User login
Caring for women with HIV: Unique needs and challenges
More than 30 years into the human immunodeficiency virus (HIV) epidemic, our understanding of the needs of women living with this virus continues to evolve. In the early years of the epidemic, managing HIV was all about preventing death and treating opportunistic infections. But now it is also about enabling patients to live long, healthy, and productive lives and preventing new HIV infections. In women, these goals can only be achieved by paying careful attention to sex-specific issues.
As a result of longer survival, HIV-infected persons are increasingly developing common health problems that also affect the general population and that require screening, management, and monitoring by primary care providers. Because people infected with HIV are typically seen by both an HIV specialist and a primary care provider, HIV specialists need to be familiar with primary care issues and primary care providers need to be familiar with HIV care recommendations in order to provide optimal care.
AFRICAN AMERICAN WOMEN BEAR A DISPROPORTIONATE BURDEN
In the United States, HIV was first reported in women in 1983 among those who had been steady sexual partners of males with acquired immune deficiency syndrome.1 Although men with HIV still outnumber women, the number of women with HIV has increased rapidly. At the end of 2010 an estimated one in four people with HIV in the United States was female.2
African American women bear a disproportionate burden of the disease (Table 1).3 In 2010, women accounted for an estimated 9,500 (20%) of the approximately 45,000 new infections occurring in the United States. Of these newly infected women, 64% were black, 18% were white, and 15% were Hispanic. Yet blacks make up only about 12% of the US population, whites make up 68%, and Hispanics 14%.
Regardless of race or ethnicity, unprotected heterosexual contact is the most common mode of transmission of HIV in women.2
Although the overall rates of HIV infection in the United States are relatively low, certain areas of the country have rates similar to those in sub-Saharan Africa, where most HIV-infected people reside.4 The HIV Prevention Trials Network found that the incidence of HIV infection in US women living in these “hot spots,” with high rates of poverty and HIV, was 0.32% per year. Compare this with the 2009 estimate of HIV incidence in the general population of US black women of similar age (0.05% per year) and the adult incidence rates in Congo (0.28% per year) and Kenya (0.53% per year).5 To better understand the epidemiology of HIV infection in women and concentrate our prevention efforts, we need to focus on these hot spots.
Misinformation abounds in these hot spots, as does disease. In a survey of residents of the South Side Chicago Housing Authority facilities,6 many were aware that effective antiretroviral therapy existed, but one-fourth thought that there was an effective HIV vaccine, and 13% thought there was a cure.
In the early years, an HIV diagnosis was essentially a death sentence. Samji et al7 estimated that life expectancy of patients who were prescribed antiretroviral therapy in the United States and Canada increased from 36.1 years in 2000–2002 to 51.4 years in 2006–2007, with the greatest increases in those who started with a baseline CD4 count above 350 cells/mm3. Now, a 20-year-old HIV-positive person with a CD4 count greater than 350 cells/mm3 can expect to live into his or her early 70s.
But not all patients achieve these benefits. In 2009, despite major advances in diagnosis and treatment, HIV was the fourth leading cause of death among African American women ages 25 to 44, causing about 800 deaths, or 9% of all deaths in this group.8
TEST ALL, UNLESS THEY OPT OUT
Testing is vital in efforts to prevent and treat HIV infection. In 2006, the US Centers for Disease Control and Prevention (CDC) recommended that everyone between the ages of 13 and 64 be screened for HIV regardless of risk.9
The CDC recommends an opt-out strategy.9 Rather than ask a patient whether he or she wants to be tested for HIV, the provider says something like, “I advise all of my patients to have an HIV test; as long as you have no objection, we will send you to the lab to have it done.” This approach reduces barriers to HIV testing by eliminating pretest counseling and by making HIV testing routine and the standard of care. Separate consent is not required—clinicians just need to document whether the patient has accepted or declined the test.
Testing should be offered at least once and can be done in any health care setting, including primary care offices and clinics, emergency rooms, health departments, and urgent care centers.9 Patients at higher risk (injection drug users and their sex partners; people who exchange sex for money or drugs; sex partners of HIV-infected people; men who have sex with men; and heterosexuals who themselves or whose sex partners have had more than one sex partner since their most recent HIV test) should receive repeat screening annually.
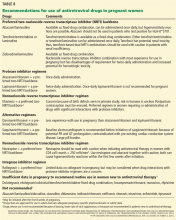
HIV testing should also be offered to all pregnant women at entry into care and again in the third trimester. This strategy is cost-effective even in areas of low prevalence.9 Since 2006, other professional organizations have made HIV testing recommendations as well (Table 2).9–12
A cost-effectiveness analysis suggested that routine opt-out testing is economically justified if the prevalence of HIV is greater than 0.2%.13
HIV-POSITIVE WOMEN NEED ROUTINE GYNECOLOGIC CARE
It is important for women with HIV to receive routine gynecologic care. Women with HIV have gynecologic problems similar to those of all women; however, they may be more vulnerable to certain conditions such as human papillomavirus (HPV) infection, which may be related to HIV disease or associated immunosuppression. In addition, pregnancy and family planning pose special challenges in this group.14
Cervical cancer screening
Effective screening and timely treatment of precancerous cervical lesions are key in preventing cervical cancer in women with or without HIV.
Persistent infection with HPV is necessary for the development of precancerous lesions as well as invasive cervical cancer. Most new cases of HPV infection in the general population resolve spontaneously within 2 years. However, in HIV-infected women, HPV infection is more likely to persist and progress to precancerous lesions of the cervix. This association is strongest in women with more compromised immune function as reflected by low CD4 cell counts and high viral loads.14 Women with HIV have higher rates of infection with high-risk HPV strains and of cervical intraepithelial neoplasia compared with their HIV-negative counterparts.14 The incidence of cervical cancer is five to six times higher in HIV-infected women in the United States than in the general population.15
According to guidelines from the Infectious Diseases Society of America,16 the American College of Obstetricians and Gynecologists,10 the CDC,17 and the American Cancer Society,18 all HIV-infected women should undergo cervical Papanicolaou (Pap) screening upon initiation into care, and this test should be repeated at 6 months and then annually if the results are normal. Patients with abnormalities on the Pap test should undergo colposcopy and, possibly, also biopsy. These abnormalities include atypical squamous cells of unknown significance and higher-grade lesions.16
Nearly one-fourth of HIV-positive women do not receive annual Pap smears despite engagement in care.19 This is unacceptable, because half of the cases of cervical cancer diagnosed in the United States are in women who never received appropriate screening, and an additional 10% are in women who have not been screened in the previous 5 years.19
In HIV-infected women who have had a total hysterectomy, whether to continue Pap testing depends on their history before the surgery. Continued vaginal Pap smear screening is recommended after hysterectomy (including removal of the cervix) in HIV-infected women who have a history of cervical intraepithelial neoplasia grades 2 or 3 or invasive cancer.10,17,20
TREATING HIV IN WOMEN: SPECIAL CONSIDERATIONS
Because it is not yet possible to eradicate the HIV virus, the goals of antiretroviral therapy are to reduce HIV-associated morbidity and mortality, to restore and preserve immune function, to suppress viral load, and to prevent sexual and, in women, perinatal transmission of the virus.21
Antiretroviral therapy is recommended for all HIV-infected patients regardless of the CD4 count, although the strength of recommendation is weaker with higher CD4 counts (Table 3).21 The recommendations for starting antiretroviral therapy and the goals of treatment are the same for men and women. Table 4 summarizes the recommendations for adolescents and adults who are new to treatment.21 For women, additional factors that should be taken into account when considering a regimen include pregnancy potential and whether the drugs chosen for the regimen are considered safe in pregnancy.
Since the early years of the HIV epidemic, researchers have debated whether women attain the same benefits from antiretroviral therapy as men. US Food and Drug Administration investigators performed a meta-analysis of the efficacy outcomes in women in studies of antiretroviral drugs published between 2000 and 2008. They included randomized clinical trials reporting at least 48-week efficacy outcomes, with viral suppression defined as HIV RNA less than 50 copies/mL. The combined database included 40 trials of 16 drugs from 7 drug classes with a total of 20,328 HIV-positive participants. Overall, there were no clinically or statistically significant differences between the sexes in 48-week efficacy outcomes or in rates of trial discontinuation due to adverse events, loss to follow-up, or death.22
Antiretroviral therapy may, however, cause different adverse effects in women than in men. For example:
Nevirapine, a nonnucleoside reverse transcriptase inhibitor, has been associated with the development of a rash and potentially life-threatening hepatotoxicity, more commonly in women than in men and at lower CD4 counts in women. This resulted in recommendations21 to avoid starting a nevirapine-containing regimen in women with CD4 counts greater than 250 cells/mm3 and in men with CD4 counts greater than 400 cells/mm3.
Ritonavir has been observed to cause a higher incidence of nausea and vomiting in women and a higher incidence of diarrhea in men. These are thought to be due to differences between men and women in weight and pharmacokinetics.23
PRECONCEPTION COUNSELING FOR HIV-POSITIVE WOMEN
Preconception counseling is an essential component of both primary and preventive care and should be considered the standard of care for all women of reproductive age who have HIV.24 Health care providers who fully understand the impact of HIV infection and associated comorbidities upon a woman’s reproductive health, fertility desires, and family planning needs are better prepared to assist in their patients’ reproductive health decisions.
The first few weeks of pregnancy are the most critical period in fetal development. During this time, a woman should be healthy and avoid any activities or substances that could cause adverse maternal or fetal outcomes. However, most patients present for prenatal care after this critical time period—thus the need for preconception counseling. Both the Infectious Diseases Society of America and the HIV Medicine Association recommend that all HIV-infected women of childbearing age be asked about their pregnancy plans and desires at the start of care and routinely thereafter.16
The goals of preconception care in women with HIV are to prevent unintended pregnancy, optimize maternal health before pregnancy, optimize pregnancy outcomes for mother and fetus, prevent perinatal HIV transmission, and prevent HIV transmission to an HIV-negative partner when trying to conceive.24
Goal 1: Prevent unintended pregnancy
Nearly half of all pregnancies in the United States are unintended.25 Moreover, the Women’s Interagency HIV Study26 showed that women with HIV are underusing effective contraception. In the Medical Monitoring Project, 85% of the women who had been pregnant since being diagnosed with HIV said that at least one pregnancy was unplanned.27
The consequences of unintended and unplanned pregnancies are serious and add significant burden to women, men, and families. Women who do not wish to become pregnant should be advised to use an effective method of contraception.
Contraception
Contraception use varies worldwide. Factors affecting its use include the methods available, patient choice, current health conditions, religious beliefs, perception of method effectiveness, and side effects.24
The Women’s Interagency HIV Study evaluated trends in contraception use from 1998 to 2010. Condoms were the most common form of contraception, and their use changed little over time. Fewer than 15% of women with HIV used no contraception. The use of long-acting reversible contraception, including injectable progestins, implants, and intrauterine devices, which minimize the need for user adherence, increased among HIV-negative women but not among HIV-positive women.28
The World Health Organization states that all available methods are safe for women with HIV except for spermicides with or without a diaphragm, as there is evidence linking the use of spermicides to an increased risk of HIV transmission (Table 5).29
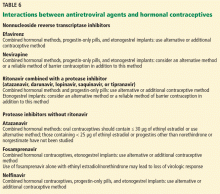
Some antiretroviral drugs may reduce the effectiveness of some contraceptives (Table 6); however, recommendations are based on pharmacokinetic studies, not on outcome studies. Condoms should be recommended not only to protect against pregnancy, but also to protect against sexually transmitted infections.
Goal 2: Optimize maternal health before pregnancy
Maternal health should be optimized before conceiving to reduce the risk of pregnancy-related morbidities and poor birth outcomes. This includes screening for other infections and ensuring that other comorbidities, such as hypertension, diabetes, substance abuse, and mental illness, are well managed with medications that are safe to use in pregnancy (Table 7).
Goal 3: Prevent perinatal HIV transmission
Educating the patient about perinatal transmission is a fundamental component of preconception counseling. Topics that need to be addressed are transmission risk and methods to reduce the risk, including not breastfeeding after delivery.
Goal 4: Prevent HIV transmission to an uninfected partner when trying to conceive
HIV-discordant couples who desire pregnancy should receive appropriate counseling about methods to minimize risk of transmission to the uninfected partner while trying to conceive. There are a number of effective methods and techniques, which are beyond the scope of this review. Key components of all methods are to screen for and treat sexually transmitted infections in both partners and to use effective antiretroviral therapy and attain maximal viral suppression in the HIV-positive partner.
Antiretroviral therapy for the HIV-infected partner significantly reduced the risk of HIV transmission by 96% in the HIV Prevention Trials Network 052 trial.30 Of note: this reduction was the result of both risk-reduction counseling and antiretroviral therapy. This was the first randomized clinical trial to demonstrate that antiretroviral therapy in those with some preserved immune function (CD4 counts 350–500 cells/mm3) in conjunction with risk-reduction counseling can reduce HIV transmission to an uninfected partner.
Vaginal insemination without intercourse is another option for female-positive couples. The man ejaculates into a condom without spermicide, and the contents are introduced with a non-needle syringe or turkey baster. This can be done at home and confers no risk to the uninfected male partner.31 Chances of pregnancy can be maximized by insemination during the most fertile days of the menstrual cycle.
Preexposure prophylaxis combined with timed intercourse. In a study in Switzerland, the infected male partner was given antiretroviral therapy to suppress his viral load to less than 50 copies/mL for at least 6 months, and luteinizing hormone was measured every day in the urine of the noninfected female partner. When the urinary luteinizing hormone level reached a peak, the woman received a dose of tenofovir in the morning, the couple had unprotected intercourse, and the woman took a second dose the next morning. In 53 cases, none of the female partners seroconverted for HIV.32
Health care providers need to document and update the relationship status, partner HIV status, and fertility desires of their HIV patients, both men and women, on a regular basis. Patient education should include awareness of referrals and options to help safely conceive when desired and achieve effective contraception when not.33
WHEN HIV-POSITIVE WOMEN BECOME PREGNANT
Screening for HIV during pregnancy
The CDC recommends prenatal screening for HIV in the first trimester or at entry into prenatal care. A repeat HIV test should be offered in the third trimester for women at risk of acquiring HIV, for women who have signs or symptoms of early HIV infection, in health care settings where prenatal testing yields at least 1 case of HIV infection per 1,000 women screened, and in areas of high HIV incidence. If women present to labor and delivery with unknown HIV status, rapid HIV testing should be done.9
If a woman acquires HIV during pregnancy, the infection may not be detected and may be transmitted to the infant at birth. From 2002 to 2006 in New York State, 3,396 HIV-exposed babies were born. Of these, 9 (22%) of 41 infants born to mothers who acquired HIV during pregnancy became infected, compared with 1.8% of those born to mothers who acquired HIV before pregnancy. Maternal acquisition of HIV during pregnancy was documented in only 1.3% of perinatal HIV exposures, but it was associated with 9 (13.8%) of the 65 perinatal transmission cases.34
Providers should be aware of the signs and symptoms of acute HIV infection and should have a low threshold for repeating HIV testing at any time during pregnancy. It has been estimated that 40% to 90% of patients with acute HIV infection experience fever, lymphadenopathy, pharyngitis, skin rash, myalgia, arthralgia, or other symptoms.35 Providers often do not recognize acute HIV infection, however, because the symptoms are similar to those of other common illnesses. Also, some individuals with the condition have no symptoms.
Antiretroviral therapy during pregnancy
In a landmark study, AIDS Clinical Trial Group 076 demonstrated that zidovudine monotherapy given during pregnancy, labor, and delivery and to the newborn reduced the risk of HIV transmission to the infant by 67%, from 25% to 8%.36 Other studies demonstrated that combination therapy further decreased the risk of HIV transmission to 1% to 2%.37
The US Department of Health and Human Services recommends that all HIV-positive women who are pregnant receive effective combination antiretroviral therapy regardless of CD4 count to minimize the risk of mother-to-child transmission.37
The goals of HIV treatment during pregnancy are to maintain the woman’s health, restore her immune system, suppress viral replication, and decrease the risk of perinatal transmission. The preferred antiretroviral therapy for pregnant women differs from that for nonpregnant women and is based on evolving experience and information about safety, efficacy, and tolerability in pregnancy (Table 8). A woman who presents for prenatal care on a suppressive regimen should continue that regimen as long as she can tolerate it because there is a risk of losing virologic control when switching regimens, and this may increase the risk of perinatal transmission.37
Physiologic changes that occur during pregnancy may alter drug disposition, which could potentially lead to decreased drug exposure. Some of the changes include an increase in total body water, decreased protein binding, induction of hepatic metabolic pathways, and increased clearance of drugs eliminated by the kidneys.38 These changes may be associated with incomplete virologic suppression, virologic failure, or development of drug resistance, so altered doses of some antiretroviral drugs or careful monitoring of viral load should be considered, particularly in the second and third trimester.
Delivery
Women who have a viral load greater than 1,000 copies/mL near the end of pregnancy should undergo a cesarean delivery at 38 weeks and, before surgery, should receive intravenous zidovudine to reduce the risk of perinatal transmission. For women with viral loads below the threshold of 1,000 copies/mL, there is no proven added benefit to cesarean delivery, and in this situation it should be performed only for standard obstetric indications. Antiretroviral regimens should be continued during labor.37
HIV IN OLDER ADULTS
By 2015, approximately 50% of people with HIV will be over age 50.39 Unfortunately, older people and their providers often underestimate their risk of acquiring HIV. Many older people are newly single and may engage in sexual activity with new partners. Also, older people may be reluctant to use condoms as the need for contraception is past.40,41
Baseline HIV RNA levels tend to be higher and CD4 cell counts lower in patients diagnosed with HIV at older ages. These observations support previous ones that older HIV-infected patients may have advanced HIV disease at the time of diagnosis, perhaps in part due to delayed testing.42 Other possible factors are limited income, comorbid illness, polypharmacy, and insufficient data on drug interactions in the elderly.41,42
A prompt diagnosis is important for older patients because HIV may accelerate aging, and aging may speed up HIV progression. Studies have shown that aging is associated with more rapid progression to AIDS, particularly among people who are older than 40 at seroconversion.43 Other studies have reported that older patients have better virologic responses to antiretroviral therapy but have a blunted immune response, more AIDS-defining events, and a higher mortality rate than younger patients.42
- Centers for Disease Control and Prevention (CDC). Immunodeficiency among female sexual partners of males with acquired immune deficiency syndrome (AIDS) - New York. MMWR Morb Mortal Wkly Rep 1983; 31:697–698.
- Centers for Disease Control and Prevention (CDC). Estimated HIV incidence in the United States, 2007–2010. HIV Surveillance Supplemental Report 2012; 17( No. 4). www.cdc.gov/hiv/topics/surveillance/resources/reports/#supplemental. Accessed October 3, 2014.
- Centers for Disease Control and Prevention. HIV in the United States: at a glance. www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed October 3, 2014.
- El-Sadr WM, Mayer KH, Hodder SL. AIDS in America—forgotten but not gone. N Engl J Med 2010; 362:967–970.
- Eshleman SH, Hughes JP, Laeyendecker O, et al. Use of a multifaceted approach to analyze HIV incidence in a cohort study of women in the United States: HIV Prevention Trials Network 064 Study. J Infect Dis 2013; 207:223–231.
- Djokic D, Englund J, Daum R, et al. HIV knowledge and attitudes toward HIV testing of South Side Chicago Housing Authority residents. AIDS Patient Care STDS 2009; 23:23–28.
- Samji H, Cescon A, Hogg RS, et al; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e81355.
- Centers for Disease Control and Prevention (CDC). HIV/AIDS. HIV mortality (through 2010). www.cdc.gov/hiv/library/slideSets/index.html. Accessed October 3, 2014.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55:1–17.
- The American College of Obstetricians and Gynecologists (ACOG). Routine Human Immunodeficiency Virus Screening Committee Opinion Number 596, May 2014. (Replaces Committee Opinion Number 411, August 2008.) www.acog.org/Resources_And_Publications/Committee_Opinions/Committee_on_Gynecologic_Practice/Routine_Human_Immunodeficiency_Virus_Screening. Accessed October 3, 2014.
- US Preventive Services Task Force. Screening for HIV. http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/human-immunodeficiency-virus-hiv-infection-screening. Accessed October 3, 2014.
- Institute of Medicine. HIV screening and access to care health care system capacity for increased HIV testing and provision of care. www.iom.edu/Reports/2011/HIV-Screening-and-Access-to-Care-Health-Care-System-Capacity-for-Increased-HIV-Testing-and-Provision-of-Care.aspx. Accessed October 3, 2014.
- Walensky RP, Freedberg KA, Weinstein MC, Paltiel AD. Cost-effectiveness of HIV testing and treatment in the United States. Clin Infect Dis 2007; 45(suppl 4):S248–S254.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 117: Gynecologic care for women with human immunodeficiency virus. Obstet Gynecol 2010; 116:1492–1509.
- Centers for Disease Control and Prevention (CDC). Invasive cancer incidence—United States, 2009. MMWR Morb Mortal Wkly Rep 2013; 62:113–118.
- Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58:e1–e34.
- Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed October 3, 2014.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin 2012; 62:147–172.
- Oster AM, Sullivan PS, Blair JM. Prevalence of cervical cancer screening of HIV-infected women in the United States. J Acquir Immune Defic Syndr 2009; 51:430–436.
- Paramsothy P, Duerr A, Heilig CM, et al; HIV Epidemiology Research (HER) Study Group. Abnormal vaginal cytology in HIV-infected and at-risk women after hysterectomy. J Acquir Immune Defic Syndr 2004; 35:484–491.
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed October 3, 2014.
- Soon GG, Min M, Struble KA, et al. Meta-analysis of gender differences in efficacy outcomes for HIV-positive subjects in randomized controlled clinical trials of antiretroviral therapy (2000–2008). AIDS Patient Care STDS 2012; 26:444–453.
- Clark RA, Squires KE. Gender-specific considerations in the antiretroviral management of HIV-infected women. Expert Rev Anti Infect Ther 2005; 3:213–227.
- Johnson K, Posner SF, Biermann J, et al; CDC/ATSDR Preconception Care Work Group; Select Panel on Preconception Care. Recommendations to improve preconception health and health care—United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. MMWR Recomm Rep 2006; 55:1–23.
- Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception 2011; 84:478–485.
- Massad LS, Evans CT, Wilson TE, et al. Contraceptive use among US women with HIV. J Womens Health (Larchmt) 2007; 16:657–666.
- Sutton MY, Patel R, Frazier EL. Unplanned pregnancies among HIV-infected women in care-United States. J Acquir Immune Defic Syndr 2014; 65:350–358.
- Sun M, Peipert JF, Zhao Q, et al. Trends in contraceptive use among women with human immunodeficiency virus. Obstet Gynecol 2012; 120:783–790.
- World Health Organization (WHO). Medical eligibility criteria for contraceptive use. 4th ed. http://whqlibdoc.who.int/publications/2010/9789241563888_eng.pdf. Accessed October 3, 2014.
- Cohen MS, Chen YQ, McCauley M, et al; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505.
- Mmeje O, Cohen CR, Cohan D. Evaluating safer conception options for HIV-serodiscordant couples (HIV-infected female/HIV-uninfected male): a closer look at vaginal insemination. Infect Dis Obstet Gynecol 2012; 2012:587–651.
- Vernazza PL, Graf I, Sonnenberg-Schwan U, Geit M, Meurer A. Preexposure prophylaxis and timed intercourse for HIV-discordant couples willing to conceive a child. AIDS 2011; 25:2005–2008.
- Lampe MA, Smith DK, Anderson GJ, Edwards AE, Nesheim SR. Achieving safe conception in HIV-discordant couples: the potential role of oral preexposure prophylaxis (PrEP) in the United States. Am J Obstet Gynecol 2011; 204:488.e1–e8.
- Birkhead GS, Pulver WP, Warren BL, Hackel S, Rodríguez D, Smith L. Acquiring human immunodeficiency virus during pregnancy and mother-to-child transmission in New York: 2002–2006. Obstet Gynecol 2010; 115:1247–1255.
- Yerly S, Hirschel B. Diagnosing acute HIV infection. Expert Rev Anti Infect Ther 2012; 10:31–41.
- Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med 1994; 331:1173–1180.
- Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Accessed October 3, 2014.
- Mirochnick M, Capparelli E. Pharmacokinetics of antiretrovirals in pregnant women. Clin Pharmacokinet 2004; 43:1071–1087.
- Smith GSenate Committee on Aging. HIV over fifty: exploring the new threat. Washington, DC; 2005. http://www.aging.senate.gov/imo/media/doc/5122005.pdf. Accessed October 3, 2014.
- Illa L, Brickman A, Saint-Jean G, et al. Sexual risk behaviors in late middle age and older HIV seropositive adults. AIDS Behav 2008; 12:935–942.
- Luther VP, Wilkin AM. HIV infection in older adults. Clin Geriatr Med 2007; 23:567–583.
- Collaboration of Observational HIV Epidemiological Research Europe (COHERE) Study Group; Sabin CA, Smith CJ, d’Arminio Monforte A, et al. Response to combination antiretroviral therapy: variation by age. AIDS 2008; 22:1463–1473.
- Pezzotti P, Phillips AN, Dorrucci M, et al. Category of exposure to HIV and age in the progression to AIDS: longitudinal study of 1,199 people with known dates of seroconversion. HIV Italian Seroconversion Study Group. BMJ 1996; 313:583–586.
More than 30 years into the human immunodeficiency virus (HIV) epidemic, our understanding of the needs of women living with this virus continues to evolve. In the early years of the epidemic, managing HIV was all about preventing death and treating opportunistic infections. But now it is also about enabling patients to live long, healthy, and productive lives and preventing new HIV infections. In women, these goals can only be achieved by paying careful attention to sex-specific issues.
As a result of longer survival, HIV-infected persons are increasingly developing common health problems that also affect the general population and that require screening, management, and monitoring by primary care providers. Because people infected with HIV are typically seen by both an HIV specialist and a primary care provider, HIV specialists need to be familiar with primary care issues and primary care providers need to be familiar with HIV care recommendations in order to provide optimal care.
AFRICAN AMERICAN WOMEN BEAR A DISPROPORTIONATE BURDEN
In the United States, HIV was first reported in women in 1983 among those who had been steady sexual partners of males with acquired immune deficiency syndrome.1 Although men with HIV still outnumber women, the number of women with HIV has increased rapidly. At the end of 2010 an estimated one in four people with HIV in the United States was female.2
African American women bear a disproportionate burden of the disease (Table 1).3 In 2010, women accounted for an estimated 9,500 (20%) of the approximately 45,000 new infections occurring in the United States. Of these newly infected women, 64% were black, 18% were white, and 15% were Hispanic. Yet blacks make up only about 12% of the US population, whites make up 68%, and Hispanics 14%.
Regardless of race or ethnicity, unprotected heterosexual contact is the most common mode of transmission of HIV in women.2
Although the overall rates of HIV infection in the United States are relatively low, certain areas of the country have rates similar to those in sub-Saharan Africa, where most HIV-infected people reside.4 The HIV Prevention Trials Network found that the incidence of HIV infection in US women living in these “hot spots,” with high rates of poverty and HIV, was 0.32% per year. Compare this with the 2009 estimate of HIV incidence in the general population of US black women of similar age (0.05% per year) and the adult incidence rates in Congo (0.28% per year) and Kenya (0.53% per year).5 To better understand the epidemiology of HIV infection in women and concentrate our prevention efforts, we need to focus on these hot spots.
Misinformation abounds in these hot spots, as does disease. In a survey of residents of the South Side Chicago Housing Authority facilities,6 many were aware that effective antiretroviral therapy existed, but one-fourth thought that there was an effective HIV vaccine, and 13% thought there was a cure.
In the early years, an HIV diagnosis was essentially a death sentence. Samji et al7 estimated that life expectancy of patients who were prescribed antiretroviral therapy in the United States and Canada increased from 36.1 years in 2000–2002 to 51.4 years in 2006–2007, with the greatest increases in those who started with a baseline CD4 count above 350 cells/mm3. Now, a 20-year-old HIV-positive person with a CD4 count greater than 350 cells/mm3 can expect to live into his or her early 70s.
But not all patients achieve these benefits. In 2009, despite major advances in diagnosis and treatment, HIV was the fourth leading cause of death among African American women ages 25 to 44, causing about 800 deaths, or 9% of all deaths in this group.8
TEST ALL, UNLESS THEY OPT OUT
Testing is vital in efforts to prevent and treat HIV infection. In 2006, the US Centers for Disease Control and Prevention (CDC) recommended that everyone between the ages of 13 and 64 be screened for HIV regardless of risk.9
The CDC recommends an opt-out strategy.9 Rather than ask a patient whether he or she wants to be tested for HIV, the provider says something like, “I advise all of my patients to have an HIV test; as long as you have no objection, we will send you to the lab to have it done.” This approach reduces barriers to HIV testing by eliminating pretest counseling and by making HIV testing routine and the standard of care. Separate consent is not required—clinicians just need to document whether the patient has accepted or declined the test.
Testing should be offered at least once and can be done in any health care setting, including primary care offices and clinics, emergency rooms, health departments, and urgent care centers.9 Patients at higher risk (injection drug users and their sex partners; people who exchange sex for money or drugs; sex partners of HIV-infected people; men who have sex with men; and heterosexuals who themselves or whose sex partners have had more than one sex partner since their most recent HIV test) should receive repeat screening annually.
HIV testing should also be offered to all pregnant women at entry into care and again in the third trimester. This strategy is cost-effective even in areas of low prevalence.9 Since 2006, other professional organizations have made HIV testing recommendations as well (Table 2).9–12
A cost-effectiveness analysis suggested that routine opt-out testing is economically justified if the prevalence of HIV is greater than 0.2%.13
HIV-POSITIVE WOMEN NEED ROUTINE GYNECOLOGIC CARE
It is important for women with HIV to receive routine gynecologic care. Women with HIV have gynecologic problems similar to those of all women; however, they may be more vulnerable to certain conditions such as human papillomavirus (HPV) infection, which may be related to HIV disease or associated immunosuppression. In addition, pregnancy and family planning pose special challenges in this group.14
Cervical cancer screening
Effective screening and timely treatment of precancerous cervical lesions are key in preventing cervical cancer in women with or without HIV.
Persistent infection with HPV is necessary for the development of precancerous lesions as well as invasive cervical cancer. Most new cases of HPV infection in the general population resolve spontaneously within 2 years. However, in HIV-infected women, HPV infection is more likely to persist and progress to precancerous lesions of the cervix. This association is strongest in women with more compromised immune function as reflected by low CD4 cell counts and high viral loads.14 Women with HIV have higher rates of infection with high-risk HPV strains and of cervical intraepithelial neoplasia compared with their HIV-negative counterparts.14 The incidence of cervical cancer is five to six times higher in HIV-infected women in the United States than in the general population.15
According to guidelines from the Infectious Diseases Society of America,16 the American College of Obstetricians and Gynecologists,10 the CDC,17 and the American Cancer Society,18 all HIV-infected women should undergo cervical Papanicolaou (Pap) screening upon initiation into care, and this test should be repeated at 6 months and then annually if the results are normal. Patients with abnormalities on the Pap test should undergo colposcopy and, possibly, also biopsy. These abnormalities include atypical squamous cells of unknown significance and higher-grade lesions.16
Nearly one-fourth of HIV-positive women do not receive annual Pap smears despite engagement in care.19 This is unacceptable, because half of the cases of cervical cancer diagnosed in the United States are in women who never received appropriate screening, and an additional 10% are in women who have not been screened in the previous 5 years.19
In HIV-infected women who have had a total hysterectomy, whether to continue Pap testing depends on their history before the surgery. Continued vaginal Pap smear screening is recommended after hysterectomy (including removal of the cervix) in HIV-infected women who have a history of cervical intraepithelial neoplasia grades 2 or 3 or invasive cancer.10,17,20
TREATING HIV IN WOMEN: SPECIAL CONSIDERATIONS
Because it is not yet possible to eradicate the HIV virus, the goals of antiretroviral therapy are to reduce HIV-associated morbidity and mortality, to restore and preserve immune function, to suppress viral load, and to prevent sexual and, in women, perinatal transmission of the virus.21
Antiretroviral therapy is recommended for all HIV-infected patients regardless of the CD4 count, although the strength of recommendation is weaker with higher CD4 counts (Table 3).21 The recommendations for starting antiretroviral therapy and the goals of treatment are the same for men and women. Table 4 summarizes the recommendations for adolescents and adults who are new to treatment.21 For women, additional factors that should be taken into account when considering a regimen include pregnancy potential and whether the drugs chosen for the regimen are considered safe in pregnancy.
Since the early years of the HIV epidemic, researchers have debated whether women attain the same benefits from antiretroviral therapy as men. US Food and Drug Administration investigators performed a meta-analysis of the efficacy outcomes in women in studies of antiretroviral drugs published between 2000 and 2008. They included randomized clinical trials reporting at least 48-week efficacy outcomes, with viral suppression defined as HIV RNA less than 50 copies/mL. The combined database included 40 trials of 16 drugs from 7 drug classes with a total of 20,328 HIV-positive participants. Overall, there were no clinically or statistically significant differences between the sexes in 48-week efficacy outcomes or in rates of trial discontinuation due to adverse events, loss to follow-up, or death.22
Antiretroviral therapy may, however, cause different adverse effects in women than in men. For example:
Nevirapine, a nonnucleoside reverse transcriptase inhibitor, has been associated with the development of a rash and potentially life-threatening hepatotoxicity, more commonly in women than in men and at lower CD4 counts in women. This resulted in recommendations21 to avoid starting a nevirapine-containing regimen in women with CD4 counts greater than 250 cells/mm3 and in men with CD4 counts greater than 400 cells/mm3.
Ritonavir has been observed to cause a higher incidence of nausea and vomiting in women and a higher incidence of diarrhea in men. These are thought to be due to differences between men and women in weight and pharmacokinetics.23
PRECONCEPTION COUNSELING FOR HIV-POSITIVE WOMEN
Preconception counseling is an essential component of both primary and preventive care and should be considered the standard of care for all women of reproductive age who have HIV.24 Health care providers who fully understand the impact of HIV infection and associated comorbidities upon a woman’s reproductive health, fertility desires, and family planning needs are better prepared to assist in their patients’ reproductive health decisions.
The first few weeks of pregnancy are the most critical period in fetal development. During this time, a woman should be healthy and avoid any activities or substances that could cause adverse maternal or fetal outcomes. However, most patients present for prenatal care after this critical time period—thus the need for preconception counseling. Both the Infectious Diseases Society of America and the HIV Medicine Association recommend that all HIV-infected women of childbearing age be asked about their pregnancy plans and desires at the start of care and routinely thereafter.16
The goals of preconception care in women with HIV are to prevent unintended pregnancy, optimize maternal health before pregnancy, optimize pregnancy outcomes for mother and fetus, prevent perinatal HIV transmission, and prevent HIV transmission to an HIV-negative partner when trying to conceive.24
Goal 1: Prevent unintended pregnancy
Nearly half of all pregnancies in the United States are unintended.25 Moreover, the Women’s Interagency HIV Study26 showed that women with HIV are underusing effective contraception. In the Medical Monitoring Project, 85% of the women who had been pregnant since being diagnosed with HIV said that at least one pregnancy was unplanned.27
The consequences of unintended and unplanned pregnancies are serious and add significant burden to women, men, and families. Women who do not wish to become pregnant should be advised to use an effective method of contraception.
Contraception
Contraception use varies worldwide. Factors affecting its use include the methods available, patient choice, current health conditions, religious beliefs, perception of method effectiveness, and side effects.24
The Women’s Interagency HIV Study evaluated trends in contraception use from 1998 to 2010. Condoms were the most common form of contraception, and their use changed little over time. Fewer than 15% of women with HIV used no contraception. The use of long-acting reversible contraception, including injectable progestins, implants, and intrauterine devices, which minimize the need for user adherence, increased among HIV-negative women but not among HIV-positive women.28
The World Health Organization states that all available methods are safe for women with HIV except for spermicides with or without a diaphragm, as there is evidence linking the use of spermicides to an increased risk of HIV transmission (Table 5).29
Some antiretroviral drugs may reduce the effectiveness of some contraceptives (Table 6); however, recommendations are based on pharmacokinetic studies, not on outcome studies. Condoms should be recommended not only to protect against pregnancy, but also to protect against sexually transmitted infections.
Goal 2: Optimize maternal health before pregnancy
Maternal health should be optimized before conceiving to reduce the risk of pregnancy-related morbidities and poor birth outcomes. This includes screening for other infections and ensuring that other comorbidities, such as hypertension, diabetes, substance abuse, and mental illness, are well managed with medications that are safe to use in pregnancy (Table 7).
Goal 3: Prevent perinatal HIV transmission
Educating the patient about perinatal transmission is a fundamental component of preconception counseling. Topics that need to be addressed are transmission risk and methods to reduce the risk, including not breastfeeding after delivery.
Goal 4: Prevent HIV transmission to an uninfected partner when trying to conceive
HIV-discordant couples who desire pregnancy should receive appropriate counseling about methods to minimize risk of transmission to the uninfected partner while trying to conceive. There are a number of effective methods and techniques, which are beyond the scope of this review. Key components of all methods are to screen for and treat sexually transmitted infections in both partners and to use effective antiretroviral therapy and attain maximal viral suppression in the HIV-positive partner.
Antiretroviral therapy for the HIV-infected partner significantly reduced the risk of HIV transmission by 96% in the HIV Prevention Trials Network 052 trial.30 Of note: this reduction was the result of both risk-reduction counseling and antiretroviral therapy. This was the first randomized clinical trial to demonstrate that antiretroviral therapy in those with some preserved immune function (CD4 counts 350–500 cells/mm3) in conjunction with risk-reduction counseling can reduce HIV transmission to an uninfected partner.
Vaginal insemination without intercourse is another option for female-positive couples. The man ejaculates into a condom without spermicide, and the contents are introduced with a non-needle syringe or turkey baster. This can be done at home and confers no risk to the uninfected male partner.31 Chances of pregnancy can be maximized by insemination during the most fertile days of the menstrual cycle.
Preexposure prophylaxis combined with timed intercourse. In a study in Switzerland, the infected male partner was given antiretroviral therapy to suppress his viral load to less than 50 copies/mL for at least 6 months, and luteinizing hormone was measured every day in the urine of the noninfected female partner. When the urinary luteinizing hormone level reached a peak, the woman received a dose of tenofovir in the morning, the couple had unprotected intercourse, and the woman took a second dose the next morning. In 53 cases, none of the female partners seroconverted for HIV.32
Health care providers need to document and update the relationship status, partner HIV status, and fertility desires of their HIV patients, both men and women, on a regular basis. Patient education should include awareness of referrals and options to help safely conceive when desired and achieve effective contraception when not.33
WHEN HIV-POSITIVE WOMEN BECOME PREGNANT
Screening for HIV during pregnancy
The CDC recommends prenatal screening for HIV in the first trimester or at entry into prenatal care. A repeat HIV test should be offered in the third trimester for women at risk of acquiring HIV, for women who have signs or symptoms of early HIV infection, in health care settings where prenatal testing yields at least 1 case of HIV infection per 1,000 women screened, and in areas of high HIV incidence. If women present to labor and delivery with unknown HIV status, rapid HIV testing should be done.9
If a woman acquires HIV during pregnancy, the infection may not be detected and may be transmitted to the infant at birth. From 2002 to 2006 in New York State, 3,396 HIV-exposed babies were born. Of these, 9 (22%) of 41 infants born to mothers who acquired HIV during pregnancy became infected, compared with 1.8% of those born to mothers who acquired HIV before pregnancy. Maternal acquisition of HIV during pregnancy was documented in only 1.3% of perinatal HIV exposures, but it was associated with 9 (13.8%) of the 65 perinatal transmission cases.34
Providers should be aware of the signs and symptoms of acute HIV infection and should have a low threshold for repeating HIV testing at any time during pregnancy. It has been estimated that 40% to 90% of patients with acute HIV infection experience fever, lymphadenopathy, pharyngitis, skin rash, myalgia, arthralgia, or other symptoms.35 Providers often do not recognize acute HIV infection, however, because the symptoms are similar to those of other common illnesses. Also, some individuals with the condition have no symptoms.
Antiretroviral therapy during pregnancy
In a landmark study, AIDS Clinical Trial Group 076 demonstrated that zidovudine monotherapy given during pregnancy, labor, and delivery and to the newborn reduced the risk of HIV transmission to the infant by 67%, from 25% to 8%.36 Other studies demonstrated that combination therapy further decreased the risk of HIV transmission to 1% to 2%.37
The US Department of Health and Human Services recommends that all HIV-positive women who are pregnant receive effective combination antiretroviral therapy regardless of CD4 count to minimize the risk of mother-to-child transmission.37
The goals of HIV treatment during pregnancy are to maintain the woman’s health, restore her immune system, suppress viral replication, and decrease the risk of perinatal transmission. The preferred antiretroviral therapy for pregnant women differs from that for nonpregnant women and is based on evolving experience and information about safety, efficacy, and tolerability in pregnancy (Table 8). A woman who presents for prenatal care on a suppressive regimen should continue that regimen as long as she can tolerate it because there is a risk of losing virologic control when switching regimens, and this may increase the risk of perinatal transmission.37
Physiologic changes that occur during pregnancy may alter drug disposition, which could potentially lead to decreased drug exposure. Some of the changes include an increase in total body water, decreased protein binding, induction of hepatic metabolic pathways, and increased clearance of drugs eliminated by the kidneys.38 These changes may be associated with incomplete virologic suppression, virologic failure, or development of drug resistance, so altered doses of some antiretroviral drugs or careful monitoring of viral load should be considered, particularly in the second and third trimester.
Delivery
Women who have a viral load greater than 1,000 copies/mL near the end of pregnancy should undergo a cesarean delivery at 38 weeks and, before surgery, should receive intravenous zidovudine to reduce the risk of perinatal transmission. For women with viral loads below the threshold of 1,000 copies/mL, there is no proven added benefit to cesarean delivery, and in this situation it should be performed only for standard obstetric indications. Antiretroviral regimens should be continued during labor.37
HIV IN OLDER ADULTS
By 2015, approximately 50% of people with HIV will be over age 50.39 Unfortunately, older people and their providers often underestimate their risk of acquiring HIV. Many older people are newly single and may engage in sexual activity with new partners. Also, older people may be reluctant to use condoms as the need for contraception is past.40,41
Baseline HIV RNA levels tend to be higher and CD4 cell counts lower in patients diagnosed with HIV at older ages. These observations support previous ones that older HIV-infected patients may have advanced HIV disease at the time of diagnosis, perhaps in part due to delayed testing.42 Other possible factors are limited income, comorbid illness, polypharmacy, and insufficient data on drug interactions in the elderly.41,42
A prompt diagnosis is important for older patients because HIV may accelerate aging, and aging may speed up HIV progression. Studies have shown that aging is associated with more rapid progression to AIDS, particularly among people who are older than 40 at seroconversion.43 Other studies have reported that older patients have better virologic responses to antiretroviral therapy but have a blunted immune response, more AIDS-defining events, and a higher mortality rate than younger patients.42
More than 30 years into the human immunodeficiency virus (HIV) epidemic, our understanding of the needs of women living with this virus continues to evolve. In the early years of the epidemic, managing HIV was all about preventing death and treating opportunistic infections. But now it is also about enabling patients to live long, healthy, and productive lives and preventing new HIV infections. In women, these goals can only be achieved by paying careful attention to sex-specific issues.
As a result of longer survival, HIV-infected persons are increasingly developing common health problems that also affect the general population and that require screening, management, and monitoring by primary care providers. Because people infected with HIV are typically seen by both an HIV specialist and a primary care provider, HIV specialists need to be familiar with primary care issues and primary care providers need to be familiar with HIV care recommendations in order to provide optimal care.
AFRICAN AMERICAN WOMEN BEAR A DISPROPORTIONATE BURDEN
In the United States, HIV was first reported in women in 1983 among those who had been steady sexual partners of males with acquired immune deficiency syndrome.1 Although men with HIV still outnumber women, the number of women with HIV has increased rapidly. At the end of 2010 an estimated one in four people with HIV in the United States was female.2
African American women bear a disproportionate burden of the disease (Table 1).3 In 2010, women accounted for an estimated 9,500 (20%) of the approximately 45,000 new infections occurring in the United States. Of these newly infected women, 64% were black, 18% were white, and 15% were Hispanic. Yet blacks make up only about 12% of the US population, whites make up 68%, and Hispanics 14%.
Regardless of race or ethnicity, unprotected heterosexual contact is the most common mode of transmission of HIV in women.2
Although the overall rates of HIV infection in the United States are relatively low, certain areas of the country have rates similar to those in sub-Saharan Africa, where most HIV-infected people reside.4 The HIV Prevention Trials Network found that the incidence of HIV infection in US women living in these “hot spots,” with high rates of poverty and HIV, was 0.32% per year. Compare this with the 2009 estimate of HIV incidence in the general population of US black women of similar age (0.05% per year) and the adult incidence rates in Congo (0.28% per year) and Kenya (0.53% per year).5 To better understand the epidemiology of HIV infection in women and concentrate our prevention efforts, we need to focus on these hot spots.
Misinformation abounds in these hot spots, as does disease. In a survey of residents of the South Side Chicago Housing Authority facilities,6 many were aware that effective antiretroviral therapy existed, but one-fourth thought that there was an effective HIV vaccine, and 13% thought there was a cure.
In the early years, an HIV diagnosis was essentially a death sentence. Samji et al7 estimated that life expectancy of patients who were prescribed antiretroviral therapy in the United States and Canada increased from 36.1 years in 2000–2002 to 51.4 years in 2006–2007, with the greatest increases in those who started with a baseline CD4 count above 350 cells/mm3. Now, a 20-year-old HIV-positive person with a CD4 count greater than 350 cells/mm3 can expect to live into his or her early 70s.
But not all patients achieve these benefits. In 2009, despite major advances in diagnosis and treatment, HIV was the fourth leading cause of death among African American women ages 25 to 44, causing about 800 deaths, or 9% of all deaths in this group.8
TEST ALL, UNLESS THEY OPT OUT
Testing is vital in efforts to prevent and treat HIV infection. In 2006, the US Centers for Disease Control and Prevention (CDC) recommended that everyone between the ages of 13 and 64 be screened for HIV regardless of risk.9
The CDC recommends an opt-out strategy.9 Rather than ask a patient whether he or she wants to be tested for HIV, the provider says something like, “I advise all of my patients to have an HIV test; as long as you have no objection, we will send you to the lab to have it done.” This approach reduces barriers to HIV testing by eliminating pretest counseling and by making HIV testing routine and the standard of care. Separate consent is not required—clinicians just need to document whether the patient has accepted or declined the test.
Testing should be offered at least once and can be done in any health care setting, including primary care offices and clinics, emergency rooms, health departments, and urgent care centers.9 Patients at higher risk (injection drug users and their sex partners; people who exchange sex for money or drugs; sex partners of HIV-infected people; men who have sex with men; and heterosexuals who themselves or whose sex partners have had more than one sex partner since their most recent HIV test) should receive repeat screening annually.
HIV testing should also be offered to all pregnant women at entry into care and again in the third trimester. This strategy is cost-effective even in areas of low prevalence.9 Since 2006, other professional organizations have made HIV testing recommendations as well (Table 2).9–12
A cost-effectiveness analysis suggested that routine opt-out testing is economically justified if the prevalence of HIV is greater than 0.2%.13
HIV-POSITIVE WOMEN NEED ROUTINE GYNECOLOGIC CARE
It is important for women with HIV to receive routine gynecologic care. Women with HIV have gynecologic problems similar to those of all women; however, they may be more vulnerable to certain conditions such as human papillomavirus (HPV) infection, which may be related to HIV disease or associated immunosuppression. In addition, pregnancy and family planning pose special challenges in this group.14
Cervical cancer screening
Effective screening and timely treatment of precancerous cervical lesions are key in preventing cervical cancer in women with or without HIV.
Persistent infection with HPV is necessary for the development of precancerous lesions as well as invasive cervical cancer. Most new cases of HPV infection in the general population resolve spontaneously within 2 years. However, in HIV-infected women, HPV infection is more likely to persist and progress to precancerous lesions of the cervix. This association is strongest in women with more compromised immune function as reflected by low CD4 cell counts and high viral loads.14 Women with HIV have higher rates of infection with high-risk HPV strains and of cervical intraepithelial neoplasia compared with their HIV-negative counterparts.14 The incidence of cervical cancer is five to six times higher in HIV-infected women in the United States than in the general population.15
According to guidelines from the Infectious Diseases Society of America,16 the American College of Obstetricians and Gynecologists,10 the CDC,17 and the American Cancer Society,18 all HIV-infected women should undergo cervical Papanicolaou (Pap) screening upon initiation into care, and this test should be repeated at 6 months and then annually if the results are normal. Patients with abnormalities on the Pap test should undergo colposcopy and, possibly, also biopsy. These abnormalities include atypical squamous cells of unknown significance and higher-grade lesions.16
Nearly one-fourth of HIV-positive women do not receive annual Pap smears despite engagement in care.19 This is unacceptable, because half of the cases of cervical cancer diagnosed in the United States are in women who never received appropriate screening, and an additional 10% are in women who have not been screened in the previous 5 years.19
In HIV-infected women who have had a total hysterectomy, whether to continue Pap testing depends on their history before the surgery. Continued vaginal Pap smear screening is recommended after hysterectomy (including removal of the cervix) in HIV-infected women who have a history of cervical intraepithelial neoplasia grades 2 or 3 or invasive cancer.10,17,20
TREATING HIV IN WOMEN: SPECIAL CONSIDERATIONS
Because it is not yet possible to eradicate the HIV virus, the goals of antiretroviral therapy are to reduce HIV-associated morbidity and mortality, to restore and preserve immune function, to suppress viral load, and to prevent sexual and, in women, perinatal transmission of the virus.21
Antiretroviral therapy is recommended for all HIV-infected patients regardless of the CD4 count, although the strength of recommendation is weaker with higher CD4 counts (Table 3).21 The recommendations for starting antiretroviral therapy and the goals of treatment are the same for men and women. Table 4 summarizes the recommendations for adolescents and adults who are new to treatment.21 For women, additional factors that should be taken into account when considering a regimen include pregnancy potential and whether the drugs chosen for the regimen are considered safe in pregnancy.
Since the early years of the HIV epidemic, researchers have debated whether women attain the same benefits from antiretroviral therapy as men. US Food and Drug Administration investigators performed a meta-analysis of the efficacy outcomes in women in studies of antiretroviral drugs published between 2000 and 2008. They included randomized clinical trials reporting at least 48-week efficacy outcomes, with viral suppression defined as HIV RNA less than 50 copies/mL. The combined database included 40 trials of 16 drugs from 7 drug classes with a total of 20,328 HIV-positive participants. Overall, there were no clinically or statistically significant differences between the sexes in 48-week efficacy outcomes or in rates of trial discontinuation due to adverse events, loss to follow-up, or death.22
Antiretroviral therapy may, however, cause different adverse effects in women than in men. For example:
Nevirapine, a nonnucleoside reverse transcriptase inhibitor, has been associated with the development of a rash and potentially life-threatening hepatotoxicity, more commonly in women than in men and at lower CD4 counts in women. This resulted in recommendations21 to avoid starting a nevirapine-containing regimen in women with CD4 counts greater than 250 cells/mm3 and in men with CD4 counts greater than 400 cells/mm3.
Ritonavir has been observed to cause a higher incidence of nausea and vomiting in women and a higher incidence of diarrhea in men. These are thought to be due to differences between men and women in weight and pharmacokinetics.23
PRECONCEPTION COUNSELING FOR HIV-POSITIVE WOMEN
Preconception counseling is an essential component of both primary and preventive care and should be considered the standard of care for all women of reproductive age who have HIV.24 Health care providers who fully understand the impact of HIV infection and associated comorbidities upon a woman’s reproductive health, fertility desires, and family planning needs are better prepared to assist in their patients’ reproductive health decisions.
The first few weeks of pregnancy are the most critical period in fetal development. During this time, a woman should be healthy and avoid any activities or substances that could cause adverse maternal or fetal outcomes. However, most patients present for prenatal care after this critical time period—thus the need for preconception counseling. Both the Infectious Diseases Society of America and the HIV Medicine Association recommend that all HIV-infected women of childbearing age be asked about their pregnancy plans and desires at the start of care and routinely thereafter.16
The goals of preconception care in women with HIV are to prevent unintended pregnancy, optimize maternal health before pregnancy, optimize pregnancy outcomes for mother and fetus, prevent perinatal HIV transmission, and prevent HIV transmission to an HIV-negative partner when trying to conceive.24
Goal 1: Prevent unintended pregnancy
Nearly half of all pregnancies in the United States are unintended.25 Moreover, the Women’s Interagency HIV Study26 showed that women with HIV are underusing effective contraception. In the Medical Monitoring Project, 85% of the women who had been pregnant since being diagnosed with HIV said that at least one pregnancy was unplanned.27
The consequences of unintended and unplanned pregnancies are serious and add significant burden to women, men, and families. Women who do not wish to become pregnant should be advised to use an effective method of contraception.
Contraception
Contraception use varies worldwide. Factors affecting its use include the methods available, patient choice, current health conditions, religious beliefs, perception of method effectiveness, and side effects.24
The Women’s Interagency HIV Study evaluated trends in contraception use from 1998 to 2010. Condoms were the most common form of contraception, and their use changed little over time. Fewer than 15% of women with HIV used no contraception. The use of long-acting reversible contraception, including injectable progestins, implants, and intrauterine devices, which minimize the need for user adherence, increased among HIV-negative women but not among HIV-positive women.28
The World Health Organization states that all available methods are safe for women with HIV except for spermicides with or without a diaphragm, as there is evidence linking the use of spermicides to an increased risk of HIV transmission (Table 5).29
Some antiretroviral drugs may reduce the effectiveness of some contraceptives (Table 6); however, recommendations are based on pharmacokinetic studies, not on outcome studies. Condoms should be recommended not only to protect against pregnancy, but also to protect against sexually transmitted infections.
Goal 2: Optimize maternal health before pregnancy
Maternal health should be optimized before conceiving to reduce the risk of pregnancy-related morbidities and poor birth outcomes. This includes screening for other infections and ensuring that other comorbidities, such as hypertension, diabetes, substance abuse, and mental illness, are well managed with medications that are safe to use in pregnancy (Table 7).
Goal 3: Prevent perinatal HIV transmission
Educating the patient about perinatal transmission is a fundamental component of preconception counseling. Topics that need to be addressed are transmission risk and methods to reduce the risk, including not breastfeeding after delivery.
Goal 4: Prevent HIV transmission to an uninfected partner when trying to conceive
HIV-discordant couples who desire pregnancy should receive appropriate counseling about methods to minimize risk of transmission to the uninfected partner while trying to conceive. There are a number of effective methods and techniques, which are beyond the scope of this review. Key components of all methods are to screen for and treat sexually transmitted infections in both partners and to use effective antiretroviral therapy and attain maximal viral suppression in the HIV-positive partner.
Antiretroviral therapy for the HIV-infected partner significantly reduced the risk of HIV transmission by 96% in the HIV Prevention Trials Network 052 trial.30 Of note: this reduction was the result of both risk-reduction counseling and antiretroviral therapy. This was the first randomized clinical trial to demonstrate that antiretroviral therapy in those with some preserved immune function (CD4 counts 350–500 cells/mm3) in conjunction with risk-reduction counseling can reduce HIV transmission to an uninfected partner.
Vaginal insemination without intercourse is another option for female-positive couples. The man ejaculates into a condom without spermicide, and the contents are introduced with a non-needle syringe or turkey baster. This can be done at home and confers no risk to the uninfected male partner.31 Chances of pregnancy can be maximized by insemination during the most fertile days of the menstrual cycle.
Preexposure prophylaxis combined with timed intercourse. In a study in Switzerland, the infected male partner was given antiretroviral therapy to suppress his viral load to less than 50 copies/mL for at least 6 months, and luteinizing hormone was measured every day in the urine of the noninfected female partner. When the urinary luteinizing hormone level reached a peak, the woman received a dose of tenofovir in the morning, the couple had unprotected intercourse, and the woman took a second dose the next morning. In 53 cases, none of the female partners seroconverted for HIV.32
Health care providers need to document and update the relationship status, partner HIV status, and fertility desires of their HIV patients, both men and women, on a regular basis. Patient education should include awareness of referrals and options to help safely conceive when desired and achieve effective contraception when not.33
WHEN HIV-POSITIVE WOMEN BECOME PREGNANT
Screening for HIV during pregnancy
The CDC recommends prenatal screening for HIV in the first trimester or at entry into prenatal care. A repeat HIV test should be offered in the third trimester for women at risk of acquiring HIV, for women who have signs or symptoms of early HIV infection, in health care settings where prenatal testing yields at least 1 case of HIV infection per 1,000 women screened, and in areas of high HIV incidence. If women present to labor and delivery with unknown HIV status, rapid HIV testing should be done.9
If a woman acquires HIV during pregnancy, the infection may not be detected and may be transmitted to the infant at birth. From 2002 to 2006 in New York State, 3,396 HIV-exposed babies were born. Of these, 9 (22%) of 41 infants born to mothers who acquired HIV during pregnancy became infected, compared with 1.8% of those born to mothers who acquired HIV before pregnancy. Maternal acquisition of HIV during pregnancy was documented in only 1.3% of perinatal HIV exposures, but it was associated with 9 (13.8%) of the 65 perinatal transmission cases.34
Providers should be aware of the signs and symptoms of acute HIV infection and should have a low threshold for repeating HIV testing at any time during pregnancy. It has been estimated that 40% to 90% of patients with acute HIV infection experience fever, lymphadenopathy, pharyngitis, skin rash, myalgia, arthralgia, or other symptoms.35 Providers often do not recognize acute HIV infection, however, because the symptoms are similar to those of other common illnesses. Also, some individuals with the condition have no symptoms.
Antiretroviral therapy during pregnancy
In a landmark study, AIDS Clinical Trial Group 076 demonstrated that zidovudine monotherapy given during pregnancy, labor, and delivery and to the newborn reduced the risk of HIV transmission to the infant by 67%, from 25% to 8%.36 Other studies demonstrated that combination therapy further decreased the risk of HIV transmission to 1% to 2%.37
The US Department of Health and Human Services recommends that all HIV-positive women who are pregnant receive effective combination antiretroviral therapy regardless of CD4 count to minimize the risk of mother-to-child transmission.37
The goals of HIV treatment during pregnancy are to maintain the woman’s health, restore her immune system, suppress viral replication, and decrease the risk of perinatal transmission. The preferred antiretroviral therapy for pregnant women differs from that for nonpregnant women and is based on evolving experience and information about safety, efficacy, and tolerability in pregnancy (Table 8). A woman who presents for prenatal care on a suppressive regimen should continue that regimen as long as she can tolerate it because there is a risk of losing virologic control when switching regimens, and this may increase the risk of perinatal transmission.37
Physiologic changes that occur during pregnancy may alter drug disposition, which could potentially lead to decreased drug exposure. Some of the changes include an increase in total body water, decreased protein binding, induction of hepatic metabolic pathways, and increased clearance of drugs eliminated by the kidneys.38 These changes may be associated with incomplete virologic suppression, virologic failure, or development of drug resistance, so altered doses of some antiretroviral drugs or careful monitoring of viral load should be considered, particularly in the second and third trimester.
Delivery
Women who have a viral load greater than 1,000 copies/mL near the end of pregnancy should undergo a cesarean delivery at 38 weeks and, before surgery, should receive intravenous zidovudine to reduce the risk of perinatal transmission. For women with viral loads below the threshold of 1,000 copies/mL, there is no proven added benefit to cesarean delivery, and in this situation it should be performed only for standard obstetric indications. Antiretroviral regimens should be continued during labor.37
HIV IN OLDER ADULTS
By 2015, approximately 50% of people with HIV will be over age 50.39 Unfortunately, older people and their providers often underestimate their risk of acquiring HIV. Many older people are newly single and may engage in sexual activity with new partners. Also, older people may be reluctant to use condoms as the need for contraception is past.40,41
Baseline HIV RNA levels tend to be higher and CD4 cell counts lower in patients diagnosed with HIV at older ages. These observations support previous ones that older HIV-infected patients may have advanced HIV disease at the time of diagnosis, perhaps in part due to delayed testing.42 Other possible factors are limited income, comorbid illness, polypharmacy, and insufficient data on drug interactions in the elderly.41,42
A prompt diagnosis is important for older patients because HIV may accelerate aging, and aging may speed up HIV progression. Studies have shown that aging is associated with more rapid progression to AIDS, particularly among people who are older than 40 at seroconversion.43 Other studies have reported that older patients have better virologic responses to antiretroviral therapy but have a blunted immune response, more AIDS-defining events, and a higher mortality rate than younger patients.42
- Centers for Disease Control and Prevention (CDC). Immunodeficiency among female sexual partners of males with acquired immune deficiency syndrome (AIDS) - New York. MMWR Morb Mortal Wkly Rep 1983; 31:697–698.
- Centers for Disease Control and Prevention (CDC). Estimated HIV incidence in the United States, 2007–2010. HIV Surveillance Supplemental Report 2012; 17( No. 4). www.cdc.gov/hiv/topics/surveillance/resources/reports/#supplemental. Accessed October 3, 2014.
- Centers for Disease Control and Prevention. HIV in the United States: at a glance. www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed October 3, 2014.
- El-Sadr WM, Mayer KH, Hodder SL. AIDS in America—forgotten but not gone. N Engl J Med 2010; 362:967–970.
- Eshleman SH, Hughes JP, Laeyendecker O, et al. Use of a multifaceted approach to analyze HIV incidence in a cohort study of women in the United States: HIV Prevention Trials Network 064 Study. J Infect Dis 2013; 207:223–231.
- Djokic D, Englund J, Daum R, et al. HIV knowledge and attitudes toward HIV testing of South Side Chicago Housing Authority residents. AIDS Patient Care STDS 2009; 23:23–28.
- Samji H, Cescon A, Hogg RS, et al; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e81355.
- Centers for Disease Control and Prevention (CDC). HIV/AIDS. HIV mortality (through 2010). www.cdc.gov/hiv/library/slideSets/index.html. Accessed October 3, 2014.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55:1–17.
- The American College of Obstetricians and Gynecologists (ACOG). Routine Human Immunodeficiency Virus Screening Committee Opinion Number 596, May 2014. (Replaces Committee Opinion Number 411, August 2008.) www.acog.org/Resources_And_Publications/Committee_Opinions/Committee_on_Gynecologic_Practice/Routine_Human_Immunodeficiency_Virus_Screening. Accessed October 3, 2014.
- US Preventive Services Task Force. Screening for HIV. http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/human-immunodeficiency-virus-hiv-infection-screening. Accessed October 3, 2014.
- Institute of Medicine. HIV screening and access to care health care system capacity for increased HIV testing and provision of care. www.iom.edu/Reports/2011/HIV-Screening-and-Access-to-Care-Health-Care-System-Capacity-for-Increased-HIV-Testing-and-Provision-of-Care.aspx. Accessed October 3, 2014.
- Walensky RP, Freedberg KA, Weinstein MC, Paltiel AD. Cost-effectiveness of HIV testing and treatment in the United States. Clin Infect Dis 2007; 45(suppl 4):S248–S254.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 117: Gynecologic care for women with human immunodeficiency virus. Obstet Gynecol 2010; 116:1492–1509.
- Centers for Disease Control and Prevention (CDC). Invasive cancer incidence—United States, 2009. MMWR Morb Mortal Wkly Rep 2013; 62:113–118.
- Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58:e1–e34.
- Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed October 3, 2014.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin 2012; 62:147–172.
- Oster AM, Sullivan PS, Blair JM. Prevalence of cervical cancer screening of HIV-infected women in the United States. J Acquir Immune Defic Syndr 2009; 51:430–436.
- Paramsothy P, Duerr A, Heilig CM, et al; HIV Epidemiology Research (HER) Study Group. Abnormal vaginal cytology in HIV-infected and at-risk women after hysterectomy. J Acquir Immune Defic Syndr 2004; 35:484–491.
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed October 3, 2014.
- Soon GG, Min M, Struble KA, et al. Meta-analysis of gender differences in efficacy outcomes for HIV-positive subjects in randomized controlled clinical trials of antiretroviral therapy (2000–2008). AIDS Patient Care STDS 2012; 26:444–453.
- Clark RA, Squires KE. Gender-specific considerations in the antiretroviral management of HIV-infected women. Expert Rev Anti Infect Ther 2005; 3:213–227.
- Johnson K, Posner SF, Biermann J, et al; CDC/ATSDR Preconception Care Work Group; Select Panel on Preconception Care. Recommendations to improve preconception health and health care—United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. MMWR Recomm Rep 2006; 55:1–23.
- Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception 2011; 84:478–485.
- Massad LS, Evans CT, Wilson TE, et al. Contraceptive use among US women with HIV. J Womens Health (Larchmt) 2007; 16:657–666.
- Sutton MY, Patel R, Frazier EL. Unplanned pregnancies among HIV-infected women in care-United States. J Acquir Immune Defic Syndr 2014; 65:350–358.
- Sun M, Peipert JF, Zhao Q, et al. Trends in contraceptive use among women with human immunodeficiency virus. Obstet Gynecol 2012; 120:783–790.
- World Health Organization (WHO). Medical eligibility criteria for contraceptive use. 4th ed. http://whqlibdoc.who.int/publications/2010/9789241563888_eng.pdf. Accessed October 3, 2014.
- Cohen MS, Chen YQ, McCauley M, et al; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505.
- Mmeje O, Cohen CR, Cohan D. Evaluating safer conception options for HIV-serodiscordant couples (HIV-infected female/HIV-uninfected male): a closer look at vaginal insemination. Infect Dis Obstet Gynecol 2012; 2012:587–651.
- Vernazza PL, Graf I, Sonnenberg-Schwan U, Geit M, Meurer A. Preexposure prophylaxis and timed intercourse for HIV-discordant couples willing to conceive a child. AIDS 2011; 25:2005–2008.
- Lampe MA, Smith DK, Anderson GJ, Edwards AE, Nesheim SR. Achieving safe conception in HIV-discordant couples: the potential role of oral preexposure prophylaxis (PrEP) in the United States. Am J Obstet Gynecol 2011; 204:488.e1–e8.
- Birkhead GS, Pulver WP, Warren BL, Hackel S, Rodríguez D, Smith L. Acquiring human immunodeficiency virus during pregnancy and mother-to-child transmission in New York: 2002–2006. Obstet Gynecol 2010; 115:1247–1255.
- Yerly S, Hirschel B. Diagnosing acute HIV infection. Expert Rev Anti Infect Ther 2012; 10:31–41.
- Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med 1994; 331:1173–1180.
- Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Accessed October 3, 2014.
- Mirochnick M, Capparelli E. Pharmacokinetics of antiretrovirals in pregnant women. Clin Pharmacokinet 2004; 43:1071–1087.
- Smith GSenate Committee on Aging. HIV over fifty: exploring the new threat. Washington, DC; 2005. http://www.aging.senate.gov/imo/media/doc/5122005.pdf. Accessed October 3, 2014.
- Illa L, Brickman A, Saint-Jean G, et al. Sexual risk behaviors in late middle age and older HIV seropositive adults. AIDS Behav 2008; 12:935–942.
- Luther VP, Wilkin AM. HIV infection in older adults. Clin Geriatr Med 2007; 23:567–583.
- Collaboration of Observational HIV Epidemiological Research Europe (COHERE) Study Group; Sabin CA, Smith CJ, d’Arminio Monforte A, et al. Response to combination antiretroviral therapy: variation by age. AIDS 2008; 22:1463–1473.
- Pezzotti P, Phillips AN, Dorrucci M, et al. Category of exposure to HIV and age in the progression to AIDS: longitudinal study of 1,199 people with known dates of seroconversion. HIV Italian Seroconversion Study Group. BMJ 1996; 313:583–586.
- Centers for Disease Control and Prevention (CDC). Immunodeficiency among female sexual partners of males with acquired immune deficiency syndrome (AIDS) - New York. MMWR Morb Mortal Wkly Rep 1983; 31:697–698.
- Centers for Disease Control and Prevention (CDC). Estimated HIV incidence in the United States, 2007–2010. HIV Surveillance Supplemental Report 2012; 17( No. 4). www.cdc.gov/hiv/topics/surveillance/resources/reports/#supplemental. Accessed October 3, 2014.
- Centers for Disease Control and Prevention. HIV in the United States: at a glance. www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed October 3, 2014.
- El-Sadr WM, Mayer KH, Hodder SL. AIDS in America—forgotten but not gone. N Engl J Med 2010; 362:967–970.
- Eshleman SH, Hughes JP, Laeyendecker O, et al. Use of a multifaceted approach to analyze HIV incidence in a cohort study of women in the United States: HIV Prevention Trials Network 064 Study. J Infect Dis 2013; 207:223–231.
- Djokic D, Englund J, Daum R, et al. HIV knowledge and attitudes toward HIV testing of South Side Chicago Housing Authority residents. AIDS Patient Care STDS 2009; 23:23–28.
- Samji H, Cescon A, Hogg RS, et al; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e81355.
- Centers for Disease Control and Prevention (CDC). HIV/AIDS. HIV mortality (through 2010). www.cdc.gov/hiv/library/slideSets/index.html. Accessed October 3, 2014.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55:1–17.
- The American College of Obstetricians and Gynecologists (ACOG). Routine Human Immunodeficiency Virus Screening Committee Opinion Number 596, May 2014. (Replaces Committee Opinion Number 411, August 2008.) www.acog.org/Resources_And_Publications/Committee_Opinions/Committee_on_Gynecologic_Practice/Routine_Human_Immunodeficiency_Virus_Screening. Accessed October 3, 2014.
- US Preventive Services Task Force. Screening for HIV. http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/human-immunodeficiency-virus-hiv-infection-screening. Accessed October 3, 2014.
- Institute of Medicine. HIV screening and access to care health care system capacity for increased HIV testing and provision of care. www.iom.edu/Reports/2011/HIV-Screening-and-Access-to-Care-Health-Care-System-Capacity-for-Increased-HIV-Testing-and-Provision-of-Care.aspx. Accessed October 3, 2014.
- Walensky RP, Freedberg KA, Weinstein MC, Paltiel AD. Cost-effectiveness of HIV testing and treatment in the United States. Clin Infect Dis 2007; 45(suppl 4):S248–S254.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 117: Gynecologic care for women with human immunodeficiency virus. Obstet Gynecol 2010; 116:1492–1509.
- Centers for Disease Control and Prevention (CDC). Invasive cancer incidence—United States, 2009. MMWR Morb Mortal Wkly Rep 2013; 62:113–118.
- Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58:e1–e34.
- Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed October 3, 2014.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin 2012; 62:147–172.
- Oster AM, Sullivan PS, Blair JM. Prevalence of cervical cancer screening of HIV-infected women in the United States. J Acquir Immune Defic Syndr 2009; 51:430–436.
- Paramsothy P, Duerr A, Heilig CM, et al; HIV Epidemiology Research (HER) Study Group. Abnormal vaginal cytology in HIV-infected and at-risk women after hysterectomy. J Acquir Immune Defic Syndr 2004; 35:484–491.
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed October 3, 2014.
- Soon GG, Min M, Struble KA, et al. Meta-analysis of gender differences in efficacy outcomes for HIV-positive subjects in randomized controlled clinical trials of antiretroviral therapy (2000–2008). AIDS Patient Care STDS 2012; 26:444–453.
- Clark RA, Squires KE. Gender-specific considerations in the antiretroviral management of HIV-infected women. Expert Rev Anti Infect Ther 2005; 3:213–227.
- Johnson K, Posner SF, Biermann J, et al; CDC/ATSDR Preconception Care Work Group; Select Panel on Preconception Care. Recommendations to improve preconception health and health care—United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. MMWR Recomm Rep 2006; 55:1–23.
- Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception 2011; 84:478–485.
- Massad LS, Evans CT, Wilson TE, et al. Contraceptive use among US women with HIV. J Womens Health (Larchmt) 2007; 16:657–666.
- Sutton MY, Patel R, Frazier EL. Unplanned pregnancies among HIV-infected women in care-United States. J Acquir Immune Defic Syndr 2014; 65:350–358.
- Sun M, Peipert JF, Zhao Q, et al. Trends in contraceptive use among women with human immunodeficiency virus. Obstet Gynecol 2012; 120:783–790.
- World Health Organization (WHO). Medical eligibility criteria for contraceptive use. 4th ed. http://whqlibdoc.who.int/publications/2010/9789241563888_eng.pdf. Accessed October 3, 2014.
- Cohen MS, Chen YQ, McCauley M, et al; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505.
- Mmeje O, Cohen CR, Cohan D. Evaluating safer conception options for HIV-serodiscordant couples (HIV-infected female/HIV-uninfected male): a closer look at vaginal insemination. Infect Dis Obstet Gynecol 2012; 2012:587–651.
- Vernazza PL, Graf I, Sonnenberg-Schwan U, Geit M, Meurer A. Preexposure prophylaxis and timed intercourse for HIV-discordant couples willing to conceive a child. AIDS 2011; 25:2005–2008.
- Lampe MA, Smith DK, Anderson GJ, Edwards AE, Nesheim SR. Achieving safe conception in HIV-discordant couples: the potential role of oral preexposure prophylaxis (PrEP) in the United States. Am J Obstet Gynecol 2011; 204:488.e1–e8.
- Birkhead GS, Pulver WP, Warren BL, Hackel S, Rodríguez D, Smith L. Acquiring human immunodeficiency virus during pregnancy and mother-to-child transmission in New York: 2002–2006. Obstet Gynecol 2010; 115:1247–1255.
- Yerly S, Hirschel B. Diagnosing acute HIV infection. Expert Rev Anti Infect Ther 2012; 10:31–41.
- Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med 1994; 331:1173–1180.
- Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Accessed October 3, 2014.
- Mirochnick M, Capparelli E. Pharmacokinetics of antiretrovirals in pregnant women. Clin Pharmacokinet 2004; 43:1071–1087.
- Smith GSenate Committee on Aging. HIV over fifty: exploring the new threat. Washington, DC; 2005. http://www.aging.senate.gov/imo/media/doc/5122005.pdf. Accessed October 3, 2014.
- Illa L, Brickman A, Saint-Jean G, et al. Sexual risk behaviors in late middle age and older HIV seropositive adults. AIDS Behav 2008; 12:935–942.
- Luther VP, Wilkin AM. HIV infection in older adults. Clin Geriatr Med 2007; 23:567–583.
- Collaboration of Observational HIV Epidemiological Research Europe (COHERE) Study Group; Sabin CA, Smith CJ, d’Arminio Monforte A, et al. Response to combination antiretroviral therapy: variation by age. AIDS 2008; 22:1463–1473.
- Pezzotti P, Phillips AN, Dorrucci M, et al. Category of exposure to HIV and age in the progression to AIDS: longitudinal study of 1,199 people with known dates of seroconversion. HIV Italian Seroconversion Study Group. BMJ 1996; 313:583–586.
KEY POINTS
- The number of women living with HIV has increased over the past 30 years, and African American women bear a disproportionate burden of disease.
- Women of all ages are at risk of acquiring HIV; therefore, HIV testing should be part of routine care.
- Preconception counseling is an essential component of both primary and preventive care and should be the standard of care for all women of reproductive age with HIV.
- Women with HIV have the same gynecologic problems as all women but may be more vulnerable to certain conditions, such as human papillomavirus infection.
Acute respiratory distress syndrome: Implications of recent studies
Continued progress in understanding the pathophysiology of acute respiratory distress syndrome (ARDS) is translating into changes in the way we diagnose and manage it. Over the past 20 years, low tidal volume,1 positive end-expiratory pressure (PEEP),2 and fluid restriction3 have become the standard of care. A multidisciplinary approach, including targeted use of sedatives, early mobilization, and protocols for weaning from the ventilator, has also brought about substantial changes in ARDS management and its outcomes.4–6
In this article, we review the most relevant articles about ARDS in the last 5 years. We include the new definition of ARDS and studies of ventilatory and nonventilatory therapies that have implications in managing patients with ARDS.
A STANDARDIZED APPROACH
ARDS is characterized by damage to the alveolar architecture, severe hypoxemia, and bilateral parenchymal opacities.
The working definition of ARDS developed in 1994 by the American-European Consensus Conference (AECC) was the basis for enrollment in most of the landmark trials and observational studies over the past 20 years.7,8 However, it was limited in its reliability and validity.
