User login
Palliative care update highlights role of nonspecialists
The new edition of providing care for critically ill patients, not just those clinicians actively specialized in palliative care.
The Clinical Practice Guidelines for Quality Palliative Care, 4th Edition, emphasizes the importance of palliative care provided by “clinicians in primary care and specialty care practices, such as oncologists,” the guideline authors stated.
The latest revision of the guideline aims to establish a foundation for “gold-standard” palliative care for people living with serious illness, regardless of diagnosis, prognosis, setting, or age, according to the National Coalition for Hospice and Palliative Care, which published the clinical practice guidelines.
The update was developed by the National Consensus Project for Quality Palliative Care (NCP), which includes 16 national organizations with palliative care and hospice expertise, and is endorsed by more than 80 national organizations, including the American Society of Hematology and the Oncology Nurses Society.
One key reason for the update, according to the NCP, was to acknowledge that today’s health care system may not be meeting patients’ palliative care needs.
Specifically, the guidelines call on all clinicians who are not palliative specialists to integrate palliative care principles into their routine assessment of seriously ill patients with conditions such as heart failure, lung disease, and cancer.
This approach differs from the way palliative care is traditionally practiced, often by fellowship-trained physicians, trained nurses, and other specialists who provide that support.
The guidelines are organized into sections covering palliative care structure and processes, care for the patient nearing the end of life, and specific aspects of palliative care, including physical, psychological, and psychiatric; social; cultural, ethical, and legal; and spiritual, religious, and existential aspects.
“The expectation is that all clinicians caring for seriously ill patients will integrate palliative care competencies, such as safe and effective pain and symptom management and expert communication skills in their practice, and palliative care specialists will provide expertise for those with the most complex needs,” the guideline authors wrote.
Implications for treatment of oncology patients
These new guidelines represent a “blueprint for what it looks like to provide high-quality, comprehensive palliative care to people with serious illness,” said Thomas W. LeBlanc, MD, who is a medical oncologist, palliative care physician, and patient experience researcher at Duke University, Durham, N.C.
“Part of this report to is about trying to raise the game of everybody in medicine and provide a higher basic level of primary palliative care to all people with serious illness, but then also to figure out who has higher levels of needs where the specialists should be applied, since they are a scarce resource,” said Dr. LeBlanc.
An issue with that traditional model is a shortage of specialized clinicians to meet palliative care needs, said Dr. LeBlanc, whose clinical practice and research focuses on palliative care needs of patients with hematologic malignancies.
“Palliative care has matured as a field such that we are now actually facing workforce shortage issues and really fundamental questions about who needs us the most, and how we increase our reach to improve the lives of more patients and families facing serious illness,” he said in an interview.
That’s a major driver behind the emphasis in these latest guidelines on providing palliative care in the community, coordinating care, and dealing with care transitions, he added.
“I hope that this document will help to demonstrate the value and the need for palliative care specialists, and for improvements in primary care in the care of patients with hematologic diseases in general,” he said. “To me, this adds increasing legitimacy to this whole field.”
Palliative care in surgical care
These guidelines are particularly useful to surgeons in part because of their focus on what’s known as primary palliative care, said to Geoffrey P. Dunn, MD, former chair of the American College of Surgeons Committee on Surgical Palliative Care. Palliative care, the new guidelines suggest, can be implemented by nonspecialists.
Primary palliative care includes diverse skills such as breaking adverse news to patients, managing uncomplicated pain, and being able to recognize signs and symptoms of imminent demise. “These are the minimum deliverables for all people dealing with seriously ill patients,” Dr. Dunn said in an interview. “It’s palliative care that any practicing physician should be able to handle.”
Dr. Dunn concurred with Dr. LaBlanc about the workforce shortage in the palliative field. The traditional model has created a shortage of specialized clinicians to meet palliative care needs. Across the board, “staffing for palliative teams is very inconsistent,” said Dr. Dunn. “It’s a classic unfunded mandate.”
While these guidelines are a step forward in recognizing the importance of palliative care outside of the palliative care specialty, there is no reference to surgery anywhere in the text of the 141-page prepublication draft provided by the NCP, Dr. Dunn noted in the interview.
“There’s still a danger of parallel universes, where surgery is developing its own understanding of this in parallel with the more general national palliative care movement,” he said. Despite that, there is a growing connection between surgery and the broader palliative care community. That linkage is especially important given the number of seriously ill patients with high symptom burden that are seen in surgery.
“I think where surgeons are beginning to find [palliative principles] very helpful is dealing with these protracted serial discussions with families in difficult circumstances, such as how long is the life support going to be prolonged in someone with a devastating head injury, or multiple system organ failure in the elderly,” Dr. Dunn added.
The new edition of providing care for critically ill patients, not just those clinicians actively specialized in palliative care.
The Clinical Practice Guidelines for Quality Palliative Care, 4th Edition, emphasizes the importance of palliative care provided by “clinicians in primary care and specialty care practices, such as oncologists,” the guideline authors stated.
The latest revision of the guideline aims to establish a foundation for “gold-standard” palliative care for people living with serious illness, regardless of diagnosis, prognosis, setting, or age, according to the National Coalition for Hospice and Palliative Care, which published the clinical practice guidelines.
The update was developed by the National Consensus Project for Quality Palliative Care (NCP), which includes 16 national organizations with palliative care and hospice expertise, and is endorsed by more than 80 national organizations, including the American Society of Hematology and the Oncology Nurses Society.
One key reason for the update, according to the NCP, was to acknowledge that today’s health care system may not be meeting patients’ palliative care needs.
Specifically, the guidelines call on all clinicians who are not palliative specialists to integrate palliative care principles into their routine assessment of seriously ill patients with conditions such as heart failure, lung disease, and cancer.
This approach differs from the way palliative care is traditionally practiced, often by fellowship-trained physicians, trained nurses, and other specialists who provide that support.
The guidelines are organized into sections covering palliative care structure and processes, care for the patient nearing the end of life, and specific aspects of palliative care, including physical, psychological, and psychiatric; social; cultural, ethical, and legal; and spiritual, religious, and existential aspects.
“The expectation is that all clinicians caring for seriously ill patients will integrate palliative care competencies, such as safe and effective pain and symptom management and expert communication skills in their practice, and palliative care specialists will provide expertise for those with the most complex needs,” the guideline authors wrote.
Implications for treatment of oncology patients
These new guidelines represent a “blueprint for what it looks like to provide high-quality, comprehensive palliative care to people with serious illness,” said Thomas W. LeBlanc, MD, who is a medical oncologist, palliative care physician, and patient experience researcher at Duke University, Durham, N.C.
“Part of this report to is about trying to raise the game of everybody in medicine and provide a higher basic level of primary palliative care to all people with serious illness, but then also to figure out who has higher levels of needs where the specialists should be applied, since they are a scarce resource,” said Dr. LeBlanc.
An issue with that traditional model is a shortage of specialized clinicians to meet palliative care needs, said Dr. LeBlanc, whose clinical practice and research focuses on palliative care needs of patients with hematologic malignancies.
“Palliative care has matured as a field such that we are now actually facing workforce shortage issues and really fundamental questions about who needs us the most, and how we increase our reach to improve the lives of more patients and families facing serious illness,” he said in an interview.
That’s a major driver behind the emphasis in these latest guidelines on providing palliative care in the community, coordinating care, and dealing with care transitions, he added.
“I hope that this document will help to demonstrate the value and the need for palliative care specialists, and for improvements in primary care in the care of patients with hematologic diseases in general,” he said. “To me, this adds increasing legitimacy to this whole field.”
Palliative care in surgical care
These guidelines are particularly useful to surgeons in part because of their focus on what’s known as primary palliative care, said to Geoffrey P. Dunn, MD, former chair of the American College of Surgeons Committee on Surgical Palliative Care. Palliative care, the new guidelines suggest, can be implemented by nonspecialists.
Primary palliative care includes diverse skills such as breaking adverse news to patients, managing uncomplicated pain, and being able to recognize signs and symptoms of imminent demise. “These are the minimum deliverables for all people dealing with seriously ill patients,” Dr. Dunn said in an interview. “It’s palliative care that any practicing physician should be able to handle.”
Dr. Dunn concurred with Dr. LaBlanc about the workforce shortage in the palliative field. The traditional model has created a shortage of specialized clinicians to meet palliative care needs. Across the board, “staffing for palliative teams is very inconsistent,” said Dr. Dunn. “It’s a classic unfunded mandate.”
While these guidelines are a step forward in recognizing the importance of palliative care outside of the palliative care specialty, there is no reference to surgery anywhere in the text of the 141-page prepublication draft provided by the NCP, Dr. Dunn noted in the interview.
“There’s still a danger of parallel universes, where surgery is developing its own understanding of this in parallel with the more general national palliative care movement,” he said. Despite that, there is a growing connection between surgery and the broader palliative care community. That linkage is especially important given the number of seriously ill patients with high symptom burden that are seen in surgery.
“I think where surgeons are beginning to find [palliative principles] very helpful is dealing with these protracted serial discussions with families in difficult circumstances, such as how long is the life support going to be prolonged in someone with a devastating head injury, or multiple system organ failure in the elderly,” Dr. Dunn added.
The new edition of providing care for critically ill patients, not just those clinicians actively specialized in palliative care.
The Clinical Practice Guidelines for Quality Palliative Care, 4th Edition, emphasizes the importance of palliative care provided by “clinicians in primary care and specialty care practices, such as oncologists,” the guideline authors stated.
The latest revision of the guideline aims to establish a foundation for “gold-standard” palliative care for people living with serious illness, regardless of diagnosis, prognosis, setting, or age, according to the National Coalition for Hospice and Palliative Care, which published the clinical practice guidelines.
The update was developed by the National Consensus Project for Quality Palliative Care (NCP), which includes 16 national organizations with palliative care and hospice expertise, and is endorsed by more than 80 national organizations, including the American Society of Hematology and the Oncology Nurses Society.
One key reason for the update, according to the NCP, was to acknowledge that today’s health care system may not be meeting patients’ palliative care needs.
Specifically, the guidelines call on all clinicians who are not palliative specialists to integrate palliative care principles into their routine assessment of seriously ill patients with conditions such as heart failure, lung disease, and cancer.
This approach differs from the way palliative care is traditionally practiced, often by fellowship-trained physicians, trained nurses, and other specialists who provide that support.
The guidelines are organized into sections covering palliative care structure and processes, care for the patient nearing the end of life, and specific aspects of palliative care, including physical, psychological, and psychiatric; social; cultural, ethical, and legal; and spiritual, religious, and existential aspects.
“The expectation is that all clinicians caring for seriously ill patients will integrate palliative care competencies, such as safe and effective pain and symptom management and expert communication skills in their practice, and palliative care specialists will provide expertise for those with the most complex needs,” the guideline authors wrote.
Implications for treatment of oncology patients
These new guidelines represent a “blueprint for what it looks like to provide high-quality, comprehensive palliative care to people with serious illness,” said Thomas W. LeBlanc, MD, who is a medical oncologist, palliative care physician, and patient experience researcher at Duke University, Durham, N.C.
“Part of this report to is about trying to raise the game of everybody in medicine and provide a higher basic level of primary palliative care to all people with serious illness, but then also to figure out who has higher levels of needs where the specialists should be applied, since they are a scarce resource,” said Dr. LeBlanc.
An issue with that traditional model is a shortage of specialized clinicians to meet palliative care needs, said Dr. LeBlanc, whose clinical practice and research focuses on palliative care needs of patients with hematologic malignancies.
“Palliative care has matured as a field such that we are now actually facing workforce shortage issues and really fundamental questions about who needs us the most, and how we increase our reach to improve the lives of more patients and families facing serious illness,” he said in an interview.
That’s a major driver behind the emphasis in these latest guidelines on providing palliative care in the community, coordinating care, and dealing with care transitions, he added.
“I hope that this document will help to demonstrate the value and the need for palliative care specialists, and for improvements in primary care in the care of patients with hematologic diseases in general,” he said. “To me, this adds increasing legitimacy to this whole field.”
Palliative care in surgical care
These guidelines are particularly useful to surgeons in part because of their focus on what’s known as primary palliative care, said to Geoffrey P. Dunn, MD, former chair of the American College of Surgeons Committee on Surgical Palliative Care. Palliative care, the new guidelines suggest, can be implemented by nonspecialists.
Primary palliative care includes diverse skills such as breaking adverse news to patients, managing uncomplicated pain, and being able to recognize signs and symptoms of imminent demise. “These are the minimum deliverables for all people dealing with seriously ill patients,” Dr. Dunn said in an interview. “It’s palliative care that any practicing physician should be able to handle.”
Dr. Dunn concurred with Dr. LaBlanc about the workforce shortage in the palliative field. The traditional model has created a shortage of specialized clinicians to meet palliative care needs. Across the board, “staffing for palliative teams is very inconsistent,” said Dr. Dunn. “It’s a classic unfunded mandate.”
While these guidelines are a step forward in recognizing the importance of palliative care outside of the palliative care specialty, there is no reference to surgery anywhere in the text of the 141-page prepublication draft provided by the NCP, Dr. Dunn noted in the interview.
“There’s still a danger of parallel universes, where surgery is developing its own understanding of this in parallel with the more general national palliative care movement,” he said. Despite that, there is a growing connection between surgery and the broader palliative care community. That linkage is especially important given the number of seriously ill patients with high symptom burden that are seen in surgery.
“I think where surgeons are beginning to find [palliative principles] very helpful is dealing with these protracted serial discussions with families in difficult circumstances, such as how long is the life support going to be prolonged in someone with a devastating head injury, or multiple system organ failure in the elderly,” Dr. Dunn added.
Game changers in pediatric cancer
Although there have been significant improvements in patient outcomes for some forms of pediatric cancer, progress has been painfully slow for others. An increasing understanding of pediatric cancers is highlighting the unique molecular drivers and challenging the assumption that drugs developed in adults can be applied to children and young adults. Here, we discuss game-changing therapeutic advances and a shifting view of childhood cancers.
Unique genomic background
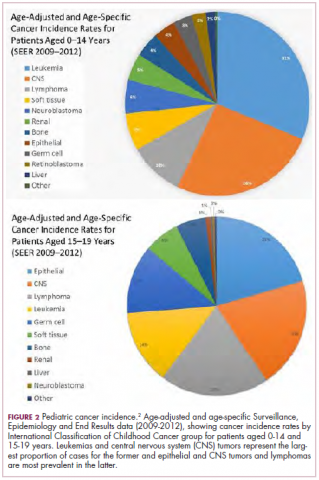
Although pediatric cancers are rare, representing just 1% of all new cancers diagnosed annually in the United States, they are the second leading cause of death in children aged 1 to 14 years. There are many different histological tumor types under the umbrella of childhood cancers, of which the most common are leukemias, central nervous system tumors, and lymphomas (Figure 1).1,2
Significant progress has been made in the treatment of certain pediatric cancers in recent decades, exemplified by pediatric acute lymphoblastic leukemia (ALL), which has been transformed from a virtually incurable cancer to one in which 5-year survival rates now reach up to 90%. In other forms of pediatric cancer, however, survival rates have stagnated and little progress has been made in the development of effective new therapies.3
Because of their rarity, pediatric cancers are difficult to study and adequate enrollment of children in clinical trials can be challenging. Pharmaceutical companies are often hesitant to test drugs in the pediatric population in patients who often cannot advocate for themselves. As a result, the activity of drugs developed in adult patients has often been inferred in pediatric patients with the same tumor type or molecular aberrations. However, as researchers have gathered more information about pediatric cancers, there has been increasing recognition of their unique attributes and the need for dedicated clinical trials in this patient population.
Pediatric cancers tend to be found in the developing mesodermic tissue, whereas adult cancers are more prevalent in the epithelial tissues. Genome sequencing studies have revealed a much lower mutational burden in pediatric cancers and the mechanisms of oncogenesis are also quite different; adult tumors can develop from a series of acquired gene mutations, but pediatric tumors tend to develop from a single catastrophic event.4,5
Even the same type of cancer in a pediatric and adult patient can be quite different, with very different underlying molecular mechanisms. In a recent genomic analysis of different types of pediatric cancer by researchers at St Jude’s Children’s Research Hospital, less than half of the identified mutated genes were found to be similar to those found in adult patients.6
A ‘magic bullet’?
Chromosomal rearrangements are common in pediatric cancers. This type of molecular abnormality can result in a fusion of 2 different genes when the chromosome breaks apart and the pieces join back together in a muddled order. If the genetic code fuses in a manner that is “readable” by the cell, then it can drive aberrant activation of one or both genes.7 Gene fusions often involve kinase enzymes that are essential players in cell signaling pathways regulating hallmark cancer processes, such as unchecked cell proliferation. The fusion drives the constitutive activation of the kinase and, thus, these downstream signaling pathways.
One of the first chromosomal rearrangements linked to cancer, BCR-ABL1 – more commonly known as the Philadelphia chromosome – results in aberrant activation of the ABL1 kinase. It is present in nearly all cases of chronic myeloid leukemia (CML) and 3% to 5% of patients with ALL, and thus became the central focus of targeted drug development. Imatinib was initially approved by the US Food and Drug Administration (FDA) in 2001 for the treatment of adult patients with CML and had such a significant impact on the treatment landscape that it made the cover of Time magazine as a “magic bullet” in the war on cancer.8
Approval was expanded into pediatric patients in 2006 and for pediatric patients with ALL in 2013. However, as with the use of most kinase inhibitors, tumors can evolve under the selective pressure of treatment, developing additional molecular abnormalities that drive resistance.9
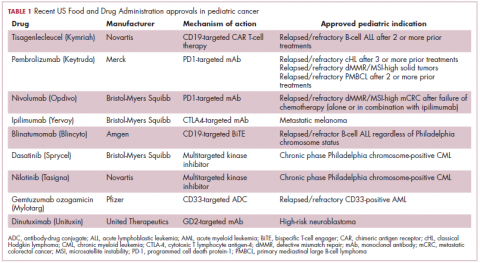
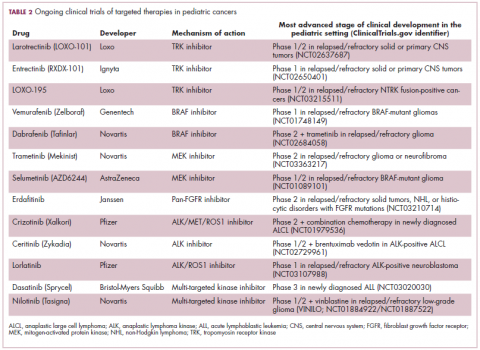
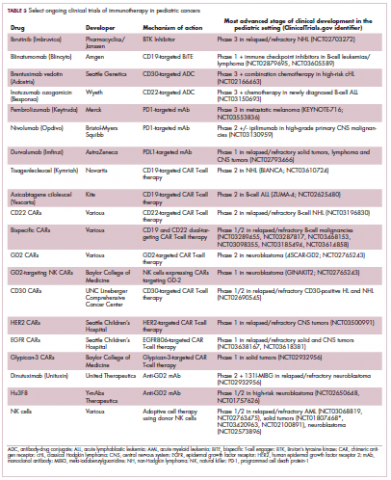
Next-generation multikinase inhibitors that more potently inhibit the BCR-ABL1 fusion protein have been developed to provide additional treatment options for patients who become resistant to imatinib. Dasatinib and nilotinib are among several drugs that have recently been approved for pediatric cancer therapy (Table 1). Both therapies were approved to treat children with Philadelphia chromosome-positive CML in the chronic phase in either the front- or second-line setting after failure of imatinib.
The approval of dasatinib was based on data from 97 patients across 2 trials, 51 of whom were newly diagnosed and 46 previously treated with imatinib. Most of the patients were treated with dasatinib 60 mg/m2 once daily. After 2 years of follow-up, more than 95% of newly diagnosed patients and 82.6% of relapsed/refractory patients had complete cytogenetic response.10
Nilotinib was approved on the basis of findings from 2 clinical trials including 69 patients – 1 trial involving patients who were refractory to or relapsed after dasatinib and imatinib treatment, and 1 that included both relapsed/refractory and newly diagnosed patients. Patients received nilotinib 230 mg/m2 twice daily, rounded to the nearest 50-mg dose, in 28-day cycles. By cycle 12, the cumulative major molecular response rate (MMR) was 47.7% in patients with relapsed/refractory disease, and 64% in newly diagnosed patients.11 Clinical trials of both drugs in the pediatric setting are ongoing.
Other prominent gene fusions
Gene fusions involving the anaplastic lymphoma kinase (ALK) occur in patients with non–small-cell lung cancer and ALK inhibitors have provided an effective new treatment option for patients whose tumors display this abnormality.
ALK fusions are also a prominent feature of several kinds of pediatric cancers and ALK inhibitors offer promise in this setting.7,12 An NPM-ALK fusion is found in 90% of pediatric anaplastic large cell lymphoma (ALCL) cases,13 whereas a variety of ALK fusions are found in up to half of patients with inflammatory myofibroblastic tumor (IMT), a rare form of soft tissue sarcoma.14 ALK inhibitors are being tested in a variety of clinical trials in pediatric patients (Table 2).
