User login
Unstable Dorsal Proximal Interphalangeal Joint Fracture-Dislocations Treated With Extension-Block Pinning
The proximal interphalangeal (PIP) joint plays a crucial role in hand function, accounting for an estimated 85% of the motion required to grasp an object.1 The anatomy and biomechanics of the PIP joint, however, make it particularly prone to injury.2,3 Dorsal PIP fracture-dislocations represent a subset of PIP injuries that often require surgical intervention.2 The stability of these fracture-dislocations largely depends on the extent of articular involvement of the base of the middle phalanx. Fractures that involve less than 30% of the joint surface typically remain stable after reduction.2,4,5 In cases in which involvement ranges from 30% to 50%, PIP joint stability is more tenuous, and more joint flexion is required to maintain concentric reduction. Fractures that involve more than 50% of the articular surface are unstable and require operative intervention.2,5,6 Fractures that require more than 30° of flexion for reduction maintenance are generally considered unstable and may benefit from surgical intervention.2
The goals of treatment for this injury are to restore a stable, concentrically reduced joint and initiate early joint mobilization to prevent stiffness, pain, recurrent instability, and posttraumatic arthritis.3,7 Numerous surgical interventions for unstable PIP fracture-dislocations have been proposed, including open reduction and internal fixation (ORIF),8-10 extension-block pinning (EBP),11-13 dynamic external fixation,14-17 volar plate arthroplasty,18,19 and hemi-hamate resurfacing arthroplasty.20,21 Many of these techniques can be technically demanding and may require prolonged immobilization. EBP can be performed easily and efficiently and allows for early joint motion.
Extension-block pinning—placing a Kirschner wire (K-wire) into the head of the proximal phalanx at an angle that blocks PIP extension and prevents joint subluxation—was first described by Sugawa and colleagues12 in 1979. In a study by Inoue and Tamura,11 patients treated with EBP had a mean PIP range of motion (ROM) of 94° at a mean follow-up of 14 months. In a series of 3 case reports, Viegas22 noted an inverse relationship between extent of articular surface involvement and postoperative ROM in patients treated with EBP.
We conducted a study to expand on previous research on pain, function, and satisfaction outcomes in addition to ROM. We hypothesized that percutaneous EBP is an effective treatment for unstable dorsal PIP fracture-dislocations and has efficacy similar to that of more complex and technically demanding methods of treatment.
Materials and Methods
We retrospectively reviewed patient charts to identify candidates for this study. Inclusion criteria were unstable dorsal PIP fracture-dislocations treated with EBP and minimum 4-month follow-up. (Fracture-dislocations were deemed unstable if they involved at least 30% of the articular surface or required more than 30° of flexion for reduction maintenance.) Exclusion criteria were open injury, neurovascular or tendon injury, or any prior injury to the PIP joint.
Twelve patients (5 females, 7 males) treated over a 4-year period (2002–2006) met the inclusion criteria. Mean age was 30 years (range, 15-64 years). Each surgery was performed by Dr. Hagberg or Dr. Balk. Half the cases involved the dominant hand. Two small fingers, 4 ring fingers, 2 long fingers, and 4 index fingers were injured. The injuries were sustained in an all-terrain vehicle accident (n = 1), in falls (n = 2), while swimming (n = 1), or while playing softball (n = 3), football (n = 4), or soccer (n = 1). Mean time from injury to surgery was 7.5 days (range, 4-27 days). Extent of articular surface involvement of the base of the fractured middle phalanx was calculated using preoperatively obtained lateral radiographs.
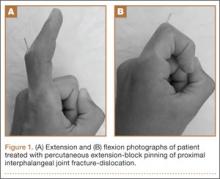
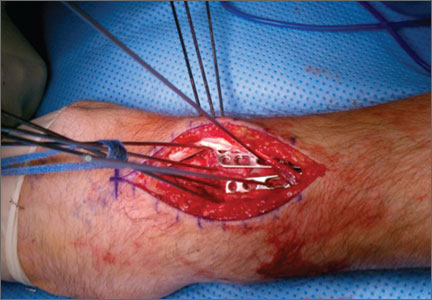
Surgical intervention was performed in a reproducible fashion. All patients were treated with closed reduction of the PIP joint under fluoroscopic guidance. Before pinning, joint stability was assessed fluoroscopically both at rest and through an arc of motion. A single smooth 0.045-in K-wire was then inserted percutaneously into the distal and dorsal aspects of the proximal phalanx in retrograde fashion (Figure 1). During wire insertion, the distal interphalangeal joint was flexed to relax the intrinsic mechanism, and the central slip tendon was pierced just proximal to its insertion. We have not noted significant adhesion formation about the central slip with this technique, likely because of limited tendon excursion in this location. Stable joint reduction was confirmed with fluoroscopy. No attempt was made to reduce the intra-articular fracture at the base of the middle phalanx.
A therapy program was initiated 2 to 9 days after surgery. At the first postoperative visit, patients were allowed to perform active ROM (AROM) with the pin in place (Figure 1). K-wires were removed a mean of 25 days (range, 17-31 days) after surgery. A static dorsal block splint was then applied, and patients were encouraged to remove it several times per day for AROM between 20° and full flexion until 6 weeks after surgery. At that time, formal occupational therapy was commenced for another 6 weeks. If there was residual flexion contracture of the PIP joint, dynamic extension splinting was initiated after fracture consolidation.
Mean follow-up was 35.5 months (range, 4-94 months). Postoperative anteroposterior and lateral radiographs were used to evaluate maintenance of joint congruity, fracture union, remodeling, and evidence of degenerative changes. At final follow-up, grip strength of injured and contralateral hands was measured with a dynamometer (Jamar; Patterson Medical, Warrenville, Illinois). AROM and passive ROM (PROM) of the PIP joint was documented at follow-up visits. In addition, patients rated their pain on a 0-to-10 visual analog scale (VAS), with 0 representing no pain and 10 representing excruciating pain. Patients also completed a questionnaire assessing satisfaction with surgical outcome. Physical function and disability were assessed with the Quick Disabilities of the Arm, Shoulder, and Hand (QuickDASH) questionnaire. Any complications, including the need for further surgeries, were documented. Pearson correlation coefficients and Student t tests (with significance set at P < .05) were used to compare outcomes.
Results
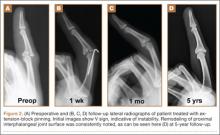
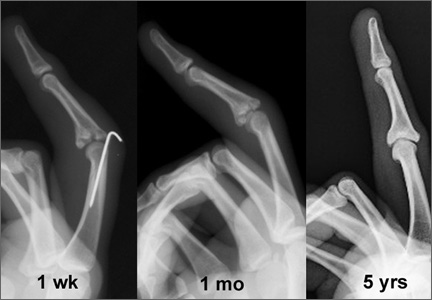
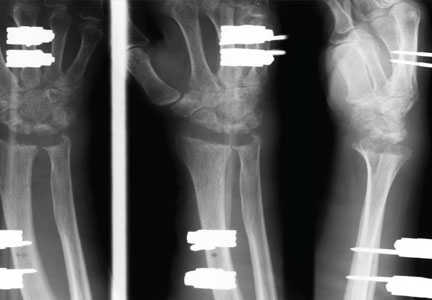
Radiographic reduction of joint dislocation was achieved and maintained in 11 of the 12 patients at a mean follow-up of 35.5 months (range, 4-94 months). Extent of joint surface involvement, based on preoperative lateral radiographs, averaged 43% (range, 25%-75%). Although no direct articular reduction was performed, remodeling of the joint surface was consistently noted at follow-up (Figure 2). Mild radiographic degenerative changes were noted at final follow-up in 4 patients, and moderate changes were noted in 1 patient. Radiographic union was achieved in all cases, and no pin-tract infections were noted.
Mean AROM of the PIP joint at final follow-up was 84° (range, 50°-110°), with patients lacking a mean of 7° of full extension and achieving mean flexion of 91°. Mean PROM was 93° (range, 75°-110°). There was no correlation between extent of articular surface involvement and ROM. Furthermore, no correlation was found between time from injury to surgery and ROM. Patients regained full grip strength in the operative hand. At final follow-up, mean grip strength was 79.4 pounds in the operative hand and 79.6 pounds in the contralateral hand, demonstrating equal grip strengths bilaterally.
Patients overall had very low levels of pain; mean VAS score was 0.64 (range, 0-3). Mean QuickDASH score was 5.7 (range, 0-30), suggesting minimal functional impairment. One patient developed a malunion of the middle phalanx fracture resulting in a rotational deformity and required corrective osteotomy. This patient’s VAS score (3) and QuickDASH score (30) were significantly higher than those of the other patients in the study. No other complications were noted by final follow-up.
A higher level of patient satisfaction was found to be directly related to length of follow-up (P < .05). Satisfaction was inversely related to higher VAS score (P < .05) and higher QuickDASH score (P < .001). Pain at work correlated with lower satisfaction level (P < .05). There was no correlation between patient satisfaction and AROM or PROM.
Discussion
The results of this study demonstrate the efficacy of EBP in the treatment of dorsal PIP joint fracture-dislocations. EBP maintained joint dislocation reduction and allowed for early mobilization, which resulted in good ROM, minimal pain, and good functional outcomes. Of note, postoperative patient satisfaction correlated with pain but not with ROM. It is possible that EBP yielded sufficient functional ROM in all patients such that improvement beyond this threshold did not lead to further improvement in satisfaction. Hume and colleagues23 found that mean PIP joint flexion of 60° is needed for activities of daily living. As mean PIP active flexion was 91° (range, 70°-105°) in the present study, it is possible that satisfaction did not correlate with ROM, as all 12 patients achieved active flexion of more than 60°. Despite the lack of correlation between ROM and satisfaction, early PIP joint mobilization is likely a key contributor to positive outcomes because of its significant role in cartilage healing.24
Postoperative ROM in the present study is consistent with that in other reports of patients with PIP joint fracture-dislocations treated with EBP.11,12,22 In a study by Inoue and Tamura,11 14 such patients had mean PIP ROM of 94° at a mean follow-up of 14 months. Viegas22 followed a series of 3 patients for a mean of 7 weeks. At final follow-up, their mean PIP arc of motion was 71°; they lacked 12° of full extension and achieved 83° of flexion. The larger PIP arc of motion (84°) found in the present study may be due to our significantly longer follow-up (35 months). Unlike us, Viegas22 noted an inverse relationship between extent of articular surface involvement and postoperative ROM. Our finding a lack of correlation may be a result of the significant amount of joint remodeling noted on follow-up radiographs.
Studies of transarticular pinning of PIP joints after dorsal PIP fracture-dislocations have reported outcomes similar to ours.25,26 Newington and colleagues25 evaluated 10 cases of transarticular pinning of the PIP joint and found mean arc of motion of 85° and equal grip strengths between injured and contralateral hands. In a series of 19 patients with PIP fracture-dislocations, Aladin and Davis26 noted similar outcomes of transarticular K-wire fixation and ORIF. In both of their treatment groups, however, there was evidence of PIP joint incongruity and subluxation. Of note, PIP arc motion was lower in their study than in ours.
Recent studies have evaluated unstable PIP fracture-dislocations treated with both EBP and percutaneous reduction and pinning with a second K-wire.13,27 At a mean follow-up of 18 months, Vitale and colleagues13 noted maintenance of concentric fracture reduction, good PIP ROM (mean range, 4°-93°), and low VAS and DASH scores (1.4 and 8, respectively). Waris and Alanen27 noted mean PIP AROM of 83° and low VAS and DASH scores (1 and 4, respectively). The EBP technique used in the present study did not involve percutaneous fracture reduction but achieved equally good ROM and VAS and QuickDASH scores.
Clinical outcomes of EBP of PIP joint fracture-dislocations are also comparable to outcomes of more complex treatment methods.8-10,15-19,21,26,28-33 Dynamic distraction external fixation has led to equally good ROM (mean AROM, 80°-85°15,16) and VAS scores, but with a higher incidence of pin-site infection.14-17 ORIF of the intra-articular middle phalanx fracture has the advantage of obtaining a direct anatomical reduction, but clinical outcomes are similar to those in the present study (mean AROM, 70°; 78% pain-free9), and flexion contractures have been noted.8-10 Furthermore, reduction of the fractured PIP joint articular surface has not been shown to be necessary for good outcomes.16,34 This may be explained in part by PIP joint remodeling, which has been routinely observed on long-term follow-up by the senior authors of the present study. Hemi-hamate autografting and volar plate arthroplasty are other options that have had promising results in the treatment of acute and chronic unstable PIP fracture-dislocations.18-21 However, the postoperative ROM (mean AROM, 61°-85°18,21), VAS scores, and patient satisfaction (91% very satisfied21) of these operations are similar to those of EBP in the present study and may not justify the longer operative times and technical challenges associated with these techniques.
We believe that our study group’s 1 complication, a malunion that was treated with corrective osteotomy, resulted from lack of appreciation of the degree of injury. The teenaged female patient’s index finger PIP joint had a rotational malalignment that was not appreciated before or during surgery. After pinning and after ROM was restored, the index finger was observed crossing over the middle finger with digital flexion. The patient returned to the operating room for corrective osteotomy.
We recommend that surgeons assess alignment carefully, before and during surgery, when considering this technique. Although complications are rare, the technique is not for patients with rotational malalignment; ORIF may be more suitable in these cases. In addition, though EBP may be appropriate for pilon-type injuries, as it allows for early AROM, our procedure of choice for pilon fracture is dynamic external fixation, which in addition to allowing for AROM provides ligamentotaxis. In the event that a large volar articular fragment extends into the middle phalanx diaphysis, we typically proceed with ORIF through a volar shotgun approach. At our institution, injuries lasting more than 3 months are often treated with volar plate arthroplasty or hemi-hamate resurfacing. Finally, we believe that caution should be exercised when using this technique in patients with more than 50% articular involvement. In the present study, though we used this treatment in cases of up to 75% surface involvement, alternative techniques, such as hemi-hamate resurfacing arthroplasty, may provide a better volar bony buttress and limit the risk for recurrent instability. Despite its relative contraindications, our technique has been appropriate for more than 90% of the acute PIP fracture-dislocations we have seen.
This study expands on prior research by demonstrating good function, satisfaction, and pain outcomes of percutaneous EBP in the treatment of unstable dorsal PIP fracture-dislocations. In addition, this study demonstrated that the efficacy of EBP is similar to that of more complex and technically demanding methods of treatment. Our technique has the advantage of simplicity. It obviates the soft-tissue damage required for ORIF and more complex fixation techniques. Furthermore, use of this simple technique may save time and costs and lead to more reproducible outcomes.
One limitation of this study is its small sample size. It is possible that outcomes may have been different with a larger sample. Furthermore, we did not make a direct comparison with other treatment methods. To better determine the optimal treatment method for this fracture type, future studies should prospectively evaluate outcomes for multiple treatment modalities in a randomized fashion.
1. Leibovic SJ, Bowers WH. Anatomy of the proximal interphalangeal joint. Hand Clin. 1994;10(2):169-178.
2. Kiefhaber TR, Stern PJ. Fracture dislocations of the proximal interphalangeal joint. J Hand Surg Am. 1998;23(3):368-380.
3. Ng CY, Oliver CW. Fractures of the proximal interphalangeal joints of the fingers. J Bone Joint Surg Br. 2009;91(6):705-712.
4. Isani A. Small joint injuries requiring surgical treatment. Orthop Clin North Am. 1986;17(3):407-419.
5. McElfresh EC, Dobyns JH, O’Brien ET. Management of fracture-dislocation of the proximal interphalangeal joints by extension-block splinting. J Bone Joint Surg Am. 1972;54(8):1705-1711.
6. Hastings H 2nd, Carroll C 4th. Treatment of closed articular fractures of the metacarpophalangeal and proximal interphalangeal joints. Hand Clin. 1988;4(3):503-527.
7. O’Rourke SK, Gaur S, Barton NJ. Long-term outcome of articular fractures of the phalanges: an eleven year follow up. J Hand Surg Br. 1989;14(2):183-193.
8. Grant I, Berger AC, Tham SK. Internal fixation of unstable fracture dislocations of the proximal interphalangeal joint. J Hand Surg Br. 2005;30(5):492-498.
9. Hamilton SC, Stern PJ, Fassler PR, Kiefhaber TR. Mini-screw fixation for the treatment of proximal interphalangeal joint dorsal fracture-dislocations. J Hand Surg Am. 2006;31(8):1349-1354.
10. Lee JY, Teoh LC. Dorsal fracture dislocations of the proximal interphalangeal joint treated by open reduction and interfragmentary screw fixation: indications, approaches and results. J Hand Surg Br. 2006;31(2):138-146.
11. Inoue G, Tamura Y. Treatment of fracture-dislocation of the proximal interphalangeal joint using extension-block Kirschner wire. Ann Chir Main Memb Super. 1991;10(6):564-568.
12. Sugawa I, Otani K, Kobayashi A. Treatment of fracture dislocation PIP-joint by Kirschner wire extension block method. Cent Jpn J Orthop Traumat. 1979;22:1409-1412.
13. Vitale MA, White NJ, Strauch RJ. A percutaneous technique to treat unstable dorsal fracture-dislocations of the proximal interphalangeal joint. J Hand Surg Am. 2011;36(9):1453-1459.
14. Badia A, Riano F, Ravikoff J, Khouri R, Gonzalez-Hernandez E, Orbay JL. Dynamic intradigital external fixation for proximal interphalangeal joint fracture dislocations. J Hand Surg Am. 2005;30(1):154-160.
15. Ellis SJ, Cheng R, Prokopis P, et al. Treatment of proximal interphalangeal dorsal fracture-dislocation injuries with dynamic external fixation: a pins and rubber band system. J Hand Surg Am. 2007;32(8):1242-1250.
16. Morgan JP, Gordon DA, Klug MS, Perry PE, Barre PS. Dynamic digital traction for unstable comminuted intra-articular fracture-dislocations of the proximal interphalangeal joint. J Hand Surg Am. 1995;20(4):565-573.
17. Ruland RT, Hogan CJ, Cannon DL, Slade JF. Use of dynamic distraction external fixation for unstable fracture-dislocations of the proximal interphalangeal joint. J Hand Surg Am. 2008;33(1):19-25.
18. Dionysian E, Eaton RG. The long-term outcome of volar plate arthroplasty of the proximal interphalangeal joint. J Hand Surg Am. 2000;25(3):429-437.
19. Durham-Smith G, McCarten GM. Volar plate arthroplasty for closed proximal interphalangeal joint injuries. J Hand Surg Br. 1992;17(4):422-428.
20. Calfee RP, Kiefhaber TR, Sommerkamp TG, Stern PJ. Hemi-hamate arthroplasty provides functional reconstruction of acute and chronic proximal interphalangeal fracture-dislocations. J Hand Surg Am. 2009;34(7):1232-1241.
21. Williams RM, Kiefhaber TR, Sommerkamp TG, Stern PJ. Treatment of unstable dorsal proximal interphalangeal fracture/dislocations using a hemi-hamate autograft. J Hand Surg Am. 2003;28(5):856-865.
22. Viegas SF. Extension block pinning for proximal interphalangeal joint fracture dislocations: preliminary report of a new technique. J Hand Surg Am. 1992;17(5):896-901.
23. Hume MC, Gellman H, McKellop H, Brumfield RH Jr. Functional range of motion of the joints of the hand. J Hand Surg Am. 1990;15(2):240-243.
24. Salter RB. The physiologic basis of continuous passive motion for articular cartilage healing and regeneration. Hand Clin. 1994;10(2):211-220.
25. Newington DP, Davis TR, Barton NJ. The treatment of dorsal fracture-dislocation of the proximal interphalangeal joint by closed reduction and Kirschner wire fixation: a 16-year follow up. J Hand Surg Br. 2001;26(6):537-540.
26. Aladin A, Davis TR. Dorsal fracture-dislocation of the proximal interphalangeal joint: a comparative study of percutaneous Kirschner wire fixation versus open reduction and internal fixation. J Hand Surg Br. 2005;30(2):120-128.
27. Waris E, Alanen V. Percutaneous, intramedullary fracture reduction and extension block pinning for dorsal proximal interphalangeal fracture-dislocations. J Hand Surg Am. 2010;35(12):2046-2052.
