User login
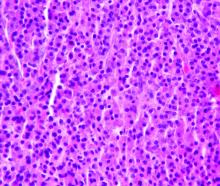
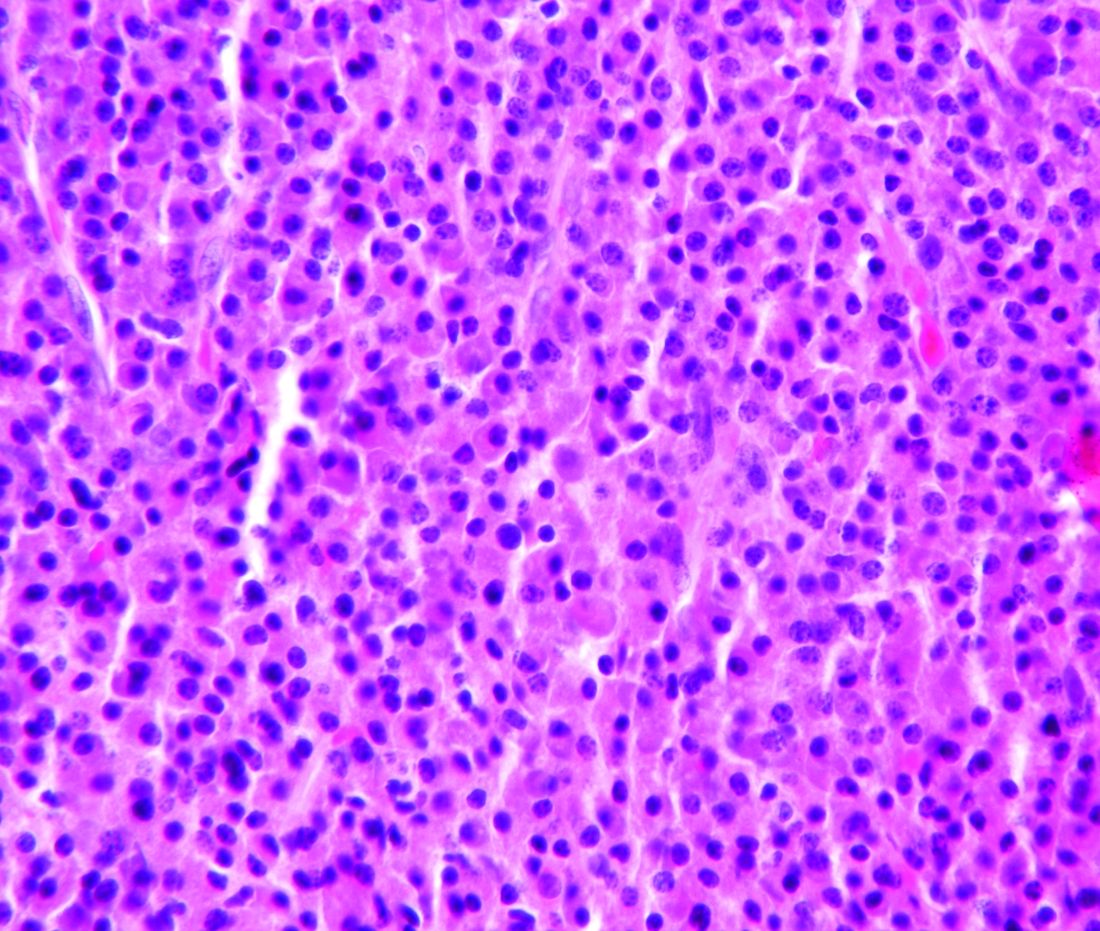
Adjuvant Pembrolizumab Improves Progression-Free Survival in Stage III Melanoma
Study Overview
Objective. To evaluate pembrolizumab as adjuvant therapy for patients with resected, high-risk stage III melanoma.
Design. International randomized phase 3 trial.
Setting and participants. This multicenter international trial enrolled patients who had histologically confirmed cutaneous melanoma with regional lymph node metastasis (stage IIIA, IIIB or IIIC with no in-transit metastases). Patients had to have undergone a complete regional lymphadenectomy within 13 weeks before the start of treatment. Exclusion criteria were: ECOG performance status score > 1, autoimmune disease, current steroid use, and prior systemic therapy for melanoma. All tumor samples from melanoma-positive lymph nodes were required to be sent to the central lab for evaluation of programmed death ligand 1 (PD-L1) expression; PD-L1 positivity was defined as a tumor proportion score (TPS) ≥ 1%.
Intervention. Patients were randomized in a 1:1 fashion and stratified according to stage and geographic region. Local pharmacies were aware of trial-group assignments. Patients received either an intravenous infusion of pembrolizumab 200 mg or placebo every 3 weeks for a total of 18 doses or until disease recurrence or unacceptable toxicity occurred. If recurrence was detected, patients were able to cross over.
Main outcome measures. The primary outcome was recurrence-free survival (RFS) in the intention-to-treat population and in the subgroup of PD-L1–positive patients. Secondary endpoints included distant metastasis–free survival, overall survival (OS), safety, and quality of life.
Results. A total of 1019 patients were recruited from 123 centers in 23 countries: 514 were assigned to the pembrolizumab group and 505 were assigned to the placebo group. In the pembrolizumab group, 70 patients (13.8%) discontinued treatment because of an adverse event; in 66 patients of these patients the event was deemed drug-related. In the placebo group, 11 (2.2%) patients discontinued treatment due to an adverse event. Discontinuation due to disease recurrence was seen in 109 (21%) patients in the pembrolizumab group and 179 (35.7%) patients in the placebo group. The median duration of follow up was 15 months. In the overall intention-to-treat population, the 12-month RFS rate was 75.4% in the pembrolizumab group versus 61% in the placebo group (P < 0.001). At 18 months the RFS rates were 71.4% and 53.2%, respectively. The 18-month incidence of distant metastasis at recurrence was lower in the pembrolizumab group (16.7% vs. 29.7%, hazard ratio [HR] 0.53; 95% confidence interval 0.37 to 0.76). In those who were PD-L1–positive (n = 853), the 12-month RFS rate was 77.1% in the pembrolizumab group versus 62.6% in the placebo group. PD-L1 status had no impact on pembrolizumab efficacy. The benefit of pembrolizumab was noted across all subgroups, and no difference was seen in patients with stage IIIA, IIIB or IIIC disease. The benefit of pembrolizumab was similar in those with macroscopic or microscopic nodal metastasis. BRAF status did not influence RFS between the pembrolizumab and placebo groups.
Adverse events of grade 3 or higher were seen in 14.7% and 3.4% of the pembrolizumab and placebo groups, respectively. Immune-related adverse events of any grade were noted in 37% of patients in the pembrolizumab group. There was 1 pembrolizumab-related death secondary to myositis. Grades 3 or 4 immune-related events in the pembrolizumab group occurred at a low rate, including colitis (2% and 0.2%), hypophysitis (0.6% and 0%), and type 1 diabetes mellitus (1% and 0%).
Conclusion. Adjuvant pembrolizumab for patients with high-risk stage III melanoma significantly improved RFS compared with placebo and should be considered as an option for adjuvant therapy in this patient population.
Commentary
Prior to the development of immune checkpoint inhibitors, high-dose interferon alfa was the sole option for adjuvant therapy in high-risk melanoma. Although adjuvant interferon alfa is associated with improvements in disease-free survival [1], it is also associated with significant toxicity, including myelosuppression, neurologic adverse effects, and hepatotoxicity. The development of checkpoint inhibition represents an important advancement in the management of patients with melanoma. In the previously reported EORTC 18071 trial, Eggermont and colleagues demonstrated that adjuvant therapy with the CTLA-4 antibody ipilimumab improved both RFS (41% vs. 30%) and OS (65% vs. 54%) at 5 years in patients with stage III melanoma [2]. In 2017, Weber and colleagues demonstrated superior RFS (70% vs. 60%) and a lower rate of grade 3 or 4 adverse events with adjuvant nivolumab compared to ipilimumab in the CheckMate-238 trial [3].
In the current article, Eggermont and colleagues present the results of the EORTC 1325/KEYNOTE-054 study comparing the use of the PD-1 antibody pembrolizumab to placebo in the adjuvant setting for stage III melanoma. This study demonstrated a 43% reduced risk of recurrence or death favoring the pembrolizumab group (HR 0.57; P < 0.001). The 12-month RFS was 75.4% in the pembrolizumab arm versus 61% in the placebo arm. Treatment-related adverse events of grade 3 or higher occurred more commonly in the pembrolizumab arm (14.7% vs. 3.4%), with approximately 7% of these patients experiencing a grade 3 or higher immune-related adverse event. The results of this study corroborate prior data on the efficacy of PD-1 inhibitors in melanoma. Also, the investigators assessed RFS based on patient’s PD-L1 status (positivity defined as TPS ≥ 1% ) as a co-primary endpoint, and found consistent efficacy regardless of PD-L1 expression, with a hazard ratio of 0.47 in the 116 patients who had no PD-L1 expression.
Although the results of this study demonstrate a significant increase in RFS associated with adjuvant pembrolizumab therapy, an OS benefit has not yet been demonstrated. As noted, the only adjuvant checkpoint inhibitor trial to demonstrate an OS advantage thus far is the EORTC 18071 study of ipilimumab. However, the toxicity profile of adjuvant ipilimumab makes it an unattractive option compared to the PD-1 inhibitors. Which of the PD-1 inhibitors should be the treatment of choice for adjuvant therapy remains unclear, although it is worth noting that only nivolumab was compared to the best alternate therapy, ipilimumab [3]. It is also important to note that EORTC 1325/KEYNOTE-054 included patients with stage IIIA disease (N1a disease with at least 1 micrometastasis > 1 mm) or stage IIIB or IIIC without in-transit metastases, while CheckMate-238 did not include stage IIIA patients. Thus, for stage IIIA patients pembrolizumab remains the only PD-1 inhibitor with randomized data demonstrating a benefit.
Applications for Clinical Practice
The results from the EORTC 1325/KEYNOTE-054 study demonstrate a 43% reduction in the risk of progression or death with the use of adjuvant pembrolizumab in patients with stage III melanoma. As of now, the only checkpoint inhibitor to demonstrate an improvement in OS is ipilimumab, and whether the RFS benefit of both pembrolizumab and nivolumab will translate into an OS benefit is yet to be demonstrated.
—Daniel Isaac, DO, MS
1. Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon alfa-2b adjuvant therapy of high-risk cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 1996;14:7–17.
2. Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med 2016;375:1845–55.
3. Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 2017;377:1824–35.
Study Overview
Objective. To evaluate pembrolizumab as adjuvant therapy for patients with resected, high-risk stage III melanoma.
Design. International randomized phase 3 trial.
Setting and participants. This multicenter international trial enrolled patients who had histologically confirmed cutaneous melanoma with regional lymph node metastasis (stage IIIA, IIIB or IIIC with no in-transit metastases). Patients had to have undergone a complete regional lymphadenectomy within 13 weeks before the start of treatment. Exclusion criteria were: ECOG performance status score > 1, autoimmune disease, current steroid use, and prior systemic therapy for melanoma. All tumor samples from melanoma-positive lymph nodes were required to be sent to the central lab for evaluation of programmed death ligand 1 (PD-L1) expression; PD-L1 positivity was defined as a tumor proportion score (TPS) ≥ 1%.
Intervention. Patients were randomized in a 1:1 fashion and stratified according to stage and geographic region. Local pharmacies were aware of trial-group assignments. Patients received either an intravenous infusion of pembrolizumab 200 mg or placebo every 3 weeks for a total of 18 doses or until disease recurrence or unacceptable toxicity occurred. If recurrence was detected, patients were able to cross over.
Main outcome measures. The primary outcome was recurrence-free survival (RFS) in the intention-to-treat population and in the subgroup of PD-L1–positive patients. Secondary endpoints included distant metastasis–free survival, overall survival (OS), safety, and quality of life.
