User login
Gynecologic surgeries linked with persistent opioid use
– showing that persistent opioid use can follow such surgeries.
For a study published in Obstetrics & Gynecology, Jason D. Wright, MD, of Columbia University, New York, and colleagues looked at insurance claims data from 729,625 opioid-naive women, median age 44 years, who had undergone a myomectomy; a minimally invasive, vaginal, or abdominal hysterectomy; an open or laparoscopic oophorectomy; endometrial ablation; tubal ligation; or dilation and curettage. The vast majority of subjects, 93%, had commercial health insurance, with the rest enrolled in Medicaid. Women undergoing multiple surgical procedures, with serious comorbidities, or who underwent another surgery within 6 months of the initial one, were excluded from the analysis.
Dr. Wright and colleagues found that 60% of patients in the cohort received an initial opioid prescription in the perioperative period. Additional opioids were then prescribed to 6.8% (P less than .001) of those women between 90 and 180 days after surgery. The rate of additional prescriptions varied by year across the study period, from 2009 to 2016, and declined to 6% by the final year of the study. The rate of further opioid prescriptions varied according to procedure: 4.8% for myomectomy, 6.6% for minimally invasive hysterectomy, 6.7% for abdominal hysterectomy, 6.3% for endometrial ablation, 7% for tubal ligation, and 7.2% for dilation and curettage (P less than .001).
Factors significantly increasing likelihood of a new prescription included younger age and a history of depression, anxiety, or a substance abuse disorder. Also, a higher total dose of opioids initially prescribed, and a greater number of days supplied, were associated with increased risk for an additional prescription.
“These data demonstrate that the rate of new persistent opioid use after common gynecologic procedures is substantial,” Dr. Wright and colleagues wrote in their analysis, noting that prior studies across a wide range of surgeries have shown rates of new persistent opioid use to be between 3% and 8%. “Careful risk assessment of patients preoperatively may be useful to mitigate opioid misuse in high risk populations,” the investigators wrote. “Women with underlying psychosocial disorders, medical comorbidities, or a history of substance use disorder are at particular risk for persistent opioid use and should be prescribed opioids with extra caution.”
Dr. Wright and colleagues’ study “provides powerful data that should cause gynecological surgeons to pause when writing an opioid prescription,” David M. Jaspan, DO, chairman of obstetrics and gynecology at Einstein Medical Center, Philadelphia, said in an interview. “Is an opioid the best first line medication for this patient? Would an NSAID work better? Is multimodal medication an option? What are the patient characteristics that may be associated with persistent use?”
Dr. Wright and colleagues noted among the study’s limitations the fact that actual opioid use could not be measured, nor could use of nonopioid painkillers.
Dr. Wright has served as a consultant for Tesaro and Clovis Oncology. Dr. Alfred I. Neugut disclosed relationships with various pharmaceutical firms. Dr. Dawn L. Hershman received a grant from the Breast Cancer Research Foundation/Conquer Cancer Foundation. The remaining coauthors had no relevant financial disclosures.
SOURCE: Wright JD et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003358.
– showing that persistent opioid use can follow such surgeries.
For a study published in Obstetrics & Gynecology, Jason D. Wright, MD, of Columbia University, New York, and colleagues looked at insurance claims data from 729,625 opioid-naive women, median age 44 years, who had undergone a myomectomy; a minimally invasive, vaginal, or abdominal hysterectomy; an open or laparoscopic oophorectomy; endometrial ablation; tubal ligation; or dilation and curettage. The vast majority of subjects, 93%, had commercial health insurance, with the rest enrolled in Medicaid. Women undergoing multiple surgical procedures, with serious comorbidities, or who underwent another surgery within 6 months of the initial one, were excluded from the analysis.
Dr. Wright and colleagues found that 60% of patients in the cohort received an initial opioid prescription in the perioperative period. Additional opioids were then prescribed to 6.8% (P less than .001) of those women between 90 and 180 days after surgery. The rate of additional prescriptions varied by year across the study period, from 2009 to 2016, and declined to 6% by the final year of the study. The rate of further opioid prescriptions varied according to procedure: 4.8% for myomectomy, 6.6% for minimally invasive hysterectomy, 6.7% for abdominal hysterectomy, 6.3% for endometrial ablation, 7% for tubal ligation, and 7.2% for dilation and curettage (P less than .001).
Factors significantly increasing likelihood of a new prescription included younger age and a history of depression, anxiety, or a substance abuse disorder. Also, a higher total dose of opioids initially prescribed, and a greater number of days supplied, were associated with increased risk for an additional prescription.