28. Bain GI, Mehta JA, Heptinstall RJ, Bria M. Dynamic external fixation for injuries of the proximal interphalangeal joint. J Bone Joint Surg Br. 1998;80(6):1014-1019.
29. Eaton RG, Malerich MM. Volar plate arthroplasty of the proximal interphalangeal joint: a review of ten years’ experience. J Hand Surg Am. 1980;5(3):260-268.
30. Green A, Smith J, Redding M, Akelman E. Acute open reduction and rigid internal fixation of proximal interphalangeal joint fracture dislocation. J Hand Surg Am. 1992;17(3):512-517.
31. Inanami H, Ninomiya S, Okutsu I, Tarui T. Dynamic external finger fixator for fracture dislocation of the proximal interphalangeal joint. J Hand Surg Am. 1993;18(1):160-164.
32. Suzuki Y, Matsunaga T, Sato S, Yokoi T. The pins and rubbers traction system for treatment of comminuted intraarticular fractures and fracture-dislocations in the hand. J Hand Surg Br. 1994;19(1):98-107.
33. Weiss AP. Cerclage fixation for fracture dislocation of the proximal interphalangeal joint. Clin Orthop. 1996;(327):21-28.
34. Agee JM. Unstable fracture dislocations of the proximal interphalangeal joint. Treatment with the force couple splint. Clin Orthop. 1987;(214):101-112.
The proximal interphalangeal (PIP) joint plays a crucial role in hand function, accounting for an estimated 85% of the motion required to grasp an object.1 The anatomy and biomechanics of the PIP joint, however, make it particularly prone to injury.2,3 Dorsal PIP fracture-dislocations represent a subset of PIP injuries that often require surgical intervention.2 The stability of these fracture-dislocations largely depends on the extent of articular involvement of the base of the middle phalanx. Fractures that involve less than 30% of the joint surface typically remain stable after reduction.2,4,5 In cases in which involvement ranges from 30% to 50%, PIP joint stability is more tenuous, and more joint flexion is required to maintain concentric reduction. Fractures that involve more than 50% of the articular surface are unstable and require operative intervention.2,5,6 Fractures that require more than 30° of flexion for reduction maintenance are generally considered unstable and may benefit from surgical intervention.2
The goals of treatment for this injury are to restore a stable, concentrically reduced joint and initiate early joint mobilization to prevent stiffness, pain, recurrent instability, and posttraumatic arthritis.3,7 Numerous surgical interventions for unstable PIP fracture-dislocations have been proposed, including open reduction and internal fixation (ORIF),8-10 extension-block pinning (EBP),11-13 dynamic external fixation,14-17 volar plate arthroplasty,18,19 and hemi-hamate resurfacing arthroplasty.20,21 Many of these techniques can be technically demanding and may require prolonged immobilization. EBP can be performed easily and efficiently and allows for early joint motion.
Extension-block pinning—placing a Kirschner wire (K-wire) into the head of the proximal phalanx at an angle that blocks PIP extension and prevents joint subluxation—was first described by Sugawa and colleagues12 in 1979. In a study by Inoue and Tamura,11 patients treated with EBP had a mean PIP range of motion (ROM) of 94° at a mean follow-up of 14 months. In a series of 3 case reports, Viegas22 noted an inverse relationship between extent of articular surface involvement and postoperative ROM in patients treated with EBP.
We conducted a study to expand on previous research on pain, function, and satisfaction outcomes in addition to ROM. We hypothesized that percutaneous EBP is an effective treatment for unstable dorsal PIP fracture-dislocations and has efficacy similar to that of more complex and technically demanding methods of treatment.
Materials and Methods
We retrospectively reviewed patient charts to identify candidates for this study. Inclusion criteria were unstable dorsal PIP fracture-dislocations treated with EBP and minimum 4-month follow-up. (Fracture-dislocations were deemed unstable if they involved at least 30% of the articular surface or required more than 30° of flexion for reduction maintenance.) Exclusion criteria were open injury, neurovascular or tendon injury, or any prior injury to the PIP joint.
Twelve patients (5 females, 7 males) treated over a 4-year period (2002–2006) met the inclusion criteria. Mean age was 30 years (range, 15-64 years). Each surgery was performed by Dr. Hagberg or Dr. Balk. Half the cases involved the dominant hand. Two small fingers, 4 ring fingers, 2 long fingers, and 4 index fingers were injured. The injuries were sustained in an all-terrain vehicle accident (n = 1), in falls (n = 2), while swimming (n = 1), or while playing softball (n = 3), football (n = 4), or soccer (n = 1). Mean time from injury to surgery was 7.5 days (range, 4-27 days). Extent of articular surface involvement of the base of the fractured middle phalanx was calculated using preoperatively obtained lateral radiographs.
Surgical intervention was performed in a reproducible fashion. All patients were treated with closed reduction of the PIP joint under fluoroscopic guidance. Before pinning, joint stability was assessed fluoroscopically both at rest and through an arc of motion. A single smooth 0.045-in K-wire was then inserted percutaneously into the distal and dorsal aspects of the proximal phalanx in retrograde fashion (Figure 1). During wire insertion, the distal interphalangeal joint was flexed to relax the intrinsic mechanism, and the central slip tendon was pierced just proximal to its insertion. We have not noted significant adhesion formation about the central slip with this technique, likely because of limited tendon excursion in this location. Stable joint reduction was confirmed with fluoroscopy. No attempt was made to reduce the intra-articular fracture at the base of the middle phalanx.
A therapy program was initiated 2 to 9 days after surgery. At the first postoperative visit, patients were allowed to perform active ROM (AROM) with the pin in place (Figure 1). K-wires were removed a mean of 25 days (range, 17-31 days) after surgery. A static dorsal block splint was then applied, and patients were encouraged to remove it several times per day for AROM between 20° and full flexion until 6 weeks after surgery. At that time, formal occupational therapy was commenced for another 6 weeks. If there was residual flexion contracture of the PIP joint, dynamic extension splinting was initiated after fracture consolidation.
Mean follow-up was 35.5 months (range, 4-94 months). Postoperative anteroposterior and lateral radiographs were used to evaluate maintenance of joint congruity, fracture union, remodeling, and evidence of degenerative changes. At final follow-up, grip strength of injured and contralateral hands was measured with a dynamometer (Jamar; Patterson Medical, Warrenville, Illinois). AROM and passive ROM (PROM) of the PIP joint was documented at follow-up visits. In addition, patients rated their pain on a 0-to-10 visual analog scale (VAS), with 0 representing no pain and 10 representing excruciating pain. Patients also completed a questionnaire assessing satisfaction with surgical outcome. Physical function and disability were assessed with the Quick Disabilities of the Arm, Shoulder, and Hand (QuickDASH) questionnaire. Any complications, including the need for further surgeries, were documented. Pearson correlation coefficients and Student t tests (with significance set at P < .05) were used to compare outcomes.
Results
Radiographic reduction of joint dislocation was achieved and maintained in 11 of the 12 patients at a mean follow-up of 35.5 months (range, 4-94 months). Extent of joint surface involvement, based on preoperative lateral radiographs, averaged 43% (range, 25%-75%). Although no direct articular reduction was performed, remodeling of the joint surface was consistently noted at follow-up (Figure 2). Mild radiographic degenerative changes were noted at final follow-up in 4 patients, and moderate changes were noted in 1 patient. Radiographic union was achieved in all cases, and no pin-tract infections were noted.
Mean AROM of the PIP joint at final follow-up was 84° (range, 50°-110°), with patients lacking a mean of 7° of full extension and achieving mean flexion of 91°. Mean PROM was 93° (range, 75°-110°). There was no correlation between extent of articular surface involvement and ROM. Furthermore, no correlation was found between time from injury to surgery and ROM. Patients regained full grip strength in the operative hand. At final follow-up, mean grip strength was 79.4 pounds in the operative hand and 79.6 pounds in the contralateral hand, demonstrating equal grip strengths bilaterally.
Patients overall had very low levels of pain; mean VAS score was 0.64 (range, 0-3). Mean QuickDASH score was 5.7 (range, 0-30), suggesting minimal functional impairment. One patient developed a malunion of the middle phalanx fracture resulting in a rotational deformity and required corrective osteotomy. This patient’s VAS score (3) and QuickDASH score (30) were significantly higher than those of the other patients in the study. No other complications were noted by final follow-up.
A higher level of patient satisfaction was found to be directly related to length of follow-up (P < .05). Satisfaction was inversely related to higher VAS score (P < .05) and higher QuickDASH score (P < .001). Pain at work correlated with lower satisfaction level (P < .05). There was no correlation between patient satisfaction and AROM or PROM.
Discussion
The results of this study demonstrate the efficacy of EBP in the treatment of dorsal PIP joint fracture-dislocations. EBP maintained joint dislocation reduction and allowed for early mobilization, which resulted in good ROM, minimal pain, and good functional outcomes. Of note, postoperative patient satisfaction correlated with pain but not with ROM. It is possible that EBP yielded sufficient functional ROM in all patients such that improvement beyond this threshold did not lead to further improvement in satisfaction. Hume and colleagues23 found that mean PIP joint flexion of 60° is needed for activities of daily living. As mean PIP active flexion was 91° (range, 70°-105°) in the present study, it is possible that satisfaction did not correlate with ROM, as all 12 patients achieved active flexion of more than 60°. Despite the lack of correlation between ROM and satisfaction, early PIP joint mobilization is likely a key contributor to positive outcomes because of its significant role in cartilage healing.24
Postoperative ROM in the present study is consistent with that in other reports of patients with PIP joint fracture-dislocations treated with EBP.11,12,22 In a study by Inoue and Tamura,11 14 such patients had mean PIP ROM of 94° at a mean follow-up of 14 months. Viegas22 followed a series of 3 patients for a mean of 7 weeks. At final follow-up, their mean PIP arc of motion was 71°; they lacked 12° of full extension and achieved 83° of flexion. The larger PIP arc of motion (84°) found in the present study may be due to our significantly longer follow-up (35 months). Unlike us, Viegas22 noted an inverse relationship between extent of articular surface involvement and postoperative ROM. Our finding a lack of correlation may be a result of the significant amount of joint remodeling noted on follow-up radiographs.
Studies of transarticular pinning of PIP joints after dorsal PIP fracture-dislocations have reported outcomes similar to ours.25,26 Newington and colleagues25 evaluated 10 cases of transarticular pinning of the PIP joint and found mean arc of motion of 85° and equal grip strengths between injured and contralateral hands. In a series of 19 patients with PIP fracture-dislocations, Aladin and Davis26 noted similar outcomes of transarticular K-wire fixation and ORIF. In both of their treatment groups, however, there was evidence of PIP joint incongruity and subluxation. Of note, PIP arc motion was lower in their study than in ours.
Recent studies have evaluated unstable PIP fracture-dislocations treated with both EBP and percutaneous reduction and pinning with a second K-wire.13,27 At a mean follow-up of 18 months, Vitale and colleagues13 noted maintenance of concentric fracture reduction, good PIP ROM (mean range, 4°-93°), and low VAS and DASH scores (1.4 and 8, respectively). Waris and Alanen27 noted mean PIP AROM of 83° and low VAS and DASH scores (1 and 4, respectively). The EBP technique used in the present study did not involve percutaneous fracture reduction but achieved equally good ROM and VAS and QuickDASH scores.
Clinical outcomes of EBP of PIP joint fracture-dislocations are also comparable to outcomes of more complex treatment methods.8-10,15-19,21,26,28-33 Dynamic distraction external fixation has led to equally good ROM (mean AROM, 80°-85°15,16) and VAS scores, but with a higher incidence of pin-site infection.14-17 ORIF of the intra-articular middle phalanx fracture has the advantage of obtaining a direct anatomical reduction, but clinical outcomes are similar to those in the present study (mean AROM, 70°; 78% pain-free9), and flexion contractures have been noted.8-10 Furthermore, reduction of the fractured PIP joint articular surface has not been shown to be necessary for good outcomes.16,34 This may be explained in part by PIP joint remodeling, which has been routinely observed on long-term follow-up by the senior authors of the present study. Hemi-hamate autografting and volar plate arthroplasty are other options that have had promising results in the treatment of acute and chronic unstable PIP fracture-dislocations.18-21 However, the postoperative ROM (mean AROM, 61°-85°18,21), VAS scores, and patient satisfaction (91% very satisfied21) of these operations are similar to those of EBP in the present study and may not justify the longer operative times and technical challenges associated with these techniques.
We believe that our study group’s 1 complication, a malunion that was treated with corrective osteotomy, resulted from lack of appreciation of the degree of injury. The teenaged female patient’s index finger PIP joint had a rotational malalignment that was not appreciated before or during surgery. After pinning and after ROM was restored, the index finger was observed crossing over the middle finger with digital flexion. The patient returned to the operating room for corrective osteotomy.
We recommend that surgeons assess alignment carefully, before and during surgery, when considering this technique. Although complications are rare, the technique is not for patients with rotational malalignment; ORIF may be more suitable in these cases. In addition, though EBP may be appropriate for pilon-type injuries, as it allows for early AROM, our procedure of choice for pilon fracture is dynamic external fixation, which in addition to allowing for AROM provides ligamentotaxis. In the event that a large volar articular fragment extends into the middle phalanx diaphysis, we typically proceed with ORIF through a volar shotgun approach. At our institution, injuries lasting more than 3 months are often treated with volar plate arthroplasty or hemi-hamate resurfacing. Finally, we believe that caution should be exercised when using this technique in patients with more than 50% articular involvement. In the present study, though we used this treatment in cases of up to 75% surface involvement, alternative techniques, such as hemi-hamate resurfacing arthroplasty, may provide a better volar bony buttress and limit the risk for recurrent instability. Despite its relative contraindications, our technique has been appropriate for more than 90% of the acute PIP fracture-dislocations we have seen.
This study expands on prior research by demonstrating good function, satisfaction, and pain outcomes of percutaneous EBP in the treatment of unstable dorsal PIP fracture-dislocations. In addition, this study demonstrated that the efficacy of EBP is similar to that of more complex and technically demanding methods of treatment. Our technique has the advantage of simplicity. It obviates the soft-tissue damage required for ORIF and more complex fixation techniques. Furthermore, use of this simple technique may save time and costs and lead to more reproducible outcomes.
One limitation of this study is its small sample size. It is possible that outcomes may have been different with a larger sample. Furthermore, we did not make a direct comparison with other treatment methods. To better determine the optimal treatment method for this fracture type, future studies should prospectively evaluate outcomes for multiple treatment modalities in a randomized fashion.
The proximal interphalangeal (PIP) joint plays a crucial role in hand function, accounting for an estimated 85% of the motion required to grasp an object.1 The anatomy and biomechanics of the PIP joint, however, make it particularly prone to injury.2,3 Dorsal PIP fracture-dislocations represent a subset of PIP injuries that often require surgical intervention.2 The stability of these fracture-dislocations largely depends on the extent of articular involvement of the base of the middle phalanx. Fractures that involve less than 30% of the joint surface typically remain stable after reduction.2,4,5 In cases in which involvement ranges from 30% to 50%, PIP joint stability is more tenuous, and more joint flexion is required to maintain concentric reduction. Fractures that involve more than 50% of the articular surface are unstable and require operative intervention.2,5,6 Fractures that require more than 30° of flexion for reduction maintenance are generally considered unstable and may benefit from surgical intervention.2
The goals of treatment for this injury are to restore a stable, concentrically reduced joint and initiate early joint mobilization to prevent stiffness, pain, recurrent instability, and posttraumatic arthritis.3,7 Numerous surgical interventions for unstable PIP fracture-dislocations have been proposed, including open reduction and internal fixation (ORIF),8-10 extension-block pinning (EBP),11-13 dynamic external fixation,14-17 volar plate arthroplasty,18,19 and hemi-hamate resurfacing arthroplasty.20,21 Many of these techniques can be technically demanding and may require prolonged immobilization. EBP can be performed easily and efficiently and allows for early joint motion.
Extension-block pinning—placing a Kirschner wire (K-wire) into the head of the proximal phalanx at an angle that blocks PIP extension and prevents joint subluxation—was first described by Sugawa and colleagues12 in 1979. In a study by Inoue and Tamura,11 patients treated with EBP had a mean PIP range of motion (ROM) of 94° at a mean follow-up of 14 months. In a series of 3 case reports, Viegas22 noted an inverse relationship between extent of articular surface involvement and postoperative ROM in patients treated with EBP.
We conducted a study to expand on previous research on pain, function, and satisfaction outcomes in addition to ROM. We hypothesized that percutaneous EBP is an effective treatment for unstable dorsal PIP fracture-dislocations and has efficacy similar to that of more complex and technically demanding methods of treatment.
Materials and Methods
We retrospectively reviewed patient charts to identify candidates for this study. Inclusion criteria were unstable dorsal PIP fracture-dislocations treated with EBP and minimum 4-month follow-up. (Fracture-dislocations were deemed unstable if they involved at least 30% of the articular surface or required more than 30° of flexion for reduction maintenance.) Exclusion criteria were open injury, neurovascular or tendon injury, or any prior injury to the PIP joint.
Twelve patients (5 females, 7 males) treated over a 4-year period (2002–2006) met the inclusion criteria. Mean age was 30 years (range, 15-64 years). Each surgery was performed by Dr. Hagberg or Dr. Balk. Half the cases involved the dominant hand. Two small fingers, 4 ring fingers, 2 long fingers, and 4 index fingers were injured. The injuries were sustained in an all-terrain vehicle accident (n = 1), in falls (n = 2), while swimming (n = 1), or while playing softball (n = 3), football (n = 4), or soccer (n = 1). Mean time from injury to surgery was 7.5 days (range, 4-27 days). Extent of articular surface involvement of the base of the fractured middle phalanx was calculated using preoperatively obtained lateral radiographs.
Surgical intervention was performed in a reproducible fashion. All patients were treated with closed reduction of the PIP joint under fluoroscopic guidance. Before pinning, joint stability was assessed fluoroscopically both at rest and through an arc of motion. A single smooth 0.045-in K-wire was then inserted percutaneously into the distal and dorsal aspects of the proximal phalanx in retrograde fashion (Figure 1). During wire insertion, the distal interphalangeal joint was flexed to relax the intrinsic mechanism, and the central slip tendon was pierced just proximal to its insertion. We have not noted significant adhesion formation about the central slip with this technique, likely because of limited tendon excursion in this location. Stable joint reduction was confirmed with fluoroscopy. No attempt was made to reduce the intra-articular fracture at the base of the middle phalanx.
A therapy program was initiated 2 to 9 days after surgery. At the first postoperative visit, patients were allowed to perform active ROM (AROM) with the pin in place (Figure 1). K-wires were removed a mean of 25 days (range, 17-31 days) after surgery. A static dorsal block splint was then applied, and patients were encouraged to remove it several times per day for AROM between 20° and full flexion until 6 weeks after surgery. At that time, formal occupational therapy was commenced for another 6 weeks. If there was residual flexion contracture of the PIP joint, dynamic extension splinting was initiated after fracture consolidation.
Mean follow-up was 35.5 months (range, 4-94 months). Postoperative anteroposterior and lateral radiographs were used to evaluate maintenance of joint congruity, fracture union, remodeling, and evidence of degenerative changes. At final follow-up, grip strength of injured and contralateral hands was measured with a dynamometer (Jamar; Patterson Medical, Warrenville, Illinois). AROM and passive ROM (PROM) of the PIP joint was documented at follow-up visits. In addition, patients rated their pain on a 0-to-10 visual analog scale (VAS), with 0 representing no pain and 10 representing excruciating pain. Patients also completed a questionnaire assessing satisfaction with surgical outcome. Physical function and disability were assessed with the Quick Disabilities of the Arm, Shoulder, and Hand (QuickDASH) questionnaire. Any complications, including the need for further surgeries, were documented. Pearson correlation coefficients and Student t tests (with significance set at P < .05) were used to compare outcomes.
Results
Radiographic reduction of joint dislocation was achieved and maintained in 11 of the 12 patients at a mean follow-up of 35.5 months (range, 4-94 months). Extent of joint surface involvement, based on preoperative lateral radiographs, averaged 43% (range, 25%-75%). Although no direct articular reduction was performed, remodeling of the joint surface was consistently noted at follow-up (Figure 2). Mild radiographic degenerative changes were noted at final follow-up in 4 patients, and moderate changes were noted in 1 patient. Radiographic union was achieved in all cases, and no pin-tract infections were noted.
