User login
Treatment for BV, trichomoniasis approved for adolescents
The antimicrobial agent, marketed as Solosec, was first approved in 2017 as a treatment for BV in adult women. In 2021, it was approved for the treatment of trichomoniasis in adult men and women.
Lupin Pharmaceuticals, which manufactures the drug, announced the expanded approval for adolescents in a news release.
The medication is meant to be taken as a single dose. It comes in a packet that should be sprinkled onto applesauce, yogurt, or pudding and consumed without chewing or crunching.
The treatment option may help “address gaps in care related to adherence,” said Tom Merriam, an executive director with Lupin.
Bacterial vaginosis is a common vaginal infection. Trichomoniasis is the most common nonviral, curable STI in the United States. Sexual partners of patients with trichomoniasis can be treated at the same time.
Vulvovaginal candidiasis is one of the possible side effects of secnidazole treatment, the drug’s label notes.
The antimicrobial agent, marketed as Solosec, was first approved in 2017 as a treatment for BV in adult women. In 2021, it was approved for the treatment of trichomoniasis in adult men and women.
Lupin Pharmaceuticals, which manufactures the drug, announced the expanded approval for adolescents in a news release.
The medication is meant to be taken as a single dose. It comes in a packet that should be sprinkled onto applesauce, yogurt, or pudding and consumed without chewing or crunching.
The treatment option may help “address gaps in care related to adherence,” said Tom Merriam, an executive director with Lupin.
Bacterial vaginosis is a common vaginal infection. Trichomoniasis is the most common nonviral, curable STI in the United States. Sexual partners of patients with trichomoniasis can be treated at the same time.
Vulvovaginal candidiasis is one of the possible side effects of secnidazole treatment, the drug’s label notes.
The antimicrobial agent, marketed as Solosec, was first approved in 2017 as a treatment for BV in adult women. In 2021, it was approved for the treatment of trichomoniasis in adult men and women.
Lupin Pharmaceuticals, which manufactures the drug, announced the expanded approval for adolescents in a news release.
The medication is meant to be taken as a single dose. It comes in a packet that should be sprinkled onto applesauce, yogurt, or pudding and consumed without chewing or crunching.
The treatment option may help “address gaps in care related to adherence,” said Tom Merriam, an executive director with Lupin.
Bacterial vaginosis is a common vaginal infection. Trichomoniasis is the most common nonviral, curable STI in the United States. Sexual partners of patients with trichomoniasis can be treated at the same time.
Vulvovaginal candidiasis is one of the possible side effects of secnidazole treatment, the drug’s label notes.
PCOS common in adolescent girls with type 2 diabetes
Polycystic ovary syndrome is common in girls with type 2 diabetes, findings of a new study suggest, and authors say screening for PCOS is critical in this group.
In a systematic review and meta-analysis involving 470 girls (average age 12.9-16.1 years) with type 2 diabetes in six studies, the prevalence of PCOS was nearly 1 in 5 (19.58%; 95% confidence interval, 12.02%-27.14%; P = .002), substantially higher than that of PCOS in the general adolescent population.
PCOS, a complex endocrine disorder, occurs in 1.14%-11.04% of adolescent girls globally, according to the paper published online in JAMA Network Open.
The secondary outcome studied links to prevalence of PCOS with race and obesity.
Insulin resistance and compensatory hyperinsulinemia are present in 44%-70% of women with PCOS, suggesting that they are more likely to develop type 2 diabetes, according to the researchers led by Milena Cioana, BHSc, with the department of pediatrics, McMaster University, Hamilton, Ont.
Kelly A. Curran, MD, an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center in Oklahoma City, where she practices adolescent medicine, said in an interview that it has been known that women with PCOS have higher rates of diabetes and many in the field have suspected the relationship is bidirectional.
“In my clinical practice, I’ve seen a high percentage of women with type 2 diabetes present with irregular menses, some of whom have gone on to be diagnosed with PCOS,” said Dr. Curran, who was not involved with the study.
However, she said, she was surprised the prevalence of PCOS reported in this paper – nearly one in five – was so high. Early diagnosis is important for PCOS to prevent complications such as hypertension, hyperglycemia, and dyslipidemia.
Psychiatric conditions are also prevalent in patients with PCOS, including anxiety (18%), depression (16%), and ADHD (9%).
Dr. Curran agreed there is a need to screen for PCOS and to evaluate for other causes of irregular periods in patients with type 2 diabetes.
“Menstrual irregularities are often overlooked in young women without further work-up, especially in patients who have chronic illnesses,” she noted.
Results come with a caveat
However, the authors said, results should be viewed with caution because “studies including the larger numbers of girls did not report the criteria used to diagnose PCOS, which is a challenge during adolescence.”
Diagnostic criteria for PCOS during adolescence include the combination of menstrual irregularities according to time since their first period and clinical or biochemical hyperandrogenism after excluding other potential causes.
Dr. Curran explained that PCOS symptoms include irregular periods and acne which can overlap with normal changes in puberty. In her experience, PCOS is often diagnosed without patients meeting full criteria. She agreed further research with standardized criteria is urgently needed.
The European Society of Human Reproduction and Embryology/American Society of Reproductive Medicine, the Pediatric Endocrine Society, and the International Consortium of Paediatric Endocrinology guidelines suggest that using ultrasound to check the size of ovaries could help diagnose PCOS, but other guidelines are more conservative, the authors noted.
They added that “there is a need for a consensus to establish the pediatric criteria for diagnosing PCOS in adolescents to ensure accurate diagnosis and lower the misclassification rates.”
Assessing links to obesity and race
Still unclear, the authors wrote, is whether and how obesity and race affect prevalence of PCOS among girls with type 2 diabetes.
The authors wrote: “Although earlier studies suggested that obesity-related insulin resistance and hyperinsulinemia can contribute to PCOS pathogenesis, insulin resistance in patients with PCOS may be present independently of [body mass index]. Obesity seems to increase the risk of PCOS only slightly and might represent a referral bias for PCOS.”
Few studies included in the meta-analysis had race-specific data, so the authors were limited in assessing associations between race and PCOS prevalence.
“However,” they wrote, “our data demonstrate that Indian girls had the highest prevalence, followed by White girls, and then Indigenous girls in Canada.”
Further studies are needed to help define at-risk subgroups and evaluate treatment strategies, the authors noted.
They reported having no relevant financial relationships. Dr. Curran had no conflicts of interest.
Polycystic ovary syndrome is common in girls with type 2 diabetes, findings of a new study suggest, and authors say screening for PCOS is critical in this group.
In a systematic review and meta-analysis involving 470 girls (average age 12.9-16.1 years) with type 2 diabetes in six studies, the prevalence of PCOS was nearly 1 in 5 (19.58%; 95% confidence interval, 12.02%-27.14%; P = .002), substantially higher than that of PCOS in the general adolescent population.
PCOS, a complex endocrine disorder, occurs in 1.14%-11.04% of adolescent girls globally, according to the paper published online in JAMA Network Open.
The secondary outcome studied links to prevalence of PCOS with race and obesity.
Insulin resistance and compensatory hyperinsulinemia are present in 44%-70% of women with PCOS, suggesting that they are more likely to develop type 2 diabetes, according to the researchers led by Milena Cioana, BHSc, with the department of pediatrics, McMaster University, Hamilton, Ont.
Kelly A. Curran, MD, an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center in Oklahoma City, where she practices adolescent medicine, said in an interview that it has been known that women with PCOS have higher rates of diabetes and many in the field have suspected the relationship is bidirectional.
“In my clinical practice, I’ve seen a high percentage of women with type 2 diabetes present with irregular menses, some of whom have gone on to be diagnosed with PCOS,” said Dr. Curran, who was not involved with the study.
However, she said, she was surprised the prevalence of PCOS reported in this paper – nearly one in five – was so high. Early diagnosis is important for PCOS to prevent complications such as hypertension, hyperglycemia, and dyslipidemia.
Psychiatric conditions are also prevalent in patients with PCOS, including anxiety (18%), depression (16%), and ADHD (9%).
Dr. Curran agreed there is a need to screen for PCOS and to evaluate for other causes of irregular periods in patients with type 2 diabetes.
“Menstrual irregularities are often overlooked in young women without further work-up, especially in patients who have chronic illnesses,” she noted.
Results come with a caveat
However, the authors said, results should be viewed with caution because “studies including the larger numbers of girls did not report the criteria used to diagnose PCOS, which is a challenge during adolescence.”
Diagnostic criteria for PCOS during adolescence include the combination of menstrual irregularities according to time since their first period and clinical or biochemical hyperandrogenism after excluding other potential causes.
Dr. Curran explained that PCOS symptoms include irregular periods and acne which can overlap with normal changes in puberty. In her experience, PCOS is often diagnosed without patients meeting full criteria. She agreed further research with standardized criteria is urgently needed.
The European Society of Human Reproduction and Embryology/American Society of Reproductive Medicine, the Pediatric Endocrine Society, and the International Consortium of Paediatric Endocrinology guidelines suggest that using ultrasound to check the size of ovaries could help diagnose PCOS, but other guidelines are more conservative, the authors noted.
They added that “there is a need for a consensus to establish the pediatric criteria for diagnosing PCOS in adolescents to ensure accurate diagnosis and lower the misclassification rates.”
Assessing links to obesity and race
Still unclear, the authors wrote, is whether and how obesity and race affect prevalence of PCOS among girls with type 2 diabetes.
The authors wrote: “Although earlier studies suggested that obesity-related insulin resistance and hyperinsulinemia can contribute to PCOS pathogenesis, insulin resistance in patients with PCOS may be present independently of [body mass index]. Obesity seems to increase the risk of PCOS only slightly and might represent a referral bias for PCOS.”
Few studies included in the meta-analysis had race-specific data, so the authors were limited in assessing associations between race and PCOS prevalence.
“However,” they wrote, “our data demonstrate that Indian girls had the highest prevalence, followed by White girls, and then Indigenous girls in Canada.”
Further studies are needed to help define at-risk subgroups and evaluate treatment strategies, the authors noted.
They reported having no relevant financial relationships. Dr. Curran had no conflicts of interest.
Polycystic ovary syndrome is common in girls with type 2 diabetes, findings of a new study suggest, and authors say screening for PCOS is critical in this group.
In a systematic review and meta-analysis involving 470 girls (average age 12.9-16.1 years) with type 2 diabetes in six studies, the prevalence of PCOS was nearly 1 in 5 (19.58%; 95% confidence interval, 12.02%-27.14%; P = .002), substantially higher than that of PCOS in the general adolescent population.
PCOS, a complex endocrine disorder, occurs in 1.14%-11.04% of adolescent girls globally, according to the paper published online in JAMA Network Open.
The secondary outcome studied links to prevalence of PCOS with race and obesity.
Insulin resistance and compensatory hyperinsulinemia are present in 44%-70% of women with PCOS, suggesting that they are more likely to develop type 2 diabetes, according to the researchers led by Milena Cioana, BHSc, with the department of pediatrics, McMaster University, Hamilton, Ont.
Kelly A. Curran, MD, an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center in Oklahoma City, where she practices adolescent medicine, said in an interview that it has been known that women with PCOS have higher rates of diabetes and many in the field have suspected the relationship is bidirectional.
“In my clinical practice, I’ve seen a high percentage of women with type 2 diabetes present with irregular menses, some of whom have gone on to be diagnosed with PCOS,” said Dr. Curran, who was not involved with the study.
However, she said, she was surprised the prevalence of PCOS reported in this paper – nearly one in five – was so high. Early diagnosis is important for PCOS to prevent complications such as hypertension, hyperglycemia, and dyslipidemia.
Psychiatric conditions are also prevalent in patients with PCOS, including anxiety (18%), depression (16%), and ADHD (9%).
Dr. Curran agreed there is a need to screen for PCOS and to evaluate for other causes of irregular periods in patients with type 2 diabetes.
“Menstrual irregularities are often overlooked in young women without further work-up, especially in patients who have chronic illnesses,” she noted.
Results come with a caveat
However, the authors said, results should be viewed with caution because “studies including the larger numbers of girls did not report the criteria used to diagnose PCOS, which is a challenge during adolescence.”
Diagnostic criteria for PCOS during adolescence include the combination of menstrual irregularities according to time since their first period and clinical or biochemical hyperandrogenism after excluding other potential causes.
Dr. Curran explained that PCOS symptoms include irregular periods and acne which can overlap with normal changes in puberty. In her experience, PCOS is often diagnosed without patients meeting full criteria. She agreed further research with standardized criteria is urgently needed.
The European Society of Human Reproduction and Embryology/American Society of Reproductive Medicine, the Pediatric Endocrine Society, and the International Consortium of Paediatric Endocrinology guidelines suggest that using ultrasound to check the size of ovaries could help diagnose PCOS, but other guidelines are more conservative, the authors noted.
They added that “there is a need for a consensus to establish the pediatric criteria for diagnosing PCOS in adolescents to ensure accurate diagnosis and lower the misclassification rates.”
Assessing links to obesity and race
Still unclear, the authors wrote, is whether and how obesity and race affect prevalence of PCOS among girls with type 2 diabetes.
The authors wrote: “Although earlier studies suggested that obesity-related insulin resistance and hyperinsulinemia can contribute to PCOS pathogenesis, insulin resistance in patients with PCOS may be present independently of [body mass index]. Obesity seems to increase the risk of PCOS only slightly and might represent a referral bias for PCOS.”
Few studies included in the meta-analysis had race-specific data, so the authors were limited in assessing associations between race and PCOS prevalence.
“However,” they wrote, “our data demonstrate that Indian girls had the highest prevalence, followed by White girls, and then Indigenous girls in Canada.”
Further studies are needed to help define at-risk subgroups and evaluate treatment strategies, the authors noted.
They reported having no relevant financial relationships. Dr. Curran had no conflicts of interest.
FROM JAMA NETWORK OPEN
Ureter identification in gynecologic surgery

Ureter identification is essential when performing pelvic surgery in order to avoid iatrogenic ureteral injuries, which can result in significant patient morbidity, especially when unrecognized. There are many modalities available to assist the surgeon with ureter identification, however, and this video highlights those options. The video objectives are:
- present the clinical significance of ureter identification in gynecologic surgical procedures
- provide anatomic review of the ureter
- outline common sites for ureteral injury during gynecologic surgery
- present ureteral physiology
- discuss ureter identification techniques
- review ureteral identification during gynecologic surgical procedures.

Ureter identification is essential when performing pelvic surgery in order to avoid iatrogenic ureteral injuries, which can result in significant patient morbidity, especially when unrecognized. There are many modalities available to assist the surgeon with ureter identification, however, and this video highlights those options. The video objectives are:
- present the clinical significance of ureter identification in gynecologic surgical procedures
- provide anatomic review of the ureter
- outline common sites for ureteral injury during gynecologic surgery
- present ureteral physiology
- discuss ureter identification techniques
- review ureteral identification during gynecologic surgical procedures.

Ureter identification is essential when performing pelvic surgery in order to avoid iatrogenic ureteral injuries, which can result in significant patient morbidity, especially when unrecognized. There are many modalities available to assist the surgeon with ureter identification, however, and this video highlights those options. The video objectives are:
- present the clinical significance of ureter identification in gynecologic surgical procedures
- provide anatomic review of the ureter
- outline common sites for ureteral injury during gynecologic surgery
- present ureteral physiology
- discuss ureter identification techniques
- review ureteral identification during gynecologic surgical procedures.
HT for women who have had BSO before the age of natural menopause: Discerning the nuances
Women who undergo bilateral salpingo-oophorectomy (BSO) for various indications prior to menopause experience a rapid decline in ovarian hormone levels and consequent vasomotor and other menopausal symptoms. In addition, the resulting estrogen deprivation is associated with such long-term adverse outcomes as osteoporosis and cardiovascular morbidity.
OBG M
Surgical vs natural menopause
Stephanie Faubion, MD, MBA, NCMP: Since the Women’s Health Initiative study was published in 2002,2 many clinicians have been fearful of using systemic HT in menopausal women, and HT use has declined dramatically such that only about 4% to 6% of menopausal women are now receiving systemic HT. Importantly, however, a group of younger menopausal women also are not receiving HT, and that is women who undergo BSO before they reach the average age of menopause, which in the United States is about age 52; this is sometimes referred to as surgical menopause or early surgical menopause. Early surgical menopause has different connotations for long-term health risks than natural menopause at the average age, and we are here to discuss these health effects and their management.
My name is Stephanie Faubion, and I am a women’s health internist and the Chair of the Department of Medicine at Mayo Clinic in Jacksonville, Florida, and Director of Mayo Clinic Women’s Health. I am here with 2 of my esteemed colleagues, Dr. Andrew Kaunitz and Dr. Ekta Kapoor.
Andrew M. Kaunitz, MD, NCMP: Hello, I am an ObGyn with the University of Florida College of Medicine in Jacksonville, with particular interests in contraception, menopause, and gynecologic ultrasonography.
Ekta Kapoor, MBBS, NCMP: And I am an endocrinologist at Mayo Clinic in Rochester with a specific interest in menopause and hormone therapy. I am also the Assistant Director for Mayo Clinic Women’s Health.
Higher-than-standard estrogen doses needed in younger menopausal women
Dr. Faubion: Let’s consider a couple of cases so that we can illustrate some important points regarding hormone management in women who have undergone BSO before the age of natural menopause.
Our first case patient is a woman who is 41 years of age and, because of adenomyosis, she will undergo a hysterectomy. She tells her clinician that she is very concerned about ovarian cancer risk because one of her good friends recently was diagnosed with ovarian cancer, and together they decide to remove her ovaries at the time of hysterectomy. Notably, her ovaries were healthy.
The patient is now menopausal postsurgery, and she is having significant hot flashes and night sweats. She visits her local internist, who is concerned about initiating HT. She is otherwise a healthy woman and does not have any contraindications to HT. Dr. Kaunitz, what would you tell her internist?
Dr. Kaunitz: We are dealing with 2 different issues in terms of decision making about systemic HT for this 41-year-old who has undergone BSO. First, as you mentioned, Dr. Faubion, she has bothersome hot flashes, or vasomotor symptoms. Unless there are contraindications, systemic HT would be appropriate. Although I might start treatment at standard doses, and the accompanying TABLE depicts standard doses for the 2 most common oral estrogen formulations as well as transdermal estradiol, it’s important to recognize that younger menopausal women often will need to use higher-than-standard doses.
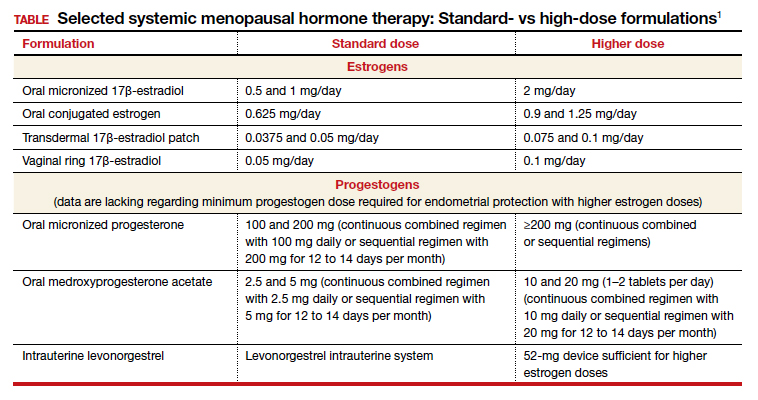
For example, for a 53-year-old woman who has been menopausal for a year or 2 and now has bothersome symptoms, I might start her on estradiol 1 mg tablets with progestin if a uterus is present. However, in this 41-year-old case patient, while I might start treatment at a standard dose, I would anticipate increasing to higher doses, such as 1.5 or 2 mg of daily estradiol until she feels her menopausal symptoms are adequately addressed.
Dr. Faubion: It is important to note that sometimes women with early BSO tend to have more severe vasomotor symptoms. Do you find that sometimes a higher dose is required just to manage symptoms, Dr. Kaunitz?
Dr. Kaunitz: Absolutely, yes. The decision whether or not to use systemic HT might be considered discretionary or elective in the classic 53-year-old woman recently menopausal with hot flashes, a so-called spontaneously or naturally menopausal woman. But my perspective is that unless there are clear contraindications, the decision to start systemic HT in the 41-year-old BSO case patient is actually not discretionary. Unless contraindications are present, it is important not only to treat symptoms but also to prevent an array of chronic major health concerns that are more likely if we don’t prescribe systemic HT.
Continue to: Health effects of not using HT...
Health effects of not using HT
Dr. Faubion: Dr. Kapoor, can you describe the potential long-term adverse health consequences of not using estrogen therapy? Say the same 41-year-old woman does not have many bothersome symptoms. What would you do?
Dr. Kapoor: Thank you for that important question. Building on what Dr. Kaunitz said, in these patients there are really 2 issues that can seem to be independent but are not: The first relates to the immediate consequences of lack of estrogen, ie, the menopause-related symptoms, but the second and perhaps the bigger issue is the long-term risk associated with estrogen deprivation.
The symptoms in these women are often obvious as they can be quite severe and abrupt; one day these women have normal hormone levels and the next day, after BSO, suddenly their hormones are very low. So if symptoms occur, they are usually hard to miss, simply because they are very drastic and very severe.
Historically, patients and their clinicians have targeted these symptoms. Patients experience menopausal symptoms, they seek treatment, and then the clinicians basically titrate the treatment to manage these symptoms. That misses the bigger issue, however, which is that premature estrogen deprivation leads to a host of chronic health conditions, as Dr. Kaunitz mentioned. These mainly include increased risk for cardiovascular disease, diabetes, hypertension, dyslipidemia, increased risk of mortality, dementia, and osteoporosis.
Fairly strong observational evidence suggests that use of estrogen therapy given in replacement doses—doses higher than those typically used in women after natural menopause, therefore considered replacement doses—helps mitigate the risk of some of these adverse health conditions.
In these women, the bigger goal really is to reinstate the hormonal milieu that exists prior to menopause. To your point, Dr. Faubion, if I have a patient who is younger than 46 years, who has her ovaries taken out, and even if she has zero symptoms (and sometimes that does happen), I would still make a case for this patient to utilize hormone therapy unless there is a contraindication such as breast cancer or other estrogen-sensitive cancers.
Dr. Faubion: Again, would you aim for those higher doses rather than treat with the “lowest dose”?
Dr. Kapoor: Absolutely. My punchline to the patients and clinicians in these discussions is that the rules of the game are different for these women. We cannot extrapolate the risks and benefits of HT use in women after natural menopause to younger women who have surgical menopause. Those rules just do not apply with respect to both benefits and risks.
