User login
FDA approves DNA-based blood type test
The for use in transfusion.
It’s the second molecular test for blood compatibility but the first to report genotype in its results, according to an announcement from the agency.
The test is important because it evaluates patients – especially those who receive repeated blood transfusions for conditions such as sickle cell anemia – for non-ABO antigens, but it does so without using antisera, which is sometimes unavailable.
A study found comparable performance between the ID CORE XT, licensed serologic reagents, and DNA sequencing tests, according to the FDA.
The ID CORE XT test is marketed by Progenika Biopharma, a Grifols company.
More information can be found in the full FDA press announcement.
The for use in transfusion.
It’s the second molecular test for blood compatibility but the first to report genotype in its results, according to an announcement from the agency.
The test is important because it evaluates patients – especially those who receive repeated blood transfusions for conditions such as sickle cell anemia – for non-ABO antigens, but it does so without using antisera, which is sometimes unavailable.
A study found comparable performance between the ID CORE XT, licensed serologic reagents, and DNA sequencing tests, according to the FDA.
The ID CORE XT test is marketed by Progenika Biopharma, a Grifols company.
More information can be found in the full FDA press announcement.
The for use in transfusion.
It’s the second molecular test for blood compatibility but the first to report genotype in its results, according to an announcement from the agency.
The test is important because it evaluates patients – especially those who receive repeated blood transfusions for conditions such as sickle cell anemia – for non-ABO antigens, but it does so without using antisera, which is sometimes unavailable.
A study found comparable performance between the ID CORE XT, licensed serologic reagents, and DNA sequencing tests, according to the FDA.
The ID CORE XT test is marketed by Progenika Biopharma, a Grifols company.
More information can be found in the full FDA press announcement.
Acute Superior Mesenteric Venous Thrombosis in a Young Patient Without Risk Factors
In this case report, the authors address the diagnostic challenges of a young, healthy patient who presented to the ED with unrelenting abdominal pain.
Acute mesenteric ischemia (AMI) results when oxygen delivery to the mesenteric artery is compromised, and is a serious diagnosis that should be considered in patients of all ages to avoid significant morbidity and mortality. The majority of cases are due to arterial embolism, arterial thrombus, or intestinal hypoperfusion (non-occlusive). Acute mesenteric venous thrombosis (MVT) accounts for only 2% to 10% of AMI cases, and only 0.01% of emergency surgery admissions.1 A large systematic review showed a 44% mortality rate for MVT, in contrast to 66% to 89% for all other forms of AMI.2 The typical age range for MVT is reported between 45 and 60 years, with a slight male predominance.3 Dull, central abdominal pain is the most frequently reported symptom of MVT, although it is generally less impressive than the pain described in other forms of AMI.3Along with the hallmark of abdominal pain out of proportion to the examination, other gastrointestinal symptoms include weight loss and non-specific altered bowel function (constipation, diarrhea, abdominal distention, and bloating), which are present in half of all patients with MVT.1 Peritoneal signs and bloody stools portend poor outcomes, as they often occur with disease progression.4
Case
A 26-year-old man presented to the ED with periumbilical and lower abdominal pain for 1 week. The pain was described as constant and dull, worsened by movement and oral intake, and improved with lying flat. He described bloating and decreased volume of bowel movements. He denied nausea, vomiting, fever, colicky pain, blood in stool, testicular pain, urinary complaints, trauma, or any similar episodes in the past. The patient had no known medical conditions or surgical history, except for a remote history of alcohol dependence (in remission) and tobacco use. There was no personal or family history of coagulopathy. Of note, he was seen by his primary care physician a few days prior to his ED presentation and had been instructed to take acetaminophen, which did not provide relief.
The patient’s vital signs at presentation were: blood pressure, 122/70 mm Hg; heart rate, 93 beats/min; respiratory rate, 18 breaths/min; and temperature, 37.5°C (99.5°F). Oxygen saturation was 99% on room air. The physical examination was remarkable only for mild abdominal tenderness diffusely, greater in the lower and central abdomen than in the upper abdomen. The remainder of the physical examination was unremarkable.
Laboratory studies ordered included a complete blood count, comprehensive metabolic profile, lipase, and urinalysis. The patient did have a mild transaminitis (aspartate aminotransferase, 48 U/L; alanine aminotransferase, 84 U/L); the remainder of the studies were normal. A serum lactate, drawn after the 1 L of normal saline was administered intravenously (IV), was within normal limits (0.7 mmol/L). No prior laboratory studies were available for comparison.
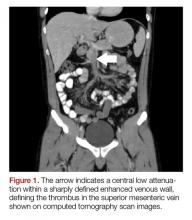
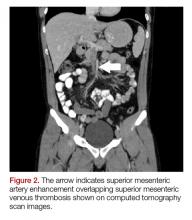
The patient’s continued abdominal pain and transaminitis prompted an ED bedside right upper quadrant ultrasound, which showed a small gallbladder polyp; no signs of gallbladder disease were present. The patient required three doses of morphine 4 mg IV without complete pain relief. Given the concern for pain out of proportion to physical examination, a computed tomography (CT) scan of the abdomen/pelvis with IV and oral contrast was ordered. The radiologist interpreted the scan as showing a superior mesenteric vein (SMV) thrombus extending into the splenic/portal vein confluence and the intrahepatic portal veins (Figures 1 and 2).
Ciprofloxacin and metronidazole were administered IV for antibiotic prophylaxis, and the patient was placed on bowel rest with advancement to regular diet as tolerated. Propranolol was given for variceal prophylaxis. The patient was discharged home the following day in stable condition. Although he still had mild abdominal tenderness, the vital signs and physical examination were within normal limits. The patient was placed on a 6-month course of rivaroxaban therapy. Coagulopathy testing was scheduled at a later date, since ongoing anticoagulation treatment could interfere with test results. Unfortunately, the patient did not attend follow-up appointments to obtain testing.
Discussion
Mesenteric venous thrombosis is seen predominantly in middle-aged patients presenting with vague symptoms, which makes this a challenging diagnosis to make in the acute care setting. Risk factors for MVT include recent injury (causing trauma to the vasculature), recent surgery (causing stagnant blood flow), inflammatory conditions, and hypercoagulable states.1 In this patient’s case, no risk factors were identified; although the majority of cases of MVT will have an identifiable risk factor.2 Still, 21% to 49% of cases of MVT are considered idiopathic.1,3It is possible that our patient had a prior undiagnosed pancreatitis associated with his history of alcoholism that contributed to his thrombosis. Pancreatitis and other inflammatory conditions, including diverticulitis or inflammatory bowel disease, are more commonly associated with thrombus formation in the large veins, as opposed to an undiagnosed hypercoagulable state, which would more likely affect distal venuoles, vasa recta, or venous arcades.1,5 The patient’s mild transaminitis was likely secondary to hepatic congestion from the venous thrombus extending to the splenic-portal vein confluence and intrahepatic portal vein. One study looked at patients with pancreatitis and found that 16.7% of their study population had an SMV thrombus, while 4.1% had a SMV thrombus with a concomitant portal vein thrombus.6
Although there are no pathognomonic laboratory findings of MVT, elevated lactate, leukocytosis, and elevated D-dimer levels may be helpful in supporting the diagnosis.7,8 A recent study found that elevated D-dimer levels may be a specific marker in the early recognition of acute SMV thrombosis, as well as predicting risk, outcomes, and treatment options.8 However, emergency physicians should maintain a high index of suspicion in patients with concerning features of the disease, since normal laboratory values, including lactate, do not reliably exclude the diagnosis.
Computed tomography scanning and CT angiography (CTA) are quite helpful in diagnosing MVT. Ultrasound of the upper abdomen may also play a role, noting dilated or thickened bowel wall with intraluminal air or echogenic material in the superior mesenteric vein or portal vein.9 Although magnetic resonance venography most reliably demonstrates thrombi, its lack of widespread availability makes CT with IV contrast the preferred initial study.3Computed tomography not only has high sensitivity, but also offers alternative diagnoses in the undifferentiated presentation.1One study found CT to be 100% sensitive in detecting any abnormality associated with MVT or bowel ischemia.10 Common CT findings of MVT include dilated and thickened bowel loops, mesenteric fat standing, ascites, a halo or target appearance of bowel, vessel filling defects from a thrombus, and pneumatosis intestinalis.11 The latter usually indicates transmural infarction, and can extend as portomesenteric vein gas.11 Of note, if the initial CT scan is non-diagnostic and a high clinical suspicion for mesenteric ischemia remains with no alternative diagnosis, CTA is the gold standard.3,7Expeditious diagnosis of MVT is imperative, given the potential complications of intestinal infarction, submucosal hemorrhage secondary to edema, and third spacing of the venous outflow into the bowel wall due to collateral vessels being unable to redirect blood flow in conjunction with complete venous occlusion.12Not all MVTs progress to infarction, given the extensive collateral circulation. Early diagnosis, however, is crucial for conservative management to be effective.9Acute MVT without signs of infarction necessitates anticoagulation therapy to decrease clot propagation and recurrence.1 In addition, prophylactic antibiotics to limit bacterial translocation, and bowel rest are advised.13,14 If the patient is unresponsive to anticoagulation, thrombolytic and endovascular therapies may be of benefit in select patients.15 Once intestinal ischemia or infarction develops, the prognosis is poor: mortality approaches 75% with infarction.1 If signs of bowel infarction are present, a laparotomy must be performed promptly, although in most cases, delayed patient presentation makes small bowel resection unavoidable.9 Further testing for hypercoagulability is recommended, particularly in isolated thrombosis, since long-term anticoagulation therapy may be necessary if a coagulopathy is discovered.1
Conclusion
Mesenteric venous thrombosis is atypical in a young, healthy patient. However, due to high mortality rates with disease progression, it is important to consider in any patient with unrelenting abdominal pain and vague gastrointestinal symptoms of uncertain cause, even in those without risk factors. Early detection and management of MVT before progression to mesenteric ischemia and infarction considerably lowers the mortality rate. Emergency physicians must be vigilant when treating a patient with abdominal pain out of proportion to physical examination, unrelenting pain despite analgesic medications, or repeat ED visits for the same abdominal complaints.
1. Harnik IG, Brandt LJ. Mesenteric venous thrombosis. Vasc Med. 2010;15(5):407-418. doi:10.1177/1358863x10379673.
2. Tilsed JV, Casamassima A, Kurihara H, et al. ESTES guidelines: acute mesenteric ischaemia. Eur J Trauma Emerg Surg. 2016;42(2):253-270. doi:10.1007/s00068-016-0634-0.
3. Tendler DA, Lamont JT, Grubel P. Mesenteric venous thrombosis in adults. UpToDate Web site. https://www.uptodate.com/contents/mesenteric-venous-thrombosis-in-adults. Accessed November 16, 2017.
4. Al-Zahrani HA, Lindsay T. Mesenteric ischemia. In: Hall JB, Schmidt GA, Kress JP, eds. Principles of Critical Care. 4th ed. New York, NY: McGraw Hill; 2015:1036-1044.
5. Kumar S, Sarr MG, Kamath PS. Mesenteric venous thrombosis. N Engl J Med. 2001;345(23):1683-1688. doi:10.1056/nejmra010076.
6. Al-Khazraji A, Hasan AQ, Patel I, Alkhawam H, Ghrair F, Lieber J. The role of abdominal computed tomography scan in acute pancreatitis. Pancreas. 2017;46(6):e52-e54. doi:10.1097/mpa.0000000000000837.
7. Bradbury MS, Kavanagh PV, Bechtold RE, et al. Mesenteric venous thrombosis: diagnosis and noninvasive imaging. Radiographics. 2002;22(3):527-541.
8. Yang S, Fan X, Ding W, et al. D-dimer as an early marker of severity in patients with acute superior mesenteric venous thrombosis. Medicine (Baltimore). 2014;93(29):e270. doi:10.1097/md.0000000000000270.
9. Matos C, Van Gansbeke D, Zalcman M, et al. Mesenteric vein thrombosis: early CT and US diagnosis and conservative management. Gastrointest Radiol. 1986;11(4):322-325.
10. Rhee RY, Gloviczki P, Mendonca CT, et al. Mesenteric venous thrombosis: still a lethal disease in the 1990s. J Vasc Surg. 1994;20(5):688-697.
11. Furukawa A, Kanasaki S, Kono N, et al. CT diagnosis of acute mesenteric ischemia from various causes. AJR Am J Roentgenol. 2009;192(2):408-416. doi:10.2214/ajr.08.1138.
12. Johnson CC, Baggenstoss AH. Mesenteric vascular occlusion; study of 99 cases of occlusion of veins. Proc Staff Meet Mayo Clin. 1949;24(25):628-636.13. Hmoud B, Singal AK, Kamath PS. Mesenteric venous thrombosis. J Clin Exp Hepatol. 2014;4(3):257-263. doi:10.1016/j.jceh.2014.03.052.
14. Schoots IG, Koffeman GI, Legemate DA, Levi M, van Gulik TM. Systematic review of survival after acute mesenteric ischaemia according to disease aetiology. Br J Surg. 2004;91(1):17-27.
15. Yang S, Fan X, Ding W, et al. Multidisciplinary stepwise management strategy for acute superior mesenteric venous thrombosis: an intestinal stroke center experience. Thromb Res. 2015;135(1):36-45. doi:10.1016/j.thromres.2014.10.018.
In this case report, the authors address the diagnostic challenges of a young, healthy patient who presented to the ED with unrelenting abdominal pain.
In this case report, the authors address the diagnostic challenges of a young, healthy patient who presented to the ED with unrelenting abdominal pain.
Acute mesenteric ischemia (AMI) results when oxygen delivery to the mesenteric artery is compromised, and is a serious diagnosis that should be considered in patients of all ages to avoid significant morbidity and mortality. The majority of cases are due to arterial embolism, arterial thrombus, or intestinal hypoperfusion (non-occlusive). Acute mesenteric venous thrombosis (MVT) accounts for only 2% to 10% of AMI cases, and only 0.01% of emergency surgery admissions.1 A large systematic review showed a 44% mortality rate for MVT, in contrast to 66% to 89% for all other forms of AMI.2 The typical age range for MVT is reported between 45 and 60 years, with a slight male predominance.3 Dull, central abdominal pain is the most frequently reported symptom of MVT, although it is generally less impressive than the pain described in other forms of AMI.3Along with the hallmark of abdominal pain out of proportion to the examination, other gastrointestinal symptoms include weight loss and non-specific altered bowel function (constipation, diarrhea, abdominal distention, and bloating), which are present in half of all patients with MVT.1 Peritoneal signs and bloody stools portend poor outcomes, as they often occur with disease progression.4
Case
A 26-year-old man presented to the ED with periumbilical and lower abdominal pain for 1 week. The pain was described as constant and dull, worsened by movement and oral intake, and improved with lying flat. He described bloating and decreased volume of bowel movements. He denied nausea, vomiting, fever, colicky pain, blood in stool, testicular pain, urinary complaints, trauma, or any similar episodes in the past. The patient had no known medical conditions or surgical history, except for a remote history of alcohol dependence (in remission) and tobacco use. There was no personal or family history of coagulopathy. Of note, he was seen by his primary care physician a few days prior to his ED presentation and had been instructed to take acetaminophen, which did not provide relief.
The patient’s vital signs at presentation were: blood pressure, 122/70 mm Hg; heart rate, 93 beats/min; respiratory rate, 18 breaths/min; and temperature, 37.5°C (99.5°F). Oxygen saturation was 99% on room air. The physical examination was remarkable only for mild abdominal tenderness diffusely, greater in the lower and central abdomen than in the upper abdomen. The remainder of the physical examination was unremarkable.
Laboratory studies ordered included a complete blood count, comprehensive metabolic profile, lipase, and urinalysis. The patient did have a mild transaminitis (aspartate aminotransferase, 48 U/L; alanine aminotransferase, 84 U/L); the remainder of the studies were normal. A serum lactate, drawn after the 1 L of normal saline was administered intravenously (IV), was within normal limits (0.7 mmol/L). No prior laboratory studies were available for comparison.
The patient’s continued abdominal pain and transaminitis prompted an ED bedside right upper quadrant ultrasound, which showed a small gallbladder polyp; no signs of gallbladder disease were present. The patient required three doses of morphine 4 mg IV without complete pain relief. Given the concern for pain out of proportion to physical examination, a computed tomography (CT) scan of the abdomen/pelvis with IV and oral contrast was ordered. The radiologist interpreted the scan as showing a superior mesenteric vein (SMV) thrombus extending into the splenic/portal vein confluence and the intrahepatic portal veins (Figures 1 and 2).
Ciprofloxacin and metronidazole were administered IV for antibiotic prophylaxis, and the patient was placed on bowel rest with advancement to regular diet as tolerated. Propranolol was given for variceal prophylaxis. The patient was discharged home the following day in stable condition. Although he still had mild abdominal tenderness, the vital signs and physical examination were within normal limits. The patient was placed on a 6-month course of rivaroxaban therapy. Coagulopathy testing was scheduled at a later date, since ongoing anticoagulation treatment could interfere with test results. Unfortunately, the patient did not attend follow-up appointments to obtain testing.
Discussion
Mesenteric venous thrombosis is seen predominantly in middle-aged patients presenting with vague symptoms, which makes this a challenging diagnosis to make in the acute care setting. Risk factors for MVT include recent injury (causing trauma to the vasculature), recent surgery (causing stagnant blood flow), inflammatory conditions, and hypercoagulable states.1 In this patient’s case, no risk factors were identified; although the majority of cases of MVT will have an identifiable risk factor.2 Still, 21% to 49% of cases of MVT are considered idiopathic.1,3It is possible that our patient had a prior undiagnosed pancreatitis associated with his history of alcoholism that contributed to his thrombosis. Pancreatitis and other inflammatory conditions, including diverticulitis or inflammatory bowel disease, are more commonly associated with thrombus formation in the large veins, as opposed to an undiagnosed hypercoagulable state, which would more likely affect distal venuoles, vasa recta, or venous arcades.1,5 The patient’s mild transaminitis was likely secondary to hepatic congestion from the venous thrombus extending to the splenic-portal vein confluence and intrahepatic portal vein. One study looked at patients with pancreatitis and found that 16.7% of their study population had an SMV thrombus, while 4.1% had a SMV thrombus with a concomitant portal vein thrombus.6
Although there are no pathognomonic laboratory findings of MVT, elevated lactate, leukocytosis, and elevated D-dimer levels may be helpful in supporting the diagnosis.7,8 A recent study found that elevated D-dimer levels may be a specific marker in the early recognition of acute SMV thrombosis, as well as predicting risk, outcomes, and treatment options.8 However, emergency physicians should maintain a high index of suspicion in patients with concerning features of the disease, since normal laboratory values, including lactate, do not reliably exclude the diagnosis.
Computed tomography scanning and CT angiography (CTA) are quite helpful in diagnosing MVT. Ultrasound of the upper abdomen may also play a role, noting dilated or thickened bowel wall with intraluminal air or echogenic material in the superior mesenteric vein or portal vein.9 Although magnetic resonance venography most reliably demonstrates thrombi, its lack of widespread availability makes CT with IV contrast the preferred initial study.3Computed tomography not only has high sensitivity, but also offers alternative diagnoses in the undifferentiated presentation.1One study found CT to be 100% sensitive in detecting any abnormality associated with MVT or bowel ischemia.10 Common CT findings of MVT include dilated and thickened bowel loops, mesenteric fat standing, ascites, a halo or target appearance of bowel, vessel filling defects from a thrombus, and pneumatosis intestinalis.11 The latter usually indicates transmural infarction, and can extend as portomesenteric vein gas.11 Of note, if the initial CT scan is non-diagnostic and a high clinical suspicion for mesenteric ischemia remains with no alternative diagnosis, CTA is the gold standard.3,7Expeditious diagnosis of MVT is imperative, given the potential complications of intestinal infarction, submucosal hemorrhage secondary to edema, and third spacing of the venous outflow into the bowel wall due to collateral vessels being unable to redirect blood flow in conjunction with complete venous occlusion.12Not all MVTs progress to infarction, given the extensive collateral circulation. Early diagnosis, however, is crucial for conservative management to be effective.9Acute MVT without signs of infarction necessitates anticoagulation therapy to decrease clot propagation and recurrence.1 In addition, prophylactic antibiotics to limit bacterial translocation, and bowel rest are advised.13,14 If the patient is unresponsive to anticoagulation, thrombolytic and endovascular therapies may be of benefit in select patients.15 Once intestinal ischemia or infarction develops, the prognosis is poor: mortality approaches 75% with infarction.1 If signs of bowel infarction are present, a laparotomy must be performed promptly, although in most cases, delayed patient presentation makes small bowel resection unavoidable.9 Further testing for hypercoagulability is recommended, particularly in isolated thrombosis, since long-term anticoagulation therapy may be necessary if a coagulopathy is discovered.1
Conclusion
Mesenteric venous thrombosis is atypical in a young, healthy patient. However, due to high mortality rates with disease progression, it is important to consider in any patient with unrelenting abdominal pain and vague gastrointestinal symptoms of uncertain cause, even in those without risk factors. Early detection and management of MVT before progression to mesenteric ischemia and infarction considerably lowers the mortality rate. Emergency physicians must be vigilant when treating a patient with abdominal pain out of proportion to physical examination, unrelenting pain despite analgesic medications, or repeat ED visits for the same abdominal complaints.
Acute mesenteric ischemia (AMI) results when oxygen delivery to the mesenteric artery is compromised, and is a serious diagnosis that should be considered in patients of all ages to avoid significant morbidity and mortality. The majority of cases are due to arterial embolism, arterial thrombus, or intestinal hypoperfusion (non-occlusive). Acute mesenteric venous thrombosis (MVT) accounts for only 2% to 10% of AMI cases, and only 0.01% of emergency surgery admissions.1 A large systematic review showed a 44% mortality rate for MVT, in contrast to 66% to 89% for all other forms of AMI.2 The typical age range for MVT is reported between 45 and 60 years, with a slight male predominance.3 Dull, central abdominal pain is the most frequently reported symptom of MVT, although it is generally less impressive than the pain described in other forms of AMI.3Along with the hallmark of abdominal pain out of proportion to the examination, other gastrointestinal symptoms include weight loss and non-specific altered bowel function (constipation, diarrhea, abdominal distention, and bloating), which are present in half of all patients with MVT.1 Peritoneal signs and bloody stools portend poor outcomes, as they often occur with disease progression.4
Case
A 26-year-old man presented to the ED with periumbilical and lower abdominal pain for 1 week. The pain was described as constant and dull, worsened by movement and oral intake, and improved with lying flat. He described bloating and decreased volume of bowel movements. He denied nausea, vomiting, fever, colicky pain, blood in stool, testicular pain, urinary complaints, trauma, or any similar episodes in the past. The patient had no known medical conditions or surgical history, except for a remote history of alcohol dependence (in remission) and tobacco use. There was no personal or family history of coagulopathy. Of note, he was seen by his primary care physician a few days prior to his ED presentation and had been instructed to take acetaminophen, which did not provide relief.
The patient’s vital signs at presentation were: blood pressure, 122/70 mm Hg; heart rate, 93 beats/min; respiratory rate, 18 breaths/min; and temperature, 37.5°C (99.5°F). Oxygen saturation was 99% on room air. The physical examination was remarkable only for mild abdominal tenderness diffusely, greater in the lower and central abdomen than in the upper abdomen. The remainder of the physical examination was unremarkable.
Laboratory studies ordered included a complete blood count, comprehensive metabolic profile, lipase, and urinalysis. The patient did have a mild transaminitis (aspartate aminotransferase, 48 U/L; alanine aminotransferase, 84 U/L); the remainder of the studies were normal. A serum lactate, drawn after the 1 L of normal saline was administered intravenously (IV), was within normal limits (0.7 mmol/L). No prior laboratory studies were available for comparison.
The patient’s continued abdominal pain and transaminitis prompted an ED bedside right upper quadrant ultrasound, which showed a small gallbladder polyp; no signs of gallbladder disease were present. The patient required three doses of morphine 4 mg IV without complete pain relief. Given the concern for pain out of proportion to physical examination, a computed tomography (CT) scan of the abdomen/pelvis with IV and oral contrast was ordered. The radiologist interpreted the scan as showing a superior mesenteric vein (SMV) thrombus extending into the splenic/portal vein confluence and the intrahepatic portal veins (Figures 1 and 2).
Ciprofloxacin and metronidazole were administered IV for antibiotic prophylaxis, and the patient was placed on bowel rest with advancement to regular diet as tolerated. Propranolol was given for variceal prophylaxis. The patient was discharged home the following day in stable condition. Although he still had mild abdominal tenderness, the vital signs and physical examination were within normal limits. The patient was placed on a 6-month course of rivaroxaban therapy. Coagulopathy testing was scheduled at a later date, since ongoing anticoagulation treatment could interfere with test results. Unfortunately, the patient did not attend follow-up appointments to obtain testing.
Discussion
Mesenteric venous thrombosis is seen predominantly in middle-aged patients presenting with vague symptoms, which makes this a challenging diagnosis to make in the acute care setting. Risk factors for MVT include recent injury (causing trauma to the vasculature), recent surgery (causing stagnant blood flow), inflammatory conditions, and hypercoagulable states.1 In this patient’s case, no risk factors were identified; although the majority of cases of MVT will have an identifiable risk factor.2 Still, 21% to 49% of cases of MVT are considered idiopathic.1,3It is possible that our patient had a prior undiagnosed pancreatitis associated with his history of alcoholism that contributed to his thrombosis. Pancreatitis and other inflammatory conditions, including diverticulitis or inflammatory bowel disease, are more commonly associated with thrombus formation in the large veins, as opposed to an undiagnosed hypercoagulable state, which would more likely affect distal venuoles, vasa recta, or venous arcades.1,5 The patient’s mild transaminitis was likely secondary to hepatic congestion from the venous thrombus extending to the splenic-portal vein confluence and intrahepatic portal vein. One study looked at patients with pancreatitis and found that 16.7% of their study population had an SMV thrombus, while 4.1% had a SMV thrombus with a concomitant portal vein thrombus.6
Although there are no pathognomonic laboratory findings of MVT, elevated lactate, leukocytosis, and elevated D-dimer levels may be helpful in supporting the diagnosis.7,8 A recent study found that elevated D-dimer levels may be a specific marker in the early recognition of acute SMV thrombosis, as well as predicting risk, outcomes, and treatment options.8 However, emergency physicians should maintain a high index of suspicion in patients with concerning features of the disease, since normal laboratory values, including lactate, do not reliably exclude the diagnosis.
Computed tomography scanning and CT angiography (CTA) are quite helpful in diagnosing MVT. Ultrasound of the upper abdomen may also play a role, noting dilated or thickened bowel wall with intraluminal air or echogenic material in the superior mesenteric vein or portal vein.9 Although magnetic resonance venography most reliably demonstrates thrombi, its lack of widespread availability makes CT with IV contrast the preferred initial study.3Computed tomography not only has high sensitivity, but also offers alternative diagnoses in the undifferentiated presentation.1One study found CT to be 100% sensitive in detecting any abnormality associated with MVT or bowel ischemia.10 Common CT findings of MVT include dilated and thickened bowel loops, mesenteric fat standing, ascites, a halo or target appearance of bowel, vessel filling defects from a thrombus, and pneumatosis intestinalis.11 The latter usually indicates transmural infarction, and can extend as portomesenteric vein gas.11 Of note, if the initial CT scan is non-diagnostic and a high clinical suspicion for mesenteric ischemia remains with no alternative diagnosis, CTA is the gold standard.3,7Expeditious diagnosis of MVT is imperative, given the potential complications of intestinal infarction, submucosal hemorrhage secondary to edema, and third spacing of the venous outflow into the bowel wall due to collateral vessels being unable to redirect blood flow in conjunction with complete venous occlusion.12Not all MVTs progress to infarction, given the extensive collateral circulation. Early diagnosis, however, is crucial for conservative management to be effective.9Acute MVT without signs of infarction necessitates anticoagulation therapy to decrease clot propagation and recurrence.1 In addition, prophylactic antibiotics to limit bacterial translocation, and bowel rest are advised.13,14 If the patient is unresponsive to anticoagulation, thrombolytic and endovascular therapies may be of benefit in select patients.15 Once intestinal ischemia or infarction develops, the prognosis is poor: mortality approaches 75% with infarction.1 If signs of bowel infarction are present, a laparotomy must be performed promptly, although in most cases, delayed patient presentation makes small bowel resection unavoidable.9 Further testing for hypercoagulability is recommended, particularly in isolated thrombosis, since long-term anticoagulation therapy may be necessary if a coagulopathy is discovered.1
Conclusion
Mesenteric venous thrombosis is atypical in a young, healthy patient. However, due to high mortality rates with disease progression, it is important to consider in any patient with unrelenting abdominal pain and vague gastrointestinal symptoms of uncertain cause, even in those without risk factors. Early detection and management of MVT before progression to mesenteric ischemia and infarction considerably lowers the mortality rate. Emergency physicians must be vigilant when treating a patient with abdominal pain out of proportion to physical examination, unrelenting pain despite analgesic medications, or repeat ED visits for the same abdominal complaints.
1. Harnik IG, Brandt LJ. Mesenteric venous thrombosis. Vasc Med. 2010;15(5):407-418. doi:10.1177/1358863x10379673.
2. Tilsed JV, Casamassima A, Kurihara H, et al. ESTES guidelines: acute mesenteric ischaemia. Eur J Trauma Emerg Surg. 2016;42(2):253-270. doi:10.1007/s00068-016-0634-0.
3. Tendler DA, Lamont JT, Grubel P. Mesenteric venous thrombosis in adults. UpToDate Web site. https://www.uptodate.com/contents/mesenteric-venous-thrombosis-in-adults. Accessed November 16, 2017.
4. Al-Zahrani HA, Lindsay T. Mesenteric ischemia. In: Hall JB, Schmidt GA, Kress JP, eds. Principles of Critical Care. 4th ed. New York, NY: McGraw Hill; 2015:1036-1044.
5. Kumar S, Sarr MG, Kamath PS. Mesenteric venous thrombosis. N Engl J Med. 2001;345(23):1683-1688. doi:10.1056/nejmra010076.
6. Al-Khazraji A, Hasan AQ, Patel I, Alkhawam H, Ghrair F, Lieber J. The role of abdominal computed tomography scan in acute pancreatitis. Pancreas. 2017;46(6):e52-e54. doi:10.1097/mpa.0000000000000837.
7. Bradbury MS, Kavanagh PV, Bechtold RE, et al. Mesenteric venous thrombosis: diagnosis and noninvasive imaging. Radiographics. 2002;22(3):527-541.
8. Yang S, Fan X, Ding W, et al. D-dimer as an early marker of severity in patients with acute superior mesenteric venous thrombosis. Medicine (Baltimore). 2014;93(29):e270. doi:10.1097/md.0000000000000270.
9. Matos C, Van Gansbeke D, Zalcman M, et al. Mesenteric vein thrombosis: early CT and US diagnosis and conservative management. Gastrointest Radiol. 1986;11(4):322-325.
10. Rhee RY, Gloviczki P, Mendonca CT, et al. Mesenteric venous thrombosis: still a lethal disease in the 1990s. J Vasc Surg. 1994;20(5):688-697.
11. Furukawa A, Kanasaki S, Kono N, et al. CT diagnosis of acute mesenteric ischemia from various causes. AJR Am J Roentgenol. 2009;192(2):408-416. doi:10.2214/ajr.08.1138.
12. Johnson CC, Baggenstoss AH. Mesenteric vascular occlusion; study of 99 cases of occlusion of veins. Proc Staff Meet Mayo Clin. 1949;24(25):628-636.13. Hmoud B, Singal AK, Kamath PS. Mesenteric venous thrombosis. J Clin Exp Hepatol. 2014;4(3):257-263. doi:10.1016/j.jceh.2014.03.052.
14. Schoots IG, Koffeman GI, Legemate DA, Levi M, van Gulik TM. Systematic review of survival after acute mesenteric ischaemia according to disease aetiology. Br J Surg. 2004;91(1):17-27.
15. Yang S, Fan X, Ding W, et al. Multidisciplinary stepwise management strategy for acute superior mesenteric venous thrombosis: an intestinal stroke center experience. Thromb Res. 2015;135(1):36-45. doi:10.1016/j.thromres.2014.10.018.
1. Harnik IG, Brandt LJ. Mesenteric venous thrombosis. Vasc Med. 2010;15(5):407-418. doi:10.1177/1358863x10379673.
2. Tilsed JV, Casamassima A, Kurihara H, et al. ESTES guidelines: acute mesenteric ischaemia. Eur J Trauma Emerg Surg. 2016;42(2):253-270. doi:10.1007/s00068-016-0634-0.
3. Tendler DA, Lamont JT, Grubel P. Mesenteric venous thrombosis in adults. UpToDate Web site. https://www.uptodate.com/contents/mesenteric-venous-thrombosis-in-adults. Accessed November 16, 2017.
4. Al-Zahrani HA, Lindsay T. Mesenteric ischemia. In: Hall JB, Schmidt GA, Kress JP, eds. Principles of Critical Care. 4th ed. New York, NY: McGraw Hill; 2015:1036-1044.
5. Kumar S, Sarr MG, Kamath PS. Mesenteric venous thrombosis. N Engl J Med. 2001;345(23):1683-1688. doi:10.1056/nejmra010076.
6. Al-Khazraji A, Hasan AQ, Patel I, Alkhawam H, Ghrair F, Lieber J. The role of abdominal computed tomography scan in acute pancreatitis. Pancreas. 2017;46(6):e52-e54. doi:10.1097/mpa.0000000000000837.
7. Bradbury MS, Kavanagh PV, Bechtold RE, et al. Mesenteric venous thrombosis: diagnosis and noninvasive imaging. Radiographics. 2002;22(3):527-541.
8. Yang S, Fan X, Ding W, et al. D-dimer as an early marker of severity in patients with acute superior mesenteric venous thrombosis. Medicine (Baltimore). 2014;93(29):e270. doi:10.1097/md.0000000000000270.
9. Matos C, Van Gansbeke D, Zalcman M, et al. Mesenteric vein thrombosis: early CT and US diagnosis and conservative management. Gastrointest Radiol. 1986;11(4):322-325.
10. Rhee RY, Gloviczki P, Mendonca CT, et al. Mesenteric venous thrombosis: still a lethal disease in the 1990s. J Vasc Surg. 1994;20(5):688-697.
11. Furukawa A, Kanasaki S, Kono N, et al. CT diagnosis of acute mesenteric ischemia from various causes. AJR Am J Roentgenol. 2009;192(2):408-416. doi:10.2214/ajr.08.1138.
12. Johnson CC, Baggenstoss AH. Mesenteric vascular occlusion; study of 99 cases of occlusion of veins. Proc Staff Meet Mayo Clin. 1949;24(25):628-636.13. Hmoud B, Singal AK, Kamath PS. Mesenteric venous thrombosis. J Clin Exp Hepatol. 2014;4(3):257-263. doi:10.1016/j.jceh.2014.03.052.
14. Schoots IG, Koffeman GI, Legemate DA, Levi M, van Gulik TM. Systematic review of survival after acute mesenteric ischaemia according to disease aetiology. Br J Surg. 2004;91(1):17-27.
15. Yang S, Fan X, Ding W, et al. Multidisciplinary stepwise management strategy for acute superior mesenteric venous thrombosis: an intestinal stroke center experience. Thromb Res. 2015;135(1):36-45. doi:10.1016/j.thromres.2014.10.018.
Crizanlizumab relieves sickle cell crises across subgroups
Crizanlizumab effectively reduced vaso-occlusive crises among patients with sickle cell disease (SCD) who have numerous crises, exhibit the HbSS genotype, and take concomitant hydroxyurea, according to investigators.
Across subgroups, crizanlizumab was safe and more effective than placebo at delaying time to first vaso-occlusive crisis (VOC) and eliminating crises, reported lead author Abdullah Kutlar, MD, of the Sickle Cell Center at the Medical College of Georgia, Augusta, and his colleagues.
The phase 2 SUSTAIN trial recently showed that crizanlizumab – a humanized, anti–P-selectin monoclonal antibody – reduced the frequency of VOCs by 45% and delayed time to first crisis by about 3 months (N Engl J Med. 2017;376:429-39).
Additionally, a subgroup analysis showed that there was a lower frequency of pain crises with crizanlizumab 5 mg/kg, compared with placebo, regardless of the number of prior VOCs, concomitant hydroxyurea use, or the SCD genotype.
The present post hoc analysis took a deeper look at these observations across the same subgroups; specifically, the investigators assessed elimination of VOCs, time to first crisis, and adverse events. They reported the findings in the American Journal of Hematology.
Crizanlizumab eliminated pain crises about seven times more frequently than did placebo in patients who had a high frequency of VOCs before the study (28.0% vs. 4.2%), and about twice as often in patients with the HbSS genotype (31.9% vs. 17.0%), and patients who were using concomitant hydroxyurea (33.3% vs. 17.5%).
Further analysis showed that crizanlizumab delayed time to first pain crisis across all subgroups, most dramatically in patients with the HbSS genotype (4.07 months for crizanlizumab vs. 1.12 months for placebo). Safety was comparable across subgroups.
“These findings provide supportive evidence that crizanlizumab provides a clinically meaningful treatment benefit when used alone or in combination with hydroxyurea for the prevention of VOCs,” the investigators wrote.
An ongoing phase 2 pharmacokinetic/pharmacodynamic study is evaluating a higher dose of crizanlizumab (7.5 mg/kg), and another trial seeks to evaluate pediatric doses of the drug.
The study was sponsored by Novartis. The authors reported financial relationships with Novartis, Bluebird Bio, AstraZeneca, and others.
SOURCE: Kutlar A et al. Am J Hematol. 2018 Oct 8. doi: 10.1002/ajh.25308.
Crizanlizumab effectively reduced vaso-occlusive crises among patients with sickle cell disease (SCD) who have numerous crises, exhibit the HbSS genotype, and take concomitant hydroxyurea, according to investigators.
Across subgroups, crizanlizumab was safe and more effective than placebo at delaying time to first vaso-occlusive crisis (VOC) and eliminating crises, reported lead author Abdullah Kutlar, MD, of the Sickle Cell Center at the Medical College of Georgia, Augusta, and his colleagues.
The phase 2 SUSTAIN trial recently showed that crizanlizumab – a humanized, anti–P-selectin monoclonal antibody – reduced the frequency of VOCs by 45% and delayed time to first crisis by about 3 months (N Engl J Med. 2017;376:429-39).
Additionally, a subgroup analysis showed that there was a lower frequency of pain crises with crizanlizumab 5 mg/kg, compared with placebo, regardless of the number of prior VOCs, concomitant hydroxyurea use, or the SCD genotype.
The present post hoc analysis took a deeper look at these observations across the same subgroups; specifically, the investigators assessed elimination of VOCs, time to first crisis, and adverse events. They reported the findings in the American Journal of Hematology.
Crizanlizumab eliminated pain crises about seven times more frequently than did placebo in patients who had a high frequency of VOCs before the study (28.0% vs. 4.2%), and about twice as often in patients with the HbSS genotype (31.9% vs. 17.0%), and patients who were using concomitant hydroxyurea (33.3% vs. 17.5%).
Further analysis showed that crizanlizumab delayed time to first pain crisis across all subgroups, most dramatically in patients with the HbSS genotype (4.07 months for crizanlizumab vs. 1.12 months for placebo). Safety was comparable across subgroups.
“These findings provide supportive evidence that crizanlizumab provides a clinically meaningful treatment benefit when used alone or in combination with hydroxyurea for the prevention of VOCs,” the investigators wrote.
An ongoing phase 2 pharmacokinetic/pharmacodynamic study is evaluating a higher dose of crizanlizumab (7.5 mg/kg), and another trial seeks to evaluate pediatric doses of the drug.
The study was sponsored by Novartis. The authors reported financial relationships with Novartis, Bluebird Bio, AstraZeneca, and others.
SOURCE: Kutlar A et al. Am J Hematol. 2018 Oct 8. doi: 10.1002/ajh.25308.
Crizanlizumab effectively reduced vaso-occlusive crises among patients with sickle cell disease (SCD) who have numerous crises, exhibit the HbSS genotype, and take concomitant hydroxyurea, according to investigators.
Across subgroups, crizanlizumab was safe and more effective than placebo at delaying time to first vaso-occlusive crisis (VOC) and eliminating crises, reported lead author Abdullah Kutlar, MD, of the Sickle Cell Center at the Medical College of Georgia, Augusta, and his colleagues.
The phase 2 SUSTAIN trial recently showed that crizanlizumab – a humanized, anti–P-selectin monoclonal antibody – reduced the frequency of VOCs by 45% and delayed time to first crisis by about 3 months (N Engl J Med. 2017;376:429-39).
Additionally, a subgroup analysis showed that there was a lower frequency of pain crises with crizanlizumab 5 mg/kg, compared with placebo, regardless of the number of prior VOCs, concomitant hydroxyurea use, or the SCD genotype.
The present post hoc analysis took a deeper look at these observations across the same subgroups; specifically, the investigators assessed elimination of VOCs, time to first crisis, and adverse events. They reported the findings in the American Journal of Hematology.
Crizanlizumab eliminated pain crises about seven times more frequently than did placebo in patients who had a high frequency of VOCs before the study (28.0% vs. 4.2%), and about twice as often in patients with the HbSS genotype (31.9% vs. 17.0%), and patients who were using concomitant hydroxyurea (33.3% vs. 17.5%).
Further analysis showed that crizanlizumab delayed time to first pain crisis across all subgroups, most dramatically in patients with the HbSS genotype (4.07 months for crizanlizumab vs. 1.12 months for placebo). Safety was comparable across subgroups.
“These findings provide supportive evidence that crizanlizumab provides a clinically meaningful treatment benefit when used alone or in combination with hydroxyurea for the prevention of VOCs,” the investigators wrote.
An ongoing phase 2 pharmacokinetic/pharmacodynamic study is evaluating a higher dose of crizanlizumab (7.5 mg/kg), and another trial seeks to evaluate pediatric doses of the drug.
The study was sponsored by Novartis. The authors reported financial relationships with Novartis, Bluebird Bio, AstraZeneca, and others.
SOURCE: Kutlar A et al. Am J Hematol. 2018 Oct 8. doi: 10.1002/ajh.25308.
FROM THE AMERICAN JOURNAL OF HEMATOLOGY
Key clinical point:
Major finding: Crizanlizumab eliminated vaso-occlusive crises about seven times more frequently than did placebo in patients with numerous crises (28.0% vs. 4.2%).
Study details: A post hoc analysis of 132 patients from the phase 2 SUSTAIN trial.
Disclosures: The study was sponsored by Novartis. The authors reported financial relationships with Novartis, Bluebird Bio, AstraZeneca, and others.
Source: Kutlar A et al. Am J Hematol. 2018 Oct 8. doi: 10.1002/ajh.25308.
Five “can’t miss” oncologic emergencies
SAN DIEGO – Acute promyelocytic leukemia is one of five “can’t miss” oncologic emergencies, Megan Boysen Osborn, MD, MHPE, told a standing-room-only crowd at the annual meeting of the American College of Emergency Physicians.
In our exclusive video interview, Dr. Osborn, vice chair of education and the residency program director in the department of emergency medicine at the University of California, Irvine, offered tips on how to recognize acute promyelocytic leukemia, leukostasis, neutropenic fever, tumor lysis syndrome, and disseminated intravascular coagulation.
“All patients with suspected leukemias should be admitted,” she said. “Time is of the essence.”
Dr. Osborn reported having no financial disclosures related to her presentation.
SAN DIEGO – Acute promyelocytic leukemia is one of five “can’t miss” oncologic emergencies, Megan Boysen Osborn, MD, MHPE, told a standing-room-only crowd at the annual meeting of the American College of Emergency Physicians.
In our exclusive video interview, Dr. Osborn, vice chair of education and the residency program director in the department of emergency medicine at the University of California, Irvine, offered tips on how to recognize acute promyelocytic leukemia, leukostasis, neutropenic fever, tumor lysis syndrome, and disseminated intravascular coagulation.
“All patients with suspected leukemias should be admitted,” she said. “Time is of the essence.”
Dr. Osborn reported having no financial disclosures related to her presentation.
SAN DIEGO – Acute promyelocytic leukemia is one of five “can’t miss” oncologic emergencies, Megan Boysen Osborn, MD, MHPE, told a standing-room-only crowd at the annual meeting of the American College of Emergency Physicians.
In our exclusive video interview, Dr. Osborn, vice chair of education and the residency program director in the department of emergency medicine at the University of California, Irvine, offered tips on how to recognize acute promyelocytic leukemia, leukostasis, neutropenic fever, tumor lysis syndrome, and disseminated intravascular coagulation.
“All patients with suspected leukemias should be admitted,” she said. “Time is of the essence.”
Dr. Osborn reported having no financial disclosures related to her presentation.
REPORTING FROM ACEP18
What’s the best VTE treatment for patients with cancer?
EVIDENCE SUMMARY
No head-to-head studies or umbrella meta-analyses assess all the main treatments for VTE against each other.
Long-term LMWH decreases VTE recurrence compared with VKA
Two meta-analyses of RCTs evaluating LMWH and VKA for long-term treatment (3-12 months) of confirmed VTE in patients with cancer found that LMWH didn’t change mortality, but reduced the rate of VTE recurrence compared with VKA (40% relative reduction).1,2 The comparison showed no differences in major or minor bleeding or thrombocytopenia between LMWH and VKA (TABLE1-5).
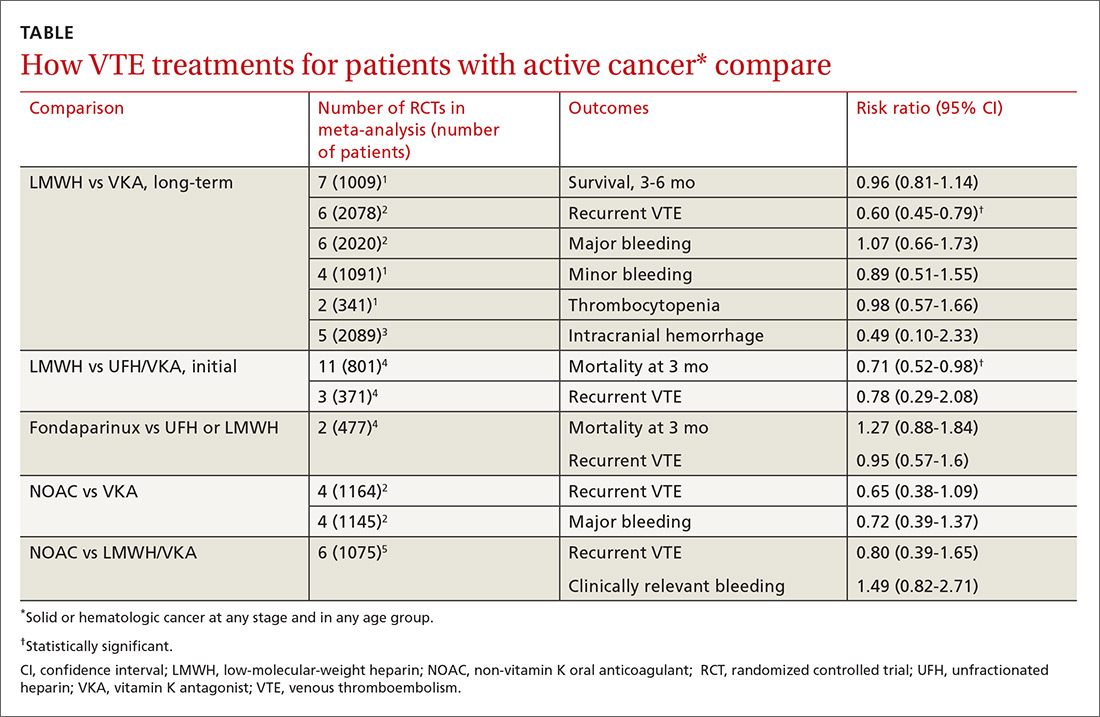
The studies included patients with any solid or hematologic cancer at any stage and from any age group, including children. Overall, the mean age of patients was in the mid 60s; approximately 50% were male when specified. Investigators rated the evidence quality as moderate for VTE, but low for the other outcomes.1
The most recent meta-analysis of the same RCTs comparing LMWH with VKA evaluated intracranial hemorrhage rates and found no difference.3
Initial therapy with LMWH: A look at mortality
A meta-analysis of RCTs that compared LMWH with UFH/VKA for initial treatment of confirmed VTE in adult cancer patients (any type or stage of cancer, mean ages not specified) found that LMWH reduced mortality by 30%, but didn’t affect VTE recurrence or major bleeding.4
The control groups received UFH for 5 to 10 days and then continued with VKA, whereas the experimental groups received different types of LMWH (reviparin, nadroparin, tinzaparin, enoxaparin) initially and for 3 months thereafter. Investigators rated all studies low quality because of imprecision and publication bias favoring LMWH.
Fondaparinux shows no advantage for initial therapy
The same meta-analysis compared initial treatment with fondaparinux and initial therapy with enoxaparin or UFH transitioning to warfarin.4 It found no differences in any outcomes at 3 months. Investigators rated both studies as low quality for recurrent VTE and moderate for mortality and bleeding.
Continue to: Non-vitamin K oral anticoagulants vs LMWH/VKA or VKA
Non-vitamin K oral anticoagulants vs LMWH/VKA or VKA: No differences
A meta-analysis of RCTs comparing NOACs (dabigatran, edoxaban, apixaban, rivaroxaban) with VKA for 6 months found no differences in recurrent VTE or major bleeding.2
A second meta-analysis of RCTs that compared NOACs (rivaroxaban, dabigatran, apixaban) with control (LMWH followed by VKA) in adult cancer patients (mean ages, 54-66 years; 50%-60% men) reported no difference in the composite outcome of recurrent VTE or VTE-related death nor clinically significant bleeding over 1 to 36 months (most RCTs ran 3-12 months).5 Separate comparisons for rivaroxaban and dabigatran found no difference in the composite outcome, and rivaroxaban also produced no difference in clinically-significant bleeding.
RECOMMENDATIONS
The 2016 CHEST guidelines recommend LMWH as first-line treatment for VTE in patients with cancer and indicate no preference between NOACs and VKA for second-line treatment.6
1. Akl EA, Kahale L, Barba M, et al. Anticoagulation for the long-term treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2014;(7):CD006650.
2. Posch F, Königsbrügge O, Zielinski C, et al. Treatment of venous thromboembolism in patients with cancer: A network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136:582-589.
3. Rojas-Hernandez CM, Oo TH, García-Perdomo HA. Risk of intracranial hemorrhage associated with therapeutic anticoagulation for venous thromboembolism in cancer patients: a systematic review and meta-analysis. J Thromb Thrombolysis. 2017;43:233-240.
4. Akl EA, Kahale L, Neumann I, et al. Anticoagulation for the initial treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2014;(6):CD006649.
5. Sardar P, Chatterjee S, Herzog E, et al. New oral anticoagulants in patients with cancer: current state of evidence. Am J Ther. 2015;22:460-468.
6. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315-352.
EVIDENCE SUMMARY
No head-to-head studies or umbrella meta-analyses assess all the main treatments for VTE against each other.
Long-term LMWH decreases VTE recurrence compared with VKA
Two meta-analyses of RCTs evaluating LMWH and VKA for long-term treatment (3-12 months) of confirmed VTE in patients with cancer found that LMWH didn’t change mortality, but reduced the rate of VTE recurrence compared with VKA (40% relative reduction).1,2 The comparison showed no differences in major or minor bleeding or thrombocytopenia between LMWH and VKA (TABLE1-5).

