User login
Immunotherapy may hold the key to defeating virally associated cancers
Infection with certain viruses has been causally linked to the development of cancer. In recent years, an improved understanding of the unique pathology and molecular underpinnings of these virally associated cancers has prompted the development of more personalized treatment strategies, with a particular focus on immunotherapy. Here, we describe some of the latest developments.
The link between viruses and cancer
Suspicions about a possible role of viral infections in the development of cancer were first aroused in the early 1900s. The seminal discovery is traced back to Peyton Rous, who showed that a malignant tumor growing in a chicken could be transferred to a healthy bird by injecting it with tumor extracts that contained no actual tumor cells.1
The infectious etiology of human cancer, however, remained controversial until many years later when the first cancer-causing virus, Epstein-Barr virus (EBV), was identified in cell cultures from patients with Burkitt lymphoma. Shortly afterward, the Rous sarcoma virus was unveiled as the oncogenic agent behind Rous’ observations.2Seven viruses have now been linked to the development of cancers and are thought to be responsible for around 12% of all cancer cases worldwide. The burden is likely to increase as technological advancements make it easier to establish a causal link between viruses and cancer development.3
In addition to making these links, researchers have also made significant headway in understanding how viruses cause cancer. Cancerous transformation of host cells occurs in only a minority of those who are infected with oncogenic viruses and often occurs in the setting of chronic infection.
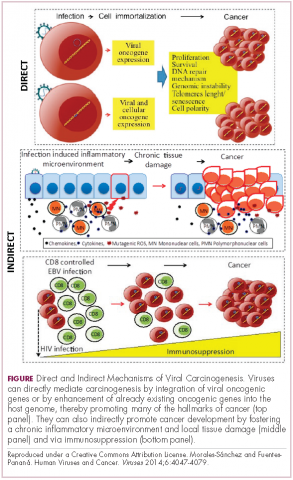
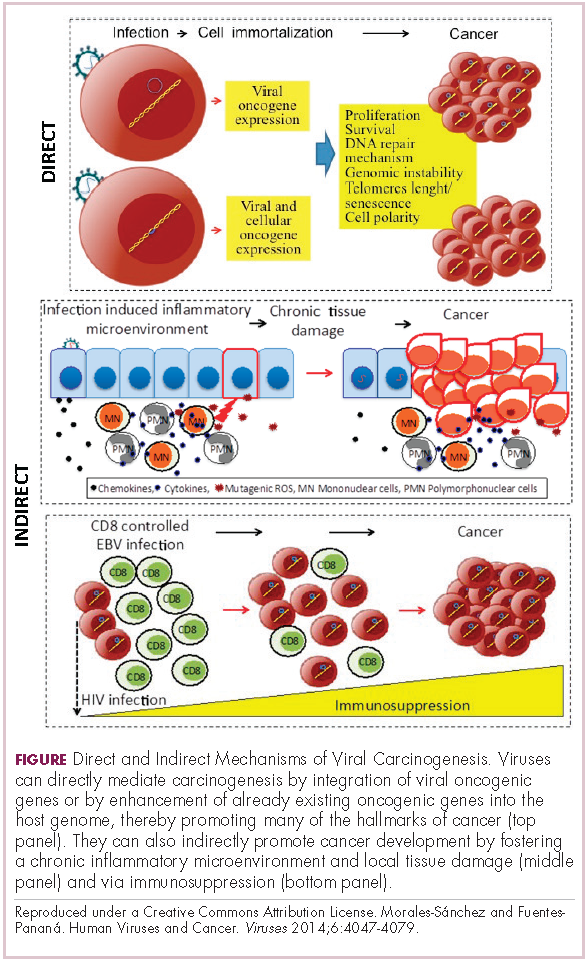
Viruses can mediate carcinogenesis by direct and/or indirect mechanisms (Figure 1). Many of the hallmarks of cancer, the key attributes that drive the transformation from a normal cell to a malignant one, are compatible with the virus’s needs, such as needing to avoid cell death, increasing cell proliferation, and avoiding detection by the immune system.
Viruses hijack the cellular machinery to meet those needs and they can do this either by producing viral proteins that have an oncogenic effect or by integrating their genetic material into the host cell genome. When the latter occurs, the process of integration can also cause damage to the DNA, which further increases the risk of cancer-promoting changes occurring in the host genome.
Viruses can indirectly contribute to carcinogenesis by fostering a microenvironment of chronic inflammation, causing oxidative stress and local tissue damage, and by suppressing the antitumor immune response.4,5
Screening and prevention efforts have helped to reduce the burden of several different virally associated cancers. However, for the substantial proportion of patients who are still affected by these cancers, there is a pressing need for new therapeutic options, particularly since genome sequencing studies have revealed that these cancers can often have distinct underlying molecular mechanisms.
Vaccines lead the charge in HPV-driven cancers
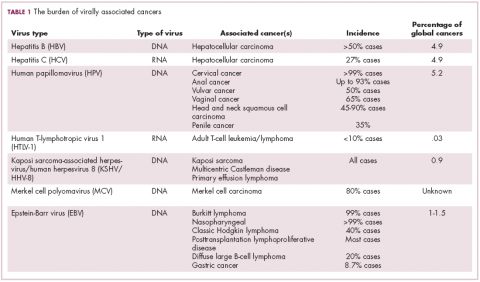
German virologist Harald zur Hausen received the Nobel Prize in 2008 for his discovery of the oncogenic role of human papillomaviruses (HPVs), a large family of more than 100 DNA viruses that infect the epithelial cells of the skin and mucous membranes. They are responsible for the largest number of virally associated cancer cases globally – around 5% (Table 1).
A number of different cancer types are linked to HPV infection, but it is best known as the cause of cervical cancer. The development of diagnostic blood tests and prophylactic vaccines for prevention and early intervention in HPV infection has helped to reduce the incidence of cervical cancer. Conversely, another type of HPV-associated cancer, head and neck squamous cell carcinoma (HNSCC), has seen increased incidence in recent years.
HPVs are categorized according to their oncogenic potential as high, intermediate, or low risk. The high-risk HPV16 and HPV18 strains are most commonly associated with cancer. They are thought to cause cancer predominantly through integration into the host genome. The HPV genome is composed of 8 genes encoding proteins that regulate viral replication and assembly. The E6 and E7 genes are the most highly oncogenic; as the HPV DNA is inserted into the host genome, the transcriptional regulator of E6/E7 is lost, leading to their increased expression. These genes have significant oncogenic potential because of their interaction with 2 tumor suppressor proteins, p53 and pRb.6,7
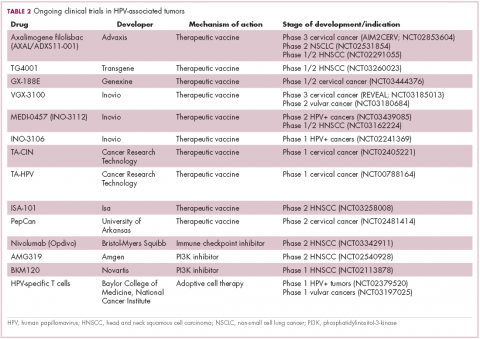
The largest investment in therapeutic development for HPV-positive cancers has been in the realm of immunotherapy in an effort to boost the anti-tumor immune response. In particular, there has been a focus on the development of therapeutic vaccines, designed to prime the anti-tumor immune response to recognize viral antigens. A variety of different types of vaccines are being developed, including live, attenuated and inactivated vaccines that are protein, DNA, or peptide based. Most developed to date target the E6/E7 proteins from the HPV16/18 strains (Table 2).8,9
Other immunotherapies are also being evaluated, including immune checkpoint inhibitors, antibodies designed to target one of the principal mechanisms of immune evasion exploited by cancer cells. The combination of immune checkpoint inhibitors with vaccines is a particularly promising strategy in HPV-associated cancers. At the European Society for Medical Oncology Congress in 2017, the results of a phase 2 trial of nivolumab in combination with ISA-101 were presented.
Among 24 patients with HPV-positive tumors, the majority oropharyngeal cancers, the combination elicited an overall response rate (ORR) of 33%, including 2 complete responses (CRs). Most adverse events (AEs) were mild to moderate in severity and included fever, injection site reactions, fatigue and nausea.14
Hepatocellular carcinoma: a tale of two viruses
The hepatitis viruses are a group of 5 unrelated viruses that causes inflammation of the liver. Hepatitis B (HBV), a DNA virus, and hepatitis C (HCV), an RNA virus, are also oncoviruses; HBV in particular is one of the main causes of hepatocellular carcinoma (HCC), the most common type of liver cancer.
The highly inflammatory environment fostered by HBV and HCV infection causes liver damage that often leads to cirrhosis. Continued infection can drive permanent damage to the hepatocytes, leading to genetic and epigenetic damage and driving oncogenesis. As an RNA virus, HCV doesn’t integrate into the genome and no confirmed viral oncoproteins have been identified to date, therefore it mostly drives cancer through these indirect mechanisms, which is also reflected in the fact that HCV-associated HCC predominantly occurs against a backdrop of liver cirrhosis.
HBV does integrate into the host genome. Genome sequencing studies revealed hundreds of integration sites, but most commonly they disrupted host genes involved in telomere stability and cell cycle regulation, providing some insight into the mechanisms by which HBV-associated HCC develops. In addition, HBV produces several oncoproteins, including HBx, which disrupts gene transcription, cell signaling pathways, cell cycle progress, apoptosis and other cellular processes.15,16
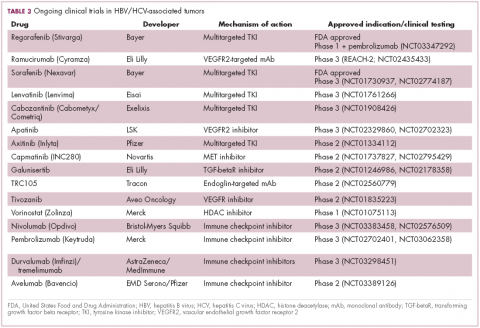
Multitargeted tyrosine kinase inhibitors (TKIs) have been the focal point of therapeutic development in HCC. However, following the approval of sorafenib in 2008, there was a dearth of effective new treatment options despite substantial efforts and numerous phase 3 trials. More recently, immunotherapy has also come to the forefront, especially immune checkpoint inhibitors.
Last year marked the first new drug approvals in nearly a decade – the TKI regorafenib (Stivarga) and immune checkpoint inhibitor nivolumab (Opdivo), both in the second-line setting after failure of sorafenib. Treatment options in this setting may continue to expand, with the TKIs cabozantinib and lenvatinib and the immune checkpoint inhibitor pembrolizumab and the combination of durvalumab and tremelimumab hot on their heels.17-20 Many of these drugs are also being evaluated in the front-line setting in comparison with sorafenib (Table 3).
At the current time, the treatment strategy for patients with HCC is independent of etiology, however, there are significant ongoing efforts to try to tease out the implications of infection for treatment efficacy. A recent meta-analysis of patients treated with sorafenib in 3 randomized phase 3 trials (n = 3,526) suggested that it improved overall survival (OS) among patients who were HCV-positive, but HBV-negative.21
Studies of the vascular endothelial growth factor receptor 2-targeting monoclonal antibody ramucirumab, on the other hand, suggested that it may have a greater OS benefit in patients with HBV, while regorafenib seemed to have a comparable OS benefit in both subgroups.22-25 The immune checkpoint inhibitors studied thus far seem to elicit responses irrespective of infection status.
A phase 2 trial of the immune checkpoint inhibitor tremelimumab was conducted specifically in patients with advanced HCC and chronic HCV infection. The disease control rate (DCR) was 76.4%, with 17.6% partial response (PR) rate. There was also a significant drop in viral load, suggesting that tremelimumab may have antiviral effects.26,27,28
Adoptive cell therapy promising in EBV-positive cancers
More than 90% of the global population is infected with EBV, making it one of the most common human viruses. It is a member of the herpesvirus family that is probably best known as the cause of infectious mononucleosis. On rare occasions, however, EBV can cause tumor development, though our understanding of its exact pathogenic role in cancer is still incomplete.
EBV is a DNA virus that doesn’t tend to integrate into the host genome, but instead remains in the nucleus in the form of episomes and produces several oncoproteins, including latent membrane protein-1. It is associated with a range of different cancer types, including Burkitt lymphoma and other B-cell malignancies. It also infects epithelial cells and can cause nasopharyngeal carcinoma and gastric cancer, however, much less is known about the molecular underpinnings of these EBV-positive cancer types.26,27Gastric cancers actually comprise the largest group of EBV-associated tumors because of the global incidence of this cancer type. The Cancer Genome Atlas Research Network recently characterized gastric cancer on a molecular level and identified an EBV-positive subgroup as a distinct clinical entity with unique molecular characteristics.29
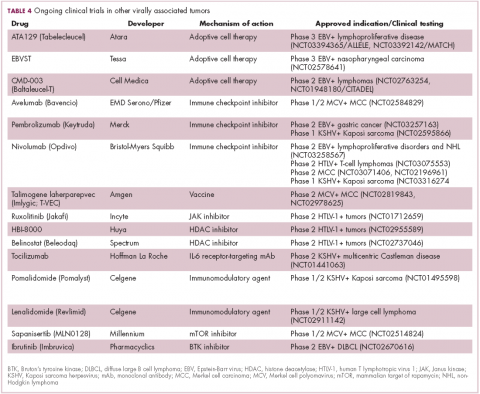
The focus of therapeutic development has again been on immunotherapy, however in this case the idea of collecting the patients T cells, engineering them to recognize EBV, and then reinfusing them into the patient – adoptive cell therapy – has gained the most traction (Table 4).
Two presentations at the American Society of Hematology annual meeting in 2017 detailed ongoing clinical trials of Atara Biotherapeutics’ ATA129 and Cell Medica’s CMD-003. ATA129 was associated with a high response rate and a low rate of serious AEs in patients with posttransplant lymphoproliferative disorder; ORR was 80% in 6 patients treated after hematopoietic stem cell transplantation, and 83% in 6 patients after solid organ transplant.30
CMD-003, meanwhile, demonstrated preliminary signs of activity and safety in patients with relapsed extranodal NK/T-cell lymphoma, according to early results from the phase 2 CITADEL trial. Among 6 evaluable patients, the ORR was 50% and the DCR was 67%.31
Newest oncovirus on the block
The most recently discovered cancer-associated virus is Merkel cell polyomavirus (MCV), a DNA virus that was identified in 2008. Like EBV, virtually the whole global adult population is infected with MCV. It is linked to the development of a highly aggressive and lethal, though rare, form of skin cancer – Merkel cell carcinoma.
MCV is found in around 80% of MCC cases and in fewer than 10% of melanomas and other skin cancers. Thus far, several direct mechanisms of oncogenesis have been described, including integration of MCV into the host genome and the production of viral oncogenes, though their precise function is as yet unclear.32-34
The American Cancer Society estimates that only 1500 cases of MCC are diagnosed each year in the United States.35 Its rarity makes it difficult to conduct clinical trials with sufficient power, yet some headway has still been made.
Around half of MCCs express the programmed cell death ligand 1 (PD-L1) on their surface, making them a logical candidate for immune checkpoint inhibition. In 2017, avelumab became the first FDA-approved drug for the treatment of MCC. Approval was based on the JAVELIN Merkel 200 study in which 88 patients received avelumab. After 1 year of follow-up the ORR was 31.8%, with a CR rate of 9%.36
Genome sequencing studies suggest that the mutational profile of MCV-positive tumors is quite different to those that are MCV-negative, which could have therapeutic implications. To date, these implications have not been delineated, given the challenge of small patient numbers, however an ongoing phase 1/2 trial is evaluating the combination of avelumab and radiation therapy or recombinant interferon beta, with or without MCV-specific cytotoxic T cells in patients with MCC and MCV infection.
The 2 other known cancer-causing viruses are human T-lymphotropic virus 1 (HTLV-1), a retrovirus associated with adult T-cell leukemia/lymphoma (ATL) and Kaposi sarcoma herpesvirus (KSHV). The latter is the causative agent of Kaposi sarcoma, often in combination with human immunodeficiency virus (HIV), a rare skin tumor that became renowned in the 1980s as an AIDS-defining illness.
The incidence of HTLV-1- and KSHV-positive tumors is substantially lower than the other virally associated cancers and, like MCC, this makes studying them and conducting clinical trials of novel therapeutic options a challenge. Nonetheless, several trials of targeted therapies and immunotherapies are underway.
1. Rous PA. Transmissible avain neoplasm. (Sarcoma of the common fowl). J Exp Med. 1910;12(5):696-705.
2. Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet. 1964;1(7335):702-703.
3. Mesri Enrique A, Feitelson MA, Munger K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host & Microbe. 2014;15(3):266-282.