An updated definition
In 2011, the ARDS Definition Task Force, using a novel consensus process, updated the ARDS definition,9 focusing on its feasibility, reliability, and validity in predicting response to therapies and outcomes in ARDS. This new “Berlin” definition is not substantially different from the old, but defines the criteria more specifically:
- Bilateral opacities, unexplained by nodules, atelectasis, or effusion, on chest radiography or computed tomography
- New or worsening respiratory symptoms, or a clinical insult associated with ARDS within 7 days of diagnosis
- Objective assessment of cardiac function (eg, with echocardiography) to exclude cardiogenic pulmonary edema
- Hypoxemia, with a partial pressure of arterial oxygen divided by the percentage of inspired oxygen (PaO2/FiO2 ratio) of 300 mm Hg or less despite noninvasive or invasive mechanical ventilation with PEEP or continuous positive airway pressure (CPAP) of at least 5 cm H2O.
In addition, the new definition classifies the severity of disease on the basis of the degree of hypoxemia, ie, the PaO2/FiO2 ratio:
- Mild: PaO2/FiO2 ratio > 200 and ≤ 300 mm Hg
- Moderate: PaO2/FiO2 ratio > 100 and ≤ 200 mm Hg
- Severe: PaO2/FiO2 ratio ≤ 100 mm Hg.
The term “acute lung injury” has been eliminated, as has the previous criterion of a pulmonary artery wedge pressure of 18 mm Hg or less.
The panel also evaluated four ancillary variables for predicting outcomes in severe ARDS:
- Compliance of the respiratory system less than or equal to 40 mL/cm H2O
- Radiographic severity (involvement of three or four quadrants on chest radiography)
- PEEP of 10 cm H2O or greater
- Corrected expired volume 10 L/min or greater.
The task force evaluated the reliability and validity of this definition in a meta-analysis of 4,400 patients previously enrolled in randomized controlled trials or observational studies.
Findings. The Berlin definition predicted the risk of death better than the AECC definition. The mortality rate increased with the severity of ARDS, from 27% with mild disease to 32% with moderate disease to 45% with severe disease. The four ancillary variables did not contribute to the predictive validity of severe ARDS for mortality and were removed from the definition.
Thille et al10 retrospectively reviewed autopsy findings from 712 patients and found that the new definition identified a homogeneous group who had severe ARDS.10
Conclusions. The new definition may overcome some of the limitations of the old one, but it needs to be validated in clinical practice, especially its ability to predict death.
VENTILATORY SUPPORT
Prompt recognition, lung-protective ventilation, and a conservative fluid strategy remain the cornerstones of ARDS management. However, other strategies are being tested.
Prone-position ventilation in severe ARDS: The right therapy in a specific population
Prone-position ventilation was first described almost 30 years ago, but it has been used inconsistently in clinical practice.
Physiologic and observational studies indicated that prone positioning might improve survival in patients with ARDS, but several randomized trials failed to demonstrate any positive effect on outcomes.11,12 Some trials also reported a higher rate of complications with this intervention.13 However, meta-analyses suggested that prone-position ventilation might have a beneficial effect in patients with severe ARDS (defined as a PaO2/FiO2 ratio ≤ 100 mm Hg).14
In view of these findings, investigators conducted a trial of prone-position ventilation exclusively in patients with severe ARDS.
The PROSEVA study
The Proning Severe ARDS Patients (PROSEVA) study was a randomized controlled trial designed to determine whether prone-position ventilation, applied early, would improve outcomes in patients with severe ARDS.15
In PROSEVA, 466 patients with severe ARDS (defined as a PaO2/FiO2 ratio < 150 mm Hg, FiO2 ≥ 60%, and PEEP ≥ 5 cm H2O) underwent either at least 16 hours of prone positioning or were left in the supine position after 12 to 24 hours of initial conventional mechanical ventilation. The patients were recruited from centers in France and Spain where prone-position ventilation had been used in daily practice for more than 5 years.
The primary outcome studied was the rate of death at 28 days. The secondary end points were the death rate at day 90, rates of successful extubation, the length of stay in the intensive care unit, and complications.
Findings. At study entry, the patients in the supine group were sicker, more of them required a vasopressor, and fewer of them were receiving neuromuscular blocking agents than those in the prone group. These baseline differences may have influenced the outcomes; the unadjusted 28-day mortality rate was 16.0% in the prone group compared with 32.8% in the supine group (P < .001). However, the hazard ratio for death with prone positioning was 0.39 (95% confidence interval [CI] 0.25–0.63) even after adjusting for severity and the use of vasopressors and neuromuscular blocking agents. Prone-position ventilation was not associated with a higher incidence of complications, and the rate of successful extubation was higher.
Conclusions. In patients with severe ARDS, early use of prolonged prone positioning significantly decreased the 28-day and 90-day mortality rates. This trial has made prone positioning one of the strategies in managing patients with early severe ARDS. To minimize complications such as pressure ulcers and line or tube dislodgement, personnel caring for these patients must follow a protocol and undergo specific training.
These results were corroborated by a meta-analysis by Beitler et al16 that found a significant decrease in mortality rate with prone-position ventilation even in older studies when lung-protective ventilation strategies were separated from high-tidal-volume ventilation.
High-frequency oscillatory ventilation: No benefit in two trials
Observational data and experimental studies suggested that high-frequency oscillatory ventilation (HFOV) is superior to conventional mechanical ventilation in ARDS patients.17,18 However, outdated and cumbersome equipment, lack of protocols, and a lack of high-quality evidence led to limited and inconsistent use of HFOV, mainly as a rescue therapy in ARDS.19
Over the last few years, HFOV has been gaining acceptance, especially earlier in the course of ARDS.20 After preliminary clinical trials reported promising results, two trials conducted in Canada and the United Kingdom compared HFOV vs conventional mechanical ventilation in patients with ARDS.
The OSCAR study
The Oscillation in ARDS (OSCAR) study21 was a “pragmatic” trial22 (ie, it had minimal exclusion criteria) of the safety and effectiveness of HFOV as a primary ventilatory strategy for ARDS. It included 795 patients randomized to receive conventional ventilation (n = 397) or HFOV (n = 398). Research centers followed detailed algorithms for HFOV management and adopted their usual practice for conventional ventilation. Medical care was given according to the clinician’s judgment.
The primary outcome studied was survival at 30 days. The secondary outcomes were all-cause mortality in the intensive care unit and the hospital, duration of mechanical ventilation, and use of antimicrobial, sedative, vasoactive, and neuromuscular-blocking drugs.
Findings. The patient baseline characteristics were similar in both groups.
There was no significant difference in intensive care unit mortality rates, hospital mortality rates, or mortality rates at 30 days (41.7% in the HFOV group vs 41.1% in the conventional ventilation group; P = .85, 95% CI 6.1–7.5) even after adjustments for center or severity of illness.
The duration of mechanical ventilation was similar in both groups (14.9 ± 13.3 days in the HFOV group vs 14.1 ± 13.4 days in the conventional ventilation group, P = .41). However, sedatives and neuromuscular-blocking drugs were used more often and longer in the HFOV group than in the conventional ventilation group. There was no difference in the use of vasoactive or antimicrobial medications.
Conclusions. This multicenter randomized control trial did not demonstrate any benefit from using HFOV for routine management of ARDS. Its pragmatic design made it less likely to reach a firm conclusion,22 but it at least made a case against routinely using HFOV in patients with ARDS.
The OSCILLATE study
The Oscillation for Acute Respiratory Distress Syndrome Treated Early (OSCILLATE) study23 assessed the safety and efficacy of HFOV as a treatment for early-onset moderate-to-severe ARDS.
The inclusion criteria were similar to those in the OSCAR trial except that pulmonary symptoms had to be present less than 2 weeks and ARDS assessment was done under standard ventilator settings. As this was an efficacy trial, it had more exclusion criteria than the OSCAR trial. A total of 548 patients were randomized to receive conventional ventilation (n = 273) or HFOV (n = 275). The baseline characteristics were similar between groups.
Conventional ventilation was given according to a protocol used in an earlier trial2 and included recruitment maneuvers. HFOV was given in centers that had experience in this treatment, and there were protocols for ventilation management, hemodynamic optimization, and weaning. All other care was left to the clinician’s choice.
The primary outcome studied was in-hospital mortality. The investigators also evaluated whether there were interactions between the treatment and baseline severity of lung injury and center experience with HFOV.
Findings. The trial was stopped after an interim analysis found that HFOV might be harmful, although the statistical threshold for stopping was not reached. The in-hospital mortality rate was 47% in the HFOV group and 35% in the control group (relative risk of death with HFOV 1.33, 95% CI 1.09–1.64, P = .005). HFOV was worse than conventional ventilation regardless of the severity of disease or center experience. The HFOV group had higher mean airway pressures but similar FiO2 compared with the conventional ventilation group.
The HFOV group received significantly more vasopressors, sedatives, and neuromuscular blockers. This group’s fluid balance was higher as well, but not significantly so. Refractory hypoxemia (defined as PaO2 < 60 mm Hg for 1 hour with an FiO2 of 1.0 and neuromuscular blockade) was more frequent in the conventional ventilation group, but the number of deaths in the subgroup with refractory hypoxemia was similar with either treatment.
Conclusions. This multicenter randomized controlled trial demonstrated that HFOV was harmful when used routinely to manage ARDS. The trial’s protocol was based on the results of a pilot study carried out by the same investigators, which provided the best evidence available regarding the safety of HFOV at that time.
The results of the OSCAR and OSCILLATE trials have quelled enthusiasm for early, routine use of HFOV in ARDS. Although there are concerns that the protocol (ie, the way HFOV was implemented) rather than HFOV itself may have led to worse outcomes, there is no signal to support its routine use. We need further studies to define if it remains a viable rescue therapy.
Extracorporeal membrane oxygenation: Is it a viable option in severe ARDS?
Extracorporeal membrane oxygenation (ECMO) uses cardiopulmonary bypass technology to provide gas exchange. In patients with severe hypoxemia, ECMO can ensure adequate oxygenation and ventilation while ensuring the optimization of lung-protective ventilation. But ECMO was never as successful in adults with ARDS as it was in children and neonates.24
The first two trials of ECMO in ARDS24,25 reported equal or worse survival rates compared with conventional ventilation, and the overall mortality rate in these studies was staggeringly high. However, these studies were carried out before the era of lung-protective ventilation and at a time when ECMO technology was relatively primitive.
With new technology such as venovenous circuits and smaller cannulas, ECMO has gained more acceptance. It was used in patients with severe or refractory hypoxemia associated with ARDS during the H1N1 pandemic.26,27
The CESAR trial
The Conventional Ventilatory Support Versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure (CESAR) trial28 assessed the safety, clinical efficacy, and cost-effectiveness of ECMO in managing severe ARDS. It compared best standard practice vs a protocol that included ECMO. The trial was conducted from 2001 to 2006.
Patients with severe ARDS, as defined by a Murray score29 greater than 3 or uncompensated hypercapnea, were prospectively randomized and recruited from an ECMO center and 148 tertiary intensive care units and referral hospitals in England. This was a pragmatic trial, with minimal exclusion criteria (essentially, mechanical ventilation with high pressures and high FiO2 for more than 7 days, intracranial bleeding, or contraindication to heparinization).
A total of 180 patients were randomized in a one-to-one ratio to receive ECMO or conventional management. The ventilator management in the conventional treatment group was not done according to a protocol but in general was low-volume and low-pressure. All patients randomized to ECMO were transferred to the ECMO center and treated according to a standardized ventilation protocol. After 12 hours, if predefined goals were not reached, venovenous ECMO was started. Patients assigned to conventional management could not cross over to ECMO.
The primary outcomes were death or severe disability at 6 months after randomization, and cost-effectiveness. The secondary outcomes were hospital resource use (eg, rescue techniques, length of stay, duration of ECMO) and health status after 6 months.
Findings. The groups were similar at baseline. Sixty-eight (75%) of the 90 patients randomized to receive ECMO actually received it. Of the 22 patients who did not receive ECMO, 16 (18% of the 90) improved on conventional therapy, 5 (6%) died during or before transfer, and 1 could not receive heparin.
Two patients had severe complications in the ECMO group: one had an arterial puncture, and one had an oxygen delivery failure during transport. In each case, these events contributed to the death of the patient.
More patients in the ECMO group received lung-protective ventilation, 84 (93%) vs 63 (70%).
The primary outcome, ie, death or severe disability at 6 months, occurred in 33 (37%) of the 90 patients in the ECMO group and in 46 (53%) of the patients in the conventional management group (relative risk 0.69, 95% CI 0.05–0.97, P = .03). More patients in the ECMO group survived, but the difference was not statistically significant (relative risk of death 0.73, 95% CI 0.52–1.03, P = .07). The most common cause of death in the ECMO group was multiorgan failure (42%), whereas in the conventional management group, the most common cause of death was respiratory failure (60%).
Length of stay in the hospital and in the critical care unit and health care costs were double for patients in the ECMO group. There was no difference in quality-of-life markers at 6 months in the survivors.
Conclusions. This pragmatic trial demonstrated that a protocol that includes ECMO could improve survival rates in ARDS.
Of note, the ECMO group got care in regional centers that used protocols. Therefore, in interpreting the results of this trial, we have to consider that being in a center with protocol-specified care for ARDS could drive some of the difference in mortality rates.
Regardless, this trial demonstrated that ECMO is feasible and led to better outcomes than expected. The findings were encouraging, and spurred the use of ECMO in severe ARDS during the 2009 H1N1 pandemic. Two propensity-matched studies and a number of case series reported a survival benefit associated with the use of ECMO in patients with severe ARDS.27,30
A recent meta-analysis also reported that ECMO might lower the mortality rate in ARDS; however, the patients in the H1N1 pandemic were younger and usually had isolated respiratory failure.31
The success of ECMO has opened new possibilities in the management of ARDS. As the technology improves and our experience increases, ECMO will likely gain more acceptance as a treatment for severe ARDS.
Airway pressure release ventilation
The use of airway pressure release ventilation and other ventilator modalities in ARDS is not supported by current evidence, though results of clinical trials may influence our practice in the future.
PHARMACOTHERAPY IN ARDS
The pathogenesis of ARDS includes damage to the alveolar-capillary membrane, with leakage of protein-rich edema fluid into alveoli. This damage is propagated by a complex inflammatory response including but not limited to neutrophil activation, free-radical formation, dysregulation of the coagulation system, and extensive release of inflammatory mediators.32,33 As a consequence, there are multiple potential targets for pharmacologic therapy in ARDS.
A variety of drugs, including corticosteroids, anti-inflammatory agents, immune-modulating agents, pulmonary vasodilators, antioxidants, and surfactants, have been studied in patients with ARDS.34 But effective pharmacotherapy for ARDS remains extremely limited.
Neuromuscular blockade in early severe ARDS
Mechanical ventilation can result in injurious stretching of the lung parenchyma, either from alveolar overdistention (volutrauma) or from continual recruitment and derecruitment of unstable lung units during the ventilator cycle (atelectrauma).35 Ventilator-induced lung injury can be exacerbated by asynchronous breathing.
In theory, neuromuscular blockers could minimize patient-ventilator asynchrony and provide much better control of tidal volume and pressure in patients with ARDS. This may result in less volutrauma and atelectrauma associated with asynchronous breathing. Data also suggest that cisatracurium (Nimbex), a neuromuscular blocking agent, may have a direct effect on the amount of inflammation in lungs with ARDS.36
The ACURASYS study
The ARDS et Curarisation Systématique (ACURASYS) study37 was a randomized trial in 340 patients undergoing mechanical ventilation for severe ARDS to evaluate the impact of neuromuscular blockade within the first 48 hours in this population.
The primary outcome was the mortality rate before hospital discharge or within 90 days of study entry. Secondary outcomes included the 28-day mortality rate, the rate of intensive care unit-acquired paresis, and the number of ventilator-free days. To be included, patients had to have been mechanically ventilated for less than 48 hours and to meet the AECC criteria for severe ARDS, with a PaO2/FiO2 ratio less than 150 mm Hg.
The intervention group received a continuous infusion of cisatracurium for 48 hours, while the control patients received placebo. Muscle strength was evaluated by clinical scoring of strength in different muscle groups.
Findings. The study groups were similar at baseline.
The crude 90-day mortality rate was lower in the cisatracurium group (31.6% vs 40.7%, P = .08). Regression analysis showed an improved 90-day survival rate with the use of this neuromuscular blocker after adjustment for severity of illness and the severity of ARDS (based on degree of hypoxemia and plateau pressures) (hazard ratio for death at 90 days 0.68; 95% CI 0.48–0.98; P = .04). The rate of paresis acquired in the intensive care unit did not differ significantly between the two groups.
Conclusion. In patients with severe ARDS, giving a neuromuscular blocking agent early improved the survival rate and increased the time off the ventilator without increasing muscle weakness.
These data are in line with similar findings from two other studies published by the same group.38,39 A meta-analysis of 432 patients showed that the use of neuromuscular blockade in early severe ARDS is associated with a statistically significant effect on early mortality (relative risk 0.66, 95% CI 0.50–0.87).40 The pooled analysis of these trials did not show any statistically significant critical-illness polyneuropathy.
These results need to be interpreted carefully, as we have inadequate data to see if they generalize to different intensive care units, and the evaluation and categorization of critical-illness polyneuropathy remains to be defined.
Cisatracurium is a promising treatment for moderate to severe ARDS and merits investigation in a large confirmatory randomized controlled trial.
Other pharmacologic agents
A number of other drugs have been studied in ARDS patients, including both inhaled and intravenous beta agonists,41,42 statins,43 and nutritional supplements.44 But as with other drugs previously studied in ARDS such as corticosteroids, N-acetylcysteine, and surfactant,34 these agents showed no effect on outcomes. In fact, a recent trial of intravenous salbutamol in ARDS patients was stopped after an interim analysis because of a higher incidence of arrhythmias and lactic acidosis with this agent.42
These findings reaffirm that pharmacologic therapy needs to be carefully considered, and potential harms associated with these therapies need to be addressed before they are introduced in the care of critically ill patients.
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. Acute Respiratory Distress Syndrome Network. N Engl J Med 2000; 342:1301–1308.
- Meade MO, Cook DJ, Guyatt GH, et al; Lung Open Ventilation Study Investigators. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008; 299:637–645.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575.
- Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008; 371:126–134.
- Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009; 373:1874–1882.
- Ely EW, Baker AM, Dunagan DP, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med 1996; 335:1864–1869.
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994; 149:818–824.
- Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med 2012; 38:1573–1582.
- ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307:2526–2533.
- Thille AW, Esteban A, Fernández-Segoviano P, et al. Comparison of the Berlin definition for acute respiratory distress syndrome with autopsy. Am J Respir Crit Care Med 2013; 187:761–767.
- Gattinoni L, Tognoni G, Pesenti A, et al; Prone-Supine Study Group. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med 2001; 345:568–573.
- Taccone P, Pesenti A, Latini R, et al; Prone-Supine II Study Group. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA 2009; 302:1977–1984.
- Mancebo J, Fernández R, Blanch L, et al. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med 2006; 173:1233–1239.
- Sud S, Friedrich JO, Taccone P, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med 2010; 36:585–599.
- Guérin C, Reignier J, Richard JC, et al; PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013; 368:2159–2168.
- Beitler JR, Shaefi S, Montesi SB, et al. Prone positioning reduces mortality from acute respiratory distress syndrome in the low tidal volume era: a meta-analysis. Intensive Care Med 2014; 40:332–341.
- Chan KP, Stewart TE, Mehta S. High-frequency oscillatory ventilation for adult patients with ARDS. Chest 2007; 131:1907–1916.
- Fessler HE, Hager DN, Brower RG. Feasibility of very high-frequency ventilation in adults with acute respiratory distress syndrome. Crit Care Med 2008; 36:1043–1048.
- Mehta S, Granton J, MacDonald RJ, et al. High-frequency oscillatory ventilation in adults: the Toronto experience. Chest 2004; 126:518–527.
- Ferguson ND, Chiche JD, Kacmarek RM, et al. Combining high-frequency oscillatory ventilation and recruitment maneuvers in adults with early acute respiratory distress syndrome: the Treatment with Oscillation and an Open Lung Strategy (TOOLS) Trial pilot study. Crit Care Med 2005; 33:479–486.
- Young D, Lamb SE, Shah S, et al; OSCAR Study Group. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med 2013; 368:806–813.
- Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol 2009; 62:464–475.
- Ferguson ND, Cook DJ, Guyatt GH, et al; OSCILLATE Trial Investigators; Canadian Critical Care Trials Group. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med 2013; 368:795–805.
- Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 1994; 149:295–305.
- Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 1979; 242:2193–2196.
- Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators; Davies A, Jones D, Bailey M, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA 2009; 302:1888–1895.
- Pham T, Combes A, Rozé H, et al; REVA Research Network. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med 2013; 187:276–285.
- Peek GJ, Mugford M, Tiruvoipati R, et al; CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009; 374:1351–1363.
- Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 1988; 138:720–723.
- Noah MA, Peek GJ, Finney SJ, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA 2011; 306:1659–1668.
- Zampieri FG, Mendes PV, Ranzani OT, et al. Extracorporeal membrane oxygenation for severe respiratory failure in adult patients: a systematic review and meta-analysis of current evidence. J Crit Care 2013; 28:998–1005.
- Raghavendran K, Pryhuber GS, Chess PR, Davidson BA, Knight PR, Notter RH. Pharmacotherapy of acute lung injury and acute respiratory distress syndrome. Curr Med Chem 2008; 15:1911–1924.
- Adhikari N, Burns KE, Meade MO. Pharmacologic treatments for acute respiratory distress syndrome and acute lung injury: systematic review and meta-analysis. Treat Respir Med 2004; 3:307–328.
- Adhikari N, Burns KE, Meade MO. Pharmacologic therapies for adults with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev 2004; 4:CD004477.
- Terragni PP, Rosboch GL, Lisi A, Viale AG, Ranieri VM. How respiratory system mechanics may help in minimising ventilator-induced lung injury in ARDS patients. Eur Respir J Suppl 2003; 42:15s–21s.
- Forel JM, Roch A, Papazian L. Paralytics in critical care: not always the bad guy. Curr Opin Crit Care 2009; 15:59–66.
- Papazian L, Forel JM, Gacouin A, et al; ACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010; 363:1107–1116.
- Gainnier M, Roch A, Forel JM, et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med 2004; 32:113–19.
- Forel JM, Roch A, Marin V, et al. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med 2006; 34:2749–2757.
- Alhazzani W, Alshahrani M, Jaeschke R, et al. Neuromuscular blocking agents in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. Crit Care 2013; 17:R43.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Matthay MA, Brower RG, Carson S, et al. Randomized, placebo-controlled clinical trial of an aerosolized beta-2-agonist for treatment of acute lung injury. Am J Respir Crit Care Med 2011; 184:561–568.
- Gao Smith F, Perkins GD, Gates S, et al; BALTI-2 study investigators. Effect of intravenous beta-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet 2012; 379:229–235.
- Craig TR, Duffy MJ, Shyamsundar M, et al. A randomized clinical trial of hydroxymethylglutaryl-coenzyme a reductase inhibition for acute lung injury (The HARP Study). Am J Respir Crit Care Med 2011; 183:620–626.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Rice TW, Wheeler AP, Thompson BT, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA 2012; 307:795–803.
Continued progress in understanding the pathophysiology of acute respiratory distress syndrome (ARDS) is translating into changes in the way we diagnose and manage it. Over the past 20 years, low tidal volume,1 positive end-expiratory pressure (PEEP),2 and fluid restriction3 have become the standard of care. A multidisciplinary approach, including targeted use of sedatives, early mobilization, and protocols for weaning from the ventilator, has also brought about substantial changes in ARDS management and its outcomes.4–6
In this article, we review the most relevant articles about ARDS in the last 5 years. We include the new definition of ARDS and studies of ventilatory and nonventilatory therapies that have implications in managing patients with ARDS.
A STANDARDIZED APPROACH
ARDS is characterized by damage to the alveolar architecture, severe hypoxemia, and bilateral parenchymal opacities.
The working definition of ARDS developed in 1994 by the American-European Consensus Conference (AECC) was the basis for enrollment in most of the landmark trials and observational studies over the past 20 years.7,8 However, it was limited in its reliability and validity.
An updated definition
In 2011, the ARDS Definition Task Force, using a novel consensus process, updated the ARDS definition,9 focusing on its feasibility, reliability, and validity in predicting response to therapies and outcomes in ARDS. This new “Berlin” definition is not substantially different from the old, but defines the criteria more specifically:
- Bilateral opacities, unexplained by nodules, atelectasis, or effusion, on chest radiography or computed tomography
- New or worsening respiratory symptoms, or a clinical insult associated with ARDS within 7 days of diagnosis
- Objective assessment of cardiac function (eg, with echocardiography) to exclude cardiogenic pulmonary edema
- Hypoxemia, with a partial pressure of arterial oxygen divided by the percentage of inspired oxygen (PaO2/FiO2 ratio) of 300 mm Hg or less despite noninvasive or invasive mechanical ventilation with PEEP or continuous positive airway pressure (CPAP) of at least 5 cm H2O.
In addition, the new definition classifies the severity of disease on the basis of the degree of hypoxemia, ie, the PaO2/FiO2 ratio:
- Mild: PaO2/FiO2 ratio > 200 and ≤ 300 mm Hg
- Moderate: PaO2/FiO2 ratio > 100 and ≤ 200 mm Hg
- Severe: PaO2/FiO2 ratio ≤ 100 mm Hg.
The term “acute lung injury” has been eliminated, as has the previous criterion of a pulmonary artery wedge pressure of 18 mm Hg or less.
The panel also evaluated four ancillary variables for predicting outcomes in severe ARDS:
- Compliance of the respiratory system less than or equal to 40 mL/cm H2O
- Radiographic severity (involvement of three or four quadrants on chest radiography)
- PEEP of 10 cm H2O or greater
- Corrected expired volume 10 L/min or greater.
The task force evaluated the reliability and validity of this definition in a meta-analysis of 4,400 patients previously enrolled in randomized controlled trials or observational studies.
Findings. The Berlin definition predicted the risk of death better than the AECC definition. The mortality rate increased with the severity of ARDS, from 27% with mild disease to 32% with moderate disease to 45% with severe disease. The four ancillary variables did not contribute to the predictive validity of severe ARDS for mortality and were removed from the definition.
Thille et al10 retrospectively reviewed autopsy findings from 712 patients and found that the new definition identified a homogeneous group who had severe ARDS.10
Conclusions. The new definition may overcome some of the limitations of the old one, but it needs to be validated in clinical practice, especially its ability to predict death.
VENTILATORY SUPPORT
Prompt recognition, lung-protective ventilation, and a conservative fluid strategy remain the cornerstones of ARDS management. However, other strategies are being tested.
Prone-position ventilation in severe ARDS: The right therapy in a specific population
Prone-position ventilation was first described almost 30 years ago, but it has been used inconsistently in clinical practice.
Physiologic and observational studies indicated that prone positioning might improve survival in patients with ARDS, but several randomized trials failed to demonstrate any positive effect on outcomes.11,12 Some trials also reported a higher rate of complications with this intervention.13 However, meta-analyses suggested that prone-position ventilation might have a beneficial effect in patients with severe ARDS (defined as a PaO2/FiO2 ratio ≤ 100 mm Hg).14
In view of these findings, investigators conducted a trial of prone-position ventilation exclusively in patients with severe ARDS.
The PROSEVA study
The Proning Severe ARDS Patients (PROSEVA) study was a randomized controlled trial designed to determine whether prone-position ventilation, applied early, would improve outcomes in patients with severe ARDS.15
In PROSEVA, 466 patients with severe ARDS (defined as a PaO2/FiO2 ratio < 150 mm Hg, FiO2 ≥ 60%, and PEEP ≥ 5 cm H2O) underwent either at least 16 hours of prone positioning or were left in the supine position after 12 to 24 hours of initial conventional mechanical ventilation. The patients were recruited from centers in France and Spain where prone-position ventilation had been used in daily practice for more than 5 years.
The primary outcome studied was the rate of death at 28 days. The secondary end points were the death rate at day 90, rates of successful extubation, the length of stay in the intensive care unit, and complications.
Findings. At study entry, the patients in the supine group were sicker, more of them required a vasopressor, and fewer of them were receiving neuromuscular blocking agents than those in the prone group. These baseline differences may have influenced the outcomes; the unadjusted 28-day mortality rate was 16.0% in the prone group compared with 32.8% in the supine group (P < .001). However, the hazard ratio for death with prone positioning was 0.39 (95% confidence interval [CI] 0.25–0.63) even after adjusting for severity and the use of vasopressors and neuromuscular blocking agents. Prone-position ventilation was not associated with a higher incidence of complications, and the rate of successful extubation was higher.
Conclusions. In patients with severe ARDS, early use of prolonged prone positioning significantly decreased the 28-day and 90-day mortality rates. This trial has made prone positioning one of the strategies in managing patients with early severe ARDS. To minimize complications such as pressure ulcers and line or tube dislodgement, personnel caring for these patients must follow a protocol and undergo specific training.
These results were corroborated by a meta-analysis by Beitler et al16 that found a significant decrease in mortality rate with prone-position ventilation even in older studies when lung-protective ventilation strategies were separated from high-tidal-volume ventilation.
High-frequency oscillatory ventilation: No benefit in two trials
Observational data and experimental studies suggested that high-frequency oscillatory ventilation (HFOV) is superior to conventional mechanical ventilation in ARDS patients.17,18 However, outdated and cumbersome equipment, lack of protocols, and a lack of high-quality evidence led to limited and inconsistent use of HFOV, mainly as a rescue therapy in ARDS.19
Over the last few years, HFOV has been gaining acceptance, especially earlier in the course of ARDS.20 After preliminary clinical trials reported promising results, two trials conducted in Canada and the United Kingdom compared HFOV vs conventional mechanical ventilation in patients with ARDS.
The OSCAR study
The Oscillation in ARDS (OSCAR) study21 was a “pragmatic” trial22 (ie, it had minimal exclusion criteria) of the safety and effectiveness of HFOV as a primary ventilatory strategy for ARDS. It included 795 patients randomized to receive conventional ventilation (n = 397) or HFOV (n = 398). Research centers followed detailed algorithms for HFOV management and adopted their usual practice for conventional ventilation. Medical care was given according to the clinician’s judgment.
The primary outcome studied was survival at 30 days. The secondary outcomes were all-cause mortality in the intensive care unit and the hospital, duration of mechanical ventilation, and use of antimicrobial, sedative, vasoactive, and neuromuscular-blocking drugs.
Findings. The patient baseline characteristics were similar in both groups.
There was no significant difference in intensive care unit mortality rates, hospital mortality rates, or mortality rates at 30 days (41.7% in the HFOV group vs 41.1% in the conventional ventilation group; P = .85, 95% CI 6.1–7.5) even after adjustments for center or severity of illness.
The duration of mechanical ventilation was similar in both groups (14.9 ± 13.3 days in the HFOV group vs 14.1 ± 13.4 days in the conventional ventilation group, P = .41). However, sedatives and neuromuscular-blocking drugs were used more often and longer in the HFOV group than in the conventional ventilation group. There was no difference in the use of vasoactive or antimicrobial medications.
Conclusions. This multicenter randomized control trial did not demonstrate any benefit from using HFOV for routine management of ARDS. Its pragmatic design made it less likely to reach a firm conclusion,22 but it at least made a case against routinely using HFOV in patients with ARDS.
The OSCILLATE study
The Oscillation for Acute Respiratory Distress Syndrome Treated Early (OSCILLATE) study23 assessed the safety and efficacy of HFOV as a treatment for early-onset moderate-to-severe ARDS.
The inclusion criteria were similar to those in the OSCAR trial except that pulmonary symptoms had to be present less than 2 weeks and ARDS assessment was done under standard ventilator settings. As this was an efficacy trial, it had more exclusion criteria than the OSCAR trial. A total of 548 patients were randomized to receive conventional ventilation (n = 273) or HFOV (n = 275). The baseline characteristics were similar between groups.
Conventional ventilation was given according to a protocol used in an earlier trial2 and included recruitment maneuvers. HFOV was given in centers that had experience in this treatment, and there were protocols for ventilation management, hemodynamic optimization, and weaning. All other care was left to the clinician’s choice.
The primary outcome studied was in-hospital mortality. The investigators also evaluated whether there were interactions between the treatment and baseline severity of lung injury and center experience with HFOV.
Findings. The trial was stopped after an interim analysis found that HFOV might be harmful, although the statistical threshold for stopping was not reached. The in-hospital mortality rate was 47% in the HFOV group and 35% in the control group (relative risk of death with HFOV 1.33, 95% CI 1.09–1.64, P = .005). HFOV was worse than conventional ventilation regardless of the severity of disease or center experience. The HFOV group had higher mean airway pressures but similar FiO2 compared with the conventional ventilation group.
The HFOV group received significantly more vasopressors, sedatives, and neuromuscular blockers. This group’s fluid balance was higher as well, but not significantly so. Refractory hypoxemia (defined as PaO2 < 60 mm Hg for 1 hour with an FiO2 of 1.0 and neuromuscular blockade) was more frequent in the conventional ventilation group, but the number of deaths in the subgroup with refractory hypoxemia was similar with either treatment.
Conclusions. This multicenter randomized controlled trial demonstrated that HFOV was harmful when used routinely to manage ARDS. The trial’s protocol was based on the results of a pilot study carried out by the same investigators, which provided the best evidence available regarding the safety of HFOV at that time.
The results of the OSCAR and OSCILLATE trials have quelled enthusiasm for early, routine use of HFOV in ARDS. Although there are concerns that the protocol (ie, the way HFOV was implemented) rather than HFOV itself may have led to worse outcomes, there is no signal to support its routine use. We need further studies to define if it remains a viable rescue therapy.
Extracorporeal membrane oxygenation: Is it a viable option in severe ARDS?
Extracorporeal membrane oxygenation (ECMO) uses cardiopulmonary bypass technology to provide gas exchange. In patients with severe hypoxemia, ECMO can ensure adequate oxygenation and ventilation while ensuring the optimization of lung-protective ventilation. But ECMO was never as successful in adults with ARDS as it was in children and neonates.24
The first two trials of ECMO in ARDS24,25 reported equal or worse survival rates compared with conventional ventilation, and the overall mortality rate in these studies was staggeringly high. However, these studies were carried out before the era of lung-protective ventilation and at a time when ECMO technology was relatively primitive.
With new technology such as venovenous circuits and smaller cannulas, ECMO has gained more acceptance. It was used in patients with severe or refractory hypoxemia associated with ARDS during the H1N1 pandemic.26,27
The CESAR trial
The Conventional Ventilatory Support Versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure (CESAR) trial28 assessed the safety, clinical efficacy, and cost-effectiveness of ECMO in managing severe ARDS. It compared best standard practice vs a protocol that included ECMO. The trial was conducted from 2001 to 2006.
Patients with severe ARDS, as defined by a Murray score29 greater than 3 or uncompensated hypercapnea, were prospectively randomized and recruited from an ECMO center and 148 tertiary intensive care units and referral hospitals in England. This was a pragmatic trial, with minimal exclusion criteria (essentially, mechanical ventilation with high pressures and high FiO2 for more than 7 days, intracranial bleeding, or contraindication to heparinization).
A total of 180 patients were randomized in a one-to-one ratio to receive ECMO or conventional management. The ventilator management in the conventional treatment group was not done according to a protocol but in general was low-volume and low-pressure. All patients randomized to ECMO were transferred to the ECMO center and treated according to a standardized ventilation protocol. After 12 hours, if predefined goals were not reached, venovenous ECMO was started. Patients assigned to conventional management could not cross over to ECMO.
The primary outcomes were death or severe disability at 6 months after randomization, and cost-effectiveness. The secondary outcomes were hospital resource use (eg, rescue techniques, length of stay, duration of ECMO) and health status after 6 months.
Findings. The groups were similar at baseline. Sixty-eight (75%) of the 90 patients randomized to receive ECMO actually received it. Of the 22 patients who did not receive ECMO, 16 (18% of the 90) improved on conventional therapy, 5 (6%) died during or before transfer, and 1 could not receive heparin.
Two patients had severe complications in the ECMO group: one had an arterial puncture, and one had an oxygen delivery failure during transport. In each case, these events contributed to the death of the patient.
More patients in the ECMO group received lung-protective ventilation, 84 (93%) vs 63 (70%).
The primary outcome, ie, death or severe disability at 6 months, occurred in 33 (37%) of the 90 patients in the ECMO group and in 46 (53%) of the patients in the conventional management group (relative risk 0.69, 95% CI 0.05–0.97, P = .03). More patients in the ECMO group survived, but the difference was not statistically significant (relative risk of death 0.73, 95% CI 0.52–1.03, P = .07). The most common cause of death in the ECMO group was multiorgan failure (42%), whereas in the conventional management group, the most common cause of death was respiratory failure (60%).
Length of stay in the hospital and in the critical care unit and health care costs were double for patients in the ECMO group. There was no difference in quality-of-life markers at 6 months in the survivors.
Conclusions. This pragmatic trial demonstrated that a protocol that includes ECMO could improve survival rates in ARDS.
Of note, the ECMO group got care in regional centers that used protocols. Therefore, in interpreting the results of this trial, we have to consider that being in a center with protocol-specified care for ARDS could drive some of the difference in mortality rates.
Regardless, this trial demonstrated that ECMO is feasible and led to better outcomes than expected. The findings were encouraging, and spurred the use of ECMO in severe ARDS during the 2009 H1N1 pandemic. Two propensity-matched studies and a number of case series reported a survival benefit associated with the use of ECMO in patients with severe ARDS.27,30
A recent meta-analysis also reported that ECMO might lower the mortality rate in ARDS; however, the patients in the H1N1 pandemic were younger and usually had isolated respiratory failure.31
The success of ECMO has opened new possibilities in the management of ARDS. As the technology improves and our experience increases, ECMO will likely gain more acceptance as a treatment for severe ARDS.
Airway pressure release ventilation
The use of airway pressure release ventilation and other ventilator modalities in ARDS is not supported by current evidence, though results of clinical trials may influence our practice in the future.
PHARMACOTHERAPY IN ARDS
The pathogenesis of ARDS includes damage to the alveolar-capillary membrane, with leakage of protein-rich edema fluid into alveoli. This damage is propagated by a complex inflammatory response including but not limited to neutrophil activation, free-radical formation, dysregulation of the coagulation system, and extensive release of inflammatory mediators.32,33 As a consequence, there are multiple potential targets for pharmacologic therapy in ARDS.
A variety of drugs, including corticosteroids, anti-inflammatory agents, immune-modulating agents, pulmonary vasodilators, antioxidants, and surfactants, have been studied in patients with ARDS.34 But effective pharmacotherapy for ARDS remains extremely limited.
Neuromuscular blockade in early severe ARDS
Mechanical ventilation can result in injurious stretching of the lung parenchyma, either from alveolar overdistention (volutrauma) or from continual recruitment and derecruitment of unstable lung units during the ventilator cycle (atelectrauma).35 Ventilator-induced lung injury can be exacerbated by asynchronous breathing.
In theory, neuromuscular blockers could minimize patient-ventilator asynchrony and provide much better control of tidal volume and pressure in patients with ARDS. This may result in less volutrauma and atelectrauma associated with asynchronous breathing. Data also suggest that cisatracurium (Nimbex), a neuromuscular blocking agent, may have a direct effect on the amount of inflammation in lungs with ARDS.36
The ACURASYS study
The ARDS et Curarisation Systématique (ACURASYS) study37 was a randomized trial in 340 patients undergoing mechanical ventilation for severe ARDS to evaluate the impact of neuromuscular blockade within the first 48 hours in this population.
The primary outcome was the mortality rate before hospital discharge or within 90 days of study entry. Secondary outcomes included the 28-day mortality rate, the rate of intensive care unit-acquired paresis, and the number of ventilator-free days. To be included, patients had to have been mechanically ventilated for less than 48 hours and to meet the AECC criteria for severe ARDS, with a PaO2/FiO2 ratio less than 150 mm Hg.
The intervention group received a continuous infusion of cisatracurium for 48 hours, while the control patients received placebo. Muscle strength was evaluated by clinical scoring of strength in different muscle groups.
Findings. The study groups were similar at baseline.
The crude 90-day mortality rate was lower in the cisatracurium group (31.6% vs 40.7%, P = .08). Regression analysis showed an improved 90-day survival rate with the use of this neuromuscular blocker after adjustment for severity of illness and the severity of ARDS (based on degree of hypoxemia and plateau pressures) (hazard ratio for death at 90 days 0.68; 95% CI 0.48–0.98; P = .04). The rate of paresis acquired in the intensive care unit did not differ significantly between the two groups.
Conclusion. In patients with severe ARDS, giving a neuromuscular blocking agent early improved the survival rate and increased the time off the ventilator without increasing muscle weakness.
These data are in line with similar findings from two other studies published by the same group.38,39 A meta-analysis of 432 patients showed that the use of neuromuscular blockade in early severe ARDS is associated with a statistically significant effect on early mortality (relative risk 0.66, 95% CI 0.50–0.87).40 The pooled analysis of these trials did not show any statistically significant critical-illness polyneuropathy.
These results need to be interpreted carefully, as we have inadequate data to see if they generalize to different intensive care units, and the evaluation and categorization of critical-illness polyneuropathy remains to be defined.
Cisatracurium is a promising treatment for moderate to severe ARDS and merits investigation in a large confirmatory randomized controlled trial.
Other pharmacologic agents
A number of other drugs have been studied in ARDS patients, including both inhaled and intravenous beta agonists,41,42 statins,43 and nutritional supplements.44 But as with other drugs previously studied in ARDS such as corticosteroids, N-acetylcysteine, and surfactant,34 these agents showed no effect on outcomes. In fact, a recent trial of intravenous salbutamol in ARDS patients was stopped after an interim analysis because of a higher incidence of arrhythmias and lactic acidosis with this agent.42
These findings reaffirm that pharmacologic therapy needs to be carefully considered, and potential harms associated with these therapies need to be addressed before they are introduced in the care of critically ill patients.
Continued progress in understanding the pathophysiology of acute respiratory distress syndrome (ARDS) is translating into changes in the way we diagnose and manage it. Over the past 20 years, low tidal volume,1 positive end-expiratory pressure (PEEP),2 and fluid restriction3 have become the standard of care. A multidisciplinary approach, including targeted use of sedatives, early mobilization, and protocols for weaning from the ventilator, has also brought about substantial changes in ARDS management and its outcomes.4–6
In this article, we review the most relevant articles about ARDS in the last 5 years. We include the new definition of ARDS and studies of ventilatory and nonventilatory therapies that have implications in managing patients with ARDS.
A STANDARDIZED APPROACH
ARDS is characterized by damage to the alveolar architecture, severe hypoxemia, and bilateral parenchymal opacities.
The working definition of ARDS developed in 1994 by the American-European Consensus Conference (AECC) was the basis for enrollment in most of the landmark trials and observational studies over the past 20 years.7,8 However, it was limited in its reliability and validity.
An updated definition
In 2011, the ARDS Definition Task Force, using a novel consensus process, updated the ARDS definition,9 focusing on its feasibility, reliability, and validity in predicting response to therapies and outcomes in ARDS. This new “Berlin” definition is not substantially different from the old, but defines the criteria more specifically:
- Bilateral opacities, unexplained by nodules, atelectasis, or effusion, on chest radiography or computed tomography
- New or worsening respiratory symptoms, or a clinical insult associated with ARDS within 7 days of diagnosis
- Objective assessment of cardiac function (eg, with echocardiography) to exclude cardiogenic pulmonary edema
- Hypoxemia, with a partial pressure of arterial oxygen divided by the percentage of inspired oxygen (PaO2/FiO2 ratio) of 300 mm Hg or less despite noninvasive or invasive mechanical ventilation with PEEP or continuous positive airway pressure (CPAP) of at least 5 cm H2O.
In addition, the new definition classifies the severity of disease on the basis of the degree of hypoxemia, ie, the PaO2/FiO2 ratio:
- Mild: PaO2/FiO2 ratio > 200 and ≤ 300 mm Hg
- Moderate: PaO2/FiO2 ratio > 100 and ≤ 200 mm Hg
- Severe: PaO2/FiO2 ratio ≤ 100 mm Hg.
The term “acute lung injury” has been eliminated, as has the previous criterion of a pulmonary artery wedge pressure of 18 mm Hg or less.
The panel also evaluated four ancillary variables for predicting outcomes in severe ARDS:
- Compliance of the respiratory system less than or equal to 40 mL/cm H2O
- Radiographic severity (involvement of three or four quadrants on chest radiography)
- PEEP of 10 cm H2O or greater
- Corrected expired volume 10 L/min or greater.
The task force evaluated the reliability and validity of this definition in a meta-analysis of 4,400 patients previously enrolled in randomized controlled trials or observational studies.
Findings. The Berlin definition predicted the risk of death better than the AECC definition. The mortality rate increased with the severity of ARDS, from 27% with mild disease to 32% with moderate disease to 45% with severe disease. The four ancillary variables did not contribute to the predictive validity of severe ARDS for mortality and were removed from the definition.
Thille et al10 retrospectively reviewed autopsy findings from 712 patients and found that the new definition identified a homogeneous group who had severe ARDS.10
Conclusions. The new definition may overcome some of the limitations of the old one, but it needs to be validated in clinical practice, especially its ability to predict death.
VENTILATORY SUPPORT
Prompt recognition, lung-protective ventilation, and a conservative fluid strategy remain the cornerstones of ARDS management. However, other strategies are being tested.
Prone-position ventilation in severe ARDS: The right therapy in a specific population
Prone-position ventilation was first described almost 30 years ago, but it has been used inconsistently in clinical practice.
Physiologic and observational studies indicated that prone positioning might improve survival in patients with ARDS, but several randomized trials failed to demonstrate any positive effect on outcomes.11,12 Some trials also reported a higher rate of complications with this intervention.13 However, meta-analyses suggested that prone-position ventilation might have a beneficial effect in patients with severe ARDS (defined as a PaO2/FiO2 ratio ≤ 100 mm Hg).14
In view of these findings, investigators conducted a trial of prone-position ventilation exclusively in patients with severe ARDS.
The PROSEVA study
The Proning Severe ARDS Patients (PROSEVA) study was a randomized controlled trial designed to determine whether prone-position ventilation, applied early, would improve outcomes in patients with severe ARDS.15
In PROSEVA, 466 patients with severe ARDS (defined as a PaO2/FiO2 ratio < 150 mm Hg, FiO2 ≥ 60%, and PEEP ≥ 5 cm H2O) underwent either at least 16 hours of prone positioning or were left in the supine position after 12 to 24 hours of initial conventional mechanical ventilation. The patients were recruited from centers in France and Spain where prone-position ventilation had been used in daily practice for more than 5 years.
The primary outcome studied was the rate of death at 28 days. The secondary end points were the death rate at day 90, rates of successful extubation, the length of stay in the intensive care unit, and complications.
Findings. At study entry, the patients in the supine group were sicker, more of them required a vasopressor, and fewer of them were receiving neuromuscular blocking agents than those in the prone group. These baseline differences may have influenced the outcomes; the unadjusted 28-day mortality rate was 16.0% in the prone group compared with 32.8% in the supine group (P < .001). However, the hazard ratio for death with prone positioning was 0.39 (95% confidence interval [CI] 0.25–0.63) even after adjusting for severity and the use of vasopressors and neuromuscular blocking agents. Prone-position ventilation was not associated with a higher incidence of complications, and the rate of successful extubation was higher.
Conclusions. In patients with severe ARDS, early use of prolonged prone positioning significantly decreased the 28-day and 90-day mortality rates. This trial has made prone positioning one of the strategies in managing patients with early severe ARDS. To minimize complications such as pressure ulcers and line or tube dislodgement, personnel caring for these patients must follow a protocol and undergo specific training.
These results were corroborated by a meta-analysis by Beitler et al16 that found a significant decrease in mortality rate with prone-position ventilation even in older studies when lung-protective ventilation strategies were separated from high-tidal-volume ventilation.
High-frequency oscillatory ventilation: No benefit in two trials
Observational data and experimental studies suggested that high-frequency oscillatory ventilation (HFOV) is superior to conventional mechanical ventilation in ARDS patients.17,18 However, outdated and cumbersome equipment, lack of protocols, and a lack of high-quality evidence led to limited and inconsistent use of HFOV, mainly as a rescue therapy in ARDS.19
Over the last few years, HFOV has been gaining acceptance, especially earlier in the course of ARDS.20 After preliminary clinical trials reported promising results, two trials conducted in Canada and the United Kingdom compared HFOV vs conventional mechanical ventilation in patients with ARDS.
The OSCAR study
The Oscillation in ARDS (OSCAR) study21 was a “pragmatic” trial22 (ie, it had minimal exclusion criteria) of the safety and effectiveness of HFOV as a primary ventilatory strategy for ARDS. It included 795 patients randomized to receive conventional ventilation (n = 397) or HFOV (n = 398). Research centers followed detailed algorithms for HFOV management and adopted their usual practice for conventional ventilation. Medical care was given according to the clinician’s judgment.
The primary outcome studied was survival at 30 days. The secondary outcomes were all-cause mortality in the intensive care unit and the hospital, duration of mechanical ventilation, and use of antimicrobial, sedative, vasoactive, and neuromuscular-blocking drugs.
Findings. The patient baseline characteristics were similar in both groups.
There was no significant difference in intensive care unit mortality rates, hospital mortality rates, or mortality rates at 30 days (41.7% in the HFOV group vs 41.1% in the conventional ventilation group; P = .85, 95% CI 6.1–7.5) even after adjustments for center or severity of illness.
The duration of mechanical ventilation was similar in both groups (14.9 ± 13.3 days in the HFOV group vs 14.1 ± 13.4 days in the conventional ventilation group, P = .41). However, sedatives and neuromuscular-blocking drugs were used more often and longer in the HFOV group than in the conventional ventilation group. There was no difference in the use of vasoactive or antimicrobial medications.
Conclusions. This multicenter randomized control trial did not demonstrate any benefit from using HFOV for routine management of ARDS. Its pragmatic design made it less likely to reach a firm conclusion,22 but it at least made a case against routinely using HFOV in patients with ARDS.
The OSCILLATE study
The Oscillation for Acute Respiratory Distress Syndrome Treated Early (OSCILLATE) study23 assessed the safety and efficacy of HFOV as a treatment for early-onset moderate-to-severe ARDS.
The inclusion criteria were similar to those in the OSCAR trial except that pulmonary symptoms had to be present less than 2 weeks and ARDS assessment was done under standard ventilator settings. As this was an efficacy trial, it had more exclusion criteria than the OSCAR trial. A total of 548 patients were randomized to receive conventional ventilation (n = 273) or HFOV (n = 275). The baseline characteristics were similar between groups.
Conventional ventilation was given according to a protocol used in an earlier trial2 and included recruitment maneuvers. HFOV was given in centers that had experience in this treatment, and there were protocols for ventilation management, hemodynamic optimization, and weaning. All other care was left to the clinician’s choice.
The primary outcome studied was in-hospital mortality. The investigators also evaluated whether there were interactions between the treatment and baseline severity of lung injury and center experience with HFOV.
Findings. The trial was stopped after an interim analysis found that HFOV might be harmful, although the statistical threshold for stopping was not reached. The in-hospital mortality rate was 47% in the HFOV group and 35% in the control group (relative risk of death with HFOV 1.33, 95% CI 1.09–1.64, P = .005). HFOV was worse than conventional ventilation regardless of the severity of disease or center experience. The HFOV group had higher mean airway pressures but similar FiO2 compared with the conventional ventilation group.
The HFOV group received significantly more vasopressors, sedatives, and neuromuscular blockers. This group’s fluid balance was higher as well, but not significantly so. Refractory hypoxemia (defined as PaO2 < 60 mm Hg for 1 hour with an FiO2 of 1.0 and neuromuscular blockade) was more frequent in the conventional ventilation group, but the number of deaths in the subgroup with refractory hypoxemia was similar with either treatment.
Conclusions. This multicenter randomized controlled trial demonstrated that HFOV was harmful when used routinely to manage ARDS. The trial’s protocol was based on the results of a pilot study carried out by the same investigators, which provided the best evidence available regarding the safety of HFOV at that time.
The results of the OSCAR and OSCILLATE trials have quelled enthusiasm for early, routine use of HFOV in ARDS. Although there are concerns that the protocol (ie, the way HFOV was implemented) rather than HFOV itself may have led to worse outcomes, there is no signal to support its routine use. We need further studies to define if it remains a viable rescue therapy.
Extracorporeal membrane oxygenation: Is it a viable option in severe ARDS?
Extracorporeal membrane oxygenation (ECMO) uses cardiopulmonary bypass technology to provide gas exchange. In patients with severe hypoxemia, ECMO can ensure adequate oxygenation and ventilation while ensuring the optimization of lung-protective ventilation. But ECMO was never as successful in adults with ARDS as it was in children and neonates.24
The first two trials of ECMO in ARDS24,25 reported equal or worse survival rates compared with conventional ventilation, and the overall mortality rate in these studies was staggeringly high. However, these studies were carried out before the era of lung-protective ventilation and at a time when ECMO technology was relatively primitive.
With new technology such as venovenous circuits and smaller cannulas, ECMO has gained more acceptance. It was used in patients with severe or refractory hypoxemia associated with ARDS during the H1N1 pandemic.26,27
The CESAR trial
The Conventional Ventilatory Support Versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure (CESAR) trial28 assessed the safety, clinical efficacy, and cost-effectiveness of ECMO in managing severe ARDS. It compared best standard practice vs a protocol that included ECMO. The trial was conducted from 2001 to 2006.
Patients with severe ARDS, as defined by a Murray score29 greater than 3 or uncompensated hypercapnea, were prospectively randomized and recruited from an ECMO center and 148 tertiary intensive care units and referral hospitals in England. This was a pragmatic trial, with minimal exclusion criteria (essentially, mechanical ventilation with high pressures and high FiO2 for more than 7 days, intracranial bleeding, or contraindication to heparinization).
A total of 180 patients were randomized in a one-to-one ratio to receive ECMO or conventional management. The ventilator management in the conventional treatment group was not done according to a protocol but in general was low-volume and low-pressure. All patients randomized to ECMO were transferred to the ECMO center and treated according to a standardized ventilation protocol. After 12 hours, if predefined goals were not reached, venovenous ECMO was started. Patients assigned to conventional management could not cross over to ECMO.
The primary outcomes were death or severe disability at 6 months after randomization, and cost-effectiveness. The secondary outcomes were hospital resource use (eg, rescue techniques, length of stay, duration of ECMO) and health status after 6 months.
Findings. The groups were similar at baseline. Sixty-eight (75%) of the 90 patients randomized to receive ECMO actually received it. Of the 22 patients who did not receive ECMO, 16 (18% of the 90) improved on conventional therapy, 5 (6%) died during or before transfer, and 1 could not receive heparin.
Two patients had severe complications in the ECMO group: one had an arterial puncture, and one had an oxygen delivery failure during transport. In each case, these events contributed to the death of the patient.
More patients in the ECMO group received lung-protective ventilation, 84 (93%) vs 63 (70%).
The primary outcome, ie, death or severe disability at 6 months, occurred in 33 (37%) of the 90 patients in the ECMO group and in 46 (53%) of the patients in the conventional management group (relative risk 0.69, 95% CI 0.05–0.97, P = .03). More patients in the ECMO group survived, but the difference was not statistically significant (relative risk of death 0.73, 95% CI 0.52–1.03, P = .07). The most common cause of death in the ECMO group was multiorgan failure (42%), whereas in the conventional management group, the most common cause of death was respiratory failure (60%).
Length of stay in the hospital and in the critical care unit and health care costs were double for patients in the ECMO group. There was no difference in quality-of-life markers at 6 months in the survivors.
Conclusions. This pragmatic trial demonstrated that a protocol that includes ECMO could improve survival rates in ARDS.
Of note, the ECMO group got care in regional centers that used protocols. Therefore, in interpreting the results of this trial, we have to consider that being in a center with protocol-specified care for ARDS could drive some of the difference in mortality rates.
Regardless, this trial demonstrated that ECMO is feasible and led to better outcomes than expected. The findings were encouraging, and spurred the use of ECMO in severe ARDS during the 2009 H1N1 pandemic. Two propensity-matched studies and a number of case series reported a survival benefit associated with the use of ECMO in patients with severe ARDS.27,30
A recent meta-analysis also reported that ECMO might lower the mortality rate in ARDS; however, the patients in the H1N1 pandemic were younger and usually had isolated respiratory failure.31
The success of ECMO has opened new possibilities in the management of ARDS. As the technology improves and our experience increases, ECMO will likely gain more acceptance as a treatment for severe ARDS.
Airway pressure release ventilation
The use of airway pressure release ventilation and other ventilator modalities in ARDS is not supported by current evidence, though results of clinical trials may influence our practice in the future.
PHARMACOTHERAPY IN ARDS
The pathogenesis of ARDS includes damage to the alveolar-capillary membrane, with leakage of protein-rich edema fluid into alveoli. This damage is propagated by a complex inflammatory response including but not limited to neutrophil activation, free-radical formation, dysregulation of the coagulation system, and extensive release of inflammatory mediators.32,33 As a consequence, there are multiple potential targets for pharmacologic therapy in ARDS.
A variety of drugs, including corticosteroids, anti-inflammatory agents, immune-modulating agents, pulmonary vasodilators, antioxidants, and surfactants, have been studied in patients with ARDS.34 But effective pharmacotherapy for ARDS remains extremely limited.
Neuromuscular blockade in early severe ARDS
Mechanical ventilation can result in injurious stretching of the lung parenchyma, either from alveolar overdistention (volutrauma) or from continual recruitment and derecruitment of unstable lung units during the ventilator cycle (atelectrauma).35 Ventilator-induced lung injury can be exacerbated by asynchronous breathing.
In theory, neuromuscular blockers could minimize patient-ventilator asynchrony and provide much better control of tidal volume and pressure in patients with ARDS. This may result in less volutrauma and atelectrauma associated with asynchronous breathing. Data also suggest that cisatracurium (Nimbex), a neuromuscular blocking agent, may have a direct effect on the amount of inflammation in lungs with ARDS.36
The ACURASYS study
The ARDS et Curarisation Systématique (ACURASYS) study37 was a randomized trial in 340 patients undergoing mechanical ventilation for severe ARDS to evaluate the impact of neuromuscular blockade within the first 48 hours in this population.
The primary outcome was the mortality rate before hospital discharge or within 90 days of study entry. Secondary outcomes included the 28-day mortality rate, the rate of intensive care unit-acquired paresis, and the number of ventilator-free days. To be included, patients had to have been mechanically ventilated for less than 48 hours and to meet the AECC criteria for severe ARDS, with a PaO2/FiO2 ratio less than 150 mm Hg.
The intervention group received a continuous infusion of cisatracurium for 48 hours, while the control patients received placebo. Muscle strength was evaluated by clinical scoring of strength in different muscle groups.
Findings. The study groups were similar at baseline.
The crude 90-day mortality rate was lower in the cisatracurium group (31.6% vs 40.7%, P = .08). Regression analysis showed an improved 90-day survival rate with the use of this neuromuscular blocker after adjustment for severity of illness and the severity of ARDS (based on degree of hypoxemia and plateau pressures) (hazard ratio for death at 90 days 0.68; 95% CI 0.48–0.98; P = .04). The rate of paresis acquired in the intensive care unit did not differ significantly between the two groups.
Conclusion. In patients with severe ARDS, giving a neuromuscular blocking agent early improved the survival rate and increased the time off the ventilator without increasing muscle weakness.
These data are in line with similar findings from two other studies published by the same group.38,39 A meta-analysis of 432 patients showed that the use of neuromuscular blockade in early severe ARDS is associated with a statistically significant effect on early mortality (relative risk 0.66, 95% CI 0.50–0.87).40 The pooled analysis of these trials did not show any statistically significant critical-illness polyneuropathy.
These results need to be interpreted carefully, as we have inadequate data to see if they generalize to different intensive care units, and the evaluation and categorization of critical-illness polyneuropathy remains to be defined.
Cisatracurium is a promising treatment for moderate to severe ARDS and merits investigation in a large confirmatory randomized controlled trial.
Other pharmacologic agents
A number of other drugs have been studied in ARDS patients, including both inhaled and intravenous beta agonists,41,42 statins,43 and nutritional supplements.44 But as with other drugs previously studied in ARDS such as corticosteroids, N-acetylcysteine, and surfactant,34 these agents showed no effect on outcomes. In fact, a recent trial of intravenous salbutamol in ARDS patients was stopped after an interim analysis because of a higher incidence of arrhythmias and lactic acidosis with this agent.42
These findings reaffirm that pharmacologic therapy needs to be carefully considered, and potential harms associated with these therapies need to be addressed before they are introduced in the care of critically ill patients.
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. Acute Respiratory Distress Syndrome Network. N Engl J Med 2000; 342:1301–1308.
- Meade MO, Cook DJ, Guyatt GH, et al; Lung Open Ventilation Study Investigators. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008; 299:637–645.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575.
- Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008; 371:126–134.
- Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009; 373:1874–1882.
- Ely EW, Baker AM, Dunagan DP, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med 1996; 335:1864–1869.
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994; 149:818–824.
- Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med 2012; 38:1573–1582.
- ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307:2526–2533.
- Thille AW, Esteban A, Fernández-Segoviano P, et al. Comparison of the Berlin definition for acute respiratory distress syndrome with autopsy. Am J Respir Crit Care Med 2013; 187:761–767.
- Gattinoni L, Tognoni G, Pesenti A, et al; Prone-Supine Study Group. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med 2001; 345:568–573.
- Taccone P, Pesenti A, Latini R, et al; Prone-Supine II Study Group. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA 2009; 302:1977–1984.
- Mancebo J, Fernández R, Blanch L, et al. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med 2006; 173:1233–1239.
- Sud S, Friedrich JO, Taccone P, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med 2010; 36:585–599.
- Guérin C, Reignier J, Richard JC, et al; PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013; 368:2159–2168.
- Beitler JR, Shaefi S, Montesi SB, et al. Prone positioning reduces mortality from acute respiratory distress syndrome in the low tidal volume era: a meta-analysis. Intensive Care Med 2014; 40:332–341.
- Chan KP, Stewart TE, Mehta S. High-frequency oscillatory ventilation for adult patients with ARDS. Chest 2007; 131:1907–1916.
- Fessler HE, Hager DN, Brower RG. Feasibility of very high-frequency ventilation in adults with acute respiratory distress syndrome. Crit Care Med 2008; 36:1043–1048.
- Mehta S, Granton J, MacDonald RJ, et al. High-frequency oscillatory ventilation in adults: the Toronto experience. Chest 2004; 126:518–527.
- Ferguson ND, Chiche JD, Kacmarek RM, et al. Combining high-frequency oscillatory ventilation and recruitment maneuvers in adults with early acute respiratory distress syndrome: the Treatment with Oscillation and an Open Lung Strategy (TOOLS) Trial pilot study. Crit Care Med 2005; 33:479–486.
- Young D, Lamb SE, Shah S, et al; OSCAR Study Group. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med 2013; 368:806–813.
- Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol 2009; 62:464–475.
- Ferguson ND, Cook DJ, Guyatt GH, et al; OSCILLATE Trial Investigators; Canadian Critical Care Trials Group. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med 2013; 368:795–805.
- Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 1994; 149:295–305.
- Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 1979; 242:2193–2196.
- Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators; Davies A, Jones D, Bailey M, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA 2009; 302:1888–1895.
- Pham T, Combes A, Rozé H, et al; REVA Research Network. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med 2013; 187:276–285.
- Peek GJ, Mugford M, Tiruvoipati R, et al; CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009; 374:1351–1363.
- Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 1988; 138:720–723.
- Noah MA, Peek GJ, Finney SJ, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA 2011; 306:1659–1668.
- Zampieri FG, Mendes PV, Ranzani OT, et al. Extracorporeal membrane oxygenation for severe respiratory failure in adult patients: a systematic review and meta-analysis of current evidence. J Crit Care 2013; 28:998–1005.
- Raghavendran K, Pryhuber GS, Chess PR, Davidson BA, Knight PR, Notter RH. Pharmacotherapy of acute lung injury and acute respiratory distress syndrome. Curr Med Chem 2008; 15:1911–1924.
- Adhikari N, Burns KE, Meade MO. Pharmacologic treatments for acute respiratory distress syndrome and acute lung injury: systematic review and meta-analysis. Treat Respir Med 2004; 3:307–328.
- Adhikari N, Burns KE, Meade MO. Pharmacologic therapies for adults with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev 2004; 4:CD004477.
- Terragni PP, Rosboch GL, Lisi A, Viale AG, Ranieri VM. How respiratory system mechanics may help in minimising ventilator-induced lung injury in ARDS patients. Eur Respir J Suppl 2003; 42:15s–21s.
- Forel JM, Roch A, Papazian L. Paralytics in critical care: not always the bad guy. Curr Opin Crit Care 2009; 15:59–66.
- Papazian L, Forel JM, Gacouin A, et al; ACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010; 363:1107–1116.
- Gainnier M, Roch A, Forel JM, et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med 2004; 32:113–19.
- Forel JM, Roch A, Marin V, et al. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med 2006; 34:2749–2757.
- Alhazzani W, Alshahrani M, Jaeschke R, et al. Neuromuscular blocking agents in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. Crit Care 2013; 17:R43.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Matthay MA, Brower RG, Carson S, et al. Randomized, placebo-controlled clinical trial of an aerosolized beta-2-agonist for treatment of acute lung injury. Am J Respir Crit Care Med 2011; 184:561–568.
- Gao Smith F, Perkins GD, Gates S, et al; BALTI-2 study investigators. Effect of intravenous beta-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet 2012; 379:229–235.
- Craig TR, Duffy MJ, Shyamsundar M, et al. A randomized clinical trial of hydroxymethylglutaryl-coenzyme a reductase inhibition for acute lung injury (The HARP Study). Am J Respir Crit Care Med 2011; 183:620–626.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Rice TW, Wheeler AP, Thompson BT, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA 2012; 307:795–803.
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. Acute Respiratory Distress Syndrome Network. N Engl J Med 2000; 342:1301–1308.
- Meade MO, Cook DJ, Guyatt GH, et al; Lung Open Ventilation Study Investigators. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008; 299:637–645.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575.
- Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008; 371:126–134.
- Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009; 373:1874–1882.
- Ely EW, Baker AM, Dunagan DP, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med 1996; 335:1864–1869.
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994; 149:818–824.
- Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med 2012; 38:1573–1582.
- ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307:2526–2533.
- Thille AW, Esteban A, Fernández-Segoviano P, et al. Comparison of the Berlin definition for acute respiratory distress syndrome with autopsy. Am J Respir Crit Care Med 2013; 187:761–767.
- Gattinoni L, Tognoni G, Pesenti A, et al; Prone-Supine Study Group. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med 2001; 345:568–573.
- Taccone P, Pesenti A, Latini R, et al; Prone-Supine II Study Group. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA 2009; 302:1977–1984.
- Mancebo J, Fernández R, Blanch L, et al. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med 2006; 173:1233–1239.
- Sud S, Friedrich JO, Taccone P, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med 2010; 36:585–599.
- Guérin C, Reignier J, Richard JC, et al; PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013; 368:2159–2168.
- Beitler JR, Shaefi S, Montesi SB, et al. Prone positioning reduces mortality from acute respiratory distress syndrome in the low tidal volume era: a meta-analysis. Intensive Care Med 2014; 40:332–341.
- Chan KP, Stewart TE, Mehta S. High-frequency oscillatory ventilation for adult patients with ARDS. Chest 2007; 131:1907–1916.
- Fessler HE, Hager DN, Brower RG. Feasibility of very high-frequency ventilation in adults with acute respiratory distress syndrome. Crit Care Med 2008; 36:1043–1048.
- Mehta S, Granton J, MacDonald RJ, et al. High-frequency oscillatory ventilation in adults: the Toronto experience. Chest 2004; 126:518–527.
- Ferguson ND, Chiche JD, Kacmarek RM, et al. Combining high-frequency oscillatory ventilation and recruitment maneuvers in adults with early acute respiratory distress syndrome: the Treatment with Oscillation and an Open Lung Strategy (TOOLS) Trial pilot study. Crit Care Med 2005; 33:479–486.
- Young D, Lamb SE, Shah S, et al; OSCAR Study Group. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med 2013; 368:806–813.
- Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol 2009; 62:464–475.
- Ferguson ND, Cook DJ, Guyatt GH, et al; OSCILLATE Trial Investigators; Canadian Critical Care Trials Group. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med 2013; 368:795–805.
- Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 1994; 149:295–305.
- Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 1979; 242:2193–2196.
- Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators; Davies A, Jones D, Bailey M, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA 2009; 302:1888–1895.
- Pham T, Combes A, Rozé H, et al; REVA Research Network. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med 2013; 187:276–285.
- Peek GJ, Mugford M, Tiruvoipati R, et al; CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009; 374:1351–1363.
- Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 1988; 138:720–723.
- Noah MA, Peek GJ, Finney SJ, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA 2011; 306:1659–1668.
- Zampieri FG, Mendes PV, Ranzani OT, et al. Extracorporeal membrane oxygenation for severe respiratory failure in adult patients: a systematic review and meta-analysis of current evidence. J Crit Care 2013; 28:998–1005.
- Raghavendran K, Pryhuber GS, Chess PR, Davidson BA, Knight PR, Notter RH. Pharmacotherapy of acute lung injury and acute respiratory distress syndrome. Curr Med Chem 2008; 15:1911–1924.
- Adhikari N, Burns KE, Meade MO. Pharmacologic treatments for acute respiratory distress syndrome and acute lung injury: systematic review and meta-analysis. Treat Respir Med 2004; 3:307–328.
- Adhikari N, Burns KE, Meade MO. Pharmacologic therapies for adults with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev 2004; 4:CD004477.
- Terragni PP, Rosboch GL, Lisi A, Viale AG, Ranieri VM. How respiratory system mechanics may help in minimising ventilator-induced lung injury in ARDS patients. Eur Respir J Suppl 2003; 42:15s–21s.
- Forel JM, Roch A, Papazian L. Paralytics in critical care: not always the bad guy. Curr Opin Crit Care 2009; 15:59–66.
- Papazian L, Forel JM, Gacouin A, et al; ACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010; 363:1107–1116.
- Gainnier M, Roch A, Forel JM, et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med 2004; 32:113–19.
- Forel JM, Roch A, Marin V, et al. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med 2006; 34:2749–2757.
- Alhazzani W, Alshahrani M, Jaeschke R, et al. Neuromuscular blocking agents in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. Crit Care 2013; 17:R43.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Matthay MA, Brower RG, Carson S, et al. Randomized, placebo-controlled clinical trial of an aerosolized beta-2-agonist for treatment of acute lung injury. Am J Respir Crit Care Med 2011; 184:561–568.
- Gao Smith F, Perkins GD, Gates S, et al; BALTI-2 study investigators. Effect of intravenous beta-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet 2012; 379:229–235.
- Craig TR, Duffy MJ, Shyamsundar M, et al. A randomized clinical trial of hydroxymethylglutaryl-coenzyme a reductase inhibition for acute lung injury (The HARP Study). Am J Respir Crit Care Med 2011; 183:620–626.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Rice TW, Wheeler AP, Thompson BT, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA 2012; 307:795–803.
KEY POINTS
- The new definition of ARDS categorizes it as mild, moderate, or severe on the basis of oxygenation, specifically, the PaO2/FiO2 ratio.
- Neuromuscular blockade and prone positioning, used early in moderate or severe cases of ARDS, have shown some promise in trials, but questions remain about their application in critically ill patients.
- Based on two large trials, HFOV is no longer recommended as a primary therapy for ARDS, but it may still be considered as a rescue therapy in patients with refractory hypoxemia.
- In light of observational studies and randomized trials, ECMO should be considered an option in cases of refractory hypoxemia.
A 61-year-old man with fluctuating hypertension
A 61-year-old man with type 2 diabetes mellitus on glimepiride therapy presented with somnolence and slurred speech. His capillary glucose level was 17 mg/dL and his serum glucose level was 28 mg/dL. He was treated with intravenous dextrose, and his glucose level promptly returned to normal.
He had been adherent to his medication regimen and denied overmedicating or accidental overdosing. Over the past 7 months, he had noted redness on his palms, a rash on his legs, intermittent moderate to severe headaches, weight loss, and decreased appetite. In addition, his blood pressure had been labile, which his physicians had attributed to autonomic instability. He had continued on the same dose of glimepiride despite losing weight.
His history included multivessel coronary artery disease treated with angioplasty and placement of multiple coronary stents; ischemic cardiomyopathy with a left ventricular ejection fraction of 28%; implantation of a cardioverter-defibrillator for secondary prevention of ventricular arrhythmia; an ischemic stroke; and multiple sclerosis complicated by bilateral blindness, with optic nerve involvement and autonomic instability, present for over a year and manifested by labile blood pressure. He was a long-time tobacco user. His daily medications included ticagrelor 90 mg, aspirin 81 mg, metoprolol 50 mg, ramipril 10 mg, simvastatin 20 mg, glimepiride 2 mg, and esomeprazole 40 mg. He needed help taking his medications.
At the time of hospital admission, his heart rate was 69 beats per minute with a regular rhythm, blood pressure 115/73 mm Hg, respiratory rate 11 breaths per minute with an oxygen saturation of 99% on room air, and oral temperature 34.7°C (94.5°F). He appeared to be in no distress.
Cardiovascular examination revealed no murmurs or gallops; there was mild nonpitting edema of the lower extremities. Pulmonary, abdominal, and neurologic examinations were unrevealing except for bilateral blindness. Vascular examination revealed no bruits. Results of a complete blood cell count and metabolic panel were normal except for a hemoglobin level of 9.9 g/dL (reference range 13.5–17.5) and a platelet count of 477 × 109/L (150–450).
Although he continued to receive the same medications he had been taking at home, his blood pressure fluctuated. On the second hospital day, it reached 186/135 mm Hg, at which time he also had palpitations, dyspnea, and crackles in the lower lobes of both lungs. Volume resuscitation on admission was suspected to have played a role, and he received furosemide, which improved his symptoms. But several hours later, his blood pressure rose again, and he became diaphoretic. Despite aggressive treatment with different antihypertensive agents, his blood pressure remained high and his symptoms persisted. Chest radiography showed no evidence of pulmonary edema. Because of his progressive dyspnea, the diagnosis of pulmonary embolism was entertained.
CAUSES OF RESISTANT HYPERTENSION
1. What could explain this patient’s high blood pressure?
- A drug effect
- Renovascular disease
- Excess circulating catecholamines
- Obstructive sleep apnea
- Primary aldosteronism
Sympathomimetic drugs such as epinephrine, norepinephrine, dopamine, and vasopressin, which are used when hemodynamic support is required, can raise both systolic and diastolic blood pressure. Nonsteroidal anti-inflammatory drugs and nasal decongestants are common culprits in the community. However, our patient was using none of these drugs.
Renovascular disease is one of many causes of resistant hypertension, accounting for 8% of all cases.1,2 Despite fluctuations, the blood pressure often remains chronically elevated, its changes are less paroxysmal than in our patient, and a precipitating factor such as a dietary indiscretion is sometimes identified.1
Excess circulating catecholamines can be a result of stress, exogenous administration, or endogenous oversecretion. Our patient’s clinical presentation is highly suspicious for a high-catecholamine state, and this should be further evaluated.
Obstructive sleep apnea is common in patients with resistant hypertension, with an estimated prevalence as high as 60% in this group.3,4
Primary aldosteronism has an estimated prevalence of about 20% in patients evaluated for resistant hypertension.5
AN ADRENAL MASS IS INCIDENTALLY DISCOVERED
Computed tomographic angiography of the chest revealed no evidence of pulmonary emboli. There was mild dilation of the central pulmonary arteries and an incidental, incompletely imaged 4.7-by-3.4-cm mass of mixed attenuation in the right adrenal gland, with macroscopic fat within the lesion.
Computed tomography (CT) of the abdomen with dedicated cuts through the adrenal glands revealed a 4.7-cm heterogeneous right adrenal mass with a density of 34 Hounsfield units (HU). The left adrenal gland appeared diffusely enlarged without a discretely seen mass, consistent with hyperplasticity (Figure 1).
2. Based on the patient’s clinical presentation and findings on CT, what would be the most likely diagnosis for this incidentally found adrenal mass?
