User login
Meet a fierce advocate for women’s health: Jen Gunter, MD
Jen Gunter, MD, refuses to stay silent when she sees misleading claims about women’s health products.
In fact, the world’s most famous – and outspoken – ob.gyn. (as described by The Guardian), is on a social media mission to speak up whenever she sees companies or governments “prey on women’s health and vaginal shame.”
With nearly 400,000 followers, Dr. Gunter never shies away from a controversy.
Recently, she railed against vitamin and supplement maker Olly’s vaginal probiotic, taking the company to task for its product premise and objectionable ad copy.
This news organization caught up with the San Francisco–based doctor and author of two books, “The Vagina Bible” and “The Menopause Manifesto.” The following interview has been lightly edited for length and clarity.
Question: So these Olly capsules purport to be “Probiotics for Your Panty Hamster.” What was your reaction to this?
Answer: Seeing the word “panty hamsters” is so egregious. I’m so used to baseline vaginal opportunism, but this was just absolutely egregious and I had to call it out.
Question: What are vaginal probiotics anyway?
Answer: These are one of these big wellness scams where companies try to sell you on somehow hacking your microbiome by taking them. They’re not inexpensive, either, and can range in price from $30 to $150 per month, depending on how bespoke they are. And yet the data isn’t good. There is little to no evidence of the value of these probiotics except to shareholders.
Question: What’s one claim made in the Olly probiotic packaging that bothers you the most?
Answer: The product claims to balance the vaginal pH. To say that is a gross misunderstanding of the vaginal ecosystem. If that tagline is what you’re leading with, what else don’t you know?
Also, if these things worked, we’d recommend them. Vaginitis is complex and often misdiagnosed, and it’s easy for a company to be predatory and swoop in and say they have a product for you.
If I think your product for the vagina is awful and you have not studied it in at least one quality clinical trial (never mind company-funded or not), and your marketing displays a stunning ignorance about vaginal health, don’t approach me about your product. Really.
Question: When there’s a pop culture reference to, say, menstruation, you’re quick to weigh in.
Answer: I saw these viral messages from a boy mom (that’s what she called herself) where she wrote about being disgusted that there were mentions of periods in Turning Red, the animated movie.
Everything is here because of menstruation. If you didn’t menstruate, you wouldn’t have a kid, we wouldn’t have the person who had the intelligence to build the computer you’re spreading this message on. Menstruation is a vital part of human reproduction, and it’s far more complex than people think. For that reason alone, people should know about it.
Question: Do you ever get worried about being so “out there” on social media?
Answer: I have my stalkers I suppose, but the trolls don’t bother me. I don’t care if some whatever art dealer in New York thinks I have mental illness for promoting masks. That’s the best you’ve got? Honestly, this doesn’t even register with me. It’s like throwing a grain of sand at a car.
Question: You also got into an exchange with Dr. Leana Wen, CNN’s medical analyst, about mask wearing.
Answer: She obviously has a different opinion than I do. I think one of the biggest issues in the pandemic is the change in messaging and this idea that somehow people aren’t living their normal lives right now. I was sad to see her promote that concept.
This weekend I went out for lunch, I went furniture shopping, I went to the movies, I took a hike. My family and I wear masks everywhere. I fail to understand how wearing a mask means you’re not living a normal life when it’s clearly linked with the reduced spread of the virus.
Almost everything in medicine is about risk reduction. You can do things to lower your risk of heart disease. It’s not 100% guaranteed, but wouldn’t we want a lower risk of bad things? I’m going to keep wearing a mask forever!
Question: Do you wish more doctors were more vocal like you?
Answer: I wish more doctors would have conversations about health outside of the office in ways they’re comfortable with. Like, you’re at the hairdresser and you share information, or you share information with 15 of your Facebook friends. If you’re a doctor and post an article about COVID-19 and how it impacts the heart, your 15 friends are more likely to read that article than if your friend who’s a lawyer puts that up.
As doctors, I believe we can often influence people in big and small ways.
A version of this article first appeared on WebMD.com.
Jen Gunter, MD, refuses to stay silent when she sees misleading claims about women’s health products.
In fact, the world’s most famous – and outspoken – ob.gyn. (as described by The Guardian), is on a social media mission to speak up whenever she sees companies or governments “prey on women’s health and vaginal shame.”
With nearly 400,000 followers, Dr. Gunter never shies away from a controversy.
Recently, she railed against vitamin and supplement maker Olly’s vaginal probiotic, taking the company to task for its product premise and objectionable ad copy.
This news organization caught up with the San Francisco–based doctor and author of two books, “The Vagina Bible” and “The Menopause Manifesto.” The following interview has been lightly edited for length and clarity.
Question: So these Olly capsules purport to be “Probiotics for Your Panty Hamster.” What was your reaction to this?
Answer: Seeing the word “panty hamsters” is so egregious. I’m so used to baseline vaginal opportunism, but this was just absolutely egregious and I had to call it out.
Question: What are vaginal probiotics anyway?
Answer: These are one of these big wellness scams where companies try to sell you on somehow hacking your microbiome by taking them. They’re not inexpensive, either, and can range in price from $30 to $150 per month, depending on how bespoke they are. And yet the data isn’t good. There is little to no evidence of the value of these probiotics except to shareholders.
Question: What’s one claim made in the Olly probiotic packaging that bothers you the most?
Answer: The product claims to balance the vaginal pH. To say that is a gross misunderstanding of the vaginal ecosystem. If that tagline is what you’re leading with, what else don’t you know?
Also, if these things worked, we’d recommend them. Vaginitis is complex and often misdiagnosed, and it’s easy for a company to be predatory and swoop in and say they have a product for you.
If I think your product for the vagina is awful and you have not studied it in at least one quality clinical trial (never mind company-funded or not), and your marketing displays a stunning ignorance about vaginal health, don’t approach me about your product. Really.
Question: When there’s a pop culture reference to, say, menstruation, you’re quick to weigh in.
Answer: I saw these viral messages from a boy mom (that’s what she called herself) where she wrote about being disgusted that there were mentions of periods in Turning Red, the animated movie.
Everything is here because of menstruation. If you didn’t menstruate, you wouldn’t have a kid, we wouldn’t have the person who had the intelligence to build the computer you’re spreading this message on. Menstruation is a vital part of human reproduction, and it’s far more complex than people think. For that reason alone, people should know about it.
Question: Do you ever get worried about being so “out there” on social media?
Answer: I have my stalkers I suppose, but the trolls don’t bother me. I don’t care if some whatever art dealer in New York thinks I have mental illness for promoting masks. That’s the best you’ve got? Honestly, this doesn’t even register with me. It’s like throwing a grain of sand at a car.
Question: You also got into an exchange with Dr. Leana Wen, CNN’s medical analyst, about mask wearing.
Answer: She obviously has a different opinion than I do. I think one of the biggest issues in the pandemic is the change in messaging and this idea that somehow people aren’t living their normal lives right now. I was sad to see her promote that concept.
This weekend I went out for lunch, I went furniture shopping, I went to the movies, I took a hike. My family and I wear masks everywhere. I fail to understand how wearing a mask means you’re not living a normal life when it’s clearly linked with the reduced spread of the virus.
Almost everything in medicine is about risk reduction. You can do things to lower your risk of heart disease. It’s not 100% guaranteed, but wouldn’t we want a lower risk of bad things? I’m going to keep wearing a mask forever!
Question: Do you wish more doctors were more vocal like you?
Answer: I wish more doctors would have conversations about health outside of the office in ways they’re comfortable with. Like, you’re at the hairdresser and you share information, or you share information with 15 of your Facebook friends. If you’re a doctor and post an article about COVID-19 and how it impacts the heart, your 15 friends are more likely to read that article than if your friend who’s a lawyer puts that up.
As doctors, I believe we can often influence people in big and small ways.
A version of this article first appeared on WebMD.com.
Jen Gunter, MD, refuses to stay silent when she sees misleading claims about women’s health products.
In fact, the world’s most famous – and outspoken – ob.gyn. (as described by The Guardian), is on a social media mission to speak up whenever she sees companies or governments “prey on women’s health and vaginal shame.”
With nearly 400,000 followers, Dr. Gunter never shies away from a controversy.
Recently, she railed against vitamin and supplement maker Olly’s vaginal probiotic, taking the company to task for its product premise and objectionable ad copy.
This news organization caught up with the San Francisco–based doctor and author of two books, “The Vagina Bible” and “The Menopause Manifesto.” The following interview has been lightly edited for length and clarity.
Question: So these Olly capsules purport to be “Probiotics for Your Panty Hamster.” What was your reaction to this?
Answer: Seeing the word “panty hamsters” is so egregious. I’m so used to baseline vaginal opportunism, but this was just absolutely egregious and I had to call it out.
Question: What are vaginal probiotics anyway?
Answer: These are one of these big wellness scams where companies try to sell you on somehow hacking your microbiome by taking them. They’re not inexpensive, either, and can range in price from $30 to $150 per month, depending on how bespoke they are. And yet the data isn’t good. There is little to no evidence of the value of these probiotics except to shareholders.
Question: What’s one claim made in the Olly probiotic packaging that bothers you the most?
Answer: The product claims to balance the vaginal pH. To say that is a gross misunderstanding of the vaginal ecosystem. If that tagline is what you’re leading with, what else don’t you know?
Also, if these things worked, we’d recommend them. Vaginitis is complex and often misdiagnosed, and it’s easy for a company to be predatory and swoop in and say they have a product for you.
If I think your product for the vagina is awful and you have not studied it in at least one quality clinical trial (never mind company-funded or not), and your marketing displays a stunning ignorance about vaginal health, don’t approach me about your product. Really.
Question: When there’s a pop culture reference to, say, menstruation, you’re quick to weigh in.
Answer: I saw these viral messages from a boy mom (that’s what she called herself) where she wrote about being disgusted that there were mentions of periods in Turning Red, the animated movie.
Everything is here because of menstruation. If you didn’t menstruate, you wouldn’t have a kid, we wouldn’t have the person who had the intelligence to build the computer you’re spreading this message on. Menstruation is a vital part of human reproduction, and it’s far more complex than people think. For that reason alone, people should know about it.
Question: Do you ever get worried about being so “out there” on social media?
Answer: I have my stalkers I suppose, but the trolls don’t bother me. I don’t care if some whatever art dealer in New York thinks I have mental illness for promoting masks. That’s the best you’ve got? Honestly, this doesn’t even register with me. It’s like throwing a grain of sand at a car.
Question: You also got into an exchange with Dr. Leana Wen, CNN’s medical analyst, about mask wearing.
Answer: She obviously has a different opinion than I do. I think one of the biggest issues in the pandemic is the change in messaging and this idea that somehow people aren’t living their normal lives right now. I was sad to see her promote that concept.
This weekend I went out for lunch, I went furniture shopping, I went to the movies, I took a hike. My family and I wear masks everywhere. I fail to understand how wearing a mask means you’re not living a normal life when it’s clearly linked with the reduced spread of the virus.
Almost everything in medicine is about risk reduction. You can do things to lower your risk of heart disease. It’s not 100% guaranteed, but wouldn’t we want a lower risk of bad things? I’m going to keep wearing a mask forever!
Question: Do you wish more doctors were more vocal like you?
Answer: I wish more doctors would have conversations about health outside of the office in ways they’re comfortable with. Like, you’re at the hairdresser and you share information, or you share information with 15 of your Facebook friends. If you’re a doctor and post an article about COVID-19 and how it impacts the heart, your 15 friends are more likely to read that article than if your friend who’s a lawyer puts that up.
As doctors, I believe we can often influence people in big and small ways.
A version of this article first appeared on WebMD.com.
Do ObGyns use intrapartum warm compresses to the perineum or perineal massage in their practices?
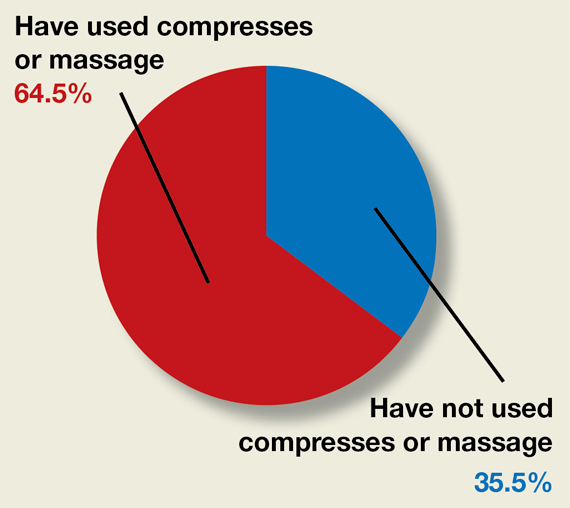
Moderate-quality evidence demonstrates a decrease in obstetric anal sphincter injury (OASIS) with the use of intrapartum warm compresses to the perineum and perineal massage, reported Editor in Chief Robert L. Barbieri, MD, in his editorial, “Obstetric anal sphincter injury: Prevention and repair” (May 2021). He also said that warm compresses may enhance the positive sensory experience of women laboring in natural childbirth. A poll for readers asked, “Do you use intrapartum or warm compresses to the perineum or perineal massage in your practice?”
A total of 200 readers cast their vote:
65.4% (129 readers)said yes
35.5% (71 readers)said no
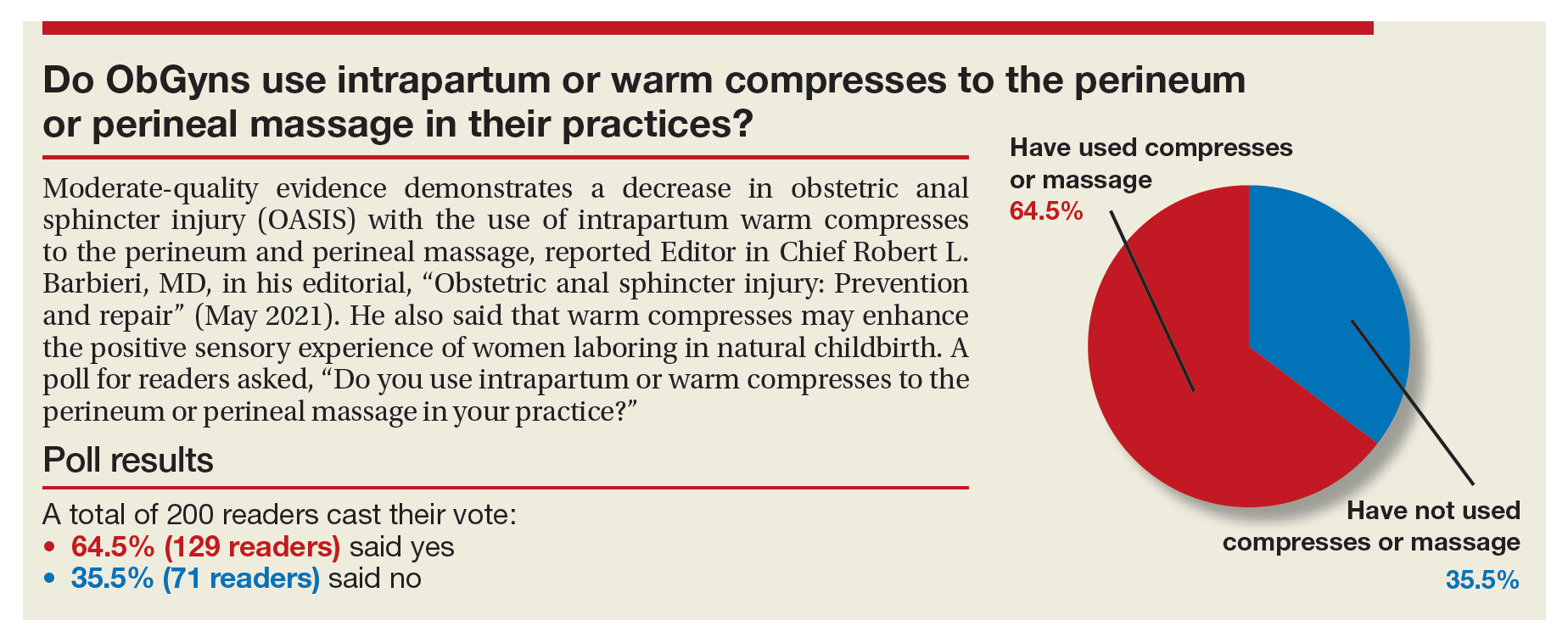
Moderate-quality evidence demonstrates a decrease in obstetric anal sphincter injury (OASIS) with the use of intrapartum warm compresses to the perineum and perineal massage, reported Editor in Chief Robert L. Barbieri, MD, in his editorial, “Obstetric anal sphincter injury: Prevention and repair” (May 2021). He also said that warm compresses may enhance the positive sensory experience of women laboring in natural childbirth. A poll for readers asked, “Do you use intrapartum or warm compresses to the perineum or perineal massage in your practice?”
A total of 200 readers cast their vote:
65.4% (129 readers)said yes
35.5% (71 readers)said no


Moderate-quality evidence demonstrates a decrease in obstetric anal sphincter injury (OASIS) with the use of intrapartum warm compresses to the perineum and perineal massage, reported Editor in Chief Robert L. Barbieri, MD, in his editorial, “Obstetric anal sphincter injury: Prevention and repair” (May 2021). He also said that warm compresses may enhance the positive sensory experience of women laboring in natural childbirth. A poll for readers asked, “Do you use intrapartum or warm compresses to the perineum or perineal massage in your practice?”
A total of 200 readers cast their vote:
65.4% (129 readers)said yes
35.5% (71 readers)said no


Doctors treat osteoporosis with hormone therapy against guidelines
This type of hormone therapy (HT) can be given as estrogen or a combination of hormones including estrogen. The physicians interviewed for this piece who prescribe HT for osteoporosis suggest the benefits outweigh the downsides to its use for some of their patients. But such doctors may be a minority group, suggests Michael R. McClung, MD, founding director of the Oregon Osteoporosis Center, Portland.
According to Dr. McClung, HT is now rarely prescribed as treatment – as opposed to prevention – for osteoporosis in the absence of additional benefits such as reducing vasomotor symptoms.
Researchers’ findings on HT use in women with osteoporosis are complex. While HT is approved for menopausal prevention of osteoporosis, it is not indicated as a treatment for the disease by the Food and Drug Administration. See the prescribing information for Premarin tablets, which contain a mixture of estrogen hormones, for an example of the FDA’s indications and usage for the type of HT addressed in this article.
Women’s Health Initiative findings
The Women’s Health Initiative (WHI) hormone therapy trials showed that HT reduces the incidence of all osteoporosis-related fractures in postmenopausal women, even those at low risk of fracture, but osteoporosis-related fractures was not a study endpoint. These trials also revealed that HT was associated with increased risks of cardiovascular and cerebrovascular events, an increased risk of breast cancer, and other adverse health outcomes.
The release of the interim results of the WHI trials in 2002 led to a fair amount of fear and confusion about the use of HT after menopause. After the WHI findings were published, estrogen use dropped dramatically, but for everything, including for vasomotor symptoms and the prevention and treatment of osteoporosis.