The results of a small phase 1 study of crizotinib in pediatric patients with ALK-positive ALCL (n = 26) or IMT (n = 14) were recently published. ALCL patients received crizotinib at a dose of 165 mg/m2, while IMT patients were given 100, 165, or 280 mg/m2. For the latter, the results were presented as a pooled cohort since safety and efficacy data were similar across dose levels. The overall response rate (ORR) was 83% for patients with ALCL and 86% for those with IMT. Grade 3/4 adverse events occurred in 83% and 71% of patients, respectively, and most commonly involved reduced neutrophil count.15
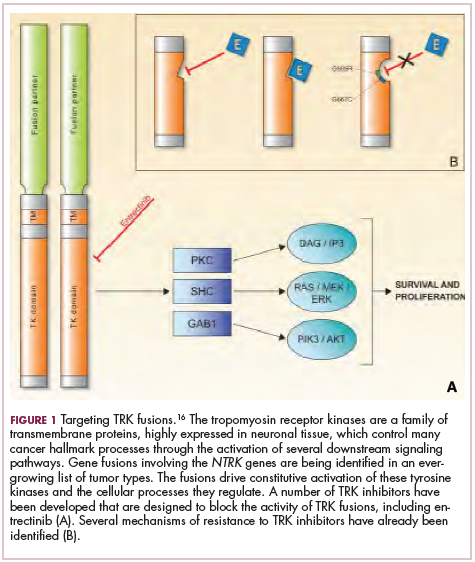
Most recently and perhaps most promisingly, fusions involving the neurotrophic tropomyosin receptor kinase (NTRK) gene have generated significant buzz. There are 3 NTRK genes, NTRK1, 2, and 3, which encode the TRKA, TRKB, and TRKC proteins, respectively.
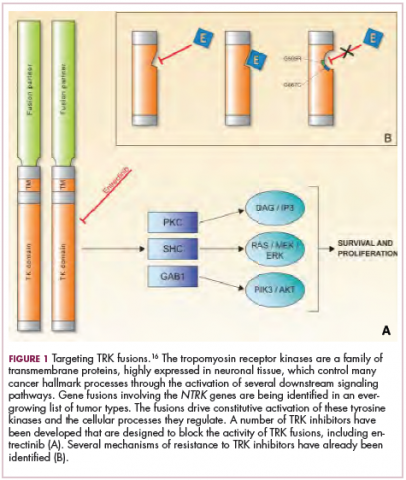
To date, 22 different partner genes have been identified that can fuse with the NTRK genes and, as with other kinase fusions, drive constitutive activation of the receptor proteins and downstream oncogenic signaling pathways, including the mitogen-activated protein kinase (MAPK) pathway (Figure 2).
NTRK fusions are being identified in an ever-growing number of cancer types, but are typically found in a small percentage of patients. However, in certain rare pediatric tumors, including congenital infantile fibrosarcoma and papillary thyroid cancer, they are found at much higher frequencies.
TRK inhibitors have been developed to target the fusion proteins and, given the spread of NTRK fusions across different types of cancers, they offer the most substantial promise as the next tumor agnostic cancer therapy – to treat patients based on the shared presence of a molecular aberration, irrespective of the type of cancer.16
The ongoing SCOUT trial is evaluating larotrectinib (LOXO-101) in pediatric patients. Among 24 patients (17 with NTRK fusions and 7 without) with infantile fibrosarcoma (47%), soft tissue sarcoma (41%) or papillary thyroid cancer (12%), the ORR was 93%, including complete response (CR) in 13% of patients.17
Preliminary results from an ongoing phase 1/2 study of entrectinib in pediatric patients with extracranial solid tumors were also recently presented at the annual meeting of the American Society for Clinical Oncology (ASCO). Among 15 evaluable patients enrolled to date, 3 have NTRK fusions and all experienced an objective response, with 1 (a patient with IMT) ongoing at 10 months.18
CAR T cells transformative in ALL
A variety of different types of immunotherapy have been tested in patients with pediatric cancers. In general, immunotherapy has proved less effective than in adult cancers, possibly because of the lower tumor mutation burden in pediatric cancers, which means there are likely fewer cancer antigens to provoke an anti-tumor immune response.
There are notable exceptions among the disappointments, however, and most exciting is the development of chimeric antigen receptor (CAR) T cells. CAR T cells fall into a category of immunotherapy known as adoptive cell therapy (ACT), in which immune cells are harvested from a patient and grown outside the body to increase their numbers before being reinfused into the patient.
In the case of CAR T-cell therapy, the cells are genetically engineered to express a CAR that endows them with tumor-targeting capabilities. To date, the development of CAR T cells has focused on the use of the CD19 antigen as a target, which is highly expressed on a variety of B-cell malignancies, including several of the most common forms of pediatric cancer. ASCO shined the spotlight on CAR T-cell therapy this year, naming it the Advance of the Year for 2018, saying that the treatment is “poised to transform childhood ALL.”19
Two CD19-targeted CAR T-cell therapies – tisagenlecleucel and axicabtagene ciloleucel – were brought to market in 2017. Only tisagenlecleucel is approved in the pediatric ALL population, however, having been awarded approval for the treatment of patients aged up to 25 years whose disease is refractory to or relapsed after receiving at least 2 prior therapies. In the pivotal trial, complete responses were observed in more than 60% of patients.20 Clinical trials of both CAR T-cell therapies in pediatric ALL and non-Hodgkin lymphoma are ongoing (Table 3).
CD19 has also proven to be a promising target for other forms of immunotherapy, including a new type of antibody known as a bispecific T-cell engager (BiTE). In 2014, blinatumomab became the first BiTE to receive regulatory approval, for the treatment of adult patients with relapsed/refractory ALL. Blinatumomab also targets the CD3 protein on T cells and helps to bring cancer cells and cytotoxic immune cells into close enough proximity that an immunological synapse can be formed between the two, facilitating tumor cell killing.21
In 2016, the approved indication was expanded into the pediatric population based on the results of a phase 1/2 study in which the safety and efficacy of blinatumomab were evaluated in 93 pediatric patients with relapsed/refractory ALL. Among the 70 patients who received the recommended dose of 5µg/m2 a day for the first 7 days, followed by 15µg/m2 a day thereafter, 51% achieved complete remission within the first 2 cycles, 52% of whom achieved minimal residual disease (MRD).22 Most recently, the FDA expanded the indication for blinatumomab to include patients (both adults and children) who are in remission, but MRD positive.23Despite the dramatic responses, many patients relapse after treatment with CD19-targeted CAR T cells, and researchers have uncovered numerous mechanisms of resistance. Among them is the loss of the CD19 antigen on the surface of target cells, such that a CD19-positive tumor becomes CD19-negative after treatment, driving relapse.24-26Several strategies for overcoming CD19-negative relapse are already being investigated, including the development of CD22-targeted CAR T cells and bispecific CAR T cells that target both CD19 and CD22. The results of a first-in-human trial of anti-CD22 CAR T-cell therapy were recently published. Among 21 pediatric and adult patients with relapsed/refractory B-cell ALL who were treated with either 3 x 105 cells/kg, 1 x 106 cells/kg, or 3 x 106 cells/kg, complete responses were observed in 57%.27
Results from 15 pediatric patients enrolled in a trial evaluating CD22-targeted CAR T cells as salvage therapy for those who relapse after CD19-targeted CAR T cell therapy were presented at the recent Congress of the European Hematology Association in Stockholm, Sweden. Patients who had undergone a stem cell transplant received the CAR T cells at a dose of 0.9 x 105 cell/kg and those who had not undergone a transplant received a dose of 8.2 x 105 cells/kg. At 30 days after CAR T cell infusion, the CR rate was 80% and the treatment was well tolerated.28
More immunotherapy approvals
The immune checkpoint inhibitors, which work by blocking inhibitory receptors on the surface of T cells, have also had recent approvals in pediatric patient populations. Pembrolizumab and nivolumab, inhibitors of the programmed cell death receptor 1 (PD-1) protein, have both been approved for use in adult and pediatric patients (older than 12 years) with relapsed/refractory metastatic colorectal cancer (and other solid tumors in the case of pembrolizumab) that display defects in the mismatch repair pathway that fixes damaged DNA or in patients that have high levels of microsatellite instability. Both deficient mismatch repair and microsatellite instability–high can indicate a high mutation burden in a tumor, which may predict increased sensitivity to immunotherapy.29
The approval in pediatric patients in both of those instances, however, was not based on data in pediatric patient populations but extrapolated from adult patients. Pembrolizumab is also approved for the treatment of adults and pediatric patients with classical Hodgkin lymphoma (cHL) after 3 or more previous treatments, but once again efficacy in the pediatric population was inferred from clinical trials performed in adults. Most recently, pembrolizumab was approved for the treatment of adult and pediatric patients with relapsed or refractory primary mediastinal large B-cell lymphoma.30Ipilimumab, which targets a different T cell receptor – cytotoxic T lymphocyte antigen-4 (CTLA-4) – has been approved for the treatment of pediatric patients aged 12 years and older with metastatic melanoma. This expanded indication, following on from its approval in adult patients in 2011, was based on data from 2 trials in which objective responses were observed in 2 out of 17 patients, including 1 partial response that lasted 16 months.31Finally, antibody-drug conjugates (ADC), in which tumor antigen-targeting monoclonal antibodies are conjugated to cytotoxic payloads to combine the specificity of an antibody with the cell-killing potency of chemotherapy, have also generated some recent successes in pediatric cancers.
Gemtuzumab ozogamicin is an ADC that targets the CD33 protein, which is highly expressed on 85%-90% of cases of acute myeloid leukemia (AML). In 2000, it was the first ADC to be brought to market in the United States, but it was subsequently voluntarily withdrawn by the manufacturer in 2010 after confirmatory trials failed to show a survival benefit.
Recently, a meta-analysis of gemtuzumab ozogamicin trials suggested that the drug likely does improve long-term overall survival (OS) and reduce the risk of relapse and researchers developed an intermittent dosing schedule to help mitigate toxicity.32 This new dosing regimen received FDA approval in 2017 for the treatment of pediatric patients aged 2 years and older on the basis of 2 clinical trials.
In the MyloFrance-1 trial, 57 patients were administered 3 mg/m2 gemtuzumab ozogamicin on days 1, 4, and 7 followed by cytarabine consolidation therapy and demonstrated a 26% CR rate and median recurrence-free survival of 11.6 months. In the phase 3 AML-19 trial, 237 patients received gemtuzumab ozogamicin at a dose of 6 mg/m2 on day 1 and 3 mg/m2 on day 8 or best supportive care. Gemtuzumab ozogamicin improved OS from 3.6 to 4.9 months.33,34
Inotuzumab ozogamicin is a CD22-targeting ADC that has been FDA approved for the treatment of adult patients with relapsed/refractory B-cell precursor ALL since last year. The therapy has been available to pediatric patients through a compassionate access program, but it has not been extensively evaluated in this population. The results of a retrospective analysis of pediatric patients who received at least 1 dose of inotuzumab ozogamicin were presented at ASCO in 2017. Among 29 patients with heavily pretreated disease the CR rate was 62%, 72% of whom achieved MRD negativity.35
1. American Cancer Society. Key statistics for childhood cancers. https://www.cancer.org/cancer/cancer-in-children/key-statistics.html. Last revised September 10, 2018. Accessed September 16, 2018.
2. NHI/National Cancer Institute website. Unusual cancers of childhood treatment (PDQ) - Health Professional Version. https://www.cancer.gov/types/childhood-cancers/hp/unusual-cancers-childhood-pdq. Last updated August 28, 2018. Accessed September 8, 2018.
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
4. Marshall GM, Carter DR, Cheung BB, et al. The prenatal origins of cancer. Nat Rev Cancer. 2014;14(4):277-289.
5. Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339(6127):1546-1558.
6. Ma X, Liu Y, Liu Y, et al. Pan-cancer genome and transcriptome analyses of 1,699 paediatric leukaemias and solid tumours. Nature. 2018;555:371.
7. Dupain C, Harttrampf AC, Urbinati G, Geoerger B, Massaad-Massade L. Relevance of fusion genes in pediatric cancers: toward precision medicine. Molec Ther Nucleic Acids. 2017;6:315-326.
8. Lemonick MD, Park A. New hope for cancer. http://content.time.com/time/world/article/0,8599,2047900-2,00.html. Published May 28, 2001. Last accessed September 13, 2018.
9. Iqbal N, Iqbal N. Imatinib: a breakthrough of targeted therapy in cancer. https://www.hindawi.com/journals/cherp/2014/357027/. Published May 19, 2014. Accessed September 16, 2018.
10. Gore L, Kearns PR, Martino MLd, et al. Dasatinib in pediatric patients with chronic myeloid leukemia in chronic phase: results from a phase II trial. J Clin Oncol. 2018;36(13):1330-1338.
11. Novartis press release. Novartis drug Tasigna approved by FDA to treat children with rare form of leukemia. 2018; https://www.novartis.com/news/media-releases/novartis-drug-tasignar-approved-fda-treat-children-rare-form-leukemia. Released March 22, 2018. Accessed September 16, 2018.
12. Takita J. The role of anaplastic lymphoma kinase in pediatric cancers. Cancer Sci. 2017;108(10):1913-1920.
13. Turner SD, Lamant L, Kenner L, Brugieres L. Anaplastic large cell lymphoma in paediatric and young adult patients. Br J Haematol. 2016;173(4):560-572.
14. Antonescu CR, Suurmeijer AJH, Zhang L, et al. Molecular characterization of inflammatory myofibroblastic tumors with frequent ALK and ROS1 fusions and rare novel RET gene rearrangement. Am J Surg Pathol. 2015;39(7):957-967.
15. Mosse YP, Voss SD, Lim MS, et al. Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: a children's oncology group study. J Clin Oncol. 2017;35(28):3215-3221.
16. Amatu A, Sartore-Bianchi A, Siena S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5070277/. Published online March 18, 2016. Accessed September 16, 2018.
17. [Behind paywall.] Laetsch TW, DuBois SG, Mascarenhas L, et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. 2018;19(5):705-714.
18. Desai AV, Brodeur GM, Foster J, et al. Phase 1 study of entrectinib (RXDX-101), a TRK, ROS1, and ALK inhibitor, in children, adolescents, and young adults with recurrent or refractory solid tumors. J Clin Oncol. 2018;36(suppl;):abstr 10536.
19. Heymach J, Krilov L, Alberg A, et al. Clinical cancer advances 2018: annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol. 2018;36(10):1020-1044.
20. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. NEJM. 2018;378(5):439-448.
21. Wu J, Fu J, Zhang M, Liu D. Blinatumomab: a bispecific T cell engager (BiTE) antibody against CD19/CD3 for refractory acute lymphoid leukemia. J Hematol Oncol. 2015;8:104.
22. Stackelberg Av, Locatelli F, Zugmaier G, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34(36):4381-4389.
23. Gokbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131(14):1522-1531.
24. Fischer J, Paret C, El Malki K, et al. CD19 isoforms enabling resistance to CART-19 immunotherapy are expressed in B-ALL patients at initial diagnosis. J Immunother. 2017;40(5):187-195.
25. Fousek K, Watanabe J, George A, et al. Targeting CD19-negative relapsed B-acute lymphoblastic leukemia using trivalent CAR T cells. J Clin Oncol. 2018;36(5_suppl):121-121.
26. Mejstríková E, Hrusak O, Borowitz MJ, et al. CD19-negative relapse of pediatric B-cell precursor acute lymphoblastic leukemia following blinatumomab treatment. Blood Cancer J. 2017;7(12):659.
27. Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20-28.
28. Pan J, Deng B, Liu S, et al. Efficacy and safety of CD22-directed CAR T-cell therapy in 15 pediatric refractory or relapsed b acute lymphoblastic leukemia patients. Paper presented at 23rd Congress of the European Hematology Association 2018; Stockholm, Sweden.
29. Boyiadzis MM, Kirkwood JM, Marshall JL, Pritchard CC, Azad NS, Gulley JL. Significance and implications of FDA approval of pembrolizumab for biomarker-defined disease. J Immunother Cancer. 2018;6:35.
30. Drugs.com. Keytruda approval history. 2018; https://www.drugs.com/history/keytruda.html. Last update information not given. Accessed September 16, 2018.
31. Bristol Myers Squibb press release. US Food and Drug Administration expands approval of Yervoy (ipilimumab) to include pediatric patients 12 years and older with unresectable or metastatic melanoma. https://news.bms.com/press-release/corporatefinancial-news/us-food-and-drug-administration-expands-approval-yervoy-ipilim. Released July 24, 2017. Accessed September 16, 2018.
32. Hills RK, Castaigne S, Appelbaum FR, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014;15(9):986-996.
33. Amadori S, Suciu S, Selleslag D, et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Oncol. 2016;34(9):972-979.
34. Taksin AL, Legrand O, Raffoux E, et al. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia. 2007;21(1):66-71.
35. Bhojwani D, Sposto R, Shah N, et al. Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia (R/R ALL). J Clin Oncol. 2017;35(15_suppl):10512-10512.
Although there have been significant improvements in patient outcomes for some forms of pediatric cancer, progress has been painfully slow for others. An increasing understanding of pediatric cancers is highlighting the unique molecular drivers and challenging the assumption that drugs developed in adults can be applied to children and young adults. Here, we discuss game-changing therapeutic advances and a shifting view of childhood cancers.
Unique genomic background
Although pediatric cancers are rare, representing just 1% of all new cancers diagnosed annually in the United States, they are the second leading cause of death in children aged 1 to 14 years. There are many different histological tumor types under the umbrella of childhood cancers, of which the most common are leukemias, central nervous system tumors, and lymphomas (Figure 1).1,2
Significant progress has been made in the treatment of certain pediatric cancers in recent decades, exemplified by pediatric acute lymphoblastic leukemia (ALL), which has been transformed from a virtually incurable cancer to one in which 5-year survival rates now reach up to 90%. In other forms of pediatric cancer, however, survival rates have stagnated and little progress has been made in the development of effective new therapies.3
Because of their rarity, pediatric cancers are difficult to study and adequate enrollment of children in clinical trials can be challenging. Pharmaceutical companies are often hesitant to test drugs in the pediatric population in patients who often cannot advocate for themselves. As a result, the activity of drugs developed in adult patients has often been inferred in pediatric patients with the same tumor type or molecular aberrations. However, as researchers have gathered more information about pediatric cancers, there has been increasing recognition of their unique attributes and the need for dedicated clinical trials in this patient population.
Pediatric cancers tend to be found in the developing mesodermic tissue, whereas adult cancers are more prevalent in the epithelial tissues. Genome sequencing studies have revealed a much lower mutational burden in pediatric cancers and the mechanisms of oncogenesis are also quite different; adult tumors can develop from a series of acquired gene mutations, but pediatric tumors tend to develop from a single catastrophic event.4,5
Even the same type of cancer in a pediatric and adult patient can be quite different, with very different underlying molecular mechanisms. In a recent genomic analysis of different types of pediatric cancer by researchers at St Jude’s Children’s Research Hospital, less than half of the identified mutated genes were found to be similar to those found in adult patients.6
A ‘magic bullet’?
Chromosomal rearrangements are common in pediatric cancers. This type of molecular abnormality can result in a fusion of 2 different genes when the chromosome breaks apart and the pieces join back together in a muddled order. If the genetic code fuses in a manner that is “readable” by the cell, then it can drive aberrant activation of one or both genes.7 Gene fusions often involve kinase enzymes that are essential players in cell signaling pathways regulating hallmark cancer processes, such as unchecked cell proliferation. The fusion drives the constitutive activation of the kinase and, thus, these downstream signaling pathways.
One of the first chromosomal rearrangements linked to cancer, BCR-ABL1 – more commonly known as the Philadelphia chromosome – results in aberrant activation of the ABL1 kinase. It is present in nearly all cases of chronic myeloid leukemia (CML) and 3% to 5% of patients with ALL, and thus became the central focus of targeted drug development. Imatinib was initially approved by the US Food and Drug Administration (FDA) in 2001 for the treatment of adult patients with CML and had such a significant impact on the treatment landscape that it made the cover of Time magazine as a “magic bullet” in the war on cancer.8
Approval was expanded into pediatric patients in 2006 and for pediatric patients with ALL in 2013. However, as with the use of most kinase inhibitors, tumors can evolve under the selective pressure of treatment, developing additional molecular abnormalities that drive resistance.9
Next-generation multikinase inhibitors that more potently inhibit the BCR-ABL1 fusion protein have been developed to provide additional treatment options for patients who become resistant to imatinib. Dasatinib and nilotinib are among several drugs that have recently been approved for pediatric cancer therapy (Table 1). Both therapies were approved to treat children with Philadelphia chromosome-positive CML in the chronic phase in either the front- or second-line setting after failure of imatinib.
The approval of dasatinib was based on data from 97 patients across 2 trials, 51 of whom were newly diagnosed and 46 previously treated with imatinib. Most of the patients were treated with dasatinib 60 mg/m2 once daily. After 2 years of follow-up, more than 95% of newly diagnosed patients and 82.6% of relapsed/refractory patients had complete cytogenetic response.10
Nilotinib was approved on the basis of findings from 2 clinical trials including 69 patients – 1 trial involving patients who were refractory to or relapsed after dasatinib and imatinib treatment, and 1 that included both relapsed/refractory and newly diagnosed patients. Patients received nilotinib 230 mg/m2 twice daily, rounded to the nearest 50-mg dose, in 28-day cycles. By cycle 12, the cumulative major molecular response rate (MMR) was 47.7% in patients with relapsed/refractory disease, and 64% in newly diagnosed patients.11 Clinical trials of both drugs in the pediatric setting are ongoing.