Mean AROM of the PIP joint at final follow-up was 84° (range, 50°-110°), with patients lacking a mean of 7° of full extension and achieving mean flexion of 91°. Mean PROM was 93° (range, 75°-110°). There was no correlation between extent of articular surface involvement and ROM. Furthermore, no correlation was found between time from injury to surgery and ROM. Patients regained full grip strength in the operative hand. At final follow-up, mean grip strength was 79.4 pounds in the operative hand and 79.6 pounds in the contralateral hand, demonstrating equal grip strengths bilaterally.
Patients overall had very low levels of pain; mean VAS score was 0.64 (range, 0-3). Mean QuickDASH score was 5.7 (range, 0-30), suggesting minimal functional impairment. One patient developed a malunion of the middle phalanx fracture resulting in a rotational deformity and required corrective osteotomy. This patient’s VAS score (3) and QuickDASH score (30) were significantly higher than those of the other patients in the study. No other complications were noted by final follow-up.
A higher level of patient satisfaction was found to be directly related to length of follow-up (P < .05). Satisfaction was inversely related to higher VAS score (P < .05) and higher QuickDASH score (P < .001). Pain at work correlated with lower satisfaction level (P < .05). There was no correlation between patient satisfaction and AROM or PROM.
Discussion
The results of this study demonstrate the efficacy of EBP in the treatment of dorsal PIP joint fracture-dislocations. EBP maintained joint dislocation reduction and allowed for early mobilization, which resulted in good ROM, minimal pain, and good functional outcomes. Of note, postoperative patient satisfaction correlated with pain but not with ROM. It is possible that EBP yielded sufficient functional ROM in all patients such that improvement beyond this threshold did not lead to further improvement in satisfaction. Hume and colleagues23 found that mean PIP joint flexion of 60° is needed for activities of daily living. As mean PIP active flexion was 91° (range, 70°-105°) in the present study, it is possible that satisfaction did not correlate with ROM, as all 12 patients achieved active flexion of more than 60°. Despite the lack of correlation between ROM and satisfaction, early PIP joint mobilization is likely a key contributor to positive outcomes because of its significant role in cartilage healing.24
Postoperative ROM in the present study is consistent with that in other reports of patients with PIP joint fracture-dislocations treated with EBP.11,12,22 In a study by Inoue and Tamura,11 14 such patients had mean PIP ROM of 94° at a mean follow-up of 14 months. Viegas22 followed a series of 3 patients for a mean of 7 weeks. At final follow-up, their mean PIP arc of motion was 71°; they lacked 12° of full extension and achieved 83° of flexion. The larger PIP arc of motion (84°) found in the present study may be due to our significantly longer follow-up (35 months). Unlike us, Viegas22 noted an inverse relationship between extent of articular surface involvement and postoperative ROM. Our finding a lack of correlation may be a result of the significant amount of joint remodeling noted on follow-up radiographs.
Studies of transarticular pinning of PIP joints after dorsal PIP fracture-dislocations have reported outcomes similar to ours.25,26 Newington and colleagues25 evaluated 10 cases of transarticular pinning of the PIP joint and found mean arc of motion of 85° and equal grip strengths between injured and contralateral hands. In a series of 19 patients with PIP fracture-dislocations, Aladin and Davis26 noted similar outcomes of transarticular K-wire fixation and ORIF. In both of their treatment groups, however, there was evidence of PIP joint incongruity and subluxation. Of note, PIP arc motion was lower in their study than in ours.
Recent studies have evaluated unstable PIP fracture-dislocations treated with both EBP and percutaneous reduction and pinning with a second K-wire.13,27 At a mean follow-up of 18 months, Vitale and colleagues13 noted maintenance of concentric fracture reduction, good PIP ROM (mean range, 4°-93°), and low VAS and DASH scores (1.4 and 8, respectively). Waris and Alanen27 noted mean PIP AROM of 83° and low VAS and DASH scores (1 and 4, respectively). The EBP technique used in the present study did not involve percutaneous fracture reduction but achieved equally good ROM and VAS and QuickDASH scores.
Clinical outcomes of EBP of PIP joint fracture-dislocations are also comparable to outcomes of more complex treatment methods.8-10,15-19,21,26,28-33 Dynamic distraction external fixation has led to equally good ROM (mean AROM, 80°-85°15,16) and VAS scores, but with a higher incidence of pin-site infection.14-17 ORIF of the intra-articular middle phalanx fracture has the advantage of obtaining a direct anatomical reduction, but clinical outcomes are similar to those in the present study (mean AROM, 70°; 78% pain-free9), and flexion contractures have been noted.8-10 Furthermore, reduction of the fractured PIP joint articular surface has not been shown to be necessary for good outcomes.16,34 This may be explained in part by PIP joint remodeling, which has been routinely observed on long-term follow-up by the senior authors of the present study. Hemi-hamate autografting and volar plate arthroplasty are other options that have had promising results in the treatment of acute and chronic unstable PIP fracture-dislocations.18-21 However, the postoperative ROM (mean AROM, 61°-85°18,21), VAS scores, and patient satisfaction (91% very satisfied21) of these operations are similar to those of EBP in the present study and may not justify the longer operative times and technical challenges associated with these techniques.
We believe that our study group’s 1 complication, a malunion that was treated with corrective osteotomy, resulted from lack of appreciation of the degree of injury. The teenaged female patient’s index finger PIP joint had a rotational malalignment that was not appreciated before or during surgery. After pinning and after ROM was restored, the index finger was observed crossing over the middle finger with digital flexion. The patient returned to the operating room for corrective osteotomy.
We recommend that surgeons assess alignment carefully, before and during surgery, when considering this technique. Although complications are rare, the technique is not for patients with rotational malalignment; ORIF may be more suitable in these cases. In addition, though EBP may be appropriate for pilon-type injuries, as it allows for early AROM, our procedure of choice for pilon fracture is dynamic external fixation, which in addition to allowing for AROM provides ligamentotaxis. In the event that a large volar articular fragment extends into the middle phalanx diaphysis, we typically proceed with ORIF through a volar shotgun approach. At our institution, injuries lasting more than 3 months are often treated with volar plate arthroplasty or hemi-hamate resurfacing. Finally, we believe that caution should be exercised when using this technique in patients with more than 50% articular involvement. In the present study, though we used this treatment in cases of up to 75% surface involvement, alternative techniques, such as hemi-hamate resurfacing arthroplasty, may provide a better volar bony buttress and limit the risk for recurrent instability. Despite its relative contraindications, our technique has been appropriate for more than 90% of the acute PIP fracture-dislocations we have seen.
This study expands on prior research by demonstrating good function, satisfaction, and pain outcomes of percutaneous EBP in the treatment of unstable dorsal PIP fracture-dislocations. In addition, this study demonstrated that the efficacy of EBP is similar to that of more complex and technically demanding methods of treatment. Our technique has the advantage of simplicity. It obviates the soft-tissue damage required for ORIF and more complex fixation techniques. Furthermore, use of this simple technique may save time and costs and lead to more reproducible outcomes.
One limitation of this study is its small sample size. It is possible that outcomes may have been different with a larger sample. Furthermore, we did not make a direct comparison with other treatment methods. To better determine the optimal treatment method for this fracture type, future studies should prospectively evaluate outcomes for multiple treatment modalities in a randomized fashion.
1. Leibovic SJ, Bowers WH. Anatomy of the proximal interphalangeal joint. Hand Clin. 1994;10(2):169-178.
2. Kiefhaber TR, Stern PJ. Fracture dislocations of the proximal interphalangeal joint. J Hand Surg Am. 1998;23(3):368-380.
3. Ng CY, Oliver CW. Fractures of the proximal interphalangeal joints of the fingers. J Bone Joint Surg Br. 2009;91(6):705-712.
4. Isani A. Small joint injuries requiring surgical treatment. Orthop Clin North Am. 1986;17(3):407-419.
5. McElfresh EC, Dobyns JH, O’Brien ET. Management of fracture-dislocation of the proximal interphalangeal joints by extension-block splinting. J Bone Joint Surg Am. 1972;54(8):1705-1711.
6. Hastings H 2nd, Carroll C 4th. Treatment of closed articular fractures of the metacarpophalangeal and proximal interphalangeal joints. Hand Clin. 1988;4(3):503-527.
7. O’Rourke SK, Gaur S, Barton NJ. Long-term outcome of articular fractures of the phalanges: an eleven year follow up. J Hand Surg Br. 1989;14(2):183-193.
8. Grant I, Berger AC, Tham SK. Internal fixation of unstable fracture dislocations of the proximal interphalangeal joint. J Hand Surg Br. 2005;30(5):492-498.
9. Hamilton SC, Stern PJ, Fassler PR, Kiefhaber TR. Mini-screw fixation for the treatment of proximal interphalangeal joint dorsal fracture-dislocations. J Hand Surg Am. 2006;31(8):1349-1354.
10. Lee JY, Teoh LC. Dorsal fracture dislocations of the proximal interphalangeal joint treated by open reduction and interfragmentary screw fixation: indications, approaches and results. J Hand Surg Br. 2006;31(2):138-146.
11. Inoue G, Tamura Y. Treatment of fracture-dislocation of the proximal interphalangeal joint using extension-block Kirschner wire. Ann Chir Main Memb Super. 1991;10(6):564-568.
12. Sugawa I, Otani K, Kobayashi A. Treatment of fracture dislocation PIP-joint by Kirschner wire extension block method. Cent Jpn J Orthop Traumat. 1979;22:1409-1412.
13. Vitale MA, White NJ, Strauch RJ. A percutaneous technique to treat unstable dorsal fracture-dislocations of the proximal interphalangeal joint. J Hand Surg Am. 2011;36(9):1453-1459.
14. Badia A, Riano F, Ravikoff J, Khouri R, Gonzalez-Hernandez E, Orbay JL. Dynamic intradigital external fixation for proximal interphalangeal joint fracture dislocations. J Hand Surg Am. 2005;30(1):154-160.
15. Ellis SJ, Cheng R, Prokopis P, et al. Treatment of proximal interphalangeal dorsal fracture-dislocation injuries with dynamic external fixation: a pins and rubber band system. J Hand Surg Am. 2007;32(8):1242-1250.
16. Morgan JP, Gordon DA, Klug MS, Perry PE, Barre PS. Dynamic digital traction for unstable comminuted intra-articular fracture-dislocations of the proximal interphalangeal joint. J Hand Surg Am. 1995;20(4):565-573.
17. Ruland RT, Hogan CJ, Cannon DL, Slade JF. Use of dynamic distraction external fixation for unstable fracture-dislocations of the proximal interphalangeal joint. J Hand Surg Am. 2008;33(1):19-25.
18. Dionysian E, Eaton RG. The long-term outcome of volar plate arthroplasty of the proximal interphalangeal joint. J Hand Surg Am. 2000;25(3):429-437.
19. Durham-Smith G, McCarten GM. Volar plate arthroplasty for closed proximal interphalangeal joint injuries. J Hand Surg Br. 1992;17(4):422-428.
20. Calfee RP, Kiefhaber TR, Sommerkamp TG, Stern PJ. Hemi-hamate arthroplasty provides functional reconstruction of acute and chronic proximal interphalangeal fracture-dislocations. J Hand Surg Am. 2009;34(7):1232-1241.
21. Williams RM, Kiefhaber TR, Sommerkamp TG, Stern PJ. Treatment of unstable dorsal proximal interphalangeal fracture/dislocations using a hemi-hamate autograft. J Hand Surg Am. 2003;28(5):856-865.
22. Viegas SF. Extension block pinning for proximal interphalangeal joint fracture dislocations: preliminary report of a new technique. J Hand Surg Am. 1992;17(5):896-901.
23. Hume MC, Gellman H, McKellop H, Brumfield RH Jr. Functional range of motion of the joints of the hand. J Hand Surg Am. 1990;15(2):240-243.
24. Salter RB. The physiologic basis of continuous passive motion for articular cartilage healing and regeneration. Hand Clin. 1994;10(2):211-220.
25. Newington DP, Davis TR, Barton NJ. The treatment of dorsal fracture-dislocation of the proximal interphalangeal joint by closed reduction and Kirschner wire fixation: a 16-year follow up. J Hand Surg Br. 2001;26(6):537-540.
26. Aladin A, Davis TR. Dorsal fracture-dislocation of the proximal interphalangeal joint: a comparative study of percutaneous Kirschner wire fixation versus open reduction and internal fixation. J Hand Surg Br. 2005;30(2):120-128.
27. Waris E, Alanen V. Percutaneous, intramedullary fracture reduction and extension block pinning for dorsal proximal interphalangeal fracture-dislocations. J Hand Surg Am. 2010;35(12):2046-2052.
28. Bain GI, Mehta JA, Heptinstall RJ, Bria M. Dynamic external fixation for injuries of the proximal interphalangeal joint. J Bone Joint Surg Br. 1998;80(6):1014-1019.
29. Eaton RG, Malerich MM. Volar plate arthroplasty of the proximal interphalangeal joint: a review of ten years’ experience. J Hand Surg Am. 1980;5(3):260-268.
30. Green A, Smith J, Redding M, Akelman E. Acute open reduction and rigid internal fixation of proximal interphalangeal joint fracture dislocation. J Hand Surg Am. 1992;17(3):512-517.
31. Inanami H, Ninomiya S, Okutsu I, Tarui T. Dynamic external finger fixator for fracture dislocation of the proximal interphalangeal joint. J Hand Surg Am. 1993;18(1):160-164.
32. Suzuki Y, Matsunaga T, Sato S, Yokoi T. The pins and rubbers traction system for treatment of comminuted intraarticular fractures and fracture-dislocations in the hand. J Hand Surg Br. 1994;19(1):98-107.
33. Weiss AP. Cerclage fixation for fracture dislocation of the proximal interphalangeal joint. Clin Orthop. 1996;(327):21-28.
34. Agee JM. Unstable fracture dislocations of the proximal interphalangeal joint. Treatment with the force couple splint. Clin Orthop. 1987;(214):101-112.
1. Leibovic SJ, Bowers WH. Anatomy of the proximal interphalangeal joint. Hand Clin. 1994;10(2):169-178.
2. Kiefhaber TR, Stern PJ. Fracture dislocations of the proximal interphalangeal joint. J Hand Surg Am. 1998;23(3):368-380.
3. Ng CY, Oliver CW. Fractures of the proximal interphalangeal joints of the fingers. J Bone Joint Surg Br. 2009;91(6):705-712.
4. Isani A. Small joint injuries requiring surgical treatment. Orthop Clin North Am. 1986;17(3):407-419.
5. McElfresh EC, Dobyns JH, O’Brien ET. Management of fracture-dislocation of the proximal interphalangeal joints by extension-block splinting. J Bone Joint Surg Am. 1972;54(8):1705-1711.
6. Hastings H 2nd, Carroll C 4th. Treatment of closed articular fractures of the metacarpophalangeal and proximal interphalangeal joints. Hand Clin. 1988;4(3):503-527.
7. O’Rourke SK, Gaur S, Barton NJ. Long-term outcome of articular fractures of the phalanges: an eleven year follow up. J Hand Surg Br. 1989;14(2):183-193.
8. Grant I, Berger AC, Tham SK. Internal fixation of unstable fracture dislocations of the proximal interphalangeal joint. J Hand Surg Br. 2005;30(5):492-498.
9. Hamilton SC, Stern PJ, Fassler PR, Kiefhaber TR. Mini-screw fixation for the treatment of proximal interphalangeal joint dorsal fracture-dislocations. J Hand Surg Am. 2006;31(8):1349-1354.
10. Lee JY, Teoh LC. Dorsal fracture dislocations of the proximal interphalangeal joint treated by open reduction and interfragmentary screw fixation: indications, approaches and results. J Hand Surg Br. 2006;31(2):138-146.
11. Inoue G, Tamura Y. Treatment of fracture-dislocation of the proximal interphalangeal joint using extension-block Kirschner wire. Ann Chir Main Memb Super. 1991;10(6):564-568.
12. Sugawa I, Otani K, Kobayashi A. Treatment of fracture dislocation PIP-joint by Kirschner wire extension block method. Cent Jpn J Orthop Traumat. 1979;22:1409-1412.
13. Vitale MA, White NJ, Strauch RJ. A percutaneous technique to treat unstable dorsal fracture-dislocations of the proximal interphalangeal joint. J Hand Surg Am. 2011;36(9):1453-1459.
14. Badia A, Riano F, Ravikoff J, Khouri R, Gonzalez-Hernandez E, Orbay JL. Dynamic intradigital external fixation for proximal interphalangeal joint fracture dislocations. J Hand Surg Am. 2005;30(1):154-160.
15. Ellis SJ, Cheng R, Prokopis P, et al. Treatment of proximal interphalangeal dorsal fracture-dislocation injuries with dynamic external fixation: a pins and rubber band system. J Hand Surg Am. 2007;32(8):1242-1250.
16. Morgan JP, Gordon DA, Klug MS, Perry PE, Barre PS. Dynamic digital traction for unstable comminuted intra-articular fracture-dislocations of the proximal interphalangeal joint. J Hand Surg Am. 1995;20(4):565-573.
17. Ruland RT, Hogan CJ, Cannon DL, Slade JF. Use of dynamic distraction external fixation for unstable fracture-dislocations of the proximal interphalangeal joint. J Hand Surg Am. 2008;33(1):19-25.
18. Dionysian E, Eaton RG. The long-term outcome of volar plate arthroplasty of the proximal interphalangeal joint. J Hand Surg Am. 2000;25(3):429-437.
19. Durham-Smith G, McCarten GM. Volar plate arthroplasty for closed proximal interphalangeal joint injuries. J Hand Surg Br. 1992;17(4):422-428.
20. Calfee RP, Kiefhaber TR, Sommerkamp TG, Stern PJ. Hemi-hamate arthroplasty provides functional reconstruction of acute and chronic proximal interphalangeal fracture-dislocations. J Hand Surg Am. 2009;34(7):1232-1241.
21. Williams RM, Kiefhaber TR, Sommerkamp TG, Stern PJ. Treatment of unstable dorsal proximal interphalangeal fracture/dislocations using a hemi-hamate autograft. J Hand Surg Am. 2003;28(5):856-865.
22. Viegas SF. Extension block pinning for proximal interphalangeal joint fracture dislocations: preliminary report of a new technique. J Hand Surg Am. 1992;17(5):896-901.
23. Hume MC, Gellman H, McKellop H, Brumfield RH Jr. Functional range of motion of the joints of the hand. J Hand Surg Am. 1990;15(2):240-243.
24. Salter RB. The physiologic basis of continuous passive motion for articular cartilage healing and regeneration. Hand Clin. 1994;10(2):211-220.
25. Newington DP, Davis TR, Barton NJ. The treatment of dorsal fracture-dislocation of the proximal interphalangeal joint by closed reduction and Kirschner wire fixation: a 16-year follow up. J Hand Surg Br. 2001;26(6):537-540.
26. Aladin A, Davis TR. Dorsal fracture-dislocation of the proximal interphalangeal joint: a comparative study of percutaneous Kirschner wire fixation versus open reduction and internal fixation. J Hand Surg Br. 2005;30(2):120-128.
27. Waris E, Alanen V. Percutaneous, intramedullary fracture reduction and extension block pinning for dorsal proximal interphalangeal fracture-dislocations. J Hand Surg Am. 2010;35(12):2046-2052.
28. Bain GI, Mehta JA, Heptinstall RJ, Bria M. Dynamic external fixation for injuries of the proximal interphalangeal joint. J Bone Joint Surg Br. 1998;80(6):1014-1019.
29. Eaton RG, Malerich MM. Volar plate arthroplasty of the proximal interphalangeal joint: a review of ten years’ experience. J Hand Surg Am. 1980;5(3):260-268.
30. Green A, Smith J, Redding M, Akelman E. Acute open reduction and rigid internal fixation of proximal interphalangeal joint fracture dislocation. J Hand Surg Am. 1992;17(3):512-517.
31. Inanami H, Ninomiya S, Okutsu I, Tarui T. Dynamic external finger fixator for fracture dislocation of the proximal interphalangeal joint. J Hand Surg Am. 1993;18(1):160-164.
