User login
Penicillin allergy: A practical guide for clinicians
Most patients who report that they are allergic to penicillin can ultimately receive penicillin or a penicillin-type antibiotic again after an appropriate evaluation and, possibly, treatment. This course of action decreases the need for broad-spectrum antibiotics,1–4 reduces health care costs, and prevents the development of multidrug-resistant pathogens.5
About 10% of the general population say that they are allergic to penicillin.1,6,7 Although the prevalence of life-threatening anaphylactic reactions to penicillin has been estimated to be between 0.02% and 0.04%,6 the most common reaction is a cutaneous eruption. Since anaphylactic reactions are mediated by immunoglobulin E (IgE), evaluation of patients with a history of penicillin allergy by penicillin skin testing is recommended to rule out IgE-mediated reactions.
This review outlines a practical approach to evaluating a suspected IgE-mediated reaction to penicillin, with key points in the history and diagnostic testing. We also review subsequent management and cross-reactivity with other beta-lactam-containing antibiotics.
EVALUATING ALLERGIC PATIENTS
Evaluation of patients with a history of penicillin allergy can be improved with an understanding of the classification of drug reactions, risk factors for allergy, and the pathophysiology of penicillin allergy.
Classification of drug reactions
Adverse drug reactions include all unintended pharmacologic effects of a drug and can be classified as predictable (type A) or unpredictable (type B). Predictable reactions are dose-dependent, are related to the known pharmacologic actions of the medication, and occur in otherwise healthy individuals. Unpredictable reactions are further classified into drug intolerance, drug idiosyncrasy, drug allergy, and pseudoallergic reactions.8,9
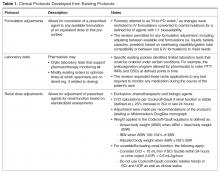
Penicillin allergy can manifest as any hypersensitivity reaction of the Gell and Coombs classification (Table 1).9 Type I (immediate) and type IV (delayed) reactions are the most common types of reactions that occur with antibiotics and should be classified based on the onset of symptoms as immediate (within 1 hour) or delayed (days or weeks).8
Risk factors for IgE-mediated reaction
Risk factors for a hypersensitivity reaction include frequent or repetitive courses of penicillin10 and high-dose parenteral (rather than oral) administration.
Age and atopy are not risk factors for penicillin allergy.7 However, atopy increases the risk of a more severe anaphylactic reaction to penicillin, and anaphylactic reactions are most commonly reported between the ages of 20 and 49.6
Pathophysiology of penicillin allergy
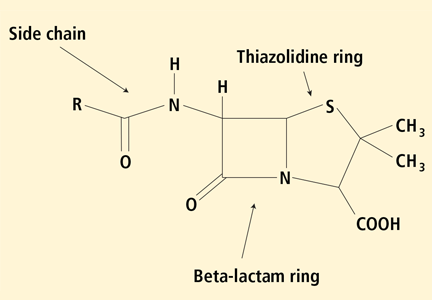
All penicillins share a common core ring structure (beta-lactam and thiazolidine rings) but differ in their side chains (R group) (Figure 1).
Under physiologic conditions, the core ring structure is metabolized into major (penicilloyl) and minor (penicillin itself, penicilloate and penilloate) antigenic determinants that may trigger an immediate IgE-dependent response.9 In the United States, commercial forms of antigenic determinates for skin testing exist in the form of penicillin G (minor determinant) and penicilloyl-polylysine, better known as Prepen (major determinant).
Immediate-type reactions to similar antibiotics such as aminopenicillins and cephalosporins may be caused by IgE antibodies against the R-group side chain rather than the core penicillin major and minor determinants.11
Questions to ask patients who have a history of penicillin allergy
Patients should be questioned closely about previous and current reactions to penicillin and should undergo skin-prick and intradermal testing, followed by graded-dose challenge or drug tolerance desensitization (Figure 2).
Questions to ask patients who have a history of penicillin allergy (Table 2)9,12 include the following:
Do you remember the details of the reaction? These include the route of administration, the time between the dose of penicillin and the appearance of symptoms, and how the reaction was managed.
Immediate reactions (ie, IgE-mediated, or Gell and Coombs type I) usually occur within the first hour after the first dose of the antibiotic, although they occasionally take up to 2 hours to occur, especially if the medication is taken orally and is taken with food. Symptoms consistent with IgE-mediated reactions include urticaria (most common), pruritus, angioedema, laryngeal edema, wheezing, shortness of breath, presyncope or syncope, hypotension, and cardiorespiratory collapse.
In contrast, symptoms of a non–IgE-mediated reaction are delayed in onset, occurring after days of treatment. They include nonpruritic maculopapular eruptions, hemolytic anemia, serum sickness, Stevens-Johnson syndrome, drug rash with eosinophilia and systemic symptoms, acute interstitial nephritis, and toxic epidermal necrolysis.9
If the patient has had severe non–IgE-mediated reactions to penicillin (eg, Stevens-Johnson syndrome, toxic epidermal necrolysis, acute interstitial nephritis, hemolytic anemia, or serum sickness) in the past, skin testing, graded-dose challenge, and desensitization are contraindicated.
How many years ago did the reaction occur? Most patients lose their sensitivity to penicillin over time.7,13–15 Nearly 50% of patients with IgE-mediated penicillin allergy lose their sensitivity within 5 years of the reaction,15 increasing to 80% or more by 10 years.13
How was the reaction managed? What was the outcome? Use of and positive response to epinephrine and histamine 1 receptor antagonists (antihistamines) with resolution or significant improvement of symptoms within a few hours may indicate an IgE-mediated reaction.
What was the indication for penicillin? Many cutaneous reactions are a result of an underlying viral or bacterial infection. For example, up to 90% of patients with Epstein-Barr virus infection develop a maculopapular rash when given penicillin.16
Have you tolerated other forms of penicillin since the reaction? Sometimes the patient has already tolerated other beta-lactams such as aminopenicillins, cephalosporins, and semisynthetic penicillins (piperacillin-tazobactam). Patients who tolerate other beta-lactams without adverse reactions are not allergic to beta-lactams.
Diagnostic tests
Skin testing. The only validated test for diagnosing IgE-mediated reactions caused by penicillin is the immediate hypersensitivity skin test,9 which should be performed by a board-certified allergist. The test consists of skin-prick and intradermal testing with the major determinant (penicilloyl-polylysine), the minor determinant (penicillin G), a negative control (normal saline), and a positive control (histamine). Minor-determinant mix is not commercially available in the United States.
Results of skin-prick testing are read 15 minutes after application. A positive response is a wheal at least 3 mm larger in diameter (with equivalent erythema) than the negative control done simultaneously. Intradermal testing is only done after a negative skin-prick test. If the allergic reaction was severe (ie, anaphylaxis), skin testing should be done at least 4 to 6 weeks after the reaction.
A history of severe non–IgE-mediated reaction to penicillin is a contraindication to skin-prick testing for penicillin allergy. The positive predictive value of penicillin skin testing is 50%, and the negative predictive value is 97%.3,7,9,13
Commercial in vitro testing (serum-specific IgE assays) for IgE-mediated hypersensitivity to penicillin is inferior to skin testing in terms of the negative predictive value and is not a suitable substitute for penicillin skin testing.
MANAGING PENICILLIN ALLERGY
If skin testing is positive, use another antibiotic, or refer for desensitization
If penicillin skin testing is positive (Figure 2), use another antibiotic that is equally efficacious. Patients who absolutely need a beta-lactam may undergo drug desensitization, performed by a board-certified allergist.
During desensitization, patients receive progressively higher doses of the drug every 15 to 20 minutes subcutaneously or intravenously, or every 20 to 30 minutes orally, until a full therapeutic dose is tolerated. Most protocols begin with a dose ranging from 1/10,000 to 1/1,000 of the final dose, depending on the severity of the allergic reaction.9,17
Using modern protocols, the success rate for tolerance induction is extremely high (75% to 100% in patients with cystic fibrosis, a group with a high rate of drug allergy18–20).
Drug desensitization is contraindicated in patients with non–IgE-mediated reactions.
If skin testing is negative, refer for graded-dose challenge
If skin testing is negative (Figure 2), graded-dose challenge is recommended. This procedure must be done by a board-certified allergist. If the original reaction was life-threatening, graded-dose challenge may entail giving 1/100 of the therapeutic dose. Then, if no reaction occurs during a brief observation period (usually 30 minutes), a full dose is given. However, many patients can start with 1/10 or even a full dose of the drug, especially if the original reaction was limited to the skin and the penicillin skin test is negative.
Graded-dose challenge is contraindicated if the original reaction was a severe non–IgE-mediated reaction.
UNDERSTANDING CROSS-REACTIVITY OF PENICILLIN
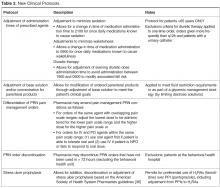
Penicillin is the only antibiotic for which skin testing is reliable and validated. If a drug that cross-reacts with penicillin is needed, it is important to know the rate of cross-reactivity (Table 3). The rate of cross-reactivity between penicillin and aminopenicillins (amoxicillin and ampicillin) is less than 1.3% in the United States.10,21 However, the cross-reactivity rate among aminopenicillins and cephalosporins is between 10% to 40%. For that reason, patients with prior reactions to aminopenicillins should avoid cephalosporins that share identical R-chain side groups with aminopenicillins.9,22
The rate of cross-reactivity between penicillin and cephalosporins was reported as 10% 40 years ago.23,24 But this was with early, first-generation cephalosporins that may have been contaminated with penicillin. The cross-reactivity rate with cephalosporins today is 3%.25 In general, first- and second-generation cephalosporins cause more allergic reactions than third- and fourth-generation cephalosporins.26
Patients with a history of penicillin allergy who require a cephalosporin should still undergo penicillin skin testing. Skin testing with cephalosporins has not been validated. However, skin testing with nonirritating concentrations of cephalosporins9 may be done to elucidate IgE reactions.
In a study by Romano et al,27 110 patients who had positive results on penicillin skin testing completed graded-dose challenge with the carbapenem antibiotic imipenem. The rate of cross-reactivity between penicillin and imipenem was less than 1%.
Monobactam antibiotics do not cross-react with other beta-lactams, except ceftazidime with aztreonam. This is probably because of similarities in their chemical structure.
- Park M, Markus P, Matesic D, Li JT. Safety and effectiveness of a preoperative allergy clinic in decreasing vancomycin use in patients with a history of penicillin allergy. Ann Allergy Asthma Immunol 2006; 97:681–687.
- Arroliga ME, Radojicic C, Gordon SM, et al. A prospective observational study of the effect of penicillin skin testing on antibiotic use in the intensive care unit. Infect Control Hosp Epidemiol 2003; 24:347–350.
- del Real GA, Rose ME, Ramirez-Atamoros MT, et al. Penicillin skin testing in patients with a history of beta-lactam allergy. Ann Allergy Asthma Immunol 2007; 98:355–359.
- Nadarajah K, Green GR, Naglak M. Clinical outcomes of penicillin skin testing. Ann Allergy Asthma Immunol 2005; 95:541–545.
- Harris AD, Sauberman L, Kabbash L, Greineder DK, Samore MH. Penicillin skin testing: a way to optimize antibiotic utilization. Am J Med 1999; 107:166–168.
- Idsoe O, Guthe T, Willcox RR, de Weck AL. Nature and extent of penicillin side-reactions, with particular reference to fatalities from anaphylactic shock. Bull World Health Organ 1968; 38:159–188.
- Gadde J, Spence M, Wheeler B, Adkinson NF Jr. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA 1993; 270:2456–2463.
- Johansson SG, Bieber T, Dahl R, et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol 2004; 113:832–836.
- Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol 2010; 105:259–273.
- Park MA, Matesic D, Markus PJ, Li JT. Female sex as a risk factor for penicillin allergy. Ann Allergy Asthma Immunol 2007; 99:54–58.
- Moreno E, Macias E, Davila I, Laffond E, Ruiz A, Lorente F. Hypersensitivity reactions to cephalosporins. Expert Opin Drug Saf 2008; 7:295–304.
- Salkind AR, Cuddy PG, Foxworth JW. The rational clinical examination. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA 2001; 285:2498–2505.
- Sullivan TJ, Wedner HJ, Shatz GS, Yecies LD, Parker CW. Skin testing to detect penicillin allergy. J Allergy Clin Immunol 1981; 68:171–180.
- Macy E, Schatz M, Lin C, Poon KY. The falling rate of positive penicillin skin tests from 1995 to 2007. Perm J 2009; 13:12–18.
- Blanca M, Torres MJ, Garcia JJ, et al. Natural evolution of skin test sensitivity in patients allergic to beta-lactam antibiotics. J Allergy Clin Immunol 1999; 103:918–924.
- Patel BM. Skin rash with infectious mononucleosis and ampicillin. Pediatrics 1967; 40:910–911.
- Liu A, Fanning L, Chong H, et al. Desensitization regimens for drug allergy: state of the art in the 21st century. Clin Exp Allergy 2011; 41:1679–1688.
- Burrows JA, Toon M, Bell SC. Antibiotic desensitization in adults with cystic fibrosis. Respirology 2003; 8:359–364.
- Turvey SE, Cronin B, Arnold AD, Dioun AF. Antibiotic desensitization for the allergic patient: 5 years of experience and practice. Ann Allergy Asthma Immunol 2004; 92:426–432.
- Legere HJ 3rd, Palis RI, Rodriguez Bouza T, Uluer AZ, Castells MC. A safe protocol for rapid desensitization in patients with cystic fibrosis and antibiotic hypersensitivity. J Cyst Fibros 2009; 8:418–424.
- Lin E, Saxon A, Riedl M. Penicillin allergy: value of including amoxicillin as a determinant in penicillin skin testing. Int Arch Allergy Immunol 2010; 152:313–318.
- Dickson SD, Salazar KC. Diagnosis and management of immediate hypersensitivity reactions to cephalosporins. Clin Rev Allergy Immunol 2013; 45:131–142.
- Dash CH. Penicillin allergy and the cephalosporins. J Antimicrob Chemother 1975; 1(suppl 3):107–118.
- Petz LD. Immunologic cross-reactivity between penicillins and cephalosporins: a review. J Infect Dis 1978; 137(suppl):S74–S79.
- American Academy of Allergy Asthma & Immunology. Cephalosporin administration to patients with a history of penicillin allergy. www.aaaai.org/Aaaai/media/MediaLibrary/PDF%20Documents/Practice%20and%20Parameters/Cephalosporin-administration-2009.pdf. Accessed April 2, 2015.
- Fonacier L, Hirschberg R, Gerson S. Adverse drug reactions to cephalosporins in hospitalized patients with a history of penicillin allergy. Allergy Asthma Proc 2005; 26:135–141.
- Romano A, Viola M, Gueant-Rodriguez RM, Gaeta F, Pettinato R, Gueant JL. Imipenem in patients with immediate hypersensitivity to penicillins. N Engl J Med 2006; 354:2835–2837.
Most patients who report that they are allergic to penicillin can ultimately receive penicillin or a penicillin-type antibiotic again after an appropriate evaluation and, possibly, treatment. This course of action decreases the need for broad-spectrum antibiotics,1–4 reduces health care costs, and prevents the development of multidrug-resistant pathogens.5
About 10% of the general population say that they are allergic to penicillin.1,6,7 Although the prevalence of life-threatening anaphylactic reactions to penicillin has been estimated to be between 0.02% and 0.04%,6 the most common reaction is a cutaneous eruption. Since anaphylactic reactions are mediated by immunoglobulin E (IgE), evaluation of patients with a history of penicillin allergy by penicillin skin testing is recommended to rule out IgE-mediated reactions.
This review outlines a practical approach to evaluating a suspected IgE-mediated reaction to penicillin, with key points in the history and diagnostic testing. We also review subsequent management and cross-reactivity with other beta-lactam-containing antibiotics.
EVALUATING ALLERGIC PATIENTS
Evaluation of patients with a history of penicillin allergy can be improved with an understanding of the classification of drug reactions, risk factors for allergy, and the pathophysiology of penicillin allergy.
Classification of drug reactions
Adverse drug reactions include all unintended pharmacologic effects of a drug and can be classified as predictable (type A) or unpredictable (type B). Predictable reactions are dose-dependent, are related to the known pharmacologic actions of the medication, and occur in otherwise healthy individuals. Unpredictable reactions are further classified into drug intolerance, drug idiosyncrasy, drug allergy, and pseudoallergic reactions.8,9
Penicillin allergy can manifest as any hypersensitivity reaction of the Gell and Coombs classification (Table 1).9 Type I (immediate) and type IV (delayed) reactions are the most common types of reactions that occur with antibiotics and should be classified based on the onset of symptoms as immediate (within 1 hour) or delayed (days or weeks).8
Risk factors for IgE-mediated reaction
Risk factors for a hypersensitivity reaction include frequent or repetitive courses of penicillin10 and high-dose parenteral (rather than oral) administration.
Age and atopy are not risk factors for penicillin allergy.7 However, atopy increases the risk of a more severe anaphylactic reaction to penicillin, and anaphylactic reactions are most commonly reported between the ages of 20 and 49.6
Pathophysiology of penicillin allergy
All penicillins share a common core ring structure (beta-lactam and thiazolidine rings) but differ in their side chains (R group) (Figure 1).
Under physiologic conditions, the core ring structure is metabolized into major (penicilloyl) and minor (penicillin itself, penicilloate and penilloate) antigenic determinants that may trigger an immediate IgE-dependent response.9 In the United States, commercial forms of antigenic determinates for skin testing exist in the form of penicillin G (minor determinant) and penicilloyl-polylysine, better known as Prepen (major determinant).
Immediate-type reactions to similar antibiotics such as aminopenicillins and cephalosporins may be caused by IgE antibodies against the R-group side chain rather than the core penicillin major and minor determinants.11
Questions to ask patients who have a history of penicillin allergy
Patients should be questioned closely about previous and current reactions to penicillin and should undergo skin-prick and intradermal testing, followed by graded-dose challenge or drug tolerance desensitization (Figure 2).
Questions to ask patients who have a history of penicillin allergy (Table 2)9,12 include the following:
Do you remember the details of the reaction? These include the route of administration, the time between the dose of penicillin and the appearance of symptoms, and how the reaction was managed.
Immediate reactions (ie, IgE-mediated, or Gell and Coombs type I) usually occur within the first hour after the first dose of the antibiotic, although they occasionally take up to 2 hours to occur, especially if the medication is taken orally and is taken with food. Symptoms consistent with IgE-mediated reactions include urticaria (most common), pruritus, angioedema, laryngeal edema, wheezing, shortness of breath, presyncope or syncope, hypotension, and cardiorespiratory collapse.
In contrast, symptoms of a non–IgE-mediated reaction are delayed in onset, occurring after days of treatment. They include nonpruritic maculopapular eruptions, hemolytic anemia, serum sickness, Stevens-Johnson syndrome, drug rash with eosinophilia and systemic symptoms, acute interstitial nephritis, and toxic epidermal necrolysis.9
If the patient has had severe non–IgE-mediated reactions to penicillin (eg, Stevens-Johnson syndrome, toxic epidermal necrolysis, acute interstitial nephritis, hemolytic anemia, or serum sickness) in the past, skin testing, graded-dose challenge, and desensitization are contraindicated.
How many years ago did the reaction occur? Most patients lose their sensitivity to penicillin over time.7,13–15 Nearly 50% of patients with IgE-mediated penicillin allergy lose their sensitivity within 5 years of the reaction,15 increasing to 80% or more by 10 years.13
How was the reaction managed? What was the outcome? Use of and positive response to epinephrine and histamine 1 receptor antagonists (antihistamines) with resolution or significant improvement of symptoms within a few hours may indicate an IgE-mediated reaction.
What was the indication for penicillin? Many cutaneous reactions are a result of an underlying viral or bacterial infection. For example, up to 90% of patients with Epstein-Barr virus infection develop a maculopapular rash when given penicillin.16
Have you tolerated other forms of penicillin since the reaction? Sometimes the patient has already tolerated other beta-lactams such as aminopenicillins, cephalosporins, and semisynthetic penicillins (piperacillin-tazobactam). Patients who tolerate other beta-lactams without adverse reactions are not allergic to beta-lactams.
Diagnostic tests
Skin testing. The only validated test for diagnosing IgE-mediated reactions caused by penicillin is the immediate hypersensitivity skin test,9 which should be performed by a board-certified allergist. The test consists of skin-prick and intradermal testing with the major determinant (penicilloyl-polylysine), the minor determinant (penicillin G), a negative control (normal saline), and a positive control (histamine). Minor-determinant mix is not commercially available in the United States.
Results of skin-prick testing are read 15 minutes after application. A positive response is a wheal at least 3 mm larger in diameter (with equivalent erythema) than the negative control done simultaneously. Intradermal testing is only done after a negative skin-prick test. If the allergic reaction was severe (ie, anaphylaxis), skin testing should be done at least 4 to 6 weeks after the reaction.
A history of severe non–IgE-mediated reaction to penicillin is a contraindication to skin-prick testing for penicillin allergy. The positive predictive value of penicillin skin testing is 50%, and the negative predictive value is 97%.3,7,9,13
Commercial in vitro testing (serum-specific IgE assays) for IgE-mediated hypersensitivity to penicillin is inferior to skin testing in terms of the negative predictive value and is not a suitable substitute for penicillin skin testing.
MANAGING PENICILLIN ALLERGY
If skin testing is positive, use another antibiotic, or refer for desensitization
If penicillin skin testing is positive (Figure 2), use another antibiotic that is equally efficacious. Patients who absolutely need a beta-lactam may undergo drug desensitization, performed by a board-certified allergist.
During desensitization, patients receive progressively higher doses of the drug every 15 to 20 minutes subcutaneously or intravenously, or every 20 to 30 minutes orally, until a full therapeutic dose is tolerated. Most protocols begin with a dose ranging from 1/10,000 to 1/1,000 of the final dose, depending on the severity of the allergic reaction.9,17
Using modern protocols, the success rate for tolerance induction is extremely high (75% to 100% in patients with cystic fibrosis, a group with a high rate of drug allergy18–20).
Drug desensitization is contraindicated in patients with non–IgE-mediated reactions.
If skin testing is negative, refer for graded-dose challenge
If skin testing is negative (Figure 2), graded-dose challenge is recommended. This procedure must be done by a board-certified allergist. If the original reaction was life-threatening, graded-dose challenge may entail giving 1/100 of the therapeutic dose. Then, if no reaction occurs during a brief observation period (usually 30 minutes), a full dose is given. However, many patients can start with 1/10 or even a full dose of the drug, especially if the original reaction was limited to the skin and the penicillin skin test is negative.
Graded-dose challenge is contraindicated if the original reaction was a severe non–IgE-mediated reaction.
UNDERSTANDING CROSS-REACTIVITY OF PENICILLIN
Penicillin is the only antibiotic for which skin testing is reliable and validated. If a drug that cross-reacts with penicillin is needed, it is important to know the rate of cross-reactivity (Table 3). The rate of cross-reactivity between penicillin and aminopenicillins (amoxicillin and ampicillin) is less than 1.3% in the United States.10,21 However, the cross-reactivity rate among aminopenicillins and cephalosporins is between 10% to 40%. For that reason, patients with prior reactions to aminopenicillins should avoid cephalosporins that share identical R-chain side groups with aminopenicillins.9,22
The rate of cross-reactivity between penicillin and cephalosporins was reported as 10% 40 years ago.23,24 But this was with early, first-generation cephalosporins that may have been contaminated with penicillin. The cross-reactivity rate with cephalosporins today is 3%.25 In general, first- and second-generation cephalosporins cause more allergic reactions than third- and fourth-generation cephalosporins.26
Patients with a history of penicillin allergy who require a cephalosporin should still undergo penicillin skin testing. Skin testing with cephalosporins has not been validated. However, skin testing with nonirritating concentrations of cephalosporins9 may be done to elucidate IgE reactions.
In a study by Romano et al,27 110 patients who had positive results on penicillin skin testing completed graded-dose challenge with the carbapenem antibiotic imipenem. The rate of cross-reactivity between penicillin and imipenem was less than 1%.
Monobactam antibiotics do not cross-react with other beta-lactams, except ceftazidime with aztreonam. This is probably because of similarities in their chemical structure.
Most patients who report that they are allergic to penicillin can ultimately receive penicillin or a penicillin-type antibiotic again after an appropriate evaluation and, possibly, treatment. This course of action decreases the need for broad-spectrum antibiotics,1–4 reduces health care costs, and prevents the development of multidrug-resistant pathogens.5
About 10% of the general population say that they are allergic to penicillin.1,6,7 Although the prevalence of life-threatening anaphylactic reactions to penicillin has been estimated to be between 0.02% and 0.04%,6 the most common reaction is a cutaneous eruption. Since anaphylactic reactions are mediated by immunoglobulin E (IgE), evaluation of patients with a history of penicillin allergy by penicillin skin testing is recommended to rule out IgE-mediated reactions.
This review outlines a practical approach to evaluating a suspected IgE-mediated reaction to penicillin, with key points in the history and diagnostic testing. We also review subsequent management and cross-reactivity with other beta-lactam-containing antibiotics.
EVALUATING ALLERGIC PATIENTS
Evaluation of patients with a history of penicillin allergy can be improved with an understanding of the classification of drug reactions, risk factors for allergy, and the pathophysiology of penicillin allergy.
Classification of drug reactions
Adverse drug reactions include all unintended pharmacologic effects of a drug and can be classified as predictable (type A) or unpredictable (type B). Predictable reactions are dose-dependent, are related to the known pharmacologic actions of the medication, and occur in otherwise healthy individuals. Unpredictable reactions are further classified into drug intolerance, drug idiosyncrasy, drug allergy, and pseudoallergic reactions.8,9
Penicillin allergy can manifest as any hypersensitivity reaction of the Gell and Coombs classification (Table 1).9 Type I (immediate) and type IV (delayed) reactions are the most common types of reactions that occur with antibiotics and should be classified based on the onset of symptoms as immediate (within 1 hour) or delayed (days or weeks).8
Risk factors for IgE-mediated reaction
Risk factors for a hypersensitivity reaction include frequent or repetitive courses of penicillin10 and high-dose parenteral (rather than oral) administration.
Age and atopy are not risk factors for penicillin allergy.7 However, atopy increases the risk of a more severe anaphylactic reaction to penicillin, and anaphylactic reactions are most commonly reported between the ages of 20 and 49.6
Pathophysiology of penicillin allergy
All penicillins share a common core ring structure (beta-lactam and thiazolidine rings) but differ in their side chains (R group) (Figure 1).
Under physiologic conditions, the core ring structure is metabolized into major (penicilloyl) and minor (penicillin itself, penicilloate and penilloate) antigenic determinants that may trigger an immediate IgE-dependent response.9 In the United States, commercial forms of antigenic determinates for skin testing exist in the form of penicillin G (minor determinant) and penicilloyl-polylysine, better known as Prepen (major determinant).
Immediate-type reactions to similar antibiotics such as aminopenicillins and cephalosporins may be caused by IgE antibodies against the R-group side chain rather than the core penicillin major and minor determinants.11
Questions to ask patients who have a history of penicillin allergy
Patients should be questioned closely about previous and current reactions to penicillin and should undergo skin-prick and intradermal testing, followed by graded-dose challenge or drug tolerance desensitization (Figure 2).
Questions to ask patients who have a history of penicillin allergy (Table 2)9,12 include the following:
Do you remember the details of the reaction? These include the route of administration, the time between the dose of penicillin and the appearance of symptoms, and how the reaction was managed.
Immediate reactions (ie, IgE-mediated, or Gell and Coombs type I) usually occur within the first hour after the first dose of the antibiotic, although they occasionally take up to 2 hours to occur, especially if the medication is taken orally and is taken with food. Symptoms consistent with IgE-mediated reactions include urticaria (most common), pruritus, angioedema, laryngeal edema, wheezing, shortness of breath, presyncope or syncope, hypotension, and cardiorespiratory collapse.
In contrast, symptoms of a non–IgE-mediated reaction are delayed in onset, occurring after days of treatment. They include nonpruritic maculopapular eruptions, hemolytic anemia, serum sickness, Stevens-Johnson syndrome, drug rash with eosinophilia and systemic symptoms, acute interstitial nephritis, and toxic epidermal necrolysis.9
If the patient has had severe non–IgE-mediated reactions to penicillin (eg, Stevens-Johnson syndrome, toxic epidermal necrolysis, acute interstitial nephritis, hemolytic anemia, or serum sickness) in the past, skin testing, graded-dose challenge, and desensitization are contraindicated.
How many years ago did the reaction occur? Most patients lose their sensitivity to penicillin over time.7,13–15 Nearly 50% of patients with IgE-mediated penicillin allergy lose their sensitivity within 5 years of the reaction,15 increasing to 80% or more by 10 years.13
How was the reaction managed? What was the outcome? Use of and positive response to epinephrine and histamine 1 receptor antagonists (antihistamines) with resolution or significant improvement of symptoms within a few hours may indicate an IgE-mediated reaction.
What was the indication for penicillin? Many cutaneous reactions are a result of an underlying viral or bacterial infection. For example, up to 90% of patients with Epstein-Barr virus infection develop a maculopapular rash when given penicillin.16
Have you tolerated other forms of penicillin since the reaction? Sometimes the patient has already tolerated other beta-lactams such as aminopenicillins, cephalosporins, and semisynthetic penicillins (piperacillin-tazobactam). Patients who tolerate other beta-lactams without adverse reactions are not allergic to beta-lactams.
Diagnostic tests
Skin testing. The only validated test for diagnosing IgE-mediated reactions caused by penicillin is the immediate hypersensitivity skin test,9 which should be performed by a board-certified allergist. The test consists of skin-prick and intradermal testing with the major determinant (penicilloyl-polylysine), the minor determinant (penicillin G), a negative control (normal saline), and a positive control (histamine). Minor-determinant mix is not commercially available in the United States.
Results of skin-prick testing are read 15 minutes after application. A positive response is a wheal at least 3 mm larger in diameter (with equivalent erythema) than the negative control done simultaneously. Intradermal testing is only done after a negative skin-prick test. If the allergic reaction was severe (ie, anaphylaxis), skin testing should be done at least 4 to 6 weeks after the reaction.
A history of severe non–IgE-mediated reaction to penicillin is a contraindication to skin-prick testing for penicillin allergy. The positive predictive value of penicillin skin testing is 50%, and the negative predictive value is 97%.3,7,9,13
Commercial in vitro testing (serum-specific IgE assays) for IgE-mediated hypersensitivity to penicillin is inferior to skin testing in terms of the negative predictive value and is not a suitable substitute for penicillin skin testing.
MANAGING PENICILLIN ALLERGY
If skin testing is positive, use another antibiotic, or refer for desensitization
If penicillin skin testing is positive (Figure 2), use another antibiotic that is equally efficacious. Patients who absolutely need a beta-lactam may undergo drug desensitization, performed by a board-certified allergist.
During desensitization, patients receive progressively higher doses of the drug every 15 to 20 minutes subcutaneously or intravenously, or every 20 to 30 minutes orally, until a full therapeutic dose is tolerated. Most protocols begin with a dose ranging from 1/10,000 to 1/1,000 of the final dose, depending on the severity of the allergic reaction.9,17
Using modern protocols, the success rate for tolerance induction is extremely high (75% to 100% in patients with cystic fibrosis, a group with a high rate of drug allergy18–20).
Drug desensitization is contraindicated in patients with non–IgE-mediated reactions.
If skin testing is negative, refer for graded-dose challenge
If skin testing is negative (Figure 2), graded-dose challenge is recommended. This procedure must be done by a board-certified allergist. If the original reaction was life-threatening, graded-dose challenge may entail giving 1/100 of the therapeutic dose. Then, if no reaction occurs during a brief observation period (usually 30 minutes), a full dose is given. However, many patients can start with 1/10 or even a full dose of the drug, especially if the original reaction was limited to the skin and the penicillin skin test is negative.
Graded-dose challenge is contraindicated if the original reaction was a severe non–IgE-mediated reaction.
UNDERSTANDING CROSS-REACTIVITY OF PENICILLIN
Penicillin is the only antibiotic for which skin testing is reliable and validated. If a drug that cross-reacts with penicillin is needed, it is important to know the rate of cross-reactivity (Table 3). The rate of cross-reactivity between penicillin and aminopenicillins (amoxicillin and ampicillin) is less than 1.3% in the United States.10,21 However, the cross-reactivity rate among aminopenicillins and cephalosporins is between 10% to 40%. For that reason, patients with prior reactions to aminopenicillins should avoid cephalosporins that share identical R-chain side groups with aminopenicillins.9,22
The rate of cross-reactivity between penicillin and cephalosporins was reported as 10% 40 years ago.23,24 But this was with early, first-generation cephalosporins that may have been contaminated with penicillin. The cross-reactivity rate with cephalosporins today is 3%.25 In general, first- and second-generation cephalosporins cause more allergic reactions than third- and fourth-generation cephalosporins.26
Patients with a history of penicillin allergy who require a cephalosporin should still undergo penicillin skin testing. Skin testing with cephalosporins has not been validated. However, skin testing with nonirritating concentrations of cephalosporins9 may be done to elucidate IgE reactions.
In a study by Romano et al,27 110 patients who had positive results on penicillin skin testing completed graded-dose challenge with the carbapenem antibiotic imipenem. The rate of cross-reactivity between penicillin and imipenem was less than 1%.
Monobactam antibiotics do not cross-react with other beta-lactams, except ceftazidime with aztreonam. This is probably because of similarities in their chemical structure.
- Park M, Markus P, Matesic D, Li JT. Safety and effectiveness of a preoperative allergy clinic in decreasing vancomycin use in patients with a history of penicillin allergy. Ann Allergy Asthma Immunol 2006; 97:681–687.
- Arroliga ME, Radojicic C, Gordon SM, et al. A prospective observational study of the effect of penicillin skin testing on antibiotic use in the intensive care unit. Infect Control Hosp Epidemiol 2003; 24:347–350.
- del Real GA, Rose ME, Ramirez-Atamoros MT, et al. Penicillin skin testing in patients with a history of beta-lactam allergy. Ann Allergy Asthma Immunol 2007; 98:355–359.
- Nadarajah K, Green GR, Naglak M. Clinical outcomes of penicillin skin testing. Ann Allergy Asthma Immunol 2005; 95:541–545.
- Harris AD, Sauberman L, Kabbash L, Greineder DK, Samore MH. Penicillin skin testing: a way to optimize antibiotic utilization. Am J Med 1999; 107:166–168.
- Idsoe O, Guthe T, Willcox RR, de Weck AL. Nature and extent of penicillin side-reactions, with particular reference to fatalities from anaphylactic shock. Bull World Health Organ 1968; 38:159–188.
- Gadde J, Spence M, Wheeler B, Adkinson NF Jr. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA 1993; 270:2456–2463.
- Johansson SG, Bieber T, Dahl R, et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol 2004; 113:832–836.
- Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol 2010; 105:259–273.
- Park MA, Matesic D, Markus PJ, Li JT. Female sex as a risk factor for penicillin allergy. Ann Allergy Asthma Immunol 2007; 99:54–58.
- Moreno E, Macias E, Davila I, Laffond E, Ruiz A, Lorente F. Hypersensitivity reactions to cephalosporins. Expert Opin Drug Saf 2008; 7:295–304.
- Salkind AR, Cuddy PG, Foxworth JW. The rational clinical examination. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA 2001; 285:2498–2505.
- Sullivan TJ, Wedner HJ, Shatz GS, Yecies LD, Parker CW. Skin testing to detect penicillin allergy. J Allergy Clin Immunol 1981; 68:171–180.
- Macy E, Schatz M, Lin C, Poon KY. The falling rate of positive penicillin skin tests from 1995 to 2007. Perm J 2009; 13:12–18.
- Blanca M, Torres MJ, Garcia JJ, et al. Natural evolution of skin test sensitivity in patients allergic to beta-lactam antibiotics. J Allergy Clin Immunol 1999; 103:918–924.
- Patel BM. Skin rash with infectious mononucleosis and ampicillin. Pediatrics 1967; 40:910–911.
- Liu A, Fanning L, Chong H, et al. Desensitization regimens for drug allergy: state of the art in the 21st century. Clin Exp Allergy 2011; 41:1679–1688.
- Burrows JA, Toon M, Bell SC. Antibiotic desensitization in adults with cystic fibrosis. Respirology 2003; 8:359–364.
- Turvey SE, Cronin B, Arnold AD, Dioun AF. Antibiotic desensitization for the allergic patient: 5 years of experience and practice. Ann Allergy Asthma Immunol 2004; 92:426–432.
- Legere HJ 3rd, Palis RI, Rodriguez Bouza T, Uluer AZ, Castells MC. A safe protocol for rapid desensitization in patients with cystic fibrosis and antibiotic hypersensitivity. J Cyst Fibros 2009; 8:418–424.
- Lin E, Saxon A, Riedl M. Penicillin allergy: value of including amoxicillin as a determinant in penicillin skin testing. Int Arch Allergy Immunol 2010; 152:313–318.
- Dickson SD, Salazar KC. Diagnosis and management of immediate hypersensitivity reactions to cephalosporins. Clin Rev Allergy Immunol 2013; 45:131–142.
- Dash CH. Penicillin allergy and the cephalosporins. J Antimicrob Chemother 1975; 1(suppl 3):107–118.
- Petz LD. Immunologic cross-reactivity between penicillins and cephalosporins: a review. J Infect Dis 1978; 137(suppl):S74–S79.
- American Academy of Allergy Asthma & Immunology. Cephalosporin administration to patients with a history of penicillin allergy. www.aaaai.org/Aaaai/media/MediaLibrary/PDF%20Documents/Practice%20and%20Parameters/Cephalosporin-administration-2009.pdf. Accessed April 2, 2015.
- Fonacier L, Hirschberg R, Gerson S. Adverse drug reactions to cephalosporins in hospitalized patients with a history of penicillin allergy. Allergy Asthma Proc 2005; 26:135–141.
- Romano A, Viola M, Gueant-Rodriguez RM, Gaeta F, Pettinato R, Gueant JL. Imipenem in patients with immediate hypersensitivity to penicillins. N Engl J Med 2006; 354:2835–2837.
- Park M, Markus P, Matesic D, Li JT. Safety and effectiveness of a preoperative allergy clinic in decreasing vancomycin use in patients with a history of penicillin allergy. Ann Allergy Asthma Immunol 2006; 97:681–687.
- Arroliga ME, Radojicic C, Gordon SM, et al. A prospective observational study of the effect of penicillin skin testing on antibiotic use in the intensive care unit. Infect Control Hosp Epidemiol 2003; 24:347–350.
- del Real GA, Rose ME, Ramirez-Atamoros MT, et al. Penicillin skin testing in patients with a history of beta-lactam allergy. Ann Allergy Asthma Immunol 2007; 98:355–359.
- Nadarajah K, Green GR, Naglak M. Clinical outcomes of penicillin skin testing. Ann Allergy Asthma Immunol 2005; 95:541–545.
- Harris AD, Sauberman L, Kabbash L, Greineder DK, Samore MH. Penicillin skin testing: a way to optimize antibiotic utilization. Am J Med 1999; 107:166–168.
- Idsoe O, Guthe T, Willcox RR, de Weck AL. Nature and extent of penicillin side-reactions, with particular reference to fatalities from anaphylactic shock. Bull World Health Organ 1968; 38:159–188.
- Gadde J, Spence M, Wheeler B, Adkinson NF Jr. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA 1993; 270:2456–2463.
- Johansson SG, Bieber T, Dahl R, et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol 2004; 113:832–836.
- Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol 2010; 105:259–273.
- Park MA, Matesic D, Markus PJ, Li JT. Female sex as a risk factor for penicillin allergy. Ann Allergy Asthma Immunol 2007; 99:54–58.
- Moreno E, Macias E, Davila I, Laffond E, Ruiz A, Lorente F. Hypersensitivity reactions to cephalosporins. Expert Opin Drug Saf 2008; 7:295–304.
- Salkind AR, Cuddy PG, Foxworth JW. The rational clinical examination. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA 2001; 285:2498–2505.
- Sullivan TJ, Wedner HJ, Shatz GS, Yecies LD, Parker CW. Skin testing to detect penicillin allergy. J Allergy Clin Immunol 1981; 68:171–180.
- Macy E, Schatz M, Lin C, Poon KY. The falling rate of positive penicillin skin tests from 1995 to 2007. Perm J 2009; 13:12–18.
- Blanca M, Torres MJ, Garcia JJ, et al. Natural evolution of skin test sensitivity in patients allergic to beta-lactam antibiotics. J Allergy Clin Immunol 1999; 103:918–924.
- Patel BM. Skin rash with infectious mononucleosis and ampicillin. Pediatrics 1967; 40:910–911.
- Liu A, Fanning L, Chong H, et al. Desensitization regimens for drug allergy: state of the art in the 21st century. Clin Exp Allergy 2011; 41:1679–1688.
- Burrows JA, Toon M, Bell SC. Antibiotic desensitization in adults with cystic fibrosis. Respirology 2003; 8:359–364.
- Turvey SE, Cronin B, Arnold AD, Dioun AF. Antibiotic desensitization for the allergic patient: 5 years of experience and practice. Ann Allergy Asthma Immunol 2004; 92:426–432.
- Legere HJ 3rd, Palis RI, Rodriguez Bouza T, Uluer AZ, Castells MC. A safe protocol for rapid desensitization in patients with cystic fibrosis and antibiotic hypersensitivity. J Cyst Fibros 2009; 8:418–424.
- Lin E, Saxon A, Riedl M. Penicillin allergy: value of including amoxicillin as a determinant in penicillin skin testing. Int Arch Allergy Immunol 2010; 152:313–318.
- Dickson SD, Salazar KC. Diagnosis and management of immediate hypersensitivity reactions to cephalosporins. Clin Rev Allergy Immunol 2013; 45:131–142.
- Dash CH. Penicillin allergy and the cephalosporins. J Antimicrob Chemother 1975; 1(suppl 3):107–118.
- Petz LD. Immunologic cross-reactivity between penicillins and cephalosporins: a review. J Infect Dis 1978; 137(suppl):S74–S79.
- American Academy of Allergy Asthma & Immunology. Cephalosporin administration to patients with a history of penicillin allergy. www.aaaai.org/Aaaai/media/MediaLibrary/PDF%20Documents/Practice%20and%20Parameters/Cephalosporin-administration-2009.pdf. Accessed April 2, 2015.
- Fonacier L, Hirschberg R, Gerson S. Adverse drug reactions to cephalosporins in hospitalized patients with a history of penicillin allergy. Allergy Asthma Proc 2005; 26:135–141.
- Romano A, Viola M, Gueant-Rodriguez RM, Gaeta F, Pettinato R, Gueant JL. Imipenem in patients with immediate hypersensitivity to penicillins. N Engl J Med 2006; 354:2835–2837.
KEY POINTS
- The prevalence of reported penicillin allergy is 10% in the general population. However, more than 90% of these patients are found not to be allergic to penicillin after skin testing.
- In patients found to have penicillin allergy, the frequency of positive results on skin testing decreases by 10% per year of avoidance. Therefore, 80% to 100% of patients are expected to test negative for penicillin allergy by 10 years after their reaction.
- Skin testing for penicillin allergy is only useful for type 1 IgE-mediated reactions. However, in properly selected patients, the negative predictive value of penicillin skin testing is nearly 97%.
- The rate of cross-reactivity between penicillin and cephalosporins is approximately 3%.
Clinical utility of warfarin pharmacogenomics
To the Editor: We previously addressed whether VKORC1 and CYP2C9 pharmacogenomic testing should be considered when prescribing warfarin.1 Our recommendation, based on available evidence at that time, was that physicians should consider pharmacogenomic testing for any patient who is started on warfarin therapy.
Since the publication of this recommendation, two major trials, COAG (Clarification of Optimal Anticoagulation Through Genetics)2 and EU-PACT (European Pharmacogenetics of Anticoagulant Therapy-Warfarin),3 were published along with commentaries debating the clinical utility of warfarin pharmacogenomics.4–15 Based on these publications, we would like to update our recommendations for pharmacogenomic testing for warfarin therapy.
COAG compared the efficacy of a clinical algorithm or a clinical algorithm plus VKORC1 and CYP2C9 genotyping to guide warfarin dosage. At the end of 4 weeks, the mean percentage of time within the therapeutic international normalized ratio (INR) range was 45.4% for those in the clinical algorithm arm and 45.2% for those in the genotyping arm (95% confidence interval [CI] –3.4 to 3.1, P = .91). For both treatment groups, clinical data that included body surface area, age, target INR, concomitantly prescribed drugs, and smoking status were used to predict warfarin dose, with the genotyping arm including VKORC1 and CYP2C9. Although VKORC1 and CYP2C9 genotyping offered no additional benefit, caution should be used when extrapolating this conclusion to clinical settings in which warfarin therapy is initiated using a standardized starting dose (eg, 5 mg daily) instead of a clinical dosing algorithm.
Of interest, in the COAG trial, among black patients, the mean percentage of time in the therapeutic INR range was significantly less for those in the genotype-guided arm than for those in the clinically guided arm—ie, 35.2% vs 43.5% (95% CI –15.0 to –2.0, P = .01). The percentage of time with therapeutic INR has been identified as a surrogate marker for poor outcomes such as death, stroke, or major hemorrhage, with those with a lower percentage of time in therapeutic INR being at greater risk of an adverse event.16 Wan et al17 demonstrated that a 6.9% improvement of time in therapeutic INR decreased the risk of major hemorrhage by one event per 100 patient-years.17 Therefore, black patients in the COAG genotyping arm may have been at greater risk for an adverse event because of a lower observed percentage of time within the therapeutic INR range.
In the COAG trial, genotyping was done for only one VKORC1 variant and for two CYP2C9 alleles (CYP2C9*2, and CYP2C9*3). Other genetic variants are of clinical importance for warfarin dosing in black patients, and the lack of genotyping for these additional variants may explain why black patients in the genotyping arm performed worse.5,7,11 In particular, CYP2C9*8 may be an important predictor of warfarin dose in black patients.18
EU-PACT compared the efficacy of standardized warfarin dosing and that of a clinical algorithm.3 Patients in the standardized dosing arm were prescribed warfarin 10 mg on the first day of treatment (5 mg for those over age 75), and 5 mg on days 2 and 3, with subsequent dosing adjustments based on INR. Patients in the genotyping arm were prescribed warfarin based on an algorithm that incorporated clinical data that included body surface area, age, and concomitantly prescribed drugs, as well as VKORC1 and CYP2C9 genotypes. At the end of 12 weeks, the mean percentage of time in the therapeutic INR range was 60.3% for those in the standardized-dosing arm and 67.4% for those in the genotyping arm (95% CI 3.3 to 10.6, P < .001).2 The approximate 7% improvement in percentage of time in the therapeutic INR range may predict a lower risk of hemorrhage for those in the genotyping arm.17 Although patients in the genotyping arm had a higher percentage of time in the therapeutic INR range, it is unclear whether genotyping alone is superior to standardized dosing because the dosing algorithm used both clinical data and genotype data.
There are substantial differences between the COAG and EU-PACT trials, including dosing schemes, racial diversity, and trial length, and these differences could have contributed to the conflicting results. Based on these two trials, a possible conclusion is that genotype-guided warfarin dosing may be superior to standardized dosing, but may be no better than utilizing a clinical algorithm in white patients. For black patients, additional studies are needed to determine which genetic variants are of importance for guiding warfarin dosing.
We would like to update the recommendations we made in our previously published article,1 to state that genotyping for CYP2C9 and VKORC1 may be of clinical utility in white patients depending on whether standardized dosing or a clinical algorithm is used to initiate warfarin therapy. Routine genotyping in black patients is not recommended until further studies clarify which genetic variants are of importance for guiding warfarin dosing.
The ongoing Genetics Informatics Trial of Warfarin to Prevent Venous Thrombosis may bring much needed clarity to the clinical utility of warfarin pharmacogenomics. We hope to publish a more detailed update of our 2013 article after completion of that trial.
- Rouse M, Cristiani C, Teng KA. Should we use pharmacogenetic testing when prescribing warfarin? Cleve Clin J Med 2013; 80:483–486.
- Kimmel SE, French B, Kasner SE, et al; COAG Investigators. A pharmacogenetic versus a clinical algorithm for warfarin dosing. N Engl J Med 2013; 369:2283–2293.
- Pirmohamed M, Burnside G, Eriksson N, et al; EU-PACT Group. A randomized trial of genotype-guided dosing of warfarin. N Engl J Med 2013; 369:2294–2303.
- Cavallari LH, Kittles RA, Perera MA. Genotype-guided dosing of vitamin K antagonists. N Engl J Med 2014; 370:1763.
- Cavallari LH, Nutescu EA. Warfarin pharmacogenetics: to genotype or not to genotype, that is the question. Clin Pharmacol Ther 2014; 96:22–24.
- Daneshjou R, Klein TE, Altman RB. Genotype-guided dosing of vitamin K antagonists. N Engl J Med 2014; 370:1762–1763.
- Hernandez W, Gamazon ER, Aquino-Michaels K, et al. Ethnicity-specific pharmacogenetics: the case of warfarin in African Americans. Pharmacogenomics J 2014; 14:223–228.
- Kimmel SE, French B, Geller NL; COAG Investigators. Genotype-guided dosing of vitamin K antagonists. N Engl J Med 2014; 370:1763–1764.
- Koller EA, Roche JC, Rollins JA. Genotype-guided dosing of vitamin K antagonists. N Engl J Med 2014; 370:1761.
- Pereira NL, Rihal CS, Weinshilboum RM. Genotype-guided dosing of vitamin K antagonists. N Engl J Med 2014; 370:1762.
- Perera MA, Cavallari LH, Johnson JA. Warfarin pharmacogenetics: an illustration of the importance of studies in minority populations. Clin Pharmacol Ther 2014; 95:242–244.
- Pirmohamed M, Wadelius M, Kamali F; EU-PACT Group. Genotype-guided dosing of vitamin K antagonists. N Engl J Med 2014; 370:1764–1765.
- Schwarz UI, Kim RB, Tirona RG. Genotype-guided dosing of vitamin K antagonists. N Engl J Med 2014; 370:1761–1762.
- Scott SA, Lubitz SA. Warfarin pharmacogenetic trials: is there a future for pharmacogenetic-guided dosing? Pharmacogenomics 2014; 15:719–722.
- Zineh I, Pacanowski M, Woodcock J. Pharmacogenetics and coumarin dosing—recalibrating expectations. N Engl J Med 2013; 369:2273–2275.
- Hylek EM. Vitamin K antagonists and time in the therapeutic range: implications, challenges, and strategies for improvement. J Thromb Thrombolysis 2013; 35:333–335.
- Wan Y, Heneghan C, Perera R, et al. Anticoagulation control and prediction of adverse events in patients with atrial fibrillation: a systematic review. Circ Cardiovasc Qual Outcomes 2008;1:84-91.
- Nagai R, Ohara M, Cavallari LH, et al. Factors influencing pharmacokinetics of warfarin in African-Americans: implications for pharmacogenetic dosing algorithms. Pharmacogenomics 2015;16:217–225.
To the Editor: We previously addressed whether VKORC1 and CYP2C9 pharmacogenomic testing should be considered when prescribing warfarin.1 Our recommendation, based on available evidence at that time, was that physicians should consider pharmacogenomic testing for any patient who is started on warfarin therapy.
Since the publication of this recommendation, two major trials, COAG (Clarification of Optimal Anticoagulation Through Genetics)2 and EU-PACT (European Pharmacogenetics of Anticoagulant Therapy-Warfarin),3 were published along with commentaries debating the clinical utility of warfarin pharmacogenomics.4–15 Based on these publications, we would like to update our recommendations for pharmacogenomic testing for warfarin therapy.
COAG compared the efficacy of a clinical algorithm or a clinical algorithm plus VKORC1 and CYP2C9 genotyping to guide warfarin dosage. At the end of 4 weeks, the mean percentage of time within the therapeutic international normalized ratio (INR) range was 45.4% for those in the clinical algorithm arm and 45.2% for those in the genotyping arm (95% confidence interval [CI] –3.4 to 3.1, P = .91). For both treatment groups, clinical data that included body surface area, age, target INR, concomitantly prescribed drugs, and smoking status were used to predict warfarin dose, with the genotyping arm including VKORC1 and CYP2C9. Although VKORC1 and CYP2C9 genotyping offered no additional benefit, caution should be used when extrapolating this conclusion to clinical settings in which warfarin therapy is initiated using a standardized starting dose (eg, 5 mg daily) instead of a clinical dosing algorithm.
Of interest, in the COAG trial, among black patients, the mean percentage of time in the therapeutic INR range was significantly less for those in the genotype-guided arm than for those in the clinically guided arm—ie, 35.2% vs 43.5% (95% CI –15.0 to –2.0, P = .01). The percentage of time with therapeutic INR has been identified as a surrogate marker for poor outcomes such as death, stroke, or major hemorrhage, with those with a lower percentage of time in therapeutic INR being at greater risk of an adverse event.16 Wan et al17 demonstrated that a 6.9% improvement of time in therapeutic INR decreased the risk of major hemorrhage by one event per 100 patient-years.17 Therefore, black patients in the COAG genotyping arm may have been at greater risk for an adverse event because of a lower observed percentage of time within the therapeutic INR range.
In the COAG trial, genotyping was done for only one VKORC1 variant and for two CYP2C9 alleles (CYP2C9*2, and CYP2C9*3). Other genetic variants are of clinical importance for warfarin dosing in black patients, and the lack of genotyping for these additional variants may explain why black patients in the genotyping arm performed worse.5,7,11 In particular, CYP2C9*8 may be an important predictor of warfarin dose in black patients.18
EU-PACT compared the efficacy of standardized warfarin dosing and that of a clinical algorithm.3 Patients in the standardized dosing arm were prescribed warfarin 10 mg on the first day of treatment (5 mg for those over age 75), and 5 mg on days 2 and 3, with subsequent dosing adjustments based on INR. Patients in the genotyping arm were prescribed warfarin based on an algorithm that incorporated clinical data that included body surface area, age, and concomitantly prescribed drugs, as well as VKORC1 and CYP2C9 genotypes. At the end of 12 weeks, the mean percentage of time in the therapeutic INR range was 60.3% for those in the standardized-dosing arm and 67.4% for those in the genotyping arm (95% CI 3.3 to 10.6, P < .001).2 The approximate 7% improvement in percentage of time in the therapeutic INR range may predict a lower risk of hemorrhage for those in the genotyping arm.17 Although patients in the genotyping arm had a higher percentage of time in the therapeutic INR range, it is unclear whether genotyping alone is superior to standardized dosing because the dosing algorithm used both clinical data and genotype data.
There are substantial differences between the COAG and EU-PACT trials, including dosing schemes, racial diversity, and trial length, and these differences could have contributed to the conflicting results. Based on these two trials, a possible conclusion is that genotype-guided warfarin dosing may be superior to standardized dosing, but may be no better than utilizing a clinical algorithm in white patients. For black patients, additional studies are needed to determine which genetic variants are of importance for guiding warfarin dosing.
We would like to update the recommendations we made in our previously published article,1 to state that genotyping for CYP2C9 and VKORC1 may be of clinical utility in white patients depending on whether standardized dosing or a clinical algorithm is used to initiate warfarin therapy. Routine genotyping in black patients is not recommended until further studies clarify which genetic variants are of importance for guiding warfarin dosing.
The ongoing Genetics Informatics Trial of Warfarin to Prevent Venous Thrombosis may bring much needed clarity to the clinical utility of warfarin pharmacogenomics. We hope to publish a more detailed update of our 2013 article after completion of that trial.
To the Editor: We previously addressed whether VKORC1 and CYP2C9 pharmacogenomic testing should be considered when prescribing warfarin.1 Our recommendation, based on available evidence at that time, was that physicians should consider pharmacogenomic testing for any patient who is started on warfarin therapy.
Since the publication of this recommendation, two major trials, COAG (Clarification of Optimal Anticoagulation Through Genetics)2 and EU-PACT (European Pharmacogenetics of Anticoagulant Therapy-Warfarin),3 were published along with commentaries debating the clinical utility of warfarin pharmacogenomics.4–15 Based on these publications, we would like to update our recommendations for pharmacogenomic testing for warfarin therapy.
COAG compared the efficacy of a clinical algorithm or a clinical algorithm plus VKORC1 and CYP2C9 genotyping to guide warfarin dosage. At the end of 4 weeks, the mean percentage of time within the therapeutic international normalized ratio (INR) range was 45.4% for those in the clinical algorithm arm and 45.2% for those in the genotyping arm (95% confidence interval [CI] –3.4 to 3.1, P = .91). For both treatment groups, clinical data that included body surface area, age, target INR, concomitantly prescribed drugs, and smoking status were used to predict warfarin dose, with the genotyping arm including VKORC1 and CYP2C9. Although VKORC1 and CYP2C9 genotyping offered no additional benefit, caution should be used when extrapolating this conclusion to clinical settings in which warfarin therapy is initiated using a standardized starting dose (eg, 5 mg daily) instead of a clinical dosing algorithm.
Of interest, in the COAG trial, among black patients, the mean percentage of time in the therapeutic INR range was significantly less for those in the genotype-guided arm than for those in the clinically guided arm—ie, 35.2% vs 43.5% (95% CI –15.0 to –2.0, P = .01). The percentage of time with therapeutic INR has been identified as a surrogate marker for poor outcomes such as death, stroke, or major hemorrhage, with those with a lower percentage of time in therapeutic INR being at greater risk of an adverse event.16 Wan et al17 demonstrated that a 6.9% improvement of time in therapeutic INR decreased the risk of major hemorrhage by one event per 100 patient-years.17 Therefore, black patients in the COAG genotyping arm may have been at greater risk for an adverse event because of a lower observed percentage of time within the therapeutic INR range.
In the COAG trial, genotyping was done for only one VKORC1 variant and for two CYP2C9 alleles (CYP2C9*2, and CYP2C9*3). Other genetic variants are of clinical importance for warfarin dosing in black patients, and the lack of genotyping for these additional variants may explain why black patients in the genotyping arm performed worse.5,7,11 In particular, CYP2C9*8 may be an important predictor of warfarin dose in black patients.18
EU-PACT compared the efficacy of standardized warfarin dosing and that of a clinical algorithm.3 Patients in the standardized dosing arm were prescribed warfarin 10 mg on the first day of treatment (5 mg for those over age 75), and 5 mg on days 2 and 3, with subsequent dosing adjustments based on INR. Patients in the genotyping arm were prescribed warfarin based on an algorithm that incorporated clinical data that included body surface area, age, and concomitantly prescribed drugs, as well as VKORC1 and CYP2C9 genotypes. At the end of 12 weeks, the mean percentage of time in the therapeutic INR range was 60.3% for those in the standardized-dosing arm and 67.4% for those in the genotyping arm (95% CI 3.3 to 10.6, P < .001).2 The approximate 7% improvement in percentage of time in the therapeutic INR range may predict a lower risk of hemorrhage for those in the genotyping arm.17 Although patients in the genotyping arm had a higher percentage of time in the therapeutic INR range, it is unclear whether genotyping alone is superior to standardized dosing because the dosing algorithm used both clinical data and genotype data.
There are substantial differences between the COAG and EU-PACT trials, including dosing schemes, racial diversity, and trial length, and these differences could have contributed to the conflicting results. Based on these two trials, a possible conclusion is that genotype-guided warfarin dosing may be superior to standardized dosing, but may be no better than utilizing a clinical algorithm in white patients. For black patients, additional studies are needed to determine which genetic variants are of importance for guiding warfarin dosing.
We would like to update the recommendations we made in our previously published article,1 to state that genotyping for CYP2C9 and VKORC1 may be of clinical utility in white patients depending on whether standardized dosing or a clinical algorithm is used to initiate warfarin therapy. Routine genotyping in black patients is not recommended until further studies clarify which genetic variants are of importance for guiding warfarin dosing.
The ongoing Genetics Informatics Trial of Warfarin to Prevent Venous Thrombosis may bring much needed clarity to the clinical utility of warfarin pharmacogenomics. We hope to publish a more detailed update of our 2013 article after completion of that trial.
- Rouse M, Cristiani C, Teng KA. Should we use pharmacogenetic testing when prescribing warfarin? Cleve Clin J Med 2013; 80:483–486.
- Kimmel SE, French B, Kasner SE, et al; COAG Investigators. A pharmacogenetic versus a clinical algorithm for warfarin dosing. N Engl J Med 2013; 369:2283–2293.
- Pirmohamed M, Burnside G, Eriksson N, et al; EU-PACT Group. A randomized trial of genotype-guided dosing of warfarin. N Engl J Med 2013; 369:2294–2303.
- Cavallari LH, Kittles RA, Perera MA. Genotype-guided dosing of vitamin K antagonists. N Engl J Med 2014; 370:1763.
- Cavallari LH, Nutescu EA. Warfarin pharmacogenetics: to genotype or not to genotype, that is the question. Clin Pharmacol Ther 2014; 96:22–24.
- Daneshjou R, Klein TE, Altman RB. Genotype-guided dosing of vitamin K antagonists. N Engl J Med 2014; 370:1762–1763.
- Hernandez W, Gamazon ER, Aquino-Michaels K, et al. Ethnicity-specific pharmacogenetics: the case of warfarin in African Americans. Pharmacogenomics J 2014; 14:223–228.
- Kimmel SE, French B, Geller NL; COAG Investigators. Genotype-guided dosing of vitamin K antagonists. N Engl J Med 2014; 370:1763–1764.
- Koller EA, Roche JC, Rollins JA. Genotype-guided dosing of vitamin K antagonists. N Engl J Med 2014; 370:1761.
- Pereira NL, Rihal CS, Weinshilboum RM. Genotype-guided dosing of vitamin K antagonists. N Engl J Med 2014; 370:1762.
- Perera MA, Cavallari LH, Johnson JA. Warfarin pharmacogenetics: an illustration of the importance of studies in minority populations. Clin Pharmacol Ther 2014; 95:242–244.
- Pirmohamed M, Wadelius M, Kamali F; EU-PACT Group. Genotype-guided dosing of vitamin K antagonists. N Engl J Med 2014; 370:1764–1765.
- Schwarz UI, Kim RB, Tirona RG. Genotype-guided dosing of vitamin K antagonists. N Engl J Med 2014; 370:1761–1762.
- Scott SA, Lubitz SA. Warfarin pharmacogenetic trials: is there a future for pharmacogenetic-guided dosing? Pharmacogenomics 2014; 15:719–722.
- Zineh I, Pacanowski M, Woodcock J. Pharmacogenetics and coumarin dosing—recalibrating expectations. N Engl J Med 2013; 369:2273–2275.
- Hylek EM. Vitamin K antagonists and time in the therapeutic range: implications, challenges, and strategies for improvement. J Thromb Thrombolysis 2013; 35:333–335.
- Wan Y, Heneghan C, Perera R, et al. Anticoagulation control and prediction of adverse events in patients with atrial fibrillation: a systematic review. Circ Cardiovasc Qual Outcomes 2008;1:84-91.
- Nagai R, Ohara M, Cavallari LH, et al. Factors influencing pharmacokinetics of warfarin in African-Americans: implications for pharmacogenetic dosing algorithms. Pharmacogenomics 2015;16:217–225.
- Rouse M, Cristiani C, Teng KA. Should we use pharmacogenetic testing when prescribing warfarin? Cleve Clin J Med 2013; 80:483–486.
- Kimmel SE, French B, Kasner SE, et al; COAG Investigators. A pharmacogenetic versus a clinical algorithm for warfarin dosing. N Engl J Med 2013; 369:2283–2293.
- Pirmohamed M, Burnside G, Eriksson N, et al; EU-PACT Group. A randomized trial of genotype-guided dosing of warfarin. N Engl J Med 2013; 369:2294–2303.
- Cavallari LH, Kittles RA, Perera MA. Genotype-guided dosing of vitamin K antagonists. N Engl J Med 2014; 370:1763.
- Cavallari LH, Nutescu EA. Warfarin pharmacogenetics: to genotype or not to genotype, that is the question. Clin Pharmacol Ther 2014; 96:22–24.
- Daneshjou R, Klein TE, Altman RB. Genotype-guided dosing of vitamin K antagonists. N Engl J Med 2014; 370:1762–1763.
- Hernandez W, Gamazon ER, Aquino-Michaels K, et al. Ethnicity-specific pharmacogenetics: the case of warfarin in African Americans. Pharmacogenomics J 2014; 14:223–228.
- Kimmel SE, French B, Geller NL; COAG Investigators. Genotype-guided dosing of vitamin K antagonists. N Engl J Med 2014; 370:1763–1764.
- Koller EA, Roche JC, Rollins JA. Genotype-guided dosing of vitamin K antagonists. N Engl J Med 2014; 370:1761.
- Pereira NL, Rihal CS, Weinshilboum RM. Genotype-guided dosing of vitamin K antagonists. N Engl J Med 2014; 370:1762.
- Perera MA, Cavallari LH, Johnson JA. Warfarin pharmacogenetics: an illustration of the importance of studies in minority populations. Clin Pharmacol Ther 2014; 95:242–244.
- Pirmohamed M, Wadelius M, Kamali F; EU-PACT Group. Genotype-guided dosing of vitamin K antagonists. N Engl J Med 2014; 370:1764–1765.
- Schwarz UI, Kim RB, Tirona RG. Genotype-guided dosing of vitamin K antagonists. N Engl J Med 2014; 370:1761–1762.
- Scott SA, Lubitz SA. Warfarin pharmacogenetic trials: is there a future for pharmacogenetic-guided dosing? Pharmacogenomics 2014; 15:719–722.
- Zineh I, Pacanowski M, Woodcock J. Pharmacogenetics and coumarin dosing—recalibrating expectations. N Engl J Med 2013; 369:2273–2275.
- Hylek EM. Vitamin K antagonists and time in the therapeutic range: implications, challenges, and strategies for improvement. J Thromb Thrombolysis 2013; 35:333–335.
- Wan Y, Heneghan C, Perera R, et al. Anticoagulation control and prediction of adverse events in patients with atrial fibrillation: a systematic review. Circ Cardiovasc Qual Outcomes 2008;1:84-91.
- Nagai R, Ohara M, Cavallari LH, et al. Factors influencing pharmacokinetics of warfarin in African-Americans: implications for pharmacogenetic dosing algorithms. Pharmacogenomics 2015;16:217–225.
Optimizing Inpatient Pharmacotherapy Using a Single Clinical Policy Streamlining Pharmacy Protocols
From the Ernest Mario School of Pharmacy, Rutgers, The State University of New Jersey, Piscataway, NJ.
Abstract
- Objectives: To describe the implementation of broadly scoped clinical pharmacy protocols positioned as a singular policy in a community hospital. These protocols were designed to expand the established benefits demonstrated using narrower, traditional protocols.
- Methods: A retrospective chart review of protocol interventions in the first year of the policy’s implementation was conducted to evaluate prescriber acceptance of protocol interventions. Interventions were identified from required email notifications. The frequency of use of each protocol was assessed, including evaluation of novel characteristics of specific protocols. Pharmacist utilization patterns were assessed for job classification, shift, and practice setting (ie, centralized or decentralized).
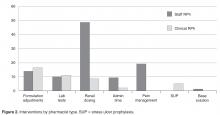
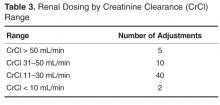
- Results: In the 1-year assessment period, 145 interventions were reported and 144 were accepted by the prescribing physicians. Interventions involved orders from hospitalists and intensivists most frequently, with the renal dosing and dose formulations protocols being the most commonly utilized. Staff pharmacists used the policy more frequently than clinical pharmacists, primarily during day shift from decentralized locations on the patient care units.
- Conclusions: The implementation of broadly scoped clinical pharmacy protocols for items our pharmacists routinely contact physicians about (and our physicians deemed were within the practice of pharmacy) instituted a cultural shift that expanded the elements considered to be part of routine pharmacy practice. As a result, pharmacists more seamlessly applied their expertise as pharmacotherapy specialists to optimize pharmacotherapy, which streamlined workflow for both pharmacists and physicians. This expanded the proven benefits of allowing professionals to work to their fullest extent, as established in the literature.
Allowing pharmacists to apply their expertise has been associated with improved outcomes in both pharmacotherapy quality (eg, reduction in mortality and length of stay [1]) and savings in health care dollars. Studies of focused protocols, including intravenous-to-oral (IV-to-PO) switch [2–20], renal dosing [21], stress ulcer prophylaxis [22] and anticoagulation management [1,23,24] demonstrate these benefits in a multitude of practice areas. While such protocols have become commonplace in the acute care setting [25–28], most continue to be singularly focused and impose patient population restrictions that preclude comprehensive patient evaluation. Many are administered as a task within the pharmacist workflow using a patient list generated by the limited protocol criteria, which are often restricted to agent or patient characteristics.
Better outcomes are associated with permitting professionals such as pharmacists to work to the fullest extent of their scope and expertise [29–31]. In specific cases, studies evaluating pharmacists’ impact within a multi-disciplinary health care team have demonstrated improved outcomes in regard to both patient care and cost [29–31]. Recognizing this, accountable care organizations (ACOs) have developed practice models that are based on this benefit. Each team member is expected to robustly apply their training and expertise to achieve the best outcomes [32,33]. As health care moves toward a more integrative approach, it is paramount that pharmacists utilize the full scope of the skills in which they are trained.
This report describes the development, implementation, and outcomes of a singular policy outlining comprehensively scoped protocols allowing acute care hospital pharmacists within Princeton HealthCare System to optimize pharmacotherapy during the course of their usual clinical practice.
Methods
Setting
The University Medical Center of Princeton at Plainsboro (UMCPP), part of the Princeton HealthCare System, is a 230-bed community acute care hospital located in central New Jersey. The hospital facility relocated in May 2012 from its previous location in Princeton to a new state-of-the-art facility in Plainsboro. As an affiliate of the Robert Wood Johnson Medical School and the Ernest Mario School of Pharmacy at Rutgers, The State University of New Jersey (ie, Rutgers), it is an academic teaching hospital with a mixed model for providing patient care. UMCPP employs both faculty physicians leading academic teams alongside hospitalists and private attendings.
