User login
AHA targets ‘low-value’ heart care in new scientific statement
Low-value health care services that provide little or no benefit to patients are “common, potentially harmful, and costly,” and there is a critical need to reduce this kind of care, the American Heart Association said in a newly released scientific statement.
Each year, nearly half of patients in the United States will receive at least one low-value test or procedure, with the attendant risk of avoidable complications from cascades of care and excess costs to individuals and society, the authors noted. Reducing low-value care is particularly important in cardiology, given the high prevalence and costs of cardiovascular disease in the United States.
The statement was published online Feb. 22, 2022, in Circulation: Cardiovascular Quality and Outcomes.
High burden with uncertain benefit
“Cardiovascular disease is common and can present suddenly, such as a heart attack or abnormal heart rhythm,” Vinay Kini, MD, chair of the statement writing group and assistant professor of medicine at Weill Cornell Medicine, New York, said in a news release.
“Our desire to be vigilant about treating and preventing cardiovascular disease may sometimes lead to use of tests and procedures where the benefits to patients may be uncertain,” Dr. Kini said. “This may impose burdens on patients, in the form of increased risk of physical harm from the low-value procedure or potential complications, as well as follow-up care and out-of-pocket financial costs.”
For example, studies have shown that up to one in five echocardiograms and up to half of all stress tests performed in the United States may be rated as rarely appropriate, based on established guidelines for their use.
In addition, up to 15% of percutaneous coronary interventions (PCIs) are classified as rarely appropriate, the writing group said.
Annually, among Medicare fee-for-service beneficiaries, low-value stress testing in patients with stable coronary artery disease is estimated to cost between $212 million and $2.1 billion, while costs of PCI for stable CAD range from $212 million to $2.8 billion, the writing group noted.
“At best, spending on low-value care potentially diverts resources from higher-value services that would benefit patients more effectively at the same or reduced cost. At worst, low-value care results in physical harm in the form of preventable morbidity and mortality,” they said.
“Thus, reducing low-value care is one of the few patient-centered solutions that directly address both the need to control health care spending and the societal imperative to devote its limited resources to beneficial health care services that improve health,” they added.
The group outlines several ways to reduce low-value cardiovascular care targeting patients, providers, and payers/policymakers.
For patients, education and shared decision-making may help reduce low-value care and dispel misconceptions about the intended purpose of test or treatment, they suggested.
For clinicians, a “layered” approach to reducing low-value care may be most effective, such as through education, audit and feedback, and behavioral science tools (“nudges”) to shift behaviors and practices, they said.
For payers and policy leaders, interventions to reduce low-value care include national insurance coverage determinations; prior authorization; alternative payment models that reward lower costs and higher-quality health care; value-based insurance designs that financially penalize low-value care; and medical liability reform to reduce defensive medical practices.
Low-value cardiovascular care is a complex problem, the writing group acknowledged, and achieving meaningful reductions in low-value cardiovascular care will require a multidisciplinary approach that includes continuous research, implementation, evaluation, and adjustment while ensuring equitable access to care.
“Each approach has benefits and drawbacks,” Dr. Kini said. “For example, prior authorization imposes a large burden on health care professionals to obtain insurance approval for tests and treatments. Prior authorization and some value-based payment models may unintentionally worsen existing racial and ethnic health care disparities.
“A one-size-fits-all approach to reducing low-value care is unlikely to succeed; rather, acting through multiple perspectives and frequently measuring impacts and potential unintended consequences is critical,” he concluded.
The scientific statement was prepared by the volunteer writing group on behalf of the AHA’s Council on Quality of Care and Outcomes Research.
The research had no commercial funding. Dr. Kini disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Low-value health care services that provide little or no benefit to patients are “common, potentially harmful, and costly,” and there is a critical need to reduce this kind of care, the American Heart Association said in a newly released scientific statement.
Each year, nearly half of patients in the United States will receive at least one low-value test or procedure, with the attendant risk of avoidable complications from cascades of care and excess costs to individuals and society, the authors noted. Reducing low-value care is particularly important in cardiology, given the high prevalence and costs of cardiovascular disease in the United States.
The statement was published online Feb. 22, 2022, in Circulation: Cardiovascular Quality and Outcomes.
High burden with uncertain benefit
“Cardiovascular disease is common and can present suddenly, such as a heart attack or abnormal heart rhythm,” Vinay Kini, MD, chair of the statement writing group and assistant professor of medicine at Weill Cornell Medicine, New York, said in a news release.
“Our desire to be vigilant about treating and preventing cardiovascular disease may sometimes lead to use of tests and procedures where the benefits to patients may be uncertain,” Dr. Kini said. “This may impose burdens on patients, in the form of increased risk of physical harm from the low-value procedure or potential complications, as well as follow-up care and out-of-pocket financial costs.”
For example, studies have shown that up to one in five echocardiograms and up to half of all stress tests performed in the United States may be rated as rarely appropriate, based on established guidelines for their use.
In addition, up to 15% of percutaneous coronary interventions (PCIs) are classified as rarely appropriate, the writing group said.
Annually, among Medicare fee-for-service beneficiaries, low-value stress testing in patients with stable coronary artery disease is estimated to cost between $212 million and $2.1 billion, while costs of PCI for stable CAD range from $212 million to $2.8 billion, the writing group noted.
“At best, spending on low-value care potentially diverts resources from higher-value services that would benefit patients more effectively at the same or reduced cost. At worst, low-value care results in physical harm in the form of preventable morbidity and mortality,” they said.
“Thus, reducing low-value care is one of the few patient-centered solutions that directly address both the need to control health care spending and the societal imperative to devote its limited resources to beneficial health care services that improve health,” they added.
The group outlines several ways to reduce low-value cardiovascular care targeting patients, providers, and payers/policymakers.
For patients, education and shared decision-making may help reduce low-value care and dispel misconceptions about the intended purpose of test or treatment, they suggested.
For clinicians, a “layered” approach to reducing low-value care may be most effective, such as through education, audit and feedback, and behavioral science tools (“nudges”) to shift behaviors and practices, they said.
For payers and policy leaders, interventions to reduce low-value care include national insurance coverage determinations; prior authorization; alternative payment models that reward lower costs and higher-quality health care; value-based insurance designs that financially penalize low-value care; and medical liability reform to reduce defensive medical practices.
Low-value cardiovascular care is a complex problem, the writing group acknowledged, and achieving meaningful reductions in low-value cardiovascular care will require a multidisciplinary approach that includes continuous research, implementation, evaluation, and adjustment while ensuring equitable access to care.
“Each approach has benefits and drawbacks,” Dr. Kini said. “For example, prior authorization imposes a large burden on health care professionals to obtain insurance approval for tests and treatments. Prior authorization and some value-based payment models may unintentionally worsen existing racial and ethnic health care disparities.
“A one-size-fits-all approach to reducing low-value care is unlikely to succeed; rather, acting through multiple perspectives and frequently measuring impacts and potential unintended consequences is critical,” he concluded.
The scientific statement was prepared by the volunteer writing group on behalf of the AHA’s Council on Quality of Care and Outcomes Research.
The research had no commercial funding. Dr. Kini disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Low-value health care services that provide little or no benefit to patients are “common, potentially harmful, and costly,” and there is a critical need to reduce this kind of care, the American Heart Association said in a newly released scientific statement.
Each year, nearly half of patients in the United States will receive at least one low-value test or procedure, with the attendant risk of avoidable complications from cascades of care and excess costs to individuals and society, the authors noted. Reducing low-value care is particularly important in cardiology, given the high prevalence and costs of cardiovascular disease in the United States.
The statement was published online Feb. 22, 2022, in Circulation: Cardiovascular Quality and Outcomes.
High burden with uncertain benefit
“Cardiovascular disease is common and can present suddenly, such as a heart attack or abnormal heart rhythm,” Vinay Kini, MD, chair of the statement writing group and assistant professor of medicine at Weill Cornell Medicine, New York, said in a news release.
“Our desire to be vigilant about treating and preventing cardiovascular disease may sometimes lead to use of tests and procedures where the benefits to patients may be uncertain,” Dr. Kini said. “This may impose burdens on patients, in the form of increased risk of physical harm from the low-value procedure or potential complications, as well as follow-up care and out-of-pocket financial costs.”
For example, studies have shown that up to one in five echocardiograms and up to half of all stress tests performed in the United States may be rated as rarely appropriate, based on established guidelines for their use.
In addition, up to 15% of percutaneous coronary interventions (PCIs) are classified as rarely appropriate, the writing group said.
Annually, among Medicare fee-for-service beneficiaries, low-value stress testing in patients with stable coronary artery disease is estimated to cost between $212 million and $2.1 billion, while costs of PCI for stable CAD range from $212 million to $2.8 billion, the writing group noted.
“At best, spending on low-value care potentially diverts resources from higher-value services that would benefit patients more effectively at the same or reduced cost. At worst, low-value care results in physical harm in the form of preventable morbidity and mortality,” they said.
“Thus, reducing low-value care is one of the few patient-centered solutions that directly address both the need to control health care spending and the societal imperative to devote its limited resources to beneficial health care services that improve health,” they added.
The group outlines several ways to reduce low-value cardiovascular care targeting patients, providers, and payers/policymakers.
For patients, education and shared decision-making may help reduce low-value care and dispel misconceptions about the intended purpose of test or treatment, they suggested.
For clinicians, a “layered” approach to reducing low-value care may be most effective, such as through education, audit and feedback, and behavioral science tools (“nudges”) to shift behaviors and practices, they said.
For payers and policy leaders, interventions to reduce low-value care include national insurance coverage determinations; prior authorization; alternative payment models that reward lower costs and higher-quality health care; value-based insurance designs that financially penalize low-value care; and medical liability reform to reduce defensive medical practices.
Low-value cardiovascular care is a complex problem, the writing group acknowledged, and achieving meaningful reductions in low-value cardiovascular care will require a multidisciplinary approach that includes continuous research, implementation, evaluation, and adjustment while ensuring equitable access to care.
“Each approach has benefits and drawbacks,” Dr. Kini said. “For example, prior authorization imposes a large burden on health care professionals to obtain insurance approval for tests and treatments. Prior authorization and some value-based payment models may unintentionally worsen existing racial and ethnic health care disparities.
“A one-size-fits-all approach to reducing low-value care is unlikely to succeed; rather, acting through multiple perspectives and frequently measuring impacts and potential unintended consequences is critical,” he concluded.
The scientific statement was prepared by the volunteer writing group on behalf of the AHA’s Council on Quality of Care and Outcomes Research.
The research had no commercial funding. Dr. Kini disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION: CARDIOVASCULAR QUALITY AND OUTCOMES
FDA okays empagliflozin for HF regardless of ejection fraction
The Food and Drug Administration has approved an expanded heart failure indication for the sodium-glucose transporter 2 inhibitor empagliflozin (Jardiance) that now includes HF with mid-range or preserved left ventricular ejection fraction (LVEF), the agency announced on Feb. 24.
That means the SGLT2 inhibitor, once considered primarily an antidiabetic agent, is approved for use in patients with HF per se without regard to ventricular function. The drug received approval for HF with reduced LVEF in August 2021.
The expanded indication, specifically for reducing the risk of cardiovascular death and HF hospitalization in adults, was widely anticipated based on the landmark results from the EMPEROR-Preserved trial. The study saw a significant 21% relative reduction in that composite endpoint over about 2 years in patients with New York Heart Association class II-IV heart failure and an LVEF greater than 40% who received empagliflozin along with other standard care.
Interestingly, the drug’s expanded indication in HF resembles that approved for sacubitril/valsartan (Entresto) in February 2021 based mostly on the PARAGON-HF trial, which entered patients with HF and an LVEF at least 45%. The trial was “negative” in that it saw no significant advantage to the drug for its primary clinical outcome but did suggest benefit for some secondary endpoints.
The FDA had used more cautionary language in its expanded indication for sacubitril/valsartan, “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure. Benefits are most clearly evident in patients with left ventricular ejection fraction below normal.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved an expanded heart failure indication for the sodium-glucose transporter 2 inhibitor empagliflozin (Jardiance) that now includes HF with mid-range or preserved left ventricular ejection fraction (LVEF), the agency announced on Feb. 24.
That means the SGLT2 inhibitor, once considered primarily an antidiabetic agent, is approved for use in patients with HF per se without regard to ventricular function. The drug received approval for HF with reduced LVEF in August 2021.
The expanded indication, specifically for reducing the risk of cardiovascular death and HF hospitalization in adults, was widely anticipated based on the landmark results from the EMPEROR-Preserved trial. The study saw a significant 21% relative reduction in that composite endpoint over about 2 years in patients with New York Heart Association class II-IV heart failure and an LVEF greater than 40% who received empagliflozin along with other standard care.
Interestingly, the drug’s expanded indication in HF resembles that approved for sacubitril/valsartan (Entresto) in February 2021 based mostly on the PARAGON-HF trial, which entered patients with HF and an LVEF at least 45%. The trial was “negative” in that it saw no significant advantage to the drug for its primary clinical outcome but did suggest benefit for some secondary endpoints.
The FDA had used more cautionary language in its expanded indication for sacubitril/valsartan, “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure. Benefits are most clearly evident in patients with left ventricular ejection fraction below normal.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved an expanded heart failure indication for the sodium-glucose transporter 2 inhibitor empagliflozin (Jardiance) that now includes HF with mid-range or preserved left ventricular ejection fraction (LVEF), the agency announced on Feb. 24.
That means the SGLT2 inhibitor, once considered primarily an antidiabetic agent, is approved for use in patients with HF per se without regard to ventricular function. The drug received approval for HF with reduced LVEF in August 2021.
The expanded indication, specifically for reducing the risk of cardiovascular death and HF hospitalization in adults, was widely anticipated based on the landmark results from the EMPEROR-Preserved trial. The study saw a significant 21% relative reduction in that composite endpoint over about 2 years in patients with New York Heart Association class II-IV heart failure and an LVEF greater than 40% who received empagliflozin along with other standard care.
Interestingly, the drug’s expanded indication in HF resembles that approved for sacubitril/valsartan (Entresto) in February 2021 based mostly on the PARAGON-HF trial, which entered patients with HF and an LVEF at least 45%. The trial was “negative” in that it saw no significant advantage to the drug for its primary clinical outcome but did suggest benefit for some secondary endpoints.
The FDA had used more cautionary language in its expanded indication for sacubitril/valsartan, “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure. Benefits are most clearly evident in patients with left ventricular ejection fraction below normal.”
A version of this article first appeared on Medscape.com.
Biden’s FDA chief nominee narrowly wins Senate confirmation
On Feb. 15, Robert Califf, MD, narrowly won Senate confirmation to once again serve as the commissioner of the Food and Drug Administration, overcoming protest votes from lawmakers about abortion and opioid issues.
The Senate voted 50-46 in favor of Dr. Califf’s nomination. A cardiologist long affiliated with Duke University and a noted expert on clinical trials, Dr. Califf also led the FDA from February 2016 through January 2017.
In 2016, the Senate confirmed him as FDA chief in an 89-4 vote. At that time, Sen. Joe Manchin, D-WV, and a few other senators said they were concerned that Dr. Califf’s links to the drug industry would hamper his ability to regulate drugmakers, particularly in terms of rules on prescription painkillers.
Sen. Manchin also objected to Dr. Califf’s second nomination as FDA commissioner, as did several fellow Democrats, including Sen. Edward Markey of Massachusetts. In a statement issued after the Feb. 15 vote, Sen. Markey said he has “consistently raised concerns about the FDA’s egregious mishandling of opioid approvals and its role in enabling the current opioid epidemic.”
“To date, the FDA still has not implemented many of the reforms necessary to ensure that it is fulfilling its role as our nation’s top pharmaceutical cop on the beat,” Sen. Markey said. “I have not received any real commitment from Dr. Califf to truly reform the FDA or to learn from the failures that fueled this public health crisis.”
This time, Dr. Califf lost support among Republican senators due to objections raised by groups seeking to end women’s access to abortion. Susan B. Anthony List and National Right to Life asked senators in a January letter to oppose Dr. Califf’s nomination, citing their objections to how the FDA handled reporting of adverse events from abortions by medication during Dr. Califf’s Tenure.
But some Republicans supported Califf in the Tuesday vote. Sens. Roy Blunt of Missouri, Richard Burr of North Carolina, Susan Collins of Maine, Lisa Murkowski of Alaska, Mitt Romney of Utah, and Pat Toomey of Pennsylvania all voted in his favor.
On Feb. 14, Sen. Patty Murray, D-WA, chairwoman of the Senate Health, Education, Labor, and Pensions Committee, urged her colleagues to vote for Dr. Califf to give the FDA strong leadership to tackle urgent health needs such as the opioid crisis, youth tobacco use, antimicrobial resistance, and inequities in health care.
“At this critical moment, we need a trusted hand to lead the FDA,” she said in a floor speech. Dr. Califf’s previous service at the FDA and his years spent as a research scientist “give him the experience to take on this challenge.”
