiple studies have shown that admitted patients who develop delirium experience prolonged hospital stays, often up to 5 to 10 days longer.12-14 The decreased incidence and duration of delirium in our postintervention group is a reasonable explanation for the observed decrease in average length of stay. Our study is in line with previously documented initiatives that show that nonpharmacologic interventions can effectively address downstream health and fiscal sequelae of hospital delirium. For example, a volunteer-based initiative named the Hospital Elder Life Program, from which elements in our order set were modeled after, demonstrated significant reductions in delirium incidence, length of stay, and health care costs.14-16 Other initiatives that focused on educational training for nurses to assess and prevent delirium have also demonstrated similar positive results.17-19 Our study provides a model for effective nursing-focused education that can be reproduced in the hospital setting.
Unlike some other studies, which identified delirium based only on physician assessments, our initiative utilized the CAM performed by floor nurses to identify delirium. While this method
Our study demonstrated an increase in the overall compliance with the CAM screening during the postintervention period, which is significant given the under-recognition of delirium by health care professionals.20 We attribute this increase to greater realized importance and a higher level of confidence from nursing staff in recognizing and addressing delirium, as supported by survey data. While the increased screening of patients should be considered a positive outcome, it also poses the possibility that the observed decrease in delirium incidence in the postintervention group was in fact due to more CAMs performed on patients without delirium. Likewise, nurses may have become more adept at recognizing true delirium, as opposed to delirium mimics, in the latter period of the study.
Perhaps the greatest limitation of our study is the variability in performing and recording CAMs, as some patients had multiple CAMs recorded while others did not have any CAMs recorded. This may have been affected in part by the increase in COVID-19 cases in our hospital towards the latter half of the study, which resulted in changes in nursing assignments as well as patient comorbidities in ways that cannot be easily quantified. Given the limited size of our patient cohorts, certain outcomes, such as the use of sitters, physical restraints, and in-hospital mortality, were unable to be assessed for changes statistically. Causative relationships between our interventions and associated outcome measures are necessarily limited in a binary comparison between control and postintervention groups.
Within these limitations, our study demonstrates promising results in core dimensions of patient care. We anticipate further quality improvement initiatives involving greater numbers of nursing staff and patients to better quantify the impact of nonpharmacologic nursing-centered interventions for preventing hospital delirium.
Conclusion
A multimodal strategy involving nursing-focused training and nonpharmacologic interventions to address hospital delirium is associated with improved patient care outcomes and nursing confidence. Nurses play an integral role in the early recognition and prevention of hospital delirium, which directly translates to reducing burdens in both patient functionality and health care costs. Education and tools to equip nurses to perform standardized delirium screening and interventions should be prioritized.
Acknowledgment: The authors thanks Olena Svetlov, NP, Oscar Abarca, Jose Chavez, and Jenita Gutierrez.
Corresponding author: Jason Ching, MD, Department of Neurology, Cedars-Sinai Medical Center, 8700 Beverly Blvd, Los Angeles, CA 90048; [email protected].
Financial disclosures: None.
Funding: This research was supported by NIH National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881.
From the Department of Neurology, Cedars-Sinai Medical Center, Los Angeles, CA (Drs. Ching, Darwish, Li, Wong, Simpson, and Funk), the Department of Anesthesia, Cedars-Sinai Medical Center, Los Angeles, CA (Keith Siegel), and the Department of Psychiatry, Cedars-Sinai Medical Center, Los Angeles, CA (Dr. Bamgbose).
Objectives: To reduce the incidence and duration of delirium among patients in a hospital ward through standardized delirium screening tools and nonpharmacologic interventions. To advance nursing-focused education on delirium-prevention strategies. To measure the efficacy of the interventions with the aim of reproducing best practices.
Background: Delirium is associated with poor patient outcomes but may be preventable in a significant percentage of hospitalized patients.
Methods: Following nursing-focused education to prevent delirium, we prospectively evaluated patient care outcomes in a consecutive series of patients who were admitted to a hospital medical-surgical ward within a 25-week period. All patients who had at least 1 Confusion Assessment Method (CAM) documented by a nurse during hospitalization met our inclusion criteria (N = 353). Standards for Quality Improvement Reporting Excellence guidelines were adhered to.
Results: There were 187 patients in the control group, and 166 in the postintervention group. Compared to the control group, the postintervention group had a significant decrease in the incidence of delirium during hospitalization (14.4% vs 4.2%) and a significant decrease in the mean percentage of tested nursing shifts with 1 or more positive CAM (4.9% vs 1.1%). Significant differences in secondary outcomes between the control and postintervention groups included median length of stay (6 days vs 4 days), mean length of stay (8.5 days vs 5.9 days), and use of an indwelling urinary catheter (9.1% vs 2.4%).
Conclusion: A multimodal strategy involving nursing-focused training and nonpharmacologic interventions to address hospital delirium is associated with improved patient care outcomes and nursing confidence. Nurses play an integral role in the early recognition and prevention of hospital delirium, which directly translates to reducing burdens in both patient functionality and health care costs.
Delirium is a disorder characterized by inattention and acute changes in cognition. It is defined by the American Psychiatric Association’s fifth edition of the Diagnostic and Statistical Manual of Mental Disorders as a disturbance in attention, awareness, and cognition over hours to a few days that is not better explained by a preexisting, established, or other evolving neurocognitive disorder.1 Delirium is common yet often under-recognized among hospitalized patients, particularly in the elderly. The incidence of delirium in elderly patients on admission is estimated to be 11% to 25%, and an additional 29% to 31% of elderly patients will develop delirium during the hospitalization.2 Delirium costs the health care system an estimated $38 billion to $152 billion per year.3 It is associated with negative outcomes, such as increased new placements to nursing homes, increased mortality, increased risk of dementia, and further cognitive deterioration among patients with dementia.4-6
Despite its prevalence, delirium may be preventable in a significant percentage of hospitalized patients. Targeted intervention strategies, such as frequent reorientation, maximizing sleep, early mobilization, restricting use of psychoactive medications, and addressing hearing or vision impairment, have been demonstrated to significantly reduce the incidence of hospital delirium.7,8 To achieve these goals, we explored the use of a multimodal strategy centered on nursing education. We integrated consistent, standardized delirium screening and nonpharmacologic interventions as part of a preventative protocol to reduce the incidence of delirium in the hospital ward.
Methods
We evaluated a consecutive series of patients who were admitted to a designated hospital medical-surgical ward within a 25-week period between October 2019 and April 2020. All patients during this period who had at least 1 Confusion Assessment Method (CAM) documented by a nurse during hospitalization met our inclusion criteria. Patients who did not have a CAM documented were excluded from the analysis. Delirium was defined according to the CAM diagnostic algorithm.9
Core nursing staff regularly assigned to the ward completed a multimodal training program designed to improve recognition, documentation, and prevention of hospital delirium. Prior to the training, the nurses completed a 5-point Likert scale survey assessing their level of confidence with recognizing delirium risk factors, preventing delirium, addressing delirium, utilizing the CAM tool, and educating others about delirium. Nurses completed the same survey after the study period ended.
The training curriculum for nurses began with an online module reviewing the epidemiology and risk factors for delirium. Nurses then participated in a series of in-service training sessions led by a team of physicians, during which the CAM and nonpharmacologic delirium prevention measures were reviewed then practiced first-hand. Nursing staff attended an in-person lecture reviewing the current body of literature on delirium risk factors and effective nursing interventions. After formal training was completed, nurses were instructed to document CAM screens

Patients admitted to the hospital unit from the start of the training program (week 1) until the order set was made available (week 15) constituted our control group. The postintervention study group consisted of patients admitted for 10 weeks after the completion of the interventions (weeks 16-25). A timeline of the study events is shown in Figure 1.

Patient demographics and hospital-stay metrics determined a priori were attained via the Cedars-Sinai Enterprise Information Services core. Age, sex, medical history, and incidence of surgery with anesthesia during hospitalization were recorded. The Charlson Comorbidity Index was calculated from patients’ listed diagnoses following discharge. Primary outcomes included incidence of patients with delirium during hospitalization, percentage of tested shifts with positive CAM screens, length of hospital stay, and survival. Secondary outcomes included measures associated with delirium, including the use of chemical restraints, physical restraints, sitters, indwelling urinary catheters, and new psychiatry and neurology consults. Chemical restraints were defined as administration of a new antipsychotic medication or benzodiazepine for the specific indication of hyperactive delirium or agitation.