The studies included patients with any solid or hematologic cancer at any stage and from any age group, including children. Overall, the mean age of patients was in the mid 60s; approximately 50% were male when specified. Investigators rated the evidence quality as moderate for VTE, but low for the other outcomes.1
The most recent meta-analysis of the same RCTs comparing LMWH with VKA evaluated intracranial hemorrhage rates and found no difference.3
Initial therapy with LMWH: A look at mortality
A meta-analysis of RCTs that compared LMWH with UFH/VKA for initial treatment of confirmed VTE in adult cancer patients (any type or stage of cancer, mean ages not specified) found that LMWH reduced mortality by 30%, but didn’t affect VTE recurrence or major bleeding.4
The control groups received UFH for 5 to 10 days and then continued with VKA, whereas the experimental groups received different types of LMWH (reviparin, nadroparin, tinzaparin, enoxaparin) initially and for 3 months thereafter. Investigators rated all studies low quality because of imprecision and publication bias favoring LMWH.
Fondaparinux shows no advantage for initial therapy
The same meta-analysis compared initial treatment with fondaparinux and initial therapy with enoxaparin or UFH transitioning to warfarin.4 It found no differences in any outcomes at 3 months. Investigators rated both studies as low quality for recurrent VTE and moderate for mortality and bleeding.
Continue to: Non-vitamin K oral anticoagulants vs LMWH/VKA or VKA
Non-vitamin K oral anticoagulants vs LMWH/VKA or VKA: No differences
A meta-analysis of RCTs comparing NOACs (dabigatran, edoxaban, apixaban, rivaroxaban) with VKA for 6 months found no differences in recurrent VTE or major bleeding.2
A second meta-analysis of RCTs that compared NOACs (rivaroxaban, dabigatran, apixaban) with control (LMWH followed by VKA) in adult cancer patients (mean ages, 54-66 years; 50%-60% men) reported no difference in the composite outcome of recurrent VTE or VTE-related death nor clinically significant bleeding over 1 to 36 months (most RCTs ran 3-12 months).5 Separate comparisons for rivaroxaban and dabigatran found no difference in the composite outcome, and rivaroxaban also produced no difference in clinically-significant bleeding.
RECOMMENDATIONS
The 2016 CHEST guidelines recommend LMWH as first-line treatment for VTE in patients with cancer and indicate no preference between NOACs and VKA for second-line treatment.6
EVIDENCE SUMMARY
No head-to-head studies or umbrella meta-analyses assess all the main treatments for VTE against each other.
Long-term LMWH decreases VTE recurrence compared with VKA
Two meta-analyses of RCTs evaluating LMWH and VKA for long-term treatment (3-12 months) of confirmed VTE in patients with cancer found that LMWH didn’t change mortality, but reduced the rate of VTE recurrence compared with VKA (40% relative reduction).1,2 The comparison showed no differences in major or minor bleeding or thrombocytopenia between LMWH and VKA (TABLE1-5).

The studies included patients with any solid or hematologic cancer at any stage and from any age group, including children. Overall, the mean age of patients was in the mid 60s; approximately 50% were male when specified. Investigators rated the evidence quality as moderate for VTE, but low for the other outcomes.1
The most recent meta-analysis of the same RCTs comparing LMWH with VKA evaluated intracranial hemorrhage rates and found no difference.3
Initial therapy with LMWH: A look at mortality
A meta-analysis of RCTs that compared LMWH with UFH/VKA for initial treatment of confirmed VTE in adult cancer patients (any type or stage of cancer, mean ages not specified) found that LMWH reduced mortality by 30%, but didn’t affect VTE recurrence or major bleeding.4
The control groups received UFH for 5 to 10 days and then continued with VKA, whereas the experimental groups received different types of LMWH (reviparin, nadroparin, tinzaparin, enoxaparin) initially and for 3 months thereafter. Investigators rated all studies low quality because of imprecision and publication bias favoring LMWH.
Fondaparinux shows no advantage for initial therapy
The same meta-analysis compared initial treatment with fondaparinux and initial therapy with enoxaparin or UFH transitioning to warfarin.4 It found no differences in any outcomes at 3 months. Investigators rated both studies as low quality for recurrent VTE and moderate for mortality and bleeding.
Continue to: Non-vitamin K oral anticoagulants vs LMWH/VKA or VKA
Non-vitamin K oral anticoagulants vs LMWH/VKA or VKA: No differences
A meta-analysis of RCTs comparing NOACs (dabigatran, edoxaban, apixaban, rivaroxaban) with VKA for 6 months found no differences in recurrent VTE or major bleeding.2
A second meta-analysis of RCTs that compared NOACs (rivaroxaban, dabigatran, apixaban) with control (LMWH followed by VKA) in adult cancer patients (mean ages, 54-66 years; 50%-60% men) reported no difference in the composite outcome of recurrent VTE or VTE-related death nor clinically significant bleeding over 1 to 36 months (most RCTs ran 3-12 months).5 Separate comparisons for rivaroxaban and dabigatran found no difference in the composite outcome, and rivaroxaban also produced no difference in clinically-significant bleeding.
RECOMMENDATIONS
The 2016 CHEST guidelines recommend LMWH as first-line treatment for VTE in patients with cancer and indicate no preference between NOACs and VKA for second-line treatment.6
1. Akl EA, Kahale L, Barba M, et al. Anticoagulation for the long-term treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2014;(7):CD006650.
2. Posch F, Königsbrügge O, Zielinski C, et al. Treatment of venous thromboembolism in patients with cancer: A network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136:582-589.
3. Rojas-Hernandez CM, Oo TH, García-Perdomo HA. Risk of intracranial hemorrhage associated with therapeutic anticoagulation for venous thromboembolism in cancer patients: a systematic review and meta-analysis. J Thromb Thrombolysis. 2017;43:233-240.
4. Akl EA, Kahale L, Neumann I, et al. Anticoagulation for the initial treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2014;(6):CD006649.
5. Sardar P, Chatterjee S, Herzog E, et al. New oral anticoagulants in patients with cancer: current state of evidence. Am J Ther. 2015;22:460-468.
6. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315-352.
1. Akl EA, Kahale L, Barba M, et al. Anticoagulation for the long-term treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2014;(7):CD006650.
2. Posch F, Königsbrügge O, Zielinski C, et al. Treatment of venous thromboembolism in patients with cancer: A network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136:582-589.
3. Rojas-Hernandez CM, Oo TH, García-Perdomo HA. Risk of intracranial hemorrhage associated with therapeutic anticoagulation for venous thromboembolism in cancer patients: a systematic review and meta-analysis. J Thromb Thrombolysis. 2017;43:233-240.
4. Akl EA, Kahale L, Neumann I, et al. Anticoagulation for the initial treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2014;(6):CD006649.
5. Sardar P, Chatterjee S, Herzog E, et al. New oral anticoagulants in patients with cancer: current state of evidence. Am J Ther. 2015;22:460-468.
6. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315-352.
EVIDENCE-BASED ANSWER:
No head-to-head studies directly compare all the main treatments for venous thromboembolism (VTE) in cancer patients. Long-term treatment (3-12 months) with low-molecular-weight heparin (LMWH) reduces recurrence of VTE by 40% compared with vitamin K antagonists (VKA), but doesn’t change rates of mortality, major or minor bleeding, or intracranial hemorrhage in patients with solid or hematologic cancer at any stage or in any age group. Initial treatment with LMWH reduces mortality by 30% compared with unfractionated heparin (UFH) for 5 to 10 days followed by warfarin, but doesn’t alter recurrent VTE or bleeding. Non-vitamin K oral anticoagulants (NOACs) have risks of recurrent VTE or VTE-related death (composite outcome) and clinically significant bleeding comparable to VKA or LMWH/VKA (strength of recommendation [SOR]: B, meta-analyses of randomized controlled trials [RCTs], mostly of low quality).
Strategies for caring for the well cancer survivor
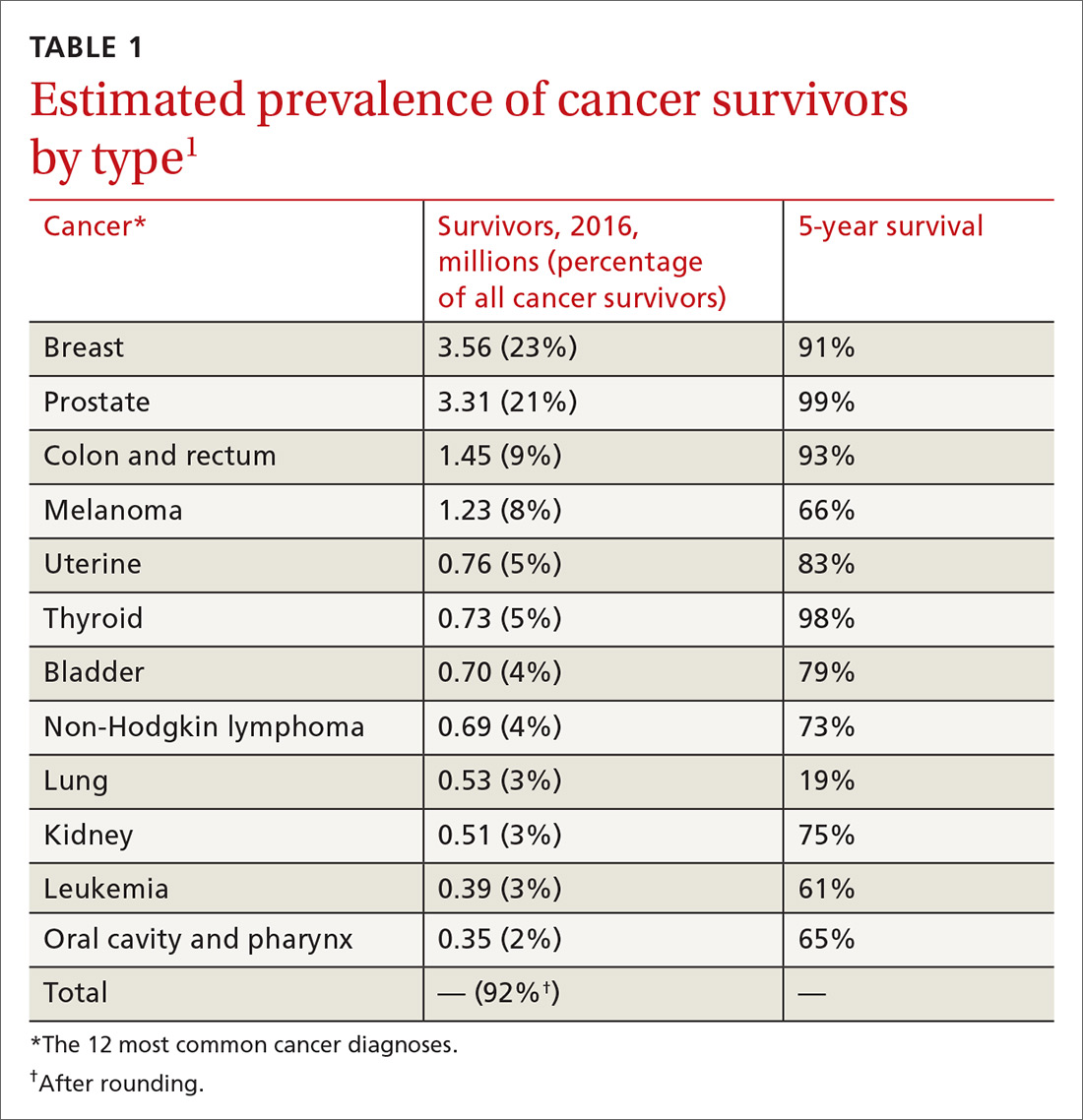
Cancer survivors represent a rapidly increasing population. In 1971, there were 3 million cancer survivors; this number increased to 15.5 million in 2016 and will reach 20 million by 2026.1TABLE 11 shows the percentage of survivors by type of cancer. Cancer survivors tend to be older,* comprising nearly 1 of every 5 people older than 65 years.2
The Institute of Medicine (IOM) identified 3 key characteristics of cancer survivors3:
- Trajectories of survivorship are variable; many cancer patients have periods of relative health between episodes of their disease.
- Survivors require careful cancer monitoring; in addition to the risk that their primary cancer will recur, they have an elevated risk for another, second cancer.
- Both cancer and its treatments increase the risk of other medical and psychiatric problems.
Family physicians (FPs) have optimal skills for navigating the chronic risks and health concerns of the well cancer survivor. This article reviews the primary care management of the functional cancer survivor, focusing on the management of chronic conditions and preventive care.
Survivorship follows any of 6 paths
Cancer survivorship is increasing in importance as treatment has steadily reduced mortality. Six trajectories of cancer survivors have been identified1:
- living cancer-free after treatment with minimal effects
- living cancer-free but suffering serious treatment complications
- Suffering late recurrence
- Developing a second cancer
- Living with intermittent cancer recurrences
- Living with cancer continuously.
Only patients in the last 2 groups are likely to be managed primarily by oncologists.
Survivors look to their FPs for ongoing care
Cancer survivors routinely see their primary care physician after initial treatment. A study of 30,000 Canadian breast cancer survivors demonstrated that follow-up care was limited to an oncologist in only 2%; 84% saw a primary care provider and an oncologist; and 14% saw a primary care provider only.4 A study of colorectal cancer survivors showed that primary care visits increased in each of the 5 years after diagnosis, during which time oncology visits decreased steadily5; in that study, primary care physicians delivered more preventive care than oncologists did.5 Similar to what is done in other chronic conditions, the various effects of cancer are best managed as a whole.
The IOM recommends that cancer survivor care comprise 4 elements2:
- coordination between oncologist and primary care physician
- surveillance for recurrence or spread of existing cancer
- screening for new cancer
- intervention for the effects of cancer and treatment.
Continue to: The following discussion summarizes...
The following discussion summarizes evidence and recommendations for each element of the IOM recommendations for survivor care.
Implementing the 4 elements of cancer survivor care
1. Coordinate care through a unified survivorship care plan
The IOM has noted that the needs of cancer survivors are rarely met2; communication between oncology and primary care is often deficient during transition of care. The IOM has recommended that oncologists provide a survivorship care plan that details the cancer (ie, tumor characteristics), the type of treatment (ie, enrollment in a clinical trial; medical, surgical, or radiation), support services, and follow-up recommendations for the primary care provider. (Examples of elements of a survivorship care plan can be found at www.mskcc.org/hcp-education-training/survivorship/survivorship-care-plan6 and http://sma.org/southern-medical-journal/article/cancer-survivors-history-physical/7).
Regrettably, survivorship care plans have been rarely and poorly employed. Studies show that fewer than one-half of oncologists provide a plan, and that when they do, the plan often lacks recommended information.8,9 Survivorship care plans may soon become common practice, however; the Commission on Cancer of the American College of Surgeons has required their use in all certified cancer centers since 2015.10
2. Provide surveillance of existing cancer
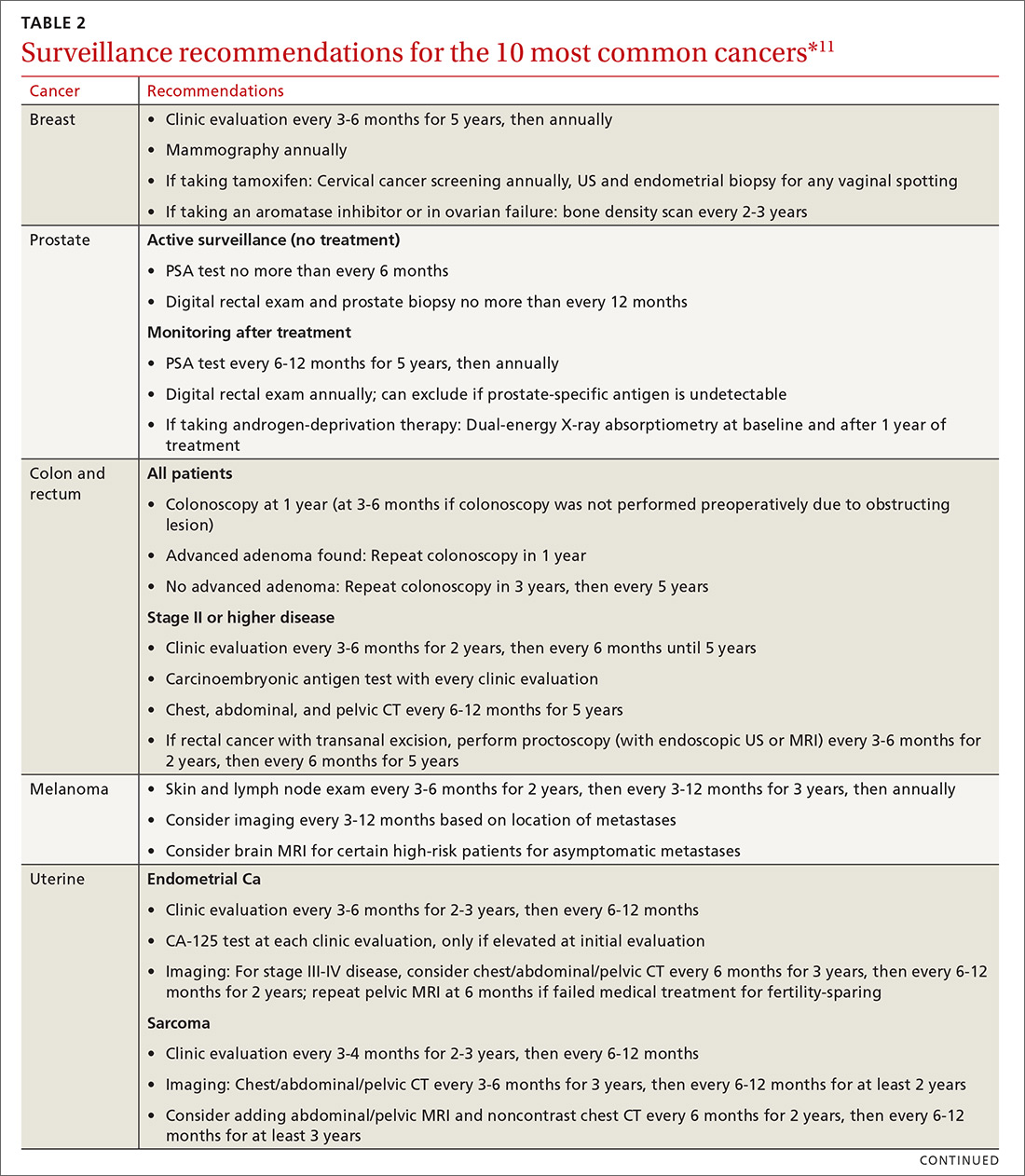
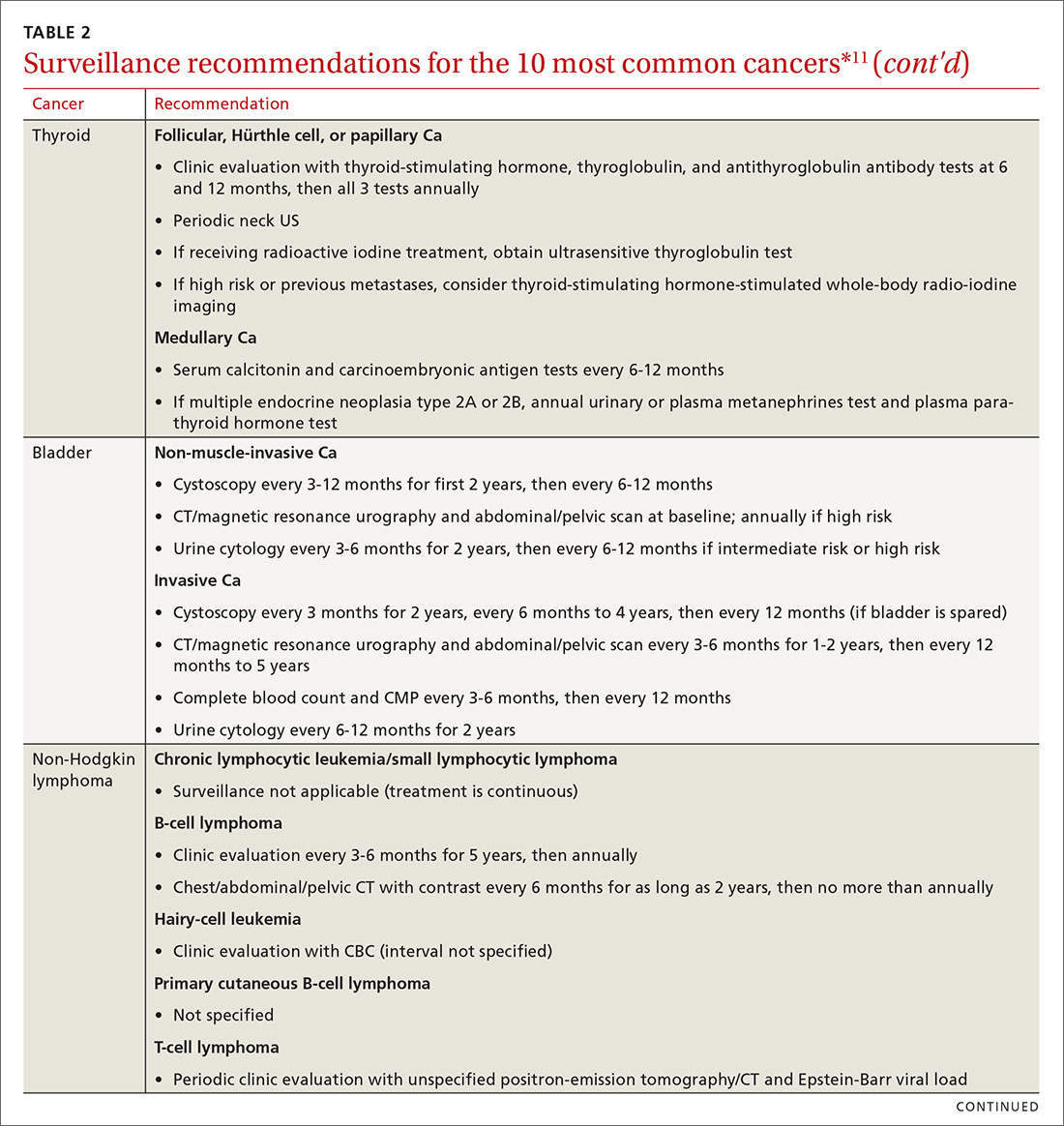
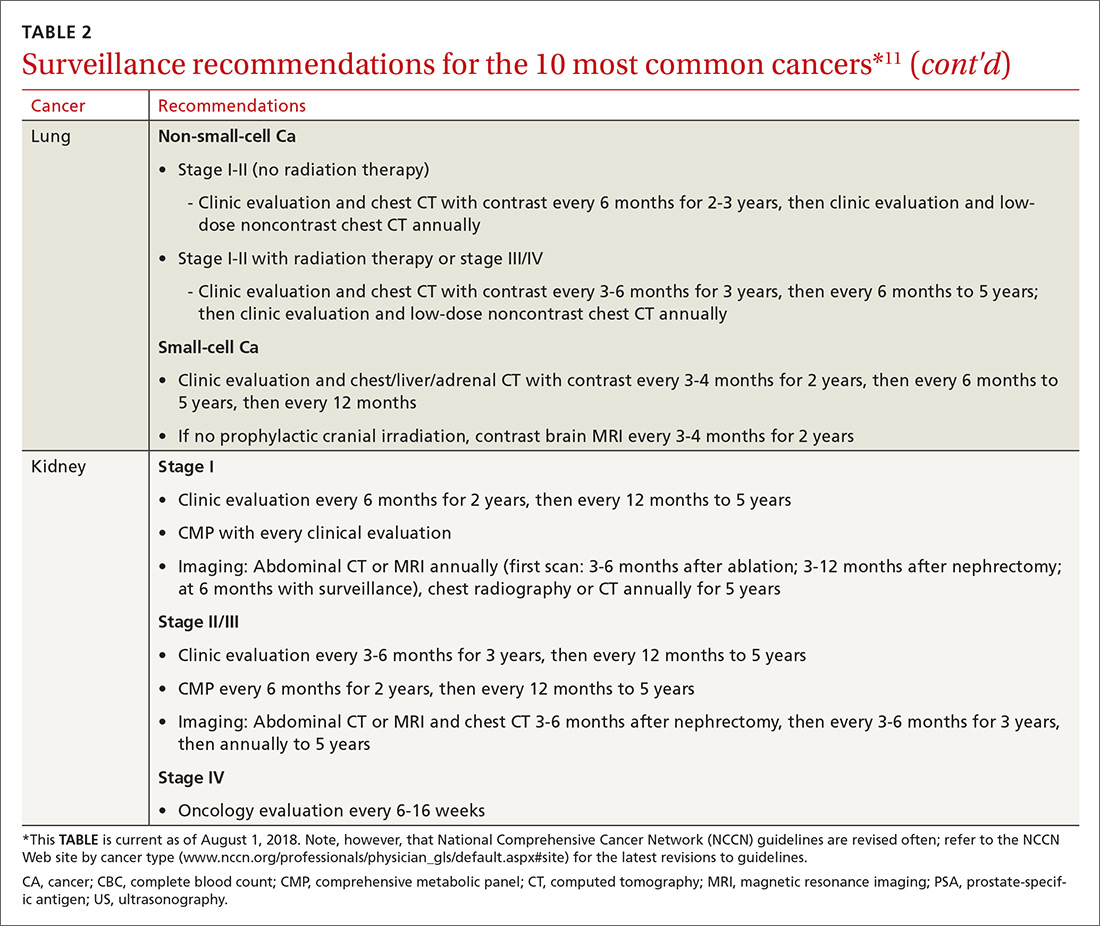
Cancer follow-up is challenging after the initial treatment phase. Although there are many conflicting guidelines for surveillance after cancer, guidelines of the National Comprehensive Cancer Network (NCCN) (summarized in TABLE 211 for the 10 most common cancers in survivors) are the ones generally accepted.12,13
Although individual surveillance recommendations are based on limited evidence, studies confirm the importance of surveillance. A systematic review showed that surveillance mammography after breast cancer reduces breast cancer mortality by 36%.14 A study showed that bladder cancer recurrence diagnosed by surveillance instead of by symptoms led to a 35% increase in 5-year survival.15
Continue to: Yet adherence to cancer surveillance...
Yet adherence to cancer surveillance recommendations is poor. A study of patients with colon cancer demonstrated that only 12% met all recommended surveillance guidelines.16 A study of patients with bladder cancer after radical cystectomy showed that only 9% met recommended surveillance more than 2 years after diagnosis.17 Those dismal statistics may be the result of provider oversight—not patient reluctance.
In the colon cancer study, for example, compliance with follow-up colonoscopy was 80% but compliance with carcinoembryonic antigen testing was only 22%.16 In the bladder cancer study, follow-up urine cytology was obtained in only 23% of patients, although 75% completed recommended imaging.17
Although surveillance remains the oncologist’s responsibility, visits to the FP provide an opportunity to review surveillance and order needed laboratory testing and other studies, including imaging.
3. Screen for new cancers
The risk of a second cancer is elevated for cancer survivors compared with the risk of a primary cancer in the healthy general population; some survivors have a lifetime risk of a second cancer as high as 36%.18 Risk varies by cancer type (TABLE 319). Some of this variation is due to the impact of smoking: Smoking-related cancers have the highest risk of second malignancy.19 Genetic predisposition to malignant transformation is also theorized to contribute to increased risk. Second malignancies are dangerous; 55% of patients die of the second cancer compared with only 13% of their initial cancer.19
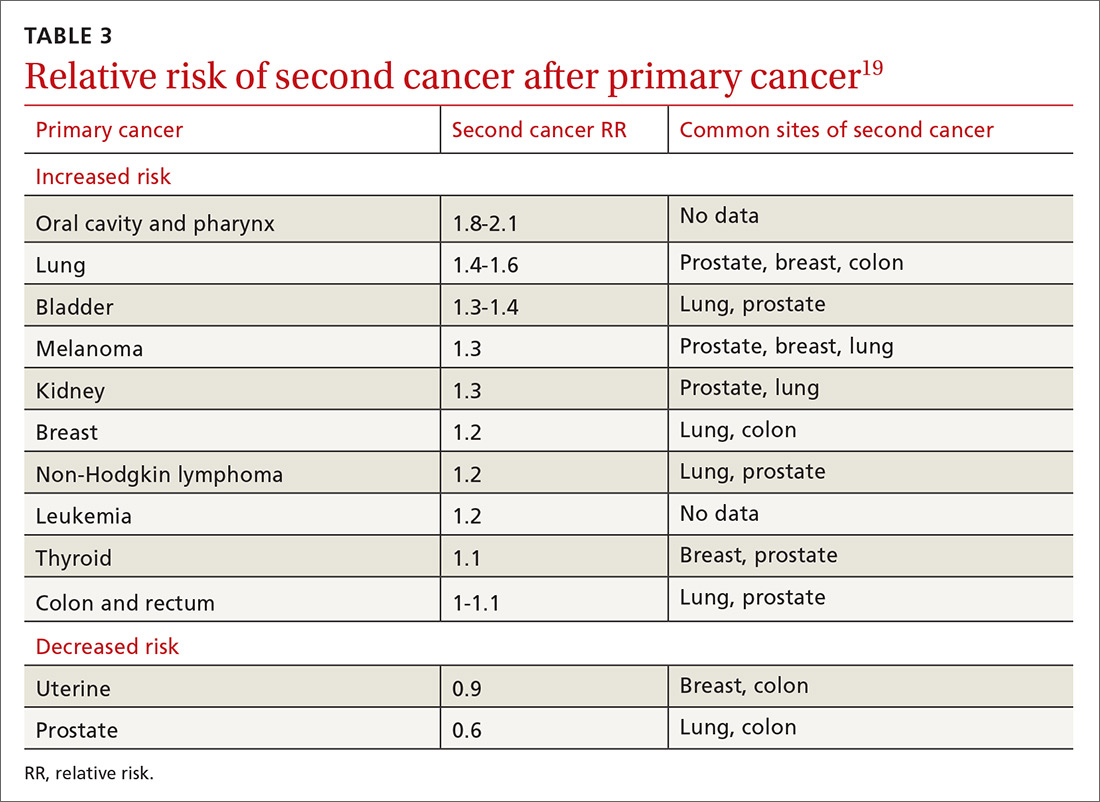
Studies show that cancer survivors display varying adherence with recommended screening for second cancers. In a study of Latina cancer survivors, depressive symptoms were associated with lower screening compliance.20 A study of survivors of hematologic cancer showed a low rate of cancer screening and high fear of cancer recurrence—suggesting avoidance due to fear.21 Other studies, however, show similar or increased compliance with screening in cancer survivors.22,23 A meta-analysis of 19 studies determined that, overall, cancer survivors receive 25% to 38% more recommended screening than the general population.24
Continue to: Few guidelines exist to guide FPs...
Few guidelines exist to guide FPs in adjusting screening for the cancer survivor. For women who received radiation therapy for a tumor in the chest, for example, the recommendation offered by several groups is to start breast cancer screening 8 to 10 years after treatment or by 30 years of age, and to consider combining magnetic resonance imaging and mammography.25 Recommendations for breast cancer screening do not account for a history of other gynecologic cancers unless genetic markers are present.25 On the other hand, the impact of a history of cancer on the risk of prostate cancer and on screening decisions has not been studied,26 and cervical cancer screening guidelines, which recommend that screening continue after 65 years of age for patients who are immunocompromised, do not address a history of other cancer.27
4. Manage the effects of both the cancer and the treatment
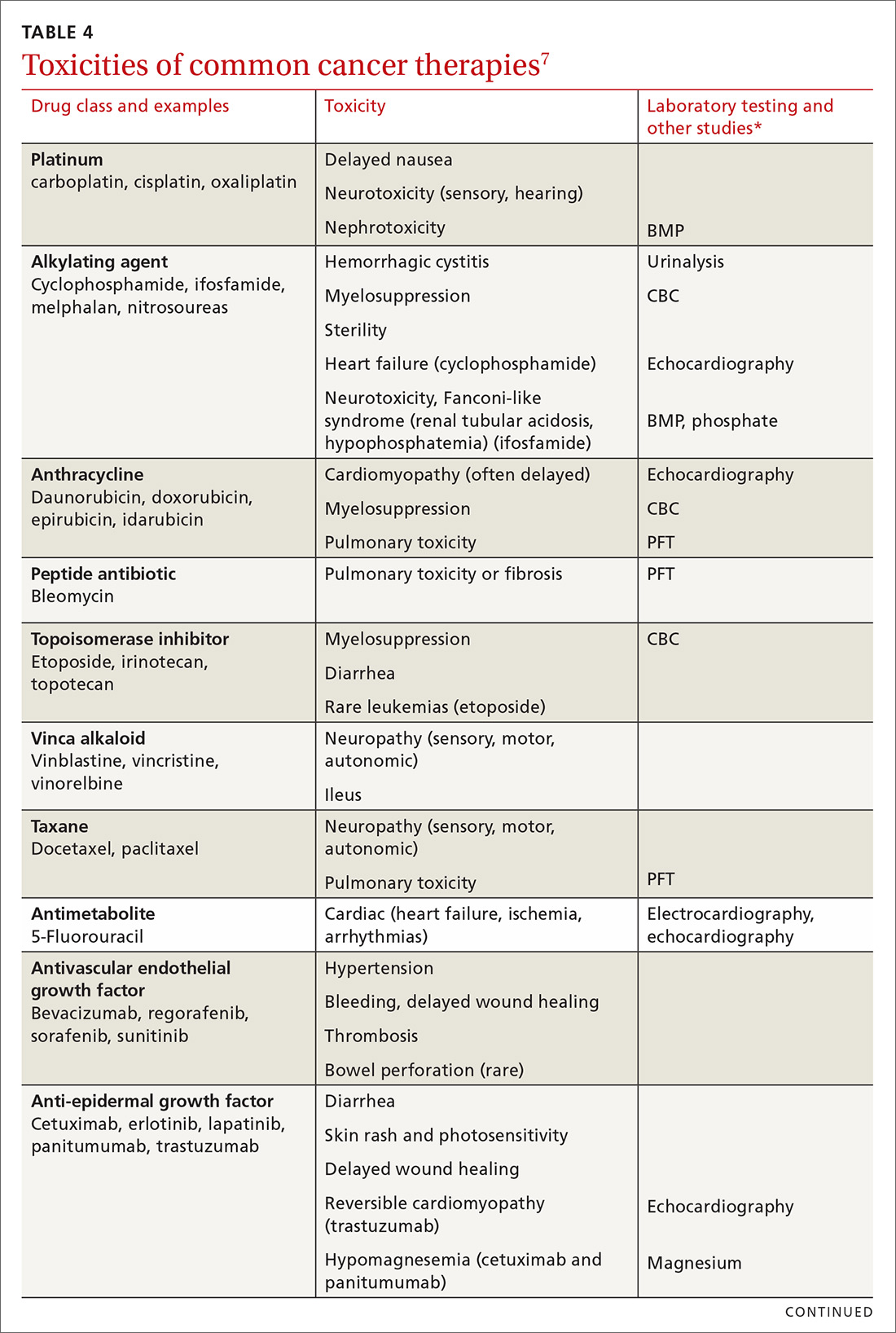
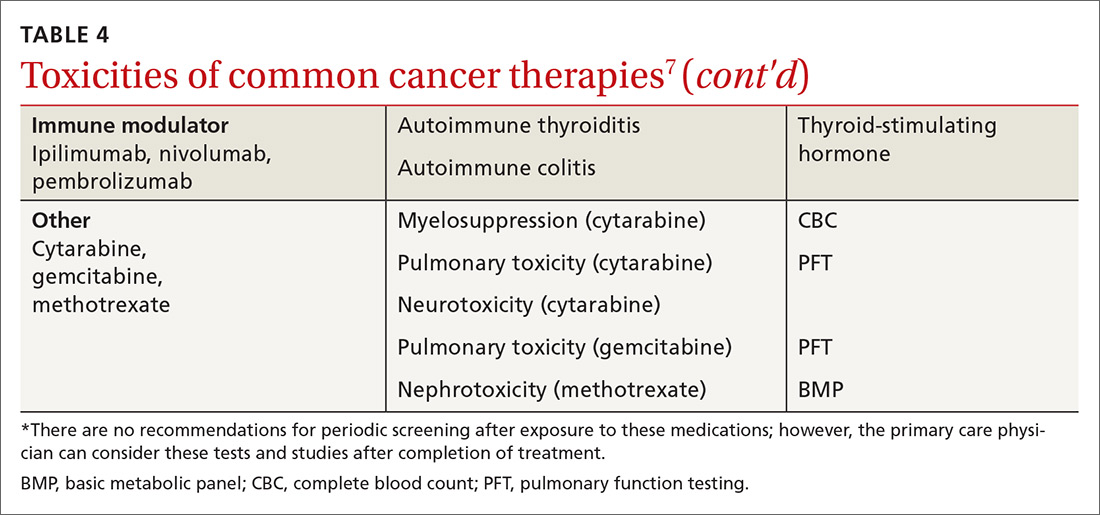
Medical issues faced by cancer survivors are familiar to FPs, but there are some specific recommendations regarding evaluation and treatment that stand in contrast to what would be considered for a healthy, or non-cancer, patient. For example, each chemotherapeutic agent has characteristic adverse effects; TABLE 47 lists the principal adverse effects of common agents and recommendations for testing when these problems develop. Common long-term problems in cancer survivors include fatigue, chronic pain, cognitive dysfunction, psychiatric illness, and cardiovascular disease. Although these symptoms and manifestations are common, the physician must be careful: New or changing symptoms could signal the spread or recurrence of disease. Fear of recurrence can lead patients to exaggerate or minimize symptoms.
Fatigue is the most common symptom seen in cancer survivors during treatment and following remission.28 More than 40% of cancer survivors report significant fatigue.29 Although fatigue is concerning for cancer recurrence, other causes are common in cancer survivors. Both depression and anxiety commonly present with worsened fatigue.30 Sleep disturbances are common, even without a psychiatric diagnosis.31 Effects of treatment, including nausea, anemia, heart failure, and medication adverse effects can cause or worsen fatigue. Pain is associated with fatigue, but to a lesser extent than are depression, anxiety, and nausea.32
Pharmacotherapy of cancer-related fatigue is challenging. Psychostimulants have been most studied. A recent systematic review shows that methylphenidate produces mild or moderate improvement in fatigue, whereas modafanil has minimal effectiveness.33 Antidepressants have not been shown to relieve fatigue.33
A recent meta-analysis showed that nonpharmaceutical treatments for cancer-related fatigue are more effective than pharmacotherapy. In this review, both exercise and pharmacotherapy had a mild-to-moderate effect on fatigue.35 Exercise is best studied in this regard, and has shown the most consistent results.31
Continue to: Chronic pain
Chronic pain. Pain is common in cancer survivors: As many as 40% experience pain for years after initial therapy.36 Treatment of some cancers—eg, thoracotomy (80%), amputation (50%-80%), neck dissection (52%), and surgical management of breast cancer (63%)—increase the likelihood of chronic pain.37 Reports of pain in cancer survivors that should be considered red flags that might signal recurrence of cancer include new or worsening pain; pain worse at night or when recumbent; new neurologic symptoms; and general symptoms of systemic illness37 (TABLE 537).