Separately, three former FDA commissioners on Feb. 15 published an opinion article that appeared in The Hill. Republican presidents nominated two of these former FDA chiefs: Scott Gottlieb, MD, and Mark McClellan, MD. The third, Margaret Hamburg, MD, was nominated by President Barack Obama, as was Dr. Califf for his first time as FDA chief.
There’s an urgent need for a confirmed leader at the FDA as the United States seeks to move beyond the pandemic, the former FDA chiefs wrote. The work ahead includes continued efforts with vaccines as well as efforts to bolster medical supply chains, they said.
Dr. Califf “knows how to advance the safe development and use of medical products and to bring a sound, science-based foundation to the FDA’s regulatory actions. Because of this, he has earned the confidence of FDA’s professional career staff, as well as a broad base of patient groups, academic experts, medical professionals, and public health organizations,” Dr. Gottlieb, Dr. Hamburg, and Dr. McClellan wrote.
The article also was signed by former Centers for Medicare and Medicaid Services Administrator Andy Slavitt, who served in the Obama administration.
Support of medical community
The American Heart Association issued a statement on Feb.15, congratulating Dr. Califf on his second confirmation after the Senate vote.
“With a distinguished career in public service and a long-time volunteer leader at the American Heart Association, Dr. Califf has honed his ability to communicate and build trust with diverse constituencies,” CEO Nancy Brown said in the statement. “He will use his experience as a cardiologist to safeguard the health and well-being of people throughout the country, and his background in research to prioritize science and evidence-based policymaking.”
Dr. Califf was also backed by the Association of American Medical Colleges, the American Academy of Pediatrics, the American Academy of Family Physicians, and the American College of Physicians when he was nominated for the role last year by President Joe Biden.
A version of this article first appeared on Medscape.com.
On Feb. 15, Robert Califf, MD, narrowly won Senate confirmation to once again serve as the commissioner of the Food and Drug Administration, overcoming protest votes from lawmakers about abortion and opioid issues.
The Senate voted 50-46 in favor of Dr. Califf’s nomination. A cardiologist long affiliated with Duke University and a noted expert on clinical trials, Dr. Califf also led the FDA from February 2016 through January 2017.
In 2016, the Senate confirmed him as FDA chief in an 89-4 vote. At that time, Sen. Joe Manchin, D-WV, and a few other senators said they were concerned that Dr. Califf’s links to the drug industry would hamper his ability to regulate drugmakers, particularly in terms of rules on prescription painkillers.
Sen. Manchin also objected to Dr. Califf’s second nomination as FDA commissioner, as did several fellow Democrats, including Sen. Edward Markey of Massachusetts. In a statement issued after the Feb. 15 vote, Sen. Markey said he has “consistently raised concerns about the FDA’s egregious mishandling of opioid approvals and its role in enabling the current opioid epidemic.”
“To date, the FDA still has not implemented many of the reforms necessary to ensure that it is fulfilling its role as our nation’s top pharmaceutical cop on the beat,” Sen. Markey said. “I have not received any real commitment from Dr. Califf to truly reform the FDA or to learn from the failures that fueled this public health crisis.”
This time, Dr. Califf lost support among Republican senators due to objections raised by groups seeking to end women’s access to abortion. Susan B. Anthony List and National Right to Life asked senators in a January letter to oppose Dr. Califf’s nomination, citing their objections to how the FDA handled reporting of adverse events from abortions by medication during Dr. Califf’s Tenure.
But some Republicans supported Califf in the Tuesday vote. Sens. Roy Blunt of Missouri, Richard Burr of North Carolina, Susan Collins of Maine, Lisa Murkowski of Alaska, Mitt Romney of Utah, and Pat Toomey of Pennsylvania all voted in his favor.
On Feb. 14, Sen. Patty Murray, D-WA, chairwoman of the Senate Health, Education, Labor, and Pensions Committee, urged her colleagues to vote for Dr. Califf to give the FDA strong leadership to tackle urgent health needs such as the opioid crisis, youth tobacco use, antimicrobial resistance, and inequities in health care.
“At this critical moment, we need a trusted hand to lead the FDA,” she said in a floor speech. Dr. Califf’s previous service at the FDA and his years spent as a research scientist “give him the experience to take on this challenge.”
Separately, three former FDA commissioners on Feb. 15 published an opinion article that appeared in The Hill. Republican presidents nominated two of these former FDA chiefs: Scott Gottlieb, MD, and Mark McClellan, MD. The third, Margaret Hamburg, MD, was nominated by President Barack Obama, as was Dr. Califf for his first time as FDA chief.
There’s an urgent need for a confirmed leader at the FDA as the United States seeks to move beyond the pandemic, the former FDA chiefs wrote. The work ahead includes continued efforts with vaccines as well as efforts to bolster medical supply chains, they said.
Dr. Califf “knows how to advance the safe development and use of medical products and to bring a sound, science-based foundation to the FDA’s regulatory actions. Because of this, he has earned the confidence of FDA’s professional career staff, as well as a broad base of patient groups, academic experts, medical professionals, and public health organizations,” Dr. Gottlieb, Dr. Hamburg, and Dr. McClellan wrote.
The article also was signed by former Centers for Medicare and Medicaid Services Administrator Andy Slavitt, who served in the Obama administration.
Support of medical community
The American Heart Association issued a statement on Feb.15, congratulating Dr. Califf on his second confirmation after the Senate vote.
“With a distinguished career in public service and a long-time volunteer leader at the American Heart Association, Dr. Califf has honed his ability to communicate and build trust with diverse constituencies,” CEO Nancy Brown said in the statement. “He will use his experience as a cardiologist to safeguard the health and well-being of people throughout the country, and his background in research to prioritize science and evidence-based policymaking.”
Dr. Califf was also backed by the Association of American Medical Colleges, the American Academy of Pediatrics, the American Academy of Family Physicians, and the American College of Physicians when he was nominated for the role last year by President Joe Biden.
A version of this article first appeared on Medscape.com.
On Feb. 15, Robert Califf, MD, narrowly won Senate confirmation to once again serve as the commissioner of the Food and Drug Administration, overcoming protest votes from lawmakers about abortion and opioid issues.
The Senate voted 50-46 in favor of Dr. Califf’s nomination. A cardiologist long affiliated with Duke University and a noted expert on clinical trials, Dr. Califf also led the FDA from February 2016 through January 2017.
In 2016, the Senate confirmed him as FDA chief in an 89-4 vote. At that time, Sen. Joe Manchin, D-WV, and a few other senators said they were concerned that Dr. Califf’s links to the drug industry would hamper his ability to regulate drugmakers, particularly in terms of rules on prescription painkillers.
Sen. Manchin also objected to Dr. Califf’s second nomination as FDA commissioner, as did several fellow Democrats, including Sen. Edward Markey of Massachusetts. In a statement issued after the Feb. 15 vote, Sen. Markey said he has “consistently raised concerns about the FDA’s egregious mishandling of opioid approvals and its role in enabling the current opioid epidemic.”
“To date, the FDA still has not implemented many of the reforms necessary to ensure that it is fulfilling its role as our nation’s top pharmaceutical cop on the beat,” Sen. Markey said. “I have not received any real commitment from Dr. Califf to truly reform the FDA or to learn from the failures that fueled this public health crisis.”
This time, Dr. Califf lost support among Republican senators due to objections raised by groups seeking to end women’s access to abortion. Susan B. Anthony List and National Right to Life asked senators in a January letter to oppose Dr. Califf’s nomination, citing their objections to how the FDA handled reporting of adverse events from abortions by medication during Dr. Califf’s Tenure.
But some Republicans supported Califf in the Tuesday vote. Sens. Roy Blunt of Missouri, Richard Burr of North Carolina, Susan Collins of Maine, Lisa Murkowski of Alaska, Mitt Romney of Utah, and Pat Toomey of Pennsylvania all voted in his favor.
On Feb. 14, Sen. Patty Murray, D-WA, chairwoman of the Senate Health, Education, Labor, and Pensions Committee, urged her colleagues to vote for Dr. Califf to give the FDA strong leadership to tackle urgent health needs such as the opioid crisis, youth tobacco use, antimicrobial resistance, and inequities in health care.
“At this critical moment, we need a trusted hand to lead the FDA,” she said in a floor speech. Dr. Califf’s previous service at the FDA and his years spent as a research scientist “give him the experience to take on this challenge.”
Separately, three former FDA commissioners on Feb. 15 published an opinion article that appeared in The Hill. Republican presidents nominated two of these former FDA chiefs: Scott Gottlieb, MD, and Mark McClellan, MD. The third, Margaret Hamburg, MD, was nominated by President Barack Obama, as was Dr. Califf for his first time as FDA chief.
There’s an urgent need for a confirmed leader at the FDA as the United States seeks to move beyond the pandemic, the former FDA chiefs wrote. The work ahead includes continued efforts with vaccines as well as efforts to bolster medical supply chains, they said.
Dr. Califf “knows how to advance the safe development and use of medical products and to bring a sound, science-based foundation to the FDA’s regulatory actions. Because of this, he has earned the confidence of FDA’s professional career staff, as well as a broad base of patient groups, academic experts, medical professionals, and public health organizations,” Dr. Gottlieb, Dr. Hamburg, and Dr. McClellan wrote.
The article also was signed by former Centers for Medicare and Medicaid Services Administrator Andy Slavitt, who served in the Obama administration.
Support of medical community
The American Heart Association issued a statement on Feb.15, congratulating Dr. Califf on his second confirmation after the Senate vote.
“With a distinguished career in public service and a long-time volunteer leader at the American Heart Association, Dr. Califf has honed his ability to communicate and build trust with diverse constituencies,” CEO Nancy Brown said in the statement. “He will use his experience as a cardiologist to safeguard the health and well-being of people throughout the country, and his background in research to prioritize science and evidence-based policymaking.”
Dr. Califf was also backed by the Association of American Medical Colleges, the American Academy of Pediatrics, the American Academy of Family Physicians, and the American College of Physicians when he was nominated for the role last year by President Joe Biden.
A version of this article first appeared on Medscape.com.
Organ transplantation: Unvaccinated need not apply
I agree with most advice given by the affable TV character Ted Lasso. “Every choice is a chance,” he said. Pandemic-era physicians must now consider whether a politically motivated choice to decline COVID-19 vaccination should negatively affect the chance to receive an organ donation.
And in confronting these choices, we have a chance to educate the public on the complexities of the organ allocation process.
A well-informed patient’s personal choice should be honored, even if clinicians disagree, if it does not affect the well-being of others. For example, I once had a patient in acute leukemic crisis who declined blood products because she was a Jehovah’s Witness. She died. Her choice affected her longevity only.
Compare that decision with awarding an organ to an individual who has declined readily available protection of that organ. Weigh that choice against the fact that said protection is against an infectious disease that has killed over 5.5 million worldwide.
Some institutions stand strong, others hedge their bets
Admirably, Loyola University Health System understands that difference. They published a firm stand on transplant candidacy and COVID-19 vaccination status in the Journal of Heart and Lung Transplant. Daniel Dilling, MD, medical director of the lung transplantation program , and Mark Kuczewski, PhD, a professor of medical ethics at Loyola University Chicago, Maywood, Ill., wrote that: “We believe that requiring vaccination against COVID-19 should not be controversial when we focus strictly on established frameworks and practices surrounding eligibility for wait-listing to receive a solid organ transplant.”
The Cleveland Clinic apparently agrees. In October 2021, they denied a liver transplant to Michelle Vitullo of Ohio, whose daughter had been deemed “a perfect match.” Her daughter, also unvaccinated, stated: “Being denied for a nonmedical reason for someone’s beliefs that are different to yours, I mean that’s not how that should be.”
But vaccination status is a medical reason, given well-established data regarding increased mortality among the immunosuppressed. Ms. Vitullo then said: “We are trying to get to UPMC [University of Pittsburgh Medical Center] as they don’t require a vaccination.”
The public information page on transplant candidacy from UPMC reads (my italics): It is recommended that all transplant candidates, transplant recipients, and their household members receive COVID-19 vaccination when the vaccine is available to them. It is preferred that transplant candidates are vaccinated more than 2 weeks before transplantation.
I reached out to UPMC for clarification and was told by email that “we do not have a policy regarding COVID-19 vaccination requirement for current transplant candidates.” Houston Methodist shares the same agnostic stance.
Compare these opinions with Brigham and Women’s Hospital, where the requirements are resolute: “Like most other transplant programs across the country, the COVID-19 vaccine is one of several vaccines and lifestyle behaviors that are required for patients awaiting solid organ transplant.”
They add that “transplant candidates must also receive the seasonal influenza and hepatitis B vaccines, follow other healthy behaviors, and demonstrate they can commit to taking the required medications following transplant.”
In January 2022, Brigham and Women’s Hospital declared 31-year-old D.J. Ferguson ineligible for a heart transplant because he declined to be vaccinated against COVID-19. According to the New York Post and ABC News, his physicians resorted to left ventricular assist device support. His mother, Tracy Ferguson, is quoted as saying: “He’s not an antivaxxer. He has all of his vaccines.” I’ll just leave that right there.
Unfortunately, Michelle Vitullo’s obituary was published in December 2021. Regardless of whether she received her liver transplant, the outcome is tragic, and whatever you think of this family’s battle playing out in the glare of the national spotlight, their loss is no less devastating.
The directed-donation aspect of this case poses an interesting question. A news anchor asked the mother and daughter: “If you both accept the risks, why doesn’t the hospital just let you try?” The answers are obvious to us clinicians. Performing a transplantation in an unvaccinated patient could lead to their early death if they became infected because of their immunocompromised state, would open the door for transplantation of any patient who is unvaccinated for anything, including influenza and hepatitis B, which could result in the preventable waste of organs, and puts other vulnerable hospitalized patients at risk during the initial transplant stay and follow-up.
That’s not to mention the potential legal suit. Never has a consent form dissuaded any party from lodging an accusation of wrongful death or medical malpractice. In the face of strong data on higher mortality in unvaccinated, immunocompromised patients, a good lawyer could charge that the institution and transplant surgeons should have known better, regardless of the donor and recipient’s willingness to accept the risks.
The Vitullo and Ferguson cases are among many similar dilemmas surrounding transplant candidacy across the United States.
University of Virginia Health in Charlottesville denied 42-year-old Shamgar Connors a kidney transplant because he is unvaccinated, despite a previous COVID-19 infection. In October 2021, Leilani Lutali of Colorado was denied a kidney by UCHealth because she declined vaccination.
As Ted Lasso says: “There’s a bunch of crazy stuff on Twitter.”
Predictably, social media is full of public outcry. “Some cold-hearted people on here” tweeted one. “What if it was one of your loved ones who needed a transplant?” Another tweeted the Hippocratic oath with the comment that “They all swore under this noble ‘oat’, but I guess it’s been forgotten.” (This was followed with a photo of a box of Quaker Oats in a failed attempt at humor.) These discussions among the Twitterati highlight the depths of misunderstanding on organ transplantation.
To be fair, unless you have been personally involved in the decision-making process for transplant candidacy, there is little opportunity to be educated. I explain to my anxious patients and their families that a donor organ is like a fumbled football. There may be well over 100 patients at all levels of transplant status in many geographic locations diving for that same organ.
The transplant team is tasked with finding the best match, determining who is the sickest, assessing time for transport of that organ, and, above all, who will be the best steward of that organ.
Take heart transplantation, for instance. Approximately 3,500 patients in the United States are awaiting one each year. Instead of facing an almost certain death within 5 years, a transplant recipient has a chance at a median survival of 12-13 years. The cost of a heart transplant is approximately $1.38 million, according to Milliman, a consulting firm. This is “an incredibly resource intensive procedure,” including expenditures for transportation, antirejection medication, office visits, physician fees, ICU stays, rejection surveillance, and acute rejection therapies.
Transplant denial is nothing new
People get turned down for organ transplants all the time. My patient with end-stage dilated cardiomyopathy was denied a heart transplant when it was discovered that he had scores of outstanding parking tickets. This was seen as a surrogate for an inability to afford his antirejection medication.
Another patient swore that her positive cotinine levels were caused by endless hours at the bingo hall where second-hand smoke swirled. She was also denied. Many potential candidates who are in acute decline hold precariously to newfound sobriety. They are denied. A patient’s boyfriend told the transplant team that he couldn’t be relied upon to drive her to her appointments. She was denied.
Many people who engage in antisocial behaviors have no idea that these actions may result in the denial of an organ transplant should their future selves need one. These are hard lines, but everyone should agree that the odds of survival are heavily in favor of the consistently adherent.
We should take this opportunity to educate the public on how complicated obtaining an organ transplant can be. More than 6,000 people die each year waiting for an organ because of the supply-and-demand disparities in the transplantation arena. I’m willing to bet that many of the loudest protesters in favor of unvaccinated transplant recipients have not signed the organ donor box on the back of their driver’s license. This conversation is an opportunity to change that and remind people that organ donation may be their only opportunity to save a fellow human’s life.