Other prominent gene fusions
Gene fusions involving the anaplastic lymphoma kinase (ALK) occur in patients with non–small-cell lung cancer and ALK inhibitors have provided an effective new treatment option for patients whose tumors display this abnormality.
ALK fusions are also a prominent feature of several kinds of pediatric cancers and ALK inhibitors offer promise in this setting.7,12 An NPM-ALK fusion is found in 90% of pediatric anaplastic large cell lymphoma (ALCL) cases,13 whereas a variety of ALK fusions are found in up to half of patients with inflammatory myofibroblastic tumor (IMT), a rare form of soft tissue sarcoma.14 ALK inhibitors are being tested in a variety of clinical trials in pediatric patients (Table 2).
The results of a small phase 1 study of crizotinib in pediatric patients with ALK-positive ALCL (n = 26) or IMT (n = 14) were recently published. ALCL patients received crizotinib at a dose of 165 mg/m2, while IMT patients were given 100, 165, or 280 mg/m2. For the latter, the results were presented as a pooled cohort since safety and efficacy data were similar across dose levels. The overall response rate (ORR) was 83% for patients with ALCL and 86% for those with IMT. Grade 3/4 adverse events occurred in 83% and 71% of patients, respectively, and most commonly involved reduced neutrophil count.15
Most recently and perhaps most promisingly, fusions involving the neurotrophic tropomyosin receptor kinase (NTRK) gene have generated significant buzz. There are 3 NTRK genes, NTRK1, 2, and 3, which encode the TRKA, TRKB, and TRKC proteins, respectively.
To date, 22 different partner genes have been identified that can fuse with the NTRK genes and, as with other kinase fusions, drive constitutive activation of the receptor proteins and downstream oncogenic signaling pathways, including the mitogen-activated protein kinase (MAPK) pathway (Figure 2).
NTRK fusions are being identified in an ever-growing number of cancer types, but are typically found in a small percentage of patients. However, in certain rare pediatric tumors, including congenital infantile fibrosarcoma and papillary thyroid cancer, they are found at much higher frequencies.
TRK inhibitors have been developed to target the fusion proteins and, given the spread of NTRK fusions across different types of cancers, they offer the most substantial promise as the next tumor agnostic cancer therapy – to treat patients based on the shared presence of a molecular aberration, irrespective of the type of cancer.16
The ongoing SCOUT trial is evaluating larotrectinib (LOXO-101) in pediatric patients. Among 24 patients (17 with NTRK fusions and 7 without) with infantile fibrosarcoma (47%), soft tissue sarcoma (41%) or papillary thyroid cancer (12%), the ORR was 93%, including complete response (CR) in 13% of patients.17
Preliminary results from an ongoing phase 1/2 study of entrectinib in pediatric patients with extracranial solid tumors were also recently presented at the annual meeting of the American Society for Clinical Oncology (ASCO). Among 15 evaluable patients enrolled to date, 3 have NTRK fusions and all experienced an objective response, with 1 (a patient with IMT) ongoing at 10 months.18
CAR T cells transformative in ALL
A variety of different types of immunotherapy have been tested in patients with pediatric cancers. In general, immunotherapy has proved less effective than in adult cancers, possibly because of the lower tumor mutation burden in pediatric cancers, which means there are likely fewer cancer antigens to provoke an anti-tumor immune response.
There are notable exceptions among the disappointments, however, and most exciting is the development of chimeric antigen receptor (CAR) T cells. CAR T cells fall into a category of immunotherapy known as adoptive cell therapy (ACT), in which immune cells are harvested from a patient and grown outside the body to increase their numbers before being reinfused into the patient.
In the case of CAR T-cell therapy, the cells are genetically engineered to express a CAR that endows them with tumor-targeting capabilities. To date, the development of CAR T cells has focused on the use of the CD19 antigen as a target, which is highly expressed on a variety of B-cell malignancies, including several of the most common forms of pediatric cancer. ASCO shined the spotlight on CAR T-cell therapy this year, naming it the Advance of the Year for 2018, saying that the treatment is “poised to transform childhood ALL.”19
Two CD19-targeted CAR T-cell therapies – tisagenlecleucel and axicabtagene ciloleucel – were brought to market in 2017. Only tisagenlecleucel is approved in the pediatric ALL population, however, having been awarded approval for the treatment of patients aged up to 25 years whose disease is refractory to or relapsed after receiving at least 2 prior therapies. In the pivotal trial, complete responses were observed in more than 60% of patients.20 Clinical trials of both CAR T-cell therapies in pediatric ALL and non-Hodgkin lymphoma are ongoing (Table 3).
CD19 has also proven to be a promising target for other forms of immunotherapy, including a new type of antibody known as a bispecific T-cell engager (BiTE). In 2014, blinatumomab became the first BiTE to receive regulatory approval, for the treatment of adult patients with relapsed/refractory ALL. Blinatumomab also targets the CD3 protein on T cells and helps to bring cancer cells and cytotoxic immune cells into close enough proximity that an immunological synapse can be formed between the two, facilitating tumor cell killing.21
In 2016, the approved indication was expanded into the pediatric population based on the results of a phase 1/2 study in which the safety and efficacy of blinatumomab were evaluated in 93 pediatric patients with relapsed/refractory ALL. Among the 70 patients who received the recommended dose of 5µg/m2 a day for the first 7 days, followed by 15µg/m2 a day thereafter, 51% achieved complete remission within the first 2 cycles, 52% of whom achieved minimal residual disease (MRD).22 Most recently, the FDA expanded the indication for blinatumomab to include patients (both adults and children) who are in remission, but MRD positive.23Despite the dramatic responses, many patients relapse after treatment with CD19-targeted CAR T cells, and researchers have uncovered numerous mechanisms of resistance. Among them is the loss of the CD19 antigen on the surface of target cells, such that a CD19-positive tumor becomes CD19-negative after treatment, driving relapse.24-26Several strategies for overcoming CD19-negative relapse are already being investigated, including the development of CD22-targeted CAR T cells and bispecific CAR T cells that target both CD19 and CD22. The results of a first-in-human trial of anti-CD22 CAR T-cell therapy were recently published. Among 21 pediatric and adult patients with relapsed/refractory B-cell ALL who were treated with either 3 x 105 cells/kg, 1 x 106 cells/kg, or 3 x 106 cells/kg, complete responses were observed in 57%.27
Results from 15 pediatric patients enrolled in a trial evaluating CD22-targeted CAR T cells as salvage therapy for those who relapse after CD19-targeted CAR T cell therapy were presented at the recent Congress of the European Hematology Association in Stockholm, Sweden. Patients who had undergone a stem cell transplant received the CAR T cells at a dose of 0.9 x 105 cell/kg and those who had not undergone a transplant received a dose of 8.2 x 105 cells/kg. At 30 days after CAR T cell infusion, the CR rate was 80% and the treatment was well tolerated.28
More immunotherapy approvals
The immune checkpoint inhibitors, which work by blocking inhibitory receptors on the surface of T cells, have also had recent approvals in pediatric patient populations. Pembrolizumab and nivolumab, inhibitors of the programmed cell death receptor 1 (PD-1) protein, have both been approved for use in adult and pediatric patients (older than 12 years) with relapsed/refractory metastatic colorectal cancer (and other solid tumors in the case of pembrolizumab) that display defects in the mismatch repair pathway that fixes damaged DNA or in patients that have high levels of microsatellite instability. Both deficient mismatch repair and microsatellite instability–high can indicate a high mutation burden in a tumor, which may predict increased sensitivity to immunotherapy.29
The approval in pediatric patients in both of those instances, however, was not based on data in pediatric patient populations but extrapolated from adult patients. Pembrolizumab is also approved for the treatment of adults and pediatric patients with classical Hodgkin lymphoma (cHL) after 3 or more previous treatments, but once again efficacy in the pediatric population was inferred from clinical trials performed in adults. Most recently, pembrolizumab was approved for the treatment of adult and pediatric patients with relapsed or refractory primary mediastinal large B-cell lymphoma.30Ipilimumab, which targets a different T cell receptor – cytotoxic T lymphocyte antigen-4 (CTLA-4) – has been approved for the treatment of pediatric patients aged 12 years and older with metastatic melanoma. This expanded indication, following on from its approval in adult patients in 2011, was based on data from 2 trials in which objective responses were observed in 2 out of 17 patients, including 1 partial response that lasted 16 months.31Finally, antibody-drug conjugates (ADC), in which tumor antigen-targeting monoclonal antibodies are conjugated to cytotoxic payloads to combine the specificity of an antibody with the cell-killing potency of chemotherapy, have also generated some recent successes in pediatric cancers.
Gemtuzumab ozogamicin is an ADC that targets the CD33 protein, which is highly expressed on 85%-90% of cases of acute myeloid leukemia (AML). In 2000, it was the first ADC to be brought to market in the United States, but it was subsequently voluntarily withdrawn by the manufacturer in 2010 after confirmatory trials failed to show a survival benefit.
Recently, a meta-analysis of gemtuzumab ozogamicin trials suggested that the drug likely does improve long-term overall survival (OS) and reduce the risk of relapse and researchers developed an intermittent dosing schedule to help mitigate toxicity.32 This new dosing regimen received FDA approval in 2017 for the treatment of pediatric patients aged 2 years and older on the basis of 2 clinical trials.
In the MyloFrance-1 trial, 57 patients were administered 3 mg/m2 gemtuzumab ozogamicin on days 1, 4, and 7 followed by cytarabine consolidation therapy and demonstrated a 26% CR rate and median recurrence-free survival of 11.6 months. In the phase 3 AML-19 trial, 237 patients received gemtuzumab ozogamicin at a dose of 6 mg/m2 on day 1 and 3 mg/m2 on day 8 or best supportive care. Gemtuzumab ozogamicin improved OS from 3.6 to 4.9 months.33,34
Inotuzumab ozogamicin is a CD22-targeting ADC that has been FDA approved for the treatment of adult patients with relapsed/refractory B-cell precursor ALL since last year. The therapy has been available to pediatric patients through a compassionate access program, but it has not been extensively evaluated in this population. The results of a retrospective analysis of pediatric patients who received at least 1 dose of inotuzumab ozogamicin were presented at ASCO in 2017. Among 29 patients with heavily pretreated disease the CR rate was 62%, 72% of whom achieved MRD negativity.35
Although there have been significant improvements in patient outcomes for some forms of pediatric cancer, progress has been painfully slow for others. An increasing understanding of pediatric cancers is highlighting the unique molecular drivers and challenging the assumption that drugs developed in adults can be applied to children and young adults. Here, we discuss game-changing therapeutic advances and a shifting view of childhood cancers.
Unique genomic background
Although pediatric cancers are rare, representing just 1% of all new cancers diagnosed annually in the United States, they are the second leading cause of death in children aged 1 to 14 years. There are many different histological tumor types under the umbrella of childhood cancers, of which the most common are leukemias, central nervous system tumors, and lymphomas (Figure 1).1,2
Significant progress has been made in the treatment of certain pediatric cancers in recent decades, exemplified by pediatric acute lymphoblastic leukemia (ALL), which has been transformed from a virtually incurable cancer to one in which 5-year survival rates now reach up to 90%. In other forms of pediatric cancer, however, survival rates have stagnated and little progress has been made in the development of effective new therapies.3
Because of their rarity, pediatric cancers are difficult to study and adequate enrollment of children in clinical trials can be challenging. Pharmaceutical companies are often hesitant to test drugs in the pediatric population in patients who often cannot advocate for themselves. As a result, the activity of drugs developed in adult patients has often been inferred in pediatric patients with the same tumor type or molecular aberrations. However, as researchers have gathered more information about pediatric cancers, there has been increasing recognition of their unique attributes and the need for dedicated clinical trials in this patient population.
Pediatric cancers tend to be found in the developing mesodermic tissue, whereas adult cancers are more prevalent in the epithelial tissues. Genome sequencing studies have revealed a much lower mutational burden in pediatric cancers and the mechanisms of oncogenesis are also quite different; adult tumors can develop from a series of acquired gene mutations, but pediatric tumors tend to develop from a single catastrophic event.4,5
Even the same type of cancer in a pediatric and adult patient can be quite different, with very different underlying molecular mechanisms. In a recent genomic analysis of different types of pediatric cancer by researchers at St Jude’s Children’s Research Hospital, less than half of the identified mutated genes were found to be similar to those found in adult patients.6
A ‘magic bullet’?
Chromosomal rearrangements are common in pediatric cancers. This type of molecular abnormality can result in a fusion of 2 different genes when the chromosome breaks apart and the pieces join back together in a muddled order. If the genetic code fuses in a manner that is “readable” by the cell, then it can drive aberrant activation of one or both genes.7 Gene fusions often involve kinase enzymes that are essential players in cell signaling pathways regulating hallmark cancer processes, such as unchecked cell proliferation. The fusion drives the constitutive activation of the kinase and, thus, these downstream signaling pathways.
One of the first chromosomal rearrangements linked to cancer, BCR-ABL1 – more commonly known as the Philadelphia chromosome – results in aberrant activation of the ABL1 kinase. It is present in nearly all cases of chronic myeloid leukemia (CML) and 3% to 5% of patients with ALL, and thus became the central focus of targeted drug development. Imatinib was initially approved by the US Food and Drug Administration (FDA) in 2001 for the treatment of adult patients with CML and had such a significant impact on the treatment landscape that it made the cover of Time magazine as a “magic bullet” in the war on cancer.8
Approval was expanded into pediatric patients in 2006 and for pediatric patients with ALL in 2013. However, as with the use of most kinase inhibitors, tumors can evolve under the selective pressure of treatment, developing additional molecular abnormalities that drive resistance.9
Next-generation multikinase inhibitors that more potently inhibit the BCR-ABL1 fusion protein have been developed to provide additional treatment options for patients who become resistant to imatinib. Dasatinib and nilotinib are among several drugs that have recently been approved for pediatric cancer therapy (Table 1). Both therapies were approved to treat children with Philadelphia chromosome-positive CML in the chronic phase in either the front- or second-line setting after failure of imatinib.
The approval of dasatinib was based on data from 97 patients across 2 trials, 51 of whom were newly diagnosed and 46 previously treated with imatinib. Most of the patients were treated with dasatinib 60 mg/m2 once daily. After 2 years of follow-up, more than 95% of newly diagnosed patients and 82.6% of relapsed/refractory patients had complete cytogenetic response.10
Nilotinib was approved on the basis of findings from 2 clinical trials including 69 patients – 1 trial involving patients who were refractory to or relapsed after dasatinib and imatinib treatment, and 1 that included both relapsed/refractory and newly diagnosed patients. Patients received nilotinib 230 mg/m2 twice daily, rounded to the nearest 50-mg dose, in 28-day cycles. By cycle 12, the cumulative major molecular response rate (MMR) was 47.7% in patients with relapsed/refractory disease, and 64% in newly diagnosed patients.11 Clinical trials of both drugs in the pediatric setting are ongoing.
Other prominent gene fusions
Gene fusions involving the anaplastic lymphoma kinase (ALK) occur in patients with non–small-cell lung cancer and ALK inhibitors have provided an effective new treatment option for patients whose tumors display this abnormality.
ALK fusions are also a prominent feature of several kinds of pediatric cancers and ALK inhibitors offer promise in this setting.7,12 An NPM-ALK fusion is found in 90% of pediatric anaplastic large cell lymphoma (ALCL) cases,13 whereas a variety of ALK fusions are found in up to half of patients with inflammatory myofibroblastic tumor (IMT), a rare form of soft tissue sarcoma.14 ALK inhibitors are being tested in a variety of clinical trials in pediatric patients (Table 2).
The results of a small phase 1 study of crizotinib in pediatric patients with ALK-positive ALCL (n = 26) or IMT (n = 14) were recently published. ALCL patients received crizotinib at a dose of 165 mg/m2, while IMT patients were given 100, 165, or 280 mg/m2. For the latter, the results were presented as a pooled cohort since safety and efficacy data were similar across dose levels. The overall response rate (ORR) was 83% for patients with ALCL and 86% for those with IMT. Grade 3/4 adverse events occurred in 83% and 71% of patients, respectively, and most commonly involved reduced neutrophil count.15
Most recently and perhaps most promisingly, fusions involving the neurotrophic tropomyosin receptor kinase (NTRK) gene have generated significant buzz. There are 3 NTRK genes, NTRK1, 2, and 3, which encode the TRKA, TRKB, and TRKC proteins, respectively.
To date, 22 different partner genes have been identified that can fuse with the NTRK genes and, as with other kinase fusions, drive constitutive activation of the receptor proteins and downstream oncogenic signaling pathways, including the mitogen-activated protein kinase (MAPK) pathway (Figure 2).
NTRK fusions are being identified in an ever-growing number of cancer types, but are typically found in a small percentage of patients. However, in certain rare pediatric tumors, including congenital infantile fibrosarcoma and papillary thyroid cancer, they are found at much higher frequencies.
TRK inhibitors have been developed to target the fusion proteins and, given the spread of NTRK fusions across different types of cancers, they offer the most substantial promise as the next tumor agnostic cancer therapy – to treat patients based on the shared presence of a molecular aberration, irrespective of the type of cancer.16
The ongoing SCOUT trial is evaluating larotrectinib (LOXO-101) in pediatric patients. Among 24 patients (17 with NTRK fusions and 7 without) with infantile fibrosarcoma (47%), soft tissue sarcoma (41%) or papillary thyroid cancer (12%), the ORR was 93%, including complete response (CR) in 13% of patients.17
Preliminary results from an ongoing phase 1/2 study of entrectinib in pediatric patients with extracranial solid tumors were also recently presented at the annual meeting of the American Society for Clinical Oncology (ASCO). Among 15 evaluable patients enrolled to date, 3 have NTRK fusions and all experienced an objective response, with 1 (a patient with IMT) ongoing at 10 months.18
CAR T cells transformative in ALL
A variety of different types of immunotherapy have been tested in patients with pediatric cancers. In general, immunotherapy has proved less effective than in adult cancers, possibly because of the lower tumor mutation burden in pediatric cancers, which means there are likely fewer cancer antigens to provoke an anti-tumor immune response.
There are notable exceptions among the disappointments, however, and most exciting is the development of chimeric antigen receptor (CAR) T cells. CAR T cells fall into a category of immunotherapy known as adoptive cell therapy (ACT), in which immune cells are harvested from a patient and grown outside the body to increase their numbers before being reinfused into the patient.
In the case of CAR T-cell therapy, the cells are genetically engineered to express a CAR that endows them with tumor-targeting capabilities. To date, the development of CAR T cells has focused on the use of the CD19 antigen as a target, which is highly expressed on a variety of B-cell malignancies, including several of the most common forms of pediatric cancer. ASCO shined the spotlight on CAR T-cell therapy this year, naming it the Advance of the Year for 2018, saying that the treatment is “poised to transform childhood ALL.”19
Two CD19-targeted CAR T-cell therapies – tisagenlecleucel and axicabtagene ciloleucel – were brought to market in 2017. Only tisagenlecleucel is approved in the pediatric ALL population, however, having been awarded approval for the treatment of patients aged up to 25 years whose disease is refractory to or relapsed after receiving at least 2 prior therapies. In the pivotal trial, complete responses were observed in more than 60% of patients.20 Clinical trials of both CAR T-cell therapies in pediatric ALL and non-Hodgkin lymphoma are ongoing (Table 3).
CD19 has also proven to be a promising target for other forms of immunotherapy, including a new type of antibody known as a bispecific T-cell engager (BiTE). In 2014, blinatumomab became the first BiTE to receive regulatory approval, for the treatment of adult patients with relapsed/refractory ALL. Blinatumomab also targets the CD3 protein on T cells and helps to bring cancer cells and cytotoxic immune cells into close enough proximity that an immunological synapse can be formed between the two, facilitating tumor cell killing.21
In 2016, the approved indication was expanded into the pediatric population based on the results of a phase 1/2 study in which the safety and efficacy of blinatumomab were evaluated in 93 pediatric patients with relapsed/refractory ALL. Among the 70 patients who received the recommended dose of 5µg/m2 a day for the first 7 days, followed by 15µg/m2 a day thereafter, 51% achieved complete remission within the first 2 cycles, 52% of whom achieved minimal residual disease (MRD).22 Most recently, the FDA expanded the indication for blinatumomab to include patients (both adults and children) who are in remission, but MRD positive.23Despite the dramatic responses, many patients relapse after treatment with CD19-targeted CAR T cells, and researchers have uncovered numerous mechanisms of resistance. Among them is the loss of the CD19 antigen on the surface of target cells, such that a CD19-positive tumor becomes CD19-negative after treatment, driving relapse.24-26Several strategies for overcoming CD19-negative relapse are already being investigated, including the development of CD22-targeted CAR T cells and bispecific CAR T cells that target both CD19 and CD22. The results of a first-in-human trial of anti-CD22 CAR T-cell therapy were recently published. Among 21 pediatric and adult patients with relapsed/refractory B-cell ALL who were treated with either 3 x 105 cells/kg, 1 x 106 cells/kg, or 3 x 106 cells/kg, complete responses were observed in 57%.27
Results from 15 pediatric patients enrolled in a trial evaluating CD22-targeted CAR T cells as salvage therapy for those who relapse after CD19-targeted CAR T cell therapy were presented at the recent Congress of the European Hematology Association in Stockholm, Sweden. Patients who had undergone a stem cell transplant received the CAR T cells at a dose of 0.9 x 105 cell/kg and those who had not undergone a transplant received a dose of 8.2 x 105 cells/kg. At 30 days after CAR T cell infusion, the CR rate was 80% and the treatment was well tolerated.28
More immunotherapy approvals
The immune checkpoint inhibitors, which work by blocking inhibitory receptors on the surface of T cells, have also had recent approvals in pediatric patient populations. Pembrolizumab and nivolumab, inhibitors of the programmed cell death receptor 1 (PD-1) protein, have both been approved for use in adult and pediatric patients (older than 12 years) with relapsed/refractory metastatic colorectal cancer (and other solid tumors in the case of pembrolizumab) that display defects in the mismatch repair pathway that fixes damaged DNA or in patients that have high levels of microsatellite instability. Both deficient mismatch repair and microsatellite instability–high can indicate a high mutation burden in a tumor, which may predict increased sensitivity to immunotherapy.29
The approval in pediatric patients in both of those instances, however, was not based on data in pediatric patient populations but extrapolated from adult patients. Pembrolizumab is also approved for the treatment of adults and pediatric patients with classical Hodgkin lymphoma (cHL) after 3 or more previous treatments, but once again efficacy in the pediatric population was inferred from clinical trials performed in adults. Most recently, pembrolizumab was approved for the treatment of adult and pediatric patients with relapsed or refractory primary mediastinal large B-cell lymphoma.30Ipilimumab, which targets a different T cell receptor – cytotoxic T lymphocyte antigen-4 (CTLA-4) – has been approved for the treatment of pediatric patients aged 12 years and older with metastatic melanoma. This expanded indication, following on from its approval in adult patients in 2011, was based on data from 2 trials in which objective responses were observed in 2 out of 17 patients, including 1 partial response that lasted 16 months.31Finally, antibody-drug conjugates (ADC), in which tumor antigen-targeting monoclonal antibodies are conjugated to cytotoxic payloads to combine the specificity of an antibody with the cell-killing potency of chemotherapy, have also generated some recent successes in pediatric cancers.