Management of pain is best approached by its cause, with neurologic, rheumatologic (including myofascial pain and arthralgia), lymphatic, and genital causes most common.37 Across all types of pain, complete relief is unlikely; functional goals provide a more effective target.
For neuropathic cancer pain, duloxetine is the only medication with evidence of benefit; anticonvulsant and topical medications are recommended on the basis of the findings of studies of noncancer pain.38 There are few data on the value of treatments for cancer-related rheumatologic and lymphatic pain, although exercise has shown benefit in both types.38 For dyspareunia and sexual dysfunction (common after gynecologic and nongynecologic cancers), vaginal lubricants and pelvic-floor physiotherapy have shown benefit.39 There is significant overlap in psychiatric comorbidities, sleep, and pain, and addressing all of a patient’s problems can reduce pain and improve function.40
Opioids are often prescribed for pain in cancer survivors. Cancer survivors have a higher rate of opioid prescribing compared with that of non-cancer patients, even 10 years after diagnosis.41 Guidelines of the Centers for Disease Control and Prevention for using opioids to manage chronic pain specifically exclude cancer patients.42 Regrettably, there is no evidence that opioids have long-term efficacy in chronic pain; in fact, evidence is accumulating that chronic opioid therapy exacerbates chronic pain.43
Cognitive dysfunction is present in 17% to 75% of cancer survivors as memory disturbance, psychological disorder, sleep dysfunction, or impairment of executive functioning.44 Cognitive deficits appear to be secondary to both cancer and treatment modalities45; as many as one-third of patients have cognitive dysfunction prior to receiving chemotherapy.46
Continue to: Chemotherapies that are more likely...
Chemotherapies that are more likely to cause cognitive symptoms include methotrexate, 5-fluorouracil, cyclophosphamide, and hormone antagonists.47 More powerful regimens and repetitive chemotherapy regimens tend to cause more cognitive effects.47
Cognitive training interventions show evidence of likely benefit,44,48 leading to recommendations for self-treatment strategies, such as written lists, wordplay, crossword puzzles, jigsaw puzzles, playing a musical instrument, and new hobbies. Small studies suggest a benefit from cognitive behavioral therapy.44,49 A study of breast cancer survivors showed that yoga led to improvement in patient-reported cognitive dysfunction.50 Physical exercise yields cognitive benefit in healthy older adults and is supported by limited evidence in cancer survivors.51
There is no effective pharmacotherapy for cancer- and cancer chemotherapy-related cognitive dysfunction unless a treatable underlying cause is found.44 Symptoms tend to subside with time after completion of chemotherapy, which might be reassuring to patients and families.45
Psychiatric problems. The most common psychiatric issues in cancer survivors are anxiety and depression; the prevalence of anxiety is nearly double that of depression.52 Anxiety often presents as fear of a recurrence of cancer or a feeling of lack of control over present or future circumstances.53 Screening for anxiety and depression is recommended at each visit, using standardized screening questionnaires.54
A small study suggests that psychiatric treatment reduces the risk of early mortality.55 Small studies also suggest that mindfulness-based therapy and cognitive behavioral therapy delivered by telehealth offer benefit.56 A meta-analysis shows that exercise interventions improve depression and anxiety in breast cancer patients.57
Continue to: There are few studies of pharmacotherapy...
There are few studies of pharmacotherapy of anxiety or depression in cancer survivors56; it is known that cancer survivors are nearly twice as likely as the general population to be taking medical therapy for anxiety and depression.58 A Cochrane systematic review of 7 small studies showed uncertain improvement in depressive symptoms in patients with cancer from antidepressant medication; however, an earlier systematic review did show benefit.59,60
In a trial of patients without depression who were being treated for head and neck cancer, escitalopram, 20 mg/d, reduced the risk of subsequent depression compared with placebo.61 A study of 420 breast cancer survivors showed that 300 mg/d and 900 mg/d dosages of gabapentin were both superior to placebo, and nearly equivalent to each other, at reducing anxiety scores.62 In both studies, however, the evidence is nonetheless insufficient to make specific recommendations about these medications.
Cardiac risk
Among chemotherapeutic agents, anthracyclines, such as doxorubicin, cause the most rapid and striking myocyte damage. This damage is dose-dependent and nearly irreversible, with 98% of injury occurring within the first year of chemotherapy.64 More than one half of cancer patients taking an anthracycline have cardiac dysfunction on imaging; 5% will be in overt heart failure 10 to 20 years, or longer, after chemotherapy.63 Following monitoring at 1 year post-therapy, regular cardiac imaging is not recommended in the absence of symptoms.62
Because other cardiotoxic chemotherapeutic agents cause partially reversible damage, imaging is not recommended in the absence of symptoms in patients taking those agents.64
Continue to: Radiation therapy to the chest leads...
Radiation therapy to the chest leads to many cardiac complications, including cardiomyopathy, valvular disease, pericardial disease, and arrhythmias. Development of cardiomyopathy can be delayed 20 to 30 years after radiation; screening echocardiography is therefore recommended every 5 to 10 years after radiation therapy.65 Recent adjustments to the dosages and delivery of radiation therapy should reduce cardiac damage, but will require decades to validate.63
For patients at risk of cardiovascular disease prior to treatment of cancer, there is evidence to support preventive treatment with angiotensin II-receptor antagonists, beta-blockers, and statins to prevent cardiomyopathy.63 Treatment of diagnosed cardiomyopathy and heart failure follows standard guidelines, with significant emphasis on aerobic exercise and smoking cessation.63
Cancer survivorship care: Your critical role
Cancer survivors constitute a large population who frequent the practices of primary care physicians. Primary care visits provide an opportunity to monitor key elements of survivorship, including surveillance of the current cancer and screening for second cancers. Similar to what is seen with diabetes and coronary artery disease, cancer increases cardiac risk, which requires preventive care and chronic management. FPs are well placed to treat common issues in cancer survivors—issues that mirror concerns seen in the general population.
CORRESPONDENCE
Michael J. Arnold, MD, CDR, USN, Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Bethesda, MD 20814; [email protected].
ACKNOWLEDGEMENT
Kristian Sanchack, MD, and James Higgins, DO, assisted with the editing of the manuscript.
1. American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2016-2017. Atlanta, GA: American Cancer Society; 2016. www.cancer.org/research/cancer-facts-statistics/survivor-facts-figures.html. Accessed July 25, 2018.
2. Survivorship. NCCN Guidelines (version 1.2017). Fort Washington, PA: National Comprehensive Cancer Network; 2017. www.nccn.org/professionals/physician_gls/default.aspx#supportive. Accessed July 26, 2018.
3. Kendall C, Decker KM, Groome PA, et al. Use of physician services during the survivorship phase: a multi-province study of women diagnosed with breast cancer. Curr Oncolog. 2017;24:81-89.
4. Snyder CF, Earle CC, Herbert RJ, et al. Preventive care for colorectal cancer survivors: a 5-year longitudinal study. J Clin Oncol. 2008;26:1073-1079.
5. Hewitt M, Greenfield S, Stovall E (eds); Institute of Medicine and National Research Council. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington DC: The National Academies Press; 2006. www.nap.edu/read/11468/chapter/1. Accessed July 25, 2018.
6. Survivorship care plan. New York, NY: Memorial Sloan Kettering Cancer Center. www.mskcc.org/hcp-education-training/survivorship/survivorship-care-plan. Accessed August 11, 2018.
7. Fuentes AC, Lambird JE, George TJ, et al. Cancer survivor’s history and physical. South Med J. 2017;110:37-44. http://sma.org/southern-medical-journal/article/cancer-survivors-history-physical/. Accessed July 26, 2018.
8. Salz T, Oeffinger KC, McCabe MS, et al. Survivorship care plans in research and practice. CA Cancer J Clin. 2012;62:101-117.
9. Birken SA, Mayer DK, Weiner BJ. Survivorship care plans: prevalence and barriers to use. J Cancer Educ. 2013;28:290-296.
10. American College of Surgeons Commission on Cancer. Cancer program standards 2012: Ensuring patient-centered care. V1.2.1. www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx. Accessed July 25, 2018.
11. NCCN guidelines for treatment of cancer by site. NCCN Guidelines (version 1.2018). Fort Washington, PA: National Comprehensive Cancer Network; 2018. www.nccn.org/professionals/physician_gls/default.aspx#site. Accessed July 25, 2018.
12. Spronka I, Korevaar JC, Burgers JS, et al. Review of guidance on recurrence risk management for general practitioners in breast cancer, colorectal cancer and melanoma guidelines. Family Pract. 2017;34:154-160.
13. Merkow RP, Korenstein D, Yeahia R, et al. Quality of cancer surveillance clinical practice guidelines: specificity and consistency of recommendations. JAMA Intern Med. 2017;177:701-709.
14. Muradali D, Kennedy EB, Eisen A, et al. Breast screening for survivors of breast cancer: a systematic review. Prev Med. 2017;103:70-75.
15. Giannarini G, Kessler TM, Thoeny HC, et al. Do patients benefit from routine follow-up to detect recurrences after radical cystectomy and ileal orthotopic bladder substitution? Eur Urol. 2010;58:486-494.
16. Sisler JJ, Seo B, Katz A, et al. Concordance with ASCO guidelines for surveillance after colorectal cancer treatment: a population-based analysis. J Oncol Pract. 2012;8:e69-e79.
17. Ehdaie B. Atoria CL, Lowrance WT, et al. Adherence to surveillance guidelines after radical cystectomy: a population-based analysis. Urol Oncol. 2014;32:779-784.
18. Travis LB, Fosså SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst. 2005;97:1354-1365.
19. Donin N, Filson C, Drakaki A, et al. Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer. 2016;122:3075-3086.
20. Holder AE, Ramirez AG, Gallion K. Depressive symptoms in Latina breast cancer survivors: a barrier to cancer screening. Health Psycholog. 2014;33:242-248.
21. Dyer G, Larsen SR, Gilroy N, et al. Adherence to cancer screening guidelines in Australian survivors of allogenic blood and marrow transplantation (BMT). Cancer Med. 2016;5:1702-1716.
22. Mandelzweig L, Chetrit A, Amitai T, et al. Primary prevention and screening practices among long-term breast cancer survivors. Cancer Causes Control. 2017;28:657-666.
23. Bishop MM, Lee SJ, Beaumont JL, et al. The preventive health behaviors of long-term survivors of cancer and hematopoietic stem cell transplantation compared with matched controls. Biol Blood Marrow Transplant. 2010;16:207-214.
24. Uhlig A, Mei J, Baik I, et al. Screening utilization among cancer survivors: a meta-analysis. J Public Health (Oxf). 2018;40:129-137.
25. Hilal T, Rudy DW. Radiation-induced breast cancer: the question of early breast cancer screening in Hodgkin’s lymphoma survivors. Oxf Med Case Reports. 2016;2016:17-18.
26. Lin K, Croswell JM, Koenig H, et al. Prostate-specific antigen-based screening for prostate cancer: an evidence update for the U.S. Preventive Services Task Force [Internet]. Evidence Syntheses No. 90. AHRQ Publication No. 12-05160-EF-1. Rockville, MD: Agency for Healthcare Research and Quality (US); October 2011. www.ncbi.nlm.nih.gov/pubmedhealth/PMH0032900/. Accessed July 25, 2018.
27. US Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
28. Hofman M, Ryan JL, Figueroa-Moseley CD, et al. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12 Suppl 1:4-10.
29. Jung JY, Lee JM, Kim MS, et al. Comparison of fatigue, depression, and anxiety as factors affecting posttreatment health-related quality of life in lung cancer survivors. Psychooncology. 2018;27:465-470.
30. Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatment. Nat Rev Clin Oncol. 2014;11:597-609.
31. Medysky ME, Temesi J, Culos-Reed SN, et al. Exercise, sleep and cancer-related fatigue: are they related? Neurophysiol Clin. 2017;47:111-122.
32. Oh HS, Sea WS. Systematic review and meta-analysis of the correlates of cancer-related fatigue. Worldviews Evid Based Nurs. 2011;8:191-201.
33. Qu D, Zhang Z, Yu X, et al. Psychotropic drugs for the management of cancer-related fatigue: a systematic review and meta-analysis. Eur J Cancer Care (Engl). 2016;25:970-979.
34. Escalante CP, Manzullo EF. Cancer-related fatigue: the approach and treatment. J Gen Intern Med. 2009;24(suppl 2):S412-S416.
35. Mustian KM, Alfano CM, Heckler C, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol. 2017;3:961-968.
36. Glare PA, Davies PS, Finlay E, et al. Pain in cancer survivors. J Clin Oncol. 2014;32:1739-1747.
37. Davies PS. Chronic pain management in the cancer survivor: tips for primary care providers. Nurse Pract. 2013;39:28-38.
38. Boland EG, Ahmedzai SH. Persistent pain in cancer survivors. Curr Opin Support Palliat Care. 2017;11:181-190.
39. Sears CS, Robinson JW, Walker LM. A comprehensive review of sexual health concerns after cancer treatment and the biopsychosocial treatment options available to female patients. Eur J Cancer Care (Engl). 2017;27:e12738.
40. Schou Bredal I, Smeby NA, Ottesen S, et al. Chronic pain in breast cancer survivors: comparison of psychological, surgical, and medical characteristics between survivors with and without pain. J Pain Symptom Manage. 2014;48:852-862.
41. Sutradhar R, Lokku A, Barbera L. Cancer survivorship and opioid prescribing rates: a population-based matched cohort study among individuals with and without a history of cancer. Cancer. 2017;123:4286-4293.
42. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
43. Davis MP, Mehta Z. Opioids and chronic pain: where is the balance? Curr Oncol Rep. 2016;18:71.
44. Von Ah D. Cognitive changes associated with cancer and cancer treatment: state of the science. Clin J Oncol Nurs. 2015;19:47-56.
45. Moore HC. An overview of chemotherapy-related cognitive dysfunction, or ‘chemobrain’. Oncology (Williston Park). 2014;28:797-804.
46. Asher A. Cognitive dysfunction among cancer survivors. Am J Phys Med Rehabil. 2011;90(suppl):S16-S26.
47. Joly F, Rigal O, Noal S, et al. Cognitive dysfunction and cancer: which consequences in terms of disease management? Psychooncology. 2011;20:1251-1258.
48. Attention, thinking or memory problems. American Society of Clinical Oncology Cancer.Net. April 2018. www.cancer.net/navigating-cancer-care/side-effects/attention-thinking-or-memory-problems. Accessed July 25, 2018.
49. Kucherer S, Ferguson RJ. Cognitive behavioral therapy for cancer-related cognitive dysfunction. Curr Opin Support Palliat Care. 2017;11:46-51.
50. Derry HM, Jaremka LM, Bennet JM, et al. Yoga and self-reported cognitive problems in breast cancer survivors: a randomized controlled trial. Psychooncology. 2015;24:958-966.
51. Treanor CJ, McMenamin UC, O’Neill RF, et al. Non-pharmacological interventions for cognitive impairment due to systemic cancer treatment. Cochrane Database Syst Rev. 2016 Aug 16;(8):CD011325.
52. Mitchell AJ, Ferguson DW, Gill J, et al. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: a systematic review and meta-analysis. Lancet Oncol. 2013;14:721-732.
53. Inhestern L, Beierlein V, Bultmann JC, et al. Anxiety and depression in working-age cancer survivors: a register-based study. BMC Cancer. 2017;17:347.
54. Partridge AH, Jacobsen PB, Andersen BL. Challenges to standardizing the care for adult cancer survivors: highlighting ASCO’s fatigue and anxiety and depression guidelines. Am Soc Clin Oncol Educ Book. 2015:188-194.
55. Andersen BL, Yang HC, Farrar WB, et al. Psychologic intervention improves survival for breast cancer patients: a randomized clinical trial. Cancer. 2008;113:3450-3458.
56. Yi JC, Syrjala KL. Anxiety and depression in cancer survivors. Med Clin N Am. 2017;101:1099-1113.
57. Zhu G, Zhang X, Wang Y, et al. Effects of exercise intervention in breast cancer survivors: a meta-analysis of 33 randomized controlled trials. Onco Targets Ther. 2016;9:2153-2168.
58. Hawkins NA, Soman A, Lunsford N, et al. Use of medications for treating anxiety and depression in cancer survivors in the United States. J Clin Oncol. 2017;35:78-85.
59. Ostuzzi G, Matcham F, Dauchy S, et al. Antidepressants for the treatment of depression in people with cancer. Cochrane Database Syst Rev. 2015 June 1;(6):CD011006.
60. Laoutidis ZG, Mathiak K. Antidepressants in the treatment of depression/depressive symptoms in cancer patients: a systematic review and meta-analysis. BMC Psychiatry. 2013;13:140.
61. Lydiatt WM, Bessette D, Schmid KK, et al. Prevention of depression with escitalopram in patients undergoing treatment for head and neck cancer: randomized, double-blind, placebo-controlled clinical trial. JAMA Otolaryngol Head Neck Surg. 2013;139:678-686.
62. Lavigne JE, Heckler C, Mathews JL, et al. A randomized, controlled, double-blinded clinical trial of gabapentin 300 versus 900 mg versus placebo for anxiety symptoms in breast cancer survivors. Breast Cancer Res Treat. 2012;136:479-486.
63. Okwuosa TM, Anzevino S, Rao R. Cardiovascular disease in cancer survivors. Postgrad Med J. 2017;93:82-90.
64. Plana, JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911-939.
65. Lancellotti, P, Nkomo VT, Badano LP, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:1013-1032.
Cancer survivors represent a rapidly increasing population. In 1971, there were 3 million cancer survivors; this number increased to 15.5 million in 2016 and will reach 20 million by 2026.1TABLE 11 shows the percentage of survivors by type of cancer. Cancer survivors tend to be older,* comprising nearly 1 of every 5 people older than 65 years.2

The Institute of Medicine (IOM) identified 3 key characteristics of cancer survivors3:
- Trajectories of survivorship are variable; many cancer patients have periods of relative health between episodes of their disease.
- Survivors require careful cancer monitoring; in addition to the risk that their primary cancer will recur, they have an elevated risk for another, second cancer.
- Both cancer and its treatments increase the risk of other medical and psychiatric problems.
Family physicians (FPs) have optimal skills for navigating the chronic risks and health concerns of the well cancer survivor. This article reviews the primary care management of the functional cancer survivor, focusing on the management of chronic conditions and preventive care.
Survivorship follows any of 6 paths
Cancer survivorship is increasing in importance as treatment has steadily reduced mortality. Six trajectories of cancer survivors have been identified1:
- living cancer-free after treatment with minimal effects
- living cancer-free but suffering serious treatment complications
- Suffering late recurrence
- Developing a second cancer
- Living with intermittent cancer recurrences
- Living with cancer continuously.
Only patients in the last 2 groups are likely to be managed primarily by oncologists.
Survivors look to their FPs for ongoing care
Cancer survivors routinely see their primary care physician after initial treatment. A study of 30,000 Canadian breast cancer survivors demonstrated that follow-up care was limited to an oncologist in only 2%; 84% saw a primary care provider and an oncologist; and 14% saw a primary care provider only.4 A study of colorectal cancer survivors showed that primary care visits increased in each of the 5 years after diagnosis, during which time oncology visits decreased steadily5; in that study, primary care physicians delivered more preventive care than oncologists did.5 Similar to what is done in other chronic conditions, the various effects of cancer are best managed as a whole.
The IOM recommends that cancer survivor care comprise 4 elements2:
- coordination between oncologist and primary care physician
- surveillance for recurrence or spread of existing cancer
- screening for new cancer
- intervention for the effects of cancer and treatment.
Continue to: The following discussion summarizes...
The following discussion summarizes evidence and recommendations for each element of the IOM recommendations for survivor care.
Implementing the 4 elements of cancer survivor care
1. Coordinate care through a unified survivorship care plan
The IOM has noted that the needs of cancer survivors are rarely met2; communication between oncology and primary care is often deficient during transition of care. The IOM has recommended that oncologists provide a survivorship care plan that details the cancer (ie, tumor characteristics), the type of treatment (ie, enrollment in a clinical trial; medical, surgical, or radiation), support services, and follow-up recommendations for the primary care provider. (Examples of elements of a survivorship care plan can be found at www.mskcc.org/hcp-education-training/survivorship/survivorship-care-plan6 and http://sma.org/southern-medical-journal/article/cancer-survivors-history-physical/7).
Regrettably, survivorship care plans have been rarely and poorly employed. Studies show that fewer than one-half of oncologists provide a plan, and that when they do, the plan often lacks recommended information.8,9 Survivorship care plans may soon become common practice, however; the Commission on Cancer of the American College of Surgeons has required their use in all certified cancer centers since 2015.10
2. Provide surveillance of existing cancer

Cancer follow-up is challenging after the initial treatment phase. Although there are many conflicting guidelines for surveillance after cancer, guidelines of the National Comprehensive Cancer Network (NCCN) (summarized in TABLE 211 for the 10 most common cancers in survivors) are the ones generally accepted.12,13

Although individual surveillance recommendations are based on limited evidence, studies confirm the importance of surveillance. A systematic review showed that surveillance mammography after breast cancer reduces breast cancer mortality by 36%.14 A study showed that bladder cancer recurrence diagnosed by surveillance instead of by symptoms led to a 35% increase in 5-year survival.15

Continue to: Yet adherence to cancer surveillance...
Yet adherence to cancer surveillance recommendations is poor. A study of patients with colon cancer demonstrated that only 12% met all recommended surveillance guidelines.16 A study of patients with bladder cancer after radical cystectomy showed that only 9% met recommended surveillance more than 2 years after diagnosis.17 Those dismal statistics may be the result of provider oversight—not patient reluctance.
In the colon cancer study, for example, compliance with follow-up colonoscopy was 80% but compliance with carcinoembryonic antigen testing was only 22%.16 In the bladder cancer study, follow-up urine cytology was obtained in only 23% of patients, although 75% completed recommended imaging.17
Although surveillance remains the oncologist’s responsibility, visits to the FP provide an opportunity to review surveillance and order needed laboratory testing and other studies, including imaging.
3. Screen for new cancers
The risk of a second cancer is elevated for cancer survivors compared with the risk of a primary cancer in the healthy general population; some survivors have a lifetime risk of a second cancer as high as 36%.18 Risk varies by cancer type (TABLE 319). Some of this variation is due to the impact of smoking: Smoking-related cancers have the highest risk of second malignancy.19 Genetic predisposition to malignant transformation is also theorized to contribute to increased risk. Second malignancies are dangerous; 55% of patients die of the second cancer compared with only 13% of their initial cancer.19

Studies show that cancer survivors display varying adherence with recommended screening for second cancers. In a study of Latina cancer survivors, depressive symptoms were associated with lower screening compliance.20 A study of survivors of hematologic cancer showed a low rate of cancer screening and high fear of cancer recurrence—suggesting avoidance due to fear.21 Other studies, however, show similar or increased compliance with screening in cancer survivors.22,23 A meta-analysis of 19 studies determined that, overall, cancer survivors receive 25% to 38% more recommended screening than the general population.24
Continue to: Few guidelines exist to guide FPs...
Few guidelines exist to guide FPs in adjusting screening for the cancer survivor. For women who received radiation therapy for a tumor in the chest, for example, the recommendation offered by several groups is to start breast cancer screening 8 to 10 years after treatment or by 30 years of age, and to consider combining magnetic resonance imaging and mammography.25 Recommendations for breast cancer screening do not account for a history of other gynecologic cancers unless genetic markers are present.25 On the other hand, the impact of a history of cancer on the risk of prostate cancer and on screening decisions has not been studied,26 and cervical cancer screening guidelines, which recommend that screening continue after 65 years of age for patients who are immunocompromised, do not address a history of other cancer.27
4. Manage the effects of both the cancer and the treatment
Medical issues faced by cancer survivors are familiar to FPs, but there are some specific recommendations regarding evaluation and treatment that stand in contrast to what would be considered for a healthy, or non-cancer, patient. For example, each chemotherapeutic agent has characteristic adverse effects; TABLE 47 lists the principal adverse effects of common agents and recommendations for testing when these problems develop. Common long-term problems in cancer survivors include fatigue, chronic pain, cognitive dysfunction, psychiatric illness, and cardiovascular disease. Although these symptoms and manifestations are common, the physician must be careful: New or changing symptoms could signal the spread or recurrence of disease. Fear of recurrence can lead patients to exaggerate or minimize symptoms.

Fatigue is the most common symptom seen in cancer survivors during treatment and following remission.28 More than 40% of cancer survivors report significant fatigue.29 Although fatigue is concerning for cancer recurrence, other causes are common in cancer survivors. Both depression and anxiety commonly present with worsened fatigue.30 Sleep disturbances are common, even without a psychiatric diagnosis.31 Effects of treatment, including nausea, anemia, heart failure, and medication adverse effects can cause or worsen fatigue. Pain is associated with fatigue, but to a lesser extent than are depression, anxiety, and nausea.32

Pharmacotherapy of cancer-related fatigue is challenging. Psychostimulants have been most studied. A recent systematic review shows that methylphenidate produces mild or moderate improvement in fatigue, whereas modafanil has minimal effectiveness.33 Antidepressants have not been shown to relieve fatigue.33
A recent meta-analysis showed that nonpharmaceutical treatments for cancer-related fatigue are more effective than pharmacotherapy. In this review, both exercise and pharmacotherapy had a mild-to-moderate effect on fatigue.35 Exercise is best studied in this regard, and has shown the most consistent results.31
Continue to: Chronic pain
Chronic pain. Pain is common in cancer survivors: As many as 40% experience pain for years after initial therapy.36 Treatment of some cancers—eg, thoracotomy (80%), amputation (50%-80%), neck dissection (52%), and surgical management of breast cancer (63%)—increase the likelihood of chronic pain.37 Reports of pain in cancer survivors that should be considered red flags that might signal recurrence of cancer include new or worsening pain; pain worse at night or when recumbent; new neurologic symptoms; and general symptoms of systemic illness37 (TABLE 537).

Management of pain is best approached by its cause, with neurologic, rheumatologic (including myofascial pain and arthralgia), lymphatic, and genital causes most common.37 Across all types of pain, complete relief is unlikely; functional goals provide a more effective target.
For neuropathic cancer pain, duloxetine is the only medication with evidence of benefit; anticonvulsant and topical medications are recommended on the basis of the findings of studies of noncancer pain.38 There are few data on the value of treatments for cancer-related rheumatologic and lymphatic pain, although exercise has shown benefit in both types.38 For dyspareunia and sexual dysfunction (common after gynecologic and nongynecologic cancers), vaginal lubricants and pelvic-floor physiotherapy have shown benefit.39 There is significant overlap in psychiatric comorbidities, sleep, and pain, and addressing all of a patient’s problems can reduce pain and improve function.40
Opioids are often prescribed for pain in cancer survivors. Cancer survivors have a higher rate of opioid prescribing compared with that of non-cancer patients, even 10 years after diagnosis.41 Guidelines of the Centers for Disease Control and Prevention for using opioids to manage chronic pain specifically exclude cancer patients.42 Regrettably, there is no evidence that opioids have long-term efficacy in chronic pain; in fact, evidence is accumulating that chronic opioid therapy exacerbates chronic pain.43
Cognitive dysfunction is present in 17% to 75% of cancer survivors as memory disturbance, psychological disorder, sleep dysfunction, or impairment of executive functioning.44 Cognitive deficits appear to be secondary to both cancer and treatment modalities45; as many as one-third of patients have cognitive dysfunction prior to receiving chemotherapy.46
Continue to: Chemotherapies that are more likely...
Chemotherapies that are more likely to cause cognitive symptoms include methotrexate, 5-fluorouracil, cyclophosphamide, and hormone antagonists.47 More powerful regimens and repetitive chemotherapy regimens tend to cause more cognitive effects.47
Cognitive training interventions show evidence of likely benefit,44,48 leading to recommendations for self-treatment strategies, such as written lists, wordplay, crossword puzzles, jigsaw puzzles, playing a musical instrument, and new hobbies. Small studies suggest a benefit from cognitive behavioral therapy.44,49 A study of breast cancer survivors showed that yoga led to improvement in patient-reported cognitive dysfunction.50 Physical exercise yields cognitive benefit in healthy older adults and is supported by limited evidence in cancer survivors.51
There is no effective pharmacotherapy for cancer- and cancer chemotherapy-related cognitive dysfunction unless a treatable underlying cause is found.44 Symptoms tend to subside with time after completion of chemotherapy, which might be reassuring to patients and families.45
Psychiatric problems. The most common psychiatric issues in cancer survivors are anxiety and depression; the prevalence of anxiety is nearly double that of depression.52 Anxiety often presents as fear of a recurrence of cancer or a feeling of lack of control over present or future circumstances.53 Screening for anxiety and depression is recommended at each visit, using standardized screening questionnaires.54
A small study suggests that psychiatric treatment reduces the risk of early mortality.55 Small studies also suggest that mindfulness-based therapy and cognitive behavioral therapy delivered by telehealth offer benefit.56 A meta-analysis shows that exercise interventions improve depression and anxiety in breast cancer patients.57
Continue to: There are few studies of pharmacotherapy...
There are few studies of pharmacotherapy of anxiety or depression in cancer survivors56; it is known that cancer survivors are nearly twice as likely as the general population to be taking medical therapy for anxiety and depression.58 A Cochrane systematic review of 7 small studies showed uncertain improvement in depressive symptoms in patients with cancer from antidepressant medication; however, an earlier systematic review did show benefit.59,60
In a trial of patients without depression who were being treated for head and neck cancer, escitalopram, 20 mg/d, reduced the risk of subsequent depression compared with placebo.61 A study of 420 breast cancer survivors showed that 300 mg/d and 900 mg/d dosages of gabapentin were both superior to placebo, and nearly equivalent to each other, at reducing anxiety scores.62 In both studies, however, the evidence is nonetheless insufficient to make specific recommendations about these medications.
Cardiac risk
Among chemotherapeutic agents, anthracyclines, such as doxorubicin, cause the most rapid and striking myocyte damage. This damage is dose-dependent and nearly irreversible, with 98% of injury occurring within the first year of chemotherapy.64 More than one half of cancer patients taking an anthracycline have cardiac dysfunction on imaging; 5% will be in overt heart failure 10 to 20 years, or longer, after chemotherapy.63 Following monitoring at 1 year post-therapy, regular cardiac imaging is not recommended in the absence of symptoms.62
Because other cardiotoxic chemotherapeutic agents cause partially reversible damage, imaging is not recommended in the absence of symptoms in patients taking those agents.64
Continue to: Radiation therapy to the chest leads...
Radiation therapy to the chest leads to many cardiac complications, including cardiomyopathy, valvular disease, pericardial disease, and arrhythmias. Development of cardiomyopathy can be delayed 20 to 30 years after radiation; screening echocardiography is therefore recommended every 5 to 10 years after radiation therapy.65 Recent adjustments to the dosages and delivery of radiation therapy should reduce cardiac damage, but will require decades to validate.63
For patients at risk of cardiovascular disease prior to treatment of cancer, there is evidence to support preventive treatment with angiotensin II-receptor antagonists, beta-blockers, and statins to prevent cardiomyopathy.63 Treatment of diagnosed cardiomyopathy and heart failure follows standard guidelines, with significant emphasis on aerobic exercise and smoking cessation.63
Cancer survivorship care: Your critical role
Cancer survivors constitute a large population who frequent the practices of primary care physicians. Primary care visits provide an opportunity to monitor key elements of survivorship, including surveillance of the current cancer and screening for second cancers. Similar to what is seen with diabetes and coronary artery disease, cancer increases cardiac risk, which requires preventive care and chronic management. FPs are well placed to treat common issues in cancer survivors—issues that mirror concerns seen in the general population.
CORRESPONDENCE
Michael J. Arnold, MD, CDR, USN, Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Bethesda, MD 20814; [email protected].
ACKNOWLEDGEMENT
Kristian Sanchack, MD, and James Higgins, DO, assisted with the editing of the manuscript.
Cancer survivors represent a rapidly increasing population. In 1971, there were 3 million cancer survivors; this number increased to 15.5 million in 2016 and will reach 20 million by 2026.1TABLE 11 shows the percentage of survivors by type of cancer. Cancer survivors tend to be older,* comprising nearly 1 of every 5 people older than 65 years.2

The Institute of Medicine (IOM) identified 3 key characteristics of cancer survivors3:
- Trajectories of survivorship are variable; many cancer patients have periods of relative health between episodes of their disease.
- Survivors require careful cancer monitoring; in addition to the risk that their primary cancer will recur, they have an elevated risk for another, second cancer.
- Both cancer and its treatments increase the risk of other medical and psychiatric problems.
Family physicians (FPs) have optimal skills for navigating the chronic risks and health concerns of the well cancer survivor. This article reviews the primary care management of the functional cancer survivor, focusing on the management of chronic conditions and preventive care.
Survivorship follows any of 6 paths
Cancer survivorship is increasing in importance as treatment has steadily reduced mortality. Six trajectories of cancer survivors have been identified1:
- living cancer-free after treatment with minimal effects
- living cancer-free but suffering serious treatment complications
- Suffering late recurrence
- Developing a second cancer
- Living with intermittent cancer recurrences
- Living with cancer continuously.
Only patients in the last 2 groups are likely to be managed primarily by oncologists.
Survivors look to their FPs for ongoing care
Cancer survivors routinely see their primary care physician after initial treatment. A study of 30,000 Canadian breast cancer survivors demonstrated that follow-up care was limited to an oncologist in only 2%; 84% saw a primary care provider and an oncologist; and 14% saw a primary care provider only.4 A study of colorectal cancer survivors showed that primary care visits increased in each of the 5 years after diagnosis, during which time oncology visits decreased steadily5; in that study, primary care physicians delivered more preventive care than oncologists did.5 Similar to what is done in other chronic conditions, the various effects of cancer are best managed as a whole.
The IOM recommends that cancer survivor care comprise 4 elements2:
- coordination between oncologist and primary care physician
- surveillance for recurrence or spread of existing cancer
- screening for new cancer
- intervention for the effects of cancer and treatment.
Continue to: The following discussion summarizes...
The following discussion summarizes evidence and recommendations for each element of the IOM recommendations for survivor care.
Implementing the 4 elements of cancer survivor care
1. Coordinate care through a unified survivorship care plan
The IOM has noted that the needs of cancer survivors are rarely met2; communication between oncology and primary care is often deficient during transition of care. The IOM has recommended that oncologists provide a survivorship care plan that details the cancer (ie, tumor characteristics), the type of treatment (ie, enrollment in a clinical trial; medical, surgical, or radiation), support services, and follow-up recommendations for the primary care provider. (Examples of elements of a survivorship care plan can be found at www.mskcc.org/hcp-education-training/survivorship/survivorship-care-plan6 and http://sma.org/southern-medical-journal/article/cancer-survivors-history-physical/7).
Regrettably, survivorship care plans have been rarely and poorly employed. Studies show that fewer than one-half of oncologists provide a plan, and that when they do, the plan often lacks recommended information.8,9 Survivorship care plans may soon become common practice, however; the Commission on Cancer of the American College of Surgeons has required their use in all certified cancer centers since 2015.10
2. Provide surveillance of existing cancer

Cancer follow-up is challenging after the initial treatment phase. Although there are many conflicting guidelines for surveillance after cancer, guidelines of the National Comprehensive Cancer Network (NCCN) (summarized in TABLE 211 for the 10 most common cancers in survivors) are the ones generally accepted.12,13

Although individual surveillance recommendations are based on limited evidence, studies confirm the importance of surveillance. A systematic review showed that surveillance mammography after breast cancer reduces breast cancer mortality by 36%.14 A study showed that bladder cancer recurrence diagnosed by surveillance instead of by symptoms led to a 35% increase in 5-year survival.15

Continue to: Yet adherence to cancer surveillance...
Yet adherence to cancer surveillance recommendations is poor. A study of patients with colon cancer demonstrated that only 12% met all recommended surveillance guidelines.16 A study of patients with bladder cancer after radical cystectomy showed that only 9% met recommended surveillance more than 2 years after diagnosis.17 Those dismal statistics may be the result of provider oversight—not patient reluctance.
In the colon cancer study, for example, compliance with follow-up colonoscopy was 80% but compliance with carcinoembryonic antigen testing was only 22%.16 In the bladder cancer study, follow-up urine cytology was obtained in only 23% of patients, although 75% completed recommended imaging.17
Although surveillance remains the oncologist’s responsibility, visits to the FP provide an opportunity to review surveillance and order needed laboratory testing and other studies, including imaging.
3. Screen for new cancers
The risk of a second cancer is elevated for cancer survivors compared with the risk of a primary cancer in the healthy general population; some survivors have a lifetime risk of a second cancer as high as 36%.18 Risk varies by cancer type (TABLE 319). Some of this variation is due to the impact of smoking: Smoking-related cancers have the highest risk of second malignancy.19 Genetic predisposition to malignant transformation is also theorized to contribute to increased risk. Second malignancies are dangerous; 55% of patients die of the second cancer compared with only 13% of their initial cancer.19

Studies show that cancer survivors display varying adherence with recommended screening for second cancers. In a study of Latina cancer survivors, depressive symptoms were associated with lower screening compliance.20 A study of survivors of hematologic cancer showed a low rate of cancer screening and high fear of cancer recurrence—suggesting avoidance due to fear.21 Other studies, however, show similar or increased compliance with screening in cancer survivors.22,23 A meta-analysis of 19 studies determined that, overall, cancer survivors receive 25% to 38% more recommended screening than the general population.24
Continue to: Few guidelines exist to guide FPs...
Few guidelines exist to guide FPs in adjusting screening for the cancer survivor. For women who received radiation therapy for a tumor in the chest, for example, the recommendation offered by several groups is to start breast cancer screening 8 to 10 years after treatment or by 30 years of age, and to consider combining magnetic resonance imaging and mammography.25 Recommendations for breast cancer screening do not account for a history of other gynecologic cancers unless genetic markers are present.25 On the other hand, the impact of a history of cancer on the risk of prostate cancer and on screening decisions has not been studied,26 and cervical cancer screening guidelines, which recommend that screening continue after 65 years of age for patients who are immunocompromised, do not address a history of other cancer.27
4. Manage the effects of both the cancer and the treatment
Medical issues faced by cancer survivors are familiar to FPs, but there are some specific recommendations regarding evaluation and treatment that stand in contrast to what would be considered for a healthy, or non-cancer, patient. For example, each chemotherapeutic agent has characteristic adverse effects; TABLE 47 lists the principal adverse effects of common agents and recommendations for testing when these problems develop. Common long-term problems in cancer survivors include fatigue, chronic pain, cognitive dysfunction, psychiatric illness, and cardiovascular disease. Although these symptoms and manifestations are common, the physician must be careful: New or changing symptoms could signal the spread or recurrence of disease. Fear of recurrence can lead patients to exaggerate or minimize symptoms.

