User login
Helping teens make the switch from pediatrics to gynecology
For many adolescents, the first visit to a gynecologist can be intimidating. The prospect of meeting a new doctor who will ask prying, deeply personal questions about sex and menstruation is scary. And, in all likelihood, a parent, older sibling, or friend has warned them about the notorious pelvic exam.
The exact timing of when adolescent patients should start seeing a gynecologist varies based on when a patient starts puberty. Primary care physicians and pediatricians can help teens transition by referring patients to an adolescent-friendly practice and clearing up some of the misconceptions that surround the first gynecology visit. Gynecologists, on the other side of the referral, can help patients transition by guaranteeing confidentiality and creating a safe space for young patients.
This news organization interviewed three experts in adolescent health about when teens should start having their gynecological needs addressed and how their physicians can help them undergo that transition.
Age-appropriate care
“Most people get very limited information about their reproductive health,” said Anne-Marie E. Amies Oelschlager, MD, a pediatric and adolescent gynecologist at Seattle Children’s, Seattle, and a member of the American College of Obstetricians and Gynecologists (ACOG) clinical consensus committee on gynecology.
Official guidelines from ACOG call for the initial reproductive health visit to take place between the ages of 13 and 15 years. The exact age may vary, however, depending on the specific needs of the patient.
For example, some patients begin menstruating early, at age 9 or 10, said Mary Romano, MD, MPH, a pediatrician and adolescent medicine specialist at Vanderbilt Children’s Hospital, Nashville, Tenn. Pediatricians who are uncomfortable educating young patients about menstruation should refer the patient to a gynecologist or a pediatric gynecologist for whom such discussions are routine.
If a patient does not have a menstrual cycle by age 14 or 15, that also should be addressed by a family physician or gynecologist, Dr. Romano added.
“The importance here is addressing the reproductive health of the teen starting really at the age of 10 or 12, or once puberty starts,” said Patricia S. Huguelet, MD, a pediatric and adolescent gynecologist at Children’s Hospital Colorado, Aurora. In those early visits, the physician can provide “anticipatory guidance,” counseling the teen on what is normal in terms of menstruation, sex, and relationships, and addressing what is not, she said.
Ideally, patients who were designated female at birth but now identify as male or nonbinary will meet with a gynecologist early on in the gender affirmation process and a gynecologist will continue to consult as part of the patient’s interdisciplinary care team, added Dr. Romano, who counsels LGBTQ+ youth as part of her practice. A gynecologist may support these patients in myriad ways, including helping those who are considering or using puberty blockers and providing reproductive and health education to patients in a way that is sensitive to the patient’s gender identity.
Patient referrals
Some pediatricians and family practice physicians may be talking with their patients about topics such as menstrual cycles and contraception. But those who are uncomfortable asking adolescent patients about their reproductive and sexual health should refer them to a gynecologist or specialist in adolescent medicine, Dr. Romano advised.
“The biggest benefit I’ve noticed is often [patients] come from a pediatrician or family medicine provider and they often appreciate the opportunity to talk to a doctor they haven’t met before about the more personal questions they may have,” Dr. Amies Oelschlager said.
Referring adolescents to a specialist who has either trained in adolescent medicine or has experience treating that age group has benefits, Dr. Romano said. Clinicians with that experience understand adolescents are not “mini-adults” but have unique developmental and medical issues. How to counsel and educate them carries unique challenges, she said.
For example, heavy menstrual bleeding is a leading reason a patient – either an adult or an adolescent – presents to a gynecologist, Dr. Huguelet said. But the pathology differs vastly for those two age groups. For patients in their 30s and 40s, polyps and fibroids are common problems associated with heavy bleeding. Those conditions are rare in adolescents, whereas bleeding disorders are common, she said.
Most patients will continue to see their pediatricians and primary care providers for other issues. And in some areas, gynecologists can reinforce advice from pediatricians, such as encouraging patients to get the HPV vaccine, Dr. Amies Oelschlager said.
Common misconceptions
Primary care physicians can also dispel common misconceptions teens – and their parents – have about gynecology. Some parents may believe that certain methods of birth control cause cancer or infertility, have concerns about the HPV vaccine, or think hormonal therapies are harmful, Dr. Amies Oelschlager said. But the biggest misconception involves the infamous pelvic exam.
“Lots of patients assume that every time they go to the gynecologist they are going to have a pelvic exam,” she said. “When I say, ‘We don’t have to do that,’ they are so relieved.”
Guidelines have changed since the parents of today’s teens were going to the gynecologist for the first time. Many patients now do not need an initial Pap smear until age 25, following a recent guideline change by the American Cancer Society. (ACOG is considering adopting the same stance but still recommends screening start at 21.) “Most patients do not need an exam, even when it comes to sexual health and screening [for sexually transmitted infections], that can be done without an exam,” Dr. Huguelet said.
Confidentiality and comfort
On the other side of the referral, gynecologists should follow several best practices to treat adolescent patients. Arguably the most important part of the initial gynecologic visit is to give patients the option of one-on-one time with the physician with no parent in the room. During that time, the physician should make it clear that what they discuss is confidential and will not be shared with their parent or guardian, Dr. Huguelet said. Patients should also have the option of having a friend or another nonparent individual in the room with them during this one-on-one time with the physician, particularly if the patient does not feel comfortable discussing sensitive subjects completely on her own.
Adolescents receive better care, disclose more, and perceive they are getting better care when the process is confidential, Dr. Romano said. Confidentiality does have limits, however, which physicians should also make sure their patients understand, according to the ACOG guidelines for the initial reproductive visit. These limitations can vary by state depending on issues related to mandatory reporting, insurance billing, and legal requirements of patient notifications of specific services such as abortion.
The use of electronic medical records has raised additional challenges when it comes to communicating privately with adolescent patients, Dr. Amies Oelschlager said. In her practice, she tries to ensure the adolescent is the one with the login information for their records. If not, her office will have the patient’s cell number to text or call securely.
“We feel strongly adolescents should be able to access reproductive health care, mental health care, and care for substance abuse disorders without parental notification,” Dr. Amies Oelschlager said.
Telehealth visits can also be helpful for adolescents coming to gynecology for the first time. And taking the time to establish a rapport with patients at the start of the visit is key, Dr. Huguelet said. By directing questions to the adolescent patient rather than the parent, Dr. Huguelet said, the physician demonstrates that the teen’s treatment needs come first.
ACOG has guidelines on other steps gynecology practices, including those that see both adults and teens, can take to make their offices and visits adolescent-friendly. These steps include asking patients about their preferred names and pronouns at the start of the visit or as part of the initial intake form, training office staff to be comfortable with issues related to adolescent sexuality and gender and sexual diversity among patients, providing a place for teens to wait separately from obstetrics patients, and having age-appropriate literature on hand for adolescents to learn about reproductive health.
After that first reproductive health visit, gynecologists and primary care providers should partner to ensure the whole health of their patients is being addressed, Dr. Huguelet said.
“Collaboration is always going to better serve patients in any area,” said Dr. Romano, “and certainly this area is no different.”
Dr. Amies Oelschlager, Dr. Romano, and Dr. Huguelet have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For many adolescents, the first visit to a gynecologist can be intimidating. The prospect of meeting a new doctor who will ask prying, deeply personal questions about sex and menstruation is scary. And, in all likelihood, a parent, older sibling, or friend has warned them about the notorious pelvic exam.
The exact timing of when adolescent patients should start seeing a gynecologist varies based on when a patient starts puberty. Primary care physicians and pediatricians can help teens transition by referring patients to an adolescent-friendly practice and clearing up some of the misconceptions that surround the first gynecology visit. Gynecologists, on the other side of the referral, can help patients transition by guaranteeing confidentiality and creating a safe space for young patients.
This news organization interviewed three experts in adolescent health about when teens should start having their gynecological needs addressed and how their physicians can help them undergo that transition.
Age-appropriate care
“Most people get very limited information about their reproductive health,” said Anne-Marie E. Amies Oelschlager, MD, a pediatric and adolescent gynecologist at Seattle Children’s, Seattle, and a member of the American College of Obstetricians and Gynecologists (ACOG) clinical consensus committee on gynecology.
Official guidelines from ACOG call for the initial reproductive health visit to take place between the ages of 13 and 15 years. The exact age may vary, however, depending on the specific needs of the patient.
For example, some patients begin menstruating early, at age 9 or 10, said Mary Romano, MD, MPH, a pediatrician and adolescent medicine specialist at Vanderbilt Children’s Hospital, Nashville, Tenn. Pediatricians who are uncomfortable educating young patients about menstruation should refer the patient to a gynecologist or a pediatric gynecologist for whom such discussions are routine.
If a patient does not have a menstrual cycle by age 14 or 15, that also should be addressed by a family physician or gynecologist, Dr. Romano added.
“The importance here is addressing the reproductive health of the teen starting really at the age of 10 or 12, or once puberty starts,” said Patricia S. Huguelet, MD, a pediatric and adolescent gynecologist at Children’s Hospital Colorado, Aurora. In those early visits, the physician can provide “anticipatory guidance,” counseling the teen on what is normal in terms of menstruation, sex, and relationships, and addressing what is not, she said.
Ideally, patients who were designated female at birth but now identify as male or nonbinary will meet with a gynecologist early on in the gender affirmation process and a gynecologist will continue to consult as part of the patient’s interdisciplinary care team, added Dr. Romano, who counsels LGBTQ+ youth as part of her practice. A gynecologist may support these patients in myriad ways, including helping those who are considering or using puberty blockers and providing reproductive and health education to patients in a way that is sensitive to the patient’s gender identity.
Patient referrals
Some pediatricians and family practice physicians may be talking with their patients about topics such as menstrual cycles and contraception. But those who are uncomfortable asking adolescent patients about their reproductive and sexual health should refer them to a gynecologist or specialist in adolescent medicine, Dr. Romano advised.
“The biggest benefit I’ve noticed is often [patients] come from a pediatrician or family medicine provider and they often appreciate the opportunity to talk to a doctor they haven’t met before about the more personal questions they may have,” Dr. Amies Oelschlager said.
Referring adolescents to a specialist who has either trained in adolescent medicine or has experience treating that age group has benefits, Dr. Romano said. Clinicians with that experience understand adolescents are not “mini-adults” but have unique developmental and medical issues. How to counsel and educate them carries unique challenges, she said.
For example, heavy menstrual bleeding is a leading reason a patient – either an adult or an adolescent – presents to a gynecologist, Dr. Huguelet said. But the pathology differs vastly for those two age groups. For patients in their 30s and 40s, polyps and fibroids are common problems associated with heavy bleeding. Those conditions are rare in adolescents, whereas bleeding disorders are common, she said.
Most patients will continue to see their pediatricians and primary care providers for other issues. And in some areas, gynecologists can reinforce advice from pediatricians, such as encouraging patients to get the HPV vaccine, Dr. Amies Oelschlager said.
Common misconceptions
Primary care physicians can also dispel common misconceptions teens – and their parents – have about gynecology. Some parents may believe that certain methods of birth control cause cancer or infertility, have concerns about the HPV vaccine, or think hormonal therapies are harmful, Dr. Amies Oelschlager said. But the biggest misconception involves the infamous pelvic exam.
“Lots of patients assume that every time they go to the gynecologist they are going to have a pelvic exam,” she said. “When I say, ‘We don’t have to do that,’ they are so relieved.”
Guidelines have changed since the parents of today’s teens were going to the gynecologist for the first time. Many patients now do not need an initial Pap smear until age 25, following a recent guideline change by the American Cancer Society. (ACOG is considering adopting the same stance but still recommends screening start at 21.) “Most patients do not need an exam, even when it comes to sexual health and screening [for sexually transmitted infections], that can be done without an exam,” Dr. Huguelet said.
Confidentiality and comfort
On the other side of the referral, gynecologists should follow several best practices to treat adolescent patients. Arguably the most important part of the initial gynecologic visit is to give patients the option of one-on-one time with the physician with no parent in the room. During that time, the physician should make it clear that what they discuss is confidential and will not be shared with their parent or guardian, Dr. Huguelet said. Patients should also have the option of having a friend or another nonparent individual in the room with them during this one-on-one time with the physician, particularly if the patient does not feel comfortable discussing sensitive subjects completely on her own.
Adolescents receive better care, disclose more, and perceive they are getting better care when the process is confidential, Dr. Romano said. Confidentiality does have limits, however, which physicians should also make sure their patients understand, according to the ACOG guidelines for the initial reproductive visit. These limitations can vary by state depending on issues related to mandatory reporting, insurance billing, and legal requirements of patient notifications of specific services such as abortion.
The use of electronic medical records has raised additional challenges when it comes to communicating privately with adolescent patients, Dr. Amies Oelschlager said. In her practice, she tries to ensure the adolescent is the one with the login information for their records. If not, her office will have the patient’s cell number to text or call securely.
“We feel strongly adolescents should be able to access reproductive health care, mental health care, and care for substance abuse disorders without parental notification,” Dr. Amies Oelschlager said.
Telehealth visits can also be helpful for adolescents coming to gynecology for the first time. And taking the time to establish a rapport with patients at the start of the visit is key, Dr. Huguelet said. By directing questions to the adolescent patient rather than the parent, Dr. Huguelet said, the physician demonstrates that the teen’s treatment needs come first.
ACOG has guidelines on other steps gynecology practices, including those that see both adults and teens, can take to make their offices and visits adolescent-friendly. These steps include asking patients about their preferred names and pronouns at the start of the visit or as part of the initial intake form, training office staff to be comfortable with issues related to adolescent sexuality and gender and sexual diversity among patients, providing a place for teens to wait separately from obstetrics patients, and having age-appropriate literature on hand for adolescents to learn about reproductive health.
After that first reproductive health visit, gynecologists and primary care providers should partner to ensure the whole health of their patients is being addressed, Dr. Huguelet said.
“Collaboration is always going to better serve patients in any area,” said Dr. Romano, “and certainly this area is no different.”
Dr. Amies Oelschlager, Dr. Romano, and Dr. Huguelet have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For many adolescents, the first visit to a gynecologist can be intimidating. The prospect of meeting a new doctor who will ask prying, deeply personal questions about sex and menstruation is scary. And, in all likelihood, a parent, older sibling, or friend has warned them about the notorious pelvic exam.
The exact timing of when adolescent patients should start seeing a gynecologist varies based on when a patient starts puberty. Primary care physicians and pediatricians can help teens transition by referring patients to an adolescent-friendly practice and clearing up some of the misconceptions that surround the first gynecology visit. Gynecologists, on the other side of the referral, can help patients transition by guaranteeing confidentiality and creating a safe space for young patients.
This news organization interviewed three experts in adolescent health about when teens should start having their gynecological needs addressed and how their physicians can help them undergo that transition.
Age-appropriate care
“Most people get very limited information about their reproductive health,” said Anne-Marie E. Amies Oelschlager, MD, a pediatric and adolescent gynecologist at Seattle Children’s, Seattle, and a member of the American College of Obstetricians and Gynecologists (ACOG) clinical consensus committee on gynecology.
Official guidelines from ACOG call for the initial reproductive health visit to take place between the ages of 13 and 15 years. The exact age may vary, however, depending on the specific needs of the patient.
For example, some patients begin menstruating early, at age 9 or 10, said Mary Romano, MD, MPH, a pediatrician and adolescent medicine specialist at Vanderbilt Children’s Hospital, Nashville, Tenn. Pediatricians who are uncomfortable educating young patients about menstruation should refer the patient to a gynecologist or a pediatric gynecologist for whom such discussions are routine.
If a patient does not have a menstrual cycle by age 14 or 15, that also should be addressed by a family physician or gynecologist, Dr. Romano added.
“The importance here is addressing the reproductive health of the teen starting really at the age of 10 or 12, or once puberty starts,” said Patricia S. Huguelet, MD, a pediatric and adolescent gynecologist at Children’s Hospital Colorado, Aurora. In those early visits, the physician can provide “anticipatory guidance,” counseling the teen on what is normal in terms of menstruation, sex, and relationships, and addressing what is not, she said.
Ideally, patients who were designated female at birth but now identify as male or nonbinary will meet with a gynecologist early on in the gender affirmation process and a gynecologist will continue to consult as part of the patient’s interdisciplinary care team, added Dr. Romano, who counsels LGBTQ+ youth as part of her practice. A gynecologist may support these patients in myriad ways, including helping those who are considering or using puberty blockers and providing reproductive and health education to patients in a way that is sensitive to the patient’s gender identity.
Patient referrals
Some pediatricians and family practice physicians may be talking with their patients about topics such as menstrual cycles and contraception. But those who are uncomfortable asking adolescent patients about their reproductive and sexual health should refer them to a gynecologist or specialist in adolescent medicine, Dr. Romano advised.
“The biggest benefit I’ve noticed is often [patients] come from a pediatrician or family medicine provider and they often appreciate the opportunity to talk to a doctor they haven’t met before about the more personal questions they may have,” Dr. Amies Oelschlager said.
Referring adolescents to a specialist who has either trained in adolescent medicine or has experience treating that age group has benefits, Dr. Romano said. Clinicians with that experience understand adolescents are not “mini-adults” but have unique developmental and medical issues. How to counsel and educate them carries unique challenges, she said.
For example, heavy menstrual bleeding is a leading reason a patient – either an adult or an adolescent – presents to a gynecologist, Dr. Huguelet said. But the pathology differs vastly for those two age groups. For patients in their 30s and 40s, polyps and fibroids are common problems associated with heavy bleeding. Those conditions are rare in adolescents, whereas bleeding disorders are common, she said.
Most patients will continue to see their pediatricians and primary care providers for other issues. And in some areas, gynecologists can reinforce advice from pediatricians, such as encouraging patients to get the HPV vaccine, Dr. Amies Oelschlager said.
Common misconceptions
Primary care physicians can also dispel common misconceptions teens – and their parents – have about gynecology. Some parents may believe that certain methods of birth control cause cancer or infertility, have concerns about the HPV vaccine, or think hormonal therapies are harmful, Dr. Amies Oelschlager said. But the biggest misconception involves the infamous pelvic exam.
“Lots of patients assume that every time they go to the gynecologist they are going to have a pelvic exam,” she said. “When I say, ‘We don’t have to do that,’ they are so relieved.”
Guidelines have changed since the parents of today’s teens were going to the gynecologist for the first time. Many patients now do not need an initial Pap smear until age 25, following a recent guideline change by the American Cancer Society. (ACOG is considering adopting the same stance but still recommends screening start at 21.) “Most patients do not need an exam, even when it comes to sexual health and screening [for sexually transmitted infections], that can be done without an exam,” Dr. Huguelet said.
Confidentiality and comfort
On the other side of the referral, gynecologists should follow several best practices to treat adolescent patients. Arguably the most important part of the initial gynecologic visit is to give patients the option of one-on-one time with the physician with no parent in the room. During that time, the physician should make it clear that what they discuss is confidential and will not be shared with their parent or guardian, Dr. Huguelet said. Patients should also have the option of having a friend or another nonparent individual in the room with them during this one-on-one time with the physician, particularly if the patient does not feel comfortable discussing sensitive subjects completely on her own.
Adolescents receive better care, disclose more, and perceive they are getting better care when the process is confidential, Dr. Romano said. Confidentiality does have limits, however, which physicians should also make sure their patients understand, according to the ACOG guidelines for the initial reproductive visit. These limitations can vary by state depending on issues related to mandatory reporting, insurance billing, and legal requirements of patient notifications of specific services such as abortion.
The use of electronic medical records has raised additional challenges when it comes to communicating privately with adolescent patients, Dr. Amies Oelschlager said. In her practice, she tries to ensure the adolescent is the one with the login information for their records. If not, her office will have the patient’s cell number to text or call securely.
“We feel strongly adolescents should be able to access reproductive health care, mental health care, and care for substance abuse disorders without parental notification,” Dr. Amies Oelschlager said.
Telehealth visits can also be helpful for adolescents coming to gynecology for the first time. And taking the time to establish a rapport with patients at the start of the visit is key, Dr. Huguelet said. By directing questions to the adolescent patient rather than the parent, Dr. Huguelet said, the physician demonstrates that the teen’s treatment needs come first.
ACOG has guidelines on other steps gynecology practices, including those that see both adults and teens, can take to make their offices and visits adolescent-friendly. These steps include asking patients about their preferred names and pronouns at the start of the visit or as part of the initial intake form, training office staff to be comfortable with issues related to adolescent sexuality and gender and sexual diversity among patients, providing a place for teens to wait separately from obstetrics patients, and having age-appropriate literature on hand for adolescents to learn about reproductive health.
After that first reproductive health visit, gynecologists and primary care providers should partner to ensure the whole health of their patients is being addressed, Dr. Huguelet said.
“Collaboration is always going to better serve patients in any area,” said Dr. Romano, “and certainly this area is no different.”