32. Suzuki Y, Matsunaga T, Sato S, Yokoi T. The pins and rubbers traction system for treatment of comminuted intraarticular fractures and fracture-dislocations in the hand. J Hand Surg Br. 1994;19(1):98-107.
33. Weiss AP. Cerclage fixation for fracture dislocation of the proximal interphalangeal joint. Clin Orthop. 1996;(327):21-28.
34. Agee JM. Unstable fracture dislocations of the proximal interphalangeal joint. Treatment with the force couple splint. Clin Orthop. 1987;(214):101-112.
Bronchogenic Squamous Cell Carcinoma With Soft-Tissue Metastasis to the Hand: An Unusual Case Presentation and Review of the Literature
Carcinoma of the lung is the most common lethal form of cancer in both men and women worldwide.1 It accounts for more deaths than the next 3 most common cancers combined. In 2012, 160,000 Americans are estimated to have died from lung cancer.1 Lung cancer is known to have a high metastatic potential for the brain, bones, adrenal glands, lungs, and liver.2 Orthopedic manifestations frequently include bony metastasis, most commonly the vertebrae (42%), ribs (20%), and pelvis (18%).3 Acral metastatic disease is defined as metastasis distal to the elbow or the knee. Bony acral metastases from lung carcinoma to the upper and lower extremities are extremely uncommon, accounting for only 1% each of total bone metastases from carcinoma of the lung.3 Metastases to the bones of the hand are even rarer. Only 0.1% of metastatic disease from any type of carcinoma or sarcoma manifests as metastasis in the hand.4 There are only a few reports in the literature of soft-tissue or muscular metastasis to the hand from a carcinoma. Of these cases, the majority are caused by metastatic lung carcinoma.5-9 There are no reports in the literature of metastatic disease of squamous cell origin affecting the soft tissues of the hand.
We present a case of a man with known metastatic squamous cell carcinoma of the lung who presented with acral soft-tissue metastatic disease. This report highlights a rare clinical scenario that has not been reported in the literature. The report also emphasizes a rare but important consideration for clinicians who encounter acral soft-tissue lesions in patients with a history of a primary carcinoma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
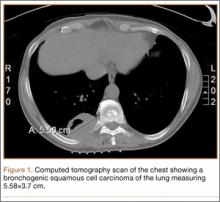
A 56-year-old man presented with right-sided pleuritic flank pain, along with a 30-lb weight loss over a 6-month period. A computed tomographic scan revealed a 5.58×3.7-cm cavitary lesion in the right lower lobe with abutment of the posterior chest wall (Figure 1). He underwent biopsy and staging, and was found to be T3N1, with biopsy-proven well-differentiated bronchogenic squamous cell carcinoma. The patient then underwent right lower and middle lobectomy with concomitant en-bloc resection of the posterior portion of ribs 7 to 11, along with mediastinal lymph-node dissection with negative margins. After surgery, he was treated with 4 cycles of adjuvant chemotherapy with cisplatin and docetaxel.
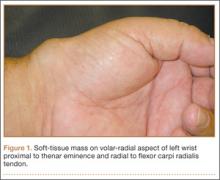
Six months after surgery, the patient began to complain of right-hand pain isolated to the thenar eminence. He also described swelling and significant pain with active or passive movement of the thumb and with relatively mild-to-moderate palpation of the area. The patient reported that the functioning of his thumb deteriorated rapidly over the course of about 1 month. On physical examination, he was neurovascularly intact with no apparent deficit in sensation of his right hand. There was no erythema or overlying skin changes. His right thenar eminence was mildly enlarged as compared with the left, and a firm, focal mass was readily palpated. Range of motion at the metacarpophalangeal joint of the thumb and index finger was limited because of pain. Thumb opposition was markedly limited. After a detailed history and physical examination, we were concerned about possible deep space infection, old hematoma, or possible metastatic disease. Magnetic resonance imaging (MRI) was ordered to evaluate the palpable mass.
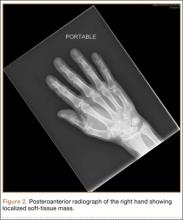
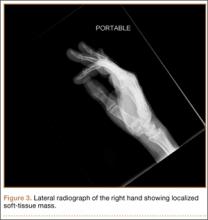
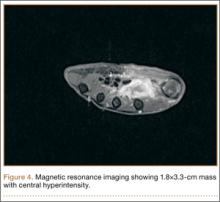
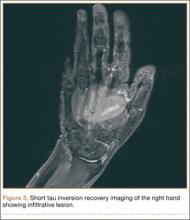
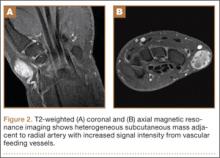
Radiographically, localized soft-tissue swelling was present on the palmar surface of the hand obliquely overlying the index finger metacarpal (Figures 2, 3). On MRI, the lesion measured approximately 1.8×3.3 cm and was isointense to slightly hyperintense diffusely with central hyperintensity on T1 images (Figure 4). On T2 and short tau inversion recovery images, the lesion was more strikingly hyperintense and infiltrative in appearance (Figure 5). Postcontrast images showed avid enhancement peripherally, with central nonenhancement consistent with necrosis in the adductor pollicis.
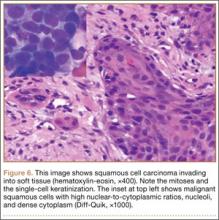
We performed a biopsy of the lesion with the aid of immediate adequacy by fine needle aspiration cytology. We saw mitotically active malignant cells with large nuclei, high nuclear-to-cytoplasmic ratios, nucleoli, and dense cytoplasm, suggesting a metastatic squamous cell carcinoma. Because infection was part of the differential, it is pertinent to note that there was no significant inflammatory infiltrate. The core biopsy was consistent with metastatic lung cancer (Figure 6).
Discussion
This patient presented an interesting diagnostic challenge, particularly because of his previous malignancy. The differential diagnosis of acute onset thenar pain without history of trauma would include encompassing soft-tissue abscess, osteomyelitis, and infectious myositis. Soft-tissue hematoma is also in the differential for this patient, especially given the malignancy. Bony metastasis should be considered in this patient given the propensity of lung carcinoma to metastasize to bone. The location would certainly be atypical, with metastasis to the bones of the forearm or hand representing only 0.1% of all metastasis of any type of primary carcinoma or sarcoma.4 Primary bone or soft-tissue sarcoma should also be considered. Some authors have also suggested that necrosis, peritumoral edema-like signal, and lobulation are more common with skeletal muscle metastasis than with a primary sarcoma.10 In this case, the degree of surrounding postcontrast enhancement made simple muscle tear with hematoma unlikely, despite the presence of increased T1 signal. The lack of evidence for localized infection and the presence of a firm focal mass on physical examination made tumor more likely than infection.
Acrometastasis
Metastatic disease distal to the elbow and knee is very rare; specifically, metastatic disease of the hands or feet accounts for approximately 0.1% of all metastases.4 Carcinoma of the lung accounts for 44% to 47% of all acrometastasis.11,12 When hand acrometastasis is considered, the right hand accounts for 55% of bony cases, likely because of hand dominance, although approximately 10% of patients had bilateral acral metastatic disease.12 The underlying mechanism of acrometastasis remains unclear; however, some authors have postulated that it may result from an increase in vascularity or a trauma to the affected extremity.12,13 Flynn and colleagues12 reviewed the literature and reported a total of 257 cases of acral metastasis to the hand; they found that the median age at presentation was 58 years. Men were more than twice as likely to be affected when compared with women. Most commonly, the primary malignancies were in the lung (44%), kidney (12%), and breast (10%). The authors also reported less common cases of acral metastasis with primary malignancies located in the stomach, liver, rectum, prostate, and colon. Most commonly, these metastases were found in the distal phalynx, followed by the metacarpals, proximal phalynx, and middle phalynx.12
Soft-Tissue Metastasis
Skeletal muscle metastasis occurs in 0.8% to 17.5% of metastatic neoplasms.14-17 Studies in lung cancer patients have also revealed a low prevalence of muscular metastasis (0% to 0.8%).16 The rarity of muscular metastatic disease has been attributed to local inhibition of tumor survival secondary to muscle contraction, increased diffusing capacity of enzymes and immune cells, and extreme variability in blood flow and pH, lactate, and oxygen concentration. Skeletal muscular metastases most commonly arise from the lung, kidneys, colon, or melanoma.16 In a recent large series of more than 1400 patients imaged for soft-tissue masses, 2.5% were metastatic.18 There are only 2 reports of soft-tissue metastatic disease involving the hand: one from a patient with a thyroid carcinoma and the other from a patient with a lung adenocarcinoma.18 Soft-tissue metastatic disease from squamous cell carcinoma distal to the wrist has never been reported in the literature.
Acral Soft-Tissue Metastasis
A review from 2012 found 264 cases of skeletal muscle metastasis from 151 articles.6 Only 2 (0.75%) of these patients, as reported above, had a soft-tissue metastasis distal to the wrist.6,17
Conclusion
We report the first known case of a soft-tissue metastasis distal to the wrist from a primary bronchogenic squamous cell carcinoma. This report highlights the extremely uncommon presentation of soft-tissue acral metastatic disease of a bronchogenic squamous cell carcinoma of the lung. Although exceedingly rare, oncologists and physicians who manage pathology of the hand should consider metastatic disease when evaluating a patient with complaints of hand pain and a soft-tissue mass, especially in a patient with a known primary malignancy.
1. American Cancer Society. Lung Cancer (Non-Small Cell). http://www.cancer.org/acs/groups/cid/documents/webcontent/003115-pdf.pdf. Revised April 30, 2014. Accessed July 22, 2014.
2. Willis RA. Pathology of Tumors. London, England: Butterworth; 1960.
3. Sugiura H, Yamada K, Sugiura T, Hida T, Mitsudomi T. Predictors of survival in patients with bone metastasis of lung cancer. Clin Orthop. 2008;466(3):729-736.
4. Kerin R. Metastatic tumors of the hand. A review of the literature. J Bone Joint Surg Am. 1983;65(9):1331-1335.
5. Alpar S. Muscle metastasis in a patient with squamous cell lung cancer. Turkish Respiratory Journal. 2002;3(2):75-78.
6. Haygood TM, Wong J, Lin JC, et al. Skeletal muscle metastases: a three-part study of a not-so-rare entity. Skeletal Radiol. 2012;41(8):899-909.
7. Tuoheti Y, Okada K, Osanai T, et al. Skeletal muscle metastases of carcinoma: a clinicopathological study of 12 cases. Jpn J Clin Oncol. 2004;34(4):210-214.
8. Chan NP, Yeo W, Ahuja AT, King AD. Multiple skeletal muscle metastases. Hong Kong Med J. 1999;5(4):410.
9. Molina-Garrido MJ, Guillen-Ponce C. Muscle metastasis of carcinoma. Clin Transl Oncol. 2011;13(2):98-101.
10. Williams JB, Youngberg RA, Bui-Mansfield LT, Pitcher JD. MR imaging of skeletal muscle metastases. AJR Am J Roentgenol. 1997;168(2):555-557.
11. Libson E, Bloom RA, Husband JE, Stoker DJ. Metastatic tumours of bones of the hand and foot. A comparative review and report of 43 additional cases. Skeletal Radiol. 1987;16(5):387-392.
12. Flynn CJ, Danjoux C, Wong J, et al. Two cases of acrometastasis to the hands and review of the literature. Curr Oncol. 2008;15(5):51-58.
13. Healey JH, Turnbull AD, Miedema B, Lane JM. Acrometastases. A study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am. 1986;68(5):743-746.
14. Sudo A, Ogihara Y, Shiokawa Y, Fujinami S, Sekiguchi S. Intramuscular metastasis of carcinoma. Clin Orthop. 1993(296):213-217.
15. Surov A, Hainz M, Holzhausen HJ, et al. Skeletal muscle metastases: primary tumours, prevalence, and radiological features. Eur Radiol. 2010;20(3):649-658.
16. Pearson CM. Incidence and type of pathologic alterations observed in muscle in a routine autopsy survey. Neurology. 1959;9:757-766.
17. Acinas Garcia O, Fernández FA, Satué EG, Beulta L, Val-Bernal JF. Metastasis of malignant neoplasms to skeletal muscle. Rev Esp Oncol. 1984;31(1):57-67.
18. Glockner JF, White LM, Sundaram M, McDonald DJ. Unsuspected metastases presenting as solitary soft tissue lesions: a fourteen-year review. Skeletal Radiol. 2000;29(5):270-274.
Carcinoma of the lung is the most common lethal form of cancer in both men and women worldwide.1 It accounts for more deaths than the next 3 most common cancers combined. In 2012, 160,000 Americans are estimated to have died from lung cancer.1 Lung cancer is known to have a high metastatic potential for the brain, bones, adrenal glands, lungs, and liver.2 Orthopedic manifestations frequently include bony metastasis, most commonly the vertebrae (42%), ribs (20%), and pelvis (18%).3 Acral metastatic disease is defined as metastasis distal to the elbow or the knee. Bony acral metastases from lung carcinoma to the upper and lower extremities are extremely uncommon, accounting for only 1% each of total bone metastases from carcinoma of the lung.3 Metastases to the bones of the hand are even rarer. Only 0.1% of metastatic disease from any type of carcinoma or sarcoma manifests as metastasis in the hand.4 There are only a few reports in the literature of soft-tissue or muscular metastasis to the hand from a carcinoma. Of these cases, the majority are caused by metastatic lung carcinoma.5-9 There are no reports in the literature of metastatic disease of squamous cell origin affecting the soft tissues of the hand.
We present a case of a man with known metastatic squamous cell carcinoma of the lung who presented with acral soft-tissue metastatic disease. This report highlights a rare clinical scenario that has not been reported in the literature. The report also emphasizes a rare but important consideration for clinicians who encounter acral soft-tissue lesions in patients with a history of a primary carcinoma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old man presented with right-sided pleuritic flank pain, along with a 30-lb weight loss over a 6-month period. A computed tomographic scan revealed a 5.58×3.7-cm cavitary lesion in the right lower lobe with abutment of the posterior chest wall (Figure 1). He underwent biopsy and staging, and was found to be T3N1, with biopsy-proven well-differentiated bronchogenic squamous cell carcinoma. The patient then underwent right lower and middle lobectomy with concomitant en-bloc resection of the posterior portion of ribs 7 to 11, along with mediastinal lymph-node dissection with negative margins. After surgery, he was treated with 4 cycles of adjuvant chemotherapy with cisplatin and docetaxel.
Six months after surgery, the patient began to complain of right-hand pain isolated to the thenar eminence. He also described swelling and significant pain with active or passive movement of the thumb and with relatively mild-to-moderate palpation of the area. The patient reported that the functioning of his thumb deteriorated rapidly over the course of about 1 month. On physical examination, he was neurovascularly intact with no apparent deficit in sensation of his right hand. There was no erythema or overlying skin changes. His right thenar eminence was mildly enlarged as compared with the left, and a firm, focal mass was readily palpated. Range of motion at the metacarpophalangeal joint of the thumb and index finger was limited because of pain. Thumb opposition was markedly limited. After a detailed history and physical examination, we were concerned about possible deep space infection, old hematoma, or possible metastatic disease. Magnetic resonance imaging (MRI) was ordered to evaluate the palpable mass.
Radiographically, localized soft-tissue swelling was present on the palmar surface of the hand obliquely overlying the index finger metacarpal (Figures 2, 3). On MRI, the lesion measured approximately 1.8×3.3 cm and was isointense to slightly hyperintense diffusely with central hyperintensity on T1 images (Figure 4). On T2 and short tau inversion recovery images, the lesion was more strikingly hyperintense and infiltrative in appearance (Figure 5). Postcontrast images showed avid enhancement peripherally, with central nonenhancement consistent with necrosis in the adductor pollicis.
We performed a biopsy of the lesion with the aid of immediate adequacy by fine needle aspiration cytology. We saw mitotically active malignant cells with large nuclei, high nuclear-to-cytoplasmic ratios, nucleoli, and dense cytoplasm, suggesting a metastatic squamous cell carcinoma. Because infection was part of the differential, it is pertinent to note that there was no significant inflammatory infiltrate. The core biopsy was consistent with metastatic lung cancer (Figure 6).
Discussion
This patient presented an interesting diagnostic challenge, particularly because of his previous malignancy. The differential diagnosis of acute onset thenar pain without history of trauma would include encompassing soft-tissue abscess, osteomyelitis, and infectious myositis. Soft-tissue hematoma is also in the differential for this patient, especially given the malignancy. Bony metastasis should be considered in this patient given the propensity of lung carcinoma to metastasize to bone. The location would certainly be atypical, with metastasis to the bones of the forearm or hand representing only 0.1% of all metastasis of any type of primary carcinoma or sarcoma.4 Primary bone or soft-tissue sarcoma should also be considered. Some authors have also suggested that necrosis, peritumoral edema-like signal, and lobulation are more common with skeletal muscle metastasis than with a primary sarcoma.10 In this case, the degree of surrounding postcontrast enhancement made simple muscle tear with hematoma unlikely, despite the presence of increased T1 signal. The lack of evidence for localized infection and the presence of a firm focal mass on physical examination made tumor more likely than infection.
Acrometastasis
Metastatic disease distal to the elbow and knee is very rare; specifically, metastatic disease of the hands or feet accounts for approximately 0.1% of all metastases.4 Carcinoma of the lung accounts for 44% to 47% of all acrometastasis.11,12 When hand acrometastasis is considered, the right hand accounts for 55% of bony cases, likely because of hand dominance, although approximately 10% of patients had bilateral acral metastatic disease.12 The underlying mechanism of acrometastasis remains unclear; however, some authors have postulated that it may result from an increase in vascularity or a trauma to the affected extremity.12,13 Flynn and colleagues12 reviewed the literature and reported a total of 257 cases of acral metastasis to the hand; they found that the median age at presentation was 58 years. Men were more than twice as likely to be affected when compared with women. Most commonly, the primary malignancies were in the lung (44%), kidney (12%), and breast (10%). The authors also reported less common cases of acral metastasis with primary malignancies located in the stomach, liver, rectum, prostate, and colon. Most commonly, these metastases were found in the distal phalynx, followed by the metacarpals, proximal phalynx, and middle phalynx.12
Soft-Tissue Metastasis
Skeletal muscle metastasis occurs in 0.8% to 17.5% of metastatic neoplasms.14-17 Studies in lung cancer patients have also revealed a low prevalence of muscular metastasis (0% to 0.8%).16 The rarity of muscular metastatic disease has been attributed to local inhibition of tumor survival secondary to muscle contraction, increased diffusing capacity of enzymes and immune cells, and extreme variability in blood flow and pH, lactate, and oxygen concentration. Skeletal muscular metastases most commonly arise from the lung, kidneys, colon, or melanoma.16 In a recent large series of more than 1400 patients imaged for soft-tissue masses, 2.5% were metastatic.18 There are only 2 reports of soft-tissue metastatic disease involving the hand: one from a patient with a thyroid carcinoma and the other from a patient with a lung adenocarcinoma.18 Soft-tissue metastatic disease from squamous cell carcinoma distal to the wrist has never been reported in the literature.
Acral Soft-Tissue Metastasis
A review from 2012 found 264 cases of skeletal muscle metastasis from 151 articles.6 Only 2 (0.75%) of these patients, as reported above, had a soft-tissue metastasis distal to the wrist.6,17
Conclusion
We report the first known case of a soft-tissue metastasis distal to the wrist from a primary bronchogenic squamous cell carcinoma. This report highlights the extremely uncommon presentation of soft-tissue acral metastatic disease of a bronchogenic squamous cell carcinoma of the lung. Although exceedingly rare, oncologists and physicians who manage pathology of the hand should consider metastatic disease when evaluating a patient with complaints of hand pain and a soft-tissue mass, especially in a patient with a known primary malignancy.