Prior to the WHI study, it was very common for hormone therapy to be prescribed as women neared or entered menopause, said Risa Kagan MD, clinical professor of obstetrics, gynecology, and reproductive sciences, University of California, San Francisco.
“When a woman turned 50, that was one of the first things we did – was to put her on hormone therapy. All that changed with the WHI, but now we are coming full circle,” noted Dr. Kagan, who currently prescribes HT as first line treatment for osteoporosis to some women.
Hormone therapy’s complex history
HT’s ability to reduce bone loss in postmenopausal women is well-documented in many papers, including one published March 8, 2018, in Osteoporosis International, by Dr. Kagan and colleagues. This reduced bone loss has been shown to significantly reduce fractures in patients with low bone mass and osteoporosis.
While a growing number of therapies are now available to treat osteoporosis, HT was traditionally viewed as a standard method of preventing fractures in this population. It was also widely used to prevent other types of symptoms associated with the menopause, such as hot flashes, night sweats, and sleep disturbances, and multiple observational studies had demonstrated that its use appeared to reduce the incidence of cardiovascular disease (CVD) in symptomatic menopausal women who initiated HT in early menopause.
Even though the WHI studies were the largest randomized trials ever performed in postmenopausal women, they had notable limitations, according to Dr. Kagan.
“The women were older – the average age was 63 years,” she said. “And they only investigated one route and one dose of estrogen.”
Since then, many different formulations and routes of administration with more favorable safety profiles than what was used in the WHI have become available.
It’s both scientifically and clinically unsound to extrapolate the unfavorable risk-benefit profile of HT seen in the WHI trials to all women regardless of age, HT dosage or formulation, or the length of time they’re on it, she added.
Today’s use of HT in women with osteoporosis
Re-analyses and follow-up studies from the WHI trials, along with data from other studies, have suggested that the benefit-risk profiles of HT are affected by a variety of factors. These include the timing of use in relation to menopause and chronological age and the type of hormone regimen.
“Clinically, many advocate for [hormone therapy] use, especially in the newer younger postmenopausal women to prevent bone loss, but also in younger women who are diagnosed with osteoporosis and then as they get older transition to more bone specific agents,” noted Dr. Kagan.
“Some advocate preserving bone mass and preventing osteoporosis and even treating the younger newly postmenopausal women who have no contraindications with hormone therapy initially, and then gradually transitioning them to a bone specific agent as they get older and at risk for fracture.
“If a woman is already fractured and/or has very low bone density with no other obvious secondary metabolic reason, we also often advocate anabolic agents for 1-2 years then consider estrogen for maintenance – again, if [there is] no contraindication to using HT,” she added.
Thus, an individualized approach is recommended to determine a woman’s risk-benefit ratio of HT use based on the absolute risk of adverse effects, Dr. Kagan noted.
“Transdermal and low/ultra-low doses of HT, have a favorable risk profile, and are effective in preserving bone mineral density and bone quality in many women,” she said.
According to Dr. McClung, HT “is most often used for treatment in women in whom hormone therapy was begun for hot flashes and then, when osteoporosis was found later, was simply continued.
“Society guidelines are cautious about recommending hormone therapy for osteoporosis treatment since estrogen is not approved for treatment, despite the clear fracture protection benefit observed in the WHI study,” he said. “Since [women in the WHI trials] were not recruited as having osteoporosis, those results do not meet the FDA requirement for treatment approval, namely the reduction in fracture risk in patients with osteoporosis. However, knowing what we know about the salutary skeletal effects of estrogen, many of us do use them in our patients with osteoporosis – although not prescribed for that purpose.”
Additional scenarios when doctors may advise HT
“I often recommend – and I think colleagues do as well – that women with recent menopause and menopausal symptoms who also have low bone mineral density or even scores showing osteoporosis see their gynecologist to discuss HT for a few years, perhaps until age 60 if no contraindications, and if it is well tolerated,” said Ethel S. Siris, MD, professor of medicine at Columbia University Medical Center in New York.
“Once they stop it we can then give one of our other bone drugs, but it delays the need to start them since on adequate estrogen the bone density should remain stable while they take it,” added Dr. Siris, an endocrinologist and internist, and director of the Toni Stabile Osteoporosis Center in New York. “They may need a bisphosphonate or another bone drug to further protect them from bone loss and future fracture [after stopping HT].”
Victor L. Roberts, MD, founder of Endocrine Associates of Florida, Lake Mary, pointed out that women now have many options for treatment of osteoporosis.
“If a woman is in early menopause and is having other symptoms, then estrogen is warranted,” he said. “If she has osteoporosis, then it’s a bonus.”
“We have better agents that are bone specific,” for a patient who presents with osteoporosis and no other symptoms, he said.
“If a woman is intolerant of alendronate or other similar drugs, or chooses not to have an injectable, then estrogen or a SERM [selective estrogen receptor modulator] would be an option.”
Dr. Roberts added that HT would be more of a niche drug.
“It has a role and documented benefit and works,” he said. “There is good scientific data for the use of estrogen.”
Dr. Kagan is a consultant for Pfizer, Therapeutics MD, Amgen, on the Medical and Scientific Advisory Board of American Bone Health. The other experts interviewed for this piece reported no conflicts.
This type of hormone therapy (HT) can be given as estrogen or a combination of hormones including estrogen. The physicians interviewed for this piece who prescribe HT for osteoporosis suggest the benefits outweigh the downsides to its use for some of their patients. But such doctors may be a minority group, suggests Michael R. McClung, MD, founding director of the Oregon Osteoporosis Center, Portland.
According to Dr. McClung, HT is now rarely prescribed as treatment – as opposed to prevention – for osteoporosis in the absence of additional benefits such as reducing vasomotor symptoms.
Researchers’ findings on HT use in women with osteoporosis are complex. While HT is approved for menopausal prevention of osteoporosis, it is not indicated as a treatment for the disease by the Food and Drug Administration. See the prescribing information for Premarin tablets, which contain a mixture of estrogen hormones, for an example of the FDA’s indications and usage for the type of HT addressed in this article.
Women’s Health Initiative findings
The Women’s Health Initiative (WHI) hormone therapy trials showed that HT reduces the incidence of all osteoporosis-related fractures in postmenopausal women, even those at low risk of fracture, but osteoporosis-related fractures was not a study endpoint. These trials also revealed that HT was associated with increased risks of cardiovascular and cerebrovascular events, an increased risk of breast cancer, and other adverse health outcomes.
The release of the interim results of the WHI trials in 2002 led to a fair amount of fear and confusion about the use of HT after menopause. After the WHI findings were published, estrogen use dropped dramatically, but for everything, including for vasomotor symptoms and the prevention and treatment of osteoporosis.
Prior to the WHI study, it was very common for hormone therapy to be prescribed as women neared or entered menopause, said Risa Kagan MD, clinical professor of obstetrics, gynecology, and reproductive sciences, University of California, San Francisco.
“When a woman turned 50, that was one of the first things we did – was to put her on hormone therapy. All that changed with the WHI, but now we are coming full circle,” noted Dr. Kagan, who currently prescribes HT as first line treatment for osteoporosis to some women.
Hormone therapy’s complex history
HT’s ability to reduce bone loss in postmenopausal women is well-documented in many papers, including one published March 8, 2018, in Osteoporosis International, by Dr. Kagan and colleagues. This reduced bone loss has been shown to significantly reduce fractures in patients with low bone mass and osteoporosis.
While a growing number of therapies are now available to treat osteoporosis, HT was traditionally viewed as a standard method of preventing fractures in this population. It was also widely used to prevent other types of symptoms associated with the menopause, such as hot flashes, night sweats, and sleep disturbances, and multiple observational studies had demonstrated that its use appeared to reduce the incidence of cardiovascular disease (CVD) in symptomatic menopausal women who initiated HT in early menopause.
Even though the WHI studies were the largest randomized trials ever performed in postmenopausal women, they had notable limitations, according to Dr. Kagan.
“The women were older – the average age was 63 years,” she said. “And they only investigated one route and one dose of estrogen.”
Since then, many different formulations and routes of administration with more favorable safety profiles than what was used in the WHI have become available.
It’s both scientifically and clinically unsound to extrapolate the unfavorable risk-benefit profile of HT seen in the WHI trials to all women regardless of age, HT dosage or formulation, or the length of time they’re on it, she added.
Today’s use of HT in women with osteoporosis
Re-analyses and follow-up studies from the WHI trials, along with data from other studies, have suggested that the benefit-risk profiles of HT are affected by a variety of factors. These include the timing of use in relation to menopause and chronological age and the type of hormone regimen.
“Clinically, many advocate for [hormone therapy] use, especially in the newer younger postmenopausal women to prevent bone loss, but also in younger women who are diagnosed with osteoporosis and then as they get older transition to more bone specific agents,” noted Dr. Kagan.
“Some advocate preserving bone mass and preventing osteoporosis and even treating the younger newly postmenopausal women who have no contraindications with hormone therapy initially, and then gradually transitioning them to a bone specific agent as they get older and at risk for fracture.
“If a woman is already fractured and/or has very low bone density with no other obvious secondary metabolic reason, we also often advocate anabolic agents for 1-2 years then consider estrogen for maintenance – again, if [there is] no contraindication to using HT,” she added.
Thus, an individualized approach is recommended to determine a woman’s risk-benefit ratio of HT use based on the absolute risk of adverse effects, Dr. Kagan noted.
“Transdermal and low/ultra-low doses of HT, have a favorable risk profile, and are effective in preserving bone mineral density and bone quality in many women,” she said.
According to Dr. McClung, HT “is most often used for treatment in women in whom hormone therapy was begun for hot flashes and then, when osteoporosis was found later, was simply continued.
“Society guidelines are cautious about recommending hormone therapy for osteoporosis treatment since estrogen is not approved for treatment, despite the clear fracture protection benefit observed in the WHI study,” he said. “Since [women in the WHI trials] were not recruited as having osteoporosis, those results do not meet the FDA requirement for treatment approval, namely the reduction in fracture risk in patients with osteoporosis. However, knowing what we know about the salutary skeletal effects of estrogen, many of us do use them in our patients with osteoporosis – although not prescribed for that purpose.”
Additional scenarios when doctors may advise HT
“I often recommend – and I think colleagues do as well – that women with recent menopause and menopausal symptoms who also have low bone mineral density or even scores showing osteoporosis see their gynecologist to discuss HT for a few years, perhaps until age 60 if no contraindications, and if it is well tolerated,” said Ethel S. Siris, MD, professor of medicine at Columbia University Medical Center in New York.
“Once they stop it we can then give one of our other bone drugs, but it delays the need to start them since on adequate estrogen the bone density should remain stable while they take it,” added Dr. Siris, an endocrinologist and internist, and director of the Toni Stabile Osteoporosis Center in New York. “They may need a bisphosphonate or another bone drug to further protect them from bone loss and future fracture [after stopping HT].”
Victor L. Roberts, MD, founder of Endocrine Associates of Florida, Lake Mary, pointed out that women now have many options for treatment of osteoporosis.
“If a woman is in early menopause and is having other symptoms, then estrogen is warranted,” he said. “If she has osteoporosis, then it’s a bonus.”
“We have better agents that are bone specific,” for a patient who presents with osteoporosis and no other symptoms, he said.
“If a woman is intolerant of alendronate or other similar drugs, or chooses not to have an injectable, then estrogen or a SERM [selective estrogen receptor modulator] would be an option.”
Dr. Roberts added that HT would be more of a niche drug.
“It has a role and documented benefit and works,” he said. “There is good scientific data for the use of estrogen.”
Dr. Kagan is a consultant for Pfizer, Therapeutics MD, Amgen, on the Medical and Scientific Advisory Board of American Bone Health. The other experts interviewed for this piece reported no conflicts.
This type of hormone therapy (HT) can be given as estrogen or a combination of hormones including estrogen. The physicians interviewed for this piece who prescribe HT for osteoporosis suggest the benefits outweigh the downsides to its use for some of their patients. But such doctors may be a minority group, suggests Michael R. McClung, MD, founding director of the Oregon Osteoporosis Center, Portland.
According to Dr. McClung, HT is now rarely prescribed as treatment – as opposed to prevention – for osteoporosis in the absence of additional benefits such as reducing vasomotor symptoms.
Researchers’ findings on HT use in women with osteoporosis are complex. While HT is approved for menopausal prevention of osteoporosis, it is not indicated as a treatment for the disease by the Food and Drug Administration. See the prescribing information for Premarin tablets, which contain a mixture of estrogen hormones, for an example of the FDA’s indications and usage for the type of HT addressed in this article.
Women’s Health Initiative findings
The Women’s Health Initiative (WHI) hormone therapy trials showed that HT reduces the incidence of all osteoporosis-related fractures in postmenopausal women, even those at low risk of fracture, but osteoporosis-related fractures was not a study endpoint. These trials also revealed that HT was associated with increased risks of cardiovascular and cerebrovascular events, an increased risk of breast cancer, and other adverse health outcomes.
The release of the interim results of the WHI trials in 2002 led to a fair amount of fear and confusion about the use of HT after menopause. After the WHI findings were published, estrogen use dropped dramatically, but for everything, including for vasomotor symptoms and the prevention and treatment of osteoporosis.
Prior to the WHI study, it was very common for hormone therapy to be prescribed as women neared or entered menopause, said Risa Kagan MD, clinical professor of obstetrics, gynecology, and reproductive sciences, University of California, San Francisco.
“When a woman turned 50, that was one of the first things we did – was to put her on hormone therapy. All that changed with the WHI, but now we are coming full circle,” noted Dr. Kagan, who currently prescribes HT as first line treatment for osteoporosis to some women.
Hormone therapy’s complex history
HT’s ability to reduce bone loss in postmenopausal women is well-documented in many papers, including one published March 8, 2018, in Osteoporosis International, by Dr. Kagan and colleagues. This reduced bone loss has been shown to significantly reduce fractures in patients with low bone mass and osteoporosis.
While a growing number of therapies are now available to treat osteoporosis, HT was traditionally viewed as a standard method of preventing fractures in this population. It was also widely used to prevent other types of symptoms associated with the menopause, such as hot flashes, night sweats, and sleep disturbances, and multiple observational studies had demonstrated that its use appeared to reduce the incidence of cardiovascular disease (CVD) in symptomatic menopausal women who initiated HT in early menopause.
Even though the WHI studies were the largest randomized trials ever performed in postmenopausal women, they had notable limitations, according to Dr. Kagan.
“The women were older – the average age was 63 years,” she said. “And they only investigated one route and one dose of estrogen.”
Since then, many different formulations and routes of administration with more favorable safety profiles than what was used in the WHI have become available.
It’s both scientifically and clinically unsound to extrapolate the unfavorable risk-benefit profile of HT seen in the WHI trials to all women regardless of age, HT dosage or formulation, or the length of time they’re on it, she added.
Today’s use of HT in women with osteoporosis
Re-analyses and follow-up studies from the WHI trials, along with data from other studies, have suggested that the benefit-risk profiles of HT are affected by a variety of factors. These include the timing of use in relation to menopause and chronological age and the type of hormone regimen.
“Clinically, many advocate for [hormone therapy] use, especially in the newer younger postmenopausal women to prevent bone loss, but also in younger women who are diagnosed with osteoporosis and then as they get older transition to more bone specific agents,” noted Dr. Kagan.
“Some advocate preserving bone mass and preventing osteoporosis and even treating the younger newly postmenopausal women who have no contraindications with hormone therapy initially, and then gradually transitioning them to a bone specific agent as they get older and at risk for fracture.
“If a woman is already fractured and/or has very low bone density with no other obvious secondary metabolic reason, we also often advocate anabolic agents for 1-2 years then consider estrogen for maintenance – again, if [there is] no contraindication to using HT,” she added.
Thus, an individualized approach is recommended to determine a woman’s risk-benefit ratio of HT use based on the absolute risk of adverse effects, Dr. Kagan noted.
“Transdermal and low/ultra-low doses of HT, have a favorable risk profile, and are effective in preserving bone mineral density and bone quality in many women,” she said.
According to Dr. McClung, HT “is most often used for treatment in women in whom hormone therapy was begun for hot flashes and then, when osteoporosis was found later, was simply continued.
“Society guidelines are cautious about recommending hormone therapy for osteoporosis treatment since estrogen is not approved for treatment, despite the clear fracture protection benefit observed in the WHI study,” he said. “Since [women in the WHI trials] were not recruited as having osteoporosis, those results do not meet the FDA requirement for treatment approval, namely the reduction in fracture risk in patients with osteoporosis. However, knowing what we know about the salutary skeletal effects of estrogen, many of us do use them in our patients with osteoporosis – although not prescribed for that purpose.”
Additional scenarios when doctors may advise HT
“I often recommend – and I think colleagues do as well – that women with recent menopause and menopausal symptoms who also have low bone mineral density or even scores showing osteoporosis see their gynecologist to discuss HT for a few years, perhaps until age 60 if no contraindications, and if it is well tolerated,” said Ethel S. Siris, MD, professor of medicine at Columbia University Medical Center in New York.
“Once they stop it we can then give one of our other bone drugs, but it delays the need to start them since on adequate estrogen the bone density should remain stable while they take it,” added Dr. Siris, an endocrinologist and internist, and director of the Toni Stabile Osteoporosis Center in New York. “They may need a bisphosphonate or another bone drug to further protect them from bone loss and future fracture [after stopping HT].”
Victor L. Roberts, MD, founder of Endocrine Associates of Florida, Lake Mary, pointed out that women now have many options for treatment of osteoporosis.
“If a woman is in early menopause and is having other symptoms, then estrogen is warranted,” he said. “If she has osteoporosis, then it’s a bonus.”
“We have better agents that are bone specific,” for a patient who presents with osteoporosis and no other symptoms, he said.
“If a woman is intolerant of alendronate or other similar drugs, or chooses not to have an injectable, then estrogen or a SERM [selective estrogen receptor modulator] would be an option.”
Dr. Roberts added that HT would be more of a niche drug.
“It has a role and documented benefit and works,” he said. “There is good scientific data for the use of estrogen.”
Dr. Kagan is a consultant for Pfizer, Therapeutics MD, Amgen, on the Medical and Scientific Advisory Board of American Bone Health. The other experts interviewed for this piece reported no conflicts.
2022 Update on gynecologic cancer
Despite the challenges of an ongoing COVID-19 pandemic, researchers in 2021 delivered practice-changing studies in gynecologic oncology. In this cancer Update, we highlight 4 studies that shed light on the surgical and systemic therapies that may improve outcomes for patients with cancers of the ovary, endometrium, and cervix. We review DESKTOP III, a trial that investigated the role of cytoreductive surgery in patients with recurrent ovarian cancer, and SENTOR, a study that evaluated the performance of sentinel lymph node biopsy in patients with high-grade endometrial cancers. Additionally, we examine 2 studies of systemic therapy that reveal the growing role of targeted therapies and immuno-oncology in the treatment of gynecologic malignancies.
A new era for patients with BRCA mutation–associated ovarian cancer
Banerjee S, Moore KN, Colombo N, et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22:1721-1731.