Dr. Amies Oelschlager, Dr. Romano, and Dr. Huguelet have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Tech can help teens connect with docs about sexual health
Maria Trent, MD, MPH, was studying ways clinicians can leverage technology to care for adolescents years before COVID-19 exposed the challenges and advantages of telehealth.
Dr. Trent, a pediatrician and adolescent medicine specialist and professor of pediatrics at Johns Hopkins University, Baltimore, has long believed that the phones in her patients’ pockets have the potential to improve the sexual health of youth. The pandemic has only made that view stronger.
“They’re a generation that’s really wired and online,” Dr. Trent told this news organization. “I think that we can meet them in that space.”
Her research has incorporated texting, apps, and videos. Out of necessity, technology increasingly became part of patient care during the pandemic. “We had to stretch our ability to do some basic triage and assessments of patients online,” Dr. Trent said.
Even when clinics are closed, doctors might be able to provide initial care remotely, such as writing prescriptions to manage symptoms or directing patients to a lab for testing.
Telemedicine could allow a clinician to guide a teenager who thinks they might be pregnant to take a store-bought test and avoid possible exposure to COVID-19 in the ED, for instance.
But doctors have concerns about the legal and practical limits of privacy and confidentiality. Who else is at home listening to a phone conversation? Are parents accessing the patient’s online portal? Will parents receive an explanation of benefits that lists testing for a sexually transmitted infection, or see a testing kit that is delivered to their home?
When a young patient needs in-person care, transportation can be a barrier. And then there’s the matter of clinicians being able to bill for telehealth services.
Practices are learning how to navigate these issues, and relevant laws vary by state.
“I think this is going to become part of standard practice,” Dr. Trent said. “I think we have to do the hard work to make sure that it’s safe, that it’s accessible, and that it is actually improving care.”
Texts, apps, videos
In one early study, Dr. Trent and colleagues found that showing adolescents with pelvic inflammatory disease a 6-minute video may improve treatment rates for their sexual partners.
Another study provided preliminary evidence that text messaging support might improve clinic attendance for moderately long-acting reversible contraception.
A third trial showed that adolescents and young adults with pelvic inflammatory disease who were randomly assigned to receive text-message prompts to take their medications and provide information about the doses they consumed had greater decreases in Neisseria gonorrhoeae and Chlamydia trachomatis infections, compared with patients who received standard care.
Dr. Trent and coinvestigators are assessing a technology-based intervention for youth with HIV, in which patients can use an app to submit videos of themselves taking antiretroviral therapy and report any side effects. The technology provides a way to monitor patients remotely and support them between visits, she said.
Will pandemic-driven options remain?
In 2020, Laura D. Lindberg, PhD, principal research scientist at the Guttmacher Institute in New York, and coauthors discussed the possible ramifications of the pandemic on the sexual and reproductive health of adolescents and young adults.
If telemedicine options driven by COVID-19 are here to stay, adolescents and young adults could be “the age group most likely to continue that approach rather than returning to traditional in-person visits,” the researchers wrote. “Innovations in health care service provision, such as use of telemedicine and obtaining contraceptives and STI testing by mail, will help expand access to [sexual and reproductive health] care for young people.”
At the 2021 annual conference of the American Academy of Pediatrics, Dr. Trent described telehealth as a viable way to provide sexual and reproductive health care to adolescents and young adults, including anticipatory guidance, contraception counseling, coordination of follow-up care and testing, and connecting patients to resources.
Her presentation cited several websites that can help patients receive testing for STIs, including Yes Means Test, the Centers for Disease Control and Prevention’s GetTested page, and I Want the Kit. Planned Parenthood has telehealth options, and the Kaiser Family Foundation compiled information about 26 online platforms that were providing contraception or STI services.
Who else is in the room?
“There’s only so much time in the day and so many patients you can see, regardless of whether you have telehealth or not,” said David L. Bell, MD, MPH, president of the Society for Adolescent Health and Medicine and a coauthor of the Perspectives on Sexual and Reproductive Health paper. In addition, “you never know who else is in the room” with the patient on the other end, added Dr. Bell, a professor of population and family health and pediatrics at the Columbia University Medical Center and medical director of the Young Men’s Clinic, both in New York.
In some respects, young patients may not be able to participate in telehealth visits the same way they would in a medical office, Dr. Trent acknowledged. Encouraging the use of headphones is one way to help protect confidentiality when talking with patients who are at home and might not be alone.
But if patients are able to find a private space for remote visits, they might be more open than usual. In that way, telemedicine could provide additional opportunities to address issues like substance use disorders and mental health, as well, she said.
“Then, if they need something, we have to problem solve,” Dr. Trent said. Next steps may involve engaging a parent or getting the patient to a lab or the clinic.
Sex ed may be lacking
The Perspectives article also raised concerns that the pandemic might exacerbate shortcomings in sex education, which already may have been lacking.
“Before the pandemic, schools were a key source of formal sex education for young people,” the authors wrote. “Sex education, which was already limited in many areas of the country, has likely not been included in the national shift to online learning. Even when in-person schooling resumes, missed sex education instruction is unlikely to be made up, given the modest attention it received prior to the pandemic.”
A recently published study in the Journal of Adolescent Health indicates that American teenagers currently receive less formal sex education than they did 25 years ago, with “troubling” inequities by race.
Researchers surveyed adolescents about what they had learned about topics such as how to say no to sex, methods of birth control and where to get them, and STIs.
Dr. Lindberg and Leslie M. Kantor, PhD, MPH, professor and chair of the department of urban-global public health at Rutgers University, Newark, N.J., conducted the analysis.
“Pediatricians and other health care providers that work with children and adolescents have a critical role to play in providing information about sexuality to both the patients and to the parents,” said Dr. Kantor, who also coauthored the Perspectives article with Dr. Lindberg and Dr. Bell. The new research “shows that doctors play an even more critical role, because they can’t assume that their patients are going to get the information that they need in a timely way from schools.”
By age 15, 21% of girls and 20% of boys have had sexual intercourse at least once, according to data from the 2015-2017 National Survey of Family Growth. By age 17, the percentages were 53% of girls and 48% of boys. By age 20, the percentages were 79% of women and 77% of men. The CDC’s 2021 guidelines on treatment and screening for STIs note that prevalence rates of certain infections – such as chlamydia and gonorrhea in females – are highest among adolescents and young adults.
Those trends underscore the importance of counseling on sexual health that clinicians can provide, but time constraints may limit how much they can discuss in a single session with a patient. To cover all topics that are important to parents and patients, doctors may need to discuss sexual and reproductive health sooner and more frequently.
Young people are getting more and more explicit information from their phones and media, yet educators are giving them less information to navigate these topics and learn what’s real, Dr. Kantor said. That mismatch can be toxic. In a December 2021 interview with Howard Stern, the pop star Billie Eilish said she started watching pornography at about age 11 and frequently watched videos that were violent. “I think it really destroyed my brain and I feel incredibly devastated that I was exposed to so much porn,” Ms. Eilish told Mr. Stern.
Researchers and a psychologist told CNN that the singer’s story may be typical. It also highlights a need to be aware of kids’ online activities and to have conversations about how pornography may not depict healthy interactions, they said.
Beyond discussing a plan for preventing pregnancy and STIs, Dr. Kantor encouraged discussions about what constitutes healthy relationships, as well as check-ins about intimate partner violence and how romantic relationships are going.
“I think for pediatricians and for parents, it’s a muscle,” she said. “As you bring up these topics more, listen, and respond, you get more comfortable with it.”
Dr. Trent has served as an advisory board member on a sexual health council for Trojan (Church & Dwight Company) and has received research funding from Hologic and research supplies from SpeeDx. Dr. Bell has received funds from the Merck Foundation, Merck, and Gilead. Dr. Kantor had no disclosures.
A version of this article first appeared on Medscape.com.
Maria Trent, MD, MPH, was studying ways clinicians can leverage technology to care for adolescents years before COVID-19 exposed the challenges and advantages of telehealth.
Dr. Trent, a pediatrician and adolescent medicine specialist and professor of pediatrics at Johns Hopkins University, Baltimore, has long believed that the phones in her patients’ pockets have the potential to improve the sexual health of youth. The pandemic has only made that view stronger.
“They’re a generation that’s really wired and online,” Dr. Trent told this news organization. “I think that we can meet them in that space.”
Her research has incorporated texting, apps, and videos. Out of necessity, technology increasingly became part of patient care during the pandemic. “We had to stretch our ability to do some basic triage and assessments of patients online,” Dr. Trent said.
Even when clinics are closed, doctors might be able to provide initial care remotely, such as writing prescriptions to manage symptoms or directing patients to a lab for testing.
Telemedicine could allow a clinician to guide a teenager who thinks they might be pregnant to take a store-bought test and avoid possible exposure to COVID-19 in the ED, for instance.
But doctors have concerns about the legal and practical limits of privacy and confidentiality. Who else is at home listening to a phone conversation? Are parents accessing the patient’s online portal? Will parents receive an explanation of benefits that lists testing for a sexually transmitted infection, or see a testing kit that is delivered to their home?
When a young patient needs in-person care, transportation can be a barrier. And then there’s the matter of clinicians being able to bill for telehealth services.
Practices are learning how to navigate these issues, and relevant laws vary by state.
“I think this is going to become part of standard practice,” Dr. Trent said. “I think we have to do the hard work to make sure that it’s safe, that it’s accessible, and that it is actually improving care.”
Texts, apps, videos
In one early study, Dr. Trent and colleagues found that showing adolescents with pelvic inflammatory disease a 6-minute video may improve treatment rates for their sexual partners.
Another study provided preliminary evidence that text messaging support might improve clinic attendance for moderately long-acting reversible contraception.
A third trial showed that adolescents and young adults with pelvic inflammatory disease who were randomly assigned to receive text-message prompts to take their medications and provide information about the doses they consumed had greater decreases in Neisseria gonorrhoeae and Chlamydia trachomatis infections, compared with patients who received standard care.
Dr. Trent and coinvestigators are assessing a technology-based intervention for youth with HIV, in which patients can use an app to submit videos of themselves taking antiretroviral therapy and report any side effects. The technology provides a way to monitor patients remotely and support them between visits, she said.
Will pandemic-driven options remain?
In 2020, Laura D. Lindberg, PhD, principal research scientist at the Guttmacher Institute in New York, and coauthors discussed the possible ramifications of the pandemic on the sexual and reproductive health of adolescents and young adults.
If telemedicine options driven by COVID-19 are here to stay, adolescents and young adults could be “the age group most likely to continue that approach rather than returning to traditional in-person visits,” the researchers wrote. “Innovations in health care service provision, such as use of telemedicine and obtaining contraceptives and STI testing by mail, will help expand access to [sexual and reproductive health] care for young people.”
At the 2021 annual conference of the American Academy of Pediatrics, Dr. Trent described telehealth as a viable way to provide sexual and reproductive health care to adolescents and young adults, including anticipatory guidance, contraception counseling, coordination of follow-up care and testing, and connecting patients to resources.
Her presentation cited several websites that can help patients receive testing for STIs, including Yes Means Test, the Centers for Disease Control and Prevention’s GetTested page, and I Want the Kit. Planned Parenthood has telehealth options, and the Kaiser Family Foundation compiled information about 26 online platforms that were providing contraception or STI services.
Who else is in the room?
“There’s only so much time in the day and so many patients you can see, regardless of whether you have telehealth or not,” said David L. Bell, MD, MPH, president of the Society for Adolescent Health and Medicine and a coauthor of the Perspectives on Sexual and Reproductive Health paper. In addition, “you never know who else is in the room” with the patient on the other end, added Dr. Bell, a professor of population and family health and pediatrics at the Columbia University Medical Center and medical director of the Young Men’s Clinic, both in New York.
In some respects, young patients may not be able to participate in telehealth visits the same way they would in a medical office, Dr. Trent acknowledged. Encouraging the use of headphones is one way to help protect confidentiality when talking with patients who are at home and might not be alone.
But if patients are able to find a private space for remote visits, they might be more open than usual. In that way, telemedicine could provide additional opportunities to address issues like substance use disorders and mental health, as well, she said.
“Then, if they need something, we have to problem solve,” Dr. Trent said. Next steps may involve engaging a parent or getting the patient to a lab or the clinic.
Sex ed may be lacking
The Perspectives article also raised concerns that the pandemic might exacerbate shortcomings in sex education, which already may have been lacking.
“Before the pandemic, schools were a key source of formal sex education for young people,” the authors wrote. “Sex education, which was already limited in many areas of the country, has likely not been included in the national shift to online learning. Even when in-person schooling resumes, missed sex education instruction is unlikely to be made up, given the modest attention it received prior to the pandemic.”
A recently published study in the Journal of Adolescent Health indicates that American teenagers currently receive less formal sex education than they did 25 years ago, with “troubling” inequities by race.
Researchers surveyed adolescents about what they had learned about topics such as how to say no to sex, methods of birth control and where to get them, and STIs.
Dr. Lindberg and Leslie M. Kantor, PhD, MPH, professor and chair of the department of urban-global public health at Rutgers University, Newark, N.J., conducted the analysis.
“Pediatricians and other health care providers that work with children and adolescents have a critical role to play in providing information about sexuality to both the patients and to the parents,” said Dr. Kantor, who also coauthored the Perspectives article with Dr. Lindberg and Dr. Bell. The new research “shows that doctors play an even more critical role, because they can’t assume that their patients are going to get the information that they need in a timely way from schools.”
By age 15, 21% of girls and 20% of boys have had sexual intercourse at least once, according to data from the 2015-2017 National Survey of Family Growth. By age 17, the percentages were 53% of girls and 48% of boys. By age 20, the percentages were 79% of women and 77% of men. The CDC’s 2021 guidelines on treatment and screening for STIs note that prevalence rates of certain infections – such as chlamydia and gonorrhea in females – are highest among adolescents and young adults.
Those trends underscore the importance of counseling on sexual health that clinicians can provide, but time constraints may limit how much they can discuss in a single session with a patient. To cover all topics that are important to parents and patients, doctors may need to discuss sexual and reproductive health sooner and more frequently.
Young people are getting more and more explicit information from their phones and media, yet educators are giving them less information to navigate these topics and learn what’s real, Dr. Kantor said. That mismatch can be toxic. In a December 2021 interview with Howard Stern, the pop star Billie Eilish said she started watching pornography at about age 11 and frequently watched videos that were violent. “I think it really destroyed my brain and I feel incredibly devastated that I was exposed to so much porn,” Ms. Eilish told Mr. Stern.
Researchers and a psychologist told CNN that the singer’s story may be typical. It also highlights a need to be aware of kids’ online activities and to have conversations about how pornography may not depict healthy interactions, they said.
Beyond discussing a plan for preventing pregnancy and STIs, Dr. Kantor encouraged discussions about what constitutes healthy relationships, as well as check-ins about intimate partner violence and how romantic relationships are going.
“I think for pediatricians and for parents, it’s a muscle,” she said. “As you bring up these topics more, listen, and respond, you get more comfortable with it.”
Dr. Trent has served as an advisory board member on a sexual health council for Trojan (Church & Dwight Company) and has received research funding from Hologic and research supplies from SpeeDx. Dr. Bell has received funds from the Merck Foundation, Merck, and Gilead. Dr. Kantor had no disclosures.
A version of this article first appeared on Medscape.com.
Maria Trent, MD, MPH, was studying ways clinicians can leverage technology to care for adolescents years before COVID-19 exposed the challenges and advantages of telehealth.
Dr. Trent, a pediatrician and adolescent medicine specialist and professor of pediatrics at Johns Hopkins University, Baltimore, has long believed that the phones in her patients’ pockets have the potential to improve the sexual health of youth. The pandemic has only made that view stronger.
“They’re a generation that’s really wired and online,” Dr. Trent told this news organization. “I think that we can meet them in that space.”
Her research has incorporated texting, apps, and videos. Out of necessity, technology increasingly became part of patient care during the pandemic. “We had to stretch our ability to do some basic triage and assessments of patients online,” Dr. Trent said.
Even when clinics are closed, doctors might be able to provide initial care remotely, such as writing prescriptions to manage symptoms or directing patients to a lab for testing.
Telemedicine could allow a clinician to guide a teenager who thinks they might be pregnant to take a store-bought test and avoid possible exposure to COVID-19 in the ED, for instance.
But doctors have concerns about the legal and practical limits of privacy and confidentiality. Who else is at home listening to a phone conversation? Are parents accessing the patient’s online portal? Will parents receive an explanation of benefits that lists testing for a sexually transmitted infection, or see a testing kit that is delivered to their home?
When a young patient needs in-person care, transportation can be a barrier. And then there’s the matter of clinicians being able to bill for telehealth services.
Practices are learning how to navigate these issues, and relevant laws vary by state.
“I think this is going to become part of standard practice,” Dr. Trent said. “I think we have to do the hard work to make sure that it’s safe, that it’s accessible, and that it is actually improving care.”
Texts, apps, videos
In one early study, Dr. Trent and colleagues found that showing adolescents with pelvic inflammatory disease a 6-minute video may improve treatment rates for their sexual partners.
Another study provided preliminary evidence that text messaging support might improve clinic attendance for moderately long-acting reversible contraception.
A third trial showed that adolescents and young adults with pelvic inflammatory disease who were randomly assigned to receive text-message prompts to take their medications and provide information about the doses they consumed had greater decreases in Neisseria gonorrhoeae and Chlamydia trachomatis infections, compared with patients who received standard care.
Dr. Trent and coinvestigators are assessing a technology-based intervention for youth with HIV, in which patients can use an app to submit videos of themselves taking antiretroviral therapy and report any side effects. The technology provides a way to monitor patients remotely and support them between visits, she said.
Will pandemic-driven options remain?
In 2020, Laura D. Lindberg, PhD, principal research scientist at the Guttmacher Institute in New York, and coauthors discussed the possible ramifications of the pandemic on the sexual and reproductive health of adolescents and young adults.
If telemedicine options driven by COVID-19 are here to stay, adolescents and young adults could be “the age group most likely to continue that approach rather than returning to traditional in-person visits,” the researchers wrote. “Innovations in health care service provision, such as use of telemedicine and obtaining contraceptives and STI testing by mail, will help expand access to [sexual and reproductive health] care for young people.”
At the 2021 annual conference of the American Academy of Pediatrics, Dr. Trent described telehealth as a viable way to provide sexual and reproductive health care to adolescents and young adults, including anticipatory guidance, contraception counseling, coordination of follow-up care and testing, and connecting patients to resources.
Her presentation cited several websites that can help patients receive testing for STIs, including Yes Means Test, the Centers for Disease Control and Prevention’s GetTested page, and I Want the Kit. Planned Parenthood has telehealth options, and the Kaiser Family Foundation compiled information about 26 online platforms that were providing contraception or STI services.
Who else is in the room?
“There’s only so much time in the day and so many patients you can see, regardless of whether you have telehealth or not,” said David L. Bell, MD, MPH, president of the Society for Adolescent Health and Medicine and a coauthor of the Perspectives on Sexual and Reproductive Health paper. In addition, “you never know who else is in the room” with the patient on the other end, added Dr. Bell, a professor of population and family health and pediatrics at the Columbia University Medical Center and medical director of the Young Men’s Clinic, both in New York.
In some respects, young patients may not be able to participate in telehealth visits the same way they would in a medical office, Dr. Trent acknowledged. Encouraging the use of headphones is one way to help protect confidentiality when talking with patients who are at home and might not be alone.
But if patients are able to find a private space for remote visits, they might be more open than usual. In that way, telemedicine could provide additional opportunities to address issues like substance use disorders and mental health, as well, she said.
“Then, if they need something, we have to problem solve,” Dr. Trent said. Next steps may involve engaging a parent or getting the patient to a lab or the clinic.
Sex ed may be lacking
The Perspectives article also raised concerns that the pandemic might exacerbate shortcomings in sex education, which already may have been lacking.
“Before the pandemic, schools were a key source of formal sex education for young people,” the authors wrote. “Sex education, which was already limited in many areas of the country, has likely not been included in the national shift to online learning. Even when in-person schooling resumes, missed sex education instruction is unlikely to be made up, given the modest attention it received prior to the pandemic.”
A recently published study in the Journal of Adolescent Health indicates that American teenagers currently receive less formal sex education than they did 25 years ago, with “troubling” inequities by race.
Researchers surveyed adolescents about what they had learned about topics such as how to say no to sex, methods of birth control and where to get them, and STIs.
Dr. Lindberg and Leslie M. Kantor, PhD, MPH, professor and chair of the department of urban-global public health at Rutgers University, Newark, N.J., conducted the analysis.
“Pediatricians and other health care providers that work with children and adolescents have a critical role to play in providing information about sexuality to both the patients and to the parents,” said Dr. Kantor, who also coauthored the Perspectives article with Dr. Lindberg and Dr. Bell. The new research “shows that doctors play an even more critical role, because they can’t assume that their patients are going to get the information that they need in a timely way from schools.”
