User login
Researchers seek more sickle cell drug research
BETHESDA, MD – While there are experimental treatments such as sevuparin and gene therapy in testing for sickle cell disease (SCD), researchers said this field is lagging because of a lack of funding.
Yogen Saunthararajah, MD, of the Cleveland Clinic, described what he called a “paltry” landscape of new drugs for SCD at Sickle Cell in Focus, a conference held by the National Institutes of Health.
There are only four main approaches taken by drugs now in clinical testing for addressing the root causes of SCD, despite decades’ worth of research of the genetic and mechanistic underpinnings of this disease, he said.
“It’s pretty sad,” Dr. Saunthararajah said, referring to the quantity of efforts, not their quality.
Within days of his presentation at the conference, one of the drugs he highlighted had officially fallen out of contention. An Oct. 26 post on NIH’s Clinicaltrials.gov site said Incyte had terminated its phase 1 study of INCB059872 in SCD “due to a business decision” not to pursue this indication. Incyte confirmed that it dropped development of INCB059872 for SCD but will continue testing it for other indications, including acute myeloid leukemia (AML).
Dr. Saunthararajah said that work on another approach, using decitabine (Dacogen), for which he has done a phase 1 study, is “struggling,” because of the search for funding.
Two other approaches that Dr. Saunthararajah cited in his presentation appear to remain on track. These are gene therapy and the once-daily voxelotor treatment from Global Blood Therapeutics.
In the field of gene therapy, Sangamo Therapeutics and Sanofi’s Bioverativ in May said the Food and Drug Administration had cleared the way for them to start a phase 1/2 clinical trial for the BIVV003 product that they are developing together. This uses zinc finger nuclease (ZFN) gene-editing technology to modifying a short sequence of the BCL11A gene, with the aim of reactivating fetal hemoglobin.
Bluebird Bio’s LentiGlobin gene therapy has advanced as far as phase 3 for transfusion-dependent beta-thalassemia and phase 1/2 for SCD. In October, the European Medicines Agency accepted the company’s application for approval of LentiGlobin gene therapy for the treatment of adolescents and adults with transfusion-dependent beta-thalassemia (TDT) and a non-beta0/beta0 genotype. The company has not said when it expects to file with the FDA for approval of this treatment.
In the field of oral therapies, Global Blood Therapeutics has said it’s in discussions with the FDA about a potential accelerated approval of voxelotor. The tablet is meant to inhibit the underlying mechanism that causes sickling of red blood cells. In June, the company completed a planned review of early data from its phase 3 trial, known as the HOPE study.
“On the primary endpoint (the proportion of patients with greater than 1 g/dL increase in hemoglobin versus baseline), a statistically significant increase was demonstrated with voxelotor at both the 1,500-mg and 900-mg doses after 12 weeks of treatment versus placebo,” Global Blood Therapeutics said in an August regulatory filing with the Securities and Exchange Commission.
The company has said that voxelotor may meet the FDA’s standard for accelerated approval under Subpart H program.
In his presentation at the NIH conference, Tom Williams, MD, PhD, of KEMRI/Wellcome Trust Research Programme highlighted a recent review of experimental SCD treatments by Marilyn Jo Telen, MD, of Duke University, Durham, N.C. (Blood. 2016;127[7]:810-9).
Also among the drugs being tested for SCD is Modus Therapeutics’ sevuparin, which Dr. Williams described as being “a heparin-like molecule without the anticoagulant complications.”
The compound originated as a “passion project” of Mats Wahlgren, MD, PhD, of the Karolinska Institute, Stockholm, who developed it for the treatment of malaria, Dr. Williams said. The company that’s developing sevuparin, Modus Therapeutics, is moving it forward first as a treatment for SCD for commercial reasons, Dr. Williams said.
“They think it’s a better first target,” he said.
An ongoing study of sevuparin for painful crisis is expected to be completed in December, with data then expected to be released in the middle of 2019, Ellen K. Donnelly, PhD, chief executive officer of Modus Therapeutics, said in an interview. She cited a mix of scientific, medical and commercial reasons for her company’s decision to advance sevuparin in SCD.
“First, and most importantly, there is proof of clinical benefit of a similar molecule (the low-molecular-weight heparin called tinzaparin) in patients with sickle cell disease,” Dr. Donnelly said. “Unfortunately, there is a bleeding risk with tinzaparin that limits use of the agent for the treatment of sickle cell disease. Sevuparin does not have the bleeding risk and thus is a strong candidate for SCD.”
Dr. Donnelly also noted the emphasis that the FDA’s Division of Hematology Products has put on development of therapeutics for SCD as a reason for proceeding first with this indication. The agency and the American Society of Hematology in early October held a workshop of experts, physicians, patients, and industry collaborators focused on identifying new endpoints for clinical studies.
Still, she noted that commercial reasons did factor into this decision.
“[I]t is possible for a small company like Modus to take an asset all the way to market when developing a therapeutic for a rare disease given the need for fewer patients and smaller trials,” Dr. Donnelly wrote. “As you can see from www.clinicaltrials.gov, we were able to run our phase 2 study with a small number of sites. In addition, in SCD it is possible to get approval with only one or two confirmatory studies.”
Financial interests
Other drugs in testing for SCD include rivipansel from GlycoMimetics, for which Pfizer is leading development. The sponsors expect to complete the so-called RESET trial by 2019, according to the clinicaltrials.gov. In this study, which is intended to enroll 350 participants, patients who have vaso-occlusive crises are randomly selected for treatment with either rivipansel or placebo.
Speaking during a question-and-answer session at the NIH conference, Robert Swift, PhD, said there’s a need for inexpensive oral drugs to treat SCD. Many other options will remain beyond the finances of people living in poor countries, he said.
“We need to focus not only on the root cause, but on something that is oral and inexpensive to solve the greater sickle cell problem,” Dr. Swift said.
Large drugmakers already have hospital-based sales forces, making SCD drugs administered in this setting attractive to them, he said.
“This is partly about where money is. The drug companies are going where the money is. It’s not oral drugs to treat everybody, it’s something else,” Dr. Swift said. “So someone else is going to have to fund the basic research” into treatments that could be more broadly used.
Dr. Swift said in an interview that he has received NIH funding for developing SCD-101, an oral drug, for which a placebo-controlled crossover study is underway.
Presenters at the NIH conference, including Dr. Saunthararajah, expressed frustration about what they see as relatively little work being done on SCD despite decades of knowledge about the root causes. Like Dr. Swift, he criticized the approach taken in selecting which treatments advance in this field.
“It’s not being driven by what is the most cost effective, what the patients need the most,” Dr. Saunthararajah said. “It’s driven by what will make the most money, not just for [the] drug company, but also for the hospital and also for the physicians.”
Dr. Saunthararajah reported having patents and patent applications around decitabine/tetrahydrouridine, 5-azacytidine/tetrahydrouridine, and differentiation therapy for oncology. He has also been a consultant for EpiDestiny, Novo Nordisk, and Takeda Oncology. Dr. Williams reported having no relevant financial disclosures. Dr. Swift is a managing member of Invenux and reported equity in Mast Therapeutics and SCD Development.
This article was updated on 11/9/2018.
BETHESDA, MD – While there are experimental treatments such as sevuparin and gene therapy in testing for sickle cell disease (SCD), researchers said this field is lagging because of a lack of funding.
Yogen Saunthararajah, MD, of the Cleveland Clinic, described what he called a “paltry” landscape of new drugs for SCD at Sickle Cell in Focus, a conference held by the National Institutes of Health.
There are only four main approaches taken by drugs now in clinical testing for addressing the root causes of SCD, despite decades’ worth of research of the genetic and mechanistic underpinnings of this disease, he said.
“It’s pretty sad,” Dr. Saunthararajah said, referring to the quantity of efforts, not their quality.
Within days of his presentation at the conference, one of the drugs he highlighted had officially fallen out of contention. An Oct. 26 post on NIH’s Clinicaltrials.gov site said Incyte had terminated its phase 1 study of INCB059872 in SCD “due to a business decision” not to pursue this indication. Incyte confirmed that it dropped development of INCB059872 for SCD but will continue testing it for other indications, including acute myeloid leukemia (AML).
Dr. Saunthararajah said that work on another approach, using decitabine (Dacogen), for which he has done a phase 1 study, is “struggling,” because of the search for funding.
Two other approaches that Dr. Saunthararajah cited in his presentation appear to remain on track. These are gene therapy and the once-daily voxelotor treatment from Global Blood Therapeutics.
In the field of gene therapy, Sangamo Therapeutics and Sanofi’s Bioverativ in May said the Food and Drug Administration had cleared the way for them to start a phase 1/2 clinical trial for the BIVV003 product that they are developing together. This uses zinc finger nuclease (ZFN) gene-editing technology to modifying a short sequence of the BCL11A gene, with the aim of reactivating fetal hemoglobin.
Bluebird Bio’s LentiGlobin gene therapy has advanced as far as phase 3 for transfusion-dependent beta-thalassemia and phase 1/2 for SCD. In October, the European Medicines Agency accepted the company’s application for approval of LentiGlobin gene therapy for the treatment of adolescents and adults with transfusion-dependent beta-thalassemia (TDT) and a non-beta0/beta0 genotype. The company has not said when it expects to file with the FDA for approval of this treatment.
In the field of oral therapies, Global Blood Therapeutics has said it’s in discussions with the FDA about a potential accelerated approval of voxelotor. The tablet is meant to inhibit the underlying mechanism that causes sickling of red blood cells. In June, the company completed a planned review of early data from its phase 3 trial, known as the HOPE study.
“On the primary endpoint (the proportion of patients with greater than 1 g/dL increase in hemoglobin versus baseline), a statistically significant increase was demonstrated with voxelotor at both the 1,500-mg and 900-mg doses after 12 weeks of treatment versus placebo,” Global Blood Therapeutics said in an August regulatory filing with the Securities and Exchange Commission.
The company has said that voxelotor may meet the FDA’s standard for accelerated approval under Subpart H program.
In his presentation at the NIH conference, Tom Williams, MD, PhD, of KEMRI/Wellcome Trust Research Programme highlighted a recent review of experimental SCD treatments by Marilyn Jo Telen, MD, of Duke University, Durham, N.C. (Blood. 2016;127[7]:810-9).
Also among the drugs being tested for SCD is Modus Therapeutics’ sevuparin, which Dr. Williams described as being “a heparin-like molecule without the anticoagulant complications.”
The compound originated as a “passion project” of Mats Wahlgren, MD, PhD, of the Karolinska Institute, Stockholm, who developed it for the treatment of malaria, Dr. Williams said. The company that’s developing sevuparin, Modus Therapeutics, is moving it forward first as a treatment for SCD for commercial reasons, Dr. Williams said.
“They think it’s a better first target,” he said.
An ongoing study of sevuparin for painful crisis is expected to be completed in December, with data then expected to be released in the middle of 2019, Ellen K. Donnelly, PhD, chief executive officer of Modus Therapeutics, said in an interview. She cited a mix of scientific, medical and commercial reasons for her company’s decision to advance sevuparin in SCD.
“First, and most importantly, there is proof of clinical benefit of a similar molecule (the low-molecular-weight heparin called tinzaparin) in patients with sickle cell disease,” Dr. Donnelly said. “Unfortunately, there is a bleeding risk with tinzaparin that limits use of the agent for the treatment of sickle cell disease. Sevuparin does not have the bleeding risk and thus is a strong candidate for SCD.”
Dr. Donnelly also noted the emphasis that the FDA’s Division of Hematology Products has put on development of therapeutics for SCD as a reason for proceeding first with this indication. The agency and the American Society of Hematology in early October held a workshop of experts, physicians, patients, and industry collaborators focused on identifying new endpoints for clinical studies.
Still, she noted that commercial reasons did factor into this decision.
“[I]t is possible for a small company like Modus to take an asset all the way to market when developing a therapeutic for a rare disease given the need for fewer patients and smaller trials,” Dr. Donnelly wrote. “As you can see from www.clinicaltrials.gov, we were able to run our phase 2 study with a small number of sites. In addition, in SCD it is possible to get approval with only one or two confirmatory studies.”
Financial interests
Other drugs in testing for SCD include rivipansel from GlycoMimetics, for which Pfizer is leading development. The sponsors expect to complete the so-called RESET trial by 2019, according to the clinicaltrials.gov. In this study, which is intended to enroll 350 participants, patients who have vaso-occlusive crises are randomly selected for treatment with either rivipansel or placebo.
Speaking during a question-and-answer session at the NIH conference, Robert Swift, PhD, said there’s a need for inexpensive oral drugs to treat SCD. Many other options will remain beyond the finances of people living in poor countries, he said.
“We need to focus not only on the root cause, but on something that is oral and inexpensive to solve the greater sickle cell problem,” Dr. Swift said.
Large drugmakers already have hospital-based sales forces, making SCD drugs administered in this setting attractive to them, he said.
“This is partly about where money is. The drug companies are going where the money is. It’s not oral drugs to treat everybody, it’s something else,” Dr. Swift said. “So someone else is going to have to fund the basic research” into treatments that could be more broadly used.
Dr. Swift said in an interview that he has received NIH funding for developing SCD-101, an oral drug, for which a placebo-controlled crossover study is underway.
Presenters at the NIH conference, including Dr. Saunthararajah, expressed frustration about what they see as relatively little work being done on SCD despite decades of knowledge about the root causes. Like Dr. Swift, he criticized the approach taken in selecting which treatments advance in this field.
“It’s not being driven by what is the most cost effective, what the patients need the most,” Dr. Saunthararajah said. “It’s driven by what will make the most money, not just for [the] drug company, but also for the hospital and also for the physicians.”
Dr. Saunthararajah reported having patents and patent applications around decitabine/tetrahydrouridine, 5-azacytidine/tetrahydrouridine, and differentiation therapy for oncology. He has also been a consultant for EpiDestiny, Novo Nordisk, and Takeda Oncology. Dr. Williams reported having no relevant financial disclosures. Dr. Swift is a managing member of Invenux and reported equity in Mast Therapeutics and SCD Development.
This article was updated on 11/9/2018.
BETHESDA, MD – While there are experimental treatments such as sevuparin and gene therapy in testing for sickle cell disease (SCD), researchers said this field is lagging because of a lack of funding.
Yogen Saunthararajah, MD, of the Cleveland Clinic, described what he called a “paltry” landscape of new drugs for SCD at Sickle Cell in Focus, a conference held by the National Institutes of Health.
There are only four main approaches taken by drugs now in clinical testing for addressing the root causes of SCD, despite decades’ worth of research of the genetic and mechanistic underpinnings of this disease, he said.
“It’s pretty sad,” Dr. Saunthararajah said, referring to the quantity of efforts, not their quality.
Within days of his presentation at the conference, one of the drugs he highlighted had officially fallen out of contention. An Oct. 26 post on NIH’s Clinicaltrials.gov site said Incyte had terminated its phase 1 study of INCB059872 in SCD “due to a business decision” not to pursue this indication. Incyte confirmed that it dropped development of INCB059872 for SCD but will continue testing it for other indications, including acute myeloid leukemia (AML).
Dr. Saunthararajah said that work on another approach, using decitabine (Dacogen), for which he has done a phase 1 study, is “struggling,” because of the search for funding.
Two other approaches that Dr. Saunthararajah cited in his presentation appear to remain on track. These are gene therapy and the once-daily voxelotor treatment from Global Blood Therapeutics.
In the field of gene therapy, Sangamo Therapeutics and Sanofi’s Bioverativ in May said the Food and Drug Administration had cleared the way for them to start a phase 1/2 clinical trial for the BIVV003 product that they are developing together. This uses zinc finger nuclease (ZFN) gene-editing technology to modifying a short sequence of the BCL11A gene, with the aim of reactivating fetal hemoglobin.
Bluebird Bio’s LentiGlobin gene therapy has advanced as far as phase 3 for transfusion-dependent beta-thalassemia and phase 1/2 for SCD. In October, the European Medicines Agency accepted the company’s application for approval of LentiGlobin gene therapy for the treatment of adolescents and adults with transfusion-dependent beta-thalassemia (TDT) and a non-beta0/beta0 genotype. The company has not said when it expects to file with the FDA for approval of this treatment.
In the field of oral therapies, Global Blood Therapeutics has said it’s in discussions with the FDA about a potential accelerated approval of voxelotor. The tablet is meant to inhibit the underlying mechanism that causes sickling of red blood cells. In June, the company completed a planned review of early data from its phase 3 trial, known as the HOPE study.
“On the primary endpoint (the proportion of patients with greater than 1 g/dL increase in hemoglobin versus baseline), a statistically significant increase was demonstrated with voxelotor at both the 1,500-mg and 900-mg doses after 12 weeks of treatment versus placebo,” Global Blood Therapeutics said in an August regulatory filing with the Securities and Exchange Commission.
The company has said that voxelotor may meet the FDA’s standard for accelerated approval under Subpart H program.
In his presentation at the NIH conference, Tom Williams, MD, PhD, of KEMRI/Wellcome Trust Research Programme highlighted a recent review of experimental SCD treatments by Marilyn Jo Telen, MD, of Duke University, Durham, N.C. (Blood. 2016;127[7]:810-9).
Also among the drugs being tested for SCD is Modus Therapeutics’ sevuparin, which Dr. Williams described as being “a heparin-like molecule without the anticoagulant complications.”
The compound originated as a “passion project” of Mats Wahlgren, MD, PhD, of the Karolinska Institute, Stockholm, who developed it for the treatment of malaria, Dr. Williams said. The company that’s developing sevuparin, Modus Therapeutics, is moving it forward first as a treatment for SCD for commercial reasons, Dr. Williams said.
“They think it’s a better first target,” he said.
An ongoing study of sevuparin for painful crisis is expected to be completed in December, with data then expected to be released in the middle of 2019, Ellen K. Donnelly, PhD, chief executive officer of Modus Therapeutics, said in an interview. She cited a mix of scientific, medical and commercial reasons for her company’s decision to advance sevuparin in SCD.
“First, and most importantly, there is proof of clinical benefit of a similar molecule (the low-molecular-weight heparin called tinzaparin) in patients with sickle cell disease,” Dr. Donnelly said. “Unfortunately, there is a bleeding risk with tinzaparin that limits use of the agent for the treatment of sickle cell disease. Sevuparin does not have the bleeding risk and thus is a strong candidate for SCD.”
Dr. Donnelly also noted the emphasis that the FDA’s Division of Hematology Products has put on development of therapeutics for SCD as a reason for proceeding first with this indication. The agency and the American Society of Hematology in early October held a workshop of experts, physicians, patients, and industry collaborators focused on identifying new endpoints for clinical studies.
Still, she noted that commercial reasons did factor into this decision.
“[I]t is possible for a small company like Modus to take an asset all the way to market when developing a therapeutic for a rare disease given the need for fewer patients and smaller trials,” Dr. Donnelly wrote. “As you can see from www.clinicaltrials.gov, we were able to run our phase 2 study with a small number of sites. In addition, in SCD it is possible to get approval with only one or two confirmatory studies.”
Financial interests
Other drugs in testing for SCD include rivipansel from GlycoMimetics, for which Pfizer is leading development. The sponsors expect to complete the so-called RESET trial by 2019, according to the clinicaltrials.gov. In this study, which is intended to enroll 350 participants, patients who have vaso-occlusive crises are randomly selected for treatment with either rivipansel or placebo.
Speaking during a question-and-answer session at the NIH conference, Robert Swift, PhD, said there’s a need for inexpensive oral drugs to treat SCD. Many other options will remain beyond the finances of people living in poor countries, he said.
“We need to focus not only on the root cause, but on something that is oral and inexpensive to solve the greater sickle cell problem,” Dr. Swift said.
Large drugmakers already have hospital-based sales forces, making SCD drugs administered in this setting attractive to them, he said.
“This is partly about where money is. The drug companies are going where the money is. It’s not oral drugs to treat everybody, it’s something else,” Dr. Swift said. “So someone else is going to have to fund the basic research” into treatments that could be more broadly used.
Dr. Swift said in an interview that he has received NIH funding for developing SCD-101, an oral drug, for which a placebo-controlled crossover study is underway.
Presenters at the NIH conference, including Dr. Saunthararajah, expressed frustration about what they see as relatively little work being done on SCD despite decades of knowledge about the root causes. Like Dr. Swift, he criticized the approach taken in selecting which treatments advance in this field.
“It’s not being driven by what is the most cost effective, what the patients need the most,” Dr. Saunthararajah said. “It’s driven by what will make the most money, not just for [the] drug company, but also for the hospital and also for the physicians.”
Dr. Saunthararajah reported having patents and patent applications around decitabine/tetrahydrouridine, 5-azacytidine/tetrahydrouridine, and differentiation therapy for oncology. He has also been a consultant for EpiDestiny, Novo Nordisk, and Takeda Oncology. Dr. Williams reported having no relevant financial disclosures. Dr. Swift is a managing member of Invenux and reported equity in Mast Therapeutics and SCD Development.
This article was updated on 11/9/2018.
REPORTING FROM SICKLE CELL IN FOCUS
Report details financial burden of blood cancers
with costs for acute leukemia almost tripling that amount, according to a new report from the Leukemia & Lymphoma Society (LLS).