Ovarian cancer remains the most lethal gynecologic malignancy due to the frequency of advanced-stage diagnosis and frequent relapse after primary therapy. But for ovarian cancer patients with inherited mutations of the BRCA1 or BRCA2 genes, poly(ADP-ribose) polymerase (PARP) inhibitors, a class of oral anticancer medicines that target DNA repair, have ushered in a new era in which the possibility of long-term remission, and even cure, is more likely than at any other time.
Olaparib trial details
The SOLO1 study was a double-blind, placebo-controlled, phase 3 trial that investigated the role of PARP inhibitor maintenance therapy with olaparib in patients with pathologic BRCA1 or BRCA2 mutations who responded to platinum-based chemotherapy administered for a newly diagnosed, advanced-stage ovarian cancer.1 The study enrolled 391 patients, with 260 randomly assigned to receive olaparib for 24 months and 131 patients randomly assigned to receive placebo tablets. Most patients in the study had a mutation in the BRCA1 gene (72%), 27% had a BRCA2 mutation, and 1% had mutations in both genes.
The primary analysis of SOLO1 was published in 2018 and was based on a median follow-up of 3.4 years.1 That study showed that olaparib maintenance therapy resulted in a large progression-free survival benefit and led to its approval by the US Food and Drug Administration (FDA) as a maintenance therapy for patients with BRCA-mutated advanced ovarian cancer who responded to first-line platinum-based chemotherapy.
In 2021, Banerjee and colleagues updated the progression-free survival results for the SOLO1 trial after 5 years of follow-up.2 In this study, the patients randomly assigned to olaparib maintenance therapy had a persistent and statistically significant progression-free survival benefit, with the median progression-free survival reaching 56 months among the olaparib group compared with 13.8 months in the placebo group (hazard ratio [HR], 0.33; 95% confidence interval [CI], 0.25–0.43).2 Olaparib maintenance therapy resulted in more clinically significant adverse events, including anemia and neutropenia. Serious adverse events occurred in 55 (21%) of the olaparib-treated patients and 17 (13%) of the placebo-treated patients, but no treatment-related adverse events were fatal.
The updated progression-free survival data from the SOLO1 study provides important and promising evidence that frontline PARP inhibitor maintenance therapy may affect long-term remission in an unprecedented proportion of patients with BRCA-related ovarian cancer. Significant, sustained benefit was seen well beyond the end of treatment, and median progression-free survival was an astonishing 3.5 years longer in the olaparib treatment group than among patients who received placebo therapy.
Continue to: Cytoreductive surgery for recurrent ovarian cancer improves survival in well-selected patients...
Cytoreductive surgery for recurrent ovarian cancer improves survival in well-selected patients
Harter P, Sehouli J, Vergote I, et al; DESKTOP III Investigators. Randomized trial of cytoreductive surgery for relapsed ovarian cancer. N Engl J Med. 2021;385:2123- 2131.
In the DESKTOP III trial, Harter and colleagues contribute results to the ongoing discourse surrounding treatment options for patients with recurrent, platinum-sensitive ovarian cancer.3 Systemic therapies continue to be the mainstay of treatment in this setting; however, several groups have attempted to evaluate the role of secondary cytoreductive surgery in this setting.4,5
Specific inclusion criteria employed
The DESKTOP III investigators randomly assigned 407 patients with platinum-sensitive recurrent ovarian cancer to secondary cytoreductive surgery followed by platinum-based chemotherapy (n = 206) or platinum-based chemotherapy alone (n = 201).3 An essential aspect of the study’s design was the use of specific inclusion criteria known to identify patients with a high likelihood of complete resection at the time of secondary cytoreduction.6,7 Patients were eligible only if they had at least a 6-month remission following platinum-based chemotherapy, had a complete resection at their previous surgery, had no restriction on physical activity, and had ascites of no more than 500 mL.
Surgery group had superior overall and progression-free survival
After a median follow-up of approximately 70 months, patients randomly assigned to surgery had superior overall survival (53.7 months) compared with those assigned to chemotherapy alone (46.0 months; HR, 0.75; 95% CI, 0.59–0.96).3 Progression-free survival also was improved among patients who underwent surgery (median 18.4 vs 12.7 months; HR, 0.66; 95% CI, 0.54–0.82). Subgroup analyses did not identify any subset of patients who did not benefit from surgery. Whether a complete resection was achieved at secondary cytoreduction was highly prognostic: Patients who had a complete resection had a median overall survival of 61.9 months compared with 27.7 months in patients with residual disease. There were no deaths within 90 days of surgery.
The DESKTOP III trial provides compelling evidence that secondary cytoreductive surgery improves overall and progression-free survival among well-selected patients with recurrent, platinum-sensitive ovarian cancer. These results differ from those of a recently reported Gynecologic Oncology Group (GOG) trial that failed to detect a survival benefit for secondary cytoreductive surgery among patients with platinum-sensitive recurrent ovarian cancer.5 Key differences, which might explain the studies’ seemingly contradictory results, were that the GOG study had fewer specific eligibility criteria than the DESKTOP III trial, and that bevacizumab was administered much more frequently in the GOG study. It is therefore reasonable to discuss the possible benefits of secondary cytoreductive surgery with patients who meet DESKTOP III eligibility criteria, with a focus toward shared decision making and a candid discussion of the potential risks and benefits of secondary cytoreduction.
Continue to: Immunotherapy enters first-line treatment regimen for advanced cervical cancer...
Immunotherapy enters first-line treatment regimen for advanced cervical cancer
Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
Persistent, recurrent, and metastatic cervical cancer carries a very poor prognosis: Most patients progress less than a year after starting treatment, and fewer than half survive for 2 years. First-line treatment in this setting has been platinum-based chemotherapy, often given with bevacizumab, a humanized monoclonal antibody that inhibits tumor growth by blocking angiogenesis.8 Pembrolizumab, an immune checkpoint inhibitor, targets cancer cells by blocking their ability to evade the immune system, and it is FDA approved and widely administered to patients with advanced cervical cancer who progress after first-line treatment.9
Addition of pembrolizumab extended survival
In the KEYNOTE-826 trial, Colombo and colleagues investigated the efficacy of incorporating an immune checkpoint inhibitor into the first-line treatment regimen for patients with persistent, recurrent, and metastatic cervical cancer.10 Researchers in this double-blinded, phase 3, randomized controlled trial assigned 617 patients to receive pembrolizumab or placebo concurrently with the investigator’s choice platinum-based chemotherapy. Bevacizumab was administered at the discretion of the treating oncologist.
The proportion of patients who survived at least 2 years following randomization was significantly higher among those assigned to pembrolizumab compared with placebo (53% vs 42%; HR, 0.67, 95% CI, 0.54–0.84).10 Similarly, median progression-free survival was superior among patients who received pembrolizumab compared with those who received placebo (10.4 months vs 8.2 months; HR, 0.65; 95% CI, 0.53–0.79). The role of bevacizumab in conjunction with pembrolizumab and platinum-based chemotherapy was not elucidated in this study because bevacizumab administration was not randomly assigned.
Anemia and neutropenia were the most common adverse events and were more frequent in the pembrolizumab group, but there were no new safety concerns related to concurrent use of pembrolizumab with cytotoxic chemotherapy and bevacizumab. Importantly, subgroup analysis results suggested that pembrolizumab was effective only in patients whose tumors expressed PD-L1 (programmed death ligand 1), a biomarker of pembrolizumab sensitivity in cervical cancer.
In light of the significant improvements in overall and progression-free survival demonstrated in the KEYNOTE-826 trial, in October 2021, the FDA approved the use of frontline pembrolizumab alongside platinum-based chemotherapy, with or without bevacizumab, for treatment of patients with persistent, recurrent, or metastatic cervical cancers that express PD-L1.
Continue to: Endometrial cancer surgical staging...
Endometrial cancer surgical staging: Is sentinel lymph node biopsy a viable option for high-risk histologies?
Cusimano MC, Vicus D, Pulman K, et al. Assessment of sentinel lymph node biopsy vs lymphadenectomy for intermediate- and high-grade endometrial cancer staging. JAMA Surg. 2021;156:157-164.
The use of intraoperative sentinel lymph node mapping and biopsy to identify lymph node metastases among patients undergoing surgical staging for endometrial cancer has become increasingly common. Lymph node status is an important prognostic factor, and it guides adjuvant treatment decisions in endometrial cancer. However, traditional pelvic and para-aortic lymphadenectomy is associated with increased risk of lower-extremity lymphedema, postoperative complications, and intraoperative injury.
Sentinel lymph node biopsy seeks to identify lymph node metastases while minimizing surgical morbidity by identifying and excising only lymph nodes that directly receive lymphatic drainage from the uterus. The combination of a fluorescent dye (indocyanine green) and near infrared cameras have led to the broad adoption of sentinel lymph node biopsy in endometrial cancer staging surgery. This practice is supported by prospective studies that demonstrate the high diagnostic accuracy of this approach.11,12 However, because most patients included in prior studies had low-grade endometrial cancer, the utility of sentinel lymph node biopsy in cases of high-grade histology has been less clear.
Sentinel lymph node biopsy vs lymphadenectomy for staging
In the SENTOR trial, Cusimano and colleagues examined the diagnostic accuracy of sentinel lymph node mapping and biopsy, using indocyanine green, in patients with intermediate- or high-grade early-stage endometrial cancer.13
All eligible patients (N = 156) underwent traditional or robot-assisted laparoscopic hysterectomy with sentinel lymph node biopsy. Subsequently, patients with grade 2 endometrioid carcinoma underwent bilateral pelvic lymphadenectomy, and those with high-grade histology (grade 3 endometrioid, serous, carcinosarcoma, clear cell, undifferentiated or dedifferentiated, and mixed high grade) underwent bilateral pelvic and para-aortic lymphadenectomy. The investigators evaluated the diagnostic characteristics of sentinel lymph node biopsy, treating complete lymphadenectomy as the gold standard.
Of the 156 patients enrolled, the median age was 65.5 and median body mass index was 27.5; 126 patients (81%) had high-grade histology. The sentinel lymph node biopsy had a sensitivity of 96% (95% CI, 81%–100%), identifying 26 of the 27 patients with nodal metastases. The false-negative rate was 4% (95% CI, 0%–9%) and the negative predictive value was 99% (95% CI, 96%–100%). Intraoperative adverse events occurred in 5 patients (3%), but none occurred during the sentinel lymph node biopsy. ●
The high sensitivity and negative predictive value of sentinel lymph node biopsy in the intermediate- and high-grade cohort included in the SENTOR trial are concordant with prior studies that predominantly included patients with low-grade endometrial cancer. These findings suggest that sentinel lymph node mapping and biopsy is a reasonable option for surgical staging, not only for patients with low-grade endometrial cancer but also for those with intermediate- and high-grade disease.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Banerjee S, Moore KN, Colombo N, et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22:1721-1731.
- Harter P, Sehouli J, Vergote I, et al; DESKTOP III Investigators. Randomized trial of cytoreductive surgery for relapsed ovarian cancer. N Engl J Med. 2021;385:2123-2131.
- Shi T, Zhu J, Feng Y, et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:439-449.
- Coleman RL, Spiritos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Harter P, du Bois A, Hahmann M, et al; Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Committee; AGO Ovarian Cancer Study Group. Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann Surg Oncol. 2006;13:1702-1710.
- Harter P, Sehouli J, Reuss A, et al. Prospective validation study of a predictive score for operability of recurrent ovarian cancer: the Multicenter Intergroup Study DESKTOP II. A project of the AGO Kommission OVAR, AGO Study Group, NOGGO, AGO-Austria, and MITO. Int J Gynecol Cancer. 2011;21: 289-295.
- Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. 2017;390:1654-1663.
- Frenel JS, Le Tourneau C, O’Neil B, et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: results from the phase Ib KEYNOTE-028 trial. J Clin Oncol. 2017;35:4035-4041.
- Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
- Rossi EC, Kowalski L, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18:384-392.
- Ballester M, Dubernard G, Lecuru F, et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: a prospective multicentre study (SENTIENDO). Lancet Oncol. 2011;12: 469-476.
- Cusimano MC, Vicus D, Pulman K, et al. Assessment of sentinel lymph node biopsy vs lymphadenectomy for intermediate- and high-grade endometrial cancer staging. JAMA Surg. 2021;156:157-164.
Despite the challenges of an ongoing COVID-19 pandemic, researchers in 2021 delivered practice-changing studies in gynecologic oncology. In this cancer Update, we highlight 4 studies that shed light on the surgical and systemic therapies that may improve outcomes for patients with cancers of the ovary, endometrium, and cervix. We review DESKTOP III, a trial that investigated the role of cytoreductive surgery in patients with recurrent ovarian cancer, and SENTOR, a study that evaluated the performance of sentinel lymph node biopsy in patients with high-grade endometrial cancers. Additionally, we examine 2 studies of systemic therapy that reveal the growing role of targeted therapies and immuno-oncology in the treatment of gynecologic malignancies.
A new era for patients with BRCA mutation–associated ovarian cancer
Banerjee S, Moore KN, Colombo N, et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22:1721-1731.
Ovarian cancer remains the most lethal gynecologic malignancy due to the frequency of advanced-stage diagnosis and frequent relapse after primary therapy. But for ovarian cancer patients with inherited mutations of the BRCA1 or BRCA2 genes, poly(ADP-ribose) polymerase (PARP) inhibitors, a class of oral anticancer medicines that target DNA repair, have ushered in a new era in which the possibility of long-term remission, and even cure, is more likely than at any other time.
Olaparib trial details
The SOLO1 study was a double-blind, placebo-controlled, phase 3 trial that investigated the role of PARP inhibitor maintenance therapy with olaparib in patients with pathologic BRCA1 or BRCA2 mutations who responded to platinum-based chemotherapy administered for a newly diagnosed, advanced-stage ovarian cancer.1 The study enrolled 391 patients, with 260 randomly assigned to receive olaparib for 24 months and 131 patients randomly assigned to receive placebo tablets. Most patients in the study had a mutation in the BRCA1 gene (72%), 27% had a BRCA2 mutation, and 1% had mutations in both genes.
The primary analysis of SOLO1 was published in 2018 and was based on a median follow-up of 3.4 years.1 That study showed that olaparib maintenance therapy resulted in a large progression-free survival benefit and led to its approval by the US Food and Drug Administration (FDA) as a maintenance therapy for patients with BRCA-mutated advanced ovarian cancer who responded to first-line platinum-based chemotherapy.
In 2021, Banerjee and colleagues updated the progression-free survival results for the SOLO1 trial after 5 years of follow-up.2 In this study, the patients randomly assigned to olaparib maintenance therapy had a persistent and statistically significant progression-free survival benefit, with the median progression-free survival reaching 56 months among the olaparib group compared with 13.8 months in the placebo group (hazard ratio [HR], 0.33; 95% confidence interval [CI], 0.25–0.43).2 Olaparib maintenance therapy resulted in more clinically significant adverse events, including anemia and neutropenia. Serious adverse events occurred in 55 (21%) of the olaparib-treated patients and 17 (13%) of the placebo-treated patients, but no treatment-related adverse events were fatal.
The updated progression-free survival data from the SOLO1 study provides important and promising evidence that frontline PARP inhibitor maintenance therapy may affect long-term remission in an unprecedented proportion of patients with BRCA-related ovarian cancer. Significant, sustained benefit was seen well beyond the end of treatment, and median progression-free survival was an astonishing 3.5 years longer in the olaparib treatment group than among patients who received placebo therapy.
Continue to: Cytoreductive surgery for recurrent ovarian cancer improves survival in well-selected patients...
Cytoreductive surgery for recurrent ovarian cancer improves survival in well-selected patients
Harter P, Sehouli J, Vergote I, et al; DESKTOP III Investigators. Randomized trial of cytoreductive surgery for relapsed ovarian cancer. N Engl J Med. 2021;385:2123- 2131.
In the DESKTOP III trial, Harter and colleagues contribute results to the ongoing discourse surrounding treatment options for patients with recurrent, platinum-sensitive ovarian cancer.3 Systemic therapies continue to be the mainstay of treatment in this setting; however, several groups have attempted to evaluate the role of secondary cytoreductive surgery in this setting.4,5
Specific inclusion criteria employed
The DESKTOP III investigators randomly assigned 407 patients with platinum-sensitive recurrent ovarian cancer to secondary cytoreductive surgery followed by platinum-based chemotherapy (n = 206) or platinum-based chemotherapy alone (n = 201).3 An essential aspect of the study’s design was the use of specific inclusion criteria known to identify patients with a high likelihood of complete resection at the time of secondary cytoreduction.6,7 Patients were eligible only if they had at least a 6-month remission following platinum-based chemotherapy, had a complete resection at their previous surgery, had no restriction on physical activity, and had ascites of no more than 500 mL.
Surgery group had superior overall and progression-free survival
After a median follow-up of approximately 70 months, patients randomly assigned to surgery had superior overall survival (53.7 months) compared with those assigned to chemotherapy alone (46.0 months; HR, 0.75; 95% CI, 0.59–0.96).3 Progression-free survival also was improved among patients who underwent surgery (median 18.4 vs 12.7 months; HR, 0.66; 95% CI, 0.54–0.82). Subgroup analyses did not identify any subset of patients who did not benefit from surgery. Whether a complete resection was achieved at secondary cytoreduction was highly prognostic: Patients who had a complete resection had a median overall survival of 61.9 months compared with 27.7 months in patients with residual disease. There were no deaths within 90 days of surgery.
The DESKTOP III trial provides compelling evidence that secondary cytoreductive surgery improves overall and progression-free survival among well-selected patients with recurrent, platinum-sensitive ovarian cancer. These results differ from those of a recently reported Gynecologic Oncology Group (GOG) trial that failed to detect a survival benefit for secondary cytoreductive surgery among patients with platinum-sensitive recurrent ovarian cancer.5 Key differences, which might explain the studies’ seemingly contradictory results, were that the GOG study had fewer specific eligibility criteria than the DESKTOP III trial, and that bevacizumab was administered much more frequently in the GOG study. It is therefore reasonable to discuss the possible benefits of secondary cytoreductive surgery with patients who meet DESKTOP III eligibility criteria, with a focus toward shared decision making and a candid discussion of the potential risks and benefits of secondary cytoreduction.
Continue to: Immunotherapy enters first-line treatment regimen for advanced cervical cancer...
Immunotherapy enters first-line treatment regimen for advanced cervical cancer
Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
Persistent, recurrent, and metastatic cervical cancer carries a very poor prognosis: Most patients progress less than a year after starting treatment, and fewer than half survive for 2 years. First-line treatment in this setting has been platinum-based chemotherapy, often given with bevacizumab, a humanized monoclonal antibody that inhibits tumor growth by blocking angiogenesis.8 Pembrolizumab, an immune checkpoint inhibitor, targets cancer cells by blocking their ability to evade the immune system, and it is FDA approved and widely administered to patients with advanced cervical cancer who progress after first-line treatment.9
Addition of pembrolizumab extended survival
In the KEYNOTE-826 trial, Colombo and colleagues investigated the efficacy of incorporating an immune checkpoint inhibitor into the first-line treatment regimen for patients with persistent, recurrent, and metastatic cervical cancer.10 Researchers in this double-blinded, phase 3, randomized controlled trial assigned 617 patients to receive pembrolizumab or placebo concurrently with the investigator’s choice platinum-based chemotherapy. Bevacizumab was administered at the discretion of the treating oncologist.
