User login
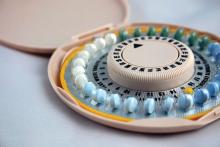
Hormonal contraceptive use linked to leukemia risk in offspring
A nationwide cohort study found an association between a woman’s use of hormonal contraceptives and a small increased risk of nonlymphoid leukemia in her offspring.
Maternal use of hormonal contraception either during pregnancy or in the 3 months beforehand was associated with a 46% higher risk of any leukemia in the children (P = .011), compared with no use, Marie Hargreave, PhD, of the Danish Cancer Society Research Center and her coauthors reported in Lancet Oncology.
The study of 1,185,157 children born between 1996 and 2014 included data from the Danish Cancer Registry and Danish National Prescription Registry and followed children for a median of 9.3 years.
Use during pregnancy was associated with a 78% higher risk of any leukemia in the offspring (P = .070), and contraception use that stopped more than 3 months before pregnancy was associated with a 25% higher risk of any leukemia (P = .039).
The researchers estimated that maternal use of hormonal contraceptives up to and including during pregnancy would have resulted in about one additional case of leukemia per 47,170 children; in other words, 25 additional cases of leukemia in Denmark from contraceptive use from 1996 to 2014.
The increased risk appeared to be limited to nonlymphoid leukemia only. The risk with recent use was more than twofold higher (HR, 2.17), compared with nonuse, and use during pregnancy was associated with a nearly fourfold increase in the risk of leukemia (HR, 3.87).
“Sex hormones are considered to be potent carcinogens, and the causal association between in-utero exposure to the oestrogen analogue diethylstilbestrol and subsequent risk for adenocarcinoma of the vagina is firmly established,” Dr. Hargreave and her colleagues wrote. “The mechanism by which maternal use of hormones increases cancer risk in children is, however, still not clear.”
Recent use of combined oral contraceptive products was associated with a more than twofold increased risk of nonlymphoid leukemia in offspring, compared with no use. However progestin-only oral contraceptives and emergency contraception did not appear to increase in the risk of lymphoid or nonlymphoid leukemia.
The association was strongest in children aged 6-10 years, which the authors suggested was likely because the incidence of nonlymphoid leukemia increases after the age of 6 years.
While acknowledging that the small increase in leukemia risk was not a major safety concern for hormonal contraceptives, the authors commented that the results suggested the intrauterine hormonal environment could be a direction for research into the causes of leukemia.
The study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
SOURCE: Hargreave M et al. Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045(18)30479-0.
Estrogenic compounds could have a number of effects on the genomic machinery, that could in turn lead to an increased risk of leukemia in offspring. It may be that oral contraceptives cause epigenetic changes to fetal hematopoietic stem cells that lead to gene rearrangements and oxidative damage, which could then influence the risk of developing childhood leukemia.
This study opens a new avenue of investigation for a risk factor that might increase a child’s susceptibility to leukemia and is important in shedding more light on dose-response associations of exposures.
Dr. Maria S. Pombo-de-Oliveira is from the pediatric hematology-oncology research program at the Instituto Nacional de Câncer in Rio de Janeiro. These comments are adapted from an accompanying editorial (Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045[18]30509-6). Dr. Pombo-de-Oliveira reported having no conflicts of interest.
Estrogenic compounds could have a number of effects on the genomic machinery, that could in turn lead to an increased risk of leukemia in offspring. It may be that oral contraceptives cause epigenetic changes to fetal hematopoietic stem cells that lead to gene rearrangements and oxidative damage, which could then influence the risk of developing childhood leukemia.
This study opens a new avenue of investigation for a risk factor that might increase a child’s susceptibility to leukemia and is important in shedding more light on dose-response associations of exposures.
Dr. Maria S. Pombo-de-Oliveira is from the pediatric hematology-oncology research program at the Instituto Nacional de Câncer in Rio de Janeiro. These comments are adapted from an accompanying editorial (Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045[18]30509-6). Dr. Pombo-de-Oliveira reported having no conflicts of interest.
Estrogenic compounds could have a number of effects on the genomic machinery, that could in turn lead to an increased risk of leukemia in offspring. It may be that oral contraceptives cause epigenetic changes to fetal hematopoietic stem cells that lead to gene rearrangements and oxidative damage, which could then influence the risk of developing childhood leukemia.
This study opens a new avenue of investigation for a risk factor that might increase a child’s susceptibility to leukemia and is important in shedding more light on dose-response associations of exposures.
Dr. Maria S. Pombo-de-Oliveira is from the pediatric hematology-oncology research program at the Instituto Nacional de Câncer in Rio de Janeiro. These comments are adapted from an accompanying editorial (Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045[18]30509-6). Dr. Pombo-de-Oliveira reported having no conflicts of interest.
A nationwide cohort study found an association between a woman’s use of hormonal contraceptives and a small increased risk of nonlymphoid leukemia in her offspring.
Maternal use of hormonal contraception either during pregnancy or in the 3 months beforehand was associated with a 46% higher risk of any leukemia in the children (P = .011), compared with no use, Marie Hargreave, PhD, of the Danish Cancer Society Research Center and her coauthors reported in Lancet Oncology.
The study of 1,185,157 children born between 1996 and 2014 included data from the Danish Cancer Registry and Danish National Prescription Registry and followed children for a median of 9.3 years.
Use during pregnancy was associated with a 78% higher risk of any leukemia in the offspring (P = .070), and contraception use that stopped more than 3 months before pregnancy was associated with a 25% higher risk of any leukemia (P = .039).
The researchers estimated that maternal use of hormonal contraceptives up to and including during pregnancy would have resulted in about one additional case of leukemia per 47,170 children; in other words, 25 additional cases of leukemia in Denmark from contraceptive use from 1996 to 2014.
The increased risk appeared to be limited to nonlymphoid leukemia only. The risk with recent use was more than twofold higher (HR, 2.17), compared with nonuse, and use during pregnancy was associated with a nearly fourfold increase in the risk of leukemia (HR, 3.87).
“Sex hormones are considered to be potent carcinogens, and the causal association between in-utero exposure to the oestrogen analogue diethylstilbestrol and subsequent risk for adenocarcinoma of the vagina is firmly established,” Dr. Hargreave and her colleagues wrote. “The mechanism by which maternal use of hormones increases cancer risk in children is, however, still not clear.”
Recent use of combined oral contraceptive products was associated with a more than twofold increased risk of nonlymphoid leukemia in offspring, compared with no use. However progestin-only oral contraceptives and emergency contraception did not appear to increase in the risk of lymphoid or nonlymphoid leukemia.
The association was strongest in children aged 6-10 years, which the authors suggested was likely because the incidence of nonlymphoid leukemia increases after the age of 6 years.
While acknowledging that the small increase in leukemia risk was not a major safety concern for hormonal contraceptives, the authors commented that the results suggested the intrauterine hormonal environment could be a direction for research into the causes of leukemia.
The study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
SOURCE: Hargreave M et al. Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045(18)30479-0.
A nationwide cohort study found an association between a woman’s use of hormonal contraceptives and a small increased risk of nonlymphoid leukemia in her offspring.
Maternal use of hormonal contraception either during pregnancy or in the 3 months beforehand was associated with a 46% higher risk of any leukemia in the children (P = .011), compared with no use, Marie Hargreave, PhD, of the Danish Cancer Society Research Center and her coauthors reported in Lancet Oncology.
The study of 1,185,157 children born between 1996 and 2014 included data from the Danish Cancer Registry and Danish National Prescription Registry and followed children for a median of 9.3 years.
Use during pregnancy was associated with a 78% higher risk of any leukemia in the offspring (P = .070), and contraception use that stopped more than 3 months before pregnancy was associated with a 25% higher risk of any leukemia (P = .039).
The researchers estimated that maternal use of hormonal contraceptives up to and including during pregnancy would have resulted in about one additional case of leukemia per 47,170 children; in other words, 25 additional cases of leukemia in Denmark from contraceptive use from 1996 to 2014.
The increased risk appeared to be limited to nonlymphoid leukemia only. The risk with recent use was more than twofold higher (HR, 2.17), compared with nonuse, and use during pregnancy was associated with a nearly fourfold increase in the risk of leukemia (HR, 3.87).
“Sex hormones are considered to be potent carcinogens, and the causal association between in-utero exposure to the oestrogen analogue diethylstilbestrol and subsequent risk for adenocarcinoma of the vagina is firmly established,” Dr. Hargreave and her colleagues wrote. “The mechanism by which maternal use of hormones increases cancer risk in children is, however, still not clear.”
Recent use of combined oral contraceptive products was associated with a more than twofold increased risk of nonlymphoid leukemia in offspring, compared with no use. However progestin-only oral contraceptives and emergency contraception did not appear to increase in the risk of lymphoid or nonlymphoid leukemia.
The association was strongest in children aged 6-10 years, which the authors suggested was likely because the incidence of nonlymphoid leukemia increases after the age of 6 years.
While acknowledging that the small increase in leukemia risk was not a major safety concern for hormonal contraceptives, the authors commented that the results suggested the intrauterine hormonal environment could be a direction for research into the causes of leukemia.
The study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
SOURCE: Hargreave M et al. Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045(18)30479-0.
FROM LANCET ONCOLOGY
Key clinical point:
Major finding: Recent maternal hormonal contraceptive use was linked to one additional case of leukemia per 47,170 children.
Study details: Danish nationwide cohort study in 1,185,157 children.
Disclosures: The study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
Source: Hargreave M et al. Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045(18)30479-0.
ASCO updates guidance on prophylaxis for adults with cancer-related immunosuppression
Fluoroquinolones are recommended for adults with cancer-related immunosuppression if they are at high risk of infection, according to an updated clinical practice guideline on antimicrobial prophylaxis.
By contrast, patients with solid tumors are not routinely recommended to receive antibiotic prophylaxis, according to the guideline, developed by the American Society of Clinical Oncology (ASCO) with the Infectious Diseases Society of America (IDSA).
The guideline includes antibacterial, antifungal, and antiviral prophylaxis recommendations, along with additional precautions such as hand hygiene that may reduce infection risk.
Released in the Journal of Clinical Oncology, the updated guidelines were developed by an expert panel cochaired by Christopher R. Flowers, MD of Emory University, Atlanta, and Randy A. Taplitz, MD of the University of California, San Diego, Health.
For the most part, the panel endorsed the previous ASCO recommendations, published in 2013. However, the panel considered six new high-quality studies and six new or updated meta-analyses to make modifications and add some new recommendations.
Fluoroquinolones, in the 2013 guideline, were recommended over trimethoprim-sulfamethoxazole because of fewer adverse events leading to treatment discontinuation. Panelists for the new guidelines said they continued to support that recommendation, based on an updated literature review.
That review showed significant reductions in both febrile neutropenia incidence and all-cause mortality, not only for patients at high risk of febrile neutropenia or profound, protracted neutropenia but also for lower-risk patients with solid tumors, they said.
However, the benefits did not sufficiently outweigh the harms to justify recommending fluoroquinolone prophylaxis for all patients with solid tumors or lymphoma, according to the report from the expert panel.
Those harms could include antibiotic-associated adverse effects, emergence of resistance, and Clostridium difficile infections, they said.
Accordingly, they recommended fluoroquinolone prophylaxis for the high-risk patients, including most patients with acute myeloid leukemia/myelodysplastic syndromes (AML/MDS) or those undergoing hematopoietic stem-cell transplantation (HSCT).
Similarly, the panel recommended that high-risk patients should receive antifungal prophylaxis with an oral triazole or parenteral echinocandin, while prophylaxis would not be routinely recommended for solid tumor patients.
By contrast, all patients undergoing chemotherapy for malignancy should receive yearly influenza vaccination with an inactivated quadrivalent vaccine, the panel said in its antiviral prophylaxis recommendations.
Family members, household contacts, and health care providers also should receive influenza vaccinations, said the panel, endorsing recommendations from the Centers for Disease Control and Prevention that were also cited in the 2013 ASCO guidelines.
Health care workers should follow hand hygiene and respiratory hygiene/cough etiquette to reduce risk of pathogen transmission, the panel said, endorsing CDC recommendations cited in the previous guideline.
However, the panel said they recommend against interventions such as neutropenic diet, footwear exchange, nutritional supplements, and surgical masks.
“Evidence of clinical benefit is lacking” for those interventions, they said.
Participants in the expert panel disclosed potential conflicts of interest related to Merck, Chimerix, GlyPharma Therapeutic, Pfizer, Cidara Therapeutics, Celgene, Astellas Pharma, Gilead Sciences, and Allergan, among other entities.
SOURCE: Taplitz RA et al. J Clin Oncol. 2018 Sept 4. doi: 10.1200/JCO.18.00374.
Fluoroquinolones are recommended for adults with cancer-related immunosuppression if they are at high risk of infection, according to an updated clinical practice guideline on antimicrobial prophylaxis.
By contrast, patients with solid tumors are not routinely recommended to receive antibiotic prophylaxis, according to the guideline, developed by the American Society of Clinical Oncology (ASCO) with the Infectious Diseases Society of America (IDSA).
The guideline includes antibacterial, antifungal, and antiviral prophylaxis recommendations, along with additional precautions such as hand hygiene that may reduce infection risk.