By age 15, 21% of girls and 20% of boys have had sexual intercourse at least once, according to data from the 2015-2017 National Survey of Family Growth. By age 17, the percentages were 53% of girls and 48% of boys. By age 20, the percentages were 79% of women and 77% of men. The CDC’s 2021 guidelines on treatment and screening for STIs note that prevalence rates of certain infections – such as chlamydia and gonorrhea in females – are highest among adolescents and young adults.
Those trends underscore the importance of counseling on sexual health that clinicians can provide, but time constraints may limit how much they can discuss in a single session with a patient. To cover all topics that are important to parents and patients, doctors may need to discuss sexual and reproductive health sooner and more frequently.
Young people are getting more and more explicit information from their phones and media, yet educators are giving them less information to navigate these topics and learn what’s real, Dr. Kantor said. That mismatch can be toxic. In a December 2021 interview with Howard Stern, the pop star Billie Eilish said she started watching pornography at about age 11 and frequently watched videos that were violent. “I think it really destroyed my brain and I feel incredibly devastated that I was exposed to so much porn,” Ms. Eilish told Mr. Stern.
Researchers and a psychologist told CNN that the singer’s story may be typical. It also highlights a need to be aware of kids’ online activities and to have conversations about how pornography may not depict healthy interactions, they said.
Beyond discussing a plan for preventing pregnancy and STIs, Dr. Kantor encouraged discussions about what constitutes healthy relationships, as well as check-ins about intimate partner violence and how romantic relationships are going.
“I think for pediatricians and for parents, it’s a muscle,” she said. “As you bring up these topics more, listen, and respond, you get more comfortable with it.”
Dr. Trent has served as an advisory board member on a sexual health council for Trojan (Church & Dwight Company) and has received research funding from Hologic and research supplies from SpeeDx. Dr. Bell has received funds from the Merck Foundation, Merck, and Gilead. Dr. Kantor had no disclosures.
A version of this article first appeared on Medscape.com.
Average-risk women with dense breasts—What breast screening is appropriate?
Text copyright DenseBreast-info.org.
Answer
A. For women with extremely dense breasts who are not otherwise at increased risk for breast cancer, screening magnetic resonance imaging (MRI) is preferred, plus her mammogram or tomosynthesis. If MRI is not an option, consider ultrasonography or contrast-enhanced mammography.
The same screening considerations apply to women with heterogeneously dense breasts; however, there is limited capacity for MRI or even ultrasound screening at many facilities. Research supports MRI in dense breasts, and abbreviated, lower-cost protocols have been validated that address some of the barriers to MRI.1 Although not yet widely available, abbreviated MRI will likely have a greater role in screening women with dense breasts who are not high risk. It is important to note that preauthorization from insurance may be required for screening MRI, and in most US states, deductibles and copays apply.
The exam
Contrast-enhanced MRI requires IV injection of gadolinium-based contrast to look at the anatomy and blood flow patterns of the breast tissue. The patient lies face down with the breasts placed in two rectangular openings, or “coils.” The exam takes place inside the tunnel of the scanner, with the head facing out.After initial images are obtained, the contrast agent is injected into a vein in the arm, and additional images are taken, which will show areas of enhancement. The exam takes about 20 to 40 minutes. An “abbreviated” MRI can be performed for screening in some centers, which uses fewer sequences and takes about 10 minutes.
Benefits
At least 40% of cancers are missed on mammography in women with dense breasts.2 MRI is the most widely studied technique using a contrast agent, and it produces the highest additional cancer detection of all the supplemental technologies to date, yielding, in the first year, 10-16 additional cancers per 1,000 women screened after mammography/tomosynthesis (reviewed in Berg et al.3). The cancer-detection benefit is seen across all breast density categories, even among average-risk women.4 There is no ionizing radiation, and it has been shown to reduce the rate of interval cancers (those detected due to symptoms after a negative screening mammogram), as well as the rate of late-stage disease. Axillary lymph nodes can be examined at the same screening exam.
While tomosynthesis improves cancer detection in women with fatty breasts, scattered fibroglandular breast tissue, and heterogeneously dense breasts, it does not significantly improve cancer detection in women with extremely dense breasts.5,6 Current American Cancer Society and National Comprehensive Cancer Network guidelines recommend annual screening MRI for women at high risk for breast cancer (regardless of breast density); however, increasingly, research supports the effectiveness of MRI in women with dense breasts who are otherwise considered average risk. A large randomized controlled trial in the Netherlands compared outcomes in women with extremely dense breasts invited to have screening MRI after negative mammography to those assigned to continue receiving screening mammography only. The incremental cancer detection rate was 16.5 per 1,000 (79/4,783) women screened with MRI in the first round7 and 6 per 1,000 women screened in the second round 2 years later.8 The interval cancer rate was 0.8 per 1,000 (4/4,783) women screened with MRI, compared with 4.9 per 1,000 (16/3,278) women who declined MRI and received mammography only.7
Screening ultrasound will show up to 3 additional cancers per 1,000 women screened after mammography/tomosynthesis (reviewed in Vourtsis and Berg9 and Berg and Vourtsis10), far lower than the added cancer-detection rate of MRI. Consider screening ultrasound for women who cannot tolerate or access screening MRI.11 Contrast-enhanced mammography (CEM) uses iodinated contrast (as in computed tomography). CEM is not widely available but appears to show cancer-detection similar to MRI. For further discussion, see Berg et al’s 2021 review.3
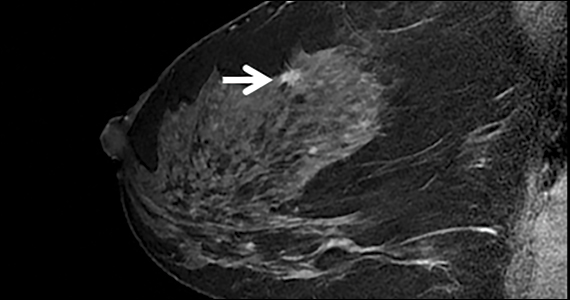
The FIGURE shows an example of an invasive cancer depicted on contrast-enhanced MRI in a 53-year-old woman with dense breasts and a family history of breast cancer that was not visible on tomosynthesis, even in retrospect, due to masking by dense tissue.

Considerations
Breast MRI increases callbacks even after mammography and ultrasound; however, such false alarms are reduced in subsequent screening rounds. MRI cannot be performed in women who have certain metal implants— some pacemakers or spinal fixation rods—and is not recommended for pregnant women. Claustrophobia may be an issue for some women. MRI is expensive and requires IV contrast. Gadolinium is known to accumulate in the brain, although the long-term effects of this are unknown and no harm has been shown.●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA. 2020;323:746-756. doi: 10.1001 /jama.2020.0572
- Kerlikowske K, Zhu W, Tosteson AN, et al. Identifying women with dense breasts at high risk for interval cancer: a cohort study. Ann Intern Med. 2015;162:673-681. doi: 10.7326/M14-1465.
- Berg WA, Rafferty EA, Friedewald SM, Hruska CB, Rahbar H. Screening Algorithms in Dense Breasts: AJR Expert Panel Narrative Review. AJR Am J Roentgenol. 2021;216:275-294. doi: 10.2214/AJR.20.24436.
- Kuhl CK, Strobel K, Bieling H, et al. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283:361-370. doi: 10.1148/radiol.2016161444.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315:1784-1786. doi: 10.1001/jama.2016.1708.
- Osteras BH, Martinsen ACT, Gullien R, et al. Digital mammography versus breast tomosynthesis: impact of breast density on diagnostic performance in population-based screening. Radiology. 2019;293:60-68. doi: 10.1148 /radiol.2019190425.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102. doi: 10.1056/NEJMoa1903986.
- Veenhuizen SGA, de Lange SV, Bakker MF, et al. Supplemental breast MRI for women with extremely dense breasts: results of the second screening round of the DENSE trial. Radiology. 2021;299:278-286. doi: 10.1148/radiol.2021203633.
- Vourtsis A, Berg WA. Breast density implications and supplemental screening. Eur Radiol. 2019;29:1762-1777. doi: 10.1007/s00330-018-5668-8.
- Berg WA, Vourtsis A. Screening ultrasound using handheld or automated technique in women with dense breasts. J Breast Imaging. 2019;1:283-296.
- National Comprehensive Cancer Network. Breast Cancer Screening and Diagnosis (Version 1.2021). https://www.nccn. org/professionals/physician_gls/pdf/breast-screening.pdf. Accessed November 18, 2021.
Text copyright DenseBreast-info.org.
Answer
A. For women with extremely dense breasts who are not otherwise at increased risk for breast cancer, screening magnetic resonance imaging (MRI) is preferred, plus her mammogram or tomosynthesis. If MRI is not an option, consider ultrasonography or contrast-enhanced mammography.
The same screening considerations apply to women with heterogeneously dense breasts; however, there is limited capacity for MRI or even ultrasound screening at many facilities. Research supports MRI in dense breasts, and abbreviated, lower-cost protocols have been validated that address some of the barriers to MRI.1 Although not yet widely available, abbreviated MRI will likely have a greater role in screening women with dense breasts who are not high risk. It is important to note that preauthorization from insurance may be required for screening MRI, and in most US states, deductibles and copays apply.
The exam
Contrast-enhanced MRI requires IV injection of gadolinium-based contrast to look at the anatomy and blood flow patterns of the breast tissue. The patient lies face down with the breasts placed in two rectangular openings, or “coils.” The exam takes place inside the tunnel of the scanner, with the head facing out.After initial images are obtained, the contrast agent is injected into a vein in the arm, and additional images are taken, which will show areas of enhancement. The exam takes about 20 to 40 minutes. An “abbreviated” MRI can be performed for screening in some centers, which uses fewer sequences and takes about 10 minutes.
Benefits
At least 40% of cancers are missed on mammography in women with dense breasts.2 MRI is the most widely studied technique using a contrast agent, and it produces the highest additional cancer detection of all the supplemental technologies to date, yielding, in the first year, 10-16 additional cancers per 1,000 women screened after mammography/tomosynthesis (reviewed in Berg et al.3). The cancer-detection benefit is seen across all breast density categories, even among average-risk women.4 There is no ionizing radiation, and it has been shown to reduce the rate of interval cancers (those detected due to symptoms after a negative screening mammogram), as well as the rate of late-stage disease. Axillary lymph nodes can be examined at the same screening exam.
While tomosynthesis improves cancer detection in women with fatty breasts, scattered fibroglandular breast tissue, and heterogeneously dense breasts, it does not significantly improve cancer detection in women with extremely dense breasts.5,6 Current American Cancer Society and National Comprehensive Cancer Network guidelines recommend annual screening MRI for women at high risk for breast cancer (regardless of breast density); however, increasingly, research supports the effectiveness of MRI in women with dense breasts who are otherwise considered average risk. A large randomized controlled trial in the Netherlands compared outcomes in women with extremely dense breasts invited to have screening MRI after negative mammography to those assigned to continue receiving screening mammography only. The incremental cancer detection rate was 16.5 per 1,000 (79/4,783) women screened with MRI in the first round7 and 6 per 1,000 women screened in the second round 2 years later.8 The interval cancer rate was 0.8 per 1,000 (4/4,783) women screened with MRI, compared with 4.9 per 1,000 (16/3,278) women who declined MRI and received mammography only.7
Screening ultrasound will show up to 3 additional cancers per 1,000 women screened after mammography/tomosynthesis (reviewed in Vourtsis and Berg9 and Berg and Vourtsis10), far lower than the added cancer-detection rate of MRI. Consider screening ultrasound for women who cannot tolerate or access screening MRI.11 Contrast-enhanced mammography (CEM) uses iodinated contrast (as in computed tomography). CEM is not widely available but appears to show cancer-detection similar to MRI. For further discussion, see Berg et al’s 2021 review.3
The FIGURE shows an example of an invasive cancer depicted on contrast-enhanced MRI in a 53-year-old woman with dense breasts and a family history of breast cancer that was not visible on tomosynthesis, even in retrospect, due to masking by dense tissue.

Considerations
Breast MRI increases callbacks even after mammography and ultrasound; however, such false alarms are reduced in subsequent screening rounds. MRI cannot be performed in women who have certain metal implants— some pacemakers or spinal fixation rods—and is not recommended for pregnant women. Claustrophobia may be an issue for some women. MRI is expensive and requires IV contrast. Gadolinium is known to accumulate in the brain, although the long-term effects of this are unknown and no harm has been shown.●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
Text copyright DenseBreast-info.org.
Answer
A. For women with extremely dense breasts who are not otherwise at increased risk for breast cancer, screening magnetic resonance imaging (MRI) is preferred, plus her mammogram or tomosynthesis. If MRI is not an option, consider ultrasonography or contrast-enhanced mammography.
The same screening considerations apply to women with heterogeneously dense breasts; however, there is limited capacity for MRI or even ultrasound screening at many facilities. Research supports MRI in dense breasts, and abbreviated, lower-cost protocols have been validated that address some of the barriers to MRI.1 Although not yet widely available, abbreviated MRI will likely have a greater role in screening women with dense breasts who are not high risk. It is important to note that preauthorization from insurance may be required for screening MRI, and in most US states, deductibles and copays apply.
The exam
Contrast-enhanced MRI requires IV injection of gadolinium-based contrast to look at the anatomy and blood flow patterns of the breast tissue. The patient lies face down with the breasts placed in two rectangular openings, or “coils.” The exam takes place inside the tunnel of the scanner, with the head facing out.After initial images are obtained, the contrast agent is injected into a vein in the arm, and additional images are taken, which will show areas of enhancement. The exam takes about 20 to 40 minutes. An “abbreviated” MRI can be performed for screening in some centers, which uses fewer sequences and takes about 10 minutes.
Benefits
At least 40% of cancers are missed on mammography in women with dense breasts.2 MRI is the most widely studied technique using a contrast agent, and it produces the highest additional cancer detection of all the supplemental technologies to date, yielding, in the first year, 10-16 additional cancers per 1,000 women screened after mammography/tomosynthesis (reviewed in Berg et al.3). The cancer-detection benefit is seen across all breast density categories, even among average-risk women.4 There is no ionizing radiation, and it has been shown to reduce the rate of interval cancers (those detected due to symptoms after a negative screening mammogram), as well as the rate of late-stage disease. Axillary lymph nodes can be examined at the same screening exam.
While tomosynthesis improves cancer detection in women with fatty breasts, scattered fibroglandular breast tissue, and heterogeneously dense breasts, it does not significantly improve cancer detection in women with extremely dense breasts.5,6 Current American Cancer Society and National Comprehensive Cancer Network guidelines recommend annual screening MRI for women at high risk for breast cancer (regardless of breast density); however, increasingly, research supports the effectiveness of MRI in women with dense breasts who are otherwise considered average risk. A large randomized controlled trial in the Netherlands compared outcomes in women with extremely dense breasts invited to have screening MRI after negative mammography to those assigned to continue receiving screening mammography only. The incremental cancer detection rate was 16.5 per 1,000 (79/4,783) women screened with MRI in the first round7 and 6 per 1,000 women screened in the second round 2 years later.8 The interval cancer rate was 0.8 per 1,000 (4/4,783) women screened with MRI, compared with 4.9 per 1,000 (16/3,278) women who declined MRI and received mammography only.7
Screening ultrasound will show up to 3 additional cancers per 1,000 women screened after mammography/tomosynthesis (reviewed in Vourtsis and Berg9 and Berg and Vourtsis10), far lower than the added cancer-detection rate of MRI. Consider screening ultrasound for women who cannot tolerate or access screening MRI.11 Contrast-enhanced mammography (CEM) uses iodinated contrast (as in computed tomography). CEM is not widely available but appears to show cancer-detection similar to MRI. For further discussion, see Berg et al’s 2021 review.3
The FIGURE shows an example of an invasive cancer depicted on contrast-enhanced MRI in a 53-year-old woman with dense breasts and a family history of breast cancer that was not visible on tomosynthesis, even in retrospect, due to masking by dense tissue.

Considerations
Breast MRI increases callbacks even after mammography and ultrasound; however, such false alarms are reduced in subsequent screening rounds. MRI cannot be performed in women who have certain metal implants— some pacemakers or spinal fixation rods—and is not recommended for pregnant women. Claustrophobia may be an issue for some women. MRI is expensive and requires IV contrast. Gadolinium is known to accumulate in the brain, although the long-term effects of this are unknown and no harm has been shown.●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA. 2020;323:746-756. doi: 10.1001 /jama.2020.0572
- Kerlikowske K, Zhu W, Tosteson AN, et al. Identifying women with dense breasts at high risk for interval cancer: a cohort study. Ann Intern Med. 2015;162:673-681. doi: 10.7326/M14-1465.
- Berg WA, Rafferty EA, Friedewald SM, Hruska CB, Rahbar H. Screening Algorithms in Dense Breasts: AJR Expert Panel Narrative Review. AJR Am J Roentgenol. 2021;216:275-294. doi: 10.2214/AJR.20.24436.
- Kuhl CK, Strobel K, Bieling H, et al. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283:361-370. doi: 10.1148/radiol.2016161444.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315:1784-1786. doi: 10.1001/jama.2016.1708.
- Osteras BH, Martinsen ACT, Gullien R, et al. Digital mammography versus breast tomosynthesis: impact of breast density on diagnostic performance in population-based screening. Radiology. 2019;293:60-68. doi: 10.1148 /radiol.2019190425.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102. doi: 10.1056/NEJMoa1903986.
- Veenhuizen SGA, de Lange SV, Bakker MF, et al. Supplemental breast MRI for women with extremely dense breasts: results of the second screening round of the DENSE trial. Radiology. 2021;299:278-286. doi: 10.1148/radiol.2021203633.
- Vourtsis A, Berg WA. Breast density implications and supplemental screening. Eur Radiol. 2019;29:1762-1777. doi: 10.1007/s00330-018-5668-8.
- Berg WA, Vourtsis A. Screening ultrasound using handheld or automated technique in women with dense breasts. J Breast Imaging. 2019;1:283-296.
- National Comprehensive Cancer Network. Breast Cancer Screening and Diagnosis (Version 1.2021). https://www.nccn. org/professionals/physician_gls/pdf/breast-screening.pdf. Accessed November 18, 2021.
- Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA. 2020;323:746-756. doi: 10.1001 /jama.2020.0572
- Kerlikowske K, Zhu W, Tosteson AN, et al. Identifying women with dense breasts at high risk for interval cancer: a cohort study. Ann Intern Med. 2015;162:673-681. doi: 10.7326/M14-1465.
- Berg WA, Rafferty EA, Friedewald SM, Hruska CB, Rahbar H. Screening Algorithms in Dense Breasts: AJR Expert Panel Narrative Review. AJR Am J Roentgenol. 2021;216:275-294. doi: 10.2214/AJR.20.24436.
- Kuhl CK, Strobel K, Bieling H, et al. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283:361-370. doi: 10.1148/radiol.2016161444.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315:1784-1786. doi: 10.1001/jama.2016.1708.
- Osteras BH, Martinsen ACT, Gullien R, et al. Digital mammography versus breast tomosynthesis: impact of breast density on diagnostic performance in population-based screening. Radiology. 2019;293:60-68. doi: 10.1148 /radiol.2019190425.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102. doi: 10.1056/NEJMoa1903986.
- Veenhuizen SGA, de Lange SV, Bakker MF, et al. Supplemental breast MRI for women with extremely dense breasts: results of the second screening round of the DENSE trial. Radiology. 2021;299:278-286. doi: 10.1148/radiol.2021203633.
- Vourtsis A, Berg WA. Breast density implications and supplemental screening. Eur Radiol. 2019;29:1762-1777. doi: 10.1007/s00330-018-5668-8.
- Berg WA, Vourtsis A. Screening ultrasound using handheld or automated technique in women with dense breasts. J Breast Imaging. 2019;1:283-296.
- National Comprehensive Cancer Network. Breast Cancer Screening and Diagnosis (Version 1.2021). https://www.nccn. org/professionals/physician_gls/pdf/breast-screening.pdf. Accessed November 18, 2021.
Quiz developed in collaboration with 
HIV testing dips during pandemic raise transmission concerns
raising concerns of a subsequent increase in transmission by people unaware of their HIV-positive status.
“Testing strategies need to be ramped up to cover this decrease in testing while adapting to the continuing COVID-19 environment,” reported Deesha Patel, MPH, and colleagues with the Centers for Disease Control and Prevention’s division of HIV prevention, Atlanta, in research presented at the annual meeting of the United States Conference on HIV/AIDS.
According to their data from the National HIV Prevention Program Monitoring and Evaluation system, the number of CDC-funded HIV tests declined by more than 1 million in 2020 amid the COVID-19 restrictions, with 1,228,142 tests reported that year, compared with 2,301,669 tests in 2019, a reduction of 46.6%.