- Adrenocortical adenoma
- Adrenocortical carcinoma
- Metastatic mass
- Pheochromocytoma
Adrenocortical adenoma can present as a small homogeneous mass of variable size, with smooth margins, and rarely containing hemorrhagic tissue or calcifications. The typical density on nonenhanced CT is less than 10 HU. On enhanced CT, it is nonvascular. T2-weighted magnetic resonance imaging (MRI) shows a lesion of the same intensity as liver tissue.6
Adrenocortical adenoma is not classically associated with autologous activity and thus is less likely to explain our patient’s symptoms.
Adrenocortical carcinoma can present as a large heterogeneous mass, usually greater than 4 cm in diameter, with irregular margins and areas of necrosis, hemorrhage, or calcification. The typical density on nonenhanced CT is greater than 10 HU. On enhanced CT, the mass is usually vascular, and T2-weighted MRI will show a lesion more intense than liver tissue.6
Adrenocortical carcinoma is also not classically associated with autologous activity, and so is not likely to explain our patient’s symptoms.6
Metastatic disease can present with masses of variable size, often bilaterally, and occasionally with cysts or areas of hemorrhage. The typical density of metastatic lesions on nonenhanced CT is greater than 10 HU. On enhanced CT, they are usually vascular, and on T2-weighted MRI they are hyperintense.6 The characteristics of the mass and the absence of a primary malignancy on CT of the chest and abdomen do not support the diagnosis of metastatic disease.
Pheochromocytoma is a neuroendocrine tumor of the adrenal medulla that can present as a large heterogeneous mass, greater than 3 cm in diameter, with clear margins and cysts or areas of hemorrhage. Extra-adrenal neuroendocrine tumors are typically called paragangliomas and have features similar to those of pheochromocytoma. The typical density of pheochromocytoma on nonenhanced CT is greater than 10 HU. On enhanced CT, it is usually vascular, and T2-weighted MRI shows a hyperintense lesion. Pheochromocytoma can be biochemically active and thus can cause signs and symptoms that will lead to the diagnosis.6
Other imaging tests may play a role in the evaluation of adrenal masses but are not required for the diagnosis of pheochromocytoma. Functional positron emission tomography using metaiodobenzylguanidine labeled with iodine 123 or-iodine 131 or using the glucose analogue F-18 fluorodeoxyglucose has been used in the initial assessment of pheochromocytoma, with good sensitivity and specificity.7,8
Our patient’s pacemaker-defibrillator precluded him from undergoing MRI.
DIAGNOSIS: PHEOCHROMOCYTOMA
Pheochromocytoma was highly suspected on the basis of the patient’s clinical presentation, and metoprolol was immediately discontinued. He was started on the calcium channel blocker verapamil and the alpha-blocker phenoxybenzamine.
Serum samples were obtained to measure metanephrines, dehydroepiandrosterone, aldosterone, and cortisol, and a 24-hour urine collection was obtained to measure creatinine, dopamine, epinephrine, norepinephrine, cortisol, and metanephrines. Based on the results (Table 1) and on the findings on imaging, the patient was diagnosed with pheochromocytoma. A surgical consultation was obtained, and surgery was recommended.
WHEN DOES PHEOCHROMOCYTOMA CALL FOR SURGERY?
3. Which criterion is most important when determining the need for surgery for pheochromocytoma?
- Findings on fine-needle aspiration biopsy
- Biochemical activity
- Size of the mass
- Bilateral masses
Fine-needle aspiration biopsy can be done when a mass is found incidentally and no evidence of biochemical activity is detected, although it is not an essential part of the diagnostic workup.9 In most cases, the sampling from fine-needle aspiration is not sufficient to achieve a diagnosis.
Biochemical activity is the most important factor when determining the need for prompt surgical intervention. The excess circulating catecholamines have been associated with increased risk of cardiovascular morbidity and death independent of the morbidity associated with hypertension alone.10 Biochemical activity can be independent of the size of the mass, but larger masses typically present with symptoms.
Bilateral masses have been associated with metastatic disease.11 In retrospect, the patient’s history of hypertension and cerebrovascular accident could be associated with the development of a catecholamine-releasing tumor.
A GOOD OUTCOME FROM SURGERY
The patient was continued on phenoxybenzamine for 7 days and responded well to this therapy.
After this preoperative preparation, he underwent laparoscopic right adrenalectomy with excision of a retroperitoneal adrenal mass. His postoperative course was complicated by transient hypotension requiring low-dose vasopressin support for less than 24 hours. He was then restarted on his previous dosage of metoprolol and was discharged home on postoperative day 5 with stable blood pressure. Follow-up 24-hour urine collection 1 month after he was discharged showed normalization of metanephrine, normetanephrine, epinephrine, and norepinephrine levels.
Despite low suspicion for an underlying genetic syndrome, he was referred for genetic testing and was scheduled to have a repeat 24-hour urine collection and imaging in 6 months to follow his enlarged left adrenal gland, which did not appear to be metabolically hyperactive.
4. What is the most common perioperative complication of pheochromocytoma excision?
- Hypoglycemia
- Hypotension
- Hypocortisolism
- Hypertension
- Tachycardia
Hypoglycemia has been observed after removal of pheochromocytoma, as levels of catecholamines (which normally inhibit pancreatic beta cells) decrease and insulin secretion consequently increases.12 Our patient developed hypoglycemia before surgery, not after, and it was likely due to the combination of his antidiabetic therapy, weight loss, and decreased oral intake.
Hypotension is the most common complication in the perioperative period. It is associated with excessive loss of catecholamine secretion. It is usually short-lived but may require aggressive administration of intravenous fluids and use of sympathomimetic agents.
Hypocortisolism is unlikely in patients with pheochromocytoma, but it is likely after removal of adrenocortical adenoma.
Hypertension and tachycardia affect up to 40% of pheochromocytoma patients in some case series.12
PHEOCHROMOCYTOMA: A CATECHOLAMINE-SECRETING TUMOR
The pathophysiology of pheochromocytoma is complex. It is characterized by accelerated growth of cells producing catecholamines, which may produce symptoms when secreted into the bloodstream. The classic triad of symptoms is headache, hypertension, and hyperglycemia, although our patient had very low blood sugar levels. Other common symptoms are nausea, orthostasis, and tremor, although not all symptoms are invariably seen.
Genetic testing recommended
Genetic associations have been described and are thought to be responsible for 20% to 30% of cases of pheochromocytoma. All associated germline mutations are autosomal dominant, some with variable penetrance. These include:
- Succinate dehydrogenase subunit B, C, and D mutations
- von Hippel-Lindau syndrome
- Multiple endocrine neoplasia type 1 and type 2 syndromes
- Neurofibromatosis type 1.13,14
The succinate dehydrogenase subunit mutations have been associated with, but not limited to, extra-adrenal adenomas (paragangliomas) and carry a worse prognosis.
Some experts recommend genetic testing in all cases of pheochromocytoma, sporadic or familial, and this testing should be followed by counseling if a mutation is found.15 Others recommend genetic testing based on the patient’s age (under age 50), history, imaging, and biochemical features of the tumor (metanephrines predominate in multiple endocrine neoplasia syndromes, and normetanephrines in von Hippel-Lindau syndrome).13
Serious consequences
A thorough evaluation is recommended, since pheochromocytoma has been associated with increased cardiovascular morbidity. In a retrospective series, Stolk et al10 reported that patients with pheochromocytoma had a higher incidence of myocardial infarction, angina, and stroke in the years preceding the diagnosis than did patients with essential hypertension (13.8% vs 1.1%, P < .001).10
Catecholamine cardiomyopathy has been described and shares clinical features with Takotsubo or stress cardiomyopathy, with global left ventricular systolic and diastolic dysfunction that improve or resolve after the adrenergic insult is removed.16
Conditions that warrant further evaluation or that may suggest pheochromocytoma are malignant hypertension, hypertensive encephalopathy, ischemic stroke, subarachnoid hemorrhage, acute pulmonary edema, angina pectoris, myocardial infarction, aortic dissection, and kidney injury.
When to suspect pheochromocytoma
Pheochromocytoma should be suspected in a patient with resistant hypertension, family history, or imaging findings that suggest an adrenal mass with a heterogeneous appearance. The diagnostic algorithm follows the same pathway as for the evaluation of an incidentally found adrenal mass, with determination of its dimension and characteristics by CT or MRI, and with biochemical testing of urine catecholamines, plasma free metanephrines, renin, aldosterone, and cortisol.
The diagnosis of pheochromocytoma is established by obtaining fractionated metanephrines and catecholamines in a 24-hour urine collection (sensitivity 90%, specificity 98%). Analysis of plasma metanephrines has a higher sensitivity (97%) but lower specificity (85%).17 The combination of typical signs, symptoms, and laboratory findings makes the diagnosis likely, especially in combination with a unilateral adrenal mass.
Laparoscopic surgery after medical preparation for active tumors
If the mass appears benign and not biochemically hyperactive, then follow-up at 1 year is recommended, with repeat testing. Surgical evaluation and intervention is recommended for lesions that appear malignant or that are biochemically active and clinically symptomatic.9
Preoperative hemodynamic control is essential in the management of pheochromocytoma to prevent or minimize hemodynamic changes that can be driven by increased catecholamines. Control is typically achieved with initial alpha-blockade and then beta-blockade to avoid worsening hypertension and to prevent an acute hypertensive crisis during surgical intervention. Phenoxybenzamine, the mainstay of therapy, is a nonselective alpha-blocker with a long duration of action that requires titration over several days up to 3 weeks.
A selective alpha-1-blocker such as doxazosin can be used to control postoperative hypotension, as it has a shorter half-life than phenoxybenzamine. Alternative strategies include calcium channel blockers, centrally acting sympathetic blockers, and magnesium.18
Laparoscopic adrenalectomy by an experienced surgeon after excellent medical preparation is often considered the treatment of choice, but for larger or malignant masses, an open procedure is recommended. The risk of perioperative morbidity and death can be reduced by adequate medical management. With successful surgical resection, the long-term prognosis is favorable.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2008; 51:1403–1419.
- Kumar N, Calhoun DA, Dudenbostel T. Management of patients with resistant hypertension: current treatment options. Integr Blood Press Control 2013; 6:139–151.
- Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension 2011; 58:811–817.
- Marcus JA, Pothineni A, Marcus CZ, Bisognano JD. The role of obesity and obstructive sleep apnea in the pathogenesis and treatment of resistant hypertension. Curr Hypertens Rep 2014; 16:411.
- Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB, Weissmann P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension 2002; 40:892–896.
- Young WF Clinical practice. The incidentally discovered adrenal mass. N Engl J Med 2007; 356:601–610.
- Lin M, Wong V, Yap J, Jin R, Leong P, Campbell P. FDG PET in the evaluation of phaeochromocytoma: a correlative study with MIBG scintigraphy and Ki-67 proliferative index. Clin Imaging 2013; 37:1084–1088.
- Raja A, Leung K, Stamm M, Girgis S, Low G. Multimodality imaging findings of pheochromocytoma with associated clinical and biochemical features in 53 patients with histologically confirmed tumors. AJR Am J Roentgenol 2013; 201:825–833.
- Nieman LK. Approach to the patient with an adrenal incidentaloma. J Clin Endocrinol Metab 2010; 95:4106–4113.
- Stolk RF, Bakx C, Mulder J, Timmers HJ, Lenders JW. Is the excess cardiovascular morbidity in pheochromocytoma related to blood pressure or to catecholamines? J Clin Endocrinol Metab 2013; 98:1100–1106.
- Grumbach MM, Biller BM, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (‘incidentaloma’). Ann Intern Med 2003; 138:424–429.
- Lentschener C, Gaujoux S, Tesniere A, Dousset B. Point of controversy: perioperative care of patients undergoing pheochromocytoma removal—time for a reappraisal? Eur J Endocrinol 2011; 165:365–373.
- Bryant J, Farmer J, Kessler LJ, Townsend RR, Nathanson KL. Pheochromocytoma: the expanding genetic differential diagnosis. J Natl Cancer Inst 2003; 95:1196–1204.
- Lee P, Leonard J. Textbook on endocrinology. BMJ 1994; 308:1512.
- Fishbein L, Merrill S, Fraker DL, Cohen DL, Nathanson KL. Inherited mutations in pheochromocytoma and paraganglioma: why all patients should be offered genetic testing. Ann Surg Oncol 2013; 20:1444–1450.
- Agarwal G, Sadacharan D, Kapoor A, et al. Cardiovascular dysfunction and catecholamine cardiomyopathy in pheochromocytoma patients and their reversal following surgical cure: results of a prospective case-control study. Surgery 2011; 150:1202–1211.
- Sawka AM, Jaeschke R, Singh RJ, Young WF A comparison of biochemical tests for pheochromocytoma: measurement of fractionated plasma metanephrines compared with the combination of 24-hour urinary metanephrines and catecholamines. J Clin Endocrinol Metab 2003; 88:553–558.
- Domi R, Laho H. Management of pheochromocytoma: old ideas and new drugs. Niger J Clin Pract 2012; 15:253–257.
A 61-year-old man with type 2 diabetes mellitus on glimepiride therapy presented with somnolence and slurred speech. His capillary glucose level was 17 mg/dL and his serum glucose level was 28 mg/dL. He was treated with intravenous dextrose, and his glucose level promptly returned to normal.
He had been adherent to his medication regimen and denied overmedicating or accidental overdosing. Over the past 7 months, he had noted redness on his palms, a rash on his legs, intermittent moderate to severe headaches, weight loss, and decreased appetite. In addition, his blood pressure had been labile, which his physicians had attributed to autonomic instability. He had continued on the same dose of glimepiride despite losing weight.
His history included multivessel coronary artery disease treated with angioplasty and placement of multiple coronary stents; ischemic cardiomyopathy with a left ventricular ejection fraction of 28%; implantation of a cardioverter-defibrillator for secondary prevention of ventricular arrhythmia; an ischemic stroke; and multiple sclerosis complicated by bilateral blindness, with optic nerve involvement and autonomic instability, present for over a year and manifested by labile blood pressure. He was a long-time tobacco user. His daily medications included ticagrelor 90 mg, aspirin 81 mg, metoprolol 50 mg, ramipril 10 mg, simvastatin 20 mg, glimepiride 2 mg, and esomeprazole 40 mg. He needed help taking his medications.
At the time of hospital admission, his heart rate was 69 beats per minute with a regular rhythm, blood pressure 115/73 mm Hg, respiratory rate 11 breaths per minute with an oxygen saturation of 99% on room air, and oral temperature 34.7°C (94.5°F). He appeared to be in no distress.
Cardiovascular examination revealed no murmurs or gallops; there was mild nonpitting edema of the lower extremities. Pulmonary, abdominal, and neurologic examinations were unrevealing except for bilateral blindness. Vascular examination revealed no bruits. Results of a complete blood cell count and metabolic panel were normal except for a hemoglobin level of 9.9 g/dL (reference range 13.5–17.5) and a platelet count of 477 × 109/L (150–450).
Although he continued to receive the same medications he had been taking at home, his blood pressure fluctuated. On the second hospital day, it reached 186/135 mm Hg, at which time he also had palpitations, dyspnea, and crackles in the lower lobes of both lungs. Volume resuscitation on admission was suspected to have played a role, and he received furosemide, which improved his symptoms. But several hours later, his blood pressure rose again, and he became diaphoretic. Despite aggressive treatment with different antihypertensive agents, his blood pressure remained high and his symptoms persisted. Chest radiography showed no evidence of pulmonary edema. Because of his progressive dyspnea, the diagnosis of pulmonary embolism was entertained.
CAUSES OF RESISTANT HYPERTENSION
1. What could explain this patient’s high blood pressure?
- A drug effect
- Renovascular disease
- Excess circulating catecholamines
- Obstructive sleep apnea
- Primary aldosteronism
Sympathomimetic drugs such as epinephrine, norepinephrine, dopamine, and vasopressin, which are used when hemodynamic support is required, can raise both systolic and diastolic blood pressure. Nonsteroidal anti-inflammatory drugs and nasal decongestants are common culprits in the community. However, our patient was using none of these drugs.
Renovascular disease is one of many causes of resistant hypertension, accounting for 8% of all cases.1,2 Despite fluctuations, the blood pressure often remains chronically elevated, its changes are less paroxysmal than in our patient, and a precipitating factor such as a dietary indiscretion is sometimes identified.1
Excess circulating catecholamines can be a result of stress, exogenous administration, or endogenous oversecretion. Our patient’s clinical presentation is highly suspicious for a high-catecholamine state, and this should be further evaluated.
Obstructive sleep apnea is common in patients with resistant hypertension, with an estimated prevalence as high as 60% in this group.3,4
Primary aldosteronism has an estimated prevalence of about 20% in patients evaluated for resistant hypertension.5
AN ADRENAL MASS IS INCIDENTALLY DISCOVERED
Computed tomographic angiography of the chest revealed no evidence of pulmonary emboli. There was mild dilation of the central pulmonary arteries and an incidental, incompletely imaged 4.7-by-3.4-cm mass of mixed attenuation in the right adrenal gland, with macroscopic fat within the lesion.
Computed tomography (CT) of the abdomen with dedicated cuts through the adrenal glands revealed a 4.7-cm heterogeneous right adrenal mass with a density of 34 Hounsfield units (HU). The left adrenal gland appeared diffusely enlarged without a discretely seen mass, consistent with hyperplasticity (Figure 1).
2. Based on the patient’s clinical presentation and findings on CT, what would be the most likely diagnosis for this incidentally found adrenal mass?
- Adrenocortical adenoma
- Adrenocortical carcinoma
- Metastatic mass
- Pheochromocytoma
Adrenocortical adenoma can present as a small homogeneous mass of variable size, with smooth margins, and rarely containing hemorrhagic tissue or calcifications. The typical density on nonenhanced CT is less than 10 HU. On enhanced CT, it is nonvascular. T2-weighted magnetic resonance imaging (MRI) shows a lesion of the same intensity as liver tissue.6
Adrenocortical adenoma is not classically associated with autologous activity and thus is less likely to explain our patient’s symptoms.
Adrenocortical carcinoma can present as a large heterogeneous mass, usually greater than 4 cm in diameter, with irregular margins and areas of necrosis, hemorrhage, or calcification. The typical density on nonenhanced CT is greater than 10 HU. On enhanced CT, the mass is usually vascular, and T2-weighted MRI will show a lesion more intense than liver tissue.6
Adrenocortical carcinoma is also not classically associated with autologous activity, and so is not likely to explain our patient’s symptoms.6
Metastatic disease can present with masses of variable size, often bilaterally, and occasionally with cysts or areas of hemorrhage. The typical density of metastatic lesions on nonenhanced CT is greater than 10 HU. On enhanced CT, they are usually vascular, and on T2-weighted MRI they are hyperintense.6 The characteristics of the mass and the absence of a primary malignancy on CT of the chest and abdomen do not support the diagnosis of metastatic disease.
Pheochromocytoma is a neuroendocrine tumor of the adrenal medulla that can present as a large heterogeneous mass, greater than 3 cm in diameter, with clear margins and cysts or areas of hemorrhage. Extra-adrenal neuroendocrine tumors are typically called paragangliomas and have features similar to those of pheochromocytoma. The typical density of pheochromocytoma on nonenhanced CT is greater than 10 HU. On enhanced CT, it is usually vascular, and T2-weighted MRI shows a hyperintense lesion. Pheochromocytoma can be biochemically active and thus can cause signs and symptoms that will lead to the diagnosis.6
Other imaging tests may play a role in the evaluation of adrenal masses but are not required for the diagnosis of pheochromocytoma. Functional positron emission tomography using metaiodobenzylguanidine labeled with iodine 123 or-iodine 131 or using the glucose analogue F-18 fluorodeoxyglucose has been used in the initial assessment of pheochromocytoma, with good sensitivity and specificity.7,8
Our patient’s pacemaker-defibrillator precluded him from undergoing MRI.
DIAGNOSIS: PHEOCHROMOCYTOMA
Pheochromocytoma was highly suspected on the basis of the patient’s clinical presentation, and metoprolol was immediately discontinued. He was started on the calcium channel blocker verapamil and the alpha-blocker phenoxybenzamine.
Serum samples were obtained to measure metanephrines, dehydroepiandrosterone, aldosterone, and cortisol, and a 24-hour urine collection was obtained to measure creatinine, dopamine, epinephrine, norepinephrine, cortisol, and metanephrines. Based on the results (Table 1) and on the findings on imaging, the patient was diagnosed with pheochromocytoma. A surgical consultation was obtained, and surgery was recommended.
WHEN DOES PHEOCHROMOCYTOMA CALL FOR SURGERY?
3. Which criterion is most important when determining the need for surgery for pheochromocytoma?
- Findings on fine-needle aspiration biopsy
- Biochemical activity
- Size of the mass
- Bilateral masses
Fine-needle aspiration biopsy can be done when a mass is found incidentally and no evidence of biochemical activity is detected, although it is not an essential part of the diagnostic workup.9 In most cases, the sampling from fine-needle aspiration is not sufficient to achieve a diagnosis.
Biochemical activity is the most important factor when determining the need for prompt surgical intervention. The excess circulating catecholamines have been associated with increased risk of cardiovascular morbidity and death independent of the morbidity associated with hypertension alone.10 Biochemical activity can be independent of the size of the mass, but larger masses typically present with symptoms.
Bilateral masses have been associated with metastatic disease.11 In retrospect, the patient’s history of hypertension and cerebrovascular accident could be associated with the development of a catecholamine-releasing tumor.
A GOOD OUTCOME FROM SURGERY
The patient was continued on phenoxybenzamine for 7 days and responded well to this therapy.
After this preoperative preparation, he underwent laparoscopic right adrenalectomy with excision of a retroperitoneal adrenal mass. His postoperative course was complicated by transient hypotension requiring low-dose vasopressin support for less than 24 hours. He was then restarted on his previous dosage of metoprolol and was discharged home on postoperative day 5 with stable blood pressure. Follow-up 24-hour urine collection 1 month after he was discharged showed normalization of metanephrine, normetanephrine, epinephrine, and norepinephrine levels.
Despite low suspicion for an underlying genetic syndrome, he was referred for genetic testing and was scheduled to have a repeat 24-hour urine collection and imaging in 6 months to follow his enlarged left adrenal gland, which did not appear to be metabolically hyperactive.
4. What is the most common perioperative complication of pheochromocytoma excision?
- Hypoglycemia
- Hypotension
- Hypocortisolism
- Hypertension
- Tachycardia
Hypoglycemia has been observed after removal of pheochromocytoma, as levels of catecholamines (which normally inhibit pancreatic beta cells) decrease and insulin secretion consequently increases.12 Our patient developed hypoglycemia before surgery, not after, and it was likely due to the combination of his antidiabetic therapy, weight loss, and decreased oral intake.
Hypotension is the most common complication in the perioperative period. It is associated with excessive loss of catecholamine secretion. It is usually short-lived but may require aggressive administration of intravenous fluids and use of sympathomimetic agents.
Hypocortisolism is unlikely in patients with pheochromocytoma, but it is likely after removal of adrenocortical adenoma.
Hypertension and tachycardia affect up to 40% of pheochromocytoma patients in some case series.12
PHEOCHROMOCYTOMA: A CATECHOLAMINE-SECRETING TUMOR
The pathophysiology of pheochromocytoma is complex. It is characterized by accelerated growth of cells producing catecholamines, which may produce symptoms when secreted into the bloodstream. The classic triad of symptoms is headache, hypertension, and hyperglycemia, although our patient had very low blood sugar levels. Other common symptoms are nausea, orthostasis, and tremor, although not all symptoms are invariably seen.
Genetic testing recommended
Genetic associations have been described and are thought to be responsible for 20% to 30% of cases of pheochromocytoma. All associated germline mutations are autosomal dominant, some with variable penetrance. These include:
- Succinate dehydrogenase subunit B, C, and D mutations
- von Hippel-Lindau syndrome
- Multiple endocrine neoplasia type 1 and type 2 syndromes
- Neurofibromatosis type 1.13,14
The succinate dehydrogenase subunit mutations have been associated with, but not limited to, extra-adrenal adenomas (paragangliomas) and carry a worse prognosis.
Some experts recommend genetic testing in all cases of pheochromocytoma, sporadic or familial, and this testing should be followed by counseling if a mutation is found.15 Others recommend genetic testing based on the patient’s age (under age 50), history, imaging, and biochemical features of the tumor (metanephrines predominate in multiple endocrine neoplasia syndromes, and normetanephrines in von Hippel-Lindau syndrome).13
Serious consequences
A thorough evaluation is recommended, since pheochromocytoma has been associated with increased cardiovascular morbidity. In a retrospective series, Stolk et al10 reported that patients with pheochromocytoma had a higher incidence of myocardial infarction, angina, and stroke in the years preceding the diagnosis than did patients with essential hypertension (13.8% vs 1.1%, P < .001).10
Catecholamine cardiomyopathy has been described and shares clinical features with Takotsubo or stress cardiomyopathy, with global left ventricular systolic and diastolic dysfunction that improve or resolve after the adrenergic insult is removed.16
Conditions that warrant further evaluation or that may suggest pheochromocytoma are malignant hypertension, hypertensive encephalopathy, ischemic stroke, subarachnoid hemorrhage, acute pulmonary edema, angina pectoris, myocardial infarction, aortic dissection, and kidney injury.
When to suspect pheochromocytoma
Pheochromocytoma should be suspected in a patient with resistant hypertension, family history, or imaging findings that suggest an adrenal mass with a heterogeneous appearance. The diagnostic algorithm follows the same pathway as for the evaluation of an incidentally found adrenal mass, with determination of its dimension and characteristics by CT or MRI, and with biochemical testing of urine catecholamines, plasma free metanephrines, renin, aldosterone, and cortisol.
The diagnosis of pheochromocytoma is established by obtaining fractionated metanephrines and catecholamines in a 24-hour urine collection (sensitivity 90%, specificity 98%). Analysis of plasma metanephrines has a higher sensitivity (97%) but lower specificity (85%).17 The combination of typical signs, symptoms, and laboratory findings makes the diagnosis likely, especially in combination with a unilateral adrenal mass.
Laparoscopic surgery after medical preparation for active tumors
If the mass appears benign and not biochemically hyperactive, then follow-up at 1 year is recommended, with repeat testing. Surgical evaluation and intervention is recommended for lesions that appear malignant or that are biochemically active and clinically symptomatic.9
Preoperative hemodynamic control is essential in the management of pheochromocytoma to prevent or minimize hemodynamic changes that can be driven by increased catecholamines. Control is typically achieved with initial alpha-blockade and then beta-blockade to avoid worsening hypertension and to prevent an acute hypertensive crisis during surgical intervention. Phenoxybenzamine, the mainstay of therapy, is a nonselective alpha-blocker with a long duration of action that requires titration over several days up to 3 weeks.
A selective alpha-1-blocker such as doxazosin can be used to control postoperative hypotension, as it has a shorter half-life than phenoxybenzamine. Alternative strategies include calcium channel blockers, centrally acting sympathetic blockers, and magnesium.18
Laparoscopic adrenalectomy by an experienced surgeon after excellent medical preparation is often considered the treatment of choice, but for larger or malignant masses, an open procedure is recommended. The risk of perioperative morbidity and death can be reduced by adequate medical management. With successful surgical resection, the long-term prognosis is favorable.
A 61-year-old man with type 2 diabetes mellitus on glimepiride therapy presented with somnolence and slurred speech. His capillary glucose level was 17 mg/dL and his serum glucose level was 28 mg/dL. He was treated with intravenous dextrose, and his glucose level promptly returned to normal.
He had been adherent to his medication regimen and denied overmedicating or accidental overdosing. Over the past 7 months, he had noted redness on his palms, a rash on his legs, intermittent moderate to severe headaches, weight loss, and decreased appetite. In addition, his blood pressure had been labile, which his physicians had attributed to autonomic instability. He had continued on the same dose of glimepiride despite losing weight.
His history included multivessel coronary artery disease treated with angioplasty and placement of multiple coronary stents; ischemic cardiomyopathy with a left ventricular ejection fraction of 28%; implantation of a cardioverter-defibrillator for secondary prevention of ventricular arrhythmia; an ischemic stroke; and multiple sclerosis complicated by bilateral blindness, with optic nerve involvement and autonomic instability, present for over a year and manifested by labile blood pressure. He was a long-time tobacco user. His daily medications included ticagrelor 90 mg, aspirin 81 mg, metoprolol 50 mg, ramipril 10 mg, simvastatin 20 mg, glimepiride 2 mg, and esomeprazole 40 mg. He needed help taking his medications.
At the time of hospital admission, his heart rate was 69 beats per minute with a regular rhythm, blood pressure 115/73 mm Hg, respiratory rate 11 breaths per minute with an oxygen saturation of 99% on room air, and oral temperature 34.7°C (94.5°F). He appeared to be in no distress.
Cardiovascular examination revealed no murmurs or gallops; there was mild nonpitting edema of the lower extremities. Pulmonary, abdominal, and neurologic examinations were unrevealing except for bilateral blindness. Vascular examination revealed no bruits. Results of a complete blood cell count and metabolic panel were normal except for a hemoglobin level of 9.9 g/dL (reference range 13.5–17.5) and a platelet count of 477 × 109/L (150–450).
Although he continued to receive the same medications he had been taking at home, his blood pressure fluctuated. On the second hospital day, it reached 186/135 mm Hg, at which time he also had palpitations, dyspnea, and crackles in the lower lobes of both lungs. Volume resuscitation on admission was suspected to have played a role, and he received furosemide, which improved his symptoms. But several hours later, his blood pressure rose again, and he became diaphoretic. Despite aggressive treatment with different antihypertensive agents, his blood pressure remained high and his symptoms persisted. Chest radiography showed no evidence of pulmonary edema. Because of his progressive dyspnea, the diagnosis of pulmonary embolism was entertained.
CAUSES OF RESISTANT HYPERTENSION
1. What could explain this patient’s high blood pressure?
- A drug effect
- Renovascular disease
- Excess circulating catecholamines
- Obstructive sleep apnea
- Primary aldosteronism
Sympathomimetic drugs such as epinephrine, norepinephrine, dopamine, and vasopressin, which are used when hemodynamic support is required, can raise both systolic and diastolic blood pressure. Nonsteroidal anti-inflammatory drugs and nasal decongestants are common culprits in the community. However, our patient was using none of these drugs.
Renovascular disease is one of many causes of resistant hypertension, accounting for 8% of all cases.1,2 Despite fluctuations, the blood pressure often remains chronically elevated, its changes are less paroxysmal than in our patient, and a precipitating factor such as a dietary indiscretion is sometimes identified.1
Excess circulating catecholamines can be a result of stress, exogenous administration, or endogenous oversecretion. Our patient’s clinical presentation is highly suspicious for a high-catecholamine state, and this should be further evaluated.
Obstructive sleep apnea is common in patients with resistant hypertension, with an estimated prevalence as high as 60% in this group.3,4
Primary aldosteronism has an estimated prevalence of about 20% in patients evaluated for resistant hypertension.5
AN ADRENAL MASS IS INCIDENTALLY DISCOVERED
Computed tomographic angiography of the chest revealed no evidence of pulmonary emboli. There was mild dilation of the central pulmonary arteries and an incidental, incompletely imaged 4.7-by-3.4-cm mass of mixed attenuation in the right adrenal gland, with macroscopic fat within the lesion.
Computed tomography (CT) of the abdomen with dedicated cuts through the adrenal glands revealed a 4.7-cm heterogeneous right adrenal mass with a density of 34 Hounsfield units (HU). The left adrenal gland appeared diffusely enlarged without a discretely seen mass, consistent with hyperplasticity (Figure 1).
2. Based on the patient’s clinical presentation and findings on CT, what would be the most likely diagnosis for this incidentally found adrenal mass?
- Adrenocortical adenoma
- Adrenocortical carcinoma
- Metastatic mass
- Pheochromocytoma
Adrenocortical adenoma can present as a small homogeneous mass of variable size, with smooth margins, and rarely containing hemorrhagic tissue or calcifications. The typical density on nonenhanced CT is less than 10 HU. On enhanced CT, it is nonvascular. T2-weighted magnetic resonance imaging (MRI) shows a lesion of the same intensity as liver tissue.6
Adrenocortical adenoma is not classically associated with autologous activity and thus is less likely to explain our patient’s symptoms.
Adrenocortical carcinoma can present as a large heterogeneous mass, usually greater than 4 cm in diameter, with irregular margins and areas of necrosis, hemorrhage, or calcification. The typical density on nonenhanced CT is greater than 10 HU. On enhanced CT, the mass is usually vascular, and T2-weighted MRI will show a lesion more intense than liver tissue.6
Adrenocortical carcinoma is also not classically associated with autologous activity, and so is not likely to explain our patient’s symptoms.6
Metastatic disease can present with masses of variable size, often bilaterally, and occasionally with cysts or areas of hemorrhage. The typical density of metastatic lesions on nonenhanced CT is greater than 10 HU. On enhanced CT, they are usually vascular, and on T2-weighted MRI they are hyperintense.6 The characteristics of the mass and the absence of a primary malignancy on CT of the chest and abdomen do not support the diagnosis of metastatic disease.
Pheochromocytoma is a neuroendocrine tumor of the adrenal medulla that can present as a large heterogeneous mass, greater than 3 cm in diameter, with clear margins and cysts or areas of hemorrhage. Extra-adrenal neuroendocrine tumors are typically called paragangliomas and have features similar to those of pheochromocytoma. The typical density of pheochromocytoma on nonenhanced CT is greater than 10 HU. On enhanced CT, it is usually vascular, and T2-weighted MRI shows a hyperintense lesion. Pheochromocytoma can be biochemically active and thus can cause signs and symptoms that will lead to the diagnosis.6
Other imaging tests may play a role in the evaluation of adrenal masses but are not required for the diagnosis of pheochromocytoma. Functional positron emission tomography using metaiodobenzylguanidine labeled with iodine 123 or-iodine 131 or using the glucose analogue F-18 fluorodeoxyglucose has been used in the initial assessment of pheochromocytoma, with good sensitivity and specificity.7,8
Our patient’s pacemaker-defibrillator precluded him from undergoing MRI.
DIAGNOSIS: PHEOCHROMOCYTOMA
Pheochromocytoma was highly suspected on the basis of the patient’s clinical presentation, and metoprolol was immediately discontinued. He was started on the calcium channel blocker verapamil and the alpha-blocker phenoxybenzamine.
Serum samples were obtained to measure metanephrines, dehydroepiandrosterone, aldosterone, and cortisol, and a 24-hour urine collection was obtained to measure creatinine, dopamine, epinephrine, norepinephrine, cortisol, and metanephrines. Based on the results (Table 1) and on the findings on imaging, the patient was diagnosed with pheochromocytoma. A surgical consultation was obtained, and surgery was recommended.
WHEN DOES PHEOCHROMOCYTOMA CALL FOR SURGERY?
3. Which criterion is most important when determining the need for surgery for pheochromocytoma?
- Findings on fine-needle aspiration biopsy
- Biochemical activity
- Size of the mass
- Bilateral masses
Fine-needle aspiration biopsy can be done when a mass is found incidentally and no evidence of biochemical activity is detected, although it is not an essential part of the diagnostic workup.9 In most cases, the sampling from fine-needle aspiration is not sufficient to achieve a diagnosis.
Biochemical activity is the most important factor when determining the need for prompt surgical intervention. The excess circulating catecholamines have been associated with increased risk of cardiovascular morbidity and death independent of the morbidity associated with hypertension alone.10 Biochemical activity can be independent of the size of the mass, but larger masses typically present with symptoms.
Bilateral masses have been associated with metastatic disease.11 In retrospect, the patient’s history of hypertension and cerebrovascular accident could be associated with the development of a catecholamine-releasing tumor.
A GOOD OUTCOME FROM SURGERY
The patient was continued on phenoxybenzamine for 7 days and responded well to this therapy.
After this preoperative preparation, he underwent laparoscopic right adrenalectomy with excision of a retroperitoneal adrenal mass. His postoperative course was complicated by transient hypotension requiring low-dose vasopressin support for less than 24 hours. He was then restarted on his previous dosage of metoprolol and was discharged home on postoperative day 5 with stable blood pressure. Follow-up 24-hour urine collection 1 month after he was discharged showed normalization of metanephrine, normetanephrine, epinephrine, and norepinephrine levels.
Despite low suspicion for an underlying genetic syndrome, he was referred for genetic testing and was scheduled to have a repeat 24-hour urine collection and imaging in 6 months to follow his enlarged left adrenal gland, which did not appear to be metabolically hyperactive.
4. What is the most common perioperative complication of pheochromocytoma excision?
- Hypoglycemia
- Hypotension
- Hypocortisolism
- Hypertension
- Tachycardia
Hypoglycemia has been observed after removal of pheochromocytoma, as levels of catecholamines (which normally inhibit pancreatic beta cells) decrease and insulin secretion consequently increases.12 Our patient developed hypoglycemia before surgery, not after, and it was likely due to the combination of his antidiabetic therapy, weight loss, and decreased oral intake.
Hypotension is the most common complication in the perioperative period. It is associated with excessive loss of catecholamine secretion. It is usually short-lived but may require aggressive administration of intravenous fluids and use of sympathomimetic agents.
Hypocortisolism is unlikely in patients with pheochromocytoma, but it is likely after removal of adrenocortical adenoma.
Hypertension and tachycardia affect up to 40% of pheochromocytoma patients in some case series.12
PHEOCHROMOCYTOMA: A CATECHOLAMINE-SECRETING TUMOR
The pathophysiology of pheochromocytoma is complex. It is characterized by accelerated growth of cells producing catecholamines, which may produce symptoms when secreted into the bloodstream. The classic triad of symptoms is headache, hypertension, and hyperglycemia, although our patient had very low blood sugar levels. Other common symptoms are nausea, orthostasis, and tremor, although not all symptoms are invariably seen.
Genetic testing recommended
Genetic associations have been described and are thought to be responsible for 20% to 30% of cases of pheochromocytoma. All associated germline mutations are autosomal dominant, some with variable penetrance. These include:
- Succinate dehydrogenase subunit B, C, and D mutations
- von Hippel-Lindau syndrome
- Multiple endocrine neoplasia type 1 and type 2 syndromes
- Neurofibromatosis type 1.13,14
The succinate dehydrogenase subunit mutations have been associated with, but not limited to, extra-adrenal adenomas (paragangliomas) and carry a worse prognosis.
Some experts recommend genetic testing in all cases of pheochromocytoma, sporadic or familial, and this testing should be followed by counseling if a mutation is found.15 Others recommend genetic testing based on the patient’s age (under age 50), history, imaging, and biochemical features of the tumor (metanephrines predominate in multiple endocrine neoplasia syndromes, and normetanephrines in von Hippel-Lindau syndrome).13
Serious consequences
A thorough evaluation is recommended, since pheochromocytoma has been associated with increased cardiovascular morbidity. In a retrospective series, Stolk et al10 reported that patients with pheochromocytoma had a higher incidence of myocardial infarction, angina, and stroke in the years preceding the diagnosis than did patients with essential hypertension (13.8% vs 1.1%, P < .001).10
Catecholamine cardiomyopathy has been described and shares clinical features with Takotsubo or stress cardiomyopathy, with global left ventricular systolic and diastolic dysfunction that improve or resolve after the adrenergic insult is removed.16
Conditions that warrant further evaluation or that may suggest pheochromocytoma are malignant hypertension, hypertensive encephalopathy, ischemic stroke, subarachnoid hemorrhage, acute pulmonary edema, angina pectoris, myocardial infarction, aortic dissection, and kidney injury.
When to suspect pheochromocytoma
Pheochromocytoma should be suspected in a patient with resistant hypertension, family history, or imaging findings that suggest an adrenal mass with a heterogeneous appearance. The diagnostic algorithm follows the same pathway as for the evaluation of an incidentally found adrenal mass, with determination of its dimension and characteristics by CT or MRI, and with biochemical testing of urine catecholamines, plasma free metanephrines, renin, aldosterone, and cortisol.
The diagnosis of pheochromocytoma is established by obtaining fractionated metanephrines and catecholamines in a 24-hour urine collection (sensitivity 90%, specificity 98%). Analysis of plasma metanephrines has a higher sensitivity (97%) but lower specificity (85%).17 The combination of typical signs, symptoms, and laboratory findings makes the diagnosis likely, especially in combination with a unilateral adrenal mass.
Laparoscopic surgery after medical preparation for active tumors
If the mass appears benign and not biochemically hyperactive, then follow-up at 1 year is recommended, with repeat testing. Surgical evaluation and intervention is recommended for lesions that appear malignant or that are biochemically active and clinically symptomatic.9
Preoperative hemodynamic control is essential in the management of pheochromocytoma to prevent or minimize hemodynamic changes that can be driven by increased catecholamines. Control is typically achieved with initial alpha-blockade and then beta-blockade to avoid worsening hypertension and to prevent an acute hypertensive crisis during surgical intervention. Phenoxybenzamine, the mainstay of therapy, is a nonselective alpha-blocker with a long duration of action that requires titration over several days up to 3 weeks.
A selective alpha-1-blocker such as doxazosin can be used to control postoperative hypotension, as it has a shorter half-life than phenoxybenzamine. Alternative strategies include calcium channel blockers, centrally acting sympathetic blockers, and magnesium.18
Laparoscopic adrenalectomy by an experienced surgeon after excellent medical preparation is often considered the treatment of choice, but for larger or malignant masses, an open procedure is recommended. The risk of perioperative morbidity and death can be reduced by adequate medical management. With successful surgical resection, the long-term prognosis is favorable.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2008; 51:1403–1419.
- Kumar N, Calhoun DA, Dudenbostel T. Management of patients with resistant hypertension: current treatment options. Integr Blood Press Control 2013; 6:139–151.
- Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension 2011; 58:811–817.
- Marcus JA, Pothineni A, Marcus CZ, Bisognano JD. The role of obesity and obstructive sleep apnea in the pathogenesis and treatment of resistant hypertension. Curr Hypertens Rep 2014; 16:411.
- Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB, Weissmann P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension 2002; 40:892–896.
- Young WF Clinical practice. The incidentally discovered adrenal mass. N Engl J Med 2007; 356:601–610.
- Lin M, Wong V, Yap J, Jin R, Leong P, Campbell P. FDG PET in the evaluation of phaeochromocytoma: a correlative study with MIBG scintigraphy and Ki-67 proliferative index. Clin Imaging 2013; 37:1084–1088.
- Raja A, Leung K, Stamm M, Girgis S, Low G. Multimodality imaging findings of pheochromocytoma with associated clinical and biochemical features in 53 patients with histologically confirmed tumors. AJR Am J Roentgenol 2013; 201:825–833.
- Nieman LK. Approach to the patient with an adrenal incidentaloma. J Clin Endocrinol Metab 2010; 95:4106–4113.
- Stolk RF, Bakx C, Mulder J, Timmers HJ, Lenders JW. Is the excess cardiovascular morbidity in pheochromocytoma related to blood pressure or to catecholamines? J Clin Endocrinol Metab 2013; 98:1100–1106.
- Grumbach MM, Biller BM, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (‘incidentaloma’). Ann Intern Med 2003; 138:424–429.
- Lentschener C, Gaujoux S, Tesniere A, Dousset B. Point of controversy: perioperative care of patients undergoing pheochromocytoma removal—time for a reappraisal? Eur J Endocrinol 2011; 165:365–373.
- Bryant J, Farmer J, Kessler LJ, Townsend RR, Nathanson KL. Pheochromocytoma: the expanding genetic differential diagnosis. J Natl Cancer Inst 2003; 95:1196–1204.
- Lee P, Leonard J. Textbook on endocrinology. BMJ 1994; 308:1512.
- Fishbein L, Merrill S, Fraker DL, Cohen DL, Nathanson KL. Inherited mutations in pheochromocytoma and paraganglioma: why all patients should be offered genetic testing. Ann Surg Oncol 2013; 20:1444–1450.
- Agarwal G, Sadacharan D, Kapoor A, et al. Cardiovascular dysfunction and catecholamine cardiomyopathy in pheochromocytoma patients and their reversal following surgical cure: results of a prospective case-control study. Surgery 2011; 150:1202–1211.
- Sawka AM, Jaeschke R, Singh RJ, Young WF A comparison of biochemical tests for pheochromocytoma: measurement of fractionated plasma metanephrines compared with the combination of 24-hour urinary metanephrines and catecholamines. J Clin Endocrinol Metab 2003; 88:553–558.
- Domi R, Laho H. Management of pheochromocytoma: old ideas and new drugs. Niger J Clin Pract 2012; 15:253–257.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2008; 51:1403–1419.
- Kumar N, Calhoun DA, Dudenbostel T. Management of patients with resistant hypertension: current treatment options. Integr Blood Press Control 2013; 6:139–151.
- Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension 2011; 58:811–817.
- Marcus JA, Pothineni A, Marcus CZ, Bisognano JD. The role of obesity and obstructive sleep apnea in the pathogenesis and treatment of resistant hypertension. Curr Hypertens Rep 2014; 16:411.
- Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB, Weissmann P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension 2002; 40:892–896.
- Young WF Clinical practice. The incidentally discovered adrenal mass. N Engl J Med 2007; 356:601–610.
- Lin M, Wong V, Yap J, Jin R, Leong P, Campbell P. FDG PET in the evaluation of phaeochromocytoma: a correlative study with MIBG scintigraphy and Ki-67 proliferative index. Clin Imaging 2013; 37:1084–1088.
- Raja A, Leung K, Stamm M, Girgis S, Low G. Multimodality imaging findings of pheochromocytoma with associated clinical and biochemical features in 53 patients with histologically confirmed tumors. AJR Am J Roentgenol 2013; 201:825–833.
- Nieman LK. Approach to the patient with an adrenal incidentaloma. J Clin Endocrinol Metab 2010; 95:4106–4113.
- Stolk RF, Bakx C, Mulder J, Timmers HJ, Lenders JW. Is the excess cardiovascular morbidity in pheochromocytoma related to blood pressure or to catecholamines? J Clin Endocrinol Metab 2013; 98:1100–1106.
- Grumbach MM, Biller BM, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (‘incidentaloma’). Ann Intern Med 2003; 138:424–429.
- Lentschener C, Gaujoux S, Tesniere A, Dousset B. Point of controversy: perioperative care of patients undergoing pheochromocytoma removal—time for a reappraisal? Eur J Endocrinol 2011; 165:365–373.
- Bryant J, Farmer J, Kessler LJ, Townsend RR, Nathanson KL. Pheochromocytoma: the expanding genetic differential diagnosis. J Natl Cancer Inst 2003; 95:1196–1204.
- Lee P, Leonard J. Textbook on endocrinology. BMJ 1994; 308:1512.
- Fishbein L, Merrill S, Fraker DL, Cohen DL, Nathanson KL. Inherited mutations in pheochromocytoma and paraganglioma: why all patients should be offered genetic testing. Ann Surg Oncol 2013; 20:1444–1450.
- Agarwal G, Sadacharan D, Kapoor A, et al. Cardiovascular dysfunction and catecholamine cardiomyopathy in pheochromocytoma patients and their reversal following surgical cure: results of a prospective case-control study. Surgery 2011; 150:1202–1211.
- Sawka AM, Jaeschke R, Singh RJ, Young WF A comparison of biochemical tests for pheochromocytoma: measurement of fractionated plasma metanephrines compared with the combination of 24-hour urinary metanephrines and catecholamines. J Clin Endocrinol Metab 2003; 88:553–558.
- Domi R, Laho H. Management of pheochromocytoma: old ideas and new drugs. Niger J Clin Pract 2012; 15:253–257.
Why are we doing cardiovascular outcome trials in type 2 diabetes?
A 50-year-old man with hypertension presents to the internal medicine clinic. He has been an active smoker for 15 years and smokes 1 pack of cigarettes a day. He was recently diagnosed with type 2 diabetes mellitus after routine blood work revealed his hemoglobin A1c level was elevated at 7.5%. He has no current complaints but is concerned about his future risk of a heart attack or stroke.
THE BURDEN OF DIABETES MELLITUS
The prevalence of diabetes mellitus in US adults (age > 20) has tripled during the last 30 years to 28.9 million, or 12% of the population in this age group.1 Globally, 382 million people had a diagnosis of diabetes in 2013, and with the increasing prevalence of obesity and adoption of a Western diet, this number is expected to escalate to 592 million by 2035.2
HOW GREAT IS THE CARDIOVASCULAR RISK IN PEOPLE WITH DIABETES?
Diabetes mellitus is linked to a twofold increase in the risk of adverse cardiovascular events even after adjusting for risk from hypertension and smoking.3 In early studies, diabetic people with no history of myocardial infarction were shown to have a lifetime risk of infarction similar to that in nondiabetic people who had already had an infarction,4 thus establishing diabetes as a “coronary artery disease equivalent.” Middle-aged men diagnosed with diabetes lose an average of 6 years of life and women lose 7 years compared with those without diabetes, with cardiovascular morbidity contributing to more than half of this reduction in life expectancy (Figure 1).5
Numerous mechanisms have been hypothesized to account for the association between diabetes and cardiovascular risk, including increased inflammation, endothelial and platelet dysfunction, and autonomic dysregulation.6
Can we modify cardiovascular risk in patients with diabetes?
Although fasting blood glucose levels strongly correlate with future cardiovascular risk, whether lowering blood glucose levels with medications will reduce cardiovascular risk has been uncertain.3 Lowering glucose beyond what is current standard practice has not been shown to significantly improve cardiovascular outcomes or mortality rates, and it comes at the price of an increased risk of hypoglycemic events.
No macrovascular benefit from lowering hemoglobin A1c beyond the standard of care
UKPDS.7 Launched in 1977, the United Kingdom Prospective Diabetes Study was designed to investigate whether intensive blood glucose control reduces the risk of macrovascular and microvascular complications in type 2 diabetes. The study randomized nearly 4,000 patients newly diagnosed with diabetes to intensive treatment (with a sulfonylurea or insulin to keep fasting blood glucose levels below 110 mg/dL) or to conventional treatment (with diet alone unless hyperglycemic symptoms or a fasting blood glucose more than 270 mg/dL arose) for 10 years.
Multivariate analysis from the overall study population revealed a direct correlation between hemoglobin A1c levels and adverse cardiovascular events. Higher hemoglobin A1c was associated with markedly more:
- Fatal and nonfatal myocardial infarctions (14% increased risk for every 1% rise in hemoglobin A1c)
- Fatal and nonfatal strokes (12% increased risk per 1% rise in hemoglobin A1c)
- Amputations or deaths from peripheral vascular disease (43% increase per 1% rise)
- Heart failure (16% increase per 1% rise).
While intensive therapy was associated with significant reductions in microvascular events (retinopathy and proteinuria), there was no significant difference in the incidence of major macrovascular events (myocardial infarction or stroke).
The mean hemoglobin A1c level at the end of the study was about 8% in the standard-treatment group and about 7% in the intensive-treatment group. Were these levels low enough to yield a significant risk reduction? Since lower hemoglobin A1c levels are associated with lower risk of myocardial infarction, it seemed reasonable to do further studies with more intensive treatment to further lower hemoglobin A1c goals.
ADVANCE.8 The Action in Diabetes and Vascular Disease trial randomized more than 11,000 participants with type 2 diabetes to either usual care or intensive therapy with a goal of achieving a hemoglobin A1c of 6.5% or less. During 5 years of follow-up, the usual-care group averaged a hemoglobin A1c of 7.3%, compared with 6.5% in the intensive-treatment group.
No significant differences between the two groups were observed in the incidence of major macrovascular events, including myocardial infarction, stroke, or death from any cause. The intensive-treatment group had fewer major microvascular events, with most of the benefit being in the form of a lower incidence of proteinuria, and with no significant effect on retinopathy. Severe hypoglycemia, although uncommon, was more frequent in the intensive-treatment group.
ACCORD.9 The Action to Control Cardiovascular Risk in Diabetes trial went one step further. This trial randomized more than 10,000 patients with type 2 diabetes to receive either intensive therapy (targeting hemoglobin A1c ≤ 6.0%) or standard therapy (hemoglobin A1c 7.0%–7.9%). At 1 year, the mean hemoglobin A1c levels were stable at 6.4% in the intensive-therapy group and 7.5% in the standard-therapy group.
The trial was stopped at 3.5 years because of a higher rate of death in the intensive-therapy group, with a hazard ratio of 1.22, predominantly from an increase in adverse cardiovascular events. The intensive-therapy group also had a significantly higher incidence of hypoglycemia.
VADT.10 The Veterans Affairs Diabetes Trial randomized 1,791 patients with type 2 diabetes who had a suboptimal response to conventional therapy to receive intensive therapy aimed at reducing hemoglobin A1c by 1.5 percentage points or standard therapy. After a follow-up of 5.6 years, median hemoglobin A1c levels were 8.4% in the standard-therapy group and 6.9% in the intensive-therapy group. No differences were found between the two groups in the incidence of major cardiovascular events, death, or microvascular complications, with the exception of a lower rate of progression of albuminuria in the intensive-therapy group. The rates of adverse events, primarily hypoglycemia, were higher in the intensive-therapy group.
Based on these negative trials and concern about potential harm with intensive glucose-lowering strategies, standard guidelines continue to recommend moderate glucose-lowering strategies for patients with diabetes. The guidelines broadly recommend targeting a hemoglobin A1c of 7% or less while advocating a less ambitious goal of lower than 7.5% or 8.0% in older patients who may be prone to hypoglycemia.11
STRATEGIES TO REDUCE CARDIOVASCULAR RISK IN DIABETES
While the incidence of diabetes mellitus has risen alarmingly, the incidence of cardiovascular complications of diabetes has declined over the years. Lowering blood glucose has not been the critical factor mediating this risk reduction. In addition to smoking cessation, proven measures that clearly reduce long-term cardiovascular risk in diabetes are blood pressure control and reduction in low-density lipoprotein cholesterol with statins.
Lower the blood pressure to less than 140 mm Hg
ADVANCE.12 In the ADVANCE trial, in addition to being randomized to usual vs intensive glucose-lowering therapy, participants were also simultaneously randomized to receive either placebo or the combination of an angiotensin-converting enzyme inhibitor and a diuretic (ie, perindopril and indapamide). Blood pressure was reduced by a mean of 5.6 mm Hg systolic and 2.2 mm Hg diastolic in the active-treatment group. This moderate reduction in blood pressure was associated with an 18% relative risk reduction in death from cardiovascular disease and a 14% relative risk reduction in death from any cause.
The ACCORD trial13 lowered systolic blood pressure even more in the intensive-treatment group, with a target systolic blood pressure of less than 120 mm Hg compared with less than 140 mm Hg in the control group. Intensive therapy did not prove to significantly reduce the risk of major cardiovascular events and was associated with a significantly higher rate of serious adverse events.
Therefore, while antihypertensive therapy lowered the mortality rate in diabetic patients, lowering blood pressure beyond conventional blood pressure targets did not decrease the risk more. The latest hypertension treatment guidelines (from the eighth Joint National Committee) emphasize a blood pressure goal of 140/90 mm Hg or less in adults with diabetes.14
Prescribe a statin regardless of the baseline lipid level
The Collaborative Atorvastatin Diabetes Study (CARDS) randomized nearly 3,000 patients with type 2 diabetes mellitus and no history of cardiovascular disease to either atorvastatin 10 mg or placebo regardless of cholesterol status. The trial was terminated 2 years early because a prespecified efficacy end point had already been met: the treatment group experienced a markedly lower incidence of major cardiovascular events, including stroke.15
A large meta-analysis of randomized trials of statins noted a 9% reduction in all-cause mortality (relative risk [RR] 0.91, 99% confidence interval 0.82–1.01; P = .02) per mmol/L reduction in low-density lipoprotein cholesterol in patients with diabetes mellitus.16 Use of statins also led to significant reductions in rates of major coronary events (RR 0.78), coronary revascularization (RR 0.75), and stroke (RR 0.79).
The latest American College of Cardiology/American Heart Association guidelines endorse moderate-intensity or high-intensity statin treatment in patients with diabetes who are over age 40.17
Encourage smoking cessation
Smoking increases the lifetime risk of developing type 2 diabetes.18 It is also associated with premature development of microvascular and macrovascular complications of diabetes,19 and it leads to increased mortality risk in people with diabetes mellitus in a dose-dependent manner.20 Therefore, smoking cessation remains paramount in reducing cardiovascular risk, and patients should be encouraged to quit as soon as possible.
Role of antiplatelet agents
Use of antiplatelet drugs such as aspirin for primary prevention of cardiovascular disease in patients with diabetes is controversial. Initial studies showed a potential reduction in the incidence of myocardial infarction in men and stroke in women with diabetes with low-dose aspirin.21,22 Subsequent randomized trials and meta-analyses, however, yielded contrasting results, showing no benefit in cardiovascular risk reduction and potential risk of bleeding and other gastrointestinal adverse effects.23,24
The US Food and Drug Administration (FDA) has not approved aspirin for primary prevention of cardiovascular disease in people who have no history of cardiovascular disease. In contrast, the American Heart Association and the American Diabetes Association endorse low-dose aspirin (75–162 mg/day) for adults with diabetes and no history of vascular disease who are at increased cardiovascular risk (estimated 10-year risk of events > 10%) and who are not at increased risk of bleeding.
In the absence of a clear consensus and given the lack of randomized data, the role of aspirin in patients with diabetes remains controversial.
WHAT IS THE ROLE OF STRESS TESTING IN ASYMPTOMATIC DIABETIC PATIENTS?
People with diabetes also have a high incidence of silent (asymptomatic) ischemia that potentially leads to worse outcomes.25 Whether screening for silent ischemia improves outcomes in these patients is debatable.
The Detection of Anemia in Asymptomatic Diabetics (DIAD) trial randomized more than 1,000 asymptomatic diabetic participants to either screening for coronary artery disease with stress testing or no screening.26 Over a 5-year follow-up, there was no significant difference in the incidence of myocardial infarction and death from cardiac causes.
The guidelines remain divided on this clinical conundrum. While the American Diabetes Association recommends against routine screening for coronary artery disease in asymptomatic patients with diabetes, the American College of Cardiology/American Heart Association guidelines recommend screening with radionuclide imaging in patients with diabetes and a high risk of coronary artery disease.27
ROLE OF REVASCULARIZATION IN DIABETIC PATIENTS WITH STABLE CORONARY ARTERY DISEASE
Patients with coronary artery disease and diabetes fare worse than those without diabetes, despite revascularization by coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI).28
The choice of CABG or PCI as the preferred revascularization strategy was recently studied in the Future Revascularization Evaluation in Patients With DM: Optimal Management of Multivessel Disease (FREEDOM) trial.29 This study randomized 1,900 patients with diabetes and multivessel coronary artery disease to revascularization with PCI or CABG. After 5 years, there was a significantly lower rate of death and myocardial infarction with CABG than with PCI.
The role of revascularization in patients with diabetes and stable coronary artery disease has also been questioned. The Bypass Angioplasty Revascularization Investigation 2 DM (BARI-2D) randomized 2,368 patients with diabetes and stable coronary artery disease to undergo revascularization (PCI or CABG) or to receive intensive medical therapy alone.30 At 5 years, there was no significant difference in the rates of death and major cardiovascular events between patients undergoing revascularization and those undergoing medical therapy alone. Subgroup analysis revealed a potential benefit with CABG over medical therapy in patients with more extensive coronary artery disease.31
CAN DIABETES THERAPY CAUSE HARM?
New diabetes drugs must show no cardiovascular harm
Several drugs that were approved purely on the basis of their potential to reduce blood glucose were reevaluated for impact on adverse cardiovascular outcomes.
Muraglitazar is a peroxisome proliferator-activated receptor agonist that was shown in phase 2 and 3 studies to dramatically lower triglyceride levels in a dose-dependent fashion while raising high-density lipoprotein levels and being neutral to low-density lipoprotein levels. It also lowers blood glucose. The FDA Advisory Committee voted to approve its use for type 2 diabetes based on phase 2 trial data. But soon after, a meta-analysis revealed that the drug was associated with more than twice the incidence of cardiovascular complications and death than standard therapy.32 Further development of this drug subsequently ceased.
A similar meta-analysis was performed on rosiglitazone, a drug that has been available since 1997 and had been used by millions of patients. Rosiglitazone was also found to be associated with a significantly increased risk of cardiovascular death, as well as death from all causes.33
In light of these findings, the FDA in 2008 issued new guidelines to the diabetes drug development industry. Henceforth, new diabetes drugs must not only lower blood glucose, they must also be shown in a large clinical trial not to increase cardiovascular risk.
Current trials will provide critical information
Numerous trials are now under way to assess cardiovascular outcomes with promising new diabetes drugs. Tens of thousands of patients are involved in trials testing dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide-1 agonists, sodium-glucose-linked transporter-2 agents, and a GPR40 agonist. Because of the change in guidelines, results over the next decade should reveal much more about the impact of lowering blood glucose on heart disease than we learned in the previous century.
Two apparently neutral but clinically relevant trials recently examined cardiovascular outcomes associated with diabetes drugs.
EXAMINE.34 The Examination of Cardiovascular Outcomes Study of Alogliptin Versus Standard of Care study randomized 5,380 patients with type 2 diabetes and a recent acute coronary syndrome event (acute myocardial infarction or unstable angina requiring hospitalization) to receive either alogliptin (a DPP-4 inhibitor) or placebo in addition to existing standard diabetes and cardiovascular therapy. Patients were followed for up to 40 months (median 18 months). Hemoglobin A1c levels were significantly lower with alogliptin than with placebo, but the time to the primary end point of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke was not significantly different between the two groups.
SAVOR.35 The Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with DM (SAVOR–TIMI 53) trial randomized more than 16,000 patients with established cardiovascular disease or multiple risk factors to either the DPP-4 inhibitor saxagliptin or placebo. The patients’ physicians were permitted to adjust all other medications, including standard diabetes medications. The median treatment period was just over 2 years. Similar to EXAMINE, this study found no difference between the two groups in the primary end point of cardiovascular death, myocardial infarction, or ischemic stroke, even though glycemic control was better in the saxagliptin group.
Thus, both drugs were shown not to increase cardiovascular risk, an FDA criterion for drug marketing and approval.
CONTROL MODIFIABLE RISK FACTORS
There has been an alarming rise in the incidence of diabetes and obesity throughout the world. Cardiovascular disease remains the leading cause of death in patients with diabetes. While elevated blood glucose in diabetic patients leads to increased cardiovascular risk, efforts to reduce blood glucose to euglycemic levels may not lead to a reduction in this risk and may even cause harm.
Success in cardiovascular risk reduction in addition to glucose-lowering remains the holy grail in the development of new diabetes drugs. But in the meantime, aggressive control of other modifiable risk factors such as hypertension, smoking, and hyperlipidemia remains critical to reducing cardiovascular risk in diabetic patients.
- Centers for Disease Control and Prevention. National diabetes statistics report. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed September 30, 2014.
- International Diabetes Federation. IDF Diabetes Atlas, 6th edition. Brussels: International Diabetes Federation, 2013.
- Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010; 375:2215–2222.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Seshasai SR, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011; 364:829–841.
- Hess K, Marx N, Lehrke M. Cardiovascular disease and diabetes: the vulnerable patient. Eur Heart J Suppl 2012; 14(suppl B):B4–B13.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007; 370:829–840.
- Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee. JAMA 2014; 311:507–520.
- Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–696.
- Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008; 371:117–125.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: synopsis of the 2013 ACC/AHA cholesterol guideline. Ann Intern Med 2014; 160:339–343.
- Benjamin RM. A report of the Surgeon General. How tobacco smoke causes disease...what it means to you. www.cdc.gov/tobacco/data_statistics/sgr/2010/consumer_booklet/pdfs/consumer.pdf. Accessed September 30, 2014.
- Haire-Joshu D, Glasgow RE, Tibbs TL. Smoking and diabetes. Diabetes Care 1999; 22:1887–1898.
- Chaturvedi N, Stevens L, Fuller JH. Which features of smoking determine mortality risk in former cigarette smokers with diabetes? The World Health Organization Multinational Study Group. Diabetes Care 1997; 20:1266–1272.
- ETDRS Investigators. Aspirin effects on mortality and morbidity in patients with diabetes mellitus. Early Treatment Diabetic Retinopathy Study report 14. JAMA 1992; 268:1292–1300.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008; 337:a1840.
- Simpson SH, Gamble JM, Mereu L, Chambers T. Effect of aspirin dose on mortality and cardiovascular events in people with diabetes: a meta-analysis. J Gen Intern Med 2011; 26:1336–1344.
- Janand-Delenne B, Savin B, Habib G, Bory M, Vague P, Lassmann-Vague V. Silent myocardial ischemia in patients with diabetes: who to screen. Diabetes Care 1999; 22:1396–1400.
- Young LH, Wackers FJ, Chyun DA, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 2009; 301:1547–1555.
- Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010; 56:e50–e103.
- Roffi M, Angiolillo DJ, Kappetein AP. Current concepts on coronary revascularization in diabetic patients. Eur Heart J 2011; 32:2748–2757.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009; 360:2503–2515.
- Chaitman BR, Hardison RM, Adler D, et al. The Bypass Angioplasty Revascularization Investigation 2 Diabetes randomized trial of different treatment strategies in type 2 diabetes mellitus with stable ischemic heart disease: impact of treatment strategy on cardiac mortality and myocardial infarction. Circulation 2009; 120:2529–2540.
- Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA 2005; 294:2581–2586.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356:2457–2471.
- White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369:1327–1335.
- Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369:1317–1326.
A 50-year-old man with hypertension presents to the internal medicine clinic. He has been an active smoker for 15 years and smokes 1 pack of cigarettes a day. He was recently diagnosed with type 2 diabetes mellitus after routine blood work revealed his hemoglobin A1c level was elevated at 7.5%. He has no current complaints but is concerned about his future risk of a heart attack or stroke.
THE BURDEN OF DIABETES MELLITUS
The prevalence of diabetes mellitus in US adults (age > 20) has tripled during the last 30 years to 28.9 million, or 12% of the population in this age group.1 Globally, 382 million people had a diagnosis of diabetes in 2013, and with the increasing prevalence of obesity and adoption of a Western diet, this number is expected to escalate to 592 million by 2035.2
HOW GREAT IS THE CARDIOVASCULAR RISK IN PEOPLE WITH DIABETES?
Diabetes mellitus is linked to a twofold increase in the risk of adverse cardiovascular events even after adjusting for risk from hypertension and smoking.3 In early studies, diabetic people with no history of myocardial infarction were shown to have a lifetime risk of infarction similar to that in nondiabetic people who had already had an infarction,4 thus establishing diabetes as a “coronary artery disease equivalent.” Middle-aged men diagnosed with diabetes lose an average of 6 years of life and women lose 7 years compared with those without diabetes, with cardiovascular morbidity contributing to more than half of this reduction in life expectancy (Figure 1).5
Numerous mechanisms have been hypothesized to account for the association between diabetes and cardiovascular risk, including increased inflammation, endothelial and platelet dysfunction, and autonomic dysregulation.6
Can we modify cardiovascular risk in patients with diabetes?
Although fasting blood glucose levels strongly correlate with future cardiovascular risk, whether lowering blood glucose levels with medications will reduce cardiovascular risk has been uncertain.3 Lowering glucose beyond what is current standard practice has not been shown to significantly improve cardiovascular outcomes or mortality rates, and it comes at the price of an increased risk of hypoglycemic events.
No macrovascular benefit from lowering hemoglobin A1c beyond the standard of care
UKPDS.7 Launched in 1977, the United Kingdom Prospective Diabetes Study was designed to investigate whether intensive blood glucose control reduces the risk of macrovascular and microvascular complications in type 2 diabetes. The study randomized nearly 4,000 patients newly diagnosed with diabetes to intensive treatment (with a sulfonylurea or insulin to keep fasting blood glucose levels below 110 mg/dL) or to conventional treatment (with diet alone unless hyperglycemic symptoms or a fasting blood glucose more than 270 mg/dL arose) for 10 years.
Multivariate analysis from the overall study population revealed a direct correlation between hemoglobin A1c levels and adverse cardiovascular events. Higher hemoglobin A1c was associated with markedly more:
- Fatal and nonfatal myocardial infarctions (14% increased risk for every 1% rise in hemoglobin A1c)
- Fatal and nonfatal strokes (12% increased risk per 1% rise in hemoglobin A1c)
- Amputations or deaths from peripheral vascular disease (43% increase per 1% rise)
- Heart failure (16% increase per 1% rise).
While intensive therapy was associated with significant reductions in microvascular events (retinopathy and proteinuria), there was no significant difference in the incidence of major macrovascular events (myocardial infarction or stroke).
The mean hemoglobin A1c level at the end of the study was about 8% in the standard-treatment group and about 7% in the intensive-treatment group. Were these levels low enough to yield a significant risk reduction? Since lower hemoglobin A1c levels are associated with lower risk of myocardial infarction, it seemed reasonable to do further studies with more intensive treatment to further lower hemoglobin A1c goals.
ADVANCE.8 The Action in Diabetes and Vascular Disease trial randomized more than 11,000 participants with type 2 diabetes to either usual care or intensive therapy with a goal of achieving a hemoglobin A1c of 6.5% or less. During 5 years of follow-up, the usual-care group averaged a hemoglobin A1c of 7.3%, compared with 6.5% in the intensive-treatment group.
No significant differences between the two groups were observed in the incidence of major macrovascular events, including myocardial infarction, stroke, or death from any cause. The intensive-treatment group had fewer major microvascular events, with most of the benefit being in the form of a lower incidence of proteinuria, and with no significant effect on retinopathy. Severe hypoglycemia, although uncommon, was more frequent in the intensive-treatment group.
ACCORD.9 The Action to Control Cardiovascular Risk in Diabetes trial went one step further. This trial randomized more than 10,000 patients with type 2 diabetes to receive either intensive therapy (targeting hemoglobin A1c ≤ 6.0%) or standard therapy (hemoglobin A1c 7.0%–7.9%). At 1 year, the mean hemoglobin A1c levels were stable at 6.4% in the intensive-therapy group and 7.5% in the standard-therapy group.
The trial was stopped at 3.5 years because of a higher rate of death in the intensive-therapy group, with a hazard ratio of 1.22, predominantly from an increase in adverse cardiovascular events. The intensive-therapy group also had a significantly higher incidence of hypoglycemia.
VADT.10 The Veterans Affairs Diabetes Trial randomized 1,791 patients with type 2 diabetes who had a suboptimal response to conventional therapy to receive intensive therapy aimed at reducing hemoglobin A1c by 1.5 percentage points or standard therapy. After a follow-up of 5.6 years, median hemoglobin A1c levels were 8.4% in the standard-therapy group and 6.9% in the intensive-therapy group. No differences were found between the two groups in the incidence of major cardiovascular events, death, or microvascular complications, with the exception of a lower rate of progression of albuminuria in the intensive-therapy group. The rates of adverse events, primarily hypoglycemia, were higher in the intensive-therapy group.
Based on these negative trials and concern about potential harm with intensive glucose-lowering strategies, standard guidelines continue to recommend moderate glucose-lowering strategies for patients with diabetes. The guidelines broadly recommend targeting a hemoglobin A1c of 7% or less while advocating a less ambitious goal of lower than 7.5% or 8.0% in older patients who may be prone to hypoglycemia.11
STRATEGIES TO REDUCE CARDIOVASCULAR RISK IN DIABETES
While the incidence of diabetes mellitus has risen alarmingly, the incidence of cardiovascular complications of diabetes has declined over the years. Lowering blood glucose has not been the critical factor mediating this risk reduction. In addition to smoking cessation, proven measures that clearly reduce long-term cardiovascular risk in diabetes are blood pressure control and reduction in low-density lipoprotein cholesterol with statins.
Lower the blood pressure to less than 140 mm Hg
ADVANCE.12 In the ADVANCE trial, in addition to being randomized to usual vs intensive glucose-lowering therapy, participants were also simultaneously randomized to receive either placebo or the combination of an angiotensin-converting enzyme inhibitor and a diuretic (ie, perindopril and indapamide). Blood pressure was reduced by a mean of 5.6 mm Hg systolic and 2.2 mm Hg diastolic in the active-treatment group. This moderate reduction in blood pressure was associated with an 18% relative risk reduction in death from cardiovascular disease and a 14% relative risk reduction in death from any cause.
The ACCORD trial13 lowered systolic blood pressure even more in the intensive-treatment group, with a target systolic blood pressure of less than 120 mm Hg compared with less than 140 mm Hg in the control group. Intensive therapy did not prove to significantly reduce the risk of major cardiovascular events and was associated with a significantly higher rate of serious adverse events.
Therefore, while antihypertensive therapy lowered the mortality rate in diabetic patients, lowering blood pressure beyond conventional blood pressure targets did not decrease the risk more. The latest hypertension treatment guidelines (from the eighth Joint National Committee) emphasize a blood pressure goal of 140/90 mm Hg or less in adults with diabetes.14
Prescribe a statin regardless of the baseline lipid level
The Collaborative Atorvastatin Diabetes Study (CARDS) randomized nearly 3,000 patients with type 2 diabetes mellitus and no history of cardiovascular disease to either atorvastatin 10 mg or placebo regardless of cholesterol status. The trial was terminated 2 years early because a prespecified efficacy end point had already been met: the treatment group experienced a markedly lower incidence of major cardiovascular events, including stroke.15
A large meta-analysis of randomized trials of statins noted a 9% reduction in all-cause mortality (relative risk [RR] 0.91, 99% confidence interval 0.82–1.01; P = .02) per mmol/L reduction in low-density lipoprotein cholesterol in patients with diabetes mellitus.16 Use of statins also led to significant reductions in rates of major coronary events (RR 0.78), coronary revascularization (RR 0.75), and stroke (RR 0.79).
The latest American College of Cardiology/American Heart Association guidelines endorse moderate-intensity or high-intensity statin treatment in patients with diabetes who are over age 40.17
Encourage smoking cessation
Smoking increases the lifetime risk of developing type 2 diabetes.18 It is also associated with premature development of microvascular and macrovascular complications of diabetes,19 and it leads to increased mortality risk in people with diabetes mellitus in a dose-dependent manner.20 Therefore, smoking cessation remains paramount in reducing cardiovascular risk, and patients should be encouraged to quit as soon as possible.
Role of antiplatelet agents
Use of antiplatelet drugs such as aspirin for primary prevention of cardiovascular disease in patients with diabetes is controversial. Initial studies showed a potential reduction in the incidence of myocardial infarction in men and stroke in women with diabetes with low-dose aspirin.21,22 Subsequent randomized trials and meta-analyses, however, yielded contrasting results, showing no benefit in cardiovascular risk reduction and potential risk of bleeding and other gastrointestinal adverse effects.23,24
The US Food and Drug Administration (FDA) has not approved aspirin for primary prevention of cardiovascular disease in people who have no history of cardiovascular disease. In contrast, the American Heart Association and the American Diabetes Association endorse low-dose aspirin (75–162 mg/day) for adults with diabetes and no history of vascular disease who are at increased cardiovascular risk (estimated 10-year risk of events > 10%) and who are not at increased risk of bleeding.
In the absence of a clear consensus and given the lack of randomized data, the role of aspirin in patients with diabetes remains controversial.
WHAT IS THE ROLE OF STRESS TESTING IN ASYMPTOMATIC DIABETIC PATIENTS?
People with diabetes also have a high incidence of silent (asymptomatic) ischemia that potentially leads to worse outcomes.25 Whether screening for silent ischemia improves outcomes in these patients is debatable.
The Detection of Anemia in Asymptomatic Diabetics (DIAD) trial randomized more than 1,000 asymptomatic diabetic participants to either screening for coronary artery disease with stress testing or no screening.26 Over a 5-year follow-up, there was no significant difference in the incidence of myocardial infarction and death from cardiac causes.
The guidelines remain divided on this clinical conundrum. While the American Diabetes Association recommends against routine screening for coronary artery disease in asymptomatic patients with diabetes, the American College of Cardiology/American Heart Association guidelines recommend screening with radionuclide imaging in patients with diabetes and a high risk of coronary artery disease.27
ROLE OF REVASCULARIZATION IN DIABETIC PATIENTS WITH STABLE CORONARY ARTERY DISEASE
Patients with coronary artery disease and diabetes fare worse than those without diabetes, despite revascularization by coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI).28
The choice of CABG or PCI as the preferred revascularization strategy was recently studied in the Future Revascularization Evaluation in Patients With DM: Optimal Management of Multivessel Disease (FREEDOM) trial.29 This study randomized 1,900 patients with diabetes and multivessel coronary artery disease to revascularization with PCI or CABG. After 5 years, there was a significantly lower rate of death and myocardial infarction with CABG than with PCI.