Dr. Faubion: I think it’s important to say that these same “rules” would apply if the women were to go through premature menopause for any other reason, too, such as chemotherapy, radiation therapy, or premature ovarian insufficiency for any number of reasons, including toxic, metabolic, or genetic causes and so on. Would that be true?
Dr. Kapoor: Yes, absolutely so.
Dr. Faubion: Dr. Kaunitz, do you want to add anything?
Dr. Kaunitz: In terms of practical or clinical issues regarding systemic HT management, for the woman in her early 50s who has experienced normal or natural spontaneous menopause, a starting dose of transdermal estradiol would be, for instance, a 0.05-mg patch, which is a patch that over 24 hours releases 0.05 mg of estradiol daily; or standard oral estrogen, including conjugated equine estrogen, a 0.625-mg tablet daily, or estradiol, a 1-mg tablet daily.
But in younger patients, we want to use higher doses. For a patch, for instance, I would aim for a 0.075- or 0.1-mg estradiol patch, which releases a higher daily dose of estradiol than the standard dose. For oral estrogen, the dose would be 0.9- or even 1.25-mg tablets of conjugated equine estrogen or 1.5 mg, which is a 1-mg plus a 0.5-mg estradiol tablet, or a 2-mg estradiol tablet. Estradiol does come in a 2-mg strength.
For oral estrogen, I prefer estradiol because it’s available as a generic medication and often available at a very low cost, sometimes as low as $4 a month from chain pharmacies.
Continue to: Usefulness of monitoring estradiol levels for dosage adjustment...
Usefulness of monitoring estradiol levels for dosage adjustment
Dr. Faubion: That’s a great point, and again it is important to emphasize that we are aiming to recreate the premenopausal hormonal milieu. If you were to check estradiol levels, that would be aiming for a premenopausal range of approximately 80 to 120 pg per mL. Dr. Kapoor, is there utility in monitoring estrogen levels?
Dr. Kapoor: Great question, Dr. Faubion, and as you know it’s a loaded one. We base this on empiric evidence. We know that if the hormonal milieu in a young patient is changed to a postmenopausal one, her risk for many chronic conditions is increased. So if we were to reinstate a premenopausal hormonal milieu, that risk would probably be reduced. It makes good sense to target an empiric goal of 80 to 120 pg per mL of estradiol, which is the average estradiol level in a premenopausal woman. If you were to ask me, however, are there randomized, controlled trial data to support this practice—that is, if you target that level, can you make sure that the risk of diabetes is lower or that the risk of heart disease is lower—that study has yet to be done, and it may not ever be done on a large scale. However, it intuitively makes good sense to target premenopausal estradiol levels.
Dr. Faubion: When might you check an estradiol level in this population? For example, if you are treating a patient with a 0.1-mg estradiol patch and she still has significant hot flashes, would it be useful to check the level?
Dr. Kapoor: It would. In my practice, I check estradiol levels on these patients on an annual basis, regardless of symptoms, but definitely in the patient who has symptoms. It makes good sense, because sometimes these patients don’t absorb the estrogen well, particularly if administered by the transdermal route.
A general rule of thumb is that in the average population, if a patient is on the 0.1-mg patch, for example, you would expect her level to be around 100. If it is much lower than that, which sometimes happens, that speaks for poor absorption. Options at that point would be to treat her with a higher dose patch, depending on what the level is, or switch to a different formulation, such as oral.
In instances in which I have treated patients with a 0.1-mg patch for example, and their estradiol levels are undetectable, that speaks for very poor absorption. For such patients I make a case for switching them to oral therapy. Most definitely that makes sense in a patient who is symptomatic despite treatment. But even for patients who don’t have symptoms, I like to target that level, acknowledging that there is no evidence as such to support this practice.
Dr. Faubion: Dr. Kaunitz, do you want to add anything?
Dr. Kaunitz: Yes, a few practical points. Although patches are available in a wider array of doses than oral estrogen formulations, the highest dose available is 0.1 mg. It’s important for clinicians to recognize that while checking serum levels when indicated can be performed in women using transdermal estradiol or patches, in women who are using oral estrogen, checking blood levels is not going to work well because serum estrogen levels have a daily peak and valley in women who use oral versus transdermal estradiol.
I also wanted to talk about progestins. Although many patients who have had a BSO prior to spontaneous menopause also have had a hysterectomy, others have an intact uterus associated with their BSO, so progestins must be used along with estrogen. And if we are using higher-than-standard doses of estrogen, we also need to use higher-than-standard doses of progestin.
In that classic 53-year-old woman I referred to who had spontaneous normal menopause, if she is taking 1 mg of estradiol daily, or a 0.05-mg patch, or 0.625 mg of conjugated equine estrogen, 2.5 mg of medroxyprogesterone is fine. In fact, that showed excellent progestational protection of the endometrium in the Women’s Health Initiative and in other studies.
However, if we are going to use double the estrogen dose, we should increase the progestin dose too. In some of my patients on higher estrogen doses who have an intact uterus, I’ll use 5 or even 10 mg of daily medroxyprogesterone acetate to ensure adequate progestational suppression.
Dr. Faubion: Another practical tip is that if one is using conjugated equine estrogens, measuring the serum estradiol levels is not useful either.
Dr. Kaunitz: I agree.
Continue to: Oral contraceptives as replacement HT...
Oral contraceptives as replacement HT
Dr. Faubion: Would you comment on use of a birth control pill in this circumstance? Would it be optimal to use a postmenopausal HT regimen as opposed to a birth control pill or combined hormonal contraception?
Dr. Kapoor: In this younger population, sometimes it seems like a more socially acceptable decision to be on a birth control option than on menopausal HT. But there are some issues with being on a contraceptive regimen. One is that we end up using estrogen doses much higher than what is really needed for replacement purposes. It is also a nonphysiologic way of replacement in another sense—as opposed to estradiol, which is the main hormone made by the ovaries, the hormonal contraceptive regimens contain the synthetic estrogen ethinyl estradiol for the most part.
The other issue that is based on some weak evidence is that it appears that the bone health outcomes are probably inferior with combined hormonal contraception. For these reasons, regimens that are based on replacement doses of estradiol are preferred.
Dr. Faubion: Right, although the data are somewhat weak, I agree that thus far it seems optimal to utilize a postmenopausal regimen for various reasons. Dr. Kaunitz, anything to add?
Dr. Kaunitz: Yes, to underscore Dr. Kapoor’s point, a common oral contraceptive that contains 20 µg of ethinyl estradiol is substantially more estrogenic than 1.0 or 2.0 mg of micronized oral estradiol.
Also consider that a 20-µg ethinyl estradiol oral contraceptive may increase the risk of venous thromboembolism more than menopausal doses of oral estradiol, whether it be a micronized estradiol or conjugated equine estrogen.
Dr. Faubion: So the risk may be greater with oral combined hormonal contraception as well?
Dr. Kaunitz: One thing we can do is explain to our patients that their ovaries, prior to surgery or prior to induced menopause, were making substantial quantities of estradiol. Whether we prescribe a patch or oral micronized estradiol, this estrogen is identical to the hormone that their ovaries were making prior to surgery or induced menopause.
Breast cancer concerns
Dr. Faubion: Let’s consider a more complicated case. A 35-year-old woman has an identified BRCA1 mutation; she has not had any cancers but has undergone risk-reducing BSO and her uterus remains. Is this woman a candidate for HT? At what dose, and for how long? Dr. Kaunitz, why don’t you start.
Dr. Kaunitz: That is a challenging case but one that I think our readers will find interesting and maybe even provocative.
We know that women with BRCA1 mutations, the more common of the 2 BRCA mutations, have a very high risk of developing epithelial ovarian cancer at a young age. For this reason, our colleagues in medical oncology who specialize in hereditary ovarian/breast cancer syndromes recommend prophylactic risk-reducing—and I would also say lifesaving—BSO with or without hysterectomy for women with BRCA1 mutations.
However, over the years there has been tremendous reluctance among physicians caring for BRCA patients and the women themselves—I use the term “previvors” to describe BRCA carriers who have not been diagnosed with breast or ovarian cancer—to use HT after BSO because of concerns that HT might increase breast cancer risk in women who are already at high risk for breast cancer.
I assume, Dr. Faubion, that in this case the woman had gynecologic surgery but continues to have intact breasts. Is that correct?
Dr. Faubion: That is correct.
Dr. Kaunitz: Although the assumption has been that it is not safe to prescribe HT in this setting, in fact, the reported cohort studies that have looked at this issue have not found an elevated risk of breast cancer when replacement estrogen, with or without progestin, is prescribed to BRCA1 previvors with intact breasts.
Given what Dr. Kapoor said regarding the morbidity that is associated with BSO without replacement of physiologic estrogen, and also given the severe symptoms that so many of these young menopausal women experience, in my practice I do prescribe estrogen or estrogen-progestin therapy and focus on the higher target doses that we discussed for the earlier case patient who had a hysterectomy for abnormal uterine bleeding with adenomyosis.
Dr. Faubion: Dr. Kapoor, do you agree with this approach? How long would you continue therapy?
Dr. Kapoor: First, in this BRCA1 case we need to appreciate that the indication for the BSO is a legitimate one, in contrast to the first case in which the ovaries were removed in a patient whose average risk of ovarian cancer was low. It is important to recognize that surgery performed in this context is the right thing to do because it does significantly reduce the risk of ovarian cancer.
The second thing to appreciate is that while we reduce the risk of ovarian cancer significantly and make sure that these patients survive longer, it’s striking a fine balance in that you want to make sure that their morbidity is not increased as a result of premature estrogen deprivation.
As Dr. Kaunitz told us, the evidence that we have so far, which granted is not very robust but is fairly strong observational evidence, suggests that the risk of breast cancer is not elevated when these patients are treated with replacement doses of HT.
Having said that, I do have very strong discussions with my patients in this category about having the risk-reducing bilateral mastectomy also, because if they were to get breast cancer because of their increased genetic predisposition, the cancer is likely to grow faster if the patient is on HT. So one of my counseling points to patients is that they strongly consider bilateral mastectomy, which reduces their breast cancer risk by more than 90%. At the same time, I also strongly endorse using HT in replacement doses for the reasons that we have already stated.
Dr. Faubion: Continue HT until age 50 or 52?
Dr. Kapoor: Definitely until that age, and possibly longer, depending on their symptoms. The indications for treating beyond the age of natural menopause are much the same as for women who experience natural menopause.
Dr. Faubion: That is assuming they had a bilateral mastectomy?
Dr. Kapoor: Yes.
Continue to: Continuing HT until the age of natural menopause...
Continuing HT until the age of natural menopause
Dr. Kaunitz: Dr. Kapoor brings up the important point of duration of systemic HT. I agree that similar considerations apply both to the healthy 41-year-old who had a hysterectomy for abnormal uterine bleeding and to the 35-year-old who had risk-reducing surgery because of her BRCA1 mutation.
In the 2 cases, both to treat symptoms and to prevent chronic diseases, it makes sense to continue HT at least until the age of natural menopause. That is consistent with 2017 guidance from The North American Menopause Society (NAMS) position statement on the use of systemic HT, that is, continuing systemic HT at least until the age of natural menopause.3 Then at that point, continuing or discontinuing systemic HT becomes discretionary, and that would be true for both cases. If the patient is slender or has a strong family history of osteoporosis, that tends to push the patient more in terms of continuing systemic HT. Those are just some examples, and Dr. Kapoor may want to detail other relevant considerations.
Dr. Kapoor: I completely agree. The decision is driven by symptoms that are not otherwise well managed, for example, with nonhormone strategies. If we have any concerns utilizing HT beyond the age of natural menopause, then nonhormonal options can be considered; but sometimes those are not as effective. And bone health is very important. You want to avoid using bisphosphonates in younger women and reserve them for older patients in their late 60s and 70s. Hormone therapy use is a very reasonable strategy to prevent bone loss.
Dr. Kaunitz: It is also worth mentioning that sometimes the woman involved in shared decision making with her clinician decides to stop systemic HT. In that setting, should the patient start developing new-onset dyspareunia, vaginal dryness, or other genital or sexuality-related concerns, it takes very little for me to advise that she start low-dose local vaginal estrogen therapy.
Dr. Faubion: In either scenario, if a woman were to develop symptoms consistent with genitourinary syndrome of menopause (GSM), would you use vaginal estrogen in addition to the systemic estrogen or alone after the woman elected to discontinue systemic therapy?
Dr. Kapoor: Yes to both, I would say.
Dr. Kaunitz: As my patients using systemic HT age, often I will lower the dose. For instance, the dose I use in a 53-year-old will be higher than when she is 59 or 62. At the same time, as we lower the dose of systemic estrogen therapy, symptoms of vaginal atrophy or GSM often will appear, and these can be effectively treated by adding low-dose vaginal estrogen therapy. A number of my patients, particularly those who are on lower-than-standard doses of systemic HT, are also using low-dose vaginal estrogen therapy.
There is a “hybrid” product available: the 90-day estradiol vaginal ring. Estring is a low-dose, 2-mg, 90-day estradiol ring that is very useful, but it is effective only for treating GSM or vaginal atrophy. A second menopausal vaginal estradiol ring, Femring, is available in 2 doses: 0.05 mg/day and 0.1 mg/day. These are very effective in treating both systemic issues, such as vasomotor symptoms or prevention of osteoporosis, and very effective in treating GSM or vaginal atrophy. One problem is that Femring, depending on insurance coverage, can be very expensive. It’s not available as a generic, so for insurance or financial reasons I don’t often prescribe it. If I could remove those financial barriers, I would prescribe Femring more often because it is very useful.
Dr. Faubion: You raise an important point, and that is, for women who have been on HT for some time, clinicians often feel the need to slowly reduce the dose. Would you do that same thing, Dr. Kapoor, for a 40-year-old woman? Would you reduce the dose as she approaches age 50? Is there pressure that “she shouldn’t be on that much estrogen”?
Dr. Kapoor: No, I would not feel pressured until the patient turns at least 46. I bring up age 46 because the average age range for menopause is 46 to 55. After that, if there is any concern, we can decrease the dose to half and keep the patient on that until she turns 50 or 51. But most of my patients are on replacement doses until the average age of menopause, which is around 51 years, and that’s when you reduce the dose to that of the typical HT regimens used after natural menopause.
Sometimes patients are told something by a friend or they have read something and they worry about the risk of 2 things. One is breast cancer and the other is venous thromboembolism (VTE), and that may be why they want to be on a lower dose. I counsel patients that while the risk of VTE is real with HT, it is the women after natural menopause who are at risk—because age itself is a risk for VTE—and it also has to do with the kind of HT regimen that a patient is on. High doses of oral estrogens and certain progestogens increase the risk. But again, for estradiol used in replacement doses and the more common progestogens that we now use in practice, such as micronized progesterone, the risk is not the same. The same goes for breast cancer. My biggest message to patients and clinicians who take care of these patients is that the rules that apply to women after natural menopause just do not apply to this very different patient population.
Dr. Faubion: Thank you, Dr. Kaunitz and Dr. Kapoor, for sharing your knowledge and experience. ●
Systemic HT past the age of 65
Dr. Kaunitz: Another practical issue relates to long-term or extended use of systemic HT. It’s not infrequent in my practice to receive mail and faxes from insurance carriers of systemic HT users who are age 65 and older in which the company refers to the American Geriatrics Society’s Beers criteria for potentially inappropriate medication use in older adults,1 suggesting that systemic HT is inappropriate for all women over age 65. In this age group, I use lower doses if I am continuing systemic HT. But the good news is that both NAMS and the American College Obstetricians and Gynecologists indicate that arbitrarily stopping systemic HT at age 65 or for any other arbitrary reason is inappropriate, and that decisions about continuing or discontinuing therapy should be made on an individualized basis using shared decision making. That’s an important message for our readers.
Counseling regarding elective BSO
Dr. Faubion: One final note about elective BSO in the absence of a genetic mutation that predisposes to increased ovarian or breast cancer risk. Fortunately, we have seen rates of oophorectomy before the age of natural menopause decline, but what would your advice be to women or clinicians of these women who say they are “just afraid of ovarian cancer and would like to have their ovaries removed before the age of natural menopause”?
Dr. Kaunitz: If patients have increased anxiety about ovarian cancer and yet they themselves are not known to be at elevated risk, I emphasize that, fortunately, ovarian cancer is uncommon. It is much less common than other cancers the patient might be familiar with, such as breast or colon or lung cancer. I also emphasize that women who have given birth, particularly multiple times; women who nursed their infants; and women who have used combination hormonal contraceptives, particularly if long term, are at markedly lower risk for ovarian cancer as they get older. We are talking about an uncommon cancer that is even less common if women have given birth, nursed their infants, or used combination contraceptives long term.
Dr. Faubion: Dr. Kapoor, what would you say regarding the increased risk they might incur if they do have their ovaries out?
Dr. Kapoor: As Dr. Kaunitz said, this is an uncommon cancer, and pursuing something to reduce the risk of an uncommon cancer does not benefit the community. That is also my counseling point to patients.
I also talk to them extensively about the risk associated with the ovaries being removed, and I tell them that although we have the option of giving them HT, it is hard to replicate the magic of nature. No matter what concoction or regimen we use, we cannot ensure reinstating health to what it was in the premenopausal state, because estrogen has such myriad effects on the body in so many different organ systems.
Reference
1. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
- Kaunitz AM, Kapoor E, Faubion S. Treatment of women after bilateral salpingo-oophorectomy performed prior to natural menopause. JAMA. 2021;326:1429-1430.
- Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
- North American Menopause Society. The 2017 hormone therapy position statement of The North American Menopause Society. J North Am Menopause Soc. 2017;24: 728-753.

Women who undergo bilateral salpingo-oophorectomy (BSO) for various indications prior to menopause experience a rapid decline in ovarian hormone levels and consequent vasomotor and other menopausal symptoms. In addition, the resulting estrogen deprivation is associated with such long-term adverse outcomes as osteoporosis and cardiovascular morbidity.
OBG M
Surgical vs natural menopause
Stephanie Faubion, MD, MBA, NCMP: Since the Women’s Health Initiative study was published in 2002,2 many clinicians have been fearful of using systemic HT in menopausal women, and HT use has declined dramatically such that only about 4% to 6% of menopausal women are now receiving systemic HT. Importantly, however, a group of younger menopausal women also are not receiving HT, and that is women who undergo BSO before they reach the average age of menopause, which in the United States is about age 52; this is sometimes referred to as surgical menopause or early surgical menopause. Early surgical menopause has different connotations for long-term health risks than natural menopause at the average age, and we are here to discuss these health effects and their management.
My name is Stephanie Faubion, and I am a women’s health internist and the Chair of the Department of Medicine at Mayo Clinic in Jacksonville, Florida, and Director of Mayo Clinic Women’s Health. I am here with 2 of my esteemed colleagues, Dr. Andrew Kaunitz and Dr. Ekta Kapoor.
Andrew M. Kaunitz, MD, NCMP: Hello, I am an ObGyn with the University of Florida College of Medicine in Jacksonville, with particular interests in contraception, menopause, and gynecologic ultrasonography.
Ekta Kapoor, MBBS, NCMP: And I am an endocrinologist at Mayo Clinic in Rochester with a specific interest in menopause and hormone therapy. I am also the Assistant Director for Mayo Clinic Women’s Health.
Higher-than-standard estrogen doses needed in younger menopausal women
Dr. Faubion: Let’s consider a couple of cases so that we can illustrate some important points regarding hormone management in women who have undergone BSO before the age of natural menopause.
Our first case patient is a woman who is 41 years of age and, because of adenomyosis, she will undergo a hysterectomy. She tells her clinician that she is very concerned about ovarian cancer risk because one of her good friends recently was diagnosed with ovarian cancer, and together they decide to remove her ovaries at the time of hysterectomy. Notably, her ovaries were healthy.
The patient is now menopausal postsurgery, and she is having significant hot flashes and night sweats. She visits her local internist, who is concerned about initiating HT. She is otherwise a healthy woman and does not have any contraindications to HT. Dr. Kaunitz, what would you tell her internist?
Dr. Kaunitz: We are dealing with 2 different issues in terms of decision making about systemic HT for this 41-year-old who has undergone BSO. First, as you mentioned, Dr. Faubion, she has bothersome hot flashes, or vasomotor symptoms. Unless there are contraindications, systemic HT would be appropriate. Although I might start treatment at standard doses, and the accompanying TABLE depicts standard doses for the 2 most common oral estrogen formulations as well as transdermal estradiol, it’s important to recognize that younger menopausal women often will need to use higher-than-standard doses.

For example, for a 53-year-old woman who has been menopausal for a year or 2 and now has bothersome symptoms, I might start her on estradiol 1 mg tablets with progestin if a uterus is present. However, in this 41-year-old case patient, while I might start treatment at a standard dose, I would anticipate increasing to higher doses, such as 1.5 or 2 mg of daily estradiol until she feels her menopausal symptoms are adequately addressed.
Dr. Faubion: It is important to note that sometimes women with early BSO tend to have more severe vasomotor symptoms. Do you find that sometimes a higher dose is required just to manage symptoms, Dr. Kaunitz?
Dr. Kaunitz: Absolutely, yes. The decision whether or not to use systemic HT might be considered discretionary or elective in the classic 53-year-old woman recently menopausal with hot flashes, a so-called spontaneously or naturally menopausal woman. But my perspective is that unless there are clear contraindications, the decision to start systemic HT in the 41-year-old BSO case patient is actually not discretionary. Unless contraindications are present, it is important not only to treat symptoms but also to prevent an array of chronic major health concerns that are more likely if we don’t prescribe systemic HT.
Continue to: Health effects of not using HT...
Health effects of not using HT
Dr. Faubion: Dr. Kapoor, can you describe the potential long-term adverse health consequences of not using estrogen therapy? Say the same 41-year-old woman does not have many bothersome symptoms. What would you do?
Dr. Kapoor: Thank you for that important question. Building on what Dr. Kaunitz said, in these patients there are really 2 issues that can seem to be independent but are not: The first relates to the immediate consequences of lack of estrogen, ie, the menopause-related symptoms, but the second and perhaps the bigger issue is the long-term risk associated with estrogen deprivation.
The symptoms in these women are often obvious as they can be quite severe and abrupt; one day these women have normal hormone levels and the next day, after BSO, suddenly their hormones are very low. So if symptoms occur, they are usually hard to miss, simply because they are very drastic and very severe.
Historically, patients and their clinicians have targeted these symptoms. Patients experience menopausal symptoms, they seek treatment, and then the clinicians basically titrate the treatment to manage these symptoms. That misses the bigger issue, however, which is that premature estrogen deprivation leads to a host of chronic health conditions, as Dr. Kaunitz mentioned. These mainly include increased risk for cardiovascular disease, diabetes, hypertension, dyslipidemia, increased risk of mortality, dementia, and osteoporosis.