4. Santana-Davila R, Bhatia S, Chow LQ. Harnessing the immune system as a therapeutic tool in virus-associated cancers. JAMA Oncol. 2017;3(1):106-112.
5. Tashiro H, Brenner MK. Immunotherapy against cancer-related viruses. Cell Res. 2017;27(1):59-73.
6. Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017;40(2):80-85.
7. Tulay P, Serakinci N. The route to HPV-associated neoplastic transformation: a review of the literature. Crit Rev Eukaryot Gene Expr. 2016;26(1):27-39.
8. Smola S. Immunopathogenesis of HPV-associated cancers and prospects for immunotherapy. Viruses. 2017;9(9).
9. Rosales R, Rosales C. Immune therapy for human papillomaviruses-related cancers. World Journal of Clinical Oncology. 2014;5(5):1002-1019.
10. Miles B, Safran HP, Monk BJ. Therapeutic options for treatment of human papillomavirus-associated cancers - novel immunologic vaccines: ADXS11-001. Gynecol Oncol Res Pract. 2017;4:10.
11. Miles BA, Monk BJ, Safran HP. Mechanistic insights into ADXS11-001 human papillomavirus-associated cancer immunotherapy. Gynecol Oncol Res Pract. 2017;4:9.
12. Huh W, Dizon D, Powell M, Landrum L, Leath C. A prospective phase II trial of the listeria-based human papillomavirus immunotherapy axalimogene filolisbac in second and third-line metastatic cervical cancer: A NRG oncology group trial. Paper presented at: Annual Meeting on Women's Cancer; March 12-15, 2017, 2017; National Harbor, MD.
13. Petit RG, Mehta A, Jain M, et al. ADXS11-001 immunotherapy targeting HPV-E7: final results from a Phase II study in Indian women with recurrent cervical cancer. Journal for Immunotherapy of Cancer. 2014;2(Suppl 3):P92-P92.
14. Glisson B, Massarelli E, William W, et al. Nivolumab and ISA 101 HPV vaccine in incurable HPV-16+ cancer. Ann Oncol. 2017;28(suppl_5):v403-v427.
15. Ding X-X, Zhu Q-G, Zhang S-M, et al. Precision medicine for hepatocellular carcinoma: driver mutations and targeted therapy. Oncotarget. 2017;8(33):55715-55730.
16. Ringehan M, McKeating JA, Protzer U. Viral hepatitis and liver cancer. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017;372(1732):20160274.
17. Abou-Alfa G, Meyer T, Cheng AL, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2017;36(Suppl 4S):abstr 207.
18. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018.
19. Zhu AX, Finn RS, Cattan S, et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36(Suppl 4S):Abstr 209.
20. Kelley RK, Abou-Alfa GK, Bendell JC, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. Journal of Clinical Oncology. 2017;35(15_suppl):4073-4073.
21. Jackson R, Psarelli E-E, Berhane S, Khan H, Johnson P. Impact of Viral Status on Survival in Patients Receiving Sorafenib for Advanced Hepatocellular Cancer: A Meta-Analysis of Randomized Phase III Trials. Journal of Clinical Oncology. 2017;35(6):622-628.
22. Kudo M. Molecular Targeted Agents for Hepatocellular Carcinoma: Current Status and Future Perspectives. Liver Cancer. 2017;6(2):101-112.
23. zur Hausen H, Meinhof W, Scheiber W, Bornkamm GW. Attempts to detect virus-secific DNA in human tumors. I. Nucleic acid hybridizations with complementary RNA of human wart virus. Int J Cancer. 1974;13(5):650-656.
24. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56-66.
25. Bruix J, Tak WY, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49(16):3412-3419.
26. Neparidze N, Lacy J. Malignancies associated with epstein-barr virus: pathobiology, clinical features, and evolving treatments. Clin Adv Hematol Oncol. 2014;12(6):358-371.
27. Ozoya OO, Sokol L, Dalia S. EBV-Related Malignancies, Outcomes and Novel Prevention Strategies. Infect Disord Drug Targets. 2016;16(1):4-21.
28. Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59(1):81-88.
29. The Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202.
30. Prockop S, Li A, Baiocchi R, et al. Efficacy and safety of ATA129, partially matched allogeneic third-party Epstein-Barr virus-targeted cytotoxic T lymphocytes in a multicenter study for post-transplant lymphoproliferative disorder. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
31. Kim W, Ardeshna K, Lin Y, et al. Autologous EBV-specific T cells (CMD-003): Early results from a multicenter, multinational Phase 2 trial for treatment of EBV-associated NK/T-cell lymphoma. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
32. Schadendorf D, Lebbé C, zur Hausen A, et al. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. European Journal of Cancer. 2017;71:53-69.
33. Spurgeon ME, Lambert PF. Merkel cell polyomavirus: a newly discovered human virus with oncogenic potential. Virology. 2013;435(1):118-130.
34. Tello TL, Coggshall K, Yom SS, Yu SS. Merkel cell carcinoma: An update and review: Current and future therapy. J Am Acad Dermatol. 2018;78(3):445-454.
35. American Cancer Society. Key Statistics for Merkel Cell Carcinoma. 2015; https://www.cancer.org/cancer/merkel-cell-skin-cancer/about/key-statistics.html#written_by. Accessed March 7th, 2017.
36. Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. The Lancet Oncology.17(10):1374-1385.
Infection with certain viruses has been causally linked to the development of cancer. In recent years, an improved understanding of the unique pathology and molecular underpinnings of these virally associated cancers has prompted the development of more personalized treatment strategies, with a particular focus on immunotherapy. Here, we describe some of the latest developments.
The link between viruses and cancer
Suspicions about a possible role of viral infections in the development of cancer were first aroused in the early 1900s. The seminal discovery is traced back to Peyton Rous, who showed that a malignant tumor growing in a chicken could be transferred to a healthy bird by injecting it with tumor extracts that contained no actual tumor cells.1
The infectious etiology of human cancer, however, remained controversial until many years later when the first cancer-causing virus, Epstein-Barr virus (EBV), was identified in cell cultures from patients with Burkitt lymphoma. Shortly afterward, the Rous sarcoma virus was unveiled as the oncogenic agent behind Rous’ observations.2Seven viruses have now been linked to the development of cancers and are thought to be responsible for around 12% of all cancer cases worldwide. The burden is likely to increase as technological advancements make it easier to establish a causal link between viruses and cancer development.3
In addition to making these links, researchers have also made significant headway in understanding how viruses cause cancer. Cancerous transformation of host cells occurs in only a minority of those who are infected with oncogenic viruses and often occurs in the setting of chronic infection.
Viruses can mediate carcinogenesis by direct and/or indirect mechanisms (Figure 1). Many of the hallmarks of cancer, the key attributes that drive the transformation from a normal cell to a malignant one, are compatible with the virus’s needs, such as needing to avoid cell death, increasing cell proliferation, and avoiding detection by the immune system.
Viruses hijack the cellular machinery to meet those needs and they can do this either by producing viral proteins that have an oncogenic effect or by integrating their genetic material into the host cell genome. When the latter occurs, the process of integration can also cause damage to the DNA, which further increases the risk of cancer-promoting changes occurring in the host genome.
Viruses can indirectly contribute to carcinogenesis by fostering a microenvironment of chronic inflammation, causing oxidative stress and local tissue damage, and by suppressing the antitumor immune response.4,5
Screening and prevention efforts have helped to reduce the burden of several different virally associated cancers. However, for the substantial proportion of patients who are still affected by these cancers, there is a pressing need for new therapeutic options, particularly since genome sequencing studies have revealed that these cancers can often have distinct underlying molecular mechanisms.
Vaccines lead the charge in HPV-driven cancers
German virologist Harald zur Hausen received the Nobel Prize in 2008 for his discovery of the oncogenic role of human papillomaviruses (HPVs), a large family of more than 100 DNA viruses that infect the epithelial cells of the skin and mucous membranes. They are responsible for the largest number of virally associated cancer cases globally – around 5% (Table 1).
A number of different cancer types are linked to HPV infection, but it is best known as the cause of cervical cancer. The development of diagnostic blood tests and prophylactic vaccines for prevention and early intervention in HPV infection has helped to reduce the incidence of cervical cancer. Conversely, another type of HPV-associated cancer, head and neck squamous cell carcinoma (HNSCC), has seen increased incidence in recent years.
HPVs are categorized according to their oncogenic potential as high, intermediate, or low risk. The high-risk HPV16 and HPV18 strains are most commonly associated with cancer. They are thought to cause cancer predominantly through integration into the host genome. The HPV genome is composed of 8 genes encoding proteins that regulate viral replication and assembly. The E6 and E7 genes are the most highly oncogenic; as the HPV DNA is inserted into the host genome, the transcriptional regulator of E6/E7 is lost, leading to their increased expression. These genes have significant oncogenic potential because of their interaction with 2 tumor suppressor proteins, p53 and pRb.6,7
The largest investment in therapeutic development for HPV-positive cancers has been in the realm of immunotherapy in an effort to boost the anti-tumor immune response. In particular, there has been a focus on the development of therapeutic vaccines, designed to prime the anti-tumor immune response to recognize viral antigens. A variety of different types of vaccines are being developed, including live, attenuated and inactivated vaccines that are protein, DNA, or peptide based. Most developed to date target the E6/E7 proteins from the HPV16/18 strains (Table 2).8,9
Other immunotherapies are also being evaluated, including immune checkpoint inhibitors, antibodies designed to target one of the principal mechanisms of immune evasion exploited by cancer cells. The combination of immune checkpoint inhibitors with vaccines is a particularly promising strategy in HPV-associated cancers. At the European Society for Medical Oncology Congress in 2017, the results of a phase 2 trial of nivolumab in combination with ISA-101 were presented.
Among 24 patients with HPV-positive tumors, the majority oropharyngeal cancers, the combination elicited an overall response rate (ORR) of 33%, including 2 complete responses (CRs). Most adverse events (AEs) were mild to moderate in severity and included fever, injection site reactions, fatigue and nausea.14
Hepatocellular carcinoma: a tale of two viruses
The hepatitis viruses are a group of 5 unrelated viruses that causes inflammation of the liver. Hepatitis B (HBV), a DNA virus, and hepatitis C (HCV), an RNA virus, are also oncoviruses; HBV in particular is one of the main causes of hepatocellular carcinoma (HCC), the most common type of liver cancer.
The highly inflammatory environment fostered by HBV and HCV infection causes liver damage that often leads to cirrhosis. Continued infection can drive permanent damage to the hepatocytes, leading to genetic and epigenetic damage and driving oncogenesis. As an RNA virus, HCV doesn’t integrate into the genome and no confirmed viral oncoproteins have been identified to date, therefore it mostly drives cancer through these indirect mechanisms, which is also reflected in the fact that HCV-associated HCC predominantly occurs against a backdrop of liver cirrhosis.
HBV does integrate into the host genome. Genome sequencing studies revealed hundreds of integration sites, but most commonly they disrupted host genes involved in telomere stability and cell cycle regulation, providing some insight into the mechanisms by which HBV-associated HCC develops. In addition, HBV produces several oncoproteins, including HBx, which disrupts gene transcription, cell signaling pathways, cell cycle progress, apoptosis and other cellular processes.15,16
Multitargeted tyrosine kinase inhibitors (TKIs) have been the focal point of therapeutic development in HCC. However, following the approval of sorafenib in 2008, there was a dearth of effective new treatment options despite substantial efforts and numerous phase 3 trials. More recently, immunotherapy has also come to the forefront, especially immune checkpoint inhibitors.
Last year marked the first new drug approvals in nearly a decade – the TKI regorafenib (Stivarga) and immune checkpoint inhibitor nivolumab (Opdivo), both in the second-line setting after failure of sorafenib. Treatment options in this setting may continue to expand, with the TKIs cabozantinib and lenvatinib and the immune checkpoint inhibitor pembrolizumab and the combination of durvalumab and tremelimumab hot on their heels.17-20 Many of these drugs are also being evaluated in the front-line setting in comparison with sorafenib (Table 3).
At the current time, the treatment strategy for patients with HCC is independent of etiology, however, there are significant ongoing efforts to try to tease out the implications of infection for treatment efficacy. A recent meta-analysis of patients treated with sorafenib in 3 randomized phase 3 trials (n = 3,526) suggested that it improved overall survival (OS) among patients who were HCV-positive, but HBV-negative.21
Studies of the vascular endothelial growth factor receptor 2-targeting monoclonal antibody ramucirumab, on the other hand, suggested that it may have a greater OS benefit in patients with HBV, while regorafenib seemed to have a comparable OS benefit in both subgroups.22-25 The immune checkpoint inhibitors studied thus far seem to elicit responses irrespective of infection status.
A phase 2 trial of the immune checkpoint inhibitor tremelimumab was conducted specifically in patients with advanced HCC and chronic HCV infection. The disease control rate (DCR) was 76.4%, with 17.6% partial response (PR) rate. There was also a significant drop in viral load, suggesting that tremelimumab may have antiviral effects.26,27,28
Adoptive cell therapy promising in EBV-positive cancers
More than 90% of the global population is infected with EBV, making it one of the most common human viruses. It is a member of the herpesvirus family that is probably best known as the cause of infectious mononucleosis. On rare occasions, however, EBV can cause tumor development, though our understanding of its exact pathogenic role in cancer is still incomplete.
EBV is a DNA virus that doesn’t tend to integrate into the host genome, but instead remains in the nucleus in the form of episomes and produces several oncoproteins, including latent membrane protein-1. It is associated with a range of different cancer types, including Burkitt lymphoma and other B-cell malignancies. It also infects epithelial cells and can cause nasopharyngeal carcinoma and gastric cancer, however, much less is known about the molecular underpinnings of these EBV-positive cancer types.26,27Gastric cancers actually comprise the largest group of EBV-associated tumors because of the global incidence of this cancer type. The Cancer Genome Atlas Research Network recently characterized gastric cancer on a molecular level and identified an EBV-positive subgroup as a distinct clinical entity with unique molecular characteristics.29
The focus of therapeutic development has again been on immunotherapy, however in this case the idea of collecting the patients T cells, engineering them to recognize EBV, and then reinfusing them into the patient – adoptive cell therapy – has gained the most traction (Table 4).
Two presentations at the American Society of Hematology annual meeting in 2017 detailed ongoing clinical trials of Atara Biotherapeutics’ ATA129 and Cell Medica’s CMD-003. ATA129 was associated with a high response rate and a low rate of serious AEs in patients with posttransplant lymphoproliferative disorder; ORR was 80% in 6 patients treated after hematopoietic stem cell transplantation, and 83% in 6 patients after solid organ transplant.30
CMD-003, meanwhile, demonstrated preliminary signs of activity and safety in patients with relapsed extranodal NK/T-cell lymphoma, according to early results from the phase 2 CITADEL trial. Among 6 evaluable patients, the ORR was 50% and the DCR was 67%.31
Newest oncovirus on the block
The most recently discovered cancer-associated virus is Merkel cell polyomavirus (MCV), a DNA virus that was identified in 2008. Like EBV, virtually the whole global adult population is infected with MCV. It is linked to the development of a highly aggressive and lethal, though rare, form of skin cancer – Merkel cell carcinoma.
MCV is found in around 80% of MCC cases and in fewer than 10% of melanomas and other skin cancers. Thus far, several direct mechanisms of oncogenesis have been described, including integration of MCV into the host genome and the production of viral oncogenes, though their precise function is as yet unclear.32-34
The American Cancer Society estimates that only 1500 cases of MCC are diagnosed each year in the United States.35 Its rarity makes it difficult to conduct clinical trials with sufficient power, yet some headway has still been made.
Around half of MCCs express the programmed cell death ligand 1 (PD-L1) on their surface, making them a logical candidate for immune checkpoint inhibition. In 2017, avelumab became the first FDA-approved drug for the treatment of MCC. Approval was based on the JAVELIN Merkel 200 study in which 88 patients received avelumab. After 1 year of follow-up the ORR was 31.8%, with a CR rate of 9%.36
Genome sequencing studies suggest that the mutational profile of MCV-positive tumors is quite different to those that are MCV-negative, which could have therapeutic implications. To date, these implications have not been delineated, given the challenge of small patient numbers, however an ongoing phase 1/2 trial is evaluating the combination of avelumab and radiation therapy or recombinant interferon beta, with or without MCV-specific cytotoxic T cells in patients with MCC and MCV infection.
The 2 other known cancer-causing viruses are human T-lymphotropic virus 1 (HTLV-1), a retrovirus associated with adult T-cell leukemia/lymphoma (ATL) and Kaposi sarcoma herpesvirus (KSHV). The latter is the causative agent of Kaposi sarcoma, often in combination with human immunodeficiency virus (HIV), a rare skin tumor that became renowned in the 1980s as an AIDS-defining illness.
