User login
New treatments promise sickle cell “cure” for all ages
SAN DIEGO –
“There is an opportunity to cure your disease no matter what age you are,” Dr. Osunkwo, medical director of the sickle cell program at Levine Cancer Institute at Atrium Health in Charlotte, N.C., said in a video interview at the annual meeting of the American Society of Hematology. “Sickle cell disease is now a disease of all ages and the treatments have to be treatments for everybody of all ages, not just for children.”
Dr. Osunkwo was the moderator of a press conference highlighting top research in sickle cell disease at ASH 2018. She pointed to findings from first-in-human trials of gene therapy using a lentiviral vector targeting BCL11A to reverse the sickle cell phenotype, as well as a study examining familial haploidentical stem cell transplantation with CD34 enrichment and mononuclear add-back in high-risk patients.
These two studies show parallel progress in curative therapies and are complementary, Dr. Osunkwo said. Improvements in transplants, and specifically in how patients are prepared and managed for them, will have a benefit in gene therapy.
But there are many other sickle cell disease studies being presented at ASH this year, she noted.
“There’s a recognition that sickle cell has been an understudied, underresourced, underexposed population,” she said. “And the suffering and the magnitude of medical problems is huge and it finally has bubbled up to the surface.”
Dr. Osunkwo reported being on advisory committees for Novartis and Pfizer and on the speaker’s bureau for Novartis. She has received honoraria from Terumo BCT and funding from the Health Resources and Services Administration and the Patient-Centered Outcomes Research Institute.
SAN DIEGO –
“There is an opportunity to cure your disease no matter what age you are,” Dr. Osunkwo, medical director of the sickle cell program at Levine Cancer Institute at Atrium Health in Charlotte, N.C., said in a video interview at the annual meeting of the American Society of Hematology. “Sickle cell disease is now a disease of all ages and the treatments have to be treatments for everybody of all ages, not just for children.”
Dr. Osunkwo was the moderator of a press conference highlighting top research in sickle cell disease at ASH 2018. She pointed to findings from first-in-human trials of gene therapy using a lentiviral vector targeting BCL11A to reverse the sickle cell phenotype, as well as a study examining familial haploidentical stem cell transplantation with CD34 enrichment and mononuclear add-back in high-risk patients.
These two studies show parallel progress in curative therapies and are complementary, Dr. Osunkwo said. Improvements in transplants, and specifically in how patients are prepared and managed for them, will have a benefit in gene therapy.
But there are many other sickle cell disease studies being presented at ASH this year, she noted.
“There’s a recognition that sickle cell has been an understudied, underresourced, underexposed population,” she said. “And the suffering and the magnitude of medical problems is huge and it finally has bubbled up to the surface.”
Dr. Osunkwo reported being on advisory committees for Novartis and Pfizer and on the speaker’s bureau for Novartis. She has received honoraria from Terumo BCT and funding from the Health Resources and Services Administration and the Patient-Centered Outcomes Research Institute.
SAN DIEGO –
“There is an opportunity to cure your disease no matter what age you are,” Dr. Osunkwo, medical director of the sickle cell program at Levine Cancer Institute at Atrium Health in Charlotte, N.C., said in a video interview at the annual meeting of the American Society of Hematology. “Sickle cell disease is now a disease of all ages and the treatments have to be treatments for everybody of all ages, not just for children.”
Dr. Osunkwo was the moderator of a press conference highlighting top research in sickle cell disease at ASH 2018. She pointed to findings from first-in-human trials of gene therapy using a lentiviral vector targeting BCL11A to reverse the sickle cell phenotype, as well as a study examining familial haploidentical stem cell transplantation with CD34 enrichment and mononuclear add-back in high-risk patients.
These two studies show parallel progress in curative therapies and are complementary, Dr. Osunkwo said. Improvements in transplants, and specifically in how patients are prepared and managed for them, will have a benefit in gene therapy.
But there are many other sickle cell disease studies being presented at ASH this year, she noted.
“There’s a recognition that sickle cell has been an understudied, underresourced, underexposed population,” she said. “And the suffering and the magnitude of medical problems is huge and it finally has bubbled up to the surface.”
Dr. Osunkwo reported being on advisory committees for Novartis and Pfizer and on the speaker’s bureau for Novartis. She has received honoraria from Terumo BCT and funding from the Health Resources and Services Administration and the Patient-Centered Outcomes Research Institute.
REPORTING FROM ASH 2018
Heavy menstrual bleeding in teens often linked to bleeding disorders
Over one-third of adolescents presenting with heavy menstrual bleeding were diagnosed with a bleeding disorder after screening, according to results of a retrospective study.
The high incidence of bleeding disorders detected argues for routine screening of adolescents with heavy menstrual bleeding (HMB), Brooke O’Brien, MD, of the University of Queensland, Brisbane, Australia, and her colleagues wrote in the Journal of Pediatric & Adolescent Gynecology.
“These findings support comprehensive and systematic hemostatic evaluation in adolescents with HMB,” Dr. O’Brien and her colleagues wrote. “A higher level of awareness of bleeding disorders as a cause for HMB in adolescence, especially [von Willebrand disease] and platelet function disorders, is needed and close multidisciplinary collaboration between the pediatric and adolescent gynecologist and hematologist in a specialized tertiary center should be established in the management of these patients.”
In their study, Dr. O’Brien and her colleagues retrospectively evaluated 124 adolescents with HMB at a pediatric and adolescent gynecology tertiary care center between July 2007 and July 2017. Of these, 77 patients (62.1%) underwent screening for blood disorders.
The researchers found 27 adolescents overall were diagnosed with a blood disorder, which consisted of 35.0% of patients screened and 21.7% of all patients studied. Specifically, 14 of 27 patients (51.6%) screened were diagnosed with von Willebrand disease, 9 of 27 patients (33.3%) screened were found to have inherited platelet function disorders, 3 of 27 patients (11.1%) had inherited or acquired thrombocytopenia, and 1 of 27 patients (3.7%) had factor IX deficiency. The researchers also screened for iron deficiency and/or anemia and found 53 of 107 patients (49.5%) who were screened received a diagnosis, and 19 of 27 patients (70.3%) who were diagnosed with a bleeding disorder also had iron deficiency and/or anemia.
“In adolescents who are already known to have a bleeding disorder, consultation with a pediatric gynecologist and/or hematologist prior to menarche may be helpful to outline abnormal patterns of menstrual bleeding and to discuss options of treatment in the event of heavy menstrual bleeding,” Dr. O’Brien and her colleagues wrote.
Potential limitations in the study include the refractory nature of referrals at a tertiary care center potentially overestimating the prevalence of HMB in this population as well as the study’s retrospective design when investigating and measuring heavy menstrual bleeding, but researchers noted patients were reviewed and classified by a specialist pediatric hematologist.
The authors reported no relevant conflicts of interest.
SOURCE: O’Brien B et al. J Pediatr Adolesc Gynecol. 2018 Nov 22. doi: 10.1016/j.jpag.2018.11.005.
Over one-third of adolescents presenting with heavy menstrual bleeding were diagnosed with a bleeding disorder after screening, according to results of a retrospective study.
The high incidence of bleeding disorders detected argues for routine screening of adolescents with heavy menstrual bleeding (HMB), Brooke O’Brien, MD, of the University of Queensland, Brisbane, Australia, and her colleagues wrote in the Journal of Pediatric & Adolescent Gynecology.
“These findings support comprehensive and systematic hemostatic evaluation in adolescents with HMB,” Dr. O’Brien and her colleagues wrote. “A higher level of awareness of bleeding disorders as a cause for HMB in adolescence, especially [von Willebrand disease] and platelet function disorders, is needed and close multidisciplinary collaboration between the pediatric and adolescent gynecologist and hematologist in a specialized tertiary center should be established in the management of these patients.”
In their study, Dr. O’Brien and her colleagues retrospectively evaluated 124 adolescents with HMB at a pediatric and adolescent gynecology tertiary care center between July 2007 and July 2017. Of these, 77 patients (62.1%) underwent screening for blood disorders.
The researchers found 27 adolescents overall were diagnosed with a blood disorder, which consisted of 35.0% of patients screened and 21.7% of all patients studied. Specifically, 14 of 27 patients (51.6%) screened were diagnosed with von Willebrand disease, 9 of 27 patients (33.3%) screened were found to have inherited platelet function disorders, 3 of 27 patients (11.1%) had inherited or acquired thrombocytopenia, and 1 of 27 patients (3.7%) had factor IX deficiency. The researchers also screened for iron deficiency and/or anemia and found 53 of 107 patients (49.5%) who were screened received a diagnosis, and 19 of 27 patients (70.3%) who were diagnosed with a bleeding disorder also had iron deficiency and/or anemia.
“In adolescents who are already known to have a bleeding disorder, consultation with a pediatric gynecologist and/or hematologist prior to menarche may be helpful to outline abnormal patterns of menstrual bleeding and to discuss options of treatment in the event of heavy menstrual bleeding,” Dr. O’Brien and her colleagues wrote.
Potential limitations in the study include the refractory nature of referrals at a tertiary care center potentially overestimating the prevalence of HMB in this population as well as the study’s retrospective design when investigating and measuring heavy menstrual bleeding, but researchers noted patients were reviewed and classified by a specialist pediatric hematologist.
The authors reported no relevant conflicts of interest.
SOURCE: O’Brien B et al. J Pediatr Adolesc Gynecol. 2018 Nov 22. doi: 10.1016/j.jpag.2018.11.005.
Over one-third of adolescents presenting with heavy menstrual bleeding were diagnosed with a bleeding disorder after screening, according to results of a retrospective study.
The high incidence of bleeding disorders detected argues for routine screening of adolescents with heavy menstrual bleeding (HMB), Brooke O’Brien, MD, of the University of Queensland, Brisbane, Australia, and her colleagues wrote in the Journal of Pediatric & Adolescent Gynecology.
“These findings support comprehensive and systematic hemostatic evaluation in adolescents with HMB,” Dr. O’Brien and her colleagues wrote. “A higher level of awareness of bleeding disorders as a cause for HMB in adolescence, especially [von Willebrand disease] and platelet function disorders, is needed and close multidisciplinary collaboration between the pediatric and adolescent gynecologist and hematologist in a specialized tertiary center should be established in the management of these patients.”
In their study, Dr. O’Brien and her colleagues retrospectively evaluated 124 adolescents with HMB at a pediatric and adolescent gynecology tertiary care center between July 2007 and July 2017. Of these, 77 patients (62.1%) underwent screening for blood disorders.
The researchers found 27 adolescents overall were diagnosed with a blood disorder, which consisted of 35.0% of patients screened and 21.7% of all patients studied. Specifically, 14 of 27 patients (51.6%) screened were diagnosed with von Willebrand disease, 9 of 27 patients (33.3%) screened were found to have inherited platelet function disorders, 3 of 27 patients (11.1%) had inherited or acquired thrombocytopenia, and 1 of 27 patients (3.7%) had factor IX deficiency. The researchers also screened for iron deficiency and/or anemia and found 53 of 107 patients (49.5%) who were screened received a diagnosis, and 19 of 27 patients (70.3%) who were diagnosed with a bleeding disorder also had iron deficiency and/or anemia.
“In adolescents who are already known to have a bleeding disorder, consultation with a pediatric gynecologist and/or hematologist prior to menarche may be helpful to outline abnormal patterns of menstrual bleeding and to discuss options of treatment in the event of heavy menstrual bleeding,” Dr. O’Brien and her colleagues wrote.
Potential limitations in the study include the refractory nature of referrals at a tertiary care center potentially overestimating the prevalence of HMB in this population as well as the study’s retrospective design when investigating and measuring heavy menstrual bleeding, but researchers noted patients were reviewed and classified by a specialist pediatric hematologist.
The authors reported no relevant conflicts of interest.
SOURCE: O’Brien B et al. J Pediatr Adolesc Gynecol. 2018 Nov 22. doi: 10.1016/j.jpag.2018.11.005.
FROM THE JOURNAL OF PEDIATRIC & ADOLESCENT GYNECOLOGY
Key clinical point: More than one-third of adolescents with heavy menstrual bleeding were diagnosed with a bleeding disorder.
Major finding: After screening, 35% of women with heavy menstrual bleeding had a bleeding disorder; over half of those screened had von Willebrand disease.
Study details: A retrospective study of 124 adolescents at the Queensland Paediatric and Adolescent Gynaecology Service between July 2007 and July 2017.
Disclosures: The authors reported no relevant conflicts of interest.
Source: O’Brien B et al. J Pediatr Adolesc Gynecol. 2018 Nov 22 . doi: 10.1016/j.jpag.2018.11.005.
Sickle cell disease phenotype reversed by gene therapy
SAN DIEGO – An adult with sickle cell disease has had significant remissions in symptoms and a near elimination of transfusion requirements after receiving an infusion of autologous stem cells genetically modified to simultaneously induce the fetal form of hemoglobin and decrease sickle hemoglobin.
In a first-in-human, proof-of-concept study, transduction of hematopoietic stem cells with a lentiviral vector targeted against the gamma globin repressor BCL11A in erythroid cells led to rapid induction of fetal hemoglobin and a reversal of the sickle cell disease (SCD) phenotype in the early phase of stem cell reconstitution, reported Erica B. Esrick, MD, from the Dana-Farber/Boston Children’s Cancer and Blood Disorders Center in Boston.
“The potential advantage of this approach over the gene-addition strategy of gene therapy is that we can harness the physiologic switch machinery that exists in the cell to simultaneously increase fetal hemoglobin and decrease sickle hemoglobin,” she said at a briefing prior to her presentation at the annual meeting of the American Society of Hematology.
Several research groups are developing autologous gene therapy for beta-hemoglobinopathies, including the use of CRISPR-Cas9 technology to mimic a rare, naturally occurring mutation that causes the fetal type of hemoglobin to persist into adulthood in some patients with SCD and beta-thalassemia.
Dr. Esrick and her colleagues are trying a different approach: Using gene therapy to knock down BCL11A expression to induce gamma globin expression.
For the treatment, autologous hematopoietic stem cells are collected from patients following mobilization with plerixafor. The cells are then transduced with a lentiviral vector consisting of a novel short hairpin RNA embedded in an endogenous micro-RNA. The investigators refer to the construct as a shmiR (“schmeer”). The construct is designed to be erythroid specific, with BCL11A knocked down only in the red cell lineage, to avoid potential off-target effects of the therapy.
Following stem cell collection and transduction, patients undergo conditioning with busulfan prior to infusion of the modified stem cells.
In three patients treated thus far, the process has been shown to be highly efficient, with approximately 96% of treated cells transduced.
In the patient mentioned before, neutrophil engraftment was confirmed on day 22 after transfusion of the modified cells. He experienced adverse events that were consistent with myeloablative conditioning, but no adverse events associated with the modified cells.
During 6 months of follow-up the patient did not experience SCD-related pain, respiratory events, or neurologic events, and did not have anemia, with a total hemoglobin of 11 g/dL at 6 months. He has not required any transfusions since engraftment.
Patients in the trial will be followed for 2 years, and then will be enrolled in a 15-year follow-up study designed to evaluate the safety and the durability of therapy.
Dr. Esrick reported receiving honoraria from Bluebird Bio, maker of the short hairpin RNA construct used in the trial.
SOURCE: Esrick EB et al. ASH 2018, Abstract 1023.
SAN DIEGO – An adult with sickle cell disease has had significant remissions in symptoms and a near elimination of transfusion requirements after receiving an infusion of autologous stem cells genetically modified to simultaneously induce the fetal form of hemoglobin and decrease sickle hemoglobin.
In a first-in-human, proof-of-concept study, transduction of hematopoietic stem cells with a lentiviral vector targeted against the gamma globin repressor BCL11A in erythroid cells led to rapid induction of fetal hemoglobin and a reversal of the sickle cell disease (SCD) phenotype in the early phase of stem cell reconstitution, reported Erica B. Esrick, MD, from the Dana-Farber/Boston Children’s Cancer and Blood Disorders Center in Boston.
“The potential advantage of this approach over the gene-addition strategy of gene therapy is that we can harness the physiologic switch machinery that exists in the cell to simultaneously increase fetal hemoglobin and decrease sickle hemoglobin,” she said at a briefing prior to her presentation at the annual meeting of the American Society of Hematology.
Several research groups are developing autologous gene therapy for beta-hemoglobinopathies, including the use of CRISPR-Cas9 technology to mimic a rare, naturally occurring mutation that causes the fetal type of hemoglobin to persist into adulthood in some patients with SCD and beta-thalassemia.