Carcinoma of the lung is the most common lethal form of cancer in both men and women worldwide.1 It accounts for more deaths than the next 3 most common cancers combined. In 2012, 160,000 Americans are estimated to have died from lung cancer.1 Lung cancer is known to have a high metastatic potential for the brain, bones, adrenal glands, lungs, and liver.2 Orthopedic manifestations frequently include bony metastasis, most commonly the vertebrae (42%), ribs (20%), and pelvis (18%).3 Acral metastatic disease is defined as metastasis distal to the elbow or the knee. Bony acral metastases from lung carcinoma to the upper and lower extremities are extremely uncommon, accounting for only 1% each of total bone metastases from carcinoma of the lung.3 Metastases to the bones of the hand are even rarer. Only 0.1% of metastatic disease from any type of carcinoma or sarcoma manifests as metastasis in the hand.4 There are only a few reports in the literature of soft-tissue or muscular metastasis to the hand from a carcinoma. Of these cases, the majority are caused by metastatic lung carcinoma.5-9 There are no reports in the literature of metastatic disease of squamous cell origin affecting the soft tissues of the hand.
We present a case of a man with known metastatic squamous cell carcinoma of the lung who presented with acral soft-tissue metastatic disease. This report highlights a rare clinical scenario that has not been reported in the literature. The report also emphasizes a rare but important consideration for clinicians who encounter acral soft-tissue lesions in patients with a history of a primary carcinoma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old man presented with right-sided pleuritic flank pain, along with a 30-lb weight loss over a 6-month period. A computed tomographic scan revealed a 5.58×3.7-cm cavitary lesion in the right lower lobe with abutment of the posterior chest wall (Figure 1). He underwent biopsy and staging, and was found to be T3N1, with biopsy-proven well-differentiated bronchogenic squamous cell carcinoma. The patient then underwent right lower and middle lobectomy with concomitant en-bloc resection of the posterior portion of ribs 7 to 11, along with mediastinal lymph-node dissection with negative margins. After surgery, he was treated with 4 cycles of adjuvant chemotherapy with cisplatin and docetaxel.
Six months after surgery, the patient began to complain of right-hand pain isolated to the thenar eminence. He also described swelling and significant pain with active or passive movement of the thumb and with relatively mild-to-moderate palpation of the area. The patient reported that the functioning of his thumb deteriorated rapidly over the course of about 1 month. On physical examination, he was neurovascularly intact with no apparent deficit in sensation of his right hand. There was no erythema or overlying skin changes. His right thenar eminence was mildly enlarged as compared with the left, and a firm, focal mass was readily palpated. Range of motion at the metacarpophalangeal joint of the thumb and index finger was limited because of pain. Thumb opposition was markedly limited. After a detailed history and physical examination, we were concerned about possible deep space infection, old hematoma, or possible metastatic disease. Magnetic resonance imaging (MRI) was ordered to evaluate the palpable mass.
Radiographically, localized soft-tissue swelling was present on the palmar surface of the hand obliquely overlying the index finger metacarpal (Figures 2, 3). On MRI, the lesion measured approximately 1.8×3.3 cm and was isointense to slightly hyperintense diffusely with central hyperintensity on T1 images (Figure 4). On T2 and short tau inversion recovery images, the lesion was more strikingly hyperintense and infiltrative in appearance (Figure 5). Postcontrast images showed avid enhancement peripherally, with central nonenhancement consistent with necrosis in the adductor pollicis.
We performed a biopsy of the lesion with the aid of immediate adequacy by fine needle aspiration cytology. We saw mitotically active malignant cells with large nuclei, high nuclear-to-cytoplasmic ratios, nucleoli, and dense cytoplasm, suggesting a metastatic squamous cell carcinoma. Because infection was part of the differential, it is pertinent to note that there was no significant inflammatory infiltrate. The core biopsy was consistent with metastatic lung cancer (Figure 6).
Discussion
This patient presented an interesting diagnostic challenge, particularly because of his previous malignancy. The differential diagnosis of acute onset thenar pain without history of trauma would include encompassing soft-tissue abscess, osteomyelitis, and infectious myositis. Soft-tissue hematoma is also in the differential for this patient, especially given the malignancy. Bony metastasis should be considered in this patient given the propensity of lung carcinoma to metastasize to bone. The location would certainly be atypical, with metastasis to the bones of the forearm or hand representing only 0.1% of all metastasis of any type of primary carcinoma or sarcoma.4 Primary bone or soft-tissue sarcoma should also be considered. Some authors have also suggested that necrosis, peritumoral edema-like signal, and lobulation are more common with skeletal muscle metastasis than with a primary sarcoma.10 In this case, the degree of surrounding postcontrast enhancement made simple muscle tear with hematoma unlikely, despite the presence of increased T1 signal. The lack of evidence for localized infection and the presence of a firm focal mass on physical examination made tumor more likely than infection.
Acrometastasis
Metastatic disease distal to the elbow and knee is very rare; specifically, metastatic disease of the hands or feet accounts for approximately 0.1% of all metastases.4 Carcinoma of the lung accounts for 44% to 47% of all acrometastasis.11,12 When hand acrometastasis is considered, the right hand accounts for 55% of bony cases, likely because of hand dominance, although approximately 10% of patients had bilateral acral metastatic disease.12 The underlying mechanism of acrometastasis remains unclear; however, some authors have postulated that it may result from an increase in vascularity or a trauma to the affected extremity.12,13 Flynn and colleagues12 reviewed the literature and reported a total of 257 cases of acral metastasis to the hand; they found that the median age at presentation was 58 years. Men were more than twice as likely to be affected when compared with women. Most commonly, the primary malignancies were in the lung (44%), kidney (12%), and breast (10%). The authors also reported less common cases of acral metastasis with primary malignancies located in the stomach, liver, rectum, prostate, and colon. Most commonly, these metastases were found in the distal phalynx, followed by the metacarpals, proximal phalynx, and middle phalynx.12
Soft-Tissue Metastasis
Skeletal muscle metastasis occurs in 0.8% to 17.5% of metastatic neoplasms.14-17 Studies in lung cancer patients have also revealed a low prevalence of muscular metastasis (0% to 0.8%).16 The rarity of muscular metastatic disease has been attributed to local inhibition of tumor survival secondary to muscle contraction, increased diffusing capacity of enzymes and immune cells, and extreme variability in blood flow and pH, lactate, and oxygen concentration. Skeletal muscular metastases most commonly arise from the lung, kidneys, colon, or melanoma.16 In a recent large series of more than 1400 patients imaged for soft-tissue masses, 2.5% were metastatic.18 There are only 2 reports of soft-tissue metastatic disease involving the hand: one from a patient with a thyroid carcinoma and the other from a patient with a lung adenocarcinoma.18 Soft-tissue metastatic disease from squamous cell carcinoma distal to the wrist has never been reported in the literature.
Acral Soft-Tissue Metastasis
A review from 2012 found 264 cases of skeletal muscle metastasis from 151 articles.6 Only 2 (0.75%) of these patients, as reported above, had a soft-tissue metastasis distal to the wrist.6,17
Conclusion
We report the first known case of a soft-tissue metastasis distal to the wrist from a primary bronchogenic squamous cell carcinoma. This report highlights the extremely uncommon presentation of soft-tissue acral metastatic disease of a bronchogenic squamous cell carcinoma of the lung. Although exceedingly rare, oncologists and physicians who manage pathology of the hand should consider metastatic disease when evaluating a patient with complaints of hand pain and a soft-tissue mass, especially in a patient with a known primary malignancy.
1. American Cancer Society. Lung Cancer (Non-Small Cell). http://www.cancer.org/acs/groups/cid/documents/webcontent/003115-pdf.pdf. Revised April 30, 2014. Accessed July 22, 2014.
2. Willis RA. Pathology of Tumors. London, England: Butterworth; 1960.
3. Sugiura H, Yamada K, Sugiura T, Hida T, Mitsudomi T. Predictors of survival in patients with bone metastasis of lung cancer. Clin Orthop. 2008;466(3):729-736.
4. Kerin R. Metastatic tumors of the hand. A review of the literature. J Bone Joint Surg Am. 1983;65(9):1331-1335.
5. Alpar S. Muscle metastasis in a patient with squamous cell lung cancer. Turkish Respiratory Journal. 2002;3(2):75-78.
6. Haygood TM, Wong J, Lin JC, et al. Skeletal muscle metastases: a three-part study of a not-so-rare entity. Skeletal Radiol. 2012;41(8):899-909.
7. Tuoheti Y, Okada K, Osanai T, et al. Skeletal muscle metastases of carcinoma: a clinicopathological study of 12 cases. Jpn J Clin Oncol. 2004;34(4):210-214.
8. Chan NP, Yeo W, Ahuja AT, King AD. Multiple skeletal muscle metastases. Hong Kong Med J. 1999;5(4):410.
9. Molina-Garrido MJ, Guillen-Ponce C. Muscle metastasis of carcinoma. Clin Transl Oncol. 2011;13(2):98-101.
10. Williams JB, Youngberg RA, Bui-Mansfield LT, Pitcher JD. MR imaging of skeletal muscle metastases. AJR Am J Roentgenol. 1997;168(2):555-557.
11. Libson E, Bloom RA, Husband JE, Stoker DJ. Metastatic tumours of bones of the hand and foot. A comparative review and report of 43 additional cases. Skeletal Radiol. 1987;16(5):387-392.
12. Flynn CJ, Danjoux C, Wong J, et al. Two cases of acrometastasis to the hands and review of the literature. Curr Oncol. 2008;15(5):51-58.
13. Healey JH, Turnbull AD, Miedema B, Lane JM. Acrometastases. A study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am. 1986;68(5):743-746.
14. Sudo A, Ogihara Y, Shiokawa Y, Fujinami S, Sekiguchi S. Intramuscular metastasis of carcinoma. Clin Orthop. 1993(296):213-217.
15. Surov A, Hainz M, Holzhausen HJ, et al. Skeletal muscle metastases: primary tumours, prevalence, and radiological features. Eur Radiol. 2010;20(3):649-658.
16. Pearson CM. Incidence and type of pathologic alterations observed in muscle in a routine autopsy survey. Neurology. 1959;9:757-766.
17. Acinas Garcia O, Fernández FA, Satué EG, Beulta L, Val-Bernal JF. Metastasis of malignant neoplasms to skeletal muscle. Rev Esp Oncol. 1984;31(1):57-67.
18. Glockner JF, White LM, Sundaram M, McDonald DJ. Unsuspected metastases presenting as solitary soft tissue lesions: a fourteen-year review. Skeletal Radiol. 2000;29(5):270-274.
1. American Cancer Society. Lung Cancer (Non-Small Cell). http://www.cancer.org/acs/groups/cid/documents/webcontent/003115-pdf.pdf. Revised April 30, 2014. Accessed July 22, 2014.
2. Willis RA. Pathology of Tumors. London, England: Butterworth; 1960.
3. Sugiura H, Yamada K, Sugiura T, Hida T, Mitsudomi T. Predictors of survival in patients with bone metastasis of lung cancer. Clin Orthop. 2008;466(3):729-736.
4. Kerin R. Metastatic tumors of the hand. A review of the literature. J Bone Joint Surg Am. 1983;65(9):1331-1335.
5. Alpar S. Muscle metastasis in a patient with squamous cell lung cancer. Turkish Respiratory Journal. 2002;3(2):75-78.
6. Haygood TM, Wong J, Lin JC, et al. Skeletal muscle metastases: a three-part study of a not-so-rare entity. Skeletal Radiol. 2012;41(8):899-909.
7. Tuoheti Y, Okada K, Osanai T, et al. Skeletal muscle metastases of carcinoma: a clinicopathological study of 12 cases. Jpn J Clin Oncol. 2004;34(4):210-214.
8. Chan NP, Yeo W, Ahuja AT, King AD. Multiple skeletal muscle metastases. Hong Kong Med J. 1999;5(4):410.
9. Molina-Garrido MJ, Guillen-Ponce C. Muscle metastasis of carcinoma. Clin Transl Oncol. 2011;13(2):98-101.
10. Williams JB, Youngberg RA, Bui-Mansfield LT, Pitcher JD. MR imaging of skeletal muscle metastases. AJR Am J Roentgenol. 1997;168(2):555-557.
11. Libson E, Bloom RA, Husband JE, Stoker DJ. Metastatic tumours of bones of the hand and foot. A comparative review and report of 43 additional cases. Skeletal Radiol. 1987;16(5):387-392.
12. Flynn CJ, Danjoux C, Wong J, et al. Two cases of acrometastasis to the hands and review of the literature. Curr Oncol. 2008;15(5):51-58.
13. Healey JH, Turnbull AD, Miedema B, Lane JM. Acrometastases. A study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am. 1986;68(5):743-746.
14. Sudo A, Ogihara Y, Shiokawa Y, Fujinami S, Sekiguchi S. Intramuscular metastasis of carcinoma. Clin Orthop. 1993(296):213-217.
15. Surov A, Hainz M, Holzhausen HJ, et al. Skeletal muscle metastases: primary tumours, prevalence, and radiological features. Eur Radiol. 2010;20(3):649-658.
16. Pearson CM. Incidence and type of pathologic alterations observed in muscle in a routine autopsy survey. Neurology. 1959;9:757-766.
17. Acinas Garcia O, Fernández FA, Satué EG, Beulta L, Val-Bernal JF. Metastasis of malignant neoplasms to skeletal muscle. Rev Esp Oncol. 1984;31(1):57-67.
18. Glockner JF, White LM, Sundaram M, McDonald DJ. Unsuspected metastases presenting as solitary soft tissue lesions: a fourteen-year review. Skeletal Radiol. 2000;29(5):270-274.
Large Solitary Glomus Tumor of the Wrist Involving the Radial Artery
Glomus tumors are neoplasms that originate from normal glomus bodies in the skin and are most commonly found in the subungual areas of the digits.1 Glomus bodies are neuromyoarterial structures in the reticular dermis that serve as specialized arteriovenous anastomoses. These bodies contain afferent arterioles and efferent veins with multiple connections, and glomus cells have contractile properties because of their similarity to smooth muscle cells.1,2 Glomus bodies help regulate blood flow and temperature of the skin and are found in their largest concentration in the fingertips, palms of the hands, and soles of the feet.3,4
Glomus tumors represent hyperplastic glomus bodies and make up 1% to 4.5% of upper extremity neoplasms, with approximately 75% in the hand and 50% in the subungual area.1,5,6 These tumors can also present in multiple locations at once and can occur in atypical and ectopic locations.3 Although generally benign, glomus tumors can also exhibit malignant and metastatic potential in rare cases.7,8 They can also be locally aggressive with bony destruction of the distal phalynx.2,9,10 Tumors typically present as painful solitary soft-tissue lesions that are exquisitely tender to palpation, dark red-purple or bluish, and hypersensitive to cold.5,10 Van Geertruyden and colleagues10 reported that the diagnosis of glomus tumor can be made clinically in 90% of cases. However, glomus tumors can easily be mistaken for other lesions, such as hemangiomas, angiomas, neuromas, neurofibromas, lipomas, and ganglion cysts. An inaccurate or incomplete workup can result in persistent pain and symptoms along with intraoperative complications.3 Magnetic resonance imaging (MRI), the most sensitive imaging modality for detecting glomus tumors of the hand, can assist in the workup.3,11,12
Extradigital glomus tumors are difficult to diagnose because of their rarity and unspecific symptoms and presentation.13 Misdiagnosis and delayed diagnosis can result in significant chronic pain, disuse syndromes, and disability.1,10 Correct diagnosis and surgical resection are generally curative with complete resolution of symptoms.
In this article, we report a case of a large atypical glomus tumor that occurred on the wrist and involved the radial artery. This tumor was successfully treated with surgical excision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 63-year-old man presented to clinic with an extremely tender soft-tissue mass on his nondominant, left wrist. The mass had been increasing in size for a year. It was painless at rest but very painful to light palpation, with referred pain proximally up to the shoulder.
The patient did not recall any traumatic or inciting event, had not undergone any prior workup or treatment for symptoms, and had no history of masses elsewhere on the body. Past medical history was significant for type 2 diabetes and colon and prostate cancer, which had been treated with chemotherapy and was now in remission.
Physical examination revealed a 2×2.5-cm well-circumscribed soft-tissue mass on the volar-radial aspect of the left wrist proximal to the thenar eminence and radial to the flexor carpi radialis tendon (Figure 1). The mass was soft, mobile, and nonfluctuant and did not transilluminate. The overlying skin was normal in color and appearance—no discoloration, erythema, wounds, or drainage. The radial artery was palpable, and the mass did not pulsate or have a bruit. The patient had normal wrist range of motion limited by pain on compression of the mass with motor and sensation intact throughout the hand. Plain radiographs of the wrist showed no bony pathology or involvement from the mass. A soft-tissue shadow was visible around the wrist without calcifications. A wrist MRI was performed to better evaluate the mass, and the T2-weighted images showed a heterogeneous subcutaneous mass adjacent to the radial artery with increased signal intensity from surrounding feeding vessels (Figure 2).
Given the clinical and imaging findings, there was concern for a possible vascular tumor. Therefore, excisional biopsy was recommended over needle biopsy because of the bleeding risk. With the patient under general anesthesia, and a tourniquet used without exsanguination, a Brunner-type zigzag incision was made centered over the mass with elevated skin flaps. The 2.7×2.6×1.1-cm mass was superficial and involved the radial artery (Figure 3). After the radial artery was dissected proximally and distally, 2 perforating vessels were found entering the mass. These vessels were ligated, which allowed the mass to be peeled completely off the artery. Histology with hematoxylin-eosin staining showed solid sheets of uniform round cells with interspersed capillaries and centrally placed nuclei without evidence of malignancy (Figure 4).
The tourniquet was released before skin closure, and adequate hemostasis was obtained. The wound was closed, and the patient was placed in a volar wrist splint for immobilization. Pain relief after excision of the mass was immediate, and the postoperative course uneventful. After surgery, immunohistochemistry of the mass showed minimal mitotic activity, with a positive immunoperoxidase stain for smooth muscle actin confirming a diagnosis of glomus tumor (Figure 5). At 3-year follow-up, the patient had no pain, symptoms, or tumor recurrence.
Discussion
Glomus tumors are an established cause of pain in the subungual areas of the hand; numerous cases have been reported.1,5,10,14 However, extradigital glomus tumors, particularly those involving the wrist, are rare, and only a few have been described. Given the lack of consistent findings and presentations, diagnosis is difficult. Case series have documented an overall 2:1 female-to-male predominance of glomus tumors,6 but extradigital tumors are more common in men (4.6:1 male-to-female ratio).3 Extradigital glomus tumors are commonly diagnosed between ages 40 and 80 years. Classic symptoms of subungual tumors include pain, localized tenderness, and cold hypersensitivity,1,10 but symptoms are much more variable with extradigital locations. Previous trauma or injury to the lesion area is reported in 20% to 30% of cases before symptom onset.3,15 Intravascular locations of glomus tumors are extremely rare; only 4 cases of tumors involving venous structures have been reported.16-19 In the present case, the patient’s main complaints were pain and localized tenderness associated with a progressively increasing mass without any history of trauma. The large size of his mass (~2.5 cm in diameter) on examination was unique, as was involvement of the radial artery.