Fatigue is the most common symptom seen in cancer survivors during treatment and following remission.28 More than 40% of cancer survivors report significant fatigue.29 Although fatigue is concerning for cancer recurrence, other causes are common in cancer survivors. Both depression and anxiety commonly present with worsened fatigue.30 Sleep disturbances are common, even without a psychiatric diagnosis.31 Effects of treatment, including nausea, anemia, heart failure, and medication adverse effects can cause or worsen fatigue. Pain is associated with fatigue, but to a lesser extent than are depression, anxiety, and nausea.32

Pharmacotherapy of cancer-related fatigue is challenging. Psychostimulants have been most studied. A recent systematic review shows that methylphenidate produces mild or moderate improvement in fatigue, whereas modafanil has minimal effectiveness.33 Antidepressants have not been shown to relieve fatigue.33
A recent meta-analysis showed that nonpharmaceutical treatments for cancer-related fatigue are more effective than pharmacotherapy. In this review, both exercise and pharmacotherapy had a mild-to-moderate effect on fatigue.35 Exercise is best studied in this regard, and has shown the most consistent results.31
Continue to: Chronic pain
Chronic pain. Pain is common in cancer survivors: As many as 40% experience pain for years after initial therapy.36 Treatment of some cancers—eg, thoracotomy (80%), amputation (50%-80%), neck dissection (52%), and surgical management of breast cancer (63%)—increase the likelihood of chronic pain.37 Reports of pain in cancer survivors that should be considered red flags that might signal recurrence of cancer include new or worsening pain; pain worse at night or when recumbent; new neurologic symptoms; and general symptoms of systemic illness37 (TABLE 537).

