User login
Meta-analysis: IVIG bests anti-D on platelet count in pediatric ITP
For patients with pediatric immune thrombocytopenia (ITP), treatment with intravenous immunoglobulins (IVIG) is more likely to raise platelet count in the short-term, compared with anti-D immunoglobulins (anti-D), according the authors of a recent systematic review and meta-analysis.
Although findings from the meta-analysis support recommendations for first-line IVIG, not all studies reported bleeding symptoms, so the clinical effects of differing platelet responses remain unknown, reported lead author Bertrand Lioger, MD, of François-Rabelais University in Tours, France, and his colleagues.
“To date, no meta-analysis has compared the efficacy and safety of IVIG vs. anti-D,” the investigators wrote in The Journal of Pediatrics.
Each treatment approach has strengths and weaknesses, the investigators noted. Namely, IVIG is more expensive, while anti-D is more likely to cause adverse drugs reactions (ADRs), such as disseminated intravascular coagulation and hemolysis.
The present review evaluated 11 studies comparing the efficacy of IVIG with that of anti-D in 704 children with ITP. Platelet response and bleeding were the main efficacy outcomes. The investigators used response thresholds defined by each study because several did not use standardized levels. Other outcomes considered were mortality, disease course, splenectomy, and ADRs. The ADRs included serious adverse reactions, infusion reactions, transfusions, hemoglobin loss, and hemolysis.
In alignment with previous guidelines, anti-D therapy was most often given to RhD positive, nonsplenectomized children at a dose of 50-75 mcg/kg, whereas IVIG was dosed at 0.8-1 g/kg for 1 or 2 consecutive days.
Results showed that patients treated with IVIG were 15% more likely to have platelet counts greater than 20 × 109/L within 24-72 hours, compared with those given anti-D. This disparity rose to 25% in favor of IVIG when using a threshold of 50 × 109/L.
Treatment risk was lower and general symptoms were less common after treatment with anti-D infusion, compared with IVIG (24.6% vs. 31.4%), but this was only true for trials foregoing premedication. Anti-D was more often associated with hemolysis, making transfusion necessary for some patients.
Although platelet count is often used as a surrogate measure of bleeding risk, the investigators decided that a lack of bleeding data among the studies precluded an accurate determination of clinical superiority between the treatments.
“Severe hemolysis remains an important issue when using anti-D immunoglobulins and premedication reduces the incidence of general symptoms observed with IVIG,” the investigators wrote. “Our conclusions should, however, be cautiously considered due to the poor overall quality of included studies and to limited data about clinically relevant outcomes.”
The study was not supported by outside funding. The investigators reported financial relationships with Amgen, Novartis, Roche Pharma, Sanofi, and others.
SOURCE: Lioger B et al. J Pediatr. 2019;204:225-33.
For patients with pediatric immune thrombocytopenia (ITP), treatment with intravenous immunoglobulins (IVIG) is more likely to raise platelet count in the short-term, compared with anti-D immunoglobulins (anti-D), according the authors of a recent systematic review and meta-analysis.
Although findings from the meta-analysis support recommendations for first-line IVIG, not all studies reported bleeding symptoms, so the clinical effects of differing platelet responses remain unknown, reported lead author Bertrand Lioger, MD, of François-Rabelais University in Tours, France, and his colleagues.
“To date, no meta-analysis has compared the efficacy and safety of IVIG vs. anti-D,” the investigators wrote in The Journal of Pediatrics.
Each treatment approach has strengths and weaknesses, the investigators noted. Namely, IVIG is more expensive, while anti-D is more likely to cause adverse drugs reactions (ADRs), such as disseminated intravascular coagulation and hemolysis.
The present review evaluated 11 studies comparing the efficacy of IVIG with that of anti-D in 704 children with ITP. Platelet response and bleeding were the main efficacy outcomes. The investigators used response thresholds defined by each study because several did not use standardized levels. Other outcomes considered were mortality, disease course, splenectomy, and ADRs. The ADRs included serious adverse reactions, infusion reactions, transfusions, hemoglobin loss, and hemolysis.
In alignment with previous guidelines, anti-D therapy was most often given to RhD positive, nonsplenectomized children at a dose of 50-75 mcg/kg, whereas IVIG was dosed at 0.8-1 g/kg for 1 or 2 consecutive days.
Results showed that patients treated with IVIG were 15% more likely to have platelet counts greater than 20 × 109/L within 24-72 hours, compared with those given anti-D. This disparity rose to 25% in favor of IVIG when using a threshold of 50 × 109/L.
Treatment risk was lower and general symptoms were less common after treatment with anti-D infusion, compared with IVIG (24.6% vs. 31.4%), but this was only true for trials foregoing premedication. Anti-D was more often associated with hemolysis, making transfusion necessary for some patients.
Although platelet count is often used as a surrogate measure of bleeding risk, the investigators decided that a lack of bleeding data among the studies precluded an accurate determination of clinical superiority between the treatments.
“Severe hemolysis remains an important issue when using anti-D immunoglobulins and premedication reduces the incidence of general symptoms observed with IVIG,” the investigators wrote. “Our conclusions should, however, be cautiously considered due to the poor overall quality of included studies and to limited data about clinically relevant outcomes.”
The study was not supported by outside funding. The investigators reported financial relationships with Amgen, Novartis, Roche Pharma, Sanofi, and others.
SOURCE: Lioger B et al. J Pediatr. 2019;204:225-33.
For patients with pediatric immune thrombocytopenia (ITP), treatment with intravenous immunoglobulins (IVIG) is more likely to raise platelet count in the short-term, compared with anti-D immunoglobulins (anti-D), according the authors of a recent systematic review and meta-analysis.
Although findings from the meta-analysis support recommendations for first-line IVIG, not all studies reported bleeding symptoms, so the clinical effects of differing platelet responses remain unknown, reported lead author Bertrand Lioger, MD, of François-Rabelais University in Tours, France, and his colleagues.
“To date, no meta-analysis has compared the efficacy and safety of IVIG vs. anti-D,” the investigators wrote in The Journal of Pediatrics.
Each treatment approach has strengths and weaknesses, the investigators noted. Namely, IVIG is more expensive, while anti-D is more likely to cause adverse drugs reactions (ADRs), such as disseminated intravascular coagulation and hemolysis.
The present review evaluated 11 studies comparing the efficacy of IVIG with that of anti-D in 704 children with ITP. Platelet response and bleeding were the main efficacy outcomes. The investigators used response thresholds defined by each study because several did not use standardized levels. Other outcomes considered were mortality, disease course, splenectomy, and ADRs. The ADRs included serious adverse reactions, infusion reactions, transfusions, hemoglobin loss, and hemolysis.
In alignment with previous guidelines, anti-D therapy was most often given to RhD positive, nonsplenectomized children at a dose of 50-75 mcg/kg, whereas IVIG was dosed at 0.8-1 g/kg for 1 or 2 consecutive days.
Results showed that patients treated with IVIG were 15% more likely to have platelet counts greater than 20 × 109/L within 24-72 hours, compared with those given anti-D. This disparity rose to 25% in favor of IVIG when using a threshold of 50 × 109/L.
Treatment risk was lower and general symptoms were less common after treatment with anti-D infusion, compared with IVIG (24.6% vs. 31.4%), but this was only true for trials foregoing premedication. Anti-D was more often associated with hemolysis, making transfusion necessary for some patients.
Although platelet count is often used as a surrogate measure of bleeding risk, the investigators decided that a lack of bleeding data among the studies precluded an accurate determination of clinical superiority between the treatments.
“Severe hemolysis remains an important issue when using anti-D immunoglobulins and premedication reduces the incidence of general symptoms observed with IVIG,” the investigators wrote. “Our conclusions should, however, be cautiously considered due to the poor overall quality of included studies and to limited data about clinically relevant outcomes.”
The study was not supported by outside funding. The investigators reported financial relationships with Amgen, Novartis, Roche Pharma, Sanofi, and others.
SOURCE: Lioger B et al. J Pediatr. 2019;204:225-33.
FROM THE JOURNAL OF PEDIATRICS
Key clinical point:
Major finding: Treatment with IVIG was 15% more likely than anti-D immunoglobulin to raise platelet counts higher than 20 × 109/L within 24-72 hours.
Study details: A systematic review and meta-analysis of 11 studies comparing the efficacy of IVIG with that of anti-D in 704 children with ITP.
Disclosures: The meta-analysis did not have outside funding. The investigators reported financial relationships with Amgen, Novartis, Roche Pharma, Sanofi, and others.
Source: Lioger B et al. J Pediatr. 2019; 204:225-33.
Risk of Cancer-Associated Thrombosis and Bleeding in Veterans With Malignancy Who Are Receiving Direct Oral Anticoagulants (FULL)
Patients with cancer are at an increased risk of both venous thromboembolism (VTE) and bleeding complications. Risk factors for development of cancer-associated thrombosis (CAT) include indwelling lines, antineoplastic therapies, lack of mobility, and physical/chemical damage from the tumor.1 Venous thromboembolism may manifest as either deep vein thrombosis (DVT) or pulmonary embolism (PE). Cancer-associated thrombosis can lead to significant mortality in patients with cancer and may increase health care costs for additional medications and hospitalizations.
Zullig and colleagues estimated that 46,666 veterans received cancer care from the US Department of Veteran Affairs (VA) health care system in 2010. This number equates to about 3% of all patients with cancer in the US who receive at least some of their health care from the VA health care system.2 In addition to cancer care, these veterans receive treatment for various comorbid conditions. One such condition that is of concern in a prothrombotic state is atrial fibrillation (AF). For this condition, patients often require anticoagulation therapy with aspirin, warfarin, or one of the recently approved direct oral anticoagulant agents (DOACs), depending on risk factors.
Background
Due to their ease of administration, limited monitoring requirements, and proven safety and efficacy in patients with AF requiring anticoagulation, the American Heart Association (AHA) and American College of Cardiology recently switched their recommendations for rivaroxaban and dabigatran for oral stroke prevention to a class 1/level B recommendation.3
The American College of Chest Physicians (ACCP) recommends treatment with DOACs over warfarin therapy for acute VTE in patients without cancer; however, the ACCP prefers low molecular-weight heparin (LMWH) over the DOACs for treatment of CAT.4 Recently, the National Comprehensive Cancer Network (NCCN) updated its guidelines for the treatment of cancer-associated thromboembolic disease to recommend 2 of the DOACs (apixaban, rivaroxaban) for treatment of acute VTE over warfarin. These guidelines also recommend LMWH over DOACs for treatment of acute VTE in patients with cancer.5 These NCCN recommendations are largely based on prespecified subgroup meta-analyses of the DOACs compared with those of LMWH or warfarin in the cancer population.
In addition to stroke prevention in patients with AF, DOACs have additional FDA-approved indications, including treatment of acute VTE, prevention of recurrent VTE, and postoperative VTE treatment and prophylaxis. Due to a lack of head-to-head, randomized controlled trials comparing LMWH with DOACs in patients with cancer, these agents have not found their formal place in the treatment or prevention of CAT. Several meta-analyses have suggested similar efficacy and safety outcomes in patients with cancer compared with those of LMWH.6-8 These meta-analysis studies largely looked at subpopulations and compared the outcomes with those of the landmark CLOT (Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer Investigators) and CATCH (Comparison of Acute Treatments in Cancer Hemostasis) trials.9,10
As it is still unclear whether the DOACs are effective and safe for treatment/prevention of CAT, some confusion remains regarding the best management of these at-risk patients. In patients with cancer on DOAC therapy for an approved indication, it is assumed that the therapeutic benefit seen in approved indications would translate to treatment and prevention of CAT. This study aims to determine the incidence of VTE and rates of major and clinically relevant nonmajor bleeding (CRNMB) in veterans with cancer who received a DOAC.
Methods
This retrospective, single-center chart review was approved by the local institutional review board and research safety committee. A search within the VA Corporate Data Warehouse identified patients who had an active prescription for one of the DOACs (apixaban, dabigatran, edoxaban, and rivaroxaban) along with an ICD 9 or ICD 10 code corresponding to a malignancy.
Patients were included in the final analysis if they were aged 18 to 89 years at time of DOAC receipt, undergoing active treatment for malignancy, had evidence of a history of malignancy (either diagnostic or charted evidence of previous treatment), or received cancer-related surgery within 30 days of DOAC prescription with curative intent. Patients were excluded from the final analysis if they did not receive a DOAC prescription or have any clear evidence of malignancy documented in the medical chart.
Patients’ charts were evaluated for the following clinical endpoints: patient age, height (cm), weight (kg), type of malignancy, type of treatment for malignancy, serum creatinine (SCr), creatinine clearance (CrCl) calculated with the Cockcroft-Gault equation using actual body weight, serum hemoglobin, aspartate aminotransferase, alanine aminotransferase, total bilirubin, indication for DOAC, type of VTE, presence of a prior VTE, and diagnostic test performed for VTE. Major bleeding and CRNMB criteria were based on the definitions provided by the International Society on Thrombosis and Haemostasis (ISTH).11 All laboratory values and demographic information were gathered at the time of initial DOAC prescription.
The primary endpoint for this study was incidence of VTE. The secondary endpoints included major bleeding and CRNMB. All data collection and statistical analysis were done using Microsoft Excel 2016 (Redmond, WA). Comparisons of data between trials were done using the chi-squared calculation.
Results
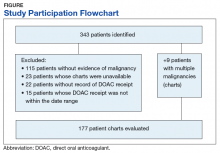
From initial FDA approval of dabigatran (first DOAC on the market) on October 15, 2012, to January 1, 2017, there were 343 patients who met initial inclusion criteria. Of those, 115 did not have any clear evidence of malignancy, 22 did not have any records of DOAC receipt, 15 did not receive a DOAC within the date range, and 23 patients’ charts were unavailable.
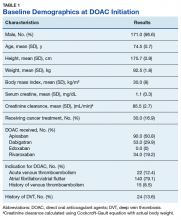
The majority of the patients were males (96.6%), with an average age of 74.5 years. The average weight of all patients was 92.5 kg, with an average SCr of 1.1 mg/dL. This equated to an average CrCl of 85.5 mL/min based on the Cockcroft-Gault equation using actual bodyweight. Of the 177 patients evaluated, 30 (16.9%) were receiving active cancer treatment at time of DOAC initiation.