Results. A total of 1019 patients were recruited from 123 centers in 23 countries: 514 were assigned to the pembrolizumab group and 505 were assigned to the placebo group. In the pembrolizumab group, 70 patients (13.8%) discontinued treatment because of an adverse event; in 66 patients of these patients the event was deemed drug-related. In the placebo group, 11 (2.2%) patients discontinued treatment due to an adverse event. Discontinuation due to disease recurrence was seen in 109 (21%) patients in the pembrolizumab group and 179 (35.7%) patients in the placebo group. The median duration of follow up was 15 months. In the overall intention-to-treat population, the 12-month RFS rate was 75.4% in the pembrolizumab group versus 61% in the placebo group (P < 0.001). At 18 months the RFS rates were 71.4% and 53.2%, respectively. The 18-month incidence of distant metastasis at recurrence was lower in the pembrolizumab group (16.7% vs. 29.7%, hazard ratio [HR] 0.53; 95% confidence interval 0.37 to 0.76). In those who were PD-L1–positive (n = 853), the 12-month RFS rate was 77.1% in the pembrolizumab group versus 62.6% in the placebo group. PD-L1 status had no impact on pembrolizumab efficacy. The benefit of pembrolizumab was noted across all subgroups, and no difference was seen in patients with stage IIIA, IIIB or IIIC disease. The benefit of pembrolizumab was similar in those with macroscopic or microscopic nodal metastasis. BRAF status did not influence RFS between the pembrolizumab and placebo groups.
Adverse events of grade 3 or higher were seen in 14.7% and 3.4% of the pembrolizumab and placebo groups, respectively. Immune-related adverse events of any grade were noted in 37% of patients in the pembrolizumab group. There was 1 pembrolizumab-related death secondary to myositis. Grades 3 or 4 immune-related events in the pembrolizumab group occurred at a low rate, including colitis (2% and 0.2%), hypophysitis (0.6% and 0%), and type 1 diabetes mellitus (1% and 0%).
Conclusion. Adjuvant pembrolizumab for patients with high-risk stage III melanoma significantly improved RFS compared with placebo and should be considered as an option for adjuvant therapy in this patient population.
Commentary
Prior to the development of immune checkpoint inhibitors, high-dose interferon alfa was the sole option for adjuvant therapy in high-risk melanoma. Although adjuvant interferon alfa is associated with improvements in disease-free survival [1], it is also associated with significant toxicity, including myelosuppression, neurologic adverse effects, and hepatotoxicity. The development of checkpoint inhibition represents an important advancement in the management of patients with melanoma. In the previously reported EORTC 18071 trial, Eggermont and colleagues demonstrated that adjuvant therapy with the CTLA-4 antibody ipilimumab improved both RFS (41% vs. 30%) and OS (65% vs. 54%) at 5 years in patients with stage III melanoma [2]. In 2017, Weber and colleagues demonstrated superior RFS (70% vs. 60%) and a lower rate of grade 3 or 4 adverse events with adjuvant nivolumab compared to ipilimumab in the CheckMate-238 trial [3].
In the current article, Eggermont and colleagues present the results of the EORTC 1325/KEYNOTE-054 study comparing the use of the PD-1 antibody pembrolizumab to placebo in the adjuvant setting for stage III melanoma. This study demonstrated a 43% reduced risk of recurrence or death favoring the pembrolizumab group (HR 0.57; P < 0.001). The 12-month RFS was 75.4% in the pembrolizumab arm versus 61% in the placebo arm. Treatment-related adverse events of grade 3 or higher occurred more commonly in the pembrolizumab arm (14.7% vs. 3.4%), with approximately 7% of these patients experiencing a grade 3 or higher immune-related adverse event. The results of this study corroborate prior data on the efficacy of PD-1 inhibitors in melanoma. Also, the investigators assessed RFS based on patient’s PD-L1 status (positivity defined as TPS ≥ 1% ) as a co-primary endpoint, and found consistent efficacy regardless of PD-L1 expression, with a hazard ratio of 0.47 in the 116 patients who had no PD-L1 expression.
Although the results of this study demonstrate a significant increase in RFS associated with adjuvant pembrolizumab therapy, an OS benefit has not yet been demonstrated. As noted, the only adjuvant checkpoint inhibitor trial to demonstrate an OS advantage thus far is the EORTC 18071 study of ipilimumab. However, the toxicity profile of adjuvant ipilimumab makes it an unattractive option compared to the PD-1 inhibitors. Which of the PD-1 inhibitors should be the treatment of choice for adjuvant therapy remains unclear, although it is worth noting that only nivolumab was compared to the best alternate therapy, ipilimumab [3]. It is also important to note that EORTC 1325/KEYNOTE-054 included patients with stage IIIA disease (N1a disease with at least 1 micrometastasis > 1 mm) or stage IIIB or IIIC without in-transit metastases, while CheckMate-238 did not include stage IIIA patients. Thus, for stage IIIA patients pembrolizumab remains the only PD-1 inhibitor with randomized data demonstrating a benefit.
Applications for Clinical Practice
The results from the EORTC 1325/KEYNOTE-054 study demonstrate a 43% reduction in the risk of progression or death with the use of adjuvant pembrolizumab in patients with stage III melanoma. As of now, the only checkpoint inhibitor to demonstrate an improvement in OS is ipilimumab, and whether the RFS benefit of both pembrolizumab and nivolumab will translate into an OS benefit is yet to be demonstrated.
—Daniel Isaac, DO, MS
Study Overview
Objective. To evaluate pembrolizumab as adjuvant therapy for patients with resected, high-risk stage III melanoma.
Design. International randomized phase 3 trial.
Setting and participants. This multicenter international trial enrolled patients who had histologically confirmed cutaneous melanoma with regional lymph node metastasis (stage IIIA, IIIB or IIIC with no in-transit metastases). Patients had to have undergone a complete regional lymphadenectomy within 13 weeks before the start of treatment. Exclusion criteria were: ECOG performance status score > 1, autoimmune disease, current steroid use, and prior systemic therapy for melanoma. All tumor samples from melanoma-positive lymph nodes were required to be sent to the central lab for evaluation of programmed death ligand 1 (PD-L1) expression; PD-L1 positivity was defined as a tumor proportion score (TPS) ≥ 1%.
Intervention. Patients were randomized in a 1:1 fashion and stratified according to stage and geographic region. Local pharmacies were aware of trial-group assignments. Patients received either an intravenous infusion of pembrolizumab 200 mg or placebo every 3 weeks for a total of 18 doses or until disease recurrence or unacceptable toxicity occurred. If recurrence was detected, patients were able to cross over.
Main outcome measures. The primary outcome was recurrence-free survival (RFS) in the intention-to-treat population and in the subgroup of PD-L1–positive patients. Secondary endpoints included distant metastasis–free survival, overall survival (OS), safety, and quality of life.
Results. A total of 1019 patients were recruited from 123 centers in 23 countries: 514 were assigned to the pembrolizumab group and 505 were assigned to the placebo group. In the pembrolizumab group, 70 patients (13.8%) discontinued treatment because of an adverse event; in 66 patients of these patients the event was deemed drug-related. In the placebo group, 11 (2.2%) patients discontinued treatment due to an adverse event. Discontinuation due to disease recurrence was seen in 109 (21%) patients in the pembrolizumab group and 179 (35.7%) patients in the placebo group. The median duration of follow up was 15 months. In the overall intention-to-treat population, the 12-month RFS rate was 75.4% in the pembrolizumab group versus 61% in the placebo group (P < 0.001). At 18 months the RFS rates were 71.4% and 53.2%, respectively. The 18-month incidence of distant metastasis at recurrence was lower in the pembrolizumab group (16.7% vs. 29.7%, hazard ratio [HR] 0.53; 95% confidence interval 0.37 to 0.76). In those who were PD-L1–positive (n = 853), the 12-month RFS rate was 77.1% in the pembrolizumab group versus 62.6% in the placebo group. PD-L1 status had no impact on pembrolizumab efficacy. The benefit of pembrolizumab was noted across all subgroups, and no difference was seen in patients with stage IIIA, IIIB or IIIC disease. The benefit of pembrolizumab was similar in those with macroscopic or microscopic nodal metastasis. BRAF status did not influence RFS between the pembrolizumab and placebo groups.
Adverse events of grade 3 or higher were seen in 14.7% and 3.4% of the pembrolizumab and placebo groups, respectively. Immune-related adverse events of any grade were noted in 37% of patients in the pembrolizumab group. There was 1 pembrolizumab-related death secondary to myositis. Grades 3 or 4 immune-related events in the pembrolizumab group occurred at a low rate, including colitis (2% and 0.2%), hypophysitis (0.6% and 0%), and type 1 diabetes mellitus (1% and 0%).
Conclusion. Adjuvant pembrolizumab for patients with high-risk stage III melanoma significantly improved RFS compared with placebo and should be considered as an option for adjuvant therapy in this patient population.
Commentary
Prior to the development of immune checkpoint inhibitors, high-dose interferon alfa was the sole option for adjuvant therapy in high-risk melanoma. Although adjuvant interferon alfa is associated with improvements in disease-free survival [1], it is also associated with significant toxicity, including myelosuppression, neurologic adverse effects, and hepatotoxicity. The development of checkpoint inhibition represents an important advancement in the management of patients with melanoma. In the previously reported EORTC 18071 trial, Eggermont and colleagues demonstrated that adjuvant therapy with the CTLA-4 antibody ipilimumab improved both RFS (41% vs. 30%) and OS (65% vs. 54%) at 5 years in patients with stage III melanoma [2]. In 2017, Weber and colleagues demonstrated superior RFS (70% vs. 60%) and a lower rate of grade 3 or 4 adverse events with adjuvant nivolumab compared to ipilimumab in the CheckMate-238 trial [3].
In the current article, Eggermont and colleagues present the results of the EORTC 1325/KEYNOTE-054 study comparing the use of the PD-1 antibody pembrolizumab to placebo in the adjuvant setting for stage III melanoma. This study demonstrated a 43% reduced risk of recurrence or death favoring the pembrolizumab group (HR 0.57; P < 0.001). The 12-month RFS was 75.4% in the pembrolizumab arm versus 61% in the placebo arm. Treatment-related adverse events of grade 3 or higher occurred more commonly in the pembrolizumab arm (14.7% vs. 3.4%), with approximately 7% of these patients experiencing a grade 3 or higher immune-related adverse event. The results of this study corroborate prior data on the efficacy of PD-1 inhibitors in melanoma. Also, the investigators assessed RFS based on patient’s PD-L1 status (positivity defined as TPS ≥ 1% ) as a co-primary endpoint, and found consistent efficacy regardless of PD-L1 expression, with a hazard ratio of 0.47 in the 116 patients who had no PD-L1 expression.
Although the results of this study demonstrate a significant increase in RFS associated with adjuvant pembrolizumab therapy, an OS benefit has not yet been demonstrated. As noted, the only adjuvant checkpoint inhibitor trial to demonstrate an OS advantage thus far is the EORTC 18071 study of ipilimumab. However, the toxicity profile of adjuvant ipilimumab makes it an unattractive option compared to the PD-1 inhibitors. Which of the PD-1 inhibitors should be the treatment of choice for adjuvant therapy remains unclear, although it is worth noting that only nivolumab was compared to the best alternate therapy, ipilimumab [3]. It is also important to note that EORTC 1325/KEYNOTE-054 included patients with stage IIIA disease (N1a disease with at least 1 micrometastasis > 1 mm) or stage IIIB or IIIC without in-transit metastases, while CheckMate-238 did not include stage IIIA patients. Thus, for stage IIIA patients pembrolizumab remains the only PD-1 inhibitor with randomized data demonstrating a benefit.
Applications for Clinical Practice
The results from the EORTC 1325/KEYNOTE-054 study demonstrate a 43% reduction in the risk of progression or death with the use of adjuvant pembrolizumab in patients with stage III melanoma. As of now, the only checkpoint inhibitor to demonstrate an improvement in OS is ipilimumab, and whether the RFS benefit of both pembrolizumab and nivolumab will translate into an OS benefit is yet to be demonstrated.
—Daniel Isaac, DO, MS
1. Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon alfa-2b adjuvant therapy of high-risk cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 1996;14:7–17.
2. Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med 2016;375:1845–55.
3. Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 2017;377:1824–35.
1. Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon alfa-2b adjuvant therapy of high-risk cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 1996;14:7–17.
2. Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med 2016;375:1845–55.
3. Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 2017;377:1824–35.
Rivaroxaban bonus: Early unmasking of occult GI cancers
MUNICH – The increased risk of major GI bleeding documented with dual-antiplatelet therapy using rivaroxaban and aspirin when compared with aspirin alone for vascular protection in the previously reported massive COMPASS trial may turn out to be a blessing in disguise.
“Among COMPASS patients with vascular disease receiving long-term antithrombotic therapy, more than 1 in 5 new diagnoses of cancer are preceded by bleeding. GI and GU bleeding are powerful and relatively specific predictors of new GI and GU cancer diagnoses, respectively, and more than 75% of these cancers diagnosed after bleeding are diagnosed within 6 months of the bleed,” John W. Eikelboom, MD, reported at the annual congress of the European Society of Cardiology.
These findings strongly suggest that rivaroxaban (Xarelto), a direct-acting oral anticoagulant (DOAC), may be unmasking occult GI and GU cancers earlier than the malignancies would otherwise have declared themselves.
“Although overall cancer rates were similar in the three treatment groups [rivaroxaban at 2.5 mg twice daily plus aspirin at 100 mg/day, rivaroxaban monotherapy at 5 mg twice daily, or aspirin alone at 100 mg/day], the early increase in GI bleeding with rivaroxaban-based therapy resulted in earlier diagnosis of GI cancer in these patients. By reducing major cardiovascular events and mortality, the combination of rivaroxaban and aspirin already produces a clear net benefit, and by unmasking GI cancers at an earlier stage, the combination could potentially lead to the added benefit of improved GI cancer outcomes,” commented Dr. Eikelboom, a hematologist at McMaster University in Hamilton, Ont., and lead investigator of the previously published COMPASS trial (N Engl J Med. 2017 Oct 5;377[14]:1319-30).
This possibility that unmasking of occult GI cancer might result in improved survival deserves to be a high research priority in light of the enormous death toll caused by colorectal cancer. Planned longer-term follow-up of the COMPASS participants should be helpful in this regard, he added.
This new COMPASS finding effectively makes a silk purse out of a sow’s ear. When the primary outcomes of COMPASS were announced, many physicians reasoned that if the other commercially available DOACs also outperform warfarin but don’t pose a significantly increased threat of serious bleeding events, why not preferentially turn to them rather than rivaroxaban in patients with high HAS-BLED scores? But now rivaroxaban’s increased GI bleeding risk has begun to look like a potentially important advantage.
As previously reported, COMPASS participants on rivaroxaban plus aspirin had a 3.1% major bleeding rate as defined according to modified International Society on Thrombosis and Hemostasis criteria, for a statistically significant 70% increase in risk, compared with the 1.9% rate in patients on aspirin alone. Rivaroxaban monotherapy also was associated with increased bleeding risk. Of note, most of the excess in bleeding involved GI bleeding, and it was front-loaded during the first year of the trial. Also, reassuringly, there was no increased incidence of intracranial or fatal bleeding in patients on rivaroxaban.
A total of 1,082 patients were diagnosed with a new cancer during 23 months of follow-up. Nearly a quarter (23%) of the new GI cancers and 45% of the new GU cancers were diagnosed after bleeding from those sites.
The incidence of GI cancer diagnosed after GI bleeding was 7.8%, whereas patients with no prior GI bleeding had a mere 0.9% rate of newly diagnosed GI cancer during the study period. Thus, roughly 1 out of every 12 cases of GI bleeding was associated with diagnosis of a new GI cancer, and GI bleeding was associated with a 13-fold increased risk of subsequent GI cancer diagnosis. Put another way, the number of cases of GI bleeding occurring in patients on rivaroxaban that needed to be investigated in order to find one new GI cancer was 12.
Similarly, 13% of COMPASS participants who developed GU bleeding were subsequently diagnosed with a new GU cancer, compared with just 0.3% of subjects without GU bleeding. That translates into an 83.4-fold increased risk of GU cancer in patients with GU bleeding.
In contrast, the incidence of non-GI cancer following a GI bleed was 1.5%, while the rate was 1.0% in patients with no prior GI bleeding.
“That relationship was much weaker, with an odds ratio of 1.77, indicating that the relationship between GI bleeding and GI cancer is not only very strong but it’s rather specific,” according to Dr. Eikelboom.
Of GI cancers associated with a prior major GI bleed, 77% were diagnosed within 6 months following the bleed, as were nearly 89% of GU cancers. Another 9%-10% were diagnosed 6-12 months after the bleeding event.
“The implication for clinical practice is certainly that in patients who have GI or GU bleeding while receiving antithrombotic therapy, we should conduct a vigorous search for underlying cancer in the same organ system,” he concluded.
Discussant Lars C. Wallentin, MD, concurred. He called it a wake-up call for cardiologists to broaden their horizons and recognize that their elderly patients with vascular disease also are at substantial competing risk for major noncardiovascular diseases – and that his colleagues may have an important role to play in earlier cancer diagnoses.
He and his coinvestigators in the RE-LY (Randomized Evaluation of Long-Term Anticoagulant Therapy) study made a similar point in their investigation of 18,113 patients with atrial fibrillation on oral anticoagulation for stroke protection. In that randomized trial of dabigatran (Pradaxa) versus warfarin, roughly 1 in 12 major GI bleeding events was found to be related to an occult colorectal or gastric cancer (Clin Gastroenterol Hepatol. 2017 May;15[5]:682-90).
“The data are very consistent. The message to cardiologists is that no bleeding should be disregarded in patients on oral anticoagulation,” declared Dr. Wallentin, professor of cardiology at Uppsala (Sweden) University.
COMPASS was sponsored by Bayer. Dr. Eikelboom reported receiving research grants from that company and more than half a dozen others.
MUNICH – The increased risk of major GI bleeding documented with dual-antiplatelet therapy using rivaroxaban and aspirin when compared with aspirin alone for vascular protection in the previously reported massive COMPASS trial may turn out to be a blessing in disguise.
“Among COMPASS patients with vascular disease receiving long-term antithrombotic therapy, more than 1 in 5 new diagnoses of cancer are preceded by bleeding. GI and GU bleeding are powerful and relatively specific predictors of new GI and GU cancer diagnoses, respectively, and more than 75% of these cancers diagnosed after bleeding are diagnosed within 6 months of the bleed,” John W. Eikelboom, MD, reported at the annual congress of the European Society of Cardiology.
These findings strongly suggest that rivaroxaban (Xarelto), a direct-acting oral anticoagulant (DOAC), may be unmasking occult GI and GU cancers earlier than the malignancies would otherwise have declared themselves.
“Although overall cancer rates were similar in the three treatment groups [rivaroxaban at 2.5 mg twice daily plus aspirin at 100 mg/day, rivaroxaban monotherapy at 5 mg twice daily, or aspirin alone at 100 mg/day], the early increase in GI bleeding with rivaroxaban-based therapy resulted in earlier diagnosis of GI cancer in these patients. By reducing major cardiovascular events and mortality, the combination of rivaroxaban and aspirin already produces a clear net benefit, and by unmasking GI cancers at an earlier stage, the combination could potentially lead to the added benefit of improved GI cancer outcomes,” commented Dr. Eikelboom, a hematologist at McMaster University in Hamilton, Ont., and lead investigator of the previously published COMPASS trial (N Engl J Med. 2017 Oct 5;377[14]:1319-30).
This possibility that unmasking of occult GI cancer might result in improved survival deserves to be a high research priority in light of the enormous death toll caused by colorectal cancer. Planned longer-term follow-up of the COMPASS participants should be helpful in this regard, he added.
This new COMPASS finding effectively makes a silk purse out of a sow’s ear. When the primary outcomes of COMPASS were announced, many physicians reasoned that if the other commercially available DOACs also outperform warfarin but don’t pose a significantly increased threat of serious bleeding events, why not preferentially turn to them rather than rivaroxaban in patients with high HAS-BLED scores? But now rivaroxaban’s increased GI bleeding risk has begun to look like a potentially important advantage.
As previously reported, COMPASS participants on rivaroxaban plus aspirin had a 3.1% major bleeding rate as defined according to modified International Society on Thrombosis and Hemostasis criteria, for a statistically significant 70% increase in risk, compared with the 1.9% rate in patients on aspirin alone. Rivaroxaban monotherapy also was associated with increased bleeding risk. Of note, most of the excess in bleeding involved GI bleeding, and it was front-loaded during the first year of the trial. Also, reassuringly, there was no increased incidence of intracranial or fatal bleeding in patients on rivaroxaban.
A total of 1,082 patients were diagnosed with a new cancer during 23 months of follow-up. Nearly a quarter (23%) of the new GI cancers and 45% of the new GU cancers were diagnosed after bleeding from those sites.
The incidence of GI cancer diagnosed after GI bleeding was 7.8%, whereas patients with no prior GI bleeding had a mere 0.9% rate of newly diagnosed GI cancer during the study period. Thus, roughly 1 out of every 12 cases of GI bleeding was associated with diagnosis of a new GI cancer, and GI bleeding was associated with a 13-fold increased risk of subsequent GI cancer diagnosis. Put another way, the number of cases of GI bleeding occurring in patients on rivaroxaban that needed to be investigated in order to find one new GI cancer was 12.
Similarly, 13% of COMPASS participants who developed GU bleeding were subsequently diagnosed with a new GU cancer, compared with just 0.3% of subjects without GU bleeding. That translates into an 83.4-fold increased risk of GU cancer in patients with GU bleeding.
In contrast, the incidence of non-GI cancer following a GI bleed was 1.5%, while the rate was 1.0% in patients with no prior GI bleeding.
“That relationship was much weaker, with an odds ratio of 1.77, indicating that the relationship between GI bleeding and GI cancer is not only very strong but it’s rather specific,” according to Dr. Eikelboom.
Of GI cancers associated with a prior major GI bleed, 77% were diagnosed within 6 months following the bleed, as were nearly 89% of GU cancers. Another 9%-10% were diagnosed 6-12 months after the bleeding event.
“The implication for clinical practice is certainly that in patients who have GI or GU bleeding while receiving antithrombotic therapy, we should conduct a vigorous search for underlying cancer in the same organ system,” he concluded.
Discussant Lars C. Wallentin, MD, concurred. He called it a wake-up call for cardiologists to broaden their horizons and recognize that their elderly patients with vascular disease also are at substantial competing risk for major noncardiovascular diseases – and that his colleagues may have an important role to play in earlier cancer diagnoses.
He and his coinvestigators in the RE-LY (Randomized Evaluation of Long-Term Anticoagulant Therapy) study made a similar point in their investigation of 18,113 patients with atrial fibrillation on oral anticoagulation for stroke protection. In that randomized trial of dabigatran (Pradaxa) versus warfarin, roughly 1 in 12 major GI bleeding events was found to be related to an occult colorectal or gastric cancer (Clin Gastroenterol Hepatol. 2017 May;15[5]:682-90).
“The data are very consistent. The message to cardiologists is that no bleeding should be disregarded in patients on oral anticoagulation,” declared Dr. Wallentin, professor of cardiology at Uppsala (Sweden) University.
COMPASS was sponsored by Bayer. Dr. Eikelboom reported receiving research grants from that company and more than half a dozen others.
MUNICH – The increased risk of major GI bleeding documented with dual-antiplatelet therapy using rivaroxaban and aspirin when compared with aspirin alone for vascular protection in the previously reported massive COMPASS trial may turn out to be a blessing in disguise.
“Among COMPASS patients with vascular disease receiving long-term antithrombotic therapy, more than 1 in 5 new diagnoses of cancer are preceded by bleeding. GI and GU bleeding are powerful and relatively specific predictors of new GI and GU cancer diagnoses, respectively, and more than 75% of these cancers diagnosed after bleeding are diagnosed within 6 months of the bleed,” John W. Eikelboom, MD, reported at the annual congress of the European Society of Cardiology.