Misdiagnosis and delayed diagnosis of extradigital glomus tumors are common, and symptoms such as chronic pain typically persist for 7 to 11 years before the correct diagnosis is made.1,10 On average, 2.5 physician consultants (including psychiatrists) evaluate the patient before glomus tumor is identified.10 There are other reports of atypical or ectopic glomus tumors taking 5 to 25 years to be diagnosed.20-22 The differential diagnosis for glomus tumors includes hemangiomas, cellular or cavernous hemangiomas, vascular tumors, neuromas, neurofibromas, lipomas, paragangliomas, ganglion cysts, pigmented nevi, Pacinian corpuscle hyperplasia, and foreign bodies. A key element of clinical diagnosis is the disproportionate amount of pain and localized tenderness caused by the lesion relative to its size. The hypersensitivity of this tumor is thought to result from enlargement of the tumor and impingement on nearby Pacinian corpuscles, nerve endings in the skin that are responsible for sensitivity to vibration and pressure.2,9
Plain radiographs can be useful in detecting glomus tumors of the hand but are less helpful with extradigital tumors, with identification rates of 24% in certain series.3 MRI is the most sensitive imaging modality for diagnosing glomus tumors of the hand; a detection rate of 80% to 100% has been reported in various case series.3,11,12 Specificity of MRI for glomus tumors has been reported at 50%.11,23 Placement of a radiographic marker directly over the area of most pain can assist in tumor localization.3 Glomus tumors typically have decreased signal intensity on T1-weighted images and increased intensity on T2-weighted images, but signal patterns are variable and particularly difficult to differentiate with small tumors. MRI is useful in the setting of recurrent glomus tumors, where incomplete excision is possible. In 24 cases of continued pain after glomus tumor excision, Theumann and colleagues24 used MRI to identify a nodule consistent with recurrent glomus tumor in all patients. Three-dimensional contrast-enhanced magnetic resonance angiography (MRA) can also help diagnose glomus tumors while providing valuable information regarding size and location for surgical planning.25,26 With MRA, it is crucial to evaluate the arterial or arteriovenous phase of imaging, as the glomus tumor is richly vascularized and shows contrast enhancement after intravenous injection of gadolinium.27 Angiography, ultrasonography, thermography, and scintigraphy have all been used to diagnose glomus tumors but have shown limited utility and accuracy.11
Treatment of glomus tumors is complete surgical excision because of their relatively small size and subcutaneous location. Resection success rates are consistently higher than 95%, with resolution of all symptoms.1,10,14 Local recurrence of tumors after excision occurs in 1% to 33% of cases, depending on series, and may be immediate or delayed, with immediate recurrence commonly caused by inadequate excision.1,10,15,28 Delayed recurrence is less common and presents several years after excision, typically with a new growth near the previous excision.10 Recurrence years after surgery may also represent multiple tumors unrecognized during initial workup and can be treated with repeat excision or radiotherapy.
Robert and colleagues29 recently reported the case of a glomus tumor, on the dorsal aspect of the wrist, discovered incidentally in a 71-year-old patient and treated with surgical excision. Several years earlier, Chim and colleagues30 described a similar case, of a large wrist glomus tumor worked up with MRI. In a retrospective review of all extradigital glomus tumors seen over a 20-year period, Schiefer and colleagues3 reported 4 glomus tumors of the wrist out of 56 tumors total. The most common sites were forearm (11 cases) and knee (10 cases), and the majority of patients presented with pain and localized tenderness. Mean tumor size was 0.66 cm (range, 0.1-0.3 cm), with 77% of tumors less than 1 cm. Our patient’s 2.7×2.6×1.1-cm tumor was large for a glomus tumor. Its involvement with the radial artery feeding vessels likely contributed to its large and progressively increasing size. It is worth noting that, in the series by Schiefer and colleagues,3 the only patient with symptoms persisting after excision had a large (3 cm in diameter) deep tumor of the foot; the entire tumor was removed, and there was no recurrence by 10-year follow-up. Folpe and colleagues7 suggested that deep tumors larger than 2 cm should be at higher suspicion for malignancy. Joseph and Posner21 reported 3 cases of glomus tumors, on the ulnar side of the wrist, diagnosed with help of a provocative test using ethyl chloride spray.
Conclusion
Overall, glomus tumors are rare and challenging to diagnosis and should be in the differential in any symptomatic patient with a painful soft-tissue mass of the wrist. Advanced imaging studies, such as MRI, can assist in localization, diagnosis, and preoperative planning. Histology and immunohistochemistry are essential to differentiate glomus tumor from other vascular tumors, and complete excision is necessary to prevent local recurrence.
1. Carroll RE, Berman AT. Glomus tumors of the hand: review of the literature and report on twenty-eight cases. J Bone Joint Surg Am. 1972;54(4):691-703.
2. Riddell DH, Martin RS. Glomus tumor of unusual size; case report. Ann Surg. 1951;133(3):401-403.
3. Schiefer TK, Parker WL, Anakwenze OA, Amadio PC, Inwards CY, Spinner RJ. Extradigital glomus tumors: a 20-year experience. Mayo Clin Proc. 2006;81(10):1337-1344.
4. Tuncali D, Yilmaz AC, Terzioglu A, Aslan G. Multiple occurrences of different histologic types of the glomus tumor. J Hand Surg Am. 2005;30(1):161-164.
5. Greene RG. Soft tissue tumors of the hand and wrist. A 10 year survey. J Med Soc N J. 1964;61:495-498.
6. Maxwell GP, Curtis RM, Wilgis EF. Multiple digital glomus tumors. J Hand Surg Am. 1979;4(4):363-367.
7. Folpe AL, Fanburg-Smith JC, Miettinen M, Weiss SW. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25(1):1-12.
8. De Chiara A, Apice G, Mori S, et al. Malignant glomus tumour: a case report and review of the literature. Sarcoma. 2003;7(2):87-91.
9. Riveros M, Pack GT. The glomus tumor; report of 20 cases. Ann Surg. 1951;133(3):394-400.
10. Van Geertruyden J, Lorea P, Goldschmidt D, et al. Glomus tumours of the hand. A retrospective study of 51 cases. J Hand Surg Br. 1996;21(2):257-260.
11. Al-Qattan MM, Al-Namla A, Al-Thunayan A, Al-Subhi F, El-Shayeb AF. Magnetic resonance imaging in the diagnosis of glomus tumours of the hand. J Hand Surg Br. 2005;30(5):535-540.
12. Drape JL, Idy-Peretti I, Goettmann S, et al. Subungual glomus tumors: evaluation with MR imaging. Radiology. 1995;195(2):507-515.
13. Heys SD, Brittenden J, Atkinson P, Eremin O. Glomus tumour: an analysis of 43 patients and review of the literature. Br J Surg. 1992;79(4):345-347.
14. Bhaskaranand K, Navadgi BC. Glomus tumour of the hand. J Hand Surg Br. 2002;27(3):229-231.
15. Rettig AC, Strickland JW. Glomus tumor of the digits. J Hand Surg Am. 1977;2(4):261-265.
16. Beham A, Fletcher CD. Intravascular glomus tumour: a previously undescribed phenomenon. Virchows Arch A Pathol Anat Histopathol. 1991;418(2):175-177.
17. Googe PB, Griffin WC. Intravenous glomus tumor of the forearm. J Cutan Pathol. 1993;20(4):359-363.
18. Koibuchi H, Fujii Y, Taniguchi N. An unusual case of a glomus tumor developing in a subcutaneous vein of the wrist. J Clin Ultrasound. 2008;36(6):369-370.
19. Acebo E, Val-Bernal JF, Arce F. Giant intravenous glomus tumor. J Cutan Pathol. 1997;24(6):384-389.
20. Ghaly RF, Ring AM. Supraclavicular glomus tumor, 20 year history of undiagnosed shoulder pain: a case report. Pain. 1999;83(2):379-382.
21. Joseph FR, Posner MA. Glomus tumors of the wrist. J Hand Surg Am. 1983;8(6):918-920.
22. Abou Jaoude JF, Roula Farah A, Sargi Z, Khairallah S, Fakih C. Glomus tumors: report on eleven cases and a review of the literature. Chir Main. 2000;19(4):243-252.
23. Jablon M, Horowitz A, Bernstein DA. Magnetic resonance imaging of a glomus tumor of the fingertip. J Hand Surg Am. 1990;15(3):507-509.
24. Theumann NH, Goettmann S, Le Viet D, et al. Recurrent glomus tumors of fingertips: MR imaging evaluation. Radiology. 2002;223(1):143-151.
25. Boudghene FP, Gouny P, Tassart M, Callard P, Le Breton C, Vayssairat M. Subungual glomus tumor: combined use of MRI and three-dimensional contrast MR angiography. J Magn Reson Imaging. 1998;8(6):1326-1328.
26. Van Ruyssevelt CE, Vranckx P. Subungual glomus tumor: emphasis on MR angiography. AJR Am J Roentgenol. 2004;182(1):263-264.
27. Connell DA, Koulouris G, Thorn DA, Potter HG. Contrast-enhanced MR angiography of the hand. Radiographics. 2002;22(3):583-599.
28. Varian JP, Cleak DK. Glomus tumours in the hand. Hand. 1980;12(3):293-299.
29. Robert G, Sawaya E, Pelissier P. Glomus tumor of the dorsal aspect of the wrist: a case report [in French]. Chir Main. 2012;31(4):214-216.
30. Chim H, Lahiri A, Chew WY. Atypical glomus tumour of the wrist: a case report. Hand Surg. 2009;14(2-3):121-123.
Glomus tumors are neoplasms that originate from normal glomus bodies in the skin and are most commonly found in the subungual areas of the digits.1 Glomus bodies are neuromyoarterial structures in the reticular dermis that serve as specialized arteriovenous anastomoses. These bodies contain afferent arterioles and efferent veins with multiple connections, and glomus cells have contractile properties because of their similarity to smooth muscle cells.1,2 Glomus bodies help regulate blood flow and temperature of the skin and are found in their largest concentration in the fingertips, palms of the hands, and soles of the feet.3,4
Glomus tumors represent hyperplastic glomus bodies and make up 1% to 4.5% of upper extremity neoplasms, with approximately 75% in the hand and 50% in the subungual area.1,5,6 These tumors can also present in multiple locations at once and can occur in atypical and ectopic locations.3 Although generally benign, glomus tumors can also exhibit malignant and metastatic potential in rare cases.7,8 They can also be locally aggressive with bony destruction of the distal phalynx.2,9,10 Tumors typically present as painful solitary soft-tissue lesions that are exquisitely tender to palpation, dark red-purple or bluish, and hypersensitive to cold.5,10 Van Geertruyden and colleagues10 reported that the diagnosis of glomus tumor can be made clinically in 90% of cases. However, glomus tumors can easily be mistaken for other lesions, such as hemangiomas, angiomas, neuromas, neurofibromas, lipomas, and ganglion cysts. An inaccurate or incomplete workup can result in persistent pain and symptoms along with intraoperative complications.3 Magnetic resonance imaging (MRI), the most sensitive imaging modality for detecting glomus tumors of the hand, can assist in the workup.3,11,12
Extradigital glomus tumors are difficult to diagnose because of their rarity and unspecific symptoms and presentation.13 Misdiagnosis and delayed diagnosis can result in significant chronic pain, disuse syndromes, and disability.1,10 Correct diagnosis and surgical resection are generally curative with complete resolution of symptoms.
In this article, we report a case of a large atypical glomus tumor that occurred on the wrist and involved the radial artery. This tumor was successfully treated with surgical excision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 63-year-old man presented to clinic with an extremely tender soft-tissue mass on his nondominant, left wrist. The mass had been increasing in size for a year. It was painless at rest but very painful to light palpation, with referred pain proximally up to the shoulder.
The patient did not recall any traumatic or inciting event, had not undergone any prior workup or treatment for symptoms, and had no history of masses elsewhere on the body. Past medical history was significant for type 2 diabetes and colon and prostate cancer, which had been treated with chemotherapy and was now in remission.
Physical examination revealed a 2×2.5-cm well-circumscribed soft-tissue mass on the volar-radial aspect of the left wrist proximal to the thenar eminence and radial to the flexor carpi radialis tendon (Figure 1). The mass was soft, mobile, and nonfluctuant and did not transilluminate. The overlying skin was normal in color and appearance—no discoloration, erythema, wounds, or drainage. The radial artery was palpable, and the mass did not pulsate or have a bruit. The patient had normal wrist range of motion limited by pain on compression of the mass with motor and sensation intact throughout the hand. Plain radiographs of the wrist showed no bony pathology or involvement from the mass. A soft-tissue shadow was visible around the wrist without calcifications. A wrist MRI was performed to better evaluate the mass, and the T2-weighted images showed a heterogeneous subcutaneous mass adjacent to the radial artery with increased signal intensity from surrounding feeding vessels (Figure 2).
Given the clinical and imaging findings, there was concern for a possible vascular tumor. Therefore, excisional biopsy was recommended over needle biopsy because of the bleeding risk. With the patient under general anesthesia, and a tourniquet used without exsanguination, a Brunner-type zigzag incision was made centered over the mass with elevated skin flaps. The 2.7×2.6×1.1-cm mass was superficial and involved the radial artery (Figure 3). After the radial artery was dissected proximally and distally, 2 perforating vessels were found entering the mass. These vessels were ligated, which allowed the mass to be peeled completely off the artery. Histology with hematoxylin-eosin staining showed solid sheets of uniform round cells with interspersed capillaries and centrally placed nuclei without evidence of malignancy (Figure 4).
The tourniquet was released before skin closure, and adequate hemostasis was obtained. The wound was closed, and the patient was placed in a volar wrist splint for immobilization. Pain relief after excision of the mass was immediate, and the postoperative course uneventful. After surgery, immunohistochemistry of the mass showed minimal mitotic activity, with a positive immunoperoxidase stain for smooth muscle actin confirming a diagnosis of glomus tumor (Figure 5). At 3-year follow-up, the patient had no pain, symptoms, or tumor recurrence.
Discussion
Glomus tumors are an established cause of pain in the subungual areas of the hand; numerous cases have been reported.1,5,10,14 However, extradigital glomus tumors, particularly those involving the wrist, are rare, and only a few have been described. Given the lack of consistent findings and presentations, diagnosis is difficult. Case series have documented an overall 2:1 female-to-male predominance of glomus tumors,6 but extradigital tumors are more common in men (4.6:1 male-to-female ratio).3 Extradigital glomus tumors are commonly diagnosed between ages 40 and 80 years. Classic symptoms of subungual tumors include pain, localized tenderness, and cold hypersensitivity,1,10 but symptoms are much more variable with extradigital locations. Previous trauma or injury to the lesion area is reported in 20% to 30% of cases before symptom onset.3,15 Intravascular locations of glomus tumors are extremely rare; only 4 cases of tumors involving venous structures have been reported.16-19 In the present case, the patient’s main complaints were pain and localized tenderness associated with a progressively increasing mass without any history of trauma. The large size of his mass (~2.5 cm in diameter) on examination was unique, as was involvement of the radial artery.
Misdiagnosis and delayed diagnosis of extradigital glomus tumors are common, and symptoms such as chronic pain typically persist for 7 to 11 years before the correct diagnosis is made.1,10 On average, 2.5 physician consultants (including psychiatrists) evaluate the patient before glomus tumor is identified.10 There are other reports of atypical or ectopic glomus tumors taking 5 to 25 years to be diagnosed.20-22 The differential diagnosis for glomus tumors includes hemangiomas, cellular or cavernous hemangiomas, vascular tumors, neuromas, neurofibromas, lipomas, paragangliomas, ganglion cysts, pigmented nevi, Pacinian corpuscle hyperplasia, and foreign bodies. A key element of clinical diagnosis is the disproportionate amount of pain and localized tenderness caused by the lesion relative to its size. The hypersensitivity of this tumor is thought to result from enlargement of the tumor and impingement on nearby Pacinian corpuscles, nerve endings in the skin that are responsible for sensitivity to vibration and pressure.2,9
Plain radiographs can be useful in detecting glomus tumors of the hand but are less helpful with extradigital tumors, with identification rates of 24% in certain series.3 MRI is the most sensitive imaging modality for diagnosing glomus tumors of the hand; a detection rate of 80% to 100% has been reported in various case series.3,11,12 Specificity of MRI for glomus tumors has been reported at 50%.11,23 Placement of a radiographic marker directly over the area of most pain can assist in tumor localization.3 Glomus tumors typically have decreased signal intensity on T1-weighted images and increased intensity on T2-weighted images, but signal patterns are variable and particularly difficult to differentiate with small tumors. MRI is useful in the setting of recurrent glomus tumors, where incomplete excision is possible. In 24 cases of continued pain after glomus tumor excision, Theumann and colleagues24 used MRI to identify a nodule consistent with recurrent glomus tumor in all patients. Three-dimensional contrast-enhanced magnetic resonance angiography (MRA) can also help diagnose glomus tumors while providing valuable information regarding size and location for surgical planning.25,26 With MRA, it is crucial to evaluate the arterial or arteriovenous phase of imaging, as the glomus tumor is richly vascularized and shows contrast enhancement after intravenous injection of gadolinium.27 Angiography, ultrasonography, thermography, and scintigraphy have all been used to diagnose glomus tumors but have shown limited utility and accuracy.11
Treatment of glomus tumors is complete surgical excision because of their relatively small size and subcutaneous location. Resection success rates are consistently higher than 95%, with resolution of all symptoms.1,10,14 Local recurrence of tumors after excision occurs in 1% to 33% of cases, depending on series, and may be immediate or delayed, with immediate recurrence commonly caused by inadequate excision.1,10,15,28 Delayed recurrence is less common and presents several years after excision, typically with a new growth near the previous excision.10 Recurrence years after surgery may also represent multiple tumors unrecognized during initial workup and can be treated with repeat excision or radiotherapy.
Robert and colleagues29 recently reported the case of a glomus tumor, on the dorsal aspect of the wrist, discovered incidentally in a 71-year-old patient and treated with surgical excision. Several years earlier, Chim and colleagues30 described a similar case, of a large wrist glomus tumor worked up with MRI. In a retrospective review of all extradigital glomus tumors seen over a 20-year period, Schiefer and colleagues3 reported 4 glomus tumors of the wrist out of 56 tumors total. The most common sites were forearm (11 cases) and knee (10 cases), and the majority of patients presented with pain and localized tenderness. Mean tumor size was 0.66 cm (range, 0.1-0.3 cm), with 77% of tumors less than 1 cm. Our patient’s 2.7×2.6×1.1-cm tumor was large for a glomus tumor. Its involvement with the radial artery feeding vessels likely contributed to its large and progressively increasing size. It is worth noting that, in the series by Schiefer and colleagues,3 the only patient with symptoms persisting after excision had a large (3 cm in diameter) deep tumor of the foot; the entire tumor was removed, and there was no recurrence by 10-year follow-up. Folpe and colleagues7 suggested that deep tumors larger than 2 cm should be at higher suspicion for malignancy. Joseph and Posner21 reported 3 cases of glomus tumors, on the ulnar side of the wrist, diagnosed with help of a provocative test using ethyl chloride spray.
Conclusion
Overall, glomus tumors are rare and challenging to diagnosis and should be in the differential in any symptomatic patient with a painful soft-tissue mass of the wrist. Advanced imaging studies, such as MRI, can assist in localization, diagnosis, and preoperative planning. Histology and immunohistochemistry are essential to differentiate glomus tumor from other vascular tumors, and complete excision is necessary to prevent local recurrence.
Glomus tumors are neoplasms that originate from normal glomus bodies in the skin and are most commonly found in the subungual areas of the digits.1 Glomus bodies are neuromyoarterial structures in the reticular dermis that serve as specialized arteriovenous anastomoses. These bodies contain afferent arterioles and efferent veins with multiple connections, and glomus cells have contractile properties because of their similarity to smooth muscle cells.1,2 Glomus bodies help regulate blood flow and temperature of the skin and are found in their largest concentration in the fingertips, palms of the hands, and soles of the feet.3,4
Glomus tumors represent hyperplastic glomus bodies and make up 1% to 4.5% of upper extremity neoplasms, with approximately 75% in the hand and 50% in the subungual area.1,5,6 These tumors can also present in multiple locations at once and can occur in atypical and ectopic locations.3 Although generally benign, glomus tumors can also exhibit malignant and metastatic potential in rare cases.7,8 They can also be locally aggressive with bony destruction of the distal phalynx.2,9,10 Tumors typically present as painful solitary soft-tissue lesions that are exquisitely tender to palpation, dark red-purple or bluish, and hypersensitive to cold.5,10 Van Geertruyden and colleagues10 reported that the diagnosis of glomus tumor can be made clinically in 90% of cases. However, glomus tumors can easily be mistaken for other lesions, such as hemangiomas, angiomas, neuromas, neurofibromas, lipomas, and ganglion cysts. An inaccurate or incomplete workup can result in persistent pain and symptoms along with intraoperative complications.3 Magnetic resonance imaging (MRI), the most sensitive imaging modality for detecting glomus tumors of the hand, can assist in the workup.3,11,12
Extradigital glomus tumors are difficult to diagnose because of their rarity and unspecific symptoms and presentation.13 Misdiagnosis and delayed diagnosis can result in significant chronic pain, disuse syndromes, and disability.1,10 Correct diagnosis and surgical resection are generally curative with complete resolution of symptoms.
In this article, we report a case of a large atypical glomus tumor that occurred on the wrist and involved the radial artery. This tumor was successfully treated with surgical excision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 63-year-old man presented to clinic with an extremely tender soft-tissue mass on his nondominant, left wrist. The mass had been increasing in size for a year. It was painless at rest but very painful to light palpation, with referred pain proximally up to the shoulder.