The incidence of HTLV-1- and KSHV-positive tumors is substantially lower than the other virally associated cancers and, like MCC, this makes studying them and conducting clinical trials of novel therapeutic options a challenge. Nonetheless, several trials of targeted therapies and immunotherapies are underway.
Infection with certain viruses has been causally linked to the development of cancer. In recent years, an improved understanding of the unique pathology and molecular underpinnings of these virally associated cancers has prompted the development of more personalized treatment strategies, with a particular focus on immunotherapy. Here, we describe some of the latest developments.
The link between viruses and cancer
Suspicions about a possible role of viral infections in the development of cancer were first aroused in the early 1900s. The seminal discovery is traced back to Peyton Rous, who showed that a malignant tumor growing in a chicken could be transferred to a healthy bird by injecting it with tumor extracts that contained no actual tumor cells.1
The infectious etiology of human cancer, however, remained controversial until many years later when the first cancer-causing virus, Epstein-Barr virus (EBV), was identified in cell cultures from patients with Burkitt lymphoma. Shortly afterward, the Rous sarcoma virus was unveiled as the oncogenic agent behind Rous’ observations.2Seven viruses have now been linked to the development of cancers and are thought to be responsible for around 12% of all cancer cases worldwide. The burden is likely to increase as technological advancements make it easier to establish a causal link between viruses and cancer development.3
In addition to making these links, researchers have also made significant headway in understanding how viruses cause cancer. Cancerous transformation of host cells occurs in only a minority of those who are infected with oncogenic viruses and often occurs in the setting of chronic infection.
Viruses can mediate carcinogenesis by direct and/or indirect mechanisms (Figure 1). Many of the hallmarks of cancer, the key attributes that drive the transformation from a normal cell to a malignant one, are compatible with the virus’s needs, such as needing to avoid cell death, increasing cell proliferation, and avoiding detection by the immune system.
Viruses hijack the cellular machinery to meet those needs and they can do this either by producing viral proteins that have an oncogenic effect or by integrating their genetic material into the host cell genome. When the latter occurs, the process of integration can also cause damage to the DNA, which further increases the risk of cancer-promoting changes occurring in the host genome.
Viruses can indirectly contribute to carcinogenesis by fostering a microenvironment of chronic inflammation, causing oxidative stress and local tissue damage, and by suppressing the antitumor immune response.4,5
Screening and prevention efforts have helped to reduce the burden of several different virally associated cancers. However, for the substantial proportion of patients who are still affected by these cancers, there is a pressing need for new therapeutic options, particularly since genome sequencing studies have revealed that these cancers can often have distinct underlying molecular mechanisms.
Vaccines lead the charge in HPV-driven cancers
German virologist Harald zur Hausen received the Nobel Prize in 2008 for his discovery of the oncogenic role of human papillomaviruses (HPVs), a large family of more than 100 DNA viruses that infect the epithelial cells of the skin and mucous membranes. They are responsible for the largest number of virally associated cancer cases globally – around 5% (Table 1).
A number of different cancer types are linked to HPV infection, but it is best known as the cause of cervical cancer. The development of diagnostic blood tests and prophylactic vaccines for prevention and early intervention in HPV infection has helped to reduce the incidence of cervical cancer. Conversely, another type of HPV-associated cancer, head and neck squamous cell carcinoma (HNSCC), has seen increased incidence in recent years.
HPVs are categorized according to their oncogenic potential as high, intermediate, or low risk. The high-risk HPV16 and HPV18 strains are most commonly associated with cancer. They are thought to cause cancer predominantly through integration into the host genome. The HPV genome is composed of 8 genes encoding proteins that regulate viral replication and assembly. The E6 and E7 genes are the most highly oncogenic; as the HPV DNA is inserted into the host genome, the transcriptional regulator of E6/E7 is lost, leading to their increased expression. These genes have significant oncogenic potential because of their interaction with 2 tumor suppressor proteins, p53 and pRb.6,7
The largest investment in therapeutic development for HPV-positive cancers has been in the realm of immunotherapy in an effort to boost the anti-tumor immune response. In particular, there has been a focus on the development of therapeutic vaccines, designed to prime the anti-tumor immune response to recognize viral antigens. A variety of different types of vaccines are being developed, including live, attenuated and inactivated vaccines that are protein, DNA, or peptide based. Most developed to date target the E6/E7 proteins from the HPV16/18 strains (Table 2).8,9
Other immunotherapies are also being evaluated, including immune checkpoint inhibitors, antibodies designed to target one of the principal mechanisms of immune evasion exploited by cancer cells. The combination of immune checkpoint inhibitors with vaccines is a particularly promising strategy in HPV-associated cancers. At the European Society for Medical Oncology Congress in 2017, the results of a phase 2 trial of nivolumab in combination with ISA-101 were presented.
Among 24 patients with HPV-positive tumors, the majority oropharyngeal cancers, the combination elicited an overall response rate (ORR) of 33%, including 2 complete responses (CRs). Most adverse events (AEs) were mild to moderate in severity and included fever, injection site reactions, fatigue and nausea.14
Hepatocellular carcinoma: a tale of two viruses
The hepatitis viruses are a group of 5 unrelated viruses that causes inflammation of the liver. Hepatitis B (HBV), a DNA virus, and hepatitis C (HCV), an RNA virus, are also oncoviruses; HBV in particular is one of the main causes of hepatocellular carcinoma (HCC), the most common type of liver cancer.
The highly inflammatory environment fostered by HBV and HCV infection causes liver damage that often leads to cirrhosis. Continued infection can drive permanent damage to the hepatocytes, leading to genetic and epigenetic damage and driving oncogenesis. As an RNA virus, HCV doesn’t integrate into the genome and no confirmed viral oncoproteins have been identified to date, therefore it mostly drives cancer through these indirect mechanisms, which is also reflected in the fact that HCV-associated HCC predominantly occurs against a backdrop of liver cirrhosis.
HBV does integrate into the host genome. Genome sequencing studies revealed hundreds of integration sites, but most commonly they disrupted host genes involved in telomere stability and cell cycle regulation, providing some insight into the mechanisms by which HBV-associated HCC develops. In addition, HBV produces several oncoproteins, including HBx, which disrupts gene transcription, cell signaling pathways, cell cycle progress, apoptosis and other cellular processes.15,16
Multitargeted tyrosine kinase inhibitors (TKIs) have been the focal point of therapeutic development in HCC. However, following the approval of sorafenib in 2008, there was a dearth of effective new treatment options despite substantial efforts and numerous phase 3 trials. More recently, immunotherapy has also come to the forefront, especially immune checkpoint inhibitors.
Last year marked the first new drug approvals in nearly a decade – the TKI regorafenib (Stivarga) and immune checkpoint inhibitor nivolumab (Opdivo), both in the second-line setting after failure of sorafenib. Treatment options in this setting may continue to expand, with the TKIs cabozantinib and lenvatinib and the immune checkpoint inhibitor pembrolizumab and the combination of durvalumab and tremelimumab hot on their heels.17-20 Many of these drugs are also being evaluated in the front-line setting in comparison with sorafenib (Table 3).
At the current time, the treatment strategy for patients with HCC is independent of etiology, however, there are significant ongoing efforts to try to tease out the implications of infection for treatment efficacy. A recent meta-analysis of patients treated with sorafenib in 3 randomized phase 3 trials (n = 3,526) suggested that it improved overall survival (OS) among patients who were HCV-positive, but HBV-negative.21
Studies of the vascular endothelial growth factor receptor 2-targeting monoclonal antibody ramucirumab, on the other hand, suggested that it may have a greater OS benefit in patients with HBV, while regorafenib seemed to have a comparable OS benefit in both subgroups.22-25 The immune checkpoint inhibitors studied thus far seem to elicit responses irrespective of infection status.
A phase 2 trial of the immune checkpoint inhibitor tremelimumab was conducted specifically in patients with advanced HCC and chronic HCV infection. The disease control rate (DCR) was 76.4%, with 17.6% partial response (PR) rate. There was also a significant drop in viral load, suggesting that tremelimumab may have antiviral effects.26,27,28
Adoptive cell therapy promising in EBV-positive cancers
More than 90% of the global population is infected with EBV, making it one of the most common human viruses. It is a member of the herpesvirus family that is probably best known as the cause of infectious mononucleosis. On rare occasions, however, EBV can cause tumor development, though our understanding of its exact pathogenic role in cancer is still incomplete.
EBV is a DNA virus that doesn’t tend to integrate into the host genome, but instead remains in the nucleus in the form of episomes and produces several oncoproteins, including latent membrane protein-1. It is associated with a range of different cancer types, including Burkitt lymphoma and other B-cell malignancies. It also infects epithelial cells and can cause nasopharyngeal carcinoma and gastric cancer, however, much less is known about the molecular underpinnings of these EBV-positive cancer types.26,27Gastric cancers actually comprise the largest group of EBV-associated tumors because of the global incidence of this cancer type. The Cancer Genome Atlas Research Network recently characterized gastric cancer on a molecular level and identified an EBV-positive subgroup as a distinct clinical entity with unique molecular characteristics.29
The focus of therapeutic development has again been on immunotherapy, however in this case the idea of collecting the patients T cells, engineering them to recognize EBV, and then reinfusing them into the patient – adoptive cell therapy – has gained the most traction (Table 4).
Two presentations at the American Society of Hematology annual meeting in 2017 detailed ongoing clinical trials of Atara Biotherapeutics’ ATA129 and Cell Medica’s CMD-003. ATA129 was associated with a high response rate and a low rate of serious AEs in patients with posttransplant lymphoproliferative disorder; ORR was 80% in 6 patients treated after hematopoietic stem cell transplantation, and 83% in 6 patients after solid organ transplant.30
CMD-003, meanwhile, demonstrated preliminary signs of activity and safety in patients with relapsed extranodal NK/T-cell lymphoma, according to early results from the phase 2 CITADEL trial. Among 6 evaluable patients, the ORR was 50% and the DCR was 67%.31
Newest oncovirus on the block
The most recently discovered cancer-associated virus is Merkel cell polyomavirus (MCV), a DNA virus that was identified in 2008. Like EBV, virtually the whole global adult population is infected with MCV. It is linked to the development of a highly aggressive and lethal, though rare, form of skin cancer – Merkel cell carcinoma.
MCV is found in around 80% of MCC cases and in fewer than 10% of melanomas and other skin cancers. Thus far, several direct mechanisms of oncogenesis have been described, including integration of MCV into the host genome and the production of viral oncogenes, though their precise function is as yet unclear.32-34
The American Cancer Society estimates that only 1500 cases of MCC are diagnosed each year in the United States.35 Its rarity makes it difficult to conduct clinical trials with sufficient power, yet some headway has still been made.
Around half of MCCs express the programmed cell death ligand 1 (PD-L1) on their surface, making them a logical candidate for immune checkpoint inhibition. In 2017, avelumab became the first FDA-approved drug for the treatment of MCC. Approval was based on the JAVELIN Merkel 200 study in which 88 patients received avelumab. After 1 year of follow-up the ORR was 31.8%, with a CR rate of 9%.36
Genome sequencing studies suggest that the mutational profile of MCV-positive tumors is quite different to those that are MCV-negative, which could have therapeutic implications. To date, these implications have not been delineated, given the challenge of small patient numbers, however an ongoing phase 1/2 trial is evaluating the combination of avelumab and radiation therapy or recombinant interferon beta, with or without MCV-specific cytotoxic T cells in patients with MCC and MCV infection.
The 2 other known cancer-causing viruses are human T-lymphotropic virus 1 (HTLV-1), a retrovirus associated with adult T-cell leukemia/lymphoma (ATL) and Kaposi sarcoma herpesvirus (KSHV). The latter is the causative agent of Kaposi sarcoma, often in combination with human immunodeficiency virus (HIV), a rare skin tumor that became renowned in the 1980s as an AIDS-defining illness.
The incidence of HTLV-1- and KSHV-positive tumors is substantially lower than the other virally associated cancers and, like MCC, this makes studying them and conducting clinical trials of novel therapeutic options a challenge. Nonetheless, several trials of targeted therapies and immunotherapies are underway.
1. Rous PA. Transmissible avain neoplasm. (Sarcoma of the common fowl). J Exp Med. 1910;12(5):696-705.
2. Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet. 1964;1(7335):702-703.
3. Mesri Enrique A, Feitelson MA, Munger K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host & Microbe. 2014;15(3):266-282.
4. Santana-Davila R, Bhatia S, Chow LQ. Harnessing the immune system as a therapeutic tool in virus-associated cancers. JAMA Oncol. 2017;3(1):106-112.
5. Tashiro H, Brenner MK. Immunotherapy against cancer-related viruses. Cell Res. 2017;27(1):59-73.
6. Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017;40(2):80-85.
7. Tulay P, Serakinci N. The route to HPV-associated neoplastic transformation: a review of the literature. Crit Rev Eukaryot Gene Expr. 2016;26(1):27-39.
8. Smola S. Immunopathogenesis of HPV-associated cancers and prospects for immunotherapy. Viruses. 2017;9(9).
9. Rosales R, Rosales C. Immune therapy for human papillomaviruses-related cancers. World Journal of Clinical Oncology. 2014;5(5):1002-1019.
10. Miles B, Safran HP, Monk BJ. Therapeutic options for treatment of human papillomavirus-associated cancers - novel immunologic vaccines: ADXS11-001. Gynecol Oncol Res Pract. 2017;4:10.
11. Miles BA, Monk BJ, Safran HP. Mechanistic insights into ADXS11-001 human papillomavirus-associated cancer immunotherapy. Gynecol Oncol Res Pract. 2017;4:9.
12. Huh W, Dizon D, Powell M, Landrum L, Leath C. A prospective phase II trial of the listeria-based human papillomavirus immunotherapy axalimogene filolisbac in second and third-line metastatic cervical cancer: A NRG oncology group trial. Paper presented at: Annual Meeting on Women's Cancer; March 12-15, 2017, 2017; National Harbor, MD.
13. Petit RG, Mehta A, Jain M, et al. ADXS11-001 immunotherapy targeting HPV-E7: final results from a Phase II study in Indian women with recurrent cervical cancer. Journal for Immunotherapy of Cancer. 2014;2(Suppl 3):P92-P92.
14. Glisson B, Massarelli E, William W, et al. Nivolumab and ISA 101 HPV vaccine in incurable HPV-16+ cancer. Ann Oncol. 2017;28(suppl_5):v403-v427.
15. Ding X-X, Zhu Q-G, Zhang S-M, et al. Precision medicine for hepatocellular carcinoma: driver mutations and targeted therapy. Oncotarget. 2017;8(33):55715-55730.
16. Ringehan M, McKeating JA, Protzer U. Viral hepatitis and liver cancer. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017;372(1732):20160274.
17. Abou-Alfa G, Meyer T, Cheng AL, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2017;36(Suppl 4S):abstr 207.
18. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018.
19. Zhu AX, Finn RS, Cattan S, et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36(Suppl 4S):Abstr 209.
20. Kelley RK, Abou-Alfa GK, Bendell JC, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. Journal of Clinical Oncology. 2017;35(15_suppl):4073-4073.
21. Jackson R, Psarelli E-E, Berhane S, Khan H, Johnson P. Impact of Viral Status on Survival in Patients Receiving Sorafenib for Advanced Hepatocellular Cancer: A Meta-Analysis of Randomized Phase III Trials. Journal of Clinical Oncology. 2017;35(6):622-628.
22. Kudo M. Molecular Targeted Agents for Hepatocellular Carcinoma: Current Status and Future Perspectives. Liver Cancer. 2017;6(2):101-112.
23. zur Hausen H, Meinhof W, Scheiber W, Bornkamm GW. Attempts to detect virus-secific DNA in human tumors. I. Nucleic acid hybridizations with complementary RNA of human wart virus. Int J Cancer. 1974;13(5):650-656.
24. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56-66.
25. Bruix J, Tak WY, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49(16):3412-3419.
26. Neparidze N, Lacy J. Malignancies associated with epstein-barr virus: pathobiology, clinical features, and evolving treatments. Clin Adv Hematol Oncol. 2014;12(6):358-371.
27. Ozoya OO, Sokol L, Dalia S. EBV-Related Malignancies, Outcomes and Novel Prevention Strategies. Infect Disord Drug Targets. 2016;16(1):4-21.
28. Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59(1):81-88.