Management of pain is best approached by its cause, with neurologic, rheumatologic (including myofascial pain and arthralgia), lymphatic, and genital causes most common.37 Across all types of pain, complete relief is unlikely; functional goals provide a more effective target.
For neuropathic cancer pain, duloxetine is the only medication with evidence of benefit; anticonvulsant and topical medications are recommended on the basis of the findings of studies of noncancer pain.38 There are few data on the value of treatments for cancer-related rheumatologic and lymphatic pain, although exercise has shown benefit in both types.38 For dyspareunia and sexual dysfunction (common after gynecologic and nongynecologic cancers), vaginal lubricants and pelvic-floor physiotherapy have shown benefit.39 There is significant overlap in psychiatric comorbidities, sleep, and pain, and addressing all of a patient’s problems can reduce pain and improve function.40
Opioids are often prescribed for pain in cancer survivors. Cancer survivors have a higher rate of opioid prescribing compared with that of non-cancer patients, even 10 years after diagnosis.41 Guidelines of the Centers for Disease Control and Prevention for using opioids to manage chronic pain specifically exclude cancer patients.42 Regrettably, there is no evidence that opioids have long-term efficacy in chronic pain; in fact, evidence is accumulating that chronic opioid therapy exacerbates chronic pain.43
Cognitive dysfunction is present in 17% to 75% of cancer survivors as memory disturbance, psychological disorder, sleep dysfunction, or impairment of executive functioning.44 Cognitive deficits appear to be secondary to both cancer and treatment modalities45; as many as one-third of patients have cognitive dysfunction prior to receiving chemotherapy.46
Continue to: Chemotherapies that are more likely...
Chemotherapies that are more likely to cause cognitive symptoms include methotrexate, 5-fluorouracil, cyclophosphamide, and hormone antagonists.47 More powerful regimens and repetitive chemotherapy regimens tend to cause more cognitive effects.47
Cognitive training interventions show evidence of likely benefit,44,48 leading to recommendations for self-treatment strategies, such as written lists, wordplay, crossword puzzles, jigsaw puzzles, playing a musical instrument, and new hobbies. Small studies suggest a benefit from cognitive behavioral therapy.44,49 A study of breast cancer survivors showed that yoga led to improvement in patient-reported cognitive dysfunction.50 Physical exercise yields cognitive benefit in healthy older adults and is supported by limited evidence in cancer survivors.51
There is no effective pharmacotherapy for cancer- and cancer chemotherapy-related cognitive dysfunction unless a treatable underlying cause is found.44 Symptoms tend to subside with time after completion of chemotherapy, which might be reassuring to patients and families.45
Psychiatric problems. The most common psychiatric issues in cancer survivors are anxiety and depression; the prevalence of anxiety is nearly double that of depression.52 Anxiety often presents as fear of a recurrence of cancer or a feeling of lack of control over present or future circumstances.53 Screening for anxiety and depression is recommended at each visit, using standardized screening questionnaires.54
A small study suggests that psychiatric treatment reduces the risk of early mortality.55 Small studies also suggest that mindfulness-based therapy and cognitive behavioral therapy delivered by telehealth offer benefit.56 A meta-analysis shows that exercise interventions improve depression and anxiety in breast cancer patients.57
Continue to: There are few studies of pharmacotherapy...
There are few studies of pharmacotherapy of anxiety or depression in cancer survivors56; it is known that cancer survivors are nearly twice as likely as the general population to be taking medical therapy for anxiety and depression.58 A Cochrane systematic review of 7 small studies showed uncertain improvement in depressive symptoms in patients with cancer from antidepressant medication; however, an earlier systematic review did show benefit.59,60
In a trial of patients without depression who were being treated for head and neck cancer, escitalopram, 20 mg/d, reduced the risk of subsequent depression compared with placebo.61 A study of 420 breast cancer survivors showed that 300 mg/d and 900 mg/d dosages of gabapentin were both superior to placebo, and nearly equivalent to each other, at reducing anxiety scores.62 In both studies, however, the evidence is nonetheless insufficient to make specific recommendations about these medications.
Cardiac risk
Among chemotherapeutic agents, anthracyclines, such as doxorubicin, cause the most rapid and striking myocyte damage. This damage is dose-dependent and nearly irreversible, with 98% of injury occurring within the first year of chemotherapy.64 More than one half of cancer patients taking an anthracycline have cardiac dysfunction on imaging; 5% will be in overt heart failure 10 to 20 years, or longer, after chemotherapy.63 Following monitoring at 1 year post-therapy, regular cardiac imaging is not recommended in the absence of symptoms.62
Because other cardiotoxic chemotherapeutic agents cause partially reversible damage, imaging is not recommended in the absence of symptoms in patients taking those agents.64
Continue to: Radiation therapy to the chest leads...
Radiation therapy to the chest leads to many cardiac complications, including cardiomyopathy, valvular disease, pericardial disease, and arrhythmias. Development of cardiomyopathy can be delayed 20 to 30 years after radiation; screening echocardiography is therefore recommended every 5 to 10 years after radiation therapy.65 Recent adjustments to the dosages and delivery of radiation therapy should reduce cardiac damage, but will require decades to validate.63
For patients at risk of cardiovascular disease prior to treatment of cancer, there is evidence to support preventive treatment with angiotensin II-receptor antagonists, beta-blockers, and statins to prevent cardiomyopathy.63 Treatment of diagnosed cardiomyopathy and heart failure follows standard guidelines, with significant emphasis on aerobic exercise and smoking cessation.63
Cancer survivorship care: Your critical role
Cancer survivors constitute a large population who frequent the practices of primary care physicians. Primary care visits provide an opportunity to monitor key elements of survivorship, including surveillance of the current cancer and screening for second cancers. Similar to what is seen with diabetes and coronary artery disease, cancer increases cardiac risk, which requires preventive care and chronic management. FPs are well placed to treat common issues in cancer survivors—issues that mirror concerns seen in the general population.
CORRESPONDENCE
Michael J. Arnold, MD, CDR, USN, Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Bethesda, MD 20814; [email protected].
ACKNOWLEDGEMENT
Kristian Sanchack, MD, and James Higgins, DO, assisted with the editing of the manuscript.
1. American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2016-2017. Atlanta, GA: American Cancer Society; 2016. www.cancer.org/research/cancer-facts-statistics/survivor-facts-figures.html. Accessed July 25, 2018.
2. Survivorship. NCCN Guidelines (version 1.2017). Fort Washington, PA: National Comprehensive Cancer Network; 2017. www.nccn.org/professionals/physician_gls/default.aspx#supportive. Accessed July 26, 2018.
3. Kendall C, Decker KM, Groome PA, et al. Use of physician services during the survivorship phase: a multi-province study of women diagnosed with breast cancer. Curr Oncolog. 2017;24:81-89.
4. Snyder CF, Earle CC, Herbert RJ, et al. Preventive care for colorectal cancer survivors: a 5-year longitudinal study. J Clin Oncol. 2008;26:1073-1079.
5. Hewitt M, Greenfield S, Stovall E (eds); Institute of Medicine and National Research Council. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington DC: The National Academies Press; 2006. www.nap.edu/read/11468/chapter/1. Accessed July 25, 2018.
6. Survivorship care plan. New York, NY: Memorial Sloan Kettering Cancer Center. www.mskcc.org/hcp-education-training/survivorship/survivorship-care-plan. Accessed August 11, 2018.
7. Fuentes AC, Lambird JE, George TJ, et al. Cancer survivor’s history and physical. South Med J. 2017;110:37-44. http://sma.org/southern-medical-journal/article/cancer-survivors-history-physical/. Accessed July 26, 2018.
8. Salz T, Oeffinger KC, McCabe MS, et al. Survivorship care plans in research and practice. CA Cancer J Clin. 2012;62:101-117.
9. Birken SA, Mayer DK, Weiner BJ. Survivorship care plans: prevalence and barriers to use. J Cancer Educ. 2013;28:290-296.
10. American College of Surgeons Commission on Cancer. Cancer program standards 2012: Ensuring patient-centered care. V1.2.1. www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx. Accessed July 25, 2018.
11. NCCN guidelines for treatment of cancer by site. NCCN Guidelines (version 1.2018). Fort Washington, PA: National Comprehensive Cancer Network; 2018. www.nccn.org/professionals/physician_gls/default.aspx#site. Accessed July 25, 2018.
12. Spronka I, Korevaar JC, Burgers JS, et al. Review of guidance on recurrence risk management for general practitioners in breast cancer, colorectal cancer and melanoma guidelines. Family Pract. 2017;34:154-160.
13. Merkow RP, Korenstein D, Yeahia R, et al. Quality of cancer surveillance clinical practice guidelines: specificity and consistency of recommendations. JAMA Intern Med. 2017;177:701-709.
14. Muradali D, Kennedy EB, Eisen A, et al. Breast screening for survivors of breast cancer: a systematic review. Prev Med. 2017;103:70-75.
15. Giannarini G, Kessler TM, Thoeny HC, et al. Do patients benefit from routine follow-up to detect recurrences after radical cystectomy and ileal orthotopic bladder substitution? Eur Urol. 2010;58:486-494.
16. Sisler JJ, Seo B, Katz A, et al. Concordance with ASCO guidelines for surveillance after colorectal cancer treatment: a population-based analysis. J Oncol Pract. 2012;8:e69-e79.
17. Ehdaie B. Atoria CL, Lowrance WT, et al. Adherence to surveillance guidelines after radical cystectomy: a population-based analysis. Urol Oncol. 2014;32:779-784.
18. Travis LB, Fosså SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst. 2005;97:1354-1365.
19. Donin N, Filson C, Drakaki A, et al. Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer. 2016;122:3075-3086.
20. Holder AE, Ramirez AG, Gallion K. Depressive symptoms in Latina breast cancer survivors: a barrier to cancer screening. Health Psycholog. 2014;33:242-248.
21. Dyer G, Larsen SR, Gilroy N, et al. Adherence to cancer screening guidelines in Australian survivors of allogenic blood and marrow transplantation (BMT). Cancer Med. 2016;5:1702-1716.
22. Mandelzweig L, Chetrit A, Amitai T, et al. Primary prevention and screening practices among long-term breast cancer survivors. Cancer Causes Control. 2017;28:657-666.
23. Bishop MM, Lee SJ, Beaumont JL, et al. The preventive health behaviors of long-term survivors of cancer and hematopoietic stem cell transplantation compared with matched controls. Biol Blood Marrow Transplant. 2010;16:207-214.
24. Uhlig A, Mei J, Baik I, et al. Screening utilization among cancer survivors: a meta-analysis. J Public Health (Oxf). 2018;40:129-137.
25. Hilal T, Rudy DW. Radiation-induced breast cancer: the question of early breast cancer screening in Hodgkin’s lymphoma survivors. Oxf Med Case Reports. 2016;2016:17-18.
26. Lin K, Croswell JM, Koenig H, et al. Prostate-specific antigen-based screening for prostate cancer: an evidence update for the U.S. Preventive Services Task Force [Internet]. Evidence Syntheses No. 90. AHRQ Publication No. 12-05160-EF-1. Rockville, MD: Agency for Healthcare Research and Quality (US); October 2011. www.ncbi.nlm.nih.gov/pubmedhealth/PMH0032900/. Accessed July 25, 2018.
27. US Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
28. Hofman M, Ryan JL, Figueroa-Moseley CD, et al. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12 Suppl 1:4-10.
29. Jung JY, Lee JM, Kim MS, et al. Comparison of fatigue, depression, and anxiety as factors affecting posttreatment health-related quality of life in lung cancer survivors. Psychooncology. 2018;27:465-470.
30. Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatment. Nat Rev Clin Oncol. 2014;11:597-609.
31. Medysky ME, Temesi J, Culos-Reed SN, et al. Exercise, sleep and cancer-related fatigue: are they related? Neurophysiol Clin. 2017;47:111-122.
32. Oh HS, Sea WS. Systematic review and meta-analysis of the correlates of cancer-related fatigue. Worldviews Evid Based Nurs. 2011;8:191-201.
33. Qu D, Zhang Z, Yu X, et al. Psychotropic drugs for the management of cancer-related fatigue: a systematic review and meta-analysis. Eur J Cancer Care (Engl). 2016;25:970-979.
34. Escalante CP, Manzullo EF. Cancer-related fatigue: the approach and treatment. J Gen Intern Med. 2009;24(suppl 2):S412-S416.
35. Mustian KM, Alfano CM, Heckler C, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol. 2017;3:961-968.
36. Glare PA, Davies PS, Finlay E, et al. Pain in cancer survivors. J Clin Oncol. 2014;32:1739-1747.
37. Davies PS. Chronic pain management in the cancer survivor: tips for primary care providers. Nurse Pract. 2013;39:28-38.
38. Boland EG, Ahmedzai SH. Persistent pain in cancer survivors. Curr Opin Support Palliat Care. 2017;11:181-190.
39. Sears CS, Robinson JW, Walker LM. A comprehensive review of sexual health concerns after cancer treatment and the biopsychosocial treatment options available to female patients. Eur J Cancer Care (Engl). 2017;27:e12738.
40. Schou Bredal I, Smeby NA, Ottesen S, et al. Chronic pain in breast cancer survivors: comparison of psychological, surgical, and medical characteristics between survivors with and without pain. J Pain Symptom Manage. 2014;48:852-862.
41. Sutradhar R, Lokku A, Barbera L. Cancer survivorship and opioid prescribing rates: a population-based matched cohort study among individuals with and without a history of cancer. Cancer. 2017;123:4286-4293.
42. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
43. Davis MP, Mehta Z. Opioids and chronic pain: where is the balance? Curr Oncol Rep. 2016;18:71.
44. Von Ah D. Cognitive changes associated with cancer and cancer treatment: state of the science. Clin J Oncol Nurs. 2015;19:47-56.
45. Moore HC. An overview of chemotherapy-related cognitive dysfunction, or ‘chemobrain’. Oncology (Williston Park). 2014;28:797-804.
46. Asher A. Cognitive dysfunction among cancer survivors. Am J Phys Med Rehabil. 2011;90(suppl):S16-S26.
47. Joly F, Rigal O, Noal S, et al. Cognitive dysfunction and cancer: which consequences in terms of disease management? Psychooncology. 2011;20:1251-1258.
48. Attention, thinking or memory problems. American Society of Clinical Oncology Cancer.Net. April 2018. www.cancer.net/navigating-cancer-care/side-effects/attention-thinking-or-memory-problems. Accessed July 25, 2018.
49. Kucherer S, Ferguson RJ. Cognitive behavioral therapy for cancer-related cognitive dysfunction. Curr Opin Support Palliat Care. 2017;11:46-51.
50. Derry HM, Jaremka LM, Bennet JM, et al. Yoga and self-reported cognitive problems in breast cancer survivors: a randomized controlled trial. Psychooncology. 2015;24:958-966.
51. Treanor CJ, McMenamin UC, O’Neill RF, et al. Non-pharmacological interventions for cognitive impairment due to systemic cancer treatment. Cochrane Database Syst Rev. 2016 Aug 16;(8):CD011325.
52. Mitchell AJ, Ferguson DW, Gill J, et al. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: a systematic review and meta-analysis. Lancet Oncol. 2013;14:721-732.
53. Inhestern L, Beierlein V, Bultmann JC, et al. Anxiety and depression in working-age cancer survivors: a register-based study. BMC Cancer. 2017;17:347.
54. Partridge AH, Jacobsen PB, Andersen BL. Challenges to standardizing the care for adult cancer survivors: highlighting ASCO’s fatigue and anxiety and depression guidelines. Am Soc Clin Oncol Educ Book. 2015:188-194.
55. Andersen BL, Yang HC, Farrar WB, et al. Psychologic intervention improves survival for breast cancer patients: a randomized clinical trial. Cancer. 2008;113:3450-3458.
56. Yi JC, Syrjala KL. Anxiety and depression in cancer survivors. Med Clin N Am. 2017;101:1099-1113.
57. Zhu G, Zhang X, Wang Y, et al. Effects of exercise intervention in breast cancer survivors: a meta-analysis of 33 randomized controlled trials. Onco Targets Ther. 2016;9:2153-2168.
58. Hawkins NA, Soman A, Lunsford N, et al. Use of medications for treating anxiety and depression in cancer survivors in the United States. J Clin Oncol. 2017;35:78-85.
59. Ostuzzi G, Matcham F, Dauchy S, et al. Antidepressants for the treatment of depression in people with cancer. Cochrane Database Syst Rev. 2015 June 1;(6):CD011006.
60. Laoutidis ZG, Mathiak K. Antidepressants in the treatment of depression/depressive symptoms in cancer patients: a systematic review and meta-analysis. BMC Psychiatry. 2013;13:140.
61. Lydiatt WM, Bessette D, Schmid KK, et al. Prevention of depression with escitalopram in patients undergoing treatment for head and neck cancer: randomized, double-blind, placebo-controlled clinical trial. JAMA Otolaryngol Head Neck Surg. 2013;139:678-686.
62. Lavigne JE, Heckler C, Mathews JL, et al. A randomized, controlled, double-blinded clinical trial of gabapentin 300 versus 900 mg versus placebo for anxiety symptoms in breast cancer survivors. Breast Cancer Res Treat. 2012;136:479-486.
63. Okwuosa TM, Anzevino S, Rao R. Cardiovascular disease in cancer survivors. Postgrad Med J. 2017;93:82-90.
64. Plana, JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911-939.
65. Lancellotti, P, Nkomo VT, Badano LP, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:1013-1032.
1. American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2016-2017. Atlanta, GA: American Cancer Society; 2016. www.cancer.org/research/cancer-facts-statistics/survivor-facts-figures.html. Accessed July 25, 2018.
2. Survivorship. NCCN Guidelines (version 1.2017). Fort Washington, PA: National Comprehensive Cancer Network; 2017. www.nccn.org/professionals/physician_gls/default.aspx#supportive. Accessed July 26, 2018.
3. Kendall C, Decker KM, Groome PA, et al. Use of physician services during the survivorship phase: a multi-province study of women diagnosed with breast cancer. Curr Oncolog. 2017;24:81-89.
4. Snyder CF, Earle CC, Herbert RJ, et al. Preventive care for colorectal cancer survivors: a 5-year longitudinal study. J Clin Oncol. 2008;26:1073-1079.
5. Hewitt M, Greenfield S, Stovall E (eds); Institute of Medicine and National Research Council. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington DC: The National Academies Press; 2006. www.nap.edu/read/11468/chapter/1. Accessed July 25, 2018.
6. Survivorship care plan. New York, NY: Memorial Sloan Kettering Cancer Center. www.mskcc.org/hcp-education-training/survivorship/survivorship-care-plan. Accessed August 11, 2018.
7. Fuentes AC, Lambird JE, George TJ, et al. Cancer survivor’s history and physical. South Med J. 2017;110:37-44. http://sma.org/southern-medical-journal/article/cancer-survivors-history-physical/. Accessed July 26, 2018.
8. Salz T, Oeffinger KC, McCabe MS, et al. Survivorship care plans in research and practice. CA Cancer J Clin. 2012;62:101-117.
9. Birken SA, Mayer DK, Weiner BJ. Survivorship care plans: prevalence and barriers to use. J Cancer Educ. 2013;28:290-296.
10. American College of Surgeons Commission on Cancer. Cancer program standards 2012: Ensuring patient-centered care. V1.2.1. www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx. Accessed July 25, 2018.
11. NCCN guidelines for treatment of cancer by site. NCCN Guidelines (version 1.2018). Fort Washington, PA: National Comprehensive Cancer Network; 2018. www.nccn.org/professionals/physician_gls/default.aspx#site. Accessed July 25, 2018.
12. Spronka I, Korevaar JC, Burgers JS, et al. Review of guidance on recurrence risk management for general practitioners in breast cancer, colorectal cancer and melanoma guidelines. Family Pract. 2017;34:154-160.
13. Merkow RP, Korenstein D, Yeahia R, et al. Quality of cancer surveillance clinical practice guidelines: specificity and consistency of recommendations. JAMA Intern Med. 2017;177:701-709.
14. Muradali D, Kennedy EB, Eisen A, et al. Breast screening for survivors of breast cancer: a systematic review. Prev Med. 2017;103:70-75.
15. Giannarini G, Kessler TM, Thoeny HC, et al. Do patients benefit from routine follow-up to detect recurrences after radical cystectomy and ileal orthotopic bladder substitution? Eur Urol. 2010;58:486-494.
16. Sisler JJ, Seo B, Katz A, et al. Concordance with ASCO guidelines for surveillance after colorectal cancer treatment: a population-based analysis. J Oncol Pract. 2012;8:e69-e79.
17. Ehdaie B. Atoria CL, Lowrance WT, et al. Adherence to surveillance guidelines after radical cystectomy: a population-based analysis. Urol Oncol. 2014;32:779-784.
18. Travis LB, Fosså SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst. 2005;97:1354-1365.
19. Donin N, Filson C, Drakaki A, et al. Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer. 2016;122:3075-3086.
20. Holder AE, Ramirez AG, Gallion K. Depressive symptoms in Latina breast cancer survivors: a barrier to cancer screening. Health Psycholog. 2014;33:242-248.
21. Dyer G, Larsen SR, Gilroy N, et al. Adherence to cancer screening guidelines in Australian survivors of allogenic blood and marrow transplantation (BMT). Cancer Med. 2016;5:1702-1716.
22. Mandelzweig L, Chetrit A, Amitai T, et al. Primary prevention and screening practices among long-term breast cancer survivors. Cancer Causes Control. 2017;28:657-666.
23. Bishop MM, Lee SJ, Beaumont JL, et al. The preventive health behaviors of long-term survivors of cancer and hematopoietic stem cell transplantation compared with matched controls. Biol Blood Marrow Transplant. 2010;16:207-214.
24. Uhlig A, Mei J, Baik I, et al. Screening utilization among cancer survivors: a meta-analysis. J Public Health (Oxf). 2018;40:129-137.
25. Hilal T, Rudy DW. Radiation-induced breast cancer: the question of early breast cancer screening in Hodgkin’s lymphoma survivors. Oxf Med Case Reports. 2016;2016:17-18.
26. Lin K, Croswell JM, Koenig H, et al. Prostate-specific antigen-based screening for prostate cancer: an evidence update for the U.S. Preventive Services Task Force [Internet]. Evidence Syntheses No. 90. AHRQ Publication No. 12-05160-EF-1. Rockville, MD: Agency for Healthcare Research and Quality (US); October 2011. www.ncbi.nlm.nih.gov/pubmedhealth/PMH0032900/. Accessed July 25, 2018.
27. US Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
28. Hofman M, Ryan JL, Figueroa-Moseley CD, et al. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12 Suppl 1:4-10.
29. Jung JY, Lee JM, Kim MS, et al. Comparison of fatigue, depression, and anxiety as factors affecting posttreatment health-related quality of life in lung cancer survivors. Psychooncology. 2018;27:465-470.
30. Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatment. Nat Rev Clin Oncol. 2014;11:597-609.
31. Medysky ME, Temesi J, Culos-Reed SN, et al. Exercise, sleep and cancer-related fatigue: are they related? Neurophysiol Clin. 2017;47:111-122.
32. Oh HS, Sea WS. Systematic review and meta-analysis of the correlates of cancer-related fatigue. Worldviews Evid Based Nurs. 2011;8:191-201.
33. Qu D, Zhang Z, Yu X, et al. Psychotropic drugs for the management of cancer-related fatigue: a systematic review and meta-analysis. Eur J Cancer Care (Engl). 2016;25:970-979.
34. Escalante CP, Manzullo EF. Cancer-related fatigue: the approach and treatment. J Gen Intern Med. 2009;24(suppl 2):S412-S416.
35. Mustian KM, Alfano CM, Heckler C, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol. 2017;3:961-968.
36. Glare PA, Davies PS, Finlay E, et al. Pain in cancer survivors. J Clin Oncol. 2014;32:1739-1747.
37. Davies PS. Chronic pain management in the cancer survivor: tips for primary care providers. Nurse Pract. 2013;39:28-38.
38. Boland EG, Ahmedzai SH. Persistent pain in cancer survivors. Curr Opin Support Palliat Care. 2017;11:181-190.
39. Sears CS, Robinson JW, Walker LM. A comprehensive review of sexual health concerns after cancer treatment and the biopsychosocial treatment options available to female patients. Eur J Cancer Care (Engl). 2017;27:e12738.
40. Schou Bredal I, Smeby NA, Ottesen S, et al. Chronic pain in breast cancer survivors: comparison of psychological, surgical, and medical characteristics between survivors with and without pain. J Pain Symptom Manage. 2014;48:852-862.
41. Sutradhar R, Lokku A, Barbera L. Cancer survivorship and opioid prescribing rates: a population-based matched cohort study among individuals with and without a history of cancer. Cancer. 2017;123:4286-4293.
42. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
43. Davis MP, Mehta Z. Opioids and chronic pain: where is the balance? Curr Oncol Rep. 2016;18:71.
44. Von Ah D. Cognitive changes associated with cancer and cancer treatment: state of the science. Clin J Oncol Nurs. 2015;19:47-56.
45. Moore HC. An overview of chemotherapy-related cognitive dysfunction, or ‘chemobrain’. Oncology (Williston Park). 2014;28:797-804.
46. Asher A. Cognitive dysfunction among cancer survivors. Am J Phys Med Rehabil. 2011;90(suppl):S16-S26.
47. Joly F, Rigal O, Noal S, et al. Cognitive dysfunction and cancer: which consequences in terms of disease management? Psychooncology. 2011;20:1251-1258.
48. Attention, thinking or memory problems. American Society of Clinical Oncology Cancer.Net. April 2018. www.cancer.net/navigating-cancer-care/side-effects/attention-thinking-or-memory-problems. Accessed July 25, 2018.
49. Kucherer S, Ferguson RJ. Cognitive behavioral therapy for cancer-related cognitive dysfunction. Curr Opin Support Palliat Care. 2017;11:46-51.
50. Derry HM, Jaremka LM, Bennet JM, et al. Yoga and self-reported cognitive problems in breast cancer survivors: a randomized controlled trial. Psychooncology. 2015;24:958-966.
51. Treanor CJ, McMenamin UC, O’Neill RF, et al. Non-pharmacological interventions for cognitive impairment due to systemic cancer treatment. Cochrane Database Syst Rev. 2016 Aug 16;(8):CD011325.
52. Mitchell AJ, Ferguson DW, Gill J, et al. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: a systematic review and meta-analysis. Lancet Oncol. 2013;14:721-732.
53. Inhestern L, Beierlein V, Bultmann JC, et al. Anxiety and depression in working-age cancer survivors: a register-based study. BMC Cancer. 2017;17:347.
54. Partridge AH, Jacobsen PB, Andersen BL. Challenges to standardizing the care for adult cancer survivors: highlighting ASCO’s fatigue and anxiety and depression guidelines. Am Soc Clin Oncol Educ Book. 2015:188-194.
55. Andersen BL, Yang HC, Farrar WB, et al. Psychologic intervention improves survival for breast cancer patients: a randomized clinical trial. Cancer. 2008;113:3450-3458.
56. Yi JC, Syrjala KL. Anxiety and depression in cancer survivors. Med Clin N Am. 2017;101:1099-1113.
57. Zhu G, Zhang X, Wang Y, et al. Effects of exercise intervention in breast cancer survivors: a meta-analysis of 33 randomized controlled trials. Onco Targets Ther. 2016;9:2153-2168.
58. Hawkins NA, Soman A, Lunsford N, et al. Use of medications for treating anxiety and depression in cancer survivors in the United States. J Clin Oncol. 2017;35:78-85.
59. Ostuzzi G, Matcham F, Dauchy S, et al. Antidepressants for the treatment of depression in people with cancer. Cochrane Database Syst Rev. 2015 June 1;(6):CD011006.
60. Laoutidis ZG, Mathiak K. Antidepressants in the treatment of depression/depressive symptoms in cancer patients: a systematic review and meta-analysis. BMC Psychiatry. 2013;13:140.
61. Lydiatt WM, Bessette D, Schmid KK, et al. Prevention of depression with escitalopram in patients undergoing treatment for head and neck cancer: randomized, double-blind, placebo-controlled clinical trial. JAMA Otolaryngol Head Neck Surg. 2013;139:678-686.
62. Lavigne JE, Heckler C, Mathews JL, et al. A randomized, controlled, double-blinded clinical trial of gabapentin 300 versus 900 mg versus placebo for anxiety symptoms in breast cancer survivors. Breast Cancer Res Treat. 2012;136:479-486.
63. Okwuosa TM, Anzevino S, Rao R. Cardiovascular disease in cancer survivors. Postgrad Med J. 2017;93:82-90.
64. Plana, JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911-939.
65. Lancellotti, P, Nkomo VT, Badano LP, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:1013-1032.
PRACTICE RECOMMENDATIONS
› Provide normal age-related cancer screening for cancer survivors because of their high risk of a second cancer. B
› Strongly encourage lifestyle changes for cancer survivors, especially smoking cessation. B
› Recommend exercise, which alleviates pain, depression, anxiety, and (more effectively than any other intervention) fatigue, for cancer survivors. B
› Remain vigilant for the development in cancer survivors of cardiovascular disease, including heart failure, which can appear long after therapy. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
*Cancer survivor care in the pediatric patients, including application of a survivorship care plan (also discussed later in this article), is reviewed in “Partnering to optimize care of childhood cancer survivors,” The Journal of Family Practice, April 2017.
Antithrombotic strategy 1 year after stenting in AF patients leans toward oral anticoagulant alone
SAN DIEGO – In patients with atrial fibrillation and stable coronary artery disease, a randomized trial of oral anticoagulation alone versus an anticoagulant plus a single antiplatelet agent failed to establish noninferiority of the single-agent approach. The trial could not demonstrate its primary endpoint of all-cause death, myocardial infarction, stroke, or systemic embolism.
But a secondary endpoint that included major bleeding did demonstrate equivalence, leading the researchers to suggest that oral anticoagulation (OAC) alone may be sufficient in most patients.
“Combined OAC and single antiplatelet therapy is unlikely to provide net clinical benefit over OAC alone. Thus, OAC alone might be reasonable for AF [atrial fibrillation] patients beyond 1 year after coronary stenting,” Yukiko Nakano, MD, of Kyoto (Japan) University Graduate School of Medicine, said during a press conference at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation. The report was simultaneously published Sept. 24 in Circulation (doi: 10.1161/CIRCULATIONAHA.118.036768).
The results support the European Society of Cardiology practice guidelines, which recommend lifelong OAC without antiplatelet therapy. But physicians often continue to prescribe antiplatelet agents out of concern that stent thrombosis could occur if the therapy is stopped.
The study was stopped prematurely because of insufficient recruitment, which may have contributed to the failed primary endpoint. It’s a shortcoming that befalls many such studies, perhaps because cardiologists tend to be set in their ways when it comes to treatment of patients after a stent implant. “Cardiologists just think they know the answer, and they don’t want to expose their patients (to a clinical trial). They say, ‘I have my patients on whatever regimen. It seems to be working, and they’re not bleeding, so I don’t want to change it.’ This study suggests that we probably can stop one of the two (antiplatelet drugs) and get by with a single agent, and in this case they got by with no agent (in the monotherapy arm),” said C. Michael Gibson, MD, chief of clinical research in the division of cardiology at Beth Israel Deaconess Medical Center, Boston, who was a discussant at the press conference.
The study recruited 696 patients who were receiving OAC plus single antiplatelet therapy (SAPT) 1 year after receiving a stent. They were randomized 1:1 to continue combined therapy or to stop SAPT and then followed for a median of 2.5 years. A total of 74% of patients who received OAC alone were taking warfarin, while 26% were taking a direct oral anticoagulant. The SAPT group took aspirin or clopidogrel.
Overall, 15.7% of OAC patients experienced the primary endpoint, compared with 13.6% of the combined group (noninferiority P = .20). None of the individual components of the primary endpoint were statistically significantly different between the groups. International Society on Thrombosis and Haemostasis major bleeding and Thrombolysis in Myocardial Infarction major bleeding trended in favor of OAC alone. The secondary endpoint (primary endpoint plus major bleeding) achieved noninferiority, occurring in 19.5% of the OAC group and 19.4% of the combined therapy group (noninferiority P = .016; superiority P = .96).
Daiichi-Sankyo funded the trial. Dr. Nakano had no conflicts of interest. Dr. Gibson reported numerous financial ties to pharmaceutical companies, including Daiichi-Sankyo.
SAN DIEGO – In patients with atrial fibrillation and stable coronary artery disease, a randomized trial of oral anticoagulation alone versus an anticoagulant plus a single antiplatelet agent failed to establish noninferiority of the single-agent approach. The trial could not demonstrate its primary endpoint of all-cause death, myocardial infarction, stroke, or systemic embolism.
But a secondary endpoint that included major bleeding did demonstrate equivalence, leading the researchers to suggest that oral anticoagulation (OAC) alone may be sufficient in most patients.
“Combined OAC and single antiplatelet therapy is unlikely to provide net clinical benefit over OAC alone. Thus, OAC alone might be reasonable for AF [atrial fibrillation] patients beyond 1 year after coronary stenting,” Yukiko Nakano, MD, of Kyoto (Japan) University Graduate School of Medicine, said during a press conference at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation. The report was simultaneously published Sept. 24 in Circulation (doi: 10.1161/CIRCULATIONAHA.118.036768).
The results support the European Society of Cardiology practice guidelines, which recommend lifelong OAC without antiplatelet therapy. But physicians often continue to prescribe antiplatelet agents out of concern that stent thrombosis could occur if the therapy is stopped.
The study was stopped prematurely because of insufficient recruitment, which may have contributed to the failed primary endpoint. It’s a shortcoming that befalls many such studies, perhaps because cardiologists tend to be set in their ways when it comes to treatment of patients after a stent implant. “Cardiologists just think they know the answer, and they don’t want to expose their patients (to a clinical trial). They say, ‘I have my patients on whatever regimen. It seems to be working, and they’re not bleeding, so I don’t want to change it.’ This study suggests that we probably can stop one of the two (antiplatelet drugs) and get by with a single agent, and in this case they got by with no agent (in the monotherapy arm),” said C. Michael Gibson, MD, chief of clinical research in the division of cardiology at Beth Israel Deaconess Medical Center, Boston, who was a discussant at the press conference.
The study recruited 696 patients who were receiving OAC plus single antiplatelet therapy (SAPT) 1 year after receiving a stent. They were randomized 1:1 to continue combined therapy or to stop SAPT and then followed for a median of 2.5 years. A total of 74% of patients who received OAC alone were taking warfarin, while 26% were taking a direct oral anticoagulant. The SAPT group took aspirin or clopidogrel.
Overall, 15.7% of OAC patients experienced the primary endpoint, compared with 13.6% of the combined group (noninferiority P = .20). None of the individual components of the primary endpoint were statistically significantly different between the groups. International Society on Thrombosis and Haemostasis major bleeding and Thrombolysis in Myocardial Infarction major bleeding trended in favor of OAC alone. The secondary endpoint (primary endpoint plus major bleeding) achieved noninferiority, occurring in 19.5% of the OAC group and 19.4% of the combined therapy group (noninferiority P = .016; superiority P = .96).
Daiichi-Sankyo funded the trial. Dr. Nakano had no conflicts of interest. Dr. Gibson reported numerous financial ties to pharmaceutical companies, including Daiichi-Sankyo.
SAN DIEGO – In patients with atrial fibrillation and stable coronary artery disease, a randomized trial of oral anticoagulation alone versus an anticoagulant plus a single antiplatelet agent failed to establish noninferiority of the single-agent approach. The trial could not demonstrate its primary endpoint of all-cause death, myocardial infarction, stroke, or systemic embolism.
But a secondary endpoint that included major bleeding did demonstrate equivalence, leading the researchers to suggest that oral anticoagulation (OAC) alone may be sufficient in most patients.
“Combined OAC and single antiplatelet therapy is unlikely to provide net clinical benefit over OAC alone. Thus, OAC alone might be reasonable for AF [atrial fibrillation] patients beyond 1 year after coronary stenting,” Yukiko Nakano, MD, of Kyoto (Japan) University Graduate School of Medicine, said during a press conference at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation. The report was simultaneously published Sept. 24 in Circulation (doi: 10.1161/CIRCULATIONAHA.118.036768).
The results support the European Society of Cardiology practice guidelines, which recommend lifelong OAC without antiplatelet therapy. But physicians often continue to prescribe antiplatelet agents out of concern that stent thrombosis could occur if the therapy is stopped.
The study was stopped prematurely because of insufficient recruitment, which may have contributed to the failed primary endpoint. It’s a shortcoming that befalls many such studies, perhaps because cardiologists tend to be set in their ways when it comes to treatment of patients after a stent implant. “Cardiologists just think they know the answer, and they don’t want to expose their patients (to a clinical trial). They say, ‘I have my patients on whatever regimen. It seems to be working, and they’re not bleeding, so I don’t want to change it.’ This study suggests that we probably can stop one of the two (antiplatelet drugs) and get by with a single agent, and in this case they got by with no agent (in the monotherapy arm),” said C. Michael Gibson, MD, chief of clinical research in the division of cardiology at Beth Israel Deaconess Medical Center, Boston, who was a discussant at the press conference.
The study recruited 696 patients who were receiving OAC plus single antiplatelet therapy (SAPT) 1 year after receiving a stent. They were randomized 1:1 to continue combined therapy or to stop SAPT and then followed for a median of 2.5 years. A total of 74% of patients who received OAC alone were taking warfarin, while 26% were taking a direct oral anticoagulant. The SAPT group took aspirin or clopidogrel.
Overall, 15.7% of OAC patients experienced the primary endpoint, compared with 13.6% of the combined group (noninferiority P = .20). None of the individual components of the primary endpoint were statistically significantly different between the groups. International Society on Thrombosis and Haemostasis major bleeding and Thrombolysis in Myocardial Infarction major bleeding trended in favor of OAC alone. The secondary endpoint (primary endpoint plus major bleeding) achieved noninferiority, occurring in 19.5% of the OAC group and 19.4% of the combined therapy group (noninferiority P = .016; superiority P = .96).
Daiichi-Sankyo funded the trial. Dr. Nakano had no conflicts of interest. Dr. Gibson reported numerous financial ties to pharmaceutical companies, including Daiichi-Sankyo.
REPORTING FROM TCT 2018
Key clinical point:
Major finding: A measure that included cardiac events plus major bleeding showed an oral anticoagulant alone was noninferior to an OAC plus antiplatelet therapy.
Study details: Randomized, controlled trial of 696 patients.
Disclosures: Daiichi-Sankyo funded the trial. Dr. Nakano had no conflicts of interest. Dr. Gibson reported numerous financial ties to pharmaceutical companies, including Daiichi-Sankyo.
Coagulopathy outbreak underscores danger of synthetic cannabinoids
Synthetic cannabinoids laced with superwarfarin were behind a recent outbreak of severe coagulopathy in Illinois.
In most cases, vitamin K replacement therapy alleviated symptoms, but four patients died after developing intracranial bleeding, said Amar H. Kelkar, MD, of the University of Illinois at Peoria.
Experts continue to look for how and why superwarfarin ended up in synthetic cannabinoids, whose street names include spice and K2, wrote Dr. Kelkar and his associates. Their report is in the New England Journal of Medicine.
Starting in March 2018, more than 150 patients across Illinois presented to hospitals with bleeding diathesis that involved persistent coagulopathy, the investigators explained. Early inquiries revealed that patients had consumed synthetic cannabinoids. Serum tests identified vitamin K antagonists, including brodifacoum, bromadiolone, and difenacoum. During arrests of suspected distributors, police confiscated synthetic cannabinoids that also tested positive for brodifacoum.
To help characterize the outbreak, the investigators reviewed admissions to Saint Francis Medical Center in Peoria, Ill., between March 28 and April 21, 2018. They identified 34 cases in which patients with vitamin K–dependent factor coagulopathy reported recent exposure to synthetic cannabinoids.
Fifteen of these patients underwent confirmatory anticoagulant testing, which universally confirmed superwarfarin poisoning. Brodifacoum was detected in all patients, difenacoum in five patients, bromadiolone in two patients, and warfarin in one patient.
Common presenting symptoms included gross hematuria (56% of patients) and abdominal pain (47%). Computed tomography identified renal abnormalities in 12 patients.
All patients received oral vitamin K1 (phytonadione). Red cell transfusions, fresh-frozen plasma infusions, and 4-factor prothrombin complex concentrate, or a combination of these treatments, were also used in some patients.
Among the four confirmed deaths in this outbreak, one occurred in a patient in this case series. The patient, a 37-year-old woman presenting to the emergency department with markedly reduced consciousness, was reported by her friends to have recently used synthetic cannabinoids and methamphetamine. She had no personal or family history of coagulopathy.
Computed tomography of the head without contrast material revealed severe acute intraparenchymal hemorrhage of the right basal nuclei and insula with intraventricular extension, a 10-mm left-sided midline shift, and herniation.
She met criteria for brain death 15 hours after hospital admission despite treatment with 10 mg of intravenous vitamin K1, four units of fresh frozen plasma, and 2,300 units of Kcentra.
Treating these patients after hospital discharge was difficult because of a lack of consensus guidelines and access to follow-up care, Dr. Kelkar and his associates noted. Some patients were quoted $24,000 to $34,000 per month for oral vitamin K1 therapy, which also made caring for them difficult and highlighted the need for confirmatory laboratory testing of suspected cases of superwarfarin poisoning.
Dr. Kelkar reported having no conflicts of interest. Two coinvestigators reported relationships outside the submitted work with Shire, CSL Behring, HEMA Biologics, and other companies.
SOURCE: Kelkar AH et al. N Engl J Med. 2018;379:1216-23.
Treating patients who are exposed to synthetic cannabinoid and a superwarfarin such as brodifacoum “requires more than the usual ‘treat ’em and street ’em’ approach,” wrote Jean M. Connors, MD.
“Brodifacoum is a successful rodenticide because of its extremely long half-life (approximately 16-36 days in humans),” Dr. Connors noted.
The drug also is lipophilic, causing tissue sequestration. Once exposed, patients often develop coagulopathy lasting 9 months or longer, she said.
Compared with warfarin poisoning, brodifacoum therefore requires substantially higher-dose and longer-term vitamin K1 therapy. Among the patients in this case series, the maximum outpatient dose was 50 mg, three times daily, and one patient was prescribed 25 mg, twice daily for 270 days, Dr. Connors noted.
“[Dr. Kelkar and his associates] highlight the resources and coordination needed for dealing with a public health crisis that has a prolonged duration of effect,” she added. “Because the synthetic cannabinoid market is lucrative, new products with new toxicity profiles are likely to crop up.”
Dr. Connors is with Brigham and Women’s Hospital, Dana-Farber Cancer Institute, and Harvard Medical School, all in Boston. She reported personal fees from Bristol-Myers Squibb, Portola, Dova Pharmaceuticals, and Unum Therapeutics outside the submitted work. These comments are from her accompanying editorial (N Engl J Med. 2018;379:1275-7).
Treating patients who are exposed to synthetic cannabinoid and a superwarfarin such as brodifacoum “requires more than the usual ‘treat ’em and street ’em’ approach,” wrote Jean M. Connors, MD.
“Brodifacoum is a successful rodenticide because of its extremely long half-life (approximately 16-36 days in humans),” Dr. Connors noted.
The drug also is lipophilic, causing tissue sequestration. Once exposed, patients often develop coagulopathy lasting 9 months or longer, she said.
Compared with warfarin poisoning, brodifacoum therefore requires substantially higher-dose and longer-term vitamin K1 therapy. Among the patients in this case series, the maximum outpatient dose was 50 mg, three times daily, and one patient was prescribed 25 mg, twice daily for 270 days, Dr. Connors noted.
“[Dr. Kelkar and his associates] highlight the resources and coordination needed for dealing with a public health crisis that has a prolonged duration of effect,” she added. “Because the synthetic cannabinoid market is lucrative, new products with new toxicity profiles are likely to crop up.”
Dr. Connors is with Brigham and Women’s Hospital, Dana-Farber Cancer Institute, and Harvard Medical School, all in Boston. She reported personal fees from Bristol-Myers Squibb, Portola, Dova Pharmaceuticals, and Unum Therapeutics outside the submitted work. These comments are from her accompanying editorial (N Engl J Med. 2018;379:1275-7).
Treating patients who are exposed to synthetic cannabinoid and a superwarfarin such as brodifacoum “requires more than the usual ‘treat ’em and street ’em’ approach,” wrote Jean M. Connors, MD.
“Brodifacoum is a successful rodenticide because of its extremely long half-life (approximately 16-36 days in humans),” Dr. Connors noted.
The drug also is lipophilic, causing tissue sequestration. Once exposed, patients often develop coagulopathy lasting 9 months or longer, she said.
Compared with warfarin poisoning, brodifacoum therefore requires substantially higher-dose and longer-term vitamin K1 therapy. Among the patients in this case series, the maximum outpatient dose was 50 mg, three times daily, and one patient was prescribed 25 mg, twice daily for 270 days, Dr. Connors noted.
“[Dr. Kelkar and his associates] highlight the resources and coordination needed for dealing with a public health crisis that has a prolonged duration of effect,” she added. “Because the synthetic cannabinoid market is lucrative, new products with new toxicity profiles are likely to crop up.”
Dr. Connors is with Brigham and Women’s Hospital, Dana-Farber Cancer Institute, and Harvard Medical School, all in Boston. She reported personal fees from Bristol-Myers Squibb, Portola, Dova Pharmaceuticals, and Unum Therapeutics outside the submitted work. These comments are from her accompanying editorial (N Engl J Med. 2018;379:1275-7).
Synthetic cannabinoids laced with superwarfarin were behind a recent outbreak of severe coagulopathy in Illinois.
In most cases, vitamin K replacement therapy alleviated symptoms, but four patients died after developing intracranial bleeding, said Amar H. Kelkar, MD, of the University of Illinois at Peoria.
Experts continue to look for how and why superwarfarin ended up in synthetic cannabinoids, whose street names include spice and K2, wrote Dr. Kelkar and his associates. Their report is in the New England Journal of Medicine.
Starting in March 2018, more than 150 patients across Illinois presented to hospitals with bleeding diathesis that involved persistent coagulopathy, the investigators explained. Early inquiries revealed that patients had consumed synthetic cannabinoids. Serum tests identified vitamin K antagonists, including brodifacoum, bromadiolone, and difenacoum. During arrests of suspected distributors, police confiscated synthetic cannabinoids that also tested positive for brodifacoum.
To help characterize the outbreak, the investigators reviewed admissions to Saint Francis Medical Center in Peoria, Ill., between March 28 and April 21, 2018. They identified 34 cases in which patients with vitamin K–dependent factor coagulopathy reported recent exposure to synthetic cannabinoids.
Fifteen of these patients underwent confirmatory anticoagulant testing, which universally confirmed superwarfarin poisoning. Brodifacoum was detected in all patients, difenacoum in five patients, bromadiolone in two patients, and warfarin in one patient.
Common presenting symptoms included gross hematuria (56% of patients) and abdominal pain (47%). Computed tomography identified renal abnormalities in 12 patients.
All patients received oral vitamin K1 (phytonadione). Red cell transfusions, fresh-frozen plasma infusions, and 4-factor prothrombin complex concentrate, or a combination of these treatments, were also used in some patients.
Among the four confirmed deaths in this outbreak, one occurred in a patient in this case series. The patient, a 37-year-old woman presenting to the emergency department with markedly reduced consciousness, was reported by her friends to have recently used synthetic cannabinoids and methamphetamine. She had no personal or family history of coagulopathy.
Computed tomography of the head without contrast material revealed severe acute intraparenchymal hemorrhage of the right basal nuclei and insula with intraventricular extension, a 10-mm left-sided midline shift, and herniation.
She met criteria for brain death 15 hours after hospital admission despite treatment with 10 mg of intravenous vitamin K1, four units of fresh frozen plasma, and 2,300 units of Kcentra.
Treating these patients after hospital discharge was difficult because of a lack of consensus guidelines and access to follow-up care, Dr. Kelkar and his associates noted. Some patients were quoted $24,000 to $34,000 per month for oral vitamin K1 therapy, which also made caring for them difficult and highlighted the need for confirmatory laboratory testing of suspected cases of superwarfarin poisoning.
Dr. Kelkar reported having no conflicts of interest. Two coinvestigators reported relationships outside the submitted work with Shire, CSL Behring, HEMA Biologics, and other companies.
SOURCE: Kelkar AH et al. N Engl J Med. 2018;379:1216-23.
Synthetic cannabinoids laced with superwarfarin were behind a recent outbreak of severe coagulopathy in Illinois.
In most cases, vitamin K replacement therapy alleviated symptoms, but four patients died after developing intracranial bleeding, said Amar H. Kelkar, MD, of the University of Illinois at Peoria.
Experts continue to look for how and why superwarfarin ended up in synthetic cannabinoids, whose street names include spice and K2, wrote Dr. Kelkar and his associates. Their report is in the New England Journal of Medicine.
Starting in March 2018, more than 150 patients across Illinois presented to hospitals with bleeding diathesis that involved persistent coagulopathy, the investigators explained. Early inquiries revealed that patients had consumed synthetic cannabinoids. Serum tests identified vitamin K antagonists, including brodifacoum, bromadiolone, and difenacoum. During arrests of suspected distributors, police confiscated synthetic cannabinoids that also tested positive for brodifacoum.
To help characterize the outbreak, the investigators reviewed admissions to Saint Francis Medical Center in Peoria, Ill., between March 28 and April 21, 2018. They identified 34 cases in which patients with vitamin K–dependent factor coagulopathy reported recent exposure to synthetic cannabinoids.
Fifteen of these patients underwent confirmatory anticoagulant testing, which universally confirmed superwarfarin poisoning. Brodifacoum was detected in all patients, difenacoum in five patients, bromadiolone in two patients, and warfarin in one patient.
Common presenting symptoms included gross hematuria (56% of patients) and abdominal pain (47%). Computed tomography identified renal abnormalities in 12 patients.
All patients received oral vitamin K1 (phytonadione). Red cell transfusions, fresh-frozen plasma infusions, and 4-factor prothrombin complex concentrate, or a combination of these treatments, were also used in some patients.
Among the four confirmed deaths in this outbreak, one occurred in a patient in this case series. The patient, a 37-year-old woman presenting to the emergency department with markedly reduced consciousness, was reported by her friends to have recently used synthetic cannabinoids and methamphetamine. She had no personal or family history of coagulopathy.