The patient did not recall any traumatic or inciting event, had not undergone any prior workup or treatment for symptoms, and had no history of masses elsewhere on the body. Past medical history was significant for type 2 diabetes and colon and prostate cancer, which had been treated with chemotherapy and was now in remission.
Physical examination revealed a 2×2.5-cm well-circumscribed soft-tissue mass on the volar-radial aspect of the left wrist proximal to the thenar eminence and radial to the flexor carpi radialis tendon (Figure 1). The mass was soft, mobile, and nonfluctuant and did not transilluminate. The overlying skin was normal in color and appearance—no discoloration, erythema, wounds, or drainage. The radial artery was palpable, and the mass did not pulsate or have a bruit. The patient had normal wrist range of motion limited by pain on compression of the mass with motor and sensation intact throughout the hand. Plain radiographs of the wrist showed no bony pathology or involvement from the mass. A soft-tissue shadow was visible around the wrist without calcifications. A wrist MRI was performed to better evaluate the mass, and the T2-weighted images showed a heterogeneous subcutaneous mass adjacent to the radial artery with increased signal intensity from surrounding feeding vessels (Figure 2).
Given the clinical and imaging findings, there was concern for a possible vascular tumor. Therefore, excisional biopsy was recommended over needle biopsy because of the bleeding risk. With the patient under general anesthesia, and a tourniquet used without exsanguination, a Brunner-type zigzag incision was made centered over the mass with elevated skin flaps. The 2.7×2.6×1.1-cm mass was superficial and involved the radial artery (Figure 3). After the radial artery was dissected proximally and distally, 2 perforating vessels were found entering the mass. These vessels were ligated, which allowed the mass to be peeled completely off the artery. Histology with hematoxylin-eosin staining showed solid sheets of uniform round cells with interspersed capillaries and centrally placed nuclei without evidence of malignancy (Figure 4).
The tourniquet was released before skin closure, and adequate hemostasis was obtained. The wound was closed, and the patient was placed in a volar wrist splint for immobilization. Pain relief after excision of the mass was immediate, and the postoperative course uneventful. After surgery, immunohistochemistry of the mass showed minimal mitotic activity, with a positive immunoperoxidase stain for smooth muscle actin confirming a diagnosis of glomus tumor (Figure 5). At 3-year follow-up, the patient had no pain, symptoms, or tumor recurrence.
Discussion
Glomus tumors are an established cause of pain in the subungual areas of the hand; numerous cases have been reported.1,5,10,14 However, extradigital glomus tumors, particularly those involving the wrist, are rare, and only a few have been described. Given the lack of consistent findings and presentations, diagnosis is difficult. Case series have documented an overall 2:1 female-to-male predominance of glomus tumors,6 but extradigital tumors are more common in men (4.6:1 male-to-female ratio).3 Extradigital glomus tumors are commonly diagnosed between ages 40 and 80 years. Classic symptoms of subungual tumors include pain, localized tenderness, and cold hypersensitivity,1,10 but symptoms are much more variable with extradigital locations. Previous trauma or injury to the lesion area is reported in 20% to 30% of cases before symptom onset.3,15 Intravascular locations of glomus tumors are extremely rare; only 4 cases of tumors involving venous structures have been reported.16-19 In the present case, the patient’s main complaints were pain and localized tenderness associated with a progressively increasing mass without any history of trauma. The large size of his mass (~2.5 cm in diameter) on examination was unique, as was involvement of the radial artery.
Misdiagnosis and delayed diagnosis of extradigital glomus tumors are common, and symptoms such as chronic pain typically persist for 7 to 11 years before the correct diagnosis is made.1,10 On average, 2.5 physician consultants (including psychiatrists) evaluate the patient before glomus tumor is identified.10 There are other reports of atypical or ectopic glomus tumors taking 5 to 25 years to be diagnosed.20-22 The differential diagnosis for glomus tumors includes hemangiomas, cellular or cavernous hemangiomas, vascular tumors, neuromas, neurofibromas, lipomas, paragangliomas, ganglion cysts, pigmented nevi, Pacinian corpuscle hyperplasia, and foreign bodies. A key element of clinical diagnosis is the disproportionate amount of pain and localized tenderness caused by the lesion relative to its size. The hypersensitivity of this tumor is thought to result from enlargement of the tumor and impingement on nearby Pacinian corpuscles, nerve endings in the skin that are responsible for sensitivity to vibration and pressure.2,9
Plain radiographs can be useful in detecting glomus tumors of the hand but are less helpful with extradigital tumors, with identification rates of 24% in certain series.3 MRI is the most sensitive imaging modality for diagnosing glomus tumors of the hand; a detection rate of 80% to 100% has been reported in various case series.3,11,12 Specificity of MRI for glomus tumors has been reported at 50%.11,23 Placement of a radiographic marker directly over the area of most pain can assist in tumor localization.3 Glomus tumors typically have decreased signal intensity on T1-weighted images and increased intensity on T2-weighted images, but signal patterns are variable and particularly difficult to differentiate with small tumors. MRI is useful in the setting of recurrent glomus tumors, where incomplete excision is possible. In 24 cases of continued pain after glomus tumor excision, Theumann and colleagues24 used MRI to identify a nodule consistent with recurrent glomus tumor in all patients. Three-dimensional contrast-enhanced magnetic resonance angiography (MRA) can also help diagnose glomus tumors while providing valuable information regarding size and location for surgical planning.25,26 With MRA, it is crucial to evaluate the arterial or arteriovenous phase of imaging, as the glomus tumor is richly vascularized and shows contrast enhancement after intravenous injection of gadolinium.27 Angiography, ultrasonography, thermography, and scintigraphy have all been used to diagnose glomus tumors but have shown limited utility and accuracy.11
Treatment of glomus tumors is complete surgical excision because of their relatively small size and subcutaneous location. Resection success rates are consistently higher than 95%, with resolution of all symptoms.1,10,14 Local recurrence of tumors after excision occurs in 1% to 33% of cases, depending on series, and may be immediate or delayed, with immediate recurrence commonly caused by inadequate excision.1,10,15,28 Delayed recurrence is less common and presents several years after excision, typically with a new growth near the previous excision.10 Recurrence years after surgery may also represent multiple tumors unrecognized during initial workup and can be treated with repeat excision or radiotherapy.
Robert and colleagues29 recently reported the case of a glomus tumor, on the dorsal aspect of the wrist, discovered incidentally in a 71-year-old patient and treated with surgical excision. Several years earlier, Chim and colleagues30 described a similar case, of a large wrist glomus tumor worked up with MRI. In a retrospective review of all extradigital glomus tumors seen over a 20-year period, Schiefer and colleagues3 reported 4 glomus tumors of the wrist out of 56 tumors total. The most common sites were forearm (11 cases) and knee (10 cases), and the majority of patients presented with pain and localized tenderness. Mean tumor size was 0.66 cm (range, 0.1-0.3 cm), with 77% of tumors less than 1 cm. Our patient’s 2.7×2.6×1.1-cm tumor was large for a glomus tumor. Its involvement with the radial artery feeding vessels likely contributed to its large and progressively increasing size. It is worth noting that, in the series by Schiefer and colleagues,3 the only patient with symptoms persisting after excision had a large (3 cm in diameter) deep tumor of the foot; the entire tumor was removed, and there was no recurrence by 10-year follow-up. Folpe and colleagues7 suggested that deep tumors larger than 2 cm should be at higher suspicion for malignancy. Joseph and Posner21 reported 3 cases of glomus tumors, on the ulnar side of the wrist, diagnosed with help of a provocative test using ethyl chloride spray.
Conclusion
Overall, glomus tumors are rare and challenging to diagnosis and should be in the differential in any symptomatic patient with a painful soft-tissue mass of the wrist. Advanced imaging studies, such as MRI, can assist in localization, diagnosis, and preoperative planning. Histology and immunohistochemistry are essential to differentiate glomus tumor from other vascular tumors, and complete excision is necessary to prevent local recurrence.
1. Carroll RE, Berman AT. Glomus tumors of the hand: review of the literature and report on twenty-eight cases. J Bone Joint Surg Am. 1972;54(4):691-703.
2. Riddell DH, Martin RS. Glomus tumor of unusual size; case report. Ann Surg. 1951;133(3):401-403.
3. Schiefer TK, Parker WL, Anakwenze OA, Amadio PC, Inwards CY, Spinner RJ. Extradigital glomus tumors: a 20-year experience. Mayo Clin Proc. 2006;81(10):1337-1344.
4. Tuncali D, Yilmaz AC, Terzioglu A, Aslan G. Multiple occurrences of different histologic types of the glomus tumor. J Hand Surg Am. 2005;30(1):161-164.
5. Greene RG. Soft tissue tumors of the hand and wrist. A 10 year survey. J Med Soc N J. 1964;61:495-498.
6. Maxwell GP, Curtis RM, Wilgis EF. Multiple digital glomus tumors. J Hand Surg Am. 1979;4(4):363-367.
7. Folpe AL, Fanburg-Smith JC, Miettinen M, Weiss SW. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25(1):1-12.
8. De Chiara A, Apice G, Mori S, et al. Malignant glomus tumour: a case report and review of the literature. Sarcoma. 2003;7(2):87-91.
9. Riveros M, Pack GT. The glomus tumor; report of 20 cases. Ann Surg. 1951;133(3):394-400.
10. Van Geertruyden J, Lorea P, Goldschmidt D, et al. Glomus tumours of the hand. A retrospective study of 51 cases. J Hand Surg Br. 1996;21(2):257-260.
11. Al-Qattan MM, Al-Namla A, Al-Thunayan A, Al-Subhi F, El-Shayeb AF. Magnetic resonance imaging in the diagnosis of glomus tumours of the hand. J Hand Surg Br. 2005;30(5):535-540.
12. Drape JL, Idy-Peretti I, Goettmann S, et al. Subungual glomus tumors: evaluation with MR imaging. Radiology. 1995;195(2):507-515.
13. Heys SD, Brittenden J, Atkinson P, Eremin O. Glomus tumour: an analysis of 43 patients and review of the literature. Br J Surg. 1992;79(4):345-347.
14. Bhaskaranand K, Navadgi BC. Glomus tumour of the hand. J Hand Surg Br. 2002;27(3):229-231.
15. Rettig AC, Strickland JW. Glomus tumor of the digits. J Hand Surg Am. 1977;2(4):261-265.
16. Beham A, Fletcher CD. Intravascular glomus tumour: a previously undescribed phenomenon. Virchows Arch A Pathol Anat Histopathol. 1991;418(2):175-177.
17. Googe PB, Griffin WC. Intravenous glomus tumor of the forearm. J Cutan Pathol. 1993;20(4):359-363.
18. Koibuchi H, Fujii Y, Taniguchi N. An unusual case of a glomus tumor developing in a subcutaneous vein of the wrist. J Clin Ultrasound. 2008;36(6):369-370.
19. Acebo E, Val-Bernal JF, Arce F. Giant intravenous glomus tumor. J Cutan Pathol. 1997;24(6):384-389.
20. Ghaly RF, Ring AM. Supraclavicular glomus tumor, 20 year history of undiagnosed shoulder pain: a case report. Pain. 1999;83(2):379-382.
21. Joseph FR, Posner MA. Glomus tumors of the wrist. J Hand Surg Am. 1983;8(6):918-920.
22. Abou Jaoude JF, Roula Farah A, Sargi Z, Khairallah S, Fakih C. Glomus tumors: report on eleven cases and a review of the literature. Chir Main. 2000;19(4):243-252.
23. Jablon M, Horowitz A, Bernstein DA. Magnetic resonance imaging of a glomus tumor of the fingertip. J Hand Surg Am. 1990;15(3):507-509.
24. Theumann NH, Goettmann S, Le Viet D, et al. Recurrent glomus tumors of fingertips: MR imaging evaluation. Radiology. 2002;223(1):143-151.
25. Boudghene FP, Gouny P, Tassart M, Callard P, Le Breton C, Vayssairat M. Subungual glomus tumor: combined use of MRI and three-dimensional contrast MR angiography. J Magn Reson Imaging. 1998;8(6):1326-1328.
26. Van Ruyssevelt CE, Vranckx P. Subungual glomus tumor: emphasis on MR angiography. AJR Am J Roentgenol. 2004;182(1):263-264.
27. Connell DA, Koulouris G, Thorn DA, Potter HG. Contrast-enhanced MR angiography of the hand. Radiographics. 2002;22(3):583-599.
28. Varian JP, Cleak DK. Glomus tumours in the hand. Hand. 1980;12(3):293-299.
29. Robert G, Sawaya E, Pelissier P. Glomus tumor of the dorsal aspect of the wrist: a case report [in French]. Chir Main. 2012;31(4):214-216.
30. Chim H, Lahiri A, Chew WY. Atypical glomus tumour of the wrist: a case report. Hand Surg. 2009;14(2-3):121-123.
1. Carroll RE, Berman AT. Glomus tumors of the hand: review of the literature and report on twenty-eight cases. J Bone Joint Surg Am. 1972;54(4):691-703.
2. Riddell DH, Martin RS. Glomus tumor of unusual size; case report. Ann Surg. 1951;133(3):401-403.
3. Schiefer TK, Parker WL, Anakwenze OA, Amadio PC, Inwards CY, Spinner RJ. Extradigital glomus tumors: a 20-year experience. Mayo Clin Proc. 2006;81(10):1337-1344.
4. Tuncali D, Yilmaz AC, Terzioglu A, Aslan G. Multiple occurrences of different histologic types of the glomus tumor. J Hand Surg Am. 2005;30(1):161-164.
5. Greene RG. Soft tissue tumors of the hand and wrist. A 10 year survey. J Med Soc N J. 1964;61:495-498.
6. Maxwell GP, Curtis RM, Wilgis EF. Multiple digital glomus tumors. J Hand Surg Am. 1979;4(4):363-367.
7. Folpe AL, Fanburg-Smith JC, Miettinen M, Weiss SW. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25(1):1-12.
8. De Chiara A, Apice G, Mori S, et al. Malignant glomus tumour: a case report and review of the literature. Sarcoma. 2003;7(2):87-91.
9. Riveros M, Pack GT. The glomus tumor; report of 20 cases. Ann Surg. 1951;133(3):394-400.
10. Van Geertruyden J, Lorea P, Goldschmidt D, et al. Glomus tumours of the hand. A retrospective study of 51 cases. J Hand Surg Br. 1996;21(2):257-260.
11. Al-Qattan MM, Al-Namla A, Al-Thunayan A, Al-Subhi F, El-Shayeb AF. Magnetic resonance imaging in the diagnosis of glomus tumours of the hand. J Hand Surg Br. 2005;30(5):535-540.
12. Drape JL, Idy-Peretti I, Goettmann S, et al. Subungual glomus tumors: evaluation with MR imaging. Radiology. 1995;195(2):507-515.
13. Heys SD, Brittenden J, Atkinson P, Eremin O. Glomus tumour: an analysis of 43 patients and review of the literature. Br J Surg. 1992;79(4):345-347.
14. Bhaskaranand K, Navadgi BC. Glomus tumour of the hand. J Hand Surg Br. 2002;27(3):229-231.
15. Rettig AC, Strickland JW. Glomus tumor of the digits. J Hand Surg Am. 1977;2(4):261-265.
16. Beham A, Fletcher CD. Intravascular glomus tumour: a previously undescribed phenomenon. Virchows Arch A Pathol Anat Histopathol. 1991;418(2):175-177.
17. Googe PB, Griffin WC. Intravenous glomus tumor of the forearm. J Cutan Pathol. 1993;20(4):359-363.
18. Koibuchi H, Fujii Y, Taniguchi N. An unusual case of a glomus tumor developing in a subcutaneous vein of the wrist. J Clin Ultrasound. 2008;36(6):369-370.
19. Acebo E, Val-Bernal JF, Arce F. Giant intravenous glomus tumor. J Cutan Pathol. 1997;24(6):384-389.
20. Ghaly RF, Ring AM. Supraclavicular glomus tumor, 20 year history of undiagnosed shoulder pain: a case report. Pain. 1999;83(2):379-382.
21. Joseph FR, Posner MA. Glomus tumors of the wrist. J Hand Surg Am. 1983;8(6):918-920.
22. Abou Jaoude JF, Roula Farah A, Sargi Z, Khairallah S, Fakih C. Glomus tumors: report on eleven cases and a review of the literature. Chir Main. 2000;19(4):243-252.
23. Jablon M, Horowitz A, Bernstein DA. Magnetic resonance imaging of a glomus tumor of the fingertip. J Hand Surg Am. 1990;15(3):507-509.
24. Theumann NH, Goettmann S, Le Viet D, et al. Recurrent glomus tumors of fingertips: MR imaging evaluation. Radiology. 2002;223(1):143-151.
25. Boudghene FP, Gouny P, Tassart M, Callard P, Le Breton C, Vayssairat M. Subungual glomus tumor: combined use of MRI and three-dimensional contrast MR angiography. J Magn Reson Imaging. 1998;8(6):1326-1328.
26. Van Ruyssevelt CE, Vranckx P. Subungual glomus tumor: emphasis on MR angiography. AJR Am J Roentgenol. 2004;182(1):263-264.
27. Connell DA, Koulouris G, Thorn DA, Potter HG. Contrast-enhanced MR angiography of the hand. Radiographics. 2002;22(3):583-599.
28. Varian JP, Cleak DK. Glomus tumours in the hand. Hand. 1980;12(3):293-299.
29. Robert G, Sawaya E, Pelissier P. Glomus tumor of the dorsal aspect of the wrist: a case report [in French]. Chir Main. 2012;31(4):214-216.
30. Chim H, Lahiri A, Chew WY. Atypical glomus tumour of the wrist: a case report. Hand Surg. 2009;14(2-3):121-123.
Volar Plate Capsulodesis for Metacarpophalangeal Hyperextension With Basal Joint Arthritis
En Bloc Joystick Reduction of a Comminuted Intra-articular Distal Radius Fracture: A Technical Trick
A Perspective on the Evolution of Distal Radius Fracture Treatment
The treatment for distal radius fractures has changed significantly over time. Initially, distal radius fractures were treated as relatively innocuous injuries that befell the elderly and the comparatively inactive, and casts were the mainstay of treatment. However, closer scrutiny of the clinical results revealed a myriad of problems with these treatments, including “cast disease,” stiffness, inability to hold skeletal position, and soft-tissue compromise that affected the overall function of the wrist and hand.
Additional techniques to improve results included the “pins and plaster” technique, with the introduction of 2 pins in the radius and metacarpals to retard collapse of the fracture while in the cast. This was in some sense an early version of external fixation, with pins giving support to the unstable wrist and the body of the cast serving as the external support. There was further evolution of the adaptation of early versions of external fixation used for the lower extremity towards the treatment of the distal radius. For example, when I was a resident at Massachusetts General Hospital, we routinely applied femoral distractors as external fixation devices for selected distal radius fractures. This was a time when more specific anatomic devices and implants were not yet available.
External fixation evolved,1 and distal radius–specific systems, with enhanced ability to adjust and achieve reduction, became available in the late 1980s. At the same time, distal radius fracture plating evolved from simple “stamped metal” plates with screws that merely fit in the screw holes, to more highly engineered implants with screws that engaged the plate at a fixed angle, much like the blade plate
technology used for lower extremity fractures.2 Over time, the volar fixed-angle plating system supplanted the other treatments and emerged as a popular treatment method.
Use of Kirschner wires or simple pins has been promoted in the past for treatment of distal radius fractures. In France, Kapandji3 described the use of “intra-focal
pinning.” In this technique, smooth Kirschner wires are introduced in the fracture site itself, and then using leverage so that the pins act like “crowbars,” the distal fragment that is malpositioned becomes adjusted into a more anatomic position.3 Kapandji’s treatment can be very effective in achieving reduction; however, as there is no fixation into the distal fragment, this technique has limitations in maintaining the reduction until healing has occurred. Interfragmentary pinning from the dorsal radial and dorsal ulnar aspects were nicely described by Clancey.4 I have found great utility in combining the Kapandji intra-focal techniques to achieve reduction with Clancey pin fixation or distal radius plating to maintain reduction.