The role of revascularization in patients with diabetes and stable coronary artery disease has also been questioned. The Bypass Angioplasty Revascularization Investigation 2 DM (BARI-2D) randomized 2,368 patients with diabetes and stable coronary artery disease to undergo revascularization (PCI or CABG) or to receive intensive medical therapy alone.30 At 5 years, there was no significant difference in the rates of death and major cardiovascular events between patients undergoing revascularization and those undergoing medical therapy alone. Subgroup analysis revealed a potential benefit with CABG over medical therapy in patients with more extensive coronary artery disease.31
CAN DIABETES THERAPY CAUSE HARM?
New diabetes drugs must show no cardiovascular harm
Several drugs that were approved purely on the basis of their potential to reduce blood glucose were reevaluated for impact on adverse cardiovascular outcomes.
Muraglitazar is a peroxisome proliferator-activated receptor agonist that was shown in phase 2 and 3 studies to dramatically lower triglyceride levels in a dose-dependent fashion while raising high-density lipoprotein levels and being neutral to low-density lipoprotein levels. It also lowers blood glucose. The FDA Advisory Committee voted to approve its use for type 2 diabetes based on phase 2 trial data. But soon after, a meta-analysis revealed that the drug was associated with more than twice the incidence of cardiovascular complications and death than standard therapy.32 Further development of this drug subsequently ceased.
A similar meta-analysis was performed on rosiglitazone, a drug that has been available since 1997 and had been used by millions of patients. Rosiglitazone was also found to be associated with a significantly increased risk of cardiovascular death, as well as death from all causes.33
In light of these findings, the FDA in 2008 issued new guidelines to the diabetes drug development industry. Henceforth, new diabetes drugs must not only lower blood glucose, they must also be shown in a large clinical trial not to increase cardiovascular risk.
Current trials will provide critical information
Numerous trials are now under way to assess cardiovascular outcomes with promising new diabetes drugs. Tens of thousands of patients are involved in trials testing dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide-1 agonists, sodium-glucose-linked transporter-2 agents, and a GPR40 agonist. Because of the change in guidelines, results over the next decade should reveal much more about the impact of lowering blood glucose on heart disease than we learned in the previous century.
Two apparently neutral but clinically relevant trials recently examined cardiovascular outcomes associated with diabetes drugs.
EXAMINE.34 The Examination of Cardiovascular Outcomes Study of Alogliptin Versus Standard of Care study randomized 5,380 patients with type 2 diabetes and a recent acute coronary syndrome event (acute myocardial infarction or unstable angina requiring hospitalization) to receive either alogliptin (a DPP-4 inhibitor) or placebo in addition to existing standard diabetes and cardiovascular therapy. Patients were followed for up to 40 months (median 18 months). Hemoglobin A1c levels were significantly lower with alogliptin than with placebo, but the time to the primary end point of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke was not significantly different between the two groups.
SAVOR.35 The Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with DM (SAVOR–TIMI 53) trial randomized more than 16,000 patients with established cardiovascular disease or multiple risk factors to either the DPP-4 inhibitor saxagliptin or placebo. The patients’ physicians were permitted to adjust all other medications, including standard diabetes medications. The median treatment period was just over 2 years. Similar to EXAMINE, this study found no difference between the two groups in the primary end point of cardiovascular death, myocardial infarction, or ischemic stroke, even though glycemic control was better in the saxagliptin group.
Thus, both drugs were shown not to increase cardiovascular risk, an FDA criterion for drug marketing and approval.
CONTROL MODIFIABLE RISK FACTORS
There has been an alarming rise in the incidence of diabetes and obesity throughout the world. Cardiovascular disease remains the leading cause of death in patients with diabetes. While elevated blood glucose in diabetic patients leads to increased cardiovascular risk, efforts to reduce blood glucose to euglycemic levels may not lead to a reduction in this risk and may even cause harm.
Success in cardiovascular risk reduction in addition to glucose-lowering remains the holy grail in the development of new diabetes drugs. But in the meantime, aggressive control of other modifiable risk factors such as hypertension, smoking, and hyperlipidemia remains critical to reducing cardiovascular risk in diabetic patients.
A 50-year-old man with hypertension presents to the internal medicine clinic. He has been an active smoker for 15 years and smokes 1 pack of cigarettes a day. He was recently diagnosed with type 2 diabetes mellitus after routine blood work revealed his hemoglobin A1c level was elevated at 7.5%. He has no current complaints but is concerned about his future risk of a heart attack or stroke.
THE BURDEN OF DIABETES MELLITUS
The prevalence of diabetes mellitus in US adults (age > 20) has tripled during the last 30 years to 28.9 million, or 12% of the population in this age group.1 Globally, 382 million people had a diagnosis of diabetes in 2013, and with the increasing prevalence of obesity and adoption of a Western diet, this number is expected to escalate to 592 million by 2035.2
HOW GREAT IS THE CARDIOVASCULAR RISK IN PEOPLE WITH DIABETES?
Diabetes mellitus is linked to a twofold increase in the risk of adverse cardiovascular events even after adjusting for risk from hypertension and smoking.3 In early studies, diabetic people with no history of myocardial infarction were shown to have a lifetime risk of infarction similar to that in nondiabetic people who had already had an infarction,4 thus establishing diabetes as a “coronary artery disease equivalent.” Middle-aged men diagnosed with diabetes lose an average of 6 years of life and women lose 7 years compared with those without diabetes, with cardiovascular morbidity contributing to more than half of this reduction in life expectancy (Figure 1).5
Numerous mechanisms have been hypothesized to account for the association between diabetes and cardiovascular risk, including increased inflammation, endothelial and platelet dysfunction, and autonomic dysregulation.6
Can we modify cardiovascular risk in patients with diabetes?
Although fasting blood glucose levels strongly correlate with future cardiovascular risk, whether lowering blood glucose levels with medications will reduce cardiovascular risk has been uncertain.3 Lowering glucose beyond what is current standard practice has not been shown to significantly improve cardiovascular outcomes or mortality rates, and it comes at the price of an increased risk of hypoglycemic events.
No macrovascular benefit from lowering hemoglobin A1c beyond the standard of care
UKPDS.7 Launched in 1977, the United Kingdom Prospective Diabetes Study was designed to investigate whether intensive blood glucose control reduces the risk of macrovascular and microvascular complications in type 2 diabetes. The study randomized nearly 4,000 patients newly diagnosed with diabetes to intensive treatment (with a sulfonylurea or insulin to keep fasting blood glucose levels below 110 mg/dL) or to conventional treatment (with diet alone unless hyperglycemic symptoms or a fasting blood glucose more than 270 mg/dL arose) for 10 years.
Multivariate analysis from the overall study population revealed a direct correlation between hemoglobin A1c levels and adverse cardiovascular events. Higher hemoglobin A1c was associated with markedly more:
- Fatal and nonfatal myocardial infarctions (14% increased risk for every 1% rise in hemoglobin A1c)
- Fatal and nonfatal strokes (12% increased risk per 1% rise in hemoglobin A1c)
- Amputations or deaths from peripheral vascular disease (43% increase per 1% rise)
- Heart failure (16% increase per 1% rise).
While intensive therapy was associated with significant reductions in microvascular events (retinopathy and proteinuria), there was no significant difference in the incidence of major macrovascular events (myocardial infarction or stroke).
The mean hemoglobin A1c level at the end of the study was about 8% in the standard-treatment group and about 7% in the intensive-treatment group. Were these levels low enough to yield a significant risk reduction? Since lower hemoglobin A1c levels are associated with lower risk of myocardial infarction, it seemed reasonable to do further studies with more intensive treatment to further lower hemoglobin A1c goals.
ADVANCE.8 The Action in Diabetes and Vascular Disease trial randomized more than 11,000 participants with type 2 diabetes to either usual care or intensive therapy with a goal of achieving a hemoglobin A1c of 6.5% or less. During 5 years of follow-up, the usual-care group averaged a hemoglobin A1c of 7.3%, compared with 6.5% in the intensive-treatment group.
No significant differences between the two groups were observed in the incidence of major macrovascular events, including myocardial infarction, stroke, or death from any cause. The intensive-treatment group had fewer major microvascular events, with most of the benefit being in the form of a lower incidence of proteinuria, and with no significant effect on retinopathy. Severe hypoglycemia, although uncommon, was more frequent in the intensive-treatment group.
ACCORD.9 The Action to Control Cardiovascular Risk in Diabetes trial went one step further. This trial randomized more than 10,000 patients with type 2 diabetes to receive either intensive therapy (targeting hemoglobin A1c ≤ 6.0%) or standard therapy (hemoglobin A1c 7.0%–7.9%). At 1 year, the mean hemoglobin A1c levels were stable at 6.4% in the intensive-therapy group and 7.5% in the standard-therapy group.
The trial was stopped at 3.5 years because of a higher rate of death in the intensive-therapy group, with a hazard ratio of 1.22, predominantly from an increase in adverse cardiovascular events. The intensive-therapy group also had a significantly higher incidence of hypoglycemia.
VADT.10 The Veterans Affairs Diabetes Trial randomized 1,791 patients with type 2 diabetes who had a suboptimal response to conventional therapy to receive intensive therapy aimed at reducing hemoglobin A1c by 1.5 percentage points or standard therapy. After a follow-up of 5.6 years, median hemoglobin A1c levels were 8.4% in the standard-therapy group and 6.9% in the intensive-therapy group. No differences were found between the two groups in the incidence of major cardiovascular events, death, or microvascular complications, with the exception of a lower rate of progression of albuminuria in the intensive-therapy group. The rates of adverse events, primarily hypoglycemia, were higher in the intensive-therapy group.
Based on these negative trials and concern about potential harm with intensive glucose-lowering strategies, standard guidelines continue to recommend moderate glucose-lowering strategies for patients with diabetes. The guidelines broadly recommend targeting a hemoglobin A1c of 7% or less while advocating a less ambitious goal of lower than 7.5% or 8.0% in older patients who may be prone to hypoglycemia.11
STRATEGIES TO REDUCE CARDIOVASCULAR RISK IN DIABETES
While the incidence of diabetes mellitus has risen alarmingly, the incidence of cardiovascular complications of diabetes has declined over the years. Lowering blood glucose has not been the critical factor mediating this risk reduction. In addition to smoking cessation, proven measures that clearly reduce long-term cardiovascular risk in diabetes are blood pressure control and reduction in low-density lipoprotein cholesterol with statins.
Lower the blood pressure to less than 140 mm Hg
ADVANCE.12 In the ADVANCE trial, in addition to being randomized to usual vs intensive glucose-lowering therapy, participants were also simultaneously randomized to receive either placebo or the combination of an angiotensin-converting enzyme inhibitor and a diuretic (ie, perindopril and indapamide). Blood pressure was reduced by a mean of 5.6 mm Hg systolic and 2.2 mm Hg diastolic in the active-treatment group. This moderate reduction in blood pressure was associated with an 18% relative risk reduction in death from cardiovascular disease and a 14% relative risk reduction in death from any cause.
The ACCORD trial13 lowered systolic blood pressure even more in the intensive-treatment group, with a target systolic blood pressure of less than 120 mm Hg compared with less than 140 mm Hg in the control group. Intensive therapy did not prove to significantly reduce the risk of major cardiovascular events and was associated with a significantly higher rate of serious adverse events.
Therefore, while antihypertensive therapy lowered the mortality rate in diabetic patients, lowering blood pressure beyond conventional blood pressure targets did not decrease the risk more. The latest hypertension treatment guidelines (from the eighth Joint National Committee) emphasize a blood pressure goal of 140/90 mm Hg or less in adults with diabetes.14
Prescribe a statin regardless of the baseline lipid level
The Collaborative Atorvastatin Diabetes Study (CARDS) randomized nearly 3,000 patients with type 2 diabetes mellitus and no history of cardiovascular disease to either atorvastatin 10 mg or placebo regardless of cholesterol status. The trial was terminated 2 years early because a prespecified efficacy end point had already been met: the treatment group experienced a markedly lower incidence of major cardiovascular events, including stroke.15
A large meta-analysis of randomized trials of statins noted a 9% reduction in all-cause mortality (relative risk [RR] 0.91, 99% confidence interval 0.82–1.01; P = .02) per mmol/L reduction in low-density lipoprotein cholesterol in patients with diabetes mellitus.16 Use of statins also led to significant reductions in rates of major coronary events (RR 0.78), coronary revascularization (RR 0.75), and stroke (RR 0.79).
The latest American College of Cardiology/American Heart Association guidelines endorse moderate-intensity or high-intensity statin treatment in patients with diabetes who are over age 40.17
Encourage smoking cessation
Smoking increases the lifetime risk of developing type 2 diabetes.18 It is also associated with premature development of microvascular and macrovascular complications of diabetes,19 and it leads to increased mortality risk in people with diabetes mellitus in a dose-dependent manner.20 Therefore, smoking cessation remains paramount in reducing cardiovascular risk, and patients should be encouraged to quit as soon as possible.
Role of antiplatelet agents
Use of antiplatelet drugs such as aspirin for primary prevention of cardiovascular disease in patients with diabetes is controversial. Initial studies showed a potential reduction in the incidence of myocardial infarction in men and stroke in women with diabetes with low-dose aspirin.21,22 Subsequent randomized trials and meta-analyses, however, yielded contrasting results, showing no benefit in cardiovascular risk reduction and potential risk of bleeding and other gastrointestinal adverse effects.23,24
The US Food and Drug Administration (FDA) has not approved aspirin for primary prevention of cardiovascular disease in people who have no history of cardiovascular disease. In contrast, the American Heart Association and the American Diabetes Association endorse low-dose aspirin (75–162 mg/day) for adults with diabetes and no history of vascular disease who are at increased cardiovascular risk (estimated 10-year risk of events > 10%) and who are not at increased risk of bleeding.
In the absence of a clear consensus and given the lack of randomized data, the role of aspirin in patients with diabetes remains controversial.
WHAT IS THE ROLE OF STRESS TESTING IN ASYMPTOMATIC DIABETIC PATIENTS?
People with diabetes also have a high incidence of silent (asymptomatic) ischemia that potentially leads to worse outcomes.25 Whether screening for silent ischemia improves outcomes in these patients is debatable.
The Detection of Anemia in Asymptomatic Diabetics (DIAD) trial randomized more than 1,000 asymptomatic diabetic participants to either screening for coronary artery disease with stress testing or no screening.26 Over a 5-year follow-up, there was no significant difference in the incidence of myocardial infarction and death from cardiac causes.
The guidelines remain divided on this clinical conundrum. While the American Diabetes Association recommends against routine screening for coronary artery disease in asymptomatic patients with diabetes, the American College of Cardiology/American Heart Association guidelines recommend screening with radionuclide imaging in patients with diabetes and a high risk of coronary artery disease.27
ROLE OF REVASCULARIZATION IN DIABETIC PATIENTS WITH STABLE CORONARY ARTERY DISEASE
Patients with coronary artery disease and diabetes fare worse than those without diabetes, despite revascularization by coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI).28
The choice of CABG or PCI as the preferred revascularization strategy was recently studied in the Future Revascularization Evaluation in Patients With DM: Optimal Management of Multivessel Disease (FREEDOM) trial.29 This study randomized 1,900 patients with diabetes and multivessel coronary artery disease to revascularization with PCI or CABG. After 5 years, there was a significantly lower rate of death and myocardial infarction with CABG than with PCI.
The role of revascularization in patients with diabetes and stable coronary artery disease has also been questioned. The Bypass Angioplasty Revascularization Investigation 2 DM (BARI-2D) randomized 2,368 patients with diabetes and stable coronary artery disease to undergo revascularization (PCI or CABG) or to receive intensive medical therapy alone.30 At 5 years, there was no significant difference in the rates of death and major cardiovascular events between patients undergoing revascularization and those undergoing medical therapy alone. Subgroup analysis revealed a potential benefit with CABG over medical therapy in patients with more extensive coronary artery disease.31
CAN DIABETES THERAPY CAUSE HARM?
New diabetes drugs must show no cardiovascular harm
Several drugs that were approved purely on the basis of their potential to reduce blood glucose were reevaluated for impact on adverse cardiovascular outcomes.
Muraglitazar is a peroxisome proliferator-activated receptor agonist that was shown in phase 2 and 3 studies to dramatically lower triglyceride levels in a dose-dependent fashion while raising high-density lipoprotein levels and being neutral to low-density lipoprotein levels. It also lowers blood glucose. The FDA Advisory Committee voted to approve its use for type 2 diabetes based on phase 2 trial data. But soon after, a meta-analysis revealed that the drug was associated with more than twice the incidence of cardiovascular complications and death than standard therapy.32 Further development of this drug subsequently ceased.
A similar meta-analysis was performed on rosiglitazone, a drug that has been available since 1997 and had been used by millions of patients. Rosiglitazone was also found to be associated with a significantly increased risk of cardiovascular death, as well as death from all causes.33
In light of these findings, the FDA in 2008 issued new guidelines to the diabetes drug development industry. Henceforth, new diabetes drugs must not only lower blood glucose, they must also be shown in a large clinical trial not to increase cardiovascular risk.
Current trials will provide critical information
Numerous trials are now under way to assess cardiovascular outcomes with promising new diabetes drugs. Tens of thousands of patients are involved in trials testing dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide-1 agonists, sodium-glucose-linked transporter-2 agents, and a GPR40 agonist. Because of the change in guidelines, results over the next decade should reveal much more about the impact of lowering blood glucose on heart disease than we learned in the previous century.
Two apparently neutral but clinically relevant trials recently examined cardiovascular outcomes associated with diabetes drugs.
EXAMINE.34 The Examination of Cardiovascular Outcomes Study of Alogliptin Versus Standard of Care study randomized 5,380 patients with type 2 diabetes and a recent acute coronary syndrome event (acute myocardial infarction or unstable angina requiring hospitalization) to receive either alogliptin (a DPP-4 inhibitor) or placebo in addition to existing standard diabetes and cardiovascular therapy. Patients were followed for up to 40 months (median 18 months). Hemoglobin A1c levels were significantly lower with alogliptin than with placebo, but the time to the primary end point of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke was not significantly different between the two groups.
SAVOR.35 The Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with DM (SAVOR–TIMI 53) trial randomized more than 16,000 patients with established cardiovascular disease or multiple risk factors to either the DPP-4 inhibitor saxagliptin or placebo. The patients’ physicians were permitted to adjust all other medications, including standard diabetes medications. The median treatment period was just over 2 years. Similar to EXAMINE, this study found no difference between the two groups in the primary end point of cardiovascular death, myocardial infarction, or ischemic stroke, even though glycemic control was better in the saxagliptin group.
Thus, both drugs were shown not to increase cardiovascular risk, an FDA criterion for drug marketing and approval.
CONTROL MODIFIABLE RISK FACTORS
There has been an alarming rise in the incidence of diabetes and obesity throughout the world. Cardiovascular disease remains the leading cause of death in patients with diabetes. While elevated blood glucose in diabetic patients leads to increased cardiovascular risk, efforts to reduce blood glucose to euglycemic levels may not lead to a reduction in this risk and may even cause harm.
Success in cardiovascular risk reduction in addition to glucose-lowering remains the holy grail in the development of new diabetes drugs. But in the meantime, aggressive control of other modifiable risk factors such as hypertension, smoking, and hyperlipidemia remains critical to reducing cardiovascular risk in diabetic patients.
- Centers for Disease Control and Prevention. National diabetes statistics report. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed September 30, 2014.
- International Diabetes Federation. IDF Diabetes Atlas, 6th edition. Brussels: International Diabetes Federation, 2013.
- Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010; 375:2215–2222.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Seshasai SR, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011; 364:829–841.
- Hess K, Marx N, Lehrke M. Cardiovascular disease and diabetes: the vulnerable patient. Eur Heart J Suppl 2012; 14(suppl B):B4–B13.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007; 370:829–840.
- Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee. JAMA 2014; 311:507–520.
- Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–696.
- Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008; 371:117–125.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: synopsis of the 2013 ACC/AHA cholesterol guideline. Ann Intern Med 2014; 160:339–343.
- Benjamin RM. A report of the Surgeon General. How tobacco smoke causes disease...what it means to you. www.cdc.gov/tobacco/data_statistics/sgr/2010/consumer_booklet/pdfs/consumer.pdf. Accessed September 30, 2014.
- Haire-Joshu D, Glasgow RE, Tibbs TL. Smoking and diabetes. Diabetes Care 1999; 22:1887–1898.
- Chaturvedi N, Stevens L, Fuller JH. Which features of smoking determine mortality risk in former cigarette smokers with diabetes? The World Health Organization Multinational Study Group. Diabetes Care 1997; 20:1266–1272.
- ETDRS Investigators. Aspirin effects on mortality and morbidity in patients with diabetes mellitus. Early Treatment Diabetic Retinopathy Study report 14. JAMA 1992; 268:1292–1300.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008; 337:a1840.
- Simpson SH, Gamble JM, Mereu L, Chambers T. Effect of aspirin dose on mortality and cardiovascular events in people with diabetes: a meta-analysis. J Gen Intern Med 2011; 26:1336–1344.
- Janand-Delenne B, Savin B, Habib G, Bory M, Vague P, Lassmann-Vague V. Silent myocardial ischemia in patients with diabetes: who to screen. Diabetes Care 1999; 22:1396–1400.
- Young LH, Wackers FJ, Chyun DA, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 2009; 301:1547–1555.
- Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010; 56:e50–e103.
- Roffi M, Angiolillo DJ, Kappetein AP. Current concepts on coronary revascularization in diabetic patients. Eur Heart J 2011; 32:2748–2757.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009; 360:2503–2515.
- Chaitman BR, Hardison RM, Adler D, et al. The Bypass Angioplasty Revascularization Investigation 2 Diabetes randomized trial of different treatment strategies in type 2 diabetes mellitus with stable ischemic heart disease: impact of treatment strategy on cardiac mortality and myocardial infarction. Circulation 2009; 120:2529–2540.
- Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA 2005; 294:2581–2586.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356:2457–2471.
- White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369:1327–1335.
- Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369:1317–1326.
- Centers for Disease Control and Prevention. National diabetes statistics report. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed September 30, 2014.
- International Diabetes Federation. IDF Diabetes Atlas, 6th edition. Brussels: International Diabetes Federation, 2013.
- Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010; 375:2215–2222.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Seshasai SR, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011; 364:829–841.
- Hess K, Marx N, Lehrke M. Cardiovascular disease and diabetes: the vulnerable patient. Eur Heart J Suppl 2012; 14(suppl B):B4–B13.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007; 370:829–840.
- Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee. JAMA 2014; 311:507–520.
- Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–696.
- Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008; 371:117–125.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: synopsis of the 2013 ACC/AHA cholesterol guideline. Ann Intern Med 2014; 160:339–343.
- Benjamin RM. A report of the Surgeon General. How tobacco smoke causes disease...what it means to you. www.cdc.gov/tobacco/data_statistics/sgr/2010/consumer_booklet/pdfs/consumer.pdf. Accessed September 30, 2014.
- Haire-Joshu D, Glasgow RE, Tibbs TL. Smoking and diabetes. Diabetes Care 1999; 22:1887–1898.
- Chaturvedi N, Stevens L, Fuller JH. Which features of smoking determine mortality risk in former cigarette smokers with diabetes? The World Health Organization Multinational Study Group. Diabetes Care 1997; 20:1266–1272.
- ETDRS Investigators. Aspirin effects on mortality and morbidity in patients with diabetes mellitus. Early Treatment Diabetic Retinopathy Study report 14. JAMA 1992; 268:1292–1300.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008; 337:a1840.
- Simpson SH, Gamble JM, Mereu L, Chambers T. Effect of aspirin dose on mortality and cardiovascular events in people with diabetes: a meta-analysis. J Gen Intern Med 2011; 26:1336–1344.
- Janand-Delenne B, Savin B, Habib G, Bory M, Vague P, Lassmann-Vague V. Silent myocardial ischemia in patients with diabetes: who to screen. Diabetes Care 1999; 22:1396–1400.
- Young LH, Wackers FJ, Chyun DA, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 2009; 301:1547–1555.
- Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010; 56:e50–e103.
- Roffi M, Angiolillo DJ, Kappetein AP. Current concepts on coronary revascularization in diabetic patients. Eur Heart J 2011; 32:2748–2757.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009; 360:2503–2515.
- Chaitman BR, Hardison RM, Adler D, et al. The Bypass Angioplasty Revascularization Investigation 2 Diabetes randomized trial of different treatment strategies in type 2 diabetes mellitus with stable ischemic heart disease: impact of treatment strategy on cardiac mortality and myocardial infarction. Circulation 2009; 120:2529–2540.
- Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA 2005; 294:2581–2586.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356:2457–2471.
- White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369:1327–1335.
- Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369:1317–1326.
KEY POINTS
- The worldwide burden of type 2 diabetes is increasing dramatically as obesity rates increase, populations age, and people around the world adopt a Western diet.
- Diabetes increases the risk of atherosclerotic cardiovascular disease, which remains the leading cause of death in diabetic patients.
- Lowering blood glucose alone may not necessarily amount to reduction in adverse cardiovascular events.
- Clinical trials of new drugs for type 2 diabetes must prove cardiovascular safety in addition to glucose-lowering potential before the drugs gain final regulatory approval.
- Aggressive risk factor modification (smoking cessation, control of hypertension, and treatment of hyperlipidemia with statins) remains paramount in reducing cardiovascular risk in people with diabetes.
Diabetes management: More than just cardiovascular risk?
Diabetes mellitus and its management have become the center of controversy in recent years. More emphasis is being placed on the potential for adverse cardiovascular outcomes with more aggressive glycemic control as well as on the potential for adverse cardiovascular events with newer antidiabetic therapies, and less on the importance of glycemic control, particularly early in the disease course.
Although it is important to take new data into consideration when managing diabetes, it appears that the results of recent clinical trials are being misinterpreted and incorrectly applied to the wrong patient populations, and in the process, the results of older landmark clinical trials are being neglected. Inadequate glycemic control not only plays a role in cardiovascular risk, it also remains the leading cause of blindness, kidney failure, and nontraumatic lower-limb amputations in the United States.1
Although we need to recognize the potential for adverse cardiovascular outcomes with diabetes and its management, we cannot lose sight of the big picture—ie, that inadequate glycemic control confers both microvascular and macrovascular risk, and that the available data show that restoring near-euglycemia in patients with diabetes considerably reduces the risk of microvascular and macrovascular complications.
Several recently published clinical trials—the Action to Control Cardiovascular Risk in Diabetes (ACCORD),2 the Veterans Affairs Diabetes Trial (VADT),3 and the Action in Diabetes and Vascular Disease (ADVANCE)4—failed to demonstrate improved cardiovascular outcomes with improved glycemic control. However, we should not take this to mean that glycemic control is unimportant.
In this article, we will discuss why the results of these recent clinical trials are not valid for the general population of patients with diabetes. We will review evidence from landmark clinical trials that clearly demonstrates that better glycemic control reduces both microvascular and macrovascular complications of diabetes (the “glucose hypothesis”). We contend that excellent glycemic control clearly decreases the microvascular complications of diabetes, and that results from long-term follow-up studies in both type 1 and type 2 diabetes show reduced rates of heart attack and stroke in patients treated intensively earlier in the course of their disease.5,6
EVIDENCE FOR THE GLUCOSE HYPOTHESIS
Diabetes Control and Complications Trial
The first major trial demonstrating that improved glycemic control provides benefit was the Diabetes Control and Complications Trial (DCCT).7 This study enrolled 1,441 patients with insulin-dependent diabetes mellitus, 726 of whom had no retinopathy at baseline (the primary-prevention cohort) and 715 of whom had mild retinopathy (the secondary-intervention cohort).
Patients were randomly assigned to intensive therapy (three or more insulin injections per day or an insulin pump) or to conventional therapy with one or two daily insulin injections. They were followed for a mean of 6.5 years, and the appearance and progression of retinopathy and other complications were assessed regularly.
During the study, the hemoglobin A1c level averaged 9% in the control group and 7% in the intensively treated group. The cumulative incidence of retinopathy was defined as a change of three steps or more on fundus photography that was sustained over a 6-month period.
Effect on retinopathy. At study completion, the cumulative incidence of retinopathy in the intensive-therapy group was approximately 50% less than in the conventional-therapy group. Intensive therapy reduced the adjusted mean risk of retinopathy by 76% (95% confidence interval [CI] 62%–85%) in the primary-prevention cohort. In the secondary-prevention cohort, intensive therapy reduced the average risk of progression by 54% (95% CI 39%–66%). Intensive therapy reduced the adjusted risk of proliferative or severe nonproliferative retinopathy by 47% (P = .011) and that of treatment with photocoagulation by 56% (P = .002).
Effect on nephropathy. Intensive therapy reduced the mean adjusted risk of microalbuminuria by 34% (P = .04) in the primary-prevention cohort and by 43% (P = .001) in the secondary-intervention cohort. The risk of macroalbuminuria was reduced by 56% (P = .01) in the secondary-intervention cohort.
Effect on neuropathy. In the patients in the primary-prevention cohort who did not have neuropathy at baseline, intensive therapy reduced the incidence of neuropathy at 5 years by 69% (to 3%, vs 10% in the conventional-therapy group; P = .006). Similarly, in the secondary-intervention cohort, intensive therapy reduced the incidence of clinical neuropathy at 5 years by 57% (to 7%, vs 16%; P < .001).
Effect on macrovascular events. In the initial trial, a nonsignificant 41% reduction in combined cardiovascular and peripheral vascular disease events was observed.
DCCT long-term follow-up
After DCCT concluded, the control and treatment groups’ hemoglobin A1c levels converged to approximately 8%. The two groups were then followed to determine the long-term effects of their prior separation of glycemic levels on micro- and macrovascular out comes.5 More than 90% of the original DCCT patients were followed for a mean of 17 years.
Intensive treatment reduced the risk of any cardiovascular disease event by 42% (95% CI 9%–63%; P = .02) and the risk of nonfatal myocardial infarction, stroke, or death from cardiovascular disease by 57% (95% CI 12%– 79%; P = .02). This result was observed despite separation of glucose control in the two groups only for the first 6.5 years. This beneficial effect of intensive early glycemic control has been termed metabolic memory.
United Kingdom Prospective Diabetes Study
A second major trial, the United Kingdom Prospective Diabetes Study (UKPDS),8 assessed the effect of excellent diabetes control on diabetes complications in patients with type 2 diabetes. A total of 3,867 patients newly diagnosed with type 2 diabetes, median age 54, who after 3 months of diet treatment had mean fasting plasma glucose concentrations of 110 to 270 mg/dL, were randomly assigned to an intensive policy (with a sulfonylurea or insulin or, if overweight, metformin) or a conventional policy with diet. The aim in the intensive group was a fasting plasma glucose less than 108 mg/dL. In the conventional group, the aim was the best achievable fasting plasma glucose with diet alone; drugs were added only if there were hyperglycemic symptoms or a fasting plasma glucose greater than 270 mg/dL.
Over 10 years, the median hemoglobin A1c level was 7.0% (interquartile range 6.2%–8.2%) in the intensive group compared with 7.9% (6.9%–8.8%) in the conventional group. Compared with the conventional group, the risk of any diabetes-related end point was 12% lower in the intensive group (95% CI 1%–21%, P = .029), the risk of any diabetes-related death was 10% lower (−11% to 27%, P = .34), and the rate of all-cause mortality was 6% lower (−10% to 20%, P = .44). Most of the reduction in risk of any diabetes-related end point was from a 25% risk reduction (95% CI 7%–40%, P = .0099) in microvascular end points, including the need for retinal photocoagulation.
UKPDS long-term follow-up
In 2008, Holman et al published the results of long-term follow-up of patients included in the UKPDS.6 In posttrial monitoring, 3,277 patients were asked to attend annual UKPDS clinics for 5 years, but no attempts were made to maintain their previously assigned therapies. Annual questionnaires were used to follow patients who were unable to attend the clinics, and all patients in years 6 to 10 were assessed through questionnaires.
Between-group differences in hemoglobin A1c levels were lost after the first year. However, in the sulfonylurea-insulin group, relative reductions in risk persisted at 10 years for any diabetes-related end point (9%, P = .04) and microvascular disease (24%, P = .001), while risk reductions for myocardial infarction (15%, P = .01) and death from any cause (13%, P = .007) emerged over time as more events occurred. In the metformin group, significant risk reductions persisted for any diabetes-related end point (21%, P = .01), myocardial infarction (33%, P = .005), and death from any cause (27%, P = .002).
The long-term follow-up to the UKPDS, like the long-term follow-up to the DCCT, demonstrated metabolic memory: that is, despite an early loss of glycemic differences after completion of the trial, a continued reduction in microvascular risk and an emergent risk reduction for myocardial infarction and death from any cause were observed.
These long-term randomized prospective trials in patients with type 1 and type 2 diabetes clearly show that the glucose hypothesis is in fact correct: intensive glucose control lowers the risk of both microvascular and macrovascular complications of diabetes.
IS THERE DISCORDANCE BETWEEN OLDER AND MORE RECENT TRIALS?
If the results of these older landmark clinical trials are true, why did the more recent clinical trials fail to show cardiovascular benefit with stricter glycemic control, and in one trial2 demonstrate the potential for harm? (ACCORD2 found an increased death rate in patients who received intensive therapy, targeting a hemoglobin A1c below 6.0%.)
The answer lies in the populations studied. ACCORD,2 VADT,3 and ADVANCE4 were performed in older patients with prior cardiac events or with several risk factors for cardiovascular events. The study populations were picked to increase the number of cardiac events in a short time frame. Therefore, extrapolating the results of these studies to the younger population of patients with diabetes, most of whom have yet to develop macrovascular disease, is inappropriate.
The available evidence suggests that early aggressive management of diabetes reduces the risk of macrovascular disease, but that this benefit is delayed. In the UKPDS and DCCT trials, it took 10 to 17 years to show cardiac benefit in younger patients.
The results of ACCORD,2 VADT,3 and ADVANCE4 are important when considered in the correct clinical context. Two of these trials did demonstrate some microvascular benefit as a result of better glycemic control in older patients, many of whom had longstanding diabetes. These studies suggest that, in patients who already have established cardiovascular disease or have several risk factors for cardiovascular events, a less-strict glycemic target may be warranted.
These trials should not be interpreted as saying that glycemic control is unimportant in older patients at higher risk. Rather, they suggest that an individualized approach to diabetes management, supported by the most recent American Diabetes Association guidelines,9 is more appropriate.
Physicians may reasonably suggest a stricter A1c goal (ie, < 6.5%) in certain patients if it can be achieved without significant hypoglycemia. Stricter glycemic targets would seem appropriate in patients recently diagnosed with diabetes, those who have a long life expectancy, and those who have not yet developed significant cardiovascular disease.9
However, in patients who already have developed advanced microvascular and macrovascular complications, who have long-standing diabetes, who have a history of severe hypoglycemia (or hypoglycemia unawareness), or who have a limited life expectancy or numerous adverse comorbidities, a less strict glycemic target (hemoglobin A1c < 8%) may be more appropriate.9
CARDIOVASCULAR RISK, HYPOGLYCEMIA, AND ATTAINING GLYCEMIC TARGETS
Metformin, in the absence of contraindications or intolerability, is generally the recommended first-line therapy to manage glycemia in patients with type 2 diabetes mellitus.10,11 However, there are only limited data to direct clinicians as to which antidiabetic medication to use if further therapy is required to obtain glycemic control.
Much of the cardiovascular and mortality risk associated with aggressive diabetes management (ie, lower A1c targets) is related to hypoglycemia. Thus, antidiabetic therapies that pose no risk or only a low risk of hypoglycemia should be chosen, particularly in older patients and in those with known cardiovascular disease. This may allow for better glycemic control without the risk of hypoglycemia and adverse cardiovascular outcomes.
However, in practice, clinicians continue to use a sulfonylurea as the second-line agent. Although sulfonylureas may appear to be a great option because of their low cost, they are associated with a higher risk of hypoglycemic episodes than other classes of diabetes drugs. We need to consider the frequency and cost of hypoglycemic episodes and the potential morbidity associated with them, because these episodes are a barrier to our efforts to achieve better glycemic control.
Budnitz et al12 reported that from 2007 through 2009, in US adults age 65 and older, insulins were implicated in 13.9% of hospitalizations related to adverse drug events, and oral hypoglycemic agents (ie, insulin secretagogues) in 10.7%.
Quilliam et al13 reported that hypoglycemia resulted in a mean cost of $17,564 for an inpatient admission, $1,387 for an emergency department visit, and $394 for an outpatient visit. Thus, the cost savings associated with prescribing a sulfonylurea vs one of the newer oral antidiabetic agents that do not increase the risk of hypoglycemia (unless used concurrently with insulin or an insulin secretagogue) can quickly be eroded by severe hypoglycemic episodes requiring medical care.
Moreover, once patients start to experience hypoglycemic episodes, they are very reluctant, as are their physicians, to intensify therapy, even if it is indicated by their elevated A1c.
There are now seven classes of oral antidiabetic therapies other than insulin secretagogues (ie, other than sulfonylureas and the meglitinides nateglinide and repaglinide), as well as a few noninsulin injectable therapies (glucagon-like peptide-1 agonists), that are not associated with hypoglycemia. We believe these agents should be tried before prescribing an agent that carries the risk of hypoglycemia (ie, sulfonylureas).
If agents that do not cause hypoglycemia are used, more-aggressive glycemic targets may be achieved safely. The ACCORD study,2 which included patients at high cardiovascular risk and aimed at an aggressive glycemic target of 6%, may have yielded much different results had agents that carry a high risk of hypoglycemia been excluded.
Of importance, cardiovascular risk is also influenced by the common comorbidities seen in patients with diabetes, such as hypertension and hypercholesterolemia. Intensive, multifactorial interventions that address not only glycemic control but also blood pressure and lipids and that include low-dose aspirin therapy have been shown to lower the risk of death from cardiovascular causes and the risk of cardiovascular events.14 Likewise, smoking cessation is very important in reducing cardiovascular risk, especially in patients with diabetes.15
CLINICAL TRIALS IN CONTEXT
In conclusion, there is more to diabetes management than cardiovascular complications. Clearly, improved glycemic control decreases the risk of retinopathy, nephropathy, and neuropathy in patients with type 1 and type 2 diabetes. The DCCT and UKPDS extension studies further found that excellent glycemic control decreases rates of cardiac events.
The best way to treat diabetes may be different in otherwise healthy younger patients who have yet to develop significant complications than it is in older patients known to have cardiovascular disease or several risk factors for cardiovascular events. The available evidence suggests it would be reasonable to aim for stricter glycemic targets in the younger patients and less stringent targets in the older patients, particularly in those with long-standing diabetes who have already developed significant micro- and macrovascular complications.
We should interpret clinical trials within their narrow clinical context, emphasizing the actual population of patients included in the study, so as to avoid the inappropriate extrapolation of the results to all.
- Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011. www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed October 7, 2014.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(Suppl 1):S11–S66.
- Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al; American Association of Clinical Endocrinologists. AACE comprehensive diabetes management algorithm 2013. Endocr Pract 2013; 19:327–336.
- Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med 2011; 365:2002–2012.
- Quilliam BJ, Simeone JC, Ozbay AB, Kogut SJ. The incidence and costs of hypoglycemia in type 2 diabetes. Am J Manag Care 2011; 17:673–680.
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358:580–591.
- Chaturvedi N, Stevens L, Fuller JH. Which features of smoking determine mortality risk in former cigarette smokers with diabetes? The World Health Organization Multinational Study Group. Diabetes Care 1997; 20:1266–1272.
Diabetes mellitus and its management have become the center of controversy in recent years. More emphasis is being placed on the potential for adverse cardiovascular outcomes with more aggressive glycemic control as well as on the potential for adverse cardiovascular events with newer antidiabetic therapies, and less on the importance of glycemic control, particularly early in the disease course.
Although it is important to take new data into consideration when managing diabetes, it appears that the results of recent clinical trials are being misinterpreted and incorrectly applied to the wrong patient populations, and in the process, the results of older landmark clinical trials are being neglected. Inadequate glycemic control not only plays a role in cardiovascular risk, it also remains the leading cause of blindness, kidney failure, and nontraumatic lower-limb amputations in the United States.1
Although we need to recognize the potential for adverse cardiovascular outcomes with diabetes and its management, we cannot lose sight of the big picture—ie, that inadequate glycemic control confers both microvascular and macrovascular risk, and that the available data show that restoring near-euglycemia in patients with diabetes considerably reduces the risk of microvascular and macrovascular complications.
Several recently published clinical trials—the Action to Control Cardiovascular Risk in Diabetes (ACCORD),2 the Veterans Affairs Diabetes Trial (VADT),3 and the Action in Diabetes and Vascular Disease (ADVANCE)4—failed to demonstrate improved cardiovascular outcomes with improved glycemic control. However, we should not take this to mean that glycemic control is unimportant.
In this article, we will discuss why the results of these recent clinical trials are not valid for the general population of patients with diabetes. We will review evidence from landmark clinical trials that clearly demonstrates that better glycemic control reduces both microvascular and macrovascular complications of diabetes (the “glucose hypothesis”). We contend that excellent glycemic control clearly decreases the microvascular complications of diabetes, and that results from long-term follow-up studies in both type 1 and type 2 diabetes show reduced rates of heart attack and stroke in patients treated intensively earlier in the course of their disease.5,6
EVIDENCE FOR THE GLUCOSE HYPOTHESIS
Diabetes Control and Complications Trial
The first major trial demonstrating that improved glycemic control provides benefit was the Diabetes Control and Complications Trial (DCCT).7 This study enrolled 1,441 patients with insulin-dependent diabetes mellitus, 726 of whom had no retinopathy at baseline (the primary-prevention cohort) and 715 of whom had mild retinopathy (the secondary-intervention cohort).
Patients were randomly assigned to intensive therapy (three or more insulin injections per day or an insulin pump) or to conventional therapy with one or two daily insulin injections. They were followed for a mean of 6.5 years, and the appearance and progression of retinopathy and other complications were assessed regularly.
During the study, the hemoglobin A1c level averaged 9% in the control group and 7% in the intensively treated group. The cumulative incidence of retinopathy was defined as a change of three steps or more on fundus photography that was sustained over a 6-month period.
Effect on retinopathy. At study completion, the cumulative incidence of retinopathy in the intensive-therapy group was approximately 50% less than in the conventional-therapy group. Intensive therapy reduced the adjusted mean risk of retinopathy by 76% (95% confidence interval [CI] 62%–85%) in the primary-prevention cohort. In the secondary-prevention cohort, intensive therapy reduced the average risk of progression by 54% (95% CI 39%–66%). Intensive therapy reduced the adjusted risk of proliferative or severe nonproliferative retinopathy by 47% (P = .011) and that of treatment with photocoagulation by 56% (P = .002).
Effect on nephropathy. Intensive therapy reduced the mean adjusted risk of microalbuminuria by 34% (P = .04) in the primary-prevention cohort and by 43% (P = .001) in the secondary-intervention cohort. The risk of macroalbuminuria was reduced by 56% (P = .01) in the secondary-intervention cohort.
Effect on neuropathy. In the patients in the primary-prevention cohort who did not have neuropathy at baseline, intensive therapy reduced the incidence of neuropathy at 5 years by 69% (to 3%, vs 10% in the conventional-therapy group; P = .006). Similarly, in the secondary-intervention cohort, intensive therapy reduced the incidence of clinical neuropathy at 5 years by 57% (to 7%, vs 16%; P < .001).
Effect on macrovascular events. In the initial trial, a nonsignificant 41% reduction in combined cardiovascular and peripheral vascular disease events was observed.
DCCT long-term follow-up
After DCCT concluded, the control and treatment groups’ hemoglobin A1c levels converged to approximately 8%. The two groups were then followed to determine the long-term effects of their prior separation of glycemic levels on micro- and macrovascular out comes.5 More than 90% of the original DCCT patients were followed for a mean of 17 years.
Intensive treatment reduced the risk of any cardiovascular disease event by 42% (95% CI 9%–63%; P = .02) and the risk of nonfatal myocardial infarction, stroke, or death from cardiovascular disease by 57% (95% CI 12%– 79%; P = .02). This result was observed despite separation of glucose control in the two groups only for the first 6.5 years. This beneficial effect of intensive early glycemic control has been termed metabolic memory.
United Kingdom Prospective Diabetes Study
A second major trial, the United Kingdom Prospective Diabetes Study (UKPDS),8 assessed the effect of excellent diabetes control on diabetes complications in patients with type 2 diabetes. A total of 3,867 patients newly diagnosed with type 2 diabetes, median age 54, who after 3 months of diet treatment had mean fasting plasma glucose concentrations of 110 to 270 mg/dL, were randomly assigned to an intensive policy (with a sulfonylurea or insulin or, if overweight, metformin) or a conventional policy with diet. The aim in the intensive group was a fasting plasma glucose less than 108 mg/dL. In the conventional group, the aim was the best achievable fasting plasma glucose with diet alone; drugs were added only if there were hyperglycemic symptoms or a fasting plasma glucose greater than 270 mg/dL.
Over 10 years, the median hemoglobin A1c level was 7.0% (interquartile range 6.2%–8.2%) in the intensive group compared with 7.9% (6.9%–8.8%) in the conventional group. Compared with the conventional group, the risk of any diabetes-related end point was 12% lower in the intensive group (95% CI 1%–21%, P = .029), the risk of any diabetes-related death was 10% lower (−11% to 27%, P = .34), and the rate of all-cause mortality was 6% lower (−10% to 20%, P = .44). Most of the reduction in risk of any diabetes-related end point was from a 25% risk reduction (95% CI 7%–40%, P = .0099) in microvascular end points, including the need for retinal photocoagulation.
UKPDS long-term follow-up
In 2008, Holman et al published the results of long-term follow-up of patients included in the UKPDS.6 In posttrial monitoring, 3,277 patients were asked to attend annual UKPDS clinics for 5 years, but no attempts were made to maintain their previously assigned therapies. Annual questionnaires were used to follow patients who were unable to attend the clinics, and all patients in years 6 to 10 were assessed through questionnaires.
Between-group differences in hemoglobin A1c levels were lost after the first year. However, in the sulfonylurea-insulin group, relative reductions in risk persisted at 10 years for any diabetes-related end point (9%, P = .04) and microvascular disease (24%, P = .001), while risk reductions for myocardial infarction (15%, P = .01) and death from any cause (13%, P = .007) emerged over time as more events occurred. In the metformin group, significant risk reductions persisted for any diabetes-related end point (21%, P = .01), myocardial infarction (33%, P = .005), and death from any cause (27%, P = .002).
The long-term follow-up to the UKPDS, like the long-term follow-up to the DCCT, demonstrated metabolic memory: that is, despite an early loss of glycemic differences after completion of the trial, a continued reduction in microvascular risk and an emergent risk reduction for myocardial infarction and death from any cause were observed.
These long-term randomized prospective trials in patients with type 1 and type 2 diabetes clearly show that the glucose hypothesis is in fact correct: intensive glucose control lowers the risk of both microvascular and macrovascular complications of diabetes.
IS THERE DISCORDANCE BETWEEN OLDER AND MORE RECENT TRIALS?
If the results of these older landmark clinical trials are true, why did the more recent clinical trials fail to show cardiovascular benefit with stricter glycemic control, and in one trial2 demonstrate the potential for harm? (ACCORD2 found an increased death rate in patients who received intensive therapy, targeting a hemoglobin A1c below 6.0%.)
The answer lies in the populations studied. ACCORD,2 VADT,3 and ADVANCE4 were performed in older patients with prior cardiac events or with several risk factors for cardiovascular events. The study populations were picked to increase the number of cardiac events in a short time frame. Therefore, extrapolating the results of these studies to the younger population of patients with diabetes, most of whom have yet to develop macrovascular disease, is inappropriate.
The available evidence suggests that early aggressive management of diabetes reduces the risk of macrovascular disease, but that this benefit is delayed. In the UKPDS and DCCT trials, it took 10 to 17 years to show cardiac benefit in younger patients.
The results of ACCORD,2 VADT,3 and ADVANCE4 are important when considered in the correct clinical context. Two of these trials did demonstrate some microvascular benefit as a result of better glycemic control in older patients, many of whom had longstanding diabetes. These studies suggest that, in patients who already have established cardiovascular disease or have several risk factors for cardiovascular events, a less-strict glycemic target may be warranted.
These trials should not be interpreted as saying that glycemic control is unimportant in older patients at higher risk. Rather, they suggest that an individualized approach to diabetes management, supported by the most recent American Diabetes Association guidelines,9 is more appropriate.
Physicians may reasonably suggest a stricter A1c goal (ie, < 6.5%) in certain patients if it can be achieved without significant hypoglycemia. Stricter glycemic targets would seem appropriate in patients recently diagnosed with diabetes, those who have a long life expectancy, and those who have not yet developed significant cardiovascular disease.9
However, in patients who already have developed advanced microvascular and macrovascular complications, who have long-standing diabetes, who have a history of severe hypoglycemia (or hypoglycemia unawareness), or who have a limited life expectancy or numerous adverse comorbidities, a less strict glycemic target (hemoglobin A1c < 8%) may be more appropriate.9
CARDIOVASCULAR RISK, HYPOGLYCEMIA, AND ATTAINING GLYCEMIC TARGETS
Metformin, in the absence of contraindications or intolerability, is generally the recommended first-line therapy to manage glycemia in patients with type 2 diabetes mellitus.10,11 However, there are only limited data to direct clinicians as to which antidiabetic medication to use if further therapy is required to obtain glycemic control.
Much of the cardiovascular and mortality risk associated with aggressive diabetes management (ie, lower A1c targets) is related to hypoglycemia. Thus, antidiabetic therapies that pose no risk or only a low risk of hypoglycemia should be chosen, particularly in older patients and in those with known cardiovascular disease. This may allow for better glycemic control without the risk of hypoglycemia and adverse cardiovascular outcomes.
However, in practice, clinicians continue to use a sulfonylurea as the second-line agent. Although sulfonylureas may appear to be a great option because of their low cost, they are associated with a higher risk of hypoglycemic episodes than other classes of diabetes drugs. We need to consider the frequency and cost of hypoglycemic episodes and the potential morbidity associated with them, because these episodes are a barrier to our efforts to achieve better glycemic control.
Budnitz et al12 reported that from 2007 through 2009, in US adults age 65 and older, insulins were implicated in 13.9% of hospitalizations related to adverse drug events, and oral hypoglycemic agents (ie, insulin secretagogues) in 10.7%.
Quilliam et al13 reported that hypoglycemia resulted in a mean cost of $17,564 for an inpatient admission, $1,387 for an emergency department visit, and $394 for an outpatient visit. Thus, the cost savings associated with prescribing a sulfonylurea vs one of the newer oral antidiabetic agents that do not increase the risk of hypoglycemia (unless used concurrently with insulin or an insulin secretagogue) can quickly be eroded by severe hypoglycemic episodes requiring medical care.
Moreover, once patients start to experience hypoglycemic episodes, they are very reluctant, as are their physicians, to intensify therapy, even if it is indicated by their elevated A1c.
There are now seven classes of oral antidiabetic therapies other than insulin secretagogues (ie, other than sulfonylureas and the meglitinides nateglinide and repaglinide), as well as a few noninsulin injectable therapies (glucagon-like peptide-1 agonists), that are not associated with hypoglycemia. We believe these agents should be tried before prescribing an agent that carries the risk of hypoglycemia (ie, sulfonylureas).
If agents that do not cause hypoglycemia are used, more-aggressive glycemic targets may be achieved safely. The ACCORD study,2 which included patients at high cardiovascular risk and aimed at an aggressive glycemic target of 6%, may have yielded much different results had agents that carry a high risk of hypoglycemia been excluded.
Of importance, cardiovascular risk is also influenced by the common comorbidities seen in patients with diabetes, such as hypertension and hypercholesterolemia. Intensive, multifactorial interventions that address not only glycemic control but also blood pressure and lipids and that include low-dose aspirin therapy have been shown to lower the risk of death from cardiovascular causes and the risk of cardiovascular events.14 Likewise, smoking cessation is very important in reducing cardiovascular risk, especially in patients with diabetes.15
CLINICAL TRIALS IN CONTEXT
In conclusion, there is more to diabetes management than cardiovascular complications. Clearly, improved glycemic control decreases the risk of retinopathy, nephropathy, and neuropathy in patients with type 1 and type 2 diabetes. The DCCT and UKPDS extension studies further found that excellent glycemic control decreases rates of cardiac events.
The best way to treat diabetes may be different in otherwise healthy younger patients who have yet to develop significant complications than it is in older patients known to have cardiovascular disease or several risk factors for cardiovascular events. The available evidence suggests it would be reasonable to aim for stricter glycemic targets in the younger patients and less stringent targets in the older patients, particularly in those with long-standing diabetes who have already developed significant micro- and macrovascular complications.
We should interpret clinical trials within their narrow clinical context, emphasizing the actual population of patients included in the study, so as to avoid the inappropriate extrapolation of the results to all.
Diabetes mellitus and its management have become the center of controversy in recent years. More emphasis is being placed on the potential for adverse cardiovascular outcomes with more aggressive glycemic control as well as on the potential for adverse cardiovascular events with newer antidiabetic therapies, and less on the importance of glycemic control, particularly early in the disease course.
Although it is important to take new data into consideration when managing diabetes, it appears that the results of recent clinical trials are being misinterpreted and incorrectly applied to the wrong patient populations, and in the process, the results of older landmark clinical trials are being neglected. Inadequate glycemic control not only plays a role in cardiovascular risk, it also remains the leading cause of blindness, kidney failure, and nontraumatic lower-limb amputations in the United States.1
Although we need to recognize the potential for adverse cardiovascular outcomes with diabetes and its management, we cannot lose sight of the big picture—ie, that inadequate glycemic control confers both microvascular and macrovascular risk, and that the available data show that restoring near-euglycemia in patients with diabetes considerably reduces the risk of microvascular and macrovascular complications.
Several recently published clinical trials—the Action to Control Cardiovascular Risk in Diabetes (ACCORD),2 the Veterans Affairs Diabetes Trial (VADT),3 and the Action in Diabetes and Vascular Disease (ADVANCE)4—failed to demonstrate improved cardiovascular outcomes with improved glycemic control. However, we should not take this to mean that glycemic control is unimportant.
In this article, we will discuss why the results of these recent clinical trials are not valid for the general population of patients with diabetes. We will review evidence from landmark clinical trials that clearly demonstrates that better glycemic control reduces both microvascular and macrovascular complications of diabetes (the “glucose hypothesis”). We contend that excellent glycemic control clearly decreases the microvascular complications of diabetes, and that results from long-term follow-up studies in both type 1 and type 2 diabetes show reduced rates of heart attack and stroke in patients treated intensively earlier in the course of their disease.5,6
EVIDENCE FOR THE GLUCOSE HYPOTHESIS
Diabetes Control and Complications Trial
The first major trial demonstrating that improved glycemic control provides benefit was the Diabetes Control and Complications Trial (DCCT).7 This study enrolled 1,441 patients with insulin-dependent diabetes mellitus, 726 of whom had no retinopathy at baseline (the primary-prevention cohort) and 715 of whom had mild retinopathy (the secondary-intervention cohort).
Patients were randomly assigned to intensive therapy (three or more insulin injections per day or an insulin pump) or to conventional therapy with one or two daily insulin injections. They were followed for a mean of 6.5 years, and the appearance and progression of retinopathy and other complications were assessed regularly.
During the study, the hemoglobin A1c level averaged 9% in the control group and 7% in the intensively treated group. The cumulative incidence of retinopathy was defined as a change of three steps or more on fundus photography that was sustained over a 6-month period.
Effect on retinopathy. At study completion, the cumulative incidence of retinopathy in the intensive-therapy group was approximately 50% less than in the conventional-therapy group. Intensive therapy reduced the adjusted mean risk of retinopathy by 76% (95% confidence interval [CI] 62%–85%) in the primary-prevention cohort. In the secondary-prevention cohort, intensive therapy reduced the average risk of progression by 54% (95% CI 39%–66%). Intensive therapy reduced the adjusted risk of proliferative or severe nonproliferative retinopathy by 47% (P = .011) and that of treatment with photocoagulation by 56% (P = .002).
Effect on nephropathy. Intensive therapy reduced the mean adjusted risk of microalbuminuria by 34% (P = .04) in the primary-prevention cohort and by 43% (P = .001) in the secondary-intervention cohort. The risk of macroalbuminuria was reduced by 56% (P = .01) in the secondary-intervention cohort.
Effect on neuropathy. In the patients in the primary-prevention cohort who did not have neuropathy at baseline, intensive therapy reduced the incidence of neuropathy at 5 years by 69% (to 3%, vs 10% in the conventional-therapy group; P = .006). Similarly, in the secondary-intervention cohort, intensive therapy reduced the incidence of clinical neuropathy at 5 years by 57% (to 7%, vs 16%; P < .001).
Effect on macrovascular events. In the initial trial, a nonsignificant 41% reduction in combined cardiovascular and peripheral vascular disease events was observed.
DCCT long-term follow-up
After DCCT concluded, the control and treatment groups’ hemoglobin A1c levels converged to approximately 8%. The two groups were then followed to determine the long-term effects of their prior separation of glycemic levels on micro- and macrovascular out comes.5 More than 90% of the original DCCT patients were followed for a mean of 17 years.
Intensive treatment reduced the risk of any cardiovascular disease event by 42% (95% CI 9%–63%; P = .02) and the risk of nonfatal myocardial infarction, stroke, or death from cardiovascular disease by 57% (95% CI 12%– 79%; P = .02). This result was observed despite separation of glucose control in the two groups only for the first 6.5 years. This beneficial effect of intensive early glycemic control has been termed metabolic memory.
United Kingdom Prospective Diabetes Study
A second major trial, the United Kingdom Prospective Diabetes Study (UKPDS),8 assessed the effect of excellent diabetes control on diabetes complications in patients with type 2 diabetes. A total of 3,867 patients newly diagnosed with type 2 diabetes, median age 54, who after 3 months of diet treatment had mean fasting plasma glucose concentrations of 110 to 270 mg/dL, were randomly assigned to an intensive policy (with a sulfonylurea or insulin or, if overweight, metformin) or a conventional policy with diet. The aim in the intensive group was a fasting plasma glucose less than 108 mg/dL. In the conventional group, the aim was the best achievable fasting plasma glucose with diet alone; drugs were added only if there were hyperglycemic symptoms or a fasting plasma glucose greater than 270 mg/dL.
Over 10 years, the median hemoglobin A1c level was 7.0% (interquartile range 6.2%–8.2%) in the intensive group compared with 7.9% (6.9%–8.8%) in the conventional group. Compared with the conventional group, the risk of any diabetes-related end point was 12% lower in the intensive group (95% CI 1%–21%, P = .029), the risk of any diabetes-related death was 10% lower (−11% to 27%, P = .34), and the rate of all-cause mortality was 6% lower (−10% to 20%, P = .44). Most of the reduction in risk of any diabetes-related end point was from a 25% risk reduction (95% CI 7%–40%, P = .0099) in microvascular end points, including the need for retinal photocoagulation.
UKPDS long-term follow-up
In 2008, Holman et al published the results of long-term follow-up of patients included in the UKPDS.6 In posttrial monitoring, 3,277 patients were asked to attend annual UKPDS clinics for 5 years, but no attempts were made to maintain their previously assigned therapies. Annual questionnaires were used to follow patients who were unable to attend the clinics, and all patients in years 6 to 10 were assessed through questionnaires.
Between-group differences in hemoglobin A1c levels were lost after the first year. However, in the sulfonylurea-insulin group, relative reductions in risk persisted at 10 years for any diabetes-related end point (9%, P = .04) and microvascular disease (24%, P = .001), while risk reductions for myocardial infarction (15%, P = .01) and death from any cause (13%, P = .007) emerged over time as more events occurred. In the metformin group, significant risk reductions persisted for any diabetes-related end point (21%, P = .01), myocardial infarction (33%, P = .005), and death from any cause (27%, P = .002).
The long-term follow-up to the UKPDS, like the long-term follow-up to the DCCT, demonstrated metabolic memory: that is, despite an early loss of glycemic differences after completion of the trial, a continued reduction in microvascular risk and an emergent risk reduction for myocardial infarction and death from any cause were observed.
These long-term randomized prospective trials in patients with type 1 and type 2 diabetes clearly show that the glucose hypothesis is in fact correct: intensive glucose control lowers the risk of both microvascular and macrovascular complications of diabetes.
IS THERE DISCORDANCE BETWEEN OLDER AND MORE RECENT TRIALS?
If the results of these older landmark clinical trials are true, why did the more recent clinical trials fail to show cardiovascular benefit with stricter glycemic control, and in one trial2 demonstrate the potential for harm? (ACCORD2 found an increased death rate in patients who received intensive therapy, targeting a hemoglobin A1c below 6.0%.)
The answer lies in the populations studied. ACCORD,2 VADT,3 and ADVANCE4 were performed in older patients with prior cardiac events or with several risk factors for cardiovascular events. The study populations were picked to increase the number of cardiac events in a short time frame. Therefore, extrapolating the results of these studies to the younger population of patients with diabetes, most of whom have yet to develop macrovascular disease, is inappropriate.
The available evidence suggests that early aggressive management of diabetes reduces the risk of macrovascular disease, but that this benefit is delayed. In the UKPDS and DCCT trials, it took 10 to 17 years to show cardiac benefit in younger patients.
The results of ACCORD,2 VADT,3 and ADVANCE4 are important when considered in the correct clinical context. Two of these trials did demonstrate some microvascular benefit as a result of better glycemic control in older patients, many of whom had longstanding diabetes. These studies suggest that, in patients who already have established cardiovascular disease or have several risk factors for cardiovascular events, a less-strict glycemic target may be warranted.
These trials should not be interpreted as saying that glycemic control is unimportant in older patients at higher risk. Rather, they suggest that an individualized approach to diabetes management, supported by the most recent American Diabetes Association guidelines,9 is more appropriate.
Physicians may reasonably suggest a stricter A1c goal (ie, < 6.5%) in certain patients if it can be achieved without significant hypoglycemia. Stricter glycemic targets would seem appropriate in patients recently diagnosed with diabetes, those who have a long life expectancy, and those who have not yet developed significant cardiovascular disease.9
However, in patients who already have developed advanced microvascular and macrovascular complications, who have long-standing diabetes, who have a history of severe hypoglycemia (or hypoglycemia unawareness), or who have a limited life expectancy or numerous adverse comorbidities, a less strict glycemic target (hemoglobin A1c < 8%) may be more appropriate.9
CARDIOVASCULAR RISK, HYPOGLYCEMIA, AND ATTAINING GLYCEMIC TARGETS
Metformin, in the absence of contraindications or intolerability, is generally the recommended first-line therapy to manage glycemia in patients with type 2 diabetes mellitus.10,11 However, there are only limited data to direct clinicians as to which antidiabetic medication to use if further therapy is required to obtain glycemic control.
Much of the cardiovascular and mortality risk associated with aggressive diabetes management (ie, lower A1c targets) is related to hypoglycemia. Thus, antidiabetic therapies that pose no risk or only a low risk of hypoglycemia should be chosen, particularly in older patients and in those with known cardiovascular disease. This may allow for better glycemic control without the risk of hypoglycemia and adverse cardiovascular outcomes.
However, in practice, clinicians continue to use a sulfonylurea as the second-line agent. Although sulfonylureas may appear to be a great option because of their low cost, they are associated with a higher risk of hypoglycemic episodes than other classes of diabetes drugs. We need to consider the frequency and cost of hypoglycemic episodes and the potential morbidity associated with them, because these episodes are a barrier to our efforts to achieve better glycemic control.
Budnitz et al12 reported that from 2007 through 2009, in US adults age 65 and older, insulins were implicated in 13.9% of hospitalizations related to adverse drug events, and oral hypoglycemic agents (ie, insulin secretagogues) in 10.7%.
Quilliam et al13 reported that hypoglycemia resulted in a mean cost of $17,564 for an inpatient admission, $1,387 for an emergency department visit, and $394 for an outpatient visit. Thus, the cost savings associated with prescribing a sulfonylurea vs one of the newer oral antidiabetic agents that do not increase the risk of hypoglycemia (unless used concurrently with insulin or an insulin secretagogue) can quickly be eroded by severe hypoglycemic episodes requiring medical care.
Moreover, once patients start to experience hypoglycemic episodes, they are very reluctant, as are their physicians, to intensify therapy, even if it is indicated by their elevated A1c.
There are now seven classes of oral antidiabetic therapies other than insulin secretagogues (ie, other than sulfonylureas and the meglitinides nateglinide and repaglinide), as well as a few noninsulin injectable therapies (glucagon-like peptide-1 agonists), that are not associated with hypoglycemia. We believe these agents should be tried before prescribing an agent that carries the risk of hypoglycemia (ie, sulfonylureas).
If agents that do not cause hypoglycemia are used, more-aggressive glycemic targets may be achieved safely. The ACCORD study,2 which included patients at high cardiovascular risk and aimed at an aggressive glycemic target of 6%, may have yielded much different results had agents that carry a high risk of hypoglycemia been excluded.
Of importance, cardiovascular risk is also influenced by the common comorbidities seen in patients with diabetes, such as hypertension and hypercholesterolemia. Intensive, multifactorial interventions that address not only glycemic control but also blood pressure and lipids and that include low-dose aspirin therapy have been shown to lower the risk of death from cardiovascular causes and the risk of cardiovascular events.14 Likewise, smoking cessation is very important in reducing cardiovascular risk, especially in patients with diabetes.15
CLINICAL TRIALS IN CONTEXT
In conclusion, there is more to diabetes management than cardiovascular complications. Clearly, improved glycemic control decreases the risk of retinopathy, nephropathy, and neuropathy in patients with type 1 and type 2 diabetes. The DCCT and UKPDS extension studies further found that excellent glycemic control decreases rates of cardiac events.
The best way to treat diabetes may be different in otherwise healthy younger patients who have yet to develop significant complications than it is in older patients known to have cardiovascular disease or several risk factors for cardiovascular events. The available evidence suggests it would be reasonable to aim for stricter glycemic targets in the younger patients and less stringent targets in the older patients, particularly in those with long-standing diabetes who have already developed significant micro- and macrovascular complications.
We should interpret clinical trials within their narrow clinical context, emphasizing the actual population of patients included in the study, so as to avoid the inappropriate extrapolation of the results to all.
- Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011. www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed October 7, 2014.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(Suppl 1):S11–S66.
- Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al; American Association of Clinical Endocrinologists. AACE comprehensive diabetes management algorithm 2013. Endocr Pract 2013; 19:327–336.
- Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med 2011; 365:2002–2012.
- Quilliam BJ, Simeone JC, Ozbay AB, Kogut SJ. The incidence and costs of hypoglycemia in type 2 diabetes. Am J Manag Care 2011; 17:673–680.
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358:580–591.
- Chaturvedi N, Stevens L, Fuller JH. Which features of smoking determine mortality risk in former cigarette smokers with diabetes? The World Health Organization Multinational Study Group. Diabetes Care 1997; 20:1266–1272.
- Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011. www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed October 7, 2014.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(Suppl 1):S11–S66.
- Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al; American Association of Clinical Endocrinologists. AACE comprehensive diabetes management algorithm 2013. Endocr Pract 2013; 19:327–336.
- Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med 2011; 365:2002–2012.
- Quilliam BJ, Simeone JC, Ozbay AB, Kogut SJ. The incidence and costs of hypoglycemia in type 2 diabetes. Am J Manag Care 2011; 17:673–680.
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358:580–591.
- Chaturvedi N, Stevens L, Fuller JH. Which features of smoking determine mortality risk in former cigarette smokers with diabetes? The World Health Organization Multinational Study Group. Diabetes Care 1997; 20:1266–1272.
Advances in autosomal dominant polycystic kidney disease—2014 and beyond
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited renal disease, has an estimated prevalence of 1:400 to 1:1,000 live births in the United States, and occurs worldwide.1,2 There are about 700,000 people living with it in the United States, and about 6,000 new cases arise annually. It accounts for nearly 5% of all patients with end-stage renal disease in the United States.3
This paper will offer an overview of the pathogenesis of renal cysts, review some of the clinical aspects of ADPKD including diagnosis and management of complications, and discuss recent drug trials and current management.
TWO TYPES—PKD1 IS MORE COMMON AND PROGRESSES MORE RAPIDLY
Two major forms of ADPKD are recognized and can usually be determined by genetic testing: PKD1, accounting for about 85% of cases, and PKD2, accounting for 15%.
The gene locus for PKD1 is on the short arm of the 16th chromosome (16p13.3), and its glycoprotein gene product is polycystin 1 (PC1), a large molecule with 4,303 amino acids.2 PC1 has a long N-terminal extracellular tail that can function as a mechanosensor. Disease progression is much faster with PKD1, and end-stage renal disease usually occurs before age 56.4
In PKD2, the gene locus is on the long arm of the fourth chromosome (4q21–23), and has a smaller glycoprotein gene product, polycystin 2 (PC2), that plays a role in calcium transport. The disease course of PKD2 tends to be slower. End-stage renal disease might not develop in the patient’s lifetime, since it typically develops when the patient is more than 70 years old.4
Although the growth rate of renal cysts is similar between the two types, patients with PKD1 develop about twice as many cysts as those with PDK2, and their cyst development starts at a younger age.5
Typically, patients have a clear phenotype and a positive family history, but in about 10% of possible ADPKD cases, there is no family history of ADPKD. Genetic variations such as incompletely penetrant PKD1 alleles,6 hypomorphic alleles,7 and trans-heterozygous mutations8 account for at least some of these cases.
IMAGING CRITERIA HAVE BROADENED
Ultrasonographic criteria for the diagnosis of ADPKD that were published in 1994 were based on patients who had a family history of PKD1.9 The criteria have since been modified (the “unified criteria”) to include patients with a family history of PKD2 who begin cyst development at a later age and with lower numbers.10 For patients ages 30 to 39, a previously difficult diagnostic group, the criterion for the minimum number of cysts visible on ultrasonography changed from four to three, improving the sensitivity of detecting disease from approximately 76% to approximately 95% (Table 1).9,10 It is important to note that these criteria apply only to patients “at risk,” ie, with a positive family history of ADPKD.
Computed tomography (CT) and magnetic resonance imaging (MRI) classically show bilaterally enlarged multicystic kidneys, though variations can be seen.
DISEASE CAN PRESENT IN MYRIAD WAYS
Although cystic kidney disease is the basic underlying problem, undiagnosed patients may present with a variety of symptoms caused by other manifestations of ADPKD (Table 2).
Hypertension is the most common presentation, occurring in about 50% of patients ages 20 to 34, and essentially 100% of those with end-stage renal disease.11 It is associated with up-regulation of the renin-angiotensin-aldosterone system.
Pain is typically located in the abdomen, flank, or back and can occur in a localized or diffuse manner. Early abdominal distress is often simply described as “fullness.” Localized pain is usually caused by bleeding into or rupture of a cyst, renal stones, or infection.12 Because renal cysts are noncommunicating, bleeding can occur into a cyst and cause pain without gross hematuria. Compression by greatly enlarged kidneys, liver, or both can cause a variety of gastrointestinal symptoms such as reflux esophagitis and varying degrees of constipation. Diffuse pain is often musculoskeletal and related to exaggerated lordosis from increasing abdominal size due to enlarging cystic kidneys and sometimes liver.12 In carefully selected cases, cyst aspiration may be helpful.11
Although renal carcinomas are rare and not more frequent than in the general population, they can occur at an earlier age and with constitutional symptoms.11
Urinary tract infections are increased in frequency. A patient may have a simple urinary tract infection that is cured with the appropriate antibiotic. However, a urinary tract infection repeatedly recurring with the same organism is a strong clue that an infected cyst is the source and requires more intensive treatment with the appropriate cyst-penetrating antibiotic. On the other hand, because cysts are noncommunicating, an infected cyst might be present despite a negative urine culture.
Identifying infected cysts can be a challenge with conventional imaging techniques, but combined positron emission tomography and CT (PET/CT) can be a valuable though expensive diagnostic tool to identify an infected kidney or liver cyst, or to identify an unsuspected source of the pain and infection.13
Jouret et al13 evaluated 27 PET/CT scans performed in 24 patients with ADPKD and suspicion of an abdominal infection. Patients were deemed to have probable cyst infection if they met all of the following criteria: temperature more than 38°C for longer than 3 days, loin or liver tenderness, plasma C-reactive protein level greater than 5 mg/dL, and no evidence of intracystic bleeding on CT. Patients with only two or three of these criteria were classified as having fever of unknown origin. Diagnosis of cyst infection was confirmed by cyst fluid analysis.
PET/CT identified a kidney or liver cyst infection in 85% of 13 infectious events in 11 patients who met all the criteria for probable cyst infection; CT alone contributed to the diagnosis in only one patient.13 In those with fever of unknown origin, PET/CT identified a source of infection in 64% of 14 events in 13 patients: two infected renal cysts, as well as one patient each with other infections that would be difficult to diagnose clinically, ie, small bowel diverticulitis, psoas abscess, diverticulitis of the right colon, pyelonephritis in a transplanted kidney, infected abdominal aortic aneurysm, prostatitis, colitis, and Helicobacter pylori gastritis. Results of PET/CT were negative in five patients with intracystic bleeding.
Kidney stones occur in 20% to 36% of patients.11,14 Uric acid stones occur at almost the same frequency as calcium oxalate stones.
Chronic kidney disease not previously diagnosed may be the presenting condition in a small percentage of patients, sometimes those in whom much earlier hypertension was not fully evaluated. ADPKD is typically not associated with significant proteinuria (eg, nephrotic range), and the presence of heavy proteinuria usually indicates the presence of a superimposed primary glomerulopathy.15
Cysts in other locations. By MRI, liver cysts are present in 58% of patients ages 15 to 24, rising to 94% in those ages 35 to 46.11 Because liver cysts are estrogen-dependent, they are more prominent in women. A small percentage of patients develop cysts in the pancreas (5%), arachnoid membranes (8%), and seminal vesicles (40% of men with ADPKD).11
Cardiovascular abnormalities occur in almost one-third of patients with ADPKD, usually as mitral and aortic valve abnormalities.16 Aneurysms of the aortic root and abdominal aorta can also occur, in addition to intracranial aneurysms (see below).17
Intracranial aneurysms are not uncommon, and size usually determines their risk.
Intracranial aneurysms are strongly influenced by family history: 16% of ADPKD patients with a family history of intracranial aneurysm also develop them, compared with 5% to 6% of patients with no family history.11 The anterior cerebral circulation is involved in about 80% of cases. A sentinel or sudden “thunderclap” headache is a classic presentation that may precede full-blown rupture in about 17% of cases.18 Patients who rupture an intracranial aneurysm have a mean age of 39, usually have normal renal function, and can be normotensive.11
For patients with no history of subarachnoid hemorrhage, the 5-year cumulative rupture rates for patients with aneurysms located in the internal carotid artery, anterior communicating or anterior cerebral artery, or middle cerebral artery were 0% for aneurysms less than 7 mm, 2.6% for those 7 to 12 mm, 14.5% for those 13 to 24 mm, and 40% for those 25 mm or larger, with higher rates for the same sizes in the posterior circulation.11
In patients without symptoms, size is correlated with risk of rupture: less than 4 mm is usually associated with very low risk, 4 to less than 7 mm with moderate risk, and 7 mm or more with increasing risk. An aneurysm larger than 10 mm is associated with roughly a 1% risk of rupture per year.19
Irazabal et al20 retrospectively studied 407 patients with ADPKD who were screened for intracranial aneurysm. Saccular aneurysms were detected in 45 patients; most were small (median diameter 3.5 mm). During cumulative imaging follow-up of 243 years, only one new intracranial aneurysm was detected (increasing from 2 to 4.4 mm over 144 months) and two previously identified aneurysms grew (one increasing 4.5 to 5.9 mm over 69 months and the other 4.7 to 6.2 mm over 184 months). No change occurred in 28 patients. Seven patients were lost to follow-up, however. During cumulative clinical follow-up of 316 years, no aneurysm ruptured. Two patients were lost to follow-up, three had surgical clipping, and five died of unrelated causes. The authors concluded that presymptomatic intracranial aneurysms are usually small, and that growth and rupture risks are no higher than for unruptured intracranial aneurysms in the general population. A 2014 study also suggests a conservative approach for managing intracranial aneurysm in the general population.21
In asymptomatic ADPKD patients, it is reasonable to reserve screening for those with a positive family history of intracranial aneurysm or subarachnoid hemorrhage, those with a previous ruptured aneurysm, those in high-risk professions (eg, pilots), and for patients prior to anticoagulant therapy or major surgery possibly associated with hemodynamic instability.11,22 Certain extremely anxious patients might also need to be studied. Screening can be performed with magnetic resonance angiography without gadolinium contrast. It is prudent to have patients with an intracranial aneurysm thoroughly evaluated by an experienced neurosurgeon with continued follow-up.