Released in the Journal of Clinical Oncology, the updated guidelines were developed by an expert panel cochaired by Christopher R. Flowers, MD of Emory University, Atlanta, and Randy A. Taplitz, MD of the University of California, San Diego, Health.
For the most part, the panel endorsed the previous ASCO recommendations, published in 2013. However, the panel considered six new high-quality studies and six new or updated meta-analyses to make modifications and add some new recommendations.
Fluoroquinolones, in the 2013 guideline, were recommended over trimethoprim-sulfamethoxazole because of fewer adverse events leading to treatment discontinuation. Panelists for the new guidelines said they continued to support that recommendation, based on an updated literature review.
That review showed significant reductions in both febrile neutropenia incidence and all-cause mortality, not only for patients at high risk of febrile neutropenia or profound, protracted neutropenia but also for lower-risk patients with solid tumors, they said.
However, the benefits did not sufficiently outweigh the harms to justify recommending fluoroquinolone prophylaxis for all patients with solid tumors or lymphoma, according to the report from the expert panel.
Those harms could include antibiotic-associated adverse effects, emergence of resistance, and Clostridium difficile infections, they said.
Accordingly, they recommended fluoroquinolone prophylaxis for the high-risk patients, including most patients with acute myeloid leukemia/myelodysplastic syndromes (AML/MDS) or those undergoing hematopoietic stem-cell transplantation (HSCT).
Similarly, the panel recommended that high-risk patients should receive antifungal prophylaxis with an oral triazole or parenteral echinocandin, while prophylaxis would not be routinely recommended for solid tumor patients.
By contrast, all patients undergoing chemotherapy for malignancy should receive yearly influenza vaccination with an inactivated quadrivalent vaccine, the panel said in its antiviral prophylaxis recommendations.
Family members, household contacts, and health care providers also should receive influenza vaccinations, said the panel, endorsing recommendations from the Centers for Disease Control and Prevention that were also cited in the 2013 ASCO guidelines.
Health care workers should follow hand hygiene and respiratory hygiene/cough etiquette to reduce risk of pathogen transmission, the panel said, endorsing CDC recommendations cited in the previous guideline.
However, the panel said they recommend against interventions such as neutropenic diet, footwear exchange, nutritional supplements, and surgical masks.
“Evidence of clinical benefit is lacking” for those interventions, they said.
Participants in the expert panel disclosed potential conflicts of interest related to Merck, Chimerix, GlyPharma Therapeutic, Pfizer, Cidara Therapeutics, Celgene, Astellas Pharma, Gilead Sciences, and Allergan, among other entities.
SOURCE: Taplitz RA et al. J Clin Oncol. 2018 Sept 4. doi: 10.1200/JCO.18.00374.
Fluoroquinolones are recommended for adults with cancer-related immunosuppression if they are at high risk of infection, according to an updated clinical practice guideline on antimicrobial prophylaxis.
By contrast, patients with solid tumors are not routinely recommended to receive antibiotic prophylaxis, according to the guideline, developed by the American Society of Clinical Oncology (ASCO) with the Infectious Diseases Society of America (IDSA).
The guideline includes antibacterial, antifungal, and antiviral prophylaxis recommendations, along with additional precautions such as hand hygiene that may reduce infection risk.
Released in the Journal of Clinical Oncology, the updated guidelines were developed by an expert panel cochaired by Christopher R. Flowers, MD of Emory University, Atlanta, and Randy A. Taplitz, MD of the University of California, San Diego, Health.
For the most part, the panel endorsed the previous ASCO recommendations, published in 2013. However, the panel considered six new high-quality studies and six new or updated meta-analyses to make modifications and add some new recommendations.
Fluoroquinolones, in the 2013 guideline, were recommended over trimethoprim-sulfamethoxazole because of fewer adverse events leading to treatment discontinuation. Panelists for the new guidelines said they continued to support that recommendation, based on an updated literature review.
That review showed significant reductions in both febrile neutropenia incidence and all-cause mortality, not only for patients at high risk of febrile neutropenia or profound, protracted neutropenia but also for lower-risk patients with solid tumors, they said.
However, the benefits did not sufficiently outweigh the harms to justify recommending fluoroquinolone prophylaxis for all patients with solid tumors or lymphoma, according to the report from the expert panel.
Those harms could include antibiotic-associated adverse effects, emergence of resistance, and Clostridium difficile infections, they said.
Accordingly, they recommended fluoroquinolone prophylaxis for the high-risk patients, including most patients with acute myeloid leukemia/myelodysplastic syndromes (AML/MDS) or those undergoing hematopoietic stem-cell transplantation (HSCT).
Similarly, the panel recommended that high-risk patients should receive antifungal prophylaxis with an oral triazole or parenteral echinocandin, while prophylaxis would not be routinely recommended for solid tumor patients.
By contrast, all patients undergoing chemotherapy for malignancy should receive yearly influenza vaccination with an inactivated quadrivalent vaccine, the panel said in its antiviral prophylaxis recommendations.
Family members, household contacts, and health care providers also should receive influenza vaccinations, said the panel, endorsing recommendations from the Centers for Disease Control and Prevention that were also cited in the 2013 ASCO guidelines.
Health care workers should follow hand hygiene and respiratory hygiene/cough etiquette to reduce risk of pathogen transmission, the panel said, endorsing CDC recommendations cited in the previous guideline.
However, the panel said they recommend against interventions such as neutropenic diet, footwear exchange, nutritional supplements, and surgical masks.
“Evidence of clinical benefit is lacking” for those interventions, they said.
Participants in the expert panel disclosed potential conflicts of interest related to Merck, Chimerix, GlyPharma Therapeutic, Pfizer, Cidara Therapeutics, Celgene, Astellas Pharma, Gilead Sciences, and Allergan, among other entities.
SOURCE: Taplitz RA et al. J Clin Oncol. 2018 Sept 4. doi: 10.1200/JCO.18.00374.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Caplacizumab approved in Europe to treat aTTP
The , a humanized bivalent nanobody that inhibits the interaction between von Willebrand factor and platelets.
Caplacizumab is now approved to treat adults with acquired thrombotic thrombocytopenic purpura (aTTP) in all member countries of the European Union as well as Norway, Iceland, and Liechtenstein.
The drug has been accepted for priority review in the United States and the Food and Drug Administration is expected to make a decision by Feb. 6, 2019.
The European Commission’s approval of caplacizumab is supported by data from the phase 2 TITAN study and the phase 3 HERCULES study.
The TITAN trial included 75 aTTP patients who were randomized to caplacizumab (n = 36) or placebo (n = 39), with all patients receiving the current standard of care – daily plasma exchange and immunosuppressive therapy (N Engl J Med. 2016;374:511-22).
Patients in the caplacizumab arm had a 39% reduction in the median time to response, compared with patients in the placebo arm (P = .005).
The rate of adverse events (AEs) thought to be related to the study drug was 17% in the caplacizumab arm and 11% in the placebo arm. The rate of AEs that were possibly related was 54% and 8%, respectively. The rate of serious AEs was 37% and 32%, respectively.
There were no deaths in the caplacizumab arm and two in the placebo arm. One death was attributable to severe, refractory TTP, and the other was attributable to cerebral hemorrhage.Results from the HERCULES trial were presented at the 2017 annual meeting of the American Society of Hematology.
The study enrolled patients with an acute episode of aTTP. They were randomized to receive caplacizumab (n = 72) or placebo (n = 73) in addition to standard care – plasma exchange and immunosuppression.
The study’s primary endpoint was the time to platelet count response (normalization). There was a significant reduction in time to platelet count response in the caplacizumab arm, compared with the placebo arm. The platelet normalization rate ratio was 1.55 (P less than .01).
A secondary endpoint was the combination of aTTP-related death, aTTP recurrence, and at least one major thromboembolic event during study treatment. The incidence of this combined endpoint was 12.7% (n = 9) in the caplacizumab arm and 49.3% (n = 36) in the placebo arm (P less than .0001).
The incidence of aTTP-related death was 0% (n = 0) in the caplacizumab arm and 4.1% (n = 3) in the placebo arm. The incidence of aTTP recurrence was 4.2% (n = 3) in the caplacizumab arm and 38.4% in the placebo arm (n = 28), and the incidence of at least one major thromboembolic event was 8.5% (n = 6) and 8.2% (n = 6), respectively.
The , a humanized bivalent nanobody that inhibits the interaction between von Willebrand factor and platelets.
Caplacizumab is now approved to treat adults with acquired thrombotic thrombocytopenic purpura (aTTP) in all member countries of the European Union as well as Norway, Iceland, and Liechtenstein.
The drug has been accepted for priority review in the United States and the Food and Drug Administration is expected to make a decision by Feb. 6, 2019.
The European Commission’s approval of caplacizumab is supported by data from the phase 2 TITAN study and the phase 3 HERCULES study.
The TITAN trial included 75 aTTP patients who were randomized to caplacizumab (n = 36) or placebo (n = 39), with all patients receiving the current standard of care – daily plasma exchange and immunosuppressive therapy (N Engl J Med. 2016;374:511-22).
Patients in the caplacizumab arm had a 39% reduction in the median time to response, compared with patients in the placebo arm (P = .005).
The rate of adverse events (AEs) thought to be related to the study drug was 17% in the caplacizumab arm and 11% in the placebo arm. The rate of AEs that were possibly related was 54% and 8%, respectively. The rate of serious AEs was 37% and 32%, respectively.
There were no deaths in the caplacizumab arm and two in the placebo arm. One death was attributable to severe, refractory TTP, and the other was attributable to cerebral hemorrhage.Results from the HERCULES trial were presented at the 2017 annual meeting of the American Society of Hematology.
The study enrolled patients with an acute episode of aTTP. They were randomized to receive caplacizumab (n = 72) or placebo (n = 73) in addition to standard care – plasma exchange and immunosuppression.
The study’s primary endpoint was the time to platelet count response (normalization). There was a significant reduction in time to platelet count response in the caplacizumab arm, compared with the placebo arm. The platelet normalization rate ratio was 1.55 (P less than .01).
A secondary endpoint was the combination of aTTP-related death, aTTP recurrence, and at least one major thromboembolic event during study treatment. The incidence of this combined endpoint was 12.7% (n = 9) in the caplacizumab arm and 49.3% (n = 36) in the placebo arm (P less than .0001).
The incidence of aTTP-related death was 0% (n = 0) in the caplacizumab arm and 4.1% (n = 3) in the placebo arm. The incidence of aTTP recurrence was 4.2% (n = 3) in the caplacizumab arm and 38.4% in the placebo arm (n = 28), and the incidence of at least one major thromboembolic event was 8.5% (n = 6) and 8.2% (n = 6), respectively.
The , a humanized bivalent nanobody that inhibits the interaction between von Willebrand factor and platelets.
Caplacizumab is now approved to treat adults with acquired thrombotic thrombocytopenic purpura (aTTP) in all member countries of the European Union as well as Norway, Iceland, and Liechtenstein.
The drug has been accepted for priority review in the United States and the Food and Drug Administration is expected to make a decision by Feb. 6, 2019.
The European Commission’s approval of caplacizumab is supported by data from the phase 2 TITAN study and the phase 3 HERCULES study.
The TITAN trial included 75 aTTP patients who were randomized to caplacizumab (n = 36) or placebo (n = 39), with all patients receiving the current standard of care – daily plasma exchange and immunosuppressive therapy (N Engl J Med. 2016;374:511-22).
Patients in the caplacizumab arm had a 39% reduction in the median time to response, compared with patients in the placebo arm (P = .005).
The rate of adverse events (AEs) thought to be related to the study drug was 17% in the caplacizumab arm and 11% in the placebo arm. The rate of AEs that were possibly related was 54% and 8%, respectively. The rate of serious AEs was 37% and 32%, respectively.
There were no deaths in the caplacizumab arm and two in the placebo arm. One death was attributable to severe, refractory TTP, and the other was attributable to cerebral hemorrhage.Results from the HERCULES trial were presented at the 2017 annual meeting of the American Society of Hematology.
The study enrolled patients with an acute episode of aTTP. They were randomized to receive caplacizumab (n = 72) or placebo (n = 73) in addition to standard care – plasma exchange and immunosuppression.
The study’s primary endpoint was the time to platelet count response (normalization). There was a significant reduction in time to platelet count response in the caplacizumab arm, compared with the placebo arm. The platelet normalization rate ratio was 1.55 (P less than .01).
A secondary endpoint was the combination of aTTP-related death, aTTP recurrence, and at least one major thromboembolic event during study treatment. The incidence of this combined endpoint was 12.7% (n = 9) in the caplacizumab arm and 49.3% (n = 36) in the placebo arm (P less than .0001).
The incidence of aTTP-related death was 0% (n = 0) in the caplacizumab arm and 4.1% (n = 3) in the placebo arm. The incidence of aTTP recurrence was 4.2% (n = 3) in the caplacizumab arm and 38.4% in the placebo arm (n = 28), and the incidence of at least one major thromboembolic event was 8.5% (n = 6) and 8.2% (n = 6), respectively.
When is the right time to stop treatment?
Martha Boxer (not her real name) had mentally prepared herself that this could happen, but the news still hit hard. Her doctors on the leukemia service broke the facts as gently as we could: The chemotherapy she had been suffering through for the last 2 weeks hadn’t worked. The results of her latest bone marrow biopsy showed it remained packed with cancer cells.