Computed tomography of the head without contrast material revealed severe acute intraparenchymal hemorrhage of the right basal nuclei and insula with intraventricular extension, a 10-mm left-sided midline shift, and herniation.
She met criteria for brain death 15 hours after hospital admission despite treatment with 10 mg of intravenous vitamin K1, four units of fresh frozen plasma, and 2,300 units of Kcentra.
Treating these patients after hospital discharge was difficult because of a lack of consensus guidelines and access to follow-up care, Dr. Kelkar and his associates noted. Some patients were quoted $24,000 to $34,000 per month for oral vitamin K1 therapy, which also made caring for them difficult and highlighted the need for confirmatory laboratory testing of suspected cases of superwarfarin poisoning.
Dr. Kelkar reported having no conflicts of interest. Two coinvestigators reported relationships outside the submitted work with Shire, CSL Behring, HEMA Biologics, and other companies.
SOURCE: Kelkar AH et al. N Engl J Med. 2018;379:1216-23.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: There were more than 150 cases in Illinois with four deaths among patients who developed spontaneous intracranial bleeding.
Study details: A single-institution case series of 15 patients.
Disclosures: Dr. Kelkar reported having no conflicts of interest. Two coinvestigators reported relationships outside the submitted work with Shire, CSL Behring, HEMA Biologics, and other companies.
Source: Kelkar AH et al. N Engl J Med. 2018;379:1216-23.
Polycythemia Vera and Essential Thrombocythemia
From the Columbia University Medical Center, New York, NY (Dr. Falchi), and the University of Texas MD Anderson Cancer Center, Houston, TX (Dr. Verstovsek).
ABSTRACT
- Objective: To review the clinical aspects and current practices in the management of polycythemia vera (PV) and essential thrombocythemia (ET).
- Methods: Review of the literature.
- Results: PV and ET are rare chronic myeloid disorders. The 2 most important life-limiting complications of PV and ET are thrombohemorrhagic events and myelofibrosis/acute myeloid leukemia (AML) transformation. Vascular events are at least in part preventable with counseling on risk factors, phlebotomy (for patients with PV), antiplatelet therapy, and cytoreduction with hydroxyurea, interferons, or anagrelide (for patients with ET). Ruxolitinib was recently approved for PV after hydroxyurea failure. PV/ET transformation into myelofibrosis or AML is part of the natural history of the disease and no therapy has been shown to prevent it. Treatment of leukemic transformation of myeloproliferative neoplasms (MPN LT) follows recommendations set forth for primary myelofibrosis and AML.
- Conclusion: With appropriate management, patients with PV and ET typically enjoy a long survival and near-normal quality of life. Transformation into myelofibrosis or AML cannot be prevented by current therapies, however. Treatment results with MPN LT are generally poor and novel strategies are needed to improve outcomes.
Key words: myeloproliferative neoplasms; myelofibrosis; leukemic transformation.
Polycythemia vera (PV) and essential thrombocythemia (ET), along with primary myelofibrosis (PMF), belong to the group of Philadelphia-negative myeloproliferative neoplasms (MPN). All these malignancies arise from the clonal proliferation of an aberrant hematopoietic stem cell, but are characterized by distinct clinical phenotypes [1,2]. Although the clinical course of PV and ET is indolent, it can be complicated by thrombohemorrhagic episodes and/or evolution into myelofibrosis and/or acute myeloid leukemia (AML) [3]. Since vascular events are the most frequent life-threatening complications of PV and ET, therapeutic strategies are aimed at reducing this risk. Treatment may also help control other symptoms associated with the disease [4]. No therapy has been shown to prevent evolution of PV or ET into myelofibrosis or AML. The discovery of the Janus kinase 2 (JAK2)/V617F mutation in most patients with PV and over half of those with ET (and PMF) [5,6] has opened new avenues of research and led to the development of targeted therapies, such as the JAK1/2 inhibitor ruxolitinib, for patients with MPN [7,8].
Epidemiology
PV and ET are typically diagnosed in the fifth to seventh decade of life [9]. Although these disorders are generally associated with a long clinical course, survival of patients with PV or ET may be shorter than that of the general population [10–13]. Estimating the incidence and prevalence of MPN is a challenge because most patients remain asymptomatic for long periods of time and do not seek medical attention [13]. The annual incidence rates of PV and ET are estimated at 0.01 to 2.61 and 0.21 to 2.53 per 100,000, respectively. PV occurs slightly more frequently in males, whereas ET has a predilection for females [14]. Given the long course and low mortality associated with these disorders, the prevalence rates of PV and ET are significantly higher than the respective incidence rates: up to 47 and 57 per 100,000, respectively [15–17].
Molecular Pathogenesis
In 2005 researchers discovered a gain-of-function mutation of the JAK2 gene in nearly all patients with PV and more than half of those with ET and PMF [5,6,18,19]. JAK2 is a non-receptor tyrosine kinase that plays a central role in normal hematopoiesis. Substitution of a valine for a phenylalanine at codon 617 (ie, V617F) leads to its constitutive activation and signaling through the JAK-STAT pathway [5,6,18,19]. More rarely (and exclusively in patients with PV), JAK2 mutations involve exon 12 [20–22]. The vast majority of JAK2-negative ET patients harbor mutations in either the myeloproliferative leukemia (MPL) gene, which encodes the thrombopoietin receptor [23–25], or the calreticulin (CALR) gene [26,27], which encodes for a chaperone protein that plays a role in cellular proliferation, differentiation, and apoptosis [28]. Both the MPL and CALR mutations ultimately result in the constitutive activation of the JAK-STAT pathway. Thus, JAK2, MPL, and CALR alterations are collectively referred to as driver mutations. Moreover, because these mutations affect the same oncogenic pathway (ie, JAK-STAT), they are almost always mutually exclusive in a given patient. Patients with ET (or myelofibrosis) who are wild-type for JAK2, MPL, and CALR are referred to as having “triple-negative” disease. Many recurrent non-driver mutations are also found in patients with MPN. These are not exclusive of each other (ie, patients may have many at the same time) and involve for example ten-eleven translocation-2 (TET2), additional sex combs like 1 (ASXL1), enhancer of zeste homolog 2 (EZH2), isocitrate dehydrogenase 1 and isocitrate dehydrogenase 2 (IDH1/2), and DNA methyltransferase 3A (DNMT3A) genes, among others [29]. The biologic and prognostic significance of these non-driver alterations remain to be fully defined in ET and PV.
Diagnostic Criteria
Diagnostic criteria for PV and ET according to the World Health Organization (WHO) classification [30] are summarized in Table 1. Criteria for the diagnosis of prefibrotic myelofibrosis are included as well since this entity was formally recognized as separate from ET and part of the PMF spectrum in the 2016 WHO classification of myeloid tumors [30]. Clinically, both PV and ET generally remain asymptomatic for a long time. PV tends to be more symptomatic than ET and can present with debilitating constitutional symptoms (fatigue, night sweats, and weight loss), microvascular symptoms (headache, lightheadedness, acral paresthesias, erythromelalgia, atypical chest pain, and pruritus) [31], or macrovascular accidents (larger vein thrombosis, stroke, or myocardial ischemia) [32]. ET is often diagnosed incidentally, but patients can suffer from similar general symptoms and vascular complications. Causes of secondary absolute erythrocytosis (altitude, chronic hypoxemia, heavy smoking, cardiomyopathy, use of corticosteroids, erythropoietin, or other anabolic hormones, familial or congenital forms) or thrombocytosis (iron deficiency, acute blood loss, trauma or injury, acute coronary syndrome, systemic autoimmune disorders, chronic kidney failure, other malignancies, splenectomy) should be considered and appropriately excluded. Once the diagnosis is made, symptom assessment tools such as the Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF) [33] or the abbreviated version, the MPN-SAF Total Symptom Score (MPN-SAF TSS) [34], are generally used to assess patients’ symptom burden and response to treatment in everyday practice.
Risk Stratification
Thrombohemorrhagic events, evolution into myelofibrosis, and leukemic transformation (LT) are the most serious complications in the course of PV or ET. Only thrombohemorrhagic events are, at least partially, preventable. Arterial or venous thrombotic complications are observed at rates of 1.8 to 10.9 per 100 patient-years in PV (arterial thrombosis being more common than venous) and 0.74 to 7.7 per 100 patient-years in ET, depending on the risk group [35] and the presence of other factors (see below).
The risk stratification of patients with PV is based on 2 factors: age ≥ 60 years and prior history of thrombosis. If either is present, the patient is assigned to the high-risk category, whereas if none is present the patient is considered at low risk [36]. In addition, high hematocrit [37] and high white blood cell (WBC) count [38], but not thrombocytosis, have been associated with the development of vascular complications. In one study, the risk of new arterial thrombosis was increased by the presence of leukoerythroblastosis, hypertension, and prior arterial thrombosis, while karyotypic abnormalities and prior venous thrombosis were predictors of new venous thrombosis [39]. Another emerging risk factor for thrombosis in patients with PV is high JAK2 allele burden (ie, the normal-to-mutated gene product ratio), although the evidence supporting this conclusion is equivocal [40].
Traditionally, in ET patients, the thrombotic risk was assessed using the same 2 factors (age ≥ 60 years and prior history of thrombosis), separating patients into low- and high-risk groups. However, the prognostication of ET patients has been refined recently with the identification of new relevant factors. In particular, the impact of JAK2 mutations on thrombotic risk has been thoroughly studied. Clinically, the presence of JAK2V617F is associated with older age, higher hemoglobin and hematocrit, lower platelet counts, more frequent need for cytoreductive treatment, and greater tendency to evolve into PV (a rare event) [41,42]. Many [41,43–46], but not all [47–51], studies suggested a correlation between JAK2 mutation and risk of both arterial and venous thrombosis. Although infrequent, a JAK2V617F homozygous state (ie, the mutation is present in both alleles) might confer an even higher thrombotic risk [52]. Moreover, the impact of the JAK2 mutation on vascular events persists over time [53], particularly in patients with high or unstable mutation burden [54]. Based on JAK2V617F’s influence on the thrombotic risk of ET patients, a new prognostic score was proposed, the International Prognostic Score for ET (IPSET)-thrombosis (Table 2). The revised version of this model is currently endorsed by the National Comprehensive Cancer Network and divides patients into 4 risk groups: high, intermediate, low, and very low. Treatment recommendations vary according to the risk group (as described below) [55].
Other thrombotic risk factors have been identified, but deemed not significant enough to be included in the model. Cardiovascular risk factors (hypercholesterolemia, hypertension, smoking, diabetes mellitus) can increase the risk of vascular events [56–59], as can splenomegaly [60] and baseline or persistent leukocytosis [61–63]. Thrombocytosis has been correlated with thrombotic risk in some studies [64–68], whereas others did not support this conclusion and/or suggested a lower rate of thrombosis and, in some cases, increased risk of bleeding in ET patients with platelet counts greater than 1000 × 103/μL (due to acquired von Willebrand syndrome) [51,61,63,68,69].
CALR mutations tend to occur in younger males with lower hemoglobin and WBC count, higher platelet count, and greater marrow megakaryocytic predominance, as compared to JAK2 mutations [26,27,70–72]. The associated incidence of thrombosis was less than 10% at 15 years in patients with CALR mutations, lower than the incidence reported for ET patients with JAK2V617F mutations [73]. The presence of the mutation per se does not appear to affect the thrombotic risk [74–76]. Information on the thrombotic risk associated with MPL mutations or a triple-negative state is scarce. In both instances, however, the risk appears to be lower than with the JAK2 mutation [73,77–79].
Venous thromboembolism (VTE) in patients with PV or ET may occur at unusual sites, such as the splanchnic or cerebral venous systems [80]. Risk factors for unusual VTE include younger age [81], female gender (especially with concomitant use of oral contraceptive pills) [82], and splenomegaly/splenectomy [83]. JAK2 mutation has also been associated with thrombosis at unusual sites. However, the prevalence of MPN or JAK2V617F in patients presenting with splanchnic VTE has varied [80]. In addition, MPN may be occult (ie, no clinical or laboratory abnormalities) in around 15% of patients [84]. Screening for JAK2V617F and underlying MPN is recommended in patients presenting with isolated unexplained splanchnic VTE. Treatment entails long-term anticoagulation therapy. JAK2V617F screening in patients with nonsplanchnic VTE is not recommended, as its prevalence in this group is low (< 3%) [85,86].
Risk-Adapted Therapy
Low-Risk PV
All patients with PV should receive counseling to mitigate cardiovascular risk factors, including smoking cessation, lifestyle modifications, and lipid-lowering therapy, as indicated. Furthermore, all PV patients should receive acetylsalicylic acid (ASA) to decrease their risk for thrombosis and control vasomotor symptoms [55,87]. Aspirin 81 to 100 mg daily is the preferred regimen because it provides adequate antithrombotic effect without the associated bleeding risk of higher-dose aspirin [88]. Low-risk PV patients should also receive periodic phlebotomies to reduce and maintain their hematocrit below 45%. This recommendation is based on the results of the Cytoreductive Therapy in Polycythemia Vera (CYTO PV) randomized controlled trial. In that study, patients receiving more intense therapy to maintain the hematocrit below 45% had a lower incidence of cardiovascular-related deaths or major thrombotic events than those with hematocrit goals of 45% to 50% (2.7% versus 9.8%) [89]. Cytoreduction is an option for low-risk patients who do not tolerate phlebotomy or require frequent phlebotomy, or who have disease-related bleeding, severe symptoms, symptomatic splenomegaly, or progressive leukocytosis [38].
High-Risk PV
Patients older than 60 years and/or with a history of thrombosis should be considered for cytoreductive therapy in addition to the above measures. Frontline cytoreductive therapies include hydroxyurea or interferon (IFN)-alfa [87]. Hydroxyurea is a potent ribonucleotide reductase inhibitor that interferes with DNA repair and is the treatment of choice for most high-risk patients with PV [90]. In a small trial, hydroxyurea reduced the risk of thrombosis compared with historical controls treated with phlebotomy alone [91]. Hydroxyurea is generally well tolerated; common side effects include cytopenias, nail changes, and mucosal and/or skin ulcers. Although never formally proven to be leukemogenic, this agent should be used with caution in younger patients [87]. Indeed, in the original study, the rates of transformation were 5.9% and 1.5% for patients receiving hydroxyurea and phlebotomy alone [92], respectively, although an independent role for hydroxyurea in LT was not supported in the much larger European Collaboration on Low-dose Aspirin in Polycythemia Vera (ECLAP) study [93]. Approximately 70% of patients will have a sustained response to hydroxyurea [94], while the remaining patients become resistant to or intolerant of the drug. Resistant individuals have a higher risk of progression to acute leukemia and death [95].
IFN-alfa is a pleiotropic antitumor agent that has found application in many types of malignancies [96] and is sometimes employed as treatment for patients with newly diagnosed high-risk PV. Early studies showed responses in up to 100% of cases [97,98], albeit at the expense of a high discontinuation rate due to adverse events, such as flu-like symptoms, fatigue, and neuropsychiatric manifestations [99]. A newer formulation of the drug obtained by adding a polyethylene glycol (PEG) moiety to the native IFN-alfa molecule (PEG-IFN alfa) was shown to have a longer half-life, greater stability, less immunogenicity, and, potentially, better tolerability [100]. Pilot phase 2 trials of PEG-IFN-alfa-2a demonstrated its remarkable activity, with symptomatic and hematologic responses seen in most patients (which, in some cases, persisted beyond discontinuation), and reasonable tolerability, with long-term discontinuation rates of 20% to 30% [101–103]. In some patients, JAK2V617F became undetectable over time [104]. Results of 2 ongoing trials, MDP-RC111 (single-arm study, PEG-IFN-alfa-2a in high-risk PV or ET [NCT01259817]) and MPD-RC112 (randomized controlled trial, PEG-IFN-alfa-2a versus hydroxyurea in the same population [NCT01258856]), will shed light on the role of PEG-IFN-alfa in the management of patients with high-risk PV or ET. In two phase 2 studies of PEG-IFN-alfa-2b, complete responses were seen in 70% to 100% of patients and discontinuation occurred in around a third of cases [105,106]. A new, longer-acting formulation of PEG-IFN-alfa-2a (peg-proline INF-alfa-2b, AOP2014) is also undergoing clinical development [107,108].
The approach to treatment of PV based on thrombotic risk level is illustrated in Figure 1.
Very Low- and Low-Risk ET
Individuals with ET should undergo rigorous cardiovascular risk management and generally receive ASA to decrease their thrombotic risk and improve symptom control. Antiplatelet therapy may not be warranted in patients with documented acquired von Willebrand syndrome, with or without extreme thrombocytosis, or in those in the very low-risk category according to the IPSET-thrombosis model [55,87]. The risk/benefit ratio of antiplatelet agents in patients with ET at different thrombotic risk levels was assessed in poor-quality studies and thus remains highly uncertain. Platelet-lowering agents are sometimes recommended in patients with low-risk disease who have platelet counts ≥ 1500 × 103/μL, due to the potential risk of acquired von Willebrand syndrome and a risk of bleeding (this would require stopping ASA) [109]. Cytoreduction may also be used in low-risk patients with progressive symptoms despite ASA, symptomatic or progressive splenomegaly, and progressive leukocytosis.
Intermediate-Risk ET
This category includes patients older than 60 years, but without thrombosis or JAK2 mutations. These individuals would have been considered high risk (and thus candidates for cytoreductive therapy) according to the traditional risk stratification. Guidelines currently recommend ASA as the sole therapy for these patients, while reserving cytoreduction for those who experience thrombosis (ie, become high-risk) or have uncontrolled vasomotor or general symptoms, symptomatic splenomegaly, symptomatic thrombocytosis, or progressive leukocytosis.
High-Risk ET
For patients with ET in need of cytoreductive therapy (ie, those with prior thrombosis or older than 60 years with a JAK2V617F mutation), first-line options include hydroxyurea, IFN, and anagrelide. Hydroxyurea remains the treatment of choice in most patients [110]. In a seminal study, 114 patients with ET were randomly assigned to either observation or hydroxyurea treatment with the goal of maintaining the platelet count below 600 × 103/μL. At a median follow-up of 27 months, patients in the hydroxyurea group had a lower thrombosis rate (3.6% versus 24%, P = 0.003) and longer thrombosis-free survival, regardless of the use of antiplatelet drugs [64].
Anagrelide, a selective inhibitor of megakaryocytic differentiation and proliferation, was compared with hydroxyurea in patients with ET in 2 randomized trials. In the first (n = 809), the group receiving anagrelide had a higher risk of arterial thrombosis, major bleeding, and fibrotic evolution, but lower incidence of venous thrombosis. Hydroxyurea was better tolerated, mainly due to anagrelide-related cardiovascular adverse events [111]. As a result of this study, hydroxyurea is often preferred to anagrelide as frontline therapy for patients with newly diagnosed high-risk ET. In the second, more recent study (n = 259), however, the 2 agents proved equivalent in terms of major or minor arterial or venous thrombosis, as well as discontinuation rate [112]. The discrepancy between the 2 trials may be partly explained by the different ET diagnostic criteria used, with the latter only enrolling patients with WHO-defined true ET and the former utilizing Polycythemia Vera Study Group-ET diagnostic criteria that included patients with increases in other blood counts or varying degrees of marrow fibrosis.
Interferons were studied in ET in parallel with PV. PEG-IFN-alfa-2a proved effective in patients with ET, with responses observed in 80% of patients [103]. PEG-IFN- alfa-2b produced similar results, with responses in 70% to 90% of patients in small studies and discontinuation observed in 20% to 38% of cases [105,106,113]. Because the very long-term leukemogenic potential of hydroxyurea has remained somewhat uncertain, anagrelide or IFN might be preferable choices in younger patients.
The approach to treatment of ET based on thrombotic risk level is illustrated in Figure 2.
Assessing Response to Therapy
For both patients with PV and ET the endpoint of treatment set forth for clinical trials has been the achievement of a clinicohematologic response. However, studies have failed to show a correlation between response and reduction of the thrombohemorrhagic risk [114]. Therefore, proposed clinical trial response criteria were revised to include absence of hemorrhagic or thrombotic events as part of the definition of response (Table 3) [94].
Approach to Patients Refractory to or Intolerant of First-line Therapy
According to the European LeukemiaNet recommendations, an inadequate response to hydroxyurea in patients with PV (or myelofibrosis) is defined as a need for phlebotomy to maintain the hematocrit below < 45%, the platelet count > 400 × 103/μL, and a WBC count > 10,000/μL, or failure to reduce splenomegaly > 10 cm by > 50% at a dose of ≥ 2 g/day or maximum tolerated dose. Historically, treatment options for patients with PV or ET who failed first-line therapy (most commonly hydroxyurea) have included alkylating agents, such as busulfan, chlorambucil, pipobroman, and phosphorus (P)-32. However, the use of these drugs is limited by the associated risk of LT [93,115,116]. IFN (or anagrelide for ET) is often considered in patients previously treated with hydroxyurea, and vice versa.
Ruxolitinib is a JAK1 and JAK2 inhibitor currently approved for the treatment of PV patients refractory to or intolerant of hydroxyurea [7]. Following promising results of a phase 2 trial [117], ruxolitinib 10 mg twice daily was compared with best available therapy in the pivotal RESPONSE trial (n = 222). Ruxolitinib proved superior in achieving hematocrit control, reduction of spleen volume, and improvement of symptoms. Grade 3-4 hematologic toxicity was infrequent and similar in the 2 arms [118]. In addition, longer follow-up of that study suggested a lower rate of thrombotic events in patients receiving ruxolitinib (1.8 versus 8.2 per 100 patient-years) [119]. In a similarly designed randomized phase 3 study in PV patients without splenomegaly (RESPONSE-2), more patients in the ruxolitinib arm had hematocrit reduction without an increase in toxicity. Based on the results of these studies, ruxolitinib can be considered a standard of care for second-line therapy in this post-hydroxyurea patient population [120]. Ruxolitinib is also being tested in patients with high-risk ET who have become resistant to, or were intolerant of hydroxyurea, but currently has no approved indication in this setting [121,122]. Common side effects of ruxolitinib include cytopenias (especially anemia), increased risk of infections, hyperlipidemia, and increased risk of non-melanoma skin cancer.
Novel agents that have been studied in patients with PV and ET are histone deacetylase inhibitors, murine double minute 2 (MDM2, or HDM2 for their human counterpart) inhibitors (which restore the function of p53), Bcl-2 homology domain 3 mimetics such as navitoclax and venetoclax, and, for patients with ET, the telomerase inhibitor imetelstat [123].
Disease Evolution
Post-PV/Post-ET Myelofibrosis
Diagnostic criteria for post-PV and post-ET myelofibrosis are outlined in Table 4. Fibrotic transformation represents a natural evolution of the clinical course of PV or ET. It occurs in up to 15% and 9% of patients with PV and ET, respectively, in western countries [124]. The true percentage of ET patients who develop myelofibrosis is confounded by the inclusion of prefibrotic myelofibrosis cases in earlier series. The survival of patients who develop myelofibrosis is shortened compared to those who do not. In patients with PV, risk factors for myelofibrosis evolution include advanced age, leukocytosis, JAK2V617F homozygosity or higher allele burden, and hydroxyurea therapy. Once post-PV myelofibrosis has occurred, hemoglobin < 10 g/dL, platelet count < 100 × 103/μL, and WBC count > 30,000/μL are associated with worse outcomes [125]. In patients with ET, risk factors for myelofibrosis transformation include age, anemia, bone marrow hypercellularity and increased reticulin, increased lactate dehydrogenase, leukocytosis, and male gender. The management of post-PV/post-ET myelofibrosis recapitulates that of PMF.
Leukemic Transformation
The presence of more than 20% blasts in peripheral blood or bone marrow in a patient with MPN defines LT. This occurs in up to 5% to 10% of patients and may or may not be preceded by a myelofibrosis phase [126]. In cases of extramedullary transformation, a lower percentage of blasts can be seen in the bone marrow compared to the peripheral blood. The pathogenesis of LT has remained elusive, but it is believed to be associated with genetic instability, which facilitates the acquisition of additional mutations, including those of TET2, ASXL1, EZH2 DNMT3, IDH1/2, and TP53 [127].
Clinical risk factors for LT include advanced age, karyotypic abnormalities, prior therapy with alkylating agents or P-32, splenectomy, increased peripheral blood or bone marrow blasts, leukocytosis, anemia, thrombocytopenia, and cytogenetic abnormalities. Hydroxyurea, IFN, and ruxolitinib have not been shown to have leukemogenic potential thus far. Prognosis of LT is uniformly poor and patient survival rarely exceeds 6 months.
There is no standard of care for MPN LT. Treatment options range from low-intensity regimens to more aggressive AML-type induction chemotherapy. No strategy appears clearly superior to others [128]. Hematopoietic stem cell transplantation is the only therapy that provides clinically meaningful benefit to patients [129], but it is applicable only to a minority of patients with chemosensitive disease and good performance status [130]. Notable experimental approaches to MPN LT include hypomethylating agents, such as decitabine [131] or azacytidine [132], with or without ruxolitinib [133–135].
Conclusion
PV and ET are rare, chronic myeloid disorders. Patients typically experience a long clinical course and enjoy near-normal quality of life if properly managed. The 2 most important life-limiting complications of PV and ET are thrombohemorrhagic events and myelofibrosis/AML transformation. Vascular events are at least in part preventable with counseling on risk factors, phlebotomy (for patients with PV), antiplatelet therapy, and cytoreduction with hydroxyurea, IFNs, or anagrelide (for patients with ET). In addition, ruxolitinib was recently approved for PV patients after hydroxyurea failure. PV/ET transformation in myelofibrosis or AML is part of the natural history of the disease and no therapy has been shown to prevent it. Treatment follows recommendations set forth for PMF and AML, but results are generally poorer and novel strategies are needed to improve outcomes.
Corresponding author: Lorenzo Falchi, MD, Columbia University Medical Center, New York, NY.
Financial disclosures: None.
1. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC; 2008.
2. Alvarez-Larran A, Pereira A, Arellano-Rodrigo E, et al. Cytoreduction plus low-dose aspirin versus cytoreduction alone as primary prophylaxis of thrombosis in patients with high-risk essential thrombocythaemia: an observational study. Br J Haematol 2013;161:865–71.
3. Tefferi A. Polycythemia vera and essential thrombocythemia: 2013 update on diagnosis, risk-stratification, and management. Am J Hematol 2013;88:507–16.
4. Michiels JJ, Berneman Z, Schroyens W, et al. Platelet-mediated erythromelalgic, cerebral, ocular and coronary microvascular ischemic and thrombotic manifestations in patients with essential thrombocythemia and polycythemia vera: a distinct aspirin-responsive and coumadin-resistant arterial thrombophilia. Platelets 2006;17:528–44.
5. James C, Ugo V, Couedic J-PL, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005;434:1144–8.
6. Kralovics R, Passamonti F, Buser A, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders.N Engl J Med 2005;352:1779–90.
7. Verstovsek S, Mesa R, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 2012;366:799–807.
8. Harrison C, Kiladjian J, Al-Ali H, et al. JAK Inhibition with ruxolitinib versus best available therapy for myelofibrosis.N Engl J Med 2012;366:787–98.
9. Berglund S, Zettervall O. Incidence of polycythemia vera in a defined population. Eur J Haematol 1992;48:20–6.
10. Rozman C, Giralt M, Feliu E, et al. Life expectancy of patients with chronic nonleukemic myeloproliferative disorders. Cancer 1991;67:2658–63.
11. Passamonti F, Rumi E, Pungolino E, et al. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med 2004;117:755–61.
12. Hultcrantz M, Kristinsson SY, Andersson TM, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol 2012;30:2995–3001.
13. Wolanskyj AP, Schwager SM, et al. Essential thrombocythemia beyond the first decade: life expectancy, long-term complication rates, and prognostic factors. Mayo Clin Proc 2006;81:159–66.
14. Srour SA, Devesa SS, Morton LM, et al. Incidence and patient survival of myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms in the United States, 2001-12. Br J Haematol 2016;174:382–96.
15. Titmarsh GJ, Duncombe AS, McMullin MF, et al. How common are myeloproliferative neoplasms? A systematic review and meta-analysis. Am J Hematol 2014;89:581–7.
16. Moulard O, Mehta J, Fryzek J, et al. Epidemiology of myelofibrosis, essential thrombocythemia, and polycythemia vera in the European Union. Eur J Haematol 2014;92:289–97.
17. Stein BL, Gotlib J, Arcasoy M, et al. Historical views, conventional approaches, and evolving management strategies for myeloproliferative neoplasms. J Natl Compr Canc Netw 2015;13:424–34.
18. Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 2005;7:387–97.
19. Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005;365:1054–61.
20. Scott LM, Tong W, Levine RL, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med 2007;356:459–68.
21. Pardanani A, Lasho TL, Finke C, et al. Prevalence and clinicopathologic correlates of JAK2 exon 12 mutations in JAK2V617F-negative polycythemia vera. Leukemia 2007;21:1960–3.
22. Passamonti F, Elena C, Schnittger S, et al. Molecular and clinical features of the myeloproliferative neoplasm associated with JAK2 exon 12 mutations. Blood 2011;117:2813–6.
23. Abe M, Suzuki K, Inagaki O, et al. A novel MPL point mutation resulting in thrombopoietin-independent activation. Leukemia 2002;16:1500–6.
24. Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med 2006;3:e270.
25. Pardanani AD, Levine RL, Lasho T, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood 2006;108:3472–6.
26. Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med 2013;369:2391–405.
27. Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med 2013;369:2379–90.
28. Wang WA, Groenendyk J, Michalak M. Calreticulin signaling in health and disease. Int J Biochem Cell Biol 2012;44:842–6.
29. Saeidi K. Myeloproliferative neoplasms: Current molecular biology and genetics. Crit Rev Oncol Hematol 2016;98:375–89.
30. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016;127: 2391–405.
31. Mesa RA, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer 2007;109:68–76.
32. Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol 2005;23:2224–32.
33. Scherber R, Dueck AC, Johansson P, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood 2011;118:401–8.
34. Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol 2012;30:4098–103.
35. Casini A, Fontana P, Lecompte TP. Thrombotic complications of myeloproliferative neoplasms: risk assessment and risk-guided management. J Thromb Haemost 2013;11:1215–27.
36. Barbui T, Finazzi G, Falanga A. Myeloproliferative neoplasms and thrombosis. Blood 2013;122:2176-–84.
37. Pearson TC, Wetherley-Mein G. Vascular occlusive episodes and venous haematocrit in primary myeloproliferative polychytemia. Lancet 1978;2:1219–22.
38. Landolfi R, Di Gennaro L, Barbui T, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood 2007;109:2446–52.
39. Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia 2013;27:1874–81.
40. Barbui T, Falanga A. Molecular biomarkers of thrombosis in myeloproliferative neoplasms. Thromb Res 2016;140 Suppl 1:S71–75.
41. Campbell PJ, Scott LM, Buck G, et al. Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study. Lancet 2005;366:1945–53.
42. Tefferi A, Guglielmelli P, Larson DR, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood 2014;124:2507–13.
43. Qin Y, Wang X, Zhao C, et al. The impact of JAK2V617F mutation on different types of thrombosis risk in patients with essential thrombocythemia: a meta-analysis. Int J Hematol 2015;102:170–80.
44. Ziakas PD. Effect of JAK2 V617F on thrombotic risk in patients with essential thrombocythemia: measuring the uncertain. Haematologica 2008;93:1412–4.
45. Lussana F, Dentali F, Abbate R, et al. Screening for thrombophilia and antithrombotic prophylaxis in pregnancy: Guidelines of the Italian Society for Haemostasis and Thrombosis (SISET). Thromb Res 2009;124: e19–25.
46. Dahabreh IJ, Zoi K, Giannouli S, et al. Is JAK2 V617F mutation more than a diagnostic index? A meta-analysis of clinical outcomes in essential thrombocythemia. Leuk Res 2009;33:67–73.
47. Cho YU, Chi HS, Lee EH, et al. Comparison of clinicopathologic findings according to JAK2 V617F mutation in patients with essential thrombocythemia. Int J Hematol 2009;89:39–44.
48. Wolanskyj AP, Lasho TL, Schwager SM, et al. JAK2 mutation in essential thrombocythaemia: clinical associations and long-term prognostic relevance. Br J Haematol 2005;131:208–13.
49. Antonioli E, Guglielmelli P, Pancrazzi A, et al. Clinical implications of the JAK2 V617F mutation in essential thrombocythemia. Leukemia 2005;19:1847–9.
50. Palandri F, Catani L, Testoni N, et al. Long-term follow-up of 386 consecutive patients with essential thrombocythemia: safety of cytoreductive therapy. Am J Hematol 2009;84:215–20.
51. Chim CS, Sim JP, Chan CC, et al. Impact of JAK2V617F mutation on thrombosis and myeloid transformation in essential thrombocythemia: a multivariate analysis by Cox regression in 141 patients. Hematology 2010;15: 187–92.
52. Vannucchi AM, Antonioli E, Guglielmelli P, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood 2007;110:840–6.
53. Carobbio A, Finazzi G, Antonioli E, et al. JAK2V617F allele burden and thrombosis: a direct comparison in essential thrombocythemia and polycythemia vera. Exp Hematol 2009;37:1016–21.
54. Alvarez-Larran A, Bellosillo B, Pereira A, et al. JAK2V617F monitoring in polycythemia vera and essential thrombocythemia: clinical usefulness for predicting myelofibrotic transformation and thrombotic events. Am J Hematol 2014;89:517–23.
55. Barbui T, Vannucchi AM, Buxhofer-Ausch V, et al. Practice-relevant revision of IPSET-thrombosis based on 1019 patients with WHO-defined essential thrombocythemia. Blood Cancer J 2015;5:e369.
56. Carobbio A, Thiele J, Passamonti F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood 2011;117:5857–9.
57. Alvarez-Larran A, Cervantes F, Bellosillo B, et al. Essential thrombocythemia in young individuals: frequency and risk factors for vascular events and evolution to myelofibrosis in 126 patients. Leukemia 2007;21:1218–23.
58. Jantunen R, Juvonen E, Ikkala E, et al. The predictive value of vascular risk factors and gender for the development of thrombotic complications in essential thrombocythemia. Ann Hematol 2001;80:74–8.
59. Besses C, Cervantes F, Pereira A, et al. Major vascular complications in essential thrombocythemia: a study of the predictive factors in a series of 148 patients. Leukemia 1999;13:150–4.
60. Haider M, Gangat N, Hanson C, Tefferi A. Splenomegaly and thrombosis risk in essential thrombocythemia: the mayo clinic experience. Am J Hematol 2016;91: E296–297.
61. Carobbio A, Finazzi G, Antonioli E, et al. Thrombocytosis and leukocytosis interaction in vascular complications of essential thrombocythemia. Blood 2008;112:3135–7.
62. Palandri F, Polverelli N, Catani L, et al. Impact of leukocytosis on thrombotic risk and survival in 532 patients with essential thrombocythemia: a retrospective study. Ann Hematol 2011;90:933–8.
63. Campbell PJ, MacLean C, Beer PA, et al. Correlation of blood counts with vascular complications in essential thrombocythemia: analysis of the prospective PT1 cohort. Blood 2012;120:1409–11.
64. Cortelazzo S, Finazzi G, Ruggeri M, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med 1995;332:1132–6.
65. van Genderen PJ, Mulder PG, Waleboer M, et al. Prevention and treatment of thrombotic complications in essential thrombocythaemia: efficacy and safety of aspirin. Br J Haematol 1997;97:179–84.
66. Storen EC, Tefferi A. Long-term use of anagrelide in young patients with essential thrombocythemia. Blood 2001;97:863–6.
67. De Stefano V, Za T, Rossi E, et al. Recurrent thrombosis in patients with polycythemia vera and essential thrombocythemia: incidence, risk factors, and effect of treatments. Haematologica 2008;93:372–80.
68. Alvarez-Larran A, Cervantes F, Pereira A, et al. Observation versus antiplatelet therapy as primary prophylaxis for thrombosis in low-risk essential thrombocythemia. Blood 2010;116:1205–10.
69. Palandri F, Polverelli N, Catani L, et al. Bleeding in essential thrombocythaemia: a retrospective analysis on 565 patients. Br J Haematol 2012;156:281–4.
70. Rotunno G, Mannarelli C, Guglielmelli P, et al. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood 2014;123:1552–5.
71. Tefferi A, Wassie EA, Lasho TL, et al. Calreticulin mutations and long-term survival in essential thrombocythemia. Leukemia 2014;28:2300–3.
72. Rumi E, Pietra D, Ferretti V, et al. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood 2014;123:1544–51.
73. Palandri F, Latagliata R, Polverelli N, et al. Mutations and long-term outcome of 217 young patients with essential thrombocythemia or early primary myelofibrosis. Leukemia 2015;29:1344–9.
74. Fu R, Xuan M, Zhou Y, et al. Analysis of calreticulin mutations in Chinese patients with essential thrombocythemia: clinical implications in diagnosis, prognosis and treatment. Leukemia 2014;28:1912–4.
75. Tefferi A, Wassie EA, Guglielmelli P, et al. Type 1 versus Type 2 calreticulin mutations in essential thrombocythemia: a collaborative study of 1027 patients. Am J Hematol 2014;89:E121–4.
76. Pietra D, Rumi E, Ferretti VV, et al. Differential clinical effects of different mutation subtypes in CALR-mutant myeloproliferative neoplasms. Leukemia 2016;30: 431–8.
77. Rumi E, Pietra D, Guglielmelli P, et al. Acquired copy-neutral loss of heterozygosity of chromosome 1p as a molecular event associated with marrow fibrosis in MPL-mutated myeloproliferative neoplasms. Blood 2013;121:4388–95.
78. Beer PA, Campbell PJ, Scott LM, et al. MPL mutations in myeloproliferative disorders: analysis of the PT-1 cohort. Blood 2008;112:141–9.
79. Gangat N, Wassie EA, Lasho TL, et al. Mutations and thrombosis in essential thrombocythemia: prognostic interaction with age and thrombosis history. Eur J Haematol 2015;94:31–6.
80. Sekhar M, McVinnie K, Burroughs AK. Splanchnic vein thrombosis in myeloproliferative neoplasms. Br J Haematol 2013;162:730–47.
81. Stein BL, Saraf S, Sobol U, et al. Age-related differences in disease characteristics and clinical outcomes in polycythemia vera. Leuk Lymph 2013;54:1989–95.
82. Landolfi R, Di Gennaro L, Nicolazzi MA, et al. Polycythemia vera: gender-related phenotypic differences. Intern Emerg Med 2012;7:509–15.
83. Winslow ER, Brunt LM, Drebin JA, et al. Portal vein thrombosis after splenectomy. Am J Surg 2002;184:631–6.
84. Smalberg JH, Arends LR, Valla DC, et al. Myeloproliferative neoplasms in Budd-Chiari syndrome and portal vein thrombosis: a meta-analysis. Blood 2012;120:4921–8.
85. Dentali F, Squizzato A, Brivio L, et al. JAK2V617F mutation for the early diagnosis of Ph- myeloproliferative neoplasms in patients with venous thromboembolism: a meta-analysis. Blood 2009;113:5617–23.
86. Pardanani A, Lasho TL, Hussein K, et al. JAK2V617F mutation screening as part of the hypercoagulable work-up in the absence of splanchnic venous thrombosis or overt myeloproliferative neoplasm: assessment of value in a series of 664 consecutive patients. Mayo Clin Proc 2008;83:457–9.
87. Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol 2011;29:761–70.
88. Landolfi R, Marchioli R, Kutti J, et al. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med 2004;350:114–24.
89. Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med 2013;368:22–33.
90. Kiladjian JJ, Chevret S, Dosquet C, et al. Treatment of polycythemia vera with hydroxyurea and pipobroman: final results of a randomized trial initiated in 1980. J Clin Oncol 2011;29:3907–13.
91. Kaplan ME, Mack K, Goldberg JD, et al. Long-term management of polycythemia vera with hydroxyurea: a progress report. Semin Hematol 1986;23:167–71.
92. Fruchtman SM, Mack K, Kaplan ME, et al. From efficacy to safety: a Polycythemia Vera Study group report on hydroxyurea in patients with polycythemia vera. Semin Hematol 1997;34:17–23.
93. Finazzi G, Caruso V, Marchioli R, et al. Acute leukemia in polycythemia vera: an analysis of 1638 patients enrolled in a prospective observational study. Blood 2005;105: 2664–70.
94. Barosi G, Mesa R, Finazzi G, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood 2013;121:4778–81.
95. Alvarez-Larran A, Pereira A, Cervantes F, et al. Assessment and prognostic value of the European LeukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia vera. Blood 2012;119:1363–9.
96. Stein BL, Tiu RV. Biological rationale and clinical use of interferon in the classical BCR-ABL-negative myeloproliferative neoplasms. J Interferon Cytokine Res 2013;33: 145–53.
97. Ludwig H, Cortelezzi A, Van Camp BG, et al. Treatment with recombinant interferon-alpha-2C: multiple myeloma and thrombocythaemia in myeloproliferative diseases. Oncology 1985;42 Suppl 1:19–25.
98. Silver RT. Long-term effects of the treatment of polycythemia vera with recombinant interferon-alpha. Cancer 2006;107:451–8.
99. Kiladjian JJ, Mesa RA, Hoffman R. The renaissance of interferon therapy for the treatment of myeloid malignancies. Blood 2011;117:4706–15.
100. Veronese FM, Mero A. The impact of PEGylation on biological therapies. BioDrugs 2008;22:315–29.
101. Kiladjian JJ, Cassinat B, Chevret S, et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood 2008;112:3065–72.
102. Turlure P, Cambier N, Roussel M, et al. Complete hematological, molecular and histological remissions without cytoreductive treatment lasting after pegylated-interferon {alpha}-2a (peg-IFN{alpha}-2a) therapy in polycythemia vera (PV): long term results of a phase 2 trial [abstract]. Blood 2011;118(21). Abstract 280.
103. Quintas-Cardama A, Kantarjian H, Manshouri T, et al. Pegylated interferon alfa-2a yields high rates of hematologic and molecular response in patients with advanced essential thrombocythemia and polycythemia vera. J Clin Oncol 2009;27:5418–24.
104. Quintas-Cardama A, Abdel-Wahab O, Manshouri T, et al. Molecular analysis of patients with polycythemia vera or essential thrombocythemia receiving pegylated interferon a-2a. Blood 2013;122:893–901.
105. Samuelsson J, Hasselbalch H, Bruserud O, et al. A phase II trial of pegylated interferon alpha-2b therapy for polycythemia vera and essential thrombocythemia: feasibility, clinical and biologic effects, and impact on quality of life. Cancer 2006;106:2397–405.
106. Jabbour E, Kantarjian H, Cortes J, et al. PEG-IFN-alpha-2b therapy in BCR-ABL-negative myeloproliferative disorders: final result of a phase 2 study. Cancer 2007; 110:2012–18.
107. Them NC, Bagienski K, Berg T, et al. Molecular responses and chromosomal aberrations in patients with polycythemia vera treated with peg-proline-interferon alpha-2b. Am J Hematol 2015;90:288–94.
108. Gisslinger H, Klade C, Georgiev P, et al. Final results from PROUD-PV a randomized controlled phase 3 trial comparing ropeginterferon alfa-2b to hydroxyurea in polycythemia vera patients [abstract]. Blood 2016;128(suppl 22). Abstract 475.
109. van Genderen PJ, van Vliet HH, Prins FJ, et al. Excessive prolongation of the bleeding time by aspirin in essential thrombocythemia is related to a decrease of large von Willebrand factor multimers in plasma. Ann Hematol 1997;75:215–20.
110. Cortelazzo S, Finazzi G, Ruggeri M, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med 1995;332:1132–7.
111. Harrison CN, Campbell PJ, Buck G, et al. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N Engl J Med 2005;353:33–45.
112. Gisslinger H, Gotic M, Holowiecki J, et al. Anagrelide compared with hydroxyurea in WHO-classified essential thrombocythemia: the ANAHYDRET Study, a randomized controlled trial. Blood 2013;121:1720–8.
113. Alvarado Y, Cortes J, Verstovsek S, et al. Pilot study of pegylated interferon-alpha 2b in patients with essential thrombocythemia. Cancer Chemother Pharmacol 2003;51:81–6.
114. Barosi G, Tefferi A, Barbui T, ad hoc committee ‘Definition of clinically relevant outcomes for contemporarily clinical trials in Ph-neg M. Do current response criteria in classical Ph-negative myeloproliferative neoplasms capture benefit for patients? Leukemia 2012;26:1148–9.
115. Bjorkholm M, Derolf AR, Hultcrantz M, et al. Treatment-related risk factors for transformation to acute myeloid leukemia and myelodysplastic syndromes in myeloproliferative neoplasms. J Clin Oncol 2011;29:2410–5.
116. Alvarez-Larran A, Martinez-Aviles L, Hernandez-Boluda JC, et al. Busulfan in patients with polycythemia vera or essential thrombocythemia refractory or intolerant to hydroxyurea. Ann Hematol 2014;93:2037–43.
117. Verstovsek S, Passamonti F, Rambaldi A, et al. A phase 2 study of ruxolitinib, an oral JAK1 and JAK2 Inhibitor, in patients with advanced polycythemia vera who are refractory or intolerant to hydroxyurea. Cancer 2014;120: 513–20.
118. Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib in polycythemia vera resistant to or intolerant of hydroxyurea. N Engl J Med 2015; 372:426–35.
119. Verstovsek S, Vannucchi AM, Griesshammer M, et al. Ruxolitinib versus best available therapy in patients with polycythemia vera: 80-week follow-up from the RESPONSE trial. Haematologica 2016;101:821–9.
120. Passamonti F, Griesshammer M, Palandri F, et al. Ruxolitinib for the treatment of inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): a randomised, open-label, phase 3b study. Lancet Oncol 2017;18:88–99.
121. Verstovsek S, Passamonti F, Rambaldi A, et al. Long-term results from a phase II open-label study of ruxolitinib in patients with essential thrombocythemia refractory to or intolerant of hydroxyurea [abstract]. Blood 2014;124. Abstract 1847.
122. Harrison CN, Mead AJ, Panchal A, et al. Ruxolitinib versus best available therapy for ET intolerant or resistant to hydroxycarbamide in a randomized trial. Blood 2017 Aug 9. pii: blood-2017-05-785790 .
123. Bose P, Verstovsek S. Drug development pipeline for myeloproliferative neoplasms: potential future impact on guidelines and management. J Natl Compr Canc Netw 2016;14:1613–24.
124. Cerquozzi S, Teffieri A. Blast transformation and fibrotic progression in polycythemia vera and essential thrombocythemia: a literature review of incidence and risk factors. Blood Cancer J 2015;Nov 13;5:e366.
125. Passamonti F, Rumi E, Caramella M, et al. A dynamic prognostic model to predict survival in post-polycythemia vera myelofibrosis. Blood 2008;111:3383–7.
126. Mesa RA, Verstovsek S, Cervantes F, et al. Primary myelofibrosis (PMF), post polycythemia vera myelofibrosis (post- PV MF), post essential thrombocythemia myelofibrosis (post-ET MF), blast phase PMF (PMF-BP): Consensus on terminology by the international working group for myelofibrosis research and treatment (IWG-MRT). Leuk Res 2007;31:737–40.
127. Rampal R, Mascarenhas J. Pathogenesis and management of acute myeloid leukemia that has evolved from a myeloproliferative neoplasm. Curr Opin Hematol 2014;21:65–71.
128. Chihara D, Kantarjian HM, Newberry KJ, et al. Survival outcome of patients with acute myeloid leukemia transformed from myeloproliferative neoplasms [abstract]. Blood 2016;128. Abstract 1940.
129. Tam CS, Nussenzveig RM, Popat U, et al. The natural history and treatment outcome of blast phase BCR-ABL-myeloproliferative neoplasms. Blood 2008;112:1628–37.
130. Kundranda MN, Tibes R, Mesa RA. Transformation of a chronic myeloproliferative neoplasm to acute myelogenous leukemia: does anything work? Curr Hematol Malig Rep 2012;7:78–86.
131. Badar T, Kantarjian HM, Ravandi F, et al. Therapeutic benefit of decitabine, a hypomethylating agent, in patients with high-risk primary myelofibrosis and myeloproliferative neoplasm in accelerated or blastic/acute myeloid leukemia phase. Leuk Res 2015;39:950–6.
132. Thepot S, Itzykson R, Seegers V, et al. Treatment of progression of Philadelphia-negative myeloproliferative neoplasms to myelodysplastic syndrome or acute myeloid leukemia by azacitidine: a report on 54 cases on the behalf of the Groupe Francophone des Myelodysplasies (GFM). Blood 2010;116:3735–42.
133. Pemmaraju N, Kantarjian H, Kadia T, et al. A phase I/II study of the Janus kinase (JAK)1 and 2 inhibitor ruxolitinib in patients with relapsed or refractory acute myeloid leukemia. Clin Lymphoma Myeloma Leuk 2015;15:171–6.
134. Rampal RK, Mascarenhas JO, Kosiorek HE, et al. Safety and efficacy of combined ruxolitinib and decitabine in patients with blast-phase MPN and post-MPN AML: results of a phase I study (Myeloproliferative Disorders Research Consortium 109 trial) [abstract]. Blood 2016;128. Abstract 1124.
135. Bose P, Verstovsek S, Gasior Y, et al. Phase I/II study of ruxolitinib (RUX) with decitabine (DAC) in patients with post-myeloproliferative neoplasm acute myeloid leukemia (post-MPN AML): phase I results [abstract]. Blood 2016;128. Abstract 4262.
From the Columbia University Medical Center, New York, NY (Dr. Falchi), and the University of Texas MD Anderson Cancer Center, Houston, TX (Dr. Verstovsek).
ABSTRACT
- Objective: To review the clinical aspects and current practices in the management of polycythemia vera (PV) and essential thrombocythemia (ET).
- Methods: Review of the literature.
- Results: PV and ET are rare chronic myeloid disorders. The 2 most important life-limiting complications of PV and ET are thrombohemorrhagic events and myelofibrosis/acute myeloid leukemia (AML) transformation. Vascular events are at least in part preventable with counseling on risk factors, phlebotomy (for patients with PV), antiplatelet therapy, and cytoreduction with hydroxyurea, interferons, or anagrelide (for patients with ET). Ruxolitinib was recently approved for PV after hydroxyurea failure. PV/ET transformation into myelofibrosis or AML is part of the natural history of the disease and no therapy has been shown to prevent it. Treatment of leukemic transformation of myeloproliferative neoplasms (MPN LT) follows recommendations set forth for primary myelofibrosis and AML.
- Conclusion: With appropriate management, patients with PV and ET typically enjoy a long survival and near-normal quality of life. Transformation into myelofibrosis or AML cannot be prevented by current therapies, however. Treatment results with MPN LT are generally poor and novel strategies are needed to improve outcomes.
Key words: myeloproliferative neoplasms; myelofibrosis; leukemic transformation.
Polycythemia vera (PV) and essential thrombocythemia (ET), along with primary myelofibrosis (PMF), belong to the group of Philadelphia-negative myeloproliferative neoplasms (MPN). All these malignancies arise from the clonal proliferation of an aberrant hematopoietic stem cell, but are characterized by distinct clinical phenotypes [1,2]. Although the clinical course of PV and ET is indolent, it can be complicated by thrombohemorrhagic episodes and/or evolution into myelofibrosis and/or acute myeloid leukemia (AML) [3]. Since vascular events are the most frequent life-threatening complications of PV and ET, therapeutic strategies are aimed at reducing this risk. Treatment may also help control other symptoms associated with the disease [4]. No therapy has been shown to prevent evolution of PV or ET into myelofibrosis or AML. The discovery of the Janus kinase 2 (JAK2)/V617F mutation in most patients with PV and over half of those with ET (and PMF) [5,6] has opened new avenues of research and led to the development of targeted therapies, such as the JAK1/2 inhibitor ruxolitinib, for patients with MPN [7,8].