The proportion of patients who survived at least 2 years following randomization was significantly higher among those assigned to pembrolizumab compared with placebo (53% vs 42%; HR, 0.67, 95% CI, 0.54–0.84).10 Similarly, median progression-free survival was superior among patients who received pembrolizumab compared with those who received placebo (10.4 months vs 8.2 months; HR, 0.65; 95% CI, 0.53–0.79). The role of bevacizumab in conjunction with pembrolizumab and platinum-based chemotherapy was not elucidated in this study because bevacizumab administration was not randomly assigned.
Anemia and neutropenia were the most common adverse events and were more frequent in the pembrolizumab group, but there were no new safety concerns related to concurrent use of pembrolizumab with cytotoxic chemotherapy and bevacizumab. Importantly, subgroup analysis results suggested that pembrolizumab was effective only in patients whose tumors expressed PD-L1 (programmed death ligand 1), a biomarker of pembrolizumab sensitivity in cervical cancer.
In light of the significant improvements in overall and progression-free survival demonstrated in the KEYNOTE-826 trial, in October 2021, the FDA approved the use of frontline pembrolizumab alongside platinum-based chemotherapy, with or without bevacizumab, for treatment of patients with persistent, recurrent, or metastatic cervical cancers that express PD-L1.
Continue to: Endometrial cancer surgical staging...
Endometrial cancer surgical staging: Is sentinel lymph node biopsy a viable option for high-risk histologies?
Cusimano MC, Vicus D, Pulman K, et al. Assessment of sentinel lymph node biopsy vs lymphadenectomy for intermediate- and high-grade endometrial cancer staging. JAMA Surg. 2021;156:157-164.
The use of intraoperative sentinel lymph node mapping and biopsy to identify lymph node metastases among patients undergoing surgical staging for endometrial cancer has become increasingly common. Lymph node status is an important prognostic factor, and it guides adjuvant treatment decisions in endometrial cancer. However, traditional pelvic and para-aortic lymphadenectomy is associated with increased risk of lower-extremity lymphedema, postoperative complications, and intraoperative injury.
Sentinel lymph node biopsy seeks to identify lymph node metastases while minimizing surgical morbidity by identifying and excising only lymph nodes that directly receive lymphatic drainage from the uterus. The combination of a fluorescent dye (indocyanine green) and near infrared cameras have led to the broad adoption of sentinel lymph node biopsy in endometrial cancer staging surgery. This practice is supported by prospective studies that demonstrate the high diagnostic accuracy of this approach.11,12 However, because most patients included in prior studies had low-grade endometrial cancer, the utility of sentinel lymph node biopsy in cases of high-grade histology has been less clear.
Sentinel lymph node biopsy vs lymphadenectomy for staging
In the SENTOR trial, Cusimano and colleagues examined the diagnostic accuracy of sentinel lymph node mapping and biopsy, using indocyanine green, in patients with intermediate- or high-grade early-stage endometrial cancer.13
All eligible patients (N = 156) underwent traditional or robot-assisted laparoscopic hysterectomy with sentinel lymph node biopsy. Subsequently, patients with grade 2 endometrioid carcinoma underwent bilateral pelvic lymphadenectomy, and those with high-grade histology (grade 3 endometrioid, serous, carcinosarcoma, clear cell, undifferentiated or dedifferentiated, and mixed high grade) underwent bilateral pelvic and para-aortic lymphadenectomy. The investigators evaluated the diagnostic characteristics of sentinel lymph node biopsy, treating complete lymphadenectomy as the gold standard.
Of the 156 patients enrolled, the median age was 65.5 and median body mass index was 27.5; 126 patients (81%) had high-grade histology. The sentinel lymph node biopsy had a sensitivity of 96% (95% CI, 81%–100%), identifying 26 of the 27 patients with nodal metastases. The false-negative rate was 4% (95% CI, 0%–9%) and the negative predictive value was 99% (95% CI, 96%–100%). Intraoperative adverse events occurred in 5 patients (3%), but none occurred during the sentinel lymph node biopsy. ●
The high sensitivity and negative predictive value of sentinel lymph node biopsy in the intermediate- and high-grade cohort included in the SENTOR trial are concordant with prior studies that predominantly included patients with low-grade endometrial cancer. These findings suggest that sentinel lymph node mapping and biopsy is a reasonable option for surgical staging, not only for patients with low-grade endometrial cancer but also for those with intermediate- and high-grade disease.
Despite the challenges of an ongoing COVID-19 pandemic, researchers in 2021 delivered practice-changing studies in gynecologic oncology. In this cancer Update, we highlight 4 studies that shed light on the surgical and systemic therapies that may improve outcomes for patients with cancers of the ovary, endometrium, and cervix. We review DESKTOP III, a trial that investigated the role of cytoreductive surgery in patients with recurrent ovarian cancer, and SENTOR, a study that evaluated the performance of sentinel lymph node biopsy in patients with high-grade endometrial cancers. Additionally, we examine 2 studies of systemic therapy that reveal the growing role of targeted therapies and immuno-oncology in the treatment of gynecologic malignancies.
A new era for patients with BRCA mutation–associated ovarian cancer
Banerjee S, Moore KN, Colombo N, et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22:1721-1731.
Ovarian cancer remains the most lethal gynecologic malignancy due to the frequency of advanced-stage diagnosis and frequent relapse after primary therapy. But for ovarian cancer patients with inherited mutations of the BRCA1 or BRCA2 genes, poly(ADP-ribose) polymerase (PARP) inhibitors, a class of oral anticancer medicines that target DNA repair, have ushered in a new era in which the possibility of long-term remission, and even cure, is more likely than at any other time.
Olaparib trial details
The SOLO1 study was a double-blind, placebo-controlled, phase 3 trial that investigated the role of PARP inhibitor maintenance therapy with olaparib in patients with pathologic BRCA1 or BRCA2 mutations who responded to platinum-based chemotherapy administered for a newly diagnosed, advanced-stage ovarian cancer.1 The study enrolled 391 patients, with 260 randomly assigned to receive olaparib for 24 months and 131 patients randomly assigned to receive placebo tablets. Most patients in the study had a mutation in the BRCA1 gene (72%), 27% had a BRCA2 mutation, and 1% had mutations in both genes.
The primary analysis of SOLO1 was published in 2018 and was based on a median follow-up of 3.4 years.1 That study showed that olaparib maintenance therapy resulted in a large progression-free survival benefit and led to its approval by the US Food and Drug Administration (FDA) as a maintenance therapy for patients with BRCA-mutated advanced ovarian cancer who responded to first-line platinum-based chemotherapy.
In 2021, Banerjee and colleagues updated the progression-free survival results for the SOLO1 trial after 5 years of follow-up.2 In this study, the patients randomly assigned to olaparib maintenance therapy had a persistent and statistically significant progression-free survival benefit, with the median progression-free survival reaching 56 months among the olaparib group compared with 13.8 months in the placebo group (hazard ratio [HR], 0.33; 95% confidence interval [CI], 0.25–0.43).2 Olaparib maintenance therapy resulted in more clinically significant adverse events, including anemia and neutropenia. Serious adverse events occurred in 55 (21%) of the olaparib-treated patients and 17 (13%) of the placebo-treated patients, but no treatment-related adverse events were fatal.
The updated progression-free survival data from the SOLO1 study provides important and promising evidence that frontline PARP inhibitor maintenance therapy may affect long-term remission in an unprecedented proportion of patients with BRCA-related ovarian cancer. Significant, sustained benefit was seen well beyond the end of treatment, and median progression-free survival was an astonishing 3.5 years longer in the olaparib treatment group than among patients who received placebo therapy.
Continue to: Cytoreductive surgery for recurrent ovarian cancer improves survival in well-selected patients...
Cytoreductive surgery for recurrent ovarian cancer improves survival in well-selected patients
Harter P, Sehouli J, Vergote I, et al; DESKTOP III Investigators. Randomized trial of cytoreductive surgery for relapsed ovarian cancer. N Engl J Med. 2021;385:2123- 2131.
In the DESKTOP III trial, Harter and colleagues contribute results to the ongoing discourse surrounding treatment options for patients with recurrent, platinum-sensitive ovarian cancer.3 Systemic therapies continue to be the mainstay of treatment in this setting; however, several groups have attempted to evaluate the role of secondary cytoreductive surgery in this setting.4,5
Specific inclusion criteria employed
The DESKTOP III investigators randomly assigned 407 patients with platinum-sensitive recurrent ovarian cancer to secondary cytoreductive surgery followed by platinum-based chemotherapy (n = 206) or platinum-based chemotherapy alone (n = 201).3 An essential aspect of the study’s design was the use of specific inclusion criteria known to identify patients with a high likelihood of complete resection at the time of secondary cytoreduction.6,7 Patients were eligible only if they had at least a 6-month remission following platinum-based chemotherapy, had a complete resection at their previous surgery, had no restriction on physical activity, and had ascites of no more than 500 mL.
Surgery group had superior overall and progression-free survival
After a median follow-up of approximately 70 months, patients randomly assigned to surgery had superior overall survival (53.7 months) compared with those assigned to chemotherapy alone (46.0 months; HR, 0.75; 95% CI, 0.59–0.96).3 Progression-free survival also was improved among patients who underwent surgery (median 18.4 vs 12.7 months; HR, 0.66; 95% CI, 0.54–0.82). Subgroup analyses did not identify any subset of patients who did not benefit from surgery. Whether a complete resection was achieved at secondary cytoreduction was highly prognostic: Patients who had a complete resection had a median overall survival of 61.9 months compared with 27.7 months in patients with residual disease. There were no deaths within 90 days of surgery.
The DESKTOP III trial provides compelling evidence that secondary cytoreductive surgery improves overall and progression-free survival among well-selected patients with recurrent, platinum-sensitive ovarian cancer. These results differ from those of a recently reported Gynecologic Oncology Group (GOG) trial that failed to detect a survival benefit for secondary cytoreductive surgery among patients with platinum-sensitive recurrent ovarian cancer.5 Key differences, which might explain the studies’ seemingly contradictory results, were that the GOG study had fewer specific eligibility criteria than the DESKTOP III trial, and that bevacizumab was administered much more frequently in the GOG study. It is therefore reasonable to discuss the possible benefits of secondary cytoreductive surgery with patients who meet DESKTOP III eligibility criteria, with a focus toward shared decision making and a candid discussion of the potential risks and benefits of secondary cytoreduction.
Continue to: Immunotherapy enters first-line treatment regimen for advanced cervical cancer...
Immunotherapy enters first-line treatment regimen for advanced cervical cancer
Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
Persistent, recurrent, and metastatic cervical cancer carries a very poor prognosis: Most patients progress less than a year after starting treatment, and fewer than half survive for 2 years. First-line treatment in this setting has been platinum-based chemotherapy, often given with bevacizumab, a humanized monoclonal antibody that inhibits tumor growth by blocking angiogenesis.8 Pembrolizumab, an immune checkpoint inhibitor, targets cancer cells by blocking their ability to evade the immune system, and it is FDA approved and widely administered to patients with advanced cervical cancer who progress after first-line treatment.9
Addition of pembrolizumab extended survival
In the KEYNOTE-826 trial, Colombo and colleagues investigated the efficacy of incorporating an immune checkpoint inhibitor into the first-line treatment regimen for patients with persistent, recurrent, and metastatic cervical cancer.10 Researchers in this double-blinded, phase 3, randomized controlled trial assigned 617 patients to receive pembrolizumab or placebo concurrently with the investigator’s choice platinum-based chemotherapy. Bevacizumab was administered at the discretion of the treating oncologist.
The proportion of patients who survived at least 2 years following randomization was significantly higher among those assigned to pembrolizumab compared with placebo (53% vs 42%; HR, 0.67, 95% CI, 0.54–0.84).10 Similarly, median progression-free survival was superior among patients who received pembrolizumab compared with those who received placebo (10.4 months vs 8.2 months; HR, 0.65; 95% CI, 0.53–0.79). The role of bevacizumab in conjunction with pembrolizumab and platinum-based chemotherapy was not elucidated in this study because bevacizumab administration was not randomly assigned.
Anemia and neutropenia were the most common adverse events and were more frequent in the pembrolizumab group, but there were no new safety concerns related to concurrent use of pembrolizumab with cytotoxic chemotherapy and bevacizumab. Importantly, subgroup analysis results suggested that pembrolizumab was effective only in patients whose tumors expressed PD-L1 (programmed death ligand 1), a biomarker of pembrolizumab sensitivity in cervical cancer.
In light of the significant improvements in overall and progression-free survival demonstrated in the KEYNOTE-826 trial, in October 2021, the FDA approved the use of frontline pembrolizumab alongside platinum-based chemotherapy, with or without bevacizumab, for treatment of patients with persistent, recurrent, or metastatic cervical cancers that express PD-L1.
Continue to: Endometrial cancer surgical staging...
Endometrial cancer surgical staging: Is sentinel lymph node biopsy a viable option for high-risk histologies?
Cusimano MC, Vicus D, Pulman K, et al. Assessment of sentinel lymph node biopsy vs lymphadenectomy for intermediate- and high-grade endometrial cancer staging. JAMA Surg. 2021;156:157-164.
The use of intraoperative sentinel lymph node mapping and biopsy to identify lymph node metastases among patients undergoing surgical staging for endometrial cancer has become increasingly common. Lymph node status is an important prognostic factor, and it guides adjuvant treatment decisions in endometrial cancer. However, traditional pelvic and para-aortic lymphadenectomy is associated with increased risk of lower-extremity lymphedema, postoperative complications, and intraoperative injury.
Sentinel lymph node biopsy seeks to identify lymph node metastases while minimizing surgical morbidity by identifying and excising only lymph nodes that directly receive lymphatic drainage from the uterus. The combination of a fluorescent dye (indocyanine green) and near infrared cameras have led to the broad adoption of sentinel lymph node biopsy in endometrial cancer staging surgery. This practice is supported by prospective studies that demonstrate the high diagnostic accuracy of this approach.11,12 However, because most patients included in prior studies had low-grade endometrial cancer, the utility of sentinel lymph node biopsy in cases of high-grade histology has been less clear.
Sentinel lymph node biopsy vs lymphadenectomy for staging
In the SENTOR trial, Cusimano and colleagues examined the diagnostic accuracy of sentinel lymph node mapping and biopsy, using indocyanine green, in patients with intermediate- or high-grade early-stage endometrial cancer.13
All eligible patients (N = 156) underwent traditional or robot-assisted laparoscopic hysterectomy with sentinel lymph node biopsy. Subsequently, patients with grade 2 endometrioid carcinoma underwent bilateral pelvic lymphadenectomy, and those with high-grade histology (grade 3 endometrioid, serous, carcinosarcoma, clear cell, undifferentiated or dedifferentiated, and mixed high grade) underwent bilateral pelvic and para-aortic lymphadenectomy. The investigators evaluated the diagnostic characteristics of sentinel lymph node biopsy, treating complete lymphadenectomy as the gold standard.
Of the 156 patients enrolled, the median age was 65.5 and median body mass index was 27.5; 126 patients (81%) had high-grade histology. The sentinel lymph node biopsy had a sensitivity of 96% (95% CI, 81%–100%), identifying 26 of the 27 patients with nodal metastases. The false-negative rate was 4% (95% CI, 0%–9%) and the negative predictive value was 99% (95% CI, 96%–100%). Intraoperative adverse events occurred in 5 patients (3%), but none occurred during the sentinel lymph node biopsy. ●
The high sensitivity and negative predictive value of sentinel lymph node biopsy in the intermediate- and high-grade cohort included in the SENTOR trial are concordant with prior studies that predominantly included patients with low-grade endometrial cancer. These findings suggest that sentinel lymph node mapping and biopsy is a reasonable option for surgical staging, not only for patients with low-grade endometrial cancer but also for those with intermediate- and high-grade disease.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Banerjee S, Moore KN, Colombo N, et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22:1721-1731.
- Harter P, Sehouli J, Vergote I, et al; DESKTOP III Investigators. Randomized trial of cytoreductive surgery for relapsed ovarian cancer. N Engl J Med. 2021;385:2123-2131.
- Shi T, Zhu J, Feng Y, et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:439-449.
- Coleman RL, Spiritos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Harter P, du Bois A, Hahmann M, et al; Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Committee; AGO Ovarian Cancer Study Group. Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann Surg Oncol. 2006;13:1702-1710.
- Harter P, Sehouli J, Reuss A, et al. Prospective validation study of a predictive score for operability of recurrent ovarian cancer: the Multicenter Intergroup Study DESKTOP II. A project of the AGO Kommission OVAR, AGO Study Group, NOGGO, AGO-Austria, and MITO. Int J Gynecol Cancer. 2011;21: 289-295.
- Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. 2017;390:1654-1663.
- Frenel JS, Le Tourneau C, O’Neil B, et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: results from the phase Ib KEYNOTE-028 trial. J Clin Oncol. 2017;35:4035-4041.
- Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
- Rossi EC, Kowalski L, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18:384-392.
- Ballester M, Dubernard G, Lecuru F, et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: a prospective multicentre study (SENTIENDO). Lancet Oncol. 2011;12: 469-476.
- Cusimano MC, Vicus D, Pulman K, et al. Assessment of sentinel lymph node biopsy vs lymphadenectomy for intermediate- and high-grade endometrial cancer staging. JAMA Surg. 2021;156:157-164.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Banerjee S, Moore KN, Colombo N, et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22:1721-1731.
- Harter P, Sehouli J, Vergote I, et al; DESKTOP III Investigators. Randomized trial of cytoreductive surgery for relapsed ovarian cancer. N Engl J Med. 2021;385:2123-2131.
- Shi T, Zhu J, Feng Y, et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:439-449.
- Coleman RL, Spiritos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Harter P, du Bois A, Hahmann M, et al; Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Committee; AGO Ovarian Cancer Study Group. Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann Surg Oncol. 2006;13:1702-1710.
- Harter P, Sehouli J, Reuss A, et al. Prospective validation study of a predictive score for operability of recurrent ovarian cancer: the Multicenter Intergroup Study DESKTOP II. A project of the AGO Kommission OVAR, AGO Study Group, NOGGO, AGO-Austria, and MITO. Int J Gynecol Cancer. 2011;21: 289-295.
- Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. 2017;390:1654-1663.
- Frenel JS, Le Tourneau C, O’Neil B, et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: results from the phase Ib KEYNOTE-028 trial. J Clin Oncol. 2017;35:4035-4041.
- Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
- Rossi EC, Kowalski L, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18:384-392.
- Ballester M, Dubernard G, Lecuru F, et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: a prospective multicentre study (SENTIENDO). Lancet Oncol. 2011;12: 469-476.
