User login
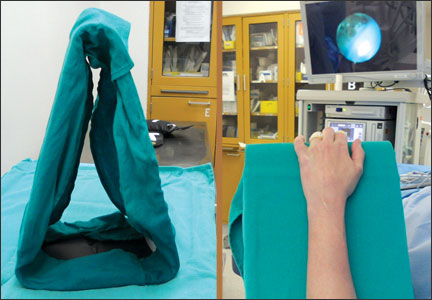
A Simple Wrist Arthroscopy Tower: The Wrist Triangle
Antirheumatic drugs don’t boost surgical infection risk
SAN DIEGO – Rheumatoid arthritis patients undergoing surgery who stayed on their antirheumatic medication perioperatively didn’t have a higher risk of early postoperative infection compared with those who temporarily stopped treatment before surgery, according to findings from a large national Veterans Affairs study.
Rheumatologists are frequently consulted about this issue. Evidence to guide practice has been scarce, however, and until now many rheumatologists and surgeons have taken a conservative approach, reasoning that the immunosuppressive drugs employed in controlling inflammation in rheumatoid arthritis might also increase the risk of surgical wound infection.
A common practice has been to have RA patients stop their medication a month ahead of elective surgery, or at least two drug half-lives beforehand, then start treatment again roughly a month after the operation, or when the wound has healed. The new Veterans Affairs (VA) study findings suggest this practice may be unnecessary, Dr. Zaki Abou Zahr said at the annual meeting of the American College of Rheumatology.
Dr. Bernard Ng, his senior coinvestigator in the study, added that temporarily stopping antirheumatic agents before surgery may actually be harmful in that it increases the risk of a flare of the RA, which in turn would impede postoperative rehabilitation.
But there is a major caveat regarding the VA study: Participation was restricted to RA patients on only a single conventional disease-modifying antirheumatic drug (DMARD) or biologic agent leading up to surgery. This restriction, imposed to make for a more clear-cut analysis, means that the study results can’t be extrapolated to patients on multidrug therapy. And multidrug therapy is quite common. Indeed, slightly more than half of RA patients in the VA health care system are on combination therapy, most often methotrexate plus a biologic agent, noted Dr. Ng, chief of rheumatology at the VA Puget Sound Health Care System, Seattle.
Dr. Abou Zahr presented the retrospective cohort study involving 6,548 RA patients in VA administrative databases, all of whom were on antirheumatic drug monotherapy prior to surgery. The surgery was of all types, including cardiothoracic, gastrointestinal, vascular, and orthopedic, as well as emergent and elective.
The primary endpoints were the rate of wound infections, both superficial and deep, within 30 days post surgery, and the general infection rate – including pneumonia, sepsis, and urinary tract infections – during the same time frame.
Sixty-two percent of the 1,480 RA patients on a single biologic agent did not stop taking it preoperatively. One key study finding was that neither their postoperative wound infection rate nor their general infection rate differed significantly from rates in patients who temporarily halted their biologic agent. The same held true among the 70% of patients on a single conventional DMARD who did not stop taking their medication preoperatively, according to Dr. Abou Zahr of Baylor College of Medicine, Houston.
Dr. Ng said the investigators plan to extend their work to include RA patients on multiple antirheumatic drugs that they do or don’t temporarily stop when undergoing surgery within the VA system. The researchers also plan to take a close look at patients undergoing specific types of surgery to see if the postoperative infection risk in patients who remain on treatment varies according to their operation.
Dr. Fehmida Zahabi, a rheumatologist from Plano, Tex., who chaired a press conference highlighting the VA study findings, said that while she’d like to see a confirmatory study, "I think we’re getting to the point where we’re saying we should cautiously keep these patients on their medications. That’s what the data suggest."
She noted that before the VA study, the very limited evidence available to guide practice in this area centered on a 12-year-old British randomized trial involving RA patients on methotrexate undergoing elective orthopedic surgery. Those assigned to stop the drug from 2 weeks before surgery to 2 weeks post surgery had significantly more infections, surgical complications, and RA flares within 6 weeks after surgery (Ann. Rheum. Dis. 2001;60:214-7).