Dr. Esrick and her colleagues are trying a different approach: Using gene therapy to knock down BCL11A expression to induce gamma globin expression.
For the treatment, autologous hematopoietic stem cells are collected from patients following mobilization with plerixafor. The cells are then transduced with a lentiviral vector consisting of a novel short hairpin RNA embedded in an endogenous micro-RNA. The investigators refer to the construct as a shmiR (“schmeer”). The construct is designed to be erythroid specific, with BCL11A knocked down only in the red cell lineage, to avoid potential off-target effects of the therapy.
Following stem cell collection and transduction, patients undergo conditioning with busulfan prior to infusion of the modified stem cells.
In three patients treated thus far, the process has been shown to be highly efficient, with approximately 96% of treated cells transduced.
In the patient mentioned before, neutrophil engraftment was confirmed on day 22 after transfusion of the modified cells. He experienced adverse events that were consistent with myeloablative conditioning, but no adverse events associated with the modified cells.
During 6 months of follow-up the patient did not experience SCD-related pain, respiratory events, or neurologic events, and did not have anemia, with a total hemoglobin of 11 g/dL at 6 months. He has not required any transfusions since engraftment.
Patients in the trial will be followed for 2 years, and then will be enrolled in a 15-year follow-up study designed to evaluate the safety and the durability of therapy.
Dr. Esrick reported receiving honoraria from Bluebird Bio, maker of the short hairpin RNA construct used in the trial.
SOURCE: Esrick EB et al. ASH 2018, Abstract 1023.
SAN DIEGO – An adult with sickle cell disease has had significant remissions in symptoms and a near elimination of transfusion requirements after receiving an infusion of autologous stem cells genetically modified to simultaneously induce the fetal form of hemoglobin and decrease sickle hemoglobin.
In a first-in-human, proof-of-concept study, transduction of hematopoietic stem cells with a lentiviral vector targeted against the gamma globin repressor BCL11A in erythroid cells led to rapid induction of fetal hemoglobin and a reversal of the sickle cell disease (SCD) phenotype in the early phase of stem cell reconstitution, reported Erica B. Esrick, MD, from the Dana-Farber/Boston Children’s Cancer and Blood Disorders Center in Boston.
“The potential advantage of this approach over the gene-addition strategy of gene therapy is that we can harness the physiologic switch machinery that exists in the cell to simultaneously increase fetal hemoglobin and decrease sickle hemoglobin,” she said at a briefing prior to her presentation at the annual meeting of the American Society of Hematology.
Several research groups are developing autologous gene therapy for beta-hemoglobinopathies, including the use of CRISPR-Cas9 technology to mimic a rare, naturally occurring mutation that causes the fetal type of hemoglobin to persist into adulthood in some patients with SCD and beta-thalassemia.
Dr. Esrick and her colleagues are trying a different approach: Using gene therapy to knock down BCL11A expression to induce gamma globin expression.
For the treatment, autologous hematopoietic stem cells are collected from patients following mobilization with plerixafor. The cells are then transduced with a lentiviral vector consisting of a novel short hairpin RNA embedded in an endogenous micro-RNA. The investigators refer to the construct as a shmiR (“schmeer”). The construct is designed to be erythroid specific, with BCL11A knocked down only in the red cell lineage, to avoid potential off-target effects of the therapy.
Following stem cell collection and transduction, patients undergo conditioning with busulfan prior to infusion of the modified stem cells.
In three patients treated thus far, the process has been shown to be highly efficient, with approximately 96% of treated cells transduced.
In the patient mentioned before, neutrophil engraftment was confirmed on day 22 after transfusion of the modified cells. He experienced adverse events that were consistent with myeloablative conditioning, but no adverse events associated with the modified cells.
During 6 months of follow-up the patient did not experience SCD-related pain, respiratory events, or neurologic events, and did not have anemia, with a total hemoglobin of 11 g/dL at 6 months. He has not required any transfusions since engraftment.
Patients in the trial will be followed for 2 years, and then will be enrolled in a 15-year follow-up study designed to evaluate the safety and the durability of therapy.
Dr. Esrick reported receiving honoraria from Bluebird Bio, maker of the short hairpin RNA construct used in the trial.
SOURCE: Esrick EB et al. ASH 2018, Abstract 1023.
REPORTING FROM ASH 2018
Key clinical point: Gene therapy to induce the fetal form of hemoglobin reversed the symptoms of sickle cell disease in an adult patient.
Major finding: During 6 months of follow-up the patient did not experience sickle cell disease–related pain, respiratory events, or neurologic events, and did not have anemia.
Study details: A first-in-human study in seven adults with sickle cell disease.
Disclosures: Dr. Esrick reported receiving honoraria from Bluebird Bio, maker of the short hairpin RNA construct used in the trial.
Source: Esrick EB et al. ASH 2018, Abstract 1023.
Daily hydroxyurea effective, safe for African children
SAN DIEGO – Daily hydroxyurea treatment for sickle cell disease is feasible, safe, and effective for children in sub-Saharan Africa, according to the results of a large open-label, phase 1-2, international trial.
Hydroxyurea was associated with reduced rates of malaria and other infections, resulting in improved survival, according to Léon Tshilolo, MD, PhD, of Centre Hospitalier Monkole in Kinshasa, the Democratic Republic of the Congo.
“Based on that data, we believe that wider access to hydroxyurea for sickle cell anemia has the potential to save millions of lives in Africa,” Dr. Tshilolo said in a press conference at the annual meeting of the American Society of Hematology.
Use of hydroxyurea has been limited in Africa because of cost, access issues, and challenges associated with laboratory monitoring, according to the researchers.
Moreover, most of the data on the efficacy of hydroxyurea come from studies conducted in the United States, Europe, and other high-income settings, said the study’s senior author Russell E. Ware, MD, PhD, of Cincinnati Children’s Hospital Center.
“Now that there’s data in an African setting, I think this will go a long way to advancing [hydroxyurea therapy] and encouraging governments, organizations, and pharmaceutical companies to bring it in,” Dr. Ware said in an interview.
In the study by Dr. Ware, Dr. Tshilolo, and their colleagues, 606 children in four sub-Saharan African countries completed a 2-month pretreatment screening phase designed to capture baseline clinical and laboratory data. The children, who were aged 1-10 years, were started at 15-20 mg/kg of hydroxyurea for 6 months, followed by escalation to the maximum tolerated dose.
With a median of 2.5 years of treatment, treated children experienced less pain and anemia, fewer cases of malaria and other infections, and lower rates of transfusions and death versus rates observed in the pretreatment screening phase of the trial.
The rate of vasoocclusive pain during hydroxyurea treatment was 44.6 events per 100 patient-years, compared with 98.3 events per 100 patient-years in the pretreatment period (incidence rate ratio, 0.45; 95% confidence interval, 0.37-0.56), according to data simultaneously published in the New England Journal of Medicine.
Malaria infection rates were 22.9 events per 100 patient-years in the hydroxyurea treatment period versus 46.9 events in the pretreatment period (IRR, 0.49; 95% CI, 0.37-0.66). Rates of nonmalaria infections were 90.0 events per 100 patient-years in the hydroxyurea treatment period versus 142.5 events per 100 patient-years in the pretreatment period (IRR, 0.62; 95% CI, 0.53-0.72).
Dr. Tshilolo said investigators were “encouraged” by the reduced infection rates, particularly in light of previous concerns that hydroxyurea could suppress the immune system and put children at risk for malaria.
Death rates were 1.1 per 100 patient-years in the hydroxyurea group and 3.6 per 100 patient-years in the pretreatment period (IR, 0.30; 95% CI, 0.10-0.88). Dose-limiting toxic events occurred in 5.1% of the children, which was below the protocol-specified threshold for safety, Dr. Tshilolo added.
Dr. Tshilolo reported grants from the National Institutes of Health/National Heart, Lung, and Blood Institute and Cincinnati Children’s Research Foundation, along with nonfinancial support from Bristol-Myers Squibb. Dr. Ware reported grants from the NIH/NHLBI and Bristol-Myers Squibb.
SOURCE: Tshilolo L et al. ASH 2018, Abstract 3.
SAN DIEGO – Daily hydroxyurea treatment for sickle cell disease is feasible, safe, and effective for children in sub-Saharan Africa, according to the results of a large open-label, phase 1-2, international trial.
Hydroxyurea was associated with reduced rates of malaria and other infections, resulting in improved survival, according to Léon Tshilolo, MD, PhD, of Centre Hospitalier Monkole in Kinshasa, the Democratic Republic of the Congo.
“Based on that data, we believe that wider access to hydroxyurea for sickle cell anemia has the potential to save millions of lives in Africa,” Dr. Tshilolo said in a press conference at the annual meeting of the American Society of Hematology.
Use of hydroxyurea has been limited in Africa because of cost, access issues, and challenges associated with laboratory monitoring, according to the researchers.
Moreover, most of the data on the efficacy of hydroxyurea come from studies conducted in the United States, Europe, and other high-income settings, said the study’s senior author Russell E. Ware, MD, PhD, of Cincinnati Children’s Hospital Center.
“Now that there’s data in an African setting, I think this will go a long way to advancing [hydroxyurea therapy] and encouraging governments, organizations, and pharmaceutical companies to bring it in,” Dr. Ware said in an interview.
In the study by Dr. Ware, Dr. Tshilolo, and their colleagues, 606 children in four sub-Saharan African countries completed a 2-month pretreatment screening phase designed to capture baseline clinical and laboratory data. The children, who were aged 1-10 years, were started at 15-20 mg/kg of hydroxyurea for 6 months, followed by escalation to the maximum tolerated dose.
With a median of 2.5 years of treatment, treated children experienced less pain and anemia, fewer cases of malaria and other infections, and lower rates of transfusions and death versus rates observed in the pretreatment screening phase of the trial.
The rate of vasoocclusive pain during hydroxyurea treatment was 44.6 events per 100 patient-years, compared with 98.3 events per 100 patient-years in the pretreatment period (incidence rate ratio, 0.45; 95% confidence interval, 0.37-0.56), according to data simultaneously published in the New England Journal of Medicine.
Malaria infection rates were 22.9 events per 100 patient-years in the hydroxyurea treatment period versus 46.9 events in the pretreatment period (IRR, 0.49; 95% CI, 0.37-0.66). Rates of nonmalaria infections were 90.0 events per 100 patient-years in the hydroxyurea treatment period versus 142.5 events per 100 patient-years in the pretreatment period (IRR, 0.62; 95% CI, 0.53-0.72).
Dr. Tshilolo said investigators were “encouraged” by the reduced infection rates, particularly in light of previous concerns that hydroxyurea could suppress the immune system and put children at risk for malaria.
Death rates were 1.1 per 100 patient-years in the hydroxyurea group and 3.6 per 100 patient-years in the pretreatment period (IR, 0.30; 95% CI, 0.10-0.88). Dose-limiting toxic events occurred in 5.1% of the children, which was below the protocol-specified threshold for safety, Dr. Tshilolo added.
Dr. Tshilolo reported grants from the National Institutes of Health/National Heart, Lung, and Blood Institute and Cincinnati Children’s Research Foundation, along with nonfinancial support from Bristol-Myers Squibb. Dr. Ware reported grants from the NIH/NHLBI and Bristol-Myers Squibb.
SOURCE: Tshilolo L et al. ASH 2018, Abstract 3.
SAN DIEGO – Daily hydroxyurea treatment for sickle cell disease is feasible, safe, and effective for children in sub-Saharan Africa, according to the results of a large open-label, phase 1-2, international trial.
Hydroxyurea was associated with reduced rates of malaria and other infections, resulting in improved survival, according to Léon Tshilolo, MD, PhD, of Centre Hospitalier Monkole in Kinshasa, the Democratic Republic of the Congo.
“Based on that data, we believe that wider access to hydroxyurea for sickle cell anemia has the potential to save millions of lives in Africa,” Dr. Tshilolo said in a press conference at the annual meeting of the American Society of Hematology.
Use of hydroxyurea has been limited in Africa because of cost, access issues, and challenges associated with laboratory monitoring, according to the researchers.
Moreover, most of the data on the efficacy of hydroxyurea come from studies conducted in the United States, Europe, and other high-income settings, said the study’s senior author Russell E. Ware, MD, PhD, of Cincinnati Children’s Hospital Center.
“Now that there’s data in an African setting, I think this will go a long way to advancing [hydroxyurea therapy] and encouraging governments, organizations, and pharmaceutical companies to bring it in,” Dr. Ware said in an interview.
In the study by Dr. Ware, Dr. Tshilolo, and their colleagues, 606 children in four sub-Saharan African countries completed a 2-month pretreatment screening phase designed to capture baseline clinical and laboratory data. The children, who were aged 1-10 years, were started at 15-20 mg/kg of hydroxyurea for 6 months, followed by escalation to the maximum tolerated dose.
With a median of 2.5 years of treatment, treated children experienced less pain and anemia, fewer cases of malaria and other infections, and lower rates of transfusions and death versus rates observed in the pretreatment screening phase of the trial.
The rate of vasoocclusive pain during hydroxyurea treatment was 44.6 events per 100 patient-years, compared with 98.3 events per 100 patient-years in the pretreatment period (incidence rate ratio, 0.45; 95% confidence interval, 0.37-0.56), according to data simultaneously published in the New England Journal of Medicine.
Malaria infection rates were 22.9 events per 100 patient-years in the hydroxyurea treatment period versus 46.9 events in the pretreatment period (IRR, 0.49; 95% CI, 0.37-0.66). Rates of nonmalaria infections were 90.0 events per 100 patient-years in the hydroxyurea treatment period versus 142.5 events per 100 patient-years in the pretreatment period (IRR, 0.62; 95% CI, 0.53-0.72).
Dr. Tshilolo said investigators were “encouraged” by the reduced infection rates, particularly in light of previous concerns that hydroxyurea could suppress the immune system and put children at risk for malaria.
Death rates were 1.1 per 100 patient-years in the hydroxyurea group and 3.6 per 100 patient-years in the pretreatment period (IR, 0.30; 95% CI, 0.10-0.88). Dose-limiting toxic events occurred in 5.1% of the children, which was below the protocol-specified threshold for safety, Dr. Tshilolo added.
Dr. Tshilolo reported grants from the National Institutes of Health/National Heart, Lung, and Blood Institute and Cincinnati Children’s Research Foundation, along with nonfinancial support from Bristol-Myers Squibb. Dr. Ware reported grants from the NIH/NHLBI and Bristol-Myers Squibb.
SOURCE: Tshilolo L et al. ASH 2018, Abstract 3.
REPORTING FROM ASH 2018
Key clinical point: Daily hydroxyurea treatment in sub-Saharan African children with sickle cell disease is feasible, safe, and effective, and has the additional benefit of reducing their rates of malaria and nonmalaria infections.
Major finding: Malaria infection rates were 22.9 versus 46.9 events per 100 patient-years in the hydroxyurea treatment period and pretreatment period, respectively (incidence rate ratio, 0.49; 95% CI, 0.37-0.66).
Study details: A phase 1-2, international, open-label trial including 606 children in four sub-Saharan African countries who completed a 2-month pretreatment screening phase and went on to receive hydroxyurea.
Disclosures: Dr. Tshilolo reported grants from the National Institutes of Health/National Heart, Lung, and Blood Institute and Cincinnati Children’s Research Foundation, along with nonfinancial support from Bristol-Myers Squibb. Dr. Ware reported grants from the NIH/NHLBI and Bristol-Myers Squibb.
Source: Tshilolo L et al. ASH 2018, Abstract 3.
Flipping the fetal hemoglobin switch reverses sickle cell symptoms
SAN DIEGO – Researchers were able to “flip the switch” from the adult to fetal form of hemoglobin using autologous stem cells genetically modified to simultaneously induce the fetal form of hemoglobin and decrease sickle hemoglobin.
The advance was announced by investigators at the Dana-Farber Cancer Institute and Boston Children’s Hospital at the annual meeting of the American Society of Hematology. At 6 months of follow-up, one adult patient in the proof-of-concept study has experienced a reversal of the sickle cell phenotype, with no pain episodes or respiratory or neurologic events.
The fetal form of hemoglobin is known to be protective against the signs and symptoms of sickle cell disease, but apart from a few rare exceptions, people with the disorder begin to experience debilitating symptoms as levels of the fetal form begin to decline in early childhood and levels of the adult form of hemoglobin steadily rise.