Epidemiology
PV and ET are typically diagnosed in the fifth to seventh decade of life [9]. Although these disorders are generally associated with a long clinical course, survival of patients with PV or ET may be shorter than that of the general population [10–13]. Estimating the incidence and prevalence of MPN is a challenge because most patients remain asymptomatic for long periods of time and do not seek medical attention [13]. The annual incidence rates of PV and ET are estimated at 0.01 to 2.61 and 0.21 to 2.53 per 100,000, respectively. PV occurs slightly more frequently in males, whereas ET has a predilection for females [14]. Given the long course and low mortality associated with these disorders, the prevalence rates of PV and ET are significantly higher than the respective incidence rates: up to 47 and 57 per 100,000, respectively [15–17].
Molecular Pathogenesis
In 2005 researchers discovered a gain-of-function mutation of the JAK2 gene in nearly all patients with PV and more than half of those with ET and PMF [5,6,18,19]. JAK2 is a non-receptor tyrosine kinase that plays a central role in normal hematopoiesis. Substitution of a valine for a phenylalanine at codon 617 (ie, V617F) leads to its constitutive activation and signaling through the JAK-STAT pathway [5,6,18,19]. More rarely (and exclusively in patients with PV), JAK2 mutations involve exon 12 [20–22]. The vast majority of JAK2-negative ET patients harbor mutations in either the myeloproliferative leukemia (MPL) gene, which encodes the thrombopoietin receptor [23–25], or the calreticulin (CALR) gene [26,27], which encodes for a chaperone protein that plays a role in cellular proliferation, differentiation, and apoptosis [28]. Both the MPL and CALR mutations ultimately result in the constitutive activation of the JAK-STAT pathway. Thus, JAK2, MPL, and CALR alterations are collectively referred to as driver mutations. Moreover, because these mutations affect the same oncogenic pathway (ie, JAK-STAT), they are almost always mutually exclusive in a given patient. Patients with ET (or myelofibrosis) who are wild-type for JAK2, MPL, and CALR are referred to as having “triple-negative” disease. Many recurrent non-driver mutations are also found in patients with MPN. These are not exclusive of each other (ie, patients may have many at the same time) and involve for example ten-eleven translocation-2 (TET2), additional sex combs like 1 (ASXL1), enhancer of zeste homolog 2 (EZH2), isocitrate dehydrogenase 1 and isocitrate dehydrogenase 2 (IDH1/2), and DNA methyltransferase 3A (DNMT3A) genes, among others [29]. The biologic and prognostic significance of these non-driver alterations remain to be fully defined in ET and PV.
Diagnostic Criteria
Diagnostic criteria for PV and ET according to the World Health Organization (WHO) classification [30] are summarized in Table 1. Criteria for the diagnosis of prefibrotic myelofibrosis are included as well since this entity was formally recognized as separate from ET and part of the PMF spectrum in the 2016 WHO classification of myeloid tumors [30]. Clinically, both PV and ET generally remain asymptomatic for a long time. PV tends to be more symptomatic than ET and can present with debilitating constitutional symptoms (fatigue, night sweats, and weight loss), microvascular symptoms (headache, lightheadedness, acral paresthesias, erythromelalgia, atypical chest pain, and pruritus) [31], or macrovascular accidents (larger vein thrombosis, stroke, or myocardial ischemia) [32]. ET is often diagnosed incidentally, but patients can suffer from similar general symptoms and vascular complications. Causes of secondary absolute erythrocytosis (altitude, chronic hypoxemia, heavy smoking, cardiomyopathy, use of corticosteroids, erythropoietin, or other anabolic hormones, familial or congenital forms) or thrombocytosis (iron deficiency, acute blood loss, trauma or injury, acute coronary syndrome, systemic autoimmune disorders, chronic kidney failure, other malignancies, splenectomy) should be considered and appropriately excluded. Once the diagnosis is made, symptom assessment tools such as the Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF) [33] or the abbreviated version, the MPN-SAF Total Symptom Score (MPN-SAF TSS) [34], are generally used to assess patients’ symptom burden and response to treatment in everyday practice.
Risk Stratification
Thrombohemorrhagic events, evolution into myelofibrosis, and leukemic transformation (LT) are the most serious complications in the course of PV or ET. Only thrombohemorrhagic events are, at least partially, preventable. Arterial or venous thrombotic complications are observed at rates of 1.8 to 10.9 per 100 patient-years in PV (arterial thrombosis being more common than venous) and 0.74 to 7.7 per 100 patient-years in ET, depending on the risk group [35] and the presence of other factors (see below).
The risk stratification of patients with PV is based on 2 factors: age ≥ 60 years and prior history of thrombosis. If either is present, the patient is assigned to the high-risk category, whereas if none is present the patient is considered at low risk [36]. In addition, high hematocrit [37] and high white blood cell (WBC) count [38], but not thrombocytosis, have been associated with the development of vascular complications. In one study, the risk of new arterial thrombosis was increased by the presence of leukoerythroblastosis, hypertension, and prior arterial thrombosis, while karyotypic abnormalities and prior venous thrombosis were predictors of new venous thrombosis [39]. Another emerging risk factor for thrombosis in patients with PV is high JAK2 allele burden (ie, the normal-to-mutated gene product ratio), although the evidence supporting this conclusion is equivocal [40].
Traditionally, in ET patients, the thrombotic risk was assessed using the same 2 factors (age ≥ 60 years and prior history of thrombosis), separating patients into low- and high-risk groups. However, the prognostication of ET patients has been refined recently with the identification of new relevant factors. In particular, the impact of JAK2 mutations on thrombotic risk has been thoroughly studied. Clinically, the presence of JAK2V617F is associated with older age, higher hemoglobin and hematocrit, lower platelet counts, more frequent need for cytoreductive treatment, and greater tendency to evolve into PV (a rare event) [41,42]. Many [41,43–46], but not all [47–51], studies suggested a correlation between JAK2 mutation and risk of both arterial and venous thrombosis. Although infrequent, a JAK2V617F homozygous state (ie, the mutation is present in both alleles) might confer an even higher thrombotic risk [52]. Moreover, the impact of the JAK2 mutation on vascular events persists over time [53], particularly in patients with high or unstable mutation burden [54]. Based on JAK2V617F’s influence on the thrombotic risk of ET patients, a new prognostic score was proposed, the International Prognostic Score for ET (IPSET)-thrombosis (Table 2). The revised version of this model is currently endorsed by the National Comprehensive Cancer Network and divides patients into 4 risk groups: high, intermediate, low, and very low. Treatment recommendations vary according to the risk group (as described below) [55].
Other thrombotic risk factors have been identified, but deemed not significant enough to be included in the model. Cardiovascular risk factors (hypercholesterolemia, hypertension, smoking, diabetes mellitus) can increase the risk of vascular events [56–59], as can splenomegaly [60] and baseline or persistent leukocytosis [61–63]. Thrombocytosis has been correlated with thrombotic risk in some studies [64–68], whereas others did not support this conclusion and/or suggested a lower rate of thrombosis and, in some cases, increased risk of bleeding in ET patients with platelet counts greater than 1000 × 103/μL (due to acquired von Willebrand syndrome) [51,61,63,68,69].
CALR mutations tend to occur in younger males with lower hemoglobin and WBC count, higher platelet count, and greater marrow megakaryocytic predominance, as compared to JAK2 mutations [26,27,70–72]. The associated incidence of thrombosis was less than 10% at 15 years in patients with CALR mutations, lower than the incidence reported for ET patients with JAK2V617F mutations [73]. The presence of the mutation per se does not appear to affect the thrombotic risk [74–76]. Information on the thrombotic risk associated with MPL mutations or a triple-negative state is scarce. In both instances, however, the risk appears to be lower than with the JAK2 mutation [73,77–79].
Venous thromboembolism (VTE) in patients with PV or ET may occur at unusual sites, such as the splanchnic or cerebral venous systems [80]. Risk factors for unusual VTE include younger age [81], female gender (especially with concomitant use of oral contraceptive pills) [82], and splenomegaly/splenectomy [83]. JAK2 mutation has also been associated with thrombosis at unusual sites. However, the prevalence of MPN or JAK2V617F in patients presenting with splanchnic VTE has varied [80]. In addition, MPN may be occult (ie, no clinical or laboratory abnormalities) in around 15% of patients [84]. Screening for JAK2V617F and underlying MPN is recommended in patients presenting with isolated unexplained splanchnic VTE. Treatment entails long-term anticoagulation therapy. JAK2V617F screening in patients with nonsplanchnic VTE is not recommended, as its prevalence in this group is low (< 3%) [85,86].
Risk-Adapted Therapy
Low-Risk PV
All patients with PV should receive counseling to mitigate cardiovascular risk factors, including smoking cessation, lifestyle modifications, and lipid-lowering therapy, as indicated. Furthermore, all PV patients should receive acetylsalicylic acid (ASA) to decrease their risk for thrombosis and control vasomotor symptoms [55,87]. Aspirin 81 to 100 mg daily is the preferred regimen because it provides adequate antithrombotic effect without the associated bleeding risk of higher-dose aspirin [88]. Low-risk PV patients should also receive periodic phlebotomies to reduce and maintain their hematocrit below 45%. This recommendation is based on the results of the Cytoreductive Therapy in Polycythemia Vera (CYTO PV) randomized controlled trial. In that study, patients receiving more intense therapy to maintain the hematocrit below 45% had a lower incidence of cardiovascular-related deaths or major thrombotic events than those with hematocrit goals of 45% to 50% (2.7% versus 9.8%) [89]. Cytoreduction is an option for low-risk patients who do not tolerate phlebotomy or require frequent phlebotomy, or who have disease-related bleeding, severe symptoms, symptomatic splenomegaly, or progressive leukocytosis [38].
High-Risk PV
Patients older than 60 years and/or with a history of thrombosis should be considered for cytoreductive therapy in addition to the above measures. Frontline cytoreductive therapies include hydroxyurea or interferon (IFN)-alfa [87]. Hydroxyurea is a potent ribonucleotide reductase inhibitor that interferes with DNA repair and is the treatment of choice for most high-risk patients with PV [90]. In a small trial, hydroxyurea reduced the risk of thrombosis compared with historical controls treated with phlebotomy alone [91]. Hydroxyurea is generally well tolerated; common side effects include cytopenias, nail changes, and mucosal and/or skin ulcers. Although never formally proven to be leukemogenic, this agent should be used with caution in younger patients [87]. Indeed, in the original study, the rates of transformation were 5.9% and 1.5% for patients receiving hydroxyurea and phlebotomy alone [92], respectively, although an independent role for hydroxyurea in LT was not supported in the much larger European Collaboration on Low-dose Aspirin in Polycythemia Vera (ECLAP) study [93]. Approximately 70% of patients will have a sustained response to hydroxyurea [94], while the remaining patients become resistant to or intolerant of the drug. Resistant individuals have a higher risk of progression to acute leukemia and death [95].
IFN-alfa is a pleiotropic antitumor agent that has found application in many types of malignancies [96] and is sometimes employed as treatment for patients with newly diagnosed high-risk PV. Early studies showed responses in up to 100% of cases [97,98], albeit at the expense of a high discontinuation rate due to adverse events, such as flu-like symptoms, fatigue, and neuropsychiatric manifestations [99]. A newer formulation of the drug obtained by adding a polyethylene glycol (PEG) moiety to the native IFN-alfa molecule (PEG-IFN alfa) was shown to have a longer half-life, greater stability, less immunogenicity, and, potentially, better tolerability [100]. Pilot phase 2 trials of PEG-IFN-alfa-2a demonstrated its remarkable activity, with symptomatic and hematologic responses seen in most patients (which, in some cases, persisted beyond discontinuation), and reasonable tolerability, with long-term discontinuation rates of 20% to 30% [101–103]. In some patients, JAK2V617F became undetectable over time [104]. Results of 2 ongoing trials, MDP-RC111 (single-arm study, PEG-IFN-alfa-2a in high-risk PV or ET [NCT01259817]) and MPD-RC112 (randomized controlled trial, PEG-IFN-alfa-2a versus hydroxyurea in the same population [NCT01258856]), will shed light on the role of PEG-IFN-alfa in the management of patients with high-risk PV or ET. In two phase 2 studies of PEG-IFN-alfa-2b, complete responses were seen in 70% to 100% of patients and discontinuation occurred in around a third of cases [105,106]. A new, longer-acting formulation of PEG-IFN-alfa-2a (peg-proline INF-alfa-2b, AOP2014) is also undergoing clinical development [107,108].
The approach to treatment of PV based on thrombotic risk level is illustrated in Figure 1.
Very Low- and Low-Risk ET
Individuals with ET should undergo rigorous cardiovascular risk management and generally receive ASA to decrease their thrombotic risk and improve symptom control. Antiplatelet therapy may not be warranted in patients with documented acquired von Willebrand syndrome, with or without extreme thrombocytosis, or in those in the very low-risk category according to the IPSET-thrombosis model [55,87]. The risk/benefit ratio of antiplatelet agents in patients with ET at different thrombotic risk levels was assessed in poor-quality studies and thus remains highly uncertain. Platelet-lowering agents are sometimes recommended in patients with low-risk disease who have platelet counts ≥ 1500 × 103/μL, due to the potential risk of acquired von Willebrand syndrome and a risk of bleeding (this would require stopping ASA) [109]. Cytoreduction may also be used in low-risk patients with progressive symptoms despite ASA, symptomatic or progressive splenomegaly, and progressive leukocytosis.
Intermediate-Risk ET
This category includes patients older than 60 years, but without thrombosis or JAK2 mutations. These individuals would have been considered high risk (and thus candidates for cytoreductive therapy) according to the traditional risk stratification. Guidelines currently recommend ASA as the sole therapy for these patients, while reserving cytoreduction for those who experience thrombosis (ie, become high-risk) or have uncontrolled vasomotor or general symptoms, symptomatic splenomegaly, symptomatic thrombocytosis, or progressive leukocytosis.
High-Risk ET
For patients with ET in need of cytoreductive therapy (ie, those with prior thrombosis or older than 60 years with a JAK2V617F mutation), first-line options include hydroxyurea, IFN, and anagrelide. Hydroxyurea remains the treatment of choice in most patients [110]. In a seminal study, 114 patients with ET were randomly assigned to either observation or hydroxyurea treatment with the goal of maintaining the platelet count below 600 × 103/μL. At a median follow-up of 27 months, patients in the hydroxyurea group had a lower thrombosis rate (3.6% versus 24%, P = 0.003) and longer thrombosis-free survival, regardless of the use of antiplatelet drugs [64].
Anagrelide, a selective inhibitor of megakaryocytic differentiation and proliferation, was compared with hydroxyurea in patients with ET in 2 randomized trials. In the first (n = 809), the group receiving anagrelide had a higher risk of arterial thrombosis, major bleeding, and fibrotic evolution, but lower incidence of venous thrombosis. Hydroxyurea was better tolerated, mainly due to anagrelide-related cardiovascular adverse events [111]. As a result of this study, hydroxyurea is often preferred to anagrelide as frontline therapy for patients with newly diagnosed high-risk ET. In the second, more recent study (n = 259), however, the 2 agents proved equivalent in terms of major or minor arterial or venous thrombosis, as well as discontinuation rate [112]. The discrepancy between the 2 trials may be partly explained by the different ET diagnostic criteria used, with the latter only enrolling patients with WHO-defined true ET and the former utilizing Polycythemia Vera Study Group-ET diagnostic criteria that included patients with increases in other blood counts or varying degrees of marrow fibrosis.
Interferons were studied in ET in parallel with PV. PEG-IFN-alfa-2a proved effective in patients with ET, with responses observed in 80% of patients [103]. PEG-IFN- alfa-2b produced similar results, with responses in 70% to 90% of patients in small studies and discontinuation observed in 20% to 38% of cases [105,106,113]. Because the very long-term leukemogenic potential of hydroxyurea has remained somewhat uncertain, anagrelide or IFN might be preferable choices in younger patients.
The approach to treatment of ET based on thrombotic risk level is illustrated in Figure 2.
Assessing Response to Therapy
For both patients with PV and ET the endpoint of treatment set forth for clinical trials has been the achievement of a clinicohematologic response. However, studies have failed to show a correlation between response and reduction of the thrombohemorrhagic risk [114]. Therefore, proposed clinical trial response criteria were revised to include absence of hemorrhagic or thrombotic events as part of the definition of response (Table 3) [94].
Approach to Patients Refractory to or Intolerant of First-line Therapy
According to the European LeukemiaNet recommendations, an inadequate response to hydroxyurea in patients with PV (or myelofibrosis) is defined as a need for phlebotomy to maintain the hematocrit below < 45%, the platelet count > 400 × 103/μL, and a WBC count > 10,000/μL, or failure to reduce splenomegaly > 10 cm by > 50% at a dose of ≥ 2 g/day or maximum tolerated dose. Historically, treatment options for patients with PV or ET who failed first-line therapy (most commonly hydroxyurea) have included alkylating agents, such as busulfan, chlorambucil, pipobroman, and phosphorus (P)-32. However, the use of these drugs is limited by the associated risk of LT [93,115,116]. IFN (or anagrelide for ET) is often considered in patients previously treated with hydroxyurea, and vice versa.
Ruxolitinib is a JAK1 and JAK2 inhibitor currently approved for the treatment of PV patients refractory to or intolerant of hydroxyurea [7]. Following promising results of a phase 2 trial [117], ruxolitinib 10 mg twice daily was compared with best available therapy in the pivotal RESPONSE trial (n = 222). Ruxolitinib proved superior in achieving hematocrit control, reduction of spleen volume, and improvement of symptoms. Grade 3-4 hematologic toxicity was infrequent and similar in the 2 arms [118]. In addition, longer follow-up of that study suggested a lower rate of thrombotic events in patients receiving ruxolitinib (1.8 versus 8.2 per 100 patient-years) [119]. In a similarly designed randomized phase 3 study in PV patients without splenomegaly (RESPONSE-2), more patients in the ruxolitinib arm had hematocrit reduction without an increase in toxicity. Based on the results of these studies, ruxolitinib can be considered a standard of care for second-line therapy in this post-hydroxyurea patient population [120]. Ruxolitinib is also being tested in patients with high-risk ET who have become resistant to, or were intolerant of hydroxyurea, but currently has no approved indication in this setting [121,122]. Common side effects of ruxolitinib include cytopenias (especially anemia), increased risk of infections, hyperlipidemia, and increased risk of non-melanoma skin cancer.
Novel agents that have been studied in patients with PV and ET are histone deacetylase inhibitors, murine double minute 2 (MDM2, or HDM2 for their human counterpart) inhibitors (which restore the function of p53), Bcl-2 homology domain 3 mimetics such as navitoclax and venetoclax, and, for patients with ET, the telomerase inhibitor imetelstat [123].
Disease Evolution
Post-PV/Post-ET Myelofibrosis
Diagnostic criteria for post-PV and post-ET myelofibrosis are outlined in Table 4. Fibrotic transformation represents a natural evolution of the clinical course of PV or ET. It occurs in up to 15% and 9% of patients with PV and ET, respectively, in western countries [124]. The true percentage of ET patients who develop myelofibrosis is confounded by the inclusion of prefibrotic myelofibrosis cases in earlier series. The survival of patients who develop myelofibrosis is shortened compared to those who do not. In patients with PV, risk factors for myelofibrosis evolution include advanced age, leukocytosis, JAK2V617F homozygosity or higher allele burden, and hydroxyurea therapy. Once post-PV myelofibrosis has occurred, hemoglobin < 10 g/dL, platelet count < 100 × 103/μL, and WBC count > 30,000/μL are associated with worse outcomes [125]. In patients with ET, risk factors for myelofibrosis transformation include age, anemia, bone marrow hypercellularity and increased reticulin, increased lactate dehydrogenase, leukocytosis, and male gender. The management of post-PV/post-ET myelofibrosis recapitulates that of PMF.
Leukemic Transformation
The presence of more than 20% blasts in peripheral blood or bone marrow in a patient with MPN defines LT. This occurs in up to 5% to 10% of patients and may or may not be preceded by a myelofibrosis phase [126]. In cases of extramedullary transformation, a lower percentage of blasts can be seen in the bone marrow compared to the peripheral blood. The pathogenesis of LT has remained elusive, but it is believed to be associated with genetic instability, which facilitates the acquisition of additional mutations, including those of TET2, ASXL1, EZH2 DNMT3, IDH1/2, and TP53 [127].
Clinical risk factors for LT include advanced age, karyotypic abnormalities, prior therapy with alkylating agents or P-32, splenectomy, increased peripheral blood or bone marrow blasts, leukocytosis, anemia, thrombocytopenia, and cytogenetic abnormalities. Hydroxyurea, IFN, and ruxolitinib have not been shown to have leukemogenic potential thus far. Prognosis of LT is uniformly poor and patient survival rarely exceeds 6 months.
There is no standard of care for MPN LT. Treatment options range from low-intensity regimens to more aggressive AML-type induction chemotherapy. No strategy appears clearly superior to others [128]. Hematopoietic stem cell transplantation is the only therapy that provides clinically meaningful benefit to patients [129], but it is applicable only to a minority of patients with chemosensitive disease and good performance status [130]. Notable experimental approaches to MPN LT include hypomethylating agents, such as decitabine [131] or azacytidine [132], with or without ruxolitinib [133–135].
Conclusion
PV and ET are rare, chronic myeloid disorders. Patients typically experience a long clinical course and enjoy near-normal quality of life if properly managed. The 2 most important life-limiting complications of PV and ET are thrombohemorrhagic events and myelofibrosis/AML transformation. Vascular events are at least in part preventable with counseling on risk factors, phlebotomy (for patients with PV), antiplatelet therapy, and cytoreduction with hydroxyurea, IFNs, or anagrelide (for patients with ET). In addition, ruxolitinib was recently approved for PV patients after hydroxyurea failure. PV/ET transformation in myelofibrosis or AML is part of the natural history of the disease and no therapy has been shown to prevent it. Treatment follows recommendations set forth for PMF and AML, but results are generally poorer and novel strategies are needed to improve outcomes.
Corresponding author: Lorenzo Falchi, MD, Columbia University Medical Center, New York, NY.
Financial disclosures: None.
From the Columbia University Medical Center, New York, NY (Dr. Falchi), and the University of Texas MD Anderson Cancer Center, Houston, TX (Dr. Verstovsek).
ABSTRACT
- Objective: To review the clinical aspects and current practices in the management of polycythemia vera (PV) and essential thrombocythemia (ET).
- Methods: Review of the literature.
- Results: PV and ET are rare chronic myeloid disorders. The 2 most important life-limiting complications of PV and ET are thrombohemorrhagic events and myelofibrosis/acute myeloid leukemia (AML) transformation. Vascular events are at least in part preventable with counseling on risk factors, phlebotomy (for patients with PV), antiplatelet therapy, and cytoreduction with hydroxyurea, interferons, or anagrelide (for patients with ET). Ruxolitinib was recently approved for PV after hydroxyurea failure. PV/ET transformation into myelofibrosis or AML is part of the natural history of the disease and no therapy has been shown to prevent it. Treatment of leukemic transformation of myeloproliferative neoplasms (MPN LT) follows recommendations set forth for primary myelofibrosis and AML.
- Conclusion: With appropriate management, patients with PV and ET typically enjoy a long survival and near-normal quality of life. Transformation into myelofibrosis or AML cannot be prevented by current therapies, however. Treatment results with MPN LT are generally poor and novel strategies are needed to improve outcomes.
Key words: myeloproliferative neoplasms; myelofibrosis; leukemic transformation.
Polycythemia vera (PV) and essential thrombocythemia (ET), along with primary myelofibrosis (PMF), belong to the group of Philadelphia-negative myeloproliferative neoplasms (MPN). All these malignancies arise from the clonal proliferation of an aberrant hematopoietic stem cell, but are characterized by distinct clinical phenotypes [1,2]. Although the clinical course of PV and ET is indolent, it can be complicated by thrombohemorrhagic episodes and/or evolution into myelofibrosis and/or acute myeloid leukemia (AML) [3]. Since vascular events are the most frequent life-threatening complications of PV and ET, therapeutic strategies are aimed at reducing this risk. Treatment may also help control other symptoms associated with the disease [4]. No therapy has been shown to prevent evolution of PV or ET into myelofibrosis or AML. The discovery of the Janus kinase 2 (JAK2)/V617F mutation in most patients with PV and over half of those with ET (and PMF) [5,6] has opened new avenues of research and led to the development of targeted therapies, such as the JAK1/2 inhibitor ruxolitinib, for patients with MPN [7,8].
Epidemiology
PV and ET are typically diagnosed in the fifth to seventh decade of life [9]. Although these disorders are generally associated with a long clinical course, survival of patients with PV or ET may be shorter than that of the general population [10–13]. Estimating the incidence and prevalence of MPN is a challenge because most patients remain asymptomatic for long periods of time and do not seek medical attention [13]. The annual incidence rates of PV and ET are estimated at 0.01 to 2.61 and 0.21 to 2.53 per 100,000, respectively. PV occurs slightly more frequently in males, whereas ET has a predilection for females [14]. Given the long course and low mortality associated with these disorders, the prevalence rates of PV and ET are significantly higher than the respective incidence rates: up to 47 and 57 per 100,000, respectively [15–17].
Molecular Pathogenesis
In 2005 researchers discovered a gain-of-function mutation of the JAK2 gene in nearly all patients with PV and more than half of those with ET and PMF [5,6,18,19]. JAK2 is a non-receptor tyrosine kinase that plays a central role in normal hematopoiesis. Substitution of a valine for a phenylalanine at codon 617 (ie, V617F) leads to its constitutive activation and signaling through the JAK-STAT pathway [5,6,18,19]. More rarely (and exclusively in patients with PV), JAK2 mutations involve exon 12 [20–22]. The vast majority of JAK2-negative ET patients harbor mutations in either the myeloproliferative leukemia (MPL) gene, which encodes the thrombopoietin receptor [23–25], or the calreticulin (CALR) gene [26,27], which encodes for a chaperone protein that plays a role in cellular proliferation, differentiation, and apoptosis [28]. Both the MPL and CALR mutations ultimately result in the constitutive activation of the JAK-STAT pathway. Thus, JAK2, MPL, and CALR alterations are collectively referred to as driver mutations. Moreover, because these mutations affect the same oncogenic pathway (ie, JAK-STAT), they are almost always mutually exclusive in a given patient. Patients with ET (or myelofibrosis) who are wild-type for JAK2, MPL, and CALR are referred to as having “triple-negative” disease. Many recurrent non-driver mutations are also found in patients with MPN. These are not exclusive of each other (ie, patients may have many at the same time) and involve for example ten-eleven translocation-2 (TET2), additional sex combs like 1 (ASXL1), enhancer of zeste homolog 2 (EZH2), isocitrate dehydrogenase 1 and isocitrate dehydrogenase 2 (IDH1/2), and DNA methyltransferase 3A (DNMT3A) genes, among others [29]. The biologic and prognostic significance of these non-driver alterations remain to be fully defined in ET and PV.
Diagnostic Criteria
Diagnostic criteria for PV and ET according to the World Health Organization (WHO) classification [30] are summarized in Table 1. Criteria for the diagnosis of prefibrotic myelofibrosis are included as well since this entity was formally recognized as separate from ET and part of the PMF spectrum in the 2016 WHO classification of myeloid tumors [30]. Clinically, both PV and ET generally remain asymptomatic for a long time. PV tends to be more symptomatic than ET and can present with debilitating constitutional symptoms (fatigue, night sweats, and weight loss), microvascular symptoms (headache, lightheadedness, acral paresthesias, erythromelalgia, atypical chest pain, and pruritus) [31], or macrovascular accidents (larger vein thrombosis, stroke, or myocardial ischemia) [32]. ET is often diagnosed incidentally, but patients can suffer from similar general symptoms and vascular complications. Causes of secondary absolute erythrocytosis (altitude, chronic hypoxemia, heavy smoking, cardiomyopathy, use of corticosteroids, erythropoietin, or other anabolic hormones, familial or congenital forms) or thrombocytosis (iron deficiency, acute blood loss, trauma or injury, acute coronary syndrome, systemic autoimmune disorders, chronic kidney failure, other malignancies, splenectomy) should be considered and appropriately excluded. Once the diagnosis is made, symptom assessment tools such as the Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF) [33] or the abbreviated version, the MPN-SAF Total Symptom Score (MPN-SAF TSS) [34], are generally used to assess patients’ symptom burden and response to treatment in everyday practice.
Risk Stratification
Thrombohemorrhagic events, evolution into myelofibrosis, and leukemic transformation (LT) are the most serious complications in the course of PV or ET. Only thrombohemorrhagic events are, at least partially, preventable. Arterial or venous thrombotic complications are observed at rates of 1.8 to 10.9 per 100 patient-years in PV (arterial thrombosis being more common than venous) and 0.74 to 7.7 per 100 patient-years in ET, depending on the risk group [35] and the presence of other factors (see below).
The risk stratification of patients with PV is based on 2 factors: age ≥ 60 years and prior history of thrombosis. If either is present, the patient is assigned to the high-risk category, whereas if none is present the patient is considered at low risk [36]. In addition, high hematocrit [37] and high white blood cell (WBC) count [38], but not thrombocytosis, have been associated with the development of vascular complications. In one study, the risk of new arterial thrombosis was increased by the presence of leukoerythroblastosis, hypertension, and prior arterial thrombosis, while karyotypic abnormalities and prior venous thrombosis were predictors of new venous thrombosis [39]. Another emerging risk factor for thrombosis in patients with PV is high JAK2 allele burden (ie, the normal-to-mutated gene product ratio), although the evidence supporting this conclusion is equivocal [40].
Traditionally, in ET patients, the thrombotic risk was assessed using the same 2 factors (age ≥ 60 years and prior history of thrombosis), separating patients into low- and high-risk groups. However, the prognostication of ET patients has been refined recently with the identification of new relevant factors. In particular, the impact of JAK2 mutations on thrombotic risk has been thoroughly studied. Clinically, the presence of JAK2V617F is associated with older age, higher hemoglobin and hematocrit, lower platelet counts, more frequent need for cytoreductive treatment, and greater tendency to evolve into PV (a rare event) [41,42]. Many [41,43–46], but not all [47–51], studies suggested a correlation between JAK2 mutation and risk of both arterial and venous thrombosis. Although infrequent, a JAK2V617F homozygous state (ie, the mutation is present in both alleles) might confer an even higher thrombotic risk [52]. Moreover, the impact of the JAK2 mutation on vascular events persists over time [53], particularly in patients with high or unstable mutation burden [54]. Based on JAK2V617F’s influence on the thrombotic risk of ET patients, a new prognostic score was proposed, the International Prognostic Score for ET (IPSET)-thrombosis (Table 2). The revised version of this model is currently endorsed by the National Comprehensive Cancer Network and divides patients into 4 risk groups: high, intermediate, low, and very low. Treatment recommendations vary according to the risk group (as described below) [55].
Other thrombotic risk factors have been identified, but deemed not significant enough to be included in the model. Cardiovascular risk factors (hypercholesterolemia, hypertension, smoking, diabetes mellitus) can increase the risk of vascular events [56–59], as can splenomegaly [60] and baseline or persistent leukocytosis [61–63]. Thrombocytosis has been correlated with thrombotic risk in some studies [64–68], whereas others did not support this conclusion and/or suggested a lower rate of thrombosis and, in some cases, increased risk of bleeding in ET patients with platelet counts greater than 1000 × 103/μL (due to acquired von Willebrand syndrome) [51,61,63,68,69].
CALR mutations tend to occur in younger males with lower hemoglobin and WBC count, higher platelet count, and greater marrow megakaryocytic predominance, as compared to JAK2 mutations [26,27,70–72]. The associated incidence of thrombosis was less than 10% at 15 years in patients with CALR mutations, lower than the incidence reported for ET patients with JAK2V617F mutations [73]. The presence of the mutation per se does not appear to affect the thrombotic risk [74–76]. Information on the thrombotic risk associated with MPL mutations or a triple-negative state is scarce. In both instances, however, the risk appears to be lower than with the JAK2 mutation [73,77–79].
Venous thromboembolism (VTE) in patients with PV or ET may occur at unusual sites, such as the splanchnic or cerebral venous systems [80]. Risk factors for unusual VTE include younger age [81], female gender (especially with concomitant use of oral contraceptive pills) [82], and splenomegaly/splenectomy [83]. JAK2 mutation has also been associated with thrombosis at unusual sites. However, the prevalence of MPN or JAK2V617F in patients presenting with splanchnic VTE has varied [80]. In addition, MPN may be occult (ie, no clinical or laboratory abnormalities) in around 15% of patients [84]. Screening for JAK2V617F and underlying MPN is recommended in patients presenting with isolated unexplained splanchnic VTE. Treatment entails long-term anticoagulation therapy. JAK2V617F screening in patients with nonsplanchnic VTE is not recommended, as its prevalence in this group is low (< 3%) [85,86].
Risk-Adapted Therapy
Low-Risk PV
All patients with PV should receive counseling to mitigate cardiovascular risk factors, including smoking cessation, lifestyle modifications, and lipid-lowering therapy, as indicated. Furthermore, all PV patients should receive acetylsalicylic acid (ASA) to decrease their risk for thrombosis and control vasomotor symptoms [55,87]. Aspirin 81 to 100 mg daily is the preferred regimen because it provides adequate antithrombotic effect without the associated bleeding risk of higher-dose aspirin [88]. Low-risk PV patients should also receive periodic phlebotomies to reduce and maintain their hematocrit below 45%. This recommendation is based on the results of the Cytoreductive Therapy in Polycythemia Vera (CYTO PV) randomized controlled trial. In that study, patients receiving more intense therapy to maintain the hematocrit below 45% had a lower incidence of cardiovascular-related deaths or major thrombotic events than those with hematocrit goals of 45% to 50% (2.7% versus 9.8%) [89]. Cytoreduction is an option for low-risk patients who do not tolerate phlebotomy or require frequent phlebotomy, or who have disease-related bleeding, severe symptoms, symptomatic splenomegaly, or progressive leukocytosis [38].
High-Risk PV
Patients older than 60 years and/or with a history of thrombosis should be considered for cytoreductive therapy in addition to the above measures. Frontline cytoreductive therapies include hydroxyurea or interferon (IFN)-alfa [87]. Hydroxyurea is a potent ribonucleotide reductase inhibitor that interferes with DNA repair and is the treatment of choice for most high-risk patients with PV [90]. In a small trial, hydroxyurea reduced the risk of thrombosis compared with historical controls treated with phlebotomy alone [91]. Hydroxyurea is generally well tolerated; common side effects include cytopenias, nail changes, and mucosal and/or skin ulcers. Although never formally proven to be leukemogenic, this agent should be used with caution in younger patients [87]. Indeed, in the original study, the rates of transformation were 5.9% and 1.5% for patients receiving hydroxyurea and phlebotomy alone [92], respectively, although an independent role for hydroxyurea in LT was not supported in the much larger European Collaboration on Low-dose Aspirin in Polycythemia Vera (ECLAP) study [93]. Approximately 70% of patients will have a sustained response to hydroxyurea [94], while the remaining patients become resistant to or intolerant of the drug. Resistant individuals have a higher risk of progression to acute leukemia and death [95].
IFN-alfa is a pleiotropic antitumor agent that has found application in many types of malignancies [96] and is sometimes employed as treatment for patients with newly diagnosed high-risk PV. Early studies showed responses in up to 100% of cases [97,98], albeit at the expense of a high discontinuation rate due to adverse events, such as flu-like symptoms, fatigue, and neuropsychiatric manifestations [99]. A newer formulation of the drug obtained by adding a polyethylene glycol (PEG) moiety to the native IFN-alfa molecule (PEG-IFN alfa) was shown to have a longer half-life, greater stability, less immunogenicity, and, potentially, better tolerability [100]. Pilot phase 2 trials of PEG-IFN-alfa-2a demonstrated its remarkable activity, with symptomatic and hematologic responses seen in most patients (which, in some cases, persisted beyond discontinuation), and reasonable tolerability, with long-term discontinuation rates of 20% to 30% [101–103]. In some patients, JAK2V617F became undetectable over time [104]. Results of 2 ongoing trials, MDP-RC111 (single-arm study, PEG-IFN-alfa-2a in high-risk PV or ET [NCT01259817]) and MPD-RC112 (randomized controlled trial, PEG-IFN-alfa-2a versus hydroxyurea in the same population [NCT01258856]), will shed light on the role of PEG-IFN-alfa in the management of patients with high-risk PV or ET. In two phase 2 studies of PEG-IFN-alfa-2b, complete responses were seen in 70% to 100% of patients and discontinuation occurred in around a third of cases [105,106]. A new, longer-acting formulation of PEG-IFN-alfa-2a (peg-proline INF-alfa-2b, AOP2014) is also undergoing clinical development [107,108].
The approach to treatment of PV based on thrombotic risk level is illustrated in Figure 1.
Very Low- and Low-Risk ET
Individuals with ET should undergo rigorous cardiovascular risk management and generally receive ASA to decrease their thrombotic risk and improve symptom control. Antiplatelet therapy may not be warranted in patients with documented acquired von Willebrand syndrome, with or without extreme thrombocytosis, or in those in the very low-risk category according to the IPSET-thrombosis model [55,87]. The risk/benefit ratio of antiplatelet agents in patients with ET at different thrombotic risk levels was assessed in poor-quality studies and thus remains highly uncertain. Platelet-lowering agents are sometimes recommended in patients with low-risk disease who have platelet counts ≥ 1500 × 103/μL, due to the potential risk of acquired von Willebrand syndrome and a risk of bleeding (this would require stopping ASA) [109]. Cytoreduction may also be used in low-risk patients with progressive symptoms despite ASA, symptomatic or progressive splenomegaly, and progressive leukocytosis.
Intermediate-Risk ET
This category includes patients older than 60 years, but without thrombosis or JAK2 mutations. These individuals would have been considered high risk (and thus candidates for cytoreductive therapy) according to the traditional risk stratification. Guidelines currently recommend ASA as the sole therapy for these patients, while reserving cytoreduction for those who experience thrombosis (ie, become high-risk) or have uncontrolled vasomotor or general symptoms, symptomatic splenomegaly, symptomatic thrombocytosis, or progressive leukocytosis.
High-Risk ET
For patients with ET in need of cytoreductive therapy (ie, those with prior thrombosis or older than 60 years with a JAK2V617F mutation), first-line options include hydroxyurea, IFN, and anagrelide. Hydroxyurea remains the treatment of choice in most patients [110]. In a seminal study, 114 patients with ET were randomly assigned to either observation or hydroxyurea treatment with the goal of maintaining the platelet count below 600 × 103/μL. At a median follow-up of 27 months, patients in the hydroxyurea group had a lower thrombosis rate (3.6% versus 24%, P = 0.003) and longer thrombosis-free survival, regardless of the use of antiplatelet drugs [64].
Anagrelide, a selective inhibitor of megakaryocytic differentiation and proliferation, was compared with hydroxyurea in patients with ET in 2 randomized trials. In the first (n = 809), the group receiving anagrelide had a higher risk of arterial thrombosis, major bleeding, and fibrotic evolution, but lower incidence of venous thrombosis. Hydroxyurea was better tolerated, mainly due to anagrelide-related cardiovascular adverse events [111]. As a result of this study, hydroxyurea is often preferred to anagrelide as frontline therapy for patients with newly diagnosed high-risk ET. In the second, more recent study (n = 259), however, the 2 agents proved equivalent in terms of major or minor arterial or venous thrombosis, as well as discontinuation rate [112]. The discrepancy between the 2 trials may be partly explained by the different ET diagnostic criteria used, with the latter only enrolling patients with WHO-defined true ET and the former utilizing Polycythemia Vera Study Group-ET diagnostic criteria that included patients with increases in other blood counts or varying degrees of marrow fibrosis.
Interferons were studied in ET in parallel with PV. PEG-IFN-alfa-2a proved effective in patients with ET, with responses observed in 80% of patients [103]. PEG-IFN- alfa-2b produced similar results, with responses in 70% to 90% of patients in small studies and discontinuation observed in 20% to 38% of cases [105,106,113]. Because the very long-term leukemogenic potential of hydroxyurea has remained somewhat uncertain, anagrelide or IFN might be preferable choices in younger patients.
The approach to treatment of ET based on thrombotic risk level is illustrated in Figure 2.
Assessing Response to Therapy
For both patients with PV and ET the endpoint of treatment set forth for clinical trials has been the achievement of a clinicohematologic response. However, studies have failed to show a correlation between response and reduction of the thrombohemorrhagic risk [114]. Therefore, proposed clinical trial response criteria were revised to include absence of hemorrhagic or thrombotic events as part of the definition of response (Table 3) [94].
Approach to Patients Refractory to or Intolerant of First-line Therapy
According to the European LeukemiaNet recommendations, an inadequate response to hydroxyurea in patients with PV (or myelofibrosis) is defined as a need for phlebotomy to maintain the hematocrit below < 45%, the platelet count > 400 × 103/μL, and a WBC count > 10,000/μL, or failure to reduce splenomegaly > 10 cm by > 50% at a dose of ≥ 2 g/day or maximum tolerated dose. Historically, treatment options for patients with PV or ET who failed first-line therapy (most commonly hydroxyurea) have included alkylating agents, such as busulfan, chlorambucil, pipobroman, and phosphorus (P)-32. However, the use of these drugs is limited by the associated risk of LT [93,115,116]. IFN (or anagrelide for ET) is often considered in patients previously treated with hydroxyurea, and vice versa.
Ruxolitinib is a JAK1 and JAK2 inhibitor currently approved for the treatment of PV patients refractory to or intolerant of hydroxyurea [7]. Following promising results of a phase 2 trial [117], ruxolitinib 10 mg twice daily was compared with best available therapy in the pivotal RESPONSE trial (n = 222). Ruxolitinib proved superior in achieving hematocrit control, reduction of spleen volume, and improvement of symptoms. Grade 3-4 hematologic toxicity was infrequent and similar in the 2 arms [118]. In addition, longer follow-up of that study suggested a lower rate of thrombotic events in patients receiving ruxolitinib (1.8 versus 8.2 per 100 patient-years) [119]. In a similarly designed randomized phase 3 study in PV patients without splenomegaly (RESPONSE-2), more patients in the ruxolitinib arm had hematocrit reduction without an increase in toxicity. Based on the results of these studies, ruxolitinib can be considered a standard of care for second-line therapy in this post-hydroxyurea patient population [120]. Ruxolitinib is also being tested in patients with high-risk ET who have become resistant to, or were intolerant of hydroxyurea, but currently has no approved indication in this setting [121,122]. Common side effects of ruxolitinib include cytopenias (especially anemia), increased risk of infections, hyperlipidemia, and increased risk of non-melanoma skin cancer.
Novel agents that have been studied in patients with PV and ET are histone deacetylase inhibitors, murine double minute 2 (MDM2, or HDM2 for their human counterpart) inhibitors (which restore the function of p53), Bcl-2 homology domain 3 mimetics such as navitoclax and venetoclax, and, for patients with ET, the telomerase inhibitor imetelstat [123].
Disease Evolution
Post-PV/Post-ET Myelofibrosis
Diagnostic criteria for post-PV and post-ET myelofibrosis are outlined in Table 4. Fibrotic transformation represents a natural evolution of the clinical course of PV or ET. It occurs in up to 15% and 9% of patients with PV and ET, respectively, in western countries [124]. The true percentage of ET patients who develop myelofibrosis is confounded by the inclusion of prefibrotic myelofibrosis cases in earlier series. The survival of patients who develop myelofibrosis is shortened compared to those who do not. In patients with PV, risk factors for myelofibrosis evolution include advanced age, leukocytosis, JAK2V617F homozygosity or higher allele burden, and hydroxyurea therapy. Once post-PV myelofibrosis has occurred, hemoglobin < 10 g/dL, platelet count < 100 × 103/μL, and WBC count > 30,000/μL are associated with worse outcomes [125]. In patients with ET, risk factors for myelofibrosis transformation include age, anemia, bone marrow hypercellularity and increased reticulin, increased lactate dehydrogenase, leukocytosis, and male gender. The management of post-PV/post-ET myelofibrosis recapitulates that of PMF.
Leukemic Transformation
The presence of more than 20% blasts in peripheral blood or bone marrow in a patient with MPN defines LT. This occurs in up to 5% to 10% of patients and may or may not be preceded by a myelofibrosis phase [126]. In cases of extramedullary transformation, a lower percentage of blasts can be seen in the bone marrow compared to the peripheral blood. The pathogenesis of LT has remained elusive, but it is believed to be associated with genetic instability, which facilitates the acquisition of additional mutations, including those of TET2, ASXL1, EZH2 DNMT3, IDH1/2, and TP53 [127].
Clinical risk factors for LT include advanced age, karyotypic abnormalities, prior therapy with alkylating agents or P-32, splenectomy, increased peripheral blood or bone marrow blasts, leukocytosis, anemia, thrombocytopenia, and cytogenetic abnormalities. Hydroxyurea, IFN, and ruxolitinib have not been shown to have leukemogenic potential thus far. Prognosis of LT is uniformly poor and patient survival rarely exceeds 6 months.
There is no standard of care for MPN LT. Treatment options range from low-intensity regimens to more aggressive AML-type induction chemotherapy. No strategy appears clearly superior to others [128]. Hematopoietic stem cell transplantation is the only therapy that provides clinically meaningful benefit to patients [129], but it is applicable only to a minority of patients with chemosensitive disease and good performance status [130]. Notable experimental approaches to MPN LT include hypomethylating agents, such as decitabine [131] or azacytidine [132], with or without ruxolitinib [133–135].
Conclusion
PV and ET are rare, chronic myeloid disorders. Patients typically experience a long clinical course and enjoy near-normal quality of life if properly managed. The 2 most important life-limiting complications of PV and ET are thrombohemorrhagic events and myelofibrosis/AML transformation. Vascular events are at least in part preventable with counseling on risk factors, phlebotomy (for patients with PV), antiplatelet therapy, and cytoreduction with hydroxyurea, IFNs, or anagrelide (for patients with ET). In addition, ruxolitinib was recently approved for PV patients after hydroxyurea failure. PV/ET transformation in myelofibrosis or AML is part of the natural history of the disease and no therapy has been shown to prevent it. Treatment follows recommendations set forth for PMF and AML, but results are generally poorer and novel strategies are needed to improve outcomes.
Corresponding author: Lorenzo Falchi, MD, Columbia University Medical Center, New York, NY.
Financial disclosures: None.
1. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC; 2008.
2. Alvarez-Larran A, Pereira A, Arellano-Rodrigo E, et al. Cytoreduction plus low-dose aspirin versus cytoreduction alone as primary prophylaxis of thrombosis in patients with high-risk essential thrombocythaemia: an observational study. Br J Haematol 2013;161:865–71.
3. Tefferi A. Polycythemia vera and essential thrombocythemia: 2013 update on diagnosis, risk-stratification, and management. Am J Hematol 2013;88:507–16.
4. Michiels JJ, Berneman Z, Schroyens W, et al. Platelet-mediated erythromelalgic, cerebral, ocular and coronary microvascular ischemic and thrombotic manifestations in patients with essential thrombocythemia and polycythemia vera: a distinct aspirin-responsive and coumadin-resistant arterial thrombophilia. Platelets 2006;17:528–44.
5. James C, Ugo V, Couedic J-PL, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005;434:1144–8.
6. Kralovics R, Passamonti F, Buser A, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders.N Engl J Med 2005;352:1779–90.
7. Verstovsek S, Mesa R, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 2012;366:799–807.
8. Harrison C, Kiladjian J, Al-Ali H, et al. JAK Inhibition with ruxolitinib versus best available therapy for myelofibrosis.N Engl J Med 2012;366:787–98.