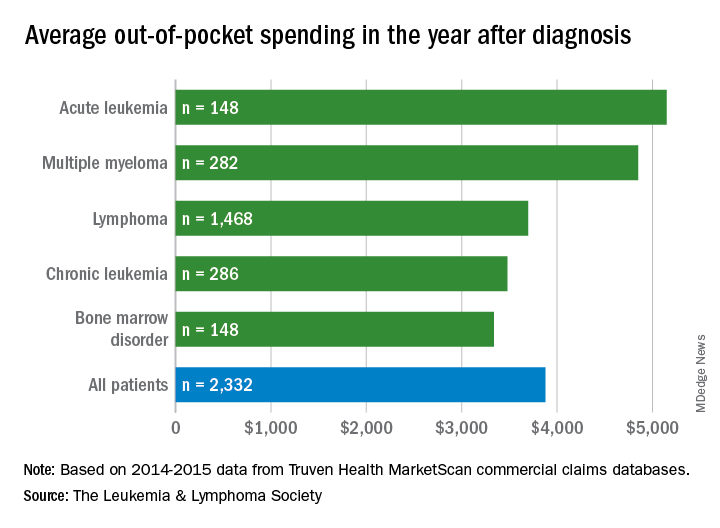
Total allowed cost – the average amount paid by the insurer and patient combined – for acute leukemia was more than $463,000 for the 12 months after initial diagnosis. Averages for the other four cancers included in the analysis came in at $214,000 for multiple myeloma, $134,000 for bone marrow disorders, $131,000 for lymphoma, and $89,000 for chronic leukemia, the LLS said.
The cost figures are drawn from claims data for 2,332 patients diagnosed in 2014.
Differences in out-of-pocket (OOP) costs were smaller, with the average for all patients at almost $3,900 in the year after diagnosis and acute leukemia coming in the highest at $5,100. Over time, however, OOP costs for multiple myeloma patients became the highest, totaling $9,100 for the 3 years after diagnosis, compared with $8,800 for acute leukemia and an average of less than $7,800 for the other blood cancers, the LLS said in the report, which was prepared by the actuarial firm Milliman.
OOP costs also varied by the type of plan. Patients in high-deductible plans averaged nearly $5,400 for the first year after diagnosis, compared with $3,300 for those with traditional insurance, the LLS noted. For acute leukemia, the OOP costs of high-deductible plans were more than twice as high as those of traditional plans.
The study was based on data for adults aged 18-64 years from the Truven Health MarketScan commercial claims databases for the years from 2013 to 2016. The LLS received support for the study from Pfizer, Genentech, and Amgen.
with costs for acute leukemia almost tripling that amount, according to a new report from the Leukemia & Lymphoma Society (LLS).

Total allowed cost – the average amount paid by the insurer and patient combined – for acute leukemia was more than $463,000 for the 12 months after initial diagnosis. Averages for the other four cancers included in the analysis came in at $214,000 for multiple myeloma, $134,000 for bone marrow disorders, $131,000 for lymphoma, and $89,000 for chronic leukemia, the LLS said.
The cost figures are drawn from claims data for 2,332 patients diagnosed in 2014.
Differences in out-of-pocket (OOP) costs were smaller, with the average for all patients at almost $3,900 in the year after diagnosis and acute leukemia coming in the highest at $5,100. Over time, however, OOP costs for multiple myeloma patients became the highest, totaling $9,100 for the 3 years after diagnosis, compared with $8,800 for acute leukemia and an average of less than $7,800 for the other blood cancers, the LLS said in the report, which was prepared by the actuarial firm Milliman.
OOP costs also varied by the type of plan. Patients in high-deductible plans averaged nearly $5,400 for the first year after diagnosis, compared with $3,300 for those with traditional insurance, the LLS noted. For acute leukemia, the OOP costs of high-deductible plans were more than twice as high as those of traditional plans.
The study was based on data for adults aged 18-64 years from the Truven Health MarketScan commercial claims databases for the years from 2013 to 2016. The LLS received support for the study from Pfizer, Genentech, and Amgen.
with costs for acute leukemia almost tripling that amount, according to a new report from the Leukemia & Lymphoma Society (LLS).

Total allowed cost – the average amount paid by the insurer and patient combined – for acute leukemia was more than $463,000 for the 12 months after initial diagnosis. Averages for the other four cancers included in the analysis came in at $214,000 for multiple myeloma, $134,000 for bone marrow disorders, $131,000 for lymphoma, and $89,000 for chronic leukemia, the LLS said.
The cost figures are drawn from claims data for 2,332 patients diagnosed in 2014.
Differences in out-of-pocket (OOP) costs were smaller, with the average for all patients at almost $3,900 in the year after diagnosis and acute leukemia coming in the highest at $5,100. Over time, however, OOP costs for multiple myeloma patients became the highest, totaling $9,100 for the 3 years after diagnosis, compared with $8,800 for acute leukemia and an average of less than $7,800 for the other blood cancers, the LLS said in the report, which was prepared by the actuarial firm Milliman.
OOP costs also varied by the type of plan. Patients in high-deductible plans averaged nearly $5,400 for the first year after diagnosis, compared with $3,300 for those with traditional insurance, the LLS noted. For acute leukemia, the OOP costs of high-deductible plans were more than twice as high as those of traditional plans.
The study was based on data for adults aged 18-64 years from the Truven Health MarketScan commercial claims databases for the years from 2013 to 2016. The LLS received support for the study from Pfizer, Genentech, and Amgen.
FDA approves second pegfilgrastim biosimilar
The Food and Drug Administration has approved a second biosimilar to pegfilgrastim (Neulasta) to decrease the chance of infection in patients with nonmyeloid cancer who are receiving myelosuppressive chemotherapy and are at risk of febrile neutropenia.
Approval of pegfilgrastim-cbqv, previously known as CHS-1701, was based on analyses establishing biosimilarity, including pharmacokinetic, pharmacodynamic, and immunogenicity studies. Clinical trial results were presented at the 2017 ASCO Annual Meeting.
The most common adverse reactions with pegfilgrastim-cbqv are bone pain and pain in extremities.
The FDA approved the first pegfilgrastim biosimilar, pegfilgrastim-jmdb (Fulphila) in June.
Pegfilgrastim-cbqv will be marketed as Udenyca by Coherus BioSciences.
“Udenyca’s robust clinical package includes a dedicated immunogenicity similarity study in over 300 healthy subjects,” Barbara Finck, MD, chief medical officer of Coherus BioSciences, said in a press release.
“In support of that study, and as part of our commitment to ensuring patient safety, we deployed a battery of sensitive immunogenicity assays. This effort not only supported the biosimilarity of Udenyca but also advanced the understanding of the immunogenic response of pegfilgrastim products.”
Coherus BioSciences plans to provide details about pricing and the launch of pegfilgrastim-cbqv during an earnings call on Nov. 8.
The Food and Drug Administration has approved a second biosimilar to pegfilgrastim (Neulasta) to decrease the chance of infection in patients with nonmyeloid cancer who are receiving myelosuppressive chemotherapy and are at risk of febrile neutropenia.
Approval of pegfilgrastim-cbqv, previously known as CHS-1701, was based on analyses establishing biosimilarity, including pharmacokinetic, pharmacodynamic, and immunogenicity studies. Clinical trial results were presented at the 2017 ASCO Annual Meeting.
The most common adverse reactions with pegfilgrastim-cbqv are bone pain and pain in extremities.
The FDA approved the first pegfilgrastim biosimilar, pegfilgrastim-jmdb (Fulphila) in June.
Pegfilgrastim-cbqv will be marketed as Udenyca by Coherus BioSciences.
“Udenyca’s robust clinical package includes a dedicated immunogenicity similarity study in over 300 healthy subjects,” Barbara Finck, MD, chief medical officer of Coherus BioSciences, said in a press release.
“In support of that study, and as part of our commitment to ensuring patient safety, we deployed a battery of sensitive immunogenicity assays. This effort not only supported the biosimilarity of Udenyca but also advanced the understanding of the immunogenic response of pegfilgrastim products.”
Coherus BioSciences plans to provide details about pricing and the launch of pegfilgrastim-cbqv during an earnings call on Nov. 8.
The Food and Drug Administration has approved a second biosimilar to pegfilgrastim (Neulasta) to decrease the chance of infection in patients with nonmyeloid cancer who are receiving myelosuppressive chemotherapy and are at risk of febrile neutropenia.
Approval of pegfilgrastim-cbqv, previously known as CHS-1701, was based on analyses establishing biosimilarity, including pharmacokinetic, pharmacodynamic, and immunogenicity studies. Clinical trial results were presented at the 2017 ASCO Annual Meeting.
The most common adverse reactions with pegfilgrastim-cbqv are bone pain and pain in extremities.
The FDA approved the first pegfilgrastim biosimilar, pegfilgrastim-jmdb (Fulphila) in June.
Pegfilgrastim-cbqv will be marketed as Udenyca by Coherus BioSciences.
“Udenyca’s robust clinical package includes a dedicated immunogenicity similarity study in over 300 healthy subjects,” Barbara Finck, MD, chief medical officer of Coherus BioSciences, said in a press release.
“In support of that study, and as part of our commitment to ensuring patient safety, we deployed a battery of sensitive immunogenicity assays. This effort not only supported the biosimilarity of Udenyca but also advanced the understanding of the immunogenic response of pegfilgrastim products.”
Coherus BioSciences plans to provide details about pricing and the launch of pegfilgrastim-cbqv during an earnings call on Nov. 8.
Platelet transfusion threshold matters for preterm infants
A lower threshold for platelet transfusions in preterm infants with severe thrombocytopenia is associated with significantly lower incidence of death and major bleeding, compared with a higher threshold, a new study suggests.
A new major bleeding episode or death occurred in 26% of infants in the high-threshold group, compared with 19% in the low-threshold group, representing a 57% higher risk of poor outcomes even after researchers adjusted for gestational age and intrauterine growth restriction (odds ratio, 1.57; P = .02).
Researchers reported the results of a trial in 660 infants with a mean gestational age of 26.6 weeks, who were randomized to a platelet infusion either at a high platelet–count threshold of 50,000/mm3 or a low threshold of 25,000/mm3.
“Although retrospective studies have suggested that platelet transfusions may cause harm in neonates independently of the disease process, data from randomized controlled trials to support this are lacking,” Anna Curley, MD, of the National Maternity Hospital in Dublin and her coauthors reported in the New England Journal of Medicine.
The rates of minor or worse bleeding were similar between the two groups, and the percentage of infants surviving with bronchopulmonary dysplasia at 36 weeks of corrected age was higher in the high-threshold group (63% vs. 54%; OR, 1.54).
The rates of serious adverse events, not including major bleeding, were similar between the high- and low-threshold groups.
The outcomes of transfusions were not influenced by other factors such as intrauterine growth restriction, gestational age, or postnatal age at randomization.
“Our trial highlights the importance of trials of platelet transfusion involving patients with conditions other than haematological malignancies,” the authors wrote.
However they acknowledged that the reasons for the differences in mortality and outcomes between the two study groups were unknown.
“Platelets have recognized immunological and inflammatory effects, outside of effects on hemostasis,” they wrote. “The effect of transfusing adult platelets to a delicately balance neonatal hemostatic system with relatively hypofunctional platelets is also poorly understood.”
The study was supported by the National Health Service Blood and Transplant Research and Development Committee and other foundations. Authors reported financial disclosures related to Sanquin and Cerus.
SOURCE: Curley A et al. N Engl J Med. 2018 Nov 2. doi: 10.1056/NEJMoa1807320.
A lower threshold for platelet transfusions in preterm infants with severe thrombocytopenia is associated with significantly lower incidence of death and major bleeding, compared with a higher threshold, a new study suggests.
A new major bleeding episode or death occurred in 26% of infants in the high-threshold group, compared with 19% in the low-threshold group, representing a 57% higher risk of poor outcomes even after researchers adjusted for gestational age and intrauterine growth restriction (odds ratio, 1.57; P = .02).
Researchers reported the results of a trial in 660 infants with a mean gestational age of 26.6 weeks, who were randomized to a platelet infusion either at a high platelet–count threshold of 50,000/mm3 or a low threshold of 25,000/mm3.
“Although retrospective studies have suggested that platelet transfusions may cause harm in neonates independently of the disease process, data from randomized controlled trials to support this are lacking,” Anna Curley, MD, of the National Maternity Hospital in Dublin and her coauthors reported in the New England Journal of Medicine.
The rates of minor or worse bleeding were similar between the two groups, and the percentage of infants surviving with bronchopulmonary dysplasia at 36 weeks of corrected age was higher in the high-threshold group (63% vs. 54%; OR, 1.54).
The rates of serious adverse events, not including major bleeding, were similar between the high- and low-threshold groups.
The outcomes of transfusions were not influenced by other factors such as intrauterine growth restriction, gestational age, or postnatal age at randomization.
“Our trial highlights the importance of trials of platelet transfusion involving patients with conditions other than haematological malignancies,” the authors wrote.
However they acknowledged that the reasons for the differences in mortality and outcomes between the two study groups were unknown.
“Platelets have recognized immunological and inflammatory effects, outside of effects on hemostasis,” they wrote. “The effect of transfusing adult platelets to a delicately balance neonatal hemostatic system with relatively hypofunctional platelets is also poorly understood.”
The study was supported by the National Health Service Blood and Transplant Research and Development Committee and other foundations. Authors reported financial disclosures related to Sanquin and Cerus.
SOURCE: Curley A et al. N Engl J Med. 2018 Nov 2. doi: 10.1056/NEJMoa1807320.
A lower threshold for platelet transfusions in preterm infants with severe thrombocytopenia is associated with significantly lower incidence of death and major bleeding, compared with a higher threshold, a new study suggests.
A new major bleeding episode or death occurred in 26% of infants in the high-threshold group, compared with 19% in the low-threshold group, representing a 57% higher risk of poor outcomes even after researchers adjusted for gestational age and intrauterine growth restriction (odds ratio, 1.57; P = .02).
Researchers reported the results of a trial in 660 infants with a mean gestational age of 26.6 weeks, who were randomized to a platelet infusion either at a high platelet–count threshold of 50,000/mm3 or a low threshold of 25,000/mm3.
“Although retrospective studies have suggested that platelet transfusions may cause harm in neonates independently of the disease process, data from randomized controlled trials to support this are lacking,” Anna Curley, MD, of the National Maternity Hospital in Dublin and her coauthors reported in the New England Journal of Medicine.
The rates of minor or worse bleeding were similar between the two groups, and the percentage of infants surviving with bronchopulmonary dysplasia at 36 weeks of corrected age was higher in the high-threshold group (63% vs. 54%; OR, 1.54).
The rates of serious adverse events, not including major bleeding, were similar between the high- and low-threshold groups.
The outcomes of transfusions were not influenced by other factors such as intrauterine growth restriction, gestational age, or postnatal age at randomization.
“Our trial highlights the importance of trials of platelet transfusion involving patients with conditions other than haematological malignancies,” the authors wrote.
However they acknowledged that the reasons for the differences in mortality and outcomes between the two study groups were unknown.
“Platelets have recognized immunological and inflammatory effects, outside of effects on hemostasis,” they wrote. “The effect of transfusing adult platelets to a delicately balance neonatal hemostatic system with relatively hypofunctional platelets is also poorly understood.”
The study was supported by the National Health Service Blood and Transplant Research and Development Committee and other foundations. Authors reported financial disclosures related to Sanquin and Cerus.
SOURCE: Curley A et al. N Engl J Med. 2018 Nov 2. doi: 10.1056/NEJMoa1807320.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: The odds of new major bleeding or death were 57% higher in preterm infants who received a platelet transfusion at a higher threshold of 50,000 per mm3 than at a lower threshold of 25,000 mm3 (P = .02).Study details: Randomized study in 660 preterm infants with severe thrombocytopenia.
Disclosures: The study was supported by the National Health Service Blood and Transplant Research and Development Committee, and other foundations. Authors reported financial disclosures related to Sanquin and Cerus.
Source: Curley A et al. N Engl J Med. 2018 Nov 2. doi: 10.1056/NEJMoa1807320.
Primary care needs pile up for sickle cell patients
BETHESDA, MD. – With many people surviving well into adulthood with sickle cell disease (SCD) because of advances in treatment, there’s a strong need for more primary care to address chronic conditions, such as obesity and the complications of the blood disorder, researchers said.
People who have lived for decades with SCD may be at higher risk for renal disease while still needing the same routine vaccinations and screening for colon, prostate, and lung cancer that the general population receives, Sophie Lanzkron, MD, of Johns Hopkins University, Baltimore, said at Sickle Cell in Focus, a conference held by the National Institutes of Health.
And obesity is an additional a concern in treating people with SCD, Dr. Lanzkron said.
“It is really hard to have a conversation for 20 minutes with a patient about pain, talk about what you’re going to do about their sickle cell disease, and then address all of” their routine health needs, she said.
People with SCD seem less likely to get renal transplants, but those with end-stage kidney disease should be encouraged to be evaluated for them, Dr. Lanzkron advised.
Older data had suggested that patients with SCD who underwent renal transplant didn’t do as well as everyone else who underwent the procedure, but new data have changed that approach. “There’s some additional data in the modern era suggesting that the outcomes for people who undergo transplant with sickle cell disease are the same as for those who undergo it with diabetes,” Dr. Lanzkron said.
She highlighted one newer study in which the kidney transplant survival rate was 73.1% among individuals with SCD, compared with 74.1% for those with diabetes (Nephrol Dial Transplant. 2013 Apr;28[4]:1039-46).
It’s unclear what the average life expectancy is at this time for someone with SCD, Dr. Lanzkron said. Research looking at death certificate data suggests a median age of death in the mid-40s, but there are limitations to this work given it may exclude many older people with SCD, she said.
“We’re hopeful that people are living into their 50s and 60s, but we don’t have a lot of great data,” she said.
One of the organizers of the NIH conference said she hoped that Dr. Lanzkron’s presentation would draw attention to the need for primary care for people with SCD. Maintaining a healthy lifestyle is particularly important for this group because they likely have had complications from the disease, as well as issues seen with normal aging, Swee Lay Thein, MBBS, of the National Heart, Lung, and Blood Institute, said in an interview.
“This is a key message for many patients with sickle cell disease,” Dr. Thein said. “It’s important to hook up with a primary care physician.”
Dr. Thein cited a recent paper, which reported on four people who had lived into their 80s with sickle cell disease. The paper said their longevity was aided by factors such as being nonsmokers, abstaining from alcohol or drinking it only on occasionally, and maintaining a normal body mass index (Blood. 2016 Nov 10;128[19]:2367-9).
Additionally, the patients had close ties with relatives. The paper said that one patient was married with a helpful husband. Others in this octogenarian set had maintained close ties with their children.
“A common factor for all of the four patients in their 80s was that they had a healthy lifestyle and very strong family support,” Dr. Thein said.
Dr. Lanzkron has been an investigator for trials sponsored by Pfizer, Global Blood Therapeutics, and Ironwood.
BETHESDA, MD. – With many people surviving well into adulthood with sickle cell disease (SCD) because of advances in treatment, there’s a strong need for more primary care to address chronic conditions, such as obesity and the complications of the blood disorder, researchers said.
People who have lived for decades with SCD may be at higher risk for renal disease while still needing the same routine vaccinations and screening for colon, prostate, and lung cancer that the general population receives, Sophie Lanzkron, MD, of Johns Hopkins University, Baltimore, said at Sickle Cell in Focus, a conference held by the National Institutes of Health.
And obesity is an additional a concern in treating people with SCD, Dr. Lanzkron said.
“It is really hard to have a conversation for 20 minutes with a patient about pain, talk about what you’re going to do about their sickle cell disease, and then address all of” their routine health needs, she said.
People with SCD seem less likely to get renal transplants, but those with end-stage kidney disease should be encouraged to be evaluated for them, Dr. Lanzkron advised.
Older data had suggested that patients with SCD who underwent renal transplant didn’t do as well as everyone else who underwent the procedure, but new data have changed that approach. “There’s some additional data in the modern era suggesting that the outcomes for people who undergo transplant with sickle cell disease are the same as for those who undergo it with diabetes,” Dr. Lanzkron said.
She highlighted one newer study in which the kidney transplant survival rate was 73.1% among individuals with SCD, compared with 74.1% for those with diabetes (Nephrol Dial Transplant. 2013 Apr;28[4]:1039-46).
It’s unclear what the average life expectancy is at this time for someone with SCD, Dr. Lanzkron said. Research looking at death certificate data suggests a median age of death in the mid-40s, but there are limitations to this work given it may exclude many older people with SCD, she said.
“We’re hopeful that people are living into their 50s and 60s, but we don’t have a lot of great data,” she said.
One of the organizers of the NIH conference said she hoped that Dr. Lanzkron’s presentation would draw attention to the need for primary care for people with SCD. Maintaining a healthy lifestyle is particularly important for this group because they likely have had complications from the disease, as well as issues seen with normal aging, Swee Lay Thein, MBBS, of the National Heart, Lung, and Blood Institute, said in an interview.
“This is a key message for many patients with sickle cell disease,” Dr. Thein said. “It’s important to hook up with a primary care physician.”
Dr. Thein cited a recent paper, which reported on four people who had lived into their 80s with sickle cell disease. The paper said their longevity was aided by factors such as being nonsmokers, abstaining from alcohol or drinking it only on occasionally, and maintaining a normal body mass index (Blood. 2016 Nov 10;128[19]:2367-9).
Additionally, the patients had close ties with relatives. The paper said that one patient was married with a helpful husband. Others in this octogenarian set had maintained close ties with their children.
“A common factor for all of the four patients in their 80s was that they had a healthy lifestyle and very strong family support,” Dr. Thein said.