Two (1.1%) patients developed a VTE while receiving a DOAC.
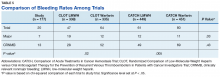
Among the 177 evaluable patients in this study, there were 7 patients (4%) who developed a major bleed and 13 patients (7.3%) who developed a clinically relevant nonmajor bleed according to the definitions provided by ISTH.11
As previously mentioned, only 30 of the patients were actively receiving treatment during DOAC administration. Most of the documented cases of malignancy were either a history of nonmelanoma skin cancer (NMSC) or prostate cancer. The most common method of treatment was surgical resection for both malignancies. Of the 30 patients who received active malignancy treatment while on a DOAC, there were 4 patients with multiple myeloma, 6 patients with NMSC, 4 patients with colon cancer, 1 patient with chronic lymphocytic leukemia (CLL), 1 patient with chronic myelogenous leukemia (CML), 1 patient with small lymphocytic leukemia (SLL), 4 patients with non-small cell lung cancer (NSCLC), 1 patient with unspecified brain cancer, and 1 patient with breast cancer. The various characteristics of these patients are presented in Table 6.
Discussion
The CLOT and CATCH trials were chosen as historic comparators. Although the active treatment interventions and comparator arms were not similar between the patients included in this study and the CLOT and CATCH trials, the authors felt the comparison was appropriate as these trials were designed specifically for patients with malignancy. Additionally, these trials sought to assess rates of VTE formation and bleeding in the patient with malignancies—outcomes that aligned with this study. Alternative trials for comparison are the subgroup analyses of patients with malignancies in the AMPLIFY, RE-COVER, and EINSTEIN trials.12-14 Although these trials were designed to stratify patients based on presence of malignancy, they were not powered to account for increased risk of VTE in patients with malignancies.
There are multiple risk factors that increase the risk of CAT. Khoranna and colleagues identified primary stomach, pancreas, brain, lung, lymphoma, gynecologic, bladder, testicular, and renal carcinomas as a high risk of VTE formation.15 Additionally, Khoranna and colleagues noted that elderly patients and patients actively receiving treatment are at an increased risk of VTE formation.15 The low rate of VTE formation (1.1%) in the patients in this study may be due to the low risk for VTE formation. As previously mentioned, only 30 of the patients (16.9%) in this study were receiving active treatment.
Additionally, there were only 42 patients (23.7%) who had a high-risk malignancy. The increased age of the patient population (74.5 years old) in this study is one risk factor that could largely skew the risks of VTE formation in the patient population. In addition to age, the average body mass index (BMI) of this study’s patient population (30 kg/m2) may further increase risk of VTE. Although Khoranna and colleagues identified a BMI of 35 kg/m2 as the cutoff for increased risk of CAT, the increased risk based on a BMI of 30 kg/m2 cannot be ignored in the patients in this study.15
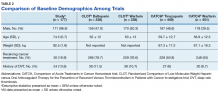
Another risk inherent in the treatment of patients with cancer is pancytopenia, which may lead to increased risks of bleeding and infection. When patients are exposed to an anticoagulant agent in the setting of decreased platelets and hemoglobin (from treatment or disease process), the risk for major bleeds and CRNMB are increased drastically. In this patient population, the combined rate of bleeding (11.3%) was relatively decreased compared with that of the CLOT (16.5% for all bleeding events) and CATCH (15.7% for all bleeding events) trials.9,10
Compared with the oncology subgroup analysis of the AMPLIFY, RE-COVER, and EINSTEIN trials, the differences are more noticeable. The AMPLIFY trial reported a 1.1% incidence of bleeding in patients with cancer on apixaban, whereas the RE-COVER trial did not report bleeding rates, and the EINSTEIN trial reported a 14% incidence of bleeding in all patients with cancer on rivaroxaban for VTE treatment.12-14 This study found a bleeding incidence of 12.2% with apixaban, 5.7% with dabigatran, and 14.7% with rivaroxaban. In this trial the incidence of bleeding with rivaroxaban were similar; however, the incidence of bleeding with apixaban was markedly higher. There is no obvious explanation for this, as the dosing of apixaban was appropriate in all patients in this trial except for one. There was no documented bleed in this patient’s medical chart.
A meta-analysis conducted by Vedovati and colleagues identified 6 studies in which patients with cancer received either a DOAC (with or without a heparin product) or vitamin K antagonist.16 That analysis found a nonsignificant reduction in VTE recurrence (odds ratio [OR], 0.63; 95% confidence interval [CI], 0.31-1.1), major bleeding (OR, 0.77; 95% CI, 0.41-1.44), and CRNMB (OR, 0.85; 95% CI, 0.62-1.18).16 The meta-analysis adds to the growing body of evidence in support of both safety and efficacy of DOACs in patients with cancer. Although the Vedovati and colleagues study does not directly compare rates between 2 treatment groups, the findings of similar rates of VTE recurrence, major bleed, and CRNMB are consistent with the current study. Despite differing patient characteristics, the meta-analysis by Vedovati and colleagues supports the ongoing use of DOACs in patients with malignancy, as does the current study.16
Limitations
Although it seems that apixaban, dabigatran, and rivaroxaban are effective in reducing the risk of VTE in veterans with malignancy, there are some inherent weaknesses in the current study. Most notably is the choice of comparator trials. The authors’ believe that the CLOT and CATCH trials were the most appropriate based on similarities in population and outcomes. Considering the CLOT and CATCH trials compared LMWH to coumarin products for treatment of VTE, future studies should compare use of these agents with DOACs in the cancer population. In addition, the study did not include outcomes that would adequately assess risks of VTE and bleeding formation. This information would have been beneficial to more effectively categorize this study’s patient population based on risks of each of its predetermined outcomes. Understanding safety and efficacy of DOACs in patients at various risks would help practitioners to choose more appropriate agents in practice. Last, this study did not assess the incidence of stroke in study patients. This is important because the DOACs were used mostly for stroke prevention in AF and atrial flutter. The increased risk of VTE in patients with cancer cannot directly correlate to risk of stroke with a comorbid cardiac condition, but the hypercoagulable state cannot be ignored in these patients.
Conclusion
This study provided some preliminary evidence for the safety and efficacy of DOACs in patients with cancer. The low incidence of VTE formation and similar rates of bleeding among other clinical trials indicate that DOACs are safe alternatives to currently recommended anticoagulation medication in patients with cancer.
1. Motykie GD, Zebala LP, Caprini JA, et al. A guide to venous thromboembolism risk factor assessment. J Thromb Thrombolysis. 2000;9(3):253-262.
2. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System: 2010 update. Mil Med. 2017;182(7):e1883-e1891.
3. January CT, Wann S, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. Circulation. 2014;130(23):2071-2104.
4. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352.
5. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Cancer-associated venous thromboembolic disease. Version 1.2018. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf. Updated March 22, 2018. Accessed April 9, 2018.
6. Brunetti ND, Gesuete E, De Gennaro L, et al. Direct-acting oral anticoagulants compared to vitamin K inhibitors and low molecular weight heparin for the prevention of venous thromboembolism in patients with cancer: a meta-analysis study. Int J Cardiol. 2017;230:214-221.
7. Posch F, Konigsbrügge O, Zielinski C, Pabinger I, Ay C. Treatment of venous thromboembolism in patients with cancer: a network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136(3):582-589.
8. van Es N, Coppens M, Schulman S, Middledorp S, Büller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124(12):1968-1975.
9. Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low molecular weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
10. Lee AY, Kamphuisen PW, Meyer G, et al; CATCH Investigators. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314(7):677-686.
11. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S; Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119-2126.
12. Agnelli G, Büller HR, Cohen A, et al. Oral apixaban for the treatment of venous thromboembolism in cancer patients: results from the AMPLIFY trial. J Thromb Haemost. 2015;13(12):2187-2191.
13. Schulman S, Goldhaber SZ, Kearon C, et al. Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer. Thromb Haemost. 2015;114(1):150-157.
14. Prins MH, Lensing AW, Brighton TA, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PF): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol. 2014;1(1):e37-e46.
15. Khoranna AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27(9):4839-4847.
16. Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest. 2015;147(2):475-483.
Patients with cancer are at an increased risk of both venous thromboembolism (VTE) and bleeding complications. Risk factors for development of cancer-associated thrombosis (CAT) include indwelling lines, antineoplastic therapies, lack of mobility, and physical/chemical damage from the tumor.1 Venous thromboembolism may manifest as either deep vein thrombosis (DVT) or pulmonary embolism (PE). Cancer-associated thrombosis can lead to significant mortality in patients with cancer and may increase health care costs for additional medications and hospitalizations.
Zullig and colleagues estimated that 46,666 veterans received cancer care from the US Department of Veteran Affairs (VA) health care system in 2010. This number equates to about 3% of all patients with cancer in the US who receive at least some of their health care from the VA health care system.2 In addition to cancer care, these veterans receive treatment for various comorbid conditions. One such condition that is of concern in a prothrombotic state is atrial fibrillation (AF). For this condition, patients often require anticoagulation therapy with aspirin, warfarin, or one of the recently approved direct oral anticoagulant agents (DOACs), depending on risk factors.
Background
Due to their ease of administration, limited monitoring requirements, and proven safety and efficacy in patients with AF requiring anticoagulation, the American Heart Association (AHA) and American College of Cardiology recently switched their recommendations for rivaroxaban and dabigatran for oral stroke prevention to a class 1/level B recommendation.3
The American College of Chest Physicians (ACCP) recommends treatment with DOACs over warfarin therapy for acute VTE in patients without cancer; however, the ACCP prefers low molecular-weight heparin (LMWH) over the DOACs for treatment of CAT.4 Recently, the National Comprehensive Cancer Network (NCCN) updated its guidelines for the treatment of cancer-associated thromboembolic disease to recommend 2 of the DOACs (apixaban, rivaroxaban) for treatment of acute VTE over warfarin. These guidelines also recommend LMWH over DOACs for treatment of acute VTE in patients with cancer.5 These NCCN recommendations are largely based on prespecified subgroup meta-analyses of the DOACs compared with those of LMWH or warfarin in the cancer population.
In addition to stroke prevention in patients with AF, DOACs have additional FDA-approved indications, including treatment of acute VTE, prevention of recurrent VTE, and postoperative VTE treatment and prophylaxis. Due to a lack of head-to-head, randomized controlled trials comparing LMWH with DOACs in patients with cancer, these agents have not found their formal place in the treatment or prevention of CAT. Several meta-analyses have suggested similar efficacy and safety outcomes in patients with cancer compared with those of LMWH.6-8 These meta-analysis studies largely looked at subpopulations and compared the outcomes with those of the landmark CLOT (Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer Investigators) and CATCH (Comparison of Acute Treatments in Cancer Hemostasis) trials.9,10
As it is still unclear whether the DOACs are effective and safe for treatment/prevention of CAT, some confusion remains regarding the best management of these at-risk patients. In patients with cancer on DOAC therapy for an approved indication, it is assumed that the therapeutic benefit seen in approved indications would translate to treatment and prevention of CAT. This study aims to determine the incidence of VTE and rates of major and clinically relevant nonmajor bleeding (CRNMB) in veterans with cancer who received a DOAC.
Methods
This retrospective, single-center chart review was approved by the local institutional review board and research safety committee. A search within the VA Corporate Data Warehouse identified patients who had an active prescription for one of the DOACs (apixaban, dabigatran, edoxaban, and rivaroxaban) along with an ICD 9 or ICD 10 code corresponding to a malignancy.
Patients were included in the final analysis if they were aged 18 to 89 years at time of DOAC receipt, undergoing active treatment for malignancy, had evidence of a history of malignancy (either diagnostic or charted evidence of previous treatment), or received cancer-related surgery within 30 days of DOAC prescription with curative intent. Patients were excluded from the final analysis if they did not receive a DOAC prescription or have any clear evidence of malignancy documented in the medical chart.
Patients’ charts were evaluated for the following clinical endpoints: patient age, height (cm), weight (kg), type of malignancy, type of treatment for malignancy, serum creatinine (SCr), creatinine clearance (CrCl) calculated with the Cockcroft-Gault equation using actual body weight, serum hemoglobin, aspartate aminotransferase, alanine aminotransferase, total bilirubin, indication for DOAC, type of VTE, presence of a prior VTE, and diagnostic test performed for VTE. Major bleeding and CRNMB criteria were based on the definitions provided by the International Society on Thrombosis and Haemostasis (ISTH).11 All laboratory values and demographic information were gathered at the time of initial DOAC prescription.
The primary endpoint for this study was incidence of VTE. The secondary endpoints included major bleeding and CRNMB. All data collection and statistical analysis were done using Microsoft Excel 2016 (Redmond, WA). Comparisons of data between trials were done using the chi-squared calculation.
Results
From initial FDA approval of dabigatran (first DOAC on the market) on October 15, 2012, to January 1, 2017, there were 343 patients who met initial inclusion criteria. Of those, 115 did not have any clear evidence of malignancy, 22 did not have any records of DOAC receipt, 15 did not receive a DOAC within the date range, and 23 patients’ charts were unavailable.
The majority of the patients were males (96.6%), with an average age of 74.5 years. The average weight of all patients was 92.5 kg, with an average SCr of 1.1 mg/dL. This equated to an average CrCl of 85.5 mL/min based on the Cockcroft-Gault equation using actual bodyweight. Of the 177 patients evaluated, 30 (16.9%) were receiving active cancer treatment at time of DOAC initiation.
Two (1.1%) patients developed a VTE while receiving a DOAC.
Among the 177 evaluable patients in this study, there were 7 patients (4%) who developed a major bleed and 13 patients (7.3%) who developed a clinically relevant nonmajor bleed according to the definitions provided by ISTH.11
As previously mentioned, only 30 of the patients were actively receiving treatment during DOAC administration. Most of the documented cases of malignancy were either a history of nonmelanoma skin cancer (NMSC) or prostate cancer. The most common method of treatment was surgical resection for both malignancies. Of the 30 patients who received active malignancy treatment while on a DOAC, there were 4 patients with multiple myeloma, 6 patients with NMSC, 4 patients with colon cancer, 1 patient with chronic lymphocytic leukemia (CLL), 1 patient with chronic myelogenous leukemia (CML), 1 patient with small lymphocytic leukemia (SLL), 4 patients with non-small cell lung cancer (NSCLC), 1 patient with unspecified brain cancer, and 1 patient with breast cancer. The various characteristics of these patients are presented in Table 6.