Gemtuzumab ozogamicin is an ADC that targets the CD33 protein, which is highly expressed on 85%-90% of cases of acute myeloid leukemia (AML). In 2000, it was the first ADC to be brought to market in the United States, but it was subsequently voluntarily withdrawn by the manufacturer in 2010 after confirmatory trials failed to show a survival benefit.
Recently, a meta-analysis of gemtuzumab ozogamicin trials suggested that the drug likely does improve long-term overall survival (OS) and reduce the risk of relapse and researchers developed an intermittent dosing schedule to help mitigate toxicity.32 This new dosing regimen received FDA approval in 2017 for the treatment of pediatric patients aged 2 years and older on the basis of 2 clinical trials.
In the MyloFrance-1 trial, 57 patients were administered 3 mg/m2 gemtuzumab ozogamicin on days 1, 4, and 7 followed by cytarabine consolidation therapy and demonstrated a 26% CR rate and median recurrence-free survival of 11.6 months. In the phase 3 AML-19 trial, 237 patients received gemtuzumab ozogamicin at a dose of 6 mg/m2 on day 1 and 3 mg/m2 on day 8 or best supportive care. Gemtuzumab ozogamicin improved OS from 3.6 to 4.9 months.33,34
Inotuzumab ozogamicin is a CD22-targeting ADC that has been FDA approved for the treatment of adult patients with relapsed/refractory B-cell precursor ALL since last year. The therapy has been available to pediatric patients through a compassionate access program, but it has not been extensively evaluated in this population. The results of a retrospective analysis of pediatric patients who received at least 1 dose of inotuzumab ozogamicin were presented at ASCO in 2017. Among 29 patients with heavily pretreated disease the CR rate was 62%, 72% of whom achieved MRD negativity.35
1. American Cancer Society. Key statistics for childhood cancers. https://www.cancer.org/cancer/cancer-in-children/key-statistics.html. Last revised September 10, 2018. Accessed September 16, 2018.
2. NHI/National Cancer Institute website. Unusual cancers of childhood treatment (PDQ) - Health Professional Version. https://www.cancer.gov/types/childhood-cancers/hp/unusual-cancers-childhood-pdq. Last updated August 28, 2018. Accessed September 8, 2018.
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
4. Marshall GM, Carter DR, Cheung BB, et al. The prenatal origins of cancer. Nat Rev Cancer. 2014;14(4):277-289.
5. Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339(6127):1546-1558.
6. Ma X, Liu Y, Liu Y, et al. Pan-cancer genome and transcriptome analyses of 1,699 paediatric leukaemias and solid tumours. Nature. 2018;555:371.
7. Dupain C, Harttrampf AC, Urbinati G, Geoerger B, Massaad-Massade L. Relevance of fusion genes in pediatric cancers: toward precision medicine. Molec Ther Nucleic Acids. 2017;6:315-326.
8. Lemonick MD, Park A. New hope for cancer. http://content.time.com/time/world/article/0,8599,2047900-2,00.html. Published May 28, 2001. Last accessed September 13, 2018.
9. Iqbal N, Iqbal N. Imatinib: a breakthrough of targeted therapy in cancer. https://www.hindawi.com/journals/cherp/2014/357027/. Published May 19, 2014. Accessed September 16, 2018.
10. Gore L, Kearns PR, Martino MLd, et al. Dasatinib in pediatric patients with chronic myeloid leukemia in chronic phase: results from a phase II trial. J Clin Oncol. 2018;36(13):1330-1338.
11. Novartis press release. Novartis drug Tasigna approved by FDA to treat children with rare form of leukemia. 2018; https://www.novartis.com/news/media-releases/novartis-drug-tasignar-approved-fda-treat-children-rare-form-leukemia. Released March 22, 2018. Accessed September 16, 2018.
12. Takita J. The role of anaplastic lymphoma kinase in pediatric cancers. Cancer Sci. 2017;108(10):1913-1920.
13. Turner SD, Lamant L, Kenner L, Brugieres L. Anaplastic large cell lymphoma in paediatric and young adult patients. Br J Haematol. 2016;173(4):560-572.
14. Antonescu CR, Suurmeijer AJH, Zhang L, et al. Molecular characterization of inflammatory myofibroblastic tumors with frequent ALK and ROS1 fusions and rare novel RET gene rearrangement. Am J Surg Pathol. 2015;39(7):957-967.
15. Mosse YP, Voss SD, Lim MS, et al. Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: a children's oncology group study. J Clin Oncol. 2017;35(28):3215-3221.
16. Amatu A, Sartore-Bianchi A, Siena S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5070277/. Published online March 18, 2016. Accessed September 16, 2018.
17. [Behind paywall.] Laetsch TW, DuBois SG, Mascarenhas L, et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. 2018;19(5):705-714.
18. Desai AV, Brodeur GM, Foster J, et al. Phase 1 study of entrectinib (RXDX-101), a TRK, ROS1, and ALK inhibitor, in children, adolescents, and young adults with recurrent or refractory solid tumors. J Clin Oncol. 2018;36(suppl;):abstr 10536.
19. Heymach J, Krilov L, Alberg A, et al. Clinical cancer advances 2018: annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol. 2018;36(10):1020-1044.
20. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. NEJM. 2018;378(5):439-448.
21. Wu J, Fu J, Zhang M, Liu D. Blinatumomab: a bispecific T cell engager (BiTE) antibody against CD19/CD3 for refractory acute lymphoid leukemia. J Hematol Oncol. 2015;8:104.
22. Stackelberg Av, Locatelli F, Zugmaier G, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34(36):4381-4389.
23. Gokbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131(14):1522-1531.
24. Fischer J, Paret C, El Malki K, et al. CD19 isoforms enabling resistance to CART-19 immunotherapy are expressed in B-ALL patients at initial diagnosis. J Immunother. 2017;40(5):187-195.
25. Fousek K, Watanabe J, George A, et al. Targeting CD19-negative relapsed B-acute lymphoblastic leukemia using trivalent CAR T cells. J Clin Oncol. 2018;36(5_suppl):121-121.
26. Mejstríková E, Hrusak O, Borowitz MJ, et al. CD19-negative relapse of pediatric B-cell precursor acute lymphoblastic leukemia following blinatumomab treatment. Blood Cancer J. 2017;7(12):659.
27. Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20-28.
28. Pan J, Deng B, Liu S, et al. Efficacy and safety of CD22-directed CAR T-cell therapy in 15 pediatric refractory or relapsed b acute lymphoblastic leukemia patients. Paper presented at 23rd Congress of the European Hematology Association 2018; Stockholm, Sweden.
29. Boyiadzis MM, Kirkwood JM, Marshall JL, Pritchard CC, Azad NS, Gulley JL. Significance and implications of FDA approval of pembrolizumab for biomarker-defined disease. J Immunother Cancer. 2018;6:35.
30. Drugs.com. Keytruda approval history. 2018; https://www.drugs.com/history/keytruda.html. Last update information not given. Accessed September 16, 2018.
31. Bristol Myers Squibb press release. US Food and Drug Administration expands approval of Yervoy (ipilimumab) to include pediatric patients 12 years and older with unresectable or metastatic melanoma. https://news.bms.com/press-release/corporatefinancial-news/us-food-and-drug-administration-expands-approval-yervoy-ipilim. Released July 24, 2017. Accessed September 16, 2018.
32. Hills RK, Castaigne S, Appelbaum FR, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014;15(9):986-996.
33. Amadori S, Suciu S, Selleslag D, et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Oncol. 2016;34(9):972-979.
34. Taksin AL, Legrand O, Raffoux E, et al. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia. 2007;21(1):66-71.
35. Bhojwani D, Sposto R, Shah N, et al. Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia (R/R ALL). J Clin Oncol. 2017;35(15_suppl):10512-10512.
1. American Cancer Society. Key statistics for childhood cancers. https://www.cancer.org/cancer/cancer-in-children/key-statistics.html. Last revised September 10, 2018. Accessed September 16, 2018.
2. NHI/National Cancer Institute website. Unusual cancers of childhood treatment (PDQ) - Health Professional Version. https://www.cancer.gov/types/childhood-cancers/hp/unusual-cancers-childhood-pdq. Last updated August 28, 2018. Accessed September 8, 2018.
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
4. Marshall GM, Carter DR, Cheung BB, et al. The prenatal origins of cancer. Nat Rev Cancer. 2014;14(4):277-289.
5. Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339(6127):1546-1558.
6. Ma X, Liu Y, Liu Y, et al. Pan-cancer genome and transcriptome analyses of 1,699 paediatric leukaemias and solid tumours. Nature. 2018;555:371.
7. Dupain C, Harttrampf AC, Urbinati G, Geoerger B, Massaad-Massade L. Relevance of fusion genes in pediatric cancers: toward precision medicine. Molec Ther Nucleic Acids. 2017;6:315-326.
8. Lemonick MD, Park A. New hope for cancer. http://content.time.com/time/world/article/0,8599,2047900-2,00.html. Published May 28, 2001. Last accessed September 13, 2018.
9. Iqbal N, Iqbal N. Imatinib: a breakthrough of targeted therapy in cancer. https://www.hindawi.com/journals/cherp/2014/357027/. Published May 19, 2014. Accessed September 16, 2018.
10. Gore L, Kearns PR, Martino MLd, et al. Dasatinib in pediatric patients with chronic myeloid leukemia in chronic phase: results from a phase II trial. J Clin Oncol. 2018;36(13):1330-1338.
11. Novartis press release. Novartis drug Tasigna approved by FDA to treat children with rare form of leukemia. 2018; https://www.novartis.com/news/media-releases/novartis-drug-tasignar-approved-fda-treat-children-rare-form-leukemia. Released March 22, 2018. Accessed September 16, 2018.
12. Takita J. The role of anaplastic lymphoma kinase in pediatric cancers. Cancer Sci. 2017;108(10):1913-1920.
13. Turner SD, Lamant L, Kenner L, Brugieres L. Anaplastic large cell lymphoma in paediatric and young adult patients. Br J Haematol. 2016;173(4):560-572.
14. Antonescu CR, Suurmeijer AJH, Zhang L, et al. Molecular characterization of inflammatory myofibroblastic tumors with frequent ALK and ROS1 fusions and rare novel RET gene rearrangement. Am J Surg Pathol. 2015;39(7):957-967.
15. Mosse YP, Voss SD, Lim MS, et al. Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: a children's oncology group study. J Clin Oncol. 2017;35(28):3215-3221.
16. Amatu A, Sartore-Bianchi A, Siena S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5070277/. Published online March 18, 2016. Accessed September 16, 2018.
17. [Behind paywall.] Laetsch TW, DuBois SG, Mascarenhas L, et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. 2018;19(5):705-714.
18. Desai AV, Brodeur GM, Foster J, et al. Phase 1 study of entrectinib (RXDX-101), a TRK, ROS1, and ALK inhibitor, in children, adolescents, and young adults with recurrent or refractory solid tumors. J Clin Oncol. 2018;36(suppl;):abstr 10536.
19. Heymach J, Krilov L, Alberg A, et al. Clinical cancer advances 2018: annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol. 2018;36(10):1020-1044.
20. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. NEJM. 2018;378(5):439-448.
21. Wu J, Fu J, Zhang M, Liu D. Blinatumomab: a bispecific T cell engager (BiTE) antibody against CD19/CD3 for refractory acute lymphoid leukemia. J Hematol Oncol. 2015;8:104.
22. Stackelberg Av, Locatelli F, Zugmaier G, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34(36):4381-4389.
23. Gokbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131(14):1522-1531.
24. Fischer J, Paret C, El Malki K, et al. CD19 isoforms enabling resistance to CART-19 immunotherapy are expressed in B-ALL patients at initial diagnosis. J Immunother. 2017;40(5):187-195.
25. Fousek K, Watanabe J, George A, et al. Targeting CD19-negative relapsed B-acute lymphoblastic leukemia using trivalent CAR T cells. J Clin Oncol. 2018;36(5_suppl):121-121.
26. Mejstríková E, Hrusak O, Borowitz MJ, et al. CD19-negative relapse of pediatric B-cell precursor acute lymphoblastic leukemia following blinatumomab treatment. Blood Cancer J. 2017;7(12):659.
27. Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20-28.
28. Pan J, Deng B, Liu S, et al. Efficacy and safety of CD22-directed CAR T-cell therapy in 15 pediatric refractory or relapsed b acute lymphoblastic leukemia patients. Paper presented at 23rd Congress of the European Hematology Association 2018; Stockholm, Sweden.
29. Boyiadzis MM, Kirkwood JM, Marshall JL, Pritchard CC, Azad NS, Gulley JL. Significance and implications of FDA approval of pembrolizumab for biomarker-defined disease. J Immunother Cancer. 2018;6:35.
30. Drugs.com. Keytruda approval history. 2018; https://www.drugs.com/history/keytruda.html. Last update information not given. Accessed September 16, 2018.
31. Bristol Myers Squibb press release. US Food and Drug Administration expands approval of Yervoy (ipilimumab) to include pediatric patients 12 years and older with unresectable or metastatic melanoma. https://news.bms.com/press-release/corporatefinancial-news/us-food-and-drug-administration-expands-approval-yervoy-ipilim. Released July 24, 2017. Accessed September 16, 2018.
32. Hills RK, Castaigne S, Appelbaum FR, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014;15(9):986-996.
33. Amadori S, Suciu S, Selleslag D, et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Oncol. 2016;34(9):972-979.
34. Taksin AL, Legrand O, Raffoux E, et al. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia. 2007;21(1):66-71.
35. Bhojwani D, Sposto R, Shah N, et al. Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia (R/R ALL). J Clin Oncol. 2017;35(15_suppl):10512-10512.
Rapid bacterial testing of platelets saves money, reduces waste
BOSTON – Rapid bacterial testing of platelets in a hospital blood bank can result in both significant cost savings and reduced wastage of blood products, investigators said.
Rapid bacterial testing of 6- or 7-day-old apheresis platelets resulted in projected annual cost savings of nearly $89,000 per year and cut the rate of platelet wastage from expiration by more than half, reported Adam L. Booth, MD, chief resident in the department of pathology at the University of Texas, Galveston, and his colleagues.
“When a person takes all this time to come in and donate, they do it under the impression that they’re going to help somebody, or several people, and you hate to see those platelets wasted. You want them to be used,” he said in an interview at AABB 2018, the annual meeting of the group formerly known as the American Association of Blood Banks.
Platelets typically have a shelf life of just 5 days because longer storage increases the risk for bacterial growth and the potential for transfusion-transmitted infections, Dr. Booth and his colleagues noted in a poster presentation.
A recently published Food and Drug Administration draft guidance for blood banks and transfusion services proposed changing regulations regarding bacterial control of blood products to allow for extended dating if the platelets are collected in an FDA-approved 7-day storage container with labeling that requires testing every product with a bacterial detection device, or if the platelets are individually tested for bacterial detection using an approved device.
To see what effect the regulations, if implemented as expected, might have on acquisition costs and wastage of apheresis platelets, the investigators reviewed their center’s platelet acquisition costs and wastage from expiration 12 months before and 6 months after implementation of a rapid bacterial testing protocol, with 6-month results projected out to 1 year for comparison purposes.
They looked at data on bacterial testing of 6-day and 7-day-old apheresis platelets, and excluded data on platelet units that were due to expire on day 5 because they were not stored in FDA-approved containers.
Prior to testing, 332 units at a mean per-unit cost of $516.96 were wasted, for an annual cost of more than $171,000. After the start of testing, however, the annualized rate of waste dropped to 117 units, for an annualized cost of more than $60,000. The difference – minus the cost of rapid bacterial testing – resulted in an annual savings for the institution of nearly $89,000.
Prior to rapid testing, the annual wastage rate was 24%; after testing, it dropped to an annualized 10% rate, the investigators reported.
The number of units transfused and the associated costs of transfusions were similar between the time periods studied.
“Our findings suggest that rapid bacterial testing can simultaneously enhance the safety of apheresis platelet transfusions and contribute to significant cost savings,” Dr. Booth and his colleagues wrote.
The study was internally funded. The authors reported having no conflicts of interest.
SOURCE: Booth AL et al. AABB18, Abstract INV4.
BOSTON – Rapid bacterial testing of platelets in a hospital blood bank can result in both significant cost savings and reduced wastage of blood products, investigators said.
Rapid bacterial testing of 6- or 7-day-old apheresis platelets resulted in projected annual cost savings of nearly $89,000 per year and cut the rate of platelet wastage from expiration by more than half, reported Adam L. Booth, MD, chief resident in the department of pathology at the University of Texas, Galveston, and his colleagues.
“When a person takes all this time to come in and donate, they do it under the impression that they’re going to help somebody, or several people, and you hate to see those platelets wasted. You want them to be used,” he said in an interview at AABB 2018, the annual meeting of the group formerly known as the American Association of Blood Banks.
Platelets typically have a shelf life of just 5 days because longer storage increases the risk for bacterial growth and the potential for transfusion-transmitted infections, Dr. Booth and his colleagues noted in a poster presentation.
A recently published Food and Drug Administration draft guidance for blood banks and transfusion services proposed changing regulations regarding bacterial control of blood products to allow for extended dating if the platelets are collected in an FDA-approved 7-day storage container with labeling that requires testing every product with a bacterial detection device, or if the platelets are individually tested for bacterial detection using an approved device.
To see what effect the regulations, if implemented as expected, might have on acquisition costs and wastage of apheresis platelets, the investigators reviewed their center’s platelet acquisition costs and wastage from expiration 12 months before and 6 months after implementation of a rapid bacterial testing protocol, with 6-month results projected out to 1 year for comparison purposes.
They looked at data on bacterial testing of 6-day and 7-day-old apheresis platelets, and excluded data on platelet units that were due to expire on day 5 because they were not stored in FDA-approved containers.
Prior to testing, 332 units at a mean per-unit cost of $516.96 were wasted, for an annual cost of more than $171,000. After the start of testing, however, the annualized rate of waste dropped to 117 units, for an annualized cost of more than $60,000. The difference – minus the cost of rapid bacterial testing – resulted in an annual savings for the institution of nearly $89,000.
Prior to rapid testing, the annual wastage rate was 24%; after testing, it dropped to an annualized 10% rate, the investigators reported.
The number of units transfused and the associated costs of transfusions were similar between the time periods studied.
“Our findings suggest that rapid bacterial testing can simultaneously enhance the safety of apheresis platelet transfusions and contribute to significant cost savings,” Dr. Booth and his colleagues wrote.
The study was internally funded. The authors reported having no conflicts of interest.
SOURCE: Booth AL et al. AABB18, Abstract INV4.
BOSTON – Rapid bacterial testing of platelets in a hospital blood bank can result in both significant cost savings and reduced wastage of blood products, investigators said.
Rapid bacterial testing of 6- or 7-day-old apheresis platelets resulted in projected annual cost savings of nearly $89,000 per year and cut the rate of platelet wastage from expiration by more than half, reported Adam L. Booth, MD, chief resident in the department of pathology at the University of Texas, Galveston, and his colleagues.
“When a person takes all this time to come in and donate, they do it under the impression that they’re going to help somebody, or several people, and you hate to see those platelets wasted. You want them to be used,” he said in an interview at AABB 2018, the annual meeting of the group formerly known as the American Association of Blood Banks.
Platelets typically have a shelf life of just 5 days because longer storage increases the risk for bacterial growth and the potential for transfusion-transmitted infections, Dr. Booth and his colleagues noted in a poster presentation.