As Martha absorbed the news quietly, her son, sitting next to her bedside with his hand on hers, spoke first. “What now?”
I looked at my attending and nodded, as we were fully ready to answer this question. From the outset, we knew that Martha’s leukemia carried a genetic mutation that unfortunately put her in a high-risk category. The chances of her cancer responding to the first round of chemotherapy were low. When this happens, what we typically do next is reinduction, we explained. It’s a different combination of chemotherapy drugs, with a somewhat different side effect profile. But it would give her the best chance of response, we believed. We could start the new chemotherapy as early as today, we said.
Martha took this in. “Okay,” she said pensively. “I’ve been thinking. And I think maybe … I won’t do chemotherapy anymore.”
Her words caught me off guard because, frankly, they seemed premature. Her leukemia had not budged with the first round of treatment. But we still had an option B, and then an option C. It was usually at a later, more dire stage – when multiple lines of treatment had not worked, and instead had only caused harm – or, when the decision was forced by the medical system’s admission that we had nothing left to offer – that I’d heard patients express similar preferences. It was then that I’d seen patients and their loved ones flip a mental switch and choose to focus the time they had left on what really mattered to them.
It didn’t feel like we were at that point.
And so, as we debriefed outside her room, my first instinct was to convince her otherwise.
However, Martha had other priorities, as I would come to learn. Above all else, she hated the hospital. She hated feeling trapped in a strange room that wasn’t hers; she hated how the chemotherapy stole her energy and made her feel too weak to even shower. She wanted to be in her own home. She wanted to eat her own food, sleep in her own bed, and be surrounded by what she recognized.
But, she also wanted to live. Two paths lay ahead of her. It was a trade-off of more suffering with a small chance at remission, versus accepting no chance of cure but feeling well for as long as she could. She soon clarified that she wasn’t definitely against chemotherapy. She couldn’t decide. She needed more information from us to make this decision, the hardest of her life.
Over the next few days, I watched as our attending physician expertly provided just that. There were actually three options, she laid out. There was aggressive chemotherapy, entailing at least 3 more weeks in the hospital and coming with significant risk of infection, nausea, vomiting, and fatigue. The chances of inducing a remission were about one in three to one in two, and that remission would likely last between several months and 2 years before the leukemia would relapse. The second option was a chemotherapy pill she could take at home, an option with fewer side effects but no longer aimed at cure. The third option was home hospice support, focused on symptoms, without any anticancer medication.
I noticed a few things during those conversations. I noticed how my attending took a navigator role, not pushing Martha in one direction or another, but rather imparting all the relevant information to empower Martha to decide for herself. I noticed how she provided realistic estimates, not hedging away from numbers, but giving the honest, nitty-gritty facts, as best as she could predict. I noticed how she took the time and never rushed, even in spite of external pressures to discharge the patient from the hospital.
There was no right or wrong answer. I no longer felt that we had something in our grasp – a clear-cut, best decision – to persuade Martha toward. Is one in three good odds, or bad odds? Is 2 years a long period of time, or a short one? Of course, there is no actual answer to these questions; the answer is as elusive and personal as if we had asked Martha: What do you think?
What I learned from Martha is that, with a devastating diagnosis, there isn’t a right time to make this decision. There isn’t one defining moment where we flip a switch and change course. It isn’t only when we run out of treatment options that the choice to forgo it makes sense. That option is on a flexible line, different for every person and priority. As my attending later said, with Martha’s diagnosis and her values, it wouldn’t have been unreasonable to decide against chemotherapy from the start.
The language we use can sometimes mask that reality. As doctors, we may casually slip in words like “need” and “have to” in response to patients’ questions about what to do next. “You need more chemotherapy,” we might say. “We’d have to treat it.” We have “treatment,” after all, and so we go down the line of offering what’s next in the medical algorithm. That word, too, can be deceivingly tempting, enticing down a road that makes it seem like the obvious answer – or the only one. If the choices are treatment versus not, who wouldn’t want the treatment? But the details are where things get murky. What does that treatment involve? What are the chances it will work, and for how long?
Surreptitiously missing from this language is the fact that there’s a choice. There’s always a choice, and it’s on the table at any point. You can start chemotherapy without committing to stick it out until the end. You can go home, if that is what’s important to you. The best treatment option is the one the patient wants.
After 4 days, Martha decided to go home with palliative chemotherapy and a bridge to hospice. Each member of our team hugged her goodbye and wished her luck. She was nervous. But she packed her hospital room, and she left.
I recently pulled up her medical chart, bracing myself for bad news. But the interesting thing about hospice is that even though the focus is no longer on prolonging life, people sometimes live longer.
She felt well, the most recent palliative note said. She was spending her time writing, getting her finances in order, and finishing a legacy project for her grandchildren.
For Martha, it seemed to be the right choice.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
Martha Boxer (not her real name) had mentally prepared herself that this could happen, but the news still hit hard. Her doctors on the leukemia service broke the facts as gently as we could: The chemotherapy she had been suffering through for the last 2 weeks hadn’t worked. The results of her latest bone marrow biopsy showed it remained packed with cancer cells.
As Martha absorbed the news quietly, her son, sitting next to her bedside with his hand on hers, spoke first. “What now?”
I looked at my attending and nodded, as we were fully ready to answer this question. From the outset, we knew that Martha’s leukemia carried a genetic mutation that unfortunately put her in a high-risk category. The chances of her cancer responding to the first round of chemotherapy were low. When this happens, what we typically do next is reinduction, we explained. It’s a different combination of chemotherapy drugs, with a somewhat different side effect profile. But it would give her the best chance of response, we believed. We could start the new chemotherapy as early as today, we said.
Martha took this in. “Okay,” she said pensively. “I’ve been thinking. And I think maybe … I won’t do chemotherapy anymore.”
Her words caught me off guard because, frankly, they seemed premature. Her leukemia had not budged with the first round of treatment. But we still had an option B, and then an option C. It was usually at a later, more dire stage – when multiple lines of treatment had not worked, and instead had only caused harm – or, when the decision was forced by the medical system’s admission that we had nothing left to offer – that I’d heard patients express similar preferences. It was then that I’d seen patients and their loved ones flip a mental switch and choose to focus the time they had left on what really mattered to them.
It didn’t feel like we were at that point.
And so, as we debriefed outside her room, my first instinct was to convince her otherwise.
However, Martha had other priorities, as I would come to learn. Above all else, she hated the hospital. She hated feeling trapped in a strange room that wasn’t hers; she hated how the chemotherapy stole her energy and made her feel too weak to even shower. She wanted to be in her own home. She wanted to eat her own food, sleep in her own bed, and be surrounded by what she recognized.
But, she also wanted to live. Two paths lay ahead of her. It was a trade-off of more suffering with a small chance at remission, versus accepting no chance of cure but feeling well for as long as she could. She soon clarified that she wasn’t definitely against chemotherapy. She couldn’t decide. She needed more information from us to make this decision, the hardest of her life.
Over the next few days, I watched as our attending physician expertly provided just that. There were actually three options, she laid out. There was aggressive chemotherapy, entailing at least 3 more weeks in the hospital and coming with significant risk of infection, nausea, vomiting, and fatigue. The chances of inducing a remission were about one in three to one in two, and that remission would likely last between several months and 2 years before the leukemia would relapse. The second option was a chemotherapy pill she could take at home, an option with fewer side effects but no longer aimed at cure. The third option was home hospice support, focused on symptoms, without any anticancer medication.
I noticed a few things during those conversations. I noticed how my attending took a navigator role, not pushing Martha in one direction or another, but rather imparting all the relevant information to empower Martha to decide for herself. I noticed how she provided realistic estimates, not hedging away from numbers, but giving the honest, nitty-gritty facts, as best as she could predict. I noticed how she took the time and never rushed, even in spite of external pressures to discharge the patient from the hospital.
There was no right or wrong answer. I no longer felt that we had something in our grasp – a clear-cut, best decision – to persuade Martha toward. Is one in three good odds, or bad odds? Is 2 years a long period of time, or a short one? Of course, there is no actual answer to these questions; the answer is as elusive and personal as if we had asked Martha: What do you think?
What I learned from Martha is that, with a devastating diagnosis, there isn’t a right time to make this decision. There isn’t one defining moment where we flip a switch and change course. It isn’t only when we run out of treatment options that the choice to forgo it makes sense. That option is on a flexible line, different for every person and priority. As my attending later said, with Martha’s diagnosis and her values, it wouldn’t have been unreasonable to decide against chemotherapy from the start.
The language we use can sometimes mask that reality. As doctors, we may casually slip in words like “need” and “have to” in response to patients’ questions about what to do next. “You need more chemotherapy,” we might say. “We’d have to treat it.” We have “treatment,” after all, and so we go down the line of offering what’s next in the medical algorithm. That word, too, can be deceivingly tempting, enticing down a road that makes it seem like the obvious answer – or the only one. If the choices are treatment versus not, who wouldn’t want the treatment? But the details are where things get murky. What does that treatment involve? What are the chances it will work, and for how long?
Surreptitiously missing from this language is the fact that there’s a choice. There’s always a choice, and it’s on the table at any point. You can start chemotherapy without committing to stick it out until the end. You can go home, if that is what’s important to you. The best treatment option is the one the patient wants.
After 4 days, Martha decided to go home with palliative chemotherapy and a bridge to hospice. Each member of our team hugged her goodbye and wished her luck. She was nervous. But she packed her hospital room, and she left.
I recently pulled up her medical chart, bracing myself for bad news. But the interesting thing about hospice is that even though the focus is no longer on prolonging life, people sometimes live longer.
She felt well, the most recent palliative note said. She was spending her time writing, getting her finances in order, and finishing a legacy project for her grandchildren.
For Martha, it seemed to be the right choice.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
Martha Boxer (not her real name) had mentally prepared herself that this could happen, but the news still hit hard. Her doctors on the leukemia service broke the facts as gently as we could: The chemotherapy she had been suffering through for the last 2 weeks hadn’t worked. The results of her latest bone marrow biopsy showed it remained packed with cancer cells.
As Martha absorbed the news quietly, her son, sitting next to her bedside with his hand on hers, spoke first. “What now?”
I looked at my attending and nodded, as we were fully ready to answer this question. From the outset, we knew that Martha’s leukemia carried a genetic mutation that unfortunately put her in a high-risk category. The chances of her cancer responding to the first round of chemotherapy were low. When this happens, what we typically do next is reinduction, we explained. It’s a different combination of chemotherapy drugs, with a somewhat different side effect profile. But it would give her the best chance of response, we believed. We could start the new chemotherapy as early as today, we said.
Martha took this in. “Okay,” she said pensively. “I’ve been thinking. And I think maybe … I won’t do chemotherapy anymore.”
Her words caught me off guard because, frankly, they seemed premature. Her leukemia had not budged with the first round of treatment. But we still had an option B, and then an option C. It was usually at a later, more dire stage – when multiple lines of treatment had not worked, and instead had only caused harm – or, when the decision was forced by the medical system’s admission that we had nothing left to offer – that I’d heard patients express similar preferences. It was then that I’d seen patients and their loved ones flip a mental switch and choose to focus the time they had left on what really mattered to them.
It didn’t feel like we were at that point.
And so, as we debriefed outside her room, my first instinct was to convince her otherwise.
However, Martha had other priorities, as I would come to learn. Above all else, she hated the hospital. She hated feeling trapped in a strange room that wasn’t hers; she hated how the chemotherapy stole her energy and made her feel too weak to even shower. She wanted to be in her own home. She wanted to eat her own food, sleep in her own bed, and be surrounded by what she recognized.
But, she also wanted to live. Two paths lay ahead of her. It was a trade-off of more suffering with a small chance at remission, versus accepting no chance of cure but feeling well for as long as she could. She soon clarified that she wasn’t definitely against chemotherapy. She couldn’t decide. She needed more information from us to make this decision, the hardest of her life.
Over the next few days, I watched as our attending physician expertly provided just that. There were actually three options, she laid out. There was aggressive chemotherapy, entailing at least 3 more weeks in the hospital and coming with significant risk of infection, nausea, vomiting, and fatigue. The chances of inducing a remission were about one in three to one in two, and that remission would likely last between several months and 2 years before the leukemia would relapse. The second option was a chemotherapy pill she could take at home, an option with fewer side effects but no longer aimed at cure. The third option was home hospice support, focused on symptoms, without any anticancer medication.
I noticed a few things during those conversations. I noticed how my attending took a navigator role, not pushing Martha in one direction or another, but rather imparting all the relevant information to empower Martha to decide for herself. I noticed how she provided realistic estimates, not hedging away from numbers, but giving the honest, nitty-gritty facts, as best as she could predict. I noticed how she took the time and never rushed, even in spite of external pressures to discharge the patient from the hospital.
There was no right or wrong answer. I no longer felt that we had something in our grasp – a clear-cut, best decision – to persuade Martha toward. Is one in three good odds, or bad odds? Is 2 years a long period of time, or a short one? Of course, there is no actual answer to these questions; the answer is as elusive and personal as if we had asked Martha: What do you think?
What I learned from Martha is that, with a devastating diagnosis, there isn’t a right time to make this decision. There isn’t one defining moment where we flip a switch and change course. It isn’t only when we run out of treatment options that the choice to forgo it makes sense. That option is on a flexible line, different for every person and priority. As my attending later said, with Martha’s diagnosis and her values, it wouldn’t have been unreasonable to decide against chemotherapy from the start.