Statistical analysis was conducted by a statistician, using R version 3.6.3.10P values of < .05 were considered significant. Categorical variables were analyzed using Fisher’s exact test. Continuous variables were analyzed with Welch’s t-test or, for highly skewed continuous variables, with Wilcoxon rank-sum test or Mood’s median test. All patient data were anonymized and stored securely in accordance with institutional guidelines.
Our project was deemed to represent nonhuman subject research and therefore did not require Institutional Review Board (IRB) approval upon review by our institution’s IRB committee and Office of Research Compliance and Quality Improvement. Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines were adhered to (Supplementary File can be found at mdedge.com/jcomjournal).
Results
We evaluated 353 patients who met our inclusion criteria: 187 in the control group, and 166 in the postintervention group. Ten patients were readmitted to the ward after their initial discharge; only the initial admission encounters were included in our analysis. Median age, sex, median Charlson Comorbidity Index, and incidence of surgery with anesthesia during hospitalization were comparable between the control and postintervention groups and are summarized in Table 2.

In the control group, 1572 CAMs were performed, with 74 positive CAMs recorded among 27 patients with delirium. In the postintervention group, 1298 CAMs were performed, with 12 positive CAMs recorded among 7 patients with delirium (Figure 2). Primary and secondary outcomes, as well as CAM compliance measures, are summarized in Table 3.

Compared to the control group, the postintervention group had a significant decrease in the incidence of delirium during hospitalization (14.4% vs 4.2%, P = .002) and a significant decrease in the mean percentage of tested nursing shifts with 1 or more positive CAM (4.9% vs 1.1%, P = .002). Significant differences in secondary outcomes between the control and postintervention groups included median length of stay (6 days vs 4 days, P = .004), mean length of stay (8.5 days vs 5.9 days, P = .003), and use of an indwelling urinary catheter (9.1% vs 2.4%, P = .012). There was a trend towards decreased incidence of chemical restraints and psychiatry consults, which did not reach statistical significance. Differences in mortality during hospitalization, physical restraint use, and sitter use could not be assessed due to low incidence.

Compliance with nursing CAM assessments was evaluated. Compared to the control group, the postintervention group saw a significant increase in the percentage of shifts with a CAM performed (54.7% vs 69.1%, P < .001). The median and mean number of CAMs performed per patient were similar between the control and postintervention groups.
Results of nursing surveys completed before and after the training program are listed in Table 4. After training, nurses had a greater level of confidence with recognizing delirium risk factors, preventing delirium, addressing delirium, utilizing the CAM tool, and educating others about delirium.

Discussion
Our study utilized a standardized delirium assessment tool to compare patient cohorts before and after nurse-targeted training interventions on delirium recognition and prevention. Our interventions emphasized nonpharmacologic intervention strategies, which are recommended as first-line in the management of patients with delirium.11 Patients were not excluded from the analysis based on preexisting medical conditions or recent surgery with anesthesia, to allow for conditions that are representative of community hospitals. We also did not use an inclusion criterion based on age; however, the majority of our patients were greater than 70 years old, representing those at highest risk for delirium.2 Significant outcomes among patients in the postintervention group include decreased incidence of delirium, lower average length of stay, decreased indwelling urinary catheter use, and increased compliance with delirium screening by nursing staff.
While the study’s focus was primarily on delirium prevention rather than treatment, these strategies may also have conferred the benefit of reversing delirium symptoms. In addition to measuring incidence of delirium, our primary outcome of percentage of tested shifts with 1 or more positive CAM was intended to assess the overall duration in which patients had delirium during their hospitalization. The reduction in shifts with positive CAMs observed in the postintervention group is notable, given that a significant percentage of patients with hospital delirium have the potential for symptom reversibility.12
Multiple studies have shown that admitted patients who develop delirium experience prolonged hospital stays, often up to 5 to 10 days longer.12-14 The decreased incidence and duration of delirium in our postintervention group is a reasonable explanation for the observed decrease in average length of stay. Our study is in line with previously documented initiatives that show that nonpharmacologic interventions can effectively address downstream health and fiscal sequelae of hospital delirium. For example, a volunteer-based initiative named the Hospital Elder Life Program, from which elements in our order set were modeled after, demonstrated significant reductions in delirium incidence, length of stay, and health care costs.14-16 Other initiatives that focused on educational training for nurses to assess and prevent delirium have also demonstrated similar positive results.17-19 Our study provides a model for effective nursing-focused education that can be reproduced in the hospital setting.
Unlike some other studies, which identified delirium based only on physician assessments, our initiative utilized the CAM performed by floor nurses to identify delirium. While this method
Our study demonstrated an increase in the overall compliance with the CAM screening during the postintervention period, which is significant given the under-recognition of delirium by health care professionals.20 We attribute this increase to greater realized importance and a higher level of confidence from nursing staff in recognizing and addressing delirium, as supported by survey data. While the increased screening of patients should be considered a positive outcome, it also poses the possibility that the observed decrease in delirium incidence in the postintervention group was in fact due to more CAMs performed on patients without delirium. Likewise, nurses may have become more adept at recognizing true delirium, as opposed to delirium mimics, in the latter period of the study.
Perhaps the greatest limitation of our study is the variability in performing and recording CAMs, as some patients had multiple CAMs recorded while others did not have any CAMs recorded. This may have been affected in part by the increase in COVID-19 cases in our hospital towards the latter half of the study, which resulted in changes in nursing assignments as well as patient comorbidities in ways that cannot be easily quantified. Given the limited size of our patient cohorts, certain outcomes, such as the use of sitters, physical restraints, and in-hospital mortality, were unable to be assessed for changes statistically. Causative relationships between our interventions and associated outcome measures are necessarily limited in a binary comparison between control and postintervention groups.
Within these limitations, our study demonstrates promising results in core dimensions of patient care. We anticipate further quality improvement initiatives involving greater numbers of nursing staff and patients to better quantify the impact of nonpharmacologic nursing-centered interventions for preventing hospital delirium.
Conclusion
A multimodal strategy involving nursing-focused training and nonpharmacologic interventions to address hospital delirium is associated with improved patient care outcomes and nursing confidence. Nurses play an integral role in the early recognition and prevention of hospital delirium, which directly translates to reducing burdens in both patient functionality and health care costs. Education and tools to equip nurses to perform standardized delirium screening and interventions should be prioritized.
Acknowledgment: The authors thanks Olena Svetlov, NP, Oscar Abarca, Jose Chavez, and Jenita Gutierrez.
Corresponding author: Jason Ching, MD, Department of Neurology, Cedars-Sinai Medical Center, 8700 Beverly Blvd, Los Angeles, CA 90048; [email protected].
Financial disclosures: None.
Funding: This research was supported by NIH National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition. American Psychiatric Association; 2013.
2. Vasilevskis EE, Han JH, Hughes CG, et al. Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol. 2012;26(3):277-287. doi:10.1016/j.bpa.2012.07003
3. Leslie DL, Marcantonio ER, Zhang Y, et al. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27-32. doi:10.1001/archinternmed.2007.4
4. McCusker J, Cole M, Abrahamowicz M, et al. Delirium predicts 12-month mortality. Arch Intern Med. 2002;162(4):457-463. doi:10.1001/archinte.162.4.457
5. Witlox J, Eurelings LS, de Jonghe JF, et al. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443-451. doi:10.1001/jama.2010.1013
6. Gross AL, Jones RN, Habtemariam DA, et al. Delirium and long-term cognitive trajectory among persons with dementia. Arch Intern Med. 2012;172(17):1324-1331. doi:10.1001/archinternmed.2012.3203
7. Inouye SK. Prevention of delirium in hospitalized older patients: risk factors and targeted intervention strategies. Ann Med. 2000;32(4):257-263. doi:10.3109/07853890009011770
8. Siddiqi N, Harrison JK, Clegg A, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2016;3:CD005563. doi:10.1002/14651858.CD005563.pub3
9. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. doi:10.7326/0003-4819-113-12-941
10. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2017.
11. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220. doi:10.1038/nrneurol.2009.24
12. Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35(4):350-364. doi:10.1093/ageing/afl005
13. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762. doi:10.1001/jama.291.14.1753
14. Chen CC, Lin MT, Tien YW, et al. Modified Hospital Elder Life Program: effects on abdominal surgery patients. J Am Coll Surg. 2011;213(2):245-252. doi:10.1016/j.jamcollsurg.2011.05.004
15. Zaubler TS, Murphy K, Rizzuto L, et al. Quality improvement and cost savings with multicomponent delirium interventions: replication of the Hospital Elder Life Program in a community hospital. Psychosomatics. 2013;54(3):219-226. doi:10.1016/j.psym.2013.01.010
16. Rubin FH, Neal K, Fenlon K, et al. Sustainability and scalability of the Hospital Elder Life Program at a community hospital. J Am Geriatr Soc. 2011;59(2):359-365. doi:10.1111/j.1532-5415.2010.03243.x
17. Milisen K, Foreman MD, Abraham IL, et al. A nurse-led interdisciplinary intervention program for delirium in elderly hip-fracture patients. J Am Geriatr Soc. 2001;49(5):523-532. doi:10.1046/j.1532-5415.2001.49109.x
18. Lundström M, Edlund A, Karlsson S, et al. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53(4):622-628. doi:10.1111/j.1532-5415.2005.53210.x
19. Tabet N, Hudson S, Sweeney V, et al. An educational intervention can prevent delirium on acute medical wards. Age Ageing. 2005;34(2):152-156. doi:10.1093/ageing/afi0320. Han JH, Zimmerman EE, Cutler N, et al. Delirium in older emergency department patients: recognition, risk factors, and psychomotor subtypes. Acad Emerg Med. 2009;16(3):193-200. doi:10.1111/j.1553-2712.2008.00339.x
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition. American Psychiatric Association; 2013.
2. Vasilevskis EE, Han JH, Hughes CG, et al. Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol. 2012;26(3):277-287. doi:10.1016/j.bpa.2012.07003
3. Leslie DL, Marcantonio ER, Zhang Y, et al. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27-32. doi:10.1001/archinternmed.2007.4
4. McCusker J, Cole M, Abrahamowicz M, et al. Delirium predicts 12-month mortality. Arch Intern Med. 2002;162(4):457-463. doi:10.1001/archinte.162.4.457
5. Witlox J, Eurelings LS, de Jonghe JF, et al. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443-451. doi:10.1001/jama.2010.1013
6. Gross AL, Jones RN, Habtemariam DA, et al. Delirium and long-term cognitive trajectory among persons with dementia. Arch Intern Med. 2012;172(17):1324-1331. doi:10.1001/archinternmed.2012.3203
7. Inouye SK. Prevention of delirium in hospitalized older patients: risk factors and targeted intervention strategies. Ann Med. 2000;32(4):257-263. doi:10.3109/07853890009011770
8. Siddiqi N, Harrison JK, Clegg A, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2016;3:CD005563. doi:10.1002/14651858.CD005563.pub3
9. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. doi:10.7326/0003-4819-113-12-941
10. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2017.
11. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220. doi:10.1038/nrneurol.2009.24
12. Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35(4):350-364. doi:10.1093/ageing/afl005
13. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762. doi:10.1001/jama.291.14.1753
14. Chen CC, Lin MT, Tien YW, et al. Modified Hospital Elder Life Program: effects on abdominal surgery patients. J Am Coll Surg. 2011;213(2):245-252. doi:10.1016/j.jamcollsurg.2011.05.004
15. Zaubler TS, Murphy K, Rizzuto L, et al. Quality improvement and cost savings with multicomponent delirium interventions: replication of the Hospital Elder Life Program in a community hospital. Psychosomatics. 2013;54(3):219-226. doi:10.1016/j.psym.2013.01.010
16. Rubin FH, Neal K, Fenlon K, et al. Sustainability and scalability of the Hospital Elder Life Program at a community hospital. J Am Geriatr Soc. 2011;59(2):359-365. doi:10.1111/j.1532-5415.2010.03243.x
17. Milisen K, Foreman MD, Abraham IL, et al. A nurse-led interdisciplinary intervention program for delirium in elderly hip-fracture patients. J Am Geriatr Soc. 2001;49(5):523-532. doi:10.1046/j.1532-5415.2001.49109.x
18. Lundström M, Edlund A, Karlsson S, et al. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53(4):622-628. doi:10.1111/j.1532-5415.2005.53210.x
19. Tabet N, Hudson S, Sweeney V, et al. An educational intervention can prevent delirium on acute medical wards. Age Ageing. 2005;34(2):152-156. doi:10.1093/ageing/afi0320. Han JH, Zimmerman EE, Cutler N, et al. Delirium in older emergency department patients: recognition, risk factors, and psychomotor subtypes. Acad Emerg Med. 2009;16(3):193-200. doi:10.1111/j.1553-2712.2008.00339.x
Predicting cardiac shock mortality in the ICU
Addition of echocardiogram measurement of biventricular dysfunction improved the accuracy of prognosis among patients with cardiac shock (CS) in the cardiac intensive care unit.
In patients in the cardiac ICU with CS, biventricular dysfunction (BVD), as assessed using transthoracic echocardiography, improves clinical risk stratification when combined with the Society for Cardiovascular Angiography and Interventions shock stage.
No improvements in risk stratification was seen with patients with left or right ventricular systolic dysfunction (LVSD or RVSD) alone, according to an article published in the journal Chest.
Ventricular systolic dysfunction is commonly seen in patients who have suffered cardiac shock, most often on the left side. Although echocardiography is often performed on these patients during diagnosis, previous studies looking at ventricular dysfunction used invasive hemodynamic parameters, which made it challenging to incorporate their findings into general cardiac ICU practice.
Pinning down cardiac shock
Although treatment of acute MI and heart failure has improved greatly, particularly with the implementation of percutaneous coronary intervention (primary PCI) for ST-segment elevation MI. This has reduced the rate of future heart failure, but cardiac shock can occur before or after the procedure, with a 30-day mortality of 30%-40%. This outcome hasn’t improved in the last 20 years.
Efforts to improve cardiac shock outcomes through percutaneous mechanical circulatory support devices have been hindered by the fact that CS patients are heterogeneous, and prognosis may depend on a range of factors.
SCAI was developed as a five-stage classification system for CS to improve communication of patient status, as well as to improve differentiation among patients participation in clinical trials. It does not include measures of ventricular dysfunction.
Simple measure boosts prognosis accuracy
The new work adds an additional layer to the SCAI shock stage. “Adding echocardiography allows discrimination between levels of risk for each SCAI stage,” said David Baran, MD, who was asked for comment. Dr. Baran was the lead author on the original SCAI study and is system director of advanced heart failure at Sentara Heart Hospital, as well as a professor of medicine at Eastern Virginia Medical School, both in Norfolk.
The work also underscores the value of repeated measures of prognosis during a patient’s stay in the ICU. “If a patient is not improving, it may prompt a consideration of whether transfer or consultation with a tertiary center may be of value. Conversely, if a patient doesn’t have high-risk features and is responding to therapy, it is reassuring to have data supporting low mortality with that care plan,” said Dr. Baran.
The study may be biased, since not every patient undergoes an echocardiogram. Still, “the authors make a convincing case that biventricular dysfunction is a powerful negative marker across the spectrum of SCAI stages,” said Dr. Baran.
Echocardiography is simple and generally available, and some are even portable and used with a smartphone. But patient body size interferes with echocardiography, as can the presence of a ventilator or multiple surgical dressings. “The key advantage of echo is that it is completely noninvasive and can be brought to the patient in the ICU, unlike other testing which involves moving the patient to the testing environment,” said Dr. Baran.
The researchers analyzed data from 3,158 patients admitted to the cardiac ICU at the Mayo Clinic Hospital St. Mary’s Campus in Rochester, Minn., 51.8% of whom had acute coronary syndromes. They defined LVSD as a left ventricular ejection fraction less than 40%, and RVSD as at least moderate systolic dysfunction determined by semiquantitative measurement. BVD constituted the presence of both LVSD and RVSD. They examined the association of in-hospital mortality with these parameters combined with SCAI stage.
BVD a risk factor
Overall in-hospital mortality was 10%. A total of 22.3% of patients had LVSD and 11.8% had RVSD; 16.4% had moderate or greater BVD. There was no association between LVSD or RVSD and in-hospital mortality after adjustment for SCAI stage, but there was a significant association for BVD (adjusted hazard ratio, 1.815; P = .0023). When combined with SCAI, BVC led to an improved ability to predict hospital mortality (area under the curve, 0.784 vs. 0.766; P < .001). Adding semiquantitative RVSD and LVSD led to more improvement (AUC, 0.794; P < .01 vs. both).
RVSD was associated with higher in-hospital mortality (adjusted odds ratio, 1.421; P = .02), and there was a trend toward greater mortality with LVSD (aOR, 1.336; P = .06). There was little change when SCAI shock stage A patients were excluded (aOR, 1.840; P < .001).