A recently published Food and Drug Administration draft guidance for blood banks and transfusion services proposed changing regulations regarding bacterial control of blood products to allow for extended dating if the platelets are collected in an FDA-approved 7-day storage container with labeling that requires testing every product with a bacterial detection device, or if the platelets are individually tested for bacterial detection using an approved device.
To see what effect the regulations, if implemented as expected, might have on acquisition costs and wastage of apheresis platelets, the investigators reviewed their center’s platelet acquisition costs and wastage from expiration 12 months before and 6 months after implementation of a rapid bacterial testing protocol, with 6-month results projected out to 1 year for comparison purposes.
They looked at data on bacterial testing of 6-day and 7-day-old apheresis platelets, and excluded data on platelet units that were due to expire on day 5 because they were not stored in FDA-approved containers.
Prior to testing, 332 units at a mean per-unit cost of $516.96 were wasted, for an annual cost of more than $171,000. After the start of testing, however, the annualized rate of waste dropped to 117 units, for an annualized cost of more than $60,000. The difference – minus the cost of rapid bacterial testing – resulted in an annual savings for the institution of nearly $89,000.
Prior to rapid testing, the annual wastage rate was 24%; after testing, it dropped to an annualized 10% rate, the investigators reported.
The number of units transfused and the associated costs of transfusions were similar between the time periods studied.
“Our findings suggest that rapid bacterial testing can simultaneously enhance the safety of apheresis platelet transfusions and contribute to significant cost savings,” Dr. Booth and his colleagues wrote.
The study was internally funded. The authors reported having no conflicts of interest.
SOURCE: Booth AL et al. AABB18, Abstract INV4.
REPORTING FROM AABB18
Key clinical point:
Major finding: Annualized cost savings with rapid bacterial testing were nearly $89,000; platelet wastage decreased from 24% to 10%.
Study details: A retrospective analysis of costs and product wastage before and after implementation of rapid bacterial testing.
Disclosures: The study was internally funded. The authors reported having no conflicts of interest.
Source: Booth AL et al. AABB18, Abstract INV4.
Few clinical outcomes convincingly linked to sickle cell trait
Although sickle cell trait (SCT) has been linked to numerous adverse clinical outcomes in multiple studies, only a handful of those associations have strong supporting evidence, results of a systematic review suggest.
Venous and renal complications had the strongest evidence supporting an association with SCT, while exertion-related sudden death – perhaps the highest-profile potential complication of SCT – had moderate-strength evidence supporting a link, according to the review.
By contrast, most other associations between SCT and clinical outcomes had either low-strength evidence or insufficient data to support a link, according to Rakhi P. Naik, MD, of Johns Hopkins University, Baltimore, and coauthors of the review.
“Future rigorous studies are needed to address potential complications of SCT and to determine modifiers of risk,” they wrote. The report in the Annals of Internal Medicine.
The systematic review by Dr. Naik and colleagues focused on 41 studies, most of which were population-based cohort or case-control studies. They rated the evidence quality of each study and grouped 24 clinical outcomes of interest into six categories: exertion-related injury, renal, vascular, pediatric, surgery- and trauma-related outcomes, and mortality.
Exercise-related injury has received considerable attention, particularly in relation to the military and athletics.
The strength of evidence for a link between SCT and exertion-related death was low in their analysis, which included two studies evaluating the outcome. However, Dr. Naik and coauthors did note that SCT may be associated with a small absolute risk of exertion-related death in extreme conditions such a highly strenuous athletic training or the military.
“We do concur with the American Society of Hematology statement recommending against routine SCT screening in athletics and supporting the consistent use of universal precautions to mitigate exertion-related risk in all persons, regardless of SCT status,” they wrote.
Similarly, the absolute risk of exertional rhabdomyolysis in SCT is small and probably occurs only in high-intensity settings, with risk modified by other genetic and environmental factors, Dr. Naik and coauthors said, based on their analysis of two studies looking at this outcome.
Venous complications had a stronger body of evidence, including several studies showing high levels of procoagulants, which makes elevated venous thromboembolism risk plausible in individuals with SCT.
High-strength evidence linked pulmonary embolism, with or without deep-vein thrombosis, to SCT. In contrast, there was no increased risk of isolated deep-vein thrombosis in these individuals.
“The cause of this paradoxical observation is unknown but may be an increased risk for clot embolization in SCT,” Dr. Naik and colleagues wrote in a discussion of the results.
Renal outcomes were often attributed to SCT, and in this review, the authors said there was evidence to support SCT as a risk factor for both proteinuria and chronic kidney disease.
Out of six studies looking at proteinuria, the one high-quality study found a 1.86-fold increased risk for baseline albuminuria in African Americans with SCT versus those without, according to the review.
Out of four studies looking at chronic kidney disease, the two high-quality studies found 1.57- to 1.89-fold increased risk of those outcomes in African Americans with SCT.
Support for the study came in part from the National Human Genome Research Institute and the National Heart, Lung, and Blood Institute. The authors reported disclosures related to Novartis, Addmedica, and Global Blood Therapeutics, among others.
SOURCE: Naik RP et al. Ann Intern Med. 2018 Oct 30. doi:10.7326/M18-1161.
Although sickle cell trait (SCT) has been linked to numerous adverse clinical outcomes in multiple studies, only a handful of those associations have strong supporting evidence, results of a systematic review suggest.
Venous and renal complications had the strongest evidence supporting an association with SCT, while exertion-related sudden death – perhaps the highest-profile potential complication of SCT – had moderate-strength evidence supporting a link, according to the review.
By contrast, most other associations between SCT and clinical outcomes had either low-strength evidence or insufficient data to support a link, according to Rakhi P. Naik, MD, of Johns Hopkins University, Baltimore, and coauthors of the review.
“Future rigorous studies are needed to address potential complications of SCT and to determine modifiers of risk,” they wrote. The report in the Annals of Internal Medicine.
The systematic review by Dr. Naik and colleagues focused on 41 studies, most of which were population-based cohort or case-control studies. They rated the evidence quality of each study and grouped 24 clinical outcomes of interest into six categories: exertion-related injury, renal, vascular, pediatric, surgery- and trauma-related outcomes, and mortality.
Exercise-related injury has received considerable attention, particularly in relation to the military and athletics.
The strength of evidence for a link between SCT and exertion-related death was low in their analysis, which included two studies evaluating the outcome. However, Dr. Naik and coauthors did note that SCT may be associated with a small absolute risk of exertion-related death in extreme conditions such a highly strenuous athletic training or the military.
“We do concur with the American Society of Hematology statement recommending against routine SCT screening in athletics and supporting the consistent use of universal precautions to mitigate exertion-related risk in all persons, regardless of SCT status,” they wrote.
Similarly, the absolute risk of exertional rhabdomyolysis in SCT is small and probably occurs only in high-intensity settings, with risk modified by other genetic and environmental factors, Dr. Naik and coauthors said, based on their analysis of two studies looking at this outcome.
Venous complications had a stronger body of evidence, including several studies showing high levels of procoagulants, which makes elevated venous thromboembolism risk plausible in individuals with SCT.
High-strength evidence linked pulmonary embolism, with or without deep-vein thrombosis, to SCT. In contrast, there was no increased risk of isolated deep-vein thrombosis in these individuals.
“The cause of this paradoxical observation is unknown but may be an increased risk for clot embolization in SCT,” Dr. Naik and colleagues wrote in a discussion of the results.
Renal outcomes were often attributed to SCT, and in this review, the authors said there was evidence to support SCT as a risk factor for both proteinuria and chronic kidney disease.
Out of six studies looking at proteinuria, the one high-quality study found a 1.86-fold increased risk for baseline albuminuria in African Americans with SCT versus those without, according to the review.
Out of four studies looking at chronic kidney disease, the two high-quality studies found 1.57- to 1.89-fold increased risk of those outcomes in African Americans with SCT.
Support for the study came in part from the National Human Genome Research Institute and the National Heart, Lung, and Blood Institute. The authors reported disclosures related to Novartis, Addmedica, and Global Blood Therapeutics, among others.
SOURCE: Naik RP et al. Ann Intern Med. 2018 Oct 30. doi:10.7326/M18-1161.
Although sickle cell trait (SCT) has been linked to numerous adverse clinical outcomes in multiple studies, only a handful of those associations have strong supporting evidence, results of a systematic review suggest.
Venous and renal complications had the strongest evidence supporting an association with SCT, while exertion-related sudden death – perhaps the highest-profile potential complication of SCT – had moderate-strength evidence supporting a link, according to the review.
By contrast, most other associations between SCT and clinical outcomes had either low-strength evidence or insufficient data to support a link, according to Rakhi P. Naik, MD, of Johns Hopkins University, Baltimore, and coauthors of the review.
“Future rigorous studies are needed to address potential complications of SCT and to determine modifiers of risk,” they wrote. The report in the Annals of Internal Medicine.
The systematic review by Dr. Naik and colleagues focused on 41 studies, most of which were population-based cohort or case-control studies. They rated the evidence quality of each study and grouped 24 clinical outcomes of interest into six categories: exertion-related injury, renal, vascular, pediatric, surgery- and trauma-related outcomes, and mortality.
Exercise-related injury has received considerable attention, particularly in relation to the military and athletics.
The strength of evidence for a link between SCT and exertion-related death was low in their analysis, which included two studies evaluating the outcome. However, Dr. Naik and coauthors did note that SCT may be associated with a small absolute risk of exertion-related death in extreme conditions such a highly strenuous athletic training or the military.
“We do concur with the American Society of Hematology statement recommending against routine SCT screening in athletics and supporting the consistent use of universal precautions to mitigate exertion-related risk in all persons, regardless of SCT status,” they wrote.
Similarly, the absolute risk of exertional rhabdomyolysis in SCT is small and probably occurs only in high-intensity settings, with risk modified by other genetic and environmental factors, Dr. Naik and coauthors said, based on their analysis of two studies looking at this outcome.
Venous complications had a stronger body of evidence, including several studies showing high levels of procoagulants, which makes elevated venous thromboembolism risk plausible in individuals with SCT.
High-strength evidence linked pulmonary embolism, with or without deep-vein thrombosis, to SCT. In contrast, there was no increased risk of isolated deep-vein thrombosis in these individuals.
“The cause of this paradoxical observation is unknown but may be an increased risk for clot embolization in SCT,” Dr. Naik and colleagues wrote in a discussion of the results.
Renal outcomes were often attributed to SCT, and in this review, the authors said there was evidence to support SCT as a risk factor for both proteinuria and chronic kidney disease.
Out of six studies looking at proteinuria, the one high-quality study found a 1.86-fold increased risk for baseline albuminuria in African Americans with SCT versus those without, according to the review.
Out of four studies looking at chronic kidney disease, the two high-quality studies found 1.57- to 1.89-fold increased risk of those outcomes in African Americans with SCT.
Support for the study came in part from the National Human Genome Research Institute and the National Heart, Lung, and Blood Institute. The authors reported disclosures related to Novartis, Addmedica, and Global Blood Therapeutics, among others.
SOURCE: Naik RP et al. Ann Intern Med. 2018 Oct 30. doi:10.7326/M18-1161.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point:
Major finding: Risks of 1.57-fold and higher were seen in high-quality studies linking SCT to venous and renal complications, while studies of moderate quality suggested small absolute risks of exertion-related mortality or rhabdomyolysis.
Study details: A systematic review including 41 mostly population-based cohort or case-control studies looking at 24 clinical outcomes of interest.
Disclosures: Support for the study came in part from the National Human Genome Research Institute and the National Heart, Lung, and Blood Institute. The authors reported disclosures related to Novartis, Addmedica, and Global Blood Therapeutics, among others.
Source: Naik RP et al. Ann Intern Med. 2018 Oct 30. doi:10.7326/M18-1161.
Improved treatments emerge for hemophilia patients with high-titer inhibitors
For patients with hemophilia and high-titer inhibitors, a new era of treatment has begun with the development of improved variants of traditional bypassing agents and novel, nonfactor-based, prophylactic agents, say authors of a recent review article.
“These new agents may transform the treatment of inhibitor patients and inhibitor-related bleeds, potentially decreasing morbidity and mortality and improving patients’ quality of life,” Amy D. Shapiro, MD, and coauthors wrote in the Journal of Thrombosis and Haemostasis.
Until recently, the only two bypassing agents available were activated prothrombin complex concentrates and recombinant factor VIIa, noted Dr. Shapiro, who is CEO and co-medical director of the Indiana Hemophilia and Thrombosis Center, Indianapolis, and her coauthors.
The first of the novel targeted agents, emicizumab, is a humanized, bispecific monoclonal antibody that was approved for prophylactic use in hemophilia A in the United States in November 2017.
Emicizumab could transform the treatment of patients with inhibitors, but it also requires reconsideration of how to treat breakthrough bleeds and eliminate the underlying inhibitor, the authors said.
In a phase 3 study, this biologic significantly decreased the annualized bleeding rate by 87% versus on-demand bypassing agent therapy, and by 79% versus a prophylactic bypassing agent regimen, they said, noting that 63% of patients had no bleeding events during the study.
Serious adverse events were seen in patients receiving emicizumab prophylaxis, including thrombosis in 2 out of 103 subjects and thrombotic microangiopathy in 3. In all cases, the patients were treating breakthrough bleeds with activated prothrombin complex concentrate, Dr. Shapiro and coauthors wrote.
Other novel agents in clinical development include fitusiran and tissue factor pathway inhibitors, which each target a different natural anticoagulant and could result in new prophylactic options, according to the study coauthors.
“The possibility of multiple therapeutic targets may allow for a highly personalized approach to prophylaxis therapy, with traditional bypassing agents providing options when breakthrough bleeds occur,” they wrote.
In the meantime, eptacog alfa is the “de facto standard” for recombinant factor VIIa, though a new variant under development, eptacog beta, has been accepted for regulatory review in the United States. In a phase 3 clinical trial, eptacog beta appeared to provide improved efficacy and decreased dosing requirements, possibly due to increased binding affinity to endothelial protein C receptor (EPCR), the authors said.
“The addition of improved rFVIIa variants with unique pharmacological and pharmacokinetic profiles will provide new tools to treat bleeding events in inhibitor patients,” Dr. Shapiro and her colleagues wrote.
The use of traditional bypassing agents is expected to decrease over time as new and improved therapeutics are developed. Traditional agents, however, will “remain a necessity” for breakthrough bleeds in hemophilia A patients with inhibitors, and until novel agents become available for hemophilia B, the authors said.
The review article was supported by an unrestricted educational grant from HEMA Biologics, LLC, which had no involvement or editorial control in research, writing or submission. Dr. Shapiro reported disclosures related to HEMA Biologics, Shire, Novo Nordisk, Kedrion Biopharma, Bioverativ, and Genentech.
SOURCE: Shapiro AD, et al. J Thromb Haemost. 2018 Sep 28.
For patients with hemophilia and high-titer inhibitors, a new era of treatment has begun with the development of improved variants of traditional bypassing agents and novel, nonfactor-based, prophylactic agents, say authors of a recent review article.
“These new agents may transform the treatment of inhibitor patients and inhibitor-related bleeds, potentially decreasing morbidity and mortality and improving patients’ quality of life,” Amy D. Shapiro, MD, and coauthors wrote in the Journal of Thrombosis and Haemostasis.
Until recently, the only two bypassing agents available were activated prothrombin complex concentrates and recombinant factor VIIa, noted Dr. Shapiro, who is CEO and co-medical director of the Indiana Hemophilia and Thrombosis Center, Indianapolis, and her coauthors.
The first of the novel targeted agents, emicizumab, is a humanized, bispecific monoclonal antibody that was approved for prophylactic use in hemophilia A in the United States in November 2017.
Emicizumab could transform the treatment of patients with inhibitors, but it also requires reconsideration of how to treat breakthrough bleeds and eliminate the underlying inhibitor, the authors said.
In a phase 3 study, this biologic significantly decreased the annualized bleeding rate by 87% versus on-demand bypassing agent therapy, and by 79% versus a prophylactic bypassing agent regimen, they said, noting that 63% of patients had no bleeding events during the study.
Serious adverse events were seen in patients receiving emicizumab prophylaxis, including thrombosis in 2 out of 103 subjects and thrombotic microangiopathy in 3. In all cases, the patients were treating breakthrough bleeds with activated prothrombin complex concentrate, Dr. Shapiro and coauthors wrote.
Other novel agents in clinical development include fitusiran and tissue factor pathway inhibitors, which each target a different natural anticoagulant and could result in new prophylactic options, according to the study coauthors.
“The possibility of multiple therapeutic targets may allow for a highly personalized approach to prophylaxis therapy, with traditional bypassing agents providing options when breakthrough bleeds occur,” they wrote.
In the meantime, eptacog alfa is the “de facto standard” for recombinant factor VIIa, though a new variant under development, eptacog beta, has been accepted for regulatory review in the United States. In a phase 3 clinical trial, eptacog beta appeared to provide improved efficacy and decreased dosing requirements, possibly due to increased binding affinity to endothelial protein C receptor (EPCR), the authors said.
“The addition of improved rFVIIa variants with unique pharmacological and pharmacokinetic profiles will provide new tools to treat bleeding events in inhibitor patients,” Dr. Shapiro and her colleagues wrote.
The use of traditional bypassing agents is expected to decrease over time as new and improved therapeutics are developed. Traditional agents, however, will “remain a necessity” for breakthrough bleeds in hemophilia A patients with inhibitors, and until novel agents become available for hemophilia B, the authors said.
The review article was supported by an unrestricted educational grant from HEMA Biologics, LLC, which had no involvement or editorial control in research, writing or submission. Dr. Shapiro reported disclosures related to HEMA Biologics, Shire, Novo Nordisk, Kedrion Biopharma, Bioverativ, and Genentech.
SOURCE: Shapiro AD, et al. J Thromb Haemost. 2018 Sep 28.
For patients with hemophilia and high-titer inhibitors, a new era of treatment has begun with the development of improved variants of traditional bypassing agents and novel, nonfactor-based, prophylactic agents, say authors of a recent review article.
“These new agents may transform the treatment of inhibitor patients and inhibitor-related bleeds, potentially decreasing morbidity and mortality and improving patients’ quality of life,” Amy D. Shapiro, MD, and coauthors wrote in the Journal of Thrombosis and Haemostasis.
Until recently, the only two bypassing agents available were activated prothrombin complex concentrates and recombinant factor VIIa, noted Dr. Shapiro, who is CEO and co-medical director of the Indiana Hemophilia and Thrombosis Center, Indianapolis, and her coauthors.
The first of the novel targeted agents, emicizumab, is a humanized, bispecific monoclonal antibody that was approved for prophylactic use in hemophilia A in the United States in November 2017.
Emicizumab could transform the treatment of patients with inhibitors, but it also requires reconsideration of how to treat breakthrough bleeds and eliminate the underlying inhibitor, the authors said.
In a phase 3 study, this biologic significantly decreased the annualized bleeding rate by 87% versus on-demand bypassing agent therapy, and by 79% versus a prophylactic bypassing agent regimen, they said, noting that 63% of patients had no bleeding events during the study.
Serious adverse events were seen in patients receiving emicizumab prophylaxis, including thrombosis in 2 out of 103 subjects and thrombotic microangiopathy in 3. In all cases, the patients were treating breakthrough bleeds with activated prothrombin complex concentrate, Dr. Shapiro and coauthors wrote.
Other novel agents in clinical development include fitusiran and tissue factor pathway inhibitors, which each target a different natural anticoagulant and could result in new prophylactic options, according to the study coauthors.
“The possibility of multiple therapeutic targets may allow for a highly personalized approach to prophylaxis therapy, with traditional bypassing agents providing options when breakthrough bleeds occur,” they wrote.
In the meantime, eptacog alfa is the “de facto standard” for recombinant factor VIIa, though a new variant under development, eptacog beta, has been accepted for regulatory review in the United States. In a phase 3 clinical trial, eptacog beta appeared to provide improved efficacy and decreased dosing requirements, possibly due to increased binding affinity to endothelial protein C receptor (EPCR), the authors said.
“The addition of improved rFVIIa variants with unique pharmacological and pharmacokinetic profiles will provide new tools to treat bleeding events in inhibitor patients,” Dr. Shapiro and her colleagues wrote.
The use of traditional bypassing agents is expected to decrease over time as new and improved therapeutics are developed. Traditional agents, however, will “remain a necessity” for breakthrough bleeds in hemophilia A patients with inhibitors, and until novel agents become available for hemophilia B, the authors said.
The review article was supported by an unrestricted educational grant from HEMA Biologics, LLC, which had no involvement or editorial control in research, writing or submission. Dr. Shapiro reported disclosures related to HEMA Biologics, Shire, Novo Nordisk, Kedrion Biopharma, Bioverativ, and Genentech.
SOURCE: Shapiro AD, et al. J Thromb Haemost. 2018 Sep 28.
FROM THE JOURNAL OF THROMBOSIS AND HAEMOSTASIS
Cardiovascular disease risk unchanged in men with hemophilia A
Concerns may be unfounded for risks of earlier-onset cardiovascular disease in men with hemophilia A, according to investigators.
Cardiovascular comorbidities between groups were generally comparable, regardless of hemophilia A status, reported lead author Thomas J. Humphries, MD, of Bayer, and his colleagues.
“To date, there have been conflicting data in the literature regarding the risks of [cardiovascular] comorbidities in patients with hemophilia A, compared with the general population,” the investigators wrote in Advances in Medical Sciences. “Some studies have reported lower mortality from [cardiovascular] diseases and/or decreased atherogenesis in patients with hemophilia … conversely, other reports indicate comparable or higher [cardiovascular] comorbidities in patients with hemophilia, compared with the general population.”