The language we use can sometimes mask that reality. As doctors, we may casually slip in words like “need” and “have to” in response to patients’ questions about what to do next. “You need more chemotherapy,” we might say. “We’d have to treat it.” We have “treatment,” after all, and so we go down the line of offering what’s next in the medical algorithm. That word, too, can be deceivingly tempting, enticing down a road that makes it seem like the obvious answer – or the only one. If the choices are treatment versus not, who wouldn’t want the treatment? But the details are where things get murky. What does that treatment involve? What are the chances it will work, and for how long?
Surreptitiously missing from this language is the fact that there’s a choice. There’s always a choice, and it’s on the table at any point. You can start chemotherapy without committing to stick it out until the end. You can go home, if that is what’s important to you. The best treatment option is the one the patient wants.
After 4 days, Martha decided to go home with palliative chemotherapy and a bridge to hospice. Each member of our team hugged her goodbye and wished her luck. She was nervous. But she packed her hospital room, and she left.
I recently pulled up her medical chart, bracing myself for bad news. But the interesting thing about hospice is that even though the focus is no longer on prolonging life, people sometimes live longer.
She felt well, the most recent palliative note said. She was spending her time writing, getting her finances in order, and finishing a legacy project for her grandchildren.
For Martha, it seemed to be the right choice.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
Abstracts Presented at the 2018 AVAHO Annual Meeting (Digital Edition)
Avatrombopag cut procedure-related transfusions in patients with thrombocytopenia, chronic liver disease
Once-daily treatment with the oral second-generation thrombopoietin agonist avatrombopag (Doptelet) significantly reduced the need for platelet transfusion and rescue therapy for up to 7 days after patients with chronic liver disease and thrombocytopenia underwent scheduled procedures, according to the results of two international, randomized, double-blind, phase III, placebo-controlled trials reported in the September issue of Gastroenterology.
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
In the ADAPT-1 trial, 66% of patients in the 60-mg arm met this primary endpoint, as did 88% of patients who received 40 mg for less severe thrombocytopenia, versus 23% and 38% of the placebo arms, respectively (P less than .001 for each comparison). In the ADAPT-2 trial, 69% of the 60-mg group met the primary endpoint, as did 88% of the 40-mg group, versus 35% and 33% of the respective placebo groups (P less than .001 for each comparison).
These results led the Food and Drug Administration to approve avatrombopag in May 2018 under its priority review process. The novel therapy “may be a safe and effective alternative to platelet transfusions” that could simplify the clinical management of patients with chronic liver disease and thrombocytopenia, Norah Terrault, MD, MPH, and her associates wrote in Gastroenterology.
The ADAPT-1 study included 231 patients, while ADAPT-2 included 204 patients. In each trial, patients were randomized on a 2:1 basis to receive oral avatrombopag or placebo once daily for 5 consecutive days. Patients in the intervention arms received 60 mg avatrombopag if their baseline platelet count was less than 40 x 109 per liter, and 40 mg if their baseline platelet count was 40-50 x 109 per liter. Procedures were scheduled for 10-13 days after treatment initiation.
“Platelet counts increased by [treatment] day 4, peaked at days 10-13, and then returned to baseline levels by day 35,” the researchers reported. Among ADAPT-1 patients with low baseline counts, 69% of avatrombopag recipients reached a prespecified target of at least 50 x 109 platelets per liter on their procedure day, versus 4% of placebo recipients (P less than .0001). Corresponding proportions in ADAPT-2 were 67% and 7%, respectively (P less than .0001). Among patients with higher baseline counts, 88% and 20% achieved the target, respectively, in ADAPT-1 (P less than .0001), as did 93% versus 39%, respectively, in ADAPT-2 (P less than .0001).
Avatrombopag and placebo produced similar rates of treatment-emergent adverse events. These most often consisted of abdominal pain, dyspepsia, nausea, pyrexia, dizziness, and headache. Only three avatrombopag patients developed platelet counts above 200 x 109 per liter, and they all remained asymptomatic, the investigators said.
Dova Pharmaceuticals makes avatrombopag and funded medical writing support. Dr. Terrault and three coinvestigators disclosed ties to AbbVie, Allergan, Bristol-Myers Squibb, Eisai, Gilead, Merck, and other pharmaceutical companies. One coinvestigator is chief medical officer of Dova, helped analyze the data and write the manuscript, and gave final approval of the submitted version.
SOURCE: Terrault N et al. Gastroenterology. 2018 May 17. doi: 10.1053/j.gastro.2018.05.025.
Thrombocytopenia in cirrhosis is frequent and multifactorial and includes sequestration in the spleen, reduced liver-derived thrombopoietin, bone marrow toxicity, and autoimmunity towards platelets. Severe thrombocytopenia (less than 50/nL) is rare in cirrhotic patients, but when it occurs may prevent required procedures from being performed or require platelet transfusions, which are associated with significant risks.
Previous attempts to increase platelets in cirrhotic patients with thrombopoietin agonists were halted because of increased frequency of portal vein thrombosis and hepatic decompensation.
Now avatrombopag has been specifically licensed with a 5-day regimen to increase platelets prior to elective interventions in severely thrombocytopenic (less than 50/nL) patients with chronic liver disease with a seemingly better safety profile than earlier treatments and good efficacy. The patient groups studied in the licensing trial had slightly milder but not significantly different liver disease, compared with those in the eltrombopag studies. The key difference was a pretreatment requirement of a portal vein flow of more than 10 cm/sec prior to enrollment, which likely reduced the risk of portal vein thrombosis. It is important that providers ready to use avatrombopag are aware of this.
Importantly, no data are currently available for patients with a Model for End-Stage Liver Disease score greater than 24, and very limited data are available for patients with Child B and Child C cirrhosis.
Given this limitation, careful judgment will be needed; a pretreatment portal vein flow may be advisable, though not a label requirement.
An observational study, NCT03554759, in patients with chronic liver disease and thrombocytopenia is ongoing and will further confirm the likely safety of avatrombopag.
Hans L. Tillmann, MD, is a clinical associate professor, East Carolina University, Greenville, and staff physician, Greenville (N.C.) VA Health Care Center. He has no relevant conflicts of interest.
Thrombocytopenia in cirrhosis is frequent and multifactorial and includes sequestration in the spleen, reduced liver-derived thrombopoietin, bone marrow toxicity, and autoimmunity towards platelets. Severe thrombocytopenia (less than 50/nL) is rare in cirrhotic patients, but when it occurs may prevent required procedures from being performed or require platelet transfusions, which are associated with significant risks.
Previous attempts to increase platelets in cirrhotic patients with thrombopoietin agonists were halted because of increased frequency of portal vein thrombosis and hepatic decompensation.
Now avatrombopag has been specifically licensed with a 5-day regimen to increase platelets prior to elective interventions in severely thrombocytopenic (less than 50/nL) patients with chronic liver disease with a seemingly better safety profile than earlier treatments and good efficacy. The patient groups studied in the licensing trial had slightly milder but not significantly different liver disease, compared with those in the eltrombopag studies. The key difference was a pretreatment requirement of a portal vein flow of more than 10 cm/sec prior to enrollment, which likely reduced the risk of portal vein thrombosis. It is important that providers ready to use avatrombopag are aware of this.
Importantly, no data are currently available for patients with a Model for End-Stage Liver Disease score greater than 24, and very limited data are available for patients with Child B and Child C cirrhosis.
Given this limitation, careful judgment will be needed; a pretreatment portal vein flow may be advisable, though not a label requirement.
An observational study, NCT03554759, in patients with chronic liver disease and thrombocytopenia is ongoing and will further confirm the likely safety of avatrombopag.
Hans L. Tillmann, MD, is a clinical associate professor, East Carolina University, Greenville, and staff physician, Greenville (N.C.) VA Health Care Center. He has no relevant conflicts of interest.
Thrombocytopenia in cirrhosis is frequent and multifactorial and includes sequestration in the spleen, reduced liver-derived thrombopoietin, bone marrow toxicity, and autoimmunity towards platelets. Severe thrombocytopenia (less than 50/nL) is rare in cirrhotic patients, but when it occurs may prevent required procedures from being performed or require platelet transfusions, which are associated with significant risks.
Previous attempts to increase platelets in cirrhotic patients with thrombopoietin agonists were halted because of increased frequency of portal vein thrombosis and hepatic decompensation.
Now avatrombopag has been specifically licensed with a 5-day regimen to increase platelets prior to elective interventions in severely thrombocytopenic (less than 50/nL) patients with chronic liver disease with a seemingly better safety profile than earlier treatments and good efficacy. The patient groups studied in the licensing trial had slightly milder but not significantly different liver disease, compared with those in the eltrombopag studies. The key difference was a pretreatment requirement of a portal vein flow of more than 10 cm/sec prior to enrollment, which likely reduced the risk of portal vein thrombosis. It is important that providers ready to use avatrombopag are aware of this.
Importantly, no data are currently available for patients with a Model for End-Stage Liver Disease score greater than 24, and very limited data are available for patients with Child B and Child C cirrhosis.
Given this limitation, careful judgment will be needed; a pretreatment portal vein flow may be advisable, though not a label requirement.
An observational study, NCT03554759, in patients with chronic liver disease and thrombocytopenia is ongoing and will further confirm the likely safety of avatrombopag.
Hans L. Tillmann, MD, is a clinical associate professor, East Carolina University, Greenville, and staff physician, Greenville (N.C.) VA Health Care Center. He has no relevant conflicts of interest.
Once-daily treatment with the oral second-generation thrombopoietin agonist avatrombopag (Doptelet) significantly reduced the need for platelet transfusion and rescue therapy for up to 7 days after patients with chronic liver disease and thrombocytopenia underwent scheduled procedures, according to the results of two international, randomized, double-blind, phase III, placebo-controlled trials reported in the September issue of Gastroenterology.
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
In the ADAPT-1 trial, 66% of patients in the 60-mg arm met this primary endpoint, as did 88% of patients who received 40 mg for less severe thrombocytopenia, versus 23% and 38% of the placebo arms, respectively (P less than .001 for each comparison). In the ADAPT-2 trial, 69% of the 60-mg group met the primary endpoint, as did 88% of the 40-mg group, versus 35% and 33% of the respective placebo groups (P less than .001 for each comparison).
These results led the Food and Drug Administration to approve avatrombopag in May 2018 under its priority review process. The novel therapy “may be a safe and effective alternative to platelet transfusions” that could simplify the clinical management of patients with chronic liver disease and thrombocytopenia, Norah Terrault, MD, MPH, and her associates wrote in Gastroenterology.
The ADAPT-1 study included 231 patients, while ADAPT-2 included 204 patients. In each trial, patients were randomized on a 2:1 basis to receive oral avatrombopag or placebo once daily for 5 consecutive days. Patients in the intervention arms received 60 mg avatrombopag if their baseline platelet count was less than 40 x 109 per liter, and 40 mg if their baseline platelet count was 40-50 x 109 per liter. Procedures were scheduled for 10-13 days after treatment initiation.
“Platelet counts increased by [treatment] day 4, peaked at days 10-13, and then returned to baseline levels by day 35,” the researchers reported. Among ADAPT-1 patients with low baseline counts, 69% of avatrombopag recipients reached a prespecified target of at least 50 x 109 platelets per liter on their procedure day, versus 4% of placebo recipients (P less than .0001). Corresponding proportions in ADAPT-2 were 67% and 7%, respectively (P less than .0001). Among patients with higher baseline counts, 88% and 20% achieved the target, respectively, in ADAPT-1 (P less than .0001), as did 93% versus 39%, respectively, in ADAPT-2 (P less than .0001).
Avatrombopag and placebo produced similar rates of treatment-emergent adverse events. These most often consisted of abdominal pain, dyspepsia, nausea, pyrexia, dizziness, and headache. Only three avatrombopag patients developed platelet counts above 200 x 109 per liter, and they all remained asymptomatic, the investigators said.
Dova Pharmaceuticals makes avatrombopag and funded medical writing support. Dr. Terrault and three coinvestigators disclosed ties to AbbVie, Allergan, Bristol-Myers Squibb, Eisai, Gilead, Merck, and other pharmaceutical companies. One coinvestigator is chief medical officer of Dova, helped analyze the data and write the manuscript, and gave final approval of the submitted version.
SOURCE: Terrault N et al. Gastroenterology. 2018 May 17. doi: 10.1053/j.gastro.2018.05.025.
Once-daily treatment with the oral second-generation thrombopoietin agonist avatrombopag (Doptelet) significantly reduced the need for platelet transfusion and rescue therapy for up to 7 days after patients with chronic liver disease and thrombocytopenia underwent scheduled procedures, according to the results of two international, randomized, double-blind, phase III, placebo-controlled trials reported in the September issue of Gastroenterology.
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
In the ADAPT-1 trial, 66% of patients in the 60-mg arm met this primary endpoint, as did 88% of patients who received 40 mg for less severe thrombocytopenia, versus 23% and 38% of the placebo arms, respectively (P less than .001 for each comparison). In the ADAPT-2 trial, 69% of the 60-mg group met the primary endpoint, as did 88% of the 40-mg group, versus 35% and 33% of the respective placebo groups (P less than .001 for each comparison).