Patients with BVD had greater in-hospital mortality than those without ventricular dysfunction (aOR, 1.815; P = .0023), but other between-group comparisons were not significant.
The researchers performed a classification and regression tree analysis using left ventricular ejection fraction (LVEF) and semiquantitative RVSD. It found that RVSD was a better predictor of in-hospital mortality than LVSD, and the best cutoff for LVSD was different among patients with RVSD and patients without RVSD.
Patients with mild or greater RVD and LVEF greater than 24% were considered high risk; those with borderline or low RVSD and LVEF less than 33%, or mild or greater RVSD with LVEF of at least 24%, were considered intermediate risk. Patients with borderline or no RVSD and LVEF of at least 33% were considered low risk. Hospital mortality was 22% in the high-risk group, 12.2% in the intermediate group, and 3.3% in the low-risk group (aOR vs. intermediate, 0.493; P = .0006; aOR vs. high risk, 0.357; P < .0001).
The study authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Addition of echocardiogram measurement of biventricular dysfunction improved the accuracy of prognosis among patients with cardiac shock (CS) in the cardiac intensive care unit.
In patients in the cardiac ICU with CS, biventricular dysfunction (BVD), as assessed using transthoracic echocardiography, improves clinical risk stratification when combined with the Society for Cardiovascular Angiography and Interventions shock stage.
No improvements in risk stratification was seen with patients with left or right ventricular systolic dysfunction (LVSD or RVSD) alone, according to an article published in the journal Chest.
Ventricular systolic dysfunction is commonly seen in patients who have suffered cardiac shock, most often on the left side. Although echocardiography is often performed on these patients during diagnosis, previous studies looking at ventricular dysfunction used invasive hemodynamic parameters, which made it challenging to incorporate their findings into general cardiac ICU practice.
Pinning down cardiac shock
Although treatment of acute MI and heart failure has improved greatly, particularly with the implementation of percutaneous coronary intervention (primary PCI) for ST-segment elevation MI. This has reduced the rate of future heart failure, but cardiac shock can occur before or after the procedure, with a 30-day mortality of 30%-40%. This outcome hasn’t improved in the last 20 years.
Efforts to improve cardiac shock outcomes through percutaneous mechanical circulatory support devices have been hindered by the fact that CS patients are heterogeneous, and prognosis may depend on a range of factors.
SCAI was developed as a five-stage classification system for CS to improve communication of patient status, as well as to improve differentiation among patients participation in clinical trials. It does not include measures of ventricular dysfunction.
Simple measure boosts prognosis accuracy
The new work adds an additional layer to the SCAI shock stage. “Adding echocardiography allows discrimination between levels of risk for each SCAI stage,” said David Baran, MD, who was asked for comment. Dr. Baran was the lead author on the original SCAI study and is system director of advanced heart failure at Sentara Heart Hospital, as well as a professor of medicine at Eastern Virginia Medical School, both in Norfolk.
The work also underscores the value of repeated measures of prognosis during a patient’s stay in the ICU. “If a patient is not improving, it may prompt a consideration of whether transfer or consultation with a tertiary center may be of value. Conversely, if a patient doesn’t have high-risk features and is responding to therapy, it is reassuring to have data supporting low mortality with that care plan,” said Dr. Baran.
The study may be biased, since not every patient undergoes an echocardiogram. Still, “the authors make a convincing case that biventricular dysfunction is a powerful negative marker across the spectrum of SCAI stages,” said Dr. Baran.
Echocardiography is simple and generally available, and some are even portable and used with a smartphone. But patient body size interferes with echocardiography, as can the presence of a ventilator or multiple surgical dressings. “The key advantage of echo is that it is completely noninvasive and can be brought to the patient in the ICU, unlike other testing which involves moving the patient to the testing environment,” said Dr. Baran.
The researchers analyzed data from 3,158 patients admitted to the cardiac ICU at the Mayo Clinic Hospital St. Mary’s Campus in Rochester, Minn., 51.8% of whom had acute coronary syndromes. They defined LVSD as a left ventricular ejection fraction less than 40%, and RVSD as at least moderate systolic dysfunction determined by semiquantitative measurement. BVD constituted the presence of both LVSD and RVSD. They examined the association of in-hospital mortality with these parameters combined with SCAI stage.
BVD a risk factor
Overall in-hospital mortality was 10%. A total of 22.3% of patients had LVSD and 11.8% had RVSD; 16.4% had moderate or greater BVD. There was no association between LVSD or RVSD and in-hospital mortality after adjustment for SCAI stage, but there was a significant association for BVD (adjusted hazard ratio, 1.815; P = .0023). When combined with SCAI, BVC led to an improved ability to predict hospital mortality (area under the curve, 0.784 vs. 0.766; P < .001). Adding semiquantitative RVSD and LVSD led to more improvement (AUC, 0.794; P < .01 vs. both).
RVSD was associated with higher in-hospital mortality (adjusted odds ratio, 1.421; P = .02), and there was a trend toward greater mortality with LVSD (aOR, 1.336; P = .06). There was little change when SCAI shock stage A patients were excluded (aOR, 1.840; P < .001).
Patients with BVD had greater in-hospital mortality than those without ventricular dysfunction (aOR, 1.815; P = .0023), but other between-group comparisons were not significant.
The researchers performed a classification and regression tree analysis using left ventricular ejection fraction (LVEF) and semiquantitative RVSD. It found that RVSD was a better predictor of in-hospital mortality than LVSD, and the best cutoff for LVSD was different among patients with RVSD and patients without RVSD.
Patients with mild or greater RVD and LVEF greater than 24% were considered high risk; those with borderline or low RVSD and LVEF less than 33%, or mild or greater RVSD with LVEF of at least 24%, were considered intermediate risk. Patients with borderline or no RVSD and LVEF of at least 33% were considered low risk. Hospital mortality was 22% in the high-risk group, 12.2% in the intermediate group, and 3.3% in the low-risk group (aOR vs. intermediate, 0.493; P = .0006; aOR vs. high risk, 0.357; P < .0001).
The study authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Addition of echocardiogram measurement of biventricular dysfunction improved the accuracy of prognosis among patients with cardiac shock (CS) in the cardiac intensive care unit.
In patients in the cardiac ICU with CS, biventricular dysfunction (BVD), as assessed using transthoracic echocardiography, improves clinical risk stratification when combined with the Society for Cardiovascular Angiography and Interventions shock stage.
No improvements in risk stratification was seen with patients with left or right ventricular systolic dysfunction (LVSD or RVSD) alone, according to an article published in the journal Chest.
Ventricular systolic dysfunction is commonly seen in patients who have suffered cardiac shock, most often on the left side. Although echocardiography is often performed on these patients during diagnosis, previous studies looking at ventricular dysfunction used invasive hemodynamic parameters, which made it challenging to incorporate their findings into general cardiac ICU practice.
Pinning down cardiac shock
Although treatment of acute MI and heart failure has improved greatly, particularly with the implementation of percutaneous coronary intervention (primary PCI) for ST-segment elevation MI. This has reduced the rate of future heart failure, but cardiac shock can occur before or after the procedure, with a 30-day mortality of 30%-40%. This outcome hasn’t improved in the last 20 years.
Efforts to improve cardiac shock outcomes through percutaneous mechanical circulatory support devices have been hindered by the fact that CS patients are heterogeneous, and prognosis may depend on a range of factors.
SCAI was developed as a five-stage classification system for CS to improve communication of patient status, as well as to improve differentiation among patients participation in clinical trials. It does not include measures of ventricular dysfunction.
Simple measure boosts prognosis accuracy
The new work adds an additional layer to the SCAI shock stage. “Adding echocardiography allows discrimination between levels of risk for each SCAI stage,” said David Baran, MD, who was asked for comment. Dr. Baran was the lead author on the original SCAI study and is system director of advanced heart failure at Sentara Heart Hospital, as well as a professor of medicine at Eastern Virginia Medical School, both in Norfolk.
The work also underscores the value of repeated measures of prognosis during a patient’s stay in the ICU. “If a patient is not improving, it may prompt a consideration of whether transfer or consultation with a tertiary center may be of value. Conversely, if a patient doesn’t have high-risk features and is responding to therapy, it is reassuring to have data supporting low mortality with that care plan,” said Dr. Baran.
The study may be biased, since not every patient undergoes an echocardiogram. Still, “the authors make a convincing case that biventricular dysfunction is a powerful negative marker across the spectrum of SCAI stages,” said Dr. Baran.