Fairly strong observational evidence suggests that use of estrogen therapy given in replacement doses—doses higher than those typically used in women after natural menopause, therefore considered replacement doses—helps mitigate the risk of some of these adverse health conditions.
In these women, the bigger goal really is to reinstate the hormonal milieu that exists prior to menopause. To your point, Dr. Faubion, if I have a patient who is younger than 46 years, who has her ovaries taken out, and even if she has zero symptoms (and sometimes that does happen), I would still make a case for this patient to utilize hormone therapy unless there is a contraindication such as breast cancer or other estrogen-sensitive cancers.
Dr. Faubion: Again, would you aim for those higher doses rather than treat with the “lowest dose”?
Dr. Kapoor: Absolutely. My punchline to the patients and clinicians in these discussions is that the rules of the game are different for these women. We cannot extrapolate the risks and benefits of HT use in women after natural menopause to younger women who have surgical menopause. Those rules just do not apply with respect to both benefits and risks.
Dr. Faubion: I think it’s important to say that these same “rules” would apply if the women were to go through premature menopause for any other reason, too, such as chemotherapy, radiation therapy, or premature ovarian insufficiency for any number of reasons, including toxic, metabolic, or genetic causes and so on. Would that be true?
Dr. Kapoor: Yes, absolutely so.
Dr. Faubion: Dr. Kaunitz, do you want to add anything?
Dr. Kaunitz: In terms of practical or clinical issues regarding systemic HT management, for the woman in her early 50s who has experienced normal or natural spontaneous menopause, a starting dose of transdermal estradiol would be, for instance, a 0.05-mg patch, which is a patch that over 24 hours releases 0.05 mg of estradiol daily; or standard oral estrogen, including conjugated equine estrogen, a 0.625-mg tablet daily, or estradiol, a 1-mg tablet daily.
But in younger patients, we want to use higher doses. For a patch, for instance, I would aim for a 0.075- or 0.1-mg estradiol patch, which releases a higher daily dose of estradiol than the standard dose. For oral estrogen, the dose would be 0.9- or even 1.25-mg tablets of conjugated equine estrogen or 1.5 mg, which is a 1-mg plus a 0.5-mg estradiol tablet, or a 2-mg estradiol tablet. Estradiol does come in a 2-mg strength.
For oral estrogen, I prefer estradiol because it’s available as a generic medication and often available at a very low cost, sometimes as low as $4 a month from chain pharmacies.
Continue to: Usefulness of monitoring estradiol levels for dosage adjustment...
Usefulness of monitoring estradiol levels for dosage adjustment
Dr. Faubion: That’s a great point, and again it is important to emphasize that we are aiming to recreate the premenopausal hormonal milieu. If you were to check estradiol levels, that would be aiming for a premenopausal range of approximately 80 to 120 pg per mL. Dr. Kapoor, is there utility in monitoring estrogen levels?
Dr. Kapoor: Great question, Dr. Faubion, and as you know it’s a loaded one. We base this on empiric evidence. We know that if the hormonal milieu in a young patient is changed to a postmenopausal one, her risk for many chronic conditions is increased. So if we were to reinstate a premenopausal hormonal milieu, that risk would probably be reduced. It makes good sense to target an empiric goal of 80 to 120 pg per mL of estradiol, which is the average estradiol level in a premenopausal woman. If you were to ask me, however, are there randomized, controlled trial data to support this practice—that is, if you target that level, can you make sure that the risk of diabetes is lower or that the risk of heart disease is lower—that study has yet to be done, and it may not ever be done on a large scale. However, it intuitively makes good sense to target premenopausal estradiol levels.
Dr. Faubion: When might you check an estradiol level in this population? For example, if you are treating a patient with a 0.1-mg estradiol patch and she still has significant hot flashes, would it be useful to check the level?
Dr. Kapoor: It would. In my practice, I check estradiol levels on these patients on an annual basis, regardless of symptoms, but definitely in the patient who has symptoms. It makes good sense, because sometimes these patients don’t absorb the estrogen well, particularly if administered by the transdermal route.
A general rule of thumb is that in the average population, if a patient is on the 0.1-mg patch, for example, you would expect her level to be around 100. If it is much lower than that, which sometimes happens, that speaks for poor absorption. Options at that point would be to treat her with a higher dose patch, depending on what the level is, or switch to a different formulation, such as oral.
In instances in which I have treated patients with a 0.1-mg patch for example, and their estradiol levels are undetectable, that speaks for very poor absorption. For such patients I make a case for switching them to oral therapy. Most definitely that makes sense in a patient who is symptomatic despite treatment. But even for patients who don’t have symptoms, I like to target that level, acknowledging that there is no evidence as such to support this practice.
Dr. Faubion: Dr. Kaunitz, do you want to add anything?
Dr. Kaunitz: Yes, a few practical points. Although patches are available in a wider array of doses than oral estrogen formulations, the highest dose available is 0.1 mg. It’s important for clinicians to recognize that while checking serum levels when indicated can be performed in women using transdermal estradiol or patches, in women who are using oral estrogen, checking blood levels is not going to work well because serum estrogen levels have a daily peak and valley in women who use oral versus transdermal estradiol.
I also wanted to talk about progestins. Although many patients who have had a BSO prior to spontaneous menopause also have had a hysterectomy, others have an intact uterus associated with their BSO, so progestins must be used along with estrogen. And if we are using higher-than-standard doses of estrogen, we also need to use higher-than-standard doses of progestin.
In that classic 53-year-old woman I referred to who had spontaneous normal menopause, if she is taking 1 mg of estradiol daily, or a 0.05-mg patch, or 0.625 mg of conjugated equine estrogen, 2.5 mg of medroxyprogesterone is fine. In fact, that showed excellent progestational protection of the endometrium in the Women’s Health Initiative and in other studies.
However, if we are going to use double the estrogen dose, we should increase the progestin dose too. In some of my patients on higher estrogen doses who have an intact uterus, I’ll use 5 or even 10 mg of daily medroxyprogesterone acetate to ensure adequate progestational suppression.
Dr. Faubion: Another practical tip is that if one is using conjugated equine estrogens, measuring the serum estradiol levels is not useful either.
Dr. Kaunitz: I agree.
Continue to: Oral contraceptives as replacement HT...
Oral contraceptives as replacement HT
Dr. Faubion: Would you comment on use of a birth control pill in this circumstance? Would it be optimal to use a postmenopausal HT regimen as opposed to a birth control pill or combined hormonal contraception?
Dr. Kapoor: In this younger population, sometimes it seems like a more socially acceptable decision to be on a birth control option than on menopausal HT. But there are some issues with being on a contraceptive regimen. One is that we end up using estrogen doses much higher than what is really needed for replacement purposes. It is also a nonphysiologic way of replacement in another sense—as opposed to estradiol, which is the main hormone made by the ovaries, the hormonal contraceptive regimens contain the synthetic estrogen ethinyl estradiol for the most part.
The other issue that is based on some weak evidence is that it appears that the bone health outcomes are probably inferior with combined hormonal contraception. For these reasons, regimens that are based on replacement doses of estradiol are preferred.
Dr. Faubion: Right, although the data are somewhat weak, I agree that thus far it seems optimal to utilize a postmenopausal regimen for various reasons. Dr. Kaunitz, anything to add?
Dr. Kaunitz: Yes, to underscore Dr. Kapoor’s point, a common oral contraceptive that contains 20 µg of ethinyl estradiol is substantially more estrogenic than 1.0 or 2.0 mg of micronized oral estradiol.
Also consider that a 20-µg ethinyl estradiol oral contraceptive may increase the risk of venous thromboembolism more than menopausal doses of oral estradiol, whether it be a micronized estradiol or conjugated equine estrogen.
Dr. Faubion: So the risk may be greater with oral combined hormonal contraception as well?
Dr. Kaunitz: One thing we can do is explain to our patients that their ovaries, prior to surgery or prior to induced menopause, were making substantial quantities of estradiol. Whether we prescribe a patch or oral micronized estradiol, this estrogen is identical to the hormone that their ovaries were making prior to surgery or induced menopause.
Breast cancer concerns
Dr. Faubion: Let’s consider a more complicated case. A 35-year-old woman has an identified BRCA1 mutation; she has not had any cancers but has undergone risk-reducing BSO and her uterus remains. Is this woman a candidate for HT? At what dose, and for how long? Dr. Kaunitz, why don’t you start.
Dr. Kaunitz: That is a challenging case but one that I think our readers will find interesting and maybe even provocative.
We know that women with BRCA1 mutations, the more common of the 2 BRCA mutations, have a very high risk of developing epithelial ovarian cancer at a young age. For this reason, our colleagues in medical oncology who specialize in hereditary ovarian/breast cancer syndromes recommend prophylactic risk-reducing—and I would also say lifesaving—BSO with or without hysterectomy for women with BRCA1 mutations.
However, over the years there has been tremendous reluctance among physicians caring for BRCA patients and the women themselves—I use the term “previvors” to describe BRCA carriers who have not been diagnosed with breast or ovarian cancer—to use HT after BSO because of concerns that HT might increase breast cancer risk in women who are already at high risk for breast cancer.
I assume, Dr. Faubion, that in this case the woman had gynecologic surgery but continues to have intact breasts. Is that correct?
Dr. Faubion: That is correct.
Dr. Kaunitz: Although the assumption has been that it is not safe to prescribe HT in this setting, in fact, the reported cohort studies that have looked at this issue have not found an elevated risk of breast cancer when replacement estrogen, with or without progestin, is prescribed to BRCA1 previvors with intact breasts.
Given what Dr. Kapoor said regarding the morbidity that is associated with BSO without replacement of physiologic estrogen, and also given the severe symptoms that so many of these young menopausal women experience, in my practice I do prescribe estrogen or estrogen-progestin therapy and focus on the higher target doses that we discussed for the earlier case patient who had a hysterectomy for abnormal uterine bleeding with adenomyosis.
Dr. Faubion: Dr. Kapoor, do you agree with this approach? How long would you continue therapy?
Dr. Kapoor: First, in this BRCA1 case we need to appreciate that the indication for the BSO is a legitimate one, in contrast to the first case in which the ovaries were removed in a patient whose average risk of ovarian cancer was low. It is important to recognize that surgery performed in this context is the right thing to do because it does significantly reduce the risk of ovarian cancer.
The second thing to appreciate is that while we reduce the risk of ovarian cancer significantly and make sure that these patients survive longer, it’s striking a fine balance in that you want to make sure that their morbidity is not increased as a result of premature estrogen deprivation.
As Dr. Kaunitz told us, the evidence that we have so far, which granted is not very robust but is fairly strong observational evidence, suggests that the risk of breast cancer is not elevated when these patients are treated with replacement doses of HT.
Having said that, I do have very strong discussions with my patients in this category about having the risk-reducing bilateral mastectomy also, because if they were to get breast cancer because of their increased genetic predisposition, the cancer is likely to grow faster if the patient is on HT. So one of my counseling points to patients is that they strongly consider bilateral mastectomy, which reduces their breast cancer risk by more than 90%. At the same time, I also strongly endorse using HT in replacement doses for the reasons that we have already stated.
Dr. Faubion: Continue HT until age 50 or 52?
Dr. Kapoor: Definitely until that age, and possibly longer, depending on their symptoms. The indications for treating beyond the age of natural menopause are much the same as for women who experience natural menopause.
Dr. Faubion: That is assuming they had a bilateral mastectomy?
Dr. Kapoor: Yes.
Continue to: Continuing HT until the age of natural menopause...
Continuing HT until the age of natural menopause
Dr. Kaunitz: Dr. Kapoor brings up the important point of duration of systemic HT. I agree that similar considerations apply both to the healthy 41-year-old who had a hysterectomy for abnormal uterine bleeding and to the 35-year-old who had risk-reducing surgery because of her BRCA1 mutation.
In the 2 cases, both to treat symptoms and to prevent chronic diseases, it makes sense to continue HT at least until the age of natural menopause. That is consistent with 2017 guidance from The North American Menopause Society (NAMS) position statement on the use of systemic HT, that is, continuing systemic HT at least until the age of natural menopause.3 Then at that point, continuing or discontinuing systemic HT becomes discretionary, and that would be true for both cases. If the patient is slender or has a strong family history of osteoporosis, that tends to push the patient more in terms of continuing systemic HT. Those are just some examples, and Dr. Kapoor may want to detail other relevant considerations.
Dr. Kapoor: I completely agree. The decision is driven by symptoms that are not otherwise well managed, for example, with nonhormone strategies. If we have any concerns utilizing HT beyond the age of natural menopause, then nonhormonal options can be considered; but sometimes those are not as effective. And bone health is very important. You want to avoid using bisphosphonates in younger women and reserve them for older patients in their late 60s and 70s. Hormone therapy use is a very reasonable strategy to prevent bone loss.
Dr. Kaunitz: It is also worth mentioning that sometimes the woman involved in shared decision making with her clinician decides to stop systemic HT. In that setting, should the patient start developing new-onset dyspareunia, vaginal dryness, or other genital or sexuality-related concerns, it takes very little for me to advise that she start low-dose local vaginal estrogen therapy.
Dr. Faubion: In either scenario, if a woman were to develop symptoms consistent with genitourinary syndrome of menopause (GSM), would you use vaginal estrogen in addition to the systemic estrogen or alone after the woman elected to discontinue systemic therapy?
Dr. Kapoor: Yes to both, I would say.
Dr. Kaunitz: As my patients using systemic HT age, often I will lower the dose. For instance, the dose I use in a 53-year-old will be higher than when she is 59 or 62. At the same time, as we lower the dose of systemic estrogen therapy, symptoms of vaginal atrophy or GSM often will appear, and these can be effectively treated by adding low-dose vaginal estrogen therapy. A number of my patients, particularly those who are on lower-than-standard doses of systemic HT, are also using low-dose vaginal estrogen therapy.
There is a “hybrid” product available: the 90-day estradiol vaginal ring. Estring is a low-dose, 2-mg, 90-day estradiol ring that is very useful, but it is effective only for treating GSM or vaginal atrophy. A second menopausal vaginal estradiol ring, Femring, is available in 2 doses: 0.05 mg/day and 0.1 mg/day. These are very effective in treating both systemic issues, such as vasomotor symptoms or prevention of osteoporosis, and very effective in treating GSM or vaginal atrophy. One problem is that Femring, depending on insurance coverage, can be very expensive. It’s not available as a generic, so for insurance or financial reasons I don’t often prescribe it. If I could remove those financial barriers, I would prescribe Femring more often because it is very useful.
Dr. Faubion: You raise an important point, and that is, for women who have been on HT for some time, clinicians often feel the need to slowly reduce the dose. Would you do that same thing, Dr. Kapoor, for a 40-year-old woman? Would you reduce the dose as she approaches age 50? Is there pressure that “she shouldn’t be on that much estrogen”?
Dr. Kapoor: No, I would not feel pressured until the patient turns at least 46. I bring up age 46 because the average age range for menopause is 46 to 55. After that, if there is any concern, we can decrease the dose to half and keep the patient on that until she turns 50 or 51. But most of my patients are on replacement doses until the average age of menopause, which is around 51 years, and that’s when you reduce the dose to that of the typical HT regimens used after natural menopause.
Sometimes patients are told something by a friend or they have read something and they worry about the risk of 2 things. One is breast cancer and the other is venous thromboembolism (VTE), and that may be why they want to be on a lower dose. I counsel patients that while the risk of VTE is real with HT, it is the women after natural menopause who are at risk—because age itself is a risk for VTE—and it also has to do with the kind of HT regimen that a patient is on. High doses of oral estrogens and certain progestogens increase the risk. But again, for estradiol used in replacement doses and the more common progestogens that we now use in practice, such as micronized progesterone, the risk is not the same. The same goes for breast cancer. My biggest message to patients and clinicians who take care of these patients is that the rules that apply to women after natural menopause just do not apply to this very different patient population.
Dr. Faubion: Thank you, Dr. Kaunitz and Dr. Kapoor, for sharing your knowledge and experience. ●
Systemic HT past the age of 65
Dr. Kaunitz: Another practical issue relates to long-term or extended use of systemic HT. It’s not infrequent in my practice to receive mail and faxes from insurance carriers of systemic HT users who are age 65 and older in which the company refers to the American Geriatrics Society’s Beers criteria for potentially inappropriate medication use in older adults,1 suggesting that systemic HT is inappropriate for all women over age 65. In this age group, I use lower doses if I am continuing systemic HT. But the good news is that both NAMS and the American College Obstetricians and Gynecologists indicate that arbitrarily stopping systemic HT at age 65 or for any other arbitrary reason is inappropriate, and that decisions about continuing or discontinuing therapy should be made on an individualized basis using shared decision making. That’s an important message for our readers.
Counseling regarding elective BSO
Dr. Faubion: One final note about elective BSO in the absence of a genetic mutation that predisposes to increased ovarian or breast cancer risk. Fortunately, we have seen rates of oophorectomy before the age of natural menopause decline, but what would your advice be to women or clinicians of these women who say they are “just afraid of ovarian cancer and would like to have their ovaries removed before the age of natural menopause”?
Dr. Kaunitz: If patients have increased anxiety about ovarian cancer and yet they themselves are not known to be at elevated risk, I emphasize that, fortunately, ovarian cancer is uncommon. It is much less common than other cancers the patient might be familiar with, such as breast or colon or lung cancer. I also emphasize that women who have given birth, particularly multiple times; women who nursed their infants; and women who have used combination hormonal contraceptives, particularly if long term, are at markedly lower risk for ovarian cancer as they get older. We are talking about an uncommon cancer that is even less common if women have given birth, nursed their infants, or used combination contraceptives long term.
Dr. Faubion: Dr. Kapoor, what would you say regarding the increased risk they might incur if they do have their ovaries out?
Dr. Kapoor: As Dr. Kaunitz said, this is an uncommon cancer, and pursuing something to reduce the risk of an uncommon cancer does not benefit the community. That is also my counseling point to patients.
I also talk to them extensively about the risk associated with the ovaries being removed, and I tell them that although we have the option of giving them HT, it is hard to replicate the magic of nature. No matter what concoction or regimen we use, we cannot ensure reinstating health to what it was in the premenopausal state, because estrogen has such myriad effects on the body in so many different organ systems.
Reference
1. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.

Women who undergo bilateral salpingo-oophorectomy (BSO) for various indications prior to menopause experience a rapid decline in ovarian hormone levels and consequent vasomotor and other menopausal symptoms. In addition, the resulting estrogen deprivation is associated with such long-term adverse outcomes as osteoporosis and cardiovascular morbidity.
OBG M
Surgical vs natural menopause
Stephanie Faubion, MD, MBA, NCMP: Since the Women’s Health Initiative study was published in 2002,2 many clinicians have been fearful of using systemic HT in menopausal women, and HT use has declined dramatically such that only about 4% to 6% of menopausal women are now receiving systemic HT. Importantly, however, a group of younger menopausal women also are not receiving HT, and that is women who undergo BSO before they reach the average age of menopause, which in the United States is about age 52; this is sometimes referred to as surgical menopause or early surgical menopause. Early surgical menopause has different connotations for long-term health risks than natural menopause at the average age, and we are here to discuss these health effects and their management.
My name is Stephanie Faubion, and I am a women’s health internist and the Chair of the Department of Medicine at Mayo Clinic in Jacksonville, Florida, and Director of Mayo Clinic Women’s Health. I am here with 2 of my esteemed colleagues, Dr. Andrew Kaunitz and Dr. Ekta Kapoor.
Andrew M. Kaunitz, MD, NCMP: Hello, I am an ObGyn with the University of Florida College of Medicine in Jacksonville, with particular interests in contraception, menopause, and gynecologic ultrasonography.
Ekta Kapoor, MBBS, NCMP: And I am an endocrinologist at Mayo Clinic in Rochester with a specific interest in menopause and hormone therapy. I am also the Assistant Director for Mayo Clinic Women’s Health.
Higher-than-standard estrogen doses needed in younger menopausal women
Dr. Faubion: Let’s consider a couple of cases so that we can illustrate some important points regarding hormone management in women who have undergone BSO before the age of natural menopause.
Our first case patient is a woman who is 41 years of age and, because of adenomyosis, she will undergo a hysterectomy. She tells her clinician that she is very concerned about ovarian cancer risk because one of her good friends recently was diagnosed with ovarian cancer, and together they decide to remove her ovaries at the time of hysterectomy. Notably, her ovaries were healthy.
The patient is now menopausal postsurgery, and she is having significant hot flashes and night sweats. She visits her local internist, who is concerned about initiating HT. She is otherwise a healthy woman and does not have any contraindications to HT. Dr. Kaunitz, what would you tell her internist?
Dr. Kaunitz: We are dealing with 2 different issues in terms of decision making about systemic HT for this 41-year-old who has undergone BSO. First, as you mentioned, Dr. Faubion, she has bothersome hot flashes, or vasomotor symptoms. Unless there are contraindications, systemic HT would be appropriate. Although I might start treatment at standard doses, and the accompanying TABLE depicts standard doses for the 2 most common oral estrogen formulations as well as transdermal estradiol, it’s important to recognize that younger menopausal women often will need to use higher-than-standard doses.

For example, for a 53-year-old woman who has been menopausal for a year or 2 and now has bothersome symptoms, I might start her on estradiol 1 mg tablets with progestin if a uterus is present. However, in this 41-year-old case patient, while I might start treatment at a standard dose, I would anticipate increasing to higher doses, such as 1.5 or 2 mg of daily estradiol until she feels her menopausal symptoms are adequately addressed.
Dr. Faubion: It is important to note that sometimes women with early BSO tend to have more severe vasomotor symptoms. Do you find that sometimes a higher dose is required just to manage symptoms, Dr. Kaunitz?
Dr. Kaunitz: Absolutely, yes. The decision whether or not to use systemic HT might be considered discretionary or elective in the classic 53-year-old woman recently menopausal with hot flashes, a so-called spontaneously or naturally menopausal woman. But my perspective is that unless there are clear contraindications, the decision to start systemic HT in the 41-year-old BSO case patient is actually not discretionary. Unless contraindications are present, it is important not only to treat symptoms but also to prevent an array of chronic major health concerns that are more likely if we don’t prescribe systemic HT.
Continue to: Health effects of not using HT...
Health effects of not using HT
Dr. Faubion: Dr. Kapoor, can you describe the potential long-term adverse health consequences of not using estrogen therapy? Say the same 41-year-old woman does not have many bothersome symptoms. What would you do?