These results led the Food and Drug Administration to approve avatrombopag in May 2018 under its priority review process. The novel therapy “may be a safe and effective alternative to platelet transfusions” that could simplify the clinical management of patients with chronic liver disease and thrombocytopenia, Norah Terrault, MD, MPH, and her associates wrote in Gastroenterology.
The ADAPT-1 study included 231 patients, while ADAPT-2 included 204 patients. In each trial, patients were randomized on a 2:1 basis to receive oral avatrombopag or placebo once daily for 5 consecutive days. Patients in the intervention arms received 60 mg avatrombopag if their baseline platelet count was less than 40 x 109 per liter, and 40 mg if their baseline platelet count was 40-50 x 109 per liter. Procedures were scheduled for 10-13 days after treatment initiation.
“Platelet counts increased by [treatment] day 4, peaked at days 10-13, and then returned to baseline levels by day 35,” the researchers reported. Among ADAPT-1 patients with low baseline counts, 69% of avatrombopag recipients reached a prespecified target of at least 50 x 109 platelets per liter on their procedure day, versus 4% of placebo recipients (P less than .0001). Corresponding proportions in ADAPT-2 were 67% and 7%, respectively (P less than .0001). Among patients with higher baseline counts, 88% and 20% achieved the target, respectively, in ADAPT-1 (P less than .0001), as did 93% versus 39%, respectively, in ADAPT-2 (P less than .0001).
Avatrombopag and placebo produced similar rates of treatment-emergent adverse events. These most often consisted of abdominal pain, dyspepsia, nausea, pyrexia, dizziness, and headache. Only three avatrombopag patients developed platelet counts above 200 x 109 per liter, and they all remained asymptomatic, the investigators said.
Dova Pharmaceuticals makes avatrombopag and funded medical writing support. Dr. Terrault and three coinvestigators disclosed ties to AbbVie, Allergan, Bristol-Myers Squibb, Eisai, Gilead, Merck, and other pharmaceutical companies. One coinvestigator is chief medical officer of Dova, helped analyze the data and write the manuscript, and gave final approval of the submitted version.
SOURCE: Terrault N et al. Gastroenterology. 2018 May 17. doi: 10.1053/j.gastro.2018.05.025.
FROM GASTROENTEROLOGY
Key clinical point: Once-daily treatment with oral avatrombopag significantly reduced the need for platelet transfusion and rescue therapy for up to 7 days after patients with chronic liver disease and thrombocytopenia underwent scheduled procedures.
Major finding: In the ADAPT-1 trial, 66% of patients in the 60-mg arm met this primary endpoint, as did 88% of patients who received 40 mg for less severe thrombocytopenia versus 23% and 38% of the placebo arms, respectively (P less than .001 for each comparison). In the ADAPT-2 trial, 69% of the 60-mg group met the primary endpoint, as did 88% of the 40-mg group versus 35% and 33% of the respective placebo groups (P less than .001 for each comparison).
Study details: ADAPT-1 and ADAPT-2, international, randomized, double-blind, placebo-controlled, phase III trials.
Disclosures: Dova Pharmaceuticals makes avatrombopag and funded medical writing support. Dr. Terrault and three coinvestigators disclosed ties to AbbVie, Allergan, BMS, Eisai, Gilead, Merck, and other pharmaceutical companies. One coinvestigator is chief medical officer of Dova, helped analyze the data and write the manuscript, and gave final approval of the submitted version.
Source: Terrault N et al. Gastroenterology. 2018 May 17. doi: 10.1053/j.gastro.2018.05.025.
Emicizumab beats factor VIII prophylaxis by a wide margin
In patients with hemophilia A who do not have factor VIII inhibitors, emicizumab therapy outperformed factor VIII prophylaxis, according to a results of a randomized, open-label, phase 3 trial.
Patients treated with emicizumab had a significantly lower bleeding rate than without prophylaxis, and more than half of the treated patients had no treated bleeding events during the trial, reported lead author Johnny Mahlangu, MD, of the University of the Witwatersrand in Johannesburg, South Africa, and his colleagues.
For patients with severe hemophilia A, factor VIII infusions are standard prophylaxis for bleeding events; however, because of the short half-life of factor VIII, multiple infusions are needed each week, and bleeding events may still occur.
“Treatments with a high efficacy and reduced burden are needed,” the authors wrote in the New England Journal of Medicine.
Emicizumab may serve as such a treatment. It is a monoclonal antibody that joins activated factor IX with factor X. This combination restores hemostasis by replacing missing factor VIII. In 2017, the FDA approved emicizumab for patients with hemophilia A and anti-factor VIII alloantibodies (inhibitors).
The HAVEN 3 trial involved 152 patients with hemophilia A who did not have inhibitors. Patients were divided into four groups. The first three groups (A, B, and C) consisted of patients who had previously been receiving episodic factor VIII therapy. During the trial, group A received emicizumab 1.5 mg/kg per week, group B received emicizumab 3.0 mg/kg every 2 weeks, and group C received no prophylaxis. The fourth group (D) consisted of patients who had previously received factor VIII prophylaxis. This group received emicizumab 1.5 mg/kg per week.
Patients who received no prophylaxis had an annualized bleeding rate of 38.2 events. For patients treated with emicizumab, the annualized bleeding rates were 1.5 events for group A (96% lower than no prophylaxis; P less than .001) and 1.3 events for group B (97% lower than no prophylaxis; P less than .001).
More than half of the patients treated with emicizumab had no treated bleeding events during the trial; in comparison, all of the patients who did not receive prophylaxis had bleeding events. An intraindividual comparison of 48 patients (group D) showed that individuals treated with emicizumab had a 68% lower annualized bleeding rate than when they were receiving factor VIII prophylaxis (decrease from 4.8 events to 1.5 events; P less than .001).
The most common adverse event associated with emicizumab was injection-site reaction in 38 patients (25%). No thrombotic events, thrombotic microangiopathies, or deaths occurred.
“Although it is difficult to predict how the treatment approach will evolve, emicizumab therapy represents one option that holds promise to improve the care of patients with hemophilia A,” the researchers wrote.
The HAVEN 3 trial was funded by F. Hoffmann-La Roche and Chugai Pharmaceutical. The researchers reported support from Bayer, Baxalta, CSL Behring, and others.
SOURCE: Mahlangu et al. N Engl J Med. 2018;379:811-22.
The HAVEN 3 trial by Mahlangu et al. showed that emicizumab can significantly decrease the incidence of bleeding events in patients with hemophilia A, but questions remain about impacts on factor VIII tolerance and the role of emicizumab in long-term management, according to Margaret V. Ragni, MD.
The HAVEN 3 results showed that emicizumab was highly effective for hemophilia A patients who did not have inhibitors. In addition, thrombosis or thrombotic microangiopathy did not occur in the trial, as breakthrough bleeding was treated with factor VIII concentrate instead of prothrombin concentrate, which was used in previous trials.
“The absence of thrombosis with factor VIII concentrate is probably related to the fact that factor VIII has an affinity for binding factors IXa and X that is 10 times as high as that of emicizumab, so it displaces emicizumab, thereby avoiding potential additive toxicity,” Dr. Ragni wrote.
A survey conducted in the trial showed that patients preferred emicizumab over factor VIII, but Dr. Ragni noted that “there was no direct comparison, nor was the basis specified for the preference, such as a simpler route of administration or a reduction in the incidence of joint bleeding events or pain severity.”
Questions surrounding joint disease, efficacy for acute bleeding, and cost should be addressed before emicizumab replaces factor VIII therapy as standard of care. Additionally, the relationship between emicizumab and inhibitor immune tolerance remains unclear. One trial participant exhibited recurrence of anti-factor VIII antibody, “a finding that suggests incomplete inhibitor suppression by emicizumab prophylaxis.”
“Until more is known, it will be important for discussions between providers and patients to focus on risk, benefit, cost, and participation in observational studies and clinical trials,” Dr. Ragni wrote.
Margaret V. Ragni, MD , is with the division of hematology/oncology at the University of Pittsburgh. Dr. Ragni reported funding from Bayer, Biomarin, NovoNordisk, and others. These comments are adapted from an accompanying editorial ( N Engl J Med. 2018;379:880-2 ).
The HAVEN 3 trial by Mahlangu et al. showed that emicizumab can significantly decrease the incidence of bleeding events in patients with hemophilia A, but questions remain about impacts on factor VIII tolerance and the role of emicizumab in long-term management, according to Margaret V. Ragni, MD.
The HAVEN 3 results showed that emicizumab was highly effective for hemophilia A patients who did not have inhibitors. In addition, thrombosis or thrombotic microangiopathy did not occur in the trial, as breakthrough bleeding was treated with factor VIII concentrate instead of prothrombin concentrate, which was used in previous trials.
“The absence of thrombosis with factor VIII concentrate is probably related to the fact that factor VIII has an affinity for binding factors IXa and X that is 10 times as high as that of emicizumab, so it displaces emicizumab, thereby avoiding potential additive toxicity,” Dr. Ragni wrote.
A survey conducted in the trial showed that patients preferred emicizumab over factor VIII, but Dr. Ragni noted that “there was no direct comparison, nor was the basis specified for the preference, such as a simpler route of administration or a reduction in the incidence of joint bleeding events or pain severity.”
Questions surrounding joint disease, efficacy for acute bleeding, and cost should be addressed before emicizumab replaces factor VIII therapy as standard of care. Additionally, the relationship between emicizumab and inhibitor immune tolerance remains unclear. One trial participant exhibited recurrence of anti-factor VIII antibody, “a finding that suggests incomplete inhibitor suppression by emicizumab prophylaxis.”
“Until more is known, it will be important for discussions between providers and patients to focus on risk, benefit, cost, and participation in observational studies and clinical trials,” Dr. Ragni wrote.
Margaret V. Ragni, MD , is with the division of hematology/oncology at the University of Pittsburgh. Dr. Ragni reported funding from Bayer, Biomarin, NovoNordisk, and others. These comments are adapted from an accompanying editorial ( N Engl J Med. 2018;379:880-2 ).
The HAVEN 3 trial by Mahlangu et al. showed that emicizumab can significantly decrease the incidence of bleeding events in patients with hemophilia A, but questions remain about impacts on factor VIII tolerance and the role of emicizumab in long-term management, according to Margaret V. Ragni, MD.
The HAVEN 3 results showed that emicizumab was highly effective for hemophilia A patients who did not have inhibitors. In addition, thrombosis or thrombotic microangiopathy did not occur in the trial, as breakthrough bleeding was treated with factor VIII concentrate instead of prothrombin concentrate, which was used in previous trials.
“The absence of thrombosis with factor VIII concentrate is probably related to the fact that factor VIII has an affinity for binding factors IXa and X that is 10 times as high as that of emicizumab, so it displaces emicizumab, thereby avoiding potential additive toxicity,” Dr. Ragni wrote.
A survey conducted in the trial showed that patients preferred emicizumab over factor VIII, but Dr. Ragni noted that “there was no direct comparison, nor was the basis specified for the preference, such as a simpler route of administration or a reduction in the incidence of joint bleeding events or pain severity.”
Questions surrounding joint disease, efficacy for acute bleeding, and cost should be addressed before emicizumab replaces factor VIII therapy as standard of care. Additionally, the relationship between emicizumab and inhibitor immune tolerance remains unclear. One trial participant exhibited recurrence of anti-factor VIII antibody, “a finding that suggests incomplete inhibitor suppression by emicizumab prophylaxis.”
“Until more is known, it will be important for discussions between providers and patients to focus on risk, benefit, cost, and participation in observational studies and clinical trials,” Dr. Ragni wrote.
Margaret V. Ragni, MD , is with the division of hematology/oncology at the University of Pittsburgh. Dr. Ragni reported funding from Bayer, Biomarin, NovoNordisk, and others. These comments are adapted from an accompanying editorial ( N Engl J Med. 2018;379:880-2 ).
In patients with hemophilia A who do not have factor VIII inhibitors, emicizumab therapy outperformed factor VIII prophylaxis, according to a results of a randomized, open-label, phase 3 trial.
Patients treated with emicizumab had a significantly lower bleeding rate than without prophylaxis, and more than half of the treated patients had no treated bleeding events during the trial, reported lead author Johnny Mahlangu, MD, of the University of the Witwatersrand in Johannesburg, South Africa, and his colleagues.
For patients with severe hemophilia A, factor VIII infusions are standard prophylaxis for bleeding events; however, because of the short half-life of factor VIII, multiple infusions are needed each week, and bleeding events may still occur.
“Treatments with a high efficacy and reduced burden are needed,” the authors wrote in the New England Journal of Medicine.
Emicizumab may serve as such a treatment. It is a monoclonal antibody that joins activated factor IX with factor X. This combination restores hemostasis by replacing missing factor VIII. In 2017, the FDA approved emicizumab for patients with hemophilia A and anti-factor VIII alloantibodies (inhibitors).
The HAVEN 3 trial involved 152 patients with hemophilia A who did not have inhibitors. Patients were divided into four groups. The first three groups (A, B, and C) consisted of patients who had previously been receiving episodic factor VIII therapy. During the trial, group A received emicizumab 1.5 mg/kg per week, group B received emicizumab 3.0 mg/kg every 2 weeks, and group C received no prophylaxis. The fourth group (D) consisted of patients who had previously received factor VIII prophylaxis. This group received emicizumab 1.5 mg/kg per week.