29. The Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202.
30. Prockop S, Li A, Baiocchi R, et al. Efficacy and safety of ATA129, partially matched allogeneic third-party Epstein-Barr virus-targeted cytotoxic T lymphocytes in a multicenter study for post-transplant lymphoproliferative disorder. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
31. Kim W, Ardeshna K, Lin Y, et al. Autologous EBV-specific T cells (CMD-003): Early results from a multicenter, multinational Phase 2 trial for treatment of EBV-associated NK/T-cell lymphoma. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
32. Schadendorf D, Lebbé C, zur Hausen A, et al. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. European Journal of Cancer. 2017;71:53-69.
33. Spurgeon ME, Lambert PF. Merkel cell polyomavirus: a newly discovered human virus with oncogenic potential. Virology. 2013;435(1):118-130.
34. Tello TL, Coggshall K, Yom SS, Yu SS. Merkel cell carcinoma: An update and review: Current and future therapy. J Am Acad Dermatol. 2018;78(3):445-454.
35. American Cancer Society. Key Statistics for Merkel Cell Carcinoma. 2015; https://www.cancer.org/cancer/merkel-cell-skin-cancer/about/key-statistics.html#written_by. Accessed March 7th, 2017.
36. Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. The Lancet Oncology.17(10):1374-1385.
1. Rous PA. Transmissible avain neoplasm. (Sarcoma of the common fowl). J Exp Med. 1910;12(5):696-705.
2. Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet. 1964;1(7335):702-703.
3. Mesri Enrique A, Feitelson MA, Munger K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host & Microbe. 2014;15(3):266-282.
4. Santana-Davila R, Bhatia S, Chow LQ. Harnessing the immune system as a therapeutic tool in virus-associated cancers. JAMA Oncol. 2017;3(1):106-112.
5. Tashiro H, Brenner MK. Immunotherapy against cancer-related viruses. Cell Res. 2017;27(1):59-73.
6. Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017;40(2):80-85.
7. Tulay P, Serakinci N. The route to HPV-associated neoplastic transformation: a review of the literature. Crit Rev Eukaryot Gene Expr. 2016;26(1):27-39.
8. Smola S. Immunopathogenesis of HPV-associated cancers and prospects for immunotherapy. Viruses. 2017;9(9).
9. Rosales R, Rosales C. Immune therapy for human papillomaviruses-related cancers. World Journal of Clinical Oncology. 2014;5(5):1002-1019.
10. Miles B, Safran HP, Monk BJ. Therapeutic options for treatment of human papillomavirus-associated cancers - novel immunologic vaccines: ADXS11-001. Gynecol Oncol Res Pract. 2017;4:10.
11. Miles BA, Monk BJ, Safran HP. Mechanistic insights into ADXS11-001 human papillomavirus-associated cancer immunotherapy. Gynecol Oncol Res Pract. 2017;4:9.
12. Huh W, Dizon D, Powell M, Landrum L, Leath C. A prospective phase II trial of the listeria-based human papillomavirus immunotherapy axalimogene filolisbac in second and third-line metastatic cervical cancer: A NRG oncology group trial. Paper presented at: Annual Meeting on Women's Cancer; March 12-15, 2017, 2017; National Harbor, MD.
13. Petit RG, Mehta A, Jain M, et al. ADXS11-001 immunotherapy targeting HPV-E7: final results from a Phase II study in Indian women with recurrent cervical cancer. Journal for Immunotherapy of Cancer. 2014;2(Suppl 3):P92-P92.
14. Glisson B, Massarelli E, William W, et al. Nivolumab and ISA 101 HPV vaccine in incurable HPV-16+ cancer. Ann Oncol. 2017;28(suppl_5):v403-v427.
15. Ding X-X, Zhu Q-G, Zhang S-M, et al. Precision medicine for hepatocellular carcinoma: driver mutations and targeted therapy. Oncotarget. 2017;8(33):55715-55730.
16. Ringehan M, McKeating JA, Protzer U. Viral hepatitis and liver cancer. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017;372(1732):20160274.
17. Abou-Alfa G, Meyer T, Cheng AL, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2017;36(Suppl 4S):abstr 207.
18. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018.
19. Zhu AX, Finn RS, Cattan S, et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36(Suppl 4S):Abstr 209.
20. Kelley RK, Abou-Alfa GK, Bendell JC, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. Journal of Clinical Oncology. 2017;35(15_suppl):4073-4073.
21. Jackson R, Psarelli E-E, Berhane S, Khan H, Johnson P. Impact of Viral Status on Survival in Patients Receiving Sorafenib for Advanced Hepatocellular Cancer: A Meta-Analysis of Randomized Phase III Trials. Journal of Clinical Oncology. 2017;35(6):622-628.
22. Kudo M. Molecular Targeted Agents for Hepatocellular Carcinoma: Current Status and Future Perspectives. Liver Cancer. 2017;6(2):101-112.
23. zur Hausen H, Meinhof W, Scheiber W, Bornkamm GW. Attempts to detect virus-secific DNA in human tumors. I. Nucleic acid hybridizations with complementary RNA of human wart virus. Int J Cancer. 1974;13(5):650-656.
24. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56-66.
25. Bruix J, Tak WY, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49(16):3412-3419.
26. Neparidze N, Lacy J. Malignancies associated with epstein-barr virus: pathobiology, clinical features, and evolving treatments. Clin Adv Hematol Oncol. 2014;12(6):358-371.
27. Ozoya OO, Sokol L, Dalia S. EBV-Related Malignancies, Outcomes and Novel Prevention Strategies. Infect Disord Drug Targets. 2016;16(1):4-21.
28. Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59(1):81-88.
29. The Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202.
30. Prockop S, Li A, Baiocchi R, et al. Efficacy and safety of ATA129, partially matched allogeneic third-party Epstein-Barr virus-targeted cytotoxic T lymphocytes in a multicenter study for post-transplant lymphoproliferative disorder. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
31. Kim W, Ardeshna K, Lin Y, et al. Autologous EBV-specific T cells (CMD-003): Early results from a multicenter, multinational Phase 2 trial for treatment of EBV-associated NK/T-cell lymphoma. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
32. Schadendorf D, Lebbé C, zur Hausen A, et al. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. European Journal of Cancer. 2017;71:53-69.
33. Spurgeon ME, Lambert PF. Merkel cell polyomavirus: a newly discovered human virus with oncogenic potential. Virology. 2013;435(1):118-130.
34. Tello TL, Coggshall K, Yom SS, Yu SS. Merkel cell carcinoma: An update and review: Current and future therapy. J Am Acad Dermatol. 2018;78(3):445-454.
35. American Cancer Society. Key Statistics for Merkel Cell Carcinoma. 2015; https://www.cancer.org/cancer/merkel-cell-skin-cancer/about/key-statistics.html#written_by. Accessed March 7th, 2017.
36. Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. The Lancet Oncology.17(10):1374-1385.
ASH 2018 coming attractions look at the big picture
In the closest thing the medical world has to movie trailers, the American Society of Hematology held a press conference offering
Shorter R-CHOP regimen for DLBCL
Under the heading “Big Trials, Big Results” will be data from the FLYER trial, a phase 3, randomized, deescalation trial in 592 patients aged 18-60 years with favorable-prognosis diffuse large B-cell lymphoma. The investigators report that both progression-free survival and overall survival with four cycles of R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone) were noninferior to those for patients treated with six cycles of R-CHOP (abstract 781).
Ibrutinib mastery in CLL
Also on the program are results of a study showing that ibrutinib (Imbruvica), either alone or in combination with rituximab, is associated with superior progression-free survival than bendamustine and rituximab in older patients with chronic lymphocytic leukemia (CLL).
The trial, the Alliance North American Intergroup Study A041202 (abstract 6) is the first major trial to pit ibrutinib against the modern standard of immunochemotherapy rather than the older standard of chlorambucil, Dr. Brodsky noted.
Anemia support in beta-thalassemia, MDS
In nonmalignant disease, investigators in the randomized, phase 3 BELIEVE trial are reporting results of their study showing that the first-in-class erythroid maturation agent luspatercept was associated with significant reductions in the need for RBC transfusion in adults with transfusion-dependent beta-thalassemia.
The investigators report that the experimental agent was “generally well tolerated” (abstract 163).
“Beyond a proof of principle, [this is] certainly a very exciting advancement in this group of patients who otherwise had very few treatment options,” said Alexis A. Thompson, MD, associate director of equity and minority health at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago, and the current ASH president.
Dr. Thompson also highlighted the MEDALIST trial (abstract 1), a phase 3, randomized study showing that luspatercept significantly reduced transfusion burden, compared with placebo, in patients with anemia caused by very low–, low-, or intermediate-risk myelodysplastic syndrome with ring sideroblasts who require RBC transfusions.
“This group of patients were individuals who were refractory or were not responders or did not tolerate erythropoietic stimulating agents and therefore were requiring regular transfusion,” Dr. Thompson said.
Worth the wait
The late-breaking abstract program was stretched from the usual six abstracts to seven this year because of the unusually high quality of the science, Dr. Brodsky said.
Among these star attractions are results of a phase 3, randomized study of daratumumab (Darzalex) plus lenalidomide and dexamethasone versus lenalidomide-dexamethasone alone for patients with newly diagnosed multiple myeloma who are ineligible for transplant.
The investigators found that adding daratumumab reduced the risk of disease progression or death by close to 50%, supporting the combination as a new standard of care in these patients, according to Thierry Facon, MD, from the Hospital Claude Huriez in Lille, France, and colleagues (abstract LBA-2).
Two other late-breakers deal with CLL. The first, a randomized, phase 3 study of ibrutinib-based therapy versus standard fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy in younger patients with untreated CLL, found that ibrutinib and rituximab provided significantly better progression-free survival and overall survival (abstract LBA-4).
“These findings have immediate practice-changing implications and establish ibrutinib-based therapy as the most efficacious first-line therapy for patients with CLL,” wrote Tait D. Shanafelt, MD, from Stanford (Calif.) University, and colleagues.
On a less positive note, Australian researchers report their discovery of a recurrent mutation in BCL2 that confers resistance to venetoclax (Venclexta) in patients with progressive CLL (abstract LBA-7).
“This mutation provides new insights into the pathobiology of venetoclax resistance and provides a potential biomarker of impending clinical relapse,” wrote Piers Blombery, MBBS, from the University of Melbourne, and colleagues.
Finally, investigators from children’s hospitals in the United States and Europe report promising findings on the safety and efficacy of emapalumab for the treatment of patients with the rare genetic disorder primary hemophagocytic lymphohistiocytosis (HLH).
The drug, newly approved by the Food and Drug Administration, was able to control HLH’s hyperinflammatory activity, and allowed a substantial proportion of patients to survive to hematopoietic stem cell transplantation, the investigators said (abstract LBA-6).
In the closest thing the medical world has to movie trailers, the American Society of Hematology held a press conference offering
Shorter R-CHOP regimen for DLBCL
Under the heading “Big Trials, Big Results” will be data from the FLYER trial, a phase 3, randomized, deescalation trial in 592 patients aged 18-60 years with favorable-prognosis diffuse large B-cell lymphoma. The investigators report that both progression-free survival and overall survival with four cycles of R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone) were noninferior to those for patients treated with six cycles of R-CHOP (abstract 781).
Ibrutinib mastery in CLL
Also on the program are results of a study showing that ibrutinib (Imbruvica), either alone or in combination with rituximab, is associated with superior progression-free survival than bendamustine and rituximab in older patients with chronic lymphocytic leukemia (CLL).
The trial, the Alliance North American Intergroup Study A041202 (abstract 6) is the first major trial to pit ibrutinib against the modern standard of immunochemotherapy rather than the older standard of chlorambucil, Dr. Brodsky noted.
Anemia support in beta-thalassemia, MDS
In nonmalignant disease, investigators in the randomized, phase 3 BELIEVE trial are reporting results of their study showing that the first-in-class erythroid maturation agent luspatercept was associated with significant reductions in the need for RBC transfusion in adults with transfusion-dependent beta-thalassemia.
The investigators report that the experimental agent was “generally well tolerated” (abstract 163).
“Beyond a proof of principle, [this is] certainly a very exciting advancement in this group of patients who otherwise had very few treatment options,” said Alexis A. Thompson, MD, associate director of equity and minority health at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago, and the current ASH president.
Dr. Thompson also highlighted the MEDALIST trial (abstract 1), a phase 3, randomized study showing that luspatercept significantly reduced transfusion burden, compared with placebo, in patients with anemia caused by very low–, low-, or intermediate-risk myelodysplastic syndrome with ring sideroblasts who require RBC transfusions.
“This group of patients were individuals who were refractory or were not responders or did not tolerate erythropoietic stimulating agents and therefore were requiring regular transfusion,” Dr. Thompson said.
Worth the wait
The late-breaking abstract program was stretched from the usual six abstracts to seven this year because of the unusually high quality of the science, Dr. Brodsky said.
Among these star attractions are results of a phase 3, randomized study of daratumumab (Darzalex) plus lenalidomide and dexamethasone versus lenalidomide-dexamethasone alone for patients with newly diagnosed multiple myeloma who are ineligible for transplant.
The investigators found that adding daratumumab reduced the risk of disease progression or death by close to 50%, supporting the combination as a new standard of care in these patients, according to Thierry Facon, MD, from the Hospital Claude Huriez in Lille, France, and colleagues (abstract LBA-2).
Two other late-breakers deal with CLL. The first, a randomized, phase 3 study of ibrutinib-based therapy versus standard fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy in younger patients with untreated CLL, found that ibrutinib and rituximab provided significantly better progression-free survival and overall survival (abstract LBA-4).
“These findings have immediate practice-changing implications and establish ibrutinib-based therapy as the most efficacious first-line therapy for patients with CLL,” wrote Tait D. Shanafelt, MD, from Stanford (Calif.) University, and colleagues.
On a less positive note, Australian researchers report their discovery of a recurrent mutation in BCL2 that confers resistance to venetoclax (Venclexta) in patients with progressive CLL (abstract LBA-7).
“This mutation provides new insights into the pathobiology of venetoclax resistance and provides a potential biomarker of impending clinical relapse,” wrote Piers Blombery, MBBS, from the University of Melbourne, and colleagues.
Finally, investigators from children’s hospitals in the United States and Europe report promising findings on the safety and efficacy of emapalumab for the treatment of patients with the rare genetic disorder primary hemophagocytic lymphohistiocytosis (HLH).
The drug, newly approved by the Food and Drug Administration, was able to control HLH’s hyperinflammatory activity, and allowed a substantial proportion of patients to survive to hematopoietic stem cell transplantation, the investigators said (abstract LBA-6).
In the closest thing the medical world has to movie trailers, the American Society of Hematology held a press conference offering
Shorter R-CHOP regimen for DLBCL
Under the heading “Big Trials, Big Results” will be data from the FLYER trial, a phase 3, randomized, deescalation trial in 592 patients aged 18-60 years with favorable-prognosis diffuse large B-cell lymphoma. The investigators report that both progression-free survival and overall survival with four cycles of R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone) were noninferior to those for patients treated with six cycles of R-CHOP (abstract 781).
Ibrutinib mastery in CLL
Also on the program are results of a study showing that ibrutinib (Imbruvica), either alone or in combination with rituximab, is associated with superior progression-free survival than bendamustine and rituximab in older patients with chronic lymphocytic leukemia (CLL).
The trial, the Alliance North American Intergroup Study A041202 (abstract 6) is the first major trial to pit ibrutinib against the modern standard of immunochemotherapy rather than the older standard of chlorambucil, Dr. Brodsky noted.
Anemia support in beta-thalassemia, MDS
In nonmalignant disease, investigators in the randomized, phase 3 BELIEVE trial are reporting results of their study showing that the first-in-class erythroid maturation agent luspatercept was associated with significant reductions in the need for RBC transfusion in adults with transfusion-dependent beta-thalassemia.
The investigators report that the experimental agent was “generally well tolerated” (abstract 163).
“Beyond a proof of principle, [this is] certainly a very exciting advancement in this group of patients who otherwise had very few treatment options,” said Alexis A. Thompson, MD, associate director of equity and minority health at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago, and the current ASH president.
Dr. Thompson also highlighted the MEDALIST trial (abstract 1), a phase 3, randomized study showing that luspatercept significantly reduced transfusion burden, compared with placebo, in patients with anemia caused by very low–, low-, or intermediate-risk myelodysplastic syndrome with ring sideroblasts who require RBC transfusions.
“This group of patients were individuals who were refractory or were not responders or did not tolerate erythropoietic stimulating agents and therefore were requiring regular transfusion,” Dr. Thompson said.
Worth the wait
The late-breaking abstract program was stretched from the usual six abstracts to seven this year because of the unusually high quality of the science, Dr. Brodsky said.
Among these star attractions are results of a phase 3, randomized study of daratumumab (Darzalex) plus lenalidomide and dexamethasone versus lenalidomide-dexamethasone alone for patients with newly diagnosed multiple myeloma who are ineligible for transplant.