Dr. Lanzkron has been an investigator for trials sponsored by Pfizer, Global Blood Therapeutics, and Ironwood.
BETHESDA, MD. – With many people surviving well into adulthood with sickle cell disease (SCD) because of advances in treatment, there’s a strong need for more primary care to address chronic conditions, such as obesity and the complications of the blood disorder, researchers said.
People who have lived for decades with SCD may be at higher risk for renal disease while still needing the same routine vaccinations and screening for colon, prostate, and lung cancer that the general population receives, Sophie Lanzkron, MD, of Johns Hopkins University, Baltimore, said at Sickle Cell in Focus, a conference held by the National Institutes of Health.
And obesity is an additional a concern in treating people with SCD, Dr. Lanzkron said.
“It is really hard to have a conversation for 20 minutes with a patient about pain, talk about what you’re going to do about their sickle cell disease, and then address all of” their routine health needs, she said.
People with SCD seem less likely to get renal transplants, but those with end-stage kidney disease should be encouraged to be evaluated for them, Dr. Lanzkron advised.
Older data had suggested that patients with SCD who underwent renal transplant didn’t do as well as everyone else who underwent the procedure, but new data have changed that approach. “There’s some additional data in the modern era suggesting that the outcomes for people who undergo transplant with sickle cell disease are the same as for those who undergo it with diabetes,” Dr. Lanzkron said.
She highlighted one newer study in which the kidney transplant survival rate was 73.1% among individuals with SCD, compared with 74.1% for those with diabetes (Nephrol Dial Transplant. 2013 Apr;28[4]:1039-46).
It’s unclear what the average life expectancy is at this time for someone with SCD, Dr. Lanzkron said. Research looking at death certificate data suggests a median age of death in the mid-40s, but there are limitations to this work given it may exclude many older people with SCD, she said.
“We’re hopeful that people are living into their 50s and 60s, but we don’t have a lot of great data,” she said.
One of the organizers of the NIH conference said she hoped that Dr. Lanzkron’s presentation would draw attention to the need for primary care for people with SCD. Maintaining a healthy lifestyle is particularly important for this group because they likely have had complications from the disease, as well as issues seen with normal aging, Swee Lay Thein, MBBS, of the National Heart, Lung, and Blood Institute, said in an interview.
“This is a key message for many patients with sickle cell disease,” Dr. Thein said. “It’s important to hook up with a primary care physician.”
Dr. Thein cited a recent paper, which reported on four people who had lived into their 80s with sickle cell disease. The paper said their longevity was aided by factors such as being nonsmokers, abstaining from alcohol or drinking it only on occasionally, and maintaining a normal body mass index (Blood. 2016 Nov 10;128[19]:2367-9).
Additionally, the patients had close ties with relatives. The paper said that one patient was married with a helpful husband. Others in this octogenarian set had maintained close ties with their children.
“A common factor for all of the four patients in their 80s was that they had a healthy lifestyle and very strong family support,” Dr. Thein said.
Dr. Lanzkron has been an investigator for trials sponsored by Pfizer, Global Blood Therapeutics, and Ironwood.
EXPERT ANALYSIS FROM SICKLE CELL IN FOCUS
Prescription Drug Benefits and Survival in Myeloma Among Medicare Beneficiaries
Study Overview
Objective. To investigate the relationship between prescription drug coverage, receipt of active myeloma therapy, and overall survival (OS) among Medicare beneficiaries with multiple myeloma.
Design. Case-control and retrospective cohort archival data research.
Setting and participants. Authors examined SEER-Medicare registry and extracted patients with histologically confirmed multiple myeloma diagnosed in the period 2006 to 2011. Availability of complete Medicare part A/B claims from 1 year before diagnosis until December 2013 was required for analysis. Patients with Medicare advantage or managed care plans did not have claims data available and hence were excluded. Beneficiaries with a diagnosis of diffuse large B-cell lymphoma (DLBCL), who typically receive parenteral drugs for lymphoma therapy, were used as a control cohort.
Main outcome measures. Association between prescription drug coverage status and OS was the primary outcome measure of interest. Authors reported 3-year restricted survival time (RMST) ratios to compare OS among the beneficiaries with different prescription drug coverages. Receipt of active myeloma therapy among beneficiaries was also studied. Relative risk, adjusting for patient and disease-related characteristics, was reported to examine receipt of active myeloma therapy.
Results. Records of 9755 Medicare beneficiaries were evaluated. Of these, 1460 (15%) had no prescription coverage at diagnosis, 3283 (34%) had part D plan prescription benefits, 3607 (37%) had sponsored prescription coverage through an employer, federal employer, or veterans plan, and 1405 (14%) had a Medicaid prescription plan. Beneficiaries without coverage had fewer comorbidities, including anemia, neuropathy, or renal disease, than those with part D prescription coverage or Medicaid. Of those without any prescription drug coverage, 41% obtained prescription plan coverage after diagnosis of myeloma by the following January. Conversely, only 19% of patients with DLBCL and no coverage obtained a prescription plan.
Patients with myeloma were followed for 4.9 years and median survival was 2.3 years, with a 3-year OS rate of 43.1% (95% confidence interval [CI], 42.1%-44.1%). Relative to the group without coverage, survival was 16% longer in the Medicare part D group and sponsored plan group (RMST 1.16; 95% CI, 1.12-1.21). Medicaid/Medicare dual beneficiaries had worse OS in both myeloma and DLBCL consistent with poor performance status and unfavorable baseline comorbidities. However, among patients with myeloma, Medicaid/Medicare dual beneficiaries had better survival (RMST 1.08; 95% CI, 1.03-1.13) compared to the group without coverage. There was no difference in OS for those with or without prescription drug coverage in the DLBCL cohort.
There were significant differences in treatment of myeloma based on types of prescription drug coverage. Due to increasing use of bortezomib following its approval by the U.S. Food and Drug Administration (FDA), parenteral chemotherapy use doubled from 24% to 48% from 2006 to 2011, and utility of active myeloma care increased from 88% to 91%. Medicare part D plan enrollees were 6% more likely to receive active myeloma care, and both Medicaid group and sponsored plan group beneficiaries were equally likely to receive active myeloma care compared to beneficiaries without prescription coverage. Medicaid enrollees were less likely to receive parenteral therapy.
Conclusion. Medicare beneficiaries with prescription drug coverage and multiple myeloma are more likely to receive myeloma therapy and have longer OS compared to those without prescription drug coverage.
Commentary
First-line therapy of multiple myeloma has evolved over the past 2 decades. Parenteral agents such as vincristine, adriamycin, dexamethasone, and cyclophosphamide and oral therapy with melphalan and prednisone were the mainstay of treatment in the past. In the past decade, the arrival of oral therapy using thalidomide or lenalidomide and parenteral therapy using bortezomib has increased OS in patients with myeloma. Most recently, a combination of lenalidomide, bortezomib, and dexamethasone has emerged as one of the frontline therapies of choice.1 Incorporation of bortezomib or an oral immunomodulatory drug is almost universal in first-line therapy.
Oral antineoplastic therapy is increasingly being approved by the FDA and being utilized in the community. During the period 2016-2018, more than half the new FDA-approved oncology drugs were in oral formulation.2 As such, access to these agents is crucial in cancer therapy. The cost of oral therapy in patients without prescription drug coverage is sometimes more than $10,000 per month, which represents a significant impediment to its adoption. Forty-three states and Washington, DC, have enacted drug parity laws that require patients to pay no more for an oral cancer treatment than they would for an infusion. However, currently there is no such federal law, and Medicare beneficiaries must participate either through part D, state Medicaid, or a sponsored program to obtain prescription drug coverage. Despite being enrolled in part D, many beneficiaries fall into the “doughnut hole” (the requirement of Part D beneficiaries with high prescription drug expenses to pay more once the total cost of their medicines reaches a certain threshold) for prescription drugs at the time of need. From 2019 onward, enrollees will see significant, yet sometimes still insufficient, coverage benefits due to ending of the doughnut hole.3 Only a very limited number of oral chemotherapy agents are covered through Medicare part B, and of those covered, only oral melphalan is used for myeloma.
The authors have acknowledged multiple limitations of their investigation, including possible unobserved clinical differences between beneficiaries. SEER-Medicare registry has limitations in obtaining individual level data and may not contain specific results of cytogenetics, laboratory risk markers, and response to therapy, which are important to determine overall outcome. A prospective evaluation may be more suitable to assess these variables independently or through a multivariate analysis in determining receipt of therapy on OS, although such a study is currently not feasible.
The indicator of active myeloma care was defined as 2 or more outpatient physician visits or receipt of parenteral chemotherapy. This definition is somewhat suboptimal, as often patients with myeloma are under surveillance and may not necessarily be receiving active treatment. Moreover, the exact prescription pattern of lenalidomide, the most active first-line oral therapy, could not be captured from this retrospective registry review. Therefore, definitive conclusions regarding use of lenalidomide and thalidomide and receipt of therapy in this population cannot be made.
A significant improvement in OS has been established using maintenance lenalidomide following high-dose chemotherapy and stem cell transplantation.4 Only 5% of this study population received stem cell transplantation. This may be due to a median age of 77 years at diagnosis in the group studied, higher than the 66 to 70 years previously published.5 Stem cell transplantation is now commonly being used even in the older population. The 3-year survival of 83% following stem cell transplantation in myeloma patients aged 75 to 84 years was nearly identical to that of the younger population.6 Since stem cell transplantation is feasible in older Medicare beneficiaries and maintenance lenalidomide for 2 years following transplant improves survival, the option of providing maintenance therapy with oral lenalidomide must be made available to Medicare beneficiaries. Due to a very limited use of transplantation in this study, the impact of oral lenalidomide maintenance in OS cannot be judged.
Of the patients reviewed in this study, 6% had a listed diagnosis of plasmacytoma. These individuals typically are treated with radiation therapy only. It is unclear if these patients also received any systemic myeloma therapy or if they ever progressed to myeloma. Availability of prescription drug coverage may not be relevant to this group. Also, the authors reported that part D participants were less likely to receive classic cytotoxic chemotherapy. This may be somewhat irrelevant in Medicare beneficiaries with a median age of 77 years for current practice, as frontline induction with old classic cytotoxic chemotherapy is less commonly used in this population.
Investigators have appropriately recognized a lack of ability to discern whether inferior survival in the group without prescription drug coverage was the result of not receiving therapy at all or inability to receive oral immunomodulatory drugs. There would have been little reason for not proceeding to parenteral therapy. As noted, 41% of beneficiaries without coverage at diagnosis subsequently obtained coverage but continued to have significantly worse survival. Cause of death, including whether related to myeloma, was not reported. The authors suggest that early separation of survival curves could therefore be reflective of suboptimal first-line therapy that lacked oral immunomodulatory drugs. During the study period 2006-2011, first-line use of lenalidomide was common.
Median survival of patients with myeloma in this study was only 27 months. According to the American Cancer Society, in 2018 median survival for stage I myeloma has not been reached, stage II myeloma is 83 months, and stage III myeloma is 43 months. A robust and dynamic landscape in myeloma therapy prevents a clear attribution to individual agents, whether oral or parenteral, in improving OS. Thus, 3-year RMST, while appropriate for 2006-2011, may not be relevant today.
Applications for Clinical Practice
The oncology community routinely encounters difficulty in initiating therapy using oral agents rapidly after diagnosis of myeloma. The retrospective data analyzed in the current study suggests that delay in initiating or unavailability of oral agents may adversely impact OS. The common approach of initiating parenteral therapy while awaiting approvals from payers or charity programs and subsequently adding oral therapy when available has not been studied in assessing OS. The oncology community should initiate plans to obtain prescription drug coverage through either Medicare part D, Medicaid, a sponsored plan, or financial assistance charity programs as soon as possible after diagnosis of myeloma. Moreover, continuation of these prescription drug plans should be strongly considered throughout the course of myeloma, as subsequent lines of treatment will quite likely involve other active and approved oral agents, such as pomalidomide, ixazomib, and panobinostat, besides other supportive therapy.
One of the mechanisms to obtain prescription drug coverage includes enrollment in state Medicaid programs for those who are eligible. Currently, 17 states have not yet adopted Medicaid expansion under the Affordable Care Act. Expansion of Medicaid in these states could increase availability of prescription drug benefits. In this study, 15.8% of Medicare and Medicaid dual enrollees with access to oral agents at low or no cost did not receive myeloma care, slightly higher than the 13.1% with no prescription drug coverage. Lower utilization in this population may be explained based on differences in comorbidities or socioeconomic conditions rather than availability of a prescription plan.
The incidence of myeloma is expected to be higher in Medicare beneficiaries, and according to one estimate, in 2030 and beyond nearly 75% of diagnosed myeloma patients will be aged 64 to 84 years, an increase from nearly 66% today.7 Changing demographics, increasing oral therapy options, and patient convenience demand attention to providing prescription drug coverage to all Medicare beneficiaries. This study lends support to that demand.
—Rakesh Gaur, MD, MPH, FACP, Cancer and Blood Center at Kansas Institute of Medicine, Lenexa, KS
1. Durie BG, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomized, open-label, phase 3 trial. Lancet. 2017;389(10068):519-527.
2. U.S. Food and Drug Administration. Hematology/Oncology (Cancer) Approvals & Safety Notifications. www.fda.gov/drugs/informationondrugs/approveddrugs/ucm279174.htm. Accessed October 11, 2018.
3. Dusetzina SB, Keating NL. Mind the gap: Why closing the doughnut hole is insufficient for increasing Medicare beneficiary access to oral chemotherapy. J Clin Oncol. 2016;34:375-380.
4. McCarthy PL, Holstein SA, Petrucci MT, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017;35:3279-3289.
5. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33.
6. Dong N, McKiernan P, Samuel D, et al. Autologous stem cell transplantation in multiple myeloma patients over age 75 [abstract]. J Clin Oncol. 2018;36(suppl): 8025.
7. Rosenberg PS, Barker KA, Anderson WF. Future distribution of multiple myeloma in the United States by sex, age, and race/ethnicity. Blood. 2015;125:410–412.
Study Overview
Objective. To investigate the relationship between prescription drug coverage, receipt of active myeloma therapy, and overall survival (OS) among Medicare beneficiaries with multiple myeloma.
Design. Case-control and retrospective cohort archival data research.
Setting and participants. Authors examined SEER-Medicare registry and extracted patients with histologically confirmed multiple myeloma diagnosed in the period 2006 to 2011. Availability of complete Medicare part A/B claims from 1 year before diagnosis until December 2013 was required for analysis. Patients with Medicare advantage or managed care plans did not have claims data available and hence were excluded. Beneficiaries with a diagnosis of diffuse large B-cell lymphoma (DLBCL), who typically receive parenteral drugs for lymphoma therapy, were used as a control cohort.
Main outcome measures. Association between prescription drug coverage status and OS was the primary outcome measure of interest. Authors reported 3-year restricted survival time (RMST) ratios to compare OS among the beneficiaries with different prescription drug coverages. Receipt of active myeloma therapy among beneficiaries was also studied. Relative risk, adjusting for patient and disease-related characteristics, was reported to examine receipt of active myeloma therapy.
Results. Records of 9755 Medicare beneficiaries were evaluated. Of these, 1460 (15%) had no prescription coverage at diagnosis, 3283 (34%) had part D plan prescription benefits, 3607 (37%) had sponsored prescription coverage through an employer, federal employer, or veterans plan, and 1405 (14%) had a Medicaid prescription plan. Beneficiaries without coverage had fewer comorbidities, including anemia, neuropathy, or renal disease, than those with part D prescription coverage or Medicaid. Of those without any prescription drug coverage, 41% obtained prescription plan coverage after diagnosis of myeloma by the following January. Conversely, only 19% of patients with DLBCL and no coverage obtained a prescription plan.
Patients with myeloma were followed for 4.9 years and median survival was 2.3 years, with a 3-year OS rate of 43.1% (95% confidence interval [CI], 42.1%-44.1%). Relative to the group without coverage, survival was 16% longer in the Medicare part D group and sponsored plan group (RMST 1.16; 95% CI, 1.12-1.21). Medicaid/Medicare dual beneficiaries had worse OS in both myeloma and DLBCL consistent with poor performance status and unfavorable baseline comorbidities. However, among patients with myeloma, Medicaid/Medicare dual beneficiaries had better survival (RMST 1.08; 95% CI, 1.03-1.13) compared to the group without coverage. There was no difference in OS for those with or without prescription drug coverage in the DLBCL cohort.
There were significant differences in treatment of myeloma based on types of prescription drug coverage. Due to increasing use of bortezomib following its approval by the U.S. Food and Drug Administration (FDA), parenteral chemotherapy use doubled from 24% to 48% from 2006 to 2011, and utility of active myeloma care increased from 88% to 91%. Medicare part D plan enrollees were 6% more likely to receive active myeloma care, and both Medicaid group and sponsored plan group beneficiaries were equally likely to receive active myeloma care compared to beneficiaries without prescription coverage. Medicaid enrollees were less likely to receive parenteral therapy.
Conclusion. Medicare beneficiaries with prescription drug coverage and multiple myeloma are more likely to receive myeloma therapy and have longer OS compared to those without prescription drug coverage.
Commentary
First-line therapy of multiple myeloma has evolved over the past 2 decades. Parenteral agents such as vincristine, adriamycin, dexamethasone, and cyclophosphamide and oral therapy with melphalan and prednisone were the mainstay of treatment in the past. In the past decade, the arrival of oral therapy using thalidomide or lenalidomide and parenteral therapy using bortezomib has increased OS in patients with myeloma. Most recently, a combination of lenalidomide, bortezomib, and dexamethasone has emerged as one of the frontline therapies of choice.1 Incorporation of bortezomib or an oral immunomodulatory drug is almost universal in first-line therapy.
Oral antineoplastic therapy is increasingly being approved by the FDA and being utilized in the community. During the period 2016-2018, more than half the new FDA-approved oncology drugs were in oral formulation.2 As such, access to these agents is crucial in cancer therapy. The cost of oral therapy in patients without prescription drug coverage is sometimes more than $10,000 per month, which represents a significant impediment to its adoption. Forty-three states and Washington, DC, have enacted drug parity laws that require patients to pay no more for an oral cancer treatment than they would for an infusion. However, currently there is no such federal law, and Medicare beneficiaries must participate either through part D, state Medicaid, or a sponsored program to obtain prescription drug coverage. Despite being enrolled in part D, many beneficiaries fall into the “doughnut hole” (the requirement of Part D beneficiaries with high prescription drug expenses to pay more once the total cost of their medicines reaches a certain threshold) for prescription drugs at the time of need. From 2019 onward, enrollees will see significant, yet sometimes still insufficient, coverage benefits due to ending of the doughnut hole.3 Only a very limited number of oral chemotherapy agents are covered through Medicare part B, and of those covered, only oral melphalan is used for myeloma.
The authors have acknowledged multiple limitations of their investigation, including possible unobserved clinical differences between beneficiaries. SEER-Medicare registry has limitations in obtaining individual level data and may not contain specific results of cytogenetics, laboratory risk markers, and response to therapy, which are important to determine overall outcome. A prospective evaluation may be more suitable to assess these variables independently or through a multivariate analysis in determining receipt of therapy on OS, although such a study is currently not feasible.
The indicator of active myeloma care was defined as 2 or more outpatient physician visits or receipt of parenteral chemotherapy. This definition is somewhat suboptimal, as often patients with myeloma are under surveillance and may not necessarily be receiving active treatment. Moreover, the exact prescription pattern of lenalidomide, the most active first-line oral therapy, could not be captured from this retrospective registry review. Therefore, definitive conclusions regarding use of lenalidomide and thalidomide and receipt of therapy in this population cannot be made.
A significant improvement in OS has been established using maintenance lenalidomide following high-dose chemotherapy and stem cell transplantation.4 Only 5% of this study population received stem cell transplantation. This may be due to a median age of 77 years at diagnosis in the group studied, higher than the 66 to 70 years previously published.5 Stem cell transplantation is now commonly being used even in the older population. The 3-year survival of 83% following stem cell transplantation in myeloma patients aged 75 to 84 years was nearly identical to that of the younger population.6 Since stem cell transplantation is feasible in older Medicare beneficiaries and maintenance lenalidomide for 2 years following transplant improves survival, the option of providing maintenance therapy with oral lenalidomide must be made available to Medicare beneficiaries. Due to a very limited use of transplantation in this study, the impact of oral lenalidomide maintenance in OS cannot be judged.
Of the patients reviewed in this study, 6% had a listed diagnosis of plasmacytoma. These individuals typically are treated with radiation therapy only. It is unclear if these patients also received any systemic myeloma therapy or if they ever progressed to myeloma. Availability of prescription drug coverage may not be relevant to this group. Also, the authors reported that part D participants were less likely to receive classic cytotoxic chemotherapy. This may be somewhat irrelevant in Medicare beneficiaries with a median age of 77 years for current practice, as frontline induction with old classic cytotoxic chemotherapy is less commonly used in this population.
Investigators have appropriately recognized a lack of ability to discern whether inferior survival in the group without prescription drug coverage was the result of not receiving therapy at all or inability to receive oral immunomodulatory drugs. There would have been little reason for not proceeding to parenteral therapy. As noted, 41% of beneficiaries without coverage at diagnosis subsequently obtained coverage but continued to have significantly worse survival. Cause of death, including whether related to myeloma, was not reported. The authors suggest that early separation of survival curves could therefore be reflective of suboptimal first-line therapy that lacked oral immunomodulatory drugs. During the study period 2006-2011, first-line use of lenalidomide was common.