In this video interview, Erica B. Esrick, MD, from the Dana-Farber/Boston Children’s Cancer and Blood Disorders Center, describes the novel approach of using RNA interference to knock down a repressor that suppresses expression of gamma globin in sickle cell disease.
SAN DIEGO – Researchers were able to “flip the switch” from the adult to fetal form of hemoglobin using autologous stem cells genetically modified to simultaneously induce the fetal form of hemoglobin and decrease sickle hemoglobin.
The advance was announced by investigators at the Dana-Farber Cancer Institute and Boston Children’s Hospital at the annual meeting of the American Society of Hematology. At 6 months of follow-up, one adult patient in the proof-of-concept study has experienced a reversal of the sickle cell phenotype, with no pain episodes or respiratory or neurologic events.
The fetal form of hemoglobin is known to be protective against the signs and symptoms of sickle cell disease, but apart from a few rare exceptions, people with the disorder begin to experience debilitating symptoms as levels of the fetal form begin to decline in early childhood and levels of the adult form of hemoglobin steadily rise.
In this video interview, Erica B. Esrick, MD, from the Dana-Farber/Boston Children’s Cancer and Blood Disorders Center, describes the novel approach of using RNA interference to knock down a repressor that suppresses expression of gamma globin in sickle cell disease.
SAN DIEGO – Researchers were able to “flip the switch” from the adult to fetal form of hemoglobin using autologous stem cells genetically modified to simultaneously induce the fetal form of hemoglobin and decrease sickle hemoglobin.
The advance was announced by investigators at the Dana-Farber Cancer Institute and Boston Children’s Hospital at the annual meeting of the American Society of Hematology. At 6 months of follow-up, one adult patient in the proof-of-concept study has experienced a reversal of the sickle cell phenotype, with no pain episodes or respiratory or neurologic events.
The fetal form of hemoglobin is known to be protective against the signs and symptoms of sickle cell disease, but apart from a few rare exceptions, people with the disorder begin to experience debilitating symptoms as levels of the fetal form begin to decline in early childhood and levels of the adult form of hemoglobin steadily rise.
In this video interview, Erica B. Esrick, MD, from the Dana-Farber/Boston Children’s Cancer and Blood Disorders Center, describes the novel approach of using RNA interference to knock down a repressor that suppresses expression of gamma globin in sickle cell disease.
REPORTING FROM ASH 2018
New data further support curability of myeloma
finds a retrospective cohort study of the International Myeloma Working Group. That figure may be even higher today because more than 90% of patients in the study – the largest yet to look at outcome predictors in this population – were treated in the era before novel therapies became available.
Investigators led by Saad Z. Usmani, MD, director/chief of plasma cell disorders and director of clinical research (hematologic malignancies) at the Levine Cancer Institute/Atrium Health in Charlotte, N.C., studied 7,291 patients with newly diagnosed multiple myeloma who were up to 75 years old and eligible for high-dose melphalan and autologous stem cell transplant. The patients were treated in clinical trials in 10 countries.
Compared with counterparts who did not achieve complete response 1 year after diagnosis, patients who did had better median progression-free survival (3.3 vs. 2.6 years; P less than .0001) and median overall survival (8.5 vs. 6.3 years; P less than .0001), according to study results report in Blood Cancer Journal.
The investigators next performed multivariate analyses to assess clinical variables at diagnosis associated with 10-year survival as compared with 2-year death.
Results here indicated that patients were less likely to be alive at 10 years if they were older than 65 years (odds ratio for death, 1.87; P = .002); had an immunoglobulin A isotype (OR, 1.53; P = .004); had a low albumin level, defined as less than 3.5 g/dL (OR, 1.36; P = .023); had an elevated beta2-microglobulin level, defined as at least 3.5 mg/dL (OR, 1.86; P less than .001); had a higher serum creatinine level, defined as at least 2 mg/dL (OR, 1.77; P = .005); had a lower hemoglobin level, defined as less than 10 g/dL (OR, 1.55; P = .003); or had a lower platelet count, defined as less than 150,000/μL (OR, 2.26; P less than .001).
Cytogenetic abnormalities did not independently predict long-term survival, but these abnormalities were obtained only by conventional band karyotyping and were not available for some patients.
Overall, the cohort had a relative survival of about 0.9 when compared with the matched general population. With follow-up out to about 20 years, the cure fraction (proportion achieving or exceeding expected survival when compared with the matched general population) was 14.3%.
Identification of early complete response as a predictor of long-term survival “underscores the importance of depth of response as we explore novel regimens for newly diagnosed [multiple myeloma] along with [minimal residual disease] endpoints,” Dr. Usmani and his colleagues wrote while acknowledging that the patients studied were a selected group eligible for transplant and treated on trials.
Recent therapeutic advances “have reignited the debate on possible functional curability of a subset MM patients,” they noted. “[T]here are perhaps more effective drugs and drug classes in the clinician’s armamentarium than [were] available for MM patients being treated in the 1990s or even early 2000s. This may mean that the depth of response after induction therapy may continue to improve over time, potentially further improving the PFS/OS of [the] biologic subset who previously achieved [partial response] yet had good long-term survival.”
Dr. Usmani disclosed that he is a consultant for AbbVie, Amgen, BMS, Celgene, Janssen, Takeda, Sanofi, and SkylineDx; receives speaker’s fees for Amgen, Celgene, Janssen, and Takeda; and receives research funding from Amgen, Array Biopharma, BMS, Celgene, Janssen, Pharmacyclics, Sanofi, and Takeda.
SOURCE: Usmani SZ et al. Blood Cancer J. 2018 Nov 23;8(12):123..
finds a retrospective cohort study of the International Myeloma Working Group. That figure may be even higher today because more than 90% of patients in the study – the largest yet to look at outcome predictors in this population – were treated in the era before novel therapies became available.
Investigators led by Saad Z. Usmani, MD, director/chief of plasma cell disorders and director of clinical research (hematologic malignancies) at the Levine Cancer Institute/Atrium Health in Charlotte, N.C., studied 7,291 patients with newly diagnosed multiple myeloma who were up to 75 years old and eligible for high-dose melphalan and autologous stem cell transplant. The patients were treated in clinical trials in 10 countries.
Compared with counterparts who did not achieve complete response 1 year after diagnosis, patients who did had better median progression-free survival (3.3 vs. 2.6 years; P less than .0001) and median overall survival (8.5 vs. 6.3 years; P less than .0001), according to study results report in Blood Cancer Journal.
The investigators next performed multivariate analyses to assess clinical variables at diagnosis associated with 10-year survival as compared with 2-year death.
Results here indicated that patients were less likely to be alive at 10 years if they were older than 65 years (odds ratio for death, 1.87; P = .002); had an immunoglobulin A isotype (OR, 1.53; P = .004); had a low albumin level, defined as less than 3.5 g/dL (OR, 1.36; P = .023); had an elevated beta2-microglobulin level, defined as at least 3.5 mg/dL (OR, 1.86; P less than .001); had a higher serum creatinine level, defined as at least 2 mg/dL (OR, 1.77; P = .005); had a lower hemoglobin level, defined as less than 10 g/dL (OR, 1.55; P = .003); or had a lower platelet count, defined as less than 150,000/μL (OR, 2.26; P less than .001).
Cytogenetic abnormalities did not independently predict long-term survival, but these abnormalities were obtained only by conventional band karyotyping and were not available for some patients.
Overall, the cohort had a relative survival of about 0.9 when compared with the matched general population. With follow-up out to about 20 years, the cure fraction (proportion achieving or exceeding expected survival when compared with the matched general population) was 14.3%.
Identification of early complete response as a predictor of long-term survival “underscores the importance of depth of response as we explore novel regimens for newly diagnosed [multiple myeloma] along with [minimal residual disease] endpoints,” Dr. Usmani and his colleagues wrote while acknowledging that the patients studied were a selected group eligible for transplant and treated on trials.
Recent therapeutic advances “have reignited the debate on possible functional curability of a subset MM patients,” they noted. “[T]here are perhaps more effective drugs and drug classes in the clinician’s armamentarium than [were] available for MM patients being treated in the 1990s or even early 2000s. This may mean that the depth of response after induction therapy may continue to improve over time, potentially further improving the PFS/OS of [the] biologic subset who previously achieved [partial response] yet had good long-term survival.”
Dr. Usmani disclosed that he is a consultant for AbbVie, Amgen, BMS, Celgene, Janssen, Takeda, Sanofi, and SkylineDx; receives speaker’s fees for Amgen, Celgene, Janssen, and Takeda; and receives research funding from Amgen, Array Biopharma, BMS, Celgene, Janssen, Pharmacyclics, Sanofi, and Takeda.
SOURCE: Usmani SZ et al. Blood Cancer J. 2018 Nov 23;8(12):123..
finds a retrospective cohort study of the International Myeloma Working Group. That figure may be even higher today because more than 90% of patients in the study – the largest yet to look at outcome predictors in this population – were treated in the era before novel therapies became available.
Investigators led by Saad Z. Usmani, MD, director/chief of plasma cell disorders and director of clinical research (hematologic malignancies) at the Levine Cancer Institute/Atrium Health in Charlotte, N.C., studied 7,291 patients with newly diagnosed multiple myeloma who were up to 75 years old and eligible for high-dose melphalan and autologous stem cell transplant. The patients were treated in clinical trials in 10 countries.
Compared with counterparts who did not achieve complete response 1 year after diagnosis, patients who did had better median progression-free survival (3.3 vs. 2.6 years; P less than .0001) and median overall survival (8.5 vs. 6.3 years; P less than .0001), according to study results report in Blood Cancer Journal.
The investigators next performed multivariate analyses to assess clinical variables at diagnosis associated with 10-year survival as compared with 2-year death.
Results here indicated that patients were less likely to be alive at 10 years if they were older than 65 years (odds ratio for death, 1.87; P = .002); had an immunoglobulin A isotype (OR, 1.53; P = .004); had a low albumin level, defined as less than 3.5 g/dL (OR, 1.36; P = .023); had an elevated beta2-microglobulin level, defined as at least 3.5 mg/dL (OR, 1.86; P less than .001); had a higher serum creatinine level, defined as at least 2 mg/dL (OR, 1.77; P = .005); had a lower hemoglobin level, defined as less than 10 g/dL (OR, 1.55; P = .003); or had a lower platelet count, defined as less than 150,000/μL (OR, 2.26; P less than .001).
Cytogenetic abnormalities did not independently predict long-term survival, but these abnormalities were obtained only by conventional band karyotyping and were not available for some patients.
Overall, the cohort had a relative survival of about 0.9 when compared with the matched general population. With follow-up out to about 20 years, the cure fraction (proportion achieving or exceeding expected survival when compared with the matched general population) was 14.3%.
Identification of early complete response as a predictor of long-term survival “underscores the importance of depth of response as we explore novel regimens for newly diagnosed [multiple myeloma] along with [minimal residual disease] endpoints,” Dr. Usmani and his colleagues wrote while acknowledging that the patients studied were a selected group eligible for transplant and treated on trials.
Recent therapeutic advances “have reignited the debate on possible functional curability of a subset MM patients,” they noted. “[T]here are perhaps more effective drugs and drug classes in the clinician’s armamentarium than [were] available for MM patients being treated in the 1990s or even early 2000s. This may mean that the depth of response after induction therapy may continue to improve over time, potentially further improving the PFS/OS of [the] biologic subset who previously achieved [partial response] yet had good long-term survival.”
Dr. Usmani disclosed that he is a consultant for AbbVie, Amgen, BMS, Celgene, Janssen, Takeda, Sanofi, and SkylineDx; receives speaker’s fees for Amgen, Celgene, Janssen, and Takeda; and receives research funding from Amgen, Array Biopharma, BMS, Celgene, Janssen, Pharmacyclics, Sanofi, and Takeda.
SOURCE: Usmani SZ et al. Blood Cancer J. 2018 Nov 23;8(12):123..
FROM BLOOD CANCER JOURNAL
Key clinical point: Some patients with newly diagnosed multiple myeloma eligible for transplant are likely now being cured.
Major finding: The cure fraction (proportion of patients achieving or exceeding expected survival compared with the matched general population) was 14.3%.
Study details: An international retrospective cohort study of 7,291 patients with newly diagnosed multiple myeloma eligible for high-dose melphalan and autologous stem cell transplant who were treated in clinical trials.
Disclosures: Dr. Usmani disclosed that he is a consultant for AbbVie, Amgen, BMS, Celgene, Janssen, Takeda, Sanofi, and SkylineDx; receives speaker’s fees for Amgen, Celgene, Janssen, and Takeda; and receives research funding from Amgen, Array Biopharma, BMS, Celgene, Janssen, Pharmacyclics, Sanofi, and Takeda.
Source: Usmani SZ et al. Blood Cancer J. 2018 Nov 23;8(12):123.
ASH releases new VTE guidelines
The new guidelines, released on Nov. 27, contain more than 150 individual recommendations, including sections devoted to managing venous thromboembolism (VTE) during pregnancy and in pediatric patients. Guideline highlights cited by some of the writing-panel participants included a high reliance on low-molecular-weight heparin (LMWH) agents as the preferred treatment for many patients, reliance on the D-dimer test to rule out VTE in patients with a low pretest probability of disease, and reliance on the 4Ts score to identify patients with heparin-induced thrombocytopenia.
The guidelines took more than 3 years to develop, an effort that began in 2015.
An updated set of VTE guidelines were needed because clinicians now have a “greater understanding of risk factors” for VTE as well as having “more options available for treating VTE, including new medications,” Adam C. Cuker, MD, cochair of the guideline-writing group and a hematologist and thrombosis specialist at the University of Pennsylvania, Philadelphia, said during a webcast to unveil the new guidelines.
Prevention
For preventing VTE in hospitalized medical patients the guidelines recommended initial assessment of the patient’s risk for both VTE and bleeding. Patients with a high bleeding risk who need VTE prevention should preferentially receive mechanical prophylaxis, either compression stockings or pneumatic sleeves. But in patients with a high VTE risk and an “acceptable” bleeding risk, prophylaxis with an anticoagulant is preferred over mechanical measures, said Mary Cushman, MD, professor and medical director of the thrombosis and hemostasis program at the University of Vermont, Burlington.
For prevention of VTE in medical inpatients, LMWH is preferred over unfractionated heparin because of its once-daily dosing and fewer complications, said Dr. Cushman, a member of the writing group. The panel also endorsed LMWH over a direct-acting oral anticoagulant, both during hospitalization and following discharge. The guidelines for prevention in medical patients explicitly “recommended against” using a direct-acting oral anticoagulant “over other treatments” both for hospitalized medical patients and after discharge, and the guidelines further recommend against extended prophylaxis after discharge with any other anticoagulant.
Another important takeaway from the prevention section was a statement that combining both mechanical and medical prophylaxis was not needed for medical inpatients. And once patients are discharged, if they take a long air trip they have no need for compression stockings or aspirin if their risk for thrombosis is not elevated. People with a “substantially increased” thrombosis risk “may benefit” from compression stockings or treatment with LMWH, Dr. Cushman said.
Diagnosis
For diagnosis, Wendy Lim, MD, highlighted the need for first categorizing patients as having a low or high probability for VTE, a judgment that can aid the accuracy of the diagnosis and helps avoid unnecessary testing.
For patients with low pretest probability, the guidelines recommended the D-dimer test as the best first step. Further testing isn’t needed when the D-dimer is negative, noted Dr. Lim, a hematologist and professor at McMaster University, Hamilton, Ont.
The guidelines also recommended using ventilation-perfusion scintigraphy (V/Q scan) for imaging a pulmonary embolism over a CT scan, which uses more radiation. But V/Q scans are not ideal for assessing older patients or patients with lung disease, Dr. Lim cautioned.
Management
Management of VTE should occur, when feasible, through a specialized anticoagulation management service center, which can provide care that is best suited to the complexities of anticoagulation therapy. But it’s a level of care that many U.S. patients don’t currently receive and hence is an area ripe for growth, said Daniel M. Witt, PharmD, professor and vice-chair of pharmacotherapy at the University of Utah, Salt Lake City.
The guidelines recommended against bridging therapy with LMWH for most patients who need to stop warfarin when undergoing an invasive procedure. The guidelines also called for “thoughtful” use of anticoagulant reversal agents and advised that patients who survive a major bleed while on anticoagulation should often resume the anticoagulant once they are stabilized.