In two previous commercial database reviews conducted by Dr. Humphries and his colleagues, cardiovascular disease appeared to occur more commonly and at a younger age in men with hemophilia A. More concerning, patients aged under 40 years showed elevated incidence of stroke and thrombosis. The authors sought to clarify these findings in the present study.
The retrospective chart review involved 74 men with hemophilia A and 222 men without the condition, matched by study year, payer type, race, and age. Patients presented at any of 31 medical facilities within the Henry Ford Health System in Detroit. Diagnoses were made between Jan. 1, 1995, and Dec. 31, 2014.
For the most part there were no significant differences in cardiovascular disease prevalence between the two cohorts. Rates of hypertension, obesity, coronary artery disease, heart failure, stroke, venous and arterial thrombosis, ventricular arrhythmias, atrial fibrillation, and chronic renal disease were numerically higher in the control group, but those differences were not statistically significant. There were significantly higher prevalence rates for diabetes (P = .0108) and hyperlipidemia (P = .0001) in the control group versus patients with hemophilia A.
The investigators pointed out that meaningful statistical differences using standardized differences were not reached for venous and arterial thrombosis and atrial fibrillation.
“It is worth noting that in the hemophilia A group, hypertension appeared first in the 18- to 29-year age group, as did venous thrombosis,” the investigators wrote, suggesting that monitoring, starting in the late teens, may be warranted.
The investigators also noted multiple study limitations, notably the small sample size, compared with commercial databases that were reviewed in previous studies. Additionally, the severity of disease was unknown for some of the hemophilia A patients and the study only followed patients for 1 year.
“The results of this retrospective chart review did not confirm diffuse statistically significant differences in [cardiovascular] comorbidities and their earlier onset in hemophilia A versus controls,” the investigators concluded.
The study was funded by Bayer. Three of the authors were employed by Bayer when the study was conducted. Other authors reported employment with Xcenda and the Henry Ford Health System and research funding from Xcenda.
SOURCE: Humphries TJ et al. Adv Med Sci. 2018;63(2):329-33.
Concerns may be unfounded for risks of earlier-onset cardiovascular disease in men with hemophilia A, according to investigators.
Cardiovascular comorbidities between groups were generally comparable, regardless of hemophilia A status, reported lead author Thomas J. Humphries, MD, of Bayer, and his colleagues.
“To date, there have been conflicting data in the literature regarding the risks of [cardiovascular] comorbidities in patients with hemophilia A, compared with the general population,” the investigators wrote in Advances in Medical Sciences. “Some studies have reported lower mortality from [cardiovascular] diseases and/or decreased atherogenesis in patients with hemophilia … conversely, other reports indicate comparable or higher [cardiovascular] comorbidities in patients with hemophilia, compared with the general population.”
In two previous commercial database reviews conducted by Dr. Humphries and his colleagues, cardiovascular disease appeared to occur more commonly and at a younger age in men with hemophilia A. More concerning, patients aged under 40 years showed elevated incidence of stroke and thrombosis. The authors sought to clarify these findings in the present study.
The retrospective chart review involved 74 men with hemophilia A and 222 men without the condition, matched by study year, payer type, race, and age. Patients presented at any of 31 medical facilities within the Henry Ford Health System in Detroit. Diagnoses were made between Jan. 1, 1995, and Dec. 31, 2014.
For the most part there were no significant differences in cardiovascular disease prevalence between the two cohorts. Rates of hypertension, obesity, coronary artery disease, heart failure, stroke, venous and arterial thrombosis, ventricular arrhythmias, atrial fibrillation, and chronic renal disease were numerically higher in the control group, but those differences were not statistically significant. There were significantly higher prevalence rates for diabetes (P = .0108) and hyperlipidemia (P = .0001) in the control group versus patients with hemophilia A.
The investigators pointed out that meaningful statistical differences using standardized differences were not reached for venous and arterial thrombosis and atrial fibrillation.
“It is worth noting that in the hemophilia A group, hypertension appeared first in the 18- to 29-year age group, as did venous thrombosis,” the investigators wrote, suggesting that monitoring, starting in the late teens, may be warranted.
The investigators also noted multiple study limitations, notably the small sample size, compared with commercial databases that were reviewed in previous studies. Additionally, the severity of disease was unknown for some of the hemophilia A patients and the study only followed patients for 1 year.
“The results of this retrospective chart review did not confirm diffuse statistically significant differences in [cardiovascular] comorbidities and their earlier onset in hemophilia A versus controls,” the investigators concluded.
The study was funded by Bayer. Three of the authors were employed by Bayer when the study was conducted. Other authors reported employment with Xcenda and the Henry Ford Health System and research funding from Xcenda.
SOURCE: Humphries TJ et al. Adv Med Sci. 2018;63(2):329-33.
Concerns may be unfounded for risks of earlier-onset cardiovascular disease in men with hemophilia A, according to investigators.
Cardiovascular comorbidities between groups were generally comparable, regardless of hemophilia A status, reported lead author Thomas J. Humphries, MD, of Bayer, and his colleagues.
“To date, there have been conflicting data in the literature regarding the risks of [cardiovascular] comorbidities in patients with hemophilia A, compared with the general population,” the investigators wrote in Advances in Medical Sciences. “Some studies have reported lower mortality from [cardiovascular] diseases and/or decreased atherogenesis in patients with hemophilia … conversely, other reports indicate comparable or higher [cardiovascular] comorbidities in patients with hemophilia, compared with the general population.”
In two previous commercial database reviews conducted by Dr. Humphries and his colleagues, cardiovascular disease appeared to occur more commonly and at a younger age in men with hemophilia A. More concerning, patients aged under 40 years showed elevated incidence of stroke and thrombosis. The authors sought to clarify these findings in the present study.
The retrospective chart review involved 74 men with hemophilia A and 222 men without the condition, matched by study year, payer type, race, and age. Patients presented at any of 31 medical facilities within the Henry Ford Health System in Detroit. Diagnoses were made between Jan. 1, 1995, and Dec. 31, 2014.
For the most part there were no significant differences in cardiovascular disease prevalence between the two cohorts. Rates of hypertension, obesity, coronary artery disease, heart failure, stroke, venous and arterial thrombosis, ventricular arrhythmias, atrial fibrillation, and chronic renal disease were numerically higher in the control group, but those differences were not statistically significant. There were significantly higher prevalence rates for diabetes (P = .0108) and hyperlipidemia (P = .0001) in the control group versus patients with hemophilia A.
The investigators pointed out that meaningful statistical differences using standardized differences were not reached for venous and arterial thrombosis and atrial fibrillation.
“It is worth noting that in the hemophilia A group, hypertension appeared first in the 18- to 29-year age group, as did venous thrombosis,” the investigators wrote, suggesting that monitoring, starting in the late teens, may be warranted.
The investigators also noted multiple study limitations, notably the small sample size, compared with commercial databases that were reviewed in previous studies. Additionally, the severity of disease was unknown for some of the hemophilia A patients and the study only followed patients for 1 year.
“The results of this retrospective chart review did not confirm diffuse statistically significant differences in [cardiovascular] comorbidities and their earlier onset in hemophilia A versus controls,” the investigators concluded.
The study was funded by Bayer. Three of the authors were employed by Bayer when the study was conducted. Other authors reported employment with Xcenda and the Henry Ford Health System and research funding from Xcenda.
SOURCE: Humphries TJ et al. Adv Med Sci. 2018;63(2):329-33.
FROM ADVANCES IN MEDICAL SCIENCES
Key clinical point:
Major finding: Prevalence rates of diabetes (P = .0108) and hyperlipidemia (P = .0001) were higher in the control group, compared with patients with hemophilia A.
Study details: A retrospective chart review involving 74 men with hemophilia A and 222 men without the condition, matched by study year, payer type, race, and age.
Disclosures: The study was funded by Bayer. Three authors were employed by Bayer when the study was conducted. Other authors reported employment by Xcenda and the Henry Ford Health System and research funding from Xcenda.
Source: Humphries TJ et al. Adv Med Sci. 2018;63(2):329-33.
Kymriah appears cost effective in analysis
The high price of chimeric antigen receptor (CAR) T-cell therapy for pediatric leukemia may prove cost effective if long-term survival benefits are realized, researchers reported.
A cost-effectiveness analysis of the CAR T-cell therapy tisagenlecleucel suggests that the $475,000 price tag is in alignment with the lifetime benefits of the treatment. The findings were published in JAMA Pediatrics.
Tisagenlecleucel – marketed as Kymriah – is a one-dose treatment for relapsed or refractory pediatric B-cell acute lymphoblastic leukemia (ALL) and the first CAR T-cell therapy approved by the Food and Drug Administration.
In this cost-effectiveness analysis, researchers used a decision analytic model that extrapolated the evidence from clinical trials over a patient’s lifetime to assess life-years gained, quality-adjusted life-years (QALYs) gained, and incremental costs per life-year and QALY gained. The comparator was the chemoimmunotherapeutic agent clofarabine.
While tisagenlecleucel has a list price of $475,000, researchers discounted the price by 3% and added several additional costs, such as hospital administration, pretreatment, and potential adverse events, to get to a total discounted cost of about $667,000. They estimated that 42.6% of patients were considered to be long-term survivors with tisagenlecleucel, 10.34 life-years would be gained, and 9.28 QALYs would be gained.
In comparison, clofarabine had a total discounted cost of approximately $337,000 (including an initial discounted price of $164,000 plus additional treatment and administrative costs), 10.8% of patients were long-term survivors, 2.43 life-years were gained, and 2.10 QALYs were gained in the model.
Overall, the mean incremental cost-effectiveness ratio was about $46,000 per QALY gained in this base-case model.
In analyses of different scenarios, such as a deeper discount, a different treatment start, or a different calculation of future treatment costs, the cost-effectiveness ratio varied from $37,000 to $78,000 per QALY gained.
“We acknowledge that considerable uncertainty remains around the long-term benefit of tisagenlecleucel owing to limited available evidence; however, with current evidence and assumptions, tisagenlecleucel meets commonly cited value thresholds over a patient lifetime horizon, assuming payment for treatment acquisition for responders at 1 month,” wrote Melanie D. Whittington, PhD, from the University of Colorado at Denver, Aurora, and her colleagues.
The authors noted that the clinical trial evidence for tisagenlecleucel came from single-arm trials, which made selection of a comparator challenging. Clofarabine was chosen because it had the most similar baseline population characteristics, but they acknowledged that blinatumomab was also frequently used as a treatment for these patients.
“We suspect that tisagenlecleucel would remain cost effective, compared with blinatumomab,” they wrote. “A study conducted by other researchers found the incremental cost-effectiveness ratio of tisagenlecleucel versus blinatumomab was similar to the incremental cost-effectiveness ratio of tisagenlecleucel versus clofarabine [i.e., $3,000 more per QALY].”
The authors suggested that uncertainties in the evidence should be considered as payers are negotiating coverage and payment for tisagenlecleucel.
“Novel payment models consistent with the present evidence may reduce the risk and uncertainty in long-term value and be more closely aligned with ensuring high-value care,” they wrote. “Financing cures in the United States is challenging, owing to the high up-front price, rapid uptake, and uncertainty in long-term outcomes; however, innovative payment models are an opportunity to address some of these challenges and to promote patient access to novel and promising therapies.”
The study was funded by the Institute for Clinical and Economic Review, which receives some funding from the pharmaceutical industry. Four authors are employees of the Institute for Clinical and Economic Review.
SOURCE: Whittington MD et al. JAMA Pediatr. 2018 Oct 8. doi: 10.1001/jamapediatrics.2018.2530.
The high price of chimeric antigen receptor (CAR) T-cell therapy for pediatric leukemia may prove cost effective if long-term survival benefits are realized, researchers reported.
A cost-effectiveness analysis of the CAR T-cell therapy tisagenlecleucel suggests that the $475,000 price tag is in alignment with the lifetime benefits of the treatment. The findings were published in JAMA Pediatrics.
Tisagenlecleucel – marketed as Kymriah – is a one-dose treatment for relapsed or refractory pediatric B-cell acute lymphoblastic leukemia (ALL) and the first CAR T-cell therapy approved by the Food and Drug Administration.
In this cost-effectiveness analysis, researchers used a decision analytic model that extrapolated the evidence from clinical trials over a patient’s lifetime to assess life-years gained, quality-adjusted life-years (QALYs) gained, and incremental costs per life-year and QALY gained. The comparator was the chemoimmunotherapeutic agent clofarabine.
While tisagenlecleucel has a list price of $475,000, researchers discounted the price by 3% and added several additional costs, such as hospital administration, pretreatment, and potential adverse events, to get to a total discounted cost of about $667,000. They estimated that 42.6% of patients were considered to be long-term survivors with tisagenlecleucel, 10.34 life-years would be gained, and 9.28 QALYs would be gained.
In comparison, clofarabine had a total discounted cost of approximately $337,000 (including an initial discounted price of $164,000 plus additional treatment and administrative costs), 10.8% of patients were long-term survivors, 2.43 life-years were gained, and 2.10 QALYs were gained in the model.
Overall, the mean incremental cost-effectiveness ratio was about $46,000 per QALY gained in this base-case model.
In analyses of different scenarios, such as a deeper discount, a different treatment start, or a different calculation of future treatment costs, the cost-effectiveness ratio varied from $37,000 to $78,000 per QALY gained.
“We acknowledge that considerable uncertainty remains around the long-term benefit of tisagenlecleucel owing to limited available evidence; however, with current evidence and assumptions, tisagenlecleucel meets commonly cited value thresholds over a patient lifetime horizon, assuming payment for treatment acquisition for responders at 1 month,” wrote Melanie D. Whittington, PhD, from the University of Colorado at Denver, Aurora, and her colleagues.
The authors noted that the clinical trial evidence for tisagenlecleucel came from single-arm trials, which made selection of a comparator challenging. Clofarabine was chosen because it had the most similar baseline population characteristics, but they acknowledged that blinatumomab was also frequently used as a treatment for these patients.
“We suspect that tisagenlecleucel would remain cost effective, compared with blinatumomab,” they wrote. “A study conducted by other researchers found the incremental cost-effectiveness ratio of tisagenlecleucel versus blinatumomab was similar to the incremental cost-effectiveness ratio of tisagenlecleucel versus clofarabine [i.e., $3,000 more per QALY].”
The authors suggested that uncertainties in the evidence should be considered as payers are negotiating coverage and payment for tisagenlecleucel.
“Novel payment models consistent with the present evidence may reduce the risk and uncertainty in long-term value and be more closely aligned with ensuring high-value care,” they wrote. “Financing cures in the United States is challenging, owing to the high up-front price, rapid uptake, and uncertainty in long-term outcomes; however, innovative payment models are an opportunity to address some of these challenges and to promote patient access to novel and promising therapies.”
The study was funded by the Institute for Clinical and Economic Review, which receives some funding from the pharmaceutical industry. Four authors are employees of the Institute for Clinical and Economic Review.
SOURCE: Whittington MD et al. JAMA Pediatr. 2018 Oct 8. doi: 10.1001/jamapediatrics.2018.2530.
The high price of chimeric antigen receptor (CAR) T-cell therapy for pediatric leukemia may prove cost effective if long-term survival benefits are realized, researchers reported.
A cost-effectiveness analysis of the CAR T-cell therapy tisagenlecleucel suggests that the $475,000 price tag is in alignment with the lifetime benefits of the treatment. The findings were published in JAMA Pediatrics.
Tisagenlecleucel – marketed as Kymriah – is a one-dose treatment for relapsed or refractory pediatric B-cell acute lymphoblastic leukemia (ALL) and the first CAR T-cell therapy approved by the Food and Drug Administration.
In this cost-effectiveness analysis, researchers used a decision analytic model that extrapolated the evidence from clinical trials over a patient’s lifetime to assess life-years gained, quality-adjusted life-years (QALYs) gained, and incremental costs per life-year and QALY gained. The comparator was the chemoimmunotherapeutic agent clofarabine.
While tisagenlecleucel has a list price of $475,000, researchers discounted the price by 3% and added several additional costs, such as hospital administration, pretreatment, and potential adverse events, to get to a total discounted cost of about $667,000. They estimated that 42.6% of patients were considered to be long-term survivors with tisagenlecleucel, 10.34 life-years would be gained, and 9.28 QALYs would be gained.
In comparison, clofarabine had a total discounted cost of approximately $337,000 (including an initial discounted price of $164,000 plus additional treatment and administrative costs), 10.8% of patients were long-term survivors, 2.43 life-years were gained, and 2.10 QALYs were gained in the model.
Overall, the mean incremental cost-effectiveness ratio was about $46,000 per QALY gained in this base-case model.
In analyses of different scenarios, such as a deeper discount, a different treatment start, or a different calculation of future treatment costs, the cost-effectiveness ratio varied from $37,000 to $78,000 per QALY gained.
“We acknowledge that considerable uncertainty remains around the long-term benefit of tisagenlecleucel owing to limited available evidence; however, with current evidence and assumptions, tisagenlecleucel meets commonly cited value thresholds over a patient lifetime horizon, assuming payment for treatment acquisition for responders at 1 month,” wrote Melanie D. Whittington, PhD, from the University of Colorado at Denver, Aurora, and her colleagues.
The authors noted that the clinical trial evidence for tisagenlecleucel came from single-arm trials, which made selection of a comparator challenging. Clofarabine was chosen because it had the most similar baseline population characteristics, but they acknowledged that blinatumomab was also frequently used as a treatment for these patients.
“We suspect that tisagenlecleucel would remain cost effective, compared with blinatumomab,” they wrote. “A study conducted by other researchers found the incremental cost-effectiveness ratio of tisagenlecleucel versus blinatumomab was similar to the incremental cost-effectiveness ratio of tisagenlecleucel versus clofarabine [i.e., $3,000 more per QALY].”
The authors suggested that uncertainties in the evidence should be considered as payers are negotiating coverage and payment for tisagenlecleucel.
“Novel payment models consistent with the present evidence may reduce the risk and uncertainty in long-term value and be more closely aligned with ensuring high-value care,” they wrote. “Financing cures in the United States is challenging, owing to the high up-front price, rapid uptake, and uncertainty in long-term outcomes; however, innovative payment models are an opportunity to address some of these challenges and to promote patient access to novel and promising therapies.”
The study was funded by the Institute for Clinical and Economic Review, which receives some funding from the pharmaceutical industry. Four authors are employees of the Institute for Clinical and Economic Review.
SOURCE: Whittington MD et al. JAMA Pediatr. 2018 Oct 8. doi: 10.1001/jamapediatrics.2018.2530.
FROM JAMA PEDIATRICS
Key clinical point:
Major finding: The incremental cost-effectiveness ratio for tisagenlecleucel versus clofarabine ranged from $37,000 to $78,000 per quality-adjusted life year gained.
Study details: A cost-effectiveness analysis comparing tisagenlecleucel with clofarabine monotherapy.
Disclosures: The study was funded by the Institute for Clinical and Economic Review, which receives some funding from the pharmaceutical industry. Four authors are employees of the Institute for Clinical and Economic Review.
Source: Whittington MD et al. JAMA Pediatr. 2018 Oct 8. doi: 10.1001/jamapediatrics.2018.2530.
Protocol violations, missed transfusions among blood delivery errors
BOSTON – Even the most vigilant hospitals experience problems with blood storage and delivery on the patient floor, particularly in pediatric units, investigators cautioned.
A review of patient safety incidents that occurred surrounding more than 1 million transfusions in U.S. hospitals showed that pediatric transfusions were associated with a higher rate of safety problems compared with adult transfusions, with errors differing by age group.
“We just looked at units transfused [and] incidents that occurred during product administration and we found that the highest incident in the pediatric population is that the protocol is not being followed, and the highest incident in the adult population is that the transfusion is not performed, in error, at all,” said Sarah Vossoughi, MD, of Columbia University and New York–Presbyterian Hospital, New York.
In both settings, the investigators observed problems with product storage on the patient floor. “It’s very common for blood banks to find platelets in the refrigerator. It doesn’t matter how old you are or what type of hospital you’re at – everyone’s putting platelets in the fridge,” she said in an interview at AABB 2018, the annual meeting of the organization formerly known as the American Association of Blood Banks.
Dr. Vossoughi and her colleagues in New York and at the University of Vermont in Burlington noted that the National Patient Safety Foundation, now a part of the Institute for Healthcare Improvement, declared preventable medical harm to be a public health crisis. In a paper published in the BMJ in 2016, researchers estimated that medical errors were the third leading cause of death in the United States, accounting for more than 250,000 fatalities annually.
Dr. Vossoughi also pointed to a study suggesting that the incidence of nonlethal medical errors may be 10- to 20-fold higher than the number of fatal errors (J Patient Saf. 2013 Sep;9[3]:122-8).
To evaluate patient safety events related to blood transfusions, Dr. Vossoughi and her colleagues drew data on events reported by three children’s hospitals and 29 adult hospitals to either the AABB Center for Patient Safety or the medical center’s own adverse event reporting system from January 2010 through September 2017.
They identified a total of 1,806 reports associated with approximately 1,088.884 transfusions. Of these reports, 249 were associated with 99,064 pediatric transfusions, and 1,577 were reported in association with 989,820 adult transfusions.
In all, 31% of the pediatric events were failure to follow the transfusion protocol.