Median survival of patients with myeloma in this study was only 27 months. According to the American Cancer Society, in 2018 median survival for stage I myeloma has not been reached, stage II myeloma is 83 months, and stage III myeloma is 43 months. A robust and dynamic landscape in myeloma therapy prevents a clear attribution to individual agents, whether oral or parenteral, in improving OS. Thus, 3-year RMST, while appropriate for 2006-2011, may not be relevant today.
Applications for Clinical Practice
The oncology community routinely encounters difficulty in initiating therapy using oral agents rapidly after diagnosis of myeloma. The retrospective data analyzed in the current study suggests that delay in initiating or unavailability of oral agents may adversely impact OS. The common approach of initiating parenteral therapy while awaiting approvals from payers or charity programs and subsequently adding oral therapy when available has not been studied in assessing OS. The oncology community should initiate plans to obtain prescription drug coverage through either Medicare part D, Medicaid, a sponsored plan, or financial assistance charity programs as soon as possible after diagnosis of myeloma. Moreover, continuation of these prescription drug plans should be strongly considered throughout the course of myeloma, as subsequent lines of treatment will quite likely involve other active and approved oral agents, such as pomalidomide, ixazomib, and panobinostat, besides other supportive therapy.
One of the mechanisms to obtain prescription drug coverage includes enrollment in state Medicaid programs for those who are eligible. Currently, 17 states have not yet adopted Medicaid expansion under the Affordable Care Act. Expansion of Medicaid in these states could increase availability of prescription drug benefits. In this study, 15.8% of Medicare and Medicaid dual enrollees with access to oral agents at low or no cost did not receive myeloma care, slightly higher than the 13.1% with no prescription drug coverage. Lower utilization in this population may be explained based on differences in comorbidities or socioeconomic conditions rather than availability of a prescription plan.
The incidence of myeloma is expected to be higher in Medicare beneficiaries, and according to one estimate, in 2030 and beyond nearly 75% of diagnosed myeloma patients will be aged 64 to 84 years, an increase from nearly 66% today.7 Changing demographics, increasing oral therapy options, and patient convenience demand attention to providing prescription drug coverage to all Medicare beneficiaries. This study lends support to that demand.
—Rakesh Gaur, MD, MPH, FACP, Cancer and Blood Center at Kansas Institute of Medicine, Lenexa, KS
Study Overview
Objective. To investigate the relationship between prescription drug coverage, receipt of active myeloma therapy, and overall survival (OS) among Medicare beneficiaries with multiple myeloma.
Design. Case-control and retrospective cohort archival data research.
Setting and participants. Authors examined SEER-Medicare registry and extracted patients with histologically confirmed multiple myeloma diagnosed in the period 2006 to 2011. Availability of complete Medicare part A/B claims from 1 year before diagnosis until December 2013 was required for analysis. Patients with Medicare advantage or managed care plans did not have claims data available and hence were excluded. Beneficiaries with a diagnosis of diffuse large B-cell lymphoma (DLBCL), who typically receive parenteral drugs for lymphoma therapy, were used as a control cohort.
Main outcome measures. Association between prescription drug coverage status and OS was the primary outcome measure of interest. Authors reported 3-year restricted survival time (RMST) ratios to compare OS among the beneficiaries with different prescription drug coverages. Receipt of active myeloma therapy among beneficiaries was also studied. Relative risk, adjusting for patient and disease-related characteristics, was reported to examine receipt of active myeloma therapy.
Results. Records of 9755 Medicare beneficiaries were evaluated. Of these, 1460 (15%) had no prescription coverage at diagnosis, 3283 (34%) had part D plan prescription benefits, 3607 (37%) had sponsored prescription coverage through an employer, federal employer, or veterans plan, and 1405 (14%) had a Medicaid prescription plan. Beneficiaries without coverage had fewer comorbidities, including anemia, neuropathy, or renal disease, than those with part D prescription coverage or Medicaid. Of those without any prescription drug coverage, 41% obtained prescription plan coverage after diagnosis of myeloma by the following January. Conversely, only 19% of patients with DLBCL and no coverage obtained a prescription plan.
Patients with myeloma were followed for 4.9 years and median survival was 2.3 years, with a 3-year OS rate of 43.1% (95% confidence interval [CI], 42.1%-44.1%). Relative to the group without coverage, survival was 16% longer in the Medicare part D group and sponsored plan group (RMST 1.16; 95% CI, 1.12-1.21). Medicaid/Medicare dual beneficiaries had worse OS in both myeloma and DLBCL consistent with poor performance status and unfavorable baseline comorbidities. However, among patients with myeloma, Medicaid/Medicare dual beneficiaries had better survival (RMST 1.08; 95% CI, 1.03-1.13) compared to the group without coverage. There was no difference in OS for those with or without prescription drug coverage in the DLBCL cohort.
There were significant differences in treatment of myeloma based on types of prescription drug coverage. Due to increasing use of bortezomib following its approval by the U.S. Food and Drug Administration (FDA), parenteral chemotherapy use doubled from 24% to 48% from 2006 to 2011, and utility of active myeloma care increased from 88% to 91%. Medicare part D plan enrollees were 6% more likely to receive active myeloma care, and both Medicaid group and sponsored plan group beneficiaries were equally likely to receive active myeloma care compared to beneficiaries without prescription coverage. Medicaid enrollees were less likely to receive parenteral therapy.
Conclusion. Medicare beneficiaries with prescription drug coverage and multiple myeloma are more likely to receive myeloma therapy and have longer OS compared to those without prescription drug coverage.
Commentary
First-line therapy of multiple myeloma has evolved over the past 2 decades. Parenteral agents such as vincristine, adriamycin, dexamethasone, and cyclophosphamide and oral therapy with melphalan and prednisone were the mainstay of treatment in the past. In the past decade, the arrival of oral therapy using thalidomide or lenalidomide and parenteral therapy using bortezomib has increased OS in patients with myeloma. Most recently, a combination of lenalidomide, bortezomib, and dexamethasone has emerged as one of the frontline therapies of choice.1 Incorporation of bortezomib or an oral immunomodulatory drug is almost universal in first-line therapy.
Oral antineoplastic therapy is increasingly being approved by the FDA and being utilized in the community. During the period 2016-2018, more than half the new FDA-approved oncology drugs were in oral formulation.2 As such, access to these agents is crucial in cancer therapy. The cost of oral therapy in patients without prescription drug coverage is sometimes more than $10,000 per month, which represents a significant impediment to its adoption. Forty-three states and Washington, DC, have enacted drug parity laws that require patients to pay no more for an oral cancer treatment than they would for an infusion. However, currently there is no such federal law, and Medicare beneficiaries must participate either through part D, state Medicaid, or a sponsored program to obtain prescription drug coverage. Despite being enrolled in part D, many beneficiaries fall into the “doughnut hole” (the requirement of Part D beneficiaries with high prescription drug expenses to pay more once the total cost of their medicines reaches a certain threshold) for prescription drugs at the time of need. From 2019 onward, enrollees will see significant, yet sometimes still insufficient, coverage benefits due to ending of the doughnut hole.3 Only a very limited number of oral chemotherapy agents are covered through Medicare part B, and of those covered, only oral melphalan is used for myeloma.
The authors have acknowledged multiple limitations of their investigation, including possible unobserved clinical differences between beneficiaries. SEER-Medicare registry has limitations in obtaining individual level data and may not contain specific results of cytogenetics, laboratory risk markers, and response to therapy, which are important to determine overall outcome. A prospective evaluation may be more suitable to assess these variables independently or through a multivariate analysis in determining receipt of therapy on OS, although such a study is currently not feasible.
The indicator of active myeloma care was defined as 2 or more outpatient physician visits or receipt of parenteral chemotherapy. This definition is somewhat suboptimal, as often patients with myeloma are under surveillance and may not necessarily be receiving active treatment. Moreover, the exact prescription pattern of lenalidomide, the most active first-line oral therapy, could not be captured from this retrospective registry review. Therefore, definitive conclusions regarding use of lenalidomide and thalidomide and receipt of therapy in this population cannot be made.
A significant improvement in OS has been established using maintenance lenalidomide following high-dose chemotherapy and stem cell transplantation.4 Only 5% of this study population received stem cell transplantation. This may be due to a median age of 77 years at diagnosis in the group studied, higher than the 66 to 70 years previously published.5 Stem cell transplantation is now commonly being used even in the older population. The 3-year survival of 83% following stem cell transplantation in myeloma patients aged 75 to 84 years was nearly identical to that of the younger population.6 Since stem cell transplantation is feasible in older Medicare beneficiaries and maintenance lenalidomide for 2 years following transplant improves survival, the option of providing maintenance therapy with oral lenalidomide must be made available to Medicare beneficiaries. Due to a very limited use of transplantation in this study, the impact of oral lenalidomide maintenance in OS cannot be judged.
Of the patients reviewed in this study, 6% had a listed diagnosis of plasmacytoma. These individuals typically are treated with radiation therapy only. It is unclear if these patients also received any systemic myeloma therapy or if they ever progressed to myeloma. Availability of prescription drug coverage may not be relevant to this group. Also, the authors reported that part D participants were less likely to receive classic cytotoxic chemotherapy. This may be somewhat irrelevant in Medicare beneficiaries with a median age of 77 years for current practice, as frontline induction with old classic cytotoxic chemotherapy is less commonly used in this population.
Investigators have appropriately recognized a lack of ability to discern whether inferior survival in the group without prescription drug coverage was the result of not receiving therapy at all or inability to receive oral immunomodulatory drugs. There would have been little reason for not proceeding to parenteral therapy. As noted, 41% of beneficiaries without coverage at diagnosis subsequently obtained coverage but continued to have significantly worse survival. Cause of death, including whether related to myeloma, was not reported. The authors suggest that early separation of survival curves could therefore be reflective of suboptimal first-line therapy that lacked oral immunomodulatory drugs. During the study period 2006-2011, first-line use of lenalidomide was common.
Median survival of patients with myeloma in this study was only 27 months. According to the American Cancer Society, in 2018 median survival for stage I myeloma has not been reached, stage II myeloma is 83 months, and stage III myeloma is 43 months. A robust and dynamic landscape in myeloma therapy prevents a clear attribution to individual agents, whether oral or parenteral, in improving OS. Thus, 3-year RMST, while appropriate for 2006-2011, may not be relevant today.
Applications for Clinical Practice
The oncology community routinely encounters difficulty in initiating therapy using oral agents rapidly after diagnosis of myeloma. The retrospective data analyzed in the current study suggests that delay in initiating or unavailability of oral agents may adversely impact OS. The common approach of initiating parenteral therapy while awaiting approvals from payers or charity programs and subsequently adding oral therapy when available has not been studied in assessing OS. The oncology community should initiate plans to obtain prescription drug coverage through either Medicare part D, Medicaid, a sponsored plan, or financial assistance charity programs as soon as possible after diagnosis of myeloma. Moreover, continuation of these prescription drug plans should be strongly considered throughout the course of myeloma, as subsequent lines of treatment will quite likely involve other active and approved oral agents, such as pomalidomide, ixazomib, and panobinostat, besides other supportive therapy.
One of the mechanisms to obtain prescription drug coverage includes enrollment in state Medicaid programs for those who are eligible. Currently, 17 states have not yet adopted Medicaid expansion under the Affordable Care Act. Expansion of Medicaid in these states could increase availability of prescription drug benefits. In this study, 15.8% of Medicare and Medicaid dual enrollees with access to oral agents at low or no cost did not receive myeloma care, slightly higher than the 13.1% with no prescription drug coverage. Lower utilization in this population may be explained based on differences in comorbidities or socioeconomic conditions rather than availability of a prescription plan.
The incidence of myeloma is expected to be higher in Medicare beneficiaries, and according to one estimate, in 2030 and beyond nearly 75% of diagnosed myeloma patients will be aged 64 to 84 years, an increase from nearly 66% today.7 Changing demographics, increasing oral therapy options, and patient convenience demand attention to providing prescription drug coverage to all Medicare beneficiaries. This study lends support to that demand.
—Rakesh Gaur, MD, MPH, FACP, Cancer and Blood Center at Kansas Institute of Medicine, Lenexa, KS
1. Durie BG, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomized, open-label, phase 3 trial. Lancet. 2017;389(10068):519-527.
2. U.S. Food and Drug Administration. Hematology/Oncology (Cancer) Approvals & Safety Notifications. www.fda.gov/drugs/informationondrugs/approveddrugs/ucm279174.htm. Accessed October 11, 2018.
3. Dusetzina SB, Keating NL. Mind the gap: Why closing the doughnut hole is insufficient for increasing Medicare beneficiary access to oral chemotherapy. J Clin Oncol. 2016;34:375-380.
4. McCarthy PL, Holstein SA, Petrucci MT, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017;35:3279-3289.
5. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33.
6. Dong N, McKiernan P, Samuel D, et al. Autologous stem cell transplantation in multiple myeloma patients over age 75 [abstract]. J Clin Oncol. 2018;36(suppl): 8025.
7. Rosenberg PS, Barker KA, Anderson WF. Future distribution of multiple myeloma in the United States by sex, age, and race/ethnicity. Blood. 2015;125:410–412.
1. Durie BG, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomized, open-label, phase 3 trial. Lancet. 2017;389(10068):519-527.
2. U.S. Food and Drug Administration. Hematology/Oncology (Cancer) Approvals & Safety Notifications. www.fda.gov/drugs/informationondrugs/approveddrugs/ucm279174.htm. Accessed October 11, 2018.
3. Dusetzina SB, Keating NL. Mind the gap: Why closing the doughnut hole is insufficient for increasing Medicare beneficiary access to oral chemotherapy. J Clin Oncol. 2016;34:375-380.
4. McCarthy PL, Holstein SA, Petrucci MT, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017;35:3279-3289.
5. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33.
6. Dong N, McKiernan P, Samuel D, et al. Autologous stem cell transplantation in multiple myeloma patients over age 75 [abstract]. J Clin Oncol. 2018;36(suppl): 8025.
7. Rosenberg PS, Barker KA, Anderson WF. Future distribution of multiple myeloma in the United States by sex, age, and race/ethnicity. Blood. 2015;125:410–412.
Combination of Ibrutinib and Rituximab Prolongs Progression-Free Survival in Waldenström Macroglobulinemia
Study Overview
Objective. To evaluate the efficacy of the combination of ibrutinib plus rituximab in patients with previously untreated or recurrent and rituximab-sensitive Waldenström macroglobulinemia.
Design. International, randomized phase 3 trial.
Setting and participants. Patients from 45 sites in 9 countries were enrolled after receiving a centrally confirmed diagnosis of Waldenström macroglobulinemia that required treatment according to current guidelines.1 Patients who were treatment-naive or had relapsed disease were eligible. Those with relapsed disease must have demonstrated response to rituximab in the past with a duration of response of at least 12 months. Patients who were rituximab resistant or those who received rituximab within the prior 12 months were excluded.
Intervention. Patients were randomized in a 1:1 fashion to receive oral ibrutinib 420 mg once daily or placebo. All patients received rituximab 375 mg/m2 at weeks 1 to 4 and 17 to 20. Treatment was continued until disease progression or intolerable adverse effects developed. Patients were stratified according to International Prognostic Scoring System for Waldenström Macroglobulinemia (IPSS) score, number of prior therapies, and performance status. Those who received placebo were permitted to crossover to receive ibrutinib at the time of progression.
Main outcome measures. The primary outcome of this study was progression-free survival (PFS). Secondary endpoints included time to next treatment, overall survival (OS), response rate, sustained hematologic improvement, quality of life, and safety. MYD88 and CXCR4 mutational status were assessed on pre-treatment bone marrow specimens.
Results. 150 patients were randomized to receive ibrutinib-rituximab (75 patients) or placebo-rituximab (75 patients). The median age was 69 years, and approximately one-third of patients were over the age of 75 years; 45% were treatment-naive. Those with relapsed disease had received a median of 2 prior treatments, and 85% of these received prior rituximab. Baseline characteristics were well balanced between the 2 groups. Mutation data was available for 136 patients enrolled, and MYD88 L265P and CXCR4 WHIM mutations were found in 85% and 36%, respectively. Rituximab therapy was completed in 93% of patients in the ibrutinib group and 71% in the placebo group.
After a median follow up of 26.5 months, the 30-month PFS was 82% in the ibrutinib group and 28% in the placebo group (median not reached vs. 20.3 months; hazard ratio 0.20, 95% confidence interval [CI] 0.11-0.38). This translated into an 80% reduction in the risk of progression or death. Overall, there was a low rate of histologic transformation to diffuse large B-cell lymphoma in the study group (2 patients in ibrutinib arm and none in placebo arm). In the treatment-naive subgroup, at 24 months the PFS rate was 84% in the ibrutinib arm compared with 59% in the placebo arm. In those with recurrent disease, the 30-month PFS was 80% in the ibrutinib arm compared with 22% in the placebo arm. Analysis across different MYD88 and CXCR4 genotypes showed consistent rates of higher PFS with ibrutinib-rituximab (Table). In addition, 30-month PFS was higher with ibrutinib regardless of IPSS score.
The 30-month OS was 94% with ibrutinib and 92% with placebo. There were 30 patients in the placebo arm that crossed over to receive ibrutinib. As assessed by the independent review committee, response rates were significantly higher with ibrutinib-rituximab (overall response rate, 92% vs. 47%). The major response rate (complete response, very good partial response, or partial response) was higher in the ibrutinib arm (72% vs. 32%). Mutation status did not affect the response rate or quality of response. Among those with at least a partial response, the median duration of response was not reached in the ibrutinib group, as compared with a median duration of response of 21.2 months in the placebo group. Serum IgM response was greater and more rapid with ibrutinib compared to placebo. Furthermore, transient increases in serum IgM levels, or “IgM flare,” was seen less frequently with the addition of ibrutinib (8% vs. 47%). No patient receiving ibrutinib required plasmapheresis. Hemoglobin response was seen more frequently with ibrutinib (73% vs. 41%).
Grade 3 or higher adverse events (AE) were seen in 60% of patients in each group. Hypertension (13% vs. 4%) and atrial fibrillation (12% vs. 1%) occurred more commonly in the ibrutinib group compared with placebo. Serious AEs were seen more frequently with ibrutinib compared to placebo (43% vs. 33%). Atrial fibrillation of any grade occurred in 15% of patients receiving ibrutinib; however, 27% of these patients had a history of atrial fibrillation prior to enrollment. Bleeding occurred more frequently with ibrutinib; however, the vast majority of these were grade 1 or grade 2. Major bleeding occurred in 3 patients in each arm. No fatal adverse events were noted in the ibrutinib group, while 3 patients in the placebo group experienced a fatal event. Discontinuation rates were similar in both arms (5% vs. 4%). Dose reduction of ibrutinib occurred in 13 patients.
Conclusion. The combination of ibrutinib and rituximab reduced the risk of disease progression by 80% compared with rituximab alone. This combination should be considered as a standard treatment option for patients with symptomatic Waldenström macroglobulinemia.
Commentary
Waldenström macroglobulinemia is a B-cell lymphoma characterized by infiltrating IgM producing clonal lymphoplasmacytic cells. Observation remains the preferred approach to asymptomatic patients; however, the presence of clinical symptoms including anemia, hyperviscosity, fatigue, or other constitutional symptoms should prompt initiation of therapy. Given the relative lack of large studies to define standard treatment strategies, rituximab monotherapy has frequently been used, with response rates of approximately 40% to 50%.2,3 Complete responses to single-agent rituximab have not been reported. Ibrutinib is an oral Bruton tyrosine kinase (BTK) inhibitor that has shown high response rates in the relapsed setting in previous studies. A study of single-agent ibrutinib in patients with relapsed disease showed overall and major response rates of 90% and 73%, respectively.4 The 2-year PFS was 69%. Additionally, such studies have suggested higher response rates in patients with mutated MYD88 genotype. This data led to the approval of ibrutinib for rituximab-refractory disease. In the treatment-naive setting, at least a minor response was seen in all patients (n = 30) in a small cohort treated with ibrutinib.5
In the reported trial, the combination of ibrutinib plus rituximab resulted in a more robust and durable response than single-agent rituximab, with significantly prolonged PFS. Of note, the response was similar for both treatment-naive and relapsed, rituximab-sensitive patients. Interestingly, a transient increase in serum IgM level was not seen in those treated with combination ibrutinib-rituximab. Improvements in PFS and response rates were independent of IPSS score. Previous studies have suggested that response to ibrutinib is related to MYD88 and CXCR4 mutational status. For example, in a phase 2 trial of ibrutinib in previously treated patients with symptomatic disease, major response rates for MYD88 L265P/CXCR WT, MYD88 L265P/CXCR4 WHIM, and MYD88 WT/CXCR4 WT groups were 91%, 62%, and 29%, respectively.4 In the current study, however, responses with ibrutinib-rituximab were seen across all genotypes at similar rates. Furthermore, PFS did not differ based on mutational status.
Similar rates of grade 3 or higher AEs were observed in each arm. Atrial fibrillation did occur in 15% of patients in the ibrutinib arm, but discontinuation rates were low. In addition, bleeding complications with ibrutinib have been increasingly recognized; however, in this cohort there did not seem to be an increased risk of major bleeding, with a vast majority of the bleeding events being grade 1 or grade 2.
Applications for Clinical Practice
The combination of ibrutinib plus rituximab represents a reasonable first-line treatment for patients with Waldenstrom macroglobulinemia. Importantly, mutational status does not appear to impact response rates and thus this combination can be considered irrespective of MYD88 status.