The number of persons who were newly diagnosed with HIV, based on the tests, declined by 29.7%, from 7,692 newly diagnosed in 2019 to 5,409 persons in 2020, the authors reported.
The reasons for the reduction in new HIV diagnoses in 2020 could be multifactorial, possibly reflecting not just the reduced rates of testing but also possibly lower rates of transmission because of the lockdowns and social distancing, Mr. Patel said in an interview.
“Both [of those] interpretations are plausible, and the reductions are likely due to a combination of reasons,” she said.
Of note, the percentage of tests that were positive did not show a decline and was in fact slightly higher in 2020 (0.4%), compared with 2019 (0.3%; rate ratio, 1.32). But the increase may reflect that those seeking testing during the pandemic were more likely to be symptomatic.
“It is plausible that the smaller pool of people getting tested represented those with a higher likelihood of receiving a positive HIV test, [for instance] having a recent exposure, exhibiting symptoms,” Mr. Patel explained. “Furthermore, it is possible that some health departments specifically focused outreach efforts to serve persons with increased potential for HIV acquisition, thus identifying a higher proportion of persons with HIV.”
The declines in testing are nevertheless of particular concern in light of recent pre-COVID data indicating that as many as 13% of people who were infected with HIV were unaware of their positive status, placing them at high risk of transmitting the virus.
And on a broader level, the declines could negatively affect the goal to eradicate HIV through the federal Ending the HIV Epidemic in the U.S. (EHE) initiative, which aims to reduce new HIV infections in the United States by 90% by 2030 through the scaling up of key HIV prevention and treatment strategies, Mr. Patel noted.
“The first pillar of EHE is to diagnose all people with HIV as early as possible, and to accomplish that, there needs to be sufficient HIV testing,” Mr. Patel explained. “With fewer HIV tests being conducted, there are missed opportunities to identify persons with newly diagnosed HIV, which affects the entire continuum of care, [including] linkage to medical care, receiving antiretroviral treatment, getting and keeping viral suppression, and reducing transmission.”
At the local level: Adaptations allowed for continued testing
In a separate report presented at the meeting detailing the experiences at a more local level, Joseph Olsen, MPH, and colleagues with CrescentCare, New Orleans, described a similar reduction of HIV testing in 2020 of 49% in their system, compared with the previous year, down from 7,952 rapid HIV tests in 2019 to 4,034 in 2020.
However, through efforts to continue to provide services during the pandemic, the program was able to link 182 patients to HIV care in 2020, which was up from 172 in 2019.
In addition to offering the rapid HIV testing in conjunction with COVID-19 testing at their urgent care centers, the center adapted to the pandemic’s challenges with strategies including a new at-home testing program; providing testing at a hotel shelter for the homeless; and testing as part of walk-in testing with a syringe access component.
Mr. Olsen credited the swift program adaptations with maintaining testing during the time of crisis.
“Without [those] measures, it would have been a near-zero number of tests provided,” he said in an interview. “It would have been easy to blame the pandemic and not try to find innovations to deliver services, but I credit our incredibly motivated team for wanting to make sure every possible resource was available.”
But now there are signs of possible fallout from the testing reductions that did occur, Mr. Olsen said.
“We are already seeing the increase with other sexually transmitted infections [STIs], and I expect that we will see this with HIV as well,” he said.
In response, clinicians should use diligence in providing HIV testing, Mr. Olsen asserted.
“The take-home message for clinicians is that anyone having sex should get tested for HIV. It’s as easy as that!” he said.
“If they are getting tested for any other STI, make sure an HIV panel is added and discussed. If someone is pregnant, make sure an HIV panel is added and discussed. If someone has never had an HIV test before in their life – and I would add if they haven’t had an HIV test since March of 2020 – make sure an HIV panel is added/discussed,” he said. “Doing this for everyone also reduces stigma around testing. It’s not because any one person or group or risk behavior is being targeted, it is just good public health practice.”
The authors disclosed no relevant financial relationships. Mr. Patel noted that the findings and conclusions of her poster are those of the authors and do not necessarily represent the official position of the CDC.
A version of this article first appeared on Medscape.com.
raising concerns of a subsequent increase in transmission by people unaware of their HIV-positive status.
“Testing strategies need to be ramped up to cover this decrease in testing while adapting to the continuing COVID-19 environment,” reported Deesha Patel, MPH, and colleagues with the Centers for Disease Control and Prevention’s division of HIV prevention, Atlanta, in research presented at the annual meeting of the United States Conference on HIV/AIDS.
According to their data from the National HIV Prevention Program Monitoring and Evaluation system, the number of CDC-funded HIV tests declined by more than 1 million in 2020 amid the COVID-19 restrictions, with 1,228,142 tests reported that year, compared with 2,301,669 tests in 2019, a reduction of 46.6%.
The number of persons who were newly diagnosed with HIV, based on the tests, declined by 29.7%, from 7,692 newly diagnosed in 2019 to 5,409 persons in 2020, the authors reported.
The reasons for the reduction in new HIV diagnoses in 2020 could be multifactorial, possibly reflecting not just the reduced rates of testing but also possibly lower rates of transmission because of the lockdowns and social distancing, Mr. Patel said in an interview.
“Both [of those] interpretations are plausible, and the reductions are likely due to a combination of reasons,” she said.
Of note, the percentage of tests that were positive did not show a decline and was in fact slightly higher in 2020 (0.4%), compared with 2019 (0.3%; rate ratio, 1.32). But the increase may reflect that those seeking testing during the pandemic were more likely to be symptomatic.
“It is plausible that the smaller pool of people getting tested represented those with a higher likelihood of receiving a positive HIV test, [for instance] having a recent exposure, exhibiting symptoms,” Mr. Patel explained. “Furthermore, it is possible that some health departments specifically focused outreach efforts to serve persons with increased potential for HIV acquisition, thus identifying a higher proportion of persons with HIV.”
The declines in testing are nevertheless of particular concern in light of recent pre-COVID data indicating that as many as 13% of people who were infected with HIV were unaware of their positive status, placing them at high risk of transmitting the virus.
And on a broader level, the declines could negatively affect the goal to eradicate HIV through the federal Ending the HIV Epidemic in the U.S. (EHE) initiative, which aims to reduce new HIV infections in the United States by 90% by 2030 through the scaling up of key HIV prevention and treatment strategies, Mr. Patel noted.
“The first pillar of EHE is to diagnose all people with HIV as early as possible, and to accomplish that, there needs to be sufficient HIV testing,” Mr. Patel explained. “With fewer HIV tests being conducted, there are missed opportunities to identify persons with newly diagnosed HIV, which affects the entire continuum of care, [including] linkage to medical care, receiving antiretroviral treatment, getting and keeping viral suppression, and reducing transmission.”
At the local level: Adaptations allowed for continued testing
In a separate report presented at the meeting detailing the experiences at a more local level, Joseph Olsen, MPH, and colleagues with CrescentCare, New Orleans, described a similar reduction of HIV testing in 2020 of 49% in their system, compared with the previous year, down from 7,952 rapid HIV tests in 2019 to 4,034 in 2020.
However, through efforts to continue to provide services during the pandemic, the program was able to link 182 patients to HIV care in 2020, which was up from 172 in 2019.
In addition to offering the rapid HIV testing in conjunction with COVID-19 testing at their urgent care centers, the center adapted to the pandemic’s challenges with strategies including a new at-home testing program; providing testing at a hotel shelter for the homeless; and testing as part of walk-in testing with a syringe access component.
Mr. Olsen credited the swift program adaptations with maintaining testing during the time of crisis.
“Without [those] measures, it would have been a near-zero number of tests provided,” he said in an interview. “It would have been easy to blame the pandemic and not try to find innovations to deliver services, but I credit our incredibly motivated team for wanting to make sure every possible resource was available.”
But now there are signs of possible fallout from the testing reductions that did occur, Mr. Olsen said.
“We are already seeing the increase with other sexually transmitted infections [STIs], and I expect that we will see this with HIV as well,” he said.
In response, clinicians should use diligence in providing HIV testing, Mr. Olsen asserted.
“The take-home message for clinicians is that anyone having sex should get tested for HIV. It’s as easy as that!” he said.
“If they are getting tested for any other STI, make sure an HIV panel is added and discussed. If someone is pregnant, make sure an HIV panel is added and discussed. If someone has never had an HIV test before in their life – and I would add if they haven’t had an HIV test since March of 2020 – make sure an HIV panel is added/discussed,” he said. “Doing this for everyone also reduces stigma around testing. It’s not because any one person or group or risk behavior is being targeted, it is just good public health practice.”
The authors disclosed no relevant financial relationships. Mr. Patel noted that the findings and conclusions of her poster are those of the authors and do not necessarily represent the official position of the CDC.
A version of this article first appeared on Medscape.com.
raising concerns of a subsequent increase in transmission by people unaware of their HIV-positive status.
“Testing strategies need to be ramped up to cover this decrease in testing while adapting to the continuing COVID-19 environment,” reported Deesha Patel, MPH, and colleagues with the Centers for Disease Control and Prevention’s division of HIV prevention, Atlanta, in research presented at the annual meeting of the United States Conference on HIV/AIDS.
According to their data from the National HIV Prevention Program Monitoring and Evaluation system, the number of CDC-funded HIV tests declined by more than 1 million in 2020 amid the COVID-19 restrictions, with 1,228,142 tests reported that year, compared with 2,301,669 tests in 2019, a reduction of 46.6%.
The number of persons who were newly diagnosed with HIV, based on the tests, declined by 29.7%, from 7,692 newly diagnosed in 2019 to 5,409 persons in 2020, the authors reported.
The reasons for the reduction in new HIV diagnoses in 2020 could be multifactorial, possibly reflecting not just the reduced rates of testing but also possibly lower rates of transmission because of the lockdowns and social distancing, Mr. Patel said in an interview.
“Both [of those] interpretations are plausible, and the reductions are likely due to a combination of reasons,” she said.
Of note, the percentage of tests that were positive did not show a decline and was in fact slightly higher in 2020 (0.4%), compared with 2019 (0.3%; rate ratio, 1.32). But the increase may reflect that those seeking testing during the pandemic were more likely to be symptomatic.
“It is plausible that the smaller pool of people getting tested represented those with a higher likelihood of receiving a positive HIV test, [for instance] having a recent exposure, exhibiting symptoms,” Mr. Patel explained. “Furthermore, it is possible that some health departments specifically focused outreach efforts to serve persons with increased potential for HIV acquisition, thus identifying a higher proportion of persons with HIV.”
The declines in testing are nevertheless of particular concern in light of recent pre-COVID data indicating that as many as 13% of people who were infected with HIV were unaware of their positive status, placing them at high risk of transmitting the virus.
And on a broader level, the declines could negatively affect the goal to eradicate HIV through the federal Ending the HIV Epidemic in the U.S. (EHE) initiative, which aims to reduce new HIV infections in the United States by 90% by 2030 through the scaling up of key HIV prevention and treatment strategies, Mr. Patel noted.
“The first pillar of EHE is to diagnose all people with HIV as early as possible, and to accomplish that, there needs to be sufficient HIV testing,” Mr. Patel explained. “With fewer HIV tests being conducted, there are missed opportunities to identify persons with newly diagnosed HIV, which affects the entire continuum of care, [including] linkage to medical care, receiving antiretroviral treatment, getting and keeping viral suppression, and reducing transmission.”
At the local level: Adaptations allowed for continued testing
In a separate report presented at the meeting detailing the experiences at a more local level, Joseph Olsen, MPH, and colleagues with CrescentCare, New Orleans, described a similar reduction of HIV testing in 2020 of 49% in their system, compared with the previous year, down from 7,952 rapid HIV tests in 2019 to 4,034 in 2020.
However, through efforts to continue to provide services during the pandemic, the program was able to link 182 patients to HIV care in 2020, which was up from 172 in 2019.
In addition to offering the rapid HIV testing in conjunction with COVID-19 testing at their urgent care centers, the center adapted to the pandemic’s challenges with strategies including a new at-home testing program; providing testing at a hotel shelter for the homeless; and testing as part of walk-in testing with a syringe access component.
Mr. Olsen credited the swift program adaptations with maintaining testing during the time of crisis.
“Without [those] measures, it would have been a near-zero number of tests provided,” he said in an interview. “It would have been easy to blame the pandemic and not try to find innovations to deliver services, but I credit our incredibly motivated team for wanting to make sure every possible resource was available.”
But now there are signs of possible fallout from the testing reductions that did occur, Mr. Olsen said.
“We are already seeing the increase with other sexually transmitted infections [STIs], and I expect that we will see this with HIV as well,” he said.
In response, clinicians should use diligence in providing HIV testing, Mr. Olsen asserted.
“The take-home message for clinicians is that anyone having sex should get tested for HIV. It’s as easy as that!” he said.
“If they are getting tested for any other STI, make sure an HIV panel is added and discussed. If someone is pregnant, make sure an HIV panel is added and discussed. If someone has never had an HIV test before in their life – and I would add if they haven’t had an HIV test since March of 2020 – make sure an HIV panel is added/discussed,” he said. “Doing this for everyone also reduces stigma around testing. It’s not because any one person or group or risk behavior is being targeted, it is just good public health practice.”
The authors disclosed no relevant financial relationships. Mr. Patel noted that the findings and conclusions of her poster are those of the authors and do not necessarily represent the official position of the CDC.
A version of this article first appeared on Medscape.com.
Unrestricted prescribing of mifepristone: Safe and effective, says study
Abortion rates remained stable and adverse events were rare after removal of mifepristone prescribing restrictions in Canada, a new study shows.
“Our study is a signal to other countries that restrictions are not necessary to ensure patient safety,” senior author Wendy V. Norman, MD, professor in the department of family practice at the University of British Columbia, Vancouver, said in a press release.
“This is the strongest evidence yet that it is safe to provide the abortion pill like most other prescriptions – meaning any doctor or nurse practitioner can prescribe, any pharmacist can dispense, and patients can take the pills if, when, and where they choose,” said lead author Laura Schummers, ScD, a postdoctoral fellow in the same department.
The findings “add to the accumulating evidence that removing restrictions from medication abortion is safe, effective, and improves access,” agreed Eve Espey, MD, professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, who was not part of the research team. “This is additional confirmation that it is safe for patients to receive abortion care medications in the ‘normal’ fashion, through a prescription available at a pharmacy,” she said in an interview.
The study, published in the New England Journal of Medicine, compared medical abortion use, safety, and effectiveness in the province of Ontario before the Canadian availability of mifepristone and after it became available without restrictions that are similar to the Risk Evaluation and Mitigation Strategy (REMS) restrictions in place for mifepristone in the United States.
Using linked administrative health data, the researchers created a population-based cohort of all Ontario residents aged 12-49 years who had received abortion services during the study period. In total, 195,183 abortions were performed in the period before mifepristone was approved (January 2012–December 2016), and 84,032 were performed after it was made available without restrictions (Nov. 7, 2017, through March 15, 2020). The vast majority of these abortions (89.3%) were surgical, with about 10% being medically induced, the authors reported.
The study found that, while the overall abortion rate declined over the study period (from 11.9 to 11.3 per 1,000 female residents), the proportion of medical abortions jumped sharply from 2.2% to 31.4%, and the rate of second-trimester abortions declined from 5.5% of all abortions to 5.1%.
Abortion safety outcomes within 6 weeks of abortion remained stable over the two study periods. This included severe adverse events (0.03% vs. 0.04%) such as blood transfusions, abdominal surgery, admission to an ICU, or sepsis during an abortion-related hospitalization; and complications (0.74% vs. 0.69%,) such as genital tract or pelvic infection, hemorrhage, embolism, shock, renal failure, damage to pelvic organs or tissues, and venous complications among other things.
There were slight declines in overall abortion effectiveness, but ongoing pregnancy rates “remained infrequent,” the authors noted. While there was a modest rise in the rates of subsequent uterine evacuation (from 1.0% to 2.2%), and ongoing intrauterine pregnancy continuing until delivery (from 0.03% to 0.08%), the rate of ectopic pregnancy diagnosed within 6 weeks after the abortion date remained stable (from 0.15% to 0.22%).
Canada was the first country in the world to remove all supplemental restrictions on the dispensing and administration of mifepristone, according to the press release. And while professional organizations have called for the removal of such restrictions “because they impede access to abortion services without improving safety,” high-quality data on this are lacking, they added.
The study’s finding are consistent with existing U.S. and U.K. data showing Food and Drug Administration REMS restrictions requiring abortion care medications to be dispensed in a clinic by a certified provider “are unnecessary and create obstacles to early abortion access,” said Dr. Espey. “For clinicians and patients in the U.S., it’s important to note that the increasing number of legislative restrictions on abortion, including medication abortion, are non–evidence based. Politically motivated false claims of safety concerns are countered by this study and others conducted during the pandemic when both the U.S. and U.K. removed REMS-type restrictions. These studies show that receiving abortion care through usual pharmacy channels and through telemedicine is safe, effective, and reduces barriers to care.”
Dr. Norman reported receiving grants from the Canadian Institutes of Health Research, providing expert witness services to the government of Ontario and Office of the Attorney General, and serving on the board of directors of the Society of Family Planning. No other researchers reported conflicts of interest. Dr. Espey reported no conflicts of interest. The Canadian Institutes of Health Research and the Women’s Health Research Institute with the support of ICES (formerly known as the Institute for Clinical Evaluative Sciences).
Abortion rates remained stable and adverse events were rare after removal of mifepristone prescribing restrictions in Canada, a new study shows.
“Our study is a signal to other countries that restrictions are not necessary to ensure patient safety,” senior author Wendy V. Norman, MD, professor in the department of family practice at the University of British Columbia, Vancouver, said in a press release.
“This is the strongest evidence yet that it is safe to provide the abortion pill like most other prescriptions – meaning any doctor or nurse practitioner can prescribe, any pharmacist can dispense, and patients can take the pills if, when, and where they choose,” said lead author Laura Schummers, ScD, a postdoctoral fellow in the same department.
The findings “add to the accumulating evidence that removing restrictions from medication abortion is safe, effective, and improves access,” agreed Eve Espey, MD, professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, who was not part of the research team. “This is additional confirmation that it is safe for patients to receive abortion care medications in the ‘normal’ fashion, through a prescription available at a pharmacy,” she said in an interview.
The study, published in the New England Journal of Medicine, compared medical abortion use, safety, and effectiveness in the province of Ontario before the Canadian availability of mifepristone and after it became available without restrictions that are similar to the Risk Evaluation and Mitigation Strategy (REMS) restrictions in place for mifepristone in the United States.
Using linked administrative health data, the researchers created a population-based cohort of all Ontario residents aged 12-49 years who had received abortion services during the study period. In total, 195,183 abortions were performed in the period before mifepristone was approved (January 2012–December 2016), and 84,032 were performed after it was made available without restrictions (Nov. 7, 2017, through March 15, 2020). The vast majority of these abortions (89.3%) were surgical, with about 10% being medically induced, the authors reported.
The study found that, while the overall abortion rate declined over the study period (from 11.9 to 11.3 per 1,000 female residents), the proportion of medical abortions jumped sharply from 2.2% to 31.4%, and the rate of second-trimester abortions declined from 5.5% of all abortions to 5.1%.
Abortion safety outcomes within 6 weeks of abortion remained stable over the two study periods. This included severe adverse events (0.03% vs. 0.04%) such as blood transfusions, abdominal surgery, admission to an ICU, or sepsis during an abortion-related hospitalization; and complications (0.74% vs. 0.69%,) such as genital tract or pelvic infection, hemorrhage, embolism, shock, renal failure, damage to pelvic organs or tissues, and venous complications among other things.
There were slight declines in overall abortion effectiveness, but ongoing pregnancy rates “remained infrequent,” the authors noted. While there was a modest rise in the rates of subsequent uterine evacuation (from 1.0% to 2.2%), and ongoing intrauterine pregnancy continuing until delivery (from 0.03% to 0.08%), the rate of ectopic pregnancy diagnosed within 6 weeks after the abortion date remained stable (from 0.15% to 0.22%).
Canada was the first country in the world to remove all supplemental restrictions on the dispensing and administration of mifepristone, according to the press release. And while professional organizations have called for the removal of such restrictions “because they impede access to abortion services without improving safety,” high-quality data on this are lacking, they added.
The study’s finding are consistent with existing U.S. and U.K. data showing Food and Drug Administration REMS restrictions requiring abortion care medications to be dispensed in a clinic by a certified provider “are unnecessary and create obstacles to early abortion access,” said Dr. Espey. “For clinicians and patients in the U.S., it’s important to note that the increasing number of legislative restrictions on abortion, including medication abortion, are non–evidence based. Politically motivated false claims of safety concerns are countered by this study and others conducted during the pandemic when both the U.S. and U.K. removed REMS-type restrictions. These studies show that receiving abortion care through usual pharmacy channels and through telemedicine is safe, effective, and reduces barriers to care.”