Pharmacy services are provided on facility 24 hours a day, 365 days a year. The department of pharmacy services provides a full scope medication services from a centralized location with 3 full-time day pharmacists and 1 oncology satellite pharmacist. During weekdays, decentralized pharmacists provide medication review, patient education, and medication reconciliation on 2 to 3 inpatient care units. Centralized support decreases to 2 pharmacists in the evening and 1 overnight. Clinical pharmacists, both hospital-based and Rutgers faculty, work in conjunction with the staff pharmacists to ensure appropriate management of patients throughout different levels of care.
Program Overview and Implementation
To enhance protocols allowing pharmacists to more holistically and robustly optimize pharmacotherapy, UMCPP implemented the Clinical Pharmacy Services policy in February 2012. The policy outlined 8 protocols through which registered pharmacists within the acute care hospital could implement outlined medication order adjustments for adults of inpatient status. Pediatric patients or those treated outside of the acute care hospital (eg, in the psychiatric hospital, surgical center or outpatient facilities) were excluded. While the hospital had existing traditional programs such as IV-to-PO conversions, the programs were restricted to specific agents or conditions. As such, pharmacists were assigned to review queues in the clinical computer system to which orders for the agents outlined by the specific program would flow. Review would occur at set intervals and focus on that detail of the patient’s care as opposed to broadly encompassing an evaluation of the patient’s comprehensive pharmacotherapy. The goal of the new policy was to better utilize the pharmacists’ expertise by broadening these assessments to all applicable agents, refine workflow (by allowing protocol management instead of requiring individual prescriber calls for each issue) and integrate holistic refinement of pharmacotherapy regimens during the usual course of the pharmacist’s clinical care.
In the state of New Jersey, the Pharmacy Practice Act (updated on 14 January 2004) formally recognizes pharmacists as health care professionals and permits for collaborative practice in the community setting [34]. However, pharmacist management by protocol in the acute care hospital setting is defined separately, requiring only medical approvals within the system [35]. In accordance, the policy and associated protocols were approved by the institution’s multidisciplinary pharmacy and therapeutics (P&T) and medical executive committee processes.
To ensure appropriate oversight, the policy required that the pharmacist making changes submit notification of protocol intervention to the patient’s attending physician, the physician who generated the original order (if other than the attending) and a designated clinical pharmacist (for auditing purposes). All notifications were made via email within the clinical computer system in “interrupt” status to ensure active recognition by the prescriber(s).
Program Evaluation
An evaluation of the first year’s interventions was conducted to validate the program, describe its utility, and provide a basis for re-evaluation and continued evolution. The aim was to evaluate the institution’s experience with the program, focusing on both specific physician and pharmacist elements. One of the primary goals was to evaluate which physician’s orders were associated with interventions as well as the rate of physician acceptance of protocol interventions, as their acceptance clearly validates the pharmacist’s ability to appropriately apply the protocols in patient-specific contexts.
To evaluate the pharmacist’s experience, trends in pharmacist utilization were captured, including which pharmacist by job classification (ie, staff or clinical pharmacist) implemented interventions, during which shift, and in what operational capacity (ie, centralized or decentralized) the pharmacist was practicing. Lastly, the study sought to characterize the frequency to which each protocol was applied. Based on the existing experiences described in the literature as well as with consideration of institutional culture and operation, we hypothesized that all pharmacists would apply protocols with equal efficacy with more interventions likely generated by staff pharmacists due to their role in primary order review and that the types of interventions would vary based on shift and location.
A retrospective review of cases throughout the first year of the policy’s implementation was conducted, including interventions made between 1 February 2012 and 31 January 2013. Cases were identified through the required email notification of the auditing clinical pharmacist. The patient’s electronic medical record for that defined visit was reviewed. To assess pharmacist utilization patterns, data captured included the agent involved in the intervention, date, day of week and shift, whether the pharmacist was centralized or decentralized, and whether that pharmacist was classified as staff or clinical. Decentralized pharmacists were defined as a pharmacist working on the patient care unit with direct access to other practitioners and patients, rather than those performing their functions from within the confines of the pharmacy department.
Prescribers were described both by status (ie, attending or resident/training) and specialty. Physician acceptance was assessed through evaluation of order trends as the electronic medical record allows for all changes to an order to be audited and tracked; a review of progress notes to capture any commentary or rationale regarding interventions or the surrounding circumstances; as well as a review of any associated laboratory or diagnostic reports and nursing notes. If the order was not altered by the physician within 24 hours (ie, the time frame in which orders must be reviewed by the prescriber per institutional standards) of the pharmacist’s protocol change it was deemed accepted by the physician. Changes made within 24 hours for clinical reasons unrelated to the protocol change as verified by documentation in the progress notes were considered as accepted. These included, for example, the discontinuation of empiric antibiotics that had been dose adjusted by the pharmacist for patients in whom infection had been ruled out or a change from the adjusted agent to one of another class (such as might occur during de-escalation of antibiotic therapy). Interventions were excluded if there were insufficient patient and/or intervention details to allow complete assessment.
For protocol evaluation, details concerning the nature of the adjustment were collected. For formulation changes, agents were classified by their bioavailability. Renal dose adjustments were classified by the patient’s estimated creatinine clearance range since interventions were not restricted to ranges or agents. Stress ulcer prophylaxis adjustments were classified as those involving initiation, changes or discontinuation of therapy. For parenteral product adjustments, the initial and final base solution and/or the change in concentration was captured. Pain management order adjustments were classified as those involving the same agent with overlapping indications or those with oral and intravenous orders for the same pain scale range. When laboratory tests were ordered, the type of test was captured.
The study was approved by the institutional review boards of Princeton HealthCare System and Rutgers.
Results
There were 145 interventions occurring between 1 February 2012 and 31 January 2013, with 144 (99.3%) of those being accepted by the prescriber. The 1 intervention that was not accepted involved an IV to oral conversion of levothyroxine. The pharmacist performed the conversion appropriately as the patient was tolerating other oral medications. However, on the day of the change, the patient refused all oral medications despite having the ability to accept them and, as a result, all medications were converted back to parenteral formulations.
Pharmacist Evaluation
Prescriber Evaluation
An evaluation of prescribers revealed that the primary physician groups (ie, order generators) involved were hospitalists (n = 32) and critical care attendings (n = 24) at 22% and 17% of all orders, respectively. The remaining 89 interventions were distributed across other attending types (including general medicine physicians, specialty physicians and surgeons) and trainees (residents and fellows) with no more than eight orders for any individual physician category.
Protocol Evaluation
The total number of laboratory tests ordered accounted for 14% (n = 21) of all interventions. Studies related to the management of anti-infective agents and blood formation, coagulation, and thrombosis agents consisted of the majority of the lab tests ordered; INR/PTT and vancomycin levels were the most commonly ordered. Thirteen percent (n = 19) of all interventions include pain management adjustments with an even distribution between pain medications.
Several protocols were less frequently used, specifically the stress ulcer prophylaxis protocol (representing 3% of all interventions or n = 5), base solution changes (< 1% of all interventions or n = 1), and adjustment of administration time (7.6% of all interventions, n = 11). Of the time adjustments, more than 50% (n = 6) involved furosemide.
Discussion
While the literature has many studies describing pharmacists improving outcomes through successful provision of clinical programs by protocol in the acute care hospital setting, the majority of studies are limited to single or focused protocols [2–24,27,37,38]. This approach fails to recognize or limits application of a pharmacist’s expertise in pharmacotherapy, as intervention is permitted only on defined agents under specific circumstances. This is the only report we are aware of that addresses a broader approach in permitting pharmacists to optimize pharmaco-therapy during the course of their usual practice through a single policy. As better outcomes are associated with allowing professionals to work to the fullest extent of their expertise, a broad range of protocols identified as pharmacy clinical services were selected and integrated into a singular policy that would be the foundation for instituting cultural change in regard to the elements considered to be routine pharmacy practice. Thus, the protocols applied here did not specify agents that could be adjusted for renal function or classes for which formulation conversion were permissible. This is also the case for dose formulation adjustments, where the protocol allowed for the pharmacist to apply their expertise beyond 1:1 conversions using standardized drug information references (Table 1 and Table 2). As such, the protocols allowed for the full application of the pharmacist’s expertise as a pharmacotherapy consultant within these intervention categories to assure that therapies are optimized. Additionally, eliminating phone calls streamlined the workflow for both the pharmacist and physicians, thus minimizing interruptions that distract from the other functions in which they are engaged.
During the approval process, physicians inquired whether all pharmacists were equally capable of making the clinical judgments involved with the protocols as described and, thusly, whether protocol management should be limited to clinical pharmacists who have less traditional dispensing roles and more experience and time at the bedside. During those discussions we contended that the nature of these protocols were fundamental and applicable to all practicing pharmacists and, if limited, would result in missed opportunities as the clinical pharmacists are focused in specialized areas during weekdays only at UMCPP. For example, a single, centralized night-shift pharmacist could make routine dose or formulation adjustments without the need to awaken a physician as the UMCPP electronic medical record makes available all progress notes, laboratory results, and diagnostics crucial to clinical decision making. All pharmacists, regardless of job title, meet the same requirements for licensure. Post-doctoral residency or fellowship training and advanced certifications in specialty areas of practice exist among both groups as well. The study results support the validity of this argument. The majority of interventions were successfully performed by staff pharmacists with involvement from all shifts, including a third that occurred overnight. This is important because, like at most hospitals, the UMCPP staffing ratio decreases throughout the course of the day presenting changing workflow challenges throughout different shifts.
Several limitations of this study should be noted. Due to its retrospective nature, it is likely that not all interventions were captured. Some decentralized pharmacists reported not emailing interventions as they had verbally communicated the adjustments prior to having the opportunity to send the email. Four interventions could not be assessed as the email notification did not contain all the required patient identifiers or intervention information to permit for appropriate evaluation. The hospital also moved to a newly built facility in the fourth month of protocol implementation, which required significant changes in drug distribution methods, and this could have contributed to the small sample size of interventions. The move temporarily shifted departmental resources to support operational needs.
Another important factor is the voluntary nature of the policy; while it was within the pharmacist’s professional judgment to apply the protocols, pharmacists were encouraged to contact prescribers if there was any ambiguity. Therefore, while one might have expected more resident physicians to be involved with orders that were adjusted, the UMCPP practice philosophy supports contacting training physicians about changes so that they may learn from the discussion to support developing stronger prescribing habits. Future development should therefore support more universal protocol application to all eligible patients to optimize the benefits described here. Lastly, data measuring the clinical outcomes and time savings or increased productivity secondary to the elimination of physician phone calls was not directly measured. We thus sought to first demonstrate to the physician base that pharmacists could successfully apply a variety of protocols that were broader than those formally studied with equal accuracy. With that effectiveness established, future studies should explore if broader protocol application produces a greater optimization of outcomes.
After the study was completed, a survey was conducted of the pharmacists to assess perceptions and guide further policy development. We received a 63.6% response rate (14 of 22 possible respondents) with a strong majority of the respondents expressing a favorable perception of the protocols. A few respondents indicated some protocols were infrequently utilized and there was limited familiarity with others. We anticipate this is largely based on various shift and unit assignments that would make some protocols more applicable than others to the populations serviced. One of the survey questions polled the respondents on the necessity of the email notification to the prescriber given that this practice is of a higher level of notification than other established hospital protocols which only requires a notation of the change within the medication order. Seventy-one percent (n = 10) of respondents favored removing the email notification, citing primarily that it would be consistent with physician comments regarding the existing notifications. Pharmacists also identified further areas of protocol development including electrocardiogram ordering for QTc monitoring, implementation of a standardized vancomycin dosing protocol, discontinuation of duplicate orders, product substitution for nonformulary items and addition of a protocol for pharmacists to order over-the-counter or nonprescription products as they would in a community setting. This input will shape the revision of the policy and its protocols.
Conclusion
Consistent with the published literature, pharmacists effectively performed pharmacotherapy interventions in a multitude of practice categories for adult inpatients of an acute care community-teaching hospital using a single, comprehensive clinical policy. Providing these broadly scoped protocols in a singular policy allowed pharmacists to increase the autonomy with which they applied their pharmacotherapy expertise during the course of their routine, prospective care and expanded the established benefit of allowing professionals to work to their fullest extent. Pharmacist protocol intervention was met with a high physician acceptance rate.
Acknowledgment: We thank all the pharmacists at UMCPP for supporting our efforts to refine pharmacy practice for our patients.
Corresponding author: Liza Barbarello Andrews, PharmD, BSPharm, BCPS, Rutgers, The State University of New Jersey, 160 Frelinghuysen Rd, Piscataway, NJ 08854, [email protected].
Financial disclosures: None.
1. Bond CA, Raehl CL. Pharmacist provided anticoagulation management in United States hospitals: death rates, length of stay, Medicare charges, bleeding complications and transfusions. Pharmacother 2004;24:953–63.
2. Yen YH, Chen HY, Wuan-Jin L, et al. Clinical and economic impact of a pharmacist-managed iv-to-po conversion service for levofloxacin in Taiwan. Int J Clin Pharmacol Ther 2012;50:136–41.
3. Buyle F, Vogelaers D, PelemanR, et al. Implementation of guidelines for sequential therapy with fluoroquinolones in a Belgian hospital. Pharm World Sci 2010;32:404–10.
4. Davis SL, Delgado G, McKinnon PS. Pharmacoeconomic consideration associated with the use of intravenous-to-oral moxifloxacin for community-acquired pneumonia Clin Infect Dis 2005;41Supp2;5:136–43.
5. Ho BP, Lau TT, Balen RM, et al. The impact of a pharmacist-managed dosage form conversion service on ciprofloxacin usage at a major Canadian teaching hospital: a pre- and post-intervention study. BMC Health Serv Res 2005;5:48.
6. Kuti JL, Le TN, Nightingale CH, et al. Pharmacoeconomics of a pharmacist-managed program for automatically converting levofloxacin route from iv to oral. Am J Health Sys Pharm 2002;59:2209–15.
7. Cohen SM, Lipsett PA, Buchman TG, et al. Comparison of intravenous/oral ciprofloxacin plus metronidazole versus piperacillin/tazobactam in the treatment of complicated intraabdominal infections. Ann Surg 2000;232:254–62.
8. Plouffe J, Schwartz DB, Kolokathis A, et al. Clinical efficacy of intravenous followed by oral azithromycin monotherapy in hospitalized patients with community-acquired pneumonia. Antimicrob Agents Chemother 2000;44:1796–802.
9. Wetzstein GA. Intravenous to oral (IV:PO) anti-infective combination therapy. Cancer Control 2000;7:170–6.
10. Paladino JA. Pharmacoeconomics of antimicrobial therapy. Am J Healthsys Pharm 1999;56(Supp3):S25–8.
11. Ahkee S, Smith S, et al. Early switch from intravenous to oral antibiotics in hospitalized patient with infections: a six-month prospective study. Pharmacotherapy 1997;17:569–75.
12. Przybylski KG, Rybak MJ, Martin PR, et al. A pharmacist-initiated program of intravenous to oral antibiotic conversion. Pharmacotherapy 1997;17:271–6.
13. Jewesson P. Cost-effectiveness and value of an IV switch. Pharmacoeconomics 1994;5(Supp2):20–6.
14. Ramirez J. Advances in antibiotic use: switch therapy. Curr Ther Res 1994;55(suppA):30–3.
15. Shepard MF. Making the switch from IV to PO. Am J Healthsys Pharm 1994;50:2510.
16. Chessin LN. When to switch from IV to oral antibiotics. Patient Care 1993;15:113–25.
17. Cohen MR. Important news! IV route not needed to justify hospitalization for antibiotics. Hospital Pharmacy 1993;28:946.
18. Schentag JJ. Changes in antimicrobial agent usage resulting from interactions among clinical pharmacy, infectious disease division and the microbiology laboratory. Diagnos Microbiol Infect Dis 1993;16:255–64.
19. Fighetto L. Intravenous to oral stepdown program: four years’ experience in a large teaching hospital. Ann Pharmacother 1992;26:1447–51.
20. Powers T. Clinical and economic effect of ciprofloxacin as an alternative to injectable antimicrobial therapy. Am J Healthsys Pharm 1990;47:1781–4.
21. Ament PW, McGuire WM. Setting up an automatic pharmacist-initiated pharmacokinetic dosing service. Hosp Formul 1993;28:589–92.
22. Mousavi M, Dashti-Khavidaki S, Khalili H, et al. Impact of clinical pharmacy services on stress ulcer prophylaxis prescribing and related cost in patients with renal insufficiency. Int J Pharm Pract 2012;Nov 9.
23. Damaske DL, Baird RW. Development and implementation of a pharmacist managed inpatient warfarin protocol. Proc (Baylor Univ Med Cent) 2005;18:397–400.
24. Radley AS, Hall J, Farrow M, et al. Evaluation of anticoagulation control in a pharmacist operated anticoagulation clinic. J Clin Pathol 1995;48:545–47.
25. Pharmacy and Therapeutics Committee. Medication policies and protocols of Nebraska Methodist Hospital, Methodist Women’s Hospital.
26. University of Kentucky Pharmacy Services. Therapeutic interchanges [Internet]. Available at www.hosp.uky.edu/pharmacy/interchange.asp
27. Bayshore Community Hospital Department of Pharmacy. Conversion of intravenous azithromycin (Zithromax), ceftriaxone (Rocephin), ciprofloxacin (Cipro), fluconazole (Diflucan), lansoprazole (Prevacid), levofloxacin (Levaquin), linezolid (Zyvox), metronidazole (Flagyl), moxifloxacin (Avelox), potassium chloride (KC), or ranitidine (Zantac) to oral medication [Internet]. [cited 2013 May 9]. Available at www.ashp.org/s_ashp/docs/files/R-IVtoPOConvPol-2.pdf.
28. Medication Education Safety Approval Committee, Massachusetts General Hospital. Automatic intravenous to oral protocol [Internet]. MESAC memo; 2005 July [cited 2013 March 9]. Available at www2.massgeneral.org/pharmacy/mesac/mesac_memo3.pdf.
29. Hanson RL, Habibi M, Khamo N, et al. Integrated clinical and specialty pharmacy practice model for management of patients with multiple sclerosis. Am J Health Syst Pharm 2014;71:463–9.
30. Making pharmacists part of the multidisciplinary team. Hosp Case Manag 2014;22:13–6.
31. Preslaski CR, Lat I, MacLaren R, et al. Pharmacist contributions as members of the multidisciplinary ICU team. Chest 2013;144:1687–95.
32. Ripley TL, Adamson PB, Hennebry TA, et al. Collaborative practice model between cardiologists and clinical pharmacist for management of patients with cardiovascular disease in an outpatient clinic. Ann Pharmacother 2014;48:412–9.
33. Smith M, Bates DW, Bodenheimer TS. Pharmacists belong in accountable care organizations and integrated care teams. Health Aff (Millwood) 2013;32:1963–70.
34. Ukens C. New Jersey rewrites its state pharmacy practice act. Drug Topics 2004;148:41.
35. New Jersey Board of Pharmacy Laws. Statute 45:14-64. Inapplicability relative to collaborative drug therapy management in hospital. [Internet] July 2011 [cited 11 September 14]. Available from: www.njconsumeraffairs.gov/laws/pharmlaws.pdf.
36. ASHP Commission on Therapeutics. ASHP therapeutic guidelines on stress ulcer prophylaxis. Am J Health Syst Pharm 1999;56:347–79.
37. Bond CA, Raehl CL. Clinical pharmacy services, pharmacy staffing, and hospital mortality rates. Pharmacotherapy 2007;27:481–93.
38. Bond CA, Raehl CL, Franke T. Interrelationships among mortality rates, drug costs, total cost of care, and length of stay in United States hospitals: summary and recommendations for clinical pharmacy services and staffing. Pharmacotherapy 2001;21:129–41.
From the Ernest Mario School of Pharmacy, Rutgers, The State University of New Jersey, Piscataway, NJ.
Abstract
- Objectives: To describe the implementation of broadly scoped clinical pharmacy protocols positioned as a singular policy in a community hospital. These protocols were designed to expand the established benefits demonstrated using narrower, traditional protocols.
- Methods: A retrospective chart review of protocol interventions in the first year of the policy’s implementation was conducted to evaluate prescriber acceptance of protocol interventions. Interventions were identified from required email notifications. The frequency of use of each protocol was assessed, including evaluation of novel characteristics of specific protocols. Pharmacist utilization patterns were assessed for job classification, shift, and practice setting (ie, centralized or decentralized).
- Results: In the 1-year assessment period, 145 interventions were reported and 144 were accepted by the prescribing physicians. Interventions involved orders from hospitalists and intensivists most frequently, with the renal dosing and dose formulations protocols being the most commonly utilized. Staff pharmacists used the policy more frequently than clinical pharmacists, primarily during day shift from decentralized locations on the patient care units.
- Conclusions: The implementation of broadly scoped clinical pharmacy protocols for items our pharmacists routinely contact physicians about (and our physicians deemed were within the practice of pharmacy) instituted a cultural shift that expanded the elements considered to be part of routine pharmacy practice. As a result, pharmacists more seamlessly applied their expertise as pharmacotherapy specialists to optimize pharmacotherapy, which streamlined workflow for both pharmacists and physicians. This expanded the proven benefits of allowing professionals to work to their fullest extent, as established in the literature.
Allowing pharmacists to apply their expertise has been associated with improved outcomes in both pharmacotherapy quality (eg, reduction in mortality and length of stay [1]) and savings in health care dollars. Studies of focused protocols, including intravenous-to-oral (IV-to-PO) switch [2–20], renal dosing [21], stress ulcer prophylaxis [22] and anticoagulation management [1,23,24] demonstrate these benefits in a multitude of practice areas. While such protocols have become commonplace in the acute care setting [25–28], most continue to be singularly focused and impose patient population restrictions that preclude comprehensive patient evaluation. Many are administered as a task within the pharmacist workflow using a patient list generated by the limited protocol criteria, which are often restricted to agent or patient characteristics.
Better outcomes are associated with permitting professionals such as pharmacists to work to the fullest extent of their scope and expertise [29–31]. In specific cases, studies evaluating pharmacists’ impact within a multi-disciplinary health care team have demonstrated improved outcomes in regard to both patient care and cost [29–31]. Recognizing this, accountable care organizations (ACOs) have developed practice models that are based on this benefit. Each team member is expected to robustly apply their training and expertise to achieve the best outcomes [32,33]. As health care moves toward a more integrative approach, it is paramount that pharmacists utilize the full scope of the skills in which they are trained.
This report describes the development, implementation, and outcomes of a singular policy outlining comprehensively scoped protocols allowing acute care hospital pharmacists within Princeton HealthCare System to optimize pharmacotherapy during the course of their usual clinical practice.
Methods
Setting
The University Medical Center of Princeton at Plainsboro (UMCPP), part of the Princeton HealthCare System, is a 230-bed community acute care hospital located in central New Jersey. The hospital facility relocated in May 2012 from its previous location in Princeton to a new state-of-the-art facility in Plainsboro. As an affiliate of the Robert Wood Johnson Medical School and the Ernest Mario School of Pharmacy at Rutgers, The State University of New Jersey (ie, Rutgers), it is an academic teaching hospital with a mixed model for providing patient care. UMCPP employs both faculty physicians leading academic teams alongside hospitalists and private attendings.
Pharmacy services are provided on facility 24 hours a day, 365 days a year. The department of pharmacy services provides a full scope medication services from a centralized location with 3 full-time day pharmacists and 1 oncology satellite pharmacist. During weekdays, decentralized pharmacists provide medication review, patient education, and medication reconciliation on 2 to 3 inpatient care units. Centralized support decreases to 2 pharmacists in the evening and 1 overnight. Clinical pharmacists, both hospital-based and Rutgers faculty, work in conjunction with the staff pharmacists to ensure appropriate management of patients throughout different levels of care.
Program Overview and Implementation
To enhance protocols allowing pharmacists to more holistically and robustly optimize pharmacotherapy, UMCPP implemented the Clinical Pharmacy Services policy in February 2012. The policy outlined 8 protocols through which registered pharmacists within the acute care hospital could implement outlined medication order adjustments for adults of inpatient status. Pediatric patients or those treated outside of the acute care hospital (eg, in the psychiatric hospital, surgical center or outpatient facilities) were excluded. While the hospital had existing traditional programs such as IV-to-PO conversions, the programs were restricted to specific agents or conditions. As such, pharmacists were assigned to review queues in the clinical computer system to which orders for the agents outlined by the specific program would flow. Review would occur at set intervals and focus on that detail of the patient’s care as opposed to broadly encompassing an evaluation of the patient’s comprehensive pharmacotherapy. The goal of the new policy was to better utilize the pharmacists’ expertise by broadening these assessments to all applicable agents, refine workflow (by allowing protocol management instead of requiring individual prescriber calls for each issue) and integrate holistic refinement of pharmacotherapy regimens during the usual course of the pharmacist’s clinical care.
In the state of New Jersey, the Pharmacy Practice Act (updated on 14 January 2004) formally recognizes pharmacists as health care professionals and permits for collaborative practice in the community setting [34]. However, pharmacist management by protocol in the acute care hospital setting is defined separately, requiring only medical approvals within the system [35]. In accordance, the policy and associated protocols were approved by the institution’s multidisciplinary pharmacy and therapeutics (P&T) and medical executive committee processes.
To ensure appropriate oversight, the policy required that the pharmacist making changes submit notification of protocol intervention to the patient’s attending physician, the physician who generated the original order (if other than the attending) and a designated clinical pharmacist (for auditing purposes). All notifications were made via email within the clinical computer system in “interrupt” status to ensure active recognition by the prescriber(s).
Program Evaluation
An evaluation of the first year’s interventions was conducted to validate the program, describe its utility, and provide a basis for re-evaluation and continued evolution. The aim was to evaluate the institution’s experience with the program, focusing on both specific physician and pharmacist elements. One of the primary goals was to evaluate which physician’s orders were associated with interventions as well as the rate of physician acceptance of protocol interventions, as their acceptance clearly validates the pharmacist’s ability to appropriately apply the protocols in patient-specific contexts.
To evaluate the pharmacist’s experience, trends in pharmacist utilization were captured, including which pharmacist by job classification (ie, staff or clinical pharmacist) implemented interventions, during which shift, and in what operational capacity (ie, centralized or decentralized) the pharmacist was practicing. Lastly, the study sought to characterize the frequency to which each protocol was applied. Based on the existing experiences described in the literature as well as with consideration of institutional culture and operation, we hypothesized that all pharmacists would apply protocols with equal efficacy with more interventions likely generated by staff pharmacists due to their role in primary order review and that the types of interventions would vary based on shift and location.
A retrospective review of cases throughout the first year of the policy’s implementation was conducted, including interventions made between 1 February 2012 and 31 January 2013. Cases were identified through the required email notification of the auditing clinical pharmacist. The patient’s electronic medical record for that defined visit was reviewed. To assess pharmacist utilization patterns, data captured included the agent involved in the intervention, date, day of week and shift, whether the pharmacist was centralized or decentralized, and whether that pharmacist was classified as staff or clinical. Decentralized pharmacists were defined as a pharmacist working on the patient care unit with direct access to other practitioners and patients, rather than those performing their functions from within the confines of the pharmacy department.
Prescribers were described both by status (ie, attending or resident/training) and specialty. Physician acceptance was assessed through evaluation of order trends as the electronic medical record allows for all changes to an order to be audited and tracked; a review of progress notes to capture any commentary or rationale regarding interventions or the surrounding circumstances; as well as a review of any associated laboratory or diagnostic reports and nursing notes. If the order was not altered by the physician within 24 hours (ie, the time frame in which orders must be reviewed by the prescriber per institutional standards) of the pharmacist’s protocol change it was deemed accepted by the physician. Changes made within 24 hours for clinical reasons unrelated to the protocol change as verified by documentation in the progress notes were considered as accepted. These included, for example, the discontinuation of empiric antibiotics that had been dose adjusted by the pharmacist for patients in whom infection had been ruled out or a change from the adjusted agent to one of another class (such as might occur during de-escalation of antibiotic therapy). Interventions were excluded if there were insufficient patient and/or intervention details to allow complete assessment.
For protocol evaluation, details concerning the nature of the adjustment were collected. For formulation changes, agents were classified by their bioavailability. Renal dose adjustments were classified by the patient’s estimated creatinine clearance range since interventions were not restricted to ranges or agents. Stress ulcer prophylaxis adjustments were classified as those involving initiation, changes or discontinuation of therapy. For parenteral product adjustments, the initial and final base solution and/or the change in concentration was captured. Pain management order adjustments were classified as those involving the same agent with overlapping indications or those with oral and intravenous orders for the same pain scale range. When laboratory tests were ordered, the type of test was captured.
The study was approved by the institutional review boards of Princeton HealthCare System and Rutgers.
Results
There were 145 interventions occurring between 1 February 2012 and 31 January 2013, with 144 (99.3%) of those being accepted by the prescriber. The 1 intervention that was not accepted involved an IV to oral conversion of levothyroxine. The pharmacist performed the conversion appropriately as the patient was tolerating other oral medications. However, on the day of the change, the patient refused all oral medications despite having the ability to accept them and, as a result, all medications were converted back to parenteral formulations.
Pharmacist Evaluation
Prescriber Evaluation
An evaluation of prescribers revealed that the primary physician groups (ie, order generators) involved were hospitalists (n = 32) and critical care attendings (n = 24) at 22% and 17% of all orders, respectively. The remaining 89 interventions were distributed across other attending types (including general medicine physicians, specialty physicians and surgeons) and trainees (residents and fellows) with no more than eight orders for any individual physician category.
Protocol Evaluation
The total number of laboratory tests ordered accounted for 14% (n = 21) of all interventions. Studies related to the management of anti-infective agents and blood formation, coagulation, and thrombosis agents consisted of the majority of the lab tests ordered; INR/PTT and vancomycin levels were the most commonly ordered. Thirteen percent (n = 19) of all interventions include pain management adjustments with an even distribution between pain medications.
Several protocols were less frequently used, specifically the stress ulcer prophylaxis protocol (representing 3% of all interventions or n = 5), base solution changes (< 1% of all interventions or n = 1), and adjustment of administration time (7.6% of all interventions, n = 11). Of the time adjustments, more than 50% (n = 6) involved furosemide.
Discussion
While the literature has many studies describing pharmacists improving outcomes through successful provision of clinical programs by protocol in the acute care hospital setting, the majority of studies are limited to single or focused protocols [2–24,27,37,38]. This approach fails to recognize or limits application of a pharmacist’s expertise in pharmacotherapy, as intervention is permitted only on defined agents under specific circumstances. This is the only report we are aware of that addresses a broader approach in permitting pharmacists to optimize pharmaco-therapy during the course of their usual practice through a single policy. As better outcomes are associated with allowing professionals to work to the fullest extent of their expertise, a broad range of protocols identified as pharmacy clinical services were selected and integrated into a singular policy that would be the foundation for instituting cultural change in regard to the elements considered to be routine pharmacy practice. Thus, the protocols applied here did not specify agents that could be adjusted for renal function or classes for which formulation conversion were permissible. This is also the case for dose formulation adjustments, where the protocol allowed for the pharmacist to apply their expertise beyond 1:1 conversions using standardized drug information references (Table 1 and Table 2). As such, the protocols allowed for the full application of the pharmacist’s expertise as a pharmacotherapy consultant within these intervention categories to assure that therapies are optimized. Additionally, eliminating phone calls streamlined the workflow for both the pharmacist and physicians, thus minimizing interruptions that distract from the other functions in which they are engaged.
During the approval process, physicians inquired whether all pharmacists were equally capable of making the clinical judgments involved with the protocols as described and, thusly, whether protocol management should be limited to clinical pharmacists who have less traditional dispensing roles and more experience and time at the bedside. During those discussions we contended that the nature of these protocols were fundamental and applicable to all practicing pharmacists and, if limited, would result in missed opportunities as the clinical pharmacists are focused in specialized areas during weekdays only at UMCPP. For example, a single, centralized night-shift pharmacist could make routine dose or formulation adjustments without the need to awaken a physician as the UMCPP electronic medical record makes available all progress notes, laboratory results, and diagnostics crucial to clinical decision making. All pharmacists, regardless of job title, meet the same requirements for licensure. Post-doctoral residency or fellowship training and advanced certifications in specialty areas of practice exist among both groups as well. The study results support the validity of this argument. The majority of interventions were successfully performed by staff pharmacists with involvement from all shifts, including a third that occurred overnight. This is important because, like at most hospitals, the UMCPP staffing ratio decreases throughout the course of the day presenting changing workflow challenges throughout different shifts.
Several limitations of this study should be noted. Due to its retrospective nature, it is likely that not all interventions were captured. Some decentralized pharmacists reported not emailing interventions as they had verbally communicated the adjustments prior to having the opportunity to send the email. Four interventions could not be assessed as the email notification did not contain all the required patient identifiers or intervention information to permit for appropriate evaluation. The hospital also moved to a newly built facility in the fourth month of protocol implementation, which required significant changes in drug distribution methods, and this could have contributed to the small sample size of interventions. The move temporarily shifted departmental resources to support operational needs.
Another important factor is the voluntary nature of the policy; while it was within the pharmacist’s professional judgment to apply the protocols, pharmacists were encouraged to contact prescribers if there was any ambiguity. Therefore, while one might have expected more resident physicians to be involved with orders that were adjusted, the UMCPP practice philosophy supports contacting training physicians about changes so that they may learn from the discussion to support developing stronger prescribing habits. Future development should therefore support more universal protocol application to all eligible patients to optimize the benefits described here. Lastly, data measuring the clinical outcomes and time savings or increased productivity secondary to the elimination of physician phone calls was not directly measured. We thus sought to first demonstrate to the physician base that pharmacists could successfully apply a variety of protocols that were broader than those formally studied with equal accuracy. With that effectiveness established, future studies should explore if broader protocol application produces a greater optimization of outcomes.
After the study was completed, a survey was conducted of the pharmacists to assess perceptions and guide further policy development. We received a 63.6% response rate (14 of 22 possible respondents) with a strong majority of the respondents expressing a favorable perception of the protocols. A few respondents indicated some protocols were infrequently utilized and there was limited familiarity with others. We anticipate this is largely based on various shift and unit assignments that would make some protocols more applicable than others to the populations serviced. One of the survey questions polled the respondents on the necessity of the email notification to the prescriber given that this practice is of a higher level of notification than other established hospital protocols which only requires a notation of the change within the medication order. Seventy-one percent (n = 10) of respondents favored removing the email notification, citing primarily that it would be consistent with physician comments regarding the existing notifications. Pharmacists also identified further areas of protocol development including electrocardiogram ordering for QTc monitoring, implementation of a standardized vancomycin dosing protocol, discontinuation of duplicate orders, product substitution for nonformulary items and addition of a protocol for pharmacists to order over-the-counter or nonprescription products as they would in a community setting. This input will shape the revision of the policy and its protocols.
Conclusion
Consistent with the published literature, pharmacists effectively performed pharmacotherapy interventions in a multitude of practice categories for adult inpatients of an acute care community-teaching hospital using a single, comprehensive clinical policy. Providing these broadly scoped protocols in a singular policy allowed pharmacists to increase the autonomy with which they applied their pharmacotherapy expertise during the course of their routine, prospective care and expanded the established benefit of allowing professionals to work to their fullest extent. Pharmacist protocol intervention was met with a high physician acceptance rate.
Acknowledgment: We thank all the pharmacists at UMCPP for supporting our efforts to refine pharmacy practice for our patients.
Corresponding author: Liza Barbarello Andrews, PharmD, BSPharm, BCPS, Rutgers, The State University of New Jersey, 160 Frelinghuysen Rd, Piscataway, NJ 08854, [email protected].
Financial disclosures: None.
From the Ernest Mario School of Pharmacy, Rutgers, The State University of New Jersey, Piscataway, NJ.
Abstract
- Objectives: To describe the implementation of broadly scoped clinical pharmacy protocols positioned as a singular policy in a community hospital. These protocols were designed to expand the established benefits demonstrated using narrower, traditional protocols.
- Methods: A retrospective chart review of protocol interventions in the first year of the policy’s implementation was conducted to evaluate prescriber acceptance of protocol interventions. Interventions were identified from required email notifications. The frequency of use of each protocol was assessed, including evaluation of novel characteristics of specific protocols. Pharmacist utilization patterns were assessed for job classification, shift, and practice setting (ie, centralized or decentralized).
- Results: In the 1-year assessment period, 145 interventions were reported and 144 were accepted by the prescribing physicians. Interventions involved orders from hospitalists and intensivists most frequently, with the renal dosing and dose formulations protocols being the most commonly utilized. Staff pharmacists used the policy more frequently than clinical pharmacists, primarily during day shift from decentralized locations on the patient care units.
- Conclusions: The implementation of broadly scoped clinical pharmacy protocols for items our pharmacists routinely contact physicians about (and our physicians deemed were within the practice of pharmacy) instituted a cultural shift that expanded the elements considered to be part of routine pharmacy practice. As a result, pharmacists more seamlessly applied their expertise as pharmacotherapy specialists to optimize pharmacotherapy, which streamlined workflow for both pharmacists and physicians. This expanded the proven benefits of allowing professionals to work to their fullest extent, as established in the literature.
Allowing pharmacists to apply their expertise has been associated with improved outcomes in both pharmacotherapy quality (eg, reduction in mortality and length of stay [1]) and savings in health care dollars. Studies of focused protocols, including intravenous-to-oral (IV-to-PO) switch [2–20], renal dosing [21], stress ulcer prophylaxis [22] and anticoagulation management [1,23,24] demonstrate these benefits in a multitude of practice areas. While such protocols have become commonplace in the acute care setting [25–28], most continue to be singularly focused and impose patient population restrictions that preclude comprehensive patient evaluation. Many are administered as a task within the pharmacist workflow using a patient list generated by the limited protocol criteria, which are often restricted to agent or patient characteristics.
Better outcomes are associated with permitting professionals such as pharmacists to work to the fullest extent of their scope and expertise [29–31]. In specific cases, studies evaluating pharmacists’ impact within a multi-disciplinary health care team have demonstrated improved outcomes in regard to both patient care and cost [29–31]. Recognizing this, accountable care organizations (ACOs) have developed practice models that are based on this benefit. Each team member is expected to robustly apply their training and expertise to achieve the best outcomes [32,33]. As health care moves toward a more integrative approach, it is paramount that pharmacists utilize the full scope of the skills in which they are trained.
This report describes the development, implementation, and outcomes of a singular policy outlining comprehensively scoped protocols allowing acute care hospital pharmacists within Princeton HealthCare System to optimize pharmacotherapy during the course of their usual clinical practice.
Methods
Setting
The University Medical Center of Princeton at Plainsboro (UMCPP), part of the Princeton HealthCare System, is a 230-bed community acute care hospital located in central New Jersey. The hospital facility relocated in May 2012 from its previous location in Princeton to a new state-of-the-art facility in Plainsboro. As an affiliate of the Robert Wood Johnson Medical School and the Ernest Mario School of Pharmacy at Rutgers, The State University of New Jersey (ie, Rutgers), it is an academic teaching hospital with a mixed model for providing patient care. UMCPP employs both faculty physicians leading academic teams alongside hospitalists and private attendings.
Pharmacy services are provided on facility 24 hours a day, 365 days a year. The department of pharmacy services provides a full scope medication services from a centralized location with 3 full-time day pharmacists and 1 oncology satellite pharmacist. During weekdays, decentralized pharmacists provide medication review, patient education, and medication reconciliation on 2 to 3 inpatient care units. Centralized support decreases to 2 pharmacists in the evening and 1 overnight. Clinical pharmacists, both hospital-based and Rutgers faculty, work in conjunction with the staff pharmacists to ensure appropriate management of patients throughout different levels of care.
Program Overview and Implementation
To enhance protocols allowing pharmacists to more holistically and robustly optimize pharmacotherapy, UMCPP implemented the Clinical Pharmacy Services policy in February 2012. The policy outlined 8 protocols through which registered pharmacists within the acute care hospital could implement outlined medication order adjustments for adults of inpatient status. Pediatric patients or those treated outside of the acute care hospital (eg, in the psychiatric hospital, surgical center or outpatient facilities) were excluded. While the hospital had existing traditional programs such as IV-to-PO conversions, the programs were restricted to specific agents or conditions. As such, pharmacists were assigned to review queues in the clinical computer system to which orders for the agents outlined by the specific program would flow. Review would occur at set intervals and focus on that detail of the patient’s care as opposed to broadly encompassing an evaluation of the patient’s comprehensive pharmacotherapy. The goal of the new policy was to better utilize the pharmacists’ expertise by broadening these assessments to all applicable agents, refine workflow (by allowing protocol management instead of requiring individual prescriber calls for each issue) and integrate holistic refinement of pharmacotherapy regimens during the usual course of the pharmacist’s clinical care.
In the state of New Jersey, the Pharmacy Practice Act (updated on 14 January 2004) formally recognizes pharmacists as health care professionals and permits for collaborative practice in the community setting [34]. However, pharmacist management by protocol in the acute care hospital setting is defined separately, requiring only medical approvals within the system [35]. In accordance, the policy and associated protocols were approved by the institution’s multidisciplinary pharmacy and therapeutics (P&T) and medical executive committee processes.
To ensure appropriate oversight, the policy required that the pharmacist making changes submit notification of protocol intervention to the patient’s attending physician, the physician who generated the original order (if other than the attending) and a designated clinical pharmacist (for auditing purposes). All notifications were made via email within the clinical computer system in “interrupt” status to ensure active recognition by the prescriber(s).
Program Evaluation
An evaluation of the first year’s interventions was conducted to validate the program, describe its utility, and provide a basis for re-evaluation and continued evolution. The aim was to evaluate the institution’s experience with the program, focusing on both specific physician and pharmacist elements. One of the primary goals was to evaluate which physician’s orders were associated with interventions as well as the rate of physician acceptance of protocol interventions, as their acceptance clearly validates the pharmacist’s ability to appropriately apply the protocols in patient-specific contexts.
To evaluate the pharmacist’s experience, trends in pharmacist utilization were captured, including which pharmacist by job classification (ie, staff or clinical pharmacist) implemented interventions, during which shift, and in what operational capacity (ie, centralized or decentralized) the pharmacist was practicing. Lastly, the study sought to characterize the frequency to which each protocol was applied. Based on the existing experiences described in the literature as well as with consideration of institutional culture and operation, we hypothesized that all pharmacists would apply protocols with equal efficacy with more interventions likely generated by staff pharmacists due to their role in primary order review and that the types of interventions would vary based on shift and location.
A retrospective review of cases throughout the first year of the policy’s implementation was conducted, including interventions made between 1 February 2012 and 31 January 2013. Cases were identified through the required email notification of the auditing clinical pharmacist. The patient’s electronic medical record for that defined visit was reviewed. To assess pharmacist utilization patterns, data captured included the agent involved in the intervention, date, day of week and shift, whether the pharmacist was centralized or decentralized, and whether that pharmacist was classified as staff or clinical. Decentralized pharmacists were defined as a pharmacist working on the patient care unit with direct access to other practitioners and patients, rather than those performing their functions from within the confines of the pharmacy department.
Prescribers were described both by status (ie, attending or resident/training) and specialty. Physician acceptance was assessed through evaluation of order trends as the electronic medical record allows for all changes to an order to be audited and tracked; a review of progress notes to capture any commentary or rationale regarding interventions or the surrounding circumstances; as well as a review of any associated laboratory or diagnostic reports and nursing notes. If the order was not altered by the physician within 24 hours (ie, the time frame in which orders must be reviewed by the prescriber per institutional standards) of the pharmacist’s protocol change it was deemed accepted by the physician. Changes made within 24 hours for clinical reasons unrelated to the protocol change as verified by documentation in the progress notes were considered as accepted. These included, for example, the discontinuation of empiric antibiotics that had been dose adjusted by the pharmacist for patients in whom infection had been ruled out or a change from the adjusted agent to one of another class (such as might occur during de-escalation of antibiotic therapy). Interventions were excluded if there were insufficient patient and/or intervention details to allow complete assessment.
For protocol evaluation, details concerning the nature of the adjustment were collected. For formulation changes, agents were classified by their bioavailability. Renal dose adjustments were classified by the patient’s estimated creatinine clearance range since interventions were not restricted to ranges or agents. Stress ulcer prophylaxis adjustments were classified as those involving initiation, changes or discontinuation of therapy. For parenteral product adjustments, the initial and final base solution and/or the change in concentration was captured. Pain management order adjustments were classified as those involving the same agent with overlapping indications or those with oral and intravenous orders for the same pain scale range. When laboratory tests were ordered, the type of test was captured.
The study was approved by the institutional review boards of Princeton HealthCare System and Rutgers.
Results
There were 145 interventions occurring between 1 February 2012 and 31 January 2013, with 144 (99.3%) of those being accepted by the prescriber. The 1 intervention that was not accepted involved an IV to oral conversion of levothyroxine. The pharmacist performed the conversion appropriately as the patient was tolerating other oral medications. However, on the day of the change, the patient refused all oral medications despite having the ability to accept them and, as a result, all medications were converted back to parenteral formulations.
Pharmacist Evaluation
Prescriber Evaluation
An evaluation of prescribers revealed that the primary physician groups (ie, order generators) involved were hospitalists (n = 32) and critical care attendings (n = 24) at 22% and 17% of all orders, respectively. The remaining 89 interventions were distributed across other attending types (including general medicine physicians, specialty physicians and surgeons) and trainees (residents and fellows) with no more than eight orders for any individual physician category.
Protocol Evaluation
The total number of laboratory tests ordered accounted for 14% (n = 21) of all interventions. Studies related to the management of anti-infective agents and blood formation, coagulation, and thrombosis agents consisted of the majority of the lab tests ordered; INR/PTT and vancomycin levels were the most commonly ordered. Thirteen percent (n = 19) of all interventions include pain management adjustments with an even distribution between pain medications.
Several protocols were less frequently used, specifically the stress ulcer prophylaxis protocol (representing 3% of all interventions or n = 5), base solution changes (< 1% of all interventions or n = 1), and adjustment of administration time (7.6% of all interventions, n = 11). Of the time adjustments, more than 50% (n = 6) involved furosemide.
Discussion
While the literature has many studies describing pharmacists improving outcomes through successful provision of clinical programs by protocol in the acute care hospital setting, the majority of studies are limited to single or focused protocols [2–24,27,37,38]. This approach fails to recognize or limits application of a pharmacist’s expertise in pharmacotherapy, as intervention is permitted only on defined agents under specific circumstances. This is the only report we are aware of that addresses a broader approach in permitting pharmacists to optimize pharmaco-therapy during the course of their usual practice through a single policy. As better outcomes are associated with allowing professionals to work to the fullest extent of their expertise, a broad range of protocols identified as pharmacy clinical services were selected and integrated into a singular policy that would be the foundation for instituting cultural change in regard to the elements considered to be routine pharmacy practice. Thus, the protocols applied here did not specify agents that could be adjusted for renal function or classes for which formulation conversion were permissible. This is also the case for dose formulation adjustments, where the protocol allowed for the pharmacist to apply their expertise beyond 1:1 conversions using standardized drug information references (Table 1 and Table 2). As such, the protocols allowed for the full application of the pharmacist’s expertise as a pharmacotherapy consultant within these intervention categories to assure that therapies are optimized. Additionally, eliminating phone calls streamlined the workflow for both the pharmacist and physicians, thus minimizing interruptions that distract from the other functions in which they are engaged.
During the approval process, physicians inquired whether all pharmacists were equally capable of making the clinical judgments involved with the protocols as described and, thusly, whether protocol management should be limited to clinical pharmacists who have less traditional dispensing roles and more experience and time at the bedside. During those discussions we contended that the nature of these protocols were fundamental and applicable to all practicing pharmacists and, if limited, would result in missed opportunities as the clinical pharmacists are focused in specialized areas during weekdays only at UMCPP. For example, a single, centralized night-shift pharmacist could make routine dose or formulation adjustments without the need to awaken a physician as the UMCPP electronic medical record makes available all progress notes, laboratory results, and diagnostics crucial to clinical decision making. All pharmacists, regardless of job title, meet the same requirements for licensure. Post-doctoral residency or fellowship training and advanced certifications in specialty areas of practice exist among both groups as well. The study results support the validity of this argument. The majority of interventions were successfully performed by staff pharmacists with involvement from all shifts, including a third that occurred overnight. This is important because, like at most hospitals, the UMCPP staffing ratio decreases throughout the course of the day presenting changing workflow challenges throughout different shifts.
Several limitations of this study should be noted. Due to its retrospective nature, it is likely that not all interventions were captured. Some decentralized pharmacists reported not emailing interventions as they had verbally communicated the adjustments prior to having the opportunity to send the email. Four interventions could not be assessed as the email notification did not contain all the required patient identifiers or intervention information to permit for appropriate evaluation. The hospital also moved to a newly built facility in the fourth month of protocol implementation, which required significant changes in drug distribution methods, and this could have contributed to the small sample size of interventions. The move temporarily shifted departmental resources to support operational needs.
Another important factor is the voluntary nature of the policy; while it was within the pharmacist’s professional judgment to apply the protocols, pharmacists were encouraged to contact prescribers if there was any ambiguity. Therefore, while one might have expected more resident physicians to be involved with orders that were adjusted, the UMCPP practice philosophy supports contacting training physicians about changes so that they may learn from the discussion to support developing stronger prescribing habits. Future development should therefore support more universal protocol application to all eligible patients to optimize the benefits described here. Lastly, data measuring the clinical outcomes and time savings or increased productivity secondary to the elimination of physician phone calls was not directly measured. We thus sought to first demonstrate to the physician base that pharmacists could successfully apply a variety of protocols that were broader than those formally studied with equal accuracy. With that effectiveness established, future studies should explore if broader protocol application produces a greater optimization of outcomes.
After the study was completed, a survey was conducted of the pharmacists to assess perceptions and guide further policy development. We received a 63.6% response rate (14 of 22 possible respondents) with a strong majority of the respondents expressing a favorable perception of the protocols. A few respondents indicated some protocols were infrequently utilized and there was limited familiarity with others. We anticipate this is largely based on various shift and unit assignments that would make some protocols more applicable than others to the populations serviced. One of the survey questions polled the respondents on the necessity of the email notification to the prescriber given that this practice is of a higher level of notification than other established hospital protocols which only requires a notation of the change within the medication order. Seventy-one percent (n = 10) of respondents favored removing the email notification, citing primarily that it would be consistent with physician comments regarding the existing notifications. Pharmacists also identified further areas of protocol development including electrocardiogram ordering for QTc monitoring, implementation of a standardized vancomycin dosing protocol, discontinuation of duplicate orders, product substitution for nonformulary items and addition of a protocol for pharmacists to order over-the-counter or nonprescription products as they would in a community setting. This input will shape the revision of the policy and its protocols.
Conclusion
Consistent with the published literature, pharmacists effectively performed pharmacotherapy interventions in a multitude of practice categories for adult inpatients of an acute care community-teaching hospital using a single, comprehensive clinical policy. Providing these broadly scoped protocols in a singular policy allowed pharmacists to increase the autonomy with which they applied their pharmacotherapy expertise during the course of their routine, prospective care and expanded the established benefit of allowing professionals to work to their fullest extent. Pharmacist protocol intervention was met with a high physician acceptance rate.
Acknowledgment: We thank all the pharmacists at UMCPP for supporting our efforts to refine pharmacy practice for our patients.
Corresponding author: Liza Barbarello Andrews, PharmD, BSPharm, BCPS, Rutgers, The State University of New Jersey, 160 Frelinghuysen Rd, Piscataway, NJ 08854, [email protected].
Financial disclosures: None.
1. Bond CA, Raehl CL. Pharmacist provided anticoagulation management in United States hospitals: death rates, length of stay, Medicare charges, bleeding complications and transfusions. Pharmacother 2004;24:953–63.
2. Yen YH, Chen HY, Wuan-Jin L, et al. Clinical and economic impact of a pharmacist-managed iv-to-po conversion service for levofloxacin in Taiwan. Int J Clin Pharmacol Ther 2012;50:136–41.
3. Buyle F, Vogelaers D, PelemanR, et al. Implementation of guidelines for sequential therapy with fluoroquinolones in a Belgian hospital. Pharm World Sci 2010;32:404–10.
4. Davis SL, Delgado G, McKinnon PS. Pharmacoeconomic consideration associated with the use of intravenous-to-oral moxifloxacin for community-acquired pneumonia Clin Infect Dis 2005;41Supp2;5:136–43.
5. Ho BP, Lau TT, Balen RM, et al. The impact of a pharmacist-managed dosage form conversion service on ciprofloxacin usage at a major Canadian teaching hospital: a pre- and post-intervention study. BMC Health Serv Res 2005;5:48.
6. Kuti JL, Le TN, Nightingale CH, et al. Pharmacoeconomics of a pharmacist-managed program for automatically converting levofloxacin route from iv to oral. Am J Health Sys Pharm 2002;59:2209–15.
7. Cohen SM, Lipsett PA, Buchman TG, et al. Comparison of intravenous/oral ciprofloxacin plus metronidazole versus piperacillin/tazobactam in the treatment of complicated intraabdominal infections. Ann Surg 2000;232:254–62.
8. Plouffe J, Schwartz DB, Kolokathis A, et al. Clinical efficacy of intravenous followed by oral azithromycin monotherapy in hospitalized patients with community-acquired pneumonia. Antimicrob Agents Chemother 2000;44:1796–802.
9. Wetzstein GA. Intravenous to oral (IV:PO) anti-infective combination therapy. Cancer Control 2000;7:170–6.
10. Paladino JA. Pharmacoeconomics of antimicrobial therapy. Am J Healthsys Pharm 1999;56(Supp3):S25–8.
11. Ahkee S, Smith S, et al. Early switch from intravenous to oral antibiotics in hospitalized patient with infections: a six-month prospective study. Pharmacotherapy 1997;17:569–75.
12. Przybylski KG, Rybak MJ, Martin PR, et al. A pharmacist-initiated program of intravenous to oral antibiotic conversion. Pharmacotherapy 1997;17:271–6.
13. Jewesson P. Cost-effectiveness and value of an IV switch. Pharmacoeconomics 1994;5(Supp2):20–6.
14. Ramirez J. Advances in antibiotic use: switch therapy. Curr Ther Res 1994;55(suppA):30–3.
15. Shepard MF. Making the switch from IV to PO. Am J Healthsys Pharm 1994;50:2510.
16. Chessin LN. When to switch from IV to oral antibiotics. Patient Care 1993;15:113–25.
17. Cohen MR. Important news! IV route not needed to justify hospitalization for antibiotics. Hospital Pharmacy 1993;28:946.
18. Schentag JJ. Changes in antimicrobial agent usage resulting from interactions among clinical pharmacy, infectious disease division and the microbiology laboratory. Diagnos Microbiol Infect Dis 1993;16:255–64.
19. Fighetto L. Intravenous to oral stepdown program: four years’ experience in a large teaching hospital. Ann Pharmacother 1992;26:1447–51.
20. Powers T. Clinical and economic effect of ciprofloxacin as an alternative to injectable antimicrobial therapy. Am J Healthsys Pharm 1990;47:1781–4.
21. Ament PW, McGuire WM. Setting up an automatic pharmacist-initiated pharmacokinetic dosing service. Hosp Formul 1993;28:589–92.
22. Mousavi M, Dashti-Khavidaki S, Khalili H, et al. Impact of clinical pharmacy services on stress ulcer prophylaxis prescribing and related cost in patients with renal insufficiency. Int J Pharm Pract 2012;Nov 9.
23. Damaske DL, Baird RW. Development and implementation of a pharmacist managed inpatient warfarin protocol. Proc (Baylor Univ Med Cent) 2005;18:397–400.
24. Radley AS, Hall J, Farrow M, et al. Evaluation of anticoagulation control in a pharmacist operated anticoagulation clinic. J Clin Pathol 1995;48:545–47.
25. Pharmacy and Therapeutics Committee. Medication policies and protocols of Nebraska Methodist Hospital, Methodist Women’s Hospital.
26. University of Kentucky Pharmacy Services. Therapeutic interchanges [Internet]. Available at www.hosp.uky.edu/pharmacy/interchange.asp
27. Bayshore Community Hospital Department of Pharmacy. Conversion of intravenous azithromycin (Zithromax), ceftriaxone (Rocephin), ciprofloxacin (Cipro), fluconazole (Diflucan), lansoprazole (Prevacid), levofloxacin (Levaquin), linezolid (Zyvox), metronidazole (Flagyl), moxifloxacin (Avelox), potassium chloride (KC), or ranitidine (Zantac) to oral medication [Internet]. [cited 2013 May 9]. Available at www.ashp.org/s_ashp/docs/files/R-IVtoPOConvPol-2.pdf.
28. Medication Education Safety Approval Committee, Massachusetts General Hospital. Automatic intravenous to oral protocol [Internet]. MESAC memo; 2005 July [cited 2013 March 9]. Available at www2.massgeneral.org/pharmacy/mesac/mesac_memo3.pdf.
29. Hanson RL, Habibi M, Khamo N, et al. Integrated clinical and specialty pharmacy practice model for management of patients with multiple sclerosis. Am J Health Syst Pharm 2014;71:463–9.
30. Making pharmacists part of the multidisciplinary team. Hosp Case Manag 2014;22:13–6.
31. Preslaski CR, Lat I, MacLaren R, et al. Pharmacist contributions as members of the multidisciplinary ICU team. Chest 2013;144:1687–95.
32. Ripley TL, Adamson PB, Hennebry TA, et al. Collaborative practice model between cardiologists and clinical pharmacist for management of patients with cardiovascular disease in an outpatient clinic. Ann Pharmacother 2014;48:412–9.
33. Smith M, Bates DW, Bodenheimer TS. Pharmacists belong in accountable care organizations and integrated care teams. Health Aff (Millwood) 2013;32:1963–70.
34. Ukens C. New Jersey rewrites its state pharmacy practice act. Drug Topics 2004;148:41.
35. New Jersey Board of Pharmacy Laws. Statute 45:14-64. Inapplicability relative to collaborative drug therapy management in hospital. [Internet] July 2011 [cited 11 September 14]. Available from: www.njconsumeraffairs.gov/laws/pharmlaws.pdf.
36. ASHP Commission on Therapeutics. ASHP therapeutic guidelines on stress ulcer prophylaxis. Am J Health Syst Pharm 1999;56:347–79.
37. Bond CA, Raehl CL. Clinical pharmacy services, pharmacy staffing, and hospital mortality rates. Pharmacotherapy 2007;27:481–93.
38. Bond CA, Raehl CL, Franke T. Interrelationships among mortality rates, drug costs, total cost of care, and length of stay in United States hospitals: summary and recommendations for clinical pharmacy services and staffing. Pharmacotherapy 2001;21:129–41.
1. Bond CA, Raehl CL. Pharmacist provided anticoagulation management in United States hospitals: death rates, length of stay, Medicare charges, bleeding complications and transfusions. Pharmacother 2004;24:953–63.
2. Yen YH, Chen HY, Wuan-Jin L, et al. Clinical and economic impact of a pharmacist-managed iv-to-po conversion service for levofloxacin in Taiwan. Int J Clin Pharmacol Ther 2012;50:136–41.
3. Buyle F, Vogelaers D, PelemanR, et al. Implementation of guidelines for sequential therapy with fluoroquinolones in a Belgian hospital. Pharm World Sci 2010;32:404–10.
4. Davis SL, Delgado G, McKinnon PS. Pharmacoeconomic consideration associated with the use of intravenous-to-oral moxifloxacin for community-acquired pneumonia Clin Infect Dis 2005;41Supp2;5:136–43.
5. Ho BP, Lau TT, Balen RM, et al. The impact of a pharmacist-managed dosage form conversion service on ciprofloxacin usage at a major Canadian teaching hospital: a pre- and post-intervention study. BMC Health Serv Res 2005;5:48.
6. Kuti JL, Le TN, Nightingale CH, et al. Pharmacoeconomics of a pharmacist-managed program for automatically converting levofloxacin route from iv to oral. Am J Health Sys Pharm 2002;59:2209–15.
7. Cohen SM, Lipsett PA, Buchman TG, et al. Comparison of intravenous/oral ciprofloxacin plus metronidazole versus piperacillin/tazobactam in the treatment of complicated intraabdominal infections. Ann Surg 2000;232:254–62.
8. Plouffe J, Schwartz DB, Kolokathis A, et al. Clinical efficacy of intravenous followed by oral azithromycin monotherapy in hospitalized patients with community-acquired pneumonia. Antimicrob Agents Chemother 2000;44:1796–802.
9. Wetzstein GA. Intravenous to oral (IV:PO) anti-infective combination therapy. Cancer Control 2000;7:170–6.
10. Paladino JA. Pharmacoeconomics of antimicrobial therapy. Am J Healthsys Pharm 1999;56(Supp3):S25–8.
11. Ahkee S, Smith S, et al. Early switch from intravenous to oral antibiotics in hospitalized patient with infections: a six-month prospective study. Pharmacotherapy 1997;17:569–75.
12. Przybylski KG, Rybak MJ, Martin PR, et al. A pharmacist-initiated program of intravenous to oral antibiotic conversion. Pharmacotherapy 1997;17:271–6.
13. Jewesson P. Cost-effectiveness and value of an IV switch. Pharmacoeconomics 1994;5(Supp2):20–6.
14. Ramirez J. Advances in antibiotic use: switch therapy. Curr Ther Res 1994;55(suppA):30–3.
15. Shepard MF. Making the switch from IV to PO. Am J Healthsys Pharm 1994;50:2510.
16. Chessin LN. When to switch from IV to oral antibiotics. Patient Care 1993;15:113–25.
17. Cohen MR. Important news! IV route not needed to justify hospitalization for antibiotics. Hospital Pharmacy 1993;28:946.
18. Schentag JJ. Changes in antimicrobial agent usage resulting from interactions among clinical pharmacy, infectious disease division and the microbiology laboratory. Diagnos Microbiol Infect Dis 1993;16:255–64.
19. Fighetto L. Intravenous to oral stepdown program: four years’ experience in a large teaching hospital. Ann Pharmacother 1992;26:1447–51.
20. Powers T. Clinical and economic effect of ciprofloxacin as an alternative to injectable antimicrobial therapy. Am J Healthsys Pharm 1990;47:1781–4.
21. Ament PW, McGuire WM. Setting up an automatic pharmacist-initiated pharmacokinetic dosing service. Hosp Formul 1993;28:589–92.
22. Mousavi M, Dashti-Khavidaki S, Khalili H, et al. Impact of clinical pharmacy services on stress ulcer prophylaxis prescribing and related cost in patients with renal insufficiency. Int J Pharm Pract 2012;Nov 9.
23. Damaske DL, Baird RW. Development and implementation of a pharmacist managed inpatient warfarin protocol. Proc (Baylor Univ Med Cent) 2005;18:397–400.
24. Radley AS, Hall J, Farrow M, et al. Evaluation of anticoagulation control in a pharmacist operated anticoagulation clinic. J Clin Pathol 1995;48:545–47.
25. Pharmacy and Therapeutics Committee. Medication policies and protocols of Nebraska Methodist Hospital, Methodist Women’s Hospital.
26. University of Kentucky Pharmacy Services. Therapeutic interchanges [Internet]. Available at www.hosp.uky.edu/pharmacy/interchange.asp
27. Bayshore Community Hospital Department of Pharmacy. Conversion of intravenous azithromycin (Zithromax), ceftriaxone (Rocephin), ciprofloxacin (Cipro), fluconazole (Diflucan), lansoprazole (Prevacid), levofloxacin (Levaquin), linezolid (Zyvox), metronidazole (Flagyl), moxifloxacin (Avelox), potassium chloride (KC), or ranitidine (Zantac) to oral medication [Internet]. [cited 2013 May 9]. Available at www.ashp.org/s_ashp/docs/files/R-IVtoPOConvPol-2.pdf.
28. Medication Education Safety Approval Committee, Massachusetts General Hospital. Automatic intravenous to oral protocol [Internet]. MESAC memo; 2005 July [cited 2013 March 9]. Available at www2.massgeneral.org/pharmacy/mesac/mesac_memo3.pdf.
29. Hanson RL, Habibi M, Khamo N, et al. Integrated clinical and specialty pharmacy practice model for management of patients with multiple sclerosis. Am J Health Syst Pharm 2014;71:463–9.
30. Making pharmacists part of the multidisciplinary team. Hosp Case Manag 2014;22:13–6.
31. Preslaski CR, Lat I, MacLaren R, et al. Pharmacist contributions as members of the multidisciplinary ICU team. Chest 2013;144:1687–95.
32. Ripley TL, Adamson PB, Hennebry TA, et al. Collaborative practice model between cardiologists and clinical pharmacist for management of patients with cardiovascular disease in an outpatient clinic. Ann Pharmacother 2014;48:412–9.
33. Smith M, Bates DW, Bodenheimer TS. Pharmacists belong in accountable care organizations and integrated care teams. Health Aff (Millwood) 2013;32:1963–70.
34. Ukens C. New Jersey rewrites its state pharmacy practice act. Drug Topics 2004;148:41.
35. New Jersey Board of Pharmacy Laws. Statute 45:14-64. Inapplicability relative to collaborative drug therapy management in hospital. [Internet] July 2011 [cited 11 September 14]. Available from: www.njconsumeraffairs.gov/laws/pharmlaws.pdf.
36. ASHP Commission on Therapeutics. ASHP therapeutic guidelines on stress ulcer prophylaxis. Am J Health Syst Pharm 1999;56:347–79.
37. Bond CA, Raehl CL. Clinical pharmacy services, pharmacy staffing, and hospital mortality rates. Pharmacotherapy 2007;27:481–93.
38. Bond CA, Raehl CL, Franke T. Interrelationships among mortality rates, drug costs, total cost of care, and length of stay in United States hospitals: summary and recommendations for clinical pharmacy services and staffing. Pharmacotherapy 2001;21:129–41.
A 57-year-old woman with abdominal pain
A 57-year-old woman presented to the emergency department with left lower quadrant pain, which had started 1 week earlier and was constant, dull, aching, and nonradiating. There were no aggravating or alleviating factors. The pain was associated with low-grade fever and nausea. She reported no vomiting, no change in bowel habits, and no hematemesis, hematochezia, or melena. She did not have urinary urgency, frequency, or dysuria. She had no cardiac, respiratory, or neurologic symptoms.
Her medical history included hypothyroidism, type 2 diabetes mellitus, diverticulosis, and chronic obstructive pulmonary disease. Her medications included metformin, insulin, levothyroxine, and inhaled tiotropium. She had no allergies. She had never undergone surgery, including cesarean delivery. She was postmenopausal. She had two children, both of whom had been born vaginally at full term. She denied using alcohol, tobacco, and illicit drugs. Her family history was noncontributory.
On examination, she was not in acute distress. Her temperature was 36.7°C (98.1°F), blood pressure 130/90 mm Hg, heart rate 86 beats per minute and regular, respiratory rate 16 breaths per minute, and oxygen saturation 98% on ambient air. Examination of her head and neck was unremarkable. Cardiopulmonary examination was normal. Abdominal examination revealed normal bowel sounds, mild tenderness in the left lower quadrant with localized guarding, and rebound tenderness. Neurologic examination was unremarkable.
Initial laboratory data showed mild leukocytosis. Computed tomography with contrast of the abdomen and pelvis suggested acute diverticulitis.
ATRIAL FIBRILLATION, AND THEN A TURN FOR THE WORSE
The patient was admitted with an initial diagnosis of acute diverticulitis. She was started on antibiotics, hydration, and pain medications, and her abdominal pain gradually improved.
On the third hospital day, she suddenly experienced shortness of breath and palpitations. At the time of admission her electrocardiogram had been normal, but it now showed atrial fibrillation with a rapid ventricular response. She also developed elevated troponin levels, which were thought to represent type 2 non-ST-elevation myocardial infarction.
She was started on aspirin, clopidogrel, and anticoagulation with heparin bridged with warfarin for the new-onset atrial fibrillation. Her heart rate was controlled with metoprolol, and her shortness of breath improved. An echocardiogram was normal.
On the seventh hospital day, she developed severe right-sided lower abdominal pain and bruising. Her blood pressure was 90/60 mm Hg, heart rate 110 beats per minute and irregularly irregular, respiratory rate 22 breaths per minute, and oxygen saturation 97% on room air. Her abdomen was diffusely tender with a palpable mass in the right lower quadrant and hypoactive bowel sounds. Ecchymosis was noted (Figure 1).
DIFFERENTIAL DIAGNOSIS
1. What is the likely cause of her decompensation?
- Acute mesenteric ischemia
- Perforation of the gastrointestinal tract
- Rectus sheath hematoma
- Abdominal compartment syndrome due to acute pancreatitis
Acute mesenteric ischemia
Signs and symptoms of acute mesenteric ischemia can be vague. Moreover, when it leads to bowel necrosis the mortality rate is high, ranging from 30% to 65%.1 Hence, one should suspect it and try to diagnose it early.
Most patients with this condition have comorbidities; risk factors include atherosclerotic disease, cardiac conditions (congestive heart failure, recent myocardial infarction, and atrial fibrillation), systemic illness, and inherited or acquired hypercoagulable states.2
The four major causes are:
- Acute thromboembolic occlusion of the superior mesenteric artery (the most common site of occlusion because of the acute angle of origin from the aorta)
- Acute thrombosis of the mesenteric vein
- Acute thrombosis of the mesenteric artery
- Nonocclusive disease affecting the mesenteric vessels2
Nonocclusive disease is seen in conditions in which the mesenteric vessels are already compromised due to background stenosis owing to atherosclerosis. Also, conditions such as septic and cardiogenic shock can compromise these arteries, leading to ischemia, which, if it persists, can lead to bowel infarction. Ischemic colitis falls under this category. It commonly involves the descending and sigmoid colon.3
The initial symptom of ischemia may be abdominal pain that is brought on by eating large meals (“postprandial intestinal angina.”2 When the ischemia worsens to infarction, patients may have a diffusely tender abdomen and constant pain that does not vary with palpation. Surprisingly, patients do not exhibit peritoneal signs early on. This gives rise to the description of “pain out of proportion to the physical findings” traditionally associated with acute mesenteric ischemia.2
Diagnosis. Supportive laboratory data include marked leukocytosis, elevated hematocrit due to hemoconcentration, metabolic acidosis, and elevated lactate.4 Newer markers such as serum alpha-glutathione S-transferase (alpha-GST) and intestinal fatty acid-binding protein (I-FABP) may be used to support the diagnosis.