Patients who received no prophylaxis had an annualized bleeding rate of 38.2 events. For patients treated with emicizumab, the annualized bleeding rates were 1.5 events for group A (96% lower than no prophylaxis; P less than .001) and 1.3 events for group B (97% lower than no prophylaxis; P less than .001).
More than half of the patients treated with emicizumab had no treated bleeding events during the trial; in comparison, all of the patients who did not receive prophylaxis had bleeding events. An intraindividual comparison of 48 patients (group D) showed that individuals treated with emicizumab had a 68% lower annualized bleeding rate than when they were receiving factor VIII prophylaxis (decrease from 4.8 events to 1.5 events; P less than .001).
The most common adverse event associated with emicizumab was injection-site reaction in 38 patients (25%). No thrombotic events, thrombotic microangiopathies, or deaths occurred.
“Although it is difficult to predict how the treatment approach will evolve, emicizumab therapy represents one option that holds promise to improve the care of patients with hemophilia A,” the researchers wrote.
The HAVEN 3 trial was funded by F. Hoffmann-La Roche and Chugai Pharmaceutical. The researchers reported support from Bayer, Baxalta, CSL Behring, and others.
SOURCE: Mahlangu et al. N Engl J Med. 2018;379:811-22.
In patients with hemophilia A who do not have factor VIII inhibitors, emicizumab therapy outperformed factor VIII prophylaxis, according to a results of a randomized, open-label, phase 3 trial.
Patients treated with emicizumab had a significantly lower bleeding rate than without prophylaxis, and more than half of the treated patients had no treated bleeding events during the trial, reported lead author Johnny Mahlangu, MD, of the University of the Witwatersrand in Johannesburg, South Africa, and his colleagues.
For patients with severe hemophilia A, factor VIII infusions are standard prophylaxis for bleeding events; however, because of the short half-life of factor VIII, multiple infusions are needed each week, and bleeding events may still occur.
“Treatments with a high efficacy and reduced burden are needed,” the authors wrote in the New England Journal of Medicine.
Emicizumab may serve as such a treatment. It is a monoclonal antibody that joins activated factor IX with factor X. This combination restores hemostasis by replacing missing factor VIII. In 2017, the FDA approved emicizumab for patients with hemophilia A and anti-factor VIII alloantibodies (inhibitors).
The HAVEN 3 trial involved 152 patients with hemophilia A who did not have inhibitors. Patients were divided into four groups. The first three groups (A, B, and C) consisted of patients who had previously been receiving episodic factor VIII therapy. During the trial, group A received emicizumab 1.5 mg/kg per week, group B received emicizumab 3.0 mg/kg every 2 weeks, and group C received no prophylaxis. The fourth group (D) consisted of patients who had previously received factor VIII prophylaxis. This group received emicizumab 1.5 mg/kg per week.
Patients who received no prophylaxis had an annualized bleeding rate of 38.2 events. For patients treated with emicizumab, the annualized bleeding rates were 1.5 events for group A (96% lower than no prophylaxis; P less than .001) and 1.3 events for group B (97% lower than no prophylaxis; P less than .001).
More than half of the patients treated with emicizumab had no treated bleeding events during the trial; in comparison, all of the patients who did not receive prophylaxis had bleeding events. An intraindividual comparison of 48 patients (group D) showed that individuals treated with emicizumab had a 68% lower annualized bleeding rate than when they were receiving factor VIII prophylaxis (decrease from 4.8 events to 1.5 events; P less than .001).
The most common adverse event associated with emicizumab was injection-site reaction in 38 patients (25%). No thrombotic events, thrombotic microangiopathies, or deaths occurred.
“Although it is difficult to predict how the treatment approach will evolve, emicizumab therapy represents one option that holds promise to improve the care of patients with hemophilia A,” the researchers wrote.
The HAVEN 3 trial was funded by F. Hoffmann-La Roche and Chugai Pharmaceutical. The researchers reported support from Bayer, Baxalta, CSL Behring, and others.
SOURCE: Mahlangu et al. N Engl J Med. 2018;379:811-22.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Treatment with emicizumab resulted in 68% fewer bleeding events, compared with factor VIII prophylaxis (P less than .001).
Study details: HAVEN 3 was a randomized, open-label phase 3 trial involving 152 patients who had hemophilia A without inhibitors.
Disclosures: The study was funded by F. Hoffmann-La Roche and Chugai Pharmaceutical. The authors reported support from Bayer, Baxalta, CSL Behring, and others.
Source: Mahlangu et al. N Engl J Med. 2018;379:811-22.
VA and NCI Join to Provide Leading-Edge Cancer Treatment
The National Cancer Institute (NCI) and VA Interagency Group to Accelerate Trials Enrollment, or NAVIGATE, is launching at 12 VA sites. At each site, it will build infrastructure that will allow more veterans to take part in cutting-edge clinical trials, such as those testing promising experimental treatments.
The VA has a robust clinical research program but VA facilities often face challenges initiating and completing externally funded trials because of the need for partners to navigate the system, says NCI. The joint program is intended to overcome those challenges with dedicated staffing and a sustainable infrastructure, and to address barriers to trial enrollment that veterans, including minority patients, often experience.
The NCI will provide infrastructure funding needed for the VA facilities to participate in the National Clinical Trials Network and the NCI Community Oncology Research Program. In turn, the VA will manage organizational and operational activities to establish a network to focus on NCI trial goals.
The program will be jointly managed for up to 3 years, during which time the VA sites will establish long-term capabilities to keep the program going. They will also establish best practices and share insights with other VA sites nationwide.
The National Cancer Institute (NCI) and VA Interagency Group to Accelerate Trials Enrollment, or NAVIGATE, is launching at 12 VA sites. At each site, it will build infrastructure that will allow more veterans to take part in cutting-edge clinical trials, such as those testing promising experimental treatments.
The VA has a robust clinical research program but VA facilities often face challenges initiating and completing externally funded trials because of the need for partners to navigate the system, says NCI. The joint program is intended to overcome those challenges with dedicated staffing and a sustainable infrastructure, and to address barriers to trial enrollment that veterans, including minority patients, often experience.
The NCI will provide infrastructure funding needed for the VA facilities to participate in the National Clinical Trials Network and the NCI Community Oncology Research Program. In turn, the VA will manage organizational and operational activities to establish a network to focus on NCI trial goals.
The program will be jointly managed for up to 3 years, during which time the VA sites will establish long-term capabilities to keep the program going. They will also establish best practices and share insights with other VA sites nationwide.
The National Cancer Institute (NCI) and VA Interagency Group to Accelerate Trials Enrollment, or NAVIGATE, is launching at 12 VA sites. At each site, it will build infrastructure that will allow more veterans to take part in cutting-edge clinical trials, such as those testing promising experimental treatments.
The VA has a robust clinical research program but VA facilities often face challenges initiating and completing externally funded trials because of the need for partners to navigate the system, says NCI. The joint program is intended to overcome those challenges with dedicated staffing and a sustainable infrastructure, and to address barriers to trial enrollment that veterans, including minority patients, often experience.
The NCI will provide infrastructure funding needed for the VA facilities to participate in the National Clinical Trials Network and the NCI Community Oncology Research Program. In turn, the VA will manage organizational and operational activities to establish a network to focus on NCI trial goals.
The program will be jointly managed for up to 3 years, during which time the VA sites will establish long-term capabilities to keep the program going. They will also establish best practices and share insights with other VA sites nationwide.
CMS finalizes CAR T-cell therapy inpatient payments
Medical associations are expressing disappointment at the new payment scheme put forward by the Centers for Medicare & Medicaid Services for inpatient administration of two chimeric antigen receptor (CAR) T-cell therapies, calling the reimbursement insufficient for use of the expensive medications.
Under its Aug. 17 final rule, CMS will now categorize CAR T-cell therapies under the umbrella of the renamed Medicare Severity–Diagnosis Related Groups (MS-DRG) 016 – Autologous Bone Marrow Transplant with CC/MCC or T-cell Immunotherapy – and assign ICD-10-PCS procedure codes XW033C3 and XW043C3 to the use of axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) in the inpatient setting for fiscal year 2019, which begins in October 2018.
CMS also approved a temporary New Technology Add-On Payment (NTAP) for use of the therapies with a maximum threshold of $186,500, according to the rule.
According to the American Society of Hematology (ASH), this payment structure is an improvement, but it hardly covers the cost of the products, nor does it account for full hospitalization costs. ASH noted that the revised MS-DRG 016 has a base payment rate of $36,000 and that the maximum NTAP payment ($186,500) is only about half of the cost for a CAR T-cell product.
“ASH is concerned that this final policy may impede access to care to this cutting-edge therapy because hospitals and academic medical centers that provide this personalized treatment will simply not be able to withstand the negative financial impact,” the society said in a statement. “While this final policy represents an improvement over current CAR T therapy reimbursement rates, ASH believes patient access to care will be jeopardized as providers and hospitals will not be able to afford to deliver the therapy at this reimbursement rate, particularly as other CAR T products receive FDA [Food and Drug Administration] approval.”
ASH and the American Society for Blood and Marrow Transplantation (ASBMT) had strongly urged CMS to develop a site-neutral, equitable payment structure that would have allowed providers to recover more product acquisition costs from CAR T-cell therapies. In its final rule, CMS stated that it was too early to develop a novel payment structure for CAR T-cell treatments and that more research is needed before such changes are made. The agency noted that in May CMS opened a national coverage determination analysis on CAR T-cell therapy for Medicare patients with advanced cancer, which is expected to be completed by May 2019.
“[CMS] is soliciting public comment … on key design considerations for developing a potential model that would test private market strategies and introduce competition to improve quality of care for beneficiaries,” the agency said in the rule. “Given the relative newness of CAR T-cell therapy, the potential model, and our request for feedback on this model approach, we believe it would be premature to adopt changes to our existing payment mechanisms.”
The payment outline by CMS is essentially the bare minimum it could have extended to CAR T-cell therapies for 2019, said Stephanie Farnia, director of health policy and strategic relations for the ASBMT.
“[ASBMT] and a number of stakeholders have been very clear in our comment letters that that would not be enough and the reasons why,” Ms. Farnia said in an interview. “It’s not going to be sufficient to cover the cost of care or the product.”
The rule also fails to address the cancer centers that are exempt from the DRG payment system, Ms. Farnia said. Eleven centers are excluded from the payment system because of past legislation that excludes exclusive cancer hospitals that do not provide noncancer services. The exempt cancer centers cannot receive additional money for new or expensive drugs and therefore will not gain any financial relief from the CAR T-cell therapy payment changes in the CMS final rule.
ASH officials plan to follow up with congressional leaders to identify ways to improve future CAR T-cell therapy payments, including a potential legislative solution. An ASH spokesperson declined to elaborate on its ideal legislative remedy.
Hospital administrators and physicians will need to have difficult conversations in the upcoming year about whether treating patients with CAR T-cell therapies is worth the cost deficits, Ms. Farnia said.
“Everyone was really counting on it being a different reimbursement scenario for the upcoming fiscal year, and it is, but again, it’s that bare minimum difference,” Ms. Farnia said. “I think a number of programs are going to be taking a look at their financial experience thus far and comparing that to the reimbursement and deciding on if they [should] continue to offer it and how to do that.”
In April 2018, CMS announced payment rates for outpatient administration of the two drugs, settling on $395,380 for axicabtagene ciloleucel and $500,839 for tisagenlecleucel. The two medications have list prices of $373,000 and $475,000, respectively.
However, physicians have raised concerns that even if the drugs are first administered in the outpatient setting, inpatient care is likely to occur with CAR T-cell therapies because some patients will need to be admitted in order to be monitored for serious side effects. In such cases, all payments will become part of the inpatient stay under CMS’s 3-day payment window rule.
Medical associations are expressing disappointment at the new payment scheme put forward by the Centers for Medicare & Medicaid Services for inpatient administration of two chimeric antigen receptor (CAR) T-cell therapies, calling the reimbursement insufficient for use of the expensive medications.
Under its Aug. 17 final rule, CMS will now categorize CAR T-cell therapies under the umbrella of the renamed Medicare Severity–Diagnosis Related Groups (MS-DRG) 016 – Autologous Bone Marrow Transplant with CC/MCC or T-cell Immunotherapy – and assign ICD-10-PCS procedure codes XW033C3 and XW043C3 to the use of axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) in the inpatient setting for fiscal year 2019, which begins in October 2018.
CMS also approved a temporary New Technology Add-On Payment (NTAP) for use of the therapies with a maximum threshold of $186,500, according to the rule.
According to the American Society of Hematology (ASH), this payment structure is an improvement, but it hardly covers the cost of the products, nor does it account for full hospitalization costs. ASH noted that the revised MS-DRG 016 has a base payment rate of $36,000 and that the maximum NTAP payment ($186,500) is only about half of the cost for a CAR T-cell product.
“ASH is concerned that this final policy may impede access to care to this cutting-edge therapy because hospitals and academic medical centers that provide this personalized treatment will simply not be able to withstand the negative financial impact,” the society said in a statement. “While this final policy represents an improvement over current CAR T therapy reimbursement rates, ASH believes patient access to care will be jeopardized as providers and hospitals will not be able to afford to deliver the therapy at this reimbursement rate, particularly as other CAR T products receive FDA [Food and Drug Administration] approval.”