Again, to quote Ted Lasso: “If you care about someone and you got a little love in your heart, there ain’t nothing you can’t get through together.” That philosophy should apply to the tasks of selecting the best organ donors as well as the best organ recipients.
And every organ should go to the one who will honor their donor and their donor’s family by taking the best care of that ultimate gift of life, including being vaccinated against COVID-19.
Dr. Walton-Shirley is a native Kentuckian who retired from full-time invasive cardiology. She enjoys locums work in Montana and is a champion of physician rights and patient safety. She disclosed no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
I agree with most advice given by the affable TV character Ted Lasso. “Every choice is a chance,” he said. Pandemic-era physicians must now consider whether a politically motivated choice to decline COVID-19 vaccination should negatively affect the chance to receive an organ donation.
And in confronting these choices, we have a chance to educate the public on the complexities of the organ allocation process.
A well-informed patient’s personal choice should be honored, even if clinicians disagree, if it does not affect the well-being of others. For example, I once had a patient in acute leukemic crisis who declined blood products because she was a Jehovah’s Witness. She died. Her choice affected her longevity only.
Compare that decision with awarding an organ to an individual who has declined readily available protection of that organ. Weigh that choice against the fact that said protection is against an infectious disease that has killed over 5.5 million worldwide.
Some institutions stand strong, others hedge their bets
Admirably, Loyola University Health System understands that difference. They published a firm stand on transplant candidacy and COVID-19 vaccination status in the Journal of Heart and Lung Transplant. Daniel Dilling, MD, medical director of the lung transplantation program , and Mark Kuczewski, PhD, a professor of medical ethics at Loyola University Chicago, Maywood, Ill., wrote that: “We believe that requiring vaccination against COVID-19 should not be controversial when we focus strictly on established frameworks and practices surrounding eligibility for wait-listing to receive a solid organ transplant.”
The Cleveland Clinic apparently agrees. In October 2021, they denied a liver transplant to Michelle Vitullo of Ohio, whose daughter had been deemed “a perfect match.” Her daughter, also unvaccinated, stated: “Being denied for a nonmedical reason for someone’s beliefs that are different to yours, I mean that’s not how that should be.”
But vaccination status is a medical reason, given well-established data regarding increased mortality among the immunosuppressed. Ms. Vitullo then said: “We are trying to get to UPMC [University of Pittsburgh Medical Center] as they don’t require a vaccination.”
The public information page on transplant candidacy from UPMC reads (my italics): It is recommended that all transplant candidates, transplant recipients, and their household members receive COVID-19 vaccination when the vaccine is available to them. It is preferred that transplant candidates are vaccinated more than 2 weeks before transplantation.
I reached out to UPMC for clarification and was told by email that “we do not have a policy regarding COVID-19 vaccination requirement for current transplant candidates.” Houston Methodist shares the same agnostic stance.
Compare these opinions with Brigham and Women’s Hospital, where the requirements are resolute: “Like most other transplant programs across the country, the COVID-19 vaccine is one of several vaccines and lifestyle behaviors that are required for patients awaiting solid organ transplant.”
They add that “transplant candidates must also receive the seasonal influenza and hepatitis B vaccines, follow other healthy behaviors, and demonstrate they can commit to taking the required medications following transplant.”
In January 2022, Brigham and Women’s Hospital declared 31-year-old D.J. Ferguson ineligible for a heart transplant because he declined to be vaccinated against COVID-19. According to the New York Post and ABC News, his physicians resorted to left ventricular assist device support. His mother, Tracy Ferguson, is quoted as saying: “He’s not an antivaxxer. He has all of his vaccines.” I’ll just leave that right there.
Unfortunately, Michelle Vitullo’s obituary was published in December 2021. Regardless of whether she received her liver transplant, the outcome is tragic, and whatever you think of this family’s battle playing out in the glare of the national spotlight, their loss is no less devastating.
The directed-donation aspect of this case poses an interesting question. A news anchor asked the mother and daughter: “If you both accept the risks, why doesn’t the hospital just let you try?” The answers are obvious to us clinicians. Performing a transplantation in an unvaccinated patient could lead to their early death if they became infected because of their immunocompromised state, would open the door for transplantation of any patient who is unvaccinated for anything, including influenza and hepatitis B, which could result in the preventable waste of organs, and puts other vulnerable hospitalized patients at risk during the initial transplant stay and follow-up.
That’s not to mention the potential legal suit. Never has a consent form dissuaded any party from lodging an accusation of wrongful death or medical malpractice. In the face of strong data on higher mortality in unvaccinated, immunocompromised patients, a good lawyer could charge that the institution and transplant surgeons should have known better, regardless of the donor and recipient’s willingness to accept the risks.
The Vitullo and Ferguson cases are among many similar dilemmas surrounding transplant candidacy across the United States.
University of Virginia Health in Charlottesville denied 42-year-old Shamgar Connors a kidney transplant because he is unvaccinated, despite a previous COVID-19 infection. In October 2021, Leilani Lutali of Colorado was denied a kidney by UCHealth because she declined vaccination.
As Ted Lasso says: “There’s a bunch of crazy stuff on Twitter.”
Predictably, social media is full of public outcry. “Some cold-hearted people on here” tweeted one. “What if it was one of your loved ones who needed a transplant?” Another tweeted the Hippocratic oath with the comment that “They all swore under this noble ‘oat’, but I guess it’s been forgotten.” (This was followed with a photo of a box of Quaker Oats in a failed attempt at humor.) These discussions among the Twitterati highlight the depths of misunderstanding on organ transplantation.
To be fair, unless you have been personally involved in the decision-making process for transplant candidacy, there is little opportunity to be educated. I explain to my anxious patients and their families that a donor organ is like a fumbled football. There may be well over 100 patients at all levels of transplant status in many geographic locations diving for that same organ.
The transplant team is tasked with finding the best match, determining who is the sickest, assessing time for transport of that organ, and, above all, who will be the best steward of that organ.
Take heart transplantation, for instance. Approximately 3,500 patients in the United States are awaiting one each year. Instead of facing an almost certain death within 5 years, a transplant recipient has a chance at a median survival of 12-13 years. The cost of a heart transplant is approximately $1.38 million, according to Milliman, a consulting firm. This is “an incredibly resource intensive procedure,” including expenditures for transportation, antirejection medication, office visits, physician fees, ICU stays, rejection surveillance, and acute rejection therapies.
Transplant denial is nothing new
People get turned down for organ transplants all the time. My patient with end-stage dilated cardiomyopathy was denied a heart transplant when it was discovered that he had scores of outstanding parking tickets. This was seen as a surrogate for an inability to afford his antirejection medication.
Another patient swore that her positive cotinine levels were caused by endless hours at the bingo hall where second-hand smoke swirled. She was also denied. Many potential candidates who are in acute decline hold precariously to newfound sobriety. They are denied. A patient’s boyfriend told the transplant team that he couldn’t be relied upon to drive her to her appointments. She was denied.
Many people who engage in antisocial behaviors have no idea that these actions may result in the denial of an organ transplant should their future selves need one. These are hard lines, but everyone should agree that the odds of survival are heavily in favor of the consistently adherent.
We should take this opportunity to educate the public on how complicated obtaining an organ transplant can be. More than 6,000 people die each year waiting for an organ because of the supply-and-demand disparities in the transplantation arena. I’m willing to bet that many of the loudest protesters in favor of unvaccinated transplant recipients have not signed the organ donor box on the back of their driver’s license. This conversation is an opportunity to change that and remind people that organ donation may be their only opportunity to save a fellow human’s life.
Again, to quote Ted Lasso: “If you care about someone and you got a little love in your heart, there ain’t nothing you can’t get through together.” That philosophy should apply to the tasks of selecting the best organ donors as well as the best organ recipients.
And every organ should go to the one who will honor their donor and their donor’s family by taking the best care of that ultimate gift of life, including being vaccinated against COVID-19.
Dr. Walton-Shirley is a native Kentuckian who retired from full-time invasive cardiology. She enjoys locums work in Montana and is a champion of physician rights and patient safety. She disclosed no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
I agree with most advice given by the affable TV character Ted Lasso. “Every choice is a chance,” he said. Pandemic-era physicians must now consider whether a politically motivated choice to decline COVID-19 vaccination should negatively affect the chance to receive an organ donation.
And in confronting these choices, we have a chance to educate the public on the complexities of the organ allocation process.
A well-informed patient’s personal choice should be honored, even if clinicians disagree, if it does not affect the well-being of others. For example, I once had a patient in acute leukemic crisis who declined blood products because she was a Jehovah’s Witness. She died. Her choice affected her longevity only.
Compare that decision with awarding an organ to an individual who has declined readily available protection of that organ. Weigh that choice against the fact that said protection is against an infectious disease that has killed over 5.5 million worldwide.
Some institutions stand strong, others hedge their bets
Admirably, Loyola University Health System understands that difference. They published a firm stand on transplant candidacy and COVID-19 vaccination status in the Journal of Heart and Lung Transplant. Daniel Dilling, MD, medical director of the lung transplantation program , and Mark Kuczewski, PhD, a professor of medical ethics at Loyola University Chicago, Maywood, Ill., wrote that: “We believe that requiring vaccination against COVID-19 should not be controversial when we focus strictly on established frameworks and practices surrounding eligibility for wait-listing to receive a solid organ transplant.”
The Cleveland Clinic apparently agrees. In October 2021, they denied a liver transplant to Michelle Vitullo of Ohio, whose daughter had been deemed “a perfect match.” Her daughter, also unvaccinated, stated: “Being denied for a nonmedical reason for someone’s beliefs that are different to yours, I mean that’s not how that should be.”
But vaccination status is a medical reason, given well-established data regarding increased mortality among the immunosuppressed. Ms. Vitullo then said: “We are trying to get to UPMC [University of Pittsburgh Medical Center] as they don’t require a vaccination.”
The public information page on transplant candidacy from UPMC reads (my italics): It is recommended that all transplant candidates, transplant recipients, and their household members receive COVID-19 vaccination when the vaccine is available to them. It is preferred that transplant candidates are vaccinated more than 2 weeks before transplantation.
I reached out to UPMC for clarification and was told by email that “we do not have a policy regarding COVID-19 vaccination requirement for current transplant candidates.” Houston Methodist shares the same agnostic stance.
Compare these opinions with Brigham and Women’s Hospital, where the requirements are resolute: “Like most other transplant programs across the country, the COVID-19 vaccine is one of several vaccines and lifestyle behaviors that are required for patients awaiting solid organ transplant.”
They add that “transplant candidates must also receive the seasonal influenza and hepatitis B vaccines, follow other healthy behaviors, and demonstrate they can commit to taking the required medications following transplant.”
In January 2022, Brigham and Women’s Hospital declared 31-year-old D.J. Ferguson ineligible for a heart transplant because he declined to be vaccinated against COVID-19. According to the New York Post and ABC News, his physicians resorted to left ventricular assist device support. His mother, Tracy Ferguson, is quoted as saying: “He’s not an antivaxxer. He has all of his vaccines.” I’ll just leave that right there.
Unfortunately, Michelle Vitullo’s obituary was published in December 2021. Regardless of whether she received her liver transplant, the outcome is tragic, and whatever you think of this family’s battle playing out in the glare of the national spotlight, their loss is no less devastating.
The directed-donation aspect of this case poses an interesting question. A news anchor asked the mother and daughter: “If you both accept the risks, why doesn’t the hospital just let you try?” The answers are obvious to us clinicians. Performing a transplantation in an unvaccinated patient could lead to their early death if they became infected because of their immunocompromised state, would open the door for transplantation of any patient who is unvaccinated for anything, including influenza and hepatitis B, which could result in the preventable waste of organs, and puts other vulnerable hospitalized patients at risk during the initial transplant stay and follow-up.
That’s not to mention the potential legal suit. Never has a consent form dissuaded any party from lodging an accusation of wrongful death or medical malpractice. In the face of strong data on higher mortality in unvaccinated, immunocompromised patients, a good lawyer could charge that the institution and transplant surgeons should have known better, regardless of the donor and recipient’s willingness to accept the risks.
The Vitullo and Ferguson cases are among many similar dilemmas surrounding transplant candidacy across the United States.
University of Virginia Health in Charlottesville denied 42-year-old Shamgar Connors a kidney transplant because he is unvaccinated, despite a previous COVID-19 infection. In October 2021, Leilani Lutali of Colorado was denied a kidney by UCHealth because she declined vaccination.
As Ted Lasso says: “There’s a bunch of crazy stuff on Twitter.”
Predictably, social media is full of public outcry. “Some cold-hearted people on here” tweeted one. “What if it was one of your loved ones who needed a transplant?” Another tweeted the Hippocratic oath with the comment that “They all swore under this noble ‘oat’, but I guess it’s been forgotten.” (This was followed with a photo of a box of Quaker Oats in a failed attempt at humor.) These discussions among the Twitterati highlight the depths of misunderstanding on organ transplantation.
To be fair, unless you have been personally involved in the decision-making process for transplant candidacy, there is little opportunity to be educated. I explain to my anxious patients and their families that a donor organ is like a fumbled football. There may be well over 100 patients at all levels of transplant status in many geographic locations diving for that same organ.
The transplant team is tasked with finding the best match, determining who is the sickest, assessing time for transport of that organ, and, above all, who will be the best steward of that organ.
Take heart transplantation, for instance. Approximately 3,500 patients in the United States are awaiting one each year. Instead of facing an almost certain death within 5 years, a transplant recipient has a chance at a median survival of 12-13 years. The cost of a heart transplant is approximately $1.38 million, according to Milliman, a consulting firm. This is “an incredibly resource intensive procedure,” including expenditures for transportation, antirejection medication, office visits, physician fees, ICU stays, rejection surveillance, and acute rejection therapies.
Transplant denial is nothing new
People get turned down for organ transplants all the time. My patient with end-stage dilated cardiomyopathy was denied a heart transplant when it was discovered that he had scores of outstanding parking tickets. This was seen as a surrogate for an inability to afford his antirejection medication.
Another patient swore that her positive cotinine levels were caused by endless hours at the bingo hall where second-hand smoke swirled. She was also denied. Many potential candidates who are in acute decline hold precariously to newfound sobriety. They are denied. A patient’s boyfriend told the transplant team that he couldn’t be relied upon to drive her to her appointments. She was denied.
Many people who engage in antisocial behaviors have no idea that these actions may result in the denial of an organ transplant should their future selves need one. These are hard lines, but everyone should agree that the odds of survival are heavily in favor of the consistently adherent.
We should take this opportunity to educate the public on how complicated obtaining an organ transplant can be. More than 6,000 people die each year waiting for an organ because of the supply-and-demand disparities in the transplantation arena. I’m willing to bet that many of the loudest protesters in favor of unvaccinated transplant recipients have not signed the organ donor box on the back of their driver’s license. This conversation is an opportunity to change that and remind people that organ donation may be their only opportunity to save a fellow human’s life.
Again, to quote Ted Lasso: “If you care about someone and you got a little love in your heart, there ain’t nothing you can’t get through together.” That philosophy should apply to the tasks of selecting the best organ donors as well as the best organ recipients.
And every organ should go to the one who will honor their donor and their donor’s family by taking the best care of that ultimate gift of life, including being vaccinated against COVID-19.
Dr. Walton-Shirley is a native Kentuckian who retired from full-time invasive cardiology. She enjoys locums work in Montana and is a champion of physician rights and patient safety. She disclosed no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
Novel drug targets raised Lp(a): topline results released
Topline results from the phase 1 APOLLO study of SLN360, a short interfering ribonucleic acid (siRNA) targeting lipoprotein(a), showed it significantly reduced Lp(a) in a dose-dependent manner from 46% to up to 98%.
Reductions of up to 81% were maintained out to 150 days, according to a release from the developer of the drug, Silence Therapeutics.
High Lp(a) affects about one in five people worldwide and is a genetic risk factor for cardiovascular disease. There are no approved medications that selectively lower Lp(a), and levels cannot be significantly modified through lifestyle changes or any approved medications.
SLN360 is a siRNA that is designed to lower Lp(a) production by using the body’s natural process of RNA interference to target and silence messenger RNA transcribed from the LPA gene in liver cells.
The first-in-human APOLLO trial evaluated 32 patients with serum Lp(a) concentrations of at least 150 nmol/L and no cardiovascular disease who received a single subcutaneous dose of SLN360 (30 mg, 100 mg, less than or equal to 300 mg, or less than or equal to 600 mg) or placebo and were followed for up to 150 days.
No clinically important safety concerns were identified, although low-grade adverse events at the injection site occurred, most prominently at the highest dose, according to the company.
Study follow-up has been extended to 1 year. Patient enrollment continues in the multiple-ascending dose portion of the phase 1 study in patients with high Lp(a) and a confirmed history of stable atherosclerotic cardiovascular disease, the company statement notes.