Dr. Kapoor: Thank you for that important question. Building on what Dr. Kaunitz said, in these patients there are really 2 issues that can seem to be independent but are not: The first relates to the immediate consequences of lack of estrogen, ie, the menopause-related symptoms, but the second and perhaps the bigger issue is the long-term risk associated with estrogen deprivation.
The symptoms in these women are often obvious as they can be quite severe and abrupt; one day these women have normal hormone levels and the next day, after BSO, suddenly their hormones are very low. So if symptoms occur, they are usually hard to miss, simply because they are very drastic and very severe.
Historically, patients and their clinicians have targeted these symptoms. Patients experience menopausal symptoms, they seek treatment, and then the clinicians basically titrate the treatment to manage these symptoms. That misses the bigger issue, however, which is that premature estrogen deprivation leads to a host of chronic health conditions, as Dr. Kaunitz mentioned. These mainly include increased risk for cardiovascular disease, diabetes, hypertension, dyslipidemia, increased risk of mortality, dementia, and osteoporosis.
Fairly strong observational evidence suggests that use of estrogen therapy given in replacement doses—doses higher than those typically used in women after natural menopause, therefore considered replacement doses—helps mitigate the risk of some of these adverse health conditions.
In these women, the bigger goal really is to reinstate the hormonal milieu that exists prior to menopause. To your point, Dr. Faubion, if I have a patient who is younger than 46 years, who has her ovaries taken out, and even if she has zero symptoms (and sometimes that does happen), I would still make a case for this patient to utilize hormone therapy unless there is a contraindication such as breast cancer or other estrogen-sensitive cancers.
Dr. Faubion: Again, would you aim for those higher doses rather than treat with the “lowest dose”?
Dr. Kapoor: Absolutely. My punchline to the patients and clinicians in these discussions is that the rules of the game are different for these women. We cannot extrapolate the risks and benefits of HT use in women after natural menopause to younger women who have surgical menopause. Those rules just do not apply with respect to both benefits and risks.
Dr. Faubion: I think it’s important to say that these same “rules” would apply if the women were to go through premature menopause for any other reason, too, such as chemotherapy, radiation therapy, or premature ovarian insufficiency for any number of reasons, including toxic, metabolic, or genetic causes and so on. Would that be true?
Dr. Kapoor: Yes, absolutely so.
Dr. Faubion: Dr. Kaunitz, do you want to add anything?
Dr. Kaunitz: In terms of practical or clinical issues regarding systemic HT management, for the woman in her early 50s who has experienced normal or natural spontaneous menopause, a starting dose of transdermal estradiol would be, for instance, a 0.05-mg patch, which is a patch that over 24 hours releases 0.05 mg of estradiol daily; or standard oral estrogen, including conjugated equine estrogen, a 0.625-mg tablet daily, or estradiol, a 1-mg tablet daily.
But in younger patients, we want to use higher doses. For a patch, for instance, I would aim for a 0.075- or 0.1-mg estradiol patch, which releases a higher daily dose of estradiol than the standard dose. For oral estrogen, the dose would be 0.9- or even 1.25-mg tablets of conjugated equine estrogen or 1.5 mg, which is a 1-mg plus a 0.5-mg estradiol tablet, or a 2-mg estradiol tablet. Estradiol does come in a 2-mg strength.
For oral estrogen, I prefer estradiol because it’s available as a generic medication and often available at a very low cost, sometimes as low as $4 a month from chain pharmacies.
Continue to: Usefulness of monitoring estradiol levels for dosage adjustment...
Usefulness of monitoring estradiol levels for dosage adjustment
Dr. Faubion: That’s a great point, and again it is important to emphasize that we are aiming to recreate the premenopausal hormonal milieu. If you were to check estradiol levels, that would be aiming for a premenopausal range of approximately 80 to 120 pg per mL. Dr. Kapoor, is there utility in monitoring estrogen levels?
Dr. Kapoor: Great question, Dr. Faubion, and as you know it’s a loaded one. We base this on empiric evidence. We know that if the hormonal milieu in a young patient is changed to a postmenopausal one, her risk for many chronic conditions is increased. So if we were to reinstate a premenopausal hormonal milieu, that risk would probably be reduced. It makes good sense to target an empiric goal of 80 to 120 pg per mL of estradiol, which is the average estradiol level in a premenopausal woman. If you were to ask me, however, are there randomized, controlled trial data to support this practice—that is, if you target that level, can you make sure that the risk of diabetes is lower or that the risk of heart disease is lower—that study has yet to be done, and it may not ever be done on a large scale. However, it intuitively makes good sense to target premenopausal estradiol levels.
Dr. Faubion: When might you check an estradiol level in this population? For example, if you are treating a patient with a 0.1-mg estradiol patch and she still has significant hot flashes, would it be useful to check the level?
Dr. Kapoor: It would. In my practice, I check estradiol levels on these patients on an annual basis, regardless of symptoms, but definitely in the patient who has symptoms. It makes good sense, because sometimes these patients don’t absorb the estrogen well, particularly if administered by the transdermal route.
A general rule of thumb is that in the average population, if a patient is on the 0.1-mg patch, for example, you would expect her level to be around 100. If it is much lower than that, which sometimes happens, that speaks for poor absorption. Options at that point would be to treat her with a higher dose patch, depending on what the level is, or switch to a different formulation, such as oral.
In instances in which I have treated patients with a 0.1-mg patch for example, and their estradiol levels are undetectable, that speaks for very poor absorption. For such patients I make a case for switching them to oral therapy. Most definitely that makes sense in a patient who is symptomatic despite treatment. But even for patients who don’t have symptoms, I like to target that level, acknowledging that there is no evidence as such to support this practice.
Dr. Faubion: Dr. Kaunitz, do you want to add anything?
Dr. Kaunitz: Yes, a few practical points. Although patches are available in a wider array of doses than oral estrogen formulations, the highest dose available is 0.1 mg. It’s important for clinicians to recognize that while checking serum levels when indicated can be performed in women using transdermal estradiol or patches, in women who are using oral estrogen, checking blood levels is not going to work well because serum estrogen levels have a daily peak and valley in women who use oral versus transdermal estradiol.
I also wanted to talk about progestins. Although many patients who have had a BSO prior to spontaneous menopause also have had a hysterectomy, others have an intact uterus associated with their BSO, so progestins must be used along with estrogen. And if we are using higher-than-standard doses of estrogen, we also need to use higher-than-standard doses of progestin.
In that classic 53-year-old woman I referred to who had spontaneous normal menopause, if she is taking 1 mg of estradiol daily, or a 0.05-mg patch, or 0.625 mg of conjugated equine estrogen, 2.5 mg of medroxyprogesterone is fine. In fact, that showed excellent progestational protection of the endometrium in the Women’s Health Initiative and in other studies.
However, if we are going to use double the estrogen dose, we should increase the progestin dose too. In some of my patients on higher estrogen doses who have an intact uterus, I’ll use 5 or even 10 mg of daily medroxyprogesterone acetate to ensure adequate progestational suppression.
Dr. Faubion: Another practical tip is that if one is using conjugated equine estrogens, measuring the serum estradiol levels is not useful either.
Dr. Kaunitz: I agree.
Continue to: Oral contraceptives as replacement HT...
Oral contraceptives as replacement HT
Dr. Faubion: Would you comment on use of a birth control pill in this circumstance? Would it be optimal to use a postmenopausal HT regimen as opposed to a birth control pill or combined hormonal contraception?
Dr. Kapoor: In this younger population, sometimes it seems like a more socially acceptable decision to be on a birth control option than on menopausal HT. But there are some issues with being on a contraceptive regimen. One is that we end up using estrogen doses much higher than what is really needed for replacement purposes. It is also a nonphysiologic way of replacement in another sense—as opposed to estradiol, which is the main hormone made by the ovaries, the hormonal contraceptive regimens contain the synthetic estrogen ethinyl estradiol for the most part.
The other issue that is based on some weak evidence is that it appears that the bone health outcomes are probably inferior with combined hormonal contraception. For these reasons, regimens that are based on replacement doses of estradiol are preferred.
Dr. Faubion: Right, although the data are somewhat weak, I agree that thus far it seems optimal to utilize a postmenopausal regimen for various reasons. Dr. Kaunitz, anything to add?
Dr. Kaunitz: Yes, to underscore Dr. Kapoor’s point, a common oral contraceptive that contains 20 µg of ethinyl estradiol is substantially more estrogenic than 1.0 or 2.0 mg of micronized oral estradiol.
Also consider that a 20-µg ethinyl estradiol oral contraceptive may increase the risk of venous thromboembolism more than menopausal doses of oral estradiol, whether it be a micronized estradiol or conjugated equine estrogen.
Dr. Faubion: So the risk may be greater with oral combined hormonal contraception as well?
Dr. Kaunitz: One thing we can do is explain to our patients that their ovaries, prior to surgery or prior to induced menopause, were making substantial quantities of estradiol. Whether we prescribe a patch or oral micronized estradiol, this estrogen is identical to the hormone that their ovaries were making prior to surgery or induced menopause.
Breast cancer concerns
Dr. Faubion: Let’s consider a more complicated case. A 35-year-old woman has an identified BRCA1 mutation; she has not had any cancers but has undergone risk-reducing BSO and her uterus remains. Is this woman a candidate for HT? At what dose, and for how long? Dr. Kaunitz, why don’t you start.
Dr. Kaunitz: That is a challenging case but one that I think our readers will find interesting and maybe even provocative.
We know that women with BRCA1 mutations, the more common of the 2 BRCA mutations, have a very high risk of developing epithelial ovarian cancer at a young age. For this reason, our colleagues in medical oncology who specialize in hereditary ovarian/breast cancer syndromes recommend prophylactic risk-reducing—and I would also say lifesaving—BSO with or without hysterectomy for women with BRCA1 mutations.
However, over the years there has been tremendous reluctance among physicians caring for BRCA patients and the women themselves—I use the term “previvors” to describe BRCA carriers who have not been diagnosed with breast or ovarian cancer—to use HT after BSO because of concerns that HT might increase breast cancer risk in women who are already at high risk for breast cancer.
I assume, Dr. Faubion, that in this case the woman had gynecologic surgery but continues to have intact breasts. Is that correct?
Dr. Faubion: That is correct.
Dr. Kaunitz: Although the assumption has been that it is not safe to prescribe HT in this setting, in fact, the reported cohort studies that have looked at this issue have not found an elevated risk of breast cancer when replacement estrogen, with or without progestin, is prescribed to BRCA1 previvors with intact breasts.
Given what Dr. Kapoor said regarding the morbidity that is associated with BSO without replacement of physiologic estrogen, and also given the severe symptoms that so many of these young menopausal women experience, in my practice I do prescribe estrogen or estrogen-progestin therapy and focus on the higher target doses that we discussed for the earlier case patient who had a hysterectomy for abnormal uterine bleeding with adenomyosis.
Dr. Faubion: Dr. Kapoor, do you agree with this approach? How long would you continue therapy?
Dr. Kapoor: First, in this BRCA1 case we need to appreciate that the indication for the BSO is a legitimate one, in contrast to the first case in which the ovaries were removed in a patient whose average risk of ovarian cancer was low. It is important to recognize that surgery performed in this context is the right thing to do because it does significantly reduce the risk of ovarian cancer.
The second thing to appreciate is that while we reduce the risk of ovarian cancer significantly and make sure that these patients survive longer, it’s striking a fine balance in that you want to make sure that their morbidity is not increased as a result of premature estrogen deprivation.
As Dr. Kaunitz told us, the evidence that we have so far, which granted is not very robust but is fairly strong observational evidence, suggests that the risk of breast cancer is not elevated when these patients are treated with replacement doses of HT.
Having said that, I do have very strong discussions with my patients in this category about having the risk-reducing bilateral mastectomy also, because if they were to get breast cancer because of their increased genetic predisposition, the cancer is likely to grow faster if the patient is on HT. So one of my counseling points to patients is that they strongly consider bilateral mastectomy, which reduces their breast cancer risk by more than 90%. At the same time, I also strongly endorse using HT in replacement doses for the reasons that we have already stated.
Dr. Faubion: Continue HT until age 50 or 52?
Dr. Kapoor: Definitely until that age, and possibly longer, depending on their symptoms. The indications for treating beyond the age of natural menopause are much the same as for women who experience natural menopause.
Dr. Faubion: That is assuming they had a bilateral mastectomy?
Dr. Kapoor: Yes.
Continue to: Continuing HT until the age of natural menopause...
Continuing HT until the age of natural menopause
Dr. Kaunitz: Dr. Kapoor brings up the important point of duration of systemic HT. I agree that similar considerations apply both to the healthy 41-year-old who had a hysterectomy for abnormal uterine bleeding and to the 35-year-old who had risk-reducing surgery because of her BRCA1 mutation.
In the 2 cases, both to treat symptoms and to prevent chronic diseases, it makes sense to continue HT at least until the age of natural menopause. That is consistent with 2017 guidance from The North American Menopause Society (NAMS) position statement on the use of systemic HT, that is, continuing systemic HT at least until the age of natural menopause.3 Then at that point, continuing or discontinuing systemic HT becomes discretionary, and that would be true for both cases. If the patient is slender or has a strong family history of osteoporosis, that tends to push the patient more in terms of continuing systemic HT. Those are just some examples, and Dr. Kapoor may want to detail other relevant considerations.
Dr. Kapoor: I completely agree. The decision is driven by symptoms that are not otherwise well managed, for example, with nonhormone strategies. If we have any concerns utilizing HT beyond the age of natural menopause, then nonhormonal options can be considered; but sometimes those are not as effective. And bone health is very important. You want to avoid using bisphosphonates in younger women and reserve them for older patients in their late 60s and 70s. Hormone therapy use is a very reasonable strategy to prevent bone loss.
Dr. Kaunitz: It is also worth mentioning that sometimes the woman involved in shared decision making with her clinician decides to stop systemic HT. In that setting, should the patient start developing new-onset dyspareunia, vaginal dryness, or other genital or sexuality-related concerns, it takes very little for me to advise that she start low-dose local vaginal estrogen therapy.
Dr. Faubion: In either scenario, if a woman were to develop symptoms consistent with genitourinary syndrome of menopause (GSM), would you use vaginal estrogen in addition to the systemic estrogen or alone after the woman elected to discontinue systemic therapy?
Dr. Kapoor: Yes to both, I would say.
Dr. Kaunitz: As my patients using systemic HT age, often I will lower the dose. For instance, the dose I use in a 53-year-old will be higher than when she is 59 or 62. At the same time, as we lower the dose of systemic estrogen therapy, symptoms of vaginal atrophy or GSM often will appear, and these can be effectively treated by adding low-dose vaginal estrogen therapy. A number of my patients, particularly those who are on lower-than-standard doses of systemic HT, are also using low-dose vaginal estrogen therapy.
There is a “hybrid” product available: the 90-day estradiol vaginal ring. Estring is a low-dose, 2-mg, 90-day estradiol ring that is very useful, but it is effective only for treating GSM or vaginal atrophy. A second menopausal vaginal estradiol ring, Femring, is available in 2 doses: 0.05 mg/day and 0.1 mg/day. These are very effective in treating both systemic issues, such as vasomotor symptoms or prevention of osteoporosis, and very effective in treating GSM or vaginal atrophy. One problem is that Femring, depending on insurance coverage, can be very expensive. It’s not available as a generic, so for insurance or financial reasons I don’t often prescribe it. If I could remove those financial barriers, I would prescribe Femring more often because it is very useful.
Dr. Faubion: You raise an important point, and that is, for women who have been on HT for some time, clinicians often feel the need to slowly reduce the dose. Would you do that same thing, Dr. Kapoor, for a 40-year-old woman? Would you reduce the dose as she approaches age 50? Is there pressure that “she shouldn’t be on that much estrogen”?
Dr. Kapoor: No, I would not feel pressured until the patient turns at least 46. I bring up age 46 because the average age range for menopause is 46 to 55. After that, if there is any concern, we can decrease the dose to half and keep the patient on that until she turns 50 or 51. But most of my patients are on replacement doses until the average age of menopause, which is around 51 years, and that’s when you reduce the dose to that of the typical HT regimens used after natural menopause.
Sometimes patients are told something by a friend or they have read something and they worry about the risk of 2 things. One is breast cancer and the other is venous thromboembolism (VTE), and that may be why they want to be on a lower dose. I counsel patients that while the risk of VTE is real with HT, it is the women after natural menopause who are at risk—because age itself is a risk for VTE—and it also has to do with the kind of HT regimen that a patient is on. High doses of oral estrogens and certain progestogens increase the risk. But again, for estradiol used in replacement doses and the more common progestogens that we now use in practice, such as micronized progesterone, the risk is not the same. The same goes for breast cancer. My biggest message to patients and clinicians who take care of these patients is that the rules that apply to women after natural menopause just do not apply to this very different patient population.
Dr. Faubion: Thank you, Dr. Kaunitz and Dr. Kapoor, for sharing your knowledge and experience. ●
Systemic HT past the age of 65
Dr. Kaunitz: Another practical issue relates to long-term or extended use of systemic HT. It’s not infrequent in my practice to receive mail and faxes from insurance carriers of systemic HT users who are age 65 and older in which the company refers to the American Geriatrics Society’s Beers criteria for potentially inappropriate medication use in older adults,1 suggesting that systemic HT is inappropriate for all women over age 65. In this age group, I use lower doses if I am continuing systemic HT. But the good news is that both NAMS and the American College Obstetricians and Gynecologists indicate that arbitrarily stopping systemic HT at age 65 or for any other arbitrary reason is inappropriate, and that decisions about continuing or discontinuing therapy should be made on an individualized basis using shared decision making. That’s an important message for our readers.
Counseling regarding elective BSO
Dr. Faubion: One final note about elective BSO in the absence of a genetic mutation that predisposes to increased ovarian or breast cancer risk. Fortunately, we have seen rates of oophorectomy before the age of natural menopause decline, but what would your advice be to women or clinicians of these women who say they are “just afraid of ovarian cancer and would like to have their ovaries removed before the age of natural menopause”?
Dr. Kaunitz: If patients have increased anxiety about ovarian cancer and yet they themselves are not known to be at elevated risk, I emphasize that, fortunately, ovarian cancer is uncommon. It is much less common than other cancers the patient might be familiar with, such as breast or colon or lung cancer. I also emphasize that women who have given birth, particularly multiple times; women who nursed their infants; and women who have used combination hormonal contraceptives, particularly if long term, are at markedly lower risk for ovarian cancer as they get older. We are talking about an uncommon cancer that is even less common if women have given birth, nursed their infants, or used combination contraceptives long term.
Dr. Faubion: Dr. Kapoor, what would you say regarding the increased risk they might incur if they do have their ovaries out?
Dr. Kapoor: As Dr. Kaunitz said, this is an uncommon cancer, and pursuing something to reduce the risk of an uncommon cancer does not benefit the community. That is also my counseling point to patients.
I also talk to them extensively about the risk associated with the ovaries being removed, and I tell them that although we have the option of giving them HT, it is hard to replicate the magic of nature. No matter what concoction or regimen we use, we cannot ensure reinstating health to what it was in the premenopausal state, because estrogen has such myriad effects on the body in so many different organ systems.
Reference
1. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
- Kaunitz AM, Kapoor E, Faubion S. Treatment of women after bilateral salpingo-oophorectomy performed prior to natural menopause. JAMA. 2021;326:1429-1430.
- Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
- North American Menopause Society. The 2017 hormone therapy position statement of The North American Menopause Society. J North Am Menopause Soc. 2017;24: 728-753.
- Kaunitz AM, Kapoor E, Faubion S. Treatment of women after bilateral salpingo-oophorectomy performed prior to natural menopause. JAMA. 2021;326:1429-1430.
- Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
- North American Menopause Society. The 2017 hormone therapy position statement of The North American Menopause Society. J North Am Menopause Soc. 2017;24: 728-753.
Researchers eye cannabis for gynecologic pain
Many women use cannabis to help manage gynecologic pain conditions.
When patients ask or tell clinicians about this treatment approach, however, few if any controlled trials exist to inform medical guidance.
A recent review of studies in this area presents a “thorough analysis of this very relevant topic,” said Erin A. Blake, MD, of Presbyterian Cancer Care, Rio Rancho, N.M..
The findings “are consistent with my anecdotal clinical findings as well as the results of my own research,” Dr. Blake said. “Cannabis products represent an underutilized but likely effective modality to relieve pain and other symptoms experienced by our patients.”
Mostly in the dark
Cannabis products “are unregulated and the data we have surrounding them is extremely limited due to outdated federal laws,” said Dr. Blake, who in 2019 described nonprescription cannabis use for symptom management by women with gynecologic malignancies. “Our ability to practice evidence-based medicine related to cannabis products will be limited until we are legally and financially able to design trials to evaluate them in a controlled fashion.”
For the new review, Jenell S. Coleman, MD, MPH, with Johns Hopkins University, Baltimore, and colleagues, identified 16 studies since 1990, including Dr. Blake’s, that examined the use of cannabinoids for managing pain from gynecologic conditions.
Dr. Coleman and her coauthors, Angela L. Liang and Erin L. Gingher, analyzed eight cross-sectional studies, six prospective studies, and two randomized controlled trials.
Patients who used cannabis tended to do so “multiple times per week, and they used a variety of delivery methods and a wide range of doses,” the authors said. “One of the most common reasons for cannabis use was pain management, and all the cross-sectional studies found that most women reported pain relief with cannabis use, especially among women who used a combination of CBD plus THC compared with either cannabinoid alone.”
Cross-sectional studies included patients with chronic pelvic pain (in two of the studies), vulvodynia (one), endometriosis (four), and gynecologic malignancy (two). These studies included between 36 and 3,426 participants and were conducted in the United States, Canada, Australia, and New Zealand.
In one Australian study, for example, Armour and colleagues asked 484 patients with endometriosis to rate the effectiveness of self-management strategies, including cannabis, heat, diet, and exercise, for reducing pelvic pain. Cannabis was used by 13% of the participants and had the highest average effectiveness rating: 7.6 on a 10-point scale.
In some cases, patients who use cannabis may decrease their use of other pain medications, the review found.
Cannabis side effects may include dry mouth, sleepiness, increased appetite, palpitations, and a “high” associated with THC.
Enhancing endogenous cannabinoids
The six prospective cohort studies and two randomized controlled trials examined the effectiveness of compounds – including palmitoylethanolamide (PEA) and a fatty acid amide hydrolase inhibitor – that can enhance endogenous cannabinoids.
Studies of PEA combined with antioxidants showed that these treatments significantly decreased pain from primary dysmenorrhea, pelvic pain, and interstitial cystitis. PEA-combination medications were well tolerated, with nausea and spotting as potential side effects.