ASH and the American Society for Blood and Marrow Transplantation (ASBMT) had strongly urged CMS to develop a site-neutral, equitable payment structure that would have allowed providers to recover more product acquisition costs from CAR T-cell therapies. In its final rule, CMS stated that it was too early to develop a novel payment structure for CAR T-cell treatments and that more research is needed before such changes are made. The agency noted that in May CMS opened a national coverage determination analysis on CAR T-cell therapy for Medicare patients with advanced cancer, which is expected to be completed by May 2019.
“[CMS] is soliciting public comment … on key design considerations for developing a potential model that would test private market strategies and introduce competition to improve quality of care for beneficiaries,” the agency said in the rule. “Given the relative newness of CAR T-cell therapy, the potential model, and our request for feedback on this model approach, we believe it would be premature to adopt changes to our existing payment mechanisms.”
The payment outline by CMS is essentially the bare minimum it could have extended to CAR T-cell therapies for 2019, said Stephanie Farnia, director of health policy and strategic relations for the ASBMT.
“[ASBMT] and a number of stakeholders have been very clear in our comment letters that that would not be enough and the reasons why,” Ms. Farnia said in an interview. “It’s not going to be sufficient to cover the cost of care or the product.”
The rule also fails to address the cancer centers that are exempt from the DRG payment system, Ms. Farnia said. Eleven centers are excluded from the payment system because of past legislation that excludes exclusive cancer hospitals that do not provide noncancer services. The exempt cancer centers cannot receive additional money for new or expensive drugs and therefore will not gain any financial relief from the CAR T-cell therapy payment changes in the CMS final rule.
ASH officials plan to follow up with congressional leaders to identify ways to improve future CAR T-cell therapy payments, including a potential legislative solution. An ASH spokesperson declined to elaborate on its ideal legislative remedy.
Hospital administrators and physicians will need to have difficult conversations in the upcoming year about whether treating patients with CAR T-cell therapies is worth the cost deficits, Ms. Farnia said.
“Everyone was really counting on it being a different reimbursement scenario for the upcoming fiscal year, and it is, but again, it’s that bare minimum difference,” Ms. Farnia said. “I think a number of programs are going to be taking a look at their financial experience thus far and comparing that to the reimbursement and deciding on if they [should] continue to offer it and how to do that.”
In April 2018, CMS announced payment rates for outpatient administration of the two drugs, settling on $395,380 for axicabtagene ciloleucel and $500,839 for tisagenlecleucel. The two medications have list prices of $373,000 and $475,000, respectively.
However, physicians have raised concerns that even if the drugs are first administered in the outpatient setting, inpatient care is likely to occur with CAR T-cell therapies because some patients will need to be admitted in order to be monitored for serious side effects. In such cases, all payments will become part of the inpatient stay under CMS’s 3-day payment window rule.
Medical associations are expressing disappointment at the new payment scheme put forward by the Centers for Medicare & Medicaid Services for inpatient administration of two chimeric antigen receptor (CAR) T-cell therapies, calling the reimbursement insufficient for use of the expensive medications.
Under its Aug. 17 final rule, CMS will now categorize CAR T-cell therapies under the umbrella of the renamed Medicare Severity–Diagnosis Related Groups (MS-DRG) 016 – Autologous Bone Marrow Transplant with CC/MCC or T-cell Immunotherapy – and assign ICD-10-PCS procedure codes XW033C3 and XW043C3 to the use of axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) in the inpatient setting for fiscal year 2019, which begins in October 2018.
CMS also approved a temporary New Technology Add-On Payment (NTAP) for use of the therapies with a maximum threshold of $186,500, according to the rule.
According to the American Society of Hematology (ASH), this payment structure is an improvement, but it hardly covers the cost of the products, nor does it account for full hospitalization costs. ASH noted that the revised MS-DRG 016 has a base payment rate of $36,000 and that the maximum NTAP payment ($186,500) is only about half of the cost for a CAR T-cell product.
“ASH is concerned that this final policy may impede access to care to this cutting-edge therapy because hospitals and academic medical centers that provide this personalized treatment will simply not be able to withstand the negative financial impact,” the society said in a statement. “While this final policy represents an improvement over current CAR T therapy reimbursement rates, ASH believes patient access to care will be jeopardized as providers and hospitals will not be able to afford to deliver the therapy at this reimbursement rate, particularly as other CAR T products receive FDA [Food and Drug Administration] approval.”
ASH and the American Society for Blood and Marrow Transplantation (ASBMT) had strongly urged CMS to develop a site-neutral, equitable payment structure that would have allowed providers to recover more product acquisition costs from CAR T-cell therapies. In its final rule, CMS stated that it was too early to develop a novel payment structure for CAR T-cell treatments and that more research is needed before such changes are made. The agency noted that in May CMS opened a national coverage determination analysis on CAR T-cell therapy for Medicare patients with advanced cancer, which is expected to be completed by May 2019.
“[CMS] is soliciting public comment … on key design considerations for developing a potential model that would test private market strategies and introduce competition to improve quality of care for beneficiaries,” the agency said in the rule. “Given the relative newness of CAR T-cell therapy, the potential model, and our request for feedback on this model approach, we believe it would be premature to adopt changes to our existing payment mechanisms.”
The payment outline by CMS is essentially the bare minimum it could have extended to CAR T-cell therapies for 2019, said Stephanie Farnia, director of health policy and strategic relations for the ASBMT.
“[ASBMT] and a number of stakeholders have been very clear in our comment letters that that would not be enough and the reasons why,” Ms. Farnia said in an interview. “It’s not going to be sufficient to cover the cost of care or the product.”
The rule also fails to address the cancer centers that are exempt from the DRG payment system, Ms. Farnia said. Eleven centers are excluded from the payment system because of past legislation that excludes exclusive cancer hospitals that do not provide noncancer services. The exempt cancer centers cannot receive additional money for new or expensive drugs and therefore will not gain any financial relief from the CAR T-cell therapy payment changes in the CMS final rule.
ASH officials plan to follow up with congressional leaders to identify ways to improve future CAR T-cell therapy payments, including a potential legislative solution. An ASH spokesperson declined to elaborate on its ideal legislative remedy.
Hospital administrators and physicians will need to have difficult conversations in the upcoming year about whether treating patients with CAR T-cell therapies is worth the cost deficits, Ms. Farnia said.
“Everyone was really counting on it being a different reimbursement scenario for the upcoming fiscal year, and it is, but again, it’s that bare minimum difference,” Ms. Farnia said. “I think a number of programs are going to be taking a look at their financial experience thus far and comparing that to the reimbursement and deciding on if they [should] continue to offer it and how to do that.”
In April 2018, CMS announced payment rates for outpatient administration of the two drugs, settling on $395,380 for axicabtagene ciloleucel and $500,839 for tisagenlecleucel. The two medications have list prices of $373,000 and $475,000, respectively.
However, physicians have raised concerns that even if the drugs are first administered in the outpatient setting, inpatient care is likely to occur with CAR T-cell therapies because some patients will need to be admitted in order to be monitored for serious side effects. In such cases, all payments will become part of the inpatient stay under CMS’s 3-day payment window rule.
Cervical cancer screening recommendations vary by age and risk
Screen women for cervical cancer with basic cytology starting at age 21 years, and consider adding high-risk human papillomavirus (hrHPV) testing alone or with cytology for women aged 30 years and older, the U.S. Preventive Services Task Force recommended in an updated statement on cervical cancer screening .
The statement, accompanying evidence report, and a modeling study were published online in JAMA.
Cervical cancer deaths in the United States have declined from 2.8 deaths per 100,000 women in 2000 to 2.3 deaths per 100,000 women in 2015 because of the adoption of widespread screening, according to Susan J. Curry, PhD., of the University of Iowa, Iowa City, and her colleagues in the USPSTF (JAMA. 2018 Aug 21. doi: 10.1001/jama.2018.10897.
Based on the latest evidence and the modeling study, the USPSTF gives an A recommendation to screening women aged 21-29 years for cervical cancer every 3 years with cervical cytology alone. The task force also gives an A to screening women aged 30-65 years every 5 years with either hrHPV testing alone or in combination with cytology.
The task force recommends against screening (D recommendation) for women younger than 21 years, older than 65 years with a history of screening and low cervical cancer risk, and women who have had hysterectomies with removal of the cervix and no history of cervical cancer risk.
To update the previous recommendations issued in 2012, the task force reviewed the latest evidence and commissioned a modeling study to help determine the best screening strategies in terms of age, screening intervals, and risks vs. benefits.
In the model, researchers assessed 19 strategies for cervical cancer screening based on a hypothetical cohort of women who began screening at 21 years of age.
Overall, the different strategies were similar in effectiveness, but primary hrHPV testing and alternative cotesting were slightly more effective: Cervical cancer deaths ranged from 0.23 to 0.29 deaths per 1,000 women in strategies involving hrHPV testing or cotesting, vs. 0.30 to 0.76 deaths per 1,000 women for strategies based on the current guidelines.
In addition, switching the age of hrHPV testing from 25 years to 30 years and using a 5-year screening interval showed the most effectiveness in terms of risks vs. harms, wrote Jane K. Kim, PhD, of Harvard University, Boston, and her colleagues (JAMA. 2018 Aug 21. doi: 10.1001/jama.2017.19872). “Switching from cytology to 5-year primary hrHPV testing at age 30 years (strategy 14) was associated with a ratio of 640 colposcopies per cancer case averted; earlier switch ages required a greater number of colposcopies per cancer case averted.”
The recommendations also were supported by an evidence report including eight randomized, controlled trials of 410,556 women, five cohort studies of 402,615 women, and a meta-analysis of individual participant data including 176,464 women.
The evidence report sought to address the benefits and harms of cervical cancer screening using hrHPV screening alone as the primary screening method or paired with cytology (cotesting), compared with primary screening using cytology alone.
Overall, both hrHPV and hrHPV plus cytology were associated with higher rates of false-positives and colposcopy compared with cytology alone, “which could lead to more treatments with potential harms,” wrote Joy Melnikow, MD, of the University of California, Davis, and her colleagues (JAMA. 2018 Aug 21. doi: 10.1001/jama.2018.10400.
In addition, hrHPV testing yielded higher rates of positive cervical intraepithelial neoplasia, compared with cytology alone as initial screening.
However, further research is needed to address the impact of any cervical cancer screening strategies in populations with limited access to health care and screening, the researchers noted.
The updated USPSTF recommendations are largely in line with those issued by leading women’s health organizations including the American College of Obstetricians and Gynecologists, ASCCP, and the Society for Gynecologic Oncology, according to a joint statement.
“With a number of screening options now available, the new guidelines emphasize the importance of the patient-provider shared decision-making process to assist women in making an informed choice about which screening method is most suitable for them,” according to the statement, “However, more importantly, there needs to be a continued effort to ensure all women are adequately screened because a significant number of women in the country are not. It’s also essential for women to have access to all of the tests and that they are appropriately covered by insurance companies.
“We hope the USPSTF recommendations foster more discussions between patients and providers about cervical cancer screening, promote opportunities for patient education on the benefits and safety of HPV vaccination for cervical cancer prevention and encourage providers to offer HPV vaccines in their offices,” the statement noted.
The USPSTF research was funded by the Agency for Healthcare Research and Quality. The researchers for the modeling report were supported in part by a National Cancer Institute grant. The researchers had no relevant financial conflicts to disclose.
SOURCES: Kim J et al. JAMA. 2018 Aug 21. doi: 10.1001/jama.2017.19872; Melnikow J et al. JAMA. 2018 Aug 21. doi: 10.1001/jama.2018.10400; Curry S et al. JAMA. 2018 Aug 21. doi: 10.1001/jama.2018.10897.
In 2016, the Society for Gynecologic Oncology (SGO) recommended screening with the newly approved hrHPV test for women aged 25 years and older, with rescreening 3 years later if the test was negative, George F. Sawaya, MD, wrote in an accompanying editorial published in JAMA Internal Medicine. The new recommendations from the U.S. Preventive Services Task Force do not endorse a single triage strategy, and do not consider costs, he said.
“Although the USPSTF sets the standard for evidence-based recommendations and acknowledges the critical value of high-quality evidence in making recommendations, it might reasonably be asked, where is the evidence of value in cervical cancer screening?” Dr. Sawaya wrote.
The updated USPSTF recommendations differ from the SGO recommendation by changing the starting age for hrHPV testing to 30 years from 25, and rescreening at 5-year intervals.
“The USPSTF recommendation that HPV testing not begin until age 30 years seems prudent,” Dr. Sawaya said, in light of the evidence report and modeling analysis of harms and benefits. He noted that the evidence reviewed by the task force showed that HPV testing and cotesting resulting in a small amount of life-years gained compared with no testing, but with the trade-off of more follow-up tests and colposcopies.
“From the perspective of society, it has been proposed that cost-effectiveness analyses be an essential part of the guideline process,” Dr. Sawaya noted. “To assist in policy decisions that many professional societies will soon face, a study that I am leading is seeking to use cost-effectiveness analyses to determine the range of reasonable options for cervical cancer screening. Such analyses may inform future screening recommendations.”