As for patients on multidrug therapy who are scheduled for surgery, her inclination until evidence becomes available for guidance is to pare down the regimen preoperatively, while keeping the patient on one or two drugs.
The VA study was funded by the Department of Veterans Affairs. Dr. Abou Zahr and Dr. Ng reported having no conflicts of interest.
SAN DIEGO – Rheumatoid arthritis patients undergoing surgery who stayed on their antirheumatic medication perioperatively didn’t have a higher risk of early postoperative infection compared with those who temporarily stopped treatment before surgery, according to findings from a large national Veterans Affairs study.
Rheumatologists are frequently consulted about this issue. Evidence to guide practice has been scarce, however, and until now many rheumatologists and surgeons have taken a conservative approach, reasoning that the immunosuppressive drugs employed in controlling inflammation in rheumatoid arthritis might also increase the risk of surgical wound infection.
A common practice has been to have RA patients stop their medication a month ahead of elective surgery, or at least two drug half-lives beforehand, then start treatment again roughly a month after the operation, or when the wound has healed. The new Veterans Affairs (VA) study findings suggest this practice may be unnecessary, Dr. Zaki Abou Zahr said at the annual meeting of the American College of Rheumatology.
Dr. Bernard Ng, his senior coinvestigator in the study, added that temporarily stopping antirheumatic agents before surgery may actually be harmful in that it increases the risk of a flare of the RA, which in turn would impede postoperative rehabilitation.
But there is a major caveat regarding the VA study: Participation was restricted to RA patients on only a single conventional disease-modifying antirheumatic drug (DMARD) or biologic agent leading up to surgery. This restriction, imposed to make for a more clear-cut analysis, means that the study results can’t be extrapolated to patients on multidrug therapy. And multidrug therapy is quite common. Indeed, slightly more than half of RA patients in the VA health care system are on combination therapy, most often methotrexate plus a biologic agent, noted Dr. Ng, chief of rheumatology at the VA Puget Sound Health Care System, Seattle.
Dr. Abou Zahr presented the retrospective cohort study involving 6,548 RA patients in VA administrative databases, all of whom were on antirheumatic drug monotherapy prior to surgery. The surgery was of all types, including cardiothoracic, gastrointestinal, vascular, and orthopedic, as well as emergent and elective.
The primary endpoints were the rate of wound infections, both superficial and deep, within 30 days post surgery, and the general infection rate – including pneumonia, sepsis, and urinary tract infections – during the same time frame.
Sixty-two percent of the 1,480 RA patients on a single biologic agent did not stop taking it preoperatively. One key study finding was that neither their postoperative wound infection rate nor their general infection rate differed significantly from rates in patients who temporarily halted their biologic agent. The same held true among the 70% of patients on a single conventional DMARD who did not stop taking their medication preoperatively, according to Dr. Abou Zahr of Baylor College of Medicine, Houston.
Dr. Ng said the investigators plan to extend their work to include RA patients on multiple antirheumatic drugs that they do or don’t temporarily stop when undergoing surgery within the VA system. The researchers also plan to take a close look at patients undergoing specific types of surgery to see if the postoperative infection risk in patients who remain on treatment varies according to their operation.
Dr. Fehmida Zahabi, a rheumatologist from Plano, Tex., who chaired a press conference highlighting the VA study findings, said that while she’d like to see a confirmatory study, "I think we’re getting to the point where we’re saying we should cautiously keep these patients on their medications. That’s what the data suggest."
She noted that before the VA study, the very limited evidence available to guide practice in this area centered on a 12-year-old British randomized trial involving RA patients on methotrexate undergoing elective orthopedic surgery. Those assigned to stop the drug from 2 weeks before surgery to 2 weeks post surgery had significantly more infections, surgical complications, and RA flares within 6 weeks after surgery (Ann. Rheum. Dis. 2001;60:214-7).
As for patients on multidrug therapy who are scheduled for surgery, her inclination until evidence becomes available for guidance is to pare down the regimen preoperatively, while keeping the patient on one or two drugs.
The VA study was funded by the Department of Veterans Affairs. Dr. Abou Zahr and Dr. Ng reported having no conflicts of interest.