For patients who develop heparin-induced thrombocytopenia, the 4Ts score is the best way to make a more accurate diagnosis and boost the prospects for recovery, said Dr. Cuker (Blood. 2012 Nov 15;120[20]:4160-7). The guidelines cite several agents now available to treat this common complication, which affects about 1% of the 12 million Americans treated with heparin annually: argatroban, bivalirudin, danaparoid, fondaparinux, apixaban, dabigatran, edoxaban, and rivaroxaban.
ASH has a VTE website with links to detailed information for each of the guideline subcategories: prophylaxis in medical patients, diagnosis, therapy, heparin-induced thrombocytopenia, VTE in pregnancy, and VTE in children. The website indicates that additional guidelines will soon be released on managing VTE in patients with cancer, in patients with thrombophilia, and for prophylaxis in surgical patients, as well as further information on treatment. A spokesperson for ASH said that these additional documents will post sometime in 2019.
At the time of the release, the guidelines panel published six articles in the journal Blood Advances that detailed the guidelines and their documentation.
The articles include prophylaxis of medical patients (Blood Advances. 2018 Nov 27;2[22]:3198-225), diagnosis (Blood Advances. 2018 Nov 27;2[22]:3226-56), anticoagulation therapy (Blood Advances. 2018 Nov 27;2[22]:3257-91), pediatrics (Blood Advances. 2018 Nov 27;2[22]:3292-316), pregnancy (Blood Advances. 2018 Nov 27;2[22]:3317-59), and heparin-induced thrombocytopenia (Blood Advances. 2018 Nov 27;2[22]:3360-92).
Dr. Cushman, Dr. Lim, and Dr. Witt reported having no relevant disclosures. Dr. Cuker reported receiving research support from T2 Biosystems.
The new guidelines, released on Nov. 27, contain more than 150 individual recommendations, including sections devoted to managing venous thromboembolism (VTE) during pregnancy and in pediatric patients. Guideline highlights cited by some of the writing-panel participants included a high reliance on low-molecular-weight heparin (LMWH) agents as the preferred treatment for many patients, reliance on the D-dimer test to rule out VTE in patients with a low pretest probability of disease, and reliance on the 4Ts score to identify patients with heparin-induced thrombocytopenia.
The guidelines took more than 3 years to develop, an effort that began in 2015.
An updated set of VTE guidelines were needed because clinicians now have a “greater understanding of risk factors” for VTE as well as having “more options available for treating VTE, including new medications,” Adam C. Cuker, MD, cochair of the guideline-writing group and a hematologist and thrombosis specialist at the University of Pennsylvania, Philadelphia, said during a webcast to unveil the new guidelines.
Prevention
For preventing VTE in hospitalized medical patients the guidelines recommended initial assessment of the patient’s risk for both VTE and bleeding. Patients with a high bleeding risk who need VTE prevention should preferentially receive mechanical prophylaxis, either compression stockings or pneumatic sleeves. But in patients with a high VTE risk and an “acceptable” bleeding risk, prophylaxis with an anticoagulant is preferred over mechanical measures, said Mary Cushman, MD, professor and medical director of the thrombosis and hemostasis program at the University of Vermont, Burlington.
For prevention of VTE in medical inpatients, LMWH is preferred over unfractionated heparin because of its once-daily dosing and fewer complications, said Dr. Cushman, a member of the writing group. The panel also endorsed LMWH over a direct-acting oral anticoagulant, both during hospitalization and following discharge. The guidelines for prevention in medical patients explicitly “recommended against” using a direct-acting oral anticoagulant “over other treatments” both for hospitalized medical patients and after discharge, and the guidelines further recommend against extended prophylaxis after discharge with any other anticoagulant.
Another important takeaway from the prevention section was a statement that combining both mechanical and medical prophylaxis was not needed for medical inpatients. And once patients are discharged, if they take a long air trip they have no need for compression stockings or aspirin if their risk for thrombosis is not elevated. People with a “substantially increased” thrombosis risk “may benefit” from compression stockings or treatment with LMWH, Dr. Cushman said.
Diagnosis
For diagnosis, Wendy Lim, MD, highlighted the need for first categorizing patients as having a low or high probability for VTE, a judgment that can aid the accuracy of the diagnosis and helps avoid unnecessary testing.
For patients with low pretest probability, the guidelines recommended the D-dimer test as the best first step. Further testing isn’t needed when the D-dimer is negative, noted Dr. Lim, a hematologist and professor at McMaster University, Hamilton, Ont.
The guidelines also recommended using ventilation-perfusion scintigraphy (V/Q scan) for imaging a pulmonary embolism over a CT scan, which uses more radiation. But V/Q scans are not ideal for assessing older patients or patients with lung disease, Dr. Lim cautioned.
Management
Management of VTE should occur, when feasible, through a specialized anticoagulation management service center, which can provide care that is best suited to the complexities of anticoagulation therapy. But it’s a level of care that many U.S. patients don’t currently receive and hence is an area ripe for growth, said Daniel M. Witt, PharmD, professor and vice-chair of pharmacotherapy at the University of Utah, Salt Lake City.
The guidelines recommended against bridging therapy with LMWH for most patients who need to stop warfarin when undergoing an invasive procedure. The guidelines also called for “thoughtful” use of anticoagulant reversal agents and advised that patients who survive a major bleed while on anticoagulation should often resume the anticoagulant once they are stabilized.
For patients who develop heparin-induced thrombocytopenia, the 4Ts score is the best way to make a more accurate diagnosis and boost the prospects for recovery, said Dr. Cuker (Blood. 2012 Nov 15;120[20]:4160-7). The guidelines cite several agents now available to treat this common complication, which affects about 1% of the 12 million Americans treated with heparin annually: argatroban, bivalirudin, danaparoid, fondaparinux, apixaban, dabigatran, edoxaban, and rivaroxaban.
ASH has a VTE website with links to detailed information for each of the guideline subcategories: prophylaxis in medical patients, diagnosis, therapy, heparin-induced thrombocytopenia, VTE in pregnancy, and VTE in children. The website indicates that additional guidelines will soon be released on managing VTE in patients with cancer, in patients with thrombophilia, and for prophylaxis in surgical patients, as well as further information on treatment. A spokesperson for ASH said that these additional documents will post sometime in 2019.
At the time of the release, the guidelines panel published six articles in the journal Blood Advances that detailed the guidelines and their documentation.
The articles include prophylaxis of medical patients (Blood Advances. 2018 Nov 27;2[22]:3198-225), diagnosis (Blood Advances. 2018 Nov 27;2[22]:3226-56), anticoagulation therapy (Blood Advances. 2018 Nov 27;2[22]:3257-91), pediatrics (Blood Advances. 2018 Nov 27;2[22]:3292-316), pregnancy (Blood Advances. 2018 Nov 27;2[22]:3317-59), and heparin-induced thrombocytopenia (Blood Advances. 2018 Nov 27;2[22]:3360-92).
Dr. Cushman, Dr. Lim, and Dr. Witt reported having no relevant disclosures. Dr. Cuker reported receiving research support from T2 Biosystems.
The new guidelines, released on Nov. 27, contain more than 150 individual recommendations, including sections devoted to managing venous thromboembolism (VTE) during pregnancy and in pediatric patients. Guideline highlights cited by some of the writing-panel participants included a high reliance on low-molecular-weight heparin (LMWH) agents as the preferred treatment for many patients, reliance on the D-dimer test to rule out VTE in patients with a low pretest probability of disease, and reliance on the 4Ts score to identify patients with heparin-induced thrombocytopenia.
The guidelines took more than 3 years to develop, an effort that began in 2015.
An updated set of VTE guidelines were needed because clinicians now have a “greater understanding of risk factors” for VTE as well as having “more options available for treating VTE, including new medications,” Adam C. Cuker, MD, cochair of the guideline-writing group and a hematologist and thrombosis specialist at the University of Pennsylvania, Philadelphia, said during a webcast to unveil the new guidelines.
Prevention
For preventing VTE in hospitalized medical patients the guidelines recommended initial assessment of the patient’s risk for both VTE and bleeding. Patients with a high bleeding risk who need VTE prevention should preferentially receive mechanical prophylaxis, either compression stockings or pneumatic sleeves. But in patients with a high VTE risk and an “acceptable” bleeding risk, prophylaxis with an anticoagulant is preferred over mechanical measures, said Mary Cushman, MD, professor and medical director of the thrombosis and hemostasis program at the University of Vermont, Burlington.
For prevention of VTE in medical inpatients, LMWH is preferred over unfractionated heparin because of its once-daily dosing and fewer complications, said Dr. Cushman, a member of the writing group. The panel also endorsed LMWH over a direct-acting oral anticoagulant, both during hospitalization and following discharge. The guidelines for prevention in medical patients explicitly “recommended against” using a direct-acting oral anticoagulant “over other treatments” both for hospitalized medical patients and after discharge, and the guidelines further recommend against extended prophylaxis after discharge with any other anticoagulant.
Another important takeaway from the prevention section was a statement that combining both mechanical and medical prophylaxis was not needed for medical inpatients. And once patients are discharged, if they take a long air trip they have no need for compression stockings or aspirin if their risk for thrombosis is not elevated. People with a “substantially increased” thrombosis risk “may benefit” from compression stockings or treatment with LMWH, Dr. Cushman said.
Diagnosis
For diagnosis, Wendy Lim, MD, highlighted the need for first categorizing patients as having a low or high probability for VTE, a judgment that can aid the accuracy of the diagnosis and helps avoid unnecessary testing.
For patients with low pretest probability, the guidelines recommended the D-dimer test as the best first step. Further testing isn’t needed when the D-dimer is negative, noted Dr. Lim, a hematologist and professor at McMaster University, Hamilton, Ont.
The guidelines also recommended using ventilation-perfusion scintigraphy (V/Q scan) for imaging a pulmonary embolism over a CT scan, which uses more radiation. But V/Q scans are not ideal for assessing older patients or patients with lung disease, Dr. Lim cautioned.
Management
Management of VTE should occur, when feasible, through a specialized anticoagulation management service center, which can provide care that is best suited to the complexities of anticoagulation therapy. But it’s a level of care that many U.S. patients don’t currently receive and hence is an area ripe for growth, said Daniel M. Witt, PharmD, professor and vice-chair of pharmacotherapy at the University of Utah, Salt Lake City.
The guidelines recommended against bridging therapy with LMWH for most patients who need to stop warfarin when undergoing an invasive procedure. The guidelines also called for “thoughtful” use of anticoagulant reversal agents and advised that patients who survive a major bleed while on anticoagulation should often resume the anticoagulant once they are stabilized.
For patients who develop heparin-induced thrombocytopenia, the 4Ts score is the best way to make a more accurate diagnosis and boost the prospects for recovery, said Dr. Cuker (Blood. 2012 Nov 15;120[20]:4160-7). The guidelines cite several agents now available to treat this common complication, which affects about 1% of the 12 million Americans treated with heparin annually: argatroban, bivalirudin, danaparoid, fondaparinux, apixaban, dabigatran, edoxaban, and rivaroxaban.
ASH has a VTE website with links to detailed information for each of the guideline subcategories: prophylaxis in medical patients, diagnosis, therapy, heparin-induced thrombocytopenia, VTE in pregnancy, and VTE in children. The website indicates that additional guidelines will soon be released on managing VTE in patients with cancer, in patients with thrombophilia, and for prophylaxis in surgical patients, as well as further information on treatment. A spokesperson for ASH said that these additional documents will post sometime in 2019.
At the time of the release, the guidelines panel published six articles in the journal Blood Advances that detailed the guidelines and their documentation.
The articles include prophylaxis of medical patients (Blood Advances. 2018 Nov 27;2[22]:3198-225), diagnosis (Blood Advances. 2018 Nov 27;2[22]:3226-56), anticoagulation therapy (Blood Advances. 2018 Nov 27;2[22]:3257-91), pediatrics (Blood Advances. 2018 Nov 27;2[22]:3292-316), pregnancy (Blood Advances. 2018 Nov 27;2[22]:3317-59), and heparin-induced thrombocytopenia (Blood Advances. 2018 Nov 27;2[22]:3360-92).
Dr. Cushman, Dr. Lim, and Dr. Witt reported having no relevant disclosures. Dr. Cuker reported receiving research support from T2 Biosystems.
Patterns of malignancies in patients with HIV-AIDS: a single institution observational study
India has the third largest HIV epidemic in the world because of its large population size, with 0.3% of the adult population infected with HIV. That translates to 2.1 million infected people, posing a significant challenge in the management of these individuals.1 In all, 43% of the infected are currently on highly active antiretroviral therapy (HAART).1 There has been a significant decrease in the number of HIV-AIDS–related deaths in recent years because of the remarkable increase in the use of antiretroviral therapy.2 However, the prolonged life expectancy in these patients has resulted in an increase in the risk of various new diseases such as cancers. With the complex interactions between altered immunity and infections, the risk of cancers is markedly increased in patients with HIV-AIDS.3 The spectrum of malignancies in this group of patients differs from that in the general population. In addition, the pattern and the magnitude of malignancies differ in different parts of the world.4 In this study, we have analyzed the pattern of malignancies in patients with HIV-AIDS in a regional cancer center in India. The aim of the study was to analyze the pattern of malignancies in patients with HIV-AIDS based on their age and sex and to document the CD4 counts at the time the malignancy was diagnosed.
Methods
We retrieved data from our institution’s medical records department on all patients who had HIV-AIDS and had been diagnosed with a malignancy. Data of all patients presenting with a malignancy and coexisting HIV-AIDS from January 2013 through December 2016 were analyzed initially. Only patients for whom there was a documented CD4 count were included in the final retrospective analysis. We analyzed the correlation between the patients’ CD4 counts and malignancies subclassified as AIDS-defining malignancies (ADMs; aggressive B-cell non-Hodgkin lymphoma [NHL] and cervical cancer) or non–AIDS-defining malignancies (NADMs; all other malignancies other than aggressive NHL and carcinoma cervix were defined as NADM). We also analyzed the correlation between the CD4 count and NHL and other malignancies. A statistical analysis was performed using SPSS Statistics for Windows, version 23 (IBM Corp, Armonk, NY). The independent sample Mann-Whitney U or Kruskal-Wallis tests were used for comparing the CD4 counts between the various subgroups of malignancies. The study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Results
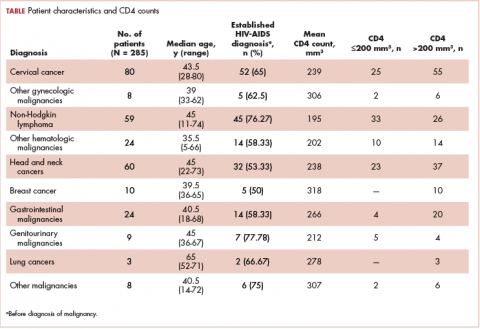
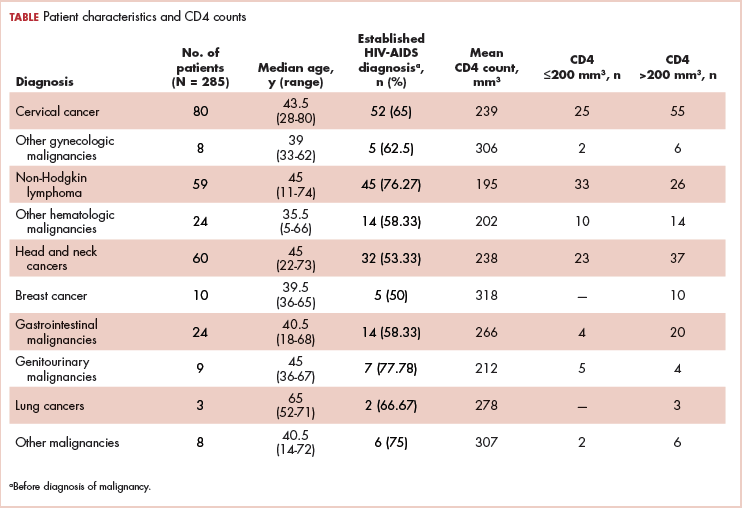
A total of 370 patients who were diagnosed with malignancy and have coexisting HIV-AIDS were identified. In all, 85 patients were excluded because there were no CD4 counts available for them, and the remaining 285 patients were included in the final analysis. Of that total, 136 patients (48%) were men, and 149 (52%) were women.