Elevated alpha-GST has 72% sensitivity and 77% specificity in the diagnosis of acute mesenteric ischemia.5 The caveat is that it cannot reliably differentiate ischemia from infarction. Its sensitivity can be improved to 97% to 100% by using the white blood cell count and lactate levels in combination.5
An I-FABP level higher than 100 ng/mL has 100% sensitivity for diagnosing mesenteric infarction but only 25% sensitivity for bowel strangulation.6
Early use of abdominal computed tomography with contrast can aid in recognizing this diagnosis.7 Thus, it should be ordered in suspected cases, even in patients who have elevated creatinine levels (which would normally preclude the use of contrast), since early diagnosis followed by endovascular therapy is associated with survival benefit, and the risk of contrast-induced nephropathy appears to be small.8 Computed tomography helps to determine the state of mesenteric vessels and bowel perfusion before ischemia progresses to infarction. It also helps to rule out other common diagnoses. Findings that suggest acute mesenteric ischemia include segmental bowel wall thickening, intestinal pneumatosis with gas in the portal vein, bowel dilation, mesenteric stranding, portomesenteric thrombosis, and solid-organ infarction.9
Treatment. If superior mesenteric artery occlusion is diagnosed on computed tomography, the next step is to determine if there is peritonitis.10 In patients who have evidence of peritonitis, exploratory laparotomy is performed. For emboli in such patients, open embolectomy followed by on-table angiography is carried out in combination with damage-control surgery. For patients with peritonitis and acute thrombosis, stenting along with damage-control surgery is preferred.10
On the other hand, if there is no peritonitis, the thrombosis may be amenable to endovascular intervention. For patients with acute embolic occlusion with no contraindications to thrombolysis, aspiration embolectomy in combination with local catheter-directed thrombolysis with recombinant tissue plasminogen activator can be performed. This can be combined with endovascular mechanical embolectomy for more complete management.10 Patients with contraindications to thrombolysis can be treated either with aspiration and mechanical embolectomy or with open embolectomy with angiography.10
During laparotomy, the surgeon carefully inspects the bowel for signs of necrosis. Signs that bowel is still viable include pink color, bleeding from cut surfaces, good peristalsis, and visible pulsations in the arterial arcade of the mesentery.
Acute mesenteric artery thrombosis arising from chronic atherosclerotic disease can be treated with stenting of the stenotic lesion.10 Patients with this condition would also benefit from aggressive management of atherosclerotic disease with statins along with antiplatelet agents.10
Mesenteric vein thrombosis requires prompt institution of anticoagulation. However, in advanced cases leading to bowel infarction, exploratory laparotomy with resection of the necrotic bowel may be required. Anticoagulation should be continued for at least 6 months, and further therapy should be determined by the underlying precipitating condition.10
Critically ill patients who develop mesenteric ischemia secondary to persistent hypotension usually respond to adequate volume resuscitation, cessation of vasopressors, and overall improvement in their hemodynamic status. These patients must be closely monitored for development of gangrene of the bowel because they may be intubated and not able to complain. Any sudden deterioration in their condition should prompt physicians to consider bowel necrosis developing in these patients. Elevation of lactate levels out of proportion to the degree of hypotension may be corroborative evidence.4
Our patient had risk factors for acute mesenteric ischemia that included atrial fibrillation and recent non-ST-elevation myocardial infarction. She could have had arterial emboli due to atrial fibrillation, in situ superior mesenteric arterial thrombosis, or splanchnic arterial vasoconstriction due to the myocardial infarction associated with transient hypotension.
Arguing against this diagnosis, although she had a grossly distended abdomen, abdominal bruising usually is not seen. Also, a palpable mass in the right lower quadrant is uncommon except when acute mesenteric ischemia occurs due to segmental intestinal strangulation, as with strangulated hernia or volvulus. She also had therapeutic international normalized ratio (INR) levels constantly while on anticoagulation. Nevertheless, acute mesenteric ischemia should be strongly considered in the initial differential diagnosis of this patient’s acute decompensation.
Perforation of the gastrointestinal tract
Diverticulitis is the acute inflammation of one or more diverticula, which are small pouches created by herniation of the mucosa into the wall of the colon. The condition is caused by microscopic or macroscopic perforation of the diverticula. Microscopic perforation is usually self-limited (uncomplicated diverticulitis) and responds to conservative treatment, whereas macroscopic perforation can be associated with fecal or purulent peritonitis, abscess, enteric fistula, bowel obstruction, and stricture (complicated diverticulitis), in which case surgery may be necessary.
Patients with peritonitis due to free perforation present with generalized tenderness with rebound tenderness and guarding on abdominal examination. The abdomen may be distended and tympanic to percussion, with diminished or absent bowel sounds. Patients may have hemodynamic compromise.
Plain upright abdominal radiographs may show free air under the diaphragm. Computed tomography may show oral contrast outside the lumen and detect even small amounts of free intraperitoneal air (more clearly seen on a lung window setting).
Our patient initially presented with acute diverticulitis. She later developed diffuse abdominal tenderness with hypoactive bowel sounds. Bowel perforation is certainly a possibility at this stage, though it is usually not associated with abdominal bruising. She would need additional imaging to rule out this complication.
Other differential diagnoses to be considered in this patient with right lower-quadrant pain include acute appendicitis, incarcerated inguinal hernia, volvulus (particularly cecal volvulus), small-bowel obstruction, pyelonephritis, and gynecologic causes such as adnexal torsion, ruptured ovarian cyst, and tubo-ovarian abscess. Computed tomography helps to differentiate most of these causes.
Rectus sheath hematoma
Rectus sheath hematoma is relatively uncommon and often not considered in the initial differential diagnosis of an acute abdomen. This gives it the rightful term “a great masquerader.” It usually results from bleeding into the rectus sheath from damage to the superior (more common) or inferior epigastric arteries and occasionally from a direct tear of the rectus abdominis muscle. Predisposing factors include anticoagulant therapy (most common), advanced age, hypertension, previous abdominal surgery, trauma, paroxysmal coughing, medication injections, pregnancy, blood dyscrasias, severe vomiting, violent physical activity, and leukemia.11
Clinical manifestations include acute abdominal pain, often associated with fever, nausea, and vomiting. Physical examination may reveal signs of hypovolemic shock, a palpable nonpulsatile abdominal mass, and signs of local peritoneal irritation. The Carnett sign11 (tenderness within the abdominal wall that persists and does not improve with raising the head) and the Fothergill sign11 (a tender abdominal mass that does not cross the midline and remains palpable with tensing of the rectus sheath) may be elicited.
Computed tomography is more sensitive than abdominal ultrasonography in differentiating rectus sheath hematoma from an intra-abdominal pathology.11 In addition, computed tomography also helps to determine if the bleeding is active or not, which has therapeutic implications.
In our patient, rectus sheath hematoma is a possibility because of her ongoing anticoagulation, findings of localized abdominal bruising, and palpable right lower-quadrant mass, and it is high on the list of differential diagnoses. Rectus sheath hematoma should be considered in the differential diagnosis of lower abdominal pain particularly in elderly women who are on anticoagulation and in whom the onset of pain coincides with a paroxysm of cough.12 Women are twice as likely as men to develop rectus sheath hematoma, owing to their different muscle mass.13 In addition, anterior abdominal wall muscles are stretched during pregnancy.13
Abdominal compartment syndrome
Abdominal compartment syndrome has been classically associated with surgical patients. However, it is being increasingly recognized in critically ill medical patients, in whom detecting and treating it early may result in significant reduction in rates of morbidity and death.14
Abdominal compartment syndrome is of three types: primary, secondary, and recurrent. Primary abdominal compartment syndrome refers to the classic surgical patients with evidence of direct injury to the abdominal or pelvic organs through major trauma or extensive abdominal surgeries. Secondary abdominal compartment syndrome refers to its development in critically ill intensive care patients in whom the pathology does not directly involve the abdominal or pelvic organs.
Various medical conditions can culminate in abdominal compartment syndrome and result in multiorgan failure. Recurrent abdominal compartment syndrome refers to its development after management of either primary or secondary intra-abdominal hypertension or abdominal compartment syndrome.15 Clinicians thus must be aware of secondary and recurrent abdominal compartment syndrome occurring in critically ill patients.
The normal intra-abdominal pressure is around 5 to 7 mm Hg, even in most critically ill patients. Persistent elevation, ie, higher than 12 mm Hg, is referred to as intra-abdominal hypertension.16–18 Intra-abdominal hypertension is subdivided into four grades:
- Grade I: 12–15 mm Hg
- Grade II: 16–20 mm Hg
- Grade III: 21–25 mm Hg
- Grade IV: > 25 mm Hg.
The World Society of the Abdominal Compartment Syndrome (WSACS) defines abdominal compartment syndrome as pressure higher than 20 mm Hg along with organ damage.18 It may or may not be associated with an abdominal perfusion pressure less than 60 mm Hg.18
Risk factors associated with abdominal compartment syndrome include conditions causing decreased gut motility (gastroparesis, ileus, and colonic pseudo-obstruction), intra-abdominal or retroperitoneal masses or abscesses, ascites, hemoperitoneum, acute pancreatitis, third-spacing due to massive fluid resuscitation with transfusions, peritoneal dialysis, and shock.18,19
Physical examination has a sensitivity of only 40% to 60% in detecting intra-abdominal hypertension.20 The gold-standard method of measuring the intra-abdominal pressure is the modified Kron technique,18 using a Foley catheter in the bladder connected to a pressure transducer. With the patient in the supine position, the transducer is zeroed at the mid-axillary line at the level of the iliac crest, and 25 mL of normal saline is instilled into the bladder and maintained for 30 to 60 seconds to let the detrusor muscle relax.15 Pressure tracings are then recorded at the end of expiration. Factors that are known to affect the transbladder pressure include patient position, respiratory movement, and body mass index, and should be taken into account when reading the pressure recordings.15,21 Other techniques that can be used include intragastric, intra-inferior vena cava, and intrarectal approaches.15
The WSACS recommends that any patient admitted to a critical care unit or in whom new organ failure develops should be screened for risk factors for intra-abdominal hypertension and abdominal compartment syndrome. If a patient has at least two of the risk factors suggested by WSACS, a baseline intra-abdominal pressure measurement should be obtained. Patients at risk for intra-abdominal hypertension should have the intra-abdominal pressure measured every 4 to 6 hours. However, in the face of hemodynamic instability and worsening multiorgan failure, the pressure may need to be measured hourly.18
Clinicians managing patients in the intensive care unit should think of intra-abdominal pressure alongside blood pressure, urine output, and mental status when evaluating hemodynamic status. Clinical manifestations of abdominal compartment syndrome reflect the underlying organ dysfunction and include hypotension, refractory shock, decreased urine output, intracranial hypertension, progressive hypoxemia and hypercarbia, elevated pulmonary peak pressures, and worsening of metabolic acidosis.22
Treatment. The standard treatment for primary abdominal compartment syndrome is surgical decompression. According to WSACS guidelines, insertion of a percutaneous drainage catheter should be advocated in patients with gross ascites and in whom decompressive surgery is not feasible. A damage-control resuscitation strategy used for patients undergoing damage-control laparotomy has been found to increase the 30-day survival rate.23 A damage-control resuscitation strategy consists of increasing the use of plasma and platelet transfusions over packed red cell transfusions, limiting the use of crystalloid solutions in early fluid resuscitation, and allowing for permissive hypotension.
Secondary abdominal compartment syndrome is treated conservatively in most cases, since patients with this condition are very poor surgical candidates owing to their comorbidities.18 However, in patients with progressive organ dysfunction in whom medical management has failed, surgical decompression should be considered.18 Medical management of secondary abdominal compartment syndrome depends on the underlying etiology. Strategies include nasogastric or colonic decompression, use of prokinetic agents, paracentesis in cases with gross ascites, and maintaining a cumulative negative fluid balance. The WSACS does not recommend routine use of diuretics, albumin infusion, or renal replacement strategies. Pain should be adequately controlled to improve abdominal wall compliance.18,24 Neuromuscular blockade agents may be used to aid this process. Neostigmine may be used to treat colonic pseudo-obstruction when other conservative methods fail. Use of enteral nutrition should be minimized.18
Our patient might have abdominal compartment syndrome, but a definitive diagnosis cannot be made at this point without measuring the intra-abdominal pressure.
WHICH IMAGING TEST WOULD BE BEST?
2. Which imaging test would be best for establishing the diagnosis in this patient?
- Plain abdominal radiography
- Abdominal ultrasonography
- Computed tomography of the abdomen and pelvis with contrast
- Magnetic resonance imaging of the abdomen and pelvis
Plain abdominal radiography
Plain abdominal radiography can help to determine if there is free gas under the diaphragm (due to bowel perforation), obstructed bowel, sentinel loop, volvulus, or fecoliths causing the abdominal pain. It cannot diagnose rectus sheath hematoma or acute mesenteric ischemia.
Abdominal ultrasonography
Abdominal ultrasonography can be used as the first diagnostic test, as it is widely available, safe, effective, and tolerable. It does not expose the patient to radiation or intravenous contrast agents. It helps to diagnose rectus sheath hematoma and helps to follow its maturation and resolution once a diagnosis is made. It can provide a rapid assessment of the size, location, extent, and physical characteristics of the mass.
Rectus sheath hematoma appears spindle-shaped on sagittal sections and ovoid on coronal sections. It often appears sonolucent in the early stages and sonodense in the late stage, but the appearance may be heterogeneous depending on the combined presence of clot and fresh blood. These findings are sufficient to make the diagnosis.
Abdominal ultrasonography has 85% to 96% sensitivity in diagnosing rectus sheath hematoma.25 It can help diagnose other causes of the abdominal pain, such as renal stones and cholecystitis. It is the preferred imaging test in pediatric patients, pregnant patients, and those with renal insufficiency.
Abdominal computed tomography
Abdominal computed tomography has a sensitivity and specificity of 100% for diagnosing acute rectus sheath hematoma with a duration of less than 5 days.25 It not only helps to determine the precise location and extent, but also helps to determine if there is active extravasation. Even in patients with renal insufficiency, noncontrast computed tomography will help to confirm the diagnosis, although it may not show extravasation or it may miss certain abdominal pathologies because of the lack of contrast.
Acute rectus sheath hematoma appears as a hyperdense mass posterior to the rectus abdominis muscle with ipsilateral anterolateral muscular enlargement. Chronic rectus sheath hematoma appears isodense or hypodense relative to the surrounding muscle. Above the arcuate line, rectus sheath hematoma has a spindle shape; below the arcuate line, it is typically spherical.
In 1996, Berná et al26 classified rectus sheath hematoma into three grades based on findings of computed tomography:
- Grade I is intramuscular and unilateral
- Grade II may involve bilateral rectus muscles without extension into the prevesicular space
- Grade III extends into the peritoneum and prevesicular space
Magnetic resonance imaging
Magnetic resonance imaging is useful to differentiate chronic rectus sheath hematoma (greater than 5-day duration) from an anterior abdominal wall mass. Chronic rectus sheath hematoma will have high signal intensity on both T1- and T2-weighted images up to 10 months after the onset of the hematoma.27
Back to our patient
Since our patient’s symptoms are acute and of less than 5 days’ duration, computed tomography of the abdomen and pelvis would be the best diagnostic test, with therapeutic implications if there is ongoing extravasation.
Computed tomography of the abdomen with contrast showed a new hematoma, measuring 25 by 14 by 13.5 cm, in the right rectus sheath (Figure 2), with no other findings. The hematoma was grade I, since it was intramuscular and unilateral without extension elsewhere.
Laboratory workup showed that the patient’s hematocrit was falling, from 34% to 24%, and her INR was elevated at 2.5. She was resuscitated with fluids, blood transfusion, and fresh-frozen plasma. Anticoagulation was withheld. In spite of resuscitation, her hematocrit kept falling, though she remained hemodynamically stable.
THE WAY FORWARD
3. At this point, what would be the best approach to management in this patient?
- Serial clinical examinations and frequent monitoring of the complete blood cell count
- Urgent surgical consult for exploratory laparotomy with evaluation of the hematoma and ligation of the bleeding vessel
- Repeat computed tomographic angiography to identify a possible bleeding vessel; consideration of radiographically guided arterial embolization
- Measuring the intra-abdominal pressure using the intrabladder pressure for abdominal compartment syndrome and consideration of surgical drainage
The key clinical concern in a patient with a rectus sheath hematoma who is hemodynamically stable is whether the hematoma is expanding. This patient responded to initial resuscitation, but her falling hematocrit was evidence of ongoing bleeding leading to an expanding rectus sheath hematoma. Thus, serial clinical examinations and frequent monitoring of the complete blood cell count would not be enough, as it could miss fatal ongoing bleeding.
Radiographically guided embolization with Gelfoam, thrombin, or coils should be attempted first, as this is less invasive than exploratory laparotomy.28 It can achieve hemostasis, decrease the size of the hematoma, limit the need for blood products, and prevent rupture into the abdomen. If this is unsuccessful, the next step is ligation of the bleeding vessel.29
Surgical treatment includes evacuation of the hematoma, repair of the rectus sheath, ligation of bleeding vessels, and abdominal wall closure. Surgical evacuation or guided drainage of a rectus sheath hematoma on its own is not normally indicated and may indeed cause persistent bleeding by diminishing a potential tamponade effect. However, it may become necessary if the hematoma is very large or infected, if it causes marked respiratory impairment, or if abdominal compartment syndrome is suspected.
Abdominal compartment syndrome is very rare but is associated with a 50% mortality rate.30 It should be suspected in patients with oliguria, low cardiac output, changes in minute ventilation, and altered splanchnic blood flow. The diagnosis is confirmed with indwelling catheter manometry of the bladder to measure intra-abdominal pressure. Intra-abominal pressure above 25 mm Hg should be treated with decompressive laparotomy.30 However, the clinical suspicion of abdominal compartment syndrome was low in this patient.
The best choice at this point would be urgent computed tomographic angiography to identify a bleeding vessel, along with consideration of radiographically guided arterial embolization.
TREATMENT IS USUALLY CONSERVATIVE
Treatment of rectus sheath hematoma is conservative in most hemodynamically stable patients, with embolization or surgical intervention reserved for unstable patients or those in whom the hematoma is expanding.
Knowledge of the grading system of Berná et al26 helps to assess the patient’s risk and to anticipate potential complications. Grade I hematomas are mild and do not necessitate admission. Patients with grade II hematoma can be admitted to the floor for 24 to 48 hours for observation. Grade III usually occurs in patients receiving anticoagulant therapy and frequently requires blood products. These patients have a prolonged hospital stay and more complications, including hypovolemic shock, myonecrosis, acute coronary syndrome, arrhythmias, acute renal failure, small-bowel infarction, and abdominal compartment syndrome—all of which increases the risk of morbidity and death. Thus, patients who are on anticoagulation who develop grade III rectus sheath hematoma should be admitted to the hospital, preferably to the intensive care unit, to ensure that the hematoma is not expanding and to plan reinstitution of anticoagulation as appropriate.
In most cases, rectus sheath hematomas resolve within 1 to 3 months. Resolution of large hematomas may be hastened with the use of pulsed ultrasound.31 However, this treatment should be used only after the acute phase is over, when there is evidence of an organized thrombus and coagulation measures have returned to the target range. This helps to reduce the risk of bleeding and to prevent symptoms from worsening.31
OUR PATIENT’S COURSE
Our patient underwent urgent computed tomographic angiography, which showed a modest increase in the size of the rectus sheath hematoma. However, no definitive blush of contrast was seen to suggest active arterial bleeding. Her hematocrit stabilized, and she remained hemodynamically stable without requiring additional intervention. Most likely her bleeding was self-contained. She had normal intra-abdominal pressure on serial monitoring. She was later transferred to acute inpatient rehabilitation in view of deconditioning and is currently doing well. The hematoma persisted, decreasing only slightly in size over the next 3 weeks.
- Kougias P, Lau D, El Sayed HF, Zhou W, Huynh TT, Lin PH. Determinants of mortality and treatment outcome following surgical interventions for acute mesenteric ischemia. J Vasc Surg 2007; 46:467–474.
- Sise MJ. Acute mesenteric ischemia. Surg Clin North Am 2014; 94:165–181.
- Scharff JR, Longo WE, Vartanian SM, Jacobs DL, Bahadursingh AN, Kaminski DL. Ischemic colitis: spectrum of disease and outcome. Surgery 2003; 134:624–629.
- Lange H, Jäckel R. Usefulness of plasma lactate concentration in the diagnosis of acute abdominal disease. Eur J Surg 1994; 160:381–384.
- Gearhart SL, Delaney CP, Senagore AJ, et al. Prospective assessment of the predictive value of alpha-glutathione S-transferase for intestinal ischemia. Am Surg 2003; 69:324–329.
- Kanda T, Fujii H, Tani T, et al. Intestinal fatty acid-binding protein is a useful diagnostic marker for mesenteric infarction in humans. Gastroenterology 1996; 110:339–343.
- Menke J. Diagnostic accuracy of multidetector CT in acute mesenteric ischemia: systematic review and meta-analysis. Radiology 2010; 256:93–101.
- Acosta S, Björnsson S, Ekberg O, Resch T. CT angiography followed by endovascular intervention for acute superior mesenteric artery occlusion does not increase risk of contrast-induced renal failure. Eur J Vasc Endovasc Surg 2010; 39:726–730.
- Clark RA. Computed tomography of bowel infarction. J Comput Assist Tomogr 1987; 11:757–762.
- Acosta S, Björck M. Modern treatment of acute mesenteric ischaemia. Br J Surg 2014; 101:e100–e108.
- Smithson A, Ruiz J, Perello R, Valverde M, Ramos J, Garzo L. Diagnostic and management of spontaneous rectus sheath hematoma. Eur J Intern Med 2013; 24:579–582.
- Moreno Gallego A, Aguayo JL, Flores B, et al. Ultrasonography and computed tomography reduce unnecessary surgery in abdominal rectus sheath haematoma. Br J Surg 1997; 84:1295–1297.
- Dubinsky IL. Hematoma of the rectus abdominis muscle: case report and review of the literature. J Emerg Med 1997; 15:165–167.
- Yi M, Yao G, Bai Y. The monitoring of intra-abdominal pressure in critically ill patients. (In Chinese.) Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2014; 26:175–178.
- Hunt L, Frost SA, Hillman K, Newton PJ, Davidson PM. Management of intra-abdominal hypertension and abdominal compartment syndrome: a review. J Trauma Manag Outcomes 2014; 8:2.
- Malbrain ML, Cheatham ML, Kirkpatrick A, et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med 2006; 32:1722–1732.
- Malbrain ML, Chiumello D, Cesana BM, et al; WAKE-Up! Investigators. A systematic review and individual patient data meta-analysis on intra-abdominal hypertension in critically ill patients: the wake-up project. World initiative on Abdominal Hypertension Epidemiology, a Unifying Project (WAKE-Up!). Minerva Anestesiol 2014; 80:293–306.
- Kirkpatrick AW, Roberts DJ, De Waele J, et al; Pediatric Guidelines Sub-Committee for the World Society of the Abdominal Compartment Syndrome. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med 2013; 39:1190–1206.
- Holodinsky JK, Roberts DJ, Ball CG, et al. Risk factors for intra-abdominal hypertension and abdominal compartment syndrome among adult intensive care unit patients: a systematic review and meta-analysis. Crit Care 2013; 17:R249.
- Sugrue M, Bauman A, Jones F, et al. Clinical examination is an inaccurate predictor of intraabdominal pressure. World J Surg 2002; 26:1428–1431.
- Cheatham ML, De Waele JJ, De Laet I, et al; World Society of the Abdominal Compartment Syndrome (WSACS) Clinical Trials Working Group. The impact of body position on intra-abdominal pressure measurement: a multicenter analysis. Crit Care Med 2009; 37:2187–2190.
- Ortiz-Diaz E, Lan CK. Intra-abdominal hypertension in medical critically ill patients: a narrative review. Shock 2014; 41:175–180.
- Cotton BA, Reddy N, Hatch QM, et al. Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg 2011; 254:598–605.
- An G, West MA. Abdominal compartment syndrome: a concise clinical review. Crit Care Med 2008; 36:1304–1310.
- Tolcher MC, Nitsche JF, Arendt KW, Rose CH. Spontaneous rectus sheath hematoma pregnancy: case report and review of the literature. Obstet Gynecol Surv 2010; 65:517–522.
- Berná JD, Garcia-Medina V, Guirao J, Garcia-Medina J. Rectus sheath hematoma: diagnostic classification by CT. Abdom Imaging 1996; 21:62–64.
- Unger EC, Glazer HS, Lee JK, Ling D. MRI of extracranial hematomas: preliminary observations. AJR Am J Roentgenol 1986; 146:403–407.
- Rimola J, Perendreu J, Falcó J, Fortuño JR, Massuet A, Branera J. Percutaneous arterial embolization in the management of rectus sheath hematoma. AJR Am J Roentgenol 2007; 188:W497–W502.
- Titone C, Lipsius M, Krakauer JS. “Spontaneous” hematoma of the rectus abdominis muscle: critical review of 50 cases with emphasis on early diagnosis and treatment. Surgery 1972; 72:568–572.
- Osinbowale O, Bartholomew JR. Rectus sheath hematoma. Vasc Med 2008; 13:275–279.
- Berná-Serna JD, Sánchez-Garre J, Madrigal M, Zuazu I, Berná-Mestre JD. Ultrasound therapy in rectus sheath hematoma. Phys Ther 2005; 85:352–357.
A 57-year-old woman presented to the emergency department with left lower quadrant pain, which had started 1 week earlier and was constant, dull, aching, and nonradiating. There were no aggravating or alleviating factors. The pain was associated with low-grade fever and nausea. She reported no vomiting, no change in bowel habits, and no hematemesis, hematochezia, or melena. She did not have urinary urgency, frequency, or dysuria. She had no cardiac, respiratory, or neurologic symptoms.
Her medical history included hypothyroidism, type 2 diabetes mellitus, diverticulosis, and chronic obstructive pulmonary disease. Her medications included metformin, insulin, levothyroxine, and inhaled tiotropium. She had no allergies. She had never undergone surgery, including cesarean delivery. She was postmenopausal. She had two children, both of whom had been born vaginally at full term. She denied using alcohol, tobacco, and illicit drugs. Her family history was noncontributory.
On examination, she was not in acute distress. Her temperature was 36.7°C (98.1°F), blood pressure 130/90 mm Hg, heart rate 86 beats per minute and regular, respiratory rate 16 breaths per minute, and oxygen saturation 98% on ambient air. Examination of her head and neck was unremarkable. Cardiopulmonary examination was normal. Abdominal examination revealed normal bowel sounds, mild tenderness in the left lower quadrant with localized guarding, and rebound tenderness. Neurologic examination was unremarkable.
Initial laboratory data showed mild leukocytosis. Computed tomography with contrast of the abdomen and pelvis suggested acute diverticulitis.
ATRIAL FIBRILLATION, AND THEN A TURN FOR THE WORSE
The patient was admitted with an initial diagnosis of acute diverticulitis. She was started on antibiotics, hydration, and pain medications, and her abdominal pain gradually improved.
On the third hospital day, she suddenly experienced shortness of breath and palpitations. At the time of admission her electrocardiogram had been normal, but it now showed atrial fibrillation with a rapid ventricular response. She also developed elevated troponin levels, which were thought to represent type 2 non-ST-elevation myocardial infarction.
She was started on aspirin, clopidogrel, and anticoagulation with heparin bridged with warfarin for the new-onset atrial fibrillation. Her heart rate was controlled with metoprolol, and her shortness of breath improved. An echocardiogram was normal.
On the seventh hospital day, she developed severe right-sided lower abdominal pain and bruising. Her blood pressure was 90/60 mm Hg, heart rate 110 beats per minute and irregularly irregular, respiratory rate 22 breaths per minute, and oxygen saturation 97% on room air. Her abdomen was diffusely tender with a palpable mass in the right lower quadrant and hypoactive bowel sounds. Ecchymosis was noted (Figure 1).
DIFFERENTIAL DIAGNOSIS
1. What is the likely cause of her decompensation?
- Acute mesenteric ischemia
- Perforation of the gastrointestinal tract
- Rectus sheath hematoma
- Abdominal compartment syndrome due to acute pancreatitis
Acute mesenteric ischemia
Signs and symptoms of acute mesenteric ischemia can be vague. Moreover, when it leads to bowel necrosis the mortality rate is high, ranging from 30% to 65%.1 Hence, one should suspect it and try to diagnose it early.
Most patients with this condition have comorbidities; risk factors include atherosclerotic disease, cardiac conditions (congestive heart failure, recent myocardial infarction, and atrial fibrillation), systemic illness, and inherited or acquired hypercoagulable states.2
The four major causes are:
- Acute thromboembolic occlusion of the superior mesenteric artery (the most common site of occlusion because of the acute angle of origin from the aorta)
- Acute thrombosis of the mesenteric vein
- Acute thrombosis of the mesenteric artery
- Nonocclusive disease affecting the mesenteric vessels2
Nonocclusive disease is seen in conditions in which the mesenteric vessels are already compromised due to background stenosis owing to atherosclerosis. Also, conditions such as septic and cardiogenic shock can compromise these arteries, leading to ischemia, which, if it persists, can lead to bowel infarction. Ischemic colitis falls under this category. It commonly involves the descending and sigmoid colon.3
The initial symptom of ischemia may be abdominal pain that is brought on by eating large meals (“postprandial intestinal angina.”2 When the ischemia worsens to infarction, patients may have a diffusely tender abdomen and constant pain that does not vary with palpation. Surprisingly, patients do not exhibit peritoneal signs early on. This gives rise to the description of “pain out of proportion to the physical findings” traditionally associated with acute mesenteric ischemia.2
Diagnosis. Supportive laboratory data include marked leukocytosis, elevated hematocrit due to hemoconcentration, metabolic acidosis, and elevated lactate.4 Newer markers such as serum alpha-glutathione S-transferase (alpha-GST) and intestinal fatty acid-binding protein (I-FABP) may be used to support the diagnosis.
Elevated alpha-GST has 72% sensitivity and 77% specificity in the diagnosis of acute mesenteric ischemia.5 The caveat is that it cannot reliably differentiate ischemia from infarction. Its sensitivity can be improved to 97% to 100% by using the white blood cell count and lactate levels in combination.5
An I-FABP level higher than 100 ng/mL has 100% sensitivity for diagnosing mesenteric infarction but only 25% sensitivity for bowel strangulation.6
Early use of abdominal computed tomography with contrast can aid in recognizing this diagnosis.7 Thus, it should be ordered in suspected cases, even in patients who have elevated creatinine levels (which would normally preclude the use of contrast), since early diagnosis followed by endovascular therapy is associated with survival benefit, and the risk of contrast-induced nephropathy appears to be small.8 Computed tomography helps to determine the state of mesenteric vessels and bowel perfusion before ischemia progresses to infarction. It also helps to rule out other common diagnoses. Findings that suggest acute mesenteric ischemia include segmental bowel wall thickening, intestinal pneumatosis with gas in the portal vein, bowel dilation, mesenteric stranding, portomesenteric thrombosis, and solid-organ infarction.9
Treatment. If superior mesenteric artery occlusion is diagnosed on computed tomography, the next step is to determine if there is peritonitis.10 In patients who have evidence of peritonitis, exploratory laparotomy is performed. For emboli in such patients, open embolectomy followed by on-table angiography is carried out in combination with damage-control surgery. For patients with peritonitis and acute thrombosis, stenting along with damage-control surgery is preferred.10
On the other hand, if there is no peritonitis, the thrombosis may be amenable to endovascular intervention. For patients with acute embolic occlusion with no contraindications to thrombolysis, aspiration embolectomy in combination with local catheter-directed thrombolysis with recombinant tissue plasminogen activator can be performed. This can be combined with endovascular mechanical embolectomy for more complete management.10 Patients with contraindications to thrombolysis can be treated either with aspiration and mechanical embolectomy or with open embolectomy with angiography.10
During laparotomy, the surgeon carefully inspects the bowel for signs of necrosis. Signs that bowel is still viable include pink color, bleeding from cut surfaces, good peristalsis, and visible pulsations in the arterial arcade of the mesentery.
Acute mesenteric artery thrombosis arising from chronic atherosclerotic disease can be treated with stenting of the stenotic lesion.10 Patients with this condition would also benefit from aggressive management of atherosclerotic disease with statins along with antiplatelet agents.10
Mesenteric vein thrombosis requires prompt institution of anticoagulation. However, in advanced cases leading to bowel infarction, exploratory laparotomy with resection of the necrotic bowel may be required. Anticoagulation should be continued for at least 6 months, and further therapy should be determined by the underlying precipitating condition.10
Critically ill patients who develop mesenteric ischemia secondary to persistent hypotension usually respond to adequate volume resuscitation, cessation of vasopressors, and overall improvement in their hemodynamic status. These patients must be closely monitored for development of gangrene of the bowel because they may be intubated and not able to complain. Any sudden deterioration in their condition should prompt physicians to consider bowel necrosis developing in these patients. Elevation of lactate levels out of proportion to the degree of hypotension may be corroborative evidence.4
Our patient had risk factors for acute mesenteric ischemia that included atrial fibrillation and recent non-ST-elevation myocardial infarction. She could have had arterial emboli due to atrial fibrillation, in situ superior mesenteric arterial thrombosis, or splanchnic arterial vasoconstriction due to the myocardial infarction associated with transient hypotension.
Arguing against this diagnosis, although she had a grossly distended abdomen, abdominal bruising usually is not seen. Also, a palpable mass in the right lower quadrant is uncommon except when acute mesenteric ischemia occurs due to segmental intestinal strangulation, as with strangulated hernia or volvulus. She also had therapeutic international normalized ratio (INR) levels constantly while on anticoagulation. Nevertheless, acute mesenteric ischemia should be strongly considered in the initial differential diagnosis of this patient’s acute decompensation.
Perforation of the gastrointestinal tract
Diverticulitis is the acute inflammation of one or more diverticula, which are small pouches created by herniation of the mucosa into the wall of the colon. The condition is caused by microscopic or macroscopic perforation of the diverticula. Microscopic perforation is usually self-limited (uncomplicated diverticulitis) and responds to conservative treatment, whereas macroscopic perforation can be associated with fecal or purulent peritonitis, abscess, enteric fistula, bowel obstruction, and stricture (complicated diverticulitis), in which case surgery may be necessary.
Patients with peritonitis due to free perforation present with generalized tenderness with rebound tenderness and guarding on abdominal examination. The abdomen may be distended and tympanic to percussion, with diminished or absent bowel sounds. Patients may have hemodynamic compromise.
Plain upright abdominal radiographs may show free air under the diaphragm. Computed tomography may show oral contrast outside the lumen and detect even small amounts of free intraperitoneal air (more clearly seen on a lung window setting).
Our patient initially presented with acute diverticulitis. She later developed diffuse abdominal tenderness with hypoactive bowel sounds. Bowel perforation is certainly a possibility at this stage, though it is usually not associated with abdominal bruising. She would need additional imaging to rule out this complication.
Other differential diagnoses to be considered in this patient with right lower-quadrant pain include acute appendicitis, incarcerated inguinal hernia, volvulus (particularly cecal volvulus), small-bowel obstruction, pyelonephritis, and gynecologic causes such as adnexal torsion, ruptured ovarian cyst, and tubo-ovarian abscess. Computed tomography helps to differentiate most of these causes.
Rectus sheath hematoma
Rectus sheath hematoma is relatively uncommon and often not considered in the initial differential diagnosis of an acute abdomen. This gives it the rightful term “a great masquerader.” It usually results from bleeding into the rectus sheath from damage to the superior (more common) or inferior epigastric arteries and occasionally from a direct tear of the rectus abdominis muscle. Predisposing factors include anticoagulant therapy (most common), advanced age, hypertension, previous abdominal surgery, trauma, paroxysmal coughing, medication injections, pregnancy, blood dyscrasias, severe vomiting, violent physical activity, and leukemia.11
Clinical manifestations include acute abdominal pain, often associated with fever, nausea, and vomiting. Physical examination may reveal signs of hypovolemic shock, a palpable nonpulsatile abdominal mass, and signs of local peritoneal irritation. The Carnett sign11 (tenderness within the abdominal wall that persists and does not improve with raising the head) and the Fothergill sign11 (a tender abdominal mass that does not cross the midline and remains palpable with tensing of the rectus sheath) may be elicited.
Computed tomography is more sensitive than abdominal ultrasonography in differentiating rectus sheath hematoma from an intra-abdominal pathology.11 In addition, computed tomography also helps to determine if the bleeding is active or not, which has therapeutic implications.
In our patient, rectus sheath hematoma is a possibility because of her ongoing anticoagulation, findings of localized abdominal bruising, and palpable right lower-quadrant mass, and it is high on the list of differential diagnoses. Rectus sheath hematoma should be considered in the differential diagnosis of lower abdominal pain particularly in elderly women who are on anticoagulation and in whom the onset of pain coincides with a paroxysm of cough.12 Women are twice as likely as men to develop rectus sheath hematoma, owing to their different muscle mass.13 In addition, anterior abdominal wall muscles are stretched during pregnancy.13
Abdominal compartment syndrome
Abdominal compartment syndrome has been classically associated with surgical patients. However, it is being increasingly recognized in critically ill medical patients, in whom detecting and treating it early may result in significant reduction in rates of morbidity and death.14
Abdominal compartment syndrome is of three types: primary, secondary, and recurrent. Primary abdominal compartment syndrome refers to the classic surgical patients with evidence of direct injury to the abdominal or pelvic organs through major trauma or extensive abdominal surgeries. Secondary abdominal compartment syndrome refers to its development in critically ill intensive care patients in whom the pathology does not directly involve the abdominal or pelvic organs.
Various medical conditions can culminate in abdominal compartment syndrome and result in multiorgan failure. Recurrent abdominal compartment syndrome refers to its development after management of either primary or secondary intra-abdominal hypertension or abdominal compartment syndrome.15 Clinicians thus must be aware of secondary and recurrent abdominal compartment syndrome occurring in critically ill patients.
The normal intra-abdominal pressure is around 5 to 7 mm Hg, even in most critically ill patients. Persistent elevation, ie, higher than 12 mm Hg, is referred to as intra-abdominal hypertension.16–18 Intra-abdominal hypertension is subdivided into four grades:
- Grade I: 12–15 mm Hg
- Grade II: 16–20 mm Hg
- Grade III: 21–25 mm Hg
- Grade IV: > 25 mm Hg.
The World Society of the Abdominal Compartment Syndrome (WSACS) defines abdominal compartment syndrome as pressure higher than 20 mm Hg along with organ damage.18 It may or may not be associated with an abdominal perfusion pressure less than 60 mm Hg.18
Risk factors associated with abdominal compartment syndrome include conditions causing decreased gut motility (gastroparesis, ileus, and colonic pseudo-obstruction), intra-abdominal or retroperitoneal masses or abscesses, ascites, hemoperitoneum, acute pancreatitis, third-spacing due to massive fluid resuscitation with transfusions, peritoneal dialysis, and shock.18,19
Physical examination has a sensitivity of only 40% to 60% in detecting intra-abdominal hypertension.20 The gold-standard method of measuring the intra-abdominal pressure is the modified Kron technique,18 using a Foley catheter in the bladder connected to a pressure transducer. With the patient in the supine position, the transducer is zeroed at the mid-axillary line at the level of the iliac crest, and 25 mL of normal saline is instilled into the bladder and maintained for 30 to 60 seconds to let the detrusor muscle relax.15 Pressure tracings are then recorded at the end of expiration. Factors that are known to affect the transbladder pressure include patient position, respiratory movement, and body mass index, and should be taken into account when reading the pressure recordings.15,21 Other techniques that can be used include intragastric, intra-inferior vena cava, and intrarectal approaches.15
The WSACS recommends that any patient admitted to a critical care unit or in whom new organ failure develops should be screened for risk factors for intra-abdominal hypertension and abdominal compartment syndrome. If a patient has at least two of the risk factors suggested by WSACS, a baseline intra-abdominal pressure measurement should be obtained. Patients at risk for intra-abdominal hypertension should have the intra-abdominal pressure measured every 4 to 6 hours. However, in the face of hemodynamic instability and worsening multiorgan failure, the pressure may need to be measured hourly.18
Clinicians managing patients in the intensive care unit should think of intra-abdominal pressure alongside blood pressure, urine output, and mental status when evaluating hemodynamic status. Clinical manifestations of abdominal compartment syndrome reflect the underlying organ dysfunction and include hypotension, refractory shock, decreased urine output, intracranial hypertension, progressive hypoxemia and hypercarbia, elevated pulmonary peak pressures, and worsening of metabolic acidosis.22
Treatment. The standard treatment for primary abdominal compartment syndrome is surgical decompression. According to WSACS guidelines, insertion of a percutaneous drainage catheter should be advocated in patients with gross ascites and in whom decompressive surgery is not feasible. A damage-control resuscitation strategy used for patients undergoing damage-control laparotomy has been found to increase the 30-day survival rate.23 A damage-control resuscitation strategy consists of increasing the use of plasma and platelet transfusions over packed red cell transfusions, limiting the use of crystalloid solutions in early fluid resuscitation, and allowing for permissive hypotension.
Secondary abdominal compartment syndrome is treated conservatively in most cases, since patients with this condition are very poor surgical candidates owing to their comorbidities.18 However, in patients with progressive organ dysfunction in whom medical management has failed, surgical decompression should be considered.18 Medical management of secondary abdominal compartment syndrome depends on the underlying etiology. Strategies include nasogastric or colonic decompression, use of prokinetic agents, paracentesis in cases with gross ascites, and maintaining a cumulative negative fluid balance. The WSACS does not recommend routine use of diuretics, albumin infusion, or renal replacement strategies. Pain should be adequately controlled to improve abdominal wall compliance.18,24 Neuromuscular blockade agents may be used to aid this process. Neostigmine may be used to treat colonic pseudo-obstruction when other conservative methods fail. Use of enteral nutrition should be minimized.18
Our patient might have abdominal compartment syndrome, but a definitive diagnosis cannot be made at this point without measuring the intra-abdominal pressure.
WHICH IMAGING TEST WOULD BE BEST?
2. Which imaging test would be best for establishing the diagnosis in this patient?
- Plain abdominal radiography
- Abdominal ultrasonography
- Computed tomography of the abdomen and pelvis with contrast
- Magnetic resonance imaging of the abdomen and pelvis
Plain abdominal radiography
Plain abdominal radiography can help to determine if there is free gas under the diaphragm (due to bowel perforation), obstructed bowel, sentinel loop, volvulus, or fecoliths causing the abdominal pain. It cannot diagnose rectus sheath hematoma or acute mesenteric ischemia.
Abdominal ultrasonography
Abdominal ultrasonography can be used as the first diagnostic test, as it is widely available, safe, effective, and tolerable. It does not expose the patient to radiation or intravenous contrast agents. It helps to diagnose rectus sheath hematoma and helps to follow its maturation and resolution once a diagnosis is made. It can provide a rapid assessment of the size, location, extent, and physical characteristics of the mass.
Rectus sheath hematoma appears spindle-shaped on sagittal sections and ovoid on coronal sections. It often appears sonolucent in the early stages and sonodense in the late stage, but the appearance may be heterogeneous depending on the combined presence of clot and fresh blood. These findings are sufficient to make the diagnosis.
Abdominal ultrasonography has 85% to 96% sensitivity in diagnosing rectus sheath hematoma.25 It can help diagnose other causes of the abdominal pain, such as renal stones and cholecystitis. It is the preferred imaging test in pediatric patients, pregnant patients, and those with renal insufficiency.
Abdominal computed tomography
Abdominal computed tomography has a sensitivity and specificity of 100% for diagnosing acute rectus sheath hematoma with a duration of less than 5 days.25 It not only helps to determine the precise location and extent, but also helps to determine if there is active extravasation. Even in patients with renal insufficiency, noncontrast computed tomography will help to confirm the diagnosis, although it may not show extravasation or it may miss certain abdominal pathologies because of the lack of contrast.
Acute rectus sheath hematoma appears as a hyperdense mass posterior to the rectus abdominis muscle with ipsilateral anterolateral muscular enlargement. Chronic rectus sheath hematoma appears isodense or hypodense relative to the surrounding muscle. Above the arcuate line, rectus sheath hematoma has a spindle shape; below the arcuate line, it is typically spherical.
In 1996, Berná et al26 classified rectus sheath hematoma into three grades based on findings of computed tomography:
- Grade I is intramuscular and unilateral
- Grade II may involve bilateral rectus muscles without extension into the prevesicular space
- Grade III extends into the peritoneum and prevesicular space
Magnetic resonance imaging
Magnetic resonance imaging is useful to differentiate chronic rectus sheath hematoma (greater than 5-day duration) from an anterior abdominal wall mass. Chronic rectus sheath hematoma will have high signal intensity on both T1- and T2-weighted images up to 10 months after the onset of the hematoma.27
Back to our patient
Since our patient’s symptoms are acute and of less than 5 days’ duration, computed tomography of the abdomen and pelvis would be the best diagnostic test, with therapeutic implications if there is ongoing extravasation.
Computed tomography of the abdomen with contrast showed a new hematoma, measuring 25 by 14 by 13.5 cm, in the right rectus sheath (Figure 2), with no other findings. The hematoma was grade I, since it was intramuscular and unilateral without extension elsewhere.
Laboratory workup showed that the patient’s hematocrit was falling, from 34% to 24%, and her INR was elevated at 2.5. She was resuscitated with fluids, blood transfusion, and fresh-frozen plasma. Anticoagulation was withheld. In spite of resuscitation, her hematocrit kept falling, though she remained hemodynamically stable.
THE WAY FORWARD
3. At this point, what would be the best approach to management in this patient?
- Serial clinical examinations and frequent monitoring of the complete blood cell count
- Urgent surgical consult for exploratory laparotomy with evaluation of the hematoma and ligation of the bleeding vessel
- Repeat computed tomographic angiography to identify a possible bleeding vessel; consideration of radiographically guided arterial embolization
- Measuring the intra-abdominal pressure using the intrabladder pressure for abdominal compartment syndrome and consideration of surgical drainage
The key clinical concern in a patient with a rectus sheath hematoma who is hemodynamically stable is whether the hematoma is expanding. This patient responded to initial resuscitation, but her falling hematocrit was evidence of ongoing bleeding leading to an expanding rectus sheath hematoma. Thus, serial clinical examinations and frequent monitoring of the complete blood cell count would not be enough, as it could miss fatal ongoing bleeding.
Radiographically guided embolization with Gelfoam, thrombin, or coils should be attempted first, as this is less invasive than exploratory laparotomy.28 It can achieve hemostasis, decrease the size of the hematoma, limit the need for blood products, and prevent rupture into the abdomen. If this is unsuccessful, the next step is ligation of the bleeding vessel.29
Surgical treatment includes evacuation of the hematoma, repair of the rectus sheath, ligation of bleeding vessels, and abdominal wall closure. Surgical evacuation or guided drainage of a rectus sheath hematoma on its own is not normally indicated and may indeed cause persistent bleeding by diminishing a potential tamponade effect. However, it may become necessary if the hematoma is very large or infected, if it causes marked respiratory impairment, or if abdominal compartment syndrome is suspected.
Abdominal compartment syndrome is very rare but is associated with a 50% mortality rate.30 It should be suspected in patients with oliguria, low cardiac output, changes in minute ventilation, and altered splanchnic blood flow. The diagnosis is confirmed with indwelling catheter manometry of the bladder to measure intra-abdominal pressure. Intra-abominal pressure above 25 mm Hg should be treated with decompressive laparotomy.30 However, the clinical suspicion of abdominal compartment syndrome was low in this patient.
The best choice at this point would be urgent computed tomographic angiography to identify a bleeding vessel, along with consideration of radiographically guided arterial embolization.
TREATMENT IS USUALLY CONSERVATIVE
Treatment of rectus sheath hematoma is conservative in most hemodynamically stable patients, with embolization or surgical intervention reserved for unstable patients or those in whom the hematoma is expanding.
Knowledge of the grading system of Berná et al26 helps to assess the patient’s risk and to anticipate potential complications. Grade I hematomas are mild and do not necessitate admission. Patients with grade II hematoma can be admitted to the floor for 24 to 48 hours for observation. Grade III usually occurs in patients receiving anticoagulant therapy and frequently requires blood products. These patients have a prolonged hospital stay and more complications, including hypovolemic shock, myonecrosis, acute coronary syndrome, arrhythmias, acute renal failure, small-bowel infarction, and abdominal compartment syndrome—all of which increases the risk of morbidity and death. Thus, patients who are on anticoagulation who develop grade III rectus sheath hematoma should be admitted to the hospital, preferably to the intensive care unit, to ensure that the hematoma is not expanding and to plan reinstitution of anticoagulation as appropriate.
In most cases, rectus sheath hematomas resolve within 1 to 3 months. Resolution of large hematomas may be hastened with the use of pulsed ultrasound.31 However, this treatment should be used only after the acute phase is over, when there is evidence of an organized thrombus and coagulation measures have returned to the target range. This helps to reduce the risk of bleeding and to prevent symptoms from worsening.31
OUR PATIENT’S COURSE
Our patient underwent urgent computed tomographic angiography, which showed a modest increase in the size of the rectus sheath hematoma. However, no definitive blush of contrast was seen to suggest active arterial bleeding. Her hematocrit stabilized, and she remained hemodynamically stable without requiring additional intervention. Most likely her bleeding was self-contained. She had normal intra-abdominal pressure on serial monitoring. She was later transferred to acute inpatient rehabilitation in view of deconditioning and is currently doing well. The hematoma persisted, decreasing only slightly in size over the next 3 weeks.
A 57-year-old woman presented to the emergency department with left lower quadrant pain, which had started 1 week earlier and was constant, dull, aching, and nonradiating. There were no aggravating or alleviating factors. The pain was associated with low-grade fever and nausea. She reported no vomiting, no change in bowel habits, and no hematemesis, hematochezia, or melena. She did not have urinary urgency, frequency, or dysuria. She had no cardiac, respiratory, or neurologic symptoms.
Her medical history included hypothyroidism, type 2 diabetes mellitus, diverticulosis, and chronic obstructive pulmonary disease. Her medications included metformin, insulin, levothyroxine, and inhaled tiotropium. She had no allergies. She had never undergone surgery, including cesarean delivery. She was postmenopausal. She had two children, both of whom had been born vaginally at full term. She denied using alcohol, tobacco, and illicit drugs. Her family history was noncontributory.
On examination, she was not in acute distress. Her temperature was 36.7°C (98.1°F), blood pressure 130/90 mm Hg, heart rate 86 beats per minute and regular, respiratory rate 16 breaths per minute, and oxygen saturation 98% on ambient air. Examination of her head and neck was unremarkable. Cardiopulmonary examination was normal. Abdominal examination revealed normal bowel sounds, mild tenderness in the left lower quadrant with localized guarding, and rebound tenderness. Neurologic examination was unremarkable.
Initial laboratory data showed mild leukocytosis. Computed tomography with contrast of the abdomen and pelvis suggested acute diverticulitis.
ATRIAL FIBRILLATION, AND THEN A TURN FOR THE WORSE
The patient was admitted with an initial diagnosis of acute diverticulitis. She was started on antibiotics, hydration, and pain medications, and her abdominal pain gradually improved.
On the third hospital day, she suddenly experienced shortness of breath and palpitations. At the time of admission her electrocardiogram had been normal, but it now showed atrial fibrillation with a rapid ventricular response. She also developed elevated troponin levels, which were thought to represent type 2 non-ST-elevation myocardial infarction.
She was started on aspirin, clopidogrel, and anticoagulation with heparin bridged with warfarin for the new-onset atrial fibrillation. Her heart rate was controlled with metoprolol, and her shortness of breath improved. An echocardiogram was normal.
On the seventh hospital day, she developed severe right-sided lower abdominal pain and bruising. Her blood pressure was 90/60 mm Hg, heart rate 110 beats per minute and irregularly irregular, respiratory rate 22 breaths per minute, and oxygen saturation 97% on room air. Her abdomen was diffusely tender with a palpable mass in the right lower quadrant and hypoactive bowel sounds. Ecchymosis was noted (Figure 1).
DIFFERENTIAL DIAGNOSIS
1. What is the likely cause of her decompensation?
- Acute mesenteric ischemia
- Perforation of the gastrointestinal tract
- Rectus sheath hematoma
- Abdominal compartment syndrome due to acute pancreatitis
Acute mesenteric ischemia
Signs and symptoms of acute mesenteric ischemia can be vague. Moreover, when it leads to bowel necrosis the mortality rate is high, ranging from 30% to 65%.1 Hence, one should suspect it and try to diagnose it early.
Most patients with this condition have comorbidities; risk factors include atherosclerotic disease, cardiac conditions (congestive heart failure, recent myocardial infarction, and atrial fibrillation), systemic illness, and inherited or acquired hypercoagulable states.2
The four major causes are:
- Acute thromboembolic occlusion of the superior mesenteric artery (the most common site of occlusion because of the acute angle of origin from the aorta)
- Acute thrombosis of the mesenteric vein
- Acute thrombosis of the mesenteric artery
- Nonocclusive disease affecting the mesenteric vessels2
Nonocclusive disease is seen in conditions in which the mesenteric vessels are already compromised due to background stenosis owing to atherosclerosis. Also, conditions such as septic and cardiogenic shock can compromise these arteries, leading to ischemia, which, if it persists, can lead to bowel infarction. Ischemic colitis falls under this category. It commonly involves the descending and sigmoid colon.3
The initial symptom of ischemia may be abdominal pain that is brought on by eating large meals (“postprandial intestinal angina.”2 When the ischemia worsens to infarction, patients may have a diffusely tender abdomen and constant pain that does not vary with palpation. Surprisingly, patients do not exhibit peritoneal signs early on. This gives rise to the description of “pain out of proportion to the physical findings” traditionally associated with acute mesenteric ischemia.2
Diagnosis. Supportive laboratory data include marked leukocytosis, elevated hematocrit due to hemoconcentration, metabolic acidosis, and elevated lactate.4 Newer markers such as serum alpha-glutathione S-transferase (alpha-GST) and intestinal fatty acid-binding protein (I-FABP) may be used to support the diagnosis.
Elevated alpha-GST has 72% sensitivity and 77% specificity in the diagnosis of acute mesenteric ischemia.5 The caveat is that it cannot reliably differentiate ischemia from infarction. Its sensitivity can be improved to 97% to 100% by using the white blood cell count and lactate levels in combination.5
An I-FABP level higher than 100 ng/mL has 100% sensitivity for diagnosing mesenteric infarction but only 25% sensitivity for bowel strangulation.6
Early use of abdominal computed tomography with contrast can aid in recognizing this diagnosis.7 Thus, it should be ordered in suspected cases, even in patients who have elevated creatinine levels (which would normally preclude the use of contrast), since early diagnosis followed by endovascular therapy is associated with survival benefit, and the risk of contrast-induced nephropathy appears to be small.8 Computed tomography helps to determine the state of mesenteric vessels and bowel perfusion before ischemia progresses to infarction. It also helps to rule out other common diagnoses. Findings that suggest acute mesenteric ischemia include segmental bowel wall thickening, intestinal pneumatosis with gas in the portal vein, bowel dilation, mesenteric stranding, portomesenteric thrombosis, and solid-organ infarction.9
Treatment. If superior mesenteric artery occlusion is diagnosed on computed tomography, the next step is to determine if there is peritonitis.10 In patients who have evidence of peritonitis, exploratory laparotomy is performed. For emboli in such patients, open embolectomy followed by on-table angiography is carried out in combination with damage-control surgery. For patients with peritonitis and acute thrombosis, stenting along with damage-control surgery is preferred.10
On the other hand, if there is no peritonitis, the thrombosis may be amenable to endovascular intervention. For patients with acute embolic occlusion with no contraindications to thrombolysis, aspiration embolectomy in combination with local catheter-directed thrombolysis with recombinant tissue plasminogen activator can be performed. This can be combined with endovascular mechanical embolectomy for more complete management.10 Patients with contraindications to thrombolysis can be treated either with aspiration and mechanical embolectomy or with open embolectomy with angiography.10
During laparotomy, the surgeon carefully inspects the bowel for signs of necrosis. Signs that bowel is still viable include pink color, bleeding from cut surfaces, good peristalsis, and visible pulsations in the arterial arcade of the mesentery.
Acute mesenteric artery thrombosis arising from chronic atherosclerotic disease can be treated with stenting of the stenotic lesion.10 Patients with this condition would also benefit from aggressive management of atherosclerotic disease with statins along with antiplatelet agents.10
Mesenteric vein thrombosis requires prompt institution of anticoagulation. However, in advanced cases leading to bowel infarction, exploratory laparotomy with resection of the necrotic bowel may be required. Anticoagulation should be continued for at least 6 months, and further therapy should be determined by the underlying precipitating condition.10
Critically ill patients who develop mesenteric ischemia secondary to persistent hypotension usually respond to adequate volume resuscitation, cessation of vasopressors, and overall improvement in their hemodynamic status. These patients must be closely monitored for development of gangrene of the bowel because they may be intubated and not able to complain. Any sudden deterioration in their condition should prompt physicians to consider bowel necrosis developing in these patients. Elevation of lactate levels out of proportion to the degree of hypotension may be corroborative evidence.4
Our patient had risk factors for acute mesenteric ischemia that included atrial fibrillation and recent non-ST-elevation myocardial infarction. She could have had arterial emboli due to atrial fibrillation, in situ superior mesenteric arterial thrombosis, or splanchnic arterial vasoconstriction due to the myocardial infarction associated with transient hypotension.
Arguing against this diagnosis, although she had a grossly distended abdomen, abdominal bruising usually is not seen. Also, a palpable mass in the right lower quadrant is uncommon except when acute mesenteric ischemia occurs due to segmental intestinal strangulation, as with strangulated hernia or volvulus. She also had therapeutic international normalized ratio (INR) levels constantly while on anticoagulation. Nevertheless, acute mesenteric ischemia should be strongly considered in the initial differential diagnosis of this patient’s acute decompensation.
Perforation of the gastrointestinal tract
Diverticulitis is the acute inflammation of one or more diverticula, which are small pouches created by herniation of the mucosa into the wall of the colon. The condition is caused by microscopic or macroscopic perforation of the diverticula. Microscopic perforation is usually self-limited (uncomplicated diverticulitis) and responds to conservative treatment, whereas macroscopic perforation can be associated with fecal or purulent peritonitis, abscess, enteric fistula, bowel obstruction, and stricture (complicated diverticulitis), in which case surgery may be necessary.
Patients with peritonitis due to free perforation present with generalized tenderness with rebound tenderness and guarding on abdominal examination. The abdomen may be distended and tympanic to percussion, with diminished or absent bowel sounds. Patients may have hemodynamic compromise.
Plain upright abdominal radiographs may show free air under the diaphragm. Computed tomography may show oral contrast outside the lumen and detect even small amounts of free intraperitoneal air (more clearly seen on a lung window setting).
Our patient initially presented with acute diverticulitis. She later developed diffuse abdominal tenderness with hypoactive bowel sounds. Bowel perforation is certainly a possibility at this stage, though it is usually not associated with abdominal bruising. She would need additional imaging to rule out this complication.
Other differential diagnoses to be considered in this patient with right lower-quadrant pain include acute appendicitis, incarcerated inguinal hernia, volvulus (particularly cecal volvulus), small-bowel obstruction, pyelonephritis, and gynecologic causes such as adnexal torsion, ruptured ovarian cyst, and tubo-ovarian abscess. Computed tomography helps to differentiate most of these causes.
Rectus sheath hematoma
Rectus sheath hematoma is relatively uncommon and often not considered in the initial differential diagnosis of an acute abdomen. This gives it the rightful term “a great masquerader.” It usually results from bleeding into the rectus sheath from damage to the superior (more common) or inferior epigastric arteries and occasionally from a direct tear of the rectus abdominis muscle. Predisposing factors include anticoagulant therapy (most common), advanced age, hypertension, previous abdominal surgery, trauma, paroxysmal coughing, medication injections, pregnancy, blood dyscrasias, severe vomiting, violent physical activity, and leukemia.11
Clinical manifestations include acute abdominal pain, often associated with fever, nausea, and vomiting. Physical examination may reveal signs of hypovolemic shock, a palpable nonpulsatile abdominal mass, and signs of local peritoneal irritation. The Carnett sign11 (tenderness within the abdominal wall that persists and does not improve with raising the head) and the Fothergill sign11 (a tender abdominal mass that does not cross the midline and remains palpable with tensing of the rectus sheath) may be elicited.
Computed tomography is more sensitive than abdominal ultrasonography in differentiating rectus sheath hematoma from an intra-abdominal pathology.11 In addition, computed tomography also helps to determine if the bleeding is active or not, which has therapeutic implications.
In our patient, rectus sheath hematoma is a possibility because of her ongoing anticoagulation, findings of localized abdominal bruising, and palpable right lower-quadrant mass, and it is high on the list of differential diagnoses. Rectus sheath hematoma should be considered in the differential diagnosis of lower abdominal pain particularly in elderly women who are on anticoagulation and in whom the onset of pain coincides with a paroxysm of cough.12 Women are twice as likely as men to develop rectus sheath hematoma, owing to their different muscle mass.13 In addition, anterior abdominal wall muscles are stretched during pregnancy.13
Abdominal compartment syndrome
Abdominal compartment syndrome has been classically associated with surgical patients. However, it is being increasingly recognized in critically ill medical patients, in whom detecting and treating it early may result in significant reduction in rates of morbidity and death.14
Abdominal compartment syndrome is of three types: primary, secondary, and recurrent. Primary abdominal compartment syndrome refers to the classic surgical patients with evidence of direct injury to the abdominal or pelvic organs through major trauma or extensive abdominal surgeries. Secondary abdominal compartment syndrome refers to its development in critically ill intensive care patients in whom the pathology does not directly involve the abdominal or pelvic organs.
Various medical conditions can culminate in abdominal compartment syndrome and result in multiorgan failure. Recurrent abdominal compartment syndrome refers to its development after management of either primary or secondary intra-abdominal hypertension or abdominal compartment syndrome.15 Clinicians thus must be aware of secondary and recurrent abdominal compartment syndrome occurring in critically ill patients.
The normal intra-abdominal pressure is around 5 to 7 mm Hg, even in most critically ill patients. Persistent elevation, ie, higher than 12 mm Hg, is referred to as intra-abdominal hypertension.16–18 Intra-abdominal hypertension is subdivided into four grades:
- Grade I: 12–15 mm Hg
- Grade II: 16–20 mm Hg
- Grade III: 21–25 mm Hg
- Grade IV: > 25 mm Hg.
The World Society of the Abdominal Compartment Syndrome (WSACS) defines abdominal compartment syndrome as pressure higher than 20 mm Hg along with organ damage.18 It may or may not be associated with an abdominal perfusion pressure less than 60 mm Hg.18
Risk factors associated with abdominal compartment syndrome include conditions causing decreased gut motility (gastroparesis, ileus, and colonic pseudo-obstruction), intra-abdominal or retroperitoneal masses or abscesses, ascites, hemoperitoneum, acute pancreatitis, third-spacing due to massive fluid resuscitation with transfusions, peritoneal dialysis, and shock.18,19
Physical examination has a sensitivity of only 40% to 60% in detecting intra-abdominal hypertension.20 The gold-standard method of measuring the intra-abdominal pressure is the modified Kron technique,18 using a Foley catheter in the bladder connected to a pressure transducer. With the patient in the supine position, the transducer is zeroed at the mid-axillary line at the level of the iliac crest, and 25 mL of normal saline is instilled into the bladder and maintained for 30 to 60 seconds to let the detrusor muscle relax.15 Pressure tracings are then recorded at the end of expiration. Factors that are known to affect the transbladder pressure include patient position, respiratory movement, and body mass index, and should be taken into account when reading the pressure recordings.15,21 Other techniques that can be used include intragastric, intra-inferior vena cava, and intrarectal approaches.15
The WSACS recommends that any patient admitted to a critical care unit or in whom new organ failure develops should be screened for risk factors for intra-abdominal hypertension and abdominal compartment syndrome. If a patient has at least two of the risk factors suggested by WSACS, a baseline intra-abdominal pressure measurement should be obtained. Patients at risk for intra-abdominal hypertension should have the intra-abdominal pressure measured every 4 to 6 hours. However, in the face of hemodynamic instability and worsening multiorgan failure, the pressure may need to be measured hourly.18
Clinicians managing patients in the intensive care unit should think of intra-abdominal pressure alongside blood pressure, urine output, and mental status when evaluating hemodynamic status. Clinical manifestations of abdominal compartment syndrome reflect the underlying organ dysfunction and include hypotension, refractory shock, decreased urine output, intracranial hypertension, progressive hypoxemia and hypercarbia, elevated pulmonary peak pressures, and worsening of metabolic acidosis.22
Treatment. The standard treatment for primary abdominal compartment syndrome is surgical decompression. According to WSACS guidelines, insertion of a percutaneous drainage catheter should be advocated in patients with gross ascites and in whom decompressive surgery is not feasible. A damage-control resuscitation strategy used for patients undergoing damage-control laparotomy has been found to increase the 30-day survival rate.23 A damage-control resuscitation strategy consists of increasing the use of plasma and platelet transfusions over packed red cell transfusions, limiting the use of crystalloid solutions in early fluid resuscitation, and allowing for permissive hypotension.
Secondary abdominal compartment syndrome is treated conservatively in most cases, since patients with this condition are very poor surgical candidates owing to their comorbidities.18 However, in patients with progressive organ dysfunction in whom medical management has failed, surgical decompression should be considered.18 Medical management of secondary abdominal compartment syndrome depends on the underlying etiology. Strategies include nasogastric or colonic decompression, use of prokinetic agents, paracentesis in cases with gross ascites, and maintaining a cumulative negative fluid balance. The WSACS does not recommend routine use of diuretics, albumin infusion, or renal replacement strategies. Pain should be adequately controlled to improve abdominal wall compliance.18,24 Neuromuscular blockade agents may be used to aid this process. Neostigmine may be used to treat colonic pseudo-obstruction when other conservative methods fail. Use of enteral nutrition should be minimized.18
Our patient might have abdominal compartment syndrome, but a definitive diagnosis cannot be made at this point without measuring the intra-abdominal pressure.
WHICH IMAGING TEST WOULD BE BEST?
2. Which imaging test would be best for establishing the diagnosis in this patient?
- Plain abdominal radiography
- Abdominal ultrasonography
- Computed tomography of the abdomen and pelvis with contrast
- Magnetic resonance imaging of the abdomen and pelvis
Plain abdominal radiography
Plain abdominal radiography can help to determine if there is free gas under the diaphragm (due to bowel perforation), obstructed bowel, sentinel loop, volvulus, or fecoliths causing the abdominal pain. It cannot diagnose rectus sheath hematoma or acute mesenteric ischemia.
Abdominal ultrasonography
Abdominal ultrasonography can be used as the first diagnostic test, as it is widely available, safe, effective, and tolerable. It does not expose the patient to radiation or intravenous contrast agents. It helps to diagnose rectus sheath hematoma and helps to follow its maturation and resolution once a diagnosis is made. It can provide a rapid assessment of the size, location, extent, and physical characteristics of the mass.
Rectus sheath hematoma appears spindle-shaped on sagittal sections and ovoid on coronal sections. It often appears sonolucent in the early stages and sonodense in the late stage, but the appearance may be heterogeneous depending on the combined presence of clot and fresh blood. These findings are sufficient to make the diagnosis.
Abdominal ultrasonography has 85% to 96% sensitivity in diagnosing rectus sheath hematoma.25 It can help diagnose other causes of the abdominal pain, such as renal stones and cholecystitis. It is the preferred imaging test in pediatric patients, pregnant patients, and those with renal insufficiency.
Abdominal computed tomography
Abdominal computed tomography has a sensitivity and specificity of 100% for diagnosing acute rectus sheath hematoma with a duration of less than 5 days.25 It not only helps to determine the precise location and extent, but also helps to determine if there is active extravasation. Even in patients with renal insufficiency, noncontrast computed tomography will help to confirm the diagnosis, although it may not show extravasation or it may miss certain abdominal pathologies because of the lack of contrast.
Acute rectus sheath hematoma appears as a hyperdense mass posterior to the rectus abdominis muscle with ipsilateral anterolateral muscular enlargement. Chronic rectus sheath hematoma appears isodense or hypodense relative to the surrounding muscle. Above the arcuate line, rectus sheath hematoma has a spindle shape; below the arcuate line, it is typically spherical.
In 1996, Berná et al26 classified rectus sheath hematoma into three grades based on findings of computed tomography:
- Grade I is intramuscular and unilateral
- Grade II may involve bilateral rectus muscles without extension into the prevesicular space
- Grade III extends into the peritoneum and prevesicular space
Magnetic resonance imaging
Magnetic resonance imaging is useful to differentiate chronic rectus sheath hematoma (greater than 5-day duration) from an anterior abdominal wall mass. Chronic rectus sheath hematoma will have high signal intensity on both T1- and T2-weighted images up to 10 months after the onset of the hematoma.27
Back to our patient
Since our patient’s symptoms are acute and of less than 5 days’ duration, computed tomography of the abdomen and pelvis would be the best diagnostic test, with therapeutic implications if there is ongoing extravasation.
Computed tomography of the abdomen with contrast showed a new hematoma, measuring 25 by 14 by 13.5 cm, in the right rectus sheath (Figure 2), with no other findings. The hematoma was grade I, since it was intramuscular and unilateral without extension elsewhere.
Laboratory workup showed that the patient’s hematocrit was falling, from 34% to 24%, and her INR was elevated at 2.5. She was resuscitated with fluids, blood transfusion, and fresh-frozen plasma. Anticoagulation was withheld. In spite of resuscitation, her hematocrit kept falling, though she remained hemodynamically stable.
THE WAY FORWARD
3. At this point, what would be the best approach to management in this patient?
- Serial clinical examinations and frequent monitoring of the complete blood cell count
- Urgent surgical consult for exploratory laparotomy with evaluation of the hematoma and ligation of the bleeding vessel
- Repeat computed tomographic angiography to identify a possible bleeding vessel; consideration of radiographically guided arterial embolization
- Measuring the intra-abdominal pressure using the intrabladder pressure for abdominal compartment syndrome and consideration of surgical drainage
The key clinical concern in a patient with a rectus sheath hematoma who is hemodynamically stable is whether the hematoma is expanding. This patient responded to initial resuscitation, but her falling hematocrit was evidence of ongoing bleeding leading to an expanding rectus sheath hematoma. Thus, serial clinical examinations and frequent monitoring of the complete blood cell count would not be enough, as it could miss fatal ongoing bleeding.