Echocardiography is simple and generally available, and some are even portable and used with a smartphone. But patient body size interferes with echocardiography, as can the presence of a ventilator or multiple surgical dressings. “The key advantage of echo is that it is completely noninvasive and can be brought to the patient in the ICU, unlike other testing which involves moving the patient to the testing environment,” said Dr. Baran.
The researchers analyzed data from 3,158 patients admitted to the cardiac ICU at the Mayo Clinic Hospital St. Mary’s Campus in Rochester, Minn., 51.8% of whom had acute coronary syndromes. They defined LVSD as a left ventricular ejection fraction less than 40%, and RVSD as at least moderate systolic dysfunction determined by semiquantitative measurement. BVD constituted the presence of both LVSD and RVSD. They examined the association of in-hospital mortality with these parameters combined with SCAI stage.
BVD a risk factor
Overall in-hospital mortality was 10%. A total of 22.3% of patients had LVSD and 11.8% had RVSD; 16.4% had moderate or greater BVD. There was no association between LVSD or RVSD and in-hospital mortality after adjustment for SCAI stage, but there was a significant association for BVD (adjusted hazard ratio, 1.815; P = .0023). When combined with SCAI, BVC led to an improved ability to predict hospital mortality (area under the curve, 0.784 vs. 0.766; P < .001). Adding semiquantitative RVSD and LVSD led to more improvement (AUC, 0.794; P < .01 vs. both).
RVSD was associated with higher in-hospital mortality (adjusted odds ratio, 1.421; P = .02), and there was a trend toward greater mortality with LVSD (aOR, 1.336; P = .06). There was little change when SCAI shock stage A patients were excluded (aOR, 1.840; P < .001).
Patients with BVD had greater in-hospital mortality than those without ventricular dysfunction (aOR, 1.815; P = .0023), but other between-group comparisons were not significant.
The researchers performed a classification and regression tree analysis using left ventricular ejection fraction (LVEF) and semiquantitative RVSD. It found that RVSD was a better predictor of in-hospital mortality than LVSD, and the best cutoff for LVSD was different among patients with RVSD and patients without RVSD.
Patients with mild or greater RVD and LVEF greater than 24% were considered high risk; those with borderline or low RVSD and LVEF less than 33%, or mild or greater RVSD with LVEF of at least 24%, were considered intermediate risk. Patients with borderline or no RVSD and LVEF of at least 33% were considered low risk. Hospital mortality was 22% in the high-risk group, 12.2% in the intermediate group, and 3.3% in the low-risk group (aOR vs. intermediate, 0.493; P = .0006; aOR vs. high risk, 0.357; P < .0001).
The study authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adjuvant Olaparib Improves Outcomes in High-Risk, HER2-Negative Early Breast Cancer Patients With Germline BRCA1 and BRCA2 Mutations
Study Overview
Objective. To assess the efficacy and safety of olaparib as an adjuvant treatment in patients with BRCA1 or BRCA2 germline mutations who are at a high-risk for relapse.
Design. A randomized, double-blind, placebo-controlled, multicenter phase III study. The published results are from the prespecified interim analysis.
Intervention. Patients were randomized in 1:1 ratio to either receive 300 mg of olaparib orally twice daily or to receive a matching placebo. Randomization was stratified by hormone receptor status (estrogen receptor and/or progesterone receptor positive/HER2-negative vs triple negative), prior neoadjuvant vs adjuvant chemotherapy, and prior platinum use for breast cancer. Treatment was continued for 52 weeks.
Setting and participants. A total of 1836 patients were randomized in a 1:1 fashion to receive olaparib or a placebo. Eligible patients had a germline BRCA1 or BRCA1 pathogenic or likely pathogenic variant. Patients had high-risk, HER2-negative primary breast cancers and all had received definitive local therapy and neoadjuvant or adjuvant chemotherapy. Patients were enrolled between 2 to 12 weeks after completion of all local therapy. Platinum chemotherapy was allowed. Patients received adjuvant endocrine therapy for hormone receptor positive disease as well as adjuvant bisphosphonates per institutional guidelines. Patients with triple negative disease who received adjuvant chemotherapy were required to be lymph node positive or have at least 2 cm invasive disease. Patients who received neoadjuvant chemotherapy were required to have residual invasive disease to be eligible. For hormone receptor positive patients receiving adjuvant chemotherapy to be eligible they had to have at least 4 pathologically confirmed lymph nodes involved. Hormone receptor positive patients who had neoadjuvant chemotherapy were required to have had residual invasive disease.
Main outcome measures. The primary endpoint for the study was invasive disease-free survival which was defined as time from randomization to date of recurrence or death from any cause. The secondary endpoints included overall survival (OS), distant disease-free survival, safety, and tolerability of olaparib.
Main results. At the time of data cutoff, 284 events had occurred with a median follow-up of 2.5 years in the intention to treat population. A total of 81% of patients had triple negative breast cancer. Most patients (94% in the olaparib group and 92% in the placebo group) received both taxane and anthracycline based chemotherapy regimens. Platinum based chemotherapy was used in 26% of patients in each group. The groups were otherwise well balanced. Germline mutations in BRCA1 were present in 72% of patients and BRCA2 in 27% of patients. These were balanced between groups.
At the time of this analysis, adjuvant olaparib reduced the risk of invasive disease-free survival by 42% compared with placebo (P < .001). At 3 years, invasive disease-free survival was 85.9% in the olaparib group and 77.1% in the placebo group (difference, 8.8 percentage points; 95% CI, 4.5-13.0; hazard ratio [HR], 0.58; 99.5% CI, 0.41-0.82; P < .001). The 3-year distant disease-free survival was 87.5% in the olaparib group and 80.4% in the placebo group (HR 0.57; 99.5% CI, 0.39-0.83; P < .001). Results also showed that olaparib was associated with fewer deaths than placebo (59 and 86, respectively) (HR, 0.68; 99% CI, 0.44-1.05; P = .02); however, there was no significant difference between treatment arms at the time of this interim analysis. Subgroup analysis showed a consistent benefit across all groups with no difference noted regarding BRCA mutation, hormone receptor status or use of neoadjuvant vs adjuvant chemotherapy.
The side effects were consistent with the safety profile of olaparib. Adverse events of grade 3 or higher more common with olaparib included anemia (8.7%), leukopenia (3%), and fatigue (1.8%). Early discontinuation of trial regimen due to adverse events of disease recurrence occurred in 25.9% in the olaparib group and 20.7% in the placebo group. Blood transfusions were required in 5.8% of patients in the olaparib group. Myelodysplasia or acute myleoid leukemia was observed in 2 patients in the olaparib group and 3 patients in the placebo group. Adverse events leading to death occurred in 1 patient in the olaparib group and 2 patients in the placebo group.
Conclusion. Among patients with high-risk, HER2-negative early breast cancer and germline BRCA1 or BRCA2 pathogenic or likely pathogenic variants, adjuvant olaparib after completion of local treatment and neoadjuvant or adjuvant chemotherapy was associated with significantly longer invasive disease-free and distant disease-free survival compared with placebo.
Commentary
The results from the current OlympiA trial provide the first evidence that adjuvant therapy with poly adenosine diphosphate-ribose polymerase (PARP) inhibitors can improve outcomes in high-risk, HER2-negative breast cancer in patients with pathogenic BRCA1 and BRCA2 mutations. The OS, while favoring olaparib, is not yet mature at the time of this analysis. Nevertheless, these results represent an important step forward in improving outcomes in this patient population. The efficacy and safety of PARP inhibitors in BRCA-mutated breast cancer has previously been shown in patients with advanced disease leading to FDA approval of both olaparib and talazoparib in this setting.1,2 With the current results, PARP inhibitors will certainly play an important role in the adjuvant setting in patients with deleterious BRCA1 or BRCA2 mutations at high risk for relapse. Importantly, the side effect profile appears acceptable with no unexpected events and a very low rate of secondary myeloid malignancies.
Subgroup analysis appears to indicate a benefit across all groups including hormone receptor–positive disease and triple negative breast cancer. Interestingly, approximately 25% of patients in both cohorts received platinum-based chemotherapy. The efficacy of adjuvant olaparib did not appear to be impacted by prior use of platinum-containing chemotherapy regimens. It is important to consider that postneoadjuvant capecitabine, per the results of the CREATE-X trial, in triple-negative patients was not permitted in the current study. Although, this has been widely adopted in clinical practice.3 The CREATE-X trial did not specify the benefit of adjuvant capecitabine in the BRCA-mutated cohort, thus, it is not clear how this subgroup fares with this approach. Thus, one cannot extrapolate the relative efficacy of olaparib compared with capecitabine, as pointed out by the authors, and whether we consider the use of capecitabine and/or olaparib in triple-negative patients with residual invasive disease after neoadjuvant chemotherapy is not clear at this time.