—Daniel Isaac, DO, MS
1. Kyle RA, Treon SP, Alexanian R, et al. Prognostic markers and criteria to initiate therapy in Waldenström’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenström’s Macroglobulinemia. Semin Oncol. 2003;30:116-120.
2. Dimopoulos MA, Zervas C, Zomas A, et al. Treatment of Waldenström’s macroglobulinemia with rituximab. J Clin Oncol. 2002;20:2327-2333.
3. Dimopoulos Ma, Alexanian R, Gika D, et al. Treatment of Waldenström’s macroglobulinemia with rituximab: prognostic factors for response and progression. Leuk Lymphoma. 2004;45:2057-2061.
4. Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated Waldenström’s macroglobulinemia. N Engl J Med. 2015;372:1430-1440.
5. Treon SP, Gustine J, Meid K, et al. Ibrutinib monotherapy in symptomatic, treatment-naïve patients with Waldenström macroglobulinemia. J Clin Oncol. 2018;36:2755-2761.
Study Overview
Objective. To evaluate the efficacy of the combination of ibrutinib plus rituximab in patients with previously untreated or recurrent and rituximab-sensitive Waldenström macroglobulinemia.
Design. International, randomized phase 3 trial.
Setting and participants. Patients from 45 sites in 9 countries were enrolled after receiving a centrally confirmed diagnosis of Waldenström macroglobulinemia that required treatment according to current guidelines.1 Patients who were treatment-naive or had relapsed disease were eligible. Those with relapsed disease must have demonstrated response to rituximab in the past with a duration of response of at least 12 months. Patients who were rituximab resistant or those who received rituximab within the prior 12 months were excluded.
Intervention. Patients were randomized in a 1:1 fashion to receive oral ibrutinib 420 mg once daily or placebo. All patients received rituximab 375 mg/m2 at weeks 1 to 4 and 17 to 20. Treatment was continued until disease progression or intolerable adverse effects developed. Patients were stratified according to International Prognostic Scoring System for Waldenström Macroglobulinemia (IPSS) score, number of prior therapies, and performance status. Those who received placebo were permitted to crossover to receive ibrutinib at the time of progression.
Main outcome measures. The primary outcome of this study was progression-free survival (PFS). Secondary endpoints included time to next treatment, overall survival (OS), response rate, sustained hematologic improvement, quality of life, and safety. MYD88 and CXCR4 mutational status were assessed on pre-treatment bone marrow specimens.
Results. 150 patients were randomized to receive ibrutinib-rituximab (75 patients) or placebo-rituximab (75 patients). The median age was 69 years, and approximately one-third of patients were over the age of 75 years; 45% were treatment-naive. Those with relapsed disease had received a median of 2 prior treatments, and 85% of these received prior rituximab. Baseline characteristics were well balanced between the 2 groups. Mutation data was available for 136 patients enrolled, and MYD88 L265P and CXCR4 WHIM mutations were found in 85% and 36%, respectively. Rituximab therapy was completed in 93% of patients in the ibrutinib group and 71% in the placebo group.
After a median follow up of 26.5 months, the 30-month PFS was 82% in the ibrutinib group and 28% in the placebo group (median not reached vs. 20.3 months; hazard ratio 0.20, 95% confidence interval [CI] 0.11-0.38). This translated into an 80% reduction in the risk of progression or death. Overall, there was a low rate of histologic transformation to diffuse large B-cell lymphoma in the study group (2 patients in ibrutinib arm and none in placebo arm). In the treatment-naive subgroup, at 24 months the PFS rate was 84% in the ibrutinib arm compared with 59% in the placebo arm. In those with recurrent disease, the 30-month PFS was 80% in the ibrutinib arm compared with 22% in the placebo arm. Analysis across different MYD88 and CXCR4 genotypes showed consistent rates of higher PFS with ibrutinib-rituximab (Table). In addition, 30-month PFS was higher with ibrutinib regardless of IPSS score.
The 30-month OS was 94% with ibrutinib and 92% with placebo. There were 30 patients in the placebo arm that crossed over to receive ibrutinib. As assessed by the independent review committee, response rates were significantly higher with ibrutinib-rituximab (overall response rate, 92% vs. 47%). The major response rate (complete response, very good partial response, or partial response) was higher in the ibrutinib arm (72% vs. 32%). Mutation status did not affect the response rate or quality of response. Among those with at least a partial response, the median duration of response was not reached in the ibrutinib group, as compared with a median duration of response of 21.2 months in the placebo group. Serum IgM response was greater and more rapid with ibrutinib compared to placebo. Furthermore, transient increases in serum IgM levels, or “IgM flare,” was seen less frequently with the addition of ibrutinib (8% vs. 47%). No patient receiving ibrutinib required plasmapheresis. Hemoglobin response was seen more frequently with ibrutinib (73% vs. 41%).
Grade 3 or higher adverse events (AE) were seen in 60% of patients in each group. Hypertension (13% vs. 4%) and atrial fibrillation (12% vs. 1%) occurred more commonly in the ibrutinib group compared with placebo. Serious AEs were seen more frequently with ibrutinib compared to placebo (43% vs. 33%). Atrial fibrillation of any grade occurred in 15% of patients receiving ibrutinib; however, 27% of these patients had a history of atrial fibrillation prior to enrollment. Bleeding occurred more frequently with ibrutinib; however, the vast majority of these were grade 1 or grade 2. Major bleeding occurred in 3 patients in each arm. No fatal adverse events were noted in the ibrutinib group, while 3 patients in the placebo group experienced a fatal event. Discontinuation rates were similar in both arms (5% vs. 4%). Dose reduction of ibrutinib occurred in 13 patients.
Conclusion. The combination of ibrutinib and rituximab reduced the risk of disease progression by 80% compared with rituximab alone. This combination should be considered as a standard treatment option for patients with symptomatic Waldenström macroglobulinemia.
Commentary
Waldenström macroglobulinemia is a B-cell lymphoma characterized by infiltrating IgM producing clonal lymphoplasmacytic cells. Observation remains the preferred approach to asymptomatic patients; however, the presence of clinical symptoms including anemia, hyperviscosity, fatigue, or other constitutional symptoms should prompt initiation of therapy. Given the relative lack of large studies to define standard treatment strategies, rituximab monotherapy has frequently been used, with response rates of approximately 40% to 50%.2,3 Complete responses to single-agent rituximab have not been reported. Ibrutinib is an oral Bruton tyrosine kinase (BTK) inhibitor that has shown high response rates in the relapsed setting in previous studies. A study of single-agent ibrutinib in patients with relapsed disease showed overall and major response rates of 90% and 73%, respectively.4 The 2-year PFS was 69%. Additionally, such studies have suggested higher response rates in patients with mutated MYD88 genotype. This data led to the approval of ibrutinib for rituximab-refractory disease. In the treatment-naive setting, at least a minor response was seen in all patients (n = 30) in a small cohort treated with ibrutinib.5
In the reported trial, the combination of ibrutinib plus rituximab resulted in a more robust and durable response than single-agent rituximab, with significantly prolonged PFS. Of note, the response was similar for both treatment-naive and relapsed, rituximab-sensitive patients. Interestingly, a transient increase in serum IgM level was not seen in those treated with combination ibrutinib-rituximab. Improvements in PFS and response rates were independent of IPSS score. Previous studies have suggested that response to ibrutinib is related to MYD88 and CXCR4 mutational status. For example, in a phase 2 trial of ibrutinib in previously treated patients with symptomatic disease, major response rates for MYD88 L265P/CXCR WT, MYD88 L265P/CXCR4 WHIM, and MYD88 WT/CXCR4 WT groups were 91%, 62%, and 29%, respectively.4 In the current study, however, responses with ibrutinib-rituximab were seen across all genotypes at similar rates. Furthermore, PFS did not differ based on mutational status.
Similar rates of grade 3 or higher AEs were observed in each arm. Atrial fibrillation did occur in 15% of patients in the ibrutinib arm, but discontinuation rates were low. In addition, bleeding complications with ibrutinib have been increasingly recognized; however, in this cohort there did not seem to be an increased risk of major bleeding, with a vast majority of the bleeding events being grade 1 or grade 2.
Applications for Clinical Practice
The combination of ibrutinib plus rituximab represents a reasonable first-line treatment for patients with Waldenstrom macroglobulinemia. Importantly, mutational status does not appear to impact response rates and thus this combination can be considered irrespective of MYD88 status.
—Daniel Isaac, DO, MS
Study Overview
Objective. To evaluate the efficacy of the combination of ibrutinib plus rituximab in patients with previously untreated or recurrent and rituximab-sensitive Waldenström macroglobulinemia.
Design. International, randomized phase 3 trial.
Setting and participants. Patients from 45 sites in 9 countries were enrolled after receiving a centrally confirmed diagnosis of Waldenström macroglobulinemia that required treatment according to current guidelines.1 Patients who were treatment-naive or had relapsed disease were eligible. Those with relapsed disease must have demonstrated response to rituximab in the past with a duration of response of at least 12 months. Patients who were rituximab resistant or those who received rituximab within the prior 12 months were excluded.
Intervention. Patients were randomized in a 1:1 fashion to receive oral ibrutinib 420 mg once daily or placebo. All patients received rituximab 375 mg/m2 at weeks 1 to 4 and 17 to 20. Treatment was continued until disease progression or intolerable adverse effects developed. Patients were stratified according to International Prognostic Scoring System for Waldenström Macroglobulinemia (IPSS) score, number of prior therapies, and performance status. Those who received placebo were permitted to crossover to receive ibrutinib at the time of progression.
Main outcome measures. The primary outcome of this study was progression-free survival (PFS). Secondary endpoints included time to next treatment, overall survival (OS), response rate, sustained hematologic improvement, quality of life, and safety. MYD88 and CXCR4 mutational status were assessed on pre-treatment bone marrow specimens.
Results. 150 patients were randomized to receive ibrutinib-rituximab (75 patients) or placebo-rituximab (75 patients). The median age was 69 years, and approximately one-third of patients were over the age of 75 years; 45% were treatment-naive. Those with relapsed disease had received a median of 2 prior treatments, and 85% of these received prior rituximab. Baseline characteristics were well balanced between the 2 groups. Mutation data was available for 136 patients enrolled, and MYD88 L265P and CXCR4 WHIM mutations were found in 85% and 36%, respectively. Rituximab therapy was completed in 93% of patients in the ibrutinib group and 71% in the placebo group.
After a median follow up of 26.5 months, the 30-month PFS was 82% in the ibrutinib group and 28% in the placebo group (median not reached vs. 20.3 months; hazard ratio 0.20, 95% confidence interval [CI] 0.11-0.38). This translated into an 80% reduction in the risk of progression or death. Overall, there was a low rate of histologic transformation to diffuse large B-cell lymphoma in the study group (2 patients in ibrutinib arm and none in placebo arm). In the treatment-naive subgroup, at 24 months the PFS rate was 84% in the ibrutinib arm compared with 59% in the placebo arm. In those with recurrent disease, the 30-month PFS was 80% in the ibrutinib arm compared with 22% in the placebo arm. Analysis across different MYD88 and CXCR4 genotypes showed consistent rates of higher PFS with ibrutinib-rituximab (Table). In addition, 30-month PFS was higher with ibrutinib regardless of IPSS score.
The 30-month OS was 94% with ibrutinib and 92% with placebo. There were 30 patients in the placebo arm that crossed over to receive ibrutinib. As assessed by the independent review committee, response rates were significantly higher with ibrutinib-rituximab (overall response rate, 92% vs. 47%). The major response rate (complete response, very good partial response, or partial response) was higher in the ibrutinib arm (72% vs. 32%). Mutation status did not affect the response rate or quality of response. Among those with at least a partial response, the median duration of response was not reached in the ibrutinib group, as compared with a median duration of response of 21.2 months in the placebo group. Serum IgM response was greater and more rapid with ibrutinib compared to placebo. Furthermore, transient increases in serum IgM levels, or “IgM flare,” was seen less frequently with the addition of ibrutinib (8% vs. 47%). No patient receiving ibrutinib required plasmapheresis. Hemoglobin response was seen more frequently with ibrutinib (73% vs. 41%).
Grade 3 or higher adverse events (AE) were seen in 60% of patients in each group. Hypertension (13% vs. 4%) and atrial fibrillation (12% vs. 1%) occurred more commonly in the ibrutinib group compared with placebo. Serious AEs were seen more frequently with ibrutinib compared to placebo (43% vs. 33%). Atrial fibrillation of any grade occurred in 15% of patients receiving ibrutinib; however, 27% of these patients had a history of atrial fibrillation prior to enrollment. Bleeding occurred more frequently with ibrutinib; however, the vast majority of these were grade 1 or grade 2. Major bleeding occurred in 3 patients in each arm. No fatal adverse events were noted in the ibrutinib group, while 3 patients in the placebo group experienced a fatal event. Discontinuation rates were similar in both arms (5% vs. 4%). Dose reduction of ibrutinib occurred in 13 patients.
Conclusion. The combination of ibrutinib and rituximab reduced the risk of disease progression by 80% compared with rituximab alone. This combination should be considered as a standard treatment option for patients with symptomatic Waldenström macroglobulinemia.
Commentary
Waldenström macroglobulinemia is a B-cell lymphoma characterized by infiltrating IgM producing clonal lymphoplasmacytic cells. Observation remains the preferred approach to asymptomatic patients; however, the presence of clinical symptoms including anemia, hyperviscosity, fatigue, or other constitutional symptoms should prompt initiation of therapy. Given the relative lack of large studies to define standard treatment strategies, rituximab monotherapy has frequently been used, with response rates of approximately 40% to 50%.2,3 Complete responses to single-agent rituximab have not been reported. Ibrutinib is an oral Bruton tyrosine kinase (BTK) inhibitor that has shown high response rates in the relapsed setting in previous studies. A study of single-agent ibrutinib in patients with relapsed disease showed overall and major response rates of 90% and 73%, respectively.4 The 2-year PFS was 69%. Additionally, such studies have suggested higher response rates in patients with mutated MYD88 genotype. This data led to the approval of ibrutinib for rituximab-refractory disease. In the treatment-naive setting, at least a minor response was seen in all patients (n = 30) in a small cohort treated with ibrutinib.5
In the reported trial, the combination of ibrutinib plus rituximab resulted in a more robust and durable response than single-agent rituximab, with significantly prolonged PFS. Of note, the response was similar for both treatment-naive and relapsed, rituximab-sensitive patients. Interestingly, a transient increase in serum IgM level was not seen in those treated with combination ibrutinib-rituximab. Improvements in PFS and response rates were independent of IPSS score. Previous studies have suggested that response to ibrutinib is related to MYD88 and CXCR4 mutational status. For example, in a phase 2 trial of ibrutinib in previously treated patients with symptomatic disease, major response rates for MYD88 L265P/CXCR WT, MYD88 L265P/CXCR4 WHIM, and MYD88 WT/CXCR4 WT groups were 91%, 62%, and 29%, respectively.4 In the current study, however, responses with ibrutinib-rituximab were seen across all genotypes at similar rates. Furthermore, PFS did not differ based on mutational status.
Similar rates of grade 3 or higher AEs were observed in each arm. Atrial fibrillation did occur in 15% of patients in the ibrutinib arm, but discontinuation rates were low. In addition, bleeding complications with ibrutinib have been increasingly recognized; however, in this cohort there did not seem to be an increased risk of major bleeding, with a vast majority of the bleeding events being grade 1 or grade 2.
Applications for Clinical Practice
The combination of ibrutinib plus rituximab represents a reasonable first-line treatment for patients with Waldenstrom macroglobulinemia. Importantly, mutational status does not appear to impact response rates and thus this combination can be considered irrespective of MYD88 status.
—Daniel Isaac, DO, MS
1. Kyle RA, Treon SP, Alexanian R, et al. Prognostic markers and criteria to initiate therapy in Waldenström’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenström’s Macroglobulinemia. Semin Oncol. 2003;30:116-120.
2. Dimopoulos MA, Zervas C, Zomas A, et al. Treatment of Waldenström’s macroglobulinemia with rituximab. J Clin Oncol. 2002;20:2327-2333.
3. Dimopoulos Ma, Alexanian R, Gika D, et al. Treatment of Waldenström’s macroglobulinemia with rituximab: prognostic factors for response and progression. Leuk Lymphoma. 2004;45:2057-2061.
4. Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated Waldenström’s macroglobulinemia. N Engl J Med. 2015;372:1430-1440.
5. Treon SP, Gustine J, Meid K, et al. Ibrutinib monotherapy in symptomatic, treatment-naïve patients with Waldenström macroglobulinemia. J Clin Oncol. 2018;36:2755-2761.
1. Kyle RA, Treon SP, Alexanian R, et al. Prognostic markers and criteria to initiate therapy in Waldenström’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenström’s Macroglobulinemia. Semin Oncol. 2003;30:116-120.
2. Dimopoulos MA, Zervas C, Zomas A, et al. Treatment of Waldenström’s macroglobulinemia with rituximab. J Clin Oncol. 2002;20:2327-2333.
3. Dimopoulos Ma, Alexanian R, Gika D, et al. Treatment of Waldenström’s macroglobulinemia with rituximab: prognostic factors for response and progression. Leuk Lymphoma. 2004;45:2057-2061.
4. Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated Waldenström’s macroglobulinemia. N Engl J Med. 2015;372:1430-1440.
5. Treon SP, Gustine J, Meid K, et al. Ibrutinib monotherapy in symptomatic, treatment-naïve patients with Waldenström macroglobulinemia. J Clin Oncol. 2018;36:2755-2761.
Sickle cell disease gene therapy seen advancing
BETHESDA, MD. – Experimental gene therapies for sickle cell disease and thalassemia appear to be advancing, with BCL11A among the most promising targets in this field, researchers said at Sickle Cell in Focus, a conference held by the National Institutes of Health.
Several highly anticipated presentations on the topic are expected for December meeting of the American Society of Hematology.
Alexis A. Thompson, MD, of Northwestern University, Chicago, reviewed highlights from a study in which the majority of patients given a gene therapy for transfusion dependent beta-thalassemia didn’t need subsequent blood transfusions. The New England Journal of Medicine in April published the results of this work done with Bluebird Bio’s LentiGlobin gene therapy (N Engl J Med. 2018; 378:1479-93).
Of the 22 patients in this trial, 15 have become transfusion independent, Dr. Thompson said in her presentation. Those patients that did not have this positive outcome still appear to have been helped by the gene therapy, she said. They had a median of 60% reduction in their transfusion volumes and nearly 60% in their number of transfusions.
“Whether it was transfusion independence or reduction in their transfusion volume or number, the vast majority of individuals in this first large-scale study had clinical benefit” from the therapy, said Dr. Thompson, who was the lead author of the study.
Dr. Thompson, the current president of the American Society of Hematology (ASH), said she’s looking forward to presentations on some of the most advanced gene therapies for sickle cell disease and thalassemia at the group’s annual meeting in December. The ASH presentations include those of John F. Tisdale, MD, who will report the latest data on LentiGlobin gene therapy in sickle cell disease, and Punam Malik, MD, of Cincinnati Children’s Hospital, who has developed a gamma globin lentivirus vector. There also will be a first readout on a particularly novel approach taken by researchers at Boston Children’s Hospital, led by David Williams, MD.
The development of CRISPR-Cas9 “has really opened up the field” of gene therapy, aiding researchers at Boston Children’s in their efforts to develop a treatment to maintain fetal hemoglobin production, Daniel E. Bauer, MD, PhD, of Boston Children’s Hospital, said during his presentation at the NIH conference.
Dr. Bauer provided an update on the BCL11A research that seeks to block what amounts to a genetic “off switch” for production of fetal hemoglobin. It’s long been known that erythrocytes of newborns with sickle cell disease are protected from sickling by high levels of fetal hemoglobin. Clinical manifestations of sickle cell disease then emerge in the first year of life as fetal hemoglobin levels decline.
“A main goal in hematology has been to understand how is it that these alternative fetal hemoglobin genes get silenced and how can we turn them back on,” said Dr. Bauer, a staff physician in pediatric hematology/oncology.
The gene BCL11A also plays key roles in the development of the central nervous system, B lymphatic lymphocyte maturation, and hematopoietic stem cell self-renewal. That led the researchers to hone in on targeting sequences around the BCL11A gene that act as erythroid enhancers, intending to limit potential complications by creating a very specific therapy for sickle cell disease.
In a related clinical trial, using lentiviral gene therapy rather than gene editing, researchers at Boston Children’s began an open-label, nonrandomized, single-center pilot study that involves a single infusion of autologous bone marrow derived CD34+ HSC cells transduced by a vector containing a short-hairpin segment of RNA targeting the gene BCL11A.
The study has a maximum accrual of seven evaluable patients, according to the NIH’s clinical trials website. The protocol is similar to bone marrow transplant, in that native blood stem cells are eliminated by myeloablative conditioning therapy. In this gene therapy, the patient’s own blood stem cells are then infused after the new genetic material has been added to counter the normal BCL11A off switch.
Swee Lay Thein, MBBS, an organizer of the NIH’s sickle cell conference, said in an interview that the “gene therapy side is really looking very optimistic.”
Dr. Thein, a senior investigator for sickle-cell genetics at the National Heart, Lung, and Blood Institute, earlier in her career discovered segments of DNA, including the BCL11A gene. She said that gaining greater understanding about genomic variation might someday aid in determining which people need more intense intervention for their sickle cell disease.