“In a lot of the pediatric hospitals, it’s kind of like the Wild West. People say, ‘well I know it’s the hospital policy, but this child is special, so I’m going to do it this way, this time.’ That seems to be a culture in pediatrics, whereas on the adult side [clinicians] seem to be much less likely to just deviate from the protocol,” Dr. Vossoughi said.
Among adults, 43% of the errors were “transfusion not performed,” which may occur because of a bungled patient hand-off during a shift change, or when a patient is being moved from one unit to another.
“The next day, they’ll check the patient’s CBC and realize that the patient didn’t respond to the infusion that it turned out they never got, and then the product will be found on the floor, expired,” Dr. Vossoughi said.
In all, 20% of pediatric errors and 24% of adult errors were associated with incorrect storage of blood products on the patient floor.
The information they presented could help inpatient blood management programs target education and interventions to providers who commit similar errors.
“If you know that a particular provider group has problems following the protocol, maybe you can make the protocol a little simpler to follow, or make the checklist less cumbersome, and then maybe they’ll follow them more often,” she said.
The study was supported by the AABB Center for Patient Safety and University of Vermont Medical Center. The authors reported no conflicts of interest.
SOURCE: Vossoughi S et al. AABB 2018, Abstract QT4.
BOSTON – Even the most vigilant hospitals experience problems with blood storage and delivery on the patient floor, particularly in pediatric units, investigators cautioned.
A review of patient safety incidents that occurred surrounding more than 1 million transfusions in U.S. hospitals showed that pediatric transfusions were associated with a higher rate of safety problems compared with adult transfusions, with errors differing by age group.
“We just looked at units transfused [and] incidents that occurred during product administration and we found that the highest incident in the pediatric population is that the protocol is not being followed, and the highest incident in the adult population is that the transfusion is not performed, in error, at all,” said Sarah Vossoughi, MD, of Columbia University and New York–Presbyterian Hospital, New York.
In both settings, the investigators observed problems with product storage on the patient floor. “It’s very common for blood banks to find platelets in the refrigerator. It doesn’t matter how old you are or what type of hospital you’re at – everyone’s putting platelets in the fridge,” she said in an interview at AABB 2018, the annual meeting of the organization formerly known as the American Association of Blood Banks.
Dr. Vossoughi and her colleagues in New York and at the University of Vermont in Burlington noted that the National Patient Safety Foundation, now a part of the Institute for Healthcare Improvement, declared preventable medical harm to be a public health crisis. In a paper published in the BMJ in 2016, researchers estimated that medical errors were the third leading cause of death in the United States, accounting for more than 250,000 fatalities annually.
Dr. Vossoughi also pointed to a study suggesting that the incidence of nonlethal medical errors may be 10- to 20-fold higher than the number of fatal errors (J Patient Saf. 2013 Sep;9[3]:122-8).
To evaluate patient safety events related to blood transfusions, Dr. Vossoughi and her colleagues drew data on events reported by three children’s hospitals and 29 adult hospitals to either the AABB Center for Patient Safety or the medical center’s own adverse event reporting system from January 2010 through September 2017.
They identified a total of 1,806 reports associated with approximately 1,088.884 transfusions. Of these reports, 249 were associated with 99,064 pediatric transfusions, and 1,577 were reported in association with 989,820 adult transfusions.
In all, 31% of the pediatric events were failure to follow the transfusion protocol.
“In a lot of the pediatric hospitals, it’s kind of like the Wild West. People say, ‘well I know it’s the hospital policy, but this child is special, so I’m going to do it this way, this time.’ That seems to be a culture in pediatrics, whereas on the adult side [clinicians] seem to be much less likely to just deviate from the protocol,” Dr. Vossoughi said.
Among adults, 43% of the errors were “transfusion not performed,” which may occur because of a bungled patient hand-off during a shift change, or when a patient is being moved from one unit to another.
“The next day, they’ll check the patient’s CBC and realize that the patient didn’t respond to the infusion that it turned out they never got, and then the product will be found on the floor, expired,” Dr. Vossoughi said.
In all, 20% of pediatric errors and 24% of adult errors were associated with incorrect storage of blood products on the patient floor.
The information they presented could help inpatient blood management programs target education and interventions to providers who commit similar errors.
“If you know that a particular provider group has problems following the protocol, maybe you can make the protocol a little simpler to follow, or make the checklist less cumbersome, and then maybe they’ll follow them more often,” she said.
The study was supported by the AABB Center for Patient Safety and University of Vermont Medical Center. The authors reported no conflicts of interest.
SOURCE: Vossoughi S et al. AABB 2018, Abstract QT4.
BOSTON – Even the most vigilant hospitals experience problems with blood storage and delivery on the patient floor, particularly in pediatric units, investigators cautioned.
A review of patient safety incidents that occurred surrounding more than 1 million transfusions in U.S. hospitals showed that pediatric transfusions were associated with a higher rate of safety problems compared with adult transfusions, with errors differing by age group.
“We just looked at units transfused [and] incidents that occurred during product administration and we found that the highest incident in the pediatric population is that the protocol is not being followed, and the highest incident in the adult population is that the transfusion is not performed, in error, at all,” said Sarah Vossoughi, MD, of Columbia University and New York–Presbyterian Hospital, New York.
In both settings, the investigators observed problems with product storage on the patient floor. “It’s very common for blood banks to find platelets in the refrigerator. It doesn’t matter how old you are or what type of hospital you’re at – everyone’s putting platelets in the fridge,” she said in an interview at AABB 2018, the annual meeting of the organization formerly known as the American Association of Blood Banks.
Dr. Vossoughi and her colleagues in New York and at the University of Vermont in Burlington noted that the National Patient Safety Foundation, now a part of the Institute for Healthcare Improvement, declared preventable medical harm to be a public health crisis. In a paper published in the BMJ in 2016, researchers estimated that medical errors were the third leading cause of death in the United States, accounting for more than 250,000 fatalities annually.
Dr. Vossoughi also pointed to a study suggesting that the incidence of nonlethal medical errors may be 10- to 20-fold higher than the number of fatal errors (J Patient Saf. 2013 Sep;9[3]:122-8).
To evaluate patient safety events related to blood transfusions, Dr. Vossoughi and her colleagues drew data on events reported by three children’s hospitals and 29 adult hospitals to either the AABB Center for Patient Safety or the medical center’s own adverse event reporting system from January 2010 through September 2017.
They identified a total of 1,806 reports associated with approximately 1,088.884 transfusions. Of these reports, 249 were associated with 99,064 pediatric transfusions, and 1,577 were reported in association with 989,820 adult transfusions.
In all, 31% of the pediatric events were failure to follow the transfusion protocol.
“In a lot of the pediatric hospitals, it’s kind of like the Wild West. People say, ‘well I know it’s the hospital policy, but this child is special, so I’m going to do it this way, this time.’ That seems to be a culture in pediatrics, whereas on the adult side [clinicians] seem to be much less likely to just deviate from the protocol,” Dr. Vossoughi said.
Among adults, 43% of the errors were “transfusion not performed,” which may occur because of a bungled patient hand-off during a shift change, or when a patient is being moved from one unit to another.
“The next day, they’ll check the patient’s CBC and realize that the patient didn’t respond to the infusion that it turned out they never got, and then the product will be found on the floor, expired,” Dr. Vossoughi said.
In all, 20% of pediatric errors and 24% of adult errors were associated with incorrect storage of blood products on the patient floor.
The information they presented could help inpatient blood management programs target education and interventions to providers who commit similar errors.
“If you know that a particular provider group has problems following the protocol, maybe you can make the protocol a little simpler to follow, or make the checklist less cumbersome, and then maybe they’ll follow them more often,” she said.
The study was supported by the AABB Center for Patient Safety and University of Vermont Medical Center. The authors reported no conflicts of interest.
SOURCE: Vossoughi S et al. AABB 2018, Abstract QT4.
REPORTING FROM AABB 2018
Key clinical point:
Major finding: In all, 31% of pediatric errors were due to protocol violation, and 43% of adult errors were due to an ordered transfusion not being performed.
Study details: Descriptive study of data from 32 U.S. hospitals that reported transfusion safety events.
Disclosures: The study was supported by the AABB Center for Patient Safety and University of Vermont. The authors reported no conflicts of interest.
Source: Vossoughi S et al. AABB 2018, Abstract QT4.
Need blood STAT? Call for a drone
BOSTON – While Amazon and other retailers are experimenting with drones to deliver toasters and toilet seats to your doorstep, drone-delivered platelets and fresh frozen plasma may be coming soon to a hospital near you, experts said at AABB 2018, the annual meeting of the group formerly known as the American Association of Blood Banks.
Using a system of completely autonomous delivery drones launched from a central location, U.S.-based Zipline International delivers blood products to treat postpartum hemorrhage, trauma, malaria, and other life-threatening conditions to patients in rural Rwanda, according to company spokesman Chris Kenney.
“In less than 2 years in Rwanda, we’ve made almost 10,000 deliveries – that’s almost 20,000 units of blood,” he said.
One-third of all deliveries are needed for urgent, life-saving interventions, he said.
The system, which delivers 30% of all blood products used in Rwanda outside the capital Kigali, has resulted in 100% availability of blood products when needed, a 98% reduction in waste (i.e., when unused blood products are discarded because of age), and a 175% increase in the use of platelets and fresh frozen plasma, Mr. Kenney said.
Setting up an airborne delivery network in the largely unregulated and uncrowded Rwandan airspace was a relatively simple process, however, compared with the myriad challenges of establishing a similar system for deliveries to urban medical centers in Boston, Chicago, New York, or Los Angeles, said Paul Eastvold, MD, chief medical officer at Vitalant, a nonprofit network of community blood banks headquartered in Spokane, Wash.
Dr. Eastvold, who is also a private pilot, described the regulatory hurdles that will need to be surmounted before blood-delivery drones are as common a sight as traffic helicopters are currently. He added, however, “I can guarantee you that in the future this is going to be an applicable technology to our industry in one way, shape, or another.”
Fast and cheap
Speed and cost are two of the most compelling arguments for blood banks to use drones. Mr. Kenney described the case of a 24-year-old Rwandan woman who had uncontrolled bleeding from complications following a cesarean section. The clinicians treating her opted to give her an immediate red blood cell transfusion, but she continued to bleed, and the hospital ran out of red blood cells in about 15 minutes.
They placed an order for more blood products – ordering can be done by text message or via WhatsApp, a free, cross-platform messaging and voiceover IP calling service – and over the course of 90 minutes Zipline was able to deliver, using multiple drone launches, 7 units of red blood cells, 4 units of plasma, and 2 units of platelets, all of which were transfused into the patient and allowed her condition to stabilize.
Deliveries that would take a minimum of 3 hours by road can be accomplished in about 15-25 minutes by air, Mr. Kenney said.
The drones – more formally known as “unmanned aerial vehicles” (UAVs) – fly a loop starting at the distribution center, find their target, descend to a height of about 10 meters and drop the package, which has a parachute attached. Packages can be delivered within a drop zone the size of two parking spaces, even in gale-force winds, Mr. Kenney said.
“The whole process is 100% autonomous. The aircraft knows where it’s going, it knows what conditions [are], it knows what its payload characteristics are and flies to the delivery point and drops its package,” he explained.
As drones return to the distribution center, they are snared from the air with a wire that catches a small tail hook on the fuselage.
Airborne deliveries are also significantly cheaper than ground-based services for local delivery, Dr. Eastvold noted. He cited a study showing that the cost of ground shipping from a local warehouse by carriers such as UPS or FedEx could be $6 or more, drones could be as cheap as 5 cents per mile with delivery within about 30 minutes, he said.
The fly in the ointment
Dr. Eastvold outlined the significant barriers to adoption of drone-based delivery systems in the United States, ranging from differences in state laws about when, where, and how drones can be used and who can operate them, to Federal Aviation Administration airspace restrictions and regulations.
For example, the FAA currently requires “line-of-sight” operation only for most drone operators, meaning that the operator must have visual contact with the drone at all times. The FAA will, however, grant waivers to individual operators for specified flying conditions on a case-by-case basis, if compelling need or extenuating circumstances can be satisfactorily explained.
In addition, federal regulations require commercial drone pilots to be 16 years old or older, be fluent in English, be in a physical and mental condition that would not interfere with safe operation of a drone, pass an aeronautical knowledge exam at an FAA-approved testing center, and undergo a Transportation Safety Administration background security screening.
Despite these challenges, at least one U.S. medical center, Johns Hopkins University, is testing the use of drones for blood delivery. In 2017, they demonstrated that a drone could successfully deliver human blood samples in temperature-controlled conditions across 161 miles of Arizona desert, in a flight lasting 3 hours.
Mr. Kenney said that his company is developing a second distribution center in Rwanda that will expand coverage to the entire country and is also working with the FAA, federal regulators, and the state of North Carolina to develop a drone-based blood delivery system in the United States.
BOSTON – While Amazon and other retailers are experimenting with drones to deliver toasters and toilet seats to your doorstep, drone-delivered platelets and fresh frozen plasma may be coming soon to a hospital near you, experts said at AABB 2018, the annual meeting of the group formerly known as the American Association of Blood Banks.
Using a system of completely autonomous delivery drones launched from a central location, U.S.-based Zipline International delivers blood products to treat postpartum hemorrhage, trauma, malaria, and other life-threatening conditions to patients in rural Rwanda, according to company spokesman Chris Kenney.
“In less than 2 years in Rwanda, we’ve made almost 10,000 deliveries – that’s almost 20,000 units of blood,” he said.
One-third of all deliveries are needed for urgent, life-saving interventions, he said.
The system, which delivers 30% of all blood products used in Rwanda outside the capital Kigali, has resulted in 100% availability of blood products when needed, a 98% reduction in waste (i.e., when unused blood products are discarded because of age), and a 175% increase in the use of platelets and fresh frozen plasma, Mr. Kenney said.
Setting up an airborne delivery network in the largely unregulated and uncrowded Rwandan airspace was a relatively simple process, however, compared with the myriad challenges of establishing a similar system for deliveries to urban medical centers in Boston, Chicago, New York, or Los Angeles, said Paul Eastvold, MD, chief medical officer at Vitalant, a nonprofit network of community blood banks headquartered in Spokane, Wash.
Dr. Eastvold, who is also a private pilot, described the regulatory hurdles that will need to be surmounted before blood-delivery drones are as common a sight as traffic helicopters are currently. He added, however, “I can guarantee you that in the future this is going to be an applicable technology to our industry in one way, shape, or another.”
Fast and cheap
Speed and cost are two of the most compelling arguments for blood banks to use drones. Mr. Kenney described the case of a 24-year-old Rwandan woman who had uncontrolled bleeding from complications following a cesarean section. The clinicians treating her opted to give her an immediate red blood cell transfusion, but she continued to bleed, and the hospital ran out of red blood cells in about 15 minutes.
They placed an order for more blood products – ordering can be done by text message or via WhatsApp, a free, cross-platform messaging and voiceover IP calling service – and over the course of 90 minutes Zipline was able to deliver, using multiple drone launches, 7 units of red blood cells, 4 units of plasma, and 2 units of platelets, all of which were transfused into the patient and allowed her condition to stabilize.
Deliveries that would take a minimum of 3 hours by road can be accomplished in about 15-25 minutes by air, Mr. Kenney said.
The drones – more formally known as “unmanned aerial vehicles” (UAVs) – fly a loop starting at the distribution center, find their target, descend to a height of about 10 meters and drop the package, which has a parachute attached. Packages can be delivered within a drop zone the size of two parking spaces, even in gale-force winds, Mr. Kenney said.
“The whole process is 100% autonomous. The aircraft knows where it’s going, it knows what conditions [are], it knows what its payload characteristics are and flies to the delivery point and drops its package,” he explained.
As drones return to the distribution center, they are snared from the air with a wire that catches a small tail hook on the fuselage.
Airborne deliveries are also significantly cheaper than ground-based services for local delivery, Dr. Eastvold noted. He cited a study showing that the cost of ground shipping from a local warehouse by carriers such as UPS or FedEx could be $6 or more, drones could be as cheap as 5 cents per mile with delivery within about 30 minutes, he said.
The fly in the ointment
Dr. Eastvold outlined the significant barriers to adoption of drone-based delivery systems in the United States, ranging from differences in state laws about when, where, and how drones can be used and who can operate them, to Federal Aviation Administration airspace restrictions and regulations.
For example, the FAA currently requires “line-of-sight” operation only for most drone operators, meaning that the operator must have visual contact with the drone at all times. The FAA will, however, grant waivers to individual operators for specified flying conditions on a case-by-case basis, if compelling need or extenuating circumstances can be satisfactorily explained.
In addition, federal regulations require commercial drone pilots to be 16 years old or older, be fluent in English, be in a physical and mental condition that would not interfere with safe operation of a drone, pass an aeronautical knowledge exam at an FAA-approved testing center, and undergo a Transportation Safety Administration background security screening.
Despite these challenges, at least one U.S. medical center, Johns Hopkins University, is testing the use of drones for blood delivery. In 2017, they demonstrated that a drone could successfully deliver human blood samples in temperature-controlled conditions across 161 miles of Arizona desert, in a flight lasting 3 hours.
Mr. Kenney said that his company is developing a second distribution center in Rwanda that will expand coverage to the entire country and is also working with the FAA, federal regulators, and the state of North Carolina to develop a drone-based blood delivery system in the United States.
BOSTON – While Amazon and other retailers are experimenting with drones to deliver toasters and toilet seats to your doorstep, drone-delivered platelets and fresh frozen plasma may be coming soon to a hospital near you, experts said at AABB 2018, the annual meeting of the group formerly known as the American Association of Blood Banks.
Using a system of completely autonomous delivery drones launched from a central location, U.S.-based Zipline International delivers blood products to treat postpartum hemorrhage, trauma, malaria, and other life-threatening conditions to patients in rural Rwanda, according to company spokesman Chris Kenney.
“In less than 2 years in Rwanda, we’ve made almost 10,000 deliveries – that’s almost 20,000 units of blood,” he said.
One-third of all deliveries are needed for urgent, life-saving interventions, he said.
The system, which delivers 30% of all blood products used in Rwanda outside the capital Kigali, has resulted in 100% availability of blood products when needed, a 98% reduction in waste (i.e., when unused blood products are discarded because of age), and a 175% increase in the use of platelets and fresh frozen plasma, Mr. Kenney said.
Setting up an airborne delivery network in the largely unregulated and uncrowded Rwandan airspace was a relatively simple process, however, compared with the myriad challenges of establishing a similar system for deliveries to urban medical centers in Boston, Chicago, New York, or Los Angeles, said Paul Eastvold, MD, chief medical officer at Vitalant, a nonprofit network of community blood banks headquartered in Spokane, Wash.
Dr. Eastvold, who is also a private pilot, described the regulatory hurdles that will need to be surmounted before blood-delivery drones are as common a sight as traffic helicopters are currently. He added, however, “I can guarantee you that in the future this is going to be an applicable technology to our industry in one way, shape, or another.”
Fast and cheap
Speed and cost are two of the most compelling arguments for blood banks to use drones. Mr. Kenney described the case of a 24-year-old Rwandan woman who had uncontrolled bleeding from complications following a cesarean section. The clinicians treating her opted to give her an immediate red blood cell transfusion, but she continued to bleed, and the hospital ran out of red blood cells in about 15 minutes.
They placed an order for more blood products – ordering can be done by text message or via WhatsApp, a free, cross-platform messaging and voiceover IP calling service – and over the course of 90 minutes Zipline was able to deliver, using multiple drone launches, 7 units of red blood cells, 4 units of plasma, and 2 units of platelets, all of which were transfused into the patient and allowed her condition to stabilize.
Deliveries that would take a minimum of 3 hours by road can be accomplished in about 15-25 minutes by air, Mr. Kenney said.
The drones – more formally known as “unmanned aerial vehicles” (UAVs) – fly a loop starting at the distribution center, find their target, descend to a height of about 10 meters and drop the package, which has a parachute attached. Packages can be delivered within a drop zone the size of two parking spaces, even in gale-force winds, Mr. Kenney said.
“The whole process is 100% autonomous. The aircraft knows where it’s going, it knows what conditions [are], it knows what its payload characteristics are and flies to the delivery point and drops its package,” he explained.
As drones return to the distribution center, they are snared from the air with a wire that catches a small tail hook on the fuselage.
Airborne deliveries are also significantly cheaper than ground-based services for local delivery, Dr. Eastvold noted. He cited a study showing that the cost of ground shipping from a local warehouse by carriers such as UPS or FedEx could be $6 or more, drones could be as cheap as 5 cents per mile with delivery within about 30 minutes, he said.
The fly in the ointment
Dr. Eastvold outlined the significant barriers to adoption of drone-based delivery systems in the United States, ranging from differences in state laws about when, where, and how drones can be used and who can operate them, to Federal Aviation Administration airspace restrictions and regulations.
For example, the FAA currently requires “line-of-sight” operation only for most drone operators, meaning that the operator must have visual contact with the drone at all times. The FAA will, however, grant waivers to individual operators for specified flying conditions on a case-by-case basis, if compelling need or extenuating circumstances can be satisfactorily explained.
In addition, federal regulations require commercial drone pilots to be 16 years old or older, be fluent in English, be in a physical and mental condition that would not interfere with safe operation of a drone, pass an aeronautical knowledge exam at an FAA-approved testing center, and undergo a Transportation Safety Administration background security screening.