Detailed results from APOLLO will be presented in a late-breaking clinical trials session at the American College of Cardiology Annual Scientific Session on April 3 by principal investigator Steven E. Nissen, MD, Cleveland Clinic.
A version of this article first appeared on Medscape.com.
Topline results from the phase 1 APOLLO study of SLN360, a short interfering ribonucleic acid (siRNA) targeting lipoprotein(a), showed it significantly reduced Lp(a) in a dose-dependent manner from 46% to up to 98%.
Reductions of up to 81% were maintained out to 150 days, according to a release from the developer of the drug, Silence Therapeutics.
High Lp(a) affects about one in five people worldwide and is a genetic risk factor for cardiovascular disease. There are no approved medications that selectively lower Lp(a), and levels cannot be significantly modified through lifestyle changes or any approved medications.
SLN360 is a siRNA that is designed to lower Lp(a) production by using the body’s natural process of RNA interference to target and silence messenger RNA transcribed from the LPA gene in liver cells.
The first-in-human APOLLO trial evaluated 32 patients with serum Lp(a) concentrations of at least 150 nmol/L and no cardiovascular disease who received a single subcutaneous dose of SLN360 (30 mg, 100 mg, less than or equal to 300 mg, or less than or equal to 600 mg) or placebo and were followed for up to 150 days.
No clinically important safety concerns were identified, although low-grade adverse events at the injection site occurred, most prominently at the highest dose, according to the company.
Study follow-up has been extended to 1 year. Patient enrollment continues in the multiple-ascending dose portion of the phase 1 study in patients with high Lp(a) and a confirmed history of stable atherosclerotic cardiovascular disease, the company statement notes.
Detailed results from APOLLO will be presented in a late-breaking clinical trials session at the American College of Cardiology Annual Scientific Session on April 3 by principal investigator Steven E. Nissen, MD, Cleveland Clinic.
A version of this article first appeared on Medscape.com.
Topline results from the phase 1 APOLLO study of SLN360, a short interfering ribonucleic acid (siRNA) targeting lipoprotein(a), showed it significantly reduced Lp(a) in a dose-dependent manner from 46% to up to 98%.
Reductions of up to 81% were maintained out to 150 days, according to a release from the developer of the drug, Silence Therapeutics.
High Lp(a) affects about one in five people worldwide and is a genetic risk factor for cardiovascular disease. There are no approved medications that selectively lower Lp(a), and levels cannot be significantly modified through lifestyle changes or any approved medications.
SLN360 is a siRNA that is designed to lower Lp(a) production by using the body’s natural process of RNA interference to target and silence messenger RNA transcribed from the LPA gene in liver cells.
The first-in-human APOLLO trial evaluated 32 patients with serum Lp(a) concentrations of at least 150 nmol/L and no cardiovascular disease who received a single subcutaneous dose of SLN360 (30 mg, 100 mg, less than or equal to 300 mg, or less than or equal to 600 mg) or placebo and were followed for up to 150 days.
No clinically important safety concerns were identified, although low-grade adverse events at the injection site occurred, most prominently at the highest dose, according to the company.
Study follow-up has been extended to 1 year. Patient enrollment continues in the multiple-ascending dose portion of the phase 1 study in patients with high Lp(a) and a confirmed history of stable atherosclerotic cardiovascular disease, the company statement notes.
Detailed results from APOLLO will be presented in a late-breaking clinical trials session at the American College of Cardiology Annual Scientific Session on April 3 by principal investigator Steven E. Nissen, MD, Cleveland Clinic.
A version of this article first appeared on Medscape.com.
SCAI refines cardiogenic shock classification system
The Society for Cardiovascular Angiography and Interventions (SCAI) has refined its cardiogenic shock (CS) classification system based on the literature and clinician feedback from real-world experience.
“In the 2 years since publication in 2019, the initial definition has been broadly accepted and eagerly appreciated, allowing a very intuitive way to stage these patients for better communication, triage, and treatment,” Srihari S. Naidu, MD, professor of medicine, New York Medical College, Valhalla, said in an interview.
“But the initial definition was based on consensus opinion, with a lack of real fundamental data on segregating patients into different stages. Now we have a lot more data utilizing the definition, and it became very clear that there were a couple of limitations in the initial definition,” Dr. Naidu explained.
The refined CS classification system – authored by Dr. Naidu and a multidisciplinary panel of experts from specialties that included cardiac critical care, interventional cardiology, surgery, nursing, emergency medicine, and heart failure – was published online Jan. 31 in the Journal of the Society for Cardiovascular Angiography and Interventions, with simultaneous publication in the Journal of the American College of Cardiology.
It maintains the five-stage pyramid of CS, starting with “at risk” and moving through “beginning,” “classic,” “deteriorating,” and “extremis” but now includes gradations of severity within each stage and pathways by which patients progress or recover.
“Progression across the SCAI shock stage continuum is a dynamic process, incorporating new information as available, and patient trajectories are important both for communication among clinicians and for decisionmaking regarding the next level of care and therapeutics,” the panel writes.
The second iteration adds a streamlined table incorporating commonly seen variables, based on lessons learned from validation studies and clinician experience.
“While keeping the same initial framework of looking at the three components of staging – the physical exam, the biochemical markers, and hemodynamics – we’ve made it very clear that there are some factors in each of these that are most typically seen. And then there are other factors that are consistent with that stage but don’t necessarily have to be seen, ... are not typically seen in that stage, or [are] not always present at that stage,” Dr. Naidu told this news organization.
The refined CS classification system provides more granularity on cardiac arrest as a risk modifier, which now excludes very brief episodes with rapid response to defibrillation and comprises only those patients who have impaired mental status with unknown neurologic recovery status after cardiopulmonary resuscitation.
Lactate level and thresholds have been highlighted to detect hypoperfusion but may be dissociated from hemodynamics in cases such as chronic heart failure.
In addition, patients may have other manifestations of end-organ hypoperfusion with a normal lactate level, and there are also important causes of an elevated lactate level other than shock.
The revision proposes a three-axis model of CS evaluation and prognostication that integrates shock severity, clinical phenotype, and risk modifiers as distinct elements that should be applied to individualize patient management.
The revision also places more emphasis on the trajectory of the patient with CS through hospitalization, including a “hub and spoke” model for transfer of higher-risk patients, including those with a deteriorating SCAI shock stage.
“It is our desire and belief that the revised SCAI SHOCK stage classification system will enhance both clinical care and CS research trial design,” the panel writes.
This statement has been endorsed by the American College of Cardiology, American College of Emergency Physicians, American Heart Association, European Society of Cardiology Association for Acute Cardiovascular Care, International Society for Heart and Lung Transplantation, Society of Critical Care Medicine, and Society of Thoracic Surgeons.
This research had no commercial funding. Dr. Naidu has disclosed no relevant financial relationships. A complete list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
The Society for Cardiovascular Angiography and Interventions (SCAI) has refined its cardiogenic shock (CS) classification system based on the literature and clinician feedback from real-world experience.
“In the 2 years since publication in 2019, the initial definition has been broadly accepted and eagerly appreciated, allowing a very intuitive way to stage these patients for better communication, triage, and treatment,” Srihari S. Naidu, MD, professor of medicine, New York Medical College, Valhalla, said in an interview.
“But the initial definition was based on consensus opinion, with a lack of real fundamental data on segregating patients into different stages. Now we have a lot more data utilizing the definition, and it became very clear that there were a couple of limitations in the initial definition,” Dr. Naidu explained.
The refined CS classification system – authored by Dr. Naidu and a multidisciplinary panel of experts from specialties that included cardiac critical care, interventional cardiology, surgery, nursing, emergency medicine, and heart failure – was published online Jan. 31 in the Journal of the Society for Cardiovascular Angiography and Interventions, with simultaneous publication in the Journal of the American College of Cardiology.
It maintains the five-stage pyramid of CS, starting with “at risk” and moving through “beginning,” “classic,” “deteriorating,” and “extremis” but now includes gradations of severity within each stage and pathways by which patients progress or recover.
“Progression across the SCAI shock stage continuum is a dynamic process, incorporating new information as available, and patient trajectories are important both for communication among clinicians and for decisionmaking regarding the next level of care and therapeutics,” the panel writes.
The second iteration adds a streamlined table incorporating commonly seen variables, based on lessons learned from validation studies and clinician experience.
“While keeping the same initial framework of looking at the three components of staging – the physical exam, the biochemical markers, and hemodynamics – we’ve made it very clear that there are some factors in each of these that are most typically seen. And then there are other factors that are consistent with that stage but don’t necessarily have to be seen, ... are not typically seen in that stage, or [are] not always present at that stage,” Dr. Naidu told this news organization.
The refined CS classification system provides more granularity on cardiac arrest as a risk modifier, which now excludes very brief episodes with rapid response to defibrillation and comprises only those patients who have impaired mental status with unknown neurologic recovery status after cardiopulmonary resuscitation.
Lactate level and thresholds have been highlighted to detect hypoperfusion but may be dissociated from hemodynamics in cases such as chronic heart failure.
In addition, patients may have other manifestations of end-organ hypoperfusion with a normal lactate level, and there are also important causes of an elevated lactate level other than shock.
The revision proposes a three-axis model of CS evaluation and prognostication that integrates shock severity, clinical phenotype, and risk modifiers as distinct elements that should be applied to individualize patient management.
The revision also places more emphasis on the trajectory of the patient with CS through hospitalization, including a “hub and spoke” model for transfer of higher-risk patients, including those with a deteriorating SCAI shock stage.
“It is our desire and belief that the revised SCAI SHOCK stage classification system will enhance both clinical care and CS research trial design,” the panel writes.
This statement has been endorsed by the American College of Cardiology, American College of Emergency Physicians, American Heart Association, European Society of Cardiology Association for Acute Cardiovascular Care, International Society for Heart and Lung Transplantation, Society of Critical Care Medicine, and Society of Thoracic Surgeons.
This research had no commercial funding. Dr. Naidu has disclosed no relevant financial relationships. A complete list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
The Society for Cardiovascular Angiography and Interventions (SCAI) has refined its cardiogenic shock (CS) classification system based on the literature and clinician feedback from real-world experience.
“In the 2 years since publication in 2019, the initial definition has been broadly accepted and eagerly appreciated, allowing a very intuitive way to stage these patients for better communication, triage, and treatment,” Srihari S. Naidu, MD, professor of medicine, New York Medical College, Valhalla, said in an interview.
“But the initial definition was based on consensus opinion, with a lack of real fundamental data on segregating patients into different stages. Now we have a lot more data utilizing the definition, and it became very clear that there were a couple of limitations in the initial definition,” Dr. Naidu explained.
The refined CS classification system – authored by Dr. Naidu and a multidisciplinary panel of experts from specialties that included cardiac critical care, interventional cardiology, surgery, nursing, emergency medicine, and heart failure – was published online Jan. 31 in the Journal of the Society for Cardiovascular Angiography and Interventions, with simultaneous publication in the Journal of the American College of Cardiology.
It maintains the five-stage pyramid of CS, starting with “at risk” and moving through “beginning,” “classic,” “deteriorating,” and “extremis” but now includes gradations of severity within each stage and pathways by which patients progress or recover.
“Progression across the SCAI shock stage continuum is a dynamic process, incorporating new information as available, and patient trajectories are important both for communication among clinicians and for decisionmaking regarding the next level of care and therapeutics,” the panel writes.
The second iteration adds a streamlined table incorporating commonly seen variables, based on lessons learned from validation studies and clinician experience.
“While keeping the same initial framework of looking at the three components of staging – the physical exam, the biochemical markers, and hemodynamics – we’ve made it very clear that there are some factors in each of these that are most typically seen. And then there are other factors that are consistent with that stage but don’t necessarily have to be seen, ... are not typically seen in that stage, or [are] not always present at that stage,” Dr. Naidu told this news organization.
The refined CS classification system provides more granularity on cardiac arrest as a risk modifier, which now excludes very brief episodes with rapid response to defibrillation and comprises only those patients who have impaired mental status with unknown neurologic recovery status after cardiopulmonary resuscitation.
Lactate level and thresholds have been highlighted to detect hypoperfusion but may be dissociated from hemodynamics in cases such as chronic heart failure.
In addition, patients may have other manifestations of end-organ hypoperfusion with a normal lactate level, and there are also important causes of an elevated lactate level other than shock.
The revision proposes a three-axis model of CS evaluation and prognostication that integrates shock severity, clinical phenotype, and risk modifiers as distinct elements that should be applied to individualize patient management.
The revision also places more emphasis on the trajectory of the patient with CS through hospitalization, including a “hub and spoke” model for transfer of higher-risk patients, including those with a deteriorating SCAI shock stage.
“It is our desire and belief that the revised SCAI SHOCK stage classification system will enhance both clinical care and CS research trial design,” the panel writes.
This statement has been endorsed by the American College of Cardiology, American College of Emergency Physicians, American Heart Association, European Society of Cardiology Association for Acute Cardiovascular Care, International Society for Heart and Lung Transplantation, Society of Critical Care Medicine, and Society of Thoracic Surgeons.
This research had no commercial funding. Dr. Naidu has disclosed no relevant financial relationships. A complete list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
‘Substantial’ CVD risks, burden up to a year after COVID-19
People who have had COVID-19 have an increased risk for, and 12-month burden of, cardiovascular disease (CVD) that is substantial and spans an array of cardiovascular disorders, a deep dive into federal data suggests.
“I went into this thinking that this is most likely happening in people to start with who have a higher risk of cardiovascular disorders, smokers, people with high BMI, diabetes, but what we found is something different,” Ziyad Al-Aly, MD, said in an interview. “It’s evident in people at high risk, but it was also as clear as the sun even in people who have no cardiovascular risk whatsoever.”
Rates were increased in younger adults, never smokers, White and Black people, and males and females, he said. “So the risk confirmed by the SARS-CoV-2 virus seems to spare almost no one.”
Although cardiovascular outcomes increased with the severity of the acute infection, the excess risks and burdens were also evident in those who never required hospitalization, a group that represents the majority of people with COVID-19, observed Dr. Al-Aly, who directs the Clinical Epidemiology Center at the Veterans Affairs St. Louis Health Care System.
“This study is very important because it underscores not just the acute cardiovascular risk associated with COVID but the increased risk of chronic cardiovascular outcomes as well,” cardiologist C. Michael Gibson, MD, professor of medicine, Harvard Medical School, Boston, said in an interview. “Given the number of patients in the U.S. who have been infected with COVID, this could represent a significant chronic burden on the health care system, particularly as health care professionals leave the profession.”
For the study, the investigators used national VA databases to build a cohort of 153,760 veterans who were alive 30 days after testing positive for COVID-19 between March 1, 2020, and January 2021. They were compared with a contemporary cohort of 5.6 million veterans with no evidence of SARS-CoV-2 infection and a historical cohort of 5.8 million veterans using the system in 2017 prior to the pandemic. Median follow-up was 347, 348, and 347 days, respectively.
As reported in Nature Medicine, the risk for a major adverse cardiovascular event, a composite of myocardial infarction, stroke, and all-cause mortality, was 4% higher in people who had been infected with COVID-19 than in those who had not.
“People say 4% is small, but actually it’s really, really big if you think about it in the context of the huge number of people who have had COVID-19 in the United States, and also globally,” Dr. Al-Aly said.
Compared with the contemporary control group, people who had COVID-19 had an increased risk (hazard ratio [HR]) and burden per 1,000 people at 1 year for the following cardiovascular outcomes:
- Stroke: HR, 1.52; burden, 4.03
- Transient ischemic attack: HR, 1.49; burden, 1.84
- Dysrhythmias: HR, 1.69; burden, 19.86
- Ischemic heart disease: HR, 1.66; burden, 7.28
- Heart failure: HR, 1.72; burden, 11.61
- Nonischemic cardiomyopathy: HR, 1.62; burden 3.56
- Pulmonary embolism: HR, 2.93; burden, 5.47
- Deep vein thrombosis: HR, 2.09; burden, 4.18
- Pericarditis: HR, 1.85, burden, 0.98
- Myocarditis: HR, 5.38; burden, 0.31
Recent reports have raised concerns about an association between COVID-19 vaccines and myocarditis and pericarditis, particularly in young males. Although very few of the participants were vaccinated prior to becoming infected, as vaccines were not yet widely available, the researchers performed two analyses censoring participants at the time of the first dose of any COVID-19 vaccine and adjusting for vaccination as a time-varying covariate.
The absolute numbers of myocarditis and pericarditis were still higher than the contemporary and historical cohorts. These numbers are much larger than those reported for myocarditis after vaccines, which are generally around 40 cases per 1 million people, observed Dr. Al-Aly.
The overall results were also consistent when compared with the historical control subjects.