Nevertheless, the magnitude of benefit seen in this trial certainly provide clinically relevant and potentially practice changing results. It will be imperative to follow these results as the survival data matures and ensure no further long-term toxicity, particularly secondary myeloid malignancies, develop. These results should be discussed with each patient and informed decisions regarding the use of adjuvant olaparib should be considered for this patient population. Lastly, these results highlight the importance of germline testing for patients with breast cancer in accordance with national guideline recommendations. Moreover, these results certainly call into question whether it is time to consider expansion of our current germline testing guidelines to detect all potential patients who may benefit from this therapy.
Application for Clinical Practice
Adjuvant olaparib in high-risk patients with germline BRCA1 or BRCA2 mutations improves invasive and distant disease-free survival and should be considered in patients who meet the enrollment criteria of the current study. Furthermore, this highlights the importance of appropriate germline genetic testing in patients with breast cancer.
Financial disclosures: None.
1. Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523-533. doi:10.1056/NEJMoa1706450
2. Litton JK, Rugo HS, Ettl J, et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med. 2018;379(8):753-763. doi:10.1056/NEJMoa1802905
3. Masuda N, Lee SJ, Ohtani S, et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med. 2017;376(22):2147-2159. doi:10.1056/NEJMoa1612645
Study Overview
Objective. To assess the efficacy and safety of olaparib as an adjuvant treatment in patients with BRCA1 or BRCA2 germline mutations who are at a high-risk for relapse.
Design. A randomized, double-blind, placebo-controlled, multicenter phase III study. The published results are from the prespecified interim analysis.
Intervention. Patients were randomized in 1:1 ratio to either receive 300 mg of olaparib orally twice daily or to receive a matching placebo. Randomization was stratified by hormone receptor status (estrogen receptor and/or progesterone receptor positive/HER2-negative vs triple negative), prior neoadjuvant vs adjuvant chemotherapy, and prior platinum use for breast cancer. Treatment was continued for 52 weeks.
Setting and participants. A total of 1836 patients were randomized in a 1:1 fashion to receive olaparib or a placebo. Eligible patients had a germline BRCA1 or BRCA1 pathogenic or likely pathogenic variant. Patients had high-risk, HER2-negative primary breast cancers and all had received definitive local therapy and neoadjuvant or adjuvant chemotherapy. Patients were enrolled between 2 to 12 weeks after completion of all local therapy. Platinum chemotherapy was allowed. Patients received adjuvant endocrine therapy for hormone receptor positive disease as well as adjuvant bisphosphonates per institutional guidelines. Patients with triple negative disease who received adjuvant chemotherapy were required to be lymph node positive or have at least 2 cm invasive disease. Patients who received neoadjuvant chemotherapy were required to have residual invasive disease to be eligible. For hormone receptor positive patients receiving adjuvant chemotherapy to be eligible they had to have at least 4 pathologically confirmed lymph nodes involved. Hormone receptor positive patients who had neoadjuvant chemotherapy were required to have had residual invasive disease.
Main outcome measures. The primary endpoint for the study was invasive disease-free survival which was defined as time from randomization to date of recurrence or death from any cause. The secondary endpoints included overall survival (OS), distant disease-free survival, safety, and tolerability of olaparib.
Main results. At the time of data cutoff, 284 events had occurred with a median follow-up of 2.5 years in the intention to treat population. A total of 81% of patients had triple negative breast cancer. Most patients (94% in the olaparib group and 92% in the placebo group) received both taxane and anthracycline based chemotherapy regimens. Platinum based chemotherapy was used in 26% of patients in each group. The groups were otherwise well balanced. Germline mutations in BRCA1 were present in 72% of patients and BRCA2 in 27% of patients. These were balanced between groups.
At the time of this analysis, adjuvant olaparib reduced the risk of invasive disease-free survival by 42% compared with placebo (P < .001). At 3 years, invasive disease-free survival was 85.9% in the olaparib group and 77.1% in the placebo group (difference, 8.8 percentage points; 95% CI, 4.5-13.0; hazard ratio [HR], 0.58; 99.5% CI, 0.41-0.82; P < .001). The 3-year distant disease-free survival was 87.5% in the olaparib group and 80.4% in the placebo group (HR 0.57; 99.5% CI, 0.39-0.83; P < .001). Results also showed that olaparib was associated with fewer deaths than placebo (59 and 86, respectively) (HR, 0.68; 99% CI, 0.44-1.05; P = .02); however, there was no significant difference between treatment arms at the time of this interim analysis. Subgroup analysis showed a consistent benefit across all groups with no difference noted regarding BRCA mutation, hormone receptor status or use of neoadjuvant vs adjuvant chemotherapy.
The side effects were consistent with the safety profile of olaparib. Adverse events of grade 3 or higher more common with olaparib included anemia (8.7%), leukopenia (3%), and fatigue (1.8%). Early discontinuation of trial regimen due to adverse events of disease recurrence occurred in 25.9% in the olaparib group and 20.7% in the placebo group. Blood transfusions were required in 5.8% of patients in the olaparib group. Myelodysplasia or acute myleoid leukemia was observed in 2 patients in the olaparib group and 3 patients in the placebo group. Adverse events leading to death occurred in 1 patient in the olaparib group and 2 patients in the placebo group.
Conclusion. Among patients with high-risk, HER2-negative early breast cancer and germline BRCA1 or BRCA2 pathogenic or likely pathogenic variants, adjuvant olaparib after completion of local treatment and neoadjuvant or adjuvant chemotherapy was associated with significantly longer invasive disease-free and distant disease-free survival compared with placebo.
Commentary
The results from the current OlympiA trial provide the first evidence that adjuvant therapy with poly adenosine diphosphate-ribose polymerase (PARP) inhibitors can improve outcomes in high-risk, HER2-negative breast cancer in patients with pathogenic BRCA1 and BRCA2 mutations. The OS, while favoring olaparib, is not yet mature at the time of this analysis. Nevertheless, these results represent an important step forward in improving outcomes in this patient population. The efficacy and safety of PARP inhibitors in BRCA-mutated breast cancer has previously been shown in patients with advanced disease leading to FDA approval of both olaparib and talazoparib in this setting.1,2 With the current results, PARP inhibitors will certainly play an important role in the adjuvant setting in patients with deleterious BRCA1 or BRCA2 mutations at high risk for relapse. Importantly, the side effect profile appears acceptable with no unexpected events and a very low rate of secondary myeloid malignancies.
Subgroup analysis appears to indicate a benefit across all groups including hormone receptor–positive disease and triple negative breast cancer. Interestingly, approximately 25% of patients in both cohorts received platinum-based chemotherapy. The efficacy of adjuvant olaparib did not appear to be impacted by prior use of platinum-containing chemotherapy regimens. It is important to consider that postneoadjuvant capecitabine, per the results of the CREATE-X trial, in triple-negative patients was not permitted in the current study. Although, this has been widely adopted in clinical practice.3 The CREATE-X trial did not specify the benefit of adjuvant capecitabine in the BRCA-mutated cohort, thus, it is not clear how this subgroup fares with this approach. Thus, one cannot extrapolate the relative efficacy of olaparib compared with capecitabine, as pointed out by the authors, and whether we consider the use of capecitabine and/or olaparib in triple-negative patients with residual invasive disease after neoadjuvant chemotherapy is not clear at this time.
Nevertheless, the magnitude of benefit seen in this trial certainly provide clinically relevant and potentially practice changing results. It will be imperative to follow these results as the survival data matures and ensure no further long-term toxicity, particularly secondary myeloid malignancies, develop. These results should be discussed with each patient and informed decisions regarding the use of adjuvant olaparib should be considered for this patient population. Lastly, these results highlight the importance of germline testing for patients with breast cancer in accordance with national guideline recommendations. Moreover, these results certainly call into question whether it is time to consider expansion of our current germline testing guidelines to detect all potential patients who may benefit from this therapy.
Application for Clinical Practice
Adjuvant olaparib in high-risk patients with germline BRCA1 or BRCA2 mutations improves invasive and distant disease-free survival and should be considered in patients who meet the enrollment criteria of the current study. Furthermore, this highlights the importance of appropriate germline genetic testing in patients with breast cancer.