On the other hand, a study that assessed a fatty acid amide hydrolase inhibitor found that it did not decrease pain from interstitial cystitis.
Dr. Coleman began reviewing the endocannabinoid system and cannabis research after hearing from patients who were using cannabis for pelvic pain.
Seeing various preclinical data that suggest cannabis could be useful for pain conditions came as a surprise.
Still, the existing evidence base for clinical effectiveness is poor quality, Dr. Coleman said in an interview. Rigorous trials are needed.
“It is a whole field that is just waiting for the U.S. to do something in terms of legalization so that we can actually study to see, does this make sense?” Dr. Coleman said.
Cannabis should not be used while pregnant
In a recent meta-analysis based on data from nearly 60,000 individuals, women who used marijuana during pregnancy were at increased risk for adverse neonatal outcomes such as low birth weight and preterm birth. Study author Greg J. Marchand, MD, of the Marchand Institute for Minimally Invasive Surgery, Mesa, Ariz., noted that the results will force some difficult decisions for mothers who use marijuana to treat medical problems, and that there may not be good substitute treatments for some of these conditions, especially chronic pain and anxiety.
Dr. Coleman disclosed investments in a cannabis exchange-traded fund. Dr. Blake and Dr. Marchand had no relevant financial disclosures.
Many women use cannabis to help manage gynecologic pain conditions.
When patients ask or tell clinicians about this treatment approach, however, few if any controlled trials exist to inform medical guidance.
A recent review of studies in this area presents a “thorough analysis of this very relevant topic,” said Erin A. Blake, MD, of Presbyterian Cancer Care, Rio Rancho, N.M..
The findings “are consistent with my anecdotal clinical findings as well as the results of my own research,” Dr. Blake said. “Cannabis products represent an underutilized but likely effective modality to relieve pain and other symptoms experienced by our patients.”
Mostly in the dark
Cannabis products “are unregulated and the data we have surrounding them is extremely limited due to outdated federal laws,” said Dr. Blake, who in 2019 described nonprescription cannabis use for symptom management by women with gynecologic malignancies. “Our ability to practice evidence-based medicine related to cannabis products will be limited until we are legally and financially able to design trials to evaluate them in a controlled fashion.”
For the new review, Jenell S. Coleman, MD, MPH, with Johns Hopkins University, Baltimore, and colleagues, identified 16 studies since 1990, including Dr. Blake’s, that examined the use of cannabinoids for managing pain from gynecologic conditions.
Dr. Coleman and her coauthors, Angela L. Liang and Erin L. Gingher, analyzed eight cross-sectional studies, six prospective studies, and two randomized controlled trials.
Patients who used cannabis tended to do so “multiple times per week, and they used a variety of delivery methods and a wide range of doses,” the authors said. “One of the most common reasons for cannabis use was pain management, and all the cross-sectional studies found that most women reported pain relief with cannabis use, especially among women who used a combination of CBD plus THC compared with either cannabinoid alone.”
Cross-sectional studies included patients with chronic pelvic pain (in two of the studies), vulvodynia (one), endometriosis (four), and gynecologic malignancy (two). These studies included between 36 and 3,426 participants and were conducted in the United States, Canada, Australia, and New Zealand.
In one Australian study, for example, Armour and colleagues asked 484 patients with endometriosis to rate the effectiveness of self-management strategies, including cannabis, heat, diet, and exercise, for reducing pelvic pain. Cannabis was used by 13% of the participants and had the highest average effectiveness rating: 7.6 on a 10-point scale.
In some cases, patients who use cannabis may decrease their use of other pain medications, the review found.
Cannabis side effects may include dry mouth, sleepiness, increased appetite, palpitations, and a “high” associated with THC.
Enhancing endogenous cannabinoids
The six prospective cohort studies and two randomized controlled trials examined the effectiveness of compounds – including palmitoylethanolamide (PEA) and a fatty acid amide hydrolase inhibitor – that can enhance endogenous cannabinoids.
Studies of PEA combined with antioxidants showed that these treatments significantly decreased pain from primary dysmenorrhea, pelvic pain, and interstitial cystitis. PEA-combination medications were well tolerated, with nausea and spotting as potential side effects.
On the other hand, a study that assessed a fatty acid amide hydrolase inhibitor found that it did not decrease pain from interstitial cystitis.
Dr. Coleman began reviewing the endocannabinoid system and cannabis research after hearing from patients who were using cannabis for pelvic pain.
Seeing various preclinical data that suggest cannabis could be useful for pain conditions came as a surprise.
Still, the existing evidence base for clinical effectiveness is poor quality, Dr. Coleman said in an interview. Rigorous trials are needed.
“It is a whole field that is just waiting for the U.S. to do something in terms of legalization so that we can actually study to see, does this make sense?” Dr. Coleman said.
Cannabis should not be used while pregnant
In a recent meta-analysis based on data from nearly 60,000 individuals, women who used marijuana during pregnancy were at increased risk for adverse neonatal outcomes such as low birth weight and preterm birth. Study author Greg J. Marchand, MD, of the Marchand Institute for Minimally Invasive Surgery, Mesa, Ariz., noted that the results will force some difficult decisions for mothers who use marijuana to treat medical problems, and that there may not be good substitute treatments for some of these conditions, especially chronic pain and anxiety.
Dr. Coleman disclosed investments in a cannabis exchange-traded fund. Dr. Blake and Dr. Marchand had no relevant financial disclosures.
Many women use cannabis to help manage gynecologic pain conditions.
When patients ask or tell clinicians about this treatment approach, however, few if any controlled trials exist to inform medical guidance.
A recent review of studies in this area presents a “thorough analysis of this very relevant topic,” said Erin A. Blake, MD, of Presbyterian Cancer Care, Rio Rancho, N.M..
The findings “are consistent with my anecdotal clinical findings as well as the results of my own research,” Dr. Blake said. “Cannabis products represent an underutilized but likely effective modality to relieve pain and other symptoms experienced by our patients.”
Mostly in the dark
Cannabis products “are unregulated and the data we have surrounding them is extremely limited due to outdated federal laws,” said Dr. Blake, who in 2019 described nonprescription cannabis use for symptom management by women with gynecologic malignancies. “Our ability to practice evidence-based medicine related to cannabis products will be limited until we are legally and financially able to design trials to evaluate them in a controlled fashion.”
For the new review, Jenell S. Coleman, MD, MPH, with Johns Hopkins University, Baltimore, and colleagues, identified 16 studies since 1990, including Dr. Blake’s, that examined the use of cannabinoids for managing pain from gynecologic conditions.
Dr. Coleman and her coauthors, Angela L. Liang and Erin L. Gingher, analyzed eight cross-sectional studies, six prospective studies, and two randomized controlled trials.
Patients who used cannabis tended to do so “multiple times per week, and they used a variety of delivery methods and a wide range of doses,” the authors said. “One of the most common reasons for cannabis use was pain management, and all the cross-sectional studies found that most women reported pain relief with cannabis use, especially among women who used a combination of CBD plus THC compared with either cannabinoid alone.”
Cross-sectional studies included patients with chronic pelvic pain (in two of the studies), vulvodynia (one), endometriosis (four), and gynecologic malignancy (two). These studies included between 36 and 3,426 participants and were conducted in the United States, Canada, Australia, and New Zealand.
In one Australian study, for example, Armour and colleagues asked 484 patients with endometriosis to rate the effectiveness of self-management strategies, including cannabis, heat, diet, and exercise, for reducing pelvic pain. Cannabis was used by 13% of the participants and had the highest average effectiveness rating: 7.6 on a 10-point scale.
In some cases, patients who use cannabis may decrease their use of other pain medications, the review found.
Cannabis side effects may include dry mouth, sleepiness, increased appetite, palpitations, and a “high” associated with THC.
Enhancing endogenous cannabinoids
The six prospective cohort studies and two randomized controlled trials examined the effectiveness of compounds – including palmitoylethanolamide (PEA) and a fatty acid amide hydrolase inhibitor – that can enhance endogenous cannabinoids.
Studies of PEA combined with antioxidants showed that these treatments significantly decreased pain from primary dysmenorrhea, pelvic pain, and interstitial cystitis. PEA-combination medications were well tolerated, with nausea and spotting as potential side effects.
On the other hand, a study that assessed a fatty acid amide hydrolase inhibitor found that it did not decrease pain from interstitial cystitis.
Dr. Coleman began reviewing the endocannabinoid system and cannabis research after hearing from patients who were using cannabis for pelvic pain.
Seeing various preclinical data that suggest cannabis could be useful for pain conditions came as a surprise.
Still, the existing evidence base for clinical effectiveness is poor quality, Dr. Coleman said in an interview. Rigorous trials are needed.
“It is a whole field that is just waiting for the U.S. to do something in terms of legalization so that we can actually study to see, does this make sense?” Dr. Coleman said.
Cannabis should not be used while pregnant
In a recent meta-analysis based on data from nearly 60,000 individuals, women who used marijuana during pregnancy were at increased risk for adverse neonatal outcomes such as low birth weight and preterm birth. Study author Greg J. Marchand, MD, of the Marchand Institute for Minimally Invasive Surgery, Mesa, Ariz., noted that the results will force some difficult decisions for mothers who use marijuana to treat medical problems, and that there may not be good substitute treatments for some of these conditions, especially chronic pain and anxiety.
Dr. Coleman disclosed investments in a cannabis exchange-traded fund. Dr. Blake and Dr. Marchand had no relevant financial disclosures.
FROM OBSTETRICS & GYNECOLOGY
Dapivirine vaginal ring for HIV prevention no longer under consideration by the FDA
Tosha Rogers, MD, is a one-woman HIV prevention evangelist. For nearly a decade now, the Atlanta-based ob/gyn has been on a mission to increase her gynecological colleagues’ awareness and prescribing of the oral HIV prevention pill. At the same time, she’s been tracking the development of a flexible vaginal ring loaded with a month’s worth of the HIV prevention medication dapivirine. That, she thought, would fit easily into women’s lives and into the toolbox of methods women already use to prevent pregnancy.
But now she’s not sure when – or if – the ring will find its way to her patients. In December, the ring’s maker, the International Partnership for Microbicides (IPM), pulled its application for FDA approval for the pre-exposure prophylaxis (PrEP) ring. Now, one year after the World Health Organization recommended the ring for member nations, there appears to be no path forward in the United States for either the dapivirine-only ring or an approach Dr. Rogers said would change the game: a vaginal ring that supplies both contraception and HIV prevention.
“It would take things to a whole other level,” she said. “It sucks that this happened, and I do think it was not anything medical. I think it was everything political.”
That leaves cisgender women – especially the Black and Latinx women who make up the vast majority of women who acquire HIV every year – with two HIV prevention options. One is the daily pill, first approved in 2012. It’s now generic but previously sold as Truvada by Gilead Sciences. The other is monthly injectable cabotegravir long-acting (Apretude). Another HIV prevention pill, tenofovir alafenamide/emtricitabine (Descovy), is approved for gay men and transgender women but not cisgender women.
Vagina-specific protection from HIV
The WHO recommendation for the vaginal ring was followed last July by a positive opinion from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) for women in low- and middle-income countries outside the European Union.
The flexible silicone ring, similar to the hormonal NuvaRing contraceptive, works by slowly releasing the antiretroviral dapivirine directly into the vaginal canal, thereby protecting women who might be exposed to the virus through vaginal sex only. Because the medicine stays where it’s delivered and doesn’t circulate through the body, it has been found to be extremely safe with few adverse events.
However, in initial studies, the ring was found to be just 27% effective overall. Later studies, where scientists divided women by how much drug was missing from the ring – a proxy for use – found that higher use was associated with higher protection (as much as 54%). By comparison, Truvada has been found to be up to 99% effective when used daily, though it can take up to 21 days to be available in the vagina in high enough concentrations to protect women from vaginal exposure. And the HIV prevention shot was found to be 90% more effective than that in a recent trial of the two methods conducted by the HIV Prevention Trials Network.
This, and an orientation away from topical HIV prevention drugs and toward systemic options, led the National Institute of Allergy and Infectious Diseases (NIAID) to discontinue funding for such projects under its Microbicide Trials Network.
“Clearly you want to counsel women to use the highest efficacy method, and that is part of our label,” Zeda Rosenberg, ScD, IPM’s founder and chief executive officer, told this news organization. “Women should not choose the ring if they can and will use oral PrEP, and I would argue it should be the same thing for [cabotegravir shots]. But if they can’t or don’t want to – and we know that especially many young women don’t want to use systemic methods – then the dapivirine ring is a great option.”
Still, Dr. Rosenberg said that the gap in efficacy, the relatively small number of women affected by HIV in the U.S. compared with gay and bisexual men, and the emergence of products like the HIV prevention shot cabotegravir, made it “very unlikely” that FDA regulators would approve the ring. And rather than be “distracted” by the FDA process, Dr. Rosenberg said IPM chose to concentrate on the countries where the ring has already been approved or where women make up the vast majority of people affected by HIV.
Zimbabwe publicly announced it has approved the ring, and three other countries may have approved it, according to Dr. Rosenberg. She declined to name them, saying they had requested silence while they formulate their new HIV prevention guidelines. Aside from Zimbabwe, the other countries where women participated in the ring clinical trials were South Africa, Malawi, and Uganda.
“The U.S. population ... has widespread access to oral PrEP, which is unlike countries in Africa, and which would have widespread access to injectable cabotegravir,” she said. “The U.S. FDA may not see choice in the same way that African women and African activists and advocates see the need for choice.”
But women’s rates of accessing HIV prevention medications in the U.S. continues to be frustratingly low. At the end of 2018, just 7% of women who could benefit from HIV prevention drugs were taking them, according to Centers for Disease Control and Prevention data.
New CDC guidelines recommend clinicians talk to every sexually active adult and adolescent about HIV prevention medications at least once and prescribe it to anyone who asks for it, whether or not they understand their patients’ HIV risks. However, research continues to show that clinicians struggle with willingness to prescribe PrEP to Black women, and the American College of Obstetrics and Gynecology’s committee opinion on managing women using HIV prevention drugs has not been updated to reflect the new guidelines. And while the HIV prevention shot is approved for women and its maker ViiV Healthcare is already initiating postmarket studies of the ring in key populations including women, there are lots of things that need to line up in order for clinicians to be willing to stock it and prescribe it to women.
From where Dázon Dixon Diallo, executive director of the nonprofit SisterLove, sits, the decision to withdraw the ring from FDA consideration and the FDA’s seeming argument that the epidemiology in the U.S. doesn’t warrant the ring’s approval is a slap in the face to the Black women who have led the movement to end HIV in the U.S. for decades.
“No matter how you slice it, we’re talking about Black women, and then we’re talking about brown women,” said Ms. Diallo. “The value [they place on us] from a government standpoint, from a political standpoint, from a public health standpoint is just woeful. It’s woeful and it’s disrespectful and it’s insulting and I’m sick of it.”
‘America sneezes and Africa catches a cold’
When she first heard the decision to pull the ring from FDA consideration, Yvette Raphael, the South Africa-based executive director of Advocates for the Prevention of HIV in Africa, started asking, “What can we do to help our sisters in America get this ring?” And then she started worrying about other women in her own country and those nearby.
“The FDA plays a big role,” she said. “You know, America sneezes and Africa catches a cold.”
She worries that IPM’s decision to withdraw the ring from FDA consideration will signal to regulators in other countries either (a) that they should not approve it or (b) in countries where it’s already been approved but guidelines have not been issued, that they won’t invest money in rolling it out to women in those countries – especially now with the U.S. approval of the prevention shot. In much of Africa, ministries of health prefer to provide injectable contraception, often giving women few or no other options. But women, she said, think about more than administration of the drug. They look at if it’s an easier option for them to manage.
“This is a long journey, an emotional one too, for women in South Africa, because the idea of a microbicide is one of the ideas that came directly from women in South Africa,” she said. “[The jab] can be seen as a solution to all. We can just give jabs to all the women. And after all, we know that women don’t adhere, so we can just grab them.”
Dr. Rosenberg pointed to the positive opinion from the EMA as another “rigorous review” process that she said ought to equally influence ministries of health in countries where women tested the ring. And she pointed to the WHO statement released last month, the same day as IPM’s announcement that it was withdrawing the ring from FDA considerations, recommitting the ring as a good option in sub-Saharan Africa: “The U.S. FDA decision is not based on any new or additional data on efficacy and safety,” it stated. “WHO will continue to support countries as they consider whether to include the [dapivirine vaginal ring]. WHO recognizes that country decisionmaking will vary based on their context and that women’s voices remain central to discussions about their prevention choices.”
Dual action ring on the horizon, but not in U.S.
What this means, though, is that the next step in the ring’s development – the combination dapivirine ring with contraceptive levonorgestrel (used in the Mirena intrauterine device) – may not come to the U.S., at least for a long while.
“It’s not out of the question,” Dr. Rosenberg said of conducting HIV/pregnancy prevention ring trials in the U.S. “But without the approval of the dapivirine-only ring by FDA, I imagine they would want to see new efficacy data on dapivirine. That is a very difficult hill to climb. There would have to be an active control group [using oral PrEP or injectable cabotegravir], and it would be very difficult for the dapivirine ring to be able to go head-to-head for either noninferiority and certainly for superiority.”
The study would need to be quite large to get enough results to prove anything, and IPM is a research organization, not a large pharmaceutical company with deep enough pockets to fund that, she said. Raising those funds “would be difficult.”
In addition to NIAID discontinuing its funding for the Microbicides Trials Network, a new 5-year, $85 million research collaboration through USAID hasn’t slated any money to fund trials of the combination HIV prevention and contraceptive ring, according to Dr. Rosenberg.
But that doesn’t mean avenues for its development are closed. NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) is currently funding a phase 1/2 trial of the combination ring, and IPM continues to receive funding from research agencies in Germany, the Netherlands, Denmark, and Ireland. And this means, she said, that the E.U. – not the U.S. – is where they would seek approval for a combination ring first.
That leaves Ms. Rafael and Ms. Diallo debating how to work together to push the FDA – and maybe IPM – to reconsider the ring. For instance, Ms. Diallo suggested that instead of seeking an indication for all women, the FDA might consider the ring for women with very high risk of HIV, such as sex workers or women with HIV positive partners not on treatment. And she said that this has to be bigger than HIV prevention. It has to be about the ways in which women’s health issues in general lag at the FDA. For instance, she pointed to the movement to get contraceptive pills available over the counter, fights against FDA rulings on hormone replacement therapy, and fights for emergency contraception.
In the meantime, ob/gyn Dr. Rogers is expecting access to the ring to follow a similar path as the copper IUD, which migrated to the U.S. from Europe, where it has been among the most popular contraceptive methods for women.
“Contrary to what we may think, we are not innovators, especially for something like this,” she said. “Once we see it is working and doing a good job – that women in Europe love it – then someone here is going to pick it up and make it as if it’s the greatest thing. But for now, I think we’re going to have to take a back seat to Europe.”
Ms. Diallo reports receiving fees from Johnson & Johnson, ViiV Healthcare, and Gilead Sciences. Dr. Rosenberg and Dr. Rogers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Tosha Rogers, MD, is a one-woman HIV prevention evangelist. For nearly a decade now, the Atlanta-based ob/gyn has been on a mission to increase her gynecological colleagues’ awareness and prescribing of the oral HIV prevention pill. At the same time, she’s been tracking the development of a flexible vaginal ring loaded with a month’s worth of the HIV prevention medication dapivirine. That, she thought, would fit easily into women’s lives and into the toolbox of methods women already use to prevent pregnancy.
But now she’s not sure when – or if – the ring will find its way to her patients. In December, the ring’s maker, the International Partnership for Microbicides (IPM), pulled its application for FDA approval for the pre-exposure prophylaxis (PrEP) ring. Now, one year after the World Health Organization recommended the ring for member nations, there appears to be no path forward in the United States for either the dapivirine-only ring or an approach Dr. Rogers said would change the game: a vaginal ring that supplies both contraception and HIV prevention.
“It would take things to a whole other level,” she said. “It sucks that this happened, and I do think it was not anything medical. I think it was everything political.”
That leaves cisgender women – especially the Black and Latinx women who make up the vast majority of women who acquire HIV every year – with two HIV prevention options. One is the daily pill, first approved in 2012. It’s now generic but previously sold as Truvada by Gilead Sciences. The other is monthly injectable cabotegravir long-acting (Apretude). Another HIV prevention pill, tenofovir alafenamide/emtricitabine (Descovy), is approved for gay men and transgender women but not cisgender women.
Vagina-specific protection from HIV
The WHO recommendation for the vaginal ring was followed last July by a positive opinion from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) for women in low- and middle-income countries outside the European Union.
The flexible silicone ring, similar to the hormonal NuvaRing contraceptive, works by slowly releasing the antiretroviral dapivirine directly into the vaginal canal, thereby protecting women who might be exposed to the virus through vaginal sex only. Because the medicine stays where it’s delivered and doesn’t circulate through the body, it has been found to be extremely safe with few adverse events.
However, in initial studies, the ring was found to be just 27% effective overall. Later studies, where scientists divided women by how much drug was missing from the ring – a proxy for use – found that higher use was associated with higher protection (as much as 54%). By comparison, Truvada has been found to be up to 99% effective when used daily, though it can take up to 21 days to be available in the vagina in high enough concentrations to protect women from vaginal exposure. And the HIV prevention shot was found to be 90% more effective than that in a recent trial of the two methods conducted by the HIV Prevention Trials Network.
This, and an orientation away from topical HIV prevention drugs and toward systemic options, led the National Institute of Allergy and Infectious Diseases (NIAID) to discontinue funding for such projects under its Microbicide Trials Network.
“Clearly you want to counsel women to use the highest efficacy method, and that is part of our label,” Zeda Rosenberg, ScD, IPM’s founder and chief executive officer, told this news organization. “Women should not choose the ring if they can and will use oral PrEP, and I would argue it should be the same thing for [cabotegravir shots]. But if they can’t or don’t want to – and we know that especially many young women don’t want to use systemic methods – then the dapivirine ring is a great option.”
Still, Dr. Rosenberg said that the gap in efficacy, the relatively small number of women affected by HIV in the U.S. compared with gay and bisexual men, and the emergence of products like the HIV prevention shot cabotegravir, made it “very unlikely” that FDA regulators would approve the ring. And rather than be “distracted” by the FDA process, Dr. Rosenberg said IPM chose to concentrate on the countries where the ring has already been approved or where women make up the vast majority of people affected by HIV.