Discussion
The CLOT and CATCH trials were chosen as historic comparators. Although the active treatment interventions and comparator arms were not similar between the patients included in this study and the CLOT and CATCH trials, the authors felt the comparison was appropriate as these trials were designed specifically for patients with malignancy. Additionally, these trials sought to assess rates of VTE formation and bleeding in the patient with malignancies—outcomes that aligned with this study. Alternative trials for comparison are the subgroup analyses of patients with malignancies in the AMPLIFY, RE-COVER, and EINSTEIN trials.12-14 Although these trials were designed to stratify patients based on presence of malignancy, they were not powered to account for increased risk of VTE in patients with malignancies.
There are multiple risk factors that increase the risk of CAT. Khoranna and colleagues identified primary stomach, pancreas, brain, lung, lymphoma, gynecologic, bladder, testicular, and renal carcinomas as a high risk of VTE formation.15 Additionally, Khoranna and colleagues noted that elderly patients and patients actively receiving treatment are at an increased risk of VTE formation.15 The low rate of VTE formation (1.1%) in the patients in this study may be due to the low risk for VTE formation. As previously mentioned, only 30 of the patients (16.9%) in this study were receiving active treatment.
Additionally, there were only 42 patients (23.7%) who had a high-risk malignancy. The increased age of the patient population (74.5 years old) in this study is one risk factor that could largely skew the risks of VTE formation in the patient population. In addition to age, the average body mass index (BMI) of this study’s patient population (30 kg/m2) may further increase risk of VTE. Although Khoranna and colleagues identified a BMI of 35 kg/m2 as the cutoff for increased risk of CAT, the increased risk based on a BMI of 30 kg/m2 cannot be ignored in the patients in this study.15
Another risk inherent in the treatment of patients with cancer is pancytopenia, which may lead to increased risks of bleeding and infection. When patients are exposed to an anticoagulant agent in the setting of decreased platelets and hemoglobin (from treatment or disease process), the risk for major bleeds and CRNMB are increased drastically. In this patient population, the combined rate of bleeding (11.3%) was relatively decreased compared with that of the CLOT (16.5% for all bleeding events) and CATCH (15.7% for all bleeding events) trials.9,10
Compared with the oncology subgroup analysis of the AMPLIFY, RE-COVER, and EINSTEIN trials, the differences are more noticeable. The AMPLIFY trial reported a 1.1% incidence of bleeding in patients with cancer on apixaban, whereas the RE-COVER trial did not report bleeding rates, and the EINSTEIN trial reported a 14% incidence of bleeding in all patients with cancer on rivaroxaban for VTE treatment.12-14 This study found a bleeding incidence of 12.2% with apixaban, 5.7% with dabigatran, and 14.7% with rivaroxaban. In this trial the incidence of bleeding with rivaroxaban were similar; however, the incidence of bleeding with apixaban was markedly higher. There is no obvious explanation for this, as the dosing of apixaban was appropriate in all patients in this trial except for one. There was no documented bleed in this patient’s medical chart.
A meta-analysis conducted by Vedovati and colleagues identified 6 studies in which patients with cancer received either a DOAC (with or without a heparin product) or vitamin K antagonist.16 That analysis found a nonsignificant reduction in VTE recurrence (odds ratio [OR], 0.63; 95% confidence interval [CI], 0.31-1.1), major bleeding (OR, 0.77; 95% CI, 0.41-1.44), and CRNMB (OR, 0.85; 95% CI, 0.62-1.18).16 The meta-analysis adds to the growing body of evidence in support of both safety and efficacy of DOACs in patients with cancer. Although the Vedovati and colleagues study does not directly compare rates between 2 treatment groups, the findings of similar rates of VTE recurrence, major bleed, and CRNMB are consistent with the current study. Despite differing patient characteristics, the meta-analysis by Vedovati and colleagues supports the ongoing use of DOACs in patients with malignancy, as does the current study.16
Limitations
Although it seems that apixaban, dabigatran, and rivaroxaban are effective in reducing the risk of VTE in veterans with malignancy, there are some inherent weaknesses in the current study. Most notably is the choice of comparator trials. The authors’ believe that the CLOT and CATCH trials were the most appropriate based on similarities in population and outcomes. Considering the CLOT and CATCH trials compared LMWH to coumarin products for treatment of VTE, future studies should compare use of these agents with DOACs in the cancer population. In addition, the study did not include outcomes that would adequately assess risks of VTE and bleeding formation. This information would have been beneficial to more effectively categorize this study’s patient population based on risks of each of its predetermined outcomes. Understanding safety and efficacy of DOACs in patients at various risks would help practitioners to choose more appropriate agents in practice. Last, this study did not assess the incidence of stroke in study patients. This is important because the DOACs were used mostly for stroke prevention in AF and atrial flutter. The increased risk of VTE in patients with cancer cannot directly correlate to risk of stroke with a comorbid cardiac condition, but the hypercoagulable state cannot be ignored in these patients.
Conclusion
This study provided some preliminary evidence for the safety and efficacy of DOACs in patients with cancer. The low incidence of VTE formation and similar rates of bleeding among other clinical trials indicate that DOACs are safe alternatives to currently recommended anticoagulation medication in patients with cancer.
Patients with cancer are at an increased risk of both venous thromboembolism (VTE) and bleeding complications. Risk factors for development of cancer-associated thrombosis (CAT) include indwelling lines, antineoplastic therapies, lack of mobility, and physical/chemical damage from the tumor.1 Venous thromboembolism may manifest as either deep vein thrombosis (DVT) or pulmonary embolism (PE). Cancer-associated thrombosis can lead to significant mortality in patients with cancer and may increase health care costs for additional medications and hospitalizations.
Zullig and colleagues estimated that 46,666 veterans received cancer care from the US Department of Veteran Affairs (VA) health care system in 2010. This number equates to about 3% of all patients with cancer in the US who receive at least some of their health care from the VA health care system.2 In addition to cancer care, these veterans receive treatment for various comorbid conditions. One such condition that is of concern in a prothrombotic state is atrial fibrillation (AF). For this condition, patients often require anticoagulation therapy with aspirin, warfarin, or one of the recently approved direct oral anticoagulant agents (DOACs), depending on risk factors.
Background
Due to their ease of administration, limited monitoring requirements, and proven safety and efficacy in patients with AF requiring anticoagulation, the American Heart Association (AHA) and American College of Cardiology recently switched their recommendations for rivaroxaban and dabigatran for oral stroke prevention to a class 1/level B recommendation.3
The American College of Chest Physicians (ACCP) recommends treatment with DOACs over warfarin therapy for acute VTE in patients without cancer; however, the ACCP prefers low molecular-weight heparin (LMWH) over the DOACs for treatment of CAT.4 Recently, the National Comprehensive Cancer Network (NCCN) updated its guidelines for the treatment of cancer-associated thromboembolic disease to recommend 2 of the DOACs (apixaban, rivaroxaban) for treatment of acute VTE over warfarin. These guidelines also recommend LMWH over DOACs for treatment of acute VTE in patients with cancer.5 These NCCN recommendations are largely based on prespecified subgroup meta-analyses of the DOACs compared with those of LMWH or warfarin in the cancer population.
In addition to stroke prevention in patients with AF, DOACs have additional FDA-approved indications, including treatment of acute VTE, prevention of recurrent VTE, and postoperative VTE treatment and prophylaxis. Due to a lack of head-to-head, randomized controlled trials comparing LMWH with DOACs in patients with cancer, these agents have not found their formal place in the treatment or prevention of CAT. Several meta-analyses have suggested similar efficacy and safety outcomes in patients with cancer compared with those of LMWH.6-8 These meta-analysis studies largely looked at subpopulations and compared the outcomes with those of the landmark CLOT (Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer Investigators) and CATCH (Comparison of Acute Treatments in Cancer Hemostasis) trials.9,10
As it is still unclear whether the DOACs are effective and safe for treatment/prevention of CAT, some confusion remains regarding the best management of these at-risk patients. In patients with cancer on DOAC therapy for an approved indication, it is assumed that the therapeutic benefit seen in approved indications would translate to treatment and prevention of CAT. This study aims to determine the incidence of VTE and rates of major and clinically relevant nonmajor bleeding (CRNMB) in veterans with cancer who received a DOAC.
Methods
This retrospective, single-center chart review was approved by the local institutional review board and research safety committee. A search within the VA Corporate Data Warehouse identified patients who had an active prescription for one of the DOACs (apixaban, dabigatran, edoxaban, and rivaroxaban) along with an ICD 9 or ICD 10 code corresponding to a malignancy.
Patients were included in the final analysis if they were aged 18 to 89 years at time of DOAC receipt, undergoing active treatment for malignancy, had evidence of a history of malignancy (either diagnostic or charted evidence of previous treatment), or received cancer-related surgery within 30 days of DOAC prescription with curative intent. Patients were excluded from the final analysis if they did not receive a DOAC prescription or have any clear evidence of malignancy documented in the medical chart.
Patients’ charts were evaluated for the following clinical endpoints: patient age, height (cm), weight (kg), type of malignancy, type of treatment for malignancy, serum creatinine (SCr), creatinine clearance (CrCl) calculated with the Cockcroft-Gault equation using actual body weight, serum hemoglobin, aspartate aminotransferase, alanine aminotransferase, total bilirubin, indication for DOAC, type of VTE, presence of a prior VTE, and diagnostic test performed for VTE. Major bleeding and CRNMB criteria were based on the definitions provided by the International Society on Thrombosis and Haemostasis (ISTH).11 All laboratory values and demographic information were gathered at the time of initial DOAC prescription.
The primary endpoint for this study was incidence of VTE. The secondary endpoints included major bleeding and CRNMB. All data collection and statistical analysis were done using Microsoft Excel 2016 (Redmond, WA). Comparisons of data between trials were done using the chi-squared calculation.
Results
From initial FDA approval of dabigatran (first DOAC on the market) on October 15, 2012, to January 1, 2017, there were 343 patients who met initial inclusion criteria. Of those, 115 did not have any clear evidence of malignancy, 22 did not have any records of DOAC receipt, 15 did not receive a DOAC within the date range, and 23 patients’ charts were unavailable.
The majority of the patients were males (96.6%), with an average age of 74.5 years. The average weight of all patients was 92.5 kg, with an average SCr of 1.1 mg/dL. This equated to an average CrCl of 85.5 mL/min based on the Cockcroft-Gault equation using actual bodyweight. Of the 177 patients evaluated, 30 (16.9%) were receiving active cancer treatment at time of DOAC initiation.
Two (1.1%) patients developed a VTE while receiving a DOAC.
Among the 177 evaluable patients in this study, there were 7 patients (4%) who developed a major bleed and 13 patients (7.3%) who developed a clinically relevant nonmajor bleed according to the definitions provided by ISTH.11
As previously mentioned, only 30 of the patients were actively receiving treatment during DOAC administration. Most of the documented cases of malignancy were either a history of nonmelanoma skin cancer (NMSC) or prostate cancer. The most common method of treatment was surgical resection for both malignancies. Of the 30 patients who received active malignancy treatment while on a DOAC, there were 4 patients with multiple myeloma, 6 patients with NMSC, 4 patients with colon cancer, 1 patient with chronic lymphocytic leukemia (CLL), 1 patient with chronic myelogenous leukemia (CML), 1 patient with small lymphocytic leukemia (SLL), 4 patients with non-small cell lung cancer (NSCLC), 1 patient with unspecified brain cancer, and 1 patient with breast cancer. The various characteristics of these patients are presented in Table 6.
Discussion
The CLOT and CATCH trials were chosen as historic comparators. Although the active treatment interventions and comparator arms were not similar between the patients included in this study and the CLOT and CATCH trials, the authors felt the comparison was appropriate as these trials were designed specifically for patients with malignancy. Additionally, these trials sought to assess rates of VTE formation and bleeding in the patient with malignancies—outcomes that aligned with this study. Alternative trials for comparison are the subgroup analyses of patients with malignancies in the AMPLIFY, RE-COVER, and EINSTEIN trials.12-14 Although these trials were designed to stratify patients based on presence of malignancy, they were not powered to account for increased risk of VTE in patients with malignancies.
There are multiple risk factors that increase the risk of CAT. Khoranna and colleagues identified primary stomach, pancreas, brain, lung, lymphoma, gynecologic, bladder, testicular, and renal carcinomas as a high risk of VTE formation.15 Additionally, Khoranna and colleagues noted that elderly patients and patients actively receiving treatment are at an increased risk of VTE formation.15 The low rate of VTE formation (1.1%) in the patients in this study may be due to the low risk for VTE formation. As previously mentioned, only 30 of the patients (16.9%) in this study were receiving active treatment.
Additionally, there were only 42 patients (23.7%) who had a high-risk malignancy. The increased age of the patient population (74.5 years old) in this study is one risk factor that could largely skew the risks of VTE formation in the patient population. In addition to age, the average body mass index (BMI) of this study’s patient population (30 kg/m2) may further increase risk of VTE. Although Khoranna and colleagues identified a BMI of 35 kg/m2 as the cutoff for increased risk of CAT, the increased risk based on a BMI of 30 kg/m2 cannot be ignored in the patients in this study.15
Another risk inherent in the treatment of patients with cancer is pancytopenia, which may lead to increased risks of bleeding and infection. When patients are exposed to an anticoagulant agent in the setting of decreased platelets and hemoglobin (from treatment or disease process), the risk for major bleeds and CRNMB are increased drastically. In this patient population, the combined rate of bleeding (11.3%) was relatively decreased compared with that of the CLOT (16.5% for all bleeding events) and CATCH (15.7% for all bleeding events) trials.9,10
Compared with the oncology subgroup analysis of the AMPLIFY, RE-COVER, and EINSTEIN trials, the differences are more noticeable. The AMPLIFY trial reported a 1.1% incidence of bleeding in patients with cancer on apixaban, whereas the RE-COVER trial did not report bleeding rates, and the EINSTEIN trial reported a 14% incidence of bleeding in all patients with cancer on rivaroxaban for VTE treatment.12-14 This study found a bleeding incidence of 12.2% with apixaban, 5.7% with dabigatran, and 14.7% with rivaroxaban. In this trial the incidence of bleeding with rivaroxaban were similar; however, the incidence of bleeding with apixaban was markedly higher. There is no obvious explanation for this, as the dosing of apixaban was appropriate in all patients in this trial except for one. There was no documented bleed in this patient’s medical chart.