These findings strongly suggest that rivaroxaban (Xarelto), a direct-acting oral anticoagulant (DOAC), may be unmasking occult GI and GU cancers earlier than the malignancies would otherwise have declared themselves.
“Although overall cancer rates were similar in the three treatment groups [rivaroxaban at 2.5 mg twice daily plus aspirin at 100 mg/day, rivaroxaban monotherapy at 5 mg twice daily, or aspirin alone at 100 mg/day], the early increase in GI bleeding with rivaroxaban-based therapy resulted in earlier diagnosis of GI cancer in these patients. By reducing major cardiovascular events and mortality, the combination of rivaroxaban and aspirin already produces a clear net benefit, and by unmasking GI cancers at an earlier stage, the combination could potentially lead to the added benefit of improved GI cancer outcomes,” commented Dr. Eikelboom, a hematologist at McMaster University in Hamilton, Ont., and lead investigator of the previously published COMPASS trial (N Engl J Med. 2017 Oct 5;377[14]:1319-30).
This possibility that unmasking of occult GI cancer might result in improved survival deserves to be a high research priority in light of the enormous death toll caused by colorectal cancer. Planned longer-term follow-up of the COMPASS participants should be helpful in this regard, he added.
This new COMPASS finding effectively makes a silk purse out of a sow’s ear. When the primary outcomes of COMPASS were announced, many physicians reasoned that if the other commercially available DOACs also outperform warfarin but don’t pose a significantly increased threat of serious bleeding events, why not preferentially turn to them rather than rivaroxaban in patients with high HAS-BLED scores? But now rivaroxaban’s increased GI bleeding risk has begun to look like a potentially important advantage.
As previously reported, COMPASS participants on rivaroxaban plus aspirin had a 3.1% major bleeding rate as defined according to modified International Society on Thrombosis and Hemostasis criteria, for a statistically significant 70% increase in risk, compared with the 1.9% rate in patients on aspirin alone. Rivaroxaban monotherapy also was associated with increased bleeding risk. Of note, most of the excess in bleeding involved GI bleeding, and it was front-loaded during the first year of the trial. Also, reassuringly, there was no increased incidence of intracranial or fatal bleeding in patients on rivaroxaban.
A total of 1,082 patients were diagnosed with a new cancer during 23 months of follow-up. Nearly a quarter (23%) of the new GI cancers and 45% of the new GU cancers were diagnosed after bleeding from those sites.
The incidence of GI cancer diagnosed after GI bleeding was 7.8%, whereas patients with no prior GI bleeding had a mere 0.9% rate of newly diagnosed GI cancer during the study period. Thus, roughly 1 out of every 12 cases of GI bleeding was associated with diagnosis of a new GI cancer, and GI bleeding was associated with a 13-fold increased risk of subsequent GI cancer diagnosis. Put another way, the number of cases of GI bleeding occurring in patients on rivaroxaban that needed to be investigated in order to find one new GI cancer was 12.
Similarly, 13% of COMPASS participants who developed GU bleeding were subsequently diagnosed with a new GU cancer, compared with just 0.3% of subjects without GU bleeding. That translates into an 83.4-fold increased risk of GU cancer in patients with GU bleeding.
In contrast, the incidence of non-GI cancer following a GI bleed was 1.5%, while the rate was 1.0% in patients with no prior GI bleeding.
“That relationship was much weaker, with an odds ratio of 1.77, indicating that the relationship between GI bleeding and GI cancer is not only very strong but it’s rather specific,” according to Dr. Eikelboom.
Of GI cancers associated with a prior major GI bleed, 77% were diagnosed within 6 months following the bleed, as were nearly 89% of GU cancers. Another 9%-10% were diagnosed 6-12 months after the bleeding event.
“The implication for clinical practice is certainly that in patients who have GI or GU bleeding while receiving antithrombotic therapy, we should conduct a vigorous search for underlying cancer in the same organ system,” he concluded.
Discussant Lars C. Wallentin, MD, concurred. He called it a wake-up call for cardiologists to broaden their horizons and recognize that their elderly patients with vascular disease also are at substantial competing risk for major noncardiovascular diseases – and that his colleagues may have an important role to play in earlier cancer diagnoses.
He and his coinvestigators in the RE-LY (Randomized Evaluation of Long-Term Anticoagulant Therapy) study made a similar point in their investigation of 18,113 patients with atrial fibrillation on oral anticoagulation for stroke protection. In that randomized trial of dabigatran (Pradaxa) versus warfarin, roughly 1 in 12 major GI bleeding events was found to be related to an occult colorectal or gastric cancer (Clin Gastroenterol Hepatol. 2017 May;15[5]:682-90).
“The data are very consistent. The message to cardiologists is that no bleeding should be disregarded in patients on oral anticoagulation,” declared Dr. Wallentin, professor of cardiology at Uppsala (Sweden) University.
COMPASS was sponsored by Bayer. Dr. Eikelboom reported receiving research grants from that company and more than half a dozen others.
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point:
Major finding: Among patients on rivaroxaban, 1 in 12 GI bleeding events was associated with an occult GI cancer.
Study details: This was a secondary analysis looking at cancers in COMPASS, a randomized trial of more than 27,000 patients on rivaroxaban and/or aspirin for vascular prevention.
Disclosures: The presenter reported receiving research grants from Bayer, which sponsored the COMPASS trial.
Researchers propose new acute leukemia subtypes
An extensive analysis of mixed phenotype acute leukemia (MPAL) has led to new insights that may have implications for disease classification and treatment.
Researchers believe they have identified new subtypes of MPAL that should be included in the World Health Organization classification for acute leukemia.
Each of these subtypes share genomic characteristics with other acute leukemias, which suggests they might respond to treatments that are already in use.
This research also has shed light on how MPAL evolves and appears to provide an explanation for why MPAL displays characteristics of both acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).
“ALL and AML have very different treatments, but MPAL has features of both, so the question of how best to treat patients with MPAL has been challenging the leukemia community worldwide, and long-term survival of patients has been poor,” said study author Charles G. Mullighan, MD, of St. Jude Children’s Research Hospital in Memphis, Tenn.
In the current study, published in Nature, Dr. Mullighan and his colleagues used whole-genome, whole-exome, and RNA sequencing to analyze 115 samples from pediatric patients with MPAL.
The analysis revealed mutations that define the two most common subtypes of MPAL – B/myeloid and T/myeloid – and suggested these subtypes share similarities with other leukemia subtypes.
The researchers found that 48% of B/myeloid MPAL cases carried rearrangements in ZNF384, a characteristic that is also found in cases of B-cell ALL. In fact, the team said the gene expression profiles of ZNF384r B-ALL and ZNF384r MPAL were indistinguishable.
“That is biologically and clinically important,” Dr. Mullighan said. “The findings suggest the ZNF384 rearrangement defines a distinct leukemia subtype, and the alteration should be used to guide treatment.”
The researchers noted that patients with ZNF384r exhibited higher FLT3 expression than that of patients with other types of B/myeloid or T/myeloid MPAL, so patients with ZNF384r MPAL might respond well to treatment with a FLT3 inhibitor.
This study also showed that cases of B/myeloid MPAL without ZNF384r shared genomic features with other B-ALL subtypes, such as Ph-like B-ALL.
In addition, the analysis showed that T/myeloid MPAL and early T-cell precursor ALL have similar gene expression profiles.
The team identified several genes that were mutated at similar frequencies in T/myeloid MPAL and early T-cell precursor ALL, including WT1, ETV6, EZH2, and FLT3.
WT1 was the most frequently mutated transcription factor gene in T/myeloid MPAL.
Based on these findings, the researchers said the WHO classification of acute leukemia should be updated to include: ZNF384r acute leukemia (either B-ALL or MPAL), WT1-mutant T/myeloid MPAL, and Ph-like B/myeloid MPAL.
This research was supported by the National Cancer Institute, the National Institutes of Health, Cookies for Kids’ Cancer, and other organizations. The researchers reported having no competing interests.
SOURCE: Alexander TB et al. Nature. 2018 Sep 12. doi: 10.1038/s41586-018-0436-0.
An extensive analysis of mixed phenotype acute leukemia (MPAL) has led to new insights that may have implications for disease classification and treatment.
Researchers believe they have identified new subtypes of MPAL that should be included in the World Health Organization classification for acute leukemia.
Each of these subtypes share genomic characteristics with other acute leukemias, which suggests they might respond to treatments that are already in use.
This research also has shed light on how MPAL evolves and appears to provide an explanation for why MPAL displays characteristics of both acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).
“ALL and AML have very different treatments, but MPAL has features of both, so the question of how best to treat patients with MPAL has been challenging the leukemia community worldwide, and long-term survival of patients has been poor,” said study author Charles G. Mullighan, MD, of St. Jude Children’s Research Hospital in Memphis, Tenn.
In the current study, published in Nature, Dr. Mullighan and his colleagues used whole-genome, whole-exome, and RNA sequencing to analyze 115 samples from pediatric patients with MPAL.
The analysis revealed mutations that define the two most common subtypes of MPAL – B/myeloid and T/myeloid – and suggested these subtypes share similarities with other leukemia subtypes.
The researchers found that 48% of B/myeloid MPAL cases carried rearrangements in ZNF384, a characteristic that is also found in cases of B-cell ALL. In fact, the team said the gene expression profiles of ZNF384r B-ALL and ZNF384r MPAL were indistinguishable.
“That is biologically and clinically important,” Dr. Mullighan said. “The findings suggest the ZNF384 rearrangement defines a distinct leukemia subtype, and the alteration should be used to guide treatment.”
The researchers noted that patients with ZNF384r exhibited higher FLT3 expression than that of patients with other types of B/myeloid or T/myeloid MPAL, so patients with ZNF384r MPAL might respond well to treatment with a FLT3 inhibitor.
This study also showed that cases of B/myeloid MPAL without ZNF384r shared genomic features with other B-ALL subtypes, such as Ph-like B-ALL.
In addition, the analysis showed that T/myeloid MPAL and early T-cell precursor ALL have similar gene expression profiles.
The team identified several genes that were mutated at similar frequencies in T/myeloid MPAL and early T-cell precursor ALL, including WT1, ETV6, EZH2, and FLT3.
WT1 was the most frequently mutated transcription factor gene in T/myeloid MPAL.
Based on these findings, the researchers said the WHO classification of acute leukemia should be updated to include: ZNF384r acute leukemia (either B-ALL or MPAL), WT1-mutant T/myeloid MPAL, and Ph-like B/myeloid MPAL.
This research was supported by the National Cancer Institute, the National Institutes of Health, Cookies for Kids’ Cancer, and other organizations. The researchers reported having no competing interests.
SOURCE: Alexander TB et al. Nature. 2018 Sep 12. doi: 10.1038/s41586-018-0436-0.
An extensive analysis of mixed phenotype acute leukemia (MPAL) has led to new insights that may have implications for disease classification and treatment.
Researchers believe they have identified new subtypes of MPAL that should be included in the World Health Organization classification for acute leukemia.
Each of these subtypes share genomic characteristics with other acute leukemias, which suggests they might respond to treatments that are already in use.
This research also has shed light on how MPAL evolves and appears to provide an explanation for why MPAL displays characteristics of both acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).
“ALL and AML have very different treatments, but MPAL has features of both, so the question of how best to treat patients with MPAL has been challenging the leukemia community worldwide, and long-term survival of patients has been poor,” said study author Charles G. Mullighan, MD, of St. Jude Children’s Research Hospital in Memphis, Tenn.
In the current study, published in Nature, Dr. Mullighan and his colleagues used whole-genome, whole-exome, and RNA sequencing to analyze 115 samples from pediatric patients with MPAL.
The analysis revealed mutations that define the two most common subtypes of MPAL – B/myeloid and T/myeloid – and suggested these subtypes share similarities with other leukemia subtypes.
The researchers found that 48% of B/myeloid MPAL cases carried rearrangements in ZNF384, a characteristic that is also found in cases of B-cell ALL. In fact, the team said the gene expression profiles of ZNF384r B-ALL and ZNF384r MPAL were indistinguishable.