The median age of the population was 44.8 years (5-80 years) at the time of diagnosis with malignancy. The mean CD4 count of the entire population was 235.4 cells/mm3 (50-734 cells/mm3). There were 104 patients with CD4 counts of ≤200 cells/mm3, and 181 patients had CD4 counts of >200 cells/mm3 (Table 1). All patients received the HAART regimen, efavirenz-lamuvidine-tenofovir (600 mg/300 mg/300 mg Telura).
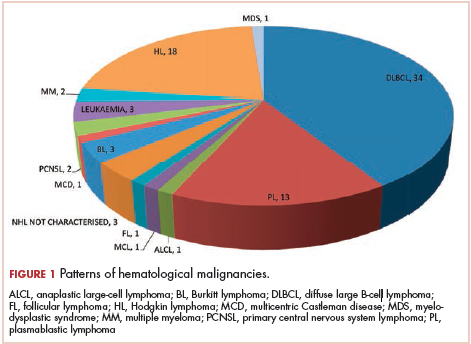
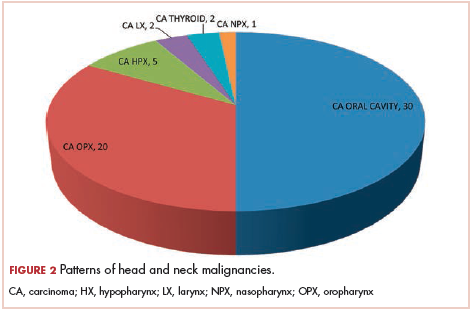
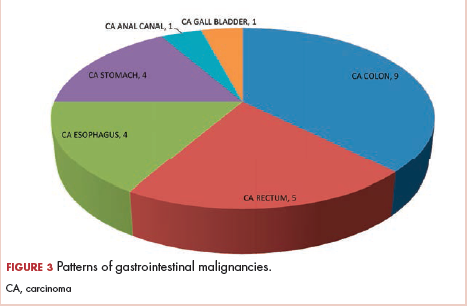
The most common malignancies in this population were gynecologic malignancies, followed by hematologic malignancies. Cervical cancer was the most common malignancy among women as well as in the overall study population. Among men, the most common malignancy was NHL. The second and third most common malignancies in men were carcinoma oral cavity and carcinoma oropharynx, respectively, whereas in women, they were NHL and breast cancer. The distribution of various hematologic, head and neck, and gastrointestinal malignancies in this group of patients is shown in Figures 1, 2, and 3.
The ADMs in the study were NHL, including 2 patients diagnosed with primary central nervous system (CNS) lymphomas, and cervical cancer. No case of Kaposi sarcoma, also considered an ADM, was identified in this study. The common NADMs include head and neck malignancies (Figure 2), gastrointestinal malignancies (Figure 3), gynecological and genitourinary malignancies, and breast cancer. The mean CD4 count in the ADM subgroup was 221 cells/mm3, and in the NADM subgroup, it was 250 cells/mm3. There was a significant difference in the distribution of CD4 counts between the ADM and NADM subgroups (P = .03; Mann-Whitney U test). A statistical difference was also noted when the CD4 counts of the patients with NHL were compared with other malignancies (P = .0001; Mann-Whitney U test) There was no statistically significant difference noted when CD4 counts of patients with cervical cancer were compared with NADMs (P = .914).
Discussion
In 2015, a report from the Indian government estimated the prevalence of HIV in the country as 0.26% (0.22%-0.32%).5 The report also noted a decreasing trend in the number of new cases of HIV diagnosed and a decrease in the number of AIDS-related deaths.5 The decrease in deaths from AIDS is primarily attributed to the widespread use of HAART. With the introduction of HAART therapy, the survival of patients diagnosed with HIV-AIDS has increased markedly.6 However, newer challenges have emerged with improved survival, such as an increasing number of patients being diagnosed with malignancies. In the current HAART era, the pattern of malignancies in people living with HIV-AIDS has changed compared with the pre-HAART era.7 The literature suggests that worldwide, malignancies are encountered in about 30% patients with HIV-AIDS, but that percentage differs sharply from that encountered in India, where it is less than 5%.8 This may partly be explained by opportunistic infections such as tuberculosis in Indian patients, which remains the leading cause of death in the HIV-AIDS population. In our study, we retrospectively analyzed the pattern of malignancies in patients with HIV-AIDS.
Although few studies have quoted NHL as the predominant malignancy in their patients with HIV-AIDS, the predominant malignancy was cervical cancer in our patient population, as seen in few other studies.8-10 Head and neck malignancies also continue to be common malignancies in men with HIV-AIDS.10 Thus, an increase in malignancies induced by the human papillomavirus (HPV) can be seen in this group of patients. Only a few pediatric malignancies were noted in our study, and all of those patients had a vertical transmission of HIV.
Kaposi sarcoma is quite rare in the Indian population, and no case of Kaposi sarcoma was diagnosed in our study population. A similar finding was seen in several earlier publications from India. In the largest published series from India by Dhir and colleagues, evaluating 251 patients with HIV-AIDS and malignancy, no case of Kaposi sarcoma was reported.10 The authors mentioned that this finding might be because of the low seroprevalence of Kaposi sarcoma-associated herpesvirus in the Asian population.10 Three different studies from southern India have also not reported the incidence of Kaposi Sarcoma in their series of HIV-AIDS patients with malignancies,11-13 and similar findings were also reported in a study from northern India.9 The incidence of other immunodeficiency-related malignancies was identical to those reported in other studies in the literature.10,14
As seen in other studies, the CD4 counts in patients with ADM were significantly lower compared with those of patients with NADM, and that difference was not seen when CD4 counts of patients with cervical cancer were compared with patients in the NADM subgroup. The risk of NHL increases proportionally to the degree of immune suppression. The increased susceptibility to various infections in patients with low CD4 counts may also contribute to the occurrence of NHL in patients with low CD4 counts. The occurrence of various other rare cancers in patients with HIV-AIDS may be because of confounding rather than a direct HIV or immunosuppression effect.
An increasing incidence of NADMs has been noted in the Western literature.7,14 ADMs remain the most common malignancies in the HIV-AIDS population, accounting for about 48% of all malignancies.8 This is in concordance with previous publications from India.8,10 With the widespread availability of generic HAART, the incidence of ADMs may decrease even more in the future. In developing countries where the screening procedures for malignancies in both the general population and patients with HIV-AIDS have not yet been implemented at a national level, premalignant lesions of the cervix are not detected.10 Cervical cancer is the most common malignancy in our study population, which underscores the importance of cervical cancer screening in patients with HIV-AIDS.
In the developed countries, following the introduction of HAART in HIV-AIDS management, the incidence of Kaposi sarcoma decreased by 60% to 70%, and the incidence of NHL decreased by 30% to 50%, whereas the rates of cervical cancer remained either stable or declined.15,16 Despite the declining trend, the incidence of these malignancies continues to be high among patients with HIV-AIDS compared with the general population.17 A study from the United States showed increasing trends in various NADMs (such as anal, lung, and liver cancers and Hodgkin lymphoma) from 2006 to 2010.17 In 2003, the number of patients with NADM were higher than the number of patients with ADM in the United States.14 In a population-based study from Brazil, ADMs were the most common malignancies diagnosed in patients with HIV-AIDS. A declining trend was noted in the incidence of ADMs in the population and an increasing trend in the incidence of NADMs. This increase in NADM incidence was contributed by anal and lung cancers.18 Studies from developing countries such as Uganda and Botswana have also shown a decrease in the incidence of Kaposi sarcoma after the introduction of HAART.19-21
Kaposi sarcoma, cervical cancer, NHL (including Burkitt lymphoma, immunoblastic lymphoma, and primary CNS lymphoma [PCNSL]) comprise ADMs. All 3 ADMs have an underlying viral infection as the causative agent.22 Kaposi sarcoma is caused by the Kaposi sarcoma herpes virus, for which seroprevalence varies worldwide.23 As already noted in this article, the incidence of Kaposi sarcoma among the HIV-AIDS population has decreased worldwide since the introduction of HAART. The preinvasive uterine cervix lesions and carcinoma cervix are caused by HPV. NHL in patients with HIV-AIDS is a predominantly aggressive B-cell neoplasm. Epstein-Barr virus is implicated for most of the ADM NHLs.24 PCNSL occurs in patients with low CD4 counts and poses a diagnostic challenge. The treatment outcomes for patients with PCNSL before the HAART era were dismal. With the widespread use of HAART, the treatment outcomes of patients with HIV-AIDS and NHL improved, and, currently, these patients are managed the same way as other patients with NHL.22
The increasing incidence of the NADM is partly attributed to the increasing incidence of these malignancies in the general population. An elevated risk of certain NADMs is also attributable to viral infections. The common NADMs in the United States are lung, anal, oropharyngeal, and hepatocellular cancers and Hodgkin lymphoma.14 The common NADMs in our study population were oral, oropharyngeal, colon, and breast cancers and Hodgkin lymphoma. One-third of head and neck cancers, including most oropharyngeal cancers, and cervical and anal cancers in patients with HIV-AIDS are related to HPV.25 Patients with HIV-AIDS are at increased risk for chronic HPV infection from immunosuppression. Chronic HPV infections and prolonged immunosuppression cause premalignant high-grade squamous intraepithelial lesions and invasive cancers.22 The initiation of and adherence to HAART leads to immune recovery and reduces high-risk HPV-associated morbidity.26 Findings from previous studies have demonstrated the benefits of screening for cervical cancer in patients with HIV-AIDS.27 The HPV vaccine is immunogenic in patients with HIV-AIDS and might help prevent HPV-associated malignancies.28
Conclusions
With the wide use of HAART by patients with HIV-AIDS, we can expect an increase in the survival of that population. The incidence of malignancies may also increase significantly in these patients, and further longitudinal studies are needed, as malignancies may emerge as the most common cause of death in patients with HIV-AIDS. In addition, the extensive use of HAART therapy and implementation of screening programs for cervical cancer in patients with HIV-AIDS could result in a decrease in the incidence of ADMs.
1. UNAIDS. Prevention gap report. http://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf. Released 2016. Accessed December 27, 2017.
3. Dubrow R, Silverberg MJ, Park LS, Crothers K, Justice AC. HIV infection, aging, and immune function: implications for cancer risk and prevention. Curr Opin Oncol. 2012;24(5):506-516.
4. Biggar RJ, Chaturvedi AK, Bhatia K, Mbulaiteye SM. Cancer risk in persons with HIV-AIDS in India: a review and future directions for research. Infect Agent Cancer. 2009;4:4.
5. National AIDS Control Organisation & National Institute of Medical Statistics, ICMR, Ministry of Health & Family Welfare, Government of India. India HIV estimations 2015, technical report. http://www.naco.gov.in/sites/default/files/India%20HIV%20Estimations%202015.pdf. Published 2015. Accessed December 27, 2017.
6. Bonnet F, Lewden C, May T, et al. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101(2):317-324.
7. Crum-Cianflone N, Hullsiek KH, Marconi V, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23(1):41-50.
8. Sharma S, Soneja M, Ranjan S. Malignancies in human immunodeficiency virus infected patients in India: initial experience in the HAART era. Indian J Med Res. 2015;142(5):563-567.
9. Sachdeva RK, Sharma A, Singh S, Varma S. Spectrum of AIDS defining & non-AIDS defining malignancies in north India. In
10. Dhir AA, Sawant S, Dikshit RP, et al. Spectrum of HIV-AIDS related cancers in India. Cancer Causes Control. 2007;19(2):147-153.
11. Venkatesh KK, Saghayam S, Devaleenal B, et al. Spectrum of malignancies among HIV-infected patients in South India. Indian J Cancer. 2012;49(1):176-180.
12. Shruti P, Narayanan G, Puthuveettil J, Jayasree K, Vijayalakshmi K. Spectrum of HIV/AIDS-associated cancers in south India. J Clin Oncol. 2014;32(suppl):e12534.
13. Paul TR, Uppin MS, Uppin SG, et al. Spectrum of malignancies in human immunodeficiency virus–positive patients at a Tertiary Care Centre in South India. Indian J Cancer. 2014;51(4):459-463.
14. Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753-762.
15. Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148(10):728-736.
16. Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187-194.
17. Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. AIDS. 2014;28(6):881-890.
18. Tanaka LF, Latorre MDRD, Gutierrez EB, Heumann C, Herbinger KH, Froeschl G. Trends in the incidence of AIDS-defining and non-AIDS-defining cancers in people living with AIDS: a population-based study from São Paulo, Brazil. Int J STD AIDS. 2017;28(12):1190-1198.
19. Mutyaba I, Phipps W, Krantz EM, et al. A population-level evaluation of the effect of antiretroviral therapy on cancer incidence in Kyadondo County, Uganda, 1999–2008. J Acquir Immune Defic Syndr. 2015;69(4):481-486.
20. Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al. Cancer incidence following expansion of HIV treatment in Botswana. PLoS ONE. 2015;10(8):e0135602.
21. Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12(1):6-11.
22. Yarchoan R, Uldrick TS. HIV-associated cancers and related diseases. N Engl J Med. 2018;378(11):1029-1041.
23. Gao SJ, Kingsley L, Li M, et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996;2(8):925-928.
24. Epstein-Barr virus and AIDS-associated lymphomas. Lancet. 1991;338(8773):979-981.
25. Picard A, Badoual C, Hourseau M, et al. Human papilloma virus prevalence in HIV patients with head and neck squamous cell carcinoma. AIDS. 2016;30(8):1257-1266.
26. Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Infect Dis. 2010;201(5):681-690.
27. Ghebre RG, Grover S, Xu MJ, Chuang LT, Simonds H. Cervical cancer control in HIV-infected women: past, present and future. Gynecol Oncol Rep. 2017;21:101-108.
28. Kojic EM, Rana AI, Cu-Uvin S. Human papillomavirus vaccination in HIV-infected women: need for increased coverage. Expert Rev Vaccines. 2016;15(1):105-117.
India has the third largest HIV epidemic in the world because of its large population size, with 0.3% of the adult population infected with HIV. That translates to 2.1 million infected people, posing a significant challenge in the management of these individuals.1 In all, 43% of the infected are currently on highly active antiretroviral therapy (HAART).1 There has been a significant decrease in the number of HIV-AIDS–related deaths in recent years because of the remarkable increase in the use of antiretroviral therapy.2 However, the prolonged life expectancy in these patients has resulted in an increase in the risk of various new diseases such as cancers. With the complex interactions between altered immunity and infections, the risk of cancers is markedly increased in patients with HIV-AIDS.3 The spectrum of malignancies in this group of patients differs from that in the general population. In addition, the pattern and the magnitude of malignancies differ in different parts of the world.4 In this study, we have analyzed the pattern of malignancies in patients with HIV-AIDS in a regional cancer center in India. The aim of the study was to analyze the pattern of malignancies in patients with HIV-AIDS based on their age and sex and to document the CD4 counts at the time the malignancy was diagnosed.
Methods
We retrieved data from our institution’s medical records department on all patients who had HIV-AIDS and had been diagnosed with a malignancy. Data of all patients presenting with a malignancy and coexisting HIV-AIDS from January 2013 through December 2016 were analyzed initially. Only patients for whom there was a documented CD4 count were included in the final retrospective analysis. We analyzed the correlation between the patients’ CD4 counts and malignancies subclassified as AIDS-defining malignancies (ADMs; aggressive B-cell non-Hodgkin lymphoma [NHL] and cervical cancer) or non–AIDS-defining malignancies (NADMs; all other malignancies other than aggressive NHL and carcinoma cervix were defined as NADM). We also analyzed the correlation between the CD4 count and NHL and other malignancies. A statistical analysis was performed using SPSS Statistics for Windows, version 23 (IBM Corp, Armonk, NY). The independent sample Mann-Whitney U or Kruskal-Wallis tests were used for comparing the CD4 counts between the various subgroups of malignancies. The study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Results
A total of 370 patients who were diagnosed with malignancy and have coexisting HIV-AIDS were identified. In all, 85 patients were excluded because there were no CD4 counts available for them, and the remaining 285 patients were included in the final analysis. Of that total, 136 patients (48%) were men, and 149 (52%) were women.
The median age of the population was 44.8 years (5-80 years) at the time of diagnosis with malignancy. The mean CD4 count of the entire population was 235.4 cells/mm3 (50-734 cells/mm3). There were 104 patients with CD4 counts of ≤200 cells/mm3, and 181 patients had CD4 counts of >200 cells/mm3 (Table 1). All patients received the HAART regimen, efavirenz-lamuvidine-tenofovir (600 mg/300 mg/300 mg Telura).