Dr. Sawaya is affiliated with the University of California, San Francisco. These comments are taken from an editorial accompanying USPSTF recommendations on cervical cancer screening (JAMA Intern Med. 2018 Aug 21. doi: 10.1001/jamainternmed.2018.4282). He disclosed serving as the principal investigator of a National Cancer Institute study on cost-effectiveness analyses to determine reasonable options for cervical cancer screening. He also served as a member of the U.S. Preventive Services Task Force from 2004 to 2008.
In 2016, the Society for Gynecologic Oncology (SGO) recommended screening with the newly approved hrHPV test for women aged 25 years and older, with rescreening 3 years later if the test was negative, George F. Sawaya, MD, wrote in an accompanying editorial published in JAMA Internal Medicine. The new recommendations from the U.S. Preventive Services Task Force do not endorse a single triage strategy, and do not consider costs, he said.
“Although the USPSTF sets the standard for evidence-based recommendations and acknowledges the critical value of high-quality evidence in making recommendations, it might reasonably be asked, where is the evidence of value in cervical cancer screening?” Dr. Sawaya wrote.
The updated USPSTF recommendations differ from the SGO recommendation by changing the starting age for hrHPV testing to 30 years from 25, and rescreening at 5-year intervals.
“The USPSTF recommendation that HPV testing not begin until age 30 years seems prudent,” Dr. Sawaya said, in light of the evidence report and modeling analysis of harms and benefits. He noted that the evidence reviewed by the task force showed that HPV testing and cotesting resulting in a small amount of life-years gained compared with no testing, but with the trade-off of more follow-up tests and colposcopies.
“From the perspective of society, it has been proposed that cost-effectiveness analyses be an essential part of the guideline process,” Dr. Sawaya noted. “To assist in policy decisions that many professional societies will soon face, a study that I am leading is seeking to use cost-effectiveness analyses to determine the range of reasonable options for cervical cancer screening. Such analyses may inform future screening recommendations.”
Dr. Sawaya is affiliated with the University of California, San Francisco. These comments are taken from an editorial accompanying USPSTF recommendations on cervical cancer screening (JAMA Intern Med. 2018 Aug 21. doi: 10.1001/jamainternmed.2018.4282). He disclosed serving as the principal investigator of a National Cancer Institute study on cost-effectiveness analyses to determine reasonable options for cervical cancer screening. He also served as a member of the U.S. Preventive Services Task Force from 2004 to 2008.
In 2016, the Society for Gynecologic Oncology (SGO) recommended screening with the newly approved hrHPV test for women aged 25 years and older, with rescreening 3 years later if the test was negative, George F. Sawaya, MD, wrote in an accompanying editorial published in JAMA Internal Medicine. The new recommendations from the U.S. Preventive Services Task Force do not endorse a single triage strategy, and do not consider costs, he said.
“Although the USPSTF sets the standard for evidence-based recommendations and acknowledges the critical value of high-quality evidence in making recommendations, it might reasonably be asked, where is the evidence of value in cervical cancer screening?” Dr. Sawaya wrote.
The updated USPSTF recommendations differ from the SGO recommendation by changing the starting age for hrHPV testing to 30 years from 25, and rescreening at 5-year intervals.
“The USPSTF recommendation that HPV testing not begin until age 30 years seems prudent,” Dr. Sawaya said, in light of the evidence report and modeling analysis of harms and benefits. He noted that the evidence reviewed by the task force showed that HPV testing and cotesting resulting in a small amount of life-years gained compared with no testing, but with the trade-off of more follow-up tests and colposcopies.
“From the perspective of society, it has been proposed that cost-effectiveness analyses be an essential part of the guideline process,” Dr. Sawaya noted. “To assist in policy decisions that many professional societies will soon face, a study that I am leading is seeking to use cost-effectiveness analyses to determine the range of reasonable options for cervical cancer screening. Such analyses may inform future screening recommendations.”
Dr. Sawaya is affiliated with the University of California, San Francisco. These comments are taken from an editorial accompanying USPSTF recommendations on cervical cancer screening (JAMA Intern Med. 2018 Aug 21. doi: 10.1001/jamainternmed.2018.4282). He disclosed serving as the principal investigator of a National Cancer Institute study on cost-effectiveness analyses to determine reasonable options for cervical cancer screening. He also served as a member of the U.S. Preventive Services Task Force from 2004 to 2008.
Screen women for cervical cancer with basic cytology starting at age 21 years, and consider adding high-risk human papillomavirus (hrHPV) testing alone or with cytology for women aged 30 years and older, the U.S. Preventive Services Task Force recommended in an updated statement on cervical cancer screening .
The statement, accompanying evidence report, and a modeling study were published online in JAMA.
Cervical cancer deaths in the United States have declined from 2.8 deaths per 100,000 women in 2000 to 2.3 deaths per 100,000 women in 2015 because of the adoption of widespread screening, according to Susan J. Curry, PhD., of the University of Iowa, Iowa City, and her colleagues in the USPSTF (JAMA. 2018 Aug 21. doi: 10.1001/jama.2018.10897.
Based on the latest evidence and the modeling study, the USPSTF gives an A recommendation to screening women aged 21-29 years for cervical cancer every 3 years with cervical cytology alone. The task force also gives an A to screening women aged 30-65 years every 5 years with either hrHPV testing alone or in combination with cytology.
The task force recommends against screening (D recommendation) for women younger than 21 years, older than 65 years with a history of screening and low cervical cancer risk, and women who have had hysterectomies with removal of the cervix and no history of cervical cancer risk.
To update the previous recommendations issued in 2012, the task force reviewed the latest evidence and commissioned a modeling study to help determine the best screening strategies in terms of age, screening intervals, and risks vs. benefits.
In the model, researchers assessed 19 strategies for cervical cancer screening based on a hypothetical cohort of women who began screening at 21 years of age.
Overall, the different strategies were similar in effectiveness, but primary hrHPV testing and alternative cotesting were slightly more effective: Cervical cancer deaths ranged from 0.23 to 0.29 deaths per 1,000 women in strategies involving hrHPV testing or cotesting, vs. 0.30 to 0.76 deaths per 1,000 women for strategies based on the current guidelines.
In addition, switching the age of hrHPV testing from 25 years to 30 years and using a 5-year screening interval showed the most effectiveness in terms of risks vs. harms, wrote Jane K. Kim, PhD, of Harvard University, Boston, and her colleagues (JAMA. 2018 Aug 21. doi: 10.1001/jama.2017.19872). “Switching from cytology to 5-year primary hrHPV testing at age 30 years (strategy 14) was associated with a ratio of 640 colposcopies per cancer case averted; earlier switch ages required a greater number of colposcopies per cancer case averted.”
The recommendations also were supported by an evidence report including eight randomized, controlled trials of 410,556 women, five cohort studies of 402,615 women, and a meta-analysis of individual participant data including 176,464 women.
The evidence report sought to address the benefits and harms of cervical cancer screening using hrHPV screening alone as the primary screening method or paired with cytology (cotesting), compared with primary screening using cytology alone.
Overall, both hrHPV and hrHPV plus cytology were associated with higher rates of false-positives and colposcopy compared with cytology alone, “which could lead to more treatments with potential harms,” wrote Joy Melnikow, MD, of the University of California, Davis, and her colleagues (JAMA. 2018 Aug 21. doi: 10.1001/jama.2018.10400.
In addition, hrHPV testing yielded higher rates of positive cervical intraepithelial neoplasia, compared with cytology alone as initial screening.
However, further research is needed to address the impact of any cervical cancer screening strategies in populations with limited access to health care and screening, the researchers noted.
The updated USPSTF recommendations are largely in line with those issued by leading women’s health organizations including the American College of Obstetricians and Gynecologists, ASCCP, and the Society for Gynecologic Oncology, according to a joint statement.
“With a number of screening options now available, the new guidelines emphasize the importance of the patient-provider shared decision-making process to assist women in making an informed choice about which screening method is most suitable for them,” according to the statement, “However, more importantly, there needs to be a continued effort to ensure all women are adequately screened because a significant number of women in the country are not. It’s also essential for women to have access to all of the tests and that they are appropriately covered by insurance companies.
“We hope the USPSTF recommendations foster more discussions between patients and providers about cervical cancer screening, promote opportunities for patient education on the benefits and safety of HPV vaccination for cervical cancer prevention and encourage providers to offer HPV vaccines in their offices,” the statement noted.
The USPSTF research was funded by the Agency for Healthcare Research and Quality. The researchers for the modeling report were supported in part by a National Cancer Institute grant. The researchers had no relevant financial conflicts to disclose.
SOURCES: Kim J et al. JAMA. 2018 Aug 21. doi: 10.1001/jama.2017.19872; Melnikow J et al. JAMA. 2018 Aug 21. doi: 10.1001/jama.2018.10400; Curry S et al. JAMA. 2018 Aug 21. doi: 10.1001/jama.2018.10897.
Screen women for cervical cancer with basic cytology starting at age 21 years, and consider adding high-risk human papillomavirus (hrHPV) testing alone or with cytology for women aged 30 years and older, the U.S. Preventive Services Task Force recommended in an updated statement on cervical cancer screening .
The statement, accompanying evidence report, and a modeling study were published online in JAMA.
Cervical cancer deaths in the United States have declined from 2.8 deaths per 100,000 women in 2000 to 2.3 deaths per 100,000 women in 2015 because of the adoption of widespread screening, according to Susan J. Curry, PhD., of the University of Iowa, Iowa City, and her colleagues in the USPSTF (JAMA. 2018 Aug 21. doi: 10.1001/jama.2018.10897.
Based on the latest evidence and the modeling study, the USPSTF gives an A recommendation to screening women aged 21-29 years for cervical cancer every 3 years with cervical cytology alone. The task force also gives an A to screening women aged 30-65 years every 5 years with either hrHPV testing alone or in combination with cytology.
The task force recommends against screening (D recommendation) for women younger than 21 years, older than 65 years with a history of screening and low cervical cancer risk, and women who have had hysterectomies with removal of the cervix and no history of cervical cancer risk.
To update the previous recommendations issued in 2012, the task force reviewed the latest evidence and commissioned a modeling study to help determine the best screening strategies in terms of age, screening intervals, and risks vs. benefits.
In the model, researchers assessed 19 strategies for cervical cancer screening based on a hypothetical cohort of women who began screening at 21 years of age.
Overall, the different strategies were similar in effectiveness, but primary hrHPV testing and alternative cotesting were slightly more effective: Cervical cancer deaths ranged from 0.23 to 0.29 deaths per 1,000 women in strategies involving hrHPV testing or cotesting, vs. 0.30 to 0.76 deaths per 1,000 women for strategies based on the current guidelines.
In addition, switching the age of hrHPV testing from 25 years to 30 years and using a 5-year screening interval showed the most effectiveness in terms of risks vs. harms, wrote Jane K. Kim, PhD, of Harvard University, Boston, and her colleagues (JAMA. 2018 Aug 21. doi: 10.1001/jama.2017.19872). “Switching from cytology to 5-year primary hrHPV testing at age 30 years (strategy 14) was associated with a ratio of 640 colposcopies per cancer case averted; earlier switch ages required a greater number of colposcopies per cancer case averted.”
The recommendations also were supported by an evidence report including eight randomized, controlled trials of 410,556 women, five cohort studies of 402,615 women, and a meta-analysis of individual participant data including 176,464 women.
The evidence report sought to address the benefits and harms of cervical cancer screening using hrHPV screening alone as the primary screening method or paired with cytology (cotesting), compared with primary screening using cytology alone.
Overall, both hrHPV and hrHPV plus cytology were associated with higher rates of false-positives and colposcopy compared with cytology alone, “which could lead to more treatments with potential harms,” wrote Joy Melnikow, MD, of the University of California, Davis, and her colleagues (JAMA. 2018 Aug 21. doi: 10.1001/jama.2018.10400.
In addition, hrHPV testing yielded higher rates of positive cervical intraepithelial neoplasia, compared with cytology alone as initial screening.
However, further research is needed to address the impact of any cervical cancer screening strategies in populations with limited access to health care and screening, the researchers noted.
The updated USPSTF recommendations are largely in line with those issued by leading women’s health organizations including the American College of Obstetricians and Gynecologists, ASCCP, and the Society for Gynecologic Oncology, according to a joint statement.
“With a number of screening options now available, the new guidelines emphasize the importance of the patient-provider shared decision-making process to assist women in making an informed choice about which screening method is most suitable for them,” according to the statement, “However, more importantly, there needs to be a continued effort to ensure all women are adequately screened because a significant number of women in the country are not. It’s also essential for women to have access to all of the tests and that they are appropriately covered by insurance companies.
“We hope the USPSTF recommendations foster more discussions between patients and providers about cervical cancer screening, promote opportunities for patient education on the benefits and safety of HPV vaccination for cervical cancer prevention and encourage providers to offer HPV vaccines in their offices,” the statement noted.
The USPSTF research was funded by the Agency for Healthcare Research and Quality. The researchers for the modeling report were supported in part by a National Cancer Institute grant. The researchers had no relevant financial conflicts to disclose.
SOURCES: Kim J et al. JAMA. 2018 Aug 21. doi: 10.1001/jama.2017.19872; Melnikow J et al. JAMA. 2018 Aug 21. doi: 10.1001/jama.2018.10400; Curry S et al. JAMA. 2018 Aug 21. doi: 10.1001/jama.2018.10897.
FROM JAMA