“That is biologically and clinically important,” Dr. Mullighan said. “The findings suggest the ZNF384 rearrangement defines a distinct leukemia subtype, and the alteration should be used to guide treatment.”
The researchers noted that patients with ZNF384r exhibited higher FLT3 expression than that of patients with other types of B/myeloid or T/myeloid MPAL, so patients with ZNF384r MPAL might respond well to treatment with a FLT3 inhibitor.
This study also showed that cases of B/myeloid MPAL without ZNF384r shared genomic features with other B-ALL subtypes, such as Ph-like B-ALL.
In addition, the analysis showed that T/myeloid MPAL and early T-cell precursor ALL have similar gene expression profiles.
The team identified several genes that were mutated at similar frequencies in T/myeloid MPAL and early T-cell precursor ALL, including WT1, ETV6, EZH2, and FLT3.
WT1 was the most frequently mutated transcription factor gene in T/myeloid MPAL.
Based on these findings, the researchers said the WHO classification of acute leukemia should be updated to include: ZNF384r acute leukemia (either B-ALL or MPAL), WT1-mutant T/myeloid MPAL, and Ph-like B/myeloid MPAL.
This research was supported by the National Cancer Institute, the National Institutes of Health, Cookies for Kids’ Cancer, and other organizations. The researchers reported having no competing interests.
SOURCE: Alexander TB et al. Nature. 2018 Sep 12. doi: 10.1038/s41586-018-0436-0.
FROM NATURE
Key clinical point:
Major finding: In total, 48% of B/myeloid MPAL cases carried rearrangements in ZNF384, a characteristic that is also found in cases of B-cell ALL.
Study details: Whole-genome, -exome, and RNA sequencing of 115 samples from pediatric patients with MPAL.
Disclosures: This research was supported by the National Cancer Institute and other organizations. The researchers reported having no competing interests.
Source: Alexander TB et al. Nature. 2018 Sep 12. doi: 10.1038/s41586-018-0436-0.
ASCO addresses financial barriers to cancer clinical trials
The American Society of Clinical Oncology (ASCO) has released a policy statement addressing financial barriers that may prevent cancer patients from participating in clinical trials.
The four main recommendations in ASCO’s policy statement are:
- Payers should create clear, consistent, streamlined, and transparent policies regarding clinical trial coverage.
- Patients should receive easy-to-understand information about potential out-of-pocket costs.
- “Ethically appropriate” financial compensation for out-of-pocket costs should be allowed.
- Researchers should be incentivized to investigate and “better characterize” costs incurred by cancer patients in clinical trials as well as identify ways to “mitigate the risk of trial-associated financial hardship.”
ASCO’s full policy statement, “Addressing Financial Barriers to Patient Participation in Clinical Trials,” is available on the Journal of Clinical Oncology website.
SOURCE: Winkfield KM et al. J Clin Oncol. 2018 Sep 13:JCO1801132. doi: 10.1200/JCO.18.01132.
The American Society of Clinical Oncology (ASCO) has released a policy statement addressing financial barriers that may prevent cancer patients from participating in clinical trials.
The four main recommendations in ASCO’s policy statement are:
- Payers should create clear, consistent, streamlined, and transparent policies regarding clinical trial coverage.
- Patients should receive easy-to-understand information about potential out-of-pocket costs.
- “Ethically appropriate” financial compensation for out-of-pocket costs should be allowed.
- Researchers should be incentivized to investigate and “better characterize” costs incurred by cancer patients in clinical trials as well as identify ways to “mitigate the risk of trial-associated financial hardship.”
ASCO’s full policy statement, “Addressing Financial Barriers to Patient Participation in Clinical Trials,” is available on the Journal of Clinical Oncology website.
SOURCE: Winkfield KM et al. J Clin Oncol. 2018 Sep 13:JCO1801132. doi: 10.1200/JCO.18.01132.
The American Society of Clinical Oncology (ASCO) has released a policy statement addressing financial barriers that may prevent cancer patients from participating in clinical trials.
The four main recommendations in ASCO’s policy statement are:
- Payers should create clear, consistent, streamlined, and transparent policies regarding clinical trial coverage.
- Patients should receive easy-to-understand information about potential out-of-pocket costs.
- “Ethically appropriate” financial compensation for out-of-pocket costs should be allowed.
- Researchers should be incentivized to investigate and “better characterize” costs incurred by cancer patients in clinical trials as well as identify ways to “mitigate the risk of trial-associated financial hardship.”
ASCO’s full policy statement, “Addressing Financial Barriers to Patient Participation in Clinical Trials,” is available on the Journal of Clinical Oncology website.
SOURCE: Winkfield KM et al. J Clin Oncol. 2018 Sep 13:JCO1801132. doi: 10.1200/JCO.18.01132.
FROM JOURNAL OF CLINICAL ONCOLOGY
Standardization of the Discharge Process for Inpatient Hematology and Oncology
Purpose/Rationale: To standardize the discharge process for the hematology/oncology inpatient service at Hines VA Hospital to improve the transition of care
Background: The landmark 1999 report from the Institute of Medicine, To Err is Human, identified the impact of medical error on mortality and morbidity. Medical errors tend to occur during transitions of care. At Hines VA Hospital, a multidisciplinary team delivers specialized care to veterans on the hematology/oncology service. However, resident physicians staffing the inpatient hematology/oncology service may be unfamiliar with the unique needs of the service and population. Currently there is no standardized discharge process in place. Prior studies have demonstrated improved outcomes following standardization of the discharge process for hematology patients. The authors aim to develop and implement a standardized discharge process to minimize risk for medical error.
Method/Approach: A multidisciplinary team of hematology and oncology staff was formed, including attending physicians, fellows, residents, advanced practice nurses, registered nurses, clinical pharmacists, and patient care coordinators, and several interviews were conducted. A standardized discharge process was developed in the form of guidelines and expectations. These include an explanation of unique features of the hematology/oncology service and expectations of medication reconciliation with emphasis placed on antiemetics, antimicrobial prophylaxis, and bowel regimen when appropriate, ambulatory hematology/oncology follow up within 1-2 weeks, primary care followup, communication with ambulatory hematology/oncology physician, written discharge instructions, and bedside teaching when appropriate. The standardized process will be taught to rotating resident physicians in the form of both online orientation and an in-person orientation. Outcome measures were identified including key components of medication reconciliation, time to hematology & oncology clinic visit, time to primary care visit, communication of discharge with outpatient hematology/oncology physician, and 30-day readmission rate.
Conclusions: All patients discharged during the twomonth period prior to and all patients discharged after the implementation of the standardized process will be reviewed; the above-mentioned variables will be recorded. Outcomes will be compared. Interim multidisciplinary team focus group meetings will be held every quarter to review and refine the process.
Purpose/Rationale: To standardize the discharge process for the hematology/oncology inpatient service at Hines VA Hospital to improve the transition of care
Background: The landmark 1999 report from the Institute of Medicine, To Err is Human, identified the impact of medical error on mortality and morbidity. Medical errors tend to occur during transitions of care. At Hines VA Hospital, a multidisciplinary team delivers specialized care to veterans on the hematology/oncology service. However, resident physicians staffing the inpatient hematology/oncology service may be unfamiliar with the unique needs of the service and population. Currently there is no standardized discharge process in place. Prior studies have demonstrated improved outcomes following standardization of the discharge process for hematology patients. The authors aim to develop and implement a standardized discharge process to minimize risk for medical error.
Method/Approach: A multidisciplinary team of hematology and oncology staff was formed, including attending physicians, fellows, residents, advanced practice nurses, registered nurses, clinical pharmacists, and patient care coordinators, and several interviews were conducted. A standardized discharge process was developed in the form of guidelines and expectations. These include an explanation of unique features of the hematology/oncology service and expectations of medication reconciliation with emphasis placed on antiemetics, antimicrobial prophylaxis, and bowel regimen when appropriate, ambulatory hematology/oncology follow up within 1-2 weeks, primary care followup, communication with ambulatory hematology/oncology physician, written discharge instructions, and bedside teaching when appropriate. The standardized process will be taught to rotating resident physicians in the form of both online orientation and an in-person orientation. Outcome measures were identified including key components of medication reconciliation, time to hematology & oncology clinic visit, time to primary care visit, communication of discharge with outpatient hematology/oncology physician, and 30-day readmission rate.
Conclusions: All patients discharged during the twomonth period prior to and all patients discharged after the implementation of the standardized process will be reviewed; the above-mentioned variables will be recorded. Outcomes will be compared. Interim multidisciplinary team focus group meetings will be held every quarter to review and refine the process.
Purpose/Rationale: To standardize the discharge process for the hematology/oncology inpatient service at Hines VA Hospital to improve the transition of care
Background: The landmark 1999 report from the Institute of Medicine, To Err is Human, identified the impact of medical error on mortality and morbidity. Medical errors tend to occur during transitions of care. At Hines VA Hospital, a multidisciplinary team delivers specialized care to veterans on the hematology/oncology service. However, resident physicians staffing the inpatient hematology/oncology service may be unfamiliar with the unique needs of the service and population. Currently there is no standardized discharge process in place. Prior studies have demonstrated improved outcomes following standardization of the discharge process for hematology patients. The authors aim to develop and implement a standardized discharge process to minimize risk for medical error.
Method/Approach: A multidisciplinary team of hematology and oncology staff was formed, including attending physicians, fellows, residents, advanced practice nurses, registered nurses, clinical pharmacists, and patient care coordinators, and several interviews were conducted. A standardized discharge process was developed in the form of guidelines and expectations. These include an explanation of unique features of the hematology/oncology service and expectations of medication reconciliation with emphasis placed on antiemetics, antimicrobial prophylaxis, and bowel regimen when appropriate, ambulatory hematology/oncology follow up within 1-2 weeks, primary care followup, communication with ambulatory hematology/oncology physician, written discharge instructions, and bedside teaching when appropriate. The standardized process will be taught to rotating resident physicians in the form of both online orientation and an in-person orientation. Outcome measures were identified including key components of medication reconciliation, time to hematology & oncology clinic visit, time to primary care visit, communication of discharge with outpatient hematology/oncology physician, and 30-day readmission rate.
Conclusions: All patients discharged during the twomonth period prior to and all patients discharged after the implementation of the standardized process will be reviewed; the above-mentioned variables will be recorded. Outcomes will be compared. Interim multidisciplinary team focus group meetings will be held every quarter to review and refine the process.
Children with BCP-ALL show inflammatory marker differences at birth
Patients who develop B-cell precursor acute lymphoblastic leukemia (BCP-ALL) in childhood may have dysregulated immune function at birth, according to a study published in Cancer Research.
Investigators evaluated neonatal concentrations of inflammatory markers and found significant differences between children who were later diagnosed with BCP-ALL and leukemia-free control subjects.
“Our findings suggest that children who develop ALL are immunologically disparate already at birth,” said study author Signe Holst Søegaard, PhD, of Statens Serum Institut in Copenhagen. “This may link to other observations suggesting that children who develop ALL respond differently to infections in early childhood, potentially promoting subsequent genetic events required for transformation to ALL, or speculations that they are unable to eliminate preleukemic cells.”
She noted that the study could not determine if the associations shown are causal or consequential so further studies will be needed both to confirm the findings and identify the underlying mechanisms.
For this study, Dr. Søegaard and her colleagues measured concentrations of 10 inflammatory markers on neonatal dried blood spots from 178 patients with BCP-ALL and 178 matched controls. The patients were diagnosed with BCP-ALL at ages 1-9 years.
Compared with controls, children who later developed BCP-ALL had significantly different neonatal concentrations of eight inflammatory markers.
Concentrations of interleukin (IL)–8, soluble receptor sIL-6R alpha, transforming growth factor (TGF)–beta 1, monocyte chemotactic protein (MCP)–1, and C-reactive protein (CRP) were significantly lower among the BCP-ALL patients.
On the other hand, concentrations of IL-6, IL-17, and IL-18 were significantly higher among BCP-ALL patients than controls.