Zimbabwe publicly announced it has approved the ring, and three other countries may have approved it, according to Dr. Rosenberg. She declined to name them, saying they had requested silence while they formulate their new HIV prevention guidelines. Aside from Zimbabwe, the other countries where women participated in the ring clinical trials were South Africa, Malawi, and Uganda.
“The U.S. population ... has widespread access to oral PrEP, which is unlike countries in Africa, and which would have widespread access to injectable cabotegravir,” she said. “The U.S. FDA may not see choice in the same way that African women and African activists and advocates see the need for choice.”
But women’s rates of accessing HIV prevention medications in the U.S. continues to be frustratingly low. At the end of 2018, just 7% of women who could benefit from HIV prevention drugs were taking them, according to Centers for Disease Control and Prevention data.
New CDC guidelines recommend clinicians talk to every sexually active adult and adolescent about HIV prevention medications at least once and prescribe it to anyone who asks for it, whether or not they understand their patients’ HIV risks. However, research continues to show that clinicians struggle with willingness to prescribe PrEP to Black women, and the American College of Obstetrics and Gynecology’s committee opinion on managing women using HIV prevention drugs has not been updated to reflect the new guidelines. And while the HIV prevention shot is approved for women and its maker ViiV Healthcare is already initiating postmarket studies of the ring in key populations including women, there are lots of things that need to line up in order for clinicians to be willing to stock it and prescribe it to women.
From where Dázon Dixon Diallo, executive director of the nonprofit SisterLove, sits, the decision to withdraw the ring from FDA consideration and the FDA’s seeming argument that the epidemiology in the U.S. doesn’t warrant the ring’s approval is a slap in the face to the Black women who have led the movement to end HIV in the U.S. for decades.
“No matter how you slice it, we’re talking about Black women, and then we’re talking about brown women,” said Ms. Diallo. “The value [they place on us] from a government standpoint, from a political standpoint, from a public health standpoint is just woeful. It’s woeful and it’s disrespectful and it’s insulting and I’m sick of it.”
‘America sneezes and Africa catches a cold’
When she first heard the decision to pull the ring from FDA consideration, Yvette Raphael, the South Africa-based executive director of Advocates for the Prevention of HIV in Africa, started asking, “What can we do to help our sisters in America get this ring?” And then she started worrying about other women in her own country and those nearby.
“The FDA plays a big role,” she said. “You know, America sneezes and Africa catches a cold.”
She worries that IPM’s decision to withdraw the ring from FDA consideration will signal to regulators in other countries either (a) that they should not approve it or (b) in countries where it’s already been approved but guidelines have not been issued, that they won’t invest money in rolling it out to women in those countries – especially now with the U.S. approval of the prevention shot. In much of Africa, ministries of health prefer to provide injectable contraception, often giving women few or no other options. But women, she said, think about more than administration of the drug. They look at if it’s an easier option for them to manage.
“This is a long journey, an emotional one too, for women in South Africa, because the idea of a microbicide is one of the ideas that came directly from women in South Africa,” she said. “[The jab] can be seen as a solution to all. We can just give jabs to all the women. And after all, we know that women don’t adhere, so we can just grab them.”
Dr. Rosenberg pointed to the positive opinion from the EMA as another “rigorous review” process that she said ought to equally influence ministries of health in countries where women tested the ring. And she pointed to the WHO statement released last month, the same day as IPM’s announcement that it was withdrawing the ring from FDA considerations, recommitting the ring as a good option in sub-Saharan Africa: “The U.S. FDA decision is not based on any new or additional data on efficacy and safety,” it stated. “WHO will continue to support countries as they consider whether to include the [dapivirine vaginal ring]. WHO recognizes that country decisionmaking will vary based on their context and that women’s voices remain central to discussions about their prevention choices.”
Dual action ring on the horizon, but not in U.S.
What this means, though, is that the next step in the ring’s development – the combination dapivirine ring with contraceptive levonorgestrel (used in the Mirena intrauterine device) – may not come to the U.S., at least for a long while.
“It’s not out of the question,” Dr. Rosenberg said of conducting HIV/pregnancy prevention ring trials in the U.S. “But without the approval of the dapivirine-only ring by FDA, I imagine they would want to see new efficacy data on dapivirine. That is a very difficult hill to climb. There would have to be an active control group [using oral PrEP or injectable cabotegravir], and it would be very difficult for the dapivirine ring to be able to go head-to-head for either noninferiority and certainly for superiority.”
The study would need to be quite large to get enough results to prove anything, and IPM is a research organization, not a large pharmaceutical company with deep enough pockets to fund that, she said. Raising those funds “would be difficult.”
In addition to NIAID discontinuing its funding for the Microbicides Trials Network, a new 5-year, $85 million research collaboration through USAID hasn’t slated any money to fund trials of the combination HIV prevention and contraceptive ring, according to Dr. Rosenberg.
But that doesn’t mean avenues for its development are closed. NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) is currently funding a phase 1/2 trial of the combination ring, and IPM continues to receive funding from research agencies in Germany, the Netherlands, Denmark, and Ireland. And this means, she said, that the E.U. – not the U.S. – is where they would seek approval for a combination ring first.
That leaves Ms. Rafael and Ms. Diallo debating how to work together to push the FDA – and maybe IPM – to reconsider the ring. For instance, Ms. Diallo suggested that instead of seeking an indication for all women, the FDA might consider the ring for women with very high risk of HIV, such as sex workers or women with HIV positive partners not on treatment. And she said that this has to be bigger than HIV prevention. It has to be about the ways in which women’s health issues in general lag at the FDA. For instance, she pointed to the movement to get contraceptive pills available over the counter, fights against FDA rulings on hormone replacement therapy, and fights for emergency contraception.
In the meantime, ob/gyn Dr. Rogers is expecting access to the ring to follow a similar path as the copper IUD, which migrated to the U.S. from Europe, where it has been among the most popular contraceptive methods for women.
“Contrary to what we may think, we are not innovators, especially for something like this,” she said. “Once we see it is working and doing a good job – that women in Europe love it – then someone here is going to pick it up and make it as if it’s the greatest thing. But for now, I think we’re going to have to take a back seat to Europe.”
Ms. Diallo reports receiving fees from Johnson & Johnson, ViiV Healthcare, and Gilead Sciences. Dr. Rosenberg and Dr. Rogers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Tosha Rogers, MD, is a one-woman HIV prevention evangelist. For nearly a decade now, the Atlanta-based ob/gyn has been on a mission to increase her gynecological colleagues’ awareness and prescribing of the oral HIV prevention pill. At the same time, she’s been tracking the development of a flexible vaginal ring loaded with a month’s worth of the HIV prevention medication dapivirine. That, she thought, would fit easily into women’s lives and into the toolbox of methods women already use to prevent pregnancy.
But now she’s not sure when – or if – the ring will find its way to her patients. In December, the ring’s maker, the International Partnership for Microbicides (IPM), pulled its application for FDA approval for the pre-exposure prophylaxis (PrEP) ring. Now, one year after the World Health Organization recommended the ring for member nations, there appears to be no path forward in the United States for either the dapivirine-only ring or an approach Dr. Rogers said would change the game: a vaginal ring that supplies both contraception and HIV prevention.
“It would take things to a whole other level,” she said. “It sucks that this happened, and I do think it was not anything medical. I think it was everything political.”
That leaves cisgender women – especially the Black and Latinx women who make up the vast majority of women who acquire HIV every year – with two HIV prevention options. One is the daily pill, first approved in 2012. It’s now generic but previously sold as Truvada by Gilead Sciences. The other is monthly injectable cabotegravir long-acting (Apretude). Another HIV prevention pill, tenofovir alafenamide/emtricitabine (Descovy), is approved for gay men and transgender women but not cisgender women.
Vagina-specific protection from HIV
The WHO recommendation for the vaginal ring was followed last July by a positive opinion from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) for women in low- and middle-income countries outside the European Union.
The flexible silicone ring, similar to the hormonal NuvaRing contraceptive, works by slowly releasing the antiretroviral dapivirine directly into the vaginal canal, thereby protecting women who might be exposed to the virus through vaginal sex only. Because the medicine stays where it’s delivered and doesn’t circulate through the body, it has been found to be extremely safe with few adverse events.
However, in initial studies, the ring was found to be just 27% effective overall. Later studies, where scientists divided women by how much drug was missing from the ring – a proxy for use – found that higher use was associated with higher protection (as much as 54%). By comparison, Truvada has been found to be up to 99% effective when used daily, though it can take up to 21 days to be available in the vagina in high enough concentrations to protect women from vaginal exposure. And the HIV prevention shot was found to be 90% more effective than that in a recent trial of the two methods conducted by the HIV Prevention Trials Network.
This, and an orientation away from topical HIV prevention drugs and toward systemic options, led the National Institute of Allergy and Infectious Diseases (NIAID) to discontinue funding for such projects under its Microbicide Trials Network.
“Clearly you want to counsel women to use the highest efficacy method, and that is part of our label,” Zeda Rosenberg, ScD, IPM’s founder and chief executive officer, told this news organization. “Women should not choose the ring if they can and will use oral PrEP, and I would argue it should be the same thing for [cabotegravir shots]. But if they can’t or don’t want to – and we know that especially many young women don’t want to use systemic methods – then the dapivirine ring is a great option.”
Still, Dr. Rosenberg said that the gap in efficacy, the relatively small number of women affected by HIV in the U.S. compared with gay and bisexual men, and the emergence of products like the HIV prevention shot cabotegravir, made it “very unlikely” that FDA regulators would approve the ring. And rather than be “distracted” by the FDA process, Dr. Rosenberg said IPM chose to concentrate on the countries where the ring has already been approved or where women make up the vast majority of people affected by HIV.
Zimbabwe publicly announced it has approved the ring, and three other countries may have approved it, according to Dr. Rosenberg. She declined to name them, saying they had requested silence while they formulate their new HIV prevention guidelines. Aside from Zimbabwe, the other countries where women participated in the ring clinical trials were South Africa, Malawi, and Uganda.
“The U.S. population ... has widespread access to oral PrEP, which is unlike countries in Africa, and which would have widespread access to injectable cabotegravir,” she said. “The U.S. FDA may not see choice in the same way that African women and African activists and advocates see the need for choice.”
But women’s rates of accessing HIV prevention medications in the U.S. continues to be frustratingly low. At the end of 2018, just 7% of women who could benefit from HIV prevention drugs were taking them, according to Centers for Disease Control and Prevention data.
New CDC guidelines recommend clinicians talk to every sexually active adult and adolescent about HIV prevention medications at least once and prescribe it to anyone who asks for it, whether or not they understand their patients’ HIV risks. However, research continues to show that clinicians struggle with willingness to prescribe PrEP to Black women, and the American College of Obstetrics and Gynecology’s committee opinion on managing women using HIV prevention drugs has not been updated to reflect the new guidelines. And while the HIV prevention shot is approved for women and its maker ViiV Healthcare is already initiating postmarket studies of the ring in key populations including women, there are lots of things that need to line up in order for clinicians to be willing to stock it and prescribe it to women.
From where Dázon Dixon Diallo, executive director of the nonprofit SisterLove, sits, the decision to withdraw the ring from FDA consideration and the FDA’s seeming argument that the epidemiology in the U.S. doesn’t warrant the ring’s approval is a slap in the face to the Black women who have led the movement to end HIV in the U.S. for decades.
“No matter how you slice it, we’re talking about Black women, and then we’re talking about brown women,” said Ms. Diallo. “The value [they place on us] from a government standpoint, from a political standpoint, from a public health standpoint is just woeful. It’s woeful and it’s disrespectful and it’s insulting and I’m sick of it.”
‘America sneezes and Africa catches a cold’
When she first heard the decision to pull the ring from FDA consideration, Yvette Raphael, the South Africa-based executive director of Advocates for the Prevention of HIV in Africa, started asking, “What can we do to help our sisters in America get this ring?” And then she started worrying about other women in her own country and those nearby.
“The FDA plays a big role,” she said. “You know, America sneezes and Africa catches a cold.”
She worries that IPM’s decision to withdraw the ring from FDA consideration will signal to regulators in other countries either (a) that they should not approve it or (b) in countries where it’s already been approved but guidelines have not been issued, that they won’t invest money in rolling it out to women in those countries – especially now with the U.S. approval of the prevention shot. In much of Africa, ministries of health prefer to provide injectable contraception, often giving women few or no other options. But women, she said, think about more than administration of the drug. They look at if it’s an easier option for them to manage.
“This is a long journey, an emotional one too, for women in South Africa, because the idea of a microbicide is one of the ideas that came directly from women in South Africa,” she said. “[The jab] can be seen as a solution to all. We can just give jabs to all the women. And after all, we know that women don’t adhere, so we can just grab them.”
Dr. Rosenberg pointed to the positive opinion from the EMA as another “rigorous review” process that she said ought to equally influence ministries of health in countries where women tested the ring. And she pointed to the WHO statement released last month, the same day as IPM’s announcement that it was withdrawing the ring from FDA considerations, recommitting the ring as a good option in sub-Saharan Africa: “The U.S. FDA decision is not based on any new or additional data on efficacy and safety,” it stated. “WHO will continue to support countries as they consider whether to include the [dapivirine vaginal ring]. WHO recognizes that country decisionmaking will vary based on their context and that women’s voices remain central to discussions about their prevention choices.”
Dual action ring on the horizon, but not in U.S.
What this means, though, is that the next step in the ring’s development – the combination dapivirine ring with contraceptive levonorgestrel (used in the Mirena intrauterine device) – may not come to the U.S., at least for a long while.
“It’s not out of the question,” Dr. Rosenberg said of conducting HIV/pregnancy prevention ring trials in the U.S. “But without the approval of the dapivirine-only ring by FDA, I imagine they would want to see new efficacy data on dapivirine. That is a very difficult hill to climb. There would have to be an active control group [using oral PrEP or injectable cabotegravir], and it would be very difficult for the dapivirine ring to be able to go head-to-head for either noninferiority and certainly for superiority.”
The study would need to be quite large to get enough results to prove anything, and IPM is a research organization, not a large pharmaceutical company with deep enough pockets to fund that, she said. Raising those funds “would be difficult.”
In addition to NIAID discontinuing its funding for the Microbicides Trials Network, a new 5-year, $85 million research collaboration through USAID hasn’t slated any money to fund trials of the combination HIV prevention and contraceptive ring, according to Dr. Rosenberg.
But that doesn’t mean avenues for its development are closed. NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) is currently funding a phase 1/2 trial of the combination ring, and IPM continues to receive funding from research agencies in Germany, the Netherlands, Denmark, and Ireland. And this means, she said, that the E.U. – not the U.S. – is where they would seek approval for a combination ring first.
That leaves Ms. Rafael and Ms. Diallo debating how to work together to push the FDA – and maybe IPM – to reconsider the ring. For instance, Ms. Diallo suggested that instead of seeking an indication for all women, the FDA might consider the ring for women with very high risk of HIV, such as sex workers or women with HIV positive partners not on treatment. And she said that this has to be bigger than HIV prevention. It has to be about the ways in which women’s health issues in general lag at the FDA. For instance, she pointed to the movement to get contraceptive pills available over the counter, fights against FDA rulings on hormone replacement therapy, and fights for emergency contraception.
In the meantime, ob/gyn Dr. Rogers is expecting access to the ring to follow a similar path as the copper IUD, which migrated to the U.S. from Europe, where it has been among the most popular contraceptive methods for women.
“Contrary to what we may think, we are not innovators, especially for something like this,” she said. “Once we see it is working and doing a good job – that women in Europe love it – then someone here is going to pick it up and make it as if it’s the greatest thing. But for now, I think we’re going to have to take a back seat to Europe.”
Ms. Diallo reports receiving fees from Johnson & Johnson, ViiV Healthcare, and Gilead Sciences. Dr. Rosenberg and Dr. Rogers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Fibroids: Growing management options for a prevalent problem
OBG Manag. 33(12). | doi 10.12788/obgm.0169
The troubling trend of repackaging feminine hygiene products for the next generation
Feminine hygiene products have been commercially available for decades. They are commonly marketed to reduce odor or clean vaginal discharge and menses. Multiple formulas are available as topical washes, wipes, creams, sprays, powders, deodorants, and douches.1 Products on the market range from those used externally on the vulva, such as wipes and sprays, to liquid solutions used intravaginally, such as washes and douches.
Who uses feminine hygiene products?
According to a 2006 study, the majority of women who use douches started using them between age 15 and 19 years, but some women initiate this practice habit as early as age 10 to 14.1 Predictably, women who douche are more likely to perceive douche products as safe.1
Demographic data on douche utilization are mixed: Some studies show that there are no significant racial differences in douching practices,2 while others have found that Black and African American women are more likely to practice douching than White and Hispanic women.1,3 Studies have shown a significant difference in attitudes toward douching and knowledge of normal vaginal symptoms among US racial demographics, although this must be examined through the historical context of racism and the lens of medical anthropology.4
Women cite that common reasons they use feminine hygiene products are to feel clean, to control odor, and to use after menses and intercourse.1,2
Modern marketing approaches
From wipes to soaps to douches, feminine hygiene products often are advertised to promote “funk-free periods”5 and “freshness,” fostering an environment in which women and men develop unrealistic standards for what is considered normal genital odor and resulting in poor body image.6
Recently, Vagisil (Combe Incorporated) marketing efforts faced backlash from the ObGyn community for targeting younger populations with a specific product line for adolescents called OMV! In addition, attention has been drawn to VCF vaginal odor eliminating film (Apothecus Pharmaceutical Corp), small stamp-sized dissolving films that are placed in the vaginal canal in contact with the epithelium. This product has entered the market of feminine hygiene products accompanied by slogans of eliminating “feminine odor” and providing “confidence for women to be intimate.”
Continue to: Effects of feminine hygiene products on the vaginal microbiome...
Effects of feminine hygiene products on the vaginal microbiome
Frequent use of feminine hygiene products has been associated with recurrent vaginitis, bacterial vaginosis, and general irritation/itch,7,8 which can cause more discharge and odor. Ironically, this may result in women using the product more frequently since they often seek out these products to eliminate odor and discharge.1,2
The pH of the vagina changes during a woman’s lifetime, but in the reproductive years, the normal pH range is typically 3.8 to 4.4.9 This range allows for a normal vaginal flora to form with bacteria such as Lactobacillus species and Gardnerella vaginalis, while feminine hygiene products have a wide range of pH.9,10
Regardless of the formulation, most feminine hygiene products contain ingredients and compositions that potentially are detrimental to the health of the vulva and vagina. Many products contain acidic ingredients, such as citric acid, lactic acid, and dehydroacetic acid, that can alter the vaginal pH and weaken the vaginal barrier by wiping out normal vaginal flora10 despite being advertised for use on “sensitive areas” (TABLE). Lactic acid also has been found to increase diverse anaerobic bacteria in the vaginal microbiome.11 Some feminine hygiene products have been shown to suppress Lactobacillus growth at 2 hours after use and to kill all lactobacilli at 24 hours.10 Shifts in microbiota numbers often occur when the vaginal pH has been altered, as is frequently the case with feminine hygiene products. In the absence of microbiome bacteria, the presence of vaginal hygiene products has been shown to increase interleukin-8 (IL-8), suggesting a proinflammatory reaction.10
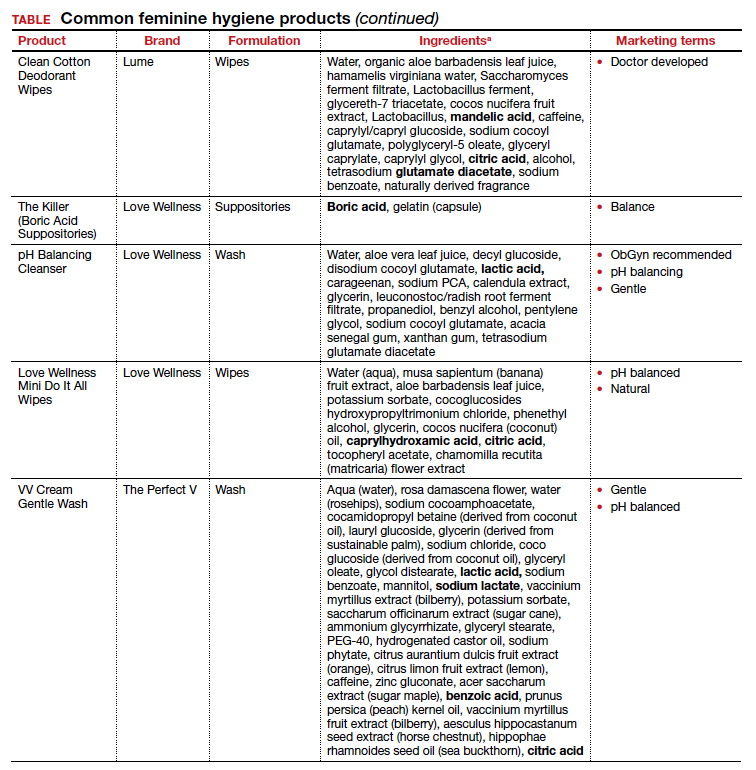
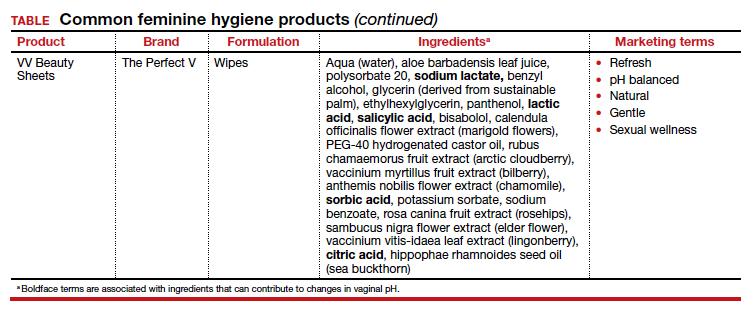
A study in the United Kingdom found that women who used bubble bath, antiseptics, or douche products had a higher incidence of bacterial vaginosis compared with women who did not use such products.7 Women in Canada who used feminine hygiene products were more likely to report adverse conditions, including yeast infections, bacterial vaginosis, urinary tract infections, and sexually transmitted diseases.8 Furthermore, a significant association exists between vaginal douching and endometrial infection by bacterial vaginosis–associated organisms.12
Additionally, a study that analyzed volatile organic compound levels in the blood with the use of feminine hygiene products revealed a significant positive dose-exposure relationship between the frequency of vaginal douching in the last 6 months and concentrations of 1,4-dichloromethane, one of the volatile organic compounds.3 This points to the issue of not only disruption of pH and vaginal flora but also to the introduction of harmful substances that can further disrupt the vaginal barrier.