PROGRESSION OF ADPKD
The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) study23 evaluated 241 patients with ADPKD (ages 15 to 46) by measuring the annual rate of change in total kidney volume, total cyst volume, and iothalamate glomerular filtration rate (GFR) over 3 years. The annual increase in total kidney volume averaged 5.3%,23 though the reported range with various imaging techniques is from 4% to 12.8% in adults.24 This study focused on macrocystic disease, ie, cysts that are visible by MRI and measurably increase total kidney volume. Although larger total kidney volume at baseline generally predicted a more rapid decline in GFR, there were wide and overlapping variations in yearly GFR declines within and among different total-kidney-volume groups.23
SPECIAL CLINICAL PROBLEMS IN ADPKD
Case 1: A man with ADPKD develops new and increasing proteinuria
A 55-year-old man with ADPKD and stage 3 chronic kidney disease developed new and increasing proteinuria, rising to 5,500 mg per 24 hours. What is the most likely explanation?
- Rapidly progressive renal failure with increasing proteinuria in ADPKD
- Bilateral renal vein thromboses because of cyst compression
- Malignant hypertension with bilateral renal artery compression
- Superimposed primary glomerulopathy
- Multiple infected renal cysts with pyonephrosis
Answer: Superimposed primary glomerulopathy.
ADPKD (similar to uncomplicated obstructive uropathy, pyelonephritis, main renal artery disease, and often cases of interstitial nephritis without secondary glomerular changes) typically does not result in nephrotic-range proteinuria. A superimposed primary glomerulopathy, focal segmental glomerulosclerosis, was the biopsy-proved diagnosis.
At least 21 cases have been reported of AD-PKD with nephrotic-range proteinuria and a renal biopsy showing a primary glomerulopathy, including focal segmental glomerulosclerosis (5 cases), minimal-change disease (5), membranous nephropathy (3), IgA nephropathy (2), and one each of crescentic glomerulonephropathy, diabetic nephropathy, membranoproliferative glomerulonephritis, postinfectious glomerulonephropathy, amyloid glomerulopathy, and mesangioproliferative glomerulopathy.15 Treatment was directed at the primary glomerulopathy, and the outcomes corresponded to the primary diagnosis (eg, with appropriate treatment, three of the five patients with focal segmental glomerulosclerosis progressed to end-stage renal disease, all of the patients with minimal-change disease went into remission, and one of the two cases with IgA nephropathy improved).15
Case 2: A woman with ADPKD and advanced renal failure develops shortness of breath
A 47-year-old woman with very large polycystic kidneys (total kidney volume 7,500 mL; normal range for a single kidney approximately 136–295 mL, mean 196)25 and estimated GFR of 25 mL/min developed new-onset shortness of breath while climbing steps and later even when making a bed. She had no chest pain, cough, or edema. She was sent directly to the emergency department and was admitted and treated; her condition improved, and she was discharged after 6 days. What did she have?
- Presentation of rare cystic pulmonary disease in ADPKD
- Onset of pneumonia with early bacteremia
- Progressive reduction in ventilatory capacity from massive polycystic kidneys and liver elevating both sides of the diaphragm
- Pulmonary emboli from an iliac vein or inferior vena cava source
- Progressive anemia accompanying rapidly worsening stage 4 chronic kidney disease
Answer: She had pulmonary emboli from an iliac vein (right) or inferior vena cava source.
Pulmonary emboli in ADPKD can be caused by thrombi in the inferior vena cava or the iliac or femoral vein because of compression by a massive right polycystic kidney. Four cases were reported at Mayo Clinic,26 three diagnosed by MRI and one with CT. One additional case occurred at Cleveland Clinic. All patients survived after treatment with anticoagulation therapy; early nephrectomy was required in two cases.
Interestingly, following kidney transplantation, the patients at greatest risk for pulmonary emboli are those with ADPKD as their original disease.27
RENAL CYSTS RESULT FROM COMBINED MUTATIONS, INJURY
The germline ADPKD mutation that occurs in one allele of all renal tubular epithelial cells is necessary but not sufficient for cystogenesis.28 One or more additional somatic mutations of the normal allele—the “second hit”—also develop within individual tubular epithelial cells.28,29 These epithelial cells undergo clonal proliferation, resulting in tubular dilatation and cyst formation. Monoclonality of cells in cysts has been documented.
Ischemia-reperfusion injury can be viewed as a “third hit.”30 In PKD1 knockout mice, which at 5 weeks of age normally develop only mild cystic kidney disease, the superimposition of unilateral ischemia-reperfusion injury at 8 weeks caused widespread and rapid cyst formation. It is believed that acute renal injury reactivates developmental signaling pathways within 48 hours that trigger epithelial cell proliferation and then cyst development detectable by MRI 2 weeks later. Although this phenomenon has not been documented in humans, it is a cautionary tale.
CYSTOGENESIS INVOLVES MULTIPLE PATHWAYS
A comprehensive description of pathways leading to renal cyst formation is beyond the scope of this article, and the reader is referred to much more detailed and extensive reviews.2,31 Disturbances in at least three major interconnected pathways promote cystogenesis in renal tubular epithelial cells:
- Normal calcium transport into the endoplasmic reticulum is disrupted by abnormal polycystins on the surface of the primary cilium
- Vasopressin and other stimuli increase the production of cyclic adenosine monophosphate (cAMP)
- The mammalian target of rapamycin (mTOR) proliferative pathway is up-regulated.
DISRUPTION OF CALCIUM TRANSPORT IN THE PRIMARY CILIUM
Primary cilia are nonmotile cellular organelles of varying size, from about 0.25 μm up to about 1 μm.32 Each primary cilium has nine peripheral pairs of microtubules but lacks a centrally located pair that is present in motile cilia. Primary cilia are ubiquitous and have been highly conserved throughout evolution. A single cilium is present on almost all vertebral cells.33
Cilial defects have been identified in autosomal dominant as well as recessive diseases and are known as ciliopathies.33 Although rare in humans, they can affect a broad spectrum of organs other than the kidney, including the eye, liver, and brain.33
Urine flow in a renal tubule is believed to exert mechanical stimulation on the extracellular flagellum-like N-terminal tail of PC1 that extends from a primary cilium into the urinary space. PC1 in concert with PC2 opens PC2 calcium channels, allowing calcium ions to flow down the microtubules to ryanodine receptors and the basal body.32,33 This leads to local release of calcium ions that regulate cell proliferation.32,34 However, in ADPKD kidneys, PC1 and PC2 molecules are sparse or mutated, resulting in defective calcium transport, increased and unregulated tubular epithelial cell proliferation, and cyst formation.
In a totally different clinical setting, biopsies of human renal transplants that sustained acute tubular necrosis during transplantation reveal that a cilium dramatically elongates in response to injury,35 possibly as a compensatory mechanism to maintain calcium transport in the presence of meager urine flow and to restore the proliferation of tubular epithelial cells in a regulated repair process.
THE ROLE OF VASOPRESSIN AND ACTIVATION OF cAMP
In classic experiments, Wang et al36 cross-bred rats having genetically inherited polycystic kidney disease (actually, autosomal recessive polycystic kidney disease) with Brattleboro rats that completely lack vasopressin. At 10 and 20 weeks of age, the offspring had virtually complete inhibition of cystogenesis because of the absence of vasopressin. However, when vasopressin was restored by exogenous administration continuously for 8 weeks, the animals formed massive renal cysts.
Vasopressin activates cAMP, which then functions as a second messenger in cell signaling. cAMP increases the activation of the protein kinase A (PKA) pathway, which in turn increases downstream activity of the B-raf/ERK pathway. Up-regulation of cAMP and PKA appears to perpetuate activation of canonical Wnt signaling, down-regulate non-canonical Wnt/planar cell polarity signaling, and lead to loss of tubular diameter control, resulting in cyst formation.31 Normally, cAMP is degraded by phosphodiesterase. However, because of the primary cilium calcium transport defect in ADPKD, phosphodiesterase is reduced and cAMP persists.37 In conjunction with the defective primary cilial calcium transport, cAMP exerts a proliferative effect on renal tubular epithelial cells that is opposite to its effect in normal kidneys.31,32 cAMP also up-regulates the cystic fibrosis transmembrane conductance regulator (CFTR) that promotes chloride ion transport. Sodium ions follow the chloride ions, leading to fluid accumulation and cyst enlargement.31
Inhibiting vasopressin by increasing water intake
A simple key mechanism for limiting vasopressin secretion is by sufficient water ingestion. Nagao et al38 found that rats with polycystic kidney disease given water with 5% glucose (resulting in 3.5-fold increased fluid intake compared with rats given tap water) had a 68% reduction in urinary vasopressin and a urine osmolality less than 290 mOsm/kg. The high-water-intake rats had dramatically reduced cystic areas in the kidney and a 28% reduction of kidney-to-body weight ratio vs controls.
In an obvious oversimplification, these findings raised the question of whether a sufficient increase in water intake could be an effective therapy for polycystic kidney disease.39 A pilot clinical study evaluated changes in urine osmolality in eight patients with ADPKD who had normal renal function.40 At baseline, 24-hour urine osmolality was typically elevated to approximately 753 mOsm/kg compared to the plasma at 285 mOsm/kg, indicating that antidiuresis is the usual state. During the 2-week study, urine volume and osmolality were measured, and additional water intake was adjusted in order to achieve a urine osmolality goal of 285 ± 45 mOsm/kg. These adjustments resulted in water intake that appeared to be in the range of 2,400 to 3,000 mL per 24 hours. The major limitations of the study were that it was very short term, and there was no opportunity to measure changes in total kidney volume or estimated GFR.
In a recent preliminary report from Japan, high water intake (2,500–3,000 mL daily) in 18 ADPKD patients was compared over 12 months with ad libitum water intake in 14 ADPKD controls (clinicaltrials.gov NCT 01348505). There was no statistically significant change in total kidney volume or cystatin-estimated GFR in those on high water intake, but serious defects in study design (patients in the high water intake group were allowed to decrease their intake if it was causing them difficulty, and patients in the ad libitum water intake group had no measurement of their actual water intake) prevent any conclusions because there was no evidence that the groups were different from one another with respect to the key element of the study, namely, water intake.
Blocking the vasopressin receptor slows disease progression
Using another approach, Gattone et al41 inhibited the effect of vasopressin by blocking the vasopressin 2 receptor (V2R) in mouse and rat models of polycystic kidney disease, using an experimental drug, OPC31260. The drug halted disease progression and, in one situation, appeared to cause regression of established disease. As noted by Torres and Harris,31 even though both increased water intake and V2R antagonists decrease cAMP in the distal tubules and collecting ducts, circulating levels of vasopressin are decreased by increased water intake but increased by V2R antagonists.
After these remarkable results in animal models, clinical trials of the V2R antagonist tolvaptan were conducted in patients with ADPKD. In the Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes 3:4 study,42 1,445 adults (ages 18 to 50) with ADPKD in 133 centers worldwide were randomized to receive either tolvaptan or placebo for 3 years. Key inclusion criteria included good renal function (estimated GFR ≥ 60 mL/min) and total kidney volume of at least 750 mL (mean 1,700 mL) as measured by MRI. Tolvaptan was titrated to the highest tolerated twice-daily dose (average total of 95 mg/day). All patients were advised to maintain good hydration and to avoid thirst by drinking a glass of water after each urination. Unfortunately, neither water intake nor urine output was measured.
The primary end point was the annual rate of change in total kidney volume, with secondary end points of clinical progression (worsening kidney function, pain, hypertension, albuminuria), and rate of decline in kidney function as measured by the slope of the reciprocal of serum creatinine.42
Patients in the tolvaptan arm had a slower annual increase in total kidney volume than controls (2.8% vs 5.5%, respectively, P < .001) and a slower annual decline in renal function (−2.61 vs −3.81 mg/mL−1, respectively, P < .001).42 More participants in the treatment group withdrew than in the placebo group (23% vs 14%, respectively).
Adverse events occurred more frequently with tolvaptan.42 Liver enzyme elevations of greater than three times the upper limit of normal occurred in 4.4% of patients in the treatment group, leading to a drug warning issued in January 2013. As expected, side effects related to diuresis (urinary frequency, nocturia, polyuria, and thirst) were more frequent in the treatment group, occurring in up to 55% of participants.
The authors noted, “Although maintaining hydration helped ensure that the blinding in the study was maintained, the suppression of vasopressin release in the placebo group may have led to an underestimation of the beneficial effect of tolvaptan and may account for the lower rates of kidney growth observed in the placebo group.”42
In 2013, the US Food and Drug Administration (FDA) denied a new drug application for tolvaptan as a treatment for ADPKD.
THE mTOR PATHWAY IS UP-REGULATED
The mTOR pathway that plays a major role in cell growth and proliferation includes interaction of the cytoplasmic tail of polycystin 1 with tuberin.43 Activation products of mTOR, including phospho-S6K, have been found in tubular epithelial cells lining cysts of ADPKD kidneys but not in normal kidneys.43 Mutant mice with polycystic disease had phospho-S6K in tubular epithelial cells of cysts, whereas those treated with the mTOR inhibitor rapamycin did not.43 But subsequent studies have shown that only a low level of mTOR activation is present in 65% to 70% of ADPKD cysts.44
Two major studies of the treatment of ADPKD with rapamycin that were published contemporaneously in 2010 failed to demonstrate any significant benefit with mTOR inhibitor treatment.45,46
Serra et al45 conducted an 18-month, open-label trial of 100 ADPKD patients ages 18 to 40 with an estimated GFR (eGFR) of at least 70 mL/min. Patients were randomized to receive rapamycin, given as sirolimus 2 mg per day, or standard care. The primary end point was the reduction in the growth rate of total kidney volume, measured by MRI. Secondary end points were eGFR and protein excretion (albumin-creatinine ratio). No significant difference was found in total kidney volume, but a nonsignificant stabilization of eGFR was noted.
Walz et al46 in a 2-year, multicenter, double-blind trial, randomized 433 patients (mean age 44; mean eGFR 54.5 mL/min) to treatment with either the short-acting mTOR inhibitor everolimus (2.5 mg twice daily) or placebo. Although patients in the treatment group had less of an increase in total kidney volume (significant at 1 year but not at 2 years), they tended to show a decline in eGFR. But further analysis showed that the only patients who had a reduction in eGFR were males who already had impaired kidney function at baseline.47
In a pilot study, 30 patients with ADPKD (mean age 49) were randomized to one of three therapies:
- Low-dose rapamycin (trough blood level 2–5 ng/mL)
- Standard-dose rapamycin (trough blood level > 5–8 ng/mL)
- Standard care without rapamycin.48
In contrast to other studies, the primary end point was the change in iothalamate GFR at 12 months, with change in total kidney volume being a secondary end point.
At 12 months, with 26 patients completing the study, the low-dose rapamycin group (n = 9) had a significant increase in iothalamate GFR of 7.7 ± 12.5 mL/min/1.73 m2, whereas the standard-dose rapamycin group (n = 8) had a nonsignificant increase of 1.6 ± 12.1 mL/min/1.73 m2, and the no-rapamycin group (n = 9) had a fall in iothalamate GFR of 11.2 ± 9.1 mL/min/1.73 m2 (P = .005 for low-dose vs no rapamycin; P = .07 for standard-dose vs no rapamycin; P = .52 for low-dose vs standard-dose rapamycin; and P = .002 for combined low-dose and standard-dose rapamycin vs no rapamycin.).48 These differences were observed despite there being no significant change in total kidney volume in any of the groups. Patients on low-dose rapamycin had fewer adverse effects than those on standard dose and were more often able to continue therapy for the entire study. This, and the use of iothalamate GFR rather than eGFR to measure GFR, are believed to be the main reasons that low-dose effects were more pronounced than those with standard doses. One may speculate that rapamycin may have its effect on microcysts and cystogenic cells, resulting in stabilization of or improvement in renal function without detectable slowing in total kidney volume enlargement. Mechanisms for this possibility involve new concepts, as discussed below.
NEW CONCEPTS
Specialized cells also promote renal cyst formation
Specialized cells that promote cyst formation have been identified by Karihaloo et al49 in a mouse model of polycystic kidney disease. In this model, alternatively activated macrophages homed to cystic areas and promoted cyst growth. These findings suggested that interrupting the homing and proliferative signals of macrophages could be a therapeutic target for ADPKD. Although rapamycin can suppress macrophage proliferation by macrophage colony-stimulating factor and inhibit macrophage function,50 alternatively activated macrophages have not been specifically studied for rapamycin responsiveness.
More promising is evidence that CD133+ progenitor cells from human ADPKD kidneys—but not from normal human kidneys—form cysts in vitro and in severe combined immunodeficient mouse models.51 Treatment with rapamycin decreased proliferation of the de-differentiated CD133+ cells from ADPKD patients and reduced cystogenesis.51
Visible cysts are the tip of the iceberg
Using ADPKD nephrectomy specimens from eight patients, Grantham et al52 compared cyst counts by MRI and by histology and found that for every renal cyst detected by MRI, about 62 smaller cysts (< 0.9 mm) are present in the kidney. For a typical patient having an average of 587 cysts in both kidneys that are detectable by MRI, this means that more than 36,000 cysts are actually present, and MRI detects less than 2% of the total cysts present.
Although microcysts are too small to contribute much to total kidney volume, they can interfere with kidney function. Microcysts can reduce GFR in two major ways: by compressing microvasculature, tubules, and glomeruli in the cortex; or by blocking the drainage of multiple upstream nephrons when they form in or block medullary collecting ducts.52 Although the growth rates of microcysts less than 1 mm in size have not yet been measured, the adult combined growth rates of the renal cyst component is approximately 12% per year, with yearly individual cyst growth rates up to 71%, and with fetal cyst growth rates even higher for cysts larger than 7.0 mm.53 Before and during an accelerated growth period, microcysts may be susceptible to certain therapies that could first improve GFR and only later change measurable total kidney volume by slowing microcyst progression to macrocysts either directly or through specialized cells that may be sensitive to rapamycin.
CURRENT MANAGEMENT OF ADPKD
Blood pressure control is essential—but too low is not good. For adult patients with hypertension caused by ADPKD, an acceptable blood pressure range is 120–130/70–80 mm Hg. However, further information from recently published blood pressure guidelines54 and the results of the Halt Progression of Polycystic Kidney Disease (HALT-PKD) study to be reported in late 201455 may provide more precise ranges for blood pressure control in ADPKD.
Although substantial experimental evidence exists for the benefits of inhibiting the up-regulation of the renin-angiotensin-aldosterone system in ADPKD, clinical proof is not yet available to confirm that angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) are preferred therapy.55 This may be determined by results of the HALT-PKD study, due for release in late 2014.55
Controlling blood pressure should be done with caution. Patients with low GFRs whose blood pressure is too low tend to have a more rapid decline of GFR, as suggested in the Modification of Diet in Renal Disease (MDRD) study in 1995.56
Experimental evidence suggests that avoiding calcium channel blockers may be advisable. Yamaguchi et al34 found that calcium channel blockers worsen the calcium transport defect and convert tubular epithelial cells to a proliferative phenotype.34
High fluid intake (2,500–3,000 mL/day), because it suppresses vasopressin, may be useful if permitted by several factors such as the patient’s cardiopulmonary and renal and electrolyte status, other medications, and diet.31 The reader is referred to a detailed description of the precautions necessary when prescribing high water intake.31 Patients should have their fluid intake managed by a physician and their renal function and serum sodium and electrolytes monitored regularly in order to avoid hyponatremia. Severe hyponatremia has occurred in patients with ADPKD and impaired kidney function who drank excessive quantities of water. Cardiac and pulmonary complications from excessive fluid intake are also possible, especially with a low GFR and compromised cardiac function.
A low-sodium diet, if not a contributing factor in hyponatremia, can be used under physician direction in the management of hypertension as well as in the prevention of calcium oxalate kidney stones.
Caffeine should be avoided because it may interfere with the activity of the phosphodiesterase that is necessary for the catabolism of cAMP to 5′AMP.
A low-protein diet is of unproven benefit,56 but it is prudent to avoid high protein intake.57
Complications such as bleeding (into or from cysts), infection (urinary tract, kidney cysts, and liver cysts), kidney stones, and urinary tract obstruction should be treated promptly and may require hospitalization.
Regular symptom reviews and physical examinations need to be performed with nonrenal concerns also in mind, such as intracranial aneurysms and cardiac valve lesions.11,58
Formal genetic counseling and molecular testing are becoming more frequently indicated as more complex inheritance patterns arise.6–8,59
Renal replacement therapy in the form of dialysis or transplantation is usually available for the patient when end-stage renal disease occurs. In the largest study thus far, ADPKD patient survival with a kidney transplant was similar to that of patients without ADPKD (about 93% at 5 years), and from 5 years to 15 years death-censored graft survival was actually better.60 Thromboembolic events are more frequent after transplantation,27,60 but they may also occur before transplantation from a massive right kidney compressing the iliac vein or the inferior vena cava, or both, leading to thrombus formation.26
Investigational as well as standard drug studies have intensified. Results from a large randomized study in approximately 1,000 adult ADPKD patients that evaluated over 6 to 8 years the effects of ACE inhibition with or without ARB treatment of hypertension, at both usual and lower blood pressure ranges in those with good renal function, are expected in late 2014.55 Outcomes from a few small clinical studies, eg, one with long-acting somatostatin31,61 and one using low-dose rapamycin48 in adults with ADPKD, will require confirmation in large randomized placebo-controlled clinical studies. In a new 3-year randomized placebo-controlled study of 91 children and young adults (ages 8 to 22) with ADPKD and essentially normal renal function who continued treatment with lisinopril, the addition of pravastatin (20 mg or 40 mg daily based on age) resulted in a significant reduction in the number of patients (46% vs 68%, respectively, P = .03) experiencing a greater than 20% change (increase) in height-adjusted total kidney volume.62 Change in GFR was not reported,62 but an earlier 4-week study in 10 patients treated with simvastatin did show an increase in renal blood flow and GFR.63 Numerous other agents that lack human studies include some described in older experimental work (eg, amiloride,31,64 citrate31,65) and many others from a growing list of potential therapeutic targets.31,66–73 It must be emphasized that there is no FDA-approved medication specifically for the treatment of ADPKD.
Future specific treatments of ADPKD may also involve minimally toxic doses of combination or sequential therapy directed at precystic and then both micro- and macrocystic/cystic fluid expansion aspects of ADPKD.48,74 Unfortunately, at the present time there is no specific FDA-approved therapy for ADPKD.
- Torres VE, Harris PC. Mechanisms of disease: autosomal dominant and recessive polycystic kidney diseases. Nat Clin Pract Nephrol 2006; 2:40–55.
- Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int 2009; 76:149–168.
- United States Renal Data System. 2013 atlas of CKD & ESRD. Volume 2 - atlas ESRD:172. www.usrds.org/atlas.aspx. Accessed June 4, 2014.
- Barua M, Cil O, Paerson AD, et al. Family history of renal disease severity predicts the mutated gene in ADPKD. J Am Soc Nephrol 2009, 20:1833–1838.
- Harris PC, Bae KT, Rossetti S, et al. Cyst number but not the rate of cystic growth is associated with the mutated gene in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2006; 17:3013–3019.
- Vujic M, Heyer CM, Ars E, et al. Incompletely penetrant PKD1 alleles mimic the renal manifestations of ARPKD. J Am Soc Nephrol 2010; 21:1097–1102.
- Harris PC. What is the role of somatic mutation in autosomal dominant polycystic kidney disease? J Am Soc Nephrol 2010; 21:1073–1076.
- Watnick T, He N, Wang K, et al. Mutations of PKD1 in ADPKD2 cysts suggest a pathogenic effect of trans-heterozygous mutations. Nat Genet 2000; 25:143–144.
- Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 1994; 343:824–827.
- Pei Y, Obaji J, Dupuis A, et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol 2009; 20:205–212.
- Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet 2007; 369:1287–1301.
- Bajwa ZH, Sial KA, Malik AB, Steinman TI. Pain patterns in patients with polycystic kidney disease. Kidney Int 2004; 66:1561–1569.
- Jouret F, Lhommel R, Beguin C, et al. Positron-emission computed tomography in cyst infection diagnosis in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2011; 6:1644–1650.
- Nishiura JL, Neves RF, Eloi SR, Cintra SM, Ajzen SA, Heilberg IP. Evaluation of nephrolithiasis in autosomal dominant polycystic kidney disease patients. Clin J Am Soc Nephrol 2009; 4:838–844.
- Hiura T, Yamazaki H, Saeki T, et al. Nephrotic syndrome and IgA nephropathy in polycystic kidney disease. Clin Exp Nephrol 2006; 10:136–139.
- Hossack KF, Leddy CL, Johnson AM, Schrier RW, Gabow PA. Echocardiographic findings in autosomal dominant polycystic kidney disease. N Engl J Med 1988; 319:907–912.
- Rossetti S, Chauveau D, Kubly V, et al. Association of mutation position in polycystic kidney disease 1 (PKD1) gene and development of a vascular phenotype. Lancet 2003; 361:2196–2201.
- Linn FH, Wijdicks EF, van der Graaf Y, Weerdesteyn-van Vliet FA, Bartelds AI, van Gijn J. Prospective study of sentinel headache in aneurismal subarachnoid haemorrhage. Lancet 1994; 344:590–593.
- Belz MM, Fick-Brosnahan GM, Hughes RL, et al. Recurrence of intracranial aneurysms in autosomal-dominant polycystic kidney disease. Kidney Int 2003; 63:1824–1830.
- Irazabal MV, Huston J, Kubly V, et al. Extended follow-up of unruptured intracranial aneurysms detected by presymptomatic screening in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2011; 6:1274–1285.
- Salman A-S, White PM, Counsell CE, et al; Scottish Audit of Intracranial Vascular Malformations Collaborators. Outcome after conservative management or intervention for unruptured brain arteriovenous malformations. JAMA 2014; 311:1661–1669.
- Vijay A, Vijay A, Pankaj P. Autosomal dominant polycystic kidney disease: a comprehensive review. Nephrourol Mon 2010; 2:172–192.
- Grantham JJ, Torres VE, Chapman AB, et al; CRISP Investigators. Volume progression in polycystic kidney disease. N Engl J Med 2006; 354:2122–2130.
- Bae KT, Grantham JJ. Imaging for the prognosis of autosomal dominant polycystic kidney disease. Nat Rev Nephrol 2010; 6:96–106.
- van den Dool SW, Wasser NM, de Fijter JW, Hoekstra J, van der Geest RJ. Functional renal volume: quantitative analysis at gadolinium-enhanced MR angiography—feasibility study in healthy potential kidney donors. Radiology 2005; 236:189–195.
- O’Sullivan DA, Torres VE, Heit JA, Liggett S, King BF. Compression of the inferior vena cava by right renal cysts: an unusual cause of IVC and/or iliofemoral thrombosis with pulmonary embolism in autosomal dominant polycystic kidney disease. Clin Nephrol 1998; 49:332–334.
- Tveit DP, Hypolite I, Bucci J, et al. Risk factors for hospitalizations resulting from pulmonary embolism after renal transplantation in the United States. J Nephrol 2001; 14:361–368.
- Pei Y. A “two-hit” model of cystogenesis in autosomal dominant polycystic kidney disease? Trends Mol Med 2001; 7:151–156.
- Qian F, Germino GG. “Mistakes happen”: somatic mutation and disease. Am J Hum Genet 1997; 61:1000–1005.
- Takakura A, Contrino L, Zhou X, et al. Renal injury is a third hit promoting rapid development of adult polycystic kidney disease. Hum Mol Genet 2009; 18:2523–2531.
- Torres VE, Harris PC. Strategies targeting cAMP signaling in the treatment of polycystic kidney disease. J Am Soc Nephrol 2014; 25:18–32.
- Nauli SM, Alenghat FJ, Luo Y, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 2003; 33:129–137.
- Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med 2011; 364:1533–1543.
- Yamaguchi T, Wallace DP, Magenheimer BS, Hempson SJ, Grantham JJ, Calvet JP. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J Biol Chem 2004; 279:40419–40430.
- Verghese E, Ricardo SD, Weidenfeld R, et al. Renal primary cilia lengthen after acute tubular necrosis. J Am Soc Nephrol 2009; 20:2147–2153.
- Wang X, Wu Y, Ward CJ, Harris PC, Torres VE. Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol 2008; 19:102–108.
- Torres VE. Cyclic AMP, at the hub of the cystic cycle. Kidney Int 2004; 66:1283–1285.
- Nagao S, Nishii K, Katsuyama M, et al. Increased water intake decreases progression of polycystic kidney disease in the PCK rat. J Am Soc Nephrol 2006; 17:2220–2227.
- Grantham JJ. Therapy for polycystic kidney disease? It’s water, stupid! J Am Soc Nephrol 2008; 19:1–7.
- Wang CJ, Creed C, Winklhofer FT, Grantham JJ. Water prescription in autosomal dominant polycystic kidney disease: a pilot study. Clin J Am Soc Nephrol 2011; 6:192–197.
- Gattone VH, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 2003; 9:1323–1326.
- Torres VE, Chapman AB, Devuyst O, et al; TEMPO 3:4 Trial Investigators. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 2012; 367:2407–2418.
- Shillingford JM, Murcia NS, Larson CH, et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A 2006; 103:5466–5471.
- Hartman TR, Liu D, Zilfou JT, et al. The tuberous sclerosis proteins regulate formation of the primary cilium via a rapamycin-insensitive and polycystin 1-independent pathway. Hum Mol Genet 2009; 18:161–163.
- Serra AL, Poster D, Kistler AD, et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med 2010; 363:820–829.
- Walz G, Budde K, Mannaa M, et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med 2010; 363:830–840. Errata in: N Engl J Med 2010; 363:1190 and N Engl J Med 2010; 363:1977.
- Walz G, Budde K, Eckardt K-U. mTOR inhibitors and autosomal dominant polycystic kidney disease (correspondence). N Engl J Med 2011; 364:287–288.
- Braun WE, Schold JD, Stephany BR, Spinko RA, Herfs BR. Low dose rapamycin (sirolimus) effects in autosomal dominant polycystic kidney disease: an open-label randomized control pilot study. Clin J Am Soc Nephrol 2014; 9:881–888.
- Karihaloo A, Koraishy F, Huen SC, et al. Macrophages promote cyst growth in polycystic kidney disease. J Am Soc Nephrol 2011; 22:1809–1814.
- Fox R, Nhan TQ, Law GL, Morris DR, Liles WC, Schwartz SM. PSGL-1 and mTOR regulate translation of ROCK-1 and physiological functions of macrophages. EMBO J 2007; 26:505–515. Erratum in: EMBO J 2007; 26:2605.
- Carvalhosa R, Deambrosis I, Carrera P, et al. Cystogenic potential of CD133+ progenitor cells of human polycystic kidneys. J Pathol 2011; 225:129–141.
- Grantham JJ, Mulamalla S, Grantham CJ, et al. Detected renal cysts are tips of the iceberg in adults with ADPKD. Clin J Am Soc Nephrol 2012; 7:1087–1093.
- Grantham JJ, Cook LT, Wetzel LH, Cadnapaphornchai MA, Bae KT. Evidence of extraordinary growth in the progressive enlargement of renal cysts. Clin J Am Soc Nephrol 2010; 5:889–896.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Chapman AB, Torres VE, Perrone RD, et al. The HALT polycystic kidney disease trials: design and implementation. Clin J Am Soc Nephrol 2010; 5:102–109.
- Klahr S, Breyer JA, Beck GJ, et al. Dietary protein restriction, blood pressure control, and the progression of polycystic kidney disease. Modification of Diet in Renal Disease Study Group. J Am Soc Nephrol 1995; 5:2037–2047.
- Thilly N. Low-protein diet in chronic kidney disease: from questions of effectiveness to those of feasibility. Nephrol Dial Transplant 2013; 28:2203–2205.
- Luciano RL, Dahl NK. Extra-renal manifestations of autosomal dominant polycystic kidney disease (ADPKD): considerations for routine screening and management. Nephrol Dial Transplant 2014; 29:247–254.
- Harris PC, Rossetti S. Molecular diagnostics for autosomal dominant polycystic kidney disease. Nat Rev Nephrol 2010; 6:197–206.
- Jacquet A, Pallet N, Kessler M, et al. Outcomes of renal transplantation in patients with autosomal dominant polycystic kidney disease: a nationwide longitudinal study. Transpl Int 2011; 24:582–587.
- Ruggenenti P, Remuzzi A, Ondei P, et al. Safety and efficacy of long-acting somatostatin treatment in autosomal-dominant polycystic kidney disease. Kidney Int 2005; 68:206–216.
- Cadnapaphornchai MA, George DM, McFann K, et al. Effect of pravastatin on total kidney volume, left ventricular mass index, and microalbuminuria in pediatric autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2014; 9:889–896.
- van Dijk MA, Kamper AM, van Veen S, Souverjin JH, Blauw GJ. Effect of simvastatin on renal function in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 2001; 16:2152–2157.
- Grantham JJ, Uchich M, Cragoe EL, et al. Chemical modification of cell proliferation and fluid secretion in renal cysts. Kidney Int 1989; 35:1379–1389.
- Tanner GA. Potassium citrate/citric acid intake improves renal function in rats with polycystic kidney disease. J Am Soc Nephrol 1998; 9:1242–1248.
- Belibi FA, Edelstein CL. Novel targets for the treatment of autosomal dominant polycystic kidney disease. Expert Opin Investig Drugs 2010; 19:315–328.
- Tao Y, Kim J, Yin Y, et al. VEGF receptor inhibition slows the progression of polycystic kidney disease. Kidney Int 2007; 72:1358–1366.
- Terryn S, Ho A, Beauwens R, Devuyst O. Fluid transport and cystogenesis in autosomal dominant polycystic kidney disease. Biochim Biophys Acta 2011; 1812:1314–1321.
- Thiagarajah JR, Verkman AS. CFTR inhibitors for treating diarrheal disease. Clin Pharmacol Ther 2012; 92:287–290.
- Boehn SN, Spahn S, Neudecker S, et al. Inhibition of Comt with tolcapone slows proression of polycystic kidney disease in the more severely affected PKD/Mhm (cy/+) substrain of the Hannover Sprague-Dawley rat. Nephrol Dial Transplant 2013; 28:2045–2058.
- Rees S, Kittikulsuth W, Roos K, Strait KA, Van Hoek A, Kohan DE. Adenylyl cyclase 6 deficiency ameliorates polycystic kidney disease. J Am Soc Nephrol 2014; 25:232–237.
- Buchholz B, Schley G, Faria D, et al. Hypoxia-inducible factor-1a causes renal cyst expansion through calcium-activated chloride secretion. J Am Soc Nephrol 2014; 25:465–474.
- Wallace DP, White C, Savinkova L, et al. Periostin promotes renal cyst growth and interstitial fibrosis in polycystic kidney disease. Kidney Int 2014; 85:845–854.
- Leuenroth SJ, Crews CM. Targeting cyst initiation in ADPKD. J Am Soc Nephrol 2009; 20:1–3.
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited renal disease, has an estimated prevalence of 1:400 to 1:1,000 live births in the United States, and occurs worldwide.1,2 There are about 700,000 people living with it in the United States, and about 6,000 new cases arise annually. It accounts for nearly 5% of all patients with end-stage renal disease in the United States.3
This paper will offer an overview of the pathogenesis of renal cysts, review some of the clinical aspects of ADPKD including diagnosis and management of complications, and discuss recent drug trials and current management.
TWO TYPES—PKD1 IS MORE COMMON AND PROGRESSES MORE RAPIDLY
Two major forms of ADPKD are recognized and can usually be determined by genetic testing: PKD1, accounting for about 85% of cases, and PKD2, accounting for 15%.
The gene locus for PKD1 is on the short arm of the 16th chromosome (16p13.3), and its glycoprotein gene product is polycystin 1 (PC1), a large molecule with 4,303 amino acids.2 PC1 has a long N-terminal extracellular tail that can function as a mechanosensor. Disease progression is much faster with PKD1, and end-stage renal disease usually occurs before age 56.4
In PKD2, the gene locus is on the long arm of the fourth chromosome (4q21–23), and has a smaller glycoprotein gene product, polycystin 2 (PC2), that plays a role in calcium transport. The disease course of PKD2 tends to be slower. End-stage renal disease might not develop in the patient’s lifetime, since it typically develops when the patient is more than 70 years old.4
Although the growth rate of renal cysts is similar between the two types, patients with PKD1 develop about twice as many cysts as those with PDK2, and their cyst development starts at a younger age.5
Typically, patients have a clear phenotype and a positive family history, but in about 10% of possible ADPKD cases, there is no family history of ADPKD. Genetic variations such as incompletely penetrant PKD1 alleles,6 hypomorphic alleles,7 and trans-heterozygous mutations8 account for at least some of these cases.
IMAGING CRITERIA HAVE BROADENED
Ultrasonographic criteria for the diagnosis of ADPKD that were published in 1994 were based on patients who had a family history of PKD1.9 The criteria have since been modified (the “unified criteria”) to include patients with a family history of PKD2 who begin cyst development at a later age and with lower numbers.10 For patients ages 30 to 39, a previously difficult diagnostic group, the criterion for the minimum number of cysts visible on ultrasonography changed from four to three, improving the sensitivity of detecting disease from approximately 76% to approximately 95% (Table 1).9,10 It is important to note that these criteria apply only to patients “at risk,” ie, with a positive family history of ADPKD.
Computed tomography (CT) and magnetic resonance imaging (MRI) classically show bilaterally enlarged multicystic kidneys, though variations can be seen.
DISEASE CAN PRESENT IN MYRIAD WAYS
Although cystic kidney disease is the basic underlying problem, undiagnosed patients may present with a variety of symptoms caused by other manifestations of ADPKD (Table 2).
Hypertension is the most common presentation, occurring in about 50% of patients ages 20 to 34, and essentially 100% of those with end-stage renal disease.11 It is associated with up-regulation of the renin-angiotensin-aldosterone system.
Pain is typically located in the abdomen, flank, or back and can occur in a localized or diffuse manner. Early abdominal distress is often simply described as “fullness.” Localized pain is usually caused by bleeding into or rupture of a cyst, renal stones, or infection.12 Because renal cysts are noncommunicating, bleeding can occur into a cyst and cause pain without gross hematuria. Compression by greatly enlarged kidneys, liver, or both can cause a variety of gastrointestinal symptoms such as reflux esophagitis and varying degrees of constipation. Diffuse pain is often musculoskeletal and related to exaggerated lordosis from increasing abdominal size due to enlarging cystic kidneys and sometimes liver.12 In carefully selected cases, cyst aspiration may be helpful.11
Although renal carcinomas are rare and not more frequent than in the general population, they can occur at an earlier age and with constitutional symptoms.11
Urinary tract infections are increased in frequency. A patient may have a simple urinary tract infection that is cured with the appropriate antibiotic. However, a urinary tract infection repeatedly recurring with the same organism is a strong clue that an infected cyst is the source and requires more intensive treatment with the appropriate cyst-penetrating antibiotic. On the other hand, because cysts are noncommunicating, an infected cyst might be present despite a negative urine culture.
Identifying infected cysts can be a challenge with conventional imaging techniques, but combined positron emission tomography and CT (PET/CT) can be a valuable though expensive diagnostic tool to identify an infected kidney or liver cyst, or to identify an unsuspected source of the pain and infection.13
Jouret et al13 evaluated 27 PET/CT scans performed in 24 patients with ADPKD and suspicion of an abdominal infection. Patients were deemed to have probable cyst infection if they met all of the following criteria: temperature more than 38°C for longer than 3 days, loin or liver tenderness, plasma C-reactive protein level greater than 5 mg/dL, and no evidence of intracystic bleeding on CT. Patients with only two or three of these criteria were classified as having fever of unknown origin. Diagnosis of cyst infection was confirmed by cyst fluid analysis.
PET/CT identified a kidney or liver cyst infection in 85% of 13 infectious events in 11 patients who met all the criteria for probable cyst infection; CT alone contributed to the diagnosis in only one patient.13 In those with fever of unknown origin, PET/CT identified a source of infection in 64% of 14 events in 13 patients: two infected renal cysts, as well as one patient each with other infections that would be difficult to diagnose clinically, ie, small bowel diverticulitis, psoas abscess, diverticulitis of the right colon, pyelonephritis in a transplanted kidney, infected abdominal aortic aneurysm, prostatitis, colitis, and Helicobacter pylori gastritis. Results of PET/CT were negative in five patients with intracystic bleeding.
Kidney stones occur in 20% to 36% of patients.11,14 Uric acid stones occur at almost the same frequency as calcium oxalate stones.
Chronic kidney disease not previously diagnosed may be the presenting condition in a small percentage of patients, sometimes those in whom much earlier hypertension was not fully evaluated. ADPKD is typically not associated with significant proteinuria (eg, nephrotic range), and the presence of heavy proteinuria usually indicates the presence of a superimposed primary glomerulopathy.15
Cysts in other locations. By MRI, liver cysts are present in 58% of patients ages 15 to 24, rising to 94% in those ages 35 to 46.11 Because liver cysts are estrogen-dependent, they are more prominent in women. A small percentage of patients develop cysts in the pancreas (5%), arachnoid membranes (8%), and seminal vesicles (40% of men with ADPKD).11
Cardiovascular abnormalities occur in almost one-third of patients with ADPKD, usually as mitral and aortic valve abnormalities.16 Aneurysms of the aortic root and abdominal aorta can also occur, in addition to intracranial aneurysms (see below).17
Intracranial aneurysms are not uncommon, and size usually determines their risk.
Intracranial aneurysms are strongly influenced by family history: 16% of ADPKD patients with a family history of intracranial aneurysm also develop them, compared with 5% to 6% of patients with no family history.11 The anterior cerebral circulation is involved in about 80% of cases. A sentinel or sudden “thunderclap” headache is a classic presentation that may precede full-blown rupture in about 17% of cases.18 Patients who rupture an intracranial aneurysm have a mean age of 39, usually have normal renal function, and can be normotensive.11
For patients with no history of subarachnoid hemorrhage, the 5-year cumulative rupture rates for patients with aneurysms located in the internal carotid artery, anterior communicating or anterior cerebral artery, or middle cerebral artery were 0% for aneurysms less than 7 mm, 2.6% for those 7 to 12 mm, 14.5% for those 13 to 24 mm, and 40% for those 25 mm or larger, with higher rates for the same sizes in the posterior circulation.11
In patients without symptoms, size is correlated with risk of rupture: less than 4 mm is usually associated with very low risk, 4 to less than 7 mm with moderate risk, and 7 mm or more with increasing risk. An aneurysm larger than 10 mm is associated with roughly a 1% risk of rupture per year.19
Irazabal et al20 retrospectively studied 407 patients with ADPKD who were screened for intracranial aneurysm. Saccular aneurysms were detected in 45 patients; most were small (median diameter 3.5 mm). During cumulative imaging follow-up of 243 years, only one new intracranial aneurysm was detected (increasing from 2 to 4.4 mm over 144 months) and two previously identified aneurysms grew (one increasing 4.5 to 5.9 mm over 69 months and the other 4.7 to 6.2 mm over 184 months). No change occurred in 28 patients. Seven patients were lost to follow-up, however. During cumulative clinical follow-up of 316 years, no aneurysm ruptured. Two patients were lost to follow-up, three had surgical clipping, and five died of unrelated causes. The authors concluded that presymptomatic intracranial aneurysms are usually small, and that growth and rupture risks are no higher than for unruptured intracranial aneurysms in the general population. A 2014 study also suggests a conservative approach for managing intracranial aneurysm in the general population.21
In asymptomatic ADPKD patients, it is reasonable to reserve screening for those with a positive family history of intracranial aneurysm or subarachnoid hemorrhage, those with a previous ruptured aneurysm, those in high-risk professions (eg, pilots), and for patients prior to anticoagulant therapy or major surgery possibly associated with hemodynamic instability.11,22 Certain extremely anxious patients might also need to be studied. Screening can be performed with magnetic resonance angiography without gadolinium contrast. It is prudent to have patients with an intracranial aneurysm thoroughly evaluated by an experienced neurosurgeon with continued follow-up.
PROGRESSION OF ADPKD
The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) study23 evaluated 241 patients with ADPKD (ages 15 to 46) by measuring the annual rate of change in total kidney volume, total cyst volume, and iothalamate glomerular filtration rate (GFR) over 3 years. The annual increase in total kidney volume averaged 5.3%,23 though the reported range with various imaging techniques is from 4% to 12.8% in adults.24 This study focused on macrocystic disease, ie, cysts that are visible by MRI and measurably increase total kidney volume. Although larger total kidney volume at baseline generally predicted a more rapid decline in GFR, there were wide and overlapping variations in yearly GFR declines within and among different total-kidney-volume groups.23
SPECIAL CLINICAL PROBLEMS IN ADPKD
Case 1: A man with ADPKD develops new and increasing proteinuria
A 55-year-old man with ADPKD and stage 3 chronic kidney disease developed new and increasing proteinuria, rising to 5,500 mg per 24 hours. What is the most likely explanation?
- Rapidly progressive renal failure with increasing proteinuria in ADPKD
- Bilateral renal vein thromboses because of cyst compression
- Malignant hypertension with bilateral renal artery compression
- Superimposed primary glomerulopathy
- Multiple infected renal cysts with pyonephrosis
Answer: Superimposed primary glomerulopathy.
ADPKD (similar to uncomplicated obstructive uropathy, pyelonephritis, main renal artery disease, and often cases of interstitial nephritis without secondary glomerular changes) typically does not result in nephrotic-range proteinuria. A superimposed primary glomerulopathy, focal segmental glomerulosclerosis, was the biopsy-proved diagnosis.
At least 21 cases have been reported of AD-PKD with nephrotic-range proteinuria and a renal biopsy showing a primary glomerulopathy, including focal segmental glomerulosclerosis (5 cases), minimal-change disease (5), membranous nephropathy (3), IgA nephropathy (2), and one each of crescentic glomerulonephropathy, diabetic nephropathy, membranoproliferative glomerulonephritis, postinfectious glomerulonephropathy, amyloid glomerulopathy, and mesangioproliferative glomerulopathy.15 Treatment was directed at the primary glomerulopathy, and the outcomes corresponded to the primary diagnosis (eg, with appropriate treatment, three of the five patients with focal segmental glomerulosclerosis progressed to end-stage renal disease, all of the patients with minimal-change disease went into remission, and one of the two cases with IgA nephropathy improved).15
Case 2: A woman with ADPKD and advanced renal failure develops shortness of breath
A 47-year-old woman with very large polycystic kidneys (total kidney volume 7,500 mL; normal range for a single kidney approximately 136–295 mL, mean 196)25 and estimated GFR of 25 mL/min developed new-onset shortness of breath while climbing steps and later even when making a bed. She had no chest pain, cough, or edema. She was sent directly to the emergency department and was admitted and treated; her condition improved, and she was discharged after 6 days. What did she have?
- Presentation of rare cystic pulmonary disease in ADPKD
- Onset of pneumonia with early bacteremia
- Progressive reduction in ventilatory capacity from massive polycystic kidneys and liver elevating both sides of the diaphragm
- Pulmonary emboli from an iliac vein or inferior vena cava source
- Progressive anemia accompanying rapidly worsening stage 4 chronic kidney disease
Answer: She had pulmonary emboli from an iliac vein (right) or inferior vena cava source.
Pulmonary emboli in ADPKD can be caused by thrombi in the inferior vena cava or the iliac or femoral vein because of compression by a massive right polycystic kidney. Four cases were reported at Mayo Clinic,26 three diagnosed by MRI and one with CT. One additional case occurred at Cleveland Clinic. All patients survived after treatment with anticoagulation therapy; early nephrectomy was required in two cases.
Interestingly, following kidney transplantation, the patients at greatest risk for pulmonary emboli are those with ADPKD as their original disease.27
RENAL CYSTS RESULT FROM COMBINED MUTATIONS, INJURY
The germline ADPKD mutation that occurs in one allele of all renal tubular epithelial cells is necessary but not sufficient for cystogenesis.28 One or more additional somatic mutations of the normal allele—the “second hit”—also develop within individual tubular epithelial cells.28,29 These epithelial cells undergo clonal proliferation, resulting in tubular dilatation and cyst formation. Monoclonality of cells in cysts has been documented.
Ischemia-reperfusion injury can be viewed as a “third hit.”30 In PKD1 knockout mice, which at 5 weeks of age normally develop only mild cystic kidney disease, the superimposition of unilateral ischemia-reperfusion injury at 8 weeks caused widespread and rapid cyst formation. It is believed that acute renal injury reactivates developmental signaling pathways within 48 hours that trigger epithelial cell proliferation and then cyst development detectable by MRI 2 weeks later. Although this phenomenon has not been documented in humans, it is a cautionary tale.
CYSTOGENESIS INVOLVES MULTIPLE PATHWAYS
A comprehensive description of pathways leading to renal cyst formation is beyond the scope of this article, and the reader is referred to much more detailed and extensive reviews.2,31 Disturbances in at least three major interconnected pathways promote cystogenesis in renal tubular epithelial cells:
- Normal calcium transport into the endoplasmic reticulum is disrupted by abnormal polycystins on the surface of the primary cilium
- Vasopressin and other stimuli increase the production of cyclic adenosine monophosphate (cAMP)
- The mammalian target of rapamycin (mTOR) proliferative pathway is up-regulated.
DISRUPTION OF CALCIUM TRANSPORT IN THE PRIMARY CILIUM
Primary cilia are nonmotile cellular organelles of varying size, from about 0.25 μm up to about 1 μm.32 Each primary cilium has nine peripheral pairs of microtubules but lacks a centrally located pair that is present in motile cilia. Primary cilia are ubiquitous and have been highly conserved throughout evolution. A single cilium is present on almost all vertebral cells.33
Cilial defects have been identified in autosomal dominant as well as recessive diseases and are known as ciliopathies.33 Although rare in humans, they can affect a broad spectrum of organs other than the kidney, including the eye, liver, and brain.33
Urine flow in a renal tubule is believed to exert mechanical stimulation on the extracellular flagellum-like N-terminal tail of PC1 that extends from a primary cilium into the urinary space. PC1 in concert with PC2 opens PC2 calcium channels, allowing calcium ions to flow down the microtubules to ryanodine receptors and the basal body.32,33 This leads to local release of calcium ions that regulate cell proliferation.32,34 However, in ADPKD kidneys, PC1 and PC2 molecules are sparse or mutated, resulting in defective calcium transport, increased and unregulated tubular epithelial cell proliferation, and cyst formation.
In a totally different clinical setting, biopsies of human renal transplants that sustained acute tubular necrosis during transplantation reveal that a cilium dramatically elongates in response to injury,35 possibly as a compensatory mechanism to maintain calcium transport in the presence of meager urine flow and to restore the proliferation of tubular epithelial cells in a regulated repair process.
THE ROLE OF VASOPRESSIN AND ACTIVATION OF cAMP
In classic experiments, Wang et al36 cross-bred rats having genetically inherited polycystic kidney disease (actually, autosomal recessive polycystic kidney disease) with Brattleboro rats that completely lack vasopressin. At 10 and 20 weeks of age, the offspring had virtually complete inhibition of cystogenesis because of the absence of vasopressin. However, when vasopressin was restored by exogenous administration continuously for 8 weeks, the animals formed massive renal cysts.
Vasopressin activates cAMP, which then functions as a second messenger in cell signaling. cAMP increases the activation of the protein kinase A (PKA) pathway, which in turn increases downstream activity of the B-raf/ERK pathway. Up-regulation of cAMP and PKA appears to perpetuate activation of canonical Wnt signaling, down-regulate non-canonical Wnt/planar cell polarity signaling, and lead to loss of tubular diameter control, resulting in cyst formation.31 Normally, cAMP is degraded by phosphodiesterase. However, because of the primary cilium calcium transport defect in ADPKD, phosphodiesterase is reduced and cAMP persists.37 In conjunction with the defective primary cilial calcium transport, cAMP exerts a proliferative effect on renal tubular epithelial cells that is opposite to its effect in normal kidneys.31,32 cAMP also up-regulates the cystic fibrosis transmembrane conductance regulator (CFTR) that promotes chloride ion transport. Sodium ions follow the chloride ions, leading to fluid accumulation and cyst enlargement.31
Inhibiting vasopressin by increasing water intake
A simple key mechanism for limiting vasopressin secretion is by sufficient water ingestion. Nagao et al38 found that rats with polycystic kidney disease given water with 5% glucose (resulting in 3.5-fold increased fluid intake compared with rats given tap water) had a 68% reduction in urinary vasopressin and a urine osmolality less than 290 mOsm/kg. The high-water-intake rats had dramatically reduced cystic areas in the kidney and a 28% reduction of kidney-to-body weight ratio vs controls.
In an obvious oversimplification, these findings raised the question of whether a sufficient increase in water intake could be an effective therapy for polycystic kidney disease.39 A pilot clinical study evaluated changes in urine osmolality in eight patients with ADPKD who had normal renal function.40 At baseline, 24-hour urine osmolality was typically elevated to approximately 753 mOsm/kg compared to the plasma at 285 mOsm/kg, indicating that antidiuresis is the usual state. During the 2-week study, urine volume and osmolality were measured, and additional water intake was adjusted in order to achieve a urine osmolality goal of 285 ± 45 mOsm/kg. These adjustments resulted in water intake that appeared to be in the range of 2,400 to 3,000 mL per 24 hours. The major limitations of the study were that it was very short term, and there was no opportunity to measure changes in total kidney volume or estimated GFR.
In a recent preliminary report from Japan, high water intake (2,500–3,000 mL daily) in 18 ADPKD patients was compared over 12 months with ad libitum water intake in 14 ADPKD controls (clinicaltrials.gov NCT 01348505). There was no statistically significant change in total kidney volume or cystatin-estimated GFR in those on high water intake, but serious defects in study design (patients in the high water intake group were allowed to decrease their intake if it was causing them difficulty, and patients in the ad libitum water intake group had no measurement of their actual water intake) prevent any conclusions because there was no evidence that the groups were different from one another with respect to the key element of the study, namely, water intake.
Blocking the vasopressin receptor slows disease progression
Using another approach, Gattone et al41 inhibited the effect of vasopressin by blocking the vasopressin 2 receptor (V2R) in mouse and rat models of polycystic kidney disease, using an experimental drug, OPC31260. The drug halted disease progression and, in one situation, appeared to cause regression of established disease. As noted by Torres and Harris,31 even though both increased water intake and V2R antagonists decrease cAMP in the distal tubules and collecting ducts, circulating levels of vasopressin are decreased by increased water intake but increased by V2R antagonists.
After these remarkable results in animal models, clinical trials of the V2R antagonist tolvaptan were conducted in patients with ADPKD. In the Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes 3:4 study,42 1,445 adults (ages 18 to 50) with ADPKD in 133 centers worldwide were randomized to receive either tolvaptan or placebo for 3 years. Key inclusion criteria included good renal function (estimated GFR ≥ 60 mL/min) and total kidney volume of at least 750 mL (mean 1,700 mL) as measured by MRI. Tolvaptan was titrated to the highest tolerated twice-daily dose (average total of 95 mg/day). All patients were advised to maintain good hydration and to avoid thirst by drinking a glass of water after each urination. Unfortunately, neither water intake nor urine output was measured.
The primary end point was the annual rate of change in total kidney volume, with secondary end points of clinical progression (worsening kidney function, pain, hypertension, albuminuria), and rate of decline in kidney function as measured by the slope of the reciprocal of serum creatinine.42
Patients in the tolvaptan arm had a slower annual increase in total kidney volume than controls (2.8% vs 5.5%, respectively, P < .001) and a slower annual decline in renal function (−2.61 vs −3.81 mg/mL−1, respectively, P < .001).42 More participants in the treatment group withdrew than in the placebo group (23% vs 14%, respectively).
Adverse events occurred more frequently with tolvaptan.42 Liver enzyme elevations of greater than three times the upper limit of normal occurred in 4.4% of patients in the treatment group, leading to a drug warning issued in January 2013. As expected, side effects related to diuresis (urinary frequency, nocturia, polyuria, and thirst) were more frequent in the treatment group, occurring in up to 55% of participants.
The authors noted, “Although maintaining hydration helped ensure that the blinding in the study was maintained, the suppression of vasopressin release in the placebo group may have led to an underestimation of the beneficial effect of tolvaptan and may account for the lower rates of kidney growth observed in the placebo group.”42
In 2013, the US Food and Drug Administration (FDA) denied a new drug application for tolvaptan as a treatment for ADPKD.
THE mTOR PATHWAY IS UP-REGULATED
The mTOR pathway that plays a major role in cell growth and proliferation includes interaction of the cytoplasmic tail of polycystin 1 with tuberin.43 Activation products of mTOR, including phospho-S6K, have been found in tubular epithelial cells lining cysts of ADPKD kidneys but not in normal kidneys.43 Mutant mice with polycystic disease had phospho-S6K in tubular epithelial cells of cysts, whereas those treated with the mTOR inhibitor rapamycin did not.43 But subsequent studies have shown that only a low level of mTOR activation is present in 65% to 70% of ADPKD cysts.44
Two major studies of the treatment of ADPKD with rapamycin that were published contemporaneously in 2010 failed to demonstrate any significant benefit with mTOR inhibitor treatment.45,46
Serra et al45 conducted an 18-month, open-label trial of 100 ADPKD patients ages 18 to 40 with an estimated GFR (eGFR) of at least 70 mL/min. Patients were randomized to receive rapamycin, given as sirolimus 2 mg per day, or standard care. The primary end point was the reduction in the growth rate of total kidney volume, measured by MRI. Secondary end points were eGFR and protein excretion (albumin-creatinine ratio). No significant difference was found in total kidney volume, but a nonsignificant stabilization of eGFR was noted.
Walz et al46 in a 2-year, multicenter, double-blind trial, randomized 433 patients (mean age 44; mean eGFR 54.5 mL/min) to treatment with either the short-acting mTOR inhibitor everolimus (2.5 mg twice daily) or placebo. Although patients in the treatment group had less of an increase in total kidney volume (significant at 1 year but not at 2 years), they tended to show a decline in eGFR. But further analysis showed that the only patients who had a reduction in eGFR were males who already had impaired kidney function at baseline.47
In a pilot study, 30 patients with ADPKD (mean age 49) were randomized to one of three therapies:
- Low-dose rapamycin (trough blood level 2–5 ng/mL)
- Standard-dose rapamycin (trough blood level > 5–8 ng/mL)
- Standard care without rapamycin.48
In contrast to other studies, the primary end point was the change in iothalamate GFR at 12 months, with change in total kidney volume being a secondary end point.
At 12 months, with 26 patients completing the study, the low-dose rapamycin group (n = 9) had a significant increase in iothalamate GFR of 7.7 ± 12.5 mL/min/1.73 m2, whereas the standard-dose rapamycin group (n = 8) had a nonsignificant increase of 1.6 ± 12.1 mL/min/1.73 m2, and the no-rapamycin group (n = 9) had a fall in iothalamate GFR of 11.2 ± 9.1 mL/min/1.73 m2 (P = .005 for low-dose vs no rapamycin; P = .07 for standard-dose vs no rapamycin; P = .52 for low-dose vs standard-dose rapamycin; and P = .002 for combined low-dose and standard-dose rapamycin vs no rapamycin.).48 These differences were observed despite there being no significant change in total kidney volume in any of the groups. Patients on low-dose rapamycin had fewer adverse effects than those on standard dose and were more often able to continue therapy for the entire study. This, and the use of iothalamate GFR rather than eGFR to measure GFR, are believed to be the main reasons that low-dose effects were more pronounced than those with standard doses. One may speculate that rapamycin may have its effect on microcysts and cystogenic cells, resulting in stabilization of or improvement in renal function without detectable slowing in total kidney volume enlargement. Mechanisms for this possibility involve new concepts, as discussed below.
NEW CONCEPTS
Specialized cells also promote renal cyst formation
Specialized cells that promote cyst formation have been identified by Karihaloo et al49 in a mouse model of polycystic kidney disease. In this model, alternatively activated macrophages homed to cystic areas and promoted cyst growth. These findings suggested that interrupting the homing and proliferative signals of macrophages could be a therapeutic target for ADPKD. Although rapamycin can suppress macrophage proliferation by macrophage colony-stimulating factor and inhibit macrophage function,50 alternatively activated macrophages have not been specifically studied for rapamycin responsiveness.
More promising is evidence that CD133+ progenitor cells from human ADPKD kidneys—but not from normal human kidneys—form cysts in vitro and in severe combined immunodeficient mouse models.51 Treatment with rapamycin decreased proliferation of the de-differentiated CD133+ cells from ADPKD patients and reduced cystogenesis.51
Visible cysts are the tip of the iceberg
Using ADPKD nephrectomy specimens from eight patients, Grantham et al52 compared cyst counts by MRI and by histology and found that for every renal cyst detected by MRI, about 62 smaller cysts (< 0.9 mm) are present in the kidney. For a typical patient having an average of 587 cysts in both kidneys that are detectable by MRI, this means that more than 36,000 cysts are actually present, and MRI detects less than 2% of the total cysts present.
Although microcysts are too small to contribute much to total kidney volume, they can interfere with kidney function. Microcysts can reduce GFR in two major ways: by compressing microvasculature, tubules, and glomeruli in the cortex; or by blocking the drainage of multiple upstream nephrons when they form in or block medullary collecting ducts.52 Although the growth rates of microcysts less than 1 mm in size have not yet been measured, the adult combined growth rates of the renal cyst component is approximately 12% per year, with yearly individual cyst growth rates up to 71%, and with fetal cyst growth rates even higher for cysts larger than 7.0 mm.53 Before and during an accelerated growth period, microcysts may be susceptible to certain therapies that could first improve GFR and only later change measurable total kidney volume by slowing microcyst progression to macrocysts either directly or through specialized cells that may be sensitive to rapamycin.
CURRENT MANAGEMENT OF ADPKD
Blood pressure control is essential—but too low is not good. For adult patients with hypertension caused by ADPKD, an acceptable blood pressure range is 120–130/70–80 mm Hg. However, further information from recently published blood pressure guidelines54 and the results of the Halt Progression of Polycystic Kidney Disease (HALT-PKD) study to be reported in late 201455 may provide more precise ranges for blood pressure control in ADPKD.
Although substantial experimental evidence exists for the benefits of inhibiting the up-regulation of the renin-angiotensin-aldosterone system in ADPKD, clinical proof is not yet available to confirm that angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) are preferred therapy.55 This may be determined by results of the HALT-PKD study, due for release in late 2014.55
Controlling blood pressure should be done with caution. Patients with low GFRs whose blood pressure is too low tend to have a more rapid decline of GFR, as suggested in the Modification of Diet in Renal Disease (MDRD) study in 1995.56
Experimental evidence suggests that avoiding calcium channel blockers may be advisable. Yamaguchi et al34 found that calcium channel blockers worsen the calcium transport defect and convert tubular epithelial cells to a proliferative phenotype.34
High fluid intake (2,500–3,000 mL/day), because it suppresses vasopressin, may be useful if permitted by several factors such as the patient’s cardiopulmonary and renal and electrolyte status, other medications, and diet.31 The reader is referred to a detailed description of the precautions necessary when prescribing high water intake.31 Patients should have their fluid intake managed by a physician and their renal function and serum sodium and electrolytes monitored regularly in order to avoid hyponatremia. Severe hyponatremia has occurred in patients with ADPKD and impaired kidney function who drank excessive quantities of water. Cardiac and pulmonary complications from excessive fluid intake are also possible, especially with a low GFR and compromised cardiac function.
A low-sodium diet, if not a contributing factor in hyponatremia, can be used under physician direction in the management of hypertension as well as in the prevention of calcium oxalate kidney stones.
Caffeine should be avoided because it may interfere with the activity of the phosphodiesterase that is necessary for the catabolism of cAMP to 5′AMP.
A low-protein diet is of unproven benefit,56 but it is prudent to avoid high protein intake.57
Complications such as bleeding (into or from cysts), infection (urinary tract, kidney cysts, and liver cysts), kidney stones, and urinary tract obstruction should be treated promptly and may require hospitalization.
Regular symptom reviews and physical examinations need to be performed with nonrenal concerns also in mind, such as intracranial aneurysms and cardiac valve lesions.11,58
Formal genetic counseling and molecular testing are becoming more frequently indicated as more complex inheritance patterns arise.6–8,59
Renal replacement therapy in the form of dialysis or transplantation is usually available for the patient when end-stage renal disease occurs. In the largest study thus far, ADPKD patient survival with a kidney transplant was similar to that of patients without ADPKD (about 93% at 5 years), and from 5 years to 15 years death-censored graft survival was actually better.60 Thromboembolic events are more frequent after transplantation,27,60 but they may also occur before transplantation from a massive right kidney compressing the iliac vein or the inferior vena cava, or both, leading to thrombus formation.26
Investigational as well as standard drug studies have intensified. Results from a large randomized study in approximately 1,000 adult ADPKD patients that evaluated over 6 to 8 years the effects of ACE inhibition with or without ARB treatment of hypertension, at both usual and lower blood pressure ranges in those with good renal function, are expected in late 2014.55 Outcomes from a few small clinical studies, eg, one with long-acting somatostatin31,61 and one using low-dose rapamycin48 in adults with ADPKD, will require confirmation in large randomized placebo-controlled clinical studies. In a new 3-year randomized placebo-controlled study of 91 children and young adults (ages 8 to 22) with ADPKD and essentially normal renal function who continued treatment with lisinopril, the addition of pravastatin (20 mg or 40 mg daily based on age) resulted in a significant reduction in the number of patients (46% vs 68%, respectively, P = .03) experiencing a greater than 20% change (increase) in height-adjusted total kidney volume.62 Change in GFR was not reported,62 but an earlier 4-week study in 10 patients treated with simvastatin did show an increase in renal blood flow and GFR.63 Numerous other agents that lack human studies include some described in older experimental work (eg, amiloride,31,64 citrate31,65) and many others from a growing list of potential therapeutic targets.31,66–73 It must be emphasized that there is no FDA-approved medication specifically for the treatment of ADPKD.
Future specific treatments of ADPKD may also involve minimally toxic doses of combination or sequential therapy directed at precystic and then both micro- and macrocystic/cystic fluid expansion aspects of ADPKD.48,74 Unfortunately, at the present time there is no specific FDA-approved therapy for ADPKD.
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited renal disease, has an estimated prevalence of 1:400 to 1:1,000 live births in the United States, and occurs worldwide.1,2 There are about 700,000 people living with it in the United States, and about 6,000 new cases arise annually. It accounts for nearly 5% of all patients with end-stage renal disease in the United States.3
This paper will offer an overview of the pathogenesis of renal cysts, review some of the clinical aspects of ADPKD including diagnosis and management of complications, and discuss recent drug trials and current management.
TWO TYPES—PKD1 IS MORE COMMON AND PROGRESSES MORE RAPIDLY
Two major forms of ADPKD are recognized and can usually be determined by genetic testing: PKD1, accounting for about 85% of cases, and PKD2, accounting for 15%.
The gene locus for PKD1 is on the short arm of the 16th chromosome (16p13.3), and its glycoprotein gene product is polycystin 1 (PC1), a large molecule with 4,303 amino acids.2 PC1 has a long N-terminal extracellular tail that can function as a mechanosensor. Disease progression is much faster with PKD1, and end-stage renal disease usually occurs before age 56.4
In PKD2, the gene locus is on the long arm of the fourth chromosome (4q21–23), and has a smaller glycoprotein gene product, polycystin 2 (PC2), that plays a role in calcium transport. The disease course of PKD2 tends to be slower. End-stage renal disease might not develop in the patient’s lifetime, since it typically develops when the patient is more than 70 years old.4
Although the growth rate of renal cysts is similar between the two types, patients with PKD1 develop about twice as many cysts as those with PDK2, and their cyst development starts at a younger age.5
Typically, patients have a clear phenotype and a positive family history, but in about 10% of possible ADPKD cases, there is no family history of ADPKD. Genetic variations such as incompletely penetrant PKD1 alleles,6 hypomorphic alleles,7 and trans-heterozygous mutations8 account for at least some of these cases.
IMAGING CRITERIA HAVE BROADENED
Ultrasonographic criteria for the diagnosis of ADPKD that were published in 1994 were based on patients who had a family history of PKD1.9 The criteria have since been modified (the “unified criteria”) to include patients with a family history of PKD2 who begin cyst development at a later age and with lower numbers.10 For patients ages 30 to 39, a previously difficult diagnostic group, the criterion for the minimum number of cysts visible on ultrasonography changed from four to three, improving the sensitivity of detecting disease from approximately 76% to approximately 95% (Table 1).9,10 It is important to note that these criteria apply only to patients “at risk,” ie, with a positive family history of ADPKD.
Computed tomography (CT) and magnetic resonance imaging (MRI) classically show bilaterally enlarged multicystic kidneys, though variations can be seen.
DISEASE CAN PRESENT IN MYRIAD WAYS
Although cystic kidney disease is the basic underlying problem, undiagnosed patients may present with a variety of symptoms caused by other manifestations of ADPKD (Table 2).
Hypertension is the most common presentation, occurring in about 50% of patients ages 20 to 34, and essentially 100% of those with end-stage renal disease.11 It is associated with up-regulation of the renin-angiotensin-aldosterone system.
Pain is typically located in the abdomen, flank, or back and can occur in a localized or diffuse manner. Early abdominal distress is often simply described as “fullness.” Localized pain is usually caused by bleeding into or rupture of a cyst, renal stones, or infection.12 Because renal cysts are noncommunicating, bleeding can occur into a cyst and cause pain without gross hematuria. Compression by greatly enlarged kidneys, liver, or both can cause a variety of gastrointestinal symptoms such as reflux esophagitis and varying degrees of constipation. Diffuse pain is often musculoskeletal and related to exaggerated lordosis from increasing abdominal size due to enlarging cystic kidneys and sometimes liver.12 In carefully selected cases, cyst aspiration may be helpful.11
Although renal carcinomas are rare and not more frequent than in the general population, they can occur at an earlier age and with constitutional symptoms.11
Urinary tract infections are increased in frequency. A patient may have a simple urinary tract infection that is cured with the appropriate antibiotic. However, a urinary tract infection repeatedly recurring with the same organism is a strong clue that an infected cyst is the source and requires more intensive treatment with the appropriate cyst-penetrating antibiotic. On the other hand, because cysts are noncommunicating, an infected cyst might be present despite a negative urine culture.
Identifying infected cysts can be a challenge with conventional imaging techniques, but combined positron emission tomography and CT (PET/CT) can be a valuable though expensive diagnostic tool to identify an infected kidney or liver cyst, or to identify an unsuspected source of the pain and infection.13
Jouret et al13 evaluated 27 PET/CT scans performed in 24 patients with ADPKD and suspicion of an abdominal infection. Patients were deemed to have probable cyst infection if they met all of the following criteria: temperature more than 38°C for longer than 3 days, loin or liver tenderness, plasma C-reactive protein level greater than 5 mg/dL, and no evidence of intracystic bleeding on CT. Patients with only two or three of these criteria were classified as having fever of unknown origin. Diagnosis of cyst infection was confirmed by cyst fluid analysis.
PET/CT identified a kidney or liver cyst infection in 85% of 13 infectious events in 11 patients who met all the criteria for probable cyst infection; CT alone contributed to the diagnosis in only one patient.13 In those with fever of unknown origin, PET/CT identified a source of infection in 64% of 14 events in 13 patients: two infected renal cysts, as well as one patient each with other infections that would be difficult to diagnose clinically, ie, small bowel diverticulitis, psoas abscess, diverticulitis of the right colon, pyelonephritis in a transplanted kidney, infected abdominal aortic aneurysm, prostatitis, colitis, and Helicobacter pylori gastritis. Results of PET/CT were negative in five patients with intracystic bleeding.
Kidney stones occur in 20% to 36% of patients.11,14 Uric acid stones occur at almost the same frequency as calcium oxalate stones.
Chronic kidney disease not previously diagnosed may be the presenting condition in a small percentage of patients, sometimes those in whom much earlier hypertension was not fully evaluated. ADPKD is typically not associated with significant proteinuria (eg, nephrotic range), and the presence of heavy proteinuria usually indicates the presence of a superimposed primary glomerulopathy.15
Cysts in other locations. By MRI, liver cysts are present in 58% of patients ages 15 to 24, rising to 94% in those ages 35 to 46.11 Because liver cysts are estrogen-dependent, they are more prominent in women. A small percentage of patients develop cysts in the pancreas (5%), arachnoid membranes (8%), and seminal vesicles (40% of men with ADPKD).11
Cardiovascular abnormalities occur in almost one-third of patients with ADPKD, usually as mitral and aortic valve abnormalities.16 Aneurysms of the aortic root and abdominal aorta can also occur, in addition to intracranial aneurysms (see below).17
Intracranial aneurysms are not uncommon, and size usually determines their risk.
Intracranial aneurysms are strongly influenced by family history: 16% of ADPKD patients with a family history of intracranial aneurysm also develop them, compared with 5% to 6% of patients with no family history.11 The anterior cerebral circulation is involved in about 80% of cases. A sentinel or sudden “thunderclap” headache is a classic presentation that may precede full-blown rupture in about 17% of cases.18 Patients who rupture an intracranial aneurysm have a mean age of 39, usually have normal renal function, and can be normotensive.11
For patients with no history of subarachnoid hemorrhage, the 5-year cumulative rupture rates for patients with aneurysms located in the internal carotid artery, anterior communicating or anterior cerebral artery, or middle cerebral artery were 0% for aneurysms less than 7 mm, 2.6% for those 7 to 12 mm, 14.5% for those 13 to 24 mm, and 40% for those 25 mm or larger, with higher rates for the same sizes in the posterior circulation.11
In patients without symptoms, size is correlated with risk of rupture: less than 4 mm is usually associated with very low risk, 4 to less than 7 mm with moderate risk, and 7 mm or more with increasing risk. An aneurysm larger than 10 mm is associated with roughly a 1% risk of rupture per year.19
Irazabal et al20 retrospectively studied 407 patients with ADPKD who were screened for intracranial aneurysm. Saccular aneurysms were detected in 45 patients; most were small (median diameter 3.5 mm). During cumulative imaging follow-up of 243 years, only one new intracranial aneurysm was detected (increasing from 2 to 4.4 mm over 144 months) and two previously identified aneurysms grew (one increasing 4.5 to 5.9 mm over 69 months and the other 4.7 to 6.2 mm over 184 months). No change occurred in 28 patients. Seven patients were lost to follow-up, however. During cumulative clinical follow-up of 316 years, no aneurysm ruptured. Two patients were lost to follow-up, three had surgical clipping, and five died of unrelated causes. The authors concluded that presymptomatic intracranial aneurysms are usually small, and that growth and rupture risks are no higher than for unruptured intracranial aneurysms in the general population. A 2014 study also suggests a conservative approach for managing intracranial aneurysm in the general population.21
In asymptomatic ADPKD patients, it is reasonable to reserve screening for those with a positive family history of intracranial aneurysm or subarachnoid hemorrhage, those with a previous ruptured aneurysm, those in high-risk professions (eg, pilots), and for patients prior to anticoagulant therapy or major surgery possibly associated with hemodynamic instability.11,22 Certain extremely anxious patients might also need to be studied. Screening can be performed with magnetic resonance angiography without gadolinium contrast. It is prudent to have patients with an intracranial aneurysm thoroughly evaluated by an experienced neurosurgeon with continued follow-up.
PROGRESSION OF ADPKD
The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) study23 evaluated 241 patients with ADPKD (ages 15 to 46) by measuring the annual rate of change in total kidney volume, total cyst volume, and iothalamate glomerular filtration rate (GFR) over 3 years. The annual increase in total kidney volume averaged 5.3%,23 though the reported range with various imaging techniques is from 4% to 12.8% in adults.24 This study focused on macrocystic disease, ie, cysts that are visible by MRI and measurably increase total kidney volume. Although larger total kidney volume at baseline generally predicted a more rapid decline in GFR, there were wide and overlapping variations in yearly GFR declines within and among different total-kidney-volume groups.23
SPECIAL CLINICAL PROBLEMS IN ADPKD
Case 1: A man with ADPKD develops new and increasing proteinuria
A 55-year-old man with ADPKD and stage 3 chronic kidney disease developed new and increasing proteinuria, rising to 5,500 mg per 24 hours. What is the most likely explanation?