I was intrigued with the article by Drs. Siegall and Ziran, “En Bloc Joystick Reduction of a Comminuted Intraarticular Distal Radius Fracture: A Technical Trick,” in this month’s issue of The American Journal of Orthopedics. In their technique, the authors introduced a series of parallel pins or screws below the articular surface from radius to ulna in parallel fashion to provide provisional fixation for the intra-articular components of their complex fracture. Once having done so, they felt more secure in manipulating the distal radius component en bloc; in fact, they used strapping to provide distal traction on the external protruding portion of the pins to help achieve and maintain reduction for their definitive fixation. Drs. Siegall and Ziran describe the use of either Kirschner wires or plating to provide definitive fixation. In the example cited, they performed (via an open method) both the scaffolding and plating without the need of an assistant to hold or maintain the reduction during the osteosynthesis. I can envision adapting the technique they describe to percutaneous treatments for placement of the scaffolding pins, and even the Kapandji/Clancey pins under fluoroscopic guidance or arthroscopeassisted placement.
Despite the popularity and utility of volar fixed-angle plating techniques to treat distal radius fractures, there remain certain situations in which these techniques are faced with challenges. Certainly one of them is the more complex intra-articular fracture with multiple components, or in the very distal fracture patterns in which there is limited bone for the surgeon to use in providing distal screw fixation in the plating systems. Additionally, the nascent malunion presents some challenges as well in terms of performing a “takedown” of the partially healed fracture without destroying the soft, partially healed distal bone that contains the all-important articular component. These are the instances where supplemental techniques such as the one described by Drs. Siegall and Ziran, as well as the
Kapandji and Clancey techniques, have their greatest utility and appeal. Despite one’s wishes and best efforts, some distal radius fractures are not easily reconstructable. In these cases, use of external fixation or temporary arthrodesis
dorsal plating with subsequent plate removal5,6 can be the best reconstructive option and a great “bailout.” The prepared surgeon should have these supplemental techniques in their armamentarium to be able to adapt to the conditions that present themselves in the operating room and to do the best job they can for the patient.
References
1. Agee JM. External fixation. Technical advances based upon multiplanar
ligamentotaxis. Orthop Clin North Am. 1993;24(2):265-274.
2. Orbay JL, Fernandez DL. Volar fixed-angle plate fixation for unstable
distal radius fractures in the elderly patient. J Hand Surg Am. 2004;29(1):96-102.
3. Kapandji A. Internal fixation by double intrafocal plate. Functional treatment
of non articular fractures of the lower end of the radius (author’s transl) [in French]. Ann Chir. 1976;30(11-12):903-908.
4. Clancey GJ. Percutaneous Kirschner-wire fixation of Colles fractures. A prospective study of thirty cases. J Bone Joint Surg Am. 1984;66(7):1008-1014.
5. Burke EF, Singer RM. Treatment of comminuted distal radius with the use of an internal distraction plate. Tech Hand Up Extrem Surg. 1998;2(4):248-252.
6. Ruch DS, Ginn TA, Yang CC, Smith BP, Rushing J, Hanel DP. Use of a distraction plate for distal radial fractures with metaphyseal and diaphyseal comminution. J Bone Joint Surg Am. 2005;87(5):945-954.
The treatment for distal radius fractures has changed significantly over time. Initially, distal radius fractures were treated as relatively innocuous injuries that befell the elderly and the comparatively inactive, and casts were the mainstay of treatment. However, closer scrutiny of the clinical results revealed a myriad of problems with these treatments, including “cast disease,” stiffness, inability to hold skeletal position, and soft-tissue compromise that affected the overall function of the wrist and hand.
Additional techniques to improve results included the “pins and plaster” technique, with the introduction of 2 pins in the radius and metacarpals to retard collapse of the fracture while in the cast. This was in some sense an early version of external fixation, with pins giving support to the unstable wrist and the body of the cast serving as the external support. There was further evolution of the adaptation of early versions of external fixation used for the lower extremity towards the treatment of the distal radius. For example, when I was a resident at Massachusetts General Hospital, we routinely applied femoral distractors as external fixation devices for selected distal radius fractures. This was a time when more specific anatomic devices and implants were not yet available.
External fixation evolved,1 and distal radius–specific systems, with enhanced ability to adjust and achieve reduction, became available in the late 1980s. At the same time, distal radius fracture plating evolved from simple “stamped metal” plates with screws that merely fit in the screw holes, to more highly engineered implants with screws that engaged the plate at a fixed angle, much like the blade plate
technology used for lower extremity fractures.2 Over time, the volar fixed-angle plating system supplanted the other treatments and emerged as a popular treatment method.
Use of Kirschner wires or simple pins has been promoted in the past for treatment of distal radius fractures. In France, Kapandji3 described the use of “intra-focal
pinning.” In this technique, smooth Kirschner wires are introduced in the fracture site itself, and then using leverage so that the pins act like “crowbars,” the distal fragment that is malpositioned becomes adjusted into a more anatomic position.3 Kapandji’s treatment can be very effective in achieving reduction; however, as there is no fixation into the distal fragment, this technique has limitations in maintaining the reduction until healing has occurred. Interfragmentary pinning from the dorsal radial and dorsal ulnar aspects were nicely described by Clancey.4 I have found great utility in combining the Kapandji intra-focal techniques to achieve reduction with Clancey pin fixation or distal radius plating to maintain reduction.
I was intrigued with the article by Drs. Siegall and Ziran, “En Bloc Joystick Reduction of a Comminuted Intraarticular Distal Radius Fracture: A Technical Trick,” in this month’s issue of The American Journal of Orthopedics. In their technique, the authors introduced a series of parallel pins or screws below the articular surface from radius to ulna in parallel fashion to provide provisional fixation for the intra-articular components of their complex fracture. Once having done so, they felt more secure in manipulating the distal radius component en bloc; in fact, they used strapping to provide distal traction on the external protruding portion of the pins to help achieve and maintain reduction for their definitive fixation. Drs. Siegall and Ziran describe the use of either Kirschner wires or plating to provide definitive fixation. In the example cited, they performed (via an open method) both the scaffolding and plating without the need of an assistant to hold or maintain the reduction during the osteosynthesis. I can envision adapting the technique they describe to percutaneous treatments for placement of the scaffolding pins, and even the Kapandji/Clancey pins under fluoroscopic guidance or arthroscopeassisted placement.
Despite the popularity and utility of volar fixed-angle plating techniques to treat distal radius fractures, there remain certain situations in which these techniques are faced with challenges. Certainly one of them is the more complex intra-articular fracture with multiple components, or in the very distal fracture patterns in which there is limited bone for the surgeon to use in providing distal screw fixation in the plating systems. Additionally, the nascent malunion presents some challenges as well in terms of performing a “takedown” of the partially healed fracture without destroying the soft, partially healed distal bone that contains the all-important articular component. These are the instances where supplemental techniques such as the one described by Drs. Siegall and Ziran, as well as the
Kapandji and Clancey techniques, have their greatest utility and appeal. Despite one’s wishes and best efforts, some distal radius fractures are not easily reconstructable. In these cases, use of external fixation or temporary arthrodesis
dorsal plating with subsequent plate removal5,6 can be the best reconstructive option and a great “bailout.” The prepared surgeon should have these supplemental techniques in their armamentarium to be able to adapt to the conditions that present themselves in the operating room and to do the best job they can for the patient.
References
1. Agee JM. External fixation. Technical advances based upon multiplanar
ligamentotaxis. Orthop Clin North Am. 1993;24(2):265-274.
2. Orbay JL, Fernandez DL. Volar fixed-angle plate fixation for unstable
distal radius fractures in the elderly patient. J Hand Surg Am. 2004;29(1):96-102.
3. Kapandji A. Internal fixation by double intrafocal plate. Functional treatment
of non articular fractures of the lower end of the radius (author’s transl) [in French]. Ann Chir. 1976;30(11-12):903-908.
4. Clancey GJ. Percutaneous Kirschner-wire fixation of Colles fractures. A prospective study of thirty cases. J Bone Joint Surg Am. 1984;66(7):1008-1014.
5. Burke EF, Singer RM. Treatment of comminuted distal radius with the use of an internal distraction plate. Tech Hand Up Extrem Surg. 1998;2(4):248-252.
6. Ruch DS, Ginn TA, Yang CC, Smith BP, Rushing J, Hanel DP. Use of a distraction plate for distal radial fractures with metaphyseal and diaphyseal comminution. J Bone Joint Surg Am. 2005;87(5):945-954.
The treatment for distal radius fractures has changed significantly over time. Initially, distal radius fractures were treated as relatively innocuous injuries that befell the elderly and the comparatively inactive, and casts were the mainstay of treatment. However, closer scrutiny of the clinical results revealed a myriad of problems with these treatments, including “cast disease,” stiffness, inability to hold skeletal position, and soft-tissue compromise that affected the overall function of the wrist and hand.
Additional techniques to improve results included the “pins and plaster” technique, with the introduction of 2 pins in the radius and metacarpals to retard collapse of the fracture while in the cast. This was in some sense an early version of external fixation, with pins giving support to the unstable wrist and the body of the cast serving as the external support. There was further evolution of the adaptation of early versions of external fixation used for the lower extremity towards the treatment of the distal radius. For example, when I was a resident at Massachusetts General Hospital, we routinely applied femoral distractors as external fixation devices for selected distal radius fractures. This was a time when more specific anatomic devices and implants were not yet available.
External fixation evolved,1 and distal radius–specific systems, with enhanced ability to adjust and achieve reduction, became available in the late 1980s. At the same time, distal radius fracture plating evolved from simple “stamped metal” plates with screws that merely fit in the screw holes, to more highly engineered implants with screws that engaged the plate at a fixed angle, much like the blade plate
technology used for lower extremity fractures.2 Over time, the volar fixed-angle plating system supplanted the other treatments and emerged as a popular treatment method.
Use of Kirschner wires or simple pins has been promoted in the past for treatment of distal radius fractures. In France, Kapandji3 described the use of “intra-focal
pinning.” In this technique, smooth Kirschner wires are introduced in the fracture site itself, and then using leverage so that the pins act like “crowbars,” the distal fragment that is malpositioned becomes adjusted into a more anatomic position.3 Kapandji’s treatment can be very effective in achieving reduction; however, as there is no fixation into the distal fragment, this technique has limitations in maintaining the reduction until healing has occurred. Interfragmentary pinning from the dorsal radial and dorsal ulnar aspects were nicely described by Clancey.4 I have found great utility in combining the Kapandji intra-focal techniques to achieve reduction with Clancey pin fixation or distal radius plating to maintain reduction.
I was intrigued with the article by Drs. Siegall and Ziran, “En Bloc Joystick Reduction of a Comminuted Intraarticular Distal Radius Fracture: A Technical Trick,” in this month’s issue of The American Journal of Orthopedics. In their technique, the authors introduced a series of parallel pins or screws below the articular surface from radius to ulna in parallel fashion to provide provisional fixation for the intra-articular components of their complex fracture. Once having done so, they felt more secure in manipulating the distal radius component en bloc; in fact, they used strapping to provide distal traction on the external protruding portion of the pins to help achieve and maintain reduction for their definitive fixation. Drs. Siegall and Ziran describe the use of either Kirschner wires or plating to provide definitive fixation. In the example cited, they performed (via an open method) both the scaffolding and plating without the need of an assistant to hold or maintain the reduction during the osteosynthesis. I can envision adapting the technique they describe to percutaneous treatments for placement of the scaffolding pins, and even the Kapandji/Clancey pins under fluoroscopic guidance or arthroscopeassisted placement.
Despite the popularity and utility of volar fixed-angle plating techniques to treat distal radius fractures, there remain certain situations in which these techniques are faced with challenges. Certainly one of them is the more complex intra-articular fracture with multiple components, or in the very distal fracture patterns in which there is limited bone for the surgeon to use in providing distal screw fixation in the plating systems. Additionally, the nascent malunion presents some challenges as well in terms of performing a “takedown” of the partially healed fracture without destroying the soft, partially healed distal bone that contains the all-important articular component. These are the instances where supplemental techniques such as the one described by Drs. Siegall and Ziran, as well as the
Kapandji and Clancey techniques, have their greatest utility and appeal. Despite one’s wishes and best efforts, some distal radius fractures are not easily reconstructable. In these cases, use of external fixation or temporary arthrodesis
dorsal plating with subsequent plate removal5,6 can be the best reconstructive option and a great “bailout.” The prepared surgeon should have these supplemental techniques in their armamentarium to be able to adapt to the conditions that present themselves in the operating room and to do the best job they can for the patient.
References
1. Agee JM. External fixation. Technical advances based upon multiplanar
ligamentotaxis. Orthop Clin North Am. 1993;24(2):265-274.
2. Orbay JL, Fernandez DL. Volar fixed-angle plate fixation for unstable
distal radius fractures in the elderly patient. J Hand Surg Am. 2004;29(1):96-102.
3. Kapandji A. Internal fixation by double intrafocal plate. Functional treatment
of non articular fractures of the lower end of the radius (author’s transl) [in French]. Ann Chir. 1976;30(11-12):903-908.
4. Clancey GJ. Percutaneous Kirschner-wire fixation of Colles fractures. A prospective study of thirty cases. J Bone Joint Surg Am. 1984;66(7):1008-1014.
5. Burke EF, Singer RM. Treatment of comminuted distal radius with the use of an internal distraction plate. Tech Hand Up Extrem Surg. 1998;2(4):248-252.
6. Ruch DS, Ginn TA, Yang CC, Smith BP, Rushing J, Hanel DP. Use of a distraction plate for distal radial fractures with metaphyseal and diaphyseal comminution. J Bone Joint Surg Am. 2005;87(5):945-954.
Bionic Arm Still in Development Stage
Within the past 13 years, roughly 2000 veterans who have returned from Afghanistan and Iraq have sustained injuries that required amputations. Of these injured veterans, 14% required upper extremity amputations. An article published in the June issue of the Journal of the American Academy of Orthopaedic Surgeons reviewed recent advancements in upper extremity bionics. Also reviewed were the challenges that linger in creating a prosthesis that meets or surpasses the abilities of the human hand and arm.
During the next 50 years, “I truly believe we will be able to make artificial arms that function better than many injured arms that doctors are saving today,” said article author Dr. Douglas T. Hutchinson, Associate Professor of Orthopedics at the University of Utah Medical School and Chief of Hand Surgery at Primary Children’s Medical Center, the Veterans Affairs Medical Center, and Shriners Intermountain Hospital.
Created more than 50 years ago, the myoelectric prosthesis continues to be the most commonly used upper extremity prostheses. This prosthesis allows residual muscles to act as natural batteries to create transcutaneous signals, to control the movements of the prosthetic hand and arm. However, the muscles used most often are the triceps and biceps, which do not inherently translate to the opening and closing of the hand. Another drawback is that sometimes the socket interface used to attach the prosthesis may interfere with the function of the residual joint, such as the elbow. Myoelectric prosthetics also do not look natural and are heavy, hot, and uncomfortable, and are not waterproof.
The current federal budget for prostheses research is $2.5 billion. The US Department of Defense Advanced Research Project (DARPA) already has invested more than $150 million for their Revolutionizing Prosthetics Program. The later program, which seeks to create an upper extremity prosthesis that can function as a normal hand and arm does, but with full sensory and motor functions.
In order for these prosthetic devices to be used effectively in a broad range for patients, adjustments still need to be made. For example, many have short-life batteries, along with being weighty and uncomfortable. Particularly challenging is the problem of accurately and efficiently sending brain signals through the peripheral nerves and muscles of the hands and arm, a feat that may warrant the creation and use of a reliable wireless device or direct wiring through an osseous-integrated implant. Current infection rates (nearly 45%) with osseous-integrated devices at the prosthesis-skin interface also pose an issue.
Suggested Reading
Hutchinson DT. The quest for the bionic arm. J Am Acad Orthop Surg. 2014;22(6):346-351.
Within the past 13 years, roughly 2000 veterans who have returned from Afghanistan and Iraq have sustained injuries that required amputations. Of these injured veterans, 14% required upper extremity amputations. An article published in the June issue of the Journal of the American Academy of Orthopaedic Surgeons reviewed recent advancements in upper extremity bionics. Also reviewed were the challenges that linger in creating a prosthesis that meets or surpasses the abilities of the human hand and arm.
During the next 50 years, “I truly believe we will be able to make artificial arms that function better than many injured arms that doctors are saving today,” said article author Dr. Douglas T. Hutchinson, Associate Professor of Orthopedics at the University of Utah Medical School and Chief of Hand Surgery at Primary Children’s Medical Center, the Veterans Affairs Medical Center, and Shriners Intermountain Hospital.
Created more than 50 years ago, the myoelectric prosthesis continues to be the most commonly used upper extremity prostheses. This prosthesis allows residual muscles to act as natural batteries to create transcutaneous signals, to control the movements of the prosthetic hand and arm. However, the muscles used most often are the triceps and biceps, which do not inherently translate to the opening and closing of the hand. Another drawback is that sometimes the socket interface used to attach the prosthesis may interfere with the function of the residual joint, such as the elbow. Myoelectric prosthetics also do not look natural and are heavy, hot, and uncomfortable, and are not waterproof.
The current federal budget for prostheses research is $2.5 billion. The US Department of Defense Advanced Research Project (DARPA) already has invested more than $150 million for their Revolutionizing Prosthetics Program. The later program, which seeks to create an upper extremity prosthesis that can function as a normal hand and arm does, but with full sensory and motor functions.
In order for these prosthetic devices to be used effectively in a broad range for patients, adjustments still need to be made. For example, many have short-life batteries, along with being weighty and uncomfortable. Particularly challenging is the problem of accurately and efficiently sending brain signals through the peripheral nerves and muscles of the hands and arm, a feat that may warrant the creation and use of a reliable wireless device or direct wiring through an osseous-integrated implant. Current infection rates (nearly 45%) with osseous-integrated devices at the prosthesis-skin interface also pose an issue.
Within the past 13 years, roughly 2000 veterans who have returned from Afghanistan and Iraq have sustained injuries that required amputations. Of these injured veterans, 14% required upper extremity amputations. An article published in the June issue of the Journal of the American Academy of Orthopaedic Surgeons reviewed recent advancements in upper extremity bionics. Also reviewed were the challenges that linger in creating a prosthesis that meets or surpasses the abilities of the human hand and arm.
During the next 50 years, “I truly believe we will be able to make artificial arms that function better than many injured arms that doctors are saving today,” said article author Dr. Douglas T. Hutchinson, Associate Professor of Orthopedics at the University of Utah Medical School and Chief of Hand Surgery at Primary Children’s Medical Center, the Veterans Affairs Medical Center, and Shriners Intermountain Hospital.
Created more than 50 years ago, the myoelectric prosthesis continues to be the most commonly used upper extremity prostheses. This prosthesis allows residual muscles to act as natural batteries to create transcutaneous signals, to control the movements of the prosthetic hand and arm. However, the muscles used most often are the triceps and biceps, which do not inherently translate to the opening and closing of the hand. Another drawback is that sometimes the socket interface used to attach the prosthesis may interfere with the function of the residual joint, such as the elbow. Myoelectric prosthetics also do not look natural and are heavy, hot, and uncomfortable, and are not waterproof.
The current federal budget for prostheses research is $2.5 billion. The US Department of Defense Advanced Research Project (DARPA) already has invested more than $150 million for their Revolutionizing Prosthetics Program. The later program, which seeks to create an upper extremity prosthesis that can function as a normal hand and arm does, but with full sensory and motor functions.
In order for these prosthetic devices to be used effectively in a broad range for patients, adjustments still need to be made. For example, many have short-life batteries, along with being weighty and uncomfortable. Particularly challenging is the problem of accurately and efficiently sending brain signals through the peripheral nerves and muscles of the hands and arm, a feat that may warrant the creation and use of a reliable wireless device or direct wiring through an osseous-integrated implant. Current infection rates (nearly 45%) with osseous-integrated devices at the prosthesis-skin interface also pose an issue.
Suggested Reading
Hutchinson DT. The quest for the bionic arm. J Am Acad Orthop Surg. 2014;22(6):346-351.
Suggested Reading
Hutchinson DT. The quest for the bionic arm. J Am Acad Orthop Surg. 2014;22(6):346-351.