Despite these challenges, at least one U.S. medical center, Johns Hopkins University, is testing the use of drones for blood delivery. In 2017, they demonstrated that a drone could successfully deliver human blood samples in temperature-controlled conditions across 161 miles of Arizona desert, in a flight lasting 3 hours.
Mr. Kenney said that his company is developing a second distribution center in Rwanda that will expand coverage to the entire country and is also working with the FAA, federal regulators, and the state of North Carolina to develop a drone-based blood delivery system in the United States.
AT AABB 2018
Tech-based cancer company raises access concerns
Oncologists are raising concerns about care access after the launch of a new company that links patients to cancer care options and clinical trials through mobile technology.
Driver, which began in September in the U.S. and China, is a global technology platform that allows patients to access treatment options across a broad network of cancer centers without leaving home. Cancer patients join the platform using a mobile app, through which Driver obtains the required consent to acquire medical records and tumor samples, and the company uses the information to recommend treatment options and clinical trials.
A separate app called Driver for Clinic enables oncologists who belong to Driver’s partner hospitals to manage their institution’s clinical trial information and quickly filter that information based on patients’ medical history to determine the patient’s eligibility for treatments.
Driver’s mission is to connect more patients to the best cancer treatments, regardless of location, said Will Polkinghorn, MD, Driver cofounder and CEO.
“Driver’s cofounders met at Harvard Medical School [in Boston] and saw firsthand the challenges of patients getting access to the latest, cutting-edge treatments available,” Dr. Polkinghorn said in an interview. “As doctors, [we] also witnessed how difficult it was for doctors to manage information in clinic and know about all the treatments that become available all around the world. Driver was created as a platform, with an app for the patient and an app for the doctor, to solve this broken marketplace.”
As part of the model, patients can review their recommended treatment options through video with an expert oncologist and select a hospital within Driver’s network for further evaluation. The company’s global network includes more than 30 leading U.S. cancer centers, including the Cleveland Clinic; multiple locations of the Mayo Clinic; the University of California, San Francisco; and Massachusetts General Hospital, Boston. The U.S. National Cancer Institute (NCI) and the Chinese National Cancer Center are founding members of Driver’s global network, according to the company.
Making more information and treatment options available is a positive for patients, said Walter Stadler, MD, chief of hematology/oncology and director of the genitourinary oncology program at the University of Chicago. However, he noted that the cost for patients to use Driver is prohibitive for many patients. Driver charges patients $3,000 up front and then a $20 monthly fee to use its service. Insurance does not subsidize the cost, nor does Driver help with travel or treatment costs, according to its website.
“It’s inequality of access,” Dr. Stadler said in an interview. “Many of us are very concerned that the clinical trials currently being conducted do not represent the general population well because they don’t represent patients with disparities … Here, we further exacerbate the problem by saying, ‘Okay, we’ll take the 5% of patients who can afford the service and expand their access, and the others, well, that’s not our problem.’ ”
Kashyap Patel, MD, secretary for the Community Oncology Alliance and CEO for the Carolina Blood and Cancer Care in Rock Hill, S.C., also sees positives and negatives about the business model. Using technology to link patients with care and clinical trials can help speed treatment and accelerate drug development, he said. But Driver’s network of large tertiary care centers in metropolitan areas poses challenges for rural cancer patients, he said.
“Access to clinical trials for patients residing in rural areas, as well as those getting their treatment in community based clinics, would not change,” Dr. Patel said in an interview. “Hence, challenges of social and demographic disparities and inequalities in clinical trial access and participation would be altered minimally. There is much greater need for such [platforms to include] community cancer clinics that would be more inclusive and encompass larger geographic areas where the majority of patients receive their care.”
Disadvantaged populations with limited access are not being overlooked by the company, according to Driver leaders. A branch of the company called Driver for All aims to increase access to optimal treatments for free through partnerships with local communities, Dr. Polkinghorn said. Driver for All has thus far partnered with Howard University Hospital in Washington to connect Howard patients to clinical trials at NCI. A partnership with Beijing Children’s Hospital and the Futang Research Center of Pediatric Development, meanwhile, is working to connect patients with rare-disease experts. Driver has funded 100% of the cost of these projects to date, according to its website.
Outside of Driver for All, Dr. Polkinghorn acknowledges that patients must bear the cost of Driver’s consumer products; however, the price should be viewed in context, he said.
“It’s important to remember that today, in order to be evaluated by 30 [plus] centers for treatment options, patients would need to fly to these centers, make appointments, and be seen by a doctor – this would require both time and resources for flights/hotels, which would cost much more than our sticker price,” he said. “So while $3,000 is a lot of money for some patients, Driver’s product is ultimately able to provide more visibility to options that simply would not be realistic today.”
James Gulley, MD, of the National Cancer Institute Center for Cancer Research, said any platform that can efficiently provide access to clinical trial options yields another source of information for patients to utilize in decision making with their health provider. Dr. Gulley, who heads the center’s genitourinary malignancies branch, declined to comment about access-to-care concerns with Driver’s model. He emphasized that patients who participate in NIH research studies are treated without charge.
“The key to finding better [cancer] treatment is to perform science-driven clinical trials,” Dr. Gulley said in an interview. “However, there are many barriers for enrollment in clinical trials. … As a government agency, NCI is open to partnering with any organization that seeks to improve access to clinical trials for cancer patients.”
NCI and Driver recently conducted a study to validate Driver’s platform; it showed that Driver’s technology successfully predicted the eligibility of patients in NCI Center for Cancer Research clinical trials. The study, presented at a recent American Society of Clinical Oncology meeting, evaluated Driver’s processing of 21 metastatic prostate cancer patients enrolled in a therapeutic NCI clinical trial within the last five years. Results showed Driver correctly predicted that 20 of the patients were “potentially eligible” for the trial in which they were enrolled, and that one was ineligible. Based on the study, a protocol is now in development for a new clinical study, which will seek to further determine the efficiency and accuracy of the clinical trial access program created by Driver, according to Dr. Gulley.
Charles Ryan, MD, director of the division of hematology, oncology, and transplantation for the University of Minnesota, Minneapolis, views Driver’s platform as a way to eliminate geographical barriers, which often keep patients from care, while at the same time enabling researchers to find the right patients for clinical trials.
“We need breakthrough technologies and opportunities for patients to be able to access the most successful and promising cancer treatments, regardless of where they live,” Dr. Ryan said in an interview. “Companies like Driver are attempting to bridge that gap by connecting patients to doctors at world class cancer institutes and direct them toward the best care for their particular condition.”
Driver’s model also allows researchers the opportunity to develop specific, unique treatment for less common cancers and remain optimistic that they can attract patients to receive such treatments as they are developed, Dr. Ryan said.
Dr. Stadler, however, worries that Driver may be giving patients the wrong perception that all it takes is a computer and medical records to determine their best treatment route.
“There’s a lot more subtlety to treatment decisions than most people would like to admit,” Dr. Stadler said. “It’s more than just a bunch of data from sophisticated laboratory tests and the written medical record. Obtaining objective information is the first step, but it’s far from the only step.”
Patients may have significant limitations in functional status that is apparent only during an in-person assessment, for example, he said. In other cases, family members may be essential in conveying information about a patient’s cognitive disabilities. Even when such information is documented, it is sometimes difficult to extract the full picture from the record alone, he said. Dr. Stadler is also bothered that the model requires physicians and hospitals to provide their skilled analyses to a for-profit company, which in turn, charges patients to review the information.
“This is our work,” he said. “I agree that patients should have the information, and I don’t mind sharing anything I have with patients, but now I’m going to share it with another business that essentially is competing with me in terms of providing guidance to patients.”
Oncologists are raising concerns about care access after the launch of a new company that links patients to cancer care options and clinical trials through mobile technology.
Driver, which began in September in the U.S. and China, is a global technology platform that allows patients to access treatment options across a broad network of cancer centers without leaving home. Cancer patients join the platform using a mobile app, through which Driver obtains the required consent to acquire medical records and tumor samples, and the company uses the information to recommend treatment options and clinical trials.
A separate app called Driver for Clinic enables oncologists who belong to Driver’s partner hospitals to manage their institution’s clinical trial information and quickly filter that information based on patients’ medical history to determine the patient’s eligibility for treatments.
Driver’s mission is to connect more patients to the best cancer treatments, regardless of location, said Will Polkinghorn, MD, Driver cofounder and CEO.
“Driver’s cofounders met at Harvard Medical School [in Boston] and saw firsthand the challenges of patients getting access to the latest, cutting-edge treatments available,” Dr. Polkinghorn said in an interview. “As doctors, [we] also witnessed how difficult it was for doctors to manage information in clinic and know about all the treatments that become available all around the world. Driver was created as a platform, with an app for the patient and an app for the doctor, to solve this broken marketplace.”
As part of the model, patients can review their recommended treatment options through video with an expert oncologist and select a hospital within Driver’s network for further evaluation. The company’s global network includes more than 30 leading U.S. cancer centers, including the Cleveland Clinic; multiple locations of the Mayo Clinic; the University of California, San Francisco; and Massachusetts General Hospital, Boston. The U.S. National Cancer Institute (NCI) and the Chinese National Cancer Center are founding members of Driver’s global network, according to the company.
Making more information and treatment options available is a positive for patients, said Walter Stadler, MD, chief of hematology/oncology and director of the genitourinary oncology program at the University of Chicago. However, he noted that the cost for patients to use Driver is prohibitive for many patients. Driver charges patients $3,000 up front and then a $20 monthly fee to use its service. Insurance does not subsidize the cost, nor does Driver help with travel or treatment costs, according to its website.
“It’s inequality of access,” Dr. Stadler said in an interview. “Many of us are very concerned that the clinical trials currently being conducted do not represent the general population well because they don’t represent patients with disparities … Here, we further exacerbate the problem by saying, ‘Okay, we’ll take the 5% of patients who can afford the service and expand their access, and the others, well, that’s not our problem.’ ”
Kashyap Patel, MD, secretary for the Community Oncology Alliance and CEO for the Carolina Blood and Cancer Care in Rock Hill, S.C., also sees positives and negatives about the business model. Using technology to link patients with care and clinical trials can help speed treatment and accelerate drug development, he said. But Driver’s network of large tertiary care centers in metropolitan areas poses challenges for rural cancer patients, he said.
“Access to clinical trials for patients residing in rural areas, as well as those getting their treatment in community based clinics, would not change,” Dr. Patel said in an interview. “Hence, challenges of social and demographic disparities and inequalities in clinical trial access and participation would be altered minimally. There is much greater need for such [platforms to include] community cancer clinics that would be more inclusive and encompass larger geographic areas where the majority of patients receive their care.”
Disadvantaged populations with limited access are not being overlooked by the company, according to Driver leaders. A branch of the company called Driver for All aims to increase access to optimal treatments for free through partnerships with local communities, Dr. Polkinghorn said. Driver for All has thus far partnered with Howard University Hospital in Washington to connect Howard patients to clinical trials at NCI. A partnership with Beijing Children’s Hospital and the Futang Research Center of Pediatric Development, meanwhile, is working to connect patients with rare-disease experts. Driver has funded 100% of the cost of these projects to date, according to its website.
Outside of Driver for All, Dr. Polkinghorn acknowledges that patients must bear the cost of Driver’s consumer products; however, the price should be viewed in context, he said.
“It’s important to remember that today, in order to be evaluated by 30 [plus] centers for treatment options, patients would need to fly to these centers, make appointments, and be seen by a doctor – this would require both time and resources for flights/hotels, which would cost much more than our sticker price,” he said. “So while $3,000 is a lot of money for some patients, Driver’s product is ultimately able to provide more visibility to options that simply would not be realistic today.”
James Gulley, MD, of the National Cancer Institute Center for Cancer Research, said any platform that can efficiently provide access to clinical trial options yields another source of information for patients to utilize in decision making with their health provider. Dr. Gulley, who heads the center’s genitourinary malignancies branch, declined to comment about access-to-care concerns with Driver’s model. He emphasized that patients who participate in NIH research studies are treated without charge.
“The key to finding better [cancer] treatment is to perform science-driven clinical trials,” Dr. Gulley said in an interview. “However, there are many barriers for enrollment in clinical trials. … As a government agency, NCI is open to partnering with any organization that seeks to improve access to clinical trials for cancer patients.”
NCI and Driver recently conducted a study to validate Driver’s platform; it showed that Driver’s technology successfully predicted the eligibility of patients in NCI Center for Cancer Research clinical trials. The study, presented at a recent American Society of Clinical Oncology meeting, evaluated Driver’s processing of 21 metastatic prostate cancer patients enrolled in a therapeutic NCI clinical trial within the last five years. Results showed Driver correctly predicted that 20 of the patients were “potentially eligible” for the trial in which they were enrolled, and that one was ineligible. Based on the study, a protocol is now in development for a new clinical study, which will seek to further determine the efficiency and accuracy of the clinical trial access program created by Driver, according to Dr. Gulley.
Charles Ryan, MD, director of the division of hematology, oncology, and transplantation for the University of Minnesota, Minneapolis, views Driver’s platform as a way to eliminate geographical barriers, which often keep patients from care, while at the same time enabling researchers to find the right patients for clinical trials.
“We need breakthrough technologies and opportunities for patients to be able to access the most successful and promising cancer treatments, regardless of where they live,” Dr. Ryan said in an interview. “Companies like Driver are attempting to bridge that gap by connecting patients to doctors at world class cancer institutes and direct them toward the best care for their particular condition.”
Driver’s model also allows researchers the opportunity to develop specific, unique treatment for less common cancers and remain optimistic that they can attract patients to receive such treatments as they are developed, Dr. Ryan said.
Dr. Stadler, however, worries that Driver may be giving patients the wrong perception that all it takes is a computer and medical records to determine their best treatment route.
“There’s a lot more subtlety to treatment decisions than most people would like to admit,” Dr. Stadler said. “It’s more than just a bunch of data from sophisticated laboratory tests and the written medical record. Obtaining objective information is the first step, but it’s far from the only step.”
Patients may have significant limitations in functional status that is apparent only during an in-person assessment, for example, he said. In other cases, family members may be essential in conveying information about a patient’s cognitive disabilities. Even when such information is documented, it is sometimes difficult to extract the full picture from the record alone, he said. Dr. Stadler is also bothered that the model requires physicians and hospitals to provide their skilled analyses to a for-profit company, which in turn, charges patients to review the information.
“This is our work,” he said. “I agree that patients should have the information, and I don’t mind sharing anything I have with patients, but now I’m going to share it with another business that essentially is competing with me in terms of providing guidance to patients.”
Oncologists are raising concerns about care access after the launch of a new company that links patients to cancer care options and clinical trials through mobile technology.
Driver, which began in September in the U.S. and China, is a global technology platform that allows patients to access treatment options across a broad network of cancer centers without leaving home. Cancer patients join the platform using a mobile app, through which Driver obtains the required consent to acquire medical records and tumor samples, and the company uses the information to recommend treatment options and clinical trials.
A separate app called Driver for Clinic enables oncologists who belong to Driver’s partner hospitals to manage their institution’s clinical trial information and quickly filter that information based on patients’ medical history to determine the patient’s eligibility for treatments.
Driver’s mission is to connect more patients to the best cancer treatments, regardless of location, said Will Polkinghorn, MD, Driver cofounder and CEO.
“Driver’s cofounders met at Harvard Medical School [in Boston] and saw firsthand the challenges of patients getting access to the latest, cutting-edge treatments available,” Dr. Polkinghorn said in an interview. “As doctors, [we] also witnessed how difficult it was for doctors to manage information in clinic and know about all the treatments that become available all around the world. Driver was created as a platform, with an app for the patient and an app for the doctor, to solve this broken marketplace.”
As part of the model, patients can review their recommended treatment options through video with an expert oncologist and select a hospital within Driver’s network for further evaluation. The company’s global network includes more than 30 leading U.S. cancer centers, including the Cleveland Clinic; multiple locations of the Mayo Clinic; the University of California, San Francisco; and Massachusetts General Hospital, Boston. The U.S. National Cancer Institute (NCI) and the Chinese National Cancer Center are founding members of Driver’s global network, according to the company.
Making more information and treatment options available is a positive for patients, said Walter Stadler, MD, chief of hematology/oncology and director of the genitourinary oncology program at the University of Chicago. However, he noted that the cost for patients to use Driver is prohibitive for many patients. Driver charges patients $3,000 up front and then a $20 monthly fee to use its service. Insurance does not subsidize the cost, nor does Driver help with travel or treatment costs, according to its website.
“It’s inequality of access,” Dr. Stadler said in an interview. “Many of us are very concerned that the clinical trials currently being conducted do not represent the general population well because they don’t represent patients with disparities … Here, we further exacerbate the problem by saying, ‘Okay, we’ll take the 5% of patients who can afford the service and expand their access, and the others, well, that’s not our problem.’ ”
Kashyap Patel, MD, secretary for the Community Oncology Alliance and CEO for the Carolina Blood and Cancer Care in Rock Hill, S.C., also sees positives and negatives about the business model. Using technology to link patients with care and clinical trials can help speed treatment and accelerate drug development, he said. But Driver’s network of large tertiary care centers in metropolitan areas poses challenges for rural cancer patients, he said.
“Access to clinical trials for patients residing in rural areas, as well as those getting their treatment in community based clinics, would not change,” Dr. Patel said in an interview. “Hence, challenges of social and demographic disparities and inequalities in clinical trial access and participation would be altered minimally. There is much greater need for such [platforms to include] community cancer clinics that would be more inclusive and encompass larger geographic areas where the majority of patients receive their care.”
Disadvantaged populations with limited access are not being overlooked by the company, according to Driver leaders. A branch of the company called Driver for All aims to increase access to optimal treatments for free through partnerships with local communities, Dr. Polkinghorn said. Driver for All has thus far partnered with Howard University Hospital in Washington to connect Howard patients to clinical trials at NCI. A partnership with Beijing Children’s Hospital and the Futang Research Center of Pediatric Development, meanwhile, is working to connect patients with rare-disease experts. Driver has funded 100% of the cost of these projects to date, according to its website.
Outside of Driver for All, Dr. Polkinghorn acknowledges that patients must bear the cost of Driver’s consumer products; however, the price should be viewed in context, he said.
“It’s important to remember that today, in order to be evaluated by 30 [plus] centers for treatment options, patients would need to fly to these centers, make appointments, and be seen by a doctor – this would require both time and resources for flights/hotels, which would cost much more than our sticker price,” he said. “So while $3,000 is a lot of money for some patients, Driver’s product is ultimately able to provide more visibility to options that simply would not be realistic today.”
James Gulley, MD, of the National Cancer Institute Center for Cancer Research, said any platform that can efficiently provide access to clinical trial options yields another source of information for patients to utilize in decision making with their health provider. Dr. Gulley, who heads the center’s genitourinary malignancies branch, declined to comment about access-to-care concerns with Driver’s model. He emphasized that patients who participate in NIH research studies are treated without charge.
“The key to finding better [cancer] treatment is to perform science-driven clinical trials,” Dr. Gulley said in an interview. “However, there are many barriers for enrollment in clinical trials. … As a government agency, NCI is open to partnering with any organization that seeks to improve access to clinical trials for cancer patients.”
NCI and Driver recently conducted a study to validate Driver’s platform; it showed that Driver’s technology successfully predicted the eligibility of patients in NCI Center for Cancer Research clinical trials. The study, presented at a recent American Society of Clinical Oncology meeting, evaluated Driver’s processing of 21 metastatic prostate cancer patients enrolled in a therapeutic NCI clinical trial within the last five years. Results showed Driver correctly predicted that 20 of the patients were “potentially eligible” for the trial in which they were enrolled, and that one was ineligible. Based on the study, a protocol is now in development for a new clinical study, which will seek to further determine the efficiency and accuracy of the clinical trial access program created by Driver, according to Dr. Gulley.
Charles Ryan, MD, director of the division of hematology, oncology, and transplantation for the University of Minnesota, Minneapolis, views Driver’s platform as a way to eliminate geographical barriers, which often keep patients from care, while at the same time enabling researchers to find the right patients for clinical trials.
“We need breakthrough technologies and opportunities for patients to be able to access the most successful and promising cancer treatments, regardless of where they live,” Dr. Ryan said in an interview. “Companies like Driver are attempting to bridge that gap by connecting patients to doctors at world class cancer institutes and direct them toward the best care for their particular condition.”
Driver’s model also allows researchers the opportunity to develop specific, unique treatment for less common cancers and remain optimistic that they can attract patients to receive such treatments as they are developed, Dr. Ryan said.
Dr. Stadler, however, worries that Driver may be giving patients the wrong perception that all it takes is a computer and medical records to determine their best treatment route.
“There’s a lot more subtlety to treatment decisions than most people would like to admit,” Dr. Stadler said. “It’s more than just a bunch of data from sophisticated laboratory tests and the written medical record. Obtaining objective information is the first step, but it’s far from the only step.”
Patients may have significant limitations in functional status that is apparent only during an in-person assessment, for example, he said. In other cases, family members may be essential in conveying information about a patient’s cognitive disabilities. Even when such information is documented, it is sometimes difficult to extract the full picture from the record alone, he said. Dr. Stadler is also bothered that the model requires physicians and hospitals to provide their skilled analyses to a for-profit company, which in turn, charges patients to review the information.
“This is our work,” he said. “I agree that patients should have the information, and I don’t mind sharing anything I have with patients, but now I’m going to share it with another business that essentially is competing with me in terms of providing guidance to patients.”