Radiographically guided embolization with Gelfoam, thrombin, or coils should be attempted first, as this is less invasive than exploratory laparotomy.28 It can achieve hemostasis, decrease the size of the hematoma, limit the need for blood products, and prevent rupture into the abdomen. If this is unsuccessful, the next step is ligation of the bleeding vessel.29
Surgical treatment includes evacuation of the hematoma, repair of the rectus sheath, ligation of bleeding vessels, and abdominal wall closure. Surgical evacuation or guided drainage of a rectus sheath hematoma on its own is not normally indicated and may indeed cause persistent bleeding by diminishing a potential tamponade effect. However, it may become necessary if the hematoma is very large or infected, if it causes marked respiratory impairment, or if abdominal compartment syndrome is suspected.
Abdominal compartment syndrome is very rare but is associated with a 50% mortality rate.30 It should be suspected in patients with oliguria, low cardiac output, changes in minute ventilation, and altered splanchnic blood flow. The diagnosis is confirmed with indwelling catheter manometry of the bladder to measure intra-abdominal pressure. Intra-abominal pressure above 25 mm Hg should be treated with decompressive laparotomy.30 However, the clinical suspicion of abdominal compartment syndrome was low in this patient.
The best choice at this point would be urgent computed tomographic angiography to identify a bleeding vessel, along with consideration of radiographically guided arterial embolization.
TREATMENT IS USUALLY CONSERVATIVE
Treatment of rectus sheath hematoma is conservative in most hemodynamically stable patients, with embolization or surgical intervention reserved for unstable patients or those in whom the hematoma is expanding.
Knowledge of the grading system of Berná et al26 helps to assess the patient’s risk and to anticipate potential complications. Grade I hematomas are mild and do not necessitate admission. Patients with grade II hematoma can be admitted to the floor for 24 to 48 hours for observation. Grade III usually occurs in patients receiving anticoagulant therapy and frequently requires blood products. These patients have a prolonged hospital stay and more complications, including hypovolemic shock, myonecrosis, acute coronary syndrome, arrhythmias, acute renal failure, small-bowel infarction, and abdominal compartment syndrome—all of which increases the risk of morbidity and death. Thus, patients who are on anticoagulation who develop grade III rectus sheath hematoma should be admitted to the hospital, preferably to the intensive care unit, to ensure that the hematoma is not expanding and to plan reinstitution of anticoagulation as appropriate.
In most cases, rectus sheath hematomas resolve within 1 to 3 months. Resolution of large hematomas may be hastened with the use of pulsed ultrasound.31 However, this treatment should be used only after the acute phase is over, when there is evidence of an organized thrombus and coagulation measures have returned to the target range. This helps to reduce the risk of bleeding and to prevent symptoms from worsening.31
OUR PATIENT’S COURSE
Our patient underwent urgent computed tomographic angiography, which showed a modest increase in the size of the rectus sheath hematoma. However, no definitive blush of contrast was seen to suggest active arterial bleeding. Her hematocrit stabilized, and she remained hemodynamically stable without requiring additional intervention. Most likely her bleeding was self-contained. She had normal intra-abdominal pressure on serial monitoring. She was later transferred to acute inpatient rehabilitation in view of deconditioning and is currently doing well. The hematoma persisted, decreasing only slightly in size over the next 3 weeks.
- Kougias P, Lau D, El Sayed HF, Zhou W, Huynh TT, Lin PH. Determinants of mortality and treatment outcome following surgical interventions for acute mesenteric ischemia. J Vasc Surg 2007; 46:467–474.
- Sise MJ. Acute mesenteric ischemia. Surg Clin North Am 2014; 94:165–181.
- Scharff JR, Longo WE, Vartanian SM, Jacobs DL, Bahadursingh AN, Kaminski DL. Ischemic colitis: spectrum of disease and outcome. Surgery 2003; 134:624–629.
- Lange H, Jäckel R. Usefulness of plasma lactate concentration in the diagnosis of acute abdominal disease. Eur J Surg 1994; 160:381–384.
- Gearhart SL, Delaney CP, Senagore AJ, et al. Prospective assessment of the predictive value of alpha-glutathione S-transferase for intestinal ischemia. Am Surg 2003; 69:324–329.
- Kanda T, Fujii H, Tani T, et al. Intestinal fatty acid-binding protein is a useful diagnostic marker for mesenteric infarction in humans. Gastroenterology 1996; 110:339–343.
- Menke J. Diagnostic accuracy of multidetector CT in acute mesenteric ischemia: systematic review and meta-analysis. Radiology 2010; 256:93–101.
- Acosta S, Björnsson S, Ekberg O, Resch T. CT angiography followed by endovascular intervention for acute superior mesenteric artery occlusion does not increase risk of contrast-induced renal failure. Eur J Vasc Endovasc Surg 2010; 39:726–730.
- Clark RA. Computed tomography of bowel infarction. J Comput Assist Tomogr 1987; 11:757–762.
- Acosta S, Björck M. Modern treatment of acute mesenteric ischaemia. Br J Surg 2014; 101:e100–e108.
- Smithson A, Ruiz J, Perello R, Valverde M, Ramos J, Garzo L. Diagnostic and management of spontaneous rectus sheath hematoma. Eur J Intern Med 2013; 24:579–582.
- Moreno Gallego A, Aguayo JL, Flores B, et al. Ultrasonography and computed tomography reduce unnecessary surgery in abdominal rectus sheath haematoma. Br J Surg 1997; 84:1295–1297.
- Dubinsky IL. Hematoma of the rectus abdominis muscle: case report and review of the literature. J Emerg Med 1997; 15:165–167.
- Yi M, Yao G, Bai Y. The monitoring of intra-abdominal pressure in critically ill patients. (In Chinese.) Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2014; 26:175–178.
- Hunt L, Frost SA, Hillman K, Newton PJ, Davidson PM. Management of intra-abdominal hypertension and abdominal compartment syndrome: a review. J Trauma Manag Outcomes 2014; 8:2.
- Malbrain ML, Cheatham ML, Kirkpatrick A, et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med 2006; 32:1722–1732.
- Malbrain ML, Chiumello D, Cesana BM, et al; WAKE-Up! Investigators. A systematic review and individual patient data meta-analysis on intra-abdominal hypertension in critically ill patients: the wake-up project. World initiative on Abdominal Hypertension Epidemiology, a Unifying Project (WAKE-Up!). Minerva Anestesiol 2014; 80:293–306.
- Kirkpatrick AW, Roberts DJ, De Waele J, et al; Pediatric Guidelines Sub-Committee for the World Society of the Abdominal Compartment Syndrome. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med 2013; 39:1190–1206.
- Holodinsky JK, Roberts DJ, Ball CG, et al. Risk factors for intra-abdominal hypertension and abdominal compartment syndrome among adult intensive care unit patients: a systematic review and meta-analysis. Crit Care 2013; 17:R249.
- Sugrue M, Bauman A, Jones F, et al. Clinical examination is an inaccurate predictor of intraabdominal pressure. World J Surg 2002; 26:1428–1431.
- Cheatham ML, De Waele JJ, De Laet I, et al; World Society of the Abdominal Compartment Syndrome (WSACS) Clinical Trials Working Group. The impact of body position on intra-abdominal pressure measurement: a multicenter analysis. Crit Care Med 2009; 37:2187–2190.
- Ortiz-Diaz E, Lan CK. Intra-abdominal hypertension in medical critically ill patients: a narrative review. Shock 2014; 41:175–180.
- Cotton BA, Reddy N, Hatch QM, et al. Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg 2011; 254:598–605.
- An G, West MA. Abdominal compartment syndrome: a concise clinical review. Crit Care Med 2008; 36:1304–1310.
- Tolcher MC, Nitsche JF, Arendt KW, Rose CH. Spontaneous rectus sheath hematoma pregnancy: case report and review of the literature. Obstet Gynecol Surv 2010; 65:517–522.
- Berná JD, Garcia-Medina V, Guirao J, Garcia-Medina J. Rectus sheath hematoma: diagnostic classification by CT. Abdom Imaging 1996; 21:62–64.
- Unger EC, Glazer HS, Lee JK, Ling D. MRI of extracranial hematomas: preliminary observations. AJR Am J Roentgenol 1986; 146:403–407.
- Rimola J, Perendreu J, Falcó J, Fortuño JR, Massuet A, Branera J. Percutaneous arterial embolization in the management of rectus sheath hematoma. AJR Am J Roentgenol 2007; 188:W497–W502.
- Titone C, Lipsius M, Krakauer JS. “Spontaneous” hematoma of the rectus abdominis muscle: critical review of 50 cases with emphasis on early diagnosis and treatment. Surgery 1972; 72:568–572.
- Osinbowale O, Bartholomew JR. Rectus sheath hematoma. Vasc Med 2008; 13:275–279.
- Berná-Serna JD, Sánchez-Garre J, Madrigal M, Zuazu I, Berná-Mestre JD. Ultrasound therapy in rectus sheath hematoma. Phys Ther 2005; 85:352–357.
- Kougias P, Lau D, El Sayed HF, Zhou W, Huynh TT, Lin PH. Determinants of mortality and treatment outcome following surgical interventions for acute mesenteric ischemia. J Vasc Surg 2007; 46:467–474.
- Sise MJ. Acute mesenteric ischemia. Surg Clin North Am 2014; 94:165–181.
- Scharff JR, Longo WE, Vartanian SM, Jacobs DL, Bahadursingh AN, Kaminski DL. Ischemic colitis: spectrum of disease and outcome. Surgery 2003; 134:624–629.
- Lange H, Jäckel R. Usefulness of plasma lactate concentration in the diagnosis of acute abdominal disease. Eur J Surg 1994; 160:381–384.
- Gearhart SL, Delaney CP, Senagore AJ, et al. Prospective assessment of the predictive value of alpha-glutathione S-transferase for intestinal ischemia. Am Surg 2003; 69:324–329.
- Kanda T, Fujii H, Tani T, et al. Intestinal fatty acid-binding protein is a useful diagnostic marker for mesenteric infarction in humans. Gastroenterology 1996; 110:339–343.
- Menke J. Diagnostic accuracy of multidetector CT in acute mesenteric ischemia: systematic review and meta-analysis. Radiology 2010; 256:93–101.
- Acosta S, Björnsson S, Ekberg O, Resch T. CT angiography followed by endovascular intervention for acute superior mesenteric artery occlusion does not increase risk of contrast-induced renal failure. Eur J Vasc Endovasc Surg 2010; 39:726–730.
- Clark RA. Computed tomography of bowel infarction. J Comput Assist Tomogr 1987; 11:757–762.
- Acosta S, Björck M. Modern treatment of acute mesenteric ischaemia. Br J Surg 2014; 101:e100–e108.
- Smithson A, Ruiz J, Perello R, Valverde M, Ramos J, Garzo L. Diagnostic and management of spontaneous rectus sheath hematoma. Eur J Intern Med 2013; 24:579–582.
- Moreno Gallego A, Aguayo JL, Flores B, et al. Ultrasonography and computed tomography reduce unnecessary surgery in abdominal rectus sheath haematoma. Br J Surg 1997; 84:1295–1297.
- Dubinsky IL. Hematoma of the rectus abdominis muscle: case report and review of the literature. J Emerg Med 1997; 15:165–167.
- Yi M, Yao G, Bai Y. The monitoring of intra-abdominal pressure in critically ill patients. (In Chinese.) Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2014; 26:175–178.
- Hunt L, Frost SA, Hillman K, Newton PJ, Davidson PM. Management of intra-abdominal hypertension and abdominal compartment syndrome: a review. J Trauma Manag Outcomes 2014; 8:2.
- Malbrain ML, Cheatham ML, Kirkpatrick A, et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med 2006; 32:1722–1732.
- Malbrain ML, Chiumello D, Cesana BM, et al; WAKE-Up! Investigators. A systematic review and individual patient data meta-analysis on intra-abdominal hypertension in critically ill patients: the wake-up project. World initiative on Abdominal Hypertension Epidemiology, a Unifying Project (WAKE-Up!). Minerva Anestesiol 2014; 80:293–306.
- Kirkpatrick AW, Roberts DJ, De Waele J, et al; Pediatric Guidelines Sub-Committee for the World Society of the Abdominal Compartment Syndrome. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med 2013; 39:1190–1206.
- Holodinsky JK, Roberts DJ, Ball CG, et al. Risk factors for intra-abdominal hypertension and abdominal compartment syndrome among adult intensive care unit patients: a systematic review and meta-analysis. Crit Care 2013; 17:R249.
- Sugrue M, Bauman A, Jones F, et al. Clinical examination is an inaccurate predictor of intraabdominal pressure. World J Surg 2002; 26:1428–1431.
- Cheatham ML, De Waele JJ, De Laet I, et al; World Society of the Abdominal Compartment Syndrome (WSACS) Clinical Trials Working Group. The impact of body position on intra-abdominal pressure measurement: a multicenter analysis. Crit Care Med 2009; 37:2187–2190.
- Ortiz-Diaz E, Lan CK. Intra-abdominal hypertension in medical critically ill patients: a narrative review. Shock 2014; 41:175–180.
- Cotton BA, Reddy N, Hatch QM, et al. Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg 2011; 254:598–605.
- An G, West MA. Abdominal compartment syndrome: a concise clinical review. Crit Care Med 2008; 36:1304–1310.
- Tolcher MC, Nitsche JF, Arendt KW, Rose CH. Spontaneous rectus sheath hematoma pregnancy: case report and review of the literature. Obstet Gynecol Surv 2010; 65:517–522.
- Berná JD, Garcia-Medina V, Guirao J, Garcia-Medina J. Rectus sheath hematoma: diagnostic classification by CT. Abdom Imaging 1996; 21:62–64.
- Unger EC, Glazer HS, Lee JK, Ling D. MRI of extracranial hematomas: preliminary observations. AJR Am J Roentgenol 1986; 146:403–407.
- Rimola J, Perendreu J, Falcó J, Fortuño JR, Massuet A, Branera J. Percutaneous arterial embolization in the management of rectus sheath hematoma. AJR Am J Roentgenol 2007; 188:W497–W502.
- Titone C, Lipsius M, Krakauer JS. “Spontaneous” hematoma of the rectus abdominis muscle: critical review of 50 cases with emphasis on early diagnosis and treatment. Surgery 1972; 72:568–572.
- Osinbowale O, Bartholomew JR. Rectus sheath hematoma. Vasc Med 2008; 13:275–279.
- Berná-Serna JD, Sánchez-Garre J, Madrigal M, Zuazu I, Berná-Mestre JD. Ultrasound therapy in rectus sheath hematoma. Phys Ther 2005; 85:352–357.
When the dissociation curve shifts to the left
A 48-year-old woman presented to the emergency department after 2 days of nonproductive cough, chest discomfort, worsening shortness of breath, and subjective fever. She had a history of systemic sclerosis. She was currently taking prednisone 20 mg daily and aspirin 81 mg daily.
Physical examination revealed tachypnea (28 breaths per minute), and bronchial breath sounds in the left lower chest posteriorly.
The initial laboratory workup revealed:
- Hemoglobin 106 g/L (reference range 115–155)
- Mean corpuscular volume 84 fL (80–100)
- White blood cell count 29.4 × 109/L (3.70–11.0), with 85% neutrophils
- Platelet count 180 × 109/L (150–350)
- Lactate dehydrogenase 312 U/L (100–220).
Chest radiography showed opacification of the lower lobe of the left lung.
She was admitted to the hospital and started treatment with intravenous azithromycin and ceftriaxone for presumed community-acquired pneumonia, based on the clinical presentation and findings on chest radiography. Because of her immunosuppression (due to chronic prednisone therapy) and her high lactate dehydrogenase level, Pneumocystis jirovecii pneumonia was suspected, and because she had a history of allergy to trimethoprim-sulfamethoxazole and pentamidine, she was started on dapsone.
During the next 24 hours, she developed worsening dyspnea, hypoxia, and cyanosis. She was placed on an air-entrainment mask, with a fraction of inspired oxygen of 0.5. Pulse oximetry showed an oxygen saturation of 85%, but arterial blood gas analysis indicated an oxyhemoglobin concentration of 95%.
THE ‘SATURATION GAP’
1. Which is most likely to have caused the discrepancy between the oxyhemoglobin concentration and the oxygen saturation by pulse oximetry in this patient?
- Methemoglobinemia
- Carbon monoxide poisoning
- Inappropriate placement of the pulse oximeter probe
- Pulmonary embolism
Methemoglobinemia is the most likely cause of the discrepancy between the oxyhemoglobin levels and the oxygen saturation by pulse oximetry, a phenomenon also known as the “saturation gap.” Other common causes are cyanide poisoning and carbon monoxide poisoning.
Carbon monoxide poisoning, however, does not explain our patient’s cyanosis. On the contrary, carbon monoxide poisoning can actually cause the patient’s lips and mucous membranes to appear unnaturally bright pink. Also, carbon monoxide poisoning raises the blood concentration of carboxyhemoglobin (which has a high affinity for oxygen), and this usually causes pulse oximetry to read inappropriately high, whereas in our patient it read low.
Incorrect placement of the pulse oximeter probe can result in an inaccurate measurement of oxygen saturation. Visualization of the waveform on the plethysmograph or the signal quality index can be used to assess adequate placement of the pulse oximeter probe. However, inadequate probe placement does not explain our patient’s dyspnea and cyanosis.
Pulmonary embolism can lead to hypoxia as a result of ventilation-perfusion mismatch. However, pulmonary embolism leading to low oxygen saturation on pulse oximetry will also lead to concomitantly low oxyhemoglobin levels as measured by arterial blood gas analysis, and this was not seen in our patient.
BACK TO OUR PATIENT
Because there was a discrepancy between our patient’s pulse oximetry reading and oxyhemoglobin concentration by arterial blood gas measurement, her methemoglobin level was checked and was found to be 30%, thus confirming the diagnosis of methemoglobinemia.
WHAT IS METHEMOGLOBINEMIA, AND WHAT CAUSES IT?
Oxygen is normally bound to iron in its ferrous (Fe2+) form in hemoglobin to form oxyhemoglobin. Oxidative stress in the body can cause iron to change from the ferrous to the ferric (Fe3+) state, forming methemoglobin. Methemoglobin is normally present in the blood in low levels (< 1% of the total hemoglobin), and ferric iron is reduced and recycled back to the ferrous form by NADH-cytochrome b5 reductase, an enzyme present in red blood cells. This protective mechanism maintains methemoglobin levels within safe limits. But increased production can lead to accumulation of methemoglobin, resulting in dyspnea and hypoxia and the condition referred to as methemoglobinemia.1
Increased levels of methemoglobin relative to normal hemoglobin cause tissue hypoxia by several mechanisms. Methemoglobin cannot efficiently carry oxygen; instead, it binds to water or to a hydroxide ion depending on the pH of the environment.2 Therefore, the hemoglobin molecule does not carry its usual load of oxygen, and hypoxia results from the reduced delivery of oxygen to tissues. In addition, an increased concentration of methemoglobin causes a leftward shift in the oxygen-hemoglobin dissociation curve, representing an increased affinity to bound oxygen in the remaining heme groups. The tightly bound oxygen is not adequately released at the tissue level, thus causing cellular hypoxia.
Methemoglobinemia is most often caused by exposure to an oxidizing chemical or drug that increases production of methemoglobin. In rare cases, it is caused by a congenital deficiency of NADH-cytochrome b5 reductase.3
2. Which of the following drugs can cause methemoglobinemia?
- Acetaminophen
- Dapsone
- Benzocaine
- Primaquine
All four of these drugs are common culprits for causing acquired methemoglobinemia; others include chloroquine, nitroglycerin, and sulfonamides.4–6
The increased production of methemoglobin caused by these drugs overwhelms the protective effect of reducing enzymes and can lead to an accumulation of methemoglobin. However, because of variability in cellular metabolism, not every person who takes these drugs develops dangerous levels of methemoglobin.
Dapsone and benzocaine are the most commonly encountered drugs known to cause methemoglobinemia (Table 1). Dapsone is an anti-inflammatory and antimicrobial agent most commonly used for treating lepromatous leprosy and dermatitis herpetiformis. It is also often prescribed for prophylaxis and treatment of P jirovecii pneumonia in immunosuppressed individuals.7 Benzocaine is a local anesthetic and was commonly used before procedures such as oral or dental surgery, transesophageal echocardiography, and endoscopy.8–10 Even low doses of benzocaine can lead to high levels of methemoglobinemia. However, the availability of other, safer anesthetics now limits the use of benzocaine in major US centers. In addition, the topical anesthetic Emla (lidocaine plus prilocaine) has been recently reported as a cause of methemoglobinemia in infants and children.11,12
Also, potentially fatal methemoglobinemia has been reported in patients with a deficiency of G-6-phosphate dehydrogenase (G6PD) who received rasburicase, a recombinant version of urate oxidase enzyme used to prevent and treat tumor lysis syndrome.13,14
Lastly, methemoglobinemia has been reported in patients with inflammatory bowel disease treated with mesalamine.
Although this adverse reaction is rare, clinicians should be aware of it, since these agents are commonly used in everyday medical practice.15
RECOGNIZING THE DANGER SIGNS
The clinical manifestations of methemoglobinemia are directly proportional to the percentage of methemoglobin in red blood cells. Cyanosis generally becomes apparent at concentrations around 15%, at which point the patient may still have no symptoms. Anxiety, lightheadedness, tachycardia, and dizziness manifest at levels of 20% to 30%. Fatigue, confusion, dizziness, tachypnea, and worsening tachycardia occur at levels of 30% to 50%. Levels of 50% to 70% cause coma, seizures, arrhythmias, and acidosis, and levels over 70% are considered lethal.16
While these levels provide a general guideline of symptomatology in an otherwise healthy person, it is important to remember that patients with underlying conditions such as anemia, lung disease (both of which our patient had), sepsis, thalassemia, G6PD deficiency, and sickle cell disease can manifest symptoms at lower concentrations of methemoglobin.1,17
Most patients who develop clinically significant levels of methemoglobin do so within the first few hours of starting one of the culprit drugs.
DIAGNOSIS: METHEMOGLOBINEMIA AND THE SATURATION GAP
In patients with methemoglobinemia, pulse oximetry gives lower values than arterial blood gas oxygen measurements. Regular pulse oximetry works by measuring light absorbance at two distinct wavelengths (660 and 940 nm) to calculate the ratio of oxyhemoglobin to deoxyhemoglobin. Methemoglobin absorbs light at both these wavelengths, thus lowering the pulse oximetry values.1
In contrast, oxygen saturation of arterial blood gas (oxyhemoglobin) is calculated indirectly from the concentration of dissolved oxygen in the blood and does not include oxygen bound to hemoglobin. Therefore, the measured arterial oxygen saturation is often normal in patients with methemoglobinemia since it relies only on inspired oxygen content and is independent of the methemoglobin concentration.18
Oxygen supplementation can raise the level of oxyhemoglobin, which is a measure of dissolved oxygen, but the oxygen saturation as measured by pulse oximetry remains largely unchanged—ie, the saturation gap. A difference of more than 5% between the oxygen saturation by pulse oximetry and blood gas analysis is abnormal. Patients with clinically significant methemoglobinemia usually have a saturation gap greater than 10%.
Several other unique features should raise suspicion of methemoglobinemia. It should be considered in a patient presenting with cyanosis out of proportion to the oxygen saturation and in a patient with low oxygen saturation and a normal chest radiograph. Other clues include blood that is chocolate-colored on gross examination, rather than the dark red of deoxygenated blood.
Co-oximetry measures oxygen saturation using different wavelengths of light to distinguish between fractions of oxyhemoglobin, deoxyhemoglobin, and methemoglobin, but it is not widely available.
THE NEXT STEP
3. What is the next step in the management of our patient?
- Discontinue the dapsone
- Start methylene blue
- Start hyperbaric oxygen
- Give sodium thiosulfate
- Discontinue dapsone and start methylene blue
The next step in her management should be to stop the dapsone and start an infusion of methylene blue. Hyperbaric oxygen is used in treating carbon monoxide poisoning, and sodium thiosulfate is used in treating cyanide toxicity. They would not be appropriate in this patient’s care.
MANAGEMENT OF ACQUIRED METHEMOGLOBINEMIA
The first, most critical step in managing acquired methemoglobinemia is to immediately discontinue the suspected offending agent. In most patients without a concomitant condition such as anemia or lung disease and with a methemoglobin level below 20%, discontinuing the offending agent may suffice. Patients with a level of 20% or greater and patients with cardiac and pulmonary disease, who develop symptoms at lower concentrations of methemoglobin, require infusion of methylene blue.
Methylene blue is converted to its reduced form, leukomethylene blue, by NADPH-methemoglobin reductase. As it is oxidized, leukomethylene blue reduces methemoglobin to hemoglobin. A dose of 1 mg/kg intravenously is given at first. The response is usually dramatic, with a reduction in methemoglobin levels and improvement in symptoms often within 30 to 60 minutes. If levels remain high, the dose can be repeated 1 hour later.19
A caveat: methylene blue therapy should be avoided in patients with complete G6PD deficiency. Methylene blue works through the enzyme NADPH-methemoglobin reductase, and since patients with G6PD deficiency lack this enzyme, methylene blue is ineffective. In fact, since it cannot be reduced, excessive methylene blue can oxidize hemoglobin to methemoglobin, further exacerbating the condition. In patients with partial G6PD deficiency, methylene blue is still recommended as a first-line treatment, but at a lower initial dose (0.3–0.5 mg/kg). However, in patients with significant hemolysis, an exchange transfusion is the only treatment option.
CASE CONCLUDED
Since dapsone was identified as the likely cause of methemoglobinemia in our patient, it was immediately discontinued. Because she was symptomatic, 70 mg of methylene blue was given intravenously. Over the next 60 minutes, her clinical condition improved significantly. A repeat methemoglobin measurement was 3%.
She was discharged home the next day on oral antibiotics to complete treatment for community-acquired pneumonia.
TAKE-HOME POINTS
- Consider methemoglobinemia in a patient with unexplained cyanosis.
- Pulse oximetry gives lower values than arterial blood gas oxygen measurements in patients with methemoglobinemia, and pulse oximetry readings do not improve with supplemental oxygen.
- A saturation gap greater than 5% strongly suggests methemoglobinemia.
- The diagnosis of methemoglobinemia is confirmed by measuring the methemoglobin concentration.
- Most healthy patients develop symptoms at methemoglobin levels of 20%, but patients with comorbidities can develop symptoms at lower levels.
- A number of drugs can cause methemoglobinemia, even at therapeutic dosages.
- Treatment is generally indicated in patients who have symptoms or in healthy patients who have a methemoglobin level of 20% or greater.
- Identifying and promptly discontinuing the causative agent and initiating methylene blue infusion (1 mg/kg over 5 minutes) is the preferred treatment.
- Cortazzo JA, Lichtman AD. Methemoglobinemia: a review and recommendations for management. J Cardiothorac Vasc Anesth 2014; 28:1055–1059.
- Margulies DR, Manookian CM. Methemoglobinemia as a cause of respiratory failure. J Trauma 2002; 52:796–797.
- Skold A, Cosco DL, Klein R. Methemoglobinemia: pathogenesis, diagnosis, and management. South Med J 2011; 104:757–761.
- Ash-Bernal R, Wise R, Wright SM. Acquired methemoglobinemia: a retrospective series of 138 cases at 2 teaching hospitals. Medicine (Baltimore) 2004; 83:265–273.
- Kanji HD, Mithani S, Boucher P, Dias VC, Yarema MC. Coma, metabolic acidosis, and methemoglobinemia in a patient with acetaminophen toxicity. J Popul Ther Clin Pharmacol 2013; 20:e207–e211.
- Kawasumi H, Tanaka E, Hoshi D, Kawaguchi Y, Yamanaka H. Methemoglobinemia induced by trimethoprim-sulfamethoxazole in a patient with systemic lupus erythematosus. Intern Med 2013; 52:1741–1743.
- Wieringa A, Bethlehem C, Hoogendoorn M, van der Maten J, van Roon EN. Very late recovery of dapsone-induced methemoglobinemia. Clin Toxicol (Phila) 2014; 52:80–81.
- Barclay JA, Ziemba SE, Ibrahim RB. Dapsone-induced methemoglobinemia: a primer for clinicians. Ann Pharmacother 2011; 45:1103–1115.
- Taleb M, Ashraf Z, Valavoor S, Tinkel J. Evaluation and management of acquired methemoglobinemia associated with topical benzocaine use. Am J Cardiovasc Drugs 2013; 13:325–330.
- Chowdhary S, Bukoye B, Bhansali AM, et al. Risk of topical anesthetic-induced methemoglobinemia: a 10-year retrospective case-control study. JAMA Intern Med 2013; 173:771–776.
- Larson A, Stidham T, Banerji S, Kaufman J. Seizures and methemoglobinemia in an infant after excessive EMLA application. Pediatr Emerg Care 2013; 29:377–379.
- Schmitt C, Matulic M, Kervégant M, et al. Methaemoglobinaemia in a child treated with Emla cream: circumstances and consequences of overdose [in French]. Ann Dermatol Venereol 2012; 139:824–827.
- Bucklin MH, Groth CM. Mortality following rasburicase-induced methemoglobinemia. Ann Pharmacother 2013; 47:1353–1358.
- Cheah CY, Lew TE, Seymour JF, Burbury K. Rasburicase causing severe oxidative hemolysis and methemoglobinemia in a patient with previously unrecognized glucose-6-phosphate dehydrogenase deficiency. Acta Haematol 2013; 130:254–259.
- Druez A, Rahier JF, Hébuterne X. Methaemoglobinaemia and renal failure following mesalazine for treatment of inflammatory bowel disease. J Crohns Colitis 2014; 8:900–901.
- Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med 1999; 34:646–656.
- Groeper K, Katcher K, Tobias JD. Anesthetic management of a patient with methemoglobinemia. South Med J 2003; 96:504–509.
- Haymond S, Cariappa R, Eby CS, Scott MG. Laboratory assessment of oxygenation in methemoglobinemia. Clin Chem 2005; 51:434–444.
- Jang DH, Nelson LS, Hoffman RS. Methylene blue for distributive shock: a potential new use of an old antidote. J Med Toxicol 2013; 9:242–249.
A 48-year-old woman presented to the emergency department after 2 days of nonproductive cough, chest discomfort, worsening shortness of breath, and subjective fever. She had a history of systemic sclerosis. She was currently taking prednisone 20 mg daily and aspirin 81 mg daily.
Physical examination revealed tachypnea (28 breaths per minute), and bronchial breath sounds in the left lower chest posteriorly.
The initial laboratory workup revealed:
- Hemoglobin 106 g/L (reference range 115–155)
- Mean corpuscular volume 84 fL (80–100)
- White blood cell count 29.4 × 109/L (3.70–11.0), with 85% neutrophils
- Platelet count 180 × 109/L (150–350)
- Lactate dehydrogenase 312 U/L (100–220).
Chest radiography showed opacification of the lower lobe of the left lung.
She was admitted to the hospital and started treatment with intravenous azithromycin and ceftriaxone for presumed community-acquired pneumonia, based on the clinical presentation and findings on chest radiography. Because of her immunosuppression (due to chronic prednisone therapy) and her high lactate dehydrogenase level, Pneumocystis jirovecii pneumonia was suspected, and because she had a history of allergy to trimethoprim-sulfamethoxazole and pentamidine, she was started on dapsone.
During the next 24 hours, she developed worsening dyspnea, hypoxia, and cyanosis. She was placed on an air-entrainment mask, with a fraction of inspired oxygen of 0.5. Pulse oximetry showed an oxygen saturation of 85%, but arterial blood gas analysis indicated an oxyhemoglobin concentration of 95%.
THE ‘SATURATION GAP’
1. Which is most likely to have caused the discrepancy between the oxyhemoglobin concentration and the oxygen saturation by pulse oximetry in this patient?
- Methemoglobinemia
- Carbon monoxide poisoning
- Inappropriate placement of the pulse oximeter probe
- Pulmonary embolism
Methemoglobinemia is the most likely cause of the discrepancy between the oxyhemoglobin levels and the oxygen saturation by pulse oximetry, a phenomenon also known as the “saturation gap.” Other common causes are cyanide poisoning and carbon monoxide poisoning.
Carbon monoxide poisoning, however, does not explain our patient’s cyanosis. On the contrary, carbon monoxide poisoning can actually cause the patient’s lips and mucous membranes to appear unnaturally bright pink. Also, carbon monoxide poisoning raises the blood concentration of carboxyhemoglobin (which has a high affinity for oxygen), and this usually causes pulse oximetry to read inappropriately high, whereas in our patient it read low.
Incorrect placement of the pulse oximeter probe can result in an inaccurate measurement of oxygen saturation. Visualization of the waveform on the plethysmograph or the signal quality index can be used to assess adequate placement of the pulse oximeter probe. However, inadequate probe placement does not explain our patient’s dyspnea and cyanosis.
Pulmonary embolism can lead to hypoxia as a result of ventilation-perfusion mismatch. However, pulmonary embolism leading to low oxygen saturation on pulse oximetry will also lead to concomitantly low oxyhemoglobin levels as measured by arterial blood gas analysis, and this was not seen in our patient.
BACK TO OUR PATIENT
Because there was a discrepancy between our patient’s pulse oximetry reading and oxyhemoglobin concentration by arterial blood gas measurement, her methemoglobin level was checked and was found to be 30%, thus confirming the diagnosis of methemoglobinemia.
WHAT IS METHEMOGLOBINEMIA, AND WHAT CAUSES IT?
Oxygen is normally bound to iron in its ferrous (Fe2+) form in hemoglobin to form oxyhemoglobin. Oxidative stress in the body can cause iron to change from the ferrous to the ferric (Fe3+) state, forming methemoglobin. Methemoglobin is normally present in the blood in low levels (< 1% of the total hemoglobin), and ferric iron is reduced and recycled back to the ferrous form by NADH-cytochrome b5 reductase, an enzyme present in red blood cells. This protective mechanism maintains methemoglobin levels within safe limits. But increased production can lead to accumulation of methemoglobin, resulting in dyspnea and hypoxia and the condition referred to as methemoglobinemia.1
Increased levels of methemoglobin relative to normal hemoglobin cause tissue hypoxia by several mechanisms. Methemoglobin cannot efficiently carry oxygen; instead, it binds to water or to a hydroxide ion depending on the pH of the environment.2 Therefore, the hemoglobin molecule does not carry its usual load of oxygen, and hypoxia results from the reduced delivery of oxygen to tissues. In addition, an increased concentration of methemoglobin causes a leftward shift in the oxygen-hemoglobin dissociation curve, representing an increased affinity to bound oxygen in the remaining heme groups. The tightly bound oxygen is not adequately released at the tissue level, thus causing cellular hypoxia.
Methemoglobinemia is most often caused by exposure to an oxidizing chemical or drug that increases production of methemoglobin. In rare cases, it is caused by a congenital deficiency of NADH-cytochrome b5 reductase.3
2. Which of the following drugs can cause methemoglobinemia?
- Acetaminophen
- Dapsone
- Benzocaine
- Primaquine
All four of these drugs are common culprits for causing acquired methemoglobinemia; others include chloroquine, nitroglycerin, and sulfonamides.4–6
The increased production of methemoglobin caused by these drugs overwhelms the protective effect of reducing enzymes and can lead to an accumulation of methemoglobin. However, because of variability in cellular metabolism, not every person who takes these drugs develops dangerous levels of methemoglobin.
Dapsone and benzocaine are the most commonly encountered drugs known to cause methemoglobinemia (Table 1). Dapsone is an anti-inflammatory and antimicrobial agent most commonly used for treating lepromatous leprosy and dermatitis herpetiformis. It is also often prescribed for prophylaxis and treatment of P jirovecii pneumonia in immunosuppressed individuals.7 Benzocaine is a local anesthetic and was commonly used before procedures such as oral or dental surgery, transesophageal echocardiography, and endoscopy.8–10 Even low doses of benzocaine can lead to high levels of methemoglobinemia. However, the availability of other, safer anesthetics now limits the use of benzocaine in major US centers. In addition, the topical anesthetic Emla (lidocaine plus prilocaine) has been recently reported as a cause of methemoglobinemia in infants and children.11,12
Also, potentially fatal methemoglobinemia has been reported in patients with a deficiency of G-6-phosphate dehydrogenase (G6PD) who received rasburicase, a recombinant version of urate oxidase enzyme used to prevent and treat tumor lysis syndrome.13,14
Lastly, methemoglobinemia has been reported in patients with inflammatory bowel disease treated with mesalamine.
Although this adverse reaction is rare, clinicians should be aware of it, since these agents are commonly used in everyday medical practice.15
RECOGNIZING THE DANGER SIGNS
The clinical manifestations of methemoglobinemia are directly proportional to the percentage of methemoglobin in red blood cells. Cyanosis generally becomes apparent at concentrations around 15%, at which point the patient may still have no symptoms. Anxiety, lightheadedness, tachycardia, and dizziness manifest at levels of 20% to 30%. Fatigue, confusion, dizziness, tachypnea, and worsening tachycardia occur at levels of 30% to 50%. Levels of 50% to 70% cause coma, seizures, arrhythmias, and acidosis, and levels over 70% are considered lethal.16
While these levels provide a general guideline of symptomatology in an otherwise healthy person, it is important to remember that patients with underlying conditions such as anemia, lung disease (both of which our patient had), sepsis, thalassemia, G6PD deficiency, and sickle cell disease can manifest symptoms at lower concentrations of methemoglobin.1,17
Most patients who develop clinically significant levels of methemoglobin do so within the first few hours of starting one of the culprit drugs.
DIAGNOSIS: METHEMOGLOBINEMIA AND THE SATURATION GAP
In patients with methemoglobinemia, pulse oximetry gives lower values than arterial blood gas oxygen measurements. Regular pulse oximetry works by measuring light absorbance at two distinct wavelengths (660 and 940 nm) to calculate the ratio of oxyhemoglobin to deoxyhemoglobin. Methemoglobin absorbs light at both these wavelengths, thus lowering the pulse oximetry values.1
In contrast, oxygen saturation of arterial blood gas (oxyhemoglobin) is calculated indirectly from the concentration of dissolved oxygen in the blood and does not include oxygen bound to hemoglobin. Therefore, the measured arterial oxygen saturation is often normal in patients with methemoglobinemia since it relies only on inspired oxygen content and is independent of the methemoglobin concentration.18
Oxygen supplementation can raise the level of oxyhemoglobin, which is a measure of dissolved oxygen, but the oxygen saturation as measured by pulse oximetry remains largely unchanged—ie, the saturation gap. A difference of more than 5% between the oxygen saturation by pulse oximetry and blood gas analysis is abnormal. Patients with clinically significant methemoglobinemia usually have a saturation gap greater than 10%.
Several other unique features should raise suspicion of methemoglobinemia. It should be considered in a patient presenting with cyanosis out of proportion to the oxygen saturation and in a patient with low oxygen saturation and a normal chest radiograph. Other clues include blood that is chocolate-colored on gross examination, rather than the dark red of deoxygenated blood.
Co-oximetry measures oxygen saturation using different wavelengths of light to distinguish between fractions of oxyhemoglobin, deoxyhemoglobin, and methemoglobin, but it is not widely available.
THE NEXT STEP
3. What is the next step in the management of our patient?
- Discontinue the dapsone
- Start methylene blue
- Start hyperbaric oxygen
- Give sodium thiosulfate
- Discontinue dapsone and start methylene blue
The next step in her management should be to stop the dapsone and start an infusion of methylene blue. Hyperbaric oxygen is used in treating carbon monoxide poisoning, and sodium thiosulfate is used in treating cyanide toxicity. They would not be appropriate in this patient’s care.
MANAGEMENT OF ACQUIRED METHEMOGLOBINEMIA
The first, most critical step in managing acquired methemoglobinemia is to immediately discontinue the suspected offending agent. In most patients without a concomitant condition such as anemia or lung disease and with a methemoglobin level below 20%, discontinuing the offending agent may suffice. Patients with a level of 20% or greater and patients with cardiac and pulmonary disease, who develop symptoms at lower concentrations of methemoglobin, require infusion of methylene blue.
Methylene blue is converted to its reduced form, leukomethylene blue, by NADPH-methemoglobin reductase. As it is oxidized, leukomethylene blue reduces methemoglobin to hemoglobin. A dose of 1 mg/kg intravenously is given at first. The response is usually dramatic, with a reduction in methemoglobin levels and improvement in symptoms often within 30 to 60 minutes. If levels remain high, the dose can be repeated 1 hour later.19
A caveat: methylene blue therapy should be avoided in patients with complete G6PD deficiency. Methylene blue works through the enzyme NADPH-methemoglobin reductase, and since patients with G6PD deficiency lack this enzyme, methylene blue is ineffective. In fact, since it cannot be reduced, excessive methylene blue can oxidize hemoglobin to methemoglobin, further exacerbating the condition. In patients with partial G6PD deficiency, methylene blue is still recommended as a first-line treatment, but at a lower initial dose (0.3–0.5 mg/kg). However, in patients with significant hemolysis, an exchange transfusion is the only treatment option.
CASE CONCLUDED
Since dapsone was identified as the likely cause of methemoglobinemia in our patient, it was immediately discontinued. Because she was symptomatic, 70 mg of methylene blue was given intravenously. Over the next 60 minutes, her clinical condition improved significantly. A repeat methemoglobin measurement was 3%.
She was discharged home the next day on oral antibiotics to complete treatment for community-acquired pneumonia.
TAKE-HOME POINTS
- Consider methemoglobinemia in a patient with unexplained cyanosis.
- Pulse oximetry gives lower values than arterial blood gas oxygen measurements in patients with methemoglobinemia, and pulse oximetry readings do not improve with supplemental oxygen.
- A saturation gap greater than 5% strongly suggests methemoglobinemia.
- The diagnosis of methemoglobinemia is confirmed by measuring the methemoglobin concentration.
- Most healthy patients develop symptoms at methemoglobin levels of 20%, but patients with comorbidities can develop symptoms at lower levels.
- A number of drugs can cause methemoglobinemia, even at therapeutic dosages.
- Treatment is generally indicated in patients who have symptoms or in healthy patients who have a methemoglobin level of 20% or greater.
- Identifying and promptly discontinuing the causative agent and initiating methylene blue infusion (1 mg/kg over 5 minutes) is the preferred treatment.
A 48-year-old woman presented to the emergency department after 2 days of nonproductive cough, chest discomfort, worsening shortness of breath, and subjective fever. She had a history of systemic sclerosis. She was currently taking prednisone 20 mg daily and aspirin 81 mg daily.
Physical examination revealed tachypnea (28 breaths per minute), and bronchial breath sounds in the left lower chest posteriorly.
The initial laboratory workup revealed:
- Hemoglobin 106 g/L (reference range 115–155)
- Mean corpuscular volume 84 fL (80–100)
- White blood cell count 29.4 × 109/L (3.70–11.0), with 85% neutrophils
- Platelet count 180 × 109/L (150–350)
- Lactate dehydrogenase 312 U/L (100–220).
Chest radiography showed opacification of the lower lobe of the left lung.
She was admitted to the hospital and started treatment with intravenous azithromycin and ceftriaxone for presumed community-acquired pneumonia, based on the clinical presentation and findings on chest radiography. Because of her immunosuppression (due to chronic prednisone therapy) and her high lactate dehydrogenase level, Pneumocystis jirovecii pneumonia was suspected, and because she had a history of allergy to trimethoprim-sulfamethoxazole and pentamidine, she was started on dapsone.
During the next 24 hours, she developed worsening dyspnea, hypoxia, and cyanosis. She was placed on an air-entrainment mask, with a fraction of inspired oxygen of 0.5. Pulse oximetry showed an oxygen saturation of 85%, but arterial blood gas analysis indicated an oxyhemoglobin concentration of 95%.
THE ‘SATURATION GAP’
1. Which is most likely to have caused the discrepancy between the oxyhemoglobin concentration and the oxygen saturation by pulse oximetry in this patient?
- Methemoglobinemia
- Carbon monoxide poisoning
- Inappropriate placement of the pulse oximeter probe
- Pulmonary embolism
Methemoglobinemia is the most likely cause of the discrepancy between the oxyhemoglobin levels and the oxygen saturation by pulse oximetry, a phenomenon also known as the “saturation gap.” Other common causes are cyanide poisoning and carbon monoxide poisoning.
Carbon monoxide poisoning, however, does not explain our patient’s cyanosis. On the contrary, carbon monoxide poisoning can actually cause the patient’s lips and mucous membranes to appear unnaturally bright pink. Also, carbon monoxide poisoning raises the blood concentration of carboxyhemoglobin (which has a high affinity for oxygen), and this usually causes pulse oximetry to read inappropriately high, whereas in our patient it read low.
Incorrect placement of the pulse oximeter probe can result in an inaccurate measurement of oxygen saturation. Visualization of the waveform on the plethysmograph or the signal quality index can be used to assess adequate placement of the pulse oximeter probe. However, inadequate probe placement does not explain our patient’s dyspnea and cyanosis.
Pulmonary embolism can lead to hypoxia as a result of ventilation-perfusion mismatch. However, pulmonary embolism leading to low oxygen saturation on pulse oximetry will also lead to concomitantly low oxyhemoglobin levels as measured by arterial blood gas analysis, and this was not seen in our patient.
BACK TO OUR PATIENT
Because there was a discrepancy between our patient’s pulse oximetry reading and oxyhemoglobin concentration by arterial blood gas measurement, her methemoglobin level was checked and was found to be 30%, thus confirming the diagnosis of methemoglobinemia.
WHAT IS METHEMOGLOBINEMIA, AND WHAT CAUSES IT?
Oxygen is normally bound to iron in its ferrous (Fe2+) form in hemoglobin to form oxyhemoglobin. Oxidative stress in the body can cause iron to change from the ferrous to the ferric (Fe3+) state, forming methemoglobin. Methemoglobin is normally present in the blood in low levels (< 1% of the total hemoglobin), and ferric iron is reduced and recycled back to the ferrous form by NADH-cytochrome b5 reductase, an enzyme present in red blood cells. This protective mechanism maintains methemoglobin levels within safe limits. But increased production can lead to accumulation of methemoglobin, resulting in dyspnea and hypoxia and the condition referred to as methemoglobinemia.1
Increased levels of methemoglobin relative to normal hemoglobin cause tissue hypoxia by several mechanisms. Methemoglobin cannot efficiently carry oxygen; instead, it binds to water or to a hydroxide ion depending on the pH of the environment.2 Therefore, the hemoglobin molecule does not carry its usual load of oxygen, and hypoxia results from the reduced delivery of oxygen to tissues. In addition, an increased concentration of methemoglobin causes a leftward shift in the oxygen-hemoglobin dissociation curve, representing an increased affinity to bound oxygen in the remaining heme groups. The tightly bound oxygen is not adequately released at the tissue level, thus causing cellular hypoxia.
Methemoglobinemia is most often caused by exposure to an oxidizing chemical or drug that increases production of methemoglobin. In rare cases, it is caused by a congenital deficiency of NADH-cytochrome b5 reductase.3
2. Which of the following drugs can cause methemoglobinemia?
- Acetaminophen
- Dapsone
- Benzocaine
- Primaquine
All four of these drugs are common culprits for causing acquired methemoglobinemia; others include chloroquine, nitroglycerin, and sulfonamides.4–6
The increased production of methemoglobin caused by these drugs overwhelms the protective effect of reducing enzymes and can lead to an accumulation of methemoglobin. However, because of variability in cellular metabolism, not every person who takes these drugs develops dangerous levels of methemoglobin.
Dapsone and benzocaine are the most commonly encountered drugs known to cause methemoglobinemia (Table 1). Dapsone is an anti-inflammatory and antimicrobial agent most commonly used for treating lepromatous leprosy and dermatitis herpetiformis. It is also often prescribed for prophylaxis and treatment of P jirovecii pneumonia in immunosuppressed individuals.7 Benzocaine is a local anesthetic and was commonly used before procedures such as oral or dental surgery, transesophageal echocardiography, and endoscopy.8–10 Even low doses of benzocaine can lead to high levels of methemoglobinemia. However, the availability of other, safer anesthetics now limits the use of benzocaine in major US centers. In addition, the topical anesthetic Emla (lidocaine plus prilocaine) has been recently reported as a cause of methemoglobinemia in infants and children.11,12
Also, potentially fatal methemoglobinemia has been reported in patients with a deficiency of G-6-phosphate dehydrogenase (G6PD) who received rasburicase, a recombinant version of urate oxidase enzyme used to prevent and treat tumor lysis syndrome.13,14
Lastly, methemoglobinemia has been reported in patients with inflammatory bowel disease treated with mesalamine.
Although this adverse reaction is rare, clinicians should be aware of it, since these agents are commonly used in everyday medical practice.15
RECOGNIZING THE DANGER SIGNS
The clinical manifestations of methemoglobinemia are directly proportional to the percentage of methemoglobin in red blood cells. Cyanosis generally becomes apparent at concentrations around 15%, at which point the patient may still have no symptoms. Anxiety, lightheadedness, tachycardia, and dizziness manifest at levels of 20% to 30%. Fatigue, confusion, dizziness, tachypnea, and worsening tachycardia occur at levels of 30% to 50%. Levels of 50% to 70% cause coma, seizures, arrhythmias, and acidosis, and levels over 70% are considered lethal.16
While these levels provide a general guideline of symptomatology in an otherwise healthy person, it is important to remember that patients with underlying conditions such as anemia, lung disease (both of which our patient had), sepsis, thalassemia, G6PD deficiency, and sickle cell disease can manifest symptoms at lower concentrations of methemoglobin.1,17
Most patients who develop clinically significant levels of methemoglobin do so within the first few hours of starting one of the culprit drugs.
DIAGNOSIS: METHEMOGLOBINEMIA AND THE SATURATION GAP
In patients with methemoglobinemia, pulse oximetry gives lower values than arterial blood gas oxygen measurements. Regular pulse oximetry works by measuring light absorbance at two distinct wavelengths (660 and 940 nm) to calculate the ratio of oxyhemoglobin to deoxyhemoglobin. Methemoglobin absorbs light at both these wavelengths, thus lowering the pulse oximetry values.1
In contrast, oxygen saturation of arterial blood gas (oxyhemoglobin) is calculated indirectly from the concentration of dissolved oxygen in the blood and does not include oxygen bound to hemoglobin. Therefore, the measured arterial oxygen saturation is often normal in patients with methemoglobinemia since it relies only on inspired oxygen content and is independent of the methemoglobin concentration.18
Oxygen supplementation can raise the level of oxyhemoglobin, which is a measure of dissolved oxygen, but the oxygen saturation as measured by pulse oximetry remains largely unchanged—ie, the saturation gap. A difference of more than 5% between the oxygen saturation by pulse oximetry and blood gas analysis is abnormal. Patients with clinically significant methemoglobinemia usually have a saturation gap greater than 10%.
Several other unique features should raise suspicion of methemoglobinemia. It should be considered in a patient presenting with cyanosis out of proportion to the oxygen saturation and in a patient with low oxygen saturation and a normal chest radiograph. Other clues include blood that is chocolate-colored on gross examination, rather than the dark red of deoxygenated blood.
Co-oximetry measures oxygen saturation using different wavelengths of light to distinguish between fractions of oxyhemoglobin, deoxyhemoglobin, and methemoglobin, but it is not widely available.
THE NEXT STEP
3. What is the next step in the management of our patient?
- Discontinue the dapsone
- Start methylene blue
- Start hyperbaric oxygen
- Give sodium thiosulfate
- Discontinue dapsone and start methylene blue
The next step in her management should be to stop the dapsone and start an infusion of methylene blue. Hyperbaric oxygen is used in treating carbon monoxide poisoning, and sodium thiosulfate is used in treating cyanide toxicity. They would not be appropriate in this patient’s care.
MANAGEMENT OF ACQUIRED METHEMOGLOBINEMIA
The first, most critical step in managing acquired methemoglobinemia is to immediately discontinue the suspected offending agent. In most patients without a concomitant condition such as anemia or lung disease and with a methemoglobin level below 20%, discontinuing the offending agent may suffice. Patients with a level of 20% or greater and patients with cardiac and pulmonary disease, who develop symptoms at lower concentrations of methemoglobin, require infusion of methylene blue.
Methylene blue is converted to its reduced form, leukomethylene blue, by NADPH-methemoglobin reductase. As it is oxidized, leukomethylene blue reduces methemoglobin to hemoglobin. A dose of 1 mg/kg intravenously is given at first. The response is usually dramatic, with a reduction in methemoglobin levels and improvement in symptoms often within 30 to 60 minutes. If levels remain high, the dose can be repeated 1 hour later.19
A caveat: methylene blue therapy should be avoided in patients with complete G6PD deficiency. Methylene blue works through the enzyme NADPH-methemoglobin reductase, and since patients with G6PD deficiency lack this enzyme, methylene blue is ineffective. In fact, since it cannot be reduced, excessive methylene blue can oxidize hemoglobin to methemoglobin, further exacerbating the condition. In patients with partial G6PD deficiency, methylene blue is still recommended as a first-line treatment, but at a lower initial dose (0.3–0.5 mg/kg). However, in patients with significant hemolysis, an exchange transfusion is the only treatment option.
CASE CONCLUDED
Since dapsone was identified as the likely cause of methemoglobinemia in our patient, it was immediately discontinued. Because she was symptomatic, 70 mg of methylene blue was given intravenously. Over the next 60 minutes, her clinical condition improved significantly. A repeat methemoglobin measurement was 3%.
She was discharged home the next day on oral antibiotics to complete treatment for community-acquired pneumonia.
TAKE-HOME POINTS
- Consider methemoglobinemia in a patient with unexplained cyanosis.
- Pulse oximetry gives lower values than arterial blood gas oxygen measurements in patients with methemoglobinemia, and pulse oximetry readings do not improve with supplemental oxygen.
- A saturation gap greater than 5% strongly suggests methemoglobinemia.
- The diagnosis of methemoglobinemia is confirmed by measuring the methemoglobin concentration.
- Most healthy patients develop symptoms at methemoglobin levels of 20%, but patients with comorbidities can develop symptoms at lower levels.
- A number of drugs can cause methemoglobinemia, even at therapeutic dosages.
- Treatment is generally indicated in patients who have symptoms or in healthy patients who have a methemoglobin level of 20% or greater.
- Identifying and promptly discontinuing the causative agent and initiating methylene blue infusion (1 mg/kg over 5 minutes) is the preferred treatment.
- Cortazzo JA, Lichtman AD. Methemoglobinemia: a review and recommendations for management. J Cardiothorac Vasc Anesth 2014; 28:1055–1059.
- Margulies DR, Manookian CM. Methemoglobinemia as a cause of respiratory failure. J Trauma 2002; 52:796–797.
- Skold A, Cosco DL, Klein R. Methemoglobinemia: pathogenesis, diagnosis, and management. South Med J 2011; 104:757–761.
- Ash-Bernal R, Wise R, Wright SM. Acquired methemoglobinemia: a retrospective series of 138 cases at 2 teaching hospitals. Medicine (Baltimore) 2004; 83:265–273.
- Kanji HD, Mithani S, Boucher P, Dias VC, Yarema MC. Coma, metabolic acidosis, and methemoglobinemia in a patient with acetaminophen toxicity. J Popul Ther Clin Pharmacol 2013; 20:e207–e211.
- Kawasumi H, Tanaka E, Hoshi D, Kawaguchi Y, Yamanaka H. Methemoglobinemia induced by trimethoprim-sulfamethoxazole in a patient with systemic lupus erythematosus. Intern Med 2013; 52:1741–1743.
- Wieringa A, Bethlehem C, Hoogendoorn M, van der Maten J, van Roon EN. Very late recovery of dapsone-induced methemoglobinemia. Clin Toxicol (Phila) 2014; 52:80–81.
- Barclay JA, Ziemba SE, Ibrahim RB. Dapsone-induced methemoglobinemia: a primer for clinicians. Ann Pharmacother 2011; 45:1103–1115.
- Taleb M, Ashraf Z, Valavoor S, Tinkel J. Evaluation and management of acquired methemoglobinemia associated with topical benzocaine use. Am J Cardiovasc Drugs 2013; 13:325–330.
- Chowdhary S, Bukoye B, Bhansali AM, et al. Risk of topical anesthetic-induced methemoglobinemia: a 10-year retrospective case-control study. JAMA Intern Med 2013; 173:771–776.
- Larson A, Stidham T, Banerji S, Kaufman J. Seizures and methemoglobinemia in an infant after excessive EMLA application. Pediatr Emerg Care 2013; 29:377–379.
- Schmitt C, Matulic M, Kervégant M, et al. Methaemoglobinaemia in a child treated with Emla cream: circumstances and consequences of overdose [in French]. Ann Dermatol Venereol 2012; 139:824–827.
- Bucklin MH, Groth CM. Mortality following rasburicase-induced methemoglobinemia. Ann Pharmacother 2013; 47:1353–1358.
- Cheah CY, Lew TE, Seymour JF, Burbury K. Rasburicase causing severe oxidative hemolysis and methemoglobinemia in a patient with previously unrecognized glucose-6-phosphate dehydrogenase deficiency. Acta Haematol 2013; 130:254–259.
- Druez A, Rahier JF, Hébuterne X. Methaemoglobinaemia and renal failure following mesalazine for treatment of inflammatory bowel disease. J Crohns Colitis 2014; 8:900–901.
- Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med 1999; 34:646–656.
- Groeper K, Katcher K, Tobias JD. Anesthetic management of a patient with methemoglobinemia. South Med J 2003; 96:504–509.
- Haymond S, Cariappa R, Eby CS, Scott MG. Laboratory assessment of oxygenation in methemoglobinemia. Clin Chem 2005; 51:434–444.
- Jang DH, Nelson LS, Hoffman RS. Methylene blue for distributive shock: a potential new use of an old antidote. J Med Toxicol 2013; 9:242–249.
- Cortazzo JA, Lichtman AD. Methemoglobinemia: a review and recommendations for management. J Cardiothorac Vasc Anesth 2014; 28:1055–1059.
- Margulies DR, Manookian CM. Methemoglobinemia as a cause of respiratory failure. J Trauma 2002; 52:796–797.
- Skold A, Cosco DL, Klein R. Methemoglobinemia: pathogenesis, diagnosis, and management. South Med J 2011; 104:757–761.
- Ash-Bernal R, Wise R, Wright SM. Acquired methemoglobinemia: a retrospective series of 138 cases at 2 teaching hospitals. Medicine (Baltimore) 2004; 83:265–273.
- Kanji HD, Mithani S, Boucher P, Dias VC, Yarema MC. Coma, metabolic acidosis, and methemoglobinemia in a patient with acetaminophen toxicity. J Popul Ther Clin Pharmacol 2013; 20:e207–e211.
- Kawasumi H, Tanaka E, Hoshi D, Kawaguchi Y, Yamanaka H. Methemoglobinemia induced by trimethoprim-sulfamethoxazole in a patient with systemic lupus erythematosus. Intern Med 2013; 52:1741–1743.
- Wieringa A, Bethlehem C, Hoogendoorn M, van der Maten J, van Roon EN. Very late recovery of dapsone-induced methemoglobinemia. Clin Toxicol (Phila) 2014; 52:80–81.
- Barclay JA, Ziemba SE, Ibrahim RB. Dapsone-induced methemoglobinemia: a primer for clinicians. Ann Pharmacother 2011; 45:1103–1115.
- Taleb M, Ashraf Z, Valavoor S, Tinkel J. Evaluation and management of acquired methemoglobinemia associated with topical benzocaine use. Am J Cardiovasc Drugs 2013; 13:325–330.
- Chowdhary S, Bukoye B, Bhansali AM, et al. Risk of topical anesthetic-induced methemoglobinemia: a 10-year retrospective case-control study. JAMA Intern Med 2013; 173:771–776.
- Larson A, Stidham T, Banerji S, Kaufman J. Seizures and methemoglobinemia in an infant after excessive EMLA application. Pediatr Emerg Care 2013; 29:377–379.
- Schmitt C, Matulic M, Kervégant M, et al. Methaemoglobinaemia in a child treated with Emla cream: circumstances and consequences of overdose [in French]. Ann Dermatol Venereol 2012; 139:824–827.
- Bucklin MH, Groth CM. Mortality following rasburicase-induced methemoglobinemia. Ann Pharmacother 2013; 47:1353–1358.
- Cheah CY, Lew TE, Seymour JF, Burbury K. Rasburicase causing severe oxidative hemolysis and methemoglobinemia in a patient with previously unrecognized glucose-6-phosphate dehydrogenase deficiency. Acta Haematol 2013; 130:254–259.
- Druez A, Rahier JF, Hébuterne X. Methaemoglobinaemia and renal failure following mesalazine for treatment of inflammatory bowel disease. J Crohns Colitis 2014; 8:900–901.
- Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med 1999; 34:646–656.
- Groeper K, Katcher K, Tobias JD. Anesthetic management of a patient with methemoglobinemia. South Med J 2003; 96:504–509.
- Haymond S, Cariappa R, Eby CS, Scott MG. Laboratory assessment of oxygenation in methemoglobinemia. Clin Chem 2005; 51:434–444.
- Jang DH, Nelson LS, Hoffman RS. Methylene blue for distributive shock: a potential new use of an old antidote. J Med Toxicol 2013; 9:242–249.
Should patients stop taking aspirin for primary prevention?
In view of current evidence, we do not recommend routinely using aspirin for primary prevention of cardiovascular disease, even in patients with diabetes mellitus. The decision must be individualized on the basis of the patient’s risks of cardiovascular disease and bleeding, especially the risk of serious bleeding events such as gastrointestinal and intracranial hemorrhage.
For example, patients with a family history of myocardial infarction at an early age and patients who smoke or have multiple cardiovascular risk factors may be most likely to benefit, whereas those with risk factors for gastrointestinal bleeding such as dyspepsia or ulcer would not be good candidates. Of note, current recommendations are mixed and confusing and will need to be reevaluated as new trial data become available.
TRIALS THAT SET THE STAGE FOR CURRENT PRACTICE
Routine use of aspirin for primary prevention of cardiovascular disease remains controversial.1,2 Aspirin’s safety and efficacy for this indication was studied in six major trials (Table 1).3–8 In the late 1980s, the first two primary prevention trials of aspirin enrolled healthy male physicians who had minimal cardiovascular risk factors3,4:
The British Doctors’ Trial3 observed no significant differences between aspirin (300–500 mg/day) and no aspirin in the rates of the primary end point of cardiovascular death or in the individual secondary end points of nonfatal myocardial infarction, nonfatal stroke, or bleeding.3
The Physicians’ Health Study4 found no differences in the rates of cardiovascular mortality or ischemic stroke between aspirin (325 mg every other day) and placebo. The rate of nonfatal myocardial infarction was significantly lower with aspirin than with placebo, but with a higher risk of bleeding. Relative risks and 95% confidence intervals with aspirin vs placebo:
- Nonfatal myocardial infarction
0.59 (0.47–0.74), P < .00001 - Bleeding
1.32 (1.25–1.40), P < .00001 - Blood transfusions
1.71 (1.09–2.69), P = .02 - Hemorrhagic stroke
2.14 (0.96–4.77), P = .06.
A subgroup analysis revealed that the benefit of aspirin for myocardial infarction in the Physicians’ Health Study was predominantly in those age 50 and older.4 This finding established the common clinical practice of routinely using aspirin for primary prevention in men age 50 and older.1
Later, aspirin for primary prevention was studied in four trials,5–8 three of which enrolled patients at higher cardiovascular risk5–7:
The Thrombosis Prevention Trial5 was conducted in men in the highest quintile of cardiovascular risk. The aspirin dosage was 75 mg/day.
The Hypertension Optimal Treatment6 trial included men and women ages 50 to 80 with hypertension. Aspirin dosage: 75 mg/day.
The Primary Prevention Project7 involved men and women age 50 and older with at least one risk factor for cardiovascular disease.1,5–7 The aspirin dosage was 100 mg/day.
In these trials (Table 1), aspirin significantly lowered the rate of ischemic events compared with placebo or control: nonfatal myocardial infarction in the Thrombosis Prevention Trial; myocardial infarction and major adverse cardiac event (ie, cardiovascular death, myocardial infarction, or stroke) in the Hypertension Optimal Treatment trial; and cardiovascular mortality and major cardiovascular events (cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, angina pectoris, transient ischemic attack, peripheral artery disease, or revascularization procedures) in the Primary Prevention Project. However, aspirin’s benefit in each trial was largely offset by a higher rate of various bleeding end points.5–7
The Women’s Health Study
A subgroup analysis of the Hypertension Optimal Treatment trial suggested that sex may influence the efficacy of aspirin—specifically, aspirin did not prevent nonfatal myocardial infarction in women.9 Given the paucity of female participants in the previous primary prevention trials, the Women’s Health Study8 was designed to determine the efficacy and safety of aspirin (100 mg every other day) in women age 45 and older with very few cardiovascular risk factors.8
Aspirin did not significantly reduce the rate of the primary end point of cardiovascular death, myocardial infarction, or stroke, though a significant effect was observed in the subgroup of women age 65 and older. Although overall the Women’s Health Study found no benefit in the rate of myocardial infarction, there was a significant reduction in the rate of ischemic stroke (which needs to be interpreted cautiously in an overall neutral trial) and a nonsignificant increase in the rate of hemorrhagic stroke. As in other trials, rates of bleeding, including gastrointestinal bleeding, were higher with aspirin.
A meta-analysis of six trials of aspirin for primary prevention
In 2009, the Antithrombotic Trialists’ Collaboration10 published a meta-analysis of six trials of aspirin for primary prevention. In this analysis, aspirin did not reduce the rate of cardiovascular death, but it did reduce the yearly risk of:
- Death from coronary heart disease or nonfatal myocardial infarction
(0.28% vs 0.34%, P < .0001) - Nonfatal myocardial infarction
(0.18% vs 0.23%, P < .0001) - Ischemic stroke
(0.11% vs 0.12%, P = .05).10
Despite aspirin’s apparent efficacy, the absolute yearly risk for major extracranial bleeding and hemorrhagic stroke was also significantly increased with aspirin use by 0.3% and 0.1%, respectively. The efficacy of aspirin for preventing all serious vascular events (vascular death, myocardial infarction, or stroke) was similar in men and women.10 The authors concluded that the net benefit of aspirin did not outweigh the increased risks of bleeding.
WHAT ABOUT PATIENTS WITH DIABETES?
When considering whether to prescribe aspirin for primary prevention, the individual patient’s risks of cardiovascular disease and bleeding must be carefully assessed. Those at highest risk of cardiovascular disease and at low risk of bleeding may still benefit, but current evidence does not clearly support this strategy.
For example, diabetes mellitus has traditionally been considered a coronary heart disease equivalent, and aspirin was routinely prescribed as “secondary prevention.”11 In the six trials of aspirin for primary prevention, the prevalence of diabetic patients ranged from 1% to 17%, the efficacy of aspirin in this subgroup was inconsistent among the trials, and aspirin did not confer a net clinical benefit according to the 2009 Antithrombotic Trialists’ Collaboration meta-analysis.1,3–8,10
Additionally, two trials of aspirin for primary prevention in diabetes12,13 failed to demonstrate significant efficacy for aspirin compared with no aspirin, either in Japanese patients with type 2 diabetes and no history of cardiovascular disease12 or in patients with asymptomatic peripheral artery disease.13
Thus, the current evidence for aspirin for primary prevention in diabetes does not demonstrate a net clinical benefit, but ongoing trials (Table 2) may provide evidence for the use of aspirin in this important subgroup.
An important finding from the 2009 Antithrombotic Trialists’ Collaboration was that traditional risk factors for cardiovascular disease also increase the risk of major bleeding, thus making it difficult to determine who will receive the maximum net clinical benefit.10 Additionally, many of the aspirin primary prevention trials predated the widespread use of statins and the current lower prevalence of smoking, which may further limit the generalizability of the positive signals seen in earlier trials.
THE DATA ARE MIXED, BUT ONE MESSAGE IS CLEAR
Based on the current available evidence, the US Food and Drug Administration recently issued a Consumer Update that does not support aspirin for primary prevention and warns patients about the risk of serious bleeding complications.14 Moreover, current guidelines and consensus panels (Table 3) for aspirin in primary prevention differ from one another,15–21 making it challenging for clinicians to determine which patients would benefit. One message is clear in the most current clinical guidelines, namely, that routine use of aspirin for primary prevention is not recommended.15–21 Several ongoing trials may resolve this important clinical dilemma.
- Depta JP, Bhatt DL. Current uses of aspirin in cardiovascular disease. Hot Topics Cardiol 2013; 32:7–21.
- Nemerovski CW, Salinitri FD, Morbitzer KA, Moser LR. Aspirin for primary prevention of cardiovascular disease events. Pharmacotherapy 2012; 32:1020–1035.
- Peto R, Gray R, Collins R, et al. Randomised trial of prophylactic daily aspirin in British male doctors. Br Med J (Clin Res Ed) 1988; 296:313–316.
- Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med 1989; 321:129–135.
- Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. The Medical Research Council’s General Practice Research Framework. Lancet 1998; 351:233–241.
- Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998; 351:1755–1762.
- de Gaetano G; Collaborative Group of the Primary Prevention Project. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Collaborative Group of the Primary Prevention Project. Lancet 2001; 357:89–95.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Kjeldsen SE, Kolloch RE, Leonetti G, et al. Influence of gender and age on preventing cardiovascular disease by antihypertensive treatment and acetylsalicylic acid. The HOT study. Hypertension Optimal Treatment. J Hypertens 2000; 18:629–642.
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373:1849–1860.
- Buse JB, Ginsberg HN, Bakris GL, et al; American Heart Association; American Diabetes Association. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2007; 115:114–126.
- Ogawa H, Nakayama M, Morimoto T, et al; Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) Trial Investigators. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA 2008; 300:2134–2141.
- Belch J, MacCuish A, Campbell I, et al; Prevention of Progression of Arterial Disease and Diabetes Study Group; Diabetes Registry Group; Royal College of Physicians Edinburgh. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008; 337:a1840.
- US Food and Drug Administration (FDA). Use of aspirin for primary prevention of heart attack and stroke. http://www.fda.gov/drugs/resourcesforyou/consumers/ucm390574.htm. Accessed January 9, 2015.
- Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e637S–e668S.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Pearson TA, Blair SN, Daniels SR, et al. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation 2002; 106:388–391.
- Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based Guidelines for the Prevention of Cardiovascular Disease in Women—2011 Update: a Guideline from the American Heart Association. Circulation 2011; 123:1243–1262.
- Bell AD, Roussin A, Cartier R, et al; Canadian Cardiovascular Society. The use of antiplatelet therapy in the outpatient setting: Canadian Cardiovascular Society Guidelines. Can J Cardiol 2011; 27(suppl A):S1–S59.
- Perk J, De Backer G, Gohlke H, et al; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J 2012; 33:1635–1701.
- US Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: US Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 150:396–404.
In view of current evidence, we do not recommend routinely using aspirin for primary prevention of cardiovascular disease, even in patients with diabetes mellitus. The decision must be individualized on the basis of the patient’s risks of cardiovascular disease and bleeding, especially the risk of serious bleeding events such as gastrointestinal and intracranial hemorrhage.
For example, patients with a family history of myocardial infarction at an early age and patients who smoke or have multiple cardiovascular risk factors may be most likely to benefit, whereas those with risk factors for gastrointestinal bleeding such as dyspepsia or ulcer would not be good candidates. Of note, current recommendations are mixed and confusing and will need to be reevaluated as new trial data become available.
TRIALS THAT SET THE STAGE FOR CURRENT PRACTICE
Routine use of aspirin for primary prevention of cardiovascular disease remains controversial.1,2 Aspirin’s safety and efficacy for this indication was studied in six major trials (Table 1).3–8 In the late 1980s, the first two primary prevention trials of aspirin enrolled healthy male physicians who had minimal cardiovascular risk factors3,4:
The British Doctors’ Trial3 observed no significant differences between aspirin (300–500 mg/day) and no aspirin in the rates of the primary end point of cardiovascular death or in the individual secondary end points of nonfatal myocardial infarction, nonfatal stroke, or bleeding.3
The Physicians’ Health Study4 found no differences in the rates of cardiovascular mortality or ischemic stroke between aspirin (325 mg every other day) and placebo. The rate of nonfatal myocardial infarction was significantly lower with aspirin than with placebo, but with a higher risk of bleeding. Relative risks and 95% confidence intervals with aspirin vs placebo:
- Nonfatal myocardial infarction
0.59 (0.47–0.74), P < .00001 - Bleeding
1.32 (1.25–1.40), P < .00001 - Blood transfusions
1.71 (1.09–2.69), P = .02 - Hemorrhagic stroke
2.14 (0.96–4.77), P = .06.
A subgroup analysis revealed that the benefit of aspirin for myocardial infarction in the Physicians’ Health Study was predominantly in those age 50 and older.4 This finding established the common clinical practice of routinely using aspirin for primary prevention in men age 50 and older.1
Later, aspirin for primary prevention was studied in four trials,5–8 three of which enrolled patients at higher cardiovascular risk5–7:
The Thrombosis Prevention Trial5 was conducted in men in the highest quintile of cardiovascular risk. The aspirin dosage was 75 mg/day.
The Hypertension Optimal Treatment6 trial included men and women ages 50 to 80 with hypertension. Aspirin dosage: 75 mg/day.
The Primary Prevention Project7 involved men and women age 50 and older with at least one risk factor for cardiovascular disease.1,5–7 The aspirin dosage was 100 mg/day.
In these trials (Table 1), aspirin significantly lowered the rate of ischemic events compared with placebo or control: nonfatal myocardial infarction in the Thrombosis Prevention Trial; myocardial infarction and major adverse cardiac event (ie, cardiovascular death, myocardial infarction, or stroke) in the Hypertension Optimal Treatment trial; and cardiovascular mortality and major cardiovascular events (cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, angina pectoris, transient ischemic attack, peripheral artery disease, or revascularization procedures) in the Primary Prevention Project. However, aspirin’s benefit in each trial was largely offset by a higher rate of various bleeding end points.5–7
The Women’s Health Study
A subgroup analysis of the Hypertension Optimal Treatment trial suggested that sex may influence the efficacy of aspirin—specifically, aspirin did not prevent nonfatal myocardial infarction in women.9 Given the paucity of female participants in the previous primary prevention trials, the Women’s Health Study8 was designed to determine the efficacy and safety of aspirin (100 mg every other day) in women age 45 and older with very few cardiovascular risk factors.8
Aspirin did not significantly reduce the rate of the primary end point of cardiovascular death, myocardial infarction, or stroke, though a significant effect was observed in the subgroup of women age 65 and older. Although overall the Women’s Health Study found no benefit in the rate of myocardial infarction, there was a significant reduction in the rate of ischemic stroke (which needs to be interpreted cautiously in an overall neutral trial) and a nonsignificant increase in the rate of hemorrhagic stroke. As in other trials, rates of bleeding, including gastrointestinal bleeding, were higher with aspirin.
A meta-analysis of six trials of aspirin for primary prevention
In 2009, the Antithrombotic Trialists’ Collaboration10 published a meta-analysis of six trials of aspirin for primary prevention. In this analysis, aspirin did not reduce the rate of cardiovascular death, but it did reduce the yearly risk of:
- Death from coronary heart disease or nonfatal myocardial infarction
(0.28% vs 0.34%, P < .0001) - Nonfatal myocardial infarction
(0.18% vs 0.23%, P < .0001) - Ischemic stroke
(0.11% vs 0.12%, P = .05).10
Despite aspirin’s apparent efficacy, the absolute yearly risk for major extracranial bleeding and hemorrhagic stroke was also significantly increased with aspirin use by 0.3% and 0.1%, respectively. The efficacy of aspirin for preventing all serious vascular events (vascular death, myocardial infarction, or stroke) was similar in men and women.10 The authors concluded that the net benefit of aspirin did not outweigh the increased risks of bleeding.
WHAT ABOUT PATIENTS WITH DIABETES?
When considering whether to prescribe aspirin for primary prevention, the individual patient’s risks of cardiovascular disease and bleeding must be carefully assessed. Those at highest risk of cardiovascular disease and at low risk of bleeding may still benefit, but current evidence does not clearly support this strategy.
For example, diabetes mellitus has traditionally been considered a coronary heart disease equivalent, and aspirin was routinely prescribed as “secondary prevention.”11 In the six trials of aspirin for primary prevention, the prevalence of diabetic patients ranged from 1% to 17%, the efficacy of aspirin in this subgroup was inconsistent among the trials, and aspirin did not confer a net clinical benefit according to the 2009 Antithrombotic Trialists’ Collaboration meta-analysis.1,3–8,10
Additionally, two trials of aspirin for primary prevention in diabetes12,13 failed to demonstrate significant efficacy for aspirin compared with no aspirin, either in Japanese patients with type 2 diabetes and no history of cardiovascular disease12 or in patients with asymptomatic peripheral artery disease.13
Thus, the current evidence for aspirin for primary prevention in diabetes does not demonstrate a net clinical benefit, but ongoing trials (Table 2) may provide evidence for the use of aspirin in this important subgroup.
An important finding from the 2009 Antithrombotic Trialists’ Collaboration was that traditional risk factors for cardiovascular disease also increase the risk of major bleeding, thus making it difficult to determine who will receive the maximum net clinical benefit.10 Additionally, many of the aspirin primary prevention trials predated the widespread use of statins and the current lower prevalence of smoking, which may further limit the generalizability of the positive signals seen in earlier trials.
THE DATA ARE MIXED, BUT ONE MESSAGE IS CLEAR
Based on the current available evidence, the US Food and Drug Administration recently issued a Consumer Update that does not support aspirin for primary prevention and warns patients about the risk of serious bleeding complications.14 Moreover, current guidelines and consensus panels (Table 3) for aspirin in primary prevention differ from one another,15–21 making it challenging for clinicians to determine which patients would benefit. One message is clear in the most current clinical guidelines, namely, that routine use of aspirin for primary prevention is not recommended.15–21 Several ongoing trials may resolve this important clinical dilemma.
In view of current evidence, we do not recommend routinely using aspirin for primary prevention of cardiovascular disease, even in patients with diabetes mellitus. The decision must be individualized on the basis of the patient’s risks of cardiovascular disease and bleeding, especially the risk of serious bleeding events such as gastrointestinal and intracranial hemorrhage.
For example, patients with a family history of myocardial infarction at an early age and patients who smoke or have multiple cardiovascular risk factors may be most likely to benefit, whereas those with risk factors for gastrointestinal bleeding such as dyspepsia or ulcer would not be good candidates. Of note, current recommendations are mixed and confusing and will need to be reevaluated as new trial data become available.
TRIALS THAT SET THE STAGE FOR CURRENT PRACTICE
Routine use of aspirin for primary prevention of cardiovascular disease remains controversial.1,2 Aspirin’s safety and efficacy for this indication was studied in six major trials (Table 1).3–8 In the late 1980s, the first two primary prevention trials of aspirin enrolled healthy male physicians who had minimal cardiovascular risk factors3,4:
The British Doctors’ Trial3 observed no significant differences between aspirin (300–500 mg/day) and no aspirin in the rates of the primary end point of cardiovascular death or in the individual secondary end points of nonfatal myocardial infarction, nonfatal stroke, or bleeding.3
The Physicians’ Health Study4 found no differences in the rates of cardiovascular mortality or ischemic stroke between aspirin (325 mg every other day) and placebo. The rate of nonfatal myocardial infarction was significantly lower with aspirin than with placebo, but with a higher risk of bleeding. Relative risks and 95% confidence intervals with aspirin vs placebo:
- Nonfatal myocardial infarction
0.59 (0.47–0.74), P < .00001 - Bleeding
1.32 (1.25–1.40), P < .00001 - Blood transfusions
1.71 (1.09–2.69), P = .02 - Hemorrhagic stroke
2.14 (0.96–4.77), P = .06.
A subgroup analysis revealed that the benefit of aspirin for myocardial infarction in the Physicians’ Health Study was predominantly in those age 50 and older.4 This finding established the common clinical practice of routinely using aspirin for primary prevention in men age 50 and older.1
Later, aspirin for primary prevention was studied in four trials,5–8 three of which enrolled patients at higher cardiovascular risk5–7:
The Thrombosis Prevention Trial5 was conducted in men in the highest quintile of cardiovascular risk. The aspirin dosage was 75 mg/day.
The Hypertension Optimal Treatment6 trial included men and women ages 50 to 80 with hypertension. Aspirin dosage: 75 mg/day.
The Primary Prevention Project7 involved men and women age 50 and older with at least one risk factor for cardiovascular disease.1,5–7 The aspirin dosage was 100 mg/day.
In these trials (Table 1), aspirin significantly lowered the rate of ischemic events compared with placebo or control: nonfatal myocardial infarction in the Thrombosis Prevention Trial; myocardial infarction and major adverse cardiac event (ie, cardiovascular death, myocardial infarction, or stroke) in the Hypertension Optimal Treatment trial; and cardiovascular mortality and major cardiovascular events (cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, angina pectoris, transient ischemic attack, peripheral artery disease, or revascularization procedures) in the Primary Prevention Project. However, aspirin’s benefit in each trial was largely offset by a higher rate of various bleeding end points.5–7
The Women’s Health Study
A subgroup analysis of the Hypertension Optimal Treatment trial suggested that sex may influence the efficacy of aspirin—specifically, aspirin did not prevent nonfatal myocardial infarction in women.9 Given the paucity of female participants in the previous primary prevention trials, the Women’s Health Study8 was designed to determine the efficacy and safety of aspirin (100 mg every other day) in women age 45 and older with very few cardiovascular risk factors.8
Aspirin did not significantly reduce the rate of the primary end point of cardiovascular death, myocardial infarction, or stroke, though a significant effect was observed in the subgroup of women age 65 and older. Although overall the Women’s Health Study found no benefit in the rate of myocardial infarction, there was a significant reduction in the rate of ischemic stroke (which needs to be interpreted cautiously in an overall neutral trial) and a nonsignificant increase in the rate of hemorrhagic stroke. As in other trials, rates of bleeding, including gastrointestinal bleeding, were higher with aspirin.
A meta-analysis of six trials of aspirin for primary prevention
In 2009, the Antithrombotic Trialists’ Collaboration10 published a meta-analysis of six trials of aspirin for primary prevention. In this analysis, aspirin did not reduce the rate of cardiovascular death, but it did reduce the yearly risk of:
- Death from coronary heart disease or nonfatal myocardial infarction
(0.28% vs 0.34%, P < .0001) - Nonfatal myocardial infarction
(0.18% vs 0.23%, P < .0001) - Ischemic stroke
(0.11% vs 0.12%, P = .05).10
Despite aspirin’s apparent efficacy, the absolute yearly risk for major extracranial bleeding and hemorrhagic stroke was also significantly increased with aspirin use by 0.3% and 0.1%, respectively. The efficacy of aspirin for preventing all serious vascular events (vascular death, myocardial infarction, or stroke) was similar in men and women.10 The authors concluded that the net benefit of aspirin did not outweigh the increased risks of bleeding.
WHAT ABOUT PATIENTS WITH DIABETES?
When considering whether to prescribe aspirin for primary prevention, the individual patient’s risks of cardiovascular disease and bleeding must be carefully assessed. Those at highest risk of cardiovascular disease and at low risk of bleeding may still benefit, but current evidence does not clearly support this strategy.
For example, diabetes mellitus has traditionally been considered a coronary heart disease equivalent, and aspirin was routinely prescribed as “secondary prevention.”11 In the six trials of aspirin for primary prevention, the prevalence of diabetic patients ranged from 1% to 17%, the efficacy of aspirin in this subgroup was inconsistent among the trials, and aspirin did not confer a net clinical benefit according to the 2009 Antithrombotic Trialists’ Collaboration meta-analysis.1,3–8,10
Additionally, two trials of aspirin for primary prevention in diabetes12,13 failed to demonstrate significant efficacy for aspirin compared with no aspirin, either in Japanese patients with type 2 diabetes and no history of cardiovascular disease12 or in patients with asymptomatic peripheral artery disease.13
Thus, the current evidence for aspirin for primary prevention in diabetes does not demonstrate a net clinical benefit, but ongoing trials (Table 2) may provide evidence for the use of aspirin in this important subgroup.
An important finding from the 2009 Antithrombotic Trialists’ Collaboration was that traditional risk factors for cardiovascular disease also increase the risk of major bleeding, thus making it difficult to determine who will receive the maximum net clinical benefit.10 Additionally, many of the aspirin primary prevention trials predated the widespread use of statins and the current lower prevalence of smoking, which may further limit the generalizability of the positive signals seen in earlier trials.
THE DATA ARE MIXED, BUT ONE MESSAGE IS CLEAR
Based on the current available evidence, the US Food and Drug Administration recently issued a Consumer Update that does not support aspirin for primary prevention and warns patients about the risk of serious bleeding complications.14 Moreover, current guidelines and consensus panels (Table 3) for aspirin in primary prevention differ from one another,15–21 making it challenging for clinicians to determine which patients would benefit. One message is clear in the most current clinical guidelines, namely, that routine use of aspirin for primary prevention is not recommended.15–21 Several ongoing trials may resolve this important clinical dilemma.
- Depta JP, Bhatt DL. Current uses of aspirin in cardiovascular disease. Hot Topics Cardiol 2013; 32:7–21.
- Nemerovski CW, Salinitri FD, Morbitzer KA, Moser LR. Aspirin for primary prevention of cardiovascular disease events. Pharmacotherapy 2012; 32:1020–1035.
- Peto R, Gray R, Collins R, et al. Randomised trial of prophylactic daily aspirin in British male doctors. Br Med J (Clin Res Ed) 1988; 296:313–316.
- Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med 1989; 321:129–135.
- Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. The Medical Research Council’s General Practice Research Framework. Lancet 1998; 351:233–241.
- Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998; 351:1755–1762.
- de Gaetano G; Collaborative Group of the Primary Prevention Project. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Collaborative Group of the Primary Prevention Project. Lancet 2001; 357:89–95.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Kjeldsen SE, Kolloch RE, Leonetti G, et al. Influence of gender and age on preventing cardiovascular disease by antihypertensive treatment and acetylsalicylic acid. The HOT study. Hypertension Optimal Treatment. J Hypertens 2000; 18:629–642.
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373:1849–1860.
- Buse JB, Ginsberg HN, Bakris GL, et al; American Heart Association; American Diabetes Association. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2007; 115:114–126.
- Ogawa H, Nakayama M, Morimoto T, et al; Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) Trial Investigators. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA 2008; 300:2134–2141.
- Belch J, MacCuish A, Campbell I, et al; Prevention of Progression of Arterial Disease and Diabetes Study Group; Diabetes Registry Group; Royal College of Physicians Edinburgh. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008; 337:a1840.
- US Food and Drug Administration (FDA). Use of aspirin for primary prevention of heart attack and stroke. http://www.fda.gov/drugs/resourcesforyou/consumers/ucm390574.htm. Accessed January 9, 2015.
- Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e637S–e668S.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Pearson TA, Blair SN, Daniels SR, et al. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation 2002; 106:388–391.
- Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based Guidelines for the Prevention of Cardiovascular Disease in Women—2011 Update: a Guideline from the American Heart Association. Circulation 2011; 123:1243–1262.
- Bell AD, Roussin A, Cartier R, et al; Canadian Cardiovascular Society. The use of antiplatelet therapy in the outpatient setting: Canadian Cardiovascular Society Guidelines. Can J Cardiol 2011; 27(suppl A):S1–S59.
- Perk J, De Backer G, Gohlke H, et al; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J 2012; 33:1635–1701.
- US Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: US Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 150:396–404.
- Depta JP, Bhatt DL. Current uses of aspirin in cardiovascular disease. Hot Topics Cardiol 2013; 32:7–21.
- Nemerovski CW, Salinitri FD, Morbitzer KA, Moser LR. Aspirin for primary prevention of cardiovascular disease events. Pharmacotherapy 2012; 32:1020–1035.
- Peto R, Gray R, Collins R, et al. Randomised trial of prophylactic daily aspirin in British male doctors. Br Med J (Clin Res Ed) 1988; 296:313–316.
- Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med 1989; 321:129–135.
- Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. The Medical Research Council’s General Practice Research Framework. Lancet 1998; 351:233–241.
- Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998; 351:1755–1762.
- de Gaetano G; Collaborative Group of the Primary Prevention Project. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Collaborative Group of the Primary Prevention Project. Lancet 2001; 357:89–95.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Kjeldsen SE, Kolloch RE, Leonetti G, et al. Influence of gender and age on preventing cardiovascular disease by antihypertensive treatment and acetylsalicylic acid. The HOT study. Hypertension Optimal Treatment. J Hypertens 2000; 18:629–642.
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373:1849–1860.
- Buse JB, Ginsberg HN, Bakris GL, et al; American Heart Association; American Diabetes Association. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2007; 115:114–126.
- Ogawa H, Nakayama M, Morimoto T, et al; Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) Trial Investigators. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA 2008; 300:2134–2141.
- Belch J, MacCuish A, Campbell I, et al; Prevention of Progression of Arterial Disease and Diabetes Study Group; Diabetes Registry Group; Royal College of Physicians Edinburgh. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008; 337:a1840.
- US Food and Drug Administration (FDA). Use of aspirin for primary prevention of heart attack and stroke. http://www.fda.gov/drugs/resourcesforyou/consumers/ucm390574.htm. Accessed January 9, 2015.
- Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e637S–e668S.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Pearson TA, Blair SN, Daniels SR, et al. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation 2002; 106:388–391.
- Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based Guidelines for the Prevention of Cardiovascular Disease in Women—2011 Update: a Guideline from the American Heart Association. Circulation 2011; 123:1243–1262.
- Bell AD, Roussin A, Cartier R, et al; Canadian Cardiovascular Society. The use of antiplatelet therapy in the outpatient setting: Canadian Cardiovascular Society Guidelines. Can J Cardiol 2011; 27(suppl A):S1–S59.
- Perk J, De Backer G, Gohlke H, et al; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J 2012; 33:1635–1701.
- US Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: US Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 150:396–404.
Denosumab: A novel antiresorptive drug for osteoporosis
A 68-year-old white woman presents with mid- thoracic back pain. Plain radiographs reveal a compression fracture of the 10th thoracic vertebra. She is diagnosed with osteoporosis on the basis of dual energy x-ray absorptiometry (DXA) scans that show T scores of –2.9 in her lumbar spine and –2.6 in her left femoral neck. Her 10-year probability of fracture is estimated as 23% for major osteoporotic fracture and 5.9% for hip fracture (based on the World Health Organization’s absolute fracture risk assessment tool, adapted for the United States, and available at www.shef.ac.uk/FRAX).
After excluding common secondary causes of osteoporosis, her physician recommends a bisphosphonate to reduce her risk of fracture, but she develops upper-gastrointestinal adverse effects with both alendronate and risedronate despite correctly following the instructions for oral administration.
What should her physician consider next?
OSTEOPOROSIS IS A MAJOR PROBLEM
Osteoporosis is a systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, predisposing to an increased risk of fragility fractures, particularly of the spine, hip, and wrist.
It is a major public health problem, affecting 200 million people throughout the world, with 9 million osteoporotic fractures reported in the year 2000.1 The incidence of hip fracture alone is predicted to rise to 2.6 million by the year 2025, and to 4.5 million by the year 2050.2 In the United States, the total burden was estimated to be about 2 million incident fractures in the year 2005, projected to rise by another 50% by the year 2025,3 primarily because of the aging of the population. Population studies have indeed suggested that about 40% of white women and 13% of white men over the age of 50 are at risk of sustaining an osteoporotic fracture during the remainder of their lifetime.4
The consequences of osteoporotic fractures can be devastating. Hip fractures are associated with a risk of death ranging from 8.4% to 36% during the first year after fracture.5 One-fifth of patients who sustain a hip fracture require long-term nursing home care, and more than half of the survivors do not regain their previous level of independence.
Patients with vertebral fractures are also at increased risk of death, although the results of some studies suggest that this could be the result of comorbid factors.6–9 Vertebral fractures can result in chronic back pain, loss of height from spinal deformity, reduced mobility, loss of self-esteem, and in severe cases, respiratory and digestive problems because of contact between the lower ribs and pelvis.
A person with one vertebral compression fracture is five times more likely to have another vertebral fracture,10 and a person with two or more compression fractures is 12 times more likely.11
The costs of treating osteoporotic fractures are greater than those of treating myocardial infarction or stroke12,13; they include not only direct costs incurred in treating the fracture, but also indirect societal costs owing to the long-term morbidity associated with the fracture. In the United States, the total cost of treating osteoporotic fractures was estimated at $19 billion in the year 2005.3 By 2025, the annual costs are projected to rise by almost 50%.3
A NEED FOR MORE OPTIONS
Until fairly recently, bisphosphonates were the only drugs of first choice, but adherence to oral bisphosphonate therapy is generally poor (< 50% at 1 year),14 most commonly because of dyspepsia,15 and poor adherence has been shown to be associated with increased fracture risk.16,17 Hence the need for additional therapeutic options.
In this review, we discuss denosumab, an antiresorptive drug approved by the US Food and Drug Administration (FDA) in 2010. First, we discuss its mechanism of action, efficacy, and safety, and then we offer recommendations for its use in clinical practice.
WHAT IS DENOSUMAB AND HOW DOES IT WORK?
Bone remodeling is a dynamic process involving a balance between bone resorption by osteoclasts on the one hand and new bone formation by osteoblasts on the other. A net gain in bone occurs when the activity of osteoblasts exceeds that of osteoclasts, and bone loss occurs when there is increased osteoclast activity or reduced osteoblast activity, or both. The activities of osteoblasts and osteoclasts are tightly coupled because of the opposing effects of two sets of proteins, namely, receptor activator of nuclear factor kappa b ligand (RANKL) and osteoprotegerin.
Both RANKL and osteoprotegerin are produced by osteoblasts. RANKL binds to its receptor (RANK) on preosteoclasts and osteoclasts and induces their differentiation and activation, respectively. Osteoprotegerin is the decoy receptor and natural antagonist for RANKL. By binding with RANKL, it blocks its interaction with RANK.18 In healthy individuals, a fine balance between RANKL and osteoprotegerin ensures that bone remodeling is regulated.
In postmenopausal women, estrogen deficiency leads to an imbalance between RANKL and osteoprotegerin (increased RANKL and reduced osteoprotegerin), resulting in net bone loss. This imbalance is also a feature of rheumatoid arthritis, myeloma bone disease, and osteolytic metastatic bone disease; it also occurs in those receiving androgen deprivation therapy for prostate cancer or aromatase inhibitors for breast cancer.
Denosumab is a fully human monoclonal antibody that targets RANKL.19 By binding to RANKL, this drug prevents the maturation and differentiation of preosteoclasts and promotes apoptosis of osteoclasts. Bone resorption is therefore slowed. It was parenteral osteoprotegerin that was initially developed by denosumab’s manufacturer,20 but this approach failed because neutralizing antibodies developed to osteoprotegerin, rendering it ineffective. Development of neutralizing antibodies has thus far not been a problem with denosumab.
Denosumab, with its property of RANKL inhibition, has also been used to prevent skeletal events in patients with bone metastases from solid tumors and to treat unresectable giant cell tumors of the bone (both FDA-approved indications) and hypercalcemia of malignancy. There is limited clinical experience in Paget disease of the bone as well.21–23 These other potential uses of denosumab are beyond the scope of this review.
HOW WELL DOES DENOSUMAB WORK FOR OSTEOPOROSIS?
Several phase 2 and phase 3 randomized controlled trials have evaluated the efficacy of denosumab, but only one, the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial, included fracture reduction as the primary outcome measure. The rest evaluated changes in bone mineral density or in markers of bone turnover, or both.
FREEDOM was a double-blind, randomized controlled trial in 7,808 postmenopausal women with T scores between –2.5 and –4.0 at the lumbar spine or hip.24 Twenty-four percent of the patients had vertebral fractures at baseline. Patients were randomized to receive either denosumab 60 mg (n = 3,902) or placebo (n = 3,906) every 6 months for up to 36 months. All patients also received adequate calcium and vitamin D supplementation.
At 36 months, compared with those who were randomized to receive placebo, those who were randomized to denosumab had lower incidence rates of:
- New vertebral fracture
(2.3% vs 7.2%, risk ratio 0.32,
95% CI 0.26–0.41, P < .001) - Nonvertebral fracture
(6.5% vs 8.0%, risk ratio 0.80,
95% CI 0.67–0.95, P = .01) - Hip fracture
(0.7% vs 1.2%, risk ratio 0.60,
95% CI 0.37–0.97, P = .04).
Increases in bone mineral density at the lumbar spine and hip, and decreases in bone turnover markers were also significantly greater in the denosumab group. The number needed to treat to prevent one new fracture over 3 years was 21 for vertebral fracture, 67 for nonvertebral fracture, and 200 for hip fracture, reflecting the relatively low event rate in the study.
In an open-label extension of the FREEDOM trial, the fracture incidence rates among participants who continued to receive denosumab for an additional 5 years remained low, and still below those projected for a “virtual placebo cohort” (total duration of exposure of 8 years). The rates among participants who switched from placebo to denosumab were similar to those of the denosumab group from the parent trial.25,26
A subgroup analysis of the FREEDOM trial suggested that denosumab reduced the risk of new vertebral fractures irrespective of age, body mass index, femoral neck bone mineral density, prevalent vertebral fractures, or prior nonvertebral fractures (risk ratio 0.32; 95% CI 0.26–0.41, P < .001), whereas the risk of nonvertebral fractures was only reduced in those women with body mass indices less than 25 kg/m2, femoral neck bone mineral density T scores less than –2.5, and in those without a prevalent vertebral fracture.27
A post hoc analysis revealed that denosumab significantly reduced the risk of new vertebral and hip fractures even in subgroups of women at higher risk of fracture.28 At 10% fracture probability (as estimated by the FRAX risk calculator), denosumab reduced the fracture risk by 11% (P = .629), whereas at 30% probability (moderate to high risk), the reduction was 50% (P = .001).29
Other phase 2 and phase 3 trials, in postmenopausal women with low bone mineral density, demonstrated that compared with placebo, denosumab significantly increased bone mineral density at all skeletal sites, increased volumetric bone mineral density at the distal radius, improved hip structural analysis parameters, and reduced bone turnover markers.30–33 Increases in bone mineral density and reductions in bone turnover markers with denosumab have been shown in men as well.34
In a randomized controlled trial,35 improvement in bone mineral density was better in those who received the combination of denosumab and teriparatide than in those who received either drug on its own.
Denosumab has also been shown to reduce the incidence of new vertebral fractures and improve bone mineral density in men receiving androgen-deprivation therapy for nonmetastatic prostate cancer,36 and to improve bone mineral density in women with metastatic breast cancer and low bone mass who were receiving adjuvant aromatase inhibitor therapy.37
HOW DOES DENOSUMAB COMPARE WITH OTHER OSTEOPOROSIS DRUGS?
A double-blind randomized controlled trial in postmenopausal women with low bone mass demonstrated that denosumab was superior to alendronate in improving bone mineral density at all skeletal sites (3.5% vs 2.6% for total hip bone mineral density, P < .0001).38
Another double-blind trial demonstrated that in patients previously treated with alendronate, switching to denosumab resulted in significantly greater increases in bone mineral density at all skeletal sites compared with continuing with alendronate (P < .0001).39
Denosumab has also been shown to be superior to alendronate in improving cortical bone mineral density, as measured by quantitative computed tomography.40
No trial has directly compared the efficacy of denosumab with other osteoporosis drugs in reducing fracture risk, but a systematic literature review of multiple databases,41 comparing the antifracture efficacy of nine osteoporosis drugs, concluded that teriparatide, zoledronic acid, and denosumab had the highest probabilities of being most efficacious for nonvertebral and vertebral fractures, with the greatest effect sizes. Indirect comparisons of the relative risk of fracture with denosumab (based on the results of FREEDOM), alendronate, risedronate, raloxifene, and strontium (based on a meta-analysis of randomized controlled trials) are presented in Table 1.42
A 2-year randomized, open-label, crossover study43 randomized patients to receive either denosumab followed by alendronate or alendronate followed by denosumab over successive 12-month periods. The results suggested that postmenopausal women with osteoporosis were more adherent, compliant, and persistent with denosumab therapy (a subcutaneous injection every 6 months) than with alendronate therapy in the form of oral tablets, self-administered weekly (7.5% nonadherence vs 36.5% at the end of 2 years). After receiving both treatments, women reported greater satisfaction with denosumab, with 92.4% preferring it over oral alendronate. Bone mineral density remained stable when patients were switched from denosumab to alendronate, but improved further when they were switched from alendronate to denosumab.
HOW SAFE IS DENOSUMAB?
The most frequent adverse events with denosumab reported in the long-term extension of one phase 2 study were upper respiratory tract infections (13.5%), arthralgia (11.5%), and back pain (9.0%).30
Increased risk of infection, cancer, and dermatologic reactions has been a concern, as RANKL and RANK are expressed by a wide variety of cells, including T lymphocytes, B cells, and dendritic cells.44 However, there were no significant differences in the overall incidences of adverse events between patients who received denosumab and those who received placebo or alendronate in any of the phase 2, phase 3, or extension studies.
In the FREEDOM trial,24 there was no significant difference between the two groups in the overall incidence of infection (52.9% with denosumab vs 54.4% with placebo, P = .17), or serious infection (4.1% with denosumab vs 3.4% with placebo, P = .14), although the incidence of “serious” cellulitis requiring hospitalization was higher in the denosumab group (0.3% vs < 0.1%, P = .002). There were more serious infections involving the gastrointestinal system, urinary tract, and ear and cases of endocarditis in the denosumab group, but the number of events was small, and there was no relationship with the timing of administration or duration of exposure to denosumab.45 Eczema was more common in the denosumab than in the placebo group (3.0% vs 1.7%, P < .001), but the extension data from the first 3 years did not provide any evidence for an increased risk of cellulitis or eczema with denosumab.26
Although randomized controlled trials reported more cases of neoplasms in the denosumab than in the placebo groups, meta-analyses have failed to detect a statistically significant difference (risk ratio 1.11, 95% CI 0.91–1.36).46 The overall incidence of adverse and serious adverse events reported in the 8-year extension of FREEDOM were consistent with data reported in the previous extension studies.25
In the FREEDOM extension trial, four events in the long-term group (n = 2,343), and two in the crossover group (n = 2,207) were adjudicated as being consistent with osteonecrosis of the jaw.26 One mid-shaft fracture in the crossover group was adjudicated as an atypical femoral fracture. There were, however, no reports of osteonecrosis of the jaw or atypical femoral fracture in the long-term phase 2 trial after 8 years of follow-up.30 By September 2013, postmarketing safety surveillance data for denosumab (estimated exposure of 1.2 million patient-years) had recorded four cases of atypical femoral fracture. All four patients had previously been on bisphosphonates. There were also 32 reports of osteonecrosis of the jaw.47
Denosumab’s manufacturer aims to communicate the risks of treatment to health care professionals and patients. Information is available online at www.proliahcp.com/risk-evaluation-mitigation-strategy/.
WHAT ARE THE PRECAUTIONS?
Several precautions need to be taken when considering treatment with denosumab.
Antiresorptives can aggravate hypocalcemia by inhibiting bone turnover. Serum calcium should therefore be checked and preexisting hypocalcemia should be corrected before starting denosumab.48
Denosumab is contraindicated in women who are pregnant or are planning to become pregnant, as fetal loss and teratogenicity have been reported in animal experiments. (Denosumab is unlikely to be used in premenopausal women, as it is not approved for use in this group.)
There are no data on excretion of denosumab in human milk, so it should not be given to nursing mothers.
Renal impairment is not a contraindication, and no dose adjustment is necessary (even for patients on renal replacement therapy), as denosumab, being an antibody, is eliminated through the reticuloendothelial system.49,50 However, in practice, any antiresorptive agent should be used with caution in patients with severe renal impairment because of the possible presence of adynamic bone disease. Further reduction of bone turnover would be detrimental in such patients. Also, severe hypocalcemia has been reported in patients with a creatinine clearance rate less than 30 mL/min and in those receiving dialysis.51,52 Postmarketing surveillance data have reported eight cases of severe symptomatic hypocalcemia, of which seven were in patients with chronic kidney disease.47
The manufacturer suggests that patients receive a dental examination with appropriate preventive dentistry before starting denosumab to reduce the incidence of osteonecrosis of the jaw, despite the lack of evidence in support of this strategy. The American Dental Association recommends regular dental visits and maintenance of good oral hygiene for patients already established on antiresorptive therapy.53,54
SHOULD PATIENTS ON DENOSUMAB BE OFFERED A DRUG HOLIDAY?
A drug holiday (temporary discontinuation of the drug after a certain duration of treatment) has been proposed for patients receiving bisphosphonates because of the risk of atypical femoral fracture and osteonecrosis of the jaw (although small) consequent to long-term continuous suppression of bone turnover.55 The antifracture efficacy of bisphosphonates is likely to persist for an unknown length of time after discontinuation because of their long skeletal half-life, while the risks gradually diminish.
By contrast, denosumab targets RANKL in the extracellular fluid and does not become embedded within the bone tissue.56 Pharmacokinetic studies have shown that denosumab has a rapid offset of action, with a half-life of only 26 days and biological activity lasting only 6 months.57 The results of a phase 2 extension study suggest that bone mineral density starts to decline and bone turnover markers start to rise within 12 months of discontinuing denosumab.58
Although fracture risk did not increase in those who were randomized to stopping the treatment and bone mineral density increased further when treatment was restarted, a drug holiday cannot presently be recommended for patients receiving denosumab because of the lack of supportive data.
HOW COST-EFFECTIVE IS DENOSUMAB?
The wholesale acquisition cost is $825 per 60-mg prefilled syringe of denosumab, although this may vary depending on where the drug is obtained. This does not include physician-related service costs associated with administration of denosumab.
Cost-effectiveness analyses conducted in the United States, the United Kingdom, and Sweden have all concluded that denosumab would offer a cost-effective alternative to other osteoporosis medications for primary prevention and secondary prevention of fractures.59–61
The Swedish study also incorporated adherence in the cost-effectiveness model and showed that denosumab was a cost-effective alternative to oral bisphosphonates, particularly for patients who were not expected to adhere well to oral treatments.61
WHICH OSTEOPOROSIS PATIENTS ARE CANDIDATES FOR DENOSUMAB?
The FDA has approved denosumab for the treatment of postmenopausal women and men at high risk of fracture (defined as having a history of osteoporotic fracture or multiple risk factors for fracture), or in those who cannot tolerate other osteoporosis medications or for whom other medications have failed.
Denosumab is also approved for men at high risk of fracture receiving androgen deprivation therapy for nonmetastatic prostate cancer, and for women at high risk of fracture receiving adjuvant aromatase inhibitor therapy for breast cancer.
WHAT DO THE GUIDELINES RECOMMEND?
The National Osteoporosis Foundation guidelines recommend pharmacologic treatment for patients with hip or vertebral fractures (clinical or asymptomatic); T scores lower than –2.5 at the femoral neck, total hip, or lumbar spine; and those with a 10-year probability of hip fracture of more than 3% or of a major osteoporotic fracture more than 20% based on the US-adapted FRAX calculator.62 The American College of Endocrinology guidelines have proposed similar thresholds for pharmacologic treatment, and they recommend alendronate, risedronate, zoledronate, and denosumab as first-line agents.63
The 2010 Osteoporosis Canada guidelines recommend denosumab, alendronate, risedronate, and zoledronate as first-line therapies for preventing hip, nonvertebral, and vertebral fractures in postmenopausal women (grade A recommendation).64 The National Institute of Health and Clinical Excellence in England and Wales, on the other hand, recommends denosumab only for patients who are unable to take a bisphosphonate.65
PRACTICAL PRESCRIBING TIPS
The patient described at the beginning of this article has already sustained a vertebral compression fracture, and her DXA scan shows T scores in the osteoporotic range. She is therefore at increased risk of another fragility fracture (with a fivefold higher risk of another vertebral fracture). Pharmacologic therapy should be considered. In addition, she should be encouraged to adhere to lifestyle measures such as a healthy diet and regular weight-bearing exercise, her risk of falling should be assessed, and adequate calcium and vitamin D supplementation should be given.
Secondary causes of osteoporosis are present in about 30% of women and 55% of men who have vertebral fractures.66 A complete blood count, erythrocyte sedimentation rate, bone biochemistry, 25-hydroxyvitamin D, thyroid-stimulating hormone, and renal and liver function tests should be requested in all patients. Further tests should be considered depending on the clinical evaluation and results of initial investigations.
Because this patient cannot tolerate oral bisphosphonates, she could be offered the option of annual intravenous zoledronic acid infusions or 6-monthly subcutaneous denosumab injections. In clinical trials, gastrointestinal adverse effects were noted with intravenous bisphosphonates as well, but the adverse effects reported were no different than those with placebo. The potential advantages with denosumab include better bone mineral density gains, adherence and patient satisfaction compared with oral bisphosphonates, convenient twice-yearly administration, safety in patients with renal impairment, and absence of gastrointestinal effects.
Raloxifene, a selective estrogen receptor modulator, has estrogen-like action on the bone and antiestrogen actions on the breast and uterus. Unlike standard hormone replacement therapy, raloxifene can therefore increase bone mineral density without increasing the risk of breast and endometrial cancers. However, it has only been shown to reduce the risk of vertebral fracture, not hip fracture. Hence, it would be a more appropriate choice for younger postmenopausal women. Moreover, it may cause troublesome menopausal symptoms.
Teriparatide, the recombinant parathyroid hormone, is an anabolic agent. It is very expensive, and because of this, guidelines in several countries restrict its use to women with severe osteoporosis and multiple fractures who fail to respond to standard treatments. It cannot be used for longer than 2 years because of its association with osteosarcoma in rats.
If our patient prefers denosumab, therapy should be initiated after appropriate counseling (see precautions above). The dose is 60 mg, given subcutaneously, once every 6 months.
Monitoring
There is no consensus regarding the optimal frequency for monitoring patients on treatment, owing to the lack of prospective trial data. The National Osteoporosis Foundation recommends repeating the bone mineral density measurements about 2 years after starting therapy, and about every 2 years thereafter.62 Some studies suggest that changes in bone mineral density correlate with reduction in fracture risk.67,68 A change in bone mineral density is considered significant when it is greater than the range of error of the densitometer (also known as the least significant change).69 If the bone mineral density is stable or improving, therapy could be continued, but if it is declining and the decline is greater than the least significant change, a change in therapy should be considered if no secondary causes for bone loss are evident (but see What are the areas of uncertainty? below).
The National Osteoporosis Foundation also recommends measuring a bone turnover marker at baseline and then 3 to 6 months later, as its suppression predicts greater bone mineral density responses and fracture risk reduction.70 If there is a decrease of more than 30% in serum carboxy-terminal collagen crosslinks (CTX) or more than 50% in urinary N-telopeptide (NTX),71 the patient can be reassured that the next bone mineral density measurement will be stable or improved. In patients on oral bisphosphonates, measurement of bone turnover markers also provides evidence of compliance.
Clinical trials suggest that a numerical increase in bone mineral density can be expected in most patients on treatment, though this depends on the measurement site and the length of time between examinations. In one phase 3 trial of denosumab in postmenopausal women, only 5% of the participants had unchanged or diminished bone mineral density at the lumbar spine, and 8% at the hip, after 36 months of treatment.72 However, the CTX levels fell to below the lower limit of the reference interval as early as 1 month after commencing treatment in all denosumab-treated patients.68
Hence, bone turnover markers may be a more sensitive indicator of treatment effect than bone mineral density, but this would ultimately need to be evaluated against fracture rates in a real-world setting.
WHAT ARE THE AREAS OF UNCERTAINTY?
There are currently no guidelines for long-term management of patients on denosumab, and also no data to suggest whether patients should be switched to a weaker antiresorptive drug after a certain number of years in order to reduce the possible risk of atypical femoral fracture or osteonecrosis of the jaw.
No head-to-head trials have directly compared the antifracture efficacy of denosumab with that of other standard osteoporosis therapies. The antifracture efficacy and safety of combination therapies involving denosumab are also uncertain. For adherent patients who have a suboptimal response, there is no evidence to guide the further course of action. The International Osteoporosis Foundation guidelines suggest replacing a stronger antiresorptive with an anabolic agent, but acknowledge that this is only based on expert opinion.71
The very-long-term effects (beyond 8 years) of continuous denosumab administration on increasing the risk of atypical femoral fracture, osteonecrosis of the jaw, malignancy, or infection or the duration after which risks would start to outweigh benefits is not known. However, postmarketing safety data continue to be collected through the voluntary Post-marketing Active Safety Surveillance Program (for prespecified adverse events) in addition to the FDA’s MedWatch program.
CASE PROGRESSION
The patient described in the vignette is presented with two options—zoledronate and denosumab. She chooses denosumab. Her renal function and serum calcium are checked and are found to be satisfactory. She undergoes a dental examination, which is also satisfactory. She is counseled about the possible increased risk of infection, and then she is started on 60 mg of denosumab subcutaneously, once every 6 months.
When reviewed after 2 years, she reports no further fractures. Her bone mineral density remains stable compared with the values obtained before starting treatment. She reports no adverse effects and is happy to continue with denosumab.
- Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006; 17:1726–1733.
- Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int 1997; 7:407–413.
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 2007; 22:465–475.
- Melton LJ 3rd, Chrischilles EA, Cooper C, Lane AW, Riggs BL. Perspective. How many women have osteoporosis? J Bone Miner Res 1992; 7:1005–1010.
- Abrahamsen B, van Staa T, Ariely R, Olson M, Cooper C. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int 2009; 20:1633–1650.
- Jalava T, Sarna S, Pylkkänen L, et al. Association between vertebral fracture and increased mortality in osteoporotic patients. J Bone Miner Res 2003; 18:1254–1260.
- Ismail AA, O’Neill TW, Cooper C, et al. Mortality associated with vertebral deformity in men and women: results from the European Prospective Osteoporosis Study (EPOS). Osteoporos Int 1998; 8:291–297.
- Ensrud KE, Thompson DE, Cauley JA, et al. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture Intervention Trial Research Group. J Am Geriatr Soc 2000; 48:241–249.
- Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med 1999; 159:1215–1220.
- Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA 2001; 285:320–323.
- Ross PD, Davis JW, Epstein RS, Wasnich RD. Pre-existing fractures and bone mass predict vertebral fracture incidence in women. Ann Intern Med 1991; 114:919–923.
- Piscitelli P, Iolascon G, Argentiero A, et al. Incidence and costs of hip fractures vs strokes and acute myocardial infarction in Italy: comparative analysis based on national hospitalization records. Clin Interv Aging 2012; 7:575–583.
- Johnell O, Kanis JA, Jonsson B, Oden A, Johansson H, De Laet C. The burden of hospitalised fractures in Sweden. Osteoporos Int 2005; 16:222–228.
- Confavreux CB, Canoui-Poitrine F, Schott AM, Ambrosi V, Tainturier V, Chapurlat RD. Persistence at 1 year of oral antiosteoporotic drugs: a prospective study in a comprehensive health insurance database. Eur J Endocrinol 2012; 166:735–741.
- Biswas PN, Wilton LV, Shakir SA. Pharmacovigilance study of alendronate in England. Osteoporos Int 2003; 14:507–514.
- Landfeldt E, Ström O, Robbins S, Borgström F. Adherence to treatment of primary osteoporosis and its association to fractures—the Swedish Adherence Register Analysis (SARA). Osteoporos Int 2012; 23:433–443.
- Sampalis JS, Adachi JD, Rampakakis E, Vaillancourt J, Karellis A, Kindundu C. Long-term impact of adherence to oral bisphosphonates on osteoporotic fracture incidence. J Bone Miner Res 2012; 27:202–210.
- Schwarz EM, Ritchlin CT. Clinical development of anti-RANKL therapy. Arthritis Res Ther 2007; 9(suppl 1):S7.
- Hanley DA, Adachi JD, Bell A, Brown V. Denosumab: mechanism of action and clinical outcomes. Int J Clin Pract 2012; 66:1139–1146.
- Bekker PJ, Holloway D, Nakanishi A, Arrighi M, Leese PT, Dunstan CR. The effect of a single dose of osteoprotegerin in postmenopausal women. J Bone Miner Res 2001; 16:348–360.
- Rizzoli R, Body JJ, Brandi ML, et al; International Osteoporosis Foundation Committee of Scientific Advisors Working Group on Cancer-Induced Bone Disease. Cancer-associated bone disease. Osteoporos Int 2013; 24:2929–2953.
- Schwarz P, Rasmussen AQ, Kvist TM, Andersen UB, Jørgensen NR. Paget’s disease of the bone after treatment with denosumab: a case report. Bone 2012; 50:1023–1025.
- Hu MI, Glezerman IG, Leboulleux S, et al. Denosumab for treatment of hypercalcemia of malignancy. J Clin Endocrinol Metab 2014; Jun 10 [Epub ahead of print].
- Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009; 361:756–765.
- Papapoulos S, Lippuner K, Roux C, et al. Eight years of denosumab treatment in postmenopausal women with osteoporosis: results from the first five years of the FREEDOM extension [abstract]. Presented at the 2013 annual meeting of the American Society for Bone and Mineral Research, Baltimore, MD, October 4–7, 2013.
- Bone HG, Chapurlat R, Brandi ML, et al. The effect of three or six years of denosumab exposure in women with postmenopausal osteoporosis: results from the FREEDOM extension. J Clin Endocrinol Metab 2013; 98:4483–4492.
- McClung MR, Boonen S, Törring O, et al. Effect of denosumab treatment on the risk of fractures in subgroups of women with postmenopausal osteoporosis. J Bone Miner Res 2012; 27:211–218.
- Boonen S, Adachi JD, Man Z, et al. Treatment with denosumab reduces the incidence of new vertebral and hip fractures in postmenopausal women at high risk. J Clin Endocrinol Metab 2011; 96:1727–1736.
- McCloskey EV, Johansson H, Oden A, et al. Denosumab reduces the risk of osteoporotic fractures in postmenopausal women, particularly in those with moderate to high fracture risk as assessed with FRAX. J Bone Miner Res 2012; 27:1480–1486.
- McClung MR, Lewiecki EM, Geller ML, et al. Effect of denosumab on bone mineral density and biochemical markers of bone turnover: 8-year results of a phase 2 clinical trial. Osteoporos Int 2013; 24:227–235.
- McClung MR, Lewiecki EM, Cohen SB, et al; AMG 162 Bone Loss Study Group. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 2006; 354:821–831.
- Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women. J Clin Endocrinol Metab 2008; 93:2149–2157.
- Genant HK, Engelke K, Hanley DA, et al. Denosumab improves density and strength parameters as measured by QCT of the radius in postmenopausal women with low bone mineral density. Bone 2010; 47:131–139.
- Orwoll E, Teglbjærg CS, Langdahl BL, et al. A randomized, placebo-controlled study of the effects of denosumab for the treatment of men with low bone mineral density. J Clin Endocrinol Metab 2012; 97:3161–3169.
- Tsai JN, Uihlein AV, Lee H, et al. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet 2013; 382:50–56.
- Smith MR, Egerdie B, Hernández Toriz N, et al; Denosumab HALT Prostate Cancer Study Group. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med 2009; 361:745–755.
- Ellis GK, Bone HG, Chlebowski R, et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol 2008; 26:4875–4882.
- Brown JP, Prince RL, Deal C, et al. Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res 2009; 24:153–161.
- Kendler DL, Roux C, Benhamou CL, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res 2010; 25:72–81.
- Seeman E, Delmas PD, Hanley DA, et al. Microarchitectural deterioration of cortical and trabecular bone: differing effects of denosumab and alendronate. J Bone Miner Res 2010; 25:1886–1894.
- Hopkins RB, Goeree R, Pullenayegum E, et al. The relative efficacy of nine osteoporosis medications for reducing the rate of fractures in post-menopausal women. BMC Musculoskelet Disord 2011; 12:209.
- National Institute for Health and Care Excellence (NICE). NICE technology appraisal guidance: TA161. Alendronate, etidronate, risedronate, raloxifene, strontium ranelate and teriparatide for the secondary prevention of osteoporotic fragility fractures in postmenopausal women (amended). http://publications.nice.org.uk/alendronate-etidronate-risedronate-raloxifene-strontium-ranelate-and-teriparatide-for-ta161. Accessed January 9, 2015.
- Freemantle N, Satram-Hoang S, Tang ET, et al; DAPS Investigators. Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int 2012; 23:317–326.
- Lewiecki EM. Safety and tolerability of denosumab for the treatment of postmenopausal osteoporosis. Drug Healthc Patient Saf 2011; 3:79–91.
- Watts NB, Roux C, Modlin JF, et al. Infections in postmenopausal women with osteoporosis treated with denosumab or placebo: coincidence or causal association? Osteoporos Int 2012; 23:327–337.
- von Keyserlingk C, Hopkins R, Anastasilakis A, et al. Clinical efficacy and safety of denosumab in postmenopausal women with low bone mineral density and osteoporosis: a meta-analysis. Semin Arthritis Rheum 2011; 41:178–186.
- Geller M, Wagman RB, Ho PR, et al. Early findings from Prolia postmarketing safety surveillance for atypical femoral fracture, osteonecrosis of the jaw, severe symptomatic hypocalcemia, and anaphylaxis (abstract). Osteoporos Int 2014; 25(suppl 2). OC40; www.wco-iof-esceo.org/sites/ecceo14/docs/wco14-abstractbook.pdf. Accessed January 9, 2015.
- McCormick BB, Davis J, Burns KD. Severe hypocalcemia following denosumab injection in a hemodialysis patient. Am J Kidney Dis 2012; 60:626–628.
- Jamal SA, Ljunggren O, Stehman-Breen C, et al. Effects of denosumab on fracture and bone mineral density by level of kidney function. J Bone Miner Res 2011; 26:1829–1835.
- Block GA, Bone HG, Fang L, Lee E, Padhi D. A single-dose study of denosumab in patients with various degrees of renal impairment. J Bone Miner Res 2012; 27:1471–1479.
- Ungprasert P, Cheungpasitporn W, Srivali N, Kittanamongkolchai W, Bischof EF. Life-threatening hypocalcemia associated with denosumab in a patient with moderate renal insufficiency. Am J Emerg Med 2013; 31:756.e1–e2.
- McCormick BB, Davis J, Burns KD. Severe hypocalcemia following denosumab injection in a hemodialysis patient. Am J Kidney Dis 2012; 60:626–628.
- Rachner TD, Platzbecker U, Felsenberg D, Hofbauer LC. Osteonecrosis of the jaw after osteoporosis therapy with denosumab following long-term bisphosphonate therapy. Mayo Clin Proc 2013; 88:418–419.
- Epstein MS, Ephros HD, Epstein JB. Review of current literature and implications of RANKL inhibitors for oral health care providers. Oral Surg Oral Med Oral Pathol Oral Radiol 2013; 116:e437–e442.
- McClung M, Harris ST, Miller PD, et al. Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med 2013; 126:13–20.
- Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone 2011; 48:677–692.
- Bekker PJ, Holloway DL, Rasmussen AS, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res 2004; 19:1059–1066.
- Miller PD, Bolognese MA, Lewiecki EM, et al; Amg Bone Loss Study Group. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone 2008; 43:222–229.
- Parthan A, Kruse M, Yurgin N, Huang J, Viswanathan HN, Taylor D. Cost effectiveness of denosumab versus oral bisphosphonates for postmenopausal osteoporosis in the US. Appl Health Econ Health Policy 2013; 11:485–497.
- Scotland G, Waugh N, Royle P, McNamee P, Henderson R, Hollick R. Denosumab for the prevention of osteoporotic fractures in post-menopausal women: a NICE single technology appraisal. Pharmacoeconomics 2011; 29:951–961.
- Jönsson B, Ström O, Eisman JA, et al. Cost-effectiveness of denosumab for the treatment of postmenopausal osteoporosis. Osteoporos Int 2011; 22:967–982.
- Clinician’s guide to prevention and treatment of osteoporosis. Washington DC: National Osteoporosis Foundation, 2013.
- Watts NB, Bilezikian JP, Camacho PM, et al; AACE Osteoporosis Task Force. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract 2010;16(suppl 3):1–37.
- Papaioannou A, Morin S, Cheung AM, et al; Scientific Advisory Council of Osteoporosis Canada. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ 2010; 182:1864–1673.
- National Institute for Health and Care Excellence (NICE). NICE technology appraisal: TA204. Denosumab for the prevention of osteoporotic fractures in postmenopausal women. http://guidance.nice.org.uk/TA204. Accessed January 9, 2015.
- Premaor MO, Compston JE. Testing for secondary causes of osteoporosis. BMJ 2010; 341:c6959.
- Hochberg MC, Ross PD, Black D, et al. Larger increases in bone mineral density during alendronate therapy are associated with a lower risk of new vertebral fractures in women with postmenopausal osteoporosis. Fracture Intervention Trial Research Group. Arthritis Rheum 1999; 42:1246–1254.
- Eastell R, Vrijens B, Cahall DL, Ringe JD, Garnero P, Watts NB. Bone turnover markers and bone mineral density response with risedronate therapy: relationship with fracture risk and patient adherence. J Bone Miner Res 2011; 26:1662–1669.
- Diez-Perez A, Adachi JD, Agnusdei D, et al; IOF CSA Inadequate Responders Working Group. Treatment failure in osteoporosis. Osteoporos Int 2012; 23:2769–2774.
- Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res 2003; 18:1051–1056.
- Rosen HN, Moses AC, Garber J, Ross DS, Lee SL, Greenspan SL. Utility of biochemical markers of bone turnover in the follow-up of patients treated with bisphosphonates. Calcif Tissue Int 1998; 63:363–368.
- Bolognese MA, Teglbjærg CS, Zanchetta JR, et al. Denosumab significantly increases DXA BMD at both trabecular and cortical sites: results from the FREEDOM study. J Clin Densitom 2013; 16:147–153.
A 68-year-old white woman presents with mid- thoracic back pain. Plain radiographs reveal a compression fracture of the 10th thoracic vertebra. She is diagnosed with osteoporosis on the basis of dual energy x-ray absorptiometry (DXA) scans that show T scores of –2.9 in her lumbar spine and –2.6 in her left femoral neck. Her 10-year probability of fracture is estimated as 23% for major osteoporotic fracture and 5.9% for hip fracture (based on the World Health Organization’s absolute fracture risk assessment tool, adapted for the United States, and available at www.shef.ac.uk/FRAX).
After excluding common secondary causes of osteoporosis, her physician recommends a bisphosphonate to reduce her risk of fracture, but she develops upper-gastrointestinal adverse effects with both alendronate and risedronate despite correctly following the instructions for oral administration.
What should her physician consider next?
OSTEOPOROSIS IS A MAJOR PROBLEM
Osteoporosis is a systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, predisposing to an increased risk of fragility fractures, particularly of the spine, hip, and wrist.
It is a major public health problem, affecting 200 million people throughout the world, with 9 million osteoporotic fractures reported in the year 2000.1 The incidence of hip fracture alone is predicted to rise to 2.6 million by the year 2025, and to 4.5 million by the year 2050.2 In the United States, the total burden was estimated to be about 2 million incident fractures in the year 2005, projected to rise by another 50% by the year 2025,3 primarily because of the aging of the population. Population studies have indeed suggested that about 40% of white women and 13% of white men over the age of 50 are at risk of sustaining an osteoporotic fracture during the remainder of their lifetime.4
The consequences of osteoporotic fractures can be devastating. Hip fractures are associated with a risk of death ranging from 8.4% to 36% during the first year after fracture.5 One-fifth of patients who sustain a hip fracture require long-term nursing home care, and more than half of the survivors do not regain their previous level of independence.
Patients with vertebral fractures are also at increased risk of death, although the results of some studies suggest that this could be the result of comorbid factors.6–9 Vertebral fractures can result in chronic back pain, loss of height from spinal deformity, reduced mobility, loss of self-esteem, and in severe cases, respiratory and digestive problems because of contact between the lower ribs and pelvis.
A person with one vertebral compression fracture is five times more likely to have another vertebral fracture,10 and a person with two or more compression fractures is 12 times more likely.11
The costs of treating osteoporotic fractures are greater than those of treating myocardial infarction or stroke12,13; they include not only direct costs incurred in treating the fracture, but also indirect societal costs owing to the long-term morbidity associated with the fracture. In the United States, the total cost of treating osteoporotic fractures was estimated at $19 billion in the year 2005.3 By 2025, the annual costs are projected to rise by almost 50%.3
A NEED FOR MORE OPTIONS
Until fairly recently, bisphosphonates were the only drugs of first choice, but adherence to oral bisphosphonate therapy is generally poor (< 50% at 1 year),14 most commonly because of dyspepsia,15 and poor adherence has been shown to be associated with increased fracture risk.16,17 Hence the need for additional therapeutic options.
In this review, we discuss denosumab, an antiresorptive drug approved by the US Food and Drug Administration (FDA) in 2010. First, we discuss its mechanism of action, efficacy, and safety, and then we offer recommendations for its use in clinical practice.
WHAT IS DENOSUMAB AND HOW DOES IT WORK?
Bone remodeling is a dynamic process involving a balance between bone resorption by osteoclasts on the one hand and new bone formation by osteoblasts on the other. A net gain in bone occurs when the activity of osteoblasts exceeds that of osteoclasts, and bone loss occurs when there is increased osteoclast activity or reduced osteoblast activity, or both. The activities of osteoblasts and osteoclasts are tightly coupled because of the opposing effects of two sets of proteins, namely, receptor activator of nuclear factor kappa b ligand (RANKL) and osteoprotegerin.
Both RANKL and osteoprotegerin are produced by osteoblasts. RANKL binds to its receptor (RANK) on preosteoclasts and osteoclasts and induces their differentiation and activation, respectively. Osteoprotegerin is the decoy receptor and natural antagonist for RANKL. By binding with RANKL, it blocks its interaction with RANK.18 In healthy individuals, a fine balance between RANKL and osteoprotegerin ensures that bone remodeling is regulated.
In postmenopausal women, estrogen deficiency leads to an imbalance between RANKL and osteoprotegerin (increased RANKL and reduced osteoprotegerin), resulting in net bone loss. This imbalance is also a feature of rheumatoid arthritis, myeloma bone disease, and osteolytic metastatic bone disease; it also occurs in those receiving androgen deprivation therapy for prostate cancer or aromatase inhibitors for breast cancer.
Denosumab is a fully human monoclonal antibody that targets RANKL.19 By binding to RANKL, this drug prevents the maturation and differentiation of preosteoclasts and promotes apoptosis of osteoclasts. Bone resorption is therefore slowed. It was parenteral osteoprotegerin that was initially developed by denosumab’s manufacturer,20 but this approach failed because neutralizing antibodies developed to osteoprotegerin, rendering it ineffective. Development of neutralizing antibodies has thus far not been a problem with denosumab.
Denosumab, with its property of RANKL inhibition, has also been used to prevent skeletal events in patients with bone metastases from solid tumors and to treat unresectable giant cell tumors of the bone (both FDA-approved indications) and hypercalcemia of malignancy. There is limited clinical experience in Paget disease of the bone as well.21–23 These other potential uses of denosumab are beyond the scope of this review.
HOW WELL DOES DENOSUMAB WORK FOR OSTEOPOROSIS?
Several phase 2 and phase 3 randomized controlled trials have evaluated the efficacy of denosumab, but only one, the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial, included fracture reduction as the primary outcome measure. The rest evaluated changes in bone mineral density or in markers of bone turnover, or both.
FREEDOM was a double-blind, randomized controlled trial in 7,808 postmenopausal women with T scores between –2.5 and –4.0 at the lumbar spine or hip.24 Twenty-four percent of the patients had vertebral fractures at baseline. Patients were randomized to receive either denosumab 60 mg (n = 3,902) or placebo (n = 3,906) every 6 months for up to 36 months. All patients also received adequate calcium and vitamin D supplementation.
At 36 months, compared with those who were randomized to receive placebo, those who were randomized to denosumab had lower incidence rates of:
- New vertebral fracture
(2.3% vs 7.2%, risk ratio 0.32,
95% CI 0.26–0.41, P < .001) - Nonvertebral fracture
(6.5% vs 8.0%, risk ratio 0.80,
95% CI 0.67–0.95, P = .01) - Hip fracture
(0.7% vs 1.2%, risk ratio 0.60,
95% CI 0.37–0.97, P = .04).
Increases in bone mineral density at the lumbar spine and hip, and decreases in bone turnover markers were also significantly greater in the denosumab group. The number needed to treat to prevent one new fracture over 3 years was 21 for vertebral fracture, 67 for nonvertebral fracture, and 200 for hip fracture, reflecting the relatively low event rate in the study.
In an open-label extension of the FREEDOM trial, the fracture incidence rates among participants who continued to receive denosumab for an additional 5 years remained low, and still below those projected for a “virtual placebo cohort” (total duration of exposure of 8 years). The rates among participants who switched from placebo to denosumab were similar to those of the denosumab group from the parent trial.25,26
A subgroup analysis of the FREEDOM trial suggested that denosumab reduced the risk of new vertebral fractures irrespective of age, body mass index, femoral neck bone mineral density, prevalent vertebral fractures, or prior nonvertebral fractures (risk ratio 0.32; 95% CI 0.26–0.41, P < .001), whereas the risk of nonvertebral fractures was only reduced in those women with body mass indices less than 25 kg/m2, femoral neck bone mineral density T scores less than –2.5, and in those without a prevalent vertebral fracture.27
A post hoc analysis revealed that denosumab significantly reduced the risk of new vertebral and hip fractures even in subgroups of women at higher risk of fracture.28 At 10% fracture probability (as estimated by the FRAX risk calculator), denosumab reduced the fracture risk by 11% (P = .629), whereas at 30% probability (moderate to high risk), the reduction was 50% (P = .001).29
Other phase 2 and phase 3 trials, in postmenopausal women with low bone mineral density, demonstrated that compared with placebo, denosumab significantly increased bone mineral density at all skeletal sites, increased volumetric bone mineral density at the distal radius, improved hip structural analysis parameters, and reduced bone turnover markers.30–33 Increases in bone mineral density and reductions in bone turnover markers with denosumab have been shown in men as well.34
In a randomized controlled trial,35 improvement in bone mineral density was better in those who received the combination of denosumab and teriparatide than in those who received either drug on its own.
Denosumab has also been shown to reduce the incidence of new vertebral fractures and improve bone mineral density in men receiving androgen-deprivation therapy for nonmetastatic prostate cancer,36 and to improve bone mineral density in women with metastatic breast cancer and low bone mass who were receiving adjuvant aromatase inhibitor therapy.37
HOW DOES DENOSUMAB COMPARE WITH OTHER OSTEOPOROSIS DRUGS?
A double-blind randomized controlled trial in postmenopausal women with low bone mass demonstrated that denosumab was superior to alendronate in improving bone mineral density at all skeletal sites (3.5% vs 2.6% for total hip bone mineral density, P < .0001).38
Another double-blind trial demonstrated that in patients previously treated with alendronate, switching to denosumab resulted in significantly greater increases in bone mineral density at all skeletal sites compared with continuing with alendronate (P < .0001).39
Denosumab has also been shown to be superior to alendronate in improving cortical bone mineral density, as measured by quantitative computed tomography.40
No trial has directly compared the efficacy of denosumab with other osteoporosis drugs in reducing fracture risk, but a systematic literature review of multiple databases,41 comparing the antifracture efficacy of nine osteoporosis drugs, concluded that teriparatide, zoledronic acid, and denosumab had the highest probabilities of being most efficacious for nonvertebral and vertebral fractures, with the greatest effect sizes. Indirect comparisons of the relative risk of fracture with denosumab (based on the results of FREEDOM), alendronate, risedronate, raloxifene, and strontium (based on a meta-analysis of randomized controlled trials) are presented in Table 1.42
A 2-year randomized, open-label, crossover study43 randomized patients to receive either denosumab followed by alendronate or alendronate followed by denosumab over successive 12-month periods. The results suggested that postmenopausal women with osteoporosis were more adherent, compliant, and persistent with denosumab therapy (a subcutaneous injection every 6 months) than with alendronate therapy in the form of oral tablets, self-administered weekly (7.5% nonadherence vs 36.5% at the end of 2 years). After receiving both treatments, women reported greater satisfaction with denosumab, with 92.4% preferring it over oral alendronate. Bone mineral density remained stable when patients were switched from denosumab to alendronate, but improved further when they were switched from alendronate to denosumab.
HOW SAFE IS DENOSUMAB?
The most frequent adverse events with denosumab reported in the long-term extension of one phase 2 study were upper respiratory tract infections (13.5%), arthralgia (11.5%), and back pain (9.0%).30
Increased risk of infection, cancer, and dermatologic reactions has been a concern, as RANKL and RANK are expressed by a wide variety of cells, including T lymphocytes, B cells, and dendritic cells.44 However, there were no significant differences in the overall incidences of adverse events between patients who received denosumab and those who received placebo or alendronate in any of the phase 2, phase 3, or extension studies.
In the FREEDOM trial,24 there was no significant difference between the two groups in the overall incidence of infection (52.9% with denosumab vs 54.4% with placebo, P = .17), or serious infection (4.1% with denosumab vs 3.4% with placebo, P = .14), although the incidence of “serious” cellulitis requiring hospitalization was higher in the denosumab group (0.3% vs < 0.1%, P = .002). There were more serious infections involving the gastrointestinal system, urinary tract, and ear and cases of endocarditis in the denosumab group, but the number of events was small, and there was no relationship with the timing of administration or duration of exposure to denosumab.45 Eczema was more common in the denosumab than in the placebo group (3.0% vs 1.7%, P < .001), but the extension data from the first 3 years did not provide any evidence for an increased risk of cellulitis or eczema with denosumab.26
Although randomized controlled trials reported more cases of neoplasms in the denosumab than in the placebo groups, meta-analyses have failed to detect a statistically significant difference (risk ratio 1.11, 95% CI 0.91–1.36).46 The overall incidence of adverse and serious adverse events reported in the 8-year extension of FREEDOM were consistent with data reported in the previous extension studies.25
In the FREEDOM extension trial, four events in the long-term group (n = 2,343), and two in the crossover group (n = 2,207) were adjudicated as being consistent with osteonecrosis of the jaw.26 One mid-shaft fracture in the crossover group was adjudicated as an atypical femoral fracture. There were, however, no reports of osteonecrosis of the jaw or atypical femoral fracture in the long-term phase 2 trial after 8 years of follow-up.30 By September 2013, postmarketing safety surveillance data for denosumab (estimated exposure of 1.2 million patient-years) had recorded four cases of atypical femoral fracture. All four patients had previously been on bisphosphonates. There were also 32 reports of osteonecrosis of the jaw.47
Denosumab’s manufacturer aims to communicate the risks of treatment to health care professionals and patients. Information is available online at www.proliahcp.com/risk-evaluation-mitigation-strategy/.
WHAT ARE THE PRECAUTIONS?
Several precautions need to be taken when considering treatment with denosumab.
Antiresorptives can aggravate hypocalcemia by inhibiting bone turnover. Serum calcium should therefore be checked and preexisting hypocalcemia should be corrected before starting denosumab.48
Denosumab is contraindicated in women who are pregnant or are planning to become pregnant, as fetal loss and teratogenicity have been reported in animal experiments. (Denosumab is unlikely to be used in premenopausal women, as it is not approved for use in this group.)
There are no data on excretion of denosumab in human milk, so it should not be given to nursing mothers.
Renal impairment is not a contraindication, and no dose adjustment is necessary (even for patients on renal replacement therapy), as denosumab, being an antibody, is eliminated through the reticuloendothelial system.49,50 However, in practice, any antiresorptive agent should be used with caution in patients with severe renal impairment because of the possible presence of adynamic bone disease. Further reduction of bone turnover would be detrimental in such patients. Also, severe hypocalcemia has been reported in patients with a creatinine clearance rate less than 30 mL/min and in those receiving dialysis.51,52 Postmarketing surveillance data have reported eight cases of severe symptomatic hypocalcemia, of which seven were in patients with chronic kidney disease.47
The manufacturer suggests that patients receive a dental examination with appropriate preventive dentistry before starting denosumab to reduce the incidence of osteonecrosis of the jaw, despite the lack of evidence in support of this strategy. The American Dental Association recommends regular dental visits and maintenance of good oral hygiene for patients already established on antiresorptive therapy.53,54
SHOULD PATIENTS ON DENOSUMAB BE OFFERED A DRUG HOLIDAY?