“What we’re seeing in our report and others is that SARS-CoV-2 can leave a sort of scar or imprint on people, and some of these conditions are likely chronic conditions,” Dr. Al-Aly said. “So you’re going to have a generation of people who will bear the scar of COVID for their lifetime and I think that requires recognition and attention, so we’re aware of the magnitude of the problem and prepared to deal with it.”
With more than 76 million COVID-19 cases in the United States, that effort will likely have to be at the federal level, similar to President Joe Biden’s recent relaunch of the “Cancer Moonshot,” he added. “We need a greater and broader recognition at the federal level to try and recognize that when you have an earthquake, you don’t just deal with the earthquake when the earth is shaking, but you also need to deal with the aftermath.”
Dr. Gibson pointed out that this was a study of predominantly males and, thus, it’s unclear if the results can be extended to females. Nevertheless, he added, “long COVID may include outcomes beyond the central nervous system and we should educate patients about the risk of late cardiovascular outcomes.”
The authors noted the largely White, male cohort may limit generalizability of the findings. Other limitations include the possibility that some people may have had COVID-19 but were not tested, the datasets lacked information on cause of death, and possible residual confounding not accounted for in the adjusted analyses.
The research was funded by the U.S. Department of Veterans Affairs and two American Society of Nephrology and Kidney Cure fellowship awards. The authors declared no competing interests. Dr. Gibson reports having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
People who have had COVID-19 have an increased risk for, and 12-month burden of, cardiovascular disease (CVD) that is substantial and spans an array of cardiovascular disorders, a deep dive into federal data suggests.
“I went into this thinking that this is most likely happening in people to start with who have a higher risk of cardiovascular disorders, smokers, people with high BMI, diabetes, but what we found is something different,” Ziyad Al-Aly, MD, said in an interview. “It’s evident in people at high risk, but it was also as clear as the sun even in people who have no cardiovascular risk whatsoever.”
Rates were increased in younger adults, never smokers, White and Black people, and males and females, he said. “So the risk confirmed by the SARS-CoV-2 virus seems to spare almost no one.”
Although cardiovascular outcomes increased with the severity of the acute infection, the excess risks and burdens were also evident in those who never required hospitalization, a group that represents the majority of people with COVID-19, observed Dr. Al-Aly, who directs the Clinical Epidemiology Center at the Veterans Affairs St. Louis Health Care System.
“This study is very important because it underscores not just the acute cardiovascular risk associated with COVID but the increased risk of chronic cardiovascular outcomes as well,” cardiologist C. Michael Gibson, MD, professor of medicine, Harvard Medical School, Boston, said in an interview. “Given the number of patients in the U.S. who have been infected with COVID, this could represent a significant chronic burden on the health care system, particularly as health care professionals leave the profession.”
For the study, the investigators used national VA databases to build a cohort of 153,760 veterans who were alive 30 days after testing positive for COVID-19 between March 1, 2020, and January 2021. They were compared with a contemporary cohort of 5.6 million veterans with no evidence of SARS-CoV-2 infection and a historical cohort of 5.8 million veterans using the system in 2017 prior to the pandemic. Median follow-up was 347, 348, and 347 days, respectively.
As reported in Nature Medicine, the risk for a major adverse cardiovascular event, a composite of myocardial infarction, stroke, and all-cause mortality, was 4% higher in people who had been infected with COVID-19 than in those who had not.
“People say 4% is small, but actually it’s really, really big if you think about it in the context of the huge number of people who have had COVID-19 in the United States, and also globally,” Dr. Al-Aly said.
Compared with the contemporary control group, people who had COVID-19 had an increased risk (hazard ratio [HR]) and burden per 1,000 people at 1 year for the following cardiovascular outcomes:
- Stroke: HR, 1.52; burden, 4.03
- Transient ischemic attack: HR, 1.49; burden, 1.84
- Dysrhythmias: HR, 1.69; burden, 19.86
- Ischemic heart disease: HR, 1.66; burden, 7.28
- Heart failure: HR, 1.72; burden, 11.61
- Nonischemic cardiomyopathy: HR, 1.62; burden 3.56
- Pulmonary embolism: HR, 2.93; burden, 5.47
- Deep vein thrombosis: HR, 2.09; burden, 4.18
- Pericarditis: HR, 1.85, burden, 0.98
- Myocarditis: HR, 5.38; burden, 0.31
Recent reports have raised concerns about an association between COVID-19 vaccines and myocarditis and pericarditis, particularly in young males. Although very few of the participants were vaccinated prior to becoming infected, as vaccines were not yet widely available, the researchers performed two analyses censoring participants at the time of the first dose of any COVID-19 vaccine and adjusting for vaccination as a time-varying covariate.
The absolute numbers of myocarditis and pericarditis were still higher than the contemporary and historical cohorts. These numbers are much larger than those reported for myocarditis after vaccines, which are generally around 40 cases per 1 million people, observed Dr. Al-Aly.
The overall results were also consistent when compared with the historical control subjects.
“What we’re seeing in our report and others is that SARS-CoV-2 can leave a sort of scar or imprint on people, and some of these conditions are likely chronic conditions,” Dr. Al-Aly said. “So you’re going to have a generation of people who will bear the scar of COVID for their lifetime and I think that requires recognition and attention, so we’re aware of the magnitude of the problem and prepared to deal with it.”
With more than 76 million COVID-19 cases in the United States, that effort will likely have to be at the federal level, similar to President Joe Biden’s recent relaunch of the “Cancer Moonshot,” he added. “We need a greater and broader recognition at the federal level to try and recognize that when you have an earthquake, you don’t just deal with the earthquake when the earth is shaking, but you also need to deal with the aftermath.”
Dr. Gibson pointed out that this was a study of predominantly males and, thus, it’s unclear if the results can be extended to females. Nevertheless, he added, “long COVID may include outcomes beyond the central nervous system and we should educate patients about the risk of late cardiovascular outcomes.”
The authors noted the largely White, male cohort may limit generalizability of the findings. Other limitations include the possibility that some people may have had COVID-19 but were not tested, the datasets lacked information on cause of death, and possible residual confounding not accounted for in the adjusted analyses.
The research was funded by the U.S. Department of Veterans Affairs and two American Society of Nephrology and Kidney Cure fellowship awards. The authors declared no competing interests. Dr. Gibson reports having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
People who have had COVID-19 have an increased risk for, and 12-month burden of, cardiovascular disease (CVD) that is substantial and spans an array of cardiovascular disorders, a deep dive into federal data suggests.
“I went into this thinking that this is most likely happening in people to start with who have a higher risk of cardiovascular disorders, smokers, people with high BMI, diabetes, but what we found is something different,” Ziyad Al-Aly, MD, said in an interview. “It’s evident in people at high risk, but it was also as clear as the sun even in people who have no cardiovascular risk whatsoever.”
Rates were increased in younger adults, never smokers, White and Black people, and males and females, he said. “So the risk confirmed by the SARS-CoV-2 virus seems to spare almost no one.”
Although cardiovascular outcomes increased with the severity of the acute infection, the excess risks and burdens were also evident in those who never required hospitalization, a group that represents the majority of people with COVID-19, observed Dr. Al-Aly, who directs the Clinical Epidemiology Center at the Veterans Affairs St. Louis Health Care System.
“This study is very important because it underscores not just the acute cardiovascular risk associated with COVID but the increased risk of chronic cardiovascular outcomes as well,” cardiologist C. Michael Gibson, MD, professor of medicine, Harvard Medical School, Boston, said in an interview. “Given the number of patients in the U.S. who have been infected with COVID, this could represent a significant chronic burden on the health care system, particularly as health care professionals leave the profession.”
For the study, the investigators used national VA databases to build a cohort of 153,760 veterans who were alive 30 days after testing positive for COVID-19 between March 1, 2020, and January 2021. They were compared with a contemporary cohort of 5.6 million veterans with no evidence of SARS-CoV-2 infection and a historical cohort of 5.8 million veterans using the system in 2017 prior to the pandemic. Median follow-up was 347, 348, and 347 days, respectively.
As reported in Nature Medicine, the risk for a major adverse cardiovascular event, a composite of myocardial infarction, stroke, and all-cause mortality, was 4% higher in people who had been infected with COVID-19 than in those who had not.
“People say 4% is small, but actually it’s really, really big if you think about it in the context of the huge number of people who have had COVID-19 in the United States, and also globally,” Dr. Al-Aly said.
Compared with the contemporary control group, people who had COVID-19 had an increased risk (hazard ratio [HR]) and burden per 1,000 people at 1 year for the following cardiovascular outcomes:
- Stroke: HR, 1.52; burden, 4.03
- Transient ischemic attack: HR, 1.49; burden, 1.84
- Dysrhythmias: HR, 1.69; burden, 19.86
- Ischemic heart disease: HR, 1.66; burden, 7.28
- Heart failure: HR, 1.72; burden, 11.61
- Nonischemic cardiomyopathy: HR, 1.62; burden 3.56
- Pulmonary embolism: HR, 2.93; burden, 5.47
- Deep vein thrombosis: HR, 2.09; burden, 4.18
- Pericarditis: HR, 1.85, burden, 0.98
- Myocarditis: HR, 5.38; burden, 0.31
Recent reports have raised concerns about an association between COVID-19 vaccines and myocarditis and pericarditis, particularly in young males. Although very few of the participants were vaccinated prior to becoming infected, as vaccines were not yet widely available, the researchers performed two analyses censoring participants at the time of the first dose of any COVID-19 vaccine and adjusting for vaccination as a time-varying covariate.
The absolute numbers of myocarditis and pericarditis were still higher than the contemporary and historical cohorts. These numbers are much larger than those reported for myocarditis after vaccines, which are generally around 40 cases per 1 million people, observed Dr. Al-Aly.
The overall results were also consistent when compared with the historical control subjects.
“What we’re seeing in our report and others is that SARS-CoV-2 can leave a sort of scar or imprint on people, and some of these conditions are likely chronic conditions,” Dr. Al-Aly said. “So you’re going to have a generation of people who will bear the scar of COVID for their lifetime and I think that requires recognition and attention, so we’re aware of the magnitude of the problem and prepared to deal with it.”
With more than 76 million COVID-19 cases in the United States, that effort will likely have to be at the federal level, similar to President Joe Biden’s recent relaunch of the “Cancer Moonshot,” he added. “We need a greater and broader recognition at the federal level to try and recognize that when you have an earthquake, you don’t just deal with the earthquake when the earth is shaking, but you also need to deal with the aftermath.”
Dr. Gibson pointed out that this was a study of predominantly males and, thus, it’s unclear if the results can be extended to females. Nevertheless, he added, “long COVID may include outcomes beyond the central nervous system and we should educate patients about the risk of late cardiovascular outcomes.”
The authors noted the largely White, male cohort may limit generalizability of the findings. Other limitations include the possibility that some people may have had COVID-19 but were not tested, the datasets lacked information on cause of death, and possible residual confounding not accounted for in the adjusted analyses.
The research was funded by the U.S. Department of Veterans Affairs and two American Society of Nephrology and Kidney Cure fellowship awards. The authors declared no competing interests. Dr. Gibson reports having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
VARC-3 TAVR technical failure definition ‘highly clinically relevant’
A new study offers early validation of the recently released Valve Academic Research Consortium 3 (VARC-3) definition of technical success after transcatheter aortic valve replacement (TAVR) and highlights its role in patient prognosis.
Results show that one in 10 patients (11.6%) undergoing TAVR with contemporary devices and techniques experiences technical failure, according to VARC-3.
At 30 days, patients with technical failure had significantly higher rates of the composite of cardiovascular (CV) death or stroke (11.5% vs. 3.5%), CV death (6.0% vs. 1.0%), and stroke (7.2% vs. 2.9%), compared with those with technical success.
Technical failure after TAVR was also independently associated with a twofold higher risk for CV death or stroke at 1 year (20.0% vs. 10.3%; hazard ratio, 2.01; 95% CI, 1.37-2.95).
Other independent predictors were history of peripheral artery disease (HR, 1.97), New York Heart Association III or IV disease (HR, 1.86), baseline moderate or greater mitral regurgitation (HR, 1.48), atrial fibrillation (HR, 1.40), and Society of Thoracic Surgeons predicted mortality risk (HR, 1.04).
“We were expecting that we were getting better over time with device iterations, with more experience, so we weren’t surprised by the result. But I think what is somewhat surprising is how much of an impact it has on the outcome,” senior study author Thomas Pilgrim, MD, Inselspital, University of Bern, Switzerland, told this news organization.
The VARC-3 document, introduced last year to some controversy, features a heavier focus on patient outcomes, as well as composite safety and efficacy endpoints. The definition of technical success after TAVR includes freedom from death; successful access, delivery of the device, and retrieval of the delivery system; correct positioning of a prosthetic heart valve into the proper anatomical location; and freedom from surgery or intervention related to the device or to an access-related or cardiac structural complication.
The composite endpoint is meant to replace the VARC-2 definition of “device success,” which also included freedom from death and correct valve positioning but required echocardiographic evaluation. With VARC-3, there is an “immediate measure” of success without having to wait for echocardiography, observed Dr. Pilgrim.
As reported in the Journal of the American College of Cardiology Cardiovascular Interventions, TAVR was a technical success in 1,435 of 1,624 (88.4%) patients. Technical failure occurred in 189 patients related to either vascular complications (8.6%) or procedural death or cardiac complications (3.0%).
The VARC-2 endpoint of device success was observed in 66.1% of patients. The high rate of device failure was largely attributed to a 28% incidence of prosthesis-patient mismatch.
“If you use the VARC-2 device success [definition], you include this patient–prosthesis mismatch, the [valve] gradients, [and] regurgitation and then device success is always lower,” Dr. Pilgrim said.
Asked whether the VARC-3 definition may be missing case failures, he replied: “At this stage, we don’t know how important these echocardiographic parameters are for hard clinical endpoints. Maybe the VARC-2 endpoint was too sensitive or the VARC-3 endpoint is not sensitive enough. This is something we just don’t know at this stage.”
Marco Barbanti, MD, an interventional cardiologist at Rodolico Polyclinic University Hospital-San Marco, Catania, Italy, and author of an accompanying editorial, said VARC-3 represents a more accurate indicator of immediate success of the procedure.
“It’s a more pertinent definition according to what really has an impact on prognosis, and, according to the results of this paper, actually, the calibration of this new definition is quite good,” Dr. Barbanti said in an interview.
Patients with VARC-3 technical failure were older, had a higher body mass index, and had more advanced heart failure symptoms than those with technical success. There were no significant differences between the two groups in echocardiographic or CT data, anesthetic strategy, valve type or size, or use of pre- or post-dilation.
All patients underwent TAVR with current balloon-expandable (Sapien 3/Sapien Ultra, Edwards Lifesciences) or self-expanding (Evolut R/PRO [Medtronic], Portico [Abbott], Symetis ACURATE/ACURATE neo [Boston Scientific]) devices between March 2012 and December 2019. A transfemoral approach was used in 92.5% of patients.
In a landmark analysis with the landmark set at 30 days, the effect of technical failure on adverse outcome was limited to the first 30 days (composite endpoint 0-30 days: HR, 3.42; P < .001; 30-360 days: HR, 1.36; P = .266; P for interaction = .002).
At 1 year, the composite of CV death and stroke endpoint occurred in 24.1% of patients with cardiac technical failure, in 18.8% of patients with vascular technical failure, and in 10.3% of patients with technical success.
In multivariate analyses, cardiac and vascular technical failures were independently associated with a 2.6-fold and 1.9-fold increased risk, respectively, for the composite of cardiovascular death and stroke at 1 year.
Female sex, larger device landing zone calcium volume, and earlier procedures (March 2012 to July 2016) were associated with a higher risk for cardiac technical failure, whereas, consistent with previous studies, higher body mass index and use of the Prostar/Manta versus the ProGlide closure device predicted vascular technical failure.
The findings “underscore that technical success is highly clinically relevant and may serve as one of the pivotal endpoints to evaluate the improvement of TAVR or for head-to-head comparisons of new devices in future clinical trials,” the authors conclude.
The findings reflect the experience of a single high-volume center with highly experienced operators in the prospective BERN TAVR registry, however, and may not be generalizable to other heart centers, they note. Although the registry has standardized follow-up, independent analysis of echocardiographic and CT, and independent event adjudication, vascular anatomy was not systematically assessed, and the potential exists for confounding from unmeasured variables.
Dr. Pilgrim reports research grants to the institution from Edwards Lifesciences, Boston Scientific, and Biotronik, personal fees from Biotronik and Boston Scientific, and other from HighLife SAS. Dr. Barbanti is a consultant for Edwards Lifesciences and Boston Scientific.
A version of this article first appeared on Medscape.com.
A new study offers early validation of the recently released Valve Academic Research Consortium 3 (VARC-3) definition of technical success after transcatheter aortic valve replacement (TAVR) and highlights its role in patient prognosis.
Results show that one in 10 patients (11.6%) undergoing TAVR with contemporary devices and techniques experiences technical failure, according to VARC-3.