A meta-analysis conducted by Vedovati and colleagues identified 6 studies in which patients with cancer received either a DOAC (with or without a heparin product) or vitamin K antagonist.16 That analysis found a nonsignificant reduction in VTE recurrence (odds ratio [OR], 0.63; 95% confidence interval [CI], 0.31-1.1), major bleeding (OR, 0.77; 95% CI, 0.41-1.44), and CRNMB (OR, 0.85; 95% CI, 0.62-1.18).16 The meta-analysis adds to the growing body of evidence in support of both safety and efficacy of DOACs in patients with cancer. Although the Vedovati and colleagues study does not directly compare rates between 2 treatment groups, the findings of similar rates of VTE recurrence, major bleed, and CRNMB are consistent with the current study. Despite differing patient characteristics, the meta-analysis by Vedovati and colleagues supports the ongoing use of DOACs in patients with malignancy, as does the current study.16
Limitations
Although it seems that apixaban, dabigatran, and rivaroxaban are effective in reducing the risk of VTE in veterans with malignancy, there are some inherent weaknesses in the current study. Most notably is the choice of comparator trials. The authors’ believe that the CLOT and CATCH trials were the most appropriate based on similarities in population and outcomes. Considering the CLOT and CATCH trials compared LMWH to coumarin products for treatment of VTE, future studies should compare use of these agents with DOACs in the cancer population. In addition, the study did not include outcomes that would adequately assess risks of VTE and bleeding formation. This information would have been beneficial to more effectively categorize this study’s patient population based on risks of each of its predetermined outcomes. Understanding safety and efficacy of DOACs in patients at various risks would help practitioners to choose more appropriate agents in practice. Last, this study did not assess the incidence of stroke in study patients. This is important because the DOACs were used mostly for stroke prevention in AF and atrial flutter. The increased risk of VTE in patients with cancer cannot directly correlate to risk of stroke with a comorbid cardiac condition, but the hypercoagulable state cannot be ignored in these patients.
Conclusion
This study provided some preliminary evidence for the safety and efficacy of DOACs in patients with cancer. The low incidence of VTE formation and similar rates of bleeding among other clinical trials indicate that DOACs are safe alternatives to currently recommended anticoagulation medication in patients with cancer.
1. Motykie GD, Zebala LP, Caprini JA, et al. A guide to venous thromboembolism risk factor assessment. J Thromb Thrombolysis. 2000;9(3):253-262.
2. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System: 2010 update. Mil Med. 2017;182(7):e1883-e1891.
3. January CT, Wann S, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. Circulation. 2014;130(23):2071-2104.
4. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352.
5. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Cancer-associated venous thromboembolic disease. Version 1.2018. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf. Updated March 22, 2018. Accessed April 9, 2018.
6. Brunetti ND, Gesuete E, De Gennaro L, et al. Direct-acting oral anticoagulants compared to vitamin K inhibitors and low molecular weight heparin for the prevention of venous thromboembolism in patients with cancer: a meta-analysis study. Int J Cardiol. 2017;230:214-221.
7. Posch F, Konigsbrügge O, Zielinski C, Pabinger I, Ay C. Treatment of venous thromboembolism in patients with cancer: a network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136(3):582-589.
8. van Es N, Coppens M, Schulman S, Middledorp S, Büller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124(12):1968-1975.
9. Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low molecular weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
10. Lee AY, Kamphuisen PW, Meyer G, et al; CATCH Investigators. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314(7):677-686.
11. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S; Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119-2126.
12. Agnelli G, Büller HR, Cohen A, et al. Oral apixaban for the treatment of venous thromboembolism in cancer patients: results from the AMPLIFY trial. J Thromb Haemost. 2015;13(12):2187-2191.
13. Schulman S, Goldhaber SZ, Kearon C, et al. Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer. Thromb Haemost. 2015;114(1):150-157.
14. Prins MH, Lensing AW, Brighton TA, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PF): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol. 2014;1(1):e37-e46.
15. Khoranna AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27(9):4839-4847.
16. Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest. 2015;147(2):475-483.
1. Motykie GD, Zebala LP, Caprini JA, et al. A guide to venous thromboembolism risk factor assessment. J Thromb Thrombolysis. 2000;9(3):253-262.
2. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System: 2010 update. Mil Med. 2017;182(7):e1883-e1891.
3. January CT, Wann S, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. Circulation. 2014;130(23):2071-2104.
4. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352.
5. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Cancer-associated venous thromboembolic disease. Version 1.2018. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf. Updated March 22, 2018. Accessed April 9, 2018.
6. Brunetti ND, Gesuete E, De Gennaro L, et al. Direct-acting oral anticoagulants compared to vitamin K inhibitors and low molecular weight heparin for the prevention of venous thromboembolism in patients with cancer: a meta-analysis study. Int J Cardiol. 2017;230:214-221.
7. Posch F, Konigsbrügge O, Zielinski C, Pabinger I, Ay C. Treatment of venous thromboembolism in patients with cancer: a network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136(3):582-589.
8. van Es N, Coppens M, Schulman S, Middledorp S, Büller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124(12):1968-1975.
9. Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low molecular weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
10. Lee AY, Kamphuisen PW, Meyer G, et al; CATCH Investigators. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314(7):677-686.
11. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S; Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119-2126.
12. Agnelli G, Büller HR, Cohen A, et al. Oral apixaban for the treatment of venous thromboembolism in cancer patients: results from the AMPLIFY trial. J Thromb Haemost. 2015;13(12):2187-2191.
13. Schulman S, Goldhaber SZ, Kearon C, et al. Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer. Thromb Haemost. 2015;114(1):150-157.
14. Prins MH, Lensing AW, Brighton TA, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PF): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol. 2014;1(1):e37-e46.
15. Khoranna AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27(9):4839-4847.
16. Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest. 2015;147(2):475-483.
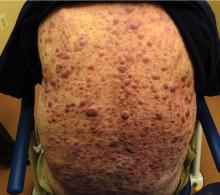
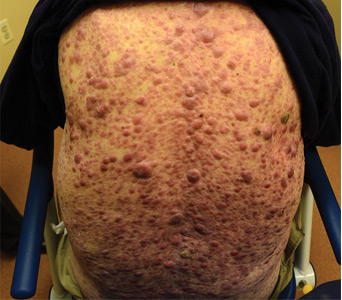
Aleukemic leukemia cutis
On examination, the numerous firm, indurated nodules ranged in size from 1 to 4 cm. There was no palpable lymphadenopathy.
Results of a peripheral blood cell count showed the following:
- Hemoglobin 12.5 g/dL (reference range 13.0–17.0)
- Platelet count 154 × 109/L (130–400)
- White blood cell count 5.0 × 109/L (4.0–11.0)
- Neutrophils 1.7 × 109/L (1.5–8.0)
- Lymphocytes 2.2 × 109/L (1.0–4.0)
- Monocytes 1.0 × 109/L (0.2–1.0)
- Eosinophils 0 (0–0.4)
- Basophils 0 (0–0.2)
- Blasts 0.
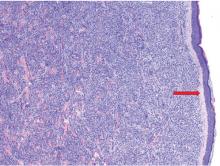
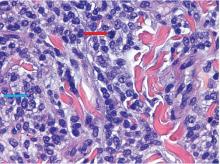
The findings were consistent with leukemic cells with monocytic differentiation. The infiltrate was unusual because leukemic infiltrates typically demonstrate a high nuclear-to-cytoplasmic ratio, but in this case the malignant cells had moderate amounts of cytoplasm due to the monocytic differentiation. Also, a grenz zone is more typically seen in B-cell lymphomas, and T cells more typically demonstrate epidermotropism.
Bone marrow aspiration was performed and revealed a hypercellular bone marrow with trilineage maturation with only 2% blasts. The fluorescence in situ hybridization testing for myelodysplastic syndrome and acute myeloid leukemia was normal. A diagnosis of aleukemic leukemia cutis was made.
After 2 months of chemotherapy with azacitidine, the nodules were less indurated. Treatment was briefly withdrawn due to the development of acute pneumonia, leading to a rapid progression of cutaneous involvement. Despite restarting chemotherapy, the patient died.
ALEUKEMIC LEUKEMIA CUTIS
The differential diagnosis of leukemia cutis is diverse and extensive. Patients often present with painless, firm, indurated nodules, papules, and plaques.1 The lesions can be small, involving a small amount of body surface area, but can also be very large and diffuse.
In our patient’s case, there were no new drugs or exposures to suggest a drug-related eruption, or pruritus or pain to suggest an inflammatory process. The rapid progression of the lesions suggested either an infectious or malignant process. The top 3 conditions in the differential diagnosis, based on his clinical presentation, were cutaneous T-cell lymphoma, cutaneous CD30+ anaplastic large-cell lymphoma, and a drug-induced cutaneous pseudolymphoma.
Skin biopsy is required to differentiate leukemia cutis from the other conditions. On skin biopsy study, leukemia cutis is characterized by infiltration of the skin by leukemic cells and is seen in 10% to 15% of patients with acute myeloid leukemia.2 In 5% of cases, leukemia cutis can present without bone marrow or peripheral signs of leukemia, hence the term aleukemic leukemia cutis.3 Cutaneous signs can occur before, after, or simultaneously with systemic leukemia.4
In the absence of systemic symptoms, the diagnosis is made when progressive cutaneous symptoms are present. The prognosis for aleukemic leukemia cutis is poor. Prompt diagnosis with skin biopsy is paramount to improve outcomes.
Acknowledgment: We would like to recognize Maanasa Devabhaktuni for her assistance in reporting this case.
- Yonal I, Hindilerden F, Coskun R, Dogan OI, Nalcaci M. Aleukemic leukemia cutis manifesting with disseminated nodular eruptions and a plaque preceding acute monocytic leukemia: a case report. Case Rep Oncol 2011; 4(3):547–554. doi:10.1159/000334745
- Cho-Vega JH, Medeiros LJ, Prieto VG, Vega F. Leukemia cutis. Am J Clin Pathol 2008; 129(1):130–142. doi:10.1309/WYACYWF6NGM3WBRT
- Kang YS, Kim HS, Park HJ, et al. Clinical characteristics of 75 patients with leukemia cutis. J Korean Med Sci 2013; 28(4):614–619. doi:10.3346/jkms.2013.28.4.614
- Obiozor C, Ganguly S, Fraga GR. Leukemia cutis with lymphoglandular bodies: a clue to acute lymphoblastic leukemia cutis. Dermatol Online J 2015; 21(8)pii:13030/qt6m18g35f. pmid:26437164
On examination, the numerous firm, indurated nodules ranged in size from 1 to 4 cm. There was no palpable lymphadenopathy.
Results of a peripheral blood cell count showed the following:
- Hemoglobin 12.5 g/dL (reference range 13.0–17.0)
- Platelet count 154 × 109/L (130–400)
- White blood cell count 5.0 × 109/L (4.0–11.0)
- Neutrophils 1.7 × 109/L (1.5–8.0)
- Lymphocytes 2.2 × 109/L (1.0–4.0)
- Monocytes 1.0 × 109/L (0.2–1.0)
- Eosinophils 0 (0–0.4)
- Basophils 0 (0–0.2)
- Blasts 0.
The findings were consistent with leukemic cells with monocytic differentiation. The infiltrate was unusual because leukemic infiltrates typically demonstrate a high nuclear-to-cytoplasmic ratio, but in this case the malignant cells had moderate amounts of cytoplasm due to the monocytic differentiation. Also, a grenz zone is more typically seen in B-cell lymphomas, and T cells more typically demonstrate epidermotropism.
Bone marrow aspiration was performed and revealed a hypercellular bone marrow with trilineage maturation with only 2% blasts. The fluorescence in situ hybridization testing for myelodysplastic syndrome and acute myeloid leukemia was normal. A diagnosis of aleukemic leukemia cutis was made.
After 2 months of chemotherapy with azacitidine, the nodules were less indurated. Treatment was briefly withdrawn due to the development of acute pneumonia, leading to a rapid progression of cutaneous involvement. Despite restarting chemotherapy, the patient died.
ALEUKEMIC LEUKEMIA CUTIS
The differential diagnosis of leukemia cutis is diverse and extensive. Patients often present with painless, firm, indurated nodules, papules, and plaques.1 The lesions can be small, involving a small amount of body surface area, but can also be very large and diffuse.
In our patient’s case, there were no new drugs or exposures to suggest a drug-related eruption, or pruritus or pain to suggest an inflammatory process. The rapid progression of the lesions suggested either an infectious or malignant process. The top 3 conditions in the differential diagnosis, based on his clinical presentation, were cutaneous T-cell lymphoma, cutaneous CD30+ anaplastic large-cell lymphoma, and a drug-induced cutaneous pseudolymphoma.
Skin biopsy is required to differentiate leukemia cutis from the other conditions. On skin biopsy study, leukemia cutis is characterized by infiltration of the skin by leukemic cells and is seen in 10% to 15% of patients with acute myeloid leukemia.2 In 5% of cases, leukemia cutis can present without bone marrow or peripheral signs of leukemia, hence the term aleukemic leukemia cutis.3 Cutaneous signs can occur before, after, or simultaneously with systemic leukemia.4
In the absence of systemic symptoms, the diagnosis is made when progressive cutaneous symptoms are present. The prognosis for aleukemic leukemia cutis is poor. Prompt diagnosis with skin biopsy is paramount to improve outcomes.
Acknowledgment: We would like to recognize Maanasa Devabhaktuni for her assistance in reporting this case.
On examination, the numerous firm, indurated nodules ranged in size from 1 to 4 cm. There was no palpable lymphadenopathy.
Results of a peripheral blood cell count showed the following:
- Hemoglobin 12.5 g/dL (reference range 13.0–17.0)
- Platelet count 154 × 109/L (130–400)
- White blood cell count 5.0 × 109/L (4.0–11.0)
- Neutrophils 1.7 × 109/L (1.5–8.0)
- Lymphocytes 2.2 × 109/L (1.0–4.0)
- Monocytes 1.0 × 109/L (0.2–1.0)
- Eosinophils 0 (0–0.4)
- Basophils 0 (0–0.2)
- Blasts 0.