- Rapidly progressive renal failure with increasing proteinuria in ADPKD
- Bilateral renal vein thromboses because of cyst compression
- Malignant hypertension with bilateral renal artery compression
- Superimposed primary glomerulopathy
- Multiple infected renal cysts with pyonephrosis
Answer: Superimposed primary glomerulopathy.
ADPKD (similar to uncomplicated obstructive uropathy, pyelonephritis, main renal artery disease, and often cases of interstitial nephritis without secondary glomerular changes) typically does not result in nephrotic-range proteinuria. A superimposed primary glomerulopathy, focal segmental glomerulosclerosis, was the biopsy-proved diagnosis.
At least 21 cases have been reported of AD-PKD with nephrotic-range proteinuria and a renal biopsy showing a primary glomerulopathy, including focal segmental glomerulosclerosis (5 cases), minimal-change disease (5), membranous nephropathy (3), IgA nephropathy (2), and one each of crescentic glomerulonephropathy, diabetic nephropathy, membranoproliferative glomerulonephritis, postinfectious glomerulonephropathy, amyloid glomerulopathy, and mesangioproliferative glomerulopathy.15 Treatment was directed at the primary glomerulopathy, and the outcomes corresponded to the primary diagnosis (eg, with appropriate treatment, three of the five patients with focal segmental glomerulosclerosis progressed to end-stage renal disease, all of the patients with minimal-change disease went into remission, and one of the two cases with IgA nephropathy improved).15
Case 2: A woman with ADPKD and advanced renal failure develops shortness of breath
A 47-year-old woman with very large polycystic kidneys (total kidney volume 7,500 mL; normal range for a single kidney approximately 136–295 mL, mean 196)25 and estimated GFR of 25 mL/min developed new-onset shortness of breath while climbing steps and later even when making a bed. She had no chest pain, cough, or edema. She was sent directly to the emergency department and was admitted and treated; her condition improved, and she was discharged after 6 days. What did she have?
- Presentation of rare cystic pulmonary disease in ADPKD
- Onset of pneumonia with early bacteremia
- Progressive reduction in ventilatory capacity from massive polycystic kidneys and liver elevating both sides of the diaphragm
- Pulmonary emboli from an iliac vein or inferior vena cava source
- Progressive anemia accompanying rapidly worsening stage 4 chronic kidney disease
Answer: She had pulmonary emboli from an iliac vein (right) or inferior vena cava source.
Pulmonary emboli in ADPKD can be caused by thrombi in the inferior vena cava or the iliac or femoral vein because of compression by a massive right polycystic kidney. Four cases were reported at Mayo Clinic,26 three diagnosed by MRI and one with CT. One additional case occurred at Cleveland Clinic. All patients survived after treatment with anticoagulation therapy; early nephrectomy was required in two cases.
Interestingly, following kidney transplantation, the patients at greatest risk for pulmonary emboli are those with ADPKD as their original disease.27
RENAL CYSTS RESULT FROM COMBINED MUTATIONS, INJURY
The germline ADPKD mutation that occurs in one allele of all renal tubular epithelial cells is necessary but not sufficient for cystogenesis.28 One or more additional somatic mutations of the normal allele—the “second hit”—also develop within individual tubular epithelial cells.28,29 These epithelial cells undergo clonal proliferation, resulting in tubular dilatation and cyst formation. Monoclonality of cells in cysts has been documented.
Ischemia-reperfusion injury can be viewed as a “third hit.”30 In PKD1 knockout mice, which at 5 weeks of age normally develop only mild cystic kidney disease, the superimposition of unilateral ischemia-reperfusion injury at 8 weeks caused widespread and rapid cyst formation. It is believed that acute renal injury reactivates developmental signaling pathways within 48 hours that trigger epithelial cell proliferation and then cyst development detectable by MRI 2 weeks later. Although this phenomenon has not been documented in humans, it is a cautionary tale.
CYSTOGENESIS INVOLVES MULTIPLE PATHWAYS
A comprehensive description of pathways leading to renal cyst formation is beyond the scope of this article, and the reader is referred to much more detailed and extensive reviews.2,31 Disturbances in at least three major interconnected pathways promote cystogenesis in renal tubular epithelial cells:
- Normal calcium transport into the endoplasmic reticulum is disrupted by abnormal polycystins on the surface of the primary cilium
- Vasopressin and other stimuli increase the production of cyclic adenosine monophosphate (cAMP)
- The mammalian target of rapamycin (mTOR) proliferative pathway is up-regulated.
DISRUPTION OF CALCIUM TRANSPORT IN THE PRIMARY CILIUM
Primary cilia are nonmotile cellular organelles of varying size, from about 0.25 μm up to about 1 μm.32 Each primary cilium has nine peripheral pairs of microtubules but lacks a centrally located pair that is present in motile cilia. Primary cilia are ubiquitous and have been highly conserved throughout evolution. A single cilium is present on almost all vertebral cells.33
Cilial defects have been identified in autosomal dominant as well as recessive diseases and are known as ciliopathies.33 Although rare in humans, they can affect a broad spectrum of organs other than the kidney, including the eye, liver, and brain.33
Urine flow in a renal tubule is believed to exert mechanical stimulation on the extracellular flagellum-like N-terminal tail of PC1 that extends from a primary cilium into the urinary space. PC1 in concert with PC2 opens PC2 calcium channels, allowing calcium ions to flow down the microtubules to ryanodine receptors and the basal body.32,33 This leads to local release of calcium ions that regulate cell proliferation.32,34 However, in ADPKD kidneys, PC1 and PC2 molecules are sparse or mutated, resulting in defective calcium transport, increased and unregulated tubular epithelial cell proliferation, and cyst formation.
In a totally different clinical setting, biopsies of human renal transplants that sustained acute tubular necrosis during transplantation reveal that a cilium dramatically elongates in response to injury,35 possibly as a compensatory mechanism to maintain calcium transport in the presence of meager urine flow and to restore the proliferation of tubular epithelial cells in a regulated repair process.
THE ROLE OF VASOPRESSIN AND ACTIVATION OF cAMP
In classic experiments, Wang et al36 cross-bred rats having genetically inherited polycystic kidney disease (actually, autosomal recessive polycystic kidney disease) with Brattleboro rats that completely lack vasopressin. At 10 and 20 weeks of age, the offspring had virtually complete inhibition of cystogenesis because of the absence of vasopressin. However, when vasopressin was restored by exogenous administration continuously for 8 weeks, the animals formed massive renal cysts.
Vasopressin activates cAMP, which then functions as a second messenger in cell signaling. cAMP increases the activation of the protein kinase A (PKA) pathway, which in turn increases downstream activity of the B-raf/ERK pathway. Up-regulation of cAMP and PKA appears to perpetuate activation of canonical Wnt signaling, down-regulate non-canonical Wnt/planar cell polarity signaling, and lead to loss of tubular diameter control, resulting in cyst formation.31 Normally, cAMP is degraded by phosphodiesterase. However, because of the primary cilium calcium transport defect in ADPKD, phosphodiesterase is reduced and cAMP persists.37 In conjunction with the defective primary cilial calcium transport, cAMP exerts a proliferative effect on renal tubular epithelial cells that is opposite to its effect in normal kidneys.31,32 cAMP also up-regulates the cystic fibrosis transmembrane conductance regulator (CFTR) that promotes chloride ion transport. Sodium ions follow the chloride ions, leading to fluid accumulation and cyst enlargement.31
Inhibiting vasopressin by increasing water intake
A simple key mechanism for limiting vasopressin secretion is by sufficient water ingestion. Nagao et al38 found that rats with polycystic kidney disease given water with 5% glucose (resulting in 3.5-fold increased fluid intake compared with rats given tap water) had a 68% reduction in urinary vasopressin and a urine osmolality less than 290 mOsm/kg. The high-water-intake rats had dramatically reduced cystic areas in the kidney and a 28% reduction of kidney-to-body weight ratio vs controls.
In an obvious oversimplification, these findings raised the question of whether a sufficient increase in water intake could be an effective therapy for polycystic kidney disease.39 A pilot clinical study evaluated changes in urine osmolality in eight patients with ADPKD who had normal renal function.40 At baseline, 24-hour urine osmolality was typically elevated to approximately 753 mOsm/kg compared to the plasma at 285 mOsm/kg, indicating that antidiuresis is the usual state. During the 2-week study, urine volume and osmolality were measured, and additional water intake was adjusted in order to achieve a urine osmolality goal of 285 ± 45 mOsm/kg. These adjustments resulted in water intake that appeared to be in the range of 2,400 to 3,000 mL per 24 hours. The major limitations of the study were that it was very short term, and there was no opportunity to measure changes in total kidney volume or estimated GFR.
In a recent preliminary report from Japan, high water intake (2,500–3,000 mL daily) in 18 ADPKD patients was compared over 12 months with ad libitum water intake in 14 ADPKD controls (clinicaltrials.gov NCT 01348505). There was no statistically significant change in total kidney volume or cystatin-estimated GFR in those on high water intake, but serious defects in study design (patients in the high water intake group were allowed to decrease their intake if it was causing them difficulty, and patients in the ad libitum water intake group had no measurement of their actual water intake) prevent any conclusions because there was no evidence that the groups were different from one another with respect to the key element of the study, namely, water intake.
Blocking the vasopressin receptor slows disease progression
Using another approach, Gattone et al41 inhibited the effect of vasopressin by blocking the vasopressin 2 receptor (V2R) in mouse and rat models of polycystic kidney disease, using an experimental drug, OPC31260. The drug halted disease progression and, in one situation, appeared to cause regression of established disease. As noted by Torres and Harris,31 even though both increased water intake and V2R antagonists decrease cAMP in the distal tubules and collecting ducts, circulating levels of vasopressin are decreased by increased water intake but increased by V2R antagonists.
After these remarkable results in animal models, clinical trials of the V2R antagonist tolvaptan were conducted in patients with ADPKD. In the Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes 3:4 study,42 1,445 adults (ages 18 to 50) with ADPKD in 133 centers worldwide were randomized to receive either tolvaptan or placebo for 3 years. Key inclusion criteria included good renal function (estimated GFR ≥ 60 mL/min) and total kidney volume of at least 750 mL (mean 1,700 mL) as measured by MRI. Tolvaptan was titrated to the highest tolerated twice-daily dose (average total of 95 mg/day). All patients were advised to maintain good hydration and to avoid thirst by drinking a glass of water after each urination. Unfortunately, neither water intake nor urine output was measured.
The primary end point was the annual rate of change in total kidney volume, with secondary end points of clinical progression (worsening kidney function, pain, hypertension, albuminuria), and rate of decline in kidney function as measured by the slope of the reciprocal of serum creatinine.42
Patients in the tolvaptan arm had a slower annual increase in total kidney volume than controls (2.8% vs 5.5%, respectively, P < .001) and a slower annual decline in renal function (−2.61 vs −3.81 mg/mL−1, respectively, P < .001).42 More participants in the treatment group withdrew than in the placebo group (23% vs 14%, respectively).
Adverse events occurred more frequently with tolvaptan.42 Liver enzyme elevations of greater than three times the upper limit of normal occurred in 4.4% of patients in the treatment group, leading to a drug warning issued in January 2013. As expected, side effects related to diuresis (urinary frequency, nocturia, polyuria, and thirst) were more frequent in the treatment group, occurring in up to 55% of participants.
The authors noted, “Although maintaining hydration helped ensure that the blinding in the study was maintained, the suppression of vasopressin release in the placebo group may have led to an underestimation of the beneficial effect of tolvaptan and may account for the lower rates of kidney growth observed in the placebo group.”42
In 2013, the US Food and Drug Administration (FDA) denied a new drug application for tolvaptan as a treatment for ADPKD.
THE mTOR PATHWAY IS UP-REGULATED
The mTOR pathway that plays a major role in cell growth and proliferation includes interaction of the cytoplasmic tail of polycystin 1 with tuberin.43 Activation products of mTOR, including phospho-S6K, have been found in tubular epithelial cells lining cysts of ADPKD kidneys but not in normal kidneys.43 Mutant mice with polycystic disease had phospho-S6K in tubular epithelial cells of cysts, whereas those treated with the mTOR inhibitor rapamycin did not.43 But subsequent studies have shown that only a low level of mTOR activation is present in 65% to 70% of ADPKD cysts.44
Two major studies of the treatment of ADPKD with rapamycin that were published contemporaneously in 2010 failed to demonstrate any significant benefit with mTOR inhibitor treatment.45,46
Serra et al45 conducted an 18-month, open-label trial of 100 ADPKD patients ages 18 to 40 with an estimated GFR (eGFR) of at least 70 mL/min. Patients were randomized to receive rapamycin, given as sirolimus 2 mg per day, or standard care. The primary end point was the reduction in the growth rate of total kidney volume, measured by MRI. Secondary end points were eGFR and protein excretion (albumin-creatinine ratio). No significant difference was found in total kidney volume, but a nonsignificant stabilization of eGFR was noted.
Walz et al46 in a 2-year, multicenter, double-blind trial, randomized 433 patients (mean age 44; mean eGFR 54.5 mL/min) to treatment with either the short-acting mTOR inhibitor everolimus (2.5 mg twice daily) or placebo. Although patients in the treatment group had less of an increase in total kidney volume (significant at 1 year but not at 2 years), they tended to show a decline in eGFR. But further analysis showed that the only patients who had a reduction in eGFR were males who already had impaired kidney function at baseline.47
In a pilot study, 30 patients with ADPKD (mean age 49) were randomized to one of three therapies:
- Low-dose rapamycin (trough blood level 2–5 ng/mL)
- Standard-dose rapamycin (trough blood level > 5–8 ng/mL)
- Standard care without rapamycin.48
In contrast to other studies, the primary end point was the change in iothalamate GFR at 12 months, with change in total kidney volume being a secondary end point.
At 12 months, with 26 patients completing the study, the low-dose rapamycin group (n = 9) had a significant increase in iothalamate GFR of 7.7 ± 12.5 mL/min/1.73 m2, whereas the standard-dose rapamycin group (n = 8) had a nonsignificant increase of 1.6 ± 12.1 mL/min/1.73 m2, and the no-rapamycin group (n = 9) had a fall in iothalamate GFR of 11.2 ± 9.1 mL/min/1.73 m2 (P = .005 for low-dose vs no rapamycin; P = .07 for standard-dose vs no rapamycin; P = .52 for low-dose vs standard-dose rapamycin; and P = .002 for combined low-dose and standard-dose rapamycin vs no rapamycin.).48 These differences were observed despite there being no significant change in total kidney volume in any of the groups. Patients on low-dose rapamycin had fewer adverse effects than those on standard dose and were more often able to continue therapy for the entire study. This, and the use of iothalamate GFR rather than eGFR to measure GFR, are believed to be the main reasons that low-dose effects were more pronounced than those with standard doses. One may speculate that rapamycin may have its effect on microcysts and cystogenic cells, resulting in stabilization of or improvement in renal function without detectable slowing in total kidney volume enlargement. Mechanisms for this possibility involve new concepts, as discussed below.
NEW CONCEPTS
Specialized cells also promote renal cyst formation
Specialized cells that promote cyst formation have been identified by Karihaloo et al49 in a mouse model of polycystic kidney disease. In this model, alternatively activated macrophages homed to cystic areas and promoted cyst growth. These findings suggested that interrupting the homing and proliferative signals of macrophages could be a therapeutic target for ADPKD. Although rapamycin can suppress macrophage proliferation by macrophage colony-stimulating factor and inhibit macrophage function,50 alternatively activated macrophages have not been specifically studied for rapamycin responsiveness.
More promising is evidence that CD133+ progenitor cells from human ADPKD kidneys—but not from normal human kidneys—form cysts in vitro and in severe combined immunodeficient mouse models.51 Treatment with rapamycin decreased proliferation of the de-differentiated CD133+ cells from ADPKD patients and reduced cystogenesis.51
Visible cysts are the tip of the iceberg
Using ADPKD nephrectomy specimens from eight patients, Grantham et al52 compared cyst counts by MRI and by histology and found that for every renal cyst detected by MRI, about 62 smaller cysts (< 0.9 mm) are present in the kidney. For a typical patient having an average of 587 cysts in both kidneys that are detectable by MRI, this means that more than 36,000 cysts are actually present, and MRI detects less than 2% of the total cysts present.
Although microcysts are too small to contribute much to total kidney volume, they can interfere with kidney function. Microcysts can reduce GFR in two major ways: by compressing microvasculature, tubules, and glomeruli in the cortex; or by blocking the drainage of multiple upstream nephrons when they form in or block medullary collecting ducts.52 Although the growth rates of microcysts less than 1 mm in size have not yet been measured, the adult combined growth rates of the renal cyst component is approximately 12% per year, with yearly individual cyst growth rates up to 71%, and with fetal cyst growth rates even higher for cysts larger than 7.0 mm.53 Before and during an accelerated growth period, microcysts may be susceptible to certain therapies that could first improve GFR and only later change measurable total kidney volume by slowing microcyst progression to macrocysts either directly or through specialized cells that may be sensitive to rapamycin.
CURRENT MANAGEMENT OF ADPKD
Blood pressure control is essential—but too low is not good. For adult patients with hypertension caused by ADPKD, an acceptable blood pressure range is 120–130/70–80 mm Hg. However, further information from recently published blood pressure guidelines54 and the results of the Halt Progression of Polycystic Kidney Disease (HALT-PKD) study to be reported in late 201455 may provide more precise ranges for blood pressure control in ADPKD.
Although substantial experimental evidence exists for the benefits of inhibiting the up-regulation of the renin-angiotensin-aldosterone system in ADPKD, clinical proof is not yet available to confirm that angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) are preferred therapy.55 This may be determined by results of the HALT-PKD study, due for release in late 2014.55
Controlling blood pressure should be done with caution. Patients with low GFRs whose blood pressure is too low tend to have a more rapid decline of GFR, as suggested in the Modification of Diet in Renal Disease (MDRD) study in 1995.56
Experimental evidence suggests that avoiding calcium channel blockers may be advisable. Yamaguchi et al34 found that calcium channel blockers worsen the calcium transport defect and convert tubular epithelial cells to a proliferative phenotype.34
High fluid intake (2,500–3,000 mL/day), because it suppresses vasopressin, may be useful if permitted by several factors such as the patient’s cardiopulmonary and renal and electrolyte status, other medications, and diet.31 The reader is referred to a detailed description of the precautions necessary when prescribing high water intake.31 Patients should have their fluid intake managed by a physician and their renal function and serum sodium and electrolytes monitored regularly in order to avoid hyponatremia. Severe hyponatremia has occurred in patients with ADPKD and impaired kidney function who drank excessive quantities of water. Cardiac and pulmonary complications from excessive fluid intake are also possible, especially with a low GFR and compromised cardiac function.
A low-sodium diet, if not a contributing factor in hyponatremia, can be used under physician direction in the management of hypertension as well as in the prevention of calcium oxalate kidney stones.
Caffeine should be avoided because it may interfere with the activity of the phosphodiesterase that is necessary for the catabolism of cAMP to 5′AMP.
A low-protein diet is of unproven benefit,56 but it is prudent to avoid high protein intake.57
Complications such as bleeding (into or from cysts), infection (urinary tract, kidney cysts, and liver cysts), kidney stones, and urinary tract obstruction should be treated promptly and may require hospitalization.
Regular symptom reviews and physical examinations need to be performed with nonrenal concerns also in mind, such as intracranial aneurysms and cardiac valve lesions.11,58
Formal genetic counseling and molecular testing are becoming more frequently indicated as more complex inheritance patterns arise.6–8,59
Renal replacement therapy in the form of dialysis or transplantation is usually available for the patient when end-stage renal disease occurs. In the largest study thus far, ADPKD patient survival with a kidney transplant was similar to that of patients without ADPKD (about 93% at 5 years), and from 5 years to 15 years death-censored graft survival was actually better.60 Thromboembolic events are more frequent after transplantation,27,60 but they may also occur before transplantation from a massive right kidney compressing the iliac vein or the inferior vena cava, or both, leading to thrombus formation.26
Investigational as well as standard drug studies have intensified. Results from a large randomized study in approximately 1,000 adult ADPKD patients that evaluated over 6 to 8 years the effects of ACE inhibition with or without ARB treatment of hypertension, at both usual and lower blood pressure ranges in those with good renal function, are expected in late 2014.55 Outcomes from a few small clinical studies, eg, one with long-acting somatostatin31,61 and one using low-dose rapamycin48 in adults with ADPKD, will require confirmation in large randomized placebo-controlled clinical studies. In a new 3-year randomized placebo-controlled study of 91 children and young adults (ages 8 to 22) with ADPKD and essentially normal renal function who continued treatment with lisinopril, the addition of pravastatin (20 mg or 40 mg daily based on age) resulted in a significant reduction in the number of patients (46% vs 68%, respectively, P = .03) experiencing a greater than 20% change (increase) in height-adjusted total kidney volume.62 Change in GFR was not reported,62 but an earlier 4-week study in 10 patients treated with simvastatin did show an increase in renal blood flow and GFR.63 Numerous other agents that lack human studies include some described in older experimental work (eg, amiloride,31,64 citrate31,65) and many others from a growing list of potential therapeutic targets.31,66–73 It must be emphasized that there is no FDA-approved medication specifically for the treatment of ADPKD.
Future specific treatments of ADPKD may also involve minimally toxic doses of combination or sequential therapy directed at precystic and then both micro- and macrocystic/cystic fluid expansion aspects of ADPKD.48,74 Unfortunately, at the present time there is no specific FDA-approved therapy for ADPKD.
- Torres VE, Harris PC. Mechanisms of disease: autosomal dominant and recessive polycystic kidney diseases. Nat Clin Pract Nephrol 2006; 2:40–55.
- Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int 2009; 76:149–168.
- United States Renal Data System. 2013 atlas of CKD & ESRD. Volume 2 - atlas ESRD:172. www.usrds.org/atlas.aspx. Accessed June 4, 2014.
- Barua M, Cil O, Paerson AD, et al. Family history of renal disease severity predicts the mutated gene in ADPKD. J Am Soc Nephrol 2009, 20:1833–1838.
- Harris PC, Bae KT, Rossetti S, et al. Cyst number but not the rate of cystic growth is associated with the mutated gene in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2006; 17:3013–3019.
- Vujic M, Heyer CM, Ars E, et al. Incompletely penetrant PKD1 alleles mimic the renal manifestations of ARPKD. J Am Soc Nephrol 2010; 21:1097–1102.
- Harris PC. What is the role of somatic mutation in autosomal dominant polycystic kidney disease? J Am Soc Nephrol 2010; 21:1073–1076.
- Watnick T, He N, Wang K, et al. Mutations of PKD1 in ADPKD2 cysts suggest a pathogenic effect of trans-heterozygous mutations. Nat Genet 2000; 25:143–144.
- Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 1994; 343:824–827.
- Pei Y, Obaji J, Dupuis A, et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol 2009; 20:205–212.
- Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet 2007; 369:1287–1301.
- Bajwa ZH, Sial KA, Malik AB, Steinman TI. Pain patterns in patients with polycystic kidney disease. Kidney Int 2004; 66:1561–1569.
- Jouret F, Lhommel R, Beguin C, et al. Positron-emission computed tomography in cyst infection diagnosis in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2011; 6:1644–1650.
- Nishiura JL, Neves RF, Eloi SR, Cintra SM, Ajzen SA, Heilberg IP. Evaluation of nephrolithiasis in autosomal dominant polycystic kidney disease patients. Clin J Am Soc Nephrol 2009; 4:838–844.
- Hiura T, Yamazaki H, Saeki T, et al. Nephrotic syndrome and IgA nephropathy in polycystic kidney disease. Clin Exp Nephrol 2006; 10:136–139.
- Hossack KF, Leddy CL, Johnson AM, Schrier RW, Gabow PA. Echocardiographic findings in autosomal dominant polycystic kidney disease. N Engl J Med 1988; 319:907–912.
- Rossetti S, Chauveau D, Kubly V, et al. Association of mutation position in polycystic kidney disease 1 (PKD1) gene and development of a vascular phenotype. Lancet 2003; 361:2196–2201.
- Linn FH, Wijdicks EF, van der Graaf Y, Weerdesteyn-van Vliet FA, Bartelds AI, van Gijn J. Prospective study of sentinel headache in aneurismal subarachnoid haemorrhage. Lancet 1994; 344:590–593.
- Belz MM, Fick-Brosnahan GM, Hughes RL, et al. Recurrence of intracranial aneurysms in autosomal-dominant polycystic kidney disease. Kidney Int 2003; 63:1824–1830.
- Irazabal MV, Huston J, Kubly V, et al. Extended follow-up of unruptured intracranial aneurysms detected by presymptomatic screening in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2011; 6:1274–1285.
- Salman A-S, White PM, Counsell CE, et al; Scottish Audit of Intracranial Vascular Malformations Collaborators. Outcome after conservative management or intervention for unruptured brain arteriovenous malformations. JAMA 2014; 311:1661–1669.
- Vijay A, Vijay A, Pankaj P. Autosomal dominant polycystic kidney disease: a comprehensive review. Nephrourol Mon 2010; 2:172–192.
- Grantham JJ, Torres VE, Chapman AB, et al; CRISP Investigators. Volume progression in polycystic kidney disease. N Engl J Med 2006; 354:2122–2130.
- Bae KT, Grantham JJ. Imaging for the prognosis of autosomal dominant polycystic kidney disease. Nat Rev Nephrol 2010; 6:96–106.
- van den Dool SW, Wasser NM, de Fijter JW, Hoekstra J, van der Geest RJ. Functional renal volume: quantitative analysis at gadolinium-enhanced MR angiography—feasibility study in healthy potential kidney donors. Radiology 2005; 236:189–195.
- O’Sullivan DA, Torres VE, Heit JA, Liggett S, King BF. Compression of the inferior vena cava by right renal cysts: an unusual cause of IVC and/or iliofemoral thrombosis with pulmonary embolism in autosomal dominant polycystic kidney disease. Clin Nephrol 1998; 49:332–334.
- Tveit DP, Hypolite I, Bucci J, et al. Risk factors for hospitalizations resulting from pulmonary embolism after renal transplantation in the United States. J Nephrol 2001; 14:361–368.
- Pei Y. A “two-hit” model of cystogenesis in autosomal dominant polycystic kidney disease? Trends Mol Med 2001; 7:151–156.
- Qian F, Germino GG. “Mistakes happen”: somatic mutation and disease. Am J Hum Genet 1997; 61:1000–1005.
- Takakura A, Contrino L, Zhou X, et al. Renal injury is a third hit promoting rapid development of adult polycystic kidney disease. Hum Mol Genet 2009; 18:2523–2531.
- Torres VE, Harris PC. Strategies targeting cAMP signaling in the treatment of polycystic kidney disease. J Am Soc Nephrol 2014; 25:18–32.
- Nauli SM, Alenghat FJ, Luo Y, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 2003; 33:129–137.
- Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med 2011; 364:1533–1543.
- Yamaguchi T, Wallace DP, Magenheimer BS, Hempson SJ, Grantham JJ, Calvet JP. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J Biol Chem 2004; 279:40419–40430.
- Verghese E, Ricardo SD, Weidenfeld R, et al. Renal primary cilia lengthen after acute tubular necrosis. J Am Soc Nephrol 2009; 20:2147–2153.
- Wang X, Wu Y, Ward CJ, Harris PC, Torres VE. Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol 2008; 19:102–108.
- Torres VE. Cyclic AMP, at the hub of the cystic cycle. Kidney Int 2004; 66:1283–1285.
- Nagao S, Nishii K, Katsuyama M, et al. Increased water intake decreases progression of polycystic kidney disease in the PCK rat. J Am Soc Nephrol 2006; 17:2220–2227.
- Grantham JJ. Therapy for polycystic kidney disease? It’s water, stupid! J Am Soc Nephrol 2008; 19:1–7.
- Wang CJ, Creed C, Winklhofer FT, Grantham JJ. Water prescription in autosomal dominant polycystic kidney disease: a pilot study. Clin J Am Soc Nephrol 2011; 6:192–197.
- Gattone VH, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 2003; 9:1323–1326.
- Torres VE, Chapman AB, Devuyst O, et al; TEMPO 3:4 Trial Investigators. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 2012; 367:2407–2418.
- Shillingford JM, Murcia NS, Larson CH, et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A 2006; 103:5466–5471.
- Hartman TR, Liu D, Zilfou JT, et al. The tuberous sclerosis proteins regulate formation of the primary cilium via a rapamycin-insensitive and polycystin 1-independent pathway. Hum Mol Genet 2009; 18:161–163.
- Serra AL, Poster D, Kistler AD, et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med 2010; 363:820–829.
- Walz G, Budde K, Mannaa M, et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med 2010; 363:830–840. Errata in: N Engl J Med 2010; 363:1190 and N Engl J Med 2010; 363:1977.
- Walz G, Budde K, Eckardt K-U. mTOR inhibitors and autosomal dominant polycystic kidney disease (correspondence). N Engl J Med 2011; 364:287–288.
- Braun WE, Schold JD, Stephany BR, Spinko RA, Herfs BR. Low dose rapamycin (sirolimus) effects in autosomal dominant polycystic kidney disease: an open-label randomized control pilot study. Clin J Am Soc Nephrol 2014; 9:881–888.
- Karihaloo A, Koraishy F, Huen SC, et al. Macrophages promote cyst growth in polycystic kidney disease. J Am Soc Nephrol 2011; 22:1809–1814.
- Fox R, Nhan TQ, Law GL, Morris DR, Liles WC, Schwartz SM. PSGL-1 and mTOR regulate translation of ROCK-1 and physiological functions of macrophages. EMBO J 2007; 26:505–515. Erratum in: EMBO J 2007; 26:2605.
- Carvalhosa R, Deambrosis I, Carrera P, et al. Cystogenic potential of CD133+ progenitor cells of human polycystic kidneys. J Pathol 2011; 225:129–141.
- Grantham JJ, Mulamalla S, Grantham CJ, et al. Detected renal cysts are tips of the iceberg in adults with ADPKD. Clin J Am Soc Nephrol 2012; 7:1087–1093.
- Grantham JJ, Cook LT, Wetzel LH, Cadnapaphornchai MA, Bae KT. Evidence of extraordinary growth in the progressive enlargement of renal cysts. Clin J Am Soc Nephrol 2010; 5:889–896.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Chapman AB, Torres VE, Perrone RD, et al. The HALT polycystic kidney disease trials: design and implementation. Clin J Am Soc Nephrol 2010; 5:102–109.
- Klahr S, Breyer JA, Beck GJ, et al. Dietary protein restriction, blood pressure control, and the progression of polycystic kidney disease. Modification of Diet in Renal Disease Study Group. J Am Soc Nephrol 1995; 5:2037–2047.
- Thilly N. Low-protein diet in chronic kidney disease: from questions of effectiveness to those of feasibility. Nephrol Dial Transplant 2013; 28:2203–2205.
- Luciano RL, Dahl NK. Extra-renal manifestations of autosomal dominant polycystic kidney disease (ADPKD): considerations for routine screening and management. Nephrol Dial Transplant 2014; 29:247–254.
- Harris PC, Rossetti S. Molecular diagnostics for autosomal dominant polycystic kidney disease. Nat Rev Nephrol 2010; 6:197–206.
- Jacquet A, Pallet N, Kessler M, et al. Outcomes of renal transplantation in patients with autosomal dominant polycystic kidney disease: a nationwide longitudinal study. Transpl Int 2011; 24:582–587.
- Ruggenenti P, Remuzzi A, Ondei P, et al. Safety and efficacy of long-acting somatostatin treatment in autosomal-dominant polycystic kidney disease. Kidney Int 2005; 68:206–216.
- Cadnapaphornchai MA, George DM, McFann K, et al. Effect of pravastatin on total kidney volume, left ventricular mass index, and microalbuminuria in pediatric autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2014; 9:889–896.
- van Dijk MA, Kamper AM, van Veen S, Souverjin JH, Blauw GJ. Effect of simvastatin on renal function in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 2001; 16:2152–2157.
- Grantham JJ, Uchich M, Cragoe EL, et al. Chemical modification of cell proliferation and fluid secretion in renal cysts. Kidney Int 1989; 35:1379–1389.
- Tanner GA. Potassium citrate/citric acid intake improves renal function in rats with polycystic kidney disease. J Am Soc Nephrol 1998; 9:1242–1248.
- Belibi FA, Edelstein CL. Novel targets for the treatment of autosomal dominant polycystic kidney disease. Expert Opin Investig Drugs 2010; 19:315–328.
- Tao Y, Kim J, Yin Y, et al. VEGF receptor inhibition slows the progression of polycystic kidney disease. Kidney Int 2007; 72:1358–1366.
- Terryn S, Ho A, Beauwens R, Devuyst O. Fluid transport and cystogenesis in autosomal dominant polycystic kidney disease. Biochim Biophys Acta 2011; 1812:1314–1321.
- Thiagarajah JR, Verkman AS. CFTR inhibitors for treating diarrheal disease. Clin Pharmacol Ther 2012; 92:287–290.
- Boehn SN, Spahn S, Neudecker S, et al. Inhibition of Comt with tolcapone slows proression of polycystic kidney disease in the more severely affected PKD/Mhm (cy/+) substrain of the Hannover Sprague-Dawley rat. Nephrol Dial Transplant 2013; 28:2045–2058.
- Rees S, Kittikulsuth W, Roos K, Strait KA, Van Hoek A, Kohan DE. Adenylyl cyclase 6 deficiency ameliorates polycystic kidney disease. J Am Soc Nephrol 2014; 25:232–237.
- Buchholz B, Schley G, Faria D, et al. Hypoxia-inducible factor-1a causes renal cyst expansion through calcium-activated chloride secretion. J Am Soc Nephrol 2014; 25:465–474.
- Wallace DP, White C, Savinkova L, et al. Periostin promotes renal cyst growth and interstitial fibrosis in polycystic kidney disease. Kidney Int 2014; 85:845–854.
- Leuenroth SJ, Crews CM. Targeting cyst initiation in ADPKD. J Am Soc Nephrol 2009; 20:1–3.
- Torres VE, Harris PC. Mechanisms of disease: autosomal dominant and recessive polycystic kidney diseases. Nat Clin Pract Nephrol 2006; 2:40–55.
- Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int 2009; 76:149–168.
- United States Renal Data System. 2013 atlas of CKD & ESRD. Volume 2 - atlas ESRD:172. www.usrds.org/atlas.aspx. Accessed June 4, 2014.
- Barua M, Cil O, Paerson AD, et al. Family history of renal disease severity predicts the mutated gene in ADPKD. J Am Soc Nephrol 2009, 20:1833–1838.
- Harris PC, Bae KT, Rossetti S, et al. Cyst number but not the rate of cystic growth is associated with the mutated gene in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2006; 17:3013–3019.
- Vujic M, Heyer CM, Ars E, et al. Incompletely penetrant PKD1 alleles mimic the renal manifestations of ARPKD. J Am Soc Nephrol 2010; 21:1097–1102.
- Harris PC. What is the role of somatic mutation in autosomal dominant polycystic kidney disease? J Am Soc Nephrol 2010; 21:1073–1076.
- Watnick T, He N, Wang K, et al. Mutations of PKD1 in ADPKD2 cysts suggest a pathogenic effect of trans-heterozygous mutations. Nat Genet 2000; 25:143–144.
- Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 1994; 343:824–827.
- Pei Y, Obaji J, Dupuis A, et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol 2009; 20:205–212.
- Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet 2007; 369:1287–1301.
- Bajwa ZH, Sial KA, Malik AB, Steinman TI. Pain patterns in patients with polycystic kidney disease. Kidney Int 2004; 66:1561–1569.
- Jouret F, Lhommel R, Beguin C, et al. Positron-emission computed tomography in cyst infection diagnosis in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2011; 6:1644–1650.
- Nishiura JL, Neves RF, Eloi SR, Cintra SM, Ajzen SA, Heilberg IP. Evaluation of nephrolithiasis in autosomal dominant polycystic kidney disease patients. Clin J Am Soc Nephrol 2009; 4:838–844.
- Hiura T, Yamazaki H, Saeki T, et al. Nephrotic syndrome and IgA nephropathy in polycystic kidney disease. Clin Exp Nephrol 2006; 10:136–139.
- Hossack KF, Leddy CL, Johnson AM, Schrier RW, Gabow PA. Echocardiographic findings in autosomal dominant polycystic kidney disease. N Engl J Med 1988; 319:907–912.
- Rossetti S, Chauveau D, Kubly V, et al. Association of mutation position in polycystic kidney disease 1 (PKD1) gene and development of a vascular phenotype. Lancet 2003; 361:2196–2201.
- Linn FH, Wijdicks EF, van der Graaf Y, Weerdesteyn-van Vliet FA, Bartelds AI, van Gijn J. Prospective study of sentinel headache in aneurismal subarachnoid haemorrhage. Lancet 1994; 344:590–593.
- Belz MM, Fick-Brosnahan GM, Hughes RL, et al. Recurrence of intracranial aneurysms in autosomal-dominant polycystic kidney disease. Kidney Int 2003; 63:1824–1830.
- Irazabal MV, Huston J, Kubly V, et al. Extended follow-up of unruptured intracranial aneurysms detected by presymptomatic screening in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2011; 6:1274–1285.
- Salman A-S, White PM, Counsell CE, et al; Scottish Audit of Intracranial Vascular Malformations Collaborators. Outcome after conservative management or intervention for unruptured brain arteriovenous malformations. JAMA 2014; 311:1661–1669.
- Vijay A, Vijay A, Pankaj P. Autosomal dominant polycystic kidney disease: a comprehensive review. Nephrourol Mon 2010; 2:172–192.
- Grantham JJ, Torres VE, Chapman AB, et al; CRISP Investigators. Volume progression in polycystic kidney disease. N Engl J Med 2006; 354:2122–2130.
- Bae KT, Grantham JJ. Imaging for the prognosis of autosomal dominant polycystic kidney disease. Nat Rev Nephrol 2010; 6:96–106.
- van den Dool SW, Wasser NM, de Fijter JW, Hoekstra J, van der Geest RJ. Functional renal volume: quantitative analysis at gadolinium-enhanced MR angiography—feasibility study in healthy potential kidney donors. Radiology 2005; 236:189–195.
- O’Sullivan DA, Torres VE, Heit JA, Liggett S, King BF. Compression of the inferior vena cava by right renal cysts: an unusual cause of IVC and/or iliofemoral thrombosis with pulmonary embolism in autosomal dominant polycystic kidney disease. Clin Nephrol 1998; 49:332–334.
- Tveit DP, Hypolite I, Bucci J, et al. Risk factors for hospitalizations resulting from pulmonary embolism after renal transplantation in the United States. J Nephrol 2001; 14:361–368.
- Pei Y. A “two-hit” model of cystogenesis in autosomal dominant polycystic kidney disease? Trends Mol Med 2001; 7:151–156.
- Qian F, Germino GG. “Mistakes happen”: somatic mutation and disease. Am J Hum Genet 1997; 61:1000–1005.
- Takakura A, Contrino L, Zhou X, et al. Renal injury is a third hit promoting rapid development of adult polycystic kidney disease. Hum Mol Genet 2009; 18:2523–2531.
- Torres VE, Harris PC. Strategies targeting cAMP signaling in the treatment of polycystic kidney disease. J Am Soc Nephrol 2014; 25:18–32.
- Nauli SM, Alenghat FJ, Luo Y, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 2003; 33:129–137.
- Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med 2011; 364:1533–1543.
- Yamaguchi T, Wallace DP, Magenheimer BS, Hempson SJ, Grantham JJ, Calvet JP. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J Biol Chem 2004; 279:40419–40430.
- Verghese E, Ricardo SD, Weidenfeld R, et al. Renal primary cilia lengthen after acute tubular necrosis. J Am Soc Nephrol 2009; 20:2147–2153.
- Wang X, Wu Y, Ward CJ, Harris PC, Torres VE. Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol 2008; 19:102–108.
- Torres VE. Cyclic AMP, at the hub of the cystic cycle. Kidney Int 2004; 66:1283–1285.
- Nagao S, Nishii K, Katsuyama M, et al. Increased water intake decreases progression of polycystic kidney disease in the PCK rat. J Am Soc Nephrol 2006; 17:2220–2227.
- Grantham JJ. Therapy for polycystic kidney disease? It’s water, stupid! J Am Soc Nephrol 2008; 19:1–7.
- Wang CJ, Creed C, Winklhofer FT, Grantham JJ. Water prescription in autosomal dominant polycystic kidney disease: a pilot study. Clin J Am Soc Nephrol 2011; 6:192–197.
- Gattone VH, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 2003; 9:1323–1326.
- Torres VE, Chapman AB, Devuyst O, et al; TEMPO 3:4 Trial Investigators. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 2012; 367:2407–2418.
- Shillingford JM, Murcia NS, Larson CH, et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A 2006; 103:5466–5471.
- Hartman TR, Liu D, Zilfou JT, et al. The tuberous sclerosis proteins regulate formation of the primary cilium via a rapamycin-insensitive and polycystin 1-independent pathway. Hum Mol Genet 2009; 18:161–163.
- Serra AL, Poster D, Kistler AD, et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med 2010; 363:820–829.
- Walz G, Budde K, Mannaa M, et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med 2010; 363:830–840. Errata in: N Engl J Med 2010; 363:1190 and N Engl J Med 2010; 363:1977.
- Walz G, Budde K, Eckardt K-U. mTOR inhibitors and autosomal dominant polycystic kidney disease (correspondence). N Engl J Med 2011; 364:287–288.
- Braun WE, Schold JD, Stephany BR, Spinko RA, Herfs BR. Low dose rapamycin (sirolimus) effects in autosomal dominant polycystic kidney disease: an open-label randomized control pilot study. Clin J Am Soc Nephrol 2014; 9:881–888.
- Karihaloo A, Koraishy F, Huen SC, et al. Macrophages promote cyst growth in polycystic kidney disease. J Am Soc Nephrol 2011; 22:1809–1814.
- Fox R, Nhan TQ, Law GL, Morris DR, Liles WC, Schwartz SM. PSGL-1 and mTOR regulate translation of ROCK-1 and physiological functions of macrophages. EMBO J 2007; 26:505–515. Erratum in: EMBO J 2007; 26:2605.
- Carvalhosa R, Deambrosis I, Carrera P, et al. Cystogenic potential of CD133+ progenitor cells of human polycystic kidneys. J Pathol 2011; 225:129–141.
- Grantham JJ, Mulamalla S, Grantham CJ, et al. Detected renal cysts are tips of the iceberg in adults with ADPKD. Clin J Am Soc Nephrol 2012; 7:1087–1093.
- Grantham JJ, Cook LT, Wetzel LH, Cadnapaphornchai MA, Bae KT. Evidence of extraordinary growth in the progressive enlargement of renal cysts. Clin J Am Soc Nephrol 2010; 5:889–896.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Chapman AB, Torres VE, Perrone RD, et al. The HALT polycystic kidney disease trials: design and implementation. Clin J Am Soc Nephrol 2010; 5:102–109.
- Klahr S, Breyer JA, Beck GJ, et al. Dietary protein restriction, blood pressure control, and the progression of polycystic kidney disease. Modification of Diet in Renal Disease Study Group. J Am Soc Nephrol 1995; 5:2037–2047.
- Thilly N. Low-protein diet in chronic kidney disease: from questions of effectiveness to those of feasibility. Nephrol Dial Transplant 2013; 28:2203–2205.
- Luciano RL, Dahl NK. Extra-renal manifestations of autosomal dominant polycystic kidney disease (ADPKD): considerations for routine screening and management. Nephrol Dial Transplant 2014; 29:247–254.
- Harris PC, Rossetti S. Molecular diagnostics for autosomal dominant polycystic kidney disease. Nat Rev Nephrol 2010; 6:197–206.
- Jacquet A, Pallet N, Kessler M, et al. Outcomes of renal transplantation in patients with autosomal dominant polycystic kidney disease: a nationwide longitudinal study. Transpl Int 2011; 24:582–587.
- Ruggenenti P, Remuzzi A, Ondei P, et al. Safety and efficacy of long-acting somatostatin treatment in autosomal-dominant polycystic kidney disease. Kidney Int 2005; 68:206–216.
- Cadnapaphornchai MA, George DM, McFann K, et al. Effect of pravastatin on total kidney volume, left ventricular mass index, and microalbuminuria in pediatric autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2014; 9:889–896.
- van Dijk MA, Kamper AM, van Veen S, Souverjin JH, Blauw GJ. Effect of simvastatin on renal function in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 2001; 16:2152–2157.
- Grantham JJ, Uchich M, Cragoe EL, et al. Chemical modification of cell proliferation and fluid secretion in renal cysts. Kidney Int 1989; 35:1379–1389.
- Tanner GA. Potassium citrate/citric acid intake improves renal function in rats with polycystic kidney disease. J Am Soc Nephrol 1998; 9:1242–1248.
- Belibi FA, Edelstein CL. Novel targets for the treatment of autosomal dominant polycystic kidney disease. Expert Opin Investig Drugs 2010; 19:315–328.
- Tao Y, Kim J, Yin Y, et al. VEGF receptor inhibition slows the progression of polycystic kidney disease. Kidney Int 2007; 72:1358–1366.
- Terryn S, Ho A, Beauwens R, Devuyst O. Fluid transport and cystogenesis in autosomal dominant polycystic kidney disease. Biochim Biophys Acta 2011; 1812:1314–1321.
- Thiagarajah JR, Verkman AS. CFTR inhibitors for treating diarrheal disease. Clin Pharmacol Ther 2012; 92:287–290.
- Boehn SN, Spahn S, Neudecker S, et al. Inhibition of Comt with tolcapone slows proression of polycystic kidney disease in the more severely affected PKD/Mhm (cy/+) substrain of the Hannover Sprague-Dawley rat. Nephrol Dial Transplant 2013; 28:2045–2058.
- Rees S, Kittikulsuth W, Roos K, Strait KA, Van Hoek A, Kohan DE. Adenylyl cyclase 6 deficiency ameliorates polycystic kidney disease. J Am Soc Nephrol 2014; 25:232–237.
- Buchholz B, Schley G, Faria D, et al. Hypoxia-inducible factor-1a causes renal cyst expansion through calcium-activated chloride secretion. J Am Soc Nephrol 2014; 25:465–474.
- Wallace DP, White C, Savinkova L, et al. Periostin promotes renal cyst growth and interstitial fibrosis in polycystic kidney disease. Kidney Int 2014; 85:845–854.
- Leuenroth SJ, Crews CM. Targeting cyst initiation in ADPKD. J Am Soc Nephrol 2009; 20:1–3.
KEY POINTS
- For at-risk patients in the previously difficult diagnostic group from 30 to 39 years of age, newer ultrasonographic criteria for diagnosing PKD1 and PKD2 now require a minimum total of three renal cysts.
- An intracranial aneurysm occurs in approximately 16% of ADPKD patients who have a family member with ADPKD plus an intracranial aneurysm or subarachnoid hemorrhage. Appropriate screening is warranted.
- Combined positron-emission and computed tomography helps identify infected renal or liver cysts and may uncover other unsuspected abdominal or pelvic infections.
- Cyst expansion and increasing total kidney volume might be slowed by increasing water intake to 2,500 to 3,000 mL per day, although formal documentation of this is not published. However, this must be done under a physician’s supervision because of possible adverse effects.
- Tolvaptan, a promising new drug for treating ADPKD, failed to receive US approval. Rapamycin is another potentially effective agent but has had mixed results in clinical trials.
Perioperative beta-blockers in noncardiac surgery: The evidence continues to evolve
Prophylactic use of beta-blockers in the perioperative period is highly controversial. Initial studies in the 1990s were favorable, but evidence has been conflicting since then.
The pendulum swung away from routinely recommending beta-blockers after the publication of negative results from several studies, including the Perioperative Ischemic Evaluation (POISE) trial in 2008.1 Highlighting this change in practice, a Canadian study2 found that the use of perioperative beta-blockade increased between 1999 and 2005 but subsequently declined from 2005 to 2010. However, there was no appreciable change in this pattern after the POISE trial or after changes in the American College of Cardiology guidelines in 2002 and 2006.3
In 2008, Harte and Jaffer reviewed the perioperative use of beta-blockers in noncardiac surgery in this journal.4 Since then, a number of meta-analyses and retrospective observational studies have reported variable findings related to specific beta-blockers and specific complications.
In this paper, we review the rationale and recent evidence for and against the perioperative use of beta-blockers as guidance for internists and hospitalists.
POTENTIAL CARDIOPROTECTIVE EFFECTS OF BETA-BLOCKERS
Myocardial infarction and unstable angina are the leading cardiovascular causes of death after surgery.5 These events are multifactorial. Some are caused by the stress of surgery, which precipitates physiologic changes related to inflammatory mediators, sympathetic tone, and oxygen supply and demand; others are caused by acute plaque rupture, thrombosis, and occlusion.6 Most perioperative infarcts are non-Q-wave events7 and occur within the first 2 days after the procedure, when the effects of anesthetics, pain, fluid shifts, and physiologic changes are greatest. Because multiple causes may contribute to perioperative myocardial infarction, a single preventive strategy may not be sufficient.8,9
Beta-blockers do several things that may be beneficial in the perioperative setting. They reduce myocardial oxygen demand by decreasing the force of contraction and by slowing the heart rate, and slowing the heart rate increases diastolic perfusion time.10 They suppress arrhythmias; they limit leukocyte recruitment, the production of free radicals, metalloproteinase activity, monocyte activation, release of growth factors, and inflammatory cytokine response; and they stabilize plaque.11 Their long-term use may also alter intracellular signaling processes, thus improving cell survival by decreasing the expression of receptors for substances that induce apoptosis.12
INITIAL POSITIVE TRIALS
Mangano et al13 began the beta-blocker trend in 1996 with a study in 200 patients known to have coronary artery disease or risk factors for it who were undergoing noncardiac surgery. Patients were randomized to receive either atenolol orally and intravenously, titrated to control the heart rate, or placebo in the immediate perioperative period.
The atenolol group had less perioperative ischemia but no difference in short-term rates of myocardial infarction and death. However, the death rate was lower in the atenolol group at 6 months after discharge and at 2 years, although patients who died in the immediate postoperative period were excluded from the analysis.
Although this finding did not appear to make sense physiologically, we now know that patients may experience myocardial injury without infarction after noncardiac surgery, a phenomenon associated with an increased risk of death in the short term and the long term.14 Preventing these episodes may be the explanation for the improved outcome.
The DECREASE trial15 (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography) provided additional support for beta-blocker use. The patients were at high risk, had abnormal dobutamine stress echocardiograms, and were undergoing vascular surgery; 112 patients were randomized to receive either oral bisoprolol (started 1 month before surgery, titrated to control the heart rate, and continued for 1 month after surgery) or placebo.
The study was stopped early because the bisoprolol group reportedly had a 90% lower rate of myocardial infarction and cardiac death 1 month after surgery. However, the study was criticized because the total number of patients enrolled was small and the benefit was much greater than usual for any pharmacologic intervention, thus calling the results into question.
In a follow-up study,16 survivors continued to be followed while receiving bisoprolol or usual care. The incidence of myocardial infarction or cardiac death at 2 years was significantly lower in the group receiving bisoprolol (12% vs 32%, odds ratio [OR] 0.30, P = .025).
Boersma et al,17 in an observational study, analyzed data from all 1,351 patients scheduled for major vascular surgery being considered for enrollment in the DECREASE trial. The DECREASE protocol required patients to undergo dobutamine stress echocardiography if they had one or more risk factors (age 70 or older, angina, prior myocardial infarction, congestive heart failure, treatment for ventricular arrhythmia, treatment for diabetes mellitus, or limited exercise capacity) or if their physician requested it. Twenty-seven percent received beta-blockers.
In multivariate analysis, clinical predictors of adverse outcome were age 70 or older; current or prior history of angina; and prior myocardial infarction, heart failure, or cerebrovascular accident.
In patients who had fewer than three clinical risk factors, beta-blocker use was associated with a lower rate of complications (0.8% vs 2.3%). Dobutamine stress echocardiography had minimal predictive value in this lower-risk group, suggesting that stress testing may not be necessary in this group if beta-blockers are used appropriately. However, in patients who had three or more risk factors, this test did provide additional prognostic information; those without stress-induced ischemia had lower event rates than those with ischemia, and beta-blocker use further reduced those rates, except in patients with extensive ischemia (more than five left ventricular segments involved).
The Revised Cardiac Risk Index. Lee et al18 devised an index to assist in preoperative cardiac risk stratification that was subsequently incorporated into the 2007 American College of Cardiology/American Heart Association preoperative risk guidelines. (It does not, however, address the beta-blocker issue.) It consists of six independent risk-predictors of major cardiac complications derived from 4,315 patients over age 50 undergoing non-cardiac surgery. The risk factors, each of which is given 1 point, are:
- Congestive heart failure based on history or examination
- Renal insufficiency (serum creatinine level > 2 mg/dL)
- Myocardial infarction, symptomatic ischemic heart disease, or a positive stress test
- History of transient ischemic attack or stroke
- Diabetes requiring insulin
- High-risk surgery (defined as intrathoracic, intra-abdominal, or suprainguinal vascular surgery).
Patients with 3 or more points are considered to be at high risk, and those with 1 or 2 points are considered to be at intermediate risk. The American College of Cardiology/American Heart Association preoperative cardiac risk algorithm subsequently included five of these six risk factors (the type of surgery was considered separately) and made recommendations concerning noninvasive stress testing and heart rate control.
On the basis of these studies, specialty societies, guideline committees, and hospitals enthusiastically recommended the prophylactic use of beta-blockers to decrease postoperative cardiac complications.
THREE NEGATIVE TRIALS OF METOPROLOL
In 2005 and 2006, two studies in vascular surgery patients and another in patients with diabetes cast doubt on the role of beta-blockers when the results failed to show a benefit. The trials used metoprolol, started shortly before surgery, and with no titration to control the heart rate.
The MaVS study19 (Metoprolol After Vascular Surgery) randomized 496 patients to receive metoprolol or placebo 2 hours before surgery and until hospital discharge or a maximum of 5 days after surgery. The metoprolol dose varied by weight: patients weighing 75 kg or more got 100 mg, those weighing between 40 and 75 kg got 50 mg, and those weighing less than 40 kg got 25 mg. Overall effects at 6 months were not significantly different, but intraoperative bradycardia and hypotension requiring intervention were more frequent in the metoprolol group.
The POBBLE study20 (Perioperative Beta Blockade) randomized 103 patients who had no history of myocardial infarction to receive either metoprolol 50 mg twice daily or placebo from admission to 7 days after surgery. Myocardial ischemia was present in one-third of the patients after surgery. Metoprolol did not reduce the 30-day cardiac mortality rate, but it was associated with a shorter length of stay.
The DIPOM trial21 (Diabetic Postoperative Mortality and Morbidity) randomized 921 diabetic patients to receive long-acting metoprolol succinate controlled-release/extended release (CR/XL) or placebo. Patients in the metoprolol group received a test dose of 50 mg the evening before surgery, another dose 2 hours before surgery (100 mg if the heart rate was more than 65 bpm, or 50 mg if between 55 and 65 bpm), and daily thereafter until discharge or a maximum of 8 days. The dose was not titrated to heart-rate control.
Metoprolol had no statistically significant effect on the composite primary outcome measures of time to death from any cause, acute myocardial infarction, unstable angina, or congestive heart failure or on the secondary outcome measures of time to death from any cause, death from a cardiac cause, and nonfatal cardiac morbidity.
ADDITIONAL POSITIVE STUDIES
Lindenauer et al22 retrospectively evaluated the use of beta-blockers in the first 2 days after surgery in 782,969 patients undergoing non-cardiac surgery. Using propensity score matching and Revised Cardiac Risk Index scores, they found a lower rate of postoperative mortality in patients with three or more risk factors who received a beta-blocker. There was no significant difference in the group with two risk factors, but in the lowest-risk group (with a score of 0 to 1), beta-blockers were not beneficial and may have been associated with harm as evidenced by a higher odds ratio for death, although this was probably artifactual and reflecting database limitations.
Feringa et al,23 in an observational cohort study of 272 patients undergoing vascular surgery, reported that higher doses of beta-blockers and tight heart-rate control were associated with less perioperative myocardial ischemia, lower troponin T levels, and better long-term outcome.
THE POISE TRIAL: MIXED RESULTS
The randomized POISE trial,1 published in 2008, compared the effects of extended-release metoprolol succinate vs placebo on the 30-day risk of major cardiovascular events in 8,351 patients with or at risk of atherosclerotic disease who were undergoing noncardiac surgery. The metoprolol regimen was 100 mg 2 to 4 hours before surgery, another 100 mg by 6 hours after surgery, and then 200 mg 12 hours later and once daily for 30 days.
The incidence of the composite primary end point of cardiovascular death, nonfatal myocardial infarction, and nonfatal cardiac arrest at 30 days was lower in the metoprolol group than in the placebo group (5.8% vs 6.9%; P = .04), primarily because of fewer nonfatal myocardial infarctions. However, more patients in the metoprolol group died of any cause (3.1% vs 2.3% P = .03) or had a stroke (1.0% vs 0.5% P = .005) than in the placebo group.
The metoprolol group had a higher incidence of clinically significant hypotension, bradycardia, and stroke, which could account for much of the increase in the mortality rate. Sepsis was the major cause of death in this group; hypotension may have increased the risk of infection, and beta-blockers may have potentiated hypotension in patients who were already septic. Also, the bradycardic and negative inotropic effects of the beta-blocker could have masked the physiologic response to systemic infection, thereby delaying recognition and treatment or impeding the normal immune response.
One of the major criticisms of the POISE trial was its aggressive dosing regimen (200 to 400 mg within a 36-hour period) in patients who had not been on beta-blockers before then. Also, the drug was started only a few hours before surgery. In addition, these patients were at higher risk of death and stroke than those in other trials based on a high baseline rate of cerebrovascular disease, and inclusion of urgent and emergency surgical procedures.
STUDIES SINCE POISE
The POISE trial results1 prompted further questioning of the prophylactic perioperative use of beta-blockers. However, proponents of beta-blockers voiced serious criticisms of the trial, particularly the dosing regimen, and continued to believe that these drugs were beneficial if used appropriately.
The DECREASE IV trial. Dunkelgrun et al,24 in a study using bisoprolol started approximately 1 month before surgery and titrated to control the heart rate, reported beneficial results in intermediate-risk patients. In their randomized open-label study with a 2 × 2 factorial design, 1,066 patients at intermediate cardiac risk were assigned to receive bisoprolol, fluvastatin, combination treatment, or control therapy at least 34 days before surgery. Bisoprolol was started at 2.5 mg orally daily and slowly titrated up to a maximum dose of 10 mg to keep the heart rate between 50 and 70 beats per minute. The group of 533 patients randomized to receive bisoprolol had a lower incidence rate of cardiac death and nonfatal myocardial infarction than the control group (2.1% vs 6.0%, HR 0.34, P = .002). A potential limitation of this study was its open-label design, which might have led to treatment bias.
Updated guidelines. Based on the results from POISE and DECREASE IV, the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines25 published a focused update on beta-blockers in 2009 as an amendment to their 2007 guidelines on perioperative evaluation and care for noncardiac surgery. The European Society of Cardiology26 released similar but somewhat more liberal guidelines (Table 1).
London et al,27 in an observational study published in 2013, found a lower 30-day overall mortality rate with beta-blockers (relative risk [RR] 0.73, 95% confidence interval [CI] 0.65–0.83, P < .001, number needed to treat [NNT] 241), as well as a lower rate of cardiac morbidity (nonfatal myocardial infarction and cardiac death), but only in nonvascular surgery patients who were on beta-blockers within 7 days of scheduled surgery. Moreover, similar to the findings of Lindenauer et al,22 only patients with a Revised Cardiac Risk Index score of 2 or more benefited from beta-blocker use in terms of a lower risk of death, whereas the lower-risk patients did not:
- Risk score of 0 or 1—no association
- Score of 2—RR 0.63, 95% CI 0.50–0.80, P < .001, NNT 105
- Score of 3—RR 0.54, 95% CI 0.39–0.73, P < .001, NNT 41
- Score of 4 or more—RR 0.40, 95% CI 0.24–0.73, P < .001, NNT 18).
Beta-blocker exposure was associated with a significantly lower rate of cardiac complications (RR 0.67, 95% CI 0.57–0.79, P < .001, NNT 339), also limited to nonvascular surgery patients with a risk score of 2 or 3.
The Danish Nationwide Cohort Study28 examined the effect of beta-blockers on major adverse cardiac events (MACE, ie, myocardial infarction, cerebrovascular accident, and death) in 28,263 patients with ischemic heart disease undergoing noncardiac surgery; 7,990 with heart failure and 20,273 without. Beta-blockers were used in 53% of patients with heart failure and 36% of those without heart failure. Outcomes for all of the beta-blocker recipients:
- MACE—HR 0.90, 95% CI 0.79–1.02
- All-cause mortality—HR 0.95, 95% CI 0.85–1.06.
Outcomes for patients with heart failure if they received beta-blockers:
- MACE—HR 0.75, 95% CI 0.70–0.87
- All-cause mortality—HR 0.80, 95% CI 0.70–0.92.
There was no significant benefit from beta-blockers in patients without heart failure. Outcomes for those patients if they received beta-blockers:
- MACE—HR 1.11, 95% CI 0.92–1.33
- All-cause mortality—HR 1.15, 95% CI 0.98–1.35.
However, in patients without heart failure but with a history of myocardial infarction within the past 2 years, beta-blockers were associated with a lower risk of MACE and all-cause mortality. In patients with neither heart failure nor a recent myocardial infarction, beta-blockers were associated with an increased risk of MACE and all-cause mortality.
This difference in efficacy depending on the presence and timing of a prior myocardial infarction is consistent with the 2012 American College of Cardiology/American Heart Association guidelines for secondary prevention, in which beta-blockers are given a class I recommendation only for patients with a myocardial infarction within the past 3 years.
Meta-analyses and outcomes
A number of meta-analyses have been published over the past 10 years, with conflicting results (Table 2). The divergent findings are primarily due to the different studies included in the analyses as well as the strong influence of the POISE trial.1 The studies varied in terms of the specific beta-blocker used, dose titration and heart rate control, time of initiation of beta-blocker use before surgery, type of surgery, patient characteristics, comorbidities, biomarkers and diagnosis of myocardial infarction, and clinical end points.
In general, these meta-analyses have found that prophylactic perioperative use of beta-blockers decreases ischemia and tends to reduce the risk of nonfatal myocardial infarction. They vary on whether the overall mortality risk is decreased. The meta-analyses that included POISE1 found an increased incidence of stroke, whereas those that excluded POISE found no significant difference, although there appeared to be slightly more strokes in the beta-blocker groups.
The beta-blocker controversy increased even further when Dr. Don Poldermans was fired by Erasmus Medical Center in November 2011 for violations of academic integrity involving his research, including the DECREASE trials. The most recent meta-analysis, by Bouri et al,29 included nine “secure trials” and excluded the DECREASE trials in view of the controversy about their authenticity. The analysis showed an increase in overall mortality as well as stroke, primarily because it was heavily influenced by POISE.1 In contrast, the DECREASE trials had reported a decreased risk of myocardial infarction and death, with no significant increase in stroke. The authors concluded that guideline bodies should “retract their recommendations based on the fictitious data without further delay.”29
Although the design of the DECREASE trials (in which beta-blockers were started well in advance of surgery and doses were titrated to achieve heart rate control) is physiologically more compelling than those of the negative trials, the results have been questioned in light of the integrity issue. However, to date, none of the published DECREASE trials have been retracted.
Two other meta-analyses,30,31 published in 2013, also found a decreased risk of myocardial infarction and increased risk of stroke but no significant difference in short-term all-cause mortality.
ARE ALL BETA-BLOCKERS EQUIVALENT?
In various studies evaluating specific beta-blockers, the more cardioselective agents bisoprolol and atenolol were associated with better outcomes than metoprolol. The affinity ratios for beta-1/beta-2 receptors range from 13.5 for bisoprolol to 4.7 for atenolol and 2.3 for metoprolol.32 Blocking beta-1 receptors blunts tachycardia, whereas blocking beta-2 receptors may block systemic or cerebral vasodilation.
In patients with anemia, beta-blockade in general may be harmful, but beta-2 blockade may be even worse. Beta-blockers were associated with an increased risk of MACE (6.5% vs 3.0%)33 in patients with acute surgical anemia if the hemoglobin concentration decreased to less than 35% of baseline, and increased risks of hospital death (OR 6.65) and multiorgan dysfunction syndrome (OR 4.18) with severe bleeding during aortic surgery.34
In addition, the pathway by which the beta-blocker is metabolized may also affect outcome, with less benefit from beta-blockers metabolized by the CYP2D6 isoenzyme of the cytochrome P450 system. Individual variations in CYP2D6 activity related to genetics or drug interactions may result in insufficient or excessive beta-blockade. Because metoprolol is the most dependent on this system, patients using it may be more susceptible to bradycardia.35
Studies comparing atenolol and metoprolol found that the atenolol groups had fewer myocardial infarctions and deaths36 and lower 30-day and 1-year mortality rates37 than the groups on metoprolol. Studies comparing the three beta-blockers found better outcomes with atenolol and bisoprolol than with metoprolol—fewer strokes,38,39 a lower mortality rate,31 and a better composite outcome39 (Table 3 and Table 4).
START THE BETA-BLOCKER EARLY, TITRATE TO CONTROL THE HEART RATE
A number of studies suggest that how long the beta-blocker is given before surgery may influence the outcome (Table 5). The best results were achieved when beta-blockers were started approximately 1 month before surgery and titrated to control the heart rate.
Because this long lead-in time is not always practical, it is important to determine the shortest time before surgery in which starting beta-blockers may be beneficial and yet safe. Some evidence suggests that results are better when the beta-blocker is started more than 1 week preoperatively compared with less than 1 week, but it is unknown what the minimum or optimal time period should be.
If a beta-blocker is started well in advance of the scheduled surgery, there is adequate time for dose titration and tighter heart rate control. Most of the studies demonstrating beneficial effects of perioperative beta-blockers used dose titration and achieved lower heart rates in the treatment group than in the control group. A criticism of the MaVs,19 POBBLE,20 and DIPOM21 trials was that the patients did not receive adequate beta-blockade. The POISE trial1 used a much higher dose of metoprolol in an attempt to assure beta-blockade without dose titration, and although the regimen decreased nonfatal myocardial infarctions, it increased strokes and the overall mortality rate, probably related to excess bradycardia and hypotension. The target heart rate should probably be between 55 and 70 beats per minute.
RISK OF STROKE
POISE1 was the first trial to note a clinically and statistically significant increase in strokes with perioperative beta-blocker use. Although no other study has shown a similar increased risk, almost all reported a higher number of strokes in the beta-blocker groups, although the absolute numbers and differences were small and not statistically significant. This risk may also vary from one beta-blocker to another (Table 4).
The usual incidence rate of postoperative stroke after noncardiac, noncarotid surgery is well under 1% in patients with no prior history of stroke but increases to approximately 3% in patients with a previous stroke.40 An observational study from the Dutch group reported a very low incidence of stroke overall (0.02%) in 186,779 patients undergoing noncardiac surgery with no significant difference in those on chronic beta-blocker therapy.41 The DECREASE trials, with a total of 3,884 patients, also found no statistically significant increase in stroke with beta-blocker use (0.46% overall vs 0.5% with a beta-blocker),42 which in this case was bisoprolol started well in advance of surgery and titrated to control the heart rate. Although the DECREASE data are under suspicion, they seem reasonable and consistent with those of observational studies.
Proposed mechanisms by which beta-blockers may increase stroke risk include the side effects of hypotension and bradycardia, particularly in the setting of anemia. They may also cause cerebral ischemia by blocking cerebral vasodilation. This effect on cerebral blood flow may be more pronounced with the less cardioselective beta-blockers, which may explain the apparent increased stroke risk associated with metoprolol.
WHAT SHOULD WE DO NOW?
The evidence for the safety and efficacy of beta-blockers in the perioperative setting continues to evolve, and new clinical trials are needed to clarify the ongoing controversy, particularly regarding the risk of stroke.
If patients have other indications for beta-blocker therapy, such as history of heart failure, myocardial infarction in the past 3 years, or atrial fibrillation for rate control, they should be receiving them if time permits.
If prophylactic beta-blockers are to be effective in minimizing perioperative complications, it appears that they may need to be more cardioselective, started at least 1 week before surgery, titrated to control heart rate, and used in high-risk patients (Revised Cardiac Risk Index score > 2 or 3) undergoing high-risk surgery.
Ideally, a large randomized controlled trial using a cardioselective beta-blocker started in advance of surgery in patients with a Revised Cardiac Risk Index score greater than 2, undergoing intermediate or high-risk procedures, is needed to fully answer the questions raised by the current data.
- POISE Study Group; Devereaux PJ, Yang H, Yusuf S, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; 371:1839–1847.
- Wijeysundera DN, Mamdani M, Laupacis A, et al. Clinical evidence, practice guidelines, and ß-blocker utilization before major noncardiac surgery. Circ Cardiovasc Qual Outcomes 2012; 5:558–565.
- American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery); American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Rhythm Society; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery: focused update on perioperative beta-blocker therapy: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society for Vascular Medicine and Biology. J Am Coll Cardiol 2006; 47:2343–2355.
- Harte B, Jaffer AK. Perioperative beta-blockers in noncardiac surgery: evolution of the evidence. Cleve Clin J Med 2008; 75:513–519.
- Mangano DT. Perioperative cardiac morbidity. Anesthesiology 1990; 72:153–184.
- London MJ, Zaugg M, Schaub MC, Spahn DR. Perioperative beta-adrenergic receptor blockade: physiologic foundations and clinical controversies. Anesthesiology 2004; 100:170–175.
- Badner NH, Knill RL, Brown JE, Novick TV, Gelb AW. Myocardial infarction after noncardiac surgery. Anesthesiology 1998; 88:572–578.
- Priebe HJ. Triggers of perioperative myocardial ischaemia and infarction. Br J Anaesth 2004; 93:9–20.
- Zaugg M, Schaub MC, Foëx P. Myocardial injury and its prevention in the perioperative setting. Br J Anaesth 2004; 93:21–33.
- Zaugg M, Schaub MC, Pasch T, Spahn DR. Modulation of beta-adrenergic receptor subtype activities in perioperative medicine: mechanisms and sites of action. Br J Anaesth 2002; 88:101–123.
- Landesberg G. The pathophysiology of perioperative myocardial infarction: facts and perspectives. J Cardiothorac Vasc Anesth 2003; 17:90–100.
- Yeager MP, Fillinger MP, Hettleman BD, Hartman GS. Perioperative beta-blockade and late cardiac outcomes: a complementary hypothesis. J Cardiothorac Vasc Anesth 2005; 19:237–241.
- Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 1996; 335:1713–1720.
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120:564–578.
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med 1999; 341:1789–1794.
- Poldermans D, Boersma E, Bax JJ, et al; Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. Bisoprolol reduces cardiac death and myocardial infarction in high-risk patients as long as 2 years after successful major vascular surgery. Eur Heart J 2001; 22:1353–1358.
- Boersma E, Poldermans D, Bax JJ, et al; DECREASE Study Group (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiogrpahy). Predictors of cardiac events after major vascular surgery: role of clinical characteristics, dobutamine echocardiography, and beta-blocker therapy. JAMA 2001; 285:1865–1873.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Yang H, Raymer K, Butler R, Parlow J, Roberts R. The effects of perioperative beta-blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J 2006; 152:983–990.
- Brady AR, Gibbs JS, Greenhalgh RM, Powell JT, Sydes MR; POBBLE trial investigators. Perioperative beta-blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double-blind controlled trial. J Vasc Surg 2005; 41:602–609.
- Juul AB, Wetterslev J, Gluud C, et al; DIPOM Trial Group. Effect of perioperative beta blockade in patients with diabetes undergoing major non-cardiac surgery: randomised placebo controlled, blinded multicentre trial. BMJ 2006; 332:1482.
- Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major non-cardiac surgery. N Engl J Med 2005; 353:349–361.
- Feringa HH, Bax JJ, Boersma E, et al. High-dose beta-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation 2006; 114(suppl 1):1344–1349.
- Dunkelgrun M, Boersma E, Schouten O, et al; Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate-risk patients undergoing noncardiovascular surgery: a randomized controlled trial (DECREASE-IV). Ann Surg 2009; 249:921–926.
- American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Rhythm Society; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine; Society for Vascular Surgery; Fleisher LA, Beckman JA, Brown KA, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol 2009; 54:e13–e118.
- Task Force for Preoperative Cardiac Risk Assessment and Perioperative Cardiac Management in Non-cardiac Surgery; European Society of Cardiology (ESC); Poldermans D, Bax JJ, Boersma E, et al. Guidelines for preoperative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery. Eur Heart J 2009; 30:2769–2812.
- London MJ, Hur K, Schwartz GG, Henderson WG. Association of perioperative beta-blockade with mortality and cardiovascular morbidity following major noncardiac surgery. JAMA 2013; 309:1704–1713.
- Andersson C, Mérie C, Jørgensen M, et al. Association of beta-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing noncardiac surgery: a Danish nationwide cohort study. JAMA Intern Med 2014; 174:336–344.
- Bouri S, Shun-Shin MJ, Cole GD, Mayet J, Francis DP. Meta-analysis of secure randomised controlled trials of beta-blockade to prevent perioperative death in non-cardiac surgery. Heart 2014; 100:456–464.
- Guay J, Ochroch EA. Beta-blocking agents for surgery: influence on mortality and major outcomes. A meta-analysis. J Cardiothorac Vasc Anesth 2013; 27:834–844.
- Dai N, Xu D, Zhang J, et al. Different beta-blockers and initiation time in patients undergoing noncardiac surgery: a meta-analysis. Am J Med Sci 2014; 347:235–244.
- Baker JG. The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br J Pharmacol 2005; 144:317–322.
- Beattie WS, Wijeysundera DN, Karkouti K, et al. Acute surgical anemia influences the cardioprotective effects of beta-blockade: a single-center, propensity-matched cohort study. Anesthesiology 2010; 112:25–33.
- Le Manach Y, Collins GS, Ibanez C, et al. Impact of perioperative bleeding on the protective effect of beta-blockers during infrarenal aortic reconstruction. Anesthesiology 2012; 117:1203–1211.
- Badgett RG, Lawrence VA, Cohn SL. Variations in pharmacology of beta-blockers may contribute to heterogeneous results in trials of perioperative beta-blockade. Anesthesiology 2010; 113:585–592.
- Redelmeier D, Scales D, Kopp A. Beta blockers for elective surgery in elderly patients: population based, retrospective cohort study. BMJ 2005; 331:932.
- Wallace AW, Au S, Cason BA. Perioperative beta-blockade: atenolol is associated with reduced mortality when compared to metoprolol. Anesthesiology 2011; 114:824–836.
- Mashour GA, Sharifpour M, Freundlich RE, et al. Perioperative metoprolol and risk of stroke after noncardiac surgery. Anesthesiology 2013; 119:1340–1346.
- Ashes C, Judelman S, Wijeysundera DN, et al. Selective beta1-antagonism with bisoprolol is associated with fewer postoperative strokes than atenolol or metoprolol: a single-center cohort study of 44,092 consecutive patients. Anesthesiology 2013; 119:777–787.
- Selim M. Perioperative stroke. N Engl J Med 2007; 356:706–713.
- van Lier F, Schouten O, van Domburg RT, et al. Effect of chronic beta-blocker use on stroke after noncardiac surgery. Am J Cardiol 2009; 104:429–433.
- van Lier F, Schouten O, Hoeks SE, et al. Impact of prophylactic beta-blocker therapy to prevent stroke after noncardiac surgery. Am J Cardiol 2010; 105:43–47.
- Devereaux PJ, Beattie WS, Choi PT, et al. How strong is the evidence for the use of perioperative beta blockers in non-cardiac surgery? Systematic review and meta-analysis of randomised controlled trials. BMJ 2005; 331:313–321.