At 30 days, patients with technical failure had significantly higher rates of the composite of cardiovascular (CV) death or stroke (11.5% vs. 3.5%), CV death (6.0% vs. 1.0%), and stroke (7.2% vs. 2.9%), compared with those with technical success.
Technical failure after TAVR was also independently associated with a twofold higher risk for CV death or stroke at 1 year (20.0% vs. 10.3%; hazard ratio, 2.01; 95% CI, 1.37-2.95).
Other independent predictors were history of peripheral artery disease (HR, 1.97), New York Heart Association III or IV disease (HR, 1.86), baseline moderate or greater mitral regurgitation (HR, 1.48), atrial fibrillation (HR, 1.40), and Society of Thoracic Surgeons predicted mortality risk (HR, 1.04).
“We were expecting that we were getting better over time with device iterations, with more experience, so we weren’t surprised by the result. But I think what is somewhat surprising is how much of an impact it has on the outcome,” senior study author Thomas Pilgrim, MD, Inselspital, University of Bern, Switzerland, told this news organization.
The VARC-3 document, introduced last year to some controversy, features a heavier focus on patient outcomes, as well as composite safety and efficacy endpoints. The definition of technical success after TAVR includes freedom from death; successful access, delivery of the device, and retrieval of the delivery system; correct positioning of a prosthetic heart valve into the proper anatomical location; and freedom from surgery or intervention related to the device or to an access-related or cardiac structural complication.
The composite endpoint is meant to replace the VARC-2 definition of “device success,” which also included freedom from death and correct valve positioning but required echocardiographic evaluation. With VARC-3, there is an “immediate measure” of success without having to wait for echocardiography, observed Dr. Pilgrim.
As reported in the Journal of the American College of Cardiology Cardiovascular Interventions, TAVR was a technical success in 1,435 of 1,624 (88.4%) patients. Technical failure occurred in 189 patients related to either vascular complications (8.6%) or procedural death or cardiac complications (3.0%).
The VARC-2 endpoint of device success was observed in 66.1% of patients. The high rate of device failure was largely attributed to a 28% incidence of prosthesis-patient mismatch.
“If you use the VARC-2 device success [definition], you include this patient–prosthesis mismatch, the [valve] gradients, [and] regurgitation and then device success is always lower,” Dr. Pilgrim said.
Asked whether the VARC-3 definition may be missing case failures, he replied: “At this stage, we don’t know how important these echocardiographic parameters are for hard clinical endpoints. Maybe the VARC-2 endpoint was too sensitive or the VARC-3 endpoint is not sensitive enough. This is something we just don’t know at this stage.”
Marco Barbanti, MD, an interventional cardiologist at Rodolico Polyclinic University Hospital-San Marco, Catania, Italy, and author of an accompanying editorial, said VARC-3 represents a more accurate indicator of immediate success of the procedure.
“It’s a more pertinent definition according to what really has an impact on prognosis, and, according to the results of this paper, actually, the calibration of this new definition is quite good,” Dr. Barbanti said in an interview.
Patients with VARC-3 technical failure were older, had a higher body mass index, and had more advanced heart failure symptoms than those with technical success. There were no significant differences between the two groups in echocardiographic or CT data, anesthetic strategy, valve type or size, or use of pre- or post-dilation.
All patients underwent TAVR with current balloon-expandable (Sapien 3/Sapien Ultra, Edwards Lifesciences) or self-expanding (Evolut R/PRO [Medtronic], Portico [Abbott], Symetis ACURATE/ACURATE neo [Boston Scientific]) devices between March 2012 and December 2019. A transfemoral approach was used in 92.5% of patients.
In a landmark analysis with the landmark set at 30 days, the effect of technical failure on adverse outcome was limited to the first 30 days (composite endpoint 0-30 days: HR, 3.42; P < .001; 30-360 days: HR, 1.36; P = .266; P for interaction = .002).
At 1 year, the composite of CV death and stroke endpoint occurred in 24.1% of patients with cardiac technical failure, in 18.8% of patients with vascular technical failure, and in 10.3% of patients with technical success.
In multivariate analyses, cardiac and vascular technical failures were independently associated with a 2.6-fold and 1.9-fold increased risk, respectively, for the composite of cardiovascular death and stroke at 1 year.
Female sex, larger device landing zone calcium volume, and earlier procedures (March 2012 to July 2016) were associated with a higher risk for cardiac technical failure, whereas, consistent with previous studies, higher body mass index and use of the Prostar/Manta versus the ProGlide closure device predicted vascular technical failure.
The findings “underscore that technical success is highly clinically relevant and may serve as one of the pivotal endpoints to evaluate the improvement of TAVR or for head-to-head comparisons of new devices in future clinical trials,” the authors conclude.
The findings reflect the experience of a single high-volume center with highly experienced operators in the prospective BERN TAVR registry, however, and may not be generalizable to other heart centers, they note. Although the registry has standardized follow-up, independent analysis of echocardiographic and CT, and independent event adjudication, vascular anatomy was not systematically assessed, and the potential exists for confounding from unmeasured variables.
Dr. Pilgrim reports research grants to the institution from Edwards Lifesciences, Boston Scientific, and Biotronik, personal fees from Biotronik and Boston Scientific, and other from HighLife SAS. Dr. Barbanti is a consultant for Edwards Lifesciences and Boston Scientific.
A version of this article first appeared on Medscape.com.
A new study offers early validation of the recently released Valve Academic Research Consortium 3 (VARC-3) definition of technical success after transcatheter aortic valve replacement (TAVR) and highlights its role in patient prognosis.
Results show that one in 10 patients (11.6%) undergoing TAVR with contemporary devices and techniques experiences technical failure, according to VARC-3.
At 30 days, patients with technical failure had significantly higher rates of the composite of cardiovascular (CV) death or stroke (11.5% vs. 3.5%), CV death (6.0% vs. 1.0%), and stroke (7.2% vs. 2.9%), compared with those with technical success.
Technical failure after TAVR was also independently associated with a twofold higher risk for CV death or stroke at 1 year (20.0% vs. 10.3%; hazard ratio, 2.01; 95% CI, 1.37-2.95).
Other independent predictors were history of peripheral artery disease (HR, 1.97), New York Heart Association III or IV disease (HR, 1.86), baseline moderate or greater mitral regurgitation (HR, 1.48), atrial fibrillation (HR, 1.40), and Society of Thoracic Surgeons predicted mortality risk (HR, 1.04).
“We were expecting that we were getting better over time with device iterations, with more experience, so we weren’t surprised by the result. But I think what is somewhat surprising is how much of an impact it has on the outcome,” senior study author Thomas Pilgrim, MD, Inselspital, University of Bern, Switzerland, told this news organization.
The VARC-3 document, introduced last year to some controversy, features a heavier focus on patient outcomes, as well as composite safety and efficacy endpoints. The definition of technical success after TAVR includes freedom from death; successful access, delivery of the device, and retrieval of the delivery system; correct positioning of a prosthetic heart valve into the proper anatomical location; and freedom from surgery or intervention related to the device or to an access-related or cardiac structural complication.
The composite endpoint is meant to replace the VARC-2 definition of “device success,” which also included freedom from death and correct valve positioning but required echocardiographic evaluation. With VARC-3, there is an “immediate measure” of success without having to wait for echocardiography, observed Dr. Pilgrim.
As reported in the Journal of the American College of Cardiology Cardiovascular Interventions, TAVR was a technical success in 1,435 of 1,624 (88.4%) patients. Technical failure occurred in 189 patients related to either vascular complications (8.6%) or procedural death or cardiac complications (3.0%).
The VARC-2 endpoint of device success was observed in 66.1% of patients. The high rate of device failure was largely attributed to a 28% incidence of prosthesis-patient mismatch.
“If you use the VARC-2 device success [definition], you include this patient–prosthesis mismatch, the [valve] gradients, [and] regurgitation and then device success is always lower,” Dr. Pilgrim said.
Asked whether the VARC-3 definition may be missing case failures, he replied: “At this stage, we don’t know how important these echocardiographic parameters are for hard clinical endpoints. Maybe the VARC-2 endpoint was too sensitive or the VARC-3 endpoint is not sensitive enough. This is something we just don’t know at this stage.”
Marco Barbanti, MD, an interventional cardiologist at Rodolico Polyclinic University Hospital-San Marco, Catania, Italy, and author of an accompanying editorial, said VARC-3 represents a more accurate indicator of immediate success of the procedure.
“It’s a more pertinent definition according to what really has an impact on prognosis, and, according to the results of this paper, actually, the calibration of this new definition is quite good,” Dr. Barbanti said in an interview.
Patients with VARC-3 technical failure were older, had a higher body mass index, and had more advanced heart failure symptoms than those with technical success. There were no significant differences between the two groups in echocardiographic or CT data, anesthetic strategy, valve type or size, or use of pre- or post-dilation.
All patients underwent TAVR with current balloon-expandable (Sapien 3/Sapien Ultra, Edwards Lifesciences) or self-expanding (Evolut R/PRO [Medtronic], Portico [Abbott], Symetis ACURATE/ACURATE neo [Boston Scientific]) devices between March 2012 and December 2019. A transfemoral approach was used in 92.5% of patients.
In a landmark analysis with the landmark set at 30 days, the effect of technical failure on adverse outcome was limited to the first 30 days (composite endpoint 0-30 days: HR, 3.42; P < .001; 30-360 days: HR, 1.36; P = .266; P for interaction = .002).
At 1 year, the composite of CV death and stroke endpoint occurred in 24.1% of patients with cardiac technical failure, in 18.8% of patients with vascular technical failure, and in 10.3% of patients with technical success.
In multivariate analyses, cardiac and vascular technical failures were independently associated with a 2.6-fold and 1.9-fold increased risk, respectively, for the composite of cardiovascular death and stroke at 1 year.
Female sex, larger device landing zone calcium volume, and earlier procedures (March 2012 to July 2016) were associated with a higher risk for cardiac technical failure, whereas, consistent with previous studies, higher body mass index and use of the Prostar/Manta versus the ProGlide closure device predicted vascular technical failure.
The findings “underscore that technical success is highly clinically relevant and may serve as one of the pivotal endpoints to evaluate the improvement of TAVR or for head-to-head comparisons of new devices in future clinical trials,” the authors conclude.
The findings reflect the experience of a single high-volume center with highly experienced operators in the prospective BERN TAVR registry, however, and may not be generalizable to other heart centers, they note. Although the registry has standardized follow-up, independent analysis of echocardiographic and CT, and independent event adjudication, vascular anatomy was not systematically assessed, and the potential exists for confounding from unmeasured variables.
Dr. Pilgrim reports research grants to the institution from Edwards Lifesciences, Boston Scientific, and Biotronik, personal fees from Biotronik and Boston Scientific, and other from HighLife SAS. Dr. Barbanti is a consultant for Edwards Lifesciences and Boston Scientific.
A version of this article first appeared on Medscape.com.
FROM JACC: CARDIOVASCULAR INTERVENTIONS
Topline data for aficamten positive in obstructive HCM
The investigational, next-generation cardiac myosin inhibitor aficamten (previously CK-274, Cytokinetics) continues to show promise as a potential treatment for hypertrophic cardiomyopathy (HCM).
Today, the company announced positive topline results from cohort 3 of the REDWOOD-HCM phase 2 clinical trial, which included 13 patients with symptomatic obstructive HCM and a resting or post-Valsalva left ventricular outflow tract pressure gradient (LVOT-G) of 50 mm Hg or greater whose background therapy included disopyramide.
Treatment with aficamten led to substantial reductions in the average resting LVOT-G, as well as the post-Valsalva LVOT-G (defined as resting gradient less than 30 mm Hg and post-Valsalva gradient less than 50 mm Hg), the company reported.
These “clinically relevant” decreases in pressure gradients were achieved with only modest decreases in average left ventricular ejection fraction (LVEF), the company said.
In no patient did LVEF fall below the prespecified safety threshold of 50%.
New York Heart Association (NYHA) functional class was improved in most patients.
The safety and tolerability of aficamten in cohort 3 were consistent with previous experience in the REDWOOD-HCM trial, with no treatment interruptions and no serious treatment-related adverse events.
The pharmacokinetic data from cohort 3 are similar to those observed in REDWOOD-HCM cohorts 1 and 2, which included HCM patients taking background medications exclusive of disopyramide, as reported previously by this news organization.
“We are encouraged by the clinically relevant reductions in the LVOT gradient observed in these medically refractory patients and are pleased with the safety profile of aficamten when administered in combination with disopyramide,” Fady Malik, MD, PhD, Cytokinetics’ executive vice president of research and development, said in a news release.
“These results represent the first report of patients with obstructive HCM treated with a combination of a cardiac myosin inhibitor and disopyramide and support our plan to include this patient population in SEQUOIA-HCM, our phase 3 trial, which is important, given these patients have exhausted other available medical therapies,” Dr. Malik said.
The results from cohort 3 of the REDWOOD-HCM trial will be presented at the upcoming American College of Cardiology Annual Meeting in April.
A version of this article first appeared on Medscape.com.
The investigational, next-generation cardiac myosin inhibitor aficamten (previously CK-274, Cytokinetics) continues to show promise as a potential treatment for hypertrophic cardiomyopathy (HCM).
Today, the company announced positive topline results from cohort 3 of the REDWOOD-HCM phase 2 clinical trial, which included 13 patients with symptomatic obstructive HCM and a resting or post-Valsalva left ventricular outflow tract pressure gradient (LVOT-G) of 50 mm Hg or greater whose background therapy included disopyramide.
Treatment with aficamten led to substantial reductions in the average resting LVOT-G, as well as the post-Valsalva LVOT-G (defined as resting gradient less than 30 mm Hg and post-Valsalva gradient less than 50 mm Hg), the company reported.
These “clinically relevant” decreases in pressure gradients were achieved with only modest decreases in average left ventricular ejection fraction (LVEF), the company said.
In no patient did LVEF fall below the prespecified safety threshold of 50%.
New York Heart Association (NYHA) functional class was improved in most patients.
The safety and tolerability of aficamten in cohort 3 were consistent with previous experience in the REDWOOD-HCM trial, with no treatment interruptions and no serious treatment-related adverse events.
The pharmacokinetic data from cohort 3 are similar to those observed in REDWOOD-HCM cohorts 1 and 2, which included HCM patients taking background medications exclusive of disopyramide, as reported previously by this news organization.
“We are encouraged by the clinically relevant reductions in the LVOT gradient observed in these medically refractory patients and are pleased with the safety profile of aficamten when administered in combination with disopyramide,” Fady Malik, MD, PhD, Cytokinetics’ executive vice president of research and development, said in a news release.
“These results represent the first report of patients with obstructive HCM treated with a combination of a cardiac myosin inhibitor and disopyramide and support our plan to include this patient population in SEQUOIA-HCM, our phase 3 trial, which is important, given these patients have exhausted other available medical therapies,” Dr. Malik said.
The results from cohort 3 of the REDWOOD-HCM trial will be presented at the upcoming American College of Cardiology Annual Meeting in April.
A version of this article first appeared on Medscape.com.
The investigational, next-generation cardiac myosin inhibitor aficamten (previously CK-274, Cytokinetics) continues to show promise as a potential treatment for hypertrophic cardiomyopathy (HCM).
Today, the company announced positive topline results from cohort 3 of the REDWOOD-HCM phase 2 clinical trial, which included 13 patients with symptomatic obstructive HCM and a resting or post-Valsalva left ventricular outflow tract pressure gradient (LVOT-G) of 50 mm Hg or greater whose background therapy included disopyramide.
Treatment with aficamten led to substantial reductions in the average resting LVOT-G, as well as the post-Valsalva LVOT-G (defined as resting gradient less than 30 mm Hg and post-Valsalva gradient less than 50 mm Hg), the company reported.
These “clinically relevant” decreases in pressure gradients were achieved with only modest decreases in average left ventricular ejection fraction (LVEF), the company said.
In no patient did LVEF fall below the prespecified safety threshold of 50%.
New York Heart Association (NYHA) functional class was improved in most patients.
The safety and tolerability of aficamten in cohort 3 were consistent with previous experience in the REDWOOD-HCM trial, with no treatment interruptions and no serious treatment-related adverse events.
The pharmacokinetic data from cohort 3 are similar to those observed in REDWOOD-HCM cohorts 1 and 2, which included HCM patients taking background medications exclusive of disopyramide, as reported previously by this news organization.
“We are encouraged by the clinically relevant reductions in the LVOT gradient observed in these medically refractory patients and are pleased with the safety profile of aficamten when administered in combination with disopyramide,” Fady Malik, MD, PhD, Cytokinetics’ executive vice president of research and development, said in a news release.
“These results represent the first report of patients with obstructive HCM treated with a combination of a cardiac myosin inhibitor and disopyramide and support our plan to include this patient population in SEQUOIA-HCM, our phase 3 trial, which is important, given these patients have exhausted other available medical therapies,” Dr. Malik said.