“You would be able to predict who will have more severe disease; we could monitor them more closely and perhaps even advocate for gene therapy or bone marrow transplant before complications have occurred rather than waiting for them to occur,” Dr. Thein said.
She referred to this as her “dream” for care of people with sickle cell disease. “This is still far off in the horizon.”
The NHLBI in September 2018 launched its Cure Sickle Cell Initiative. The agency estimates that it spends about $100 million on sickle cell disease research each year. The inherited blood disorder affects about 100,000 people in the United States and 20 million individuals worldwide. In sickle cell disease, a single genetic mutation causes red blood cells to form abnormal, sickle shapes that can clog the blood vessels and deprive cells of oxygen.
Dr. Thompson reported research funding and consulting agreements with Biomarin, Bluebird Bio, Celgene, Novartis, and Shire. Dr. Bauer reported patents related to BCL11A enhancer editing, consulting agreements with Merck and Pfizer, and research support from Bioverativ.
BETHESDA, MD. – Experimental gene therapies for sickle cell disease and thalassemia appear to be advancing, with BCL11A among the most promising targets in this field, researchers said at Sickle Cell in Focus, a conference held by the National Institutes of Health.
Several highly anticipated presentations on the topic are expected for December meeting of the American Society of Hematology.
Alexis A. Thompson, MD, of Northwestern University, Chicago, reviewed highlights from a study in which the majority of patients given a gene therapy for transfusion dependent beta-thalassemia didn’t need subsequent blood transfusions. The New England Journal of Medicine in April published the results of this work done with Bluebird Bio’s LentiGlobin gene therapy (N Engl J Med. 2018; 378:1479-93).
Of the 22 patients in this trial, 15 have become transfusion independent, Dr. Thompson said in her presentation. Those patients that did not have this positive outcome still appear to have been helped by the gene therapy, she said. They had a median of 60% reduction in their transfusion volumes and nearly 60% in their number of transfusions.
“Whether it was transfusion independence or reduction in their transfusion volume or number, the vast majority of individuals in this first large-scale study had clinical benefit” from the therapy, said Dr. Thompson, who was the lead author of the study.
Dr. Thompson, the current president of the American Society of Hematology (ASH), said she’s looking forward to presentations on some of the most advanced gene therapies for sickle cell disease and thalassemia at the group’s annual meeting in December. The ASH presentations include those of John F. Tisdale, MD, who will report the latest data on LentiGlobin gene therapy in sickle cell disease, and Punam Malik, MD, of Cincinnati Children’s Hospital, who has developed a gamma globin lentivirus vector. There also will be a first readout on a particularly novel approach taken by researchers at Boston Children’s Hospital, led by David Williams, MD.
The development of CRISPR-Cas9 “has really opened up the field” of gene therapy, aiding researchers at Boston Children’s in their efforts to develop a treatment to maintain fetal hemoglobin production, Daniel E. Bauer, MD, PhD, of Boston Children’s Hospital, said during his presentation at the NIH conference.
Dr. Bauer provided an update on the BCL11A research that seeks to block what amounts to a genetic “off switch” for production of fetal hemoglobin. It’s long been known that erythrocytes of newborns with sickle cell disease are protected from sickling by high levels of fetal hemoglobin. Clinical manifestations of sickle cell disease then emerge in the first year of life as fetal hemoglobin levels decline.
“A main goal in hematology has been to understand how is it that these alternative fetal hemoglobin genes get silenced and how can we turn them back on,” said Dr. Bauer, a staff physician in pediatric hematology/oncology.
The gene BCL11A also plays key roles in the development of the central nervous system, B lymphatic lymphocyte maturation, and hematopoietic stem cell self-renewal. That led the researchers to hone in on targeting sequences around the BCL11A gene that act as erythroid enhancers, intending to limit potential complications by creating a very specific therapy for sickle cell disease.
In a related clinical trial, using lentiviral gene therapy rather than gene editing, researchers at Boston Children’s began an open-label, nonrandomized, single-center pilot study that involves a single infusion of autologous bone marrow derived CD34+ HSC cells transduced by a vector containing a short-hairpin segment of RNA targeting the gene BCL11A.
The study has a maximum accrual of seven evaluable patients, according to the NIH’s clinical trials website. The protocol is similar to bone marrow transplant, in that native blood stem cells are eliminated by myeloablative conditioning therapy. In this gene therapy, the patient’s own blood stem cells are then infused after the new genetic material has been added to counter the normal BCL11A off switch.
Swee Lay Thein, MBBS, an organizer of the NIH’s sickle cell conference, said in an interview that the “gene therapy side is really looking very optimistic.”
Dr. Thein, a senior investigator for sickle-cell genetics at the National Heart, Lung, and Blood Institute, earlier in her career discovered segments of DNA, including the BCL11A gene. She said that gaining greater understanding about genomic variation might someday aid in determining which people need more intense intervention for their sickle cell disease.
“You would be able to predict who will have more severe disease; we could monitor them more closely and perhaps even advocate for gene therapy or bone marrow transplant before complications have occurred rather than waiting for them to occur,” Dr. Thein said.
She referred to this as her “dream” for care of people with sickle cell disease. “This is still far off in the horizon.”
The NHLBI in September 2018 launched its Cure Sickle Cell Initiative. The agency estimates that it spends about $100 million on sickle cell disease research each year. The inherited blood disorder affects about 100,000 people in the United States and 20 million individuals worldwide. In sickle cell disease, a single genetic mutation causes red blood cells to form abnormal, sickle shapes that can clog the blood vessels and deprive cells of oxygen.
Dr. Thompson reported research funding and consulting agreements with Biomarin, Bluebird Bio, Celgene, Novartis, and Shire. Dr. Bauer reported patents related to BCL11A enhancer editing, consulting agreements with Merck and Pfizer, and research support from Bioverativ.
BETHESDA, MD. – Experimental gene therapies for sickle cell disease and thalassemia appear to be advancing, with BCL11A among the most promising targets in this field, researchers said at Sickle Cell in Focus, a conference held by the National Institutes of Health.
Several highly anticipated presentations on the topic are expected for December meeting of the American Society of Hematology.
Alexis A. Thompson, MD, of Northwestern University, Chicago, reviewed highlights from a study in which the majority of patients given a gene therapy for transfusion dependent beta-thalassemia didn’t need subsequent blood transfusions. The New England Journal of Medicine in April published the results of this work done with Bluebird Bio’s LentiGlobin gene therapy (N Engl J Med. 2018; 378:1479-93).
Of the 22 patients in this trial, 15 have become transfusion independent, Dr. Thompson said in her presentation. Those patients that did not have this positive outcome still appear to have been helped by the gene therapy, she said. They had a median of 60% reduction in their transfusion volumes and nearly 60% in their number of transfusions.
“Whether it was transfusion independence or reduction in their transfusion volume or number, the vast majority of individuals in this first large-scale study had clinical benefit” from the therapy, said Dr. Thompson, who was the lead author of the study.
Dr. Thompson, the current president of the American Society of Hematology (ASH), said she’s looking forward to presentations on some of the most advanced gene therapies for sickle cell disease and thalassemia at the group’s annual meeting in December. The ASH presentations include those of John F. Tisdale, MD, who will report the latest data on LentiGlobin gene therapy in sickle cell disease, and Punam Malik, MD, of Cincinnati Children’s Hospital, who has developed a gamma globin lentivirus vector. There also will be a first readout on a particularly novel approach taken by researchers at Boston Children’s Hospital, led by David Williams, MD.
The development of CRISPR-Cas9 “has really opened up the field” of gene therapy, aiding researchers at Boston Children’s in their efforts to develop a treatment to maintain fetal hemoglobin production, Daniel E. Bauer, MD, PhD, of Boston Children’s Hospital, said during his presentation at the NIH conference.
Dr. Bauer provided an update on the BCL11A research that seeks to block what amounts to a genetic “off switch” for production of fetal hemoglobin. It’s long been known that erythrocytes of newborns with sickle cell disease are protected from sickling by high levels of fetal hemoglobin. Clinical manifestations of sickle cell disease then emerge in the first year of life as fetal hemoglobin levels decline.
“A main goal in hematology has been to understand how is it that these alternative fetal hemoglobin genes get silenced and how can we turn them back on,” said Dr. Bauer, a staff physician in pediatric hematology/oncology.
The gene BCL11A also plays key roles in the development of the central nervous system, B lymphatic lymphocyte maturation, and hematopoietic stem cell self-renewal. That led the researchers to hone in on targeting sequences around the BCL11A gene that act as erythroid enhancers, intending to limit potential complications by creating a very specific therapy for sickle cell disease.
In a related clinical trial, using lentiviral gene therapy rather than gene editing, researchers at Boston Children’s began an open-label, nonrandomized, single-center pilot study that involves a single infusion of autologous bone marrow derived CD34+ HSC cells transduced by a vector containing a short-hairpin segment of RNA targeting the gene BCL11A.
The study has a maximum accrual of seven evaluable patients, according to the NIH’s clinical trials website. The protocol is similar to bone marrow transplant, in that native blood stem cells are eliminated by myeloablative conditioning therapy. In this gene therapy, the patient’s own blood stem cells are then infused after the new genetic material has been added to counter the normal BCL11A off switch.
Swee Lay Thein, MBBS, an organizer of the NIH’s sickle cell conference, said in an interview that the “gene therapy side is really looking very optimistic.”
Dr. Thein, a senior investigator for sickle-cell genetics at the National Heart, Lung, and Blood Institute, earlier in her career discovered segments of DNA, including the BCL11A gene. She said that gaining greater understanding about genomic variation might someday aid in determining which people need more intense intervention for their sickle cell disease.
“You would be able to predict who will have more severe disease; we could monitor them more closely and perhaps even advocate for gene therapy or bone marrow transplant before complications have occurred rather than waiting for them to occur,” Dr. Thein said.
She referred to this as her “dream” for care of people with sickle cell disease. “This is still far off in the horizon.”
The NHLBI in September 2018 launched its Cure Sickle Cell Initiative. The agency estimates that it spends about $100 million on sickle cell disease research each year. The inherited blood disorder affects about 100,000 people in the United States and 20 million individuals worldwide. In sickle cell disease, a single genetic mutation causes red blood cells to form abnormal, sickle shapes that can clog the blood vessels and deprive cells of oxygen.
Dr. Thompson reported research funding and consulting agreements with Biomarin, Bluebird Bio, Celgene, Novartis, and Shire. Dr. Bauer reported patents related to BCL11A enhancer editing, consulting agreements with Merck and Pfizer, and research support from Bioverativ.
REPORTING FROM SICKLE CELL IN FOCUS
Collaboration is key to bridging the AYA cancer care divide
Survival gains among adolescents and young adults (AYAs) with cancer continue to lag behind outcomes for children and older adult patients. It’s a trend that spans decades, but clinicians and researchers are finally getting serious about trying to understand the underlying causes and are re-examining prevailing practices in an effort to address the discrepancies.
“This is a very heterogeneous group of disorders,” Rabi Hanna, MD, a pediatric hematologist and oncologist at Cleveland Clinic Children’s Hospital, Ohio, said in an interview. He’s specifically referring to the cancers that affect AYAs, who are broadly defined as patients aged 15 through 39 years. “A few cancers, such as [acute lymphoblastic leukemia], are more common in children, and others, such as breast cancer, are more common in adults. The biology may be different in the adolescent and young adult patients, which may lead to different outcomes.”
In addition, the psychosocial needs in this age group differ vastly from those in other groups. “Many of these patients are in college or have just started their families, so we have to pay more attention to [issues related to] financial toxicity and fertility, for example,” said Dr Hanna, who is the director of pediatric bone marrow transplantation at the clinic. (The term “financial toxicity” describes the cumulative negative impact of the high cost of care, lost work time, and delays in reaching educational and career goals on patients with cancer and their families.)
Another factor that likely contributes to the outcome disparities between AYAs and other populations with cancer is the relative lack of clinical trial involvement among AYAs.
A recent series of articles published in the journal Blood addressed these and other issues, among them, whether AYAs with acute lymphoblastic leukemia (ALL)1 or aggressive B-cell non-Hodgkin lymphomas (NHLs) 2 should be treated as children or adults; treatment strategies for those with acute myeloid leukemias (AMLs); 3 management of Hodgkin lymphoma;4 and psychosocial challenges and health-related quality of life (QoL) in AYAs with hematologic malignancies.5
In the introduction to the series, Jorge Cortes, MD, an assistant editor on the journal, wrote that hematologic malignancies in AYAs “represent a unique challenge because of their special biological features and distinctive therapeutic requirements, as well as the unique medical, social, and psychological characteristics of this patient population.”6
He noted, however, that “not much has been done to explore unique molecular and biological features of AYA hematologic malignancies. The discussion on the management of AYAs often centers on whether these patients should be treated in a pediatric setting or an adult setting, or with regimens designed for children or for adults,” noted Dr Cortes, professor and chair of the chronic myeloid leukemia section in the department of leukemia at the University of Texas MD Anderson Cancer Center, Houston.
Therapeutic options: pediatric or adult protocols?
In their article on ALL in AYAs, Nicolas Boissel, MD, and André Baruchel, MD, note that the use of “fully pediatric protocols” in patients aged 15 through 20 years is supported by findings from numerous studies. In young adults, evidence increasingly supports “pediatric-inspired or even fully pediatric approaches” because they have been shown to significantly improve outcomes, with long-term survival rates nearing 70%.1 Patients in these age groups require specific programs that factor in access to care and to trials, an increased risk of acute toxicities, and treatment adherence, which can be particularly problematic in AYAs, they concluded.
However, Kristen O’Dwyer, MD, and colleagues, argue in an article on AML treatment in AYAs that neither the pediatric nor adult approaches are ideally suited for AYAs because of the “distinguishing characteristics of AYAs with AML.” Rather, they conclude that AYA-specific approaches merit consideration.3
Similarly, Kieron Dunleavy, MD, and Thomas G Gross, MD, note in an article on managing aggressive B-cell NHLs in AYAs that there is a “remarkable divide” in the treatment of patients younger than 18 years with lymphoma compared with their young adult counterparts, and that it underscores the need for collaboration in developing consensus regarding treatment of AYAs.2
Clinical setting: pediatric or adult?
Consideration is also being given to the clinical setting in which AYA patients receive their treatment. Lori Muffly, MD, MS, and colleagues have reported that survival was superior for AYA patients with ALL who were treated in pediatric cancer settings,7 and other researchers have reported similar findings.
However, those improved outcomes in the pediatric setting might be offset by a higher use of resources and therefore higher costs, based on recent findings in a Canadian study by Paul C Nathan, MD, and colleagues.8 Among 1,356 patients aged 15-17 years who were diagnosed with cancer between 1996 and 2010, the authors found that the cost of care was higher when treatment took place in a pediatric setting compared with in an adult institution, and that it was driven in part by higher hospitalization rates and longer hospital stays. These findings were true across different diagnoses, including leukemias, lymphomas, sarcomas, and germ cell tumors, but only during the initial treatment phase.
In an accompanying editorial, Helen M Parsons, PhD, and her co-authors wrote that adolescents who receive treatment in the pediatric setting “tended to seek more [emergency department (ED)] care immediately before diagnosis and during the initial treatment phase; these adolescents also used more home care services during initial treatment and survivorship.9 They pointed out that the findings of higher inpatient days in the pediatric setting was not surprising given that induction therapies for pediatric ALL tend to be more complex and intensive than therapies commonly used in adults with ALL, and that pediatric cancer hospitals tend to have a wider array of services, including psychosocial and family support services.
“What is less clear is why individuals seen in pediatric settings have higher rates of ED care directly before diagnosis and during the initial treatment phase,” they wrote, adding that further investigation was needed on this topic to better understand those trends. “The finding that adolescents treated in pediatric institutions had higher resource use across diagnostic groups demonstrates that resource utilization may be driven just as much by care setting as diagnosis.” 9
The authors of the editorial emphasized that because of the differences in health care delivery and payment structures between the United States and Canada, where the Nathan study was done, it was important that similar studies are done in the United States to confirm these findings.
Disease and developmental biology
As Dr Hanna noted, biological differences and changes over time suggest that different age groups need varying approaches to treatment and that they may have different outcomes with the same treatments.
For example, the biology of AML is known to change with age, Dr O'Dwyer and her colleagues noted,3 citing a recent European study of 5,564 patients with de novo AML that showed that the frequency of favorable cytogenetics was low in infants (13.7%), increased in children (25%) and young adults (44%), and decreased again in middle age and older patients.10
“Most unfavorable cytogenetic abnormalities are rare across all age groups, though complex cytogenetics are relatively more frequent in infants, decrease in frequency in AYAs, and then increase in frequency beyond AYA,” Dr O'Dwyer and her colleagues wrote.3 It was also becoming more apparent that age influences the presence of AML-related molecular abnormalities, and recognition of age-related differences in disease biology “will provide the best opportunity to improve the clinical outcomes that have been static for decades.”
Dr Boissel and Dr Baruchel also noted in their report that light was finally being shed on the “black hole” of understanding ALL biology in AYAs, and research has shown that there is a continuum between childhood and adult ALL.1 They concluded that “risk stratification based on recent biology findings and sequential [minimum residual disease] evaluations should now be implemented, as well as new therapeutic options including immunotherapy and targeted therapies, at best within the setting of integrated pediatric and AYA protocols.”
Psychosocial factors
“Cancer is a non-normative event for AYAs. It is extremely disruptive to them physically, psychologically, and vocationally ... and this poses significant challenges,” John Salsman, PhD, director of clinical research in AYA oncology at Wake Forest University, Winston-Salem, NC, said in an interview.
These patients have 5-year survival rates that haven’t improved in tandem with those in pediatric and adult populations over the last 3 decades, and in addition to the financial toxicity and strain, they also have higher rates of depression and anxiety, including fear of recurrence, he added. “Quality of life is incredibly important, and these things need to be addressed because of the developmental changes AYAs are navigating; there are issues of positive body image, family and career decisions ... these are challenging for anyone, and when you throw a cancer diagnosis into the mix they become disproportionately so.”
In a 2014 study, Dr Salsman and his colleagues found that AYAs with cancer had poorer physical and emotional quality of life when compared with matched controls, but better social quality of life.11 The latter finding was surprising and highlights the importance of the social dimension in the lives of AYAs. “Patient after patient will say ‘I found out who my real friends are,’ ” he said. “There’s this refinement and deepening of the social network among some posttreatment survivors.”
Dr Salsman and his colleagues are using those findings to develop interventions that can maximize self-care in posttreatment survivorship – a time when AYAs may feel they have a new lease on life and may be more motivated to adhere to recommendations and take care of themselves. For example, a randomized controlled pilot study that incorporates social media apps and other technologies to build on the positive social components of their lives in promoting physical activity interventions is underway.
Another intervention targets emotional well-being through the use of web-based tools to increase positive affect. A proof-of-concept study showed that the approach was feasible and well received, and a larger-scale randomized controlled trial is being planned, he said.
Dr Salsman also praised the PRISM (Promoting Resilience in Stress Management) tool developed by researchers at Seattle Children’s Hospital. It was created to help AYAs with cancer and other illnesses learn coping skills to manage stress after their diagnosis and to boost quality of life beyond treatment. A digital app has also been developed to be used in conjunction with the program.
Trial enrollment
In his editorial introducing the Blood series on AYAs and cancer, Dr Cortes noted a paucity of clinical trials specifically designed for this population. “At the time of this writing, I could identify four therapeutic trials registered at www.clinicaltrials.gov that appeared to be somewhat specifically designed for AYAs (some included children also),” he wrote, describing AYA enrollment in clinical trials in cancer as “suboptimal at best.”6
Dr Salsman said these dismal enrolment numbers could in part be related to treatment setting. Data suggest that most AYAs with cancer are treated in community-based practices rather than comprehensive cancer centers where the bulk of research is being done, he explained.
Dr Hanna agreed that more research involving AYAs was needed as is a better understanding of why enrollment is so much lower in this population. He pointed out that in 2017 the American Society of Clinical Oncology and Friends of Cancer Research released a statement recommending that pediatric patients be considered for enrollment in later-phase trials for cancer types that span both adults and children.12 The organizations said that individuals aged 12 years and older should routinely be included in such trials because their drug metabolism is similar to adults, and inclusion of younger patients may also be appropriate if they are part of the population affected by the disease, depending on specific disease biology, action of the drug, and available safety information.
Officials at the Food and Drug Administration are considering that possibility, Dr Hanna said.
Dr Salsman added there has been an increase in recent years in the attention paid to disparities in survival improvements and trial involvement among AYAs with cancer, compared with other age groups. For example, about 5 years ago, the National Clinical Trials Network formed a working group that developed a number of specific objectives for incorporating more AYAs into cancer trials and finding better ways to study this population;13 the Institute of Medicine held a forum on the care of AYAs with cancer;14 and the National Cancer Institute held a state-of-the-science meeting that focused on identifying strategic priorities for AYA oncology,15 he noted.
Dr Hanna added that “scientific groups such as Southwest Oncology Group (SWOG) and Children’s Oncology Group (COG) also have AYA committees now. One of the success stories of working together between SWOG and COG was the intergroup study C10403 for patients with ALL. And now there are efforts for an intergroup AYA-AML task force to include representatives from each of the cooperative groups that historically co-ordinated myeloid disease clinical trials – COG, SWOG, Alliance, and ECOG-ACRIN,” he said.