The investigators found that adding daratumumab reduced the risk of disease progression or death by close to 50%, supporting the combination as a new standard of care in these patients, according to Thierry Facon, MD, from the Hospital Claude Huriez in Lille, France, and colleagues (abstract LBA-2).
Two other late-breakers deal with CLL. The first, a randomized, phase 3 study of ibrutinib-based therapy versus standard fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy in younger patients with untreated CLL, found that ibrutinib and rituximab provided significantly better progression-free survival and overall survival (abstract LBA-4).
“These findings have immediate practice-changing implications and establish ibrutinib-based therapy as the most efficacious first-line therapy for patients with CLL,” wrote Tait D. Shanafelt, MD, from Stanford (Calif.) University, and colleagues.
On a less positive note, Australian researchers report their discovery of a recurrent mutation in BCL2 that confers resistance to venetoclax (Venclexta) in patients with progressive CLL (abstract LBA-7).
“This mutation provides new insights into the pathobiology of venetoclax resistance and provides a potential biomarker of impending clinical relapse,” wrote Piers Blombery, MBBS, from the University of Melbourne, and colleagues.
Finally, investigators from children’s hospitals in the United States and Europe report promising findings on the safety and efficacy of emapalumab for the treatment of patients with the rare genetic disorder primary hemophagocytic lymphohistiocytosis (HLH).
The drug, newly approved by the Food and Drug Administration, was able to control HLH’s hyperinflammatory activity, and allowed a substantial proportion of patients to survive to hematopoietic stem cell transplantation, the investigators said (abstract LBA-6).
ASH preview: Studies target CAR T-cell improvements
, according to investigators in two separate studies.
Meanwhile, responses to tisagenlecleucel appear to be even more durable with longer follow-up, according to preliminary results from two more CAR T-cell therapy studies slated for presentation at the annual meeting of the American Society of Hematology.
A fifth study will show that bone marrow transplant may effectively consolidate remission after CAR T-cell therapy, according to Robert A. Brodsky, MD, ASH secretary, who highlighted the studies during a media briefing.
The ibrutinib study (abstract 299) shows that administering this BTK inhibitor starting 2 weeks prior to leukapheresis and continuing until 3 months after JCAR014 could improve responses and may decrease the incidence of severe cytokine release syndrome in patients with relapsed or refractory chronic lymphocytic leukemia (CLL).
Of 16 patients in an ibrutinib cohort and 18 patients in a no-ibrutinib cohort, the proportion of responders was 88% and 56%, respectively, according to preliminary data reported in the abstract. Grade 3-5 cytokine release syndrome occurred in 5 of 19 patients in the no-ibrutinib cohort, and 0 of 17 patients in the ibrutinib cohort.
Those findings are “early and preliminary, but very exciting” for ibrutinib in combination with this CD-19 specific CAR T-cell therapy said Dr. Brodsky, director of the division of hematology at Johns Hopkins University in Baltimore.
Early results of the checkpoint inhibitor study (abstract 556) suggest that pembrolizumab or nivolumab may augment CD19-directed CAR T-cell therapy in patients with relapsed B-cell acute lymphoblastic leukemia (ALL).
In data to date, 14 patients with early CAR T-cell loss, partial response, or no response to CAR T-cell therapy received a PD-1 inhibitor.
“The idea was if you can give pembrolizumab, you can take the brakes off, and maybe you can reinitiate the immune attack,” Dr. Brodsky said. “Sure enough, they were able to see that in roughly half of the patients. So again, very small, preliminary data, but very exciting that it is safe to give checkpoint inhibitors with CAR T-cells and it may be efficacious at getting the immune response back.”
One of the two tisagenlecleucel updates (abstract 895) showed that in the ELIANA trial, which included pediatric and young adults patients with relapsed/refractory ALL, the probability of relapse-free survival at 18 months was 66%.
“These are some very fast-growing tumors and these are refractory resistant patients, so as we get further and further out, it’s more encouraging to see that there are durable responses,” Dr. Brodsky said.
In the other tisagenlecleucel update (abstract 1684), investigators showed sustained disease control in Juliet, the global trial including adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), with 40% of patients still in remission at 18 months, according to Dr. Brodsky.
One more report Dr. Brodsky highlighted (abstract 967) will look at long-term follow-up after administration of SCRI-CAR19v1, a CD19-specific CAR T-cell product. Preliminary data suggest a survival advantage when hematopoietic stem cell transplantation is done after CAR T-cell induced remission.
“This study is very small and it’s retrospective, but it suggests that bone marrow transplant is a good way to consolidate the remission after CAR T-cell therapy,” Dr. Brodsky said.
, according to investigators in two separate studies.
Meanwhile, responses to tisagenlecleucel appear to be even more durable with longer follow-up, according to preliminary results from two more CAR T-cell therapy studies slated for presentation at the annual meeting of the American Society of Hematology.
A fifth study will show that bone marrow transplant may effectively consolidate remission after CAR T-cell therapy, according to Robert A. Brodsky, MD, ASH secretary, who highlighted the studies during a media briefing.
The ibrutinib study (abstract 299) shows that administering this BTK inhibitor starting 2 weeks prior to leukapheresis and continuing until 3 months after JCAR014 could improve responses and may decrease the incidence of severe cytokine release syndrome in patients with relapsed or refractory chronic lymphocytic leukemia (CLL).
Of 16 patients in an ibrutinib cohort and 18 patients in a no-ibrutinib cohort, the proportion of responders was 88% and 56%, respectively, according to preliminary data reported in the abstract. Grade 3-5 cytokine release syndrome occurred in 5 of 19 patients in the no-ibrutinib cohort, and 0 of 17 patients in the ibrutinib cohort.
Those findings are “early and preliminary, but very exciting” for ibrutinib in combination with this CD-19 specific CAR T-cell therapy said Dr. Brodsky, director of the division of hematology at Johns Hopkins University in Baltimore.
Early results of the checkpoint inhibitor study (abstract 556) suggest that pembrolizumab or nivolumab may augment CD19-directed CAR T-cell therapy in patients with relapsed B-cell acute lymphoblastic leukemia (ALL).
In data to date, 14 patients with early CAR T-cell loss, partial response, or no response to CAR T-cell therapy received a PD-1 inhibitor.
“The idea was if you can give pembrolizumab, you can take the brakes off, and maybe you can reinitiate the immune attack,” Dr. Brodsky said. “Sure enough, they were able to see that in roughly half of the patients. So again, very small, preliminary data, but very exciting that it is safe to give checkpoint inhibitors with CAR T-cells and it may be efficacious at getting the immune response back.”
One of the two tisagenlecleucel updates (abstract 895) showed that in the ELIANA trial, which included pediatric and young adults patients with relapsed/refractory ALL, the probability of relapse-free survival at 18 months was 66%.
“These are some very fast-growing tumors and these are refractory resistant patients, so as we get further and further out, it’s more encouraging to see that there are durable responses,” Dr. Brodsky said.
In the other tisagenlecleucel update (abstract 1684), investigators showed sustained disease control in Juliet, the global trial including adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), with 40% of patients still in remission at 18 months, according to Dr. Brodsky.
One more report Dr. Brodsky highlighted (abstract 967) will look at long-term follow-up after administration of SCRI-CAR19v1, a CD19-specific CAR T-cell product. Preliminary data suggest a survival advantage when hematopoietic stem cell transplantation is done after CAR T-cell induced remission.
“This study is very small and it’s retrospective, but it suggests that bone marrow transplant is a good way to consolidate the remission after CAR T-cell therapy,” Dr. Brodsky said.
, according to investigators in two separate studies.
Meanwhile, responses to tisagenlecleucel appear to be even more durable with longer follow-up, according to preliminary results from two more CAR T-cell therapy studies slated for presentation at the annual meeting of the American Society of Hematology.
A fifth study will show that bone marrow transplant may effectively consolidate remission after CAR T-cell therapy, according to Robert A. Brodsky, MD, ASH secretary, who highlighted the studies during a media briefing.
The ibrutinib study (abstract 299) shows that administering this BTK inhibitor starting 2 weeks prior to leukapheresis and continuing until 3 months after JCAR014 could improve responses and may decrease the incidence of severe cytokine release syndrome in patients with relapsed or refractory chronic lymphocytic leukemia (CLL).
Of 16 patients in an ibrutinib cohort and 18 patients in a no-ibrutinib cohort, the proportion of responders was 88% and 56%, respectively, according to preliminary data reported in the abstract. Grade 3-5 cytokine release syndrome occurred in 5 of 19 patients in the no-ibrutinib cohort, and 0 of 17 patients in the ibrutinib cohort.
Those findings are “early and preliminary, but very exciting” for ibrutinib in combination with this CD-19 specific CAR T-cell therapy said Dr. Brodsky, director of the division of hematology at Johns Hopkins University in Baltimore.
Early results of the checkpoint inhibitor study (abstract 556) suggest that pembrolizumab or nivolumab may augment CD19-directed CAR T-cell therapy in patients with relapsed B-cell acute lymphoblastic leukemia (ALL).
In data to date, 14 patients with early CAR T-cell loss, partial response, or no response to CAR T-cell therapy received a PD-1 inhibitor.
“The idea was if you can give pembrolizumab, you can take the brakes off, and maybe you can reinitiate the immune attack,” Dr. Brodsky said. “Sure enough, they were able to see that in roughly half of the patients. So again, very small, preliminary data, but very exciting that it is safe to give checkpoint inhibitors with CAR T-cells and it may be efficacious at getting the immune response back.”
One of the two tisagenlecleucel updates (abstract 895) showed that in the ELIANA trial, which included pediatric and young adults patients with relapsed/refractory ALL, the probability of relapse-free survival at 18 months was 66%.
“These are some very fast-growing tumors and these are refractory resistant patients, so as we get further and further out, it’s more encouraging to see that there are durable responses,” Dr. Brodsky said.
In the other tisagenlecleucel update (abstract 1684), investigators showed sustained disease control in Juliet, the global trial including adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), with 40% of patients still in remission at 18 months, according to Dr. Brodsky.
One more report Dr. Brodsky highlighted (abstract 967) will look at long-term follow-up after administration of SCRI-CAR19v1, a CD19-specific CAR T-cell product. Preliminary data suggest a survival advantage when hematopoietic stem cell transplantation is done after CAR T-cell induced remission.
“This study is very small and it’s retrospective, but it suggests that bone marrow transplant is a good way to consolidate the remission after CAR T-cell therapy,” Dr. Brodsky said.
Tofacitinib and TNF inhibitors show similar VTE rates
CHICAGO – Rheumatoid arthritis patients treated with tofacitinib did not have a significantly increased incidence of hospitalization for venous thromboembolism, compared with patients treated with a tumor necrosis factor (TNF) inhibitor, in a study of more than 50,000 U.S. patients culled from a pair of health insurance databases.
The Janus kinase inhibitor class of agents, including tofacitinib (Xeljanz), has acquired a reputation for causing an excess of venous thromboembolic events (VTE) (Drug Saf. 2018 Jul;41[7]:645-53). To assess this in a real-world setting Seoyoung C. Kim, MD, and her associates took data from Medicare patients during 2012-2015 and from Truven MarketScan for commercially insured patients during 2012-2016 and derived a database of 16,091 RA patients on newly begun treatment with a TNF inhibitor and 995 newly begun on tofacitinib in the Medicare data, and 32,164 RA patients newly started on a TNF inhibitor and 1,910 on tofacitinib in the Truven database. The analysis excluded patients with a history of VTE.
Using propensity score–adjusted matching of patients in the two treatment arms in both of these databases, and using a VTE event – either a pulmonary embolism or deep-vein thrombosis that resulted in hospitalization – as the primary endpoint, the results showed statistically nonsignificant excesses of VTE in the patients treated with tofacitinib, compared with a TNF inhibitor, Dr. Kim reported in a poster she presented at the annual meeting of the American College of Rheumatology.
In the adjusted comparison, the Medicare data showed a nonsignificant 12% higher VTE rate in the tofacitinib-treated patients, while the Truven data showed a nonsignificant 55% higher rate of VTE during tofacitinib treatment. When the data were pooled, the result was a 33% higher rate of VTE while on tofacitinib treatment, which was not statistically significant.
Dr. Kim cautioned that the low rate of VTE events, especially among the patients on tofacitinib, limited the precision of the results. The combined data included 2,905 patients on tofacitinib treatment who had 15 VTE events, a rate of 0.77 events/100 person-years of follow-up. This compared with a rate of 0.52/100 person-years among patients on a TNF inhibitor. Thus, in both treatment groups the absolute VTE rate was low.
The most reliable finding from the analysis is that it “rules out a large increase in the risk for VTE events with tofacitinib,” said Dr. Kim, a rheumatologist at Brigham and Women’s Hospital in Boston.
The researchers also ran an analysis that included not only VTE events that resulted in hospitalization but also VTE events managed on an outpatient basis. Dr. Kim did not report the specific numbers involved in this calculation, but she reported that, when her group included both types of VTE events, the patients treated with tofacitinib had a nonsignificant 12% lower rate of events, compared with patients treated with a TNF inhibitor.
Dr. Kim has received research support from Bristol-Myers Squibb, Pfizer, and Roche.
SOURCE: Desai RJ et al. Arthritis Rheumatol. 2018;70(suppl 10), Abstract L09.
CHICAGO – Rheumatoid arthritis patients treated with tofacitinib did not have a significantly increased incidence of hospitalization for venous thromboembolism, compared with patients treated with a tumor necrosis factor (TNF) inhibitor, in a study of more than 50,000 U.S. patients culled from a pair of health insurance databases.
The Janus kinase inhibitor class of agents, including tofacitinib (Xeljanz), has acquired a reputation for causing an excess of venous thromboembolic events (VTE) (Drug Saf. 2018 Jul;41[7]:645-53). To assess this in a real-world setting Seoyoung C. Kim, MD, and her associates took data from Medicare patients during 2012-2015 and from Truven MarketScan for commercially insured patients during 2012-2016 and derived a database of 16,091 RA patients on newly begun treatment with a TNF inhibitor and 995 newly begun on tofacitinib in the Medicare data, and 32,164 RA patients newly started on a TNF inhibitor and 1,910 on tofacitinib in the Truven database. The analysis excluded patients with a history of VTE.
Using propensity score–adjusted matching of patients in the two treatment arms in both of these databases, and using a VTE event – either a pulmonary embolism or deep-vein thrombosis that resulted in hospitalization – as the primary endpoint, the results showed statistically nonsignificant excesses of VTE in the patients treated with tofacitinib, compared with a TNF inhibitor, Dr. Kim reported in a poster she presented at the annual meeting of the American College of Rheumatology.
In the adjusted comparison, the Medicare data showed a nonsignificant 12% higher VTE rate in the tofacitinib-treated patients, while the Truven data showed a nonsignificant 55% higher rate of VTE during tofacitinib treatment. When the data were pooled, the result was a 33% higher rate of VTE while on tofacitinib treatment, which was not statistically significant.
Dr. Kim cautioned that the low rate of VTE events, especially among the patients on tofacitinib, limited the precision of the results. The combined data included 2,905 patients on tofacitinib treatment who had 15 VTE events, a rate of 0.77 events/100 person-years of follow-up. This compared with a rate of 0.52/100 person-years among patients on a TNF inhibitor. Thus, in both treatment groups the absolute VTE rate was low.
The most reliable finding from the analysis is that it “rules out a large increase in the risk for VTE events with tofacitinib,” said Dr. Kim, a rheumatologist at Brigham and Women’s Hospital in Boston.
The researchers also ran an analysis that included not only VTE events that resulted in hospitalization but also VTE events managed on an outpatient basis. Dr. Kim did not report the specific numbers involved in this calculation, but she reported that, when her group included both types of VTE events, the patients treated with tofacitinib had a nonsignificant 12% lower rate of events, compared with patients treated with a TNF inhibitor.
Dr. Kim has received research support from Bristol-Myers Squibb, Pfizer, and Roche.
SOURCE: Desai RJ et al. Arthritis Rheumatol. 2018;70(suppl 10), Abstract L09.
CHICAGO – Rheumatoid arthritis patients treated with tofacitinib did not have a significantly increased incidence of hospitalization for venous thromboembolism, compared with patients treated with a tumor necrosis factor (TNF) inhibitor, in a study of more than 50,000 U.S. patients culled from a pair of health insurance databases.
The Janus kinase inhibitor class of agents, including tofacitinib (Xeljanz), has acquired a reputation for causing an excess of venous thromboembolic events (VTE) (Drug Saf. 2018 Jul;41[7]:645-53). To assess this in a real-world setting Seoyoung C. Kim, MD, and her associates took data from Medicare patients during 2012-2015 and from Truven MarketScan for commercially insured patients during 2012-2016 and derived a database of 16,091 RA patients on newly begun treatment with a TNF inhibitor and 995 newly begun on tofacitinib in the Medicare data, and 32,164 RA patients newly started on a TNF inhibitor and 1,910 on tofacitinib in the Truven database. The analysis excluded patients with a history of VTE.