The results from cohort 3 of the REDWOOD-HCM trial will be presented at the upcoming American College of Cardiology Annual Meeting in April.
A version of this article first appeared on Medscape.com.
Hong Kong, U.S., Israeli data illuminate COVID vaccine myocarditis
Why some COVID-19 vaccines seem occasionally to cause a distinctive form of myocarditis, and why adolescent boys and young men appear most vulnerable, remain a mystery. But the entity’s prevalence, nuances of presentation, and likely clinical course have come into sharper view after recent additions to the literature.
Two new publications all but confirm that the rare cases of myocarditis closely following vaccination against SARS-CoV-2, primarily with one of the mRNA-based vaccines from Pfizer-BioNTech and Moderna, is a clinically different creature from myocarditis physicians were likely to see before the pandemic.
A third report unveils rates of hospitalization for myocarditis linked to Pfizer-BioNTech vaccination in the 12- to 15-year age group, based on active surveillance across Israel. Of note, the rates were lower than corresponding numbers among the country’s 16- to 19-year-olds published in late 2021 by the same authors.
No link with CoronaVac
A case-control study covering almost the entire population of Hong Kong from February to August 2021 confirms a slight but significant excess risk for myocarditis and, to a lesser degree, pericarditis, after injections of the Pfizer-BioNTech vaccine. As consistently reported from other studies, the risks were highest in adolescent and young adult males and after a second dose.
The study estimated an overall carditis incidence of 5.7 cases per million doses of Pfizer-BioNTech, for a risk 3.5 times that in the unvaccinated Hong Kong population. Carditis rates after a first dose were about 2.5 per million and 10 per million after a second dose.
Hong Kong launched its public SARS-CoV-2 immunization program in late February 2021 with the Chinese-made CoronaVac (Sinovac) inactivated-virus vaccine, and introduced the mRNA-based alternative several weeks later. By August 2021, the vaccines had reached about 3.3 million people in the region – 49% of the Hong Kong population at least 12 years of age.
In a novel finding, there were no excesses in carditis cases after CoronaVac vaccination. The difference between vaccines likely isn’t caused by chance, because three-fourths of the carditis-associated Pfizer-BioNTech injections arose within a week, whereas “71% of cases following the use of CoronaVac occurred more than 30 days after vaccination,” senior author Ian Chi Kei Wong, PhD, University of Hong Kong, said in an interview.
“This onset distribution for cases having received CoronaVac demonstrates that it is highly unlikely the carditis cases are related to the vaccine,” he said. And that “plausibly implies a specific underlying mechanism between vaccination and carditis that may only be applicable to mRNA vaccines.”
That inference is in line with case reports and other research, including large population-based studies from Israel and Denmark, although a recent study from the United Kingdom hinted at a potential excess myocarditis risk associated with the adenovirus-based AstraZeneca-Oxford vaccine.
The Hong Kong study identified 160 patients age 12 or older with a first diagnosis of carditis during February to August 2021, in electronic health records covering nearly the entire region.
“We used laboratory test results of troponin levels to further eliminate unlikely cases of carditis,” Dr. Wong said. The health records were linked to a “population-based vaccination record” maintained by the government’s department of health.
About 10 control patients from among all hospitalized patients without carditis were matched by age, sex, and admission date to each of the 160 carditis cases. About 83% of cases and 92% of the controls were unvaccinated.
Among those who received the Pfizer-BioNTech vaccine, representing 12.5% of cases and 4.2% of controls, the estimated carditis incidence was 0.57 per 100,000 doses. For those who received CoronaVac, representing 4.4% of cases and 3.9% of controls, it was 0.31 per 100,000 doses.
In adjusted analysis, the odds ratios for carditis among Pfizer-BioNTech vaccine recipients, compared with unvaccinated controls, were 3.57 (95% confidence interval, 1.93-6.60) overall, 4.68 (95% CI, 2.25-9.71) for males, 2.22 (95% CI, 0.57-8.69) for females, 2.41 (95% CI, 1.18-4.90) for ages 18 and older, and 13.8 (95% CI, 2.86-110.4) for ages 12-17
Myocarditis accounted for most of the excess cases, with an overall OR of 9.29 (95% CI, 3.94-21.9). The OR reached only 1.06 (95% CI, 0.35-3.22) for pericarditis alone.
The case-control study is noteworthy for its design, which contrasts with the many recent case series and passive or active surveillance studies, and even the more robust population-based studies of vaccine-related myocarditis, observed Dongngan Truong, MD, University of Utah and Primary Children’s Hospital, both in Salt Lake City, who wasn’t part of the study.
Among its strengths, she said in an interview, are its linkage of comprehensive hospital and vaccination data sets for two different vaccines; and that it corroborates other research suggesting there is “something in particular about mRNA vaccination that seems to be associated with the development of myocarditis.”
Active surveillance in Israel
In an October 2021 report based on an Israeli Ministry of Health database covering up to May 2021, rates of myocarditis arising within 21 days of a second Pfizer-BioNTech dose in 16- to 19-year-olds reached about 1 per 6,637 males and 1 per 99,853 females. Those numbers compared with 1 per 26,000 males and 1 per 218,000 females across all age groups.
Now authors led by Dror Mevorach, MD, Hadassah Medical Center, Jerusalem, have published corresponding numbers from the same data base for myocarditis associated with the same vaccine in males and females aged 12-15.
Their research covers 404,407 people in that age group who received a first dose of the mRNA-based vaccine and 326,463 who received the second dose from June to October, 2021. Only 18 cases of myocarditis were observed within 21 days of either dose.
The estimated rates for males were 0.56 cases per 100,000 after a first dose and 8.09 cases per 100,000 after a second dose.
For females, the estimates were 0 cases per 100,000 after a first dose and 0.69 cases per 100,000 after a second dose.
“The pattern observed, mainly following the second vaccination in males, suggests causality,” the group wrote.
Leveraging passive surveillance reports
Another new report adds a twist to updated numbers from the U.S. Vaccine Adverse Event Reporting System (VAERS).
Prevalences derived from the passive-surveillance data base, known for including case records of inconsistent quality or completeness, are considered especially prone to reporting bias, the authors acknowledged.
The current analysis, however, plunges deep into VAERS-reported cases of presumed SARS-CoV-2 vaccine-associated myocarditis to help clarify “more of the characteristics of the patients and some of the treatments and short-term outcomes,” Matthew E. Oster, MD, MPH, said in an interview.
Dr. Oster, from the Centers for Disease Control and Prevention and Emory University, Atlanta, is lead author on the study’s Jan. 25, 2022, publication in JAMA.
The group reviewed charts and interviewed involved clinicians to adjudicate and document presentations, therapies, and the clinical course of cases reported as SARS-CoV-2 vaccine–associated myocarditis from December 2020 to August 2021. Out of the nearly 2000 reports, which were limited to patients younger than 30, the group identified 1,626 likely cases of such myocarditis arising within 7 days of a second mRNA vaccine dose.
The confirmed cases consistently represented higher prevalences than expected compared with prepandemic myocarditis claims data for both sexes and across age groups spanning 12-29 years.
For example, rates were highest for adolescent males – about 106 and 71 cases per million second doses of the Pfizer-BioNTech vaccine in those aged 16-17 and 12-16, respectively, for example. They were lowest for women aged 25-29, at 2.23 cases per million second Pfizer-BioNTech doses; the highest rate among females was about 11 per million for the 16-17 age group.
The observed rates, Dr. Oster said, represent an update to VAERS numbers published June 2021 in Morbidity and Mortality Weekly Report covering cases through June 2021.
“Overall, the general risk of having myocarditis from the vaccines is still extremely low. Even in the highest risk groups, it is still extremely low, and still lower than the risk of having cardiac complications from COVID,” he noted.
How do patients fare clinically?
From their chart reviews and interviews with case clinicians, Dr. Oster said, “we started to learn quickly that this is really a different type of myocarditis.”
For example, its onset, typically within a few days of the potential immunologic cause, was more rapid than in viral myocarditis, and its symptoms resolved faster, the report notes. Clinical presentations tended to be less severe, treatments not as intensive, and outcomes not as serious, compared with “the kind of typical viral myocarditis that most of the providers were used to taking care of in the past,” he said. “The pattern for these cases was very consistent.”
The study covered VAERS reports of suspected myocarditis arising within a week of first dose of a mRNA-based vaccine from the United States launch of public vaccination in December 2020 to August 2021, the CDC-based group reported. By then, more than 192 million people in the country had received either the Pfizer-BioNTech (age 12 or older) or Moderna (age 18 or older) vaccines.
Of the 1,991 reports of myocarditis, including 391 also involving pericarditis, 1,626 met the study’s definition for myocarditis on adjudication; about 82% of the latter cases were in males.
Based on the investigators’ review of charts and clinician interviews connected with 826 cases that met their definition of myocarditis in patients younger than 30, 89% reported “chest pain, pressure, or discomfort” and 30% reported dyspnea or shortness of breath. Troponin levels were elevated in 98%, 72% of patients who underwent electrocardiography showed abnormalities, and 12% of those with echocardiography had left ventricular ejection fractions less than 50%.
About 96% were hospitalized, and presenting symptoms resolved by discharge in 87% of those with available data, the group noted. Among patients with data on in-hospital therapy, they wrote, NSAIDs were the most common therapy, in 87%.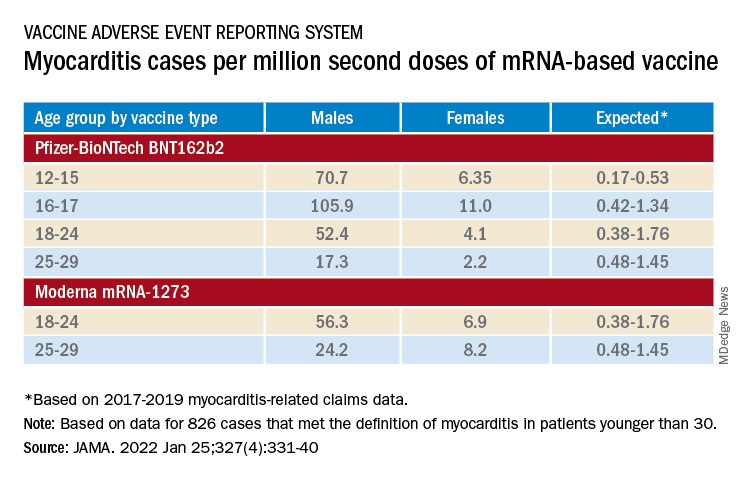
‘Mild and self-limiting’
The case-control study from Hong Kong didn’t specifically examine patients’ treatment and clinical course, but it does portray their vaccine-associated myocarditis as contrasting with more familiar viral myocarditis.
Patients with “typical” myocarditis tend to be “overall much sicker than what we’re seeing with myocarditis following vaccination,” Dr. Truong agreed. None of the 20 patients with myocarditis after Pfizer-BioNTech vaccination in Hong Kong were admitted to the intensive care unit. That, she added, suggests none required extracorporeal membrane oxygenation or vasoactive support, often necessary in viral myocarditis. “And they had shorter hospital stays.”
In contrast, Dr. Wong noted, 14 of the study’s unvaccinated patients required ICU admission; 12 of them died during the follow-up period. None with vaccine-related carditis died during the study’s follow-up. “We also showed that cases following [Pfizer-BioNTech] vaccination were all mild and self-limiting.”
Dr. Truong largely agreed that SARS-CoV-2 vaccine myocarditis and most myocarditis seen before the pandemic can be viewed as distinct clinical entities, “at least in the short term. I think we do need to follow these patients to look at more long-term outcomes, because at this point I don’t think we know the long-term implications. But at least in the short term, it seems like these patients are different, are much less sick, and recover pretty quickly overall.”
Dr. Oster emphasized that the many and varied acute and long-term hazards from contracting COVID-19 far outweigh any risk for myocarditis from vaccination. But for individuals who were hit with myocarditis soon after their first mRNA vaccine dose, who have already established their susceptibility, he and his colleagues would recommend that they “consider alternatives and not get the vaccine again.”
Dr. Oster reported no relevant financial relationships. Dr. Wong and colleagues did not report any relevant disclosures. Dr. Truong has previously disclosed serving as a consultant to Pfizer.
A version of this article first appeared on Medscape.com.
Why some COVID-19 vaccines seem occasionally to cause a distinctive form of myocarditis, and why adolescent boys and young men appear most vulnerable, remain a mystery. But the entity’s prevalence, nuances of presentation, and likely clinical course have come into sharper view after recent additions to the literature.
Two new publications all but confirm that the rare cases of myocarditis closely following vaccination against SARS-CoV-2, primarily with one of the mRNA-based vaccines from Pfizer-BioNTech and Moderna, is a clinically different creature from myocarditis physicians were likely to see before the pandemic.
A third report unveils rates of hospitalization for myocarditis linked to Pfizer-BioNTech vaccination in the 12- to 15-year age group, based on active surveillance across Israel. Of note, the rates were lower than corresponding numbers among the country’s 16- to 19-year-olds published in late 2021 by the same authors.
No link with CoronaVac
A case-control study covering almost the entire population of Hong Kong from February to August 2021 confirms a slight but significant excess risk for myocarditis and, to a lesser degree, pericarditis, after injections of the Pfizer-BioNTech vaccine. As consistently reported from other studies, the risks were highest in adolescent and young adult males and after a second dose.
The study estimated an overall carditis incidence of 5.7 cases per million doses of Pfizer-BioNTech, for a risk 3.5 times that in the unvaccinated Hong Kong population. Carditis rates after a first dose were about 2.5 per million and 10 per million after a second dose.
Hong Kong launched its public SARS-CoV-2 immunization program in late February 2021 with the Chinese-made CoronaVac (Sinovac) inactivated-virus vaccine, and introduced the mRNA-based alternative several weeks later. By August 2021, the vaccines had reached about 3.3 million people in the region – 49% of the Hong Kong population at least 12 years of age.
In a novel finding, there were no excesses in carditis cases after CoronaVac vaccination. The difference between vaccines likely isn’t caused by chance, because three-fourths of the carditis-associated Pfizer-BioNTech injections arose within a week, whereas “71% of cases following the use of CoronaVac occurred more than 30 days after vaccination,” senior author Ian Chi Kei Wong, PhD, University of Hong Kong, said in an interview.
“This onset distribution for cases having received CoronaVac demonstrates that it is highly unlikely the carditis cases are related to the vaccine,” he said. And that “plausibly implies a specific underlying mechanism between vaccination and carditis that may only be applicable to mRNA vaccines.”
That inference is in line with case reports and other research, including large population-based studies from Israel and Denmark, although a recent study from the United Kingdom hinted at a potential excess myocarditis risk associated with the adenovirus-based AstraZeneca-Oxford vaccine.
The Hong Kong study identified 160 patients age 12 or older with a first diagnosis of carditis during February to August 2021, in electronic health records covering nearly the entire region.
“We used laboratory test results of troponin levels to further eliminate unlikely cases of carditis,” Dr. Wong said. The health records were linked to a “population-based vaccination record” maintained by the government’s department of health.
About 10 control patients from among all hospitalized patients without carditis were matched by age, sex, and admission date to each of the 160 carditis cases. About 83% of cases and 92% of the controls were unvaccinated.
Among those who received the Pfizer-BioNTech vaccine, representing 12.5% of cases and 4.2% of controls, the estimated carditis incidence was 0.57 per 100,000 doses. For those who received CoronaVac, representing 4.4% of cases and 3.9% of controls, it was 0.31 per 100,000 doses.
In adjusted analysis, the odds ratios for carditis among Pfizer-BioNTech vaccine recipients, compared with unvaccinated controls, were 3.57 (95% confidence interval, 1.93-6.60) overall, 4.68 (95% CI, 2.25-9.71) for males, 2.22 (95% CI, 0.57-8.69) for females, 2.41 (95% CI, 1.18-4.90) for ages 18 and older, and 13.8 (95% CI, 2.86-110.4) for ages 12-17
Myocarditis accounted for most of the excess cases, with an overall OR of 9.29 (95% CI, 3.94-21.9). The OR reached only 1.06 (95% CI, 0.35-3.22) for pericarditis alone.
The case-control study is noteworthy for its design, which contrasts with the many recent case series and passive or active surveillance studies, and even the more robust population-based studies of vaccine-related myocarditis, observed Dongngan Truong, MD, University of Utah and Primary Children’s Hospital, both in Salt Lake City, who wasn’t part of the study.
Among its strengths, she said in an interview, are its linkage of comprehensive hospital and vaccination data sets for two different vaccines; and that it corroborates other research suggesting there is “something in particular about mRNA vaccination that seems to be associated with the development of myocarditis.”
Active surveillance in Israel
In an October 2021 report based on an Israeli Ministry of Health database covering up to May 2021, rates of myocarditis arising within 21 days of a second Pfizer-BioNTech dose in 16- to 19-year-olds reached about 1 per 6,637 males and 1 per 99,853 females. Those numbers compared with 1 per 26,000 males and 1 per 218,000 females across all age groups.