The investigators noted that IL-10 concentrations were too low for accurate measurement in all patients and controls. Additionally, a “large proportion” of patients and controls had IL-6 and IL-17 concentrations that were below the limit of detection.
“We also demonstrated that several previously shown ALL risk factors – namely, birth order, gestational age, and sex – were associated with the neonatal concentrations of inflammatory markers,” Dr. Søegaard said. “These findings raise the interesting possibility that the effects of some known ALL risk factors partly act through prenatal programming of immune function.”
The investigators found that increasing birth order was associated with significantly higher IL-18 and lower CRP concentrations.
Increasing gestational age was associated with significantly lower sIL-6R alpha and TGF-beta 1 concentrations and higher CRP concentrations. And boys had significantly lower sIL-6R alpha and IL-8 concentrations and higher CRP concentrations than girls.
However, none of the following factors were significantly associated with concentrations of inflammatory biomarkers: maternal age at delivery, maternal hospital contact attributable to infection during pregnancy, maternal prescription for antimicrobials during pregnancy, birth weight, and mode of delivery.
“Our findings underline the role the child’s baseline immune characteristics may play in the development of ALL,” Dr. Søegaard said. “However, we cannot yet use our research results to predict who will develop childhood ALL.”
The study was sponsored by the Dagmar Marshall Foundation, the A.P. Møller Foundation, the Danish Childhood Cancer Foundation, the Arvid Nilsson Foundation, and the Danish Cancer Research Foundation. The investigators reported having no conflicts of interest.
SOURCE: Søegaard SH et al. Cancer Res. 2018;78(18);5458-63.
Patients who develop B-cell precursor acute lymphoblastic leukemia (BCP-ALL) in childhood may have dysregulated immune function at birth, according to a study published in Cancer Research.
Investigators evaluated neonatal concentrations of inflammatory markers and found significant differences between children who were later diagnosed with BCP-ALL and leukemia-free control subjects.
“Our findings suggest that children who develop ALL are immunologically disparate already at birth,” said study author Signe Holst Søegaard, PhD, of Statens Serum Institut in Copenhagen. “This may link to other observations suggesting that children who develop ALL respond differently to infections in early childhood, potentially promoting subsequent genetic events required for transformation to ALL, or speculations that they are unable to eliminate preleukemic cells.”
She noted that the study could not determine if the associations shown are causal or consequential so further studies will be needed both to confirm the findings and identify the underlying mechanisms.
For this study, Dr. Søegaard and her colleagues measured concentrations of 10 inflammatory markers on neonatal dried blood spots from 178 patients with BCP-ALL and 178 matched controls. The patients were diagnosed with BCP-ALL at ages 1-9 years.
Compared with controls, children who later developed BCP-ALL had significantly different neonatal concentrations of eight inflammatory markers.
Concentrations of interleukin (IL)–8, soluble receptor sIL-6R alpha, transforming growth factor (TGF)–beta 1, monocyte chemotactic protein (MCP)–1, and C-reactive protein (CRP) were significantly lower among the BCP-ALL patients.
On the other hand, concentrations of IL-6, IL-17, and IL-18 were significantly higher among BCP-ALL patients than controls.
The investigators noted that IL-10 concentrations were too low for accurate measurement in all patients and controls. Additionally, a “large proportion” of patients and controls had IL-6 and IL-17 concentrations that were below the limit of detection.
“We also demonstrated that several previously shown ALL risk factors – namely, birth order, gestational age, and sex – were associated with the neonatal concentrations of inflammatory markers,” Dr. Søegaard said. “These findings raise the interesting possibility that the effects of some known ALL risk factors partly act through prenatal programming of immune function.”
The investigators found that increasing birth order was associated with significantly higher IL-18 and lower CRP concentrations.
Increasing gestational age was associated with significantly lower sIL-6R alpha and TGF-beta 1 concentrations and higher CRP concentrations. And boys had significantly lower sIL-6R alpha and IL-8 concentrations and higher CRP concentrations than girls.
However, none of the following factors were significantly associated with concentrations of inflammatory biomarkers: maternal age at delivery, maternal hospital contact attributable to infection during pregnancy, maternal prescription for antimicrobials during pregnancy, birth weight, and mode of delivery.
“Our findings underline the role the child’s baseline immune characteristics may play in the development of ALL,” Dr. Søegaard said. “However, we cannot yet use our research results to predict who will develop childhood ALL.”
The study was sponsored by the Dagmar Marshall Foundation, the A.P. Møller Foundation, the Danish Childhood Cancer Foundation, the Arvid Nilsson Foundation, and the Danish Cancer Research Foundation. The investigators reported having no conflicts of interest.
SOURCE: Søegaard SH et al. Cancer Res. 2018;78(18);5458-63.
Patients who develop B-cell precursor acute lymphoblastic leukemia (BCP-ALL) in childhood may have dysregulated immune function at birth, according to a study published in Cancer Research.
Investigators evaluated neonatal concentrations of inflammatory markers and found significant differences between children who were later diagnosed with BCP-ALL and leukemia-free control subjects.
“Our findings suggest that children who develop ALL are immunologically disparate already at birth,” said study author Signe Holst Søegaard, PhD, of Statens Serum Institut in Copenhagen. “This may link to other observations suggesting that children who develop ALL respond differently to infections in early childhood, potentially promoting subsequent genetic events required for transformation to ALL, or speculations that they are unable to eliminate preleukemic cells.”
She noted that the study could not determine if the associations shown are causal or consequential so further studies will be needed both to confirm the findings and identify the underlying mechanisms.
For this study, Dr. Søegaard and her colleagues measured concentrations of 10 inflammatory markers on neonatal dried blood spots from 178 patients with BCP-ALL and 178 matched controls. The patients were diagnosed with BCP-ALL at ages 1-9 years.
Compared with controls, children who later developed BCP-ALL had significantly different neonatal concentrations of eight inflammatory markers.
Concentrations of interleukin (IL)–8, soluble receptor sIL-6R alpha, transforming growth factor (TGF)–beta 1, monocyte chemotactic protein (MCP)–1, and C-reactive protein (CRP) were significantly lower among the BCP-ALL patients.
On the other hand, concentrations of IL-6, IL-17, and IL-18 were significantly higher among BCP-ALL patients than controls.
The investigators noted that IL-10 concentrations were too low for accurate measurement in all patients and controls. Additionally, a “large proportion” of patients and controls had IL-6 and IL-17 concentrations that were below the limit of detection.
“We also demonstrated that several previously shown ALL risk factors – namely, birth order, gestational age, and sex – were associated with the neonatal concentrations of inflammatory markers,” Dr. Søegaard said. “These findings raise the interesting possibility that the effects of some known ALL risk factors partly act through prenatal programming of immune function.”
The investigators found that increasing birth order was associated with significantly higher IL-18 and lower CRP concentrations.
Increasing gestational age was associated with significantly lower sIL-6R alpha and TGF-beta 1 concentrations and higher CRP concentrations. And boys had significantly lower sIL-6R alpha and IL-8 concentrations and higher CRP concentrations than girls.
However, none of the following factors were significantly associated with concentrations of inflammatory biomarkers: maternal age at delivery, maternal hospital contact attributable to infection during pregnancy, maternal prescription for antimicrobials during pregnancy, birth weight, and mode of delivery.
“Our findings underline the role the child’s baseline immune characteristics may play in the development of ALL,” Dr. Søegaard said. “However, we cannot yet use our research results to predict who will develop childhood ALL.”
The study was sponsored by the Dagmar Marshall Foundation, the A.P. Møller Foundation, the Danish Childhood Cancer Foundation, the Arvid Nilsson Foundation, and the Danish Cancer Research Foundation. The investigators reported having no conflicts of interest.
SOURCE: Søegaard SH et al. Cancer Res. 2018;78(18);5458-63.
FROM CANCER RESEARCH
Key clinical point:
Major finding: Neonatal concentrations of some inflammatory markers were significantly different between BCP-ALL patients and controls.
Study details: Ten markers were measured in 178 patients with BCP-ALL and 178 matched controls.
Disclosures: The study was sponsored by the Dagmar Marshall Foundation, the A.P. Møller Foundation, the Danish Childhood Cancer Foundation, the Arvid Nilsson Foundation, and the Danish Cancer Research Foundation. The investigators reported having no conflicts of interest.
Source: Søegaard SH et al. Cancer Res. 2018;78(18);5458-63.
STORM trial shows response in penta-refractory myeloma
Treatment with selinexor and low-dose dexamethasone can provide a “meaningful clinical benefit” in patients with penta-refractory multiple myeloma, according to the principal investigator of the STORM trial.
Updated results from this phase 2 trial showed that selinexor and low-dose dexamethasone produced an overall response rate of 26.2% and a clinical benefit rate of 39.3%. The median progression-free survival was 3.7 months and the median overall survival was 8.6 months.
The trial’s principal investigator, Sundar Jagannath, MBBS, of the Icahn School of Medicine at Mount Sinai, New York, presented these results at the annual meeting of the Society of Hematologic Oncology.
“The additional phase 2b clinical results… are very encouraging for the patients suffering from penta-refractory multiple myeloma and their families,” Dr. Jagannath said in a statement. “Of particular significance, for the nearly 40% of patients who had a minimal response or better, the median survival was 15.6 months, which provided the opportunity for a meaningful clinical benefit for patients on the STORM [Selinexor Treatment of Refractory Myeloma] study.”
STORM (NCT02336815) included 122 patients with penta-refractory multiple myeloma. They had previously received bortezomib, carfilzomib, lenalidomide, pomalidomide, daratumumab, alkylating agents, and glucocorticoids. Their disease was refractory to glucocorticoids, at least one proteasome inhibitor, at least one immunomodulatory drug, daratumumab, and their most recent therapy.
The patients had received a median of seven prior treatment regimens. Their median age was 65 years, a little more than half were men, and more than half had high-risk cytogenetics. Patients received oral selinexor at 80 mg twice weekly plus dexamethasone at 20 mg twice weekly until disease progression.Two patients (1.6%) achieved stringent complete responses. They also had minimal residual disease negativity, one at the level of 1 x 10–6 and one at 1 x 10–4.
Very good partial responses were seen in 4.9% of patients, 19.7% had partial responses, 13.1% had minimal responses (MRs), and 39.3% had stable disease. Progressive disease occurred in 13.1% of patients; 8.2% were not evaluable for response.
The overall response rate (partial response or better) was 26.2%, the clinical benefit rate (MR or better) was 39.3%, and the disease control rate (stable disease or better) was 78.7%.
The median duration of response was 4.4 months. The median progression-free survival was 3.7 months overall, 4.6 months in patients with an MR or better, and 1.1 months in patients who had progressive disease or were not evaluable.
The median overall survival was 8.6 months for the entire cohort. Overall survival was 15.6 months in patients with an MR or better and 1.7 months in patients who had progressive disease or were not evaluable (P less than .0001).
The “most important” grade 3/4 adverse events, according to Dr. Jagannath, were thrombocytopenia (53.7%), anemia (29.3%), fatigue (22.8%), hyponatremia (16.3%), nausea (9.8%), diarrhea (6.5%), anorexia (3.3%), and emesis (3.3%). A total of 23 patients (19.5%) discontinued treatment because of a related adverse.
This study was sponsored by Karyopharm Therapeutics. Dr. Jagannath reported relationships with Karyopharm, Janssen, Celgene, Amgen, and GlaxoSmithKline.
SOURCE: Jagannath S et al. SOHO 2018, Abstract MM-255
Treatment with selinexor and low-dose dexamethasone can provide a “meaningful clinical benefit” in patients with penta-refractory multiple myeloma, according to the principal investigator of the STORM trial.
Updated results from this phase 2 trial showed that selinexor and low-dose dexamethasone produced an overall response rate of 26.2% and a clinical benefit rate of 39.3%. The median progression-free survival was 3.7 months and the median overall survival was 8.6 months.
The trial’s principal investigator, Sundar Jagannath, MBBS, of the Icahn School of Medicine at Mount Sinai, New York, presented these results at the annual meeting of the Society of Hematologic Oncology.