In fact, all of the National Clinical Trials Network groups have some initiative in place to address AYA concerns, said Dr Salsman, who chairs the ECOG-ACRIN AYA oncology subcommittee.
Despite these efforts, and many others, long-term survival improvements among AYAs with cancer still fall short, compared with those of other age groups.16
Next steps
Among the recommendations from authors in the AYA series in Blood is a call for assessing AYA-specific therapy in future clinical trials, as well as improved collaboration between adult and pediatric teams and the involvement of multidisciplinary teams in care for this population.
Many centers are already working on models for collaborative care, Dr Salsman said, citing the Fort Worth AYA Oncology Coalition led by medical director Karen Albritton, MD, as an example of a program that has been successful in helping clinical and supportive caregivers and their AYA patients “have a shared vision” as they work to maximize improvements in outcomes.
Patients are also taking the lead in demanding better care and attention to their psychosocial needs, Dr Hanna said. In the case of the community-powered advocacy organization Critical Mass, members have succeeded in getting lawmakers to introduce a bill in the US House of Representatives that would allow college students to defer loan payments while undergoing cancer treatment.
1. Boissel N, Baruchel A. Acute lymphoblastic leukemia in adolescent and young adults: treat as adults or as children? Blood. 2018;132:351-361.
2. Dunleavy K, Gross TG. Management of aggressive B-cell NHLs in the AYA population: an adult vs pediatric perspective. Blood. 2018;132:369-375.
3. O’Dwyer K, Freyer DR, Horan JT. Treatment strategies for adolescent and young adult patients with acute myeloid leukemia. Blood. 2018;132:362-368.
4. Flerlage JE, Metzger ML, Bhakta N. The management of Hodgkin lymphoma in adolescents and young adults: burden of disease or burden of choice? Blood. 2018;132:376-384.
5. Husson O, Huijgens PC, van der Graaf WTA. Psychosocial challenges and health-related quality of life of adolescents and young adults with hematologic malignancies. Blood. 2018;132:385-392.
6. Cortes J. Introduction to a review series on adolescent and young adult malignant hematology. Blood. 2018;132:345-346.
7. Muffly L, Alvarez E, Lichtensztajn D, Abrahão R, Gomez SL, Keegan T. Patterns of care and outcomes in adolescent and young adult acute lymphoblastic leukemia: a population-based study. Blood Adv. 2018;2(8):895-903.
8. Nathan PC, Bremner KE, Liu N, et al. Resource utilization and costs in adolescents treated for cancer in pediatric vs adult institutions. J Natl Cancer Inst. July 19, 2018. [Epub ahead of print.]
9. Parsons HM, Muffly L, Alvarez EM, Keegan THM. Does treatment setting matter? Evaluating resource utilization for adolescents treated in pediatric vs adult cancer institutions. https://academic.oup.com/jnci/advance-article/doi/10.1093/jnci/djy123/5056313?searchresult=1. Published July 19, 2018. Last accessed October 12, 2018.
10. Creutzig U, Zimmermann M, Reinhardt D, et al. Changes in cytogenetics and molecular genetics in acute myeloid leukemia from childhood to adult age groups. Cancer. 2016;122(24):3821-3830.
11. Salsman JM, Garcia SF, Yanez B, et al. Physical, emotional, and social health differences between posttreatment young adults with cancer and matched healthy controls. Cancer. 2014;120(15):2247-2254.
12. Kim ES, Bruinooge SS, Roberts S, et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research joint research statement. J Clin Oncol. 2017;35(33):3737-3744.
13. Freyer DR, Seibel NL. The clinical trials gap for adolescents and young adults with cancer: recent progress and conceptual framework for continued research. Curr Pediatr Rep. Published online February 18, 2015. DOI 10.1007/s40124-015-0075-y.
14. Nass SJ, Beaupin LK, Demark-Wahnefried W, et al. Identifying and addressing the needs of adolescents and young adults with cancer: summary of an Institute of Medicine workshop. Oncologist. 2015;20(2):186-195.
15. Wilder Smith A, Seibel NL, Lewis DR, et al. Next steps for adolescent and young adult oncology workshop: An update on progress and recommendations for the future. Cancer. 2016;122(7):988-999.
16. Keegan THM, Ries LAG, Barr RD, et al. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer. 2016;122(7):1009-1016.
Survival gains among adolescents and young adults (AYAs) with cancer continue to lag behind outcomes for children and older adult patients. It’s a trend that spans decades, but clinicians and researchers are finally getting serious about trying to understand the underlying causes and are re-examining prevailing practices in an effort to address the discrepancies.
“This is a very heterogeneous group of disorders,” Rabi Hanna, MD, a pediatric hematologist and oncologist at Cleveland Clinic Children’s Hospital, Ohio, said in an interview. He’s specifically referring to the cancers that affect AYAs, who are broadly defined as patients aged 15 through 39 years. “A few cancers, such as [acute lymphoblastic leukemia], are more common in children, and others, such as breast cancer, are more common in adults. The biology may be different in the adolescent and young adult patients, which may lead to different outcomes.”
In addition, the psychosocial needs in this age group differ vastly from those in other groups. “Many of these patients are in college or have just started their families, so we have to pay more attention to [issues related to] financial toxicity and fertility, for example,” said Dr Hanna, who is the director of pediatric bone marrow transplantation at the clinic. (The term “financial toxicity” describes the cumulative negative impact of the high cost of care, lost work time, and delays in reaching educational and career goals on patients with cancer and their families.)
Another factor that likely contributes to the outcome disparities between AYAs and other populations with cancer is the relative lack of clinical trial involvement among AYAs.
A recent series of articles published in the journal Blood addressed these and other issues, among them, whether AYAs with acute lymphoblastic leukemia (ALL)1 or aggressive B-cell non-Hodgkin lymphomas (NHLs) 2 should be treated as children or adults; treatment strategies for those with acute myeloid leukemias (AMLs); 3 management of Hodgkin lymphoma;4 and psychosocial challenges and health-related quality of life (QoL) in AYAs with hematologic malignancies.5
In the introduction to the series, Jorge Cortes, MD, an assistant editor on the journal, wrote that hematologic malignancies in AYAs “represent a unique challenge because of their special biological features and distinctive therapeutic requirements, as well as the unique medical, social, and psychological characteristics of this patient population.”6
He noted, however, that “not much has been done to explore unique molecular and biological features of AYA hematologic malignancies. The discussion on the management of AYAs often centers on whether these patients should be treated in a pediatric setting or an adult setting, or with regimens designed for children or for adults,” noted Dr Cortes, professor and chair of the chronic myeloid leukemia section in the department of leukemia at the University of Texas MD Anderson Cancer Center, Houston.
Therapeutic options: pediatric or adult protocols?
In their article on ALL in AYAs, Nicolas Boissel, MD, and André Baruchel, MD, note that the use of “fully pediatric protocols” in patients aged 15 through 20 years is supported by findings from numerous studies. In young adults, evidence increasingly supports “pediatric-inspired or even fully pediatric approaches” because they have been shown to significantly improve outcomes, with long-term survival rates nearing 70%.1 Patients in these age groups require specific programs that factor in access to care and to trials, an increased risk of acute toxicities, and treatment adherence, which can be particularly problematic in AYAs, they concluded.
However, Kristen O’Dwyer, MD, and colleagues, argue in an article on AML treatment in AYAs that neither the pediatric nor adult approaches are ideally suited for AYAs because of the “distinguishing characteristics of AYAs with AML.” Rather, they conclude that AYA-specific approaches merit consideration.3
Similarly, Kieron Dunleavy, MD, and Thomas G Gross, MD, note in an article on managing aggressive B-cell NHLs in AYAs that there is a “remarkable divide” in the treatment of patients younger than 18 years with lymphoma compared with their young adult counterparts, and that it underscores the need for collaboration in developing consensus regarding treatment of AYAs.2
Clinical setting: pediatric or adult?
Consideration is also being given to the clinical setting in which AYA patients receive their treatment. Lori Muffly, MD, MS, and colleagues have reported that survival was superior for AYA patients with ALL who were treated in pediatric cancer settings,7 and other researchers have reported similar findings.
However, those improved outcomes in the pediatric setting might be offset by a higher use of resources and therefore higher costs, based on recent findings in a Canadian study by Paul C Nathan, MD, and colleagues.8 Among 1,356 patients aged 15-17 years who were diagnosed with cancer between 1996 and 2010, the authors found that the cost of care was higher when treatment took place in a pediatric setting compared with in an adult institution, and that it was driven in part by higher hospitalization rates and longer hospital stays. These findings were true across different diagnoses, including leukemias, lymphomas, sarcomas, and germ cell tumors, but only during the initial treatment phase.
In an accompanying editorial, Helen M Parsons, PhD, and her co-authors wrote that adolescents who receive treatment in the pediatric setting “tended to seek more [emergency department (ED)] care immediately before diagnosis and during the initial treatment phase; these adolescents also used more home care services during initial treatment and survivorship.9 They pointed out that the findings of higher inpatient days in the pediatric setting was not surprising given that induction therapies for pediatric ALL tend to be more complex and intensive than therapies commonly used in adults with ALL, and that pediatric cancer hospitals tend to have a wider array of services, including psychosocial and family support services.
“What is less clear is why individuals seen in pediatric settings have higher rates of ED care directly before diagnosis and during the initial treatment phase,” they wrote, adding that further investigation was needed on this topic to better understand those trends. “The finding that adolescents treated in pediatric institutions had higher resource use across diagnostic groups demonstrates that resource utilization may be driven just as much by care setting as diagnosis.” 9
The authors of the editorial emphasized that because of the differences in health care delivery and payment structures between the United States and Canada, where the Nathan study was done, it was important that similar studies are done in the United States to confirm these findings.
Disease and developmental biology
As Dr Hanna noted, biological differences and changes over time suggest that different age groups need varying approaches to treatment and that they may have different outcomes with the same treatments.
For example, the biology of AML is known to change with age, Dr O'Dwyer and her colleagues noted,3 citing a recent European study of 5,564 patients with de novo AML that showed that the frequency of favorable cytogenetics was low in infants (13.7%), increased in children (25%) and young adults (44%), and decreased again in middle age and older patients.10
“Most unfavorable cytogenetic abnormalities are rare across all age groups, though complex cytogenetics are relatively more frequent in infants, decrease in frequency in AYAs, and then increase in frequency beyond AYA,” Dr O'Dwyer and her colleagues wrote.3 It was also becoming more apparent that age influences the presence of AML-related molecular abnormalities, and recognition of age-related differences in disease biology “will provide the best opportunity to improve the clinical outcomes that have been static for decades.”
Dr Boissel and Dr Baruchel also noted in their report that light was finally being shed on the “black hole” of understanding ALL biology in AYAs, and research has shown that there is a continuum between childhood and adult ALL.1 They concluded that “risk stratification based on recent biology findings and sequential [minimum residual disease] evaluations should now be implemented, as well as new therapeutic options including immunotherapy and targeted therapies, at best within the setting of integrated pediatric and AYA protocols.”
Psychosocial factors
“Cancer is a non-normative event for AYAs. It is extremely disruptive to them physically, psychologically, and vocationally ... and this poses significant challenges,” John Salsman, PhD, director of clinical research in AYA oncology at Wake Forest University, Winston-Salem, NC, said in an interview.
These patients have 5-year survival rates that haven’t improved in tandem with those in pediatric and adult populations over the last 3 decades, and in addition to the financial toxicity and strain, they also have higher rates of depression and anxiety, including fear of recurrence, he added. “Quality of life is incredibly important, and these things need to be addressed because of the developmental changes AYAs are navigating; there are issues of positive body image, family and career decisions ... these are challenging for anyone, and when you throw a cancer diagnosis into the mix they become disproportionately so.”
In a 2014 study, Dr Salsman and his colleagues found that AYAs with cancer had poorer physical and emotional quality of life when compared with matched controls, but better social quality of life.11 The latter finding was surprising and highlights the importance of the social dimension in the lives of AYAs. “Patient after patient will say ‘I found out who my real friends are,’ ” he said. “There’s this refinement and deepening of the social network among some posttreatment survivors.”
Dr Salsman and his colleagues are using those findings to develop interventions that can maximize self-care in posttreatment survivorship – a time when AYAs may feel they have a new lease on life and may be more motivated to adhere to recommendations and take care of themselves. For example, a randomized controlled pilot study that incorporates social media apps and other technologies to build on the positive social components of their lives in promoting physical activity interventions is underway.
Another intervention targets emotional well-being through the use of web-based tools to increase positive affect. A proof-of-concept study showed that the approach was feasible and well received, and a larger-scale randomized controlled trial is being planned, he said.
Dr Salsman also praised the PRISM (Promoting Resilience in Stress Management) tool developed by researchers at Seattle Children’s Hospital. It was created to help AYAs with cancer and other illnesses learn coping skills to manage stress after their diagnosis and to boost quality of life beyond treatment. A digital app has also been developed to be used in conjunction with the program.
Trial enrollment
In his editorial introducing the Blood series on AYAs and cancer, Dr Cortes noted a paucity of clinical trials specifically designed for this population. “At the time of this writing, I could identify four therapeutic trials registered at www.clinicaltrials.gov that appeared to be somewhat specifically designed for AYAs (some included children also),” he wrote, describing AYA enrollment in clinical trials in cancer as “suboptimal at best.”6
Dr Salsman said these dismal enrolment numbers could in part be related to treatment setting. Data suggest that most AYAs with cancer are treated in community-based practices rather than comprehensive cancer centers where the bulk of research is being done, he explained.
Dr Hanna agreed that more research involving AYAs was needed as is a better understanding of why enrollment is so much lower in this population. He pointed out that in 2017 the American Society of Clinical Oncology and Friends of Cancer Research released a statement recommending that pediatric patients be considered for enrollment in later-phase trials for cancer types that span both adults and children.12 The organizations said that individuals aged 12 years and older should routinely be included in such trials because their drug metabolism is similar to adults, and inclusion of younger patients may also be appropriate if they are part of the population affected by the disease, depending on specific disease biology, action of the drug, and available safety information.
Officials at the Food and Drug Administration are considering that possibility, Dr Hanna said.
Dr Salsman added there has been an increase in recent years in the attention paid to disparities in survival improvements and trial involvement among AYAs with cancer, compared with other age groups. For example, about 5 years ago, the National Clinical Trials Network formed a working group that developed a number of specific objectives for incorporating more AYAs into cancer trials and finding better ways to study this population;13 the Institute of Medicine held a forum on the care of AYAs with cancer;14 and the National Cancer Institute held a state-of-the-science meeting that focused on identifying strategic priorities for AYA oncology,15 he noted.
Dr Hanna added that “scientific groups such as Southwest Oncology Group (SWOG) and Children’s Oncology Group (COG) also have AYA committees now. One of the success stories of working together between SWOG and COG was the intergroup study C10403 for patients with ALL. And now there are efforts for an intergroup AYA-AML task force to include representatives from each of the cooperative groups that historically co-ordinated myeloid disease clinical trials – COG, SWOG, Alliance, and ECOG-ACRIN,” he said.
In fact, all of the National Clinical Trials Network groups have some initiative in place to address AYA concerns, said Dr Salsman, who chairs the ECOG-ACRIN AYA oncology subcommittee.
Despite these efforts, and many others, long-term survival improvements among AYAs with cancer still fall short, compared with those of other age groups.16
Next steps
Among the recommendations from authors in the AYA series in Blood is a call for assessing AYA-specific therapy in future clinical trials, as well as improved collaboration between adult and pediatric teams and the involvement of multidisciplinary teams in care for this population.
Many centers are already working on models for collaborative care, Dr Salsman said, citing the Fort Worth AYA Oncology Coalition led by medical director Karen Albritton, MD, as an example of a program that has been successful in helping clinical and supportive caregivers and their AYA patients “have a shared vision” as they work to maximize improvements in outcomes.
Patients are also taking the lead in demanding better care and attention to their psychosocial needs, Dr Hanna said. In the case of the community-powered advocacy organization Critical Mass, members have succeeded in getting lawmakers to introduce a bill in the US House of Representatives that would allow college students to defer loan payments while undergoing cancer treatment.
Survival gains among adolescents and young adults (AYAs) with cancer continue to lag behind outcomes for children and older adult patients. It’s a trend that spans decades, but clinicians and researchers are finally getting serious about trying to understand the underlying causes and are re-examining prevailing practices in an effort to address the discrepancies.
“This is a very heterogeneous group of disorders,” Rabi Hanna, MD, a pediatric hematologist and oncologist at Cleveland Clinic Children’s Hospital, Ohio, said in an interview. He’s specifically referring to the cancers that affect AYAs, who are broadly defined as patients aged 15 through 39 years. “A few cancers, such as [acute lymphoblastic leukemia], are more common in children, and others, such as breast cancer, are more common in adults. The biology may be different in the adolescent and young adult patients, which may lead to different outcomes.”
In addition, the psychosocial needs in this age group differ vastly from those in other groups. “Many of these patients are in college or have just started their families, so we have to pay more attention to [issues related to] financial toxicity and fertility, for example,” said Dr Hanna, who is the director of pediatric bone marrow transplantation at the clinic. (The term “financial toxicity” describes the cumulative negative impact of the high cost of care, lost work time, and delays in reaching educational and career goals on patients with cancer and their families.)
Another factor that likely contributes to the outcome disparities between AYAs and other populations with cancer is the relative lack of clinical trial involvement among AYAs.
A recent series of articles published in the journal Blood addressed these and other issues, among them, whether AYAs with acute lymphoblastic leukemia (ALL)1 or aggressive B-cell non-Hodgkin lymphomas (NHLs) 2 should be treated as children or adults; treatment strategies for those with acute myeloid leukemias (AMLs); 3 management of Hodgkin lymphoma;4 and psychosocial challenges and health-related quality of life (QoL) in AYAs with hematologic malignancies.5
In the introduction to the series, Jorge Cortes, MD, an assistant editor on the journal, wrote that hematologic malignancies in AYAs “represent a unique challenge because of their special biological features and distinctive therapeutic requirements, as well as the unique medical, social, and psychological characteristics of this patient population.”6
He noted, however, that “not much has been done to explore unique molecular and biological features of AYA hematologic malignancies. The discussion on the management of AYAs often centers on whether these patients should be treated in a pediatric setting or an adult setting, or with regimens designed for children or for adults,” noted Dr Cortes, professor and chair of the chronic myeloid leukemia section in the department of leukemia at the University of Texas MD Anderson Cancer Center, Houston.
Therapeutic options: pediatric or adult protocols?
In their article on ALL in AYAs, Nicolas Boissel, MD, and André Baruchel, MD, note that the use of “fully pediatric protocols” in patients aged 15 through 20 years is supported by findings from numerous studies. In young adults, evidence increasingly supports “pediatric-inspired or even fully pediatric approaches” because they have been shown to significantly improve outcomes, with long-term survival rates nearing 70%.1 Patients in these age groups require specific programs that factor in access to care and to trials, an increased risk of acute toxicities, and treatment adherence, which can be particularly problematic in AYAs, they concluded.
However, Kristen O’Dwyer, MD, and colleagues, argue in an article on AML treatment in AYAs that neither the pediatric nor adult approaches are ideally suited for AYAs because of the “distinguishing characteristics of AYAs with AML.” Rather, they conclude that AYA-specific approaches merit consideration.3
Similarly, Kieron Dunleavy, MD, and Thomas G Gross, MD, note in an article on managing aggressive B-cell NHLs in AYAs that there is a “remarkable divide” in the treatment of patients younger than 18 years with lymphoma compared with their young adult counterparts, and that it underscores the need for collaboration in developing consensus regarding treatment of AYAs.2
Clinical setting: pediatric or adult?
Consideration is also being given to the clinical setting in which AYA patients receive their treatment. Lori Muffly, MD, MS, and colleagues have reported that survival was superior for AYA patients with ALL who were treated in pediatric cancer settings,7 and other researchers have reported similar findings.
However, those improved outcomes in the pediatric setting might be offset by a higher use of resources and therefore higher costs, based on recent findings in a Canadian study by Paul C Nathan, MD, and colleagues.8 Among 1,356 patients aged 15-17 years who were diagnosed with cancer between 1996 and 2010, the authors found that the cost of care was higher when treatment took place in a pediatric setting compared with in an adult institution, and that it was driven in part by higher hospitalization rates and longer hospital stays. These findings were true across different diagnoses, including leukemias, lymphomas, sarcomas, and germ cell tumors, but only during the initial treatment phase.
In an accompanying editorial, Helen M Parsons, PhD, and her co-authors wrote that adolescents who receive treatment in the pediatric setting “tended to seek more [emergency department (ED)] care immediately before diagnosis and during the initial treatment phase; these adolescents also used more home care services during initial treatment and survivorship.9 They pointed out that the findings of higher inpatient days in the pediatric setting was not surprising given that induction therapies for pediatric ALL tend to be more complex and intensive than therapies commonly used in adults with ALL, and that pediatric cancer hospitals tend to have a wider array of services, including psychosocial and family support services.
“What is less clear is why individuals seen in pediatric settings have higher rates of ED care directly before diagnosis and during the initial treatment phase,” they wrote, adding that further investigation was needed on this topic to better understand those trends. “The finding that adolescents treated in pediatric institutions had higher resource use across diagnostic groups demonstrates that resource utilization may be driven just as much by care setting as diagnosis.” 9
The authors of the editorial emphasized that because of the differences in health care delivery and payment structures between the United States and Canada, where the Nathan study was done, it was important that similar studies are done in the United States to confirm these findings.