SAN DIEGO – Rheumatoid arthritis patients undergoing surgery who stayed on their antirheumatic medication perioperatively didn’t have a higher risk of early postoperative infection compared with those who temporarily stopped treatment before surgery, according to findings from a large national Veterans Affairs study.
Rheumatologists are frequently consulted about this issue. Evidence to guide practice has been scarce, however, and until now many rheumatologists and surgeons have taken a conservative approach, reasoning that the immunosuppressive drugs employed in controlling inflammation in rheumatoid arthritis might also increase the risk of surgical wound infection.
A common practice has been to have RA patients stop their medication a month ahead of elective surgery, or at least two drug half-lives beforehand, then start treatment again roughly a month after the operation, or when the wound has healed. The new Veterans Affairs (VA) study findings suggest this practice may be unnecessary, Dr. Zaki Abou Zahr said at the annual meeting of the American College of Rheumatology.
Dr. Bernard Ng, his senior coinvestigator in the study, added that temporarily stopping antirheumatic agents before surgery may actually be harmful in that it increases the risk of a flare of the RA, which in turn would impede postoperative rehabilitation.
But there is a major caveat regarding the VA study: Participation was restricted to RA patients on only a single conventional disease-modifying antirheumatic drug (DMARD) or biologic agent leading up to surgery. This restriction, imposed to make for a more clear-cut analysis, means that the study results can’t be extrapolated to patients on multidrug therapy. And multidrug therapy is quite common. Indeed, slightly more than half of RA patients in the VA health care system are on combination therapy, most often methotrexate plus a biologic agent, noted Dr. Ng, chief of rheumatology at the VA Puget Sound Health Care System, Seattle.
Dr. Abou Zahr presented the retrospective cohort study involving 6,548 RA patients in VA administrative databases, all of whom were on antirheumatic drug monotherapy prior to surgery. The surgery was of all types, including cardiothoracic, gastrointestinal, vascular, and orthopedic, as well as emergent and elective.
The primary endpoints were the rate of wound infections, both superficial and deep, within 30 days post surgery, and the general infection rate – including pneumonia, sepsis, and urinary tract infections – during the same time frame.
Sixty-two percent of the 1,480 RA patients on a single biologic agent did not stop taking it preoperatively. One key study finding was that neither their postoperative wound infection rate nor their general infection rate differed significantly from rates in patients who temporarily halted their biologic agent. The same held true among the 70% of patients on a single conventional DMARD who did not stop taking their medication preoperatively, according to Dr. Abou Zahr of Baylor College of Medicine, Houston.
Dr. Ng said the investigators plan to extend their work to include RA patients on multiple antirheumatic drugs that they do or don’t temporarily stop when undergoing surgery within the VA system. The researchers also plan to take a close look at patients undergoing specific types of surgery to see if the postoperative infection risk in patients who remain on treatment varies according to their operation.
Dr. Fehmida Zahabi, a rheumatologist from Plano, Tex., who chaired a press conference highlighting the VA study findings, said that while she’d like to see a confirmatory study, "I think we’re getting to the point where we’re saying we should cautiously keep these patients on their medications. That’s what the data suggest."
She noted that before the VA study, the very limited evidence available to guide practice in this area centered on a 12-year-old British randomized trial involving RA patients on methotrexate undergoing elective orthopedic surgery. Those assigned to stop the drug from 2 weeks before surgery to 2 weeks post surgery had significantly more infections, surgical complications, and RA flares within 6 weeks after surgery (Ann. Rheum. Dis. 2001;60:214-7).
As for patients on multidrug therapy who are scheduled for surgery, her inclination until evidence becomes available for guidance is to pare down the regimen preoperatively, while keeping the patient on one or two drugs.
The VA study was funded by the Department of Veterans Affairs. Dr. Abou Zahr and Dr. Ng reported having no conflicts of interest.
AT THE ACR ANNUAL MEETING
Major finding: Rheumatoid arthritis patients who remained on their antirheumatic medication while they underwent various types of surgery did not have a significantly different 30-day wound infection rate than those who stopped treatment temporarily prior to surgery.
Data source: This was a retrospective observational cohort study involving 6,548 rheumatoid arthritis patients undergoing various types of surgery.