“The additional phase 2b clinical results… are very encouraging for the patients suffering from penta-refractory multiple myeloma and their families,” Dr. Jagannath said in a statement. “Of particular significance, for the nearly 40% of patients who had a minimal response or better, the median survival was 15.6 months, which provided the opportunity for a meaningful clinical benefit for patients on the STORM [Selinexor Treatment of Refractory Myeloma] study.”
STORM (NCT02336815) included 122 patients with penta-refractory multiple myeloma. They had previously received bortezomib, carfilzomib, lenalidomide, pomalidomide, daratumumab, alkylating agents, and glucocorticoids. Their disease was refractory to glucocorticoids, at least one proteasome inhibitor, at least one immunomodulatory drug, daratumumab, and their most recent therapy.
The patients had received a median of seven prior treatment regimens. Their median age was 65 years, a little more than half were men, and more than half had high-risk cytogenetics. Patients received oral selinexor at 80 mg twice weekly plus dexamethasone at 20 mg twice weekly until disease progression.Two patients (1.6%) achieved stringent complete responses. They also had minimal residual disease negativity, one at the level of 1 x 10–6 and one at 1 x 10–4.
Very good partial responses were seen in 4.9% of patients, 19.7% had partial responses, 13.1% had minimal responses (MRs), and 39.3% had stable disease. Progressive disease occurred in 13.1% of patients; 8.2% were not evaluable for response.
The overall response rate (partial response or better) was 26.2%, the clinical benefit rate (MR or better) was 39.3%, and the disease control rate (stable disease or better) was 78.7%.
The median duration of response was 4.4 months. The median progression-free survival was 3.7 months overall, 4.6 months in patients with an MR or better, and 1.1 months in patients who had progressive disease or were not evaluable.
The median overall survival was 8.6 months for the entire cohort. Overall survival was 15.6 months in patients with an MR or better and 1.7 months in patients who had progressive disease or were not evaluable (P less than .0001).
The “most important” grade 3/4 adverse events, according to Dr. Jagannath, were thrombocytopenia (53.7%), anemia (29.3%), fatigue (22.8%), hyponatremia (16.3%), nausea (9.8%), diarrhea (6.5%), anorexia (3.3%), and emesis (3.3%). A total of 23 patients (19.5%) discontinued treatment because of a related adverse.
This study was sponsored by Karyopharm Therapeutics. Dr. Jagannath reported relationships with Karyopharm, Janssen, Celgene, Amgen, and GlaxoSmithKline.
SOURCE: Jagannath S et al. SOHO 2018, Abstract MM-255
Treatment with selinexor and low-dose dexamethasone can provide a “meaningful clinical benefit” in patients with penta-refractory multiple myeloma, according to the principal investigator of the STORM trial.
Updated results from this phase 2 trial showed that selinexor and low-dose dexamethasone produced an overall response rate of 26.2% and a clinical benefit rate of 39.3%. The median progression-free survival was 3.7 months and the median overall survival was 8.6 months.
The trial’s principal investigator, Sundar Jagannath, MBBS, of the Icahn School of Medicine at Mount Sinai, New York, presented these results at the annual meeting of the Society of Hematologic Oncology.
“The additional phase 2b clinical results… are very encouraging for the patients suffering from penta-refractory multiple myeloma and their families,” Dr. Jagannath said in a statement. “Of particular significance, for the nearly 40% of patients who had a minimal response or better, the median survival was 15.6 months, which provided the opportunity for a meaningful clinical benefit for patients on the STORM [Selinexor Treatment of Refractory Myeloma] study.”
STORM (NCT02336815) included 122 patients with penta-refractory multiple myeloma. They had previously received bortezomib, carfilzomib, lenalidomide, pomalidomide, daratumumab, alkylating agents, and glucocorticoids. Their disease was refractory to glucocorticoids, at least one proteasome inhibitor, at least one immunomodulatory drug, daratumumab, and their most recent therapy.
The patients had received a median of seven prior treatment regimens. Their median age was 65 years, a little more than half were men, and more than half had high-risk cytogenetics. Patients received oral selinexor at 80 mg twice weekly plus dexamethasone at 20 mg twice weekly until disease progression.Two patients (1.6%) achieved stringent complete responses. They also had minimal residual disease negativity, one at the level of 1 x 10–6 and one at 1 x 10–4.
Very good partial responses were seen in 4.9% of patients, 19.7% had partial responses, 13.1% had minimal responses (MRs), and 39.3% had stable disease. Progressive disease occurred in 13.1% of patients; 8.2% were not evaluable for response.
The overall response rate (partial response or better) was 26.2%, the clinical benefit rate (MR or better) was 39.3%, and the disease control rate (stable disease or better) was 78.7%.
The median duration of response was 4.4 months. The median progression-free survival was 3.7 months overall, 4.6 months in patients with an MR or better, and 1.1 months in patients who had progressive disease or were not evaluable.
The median overall survival was 8.6 months for the entire cohort. Overall survival was 15.6 months in patients with an MR or better and 1.7 months in patients who had progressive disease or were not evaluable (P less than .0001).
The “most important” grade 3/4 adverse events, according to Dr. Jagannath, were thrombocytopenia (53.7%), anemia (29.3%), fatigue (22.8%), hyponatremia (16.3%), nausea (9.8%), diarrhea (6.5%), anorexia (3.3%), and emesis (3.3%). A total of 23 patients (19.5%) discontinued treatment because of a related adverse.
This study was sponsored by Karyopharm Therapeutics. Dr. Jagannath reported relationships with Karyopharm, Janssen, Celgene, Amgen, and GlaxoSmithKline.
SOURCE: Jagannath S et al. SOHO 2018, Abstract MM-255
FROM SOHO 2018
Key clinical point:
Major finding: The overall response rate was 26.2% and the clinical benefit rate was 39.3%.
Study details: A phase 2 trial of 122 patients with penta-refractory multiple myeloma.
Disclosures: This study was sponsored by Karyopharm Therapeutics. Dr. Jagannath reported relationships with Karyopharm, Janssen, Celgene, Amgen, and GlaxoSmithKline.
Source: Jagannath S et al. SOHO 2018, Abstract MM-255.
Impact of an Educational Seminar Series for VA Providers in Personalized Cancer Care Across Hematologic and Solid Tumors
Purpose/Rationale: To address educational needs of hematology/oncology providers in VA and other federal settings, we conducted a national series of accredited 6-hour seminars. Through surveys, we assessed baseline barriers and educational outcomes.
Background: Recent landmark advances in cancer therapies engender pressing needs for education among VA providers.
Methods: The educational seminars were held in 9 US cities with large VA facilities between November 2017 and March 2018. The agenda, covering hematologic malignancies (3 hours) and solid tumors (3 hours), emphasized evidenced-based and guideline-directed uses of new cancer therapies. Before and after the seminars, participants completed surveys designed to assess self-reported barriers, confidence, and competence regarding personalized medicine approaches to implementing the therapies.
Results: Survey respondents (n = 639) were physicians (29%), pharmacists (23%), nurses (21%), physician assistants (18%), and nurse practitioners (9%) who practice in VA clinics and other federal settings; providers reported seeing an average of 103 oncology patients per month. On the pre-seminar survey, gaps were indicated by relatively small proportions of respondents who reported that their decision-making involving new cancer therapies is guided by genetic/prognostic testing (21%) and assessing patientspecific characteristics including comorbidities (38%); 42% reported having inadequate staff training for personalized hematology/oncology care.
Across the pre- to post-seminar surveys, there were significant increases (P < .0001 for all comparisons) in the proportions of respondents who reported: (1) high confidence in using immunotherapies (17% to 38%), targeted therapies (19% to 37%), and hormonal therapies (20% to 36%); and (2) high competence in performing various clinical skills, including identifying genetic tests for patients with acute myeloid leukemia (8% to 42%), interpreting genetic tests to support personalized treatment decision-making for patients with chronic lymphocytic leukemia (7% to 42%), recognizing and managing adverse events associated with targeted therapies (15% to 48%), and applying precision medicine principles in managing patients with highgrade gliomas (17% to 44%).
Conclusions/Implications: These findings indicate the positive impact of intensive education on self-reported confidence and competence among VA providers in applying personalized medicine approaches to implementing new cancer therapies. We will present additional baseline barriers and educational outcomes, as well as the seminar participants’ gap-targeted action plans for improvement.
Purpose/Rationale: To address educational needs of hematology/oncology providers in VA and other federal settings, we conducted a national series of accredited 6-hour seminars. Through surveys, we assessed baseline barriers and educational outcomes.
Background: Recent landmark advances in cancer therapies engender pressing needs for education among VA providers.
Methods: The educational seminars were held in 9 US cities with large VA facilities between November 2017 and March 2018. The agenda, covering hematologic malignancies (3 hours) and solid tumors (3 hours), emphasized evidenced-based and guideline-directed uses of new cancer therapies. Before and after the seminars, participants completed surveys designed to assess self-reported barriers, confidence, and competence regarding personalized medicine approaches to implementing the therapies.
Results: Survey respondents (n = 639) were physicians (29%), pharmacists (23%), nurses (21%), physician assistants (18%), and nurse practitioners (9%) who practice in VA clinics and other federal settings; providers reported seeing an average of 103 oncology patients per month. On the pre-seminar survey, gaps were indicated by relatively small proportions of respondents who reported that their decision-making involving new cancer therapies is guided by genetic/prognostic testing (21%) and assessing patientspecific characteristics including comorbidities (38%); 42% reported having inadequate staff training for personalized hematology/oncology care.
Across the pre- to post-seminar surveys, there were significant increases (P < .0001 for all comparisons) in the proportions of respondents who reported: (1) high confidence in using immunotherapies (17% to 38%), targeted therapies (19% to 37%), and hormonal therapies (20% to 36%); and (2) high competence in performing various clinical skills, including identifying genetic tests for patients with acute myeloid leukemia (8% to 42%), interpreting genetic tests to support personalized treatment decision-making for patients with chronic lymphocytic leukemia (7% to 42%), recognizing and managing adverse events associated with targeted therapies (15% to 48%), and applying precision medicine principles in managing patients with highgrade gliomas (17% to 44%).
Conclusions/Implications: These findings indicate the positive impact of intensive education on self-reported confidence and competence among VA providers in applying personalized medicine approaches to implementing new cancer therapies. We will present additional baseline barriers and educational outcomes, as well as the seminar participants’ gap-targeted action plans for improvement.
Purpose/Rationale: To address educational needs of hematology/oncology providers in VA and other federal settings, we conducted a national series of accredited 6-hour seminars. Through surveys, we assessed baseline barriers and educational outcomes.
Background: Recent landmark advances in cancer therapies engender pressing needs for education among VA providers.
Methods: The educational seminars were held in 9 US cities with large VA facilities between November 2017 and March 2018. The agenda, covering hematologic malignancies (3 hours) and solid tumors (3 hours), emphasized evidenced-based and guideline-directed uses of new cancer therapies. Before and after the seminars, participants completed surveys designed to assess self-reported barriers, confidence, and competence regarding personalized medicine approaches to implementing the therapies.
Results: Survey respondents (n = 639) were physicians (29%), pharmacists (23%), nurses (21%), physician assistants (18%), and nurse practitioners (9%) who practice in VA clinics and other federal settings; providers reported seeing an average of 103 oncology patients per month. On the pre-seminar survey, gaps were indicated by relatively small proportions of respondents who reported that their decision-making involving new cancer therapies is guided by genetic/prognostic testing (21%) and assessing patientspecific characteristics including comorbidities (38%); 42% reported having inadequate staff training for personalized hematology/oncology care.
Across the pre- to post-seminar surveys, there were significant increases (P < .0001 for all comparisons) in the proportions of respondents who reported: (1) high confidence in using immunotherapies (17% to 38%), targeted therapies (19% to 37%), and hormonal therapies (20% to 36%); and (2) high competence in performing various clinical skills, including identifying genetic tests for patients with acute myeloid leukemia (8% to 42%), interpreting genetic tests to support personalized treatment decision-making for patients with chronic lymphocytic leukemia (7% to 42%), recognizing and managing adverse events associated with targeted therapies (15% to 48%), and applying precision medicine principles in managing patients with highgrade gliomas (17% to 44%).