Using propensity score–adjusted matching of patients in the two treatment arms in both of these databases, and using a VTE event – either a pulmonary embolism or deep-vein thrombosis that resulted in hospitalization – as the primary endpoint, the results showed statistically nonsignificant excesses of VTE in the patients treated with tofacitinib, compared with a TNF inhibitor, Dr. Kim reported in a poster she presented at the annual meeting of the American College of Rheumatology.
In the adjusted comparison, the Medicare data showed a nonsignificant 12% higher VTE rate in the tofacitinib-treated patients, while the Truven data showed a nonsignificant 55% higher rate of VTE during tofacitinib treatment. When the data were pooled, the result was a 33% higher rate of VTE while on tofacitinib treatment, which was not statistically significant.
Dr. Kim cautioned that the low rate of VTE events, especially among the patients on tofacitinib, limited the precision of the results. The combined data included 2,905 patients on tofacitinib treatment who had 15 VTE events, a rate of 0.77 events/100 person-years of follow-up. This compared with a rate of 0.52/100 person-years among patients on a TNF inhibitor. Thus, in both treatment groups the absolute VTE rate was low.
The most reliable finding from the analysis is that it “rules out a large increase in the risk for VTE events with tofacitinib,” said Dr. Kim, a rheumatologist at Brigham and Women’s Hospital in Boston.
The researchers also ran an analysis that included not only VTE events that resulted in hospitalization but also VTE events managed on an outpatient basis. Dr. Kim did not report the specific numbers involved in this calculation, but she reported that, when her group included both types of VTE events, the patients treated with tofacitinib had a nonsignificant 12% lower rate of events, compared with patients treated with a TNF inhibitor.
Dr. Kim has received research support from Bristol-Myers Squibb, Pfizer, and Roche.
SOURCE: Desai RJ et al. Arthritis Rheumatol. 2018;70(suppl 10), Abstract L09.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: Rheumatoid arthritis patients treated with tofacitinib showed no excess incidence of venous thromboembolism, compared with patients on a tumor necrosis factor inhibitor.
Major finding: Propensity score–adjusted rates of VTE were 33% higher with tofacitinib, compared with TNF inhibition, which was not a statistically significant difference.
Study details: Review of 51,160 rheumatoid arthritis patients from U.S. health insurance databases.
Disclosures: Dr. Kim has received research support from Bristol-Myers Squibb, Pfizer, and Roche.
Source: Desai RJ et al. Arthritis Rheumatol. 2018;70(suppl 10), Abstract L09.
All patients with VTE have a high risk of recurrence
Recurrence risk is significant among all patients with venous thromboembolism (VTE), though recurrence is most frequent in patients with cancer-related VTE, according to a nationwide Danish study.
Ida Ehlers Albertsen, MD, of Aalborg (Denmark) University Hospital and her coauthors followed 73,993 patients who were diagnosed with incident VTE during January 2000–December 2015. The patients’ VTEs were classified as either cancer-related, unprovoked (occurring in patients without any provoking factors), or provoked (occurring in patients with one or more provoking factors, such as recent major surgery, recent fracture/trauma, obesity, or hormone replacement therapy).
The researchers found similar risks of recurrence among patients with unprovoked and provoked VTE at 6-month follow-up, with rates per 100 person-years of 6.80 and 6.92, respectively. By comparison, the recurrence rate for cancer-related VTE at 6 months was 9.06. The findings were reported in the American Journal of Medicine.
However, at 10-year follow-up the rates were 3.70 for cancer-related VTE, 2.84 for unprovoked VTE, and 2.22 for provoked VTE, which reinforces the belief that “unprovoked venous thromboembolism is associated with long-term higher risk of recurrence than provoked venous thromboembolism.”
Additionally, at 10-year follow-up, the absolute recurrence risk of cancer-related VTE and unprovoked VTE were both at approximately 20%, with recurrence risk of provoked VTE at just above 15%. Compared with the recurrence risk of provoked VTE at 10-year follow-up, the hazard ratios of cancer-related VTE and unprovoked VTE recurrence risk were 1.23 (95% confidence interval, 1.13-1.33) and 1.18 (95% CI, 1.13-1.24), respectively.
The coauthors observed several challenges in comparing their study to previous analyses on recurrent risk, noting that the definition of provoked VTE “varies throughout the literature” and that the majority of VTE studies “provide cumulative incidence proportions and not the actual rates.” They also stated that indefinite or extended therapy for all VTE patients comes with its own potential complications, even with the improved safety of non–vitamin K antagonist oral anticoagulants, writing that “treatment should be given to patients where the benefits outweigh the risks.”
Despite the differences in recurrence rates at 6-month and 10-year follow-up, the coauthors suggested that enough risk was present in all types to warrant additional studies and reconsider how VTE patients are categorized.
“A high recurrence risk in all types of venous thromboembolism indicates that further research is needed to optimize risk stratification for venous thromboembolism patients,” they wrote.
The study was partially funded by a grant from the Obel Family Foundation. Some authors reported financial disclosures related to Janssen, Bayer, Roche, and others.
SOURCE: Albertsen IE et al. Am J Med. 2018 Sep;131(9):1067-74.e4.
Recurrence risk is significant among all patients with venous thromboembolism (VTE), though recurrence is most frequent in patients with cancer-related VTE, according to a nationwide Danish study.
Ida Ehlers Albertsen, MD, of Aalborg (Denmark) University Hospital and her coauthors followed 73,993 patients who were diagnosed with incident VTE during January 2000–December 2015. The patients’ VTEs were classified as either cancer-related, unprovoked (occurring in patients without any provoking factors), or provoked (occurring in patients with one or more provoking factors, such as recent major surgery, recent fracture/trauma, obesity, or hormone replacement therapy).
The researchers found similar risks of recurrence among patients with unprovoked and provoked VTE at 6-month follow-up, with rates per 100 person-years of 6.80 and 6.92, respectively. By comparison, the recurrence rate for cancer-related VTE at 6 months was 9.06. The findings were reported in the American Journal of Medicine.
However, at 10-year follow-up the rates were 3.70 for cancer-related VTE, 2.84 for unprovoked VTE, and 2.22 for provoked VTE, which reinforces the belief that “unprovoked venous thromboembolism is associated with long-term higher risk of recurrence than provoked venous thromboembolism.”
Additionally, at 10-year follow-up, the absolute recurrence risk of cancer-related VTE and unprovoked VTE were both at approximately 20%, with recurrence risk of provoked VTE at just above 15%. Compared with the recurrence risk of provoked VTE at 10-year follow-up, the hazard ratios of cancer-related VTE and unprovoked VTE recurrence risk were 1.23 (95% confidence interval, 1.13-1.33) and 1.18 (95% CI, 1.13-1.24), respectively.
The coauthors observed several challenges in comparing their study to previous analyses on recurrent risk, noting that the definition of provoked VTE “varies throughout the literature” and that the majority of VTE studies “provide cumulative incidence proportions and not the actual rates.” They also stated that indefinite or extended therapy for all VTE patients comes with its own potential complications, even with the improved safety of non–vitamin K antagonist oral anticoagulants, writing that “treatment should be given to patients where the benefits outweigh the risks.”
Despite the differences in recurrence rates at 6-month and 10-year follow-up, the coauthors suggested that enough risk was present in all types to warrant additional studies and reconsider how VTE patients are categorized.
“A high recurrence risk in all types of venous thromboembolism indicates that further research is needed to optimize risk stratification for venous thromboembolism patients,” they wrote.
The study was partially funded by a grant from the Obel Family Foundation. Some authors reported financial disclosures related to Janssen, Bayer, Roche, and others.
SOURCE: Albertsen IE et al. Am J Med. 2018 Sep;131(9):1067-74.e4.
Recurrence risk is significant among all patients with venous thromboembolism (VTE), though recurrence is most frequent in patients with cancer-related VTE, according to a nationwide Danish study.
Ida Ehlers Albertsen, MD, of Aalborg (Denmark) University Hospital and her coauthors followed 73,993 patients who were diagnosed with incident VTE during January 2000–December 2015. The patients’ VTEs were classified as either cancer-related, unprovoked (occurring in patients without any provoking factors), or provoked (occurring in patients with one or more provoking factors, such as recent major surgery, recent fracture/trauma, obesity, or hormone replacement therapy).
The researchers found similar risks of recurrence among patients with unprovoked and provoked VTE at 6-month follow-up, with rates per 100 person-years of 6.80 and 6.92, respectively. By comparison, the recurrence rate for cancer-related VTE at 6 months was 9.06. The findings were reported in the American Journal of Medicine.
However, at 10-year follow-up the rates were 3.70 for cancer-related VTE, 2.84 for unprovoked VTE, and 2.22 for provoked VTE, which reinforces the belief that “unprovoked venous thromboembolism is associated with long-term higher risk of recurrence than provoked venous thromboembolism.”
Additionally, at 10-year follow-up, the absolute recurrence risk of cancer-related VTE and unprovoked VTE were both at approximately 20%, with recurrence risk of provoked VTE at just above 15%. Compared with the recurrence risk of provoked VTE at 10-year follow-up, the hazard ratios of cancer-related VTE and unprovoked VTE recurrence risk were 1.23 (95% confidence interval, 1.13-1.33) and 1.18 (95% CI, 1.13-1.24), respectively.
The coauthors observed several challenges in comparing their study to previous analyses on recurrent risk, noting that the definition of provoked VTE “varies throughout the literature” and that the majority of VTE studies “provide cumulative incidence proportions and not the actual rates.” They also stated that indefinite or extended therapy for all VTE patients comes with its own potential complications, even with the improved safety of non–vitamin K antagonist oral anticoagulants, writing that “treatment should be given to patients where the benefits outweigh the risks.”
Despite the differences in recurrence rates at 6-month and 10-year follow-up, the coauthors suggested that enough risk was present in all types to warrant additional studies and reconsider how VTE patients are categorized.
“A high recurrence risk in all types of venous thromboembolism indicates that further research is needed to optimize risk stratification for venous thromboembolism patients,” they wrote.
The study was partially funded by a grant from the Obel Family Foundation. Some authors reported financial disclosures related to Janssen, Bayer, Roche, and others.
SOURCE: Albertsen IE et al. Am J Med. 2018 Sep;131(9):1067-74.e4.
FROM THE AMERICAN JOURNAL OF MEDICINE
Key clinical point:
Major finding: At 10-year follow-up, recurrence rates per 100 person-years were 3.70 for patients with cancer-related VTE, 2.84 for patients with unprovoked VTE, and 2.22 for patients with provoked VTE.
Study details: An observational cohort study of 73,993 Danish patients with incident venous thromboembolism during January 2000–December 2015.
Disclosures: The study was partially funded by a grant from the Obel Family Foundation. Some authors reported financial disclosures related to Janssen, Bayer, Roche, and others.
Source: Albertsen IE et al. Am J Med. 2018 Sep;131(9):1067-74.e4.
FDA approves emapalumab for primary HLH
The (HLH).
Emapalumab, an interferon gamma-blocking antibody, is approved to treat patients of all ages (newborn and older) with primary HLH who have refractory, recurrent, or progressive disease or who cannot tolerate conventional HLH therapy. Emapalumab is the first treatment to be FDA approved for primary HLH, and it is expected to be available in the United States in the first quarter of 2019. The FDA previously granted emapalumab priority review, breakthrough therapy designation, orphan drug designation, and rare pediatric disease designation. The FDA’s approval of emapalumab is based on results from a phase 2/3 trial (NCT01818492).
The trial included 34 patients, 27 of whom had refractory, recurrent, or progressive disease or could not tolerate conventional HLH therapy. Patients received emapalumab in combination with dexamethasone. At the end of treatment, 63% (17/27) of patients had achieved a response, which was defined as complete response (n = 7), partial response (n=8), or HLH improvement (n = 2). A total of 70% (n=19) of patients went on to hematopoietic stem cell transplant. The most common adverse events were infections (56%), hypertension (41%), infusion-related reactions (27%), and pyrexia (24%).
Results also are scheduled to be presented at the 2018 annual meeting of the American Society of Hematology in the late-breaker abstract session (Abstract LBA-6).
Emapalumab was developed by Novimmune SA. Sobi acquired global rights to the drug in August 2018.
The (HLH).
Emapalumab, an interferon gamma-blocking antibody, is approved to treat patients of all ages (newborn and older) with primary HLH who have refractory, recurrent, or progressive disease or who cannot tolerate conventional HLH therapy. Emapalumab is the first treatment to be FDA approved for primary HLH, and it is expected to be available in the United States in the first quarter of 2019. The FDA previously granted emapalumab priority review, breakthrough therapy designation, orphan drug designation, and rare pediatric disease designation. The FDA’s approval of emapalumab is based on results from a phase 2/3 trial (NCT01818492).
The trial included 34 patients, 27 of whom had refractory, recurrent, or progressive disease or could not tolerate conventional HLH therapy. Patients received emapalumab in combination with dexamethasone. At the end of treatment, 63% (17/27) of patients had achieved a response, which was defined as complete response (n = 7), partial response (n=8), or HLH improvement (n = 2). A total of 70% (n=19) of patients went on to hematopoietic stem cell transplant. The most common adverse events were infections (56%), hypertension (41%), infusion-related reactions (27%), and pyrexia (24%).
Results also are scheduled to be presented at the 2018 annual meeting of the American Society of Hematology in the late-breaker abstract session (Abstract LBA-6).
Emapalumab was developed by Novimmune SA. Sobi acquired global rights to the drug in August 2018.
The (HLH).
Emapalumab, an interferon gamma-blocking antibody, is approved to treat patients of all ages (newborn and older) with primary HLH who have refractory, recurrent, or progressive disease or who cannot tolerate conventional HLH therapy. Emapalumab is the first treatment to be FDA approved for primary HLH, and it is expected to be available in the United States in the first quarter of 2019. The FDA previously granted emapalumab priority review, breakthrough therapy designation, orphan drug designation, and rare pediatric disease designation. The FDA’s approval of emapalumab is based on results from a phase 2/3 trial (NCT01818492).
The trial included 34 patients, 27 of whom had refractory, recurrent, or progressive disease or could not tolerate conventional HLH therapy. Patients received emapalumab in combination with dexamethasone. At the end of treatment, 63% (17/27) of patients had achieved a response, which was defined as complete response (n = 7), partial response (n=8), or HLH improvement (n = 2). A total of 70% (n=19) of patients went on to hematopoietic stem cell transplant. The most common adverse events were infections (56%), hypertension (41%), infusion-related reactions (27%), and pyrexia (24%).
Results also are scheduled to be presented at the 2018 annual meeting of the American Society of Hematology in the late-breaker abstract session (Abstract LBA-6).
Emapalumab was developed by Novimmune SA. Sobi acquired global rights to the drug in August 2018.
Preop anemia management saves blood, costs
BOSTON – A pilot anemia optimization program resulted in significant increases in day-of-surgery hemoglobin levels and reductions in RBC transfusion rates and costs in one center, but whether patient outcomes also improved is still not known.
By diagnosing anemia at the preanesthesia visit and providing anemic patients with dietary guidance and supplementation prior to cardiac surgery, blood program managers noticed a more than $360 reduction in per-patient blood-product acquisition costs, a more than $1,800 average reduction per patient in transfusion costs, and overall cost savings of more than $100,000 over 18 months, compared with historical data.
The findings were reported by Christine M. Cahill, RN, from Strong Memorial Hospital in Rochester, N.Y., and the University of Rochester (N.Y.), at AABB 2018, the annual meeting of the group formerly known as the American Association of Blood Banks.
“Anemia has been thought of as a relatively benign thing our patients live with traditionally, but what we have been finding lately is that anemia is actually more serious than we once thought, and is an independent risk factor for hospitalization, readmission, increased patient length of stay, loss of function, and diminished quality of life,” she said.
Anemia also increases the likelihood that a patient will require allogeneic transfusions and is an independent risk factor for morbidity and mortality, she added.
The pilot program, which ran from February 2016 to September 2017, was designed to test the feasibility of diagnosing anemia during a cardiology consult visit and implementing a management plan.
During the study period, 240 patients presenting for elective cardiac surgery were screened for anemia, and 58 were diagnosed as anemic, defined as a hemoglobin level of less than 12 g/dL. These patients were referred for anemia work-ups, which found that 33 patients had iron-deficient anemia and 25 had anemia from other causes. Controls were patients who underwent cardiac surgery from March to July 2015, matched by age, sex, and procedures.