- Cusimano MC, Vicus D, Pulman K, et al. Assessment of sentinel lymph node biopsy vs lymphadenectomy for intermediate- and high-grade endometrial cancer staging. JAMA Surg. 2021;156:157-164.
Antiseptic as good as antibiotics for preventing recurrent UTI
The antiseptic methenamine hippurate (MH) is known to sterilize urine and has been suggested to be of use in preventing urinary tract infections (UTIs), but firm evidence has so far been lacking. Now researchers led by clinicians and scientists from Newcastle-upon-Tyne, England, have provided the ALTAR trial (Alternative to Prophylactic Antibiotics for the Treatment of Recurrent UTIs in Women).
Daily low-dose antibiotics as recommended by current guidelines for prophylactic treatment of recurrent UTI have been linked to antibiotic resistance. Using MH as an alternative could play an important role in helping to tackle the global problem of increasing antibiotic resistance, the team said.
Study details
They recruited 240 women aged 18 or over with recurrent UTIs requiring prophylactic treatment from eight secondary care urology and urogynecology centers in the United Kingdom from June 2016 to June 2018. Women were randomized to receive MH or daily low-dose antibiotics for 12 months, with follow up for a further 6 months beyond that.
Before trial entry the women had experienced an average of more than six UTI episodes per year. During the 12-month treatment period, in the modified intention-to-treat population, there were 90 symptomatic, antibiotic-treated UTI episodes reported over 101 person-years of follow-up in the antibiotic group, and 141 episodes over 102 person-years in the MH group.
This yielded a UTI rate of 0.89 episodes per person-year in the antibiotic group, compared with 1.38 in the MH group, an absolute difference of 0.49 episodes per person-year. In the 6-month posttreatment follow-up period, the UTI incidence rate was 1.19 episodes per person-year in the antibiotic prophylaxis group versus 1.72 in the MH group, an absolute difference of 0.53.
Before the trial, a patient and public involvement group had predefined the noninferiority margin as one episode of UTI per person-year. The small difference between the two groups was less than this, confirming noninferiority of MH to antibiotic prophylaxis in this setting. This finding was consistent across the modified intention-to-treat, strict intention-to-treat, per protocol, and modified per protocol (post hoc) analyses.
Thus the ALTAR results showed that MH was no worse than antibiotics at preventing UTIs, and MH was also associated with reduced antibiotic consumption.
The vast majority of participants were over 90% adherent with the allocated treatment. Patient satisfaction was generally high and rates of adverse events and adverse reactions generally low, and both were comparable between treatment groups. Adverse reactions were reported by 34/142 (24%) in the antibiotic group and 35/127 (28%) in the MH group, and most reactions were mild. In the antibiotic group there were two serious adverse reactions (severe abdominal pain and raised alanine transaminase), whereas six participants in the MH group reported an episode of febrile UTI and four were admitted to hospital because of UTI.
Substantial global health care problem
At least 50% and up to 80% of all women have at least one acute UTI in their lifetime, most often uncomplicated acute cystitis. About a quarter of them go on to suffer recurrent infection, defined as three or more repeat infections in the past year, or two infections in the preceding 6 months. Frequent recurrences thus represent “a substantial global health care problem,” the authors say.
Guidelines from the United Kingdom, Europe, and the United States acknowledge the need for preventive strategies and strongly recommend the use of daily, low-dose antibiotics as standard prophylactic treatment. However, the United Kingdom’s antimicrobial resistance strategy recommends a “strong focus on infection prevention,” and aims to reduce antimicrobial use in humans by 15% before 2024.
“To achieve that, exploration of nonantibiotic preventive treatments in common conditions such as UTI is essential,” the team said.
MH is one such nonantibiotic treatment. It is bactericidal and works by denaturing bacterial proteins and nucleic acids. Although previous Cochrane systematic reviews had concluded that it could be effective for preventing UTI, further large trials were needed.
“This trial adds to the evidence base for the use of MH for prophylactic treatment in adult women with recurrent UTI. Although the MH group had a 55% higher rate of UTI episodes than the antibiotics group, the absolute difference was just 0.49 UTI episodes per year, which has limited clinical consequence,” the team concluded.
Results could ‘support a change in practice’
In older patients, particularly, the risks of long-term antibiotic prophylaxis might outweigh the benefits, and the authors said that their results “could support a change in practice in terms of preventive treatments for recurrent UTI and provide patients and clinicians with a credible alternative to daily antibiotics, giving them the confidence to pursue strategies that avoid long-term antibiotic use.”
They acknowledged limitations of the study, including that treatment allocation was not masked, crossover between arms was allowed, and differences in antibiotics prescribed may have affected the results. In addition, data regarding long-term safety of MH are scarce.
However, they said that the trial accurately represented the broad range of women with recurrent UTI, and that its results “might encourage patients and clinicians to consider MH as a first line treatment for UTI prevention in women.”
In a linked editorial, scientists from the Institute for Evidence-Based Healthcare at Bond University in Queensland, Australia, commented: “Although the results need cautious interpretation, they align with others, and this new research increases the confidence with which MH can be offered as an option to women needing prophylaxis against recurrent urinary tract infection.”
References
Harding C et al. Alternative to prophylactic antibiotics for the treatment of recurrent urinary tract infections in women: multicentre, open label, randomised, noninferiority trial. BMJ 2022 Mar 9;376:e068229.
Hoffmann TC et al. Methenamine hippurate for recurrent urinary tract infections. BMJ 2022 Mar 9;376:o533.
A version of this article first appeared on Medscape.co.uk.
The antiseptic methenamine hippurate (MH) is known to sterilize urine and has been suggested to be of use in preventing urinary tract infections (UTIs), but firm evidence has so far been lacking. Now researchers led by clinicians and scientists from Newcastle-upon-Tyne, England, have provided the ALTAR trial (Alternative to Prophylactic Antibiotics for the Treatment of Recurrent UTIs in Women).
Daily low-dose antibiotics as recommended by current guidelines for prophylactic treatment of recurrent UTI have been linked to antibiotic resistance. Using MH as an alternative could play an important role in helping to tackle the global problem of increasing antibiotic resistance, the team said.
Study details
They recruited 240 women aged 18 or over with recurrent UTIs requiring prophylactic treatment from eight secondary care urology and urogynecology centers in the United Kingdom from June 2016 to June 2018. Women were randomized to receive MH or daily low-dose antibiotics for 12 months, with follow up for a further 6 months beyond that.
Before trial entry the women had experienced an average of more than six UTI episodes per year. During the 12-month treatment period, in the modified intention-to-treat population, there were 90 symptomatic, antibiotic-treated UTI episodes reported over 101 person-years of follow-up in the antibiotic group, and 141 episodes over 102 person-years in the MH group.
This yielded a UTI rate of 0.89 episodes per person-year in the antibiotic group, compared with 1.38 in the MH group, an absolute difference of 0.49 episodes per person-year. In the 6-month posttreatment follow-up period, the UTI incidence rate was 1.19 episodes per person-year in the antibiotic prophylaxis group versus 1.72 in the MH group, an absolute difference of 0.53.
Before the trial, a patient and public involvement group had predefined the noninferiority margin as one episode of UTI per person-year. The small difference between the two groups was less than this, confirming noninferiority of MH to antibiotic prophylaxis in this setting. This finding was consistent across the modified intention-to-treat, strict intention-to-treat, per protocol, and modified per protocol (post hoc) analyses.
Thus the ALTAR results showed that MH was no worse than antibiotics at preventing UTIs, and MH was also associated with reduced antibiotic consumption.
The vast majority of participants were over 90% adherent with the allocated treatment. Patient satisfaction was generally high and rates of adverse events and adverse reactions generally low, and both were comparable between treatment groups. Adverse reactions were reported by 34/142 (24%) in the antibiotic group and 35/127 (28%) in the MH group, and most reactions were mild. In the antibiotic group there were two serious adverse reactions (severe abdominal pain and raised alanine transaminase), whereas six participants in the MH group reported an episode of febrile UTI and four were admitted to hospital because of UTI.
Substantial global health care problem
At least 50% and up to 80% of all women have at least one acute UTI in their lifetime, most often uncomplicated acute cystitis. About a quarter of them go on to suffer recurrent infection, defined as three or more repeat infections in the past year, or two infections in the preceding 6 months. Frequent recurrences thus represent “a substantial global health care problem,” the authors say.
Guidelines from the United Kingdom, Europe, and the United States acknowledge the need for preventive strategies and strongly recommend the use of daily, low-dose antibiotics as standard prophylactic treatment. However, the United Kingdom’s antimicrobial resistance strategy recommends a “strong focus on infection prevention,” and aims to reduce antimicrobial use in humans by 15% before 2024.
“To achieve that, exploration of nonantibiotic preventive treatments in common conditions such as UTI is essential,” the team said.
MH is one such nonantibiotic treatment. It is bactericidal and works by denaturing bacterial proteins and nucleic acids. Although previous Cochrane systematic reviews had concluded that it could be effective for preventing UTI, further large trials were needed.
“This trial adds to the evidence base for the use of MH for prophylactic treatment in adult women with recurrent UTI. Although the MH group had a 55% higher rate of UTI episodes than the antibiotics group, the absolute difference was just 0.49 UTI episodes per year, which has limited clinical consequence,” the team concluded.
Results could ‘support a change in practice’
In older patients, particularly, the risks of long-term antibiotic prophylaxis might outweigh the benefits, and the authors said that their results “could support a change in practice in terms of preventive treatments for recurrent UTI and provide patients and clinicians with a credible alternative to daily antibiotics, giving them the confidence to pursue strategies that avoid long-term antibiotic use.”
They acknowledged limitations of the study, including that treatment allocation was not masked, crossover between arms was allowed, and differences in antibiotics prescribed may have affected the results. In addition, data regarding long-term safety of MH are scarce.
However, they said that the trial accurately represented the broad range of women with recurrent UTI, and that its results “might encourage patients and clinicians to consider MH as a first line treatment for UTI prevention in women.”
In a linked editorial, scientists from the Institute for Evidence-Based Healthcare at Bond University in Queensland, Australia, commented: “Although the results need cautious interpretation, they align with others, and this new research increases the confidence with which MH can be offered as an option to women needing prophylaxis against recurrent urinary tract infection.”
References
Harding C et al. Alternative to prophylactic antibiotics for the treatment of recurrent urinary tract infections in women: multicentre, open label, randomised, noninferiority trial. BMJ 2022 Mar 9;376:e068229.
Hoffmann TC et al. Methenamine hippurate for recurrent urinary tract infections. BMJ 2022 Mar 9;376:o533.
A version of this article first appeared on Medscape.co.uk.
The antiseptic methenamine hippurate (MH) is known to sterilize urine and has been suggested to be of use in preventing urinary tract infections (UTIs), but firm evidence has so far been lacking. Now researchers led by clinicians and scientists from Newcastle-upon-Tyne, England, have provided the ALTAR trial (Alternative to Prophylactic Antibiotics for the Treatment of Recurrent UTIs in Women).
Daily low-dose antibiotics as recommended by current guidelines for prophylactic treatment of recurrent UTI have been linked to antibiotic resistance. Using MH as an alternative could play an important role in helping to tackle the global problem of increasing antibiotic resistance, the team said.
Study details
They recruited 240 women aged 18 or over with recurrent UTIs requiring prophylactic treatment from eight secondary care urology and urogynecology centers in the United Kingdom from June 2016 to June 2018. Women were randomized to receive MH or daily low-dose antibiotics for 12 months, with follow up for a further 6 months beyond that.
Before trial entry the women had experienced an average of more than six UTI episodes per year. During the 12-month treatment period, in the modified intention-to-treat population, there were 90 symptomatic, antibiotic-treated UTI episodes reported over 101 person-years of follow-up in the antibiotic group, and 141 episodes over 102 person-years in the MH group.
This yielded a UTI rate of 0.89 episodes per person-year in the antibiotic group, compared with 1.38 in the MH group, an absolute difference of 0.49 episodes per person-year. In the 6-month posttreatment follow-up period, the UTI incidence rate was 1.19 episodes per person-year in the antibiotic prophylaxis group versus 1.72 in the MH group, an absolute difference of 0.53.
Before the trial, a patient and public involvement group had predefined the noninferiority margin as one episode of UTI per person-year. The small difference between the two groups was less than this, confirming noninferiority of MH to antibiotic prophylaxis in this setting. This finding was consistent across the modified intention-to-treat, strict intention-to-treat, per protocol, and modified per protocol (post hoc) analyses.
Thus the ALTAR results showed that MH was no worse than antibiotics at preventing UTIs, and MH was also associated with reduced antibiotic consumption.
The vast majority of participants were over 90% adherent with the allocated treatment. Patient satisfaction was generally high and rates of adverse events and adverse reactions generally low, and both were comparable between treatment groups. Adverse reactions were reported by 34/142 (24%) in the antibiotic group and 35/127 (28%) in the MH group, and most reactions were mild. In the antibiotic group there were two serious adverse reactions (severe abdominal pain and raised alanine transaminase), whereas six participants in the MH group reported an episode of febrile UTI and four were admitted to hospital because of UTI.
Substantial global health care problem
At least 50% and up to 80% of all women have at least one acute UTI in their lifetime, most often uncomplicated acute cystitis. About a quarter of them go on to suffer recurrent infection, defined as three or more repeat infections in the past year, or two infections in the preceding 6 months. Frequent recurrences thus represent “a substantial global health care problem,” the authors say.
Guidelines from the United Kingdom, Europe, and the United States acknowledge the need for preventive strategies and strongly recommend the use of daily, low-dose antibiotics as standard prophylactic treatment. However, the United Kingdom’s antimicrobial resistance strategy recommends a “strong focus on infection prevention,” and aims to reduce antimicrobial use in humans by 15% before 2024.
“To achieve that, exploration of nonantibiotic preventive treatments in common conditions such as UTI is essential,” the team said.
MH is one such nonantibiotic treatment. It is bactericidal and works by denaturing bacterial proteins and nucleic acids. Although previous Cochrane systematic reviews had concluded that it could be effective for preventing UTI, further large trials were needed.
“This trial adds to the evidence base for the use of MH for prophylactic treatment in adult women with recurrent UTI. Although the MH group had a 55% higher rate of UTI episodes than the antibiotics group, the absolute difference was just 0.49 UTI episodes per year, which has limited clinical consequence,” the team concluded.
Results could ‘support a change in practice’
In older patients, particularly, the risks of long-term antibiotic prophylaxis might outweigh the benefits, and the authors said that their results “could support a change in practice in terms of preventive treatments for recurrent UTI and provide patients and clinicians with a credible alternative to daily antibiotics, giving them the confidence to pursue strategies that avoid long-term antibiotic use.”
They acknowledged limitations of the study, including that treatment allocation was not masked, crossover between arms was allowed, and differences in antibiotics prescribed may have affected the results. In addition, data regarding long-term safety of MH are scarce.
However, they said that the trial accurately represented the broad range of women with recurrent UTI, and that its results “might encourage patients and clinicians to consider MH as a first line treatment for UTI prevention in women.”
In a linked editorial, scientists from the Institute for Evidence-Based Healthcare at Bond University in Queensland, Australia, commented: “Although the results need cautious interpretation, they align with others, and this new research increases the confidence with which MH can be offered as an option to women needing prophylaxis against recurrent urinary tract infection.”
References
Harding C et al. Alternative to prophylactic antibiotics for the treatment of recurrent urinary tract infections in women: multicentre, open label, randomised, noninferiority trial. BMJ 2022 Mar 9;376:e068229.
Hoffmann TC et al. Methenamine hippurate for recurrent urinary tract infections. BMJ 2022 Mar 9;376:o533.
A version of this article first appeared on Medscape.co.uk.
Irregular and long periods linked to NAFLD
Long or irregular menstrual cycles in relatively young women are linked an increased risk of both prevalent and incident nonalcoholic fatty liver disease (NAFLD), according to a cross-sectional study that included data on more than 70,000 women.
“Our results indicate that menstrual irregularity, which is easier to diagnose and usually presented earlier than PCOS [polycystic ovary syndrome] highlights the possibility of identifying premenopausal women at risk of developing NAFLD,” reported a team of authors primarily from Sungkyunkwan University, Seoul, South Korea.
The study evaluated women aged younger than 40 years who were participating in the Kangbuk Samsung Health Study, which involves a comprehensive biennial health examination at health centers in South Korea. Of the 135,090 women enrolled over a 6-year period who had at least one follow-up examination, 72,092 were available for analysis after excluding for a sizable list of confounding factors such as liver disease and infections; exposure to steatogenic medications, such as corticosteroids; hysterectomy; and pregnancy.
NAFLD prevalence climbs with longer menses
Of these women, 36.378 (27.7%) had menstrual cycles of 26-30 days and were identified as the index group. The prevalence of NAFLD in this group was 5.8%. For those with a menstrual cycle of 31-39 days, the prevalence rate climbed to 7.2%. For those with a menstrual cycle of at least 40 days or too irregular to estimate, the prevalence was 9.7%. The prevalence was 7.1% for those with a menstrual cycle less than 21 days.
The results of this study were published in the Journal of Clinical Endocrinology & Metabolism.
In those without NAFLD at baseline who were then followed for a mean of 4.4 years, there were 4,524 incident cases of NAFLD. Incidence density was calculated per 103 patient-years. In the index group, the rate was 18.4. It climbed to 20.2 for those with a menstrual cycle of 31-39 days and then to 22.9 for those with a menstrual cycle of at least 40 days. For those with a cycle of fewer than 21 days, the rate was 26.8.
After adjusting for age, body mass index, insulin resistance, and other confounders, the hazard ratio for incident NAFLD for those with long or irregular menstrual cycles compared with the incident group corresponded with a 22% increased risk (HR, 1.22; 95% confidence interval, 1.14-1.31). When calculated in a time-dependent analysis, the risk of NAFLD was increased by almost 50% (HR, 1.49; 95% CI, 1.38-1.60).
Risk persists with PCOS exclusion
PCOS has previously been associated with increased risk of NAFLD, but the association between long or irregular menstrual cycles and NAFLD persisted after women with PCOS were excluded.
The mechanism that links menstrual irregularity with NAFLD is unclear, but the investigators said that estrogen exposure is implicated. In addition to a previously reported associated between low estradiol levels and antiestrogens such as tamoxifen with increased risk of NAFLD, they cited studies associating estrogen replacement therapy with a reduced risk of NAFLD. The role of estrogen in suppressing inflammation, oxidative stress, and insulin resistance are all activities that might link more regular menses with a reduced risk of NAFLD, the authors contended.
Women older than 40 years were excluded from this analysis to reduce the possibility of perimenopausal changes as a confounding factor.
Of study limitations acknowledged by the investigators, the presence of NAFLD was diagnosed on ultrasonography rather than histology. Information on sex hormone or prolactin levels was not captured in relation to NAFLD incidence, and the lack of exposure to estrogen replacement therapy and oral contraceptives was based on self-reports from the participants.
Still, the large study size and the consistency of results after adjustment for multiple risk factors argue that long and irregular menstrual cycles do identify women at risk for NAFLD. One implication is that irregular menses can be a marker for NAFLD risk.