Conclusions/Implications: These findings indicate the positive impact of intensive education on self-reported confidence and competence among VA providers in applying personalized medicine approaches to implementing new cancer therapies. We will present additional baseline barriers and educational outcomes, as well as the seminar participants’ gap-targeted action plans for improvement.
Implementation of a Hematology VA-ECHO Program
Purpose/Rationale: Lack of access to a hematology specialist is a barrier to care for veterans in rural and underserved areas. In light of the increasingly short supply of sub-specialists, there is a need to provide hematology education and outreach to primary care providers within the VA system.
Background: The ECHO program (Extension for Community Healthcare Outcomes) is a well-established and successful platform that audibly and visually links an area specialist to primary care providers and interdisciplinary team members, allowing for two-way specialty consultation combined with continuing education. Overall, our service network (VISN 20) has provided specialty education to more than 10 specialties and 171 clinical sites (26% rural or highly rural) through the VA-ECHO program. In fiscal year 2016, VISN 20 estimated that over 280,000 potential patient travel miles saved as a result of VA-ECHO.
Methods/Approach: As we could not identify an existing Hematology VA-ECHO program within VISN 20, nor to our knowledge nationally, we sought to develop and implement a Hematology VA-ECHO program based at the Puget Sound VA (Seattle, WA).
Results: We delivered an introductory 3-part VA-ECHO series to assess demand and solicit feedback about Hematology VA-ECHO. Session topics were iron deficiency anemia, polycythemia, and deep vein thrombosis/pulmonary embolism. We had participation from 17 sites in 9 states; 10 sites within VISN 20. Attendees included pharmacists, MDs, APRNs and RNs. We averaged 20 participants per session and welcomed 43 unique participants over 3 sessions. The vast majority (94%) of respondents (n=34) agreed or strongly agreed that the content in the sessions were relevant to their practice and 80% anticipated changing their practice as a result of session participation
Conclusion/Implications: A successful Hematology VA-ECHO program stands to de-monopolize specialty knowledge and help primary providers evaluate and manage common hematologic abnormalities, especially in underserved areas. We aim to expand Hematology VA-ECHO to include 8-9 sessions over the next calendar year. Uptake of Hematology VA-ECHO at additional VA sites in different geographical areas would help further increase hematology access for primary providers.
Purpose/Rationale: Lack of access to a hematology specialist is a barrier to care for veterans in rural and underserved areas. In light of the increasingly short supply of sub-specialists, there is a need to provide hematology education and outreach to primary care providers within the VA system.
Background: The ECHO program (Extension for Community Healthcare Outcomes) is a well-established and successful platform that audibly and visually links an area specialist to primary care providers and interdisciplinary team members, allowing for two-way specialty consultation combined with continuing education. Overall, our service network (VISN 20) has provided specialty education to more than 10 specialties and 171 clinical sites (26% rural or highly rural) through the VA-ECHO program. In fiscal year 2016, VISN 20 estimated that over 280,000 potential patient travel miles saved as a result of VA-ECHO.
Methods/Approach: As we could not identify an existing Hematology VA-ECHO program within VISN 20, nor to our knowledge nationally, we sought to develop and implement a Hematology VA-ECHO program based at the Puget Sound VA (Seattle, WA).
Results: We delivered an introductory 3-part VA-ECHO series to assess demand and solicit feedback about Hematology VA-ECHO. Session topics were iron deficiency anemia, polycythemia, and deep vein thrombosis/pulmonary embolism. We had participation from 17 sites in 9 states; 10 sites within VISN 20. Attendees included pharmacists, MDs, APRNs and RNs. We averaged 20 participants per session and welcomed 43 unique participants over 3 sessions. The vast majority (94%) of respondents (n=34) agreed or strongly agreed that the content in the sessions were relevant to their practice and 80% anticipated changing their practice as a result of session participation
Conclusion/Implications: A successful Hematology VA-ECHO program stands to de-monopolize specialty knowledge and help primary providers evaluate and manage common hematologic abnormalities, especially in underserved areas. We aim to expand Hematology VA-ECHO to include 8-9 sessions over the next calendar year. Uptake of Hematology VA-ECHO at additional VA sites in different geographical areas would help further increase hematology access for primary providers.
Purpose/Rationale: Lack of access to a hematology specialist is a barrier to care for veterans in rural and underserved areas. In light of the increasingly short supply of sub-specialists, there is a need to provide hematology education and outreach to primary care providers within the VA system.
Background: The ECHO program (Extension for Community Healthcare Outcomes) is a well-established and successful platform that audibly and visually links an area specialist to primary care providers and interdisciplinary team members, allowing for two-way specialty consultation combined with continuing education. Overall, our service network (VISN 20) has provided specialty education to more than 10 specialties and 171 clinical sites (26% rural or highly rural) through the VA-ECHO program. In fiscal year 2016, VISN 20 estimated that over 280,000 potential patient travel miles saved as a result of VA-ECHO.
Methods/Approach: As we could not identify an existing Hematology VA-ECHO program within VISN 20, nor to our knowledge nationally, we sought to develop and implement a Hematology VA-ECHO program based at the Puget Sound VA (Seattle, WA).
Results: We delivered an introductory 3-part VA-ECHO series to assess demand and solicit feedback about Hematology VA-ECHO. Session topics were iron deficiency anemia, polycythemia, and deep vein thrombosis/pulmonary embolism. We had participation from 17 sites in 9 states; 10 sites within VISN 20. Attendees included pharmacists, MDs, APRNs and RNs. We averaged 20 participants per session and welcomed 43 unique participants over 3 sessions. The vast majority (94%) of respondents (n=34) agreed or strongly agreed that the content in the sessions were relevant to their practice and 80% anticipated changing their practice as a result of session participation
Conclusion/Implications: A successful Hematology VA-ECHO program stands to de-monopolize specialty knowledge and help primary providers evaluate and manage common hematologic abnormalities, especially in underserved areas. We aim to expand Hematology VA-ECHO to include 8-9 sessions over the next calendar year. Uptake of Hematology VA-ECHO at additional VA sites in different geographical areas would help further increase hematology access for primary providers.
Cardiovascular Effects of Tyrosine Kinase Inhibitors in Advanced Renal Cell Carcinoma (RCC) Patients at VA San Diego Healthcare System (VASDHS)
Purpose: The purpose of this study was to characterize the incidence of cardiovascular events and time to first cardiovascular event due to tyrosine kinase inhibitors (TKIs) in patients with advanced RCC at VASDHS. Additional aims were to describe the current clinical practice management of cardiac monitoring via EKG or ECHO in this patient population.
Background: Over the past 12 years, the US Food and Drug Administration has approved six multitargeted TKIs for the treatment of RCC: axitinib, cabozantinib, lenvatinib, pazopanib, sorafenib and sunitinib. Other than hypertension, most studies of these therapies did not report the incidence of cardiovascular events that occurred during early clinical trials.
Methodology: This was a retrospective study that evaluated the incidence of cardiovascular events in patients diagnosed with advanced RCC who received oral TKI therapy for at least 30 days. The incidence of cardiovascular events was collected in accordance with the Common Terminology Criteria for Adverse Events (CTCAE) grading at predetermined time points from the start of each TKI therapy. Cardiovascular events were defined as hypertension, QT prolongation, congestive heart failure (CHF), stroke, myocardial infarction (MI) and pulmonary hypertension.
Results: Fifty-four patients were included in this study with a median age of 66 years. There were no cardiac events in patients without a history of cardiovascular disease. Eleven patients with a history of cardiovascular disease experienced an adverse cardiac event while taking pazopanib, sorafenib, or sunitinib. The most common adverse events were hypertension (n=6) and MI (n=2). The remaining three patients experienced a stroke, QT prolongation, or CHF. Seventy-three percent of events occurred within four months of starting therapy. Baseline EKG or ECHO were not available for most patients started on TKI therapy and obtained only when patients were symptomatic.
Conclusions: The results of this study indicate a need for baseline and routine monitoring in patients started on oral TKIs with a history of cardiovascular disease to align with the current recommendations as listed in the medication package inserts. Based on the results of this study, baseline and routine monitoring should be considered during the first four months of therapy in patients with a history of cardiovascular disease.
Purpose: The purpose of this study was to characterize the incidence of cardiovascular events and time to first cardiovascular event due to tyrosine kinase inhibitors (TKIs) in patients with advanced RCC at VASDHS. Additional aims were to describe the current clinical practice management of cardiac monitoring via EKG or ECHO in this patient population.
Background: Over the past 12 years, the US Food and Drug Administration has approved six multitargeted TKIs for the treatment of RCC: axitinib, cabozantinib, lenvatinib, pazopanib, sorafenib and sunitinib. Other than hypertension, most studies of these therapies did not report the incidence of cardiovascular events that occurred during early clinical trials.
Methodology: This was a retrospective study that evaluated the incidence of cardiovascular events in patients diagnosed with advanced RCC who received oral TKI therapy for at least 30 days. The incidence of cardiovascular events was collected in accordance with the Common Terminology Criteria for Adverse Events (CTCAE) grading at predetermined time points from the start of each TKI therapy. Cardiovascular events were defined as hypertension, QT prolongation, congestive heart failure (CHF), stroke, myocardial infarction (MI) and pulmonary hypertension.
Results: Fifty-four patients were included in this study with a median age of 66 years. There were no cardiac events in patients without a history of cardiovascular disease. Eleven patients with a history of cardiovascular disease experienced an adverse cardiac event while taking pazopanib, sorafenib, or sunitinib. The most common adverse events were hypertension (n=6) and MI (n=2). The remaining three patients experienced a stroke, QT prolongation, or CHF. Seventy-three percent of events occurred within four months of starting therapy. Baseline EKG or ECHO were not available for most patients started on TKI therapy and obtained only when patients were symptomatic.
Conclusions: The results of this study indicate a need for baseline and routine monitoring in patients started on oral TKIs with a history of cardiovascular disease to align with the current recommendations as listed in the medication package inserts. Based on the results of this study, baseline and routine monitoring should be considered during the first four months of therapy in patients with a history of cardiovascular disease.
Purpose: The purpose of this study was to characterize the incidence of cardiovascular events and time to first cardiovascular event due to tyrosine kinase inhibitors (TKIs) in patients with advanced RCC at VASDHS. Additional aims were to describe the current clinical practice management of cardiac monitoring via EKG or ECHO in this patient population.
Background: Over the past 12 years, the US Food and Drug Administration has approved six multitargeted TKIs for the treatment of RCC: axitinib, cabozantinib, lenvatinib, pazopanib, sorafenib and sunitinib. Other than hypertension, most studies of these therapies did not report the incidence of cardiovascular events that occurred during early clinical trials.
Methodology: This was a retrospective study that evaluated the incidence of cardiovascular events in patients diagnosed with advanced RCC who received oral TKI therapy for at least 30 days. The incidence of cardiovascular events was collected in accordance with the Common Terminology Criteria for Adverse Events (CTCAE) grading at predetermined time points from the start of each TKI therapy. Cardiovascular events were defined as hypertension, QT prolongation, congestive heart failure (CHF), stroke, myocardial infarction (MI) and pulmonary hypertension.
Results: Fifty-four patients were included in this study with a median age of 66 years. There were no cardiac events in patients without a history of cardiovascular disease. Eleven patients with a history of cardiovascular disease experienced an adverse cardiac event while taking pazopanib, sorafenib, or sunitinib. The most common adverse events were hypertension (n=6) and MI (n=2). The remaining three patients experienced a stroke, QT prolongation, or CHF. Seventy-three percent of events occurred within four months of starting therapy. Baseline EKG or ECHO were not available for most patients started on TKI therapy and obtained only when patients were symptomatic.
Conclusions: The results of this study indicate a need for baseline and routine monitoring in patients started on oral TKIs with a history of cardiovascular disease to align with the current recommendations as listed in the medication package inserts. Based on the results of this study, baseline and routine monitoring should be considered during the first four months of therapy in patients with a history of cardiovascular disease.