Now authors led by Dror Mevorach, MD, Hadassah Medical Center, Jerusalem, have published corresponding numbers from the same data base for myocarditis associated with the same vaccine in males and females aged 12-15.
Their research covers 404,407 people in that age group who received a first dose of the mRNA-based vaccine and 326,463 who received the second dose from June to October, 2021. Only 18 cases of myocarditis were observed within 21 days of either dose.
The estimated rates for males were 0.56 cases per 100,000 after a first dose and 8.09 cases per 100,000 after a second dose.
For females, the estimates were 0 cases per 100,000 after a first dose and 0.69 cases per 100,000 after a second dose.
“The pattern observed, mainly following the second vaccination in males, suggests causality,” the group wrote.
Leveraging passive surveillance reports
Another new report adds a twist to updated numbers from the U.S. Vaccine Adverse Event Reporting System (VAERS).
Prevalences derived from the passive-surveillance data base, known for including case records of inconsistent quality or completeness, are considered especially prone to reporting bias, the authors acknowledged.
The current analysis, however, plunges deep into VAERS-reported cases of presumed SARS-CoV-2 vaccine-associated myocarditis to help clarify “more of the characteristics of the patients and some of the treatments and short-term outcomes,” Matthew E. Oster, MD, MPH, said in an interview.
Dr. Oster, from the Centers for Disease Control and Prevention and Emory University, Atlanta, is lead author on the study’s Jan. 25, 2022, publication in JAMA.
The group reviewed charts and interviewed involved clinicians to adjudicate and document presentations, therapies, and the clinical course of cases reported as SARS-CoV-2 vaccine–associated myocarditis from December 2020 to August 2021. Out of the nearly 2000 reports, which were limited to patients younger than 30, the group identified 1,626 likely cases of such myocarditis arising within 7 days of a second mRNA vaccine dose.
The confirmed cases consistently represented higher prevalences than expected compared with prepandemic myocarditis claims data for both sexes and across age groups spanning 12-29 years.
For example, rates were highest for adolescent males – about 106 and 71 cases per million second doses of the Pfizer-BioNTech vaccine in those aged 16-17 and 12-16, respectively, for example. They were lowest for women aged 25-29, at 2.23 cases per million second Pfizer-BioNTech doses; the highest rate among females was about 11 per million for the 16-17 age group.
The observed rates, Dr. Oster said, represent an update to VAERS numbers published June 2021 in Morbidity and Mortality Weekly Report covering cases through June 2021.
“Overall, the general risk of having myocarditis from the vaccines is still extremely low. Even in the highest risk groups, it is still extremely low, and still lower than the risk of having cardiac complications from COVID,” he noted.
How do patients fare clinically?
From their chart reviews and interviews with case clinicians, Dr. Oster said, “we started to learn quickly that this is really a different type of myocarditis.”
For example, its onset, typically within a few days of the potential immunologic cause, was more rapid than in viral myocarditis, and its symptoms resolved faster, the report notes. Clinical presentations tended to be less severe, treatments not as intensive, and outcomes not as serious, compared with “the kind of typical viral myocarditis that most of the providers were used to taking care of in the past,” he said. “The pattern for these cases was very consistent.”
The study covered VAERS reports of suspected myocarditis arising within a week of first dose of a mRNA-based vaccine from the United States launch of public vaccination in December 2020 to August 2021, the CDC-based group reported. By then, more than 192 million people in the country had received either the Pfizer-BioNTech (age 12 or older) or Moderna (age 18 or older) vaccines.
Of the 1,991 reports of myocarditis, including 391 also involving pericarditis, 1,626 met the study’s definition for myocarditis on adjudication; about 82% of the latter cases were in males.
Based on the investigators’ review of charts and clinician interviews connected with 826 cases that met their definition of myocarditis in patients younger than 30, 89% reported “chest pain, pressure, or discomfort” and 30% reported dyspnea or shortness of breath. Troponin levels were elevated in 98%, 72% of patients who underwent electrocardiography showed abnormalities, and 12% of those with echocardiography had left ventricular ejection fractions less than 50%.
About 96% were hospitalized, and presenting symptoms resolved by discharge in 87% of those with available data, the group noted. Among patients with data on in-hospital therapy, they wrote, NSAIDs were the most common therapy, in 87%.
‘Mild and self-limiting’
The case-control study from Hong Kong didn’t specifically examine patients’ treatment and clinical course, but it does portray their vaccine-associated myocarditis as contrasting with more familiar viral myocarditis.
Patients with “typical” myocarditis tend to be “overall much sicker than what we’re seeing with myocarditis following vaccination,” Dr. Truong agreed. None of the 20 patients with myocarditis after Pfizer-BioNTech vaccination in Hong Kong were admitted to the intensive care unit. That, she added, suggests none required extracorporeal membrane oxygenation or vasoactive support, often necessary in viral myocarditis. “And they had shorter hospital stays.”
In contrast, Dr. Wong noted, 14 of the study’s unvaccinated patients required ICU admission; 12 of them died during the follow-up period. None with vaccine-related carditis died during the study’s follow-up. “We also showed that cases following [Pfizer-BioNTech] vaccination were all mild and self-limiting.”
Dr. Truong largely agreed that SARS-CoV-2 vaccine myocarditis and most myocarditis seen before the pandemic can be viewed as distinct clinical entities, “at least in the short term. I think we do need to follow these patients to look at more long-term outcomes, because at this point I don’t think we know the long-term implications. But at least in the short term, it seems like these patients are different, are much less sick, and recover pretty quickly overall.”
Dr. Oster emphasized that the many and varied acute and long-term hazards from contracting COVID-19 far outweigh any risk for myocarditis from vaccination. But for individuals who were hit with myocarditis soon after their first mRNA vaccine dose, who have already established their susceptibility, he and his colleagues would recommend that they “consider alternatives and not get the vaccine again.”
Dr. Oster reported no relevant financial relationships. Dr. Wong and colleagues did not report any relevant disclosures. Dr. Truong has previously disclosed serving as a consultant to Pfizer.
A version of this article first appeared on Medscape.com.
Why some COVID-19 vaccines seem occasionally to cause a distinctive form of myocarditis, and why adolescent boys and young men appear most vulnerable, remain a mystery. But the entity’s prevalence, nuances of presentation, and likely clinical course have come into sharper view after recent additions to the literature.
Two new publications all but confirm that the rare cases of myocarditis closely following vaccination against SARS-CoV-2, primarily with one of the mRNA-based vaccines from Pfizer-BioNTech and Moderna, is a clinically different creature from myocarditis physicians were likely to see before the pandemic.
A third report unveils rates of hospitalization for myocarditis linked to Pfizer-BioNTech vaccination in the 12- to 15-year age group, based on active surveillance across Israel. Of note, the rates were lower than corresponding numbers among the country’s 16- to 19-year-olds published in late 2021 by the same authors.
No link with CoronaVac
A case-control study covering almost the entire population of Hong Kong from February to August 2021 confirms a slight but significant excess risk for myocarditis and, to a lesser degree, pericarditis, after injections of the Pfizer-BioNTech vaccine. As consistently reported from other studies, the risks were highest in adolescent and young adult males and after a second dose.
The study estimated an overall carditis incidence of 5.7 cases per million doses of Pfizer-BioNTech, for a risk 3.5 times that in the unvaccinated Hong Kong population. Carditis rates after a first dose were about 2.5 per million and 10 per million after a second dose.
Hong Kong launched its public SARS-CoV-2 immunization program in late February 2021 with the Chinese-made CoronaVac (Sinovac) inactivated-virus vaccine, and introduced the mRNA-based alternative several weeks later. By August 2021, the vaccines had reached about 3.3 million people in the region – 49% of the Hong Kong population at least 12 years of age.
In a novel finding, there were no excesses in carditis cases after CoronaVac vaccination. The difference between vaccines likely isn’t caused by chance, because three-fourths of the carditis-associated Pfizer-BioNTech injections arose within a week, whereas “71% of cases following the use of CoronaVac occurred more than 30 days after vaccination,” senior author Ian Chi Kei Wong, PhD, University of Hong Kong, said in an interview.
“This onset distribution for cases having received CoronaVac demonstrates that it is highly unlikely the carditis cases are related to the vaccine,” he said. And that “plausibly implies a specific underlying mechanism between vaccination and carditis that may only be applicable to mRNA vaccines.”
That inference is in line with case reports and other research, including large population-based studies from Israel and Denmark, although a recent study from the United Kingdom hinted at a potential excess myocarditis risk associated with the adenovirus-based AstraZeneca-Oxford vaccine.
The Hong Kong study identified 160 patients age 12 or older with a first diagnosis of carditis during February to August 2021, in electronic health records covering nearly the entire region.
“We used laboratory test results of troponin levels to further eliminate unlikely cases of carditis,” Dr. Wong said. The health records were linked to a “population-based vaccination record” maintained by the government’s department of health.
About 10 control patients from among all hospitalized patients without carditis were matched by age, sex, and admission date to each of the 160 carditis cases. About 83% of cases and 92% of the controls were unvaccinated.
Among those who received the Pfizer-BioNTech vaccine, representing 12.5% of cases and 4.2% of controls, the estimated carditis incidence was 0.57 per 100,000 doses. For those who received CoronaVac, representing 4.4% of cases and 3.9% of controls, it was 0.31 per 100,000 doses.
In adjusted analysis, the odds ratios for carditis among Pfizer-BioNTech vaccine recipients, compared with unvaccinated controls, were 3.57 (95% confidence interval, 1.93-6.60) overall, 4.68 (95% CI, 2.25-9.71) for males, 2.22 (95% CI, 0.57-8.69) for females, 2.41 (95% CI, 1.18-4.90) for ages 18 and older, and 13.8 (95% CI, 2.86-110.4) for ages 12-17
Myocarditis accounted for most of the excess cases, with an overall OR of 9.29 (95% CI, 3.94-21.9). The OR reached only 1.06 (95% CI, 0.35-3.22) for pericarditis alone.
The case-control study is noteworthy for its design, which contrasts with the many recent case series and passive or active surveillance studies, and even the more robust population-based studies of vaccine-related myocarditis, observed Dongngan Truong, MD, University of Utah and Primary Children’s Hospital, both in Salt Lake City, who wasn’t part of the study.
Among its strengths, she said in an interview, are its linkage of comprehensive hospital and vaccination data sets for two different vaccines; and that it corroborates other research suggesting there is “something in particular about mRNA vaccination that seems to be associated with the development of myocarditis.”
Active surveillance in Israel
In an October 2021 report based on an Israeli Ministry of Health database covering up to May 2021, rates of myocarditis arising within 21 days of a second Pfizer-BioNTech dose in 16- to 19-year-olds reached about 1 per 6,637 males and 1 per 99,853 females. Those numbers compared with 1 per 26,000 males and 1 per 218,000 females across all age groups.
Now authors led by Dror Mevorach, MD, Hadassah Medical Center, Jerusalem, have published corresponding numbers from the same data base for myocarditis associated with the same vaccine in males and females aged 12-15.
Their research covers 404,407 people in that age group who received a first dose of the mRNA-based vaccine and 326,463 who received the second dose from June to October, 2021. Only 18 cases of myocarditis were observed within 21 days of either dose.
The estimated rates for males were 0.56 cases per 100,000 after a first dose and 8.09 cases per 100,000 after a second dose.
For females, the estimates were 0 cases per 100,000 after a first dose and 0.69 cases per 100,000 after a second dose.
“The pattern observed, mainly following the second vaccination in males, suggests causality,” the group wrote.
Leveraging passive surveillance reports
Another new report adds a twist to updated numbers from the U.S. Vaccine Adverse Event Reporting System (VAERS).
Prevalences derived from the passive-surveillance data base, known for including case records of inconsistent quality or completeness, are considered especially prone to reporting bias, the authors acknowledged.
The current analysis, however, plunges deep into VAERS-reported cases of presumed SARS-CoV-2 vaccine-associated myocarditis to help clarify “more of the characteristics of the patients and some of the treatments and short-term outcomes,” Matthew E. Oster, MD, MPH, said in an interview.
Dr. Oster, from the Centers for Disease Control and Prevention and Emory University, Atlanta, is lead author on the study’s Jan. 25, 2022, publication in JAMA.
The group reviewed charts and interviewed involved clinicians to adjudicate and document presentations, therapies, and the clinical course of cases reported as SARS-CoV-2 vaccine–associated myocarditis from December 2020 to August 2021. Out of the nearly 2000 reports, which were limited to patients younger than 30, the group identified 1,626 likely cases of such myocarditis arising within 7 days of a second mRNA vaccine dose.
The confirmed cases consistently represented higher prevalences than expected compared with prepandemic myocarditis claims data for both sexes and across age groups spanning 12-29 years.
For example, rates were highest for adolescent males – about 106 and 71 cases per million second doses of the Pfizer-BioNTech vaccine in those aged 16-17 and 12-16, respectively, for example. They were lowest for women aged 25-29, at 2.23 cases per million second Pfizer-BioNTech doses; the highest rate among females was about 11 per million for the 16-17 age group.
The observed rates, Dr. Oster said, represent an update to VAERS numbers published June 2021 in Morbidity and Mortality Weekly Report covering cases through June 2021.
“Overall, the general risk of having myocarditis from the vaccines is still extremely low. Even in the highest risk groups, it is still extremely low, and still lower than the risk of having cardiac complications from COVID,” he noted.
How do patients fare clinically?
From their chart reviews and interviews with case clinicians, Dr. Oster said, “we started to learn quickly that this is really a different type of myocarditis.”
For example, its onset, typically within a few days of the potential immunologic cause, was more rapid than in viral myocarditis, and its symptoms resolved faster, the report notes. Clinical presentations tended to be less severe, treatments not as intensive, and outcomes not as serious, compared with “the kind of typical viral myocarditis that most of the providers were used to taking care of in the past,” he said. “The pattern for these cases was very consistent.”
The study covered VAERS reports of suspected myocarditis arising within a week of first dose of a mRNA-based vaccine from the United States launch of public vaccination in December 2020 to August 2021, the CDC-based group reported. By then, more than 192 million people in the country had received either the Pfizer-BioNTech (age 12 or older) or Moderna (age 18 or older) vaccines.
Of the 1,991 reports of myocarditis, including 391 also involving pericarditis, 1,626 met the study’s definition for myocarditis on adjudication; about 82% of the latter cases were in males.
Based on the investigators’ review of charts and clinician interviews connected with 826 cases that met their definition of myocarditis in patients younger than 30, 89% reported “chest pain, pressure, or discomfort” and 30% reported dyspnea or shortness of breath. Troponin levels were elevated in 98%, 72% of patients who underwent electrocardiography showed abnormalities, and 12% of those with echocardiography had left ventricular ejection fractions less than 50%.
About 96% were hospitalized, and presenting symptoms resolved by discharge in 87% of those with available data, the group noted. Among patients with data on in-hospital therapy, they wrote, NSAIDs were the most common therapy, in 87%.
‘Mild and self-limiting’
The case-control study from Hong Kong didn’t specifically examine patients’ treatment and clinical course, but it does portray their vaccine-associated myocarditis as contrasting with more familiar viral myocarditis.
Patients with “typical” myocarditis tend to be “overall much sicker than what we’re seeing with myocarditis following vaccination,” Dr. Truong agreed. None of the 20 patients with myocarditis after Pfizer-BioNTech vaccination in Hong Kong were admitted to the intensive care unit. That, she added, suggests none required extracorporeal membrane oxygenation or vasoactive support, often necessary in viral myocarditis. “And they had shorter hospital stays.”
In contrast, Dr. Wong noted, 14 of the study’s unvaccinated patients required ICU admission; 12 of them died during the follow-up period. None with vaccine-related carditis died during the study’s follow-up. “We also showed that cases following [Pfizer-BioNTech] vaccination were all mild and self-limiting.”
Dr. Truong largely agreed that SARS-CoV-2 vaccine myocarditis and most myocarditis seen before the pandemic can be viewed as distinct clinical entities, “at least in the short term. I think we do need to follow these patients to look at more long-term outcomes, because at this point I don’t think we know the long-term implications. But at least in the short term, it seems like these patients are different, are much less sick, and recover pretty quickly overall.”
Dr. Oster emphasized that the many and varied acute and long-term hazards from contracting COVID-19 far outweigh any risk for myocarditis from vaccination. But for individuals who were hit with myocarditis soon after their first mRNA vaccine dose, who have already established their susceptibility, he and his colleagues would recommend that they “consider alternatives and not get the vaccine again.”
Dr. Oster reported no relevant financial relationships. Dr. Wong and colleagues did not report any relevant disclosures. Dr. Truong has previously disclosed serving as a consultant to Pfizer.
A version of this article first appeared on Medscape.com.