“Our findings do not prove a causal relationship, but they show that long or irregular menstrual cycles were significantly associated with an increased risk of developing NAFLD,” said Seungho Ryu, MD, PhD, a professor at the Sungkyunkwan University. Senior author of this study, Dr. Ryu emphasized in an interview that the association “was not explained by obesity or any other risk factor for NAFLD.”
Lifestyle changes may lower risk
The message is that “young women with long or irregular menstrual cycles may benefit from lifestyle changes to reduce the risk of NAFLD,” Dr. Ryu stated.
The Study of Women’s Health Across the Nation, which was started in 1994, has not evaluated NAFLD, but it did show a relationship between longer menstrual cycles and more cardiometabolic risk factors, according to Nanette Santoro MD, professor and chair, department of obstetrics & gynecology, University of Colorado at Denver, Aurora.
This suggests that others are “thinking along the same lines,” but in discussing this study with this news organization, she characterized some of the design elements as well as some of the findings in this study as “peculiar.”
In addition to a “very, very narrow definition of regular cycles,” she questioned the consistent hazard ratio for NAFLD for those with long cycles relative to other types of irregular menses. Presuming that the group with longer cycles would have included at least some patients with undiagnosed PCOS, she was would have expected that the risk would have been highest in this group. While conceding that differences in body composition of Korean women is a potential explanation for this apparent discrepancy, “I would like to see confirmed in other samples of women with more detailed metabolic assessments to understand who is at risk,” she said.
Not least problematic for the strength of the conclusions, the hazard ratio for NAFLD among women with long or irregular menstrual cycles was “pretty low.” She described this as a level at which the risk “is very susceptible to confounding and unlikely to influence clinical practice.”
Anuja Dokras, MD, PHD, a professor of obstetrics and gynecology and director of the PCOS Center at the University of Pennsylvania, Philadelphia, also questioned whether undiagnosed PCOS might have skewed the data.
“There is increasing data on the association between PCOS and NAFLD. Irregular menses is a key criterion for PCOS, and PCOS is the commonest reason for anovulation,” she said. Dr. Dokras therefore considered it possible that patients with unrecognized PCOS were included in the study, weakening the claim that risk of NAFLD and long menstrual cycles remains significant after controlling for PCOS.
Dr. Ryu and coinvestigators, Dr. Santoro, and Dr. Dokras reported no potential conflicts of interest.
Long or irregular menstrual cycles in relatively young women are linked an increased risk of both prevalent and incident nonalcoholic fatty liver disease (NAFLD), according to a cross-sectional study that included data on more than 70,000 women.
“Our results indicate that menstrual irregularity, which is easier to diagnose and usually presented earlier than PCOS [polycystic ovary syndrome] highlights the possibility of identifying premenopausal women at risk of developing NAFLD,” reported a team of authors primarily from Sungkyunkwan University, Seoul, South Korea.
The study evaluated women aged younger than 40 years who were participating in the Kangbuk Samsung Health Study, which involves a comprehensive biennial health examination at health centers in South Korea. Of the 135,090 women enrolled over a 6-year period who had at least one follow-up examination, 72,092 were available for analysis after excluding for a sizable list of confounding factors such as liver disease and infections; exposure to steatogenic medications, such as corticosteroids; hysterectomy; and pregnancy.
NAFLD prevalence climbs with longer menses
Of these women, 36.378 (27.7%) had menstrual cycles of 26-30 days and were identified as the index group. The prevalence of NAFLD in this group was 5.8%. For those with a menstrual cycle of 31-39 days, the prevalence rate climbed to 7.2%. For those with a menstrual cycle of at least 40 days or too irregular to estimate, the prevalence was 9.7%. The prevalence was 7.1% for those with a menstrual cycle less than 21 days.
The results of this study were published in the Journal of Clinical Endocrinology & Metabolism.
In those without NAFLD at baseline who were then followed for a mean of 4.4 years, there were 4,524 incident cases of NAFLD. Incidence density was calculated per 103 patient-years. In the index group, the rate was 18.4. It climbed to 20.2 for those with a menstrual cycle of 31-39 days and then to 22.9 for those with a menstrual cycle of at least 40 days. For those with a cycle of fewer than 21 days, the rate was 26.8.
After adjusting for age, body mass index, insulin resistance, and other confounders, the hazard ratio for incident NAFLD for those with long or irregular menstrual cycles compared with the incident group corresponded with a 22% increased risk (HR, 1.22; 95% confidence interval, 1.14-1.31). When calculated in a time-dependent analysis, the risk of NAFLD was increased by almost 50% (HR, 1.49; 95% CI, 1.38-1.60).
Risk persists with PCOS exclusion
PCOS has previously been associated with increased risk of NAFLD, but the association between long or irregular menstrual cycles and NAFLD persisted after women with PCOS were excluded.
The mechanism that links menstrual irregularity with NAFLD is unclear, but the investigators said that estrogen exposure is implicated. In addition to a previously reported associated between low estradiol levels and antiestrogens such as tamoxifen with increased risk of NAFLD, they cited studies associating estrogen replacement therapy with a reduced risk of NAFLD. The role of estrogen in suppressing inflammation, oxidative stress, and insulin resistance are all activities that might link more regular menses with a reduced risk of NAFLD, the authors contended.
Women older than 40 years were excluded from this analysis to reduce the possibility of perimenopausal changes as a confounding factor.
Of study limitations acknowledged by the investigators, the presence of NAFLD was diagnosed on ultrasonography rather than histology. Information on sex hormone or prolactin levels was not captured in relation to NAFLD incidence, and the lack of exposure to estrogen replacement therapy and oral contraceptives was based on self-reports from the participants.
Still, the large study size and the consistency of results after adjustment for multiple risk factors argue that long and irregular menstrual cycles do identify women at risk for NAFLD. One implication is that irregular menses can be a marker for NAFLD risk.
“Our findings do not prove a causal relationship, but they show that long or irregular menstrual cycles were significantly associated with an increased risk of developing NAFLD,” said Seungho Ryu, MD, PhD, a professor at the Sungkyunkwan University. Senior author of this study, Dr. Ryu emphasized in an interview that the association “was not explained by obesity or any other risk factor for NAFLD.”
Lifestyle changes may lower risk
The message is that “young women with long or irregular menstrual cycles may benefit from lifestyle changes to reduce the risk of NAFLD,” Dr. Ryu stated.
The Study of Women’s Health Across the Nation, which was started in 1994, has not evaluated NAFLD, but it did show a relationship between longer menstrual cycles and more cardiometabolic risk factors, according to Nanette Santoro MD, professor and chair, department of obstetrics & gynecology, University of Colorado at Denver, Aurora.
This suggests that others are “thinking along the same lines,” but in discussing this study with this news organization, she characterized some of the design elements as well as some of the findings in this study as “peculiar.”
In addition to a “very, very narrow definition of regular cycles,” she questioned the consistent hazard ratio for NAFLD for those with long cycles relative to other types of irregular menses. Presuming that the group with longer cycles would have included at least some patients with undiagnosed PCOS, she was would have expected that the risk would have been highest in this group. While conceding that differences in body composition of Korean women is a potential explanation for this apparent discrepancy, “I would like to see confirmed in other samples of women with more detailed metabolic assessments to understand who is at risk,” she said.
Not least problematic for the strength of the conclusions, the hazard ratio for NAFLD among women with long or irregular menstrual cycles was “pretty low.” She described this as a level at which the risk “is very susceptible to confounding and unlikely to influence clinical practice.”
Anuja Dokras, MD, PHD, a professor of obstetrics and gynecology and director of the PCOS Center at the University of Pennsylvania, Philadelphia, also questioned whether undiagnosed PCOS might have skewed the data.
“There is increasing data on the association between PCOS and NAFLD. Irregular menses is a key criterion for PCOS, and PCOS is the commonest reason for anovulation,” she said. Dr. Dokras therefore considered it possible that patients with unrecognized PCOS were included in the study, weakening the claim that risk of NAFLD and long menstrual cycles remains significant after controlling for PCOS.
Dr. Ryu and coinvestigators, Dr. Santoro, and Dr. Dokras reported no potential conflicts of interest.
Long or irregular menstrual cycles in relatively young women are linked an increased risk of both prevalent and incident nonalcoholic fatty liver disease (NAFLD), according to a cross-sectional study that included data on more than 70,000 women.
“Our results indicate that menstrual irregularity, which is easier to diagnose and usually presented earlier than PCOS [polycystic ovary syndrome] highlights the possibility of identifying premenopausal women at risk of developing NAFLD,” reported a team of authors primarily from Sungkyunkwan University, Seoul, South Korea.
The study evaluated women aged younger than 40 years who were participating in the Kangbuk Samsung Health Study, which involves a comprehensive biennial health examination at health centers in South Korea. Of the 135,090 women enrolled over a 6-year period who had at least one follow-up examination, 72,092 were available for analysis after excluding for a sizable list of confounding factors such as liver disease and infections; exposure to steatogenic medications, such as corticosteroids; hysterectomy; and pregnancy.
NAFLD prevalence climbs with longer menses
Of these women, 36.378 (27.7%) had menstrual cycles of 26-30 days and were identified as the index group. The prevalence of NAFLD in this group was 5.8%. For those with a menstrual cycle of 31-39 days, the prevalence rate climbed to 7.2%. For those with a menstrual cycle of at least 40 days or too irregular to estimate, the prevalence was 9.7%. The prevalence was 7.1% for those with a menstrual cycle less than 21 days.
The results of this study were published in the Journal of Clinical Endocrinology & Metabolism.
In those without NAFLD at baseline who were then followed for a mean of 4.4 years, there were 4,524 incident cases of NAFLD. Incidence density was calculated per 103 patient-years. In the index group, the rate was 18.4. It climbed to 20.2 for those with a menstrual cycle of 31-39 days and then to 22.9 for those with a menstrual cycle of at least 40 days. For those with a cycle of fewer than 21 days, the rate was 26.8.
After adjusting for age, body mass index, insulin resistance, and other confounders, the hazard ratio for incident NAFLD for those with long or irregular menstrual cycles compared with the incident group corresponded with a 22% increased risk (HR, 1.22; 95% confidence interval, 1.14-1.31). When calculated in a time-dependent analysis, the risk of NAFLD was increased by almost 50% (HR, 1.49; 95% CI, 1.38-1.60).
Risk persists with PCOS exclusion
PCOS has previously been associated with increased risk of NAFLD, but the association between long or irregular menstrual cycles and NAFLD persisted after women with PCOS were excluded.
The mechanism that links menstrual irregularity with NAFLD is unclear, but the investigators said that estrogen exposure is implicated. In addition to a previously reported associated between low estradiol levels and antiestrogens such as tamoxifen with increased risk of NAFLD, they cited studies associating estrogen replacement therapy with a reduced risk of NAFLD. The role of estrogen in suppressing inflammation, oxidative stress, and insulin resistance are all activities that might link more regular menses with a reduced risk of NAFLD, the authors contended.
Women older than 40 years were excluded from this analysis to reduce the possibility of perimenopausal changes as a confounding factor.
Of study limitations acknowledged by the investigators, the presence of NAFLD was diagnosed on ultrasonography rather than histology. Information on sex hormone or prolactin levels was not captured in relation to NAFLD incidence, and the lack of exposure to estrogen replacement therapy and oral contraceptives was based on self-reports from the participants.
Still, the large study size and the consistency of results after adjustment for multiple risk factors argue that long and irregular menstrual cycles do identify women at risk for NAFLD. One implication is that irregular menses can be a marker for NAFLD risk.
“Our findings do not prove a causal relationship, but they show that long or irregular menstrual cycles were significantly associated with an increased risk of developing NAFLD,” said Seungho Ryu, MD, PhD, a professor at the Sungkyunkwan University. Senior author of this study, Dr. Ryu emphasized in an interview that the association “was not explained by obesity or any other risk factor for NAFLD.”
Lifestyle changes may lower risk
The message is that “young women with long or irregular menstrual cycles may benefit from lifestyle changes to reduce the risk of NAFLD,” Dr. Ryu stated.
The Study of Women’s Health Across the Nation, which was started in 1994, has not evaluated NAFLD, but it did show a relationship between longer menstrual cycles and more cardiometabolic risk factors, according to Nanette Santoro MD, professor and chair, department of obstetrics & gynecology, University of Colorado at Denver, Aurora.
This suggests that others are “thinking along the same lines,” but in discussing this study with this news organization, she characterized some of the design elements as well as some of the findings in this study as “peculiar.”
In addition to a “very, very narrow definition of regular cycles,” she questioned the consistent hazard ratio for NAFLD for those with long cycles relative to other types of irregular menses. Presuming that the group with longer cycles would have included at least some patients with undiagnosed PCOS, she was would have expected that the risk would have been highest in this group. While conceding that differences in body composition of Korean women is a potential explanation for this apparent discrepancy, “I would like to see confirmed in other samples of women with more detailed metabolic assessments to understand who is at risk,” she said.
Not least problematic for the strength of the conclusions, the hazard ratio for NAFLD among women with long or irregular menstrual cycles was “pretty low.” She described this as a level at which the risk “is very susceptible to confounding and unlikely to influence clinical practice.”
Anuja Dokras, MD, PHD, a professor of obstetrics and gynecology and director of the PCOS Center at the University of Pennsylvania, Philadelphia, also questioned whether undiagnosed PCOS might have skewed the data.
“There is increasing data on the association between PCOS and NAFLD. Irregular menses is a key criterion for PCOS, and PCOS is the commonest reason for anovulation,” she said. Dr. Dokras therefore considered it possible that patients with unrecognized PCOS were included in the study, weakening the claim that risk of NAFLD and long menstrual cycles remains significant after controlling for PCOS.
Dr. Ryu and coinvestigators, Dr. Santoro, and Dr. Dokras reported no potential conflicts of interest.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Into the unknown: Are vulvar cysts so simple?
Immediate postpartum IUD insertion increases expulsion risk
Expulsion of intrauterine devices was significantly more likely when the devices were inserted within the first 3 days after delivery compared with later insertions, based on data from more than 300,000 women.
Intrauterine devices are effective contraception, and current guidelines support immediate postpartum IUD insertion as a safe, effective, and convenient option, Mary Anne Armstrong, MA, of Kaiser Permanente Northern California, Oakland, and colleagues wrote. Although IUD expulsion rates are low overall, data from previous studies suggest that timing of insertion may affect expulsion rates, and that breastfeeding may play a role.
In the Association of Perforation and Expulsion of Intrauterine Devices (APEX-IUD) cohort study published in JAMA Network Open, the researchers reviewed data from the electronic health records at four sites; the study population included women aged 50 years and younger who underwent IUD insertion between 2001 and 2018.
The women were grouped by postpartum status and timing of IUD placement: 0-3 days, 4 days to 6 weeks, 6-14 weeks, 14-52 weeks, and nonpostpartum (defined as more than 52 weeks or no evidence of delivery).
The researchers also compared expulsion rates in postpartum women who were and were not breastfeeding at the time of IUD insertion based on clinical records, diagnostic codes, or questionnaires at well-baby visits.
The total study population included 326,658 women with a mean age of 32.0 years; 42% were non-Hispanic White, 17.2% were Hispanic other, 13.0% were Hispanic White, 11.9% were Asian or Pacific Islander, 8.7% were non-Hispanic Black, and 0.2% were Hispanic Black. Approximately 80% of the IUDs were levonorgestrel releasing.
A total of 8,943 expulsions were reported, for an overall expulsion rate of 13.94 per 1,000 person-years.
The adjusted hazard ratios for IUD expulsion were 5.34, 1.22, 1.06, and 1.43 for women with insertion times, respectively, of 0-3 days, 4 days to 6 or fewer weeks, 6-14 weeks, and 14-52 weeks. Women with nonpostpartum IUD insertion served as the referent.
The 5-year cumulative incidence of IUD expulsion was highest with placement between 0 and 3 days post partum and lowest with placement at 6-14 weeks postpartum (10.73% and 3.18%, respectively).
“Within the group with IUD insertions 0-3 days postpartum, the highest expulsion rates were discovered within 12 weeks of insertion, with the highest incidence rate occurring at week 6 (844 per 1,000 person-years), a time women are commonly seen post delivery,” the researchers noted.
In a subcohort of 94,817 women with known breastfeeding status, the 5-year cumulative incidence of expulsion was 3.49% for breastfeeding women and 4.57% for nonbreastfeeding women, with an adjusted HR of 0.71 for breastfeeding versus not breastfeeding.
“While women who accept immediate postpartum IUD placement report high satisfaction rates, information on women’s preferences and satisfaction associated with different timing of postpartum placement would also be helpful to understand the benefit-risk profile,” the researchers wrote in their discussion of the findings. “The fact that most expulsions in the immediate postpartum group occurred early presents an opportunity to mitigate risk of unrecognized expulsion and unintended pregnancy via counseling on signs of expulsion and follow-up examination.”
The study findings were limited by several factors including the potential misclassification of exposures and the primary outcome of expulsion, especially since some postpartum women may be lactating whether or not they are breastfeeding, the researchers noted. Other limitations included the combination of complete and partial expulsions, and the dating of IUD expulsion based on when it came to medical attention, which was not necessarily when it occurred. More data are needed on the potential association between lactational amenorrhea and lower expulsion risk among postpartum women who are breastfeeding.
However, the results were strengthened by the large and diverse study population, the use of linked mother-infant records to identify exposures, and the use electronic health records to identify outcomes, and the data can inform patient counseling for postpartum IUDs, the researchers concluded.
Study reflects findings from Europe
“The FDA mandated this study in response to a European study, EURAS-IUD1, a European prospective observational study that enrolled 61,448 participants between 2006 and 2012,” Ms. Armstrong said in an interview. In the European study “women breastfeeding at the time of device insertion or with the device inserted at 36 weeks’ postpartum or less had higher risk of uterine perforation. The FDA wanted to know if the risks were similar in the United States population”
The APEX-IUD study was designed to reflect current United States clinical practice. “The aims of APEX-IUD are to evaluate risk of IUD-related uterine perforation and device expulsion among women who are breastfeeding or within 12 months postpartum at insertion. The perforation outcome is addressed in a separate paper,” Ms. Armstrong noted.
“We were not surprised by the findings; they aligned with previous findings and confirm the overall safety of intrauterine devices,” said Ms. Armstrong. “Data from this study provides IUD expulsion risk estimates that can be used to inform clinical practice and preinsertion counseling. IUD insertions 0-3 days postpartum might decrease the risk of unintended pregnancy and provide more convenience and efficiency for new mothers. This has proven to be especially important during the pandemic. The higher risk of expulsion at 0-3 days post partum must be balanced with the low IUD-related uterine perforation risk to provide a comprehensive picture that aids in clinical decision-making.
“Potential barriers to postpartum IUD placement include lack of provision of education on the range of contraceptive options available during prenatal care and failure or inability of hospital inpatient units to stock the intrauterine devices for use when needed,” said Ms. Armstrong.