Financial disclosures: None.
Study Overview
Objective. To assess the efficacy and safety of olaparib as an adjuvant treatment in patients with BRCA1 or BRCA2 germline mutations who are at a high-risk for relapse.
Design. A randomized, double-blind, placebo-controlled, multicenter phase III study. The published results are from the prespecified interim analysis.
Intervention. Patients were randomized in 1:1 ratio to either receive 300 mg of olaparib orally twice daily or to receive a matching placebo. Randomization was stratified by hormone receptor status (estrogen receptor and/or progesterone receptor positive/HER2-negative vs triple negative), prior neoadjuvant vs adjuvant chemotherapy, and prior platinum use for breast cancer. Treatment was continued for 52 weeks.
Setting and participants. A total of 1836 patients were randomized in a 1:1 fashion to receive olaparib or a placebo. Eligible patients had a germline BRCA1 or BRCA1 pathogenic or likely pathogenic variant. Patients had high-risk, HER2-negative primary breast cancers and all had received definitive local therapy and neoadjuvant or adjuvant chemotherapy. Patients were enrolled between 2 to 12 weeks after completion of all local therapy. Platinum chemotherapy was allowed. Patients received adjuvant endocrine therapy for hormone receptor positive disease as well as adjuvant bisphosphonates per institutional guidelines. Patients with triple negative disease who received adjuvant chemotherapy were required to be lymph node positive or have at least 2 cm invasive disease. Patients who received neoadjuvant chemotherapy were required to have residual invasive disease to be eligible. For hormone receptor positive patients receiving adjuvant chemotherapy to be eligible they had to have at least 4 pathologically confirmed lymph nodes involved. Hormone receptor positive patients who had neoadjuvant chemotherapy were required to have had residual invasive disease.
Main outcome measures. The primary endpoint for the study was invasive disease-free survival which was defined as time from randomization to date of recurrence or death from any cause. The secondary endpoints included overall survival (OS), distant disease-free survival, safety, and tolerability of olaparib.
Main results. At the time of data cutoff, 284 events had occurred with a median follow-up of 2.5 years in the intention to treat population. A total of 81% of patients had triple negative breast cancer. Most patients (94% in the olaparib group and 92% in the placebo group) received both taxane and anthracycline based chemotherapy regimens. Platinum based chemotherapy was used in 26% of patients in each group. The groups were otherwise well balanced. Germline mutations in BRCA1 were present in 72% of patients and BRCA2 in 27% of patients. These were balanced between groups.
At the time of this analysis, adjuvant olaparib reduced the risk of invasive disease-free survival by 42% compared with placebo (P < .001). At 3 years, invasive disease-free survival was 85.9% in the olaparib group and 77.1% in the placebo group (difference, 8.8 percentage points; 95% CI, 4.5-13.0; hazard ratio [HR], 0.58; 99.5% CI, 0.41-0.82; P < .001). The 3-year distant disease-free survival was 87.5% in the olaparib group and 80.4% in the placebo group (HR 0.57; 99.5% CI, 0.39-0.83; P < .001). Results also showed that olaparib was associated with fewer deaths than placebo (59 and 86, respectively) (HR, 0.68; 99% CI, 0.44-1.05; P = .02); however, there was no significant difference between treatment arms at the time of this interim analysis. Subgroup analysis showed a consistent benefit across all groups with no difference noted regarding BRCA mutation, hormone receptor status or use of neoadjuvant vs adjuvant chemotherapy.
The side effects were consistent with the safety profile of olaparib. Adverse events of grade 3 or higher more common with olaparib included anemia (8.7%), leukopenia (3%), and fatigue (1.8%). Early discontinuation of trial regimen due to adverse events of disease recurrence occurred in 25.9% in the olaparib group and 20.7% in the placebo group. Blood transfusions were required in 5.8% of patients in the olaparib group. Myelodysplasia or acute myleoid leukemia was observed in 2 patients in the olaparib group and 3 patients in the placebo group. Adverse events leading to death occurred in 1 patient in the olaparib group and 2 patients in the placebo group.
Conclusion. Among patients with high-risk, HER2-negative early breast cancer and germline BRCA1 or BRCA2 pathogenic or likely pathogenic variants, adjuvant olaparib after completion of local treatment and neoadjuvant or adjuvant chemotherapy was associated with significantly longer invasive disease-free and distant disease-free survival compared with placebo.
Commentary
The results from the current OlympiA trial provide the first evidence that adjuvant therapy with poly adenosine diphosphate-ribose polymerase (PARP) inhibitors can improve outcomes in high-risk, HER2-negative breast cancer in patients with pathogenic BRCA1 and BRCA2 mutations. The OS, while favoring olaparib, is not yet mature at the time of this analysis. Nevertheless, these results represent an important step forward in improving outcomes in this patient population. The efficacy and safety of PARP inhibitors in BRCA-mutated breast cancer has previously been shown in patients with advanced disease leading to FDA approval of both olaparib and talazoparib in this setting.1,2 With the current results, PARP inhibitors will certainly play an important role in the adjuvant setting in patients with deleterious BRCA1 or BRCA2 mutations at high risk for relapse. Importantly, the side effect profile appears acceptable with no unexpected events and a very low rate of secondary myeloid malignancies.
Subgroup analysis appears to indicate a benefit across all groups including hormone receptor–positive disease and triple negative breast cancer. Interestingly, approximately 25% of patients in both cohorts received platinum-based chemotherapy. The efficacy of adjuvant olaparib did not appear to be impacted by prior use of platinum-containing chemotherapy regimens. It is important to consider that postneoadjuvant capecitabine, per the results of the CREATE-X trial, in triple-negative patients was not permitted in the current study. Although, this has been widely adopted in clinical practice.3 The CREATE-X trial did not specify the benefit of adjuvant capecitabine in the BRCA-mutated cohort, thus, it is not clear how this subgroup fares with this approach. Thus, one cannot extrapolate the relative efficacy of olaparib compared with capecitabine, as pointed out by the authors, and whether we consider the use of capecitabine and/or olaparib in triple-negative patients with residual invasive disease after neoadjuvant chemotherapy is not clear at this time.
Nevertheless, the magnitude of benefit seen in this trial certainly provide clinically relevant and potentially practice changing results. It will be imperative to follow these results as the survival data matures and ensure no further long-term toxicity, particularly secondary myeloid malignancies, develop. These results should be discussed with each patient and informed decisions regarding the use of adjuvant olaparib should be considered for this patient population. Lastly, these results highlight the importance of germline testing for patients with breast cancer in accordance with national guideline recommendations. Moreover, these results certainly call into question whether it is time to consider expansion of our current germline testing guidelines to detect all potential patients who may benefit from this therapy.
Application for Clinical Practice
Adjuvant olaparib in high-risk patients with germline BRCA1 or BRCA2 mutations improves invasive and distant disease-free survival and should be considered in patients who meet the enrollment criteria of the current study. Furthermore, this highlights the importance of appropriate germline genetic testing in patients with breast cancer.
Financial disclosures: None.
1. Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523-533. doi:10.1056/NEJMoa1706450
2. Litton JK, Rugo HS, Ettl J, et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med. 2018;379(8):753-763. doi:10.1056/NEJMoa1802905
3. Masuda N, Lee SJ, Ohtani S, et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med. 2017;376(22):2147-2159. doi:10.1056/NEJMoa1612645
1. Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523-533. doi:10.1056/NEJMoa1706450
2. Litton JK, Rugo HS, Ettl J, et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med. 2018;379(8):753-763. doi:10.1056/NEJMoa1802905
3. Masuda N, Lee SJ, Ohtani S, et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med. 2017;376(22):2147-2159. doi:10.1056/NEJMoa1612645
Leadership & Professional Development: Relational Leadership—It’s Not About You
“Lead me, follow me, or get the hell out of my way.”
—George Patton
The concept of leadership is often viewed through the lens of the individual. Terms such as “born leader” are canon in our lexicon, and motivational images are common, frequently paired with a singular majestic animal on a mountain peak, meant to inspire awe in the value of the individual leader. This mindset can be problematically reductive, suggesting that leadership is binary and mutually exclusive: we are either leaders or followers. The terminology can also be pejorative, as few are likely to populate a curriculum vitae with examples of being a great follower.