Treatments for iron-deficient patients included oral iron (7 patients), intravenous iron with or without folate (20 patients), or oral folate with or without vitamin B12 (5 patients). One iron-deficient patient could not have surgery delayed for anemia management.
Of the iron-replete patients, one received oral iron and 17 received folate plus or minus vitamin B12. The remaining seven iron-replete patients were not treated for anemia.
One iron-deficient patient had a reaction to the infusion and did not receive a scheduled second dose due to the need for immediate surgery. A second patient scheduled for intravenous iron and folate broke an arm and therefore missed an intravenous infusion appointment. No other complications or reactions occurred.
Intraoperative transfusion units used in the anemia management group totaled 10, compared with 68 for controls. Postoperative transfusion units used were also significantly lower following anemia management at 13 versus 122, respectively.
The rate of RBC transfusions among patients with anemia management was 24%, compared with 60% for controls (P less than .0001). Patients in the management program also had significantly higher day-of-surgery hemoglobin, at 11.01 g/dL versus 10.16 g/dL (P less than .001), and less RBC utilization, at an average 0.40 units per patient versus 2.07 for controls (P less than .0001).
The average per patient savings in acquisition costs was $367.40, the average transfusion cost saving was $1,837, and the total cost savings over the life of the pilot program was $106,546.
The keys to success for similar programs is “to make sure you do your homework,” Ms. Cahill said. Specifically, she recommended feasibility studies, evaluation of the potential impact of infusions on the service, work flow analyses, and cost analyses. It’s also important to get high-level administrative support as well as buy-in from surgeons and patients.
Future studies should include assessment of patient outcomes, safety, and length of ICU and hospital stay, she emphasized.
The study was internally funded. Ms. Cahill reported having no conflicts of interest.
SOURCE: Cahill CM et al. AABB 2018, Abstract PBM4-ST4-22.
BOSTON – A pilot anemia optimization program resulted in significant increases in day-of-surgery hemoglobin levels and reductions in RBC transfusion rates and costs in one center, but whether patient outcomes also improved is still not known.
By diagnosing anemia at the preanesthesia visit and providing anemic patients with dietary guidance and supplementation prior to cardiac surgery, blood program managers noticed a more than $360 reduction in per-patient blood-product acquisition costs, a more than $1,800 average reduction per patient in transfusion costs, and overall cost savings of more than $100,000 over 18 months, compared with historical data.
The findings were reported by Christine M. Cahill, RN, from Strong Memorial Hospital in Rochester, N.Y., and the University of Rochester (N.Y.), at AABB 2018, the annual meeting of the group formerly known as the American Association of Blood Banks.
“Anemia has been thought of as a relatively benign thing our patients live with traditionally, but what we have been finding lately is that anemia is actually more serious than we once thought, and is an independent risk factor for hospitalization, readmission, increased patient length of stay, loss of function, and diminished quality of life,” she said.
Anemia also increases the likelihood that a patient will require allogeneic transfusions and is an independent risk factor for morbidity and mortality, she added.
The pilot program, which ran from February 2016 to September 2017, was designed to test the feasibility of diagnosing anemia during a cardiology consult visit and implementing a management plan.
During the study period, 240 patients presenting for elective cardiac surgery were screened for anemia, and 58 were diagnosed as anemic, defined as a hemoglobin level of less than 12 g/dL. These patients were referred for anemia work-ups, which found that 33 patients had iron-deficient anemia and 25 had anemia from other causes. Controls were patients who underwent cardiac surgery from March to July 2015, matched by age, sex, and procedures.
Treatments for iron-deficient patients included oral iron (7 patients), intravenous iron with or without folate (20 patients), or oral folate with or without vitamin B12 (5 patients). One iron-deficient patient could not have surgery delayed for anemia management.
Of the iron-replete patients, one received oral iron and 17 received folate plus or minus vitamin B12. The remaining seven iron-replete patients were not treated for anemia.
One iron-deficient patient had a reaction to the infusion and did not receive a scheduled second dose due to the need for immediate surgery. A second patient scheduled for intravenous iron and folate broke an arm and therefore missed an intravenous infusion appointment. No other complications or reactions occurred.
Intraoperative transfusion units used in the anemia management group totaled 10, compared with 68 for controls. Postoperative transfusion units used were also significantly lower following anemia management at 13 versus 122, respectively.
The rate of RBC transfusions among patients with anemia management was 24%, compared with 60% for controls (P less than .0001). Patients in the management program also had significantly higher day-of-surgery hemoglobin, at 11.01 g/dL versus 10.16 g/dL (P less than .001), and less RBC utilization, at an average 0.40 units per patient versus 2.07 for controls (P less than .0001).
The average per patient savings in acquisition costs was $367.40, the average transfusion cost saving was $1,837, and the total cost savings over the life of the pilot program was $106,546.
The keys to success for similar programs is “to make sure you do your homework,” Ms. Cahill said. Specifically, she recommended feasibility studies, evaluation of the potential impact of infusions on the service, work flow analyses, and cost analyses. It’s also important to get high-level administrative support as well as buy-in from surgeons and patients.
Future studies should include assessment of patient outcomes, safety, and length of ICU and hospital stay, she emphasized.
The study was internally funded. Ms. Cahill reported having no conflicts of interest.
SOURCE: Cahill CM et al. AABB 2018, Abstract PBM4-ST4-22.
BOSTON – A pilot anemia optimization program resulted in significant increases in day-of-surgery hemoglobin levels and reductions in RBC transfusion rates and costs in one center, but whether patient outcomes also improved is still not known.
By diagnosing anemia at the preanesthesia visit and providing anemic patients with dietary guidance and supplementation prior to cardiac surgery, blood program managers noticed a more than $360 reduction in per-patient blood-product acquisition costs, a more than $1,800 average reduction per patient in transfusion costs, and overall cost savings of more than $100,000 over 18 months, compared with historical data.
The findings were reported by Christine M. Cahill, RN, from Strong Memorial Hospital in Rochester, N.Y., and the University of Rochester (N.Y.), at AABB 2018, the annual meeting of the group formerly known as the American Association of Blood Banks.
“Anemia has been thought of as a relatively benign thing our patients live with traditionally, but what we have been finding lately is that anemia is actually more serious than we once thought, and is an independent risk factor for hospitalization, readmission, increased patient length of stay, loss of function, and diminished quality of life,” she said.
Anemia also increases the likelihood that a patient will require allogeneic transfusions and is an independent risk factor for morbidity and mortality, she added.
The pilot program, which ran from February 2016 to September 2017, was designed to test the feasibility of diagnosing anemia during a cardiology consult visit and implementing a management plan.
During the study period, 240 patients presenting for elective cardiac surgery were screened for anemia, and 58 were diagnosed as anemic, defined as a hemoglobin level of less than 12 g/dL. These patients were referred for anemia work-ups, which found that 33 patients had iron-deficient anemia and 25 had anemia from other causes. Controls were patients who underwent cardiac surgery from March to July 2015, matched by age, sex, and procedures.
Treatments for iron-deficient patients included oral iron (7 patients), intravenous iron with or without folate (20 patients), or oral folate with or without vitamin B12 (5 patients). One iron-deficient patient could not have surgery delayed for anemia management.
Of the iron-replete patients, one received oral iron and 17 received folate plus or minus vitamin B12. The remaining seven iron-replete patients were not treated for anemia.
One iron-deficient patient had a reaction to the infusion and did not receive a scheduled second dose due to the need for immediate surgery. A second patient scheduled for intravenous iron and folate broke an arm and therefore missed an intravenous infusion appointment. No other complications or reactions occurred.
Intraoperative transfusion units used in the anemia management group totaled 10, compared with 68 for controls. Postoperative transfusion units used were also significantly lower following anemia management at 13 versus 122, respectively.
The rate of RBC transfusions among patients with anemia management was 24%, compared with 60% for controls (P less than .0001). Patients in the management program also had significantly higher day-of-surgery hemoglobin, at 11.01 g/dL versus 10.16 g/dL (P less than .001), and less RBC utilization, at an average 0.40 units per patient versus 2.07 for controls (P less than .0001).
The average per patient savings in acquisition costs was $367.40, the average transfusion cost saving was $1,837, and the total cost savings over the life of the pilot program was $106,546.
The keys to success for similar programs is “to make sure you do your homework,” Ms. Cahill said. Specifically, she recommended feasibility studies, evaluation of the potential impact of infusions on the service, work flow analyses, and cost analyses. It’s also important to get high-level administrative support as well as buy-in from surgeons and patients.
Future studies should include assessment of patient outcomes, safety, and length of ICU and hospital stay, she emphasized.
The study was internally funded. Ms. Cahill reported having no conflicts of interest.
SOURCE: Cahill CM et al. AABB 2018, Abstract PBM4-ST4-22.
REPORTING FROM AABB 2018
Key clinical point:
Major finding: The total cost savings over the life of a pilot anemia management program was $106,546.
Study details: A case-control study with 58 patients scheduled for elective cardiac surgery and matched historical controls.
Disclosures: The study was internally funded. Ms. Cahill reported having no conflicts of interest.
Source: Cahill CM et al. AABB 2018, Abstract PBM4-ST4-22.
Scleroderma SCOT trial findings hold similar in lung disease
CHICAGO – Changes in quantitative lung CT scores for scleroderma-related interstitial lung disease independently validate the superiority of hematopoietic stem cell transplantation versus cyclophosphamide for severe systemic sclerosis, according to findings in a subset of patients from the SCOT (Scleroderma: Cyclophosphamide or Transplantation) trial.
The recently published findings from the SCOT trial showed that myeloablation followed by autologous hematopoietic stem cell transplant (HSCT) significantly improved event-free and overall survival of systemic sclerosis patients at 54 months, compared with 12 monthly treatments with intravenous cyclophosphamide (N Engl J Med. 2018;378:35-47).
In a subset of 75 patients from the SCOT trial, the investigators analyzed changes in lung parenchymal abnormalities on high-resolution CT scans between baseline and serial follow-up exams performed yearly for up to 5 years. Follow-up scans at 14, 26, 48, and 54 months in available patients at each time point showed that whole-lung quantitative interstitial lung disease (QILD) scores – a validated measure that combines various CT texture-based characteristics to determine disease extent – decreased significantly by 7% at 54 months in patients who underwent HSCT, compared with no change in those who received cyclophosphamide (CYC; P = .024), Keith M. Sullivan, MD, reported at the annual meeting of the American College of Rheumatology.
Additionally, whole-lung quantitative lung fibrosis (QLF) scores were stable (–1%) in the HSCT patients, but increased 3% in the CYC patients (P = .047), said Dr. Sullivan, a professor of medicine at Duke University, Durham, N.C.
Dr. Sullivan was the first author on the SCOT trial, and he reported the current study results on behalf of lead investigator Jonathan Goldin, MD, PhD, of the department of radiologic sciences at the University of California, Los Angeles.
“These are really kind of meaningful associations, especially since the worst of the [CYC] treatment group didn’t make it to month 54,” Dr. Sullivan said.
Quantitative scores of scleroderma-related interstitial lung disease were measured using computer-based quantitative image analysis of standardized, noncontrast, volumetric, thin-section, thoracic, high-resolution CT. The same CT machine was used for all time points (except for one subject) with careful attention to breath hold reproducibility and image quality. Baseline characteristics were not different between the HSCT and CYC groups, he noted, stressing the rigorous study design.
CT assessments were also compared for the most severe lobe in each patient and showed similar findings, with both QILD and QLF scores for that lobe improving in the HSCT patients relative to the CYC patients (P = .004 and P = .002, respectively), Dr. Sullivan said, adding that the direction of change in structural measures of QILD and QLF for both whole lung and most severe lobe CTs tracked with physiological pulmonary function tests, including forced vital capacity (FVC), forced expiratory volume in 1 second, and diffusing capacity of the lungs for carbon monoxide.
“The FVC improved while QILD decreased, and that’s what you would expect to see,” he said. “So for each of these ways of displaying data, there was an expected and sensible inverse correlation.”
Scleroderma-related interstitial lung disease is a major cause of morbidity and mortality in severe systemic sclerosis. In the wake of the SCOT trial findings, questions remained with respect to correlation between those findings and pulmonary function; if the improvements with HSCT are real and meaningful, they should have meaningful correlation with pulmonary function, and these findings demonstrate those correlates, he said.
“Changes in quantitative lung CT scoring of scleroderma lung disease provide an objective radiologic validation of the long-term benefits of transplant compared to cyclophosphamide in individuals with severe scleroderma and lung involvement. Improvement in imaging after transplant continues for up to 54 months after randomization, giving radiologic confirmation of a durable treatment benefit,” Dr. Sullivan concluded.
The investigators reported having no relevant disclosures.
SOURCE: Goldin J et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 901.
CHICAGO – Changes in quantitative lung CT scores for scleroderma-related interstitial lung disease independently validate the superiority of hematopoietic stem cell transplantation versus cyclophosphamide for severe systemic sclerosis, according to findings in a subset of patients from the SCOT (Scleroderma: Cyclophosphamide or Transplantation) trial.
The recently published findings from the SCOT trial showed that myeloablation followed by autologous hematopoietic stem cell transplant (HSCT) significantly improved event-free and overall survival of systemic sclerosis patients at 54 months, compared with 12 monthly treatments with intravenous cyclophosphamide (N Engl J Med. 2018;378:35-47).
In a subset of 75 patients from the SCOT trial, the investigators analyzed changes in lung parenchymal abnormalities on high-resolution CT scans between baseline and serial follow-up exams performed yearly for up to 5 years. Follow-up scans at 14, 26, 48, and 54 months in available patients at each time point showed that whole-lung quantitative interstitial lung disease (QILD) scores – a validated measure that combines various CT texture-based characteristics to determine disease extent – decreased significantly by 7% at 54 months in patients who underwent HSCT, compared with no change in those who received cyclophosphamide (CYC; P = .024), Keith M. Sullivan, MD, reported at the annual meeting of the American College of Rheumatology.
Additionally, whole-lung quantitative lung fibrosis (QLF) scores were stable (–1%) in the HSCT patients, but increased 3% in the CYC patients (P = .047), said Dr. Sullivan, a professor of medicine at Duke University, Durham, N.C.
Dr. Sullivan was the first author on the SCOT trial, and he reported the current study results on behalf of lead investigator Jonathan Goldin, MD, PhD, of the department of radiologic sciences at the University of California, Los Angeles.
“These are really kind of meaningful associations, especially since the worst of the [CYC] treatment group didn’t make it to month 54,” Dr. Sullivan said.
Quantitative scores of scleroderma-related interstitial lung disease were measured using computer-based quantitative image analysis of standardized, noncontrast, volumetric, thin-section, thoracic, high-resolution CT. The same CT machine was used for all time points (except for one subject) with careful attention to breath hold reproducibility and image quality. Baseline characteristics were not different between the HSCT and CYC groups, he noted, stressing the rigorous study design.
CT assessments were also compared for the most severe lobe in each patient and showed similar findings, with both QILD and QLF scores for that lobe improving in the HSCT patients relative to the CYC patients (P = .004 and P = .002, respectively), Dr. Sullivan said, adding that the direction of change in structural measures of QILD and QLF for both whole lung and most severe lobe CTs tracked with physiological pulmonary function tests, including forced vital capacity (FVC), forced expiratory volume in 1 second, and diffusing capacity of the lungs for carbon monoxide.
“The FVC improved while QILD decreased, and that’s what you would expect to see,” he said. “So for each of these ways of displaying data, there was an expected and sensible inverse correlation.”
Scleroderma-related interstitial lung disease is a major cause of morbidity and mortality in severe systemic sclerosis. In the wake of the SCOT trial findings, questions remained with respect to correlation between those findings and pulmonary function; if the improvements with HSCT are real and meaningful, they should have meaningful correlation with pulmonary function, and these findings demonstrate those correlates, he said.
“Changes in quantitative lung CT scoring of scleroderma lung disease provide an objective radiologic validation of the long-term benefits of transplant compared to cyclophosphamide in individuals with severe scleroderma and lung involvement. Improvement in imaging after transplant continues for up to 54 months after randomization, giving radiologic confirmation of a durable treatment benefit,” Dr. Sullivan concluded.
The investigators reported having no relevant disclosures.
SOURCE: Goldin J et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 901.
CHICAGO – Changes in quantitative lung CT scores for scleroderma-related interstitial lung disease independently validate the superiority of hematopoietic stem cell transplantation versus cyclophosphamide for severe systemic sclerosis, according to findings in a subset of patients from the SCOT (Scleroderma: Cyclophosphamide or Transplantation) trial.
The recently published findings from the SCOT trial showed that myeloablation followed by autologous hematopoietic stem cell transplant (HSCT) significantly improved event-free and overall survival of systemic sclerosis patients at 54 months, compared with 12 monthly treatments with intravenous cyclophosphamide (N Engl J Med. 2018;378:35-47).