Looking ahead, “future research could evaluate risk factors for partial versus complete expulsions, the association of preinsertion counseling with recognition of potential expulsions and corresponding IUD failure rates, and whether ultrasound verification of IUD position in the uterus after insertion is associated with expulsion risk,” she said.
Identifying risk factors informs patient counseling
“The current study examines breastfeeding at time of IUD insertion as a risk factor for expulsion,” Iris Krishna, MD, of Emory University, Atlanta, said in an interview. “There is biologic plausibility that breastfeeding may be a risk factor of IUD expulsion. Breastfeeding stimulates secretion of oxytocin, a hormone which plays a key role in the contraction of the uterus during labor and uterine involution postpartum. It also plays a key role in the contraction of milk ducts to allow for milk letdown. Because of its dual role some mothers may occasionally report uterine cramping with breastfeeding. Prior studies have suggested that breastfeeding may be associated with an increased risk of uterine perforation with postpartum IUD placement, but how breastfeeding may contribute to risk of IUD expulsion has not been studied extensively.”
The current data are consistent with previous studies suggesting the highest risk of IUD expulsion is with placement in the immediate postpartum period (0-3 days). “In a subcohort analysis by breastfeeding status, the risk of IUD expulsion was lower for women who were breastfeeding versus not breastfeeding;” however, “these findings may be due to amenorrhea that can also be seen with breastfeeding,” Dr. Krishna said. “Menstrual bleeding is an independent risk factor for IUD expulsion and not having menstrual bleeding while breastfeeding may lower risk of expulsion.
“Patients should be counseled on the benefits of immediate postpartum IUD placement, the risk of IUD expulsion, and alternative contraception options to be able to make an informed decision about the right contraception for them,” Dr. Krishna emphasized. “Clinicians can reassure patients that the uterine cramping they may feel while breastfeeding does not appear to increase the risk of IUD expulsion and that the amenorrhea that may result from breastfeeding also may lower the risk of IUD expulsion.”
The study was supported by Bayer through support to RTI Health Solutions, Kaiser Permanente Northern California, Kaiser Permanente Southern California, Kaiser Permanente Washington, and the Regenstrief Institute. Ms. Armstrong and several coauthors disclosed support from Bayer during the study. Dr. Krishna had no relevant disclosures.
Expulsion of intrauterine devices was significantly more likely when the devices were inserted within the first 3 days after delivery compared with later insertions, based on data from more than 300,000 women.
Intrauterine devices are effective contraception, and current guidelines support immediate postpartum IUD insertion as a safe, effective, and convenient option, Mary Anne Armstrong, MA, of Kaiser Permanente Northern California, Oakland, and colleagues wrote. Although IUD expulsion rates are low overall, data from previous studies suggest that timing of insertion may affect expulsion rates, and that breastfeeding may play a role.
In the Association of Perforation and Expulsion of Intrauterine Devices (APEX-IUD) cohort study published in JAMA Network Open, the researchers reviewed data from the electronic health records at four sites; the study population included women aged 50 years and younger who underwent IUD insertion between 2001 and 2018.
The women were grouped by postpartum status and timing of IUD placement: 0-3 days, 4 days to 6 weeks, 6-14 weeks, 14-52 weeks, and nonpostpartum (defined as more than 52 weeks or no evidence of delivery).
The researchers also compared expulsion rates in postpartum women who were and were not breastfeeding at the time of IUD insertion based on clinical records, diagnostic codes, or questionnaires at well-baby visits.
The total study population included 326,658 women with a mean age of 32.0 years; 42% were non-Hispanic White, 17.2% were Hispanic other, 13.0% were Hispanic White, 11.9% were Asian or Pacific Islander, 8.7% were non-Hispanic Black, and 0.2% were Hispanic Black. Approximately 80% of the IUDs were levonorgestrel releasing.
A total of 8,943 expulsions were reported, for an overall expulsion rate of 13.94 per 1,000 person-years.
The adjusted hazard ratios for IUD expulsion were 5.34, 1.22, 1.06, and 1.43 for women with insertion times, respectively, of 0-3 days, 4 days to 6 or fewer weeks, 6-14 weeks, and 14-52 weeks. Women with nonpostpartum IUD insertion served as the referent.
The 5-year cumulative incidence of IUD expulsion was highest with placement between 0 and 3 days post partum and lowest with placement at 6-14 weeks postpartum (10.73% and 3.18%, respectively).
“Within the group with IUD insertions 0-3 days postpartum, the highest expulsion rates were discovered within 12 weeks of insertion, with the highest incidence rate occurring at week 6 (844 per 1,000 person-years), a time women are commonly seen post delivery,” the researchers noted.
In a subcohort of 94,817 women with known breastfeeding status, the 5-year cumulative incidence of expulsion was 3.49% for breastfeeding women and 4.57% for nonbreastfeeding women, with an adjusted HR of 0.71 for breastfeeding versus not breastfeeding.
“While women who accept immediate postpartum IUD placement report high satisfaction rates, information on women’s preferences and satisfaction associated with different timing of postpartum placement would also be helpful to understand the benefit-risk profile,” the researchers wrote in their discussion of the findings. “The fact that most expulsions in the immediate postpartum group occurred early presents an opportunity to mitigate risk of unrecognized expulsion and unintended pregnancy via counseling on signs of expulsion and follow-up examination.”
The study findings were limited by several factors including the potential misclassification of exposures and the primary outcome of expulsion, especially since some postpartum women may be lactating whether or not they are breastfeeding, the researchers noted. Other limitations included the combination of complete and partial expulsions, and the dating of IUD expulsion based on when it came to medical attention, which was not necessarily when it occurred. More data are needed on the potential association between lactational amenorrhea and lower expulsion risk among postpartum women who are breastfeeding.
However, the results were strengthened by the large and diverse study population, the use of linked mother-infant records to identify exposures, and the use electronic health records to identify outcomes, and the data can inform patient counseling for postpartum IUDs, the researchers concluded.
Study reflects findings from Europe
“The FDA mandated this study in response to a European study, EURAS-IUD1, a European prospective observational study that enrolled 61,448 participants between 2006 and 2012,” Ms. Armstrong said in an interview. In the European study “women breastfeeding at the time of device insertion or with the device inserted at 36 weeks’ postpartum or less had higher risk of uterine perforation. The FDA wanted to know if the risks were similar in the United States population”
The APEX-IUD study was designed to reflect current United States clinical practice. “The aims of APEX-IUD are to evaluate risk of IUD-related uterine perforation and device expulsion among women who are breastfeeding or within 12 months postpartum at insertion. The perforation outcome is addressed in a separate paper,” Ms. Armstrong noted.
“We were not surprised by the findings; they aligned with previous findings and confirm the overall safety of intrauterine devices,” said Ms. Armstrong. “Data from this study provides IUD expulsion risk estimates that can be used to inform clinical practice and preinsertion counseling. IUD insertions 0-3 days postpartum might decrease the risk of unintended pregnancy and provide more convenience and efficiency for new mothers. This has proven to be especially important during the pandemic. The higher risk of expulsion at 0-3 days post partum must be balanced with the low IUD-related uterine perforation risk to provide a comprehensive picture that aids in clinical decision-making.
“Potential barriers to postpartum IUD placement include lack of provision of education on the range of contraceptive options available during prenatal care and failure or inability of hospital inpatient units to stock the intrauterine devices for use when needed,” said Ms. Armstrong.
Looking ahead, “future research could evaluate risk factors for partial versus complete expulsions, the association of preinsertion counseling with recognition of potential expulsions and corresponding IUD failure rates, and whether ultrasound verification of IUD position in the uterus after insertion is associated with expulsion risk,” she said.
Identifying risk factors informs patient counseling
“The current study examines breastfeeding at time of IUD insertion as a risk factor for expulsion,” Iris Krishna, MD, of Emory University, Atlanta, said in an interview. “There is biologic plausibility that breastfeeding may be a risk factor of IUD expulsion. Breastfeeding stimulates secretion of oxytocin, a hormone which plays a key role in the contraction of the uterus during labor and uterine involution postpartum. It also plays a key role in the contraction of milk ducts to allow for milk letdown. Because of its dual role some mothers may occasionally report uterine cramping with breastfeeding. Prior studies have suggested that breastfeeding may be associated with an increased risk of uterine perforation with postpartum IUD placement, but how breastfeeding may contribute to risk of IUD expulsion has not been studied extensively.”
The current data are consistent with previous studies suggesting the highest risk of IUD expulsion is with placement in the immediate postpartum period (0-3 days). “In a subcohort analysis by breastfeeding status, the risk of IUD expulsion was lower for women who were breastfeeding versus not breastfeeding;” however, “these findings may be due to amenorrhea that can also be seen with breastfeeding,” Dr. Krishna said. “Menstrual bleeding is an independent risk factor for IUD expulsion and not having menstrual bleeding while breastfeeding may lower risk of expulsion.
“Patients should be counseled on the benefits of immediate postpartum IUD placement, the risk of IUD expulsion, and alternative contraception options to be able to make an informed decision about the right contraception for them,” Dr. Krishna emphasized. “Clinicians can reassure patients that the uterine cramping they may feel while breastfeeding does not appear to increase the risk of IUD expulsion and that the amenorrhea that may result from breastfeeding also may lower the risk of IUD expulsion.”
The study was supported by Bayer through support to RTI Health Solutions, Kaiser Permanente Northern California, Kaiser Permanente Southern California, Kaiser Permanente Washington, and the Regenstrief Institute. Ms. Armstrong and several coauthors disclosed support from Bayer during the study. Dr. Krishna had no relevant disclosures.
Expulsion of intrauterine devices was significantly more likely when the devices were inserted within the first 3 days after delivery compared with later insertions, based on data from more than 300,000 women.
Intrauterine devices are effective contraception, and current guidelines support immediate postpartum IUD insertion as a safe, effective, and convenient option, Mary Anne Armstrong, MA, of Kaiser Permanente Northern California, Oakland, and colleagues wrote. Although IUD expulsion rates are low overall, data from previous studies suggest that timing of insertion may affect expulsion rates, and that breastfeeding may play a role.
In the Association of Perforation and Expulsion of Intrauterine Devices (APEX-IUD) cohort study published in JAMA Network Open, the researchers reviewed data from the electronic health records at four sites; the study population included women aged 50 years and younger who underwent IUD insertion between 2001 and 2018.
The women were grouped by postpartum status and timing of IUD placement: 0-3 days, 4 days to 6 weeks, 6-14 weeks, 14-52 weeks, and nonpostpartum (defined as more than 52 weeks or no evidence of delivery).
The researchers also compared expulsion rates in postpartum women who were and were not breastfeeding at the time of IUD insertion based on clinical records, diagnostic codes, or questionnaires at well-baby visits.
The total study population included 326,658 women with a mean age of 32.0 years; 42% were non-Hispanic White, 17.2% were Hispanic other, 13.0% were Hispanic White, 11.9% were Asian or Pacific Islander, 8.7% were non-Hispanic Black, and 0.2% were Hispanic Black. Approximately 80% of the IUDs were levonorgestrel releasing.
A total of 8,943 expulsions were reported, for an overall expulsion rate of 13.94 per 1,000 person-years.
The adjusted hazard ratios for IUD expulsion were 5.34, 1.22, 1.06, and 1.43 for women with insertion times, respectively, of 0-3 days, 4 days to 6 or fewer weeks, 6-14 weeks, and 14-52 weeks. Women with nonpostpartum IUD insertion served as the referent.
The 5-year cumulative incidence of IUD expulsion was highest with placement between 0 and 3 days post partum and lowest with placement at 6-14 weeks postpartum (10.73% and 3.18%, respectively).
“Within the group with IUD insertions 0-3 days postpartum, the highest expulsion rates were discovered within 12 weeks of insertion, with the highest incidence rate occurring at week 6 (844 per 1,000 person-years), a time women are commonly seen post delivery,” the researchers noted.
In a subcohort of 94,817 women with known breastfeeding status, the 5-year cumulative incidence of expulsion was 3.49% for breastfeeding women and 4.57% for nonbreastfeeding women, with an adjusted HR of 0.71 for breastfeeding versus not breastfeeding.
“While women who accept immediate postpartum IUD placement report high satisfaction rates, information on women’s preferences and satisfaction associated with different timing of postpartum placement would also be helpful to understand the benefit-risk profile,” the researchers wrote in their discussion of the findings. “The fact that most expulsions in the immediate postpartum group occurred early presents an opportunity to mitigate risk of unrecognized expulsion and unintended pregnancy via counseling on signs of expulsion and follow-up examination.”
The study findings were limited by several factors including the potential misclassification of exposures and the primary outcome of expulsion, especially since some postpartum women may be lactating whether or not they are breastfeeding, the researchers noted. Other limitations included the combination of complete and partial expulsions, and the dating of IUD expulsion based on when it came to medical attention, which was not necessarily when it occurred. More data are needed on the potential association between lactational amenorrhea and lower expulsion risk among postpartum women who are breastfeeding.
However, the results were strengthened by the large and diverse study population, the use of linked mother-infant records to identify exposures, and the use electronic health records to identify outcomes, and the data can inform patient counseling for postpartum IUDs, the researchers concluded.
Study reflects findings from Europe
“The FDA mandated this study in response to a European study, EURAS-IUD1, a European prospective observational study that enrolled 61,448 participants between 2006 and 2012,” Ms. Armstrong said in an interview. In the European study “women breastfeeding at the time of device insertion or with the device inserted at 36 weeks’ postpartum or less had higher risk of uterine perforation. The FDA wanted to know if the risks were similar in the United States population”
The APEX-IUD study was designed to reflect current United States clinical practice. “The aims of APEX-IUD are to evaluate risk of IUD-related uterine perforation and device expulsion among women who are breastfeeding or within 12 months postpartum at insertion. The perforation outcome is addressed in a separate paper,” Ms. Armstrong noted.
“We were not surprised by the findings; they aligned with previous findings and confirm the overall safety of intrauterine devices,” said Ms. Armstrong. “Data from this study provides IUD expulsion risk estimates that can be used to inform clinical practice and preinsertion counseling. IUD insertions 0-3 days postpartum might decrease the risk of unintended pregnancy and provide more convenience and efficiency for new mothers. This has proven to be especially important during the pandemic. The higher risk of expulsion at 0-3 days post partum must be balanced with the low IUD-related uterine perforation risk to provide a comprehensive picture that aids in clinical decision-making.
“Potential barriers to postpartum IUD placement include lack of provision of education on the range of contraceptive options available during prenatal care and failure or inability of hospital inpatient units to stock the intrauterine devices for use when needed,” said Ms. Armstrong.
Looking ahead, “future research could evaluate risk factors for partial versus complete expulsions, the association of preinsertion counseling with recognition of potential expulsions and corresponding IUD failure rates, and whether ultrasound verification of IUD position in the uterus after insertion is associated with expulsion risk,” she said.
Identifying risk factors informs patient counseling
“The current study examines breastfeeding at time of IUD insertion as a risk factor for expulsion,” Iris Krishna, MD, of Emory University, Atlanta, said in an interview. “There is biologic plausibility that breastfeeding may be a risk factor of IUD expulsion. Breastfeeding stimulates secretion of oxytocin, a hormone which plays a key role in the contraction of the uterus during labor and uterine involution postpartum. It also plays a key role in the contraction of milk ducts to allow for milk letdown. Because of its dual role some mothers may occasionally report uterine cramping with breastfeeding. Prior studies have suggested that breastfeeding may be associated with an increased risk of uterine perforation with postpartum IUD placement, but how breastfeeding may contribute to risk of IUD expulsion has not been studied extensively.”
The current data are consistent with previous studies suggesting the highest risk of IUD expulsion is with placement in the immediate postpartum period (0-3 days). “In a subcohort analysis by breastfeeding status, the risk of IUD expulsion was lower for women who were breastfeeding versus not breastfeeding;” however, “these findings may be due to amenorrhea that can also be seen with breastfeeding,” Dr. Krishna said. “Menstrual bleeding is an independent risk factor for IUD expulsion and not having menstrual bleeding while breastfeeding may lower risk of expulsion.
“Patients should be counseled on the benefits of immediate postpartum IUD placement, the risk of IUD expulsion, and alternative contraception options to be able to make an informed decision about the right contraception for them,” Dr. Krishna emphasized. “Clinicians can reassure patients that the uterine cramping they may feel while breastfeeding does not appear to increase the risk of IUD expulsion and that the amenorrhea that may result from breastfeeding also may lower the risk of IUD expulsion.”
The study was supported by Bayer through support to RTI Health Solutions, Kaiser Permanente Northern California, Kaiser Permanente Southern California, Kaiser Permanente Washington, and the Regenstrief Institute. Ms. Armstrong and several coauthors disclosed support from Bayer during the study. Dr. Krishna had no relevant disclosures.
FROM JAMA NETWORK OPEN
FDA OKs first condom for anal sex
specifically designed for use during anal sex has gained Food and Drug Administration approval.
Anal intercourse is considered to be much riskier than vaginal sex for the transmission of infections such as HIV and HPV, a risk factor for anal cancer, agency officials said in a statement Feb. 23 announcing the decision. And though the Centers for Disease Control and Prevention has long encouraged the use of a condom during anal intercourse, the FDA had not until now deemed this practice safe.
The latex ONE Male Condom, from prophylactic maker Global Protection Corp. of Boston, has already been available for vaginal sex. The FDA action now allows the company to market the product for anal intercourse.
“This authorization helps us accomplish our priority to advance health equity through the development of safe and effective products that meet the needs of diverse populations,” Courtney Lias, PhD, the director of the FDA’s Office of GastroRenal, ObGyn, General Hospital, and Urology Devices, said in a statement.
The FDA said it relied on an Emory University clinical study of condom safety of more than 500 men. Those who took part in the study were evenly divided between men who have sex with men and men who have sex with women. The condom failure rate, meaning that a condom either broke or slipped, was less than 1% during anal sex. The failure rate was 3 times higher during vaginal intercourse.
The Emory researchers also found that roughly 70% of men who have sex with men would be more likely to use condoms marked as safe for anal sex, according to a survey of 10,000 people.
ONE Male Condoms sell for between $3.48 for a three-pack and $14.48 for a 24-pack, according to Milla Impola, Global Protection’s director of marketing and communications. The FDA said the condom should be used with a condom-compatible lubricant when used during anal sex.
A version of this article first appeared on WebMD.com.
specifically designed for use during anal sex has gained Food and Drug Administration approval.
Anal intercourse is considered to be much riskier than vaginal sex for the transmission of infections such as HIV and HPV, a risk factor for anal cancer, agency officials said in a statement Feb. 23 announcing the decision. And though the Centers for Disease Control and Prevention has long encouraged the use of a condom during anal intercourse, the FDA had not until now deemed this practice safe.
The latex ONE Male Condom, from prophylactic maker Global Protection Corp. of Boston, has already been available for vaginal sex. The FDA action now allows the company to market the product for anal intercourse.
“This authorization helps us accomplish our priority to advance health equity through the development of safe and effective products that meet the needs of diverse populations,” Courtney Lias, PhD, the director of the FDA’s Office of GastroRenal, ObGyn, General Hospital, and Urology Devices, said in a statement.