A drug holiday (temporary discontinuation of the drug after a certain duration of treatment) has been proposed for patients receiving bisphosphonates because of the risk of atypical femoral fracture and osteonecrosis of the jaw (although small) consequent to long-term continuous suppression of bone turnover.55 The antifracture efficacy of bisphosphonates is likely to persist for an unknown length of time after discontinuation because of their long skeletal half-life, while the risks gradually diminish.
By contrast, denosumab targets RANKL in the extracellular fluid and does not become embedded within the bone tissue.56 Pharmacokinetic studies have shown that denosumab has a rapid offset of action, with a half-life of only 26 days and biological activity lasting only 6 months.57 The results of a phase 2 extension study suggest that bone mineral density starts to decline and bone turnover markers start to rise within 12 months of discontinuing denosumab.58
Although fracture risk did not increase in those who were randomized to stopping the treatment and bone mineral density increased further when treatment was restarted, a drug holiday cannot presently be recommended for patients receiving denosumab because of the lack of supportive data.
HOW COST-EFFECTIVE IS DENOSUMAB?
The wholesale acquisition cost is $825 per 60-mg prefilled syringe of denosumab, although this may vary depending on where the drug is obtained. This does not include physician-related service costs associated with administration of denosumab.
Cost-effectiveness analyses conducted in the United States, the United Kingdom, and Sweden have all concluded that denosumab would offer a cost-effective alternative to other osteoporosis medications for primary prevention and secondary prevention of fractures.59–61
The Swedish study also incorporated adherence in the cost-effectiveness model and showed that denosumab was a cost-effective alternative to oral bisphosphonates, particularly for patients who were not expected to adhere well to oral treatments.61
WHICH OSTEOPOROSIS PATIENTS ARE CANDIDATES FOR DENOSUMAB?
The FDA has approved denosumab for the treatment of postmenopausal women and men at high risk of fracture (defined as having a history of osteoporotic fracture or multiple risk factors for fracture), or in those who cannot tolerate other osteoporosis medications or for whom other medications have failed.
Denosumab is also approved for men at high risk of fracture receiving androgen deprivation therapy for nonmetastatic prostate cancer, and for women at high risk of fracture receiving adjuvant aromatase inhibitor therapy for breast cancer.
WHAT DO THE GUIDELINES RECOMMEND?
The National Osteoporosis Foundation guidelines recommend pharmacologic treatment for patients with hip or vertebral fractures (clinical or asymptomatic); T scores lower than –2.5 at the femoral neck, total hip, or lumbar spine; and those with a 10-year probability of hip fracture of more than 3% or of a major osteoporotic fracture more than 20% based on the US-adapted FRAX calculator.62 The American College of Endocrinology guidelines have proposed similar thresholds for pharmacologic treatment, and they recommend alendronate, risedronate, zoledronate, and denosumab as first-line agents.63
The 2010 Osteoporosis Canada guidelines recommend denosumab, alendronate, risedronate, and zoledronate as first-line therapies for preventing hip, nonvertebral, and vertebral fractures in postmenopausal women (grade A recommendation).64 The National Institute of Health and Clinical Excellence in England and Wales, on the other hand, recommends denosumab only for patients who are unable to take a bisphosphonate.65
PRACTICAL PRESCRIBING TIPS
The patient described at the beginning of this article has already sustained a vertebral compression fracture, and her DXA scan shows T scores in the osteoporotic range. She is therefore at increased risk of another fragility fracture (with a fivefold higher risk of another vertebral fracture). Pharmacologic therapy should be considered. In addition, she should be encouraged to adhere to lifestyle measures such as a healthy diet and regular weight-bearing exercise, her risk of falling should be assessed, and adequate calcium and vitamin D supplementation should be given.
Secondary causes of osteoporosis are present in about 30% of women and 55% of men who have vertebral fractures.66 A complete blood count, erythrocyte sedimentation rate, bone biochemistry, 25-hydroxyvitamin D, thyroid-stimulating hormone, and renal and liver function tests should be requested in all patients. Further tests should be considered depending on the clinical evaluation and results of initial investigations.
Because this patient cannot tolerate oral bisphosphonates, she could be offered the option of annual intravenous zoledronic acid infusions or 6-monthly subcutaneous denosumab injections. In clinical trials, gastrointestinal adverse effects were noted with intravenous bisphosphonates as well, but the adverse effects reported were no different than those with placebo. The potential advantages with denosumab include better bone mineral density gains, adherence and patient satisfaction compared with oral bisphosphonates, convenient twice-yearly administration, safety in patients with renal impairment, and absence of gastrointestinal effects.
Raloxifene, a selective estrogen receptor modulator, has estrogen-like action on the bone and antiestrogen actions on the breast and uterus. Unlike standard hormone replacement therapy, raloxifene can therefore increase bone mineral density without increasing the risk of breast and endometrial cancers. However, it has only been shown to reduce the risk of vertebral fracture, not hip fracture. Hence, it would be a more appropriate choice for younger postmenopausal women. Moreover, it may cause troublesome menopausal symptoms.
Teriparatide, the recombinant parathyroid hormone, is an anabolic agent. It is very expensive, and because of this, guidelines in several countries restrict its use to women with severe osteoporosis and multiple fractures who fail to respond to standard treatments. It cannot be used for longer than 2 years because of its association with osteosarcoma in rats.
If our patient prefers denosumab, therapy should be initiated after appropriate counseling (see precautions above). The dose is 60 mg, given subcutaneously, once every 6 months.
Monitoring
There is no consensus regarding the optimal frequency for monitoring patients on treatment, owing to the lack of prospective trial data. The National Osteoporosis Foundation recommends repeating the bone mineral density measurements about 2 years after starting therapy, and about every 2 years thereafter.62 Some studies suggest that changes in bone mineral density correlate with reduction in fracture risk.67,68 A change in bone mineral density is considered significant when it is greater than the range of error of the densitometer (also known as the least significant change).69 If the bone mineral density is stable or improving, therapy could be continued, but if it is declining and the decline is greater than the least significant change, a change in therapy should be considered if no secondary causes for bone loss are evident (but see What are the areas of uncertainty? below).
The National Osteoporosis Foundation also recommends measuring a bone turnover marker at baseline and then 3 to 6 months later, as its suppression predicts greater bone mineral density responses and fracture risk reduction.70 If there is a decrease of more than 30% in serum carboxy-terminal collagen crosslinks (CTX) or more than 50% in urinary N-telopeptide (NTX),71 the patient can be reassured that the next bone mineral density measurement will be stable or improved. In patients on oral bisphosphonates, measurement of bone turnover markers also provides evidence of compliance.
Clinical trials suggest that a numerical increase in bone mineral density can be expected in most patients on treatment, though this depends on the measurement site and the length of time between examinations. In one phase 3 trial of denosumab in postmenopausal women, only 5% of the participants had unchanged or diminished bone mineral density at the lumbar spine, and 8% at the hip, after 36 months of treatment.72 However, the CTX levels fell to below the lower limit of the reference interval as early as 1 month after commencing treatment in all denosumab-treated patients.68
Hence, bone turnover markers may be a more sensitive indicator of treatment effect than bone mineral density, but this would ultimately need to be evaluated against fracture rates in a real-world setting.
WHAT ARE THE AREAS OF UNCERTAINTY?
There are currently no guidelines for long-term management of patients on denosumab, and also no data to suggest whether patients should be switched to a weaker antiresorptive drug after a certain number of years in order to reduce the possible risk of atypical femoral fracture or osteonecrosis of the jaw.
No head-to-head trials have directly compared the antifracture efficacy of denosumab with that of other standard osteoporosis therapies. The antifracture efficacy and safety of combination therapies involving denosumab are also uncertain. For adherent patients who have a suboptimal response, there is no evidence to guide the further course of action. The International Osteoporosis Foundation guidelines suggest replacing a stronger antiresorptive with an anabolic agent, but acknowledge that this is only based on expert opinion.71
The very-long-term effects (beyond 8 years) of continuous denosumab administration on increasing the risk of atypical femoral fracture, osteonecrosis of the jaw, malignancy, or infection or the duration after which risks would start to outweigh benefits is not known. However, postmarketing safety data continue to be collected through the voluntary Post-marketing Active Safety Surveillance Program (for prespecified adverse events) in addition to the FDA’s MedWatch program.
CASE PROGRESSION
The patient described in the vignette is presented with two options—zoledronate and denosumab. She chooses denosumab. Her renal function and serum calcium are checked and are found to be satisfactory. She undergoes a dental examination, which is also satisfactory. She is counseled about the possible increased risk of infection, and then she is started on 60 mg of denosumab subcutaneously, once every 6 months.
When reviewed after 2 years, she reports no further fractures. Her bone mineral density remains stable compared with the values obtained before starting treatment. She reports no adverse effects and is happy to continue with denosumab.
A 68-year-old white woman presents with mid- thoracic back pain. Plain radiographs reveal a compression fracture of the 10th thoracic vertebra. She is diagnosed with osteoporosis on the basis of dual energy x-ray absorptiometry (DXA) scans that show T scores of –2.9 in her lumbar spine and –2.6 in her left femoral neck. Her 10-year probability of fracture is estimated as 23% for major osteoporotic fracture and 5.9% for hip fracture (based on the World Health Organization’s absolute fracture risk assessment tool, adapted for the United States, and available at www.shef.ac.uk/FRAX).
After excluding common secondary causes of osteoporosis, her physician recommends a bisphosphonate to reduce her risk of fracture, but she develops upper-gastrointestinal adverse effects with both alendronate and risedronate despite correctly following the instructions for oral administration.
What should her physician consider next?
OSTEOPOROSIS IS A MAJOR PROBLEM
Osteoporosis is a systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, predisposing to an increased risk of fragility fractures, particularly of the spine, hip, and wrist.
It is a major public health problem, affecting 200 million people throughout the world, with 9 million osteoporotic fractures reported in the year 2000.1 The incidence of hip fracture alone is predicted to rise to 2.6 million by the year 2025, and to 4.5 million by the year 2050.2 In the United States, the total burden was estimated to be about 2 million incident fractures in the year 2005, projected to rise by another 50% by the year 2025,3 primarily because of the aging of the population. Population studies have indeed suggested that about 40% of white women and 13% of white men over the age of 50 are at risk of sustaining an osteoporotic fracture during the remainder of their lifetime.4
The consequences of osteoporotic fractures can be devastating. Hip fractures are associated with a risk of death ranging from 8.4% to 36% during the first year after fracture.5 One-fifth of patients who sustain a hip fracture require long-term nursing home care, and more than half of the survivors do not regain their previous level of independence.
Patients with vertebral fractures are also at increased risk of death, although the results of some studies suggest that this could be the result of comorbid factors.6–9 Vertebral fractures can result in chronic back pain, loss of height from spinal deformity, reduced mobility, loss of self-esteem, and in severe cases, respiratory and digestive problems because of contact between the lower ribs and pelvis.
A person with one vertebral compression fracture is five times more likely to have another vertebral fracture,10 and a person with two or more compression fractures is 12 times more likely.11
The costs of treating osteoporotic fractures are greater than those of treating myocardial infarction or stroke12,13; they include not only direct costs incurred in treating the fracture, but also indirect societal costs owing to the long-term morbidity associated with the fracture. In the United States, the total cost of treating osteoporotic fractures was estimated at $19 billion in the year 2005.3 By 2025, the annual costs are projected to rise by almost 50%.3
A NEED FOR MORE OPTIONS
Until fairly recently, bisphosphonates were the only drugs of first choice, but adherence to oral bisphosphonate therapy is generally poor (< 50% at 1 year),14 most commonly because of dyspepsia,15 and poor adherence has been shown to be associated with increased fracture risk.16,17 Hence the need for additional therapeutic options.
In this review, we discuss denosumab, an antiresorptive drug approved by the US Food and Drug Administration (FDA) in 2010. First, we discuss its mechanism of action, efficacy, and safety, and then we offer recommendations for its use in clinical practice.
WHAT IS DENOSUMAB AND HOW DOES IT WORK?
Bone remodeling is a dynamic process involving a balance between bone resorption by osteoclasts on the one hand and new bone formation by osteoblasts on the other. A net gain in bone occurs when the activity of osteoblasts exceeds that of osteoclasts, and bone loss occurs when there is increased osteoclast activity or reduced osteoblast activity, or both. The activities of osteoblasts and osteoclasts are tightly coupled because of the opposing effects of two sets of proteins, namely, receptor activator of nuclear factor kappa b ligand (RANKL) and osteoprotegerin.
Both RANKL and osteoprotegerin are produced by osteoblasts. RANKL binds to its receptor (RANK) on preosteoclasts and osteoclasts and induces their differentiation and activation, respectively. Osteoprotegerin is the decoy receptor and natural antagonist for RANKL. By binding with RANKL, it blocks its interaction with RANK.18 In healthy individuals, a fine balance between RANKL and osteoprotegerin ensures that bone remodeling is regulated.
In postmenopausal women, estrogen deficiency leads to an imbalance between RANKL and osteoprotegerin (increased RANKL and reduced osteoprotegerin), resulting in net bone loss. This imbalance is also a feature of rheumatoid arthritis, myeloma bone disease, and osteolytic metastatic bone disease; it also occurs in those receiving androgen deprivation therapy for prostate cancer or aromatase inhibitors for breast cancer.
Denosumab is a fully human monoclonal antibody that targets RANKL.19 By binding to RANKL, this drug prevents the maturation and differentiation of preosteoclasts and promotes apoptosis of osteoclasts. Bone resorption is therefore slowed. It was parenteral osteoprotegerin that was initially developed by denosumab’s manufacturer,20 but this approach failed because neutralizing antibodies developed to osteoprotegerin, rendering it ineffective. Development of neutralizing antibodies has thus far not been a problem with denosumab.
Denosumab, with its property of RANKL inhibition, has also been used to prevent skeletal events in patients with bone metastases from solid tumors and to treat unresectable giant cell tumors of the bone (both FDA-approved indications) and hypercalcemia of malignancy. There is limited clinical experience in Paget disease of the bone as well.21–23 These other potential uses of denosumab are beyond the scope of this review.
HOW WELL DOES DENOSUMAB WORK FOR OSTEOPOROSIS?
Several phase 2 and phase 3 randomized controlled trials have evaluated the efficacy of denosumab, but only one, the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial, included fracture reduction as the primary outcome measure. The rest evaluated changes in bone mineral density or in markers of bone turnover, or both.
FREEDOM was a double-blind, randomized controlled trial in 7,808 postmenopausal women with T scores between –2.5 and –4.0 at the lumbar spine or hip.24 Twenty-four percent of the patients had vertebral fractures at baseline. Patients were randomized to receive either denosumab 60 mg (n = 3,902) or placebo (n = 3,906) every 6 months for up to 36 months. All patients also received adequate calcium and vitamin D supplementation.
At 36 months, compared with those who were randomized to receive placebo, those who were randomized to denosumab had lower incidence rates of:
- New vertebral fracture
(2.3% vs 7.2%, risk ratio 0.32,
95% CI 0.26–0.41, P < .001) - Nonvertebral fracture
(6.5% vs 8.0%, risk ratio 0.80,
95% CI 0.67–0.95, P = .01) - Hip fracture
(0.7% vs 1.2%, risk ratio 0.60,
95% CI 0.37–0.97, P = .04).
Increases in bone mineral density at the lumbar spine and hip, and decreases in bone turnover markers were also significantly greater in the denosumab group. The number needed to treat to prevent one new fracture over 3 years was 21 for vertebral fracture, 67 for nonvertebral fracture, and 200 for hip fracture, reflecting the relatively low event rate in the study.
In an open-label extension of the FREEDOM trial, the fracture incidence rates among participants who continued to receive denosumab for an additional 5 years remained low, and still below those projected for a “virtual placebo cohort” (total duration of exposure of 8 years). The rates among participants who switched from placebo to denosumab were similar to those of the denosumab group from the parent trial.25,26
A subgroup analysis of the FREEDOM trial suggested that denosumab reduced the risk of new vertebral fractures irrespective of age, body mass index, femoral neck bone mineral density, prevalent vertebral fractures, or prior nonvertebral fractures (risk ratio 0.32; 95% CI 0.26–0.41, P < .001), whereas the risk of nonvertebral fractures was only reduced in those women with body mass indices less than 25 kg/m2, femoral neck bone mineral density T scores less than –2.5, and in those without a prevalent vertebral fracture.27
A post hoc analysis revealed that denosumab significantly reduced the risk of new vertebral and hip fractures even in subgroups of women at higher risk of fracture.28 At 10% fracture probability (as estimated by the FRAX risk calculator), denosumab reduced the fracture risk by 11% (P = .629), whereas at 30% probability (moderate to high risk), the reduction was 50% (P = .001).29
Other phase 2 and phase 3 trials, in postmenopausal women with low bone mineral density, demonstrated that compared with placebo, denosumab significantly increased bone mineral density at all skeletal sites, increased volumetric bone mineral density at the distal radius, improved hip structural analysis parameters, and reduced bone turnover markers.30–33 Increases in bone mineral density and reductions in bone turnover markers with denosumab have been shown in men as well.34
In a randomized controlled trial,35 improvement in bone mineral density was better in those who received the combination of denosumab and teriparatide than in those who received either drug on its own.
Denosumab has also been shown to reduce the incidence of new vertebral fractures and improve bone mineral density in men receiving androgen-deprivation therapy for nonmetastatic prostate cancer,36 and to improve bone mineral density in women with metastatic breast cancer and low bone mass who were receiving adjuvant aromatase inhibitor therapy.37
HOW DOES DENOSUMAB COMPARE WITH OTHER OSTEOPOROSIS DRUGS?
A double-blind randomized controlled trial in postmenopausal women with low bone mass demonstrated that denosumab was superior to alendronate in improving bone mineral density at all skeletal sites (3.5% vs 2.6% for total hip bone mineral density, P < .0001).38
Another double-blind trial demonstrated that in patients previously treated with alendronate, switching to denosumab resulted in significantly greater increases in bone mineral density at all skeletal sites compared with continuing with alendronate (P < .0001).39
Denosumab has also been shown to be superior to alendronate in improving cortical bone mineral density, as measured by quantitative computed tomography.40
No trial has directly compared the efficacy of denosumab with other osteoporosis drugs in reducing fracture risk, but a systematic literature review of multiple databases,41 comparing the antifracture efficacy of nine osteoporosis drugs, concluded that teriparatide, zoledronic acid, and denosumab had the highest probabilities of being most efficacious for nonvertebral and vertebral fractures, with the greatest effect sizes. Indirect comparisons of the relative risk of fracture with denosumab (based on the results of FREEDOM), alendronate, risedronate, raloxifene, and strontium (based on a meta-analysis of randomized controlled trials) are presented in Table 1.42
A 2-year randomized, open-label, crossover study43 randomized patients to receive either denosumab followed by alendronate or alendronate followed by denosumab over successive 12-month periods. The results suggested that postmenopausal women with osteoporosis were more adherent, compliant, and persistent with denosumab therapy (a subcutaneous injection every 6 months) than with alendronate therapy in the form of oral tablets, self-administered weekly (7.5% nonadherence vs 36.5% at the end of 2 years). After receiving both treatments, women reported greater satisfaction with denosumab, with 92.4% preferring it over oral alendronate. Bone mineral density remained stable when patients were switched from denosumab to alendronate, but improved further when they were switched from alendronate to denosumab.
HOW SAFE IS DENOSUMAB?
The most frequent adverse events with denosumab reported in the long-term extension of one phase 2 study were upper respiratory tract infections (13.5%), arthralgia (11.5%), and back pain (9.0%).30
Increased risk of infection, cancer, and dermatologic reactions has been a concern, as RANKL and RANK are expressed by a wide variety of cells, including T lymphocytes, B cells, and dendritic cells.44 However, there were no significant differences in the overall incidences of adverse events between patients who received denosumab and those who received placebo or alendronate in any of the phase 2, phase 3, or extension studies.
In the FREEDOM trial,24 there was no significant difference between the two groups in the overall incidence of infection (52.9% with denosumab vs 54.4% with placebo, P = .17), or serious infection (4.1% with denosumab vs 3.4% with placebo, P = .14), although the incidence of “serious” cellulitis requiring hospitalization was higher in the denosumab group (0.3% vs < 0.1%, P = .002). There were more serious infections involving the gastrointestinal system, urinary tract, and ear and cases of endocarditis in the denosumab group, but the number of events was small, and there was no relationship with the timing of administration or duration of exposure to denosumab.45 Eczema was more common in the denosumab than in the placebo group (3.0% vs 1.7%, P < .001), but the extension data from the first 3 years did not provide any evidence for an increased risk of cellulitis or eczema with denosumab.26
Although randomized controlled trials reported more cases of neoplasms in the denosumab than in the placebo groups, meta-analyses have failed to detect a statistically significant difference (risk ratio 1.11, 95% CI 0.91–1.36).46 The overall incidence of adverse and serious adverse events reported in the 8-year extension of FREEDOM were consistent with data reported in the previous extension studies.25
In the FREEDOM extension trial, four events in the long-term group (n = 2,343), and two in the crossover group (n = 2,207) were adjudicated as being consistent with osteonecrosis of the jaw.26 One mid-shaft fracture in the crossover group was adjudicated as an atypical femoral fracture. There were, however, no reports of osteonecrosis of the jaw or atypical femoral fracture in the long-term phase 2 trial after 8 years of follow-up.30 By September 2013, postmarketing safety surveillance data for denosumab (estimated exposure of 1.2 million patient-years) had recorded four cases of atypical femoral fracture. All four patients had previously been on bisphosphonates. There were also 32 reports of osteonecrosis of the jaw.47
Denosumab’s manufacturer aims to communicate the risks of treatment to health care professionals and patients. Information is available online at www.proliahcp.com/risk-evaluation-mitigation-strategy/.
WHAT ARE THE PRECAUTIONS?
Several precautions need to be taken when considering treatment with denosumab.
Antiresorptives can aggravate hypocalcemia by inhibiting bone turnover. Serum calcium should therefore be checked and preexisting hypocalcemia should be corrected before starting denosumab.48
Denosumab is contraindicated in women who are pregnant or are planning to become pregnant, as fetal loss and teratogenicity have been reported in animal experiments. (Denosumab is unlikely to be used in premenopausal women, as it is not approved for use in this group.)
There are no data on excretion of denosumab in human milk, so it should not be given to nursing mothers.
Renal impairment is not a contraindication, and no dose adjustment is necessary (even for patients on renal replacement therapy), as denosumab, being an antibody, is eliminated through the reticuloendothelial system.49,50 However, in practice, any antiresorptive agent should be used with caution in patients with severe renal impairment because of the possible presence of adynamic bone disease. Further reduction of bone turnover would be detrimental in such patients. Also, severe hypocalcemia has been reported in patients with a creatinine clearance rate less than 30 mL/min and in those receiving dialysis.51,52 Postmarketing surveillance data have reported eight cases of severe symptomatic hypocalcemia, of which seven were in patients with chronic kidney disease.47
The manufacturer suggests that patients receive a dental examination with appropriate preventive dentistry before starting denosumab to reduce the incidence of osteonecrosis of the jaw, despite the lack of evidence in support of this strategy. The American Dental Association recommends regular dental visits and maintenance of good oral hygiene for patients already established on antiresorptive therapy.53,54
SHOULD PATIENTS ON DENOSUMAB BE OFFERED A DRUG HOLIDAY?
A drug holiday (temporary discontinuation of the drug after a certain duration of treatment) has been proposed for patients receiving bisphosphonates because of the risk of atypical femoral fracture and osteonecrosis of the jaw (although small) consequent to long-term continuous suppression of bone turnover.55 The antifracture efficacy of bisphosphonates is likely to persist for an unknown length of time after discontinuation because of their long skeletal half-life, while the risks gradually diminish.
By contrast, denosumab targets RANKL in the extracellular fluid and does not become embedded within the bone tissue.56 Pharmacokinetic studies have shown that denosumab has a rapid offset of action, with a half-life of only 26 days and biological activity lasting only 6 months.57 The results of a phase 2 extension study suggest that bone mineral density starts to decline and bone turnover markers start to rise within 12 months of discontinuing denosumab.58
Although fracture risk did not increase in those who were randomized to stopping the treatment and bone mineral density increased further when treatment was restarted, a drug holiday cannot presently be recommended for patients receiving denosumab because of the lack of supportive data.
HOW COST-EFFECTIVE IS DENOSUMAB?
The wholesale acquisition cost is $825 per 60-mg prefilled syringe of denosumab, although this may vary depending on where the drug is obtained. This does not include physician-related service costs associated with administration of denosumab.
Cost-effectiveness analyses conducted in the United States, the United Kingdom, and Sweden have all concluded that denosumab would offer a cost-effective alternative to other osteoporosis medications for primary prevention and secondary prevention of fractures.59–61
The Swedish study also incorporated adherence in the cost-effectiveness model and showed that denosumab was a cost-effective alternative to oral bisphosphonates, particularly for patients who were not expected to adhere well to oral treatments.61
WHICH OSTEOPOROSIS PATIENTS ARE CANDIDATES FOR DENOSUMAB?
The FDA has approved denosumab for the treatment of postmenopausal women and men at high risk of fracture (defined as having a history of osteoporotic fracture or multiple risk factors for fracture), or in those who cannot tolerate other osteoporosis medications or for whom other medications have failed.
Denosumab is also approved for men at high risk of fracture receiving androgen deprivation therapy for nonmetastatic prostate cancer, and for women at high risk of fracture receiving adjuvant aromatase inhibitor therapy for breast cancer.
WHAT DO THE GUIDELINES RECOMMEND?
The National Osteoporosis Foundation guidelines recommend pharmacologic treatment for patients with hip or vertebral fractures (clinical or asymptomatic); T scores lower than –2.5 at the femoral neck, total hip, or lumbar spine; and those with a 10-year probability of hip fracture of more than 3% or of a major osteoporotic fracture more than 20% based on the US-adapted FRAX calculator.62 The American College of Endocrinology guidelines have proposed similar thresholds for pharmacologic treatment, and they recommend alendronate, risedronate, zoledronate, and denosumab as first-line agents.63
The 2010 Osteoporosis Canada guidelines recommend denosumab, alendronate, risedronate, and zoledronate as first-line therapies for preventing hip, nonvertebral, and vertebral fractures in postmenopausal women (grade A recommendation).64 The National Institute of Health and Clinical Excellence in England and Wales, on the other hand, recommends denosumab only for patients who are unable to take a bisphosphonate.65
PRACTICAL PRESCRIBING TIPS
The patient described at the beginning of this article has already sustained a vertebral compression fracture, and her DXA scan shows T scores in the osteoporotic range. She is therefore at increased risk of another fragility fracture (with a fivefold higher risk of another vertebral fracture). Pharmacologic therapy should be considered. In addition, she should be encouraged to adhere to lifestyle measures such as a healthy diet and regular weight-bearing exercise, her risk of falling should be assessed, and adequate calcium and vitamin D supplementation should be given.
Secondary causes of osteoporosis are present in about 30% of women and 55% of men who have vertebral fractures.66 A complete blood count, erythrocyte sedimentation rate, bone biochemistry, 25-hydroxyvitamin D, thyroid-stimulating hormone, and renal and liver function tests should be requested in all patients. Further tests should be considered depending on the clinical evaluation and results of initial investigations.
Because this patient cannot tolerate oral bisphosphonates, she could be offered the option of annual intravenous zoledronic acid infusions or 6-monthly subcutaneous denosumab injections. In clinical trials, gastrointestinal adverse effects were noted with intravenous bisphosphonates as well, but the adverse effects reported were no different than those with placebo. The potential advantages with denosumab include better bone mineral density gains, adherence and patient satisfaction compared with oral bisphosphonates, convenient twice-yearly administration, safety in patients with renal impairment, and absence of gastrointestinal effects.
Raloxifene, a selective estrogen receptor modulator, has estrogen-like action on the bone and antiestrogen actions on the breast and uterus. Unlike standard hormone replacement therapy, raloxifene can therefore increase bone mineral density without increasing the risk of breast and endometrial cancers. However, it has only been shown to reduce the risk of vertebral fracture, not hip fracture. Hence, it would be a more appropriate choice for younger postmenopausal women. Moreover, it may cause troublesome menopausal symptoms.
Teriparatide, the recombinant parathyroid hormone, is an anabolic agent. It is very expensive, and because of this, guidelines in several countries restrict its use to women with severe osteoporosis and multiple fractures who fail to respond to standard treatments. It cannot be used for longer than 2 years because of its association with osteosarcoma in rats.
If our patient prefers denosumab, therapy should be initiated after appropriate counseling (see precautions above). The dose is 60 mg, given subcutaneously, once every 6 months.
Monitoring
There is no consensus regarding the optimal frequency for monitoring patients on treatment, owing to the lack of prospective trial data. The National Osteoporosis Foundation recommends repeating the bone mineral density measurements about 2 years after starting therapy, and about every 2 years thereafter.62 Some studies suggest that changes in bone mineral density correlate with reduction in fracture risk.67,68 A change in bone mineral density is considered significant when it is greater than the range of error of the densitometer (also known as the least significant change).69 If the bone mineral density is stable or improving, therapy could be continued, but if it is declining and the decline is greater than the least significant change, a change in therapy should be considered if no secondary causes for bone loss are evident (but see What are the areas of uncertainty? below).
The National Osteoporosis Foundation also recommends measuring a bone turnover marker at baseline and then 3 to 6 months later, as its suppression predicts greater bone mineral density responses and fracture risk reduction.70 If there is a decrease of more than 30% in serum carboxy-terminal collagen crosslinks (CTX) or more than 50% in urinary N-telopeptide (NTX),71 the patient can be reassured that the next bone mineral density measurement will be stable or improved. In patients on oral bisphosphonates, measurement of bone turnover markers also provides evidence of compliance.
Clinical trials suggest that a numerical increase in bone mineral density can be expected in most patients on treatment, though this depends on the measurement site and the length of time between examinations. In one phase 3 trial of denosumab in postmenopausal women, only 5% of the participants had unchanged or diminished bone mineral density at the lumbar spine, and 8% at the hip, after 36 months of treatment.72 However, the CTX levels fell to below the lower limit of the reference interval as early as 1 month after commencing treatment in all denosumab-treated patients.68
Hence, bone turnover markers may be a more sensitive indicator of treatment effect than bone mineral density, but this would ultimately need to be evaluated against fracture rates in a real-world setting.
WHAT ARE THE AREAS OF UNCERTAINTY?
There are currently no guidelines for long-term management of patients on denosumab, and also no data to suggest whether patients should be switched to a weaker antiresorptive drug after a certain number of years in order to reduce the possible risk of atypical femoral fracture or osteonecrosis of the jaw.
No head-to-head trials have directly compared the antifracture efficacy of denosumab with that of other standard osteoporosis therapies. The antifracture efficacy and safety of combination therapies involving denosumab are also uncertain. For adherent patients who have a suboptimal response, there is no evidence to guide the further course of action. The International Osteoporosis Foundation guidelines suggest replacing a stronger antiresorptive with an anabolic agent, but acknowledge that this is only based on expert opinion.71
The very-long-term effects (beyond 8 years) of continuous denosumab administration on increasing the risk of atypical femoral fracture, osteonecrosis of the jaw, malignancy, or infection or the duration after which risks would start to outweigh benefits is not known. However, postmarketing safety data continue to be collected through the voluntary Post-marketing Active Safety Surveillance Program (for prespecified adverse events) in addition to the FDA’s MedWatch program.
CASE PROGRESSION
The patient described in the vignette is presented with two options—zoledronate and denosumab. She chooses denosumab. Her renal function and serum calcium are checked and are found to be satisfactory. She undergoes a dental examination, which is also satisfactory. She is counseled about the possible increased risk of infection, and then she is started on 60 mg of denosumab subcutaneously, once every 6 months.
When reviewed after 2 years, she reports no further fractures. Her bone mineral density remains stable compared with the values obtained before starting treatment. She reports no adverse effects and is happy to continue with denosumab.
- Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006; 17:1726–1733.
- Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int 1997; 7:407–413.
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 2007; 22:465–475.
- Melton LJ 3rd, Chrischilles EA, Cooper C, Lane AW, Riggs BL. Perspective. How many women have osteoporosis? J Bone Miner Res 1992; 7:1005–1010.
- Abrahamsen B, van Staa T, Ariely R, Olson M, Cooper C. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int 2009; 20:1633–1650.
- Jalava T, Sarna S, Pylkkänen L, et al. Association between vertebral fracture and increased mortality in osteoporotic patients. J Bone Miner Res 2003; 18:1254–1260.
- Ismail AA, O’Neill TW, Cooper C, et al. Mortality associated with vertebral deformity in men and women: results from the European Prospective Osteoporosis Study (EPOS). Osteoporos Int 1998; 8:291–297.
- Ensrud KE, Thompson DE, Cauley JA, et al. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture Intervention Trial Research Group. J Am Geriatr Soc 2000; 48:241–249.
- Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med 1999; 159:1215–1220.
- Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA 2001; 285:320–323.
- Ross PD, Davis JW, Epstein RS, Wasnich RD. Pre-existing fractures and bone mass predict vertebral fracture incidence in women. Ann Intern Med 1991; 114:919–923.
- Piscitelli P, Iolascon G, Argentiero A, et al. Incidence and costs of hip fractures vs strokes and acute myocardial infarction in Italy: comparative analysis based on national hospitalization records. Clin Interv Aging 2012; 7:575–583.
- Johnell O, Kanis JA, Jonsson B, Oden A, Johansson H, De Laet C. The burden of hospitalised fractures in Sweden. Osteoporos Int 2005; 16:222–228.
- Confavreux CB, Canoui-Poitrine F, Schott AM, Ambrosi V, Tainturier V, Chapurlat RD. Persistence at 1 year of oral antiosteoporotic drugs: a prospective study in a comprehensive health insurance database. Eur J Endocrinol 2012; 166:735–741.
- Biswas PN, Wilton LV, Shakir SA. Pharmacovigilance study of alendronate in England. Osteoporos Int 2003; 14:507–514.
- Landfeldt E, Ström O, Robbins S, Borgström F. Adherence to treatment of primary osteoporosis and its association to fractures—the Swedish Adherence Register Analysis (SARA). Osteoporos Int 2012; 23:433–443.
- Sampalis JS, Adachi JD, Rampakakis E, Vaillancourt J, Karellis A, Kindundu C. Long-term impact of adherence to oral bisphosphonates on osteoporotic fracture incidence. J Bone Miner Res 2012; 27:202–210.
- Schwarz EM, Ritchlin CT. Clinical development of anti-RANKL therapy. Arthritis Res Ther 2007; 9(suppl 1):S7.
- Hanley DA, Adachi JD, Bell A, Brown V. Denosumab: mechanism of action and clinical outcomes. Int J Clin Pract 2012; 66:1139–1146.
- Bekker PJ, Holloway D, Nakanishi A, Arrighi M, Leese PT, Dunstan CR. The effect of a single dose of osteoprotegerin in postmenopausal women. J Bone Miner Res 2001; 16:348–360.
- Rizzoli R, Body JJ, Brandi ML, et al; International Osteoporosis Foundation Committee of Scientific Advisors Working Group on Cancer-Induced Bone Disease. Cancer-associated bone disease. Osteoporos Int 2013; 24:2929–2953.
- Schwarz P, Rasmussen AQ, Kvist TM, Andersen UB, Jørgensen NR. Paget’s disease of the bone after treatment with denosumab: a case report. Bone 2012; 50:1023–1025.
- Hu MI, Glezerman IG, Leboulleux S, et al. Denosumab for treatment of hypercalcemia of malignancy. J Clin Endocrinol Metab 2014; Jun 10 [Epub ahead of print].
- Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009; 361:756–765.
- Papapoulos S, Lippuner K, Roux C, et al. Eight years of denosumab treatment in postmenopausal women with osteoporosis: results from the first five years of the FREEDOM extension [abstract]. Presented at the 2013 annual meeting of the American Society for Bone and Mineral Research, Baltimore, MD, October 4–7, 2013.
- Bone HG, Chapurlat R, Brandi ML, et al. The effect of three or six years of denosumab exposure in women with postmenopausal osteoporosis: results from the FREEDOM extension. J Clin Endocrinol Metab 2013; 98:4483–4492.
- McClung MR, Boonen S, Törring O, et al. Effect of denosumab treatment on the risk of fractures in subgroups of women with postmenopausal osteoporosis. J Bone Miner Res 2012; 27:211–218.
- Boonen S, Adachi JD, Man Z, et al. Treatment with denosumab reduces the incidence of new vertebral and hip fractures in postmenopausal women at high risk. J Clin Endocrinol Metab 2011; 96:1727–1736.
- McCloskey EV, Johansson H, Oden A, et al. Denosumab reduces the risk of osteoporotic fractures in postmenopausal women, particularly in those with moderate to high fracture risk as assessed with FRAX. J Bone Miner Res 2012; 27:1480–1486.
- McClung MR, Lewiecki EM, Geller ML, et al. Effect of denosumab on bone mineral density and biochemical markers of bone turnover: 8-year results of a phase 2 clinical trial. Osteoporos Int 2013; 24:227–235.
- McClung MR, Lewiecki EM, Cohen SB, et al; AMG 162 Bone Loss Study Group. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 2006; 354:821–831.
- Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women. J Clin Endocrinol Metab 2008; 93:2149–2157.
- Genant HK, Engelke K, Hanley DA, et al. Denosumab improves density and strength parameters as measured by QCT of the radius in postmenopausal women with low bone mineral density. Bone 2010; 47:131–139.
- Orwoll E, Teglbjærg CS, Langdahl BL, et al. A randomized, placebo-controlled study of the effects of denosumab for the treatment of men with low bone mineral density. J Clin Endocrinol Metab 2012; 97:3161–3169.
- Tsai JN, Uihlein AV, Lee H, et al. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet 2013; 382:50–56.
- Smith MR, Egerdie B, Hernández Toriz N, et al; Denosumab HALT Prostate Cancer Study Group. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med 2009; 361:745–755.
- Ellis GK, Bone HG, Chlebowski R, et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol 2008; 26:4875–4882.
- Brown JP, Prince RL, Deal C, et al. Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res 2009; 24:153–161.
- Kendler DL, Roux C, Benhamou CL, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res 2010; 25:72–81.
- Seeman E, Delmas PD, Hanley DA, et al. Microarchitectural deterioration of cortical and trabecular bone: differing effects of denosumab and alendronate. J Bone Miner Res 2010; 25:1886–1894.
- Hopkins RB, Goeree R, Pullenayegum E, et al. The relative efficacy of nine osteoporosis medications for reducing the rate of fractures in post-menopausal women. BMC Musculoskelet Disord 2011; 12:209.
- National Institute for Health and Care Excellence (NICE). NICE technology appraisal guidance: TA161. Alendronate, etidronate, risedronate, raloxifene, strontium ranelate and teriparatide for the secondary prevention of osteoporotic fragility fractures in postmenopausal women (amended). http://publications.nice.org.uk/alendronate-etidronate-risedronate-raloxifene-strontium-ranelate-and-teriparatide-for-ta161. Accessed January 9, 2015.
- Freemantle N, Satram-Hoang S, Tang ET, et al; DAPS Investigators. Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int 2012; 23:317–326.
- Lewiecki EM. Safety and tolerability of denosumab for the treatment of postmenopausal osteoporosis. Drug Healthc Patient Saf 2011; 3:79–91.
- Watts NB, Roux C, Modlin JF, et al. Infections in postmenopausal women with osteoporosis treated with denosumab or placebo: coincidence or causal association? Osteoporos Int 2012; 23:327–337.
- von Keyserlingk C, Hopkins R, Anastasilakis A, et al. Clinical efficacy and safety of denosumab in postmenopausal women with low bone mineral density and osteoporosis: a meta-analysis. Semin Arthritis Rheum 2011; 41:178–186.
- Geller M, Wagman RB, Ho PR, et al. Early findings from Prolia postmarketing safety surveillance for atypical femoral fracture, osteonecrosis of the jaw, severe symptomatic hypocalcemia, and anaphylaxis (abstract). Osteoporos Int 2014; 25(suppl 2). OC40; www.wco-iof-esceo.org/sites/ecceo14/docs/wco14-abstractbook.pdf. Accessed January 9, 2015.
- McCormick BB, Davis J, Burns KD. Severe hypocalcemia following denosumab injection in a hemodialysis patient. Am J Kidney Dis 2012; 60:626–628.
- Jamal SA, Ljunggren O, Stehman-Breen C, et al. Effects of denosumab on fracture and bone mineral density by level of kidney function. J Bone Miner Res 2011; 26:1829–1835.
- Block GA, Bone HG, Fang L, Lee E, Padhi D. A single-dose study of denosumab in patients with various degrees of renal impairment. J Bone Miner Res 2012; 27:1471–1479.
- Ungprasert P, Cheungpasitporn W, Srivali N, Kittanamongkolchai W, Bischof EF. Life-threatening hypocalcemia associated with denosumab in a patient with moderate renal insufficiency. Am J Emerg Med 2013; 31:756.e1–e2.
- McCormick BB, Davis J, Burns KD. Severe hypocalcemia following denosumab injection in a hemodialysis patient. Am J Kidney Dis 2012; 60:626–628.
- Rachner TD, Platzbecker U, Felsenberg D, Hofbauer LC. Osteonecrosis of the jaw after osteoporosis therapy with denosumab following long-term bisphosphonate therapy. Mayo Clin Proc 2013; 88:418–419.
- Epstein MS, Ephros HD, Epstein JB. Review of current literature and implications of RANKL inhibitors for oral health care providers. Oral Surg Oral Med Oral Pathol Oral Radiol 2013; 116:e437–e442.
- McClung M, Harris ST, Miller PD, et al. Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med 2013; 126:13–20.
- Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone 2011; 48:677–692.
- Bekker PJ, Holloway DL, Rasmussen AS, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res 2004; 19:1059–1066.
- Miller PD, Bolognese MA, Lewiecki EM, et al; Amg Bone Loss Study Group. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone 2008; 43:222–229.
- Parthan A, Kruse M, Yurgin N, Huang J, Viswanathan HN, Taylor D. Cost effectiveness of denosumab versus oral bisphosphonates for postmenopausal osteoporosis in the US. Appl Health Econ Health Policy 2013; 11:485–497.
- Scotland G, Waugh N, Royle P, McNamee P, Henderson R, Hollick R. Denosumab for the prevention of osteoporotic fractures in post-menopausal women: a NICE single technology appraisal. Pharmacoeconomics 2011; 29:951–961.
- Jönsson B, Ström O, Eisman JA, et al. Cost-effectiveness of denosumab for the treatment of postmenopausal osteoporosis. Osteoporos Int 2011; 22:967–982.
- Clinician’s guide to prevention and treatment of osteoporosis. Washington DC: National Osteoporosis Foundation, 2013.
- Watts NB, Bilezikian JP, Camacho PM, et al; AACE Osteoporosis Task Force. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract 2010;16(suppl 3):1–37.
- Papaioannou A, Morin S, Cheung AM, et al; Scientific Advisory Council of Osteoporosis Canada. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ 2010; 182:1864–1673.
- National Institute for Health and Care Excellence (NICE). NICE technology appraisal: TA204. Denosumab for the prevention of osteoporotic fractures in postmenopausal women. http://guidance.nice.org.uk/TA204. Accessed January 9, 2015.
- Premaor MO, Compston JE. Testing for secondary causes of osteoporosis. BMJ 2010; 341:c6959.
- Hochberg MC, Ross PD, Black D, et al. Larger increases in bone mineral density during alendronate therapy are associated with a lower risk of new vertebral fractures in women with postmenopausal osteoporosis. Fracture Intervention Trial Research Group. Arthritis Rheum 1999; 42:1246–1254.
- Eastell R, Vrijens B, Cahall DL, Ringe JD, Garnero P, Watts NB. Bone turnover markers and bone mineral density response with risedronate therapy: relationship with fracture risk and patient adherence. J Bone Miner Res 2011; 26:1662–1669.
- Diez-Perez A, Adachi JD, Agnusdei D, et al; IOF CSA Inadequate Responders Working Group. Treatment failure in osteoporosis. Osteoporos Int 2012; 23:2769–2774.
- Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res 2003; 18:1051–1056.
- Rosen HN, Moses AC, Garber J, Ross DS, Lee SL, Greenspan SL. Utility of biochemical markers of bone turnover in the follow-up of patients treated with bisphosphonates. Calcif Tissue Int 1998; 63:363–368.
- Bolognese MA, Teglbjærg CS, Zanchetta JR, et al. Denosumab significantly increases DXA BMD at both trabecular and cortical sites: results from the FREEDOM study. J Clin Densitom 2013; 16:147–153.
- Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006; 17:1726–1733.
- Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int 1997; 7:407–413.
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 2007; 22:465–475.
- Melton LJ 3rd, Chrischilles EA, Cooper C, Lane AW, Riggs BL. Perspective. How many women have osteoporosis? J Bone Miner Res 1992; 7:1005–1010.
- Abrahamsen B, van Staa T, Ariely R, Olson M, Cooper C. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int 2009; 20:1633–1650.
- Jalava T, Sarna S, Pylkkänen L, et al. Association between vertebral fracture and increased mortality in osteoporotic patients. J Bone Miner Res 2003; 18:1254–1260.
- Ismail AA, O’Neill TW, Cooper C, et al. Mortality associated with vertebral deformity in men and women: results from the European Prospective Osteoporosis Study (EPOS). Osteoporos Int 1998; 8:291–297.
- Ensrud KE, Thompson DE, Cauley JA, et al. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture Intervention Trial Research Group. J Am Geriatr Soc 2000; 48:241–249.
- Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med 1999; 159:1215–1220.
- Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA 2001; 285:320–323.
- Ross PD, Davis JW, Epstein RS, Wasnich RD. Pre-existing fractures and bone mass predict vertebral fracture incidence in women. Ann Intern Med 1991; 114:919–923.
- Piscitelli P, Iolascon G, Argentiero A, et al. Incidence and costs of hip fractures vs strokes and acute myocardial infarction in Italy: comparative analysis based on national hospitalization records. Clin Interv Aging 2012; 7:575–583.
- Johnell O, Kanis JA, Jonsson B, Oden A, Johansson H, De Laet C. The burden of hospitalised fractures in Sweden. Osteoporos Int 2005; 16:222–228.
- Confavreux CB, Canoui-Poitrine F, Schott AM, Ambrosi V, Tainturier V, Chapurlat RD. Persistence at 1 year of oral antiosteoporotic drugs: a prospective study in a comprehensive health insurance database. Eur J Endocrinol 2012; 166:735–741.
- Biswas PN, Wilton LV, Shakir SA. Pharmacovigilance study of alendronate in England. Osteoporos Int 2003; 14:507–514.
- Landfeldt E, Ström O, Robbins S, Borgström F. Adherence to treatment of primary osteoporosis and its association to fractures—the Swedish Adherence Register Analysis (SARA). Osteoporos Int 2012; 23:433–443.
- Sampalis JS, Adachi JD, Rampakakis E, Vaillancourt J, Karellis A, Kindundu C. Long-term impact of adherence to oral bisphosphonates on osteoporotic fracture incidence. J Bone Miner Res 2012; 27:202–210.
- Schwarz EM, Ritchlin CT. Clinical development of anti-RANKL therapy. Arthritis Res Ther 2007; 9(suppl 1):S7.
- Hanley DA, Adachi JD, Bell A, Brown V. Denosumab: mechanism of action and clinical outcomes. Int J Clin Pract 2012; 66:1139–1146.
- Bekker PJ, Holloway D, Nakanishi A, Arrighi M, Leese PT, Dunstan CR. The effect of a single dose of osteoprotegerin in postmenopausal women. J Bone Miner Res 2001; 16:348–360.
- Rizzoli R, Body JJ, Brandi ML, et al; International Osteoporosis Foundation Committee of Scientific Advisors Working Group on Cancer-Induced Bone Disease. Cancer-associated bone disease. Osteoporos Int 2013; 24:2929–2953.
- Schwarz P, Rasmussen AQ, Kvist TM, Andersen UB, Jørgensen NR. Paget’s disease of the bone after treatment with denosumab: a case report. Bone 2012; 50:1023–1025.
- Hu MI, Glezerman IG, Leboulleux S, et al. Denosumab for treatment of hypercalcemia of malignancy. J Clin Endocrinol Metab 2014; Jun 10 [Epub ahead of print].
- Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009; 361:756–765.
- Papapoulos S, Lippuner K, Roux C, et al. Eight years of denosumab treatment in postmenopausal women with osteoporosis: results from the first five years of the FREEDOM extension [abstract]. Presented at the 2013 annual meeting of the American Society for Bone and Mineral Research, Baltimore, MD, October 4–7, 2013.
- Bone HG, Chapurlat R, Brandi ML, et al. The effect of three or six years of denosumab exposure in women with postmenopausal osteoporosis: results from the FREEDOM extension. J Clin Endocrinol Metab 2013; 98:4483–4492.
- McClung MR, Boonen S, Törring O, et al. Effect of denosumab treatment on the risk of fractures in subgroups of women with postmenopausal osteoporosis. J Bone Miner Res 2012; 27:211–218.
- Boonen S, Adachi JD, Man Z, et al. Treatment with denosumab reduces the incidence of new vertebral and hip fractures in postmenopausal women at high risk. J Clin Endocrinol Metab 2011; 96:1727–1736.
- McCloskey EV, Johansson H, Oden A, et al. Denosumab reduces the risk of osteoporotic fractures in postmenopausal women, particularly in those with moderate to high fracture risk as assessed with FRAX. J Bone Miner Res 2012; 27:1480–1486.
- McClung MR, Lewiecki EM, Geller ML, et al. Effect of denosumab on bone mineral density and biochemical markers of bone turnover: 8-year results of a phase 2 clinical trial. Osteoporos Int 2013; 24:227–235.
- McClung MR, Lewiecki EM, Cohen SB, et al; AMG 162 Bone Loss Study Group. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 2006; 354:821–831.
- Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women. J Clin Endocrinol Metab 2008; 93:2149–2157.
- Genant HK, Engelke K, Hanley DA, et al. Denosumab improves density and strength parameters as measured by QCT of the radius in postmenopausal women with low bone mineral density. Bone 2010; 47:131–139.
- Orwoll E, Teglbjærg CS, Langdahl BL, et al. A randomized, placebo-controlled study of the effects of denosumab for the treatment of men with low bone mineral density. J Clin Endocrinol Metab 2012; 97:3161–3169.
- Tsai JN, Uihlein AV, Lee H, et al. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet 2013; 382:50–56.
- Smith MR, Egerdie B, Hernández Toriz N, et al; Denosumab HALT Prostate Cancer Study Group. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med 2009; 361:745–755.
- Ellis GK, Bone HG, Chlebowski R, et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol 2008; 26:4875–4882.
- Brown JP, Prince RL, Deal C, et al. Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res 2009; 24:153–161.
- Kendler DL, Roux C, Benhamou CL, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res 2010; 25:72–81.
- Seeman E, Delmas PD, Hanley DA, et al. Microarchitectural deterioration of cortical and trabecular bone: differing effects of denosumab and alendronate. J Bone Miner Res 2010; 25:1886–1894.
- Hopkins RB, Goeree R, Pullenayegum E, et al. The relative efficacy of nine osteoporosis medications for reducing the rate of fractures in post-menopausal women. BMC Musculoskelet Disord 2011; 12:209.
- National Institute for Health and Care Excellence (NICE). NICE technology appraisal guidance: TA161. Alendronate, etidronate, risedronate, raloxifene, strontium ranelate and teriparatide for the secondary prevention of osteoporotic fragility fractures in postmenopausal women (amended). http://publications.nice.org.uk/alendronate-etidronate-risedronate-raloxifene-strontium-ranelate-and-teriparatide-for-ta161. Accessed January 9, 2015.
- Freemantle N, Satram-Hoang S, Tang ET, et al; DAPS Investigators. Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int 2012; 23:317–326.
- Lewiecki EM. Safety and tolerability of denosumab for the treatment of postmenopausal osteoporosis. Drug Healthc Patient Saf 2011; 3:79–91.
- Watts NB, Roux C, Modlin JF, et al. Infections in postmenopausal women with osteoporosis treated with denosumab or placebo: coincidence or causal association? Osteoporos Int 2012; 23:327–337.
- von Keyserlingk C, Hopkins R, Anastasilakis A, et al. Clinical efficacy and safety of denosumab in postmenopausal women with low bone mineral density and osteoporosis: a meta-analysis. Semin Arthritis Rheum 2011; 41:178–186.
- Geller M, Wagman RB, Ho PR, et al. Early findings from Prolia postmarketing safety surveillance for atypical femoral fracture, osteonecrosis of the jaw, severe symptomatic hypocalcemia, and anaphylaxis (abstract). Osteoporos Int 2014; 25(suppl 2). OC40; www.wco-iof-esceo.org/sites/ecceo14/docs/wco14-abstractbook.pdf. Accessed January 9, 2015.
- McCormick BB, Davis J, Burns KD. Severe hypocalcemia following denosumab injection in a hemodialysis patient. Am J Kidney Dis 2012; 60:626–628.
- Jamal SA, Ljunggren O, Stehman-Breen C, et al. Effects of denosumab on fracture and bone mineral density by level of kidney function. J Bone Miner Res 2011; 26:1829–1835.
- Block GA, Bone HG, Fang L, Lee E, Padhi D. A single-dose study of denosumab in patients with various degrees of renal impairment. J Bone Miner Res 2012; 27:1471–1479.
- Ungprasert P, Cheungpasitporn W, Srivali N, Kittanamongkolchai W, Bischof EF. Life-threatening hypocalcemia associated with denosumab in a patient with moderate renal insufficiency. Am J Emerg Med 2013; 31:756.e1–e2.
- McCormick BB, Davis J, Burns KD. Severe hypocalcemia following denosumab injection in a hemodialysis patient. Am J Kidney Dis 2012; 60:626–628.
- Rachner TD, Platzbecker U, Felsenberg D, Hofbauer LC. Osteonecrosis of the jaw after osteoporosis therapy with denosumab following long-term bisphosphonate therapy. Mayo Clin Proc 2013; 88:418–419.
- Epstein MS, Ephros HD, Epstein JB. Review of current literature and implications of RANKL inhibitors for oral health care providers. Oral Surg Oral Med Oral Pathol Oral Radiol 2013; 116:e437–e442.
- McClung M, Harris ST, Miller PD, et al. Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med 2013; 126:13–20.
- Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone 2011; 48:677–692.
- Bekker PJ, Holloway DL, Rasmussen AS, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res 2004; 19:1059–1066.
- Miller PD, Bolognese MA, Lewiecki EM, et al; Amg Bone Loss Study Group. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone 2008; 43:222–229.
- Parthan A, Kruse M, Yurgin N, Huang J, Viswanathan HN, Taylor D. Cost effectiveness of denosumab versus oral bisphosphonates for postmenopausal osteoporosis in the US. Appl Health Econ Health Policy 2013; 11:485–497.
- Scotland G, Waugh N, Royle P, McNamee P, Henderson R, Hollick R. Denosumab for the prevention of osteoporotic fractures in post-menopausal women: a NICE single technology appraisal. Pharmacoeconomics 2011; 29:951–961.
- Jönsson B, Ström O, Eisman JA, et al. Cost-effectiveness of denosumab for the treatment of postmenopausal osteoporosis. Osteoporos Int 2011; 22:967–982.
- Clinician’s guide to prevention and treatment of osteoporosis. Washington DC: National Osteoporosis Foundation, 2013.
- Watts NB, Bilezikian JP, Camacho PM, et al; AACE Osteoporosis Task Force. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract 2010;16(suppl 3):1–37.
- Papaioannou A, Morin S, Cheung AM, et al; Scientific Advisory Council of Osteoporosis Canada. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ 2010; 182:1864–1673.
- National Institute for Health and Care Excellence (NICE). NICE technology appraisal: TA204. Denosumab for the prevention of osteoporotic fractures in postmenopausal women. http://guidance.nice.org.uk/TA204. Accessed January 9, 2015.
- Premaor MO, Compston JE. Testing for secondary causes of osteoporosis. BMJ 2010; 341:c6959.
- Hochberg MC, Ross PD, Black D, et al. Larger increases in bone mineral density during alendronate therapy are associated with a lower risk of new vertebral fractures in women with postmenopausal osteoporosis. Fracture Intervention Trial Research Group. Arthritis Rheum 1999; 42:1246–1254.
- Eastell R, Vrijens B, Cahall DL, Ringe JD, Garnero P, Watts NB. Bone turnover markers and bone mineral density response with risedronate therapy: relationship with fracture risk and patient adherence. J Bone Miner Res 2011; 26:1662–1669.
- Diez-Perez A, Adachi JD, Agnusdei D, et al; IOF CSA Inadequate Responders Working Group. Treatment failure in osteoporosis. Osteoporos Int 2012; 23:2769–2774.
- Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res 2003; 18:1051–1056.
- Rosen HN, Moses AC, Garber J, Ross DS, Lee SL, Greenspan SL. Utility of biochemical markers of bone turnover in the follow-up of patients treated with bisphosphonates. Calcif Tissue Int 1998; 63:363–368.
- Bolognese MA, Teglbjærg CS, Zanchetta JR, et al. Denosumab significantly increases DXA BMD at both trabecular and cortical sites: results from the FREEDOM study. J Clin Densitom 2013; 16:147–153.
KEY POINTS
- Denosumab is a fully human monoclonal antibody that targets the receptor activator of nuclear factor kappa b ligand, a key mediator of osteoclastic bone resorption.
- Commpared with placebo, denosumab has been shown to significantly reduce the risk of vertebral, nonvertebral, and hip fractures in postmenopausal women with osteoporosis.
- Patients taking denosumab are more adherent, compliant, and persistent with therapy than those taking alendronate. Denosumab is also superior to alendronate in improving bone mineral density at all skeletal sites.
- Denosumab is safe, with safety data now available for up to 8 years of exposure.
Genetics and hepatitis C: It’s good to be ‘CC’
What a difference a single nucleotide can make! The human genome contains more than 3 billion base pairs. Yet having a different nucleotide in only one pair can make a big difference in how we respond to a disease or its treatment.
Specifically, in hepatitis C virus infection, people born with the nucleotide cytosine (C) at location rs12979860 in both alleles of the gene that codes for interleukin 28B (the IL28B CC genotype) can count themselves luckier than those born with thymine (T) in this location in one of their alleles (the CT genotype) or both of their alleles (the TT genotype). Those with the CC genotype are more likely to clear the virus spontaneously, and even if the infection persists, it is less likely to progress to liver cancer and more likely to respond to treatment with interferon.
Here, we review the IL28B polymorphism and its implications in treating hepatitis C.
GENETIC POLYMORPHISM AND HUMAN DISEASE
Of the 3 billion base pairs of nucleotides, fewer than 1% differ between individuals, but this 1% is responsible for the diversity of human beings. Differences in genetic sequences among individuals are called genetic polymorphisms. A single-nucleotide polymorphism is a DNA sequence variation that occurs in a single nucleotide in the genome. For example, two sequenced DNA fragments from different individuals, AAGCCTA and AAGCTTA, contain a difference in a single nucleotide.
Genetic variations such as these underlie some of the differences in our susceptibility to disease, the severity of illness we develop, and our response to treatments. Therefore, identifying genetic polymorphisms may shed light on biologic pathways involved in diseases and may uncover new targets for therapy.1
Genome-wide association studies have looked at hundreds of thousands of single-nucleotide polymorphisms to try to identify most of the common genetic differences among people and relate them to common chronic diseases such as coronary artery disease,2 type 2 diabetes,3 stroke,4 breast cancer,5 rheumatoid arthritis,6 Alzheimer disease,7 and, more recently, hepatitis C virus infection.8
HEPATITIS C VIRUS: A MAJOR CAUSE OF LIVER DISEASE
Hepatitis C virus infection is a major cause of chronic liver disease and hepatocellular carcinoma and has become the most common indication for liver transplantation in the United States.9
This virus has six distinct genotypes throughout the world, with multiple subtypes in each genotype. (A genotype is a classification of a virus based on its RNA.9) In this review, we will focus on genotype 1; hence, “hepatitis C virus” will refer to hepatitis C virus genotype 1.
Our knowledge of the biology, pathogenesis, and treatment of hepatitis C has been advancing. Originally, fewer than 50% of patients responded to therapy with the combination of pegylated interferon and ribavirin,10,11 but since 2011 the response rate has increased to approximately 70% with the approval of the protease inhibitors telaprevir and boceprevir, used in combination with pegylated interferon and ribavirin.12–15
Unfortunately, interferon-based treatment is often complicated by side effects such as fatigue, influenza-like symptoms, hematologic abnormalities, and neuropsychiatric symptoms. An accurate way to predict response would help patients make informed decisions about antiviral treatment, taking into account the risk and possible benefit for individual patients.
GENETIC POLYMORPHISM AND HEPATITIS C VIRUS INFECTION
Genome-wide association studies have identified single-nucleotide polymorphisms in the IL28B gene that are associated with differences in response to hepatitis C treatment.8
Studying 565,759 polymorphisms in 1,137 patients, researchers at Duke University identified a single-nucleotide polymorphism at location rs12979860 in IL28B (Figure 1) that was strongly associated with response to combination therapy with pegylated interferon and ribavirin.8 The chance of cure with this standard treatment is twice as high in patients who are homozygous for cytosine in this location (the CC genotype) than in those who are heterozygous (CT) or homozygous for thymine in this location (the TT genotype) (Table 1).
Adding one of the new protease inhibitors, telaprevir or boceprevir, to the standard hepatitis C treatment substantially improves the cure rates in all three IL28B genotypes, but especially in people with CT or TT, in whom the response rate almost triples with the addition of one of these drugs. Those with the CC genotype (who are more likely to be cured with pegylated interferon and ribavirin alone) also achieve an increase (although minimal) in cure rates when a protease inhibitor is included in the regimen (TABLE 1).13–15 Thus, it remains unclear if adding a protease inhibitor to pegylated interferon plus ribavirin in patients with the IL28B CC genotype translates into added effectiveness worth the additional cost of the protease inhibitor in previously untreated patients.
Additionally, the effect of the IL28B genotype on telaprevir-based triple therapy has been disputed in more recent studies. In a subgroup analysis of the results of a trial that evaluated telaprevir in the treatment of hepatitis C, researchers found that sustained virologic response rates were significantly higher in the telaprevir group, and this was similar across the different IL28B polymorphisms.16
The favorable IL28B CC genotype is associated with higher rates of rapid virologic response to antiviral therapy.13–15 Of note, almost all patients who achieve a rapid virologic response do well, with a high rate of sustained virologic response even after a shorter duration of therapy (24 vs 48 weeks). Therefore, in addition to predicting response to interferon before starting treatment, the IL28B CC genotype may also identify patients who need only a shorter duration of therapy.
Interestingly, the C allele is much more frequent in white than in African American populations, an important observation that explains the racial difference in response to hepatitis C therapy.8
Two other research groups, from Asia and Australia, performed independent genome-wide association studies that identified different single-nucleotide polymorphisms (eg, rs8099917) in the same IL28B gene as predictors of response to treatment in patients with hepatitis C virus infection.17,18 These findings may be explained by linkage disequilibrium, which means that these single-nucleotide polymorphisms are found more frequently together in the same patient due to their proximity to each other. In this review, we will focus on the rs12979860 polymorphism; hence “IL28B genotype” will refer to the single-nucleotide polymorphism at rs12979860, unless otherwise specified.
The favorable CC genotype is less common in African Americans than in patients of other ethnicities.19 Moreover, although IL28B CC is associated with a better response rate to interferon-based antiviral therapy across all ethnicities, those of African American descent with the CC genotype are less likely to achieve a sustained virologic response than white or Hispanic Americans.8
BIOLOGIC ASSOCIATION: IL28B POLYMORPHISM AND HEPATITIS C
The interferon lambda family consists of three cytokines:
- Interleukin 29 (interferon lambda 1)
- Interleukin 28A (interferon lambda 2)
- Interleukin 28B (interferon lambda 3).
Production of these three molecules can be triggered by viral infection, and they induce antiviral activity through both innate and adaptive immune pathways. They signal through the IL10R-IL28R receptor complex.20–22 This receptor activates the JAK-STAT (Janus kinase-signal transducer and activator of transcription) pathway, which regulates a large number of interferon-stimulated genes, primarily through the interferon-stimulated response element (Figure 2).
A 2013 study found that interferon-stimulated gene expression levels in patients with normal livers were highest in those with the CC genotype, intermediate with CT, and lowest with TT. Interestingly, this pattern was reversed in those with hepatitis C virus infection, indicating a relationship between the IL28B genotype and gene expression before infection.23
The mechanism underlying the association between the IL28B polymorphism and response to hepatitis C treatment is not well understood. The unfavorable TT genotype seems to lead to continuous activation of a subset of interferon-stimulated genes in the presence of intracellular hepatitis C viral RNA. But this level of expression is not sufficient to eliminate the virus from the cells. Instead, it might lead to up-regulation of interferon-inhibitory molecules that suppress JAK-STAT signaling, thereby reducing sensitivity to interferon signaling. Therefore, the hepatocyte not only cannot clear the virus by itself, but also cannot induce strong interferon-stimulated gene expression when interferon is given during therapy.20–22
The recently identified ss469425590 polymorphism, which is located in close proximity to rs12979860 in the IL28B gene, is particularly interesting, as it suggests a possible molecular mechanism. The delta G frameshift variant creates a novel gene called IFNL4, which is transiently activated in response to hepatitis C virus infection.24IFNL4 stimulates STAT1 and STAT2 phosphorylation and induces the expression of interferon-stimulated genes. Increased interferon-stimulated gene expression has been shown to be associated with decreased response to pegylated interferon-ribavirin treatment. These observations suggest that the ss469425590 delta G allele is responsible for the increased activation of interferon-stimulated genes and the lower sustained virologic response rate observed in patients who receive pegylated interferon-ribavirin treatment. It is possible that the activation of interferon-stimulated genes in patients with the ss469425590 delta G/delta G genotype reduces interferon-stimulated gene responsiveness to interferon alpha, which normally activates interferon-stimulated genes and inhibits hepatitis C progression.24
IL28B POLYMORPHISM AND ACUTE HEPATITIS C VIRUS INFECTION
From 70% to 80% of acute hepatitis C virus infections persist and become chronic, while 20% to 30% spontaneously resolve. Epidemiologic, viral, and host factors have been associated with the differences in viral clearance or persistence, and studies have found that a strong host immune response against the virus favors viral clearance. Thus, variation in the genes involved in the immune response may contribute to one’s ability to clear the virus. Consistent with these observations, recent studies have shown that the polymorphism in the IL28 gene region encoding interferon lambda 3 strongly predicts spontaneous resolution of acute hepatitis C virus infection. People who have the IL28B CC genotype are three times more likely to spontaneously clear the virus than those with the CT or TT genotype (Figure 3).24
IL28B POLYMORPHISM AND THE NATURAL HISTORY OF HEPATITIS C
In people in whom hepatitis C virus infection persists, up to 20% develop progressive liver fibrosis and eventually cirrhosis over 10 to 20 years.19,25,26 The speed at which fibrosis develops in these patients is variable and unpredictable.25 The relationship between IL28B polymorphisms and hepatic fibrosis in patients with chronic hepatitis C virus infection has not been clearly established, although a study indicated that in patients with a known date of infection, the IL28B genotype is not associated with progression of hepatic fibrosis.27 Obstacles in this field of study are that it is difficult to determine accurately when the patient contracted the virus, and that serial liver biopsies are needed to investigate the progression of hepatic fibrosis.
Patients with chronic hepatitis C virus infection are also at higher risk of hepatocellular carcinoma compared with the general population.28 An analysis of explanted livers of patients with hepatitis C found that the prevalence of hepatocellular carcinoma in those with the unfavorable TT genotype was significantly higher than with the other genotypes.29 Similarly, an earlier study demonstrated that patients with hepatitis C-associated hepatocellular carcinoma carried the T allele more frequently.30 As with other aspects of IL28B associations with hepatitis C, these findings indicate that the C allele confers a certain degree of protection.
An important implication of these relationships is that they may eventually help identify patients at greater risk, who therefore need earlier intervention.
IL28B POLYMORPHISM AND LIVER TRANSPLANTATION
Hepatitis C virus infection always recurs after liver transplantation, with serious consequences that include cirrhosis and liver failure. Recurrent hepatitis C virus infection has become an important reason for repeat transplantation in the United States.
Results of treatment with pegylated interferon and ribavirin for recurrent hepatitis C after liver transplantation have been disappointing, with response rates lower than 30% and significant side effects.31 Identifying the factors that predict the response to therapy allows for better selection of treatment candidates.
Similar to the way the IL28B genotype predicts response to antiviral therapy in the nontransplant setting, the IL28B genotypes of both the recipient and the donor are strongly and independently associated with response to interferon-based treatment in patients with hepatitis C after liver transplantation. The IL28B CC genotype in either the recipient or the donor is associated with a higher rate of response to pegylated interferon and ribavirin combination therapy after liver transplantation.30,32 For example, the response rate to therapy after liver transplantation reaches 86% in CC-donor and CC-recipient livers, compared with 0% in TT-donor and TT-recipient livers.
Additionally, the IL28B genotype of the recipient may determine the severity of histologic recurrence of hepatitis C, as indicated by progressive hepatic fibrosis. A recipient IL28B TT genotype is associated with more severe histologic recurrence of hepatitis C.33
These data suggest that CC donor livers might be preferentially allocated to patients with hepatitis C virus infection.
IL28B AND OTHER FACTORS IN HEPATITIS C VIRUS INFECTION
Although it is tempting to think that the IL28B polymorphism is the sole predictor of response to antiviral therapy, it is but one of several known factors in the virus and the host.
While IL28B polymorphisms are the most important predictor of sustained virologic response with an interferon-based regimen, a rapid virologic response (undetectable viral load at 4 weeks) had superior predictive value and specificity in one study.34 In fact, for patients with chronic hepatitis C infection who achieved a rapid virologic response with pegylated interferon and ribavirin, the IL28B polymorphism had no effect on the rate of sustained virologic response. However, it did predict a sustained virologic response in the group who did not achieve rapid virologic response.
In a study of patients with acute hepatitis C infection,35 jaundice and the IL28 rs12979860 CC genotype both predicted spontaneous clearance. The best predictor of viral persistence was the combination of the CT or TT genotype plus the absence of jaundice, which had a predictive value of 98%.