Dr. Norman reported receiving grants from the Canadian Institutes of Health Research, providing expert witness services to the government of Ontario and Office of the Attorney General, and serving on the board of directors of the Society of Family Planning. No other researchers reported conflicts of interest. Dr. Espey reported no conflicts of interest. The Canadian Institutes of Health Research and the Women’s Health Research Institute with the support of ICES (formerly known as the Institute for Clinical Evaluative Sciences).
Abortion rates remained stable and adverse events were rare after removal of mifepristone prescribing restrictions in Canada, a new study shows.
“Our study is a signal to other countries that restrictions are not necessary to ensure patient safety,” senior author Wendy V. Norman, MD, professor in the department of family practice at the University of British Columbia, Vancouver, said in a press release.
“This is the strongest evidence yet that it is safe to provide the abortion pill like most other prescriptions – meaning any doctor or nurse practitioner can prescribe, any pharmacist can dispense, and patients can take the pills if, when, and where they choose,” said lead author Laura Schummers, ScD, a postdoctoral fellow in the same department.
The findings “add to the accumulating evidence that removing restrictions from medication abortion is safe, effective, and improves access,” agreed Eve Espey, MD, professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, who was not part of the research team. “This is additional confirmation that it is safe for patients to receive abortion care medications in the ‘normal’ fashion, through a prescription available at a pharmacy,” she said in an interview.
The study, published in the New England Journal of Medicine, compared medical abortion use, safety, and effectiveness in the province of Ontario before the Canadian availability of mifepristone and after it became available without restrictions that are similar to the Risk Evaluation and Mitigation Strategy (REMS) restrictions in place for mifepristone in the United States.
Using linked administrative health data, the researchers created a population-based cohort of all Ontario residents aged 12-49 years who had received abortion services during the study period. In total, 195,183 abortions were performed in the period before mifepristone was approved (January 2012–December 2016), and 84,032 were performed after it was made available without restrictions (Nov. 7, 2017, through March 15, 2020). The vast majority of these abortions (89.3%) were surgical, with about 10% being medically induced, the authors reported.
The study found that, while the overall abortion rate declined over the study period (from 11.9 to 11.3 per 1,000 female residents), the proportion of medical abortions jumped sharply from 2.2% to 31.4%, and the rate of second-trimester abortions declined from 5.5% of all abortions to 5.1%.
Abortion safety outcomes within 6 weeks of abortion remained stable over the two study periods. This included severe adverse events (0.03% vs. 0.04%) such as blood transfusions, abdominal surgery, admission to an ICU, or sepsis during an abortion-related hospitalization; and complications (0.74% vs. 0.69%,) such as genital tract or pelvic infection, hemorrhage, embolism, shock, renal failure, damage to pelvic organs or tissues, and venous complications among other things.
There were slight declines in overall abortion effectiveness, but ongoing pregnancy rates “remained infrequent,” the authors noted. While there was a modest rise in the rates of subsequent uterine evacuation (from 1.0% to 2.2%), and ongoing intrauterine pregnancy continuing until delivery (from 0.03% to 0.08%), the rate of ectopic pregnancy diagnosed within 6 weeks after the abortion date remained stable (from 0.15% to 0.22%).
Canada was the first country in the world to remove all supplemental restrictions on the dispensing and administration of mifepristone, according to the press release. And while professional organizations have called for the removal of such restrictions “because they impede access to abortion services without improving safety,” high-quality data on this are lacking, they added.
The study’s finding are consistent with existing U.S. and U.K. data showing Food and Drug Administration REMS restrictions requiring abortion care medications to be dispensed in a clinic by a certified provider “are unnecessary and create obstacles to early abortion access,” said Dr. Espey. “For clinicians and patients in the U.S., it’s important to note that the increasing number of legislative restrictions on abortion, including medication abortion, are non–evidence based. Politically motivated false claims of safety concerns are countered by this study and others conducted during the pandemic when both the U.S. and U.K. removed REMS-type restrictions. These studies show that receiving abortion care through usual pharmacy channels and through telemedicine is safe, effective, and reduces barriers to care.”
Dr. Norman reported receiving grants from the Canadian Institutes of Health Research, providing expert witness services to the government of Ontario and Office of the Attorney General, and serving on the board of directors of the Society of Family Planning. No other researchers reported conflicts of interest. Dr. Espey reported no conflicts of interest. The Canadian Institutes of Health Research and the Women’s Health Research Institute with the support of ICES (formerly known as the Institute for Clinical Evaluative Sciences).
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Study shows wider gaps, broader inequities in U.S. sex education than 25 years ago
American teenagers receive less formal sex education today than they did 25 years ago, with “troubling” racial inequities that leave youth of color and queer youth at greater risk than other teens for sexually transmitted diseases and unintended pregnancy, according to a new study.
“Many adolescents do not receive any instruction on essential topics or do not receive this instruction until after the first sex,” wrote Laura D. Lindberg, PhD, and Leslie M. Kantor, PhD, MPH, from the Guttmacher Institute, New York, and the department of urban-global public health at Rutgers University, Piscataway, N.J., respectively. “These gaps in sex education in the U.S. are uneven, and gender, racial, and other disparities are widespread,” they added, calling for “robust efforts ... to ensure equity and reduce health disparities.”
The study used cross-sectional data from the 2011-2015 and 2015-2019 National Surveys of Family Growth (NSFG) to examine content, timing, and location of formal sex education among 15- to 19-year-olds in the United States. The data came from samples of 2,047 females and 2,087 males in 2011-2015, and 1,894 females and 1,918 males in 2015-2019. The majority of respondents were aged 15-17 years and non-Hispanic White, with another quarter being Hispanic, and 14% Black.
The survey asked respondents whether, before they turned 18, they had ever received formal instruction at school, church, a community center, “or some other place” about how to say no to sex, methods of birth control, STDs, how to prevent HIV/AIDS, abstaining until marriage to have sex, where to get birth control, and how to use a condom.
Follow-up questions asked about what grade instruction was first received and whether it had occurred before first penile-vaginal intercourse. The 2015-2019 survey also asked about the location of instruction, but only concerning methods of birth control and abstinence until marriage.
The results showed that HIV and STD prevention was the most commonly reported area of instruction, received by more than 90% of both males and females. However, beyond this there were imbalances, with only about half (49%-55%) of respondents receiving instruction meeting the Surgeon General’s Healthy People 2030 composite sex education goal. Lack of instruction on birth control drove this result for 80% of respondents. Specifically, there was a strong slant emphasizing abstinence over birth control instruction. Over both survey periods and both genders, more respondents reported instruction on how to say no to sex (79%-84%) and abstaining until marriage (58%-73%), compared with where to obtain birth control (40%-53%) or how to use a condom (54%-60%). “Overall, about 20% of adolescents received instruction from multiple sources about waiting until marriage, but only 5%-8% received birth control information from multiple settings,” they reported.
There were racial/ethnic and sexual orientation differences in the scope and balance of instruction reported by teens. Less than half of Black (45%) and Hispanic (47%) males received instruction on the combined Healthy People topics, compared with 57% of White males. Black females were less likely (30%) than White females (45%) to receive information on where to get birth control before the first sex. Nonstraight males were less likely than straight males to receive instruction about STIs or HIV/AIDS (83% vs. 93%).
In addition, religious attendance emerged as a key factor in the receipt of sex education, “with more frequent religious attendance associated with a greater likelihood of instruction about delaying sex and less likelihood of instruction about contraception,” the authors noted.
Comparing their findings to previous NSFG surveys, the researchers commented that “the share of adolescents receiving instruction about birth control was higher in 1995 than in 2015-2019 for both the genders; in 1995, 87% of females and 81% of males reported sex education about birth control methods, compared with 64% and 63% in 2015-2019, respectively.” The findings “should spur policy makers at the national, state, and local levels to ensure the broader provision of sex education and that school districts serving young people of color are the focus of additional efforts and funding.”
Asked for comment, John Santelli, MD, MPH, professor of population and family health and pediatrics at Columbia University, New York, who was not involved with the study, said the findings fit into a series of studies by Lindberg going back to 1988 showing that receipt of formal sex education before age 18 has declined over time.
“We, the adults, in America can do better by our young people,” he said in an interview. “Adolescents need sex education that is science based, medically accurate, and developmentally appropriate. Many adolescents are not receiving education that the CDC and health professionals recommend including information about where to get birth control, condom skills, and even, how to say no to sex. The neglect of young Black and Hispanic men is very concerning. However, we are not doing a great job in educating most of our adolescents. Health care providers can be influential in speaking with parents about their children’s education about sex. We need to activate parents, health care providers, and members of the faith community to investigate what is happening about sex education in their own communities.”
Dr. Santelli noted that there are multiple ways to strengthen the provision of sex education in the United States. In a recent commentary, he and his coauthors highlighted the National Sex Education Standards (NSES), which, “developed in partnership between sex education organizations and health professionals, provide clear, consistent, and straightforward guidance on the essential content for students in grades K-12.” The NSES were also used in the development of the CDC’s recently released Health Education Curriculum Analysis Tool.
The commentary takes a strong stand against the recently released revised Medical Institute for Sexual Heath K-12 Standards for Optimal Sexual Development, which, compared with the NSES, are “seriously flawed from both scientific and human rights’ perspectives,” they wrote. “States and local communities aiming to improve adolescent sexual and reproductive health and looking for national standards on sex education should adopt the NSES.”
Dr. Lindberg and Dr. Kantor disclosed no conflicts of interest. Dr. Santelli teaches public health students about adolescent health and chairs the board of directors of the Sexuality Information and Education Council of the United States. He disclosed no financial conflicts.
American teenagers receive less formal sex education today than they did 25 years ago, with “troubling” racial inequities that leave youth of color and queer youth at greater risk than other teens for sexually transmitted diseases and unintended pregnancy, according to a new study.
“Many adolescents do not receive any instruction on essential topics or do not receive this instruction until after the first sex,” wrote Laura D. Lindberg, PhD, and Leslie M. Kantor, PhD, MPH, from the Guttmacher Institute, New York, and the department of urban-global public health at Rutgers University, Piscataway, N.J., respectively. “These gaps in sex education in the U.S. are uneven, and gender, racial, and other disparities are widespread,” they added, calling for “robust efforts ... to ensure equity and reduce health disparities.”
The study used cross-sectional data from the 2011-2015 and 2015-2019 National Surveys of Family Growth (NSFG) to examine content, timing, and location of formal sex education among 15- to 19-year-olds in the United States. The data came from samples of 2,047 females and 2,087 males in 2011-2015, and 1,894 females and 1,918 males in 2015-2019. The majority of respondents were aged 15-17 years and non-Hispanic White, with another quarter being Hispanic, and 14% Black.
The survey asked respondents whether, before they turned 18, they had ever received formal instruction at school, church, a community center, “or some other place” about how to say no to sex, methods of birth control, STDs, how to prevent HIV/AIDS, abstaining until marriage to have sex, where to get birth control, and how to use a condom.
Follow-up questions asked about what grade instruction was first received and whether it had occurred before first penile-vaginal intercourse. The 2015-2019 survey also asked about the location of instruction, but only concerning methods of birth control and abstinence until marriage.
The results showed that HIV and STD prevention was the most commonly reported area of instruction, received by more than 90% of both males and females. However, beyond this there were imbalances, with only about half (49%-55%) of respondents receiving instruction meeting the Surgeon General’s Healthy People 2030 composite sex education goal. Lack of instruction on birth control drove this result for 80% of respondents. Specifically, there was a strong slant emphasizing abstinence over birth control instruction. Over both survey periods and both genders, more respondents reported instruction on how to say no to sex (79%-84%) and abstaining until marriage (58%-73%), compared with where to obtain birth control (40%-53%) or how to use a condom (54%-60%). “Overall, about 20% of adolescents received instruction from multiple sources about waiting until marriage, but only 5%-8% received birth control information from multiple settings,” they reported.
There were racial/ethnic and sexual orientation differences in the scope and balance of instruction reported by teens. Less than half of Black (45%) and Hispanic (47%) males received instruction on the combined Healthy People topics, compared with 57% of White males. Black females were less likely (30%) than White females (45%) to receive information on where to get birth control before the first sex. Nonstraight males were less likely than straight males to receive instruction about STIs or HIV/AIDS (83% vs. 93%).
In addition, religious attendance emerged as a key factor in the receipt of sex education, “with more frequent religious attendance associated with a greater likelihood of instruction about delaying sex and less likelihood of instruction about contraception,” the authors noted.
Comparing their findings to previous NSFG surveys, the researchers commented that “the share of adolescents receiving instruction about birth control was higher in 1995 than in 2015-2019 for both the genders; in 1995, 87% of females and 81% of males reported sex education about birth control methods, compared with 64% and 63% in 2015-2019, respectively.” The findings “should spur policy makers at the national, state, and local levels to ensure the broader provision of sex education and that school districts serving young people of color are the focus of additional efforts and funding.”
Asked for comment, John Santelli, MD, MPH, professor of population and family health and pediatrics at Columbia University, New York, who was not involved with the study, said the findings fit into a series of studies by Lindberg going back to 1988 showing that receipt of formal sex education before age 18 has declined over time.
“We, the adults, in America can do better by our young people,” he said in an interview. “Adolescents need sex education that is science based, medically accurate, and developmentally appropriate. Many adolescents are not receiving education that the CDC and health professionals recommend including information about where to get birth control, condom skills, and even, how to say no to sex. The neglect of young Black and Hispanic men is very concerning. However, we are not doing a great job in educating most of our adolescents. Health care providers can be influential in speaking with parents about their children’s education about sex. We need to activate parents, health care providers, and members of the faith community to investigate what is happening about sex education in their own communities.”
Dr. Santelli noted that there are multiple ways to strengthen the provision of sex education in the United States. In a recent commentary, he and his coauthors highlighted the National Sex Education Standards (NSES), which, “developed in partnership between sex education organizations and health professionals, provide clear, consistent, and straightforward guidance on the essential content for students in grades K-12.” The NSES were also used in the development of the CDC’s recently released Health Education Curriculum Analysis Tool.
The commentary takes a strong stand against the recently released revised Medical Institute for Sexual Heath K-12 Standards for Optimal Sexual Development, which, compared with the NSES, are “seriously flawed from both scientific and human rights’ perspectives,” they wrote. “States and local communities aiming to improve adolescent sexual and reproductive health and looking for national standards on sex education should adopt the NSES.”
Dr. Lindberg and Dr. Kantor disclosed no conflicts of interest. Dr. Santelli teaches public health students about adolescent health and chairs the board of directors of the Sexuality Information and Education Council of the United States. He disclosed no financial conflicts.
American teenagers receive less formal sex education today than they did 25 years ago, with “troubling” racial inequities that leave youth of color and queer youth at greater risk than other teens for sexually transmitted diseases and unintended pregnancy, according to a new study.
“Many adolescents do not receive any instruction on essential topics or do not receive this instruction until after the first sex,” wrote Laura D. Lindberg, PhD, and Leslie M. Kantor, PhD, MPH, from the Guttmacher Institute, New York, and the department of urban-global public health at Rutgers University, Piscataway, N.J., respectively. “These gaps in sex education in the U.S. are uneven, and gender, racial, and other disparities are widespread,” they added, calling for “robust efforts ... to ensure equity and reduce health disparities.”
The study used cross-sectional data from the 2011-2015 and 2015-2019 National Surveys of Family Growth (NSFG) to examine content, timing, and location of formal sex education among 15- to 19-year-olds in the United States. The data came from samples of 2,047 females and 2,087 males in 2011-2015, and 1,894 females and 1,918 males in 2015-2019. The majority of respondents were aged 15-17 years and non-Hispanic White, with another quarter being Hispanic, and 14% Black.
The survey asked respondents whether, before they turned 18, they had ever received formal instruction at school, church, a community center, “or some other place” about how to say no to sex, methods of birth control, STDs, how to prevent HIV/AIDS, abstaining until marriage to have sex, where to get birth control, and how to use a condom.
Follow-up questions asked about what grade instruction was first received and whether it had occurred before first penile-vaginal intercourse. The 2015-2019 survey also asked about the location of instruction, but only concerning methods of birth control and abstinence until marriage.
The results showed that HIV and STD prevention was the most commonly reported area of instruction, received by more than 90% of both males and females. However, beyond this there were imbalances, with only about half (49%-55%) of respondents receiving instruction meeting the Surgeon General’s Healthy People 2030 composite sex education goal. Lack of instruction on birth control drove this result for 80% of respondents. Specifically, there was a strong slant emphasizing abstinence over birth control instruction. Over both survey periods and both genders, more respondents reported instruction on how to say no to sex (79%-84%) and abstaining until marriage (58%-73%), compared with where to obtain birth control (40%-53%) or how to use a condom (54%-60%). “Overall, about 20% of adolescents received instruction from multiple sources about waiting until marriage, but only 5%-8% received birth control information from multiple settings,” they reported.
There were racial/ethnic and sexual orientation differences in the scope and balance of instruction reported by teens. Less than half of Black (45%) and Hispanic (47%) males received instruction on the combined Healthy People topics, compared with 57% of White males. Black females were less likely (30%) than White females (45%) to receive information on where to get birth control before the first sex. Nonstraight males were less likely than straight males to receive instruction about STIs or HIV/AIDS (83% vs. 93%).
In addition, religious attendance emerged as a key factor in the receipt of sex education, “with more frequent religious attendance associated with a greater likelihood of instruction about delaying sex and less likelihood of instruction about contraception,” the authors noted.
Comparing their findings to previous NSFG surveys, the researchers commented that “the share of adolescents receiving instruction about birth control was higher in 1995 than in 2015-2019 for both the genders; in 1995, 87% of females and 81% of males reported sex education about birth control methods, compared with 64% and 63% in 2015-2019, respectively.” The findings “should spur policy makers at the national, state, and local levels to ensure the broader provision of sex education and that school districts serving young people of color are the focus of additional efforts and funding.”
Asked for comment, John Santelli, MD, MPH, professor of population and family health and pediatrics at Columbia University, New York, who was not involved with the study, said the findings fit into a series of studies by Lindberg going back to 1988 showing that receipt of formal sex education before age 18 has declined over time.
“We, the adults, in America can do better by our young people,” he said in an interview. “Adolescents need sex education that is science based, medically accurate, and developmentally appropriate. Many adolescents are not receiving education that the CDC and health professionals recommend including information about where to get birth control, condom skills, and even, how to say no to sex. The neglect of young Black and Hispanic men is very concerning. However, we are not doing a great job in educating most of our adolescents. Health care providers can be influential in speaking with parents about their children’s education about sex. We need to activate parents, health care providers, and members of the faith community to investigate what is happening about sex education in their own communities.”
Dr. Santelli noted that there are multiple ways to strengthen the provision of sex education in the United States. In a recent commentary, he and his coauthors highlighted the National Sex Education Standards (NSES), which, “developed in partnership between sex education organizations and health professionals, provide clear, consistent, and straightforward guidance on the essential content for students in grades K-12.” The NSES were also used in the development of the CDC’s recently released Health Education Curriculum Analysis Tool.
The commentary takes a strong stand against the recently released revised Medical Institute for Sexual Heath K-12 Standards for Optimal Sexual Development, which, compared with the NSES, are “seriously flawed from both scientific and human rights’ perspectives,” they wrote. “States and local communities aiming to improve adolescent sexual and reproductive health and looking for national standards on sex education should adopt the NSES.”
Dr. Lindberg and Dr. Kantor disclosed no conflicts of interest. Dr. Santelli teaches public health students about adolescent health and chairs the board of directors of the Sexuality Information and Education Council of the United States. He disclosed no financial conflicts.
FROM THE JOURNAL OF ADOLESCENT HEALTH
Big drop in U.S. cervical cancer rates, mortality in younger women
The analysis adds to a growing body of evidence demonstrating vaccine-associated changes in cervical cancer incidence and mortality.
Previous data from the United Kingdom, published earlier in November, showed that cervical cancer rates were 87% lower among girls who received the HPV vaccine compared to previously unvaccinated generations. Based on the analysis, the authors concluded that the UK’s HPV immunization program “almost eliminated cervical cancer” in women born since September 1995.
The latest study, published Nov. 29 in JAMA Pediatrics , reports a 38% drop in cervical cancer incidence and a 43% decline in mortality among young women and girls after HPV vaccination was introduced in the United States.
“These results are encouraging,” Peter Sasieni, MD, of King’s College London, and senior author on the U.K. study, told this news organization in an email.
The difference in incidence rates between the U.K. and U.S. studies, Dr. Sasieni explained, is likely due to HPV vaccine coverage not expanding as significantly in the United States as it has in the United Kingdom, and “thus one would anticipate a lower impact on the population in the U.S.”
In the U.S. analysis, Justin Barnes, MD, a radiation oncology resident at Washington University, St. Louis, and colleagues examined cervical cancer incidence between January 2001 and December 2017 using Surveillance, Epidemiology, and End Results and National Program of Cancer Registries data as well as mortality data from the National Center for Health Statistics.