Leadership can instead be regarded as a role rather than a personality trait or superpower. Many of us inhabit multiple leadership roles in our professional lives. Whether participating on a committee, designing an educational curriculum, overseeing a clinical service line, or supervising learners as ward teaching attending, we function as leaders in the context of our relationships with other members of the numerous cohorts within which we work. As leaders, we must consider our relationships to others in a group as opposed to our intrinsic personalities.1
The following pearls can help operationalize relational leadership concepts2,3:
We are not alone. In any given leadership role, we must understand with whom we work (and often depend upon) and what we need to do to allow others to help us succeed. When entering a leadership role with a new group, it is important to assess the interests and skill sets of the rest of the team by either formal or informal means. Many are used to doing so on the first day of attending on a new ward service, but this concept also applies to other roles, such as chairing a new committee.
Work with individuals and groups whose knowledge, experience, skills, and/or attitudes are complementary to our own. This is not as easy as it sounds; when hiring individuals or assembling groups, we tend to gravitate to those like ourselves. Seeking different opinions and styles can be valuable, and promoting diversity, inclusion, and equity is paramount. To do so, we must make efforts to understand our own personal strengths and limitations, ideally supplemented with observation and feedback from a trusted mentor or coach. Taking an honest look at our preconceptions and assumptions is crucial. Consider how we view other silos with which we interact and the presuppositions we make, such as the “typical” surgeon or emergency department practitioner.
Recognize and publicly share shortcomings. Transparency about our limitations allows us to build relationships that are more effective and impactful. A leader who meaningfully reveals a weakness for which they need other group members to contribute specific expertise can allow team members to feel more connected or engaged with that leader or group by shifting from interpreting an ask as “Do this task” to the more empowering “I need your help.”
Leadership can be effectively conceptualized as a relational skill, fulfilled by various roles in our professional lives. Collaboration, introspection, and transparency are essential to becoming a successful leader.
Acknowledgments
The author gratefully acknowledges Dr David Berg for his invaluable mentorship as well as the core faculty of the SHM-SGIM Academic Hospitalist Academy 2.0 for their support and encouragement.
1. Wood M, Dibben M. Leadership as a relational process. Process Studies. 2015;44(1): 24-47. https://doi.org/10.5840/process20154412
2. Berg DN. Resurrecting the muse: followership in organizations. In: Klein EB, Gabelnick E, Herr R, eds. Psychodynamics of Leadership. Psychosocial Press; 1998.
3. Berg DN, Bradley EH. Leadership: Rhetoric vs. Reality. 2015. Accessed September 22, 2021. https://www.youtube.com/watch?v=77IwJ8wXaM8
“Lead me, follow me, or get the hell out of my way.”
—George Patton
The concept of leadership is often viewed through the lens of the individual. Terms such as “born leader” are canon in our lexicon, and motivational images are common, frequently paired with a singular majestic animal on a mountain peak, meant to inspire awe in the value of the individual leader. This mindset can be problematically reductive, suggesting that leadership is binary and mutually exclusive: we are either leaders or followers. The terminology can also be pejorative, as few are likely to populate a curriculum vitae with examples of being a great follower.
Leadership can instead be regarded as a role rather than a personality trait or superpower. Many of us inhabit multiple leadership roles in our professional lives. Whether participating on a committee, designing an educational curriculum, overseeing a clinical service line, or supervising learners as ward teaching attending, we function as leaders in the context of our relationships with other members of the numerous cohorts within which we work. As leaders, we must consider our relationships to others in a group as opposed to our intrinsic personalities.1
The following pearls can help operationalize relational leadership concepts2,3:
We are not alone. In any given leadership role, we must understand with whom we work (and often depend upon) and what we need to do to allow others to help us succeed. When entering a leadership role with a new group, it is important to assess the interests and skill sets of the rest of the team by either formal or informal means. Many are used to doing so on the first day of attending on a new ward service, but this concept also applies to other roles, such as chairing a new committee.
Work with individuals and groups whose knowledge, experience, skills, and/or attitudes are complementary to our own. This is not as easy as it sounds; when hiring individuals or assembling groups, we tend to gravitate to those like ourselves. Seeking different opinions and styles can be valuable, and promoting diversity, inclusion, and equity is paramount. To do so, we must make efforts to understand our own personal strengths and limitations, ideally supplemented with observation and feedback from a trusted mentor or coach. Taking an honest look at our preconceptions and assumptions is crucial. Consider how we view other silos with which we interact and the presuppositions we make, such as the “typical” surgeon or emergency department practitioner.
Recognize and publicly share shortcomings. Transparency about our limitations allows us to build relationships that are more effective and impactful. A leader who meaningfully reveals a weakness for which they need other group members to contribute specific expertise can allow team members to feel more connected or engaged with that leader or group by shifting from interpreting an ask as “Do this task” to the more empowering “I need your help.”
Leadership can be effectively conceptualized as a relational skill, fulfilled by various roles in our professional lives. Collaboration, introspection, and transparency are essential to becoming a successful leader.
Acknowledgments
The author gratefully acknowledges Dr David Berg for his invaluable mentorship as well as the core faculty of the SHM-SGIM Academic Hospitalist Academy 2.0 for their support and encouragement.
“Lead me, follow me, or get the hell out of my way.”
—George Patton
The concept of leadership is often viewed through the lens of the individual. Terms such as “born leader” are canon in our lexicon, and motivational images are common, frequently paired with a singular majestic animal on a mountain peak, meant to inspire awe in the value of the individual leader. This mindset can be problematically reductive, suggesting that leadership is binary and mutually exclusive: we are either leaders or followers. The terminology can also be pejorative, as few are likely to populate a curriculum vitae with examples of being a great follower.
Leadership can instead be regarded as a role rather than a personality trait or superpower. Many of us inhabit multiple leadership roles in our professional lives. Whether participating on a committee, designing an educational curriculum, overseeing a clinical service line, or supervising learners as ward teaching attending, we function as leaders in the context of our relationships with other members of the numerous cohorts within which we work. As leaders, we must consider our relationships to others in a group as opposed to our intrinsic personalities.1
The following pearls can help operationalize relational leadership concepts2,3:
We are not alone. In any given leadership role, we must understand with whom we work (and often depend upon) and what we need to do to allow others to help us succeed. When entering a leadership role with a new group, it is important to assess the interests and skill sets of the rest of the team by either formal or informal means. Many are used to doing so on the first day of attending on a new ward service, but this concept also applies to other roles, such as chairing a new committee.
Work with individuals and groups whose knowledge, experience, skills, and/or attitudes are complementary to our own. This is not as easy as it sounds; when hiring individuals or assembling groups, we tend to gravitate to those like ourselves. Seeking different opinions and styles can be valuable, and promoting diversity, inclusion, and equity is paramount. To do so, we must make efforts to understand our own personal strengths and limitations, ideally supplemented with observation and feedback from a trusted mentor or coach. Taking an honest look at our preconceptions and assumptions is crucial. Consider how we view other silos with which we interact and the presuppositions we make, such as the “typical” surgeon or emergency department practitioner.
Recognize and publicly share shortcomings. Transparency about our limitations allows us to build relationships that are more effective and impactful. A leader who meaningfully reveals a weakness for which they need other group members to contribute specific expertise can allow team members to feel more connected or engaged with that leader or group by shifting from interpreting an ask as “Do this task” to the more empowering “I need your help.”
Leadership can be effectively conceptualized as a relational skill, fulfilled by various roles in our professional lives. Collaboration, introspection, and transparency are essential to becoming a successful leader.
Acknowledgments
The author gratefully acknowledges Dr David Berg for his invaluable mentorship as well as the core faculty of the SHM-SGIM Academic Hospitalist Academy 2.0 for their support and encouragement.
1. Wood M, Dibben M. Leadership as a relational process. Process Studies. 2015;44(1): 24-47. https://doi.org/10.5840/process20154412
2. Berg DN. Resurrecting the muse: followership in organizations. In: Klein EB, Gabelnick E, Herr R, eds. Psychodynamics of Leadership. Psychosocial Press; 1998.
3. Berg DN, Bradley EH. Leadership: Rhetoric vs. Reality. 2015. Accessed September 22, 2021. https://www.youtube.com/watch?v=77IwJ8wXaM8
1. Wood M, Dibben M. Leadership as a relational process. Process Studies. 2015;44(1): 24-47. https://doi.org/10.5840/process20154412
2. Berg DN. Resurrecting the muse: followership in organizations. In: Klein EB, Gabelnick E, Herr R, eds. Psychodynamics of Leadership. Psychosocial Press; 1998.
3. Berg DN, Bradley EH. Leadership: Rhetoric vs. Reality. 2015. Accessed September 22, 2021. https://www.youtube.com/watch?v=77IwJ8wXaM8