Understand the products to help educate patients
Use of feminine hygiene products is common among women. While women depend on the market to filter out products that are considered unsafe or may have harmful side effects,1 unfortunately that is not necessarily the case. With increasingly more feminine products on the market and the target demographic becoming younger, women of all ages are susceptible to misinformation that could affect their vaginal health long term.
It is vital that clinicians understand the topical effects of these products in order to properly educate and counsel patients. Ultimately, research on feminine hygiene products is limited and, as more products come to market, we must continue to reassess the effects of topical products on the vaginal epithelium and vulvar tissues. ●
- Grimley DM, Annang L, Foushee HR, et al. Vaginal douches and other feminine hygiene products: women’s practices and perceptions of product safety. Matern Child Health J. 2006;10:303-310. doi: 10.1007/s10995-005-0054-y.
- Foch BJ, McDaniel ND, Chacko MR. Racial differences in vaginal douching knowledge, attitude, and practices among sexually active adolescents. J Pediatr Adolesc Gynecol. 2001;14:29-33. doi: 10.1016/S1083-3188(00)00080-2.
- Lin N, Ding N, Meza-Wilson E, et al. Volatile organic compounds in feminine hygiene products sold in the US market: a survey of products and health risks. Environ Int. 2020;144:105740. doi: 10.1016/j.envint.2020.105740.
- Wayne State University Digital Commons. Guy-Lee AK. Rituals reproducing race: African American women’s feminine hygiene practices, shared experiences, and power. 2017. http://digitalcommons.wayne.edu/oa_dissertations/1806. Accessed December 13, 2021.
- YouTube. OMV! by Vagisil—Intimate care products designed by teens. July 10, 2020. www.youtube.com/ watch?v=VkVsCagrAw0. Accessed December 13, 2021.
- Jenkins A, O’Doherty KC. The clean vagina, the healthy vagina, and the dirty vagina: exploring women’s portrayals of the vagina in relation to vaginal cleansing product use. Fem Psychol. 2021;31:192-211. doi: 10.1177/0959353520944144.
- Rajamanoharan S, Low N, Jones SB, et al. Bacterial vaginosis, ethnicity, and the use of genital cleansing agents: a case control study. Sex Transm Dis. 1999;26:404-409.
- Crann SE, Cunningham S, Albert A, et al. Vaginal health and hygiene practices and product use in Canada: a national cross-sectional survey. BMC Womens Health. 2018;18:52. doi: 10.1186/s12905-018-0543-y.
- Chen Y, Bruning E, Rubino J, et al. Role of female intimate hygiene in vulvovaginal health: global hygiene practices and product usage. Womens Health (London). 2017;13:58-67. doi: 10.1177/1745505717731011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd. v24i0.19703.
- Van der Veer C, Bruisten SM, Van Houdt R, et al. Effects of an over-the-counter lactic-acid containing intra-vaginal douching product on the vaginal microbiota. BMC Microbiol. 2019;19:168. doi: 10.1186/s12866-019-1545-0.
- Gondwe T, Ness R, Totten PA, et al. Novel bacterial vaginosis-associated organisms mediate the relationship between vaginal douching and pelvic inflammatory disease. Sex Transm Infect. 2020;96:439-444. doi: 10.1136/ sextrans-2019-054191.
Feminine hygiene products have been commercially available for decades. They are commonly marketed to reduce odor or clean vaginal discharge and menses. Multiple formulas are available as topical washes, wipes, creams, sprays, powders, deodorants, and douches.1 Products on the market range from those used externally on the vulva, such as wipes and sprays, to liquid solutions used intravaginally, such as washes and douches.
Who uses feminine hygiene products?
According to a 2006 study, the majority of women who use douches started using them between age 15 and 19 years, but some women initiate this practice habit as early as age 10 to 14.1 Predictably, women who douche are more likely to perceive douche products as safe.1
Demographic data on douche utilization are mixed: Some studies show that there are no significant racial differences in douching practices,2 while others have found that Black and African American women are more likely to practice douching than White and Hispanic women.1,3 Studies have shown a significant difference in attitudes toward douching and knowledge of normal vaginal symptoms among US racial demographics, although this must be examined through the historical context of racism and the lens of medical anthropology.4
Women cite that common reasons they use feminine hygiene products are to feel clean, to control odor, and to use after menses and intercourse.1,2
Modern marketing approaches
From wipes to soaps to douches, feminine hygiene products often are advertised to promote “funk-free periods”5 and “freshness,” fostering an environment in which women and men develop unrealistic standards for what is considered normal genital odor and resulting in poor body image.6
Recently, Vagisil (Combe Incorporated) marketing efforts faced backlash from the ObGyn community for targeting younger populations with a specific product line for adolescents called OMV! In addition, attention has been drawn to VCF vaginal odor eliminating film (Apothecus Pharmaceutical Corp), small stamp-sized dissolving films that are placed in the vaginal canal in contact with the epithelium. This product has entered the market of feminine hygiene products accompanied by slogans of eliminating “feminine odor” and providing “confidence for women to be intimate.”
Continue to: Effects of feminine hygiene products on the vaginal microbiome...
Effects of feminine hygiene products on the vaginal microbiome
Frequent use of feminine hygiene products has been associated with recurrent vaginitis, bacterial vaginosis, and general irritation/itch,7,8 which can cause more discharge and odor. Ironically, this may result in women using the product more frequently since they often seek out these products to eliminate odor and discharge.1,2
The pH of the vagina changes during a woman’s lifetime, but in the reproductive years, the normal pH range is typically 3.8 to 4.4.9 This range allows for a normal vaginal flora to form with bacteria such as Lactobacillus species and Gardnerella vaginalis, while feminine hygiene products have a wide range of pH.9,10
Regardless of the formulation, most feminine hygiene products contain ingredients and compositions that potentially are detrimental to the health of the vulva and vagina. Many products contain acidic ingredients, such as citric acid, lactic acid, and dehydroacetic acid, that can alter the vaginal pH and weaken the vaginal barrier by wiping out normal vaginal flora10 despite being advertised for use on “sensitive areas” (TABLE). Lactic acid also has been found to increase diverse anaerobic bacteria in the vaginal microbiome.11 Some feminine hygiene products have been shown to suppress Lactobacillus growth at 2 hours after use and to kill all lactobacilli at 24 hours.10 Shifts in microbiota numbers often occur when the vaginal pH has been altered, as is frequently the case with feminine hygiene products. In the absence of microbiome bacteria, the presence of vaginal hygiene products has been shown to increase interleukin-8 (IL-8), suggesting a proinflammatory reaction.10



A study in the United Kingdom found that women who used bubble bath, antiseptics, or douche products had a higher incidence of bacterial vaginosis compared with women who did not use such products.7 Women in Canada who used feminine hygiene products were more likely to report adverse conditions, including yeast infections, bacterial vaginosis, urinary tract infections, and sexually transmitted diseases.8 Furthermore, a significant association exists between vaginal douching and endometrial infection by bacterial vaginosis–associated organisms.12
Additionally, a study that analyzed volatile organic compound levels in the blood with the use of feminine hygiene products revealed a significant positive dose-exposure relationship between the frequency of vaginal douching in the last 6 months and concentrations of 1,4-dichloromethane, one of the volatile organic compounds.3 This points to the issue of not only disruption of pH and vaginal flora but also to the introduction of harmful substances that can further disrupt the vaginal barrier.
Understand the products to help educate patients
Use of feminine hygiene products is common among women. While women depend on the market to filter out products that are considered unsafe or may have harmful side effects,1 unfortunately that is not necessarily the case. With increasingly more feminine products on the market and the target demographic becoming younger, women of all ages are susceptible to misinformation that could affect their vaginal health long term.
It is vital that clinicians understand the topical effects of these products in order to properly educate and counsel patients. Ultimately, research on feminine hygiene products is limited and, as more products come to market, we must continue to reassess the effects of topical products on the vaginal epithelium and vulvar tissues. ●
Feminine hygiene products have been commercially available for decades. They are commonly marketed to reduce odor or clean vaginal discharge and menses. Multiple formulas are available as topical washes, wipes, creams, sprays, powders, deodorants, and douches.1 Products on the market range from those used externally on the vulva, such as wipes and sprays, to liquid solutions used intravaginally, such as washes and douches.
Who uses feminine hygiene products?
According to a 2006 study, the majority of women who use douches started using them between age 15 and 19 years, but some women initiate this practice habit as early as age 10 to 14.1 Predictably, women who douche are more likely to perceive douche products as safe.1
Demographic data on douche utilization are mixed: Some studies show that there are no significant racial differences in douching practices,2 while others have found that Black and African American women are more likely to practice douching than White and Hispanic women.1,3 Studies have shown a significant difference in attitudes toward douching and knowledge of normal vaginal symptoms among US racial demographics, although this must be examined through the historical context of racism and the lens of medical anthropology.4
Women cite that common reasons they use feminine hygiene products are to feel clean, to control odor, and to use after menses and intercourse.1,2
Modern marketing approaches
From wipes to soaps to douches, feminine hygiene products often are advertised to promote “funk-free periods”5 and “freshness,” fostering an environment in which women and men develop unrealistic standards for what is considered normal genital odor and resulting in poor body image.6
Recently, Vagisil (Combe Incorporated) marketing efforts faced backlash from the ObGyn community for targeting younger populations with a specific product line for adolescents called OMV! In addition, attention has been drawn to VCF vaginal odor eliminating film (Apothecus Pharmaceutical Corp), small stamp-sized dissolving films that are placed in the vaginal canal in contact with the epithelium. This product has entered the market of feminine hygiene products accompanied by slogans of eliminating “feminine odor” and providing “confidence for women to be intimate.”
Continue to: Effects of feminine hygiene products on the vaginal microbiome...
Effects of feminine hygiene products on the vaginal microbiome
Frequent use of feminine hygiene products has been associated with recurrent vaginitis, bacterial vaginosis, and general irritation/itch,7,8 which can cause more discharge and odor. Ironically, this may result in women using the product more frequently since they often seek out these products to eliminate odor and discharge.1,2
The pH of the vagina changes during a woman’s lifetime, but in the reproductive years, the normal pH range is typically 3.8 to 4.4.9 This range allows for a normal vaginal flora to form with bacteria such as Lactobacillus species and Gardnerella vaginalis, while feminine hygiene products have a wide range of pH.9,10
Regardless of the formulation, most feminine hygiene products contain ingredients and compositions that potentially are detrimental to the health of the vulva and vagina. Many products contain acidic ingredients, such as citric acid, lactic acid, and dehydroacetic acid, that can alter the vaginal pH and weaken the vaginal barrier by wiping out normal vaginal flora10 despite being advertised for use on “sensitive areas” (TABLE). Lactic acid also has been found to increase diverse anaerobic bacteria in the vaginal microbiome.11 Some feminine hygiene products have been shown to suppress Lactobacillus growth at 2 hours after use and to kill all lactobacilli at 24 hours.10 Shifts in microbiota numbers often occur when the vaginal pH has been altered, as is frequently the case with feminine hygiene products. In the absence of microbiome bacteria, the presence of vaginal hygiene products has been shown to increase interleukin-8 (IL-8), suggesting a proinflammatory reaction.10



A study in the United Kingdom found that women who used bubble bath, antiseptics, or douche products had a higher incidence of bacterial vaginosis compared with women who did not use such products.7 Women in Canada who used feminine hygiene products were more likely to report adverse conditions, including yeast infections, bacterial vaginosis, urinary tract infections, and sexually transmitted diseases.8 Furthermore, a significant association exists between vaginal douching and endometrial infection by bacterial vaginosis–associated organisms.12
Additionally, a study that analyzed volatile organic compound levels in the blood with the use of feminine hygiene products revealed a significant positive dose-exposure relationship between the frequency of vaginal douching in the last 6 months and concentrations of 1,4-dichloromethane, one of the volatile organic compounds.3 This points to the issue of not only disruption of pH and vaginal flora but also to the introduction of harmful substances that can further disrupt the vaginal barrier.
Understand the products to help educate patients
Use of feminine hygiene products is common among women. While women depend on the market to filter out products that are considered unsafe or may have harmful side effects,1 unfortunately that is not necessarily the case. With increasingly more feminine products on the market and the target demographic becoming younger, women of all ages are susceptible to misinformation that could affect their vaginal health long term.
It is vital that clinicians understand the topical effects of these products in order to properly educate and counsel patients. Ultimately, research on feminine hygiene products is limited and, as more products come to market, we must continue to reassess the effects of topical products on the vaginal epithelium and vulvar tissues. ●
- Grimley DM, Annang L, Foushee HR, et al. Vaginal douches and other feminine hygiene products: women’s practices and perceptions of product safety. Matern Child Health J. 2006;10:303-310. doi: 10.1007/s10995-005-0054-y.
- Foch BJ, McDaniel ND, Chacko MR. Racial differences in vaginal douching knowledge, attitude, and practices among sexually active adolescents. J Pediatr Adolesc Gynecol. 2001;14:29-33. doi: 10.1016/S1083-3188(00)00080-2.
- Lin N, Ding N, Meza-Wilson E, et al. Volatile organic compounds in feminine hygiene products sold in the US market: a survey of products and health risks. Environ Int. 2020;144:105740. doi: 10.1016/j.envint.2020.105740.
- Wayne State University Digital Commons. Guy-Lee AK. Rituals reproducing race: African American women’s feminine hygiene practices, shared experiences, and power. 2017. http://digitalcommons.wayne.edu/oa_dissertations/1806. Accessed December 13, 2021.
- YouTube. OMV! by Vagisil—Intimate care products designed by teens. July 10, 2020. www.youtube.com/ watch?v=VkVsCagrAw0. Accessed December 13, 2021.
- Jenkins A, O’Doherty KC. The clean vagina, the healthy vagina, and the dirty vagina: exploring women’s portrayals of the vagina in relation to vaginal cleansing product use. Fem Psychol. 2021;31:192-211. doi: 10.1177/0959353520944144.
- Rajamanoharan S, Low N, Jones SB, et al. Bacterial vaginosis, ethnicity, and the use of genital cleansing agents: a case control study. Sex Transm Dis. 1999;26:404-409.
- Crann SE, Cunningham S, Albert A, et al. Vaginal health and hygiene practices and product use in Canada: a national cross-sectional survey. BMC Womens Health. 2018;18:52. doi: 10.1186/s12905-018-0543-y.
- Chen Y, Bruning E, Rubino J, et al. Role of female intimate hygiene in vulvovaginal health: global hygiene practices and product usage. Womens Health (London). 2017;13:58-67. doi: 10.1177/1745505717731011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd. v24i0.19703.
- Van der Veer C, Bruisten SM, Van Houdt R, et al. Effects of an over-the-counter lactic-acid containing intra-vaginal douching product on the vaginal microbiota. BMC Microbiol. 2019;19:168. doi: 10.1186/s12866-019-1545-0.
- Gondwe T, Ness R, Totten PA, et al. Novel bacterial vaginosis-associated organisms mediate the relationship between vaginal douching and pelvic inflammatory disease. Sex Transm Infect. 2020;96:439-444. doi: 10.1136/ sextrans-2019-054191.
- Grimley DM, Annang L, Foushee HR, et al. Vaginal douches and other feminine hygiene products: women’s practices and perceptions of product safety. Matern Child Health J. 2006;10:303-310. doi: 10.1007/s10995-005-0054-y.
- Foch BJ, McDaniel ND, Chacko MR. Racial differences in vaginal douching knowledge, attitude, and practices among sexually active adolescents. J Pediatr Adolesc Gynecol. 2001;14:29-33. doi: 10.1016/S1083-3188(00)00080-2.
- Lin N, Ding N, Meza-Wilson E, et al. Volatile organic compounds in feminine hygiene products sold in the US market: a survey of products and health risks. Environ Int. 2020;144:105740. doi: 10.1016/j.envint.2020.105740.
- Wayne State University Digital Commons. Guy-Lee AK. Rituals reproducing race: African American women’s feminine hygiene practices, shared experiences, and power. 2017. http://digitalcommons.wayne.edu/oa_dissertations/1806. Accessed December 13, 2021.
- YouTube. OMV! by Vagisil—Intimate care products designed by teens. July 10, 2020. www.youtube.com/ watch?v=VkVsCagrAw0. Accessed December 13, 2021.
- Jenkins A, O’Doherty KC. The clean vagina, the healthy vagina, and the dirty vagina: exploring women’s portrayals of the vagina in relation to vaginal cleansing product use. Fem Psychol. 2021;31:192-211. doi: 10.1177/0959353520944144.
- Rajamanoharan S, Low N, Jones SB, et al. Bacterial vaginosis, ethnicity, and the use of genital cleansing agents: a case control study. Sex Transm Dis. 1999;26:404-409.
- Crann SE, Cunningham S, Albert A, et al. Vaginal health and hygiene practices and product use in Canada: a national cross-sectional survey. BMC Womens Health. 2018;18:52. doi: 10.1186/s12905-018-0543-y.
- Chen Y, Bruning E, Rubino J, et al. Role of female intimate hygiene in vulvovaginal health: global hygiene practices and product usage. Womens Health (London). 2017;13:58-67. doi: 10.1177/1745505717731011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd. v24i0.19703.
- Van der Veer C, Bruisten SM, Van Houdt R, et al. Effects of an over-the-counter lactic-acid containing intra-vaginal douching product on the vaginal microbiota. BMC Microbiol. 2019;19:168. doi: 10.1186/s12866-019-1545-0.
- Gondwe T, Ness R, Totten PA, et al. Novel bacterial vaginosis-associated organisms mediate the relationship between vaginal douching and pelvic inflammatory disease. Sex Transm Infect. 2020;96:439-444. doi: 10.1136/ sextrans-2019-054191.
HPV testing plus cytology catches two times more cervical lesions
, according to a new study.
The study, which analyzed data from Mexico’s population-based hrHPV screening program over 6 years, confirms the importance of HPV screening for catching high-grade cervical lesions early.
“Our results provide evidence that hrHPV testing is the best strategy for a timely diagnosis of CIN2+ lesions while avoiding overtreatment of young women,” the study authors write. “Many countries now use hrHPV testing as the primary screening method, given it has higher sensitivity and detects more cervical cancer precursor lesions, such as CIN2+.”
According to Erik Jansen, MSc, the analysis supports recent updates to U.S. screening standards and confirms findings from previous trials, which show that HPV testing significantly improves prevention of cervical cancer.
“The significance of this paper is that the data reported is from a long follow-up in a country that implemented HPV screening on a large scale,” Mr. Jansen, PhD candidate in the Department of Public Health, Erasmus University Medical Center, Rotterdam, the Netherlands, told this news organization.
The study, conducted by Mexico’s National Institute of Public Health, analyzed screening data from the country’s public cervical cancer prevention program from 2010 to 2015. More than 2 million women aged 34 to 65 who had hrHPV-based screening tests followed by cytologic triage if they were HPV positive were included, as were 2.8 million women of the same age who received cytologic testing alone.
In the hrHPV group, 1.2% of women (n = 24,276) received referrals to colposcopy versus 3.1% of women (n = 90,980) in the cytology group. And among all women, only 0.8% who had abnormal results (n = 16,459) in the HPV went for a colposcopy versus 1.5% (n = 43,638) in the cytology group.
Overall, the authors found that 13.3 colposcopies were required to detect a single CIN2+ case in the cytology group compared to 5.7 colposcopies in the hrHPV with cytologic triage group.
The authors also note that the cost of colposcopies was three times lower in the HPV testing group and that the positive predictive value of hrHPV testing with cytologic triage was 17.5% versus 7.5% for cytology alone.
“The positive predictive value did not change for either screening strategy whether or not women lost to follow-up were taken into account,” the authors write.
Although Mr. Jansen noted that the findings are important, he also pointed to several limitations – namely, the significant loss to follow-up in the HPV group.
The HPV testing and cytologic triage happened in separate visits, and under the two-visit protocol, more than 50% of women who tested positive for HPV didn’t return for cytology. Such a significant loss to follow-up may call some of the findings into question, Mr. Jansen noted.
For instance, the rate of colposcopy referrals does not account for HPV-positive women who skipped their cytology screening. Assuming the same HPV risk for women who received cytology and those who did not, Mr. Jansen calculated that without any loss to follow-up, the colposcopy referral rate would have increased from the reported 1.2% to 2.6%, which is much closer to the 3.1% of the women referred in the cytology arm.
The lower colposcopy costs in the HPV group were also likely due, in part, to the loss to follow-up, which is not necessarily a good thing, Mr. Jansen said.
Still, “this study does confirm the finding that a primary HPV screening program is more effective than cytology [alone],” Mr. Jansen said.
Co-author Eduardo Franco reported receiving grants and personal fees from MSD and has a pending patent, “Methylation Markers in Cervical Cancer.” All other authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
, according to a new study.
The study, which analyzed data from Mexico’s population-based hrHPV screening program over 6 years, confirms the importance of HPV screening for catching high-grade cervical lesions early.
“Our results provide evidence that hrHPV testing is the best strategy for a timely diagnosis of CIN2+ lesions while avoiding overtreatment of young women,” the study authors write. “Many countries now use hrHPV testing as the primary screening method, given it has higher sensitivity and detects more cervical cancer precursor lesions, such as CIN2+.”
According to Erik Jansen, MSc, the analysis supports recent updates to U.S. screening standards and confirms findings from previous trials, which show that HPV testing significantly improves prevention of cervical cancer.
“The significance of this paper is that the data reported is from a long follow-up in a country that implemented HPV screening on a large scale,” Mr. Jansen, PhD candidate in the Department of Public Health, Erasmus University Medical Center, Rotterdam, the Netherlands, told this news organization.
The study, conducted by Mexico’s National Institute of Public Health, analyzed screening data from the country’s public cervical cancer prevention program from 2010 to 2015. More than 2 million women aged 34 to 65 who had hrHPV-based screening tests followed by cytologic triage if they were HPV positive were included, as were 2.8 million women of the same age who received cytologic testing alone.
In the hrHPV group, 1.2% of women (n = 24,276) received referrals to colposcopy versus 3.1% of women (n = 90,980) in the cytology group. And among all women, only 0.8% who had abnormal results (n = 16,459) in the HPV went for a colposcopy versus 1.5% (n = 43,638) in the cytology group.
Overall, the authors found that 13.3 colposcopies were required to detect a single CIN2+ case in the cytology group compared to 5.7 colposcopies in the hrHPV with cytologic triage group.
The authors also note that the cost of colposcopies was three times lower in the HPV testing group and that the positive predictive value of hrHPV testing with cytologic triage was 17.5% versus 7.5% for cytology alone.
“The positive predictive value did not change for either screening strategy whether or not women lost to follow-up were taken into account,” the authors write.