- McGory ML, Maggard MA, Ko CY. A meta-analysis of perioperative beta blockade: what is the actual risk reduction? Surgery 2005; 138:171–179.
- Schouten O, Shaw LJ, Boersma E, et al. A meta-analysis of safety and effectiveness of perioperative beta-blocker use for the prevention of cardiac events in different types of noncardiac surgery. Coron Artery Dis 2006; 17:173–179.
- Wiesbauer F, Schlager O, Domanovits H, et al. Perioperative beta-blockers for preventing surgery-related mortality and morbidity: a systematic review and meta-analysis. Anesth Analg 2007; 104:27–41.
- Bangalore S, Wetterslev J, Pranesh S, Sawhney S, Gluud C, Messerli FH. Perioperative beta blockers in patients having non-cardiac surgery: a meta-analysis. Lancet 2008; 372:1962–1976.
- Flu WJ, van Kuijk JP, Chonchol M, et al. Timing of preoperative beta-blocker treatment in vascular surgery patients: influence on postoperative outcome. J Am Coll Cardiol 2010; 56:1922–1929.
- Wijeysundera DN, Beattie WS, Wijeysundera HC, Yun L, Austin PC, Ko DT. Duration of preoperative beta-blockade and outcomes after major elective noncardiac surgery. Can J Cardiol 2014; 30:217–223.
Prophylactic use of beta-blockers in the perioperative period is highly controversial. Initial studies in the 1990s were favorable, but evidence has been conflicting since then.
The pendulum swung away from routinely recommending beta-blockers after the publication of negative results from several studies, including the Perioperative Ischemic Evaluation (POISE) trial in 2008.1 Highlighting this change in practice, a Canadian study2 found that the use of perioperative beta-blockade increased between 1999 and 2005 but subsequently declined from 2005 to 2010. However, there was no appreciable change in this pattern after the POISE trial or after changes in the American College of Cardiology guidelines in 2002 and 2006.3
In 2008, Harte and Jaffer reviewed the perioperative use of beta-blockers in noncardiac surgery in this journal.4 Since then, a number of meta-analyses and retrospective observational studies have reported variable findings related to specific beta-blockers and specific complications.
In this paper, we review the rationale and recent evidence for and against the perioperative use of beta-blockers as guidance for internists and hospitalists.
POTENTIAL CARDIOPROTECTIVE EFFECTS OF BETA-BLOCKERS
Myocardial infarction and unstable angina are the leading cardiovascular causes of death after surgery.5 These events are multifactorial. Some are caused by the stress of surgery, which precipitates physiologic changes related to inflammatory mediators, sympathetic tone, and oxygen supply and demand; others are caused by acute plaque rupture, thrombosis, and occlusion.6 Most perioperative infarcts are non-Q-wave events7 and occur within the first 2 days after the procedure, when the effects of anesthetics, pain, fluid shifts, and physiologic changes are greatest. Because multiple causes may contribute to perioperative myocardial infarction, a single preventive strategy may not be sufficient.8,9
Beta-blockers do several things that may be beneficial in the perioperative setting. They reduce myocardial oxygen demand by decreasing the force of contraction and by slowing the heart rate, and slowing the heart rate increases diastolic perfusion time.10 They suppress arrhythmias; they limit leukocyte recruitment, the production of free radicals, metalloproteinase activity, monocyte activation, release of growth factors, and inflammatory cytokine response; and they stabilize plaque.11 Their long-term use may also alter intracellular signaling processes, thus improving cell survival by decreasing the expression of receptors for substances that induce apoptosis.12
INITIAL POSITIVE TRIALS
Mangano et al13 began the beta-blocker trend in 1996 with a study in 200 patients known to have coronary artery disease or risk factors for it who were undergoing noncardiac surgery. Patients were randomized to receive either atenolol orally and intravenously, titrated to control the heart rate, or placebo in the immediate perioperative period.
The atenolol group had less perioperative ischemia but no difference in short-term rates of myocardial infarction and death. However, the death rate was lower in the atenolol group at 6 months after discharge and at 2 years, although patients who died in the immediate postoperative period were excluded from the analysis.
Although this finding did not appear to make sense physiologically, we now know that patients may experience myocardial injury without infarction after noncardiac surgery, a phenomenon associated with an increased risk of death in the short term and the long term.14 Preventing these episodes may be the explanation for the improved outcome.
The DECREASE trial15 (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography) provided additional support for beta-blocker use. The patients were at high risk, had abnormal dobutamine stress echocardiograms, and were undergoing vascular surgery; 112 patients were randomized to receive either oral bisoprolol (started 1 month before surgery, titrated to control the heart rate, and continued for 1 month after surgery) or placebo.
The study was stopped early because the bisoprolol group reportedly had a 90% lower rate of myocardial infarction and cardiac death 1 month after surgery. However, the study was criticized because the total number of patients enrolled was small and the benefit was much greater than usual for any pharmacologic intervention, thus calling the results into question.
In a follow-up study,16 survivors continued to be followed while receiving bisoprolol or usual care. The incidence of myocardial infarction or cardiac death at 2 years was significantly lower in the group receiving bisoprolol (12% vs 32%, odds ratio [OR] 0.30, P = .025).
Boersma et al,17 in an observational study, analyzed data from all 1,351 patients scheduled for major vascular surgery being considered for enrollment in the DECREASE trial. The DECREASE protocol required patients to undergo dobutamine stress echocardiography if they had one or more risk factors (age 70 or older, angina, prior myocardial infarction, congestive heart failure, treatment for ventricular arrhythmia, treatment for diabetes mellitus, or limited exercise capacity) or if their physician requested it. Twenty-seven percent received beta-blockers.
In multivariate analysis, clinical predictors of adverse outcome were age 70 or older; current or prior history of angina; and prior myocardial infarction, heart failure, or cerebrovascular accident.
In patients who had fewer than three clinical risk factors, beta-blocker use was associated with a lower rate of complications (0.8% vs 2.3%). Dobutamine stress echocardiography had minimal predictive value in this lower-risk group, suggesting that stress testing may not be necessary in this group if beta-blockers are used appropriately. However, in patients who had three or more risk factors, this test did provide additional prognostic information; those without stress-induced ischemia had lower event rates than those with ischemia, and beta-blocker use further reduced those rates, except in patients with extensive ischemia (more than five left ventricular segments involved).
The Revised Cardiac Risk Index. Lee et al18 devised an index to assist in preoperative cardiac risk stratification that was subsequently incorporated into the 2007 American College of Cardiology/American Heart Association preoperative risk guidelines. (It does not, however, address the beta-blocker issue.) It consists of six independent risk-predictors of major cardiac complications derived from 4,315 patients over age 50 undergoing non-cardiac surgery. The risk factors, each of which is given 1 point, are:
- Congestive heart failure based on history or examination
- Renal insufficiency (serum creatinine level > 2 mg/dL)
- Myocardial infarction, symptomatic ischemic heart disease, or a positive stress test
- History of transient ischemic attack or stroke
- Diabetes requiring insulin
- High-risk surgery (defined as intrathoracic, intra-abdominal, or suprainguinal vascular surgery).
Patients with 3 or more points are considered to be at high risk, and those with 1 or 2 points are considered to be at intermediate risk. The American College of Cardiology/American Heart Association preoperative cardiac risk algorithm subsequently included five of these six risk factors (the type of surgery was considered separately) and made recommendations concerning noninvasive stress testing and heart rate control.
On the basis of these studies, specialty societies, guideline committees, and hospitals enthusiastically recommended the prophylactic use of beta-blockers to decrease postoperative cardiac complications.
THREE NEGATIVE TRIALS OF METOPROLOL
In 2005 and 2006, two studies in vascular surgery patients and another in patients with diabetes cast doubt on the role of beta-blockers when the results failed to show a benefit. The trials used metoprolol, started shortly before surgery, and with no titration to control the heart rate.
The MaVS study19 (Metoprolol After Vascular Surgery) randomized 496 patients to receive metoprolol or placebo 2 hours before surgery and until hospital discharge or a maximum of 5 days after surgery. The metoprolol dose varied by weight: patients weighing 75 kg or more got 100 mg, those weighing between 40 and 75 kg got 50 mg, and those weighing less than 40 kg got 25 mg. Overall effects at 6 months were not significantly different, but intraoperative bradycardia and hypotension requiring intervention were more frequent in the metoprolol group.
The POBBLE study20 (Perioperative Beta Blockade) randomized 103 patients who had no history of myocardial infarction to receive either metoprolol 50 mg twice daily or placebo from admission to 7 days after surgery. Myocardial ischemia was present in one-third of the patients after surgery. Metoprolol did not reduce the 30-day cardiac mortality rate, but it was associated with a shorter length of stay.
The DIPOM trial21 (Diabetic Postoperative Mortality and Morbidity) randomized 921 diabetic patients to receive long-acting metoprolol succinate controlled-release/extended release (CR/XL) or placebo. Patients in the metoprolol group received a test dose of 50 mg the evening before surgery, another dose 2 hours before surgery (100 mg if the heart rate was more than 65 bpm, or 50 mg if between 55 and 65 bpm), and daily thereafter until discharge or a maximum of 8 days. The dose was not titrated to heart-rate control.
Metoprolol had no statistically significant effect on the composite primary outcome measures of time to death from any cause, acute myocardial infarction, unstable angina, or congestive heart failure or on the secondary outcome measures of time to death from any cause, death from a cardiac cause, and nonfatal cardiac morbidity.
ADDITIONAL POSITIVE STUDIES
Lindenauer et al22 retrospectively evaluated the use of beta-blockers in the first 2 days after surgery in 782,969 patients undergoing non-cardiac surgery. Using propensity score matching and Revised Cardiac Risk Index scores, they found a lower rate of postoperative mortality in patients with three or more risk factors who received a beta-blocker. There was no significant difference in the group with two risk factors, but in the lowest-risk group (with a score of 0 to 1), beta-blockers were not beneficial and may have been associated with harm as evidenced by a higher odds ratio for death, although this was probably artifactual and reflecting database limitations.
Feringa et al,23 in an observational cohort study of 272 patients undergoing vascular surgery, reported that higher doses of beta-blockers and tight heart-rate control were associated with less perioperative myocardial ischemia, lower troponin T levels, and better long-term outcome.
THE POISE TRIAL: MIXED RESULTS
The randomized POISE trial,1 published in 2008, compared the effects of extended-release metoprolol succinate vs placebo on the 30-day risk of major cardiovascular events in 8,351 patients with or at risk of atherosclerotic disease who were undergoing noncardiac surgery. The metoprolol regimen was 100 mg 2 to 4 hours before surgery, another 100 mg by 6 hours after surgery, and then 200 mg 12 hours later and once daily for 30 days.
The incidence of the composite primary end point of cardiovascular death, nonfatal myocardial infarction, and nonfatal cardiac arrest at 30 days was lower in the metoprolol group than in the placebo group (5.8% vs 6.9%; P = .04), primarily because of fewer nonfatal myocardial infarctions. However, more patients in the metoprolol group died of any cause (3.1% vs 2.3% P = .03) or had a stroke (1.0% vs 0.5% P = .005) than in the placebo group.
The metoprolol group had a higher incidence of clinically significant hypotension, bradycardia, and stroke, which could account for much of the increase in the mortality rate. Sepsis was the major cause of death in this group; hypotension may have increased the risk of infection, and beta-blockers may have potentiated hypotension in patients who were already septic. Also, the bradycardic and negative inotropic effects of the beta-blocker could have masked the physiologic response to systemic infection, thereby delaying recognition and treatment or impeding the normal immune response.
One of the major criticisms of the POISE trial was its aggressive dosing regimen (200 to 400 mg within a 36-hour period) in patients who had not been on beta-blockers before then. Also, the drug was started only a few hours before surgery. In addition, these patients were at higher risk of death and stroke than those in other trials based on a high baseline rate of cerebrovascular disease, and inclusion of urgent and emergency surgical procedures.
STUDIES SINCE POISE
The POISE trial results1 prompted further questioning of the prophylactic perioperative use of beta-blockers. However, proponents of beta-blockers voiced serious criticisms of the trial, particularly the dosing regimen, and continued to believe that these drugs were beneficial if used appropriately.
The DECREASE IV trial. Dunkelgrun et al,24 in a study using bisoprolol started approximately 1 month before surgery and titrated to control the heart rate, reported beneficial results in intermediate-risk patients. In their randomized open-label study with a 2 × 2 factorial design, 1,066 patients at intermediate cardiac risk were assigned to receive bisoprolol, fluvastatin, combination treatment, or control therapy at least 34 days before surgery. Bisoprolol was started at 2.5 mg orally daily and slowly titrated up to a maximum dose of 10 mg to keep the heart rate between 50 and 70 beats per minute. The group of 533 patients randomized to receive bisoprolol had a lower incidence rate of cardiac death and nonfatal myocardial infarction than the control group (2.1% vs 6.0%, HR 0.34, P = .002). A potential limitation of this study was its open-label design, which might have led to treatment bias.
Updated guidelines. Based on the results from POISE and DECREASE IV, the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines25 published a focused update on beta-blockers in 2009 as an amendment to their 2007 guidelines on perioperative evaluation and care for noncardiac surgery. The European Society of Cardiology26 released similar but somewhat more liberal guidelines (Table 1).
London et al,27 in an observational study published in 2013, found a lower 30-day overall mortality rate with beta-blockers (relative risk [RR] 0.73, 95% confidence interval [CI] 0.65–0.83, P < .001, number needed to treat [NNT] 241), as well as a lower rate of cardiac morbidity (nonfatal myocardial infarction and cardiac death), but only in nonvascular surgery patients who were on beta-blockers within 7 days of scheduled surgery. Moreover, similar to the findings of Lindenauer et al,22 only patients with a Revised Cardiac Risk Index score of 2 or more benefited from beta-blocker use in terms of a lower risk of death, whereas the lower-risk patients did not:
- Risk score of 0 or 1—no association
- Score of 2—RR 0.63, 95% CI 0.50–0.80, P < .001, NNT 105
- Score of 3—RR 0.54, 95% CI 0.39–0.73, P < .001, NNT 41
- Score of 4 or more—RR 0.40, 95% CI 0.24–0.73, P < .001, NNT 18).
Beta-blocker exposure was associated with a significantly lower rate of cardiac complications (RR 0.67, 95% CI 0.57–0.79, P < .001, NNT 339), also limited to nonvascular surgery patients with a risk score of 2 or 3.
The Danish Nationwide Cohort Study28 examined the effect of beta-blockers on major adverse cardiac events (MACE, ie, myocardial infarction, cerebrovascular accident, and death) in 28,263 patients with ischemic heart disease undergoing noncardiac surgery; 7,990 with heart failure and 20,273 without. Beta-blockers were used in 53% of patients with heart failure and 36% of those without heart failure. Outcomes for all of the beta-blocker recipients:
- MACE—HR 0.90, 95% CI 0.79–1.02
- All-cause mortality—HR 0.95, 95% CI 0.85–1.06.
Outcomes for patients with heart failure if they received beta-blockers:
- MACE—HR 0.75, 95% CI 0.70–0.87
- All-cause mortality—HR 0.80, 95% CI 0.70–0.92.
There was no significant benefit from beta-blockers in patients without heart failure. Outcomes for those patients if they received beta-blockers:
- MACE—HR 1.11, 95% CI 0.92–1.33
- All-cause mortality—HR 1.15, 95% CI 0.98–1.35.
However, in patients without heart failure but with a history of myocardial infarction within the past 2 years, beta-blockers were associated with a lower risk of MACE and all-cause mortality. In patients with neither heart failure nor a recent myocardial infarction, beta-blockers were associated with an increased risk of MACE and all-cause mortality.
This difference in efficacy depending on the presence and timing of a prior myocardial infarction is consistent with the 2012 American College of Cardiology/American Heart Association guidelines for secondary prevention, in which beta-blockers are given a class I recommendation only for patients with a myocardial infarction within the past 3 years.
Meta-analyses and outcomes
A number of meta-analyses have been published over the past 10 years, with conflicting results (Table 2). The divergent findings are primarily due to the different studies included in the analyses as well as the strong influence of the POISE trial.1 The studies varied in terms of the specific beta-blocker used, dose titration and heart rate control, time of initiation of beta-blocker use before surgery, type of surgery, patient characteristics, comorbidities, biomarkers and diagnosis of myocardial infarction, and clinical end points.
In general, these meta-analyses have found that prophylactic perioperative use of beta-blockers decreases ischemia and tends to reduce the risk of nonfatal myocardial infarction. They vary on whether the overall mortality risk is decreased. The meta-analyses that included POISE1 found an increased incidence of stroke, whereas those that excluded POISE found no significant difference, although there appeared to be slightly more strokes in the beta-blocker groups.
The beta-blocker controversy increased even further when Dr. Don Poldermans was fired by Erasmus Medical Center in November 2011 for violations of academic integrity involving his research, including the DECREASE trials. The most recent meta-analysis, by Bouri et al,29 included nine “secure trials” and excluded the DECREASE trials in view of the controversy about their authenticity. The analysis showed an increase in overall mortality as well as stroke, primarily because it was heavily influenced by POISE.1 In contrast, the DECREASE trials had reported a decreased risk of myocardial infarction and death, with no significant increase in stroke. The authors concluded that guideline bodies should “retract their recommendations based on the fictitious data without further delay.”29
Although the design of the DECREASE trials (in which beta-blockers were started well in advance of surgery and doses were titrated to achieve heart rate control) is physiologically more compelling than those of the negative trials, the results have been questioned in light of the integrity issue. However, to date, none of the published DECREASE trials have been retracted.
Two other meta-analyses,30,31 published in 2013, also found a decreased risk of myocardial infarction and increased risk of stroke but no significant difference in short-term all-cause mortality.
ARE ALL BETA-BLOCKERS EQUIVALENT?
In various studies evaluating specific beta-blockers, the more cardioselective agents bisoprolol and atenolol were associated with better outcomes than metoprolol. The affinity ratios for beta-1/beta-2 receptors range from 13.5 for bisoprolol to 4.7 for atenolol and 2.3 for metoprolol.32 Blocking beta-1 receptors blunts tachycardia, whereas blocking beta-2 receptors may block systemic or cerebral vasodilation.
In patients with anemia, beta-blockade in general may be harmful, but beta-2 blockade may be even worse. Beta-blockers were associated with an increased risk of MACE (6.5% vs 3.0%)33 in patients with acute surgical anemia if the hemoglobin concentration decreased to less than 35% of baseline, and increased risks of hospital death (OR 6.65) and multiorgan dysfunction syndrome (OR 4.18) with severe bleeding during aortic surgery.34
In addition, the pathway by which the beta-blocker is metabolized may also affect outcome, with less benefit from beta-blockers metabolized by the CYP2D6 isoenzyme of the cytochrome P450 system. Individual variations in CYP2D6 activity related to genetics or drug interactions may result in insufficient or excessive beta-blockade. Because metoprolol is the most dependent on this system, patients using it may be more susceptible to bradycardia.35
Studies comparing atenolol and metoprolol found that the atenolol groups had fewer myocardial infarctions and deaths36 and lower 30-day and 1-year mortality rates37 than the groups on metoprolol. Studies comparing the three beta-blockers found better outcomes with atenolol and bisoprolol than with metoprolol—fewer strokes,38,39 a lower mortality rate,31 and a better composite outcome39 (Table 3 and Table 4).
START THE BETA-BLOCKER EARLY, TITRATE TO CONTROL THE HEART RATE
A number of studies suggest that how long the beta-blocker is given before surgery may influence the outcome (Table 5). The best results were achieved when beta-blockers were started approximately 1 month before surgery and titrated to control the heart rate.
Because this long lead-in time is not always practical, it is important to determine the shortest time before surgery in which starting beta-blockers may be beneficial and yet safe. Some evidence suggests that results are better when the beta-blocker is started more than 1 week preoperatively compared with less than 1 week, but it is unknown what the minimum or optimal time period should be.
If a beta-blocker is started well in advance of the scheduled surgery, there is adequate time for dose titration and tighter heart rate control. Most of the studies demonstrating beneficial effects of perioperative beta-blockers used dose titration and achieved lower heart rates in the treatment group than in the control group. A criticism of the MaVs,19 POBBLE,20 and DIPOM21 trials was that the patients did not receive adequate beta-blockade. The POISE trial1 used a much higher dose of metoprolol in an attempt to assure beta-blockade without dose titration, and although the regimen decreased nonfatal myocardial infarctions, it increased strokes and the overall mortality rate, probably related to excess bradycardia and hypotension. The target heart rate should probably be between 55 and 70 beats per minute.
RISK OF STROKE
POISE1 was the first trial to note a clinically and statistically significant increase in strokes with perioperative beta-blocker use. Although no other study has shown a similar increased risk, almost all reported a higher number of strokes in the beta-blocker groups, although the absolute numbers and differences were small and not statistically significant. This risk may also vary from one beta-blocker to another (Table 4).
The usual incidence rate of postoperative stroke after noncardiac, noncarotid surgery is well under 1% in patients with no prior history of stroke but increases to approximately 3% in patients with a previous stroke.40 An observational study from the Dutch group reported a very low incidence of stroke overall (0.02%) in 186,779 patients undergoing noncardiac surgery with no significant difference in those on chronic beta-blocker therapy.41 The DECREASE trials, with a total of 3,884 patients, also found no statistically significant increase in stroke with beta-blocker use (0.46% overall vs 0.5% with a beta-blocker),42 which in this case was bisoprolol started well in advance of surgery and titrated to control the heart rate. Although the DECREASE data are under suspicion, they seem reasonable and consistent with those of observational studies.
Proposed mechanisms by which beta-blockers may increase stroke risk include the side effects of hypotension and bradycardia, particularly in the setting of anemia. They may also cause cerebral ischemia by blocking cerebral vasodilation. This effect on cerebral blood flow may be more pronounced with the less cardioselective beta-blockers, which may explain the apparent increased stroke risk associated with metoprolol.
WHAT SHOULD WE DO NOW?
The evidence for the safety and efficacy of beta-blockers in the perioperative setting continues to evolve, and new clinical trials are needed to clarify the ongoing controversy, particularly regarding the risk of stroke.
If patients have other indications for beta-blocker therapy, such as history of heart failure, myocardial infarction in the past 3 years, or atrial fibrillation for rate control, they should be receiving them if time permits.
If prophylactic beta-blockers are to be effective in minimizing perioperative complications, it appears that they may need to be more cardioselective, started at least 1 week before surgery, titrated to control heart rate, and used in high-risk patients (Revised Cardiac Risk Index score > 2 or 3) undergoing high-risk surgery.
Ideally, a large randomized controlled trial using a cardioselective beta-blocker started in advance of surgery in patients with a Revised Cardiac Risk Index score greater than 2, undergoing intermediate or high-risk procedures, is needed to fully answer the questions raised by the current data.
Prophylactic use of beta-blockers in the perioperative period is highly controversial. Initial studies in the 1990s were favorable, but evidence has been conflicting since then.
The pendulum swung away from routinely recommending beta-blockers after the publication of negative results from several studies, including the Perioperative Ischemic Evaluation (POISE) trial in 2008.1 Highlighting this change in practice, a Canadian study2 found that the use of perioperative beta-blockade increased between 1999 and 2005 but subsequently declined from 2005 to 2010. However, there was no appreciable change in this pattern after the POISE trial or after changes in the American College of Cardiology guidelines in 2002 and 2006.3
In 2008, Harte and Jaffer reviewed the perioperative use of beta-blockers in noncardiac surgery in this journal.4 Since then, a number of meta-analyses and retrospective observational studies have reported variable findings related to specific beta-blockers and specific complications.
In this paper, we review the rationale and recent evidence for and against the perioperative use of beta-blockers as guidance for internists and hospitalists.
POTENTIAL CARDIOPROTECTIVE EFFECTS OF BETA-BLOCKERS
Myocardial infarction and unstable angina are the leading cardiovascular causes of death after surgery.5 These events are multifactorial. Some are caused by the stress of surgery, which precipitates physiologic changes related to inflammatory mediators, sympathetic tone, and oxygen supply and demand; others are caused by acute plaque rupture, thrombosis, and occlusion.6 Most perioperative infarcts are non-Q-wave events7 and occur within the first 2 days after the procedure, when the effects of anesthetics, pain, fluid shifts, and physiologic changes are greatest. Because multiple causes may contribute to perioperative myocardial infarction, a single preventive strategy may not be sufficient.8,9
Beta-blockers do several things that may be beneficial in the perioperative setting. They reduce myocardial oxygen demand by decreasing the force of contraction and by slowing the heart rate, and slowing the heart rate increases diastolic perfusion time.10 They suppress arrhythmias; they limit leukocyte recruitment, the production of free radicals, metalloproteinase activity, monocyte activation, release of growth factors, and inflammatory cytokine response; and they stabilize plaque.11 Their long-term use may also alter intracellular signaling processes, thus improving cell survival by decreasing the expression of receptors for substances that induce apoptosis.12
INITIAL POSITIVE TRIALS
Mangano et al13 began the beta-blocker trend in 1996 with a study in 200 patients known to have coronary artery disease or risk factors for it who were undergoing noncardiac surgery. Patients were randomized to receive either atenolol orally and intravenously, titrated to control the heart rate, or placebo in the immediate perioperative period.
The atenolol group had less perioperative ischemia but no difference in short-term rates of myocardial infarction and death. However, the death rate was lower in the atenolol group at 6 months after discharge and at 2 years, although patients who died in the immediate postoperative period were excluded from the analysis.
Although this finding did not appear to make sense physiologically, we now know that patients may experience myocardial injury without infarction after noncardiac surgery, a phenomenon associated with an increased risk of death in the short term and the long term.14 Preventing these episodes may be the explanation for the improved outcome.
The DECREASE trial15 (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography) provided additional support for beta-blocker use. The patients were at high risk, had abnormal dobutamine stress echocardiograms, and were undergoing vascular surgery; 112 patients were randomized to receive either oral bisoprolol (started 1 month before surgery, titrated to control the heart rate, and continued for 1 month after surgery) or placebo.
The study was stopped early because the bisoprolol group reportedly had a 90% lower rate of myocardial infarction and cardiac death 1 month after surgery. However, the study was criticized because the total number of patients enrolled was small and the benefit was much greater than usual for any pharmacologic intervention, thus calling the results into question.
In a follow-up study,16 survivors continued to be followed while receiving bisoprolol or usual care. The incidence of myocardial infarction or cardiac death at 2 years was significantly lower in the group receiving bisoprolol (12% vs 32%, odds ratio [OR] 0.30, P = .025).
Boersma et al,17 in an observational study, analyzed data from all 1,351 patients scheduled for major vascular surgery being considered for enrollment in the DECREASE trial. The DECREASE protocol required patients to undergo dobutamine stress echocardiography if they had one or more risk factors (age 70 or older, angina, prior myocardial infarction, congestive heart failure, treatment for ventricular arrhythmia, treatment for diabetes mellitus, or limited exercise capacity) or if their physician requested it. Twenty-seven percent received beta-blockers.
In multivariate analysis, clinical predictors of adverse outcome were age 70 or older; current or prior history of angina; and prior myocardial infarction, heart failure, or cerebrovascular accident.
In patients who had fewer than three clinical risk factors, beta-blocker use was associated with a lower rate of complications (0.8% vs 2.3%). Dobutamine stress echocardiography had minimal predictive value in this lower-risk group, suggesting that stress testing may not be necessary in this group if beta-blockers are used appropriately. However, in patients who had three or more risk factors, this test did provide additional prognostic information; those without stress-induced ischemia had lower event rates than those with ischemia, and beta-blocker use further reduced those rates, except in patients with extensive ischemia (more than five left ventricular segments involved).
The Revised Cardiac Risk Index. Lee et al18 devised an index to assist in preoperative cardiac risk stratification that was subsequently incorporated into the 2007 American College of Cardiology/American Heart Association preoperative risk guidelines. (It does not, however, address the beta-blocker issue.) It consists of six independent risk-predictors of major cardiac complications derived from 4,315 patients over age 50 undergoing non-cardiac surgery. The risk factors, each of which is given 1 point, are:
- Congestive heart failure based on history or examination
- Renal insufficiency (serum creatinine level > 2 mg/dL)
- Myocardial infarction, symptomatic ischemic heart disease, or a positive stress test
- History of transient ischemic attack or stroke
- Diabetes requiring insulin
- High-risk surgery (defined as intrathoracic, intra-abdominal, or suprainguinal vascular surgery).
Patients with 3 or more points are considered to be at high risk, and those with 1 or 2 points are considered to be at intermediate risk. The American College of Cardiology/American Heart Association preoperative cardiac risk algorithm subsequently included five of these six risk factors (the type of surgery was considered separately) and made recommendations concerning noninvasive stress testing and heart rate control.
On the basis of these studies, specialty societies, guideline committees, and hospitals enthusiastically recommended the prophylactic use of beta-blockers to decrease postoperative cardiac complications.
THREE NEGATIVE TRIALS OF METOPROLOL
In 2005 and 2006, two studies in vascular surgery patients and another in patients with diabetes cast doubt on the role of beta-blockers when the results failed to show a benefit. The trials used metoprolol, started shortly before surgery, and with no titration to control the heart rate.
The MaVS study19 (Metoprolol After Vascular Surgery) randomized 496 patients to receive metoprolol or placebo 2 hours before surgery and until hospital discharge or a maximum of 5 days after surgery. The metoprolol dose varied by weight: patients weighing 75 kg or more got 100 mg, those weighing between 40 and 75 kg got 50 mg, and those weighing less than 40 kg got 25 mg. Overall effects at 6 months were not significantly different, but intraoperative bradycardia and hypotension requiring intervention were more frequent in the metoprolol group.
The POBBLE study20 (Perioperative Beta Blockade) randomized 103 patients who had no history of myocardial infarction to receive either metoprolol 50 mg twice daily or placebo from admission to 7 days after surgery. Myocardial ischemia was present in one-third of the patients after surgery. Metoprolol did not reduce the 30-day cardiac mortality rate, but it was associated with a shorter length of stay.
The DIPOM trial21 (Diabetic Postoperative Mortality and Morbidity) randomized 921 diabetic patients to receive long-acting metoprolol succinate controlled-release/extended release (CR/XL) or placebo. Patients in the metoprolol group received a test dose of 50 mg the evening before surgery, another dose 2 hours before surgery (100 mg if the heart rate was more than 65 bpm, or 50 mg if between 55 and 65 bpm), and daily thereafter until discharge or a maximum of 8 days. The dose was not titrated to heart-rate control.
Metoprolol had no statistically significant effect on the composite primary outcome measures of time to death from any cause, acute myocardial infarction, unstable angina, or congestive heart failure or on the secondary outcome measures of time to death from any cause, death from a cardiac cause, and nonfatal cardiac morbidity.
ADDITIONAL POSITIVE STUDIES
Lindenauer et al22 retrospectively evaluated the use of beta-blockers in the first 2 days after surgery in 782,969 patients undergoing non-cardiac surgery. Using propensity score matching and Revised Cardiac Risk Index scores, they found a lower rate of postoperative mortality in patients with three or more risk factors who received a beta-blocker. There was no significant difference in the group with two risk factors, but in the lowest-risk group (with a score of 0 to 1), beta-blockers were not beneficial and may have been associated with harm as evidenced by a higher odds ratio for death, although this was probably artifactual and reflecting database limitations.
Feringa et al,23 in an observational cohort study of 272 patients undergoing vascular surgery, reported that higher doses of beta-blockers and tight heart-rate control were associated with less perioperative myocardial ischemia, lower troponin T levels, and better long-term outcome.
THE POISE TRIAL: MIXED RESULTS
The randomized POISE trial,1 published in 2008, compared the effects of extended-release metoprolol succinate vs placebo on the 30-day risk of major cardiovascular events in 8,351 patients with or at risk of atherosclerotic disease who were undergoing noncardiac surgery. The metoprolol regimen was 100 mg 2 to 4 hours before surgery, another 100 mg by 6 hours after surgery, and then 200 mg 12 hours later and once daily for 30 days.
The incidence of the composite primary end point of cardiovascular death, nonfatal myocardial infarction, and nonfatal cardiac arrest at 30 days was lower in the metoprolol group than in the placebo group (5.8% vs 6.9%; P = .04), primarily because of fewer nonfatal myocardial infarctions. However, more patients in the metoprolol group died of any cause (3.1% vs 2.3% P = .03) or had a stroke (1.0% vs 0.5% P = .005) than in the placebo group.
The metoprolol group had a higher incidence of clinically significant hypotension, bradycardia, and stroke, which could account for much of the increase in the mortality rate. Sepsis was the major cause of death in this group; hypotension may have increased the risk of infection, and beta-blockers may have potentiated hypotension in patients who were already septic. Also, the bradycardic and negative inotropic effects of the beta-blocker could have masked the physiologic response to systemic infection, thereby delaying recognition and treatment or impeding the normal immune response.
One of the major criticisms of the POISE trial was its aggressive dosing regimen (200 to 400 mg within a 36-hour period) in patients who had not been on beta-blockers before then. Also, the drug was started only a few hours before surgery. In addition, these patients were at higher risk of death and stroke than those in other trials based on a high baseline rate of cerebrovascular disease, and inclusion of urgent and emergency surgical procedures.
STUDIES SINCE POISE
The POISE trial results1 prompted further questioning of the prophylactic perioperative use of beta-blockers. However, proponents of beta-blockers voiced serious criticisms of the trial, particularly the dosing regimen, and continued to believe that these drugs were beneficial if used appropriately.
The DECREASE IV trial. Dunkelgrun et al,24 in a study using bisoprolol started approximately 1 month before surgery and titrated to control the heart rate, reported beneficial results in intermediate-risk patients. In their randomized open-label study with a 2 × 2 factorial design, 1,066 patients at intermediate cardiac risk were assigned to receive bisoprolol, fluvastatin, combination treatment, or control therapy at least 34 days before surgery. Bisoprolol was started at 2.5 mg orally daily and slowly titrated up to a maximum dose of 10 mg to keep the heart rate between 50 and 70 beats per minute. The group of 533 patients randomized to receive bisoprolol had a lower incidence rate of cardiac death and nonfatal myocardial infarction than the control group (2.1% vs 6.0%, HR 0.34, P = .002). A potential limitation of this study was its open-label design, which might have led to treatment bias.
Updated guidelines. Based on the results from POISE and DECREASE IV, the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines25 published a focused update on beta-blockers in 2009 as an amendment to their 2007 guidelines on perioperative evaluation and care for noncardiac surgery. The European Society of Cardiology26 released similar but somewhat more liberal guidelines (Table 1).
London et al,27 in an observational study published in 2013, found a lower 30-day overall mortality rate with beta-blockers (relative risk [RR] 0.73, 95% confidence interval [CI] 0.65–0.83, P < .001, number needed to treat [NNT] 241), as well as a lower rate of cardiac morbidity (nonfatal myocardial infarction and cardiac death), but only in nonvascular surgery patients who were on beta-blockers within 7 days of scheduled surgery. Moreover, similar to the findings of Lindenauer et al,22 only patients with a Revised Cardiac Risk Index score of 2 or more benefited from beta-blocker use in terms of a lower risk of death, whereas the lower-risk patients did not:
- Risk score of 0 or 1—no association
- Score of 2—RR 0.63, 95% CI 0.50–0.80, P < .001, NNT 105
- Score of 3—RR 0.54, 95% CI 0.39–0.73, P < .001, NNT 41
- Score of 4 or more—RR 0.40, 95% CI 0.24–0.73, P < .001, NNT 18).
Beta-blocker exposure was associated with a significantly lower rate of cardiac complications (RR 0.67, 95% CI 0.57–0.79, P < .001, NNT 339), also limited to nonvascular surgery patients with a risk score of 2 or 3.
The Danish Nationwide Cohort Study28 examined the effect of beta-blockers on major adverse cardiac events (MACE, ie, myocardial infarction, cerebrovascular accident, and death) in 28,263 patients with ischemic heart disease undergoing noncardiac surgery; 7,990 with heart failure and 20,273 without. Beta-blockers were used in 53% of patients with heart failure and 36% of those without heart failure. Outcomes for all of the beta-blocker recipients:
- MACE—HR 0.90, 95% CI 0.79–1.02
- All-cause mortality—HR 0.95, 95% CI 0.85–1.06.
Outcomes for patients with heart failure if they received beta-blockers:
- MACE—HR 0.75, 95% CI 0.70–0.87
- All-cause mortality—HR 0.80, 95% CI 0.70–0.92.
There was no significant benefit from beta-blockers in patients without heart failure. Outcomes for those patients if they received beta-blockers:
- MACE—HR 1.11, 95% CI 0.92–1.33
- All-cause mortality—HR 1.15, 95% CI 0.98–1.35.
However, in patients without heart failure but with a history of myocardial infarction within the past 2 years, beta-blockers were associated with a lower risk of MACE and all-cause mortality. In patients with neither heart failure nor a recent myocardial infarction, beta-blockers were associated with an increased risk of MACE and all-cause mortality.
This difference in efficacy depending on the presence and timing of a prior myocardial infarction is consistent with the 2012 American College of Cardiology/American Heart Association guidelines for secondary prevention, in which beta-blockers are given a class I recommendation only for patients with a myocardial infarction within the past 3 years.
Meta-analyses and outcomes
A number of meta-analyses have been published over the past 10 years, with conflicting results (Table 2). The divergent findings are primarily due to the different studies included in the analyses as well as the strong influence of the POISE trial.1 The studies varied in terms of the specific beta-blocker used, dose titration and heart rate control, time of initiation of beta-blocker use before surgery, type of surgery, patient characteristics, comorbidities, biomarkers and diagnosis of myocardial infarction, and clinical end points.
In general, these meta-analyses have found that prophylactic perioperative use of beta-blockers decreases ischemia and tends to reduce the risk of nonfatal myocardial infarction. They vary on whether the overall mortality risk is decreased. The meta-analyses that included POISE1 found an increased incidence of stroke, whereas those that excluded POISE found no significant difference, although there appeared to be slightly more strokes in the beta-blocker groups.
The beta-blocker controversy increased even further when Dr. Don Poldermans was fired by Erasmus Medical Center in November 2011 for violations of academic integrity involving his research, including the DECREASE trials. The most recent meta-analysis, by Bouri et al,29 included nine “secure trials” and excluded the DECREASE trials in view of the controversy about their authenticity. The analysis showed an increase in overall mortality as well as stroke, primarily because it was heavily influenced by POISE.1 In contrast, the DECREASE trials had reported a decreased risk of myocardial infarction and death, with no significant increase in stroke. The authors concluded that guideline bodies should “retract their recommendations based on the fictitious data without further delay.”29
Although the design of the DECREASE trials (in which beta-blockers were started well in advance of surgery and doses were titrated to achieve heart rate control) is physiologically more compelling than those of the negative trials, the results have been questioned in light of the integrity issue. However, to date, none of the published DECREASE trials have been retracted.
Two other meta-analyses,30,31 published in 2013, also found a decreased risk of myocardial infarction and increased risk of stroke but no significant difference in short-term all-cause mortality.
ARE ALL BETA-BLOCKERS EQUIVALENT?
In various studies evaluating specific beta-blockers, the more cardioselective agents bisoprolol and atenolol were associated with better outcomes than metoprolol. The affinity ratios for beta-1/beta-2 receptors range from 13.5 for bisoprolol to 4.7 for atenolol and 2.3 for metoprolol.32 Blocking beta-1 receptors blunts tachycardia, whereas blocking beta-2 receptors may block systemic or cerebral vasodilation.
In patients with anemia, beta-blockade in general may be harmful, but beta-2 blockade may be even worse. Beta-blockers were associated with an increased risk of MACE (6.5% vs 3.0%)33 in patients with acute surgical anemia if the hemoglobin concentration decreased to less than 35% of baseline, and increased risks of hospital death (OR 6.65) and multiorgan dysfunction syndrome (OR 4.18) with severe bleeding during aortic surgery.34
In addition, the pathway by which the beta-blocker is metabolized may also affect outcome, with less benefit from beta-blockers metabolized by the CYP2D6 isoenzyme of the cytochrome P450 system. Individual variations in CYP2D6 activity related to genetics or drug interactions may result in insufficient or excessive beta-blockade. Because metoprolol is the most dependent on this system, patients using it may be more susceptible to bradycardia.35
Studies comparing atenolol and metoprolol found that the atenolol groups had fewer myocardial infarctions and deaths36 and lower 30-day and 1-year mortality rates37 than the groups on metoprolol. Studies comparing the three beta-blockers found better outcomes with atenolol and bisoprolol than with metoprolol—fewer strokes,38,39 a lower mortality rate,31 and a better composite outcome39 (Table 3 and Table 4).
START THE BETA-BLOCKER EARLY, TITRATE TO CONTROL THE HEART RATE
A number of studies suggest that how long the beta-blocker is given before surgery may influence the outcome (Table 5). The best results were achieved when beta-blockers were started approximately 1 month before surgery and titrated to control the heart rate.
Because this long lead-in time is not always practical, it is important to determine the shortest time before surgery in which starting beta-blockers may be beneficial and yet safe. Some evidence suggests that results are better when the beta-blocker is started more than 1 week preoperatively compared with less than 1 week, but it is unknown what the minimum or optimal time period should be.
If a beta-blocker is started well in advance of the scheduled surgery, there is adequate time for dose titration and tighter heart rate control. Most of the studies demonstrating beneficial effects of perioperative beta-blockers used dose titration and achieved lower heart rates in the treatment group than in the control group. A criticism of the MaVs,19 POBBLE,20 and DIPOM21 trials was that the patients did not receive adequate beta-blockade. The POISE trial1 used a much higher dose of metoprolol in an attempt to assure beta-blockade without dose titration, and although the regimen decreased nonfatal myocardial infarctions, it increased strokes and the overall mortality rate, probably related to excess bradycardia and hypotension. The target heart rate should probably be between 55 and 70 beats per minute.
RISK OF STROKE
POISE1 was the first trial to note a clinically and statistically significant increase in strokes with perioperative beta-blocker use. Although no other study has shown a similar increased risk, almost all reported a higher number of strokes in the beta-blocker groups, although the absolute numbers and differences were small and not statistically significant. This risk may also vary from one beta-blocker to another (Table 4).
The usual incidence rate of postoperative stroke after noncardiac, noncarotid surgery is well under 1% in patients with no prior history of stroke but increases to approximately 3% in patients with a previous stroke.40 An observational study from the Dutch group reported a very low incidence of stroke overall (0.02%) in 186,779 patients undergoing noncardiac surgery with no significant difference in those on chronic beta-blocker therapy.41 The DECREASE trials, with a total of 3,884 patients, also found no statistically significant increase in stroke with beta-blocker use (0.46% overall vs 0.5% with a beta-blocker),42 which in this case was bisoprolol started well in advance of surgery and titrated to control the heart rate. Although the DECREASE data are under suspicion, they seem reasonable and consistent with those of observational studies.
Proposed mechanisms by which beta-blockers may increase stroke risk include the side effects of hypotension and bradycardia, particularly in the setting of anemia. They may also cause cerebral ischemia by blocking cerebral vasodilation. This effect on cerebral blood flow may be more pronounced with the less cardioselective beta-blockers, which may explain the apparent increased stroke risk associated with metoprolol.
WHAT SHOULD WE DO NOW?
The evidence for the safety and efficacy of beta-blockers in the perioperative setting continues to evolve, and new clinical trials are needed to clarify the ongoing controversy, particularly regarding the risk of stroke.
If patients have other indications for beta-blocker therapy, such as history of heart failure, myocardial infarction in the past 3 years, or atrial fibrillation for rate control, they should be receiving them if time permits.
If prophylactic beta-blockers are to be effective in minimizing perioperative complications, it appears that they may need to be more cardioselective, started at least 1 week before surgery, titrated to control heart rate, and used in high-risk patients (Revised Cardiac Risk Index score > 2 or 3) undergoing high-risk surgery.
Ideally, a large randomized controlled trial using a cardioselective beta-blocker started in advance of surgery in patients with a Revised Cardiac Risk Index score greater than 2, undergoing intermediate or high-risk procedures, is needed to fully answer the questions raised by the current data.
- POISE Study Group; Devereaux PJ, Yang H, Yusuf S, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; 371:1839–1847.
- Wijeysundera DN, Mamdani M, Laupacis A, et al. Clinical evidence, practice guidelines, and ß-blocker utilization before major noncardiac surgery. Circ Cardiovasc Qual Outcomes 2012; 5:558–565.
- American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery); American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Rhythm Society; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery: focused update on perioperative beta-blocker therapy: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society for Vascular Medicine and Biology. J Am Coll Cardiol 2006; 47:2343–2355.
- Harte B, Jaffer AK. Perioperative beta-blockers in noncardiac surgery: evolution of the evidence. Cleve Clin J Med 2008; 75:513–519.
- Mangano DT. Perioperative cardiac morbidity. Anesthesiology 1990; 72:153–184.
- London MJ, Zaugg M, Schaub MC, Spahn DR. Perioperative beta-adrenergic receptor blockade: physiologic foundations and clinical controversies. Anesthesiology 2004; 100:170–175.
- Badner NH, Knill RL, Brown JE, Novick TV, Gelb AW. Myocardial infarction after noncardiac surgery. Anesthesiology 1998; 88:572–578.
- Priebe HJ. Triggers of perioperative myocardial ischaemia and infarction. Br J Anaesth 2004; 93:9–20.
- Zaugg M, Schaub MC, Foëx P. Myocardial injury and its prevention in the perioperative setting. Br J Anaesth 2004; 93:21–33.
- Zaugg M, Schaub MC, Pasch T, Spahn DR. Modulation of beta-adrenergic receptor subtype activities in perioperative medicine: mechanisms and sites of action. Br J Anaesth 2002; 88:101–123.
- Landesberg G. The pathophysiology of perioperative myocardial infarction: facts and perspectives. J Cardiothorac Vasc Anesth 2003; 17:90–100.
- Yeager MP, Fillinger MP, Hettleman BD, Hartman GS. Perioperative beta-blockade and late cardiac outcomes: a complementary hypothesis. J Cardiothorac Vasc Anesth 2005; 19:237–241.
- Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 1996; 335:1713–1720.
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120:564–578.
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med 1999; 341:1789–1794.
- Poldermans D, Boersma E, Bax JJ, et al; Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. Bisoprolol reduces cardiac death and myocardial infarction in high-risk patients as long as 2 years after successful major vascular surgery. Eur Heart J 2001; 22:1353–1358.
- Boersma E, Poldermans D, Bax JJ, et al; DECREASE Study Group (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiogrpahy). Predictors of cardiac events after major vascular surgery: role of clinical characteristics, dobutamine echocardiography, and beta-blocker therapy. JAMA 2001; 285:1865–1873.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Yang H, Raymer K, Butler R, Parlow J, Roberts R. The effects of perioperative beta-blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J 2006; 152:983–990.
- Brady AR, Gibbs JS, Greenhalgh RM, Powell JT, Sydes MR; POBBLE trial investigators. Perioperative beta-blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double-blind controlled trial. J Vasc Surg 2005; 41:602–609.
- Juul AB, Wetterslev J, Gluud C, et al; DIPOM Trial Group. Effect of perioperative beta blockade in patients with diabetes undergoing major non-cardiac surgery: randomised placebo controlled, blinded multicentre trial. BMJ 2006; 332:1482.
- Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major non-cardiac surgery. N Engl J Med 2005; 353:349–361.
- Feringa HH, Bax JJ, Boersma E, et al. High-dose beta-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation 2006; 114(suppl 1):1344–1349.
- Dunkelgrun M, Boersma E, Schouten O, et al; Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate-risk patients undergoing noncardiovascular surgery: a randomized controlled trial (DECREASE-IV). Ann Surg 2009; 249:921–926.
- American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Rhythm Society; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine; Society for Vascular Surgery; Fleisher LA, Beckman JA, Brown KA, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol 2009; 54:e13–e118.
- Task Force for Preoperative Cardiac Risk Assessment and Perioperative Cardiac Management in Non-cardiac Surgery; European Society of Cardiology (ESC); Poldermans D, Bax JJ, Boersma E, et al. Guidelines for preoperative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery. Eur Heart J 2009; 30:2769–2812.
- London MJ, Hur K, Schwartz GG, Henderson WG. Association of perioperative beta-blockade with mortality and cardiovascular morbidity following major noncardiac surgery. JAMA 2013; 309:1704–1713.
- Andersson C, Mérie C, Jørgensen M, et al. Association of beta-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing noncardiac surgery: a Danish nationwide cohort study. JAMA Intern Med 2014; 174:336–344.
- Bouri S, Shun-Shin MJ, Cole GD, Mayet J, Francis DP. Meta-analysis of secure randomised controlled trials of beta-blockade to prevent perioperative death in non-cardiac surgery. Heart 2014; 100:456–464.
- Guay J, Ochroch EA. Beta-blocking agents for surgery: influence on mortality and major outcomes. A meta-analysis. J Cardiothorac Vasc Anesth 2013; 27:834–844.
- Dai N, Xu D, Zhang J, et al. Different beta-blockers and initiation time in patients undergoing noncardiac surgery: a meta-analysis. Am J Med Sci 2014; 347:235–244.
- Baker JG. The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br J Pharmacol 2005; 144:317–322.
- Beattie WS, Wijeysundera DN, Karkouti K, et al. Acute surgical anemia influences the cardioprotective effects of beta-blockade: a single-center, propensity-matched cohort study. Anesthesiology 2010; 112:25–33.
- Le Manach Y, Collins GS, Ibanez C, et al. Impact of perioperative bleeding on the protective effect of beta-blockers during infrarenal aortic reconstruction. Anesthesiology 2012; 117:1203–1211.
- Badgett RG, Lawrence VA, Cohn SL. Variations in pharmacology of beta-blockers may contribute to heterogeneous results in trials of perioperative beta-blockade. Anesthesiology 2010; 113:585–592.
- Redelmeier D, Scales D, Kopp A. Beta blockers for elective surgery in elderly patients: population based, retrospective cohort study. BMJ 2005; 331:932.
- Wallace AW, Au S, Cason BA. Perioperative beta-blockade: atenolol is associated with reduced mortality when compared to metoprolol. Anesthesiology 2011; 114:824–836.
- Mashour GA, Sharifpour M, Freundlich RE, et al. Perioperative metoprolol and risk of stroke after noncardiac surgery. Anesthesiology 2013; 119:1340–1346.
- Ashes C, Judelman S, Wijeysundera DN, et al. Selective beta1-antagonism with bisoprolol is associated with fewer postoperative strokes than atenolol or metoprolol: a single-center cohort study of 44,092 consecutive patients. Anesthesiology 2013; 119:777–787.
- Selim M. Perioperative stroke. N Engl J Med 2007; 356:706–713.
- van Lier F, Schouten O, van Domburg RT, et al. Effect of chronic beta-blocker use on stroke after noncardiac surgery. Am J Cardiol 2009; 104:429–433.
- van Lier F, Schouten O, Hoeks SE, et al. Impact of prophylactic beta-blocker therapy to prevent stroke after noncardiac surgery. Am J Cardiol 2010; 105:43–47.
- Devereaux PJ, Beattie WS, Choi PT, et al. How strong is the evidence for the use of perioperative beta blockers in non-cardiac surgery? Systematic review and meta-analysis of randomised controlled trials. BMJ 2005; 331:313–321.
- McGory ML, Maggard MA, Ko CY. A meta-analysis of perioperative beta blockade: what is the actual risk reduction? Surgery 2005; 138:171–179.
- Schouten O, Shaw LJ, Boersma E, et al. A meta-analysis of safety and effectiveness of perioperative beta-blocker use for the prevention of cardiac events in different types of noncardiac surgery. Coron Artery Dis 2006; 17:173–179.
- Wiesbauer F, Schlager O, Domanovits H, et al. Perioperative beta-blockers for preventing surgery-related mortality and morbidity: a systematic review and meta-analysis. Anesth Analg 2007; 104:27–41.
- Bangalore S, Wetterslev J, Pranesh S, Sawhney S, Gluud C, Messerli FH. Perioperative beta blockers in patients having non-cardiac surgery: a meta-analysis. Lancet 2008; 372:1962–1976.
- Flu WJ, van Kuijk JP, Chonchol M, et al. Timing of preoperative beta-blocker treatment in vascular surgery patients: influence on postoperative outcome. J Am Coll Cardiol 2010; 56:1922–1929.
- Wijeysundera DN, Beattie WS, Wijeysundera HC, Yun L, Austin PC, Ko DT. Duration of preoperative beta-blockade and outcomes after major elective noncardiac surgery. Can J Cardiol 2014; 30:217–223.
- POISE Study Group; Devereaux PJ, Yang H, Yusuf S, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; 371:1839–1847.
- Wijeysundera DN, Mamdani M, Laupacis A, et al. Clinical evidence, practice guidelines, and ß-blocker utilization before major noncardiac surgery. Circ Cardiovasc Qual Outcomes 2012; 5:558–565.
- American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery); American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Rhythm Society; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery: focused update on perioperative beta-blocker therapy: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society for Vascular Medicine and Biology. J Am Coll Cardiol 2006; 47:2343–2355.
- Harte B, Jaffer AK. Perioperative beta-blockers in noncardiac surgery: evolution of the evidence. Cleve Clin J Med 2008; 75:513–519.
- Mangano DT. Perioperative cardiac morbidity. Anesthesiology 1990; 72:153–184.
- London MJ, Zaugg M, Schaub MC, Spahn DR. Perioperative beta-adrenergic receptor blockade: physiologic foundations and clinical controversies. Anesthesiology 2004; 100:170–175.
- Badner NH, Knill RL, Brown JE, Novick TV, Gelb AW. Myocardial infarction after noncardiac surgery. Anesthesiology 1998; 88:572–578.
- Priebe HJ. Triggers of perioperative myocardial ischaemia and infarction. Br J Anaesth 2004; 93:9–20.
- Zaugg M, Schaub MC, Foëx P. Myocardial injury and its prevention in the perioperative setting. Br J Anaesth 2004; 93:21–33.
- Zaugg M, Schaub MC, Pasch T, Spahn DR. Modulation of beta-adrenergic receptor subtype activities in perioperative medicine: mechanisms and sites of action. Br J Anaesth 2002; 88:101–123.
- Landesberg G. The pathophysiology of perioperative myocardial infarction: facts and perspectives. J Cardiothorac Vasc Anesth 2003; 17:90–100.
- Yeager MP, Fillinger MP, Hettleman BD, Hartman GS. Perioperative beta-blockade and late cardiac outcomes: a complementary hypothesis. J Cardiothorac Vasc Anesth 2005; 19:237–241.
- Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 1996; 335:1713–1720.
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120:564–578.
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med 1999; 341:1789–1794.
- Poldermans D, Boersma E, Bax JJ, et al; Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. Bisoprolol reduces cardiac death and myocardial infarction in high-risk patients as long as 2 years after successful major vascular surgery. Eur Heart J 2001; 22:1353–1358.
- Boersma E, Poldermans D, Bax JJ, et al; DECREASE Study Group (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiogrpahy). Predictors of cardiac events after major vascular surgery: role of clinical characteristics, dobutamine echocardiography, and beta-blocker therapy. JAMA 2001; 285:1865–1873.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Yang H, Raymer K, Butler R, Parlow J, Roberts R. The effects of perioperative beta-blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J 2006; 152:983–990.
- Brady AR, Gibbs JS, Greenhalgh RM, Powell JT, Sydes MR; POBBLE trial investigators. Perioperative beta-blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double-blind controlled trial. J Vasc Surg 2005; 41:602–609.
- Juul AB, Wetterslev J, Gluud C, et al; DIPOM Trial Group. Effect of perioperative beta blockade in patients with diabetes undergoing major non-cardiac surgery: randomised placebo controlled, blinded multicentre trial. BMJ 2006; 332:1482.
- Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major non-cardiac surgery. N Engl J Med 2005; 353:349–361.
- Feringa HH, Bax JJ, Boersma E, et al. High-dose beta-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation 2006; 114(suppl 1):1344–1349.
- Dunkelgrun M, Boersma E, Schouten O, et al; Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate-risk patients undergoing noncardiovascular surgery: a randomized controlled trial (DECREASE-IV). Ann Surg 2009; 249:921–926.
- American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Rhythm Society; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine; Society for Vascular Surgery; Fleisher LA, Beckman JA, Brown KA, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol 2009; 54:e13–e118.
- Task Force for Preoperative Cardiac Risk Assessment and Perioperative Cardiac Management in Non-cardiac Surgery; European Society of Cardiology (ESC); Poldermans D, Bax JJ, Boersma E, et al. Guidelines for preoperative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery. Eur Heart J 2009; 30:2769–2812.
- London MJ, Hur K, Schwartz GG, Henderson WG. Association of perioperative beta-blockade with mortality and cardiovascular morbidity following major noncardiac surgery. JAMA 2013; 309:1704–1713.
- Andersson C, Mérie C, Jørgensen M, et al. Association of beta-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing noncardiac surgery: a Danish nationwide cohort study. JAMA Intern Med 2014; 174:336–344.
- Bouri S, Shun-Shin MJ, Cole GD, Mayet J, Francis DP. Meta-analysis of secure randomised controlled trials of beta-blockade to prevent perioperative death in non-cardiac surgery. Heart 2014; 100:456–464.
- Guay J, Ochroch EA. Beta-blocking agents for surgery: influence on mortality and major outcomes. A meta-analysis. J Cardiothorac Vasc Anesth 2013; 27:834–844.
- Dai N, Xu D, Zhang J, et al. Different beta-blockers and initiation time in patients undergoing noncardiac surgery: a meta-analysis. Am J Med Sci 2014; 347:235–244.
- Baker JG. The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br J Pharmacol 2005; 144:317–322.
- Beattie WS, Wijeysundera DN, Karkouti K, et al. Acute surgical anemia influences the cardioprotective effects of beta-blockade: a single-center, propensity-matched cohort study. Anesthesiology 2010; 112:25–33.
- Le Manach Y, Collins GS, Ibanez C, et al. Impact of perioperative bleeding on the protective effect of beta-blockers during infrarenal aortic reconstruction. Anesthesiology 2012; 117:1203–1211.
- Badgett RG, Lawrence VA, Cohn SL. Variations in pharmacology of beta-blockers may contribute to heterogeneous results in trials of perioperative beta-blockade. Anesthesiology 2010; 113:585–592.
- Redelmeier D, Scales D, Kopp A. Beta blockers for elective surgery in elderly patients: population based, retrospective cohort study. BMJ 2005; 331:932.
- Wallace AW, Au S, Cason BA. Perioperative beta-blockade: atenolol is associated with reduced mortality when compared to metoprolol. Anesthesiology 2011; 114:824–836.
- Mashour GA, Sharifpour M, Freundlich RE, et al. Perioperative metoprolol and risk of stroke after noncardiac surgery. Anesthesiology 2013; 119:1340–1346.
- Ashes C, Judelman S, Wijeysundera DN, et al. Selective beta1-antagonism with bisoprolol is associated with fewer postoperative strokes than atenolol or metoprolol: a single-center cohort study of 44,092 consecutive patients. Anesthesiology 2013; 119:777–787.
- Selim M. Perioperative stroke. N Engl J Med 2007; 356:706–713.
- van Lier F, Schouten O, van Domburg RT, et al. Effect of chronic beta-blocker use on stroke after noncardiac surgery. Am J Cardiol 2009; 104:429–433.
- van Lier F, Schouten O, Hoeks SE, et al. Impact of prophylactic beta-blocker therapy to prevent stroke after noncardiac surgery. Am J Cardiol 2010; 105:43–47.
- Devereaux PJ, Beattie WS, Choi PT, et al. How strong is the evidence for the use of perioperative beta blockers in non-cardiac surgery? Systematic review and meta-analysis of randomised controlled trials. BMJ 2005; 331:313–321.
- McGory ML, Maggard MA, Ko CY. A meta-analysis of perioperative beta blockade: what is the actual risk reduction? Surgery 2005; 138:171–179.
- Schouten O, Shaw LJ, Boersma E, et al. A meta-analysis of safety and effectiveness of perioperative beta-blocker use for the prevention of cardiac events in different types of noncardiac surgery. Coron Artery Dis 2006; 17:173–179.
- Wiesbauer F, Schlager O, Domanovits H, et al. Perioperative beta-blockers for preventing surgery-related mortality and morbidity: a systematic review and meta-analysis. Anesth Analg 2007; 104:27–41.
- Bangalore S, Wetterslev J, Pranesh S, Sawhney S, Gluud C, Messerli FH. Perioperative beta blockers in patients having non-cardiac surgery: a meta-analysis. Lancet 2008; 372:1962–1976.
- Flu WJ, van Kuijk JP, Chonchol M, et al. Timing of preoperative beta-blocker treatment in vascular surgery patients: influence on postoperative outcome. J Am Coll Cardiol 2010; 56:1922–1929.
- Wijeysundera DN, Beattie WS, Wijeysundera HC, Yun L, Austin PC, Ko DT. Duration of preoperative beta-blockade and outcomes after major elective noncardiac surgery. Can J Cardiol 2014; 30:217–223.
KEY POINTS
- If patients have other indications for beta-blocker therapy, such as a history of heart failure, myocardial infarction in the past 3 years, or atrial fibrillation, they should be started on a beta-blocker before surgery if time permits.
- Of the various beta-blockers, the cardioselective ones appear to be preferable in the perioperative setting.
- Beta-blockers may need to be started at least 1 week before surgery, titrated to control the heart rate, and used only in patients at high risk (Revised Cardiac Risk Index score > 2 or 3) undergoing high-risk surgery.
- Further clinical trials are necessary to clarify the ongoing controversy, particularly regarding the risk of stroke, which was increased in the large Perioperative Ischemic Evaluation (POISE) trial.
A serious complication of a common stress test
To the Editor: We read with interest the article by Drs. Buitrago et al in the May 2014 issue of Cleveland Clinic Journal of Medicine, “Syncope during a pharmacologic nuclear stress test.”1 It highlights a known, serious interaction between adenosine and dipyridamole (the latter contained in the aspirin-dipyridamole combination Aggrenox) and associated asystole in patients undergoing pharmacologic cardiac stress testing. This interaction is known in the cardiology literature, as it was noted in the current guidelines for pharmacologic stress testing.2 However, I would like to discuss a few points with the authors for a better understanding of the case.
First, the underlying rhythm before the development of complete atrioventricular (AV) dissociation and asystole was significant for second-degree AV block (Mobitz type I, Wenckebach). Second- or third-degree AV block is considered a contraindication to adenosine because of the risk of exacerbating these conditions. This underlying AV nodal disease made dipyridamole not the only culprit. In addition, the patient had been on two agents (labetalol and clonidine) that have AV nodal-blocking properties. Electrolyte imbalances such as hypokalemia, hypomagnesemia, and hypocalcemia are another reason for delayed conduction and PR prolongation, and electrolyte levels should be checked and corrected properly before the stress test or coronary angiography. It would have been helpful if the authors had discussed these points for a better understanding of the drug-drug interaction.
Because of the increasing trend to admit patients with chest pain to observation units to rule out myocardial infarction, the case has a valuable teaching point, especially for hospitalists and emergency physicians in charge of patients admitted with chest pain.3 Since cardiologists rarely get involved in the care of these patients, careful review of medications before scheduling stress testing is of ultimate importance and should be emphasized in the discussion.
Lastly, the number of combined medications that are available commercially is increasing, which puts patients at higher risk of drug interactions. Hospitalists and internists taking care of patients, especially elderly patients, admitted from nursing homes and taking multiple medications should pay extra attention when reviewing medications with brand names.4,5 Furthermore, a 12-lead electrocardiogram should be reviewed, with special attention to the PR interval and QT segment. A pharmacy consultation could be valuable, especially in patients taking multiple drugs.6
- Buitrago I, Wolinsky D, Asher CR. Syncope during a pharmacologic nuclear stress test. Cleve Clin J Med 2014; 81:279–280.
- Henzlova MJ, Cerqueira MD, Mahmarian JJ, Yao SS; Quality Assurance Committee of the American Society of Nuclear Cardiology. Stress protocols and tracers. J Nucl Cardiol 2006; 13:e80–e90.
- Graff LG, Dallara J, Ross MA, et al. Impact on the care of the emergency department chest pain patient from the chest pain evaluation registry (CHEPER) study. Am J Cardiol 1997; 80:563–568.
- Samaras N, Chevalley T, Samaras D, Gold G. Older patients in the emergency department: a review. Ann Emerg Med 2010; 56:261–269.
- Steinman MA, Hanlon JT. Managing medications in clinically complex elders: “There’s got to be a happy medium.” JAMA 2010; 304:1592–1601.
- Scott IA, Gray LC, Martin JH, Mitchell CA. Minimizing inappropriate medications in older populations: a 10-step conceptual framework. Am J Med 2012; 125:529–537.
To the Editor: We read with interest the article by Drs. Buitrago et al in the May 2014 issue of Cleveland Clinic Journal of Medicine, “Syncope during a pharmacologic nuclear stress test.”1 It highlights a known, serious interaction between adenosine and dipyridamole (the latter contained in the aspirin-dipyridamole combination Aggrenox) and associated asystole in patients undergoing pharmacologic cardiac stress testing. This interaction is known in the cardiology literature, as it was noted in the current guidelines for pharmacologic stress testing.2 However, I would like to discuss a few points with the authors for a better understanding of the case.
First, the underlying rhythm before the development of complete atrioventricular (AV) dissociation and asystole was significant for second-degree AV block (Mobitz type I, Wenckebach). Second- or third-degree AV block is considered a contraindication to adenosine because of the risk of exacerbating these conditions. This underlying AV nodal disease made dipyridamole not the only culprit. In addition, the patient had been on two agents (labetalol and clonidine) that have AV nodal-blocking properties. Electrolyte imbalances such as hypokalemia, hypomagnesemia, and hypocalcemia are another reason for delayed conduction and PR prolongation, and electrolyte levels should be checked and corrected properly before the stress test or coronary angiography. It would have been helpful if the authors had discussed these points for a better understanding of the drug-drug interaction.
Because of the increasing trend to admit patients with chest pain to observation units to rule out myocardial infarction, the case has a valuable teaching point, especially for hospitalists and emergency physicians in charge of patients admitted with chest pain.3 Since cardiologists rarely get involved in the care of these patients, careful review of medications before scheduling stress testing is of ultimate importance and should be emphasized in the discussion.
Lastly, the number of combined medications that are available commercially is increasing, which puts patients at higher risk of drug interactions. Hospitalists and internists taking care of patients, especially elderly patients, admitted from nursing homes and taking multiple medications should pay extra attention when reviewing medications with brand names.4,5 Furthermore, a 12-lead electrocardiogram should be reviewed, with special attention to the PR interval and QT segment. A pharmacy consultation could be valuable, especially in patients taking multiple drugs.6
To the Editor: We read with interest the article by Drs. Buitrago et al in the May 2014 issue of Cleveland Clinic Journal of Medicine, “Syncope during a pharmacologic nuclear stress test.”1 It highlights a known, serious interaction between adenosine and dipyridamole (the latter contained in the aspirin-dipyridamole combination Aggrenox) and associated asystole in patients undergoing pharmacologic cardiac stress testing. This interaction is known in the cardiology literature, as it was noted in the current guidelines for pharmacologic stress testing.2 However, I would like to discuss a few points with the authors for a better understanding of the case.
First, the underlying rhythm before the development of complete atrioventricular (AV) dissociation and asystole was significant for second-degree AV block (Mobitz type I, Wenckebach). Second- or third-degree AV block is considered a contraindication to adenosine because of the risk of exacerbating these conditions. This underlying AV nodal disease made dipyridamole not the only culprit. In addition, the patient had been on two agents (labetalol and clonidine) that have AV nodal-blocking properties. Electrolyte imbalances such as hypokalemia, hypomagnesemia, and hypocalcemia are another reason for delayed conduction and PR prolongation, and electrolyte levels should be checked and corrected properly before the stress test or coronary angiography. It would have been helpful if the authors had discussed these points for a better understanding of the drug-drug interaction.
Because of the increasing trend to admit patients with chest pain to observation units to rule out myocardial infarction, the case has a valuable teaching point, especially for hospitalists and emergency physicians in charge of patients admitted with chest pain.3 Since cardiologists rarely get involved in the care of these patients, careful review of medications before scheduling stress testing is of ultimate importance and should be emphasized in the discussion.
Lastly, the number of combined medications that are available commercially is increasing, which puts patients at higher risk of drug interactions. Hospitalists and internists taking care of patients, especially elderly patients, admitted from nursing homes and taking multiple medications should pay extra attention when reviewing medications with brand names.4,5 Furthermore, a 12-lead electrocardiogram should be reviewed, with special attention to the PR interval and QT segment. A pharmacy consultation could be valuable, especially in patients taking multiple drugs.6
- Buitrago I, Wolinsky D, Asher CR. Syncope during a pharmacologic nuclear stress test. Cleve Clin J Med 2014; 81:279–280.
- Henzlova MJ, Cerqueira MD, Mahmarian JJ, Yao SS; Quality Assurance Committee of the American Society of Nuclear Cardiology. Stress protocols and tracers. J Nucl Cardiol 2006; 13:e80–e90.
- Graff LG, Dallara J, Ross MA, et al. Impact on the care of the emergency department chest pain patient from the chest pain evaluation registry (CHEPER) study. Am J Cardiol 1997; 80:563–568.
- Samaras N, Chevalley T, Samaras D, Gold G. Older patients in the emergency department: a review. Ann Emerg Med 2010; 56:261–269.
- Steinman MA, Hanlon JT. Managing medications in clinically complex elders: “There’s got to be a happy medium.” JAMA 2010; 304:1592–1601.
- Scott IA, Gray LC, Martin JH, Mitchell CA. Minimizing inappropriate medications in older populations: a 10-step conceptual framework. Am J Med 2012; 125:529–537.
- Buitrago I, Wolinsky D, Asher CR. Syncope during a pharmacologic nuclear stress test. Cleve Clin J Med 2014; 81:279–280.
- Henzlova MJ, Cerqueira MD, Mahmarian JJ, Yao SS; Quality Assurance Committee of the American Society of Nuclear Cardiology. Stress protocols and tracers. J Nucl Cardiol 2006; 13:e80–e90.
- Graff LG, Dallara J, Ross MA, et al. Impact on the care of the emergency department chest pain patient from the chest pain evaluation registry (CHEPER) study. Am J Cardiol 1997; 80:563–568.
- Samaras N, Chevalley T, Samaras D, Gold G. Older patients in the emergency department: a review. Ann Emerg Med 2010; 56:261–269.
- Steinman MA, Hanlon JT. Managing medications in clinically complex elders: “There’s got to be a happy medium.” JAMA 2010; 304:1592–1601.
- Scott IA, Gray LC, Martin JH, Mitchell CA. Minimizing inappropriate medications in older populations: a 10-step conceptual framework. Am J Med 2012; 125:529–537.
In reply: A serious complication of a common stress test
In Reply: We appreciate the interest and comments of Dr. Alraies. We would like to clarify that the patient’s baseline electrocardiogram before the nuclear stress test was normal. Second-degree atrioventricular (AV) block (Mobitz type I) was evident only during adenosine infusion before ventricular asystole. The patient was on two AV nodal blockers (labetalol and clonidine) but had no underlying conduction disease. There is no contraindication to continuing these agents before pharmacologic stress testing. In addition, the patient’s electrolyte levels were within normal ranges before testing.
We agree that the valuable teaching point for clinicians is to appreciate the contraindication to and consequences of the use of dipyridamole-containing oral medications and either adenosine or regadenoson during pharmacologic stress testing. As Dr. Alraies points out, most cardiologists may be familiar with this interaction, but a large proportion of stress tests are ordered by emergency room physicians, internists, and hospitalists who are not. Still, the overall incidence of side effects with pharmacologic stress testing is very low and comparable to that with exercise testing, with safety enhanced by following the American Society of Nuclear Cardiology (ASNC) guidelines for performing stress myocardial perfusion imaging.1 Avoidance of this interaction may be enhanced through education, but also by using checklists and building notifications into the electronic medical record when ordering pharmacologic stress testing. Of note, according to the ASNC guidelines, the use of intravenous dipyridamole as a stress agent is a safe alternative for pharmacologic stress testing in patients taking oral dipyridamole-containing medications.
- Henzlova MJ, Cerqueira MD, Mahmarian JJ, Yao SS; Quality Assurance Committee of the American Society of Nuclear Cardiology. Stress protocols and tracers. J Nucl Cardiol 2006; 13:e80–e90.
In Reply: We appreciate the interest and comments of Dr. Alraies. We would like to clarify that the patient’s baseline electrocardiogram before the nuclear stress test was normal. Second-degree atrioventricular (AV) block (Mobitz type I) was evident only during adenosine infusion before ventricular asystole. The patient was on two AV nodal blockers (labetalol and clonidine) but had no underlying conduction disease. There is no contraindication to continuing these agents before pharmacologic stress testing. In addition, the patient’s electrolyte levels were within normal ranges before testing.
We agree that the valuable teaching point for clinicians is to appreciate the contraindication to and consequences of the use of dipyridamole-containing oral medications and either adenosine or regadenoson during pharmacologic stress testing. As Dr. Alraies points out, most cardiologists may be familiar with this interaction, but a large proportion of stress tests are ordered by emergency room physicians, internists, and hospitalists who are not. Still, the overall incidence of side effects with pharmacologic stress testing is very low and comparable to that with exercise testing, with safety enhanced by following the American Society of Nuclear Cardiology (ASNC) guidelines for performing stress myocardial perfusion imaging.1 Avoidance of this interaction may be enhanced through education, but also by using checklists and building notifications into the electronic medical record when ordering pharmacologic stress testing. Of note, according to the ASNC guidelines, the use of intravenous dipyridamole as a stress agent is a safe alternative for pharmacologic stress testing in patients taking oral dipyridamole-containing medications.
In Reply: We appreciate the interest and comments of Dr. Alraies. We would like to clarify that the patient’s baseline electrocardiogram before the nuclear stress test was normal. Second-degree atrioventricular (AV) block (Mobitz type I) was evident only during adenosine infusion before ventricular asystole. The patient was on two AV nodal blockers (labetalol and clonidine) but had no underlying conduction disease. There is no contraindication to continuing these agents before pharmacologic stress testing. In addition, the patient’s electrolyte levels were within normal ranges before testing.
We agree that the valuable teaching point for clinicians is to appreciate the contraindication to and consequences of the use of dipyridamole-containing oral medications and either adenosine or regadenoson during pharmacologic stress testing. As Dr. Alraies points out, most cardiologists may be familiar with this interaction, but a large proportion of stress tests are ordered by emergency room physicians, internists, and hospitalists who are not. Still, the overall incidence of side effects with pharmacologic stress testing is very low and comparable to that with exercise testing, with safety enhanced by following the American Society of Nuclear Cardiology (ASNC) guidelines for performing stress myocardial perfusion imaging.1 Avoidance of this interaction may be enhanced through education, but also by using checklists and building notifications into the electronic medical record when ordering pharmacologic stress testing. Of note, according to the ASNC guidelines, the use of intravenous dipyridamole as a stress agent is a safe alternative for pharmacologic stress testing in patients taking oral dipyridamole-containing medications.
- Henzlova MJ, Cerqueira MD, Mahmarian JJ, Yao SS; Quality Assurance Committee of the American Society of Nuclear Cardiology. Stress protocols and tracers. J Nucl Cardiol 2006; 13:e80–e90.
- Henzlova MJ, Cerqueira MD, Mahmarian JJ, Yao SS; Quality Assurance Committee of the American Society of Nuclear Cardiology. Stress protocols and tracers. J Nucl Cardiol 2006; 13:e80–e90.
Promoting higher blood pressure targets for frail older adults: A consensus guideline from Canada
Frail older adults deserve guidelines that take frailty into account while assessing the potential benefit and risks of treatment.
Specifically, our group—the Dalhousie Academic Detailing Service (ADS) and the Palliative and Therapeutic Harmonization (PATH) program—recommends that physicians strive to achieve more liberal treatment targets for elderly frail patients who have high blood pressure,1 as evidence does not support an aggressive approach in the frail elderly and the potential exists for harm.
This article reviews the evidence and reasoning that were used to develop and promote a guideline for drug treatment of hypertension in frail older adults. Our recommendations differ from other guidelines in that they focus as much on stopping or decreasing therapy as on starting or increasing it.