Dr. Barnes and colleagues then compared changes in cervical cancer incidence and mortality between prevaccination years (January 2001 to December 2005) and postvaccination years (January 2010 to December 2017) among three age cohorts – 15-24 years, 25-29 years, and 30-39 years.
“The older 2 groups were included as comparison, given their low vaccination rates,” Dr. Barnes and colleagues explained.
Results show that between the prevaccination and postvaccination periods, the incidence of cervical cancer dropped by 38% in the youngest cohort and by only 16% in the middle-aged group and 8% in the oldest cohort.
Women and girls in the youngest group saw a striking drop in mortality: a 43% decline, which translated to a mortality rate of 0.6 per 100,000.
On the other hand, the authors report a 4.7% decline in mortality in the oldest group and a 4.3% increase in mortality in the middle-aged group – translating to a mortality rate of 1.89 per 100,000 and 0.57 per 100,000, respectively.
Overall, “these nationwide data showed decreased cervical cancer incidence and mortality among women and girls aged 15-24 years after HPV vaccine introduction,” Dr. Barnes and colleagues wrote. The changes in cervical cancer incidence and mortality observed in the youngest age group “were greater than changes in those aged 25 to 29 years and 30 to 39 years, suggesting possible associations with HPV vaccination.”
This analysis lines up with previous evidence from U.S. epidemiologic data, which “have shown decreased cervical cancer incidence after vaccine implementation in women and girls aged 15 to 24 years but not older women.”
Although “the number of deaths and hence the number of potentially averted deaths in young women and girls was small,” the study adds to the current literature by “providing suggestive evidence for vaccine-associated decreases in cervical cancer mortality,” investigators concluded.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The analysis adds to a growing body of evidence demonstrating vaccine-associated changes in cervical cancer incidence and mortality.
Previous data from the United Kingdom, published earlier in November, showed that cervical cancer rates were 87% lower among girls who received the HPV vaccine compared to previously unvaccinated generations. Based on the analysis, the authors concluded that the UK’s HPV immunization program “almost eliminated cervical cancer” in women born since September 1995.
The latest study, published Nov. 29 in JAMA Pediatrics , reports a 38% drop in cervical cancer incidence and a 43% decline in mortality among young women and girls after HPV vaccination was introduced in the United States.
“These results are encouraging,” Peter Sasieni, MD, of King’s College London, and senior author on the U.K. study, told this news organization in an email.
The difference in incidence rates between the U.K. and U.S. studies, Dr. Sasieni explained, is likely due to HPV vaccine coverage not expanding as significantly in the United States as it has in the United Kingdom, and “thus one would anticipate a lower impact on the population in the U.S.”
In the U.S. analysis, Justin Barnes, MD, a radiation oncology resident at Washington University, St. Louis, and colleagues examined cervical cancer incidence between January 2001 and December 2017 using Surveillance, Epidemiology, and End Results and National Program of Cancer Registries data as well as mortality data from the National Center for Health Statistics.
Dr. Barnes and colleagues then compared changes in cervical cancer incidence and mortality between prevaccination years (January 2001 to December 2005) and postvaccination years (January 2010 to December 2017) among three age cohorts – 15-24 years, 25-29 years, and 30-39 years.
“The older 2 groups were included as comparison, given their low vaccination rates,” Dr. Barnes and colleagues explained.
Results show that between the prevaccination and postvaccination periods, the incidence of cervical cancer dropped by 38% in the youngest cohort and by only 16% in the middle-aged group and 8% in the oldest cohort.
Women and girls in the youngest group saw a striking drop in mortality: a 43% decline, which translated to a mortality rate of 0.6 per 100,000.
On the other hand, the authors report a 4.7% decline in mortality in the oldest group and a 4.3% increase in mortality in the middle-aged group – translating to a mortality rate of 1.89 per 100,000 and 0.57 per 100,000, respectively.
Overall, “these nationwide data showed decreased cervical cancer incidence and mortality among women and girls aged 15-24 years after HPV vaccine introduction,” Dr. Barnes and colleagues wrote. The changes in cervical cancer incidence and mortality observed in the youngest age group “were greater than changes in those aged 25 to 29 years and 30 to 39 years, suggesting possible associations with HPV vaccination.”
This analysis lines up with previous evidence from U.S. epidemiologic data, which “have shown decreased cervical cancer incidence after vaccine implementation in women and girls aged 15 to 24 years but not older women.”
Although “the number of deaths and hence the number of potentially averted deaths in young women and girls was small,” the study adds to the current literature by “providing suggestive evidence for vaccine-associated decreases in cervical cancer mortality,” investigators concluded.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The analysis adds to a growing body of evidence demonstrating vaccine-associated changes in cervical cancer incidence and mortality.
Previous data from the United Kingdom, published earlier in November, showed that cervical cancer rates were 87% lower among girls who received the HPV vaccine compared to previously unvaccinated generations. Based on the analysis, the authors concluded that the UK’s HPV immunization program “almost eliminated cervical cancer” in women born since September 1995.
The latest study, published Nov. 29 in JAMA Pediatrics , reports a 38% drop in cervical cancer incidence and a 43% decline in mortality among young women and girls after HPV vaccination was introduced in the United States.
“These results are encouraging,” Peter Sasieni, MD, of King’s College London, and senior author on the U.K. study, told this news organization in an email.
The difference in incidence rates between the U.K. and U.S. studies, Dr. Sasieni explained, is likely due to HPV vaccine coverage not expanding as significantly in the United States as it has in the United Kingdom, and “thus one would anticipate a lower impact on the population in the U.S.”
In the U.S. analysis, Justin Barnes, MD, a radiation oncology resident at Washington University, St. Louis, and colleagues examined cervical cancer incidence between January 2001 and December 2017 using Surveillance, Epidemiology, and End Results and National Program of Cancer Registries data as well as mortality data from the National Center for Health Statistics.
Dr. Barnes and colleagues then compared changes in cervical cancer incidence and mortality between prevaccination years (January 2001 to December 2005) and postvaccination years (January 2010 to December 2017) among three age cohorts – 15-24 years, 25-29 years, and 30-39 years.
“The older 2 groups were included as comparison, given their low vaccination rates,” Dr. Barnes and colleagues explained.
Results show that between the prevaccination and postvaccination periods, the incidence of cervical cancer dropped by 38% in the youngest cohort and by only 16% in the middle-aged group and 8% in the oldest cohort.
Women and girls in the youngest group saw a striking drop in mortality: a 43% decline, which translated to a mortality rate of 0.6 per 100,000.
On the other hand, the authors report a 4.7% decline in mortality in the oldest group and a 4.3% increase in mortality in the middle-aged group – translating to a mortality rate of 1.89 per 100,000 and 0.57 per 100,000, respectively.
Overall, “these nationwide data showed decreased cervical cancer incidence and mortality among women and girls aged 15-24 years after HPV vaccine introduction,” Dr. Barnes and colleagues wrote. The changes in cervical cancer incidence and mortality observed in the youngest age group “were greater than changes in those aged 25 to 29 years and 30 to 39 years, suggesting possible associations with HPV vaccination.”
This analysis lines up with previous evidence from U.S. epidemiologic data, which “have shown decreased cervical cancer incidence after vaccine implementation in women and girls aged 15 to 24 years but not older women.”
Although “the number of deaths and hence the number of potentially averted deaths in young women and girls was small,” the study adds to the current literature by “providing suggestive evidence for vaccine-associated decreases in cervical cancer mortality,” investigators concluded.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PEDIATRICS
Should gynecologists receive the HPV vaccine?
Gynecologists have experience managing human papillomavirus–associated diseases of the lower genital tract. However, HPV also causes warty disease, dysplasia, and carcinoma of the head and neck. Risk factors for head and neck cancer include smoking and smokeless tobacco use, alcohol consumption, periodontal disease, radiation exposure, and HPV. The incidence of HPV-associated head and neck cancer is rising, particularly among men, at a rate of 2.7% per year.1 The incidence of HPV-associated squamous cell carcinoma of the oropharynx now surpasses that of cervical cancer. Concerns exist regarding occupational exposure to HPV by health care providers (HCP) who perform smoke-generating procedures on HPV-infected tissues, and the potential for them to develop head and neck pathology.
In March of 2020, the American Society for Colposcopy and Cervical Pathology made the recommendation that clinicians who are routinely exposed to HPV should protect themselves against the sequela of occupationally acquired HPV by receiving the HPV vaccine.2 They advocate for the “complete provider team” including physicians, advanced practice providers, nurses, operative technicians, and residents and fellows to be considered for protective vaccination.
Similar to disease patterns in the genital tract, different strains of HPV have differing propensity to cause benign, premalignant, and malignant disease states. HPV 6 and 11 are more commonly associated with warty disease in the nares, pharynx, and tonsillar tissues. HPV 16, 18, 31, and 33 (most commonly 16) are considered high risk for carcinoma formation, particularly of the tonsils and base of the tongue.
The procedures most implicated in occupational HPV exposure include ablative procedures for anogenital warts, laser ablation of vaginal and vulvar dysplasia, and electrosurgical excisional procedures for cervical dysplasia. Smoke plumes from HPV-associated procedures are known to contain HPV for both laser and electrocautery sources.3 A study of 134 patients undergoing surgical procedures for laser ablation of HPV-infected tissues detected concordant strains of HPV in approximately 30% of smoke plumes and approximately 1.5% of surgeons’ nares.4 Not all procedures appear to carry the same risk. Electrocoagulation procedures appear to yield fewer postprocedural positive mucosal swabs for HPV, compared with those taken after CO2 laser.5
Animal studies have shown that papilloma virus procured from smoke plume has the capacity to generate disease. When 10 calves were inoculated with bovine papillary virus obtained from smoke plumes from laser ablation of bovine papillomavirus lesions, all calves manifested BPV fibropapilloma lesions at the sites of inoculation.6
There appears to be an increased incidence of HPV-associated head and neck disease among surgeons who perform procedures on HPV tissues, and there have been multiple case reports that have cited examples of HPV-associated benign and malignant disease among HCPs with frequent occupational exposure to HPV anogenital ablative and excisional procedures.7 While these observations are not proof of causation, they are cause for concern.
While the ASCCP guidelines advocate for HPV vaccination as a strategy for prevention of occupationally related HPV-associated disease, there are other strategies in place to minimize risk. The CDC guidelines for environmental infection control in health care facilities include the following recommendations:
- In settings where surgical lasers are used, wear appropriate personnel protective equipment (PPE), including N95 or N100 respirators to minimize exposure to laser plumes.
- Use central wall suction units with in-line filters to evacuate minimal laser plumes.
- Use a mechanical smoke evaluation system with a high efficiency filter to manage the generation of large amounts of laser plume, when ablating tissue infected with HPV.
- Use local exhaust ventilation (LEV).8
When closely adhered to, these methods appear to provide high-level protection. Data suggest that, when HCPs can access appropriate protective equipment, risks for HPV exposure are low. However, this is more feasible for larger hospital facilities, and may be more limited in outpatient settings. This has led to the consideration of background protection in the form of HPV vaccination for at-risk HCPs. This is analogous to mandates for HCPs to receive hepatitis B vaccination despite the concomitant practice of universal precautions in health care settings. Preventative strategies are typically most efficacious when performed in concert.
After nearly 2 decades of widespread use, we have confidence in the safety of the HPV vaccination. Its benefit through age 45 has been established, leading to the 2018 FDA approval for the 9-valent HPV vaccine, Guardisil-9, for this expanded age group. It would seem logical that systematic administration of the HPV vaccine for at-risk HCPs would be both feasible and safe. There are well-established systems for administering vaccines for HCPs in all health care systems. Perhaps health system administrators should consider routinely offering HPV vaccination for at-risk employees as part of their occupational health care responsibilities. One important caveat being the cost and efficacy of HPV vaccination in this group has not been not established.
In the meantime, it is critical that gynecology providers be aware of their risk for occupational exposure to HPV when using laser and electrocautery techniques on HPV-infected tissues and the potential for them developing head and neck pathology. They should strictly adhere to preventative measures such as use of fit-tested N-95 respirators, mechanical smoke evacuators with high-efficiency filters and work in environments with adequate room ventilation. We all should individually evaluate what role HPV vaccination may play for us in augmenting our own safety.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Van Dyne EA et al. MMWR Morb Mortal Wkly Rep. 2018 Aug 24;67(33):918-24.
2. ASCCP. ASCCP recommends HPV vaccination for providers.
3. Fox-Lewis A et al. Occup Environ Med. 2020 Dec;77(12):809-17.
4. Zhou Q et al. Cancer Manag Res. 2019;11:3643-54
5. Bergbrant I et al. Acta Derm Venereol. 1994 Sep;74(5):393-5.
6. Garden J et al. Arch Dermatol. 2002 Oct;138(10):1303-7.
7. Harrison R, Huh W. Obstet Gynecol. 2020;136:663-5.
8. CDC. 1996. DHHS (NIOSH) Publication Number 96-128.
Gynecologists have experience managing human papillomavirus–associated diseases of the lower genital tract. However, HPV also causes warty disease, dysplasia, and carcinoma of the head and neck. Risk factors for head and neck cancer include smoking and smokeless tobacco use, alcohol consumption, periodontal disease, radiation exposure, and HPV. The incidence of HPV-associated head and neck cancer is rising, particularly among men, at a rate of 2.7% per year.1 The incidence of HPV-associated squamous cell carcinoma of the oropharynx now surpasses that of cervical cancer. Concerns exist regarding occupational exposure to HPV by health care providers (HCP) who perform smoke-generating procedures on HPV-infected tissues, and the potential for them to develop head and neck pathology.
In March of 2020, the American Society for Colposcopy and Cervical Pathology made the recommendation that clinicians who are routinely exposed to HPV should protect themselves against the sequela of occupationally acquired HPV by receiving the HPV vaccine.2 They advocate for the “complete provider team” including physicians, advanced practice providers, nurses, operative technicians, and residents and fellows to be considered for protective vaccination.
Similar to disease patterns in the genital tract, different strains of HPV have differing propensity to cause benign, premalignant, and malignant disease states. HPV 6 and 11 are more commonly associated with warty disease in the nares, pharynx, and tonsillar tissues. HPV 16, 18, 31, and 33 (most commonly 16) are considered high risk for carcinoma formation, particularly of the tonsils and base of the tongue.
The procedures most implicated in occupational HPV exposure include ablative procedures for anogenital warts, laser ablation of vaginal and vulvar dysplasia, and electrosurgical excisional procedures for cervical dysplasia. Smoke plumes from HPV-associated procedures are known to contain HPV for both laser and electrocautery sources.3 A study of 134 patients undergoing surgical procedures for laser ablation of HPV-infected tissues detected concordant strains of HPV in approximately 30% of smoke plumes and approximately 1.5% of surgeons’ nares.4 Not all procedures appear to carry the same risk. Electrocoagulation procedures appear to yield fewer postprocedural positive mucosal swabs for HPV, compared with those taken after CO2 laser.5
Animal studies have shown that papilloma virus procured from smoke plume has the capacity to generate disease. When 10 calves were inoculated with bovine papillary virus obtained from smoke plumes from laser ablation of bovine papillomavirus lesions, all calves manifested BPV fibropapilloma lesions at the sites of inoculation.6
There appears to be an increased incidence of HPV-associated head and neck disease among surgeons who perform procedures on HPV tissues, and there have been multiple case reports that have cited examples of HPV-associated benign and malignant disease among HCPs with frequent occupational exposure to HPV anogenital ablative and excisional procedures.7 While these observations are not proof of causation, they are cause for concern.
While the ASCCP guidelines advocate for HPV vaccination as a strategy for prevention of occupationally related HPV-associated disease, there are other strategies in place to minimize risk. The CDC guidelines for environmental infection control in health care facilities include the following recommendations:
- In settings where surgical lasers are used, wear appropriate personnel protective equipment (PPE), including N95 or N100 respirators to minimize exposure to laser plumes.
- Use central wall suction units with in-line filters to evacuate minimal laser plumes.
- Use a mechanical smoke evaluation system with a high efficiency filter to manage the generation of large amounts of laser plume, when ablating tissue infected with HPV.
- Use local exhaust ventilation (LEV).8
When closely adhered to, these methods appear to provide high-level protection. Data suggest that, when HCPs can access appropriate protective equipment, risks for HPV exposure are low. However, this is more feasible for larger hospital facilities, and may be more limited in outpatient settings. This has led to the consideration of background protection in the form of HPV vaccination for at-risk HCPs. This is analogous to mandates for HCPs to receive hepatitis B vaccination despite the concomitant practice of universal precautions in health care settings. Preventative strategies are typically most efficacious when performed in concert.
After nearly 2 decades of widespread use, we have confidence in the safety of the HPV vaccination. Its benefit through age 45 has been established, leading to the 2018 FDA approval for the 9-valent HPV vaccine, Guardisil-9, for this expanded age group. It would seem logical that systematic administration of the HPV vaccine for at-risk HCPs would be both feasible and safe. There are well-established systems for administering vaccines for HCPs in all health care systems. Perhaps health system administrators should consider routinely offering HPV vaccination for at-risk employees as part of their occupational health care responsibilities. One important caveat being the cost and efficacy of HPV vaccination in this group has not been not established.
In the meantime, it is critical that gynecology providers be aware of their risk for occupational exposure to HPV when using laser and electrocautery techniques on HPV-infected tissues and the potential for them developing head and neck pathology. They should strictly adhere to preventative measures such as use of fit-tested N-95 respirators, mechanical smoke evacuators with high-efficiency filters and work in environments with adequate room ventilation. We all should individually evaluate what role HPV vaccination may play for us in augmenting our own safety.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Van Dyne EA et al. MMWR Morb Mortal Wkly Rep. 2018 Aug 24;67(33):918-24.
2. ASCCP. ASCCP recommends HPV vaccination for providers.
3. Fox-Lewis A et al. Occup Environ Med. 2020 Dec;77(12):809-17.
4. Zhou Q et al. Cancer Manag Res. 2019;11:3643-54
5. Bergbrant I et al. Acta Derm Venereol. 1994 Sep;74(5):393-5.
6. Garden J et al. Arch Dermatol. 2002 Oct;138(10):1303-7.
7. Harrison R, Huh W. Obstet Gynecol. 2020;136:663-5.
8. CDC. 1996. DHHS (NIOSH) Publication Number 96-128.
Gynecologists have experience managing human papillomavirus–associated diseases of the lower genital tract. However, HPV also causes warty disease, dysplasia, and carcinoma of the head and neck. Risk factors for head and neck cancer include smoking and smokeless tobacco use, alcohol consumption, periodontal disease, radiation exposure, and HPV. The incidence of HPV-associated head and neck cancer is rising, particularly among men, at a rate of 2.7% per year.1 The incidence of HPV-associated squamous cell carcinoma of the oropharynx now surpasses that of cervical cancer. Concerns exist regarding occupational exposure to HPV by health care providers (HCP) who perform smoke-generating procedures on HPV-infected tissues, and the potential for them to develop head and neck pathology.
In March of 2020, the American Society for Colposcopy and Cervical Pathology made the recommendation that clinicians who are routinely exposed to HPV should protect themselves against the sequela of occupationally acquired HPV by receiving the HPV vaccine.2 They advocate for the “complete provider team” including physicians, advanced practice providers, nurses, operative technicians, and residents and fellows to be considered for protective vaccination.
Similar to disease patterns in the genital tract, different strains of HPV have differing propensity to cause benign, premalignant, and malignant disease states. HPV 6 and 11 are more commonly associated with warty disease in the nares, pharynx, and tonsillar tissues. HPV 16, 18, 31, and 33 (most commonly 16) are considered high risk for carcinoma formation, particularly of the tonsils and base of the tongue.
The procedures most implicated in occupational HPV exposure include ablative procedures for anogenital warts, laser ablation of vaginal and vulvar dysplasia, and electrosurgical excisional procedures for cervical dysplasia. Smoke plumes from HPV-associated procedures are known to contain HPV for both laser and electrocautery sources.3 A study of 134 patients undergoing surgical procedures for laser ablation of HPV-infected tissues detected concordant strains of HPV in approximately 30% of smoke plumes and approximately 1.5% of surgeons’ nares.4 Not all procedures appear to carry the same risk. Electrocoagulation procedures appear to yield fewer postprocedural positive mucosal swabs for HPV, compared with those taken after CO2 laser.5
Animal studies have shown that papilloma virus procured from smoke plume has the capacity to generate disease. When 10 calves were inoculated with bovine papillary virus obtained from smoke plumes from laser ablation of bovine papillomavirus lesions, all calves manifested BPV fibropapilloma lesions at the sites of inoculation.6
There appears to be an increased incidence of HPV-associated head and neck disease among surgeons who perform procedures on HPV tissues, and there have been multiple case reports that have cited examples of HPV-associated benign and malignant disease among HCPs with frequent occupational exposure to HPV anogenital ablative and excisional procedures.7 While these observations are not proof of causation, they are cause for concern.