The findings were consistent with leukemic cells with monocytic differentiation. The infiltrate was unusual because leukemic infiltrates typically demonstrate a high nuclear-to-cytoplasmic ratio, but in this case the malignant cells had moderate amounts of cytoplasm due to the monocytic differentiation. Also, a grenz zone is more typically seen in B-cell lymphomas, and T cells more typically demonstrate epidermotropism.
Bone marrow aspiration was performed and revealed a hypercellular bone marrow with trilineage maturation with only 2% blasts. The fluorescence in situ hybridization testing for myelodysplastic syndrome and acute myeloid leukemia was normal. A diagnosis of aleukemic leukemia cutis was made.
After 2 months of chemotherapy with azacitidine, the nodules were less indurated. Treatment was briefly withdrawn due to the development of acute pneumonia, leading to a rapid progression of cutaneous involvement. Despite restarting chemotherapy, the patient died.
ALEUKEMIC LEUKEMIA CUTIS
The differential diagnosis of leukemia cutis is diverse and extensive. Patients often present with painless, firm, indurated nodules, papules, and plaques.1 The lesions can be small, involving a small amount of body surface area, but can also be very large and diffuse.
In our patient’s case, there were no new drugs or exposures to suggest a drug-related eruption, or pruritus or pain to suggest an inflammatory process. The rapid progression of the lesions suggested either an infectious or malignant process. The top 3 conditions in the differential diagnosis, based on his clinical presentation, were cutaneous T-cell lymphoma, cutaneous CD30+ anaplastic large-cell lymphoma, and a drug-induced cutaneous pseudolymphoma.
Skin biopsy is required to differentiate leukemia cutis from the other conditions. On skin biopsy study, leukemia cutis is characterized by infiltration of the skin by leukemic cells and is seen in 10% to 15% of patients with acute myeloid leukemia.2 In 5% of cases, leukemia cutis can present without bone marrow or peripheral signs of leukemia, hence the term aleukemic leukemia cutis.3 Cutaneous signs can occur before, after, or simultaneously with systemic leukemia.4
In the absence of systemic symptoms, the diagnosis is made when progressive cutaneous symptoms are present. The prognosis for aleukemic leukemia cutis is poor. Prompt diagnosis with skin biopsy is paramount to improve outcomes.
Acknowledgment: We would like to recognize Maanasa Devabhaktuni for her assistance in reporting this case.
- Yonal I, Hindilerden F, Coskun R, Dogan OI, Nalcaci M. Aleukemic leukemia cutis manifesting with disseminated nodular eruptions and a plaque preceding acute monocytic leukemia: a case report. Case Rep Oncol 2011; 4(3):547–554. doi:10.1159/000334745
- Cho-Vega JH, Medeiros LJ, Prieto VG, Vega F. Leukemia cutis. Am J Clin Pathol 2008; 129(1):130–142. doi:10.1309/WYACYWF6NGM3WBRT
- Kang YS, Kim HS, Park HJ, et al. Clinical characteristics of 75 patients with leukemia cutis. J Korean Med Sci 2013; 28(4):614–619. doi:10.3346/jkms.2013.28.4.614
- Obiozor C, Ganguly S, Fraga GR. Leukemia cutis with lymphoglandular bodies: a clue to acute lymphoblastic leukemia cutis. Dermatol Online J 2015; 21(8)pii:13030/qt6m18g35f. pmid:26437164
- Yonal I, Hindilerden F, Coskun R, Dogan OI, Nalcaci M. Aleukemic leukemia cutis manifesting with disseminated nodular eruptions and a plaque preceding acute monocytic leukemia: a case report. Case Rep Oncol 2011; 4(3):547–554. doi:10.1159/000334745
- Cho-Vega JH, Medeiros LJ, Prieto VG, Vega F. Leukemia cutis. Am J Clin Pathol 2008; 129(1):130–142. doi:10.1309/WYACYWF6NGM3WBRT
- Kang YS, Kim HS, Park HJ, et al. Clinical characteristics of 75 patients with leukemia cutis. J Korean Med Sci 2013; 28(4):614–619. doi:10.3346/jkms.2013.28.4.614
- Obiozor C, Ganguly S, Fraga GR. Leukemia cutis with lymphoglandular bodies: a clue to acute lymphoblastic leukemia cutis. Dermatol Online J 2015; 21(8)pii:13030/qt6m18g35f. pmid:26437164
Diagnosing Anemia
This clinical puzzle is based on O’Neil. Diagnosing and classifying anemia in adult primary care. Clinician Reviews. 2017; 27(8):28-35.
For the best mobile experience, rotate screen to landscape mode.
Have feedback on our new crossword puzzle? Share your thoughts at [email protected].
This clinical puzzle is based on O’Neil. Diagnosing and classifying anemia in adult primary care. Clinician Reviews. 2017; 27(8):28-35.
For the best mobile experience, rotate screen to landscape mode.
Have feedback on our new crossword puzzle? Share your thoughts at [email protected].
This clinical puzzle is based on O’Neil. Diagnosing and classifying anemia in adult primary care. Clinician Reviews. 2017; 27(8):28-35.
For the best mobile experience, rotate screen to landscape mode.
Have feedback on our new crossword puzzle? Share your thoughts at [email protected].
Matched transplant improves stroke risk indicator in sickle cell anemia
, suggesting that this intervention may improve outcomes related to cerebral vasculopathy.
Matched sibling donor hematopoietic stem cell transplantation (MSD-HSCT) was linked to significantly lower transcranial Doppler (TCD) velocities at one year compared to standard care in the 9-center study, investigators reported in JAMA.
The study enrolled children with sickle cell anemia who required chronic transfusion due to persistently high TCD velocities, which are associated with increased stroke risk, researchers said.
“Further research is warranted to assess the effects of MSD-HSCT on clinical outcomes and over longer follow-up,” said the researchers, led by Françoise Bernaudin, MD, of Centre Hospitalier Intercommunal de Créteil, Créteil, France
In the non-randomized, prospective DREPAGREFFE study by Dr. Bernaudin and colleagues, 32 children with sickle cell anemia who had a matched sibling donor underwent transplantation, while another 35 children received standard therapy. The primary end point of the study was time-averaged mean of maximum velocities (TAMV) in cerebral arteries at one year.
The highest TAMV at one year was on average 129.6 cm/s in the MSD-HSCT group, versus 170.4 cm/s in the standard care group, for a difference of -40.8 cm/s (P less than .001), Dr. Bernaudin and co-investigators reported.
The improvement persisted at 3 years, with a TAMV of 112.4 cm/s in the transplantation group and 156.7 cm/s in the standard care group (P less than .001), which they also reported as a secondary outcome of the study.
These findings indicate that MSD-HSCT may allow patients with a history of abnormal TCD velocities to stop transfusions and hydroxyurea, Dr. Bernaudin and colleagues said.
The improvement in TCD velocities may be due in part to anemia correction, but also to the “exclusive presence” of normal red blood cells following transplantation, as opposed to simultaneous presence of normal and sickled cells as would be seen after transfusion, they added.
This study wasn’t powered to determine whether a 40 cm/s reduction in TCD velocities would translate into clinical benefits such as reduction in stenosis and silent infarct, or improved cognitive function, they said. Even so, there were no infarcts or stenoses in the MSD-HSCT group, whereas those event occurred in 9% and 6% of patients in the standard care group, respectively, they added.
Dr. Bernaudin reported disclosures related to Addmedica and bluebird bio. Co-authors reported disclosures with Addmedica, Novartis, Alexion, Amgen, Jazz Pharmaceuticals, and others.
SOURCE: Bernaudin F, et al. JAMA. 2019;321(3):266-276.
Results of DREPAGREFFE illustrate the benefits of matched sibling donor hematopoietic stem cell transplantation (HSCT) for a select group of children with sickle cell anemia, according to the author of an editorial on the study.
Matched sibling donor HSCT was well-tolerated in the study and linked to improved control of transcranial Doppler velocities compared to standard care, Janet L. Kwiatkowski, MD, said in the editorial.
“As a curative therapy, it also obviates the need for long-term treatment wrought with adherence challenges with the potential consequence of stroke, and morbidity from iron overload with transfusion therapy,” wrote Dr. Kwiatkowski.
Only a certain proportion of patients have matched sibling donor HSCT as a potential treatment choice, however, she added.
In this particular study, conducted at 9 sites in France, a higher-than-expected 48% of children with sickle cell anemia had a matched sibling donor, whereas in the United States, she said, less than 1 out of 5 such children would be expected to have an HLA-identical sibling donor.
Because many children don’t have an appropriate matched sibling donor, additional studies are needed not only to evaluate the role of HSCT using matched unrelated and haploidentical donors, Dr. Kwiatkowski said, but also to assess how gene therapy interventions impact cerebrovascular outcomes.
These comments are taken from the accompanying editorial in JAMA by Janet L. Kwiatkowski, MD, MSCE, of Children’s Hospital of Philadelphia, and the Department of Pediatrics at Perelman School of Medicine, University of Pennsylvania, Philadelphia. Dr. Kwiatowski disclosed relationships with bluebird bio, Apopharma, and Novartis.
Results of DREPAGREFFE illustrate the benefits of matched sibling donor hematopoietic stem cell transplantation (HSCT) for a select group of children with sickle cell anemia, according to the author of an editorial on the study.
Matched sibling donor HSCT was well-tolerated in the study and linked to improved control of transcranial Doppler velocities compared to standard care, Janet L. Kwiatkowski, MD, said in the editorial.
“As a curative therapy, it also obviates the need for long-term treatment wrought with adherence challenges with the potential consequence of stroke, and morbidity from iron overload with transfusion therapy,” wrote Dr. Kwiatkowski.
Only a certain proportion of patients have matched sibling donor HSCT as a potential treatment choice, however, she added.
In this particular study, conducted at 9 sites in France, a higher-than-expected 48% of children with sickle cell anemia had a matched sibling donor, whereas in the United States, she said, less than 1 out of 5 such children would be expected to have an HLA-identical sibling donor.
Because many children don’t have an appropriate matched sibling donor, additional studies are needed not only to evaluate the role of HSCT using matched unrelated and haploidentical donors, Dr. Kwiatkowski said, but also to assess how gene therapy interventions impact cerebrovascular outcomes.
These comments are taken from the accompanying editorial in JAMA by Janet L. Kwiatkowski, MD, MSCE, of Children’s Hospital of Philadelphia, and the Department of Pediatrics at Perelman School of Medicine, University of Pennsylvania, Philadelphia. Dr. Kwiatowski disclosed relationships with bluebird bio, Apopharma, and Novartis.
Results of DREPAGREFFE illustrate the benefits of matched sibling donor hematopoietic stem cell transplantation (HSCT) for a select group of children with sickle cell anemia, according to the author of an editorial on the study.
Matched sibling donor HSCT was well-tolerated in the study and linked to improved control of transcranial Doppler velocities compared to standard care, Janet L. Kwiatkowski, MD, said in the editorial.
“As a curative therapy, it also obviates the need for long-term treatment wrought with adherence challenges with the potential consequence of stroke, and morbidity from iron overload with transfusion therapy,” wrote Dr. Kwiatkowski.
Only a certain proportion of patients have matched sibling donor HSCT as a potential treatment choice, however, she added.
In this particular study, conducted at 9 sites in France, a higher-than-expected 48% of children with sickle cell anemia had a matched sibling donor, whereas in the United States, she said, less than 1 out of 5 such children would be expected to have an HLA-identical sibling donor.
Because many children don’t have an appropriate matched sibling donor, additional studies are needed not only to evaluate the role of HSCT using matched unrelated and haploidentical donors, Dr. Kwiatkowski said, but also to assess how gene therapy interventions impact cerebrovascular outcomes.
These comments are taken from the accompanying editorial in JAMA by Janet L. Kwiatkowski, MD, MSCE, of Children’s Hospital of Philadelphia, and the Department of Pediatrics at Perelman School of Medicine, University of Pennsylvania, Philadelphia. Dr. Kwiatowski disclosed relationships with bluebird bio, Apopharma, and Novartis.
, suggesting that this intervention may improve outcomes related to cerebral vasculopathy.
Matched sibling donor hematopoietic stem cell transplantation (MSD-HSCT) was linked to significantly lower transcranial Doppler (TCD) velocities at one year compared to standard care in the 9-center study, investigators reported in JAMA.
The study enrolled children with sickle cell anemia who required chronic transfusion due to persistently high TCD velocities, which are associated with increased stroke risk, researchers said.
“Further research is warranted to assess the effects of MSD-HSCT on clinical outcomes and over longer follow-up,” said the researchers, led by Françoise Bernaudin, MD, of Centre Hospitalier Intercommunal de Créteil, Créteil, France
In the non-randomized, prospective DREPAGREFFE study by Dr. Bernaudin and colleagues, 32 children with sickle cell anemia who had a matched sibling donor underwent transplantation, while another 35 children received standard therapy. The primary end point of the study was time-averaged mean of maximum velocities (TAMV) in cerebral arteries at one year.
The highest TAMV at one year was on average 129.6 cm/s in the MSD-HSCT group, versus 170.4 cm/s in the standard care group, for a difference of -40.8 cm/s (P less than .001), Dr. Bernaudin and co-investigators reported.
The improvement persisted at 3 years, with a TAMV of 112.4 cm/s in the transplantation group and 156.7 cm/s in the standard care group (P less than .001), which they also reported as a secondary outcome of the study.
These findings indicate that MSD-HSCT may allow patients with a history of abnormal TCD velocities to stop transfusions and hydroxyurea, Dr. Bernaudin and colleagues said.
The improvement in TCD velocities may be due in part to anemia correction, but also to the “exclusive presence” of normal red blood cells following transplantation, as opposed to simultaneous presence of normal and sickled cells as would be seen after transfusion, they added.
This study wasn’t powered to determine whether a 40 cm/s reduction in TCD velocities would translate into clinical benefits such as reduction in stenosis and silent infarct, or improved cognitive function, they said. Even so, there were no infarcts or stenoses in the MSD-HSCT group, whereas those event occurred in 9% and 6% of patients in the standard care group, respectively, they added.
Dr. Bernaudin reported disclosures related to Addmedica and bluebird bio. Co-authors reported disclosures with Addmedica, Novartis, Alexion, Amgen, Jazz Pharmaceuticals, and others.
SOURCE: Bernaudin F, et al. JAMA. 2019;321(3):266-276.