FRAILTY INCREASES THE RISK OF ADVERSE EFFECTS
The word frail, applied to older adults, describes those who have complex medical illnesses severe enough to compromise their ability to live independently.2 Many have multiple coexisting medical problems for which they take numerous drugs, in addition to dementia, impaired mobility, compromised functional ability, or a history of falling.
Frailty denotes vulnerability; it increases the risk of adverse effects from medical and surgical procedures,3 complicates drug therapy,4 prolongs hospital length of stay,5 leads to functional and cognitive decline,6 increases the risk of institutionalization,7 and reduces life expectancy8—all of which affect the benefit and harm of medical treatments.
Guidelines for treating hypertension9–11 now acknowledge that little evidence exists to support starting treatment for systolic blood pressure between 140 and 160 mm Hg or aiming for a target of less than 140 mm Hg for “very old” adults, commonly defined as over the age of 80. New guidelines loosen the treatment targets for the very old, but they do not specify targets for the frail and do not describe how to recognize or measure frailty.
RECOGNIZING AND MEASURING FRAILTY
A number of tools are available to recognize and measure frailty.12
The Fried frailty assessment13 has five items:
- Unintentional weight loss
- Self-reported exhaustion
- Weakness in grip
- Slow walking speed
- Low physical activity and energy expenditure.
People are deemed frail if they have three or more of these five. However, experts disagree about whether this system is too sensitive14 or not sensitive enough.15,16
The FRAIL questionnaire17 also has five items:
- Fatigue
- Resistance (inability to climb stairs)
- Ambulation (inability to walk 1 city block)
- Illness (more than 5 major illnesses)
- Weight loss.
People are deemed frail if they have at least three of these five items, and “prefrail” if they have two.
These and other tools are limited by being dichotomous: they classify people as being either frail or not frail18–20 but do not define the spectrum of frailty.
Other frailty assessments such as the Frailty Index21 identify frailty based on the number of accumulated health deficits but take a long time to complete, making them difficult to use in busy clinical settings.22–24
The Clinical Frailty Scale7 is a validated scale that categorizes frailty based on physical and functional indicators of health, such as cognition, function, and mobility, with scores that range from 1 (very fit) to 9 (terminally ill).7,12
The Frailty Assessment for Care-planning Tool (FACT) uses scaling compatible with the Clinical Frailty Scale but has been developed for use as a practical and interpretable frailty screening tool for nonexperts (Table 1). The FACT assesses cognition, mobility, function, and the social situation, using a combination of caregiver report and objective measures. To assess cognition, a health care professional uses items from the Mini-Cog25 (ie, the ability to draw an analog clock face and then recall three unrelated items following the clock-drawing test) and the memory axis of the Brief Cognitive Rating Scale26 (ie, the ability to recall current events, the current US president, and the names of children or spouse). Mobility, function, and social circumstance scores are assigned according to the caregiver report of the patient’s baseline status.
The FACT can be completed in busy clinical settings. Once a caregiver is identified, it takes about 5 minutes to complete.
Our guideline27–31 is intended for those with a score of 7 or more on the Clinical Frailty Scale or FACT,7,12 a score we chose because it describes people who are severely frail with shortened life expectancy.8 At this level, people need help with all instrumental activities of daily living (eg, handling finances, medication management, household chores, and shopping) as well as with basic activities of daily living such as bathing or dressing.
REVIEWING THE LIMITED EVIDENCE
We found no studies that addressed the risks and benefits of treating hypertension in frail older adults; therefore, we concentrated on studies that enrolled individuals who were chronologically old but not frail. We reviewed prominent guidelines,9–11,32,33 the evidence base for these guidelines,34–44 and Cochrane reviews.45,46 A detailed description of the evidence used to build our recommendation can be found online.31
When we deliberated on treatment targets, we reviewed evidence from two types of randomized controlled trials47:
Drug treatment trials randomize patients to different treatments, such as placebo versus a drug or one drug compared with another drug. Patients in different treatment groups may achieve different blood pressures and clinical outcomes, and this information is then used to define optimal targets. However, it may be difficult to determine if the benefit came from lowering blood pressure or from some other effect of the drug, which can be independent of blood pressure lowering.
Treat-to-target trials randomize patients to different blood pressure goals, but the groups are treated with the same or similar drugs. Therefore, any identified benefit can be attributed to the differences in blood pressure rather than the medications used. Compared with a drug treatment trial, this type of trial provides stronger evidence about optimal targets.
We also considered the characteristics of frailty, the dilemma of polypharmacy, and the relevance of the available scientific evidence to those who are frail.
Drug treatment trials
A Cochrane review45 of 15 studies with approximately 24,000 elderly participants found that treating hypertension decreased the rates of cardiovascular morbidity and mortality as well as fatal and nonfatal stroke in the “elderly” (defined as age ≥ 60) and “very elderly” (age ≥ 80). However, in the very elderly, all-cause mortality rates were not statistically significantly different with treatment compared with placebo. The mean duration of treatment was 4.5 years in the elderly and 2.2 years in the very elderly (Table 2). Of importance, all the trials enrolled only those individuals whose systolic blood pressure was at least 160 mm Hg at baseline.
None of the studies were treat-to-target trials—patients were assigned either active medication or placebo. Thus, these trials provide evidence of benefit for treating hypertension in the elderly and very elderly but do not identify the optimal target. All of the drug treatment trials showed benefit, but none achieved a systolic pressure lower than 140 mm Hg with active treatment (Table 3). Therefore, these studies do not support a systolic target of less than 140 mm Hg in the elderly.
Treat-to-target trials: JATOS and VALISH
The Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS)42 and the Valsartan in Elderly Isolated Systolic Hypertension (VALISH) study43 each enrolled more than 3,000 people age 65 or older (mean age approximately 75). Patients were randomized to either a strict systolic target of less than 140 mm Hg or a higher (more permissive) target of 140 to 160 mm Hg in JATOS and 140 to 149 mm Hg in VALISH.
In both trials, the group with strict targets achieved a systolic pressure of approximately 136 mm Hg, while the group with higher blood pressure targets achieved a systolic pressure of 146 mm Hg in JATOS and 142 mm Hg in VALISH. Despite these differences, there was no statistically significant difference in the primary outcome.
Thus, treat-to-target studies also fail to support a systolic target of less than 140 mm Hg in the elderly, although it is important to recognize the limitations of the studies. Approximately 15% of the participants had cardiovascular disease, so the applicability of the findings to patients with target-organ damage is uncertain. In addition, there were fewer efficacy outcome events than expected, which suggests that the studies were underpowered.
When to start drug treatment
In each of the drug treatment and treat-to-target trials, the inclusion criterion for study entry was a systolic blood pressure above 160 mm Hg, with a mean blood pressure at entry into the drug treatment trials of 182/95 mm Hg.46 Thus, data support starting treatment if the systolic blood pressure is above 160 mm Hg, but not lower.
Notably, in all but one study,46 at least two-thirds of the participants took no more than two antihypertensive medications. Since adverse events become more common as the number of medications increases, the benefit of adding a third drug to lower blood pressure is uncertain.
Evidence in the ‘very elderly’: HYVET
With the exception of the Hypertension in the Very Elderly Trial (HYVET),44 the mean age of elderly patients in the reported studies was between 67 and 76.
HYVET patients were age 80 and older (mean age 84) and were randomized to receive either indapamide (with or without perindopril) or placebo. The trial was stopped early at 2 years because the mortality rate was lower in the treatment group (10.1%) than in the placebo group (12.3%) (number needed to treat 46, 95% confidence interval 24–637, P = .02). There was no significant difference in the primary outcome of fatal and nonfatal stroke.
Notably, trials that are stopped early may overestimate treatment benefit.48
Evidence in frail older adults
While the above studies provide some information about managing hypertension in the elderly, the participants were generally healthy. HYVET44 specifically excluded those with a standing systolic blood pressure of less than 140 mm Hg and enrolled few patients with orthostasis (7.9% in the placebo group and 8.8% in the treatment group), a condition commonly associated with frailty. As such, these studies may be less relevant to the frail elderly, who are at higher risk of adverse drug events and have competing risks for morbidity and mortality.
Observational studies, in fact, raise questions about whether tight blood pressure control improves clinical outcomes for the very elderly. In the Leiden 85-plus study, lower systolic blood pressure was associated with lower cognitive scores, worse functional ability,49,50 and a higher mortality rate51 compared with higher systolic pressure, although it is uncertain whether these outcomes were indicative of underlying disease that could result in lower blood pressure or an effect of blood pressure-lowering.
The National Health and Nutrition Examination Survey52 found an association between blood pressure and mortality rate that varied by walking speed. For slower walkers (based on the 6-minute walk test), higher systolic pressures were not associated with a higher risk of death, suggesting that when older adults are frail (as indicated by their slow walking speed) they are less likely to benefit from aggressive treatment of hypertension.
People at high risk because of stroke
Because the evidence is limited, it is even more difficult to judge whether lowering blood pressure below 140 mm Hg is beneficial for frail patients who have a history of stroke, compared with the possibility that medications will cause adverse effects such as weakness, orthostasis, and falls. When reviewing the evidence to answer this question, we especially looked at outcomes that affect quality of life, such as nonfatal stroke leading to disability. In contrast, because the frail elderly have competing causes of mortality, we could not assume that a mortality benefit shown in nonfrail populations could be applied to frail populations.
The PROGRESS trial (Perindopril Protection Against Recurrent Stroke Study)53 was in patients with a history of stroke or transient ischemic attack and a mean age of 64, who were treated with either perindopril (with or without indapamide) or placebo.
At almost 4 years, the rate of disabling stroke was 2.7% in the treatment group and 4.3% in the placebo group, a relative risk reduction of 38% and an absolute risk reduction of 1.64% (number needed to treat 61, 95% confidence interval 39–139). The relative risk reduction for all strokes (fatal and nonfatal) was similar across a range of baseline systolic pressures, but the absolute risk reduction was greater in the prespecified subgroup that had hypertension at baseline (mean blood pressure 159/94 mm Hg) than in the normotensive subgroup (mean blood pressure 136/79 mm Hg), suggesting that treatment is most beneficial for those with higher systolic blood pressures. Also, the benefit was only demonstrated in the subgroup that received two antihypertensive medications; those who received perindopril alone showed no benefit.
This study involved relatively young patients in relatively good health except for their strokes. The extent to which the results can be extrapolated to older, frail adults is uncertain because of the time needed to achieve benefit and because of the added vulnerability of frailty, which could make treatment with two antihypertensive medications riskier.
PRoFESS (Prevention Regimen for Effectively Avoiding Second Strokes),54 another study in patients with previous stroke (mean age 66) showed no benefit over 2.5 years in the primary outcome of stroke using telmesartan 80 mg daily compared with placebo. This result is concordant with that of PROGRESS,53 in which patients who took only one medication did not show a significant decrease in the rate of stroke.
A possible reason for the lack of benefit from monotherapy was that the differences in blood pressure between the placebo group and the treatment group on monotherapy were small in both studies (3.8/2.0 mm Hg in PRoFESS, 5/3 mm Hg in PROGRESS). In contrast, patients on dual therapy in PROGRESS decreased their blood pressure by 12/5 mm Hg compared with placebo.
CURRENT HYPERTENSION GUIDELINES
Current guidelines make reference to the elderly, but we found none that made specific recommendations for the frail elderly.
JNC 8
In December 2013, members of the Eighth Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 8) released new recommendations.32 One significant revision was to support higher blood pressure targets for older adults (age 60 and older). Whereas JNC 7 stated that lowering blood pressure below 140/90 mm Hg reduced cardiovascular complications,33 JNC 8 now acknowledges that there is no strong evidence to support blood pressure targets below 150/90 mm Hg for hypertensive persons without kidney disease or diabetes age 60 and older. Thus, in the general population age 60 and older, JNC 8 recommends starting antihypertensive treatment when blood pressure is 150/90 mm Hg or higher, and treating to a goal blood pressure of less than 150/90 mm Hg. JNC 8 makes no recommendation about how to adjust blood pressure targets for frailty or how to measure blood pressure.
American College of Cardiology and American Heart Association
In 2011, the American College of Cardiology and American Heart Association published a consensus document on the management of hypertension in the elderly.9
They acknowledged that the generally recommended blood pressure goal of lower than 140/90 mm Hg in uncomplicated elderly patients is based on expert opinion rather than on data from randomized controlled trials, but nevertheless recommended a target systolic pressure lower than 140 mm Hg for older adults, except for octogenarians.
For those over age 80, systolic levels of 140 to 145 mm Hg can be acceptable if tolerated and if the patient does not experience orthostasis when standing. Systolic pressure lower than 130 mm Hg and diastolic pressures lower than 65 mm Hg should be avoided in this age group.
The document acknowledges that systolic pressure may have to remain above 150 mm Hg if there is no response to four “well-selected drugs” or if there are unacceptable side effects. In these cases, the lowest “safely achieved” systolic blood pressure should be the goal.
Canadian Hypertension Education Program
The 2014 Canadian Hypertension Education Program (CHEP) report makes several recommendations for the “very elderly,” a group they define as over the age of 80. The CHEP website and resources include the following recommendations10:
- For the very elderly without diabetes or target-organ damage, drug therapy should be initiated when systolic blood pressure is higher than 160 mm Hg to reach a systolic blood pressure target lower than 150 mm Hg. This is a grade C level recommendation, indicating that it is based on low-quality trials, unvalidated surrogate outcomes, or results from nonrandomized observational studies.
- For the very elderly with macrovascular target-organ damage, antihypertensive therapy should be considered if systolic blood pressure readings average 140 mm Hg or higher (grade D for 140 to 160 mm Hg; grade A for higher than 160 mm Hg), although caution should be exercised in elderly patients who are frail. (Grade D recommendations are the weakest, as they are based on low-powered, imprecise studies or expert opinion, whereas grade A recommendations are based on the strongest evidence from high-quality randomized clinical trials.)
- Decisions regarding initiating and intensifying pharmacotherapy in the very elderly should be based on an individualized risk-benefit analysis.
The European Society of Hypertension and European Society of Cardiology
The 2013 guidelines from the European Society of Hypertension and the European Society of Cardiology11 recommend that for elderly patients under age 80, antihypertensive treatment may be considered at systolic values higher than 140 mm Hg and aimed at values lower than 140 mm Hg if the patient is fit and treatment is well tolerated.
For those over age 80 with an initial systolic pressure of 160 mm Hg or higher, the guidelines recommend lowering systolic pressure to between 150 and 140 mm Hg, provided the patient is in good physical and mental condition. In frail elderly patients, they recommend leaving decisions on antihypertensive therapy to the treating physician, based on monitoring of the clinical effects of treatment.11
The ADS/PATH guidelines
When finalizing our recommendations,1 we considered the characteristics of frailty and the following key points from the evidence:
- Although evidence from drug treatment trials indicates that there is benefit in treating healthy older adults who have hypertension, the benefit of treating frail older adults is unknown.
- Major trials enrolled elderly patients only if they had systolic blood pressures of at least 160 mm Hg. Therefore, evidence supports initiating pharmacotherapy at a systolic pressure of 160 mm Hg or higher.
- No evidence from randomized controlled trials supports a systolic target lower than 140 mm Hg in the elderly, and there is some evidence that such a target does not benefit.
- The benefit of adding a third medication to lower blood pressure has not been studied.
- Frailty makes the potential benefits of strict blood pressure targets even less certain and increases the possibility of harm from adverse drug events.
- The only study of very old adults, HYVET,44 enrolled relatively healthy older adults and few with orthostasis, while excluding those with a standing systolic blood pressure lower than 140 mm Hg.
OUR RECOMMENDATIONS
Based on the above, we advise against unnecessarily strict targets and recommend stopping antihypertensive medications that are used for the sole purpose of keeping the systolic blood pressure below 140 mm Hg. Our guidelines are unique in that they focus equally on when to stop and when to start medications. We concluded that without evidence of definitive benefit, “less is more” with frailty.55 We believe that if physicians and health professionals understand the limitations of the evidence, they can be more confident in stopping medications that lower blood pressure to an unnecessarily low level.
We recommend the following (Table 4):
Before treating
- Carefully review the risks and the potential but unproven benefits of treatment.
- To avoid overtreatment, treatment decisions should be based on blood pressure measurements in the seated (not supine) position, while also considering the presence of orthostasis.
- To evaluate orthostasis, measure blood pressure in the supine position, then immediately on standing, and again after 2 minutes. Ask the patient if he or she feels light-headed or dizzy when standing.
Stop treatment
- If the seated systolic blood pressure is less than 140 mm Hg, medications can be tapered and discontinued to achieve the targets described below.
- Before discontinuation, consider whether the medications are treating additional conditions such as rate control for atrial fibrillation or symptomatic management of heart failure.
- It is uncertain whether to discontinue treatment when there is a history of stroke. Consider that treatment with two medications resulted in an absolute risk reduction for disabling stroke of 1.64% over approximately 4 years for adults with previous stroke and a mean age of 64,57 an effect that may be more prominent at higher systolic pressures.
Start treatment
- Consider starting treatment when systolic pressure is 160 mm Hg or higher.
- Aim for a seated systolic pressure between 140 and 160 mm Hg if there are no adverse effects from treatment that affect quality of life.
- If there is symptomatic orthostasis or if standing systolic pressure is lower than 140 mm Hg, the target seated systolic pressure can be adjusted upwards.
- In the severely frail nearing the end of life, a target systolic pressure of 160 to 190 mm Hg is reasonable.
- The blood pressure target is the same in people with diabetes.
- In general, use no more than two medications.
Dissemination and implementation
The ADS/PATH guideline is intended for use by physicians and other health professionals (eg, pharmacists and nurses) who care for frail older adults or who work in long-term care facilities. Since creating our guideline, we have disseminated it to physicians, pharmacists, and other health professionals through academic detailing, large conferences, and interactive webinars.
While we do not have objective evidence of practice change, our evaluation data found that 34% of 403 family physicians who received academic detailing indicated that the guideline would change their practice, while 36% stated that the guideline confirmed their practice, an indication that family physicians are sensitive to the needs of the frail elderly.
Because health professionals may be wary of stopping medications and not meeting recommended targets, there may be barriers to adopting this guideline. However, our experience with the PATH program indicates that these barriers can be overcome using effective communication strategies between health professionals and consumers.
AN APPROACH APPROPRIATE TO FRAILTY
There is no direct evidence for systolic blood pressure targets in the frail elderly, so we applied evidence from the nonfrail elderly. Our recommendations differ somewhat from those of other groups, which recommend targets below 140 to 150 mm Hg for older adults, although some do advise caution in the elderly for whom a substantial fall in blood pressure might be poorly tolerated. Despite these messages, we believe that clearer guidance is needed to direct health practitioners toward models that acknowledge that frail patients are in a precarious balance of health and may be harmed by treatments that strive to lower blood pressure to unproven targets. For this reason, our guideline clearly indicates when to decrease or stop drug treatment.
After physicians and health professionals examine the evidence and more fully understand the benefits and harms of treating frail older adults, we are confident that they will be more comfortable stopping medications that lower blood pressure to an unnecessarily low level and instead use an approach that is more appropriate to frailty. We hope clinicians can use this guideline with the same enthusiasm applied to other guidelines, and we welcome discussion.
Acknowledgments: We would like to thank and acknowledge Tanya MacLeod and Kathryn Yuill for their review of and advice about the manuscript.
- Palliative and Therapeutic Harmonization program. Hypertension guidelines. Treating hypertension in frailty. http://pathclinic.ca/resources/hypertension/. Accessed May 2, 2014.
- Theou O, Rockwood MR, Mitnitski A, Rockwood K. Disability and co-morbidity in relation to frailty: how much do they overlap? Arch Gerontol Geriatr 2012; 55:e1–e8.
- Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 2010; 210:901–908.
- Tinetti ME, Bogardus ST, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351:2870–2874.
- Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation 2011; 124:2397–2404.
- Theou O, Rockwood K. Should frailty status always be considered when treating the elderly patient? Aging Health 2012; 8:261–271.
- Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173:489–495.
- Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008; 8:24.
- Aronow WS, Fleg JL, Pepine CJ, et al; ACCF Task Force. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2011; 123:2434–2506.
- The Canadian Hypertension Education Program (CHEP). 2014 CHEP recommendations. www.hypertension.ca/en/. Accessed May 2, 2014.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34:2159–2219.
- Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013; 14:392–397.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Ensrud KE, Ewing SK, Cawthon PM, et al; Osteoporotic Fractures in Men Research Group. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc 2009; 57:492–498.
- Avila-Funes JA, Amieva H, Barberger-Gateau P, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc 2009; 57:453–461.
- Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A Biol Sci Med Sci 2007; 62:731–737.
- Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 2012; 16:601–608.
- Strawbridge WJ, Shema SJ, Balfour JL, Higby HR, Kaplan GA. Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci 1998; 53:S9–S16.
- Matthews M, Lucas A, Boland R, et al. Use of a questionnaire to screen for frailty in the elderly: an exploratory study. Aging Clin Exp Res 2004; 16:34–40.
- Salvi F, Morichi V, Grilli A, et al. Screening for frailty in elderly emergency department patients by using the Identification of Seniors At Risk (ISAR). J Nutr Health Aging 2012; 16:313–318.
- Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 2001; 1:323–336.
- Kellen E, Bulens P, Deckx L, et al. Identifying an accurate pre-screening tool in geriatric oncology. Crit Rev Oncol Hematol 2010; 75:243–248.
- Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006; 35:526–529.
- Martin FC, Brighton P. Frailty: different tools for different purposes? Age Ageing 2008; 37:129–131.
- Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry 2000; 15:1021–1027.
- Reisberg B, Ferris SH. Brief Cognitive Rating Scale (BCRS). Psychopharmacol Bull 1988; 24:629–636.
- Moorhouse P, Mallery LH. Palliative and therapeutic harmonization: a model for appropriate decision-making in frail older adults. J Am Geriatr Soc 2012; 60:2326–2332.
- Palliative and Therapeutic Harmonization Clinic (PATH). www.pathclinic.ca. Accessed May 2, 2014.
- Dalhousie University Faculty of Medicine: Continuing Medical Education. http://cme.medicine.dal.ca/ADS.htm. Accessed January 8, 2014.
- Mallery LH, Moorhouse P. Respecting frailty. J Med Ethics 2011; 37:126–128.
- Dalhousie University Faculty of Medicine: Continuing Medical Education. Issues in hypertension 2011. http://cme.medicine.dal.ca/files/Hypertension%20book.pdf. Accessed May 2, 2014.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Amery A, Birkenhäger W, Brixko P, et al. Mortality and morbidity results from the European Working Party on High Blood Pressure in the Elderly trial. Lancet 1985; 1:1349–1354.
- Coope J, Warrender TS. Randomised trial of treatment of hypertension in elderly patients in primary care. Br Med J (Clin Res Ed) 1986; 293:1145–1151.
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991; 265:3255–3264.
- Dahlöf B, Lindholm LH, Hansson L, Scherstén B, Ekbom T, Wester PO. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension). Lancet 1991; 338:1281–1285.
- Medical Research Council trial of treatment of hypertension in older adults: principal results. MRC Working Party. BMJ 1992; 304:405–412.
- Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997; 350:757–764.
- Liu L, Wang JG, Gong L, Liu G, Staessen JA. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. Systolic Hypertension in China (Syst-China) Collaborative Group. J Hypertens 1998; 16:1823–1829.
- Lithell H, Hansson L, Skoog I, et al; SCOPE Study Group. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens 2003; 21:875–886.
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Oparil S, Yarows SA, Patel S, Fang H, Zhang J, Satlin A. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial. Lancet 2007; 370:221–229.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Musini VM, Tejani AM, Bassett K, Wright JM. Pharmacotherapy for hypertension in the elderly. Cochrane Database Syst Rev 2009;CD000028.
- He FJ, MacGregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev 2004;CD004937.
- Allen M, Kelly K, Fleming I. Hypertension in elderly patients: recommended systolic targets are not evidence based [in French]. Can Fam Physician 2013; 59:19–24.
- Guyatt GH, Briel M, Glasziou P, Bassler D, Montori VM. Problems of stopping trials early. BMJ 2012; 344:e3863.
- Sabayan B, Oleksik AM, Maier AB, et al. High blood pressure and resilience to physical and cognitive decline in the oldest old: the Leiden 85-plus Study. J Am Geriatr Soc 2012; 60:2014–2019.
- Sabayan B, van Vliet P, de Ruijter W, Gussekloo J, de Craen AJ, Westendorp RG. High blood pressure, physical and cognitive function, and risk of stroke in the oldest old: the Leiden 85-plus Study. Stroke 2013; 44:15–20.
- Poortvliet RK, Blom JW, de Craen AJ, et al. Low blood pressure predicts increased mortality in very old age even without heart failure: the Leiden 85-plus Study. Eur J Heart Fail 2013; 15:528–533.
- Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med 2012; 172:1162–1168.
- PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001; 358:1033–1041.
- Yusuf S, Diener HC, Sacco RL, et al; PRoFESS Study Group. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med 2008; 359:1225–1237.
- Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med 2010; 170:1648–1654.
Frail older adults deserve guidelines that take frailty into account while assessing the potential benefit and risks of treatment.
Specifically, our group—the Dalhousie Academic Detailing Service (ADS) and the Palliative and Therapeutic Harmonization (PATH) program—recommends that physicians strive to achieve more liberal treatment targets for elderly frail patients who have high blood pressure,1 as evidence does not support an aggressive approach in the frail elderly and the potential exists for harm.
This article reviews the evidence and reasoning that were used to develop and promote a guideline for drug treatment of hypertension in frail older adults. Our recommendations differ from other guidelines in that they focus as much on stopping or decreasing therapy as on starting or increasing it.
FRAILTY INCREASES THE RISK OF ADVERSE EFFECTS
The word frail, applied to older adults, describes those who have complex medical illnesses severe enough to compromise their ability to live independently.2 Many have multiple coexisting medical problems for which they take numerous drugs, in addition to dementia, impaired mobility, compromised functional ability, or a history of falling.
Frailty denotes vulnerability; it increases the risk of adverse effects from medical and surgical procedures,3 complicates drug therapy,4 prolongs hospital length of stay,5 leads to functional and cognitive decline,6 increases the risk of institutionalization,7 and reduces life expectancy8—all of which affect the benefit and harm of medical treatments.
Guidelines for treating hypertension9–11 now acknowledge that little evidence exists to support starting treatment for systolic blood pressure between 140 and 160 mm Hg or aiming for a target of less than 140 mm Hg for “very old” adults, commonly defined as over the age of 80. New guidelines loosen the treatment targets for the very old, but they do not specify targets for the frail and do not describe how to recognize or measure frailty.
RECOGNIZING AND MEASURING FRAILTY
A number of tools are available to recognize and measure frailty.12
The Fried frailty assessment13 has five items:
- Unintentional weight loss
- Self-reported exhaustion
- Weakness in grip
- Slow walking speed
- Low physical activity and energy expenditure.
People are deemed frail if they have three or more of these five. However, experts disagree about whether this system is too sensitive14 or not sensitive enough.15,16
The FRAIL questionnaire17 also has five items:
- Fatigue
- Resistance (inability to climb stairs)
- Ambulation (inability to walk 1 city block)
- Illness (more than 5 major illnesses)
- Weight loss.
People are deemed frail if they have at least three of these five items, and “prefrail” if they have two.
These and other tools are limited by being dichotomous: they classify people as being either frail or not frail18–20 but do not define the spectrum of frailty.
Other frailty assessments such as the Frailty Index21 identify frailty based on the number of accumulated health deficits but take a long time to complete, making them difficult to use in busy clinical settings.22–24
The Clinical Frailty Scale7 is a validated scale that categorizes frailty based on physical and functional indicators of health, such as cognition, function, and mobility, with scores that range from 1 (very fit) to 9 (terminally ill).7,12
The Frailty Assessment for Care-planning Tool (FACT) uses scaling compatible with the Clinical Frailty Scale but has been developed for use as a practical and interpretable frailty screening tool for nonexperts (Table 1). The FACT assesses cognition, mobility, function, and the social situation, using a combination of caregiver report and objective measures. To assess cognition, a health care professional uses items from the Mini-Cog25 (ie, the ability to draw an analog clock face and then recall three unrelated items following the clock-drawing test) and the memory axis of the Brief Cognitive Rating Scale26 (ie, the ability to recall current events, the current US president, and the names of children or spouse). Mobility, function, and social circumstance scores are assigned according to the caregiver report of the patient’s baseline status.
The FACT can be completed in busy clinical settings. Once a caregiver is identified, it takes about 5 minutes to complete.
Our guideline27–31 is intended for those with a score of 7 or more on the Clinical Frailty Scale or FACT,7,12 a score we chose because it describes people who are severely frail with shortened life expectancy.8 At this level, people need help with all instrumental activities of daily living (eg, handling finances, medication management, household chores, and shopping) as well as with basic activities of daily living such as bathing or dressing.
REVIEWING THE LIMITED EVIDENCE
We found no studies that addressed the risks and benefits of treating hypertension in frail older adults; therefore, we concentrated on studies that enrolled individuals who were chronologically old but not frail. We reviewed prominent guidelines,9–11,32,33 the evidence base for these guidelines,34–44 and Cochrane reviews.45,46 A detailed description of the evidence used to build our recommendation can be found online.31
When we deliberated on treatment targets, we reviewed evidence from two types of randomized controlled trials47:
Drug treatment trials randomize patients to different treatments, such as placebo versus a drug or one drug compared with another drug. Patients in different treatment groups may achieve different blood pressures and clinical outcomes, and this information is then used to define optimal targets. However, it may be difficult to determine if the benefit came from lowering blood pressure or from some other effect of the drug, which can be independent of blood pressure lowering.
Treat-to-target trials randomize patients to different blood pressure goals, but the groups are treated with the same or similar drugs. Therefore, any identified benefit can be attributed to the differences in blood pressure rather than the medications used. Compared with a drug treatment trial, this type of trial provides stronger evidence about optimal targets.
We also considered the characteristics of frailty, the dilemma of polypharmacy, and the relevance of the available scientific evidence to those who are frail.
Drug treatment trials
A Cochrane review45 of 15 studies with approximately 24,000 elderly participants found that treating hypertension decreased the rates of cardiovascular morbidity and mortality as well as fatal and nonfatal stroke in the “elderly” (defined as age ≥ 60) and “very elderly” (age ≥ 80). However, in the very elderly, all-cause mortality rates were not statistically significantly different with treatment compared with placebo. The mean duration of treatment was 4.5 years in the elderly and 2.2 years in the very elderly (Table 2). Of importance, all the trials enrolled only those individuals whose systolic blood pressure was at least 160 mm Hg at baseline.
None of the studies were treat-to-target trials—patients were assigned either active medication or placebo. Thus, these trials provide evidence of benefit for treating hypertension in the elderly and very elderly but do not identify the optimal target. All of the drug treatment trials showed benefit, but none achieved a systolic pressure lower than 140 mm Hg with active treatment (Table 3). Therefore, these studies do not support a systolic target of less than 140 mm Hg in the elderly.
Treat-to-target trials: JATOS and VALISH
The Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS)42 and the Valsartan in Elderly Isolated Systolic Hypertension (VALISH) study43 each enrolled more than 3,000 people age 65 or older (mean age approximately 75). Patients were randomized to either a strict systolic target of less than 140 mm Hg or a higher (more permissive) target of 140 to 160 mm Hg in JATOS and 140 to 149 mm Hg in VALISH.
In both trials, the group with strict targets achieved a systolic pressure of approximately 136 mm Hg, while the group with higher blood pressure targets achieved a systolic pressure of 146 mm Hg in JATOS and 142 mm Hg in VALISH. Despite these differences, there was no statistically significant difference in the primary outcome.
Thus, treat-to-target studies also fail to support a systolic target of less than 140 mm Hg in the elderly, although it is important to recognize the limitations of the studies. Approximately 15% of the participants had cardiovascular disease, so the applicability of the findings to patients with target-organ damage is uncertain. In addition, there were fewer efficacy outcome events than expected, which suggests that the studies were underpowered.
When to start drug treatment
In each of the drug treatment and treat-to-target trials, the inclusion criterion for study entry was a systolic blood pressure above 160 mm Hg, with a mean blood pressure at entry into the drug treatment trials of 182/95 mm Hg.46 Thus, data support starting treatment if the systolic blood pressure is above 160 mm Hg, but not lower.
Notably, in all but one study,46 at least two-thirds of the participants took no more than two antihypertensive medications. Since adverse events become more common as the number of medications increases, the benefit of adding a third drug to lower blood pressure is uncertain.
Evidence in the ‘very elderly’: HYVET
With the exception of the Hypertension in the Very Elderly Trial (HYVET),44 the mean age of elderly patients in the reported studies was between 67 and 76.
HYVET patients were age 80 and older (mean age 84) and were randomized to receive either indapamide (with or without perindopril) or placebo. The trial was stopped early at 2 years because the mortality rate was lower in the treatment group (10.1%) than in the placebo group (12.3%) (number needed to treat 46, 95% confidence interval 24–637, P = .02). There was no significant difference in the primary outcome of fatal and nonfatal stroke.
Notably, trials that are stopped early may overestimate treatment benefit.48
Evidence in frail older adults
While the above studies provide some information about managing hypertension in the elderly, the participants were generally healthy. HYVET44 specifically excluded those with a standing systolic blood pressure of less than 140 mm Hg and enrolled few patients with orthostasis (7.9% in the placebo group and 8.8% in the treatment group), a condition commonly associated with frailty. As such, these studies may be less relevant to the frail elderly, who are at higher risk of adverse drug events and have competing risks for morbidity and mortality.
Observational studies, in fact, raise questions about whether tight blood pressure control improves clinical outcomes for the very elderly. In the Leiden 85-plus study, lower systolic blood pressure was associated with lower cognitive scores, worse functional ability,49,50 and a higher mortality rate51 compared with higher systolic pressure, although it is uncertain whether these outcomes were indicative of underlying disease that could result in lower blood pressure or an effect of blood pressure-lowering.
The National Health and Nutrition Examination Survey52 found an association between blood pressure and mortality rate that varied by walking speed. For slower walkers (based on the 6-minute walk test), higher systolic pressures were not associated with a higher risk of death, suggesting that when older adults are frail (as indicated by their slow walking speed) they are less likely to benefit from aggressive treatment of hypertension.
People at high risk because of stroke
Because the evidence is limited, it is even more difficult to judge whether lowering blood pressure below 140 mm Hg is beneficial for frail patients who have a history of stroke, compared with the possibility that medications will cause adverse effects such as weakness, orthostasis, and falls. When reviewing the evidence to answer this question, we especially looked at outcomes that affect quality of life, such as nonfatal stroke leading to disability. In contrast, because the frail elderly have competing causes of mortality, we could not assume that a mortality benefit shown in nonfrail populations could be applied to frail populations.
The PROGRESS trial (Perindopril Protection Against Recurrent Stroke Study)53 was in patients with a history of stroke or transient ischemic attack and a mean age of 64, who were treated with either perindopril (with or without indapamide) or placebo.
At almost 4 years, the rate of disabling stroke was 2.7% in the treatment group and 4.3% in the placebo group, a relative risk reduction of 38% and an absolute risk reduction of 1.64% (number needed to treat 61, 95% confidence interval 39–139). The relative risk reduction for all strokes (fatal and nonfatal) was similar across a range of baseline systolic pressures, but the absolute risk reduction was greater in the prespecified subgroup that had hypertension at baseline (mean blood pressure 159/94 mm Hg) than in the normotensive subgroup (mean blood pressure 136/79 mm Hg), suggesting that treatment is most beneficial for those with higher systolic blood pressures. Also, the benefit was only demonstrated in the subgroup that received two antihypertensive medications; those who received perindopril alone showed no benefit.
This study involved relatively young patients in relatively good health except for their strokes. The extent to which the results can be extrapolated to older, frail adults is uncertain because of the time needed to achieve benefit and because of the added vulnerability of frailty, which could make treatment with two antihypertensive medications riskier.
PRoFESS (Prevention Regimen for Effectively Avoiding Second Strokes),54 another study in patients with previous stroke (mean age 66) showed no benefit over 2.5 years in the primary outcome of stroke using telmesartan 80 mg daily compared with placebo. This result is concordant with that of PROGRESS,53 in which patients who took only one medication did not show a significant decrease in the rate of stroke.
A possible reason for the lack of benefit from monotherapy was that the differences in blood pressure between the placebo group and the treatment group on monotherapy were small in both studies (3.8/2.0 mm Hg in PRoFESS, 5/3 mm Hg in PROGRESS). In contrast, patients on dual therapy in PROGRESS decreased their blood pressure by 12/5 mm Hg compared with placebo.
CURRENT HYPERTENSION GUIDELINES
Current guidelines make reference to the elderly, but we found none that made specific recommendations for the frail elderly.
JNC 8
In December 2013, members of the Eighth Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 8) released new recommendations.32 One significant revision was to support higher blood pressure targets for older adults (age 60 and older). Whereas JNC 7 stated that lowering blood pressure below 140/90 mm Hg reduced cardiovascular complications,33 JNC 8 now acknowledges that there is no strong evidence to support blood pressure targets below 150/90 mm Hg for hypertensive persons without kidney disease or diabetes age 60 and older. Thus, in the general population age 60 and older, JNC 8 recommends starting antihypertensive treatment when blood pressure is 150/90 mm Hg or higher, and treating to a goal blood pressure of less than 150/90 mm Hg. JNC 8 makes no recommendation about how to adjust blood pressure targets for frailty or how to measure blood pressure.
American College of Cardiology and American Heart Association
In 2011, the American College of Cardiology and American Heart Association published a consensus document on the management of hypertension in the elderly.9
They acknowledged that the generally recommended blood pressure goal of lower than 140/90 mm Hg in uncomplicated elderly patients is based on expert opinion rather than on data from randomized controlled trials, but nevertheless recommended a target systolic pressure lower than 140 mm Hg for older adults, except for octogenarians.
For those over age 80, systolic levels of 140 to 145 mm Hg can be acceptable if tolerated and if the patient does not experience orthostasis when standing. Systolic pressure lower than 130 mm Hg and diastolic pressures lower than 65 mm Hg should be avoided in this age group.
The document acknowledges that systolic pressure may have to remain above 150 mm Hg if there is no response to four “well-selected drugs” or if there are unacceptable side effects. In these cases, the lowest “safely achieved” systolic blood pressure should be the goal.
Canadian Hypertension Education Program
The 2014 Canadian Hypertension Education Program (CHEP) report makes several recommendations for the “very elderly,” a group they define as over the age of 80. The CHEP website and resources include the following recommendations10:
- For the very elderly without diabetes or target-organ damage, drug therapy should be initiated when systolic blood pressure is higher than 160 mm Hg to reach a systolic blood pressure target lower than 150 mm Hg. This is a grade C level recommendation, indicating that it is based on low-quality trials, unvalidated surrogate outcomes, or results from nonrandomized observational studies.
- For the very elderly with macrovascular target-organ damage, antihypertensive therapy should be considered if systolic blood pressure readings average 140 mm Hg or higher (grade D for 140 to 160 mm Hg; grade A for higher than 160 mm Hg), although caution should be exercised in elderly patients who are frail. (Grade D recommendations are the weakest, as they are based on low-powered, imprecise studies or expert opinion, whereas grade A recommendations are based on the strongest evidence from high-quality randomized clinical trials.)
- Decisions regarding initiating and intensifying pharmacotherapy in the very elderly should be based on an individualized risk-benefit analysis.
The European Society of Hypertension and European Society of Cardiology
The 2013 guidelines from the European Society of Hypertension and the European Society of Cardiology11 recommend that for elderly patients under age 80, antihypertensive treatment may be considered at systolic values higher than 140 mm Hg and aimed at values lower than 140 mm Hg if the patient is fit and treatment is well tolerated.
For those over age 80 with an initial systolic pressure of 160 mm Hg or higher, the guidelines recommend lowering systolic pressure to between 150 and 140 mm Hg, provided the patient is in good physical and mental condition. In frail elderly patients, they recommend leaving decisions on antihypertensive therapy to the treating physician, based on monitoring of the clinical effects of treatment.11
The ADS/PATH guidelines
When finalizing our recommendations,1 we considered the characteristics of frailty and the following key points from the evidence:
- Although evidence from drug treatment trials indicates that there is benefit in treating healthy older adults who have hypertension, the benefit of treating frail older adults is unknown.
- Major trials enrolled elderly patients only if they had systolic blood pressures of at least 160 mm Hg. Therefore, evidence supports initiating pharmacotherapy at a systolic pressure of 160 mm Hg or higher.
- No evidence from randomized controlled trials supports a systolic target lower than 140 mm Hg in the elderly, and there is some evidence that such a target does not benefit.
- The benefit of adding a third medication to lower blood pressure has not been studied.
- Frailty makes the potential benefits of strict blood pressure targets even less certain and increases the possibility of harm from adverse drug events.
- The only study of very old adults, HYVET,44 enrolled relatively healthy older adults and few with orthostasis, while excluding those with a standing systolic blood pressure lower than 140 mm Hg.
OUR RECOMMENDATIONS
Based on the above, we advise against unnecessarily strict targets and recommend stopping antihypertensive medications that are used for the sole purpose of keeping the systolic blood pressure below 140 mm Hg. Our guidelines are unique in that they focus equally on when to stop and when to start medications. We concluded that without evidence of definitive benefit, “less is more” with frailty.55 We believe that if physicians and health professionals understand the limitations of the evidence, they can be more confident in stopping medications that lower blood pressure to an unnecessarily low level.
We recommend the following (Table 4):
Before treating
- Carefully review the risks and the potential but unproven benefits of treatment.
- To avoid overtreatment, treatment decisions should be based on blood pressure measurements in the seated (not supine) position, while also considering the presence of orthostasis.
- To evaluate orthostasis, measure blood pressure in the supine position, then immediately on standing, and again after 2 minutes. Ask the patient if he or she feels light-headed or dizzy when standing.
Stop treatment
- If the seated systolic blood pressure is less than 140 mm Hg, medications can be tapered and discontinued to achieve the targets described below.
- Before discontinuation, consider whether the medications are treating additional conditions such as rate control for atrial fibrillation or symptomatic management of heart failure.
- It is uncertain whether to discontinue treatment when there is a history of stroke. Consider that treatment with two medications resulted in an absolute risk reduction for disabling stroke of 1.64% over approximately 4 years for adults with previous stroke and a mean age of 64,57 an effect that may be more prominent at higher systolic pressures.
Start treatment
- Consider starting treatment when systolic pressure is 160 mm Hg or higher.
- Aim for a seated systolic pressure between 140 and 160 mm Hg if there are no adverse effects from treatment that affect quality of life.
- If there is symptomatic orthostasis or if standing systolic pressure is lower than 140 mm Hg, the target seated systolic pressure can be adjusted upwards.
- In the severely frail nearing the end of life, a target systolic pressure of 160 to 190 mm Hg is reasonable.
- The blood pressure target is the same in people with diabetes.
- In general, use no more than two medications.
Dissemination and implementation
The ADS/PATH guideline is intended for use by physicians and other health professionals (eg, pharmacists and nurses) who care for frail older adults or who work in long-term care facilities. Since creating our guideline, we have disseminated it to physicians, pharmacists, and other health professionals through academic detailing, large conferences, and interactive webinars.
While we do not have objective evidence of practice change, our evaluation data found that 34% of 403 family physicians who received academic detailing indicated that the guideline would change their practice, while 36% stated that the guideline confirmed their practice, an indication that family physicians are sensitive to the needs of the frail elderly.
Because health professionals may be wary of stopping medications and not meeting recommended targets, there may be barriers to adopting this guideline. However, our experience with the PATH program indicates that these barriers can be overcome using effective communication strategies between health professionals and consumers.
AN APPROACH APPROPRIATE TO FRAILTY
There is no direct evidence for systolic blood pressure targets in the frail elderly, so we applied evidence from the nonfrail elderly. Our recommendations differ somewhat from those of other groups, which recommend targets below 140 to 150 mm Hg for older adults, although some do advise caution in the elderly for whom a substantial fall in blood pressure might be poorly tolerated. Despite these messages, we believe that clearer guidance is needed to direct health practitioners toward models that acknowledge that frail patients are in a precarious balance of health and may be harmed by treatments that strive to lower blood pressure to unproven targets. For this reason, our guideline clearly indicates when to decrease or stop drug treatment.
After physicians and health professionals examine the evidence and more fully understand the benefits and harms of treating frail older adults, we are confident that they will be more comfortable stopping medications that lower blood pressure to an unnecessarily low level and instead use an approach that is more appropriate to frailty. We hope clinicians can use this guideline with the same enthusiasm applied to other guidelines, and we welcome discussion.
Acknowledgments: We would like to thank and acknowledge Tanya MacLeod and Kathryn Yuill for their review of and advice about the manuscript.
Frail older adults deserve guidelines that take frailty into account while assessing the potential benefit and risks of treatment.
Specifically, our group—the Dalhousie Academic Detailing Service (ADS) and the Palliative and Therapeutic Harmonization (PATH) program—recommends that physicians strive to achieve more liberal treatment targets for elderly frail patients who have high blood pressure,1 as evidence does not support an aggressive approach in the frail elderly and the potential exists for harm.
This article reviews the evidence and reasoning that were used to develop and promote a guideline for drug treatment of hypertension in frail older adults. Our recommendations differ from other guidelines in that they focus as much on stopping or decreasing therapy as on starting or increasing it.
FRAILTY INCREASES THE RISK OF ADVERSE EFFECTS
The word frail, applied to older adults, describes those who have complex medical illnesses severe enough to compromise their ability to live independently.2 Many have multiple coexisting medical problems for which they take numerous drugs, in addition to dementia, impaired mobility, compromised functional ability, or a history of falling.
Frailty denotes vulnerability; it increases the risk of adverse effects from medical and surgical procedures,3 complicates drug therapy,4 prolongs hospital length of stay,5 leads to functional and cognitive decline,6 increases the risk of institutionalization,7 and reduces life expectancy8—all of which affect the benefit and harm of medical treatments.
Guidelines for treating hypertension9–11 now acknowledge that little evidence exists to support starting treatment for systolic blood pressure between 140 and 160 mm Hg or aiming for a target of less than 140 mm Hg for “very old” adults, commonly defined as over the age of 80. New guidelines loosen the treatment targets for the very old, but they do not specify targets for the frail and do not describe how to recognize or measure frailty.
RECOGNIZING AND MEASURING FRAILTY
A number of tools are available to recognize and measure frailty.12
The Fried frailty assessment13 has five items:
- Unintentional weight loss
- Self-reported exhaustion
- Weakness in grip
- Slow walking speed
- Low physical activity and energy expenditure.
People are deemed frail if they have three or more of these five. However, experts disagree about whether this system is too sensitive14 or not sensitive enough.15,16
The FRAIL questionnaire17 also has five items:
- Fatigue
- Resistance (inability to climb stairs)
- Ambulation (inability to walk 1 city block)
- Illness (more than 5 major illnesses)
- Weight loss.
People are deemed frail if they have at least three of these five items, and “prefrail” if they have two.
These and other tools are limited by being dichotomous: they classify people as being either frail or not frail18–20 but do not define the spectrum of frailty.
Other frailty assessments such as the Frailty Index21 identify frailty based on the number of accumulated health deficits but take a long time to complete, making them difficult to use in busy clinical settings.22–24
The Clinical Frailty Scale7 is a validated scale that categorizes frailty based on physical and functional indicators of health, such as cognition, function, and mobility, with scores that range from 1 (very fit) to 9 (terminally ill).7,12
The Frailty Assessment for Care-planning Tool (FACT) uses scaling compatible with the Clinical Frailty Scale but has been developed for use as a practical and interpretable frailty screening tool for nonexperts (Table 1). The FACT assesses cognition, mobility, function, and the social situation, using a combination of caregiver report and objective measures. To assess cognition, a health care professional uses items from the Mini-Cog25 (ie, the ability to draw an analog clock face and then recall three unrelated items following the clock-drawing test) and the memory axis of the Brief Cognitive Rating Scale26 (ie, the ability to recall current events, the current US president, and the names of children or spouse). Mobility, function, and social circumstance scores are assigned according to the caregiver report of the patient’s baseline status.
The FACT can be completed in busy clinical settings. Once a caregiver is identified, it takes about 5 minutes to complete.
Our guideline27–31 is intended for those with a score of 7 or more on the Clinical Frailty Scale or FACT,7,12 a score we chose because it describes people who are severely frail with shortened life expectancy.8 At this level, people need help with all instrumental activities of daily living (eg, handling finances, medication management, household chores, and shopping) as well as with basic activities of daily living such as bathing or dressing.
REVIEWING THE LIMITED EVIDENCE
We found no studies that addressed the risks and benefits of treating hypertension in frail older adults; therefore, we concentrated on studies that enrolled individuals who were chronologically old but not frail. We reviewed prominent guidelines,9–11,32,33 the evidence base for these guidelines,34–44 and Cochrane reviews.45,46 A detailed description of the evidence used to build our recommendation can be found online.31
When we deliberated on treatment targets, we reviewed evidence from two types of randomized controlled trials47:
Drug treatment trials randomize patients to different treatments, such as placebo versus a drug or one drug compared with another drug. Patients in different treatment groups may achieve different blood pressures and clinical outcomes, and this information is then used to define optimal targets. However, it may be difficult to determine if the benefit came from lowering blood pressure or from some other effect of the drug, which can be independent of blood pressure lowering.
Treat-to-target trials randomize patients to different blood pressure goals, but the groups are treated with the same or similar drugs. Therefore, any identified benefit can be attributed to the differences in blood pressure rather than the medications used. Compared with a drug treatment trial, this type of trial provides stronger evidence about optimal targets.
We also considered the characteristics of frailty, the dilemma of polypharmacy, and the relevance of the available scientific evidence to those who are frail.
Drug treatment trials
A Cochrane review45 of 15 studies with approximately 24,000 elderly participants found that treating hypertension decreased the rates of cardiovascular morbidity and mortality as well as fatal and nonfatal stroke in the “elderly” (defined as age ≥ 60) and “very elderly” (age ≥ 80). However, in the very elderly, all-cause mortality rates were not statistically significantly different with treatment compared with placebo. The mean duration of treatment was 4.5 years in the elderly and 2.2 years in the very elderly (Table 2). Of importance, all the trials enrolled only those individuals whose systolic blood pressure was at least 160 mm Hg at baseline.
None of the studies were treat-to-target trials—patients were assigned either active medication or placebo. Thus, these trials provide evidence of benefit for treating hypertension in the elderly and very elderly but do not identify the optimal target. All of the drug treatment trials showed benefit, but none achieved a systolic pressure lower than 140 mm Hg with active treatment (Table 3). Therefore, these studies do not support a systolic target of less than 140 mm Hg in the elderly.
Treat-to-target trials: JATOS and VALISH
The Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS)42 and the Valsartan in Elderly Isolated Systolic Hypertension (VALISH) study43 each enrolled more than 3,000 people age 65 or older (mean age approximately 75). Patients were randomized to either a strict systolic target of less than 140 mm Hg or a higher (more permissive) target of 140 to 160 mm Hg in JATOS and 140 to 149 mm Hg in VALISH.
In both trials, the group with strict targets achieved a systolic pressure of approximately 136 mm Hg, while the group with higher blood pressure targets achieved a systolic pressure of 146 mm Hg in JATOS and 142 mm Hg in VALISH. Despite these differences, there was no statistically significant difference in the primary outcome.
Thus, treat-to-target studies also fail to support a systolic target of less than 140 mm Hg in the elderly, although it is important to recognize the limitations of the studies. Approximately 15% of the participants had cardiovascular disease, so the applicability of the findings to patients with target-organ damage is uncertain. In addition, there were fewer efficacy outcome events than expected, which suggests that the studies were underpowered.
When to start drug treatment
In each of the drug treatment and treat-to-target trials, the inclusion criterion for study entry was a systolic blood pressure above 160 mm Hg, with a mean blood pressure at entry into the drug treatment trials of 182/95 mm Hg.46 Thus, data support starting treatment if the systolic blood pressure is above 160 mm Hg, but not lower.
Notably, in all but one study,46 at least two-thirds of the participants took no more than two antihypertensive medications. Since adverse events become more common as the number of medications increases, the benefit of adding a third drug to lower blood pressure is uncertain.
Evidence in the ‘very elderly’: HYVET
With the exception of the Hypertension in the Very Elderly Trial (HYVET),44 the mean age of elderly patients in the reported studies was between 67 and 76.
HYVET patients were age 80 and older (mean age 84) and were randomized to receive either indapamide (with or without perindopril) or placebo. The trial was stopped early at 2 years because the mortality rate was lower in the treatment group (10.1%) than in the placebo group (12.3%) (number needed to treat 46, 95% confidence interval 24–637, P = .02). There was no significant difference in the primary outcome of fatal and nonfatal stroke.
Notably, trials that are stopped early may overestimate treatment benefit.48
Evidence in frail older adults
While the above studies provide some information about managing hypertension in the elderly, the participants were generally healthy. HYVET44 specifically excluded those with a standing systolic blood pressure of less than 140 mm Hg and enrolled few patients with orthostasis (7.9% in the placebo group and 8.8% in the treatment group), a condition commonly associated with frailty. As such, these studies may be less relevant to the frail elderly, who are at higher risk of adverse drug events and have competing risks for morbidity and mortality.
Observational studies, in fact, raise questions about whether tight blood pressure control improves clinical outcomes for the very elderly. In the Leiden 85-plus study, lower systolic blood pressure was associated with lower cognitive scores, worse functional ability,49,50 and a higher mortality rate51 compared with higher systolic pressure, although it is uncertain whether these outcomes were indicative of underlying disease that could result in lower blood pressure or an effect of blood pressure-lowering.
The National Health and Nutrition Examination Survey52 found an association between blood pressure and mortality rate that varied by walking speed. For slower walkers (based on the 6-minute walk test), higher systolic pressures were not associated with a higher risk of death, suggesting that when older adults are frail (as indicated by their slow walking speed) they are less likely to benefit from aggressive treatment of hypertension.
People at high risk because of stroke
Because the evidence is limited, it is even more difficult to judge whether lowering blood pressure below 140 mm Hg is beneficial for frail patients who have a history of stroke, compared with the possibility that medications will cause adverse effects such as weakness, orthostasis, and falls. When reviewing the evidence to answer this question, we especially looked at outcomes that affect quality of life, such as nonfatal stroke leading to disability. In contrast, because the frail elderly have competing causes of mortality, we could not assume that a mortality benefit shown in nonfrail populations could be applied to frail populations.
The PROGRESS trial (Perindopril Protection Against Recurrent Stroke Study)53 was in patients with a history of stroke or transient ischemic attack and a mean age of 64, who were treated with either perindopril (with or without indapamide) or placebo.
At almost 4 years, the rate of disabling stroke was 2.7% in the treatment group and 4.3% in the placebo group, a relative risk reduction of 38% and an absolute risk reduction of 1.64% (number needed to treat 61, 95% confidence interval 39–139). The relative risk reduction for all strokes (fatal and nonfatal) was similar across a range of baseline systolic pressures, but the absolute risk reduction was greater in the prespecified subgroup that had hypertension at baseline (mean blood pressure 159/94 mm Hg) than in the normotensive subgroup (mean blood pressure 136/79 mm Hg), suggesting that treatment is most beneficial for those with higher systolic blood pressures. Also, the benefit was only demonstrated in the subgroup that received two antihypertensive medications; those who received perindopril alone showed no benefit.
This study involved relatively young patients in relatively good health except for their strokes. The extent to which the results can be extrapolated to older, frail adults is uncertain because of the time needed to achieve benefit and because of the added vulnerability of frailty, which could make treatment with two antihypertensive medications riskier.
PRoFESS (Prevention Regimen for Effectively Avoiding Second Strokes),54 another study in patients with previous stroke (mean age 66) showed no benefit over 2.5 years in the primary outcome of stroke using telmesartan 80 mg daily compared with placebo. This result is concordant with that of PROGRESS,53 in which patients who took only one medication did not show a significant decrease in the rate of stroke.
A possible reason for the lack of benefit from monotherapy was that the differences in blood pressure between the placebo group and the treatment group on monotherapy were small in both studies (3.8/2.0 mm Hg in PRoFESS, 5/3 mm Hg in PROGRESS). In contrast, patients on dual therapy in PROGRESS decreased their blood pressure by 12/5 mm Hg compared with placebo.
CURRENT HYPERTENSION GUIDELINES
Current guidelines make reference to the elderly, but we found none that made specific recommendations for the frail elderly.
JNC 8
In December 2013, members of the Eighth Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 8) released new recommendations.32 One significant revision was to support higher blood pressure targets for older adults (age 60 and older). Whereas JNC 7 stated that lowering blood pressure below 140/90 mm Hg reduced cardiovascular complications,33 JNC 8 now acknowledges that there is no strong evidence to support blood pressure targets below 150/90 mm Hg for hypertensive persons without kidney disease or diabetes age 60 and older. Thus, in the general population age 60 and older, JNC 8 recommends starting antihypertensive treatment when blood pressure is 150/90 mm Hg or higher, and treating to a goal blood pressure of less than 150/90 mm Hg. JNC 8 makes no recommendation about how to adjust blood pressure targets for frailty or how to measure blood pressure.
American College of Cardiology and American Heart Association
In 2011, the American College of Cardiology and American Heart Association published a consensus document on the management of hypertension in the elderly.9
They acknowledged that the generally recommended blood pressure goal of lower than 140/90 mm Hg in uncomplicated elderly patients is based on expert opinion rather than on data from randomized controlled trials, but nevertheless recommended a target systolic pressure lower than 140 mm Hg for older adults, except for octogenarians.
For those over age 80, systolic levels of 140 to 145 mm Hg can be acceptable if tolerated and if the patient does not experience orthostasis when standing. Systolic pressure lower than 130 mm Hg and diastolic pressures lower than 65 mm Hg should be avoided in this age group.
The document acknowledges that systolic pressure may have to remain above 150 mm Hg if there is no response to four “well-selected drugs” or if there are unacceptable side effects. In these cases, the lowest “safely achieved” systolic blood pressure should be the goal.
Canadian Hypertension Education Program
The 2014 Canadian Hypertension Education Program (CHEP) report makes several recommendations for the “very elderly,” a group they define as over the age of 80. The CHEP website and resources include the following recommendations10:
- For the very elderly without diabetes or target-organ damage, drug therapy should be initiated when systolic blood pressure is higher than 160 mm Hg to reach a systolic blood pressure target lower than 150 mm Hg. This is a grade C level recommendation, indicating that it is based on low-quality trials, unvalidated surrogate outcomes, or results from nonrandomized observational studies.
- For the very elderly with macrovascular target-organ damage, antihypertensive therapy should be considered if systolic blood pressure readings average 140 mm Hg or higher (grade D for 140 to 160 mm Hg; grade A for higher than 160 mm Hg), although caution should be exercised in elderly patients who are frail. (Grade D recommendations are the weakest, as they are based on low-powered, imprecise studies or expert opinion, whereas grade A recommendations are based on the strongest evidence from high-quality randomized clinical trials.)
- Decisions regarding initiating and intensifying pharmacotherapy in the very elderly should be based on an individualized risk-benefit analysis.
The European Society of Hypertension and European Society of Cardiology
The 2013 guidelines from the European Society of Hypertension and the European Society of Cardiology11 recommend that for elderly patients under age 80, antihypertensive treatment may be considered at systolic values higher than 140 mm Hg and aimed at values lower than 140 mm Hg if the patient is fit and treatment is well tolerated.
For those over age 80 with an initial systolic pressure of 160 mm Hg or higher, the guidelines recommend lowering systolic pressure to between 150 and 140 mm Hg, provided the patient is in good physical and mental condition. In frail elderly patients, they recommend leaving decisions on antihypertensive therapy to the treating physician, based on monitoring of the clinical effects of treatment.11
The ADS/PATH guidelines
When finalizing our recommendations,1 we considered the characteristics of frailty and the following key points from the evidence:
- Although evidence from drug treatment trials indicates that there is benefit in treating healthy older adults who have hypertension, the benefit of treating frail older adults is unknown.
- Major trials enrolled elderly patients only if they had systolic blood pressures of at least 160 mm Hg. Therefore, evidence supports initiating pharmacotherapy at a systolic pressure of 160 mm Hg or higher.
- No evidence from randomized controlled trials supports a systolic target lower than 140 mm Hg in the elderly, and there is some evidence that such a target does not benefit.
- The benefit of adding a third medication to lower blood pressure has not been studied.
- Frailty makes the potential benefits of strict blood pressure targets even less certain and increases the possibility of harm from adverse drug events.
- The only study of very old adults, HYVET,44 enrolled relatively healthy older adults and few with orthostasis, while excluding those with a standing systolic blood pressure lower than 140 mm Hg.
OUR RECOMMENDATIONS
Based on the above, we advise against unnecessarily strict targets and recommend stopping antihypertensive medications that are used for the sole purpose of keeping the systolic blood pressure below 140 mm Hg. Our guidelines are unique in that they focus equally on when to stop and when to start medications. We concluded that without evidence of definitive benefit, “less is more” with frailty.55 We believe that if physicians and health professionals understand the limitations of the evidence, they can be more confident in stopping medications that lower blood pressure to an unnecessarily low level.
We recommend the following (Table 4):
Before treating
- Carefully review the risks and the potential but unproven benefits of treatment.
- To avoid overtreatment, treatment decisions should be based on blood pressure measurements in the seated (not supine) position, while also considering the presence of orthostasis.
- To evaluate orthostasis, measure blood pressure in the supine position, then immediately on standing, and again after 2 minutes. Ask the patient if he or she feels light-headed or dizzy when standing.
Stop treatment
- If the seated systolic blood pressure is less than 140 mm Hg, medications can be tapered and discontinued to achieve the targets described below.
- Before discontinuation, consider whether the medications are treating additional conditions such as rate control for atrial fibrillation or symptomatic management of heart failure.
- It is uncertain whether to discontinue treatment when there is a history of stroke. Consider that treatment with two medications resulted in an absolute risk reduction for disabling stroke of 1.64% over approximately 4 years for adults with previous stroke and a mean age of 64,57 an effect that may be more prominent at higher systolic pressures.
Start treatment
- Consider starting treatment when systolic pressure is 160 mm Hg or higher.
- Aim for a seated systolic pressure between 140 and 160 mm Hg if there are no adverse effects from treatment that affect quality of life.
- If there is symptomatic orthostasis or if standing systolic pressure is lower than 140 mm Hg, the target seated systolic pressure can be adjusted upwards.
- In the severely frail nearing the end of life, a target systolic pressure of 160 to 190 mm Hg is reasonable.
- The blood pressure target is the same in people with diabetes.
- In general, use no more than two medications.
Dissemination and implementation
The ADS/PATH guideline is intended for use by physicians and other health professionals (eg, pharmacists and nurses) who care for frail older adults or who work in long-term care facilities. Since creating our guideline, we have disseminated it to physicians, pharmacists, and other health professionals through academic detailing, large conferences, and interactive webinars.
While we do not have objective evidence of practice change, our evaluation data found that 34% of 403 family physicians who received academic detailing indicated that the guideline would change their practice, while 36% stated that the guideline confirmed their practice, an indication that family physicians are sensitive to the needs of the frail elderly.
Because health professionals may be wary of stopping medications and not meeting recommended targets, there may be barriers to adopting this guideline. However, our experience with the PATH program indicates that these barriers can be overcome using effective communication strategies between health professionals and consumers.
AN APPROACH APPROPRIATE TO FRAILTY
There is no direct evidence for systolic blood pressure targets in the frail elderly, so we applied evidence from the nonfrail elderly. Our recommendations differ somewhat from those of other groups, which recommend targets below 140 to 150 mm Hg for older adults, although some do advise caution in the elderly for whom a substantial fall in blood pressure might be poorly tolerated. Despite these messages, we believe that clearer guidance is needed to direct health practitioners toward models that acknowledge that frail patients are in a precarious balance of health and may be harmed by treatments that strive to lower blood pressure to unproven targets. For this reason, our guideline clearly indicates when to decrease or stop drug treatment.
After physicians and health professionals examine the evidence and more fully understand the benefits and harms of treating frail older adults, we are confident that they will be more comfortable stopping medications that lower blood pressure to an unnecessarily low level and instead use an approach that is more appropriate to frailty. We hope clinicians can use this guideline with the same enthusiasm applied to other guidelines, and we welcome discussion.
Acknowledgments: We would like to thank and acknowledge Tanya MacLeod and Kathryn Yuill for their review of and advice about the manuscript.
- Palliative and Therapeutic Harmonization program. Hypertension guidelines. Treating hypertension in frailty. http://pathclinic.ca/resources/hypertension/. Accessed May 2, 2014.
- Theou O, Rockwood MR, Mitnitski A, Rockwood K. Disability and co-morbidity in relation to frailty: how much do they overlap? Arch Gerontol Geriatr 2012; 55:e1–e8.
- Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 2010; 210:901–908.
- Tinetti ME, Bogardus ST, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351:2870–2874.
- Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation 2011; 124:2397–2404.
- Theou O, Rockwood K. Should frailty status always be considered when treating the elderly patient? Aging Health 2012; 8:261–271.
- Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173:489–495.
- Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008; 8:24.
- Aronow WS, Fleg JL, Pepine CJ, et al; ACCF Task Force. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2011; 123:2434–2506.
- The Canadian Hypertension Education Program (CHEP). 2014 CHEP recommendations. www.hypertension.ca/en/. Accessed May 2, 2014.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34:2159–2219.
- Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013; 14:392–397.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Ensrud KE, Ewing SK, Cawthon PM, et al; Osteoporotic Fractures in Men Research Group. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc 2009; 57:492–498.
- Avila-Funes JA, Amieva H, Barberger-Gateau P, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc 2009; 57:453–461.
- Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A Biol Sci Med Sci 2007; 62:731–737.
- Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 2012; 16:601–608.
- Strawbridge WJ, Shema SJ, Balfour JL, Higby HR, Kaplan GA. Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci 1998; 53:S9–S16.
- Matthews M, Lucas A, Boland R, et al. Use of a questionnaire to screen for frailty in the elderly: an exploratory study. Aging Clin Exp Res 2004; 16:34–40.
- Salvi F, Morichi V, Grilli A, et al. Screening for frailty in elderly emergency department patients by using the Identification of Seniors At Risk (ISAR). J Nutr Health Aging 2012; 16:313–318.
- Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 2001; 1:323–336.
- Kellen E, Bulens P, Deckx L, et al. Identifying an accurate pre-screening tool in geriatric oncology. Crit Rev Oncol Hematol 2010; 75:243–248.
- Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006; 35:526–529.
- Martin FC, Brighton P. Frailty: different tools for different purposes? Age Ageing 2008; 37:129–131.
- Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry 2000; 15:1021–1027.
- Reisberg B, Ferris SH. Brief Cognitive Rating Scale (BCRS). Psychopharmacol Bull 1988; 24:629–636.
- Moorhouse P, Mallery LH. Palliative and therapeutic harmonization: a model for appropriate decision-making in frail older adults. J Am Geriatr Soc 2012; 60:2326–2332.
- Palliative and Therapeutic Harmonization Clinic (PATH). www.pathclinic.ca. Accessed May 2, 2014.
- Dalhousie University Faculty of Medicine: Continuing Medical Education. http://cme.medicine.dal.ca/ADS.htm. Accessed January 8, 2014.
- Mallery LH, Moorhouse P. Respecting frailty. J Med Ethics 2011; 37:126–128.
- Dalhousie University Faculty of Medicine: Continuing Medical Education. Issues in hypertension 2011. http://cme.medicine.dal.ca/files/Hypertension%20book.pdf. Accessed May 2, 2014.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Amery A, Birkenhäger W, Brixko P, et al. Mortality and morbidity results from the European Working Party on High Blood Pressure in the Elderly trial. Lancet 1985; 1:1349–1354.
- Coope J, Warrender TS. Randomised trial of treatment of hypertension in elderly patients in primary care. Br Med J (Clin Res Ed) 1986; 293:1145–1151.
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991; 265:3255–3264.
- Dahlöf B, Lindholm LH, Hansson L, Scherstén B, Ekbom T, Wester PO. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension). Lancet 1991; 338:1281–1285.
- Medical Research Council trial of treatment of hypertension in older adults: principal results. MRC Working Party. BMJ 1992; 304:405–412.
- Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997; 350:757–764.
- Liu L, Wang JG, Gong L, Liu G, Staessen JA. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. Systolic Hypertension in China (Syst-China) Collaborative Group. J Hypertens 1998; 16:1823–1829.
- Lithell H, Hansson L, Skoog I, et al; SCOPE Study Group. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens 2003; 21:875–886.
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Oparil S, Yarows SA, Patel S, Fang H, Zhang J, Satlin A. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial. Lancet 2007; 370:221–229.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Musini VM, Tejani AM, Bassett K, Wright JM. Pharmacotherapy for hypertension in the elderly. Cochrane Database Syst Rev 2009;CD000028.
- He FJ, MacGregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev 2004;CD004937.
- Allen M, Kelly K, Fleming I. Hypertension in elderly patients: recommended systolic targets are not evidence based [in French]. Can Fam Physician 2013; 59:19–24.
- Guyatt GH, Briel M, Glasziou P, Bassler D, Montori VM. Problems of stopping trials early. BMJ 2012; 344:e3863.
- Sabayan B, Oleksik AM, Maier AB, et al. High blood pressure and resilience to physical and cognitive decline in the oldest old: the Leiden 85-plus Study. J Am Geriatr Soc 2012; 60:2014–2019.
- Sabayan B, van Vliet P, de Ruijter W, Gussekloo J, de Craen AJ, Westendorp RG. High blood pressure, physical and cognitive function, and risk of stroke in the oldest old: the Leiden 85-plus Study. Stroke 2013; 44:15–20.
- Poortvliet RK, Blom JW, de Craen AJ, et al. Low blood pressure predicts increased mortality in very old age even without heart failure: the Leiden 85-plus Study. Eur J Heart Fail 2013; 15:528–533.
- Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med 2012; 172:1162–1168.
- PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001; 358:1033–1041.
- Yusuf S, Diener HC, Sacco RL, et al; PRoFESS Study Group. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med 2008; 359:1225–1237.
- Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med 2010; 170:1648–1654.
- Palliative and Therapeutic Harmonization program. Hypertension guidelines. Treating hypertension in frailty. http://pathclinic.ca/resources/hypertension/. Accessed May 2, 2014.
- Theou O, Rockwood MR, Mitnitski A, Rockwood K. Disability and co-morbidity in relation to frailty: how much do they overlap? Arch Gerontol Geriatr 2012; 55:e1–e8.
- Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 2010; 210:901–908.
- Tinetti ME, Bogardus ST, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351:2870–2874.
- Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation 2011; 124:2397–2404.
- Theou O, Rockwood K. Should frailty status always be considered when treating the elderly patient? Aging Health 2012; 8:261–271.
- Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173:489–495.
- Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008; 8:24.
- Aronow WS, Fleg JL, Pepine CJ, et al; ACCF Task Force. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2011; 123:2434–2506.
- The Canadian Hypertension Education Program (CHEP). 2014 CHEP recommendations. www.hypertension.ca/en/. Accessed May 2, 2014.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34:2159–2219.
- Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013; 14:392–397.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Ensrud KE, Ewing SK, Cawthon PM, et al; Osteoporotic Fractures in Men Research Group. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc 2009; 57:492–498.
- Avila-Funes JA, Amieva H, Barberger-Gateau P, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc 2009; 57:453–461.
- Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A Biol Sci Med Sci 2007; 62:731–737.
- Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 2012; 16:601–608.
- Strawbridge WJ, Shema SJ, Balfour JL, Higby HR, Kaplan GA. Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci 1998; 53:S9–S16.
- Matthews M, Lucas A, Boland R, et al. Use of a questionnaire to screen for frailty in the elderly: an exploratory study. Aging Clin Exp Res 2004; 16:34–40.
- Salvi F, Morichi V, Grilli A, et al. Screening for frailty in elderly emergency department patients by using the Identification of Seniors At Risk (ISAR). J Nutr Health Aging 2012; 16:313–318.
- Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 2001; 1:323–336.
- Kellen E, Bulens P, Deckx L, et al. Identifying an accurate pre-screening tool in geriatric oncology. Crit Rev Oncol Hematol 2010; 75:243–248.
- Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006; 35:526–529.
- Martin FC, Brighton P. Frailty: different tools for different purposes? Age Ageing 2008; 37:129–131.
- Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry 2000; 15:1021–1027.
- Reisberg B, Ferris SH. Brief Cognitive Rating Scale (BCRS). Psychopharmacol Bull 1988; 24:629–636.
- Moorhouse P, Mallery LH. Palliative and therapeutic harmonization: a model for appropriate decision-making in frail older adults. J Am Geriatr Soc 2012; 60:2326–2332.
- Palliative and Therapeutic Harmonization Clinic (PATH). www.pathclinic.ca. Accessed May 2, 2014.
- Dalhousie University Faculty of Medicine: Continuing Medical Education. http://cme.medicine.dal.ca/ADS.htm. Accessed January 8, 2014.
- Mallery LH, Moorhouse P. Respecting frailty. J Med Ethics 2011; 37:126–128.
- Dalhousie University Faculty of Medicine: Continuing Medical Education. Issues in hypertension 2011. http://cme.medicine.dal.ca/files/Hypertension%20book.pdf. Accessed May 2, 2014.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Amery A, Birkenhäger W, Brixko P, et al. Mortality and morbidity results from the European Working Party on High Blood Pressure in the Elderly trial. Lancet 1985; 1:1349–1354.
- Coope J, Warrender TS. Randomised trial of treatment of hypertension in elderly patients in primary care. Br Med J (Clin Res Ed) 1986; 293:1145–1151.
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991; 265:3255–3264.
- Dahlöf B, Lindholm LH, Hansson L, Scherstén B, Ekbom T, Wester PO. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension). Lancet 1991; 338:1281–1285.
- Medical Research Council trial of treatment of hypertension in older adults: principal results. MRC Working Party. BMJ 1992; 304:405–412.
- Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997; 350:757–764.
- Liu L, Wang JG, Gong L, Liu G, Staessen JA. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. Systolic Hypertension in China (Syst-China) Collaborative Group. J Hypertens 1998; 16:1823–1829.
- Lithell H, Hansson L, Skoog I, et al; SCOPE Study Group. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens 2003; 21:875–886.
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Oparil S, Yarows SA, Patel S, Fang H, Zhang J, Satlin A. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial. Lancet 2007; 370:221–229.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Musini VM, Tejani AM, Bassett K, Wright JM. Pharmacotherapy for hypertension in the elderly. Cochrane Database Syst Rev 2009;CD000028.
- He FJ, MacGregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev 2004;CD004937.
- Allen M, Kelly K, Fleming I. Hypertension in elderly patients: recommended systolic targets are not evidence based [in French]. Can Fam Physician 2013; 59:19–24.
- Guyatt GH, Briel M, Glasziou P, Bassler D, Montori VM. Problems of stopping trials early. BMJ 2012; 344:e3863.
- Sabayan B, Oleksik AM, Maier AB, et al. High blood pressure and resilience to physical and cognitive decline in the oldest old: the Leiden 85-plus Study. J Am Geriatr Soc 2012; 60:2014–2019.
- Sabayan B, van Vliet P, de Ruijter W, Gussekloo J, de Craen AJ, Westendorp RG. High blood pressure, physical and cognitive function, and risk of stroke in the oldest old: the Leiden 85-plus Study. Stroke 2013; 44:15–20.
- Poortvliet RK, Blom JW, de Craen AJ, et al. Low blood pressure predicts increased mortality in very old age even without heart failure: the Leiden 85-plus Study. Eur J Heart Fail 2013; 15:528–533.
- Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med 2012; 172:1162–1168.
- PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001; 358:1033–1041.
- Yusuf S, Diener HC, Sacco RL, et al; PRoFESS Study Group. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med 2008; 359:1225–1237.
- Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med 2010; 170:1648–1654.
KEY POINTS
- For frail elderly patients, consider starting treatment if the systolic blood pressure is 160 mm Hg or higher.
- An appropriate target in this population is a seated systolic pressure between 140 and 160 mm Hg, as long as there is no orthostatic drop to less than 140 mm Hg upon standing from a lying position and treatment does not adversely affect quality of life.
- The blood pressure target does not need to be lower if the patient has diabetes. If the patient is severely frail and has a short life expectancy, a systolic target of 160 to 190 mm Hg may be reasonable.
- If the systolic pressure is below 140 mm Hg, antihypertensive medications can be reduced as long as they are not indicated for other conditions.
- In general, one should prescribe no more than two antihypertensive medications.