The most common malignancies in this population were gynecologic malignancies, followed by hematologic malignancies. Cervical cancer was the most common malignancy among women as well as in the overall study population. Among men, the most common malignancy was NHL. The second and third most common malignancies in men were carcinoma oral cavity and carcinoma oropharynx, respectively, whereas in women, they were NHL and breast cancer. The distribution of various hematologic, head and neck, and gastrointestinal malignancies in this group of patients is shown in Figures 1, 2, and 3.
The ADMs in the study were NHL, including 2 patients diagnosed with primary central nervous system (CNS) lymphomas, and cervical cancer. No case of Kaposi sarcoma, also considered an ADM, was identified in this study. The common NADMs include head and neck malignancies (Figure 2), gastrointestinal malignancies (Figure 3), gynecological and genitourinary malignancies, and breast cancer. The mean CD4 count in the ADM subgroup was 221 cells/mm3, and in the NADM subgroup, it was 250 cells/mm3. There was a significant difference in the distribution of CD4 counts between the ADM and NADM subgroups (P = .03; Mann-Whitney U test). A statistical difference was also noted when the CD4 counts of the patients with NHL were compared with other malignancies (P = .0001; Mann-Whitney U test) There was no statistically significant difference noted when CD4 counts of patients with cervical cancer were compared with NADMs (P = .914).
Discussion
In 2015, a report from the Indian government estimated the prevalence of HIV in the country as 0.26% (0.22%-0.32%).5 The report also noted a decreasing trend in the number of new cases of HIV diagnosed and a decrease in the number of AIDS-related deaths.5 The decrease in deaths from AIDS is primarily attributed to the widespread use of HAART. With the introduction of HAART therapy, the survival of patients diagnosed with HIV-AIDS has increased markedly.6 However, newer challenges have emerged with improved survival, such as an increasing number of patients being diagnosed with malignancies. In the current HAART era, the pattern of malignancies in people living with HIV-AIDS has changed compared with the pre-HAART era.7 The literature suggests that worldwide, malignancies are encountered in about 30% patients with HIV-AIDS, but that percentage differs sharply from that encountered in India, where it is less than 5%.8 This may partly be explained by opportunistic infections such as tuberculosis in Indian patients, which remains the leading cause of death in the HIV-AIDS population. In our study, we retrospectively analyzed the pattern of malignancies in patients with HIV-AIDS.
Although few studies have quoted NHL as the predominant malignancy in their patients with HIV-AIDS, the predominant malignancy was cervical cancer in our patient population, as seen in few other studies.8-10 Head and neck malignancies also continue to be common malignancies in men with HIV-AIDS.10 Thus, an increase in malignancies induced by the human papillomavirus (HPV) can be seen in this group of patients. Only a few pediatric malignancies were noted in our study, and all of those patients had a vertical transmission of HIV.
Kaposi sarcoma is quite rare in the Indian population, and no case of Kaposi sarcoma was diagnosed in our study population. A similar finding was seen in several earlier publications from India. In the largest published series from India by Dhir and colleagues, evaluating 251 patients with HIV-AIDS and malignancy, no case of Kaposi sarcoma was reported.10 The authors mentioned that this finding might be because of the low seroprevalence of Kaposi sarcoma-associated herpesvirus in the Asian population.10 Three different studies from southern India have also not reported the incidence of Kaposi Sarcoma in their series of HIV-AIDS patients with malignancies,11-13 and similar findings were also reported in a study from northern India.9 The incidence of other immunodeficiency-related malignancies was identical to those reported in other studies in the literature.10,14
As seen in other studies, the CD4 counts in patients with ADM were significantly lower compared with those of patients with NADM, and that difference was not seen when CD4 counts of patients with cervical cancer were compared with patients in the NADM subgroup. The risk of NHL increases proportionally to the degree of immune suppression. The increased susceptibility to various infections in patients with low CD4 counts may also contribute to the occurrence of NHL in patients with low CD4 counts. The occurrence of various other rare cancers in patients with HIV-AIDS may be because of confounding rather than a direct HIV or immunosuppression effect.
An increasing incidence of NADMs has been noted in the Western literature.7,14 ADMs remain the most common malignancies in the HIV-AIDS population, accounting for about 48% of all malignancies.8 This is in concordance with previous publications from India.8,10 With the widespread availability of generic HAART, the incidence of ADMs may decrease even more in the future. In developing countries where the screening procedures for malignancies in both the general population and patients with HIV-AIDS have not yet been implemented at a national level, premalignant lesions of the cervix are not detected.10 Cervical cancer is the most common malignancy in our study population, which underscores the importance of cervical cancer screening in patients with HIV-AIDS.
In the developed countries, following the introduction of HAART in HIV-AIDS management, the incidence of Kaposi sarcoma decreased by 60% to 70%, and the incidence of NHL decreased by 30% to 50%, whereas the rates of cervical cancer remained either stable or declined.15,16 Despite the declining trend, the incidence of these malignancies continues to be high among patients with HIV-AIDS compared with the general population.17 A study from the United States showed increasing trends in various NADMs (such as anal, lung, and liver cancers and Hodgkin lymphoma) from 2006 to 2010.17 In 2003, the number of patients with NADM were higher than the number of patients with ADM in the United States.14 In a population-based study from Brazil, ADMs were the most common malignancies diagnosed in patients with HIV-AIDS. A declining trend was noted in the incidence of ADMs in the population and an increasing trend in the incidence of NADMs. This increase in NADM incidence was contributed by anal and lung cancers.18 Studies from developing countries such as Uganda and Botswana have also shown a decrease in the incidence of Kaposi sarcoma after the introduction of HAART.19-21
Kaposi sarcoma, cervical cancer, NHL (including Burkitt lymphoma, immunoblastic lymphoma, and primary CNS lymphoma [PCNSL]) comprise ADMs. All 3 ADMs have an underlying viral infection as the causative agent.22 Kaposi sarcoma is caused by the Kaposi sarcoma herpes virus, for which seroprevalence varies worldwide.23 As already noted in this article, the incidence of Kaposi sarcoma among the HIV-AIDS population has decreased worldwide since the introduction of HAART. The preinvasive uterine cervix lesions and carcinoma cervix are caused by HPV. NHL in patients with HIV-AIDS is a predominantly aggressive B-cell neoplasm. Epstein-Barr virus is implicated for most of the ADM NHLs.24 PCNSL occurs in patients with low CD4 counts and poses a diagnostic challenge. The treatment outcomes for patients with PCNSL before the HAART era were dismal. With the widespread use of HAART, the treatment outcomes of patients with HIV-AIDS and NHL improved, and, currently, these patients are managed the same way as other patients with NHL.22
The increasing incidence of the NADM is partly attributed to the increasing incidence of these malignancies in the general population. An elevated risk of certain NADMs is also attributable to viral infections. The common NADMs in the United States are lung, anal, oropharyngeal, and hepatocellular cancers and Hodgkin lymphoma.14 The common NADMs in our study population were oral, oropharyngeal, colon, and breast cancers and Hodgkin lymphoma. One-third of head and neck cancers, including most oropharyngeal cancers, and cervical and anal cancers in patients with HIV-AIDS are related to HPV.25 Patients with HIV-AIDS are at increased risk for chronic HPV infection from immunosuppression. Chronic HPV infections and prolonged immunosuppression cause premalignant high-grade squamous intraepithelial lesions and invasive cancers.22 The initiation of and adherence to HAART leads to immune recovery and reduces high-risk HPV-associated morbidity.26 Findings from previous studies have demonstrated the benefits of screening for cervical cancer in patients with HIV-AIDS.27 The HPV vaccine is immunogenic in patients with HIV-AIDS and might help prevent HPV-associated malignancies.28
Conclusions
With the wide use of HAART by patients with HIV-AIDS, we can expect an increase in the survival of that population. The incidence of malignancies may also increase significantly in these patients, and further longitudinal studies are needed, as malignancies may emerge as the most common cause of death in patients with HIV-AIDS. In addition, the extensive use of HAART therapy and implementation of screening programs for cervical cancer in patients with HIV-AIDS could result in a decrease in the incidence of ADMs.
India has the third largest HIV epidemic in the world because of its large population size, with 0.3% of the adult population infected with HIV. That translates to 2.1 million infected people, posing a significant challenge in the management of these individuals.1 In all, 43% of the infected are currently on highly active antiretroviral therapy (HAART).1 There has been a significant decrease in the number of HIV-AIDS–related deaths in recent years because of the remarkable increase in the use of antiretroviral therapy.2 However, the prolonged life expectancy in these patients has resulted in an increase in the risk of various new diseases such as cancers. With the complex interactions between altered immunity and infections, the risk of cancers is markedly increased in patients with HIV-AIDS.3 The spectrum of malignancies in this group of patients differs from that in the general population. In addition, the pattern and the magnitude of malignancies differ in different parts of the world.4 In this study, we have analyzed the pattern of malignancies in patients with HIV-AIDS in a regional cancer center in India. The aim of the study was to analyze the pattern of malignancies in patients with HIV-AIDS based on their age and sex and to document the CD4 counts at the time the malignancy was diagnosed.
Methods
We retrieved data from our institution’s medical records department on all patients who had HIV-AIDS and had been diagnosed with a malignancy. Data of all patients presenting with a malignancy and coexisting HIV-AIDS from January 2013 through December 2016 were analyzed initially. Only patients for whom there was a documented CD4 count were included in the final retrospective analysis. We analyzed the correlation between the patients’ CD4 counts and malignancies subclassified as AIDS-defining malignancies (ADMs; aggressive B-cell non-Hodgkin lymphoma [NHL] and cervical cancer) or non–AIDS-defining malignancies (NADMs; all other malignancies other than aggressive NHL and carcinoma cervix were defined as NADM). We also analyzed the correlation between the CD4 count and NHL and other malignancies. A statistical analysis was performed using SPSS Statistics for Windows, version 23 (IBM Corp, Armonk, NY). The independent sample Mann-Whitney U or Kruskal-Wallis tests were used for comparing the CD4 counts between the various subgroups of malignancies. The study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Results
A total of 370 patients who were diagnosed with malignancy and have coexisting HIV-AIDS were identified. In all, 85 patients were excluded because there were no CD4 counts available for them, and the remaining 285 patients were included in the final analysis. Of that total, 136 patients (48%) were men, and 149 (52%) were women.
The median age of the population was 44.8 years (5-80 years) at the time of diagnosis with malignancy. The mean CD4 count of the entire population was 235.4 cells/mm3 (50-734 cells/mm3). There were 104 patients with CD4 counts of ≤200 cells/mm3, and 181 patients had CD4 counts of >200 cells/mm3 (Table 1). All patients received the HAART regimen, efavirenz-lamuvidine-tenofovir (600 mg/300 mg/300 mg Telura).
The most common malignancies in this population were gynecologic malignancies, followed by hematologic malignancies. Cervical cancer was the most common malignancy among women as well as in the overall study population. Among men, the most common malignancy was NHL. The second and third most common malignancies in men were carcinoma oral cavity and carcinoma oropharynx, respectively, whereas in women, they were NHL and breast cancer. The distribution of various hematologic, head and neck, and gastrointestinal malignancies in this group of patients is shown in Figures 1, 2, and 3.
The ADMs in the study were NHL, including 2 patients diagnosed with primary central nervous system (CNS) lymphomas, and cervical cancer. No case of Kaposi sarcoma, also considered an ADM, was identified in this study. The common NADMs include head and neck malignancies (Figure 2), gastrointestinal malignancies (Figure 3), gynecological and genitourinary malignancies, and breast cancer. The mean CD4 count in the ADM subgroup was 221 cells/mm3, and in the NADM subgroup, it was 250 cells/mm3. There was a significant difference in the distribution of CD4 counts between the ADM and NADM subgroups (P = .03; Mann-Whitney U test). A statistical difference was also noted when the CD4 counts of the patients with NHL were compared with other malignancies (P = .0001; Mann-Whitney U test) There was no statistically significant difference noted when CD4 counts of patients with cervical cancer were compared with NADMs (P = .914).
Discussion
In 2015, a report from the Indian government estimated the prevalence of HIV in the country as 0.26% (0.22%-0.32%).5 The report also noted a decreasing trend in the number of new cases of HIV diagnosed and a decrease in the number of AIDS-related deaths.5 The decrease in deaths from AIDS is primarily attributed to the widespread use of HAART. With the introduction of HAART therapy, the survival of patients diagnosed with HIV-AIDS has increased markedly.6 However, newer challenges have emerged with improved survival, such as an increasing number of patients being diagnosed with malignancies. In the current HAART era, the pattern of malignancies in people living with HIV-AIDS has changed compared with the pre-HAART era.7 The literature suggests that worldwide, malignancies are encountered in about 30% patients with HIV-AIDS, but that percentage differs sharply from that encountered in India, where it is less than 5%.8 This may partly be explained by opportunistic infections such as tuberculosis in Indian patients, which remains the leading cause of death in the HIV-AIDS population. In our study, we retrospectively analyzed the pattern of malignancies in patients with HIV-AIDS.
Although few studies have quoted NHL as the predominant malignancy in their patients with HIV-AIDS, the predominant malignancy was cervical cancer in our patient population, as seen in few other studies.8-10 Head and neck malignancies also continue to be common malignancies in men with HIV-AIDS.10 Thus, an increase in malignancies induced by the human papillomavirus (HPV) can be seen in this group of patients. Only a few pediatric malignancies were noted in our study, and all of those patients had a vertical transmission of HIV.
Kaposi sarcoma is quite rare in the Indian population, and no case of Kaposi sarcoma was diagnosed in our study population. A similar finding was seen in several earlier publications from India. In the largest published series from India by Dhir and colleagues, evaluating 251 patients with HIV-AIDS and malignancy, no case of Kaposi sarcoma was reported.10 The authors mentioned that this finding might be because of the low seroprevalence of Kaposi sarcoma-associated herpesvirus in the Asian population.10 Three different studies from southern India have also not reported the incidence of Kaposi Sarcoma in their series of HIV-AIDS patients with malignancies,11-13 and similar findings were also reported in a study from northern India.9 The incidence of other immunodeficiency-related malignancies was identical to those reported in other studies in the literature.10,14
As seen in other studies, the CD4 counts in patients with ADM were significantly lower compared with those of patients with NADM, and that difference was not seen when CD4 counts of patients with cervical cancer were compared with patients in the NADM subgroup. The risk of NHL increases proportionally to the degree of immune suppression. The increased susceptibility to various infections in patients with low CD4 counts may also contribute to the occurrence of NHL in patients with low CD4 counts. The occurrence of various other rare cancers in patients with HIV-AIDS may be because of confounding rather than a direct HIV or immunosuppression effect.
An increasing incidence of NADMs has been noted in the Western literature.7,14 ADMs remain the most common malignancies in the HIV-AIDS population, accounting for about 48% of all malignancies.8 This is in concordance with previous publications from India.8,10 With the widespread availability of generic HAART, the incidence of ADMs may decrease even more in the future. In developing countries where the screening procedures for malignancies in both the general population and patients with HIV-AIDS have not yet been implemented at a national level, premalignant lesions of the cervix are not detected.10 Cervical cancer is the most common malignancy in our study population, which underscores the importance of cervical cancer screening in patients with HIV-AIDS.