, suggesting that this intervention may improve outcomes related to cerebral vasculopathy.
Matched sibling donor hematopoietic stem cell transplantation (MSD-HSCT) was linked to significantly lower transcranial Doppler (TCD) velocities at one year compared to standard care in the 9-center study, investigators reported in JAMA.
The study enrolled children with sickle cell anemia who required chronic transfusion due to persistently high TCD velocities, which are associated with increased stroke risk, researchers said.
“Further research is warranted to assess the effects of MSD-HSCT on clinical outcomes and over longer follow-up,” said the researchers, led by Françoise Bernaudin, MD, of Centre Hospitalier Intercommunal de Créteil, Créteil, France
In the non-randomized, prospective DREPAGREFFE study by Dr. Bernaudin and colleagues, 32 children with sickle cell anemia who had a matched sibling donor underwent transplantation, while another 35 children received standard therapy. The primary end point of the study was time-averaged mean of maximum velocities (TAMV) in cerebral arteries at one year.
The highest TAMV at one year was on average 129.6 cm/s in the MSD-HSCT group, versus 170.4 cm/s in the standard care group, for a difference of -40.8 cm/s (P less than .001), Dr. Bernaudin and co-investigators reported.
The improvement persisted at 3 years, with a TAMV of 112.4 cm/s in the transplantation group and 156.7 cm/s in the standard care group (P less than .001), which they also reported as a secondary outcome of the study.
These findings indicate that MSD-HSCT may allow patients with a history of abnormal TCD velocities to stop transfusions and hydroxyurea, Dr. Bernaudin and colleagues said.
The improvement in TCD velocities may be due in part to anemia correction, but also to the “exclusive presence” of normal red blood cells following transplantation, as opposed to simultaneous presence of normal and sickled cells as would be seen after transfusion, they added.
This study wasn’t powered to determine whether a 40 cm/s reduction in TCD velocities would translate into clinical benefits such as reduction in stenosis and silent infarct, or improved cognitive function, they said. Even so, there were no infarcts or stenoses in the MSD-HSCT group, whereas those event occurred in 9% and 6% of patients in the standard care group, respectively, they added.
Dr. Bernaudin reported disclosures related to Addmedica and bluebird bio. Co-authors reported disclosures with Addmedica, Novartis, Alexion, Amgen, Jazz Pharmaceuticals, and others.
SOURCE: Bernaudin F, et al. JAMA. 2019;321(3):266-276.
FROM JAMA
Key clinical point: In children with sickle cell anemia, matched sibling donor hematopoietic stem cell transplants (HSCT) reduced an indicator of stroke risk, suggesting that the intervention may improve cerebrovascular outcomes.
Major finding: The primary end point, time-averaged mean of maximum velocities in cerebral arteries at one year, was on average 129.6 cm/s in the MSD-HSCT group, versus 170.4 cm/s in the standard care group (P less than .001).
Study details: A multicenter, non-randomized, prospective study (DREPAGREFFE) including 32 children with sickle cell anemia who underwent MSD-HSCT and 35 who received standard therapy.
Disclosures: Study authors provided disclosures related to Addmedica, bluebird bio, Novartis, Alexion, Amgen, Jazz Pharmaceuticals, and others.
Source: Bernaudin F, et al. JAMA. 2019;321(3):266-276.
Plerixafor produced dramatic responses in severe WHIM syndrome
Low-dose treatment with plerixafor, a CXC chemokine receptor 4 antagonist, was well tolerated and markedly improved severe presentations of warts, hypogammaglobulinemia, infections, and myelokathexis (WHIM) syndrome in three patients who could not receive granulocyte colony-stimulating factor therapy, investigators reported.
“Myelofibrosis, panleukopenia, anemia, and thrombocytopenia were ameliorated, the wart burden and frequency of infection declined, human papillomavirus–associated oropharyngeal squamous-cell carcinoma stabilized, and quality of life improved markedly,” David H. McDermott, MD, of the National Institute of Allergy and Infectious Diseases and his colleagues wrote in the New England Journal of Medicine.
WHIM syndrome is a primary immunodeficiency disorder characterized by panleukopenia and caused by autosomal dominant gain-of-function mutations in CXC chemokine receptor 4 (CXCR4). Granulocyte colony-stimulating factor (G-CSF) therapy improves neutropenia in these patients, but not other cytopenias.
Previously, the investigators treated three WHIM syndrome patients with plerixafor (Mozobil), which was well tolerated and led to sustained increases in circulating neutrophils, lymphocytes, and monocytes. The current report is of three patients with advanced WHIM syndrome who received open-label plerixafor because they were ineligible for a randomized trial of this drug.
After treatment initiation, infection frequency dropped by 85% in one patient and declined markedly in all three patients. Lymphocyte counts improved the most in two patients while neutrophils were most responsive in the third patient. Warts partially resolved in two patients, of which one patient also experienced partial resolution of head and neck squamous cell carcinoma. This patient later died of a multidrug-resistant Pseudomonas aeruginosa infection after undergoing a 9-hour surgery.
In the third patient, plerixafor therapy led to clearance of TSPyV and 17 human papillomavirus (HPV) infections, with consequent resolution of chronic, progressive, multifocal eczematoid and follicular lesions, the researchers reported. The study dose was relatively low – about 10% of the stem-cell mobilization dose – and did not cause bone pain or other treatment-emergent adverse events, despite the relatively long treatment course (19-52 months).
A separate, phase 3 trial (NCT02231879) has enrolled 19 patients. Primary results are expected in 2020.
The National Institutes of Health funded the work. Dr. McDermott reported a pending patent to reduce CXCR4 expression and/or function to enhance engraftment of hematopoietic stem cells.
SOURCE: McDermott DH et al. N Engl J Med. 2019;380:163-70.
Low-dose treatment with plerixafor, a CXC chemokine receptor 4 antagonist, was well tolerated and markedly improved severe presentations of warts, hypogammaglobulinemia, infections, and myelokathexis (WHIM) syndrome in three patients who could not receive granulocyte colony-stimulating factor therapy, investigators reported.
“Myelofibrosis, panleukopenia, anemia, and thrombocytopenia were ameliorated, the wart burden and frequency of infection declined, human papillomavirus–associated oropharyngeal squamous-cell carcinoma stabilized, and quality of life improved markedly,” David H. McDermott, MD, of the National Institute of Allergy and Infectious Diseases and his colleagues wrote in the New England Journal of Medicine.
WHIM syndrome is a primary immunodeficiency disorder characterized by panleukopenia and caused by autosomal dominant gain-of-function mutations in CXC chemokine receptor 4 (CXCR4). Granulocyte colony-stimulating factor (G-CSF) therapy improves neutropenia in these patients, but not other cytopenias.
Previously, the investigators treated three WHIM syndrome patients with plerixafor (Mozobil), which was well tolerated and led to sustained increases in circulating neutrophils, lymphocytes, and monocytes. The current report is of three patients with advanced WHIM syndrome who received open-label plerixafor because they were ineligible for a randomized trial of this drug.
After treatment initiation, infection frequency dropped by 85% in one patient and declined markedly in all three patients. Lymphocyte counts improved the most in two patients while neutrophils were most responsive in the third patient. Warts partially resolved in two patients, of which one patient also experienced partial resolution of head and neck squamous cell carcinoma. This patient later died of a multidrug-resistant Pseudomonas aeruginosa infection after undergoing a 9-hour surgery.
In the third patient, plerixafor therapy led to clearance of TSPyV and 17 human papillomavirus (HPV) infections, with consequent resolution of chronic, progressive, multifocal eczematoid and follicular lesions, the researchers reported. The study dose was relatively low – about 10% of the stem-cell mobilization dose – and did not cause bone pain or other treatment-emergent adverse events, despite the relatively long treatment course (19-52 months).
A separate, phase 3 trial (NCT02231879) has enrolled 19 patients. Primary results are expected in 2020.
The National Institutes of Health funded the work. Dr. McDermott reported a pending patent to reduce CXCR4 expression and/or function to enhance engraftment of hematopoietic stem cells.
SOURCE: McDermott DH et al. N Engl J Med. 2019;380:163-70.
Low-dose treatment with plerixafor, a CXC chemokine receptor 4 antagonist, was well tolerated and markedly improved severe presentations of warts, hypogammaglobulinemia, infections, and myelokathexis (WHIM) syndrome in three patients who could not receive granulocyte colony-stimulating factor therapy, investigators reported.
“Myelofibrosis, panleukopenia, anemia, and thrombocytopenia were ameliorated, the wart burden and frequency of infection declined, human papillomavirus–associated oropharyngeal squamous-cell carcinoma stabilized, and quality of life improved markedly,” David H. McDermott, MD, of the National Institute of Allergy and Infectious Diseases and his colleagues wrote in the New England Journal of Medicine.
WHIM syndrome is a primary immunodeficiency disorder characterized by panleukopenia and caused by autosomal dominant gain-of-function mutations in CXC chemokine receptor 4 (CXCR4). Granulocyte colony-stimulating factor (G-CSF) therapy improves neutropenia in these patients, but not other cytopenias.
Previously, the investigators treated three WHIM syndrome patients with plerixafor (Mozobil), which was well tolerated and led to sustained increases in circulating neutrophils, lymphocytes, and monocytes. The current report is of three patients with advanced WHIM syndrome who received open-label plerixafor because they were ineligible for a randomized trial of this drug.
After treatment initiation, infection frequency dropped by 85% in one patient and declined markedly in all three patients. Lymphocyte counts improved the most in two patients while neutrophils were most responsive in the third patient. Warts partially resolved in two patients, of which one patient also experienced partial resolution of head and neck squamous cell carcinoma. This patient later died of a multidrug-resistant Pseudomonas aeruginosa infection after undergoing a 9-hour surgery.
In the third patient, plerixafor therapy led to clearance of TSPyV and 17 human papillomavirus (HPV) infections, with consequent resolution of chronic, progressive, multifocal eczematoid and follicular lesions, the researchers reported. The study dose was relatively low – about 10% of the stem-cell mobilization dose – and did not cause bone pain or other treatment-emergent adverse events, despite the relatively long treatment course (19-52 months).
A separate, phase 3 trial (NCT02231879) has enrolled 19 patients. Primary results are expected in 2020.
The National Institutes of Health funded the work. Dr. McDermott reported a pending patent to reduce CXCR4 expression and/or function to enhance engraftment of hematopoietic stem cells.
SOURCE: McDermott DH et al. N Engl J Med. 2019;380:163-70.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Infection frequency dropped by 85% in one patient and showed marked declines in all three patients.
Study details: Open-label study of three patients who were ineligible to receive G-CSF therapy.
Disclosures: The National Institutes of Health funded the work. Dr. McDermott reported a pending patent on reducing CXCR4 expression and/or function to enhance engraftment of hematopoietic stem cells.
Source: McDermott DH et al. N Engl J Med. 2019;380:163-70.
Sickle cell infusion gains FDA breakthrough designation
The in patients with sickle cell disease of all genotypes.
The designation allows the treatment to be reviewed on an expedited schedule.
Crizanlizumab, marketed by Novartis, is a humanized anti–P-selectin monoclonal antibody that has been shown to inhibit interactions between endothelial cells, platelets, red blood cells, sickled red blood cells, and leukocytes.
In the phase 2 SUSTAIN trial, crizanlizumab reduced the median annual rate of vasoocclusive crises that resulted in health care visits by about 45%, compared with placebo (1.63 vs. 2.98; P = .010). The drug also increased the percentage of patients who did not experience any vasoocclusive crises, compared with placebo (35.8% vs. 16.9%; P = .010).
The rates of treatment-emergent and serious adverse events was similar in the drug and placebo arms of the trial.
The in patients with sickle cell disease of all genotypes.
The designation allows the treatment to be reviewed on an expedited schedule.
Crizanlizumab, marketed by Novartis, is a humanized anti–P-selectin monoclonal antibody that has been shown to inhibit interactions between endothelial cells, platelets, red blood cells, sickled red blood cells, and leukocytes.
In the phase 2 SUSTAIN trial, crizanlizumab reduced the median annual rate of vasoocclusive crises that resulted in health care visits by about 45%, compared with placebo (1.63 vs. 2.98; P = .010). The drug also increased the percentage of patients who did not experience any vasoocclusive crises, compared with placebo (35.8% vs. 16.9%; P = .010).
The rates of treatment-emergent and serious adverse events was similar in the drug and placebo arms of the trial.
The in patients with sickle cell disease of all genotypes.
The designation allows the treatment to be reviewed on an expedited schedule.
Crizanlizumab, marketed by Novartis, is a humanized anti–P-selectin monoclonal antibody that has been shown to inhibit interactions between endothelial cells, platelets, red blood cells, sickled red blood cells, and leukocytes.
In the phase 2 SUSTAIN trial, crizanlizumab reduced the median annual rate of vasoocclusive crises that resulted in health care visits by about 45%, compared with placebo (1.63 vs. 2.98; P = .010). The drug also increased the percentage of patients who did not experience any vasoocclusive crises, compared with placebo (35.8% vs. 16.9%; P = .010).
The rates of treatment-emergent and serious adverse events was similar in the drug and placebo arms of the trial.
Poor-prognosis cancers linked to highest suicide risk in first year
Suicide risk significantly increases within the first year of a cancer diagnosis, with risk varying by type of cancer, according to investigators who conducted a retrospective analysis representing nearly 4.7 million patients.
Risk of suicide in that first year after diagnosis was especially high in pancreatic and lung cancers, while by contrast, breast and prostate cancer did not increase suicide risk, reported the researchers, led by Hesham Hamoda, MD, MPH, of Boston Children’s Hospital/Harvard Medical School, and Ahmad Alfaar, MBBCh, MSc, of Charité–Universitätsmedizin Berlin.
That variation in suicide risk by cancer type suggests that prognosis and 5-year relative survival play a role in increasing suicide rates, according to Dr. Hamoda, Dr. Alfaar, and their coauthors.
“After the diagnosis, it is important that health care providers be vigilant in screening for suicide and ensuring that patients have access to social and emotional support,” they wrote in a report published in Cancer. Their analysis was based on 4,671,989 patients with a diagnosis of cancer in the Surveillance, Epidemiology, and End Results (SEER) database between 2000 and 2014. Out of 1,005,825 of those patients who died within the first year of diagnosis, the cause of death was suicide for 1,585, or 0.16%.