Disclosures: The study was funded by the Department of Veterans Affairs. The presenters reported having no financial conflicts.
Orthopaedic surgeons’ ‘Choosing Wisely’ list centers on osteoarthritis treatments
Three of the five recommendations in the first-ever list developed by the American Academy of Orthopaedic Surgeons for the American Board of Internal Medicine Foundation’s "Choosing Wisely" campaign focus primarily on treatments for symptomatic osteoarthritis.
The campaign is meant to educate patients and physicians about unnecessary and potentially harmful testing and treatment.
According to the Choosing Wisely website, the AAOS’s list was formed based on a review of the most recent approved clinical practice guidelines previously developed by AAOS physician volunteer work groups and a selection of a variety of topics frequently used in orthopaedic surgical practice with input from specialty society leaders and the Academy’s presidential leadership and board of directors. The list was created with the intent to "serve as an educational tool based on an assessment of the current scientific and clinical information and accepted approaches to treatment."
However, some specialists find fault with the recommendations. For example, Dr. Roy Altman, a professor of medicine in the division of rheumatology and immunology at the University of California, Los Angeles, said the methodology used to create the guidelines overlooks a number of treatments, including multimodal therapy, and could have the unintended consequence of allowing specialists to deny effective care to patients.
"These guidelines are not consistent with my clinical experience," Dr. Altman said. In particular, he noted that many of his patients react positively to injection treatments for osteoarthritis (OA), which the Choosing Wisely recommendations specifically discourage.
AAOS’s recommendations are as follows:
• Avoid using postoperative ultrasonography screening for deep vein thrombosis on patients receiving hip or knee arthroplasty because it is not effective at diagnosing unsuspected cases.
• Don’t use needle lavage for long-term relief in symptomatic OA treatment, as the procedure "does not lead to measurable improvements in pain, function, 50-foot walking time, stiffness, tenderness, or swelling."
• Do not use glucosamine and chondroitin sulfate to treat patients with symptomatic knee OA.
• Lateral wedge or neutral insoles do not improve pain or functional outcomes in patients; on the contrary, patients with OA of the knee may experience fewer symptoms without insoles.
• Routine postoperative splinting of the wrist after the carpal tunnel release procedure does not improve subjective outcomes, and may lead to detrimental effects, including adhesion formation, stiffness, and prevention of nerve and tendon movement.
Three of the five recommendations in the first-ever list developed by the American Academy of Orthopaedic Surgeons for the American Board of Internal Medicine Foundation’s "Choosing Wisely" campaign focus primarily on treatments for symptomatic osteoarthritis.
The campaign is meant to educate patients and physicians about unnecessary and potentially harmful testing and treatment.
According to the Choosing Wisely website, the AAOS’s list was formed based on a review of the most recent approved clinical practice guidelines previously developed by AAOS physician volunteer work groups and a selection of a variety of topics frequently used in orthopaedic surgical practice with input from specialty society leaders and the Academy’s presidential leadership and board of directors. The list was created with the intent to "serve as an educational tool based on an assessment of the current scientific and clinical information and accepted approaches to treatment."
However, some specialists find fault with the recommendations. For example, Dr. Roy Altman, a professor of medicine in the division of rheumatology and immunology at the University of California, Los Angeles, said the methodology used to create the guidelines overlooks a number of treatments, including multimodal therapy, and could have the unintended consequence of allowing specialists to deny effective care to patients.
"These guidelines are not consistent with my clinical experience," Dr. Altman said. In particular, he noted that many of his patients react positively to injection treatments for osteoarthritis (OA), which the Choosing Wisely recommendations specifically discourage.
AAOS’s recommendations are as follows:
• Avoid using postoperative ultrasonography screening for deep vein thrombosis on patients receiving hip or knee arthroplasty because it is not effective at diagnosing unsuspected cases.
• Don’t use needle lavage for long-term relief in symptomatic OA treatment, as the procedure "does not lead to measurable improvements in pain, function, 50-foot walking time, stiffness, tenderness, or swelling."
• Do not use glucosamine and chondroitin sulfate to treat patients with symptomatic knee OA.