In a subset of 75 patients from the SCOT trial, the investigators analyzed changes in lung parenchymal abnormalities on high-resolution CT scans between baseline and serial follow-up exams performed yearly for up to 5 years. Follow-up scans at 14, 26, 48, and 54 months in available patients at each time point showed that whole-lung quantitative interstitial lung disease (QILD) scores – a validated measure that combines various CT texture-based characteristics to determine disease extent – decreased significantly by 7% at 54 months in patients who underwent HSCT, compared with no change in those who received cyclophosphamide (CYC; P = .024), Keith M. Sullivan, MD, reported at the annual meeting of the American College of Rheumatology.
Additionally, whole-lung quantitative lung fibrosis (QLF) scores were stable (–1%) in the HSCT patients, but increased 3% in the CYC patients (P = .047), said Dr. Sullivan, a professor of medicine at Duke University, Durham, N.C.
Dr. Sullivan was the first author on the SCOT trial, and he reported the current study results on behalf of lead investigator Jonathan Goldin, MD, PhD, of the department of radiologic sciences at the University of California, Los Angeles.
“These are really kind of meaningful associations, especially since the worst of the [CYC] treatment group didn’t make it to month 54,” Dr. Sullivan said.
Quantitative scores of scleroderma-related interstitial lung disease were measured using computer-based quantitative image analysis of standardized, noncontrast, volumetric, thin-section, thoracic, high-resolution CT. The same CT machine was used for all time points (except for one subject) with careful attention to breath hold reproducibility and image quality. Baseline characteristics were not different between the HSCT and CYC groups, he noted, stressing the rigorous study design.
CT assessments were also compared for the most severe lobe in each patient and showed similar findings, with both QILD and QLF scores for that lobe improving in the HSCT patients relative to the CYC patients (P = .004 and P = .002, respectively), Dr. Sullivan said, adding that the direction of change in structural measures of QILD and QLF for both whole lung and most severe lobe CTs tracked with physiological pulmonary function tests, including forced vital capacity (FVC), forced expiratory volume in 1 second, and diffusing capacity of the lungs for carbon monoxide.
“The FVC improved while QILD decreased, and that’s what you would expect to see,” he said. “So for each of these ways of displaying data, there was an expected and sensible inverse correlation.”
Scleroderma-related interstitial lung disease is a major cause of morbidity and mortality in severe systemic sclerosis. In the wake of the SCOT trial findings, questions remained with respect to correlation between those findings and pulmonary function; if the improvements with HSCT are real and meaningful, they should have meaningful correlation with pulmonary function, and these findings demonstrate those correlates, he said.
“Changes in quantitative lung CT scoring of scleroderma lung disease provide an objective radiologic validation of the long-term benefits of transplant compared to cyclophosphamide in individuals with severe scleroderma and lung involvement. Improvement in imaging after transplant continues for up to 54 months after randomization, giving radiologic confirmation of a durable treatment benefit,” Dr. Sullivan concluded.
The investigators reported having no relevant disclosures.
SOURCE: Goldin J et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 901.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: Lung CT scores remain stable or improve following hematopoietic stem cell transplantation in patients with scleroderma-related interstitial lung disease when compared against monthly cyclophosphamide treatments.
Major finding: Quantitative interstitial lung disease scores decreased by 7% at 54 months in hematopoietic stem cell transplant patients versus no change in those who received cyclophosphamide (P = .024).
Study details: A study of 75 patients from the SCOT trial.
Disclosures: The investigators reported having no relevant disclosures.
Source: Goldin J et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 901.
Palliative-rehab combo may improve QoL in newly diagnosed cancer patients
In patients with a new diagnosis of advanced cancer, an intervention that combined palliative care with rehabilitation helped improve quality of life, results of a randomized, single-center study suggest.
Patients had a significant improvement in their most pressing quality-of-life issues after participating in the intervention, which included individualized palliative care consultations and a patient/caregiver “school” of lectures, discussion, and physical exercise, investigators said.
These findings suggest that every patient facing an advanced cancer diagnosis should at least have an initial exploratory consultation with a specialized palliative care team, and should be offered not only the usual components of palliative care, but also cancer rehabilitation, said Lise Nottelmann, MD, of the department of oncology at Vejle Hospital in Denmark.
“We should be active as a health care system in approaching these patients and offering them this intervention, or at least a consultation exploring these aspects of quality of life,” Dr. Nottelmann said in an interview at the 2018 Palliative and Supportive Care in Oncology Symposium.
The study by Dr. Nottelmann and her colleagues, presented at the symposium, comprised 301 patients with nonresectable solid tumors, including lung, gastrointestinal, prostate, and others. Those patients were randomly allocated to the palliative rehabilitation intervention or to standard care only.
Every patient participated in two consultations with a specialized palliative care team, and then had the opportunity for individualized contact with the team in a 12-week open contact period. They were also invited to participate in the school sessions, each of which included a 20-minute lecture on topics such as physical activity and good nutrition plus a 40-minute discussion period, followed by an exercise session.
Of the patients randomized to the palliative rehabilitation intervention, 26 participated only in the initial consultations, while 59 participated in the group program, and 47 had individual consultations, Dr. Nottelmann reported.
To measure quality of life, the investigators asked patients to identify a “primary problem” that corresponded to one of 12 scales in the EORTC QLQ-C30 questionnaire related to physical and role functioning, emotional and cognitive functioning, or symptoms.
The primary endpoint of the analysis was improvement in QLQ-C30 scores at 12 weeks. The analysis was done on specific scales in the patients who identified a primary problem, combined with global QLQ-C30 scores for the remaining one-quarter of the patients who did not, Dr. Nottelmann said.
After 12 weeks, the patients in the intervention arm had a significant improvement versus the no-intervention arm as measured by a version of the EORTC QLQ-C30 questionnaire. The absolute between-group difference in scores was 3.0 (95% confidence interval, 0.0-6.0; P less than .047), according to researchers.
Starting palliative care earlier in the course of cancer, as done in this intervention, is an increasingly accepted practice, supported by large studies and recent clinical practice guidelines that recommend early integration of palliative care into the seriously ill patient’s care plan.
What was different about this intervention was the integration of rehabilitation aspects into palliative care, Dr. Nottelmann said in the interview. While not traditionally thought of as a component of palliative care, the concept of palliative rehabilitation is gaining ground, she said.
The goal of rehabilitative palliative care is to help individuals with life-limiting or terminal conditions actively self-manage their conditions so they can “live fully” and enjoy the best quality of life possible, according to Hospice UK, a national charity for hospice care in the United Kingdom.
The symposium was cosponsored by AAHPM, ASCO, ASTRO, and MASCC. Dr. Nottelmann and her colleagues reported research funding from the Danish Cancer Society. Dr. Nottelmann had no disclosures related to the presentation. One coauthor provided disclosures related to Roche, Amgen, Bayer, and Merck Sharp & Dohme.
SOURCE: Nottelmann L et al. PallOnc 2018, Abstract 75.
In patients with a new diagnosis of advanced cancer, an intervention that combined palliative care with rehabilitation helped improve quality of life, results of a randomized, single-center study suggest.
Patients had a significant improvement in their most pressing quality-of-life issues after participating in the intervention, which included individualized palliative care consultations and a patient/caregiver “school” of lectures, discussion, and physical exercise, investigators said.
These findings suggest that every patient facing an advanced cancer diagnosis should at least have an initial exploratory consultation with a specialized palliative care team, and should be offered not only the usual components of palliative care, but also cancer rehabilitation, said Lise Nottelmann, MD, of the department of oncology at Vejle Hospital in Denmark.
“We should be active as a health care system in approaching these patients and offering them this intervention, or at least a consultation exploring these aspects of quality of life,” Dr. Nottelmann said in an interview at the 2018 Palliative and Supportive Care in Oncology Symposium.
The study by Dr. Nottelmann and her colleagues, presented at the symposium, comprised 301 patients with nonresectable solid tumors, including lung, gastrointestinal, prostate, and others. Those patients were randomly allocated to the palliative rehabilitation intervention or to standard care only.
Every patient participated in two consultations with a specialized palliative care team, and then had the opportunity for individualized contact with the team in a 12-week open contact period. They were also invited to participate in the school sessions, each of which included a 20-minute lecture on topics such as physical activity and good nutrition plus a 40-minute discussion period, followed by an exercise session.
Of the patients randomized to the palliative rehabilitation intervention, 26 participated only in the initial consultations, while 59 participated in the group program, and 47 had individual consultations, Dr. Nottelmann reported.
To measure quality of life, the investigators asked patients to identify a “primary problem” that corresponded to one of 12 scales in the EORTC QLQ-C30 questionnaire related to physical and role functioning, emotional and cognitive functioning, or symptoms.
The primary endpoint of the analysis was improvement in QLQ-C30 scores at 12 weeks. The analysis was done on specific scales in the patients who identified a primary problem, combined with global QLQ-C30 scores for the remaining one-quarter of the patients who did not, Dr. Nottelmann said.
After 12 weeks, the patients in the intervention arm had a significant improvement versus the no-intervention arm as measured by a version of the EORTC QLQ-C30 questionnaire. The absolute between-group difference in scores was 3.0 (95% confidence interval, 0.0-6.0; P less than .047), according to researchers.
Starting palliative care earlier in the course of cancer, as done in this intervention, is an increasingly accepted practice, supported by large studies and recent clinical practice guidelines that recommend early integration of palliative care into the seriously ill patient’s care plan.
What was different about this intervention was the integration of rehabilitation aspects into palliative care, Dr. Nottelmann said in the interview. While not traditionally thought of as a component of palliative care, the concept of palliative rehabilitation is gaining ground, she said.
The goal of rehabilitative palliative care is to help individuals with life-limiting or terminal conditions actively self-manage their conditions so they can “live fully” and enjoy the best quality of life possible, according to Hospice UK, a national charity for hospice care in the United Kingdom.
The symposium was cosponsored by AAHPM, ASCO, ASTRO, and MASCC. Dr. Nottelmann and her colleagues reported research funding from the Danish Cancer Society. Dr. Nottelmann had no disclosures related to the presentation. One coauthor provided disclosures related to Roche, Amgen, Bayer, and Merck Sharp & Dohme.
SOURCE: Nottelmann L et al. PallOnc 2018, Abstract 75.
In patients with a new diagnosis of advanced cancer, an intervention that combined palliative care with rehabilitation helped improve quality of life, results of a randomized, single-center study suggest.
Patients had a significant improvement in their most pressing quality-of-life issues after participating in the intervention, which included individualized palliative care consultations and a patient/caregiver “school” of lectures, discussion, and physical exercise, investigators said.
These findings suggest that every patient facing an advanced cancer diagnosis should at least have an initial exploratory consultation with a specialized palliative care team, and should be offered not only the usual components of palliative care, but also cancer rehabilitation, said Lise Nottelmann, MD, of the department of oncology at Vejle Hospital in Denmark.
“We should be active as a health care system in approaching these patients and offering them this intervention, or at least a consultation exploring these aspects of quality of life,” Dr. Nottelmann said in an interview at the 2018 Palliative and Supportive Care in Oncology Symposium.
The study by Dr. Nottelmann and her colleagues, presented at the symposium, comprised 301 patients with nonresectable solid tumors, including lung, gastrointestinal, prostate, and others. Those patients were randomly allocated to the palliative rehabilitation intervention or to standard care only.
Every patient participated in two consultations with a specialized palliative care team, and then had the opportunity for individualized contact with the team in a 12-week open contact period. They were also invited to participate in the school sessions, each of which included a 20-minute lecture on topics such as physical activity and good nutrition plus a 40-minute discussion period, followed by an exercise session.
Of the patients randomized to the palliative rehabilitation intervention, 26 participated only in the initial consultations, while 59 participated in the group program, and 47 had individual consultations, Dr. Nottelmann reported.
To measure quality of life, the investigators asked patients to identify a “primary problem” that corresponded to one of 12 scales in the EORTC QLQ-C30 questionnaire related to physical and role functioning, emotional and cognitive functioning, or symptoms.
The primary endpoint of the analysis was improvement in QLQ-C30 scores at 12 weeks. The analysis was done on specific scales in the patients who identified a primary problem, combined with global QLQ-C30 scores for the remaining one-quarter of the patients who did not, Dr. Nottelmann said.
After 12 weeks, the patients in the intervention arm had a significant improvement versus the no-intervention arm as measured by a version of the EORTC QLQ-C30 questionnaire. The absolute between-group difference in scores was 3.0 (95% confidence interval, 0.0-6.0; P less than .047), according to researchers.
Starting palliative care earlier in the course of cancer, as done in this intervention, is an increasingly accepted practice, supported by large studies and recent clinical practice guidelines that recommend early integration of palliative care into the seriously ill patient’s care plan.
What was different about this intervention was the integration of rehabilitation aspects into palliative care, Dr. Nottelmann said in the interview. While not traditionally thought of as a component of palliative care, the concept of palliative rehabilitation is gaining ground, she said.
The goal of rehabilitative palliative care is to help individuals with life-limiting or terminal conditions actively self-manage their conditions so they can “live fully” and enjoy the best quality of life possible, according to Hospice UK, a national charity for hospice care in the United Kingdom.
The symposium was cosponsored by AAHPM, ASCO, ASTRO, and MASCC. Dr. Nottelmann and her colleagues reported research funding from the Danish Cancer Society. Dr. Nottelmann had no disclosures related to the presentation. One coauthor provided disclosures related to Roche, Amgen, Bayer, and Merck Sharp & Dohme.
SOURCE: Nottelmann L et al. PallOnc 2018, Abstract 75.
FROM PALLONC 2018
Key clinical point: An intervention combining palliative care and rehabilitation aspects improved quality of life in patients with newly diagnosed, advanced cancers.
Major finding: Patients in the rehabilitative palliative care program had a significant improvement, compared with no intervention (absolute between-group difference in EORTC QLQ-30 scores, 3.0; 95% CI, 0.0-6.0; P less than .047).
Study details: A single-center randomized study of 301 patients with a newly diagnosed advanced solid tumor cancers.
Disclosures: Research funding came from the Danish Cancer Society. One study coauthor had disclosures related to Roche, Amgen, Bayer, and Merck Sharp & Dohme.
Source: Nottelmann L et al. 2018 Palliative and Supportive Care in Oncology Symposium Abstract 75.
Portable hematology analyzer gets FDA nod
The Food and Drug Administration has granted .
HemoScreen requires a single drop of blood and uses disposable cartridges that provide automatic sample preparation.
HemoScreen can analyze 20 standard complete blood count parameters and produces results within 5 minutes.
Study results suggested that HemoScreen provides results comparable to those of another hematology analyzer, Sysmex XE-2100 (J Clin Pathol. 2016 Aug;69[8]:720-5).
“The HemoScreen delivers lab accurate results,” Avishay Bransky, PhD, CEO of PixCell, said in a statement.
HemoScreen “would be especially useful” in physicians’ offices, emergency rooms, intensive care units, oncology clinics, and remote locations, he added.
HemoScreen makes use of a technology called viscoelastic focusing, which employs microfluidics and machine vision algorithms to analyze cells.
The Food and Drug Administration has granted .
HemoScreen requires a single drop of blood and uses disposable cartridges that provide automatic sample preparation.
HemoScreen can analyze 20 standard complete blood count parameters and produces results within 5 minutes.
Study results suggested that HemoScreen provides results comparable to those of another hematology analyzer, Sysmex XE-2100 (J Clin Pathol. 2016 Aug;69[8]:720-5).
“The HemoScreen delivers lab accurate results,” Avishay Bransky, PhD, CEO of PixCell, said in a statement.
HemoScreen “would be especially useful” in physicians’ offices, emergency rooms, intensive care units, oncology clinics, and remote locations, he added.
HemoScreen makes use of a technology called viscoelastic focusing, which employs microfluidics and machine vision algorithms to analyze cells.
The Food and Drug Administration has granted .
HemoScreen requires a single drop of blood and uses disposable cartridges that provide automatic sample preparation.
HemoScreen can analyze 20 standard complete blood count parameters and produces results within 5 minutes.
Study results suggested that HemoScreen provides results comparable to those of another hematology analyzer, Sysmex XE-2100 (J Clin Pathol. 2016 Aug;69[8]:720-5).
“The HemoScreen delivers lab accurate results,” Avishay Bransky, PhD, CEO of PixCell, said in a statement.
HemoScreen “would be especially useful” in physicians’ offices, emergency rooms, intensive care units, oncology clinics, and remote locations, he added.
HemoScreen makes use of a technology called viscoelastic focusing, which employs microfluidics and machine vision algorithms to analyze cells.