Although Mr. Jansen noted that the findings are important, he also pointed to several limitations – namely, the significant loss to follow-up in the HPV group.
The HPV testing and cytologic triage happened in separate visits, and under the two-visit protocol, more than 50% of women who tested positive for HPV didn’t return for cytology. Such a significant loss to follow-up may call some of the findings into question, Mr. Jansen noted.
For instance, the rate of colposcopy referrals does not account for HPV-positive women who skipped their cytology screening. Assuming the same HPV risk for women who received cytology and those who did not, Mr. Jansen calculated that without any loss to follow-up, the colposcopy referral rate would have increased from the reported 1.2% to 2.6%, which is much closer to the 3.1% of the women referred in the cytology arm.
The lower colposcopy costs in the HPV group were also likely due, in part, to the loss to follow-up, which is not necessarily a good thing, Mr. Jansen said.
Still, “this study does confirm the finding that a primary HPV screening program is more effective than cytology [alone],” Mr. Jansen said.
Co-author Eduardo Franco reported receiving grants and personal fees from MSD and has a pending patent, “Methylation Markers in Cervical Cancer.” All other authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
, according to a new study.
The study, which analyzed data from Mexico’s population-based hrHPV screening program over 6 years, confirms the importance of HPV screening for catching high-grade cervical lesions early.
“Our results provide evidence that hrHPV testing is the best strategy for a timely diagnosis of CIN2+ lesions while avoiding overtreatment of young women,” the study authors write. “Many countries now use hrHPV testing as the primary screening method, given it has higher sensitivity and detects more cervical cancer precursor lesions, such as CIN2+.”
According to Erik Jansen, MSc, the analysis supports recent updates to U.S. screening standards and confirms findings from previous trials, which show that HPV testing significantly improves prevention of cervical cancer.
“The significance of this paper is that the data reported is from a long follow-up in a country that implemented HPV screening on a large scale,” Mr. Jansen, PhD candidate in the Department of Public Health, Erasmus University Medical Center, Rotterdam, the Netherlands, told this news organization.
The study, conducted by Mexico’s National Institute of Public Health, analyzed screening data from the country’s public cervical cancer prevention program from 2010 to 2015. More than 2 million women aged 34 to 65 who had hrHPV-based screening tests followed by cytologic triage if they were HPV positive were included, as were 2.8 million women of the same age who received cytologic testing alone.
In the hrHPV group, 1.2% of women (n = 24,276) received referrals to colposcopy versus 3.1% of women (n = 90,980) in the cytology group. And among all women, only 0.8% who had abnormal results (n = 16,459) in the HPV went for a colposcopy versus 1.5% (n = 43,638) in the cytology group.
Overall, the authors found that 13.3 colposcopies were required to detect a single CIN2+ case in the cytology group compared to 5.7 colposcopies in the hrHPV with cytologic triage group.
The authors also note that the cost of colposcopies was three times lower in the HPV testing group and that the positive predictive value of hrHPV testing with cytologic triage was 17.5% versus 7.5% for cytology alone.
“The positive predictive value did not change for either screening strategy whether or not women lost to follow-up were taken into account,” the authors write.
Although Mr. Jansen noted that the findings are important, he also pointed to several limitations – namely, the significant loss to follow-up in the HPV group.
The HPV testing and cytologic triage happened in separate visits, and under the two-visit protocol, more than 50% of women who tested positive for HPV didn’t return for cytology. Such a significant loss to follow-up may call some of the findings into question, Mr. Jansen noted.
For instance, the rate of colposcopy referrals does not account for HPV-positive women who skipped their cytology screening. Assuming the same HPV risk for women who received cytology and those who did not, Mr. Jansen calculated that without any loss to follow-up, the colposcopy referral rate would have increased from the reported 1.2% to 2.6%, which is much closer to the 3.1% of the women referred in the cytology arm.
The lower colposcopy costs in the HPV group were also likely due, in part, to the loss to follow-up, which is not necessarily a good thing, Mr. Jansen said.
Still, “this study does confirm the finding that a primary HPV screening program is more effective than cytology [alone],” Mr. Jansen said.
Co-author Eduardo Franco reported receiving grants and personal fees from MSD and has a pending patent, “Methylation Markers in Cervical Cancer.” All other authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
With sexually transmitted infections off the charts, California pushes at-home tests
SACRAMENTO, CALIF. – California has become the first state to require health insurance plans to cover at-home tests for sexually transmitted infections such as HIV, chlamydia, and syphilis – which could help quell the STI epidemic that has raged nearly unchecked as public health departments have focused on COVID-19.
The rule, part of a broader law addressing the STI epidemic, took effect Jan. 1 for people with state-regulated private insurance plans and will kick in sometime later for the millions of low-income Californians enrolled in the state’s Medicaid program.
By making it easier and cheaper for Californians to self-administer tests in the privacy of their homes, the provision could bring better disease monitoring to rural and underserved parts of the state, reduce the stigma patients experience when seeking care, and give them more control over their health, say experts on infectious diseases.
“This is the first law of its kind, and I’d say it’s kind of cutting-edge,” said Stephanie Arnold Pang, senior director of policy and government relations for the National Coalition of STD Directors. “We want to bring down every single barrier for someone to get STI testing, and out-of-pocket cost is a huge factor.”
But being first has its downsides. Because the concept of insurance coverage for home STI tests is so new, the state’s Medicaid program, Medi-Cal, could not establish by Jan. 1 the billing codes it needs to start paying for tests. Federal regulators also haven’t approved the tests for home use, which could make labs reluctant to process them. And a state analysis predicts most in-network health care providers won’t start prescribing home tests for at least a year until they adjust their billing and other practices.
Nevertheless, the situation is urgent and requires action, said state Sen. Richard Pan (D-Sacramento), a pediatrician who wrote the law.
“We have children born in California with syphilis,” Dr. Pan said. “You’d think that went away in the Victorian era.”
Even before COVID, sexually transmitted infections hit all-time highs in the United States and California for 6 years in a row, according to 2019 data from the Centers for Disease Control and Prevention. Rates of congenital syphilis, which babies contract from their mothers, illustrate the severity of the STI epidemic: Cases were up 279% from 2015 to 2019 nationally and 232% in California. Of the 445 cases of congenital syphilis in California in 2019, 37 were stillbirths.
The pandemic only worsened the problem because health departments were overwhelmed responding to the COVID emergency, and stay-at-home orders kept people away from clinics.
In surveys of public health programs across the country since May 2020, the National Coalition of STD Directors found that most respondents – up to 78% in one survey – have diverted some of their STI workforces to test and monitor COVID. A report that accompanied the most recent survey found that some STIs were “completely unchecked” because of reductions in clinic hours, diversion of resources, shortages of testing kits and staff burnout.
Some at-home STI tests screen for a single disease but other kits can collect and send samples to check for a variety of infections. Depending on the test, patients collect a drop of blood with a lancet, or swab their mouth, vagina, anus, or penis.
Some tests require patients to send samples to a lab for analysis, while some oral HIV tests give results at home in a few minutes.
Ivan Beas, a 25-year-old graduate student at University of California, Los Angeles, was getting tested frequently as part of a 2-year research study. When clinics closed during the pandemic, researchers sent him a home kit.
The kit, which tests for HIV, hepatitis C, herpes, syphilis, chlamydia, gonorrhea, and trichomoniasis, was packaged discreetly and came with easy instructions. It took Mr. Beas about 10 minutes to prick his finger, swab his mouth and send the samples to the lab.
Mr. Beas wanted to continue screening himself every few months after the study ended, he said, but the kit he used retails for $289, which is out of reach for him.
The last time he went to a clinic in person, “I spent 2 hours waiting to even be seen by a doctor because of how busy they are,” he said. Until Medi-Cal begins covering home tests, he said, he will have to find time to get tested for free at a Planned Parenthood clinic.
“If insurance were to cover it, I’d definitely do it more,” he said.
Under California’s new law, plans regulated by the state must cover home STI tests when ordered by a health care provider.
Privately insured Californians can take advantage of the coverage immediately. How much they will owe out-of-pocket for the tests – if anything – depends on the type of plan they have, whether their provider is in-network, and whether they fall into a category the federal government has designated for free screening.
Medi-Cal patients almost never face out-of-pocket expenses, but they will have to wait for coverage because the Department of Health Care Services, which administers Medi-Cal, is working with the American Medical Association and the federal government to create billing codes. The reimbursement rates for those codes will then need federal approval.
The state doesn’t know how long that process will take, according to department spokesperson Anthony Cava.
The rule does not apply to the millions of Californians whose job-based health insurance plans are regulated by the federal government.
Other states and organizations have experimented with at-home STI tests. The public health departments in Alabama and the District of Columbia send free kits to residents who request them, but neither jurisdiction requires insurance coverage for them. The National Coalition of STD Directors is sending free kits to people through health departments in Philadelphia; Iowa; Virginia; Indiana; Puerto Rico; and Navajo County, Arizona. The list of recipients is expected to grow this month.
Iwantthekit.org, a project of Johns Hopkins University, has been sending free kits to Maryland residents since 2004, and to Alaskans since 2011. The program is funded by grants and works with local health departments.
Charlotte Gaydos, cofounder of the project, said that requests for test kits during the pandemic nearly tripled – and that she would expand to every state if she could bill insurance the way the California law mandates.
The tests fall into a murky regulatory area. While they have been approved by the Food and Drug Administration, none have been cleared for use at home. Patients are supposed to collect their own samples within the walls of a health facility, and some labs may not analyze samples collected at home.
Public health officials cited other potential challenges: Patients may not have the same access to counseling, treatment, or referrals to other services such as food banks that they would receive at clinics. And although patients are supposed to self-report the results of their tests to public health authorities, some people won’t follow through.
Vlad Carrillo, 31, experienced such trade-offs recently. Mr. Carrillo used to get tested at a San Francisco clinic, where they could get counseling and other services. But Carrillo lost their apartment during the pandemic and moved about 7 hours away to Bishop, the only incorporated city in rural Inyo County.
“Being away from the city, it took me a whole year to find a way to get tested,” Carrillo said.
Carrillo eventually got the kit through the mail, avoiding the stigma of going to the clinic in Bishop, which is “more focused on straight stuff,” like preventing pregnancy. Without the test, Carrillo couldn’t get PrEP, a medication to prevent HIV.
“Going without it for so long was really hard on me,” Carrillo said.
This story was produced by Kaiser Health News (KHN), which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KHN is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
SACRAMENTO, CALIF. – California has become the first state to require health insurance plans to cover at-home tests for sexually transmitted infections such as HIV, chlamydia, and syphilis – which could help quell the STI epidemic that has raged nearly unchecked as public health departments have focused on COVID-19.
The rule, part of a broader law addressing the STI epidemic, took effect Jan. 1 for people with state-regulated private insurance plans and will kick in sometime later for the millions of low-income Californians enrolled in the state’s Medicaid program.
By making it easier and cheaper for Californians to self-administer tests in the privacy of their homes, the provision could bring better disease monitoring to rural and underserved parts of the state, reduce the stigma patients experience when seeking care, and give them more control over their health, say experts on infectious diseases.
“This is the first law of its kind, and I’d say it’s kind of cutting-edge,” said Stephanie Arnold Pang, senior director of policy and government relations for the National Coalition of STD Directors. “We want to bring down every single barrier for someone to get STI testing, and out-of-pocket cost is a huge factor.”
But being first has its downsides. Because the concept of insurance coverage for home STI tests is so new, the state’s Medicaid program, Medi-Cal, could not establish by Jan. 1 the billing codes it needs to start paying for tests. Federal regulators also haven’t approved the tests for home use, which could make labs reluctant to process them. And a state analysis predicts most in-network health care providers won’t start prescribing home tests for at least a year until they adjust their billing and other practices.
Nevertheless, the situation is urgent and requires action, said state Sen. Richard Pan (D-Sacramento), a pediatrician who wrote the law.
“We have children born in California with syphilis,” Dr. Pan said. “You’d think that went away in the Victorian era.”
Even before COVID, sexually transmitted infections hit all-time highs in the United States and California for 6 years in a row, according to 2019 data from the Centers for Disease Control and Prevention. Rates of congenital syphilis, which babies contract from their mothers, illustrate the severity of the STI epidemic: Cases were up 279% from 2015 to 2019 nationally and 232% in California. Of the 445 cases of congenital syphilis in California in 2019, 37 were stillbirths.
The pandemic only worsened the problem because health departments were overwhelmed responding to the COVID emergency, and stay-at-home orders kept people away from clinics.
In surveys of public health programs across the country since May 2020, the National Coalition of STD Directors found that most respondents – up to 78% in one survey – have diverted some of their STI workforces to test and monitor COVID. A report that accompanied the most recent survey found that some STIs were “completely unchecked” because of reductions in clinic hours, diversion of resources, shortages of testing kits and staff burnout.
Some at-home STI tests screen for a single disease but other kits can collect and send samples to check for a variety of infections. Depending on the test, patients collect a drop of blood with a lancet, or swab their mouth, vagina, anus, or penis.
Some tests require patients to send samples to a lab for analysis, while some oral HIV tests give results at home in a few minutes.
Ivan Beas, a 25-year-old graduate student at University of California, Los Angeles, was getting tested frequently as part of a 2-year research study. When clinics closed during the pandemic, researchers sent him a home kit.
The kit, which tests for HIV, hepatitis C, herpes, syphilis, chlamydia, gonorrhea, and trichomoniasis, was packaged discreetly and came with easy instructions. It took Mr. Beas about 10 minutes to prick his finger, swab his mouth and send the samples to the lab.
Mr. Beas wanted to continue screening himself every few months after the study ended, he said, but the kit he used retails for $289, which is out of reach for him.
The last time he went to a clinic in person, “I spent 2 hours waiting to even be seen by a doctor because of how busy they are,” he said. Until Medi-Cal begins covering home tests, he said, he will have to find time to get tested for free at a Planned Parenthood clinic.
“If insurance were to cover it, I’d definitely do it more,” he said.
Under California’s new law, plans regulated by the state must cover home STI tests when ordered by a health care provider.
Privately insured Californians can take advantage of the coverage immediately. How much they will owe out-of-pocket for the tests – if anything – depends on the type of plan they have, whether their provider is in-network, and whether they fall into a category the federal government has designated for free screening.
Medi-Cal patients almost never face out-of-pocket expenses, but they will have to wait for coverage because the Department of Health Care Services, which administers Medi-Cal, is working with the American Medical Association and the federal government to create billing codes. The reimbursement rates for those codes will then need federal approval.
The state doesn’t know how long that process will take, according to department spokesperson Anthony Cava.
The rule does not apply to the millions of Californians whose job-based health insurance plans are regulated by the federal government.
Other states and organizations have experimented with at-home STI tests. The public health departments in Alabama and the District of Columbia send free kits to residents who request them, but neither jurisdiction requires insurance coverage for them. The National Coalition of STD Directors is sending free kits to people through health departments in Philadelphia; Iowa; Virginia; Indiana; Puerto Rico; and Navajo County, Arizona. The list of recipients is expected to grow this month.
Iwantthekit.org, a project of Johns Hopkins University, has been sending free kits to Maryland residents since 2004, and to Alaskans since 2011. The program is funded by grants and works with local health departments.
Charlotte Gaydos, cofounder of the project, said that requests for test kits during the pandemic nearly tripled – and that she would expand to every state if she could bill insurance the way the California law mandates.
The tests fall into a murky regulatory area. While they have been approved by the Food and Drug Administration, none have been cleared for use at home. Patients are supposed to collect their own samples within the walls of a health facility, and some labs may not analyze samples collected at home.
Public health officials cited other potential challenges: Patients may not have the same access to counseling, treatment, or referrals to other services such as food banks that they would receive at clinics. And although patients are supposed to self-report the results of their tests to public health authorities, some people won’t follow through.
Vlad Carrillo, 31, experienced such trade-offs recently. Mr. Carrillo used to get tested at a San Francisco clinic, where they could get counseling and other services. But Carrillo lost their apartment during the pandemic and moved about 7 hours away to Bishop, the only incorporated city in rural Inyo County.
“Being away from the city, it took me a whole year to find a way to get tested,” Carrillo said.
Carrillo eventually got the kit through the mail, avoiding the stigma of going to the clinic in Bishop, which is “more focused on straight stuff,” like preventing pregnancy. Without the test, Carrillo couldn’t get PrEP, a medication to prevent HIV.
“Going without it for so long was really hard on me,” Carrillo said.
This story was produced by Kaiser Health News (KHN), which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KHN is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
SACRAMENTO, CALIF. – California has become the first state to require health insurance plans to cover at-home tests for sexually transmitted infections such as HIV, chlamydia, and syphilis – which could help quell the STI epidemic that has raged nearly unchecked as public health departments have focused on COVID-19.
The rule, part of a broader law addressing the STI epidemic, took effect Jan. 1 for people with state-regulated private insurance plans and will kick in sometime later for the millions of low-income Californians enrolled in the state’s Medicaid program.
By making it easier and cheaper for Californians to self-administer tests in the privacy of their homes, the provision could bring better disease monitoring to rural and underserved parts of the state, reduce the stigma patients experience when seeking care, and give them more control over their health, say experts on infectious diseases.
“This is the first law of its kind, and I’d say it’s kind of cutting-edge,” said Stephanie Arnold Pang, senior director of policy and government relations for the National Coalition of STD Directors. “We want to bring down every single barrier for someone to get STI testing, and out-of-pocket cost is a huge factor.”
But being first has its downsides. Because the concept of insurance coverage for home STI tests is so new, the state’s Medicaid program, Medi-Cal, could not establish by Jan. 1 the billing codes it needs to start paying for tests. Federal regulators also haven’t approved the tests for home use, which could make labs reluctant to process them. And a state analysis predicts most in-network health care providers won’t start prescribing home tests for at least a year until they adjust their billing and other practices.
Nevertheless, the situation is urgent and requires action, said state Sen. Richard Pan (D-Sacramento), a pediatrician who wrote the law.
“We have children born in California with syphilis,” Dr. Pan said. “You’d think that went away in the Victorian era.”
Even before COVID, sexually transmitted infections hit all-time highs in the United States and California for 6 years in a row, according to 2019 data from the Centers for Disease Control and Prevention. Rates of congenital syphilis, which babies contract from their mothers, illustrate the severity of the STI epidemic: Cases were up 279% from 2015 to 2019 nationally and 232% in California. Of the 445 cases of congenital syphilis in California in 2019, 37 were stillbirths.
The pandemic only worsened the problem because health departments were overwhelmed responding to the COVID emergency, and stay-at-home orders kept people away from clinics.
In surveys of public health programs across the country since May 2020, the National Coalition of STD Directors found that most respondents – up to 78% in one survey – have diverted some of their STI workforces to test and monitor COVID. A report that accompanied the most recent survey found that some STIs were “completely unchecked” because of reductions in clinic hours, diversion of resources, shortages of testing kits and staff burnout.
Some at-home STI tests screen for a single disease but other kits can collect and send samples to check for a variety of infections. Depending on the test, patients collect a drop of blood with a lancet, or swab their mouth, vagina, anus, or penis.
Some tests require patients to send samples to a lab for analysis, while some oral HIV tests give results at home in a few minutes.
Ivan Beas, a 25-year-old graduate student at University of California, Los Angeles, was getting tested frequently as part of a 2-year research study. When clinics closed during the pandemic, researchers sent him a home kit.
The kit, which tests for HIV, hepatitis C, herpes, syphilis, chlamydia, gonorrhea, and trichomoniasis, was packaged discreetly and came with easy instructions. It took Mr. Beas about 10 minutes to prick his finger, swab his mouth and send the samples to the lab.
Mr. Beas wanted to continue screening himself every few months after the study ended, he said, but the kit he used retails for $289, which is out of reach for him.
The last time he went to a clinic in person, “I spent 2 hours waiting to even be seen by a doctor because of how busy they are,” he said. Until Medi-Cal begins covering home tests, he said, he will have to find time to get tested for free at a Planned Parenthood clinic.
“If insurance were to cover it, I’d definitely do it more,” he said.
Under California’s new law, plans regulated by the state must cover home STI tests when ordered by a health care provider.
Privately insured Californians can take advantage of the coverage immediately. How much they will owe out-of-pocket for the tests – if anything – depends on the type of plan they have, whether their provider is in-network, and whether they fall into a category the federal government has designated for free screening.
Medi-Cal patients almost never face out-of-pocket expenses, but they will have to wait for coverage because the Department of Health Care Services, which administers Medi-Cal, is working with the American Medical Association and the federal government to create billing codes. The reimbursement rates for those codes will then need federal approval.
The state doesn’t know how long that process will take, according to department spokesperson Anthony Cava.
The rule does not apply to the millions of Californians whose job-based health insurance plans are regulated by the federal government.
Other states and organizations have experimented with at-home STI tests. The public health departments in Alabama and the District of Columbia send free kits to residents who request them, but neither jurisdiction requires insurance coverage for them. The National Coalition of STD Directors is sending free kits to people through health departments in Philadelphia; Iowa; Virginia; Indiana; Puerto Rico; and Navajo County, Arizona. The list of recipients is expected to grow this month.
Iwantthekit.org, a project of Johns Hopkins University, has been sending free kits to Maryland residents since 2004, and to Alaskans since 2011. The program is funded by grants and works with local health departments.
Charlotte Gaydos, cofounder of the project, said that requests for test kits during the pandemic nearly tripled – and that she would expand to every state if she could bill insurance the way the California law mandates.
The tests fall into a murky regulatory area. While they have been approved by the Food and Drug Administration, none have been cleared for use at home. Patients are supposed to collect their own samples within the walls of a health facility, and some labs may not analyze samples collected at home.
Public health officials cited other potential challenges: Patients may not have the same access to counseling, treatment, or referrals to other services such as food banks that they would receive at clinics. And although patients are supposed to self-report the results of their tests to public health authorities, some people won’t follow through.
Vlad Carrillo, 31, experienced such trade-offs recently. Mr. Carrillo used to get tested at a San Francisco clinic, where they could get counseling and other services. But Carrillo lost their apartment during the pandemic and moved about 7 hours away to Bishop, the only incorporated city in rural Inyo County.
“Being away from the city, it took me a whole year to find a way to get tested,” Carrillo said.
Carrillo eventually got the kit through the mail, avoiding the stigma of going to the clinic in Bishop, which is “more focused on straight stuff,” like preventing pregnancy. Without the test, Carrillo couldn’t get PrEP, a medication to prevent HIV.
“Going without it for so long was really hard on me,” Carrillo said.
This story was produced by Kaiser Health News (KHN), which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KHN is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.