While the ASCCP guidelines advocate for HPV vaccination as a strategy for prevention of occupationally related HPV-associated disease, there are other strategies in place to minimize risk. The CDC guidelines for environmental infection control in health care facilities include the following recommendations:
- In settings where surgical lasers are used, wear appropriate personnel protective equipment (PPE), including N95 or N100 respirators to minimize exposure to laser plumes.
- Use central wall suction units with in-line filters to evacuate minimal laser plumes.
- Use a mechanical smoke evaluation system with a high efficiency filter to manage the generation of large amounts of laser plume, when ablating tissue infected with HPV.
- Use local exhaust ventilation (LEV).8
When closely adhered to, these methods appear to provide high-level protection. Data suggest that, when HCPs can access appropriate protective equipment, risks for HPV exposure are low. However, this is more feasible for larger hospital facilities, and may be more limited in outpatient settings. This has led to the consideration of background protection in the form of HPV vaccination for at-risk HCPs. This is analogous to mandates for HCPs to receive hepatitis B vaccination despite the concomitant practice of universal precautions in health care settings. Preventative strategies are typically most efficacious when performed in concert.
After nearly 2 decades of widespread use, we have confidence in the safety of the HPV vaccination. Its benefit through age 45 has been established, leading to the 2018 FDA approval for the 9-valent HPV vaccine, Guardisil-9, for this expanded age group. It would seem logical that systematic administration of the HPV vaccine for at-risk HCPs would be both feasible and safe. There are well-established systems for administering vaccines for HCPs in all health care systems. Perhaps health system administrators should consider routinely offering HPV vaccination for at-risk employees as part of their occupational health care responsibilities. One important caveat being the cost and efficacy of HPV vaccination in this group has not been not established.
In the meantime, it is critical that gynecology providers be aware of their risk for occupational exposure to HPV when using laser and electrocautery techniques on HPV-infected tissues and the potential for them developing head and neck pathology. They should strictly adhere to preventative measures such as use of fit-tested N-95 respirators, mechanical smoke evacuators with high-efficiency filters and work in environments with adequate room ventilation. We all should individually evaluate what role HPV vaccination may play for us in augmenting our own safety.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Van Dyne EA et al. MMWR Morb Mortal Wkly Rep. 2018 Aug 24;67(33):918-24.
2. ASCCP. ASCCP recommends HPV vaccination for providers.
3. Fox-Lewis A et al. Occup Environ Med. 2020 Dec;77(12):809-17.
4. Zhou Q et al. Cancer Manag Res. 2019;11:3643-54
5. Bergbrant I et al. Acta Derm Venereol. 1994 Sep;74(5):393-5.
6. Garden J et al. Arch Dermatol. 2002 Oct;138(10):1303-7.
7. Harrison R, Huh W. Obstet Gynecol. 2020;136:663-5.
8. CDC. 1996. DHHS (NIOSH) Publication Number 96-128.
Premenopausal bilateral oophorectomy linked to later cognitive impairment
Women whose ovaries were surgically removed before the age of 46 had a higher risk of mild cognitive impairment (MCI) around 30 years later, compared with those who did not undergo bilateral oophorectomy, according to a population-based linkage study published in JAMA Network Open.
The findings suggest that “physicians treating women with premenopausal bilateral oophorectomy need to be aware of their patients’ risk of cognitive impairment or MCI and should consider implementing treatment-monitoring plans,” noted lead author Walter A. Rocca, MD, MPH, from the division of epidemiology, department of quantitative health sciences, at the Mayo Clinic, Rochester, Minn. and colleagues.
The results may particularly “help women at mean risk levels of ovarian cancer to better evaluate the risk-to-benefit ratio of undergoing bilateral oophorectomy prior to spontaneous menopause for the prevention of ovarian cancer,” they emphasized.
While the link between premenopausal bilateral oophorectomy and higher risk of cognitive impairment has been previously suggested, this new study “contributes valuable new data to a major public health importance issue and addresses a number of important shortcomings of existing literature,” Marios K. Georgakis, MD, PhD, and Eleni T. Petridou, MD, PhD, noted in an accompanying commentary.
“As bilateral oophorectomy is still a common procedure at least in well-resourced countries, the results of these studies should alert clinicians about its potential public health consequences. Given that the abrupt cessation of ovarian hormones might be accompanied by previously underestimated long-term adverse effects, treating physicians proposing the operation should weigh its benefits against potential long-term harmful effects, especially among women without an absolute indication,” noted Dr. Georgakis and Dr. Petridou, respectively from the Center for Genomic Medicine at Massachusetts General Hospital in Boston and the National and Kapodistrian University of Athens.
The case-control cross-sectional study used data from the Mayo Clinic Study of Aging (MCSA), a prospective, population-based study examining risk factors for, as well as prevalence and incidence of cognitive decline and MCI among a representative sample of women in Olmsted County, Minn. It included 2,732 women aged 50-89 years who participated in the MCSA study from 2004 to 2019 and underwent a clinical evaluation and comprehensive cognitive testing including nine tests covering four cognitive domains. Almost all of the subjects (98.4%) were White. The mean age of cognitive evaluation was 74 years – at which time 283 women (10.4%) were diagnosed with MCI (197 with amnestic and 86 with nonamnestic MCI). Data from the Rochester Epidemiology Project medical record–linkage system showed a total of 625 women (22.9%) had a history of bilateral oophorectomy. Among this group, 161 women underwent the procedure both before age 46, and before menopause, with 46 (28.6%) receiving oral conjugated equine estrogen (unopposed) and the remaining 95 (59.0%) receiving no estrogen therapy.
The study found that, compared with women who did not undergo bilateral oophorectomy, those who did so before age 46, but not after this age, had statistically significantly increased odds of MCI (adjusted odds ratio, 2.21; P < .001). When type of MCI was examined, the risk was statistically significant for nonamnestic MCI (aOR, 2.96; P < .001), and amnestic (aOR, 1.87; P =.03). The study also found no evidence that estrogen therapy was associated with decreased risk of MCI among women aged less than 46 years, with an aOR of 2.56 in those who received estrogen therapy and 2.05 in those who did not (P = .01 for both).
Finally, in women who had bilateral oophorectomy before menopause and before age 50, surgical indication for the procedure affected the association with MCI. Indications of either cancer or “no ovarian condition” (i.e., performed at the time of hysterectomy) were associated with no increased risk, whereas there was a statistically significantly increased risk associated with benign indications such as an adnexal mass, cyst or endometriosis (aOR, 2.43; P = .003). “This is important,” noted the commentators, “because in many of those cases removal of both ovaries could be avoided.”
The study also found that, compared with women who had not undergone bilateral oophorectomy, those who had also had increased frequency of cardiovascular risk factors, heart disease, and stroke at the time of their cognitive evaluation. “Additional research is needed to clarify the biological explanation of the association,” the investigators said.
The prevailing hypothesis for why premenopausal bilateral oophorectomy is associated with cognitive decline “is that the abrupt endocrine cessation of exposure to ovarian hormones accelerates the aging process,” the commentators noted. “Most important from a clinical perspective is whether these women would benefit from specific hormone replacement therapy schemes. Observational studies cannot reliably answer this question, and possibly it is time to rethink designing trials in specific groups of women who underwent bilateral oophorectomy before 46 years of age starting treatment immediately thereafter.”
In an interview Dr. Georgakis elaborated on this point, saying that, while the Women’s Health Study clearly showed no benefit of hormone replacement therapy for preventing dementia, it recruited women who were aged 65 years or older and had therefore undergone menopause more than 10-15 years earlier. “A hypothesis suggests that a critical vulnerability window exists shortly after menopause during which hormone replacement therapy might be needed to ameliorate any elevated risk,” he said. “Thus, it might make sense to reconsider a trial focused on this group of premenopausal women, who need to undergo oophorectomy at a young age (<46 years). Early initiation would be important. Unfortunately, such a trial would be difficult to conduct, because these women would need to be followed up for very long periods, as cognitive decline usually does not occur before the age of 65.”
Asked to comment on the study, Meadow Good, DO, an ob.gyn., female pelvic medicine and reconstructive surgeon, and physician adviser for Winnie Palmer Hospital for Women & Babies in Orlando, said this study adds credibility to previous studies showing the cognitive risk associated with premenopausal bilateral oophorectomy. “The literature is now pointing to a need to refrain from elective bilateral oophorectomy in women less than 60,” she said in an interview. “It should not be common that a women receives a bilateral oophorectomy before 60 for benign reasons.”
She added that cognition is not the only think at stake. “Bilateral oophorectomy before the age of 60 has a higher risk of incident heart disease, stroke, lung cancer and total cancers,” she said, citing a prospective cohort study within the Nurses’ Health Study.
Dr. Rocca reported financial support from the Mayo Clinic Research Committee during the conduct of the study. One coauthor reported unrestricted grants from Biogen and consulting fees from Brain Protection outside the submitted work. No other disclosures were reported from the authors. Dr. Georgakis, Dr. Petridou, and Dr. Good reported no conflicts of interest. The study was funded by the National Institute on Aging. It also used resources of the Rochester Epidemiology Project medical record–linkage system, which is supported by the NIA, the Mayo Clinic Research Committee, and user fees. Dr. Rocca was partly funded by the Ralph S. and Beverley E. Caulkins Professorship of Neurodegenerative Diseases Research of the Mayo Clinic.
Women whose ovaries were surgically removed before the age of 46 had a higher risk of mild cognitive impairment (MCI) around 30 years later, compared with those who did not undergo bilateral oophorectomy, according to a population-based linkage study published in JAMA Network Open.
The findings suggest that “physicians treating women with premenopausal bilateral oophorectomy need to be aware of their patients’ risk of cognitive impairment or MCI and should consider implementing treatment-monitoring plans,” noted lead author Walter A. Rocca, MD, MPH, from the division of epidemiology, department of quantitative health sciences, at the Mayo Clinic, Rochester, Minn. and colleagues.
The results may particularly “help women at mean risk levels of ovarian cancer to better evaluate the risk-to-benefit ratio of undergoing bilateral oophorectomy prior to spontaneous menopause for the prevention of ovarian cancer,” they emphasized.
While the link between premenopausal bilateral oophorectomy and higher risk of cognitive impairment has been previously suggested, this new study “contributes valuable new data to a major public health importance issue and addresses a number of important shortcomings of existing literature,” Marios K. Georgakis, MD, PhD, and Eleni T. Petridou, MD, PhD, noted in an accompanying commentary.
“As bilateral oophorectomy is still a common procedure at least in well-resourced countries, the results of these studies should alert clinicians about its potential public health consequences. Given that the abrupt cessation of ovarian hormones might be accompanied by previously underestimated long-term adverse effects, treating physicians proposing the operation should weigh its benefits against potential long-term harmful effects, especially among women without an absolute indication,” noted Dr. Georgakis and Dr. Petridou, respectively from the Center for Genomic Medicine at Massachusetts General Hospital in Boston and the National and Kapodistrian University of Athens.
The case-control cross-sectional study used data from the Mayo Clinic Study of Aging (MCSA), a prospective, population-based study examining risk factors for, as well as prevalence and incidence of cognitive decline and MCI among a representative sample of women in Olmsted County, Minn. It included 2,732 women aged 50-89 years who participated in the MCSA study from 2004 to 2019 and underwent a clinical evaluation and comprehensive cognitive testing including nine tests covering four cognitive domains. Almost all of the subjects (98.4%) were White. The mean age of cognitive evaluation was 74 years – at which time 283 women (10.4%) were diagnosed with MCI (197 with amnestic and 86 with nonamnestic MCI). Data from the Rochester Epidemiology Project medical record–linkage system showed a total of 625 women (22.9%) had a history of bilateral oophorectomy. Among this group, 161 women underwent the procedure both before age 46, and before menopause, with 46 (28.6%) receiving oral conjugated equine estrogen (unopposed) and the remaining 95 (59.0%) receiving no estrogen therapy.
The study found that, compared with women who did not undergo bilateral oophorectomy, those who did so before age 46, but not after this age, had statistically significantly increased odds of MCI (adjusted odds ratio, 2.21; P < .001). When type of MCI was examined, the risk was statistically significant for nonamnestic MCI (aOR, 2.96; P < .001), and amnestic (aOR, 1.87; P =.03). The study also found no evidence that estrogen therapy was associated with decreased risk of MCI among women aged less than 46 years, with an aOR of 2.56 in those who received estrogen therapy and 2.05 in those who did not (P = .01 for both).
Finally, in women who had bilateral oophorectomy before menopause and before age 50, surgical indication for the procedure affected the association with MCI. Indications of either cancer or “no ovarian condition” (i.e., performed at the time of hysterectomy) were associated with no increased risk, whereas there was a statistically significantly increased risk associated with benign indications such as an adnexal mass, cyst or endometriosis (aOR, 2.43; P = .003). “This is important,” noted the commentators, “because in many of those cases removal of both ovaries could be avoided.”
The study also found that, compared with women who had not undergone bilateral oophorectomy, those who had also had increased frequency of cardiovascular risk factors, heart disease, and stroke at the time of their cognitive evaluation. “Additional research is needed to clarify the biological explanation of the association,” the investigators said.
The prevailing hypothesis for why premenopausal bilateral oophorectomy is associated with cognitive decline “is that the abrupt endocrine cessation of exposure to ovarian hormones accelerates the aging process,” the commentators noted. “Most important from a clinical perspective is whether these women would benefit from specific hormone replacement therapy schemes. Observational studies cannot reliably answer this question, and possibly it is time to rethink designing trials in specific groups of women who underwent bilateral oophorectomy before 46 years of age starting treatment immediately thereafter.”
In an interview Dr. Georgakis elaborated on this point, saying that, while the Women’s Health Study clearly showed no benefit of hormone replacement therapy for preventing dementia, it recruited women who were aged 65 years or older and had therefore undergone menopause more than 10-15 years earlier. “A hypothesis suggests that a critical vulnerability window exists shortly after menopause during which hormone replacement therapy might be needed to ameliorate any elevated risk,” he said. “Thus, it might make sense to reconsider a trial focused on this group of premenopausal women, who need to undergo oophorectomy at a young age (<46 years). Early initiation would be important. Unfortunately, such a trial would be difficult to conduct, because these women would need to be followed up for very long periods, as cognitive decline usually does not occur before the age of 65.”
Asked to comment on the study, Meadow Good, DO, an ob.gyn., female pelvic medicine and reconstructive surgeon, and physician adviser for Winnie Palmer Hospital for Women & Babies in Orlando, said this study adds credibility to previous studies showing the cognitive risk associated with premenopausal bilateral oophorectomy. “The literature is now pointing to a need to refrain from elective bilateral oophorectomy in women less than 60,” she said in an interview. “It should not be common that a women receives a bilateral oophorectomy before 60 for benign reasons.”
She added that cognition is not the only think at stake. “Bilateral oophorectomy before the age of 60 has a higher risk of incident heart disease, stroke, lung cancer and total cancers,” she said, citing a prospective cohort study within the Nurses’ Health Study.
Dr. Rocca reported financial support from the Mayo Clinic Research Committee during the conduct of the study. One coauthor reported unrestricted grants from Biogen and consulting fees from Brain Protection outside the submitted work. No other disclosures were reported from the authors. Dr. Georgakis, Dr. Petridou, and Dr. Good reported no conflicts of interest. The study was funded by the National Institute on Aging. It also used resources of the Rochester Epidemiology Project medical record–linkage system, which is supported by the NIA, the Mayo Clinic Research Committee, and user fees. Dr. Rocca was partly funded by the Ralph S. and Beverley E. Caulkins Professorship of Neurodegenerative Diseases Research of the Mayo Clinic.
Women whose ovaries were surgically removed before the age of 46 had a higher risk of mild cognitive impairment (MCI) around 30 years later, compared with those who did not undergo bilateral oophorectomy, according to a population-based linkage study published in JAMA Network Open.
The findings suggest that “physicians treating women with premenopausal bilateral oophorectomy need to be aware of their patients’ risk of cognitive impairment or MCI and should consider implementing treatment-monitoring plans,” noted lead author Walter A. Rocca, MD, MPH, from the division of epidemiology, department of quantitative health sciences, at the Mayo Clinic, Rochester, Minn. and colleagues.
The results may particularly “help women at mean risk levels of ovarian cancer to better evaluate the risk-to-benefit ratio of undergoing bilateral oophorectomy prior to spontaneous menopause for the prevention of ovarian cancer,” they emphasized.
While the link between premenopausal bilateral oophorectomy and higher risk of cognitive impairment has been previously suggested, this new study “contributes valuable new data to a major public health importance issue and addresses a number of important shortcomings of existing literature,” Marios K. Georgakis, MD, PhD, and Eleni T. Petridou, MD, PhD, noted in an accompanying commentary.
“As bilateral oophorectomy is still a common procedure at least in well-resourced countries, the results of these studies should alert clinicians about its potential public health consequences. Given that the abrupt cessation of ovarian hormones might be accompanied by previously underestimated long-term adverse effects, treating physicians proposing the operation should weigh its benefits against potential long-term harmful effects, especially among women without an absolute indication,” noted Dr. Georgakis and Dr. Petridou, respectively from the Center for Genomic Medicine at Massachusetts General Hospital in Boston and the National and Kapodistrian University of Athens.
The case-control cross-sectional study used data from the Mayo Clinic Study of Aging (MCSA), a prospective, population-based study examining risk factors for, as well as prevalence and incidence of cognitive decline and MCI among a representative sample of women in Olmsted County, Minn. It included 2,732 women aged 50-89 years who participated in the MCSA study from 2004 to 2019 and underwent a clinical evaluation and comprehensive cognitive testing including nine tests covering four cognitive domains. Almost all of the subjects (98.4%) were White. The mean age of cognitive evaluation was 74 years – at which time 283 women (10.4%) were diagnosed with MCI (197 with amnestic and 86 with nonamnestic MCI). Data from the Rochester Epidemiology Project medical record–linkage system showed a total of 625 women (22.9%) had a history of bilateral oophorectomy. Among this group, 161 women underwent the procedure both before age 46, and before menopause, with 46 (28.6%) receiving oral conjugated equine estrogen (unopposed) and the remaining 95 (59.0%) receiving no estrogen therapy.
The study found that, compared with women who did not undergo bilateral oophorectomy, those who did so before age 46, but not after this age, had statistically significantly increased odds of MCI (adjusted odds ratio, 2.21; P < .001). When type of MCI was examined, the risk was statistically significant for nonamnestic MCI (aOR, 2.96; P < .001), and amnestic (aOR, 1.87; P =.03). The study also found no evidence that estrogen therapy was associated with decreased risk of MCI among women aged less than 46 years, with an aOR of 2.56 in those who received estrogen therapy and 2.05 in those who did not (P = .01 for both).
Finally, in women who had bilateral oophorectomy before menopause and before age 50, surgical indication for the procedure affected the association with MCI. Indications of either cancer or “no ovarian condition” (i.e., performed at the time of hysterectomy) were associated with no increased risk, whereas there was a statistically significantly increased risk associated with benign indications such as an adnexal mass, cyst or endometriosis (aOR, 2.43; P = .003). “This is important,” noted the commentators, “because in many of those cases removal of both ovaries could be avoided.”
The study also found that, compared with women who had not undergone bilateral oophorectomy, those who had also had increased frequency of cardiovascular risk factors, heart disease, and stroke at the time of their cognitive evaluation. “Additional research is needed to clarify the biological explanation of the association,” the investigators said.
The prevailing hypothesis for why premenopausal bilateral oophorectomy is associated with cognitive decline “is that the abrupt endocrine cessation of exposure to ovarian hormones accelerates the aging process,” the commentators noted. “Most important from a clinical perspective is whether these women would benefit from specific hormone replacement therapy schemes. Observational studies cannot reliably answer this question, and possibly it is time to rethink designing trials in specific groups of women who underwent bilateral oophorectomy before 46 years of age starting treatment immediately thereafter.”
In an interview Dr. Georgakis elaborated on this point, saying that, while the Women’s Health Study clearly showed no benefit of hormone replacement therapy for preventing dementia, it recruited women who were aged 65 years or older and had therefore undergone menopause more than 10-15 years earlier. “A hypothesis suggests that a critical vulnerability window exists shortly after menopause during which hormone replacement therapy might be needed to ameliorate any elevated risk,” he said. “Thus, it might make sense to reconsider a trial focused on this group of premenopausal women, who need to undergo oophorectomy at a young age (<46 years). Early initiation would be important. Unfortunately, such a trial would be difficult to conduct, because these women would need to be followed up for very long periods, as cognitive decline usually does not occur before the age of 65.”