Disease and developmental biology
As Dr Hanna noted, biological differences and changes over time suggest that different age groups need varying approaches to treatment and that they may have different outcomes with the same treatments.
For example, the biology of AML is known to change with age, Dr O'Dwyer and her colleagues noted,3 citing a recent European study of 5,564 patients with de novo AML that showed that the frequency of favorable cytogenetics was low in infants (13.7%), increased in children (25%) and young adults (44%), and decreased again in middle age and older patients.10
“Most unfavorable cytogenetic abnormalities are rare across all age groups, though complex cytogenetics are relatively more frequent in infants, decrease in frequency in AYAs, and then increase in frequency beyond AYA,” Dr O'Dwyer and her colleagues wrote.3 It was also becoming more apparent that age influences the presence of AML-related molecular abnormalities, and recognition of age-related differences in disease biology “will provide the best opportunity to improve the clinical outcomes that have been static for decades.”
Dr Boissel and Dr Baruchel also noted in their report that light was finally being shed on the “black hole” of understanding ALL biology in AYAs, and research has shown that there is a continuum between childhood and adult ALL.1 They concluded that “risk stratification based on recent biology findings and sequential [minimum residual disease] evaluations should now be implemented, as well as new therapeutic options including immunotherapy and targeted therapies, at best within the setting of integrated pediatric and AYA protocols.”
Psychosocial factors
“Cancer is a non-normative event for AYAs. It is extremely disruptive to them physically, psychologically, and vocationally ... and this poses significant challenges,” John Salsman, PhD, director of clinical research in AYA oncology at Wake Forest University, Winston-Salem, NC, said in an interview.
These patients have 5-year survival rates that haven’t improved in tandem with those in pediatric and adult populations over the last 3 decades, and in addition to the financial toxicity and strain, they also have higher rates of depression and anxiety, including fear of recurrence, he added. “Quality of life is incredibly important, and these things need to be addressed because of the developmental changes AYAs are navigating; there are issues of positive body image, family and career decisions ... these are challenging for anyone, and when you throw a cancer diagnosis into the mix they become disproportionately so.”
In a 2014 study, Dr Salsman and his colleagues found that AYAs with cancer had poorer physical and emotional quality of life when compared with matched controls, but better social quality of life.11 The latter finding was surprising and highlights the importance of the social dimension in the lives of AYAs. “Patient after patient will say ‘I found out who my real friends are,’ ” he said. “There’s this refinement and deepening of the social network among some posttreatment survivors.”
Dr Salsman and his colleagues are using those findings to develop interventions that can maximize self-care in posttreatment survivorship – a time when AYAs may feel they have a new lease on life and may be more motivated to adhere to recommendations and take care of themselves. For example, a randomized controlled pilot study that incorporates social media apps and other technologies to build on the positive social components of their lives in promoting physical activity interventions is underway.
Another intervention targets emotional well-being through the use of web-based tools to increase positive affect. A proof-of-concept study showed that the approach was feasible and well received, and a larger-scale randomized controlled trial is being planned, he said.
Dr Salsman also praised the PRISM (Promoting Resilience in Stress Management) tool developed by researchers at Seattle Children’s Hospital. It was created to help AYAs with cancer and other illnesses learn coping skills to manage stress after their diagnosis and to boost quality of life beyond treatment. A digital app has also been developed to be used in conjunction with the program.
Trial enrollment
In his editorial introducing the Blood series on AYAs and cancer, Dr Cortes noted a paucity of clinical trials specifically designed for this population. “At the time of this writing, I could identify four therapeutic trials registered at www.clinicaltrials.gov that appeared to be somewhat specifically designed for AYAs (some included children also),” he wrote, describing AYA enrollment in clinical trials in cancer as “suboptimal at best.”6
Dr Salsman said these dismal enrolment numbers could in part be related to treatment setting. Data suggest that most AYAs with cancer are treated in community-based practices rather than comprehensive cancer centers where the bulk of research is being done, he explained.
Dr Hanna agreed that more research involving AYAs was needed as is a better understanding of why enrollment is so much lower in this population. He pointed out that in 2017 the American Society of Clinical Oncology and Friends of Cancer Research released a statement recommending that pediatric patients be considered for enrollment in later-phase trials for cancer types that span both adults and children.12 The organizations said that individuals aged 12 years and older should routinely be included in such trials because their drug metabolism is similar to adults, and inclusion of younger patients may also be appropriate if they are part of the population affected by the disease, depending on specific disease biology, action of the drug, and available safety information.
Officials at the Food and Drug Administration are considering that possibility, Dr Hanna said.
Dr Salsman added there has been an increase in recent years in the attention paid to disparities in survival improvements and trial involvement among AYAs with cancer, compared with other age groups. For example, about 5 years ago, the National Clinical Trials Network formed a working group that developed a number of specific objectives for incorporating more AYAs into cancer trials and finding better ways to study this population;13 the Institute of Medicine held a forum on the care of AYAs with cancer;14 and the National Cancer Institute held a state-of-the-science meeting that focused on identifying strategic priorities for AYA oncology,15 he noted.
Dr Hanna added that “scientific groups such as Southwest Oncology Group (SWOG) and Children’s Oncology Group (COG) also have AYA committees now. One of the success stories of working together between SWOG and COG was the intergroup study C10403 for patients with ALL. And now there are efforts for an intergroup AYA-AML task force to include representatives from each of the cooperative groups that historically co-ordinated myeloid disease clinical trials – COG, SWOG, Alliance, and ECOG-ACRIN,” he said.
In fact, all of the National Clinical Trials Network groups have some initiative in place to address AYA concerns, said Dr Salsman, who chairs the ECOG-ACRIN AYA oncology subcommittee.
Despite these efforts, and many others, long-term survival improvements among AYAs with cancer still fall short, compared with those of other age groups.16
Next steps
Among the recommendations from authors in the AYA series in Blood is a call for assessing AYA-specific therapy in future clinical trials, as well as improved collaboration between adult and pediatric teams and the involvement of multidisciplinary teams in care for this population.
Many centers are already working on models for collaborative care, Dr Salsman said, citing the Fort Worth AYA Oncology Coalition led by medical director Karen Albritton, MD, as an example of a program that has been successful in helping clinical and supportive caregivers and their AYA patients “have a shared vision” as they work to maximize improvements in outcomes.
Patients are also taking the lead in demanding better care and attention to their psychosocial needs, Dr Hanna said. In the case of the community-powered advocacy organization Critical Mass, members have succeeded in getting lawmakers to introduce a bill in the US House of Representatives that would allow college students to defer loan payments while undergoing cancer treatment.
1. Boissel N, Baruchel A. Acute lymphoblastic leukemia in adolescent and young adults: treat as adults or as children? Blood. 2018;132:351-361.
2. Dunleavy K, Gross TG. Management of aggressive B-cell NHLs in the AYA population: an adult vs pediatric perspective. Blood. 2018;132:369-375.
3. O’Dwyer K, Freyer DR, Horan JT. Treatment strategies for adolescent and young adult patients with acute myeloid leukemia. Blood. 2018;132:362-368.
4. Flerlage JE, Metzger ML, Bhakta N. The management of Hodgkin lymphoma in adolescents and young adults: burden of disease or burden of choice? Blood. 2018;132:376-384.
5. Husson O, Huijgens PC, van der Graaf WTA. Psychosocial challenges and health-related quality of life of adolescents and young adults with hematologic malignancies. Blood. 2018;132:385-392.
6. Cortes J. Introduction to a review series on adolescent and young adult malignant hematology. Blood. 2018;132:345-346.
7. Muffly L, Alvarez E, Lichtensztajn D, Abrahão R, Gomez SL, Keegan T. Patterns of care and outcomes in adolescent and young adult acute lymphoblastic leukemia: a population-based study. Blood Adv. 2018;2(8):895-903.
8. Nathan PC, Bremner KE, Liu N, et al. Resource utilization and costs in adolescents treated for cancer in pediatric vs adult institutions. J Natl Cancer Inst. July 19, 2018. [Epub ahead of print.]
9. Parsons HM, Muffly L, Alvarez EM, Keegan THM. Does treatment setting matter? Evaluating resource utilization for adolescents treated in pediatric vs adult cancer institutions. https://academic.oup.com/jnci/advance-article/doi/10.1093/jnci/djy123/5056313?searchresult=1. Published July 19, 2018. Last accessed October 12, 2018.
10. Creutzig U, Zimmermann M, Reinhardt D, et al. Changes in cytogenetics and molecular genetics in acute myeloid leukemia from childhood to adult age groups. Cancer. 2016;122(24):3821-3830.
11. Salsman JM, Garcia SF, Yanez B, et al. Physical, emotional, and social health differences between posttreatment young adults with cancer and matched healthy controls. Cancer. 2014;120(15):2247-2254.
12. Kim ES, Bruinooge SS, Roberts S, et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research joint research statement. J Clin Oncol. 2017;35(33):3737-3744.
13. Freyer DR, Seibel NL. The clinical trials gap for adolescents and young adults with cancer: recent progress and conceptual framework for continued research. Curr Pediatr Rep. Published online February 18, 2015. DOI 10.1007/s40124-015-0075-y.
14. Nass SJ, Beaupin LK, Demark-Wahnefried W, et al. Identifying and addressing the needs of adolescents and young adults with cancer: summary of an Institute of Medicine workshop. Oncologist. 2015;20(2):186-195.
15. Wilder Smith A, Seibel NL, Lewis DR, et al. Next steps for adolescent and young adult oncology workshop: An update on progress and recommendations for the future. Cancer. 2016;122(7):988-999.
16. Keegan THM, Ries LAG, Barr RD, et al. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer. 2016;122(7):1009-1016.
1. Boissel N, Baruchel A. Acute lymphoblastic leukemia in adolescent and young adults: treat as adults or as children? Blood. 2018;132:351-361.
2. Dunleavy K, Gross TG. Management of aggressive B-cell NHLs in the AYA population: an adult vs pediatric perspective. Blood. 2018;132:369-375.
3. O’Dwyer K, Freyer DR, Horan JT. Treatment strategies for adolescent and young adult patients with acute myeloid leukemia. Blood. 2018;132:362-368.
4. Flerlage JE, Metzger ML, Bhakta N. The management of Hodgkin lymphoma in adolescents and young adults: burden of disease or burden of choice? Blood. 2018;132:376-384.
5. Husson O, Huijgens PC, van der Graaf WTA. Psychosocial challenges and health-related quality of life of adolescents and young adults with hematologic malignancies. Blood. 2018;132:385-392.
6. Cortes J. Introduction to a review series on adolescent and young adult malignant hematology. Blood. 2018;132:345-346.
7. Muffly L, Alvarez E, Lichtensztajn D, Abrahão R, Gomez SL, Keegan T. Patterns of care and outcomes in adolescent and young adult acute lymphoblastic leukemia: a population-based study. Blood Adv. 2018;2(8):895-903.
8. Nathan PC, Bremner KE, Liu N, et al. Resource utilization and costs in adolescents treated for cancer in pediatric vs adult institutions. J Natl Cancer Inst. July 19, 2018. [Epub ahead of print.]
9. Parsons HM, Muffly L, Alvarez EM, Keegan THM. Does treatment setting matter? Evaluating resource utilization for adolescents treated in pediatric vs adult cancer institutions. https://academic.oup.com/jnci/advance-article/doi/10.1093/jnci/djy123/5056313?searchresult=1. Published July 19, 2018. Last accessed October 12, 2018.
10. Creutzig U, Zimmermann M, Reinhardt D, et al. Changes in cytogenetics and molecular genetics in acute myeloid leukemia from childhood to adult age groups. Cancer. 2016;122(24):3821-3830.
11. Salsman JM, Garcia SF, Yanez B, et al. Physical, emotional, and social health differences between posttreatment young adults with cancer and matched healthy controls. Cancer. 2014;120(15):2247-2254.
12. Kim ES, Bruinooge SS, Roberts S, et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research joint research statement. J Clin Oncol. 2017;35(33):3737-3744.
13. Freyer DR, Seibel NL. The clinical trials gap for adolescents and young adults with cancer: recent progress and conceptual framework for continued research. Curr Pediatr Rep. Published online February 18, 2015. DOI 10.1007/s40124-015-0075-y.
14. Nass SJ, Beaupin LK, Demark-Wahnefried W, et al. Identifying and addressing the needs of adolescents and young adults with cancer: summary of an Institute of Medicine workshop. Oncologist. 2015;20(2):186-195.
15. Wilder Smith A, Seibel NL, Lewis DR, et al. Next steps for adolescent and young adult oncology workshop: An update on progress and recommendations for the future. Cancer. 2016;122(7):988-999.
16. Keegan THM, Ries LAG, Barr RD, et al. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer. 2016;122(7):1009-1016.
Bleeding score could help identify hemophilia
Bleeding scores may be helpful in identifying hemophilia patients, regardless of whether or not clotting factor levels are known, results of a recent investigation suggest.
Both hemophilia A and B patients had significantly higher bleeding scores as assessed by the ISTH-BAT (International Society on Thrombosis and Hemostasis–Bleeding Assessment Tool), compared with control subjects, according to results of the study.
Moreover, hemophilia patients classified as severe had significantly higher ISTH-BAT scores compared with those classified as mild, reported by Munira Borhany, MD, of the National Institute of Blood Disease and Bone Marrow Transplantation, Karachi, Pakistan, and her colleagues.
“The ISTH-BAT can be easily used in the clinics by physicians and can help to identify those patients who should be further investigated,” Dr. Borhany and her coauthors reported in the journal Transfusion and Apheresis Science.
The ISTH-BAT, established to standardize the reporting of bleeding symptoms, scores symptoms from 0, which indicates absent or trivial, to 4, meaning a symptom that requires medical intervention. Total scores considered abnormal are 4 or greater in men, 6 and greater in women, and 3 and greater in children, according to previously published reports.
In the present cross-sectional study, Dr. Borhany and her colleagues evaluated bleeding scores for 115 adult and pediatric patients – 78 with hemophilia A and 37 with hemophilia B – who were treated in Pakistan between 2014 and 2016.
Bleeding scores were a mean of 13.5 and 13.2 for hemophilia A and B patients, respectively, and 0.8 for 100 healthy male controls also included in the study. Scores were significantly higher in hemophilia patients versus controls (P less than .001), but not different between hemophilia A and B patients, the investigators reported.
Further results suggested a correlation between factor levels and clinical presentation of bleeding symptoms, according to the investigators. Statistically significant differences in bleeding scores also were seen between patients with severe and mild disease, and between severe and moderate disease, but not between the mild and moderate groups, they added.
Most studies of bleeding questionnaires to date have been in patients with von Willebrand disease or platelet disorders, with very little data on hemophilia.
“Apart from one recent study using ISTH-BAT in hemophilia carriers as part of assessing quality of life, we are unaware of other studies examining this assessment tool in hemophilia,” the researchers wrote.
This study cohort was unique, according to the investigators, because it included a substantial number of adults who were new patients with bleeding symptoms who had no previous diagnosis of hemophilia. “This allowed assessing whether investigators may tend to apply a higher score when knowing very low factor levels in hemophilia patients,” they said.
In fact, there was no major difference in bleeding scores for those newly diagnosed patients versus already diagnosed patients.
Results of an ongoing study will determine whether the ISTH BAT bleeding score can predict risk of bleeding in hemophilia patients, according to Dr. Borhany and her coauthors.
They reported having no conflicts of interest.
SOURCE: Borhany M et al. Transfus Apher Sci. 2018 Aug;57(4):556-60.
Bleeding scores may be helpful in identifying hemophilia patients, regardless of whether or not clotting factor levels are known, results of a recent investigation suggest.
Both hemophilia A and B patients had significantly higher bleeding scores as assessed by the ISTH-BAT (International Society on Thrombosis and Hemostasis–Bleeding Assessment Tool), compared with control subjects, according to results of the study.
Moreover, hemophilia patients classified as severe had significantly higher ISTH-BAT scores compared with those classified as mild, reported by Munira Borhany, MD, of the National Institute of Blood Disease and Bone Marrow Transplantation, Karachi, Pakistan, and her colleagues.
“The ISTH-BAT can be easily used in the clinics by physicians and can help to identify those patients who should be further investigated,” Dr. Borhany and her coauthors reported in the journal Transfusion and Apheresis Science.
The ISTH-BAT, established to standardize the reporting of bleeding symptoms, scores symptoms from 0, which indicates absent or trivial, to 4, meaning a symptom that requires medical intervention. Total scores considered abnormal are 4 or greater in men, 6 and greater in women, and 3 and greater in children, according to previously published reports.
In the present cross-sectional study, Dr. Borhany and her colleagues evaluated bleeding scores for 115 adult and pediatric patients – 78 with hemophilia A and 37 with hemophilia B – who were treated in Pakistan between 2014 and 2016.
Bleeding scores were a mean of 13.5 and 13.2 for hemophilia A and B patients, respectively, and 0.8 for 100 healthy male controls also included in the study. Scores were significantly higher in hemophilia patients versus controls (P less than .001), but not different between hemophilia A and B patients, the investigators reported.
Further results suggested a correlation between factor levels and clinical presentation of bleeding symptoms, according to the investigators. Statistically significant differences in bleeding scores also were seen between patients with severe and mild disease, and between severe and moderate disease, but not between the mild and moderate groups, they added.
Most studies of bleeding questionnaires to date have been in patients with von Willebrand disease or platelet disorders, with very little data on hemophilia.
“Apart from one recent study using ISTH-BAT in hemophilia carriers as part of assessing quality of life, we are unaware of other studies examining this assessment tool in hemophilia,” the researchers wrote.
This study cohort was unique, according to the investigators, because it included a substantial number of adults who were new patients with bleeding symptoms who had no previous diagnosis of hemophilia. “This allowed assessing whether investigators may tend to apply a higher score when knowing very low factor levels in hemophilia patients,” they said.
In fact, there was no major difference in bleeding scores for those newly diagnosed patients versus already diagnosed patients.
Results of an ongoing study will determine whether the ISTH BAT bleeding score can predict risk of bleeding in hemophilia patients, according to Dr. Borhany and her coauthors.
They reported having no conflicts of interest.
SOURCE: Borhany M et al. Transfus Apher Sci. 2018 Aug;57(4):556-60.
Bleeding scores may be helpful in identifying hemophilia patients, regardless of whether or not clotting factor levels are known, results of a recent investigation suggest.
Both hemophilia A and B patients had significantly higher bleeding scores as assessed by the ISTH-BAT (International Society on Thrombosis and Hemostasis–Bleeding Assessment Tool), compared with control subjects, according to results of the study.
Moreover, hemophilia patients classified as severe had significantly higher ISTH-BAT scores compared with those classified as mild, reported by Munira Borhany, MD, of the National Institute of Blood Disease and Bone Marrow Transplantation, Karachi, Pakistan, and her colleagues.
“The ISTH-BAT can be easily used in the clinics by physicians and can help to identify those patients who should be further investigated,” Dr. Borhany and her coauthors reported in the journal Transfusion and Apheresis Science.
The ISTH-BAT, established to standardize the reporting of bleeding symptoms, scores symptoms from 0, which indicates absent or trivial, to 4, meaning a symptom that requires medical intervention. Total scores considered abnormal are 4 or greater in men, 6 and greater in women, and 3 and greater in children, according to previously published reports.
In the present cross-sectional study, Dr. Borhany and her colleagues evaluated bleeding scores for 115 adult and pediatric patients – 78 with hemophilia A and 37 with hemophilia B – who were treated in Pakistan between 2014 and 2016.
Bleeding scores were a mean of 13.5 and 13.2 for hemophilia A and B patients, respectively, and 0.8 for 100 healthy male controls also included in the study. Scores were significantly higher in hemophilia patients versus controls (P less than .001), but not different between hemophilia A and B patients, the investigators reported.
Further results suggested a correlation between factor levels and clinical presentation of bleeding symptoms, according to the investigators. Statistically significant differences in bleeding scores also were seen between patients with severe and mild disease, and between severe and moderate disease, but not between the mild and moderate groups, they added.
Most studies of bleeding questionnaires to date have been in patients with von Willebrand disease or platelet disorders, with very little data on hemophilia.
“Apart from one recent study using ISTH-BAT in hemophilia carriers as part of assessing quality of life, we are unaware of other studies examining this assessment tool in hemophilia,” the researchers wrote.
This study cohort was unique, according to the investigators, because it included a substantial number of adults who were new patients with bleeding symptoms who had no previous diagnosis of hemophilia. “This allowed assessing whether investigators may tend to apply a higher score when knowing very low factor levels in hemophilia patients,” they said.
In fact, there was no major difference in bleeding scores for those newly diagnosed patients versus already diagnosed patients.
Results of an ongoing study will determine whether the ISTH BAT bleeding score can predict risk of bleeding in hemophilia patients, according to Dr. Borhany and her coauthors.
They reported having no conflicts of interest.
SOURCE: Borhany M et al. Transfus Apher Sci. 2018 Aug;57(4):556-60.
FROM TRANSFUSION AND APHERESIS SCIENCE
Key clinical point:
Major finding: Bleeding scores were a mean of 13.5 and 13.2 for hemophilia A and B patients, respectively, and 0.8 for healthy male controls (P less than .001).
Study details: A cross-sectional study included 115 adult and pediatric patients with hemophilia A or B treated in Pakistan between 2014 and 2016.
Disclosures: The authors reported having no conflicts of interest.
Source: Borhany M et al. Transfus Apher Sci. 2018 Aug;57(4):556-60.