Overall, the risk of suicide increased significantly among cancer patients versus the general population, with an observed-to-expected (O/E) ratio of 2.51 per 10,000 person-years, the investigators found. The risk was highest in the first 6 months, with an O/E mortality of 3.13 versus 1.8 in the latter 6 months.
The highest ratios were seen for pancreatic cancer, with an O/E ratio of 8.01, and lung cancer, with a ratio of 6.05, the researchers found in further analysis.
Significant increases in suicide risk were also seen for colorectal cancer (2.08) and melanoma (1.45), though rates were not significantly different versus the general population for breast (1.23) and prostate (0.99), according to the reported data.
Suicide risk was relatively high for any cancer with distant metastases (5.63), though still significantly higher at 1.65 in persons with localized/regional disease, the data show.
The increased suicide risk persisted more than 1 year after the cancer diagnosis, though not to the degree observed within that first year, they added.
Most patients with suicide as a cause of death were white (90.2%) and male (87%). Nearly 60% were between the ages of 65 and 84 at the time of suicide.
Social support plays an integral role in suicide prevention among cancer patients, the researchers noted.
Previous studies suggest that support programs may decrease suicide risk by making patients better aware of their prognosis, receptive to decreased social stigma, or less likely to have stress related to cost of care, they said.
“Discussing the quality of life after diagnosis, the effectiveness of therapy, and the prognosis of the disease and maintaining a trusting relationship with health care professionals all decrease the likelihood of suicide immediately after a diagnosis of cancer,” they said.
Dr. Hamoda, Dr. Alfaar, and their coauthors reported no conflicts of interest. Funding for the study came in part from the German Academic Exchange Service (Dr. Alfaar).
SOURCE: Saad AM, et al. Cancer 2019 Jan 7. doi: 10.1002/cncr.31876.
Suicide risk significantly increases within the first year of a cancer diagnosis, with risk varying by type of cancer, according to investigators who conducted a retrospective analysis representing nearly 4.7 million patients.
Risk of suicide in that first year after diagnosis was especially high in pancreatic and lung cancers, while by contrast, breast and prostate cancer did not increase suicide risk, reported the researchers, led by Hesham Hamoda, MD, MPH, of Boston Children’s Hospital/Harvard Medical School, and Ahmad Alfaar, MBBCh, MSc, of Charité–Universitätsmedizin Berlin.
That variation in suicide risk by cancer type suggests that prognosis and 5-year relative survival play a role in increasing suicide rates, according to Dr. Hamoda, Dr. Alfaar, and their coauthors.
“After the diagnosis, it is important that health care providers be vigilant in screening for suicide and ensuring that patients have access to social and emotional support,” they wrote in a report published in Cancer. Their analysis was based on 4,671,989 patients with a diagnosis of cancer in the Surveillance, Epidemiology, and End Results (SEER) database between 2000 and 2014. Out of 1,005,825 of those patients who died within the first year of diagnosis, the cause of death was suicide for 1,585, or 0.16%.
Overall, the risk of suicide increased significantly among cancer patients versus the general population, with an observed-to-expected (O/E) ratio of 2.51 per 10,000 person-years, the investigators found. The risk was highest in the first 6 months, with an O/E mortality of 3.13 versus 1.8 in the latter 6 months.
The highest ratios were seen for pancreatic cancer, with an O/E ratio of 8.01, and lung cancer, with a ratio of 6.05, the researchers found in further analysis.
Significant increases in suicide risk were also seen for colorectal cancer (2.08) and melanoma (1.45), though rates were not significantly different versus the general population for breast (1.23) and prostate (0.99), according to the reported data.
Suicide risk was relatively high for any cancer with distant metastases (5.63), though still significantly higher at 1.65 in persons with localized/regional disease, the data show.
The increased suicide risk persisted more than 1 year after the cancer diagnosis, though not to the degree observed within that first year, they added.
Most patients with suicide as a cause of death were white (90.2%) and male (87%). Nearly 60% were between the ages of 65 and 84 at the time of suicide.
Social support plays an integral role in suicide prevention among cancer patients, the researchers noted.
Previous studies suggest that support programs may decrease suicide risk by making patients better aware of their prognosis, receptive to decreased social stigma, or less likely to have stress related to cost of care, they said.
“Discussing the quality of life after diagnosis, the effectiveness of therapy, and the prognosis of the disease and maintaining a trusting relationship with health care professionals all decrease the likelihood of suicide immediately after a diagnosis of cancer,” they said.
Dr. Hamoda, Dr. Alfaar, and their coauthors reported no conflicts of interest. Funding for the study came in part from the German Academic Exchange Service (Dr. Alfaar).
SOURCE: Saad AM, et al. Cancer 2019 Jan 7. doi: 10.1002/cncr.31876.
Suicide risk significantly increases within the first year of a cancer diagnosis, with risk varying by type of cancer, according to investigators who conducted a retrospective analysis representing nearly 4.7 million patients.
Risk of suicide in that first year after diagnosis was especially high in pancreatic and lung cancers, while by contrast, breast and prostate cancer did not increase suicide risk, reported the researchers, led by Hesham Hamoda, MD, MPH, of Boston Children’s Hospital/Harvard Medical School, and Ahmad Alfaar, MBBCh, MSc, of Charité–Universitätsmedizin Berlin.
That variation in suicide risk by cancer type suggests that prognosis and 5-year relative survival play a role in increasing suicide rates, according to Dr. Hamoda, Dr. Alfaar, and their coauthors.
“After the diagnosis, it is important that health care providers be vigilant in screening for suicide and ensuring that patients have access to social and emotional support,” they wrote in a report published in Cancer. Their analysis was based on 4,671,989 patients with a diagnosis of cancer in the Surveillance, Epidemiology, and End Results (SEER) database between 2000 and 2014. Out of 1,005,825 of those patients who died within the first year of diagnosis, the cause of death was suicide for 1,585, or 0.16%.
Overall, the risk of suicide increased significantly among cancer patients versus the general population, with an observed-to-expected (O/E) ratio of 2.51 per 10,000 person-years, the investigators found. The risk was highest in the first 6 months, with an O/E mortality of 3.13 versus 1.8 in the latter 6 months.
The highest ratios were seen for pancreatic cancer, with an O/E ratio of 8.01, and lung cancer, with a ratio of 6.05, the researchers found in further analysis.
Significant increases in suicide risk were also seen for colorectal cancer (2.08) and melanoma (1.45), though rates were not significantly different versus the general population for breast (1.23) and prostate (0.99), according to the reported data.
Suicide risk was relatively high for any cancer with distant metastases (5.63), though still significantly higher at 1.65 in persons with localized/regional disease, the data show.
The increased suicide risk persisted more than 1 year after the cancer diagnosis, though not to the degree observed within that first year, they added.
Most patients with suicide as a cause of death were white (90.2%) and male (87%). Nearly 60% were between the ages of 65 and 84 at the time of suicide.
Social support plays an integral role in suicide prevention among cancer patients, the researchers noted.
Previous studies suggest that support programs may decrease suicide risk by making patients better aware of their prognosis, receptive to decreased social stigma, or less likely to have stress related to cost of care, they said.
“Discussing the quality of life after diagnosis, the effectiveness of therapy, and the prognosis of the disease and maintaining a trusting relationship with health care professionals all decrease the likelihood of suicide immediately after a diagnosis of cancer,” they said.
Dr. Hamoda, Dr. Alfaar, and their coauthors reported no conflicts of interest. Funding for the study came in part from the German Academic Exchange Service (Dr. Alfaar).
SOURCE: Saad AM, et al. Cancer 2019 Jan 7. doi: 10.1002/cncr.31876.
FROM CANCER
Key clinical point: A cancer diagnosis significantly increases risk of suicide in comparison to the general population, particularly for poorer-prognosis cancers.
Major finding: The observed-to-expected mortality ratio was substantially higher for pancreatic cancer (8.01), and lung cancer (6.05), but not significantly increased for breast (1.23) and prostate (0.99).
Study details: A retrospective population-based study of 4,671,989 cancer patients.
Disclosures: The authors reported no conflicts of interest. Funding for the study came in part from the German Academic Exchange Service.
Source: Saad AM et al. Cancer. 2019 Jan 7. doi: 10.1002/cncr.31876.
Tests can identify leukemia risk in newborns with Down syndrome
SAN DIEGO – Research into hundreds of babies with Down syndrome is providing valuable insight into the genetic roots of leukemia and offering a route to identify newborns at high risk.
“We can now identify children at high risk of developing myeloid leukemia within 4 years” through blood or genetic tests, Irene Roberts, MD, a pediatric hematologist at the University of Oxford’s (England) MRC Weatherall Institute of Molecular Medicine, said at the annual meeting of the American Society of Hematology.
About 2%-3% of children with Down syndrome will develop acute lymphocytic leukemia (ALL) or acute myeloid leukemia (AML), according to the National Cancer Institute, rates that are much higher than in the general population.
Research suggests that among children aged 0-4 years with Down syndrome, the standardized incidence ratio (SIR) of AML is 114, compared with other children, Dr. Roberts said. The SIR of ALL is 27 in children aged 1-4 years, she said.
For people with Down syndrome aged 0-60 years, the SIRs are 12 and 13 in AML and ALL, respectively, she said.
In her presentation, Dr. Roberts focused on AML that appears before age 4 years and is preceded by a neonatal preleukemia – transient abnormal myelopoiesis (TAM) – that only occurs in Down syndrome. In most cases, TAM, which occurs with GATA1 mutations, resolves on its own after birth, she said. But in others, the GATA1 mutations continue and cause AML to develop.
Dr. Roberts highlighted her institution’s Oxford Down Syndrome Cohort Study and offered an update to a 2013 report (Blood. 2013 Dec 5;122[24]:3908–17). The study recruited 471 neonates with Down syndrome and followed them for up to 4 years: 341 with no GATA1 mutation and 130 (28%) with the mutation. Dr. Roberts called the latter number a “very high frequency.”
Of those with the mutation, 7 patients (5%) developed AML at a median age of 16 months. None of those without the mutation developed AML.
Also, among the 130 neonates with the mutation, 42% were considered to have “clinical” TAM (more than 10% blasts) and 58% were considered to have “silent” TAM (fewer than 10% blasts).
“We predicted that these babies with clinical TAM would have more severe clinical disease ... and that in fact turned out to be the case,” Dr. Roberts said.
Why is the GATA1 mutation so significant? Research suggests that platelet production is abnormal in neonates with Down syndrome, compared with neonates without it, regardless of whether they have the mutation, Dr. Roberts said.
The mutation doesn’t reduce further platelet count, but does disrupt megakaryopoiesis – the process of the production of platelets. As a result, giant platelets and megakaryocyte fragments are more common, she explained.
Moving forward, research data can be used to identify which children are most at risk, Dr. Roberts said. Newborns with Down syndrome are more likely to survive without leukemia if they have silent TAM, compared with those who have clinical TAM, and if they have an estimated variant allele frequency above 15%, according to findings from the Oxford study.
Children at high risk of AML before age 4 years can be identified by analyzing the percentage of blasts on a smear and/or by analyzing mutation of GATA1, according to Dr. Roberts. However, this cannot be accomplished by the use of a complete blood count (CBC) test, she said, which is used to check for leukemia.
Dr. Roberts called for the development of more guidelines for screening newborns with Down syndrome for leukemia risk. The British Society for Haematology issued testing guidelines, coauthored by Dr. Roberts, in 2018 (Br J Haematol. 2018 Jul;182[2]:200-11).
Dr. Roberts reported having no financial disclosures.
SAN DIEGO – Research into hundreds of babies with Down syndrome is providing valuable insight into the genetic roots of leukemia and offering a route to identify newborns at high risk.
“We can now identify children at high risk of developing myeloid leukemia within 4 years” through blood or genetic tests, Irene Roberts, MD, a pediatric hematologist at the University of Oxford’s (England) MRC Weatherall Institute of Molecular Medicine, said at the annual meeting of the American Society of Hematology.
About 2%-3% of children with Down syndrome will develop acute lymphocytic leukemia (ALL) or acute myeloid leukemia (AML), according to the National Cancer Institute, rates that are much higher than in the general population.
Research suggests that among children aged 0-4 years with Down syndrome, the standardized incidence ratio (SIR) of AML is 114, compared with other children, Dr. Roberts said. The SIR of ALL is 27 in children aged 1-4 years, she said.
For people with Down syndrome aged 0-60 years, the SIRs are 12 and 13 in AML and ALL, respectively, she said.
In her presentation, Dr. Roberts focused on AML that appears before age 4 years and is preceded by a neonatal preleukemia – transient abnormal myelopoiesis (TAM) – that only occurs in Down syndrome. In most cases, TAM, which occurs with GATA1 mutations, resolves on its own after birth, she said. But in others, the GATA1 mutations continue and cause AML to develop.
Dr. Roberts highlighted her institution’s Oxford Down Syndrome Cohort Study and offered an update to a 2013 report (Blood. 2013 Dec 5;122[24]:3908–17). The study recruited 471 neonates with Down syndrome and followed them for up to 4 years: 341 with no GATA1 mutation and 130 (28%) with the mutation. Dr. Roberts called the latter number a “very high frequency.”
Of those with the mutation, 7 patients (5%) developed AML at a median age of 16 months. None of those without the mutation developed AML.
Also, among the 130 neonates with the mutation, 42% were considered to have “clinical” TAM (more than 10% blasts) and 58% were considered to have “silent” TAM (fewer than 10% blasts).
“We predicted that these babies with clinical TAM would have more severe clinical disease ... and that in fact turned out to be the case,” Dr. Roberts said.
Why is the GATA1 mutation so significant? Research suggests that platelet production is abnormal in neonates with Down syndrome, compared with neonates without it, regardless of whether they have the mutation, Dr. Roberts said.
The mutation doesn’t reduce further platelet count, but does disrupt megakaryopoiesis – the process of the production of platelets. As a result, giant platelets and megakaryocyte fragments are more common, she explained.
Moving forward, research data can be used to identify which children are most at risk, Dr. Roberts said. Newborns with Down syndrome are more likely to survive without leukemia if they have silent TAM, compared with those who have clinical TAM, and if they have an estimated variant allele frequency above 15%, according to findings from the Oxford study.