The FDA said it relied on an Emory University clinical study of condom safety of more than 500 men. Those who took part in the study were evenly divided between men who have sex with men and men who have sex with women. The condom failure rate, meaning that a condom either broke or slipped, was less than 1% during anal sex. The failure rate was 3 times higher during vaginal intercourse.
The Emory researchers also found that roughly 70% of men who have sex with men would be more likely to use condoms marked as safe for anal sex, according to a survey of 10,000 people.
ONE Male Condoms sell for between $3.48 for a three-pack and $14.48 for a 24-pack, according to Milla Impola, Global Protection’s director of marketing and communications. The FDA said the condom should be used with a condom-compatible lubricant when used during anal sex.
A version of this article first appeared on WebMD.com.
specifically designed for use during anal sex has gained Food and Drug Administration approval.
Anal intercourse is considered to be much riskier than vaginal sex for the transmission of infections such as HIV and HPV, a risk factor for anal cancer, agency officials said in a statement Feb. 23 announcing the decision. And though the Centers for Disease Control and Prevention has long encouraged the use of a condom during anal intercourse, the FDA had not until now deemed this practice safe.
The latex ONE Male Condom, from prophylactic maker Global Protection Corp. of Boston, has already been available for vaginal sex. The FDA action now allows the company to market the product for anal intercourse.
“This authorization helps us accomplish our priority to advance health equity through the development of safe and effective products that meet the needs of diverse populations,” Courtney Lias, PhD, the director of the FDA’s Office of GastroRenal, ObGyn, General Hospital, and Urology Devices, said in a statement.
The FDA said it relied on an Emory University clinical study of condom safety of more than 500 men. Those who took part in the study were evenly divided between men who have sex with men and men who have sex with women. The condom failure rate, meaning that a condom either broke or slipped, was less than 1% during anal sex. The failure rate was 3 times higher during vaginal intercourse.
The Emory researchers also found that roughly 70% of men who have sex with men would be more likely to use condoms marked as safe for anal sex, according to a survey of 10,000 people.
ONE Male Condoms sell for between $3.48 for a three-pack and $14.48 for a 24-pack, according to Milla Impola, Global Protection’s director of marketing and communications. The FDA said the condom should be used with a condom-compatible lubricant when used during anal sex.
A version of this article first appeared on WebMD.com.
Women with von Willebrand disease: Managing menstrual and postpartum bleeding
Women with von Willebrand disease (VWD) experience many obstetric and gynecologic challenges, including higher levels of von Willebrand factor (VWF) in pregnancy, Romina Brignardello-Petersen, PhD, of McMaster University, Hamilton, Ont., and colleagues wrote.
The American Society of Hematology, the International Society on Thrombosis and Haemostasis, the National Hemophilia Foundation, and the World Federation of Hemophilia convened a working group in 2017 to address updated guidelines on VWD with a focus on women, the researchers said.
In an article published in Blood Advances, the researchers described the evidence from three systematic reviews conducted to inform three recommendations for the guidelines: first-line management of heavy menstrual bleeding (HMB), treatment of women requiring or desiring neuraxial analgesia, and management of postpartum hemorrhage. The authors identified studies published through October 2019.
The first systematic review of first-line therapies for HMB included five case series, one retrospective cohort study, and one randomized controlled trial. In the randomized controlled trial of 232 patients, low-certainty evidence suggested less reduction of blood loss with desmopressin, compared with tranexamic acid (TxA), with no significant differences in side effects. Very-low-certainty evidence from an observational study also supported lower effectiveness of desmopressin versus hormonal therapy. Finally, the case series showed very-low-certainty evidence for the comparative effectiveness of hormonal therapy delivered via a levonorgestrel-releasing intrauterine system (LNG-IUS) and other therapies for HMB control.
The second systematic review compared VWF levels in women who received neuraxial anesthesia during labor.
The review included five case series that described outcomes of women with VWF levels greater than 0.50 IU/mL; however, the studies did not describe outcomes according to VWF levels and did not cite the proportion of women with VWF levels greater than 1.50 IU/mL. Consequently, the evidence for the effects of increasing VWF levels was very low certainty, the authors said. In a meta-analysis, the proportion of anesthesia complications in these women was 6% (very low certainty). The complications included hypotension, accidental dural puncture, inadequate analgesia, bloody tap with no further complications, and failed block requiring general anesthesia.
The third systemic review included two retrospective cohort studies on the use of TxA during the postpartum period. In these studies, the authors found very-low-certainty evidence that TxA reduced the risk of severe primary postpartum hemorrhage, primary postpartum hemorrhage, and secondary postpartum hemorrhage (risk ratios, 0.36, 0.25, and 0.42, respectively). The effects of TxA on blood transfusions, vaginal hematoma, blood loss, and thrombotic complications also showed very-low-certainty evidence.
The currently available evidence for treatment options in women with VWD remains very low certainty, the researchers wrote in their discussion. “Because hormonal therapy is effective in controlling HMB (based on data from women without bleeding disorders), we believe the most effective strategy to be hormonal therapy with a LNG-IUS or combined oral contraceptives, followed by TxA and desmopressin.”
The study findings were limited by several factors including scarce evidence, the risk of bias in the observational studies, and lack of comparisons/controls in the case series, the researchers noted. Notable literature gaps included data on outcomes including major bleeding and the need for surgery or additional treatments in the first review; mortality, major bleeding, spinal hematoma, transfusion, and thrombotic events in the second review; and mortality, major bleeding, and the need for other procedures in the third review.
However, the findings were strengthened by the use of broad eligibility criteria to include any studies with potential useful advice, including case series, if these were the only available options. In developing recommendations, “the guideline panel interpreted the evidence adding their experience and knowledge of indirect evidence,” the authors noted.
The current evidence, though mainly very low certainty, “is the best available to inform decisions about management. Clinicians seeking advice on how to manage their patients with VWD should refer to the practice guidelines and assess to what extent they are applicable to their patients,” the researchers concluded.
Meeting the need for evidence-based guidelines
The review is important at this time because current evidence-based guidelines are limited, said coauthor Veronica Flood, MD, a pediatric hematologist at the Medical College of Wisconsin, Milwaukee, and a VWD researcher.
“While we have some guidelines that address von Willebrand disease, these were primarily based on expert opinion and not necessarily based on the best available evidence,” said Dr. Flood.
“Given how many people have von Willebrand disease, it is important that we actually base our recommendations on the data,” she emphasized. The new guidelines also incorporate patient feedback, with the inclusion of multiple panelists who are individuals living with VWD. “The final recommendations looked at not only the evidence, but the cost effectiveness, feasibility, and patient values and preferences,” she added.
“I was surprised we did not have better evidence for some of these common issues for patients with VWD,” said Dr. Flood. “I think that speaks to the need to do more high-quality research in this area.”
From a clinical standpoint, “we now have evidence-based guidelines that support the use of prophylaxis in patients with VWD and significant bleeding, as well as recommendations for surgery and bleeding issues around menstruation,” said Dr. Flood. “I do think it is also important to recognize that many of these are conditional recommendations, meaning there is room for patient preferences in implementation, which is helpful since we know that some people will have different priorities.”
Dr. Flood noted that more research is needed in many aspects of VWD. “We definitely need to better understand best options for surgical treatment, and I consider that a high priority. We are also hoping, along with the National Hemophilia Foundation, to develop some patient decision aids to help with some of these issues.”
Coauthor Nathan T. Connell, MD, an adult hematologist at the Brigham and Women’s Hospital and Harvard Medical School, both in Boston, served as the vice chair for the guideline panel. Dr. Connell agreed with the importance of the reviews and the need for additional research. “I, too, was surprised to see the lack of robust data to answer many of the basic questions about how to manage people living with VWD. Regarding the systematic reviews, I was surprised to see the power of combining the limited data in this way to come up with an evidence base for the panels to review,” he added.
The study was supported by the ASH, ISTH, NHF, and the WFH 2020 Guidelines for Management of VWD. The researchers had no financial conflicts to disclose.
Women with von Willebrand disease (VWD) experience many obstetric and gynecologic challenges, including higher levels of von Willebrand factor (VWF) in pregnancy, Romina Brignardello-Petersen, PhD, of McMaster University, Hamilton, Ont., and colleagues wrote.
The American Society of Hematology, the International Society on Thrombosis and Haemostasis, the National Hemophilia Foundation, and the World Federation of Hemophilia convened a working group in 2017 to address updated guidelines on VWD with a focus on women, the researchers said.
In an article published in Blood Advances, the researchers described the evidence from three systematic reviews conducted to inform three recommendations for the guidelines: first-line management of heavy menstrual bleeding (HMB), treatment of women requiring or desiring neuraxial analgesia, and management of postpartum hemorrhage. The authors identified studies published through October 2019.
The first systematic review of first-line therapies for HMB included five case series, one retrospective cohort study, and one randomized controlled trial. In the randomized controlled trial of 232 patients, low-certainty evidence suggested less reduction of blood loss with desmopressin, compared with tranexamic acid (TxA), with no significant differences in side effects. Very-low-certainty evidence from an observational study also supported lower effectiveness of desmopressin versus hormonal therapy. Finally, the case series showed very-low-certainty evidence for the comparative effectiveness of hormonal therapy delivered via a levonorgestrel-releasing intrauterine system (LNG-IUS) and other therapies for HMB control.
The second systematic review compared VWF levels in women who received neuraxial anesthesia during labor.
The review included five case series that described outcomes of women with VWF levels greater than 0.50 IU/mL; however, the studies did not describe outcomes according to VWF levels and did not cite the proportion of women with VWF levels greater than 1.50 IU/mL. Consequently, the evidence for the effects of increasing VWF levels was very low certainty, the authors said. In a meta-analysis, the proportion of anesthesia complications in these women was 6% (very low certainty). The complications included hypotension, accidental dural puncture, inadequate analgesia, bloody tap with no further complications, and failed block requiring general anesthesia.
The third systemic review included two retrospective cohort studies on the use of TxA during the postpartum period. In these studies, the authors found very-low-certainty evidence that TxA reduced the risk of severe primary postpartum hemorrhage, primary postpartum hemorrhage, and secondary postpartum hemorrhage (risk ratios, 0.36, 0.25, and 0.42, respectively). The effects of TxA on blood transfusions, vaginal hematoma, blood loss, and thrombotic complications also showed very-low-certainty evidence.
The currently available evidence for treatment options in women with VWD remains very low certainty, the researchers wrote in their discussion. “Because hormonal therapy is effective in controlling HMB (based on data from women without bleeding disorders), we believe the most effective strategy to be hormonal therapy with a LNG-IUS or combined oral contraceptives, followed by TxA and desmopressin.”
The study findings were limited by several factors including scarce evidence, the risk of bias in the observational studies, and lack of comparisons/controls in the case series, the researchers noted. Notable literature gaps included data on outcomes including major bleeding and the need for surgery or additional treatments in the first review; mortality, major bleeding, spinal hematoma, transfusion, and thrombotic events in the second review; and mortality, major bleeding, and the need for other procedures in the third review.
However, the findings were strengthened by the use of broad eligibility criteria to include any studies with potential useful advice, including case series, if these were the only available options. In developing recommendations, “the guideline panel interpreted the evidence adding their experience and knowledge of indirect evidence,” the authors noted.
The current evidence, though mainly very low certainty, “is the best available to inform decisions about management. Clinicians seeking advice on how to manage their patients with VWD should refer to the practice guidelines and assess to what extent they are applicable to their patients,” the researchers concluded.
Meeting the need for evidence-based guidelines
The review is important at this time because current evidence-based guidelines are limited, said coauthor Veronica Flood, MD, a pediatric hematologist at the Medical College of Wisconsin, Milwaukee, and a VWD researcher.
“While we have some guidelines that address von Willebrand disease, these were primarily based on expert opinion and not necessarily based on the best available evidence,” said Dr. Flood.
“Given how many people have von Willebrand disease, it is important that we actually base our recommendations on the data,” she emphasized. The new guidelines also incorporate patient feedback, with the inclusion of multiple panelists who are individuals living with VWD. “The final recommendations looked at not only the evidence, but the cost effectiveness, feasibility, and patient values and preferences,” she added.
“I was surprised we did not have better evidence for some of these common issues for patients with VWD,” said Dr. Flood. “I think that speaks to the need to do more high-quality research in this area.”
From a clinical standpoint, “we now have evidence-based guidelines that support the use of prophylaxis in patients with VWD and significant bleeding, as well as recommendations for surgery and bleeding issues around menstruation,” said Dr. Flood. “I do think it is also important to recognize that many of these are conditional recommendations, meaning there is room for patient preferences in implementation, which is helpful since we know that some people will have different priorities.”
Dr. Flood noted that more research is needed in many aspects of VWD. “We definitely need to better understand best options for surgical treatment, and I consider that a high priority. We are also hoping, along with the National Hemophilia Foundation, to develop some patient decision aids to help with some of these issues.”
Coauthor Nathan T. Connell, MD, an adult hematologist at the Brigham and Women’s Hospital and Harvard Medical School, both in Boston, served as the vice chair for the guideline panel. Dr. Connell agreed with the importance of the reviews and the need for additional research. “I, too, was surprised to see the lack of robust data to answer many of the basic questions about how to manage people living with VWD. Regarding the systematic reviews, I was surprised to see the power of combining the limited data in this way to come up with an evidence base for the panels to review,” he added.
The study was supported by the ASH, ISTH, NHF, and the WFH 2020 Guidelines for Management of VWD. The researchers had no financial conflicts to disclose.
Women with von Willebrand disease (VWD) experience many obstetric and gynecologic challenges, including higher levels of von Willebrand factor (VWF) in pregnancy, Romina Brignardello-Petersen, PhD, of McMaster University, Hamilton, Ont., and colleagues wrote.
The American Society of Hematology, the International Society on Thrombosis and Haemostasis, the National Hemophilia Foundation, and the World Federation of Hemophilia convened a working group in 2017 to address updated guidelines on VWD with a focus on women, the researchers said.
In an article published in Blood Advances, the researchers described the evidence from three systematic reviews conducted to inform three recommendations for the guidelines: first-line management of heavy menstrual bleeding (HMB), treatment of women requiring or desiring neuraxial analgesia, and management of postpartum hemorrhage. The authors identified studies published through October 2019.
The first systematic review of first-line therapies for HMB included five case series, one retrospective cohort study, and one randomized controlled trial. In the randomized controlled trial of 232 patients, low-certainty evidence suggested less reduction of blood loss with desmopressin, compared with tranexamic acid (TxA), with no significant differences in side effects. Very-low-certainty evidence from an observational study also supported lower effectiveness of desmopressin versus hormonal therapy. Finally, the case series showed very-low-certainty evidence for the comparative effectiveness of hormonal therapy delivered via a levonorgestrel-releasing intrauterine system (LNG-IUS) and other therapies for HMB control.
The second systematic review compared VWF levels in women who received neuraxial anesthesia during labor.
The review included five case series that described outcomes of women with VWF levels greater than 0.50 IU/mL; however, the studies did not describe outcomes according to VWF levels and did not cite the proportion of women with VWF levels greater than 1.50 IU/mL. Consequently, the evidence for the effects of increasing VWF levels was very low certainty, the authors said. In a meta-analysis, the proportion of anesthesia complications in these women was 6% (very low certainty). The complications included hypotension, accidental dural puncture, inadequate analgesia, bloody tap with no further complications, and failed block requiring general anesthesia.
The third systemic review included two retrospective cohort studies on the use of TxA during the postpartum period. In these studies, the authors found very-low-certainty evidence that TxA reduced the risk of severe primary postpartum hemorrhage, primary postpartum hemorrhage, and secondary postpartum hemorrhage (risk ratios, 0.36, 0.25, and 0.42, respectively). The effects of TxA on blood transfusions, vaginal hematoma, blood loss, and thrombotic complications also showed very-low-certainty evidence.
The currently available evidence for treatment options in women with VWD remains very low certainty, the researchers wrote in their discussion. “Because hormonal therapy is effective in controlling HMB (based on data from women without bleeding disorders), we believe the most effective strategy to be hormonal therapy with a LNG-IUS or combined oral contraceptives, followed by TxA and desmopressin.”
The study findings were limited by several factors including scarce evidence, the risk of bias in the observational studies, and lack of comparisons/controls in the case series, the researchers noted. Notable literature gaps included data on outcomes including major bleeding and the need for surgery or additional treatments in the first review; mortality, major bleeding, spinal hematoma, transfusion, and thrombotic events in the second review; and mortality, major bleeding, and the need for other procedures in the third review.
However, the findings were strengthened by the use of broad eligibility criteria to include any studies with potential useful advice, including case series, if these were the only available options. In developing recommendations, “the guideline panel interpreted the evidence adding their experience and knowledge of indirect evidence,” the authors noted.
The current evidence, though mainly very low certainty, “is the best available to inform decisions about management. Clinicians seeking advice on how to manage their patients with VWD should refer to the practice guidelines and assess to what extent they are applicable to their patients,” the researchers concluded.
Meeting the need for evidence-based guidelines
The review is important at this time because current evidence-based guidelines are limited, said coauthor Veronica Flood, MD, a pediatric hematologist at the Medical College of Wisconsin, Milwaukee, and a VWD researcher.
“While we have some guidelines that address von Willebrand disease, these were primarily based on expert opinion and not necessarily based on the best available evidence,” said Dr. Flood.
“Given how many people have von Willebrand disease, it is important that we actually base our recommendations on the data,” she emphasized. The new guidelines also incorporate patient feedback, with the inclusion of multiple panelists who are individuals living with VWD. “The final recommendations looked at not only the evidence, but the cost effectiveness, feasibility, and patient values and preferences,” she added.
“I was surprised we did not have better evidence for some of these common issues for patients with VWD,” said Dr. Flood. “I think that speaks to the need to do more high-quality research in this area.”
From a clinical standpoint, “we now have evidence-based guidelines that support the use of prophylaxis in patients with VWD and significant bleeding, as well as recommendations for surgery and bleeding issues around menstruation,” said Dr. Flood. “I do think it is also important to recognize that many of these are conditional recommendations, meaning there is room for patient preferences in implementation, which is helpful since we know that some people will have different priorities.”
Dr. Flood noted that more research is needed in many aspects of VWD. “We definitely need to better understand best options for surgical treatment, and I consider that a high priority. We are also hoping, along with the National Hemophilia Foundation, to develop some patient decision aids to help with some of these issues.”
Coauthor Nathan T. Connell, MD, an adult hematologist at the Brigham and Women’s Hospital and Harvard Medical School, both in Boston, served as the vice chair for the guideline panel. Dr. Connell agreed with the importance of the reviews and the need for additional research. “I, too, was surprised to see the lack of robust data to answer many of the basic questions about how to manage people living with VWD. Regarding the systematic reviews, I was surprised to see the power of combining the limited data in this way to come up with an evidence base for the panels to review,” he added.
The study was supported by the ASH, ISTH, NHF, and the WFH 2020 Guidelines for Management of VWD. The researchers had no financial conflicts to disclose.
FROM BLOOD ADVANCES