IL28B AND THE FUTURE OF HEPATITIS C VIRUS THERAPY
New oral agents were recently approved for treating hepatitis C. As of November 2014, these included simeprevir, sofosbuvir, and ledipasvir.
Simeprevir is a second-generation NS3/4A protease inhibitor approved for use in combination with pegylated interferon and ribavirin. A recent phase 3 trial evaluating simeprevir in patients who had relapsed after prior therapy found sustained virologic response rates to be higher with simeprevir than with placebo, irrespective of IL28B status.36 This finding was similar to that of a trial of telaprevir.16
Sofosbuvir is a nucleotide analogue NS5B polymerase inhibitor that becomes incorporated into the growing RNA, inducing a chain termination event.37 In phase 3 trials,38,39 researchers found an initial rapid decrease in viral load for patients treated with this agent regardless of IL28B status.
In the NEUTRINO trial (Sofosbuvir With Peginterferon Alfa 2a and Ribavirin for 12 Weeks in Treatment-Naive Subjects With Chronic Genotype 1, 4, 5, or 6 HCV Infection),38 which used sofusbuvir in combination with interferon and ribavirin, the rate of sustained virologic response was higher in those with the favorable CC genotype (98%) than with a non-CC genotype (87%).
In COSMOS (A Study of TMC435 in Combination With PSI-7977 [GS7977] in Chronic Hepatitis C Genotype 1-Infected Prior Null Responders to Peginterferon/Ribavirin Therapy or HCV Treatment-Naive Patients),39 which used a combination of simeprevir, sofosbuvir, and ribavirin, the rate of sustained virologic response was higher in those with the CC genotype (100%) than with the TT genotype (83%; Table 1).
These new medications have radically changed the landscape of hepatitis C therapy and have also unlocked the potential for developing completely interferon-free regimens.
Other new interferon-free regimens such as ledipasvir, daclatasvir, and asunaprevir promise high rates of sustained virologic response, which makes the utility of testing for IL28B polymorphisms to predict sustained virologic response very much diminished (Table 1).40,41 However, these new drugs are expected to be expensive, and IL28B polymorphisms may be used to identify candidates who are more likely to respond to pegylated interferon and ribavirin, particularly in resource-poor settings and in developing countries. Additionally, patients who have contraindications to these newer therapies will still likely need an interferon-based regimen, and thus the IL28B polymorphism will still be important in predicting treatment response and prognosis.
IL28B WILL STILL BE RELEVANT IN THE INTERFERON-FREE AGE
The IL28B polymorphism is a strong predictor of spontaneous clearance of hepatitis C virus and responsiveness to interferon-based therapy, and testing for it has demonstrated a great potential to improve patient care. IL28B testing has become available for clinical use and may optimize the outcome of hepatitis C treatment by helping us to select the best treatment for individual patients and minimizing the duration of therapy and the side effects associated with interferon-based antiviral medications.
As newer therapies have shifted toward interferon-free regimens that offer very high sustained virologic response rates, the usefulness of IL28B polymorphism as a clinical test to predict the response rate to antiviral therapy is minimized substantially. It may remain clinically relevant in resource-poor settings and in developing countries, especially in light of the potentially prohibitive costs of the newer regimens, and for patients in whom these treatments are contraindicated. This does not minimize the lesson we learned from the discovery of the IL28B gene and the impact on our understanding of the pathogenesis of hepatitis C virus infection.
- Attia J, Ioannidis JP, Thakkinstian A, et al. How to use an article about genetic association: A: background concepts. JAMA 2009; 301:74–81.
- Samani NJ, Erdmann J, Hall AS, et al; WTCCC and the Cardiogenics Consortium. Genomewide association analysis of coronary artery disease. N Engl J Med 2007; 357:443–453.
- Zeggini E, Weedon MN, Lindgren CM, et al; Wellcome Trust Case Control Consortium (WTCCC). Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 2007; 316:1336–1341.
- Matarín M, Brown WM, Scholz S, et al. A genome-wide genotyping study in patients with ischaemic stroke: initial analysis and data release. Lancet Neurol 2007; 6:414–420.
- Easton DF, Pooley KA, Dunning AM, et al; AOCS Management Group. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature 2007; 447:1087–1093.
- Plenge RM, Seielstad M, Padyukov L, et al. TRAF1-C5 as a risk locus for rheumatoid arthritis—a genomewide study. N Engl J Med 2007; 357:1199–1209.
- Coon KD, Myers AJ, Craig DW, et al. A high-density whole-genome association study reveals that APOE is the major susceptibility gene for sporadic late-onset Alzheimer’s disease. J Clin Psychiatry 2007; 68:613–618.
- Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance (letter). Nature 2009; 461:399–401.
- Ali A, Zein NN. Hepatitis C infection: a systemic disease with extrahepatic manifestations. Cleve Clin J Med 2005; 72:1005-1019.
- Hanouneh IA, Feldstein AE, Lopez R, et al. Clinical significance of metabolic syndrome in the setting of chronic hepatitis C virus infection. Clin Gastroenterol Hepatol 2008; 6:584–589.
- Elgouhari HM, Zein CO, Hanouneh I, Feldstein AE, Zein NN. Diabetes mellitus is associated with impaired response to antiviral therapy in chronic hepatitis C infection. Dig Dis Sci 2009; 54:2699–2705.
- Alkhouri N, Zein NN. Protease inhibitors: silver bullets for chronic hepatitis C infection? Cleve Clin J Med 2012; 79:213–222.
- McHutchison JG, Everson GT, Gordon SC, et al; PROVE1 Study Team. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med 2009; 360:1827–1838.
- Jacobson IM, McHutchison JG, Dusheiko G, et al; ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 2011; 364:2405–2416.
- Jacobson IM, Catlett I, Marcellin P, et al. Telaprevir substantially improved SVR rates across all IL28B genotypes in the ADVANCE trial. J Hepatol 2011; 54(suppl 1):S542–S543.
- Pol S, Aerssens J, Zeuzem S, et al. Limited impact of IL28B genotype on response rates in telaprevir-treated patients with prior treatment failure. J Hepatol 2013; 58:883–889.
- Suppiah V, Moldovan M, Ahlenstiel G, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet 2009; 41:1100–1104.
- Tanaka Y, Nishida N, Sugiyama M, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet 2009; 41:1105–1109.
- Thomas DL, Thio CL, Martin MP, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 2009; 461:798–801.
- Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest 2009; 119:1745–1754.
- Marcello T, Grakoui A, Barba-Spaeth G, et al. Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology 2006; 131:1887–1898.
- Doyle SE, Schreckhise H, Khuu-Duong K, et al. Interleukin-29 uses a type 1 interferon-like program to promote antiviral responses in human hepatocytes. Hepatology 2006; 44:896–906.
- Raglow Z, Thoma-Perry C, Gilroy R, Wan YJ. IL28B genotype and the expression of ISGs in normal liver. Liver Int 2013; 33:991–998.
- Prokunina-Olsson L, Muchmore B, Tang W, et al. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat Genet 2013; 45:164–171.
- Hanouneh IA, Zein NN, Askar M, Lopez R, John B. Interleukin-28B polymorphisms are associated with fibrosing cholestatic hepatitis in recurrent hepatitis C after liver transplantation. Clin Transplant 2012; 26:E335–E336.
- Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet 1997; 349:825–832.
- Thomas DL, Astemborski J, Rai RM, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA 2000; 284:450–456.
- Bochud PY, Cai T, Overbeck K, et al; Swiss Hepatitis C Cohort Study Group. Genotype 3 is associated with accelerated fibrosis progression in chronic hepatitis C. J Hepatol 2009; 51:655–666.
- Marabita F, Aghemo A, De Nicola S, et al. Genetic variation in the interleukin-28B gene is not associated with fibrosis progression in patients with chronic hepatitis C and known date of infection. Hepatology 2011; 54:1127–1134.
- Fabris C, Falleti E, Cussigh A, et al. IL-28B rs12979860 C/T allele distribution in patients with liver cirrhosis: role in the course of chronic viral hepatitis and the development of HCC. J Hepatol 2011; 54:716–722.
- Eurich D, Boas-Knoop S, Bahra M, et al. Role of IL28B polymorphism in the development of hepatitis C virus-induced hepatocellular carcinoma, graft fibrosis, and posttransplant antiviral therapy. Transplantation 2012; 93:644–649.
- Hanouneh IA, Miller C, Aucejo F, Lopez R, Quinn MK, Zein NN. Recurrent hepatitis C after liver transplantation: on-treatment prediction of response to peginterferon/ribavirin therapy. Liver Transpl 2008; 14:53–58.
- Charlton MR, Thompson A, Veldt BJ, et al. Interleukin-28B polymorphisms are associated with histological recurrence and treatment response following liver transplantation in patients with hepatitis C virus infection. Hepatology 2011; 53:317–324.
- Thompson AJ, Muir AJ, Sulkowski MS, et al. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology 2010; 139:120–129.e18.
- Beinhardt S, Payer BA, Datz C, et al. A diagnostic score for the prediction of spontaneous resolution of acute hepatitis C virus infection. J Hepatol 2013; 59:972–977.
- Forns X, Lawitz E, Zeuzem S, et al. Simeprevir with peginterferon and ribavirin leads to high rates of SVR in patients with HCV genotype 1 who relapsed after previous therapy: a phase 3 trial. Gastroenterology 2014; 146:1669–1679.e3.
- Sofia MJ, Bao D, Chang W, et al. Discovery of a ß-d-2’-deoxy-2’-ß-fluoro-2’-ß-C-methyluridine nucleotide prodrug (PSI-7977) for the treatment of hepatitis C virus. J Med Chem 2010; 53:7202–7218.
- Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013; 368:1878–1887.
- Sulkowski MS, Jacobson IM, Ghalib R, et al. Once-daily simeprevir (TMC435) plus sofosbuvir (GS-7977) with or without ribavirin in HCV genotype 1 prior null responders with metavir F0-2: COSMOS study subgroup analysis. 49th EASL, April 2014, London. Oral abstract O7. www.natap.org/2014/EASL/EASL_46.htm. Accesed January 9, 2015.
- Lok AS, Gardiner DF, Lawitz E, et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med 2012; 366:216–224.
- Afdhal N, Zeuzem S, Kwo P, et al; ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014; 370:1889–1898.
What a difference a single nucleotide can make! The human genome contains more than 3 billion base pairs. Yet having a different nucleotide in only one pair can make a big difference in how we respond to a disease or its treatment.
Specifically, in hepatitis C virus infection, people born with the nucleotide cytosine (C) at location rs12979860 in both alleles of the gene that codes for interleukin 28B (the IL28B CC genotype) can count themselves luckier than those born with thymine (T) in this location in one of their alleles (the CT genotype) or both of their alleles (the TT genotype). Those with the CC genotype are more likely to clear the virus spontaneously, and even if the infection persists, it is less likely to progress to liver cancer and more likely to respond to treatment with interferon.
Here, we review the IL28B polymorphism and its implications in treating hepatitis C.
GENETIC POLYMORPHISM AND HUMAN DISEASE
Of the 3 billion base pairs of nucleotides, fewer than 1% differ between individuals, but this 1% is responsible for the diversity of human beings. Differences in genetic sequences among individuals are called genetic polymorphisms. A single-nucleotide polymorphism is a DNA sequence variation that occurs in a single nucleotide in the genome. For example, two sequenced DNA fragments from different individuals, AAGCCTA and AAGCTTA, contain a difference in a single nucleotide.
Genetic variations such as these underlie some of the differences in our susceptibility to disease, the severity of illness we develop, and our response to treatments. Therefore, identifying genetic polymorphisms may shed light on biologic pathways involved in diseases and may uncover new targets for therapy.1
Genome-wide association studies have looked at hundreds of thousands of single-nucleotide polymorphisms to try to identify most of the common genetic differences among people and relate them to common chronic diseases such as coronary artery disease,2 type 2 diabetes,3 stroke,4 breast cancer,5 rheumatoid arthritis,6 Alzheimer disease,7 and, more recently, hepatitis C virus infection.8
HEPATITIS C VIRUS: A MAJOR CAUSE OF LIVER DISEASE
Hepatitis C virus infection is a major cause of chronic liver disease and hepatocellular carcinoma and has become the most common indication for liver transplantation in the United States.9
This virus has six distinct genotypes throughout the world, with multiple subtypes in each genotype. (A genotype is a classification of a virus based on its RNA.9) In this review, we will focus on genotype 1; hence, “hepatitis C virus” will refer to hepatitis C virus genotype 1.
Our knowledge of the biology, pathogenesis, and treatment of hepatitis C has been advancing. Originally, fewer than 50% of patients responded to therapy with the combination of pegylated interferon and ribavirin,10,11 but since 2011 the response rate has increased to approximately 70% with the approval of the protease inhibitors telaprevir and boceprevir, used in combination with pegylated interferon and ribavirin.12–15
Unfortunately, interferon-based treatment is often complicated by side effects such as fatigue, influenza-like symptoms, hematologic abnormalities, and neuropsychiatric symptoms. An accurate way to predict response would help patients make informed decisions about antiviral treatment, taking into account the risk and possible benefit for individual patients.
GENETIC POLYMORPHISM AND HEPATITIS C VIRUS INFECTION
Genome-wide association studies have identified single-nucleotide polymorphisms in the IL28B gene that are associated with differences in response to hepatitis C treatment.8
Studying 565,759 polymorphisms in 1,137 patients, researchers at Duke University identified a single-nucleotide polymorphism at location rs12979860 in IL28B (Figure 1) that was strongly associated with response to combination therapy with pegylated interferon and ribavirin.8 The chance of cure with this standard treatment is twice as high in patients who are homozygous for cytosine in this location (the CC genotype) than in those who are heterozygous (CT) or homozygous for thymine in this location (the TT genotype) (Table 1).
Adding one of the new protease inhibitors, telaprevir or boceprevir, to the standard hepatitis C treatment substantially improves the cure rates in all three IL28B genotypes, but especially in people with CT or TT, in whom the response rate almost triples with the addition of one of these drugs. Those with the CC genotype (who are more likely to be cured with pegylated interferon and ribavirin alone) also achieve an increase (although minimal) in cure rates when a protease inhibitor is included in the regimen (TABLE 1).13–15 Thus, it remains unclear if adding a protease inhibitor to pegylated interferon plus ribavirin in patients with the IL28B CC genotype translates into added effectiveness worth the additional cost of the protease inhibitor in previously untreated patients.
Additionally, the effect of the IL28B genotype on telaprevir-based triple therapy has been disputed in more recent studies. In a subgroup analysis of the results of a trial that evaluated telaprevir in the treatment of hepatitis C, researchers found that sustained virologic response rates were significantly higher in the telaprevir group, and this was similar across the different IL28B polymorphisms.16
The favorable IL28B CC genotype is associated with higher rates of rapid virologic response to antiviral therapy.13–15 Of note, almost all patients who achieve a rapid virologic response do well, with a high rate of sustained virologic response even after a shorter duration of therapy (24 vs 48 weeks). Therefore, in addition to predicting response to interferon before starting treatment, the IL28B CC genotype may also identify patients who need only a shorter duration of therapy.
Interestingly, the C allele is much more frequent in white than in African American populations, an important observation that explains the racial difference in response to hepatitis C therapy.8
Two other research groups, from Asia and Australia, performed independent genome-wide association studies that identified different single-nucleotide polymorphisms (eg, rs8099917) in the same IL28B gene as predictors of response to treatment in patients with hepatitis C virus infection.17,18 These findings may be explained by linkage disequilibrium, which means that these single-nucleotide polymorphisms are found more frequently together in the same patient due to their proximity to each other. In this review, we will focus on the rs12979860 polymorphism; hence “IL28B genotype” will refer to the single-nucleotide polymorphism at rs12979860, unless otherwise specified.
The favorable CC genotype is less common in African Americans than in patients of other ethnicities.19 Moreover, although IL28B CC is associated with a better response rate to interferon-based antiviral therapy across all ethnicities, those of African American descent with the CC genotype are less likely to achieve a sustained virologic response than white or Hispanic Americans.8
BIOLOGIC ASSOCIATION: IL28B POLYMORPHISM AND HEPATITIS C
The interferon lambda family consists of three cytokines:
- Interleukin 29 (interferon lambda 1)
- Interleukin 28A (interferon lambda 2)
- Interleukin 28B (interferon lambda 3).
Production of these three molecules can be triggered by viral infection, and they induce antiviral activity through both innate and adaptive immune pathways. They signal through the IL10R-IL28R receptor complex.20–22 This receptor activates the JAK-STAT (Janus kinase-signal transducer and activator of transcription) pathway, which regulates a large number of interferon-stimulated genes, primarily through the interferon-stimulated response element (Figure 2).
A 2013 study found that interferon-stimulated gene expression levels in patients with normal livers were highest in those with the CC genotype, intermediate with CT, and lowest with TT. Interestingly, this pattern was reversed in those with hepatitis C virus infection, indicating a relationship between the IL28B genotype and gene expression before infection.23
The mechanism underlying the association between the IL28B polymorphism and response to hepatitis C treatment is not well understood. The unfavorable TT genotype seems to lead to continuous activation of a subset of interferon-stimulated genes in the presence of intracellular hepatitis C viral RNA. But this level of expression is not sufficient to eliminate the virus from the cells. Instead, it might lead to up-regulation of interferon-inhibitory molecules that suppress JAK-STAT signaling, thereby reducing sensitivity to interferon signaling. Therefore, the hepatocyte not only cannot clear the virus by itself, but also cannot induce strong interferon-stimulated gene expression when interferon is given during therapy.20–22
The recently identified ss469425590 polymorphism, which is located in close proximity to rs12979860 in the IL28B gene, is particularly interesting, as it suggests a possible molecular mechanism. The delta G frameshift variant creates a novel gene called IFNL4, which is transiently activated in response to hepatitis C virus infection.24IFNL4 stimulates STAT1 and STAT2 phosphorylation and induces the expression of interferon-stimulated genes. Increased interferon-stimulated gene expression has been shown to be associated with decreased response to pegylated interferon-ribavirin treatment. These observations suggest that the ss469425590 delta G allele is responsible for the increased activation of interferon-stimulated genes and the lower sustained virologic response rate observed in patients who receive pegylated interferon-ribavirin treatment. It is possible that the activation of interferon-stimulated genes in patients with the ss469425590 delta G/delta G genotype reduces interferon-stimulated gene responsiveness to interferon alpha, which normally activates interferon-stimulated genes and inhibits hepatitis C progression.24
IL28B POLYMORPHISM AND ACUTE HEPATITIS C VIRUS INFECTION
From 70% to 80% of acute hepatitis C virus infections persist and become chronic, while 20% to 30% spontaneously resolve. Epidemiologic, viral, and host factors have been associated with the differences in viral clearance or persistence, and studies have found that a strong host immune response against the virus favors viral clearance. Thus, variation in the genes involved in the immune response may contribute to one’s ability to clear the virus. Consistent with these observations, recent studies have shown that the polymorphism in the IL28 gene region encoding interferon lambda 3 strongly predicts spontaneous resolution of acute hepatitis C virus infection. People who have the IL28B CC genotype are three times more likely to spontaneously clear the virus than those with the CT or TT genotype (Figure 3).24
IL28B POLYMORPHISM AND THE NATURAL HISTORY OF HEPATITIS C
In people in whom hepatitis C virus infection persists, up to 20% develop progressive liver fibrosis and eventually cirrhosis over 10 to 20 years.19,25,26 The speed at which fibrosis develops in these patients is variable and unpredictable.25 The relationship between IL28B polymorphisms and hepatic fibrosis in patients with chronic hepatitis C virus infection has not been clearly established, although a study indicated that in patients with a known date of infection, the IL28B genotype is not associated with progression of hepatic fibrosis.27 Obstacles in this field of study are that it is difficult to determine accurately when the patient contracted the virus, and that serial liver biopsies are needed to investigate the progression of hepatic fibrosis.
Patients with chronic hepatitis C virus infection are also at higher risk of hepatocellular carcinoma compared with the general population.28 An analysis of explanted livers of patients with hepatitis C found that the prevalence of hepatocellular carcinoma in those with the unfavorable TT genotype was significantly higher than with the other genotypes.29 Similarly, an earlier study demonstrated that patients with hepatitis C-associated hepatocellular carcinoma carried the T allele more frequently.30 As with other aspects of IL28B associations with hepatitis C, these findings indicate that the C allele confers a certain degree of protection.
An important implication of these relationships is that they may eventually help identify patients at greater risk, who therefore need earlier intervention.
IL28B POLYMORPHISM AND LIVER TRANSPLANTATION
Hepatitis C virus infection always recurs after liver transplantation, with serious consequences that include cirrhosis and liver failure. Recurrent hepatitis C virus infection has become an important reason for repeat transplantation in the United States.
Results of treatment with pegylated interferon and ribavirin for recurrent hepatitis C after liver transplantation have been disappointing, with response rates lower than 30% and significant side effects.31 Identifying the factors that predict the response to therapy allows for better selection of treatment candidates.
Similar to the way the IL28B genotype predicts response to antiviral therapy in the nontransplant setting, the IL28B genotypes of both the recipient and the donor are strongly and independently associated with response to interferon-based treatment in patients with hepatitis C after liver transplantation. The IL28B CC genotype in either the recipient or the donor is associated with a higher rate of response to pegylated interferon and ribavirin combination therapy after liver transplantation.30,32 For example, the response rate to therapy after liver transplantation reaches 86% in CC-donor and CC-recipient livers, compared with 0% in TT-donor and TT-recipient livers.
Additionally, the IL28B genotype of the recipient may determine the severity of histologic recurrence of hepatitis C, as indicated by progressive hepatic fibrosis. A recipient IL28B TT genotype is associated with more severe histologic recurrence of hepatitis C.33
These data suggest that CC donor livers might be preferentially allocated to patients with hepatitis C virus infection.
IL28B AND OTHER FACTORS IN HEPATITIS C VIRUS INFECTION
Although it is tempting to think that the IL28B polymorphism is the sole predictor of response to antiviral therapy, it is but one of several known factors in the virus and the host.
While IL28B polymorphisms are the most important predictor of sustained virologic response with an interferon-based regimen, a rapid virologic response (undetectable viral load at 4 weeks) had superior predictive value and specificity in one study.34 In fact, for patients with chronic hepatitis C infection who achieved a rapid virologic response with pegylated interferon and ribavirin, the IL28B polymorphism had no effect on the rate of sustained virologic response. However, it did predict a sustained virologic response in the group who did not achieve rapid virologic response.
In a study of patients with acute hepatitis C infection,35 jaundice and the IL28 rs12979860 CC genotype both predicted spontaneous clearance. The best predictor of viral persistence was the combination of the CT or TT genotype plus the absence of jaundice, which had a predictive value of 98%.
IL28B AND THE FUTURE OF HEPATITIS C VIRUS THERAPY
New oral agents were recently approved for treating hepatitis C. As of November 2014, these included simeprevir, sofosbuvir, and ledipasvir.
Simeprevir is a second-generation NS3/4A protease inhibitor approved for use in combination with pegylated interferon and ribavirin. A recent phase 3 trial evaluating simeprevir in patients who had relapsed after prior therapy found sustained virologic response rates to be higher with simeprevir than with placebo, irrespective of IL28B status.36 This finding was similar to that of a trial of telaprevir.16
Sofosbuvir is a nucleotide analogue NS5B polymerase inhibitor that becomes incorporated into the growing RNA, inducing a chain termination event.37 In phase 3 trials,38,39 researchers found an initial rapid decrease in viral load for patients treated with this agent regardless of IL28B status.
In the NEUTRINO trial (Sofosbuvir With Peginterferon Alfa 2a and Ribavirin for 12 Weeks in Treatment-Naive Subjects With Chronic Genotype 1, 4, 5, or 6 HCV Infection),38 which used sofusbuvir in combination with interferon and ribavirin, the rate of sustained virologic response was higher in those with the favorable CC genotype (98%) than with a non-CC genotype (87%).
In COSMOS (A Study of TMC435 in Combination With PSI-7977 [GS7977] in Chronic Hepatitis C Genotype 1-Infected Prior Null Responders to Peginterferon/Ribavirin Therapy or HCV Treatment-Naive Patients),39 which used a combination of simeprevir, sofosbuvir, and ribavirin, the rate of sustained virologic response was higher in those with the CC genotype (100%) than with the TT genotype (83%; Table 1).
These new medications have radically changed the landscape of hepatitis C therapy and have also unlocked the potential for developing completely interferon-free regimens.
Other new interferon-free regimens such as ledipasvir, daclatasvir, and asunaprevir promise high rates of sustained virologic response, which makes the utility of testing for IL28B polymorphisms to predict sustained virologic response very much diminished (Table 1).40,41 However, these new drugs are expected to be expensive, and IL28B polymorphisms may be used to identify candidates who are more likely to respond to pegylated interferon and ribavirin, particularly in resource-poor settings and in developing countries. Additionally, patients who have contraindications to these newer therapies will still likely need an interferon-based regimen, and thus the IL28B polymorphism will still be important in predicting treatment response and prognosis.
IL28B WILL STILL BE RELEVANT IN THE INTERFERON-FREE AGE
The IL28B polymorphism is a strong predictor of spontaneous clearance of hepatitis C virus and responsiveness to interferon-based therapy, and testing for it has demonstrated a great potential to improve patient care. IL28B testing has become available for clinical use and may optimize the outcome of hepatitis C treatment by helping us to select the best treatment for individual patients and minimizing the duration of therapy and the side effects associated with interferon-based antiviral medications.
As newer therapies have shifted toward interferon-free regimens that offer very high sustained virologic response rates, the usefulness of IL28B polymorphism as a clinical test to predict the response rate to antiviral therapy is minimized substantially. It may remain clinically relevant in resource-poor settings and in developing countries, especially in light of the potentially prohibitive costs of the newer regimens, and for patients in whom these treatments are contraindicated. This does not minimize the lesson we learned from the discovery of the IL28B gene and the impact on our understanding of the pathogenesis of hepatitis C virus infection.
What a difference a single nucleotide can make! The human genome contains more than 3 billion base pairs. Yet having a different nucleotide in only one pair can make a big difference in how we respond to a disease or its treatment.
Specifically, in hepatitis C virus infection, people born with the nucleotide cytosine (C) at location rs12979860 in both alleles of the gene that codes for interleukin 28B (the IL28B CC genotype) can count themselves luckier than those born with thymine (T) in this location in one of their alleles (the CT genotype) or both of their alleles (the TT genotype). Those with the CC genotype are more likely to clear the virus spontaneously, and even if the infection persists, it is less likely to progress to liver cancer and more likely to respond to treatment with interferon.
Here, we review the IL28B polymorphism and its implications in treating hepatitis C.
GENETIC POLYMORPHISM AND HUMAN DISEASE
Of the 3 billion base pairs of nucleotides, fewer than 1% differ between individuals, but this 1% is responsible for the diversity of human beings. Differences in genetic sequences among individuals are called genetic polymorphisms. A single-nucleotide polymorphism is a DNA sequence variation that occurs in a single nucleotide in the genome. For example, two sequenced DNA fragments from different individuals, AAGCCTA and AAGCTTA, contain a difference in a single nucleotide.
Genetic variations such as these underlie some of the differences in our susceptibility to disease, the severity of illness we develop, and our response to treatments. Therefore, identifying genetic polymorphisms may shed light on biologic pathways involved in diseases and may uncover new targets for therapy.1
Genome-wide association studies have looked at hundreds of thousands of single-nucleotide polymorphisms to try to identify most of the common genetic differences among people and relate them to common chronic diseases such as coronary artery disease,2 type 2 diabetes,3 stroke,4 breast cancer,5 rheumatoid arthritis,6 Alzheimer disease,7 and, more recently, hepatitis C virus infection.8
HEPATITIS C VIRUS: A MAJOR CAUSE OF LIVER DISEASE
Hepatitis C virus infection is a major cause of chronic liver disease and hepatocellular carcinoma and has become the most common indication for liver transplantation in the United States.9
This virus has six distinct genotypes throughout the world, with multiple subtypes in each genotype. (A genotype is a classification of a virus based on its RNA.9) In this review, we will focus on genotype 1; hence, “hepatitis C virus” will refer to hepatitis C virus genotype 1.
Our knowledge of the biology, pathogenesis, and treatment of hepatitis C has been advancing. Originally, fewer than 50% of patients responded to therapy with the combination of pegylated interferon and ribavirin,10,11 but since 2011 the response rate has increased to approximately 70% with the approval of the protease inhibitors telaprevir and boceprevir, used in combination with pegylated interferon and ribavirin.12–15
Unfortunately, interferon-based treatment is often complicated by side effects such as fatigue, influenza-like symptoms, hematologic abnormalities, and neuropsychiatric symptoms. An accurate way to predict response would help patients make informed decisions about antiviral treatment, taking into account the risk and possible benefit for individual patients.
GENETIC POLYMORPHISM AND HEPATITIS C VIRUS INFECTION
Genome-wide association studies have identified single-nucleotide polymorphisms in the IL28B gene that are associated with differences in response to hepatitis C treatment.8
Studying 565,759 polymorphisms in 1,137 patients, researchers at Duke University identified a single-nucleotide polymorphism at location rs12979860 in IL28B (Figure 1) that was strongly associated with response to combination therapy with pegylated interferon and ribavirin.8 The chance of cure with this standard treatment is twice as high in patients who are homozygous for cytosine in this location (the CC genotype) than in those who are heterozygous (CT) or homozygous for thymine in this location (the TT genotype) (Table 1).
Adding one of the new protease inhibitors, telaprevir or boceprevir, to the standard hepatitis C treatment substantially improves the cure rates in all three IL28B genotypes, but especially in people with CT or TT, in whom the response rate almost triples with the addition of one of these drugs. Those with the CC genotype (who are more likely to be cured with pegylated interferon and ribavirin alone) also achieve an increase (although minimal) in cure rates when a protease inhibitor is included in the regimen (TABLE 1).13–15 Thus, it remains unclear if adding a protease inhibitor to pegylated interferon plus ribavirin in patients with the IL28B CC genotype translates into added effectiveness worth the additional cost of the protease inhibitor in previously untreated patients.
Additionally, the effect of the IL28B genotype on telaprevir-based triple therapy has been disputed in more recent studies. In a subgroup analysis of the results of a trial that evaluated telaprevir in the treatment of hepatitis C, researchers found that sustained virologic response rates were significantly higher in the telaprevir group, and this was similar across the different IL28B polymorphisms.16
The favorable IL28B CC genotype is associated with higher rates of rapid virologic response to antiviral therapy.13–15 Of note, almost all patients who achieve a rapid virologic response do well, with a high rate of sustained virologic response even after a shorter duration of therapy (24 vs 48 weeks). Therefore, in addition to predicting response to interferon before starting treatment, the IL28B CC genotype may also identify patients who need only a shorter duration of therapy.
Interestingly, the C allele is much more frequent in white than in African American populations, an important observation that explains the racial difference in response to hepatitis C therapy.8
Two other research groups, from Asia and Australia, performed independent genome-wide association studies that identified different single-nucleotide polymorphisms (eg, rs8099917) in the same IL28B gene as predictors of response to treatment in patients with hepatitis C virus infection.17,18 These findings may be explained by linkage disequilibrium, which means that these single-nucleotide polymorphisms are found more frequently together in the same patient due to their proximity to each other. In this review, we will focus on the rs12979860 polymorphism; hence “IL28B genotype” will refer to the single-nucleotide polymorphism at rs12979860, unless otherwise specified.
The favorable CC genotype is less common in African Americans than in patients of other ethnicities.19 Moreover, although IL28B CC is associated with a better response rate to interferon-based antiviral therapy across all ethnicities, those of African American descent with the CC genotype are less likely to achieve a sustained virologic response than white or Hispanic Americans.8
BIOLOGIC ASSOCIATION: IL28B POLYMORPHISM AND HEPATITIS C
The interferon lambda family consists of three cytokines:
- Interleukin 29 (interferon lambda 1)
- Interleukin 28A (interferon lambda 2)
- Interleukin 28B (interferon lambda 3).
Production of these three molecules can be triggered by viral infection, and they induce antiviral activity through both innate and adaptive immune pathways. They signal through the IL10R-IL28R receptor complex.20–22 This receptor activates the JAK-STAT (Janus kinase-signal transducer and activator of transcription) pathway, which regulates a large number of interferon-stimulated genes, primarily through the interferon-stimulated response element (Figure 2).
A 2013 study found that interferon-stimulated gene expression levels in patients with normal livers were highest in those with the CC genotype, intermediate with CT, and lowest with TT. Interestingly, this pattern was reversed in those with hepatitis C virus infection, indicating a relationship between the IL28B genotype and gene expression before infection.23
The mechanism underlying the association between the IL28B polymorphism and response to hepatitis C treatment is not well understood. The unfavorable TT genotype seems to lead to continuous activation of a subset of interferon-stimulated genes in the presence of intracellular hepatitis C viral RNA. But this level of expression is not sufficient to eliminate the virus from the cells. Instead, it might lead to up-regulation of interferon-inhibitory molecules that suppress JAK-STAT signaling, thereby reducing sensitivity to interferon signaling. Therefore, the hepatocyte not only cannot clear the virus by itself, but also cannot induce strong interferon-stimulated gene expression when interferon is given during therapy.20–22
The recently identified ss469425590 polymorphism, which is located in close proximity to rs12979860 in the IL28B gene, is particularly interesting, as it suggests a possible molecular mechanism. The delta G frameshift variant creates a novel gene called IFNL4, which is transiently activated in response to hepatitis C virus infection.24IFNL4 stimulates STAT1 and STAT2 phosphorylation and induces the expression of interferon-stimulated genes. Increased interferon-stimulated gene expression has been shown to be associated with decreased response to pegylated interferon-ribavirin treatment. These observations suggest that the ss469425590 delta G allele is responsible for the increased activation of interferon-stimulated genes and the lower sustained virologic response rate observed in patients who receive pegylated interferon-ribavirin treatment. It is possible that the activation of interferon-stimulated genes in patients with the ss469425590 delta G/delta G genotype reduces interferon-stimulated gene responsiveness to interferon alpha, which normally activates interferon-stimulated genes and inhibits hepatitis C progression.24
IL28B POLYMORPHISM AND ACUTE HEPATITIS C VIRUS INFECTION
From 70% to 80% of acute hepatitis C virus infections persist and become chronic, while 20% to 30% spontaneously resolve. Epidemiologic, viral, and host factors have been associated with the differences in viral clearance or persistence, and studies have found that a strong host immune response against the virus favors viral clearance. Thus, variation in the genes involved in the immune response may contribute to one’s ability to clear the virus. Consistent with these observations, recent studies have shown that the polymorphism in the IL28 gene region encoding interferon lambda 3 strongly predicts spontaneous resolution of acute hepatitis C virus infection. People who have the IL28B CC genotype are three times more likely to spontaneously clear the virus than those with the CT or TT genotype (Figure 3).24
IL28B POLYMORPHISM AND THE NATURAL HISTORY OF HEPATITIS C
In people in whom hepatitis C virus infection persists, up to 20% develop progressive liver fibrosis and eventually cirrhosis over 10 to 20 years.19,25,26 The speed at which fibrosis develops in these patients is variable and unpredictable.25 The relationship between IL28B polymorphisms and hepatic fibrosis in patients with chronic hepatitis C virus infection has not been clearly established, although a study indicated that in patients with a known date of infection, the IL28B genotype is not associated with progression of hepatic fibrosis.27 Obstacles in this field of study are that it is difficult to determine accurately when the patient contracted the virus, and that serial liver biopsies are needed to investigate the progression of hepatic fibrosis.
Patients with chronic hepatitis C virus infection are also at higher risk of hepatocellular carcinoma compared with the general population.28 An analysis of explanted livers of patients with hepatitis C found that the prevalence of hepatocellular carcinoma in those with the unfavorable TT genotype was significantly higher than with the other genotypes.29 Similarly, an earlier study demonstrated that patients with hepatitis C-associated hepatocellular carcinoma carried the T allele more frequently.30 As with other aspects of IL28B associations with hepatitis C, these findings indicate that the C allele confers a certain degree of protection.
An important implication of these relationships is that they may eventually help identify patients at greater risk, who therefore need earlier intervention.
IL28B POLYMORPHISM AND LIVER TRANSPLANTATION
Hepatitis C virus infection always recurs after liver transplantation, with serious consequences that include cirrhosis and liver failure. Recurrent hepatitis C virus infection has become an important reason for repeat transplantation in the United States.
Results of treatment with pegylated interferon and ribavirin for recurrent hepatitis C after liver transplantation have been disappointing, with response rates lower than 30% and significant side effects.31 Identifying the factors that predict the response to therapy allows for better selection of treatment candidates.
Similar to the way the IL28B genotype predicts response to antiviral therapy in the nontransplant setting, the IL28B genotypes of both the recipient and the donor are strongly and independently associated with response to interferon-based treatment in patients with hepatitis C after liver transplantation. The IL28B CC genotype in either the recipient or the donor is associated with a higher rate of response to pegylated interferon and ribavirin combination therapy after liver transplantation.30,32 For example, the response rate to therapy after liver transplantation reaches 86% in CC-donor and CC-recipient livers, compared with 0% in TT-donor and TT-recipient livers.
Additionally, the IL28B genotype of the recipient may determine the severity of histologic recurrence of hepatitis C, as indicated by progressive hepatic fibrosis. A recipient IL28B TT genotype is associated with more severe histologic recurrence of hepatitis C.33
These data suggest that CC donor livers might be preferentially allocated to patients with hepatitis C virus infection.
IL28B AND OTHER FACTORS IN HEPATITIS C VIRUS INFECTION
Although it is tempting to think that the IL28B polymorphism is the sole predictor of response to antiviral therapy, it is but one of several known factors in the virus and the host.
While IL28B polymorphisms are the most important predictor of sustained virologic response with an interferon-based regimen, a rapid virologic response (undetectable viral load at 4 weeks) had superior predictive value and specificity in one study.34 In fact, for patients with chronic hepatitis C infection who achieved a rapid virologic response with pegylated interferon and ribavirin, the IL28B polymorphism had no effect on the rate of sustained virologic response. However, it did predict a sustained virologic response in the group who did not achieve rapid virologic response.
In a study of patients with acute hepatitis C infection,35 jaundice and the IL28 rs12979860 CC genotype both predicted spontaneous clearance. The best predictor of viral persistence was the combination of the CT or TT genotype plus the absence of jaundice, which had a predictive value of 98%.
IL28B AND THE FUTURE OF HEPATITIS C VIRUS THERAPY
New oral agents were recently approved for treating hepatitis C. As of November 2014, these included simeprevir, sofosbuvir, and ledipasvir.
Simeprevir is a second-generation NS3/4A protease inhibitor approved for use in combination with pegylated interferon and ribavirin. A recent phase 3 trial evaluating simeprevir in patients who had relapsed after prior therapy found sustained virologic response rates to be higher with simeprevir than with placebo, irrespective of IL28B status.36 This finding was similar to that of a trial of telaprevir.16
Sofosbuvir is a nucleotide analogue NS5B polymerase inhibitor that becomes incorporated into the growing RNA, inducing a chain termination event.37 In phase 3 trials,38,39 researchers found an initial rapid decrease in viral load for patients treated with this agent regardless of IL28B status.
In the NEUTRINO trial (Sofosbuvir With Peginterferon Alfa 2a and Ribavirin for 12 Weeks in Treatment-Naive Subjects With Chronic Genotype 1, 4, 5, or 6 HCV Infection),38 which used sofusbuvir in combination with interferon and ribavirin, the rate of sustained virologic response was higher in those with the favorable CC genotype (98%) than with a non-CC genotype (87%).
In COSMOS (A Study of TMC435 in Combination With PSI-7977 [GS7977] in Chronic Hepatitis C Genotype 1-Infected Prior Null Responders to Peginterferon/Ribavirin Therapy or HCV Treatment-Naive Patients),39 which used a combination of simeprevir, sofosbuvir, and ribavirin, the rate of sustained virologic response was higher in those with the CC genotype (100%) than with the TT genotype (83%; Table 1).
These new medications have radically changed the landscape of hepatitis C therapy and have also unlocked the potential for developing completely interferon-free regimens.
Other new interferon-free regimens such as ledipasvir, daclatasvir, and asunaprevir promise high rates of sustained virologic response, which makes the utility of testing for IL28B polymorphisms to predict sustained virologic response very much diminished (Table 1).40,41 However, these new drugs are expected to be expensive, and IL28B polymorphisms may be used to identify candidates who are more likely to respond to pegylated interferon and ribavirin, particularly in resource-poor settings and in developing countries. Additionally, patients who have contraindications to these newer therapies will still likely need an interferon-based regimen, and thus the IL28B polymorphism will still be important in predicting treatment response and prognosis.
IL28B WILL STILL BE RELEVANT IN THE INTERFERON-FREE AGE
The IL28B polymorphism is a strong predictor of spontaneous clearance of hepatitis C virus and responsiveness to interferon-based therapy, and testing for it has demonstrated a great potential to improve patient care. IL28B testing has become available for clinical use and may optimize the outcome of hepatitis C treatment by helping us to select the best treatment for individual patients and minimizing the duration of therapy and the side effects associated with interferon-based antiviral medications.
As newer therapies have shifted toward interferon-free regimens that offer very high sustained virologic response rates, the usefulness of IL28B polymorphism as a clinical test to predict the response rate to antiviral therapy is minimized substantially. It may remain clinically relevant in resource-poor settings and in developing countries, especially in light of the potentially prohibitive costs of the newer regimens, and for patients in whom these treatments are contraindicated. This does not minimize the lesson we learned from the discovery of the IL28B gene and the impact on our understanding of the pathogenesis of hepatitis C virus infection.
- Attia J, Ioannidis JP, Thakkinstian A, et al. How to use an article about genetic association: A: background concepts. JAMA 2009; 301:74–81.
- Samani NJ, Erdmann J, Hall AS, et al; WTCCC and the Cardiogenics Consortium. Genomewide association analysis of coronary artery disease. N Engl J Med 2007; 357:443–453.
- Zeggini E, Weedon MN, Lindgren CM, et al; Wellcome Trust Case Control Consortium (WTCCC). Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 2007; 316:1336–1341.
- Matarín M, Brown WM, Scholz S, et al. A genome-wide genotyping study in patients with ischaemic stroke: initial analysis and data release. Lancet Neurol 2007; 6:414–420.
- Easton DF, Pooley KA, Dunning AM, et al; AOCS Management Group. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature 2007; 447:1087–1093.
- Plenge RM, Seielstad M, Padyukov L, et al. TRAF1-C5 as a risk locus for rheumatoid arthritis—a genomewide study. N Engl J Med 2007; 357:1199–1209.
- Coon KD, Myers AJ, Craig DW, et al. A high-density whole-genome association study reveals that APOE is the major susceptibility gene for sporadic late-onset Alzheimer’s disease. J Clin Psychiatry 2007; 68:613–618.
- Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance (letter). Nature 2009; 461:399–401.
- Ali A, Zein NN. Hepatitis C infection: a systemic disease with extrahepatic manifestations. Cleve Clin J Med 2005; 72:1005-1019.
- Hanouneh IA, Feldstein AE, Lopez R, et al. Clinical significance of metabolic syndrome in the setting of chronic hepatitis C virus infection. Clin Gastroenterol Hepatol 2008; 6:584–589.
- Elgouhari HM, Zein CO, Hanouneh I, Feldstein AE, Zein NN. Diabetes mellitus is associated with impaired response to antiviral therapy in chronic hepatitis C infection. Dig Dis Sci 2009; 54:2699–2705.
- Alkhouri N, Zein NN. Protease inhibitors: silver bullets for chronic hepatitis C infection? Cleve Clin J Med 2012; 79:213–222.
- McHutchison JG, Everson GT, Gordon SC, et al; PROVE1 Study Team. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med 2009; 360:1827–1838.
- Jacobson IM, McHutchison JG, Dusheiko G, et al; ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 2011; 364:2405–2416.
- Jacobson IM, Catlett I, Marcellin P, et al. Telaprevir substantially improved SVR rates across all IL28B genotypes in the ADVANCE trial. J Hepatol 2011; 54(suppl 1):S542–S543.
- Pol S, Aerssens J, Zeuzem S, et al. Limited impact of IL28B genotype on response rates in telaprevir-treated patients with prior treatment failure. J Hepatol 2013; 58:883–889.
- Suppiah V, Moldovan M, Ahlenstiel G, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet 2009; 41:1100–1104.
- Tanaka Y, Nishida N, Sugiyama M, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet 2009; 41:1105–1109.
- Thomas DL, Thio CL, Martin MP, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 2009; 461:798–801.
- Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest 2009; 119:1745–1754.
- Marcello T, Grakoui A, Barba-Spaeth G, et al. Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology 2006; 131:1887–1898.
- Doyle SE, Schreckhise H, Khuu-Duong K, et al. Interleukin-29 uses a type 1 interferon-like program to promote antiviral responses in human hepatocytes. Hepatology 2006; 44:896–906.
- Raglow Z, Thoma-Perry C, Gilroy R, Wan YJ. IL28B genotype and the expression of ISGs in normal liver. Liver Int 2013; 33:991–998.
- Prokunina-Olsson L, Muchmore B, Tang W, et al. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat Genet 2013; 45:164–171.
- Hanouneh IA, Zein NN, Askar M, Lopez R, John B. Interleukin-28B polymorphisms are associated with fibrosing cholestatic hepatitis in recurrent hepatitis C after liver transplantation. Clin Transplant 2012; 26:E335–E336.
- Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet 1997; 349:825–832.
- Thomas DL, Astemborski J, Rai RM, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA 2000; 284:450–456.
- Bochud PY, Cai T, Overbeck K, et al; Swiss Hepatitis C Cohort Study Group. Genotype 3 is associated with accelerated fibrosis progression in chronic hepatitis C. J Hepatol 2009; 51:655–666.
- Marabita F, Aghemo A, De Nicola S, et al. Genetic variation in the interleukin-28B gene is not associated with fibrosis progression in patients with chronic hepatitis C and known date of infection. Hepatology 2011; 54:1127–1134.
- Fabris C, Falleti E, Cussigh A, et al. IL-28B rs12979860 C/T allele distribution in patients with liver cirrhosis: role in the course of chronic viral hepatitis and the development of HCC. J Hepatol 2011; 54:716–722.
- Eurich D, Boas-Knoop S, Bahra M, et al. Role of IL28B polymorphism in the development of hepatitis C virus-induced hepatocellular carcinoma, graft fibrosis, and posttransplant antiviral therapy. Transplantation 2012; 93:644–649.
- Hanouneh IA, Miller C, Aucejo F, Lopez R, Quinn MK, Zein NN. Recurrent hepatitis C after liver transplantation: on-treatment prediction of response to peginterferon/ribavirin therapy. Liver Transpl 2008; 14:53–58.
- Charlton MR, Thompson A, Veldt BJ, et al. Interleukin-28B polymorphisms are associated with histological recurrence and treatment response following liver transplantation in patients with hepatitis C virus infection. Hepatology 2011; 53:317–324.
- Thompson AJ, Muir AJ, Sulkowski MS, et al. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology 2010; 139:120–129.e18.
- Beinhardt S, Payer BA, Datz C, et al. A diagnostic score for the prediction of spontaneous resolution of acute hepatitis C virus infection. J Hepatol 2013; 59:972–977.
- Forns X, Lawitz E, Zeuzem S, et al. Simeprevir with peginterferon and ribavirin leads to high rates of SVR in patients with HCV genotype 1 who relapsed after previous therapy: a phase 3 trial. Gastroenterology 2014; 146:1669–1679.e3.
- Sofia MJ, Bao D, Chang W, et al. Discovery of a ß-d-2’-deoxy-2’-ß-fluoro-2’-ß-C-methyluridine nucleotide prodrug (PSI-7977) for the treatment of hepatitis C virus. J Med Chem 2010; 53:7202–7218.
- Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013; 368:1878–1887.
- Sulkowski MS, Jacobson IM, Ghalib R, et al. Once-daily simeprevir (TMC435) plus sofosbuvir (GS-7977) with or without ribavirin in HCV genotype 1 prior null responders with metavir F0-2: COSMOS study subgroup analysis. 49th EASL, April 2014, London. Oral abstract O7. www.natap.org/2014/EASL/EASL_46.htm. Accesed January 9, 2015.
- Lok AS, Gardiner DF, Lawitz E, et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med 2012; 366:216–224.
- Afdhal N, Zeuzem S, Kwo P, et al; ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014; 370:1889–1898.
- Attia J, Ioannidis JP, Thakkinstian A, et al. How to use an article about genetic association: A: background concepts. JAMA 2009; 301:74–81.
- Samani NJ, Erdmann J, Hall AS, et al; WTCCC and the Cardiogenics Consortium. Genomewide association analysis of coronary artery disease. N Engl J Med 2007; 357:443–453.
- Zeggini E, Weedon MN, Lindgren CM, et al; Wellcome Trust Case Control Consortium (WTCCC). Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 2007; 316:1336–1341.
- Matarín M, Brown WM, Scholz S, et al. A genome-wide genotyping study in patients with ischaemic stroke: initial analysis and data release. Lancet Neurol 2007; 6:414–420.
- Easton DF, Pooley KA, Dunning AM, et al; AOCS Management Group. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature 2007; 447:1087–1093.
- Plenge RM, Seielstad M, Padyukov L, et al. TRAF1-C5 as a risk locus for rheumatoid arthritis—a genomewide study. N Engl J Med 2007; 357:1199–1209.
- Coon KD, Myers AJ, Craig DW, et al. A high-density whole-genome association study reveals that APOE is the major susceptibility gene for sporadic late-onset Alzheimer’s disease. J Clin Psychiatry 2007; 68:613–618.
- Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance (letter). Nature 2009; 461:399–401.
- Ali A, Zein NN. Hepatitis C infection: a systemic disease with extrahepatic manifestations. Cleve Clin J Med 2005; 72:1005-1019.
- Hanouneh IA, Feldstein AE, Lopez R, et al. Clinical significance of metabolic syndrome in the setting of chronic hepatitis C virus infection. Clin Gastroenterol Hepatol 2008; 6:584–589.
- Elgouhari HM, Zein CO, Hanouneh I, Feldstein AE, Zein NN. Diabetes mellitus is associated with impaired response to antiviral therapy in chronic hepatitis C infection. Dig Dis Sci 2009; 54:2699–2705.
- Alkhouri N, Zein NN. Protease inhibitors: silver bullets for chronic hepatitis C infection? Cleve Clin J Med 2012; 79:213–222.
- McHutchison JG, Everson GT, Gordon SC, et al; PROVE1 Study Team. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med 2009; 360:1827–1838.
- Jacobson IM, McHutchison JG, Dusheiko G, et al; ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 2011; 364:2405–2416.
- Jacobson IM, Catlett I, Marcellin P, et al. Telaprevir substantially improved SVR rates across all IL28B genotypes in the ADVANCE trial. J Hepatol 2011; 54(suppl 1):S542–S543.
- Pol S, Aerssens J, Zeuzem S, et al. Limited impact of IL28B genotype on response rates in telaprevir-treated patients with prior treatment failure. J Hepatol 2013; 58:883–889.
- Suppiah V, Moldovan M, Ahlenstiel G, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet 2009; 41:1100–1104.
- Tanaka Y, Nishida N, Sugiyama M, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet 2009; 41:1105–1109.
- Thomas DL, Thio CL, Martin MP, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 2009; 461:798–801.
- Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest 2009; 119:1745–1754.
- Marcello T, Grakoui A, Barba-Spaeth G, et al. Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology 2006; 131:1887–1898.
- Doyle SE, Schreckhise H, Khuu-Duong K, et al. Interleukin-29 uses a type 1 interferon-like program to promote antiviral responses in human hepatocytes. Hepatology 2006; 44:896–906.
- Raglow Z, Thoma-Perry C, Gilroy R, Wan YJ. IL28B genotype and the expression of ISGs in normal liver. Liver Int 2013; 33:991–998.
- Prokunina-Olsson L, Muchmore B, Tang W, et al. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat Genet 2013; 45:164–171.
- Hanouneh IA, Zein NN, Askar M, Lopez R, John B. Interleukin-28B polymorphisms are associated with fibrosing cholestatic hepatitis in recurrent hepatitis C after liver transplantation. Clin Transplant 2012; 26:E335–E336.
- Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet 1997; 349:825–832.
- Thomas DL, Astemborski J, Rai RM, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA 2000; 284:450–456.
- Bochud PY, Cai T, Overbeck K, et al; Swiss Hepatitis C Cohort Study Group. Genotype 3 is associated with accelerated fibrosis progression in chronic hepatitis C. J Hepatol 2009; 51:655–666.
- Marabita F, Aghemo A, De Nicola S, et al. Genetic variation in the interleukin-28B gene is not associated with fibrosis progression in patients with chronic hepatitis C and known date of infection. Hepatology 2011; 54:1127–1134.
- Fabris C, Falleti E, Cussigh A, et al. IL-28B rs12979860 C/T allele distribution in patients with liver cirrhosis: role in the course of chronic viral hepatitis and the development of HCC. J Hepatol 2011; 54:716–722.
- Eurich D, Boas-Knoop S, Bahra M, et al. Role of IL28B polymorphism in the development of hepatitis C virus-induced hepatocellular carcinoma, graft fibrosis, and posttransplant antiviral therapy. Transplantation 2012; 93:644–649.
- Hanouneh IA, Miller C, Aucejo F, Lopez R, Quinn MK, Zein NN. Recurrent hepatitis C after liver transplantation: on-treatment prediction of response to peginterferon/ribavirin therapy. Liver Transpl 2008; 14:53–58.
- Charlton MR, Thompson A, Veldt BJ, et al. Interleukin-28B polymorphisms are associated with histological recurrence and treatment response following liver transplantation in patients with hepatitis C virus infection. Hepatology 2011; 53:317–324.
- Thompson AJ, Muir AJ, Sulkowski MS, et al. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology 2010; 139:120–129.e18.
- Beinhardt S, Payer BA, Datz C, et al. A diagnostic score for the prediction of spontaneous resolution of acute hepatitis C virus infection. J Hepatol 2013; 59:972–977.
- Forns X, Lawitz E, Zeuzem S, et al. Simeprevir with peginterferon and ribavirin leads to high rates of SVR in patients with HCV genotype 1 who relapsed after previous therapy: a phase 3 trial. Gastroenterology 2014; 146:1669–1679.e3.
- Sofia MJ, Bao D, Chang W, et al. Discovery of a ß-d-2’-deoxy-2’-ß-fluoro-2’-ß-C-methyluridine nucleotide prodrug (PSI-7977) for the treatment of hepatitis C virus. J Med Chem 2010; 53:7202–7218.
- Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013; 368:1878–1887.
- Sulkowski MS, Jacobson IM, Ghalib R, et al. Once-daily simeprevir (TMC435) plus sofosbuvir (GS-7977) with or without ribavirin in HCV genotype 1 prior null responders with metavir F0-2: COSMOS study subgroup analysis. 49th EASL, April 2014, London. Oral abstract O7. www.natap.org/2014/EASL/EASL_46.htm. Accesed January 9, 2015.
- Lok AS, Gardiner DF, Lawitz E, et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med 2012; 366:216–224.
- Afdhal N, Zeuzem S, Kwo P, et al; ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014; 370:1889–1898.
KEY POINTS
- In IL28B, the rs12979860 location can be occupied by either cytosine (C) or thymine (T). The CC genotype is more favorable than the CT or TT genotype.
- Testing for the IL28B polymorphism is currently available and allows for better outcomes through proper selection of treatment, particularly with interferon-based treatment.
- Although newer therapies have shifted toward regimens that do not use interferon, the IL28B polymorphism remains clinically significant, especially in light of the potentially prohibitive costs of the newer regimens, and for patients in whom these treatments are contraindicated.
Identifying statin-associated autoimmune necrotizing myopathy
Statins are among the most widely prescribed drugs, as they reduce cardiovascular risk very effectively. They work by inhibiting 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), a key enzyme in cholesterol biosynthesis. Although most patients tolerate statins well, muscle-related toxicity can limit the use of these drugs.
Recently, progressive necrotizing myopathy leading to profound weakness has been directly linked to statin therapy. Proper recognition of this ominous complication is important to prevent further damage from statin use.
STATIN-ASSOCIATED MUSCLE EFFECTS: A SPECTRUM
Muscle symptoms are among the best known and most important side effects of statins, ranging from asymptomatic, mild elevation of creatine kinase and benign myalgias to life-threatening rhabdomyolysis. As the various terms are often used inconsistently in the literature, we will briefly review each entity to put immune-necrotizing myopathy in its proper context on the spectrum of statin-associated muscle symptoms.
Myalgia
Myalgia, ie, muscle pain without elevation of muscle enzymes, is the most common side effect of statins. The incidence is about 10% in observational studies1,2; however, the incidence in the real world of clinical practice seems much higher. Myalgias can present as widespread pain or as localized pain, usually in the lower extremities. Muscle cramps and tendonitis-related pain are commonly reported.
Several clinical predictors of increased risk of statin-associated muscle pain were noted in the Prediction of Muscular Risk in Observational Conditions (PRIMO) study,2 assessing mild to moderate muscular symptoms in patients on high-dose statins. These included a history of muscle pain with another lipid-lowering agent, a history of cramps, a history of elevated creatine kinase levels, a personal or family history of muscle symptoms, untreated hypothyroidism, and a background of fibromyalgia-like symptoms. The incidence of muscle symptoms increased with the level of physical activity, and the median time of symptom onset was 1 month after starting statin therapy or titrating to a high dose.
In patients with myalgia alone, symptoms often improve when the statin is stopped.
Myopathy, myositis
The general terms myopathy and myositis have been used to refer to elevated muscle enzymes together with the muscle symptoms of pain, cramps, soreness, or weakness. An analysis of 21 clinical trials of statin therapy found that myopathy (creatine kinase level more than 10 times the upper limit of normal) occurred in 5 patients per 100,000 person-years.3 The incidence of myopathy increases when statins are used in high doses.
In another cohort of patients seen for statin intolerance,1 conventional risk factors for overt myositis included renal disease, diabetes, and thyroid disease. Electrolyte abnormalities did not differ between statin-tolerant and statin-intolerant patients.1
The search for genetic indicators of risk
In 2008, the Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group conducted a genome-wide association study to identify genetic variants associated with myopathy with high-dose statin therapy.4 Eighty-five patients who had developed definite myopathy (muscle symptoms with enzyme levels more than 10 times the upper limit of normal) or incipient myopathy (asymptomatic or symptomatic, but enzyme levels at least three times the upper limit of normal) while taking simvastatin 80 mg were compared with 90 controls taking the same daily dose. The study reported variants in the SLCO1B1 gene as “strongly associated with an increased risk of statin-associated myopathy.”4
SLCO1B1 encodes the peptide responsible for hepatic uptake of statins, thus affecting the blood level of these drugs. Patients in the study who had the C variant, which predisposes to higher blood levels of statins, were at higher risk of myopathy than those with the T variant. The odds ratio for myopathy increased from 4.4 in heterozygotes for the C allele to 17.4 for homozygotes. This effect was similar even with lower doses of statins.4
We believe that these findings provide a strong basis for genetic testing of patients who may be at risk of statin-associated myopathy.
Rechallenging with a different statin
Often, patients with myopathy can be rechallenged with a different statin agent. In a study done in a lipid clinic, patients identified as having simvastatin-associated myopathy were given another statin.5 Between 15% and 42% tolerated the second statin, with no statistically significant difference between the tolerability rates with the different agents used (atorvastatin, rosuvastatin, pravastatin, and fluvastatin).
Rhabdomyolysis
Rhabdomyolysis, the most devastating complication of statin use, is marked by a creatine kinase level more than 10 times the upper limit of normal or greater than 10,000 IU/L, resulting from acute and massive destruction of muscle fibers and release of their contents into the bloodstream.
But rhabdomyolysis is a clinical syndrome, not solely an alarming increase in muscle enzyme levels. It can include renal failure and death. The rate of occurrence is low (1/100,000), but it can occur at any point in treatment.6 An analysis of Canadian and US case reports of statin-associated rhabdomyolysis showed an average of 824 cases each year.7 A dose-response relationship was observed with higher statin doses.
But statin-associated myopathy may not stop when the drug is stopped
The types of muscle toxicity discussed above stem from direct myotoxic effects of statins and are thought to be related to the blood concentration of the statin.6,8 They may be limited by genetic susceptibility. Mechanisms may include a change in muscle membrane excitability caused by modulation in membrane cholesterol levels, impaired mitochondrial function and calcium signaling, induction of apoptosis, and increased lipid peroxidation. Stopping the offending drug can often halt these downstream effects—hence the dictum that statin myopathy is self-limiting and should resolve with cessation of the drug.
But as we discuss in the following sections, some patients on statin therapy develop an immune-mediated myopathy that does not resolve with discontinuation of the statin and that may only resolve with immunosuppressive therapy.
STATINS AND AUTOIMMUNE NECROTIZING MYOPATHY
At the Johns Hopkins Myositis Center, a group of patients was identified who had necrotizing myopathy on biopsy but no known underlying condition or associated autoantibodies. In an attempt to establish an autoimmune basis for their disease, the sera from 26 patients were screened for novel antibodies.9 Sera from 16 of the patients immunoprecipitated a pair of proteins (sizes 100 kd and 200 kd), indicating these patients had an antibody to these proteins. This finding was highly specific for necrotizing myopathy when compared with controls, ie, other patients with myositis. Patients who had this finding displayed proximal muscle weakness, elevated muscle enzyme levels, and myopathic findings on electromyography; 63% had been exposed to statins before the onset of weakness, and when only patients over age 50 were included, the number rose to 83%.
This association of statins with necrotizing myopathy had been previously noted by two other groups. Needham et al10 described eight patients who, while on statins, developed myopathy that continued to worsen despite cessation of the drugs. An analysis of their muscle pathology revealed myofiber necrosis with little inflammatory infiltrate, as well as widespread up-regulation of expression of major histocompatibility complex class 1. These patients required immunosuppressive treatment (prednisone and methotrexate) to control their disease.
Grable-Esposito et al11 corroborated this finding by identifying 25 additional patients who developed a similar necrotizing myopathy while on statins.11 They also noted a significantly higher frequency of statin use in patients with necrotizing myopathy than in age-matched controls with polymyositis or inclusion-body myositis.
The researchers at the Johns Hopkins Myositis Center noted the similarity between these two patient groups and their own group of patients with necrotizing myopathy. Thus, a follow-up study was done to identify the 100-kg and 200-kd autoantigens observed in their earlier study. Exposure to a statin was found to up-regulate the expression of the two molecules.12 HMGCR was hypothesized as being the 100-kd antigen, because of its 97-kd molecular weight, and also because statin treatment had already been shown to up-regulate the expression of HMGCR.13 The researchers concluded that HMGCR was indeed the 100-kd antigen, with no distinctive antibodies recognizing the 200-kd protein. Although the 200-kd protein was once postulated to be a dimer of the 100-kd protein, its identity remains unknown.
The anti-HMGCR antibody was then screened for in a cohort of 750 myositis patients. The 16 patients previously found to have anti-200/100 were all positive for anti-HMGCR antibody. An additional 45 patients from the cohort (6%) were anti-HMGCR-positive by enzyme-linked immunosorbent assay, and all had necrotizing myopathy. Patients with other types of myopathy, including inflammatory myopathy, do not possess this antibody.12
The HMGCR antibody was quite specific for immune-mediated necrotizing myopathy, and this suggested that statins were capable of triggering an immune-mediated myopathy that is then perpetuated even if the drug is discontinued. As it was also demonstrated that statins increase the expression of HMGCR in muscle as well as in regenerating cells, the process may be sustained through persistently increased HMGCR expression associated with muscle repair.12
The C allele of the SLCO1B1 gene, which has been associated with statin-associated myopathy, was not increased in this population of patients positive for anti-HMGCR. Follow-up studies of the prevalence of anti-HMGCR in statin users in the Atherosclerosis Risk in Communities (ARIC) cohort, including those with self-limited statin myotoxicity, have also shown the absence of this antibody.14 This shows that anti-HMGCR is not found in the majority of statin-exposed patients and is highly specific for autoimmune myopathy. This also suggests that statin-associated autoimmune myopathy represents a pathologic process that is distinct from self-limited statin intolerance.
HOW THE CONDITION PRESENTS
Immune-mediated statin myopathy presents similarly to other idiopathic inflammatory myopathies such as polymyositis (Table 1). Symptoms often develop in a subacute to chronic course and can occur at any time with statin treatment. In one study,10 the average duration of statin use before the onset of weakness was 3 years (range 2 months to 10 years). In some patients whose statin had been stopped because of abnormal creatine kinase levels, weakness developed later, at a range of 0.5 to 20 months. Even low doses of statins (such as 10 mg of simvastatin) have been found to trigger this condition.10
Patients uniformly develop symmetric proximal arm and leg weakness, and distal weakness can also occur.11 Other features have included dysphagia, arthralgias, myalgias, and Raynaud phenomenon.9 Men and women are represented in roughly equal numbers.
The muscle enzymes are strikingly elevated in this disease, with a mean creatine kinase value of 10,333 IU/L at initial presentation.9 Although the creatine kinase level may be very elevated, patients often do not present with weakness until a certain threshold value is reached, in contrast with patients with anti-signal recognition particle necrotizing myopathy, who can present with profound weakness at a lower level. Hence, by the time patients are clinically symptomatic, the process may have been going on for some time. Despite the seemingly massive leak in muscle enzymes, the patients do not develop rhabdomyolysis. Inflammatory markers need not be elevated, and an association with other antibodies such as antinuclear antibody is not often seen.
Magnetic resonance imaging of the thigh has shown muscle edema in all patients.9 In decreasing order of frequency, other findings are atrophy, fatty replacement, and fascial edema.
Electromyography of involved muscle has shown irritable myopathy in most patients (88%) and nonirritable myopathy in a few.
Muscle biopsy studies have shown prominent necrotic and regenerating fibers without significant inflammatory infiltrate.11 There is also myophagocytosis of necrotic fibers and diffuse or focal up-regulation of major histocompatability complex class I expression.10
PATIENTS WITH ANTI-HMGCR WHO HAVE AND WHO HAVE NEVER TAKEN STATINS
When the anti-HMGCR antibody was tested for in the Johns Hopkins cohort,12 33% of patients with a necrotizing myopathy associated with this antibody had never taken a statin. The two groups were clinically indistinguishable, save for a few aspects. Compared with patients who had taken a statin, those who had never taken a statin were younger (mean age 37 vs 59), had higher levels of creatine kinase (13,392 vs 7,881 IU/L), and had a different race distribution (46.7% vs 86.7% white). Initial HMGCR antibody levels were also noted to correlate with creatine kinase levels and strength in statin-exposed patients but not in those who had never taken a statin.15
We hypothesize that in patients who have never taken a statin, other genetic or environmental factors may be the cause of the increased HMGCR expression, which then triggers the autoimmune response. Until further data are gathered, we should probably treat these patients as we treat those who develop this disease after taking a statin, and avoid giving them statins altogether.
MANAGEMENT OF STATIN-ASSOCIATED AUTOIMMUNE NECROTIZING MYOPATHY
Treatment of statin-associated autoimmune necrotizing myopathy can be challenging and requires immunosuppressive drugs.
Statin therapy should be stopped once this condition is suspected. Patients who continue to have elevated muscle enzymes or weakness should undergo further testing with electromyography, magnetic resonance imaging, and muscle biopsy. Electromyography detects myopathy and shows chronicity, distribution, and degree of severity. Although not necessary for diagnosis, magnetic resonance imaging helps to evaluate the extent of muscle involvement and damage and provides guidance when choosing a site for muscle biopsy. Muscle biopsy is necessary to determine the actual pathology and to exclude mimics such as dystrophy or metabolic myopathies.
When an immune-mediated myopathy is confirmed, prompt referral to a rheumatologist or a neuromuscular specialist is recommended.
Steroids are usually the first-line treatment for this disease. Other immunosuppressives, such as methotrexate, azathioprine, mycophenolate mofetil, and rituximab have been used with varying levels of success. In our experience, intravenous immunoglobulin has been particularly beneficial for refractory cases. With treatment, muscle enzyme levels and weakness improve, but relapses can occur. The ideal choice of immunosuppressive therapy and the duration of therapy are currently under investigation.
Rechallenge with another statin
At this time, the issue of rechallenging the patient with another statin has not been clarified. Given the autoimmune nature of the disease, we would avoid exposing the patient to a known trigger. However, this may be a difficult decision in patients with cardiovascular risk factors who require statins for primary or secondary prevention. We suggest using alternative cholesterol-lowering agents first and using them in combination if needed.
We have had some success in maintaining a handful of patients on a statin while treating them concurrently with immunosuppression. This is not ideal because they are constantly being exposed to the likely trigger for their disease, but it may be unavoidable if statins are deemed absolutely necessary. We have also had a patient with known statin-associated immune-mediated necrotizing myopathy who later became profoundly weak after another physician started her on a newer-generation agent, pitavastatin. This suggests to us that rechallenging patients, even with a different statin, can have deleterious effects.
IMPLICATIONS FOR CLINICAL PRACTICE
The true prevalence of statin-associated autoimmune myopathy in practice is unknown. In the Johns Hopkins Myositis Center cohort of patients with suspected myopathy, anti-HMGCR was found in 6% of the patients and was the second most frequent antibody found after anti-Jo1.12
Given the frequency of muscle-related complaints in patients on statins, we recommend obtaining baseline muscle enzyme measurements before starting statin therapy. As recommended by the National Lipid Association Statin Safety Assessment Task Force, the creatine kinase level should be measured when a patient develops muscular complaints, to help gauge the severity of the disease and to help decide whether to continue therapy.8 Random testing of the creatine kinase level in asymptomatic patients is not recommended.
At present, the diagnosis of statin-associated autoimmune necrotizing myopathy is based on a combination of findings—elevated muscle enzyme levels, muscle weakness, irritable findings on electromyography, and necrotizing myopathy on biopsy in a patient on a statin. The finding of the HMGCR antibody confirms the diagnosis. A test for this antibody is now commercially available in the United States. We suggest testing for the antibody in the following scenarios:
- A persistently elevated or rising creatine kinase, aspartate aminotransferase, alanine aminotransferase, or aldolase level after the statin is stopped; although no fixed creatine kinase level has been determined, a level above 1,000 U/L would be a reasonable cutoff at which to test
- Muscle symptoms (proximal or distal weakness) that persist 12 weeks after statin cessation regardless of the creatine kinase level, especially if the patient has dysphagia
- The finding of muscle irritability on electromyography or diffuse muscle edema on magnetic resonance imaging when testing for other myositis-specific antibodies is negative
- Muscle biopsy showing necrotizing myopathy with little or no inflammation.