Asked to comment on the study, Meadow Good, DO, an ob.gyn., female pelvic medicine and reconstructive surgeon, and physician adviser for Winnie Palmer Hospital for Women & Babies in Orlando, said this study adds credibility to previous studies showing the cognitive risk associated with premenopausal bilateral oophorectomy. “The literature is now pointing to a need to refrain from elective bilateral oophorectomy in women less than 60,” she said in an interview. “It should not be common that a women receives a bilateral oophorectomy before 60 for benign reasons.”
She added that cognition is not the only think at stake. “Bilateral oophorectomy before the age of 60 has a higher risk of incident heart disease, stroke, lung cancer and total cancers,” she said, citing a prospective cohort study within the Nurses’ Health Study.
Dr. Rocca reported financial support from the Mayo Clinic Research Committee during the conduct of the study. One coauthor reported unrestricted grants from Biogen and consulting fees from Brain Protection outside the submitted work. No other disclosures were reported from the authors. Dr. Georgakis, Dr. Petridou, and Dr. Good reported no conflicts of interest. The study was funded by the National Institute on Aging. It also used resources of the Rochester Epidemiology Project medical record–linkage system, which is supported by the NIA, the Mayo Clinic Research Committee, and user fees. Dr. Rocca was partly funded by the Ralph S. and Beverley E. Caulkins Professorship of Neurodegenerative Diseases Research of the Mayo Clinic.
FROM JAMA NETWORK OPEN
California plans for a post-Roe world as abortion access shrinks elsewhere
SACRAMENTO – With access to abortion at stake across America, California is preparing to become the nation’s abortion provider.
Democratic Gov. Gavin Newsom and legislative leaders have asked a group of reproductive health experts to propose policies to bolster the state’s abortion infrastructure and ready it for more patients. Lawmakers plan to begin debating the ideas when they reconvene in January.
Abortion clinics are already girding themselves for a surge in demand.
Janet Jacobson, MD, medical director of Planned Parenthood of Orange and San Bernardino Counties, said three or four out-of-state patients visit her clinics each day – about double the number that sought treatment before a near-total ban on abortion took effect in Texas in September.
While the nine clinics can absorb that slow trickle, they expect up to 50 out-of-state patients a week if the U.S. Supreme Court’s conservative majority guts abortion rights nationally, Dr. Jacobson said. She bases her estimate on new data from the Guttmacher Institute, a research organization that supports abortion and reproductive health rights.
She is adding staff members and appointment capacity, hoping to accommodate everyone.
“We have to make sure we can still continue to care for all of our California patients,” Dr. Jacobson said. “We don’t want them getting squeezed out” of appointments.
The Texas law banned nearly all abortions after about 6 weeks of pregnancy and empowered private citizens to sue anyone who performs or “aids and abets” an abortion after that time. The Supreme Court heard arguments in that case on Nov. 1 and is expected to announce a ruling on its constitutionality in June. Nonetheless, Florida and Ohio have announced plans for copycat laws.
Next month the high court will hear another abortion case with even broader implications, Dobbs v. Jackson Women’s Health Organization, a lawsuit challenging the constitutionality of a 2018 Mississippi law that prohibited abortion after 15 weeks. If the court sides with Mississippi, its decision could overturn existing abortion rights set by the landmark Roe v. Wade case.
Should that happen, reproductive rights experts predict, 26 states will ban the procedure altogether, and states with stronger protections for abortion, like California, will draw even more patients. There could be up to a 3,000% increase in people who “may drive to California for abortion care” each year, according to the Guttmacher data.
In 2017, the most recent year for which data are available from Guttmacher, California – by far the nation’s most populous state – had more abortion providers than any other state, with 419 hospitals, clinics, or doctors’ offices performing the procedure. The next highest were New York, with 252, and Florida, with 85. Neighboring Arizona and Nevada each had 11. Of the 862,320 abortions performed in the United States that year, 132.680, about 15% were in California.
Planned Parenthood clinics in California say they already serve about 7,000 out-of-state patients a year and are expecting a surge of new ones, especially in travel hubs like the Los Angeles area.
In September, Planned Parenthood and groups such as Black Women for Wellness convened the California Future of Abortion Council with backing from influential Democratic leaders including Gov. Newsom, state Senate leader Toni Atkins, and Assembly Speaker Anthony Rendon.
Ms. Atkins, who was the director of a San Diego women’s health clinic in the 1980s, said she spent time with women from states where it was hard to get an abortion. She said California is committed to ensuring abortion access in the state and beyond.
The council is focused on increasing funding for abortion services, providing logistical and financial help for women who need to travel, increasing the number of health care providers who perform abortions, and strengthening legal protections for them.
Increasing capacity could mean licensing more practitioners to provide abortions or pumping more resources into telehealth so people can see a doctor online to prescribe pills for a medical abortion – a service California doctors currently can provide to patients only in California.
The most important thing the state should do is fix its shortage of providers, especially those who perform second-trimester abortions, which are more expensive and complicated than first-trimester abortions, said council member Daniel Grossman, MD, director of the Advancing New Standards in Reproductive Health program at the University of California, San Francisco.
It’s not feasible to place an abortion provider in every corner of the state, Dr. Grossman said. Instead, the council should focus on creating “hubs that can provide abortion care for large numbers of people” in easy-to-get-to locations.
California already struggles to provide abortions to all who seek them, especially low-income women covered by Medi-Cal, California’s Medicaid program. For example, 28 counties – home to 10% of Medi-Cal recipients of childbearing age – don’t have facilities that provide abortions to Medi-Cal patients.
A medical abortion, in which pills are used to terminate a pregnancy, costs California patients an average of $306 out-of-pocket, according to an analysis by the California Health Benefits Review Program, but isn’t available after 10 weeks. After that, the only option is a surgical abortion, which costs an average of $887 out-of-pocket in California.
One of the council’s recommendations will likely be to increase the rate Medi-Cal payments for abortions so more providers will perform them, said council member Fabiola Carrión, interim director for reproductive and sexual health at the National Health Law Program.
Medi-Cal pays $354.43 for a second-trimester abortion. A 2020 study in the journal Contraception found that states paid between $79 and $626 for a second-trimester abortion in 2017.
Increasing Medi-Cal rates won’t help patients traveling from outside California. Generally, private insurance doesn’t cover out-of-state abortions, so most women will be on the hook for the full cost, and those enrolled in other states’ Medicaid programs must pay out-of-pocket, too.
The council hopes to reduce costs for state residents and visitors, said Brandon Richards, director of communications for Planned Parenthood Affiliates of California. “It’s about making it easy for people to access abortion in California, whether they reside here or are coming in from out of state,” he said.
One way to target costs is by funding the practical support, like helping to pay for transportation, child care, hotels, or time off work, said council member Jessica Pinckney, executive director of Access Reproductive Justice, a fund that helps people pay for abortions.
Ms. Pinckney said she’s working with Los Angeles County to set up a public abortion fund to cover some of those costs for anyone seeking an abortion in the county. It would be modeled after similar pots maintained by the cities of New York; Austin, Tex.; and Portland, Ore., and could eventually be a template for the first statewide fund, Ms. Pinckney said.
Most Texans seeking abortions since that state’s law took effect are going to nearby states like Colorado, New Mexico, and Oklahoma, said Sierra Harris, deputy director of network strategies for the National Network of Abortion Funds. Women in those states, in turn, are having trouble getting care and are looking to California for appointments.
Practical support is important for out-of-state patients, said Alissa Perrucci, PhD, MPH, operations manager at the Women’s Options Center at Zuckerberg San Francisco General Hospital, one of five abortion clinics inside California hospitals.
Dr. Perrucci’s clinic is focusing on telemedicine, phone counseling, and other ways to save time so it can add appointments for out-of-state patients if necessary.
But more slots are useless if women can’t make it to California. The clinic has booked about 10 appointments for Texans since the state’s ban went into effect, but only half have shown up, mostly women with family connections in California.
“Most people just don’t have the money to get here,” she said. “If the burden of abortion was borne predominantly by the wealthy, yeah, they’d just fly here.”
This story was produced by KHN, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
SACRAMENTO – With access to abortion at stake across America, California is preparing to become the nation’s abortion provider.
Democratic Gov. Gavin Newsom and legislative leaders have asked a group of reproductive health experts to propose policies to bolster the state’s abortion infrastructure and ready it for more patients. Lawmakers plan to begin debating the ideas when they reconvene in January.
Abortion clinics are already girding themselves for a surge in demand.
Janet Jacobson, MD, medical director of Planned Parenthood of Orange and San Bernardino Counties, said three or four out-of-state patients visit her clinics each day – about double the number that sought treatment before a near-total ban on abortion took effect in Texas in September.
While the nine clinics can absorb that slow trickle, they expect up to 50 out-of-state patients a week if the U.S. Supreme Court’s conservative majority guts abortion rights nationally, Dr. Jacobson said. She bases her estimate on new data from the Guttmacher Institute, a research organization that supports abortion and reproductive health rights.
She is adding staff members and appointment capacity, hoping to accommodate everyone.
“We have to make sure we can still continue to care for all of our California patients,” Dr. Jacobson said. “We don’t want them getting squeezed out” of appointments.
The Texas law banned nearly all abortions after about 6 weeks of pregnancy and empowered private citizens to sue anyone who performs or “aids and abets” an abortion after that time. The Supreme Court heard arguments in that case on Nov. 1 and is expected to announce a ruling on its constitutionality in June. Nonetheless, Florida and Ohio have announced plans for copycat laws.
Next month the high court will hear another abortion case with even broader implications, Dobbs v. Jackson Women’s Health Organization, a lawsuit challenging the constitutionality of a 2018 Mississippi law that prohibited abortion after 15 weeks. If the court sides with Mississippi, its decision could overturn existing abortion rights set by the landmark Roe v. Wade case.
Should that happen, reproductive rights experts predict, 26 states will ban the procedure altogether, and states with stronger protections for abortion, like California, will draw even more patients. There could be up to a 3,000% increase in people who “may drive to California for abortion care” each year, according to the Guttmacher data.
In 2017, the most recent year for which data are available from Guttmacher, California – by far the nation’s most populous state – had more abortion providers than any other state, with 419 hospitals, clinics, or doctors’ offices performing the procedure. The next highest were New York, with 252, and Florida, with 85. Neighboring Arizona and Nevada each had 11. Of the 862,320 abortions performed in the United States that year, 132.680, about 15% were in California.
Planned Parenthood clinics in California say they already serve about 7,000 out-of-state patients a year and are expecting a surge of new ones, especially in travel hubs like the Los Angeles area.
In September, Planned Parenthood and groups such as Black Women for Wellness convened the California Future of Abortion Council with backing from influential Democratic leaders including Gov. Newsom, state Senate leader Toni Atkins, and Assembly Speaker Anthony Rendon.
Ms. Atkins, who was the director of a San Diego women’s health clinic in the 1980s, said she spent time with women from states where it was hard to get an abortion. She said California is committed to ensuring abortion access in the state and beyond.
The council is focused on increasing funding for abortion services, providing logistical and financial help for women who need to travel, increasing the number of health care providers who perform abortions, and strengthening legal protections for them.
Increasing capacity could mean licensing more practitioners to provide abortions or pumping more resources into telehealth so people can see a doctor online to prescribe pills for a medical abortion – a service California doctors currently can provide to patients only in California.
The most important thing the state should do is fix its shortage of providers, especially those who perform second-trimester abortions, which are more expensive and complicated than first-trimester abortions, said council member Daniel Grossman, MD, director of the Advancing New Standards in Reproductive Health program at the University of California, San Francisco.
It’s not feasible to place an abortion provider in every corner of the state, Dr. Grossman said. Instead, the council should focus on creating “hubs that can provide abortion care for large numbers of people” in easy-to-get-to locations.
California already struggles to provide abortions to all who seek them, especially low-income women covered by Medi-Cal, California’s Medicaid program. For example, 28 counties – home to 10% of Medi-Cal recipients of childbearing age – don’t have facilities that provide abortions to Medi-Cal patients.
A medical abortion, in which pills are used to terminate a pregnancy, costs California patients an average of $306 out-of-pocket, according to an analysis by the California Health Benefits Review Program, but isn’t available after 10 weeks. After that, the only option is a surgical abortion, which costs an average of $887 out-of-pocket in California.
One of the council’s recommendations will likely be to increase the rate Medi-Cal payments for abortions so more providers will perform them, said council member Fabiola Carrión, interim director for reproductive and sexual health at the National Health Law Program.
Medi-Cal pays $354.43 for a second-trimester abortion. A 2020 study in the journal Contraception found that states paid between $79 and $626 for a second-trimester abortion in 2017.
Increasing Medi-Cal rates won’t help patients traveling from outside California. Generally, private insurance doesn’t cover out-of-state abortions, so most women will be on the hook for the full cost, and those enrolled in other states’ Medicaid programs must pay out-of-pocket, too.
The council hopes to reduce costs for state residents and visitors, said Brandon Richards, director of communications for Planned Parenthood Affiliates of California. “It’s about making it easy for people to access abortion in California, whether they reside here or are coming in from out of state,” he said.
One way to target costs is by funding the practical support, like helping to pay for transportation, child care, hotels, or time off work, said council member Jessica Pinckney, executive director of Access Reproductive Justice, a fund that helps people pay for abortions.
Ms. Pinckney said she’s working with Los Angeles County to set up a public abortion fund to cover some of those costs for anyone seeking an abortion in the county. It would be modeled after similar pots maintained by the cities of New York; Austin, Tex.; and Portland, Ore., and could eventually be a template for the first statewide fund, Ms. Pinckney said.
Most Texans seeking abortions since that state’s law took effect are going to nearby states like Colorado, New Mexico, and Oklahoma, said Sierra Harris, deputy director of network strategies for the National Network of Abortion Funds. Women in those states, in turn, are having trouble getting care and are looking to California for appointments.
Practical support is important for out-of-state patients, said Alissa Perrucci, PhD, MPH, operations manager at the Women’s Options Center at Zuckerberg San Francisco General Hospital, one of five abortion clinics inside California hospitals.
Dr. Perrucci’s clinic is focusing on telemedicine, phone counseling, and other ways to save time so it can add appointments for out-of-state patients if necessary.
But more slots are useless if women can’t make it to California. The clinic has booked about 10 appointments for Texans since the state’s ban went into effect, but only half have shown up, mostly women with family connections in California.
“Most people just don’t have the money to get here,” she said. “If the burden of abortion was borne predominantly by the wealthy, yeah, they’d just fly here.”
This story was produced by KHN, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
SACRAMENTO – With access to abortion at stake across America, California is preparing to become the nation’s abortion provider.
Democratic Gov. Gavin Newsom and legislative leaders have asked a group of reproductive health experts to propose policies to bolster the state’s abortion infrastructure and ready it for more patients. Lawmakers plan to begin debating the ideas when they reconvene in January.
Abortion clinics are already girding themselves for a surge in demand.
Janet Jacobson, MD, medical director of Planned Parenthood of Orange and San Bernardino Counties, said three or four out-of-state patients visit her clinics each day – about double the number that sought treatment before a near-total ban on abortion took effect in Texas in September.
While the nine clinics can absorb that slow trickle, they expect up to 50 out-of-state patients a week if the U.S. Supreme Court’s conservative majority guts abortion rights nationally, Dr. Jacobson said. She bases her estimate on new data from the Guttmacher Institute, a research organization that supports abortion and reproductive health rights.
She is adding staff members and appointment capacity, hoping to accommodate everyone.
“We have to make sure we can still continue to care for all of our California patients,” Dr. Jacobson said. “We don’t want them getting squeezed out” of appointments.
The Texas law banned nearly all abortions after about 6 weeks of pregnancy and empowered private citizens to sue anyone who performs or “aids and abets” an abortion after that time. The Supreme Court heard arguments in that case on Nov. 1 and is expected to announce a ruling on its constitutionality in June. Nonetheless, Florida and Ohio have announced plans for copycat laws.
Next month the high court will hear another abortion case with even broader implications, Dobbs v. Jackson Women’s Health Organization, a lawsuit challenging the constitutionality of a 2018 Mississippi law that prohibited abortion after 15 weeks. If the court sides with Mississippi, its decision could overturn existing abortion rights set by the landmark Roe v. Wade case.
Should that happen, reproductive rights experts predict, 26 states will ban the procedure altogether, and states with stronger protections for abortion, like California, will draw even more patients. There could be up to a 3,000% increase in people who “may drive to California for abortion care” each year, according to the Guttmacher data.
In 2017, the most recent year for which data are available from Guttmacher, California – by far the nation’s most populous state – had more abortion providers than any other state, with 419 hospitals, clinics, or doctors’ offices performing the procedure. The next highest were New York, with 252, and Florida, with 85. Neighboring Arizona and Nevada each had 11. Of the 862,320 abortions performed in the United States that year, 132.680, about 15% were in California.
Planned Parenthood clinics in California say they already serve about 7,000 out-of-state patients a year and are expecting a surge of new ones, especially in travel hubs like the Los Angeles area.
In September, Planned Parenthood and groups such as Black Women for Wellness convened the California Future of Abortion Council with backing from influential Democratic leaders including Gov. Newsom, state Senate leader Toni Atkins, and Assembly Speaker Anthony Rendon.
Ms. Atkins, who was the director of a San Diego women’s health clinic in the 1980s, said she spent time with women from states where it was hard to get an abortion. She said California is committed to ensuring abortion access in the state and beyond.
The council is focused on increasing funding for abortion services, providing logistical and financial help for women who need to travel, increasing the number of health care providers who perform abortions, and strengthening legal protections for them.
Increasing capacity could mean licensing more practitioners to provide abortions or pumping more resources into telehealth so people can see a doctor online to prescribe pills for a medical abortion – a service California doctors currently can provide to patients only in California.
The most important thing the state should do is fix its shortage of providers, especially those who perform second-trimester abortions, which are more expensive and complicated than first-trimester abortions, said council member Daniel Grossman, MD, director of the Advancing New Standards in Reproductive Health program at the University of California, San Francisco.
It’s not feasible to place an abortion provider in every corner of the state, Dr. Grossman said. Instead, the council should focus on creating “hubs that can provide abortion care for large numbers of people” in easy-to-get-to locations.
California already struggles to provide abortions to all who seek them, especially low-income women covered by Medi-Cal, California’s Medicaid program. For example, 28 counties – home to 10% of Medi-Cal recipients of childbearing age – don’t have facilities that provide abortions to Medi-Cal patients.
A medical abortion, in which pills are used to terminate a pregnancy, costs California patients an average of $306 out-of-pocket, according to an analysis by the California Health Benefits Review Program, but isn’t available after 10 weeks. After that, the only option is a surgical abortion, which costs an average of $887 out-of-pocket in California.
One of the council’s recommendations will likely be to increase the rate Medi-Cal payments for abortions so more providers will perform them, said council member Fabiola Carrión, interim director for reproductive and sexual health at the National Health Law Program.
Medi-Cal pays $354.43 for a second-trimester abortion. A 2020 study in the journal Contraception found that states paid between $79 and $626 for a second-trimester abortion in 2017.
Increasing Medi-Cal rates won’t help patients traveling from outside California. Generally, private insurance doesn’t cover out-of-state abortions, so most women will be on the hook for the full cost, and those enrolled in other states’ Medicaid programs must pay out-of-pocket, too.
The council hopes to reduce costs for state residents and visitors, said Brandon Richards, director of communications for Planned Parenthood Affiliates of California. “It’s about making it easy for people to access abortion in California, whether they reside here or are coming in from out of state,” he said.
One way to target costs is by funding the practical support, like helping to pay for transportation, child care, hotels, or time off work, said council member Jessica Pinckney, executive director of Access Reproductive Justice, a fund that helps people pay for abortions.
Ms. Pinckney said she’s working with Los Angeles County to set up a public abortion fund to cover some of those costs for anyone seeking an abortion in the county. It would be modeled after similar pots maintained by the cities of New York; Austin, Tex.; and Portland, Ore., and could eventually be a template for the first statewide fund, Ms. Pinckney said.
Most Texans seeking abortions since that state’s law took effect are going to nearby states like Colorado, New Mexico, and Oklahoma, said Sierra Harris, deputy director of network strategies for the National Network of Abortion Funds. Women in those states, in turn, are having trouble getting care and are looking to California for appointments.
Practical support is important for out-of-state patients, said Alissa Perrucci, PhD, MPH, operations manager at the Women’s Options Center at Zuckerberg San Francisco General Hospital, one of five abortion clinics inside California hospitals.
Dr. Perrucci’s clinic is focusing on telemedicine, phone counseling, and other ways to save time so it can add appointments for out-of-state patients if necessary.
But more slots are useless if women can’t make it to California. The clinic has booked about 10 appointments for Texans since the state’s ban went into effect, but only half have shown up, mostly women with family connections in California.
“Most people just don’t have the money to get here,” she said. “If the burden of abortion was borne predominantly by the wealthy, yeah, they’d just fly here.”
This story was produced by KHN, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.