• Lateral wedge or neutral insoles do not improve pain or functional outcomes in patients; on the contrary, patients with OA of the knee may experience fewer symptoms without insoles.
• Routine postoperative splinting of the wrist after the carpal tunnel release procedure does not improve subjective outcomes, and may lead to detrimental effects, including adhesion formation, stiffness, and prevention of nerve and tendon movement.
Three of the five recommendations in the first-ever list developed by the American Academy of Orthopaedic Surgeons for the American Board of Internal Medicine Foundation’s "Choosing Wisely" campaign focus primarily on treatments for symptomatic osteoarthritis.
The campaign is meant to educate patients and physicians about unnecessary and potentially harmful testing and treatment.
According to the Choosing Wisely website, the AAOS’s list was formed based on a review of the most recent approved clinical practice guidelines previously developed by AAOS physician volunteer work groups and a selection of a variety of topics frequently used in orthopaedic surgical practice with input from specialty society leaders and the Academy’s presidential leadership and board of directors. The list was created with the intent to "serve as an educational tool based on an assessment of the current scientific and clinical information and accepted approaches to treatment."
However, some specialists find fault with the recommendations. For example, Dr. Roy Altman, a professor of medicine in the division of rheumatology and immunology at the University of California, Los Angeles, said the methodology used to create the guidelines overlooks a number of treatments, including multimodal therapy, and could have the unintended consequence of allowing specialists to deny effective care to patients.
"These guidelines are not consistent with my clinical experience," Dr. Altman said. In particular, he noted that many of his patients react positively to injection treatments for osteoarthritis (OA), which the Choosing Wisely recommendations specifically discourage.
AAOS’s recommendations are as follows:
• Avoid using postoperative ultrasonography screening for deep vein thrombosis on patients receiving hip or knee arthroplasty because it is not effective at diagnosing unsuspected cases.
• Don’t use needle lavage for long-term relief in symptomatic OA treatment, as the procedure "does not lead to measurable improvements in pain, function, 50-foot walking time, stiffness, tenderness, or swelling."
• Do not use glucosamine and chondroitin sulfate to treat patients with symptomatic knee OA.
• Lateral wedge or neutral insoles do not improve pain or functional outcomes in patients; on the contrary, patients with OA of the knee may experience fewer symptoms without insoles.
• Routine postoperative splinting of the wrist after the carpal tunnel release procedure does not improve subjective outcomes, and may lead to detrimental effects, including adhesion formation, stiffness, and prevention of nerve and tendon movement.
Avoiding Unplanned Resections of Wrist Sarcomas: An Algorithm for Evaluating Dorsal Wrist Masses
Pancarpal Synovial and Tenosynovial Chondromatosis in a 65-Year-Old Man: A Highly Unusual Presentation of a Common Condition
The Pros and Cons of Using Larger Femoral Heads in Total Hip Arthroplasty
High-Pressure Paint Gun Injection Injury to the Palm
Cartilage Defect of Lunate Facet of Distal Radius After Fracture Treated With Osteochondral Autograft From Knee
Type IIb Bony Mallet Finger: Is Anatomical Reduction of the Fracture Necessary?
FDA approves denosumab for giant cell tumors
Denosumab is approved for the treatment of giant cell tumors of the bone, the Food and Drug Administration announced June 13.
Rare and usually noncancerous, giant cell tumors of the bone generally affect 20- to 40-year-olds. Most of these tumors destroy growing bone, causing pain, limited range of motion, and bone fractures. Rarely, the tumors become cancerous and spread to the lungs.
Denosumab (Xgeva) is indicated for use in patients who are not candidates for surgical resection of their tumors or when surgery is likely to result in severe morbidity, such as loss of limbs or joint removal. It should be used only in adolescents whose bones have matured, the FDA said in a statement.
"Today’s approval of Xgeva provides a needed treatment option for patients with GCTB who are not surgical candidates or who would otherwise have to undergo extensive, life-altering surgery," said Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research.
The FDA reviewed denosumab under its priority review program as the drug was granted orphan product designation because GCTB is a rare disease.
The safety and effectiveness of denosumab for GCTB were established in two clinical trials that enrolled 305 adult or adolescent patients with confirmed GCTB that was recurrent, unresectable, or would be associated with severe morbidity if surgically managed.