In the developed countries, following the introduction of HAART in HIV-AIDS management, the incidence of Kaposi sarcoma decreased by 60% to 70%, and the incidence of NHL decreased by 30% to 50%, whereas the rates of cervical cancer remained either stable or declined.15,16 Despite the declining trend, the incidence of these malignancies continues to be high among patients with HIV-AIDS compared with the general population.17 A study from the United States showed increasing trends in various NADMs (such as anal, lung, and liver cancers and Hodgkin lymphoma) from 2006 to 2010.17 In 2003, the number of patients with NADM were higher than the number of patients with ADM in the United States.14 In a population-based study from Brazil, ADMs were the most common malignancies diagnosed in patients with HIV-AIDS. A declining trend was noted in the incidence of ADMs in the population and an increasing trend in the incidence of NADMs. This increase in NADM incidence was contributed by anal and lung cancers.18 Studies from developing countries such as Uganda and Botswana have also shown a decrease in the incidence of Kaposi sarcoma after the introduction of HAART.19-21
Kaposi sarcoma, cervical cancer, NHL (including Burkitt lymphoma, immunoblastic lymphoma, and primary CNS lymphoma [PCNSL]) comprise ADMs. All 3 ADMs have an underlying viral infection as the causative agent.22 Kaposi sarcoma is caused by the Kaposi sarcoma herpes virus, for which seroprevalence varies worldwide.23 As already noted in this article, the incidence of Kaposi sarcoma among the HIV-AIDS population has decreased worldwide since the introduction of HAART. The preinvasive uterine cervix lesions and carcinoma cervix are caused by HPV. NHL in patients with HIV-AIDS is a predominantly aggressive B-cell neoplasm. Epstein-Barr virus is implicated for most of the ADM NHLs.24 PCNSL occurs in patients with low CD4 counts and poses a diagnostic challenge. The treatment outcomes for patients with PCNSL before the HAART era were dismal. With the widespread use of HAART, the treatment outcomes of patients with HIV-AIDS and NHL improved, and, currently, these patients are managed the same way as other patients with NHL.22
The increasing incidence of the NADM is partly attributed to the increasing incidence of these malignancies in the general population. An elevated risk of certain NADMs is also attributable to viral infections. The common NADMs in the United States are lung, anal, oropharyngeal, and hepatocellular cancers and Hodgkin lymphoma.14 The common NADMs in our study population were oral, oropharyngeal, colon, and breast cancers and Hodgkin lymphoma. One-third of head and neck cancers, including most oropharyngeal cancers, and cervical and anal cancers in patients with HIV-AIDS are related to HPV.25 Patients with HIV-AIDS are at increased risk for chronic HPV infection from immunosuppression. Chronic HPV infections and prolonged immunosuppression cause premalignant high-grade squamous intraepithelial lesions and invasive cancers.22 The initiation of and adherence to HAART leads to immune recovery and reduces high-risk HPV-associated morbidity.26 Findings from previous studies have demonstrated the benefits of screening for cervical cancer in patients with HIV-AIDS.27 The HPV vaccine is immunogenic in patients with HIV-AIDS and might help prevent HPV-associated malignancies.28
Conclusions
With the wide use of HAART by patients with HIV-AIDS, we can expect an increase in the survival of that population. The incidence of malignancies may also increase significantly in these patients, and further longitudinal studies are needed, as malignancies may emerge as the most common cause of death in patients with HIV-AIDS. In addition, the extensive use of HAART therapy and implementation of screening programs for cervical cancer in patients with HIV-AIDS could result in a decrease in the incidence of ADMs.
1. UNAIDS. Prevention gap report. http://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf. Released 2016. Accessed December 27, 2017.
3. Dubrow R, Silverberg MJ, Park LS, Crothers K, Justice AC. HIV infection, aging, and immune function: implications for cancer risk and prevention. Curr Opin Oncol. 2012;24(5):506-516.
4. Biggar RJ, Chaturvedi AK, Bhatia K, Mbulaiteye SM. Cancer risk in persons with HIV-AIDS in India: a review and future directions for research. Infect Agent Cancer. 2009;4:4.
5. National AIDS Control Organisation & National Institute of Medical Statistics, ICMR, Ministry of Health & Family Welfare, Government of India. India HIV estimations 2015, technical report. http://www.naco.gov.in/sites/default/files/India%20HIV%20Estimations%202015.pdf. Published 2015. Accessed December 27, 2017.
6. Bonnet F, Lewden C, May T, et al. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101(2):317-324.
7. Crum-Cianflone N, Hullsiek KH, Marconi V, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23(1):41-50.
8. Sharma S, Soneja M, Ranjan S. Malignancies in human immunodeficiency virus infected patients in India: initial experience in the HAART era. Indian J Med Res. 2015;142(5):563-567.
9. Sachdeva RK, Sharma A, Singh S, Varma S. Spectrum of AIDS defining & non-AIDS defining malignancies in north India. In
10. Dhir AA, Sawant S, Dikshit RP, et al. Spectrum of HIV-AIDS related cancers in India. Cancer Causes Control. 2007;19(2):147-153.
11. Venkatesh KK, Saghayam S, Devaleenal B, et al. Spectrum of malignancies among HIV-infected patients in South India. Indian J Cancer. 2012;49(1):176-180.
12. Shruti P, Narayanan G, Puthuveettil J, Jayasree K, Vijayalakshmi K. Spectrum of HIV/AIDS-associated cancers in south India. J Clin Oncol. 2014;32(suppl):e12534.
13. Paul TR, Uppin MS, Uppin SG, et al. Spectrum of malignancies in human immunodeficiency virus–positive patients at a Tertiary Care Centre in South India. Indian J Cancer. 2014;51(4):459-463.
14. Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753-762.
15. Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148(10):728-736.
16. Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187-194.
17. Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. AIDS. 2014;28(6):881-890.
18. Tanaka LF, Latorre MDRD, Gutierrez EB, Heumann C, Herbinger KH, Froeschl G. Trends in the incidence of AIDS-defining and non-AIDS-defining cancers in people living with AIDS: a population-based study from São Paulo, Brazil. Int J STD AIDS. 2017;28(12):1190-1198.
19. Mutyaba I, Phipps W, Krantz EM, et al. A population-level evaluation of the effect of antiretroviral therapy on cancer incidence in Kyadondo County, Uganda, 1999–2008. J Acquir Immune Defic Syndr. 2015;69(4):481-486.
20. Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al. Cancer incidence following expansion of HIV treatment in Botswana. PLoS ONE. 2015;10(8):e0135602.
21. Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12(1):6-11.
22. Yarchoan R, Uldrick TS. HIV-associated cancers and related diseases. N Engl J Med. 2018;378(11):1029-1041.
23. Gao SJ, Kingsley L, Li M, et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996;2(8):925-928.
24. Epstein-Barr virus and AIDS-associated lymphomas. Lancet. 1991;338(8773):979-981.
25. Picard A, Badoual C, Hourseau M, et al. Human papilloma virus prevalence in HIV patients with head and neck squamous cell carcinoma. AIDS. 2016;30(8):1257-1266.
26. Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Infect Dis. 2010;201(5):681-690.
27. Ghebre RG, Grover S, Xu MJ, Chuang LT, Simonds H. Cervical cancer control in HIV-infected women: past, present and future. Gynecol Oncol Rep. 2017;21:101-108.
28. Kojic EM, Rana AI, Cu-Uvin S. Human papillomavirus vaccination in HIV-infected women: need for increased coverage. Expert Rev Vaccines. 2016;15(1):105-117.
1. UNAIDS. Prevention gap report. http://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf. Released 2016. Accessed December 27, 2017.
3. Dubrow R, Silverberg MJ, Park LS, Crothers K, Justice AC. HIV infection, aging, and immune function: implications for cancer risk and prevention. Curr Opin Oncol. 2012;24(5):506-516.
4. Biggar RJ, Chaturvedi AK, Bhatia K, Mbulaiteye SM. Cancer risk in persons with HIV-AIDS in India: a review and future directions for research. Infect Agent Cancer. 2009;4:4.
5. National AIDS Control Organisation & National Institute of Medical Statistics, ICMR, Ministry of Health & Family Welfare, Government of India. India HIV estimations 2015, technical report. http://www.naco.gov.in/sites/default/files/India%20HIV%20Estimations%202015.pdf. Published 2015. Accessed December 27, 2017.
6. Bonnet F, Lewden C, May T, et al. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101(2):317-324.
7. Crum-Cianflone N, Hullsiek KH, Marconi V, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23(1):41-50.
8. Sharma S, Soneja M, Ranjan S. Malignancies in human immunodeficiency virus infected patients in India: initial experience in the HAART era. Indian J Med Res. 2015;142(5):563-567.
9. Sachdeva RK, Sharma A, Singh S, Varma S. Spectrum of AIDS defining & non-AIDS defining malignancies in north India. In
10. Dhir AA, Sawant S, Dikshit RP, et al. Spectrum of HIV-AIDS related cancers in India. Cancer Causes Control. 2007;19(2):147-153.
11. Venkatesh KK, Saghayam S, Devaleenal B, et al. Spectrum of malignancies among HIV-infected patients in South India. Indian J Cancer. 2012;49(1):176-180.
12. Shruti P, Narayanan G, Puthuveettil J, Jayasree K, Vijayalakshmi K. Spectrum of HIV/AIDS-associated cancers in south India. J Clin Oncol. 2014;32(suppl):e12534.
13. Paul TR, Uppin MS, Uppin SG, et al. Spectrum of malignancies in human immunodeficiency virus–positive patients at a Tertiary Care Centre in South India. Indian J Cancer. 2014;51(4):459-463.
14. Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753-762.
15. Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148(10):728-736.
16. Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187-194.
17. Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. AIDS. 2014;28(6):881-890.
18. Tanaka LF, Latorre MDRD, Gutierrez EB, Heumann C, Herbinger KH, Froeschl G. Trends in the incidence of AIDS-defining and non-AIDS-defining cancers in people living with AIDS: a population-based study from São Paulo, Brazil. Int J STD AIDS. 2017;28(12):1190-1198.
19. Mutyaba I, Phipps W, Krantz EM, et al. A population-level evaluation of the effect of antiretroviral therapy on cancer incidence in Kyadondo County, Uganda, 1999–2008. J Acquir Immune Defic Syndr. 2015;69(4):481-486.
20. Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al. Cancer incidence following expansion of HIV treatment in Botswana. PLoS ONE. 2015;10(8):e0135602.
21. Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12(1):6-11.
22. Yarchoan R, Uldrick TS. HIV-associated cancers and related diseases. N Engl J Med. 2018;378(11):1029-1041.
23. Gao SJ, Kingsley L, Li M, et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996;2(8):925-928.
24. Epstein-Barr virus and AIDS-associated lymphomas. Lancet. 1991;338(8773):979-981.
25. Picard A, Badoual C, Hourseau M, et al. Human papilloma virus prevalence in HIV patients with head and neck squamous cell carcinoma. AIDS. 2016;30(8):1257-1266.
26. Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Infect Dis. 2010;201(5):681-690.
27. Ghebre RG, Grover S, Xu MJ, Chuang LT, Simonds H. Cervical cancer control in HIV-infected women: past, present and future. Gynecol Oncol Rep. 2017;21:101-108.
28. Kojic EM, Rana AI, Cu-Uvin S. Human papillomavirus vaccination in HIV-infected women: need for increased coverage. Expert Rev Vaccines. 2016;15(1):105-117.
Use of smartphone app improves pain outcomes
SAN DIEGO – A smartphone app that included artificial intelligence elements was associated with improved pain outcomes and reduced hospital admissions in patients with advanced cancers.
Pain severity significantly decreased among patients randomized to use the app versus control patients who received only palliative care, researchers reported at the Palliative and Supportive Care in Oncology Symposium.
The risk of pain-related hospital admissions was significantly lower for those who used the pain tracking app, called ePAL, though anxiety scores were higher in the app users, the investigators said, and no difference between arms was noted in quality of life or global symptom scores.
The ePAL app prompts patients three times per week to track their pain levels and, depending on the severity of pain, will use an algorithm to guide patients through their symptoms, or, in patients with persistent or worsening pain, connect them directly with the palliative care service for additional assessment.
The app also includes pain management tips, among other educational content, provides the ability to request pain prescription refills, and creates a summary of the patient’s pain condition for the provider, said Mihir M. Kamdar, MD, associate director of palliative care at Massachusetts General Hospital in Boston.
“The provider can actually start the visit with that information, instead of having to spend several minutes trying to recap what might or might not have happened since the last clinic visit,” Dr. Kamdar said.
The study included 112 English-speaking adult patients with stage IV solid cancers and moderate to severe pain who were being followed in a palliative care clinic. They were randomly assigned to receive the ePAL app plus standard of care or standard of care alone; 39 patients in the app group and 40 in the control arm completed the 8-week evaluation.
Pain severity, the primary study endpoint, decreased over time in the intervention group, from a composite Brief Pain Inventory score of 3.74 at enrollment to 2.99 at 8 weeks, while in the control group, the scores were 4.02 at enrollment and 4.05 at 8 weeks (P = .017 for intervention versus control), Dr. Kamdar reported.
Risk of pain-related hospital admissions was significantly lower in the intervention group, according to Dr. Kamdar. The per-patient risk of an inpatient admission was 0.071 and 0.232 for the intervention and controls groups, respectively, with a risk ratio of 0.31 (95% CI, 0.11-0.89; P = .018).
Anxiety was increased in the app users, as measured by the Generalized Anxiety Disorder 7-item scale, with a significant difference between the app and control groups at 8 weeks (P = .015). However, the change was in a range considered mild and was not seen in patients who used the app more than two times per week.
Negative attitudes toward cancer pain treatment decreased significantly in the app group, as shown by a lower score on the Barriers Questionnaire II at 8 weeks (P = .042), Dr. Kamdar reported.
The app and study were supported by the McKesson Foundation’s Mobilizing for Health Initiative. Dr. Kamdar reported stock/ownership and consulting/advisory role disclosures related to Amorsa Therapeutics.
SOURCE: Kamdar MM et al. PallOnc 2018, Abstract 76.
SAN DIEGO – A smartphone app that included artificial intelligence elements was associated with improved pain outcomes and reduced hospital admissions in patients with advanced cancers.
Pain severity significantly decreased among patients randomized to use the app versus control patients who received only palliative care, researchers reported at the Palliative and Supportive Care in Oncology Symposium.
The risk of pain-related hospital admissions was significantly lower for those who used the pain tracking app, called ePAL, though anxiety scores were higher in the app users, the investigators said, and no difference between arms was noted in quality of life or global symptom scores.
The ePAL app prompts patients three times per week to track their pain levels and, depending on the severity of pain, will use an algorithm to guide patients through their symptoms, or, in patients with persistent or worsening pain, connect them directly with the palliative care service for additional assessment.
The app also includes pain management tips, among other educational content, provides the ability to request pain prescription refills, and creates a summary of the patient’s pain condition for the provider, said Mihir M. Kamdar, MD, associate director of palliative care at Massachusetts General Hospital in Boston.
“The provider can actually start the visit with that information, instead of having to spend several minutes trying to recap what might or might not have happened since the last clinic visit,” Dr. Kamdar said.
The study included 112 English-speaking adult patients with stage IV solid cancers and moderate to severe pain who were being followed in a palliative care clinic. They were randomly assigned to receive the ePAL app plus standard of care or standard of care alone; 39 patients in the app group and 40 in the control arm completed the 8-week evaluation.
Pain severity, the primary study endpoint, decreased over time in the intervention group, from a composite Brief Pain Inventory score of 3.74 at enrollment to 2.99 at 8 weeks, while in the control group, the scores were 4.02 at enrollment and 4.05 at 8 weeks (P = .017 for intervention versus control), Dr. Kamdar reported.
Risk of pain-related hospital admissions was significantly lower in the intervention group, according to Dr. Kamdar. The per-patient risk of an inpatient admission was 0.071 and 0.232 for the intervention and controls groups, respectively, with a risk ratio of 0.31 (95% CI, 0.11-0.89; P = .018).
Anxiety was increased in the app users, as measured by the Generalized Anxiety Disorder 7-item scale, with a significant difference between the app and control groups at 8 weeks (P = .015). However, the change was in a range considered mild and was not seen in patients who used the app more than two times per week.
Negative attitudes toward cancer pain treatment decreased significantly in the app group, as shown by a lower score on the Barriers Questionnaire II at 8 weeks (P = .042), Dr. Kamdar reported.
The app and study were supported by the McKesson Foundation’s Mobilizing for Health Initiative. Dr. Kamdar reported stock/ownership and consulting/advisory role disclosures related to Amorsa Therapeutics.
SOURCE: Kamdar MM et al. PallOnc 2018, Abstract 76.
SAN DIEGO – A smartphone app that included artificial intelligence elements was associated with improved pain outcomes and reduced hospital admissions in patients with advanced cancers.
Pain severity significantly decreased among patients randomized to use the app versus control patients who received only palliative care, researchers reported at the Palliative and Supportive Care in Oncology Symposium.
The risk of pain-related hospital admissions was significantly lower for those who used the pain tracking app, called ePAL, though anxiety scores were higher in the app users, the investigators said, and no difference between arms was noted in quality of life or global symptom scores.
The ePAL app prompts patients three times per week to track their pain levels and, depending on the severity of pain, will use an algorithm to guide patients through their symptoms, or, in patients with persistent or worsening pain, connect them directly with the palliative care service for additional assessment.