Children at high risk of AML before age 4 years can be identified by analyzing the percentage of blasts on a smear and/or by analyzing mutation of GATA1, according to Dr. Roberts. However, this cannot be accomplished by the use of a complete blood count (CBC) test, she said, which is used to check for leukemia.
Dr. Roberts called for the development of more guidelines for screening newborns with Down syndrome for leukemia risk. The British Society for Haematology issued testing guidelines, coauthored by Dr. Roberts, in 2018 (Br J Haematol. 2018 Jul;182[2]:200-11).
Dr. Roberts reported having no financial disclosures.
SAN DIEGO – Research into hundreds of babies with Down syndrome is providing valuable insight into the genetic roots of leukemia and offering a route to identify newborns at high risk.
“We can now identify children at high risk of developing myeloid leukemia within 4 years” through blood or genetic tests, Irene Roberts, MD, a pediatric hematologist at the University of Oxford’s (England) MRC Weatherall Institute of Molecular Medicine, said at the annual meeting of the American Society of Hematology.
About 2%-3% of children with Down syndrome will develop acute lymphocytic leukemia (ALL) or acute myeloid leukemia (AML), according to the National Cancer Institute, rates that are much higher than in the general population.
Research suggests that among children aged 0-4 years with Down syndrome, the standardized incidence ratio (SIR) of AML is 114, compared with other children, Dr. Roberts said. The SIR of ALL is 27 in children aged 1-4 years, she said.
For people with Down syndrome aged 0-60 years, the SIRs are 12 and 13 in AML and ALL, respectively, she said.
In her presentation, Dr. Roberts focused on AML that appears before age 4 years and is preceded by a neonatal preleukemia – transient abnormal myelopoiesis (TAM) – that only occurs in Down syndrome. In most cases, TAM, which occurs with GATA1 mutations, resolves on its own after birth, she said. But in others, the GATA1 mutations continue and cause AML to develop.
Dr. Roberts highlighted her institution’s Oxford Down Syndrome Cohort Study and offered an update to a 2013 report (Blood. 2013 Dec 5;122[24]:3908–17). The study recruited 471 neonates with Down syndrome and followed them for up to 4 years: 341 with no GATA1 mutation and 130 (28%) with the mutation. Dr. Roberts called the latter number a “very high frequency.”
Of those with the mutation, 7 patients (5%) developed AML at a median age of 16 months. None of those without the mutation developed AML.
Also, among the 130 neonates with the mutation, 42% were considered to have “clinical” TAM (more than 10% blasts) and 58% were considered to have “silent” TAM (fewer than 10% blasts).
“We predicted that these babies with clinical TAM would have more severe clinical disease ... and that in fact turned out to be the case,” Dr. Roberts said.
Why is the GATA1 mutation so significant? Research suggests that platelet production is abnormal in neonates with Down syndrome, compared with neonates without it, regardless of whether they have the mutation, Dr. Roberts said.
The mutation doesn’t reduce further platelet count, but does disrupt megakaryopoiesis – the process of the production of platelets. As a result, giant platelets and megakaryocyte fragments are more common, she explained.
Moving forward, research data can be used to identify which children are most at risk, Dr. Roberts said. Newborns with Down syndrome are more likely to survive without leukemia if they have silent TAM, compared with those who have clinical TAM, and if they have an estimated variant allele frequency above 15%, according to findings from the Oxford study.
Children at high risk of AML before age 4 years can be identified by analyzing the percentage of blasts on a smear and/or by analyzing mutation of GATA1, according to Dr. Roberts. However, this cannot be accomplished by the use of a complete blood count (CBC) test, she said, which is used to check for leukemia.
Dr. Roberts called for the development of more guidelines for screening newborns with Down syndrome for leukemia risk. The British Society for Haematology issued testing guidelines, coauthored by Dr. Roberts, in 2018 (Br J Haematol. 2018 Jul;182[2]:200-11).
Dr. Roberts reported having no financial disclosures.
EXPERT ANALYSIS FROM ASH 2018
ALL chemotherapy looks effective in mixed phenotype leukemia
SAN DIEGO – The majority of pediatric patients with mixed phenotype acute leukemia (MPAL) who were treated with acute lymphoblastic leukemia (ALL)–directed chemotherapy achieved a minimum residual disease (MRD)–negative complete response by the end of consolidation, according to findings from a multicenter retrospective cohort study.
The cohort included 94 patients aged 1-21 years who met strict World Health Organization MPAL criteria and were treated between 2008 and 2016 at one of six U.S. institutions. Most had B/myeloid phenotype (89%), and 87 patients were treated with an ALL regimen, Etan Orgel, MD, reported at the annual meeting of the American Society of Hematology.
Of those 87 patients, 81 (93%) experienced an end-of-induction (EOI) complete response. One patient died during induction and six had induction failures, defined as either disease progression before EOI (two patients) or EOI MRD of 5% or greater (three patients), said Dr. Orgel of the University of Southern California, Los Angeles, and Children’s Hospital Los Angeles.
The MRD-negative rates, defined as MRD less than 0.01%, were 70% at EOI and 86% at EOI or end of consolidation (EOC); 12 of 14 patients who were MRD positive at EOI and continued on ALL therapy achieved an EOC MRD-negative complete response, including 8 of 8 with EOI MRD of 0.01%-0.09% and 4 of 6 with EOI MRD of 1% or greater.
Event-free survival at 5 years in the 78 patients without hematopoietic stem cell transplant at first remission was 75%, and 5-year overall survival was 89%, “thus demonstrating that, for a majority of patients, transplant in first remission may not be necessary,” Dr. Orgel said. “This is very different from the approach used at many adult centers and many of the adult recommendations.”
Overall 5-year EOI event-free survival was 80% in the 59 patients who were MRD negative at EOI, and 13% in 25 patients who were MRD-positive at EOI. The corresponding overall survival rates were 91% and 84%.
Overall 5-year EOC event-free survival was 77% in 74 patients who were MRD negative at EOC and was unavailable in 3 patients who were MRD positive at EOC, although all three were salvaged. The corresponding EOC overall survival rates were 89% and “not available,” Dr. Orgel reported.
Multivariable analysis confirmed the predictive value of MRD at EOI (hazard ratio for event-free survival and overall survival, 3.77 and 3.54, respectively).
Of note, there was a possible trend toward earlier failure and a trend toward worse overall survival (HR, 4.49, P = .074) for T-lineage–containing MPAL.
“That indicates that this might be a group that needs careful scrutiny of which form of ALL therapy they receive,” he said.
MRD in pediatric MPAL is rare. Recent studies of MPAL biology show areas of similarity with ALL and AML, and while this could eventually help further subcategorize or classify the disease and lead to biology-driven therapies, it is important to know how to treat the disease today, Dr. Orgel said.
The evolving consensus is that ALL therapy is adequate for most MPAL, but there is no established threshold for MRD to enable a risk-stratified MPAL approach, he added.
The current findings suggest that ALL therapy – without hematopoietic stem cell transplant – may be sufficient to treat most patients with pediatric MPAL, Dr. Orgen reported, noting that clinical trials are necessary to prospectively validate MRD thresholds at EOI and EOC and to establish the threshold for favorable survival.
“Future research should explore either intensification of therapy or different therapies for patients with persistent MRD,” he said.
Dr. Orgel reported having no financial disclosures.
SOURCE: Oberley M et al. ASH 2018, Abstract 558.
SAN DIEGO – The majority of pediatric patients with mixed phenotype acute leukemia (MPAL) who were treated with acute lymphoblastic leukemia (ALL)–directed chemotherapy achieved a minimum residual disease (MRD)–negative complete response by the end of consolidation, according to findings from a multicenter retrospective cohort study.
The cohort included 94 patients aged 1-21 years who met strict World Health Organization MPAL criteria and were treated between 2008 and 2016 at one of six U.S. institutions. Most had B/myeloid phenotype (89%), and 87 patients were treated with an ALL regimen, Etan Orgel, MD, reported at the annual meeting of the American Society of Hematology.
Of those 87 patients, 81 (93%) experienced an end-of-induction (EOI) complete response. One patient died during induction and six had induction failures, defined as either disease progression before EOI (two patients) or EOI MRD of 5% or greater (three patients), said Dr. Orgel of the University of Southern California, Los Angeles, and Children’s Hospital Los Angeles.
The MRD-negative rates, defined as MRD less than 0.01%, were 70% at EOI and 86% at EOI or end of consolidation (EOC); 12 of 14 patients who were MRD positive at EOI and continued on ALL therapy achieved an EOC MRD-negative complete response, including 8 of 8 with EOI MRD of 0.01%-0.09% and 4 of 6 with EOI MRD of 1% or greater.
Event-free survival at 5 years in the 78 patients without hematopoietic stem cell transplant at first remission was 75%, and 5-year overall survival was 89%, “thus demonstrating that, for a majority of patients, transplant in first remission may not be necessary,” Dr. Orgel said. “This is very different from the approach used at many adult centers and many of the adult recommendations.”
Overall 5-year EOI event-free survival was 80% in the 59 patients who were MRD negative at EOI, and 13% in 25 patients who were MRD-positive at EOI. The corresponding overall survival rates were 91% and 84%.
Overall 5-year EOC event-free survival was 77% in 74 patients who were MRD negative at EOC and was unavailable in 3 patients who were MRD positive at EOC, although all three were salvaged. The corresponding EOC overall survival rates were 89% and “not available,” Dr. Orgel reported.
Multivariable analysis confirmed the predictive value of MRD at EOI (hazard ratio for event-free survival and overall survival, 3.77 and 3.54, respectively).
Of note, there was a possible trend toward earlier failure and a trend toward worse overall survival (HR, 4.49, P = .074) for T-lineage–containing MPAL.
“That indicates that this might be a group that needs careful scrutiny of which form of ALL therapy they receive,” he said.
MRD in pediatric MPAL is rare. Recent studies of MPAL biology show areas of similarity with ALL and AML, and while this could eventually help further subcategorize or classify the disease and lead to biology-driven therapies, it is important to know how to treat the disease today, Dr. Orgel said.
The evolving consensus is that ALL therapy is adequate for most MPAL, but there is no established threshold for MRD to enable a risk-stratified MPAL approach, he added.
The current findings suggest that ALL therapy – without hematopoietic stem cell transplant – may be sufficient to treat most patients with pediatric MPAL, Dr. Orgen reported, noting that clinical trials are necessary to prospectively validate MRD thresholds at EOI and EOC and to establish the threshold for favorable survival.
“Future research should explore either intensification of therapy or different therapies for patients with persistent MRD,” he said.
Dr. Orgel reported having no financial disclosures.
SOURCE: Oberley M et al. ASH 2018, Abstract 558.
SAN DIEGO – The majority of pediatric patients with mixed phenotype acute leukemia (MPAL) who were treated with acute lymphoblastic leukemia (ALL)–directed chemotherapy achieved a minimum residual disease (MRD)–negative complete response by the end of consolidation, according to findings from a multicenter retrospective cohort study.
The cohort included 94 patients aged 1-21 years who met strict World Health Organization MPAL criteria and were treated between 2008 and 2016 at one of six U.S. institutions. Most had B/myeloid phenotype (89%), and 87 patients were treated with an ALL regimen, Etan Orgel, MD, reported at the annual meeting of the American Society of Hematology.
Of those 87 patients, 81 (93%) experienced an end-of-induction (EOI) complete response. One patient died during induction and six had induction failures, defined as either disease progression before EOI (two patients) or EOI MRD of 5% or greater (three patients), said Dr. Orgel of the University of Southern California, Los Angeles, and Children’s Hospital Los Angeles.
The MRD-negative rates, defined as MRD less than 0.01%, were 70% at EOI and 86% at EOI or end of consolidation (EOC); 12 of 14 patients who were MRD positive at EOI and continued on ALL therapy achieved an EOC MRD-negative complete response, including 8 of 8 with EOI MRD of 0.01%-0.09% and 4 of 6 with EOI MRD of 1% or greater.
Event-free survival at 5 years in the 78 patients without hematopoietic stem cell transplant at first remission was 75%, and 5-year overall survival was 89%, “thus demonstrating that, for a majority of patients, transplant in first remission may not be necessary,” Dr. Orgel said. “This is very different from the approach used at many adult centers and many of the adult recommendations.”
Overall 5-year EOI event-free survival was 80% in the 59 patients who were MRD negative at EOI, and 13% in 25 patients who were MRD-positive at EOI. The corresponding overall survival rates were 91% and 84%.
Overall 5-year EOC event-free survival was 77% in 74 patients who were MRD negative at EOC and was unavailable in 3 patients who were MRD positive at EOC, although all three were salvaged. The corresponding EOC overall survival rates were 89% and “not available,” Dr. Orgel reported.
Multivariable analysis confirmed the predictive value of MRD at EOI (hazard ratio for event-free survival and overall survival, 3.77 and 3.54, respectively).
Of note, there was a possible trend toward earlier failure and a trend toward worse overall survival (HR, 4.49, P = .074) for T-lineage–containing MPAL.
“That indicates that this might be a group that needs careful scrutiny of which form of ALL therapy they receive,” he said.
MRD in pediatric MPAL is rare. Recent studies of MPAL biology show areas of similarity with ALL and AML, and while this could eventually help further subcategorize or classify the disease and lead to biology-driven therapies, it is important to know how to treat the disease today, Dr. Orgel said.
The evolving consensus is that ALL therapy is adequate for most MPAL, but there is no established threshold for MRD to enable a risk-stratified MPAL approach, he added.
The current findings suggest that ALL therapy – without hematopoietic stem cell transplant – may be sufficient to treat most patients with pediatric MPAL, Dr. Orgen reported, noting that clinical trials are necessary to prospectively validate MRD thresholds at EOI and EOC and to establish the threshold for favorable survival.
“Future research should explore either intensification of therapy or different therapies for patients with persistent MRD,” he said.
Dr. Orgel reported having no financial disclosures.
SOURCE: Oberley M et al. ASH 2018, Abstract 558.
REPORTING FROM ASH 2018
Key clinical point:
Major finding: MRD-negative rates were 70% at end of induction and 86% at end of induction or consolidation.
Study details: A retrospective cohort study of 87 pediatric MPAL patients.
Disclosures: Dr. Orgel reported having no financial disclosures.
Source: Oberley M et al. ASH 2018, Abstract 558.