In addition, since necrotizing myopathy is known to be associated with malignancy and since necrotizing myopathy is more common in older people, who are also more likely to be taking a statin, an age-appropriate malignancy evaluation is warranted as well.
- Harris LJ, Thapa R, Brown M, et al. Clinical and laboratory phenotype of patients experiencing statin intolerance attributable to myalgia. J Clin Lipidol 2011; 5:299–307.
- Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther 2005; 19:403–414.
- Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol 2006; 97:52C–60C.
- SEARCH Collaborative Group; Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med 2008; 359:789–799.
- Fung EC, Crook MA. Statin myopathy: a lipid clinic experience on the tolerability of statin rechallenge. Cardiovasc Ther 2012; 30:e212–e218.
- Sirvent P, Mercier J, Lacampagne A. New insights into mechanisms of statin-associated myotoxicity. Curr Opin Pharmacol 2008; 8:333–338.
- Holbrook A, Wright M, Sung M, Ribic C, Baker S. Statin-associated rhabdomyolysis: is there a dose-response relationship? Can J Cardiol 2011; 27:146–151.
- McKenney JM, Davidson MH, Jacobson TA, Guyton JR; National Lipid Association Statin Safety Assessment Task Force. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol 2006; 97:89C–94C.
- Christopher-Stine L, Casciola-Rosen LA, Hong G, Chung T, Corse AM, Mammen AL. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum 2010; 62:2757–2766.
- Needham M, Fabian V, Knezevic W, Panegyres P, Zilko P, Mastaglia FL. Progressive myopathy with up-regulation of MHC-I associated with statin therapy. Neuromuscul Disord 2007; 17:194–200.
- Grable-Esposito P, Katzberg HD, Greenberg SA, Srinivasan J, Katz J, Amato AA. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve 2010; 41:185–190.
- Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum 2011; 63:713–721.
- Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature 1990; 343:425–430.
- Mammen AL, Pak K, Williams EK, et al. Rarity of anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase antibodies in statin users, including those with self-limited musculoskeletal side effects. Arthritis Care Res (Hoboken) 2012; 64:269–272.
- Werner JL, Christopher-Stine L, Ghazarian SR, et al. Antibody levels correlate with creatine kinase levels and strength in anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Rheum 2012; 64:4087–4093.
Statins are among the most widely prescribed drugs, as they reduce cardiovascular risk very effectively. They work by inhibiting 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), a key enzyme in cholesterol biosynthesis. Although most patients tolerate statins well, muscle-related toxicity can limit the use of these drugs.
Recently, progressive necrotizing myopathy leading to profound weakness has been directly linked to statin therapy. Proper recognition of this ominous complication is important to prevent further damage from statin use.
STATIN-ASSOCIATED MUSCLE EFFECTS: A SPECTRUM
Muscle symptoms are among the best known and most important side effects of statins, ranging from asymptomatic, mild elevation of creatine kinase and benign myalgias to life-threatening rhabdomyolysis. As the various terms are often used inconsistently in the literature, we will briefly review each entity to put immune-necrotizing myopathy in its proper context on the spectrum of statin-associated muscle symptoms.
Myalgia
Myalgia, ie, muscle pain without elevation of muscle enzymes, is the most common side effect of statins. The incidence is about 10% in observational studies1,2; however, the incidence in the real world of clinical practice seems much higher. Myalgias can present as widespread pain or as localized pain, usually in the lower extremities. Muscle cramps and tendonitis-related pain are commonly reported.
Several clinical predictors of increased risk of statin-associated muscle pain were noted in the Prediction of Muscular Risk in Observational Conditions (PRIMO) study,2 assessing mild to moderate muscular symptoms in patients on high-dose statins. These included a history of muscle pain with another lipid-lowering agent, a history of cramps, a history of elevated creatine kinase levels, a personal or family history of muscle symptoms, untreated hypothyroidism, and a background of fibromyalgia-like symptoms. The incidence of muscle symptoms increased with the level of physical activity, and the median time of symptom onset was 1 month after starting statin therapy or titrating to a high dose.
In patients with myalgia alone, symptoms often improve when the statin is stopped.
Myopathy, myositis
The general terms myopathy and myositis have been used to refer to elevated muscle enzymes together with the muscle symptoms of pain, cramps, soreness, or weakness. An analysis of 21 clinical trials of statin therapy found that myopathy (creatine kinase level more than 10 times the upper limit of normal) occurred in 5 patients per 100,000 person-years.3 The incidence of myopathy increases when statins are used in high doses.
In another cohort of patients seen for statin intolerance,1 conventional risk factors for overt myositis included renal disease, diabetes, and thyroid disease. Electrolyte abnormalities did not differ between statin-tolerant and statin-intolerant patients.1
The search for genetic indicators of risk
In 2008, the Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group conducted a genome-wide association study to identify genetic variants associated with myopathy with high-dose statin therapy.4 Eighty-five patients who had developed definite myopathy (muscle symptoms with enzyme levels more than 10 times the upper limit of normal) or incipient myopathy (asymptomatic or symptomatic, but enzyme levels at least three times the upper limit of normal) while taking simvastatin 80 mg were compared with 90 controls taking the same daily dose. The study reported variants in the SLCO1B1 gene as “strongly associated with an increased risk of statin-associated myopathy.”4
SLCO1B1 encodes the peptide responsible for hepatic uptake of statins, thus affecting the blood level of these drugs. Patients in the study who had the C variant, which predisposes to higher blood levels of statins, were at higher risk of myopathy than those with the T variant. The odds ratio for myopathy increased from 4.4 in heterozygotes for the C allele to 17.4 for homozygotes. This effect was similar even with lower doses of statins.4
We believe that these findings provide a strong basis for genetic testing of patients who may be at risk of statin-associated myopathy.
Rechallenging with a different statin
Often, patients with myopathy can be rechallenged with a different statin agent. In a study done in a lipid clinic, patients identified as having simvastatin-associated myopathy were given another statin.5 Between 15% and 42% tolerated the second statin, with no statistically significant difference between the tolerability rates with the different agents used (atorvastatin, rosuvastatin, pravastatin, and fluvastatin).
Rhabdomyolysis
Rhabdomyolysis, the most devastating complication of statin use, is marked by a creatine kinase level more than 10 times the upper limit of normal or greater than 10,000 IU/L, resulting from acute and massive destruction of muscle fibers and release of their contents into the bloodstream.
But rhabdomyolysis is a clinical syndrome, not solely an alarming increase in muscle enzyme levels. It can include renal failure and death. The rate of occurrence is low (1/100,000), but it can occur at any point in treatment.6 An analysis of Canadian and US case reports of statin-associated rhabdomyolysis showed an average of 824 cases each year.7 A dose-response relationship was observed with higher statin doses.
But statin-associated myopathy may not stop when the drug is stopped
The types of muscle toxicity discussed above stem from direct myotoxic effects of statins and are thought to be related to the blood concentration of the statin.6,8 They may be limited by genetic susceptibility. Mechanisms may include a change in muscle membrane excitability caused by modulation in membrane cholesterol levels, impaired mitochondrial function and calcium signaling, induction of apoptosis, and increased lipid peroxidation. Stopping the offending drug can often halt these downstream effects—hence the dictum that statin myopathy is self-limiting and should resolve with cessation of the drug.
But as we discuss in the following sections, some patients on statin therapy develop an immune-mediated myopathy that does not resolve with discontinuation of the statin and that may only resolve with immunosuppressive therapy.
STATINS AND AUTOIMMUNE NECROTIZING MYOPATHY
At the Johns Hopkins Myositis Center, a group of patients was identified who had necrotizing myopathy on biopsy but no known underlying condition or associated autoantibodies. In an attempt to establish an autoimmune basis for their disease, the sera from 26 patients were screened for novel antibodies.9 Sera from 16 of the patients immunoprecipitated a pair of proteins (sizes 100 kd and 200 kd), indicating these patients had an antibody to these proteins. This finding was highly specific for necrotizing myopathy when compared with controls, ie, other patients with myositis. Patients who had this finding displayed proximal muscle weakness, elevated muscle enzyme levels, and myopathic findings on electromyography; 63% had been exposed to statins before the onset of weakness, and when only patients over age 50 were included, the number rose to 83%.
This association of statins with necrotizing myopathy had been previously noted by two other groups. Needham et al10 described eight patients who, while on statins, developed myopathy that continued to worsen despite cessation of the drugs. An analysis of their muscle pathology revealed myofiber necrosis with little inflammatory infiltrate, as well as widespread up-regulation of expression of major histocompatibility complex class 1. These patients required immunosuppressive treatment (prednisone and methotrexate) to control their disease.
Grable-Esposito et al11 corroborated this finding by identifying 25 additional patients who developed a similar necrotizing myopathy while on statins.11 They also noted a significantly higher frequency of statin use in patients with necrotizing myopathy than in age-matched controls with polymyositis or inclusion-body myositis.
The researchers at the Johns Hopkins Myositis Center noted the similarity between these two patient groups and their own group of patients with necrotizing myopathy. Thus, a follow-up study was done to identify the 100-kg and 200-kd autoantigens observed in their earlier study. Exposure to a statin was found to up-regulate the expression of the two molecules.12 HMGCR was hypothesized as being the 100-kd antigen, because of its 97-kd molecular weight, and also because statin treatment had already been shown to up-regulate the expression of HMGCR.13 The researchers concluded that HMGCR was indeed the 100-kd antigen, with no distinctive antibodies recognizing the 200-kd protein. Although the 200-kd protein was once postulated to be a dimer of the 100-kd protein, its identity remains unknown.
The anti-HMGCR antibody was then screened for in a cohort of 750 myositis patients. The 16 patients previously found to have anti-200/100 were all positive for anti-HMGCR antibody. An additional 45 patients from the cohort (6%) were anti-HMGCR-positive by enzyme-linked immunosorbent assay, and all had necrotizing myopathy. Patients with other types of myopathy, including inflammatory myopathy, do not possess this antibody.12
The HMGCR antibody was quite specific for immune-mediated necrotizing myopathy, and this suggested that statins were capable of triggering an immune-mediated myopathy that is then perpetuated even if the drug is discontinued. As it was also demonstrated that statins increase the expression of HMGCR in muscle as well as in regenerating cells, the process may be sustained through persistently increased HMGCR expression associated with muscle repair.12
The C allele of the SLCO1B1 gene, which has been associated with statin-associated myopathy, was not increased in this population of patients positive for anti-HMGCR. Follow-up studies of the prevalence of anti-HMGCR in statin users in the Atherosclerosis Risk in Communities (ARIC) cohort, including those with self-limited statin myotoxicity, have also shown the absence of this antibody.14 This shows that anti-HMGCR is not found in the majority of statin-exposed patients and is highly specific for autoimmune myopathy. This also suggests that statin-associated autoimmune myopathy represents a pathologic process that is distinct from self-limited statin intolerance.
HOW THE CONDITION PRESENTS
Immune-mediated statin myopathy presents similarly to other idiopathic inflammatory myopathies such as polymyositis (Table 1). Symptoms often develop in a subacute to chronic course and can occur at any time with statin treatment. In one study,10 the average duration of statin use before the onset of weakness was 3 years (range 2 months to 10 years). In some patients whose statin had been stopped because of abnormal creatine kinase levels, weakness developed later, at a range of 0.5 to 20 months. Even low doses of statins (such as 10 mg of simvastatin) have been found to trigger this condition.10
Patients uniformly develop symmetric proximal arm and leg weakness, and distal weakness can also occur.11 Other features have included dysphagia, arthralgias, myalgias, and Raynaud phenomenon.9 Men and women are represented in roughly equal numbers.
The muscle enzymes are strikingly elevated in this disease, with a mean creatine kinase value of 10,333 IU/L at initial presentation.9 Although the creatine kinase level may be very elevated, patients often do not present with weakness until a certain threshold value is reached, in contrast with patients with anti-signal recognition particle necrotizing myopathy, who can present with profound weakness at a lower level. Hence, by the time patients are clinically symptomatic, the process may have been going on for some time. Despite the seemingly massive leak in muscle enzymes, the patients do not develop rhabdomyolysis. Inflammatory markers need not be elevated, and an association with other antibodies such as antinuclear antibody is not often seen.
Magnetic resonance imaging of the thigh has shown muscle edema in all patients.9 In decreasing order of frequency, other findings are atrophy, fatty replacement, and fascial edema.
Electromyography of involved muscle has shown irritable myopathy in most patients (88%) and nonirritable myopathy in a few.
Muscle biopsy studies have shown prominent necrotic and regenerating fibers without significant inflammatory infiltrate.11 There is also myophagocytosis of necrotic fibers and diffuse or focal up-regulation of major histocompatability complex class I expression.10
PATIENTS WITH ANTI-HMGCR WHO HAVE AND WHO HAVE NEVER TAKEN STATINS
When the anti-HMGCR antibody was tested for in the Johns Hopkins cohort,12 33% of patients with a necrotizing myopathy associated with this antibody had never taken a statin. The two groups were clinically indistinguishable, save for a few aspects. Compared with patients who had taken a statin, those who had never taken a statin were younger (mean age 37 vs 59), had higher levels of creatine kinase (13,392 vs 7,881 IU/L), and had a different race distribution (46.7% vs 86.7% white). Initial HMGCR antibody levels were also noted to correlate with creatine kinase levels and strength in statin-exposed patients but not in those who had never taken a statin.15
We hypothesize that in patients who have never taken a statin, other genetic or environmental factors may be the cause of the increased HMGCR expression, which then triggers the autoimmune response. Until further data are gathered, we should probably treat these patients as we treat those who develop this disease after taking a statin, and avoid giving them statins altogether.
MANAGEMENT OF STATIN-ASSOCIATED AUTOIMMUNE NECROTIZING MYOPATHY
Treatment of statin-associated autoimmune necrotizing myopathy can be challenging and requires immunosuppressive drugs.
Statin therapy should be stopped once this condition is suspected. Patients who continue to have elevated muscle enzymes or weakness should undergo further testing with electromyography, magnetic resonance imaging, and muscle biopsy. Electromyography detects myopathy and shows chronicity, distribution, and degree of severity. Although not necessary for diagnosis, magnetic resonance imaging helps to evaluate the extent of muscle involvement and damage and provides guidance when choosing a site for muscle biopsy. Muscle biopsy is necessary to determine the actual pathology and to exclude mimics such as dystrophy or metabolic myopathies.
When an immune-mediated myopathy is confirmed, prompt referral to a rheumatologist or a neuromuscular specialist is recommended.
Steroids are usually the first-line treatment for this disease. Other immunosuppressives, such as methotrexate, azathioprine, mycophenolate mofetil, and rituximab have been used with varying levels of success. In our experience, intravenous immunoglobulin has been particularly beneficial for refractory cases. With treatment, muscle enzyme levels and weakness improve, but relapses can occur. The ideal choice of immunosuppressive therapy and the duration of therapy are currently under investigation.
Rechallenge with another statin
At this time, the issue of rechallenging the patient with another statin has not been clarified. Given the autoimmune nature of the disease, we would avoid exposing the patient to a known trigger. However, this may be a difficult decision in patients with cardiovascular risk factors who require statins for primary or secondary prevention. We suggest using alternative cholesterol-lowering agents first and using them in combination if needed.
We have had some success in maintaining a handful of patients on a statin while treating them concurrently with immunosuppression. This is not ideal because they are constantly being exposed to the likely trigger for their disease, but it may be unavoidable if statins are deemed absolutely necessary. We have also had a patient with known statin-associated immune-mediated necrotizing myopathy who later became profoundly weak after another physician started her on a newer-generation agent, pitavastatin. This suggests to us that rechallenging patients, even with a different statin, can have deleterious effects.
IMPLICATIONS FOR CLINICAL PRACTICE
The true prevalence of statin-associated autoimmune myopathy in practice is unknown. In the Johns Hopkins Myositis Center cohort of patients with suspected myopathy, anti-HMGCR was found in 6% of the patients and was the second most frequent antibody found after anti-Jo1.12
Given the frequency of muscle-related complaints in patients on statins, we recommend obtaining baseline muscle enzyme measurements before starting statin therapy. As recommended by the National Lipid Association Statin Safety Assessment Task Force, the creatine kinase level should be measured when a patient develops muscular complaints, to help gauge the severity of the disease and to help decide whether to continue therapy.8 Random testing of the creatine kinase level in asymptomatic patients is not recommended.
At present, the diagnosis of statin-associated autoimmune necrotizing myopathy is based on a combination of findings—elevated muscle enzyme levels, muscle weakness, irritable findings on electromyography, and necrotizing myopathy on biopsy in a patient on a statin. The finding of the HMGCR antibody confirms the diagnosis. A test for this antibody is now commercially available in the United States. We suggest testing for the antibody in the following scenarios:
- A persistently elevated or rising creatine kinase, aspartate aminotransferase, alanine aminotransferase, or aldolase level after the statin is stopped; although no fixed creatine kinase level has been determined, a level above 1,000 U/L would be a reasonable cutoff at which to test
- Muscle symptoms (proximal or distal weakness) that persist 12 weeks after statin cessation regardless of the creatine kinase level, especially if the patient has dysphagia
- The finding of muscle irritability on electromyography or diffuse muscle edema on magnetic resonance imaging when testing for other myositis-specific antibodies is negative
- Muscle biopsy showing necrotizing myopathy with little or no inflammation.
In addition, since necrotizing myopathy is known to be associated with malignancy and since necrotizing myopathy is more common in older people, who are also more likely to be taking a statin, an age-appropriate malignancy evaluation is warranted as well.
Statins are among the most widely prescribed drugs, as they reduce cardiovascular risk very effectively. They work by inhibiting 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), a key enzyme in cholesterol biosynthesis. Although most patients tolerate statins well, muscle-related toxicity can limit the use of these drugs.
Recently, progressive necrotizing myopathy leading to profound weakness has been directly linked to statin therapy. Proper recognition of this ominous complication is important to prevent further damage from statin use.
STATIN-ASSOCIATED MUSCLE EFFECTS: A SPECTRUM
Muscle symptoms are among the best known and most important side effects of statins, ranging from asymptomatic, mild elevation of creatine kinase and benign myalgias to life-threatening rhabdomyolysis. As the various terms are often used inconsistently in the literature, we will briefly review each entity to put immune-necrotizing myopathy in its proper context on the spectrum of statin-associated muscle symptoms.
Myalgia
Myalgia, ie, muscle pain without elevation of muscle enzymes, is the most common side effect of statins. The incidence is about 10% in observational studies1,2; however, the incidence in the real world of clinical practice seems much higher. Myalgias can present as widespread pain or as localized pain, usually in the lower extremities. Muscle cramps and tendonitis-related pain are commonly reported.
Several clinical predictors of increased risk of statin-associated muscle pain were noted in the Prediction of Muscular Risk in Observational Conditions (PRIMO) study,2 assessing mild to moderate muscular symptoms in patients on high-dose statins. These included a history of muscle pain with another lipid-lowering agent, a history of cramps, a history of elevated creatine kinase levels, a personal or family history of muscle symptoms, untreated hypothyroidism, and a background of fibromyalgia-like symptoms. The incidence of muscle symptoms increased with the level of physical activity, and the median time of symptom onset was 1 month after starting statin therapy or titrating to a high dose.
In patients with myalgia alone, symptoms often improve when the statin is stopped.
Myopathy, myositis
The general terms myopathy and myositis have been used to refer to elevated muscle enzymes together with the muscle symptoms of pain, cramps, soreness, or weakness. An analysis of 21 clinical trials of statin therapy found that myopathy (creatine kinase level more than 10 times the upper limit of normal) occurred in 5 patients per 100,000 person-years.3 The incidence of myopathy increases when statins are used in high doses.
In another cohort of patients seen for statin intolerance,1 conventional risk factors for overt myositis included renal disease, diabetes, and thyroid disease. Electrolyte abnormalities did not differ between statin-tolerant and statin-intolerant patients.1
The search for genetic indicators of risk
In 2008, the Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group conducted a genome-wide association study to identify genetic variants associated with myopathy with high-dose statin therapy.4 Eighty-five patients who had developed definite myopathy (muscle symptoms with enzyme levels more than 10 times the upper limit of normal) or incipient myopathy (asymptomatic or symptomatic, but enzyme levels at least three times the upper limit of normal) while taking simvastatin 80 mg were compared with 90 controls taking the same daily dose. The study reported variants in the SLCO1B1 gene as “strongly associated with an increased risk of statin-associated myopathy.”4
SLCO1B1 encodes the peptide responsible for hepatic uptake of statins, thus affecting the blood level of these drugs. Patients in the study who had the C variant, which predisposes to higher blood levels of statins, were at higher risk of myopathy than those with the T variant. The odds ratio for myopathy increased from 4.4 in heterozygotes for the C allele to 17.4 for homozygotes. This effect was similar even with lower doses of statins.4
We believe that these findings provide a strong basis for genetic testing of patients who may be at risk of statin-associated myopathy.
Rechallenging with a different statin
Often, patients with myopathy can be rechallenged with a different statin agent. In a study done in a lipid clinic, patients identified as having simvastatin-associated myopathy were given another statin.5 Between 15% and 42% tolerated the second statin, with no statistically significant difference between the tolerability rates with the different agents used (atorvastatin, rosuvastatin, pravastatin, and fluvastatin).
Rhabdomyolysis
Rhabdomyolysis, the most devastating complication of statin use, is marked by a creatine kinase level more than 10 times the upper limit of normal or greater than 10,000 IU/L, resulting from acute and massive destruction of muscle fibers and release of their contents into the bloodstream.
But rhabdomyolysis is a clinical syndrome, not solely an alarming increase in muscle enzyme levels. It can include renal failure and death. The rate of occurrence is low (1/100,000), but it can occur at any point in treatment.6 An analysis of Canadian and US case reports of statin-associated rhabdomyolysis showed an average of 824 cases each year.7 A dose-response relationship was observed with higher statin doses.
But statin-associated myopathy may not stop when the drug is stopped
The types of muscle toxicity discussed above stem from direct myotoxic effects of statins and are thought to be related to the blood concentration of the statin.6,8 They may be limited by genetic susceptibility. Mechanisms may include a change in muscle membrane excitability caused by modulation in membrane cholesterol levels, impaired mitochondrial function and calcium signaling, induction of apoptosis, and increased lipid peroxidation. Stopping the offending drug can often halt these downstream effects—hence the dictum that statin myopathy is self-limiting and should resolve with cessation of the drug.
But as we discuss in the following sections, some patients on statin therapy develop an immune-mediated myopathy that does not resolve with discontinuation of the statin and that may only resolve with immunosuppressive therapy.
STATINS AND AUTOIMMUNE NECROTIZING MYOPATHY
At the Johns Hopkins Myositis Center, a group of patients was identified who had necrotizing myopathy on biopsy but no known underlying condition or associated autoantibodies. In an attempt to establish an autoimmune basis for their disease, the sera from 26 patients were screened for novel antibodies.9 Sera from 16 of the patients immunoprecipitated a pair of proteins (sizes 100 kd and 200 kd), indicating these patients had an antibody to these proteins. This finding was highly specific for necrotizing myopathy when compared with controls, ie, other patients with myositis. Patients who had this finding displayed proximal muscle weakness, elevated muscle enzyme levels, and myopathic findings on electromyography; 63% had been exposed to statins before the onset of weakness, and when only patients over age 50 were included, the number rose to 83%.
This association of statins with necrotizing myopathy had been previously noted by two other groups. Needham et al10 described eight patients who, while on statins, developed myopathy that continued to worsen despite cessation of the drugs. An analysis of their muscle pathology revealed myofiber necrosis with little inflammatory infiltrate, as well as widespread up-regulation of expression of major histocompatibility complex class 1. These patients required immunosuppressive treatment (prednisone and methotrexate) to control their disease.
Grable-Esposito et al11 corroborated this finding by identifying 25 additional patients who developed a similar necrotizing myopathy while on statins.11 They also noted a significantly higher frequency of statin use in patients with necrotizing myopathy than in age-matched controls with polymyositis or inclusion-body myositis.
The researchers at the Johns Hopkins Myositis Center noted the similarity between these two patient groups and their own group of patients with necrotizing myopathy. Thus, a follow-up study was done to identify the 100-kg and 200-kd autoantigens observed in their earlier study. Exposure to a statin was found to up-regulate the expression of the two molecules.12 HMGCR was hypothesized as being the 100-kd antigen, because of its 97-kd molecular weight, and also because statin treatment had already been shown to up-regulate the expression of HMGCR.13 The researchers concluded that HMGCR was indeed the 100-kd antigen, with no distinctive antibodies recognizing the 200-kd protein. Although the 200-kd protein was once postulated to be a dimer of the 100-kd protein, its identity remains unknown.
The anti-HMGCR antibody was then screened for in a cohort of 750 myositis patients. The 16 patients previously found to have anti-200/100 were all positive for anti-HMGCR antibody. An additional 45 patients from the cohort (6%) were anti-HMGCR-positive by enzyme-linked immunosorbent assay, and all had necrotizing myopathy. Patients with other types of myopathy, including inflammatory myopathy, do not possess this antibody.12
The HMGCR antibody was quite specific for immune-mediated necrotizing myopathy, and this suggested that statins were capable of triggering an immune-mediated myopathy that is then perpetuated even if the drug is discontinued. As it was also demonstrated that statins increase the expression of HMGCR in muscle as well as in regenerating cells, the process may be sustained through persistently increased HMGCR expression associated with muscle repair.12
The C allele of the SLCO1B1 gene, which has been associated with statin-associated myopathy, was not increased in this population of patients positive for anti-HMGCR. Follow-up studies of the prevalence of anti-HMGCR in statin users in the Atherosclerosis Risk in Communities (ARIC) cohort, including those with self-limited statin myotoxicity, have also shown the absence of this antibody.14 This shows that anti-HMGCR is not found in the majority of statin-exposed patients and is highly specific for autoimmune myopathy. This also suggests that statin-associated autoimmune myopathy represents a pathologic process that is distinct from self-limited statin intolerance.
HOW THE CONDITION PRESENTS
Immune-mediated statin myopathy presents similarly to other idiopathic inflammatory myopathies such as polymyositis (Table 1). Symptoms often develop in a subacute to chronic course and can occur at any time with statin treatment. In one study,10 the average duration of statin use before the onset of weakness was 3 years (range 2 months to 10 years). In some patients whose statin had been stopped because of abnormal creatine kinase levels, weakness developed later, at a range of 0.5 to 20 months. Even low doses of statins (such as 10 mg of simvastatin) have been found to trigger this condition.10
Patients uniformly develop symmetric proximal arm and leg weakness, and distal weakness can also occur.11 Other features have included dysphagia, arthralgias, myalgias, and Raynaud phenomenon.9 Men and women are represented in roughly equal numbers.
The muscle enzymes are strikingly elevated in this disease, with a mean creatine kinase value of 10,333 IU/L at initial presentation.9 Although the creatine kinase level may be very elevated, patients often do not present with weakness until a certain threshold value is reached, in contrast with patients with anti-signal recognition particle necrotizing myopathy, who can present with profound weakness at a lower level. Hence, by the time patients are clinically symptomatic, the process may have been going on for some time. Despite the seemingly massive leak in muscle enzymes, the patients do not develop rhabdomyolysis. Inflammatory markers need not be elevated, and an association with other antibodies such as antinuclear antibody is not often seen.
Magnetic resonance imaging of the thigh has shown muscle edema in all patients.9 In decreasing order of frequency, other findings are atrophy, fatty replacement, and fascial edema.
Electromyography of involved muscle has shown irritable myopathy in most patients (88%) and nonirritable myopathy in a few.
Muscle biopsy studies have shown prominent necrotic and regenerating fibers without significant inflammatory infiltrate.11 There is also myophagocytosis of necrotic fibers and diffuse or focal up-regulation of major histocompatability complex class I expression.10
PATIENTS WITH ANTI-HMGCR WHO HAVE AND WHO HAVE NEVER TAKEN STATINS
When the anti-HMGCR antibody was tested for in the Johns Hopkins cohort,12 33% of patients with a necrotizing myopathy associated with this antibody had never taken a statin. The two groups were clinically indistinguishable, save for a few aspects. Compared with patients who had taken a statin, those who had never taken a statin were younger (mean age 37 vs 59), had higher levels of creatine kinase (13,392 vs 7,881 IU/L), and had a different race distribution (46.7% vs 86.7% white). Initial HMGCR antibody levels were also noted to correlate with creatine kinase levels and strength in statin-exposed patients but not in those who had never taken a statin.15
We hypothesize that in patients who have never taken a statin, other genetic or environmental factors may be the cause of the increased HMGCR expression, which then triggers the autoimmune response. Until further data are gathered, we should probably treat these patients as we treat those who develop this disease after taking a statin, and avoid giving them statins altogether.
MANAGEMENT OF STATIN-ASSOCIATED AUTOIMMUNE NECROTIZING MYOPATHY
Treatment of statin-associated autoimmune necrotizing myopathy can be challenging and requires immunosuppressive drugs.
Statin therapy should be stopped once this condition is suspected. Patients who continue to have elevated muscle enzymes or weakness should undergo further testing with electromyography, magnetic resonance imaging, and muscle biopsy. Electromyography detects myopathy and shows chronicity, distribution, and degree of severity. Although not necessary for diagnosis, magnetic resonance imaging helps to evaluate the extent of muscle involvement and damage and provides guidance when choosing a site for muscle biopsy. Muscle biopsy is necessary to determine the actual pathology and to exclude mimics such as dystrophy or metabolic myopathies.
When an immune-mediated myopathy is confirmed, prompt referral to a rheumatologist or a neuromuscular specialist is recommended.
Steroids are usually the first-line treatment for this disease. Other immunosuppressives, such as methotrexate, azathioprine, mycophenolate mofetil, and rituximab have been used with varying levels of success. In our experience, intravenous immunoglobulin has been particularly beneficial for refractory cases. With treatment, muscle enzyme levels and weakness improve, but relapses can occur. The ideal choice of immunosuppressive therapy and the duration of therapy are currently under investigation.
Rechallenge with another statin
At this time, the issue of rechallenging the patient with another statin has not been clarified. Given the autoimmune nature of the disease, we would avoid exposing the patient to a known trigger. However, this may be a difficult decision in patients with cardiovascular risk factors who require statins for primary or secondary prevention. We suggest using alternative cholesterol-lowering agents first and using them in combination if needed.
We have had some success in maintaining a handful of patients on a statin while treating them concurrently with immunosuppression. This is not ideal because they are constantly being exposed to the likely trigger for their disease, but it may be unavoidable if statins are deemed absolutely necessary. We have also had a patient with known statin-associated immune-mediated necrotizing myopathy who later became profoundly weak after another physician started her on a newer-generation agent, pitavastatin. This suggests to us that rechallenging patients, even with a different statin, can have deleterious effects.
IMPLICATIONS FOR CLINICAL PRACTICE
The true prevalence of statin-associated autoimmune myopathy in practice is unknown. In the Johns Hopkins Myositis Center cohort of patients with suspected myopathy, anti-HMGCR was found in 6% of the patients and was the second most frequent antibody found after anti-Jo1.12
Given the frequency of muscle-related complaints in patients on statins, we recommend obtaining baseline muscle enzyme measurements before starting statin therapy. As recommended by the National Lipid Association Statin Safety Assessment Task Force, the creatine kinase level should be measured when a patient develops muscular complaints, to help gauge the severity of the disease and to help decide whether to continue therapy.8 Random testing of the creatine kinase level in asymptomatic patients is not recommended.
At present, the diagnosis of statin-associated autoimmune necrotizing myopathy is based on a combination of findings—elevated muscle enzyme levels, muscle weakness, irritable findings on electromyography, and necrotizing myopathy on biopsy in a patient on a statin. The finding of the HMGCR antibody confirms the diagnosis. A test for this antibody is now commercially available in the United States. We suggest testing for the antibody in the following scenarios:
- A persistently elevated or rising creatine kinase, aspartate aminotransferase, alanine aminotransferase, or aldolase level after the statin is stopped; although no fixed creatine kinase level has been determined, a level above 1,000 U/L would be a reasonable cutoff at which to test
- Muscle symptoms (proximal or distal weakness) that persist 12 weeks after statin cessation regardless of the creatine kinase level, especially if the patient has dysphagia
- The finding of muscle irritability on electromyography or diffuse muscle edema on magnetic resonance imaging when testing for other myositis-specific antibodies is negative
- Muscle biopsy showing necrotizing myopathy with little or no inflammation.
In addition, since necrotizing myopathy is known to be associated with malignancy and since necrotizing myopathy is more common in older people, who are also more likely to be taking a statin, an age-appropriate malignancy evaluation is warranted as well.
- Harris LJ, Thapa R, Brown M, et al. Clinical and laboratory phenotype of patients experiencing statin intolerance attributable to myalgia. J Clin Lipidol 2011; 5:299–307.
- Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther 2005; 19:403–414.
- Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol 2006; 97:52C–60C.
- SEARCH Collaborative Group; Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med 2008; 359:789–799.
- Fung EC, Crook MA. Statin myopathy: a lipid clinic experience on the tolerability of statin rechallenge. Cardiovasc Ther 2012; 30:e212–e218.
- Sirvent P, Mercier J, Lacampagne A. New insights into mechanisms of statin-associated myotoxicity. Curr Opin Pharmacol 2008; 8:333–338.
- Holbrook A, Wright M, Sung M, Ribic C, Baker S. Statin-associated rhabdomyolysis: is there a dose-response relationship? Can J Cardiol 2011; 27:146–151.
- McKenney JM, Davidson MH, Jacobson TA, Guyton JR; National Lipid Association Statin Safety Assessment Task Force. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol 2006; 97:89C–94C.
- Christopher-Stine L, Casciola-Rosen LA, Hong G, Chung T, Corse AM, Mammen AL. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum 2010; 62:2757–2766.
- Needham M, Fabian V, Knezevic W, Panegyres P, Zilko P, Mastaglia FL. Progressive myopathy with up-regulation of MHC-I associated with statin therapy. Neuromuscul Disord 2007; 17:194–200.
- Grable-Esposito P, Katzberg HD, Greenberg SA, Srinivasan J, Katz J, Amato AA. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve 2010; 41:185–190.
- Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum 2011; 63:713–721.
- Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature 1990; 343:425–430.
- Mammen AL, Pak K, Williams EK, et al. Rarity of anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase antibodies in statin users, including those with self-limited musculoskeletal side effects. Arthritis Care Res (Hoboken) 2012; 64:269–272.
- Werner JL, Christopher-Stine L, Ghazarian SR, et al. Antibody levels correlate with creatine kinase levels and strength in anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Rheum 2012; 64:4087–4093.
- Harris LJ, Thapa R, Brown M, et al. Clinical and laboratory phenotype of patients experiencing statin intolerance attributable to myalgia. J Clin Lipidol 2011; 5:299–307.
- Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther 2005; 19:403–414.
- Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol 2006; 97:52C–60C.
- SEARCH Collaborative Group; Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med 2008; 359:789–799.
- Fung EC, Crook MA. Statin myopathy: a lipid clinic experience on the tolerability of statin rechallenge. Cardiovasc Ther 2012; 30:e212–e218.
- Sirvent P, Mercier J, Lacampagne A. New insights into mechanisms of statin-associated myotoxicity. Curr Opin Pharmacol 2008; 8:333–338.
- Holbrook A, Wright M, Sung M, Ribic C, Baker S. Statin-associated rhabdomyolysis: is there a dose-response relationship? Can J Cardiol 2011; 27:146–151.
- McKenney JM, Davidson MH, Jacobson TA, Guyton JR; National Lipid Association Statin Safety Assessment Task Force. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol 2006; 97:89C–94C.
- Christopher-Stine L, Casciola-Rosen LA, Hong G, Chung T, Corse AM, Mammen AL. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum 2010; 62:2757–2766.
- Needham M, Fabian V, Knezevic W, Panegyres P, Zilko P, Mastaglia FL. Progressive myopathy with up-regulation of MHC-I associated with statin therapy. Neuromuscul Disord 2007; 17:194–200.
- Grable-Esposito P, Katzberg HD, Greenberg SA, Srinivasan J, Katz J, Amato AA. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve 2010; 41:185–190.
- Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum 2011; 63:713–721.
- Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature 1990; 343:425–430.
- Mammen AL, Pak K, Williams EK, et al. Rarity of anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase antibodies in statin users, including those with self-limited musculoskeletal side effects. Arthritis Care Res (Hoboken) 2012; 64:269–272.
- Werner JL, Christopher-Stine L, Ghazarian SR, et al. Antibody levels correlate with creatine kinase levels and strength in anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Rheum 2012; 64:4087–4093.
KEY POINTS
- Most cases of muscle symptoms associated with statin use are a direct effect of the statin on the muscle and resolve after the statin is discontinued.
- In contrast to simple myalgia or myositis, statin-associated autoimmune necrotizing myopathy can persist or even arise de novo after the statin is stopped.
- This condition presents with symmetric proximal arm and leg weakness and striking elevations of muscle enzymes such as creatine kinase.
- Treatment can be challenging and requires immunosuppressive drugs; referral to a specialist is recommended.
- Statin therapy should be discontinued once this condition is suspected. Patients who continue to have elevated muscle enzymes or weakness should undergo further testing with electromyography, magnetic resonance imaging, and muscle biopsy.
Is antibiotic treatment indicated in a patient with a positive urine culture but no symptoms?
The 2005 Infectious Diseases Society of America (IDSA) guidelines1 recommend screening pregnant women and patients who will undergo an invasive urologic procedure with a urine culture and treating them with antibiotics if bacteriuria is significant. The IDSA recommends against screening for or treating asymptomatic bacteriuria in other populations.
WHAT IS ASYMPTOMATIC BACTERIURIA?
A positive urine culture can represent three different conditions:
- Symptomatic urinary tract infection
- Contamination of the sample by organisms that are present distal to the bladder and that enter the urine at the time the specimen is collected
- Asymptomatic bacteriuria, defined as the isolation of a specified quantitative count of a single uropathogen in an appropriately collected urine specimen obtained from someone without symptoms or signs attributable to a urinary tract infection (Table 1). It represents the true presence of bacteria in the bladder and may be thought of as a state of colonization.
HOW COMMON IS ASYMPTOMATIC BACTERIURIA?
Rates vary depending on the age (higher in older persons), sex (higher in women), and presence of genitourinary abnormalities of the population studied. Prevalence rates are estimated to be 1% to 5% in healthy premenopausal women, 2% to 9% in pregnant women, 9% to 27% in diabetic women, 15% to 50% in elderly men and women in long-term care facilities, and 28% in patients undergoing hemodialysis.2–6 In patients with an indwelling urinary catheter, the rate goes up by 3% to 8% per day, and bacteriuria is nearly universal at 30 days.7,8 Asymptomatic bacteriuria can be transient, as commonly occurs in healthy young women, or it may be more prolonged, as commonly occurs in elderly patients or those with a chronic indwelling urinary catheter.
WHOM SHOULD WE SCREEN?
Screening for asymptomatic bacteriuria and treating it are strongly recommended (grade A-I recommendation) in pregnant women and in men who will undergo transurethral resection of the prostate.
Pregnant women have a risk of pyelonephritis 20 to 30 times higher if they have asymptomatic bacteriuria.9 Cohort studies and randomized clinical trials have consistently reported significant reductions in rates of pyelonephritis and low birth weight when antibiotic therapy is given for asymptomatic bacteriuria during pregnancy.
The ideal time to screen for this in pregnancy is between the 9th and 16th weeks of gestation. The appropriate screening test is a urine culture, since screening for pyuria has a low sensitivity and specificity. The choice of antibiotic is based on the results of culture. Antibiotics that have been safely used in these patients include nitrofurantoin, cephalexin, amoxicillin, and fosfomycin.10 The recommended treatment duration is between 3 and 7 days. Periodic screening for recurrent bacteriuria should be performed during the remainder of the pregnancy.
Men about to undergo transurethral resection of the prostate1 who have asymptomatic bacteriuria before the procedure have a 60% rate of bacteremia and a 6% to 10% rate of sepsis after the procedure if they do not receive antibiotic therapy. Clinical trials have documented significant reductions in these complications when antimicrobial therapy is given before the procedure.
The optimal time for obtaining the urine culture, the optimal time for starting antimicrobial therapy, and the optimal duration of antimicrobial therapy are not well defined, although some data support giving antibiotics the night before or just before the procedure.
The recommendation has been extrapolated to include not only men undergoing transurethral resection of the prostate but also any patient undergoing a urologic procedure associated with significant mucosal bleeding.
Women with catheter-acquired asymptomatic bacteriuria. If the bacteriuria persists 48 hours after catheter removal, the IDSA guidelines state that antibiotic therapy may be considered (grade B-I recommendation). However, there are no recommendations to screen women 48 hours after catheter removal.
WHAT IS THE EVIDENCE FOR NO TREATMENT?
Asymptomatic bacteriuria should not be screened for or treated in:
- Premenopausal women who are not pregnant (grade A-I recommendation)
- Diabetic women (A-I)
- Older persons residing in the community (A-II)
- Elderly residents of long-term care facilities (A-I)
- Patients with spinal cord injury (A-I)
- Patients with an indwelling urethral catheter (A-I).
Randomized controlled trials comparing antibiotic therapy with no therapy in these groups showed no benefit of antibiotic treatment in reducing the frequency of symptomatic urinary tract infection11–16 and no decrease in rates of fever or reinfection in patients with a long-term catheter.17 Moreover, in a number of trials,12,14,17 antibiotic therapy for asymptomatic bacteriuria was associated with an increase in adverse antimicrobial effects and reinfection with resistant organisms.
In transplant recipients. Because of lack of evidence, the 2005 IDSA guidelines could not make a recommendation for or against screening for or treatment of asymptomatic bacteriuria in renal transplant or other solid-organ transplant recipients (C-III). A more recent review18 noted a lack of consensus as to whether asymptomatic bacteriuria should be treated in renal transplant recipients. Based on available data, the authors recommended limiting routine screening for it to the first 1 to 3 months after renal transplantation and limiting treatment to 5 to 7 days, using the narrowest-spectrum antibiotic available.18
In prosthetic joint recipients. The 2005 IDSA guidelines recommended further research to determine if screening and treatment before surgical procedures with prosthetic implantation have clinical benefit.
Since then, two studies19,20 have suggested no benefit of screening or treatment before prosthetic joint implantation. Rates of prosthetic joint infection were not different in patients with asymptomatic bacteriuria before hip arthroplasty randomized to receive no antibiotic therapy vs those receiving antibiotic therapy specific for organisms cultured from the urine.19 Asymptomatic bacteriuria was found to be an independent risk factor for prosthetic joint infection.20 However, rates of joint infection were not different in those treated with antibiotics than in those not treated, and in no case were the microorganisms isolated in the prosthetic joint infection the same as in their preoperative urine culture.20
The authors concluded that asymptomatic bacteriuria may be a surrogate marker for increased risk of infection, but that preoperative antibiotic treatment was not beneficial.20
WHAT DOES 'ASYMPTOMATIC' MEAN?
According to the definition, asymptomatic refers to patients who do not have symptoms or signs attributable to a urinary tract infection. Thus, in patients who have symptoms or signs clearly attributable to another condition, screening with urine culture testing and treatment are not indicated. In nursing home residents, nonspecific symptoms such as a change in mental status, fever, and leukocytosis should not automatically be attributed to a positive urine culture without a careful evaluation for another cause, given the high prevalence of asymptomatic bacteriuria in this population.21 Screening with urine culture testing in this population is also not recommended for isolated foul-smelling or cloudy urine, after every urethral catheter change, upon admission, or after treatment to document cure.22
Finally, pyuria (defined as the presence of at least 5 to 10 white blood cells per high-power field) is not by itself a reason to perform a urine culture or to treat a positive urine culture, since pyuria is common in asymptomatic bacteriuria, as well as in other conditions associated with inflammation in the genitourinary system.1
TAKE-HOME POINTS
- Screening for and treating asymptomatic bacteriuria is recommended for pregnant women and for patients about to undergo an invasive urologic procedure associated with significant mucosal injury
- Screening and treatment are not recommended for premenopausal nonpregnant women, diabetic women, older persons residing in the community, elderly residents of long-term care facilities, patients with spinal cord injury, or patients with an indwelling urethral catheter.
- A urine culture should not be ordered, but if it is ordered, a positive culture should not be treated in a patient whose symptoms are attributable to another cause.
- Pyuria is not helpful in distinguishing symptomatic from asymptomatic bacteriuria.
- Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005; 40:643–654.
- Hooton TM, Scholes D, Stapleton AE, et al. A prospective study of asymptomatic bacteriuria in sexually active young women. N Engl J Med 2000; 343:992–997.
- Whalley P. Bacteriuria of pregnancy. Am J Obstet Gynecol 1967; 97:723–738.
- Zhanel GG, Nicolle LE, Harding GK. Prevalence of asymptomatic bacteriuria and associated host factors in women with diabetes mellitus. The Manitoba Diabetic Urinary Infection Study Group. Clin Infect Dis 1995; 21:316–322.
- Nicolle LE. Asymptomatic bacteriuria in the elderly. Infect Dis Clin North Am 1997; 11:647–662.
- Chaudhry A, Stone WJ, Breyer JA. Occurrence of pyuria and bacteriuria in asymptomatic hemodialysis patients. Am J Kidney Dis 1993; 21:180–183.
- Garibaldi RA, Burke JP, Dickman ML, Smith CB. Factors predisposing to bacteriuria during indwelling urethral catheterization. N Engl J Med 1974; 291:215–219.
- Warren JW, Tenney JH, Hoopes JM, Muncie HL, Anthony WC. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis 1982; 146:719–723.
- Smaill F, Vazquez JC. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev 2007; 2:CD000490.
- Guinto VT, De Guia B, Festin MR, Dowswell T. Different antibiotic regimens for treating asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev 2010; 9:CD007855.
- Asscher AW, Sussman M, Waters WE, et al. Asymptomatic significant bacteriuria in the non-pregnant woman. II. Response to treatment and follow-up. Br Med J 1969; 1:804–806.
- Harding GK, Zhanel GG, Nicolle LE, Cheang M; Manitoba Diabetes Urinary Tract Infection Study Group. Antimicrobial treatment in diabetic women with asymptomatic bacteriuria. N Engl J Med 2002; 347:1576–1583.
- Boscia JA, Kobasa WD, Knight RA, Abrutyn E, Levison ME, Kaye D. Therapy vs no therapy for bacteriuria in elderly ambulatory nonhospitalized women. JAMA 1987; 257:1067–1071.
- Nicolle LE, Mayhew WJ, Bryan L. Prospective randomized comparison of therapy and no therapy for asymptomatic bacteriuria in institutionalized elderly women. Am J Med 1987; 83:27–33.
- Nicolle LE, Bjornson J, Harding GK, MacDonell JA. Bacteriuria in elderly institutionalized men. N Engl J Med 1983; 309:1420–1425
- Mohler JL, Cowen DL, Flanigan RC. Suppression and treatment of urinary tract infection in patients with an intermittently catheterized neurogenic bladder. J Urol 1987; 138:336–340.
- Warren JW, Anthony WC, Hoopes JM, Muncie HL Jr. Cephalexin for susceptible bacteriuria in afebrile, long-term catheterized patients. JAMA 1982; 248:454–458.
- Parasuraman R, Julian K; AST Infectious Diseases Community of Practice. Urinary tract infections in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):327–336.
- Cordero-Ampuero J, González-Fernández E, Martínez-Vélez D, Esteban J. Are antibiotics necessary in hip arthroplasty with asymptomatic bacteriuria? Seeding risk with/without treatment. Clin Orthop Relat Res 2013; 471:3822–3829.
- Sousa R, Muñoz-Mahamud E, Quayle J, et al. Is asymptomatic bacteriuria a risk factor for prosthetic joint infection? Clin Infect Dis 2014: ciu235. Epub ahead of print.
- Orr PH, Nicolle LE, Duckworth H, et al. Febrile urinary infection in the institutionalized elderly. Am J Med 1996; 100:71–77.
- Zabarsky TF, Sethi AK, Donskey CJ. Sustained reduction in inappropriate treatment of asymptomatic bacteriuria in a long-term care facility through an educational intervention. Am J Infect Control 2008; 36:476–480.
The 2005 Infectious Diseases Society of America (IDSA) guidelines1 recommend screening pregnant women and patients who will undergo an invasive urologic procedure with a urine culture and treating them with antibiotics if bacteriuria is significant. The IDSA recommends against screening for or treating asymptomatic bacteriuria in other populations.
WHAT IS ASYMPTOMATIC BACTERIURIA?
A positive urine culture can represent three different conditions:
- Symptomatic urinary tract infection
- Contamination of the sample by organisms that are present distal to the bladder and that enter the urine at the time the specimen is collected
- Asymptomatic bacteriuria, defined as the isolation of a specified quantitative count of a single uropathogen in an appropriately collected urine specimen obtained from someone without symptoms or signs attributable to a urinary tract infection (Table 1). It represents the true presence of bacteria in the bladder and may be thought of as a state of colonization.
HOW COMMON IS ASYMPTOMATIC BACTERIURIA?
Rates vary depending on the age (higher in older persons), sex (higher in women), and presence of genitourinary abnormalities of the population studied. Prevalence rates are estimated to be 1% to 5% in healthy premenopausal women, 2% to 9% in pregnant women, 9% to 27% in diabetic women, 15% to 50% in elderly men and women in long-term care facilities, and 28% in patients undergoing hemodialysis.2–6 In patients with an indwelling urinary catheter, the rate goes up by 3% to 8% per day, and bacteriuria is nearly universal at 30 days.7,8 Asymptomatic bacteriuria can be transient, as commonly occurs in healthy young women, or it may be more prolonged, as commonly occurs in elderly patients or those with a chronic indwelling urinary catheter.
WHOM SHOULD WE SCREEN?
Screening for asymptomatic bacteriuria and treating it are strongly recommended (grade A-I recommendation) in pregnant women and in men who will undergo transurethral resection of the prostate.
Pregnant women have a risk of pyelonephritis 20 to 30 times higher if they have asymptomatic bacteriuria.9 Cohort studies and randomized clinical trials have consistently reported significant reductions in rates of pyelonephritis and low birth weight when antibiotic therapy is given for asymptomatic bacteriuria during pregnancy.
The ideal time to screen for this in pregnancy is between the 9th and 16th weeks of gestation. The appropriate screening test is a urine culture, since screening for pyuria has a low sensitivity and specificity. The choice of antibiotic is based on the results of culture. Antibiotics that have been safely used in these patients include nitrofurantoin, cephalexin, amoxicillin, and fosfomycin.10 The recommended treatment duration is between 3 and 7 days. Periodic screening for recurrent bacteriuria should be performed during the remainder of the pregnancy.
Men about to undergo transurethral resection of the prostate1 who have asymptomatic bacteriuria before the procedure have a 60% rate of bacteremia and a 6% to 10% rate of sepsis after the procedure if they do not receive antibiotic therapy. Clinical trials have documented significant reductions in these complications when antimicrobial therapy is given before the procedure.
The optimal time for obtaining the urine culture, the optimal time for starting antimicrobial therapy, and the optimal duration of antimicrobial therapy are not well defined, although some data support giving antibiotics the night before or just before the procedure.
The recommendation has been extrapolated to include not only men undergoing transurethral resection of the prostate but also any patient undergoing a urologic procedure associated with significant mucosal bleeding.
Women with catheter-acquired asymptomatic bacteriuria. If the bacteriuria persists 48 hours after catheter removal, the IDSA guidelines state that antibiotic therapy may be considered (grade B-I recommendation). However, there are no recommendations to screen women 48 hours after catheter removal.
WHAT IS THE EVIDENCE FOR NO TREATMENT?
Asymptomatic bacteriuria should not be screened for or treated in:
- Premenopausal women who are not pregnant (grade A-I recommendation)
- Diabetic women (A-I)
- Older persons residing in the community (A-II)
- Elderly residents of long-term care facilities (A-I)
- Patients with spinal cord injury (A-I)
- Patients with an indwelling urethral catheter (A-I).
Randomized controlled trials comparing antibiotic therapy with no therapy in these groups showed no benefit of antibiotic treatment in reducing the frequency of symptomatic urinary tract infection11–16 and no decrease in rates of fever or reinfection in patients with a long-term catheter.17 Moreover, in a number of trials,12,14,17 antibiotic therapy for asymptomatic bacteriuria was associated with an increase in adverse antimicrobial effects and reinfection with resistant organisms.
In transplant recipients. Because of lack of evidence, the 2005 IDSA guidelines could not make a recommendation for or against screening for or treatment of asymptomatic bacteriuria in renal transplant or other solid-organ transplant recipients (C-III). A more recent review18 noted a lack of consensus as to whether asymptomatic bacteriuria should be treated in renal transplant recipients. Based on available data, the authors recommended limiting routine screening for it to the first 1 to 3 months after renal transplantation and limiting treatment to 5 to 7 days, using the narrowest-spectrum antibiotic available.18
In prosthetic joint recipients. The 2005 IDSA guidelines recommended further research to determine if screening and treatment before surgical procedures with prosthetic implantation have clinical benefit.
Since then, two studies19,20 have suggested no benefit of screening or treatment before prosthetic joint implantation. Rates of prosthetic joint infection were not different in patients with asymptomatic bacteriuria before hip arthroplasty randomized to receive no antibiotic therapy vs those receiving antibiotic therapy specific for organisms cultured from the urine.19 Asymptomatic bacteriuria was found to be an independent risk factor for prosthetic joint infection.20 However, rates of joint infection were not different in those treated with antibiotics than in those not treated, and in no case were the microorganisms isolated in the prosthetic joint infection the same as in their preoperative urine culture.20
The authors concluded that asymptomatic bacteriuria may be a surrogate marker for increased risk of infection, but that preoperative antibiotic treatment was not beneficial.20
WHAT DOES 'ASYMPTOMATIC' MEAN?
According to the definition, asymptomatic refers to patients who do not have symptoms or signs attributable to a urinary tract infection. Thus, in patients who have symptoms or signs clearly attributable to another condition, screening with urine culture testing and treatment are not indicated. In nursing home residents, nonspecific symptoms such as a change in mental status, fever, and leukocytosis should not automatically be attributed to a positive urine culture without a careful evaluation for another cause, given the high prevalence of asymptomatic bacteriuria in this population.21 Screening with urine culture testing in this population is also not recommended for isolated foul-smelling or cloudy urine, after every urethral catheter change, upon admission, or after treatment to document cure.22
Finally, pyuria (defined as the presence of at least 5 to 10 white blood cells per high-power field) is not by itself a reason to perform a urine culture or to treat a positive urine culture, since pyuria is common in asymptomatic bacteriuria, as well as in other conditions associated with inflammation in the genitourinary system.1
TAKE-HOME POINTS
- Screening for and treating asymptomatic bacteriuria is recommended for pregnant women and for patients about to undergo an invasive urologic procedure associated with significant mucosal injury
- Screening and treatment are not recommended for premenopausal nonpregnant women, diabetic women, older persons residing in the community, elderly residents of long-term care facilities, patients with spinal cord injury, or patients with an indwelling urethral catheter.
- A urine culture should not be ordered, but if it is ordered, a positive culture should not be treated in a patient whose symptoms are attributable to another cause.
- Pyuria is not helpful in distinguishing symptomatic from asymptomatic bacteriuria.
The 2005 Infectious Diseases Society of America (IDSA) guidelines1 recommend screening pregnant women and patients who will undergo an invasive urologic procedure with a urine culture and treating them with antibiotics if bacteriuria is significant. The IDSA recommends against screening for or treating asymptomatic bacteriuria in other populations.
WHAT IS ASYMPTOMATIC BACTERIURIA?
A positive urine culture can represent three different conditions:
- Symptomatic urinary tract infection
- Contamination of the sample by organisms that are present distal to the bladder and that enter the urine at the time the specimen is collected
- Asymptomatic bacteriuria, defined as the isolation of a specified quantitative count of a single uropathogen in an appropriately collected urine specimen obtained from someone without symptoms or signs attributable to a urinary tract infection (Table 1). It represents the true presence of bacteria in the bladder and may be thought of as a state of colonization.
HOW COMMON IS ASYMPTOMATIC BACTERIURIA?
Rates vary depending on the age (higher in older persons), sex (higher in women), and presence of genitourinary abnormalities of the population studied. Prevalence rates are estimated to be 1% to 5% in healthy premenopausal women, 2% to 9% in pregnant women, 9% to 27% in diabetic women, 15% to 50% in elderly men and women in long-term care facilities, and 28% in patients undergoing hemodialysis.2–6 In patients with an indwelling urinary catheter, the rate goes up by 3% to 8% per day, and bacteriuria is nearly universal at 30 days.7,8 Asymptomatic bacteriuria can be transient, as commonly occurs in healthy young women, or it may be more prolonged, as commonly occurs in elderly patients or those with a chronic indwelling urinary catheter.
WHOM SHOULD WE SCREEN?
Screening for asymptomatic bacteriuria and treating it are strongly recommended (grade A-I recommendation) in pregnant women and in men who will undergo transurethral resection of the prostate.
Pregnant women have a risk of pyelonephritis 20 to 30 times higher if they have asymptomatic bacteriuria.9 Cohort studies and randomized clinical trials have consistently reported significant reductions in rates of pyelonephritis and low birth weight when antibiotic therapy is given for asymptomatic bacteriuria during pregnancy.
The ideal time to screen for this in pregnancy is between the 9th and 16th weeks of gestation. The appropriate screening test is a urine culture, since screening for pyuria has a low sensitivity and specificity. The choice of antibiotic is based on the results of culture. Antibiotics that have been safely used in these patients include nitrofurantoin, cephalexin, amoxicillin, and fosfomycin.10 The recommended treatment duration is between 3 and 7 days. Periodic screening for recurrent bacteriuria should be performed during the remainder of the pregnancy.
Men about to undergo transurethral resection of the prostate1 who have asymptomatic bacteriuria before the procedure have a 60% rate of bacteremia and a 6% to 10% rate of sepsis after the procedure if they do not receive antibiotic therapy. Clinical trials have documented significant reductions in these complications when antimicrobial therapy is given before the procedure.
The optimal time for obtaining the urine culture, the optimal time for starting antimicrobial therapy, and the optimal duration of antimicrobial therapy are not well defined, although some data support giving antibiotics the night before or just before the procedure.
The recommendation has been extrapolated to include not only men undergoing transurethral resection of the prostate but also any patient undergoing a urologic procedure associated with significant mucosal bleeding.
Women with catheter-acquired asymptomatic bacteriuria. If the bacteriuria persists 48 hours after catheter removal, the IDSA guidelines state that antibiotic therapy may be considered (grade B-I recommendation). However, there are no recommendations to screen women 48 hours after catheter removal.
WHAT IS THE EVIDENCE FOR NO TREATMENT?
Asymptomatic bacteriuria should not be screened for or treated in:
- Premenopausal women who are not pregnant (grade A-I recommendation)
- Diabetic women (A-I)
- Older persons residing in the community (A-II)
- Elderly residents of long-term care facilities (A-I)
- Patients with spinal cord injury (A-I)
- Patients with an indwelling urethral catheter (A-I).
Randomized controlled trials comparing antibiotic therapy with no therapy in these groups showed no benefit of antibiotic treatment in reducing the frequency of symptomatic urinary tract infection11–16 and no decrease in rates of fever or reinfection in patients with a long-term catheter.17 Moreover, in a number of trials,12,14,17 antibiotic therapy for asymptomatic bacteriuria was associated with an increase in adverse antimicrobial effects and reinfection with resistant organisms.
In transplant recipients. Because of lack of evidence, the 2005 IDSA guidelines could not make a recommendation for or against screening for or treatment of asymptomatic bacteriuria in renal transplant or other solid-organ transplant recipients (C-III). A more recent review18 noted a lack of consensus as to whether asymptomatic bacteriuria should be treated in renal transplant recipients. Based on available data, the authors recommended limiting routine screening for it to the first 1 to 3 months after renal transplantation and limiting treatment to 5 to 7 days, using the narrowest-spectrum antibiotic available.18
In prosthetic joint recipients. The 2005 IDSA guidelines recommended further research to determine if screening and treatment before surgical procedures with prosthetic implantation have clinical benefit.
Since then, two studies19,20 have suggested no benefit of screening or treatment before prosthetic joint implantation. Rates of prosthetic joint infection were not different in patients with asymptomatic bacteriuria before hip arthroplasty randomized to receive no antibiotic therapy vs those receiving antibiotic therapy specific for organisms cultured from the urine.19 Asymptomatic bacteriuria was found to be an independent risk factor for prosthetic joint infection.20 However, rates of joint infection were not different in those treated with antibiotics than in those not treated, and in no case were the microorganisms isolated in the prosthetic joint infection the same as in their preoperative urine culture.20
The authors concluded that asymptomatic bacteriuria may be a surrogate marker for increased risk of infection, but that preoperative antibiotic treatment was not beneficial.20
WHAT DOES 'ASYMPTOMATIC' MEAN?
According to the definition, asymptomatic refers to patients who do not have symptoms or signs attributable to a urinary tract infection. Thus, in patients who have symptoms or signs clearly attributable to another condition, screening with urine culture testing and treatment are not indicated. In nursing home residents, nonspecific symptoms such as a change in mental status, fever, and leukocytosis should not automatically be attributed to a positive urine culture without a careful evaluation for another cause, given the high prevalence of asymptomatic bacteriuria in this population.21 Screening with urine culture testing in this population is also not recommended for isolated foul-smelling or cloudy urine, after every urethral catheter change, upon admission, or after treatment to document cure.22
Finally, pyuria (defined as the presence of at least 5 to 10 white blood cells per high-power field) is not by itself a reason to perform a urine culture or to treat a positive urine culture, since pyuria is common in asymptomatic bacteriuria, as well as in other conditions associated with inflammation in the genitourinary system.1
TAKE-HOME POINTS
- Screening for and treating asymptomatic bacteriuria is recommended for pregnant women and for patients about to undergo an invasive urologic procedure associated with significant mucosal injury
- Screening and treatment are not recommended for premenopausal nonpregnant women, diabetic women, older persons residing in the community, elderly residents of long-term care facilities, patients with spinal cord injury, or patients with an indwelling urethral catheter.
- A urine culture should not be ordered, but if it is ordered, a positive culture should not be treated in a patient whose symptoms are attributable to another cause.
- Pyuria is not helpful in distinguishing symptomatic from asymptomatic bacteriuria.
- Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005; 40:643–654.
- Hooton TM, Scholes D, Stapleton AE, et al. A prospective study of asymptomatic bacteriuria in sexually active young women. N Engl J Med 2000; 343:992–997.
- Whalley P. Bacteriuria of pregnancy. Am J Obstet Gynecol 1967; 97:723–738.
- Zhanel GG, Nicolle LE, Harding GK. Prevalence of asymptomatic bacteriuria and associated host factors in women with diabetes mellitus. The Manitoba Diabetic Urinary Infection Study Group. Clin Infect Dis 1995; 21:316–322.
- Nicolle LE. Asymptomatic bacteriuria in the elderly. Infect Dis Clin North Am 1997; 11:647–662.
- Chaudhry A, Stone WJ, Breyer JA. Occurrence of pyuria and bacteriuria in asymptomatic hemodialysis patients. Am J Kidney Dis 1993; 21:180–183.
- Garibaldi RA, Burke JP, Dickman ML, Smith CB. Factors predisposing to bacteriuria during indwelling urethral catheterization. N Engl J Med 1974; 291:215–219.
- Warren JW, Tenney JH, Hoopes JM, Muncie HL, Anthony WC. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis 1982; 146:719–723.
- Smaill F, Vazquez JC. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev 2007; 2:CD000490.
- Guinto VT, De Guia B, Festin MR, Dowswell T. Different antibiotic regimens for treating asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev 2010; 9:CD007855.
- Asscher AW, Sussman M, Waters WE, et al. Asymptomatic significant bacteriuria in the non-pregnant woman. II. Response to treatment and follow-up. Br Med J 1969; 1:804–806.
- Harding GK, Zhanel GG, Nicolle LE, Cheang M; Manitoba Diabetes Urinary Tract Infection Study Group. Antimicrobial treatment in diabetic women with asymptomatic bacteriuria. N Engl J Med 2002; 347:1576–1583.
- Boscia JA, Kobasa WD, Knight RA, Abrutyn E, Levison ME, Kaye D. Therapy vs no therapy for bacteriuria in elderly ambulatory nonhospitalized women. JAMA 1987; 257:1067–1071.
- Nicolle LE, Mayhew WJ, Bryan L. Prospective randomized comparison of therapy and no therapy for asymptomatic bacteriuria in institutionalized elderly women. Am J Med 1987; 83:27–33.
- Nicolle LE, Bjornson J, Harding GK, MacDonell JA. Bacteriuria in elderly institutionalized men. N Engl J Med 1983; 309:1420–1425
- Mohler JL, Cowen DL, Flanigan RC. Suppression and treatment of urinary tract infection in patients with an intermittently catheterized neurogenic bladder. J Urol 1987; 138:336–340.
- Warren JW, Anthony WC, Hoopes JM, Muncie HL Jr. Cephalexin for susceptible bacteriuria in afebrile, long-term catheterized patients. JAMA 1982; 248:454–458.
- Parasuraman R, Julian K; AST Infectious Diseases Community of Practice. Urinary tract infections in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):327–336.
- Cordero-Ampuero J, González-Fernández E, Martínez-Vélez D, Esteban J. Are antibiotics necessary in hip arthroplasty with asymptomatic bacteriuria? Seeding risk with/without treatment. Clin Orthop Relat Res 2013; 471:3822–3829.
- Sousa R, Muñoz-Mahamud E, Quayle J, et al. Is asymptomatic bacteriuria a risk factor for prosthetic joint infection? Clin Infect Dis 2014: ciu235. Epub ahead of print.
- Orr PH, Nicolle LE, Duckworth H, et al. Febrile urinary infection in the institutionalized elderly. Am J Med 1996; 100:71–77.
- Zabarsky TF, Sethi AK, Donskey CJ. Sustained reduction in inappropriate treatment of asymptomatic bacteriuria in a long-term care facility through an educational intervention. Am J Infect Control 2008; 36:476–480.
- Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005; 40:643–654.
- Hooton TM, Scholes D, Stapleton AE, et al. A prospective study of asymptomatic bacteriuria in sexually active young women. N Engl J Med 2000; 343:992–997.
- Whalley P. Bacteriuria of pregnancy. Am J Obstet Gynecol 1967; 97:723–738.
- Zhanel GG, Nicolle LE, Harding GK. Prevalence of asymptomatic bacteriuria and associated host factors in women with diabetes mellitus. The Manitoba Diabetic Urinary Infection Study Group. Clin Infect Dis 1995; 21:316–322.
- Nicolle LE. Asymptomatic bacteriuria in the elderly. Infect Dis Clin North Am 1997; 11:647–662.
- Chaudhry A, Stone WJ, Breyer JA. Occurrence of pyuria and bacteriuria in asymptomatic hemodialysis patients. Am J Kidney Dis 1993; 21:180–183.
- Garibaldi RA, Burke JP, Dickman ML, Smith CB. Factors predisposing to bacteriuria during indwelling urethral catheterization. N Engl J Med 1974; 291:215–219.
- Warren JW, Tenney JH, Hoopes JM, Muncie HL, Anthony WC. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis 1982; 146:719–723.
- Smaill F, Vazquez JC. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev 2007; 2:CD000490.
- Guinto VT, De Guia B, Festin MR, Dowswell T. Different antibiotic regimens for treating asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev 2010; 9:CD007855.
- Asscher AW, Sussman M, Waters WE, et al. Asymptomatic significant bacteriuria in the non-pregnant woman. II. Response to treatment and follow-up. Br Med J 1969; 1:804–806.
- Harding GK, Zhanel GG, Nicolle LE, Cheang M; Manitoba Diabetes Urinary Tract Infection Study Group. Antimicrobial treatment in diabetic women with asymptomatic bacteriuria. N Engl J Med 2002; 347:1576–1583.
- Boscia JA, Kobasa WD, Knight RA, Abrutyn E, Levison ME, Kaye D. Therapy vs no therapy for bacteriuria in elderly ambulatory nonhospitalized women. JAMA 1987; 257:1067–1071.
- Nicolle LE, Mayhew WJ, Bryan L. Prospective randomized comparison of therapy and no therapy for asymptomatic bacteriuria in institutionalized elderly women. Am J Med 1987; 83:27–33.
- Nicolle LE, Bjornson J, Harding GK, MacDonell JA. Bacteriuria in elderly institutionalized men. N Engl J Med 1983; 309:1420–1425
- Mohler JL, Cowen DL, Flanigan RC. Suppression and treatment of urinary tract infection in patients with an intermittently catheterized neurogenic bladder. J Urol 1987; 138:336–340.
- Warren JW, Anthony WC, Hoopes JM, Muncie HL Jr. Cephalexin for susceptible bacteriuria in afebrile, long-term catheterized patients. JAMA 1982; 248:454–458.
- Parasuraman R, Julian K; AST Infectious Diseases Community of Practice. Urinary tract infections in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):327–336.
- Cordero-Ampuero J, González-Fernández E, Martínez-Vélez D, Esteban J. Are antibiotics necessary in hip arthroplasty with asymptomatic bacteriuria? Seeding risk with/without treatment. Clin Orthop Relat Res 2013; 471:3822–3829.
- Sousa R, Muñoz-Mahamud E, Quayle J, et al. Is asymptomatic bacteriuria a risk factor for prosthetic joint infection? Clin Infect Dis 2014: ciu235. Epub ahead of print.
- Orr PH, Nicolle LE, Duckworth H, et al. Febrile urinary infection in the institutionalized elderly. Am J Med 1996; 100:71–77.
- Zabarsky TF, Sethi AK, Donskey CJ. Sustained reduction in inappropriate treatment of asymptomatic bacteriuria in a long-term care facility through an educational intervention. Am J Infect Control 2008; 36:476–480.