9. Berglund S, Zettervall O. Incidence of polycythemia vera in a defined population. Eur J Haematol 1992;48:20–6.
10. Rozman C, Giralt M, Feliu E, et al. Life expectancy of patients with chronic nonleukemic myeloproliferative disorders. Cancer 1991;67:2658–63.
11. Passamonti F, Rumi E, Pungolino E, et al. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med 2004;117:755–61.
12. Hultcrantz M, Kristinsson SY, Andersson TM, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol 2012;30:2995–3001.
13. Wolanskyj AP, Schwager SM, et al. Essential thrombocythemia beyond the first decade: life expectancy, long-term complication rates, and prognostic factors. Mayo Clin Proc 2006;81:159–66.
14. Srour SA, Devesa SS, Morton LM, et al. Incidence and patient survival of myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms in the United States, 2001-12. Br J Haematol 2016;174:382–96.
15. Titmarsh GJ, Duncombe AS, McMullin MF, et al. How common are myeloproliferative neoplasms? A systematic review and meta-analysis. Am J Hematol 2014;89:581–7.
16. Moulard O, Mehta J, Fryzek J, et al. Epidemiology of myelofibrosis, essential thrombocythemia, and polycythemia vera in the European Union. Eur J Haematol 2014;92:289–97.
17. Stein BL, Gotlib J, Arcasoy M, et al. Historical views, conventional approaches, and evolving management strategies for myeloproliferative neoplasms. J Natl Compr Canc Netw 2015;13:424–34.
18. Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 2005;7:387–97.
19. Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005;365:1054–61.
20. Scott LM, Tong W, Levine RL, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med 2007;356:459–68.
21. Pardanani A, Lasho TL, Finke C, et al. Prevalence and clinicopathologic correlates of JAK2 exon 12 mutations in JAK2V617F-negative polycythemia vera. Leukemia 2007;21:1960–3.
22. Passamonti F, Elena C, Schnittger S, et al. Molecular and clinical features of the myeloproliferative neoplasm associated with JAK2 exon 12 mutations. Blood 2011;117:2813–6.
23. Abe M, Suzuki K, Inagaki O, et al. A novel MPL point mutation resulting in thrombopoietin-independent activation. Leukemia 2002;16:1500–6.
24. Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med 2006;3:e270.
25. Pardanani AD, Levine RL, Lasho T, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood 2006;108:3472–6.
26. Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med 2013;369:2391–405.
27. Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med 2013;369:2379–90.
28. Wang WA, Groenendyk J, Michalak M. Calreticulin signaling in health and disease. Int J Biochem Cell Biol 2012;44:842–6.
29. Saeidi K. Myeloproliferative neoplasms: Current molecular biology and genetics. Crit Rev Oncol Hematol 2016;98:375–89.
30. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016;127: 2391–405.
31. Mesa RA, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer 2007;109:68–76.
32. Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol 2005;23:2224–32.
33. Scherber R, Dueck AC, Johansson P, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood 2011;118:401–8.
34. Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol 2012;30:4098–103.
35. Casini A, Fontana P, Lecompte TP. Thrombotic complications of myeloproliferative neoplasms: risk assessment and risk-guided management. J Thromb Haemost 2013;11:1215–27.
36. Barbui T, Finazzi G, Falanga A. Myeloproliferative neoplasms and thrombosis. Blood 2013;122:2176-–84.
37. Pearson TC, Wetherley-Mein G. Vascular occlusive episodes and venous haematocrit in primary myeloproliferative polychytemia. Lancet 1978;2:1219–22.
38. Landolfi R, Di Gennaro L, Barbui T, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood 2007;109:2446–52.
39. Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia 2013;27:1874–81.
40. Barbui T, Falanga A. Molecular biomarkers of thrombosis in myeloproliferative neoplasms. Thromb Res 2016;140 Suppl 1:S71–75.
41. Campbell PJ, Scott LM, Buck G, et al. Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study. Lancet 2005;366:1945–53.
42. Tefferi A, Guglielmelli P, Larson DR, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood 2014;124:2507–13.
43. Qin Y, Wang X, Zhao C, et al. The impact of JAK2V617F mutation on different types of thrombosis risk in patients with essential thrombocythemia: a meta-analysis. Int J Hematol 2015;102:170–80.
44. Ziakas PD. Effect of JAK2 V617F on thrombotic risk in patients with essential thrombocythemia: measuring the uncertain. Haematologica 2008;93:1412–4.
45. Lussana F, Dentali F, Abbate R, et al. Screening for thrombophilia and antithrombotic prophylaxis in pregnancy: Guidelines of the Italian Society for Haemostasis and Thrombosis (SISET). Thromb Res 2009;124: e19–25.
46. Dahabreh IJ, Zoi K, Giannouli S, et al. Is JAK2 V617F mutation more than a diagnostic index? A meta-analysis of clinical outcomes in essential thrombocythemia. Leuk Res 2009;33:67–73.
47. Cho YU, Chi HS, Lee EH, et al. Comparison of clinicopathologic findings according to JAK2 V617F mutation in patients with essential thrombocythemia. Int J Hematol 2009;89:39–44.
48. Wolanskyj AP, Lasho TL, Schwager SM, et al. JAK2 mutation in essential thrombocythaemia: clinical associations and long-term prognostic relevance. Br J Haematol 2005;131:208–13.
49. Antonioli E, Guglielmelli P, Pancrazzi A, et al. Clinical implications of the JAK2 V617F mutation in essential thrombocythemia. Leukemia 2005;19:1847–9.
50. Palandri F, Catani L, Testoni N, et al. Long-term follow-up of 386 consecutive patients with essential thrombocythemia: safety of cytoreductive therapy. Am J Hematol 2009;84:215–20.
51. Chim CS, Sim JP, Chan CC, et al. Impact of JAK2V617F mutation on thrombosis and myeloid transformation in essential thrombocythemia: a multivariate analysis by Cox regression in 141 patients. Hematology 2010;15: 187–92.
52. Vannucchi AM, Antonioli E, Guglielmelli P, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood 2007;110:840–6.
53. Carobbio A, Finazzi G, Antonioli E, et al. JAK2V617F allele burden and thrombosis: a direct comparison in essential thrombocythemia and polycythemia vera. Exp Hematol 2009;37:1016–21.
54. Alvarez-Larran A, Bellosillo B, Pereira A, et al. JAK2V617F monitoring in polycythemia vera and essential thrombocythemia: clinical usefulness for predicting myelofibrotic transformation and thrombotic events. Am J Hematol 2014;89:517–23.
55. Barbui T, Vannucchi AM, Buxhofer-Ausch V, et al. Practice-relevant revision of IPSET-thrombosis based on 1019 patients with WHO-defined essential thrombocythemia. Blood Cancer J 2015;5:e369.
56. Carobbio A, Thiele J, Passamonti F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood 2011;117:5857–9.
57. Alvarez-Larran A, Cervantes F, Bellosillo B, et al. Essential thrombocythemia in young individuals: frequency and risk factors for vascular events and evolution to myelofibrosis in 126 patients. Leukemia 2007;21:1218–23.
58. Jantunen R, Juvonen E, Ikkala E, et al. The predictive value of vascular risk factors and gender for the development of thrombotic complications in essential thrombocythemia. Ann Hematol 2001;80:74–8.
59. Besses C, Cervantes F, Pereira A, et al. Major vascular complications in essential thrombocythemia: a study of the predictive factors in a series of 148 patients. Leukemia 1999;13:150–4.
60. Haider M, Gangat N, Hanson C, Tefferi A. Splenomegaly and thrombosis risk in essential thrombocythemia: the mayo clinic experience. Am J Hematol 2016;91: E296–297.
61. Carobbio A, Finazzi G, Antonioli E, et al. Thrombocytosis and leukocytosis interaction in vascular complications of essential thrombocythemia. Blood 2008;112:3135–7.
62. Palandri F, Polverelli N, Catani L, et al. Impact of leukocytosis on thrombotic risk and survival in 532 patients with essential thrombocythemia: a retrospective study. Ann Hematol 2011;90:933–8.
63. Campbell PJ, MacLean C, Beer PA, et al. Correlation of blood counts with vascular complications in essential thrombocythemia: analysis of the prospective PT1 cohort. Blood 2012;120:1409–11.
64. Cortelazzo S, Finazzi G, Ruggeri M, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med 1995;332:1132–6.
65. van Genderen PJ, Mulder PG, Waleboer M, et al. Prevention and treatment of thrombotic complications in essential thrombocythaemia: efficacy and safety of aspirin. Br J Haematol 1997;97:179–84.
66. Storen EC, Tefferi A. Long-term use of anagrelide in young patients with essential thrombocythemia. Blood 2001;97:863–6.
67. De Stefano V, Za T, Rossi E, et al. Recurrent thrombosis in patients with polycythemia vera and essential thrombocythemia: incidence, risk factors, and effect of treatments. Haematologica 2008;93:372–80.
68. Alvarez-Larran A, Cervantes F, Pereira A, et al. Observation versus antiplatelet therapy as primary prophylaxis for thrombosis in low-risk essential thrombocythemia. Blood 2010;116:1205–10.
69. Palandri F, Polverelli N, Catani L, et al. Bleeding in essential thrombocythaemia: a retrospective analysis on 565 patients. Br J Haematol 2012;156:281–4.
70. Rotunno G, Mannarelli C, Guglielmelli P, et al. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood 2014;123:1552–5.
71. Tefferi A, Wassie EA, Lasho TL, et al. Calreticulin mutations and long-term survival in essential thrombocythemia. Leukemia 2014;28:2300–3.
72. Rumi E, Pietra D, Ferretti V, et al. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood 2014;123:1544–51.
73. Palandri F, Latagliata R, Polverelli N, et al. Mutations and long-term outcome of 217 young patients with essential thrombocythemia or early primary myelofibrosis. Leukemia 2015;29:1344–9.
74. Fu R, Xuan M, Zhou Y, et al. Analysis of calreticulin mutations in Chinese patients with essential thrombocythemia: clinical implications in diagnosis, prognosis and treatment. Leukemia 2014;28:1912–4.
75. Tefferi A, Wassie EA, Guglielmelli P, et al. Type 1 versus Type 2 calreticulin mutations in essential thrombocythemia: a collaborative study of 1027 patients. Am J Hematol 2014;89:E121–4.
76. Pietra D, Rumi E, Ferretti VV, et al. Differential clinical effects of different mutation subtypes in CALR-mutant myeloproliferative neoplasms. Leukemia 2016;30: 431–8.
77. Rumi E, Pietra D, Guglielmelli P, et al. Acquired copy-neutral loss of heterozygosity of chromosome 1p as a molecular event associated with marrow fibrosis in MPL-mutated myeloproliferative neoplasms. Blood 2013;121:4388–95.
78. Beer PA, Campbell PJ, Scott LM, et al. MPL mutations in myeloproliferative disorders: analysis of the PT-1 cohort. Blood 2008;112:141–9.
79. Gangat N, Wassie EA, Lasho TL, et al. Mutations and thrombosis in essential thrombocythemia: prognostic interaction with age and thrombosis history. Eur J Haematol 2015;94:31–6.
80. Sekhar M, McVinnie K, Burroughs AK. Splanchnic vein thrombosis in myeloproliferative neoplasms. Br J Haematol 2013;162:730–47.
81. Stein BL, Saraf S, Sobol U, et al. Age-related differences in disease characteristics and clinical outcomes in polycythemia vera. Leuk Lymph 2013;54:1989–95.
82. Landolfi R, Di Gennaro L, Nicolazzi MA, et al. Polycythemia vera: gender-related phenotypic differences. Intern Emerg Med 2012;7:509–15.
83. Winslow ER, Brunt LM, Drebin JA, et al. Portal vein thrombosis after splenectomy. Am J Surg 2002;184:631–6.
84. Smalberg JH, Arends LR, Valla DC, et al. Myeloproliferative neoplasms in Budd-Chiari syndrome and portal vein thrombosis: a meta-analysis. Blood 2012;120:4921–8.
85. Dentali F, Squizzato A, Brivio L, et al. JAK2V617F mutation for the early diagnosis of Ph- myeloproliferative neoplasms in patients with venous thromboembolism: a meta-analysis. Blood 2009;113:5617–23.
86. Pardanani A, Lasho TL, Hussein K, et al. JAK2V617F mutation screening as part of the hypercoagulable work-up in the absence of splanchnic venous thrombosis or overt myeloproliferative neoplasm: assessment of value in a series of 664 consecutive patients. Mayo Clin Proc 2008;83:457–9.
87. Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol 2011;29:761–70.
88. Landolfi R, Marchioli R, Kutti J, et al. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med 2004;350:114–24.
89. Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med 2013;368:22–33.
90. Kiladjian JJ, Chevret S, Dosquet C, et al. Treatment of polycythemia vera with hydroxyurea and pipobroman: final results of a randomized trial initiated in 1980. J Clin Oncol 2011;29:3907–13.
91. Kaplan ME, Mack K, Goldberg JD, et al. Long-term management of polycythemia vera with hydroxyurea: a progress report. Semin Hematol 1986;23:167–71.
92. Fruchtman SM, Mack K, Kaplan ME, et al. From efficacy to safety: a Polycythemia Vera Study group report on hydroxyurea in patients with polycythemia vera. Semin Hematol 1997;34:17–23.
93. Finazzi G, Caruso V, Marchioli R, et al. Acute leukemia in polycythemia vera: an analysis of 1638 patients enrolled in a prospective observational study. Blood 2005;105: 2664–70.
94. Barosi G, Mesa R, Finazzi G, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood 2013;121:4778–81.
95. Alvarez-Larran A, Pereira A, Cervantes F, et al. Assessment and prognostic value of the European LeukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia vera. Blood 2012;119:1363–9.
96. Stein BL, Tiu RV. Biological rationale and clinical use of interferon in the classical BCR-ABL-negative myeloproliferative neoplasms. J Interferon Cytokine Res 2013;33: 145–53.
97. Ludwig H, Cortelezzi A, Van Camp BG, et al. Treatment with recombinant interferon-alpha-2C: multiple myeloma and thrombocythaemia in myeloproliferative diseases. Oncology 1985;42 Suppl 1:19–25.
98. Silver RT. Long-term effects of the treatment of polycythemia vera with recombinant interferon-alpha. Cancer 2006;107:451–8.
99. Kiladjian JJ, Mesa RA, Hoffman R. The renaissance of interferon therapy for the treatment of myeloid malignancies. Blood 2011;117:4706–15.
100. Veronese FM, Mero A. The impact of PEGylation on biological therapies. BioDrugs 2008;22:315–29.
101. Kiladjian JJ, Cassinat B, Chevret S, et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood 2008;112:3065–72.
102. Turlure P, Cambier N, Roussel M, et al. Complete hematological, molecular and histological remissions without cytoreductive treatment lasting after pegylated-interferon {alpha}-2a (peg-IFN{alpha}-2a) therapy in polycythemia vera (PV): long term results of a phase 2 trial [abstract]. Blood 2011;118(21). Abstract 280.
103. Quintas-Cardama A, Kantarjian H, Manshouri T, et al. Pegylated interferon alfa-2a yields high rates of hematologic and molecular response in patients with advanced essential thrombocythemia and polycythemia vera. J Clin Oncol 2009;27:5418–24.
104. Quintas-Cardama A, Abdel-Wahab O, Manshouri T, et al. Molecular analysis of patients with polycythemia vera or essential thrombocythemia receiving pegylated interferon a-2a. Blood 2013;122:893–901.
105. Samuelsson J, Hasselbalch H, Bruserud O, et al. A phase II trial of pegylated interferon alpha-2b therapy for polycythemia vera and essential thrombocythemia: feasibility, clinical and biologic effects, and impact on quality of life. Cancer 2006;106:2397–405.
106. Jabbour E, Kantarjian H, Cortes J, et al. PEG-IFN-alpha-2b therapy in BCR-ABL-negative myeloproliferative disorders: final result of a phase 2 study. Cancer 2007; 110:2012–18.
107. Them NC, Bagienski K, Berg T, et al. Molecular responses and chromosomal aberrations in patients with polycythemia vera treated with peg-proline-interferon alpha-2b. Am J Hematol 2015;90:288–94.
108. Gisslinger H, Klade C, Georgiev P, et al. Final results from PROUD-PV a randomized controlled phase 3 trial comparing ropeginterferon alfa-2b to hydroxyurea in polycythemia vera patients [abstract]. Blood 2016;128(suppl 22). Abstract 475.
109. van Genderen PJ, van Vliet HH, Prins FJ, et al. Excessive prolongation of the bleeding time by aspirin in essential thrombocythemia is related to a decrease of large von Willebrand factor multimers in plasma. Ann Hematol 1997;75:215–20.
110. Cortelazzo S, Finazzi G, Ruggeri M, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med 1995;332:1132–7.
111. Harrison CN, Campbell PJ, Buck G, et al. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N Engl J Med 2005;353:33–45.
112. Gisslinger H, Gotic M, Holowiecki J, et al. Anagrelide compared with hydroxyurea in WHO-classified essential thrombocythemia: the ANAHYDRET Study, a randomized controlled trial. Blood 2013;121:1720–8.
113. Alvarado Y, Cortes J, Verstovsek S, et al. Pilot study of pegylated interferon-alpha 2b in patients with essential thrombocythemia. Cancer Chemother Pharmacol 2003;51:81–6.
114. Barosi G, Tefferi A, Barbui T, ad hoc committee ‘Definition of clinically relevant outcomes for contemporarily clinical trials in Ph-neg M. Do current response criteria in classical Ph-negative myeloproliferative neoplasms capture benefit for patients? Leukemia 2012;26:1148–9.
115. Bjorkholm M, Derolf AR, Hultcrantz M, et al. Treatment-related risk factors for transformation to acute myeloid leukemia and myelodysplastic syndromes in myeloproliferative neoplasms. J Clin Oncol 2011;29:2410–5.
116. Alvarez-Larran A, Martinez-Aviles L, Hernandez-Boluda JC, et al. Busulfan in patients with polycythemia vera or essential thrombocythemia refractory or intolerant to hydroxyurea. Ann Hematol 2014;93:2037–43.
117. Verstovsek S, Passamonti F, Rambaldi A, et al. A phase 2 study of ruxolitinib, an oral JAK1 and JAK2 Inhibitor, in patients with advanced polycythemia vera who are refractory or intolerant to hydroxyurea. Cancer 2014;120: 513–20.
118. Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib in polycythemia vera resistant to or intolerant of hydroxyurea. N Engl J Med 2015; 372:426–35.
119. Verstovsek S, Vannucchi AM, Griesshammer M, et al. Ruxolitinib versus best available therapy in patients with polycythemia vera: 80-week follow-up from the RESPONSE trial. Haematologica 2016;101:821–9.
120. Passamonti F, Griesshammer M, Palandri F, et al. Ruxolitinib for the treatment of inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): a randomised, open-label, phase 3b study. Lancet Oncol 2017;18:88–99.
121. Verstovsek S, Passamonti F, Rambaldi A, et al. Long-term results from a phase II open-label study of ruxolitinib in patients with essential thrombocythemia refractory to or intolerant of hydroxyurea [abstract]. Blood 2014;124. Abstract 1847.
122. Harrison CN, Mead AJ, Panchal A, et al. Ruxolitinib versus best available therapy for ET intolerant or resistant to hydroxycarbamide in a randomized trial. Blood 2017 Aug 9. pii: blood-2017-05-785790 .
123. Bose P, Verstovsek S. Drug development pipeline for myeloproliferative neoplasms: potential future impact on guidelines and management. J Natl Compr Canc Netw 2016;14:1613–24.
124. Cerquozzi S, Teffieri A. Blast transformation and fibrotic progression in polycythemia vera and essential thrombocythemia: a literature review of incidence and risk factors. Blood Cancer J 2015;Nov 13;5:e366.
125. Passamonti F, Rumi E, Caramella M, et al. A dynamic prognostic model to predict survival in post-polycythemia vera myelofibrosis. Blood 2008;111:3383–7.
126. Mesa RA, Verstovsek S, Cervantes F, et al. Primary myelofibrosis (PMF), post polycythemia vera myelofibrosis (post- PV MF), post essential thrombocythemia myelofibrosis (post-ET MF), blast phase PMF (PMF-BP): Consensus on terminology by the international working group for myelofibrosis research and treatment (IWG-MRT). Leuk Res 2007;31:737–40.
127. Rampal R, Mascarenhas J. Pathogenesis and management of acute myeloid leukemia that has evolved from a myeloproliferative neoplasm. Curr Opin Hematol 2014;21:65–71.
128. Chihara D, Kantarjian HM, Newberry KJ, et al. Survival outcome of patients with acute myeloid leukemia transformed from myeloproliferative neoplasms [abstract]. Blood 2016;128. Abstract 1940.
129. Tam CS, Nussenzveig RM, Popat U, et al. The natural history and treatment outcome of blast phase BCR-ABL-myeloproliferative neoplasms. Blood 2008;112:1628–37.
130. Kundranda MN, Tibes R, Mesa RA. Transformation of a chronic myeloproliferative neoplasm to acute myelogenous leukemia: does anything work? Curr Hematol Malig Rep 2012;7:78–86.
131. Badar T, Kantarjian HM, Ravandi F, et al. Therapeutic benefit of decitabine, a hypomethylating agent, in patients with high-risk primary myelofibrosis and myeloproliferative neoplasm in accelerated or blastic/acute myeloid leukemia phase. Leuk Res 2015;39:950–6.
132. Thepot S, Itzykson R, Seegers V, et al. Treatment of progression of Philadelphia-negative myeloproliferative neoplasms to myelodysplastic syndrome or acute myeloid leukemia by azacitidine: a report on 54 cases on the behalf of the Groupe Francophone des Myelodysplasies (GFM). Blood 2010;116:3735–42.
133. Pemmaraju N, Kantarjian H, Kadia T, et al. A phase I/II study of the Janus kinase (JAK)1 and 2 inhibitor ruxolitinib in patients with relapsed or refractory acute myeloid leukemia. Clin Lymphoma Myeloma Leuk 2015;15:171–6.
134. Rampal RK, Mascarenhas JO, Kosiorek HE, et al. Safety and efficacy of combined ruxolitinib and decitabine in patients with blast-phase MPN and post-MPN AML: results of a phase I study (Myeloproliferative Disorders Research Consortium 109 trial) [abstract]. Blood 2016;128. Abstract 1124.
135. Bose P, Verstovsek S, Gasior Y, et al. Phase I/II study of ruxolitinib (RUX) with decitabine (DAC) in patients with post-myeloproliferative neoplasm acute myeloid leukemia (post-MPN AML): phase I results [abstract]. Blood 2016;128. Abstract 4262.
1. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC; 2008.
2. Alvarez-Larran A, Pereira A, Arellano-Rodrigo E, et al. Cytoreduction plus low-dose aspirin versus cytoreduction alone as primary prophylaxis of thrombosis in patients with high-risk essential thrombocythaemia: an observational study. Br J Haematol 2013;161:865–71.
3. Tefferi A. Polycythemia vera and essential thrombocythemia: 2013 update on diagnosis, risk-stratification, and management. Am J Hematol 2013;88:507–16.
4. Michiels JJ, Berneman Z, Schroyens W, et al. Platelet-mediated erythromelalgic, cerebral, ocular and coronary microvascular ischemic and thrombotic manifestations in patients with essential thrombocythemia and polycythemia vera: a distinct aspirin-responsive and coumadin-resistant arterial thrombophilia. Platelets 2006;17:528–44.
5. James C, Ugo V, Couedic J-PL, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005;434:1144–8.
6. Kralovics R, Passamonti F, Buser A, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders.N Engl J Med 2005;352:1779–90.
7. Verstovsek S, Mesa R, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 2012;366:799–807.
8. Harrison C, Kiladjian J, Al-Ali H, et al. JAK Inhibition with ruxolitinib versus best available therapy for myelofibrosis.N Engl J Med 2012;366:787–98.
9. Berglund S, Zettervall O. Incidence of polycythemia vera in a defined population. Eur J Haematol 1992;48:20–6.
10. Rozman C, Giralt M, Feliu E, et al. Life expectancy of patients with chronic nonleukemic myeloproliferative disorders. Cancer 1991;67:2658–63.
11. Passamonti F, Rumi E, Pungolino E, et al. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med 2004;117:755–61.
12. Hultcrantz M, Kristinsson SY, Andersson TM, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol 2012;30:2995–3001.
13. Wolanskyj AP, Schwager SM, et al. Essential thrombocythemia beyond the first decade: life expectancy, long-term complication rates, and prognostic factors. Mayo Clin Proc 2006;81:159–66.
14. Srour SA, Devesa SS, Morton LM, et al. Incidence and patient survival of myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms in the United States, 2001-12. Br J Haematol 2016;174:382–96.
15. Titmarsh GJ, Duncombe AS, McMullin MF, et al. How common are myeloproliferative neoplasms? A systematic review and meta-analysis. Am J Hematol 2014;89:581–7.
16. Moulard O, Mehta J, Fryzek J, et al. Epidemiology of myelofibrosis, essential thrombocythemia, and polycythemia vera in the European Union. Eur J Haematol 2014;92:289–97.
17. Stein BL, Gotlib J, Arcasoy M, et al. Historical views, conventional approaches, and evolving management strategies for myeloproliferative neoplasms. J Natl Compr Canc Netw 2015;13:424–34.
18. Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 2005;7:387–97.
19. Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005;365:1054–61.
20. Scott LM, Tong W, Levine RL, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med 2007;356:459–68.
21. Pardanani A, Lasho TL, Finke C, et al. Prevalence and clinicopathologic correlates of JAK2 exon 12 mutations in JAK2V617F-negative polycythemia vera. Leukemia 2007;21:1960–3.
22. Passamonti F, Elena C, Schnittger S, et al. Molecular and clinical features of the myeloproliferative neoplasm associated with JAK2 exon 12 mutations. Blood 2011;117:2813–6.
23. Abe M, Suzuki K, Inagaki O, et al. A novel MPL point mutation resulting in thrombopoietin-independent activation. Leukemia 2002;16:1500–6.
24. Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med 2006;3:e270.
25. Pardanani AD, Levine RL, Lasho T, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood 2006;108:3472–6.
26. Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med 2013;369:2391–405.
27. Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med 2013;369:2379–90.
28. Wang WA, Groenendyk J, Michalak M. Calreticulin signaling in health and disease. Int J Biochem Cell Biol 2012;44:842–6.
29. Saeidi K. Myeloproliferative neoplasms: Current molecular biology and genetics. Crit Rev Oncol Hematol 2016;98:375–89.
30. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016;127: 2391–405.
31. Mesa RA, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer 2007;109:68–76.
32. Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol 2005;23:2224–32.
33. Scherber R, Dueck AC, Johansson P, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood 2011;118:401–8.
34. Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol 2012;30:4098–103.
35. Casini A, Fontana P, Lecompte TP. Thrombotic complications of myeloproliferative neoplasms: risk assessment and risk-guided management. J Thromb Haemost 2013;11:1215–27.
36. Barbui T, Finazzi G, Falanga A. Myeloproliferative neoplasms and thrombosis. Blood 2013;122:2176-–84.
37. Pearson TC, Wetherley-Mein G. Vascular occlusive episodes and venous haematocrit in primary myeloproliferative polychytemia. Lancet 1978;2:1219–22.
38. Landolfi R, Di Gennaro L, Barbui T, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood 2007;109:2446–52.
39. Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia 2013;27:1874–81.
40. Barbui T, Falanga A. Molecular biomarkers of thrombosis in myeloproliferative neoplasms. Thromb Res 2016;140 Suppl 1:S71–75.
41. Campbell PJ, Scott LM, Buck G, et al. Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study. Lancet 2005;366:1945–53.
42. Tefferi A, Guglielmelli P, Larson DR, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood 2014;124:2507–13.
43. Qin Y, Wang X, Zhao C, et al. The impact of JAK2V617F mutation on different types of thrombosis risk in patients with essential thrombocythemia: a meta-analysis. Int J Hematol 2015;102:170–80.
44. Ziakas PD. Effect of JAK2 V617F on thrombotic risk in patients with essential thrombocythemia: measuring the uncertain. Haematologica 2008;93:1412–4.
45. Lussana F, Dentali F, Abbate R, et al. Screening for thrombophilia and antithrombotic prophylaxis in pregnancy: Guidelines of the Italian Society for Haemostasis and Thrombosis (SISET). Thromb Res 2009;124: e19–25.
46. Dahabreh IJ, Zoi K, Giannouli S, et al. Is JAK2 V617F mutation more than a diagnostic index? A meta-analysis of clinical outcomes in essential thrombocythemia. Leuk Res 2009;33:67–73.
47. Cho YU, Chi HS, Lee EH, et al. Comparison of clinicopathologic findings according to JAK2 V617F mutation in patients with essential thrombocythemia. Int J Hematol 2009;89:39–44.
48. Wolanskyj AP, Lasho TL, Schwager SM, et al. JAK2 mutation in essential thrombocythaemia: clinical associations and long-term prognostic relevance. Br J Haematol 2005;131:208–13.
49. Antonioli E, Guglielmelli P, Pancrazzi A, et al. Clinical implications of the JAK2 V617F mutation in essential thrombocythemia. Leukemia 2005;19:1847–9.
50. Palandri F, Catani L, Testoni N, et al. Long-term follow-up of 386 consecutive patients with essential thrombocythemia: safety of cytoreductive therapy. Am J Hematol 2009;84:215–20.
51. Chim CS, Sim JP, Chan CC, et al. Impact of JAK2V617F mutation on thrombosis and myeloid transformation in essential thrombocythemia: a multivariate analysis by Cox regression in 141 patients. Hematology 2010;15: 187–92.
52. Vannucchi AM, Antonioli E, Guglielmelli P, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood 2007;110:840–6.
53. Carobbio A, Finazzi G, Antonioli E, et al. JAK2V617F allele burden and thrombosis: a direct comparison in essential thrombocythemia and polycythemia vera. Exp Hematol 2009;37:1016–21.
54. Alvarez-Larran A, Bellosillo B, Pereira A, et al. JAK2V617F monitoring in polycythemia vera and essential thrombocythemia: clinical usefulness for predicting myelofibrotic transformation and thrombotic events. Am J Hematol 2014;89:517–23.
55. Barbui T, Vannucchi AM, Buxhofer-Ausch V, et al. Practice-relevant revision of IPSET-thrombosis based on 1019 patients with WHO-defined essential thrombocythemia. Blood Cancer J 2015;5:e369.
56. Carobbio A, Thiele J, Passamonti F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood 2011;117:5857–9.
57. Alvarez-Larran A, Cervantes F, Bellosillo B, et al. Essential thrombocythemia in young individuals: frequency and risk factors for vascular events and evolution to myelofibrosis in 126 patients. Leukemia 2007;21:1218–23.
58. Jantunen R, Juvonen E, Ikkala E, et al. The predictive value of vascular risk factors and gender for the development of thrombotic complications in essential thrombocythemia. Ann Hematol 2001;80:74–8.
59. Besses C, Cervantes F, Pereira A, et al. Major vascular complications in essential thrombocythemia: a study of the predictive factors in a series of 148 patients. Leukemia 1999;13:150–4.
60. Haider M, Gangat N, Hanson C, Tefferi A. Splenomegaly and thrombosis risk in essential thrombocythemia: the mayo clinic experience. Am J Hematol 2016;91: E296–297.
61. Carobbio A, Finazzi G, Antonioli E, et al. Thrombocytosis and leukocytosis interaction in vascular complications of essential thrombocythemia. Blood 2008;112:3135–7.
62. Palandri F, Polverelli N, Catani L, et al. Impact of leukocytosis on thrombotic risk and survival in 532 patients with essential thrombocythemia: a retrospective study. Ann Hematol 2011;90:933–8.
63. Campbell PJ, MacLean C, Beer PA, et al. Correlation of blood counts with vascular complications in essential thrombocythemia: analysis of the prospective PT1 cohort. Blood 2012;120:1409–11.
64. Cortelazzo S, Finazzi G, Ruggeri M, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med 1995;332:1132–6.
65. van Genderen PJ, Mulder PG, Waleboer M, et al. Prevention and treatment of thrombotic complications in essential thrombocythaemia: efficacy and safety of aspirin. Br J Haematol 1997;97:179–84.
66. Storen EC, Tefferi A. Long-term use of anagrelide in young patients with essential thrombocythemia. Blood 2001;97:863–6.
67. De Stefano V, Za T, Rossi E, et al. Recurrent thrombosis in patients with polycythemia vera and essential thrombocythemia: incidence, risk factors, and effect of treatments. Haematologica 2008;93:372–80.
68. Alvarez-Larran A, Cervantes F, Pereira A, et al. Observation versus antiplatelet therapy as primary prophylaxis for thrombosis in low-risk essential thrombocythemia. Blood 2010;116:1205–10.
69. Palandri F, Polverelli N, Catani L, et al. Bleeding in essential thrombocythaemia: a retrospective analysis on 565 patients. Br J Haematol 2012;156:281–4.
70. Rotunno G, Mannarelli C, Guglielmelli P, et al. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood 2014;123:1552–5.
71. Tefferi A, Wassie EA, Lasho TL, et al. Calreticulin mutations and long-term survival in essential thrombocythemia. Leukemia 2014;28:2300–3.
72. Rumi E, Pietra D, Ferretti V, et al. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood 2014;123:1544–51.
73. Palandri F, Latagliata R, Polverelli N, et al. Mutations and long-term outcome of 217 young patients with essential thrombocythemia or early primary myelofibrosis. Leukemia 2015;29:1344–9.
74. Fu R, Xuan M, Zhou Y, et al. Analysis of calreticulin mutations in Chinese patients with essential thrombocythemia: clinical implications in diagnosis, prognosis and treatment. Leukemia 2014;28:1912–4.
75. Tefferi A, Wassie EA, Guglielmelli P, et al. Type 1 versus Type 2 calreticulin mutations in essential thrombocythemia: a collaborative study of 1027 patients. Am J Hematol 2014;89:E121–4.
76. Pietra D, Rumi E, Ferretti VV, et al. Differential clinical effects of different mutation subtypes in CALR-mutant myeloproliferative neoplasms. Leukemia 2016;30: 431–8.
77. Rumi E, Pietra D, Guglielmelli P, et al. Acquired copy-neutral loss of heterozygosity of chromosome 1p as a molecular event associated with marrow fibrosis in MPL-mutated myeloproliferative neoplasms. Blood 2013;121:4388–95.
78. Beer PA, Campbell PJ, Scott LM, et al. MPL mutations in myeloproliferative disorders: analysis of the PT-1 cohort. Blood 2008;112:141–9.
79. Gangat N, Wassie EA, Lasho TL, et al. Mutations and thrombosis in essential thrombocythemia: prognostic interaction with age and thrombosis history. Eur J Haematol 2015;94:31–6.
80. Sekhar M, McVinnie K, Burroughs AK. Splanchnic vein thrombosis in myeloproliferative neoplasms. Br J Haematol 2013;162:730–47.
81. Stein BL, Saraf S, Sobol U, et al. Age-related differences in disease characteristics and clinical outcomes in polycythemia vera. Leuk Lymph 2013;54:1989–95.
82. Landolfi R, Di Gennaro L, Nicolazzi MA, et al. Polycythemia vera: gender-related phenotypic differences. Intern Emerg Med 2012;7:509–15.
83. Winslow ER, Brunt LM, Drebin JA, et al. Portal vein thrombosis after splenectomy. Am J Surg 2002;184:631–6.
84. Smalberg JH, Arends LR, Valla DC, et al. Myeloproliferative neoplasms in Budd-Chiari syndrome and portal vein thrombosis: a meta-analysis. Blood 2012;120:4921–8.
85. Dentali F, Squizzato A, Brivio L, et al. JAK2V617F mutation for the early diagnosis of Ph- myeloproliferative neoplasms in patients with venous thromboembolism: a meta-analysis. Blood 2009;113:5617–23.
86. Pardanani A, Lasho TL, Hussein K, et al. JAK2V617F mutation screening as part of the hypercoagulable work-up in the absence of splanchnic venous thrombosis or overt myeloproliferative neoplasm: assessment of value in a series of 664 consecutive patients. Mayo Clin Proc 2008;83:457–9.
87. Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol 2011;29:761–70.
88. Landolfi R, Marchioli R, Kutti J, et al. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med 2004;350:114–24.
89. Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med 2013;368:22–33.
90. Kiladjian JJ, Chevret S, Dosquet C, et al. Treatment of polycythemia vera with hydroxyurea and pipobroman: final results of a randomized trial initiated in 1980. J Clin Oncol 2011;29:3907–13.
91. Kaplan ME, Mack K, Goldberg JD, et al. Long-term management of polycythemia vera with hydroxyurea: a progress report. Semin Hematol 1986;23:167–71.
92. Fruchtman SM, Mack K, Kaplan ME, et al. From efficacy to safety: a Polycythemia Vera Study group report on hydroxyurea in patients with polycythemia vera. Semin Hematol 1997;34:17–23.
93. Finazzi G, Caruso V, Marchioli R, et al. Acute leukemia in polycythemia vera: an analysis of 1638 patients enrolled in a prospective observational study. Blood 2005;105: 2664–70.
94. Barosi G, Mesa R, Finazzi G, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood 2013;121:4778–81.
95. Alvarez-Larran A, Pereira A, Cervantes F, et al. Assessment and prognostic value of the European LeukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia vera. Blood 2012;119:1363–9.
96. Stein BL, Tiu RV. Biological rationale and clinical use of interferon in the classical BCR-ABL-negative myeloproliferative neoplasms. J Interferon Cytokine Res 2013;33: 145–53.
97. Ludwig H, Cortelezzi A, Van Camp BG, et al. Treatment with recombinant interferon-alpha-2C: multiple myeloma and thrombocythaemia in myeloproliferative diseases. Oncology 1985;42 Suppl 1:19–25.
98. Silver RT. Long-term effects of the treatment of polycythemia vera with recombinant interferon-alpha. Cancer 2006;107:451–8.
99. Kiladjian JJ, Mesa RA, Hoffman R. The renaissance of interferon therapy for the treatment of myeloid malignancies. Blood 2011;117:4706–15.
100. Veronese FM, Mero A. The impact of PEGylation on biological therapies. BioDrugs 2008;22:315–29.
101. Kiladjian JJ, Cassinat B, Chevret S, et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood 2008;112:3065–72.
102. Turlure P, Cambier N, Roussel M, et al. Complete hematological, molecular and histological remissions without cytoreductive treatment lasting after pegylated-interferon {alpha}-2a (peg-IFN{alpha}-2a) therapy in polycythemia vera (PV): long term results of a phase 2 trial [abstract]. Blood 2011;118(21). Abstract 280.
103. Quintas-Cardama A, Kantarjian H, Manshouri T, et al. Pegylated interferon alfa-2a yields high rates of hematologic and molecular response in patients with advanced essential thrombocythemia and polycythemia vera. J Clin Oncol 2009;27:5418–24.
104. Quintas-Cardama A, Abdel-Wahab O, Manshouri T, et al. Molecular analysis of patients with polycythemia vera or essential thrombocythemia receiving pegylated interferon a-2a. Blood 2013;122:893–901.
105. Samuelsson J, Hasselbalch H, Bruserud O, et al. A phase II trial of pegylated interferon alpha-2b therapy for polycythemia vera and essential thrombocythemia: feasibility, clinical and biologic effects, and impact on quality of life. Cancer 2006;106:2397–405.
106. Jabbour E, Kantarjian H, Cortes J, et al. PEG-IFN-alpha-2b therapy in BCR-ABL-negative myeloproliferative disorders: final result of a phase 2 study. Cancer 2007; 110:2012–18.
107. Them NC, Bagienski K, Berg T, et al. Molecular responses and chromosomal aberrations in patients with polycythemia vera treated with peg-proline-interferon alpha-2b. Am J Hematol 2015;90:288–94.
108. Gisslinger H, Klade C, Georgiev P, et al. Final results from PROUD-PV a randomized controlled phase 3 trial comparing ropeginterferon alfa-2b to hydroxyurea in polycythemia vera patients [abstract]. Blood 2016;128(suppl 22). Abstract 475.
109. van Genderen PJ, van Vliet HH, Prins FJ, et al. Excessive prolongation of the bleeding time by aspirin in essential thrombocythemia is related to a decrease of large von Willebrand factor multimers in plasma. Ann Hematol 1997;75:215–20.
110. Cortelazzo S, Finazzi G, Ruggeri M, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med 1995;332:1132–7.
111. Harrison CN, Campbell PJ, Buck G, et al. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N Engl J Med 2005;353:33–45.
112. Gisslinger H, Gotic M, Holowiecki J, et al. Anagrelide compared with hydroxyurea in WHO-classified essential thrombocythemia: the ANAHYDRET Study, a randomized controlled trial. Blood 2013;121:1720–8.
113. Alvarado Y, Cortes J, Verstovsek S, et al. Pilot study of pegylated interferon-alpha 2b in patients with essential thrombocythemia. Cancer Chemother Pharmacol 2003;51:81–6.
114. Barosi G, Tefferi A, Barbui T, ad hoc committee ‘Definition of clinically relevant outcomes for contemporarily clinical trials in Ph-neg M. Do current response criteria in classical Ph-negative myeloproliferative neoplasms capture benefit for patients? Leukemia 2012;26:1148–9.
115. Bjorkholm M, Derolf AR, Hultcrantz M, et al. Treatment-related risk factors for transformation to acute myeloid leukemia and myelodysplastic syndromes in myeloproliferative neoplasms. J Clin Oncol 2011;29:2410–5.
116. Alvarez-Larran A, Martinez-Aviles L, Hernandez-Boluda JC, et al. Busulfan in patients with polycythemia vera or essential thrombocythemia refractory or intolerant to hydroxyurea. Ann Hematol 2014;93:2037–43.
117. Verstovsek S, Passamonti F, Rambaldi A, et al. A phase 2 study of ruxolitinib, an oral JAK1 and JAK2 Inhibitor, in patients with advanced polycythemia vera who are refractory or intolerant to hydroxyurea. Cancer 2014;120: 513–20.
118. Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib in polycythemia vera resistant to or intolerant of hydroxyurea. N Engl J Med 2015; 372:426–35.
119. Verstovsek S, Vannucchi AM, Griesshammer M, et al. Ruxolitinib versus best available therapy in patients with polycythemia vera: 80-week follow-up from the RESPONSE trial. Haematologica 2016;101:821–9.
120. Passamonti F, Griesshammer M, Palandri F, et al. Ruxolitinib for the treatment of inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): a randomised, open-label, phase 3b study. Lancet Oncol 2017;18:88–99.
121. Verstovsek S, Passamonti F, Rambaldi A, et al. Long-term results from a phase II open-label study of ruxolitinib in patients with essential thrombocythemia refractory to or intolerant of hydroxyurea [abstract]. Blood 2014;124. Abstract 1847.
122. Harrison CN, Mead AJ, Panchal A, et al. Ruxolitinib versus best available therapy for ET intolerant or resistant to hydroxycarbamide in a randomized trial. Blood 2017 Aug 9. pii: blood-2017-05-785790 .
123. Bose P, Verstovsek S. Drug development pipeline for myeloproliferative neoplasms: potential future impact on guidelines and management. J Natl Compr Canc Netw 2016;14:1613–24.
124. Cerquozzi S, Teffieri A. Blast transformation and fibrotic progression in polycythemia vera and essential thrombocythemia: a literature review of incidence and risk factors. Blood Cancer J 2015;Nov 13;5:e366.
125. Passamonti F, Rumi E, Caramella M, et al. A dynamic prognostic model to predict survival in post-polycythemia vera myelofibrosis. Blood 2008;111:3383–7.
126. Mesa RA, Verstovsek S, Cervantes F, et al. Primary myelofibrosis (PMF), post polycythemia vera myelofibrosis (post- PV MF), post essential thrombocythemia myelofibrosis (post-ET MF), blast phase PMF (PMF-BP): Consensus on terminology by the international working group for myelofibrosis research and treatment (IWG-MRT). Leuk Res 2007;31:737–40.
127. Rampal R, Mascarenhas J. Pathogenesis and management of acute myeloid leukemia that has evolved from a myeloproliferative neoplasm. Curr Opin Hematol 2014;21:65–71.
128. Chihara D, Kantarjian HM, Newberry KJ, et al. Survival outcome of patients with acute myeloid leukemia transformed from myeloproliferative neoplasms [abstract]. Blood 2016;128. Abstract 1940.
129. Tam CS, Nussenzveig RM, Popat U, et al. The natural history and treatment outcome of blast phase BCR-ABL-myeloproliferative neoplasms. Blood 2008;112:1628–37.
130. Kundranda MN, Tibes R, Mesa RA. Transformation of a chronic myeloproliferative neoplasm to acute myelogenous leukemia: does anything work? Curr Hematol Malig Rep 2012;7:78–86.
131. Badar T, Kantarjian HM, Ravandi F, et al. Therapeutic benefit of decitabine, a hypomethylating agent, in patients with high-risk primary myelofibrosis and myeloproliferative neoplasm in accelerated or blastic/acute myeloid leukemia phase. Leuk Res 2015;39:950–6.
132. Thepot S, Itzykson R, Seegers V, et al. Treatment of progression of Philadelphia-negative myeloproliferative neoplasms to myelodysplastic syndrome or acute myeloid leukemia by azacitidine: a report on 54 cases on the behalf of the Groupe Francophone des Myelodysplasies (GFM). Blood 2010;116:3735–42.
133. Pemmaraju N, Kantarjian H, Kadia T, et al. A phase I/II study of the Janus kinase (JAK)1 and 2 inhibitor ruxolitinib in patients with relapsed or refractory acute myeloid leukemia. Clin Lymphoma Myeloma Leuk 2015;15:171–6.
134. Rampal RK, Mascarenhas JO, Kosiorek HE, et al. Safety and efficacy of combined ruxolitinib and decitabine in patients with blast-phase MPN and post-MPN AML: results of a phase I study (Myeloproliferative Disorders Research Consortium 109 trial) [abstract]. Blood 2016;128. Abstract 1124.
135. Bose P, Verstovsek S, Gasior Y, et al. Phase I/II study of ruxolitinib (RUX) with decitabine (DAC) in patients with post-myeloproliferative neoplasm acute myeloid leukemia (post-MPN AML): phase I results [abstract]. Blood 2016;128. Abstract 4262.
Bacteremic sepsis in ALL linked to later cognitive issues
Bacteremic sepsis during acute lymphoblastic leukemia (ALL) treatment may contribute to neurocognitive dysfunction later in life, results of a cohort study suggest.
Pediatric ALL survivors who had sepsis while on treatment performed worse on measures of intelligence, attention, executive function, and processing speed than survivors with no sepsis history, according to study results.
Links between sepsis and impaired neurocognitive function found in this study have “practice-changing implications” for cancer survivors, investigators reported in JAMA Pediatrics.
“Prevention of infection, early recognition and appropriate management of sepsis, and preemptive neurocognitive interventions should be prioritized, because these might prevent or ameliorate neurologic damage,” said Joshua Wolf, MBBS, of St. Jude Children’s Research Hospital, Memphis, and the coauthors of the report.
The study included 212 children who, at a median age of 5 years, had received risk-adapted chemotherapy for ALL with no hematopoietic cell transplant or cranial irradiation.
Sixteen of the patients (7.5%) had a history of bacteremic sepsis during ALL therapy, according to retrospectively obtained data.
As a part of the study, all participants participated in neurocognitive testing, which was done at a median of 7.7 years after diagnosis.
Patients with a history of bacteremic sepsis performed poorly on multiple measures of neurocognitive function, as compared with all other participants, according to results of analyses that were adjusted for multiple potentially confounding factors, such as age, race, and leukemia risk category.
Although not all neurocognitive measures were significantly different between groups, survivors with a sepsis history performed worse on evaluations of spatial planning (difference, 0.78; 95% confidence interval, 0.57-1.00), verbal fluency (0.38; 95% CI, 0.14-0.62), and attention (0.63; 95% CI, 0.30-0.95), among other measures, investigators said.
This is believed to be the first published study looking at potential links between sepsis during ALL treatment and long-term neurocognitive dysfunction, investigators said. However, similar observations have been made in other patient populations, they added.
Exactly how sepsis might lead to neurocognitive deficits remains unclear. “In the population of children with cancer, these mechanisms might be augmented by increased blood-brain barrier permeability to neurotoxic chemotherapy drugs,” they said in their report.
Further study is needed to look at potential brain injury mechanisms, and to validate the current findings in other ALL patient cohorts, they concluded.
The study was supported by the National Institute of Mental Health, the National Cancer Institute, and the American Lebanese Syrian Associated Charities. The researchers reported having no conflicts of interest.
SOURCE: Cheung YT et al. JAMA Pediatr. 2018 Sep 24. doi:10.1001/jamapediatrics.2018.2500.
Bacteremic sepsis during acute lymphoblastic leukemia (ALL) treatment may contribute to neurocognitive dysfunction later in life, results of a cohort study suggest.
Pediatric ALL survivors who had sepsis while on treatment performed worse on measures of intelligence, attention, executive function, and processing speed than survivors with no sepsis history, according to study results.
Links between sepsis and impaired neurocognitive function found in this study have “practice-changing implications” for cancer survivors, investigators reported in JAMA Pediatrics.
“Prevention of infection, early recognition and appropriate management of sepsis, and preemptive neurocognitive interventions should be prioritized, because these might prevent or ameliorate neurologic damage,” said Joshua Wolf, MBBS, of St. Jude Children’s Research Hospital, Memphis, and the coauthors of the report.
The study included 212 children who, at a median age of 5 years, had received risk-adapted chemotherapy for ALL with no hematopoietic cell transplant or cranial irradiation.
Sixteen of the patients (7.5%) had a history of bacteremic sepsis during ALL therapy, according to retrospectively obtained data.
As a part of the study, all participants participated in neurocognitive testing, which was done at a median of 7.7 years after diagnosis.
Patients with a history of bacteremic sepsis performed poorly on multiple measures of neurocognitive function, as compared with all other participants, according to results of analyses that were adjusted for multiple potentially confounding factors, such as age, race, and leukemia risk category.
Although not all neurocognitive measures were significantly different between groups, survivors with a sepsis history performed worse on evaluations of spatial planning (difference, 0.78; 95% confidence interval, 0.57-1.00), verbal fluency (0.38; 95% CI, 0.14-0.62), and attention (0.63; 95% CI, 0.30-0.95), among other measures, investigators said.
This is believed to be the first published study looking at potential links between sepsis during ALL treatment and long-term neurocognitive dysfunction, investigators said. However, similar observations have been made in other patient populations, they added.
Exactly how sepsis might lead to neurocognitive deficits remains unclear. “In the population of children with cancer, these mechanisms might be augmented by increased blood-brain barrier permeability to neurotoxic chemotherapy drugs,” they said in their report.
Further study is needed to look at potential brain injury mechanisms, and to validate the current findings in other ALL patient cohorts, they concluded.
The study was supported by the National Institute of Mental Health, the National Cancer Institute, and the American Lebanese Syrian Associated Charities. The researchers reported having no conflicts of interest.
SOURCE: Cheung YT et al. JAMA Pediatr. 2018 Sep 24. doi:10.1001/jamapediatrics.2018.2500.
Bacteremic sepsis during acute lymphoblastic leukemia (ALL) treatment may contribute to neurocognitive dysfunction later in life, results of a cohort study suggest.
Pediatric ALL survivors who had sepsis while on treatment performed worse on measures of intelligence, attention, executive function, and processing speed than survivors with no sepsis history, according to study results.
Links between sepsis and impaired neurocognitive function found in this study have “practice-changing implications” for cancer survivors, investigators reported in JAMA Pediatrics.
“Prevention of infection, early recognition and appropriate management of sepsis, and preemptive neurocognitive interventions should be prioritized, because these might prevent or ameliorate neurologic damage,” said Joshua Wolf, MBBS, of St. Jude Children’s Research Hospital, Memphis, and the coauthors of the report.
The study included 212 children who, at a median age of 5 years, had received risk-adapted chemotherapy for ALL with no hematopoietic cell transplant or cranial irradiation.
Sixteen of the patients (7.5%) had a history of bacteremic sepsis during ALL therapy, according to retrospectively obtained data.
As a part of the study, all participants participated in neurocognitive testing, which was done at a median of 7.7 years after diagnosis.
Patients with a history of bacteremic sepsis performed poorly on multiple measures of neurocognitive function, as compared with all other participants, according to results of analyses that were adjusted for multiple potentially confounding factors, such as age, race, and leukemia risk category.
Although not all neurocognitive measures were significantly different between groups, survivors with a sepsis history performed worse on evaluations of spatial planning (difference, 0.78; 95% confidence interval, 0.57-1.00), verbal fluency (0.38; 95% CI, 0.14-0.62), and attention (0.63; 95% CI, 0.30-0.95), among other measures, investigators said.
This is believed to be the first published study looking at potential links between sepsis during ALL treatment and long-term neurocognitive dysfunction, investigators said. However, similar observations have been made in other patient populations, they added.
Exactly how sepsis might lead to neurocognitive deficits remains unclear. “In the population of children with cancer, these mechanisms might be augmented by increased blood-brain barrier permeability to neurotoxic chemotherapy drugs,” they said in their report.
Further study is needed to look at potential brain injury mechanisms, and to validate the current findings in other ALL patient cohorts, they concluded.
The study was supported by the National Institute of Mental Health, the National Cancer Institute, and the American Lebanese Syrian Associated Charities. The researchers reported having no conflicts of interest.
SOURCE: Cheung YT et al. JAMA Pediatr. 2018 Sep 24. doi:10.1001/jamapediatrics.2018.2500.
FROM JAMA PEDIATRICS
Key clinical point:
Major finding: ALL survivors with a sepsis history performed worse than did those with no sepsis history on evaluations of spatial planning (difference, 0.78), verbal fluency (0.38), and attention (0.63).
Study details: Prospective cohort study of 212 ALL survivors who underwent neurocognitive testing at a median of nearly 8 years after diagnosis.
Disclosures: The study was supported by the National Institute of Mental Health, the National Cancer Institute, and the American Lebanese Syrian Associated Charities. The researchers reported having no conflicts of interest.
Source: Cheung YT et al. JAMA Pediatr. 2018 Sep 24. doi:10.1001/jamapediatrics.2018.2500.