The app also includes pain management tips, among other educational content, provides the ability to request pain prescription refills, and creates a summary of the patient’s pain condition for the provider, said Mihir M. Kamdar, MD, associate director of palliative care at Massachusetts General Hospital in Boston.
“The provider can actually start the visit with that information, instead of having to spend several minutes trying to recap what might or might not have happened since the last clinic visit,” Dr. Kamdar said.
The study included 112 English-speaking adult patients with stage IV solid cancers and moderate to severe pain who were being followed in a palliative care clinic. They were randomly assigned to receive the ePAL app plus standard of care or standard of care alone; 39 patients in the app group and 40 in the control arm completed the 8-week evaluation.
Pain severity, the primary study endpoint, decreased over time in the intervention group, from a composite Brief Pain Inventory score of 3.74 at enrollment to 2.99 at 8 weeks, while in the control group, the scores were 4.02 at enrollment and 4.05 at 8 weeks (P = .017 for intervention versus control), Dr. Kamdar reported.
Risk of pain-related hospital admissions was significantly lower in the intervention group, according to Dr. Kamdar. The per-patient risk of an inpatient admission was 0.071 and 0.232 for the intervention and controls groups, respectively, with a risk ratio of 0.31 (95% CI, 0.11-0.89; P = .018).
Anxiety was increased in the app users, as measured by the Generalized Anxiety Disorder 7-item scale, with a significant difference between the app and control groups at 8 weeks (P = .015). However, the change was in a range considered mild and was not seen in patients who used the app more than two times per week.
Negative attitudes toward cancer pain treatment decreased significantly in the app group, as shown by a lower score on the Barriers Questionnaire II at 8 weeks (P = .042), Dr. Kamdar reported.
The app and study were supported by the McKesson Foundation’s Mobilizing for Health Initiative. Dr. Kamdar reported stock/ownership and consulting/advisory role disclosures related to Amorsa Therapeutics.
SOURCE: Kamdar MM et al. PallOnc 2018, Abstract 76.
REPORTING FROM PALLONC 2018
Key clinical point: Use of a smartphone app with artificial intelligence elements improved pain outcomes and reduced hospital admissions in patients with advanced cancers.
Major finding: Pain severity decreased over time from a composite Brief Pain Inventory score of 3.74 at enrollment to 2.99 at 8 weeks, compared with baseline and 8-week values of 4.02 and 4.05 in the control group (P = .017).
Study details: A randomized study including 112 English-speaking adult patients with stage IV solid cancers and moderate to severe pain who were followed in a palliative care clinic.
Disclosures: The research was supported by the McKesson Foundation. The presenting author reported stock/ownership and consulting/advisory role disclosures related to Amorsa Therapeutic.
Source: Kamdar MM et al. PallOnc 2018, Abstract 76.
Staying up to date on screening may cut risk of death from CRC
according to the results of a large retrospective case-control study.
Source: American Gastroenterological Association
The findings signify “potentially modifiable” screening failures in a population known for relatively high uptake of colorectal cancer screening, wrote Chyke A. Doubeni, MD, MPH, of the University of Pennsylvania, Philadelphia, and his associates in Gastroenterology. Strikingly, 76% of patients who died from colorectal cancer were not current on screening versus 55% of cancer-free patients, they said. Being up to date on screening decreased the odds of dying from colorectal cancer by 62% (odds ratio, 0.38; 95% confidence interval, 0.33-0.44), even after adjustment for race, ethnicity, socioeconomic status, comorbidities, and frequency of contact with primary care providers, they added.
Colonoscopy, sigmoidoscopy, and fecal testing are effective and recommended screening techniques that help prevent deaths from colorectal cancer. Therefore, most such deaths are thought to result from “breakdowns in the screening process,” the researchers wrote. However, interval cancers and missed lesions also play a role, and no prior study has examined detailed screening histories and their association with colorectal cancer mortality.
Accordingly, the researchers reviewed medical records and registry data for 1,750 enrollees in the Kaiser Permanente Northern and Southern California systems who died from colorectal cancer during 2002-2012 and were part of the health plan for at least 5 years before their cancer diagnosis. They compared these patients with 3,486 cancer-free controls matched by age, sex, study site, and numbers of years enrolled in the health plan. Patients were considered up to date on screening if they were screened at intervals recommended by the 2008 multisociety colorectal cancer screening guidelines – that is, if they had received a colonoscopy within 10 years of colorectal cancer diagnosis or sigmoidoscopy or barium enema within 5 years of it. For fecal testing, the investigators used a 2-year interval based on its efficacy in clinical trials.
Among patients who died from colorectal cancer, only 24% were up to date on screening versus 45% of cancer-free-patients, the investigators determined. Furthermore, 68% of patients who died from colorectal cancer were never screened or were not screened at appropriate intervals, compared with 53% of cancer-free patients.
Additionally, while 8% of colorectal cancer deaths occurred in patients who had not followed up on abnormal screening results, only 2% of controls who had received abnormal screening results had failed to follow up.
“In two health systems with high rates of screening, we observed that most patients dying from colorectal cancer had potentially modifiable failures of the screening process,” the researchers concluded. “This study suggests that, even in settings with high screening uptake, access to and timely uptake of screening, regular rescreening, appropriate use of testing given patient characteristics, completion of timely diagnostic testing when screening is positive, and improving the effectiveness of screening tests, particularly for right colon cancer, remain important areas of focus for further decreasing colorectal cancer deaths.”
The National Institutes of Health funded the work. The investigators reported having no conflicts of interest except that one coinvestigator is editor in chief of the journal Gastroenterology.
SOURCE: Doubeni CA et al. Gastroenterology. 2018 Sep 27. doi: 10.1053/j.gastro.2018.09.040.
Screening for colorectal cancer (CRC) is a major success story – one of only two cancers (the other being cervical cancer) with an A recommendation for screening from the U.S. Preventive Services Task Force. Multiple randomized trials for two CRC screening modalities, stool-based tests and sigmoidoscopy, have shown significant reductions in CRC incidence and mortality.
Within this context, Doubeni et al. examined the association of CRC screening with death from CRC in a real-world HMO setting. Their study is notable for several reasons. First, it showed a highly protective effect on CRC mortality of being up to date with screening (odds ratio, 0.38; 95% confidence interval, 0.33-0.44). Second, it examined CRC screening as a process, with various steps of that process related to CRC mortality. Finally, methodologically, the study’s utilization of electronic medical records and cancer registry linkages highlights the importance of integrated data systems in the efficient performance of epidemiologic research.
Of note, screening was primarily stool-based tests (fecal occult blood test/fecal immunochemical test ) and sigmoidoscopy, in contrast to most of the U.S., where colonoscopy is predominant. Randomized trials of these modalities show mortality reductions of 15%-20% (FOBT/FIT) and 25%-30% (sigmoidoscopy), respectively. Therefore, some of the reported effect is likely due to selection bias, with healthier persons more likely to choose screening.
It would be of interest to see similar studies performed in a colonoscopy-predominant screening setting and with the effect on CRC incidence as well as mortality examined.
Paul F. Pinsky, PhD, chief of the Early Detection Research Branch, National Cancer Institute, Bethesda, MD. He has no conflicts of interest.
Screening for colorectal cancer (CRC) is a major success story – one of only two cancers (the other being cervical cancer) with an A recommendation for screening from the U.S. Preventive Services Task Force. Multiple randomized trials for two CRC screening modalities, stool-based tests and sigmoidoscopy, have shown significant reductions in CRC incidence and mortality.
Within this context, Doubeni et al. examined the association of CRC screening with death from CRC in a real-world HMO setting. Their study is notable for several reasons. First, it showed a highly protective effect on CRC mortality of being up to date with screening (odds ratio, 0.38; 95% confidence interval, 0.33-0.44). Second, it examined CRC screening as a process, with various steps of that process related to CRC mortality. Finally, methodologically, the study’s utilization of electronic medical records and cancer registry linkages highlights the importance of integrated data systems in the efficient performance of epidemiologic research.
Of note, screening was primarily stool-based tests (fecal occult blood test/fecal immunochemical test ) and sigmoidoscopy, in contrast to most of the U.S., where colonoscopy is predominant. Randomized trials of these modalities show mortality reductions of 15%-20% (FOBT/FIT) and 25%-30% (sigmoidoscopy), respectively. Therefore, some of the reported effect is likely due to selection bias, with healthier persons more likely to choose screening.
It would be of interest to see similar studies performed in a colonoscopy-predominant screening setting and with the effect on CRC incidence as well as mortality examined.
Paul F. Pinsky, PhD, chief of the Early Detection Research Branch, National Cancer Institute, Bethesda, MD. He has no conflicts of interest.
Screening for colorectal cancer (CRC) is a major success story – one of only two cancers (the other being cervical cancer) with an A recommendation for screening from the U.S. Preventive Services Task Force. Multiple randomized trials for two CRC screening modalities, stool-based tests and sigmoidoscopy, have shown significant reductions in CRC incidence and mortality.
Within this context, Doubeni et al. examined the association of CRC screening with death from CRC in a real-world HMO setting. Their study is notable for several reasons. First, it showed a highly protective effect on CRC mortality of being up to date with screening (odds ratio, 0.38; 95% confidence interval, 0.33-0.44). Second, it examined CRC screening as a process, with various steps of that process related to CRC mortality. Finally, methodologically, the study’s utilization of electronic medical records and cancer registry linkages highlights the importance of integrated data systems in the efficient performance of epidemiologic research.
Of note, screening was primarily stool-based tests (fecal occult blood test/fecal immunochemical test ) and sigmoidoscopy, in contrast to most of the U.S., where colonoscopy is predominant. Randomized trials of these modalities show mortality reductions of 15%-20% (FOBT/FIT) and 25%-30% (sigmoidoscopy), respectively. Therefore, some of the reported effect is likely due to selection bias, with healthier persons more likely to choose screening.
It would be of interest to see similar studies performed in a colonoscopy-predominant screening setting and with the effect on CRC incidence as well as mortality examined.
Paul F. Pinsky, PhD, chief of the Early Detection Research Branch, National Cancer Institute, Bethesda, MD. He has no conflicts of interest.
according to the results of a large retrospective case-control study.
Source: American Gastroenterological Association
The findings signify “potentially modifiable” screening failures in a population known for relatively high uptake of colorectal cancer screening, wrote Chyke A. Doubeni, MD, MPH, of the University of Pennsylvania, Philadelphia, and his associates in Gastroenterology. Strikingly, 76% of patients who died from colorectal cancer were not current on screening versus 55% of cancer-free patients, they said. Being up to date on screening decreased the odds of dying from colorectal cancer by 62% (odds ratio, 0.38; 95% confidence interval, 0.33-0.44), even after adjustment for race, ethnicity, socioeconomic status, comorbidities, and frequency of contact with primary care providers, they added.
Colonoscopy, sigmoidoscopy, and fecal testing are effective and recommended screening techniques that help prevent deaths from colorectal cancer. Therefore, most such deaths are thought to result from “breakdowns in the screening process,” the researchers wrote. However, interval cancers and missed lesions also play a role, and no prior study has examined detailed screening histories and their association with colorectal cancer mortality.
Accordingly, the researchers reviewed medical records and registry data for 1,750 enrollees in the Kaiser Permanente Northern and Southern California systems who died from colorectal cancer during 2002-2012 and were part of the health plan for at least 5 years before their cancer diagnosis. They compared these patients with 3,486 cancer-free controls matched by age, sex, study site, and numbers of years enrolled in the health plan. Patients were considered up to date on screening if they were screened at intervals recommended by the 2008 multisociety colorectal cancer screening guidelines – that is, if they had received a colonoscopy within 10 years of colorectal cancer diagnosis or sigmoidoscopy or barium enema within 5 years of it. For fecal testing, the investigators used a 2-year interval based on its efficacy in clinical trials.
Among patients who died from colorectal cancer, only 24% were up to date on screening versus 45% of cancer-free-patients, the investigators determined. Furthermore, 68% of patients who died from colorectal cancer were never screened or were not screened at appropriate intervals, compared with 53% of cancer-free patients.
Additionally, while 8% of colorectal cancer deaths occurred in patients who had not followed up on abnormal screening results, only 2% of controls who had received abnormal screening results had failed to follow up.
“In two health systems with high rates of screening, we observed that most patients dying from colorectal cancer had potentially modifiable failures of the screening process,” the researchers concluded. “This study suggests that, even in settings with high screening uptake, access to and timely uptake of screening, regular rescreening, appropriate use of testing given patient characteristics, completion of timely diagnostic testing when screening is positive, and improving the effectiveness of screening tests, particularly for right colon cancer, remain important areas of focus for further decreasing colorectal cancer deaths.”
The National Institutes of Health funded the work. The investigators reported having no conflicts of interest except that one coinvestigator is editor in chief of the journal Gastroenterology.
SOURCE: Doubeni CA et al. Gastroenterology. 2018 Sep 27. doi: 10.1053/j.gastro.2018.09.040.
according to the results of a large retrospective case-control study.
Source: American Gastroenterological Association
The findings signify “potentially modifiable” screening failures in a population known for relatively high uptake of colorectal cancer screening, wrote Chyke A. Doubeni, MD, MPH, of the University of Pennsylvania, Philadelphia, and his associates in Gastroenterology. Strikingly, 76% of patients who died from colorectal cancer were not current on screening versus 55% of cancer-free patients, they said. Being up to date on screening decreased the odds of dying from colorectal cancer by 62% (odds ratio, 0.38; 95% confidence interval, 0.33-0.44), even after adjustment for race, ethnicity, socioeconomic status, comorbidities, and frequency of contact with primary care providers, they added.
Colonoscopy, sigmoidoscopy, and fecal testing are effective and recommended screening techniques that help prevent deaths from colorectal cancer. Therefore, most such deaths are thought to result from “breakdowns in the screening process,” the researchers wrote. However, interval cancers and missed lesions also play a role, and no prior study has examined detailed screening histories and their association with colorectal cancer mortality.
Accordingly, the researchers reviewed medical records and registry data for 1,750 enrollees in the Kaiser Permanente Northern and Southern California systems who died from colorectal cancer during 2002-2012 and were part of the health plan for at least 5 years before their cancer diagnosis. They compared these patients with 3,486 cancer-free controls matched by age, sex, study site, and numbers of years enrolled in the health plan. Patients were considered up to date on screening if they were screened at intervals recommended by the 2008 multisociety colorectal cancer screening guidelines – that is, if they had received a colonoscopy within 10 years of colorectal cancer diagnosis or sigmoidoscopy or barium enema within 5 years of it. For fecal testing, the investigators used a 2-year interval based on its efficacy in clinical trials.
Among patients who died from colorectal cancer, only 24% were up to date on screening versus 45% of cancer-free-patients, the investigators determined. Furthermore, 68% of patients who died from colorectal cancer were never screened or were not screened at appropriate intervals, compared with 53% of cancer-free patients.
Additionally, while 8% of colorectal cancer deaths occurred in patients who had not followed up on abnormal screening results, only 2% of controls who had received abnormal screening results had failed to follow up.
“In two health systems with high rates of screening, we observed that most patients dying from colorectal cancer had potentially modifiable failures of the screening process,” the researchers concluded. “This study suggests that, even in settings with high screening uptake, access to and timely uptake of screening, regular rescreening, appropriate use of testing given patient characteristics, completion of timely diagnostic testing when screening is positive, and improving the effectiveness of screening tests, particularly for right colon cancer, remain important areas of focus for further decreasing colorectal cancer deaths.”
The National Institutes of Health funded the work. The investigators reported having no conflicts of interest except that one coinvestigator is editor in chief of the journal Gastroenterology.
SOURCE: Doubeni CA et al. Gastroenterology. 2018 Sep 27. doi: 10.1053/j.gastro.2018.09.040.
FROM GASTROENTEROLOGY
Key clinical point: Being up to date on screening was associated with a significant reduction in the risk of dying from colon cancer.
Major finding: Being up to date on screening decreased the odds of dying from colorectal cancer by 62% (odds ratio, 0.38; 95% confidence interval, 0.33-0.44).
Study details: Retrospective cohort study of 1,750 patients who died from colorectal cancer during 2002-2012 and 3,486 matched controls.
Disclosures: The National Institutes of Health funded the work. The investigators reported having no conflicts of interest except that one coinvestigator is editor in chief of Gastroenterology.
Source: Doubeni CA et al. Gastroenterology. 2018 Sep 27. doi: 10.1053/j.gastro.2018.09.040.