After an average of 3 months, tumors reduced in size among 47 of 187 patients whose tumors could be measured. Over an average follow-up of 20 months, GCTBs regrew in three patients whose tumors originally became smaller during treatment.
Common side effects of denosumab included joint pain, headache, nausea, fatigue, back pain, and extremity pain. The most common serious side effects were osteonecrosis of the jaw and osteomyelitis. Women of reproductive potential should use highly effective contraception while taking denosumab because of potential fetal harm, according to the FDA.
Denosumab was approved in 2010 to prevent fractures when cancer has spread to the bones. It is marketed by Amgen.
On Twitter @maryjodales
Denosumab is approved for the treatment of giant cell tumors of the bone, the Food and Drug Administration announced June 13.
Rare and usually noncancerous, giant cell tumors of the bone generally affect 20- to 40-year-olds. Most of these tumors destroy growing bone, causing pain, limited range of motion, and bone fractures. Rarely, the tumors become cancerous and spread to the lungs.
Denosumab (Xgeva) is indicated for use in patients who are not candidates for surgical resection of their tumors or when surgery is likely to result in severe morbidity, such as loss of limbs or joint removal. It should be used only in adolescents whose bones have matured, the FDA said in a statement.
"Today’s approval of Xgeva provides a needed treatment option for patients with GCTB who are not surgical candidates or who would otherwise have to undergo extensive, life-altering surgery," said Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research.
The FDA reviewed denosumab under its priority review program as the drug was granted orphan product designation because GCTB is a rare disease.
The safety and effectiveness of denosumab for GCTB were established in two clinical trials that enrolled 305 adult or adolescent patients with confirmed GCTB that was recurrent, unresectable, or would be associated with severe morbidity if surgically managed.
After an average of 3 months, tumors reduced in size among 47 of 187 patients whose tumors could be measured. Over an average follow-up of 20 months, GCTBs regrew in three patients whose tumors originally became smaller during treatment.
Common side effects of denosumab included joint pain, headache, nausea, fatigue, back pain, and extremity pain. The most common serious side effects were osteonecrosis of the jaw and osteomyelitis. Women of reproductive potential should use highly effective contraception while taking denosumab because of potential fetal harm, according to the FDA.
Denosumab was approved in 2010 to prevent fractures when cancer has spread to the bones. It is marketed by Amgen.
On Twitter @maryjodales
Denosumab is approved for the treatment of giant cell tumors of the bone, the Food and Drug Administration announced June 13.
Rare and usually noncancerous, giant cell tumors of the bone generally affect 20- to 40-year-olds. Most of these tumors destroy growing bone, causing pain, limited range of motion, and bone fractures. Rarely, the tumors become cancerous and spread to the lungs.
Denosumab (Xgeva) is indicated for use in patients who are not candidates for surgical resection of their tumors or when surgery is likely to result in severe morbidity, such as loss of limbs or joint removal. It should be used only in adolescents whose bones have matured, the FDA said in a statement.
"Today’s approval of Xgeva provides a needed treatment option for patients with GCTB who are not surgical candidates or who would otherwise have to undergo extensive, life-altering surgery," said Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research.
The FDA reviewed denosumab under its priority review program as the drug was granted orphan product designation because GCTB is a rare disease.
The safety and effectiveness of denosumab for GCTB were established in two clinical trials that enrolled 305 adult or adolescent patients with confirmed GCTB that was recurrent, unresectable, or would be associated with severe morbidity if surgically managed.
After an average of 3 months, tumors reduced in size among 47 of 187 patients whose tumors could be measured. Over an average follow-up of 20 months, GCTBs regrew in three patients whose tumors originally became smaller during treatment.
Common side effects of denosumab included joint pain, headache, nausea, fatigue, back pain, and extremity pain. The most common serious side effects were osteonecrosis of the jaw and osteomyelitis. Women of reproductive potential should use highly effective contraception while taking denosumab because of potential fetal harm, according to the FDA.
Denosumab was approved in 2010 to prevent fractures when cancer has spread to the bones. It is marketed by Amgen.
On Twitter @maryjodales