“These data demonstrate that the rate of new persistent opioid use after common gynecologic procedures is substantial,” Dr. Wright and colleagues wrote in their analysis, noting that prior studies across a wide range of surgeries have shown rates of new persistent opioid use to be between 3% and 8%. “Careful risk assessment of patients preoperatively may be useful to mitigate opioid misuse in high risk populations,” the investigators wrote. “Women with underlying psychosocial disorders, medical comorbidities, or a history of substance use disorder are at particular risk for persistent opioid use and should be prescribed opioids with extra caution.”
Dr. Wright and colleagues’ study “provides powerful data that should cause gynecological surgeons to pause when writing an opioid prescription,” David M. Jaspan, DO, chairman of obstetrics and gynecology at Einstein Medical Center, Philadelphia, said in an interview. “Is an opioid the best first line medication for this patient? Would an NSAID work better? Is multimodal medication an option? What are the patient characteristics that may be associated with persistent use?”
Dr. Wright and colleagues noted among the study’s limitations the fact that actual opioid use could not be measured, nor could use of nonopioid painkillers.
Dr. Wright has served as a consultant for Tesaro and Clovis Oncology. Dr. Alfred I. Neugut disclosed relationships with various pharmaceutical firms. Dr. Dawn L. Hershman received a grant from the Breast Cancer Research Foundation/Conquer Cancer Foundation. The remaining coauthors had no relevant financial disclosures.
SOURCE: Wright JD et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003358.
– showing that persistent opioid use can follow such surgeries.
For a study published in Obstetrics & Gynecology, Jason D. Wright, MD, of Columbia University, New York, and colleagues looked at insurance claims data from 729,625 opioid-naive women, median age 44 years, who had undergone a myomectomy; a minimally invasive, vaginal, or abdominal hysterectomy; an open or laparoscopic oophorectomy; endometrial ablation; tubal ligation; or dilation and curettage. The vast majority of subjects, 93%, had commercial health insurance, with the rest enrolled in Medicaid. Women undergoing multiple surgical procedures, with serious comorbidities, or who underwent another surgery within 6 months of the initial one, were excluded from the analysis.
Dr. Wright and colleagues found that 60% of patients in the cohort received an initial opioid prescription in the perioperative period. Additional opioids were then prescribed to 6.8% (P less than .001) of those women between 90 and 180 days after surgery. The rate of additional prescriptions varied by year across the study period, from 2009 to 2016, and declined to 6% by the final year of the study. The rate of further opioid prescriptions varied according to procedure: 4.8% for myomectomy, 6.6% for minimally invasive hysterectomy, 6.7% for abdominal hysterectomy, 6.3% for endometrial ablation, 7% for tubal ligation, and 7.2% for dilation and curettage (P less than .001).
Factors significantly increasing likelihood of a new prescription included younger age and a history of depression, anxiety, or a substance abuse disorder. Also, a higher total dose of opioids initially prescribed, and a greater number of days supplied, were associated with increased risk for an additional prescription.
“These data demonstrate that the rate of new persistent opioid use after common gynecologic procedures is substantial,” Dr. Wright and colleagues wrote in their analysis, noting that prior studies across a wide range of surgeries have shown rates of new persistent opioid use to be between 3% and 8%. “Careful risk assessment of patients preoperatively may be useful to mitigate opioid misuse in high risk populations,” the investigators wrote. “Women with underlying psychosocial disorders, medical comorbidities, or a history of substance use disorder are at particular risk for persistent opioid use and should be prescribed opioids with extra caution.”
Dr. Wright and colleagues’ study “provides powerful data that should cause gynecological surgeons to pause when writing an opioid prescription,” David M. Jaspan, DO, chairman of obstetrics and gynecology at Einstein Medical Center, Philadelphia, said in an interview. “Is an opioid the best first line medication for this patient? Would an NSAID work better? Is multimodal medication an option? What are the patient characteristics that may be associated with persistent use?”
Dr. Wright and colleagues noted among the study’s limitations the fact that actual opioid use could not be measured, nor could use of nonopioid painkillers.
Dr. Wright has served as a consultant for Tesaro and Clovis Oncology. Dr. Alfred I. Neugut disclosed relationships with various pharmaceutical firms. Dr. Dawn L. Hershman received a grant from the Breast Cancer Research Foundation/Conquer Cancer Foundation. The remaining coauthors had no relevant financial disclosures.
SOURCE: Wright JD et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003358.
FROM OBSTETRICS & GYNECOLOGY
Nearly 20% of migraineurs use opioids for migraine
PHILADELPHIA – People with 4 or more migraine headache days per month are more likely to use opioids, compared with people with fewer migraine headache days per month, researchers said. Opioid use for migraine “remains alarmingly high,” the investigators said at the annual meeting of the American Headache Society.
Although opioid use for the treatment of migraine typically is discouraged, studies indicate that it is common. Evidence suggests that opioids may increase the risk of progression from episodic to chronic migraine.
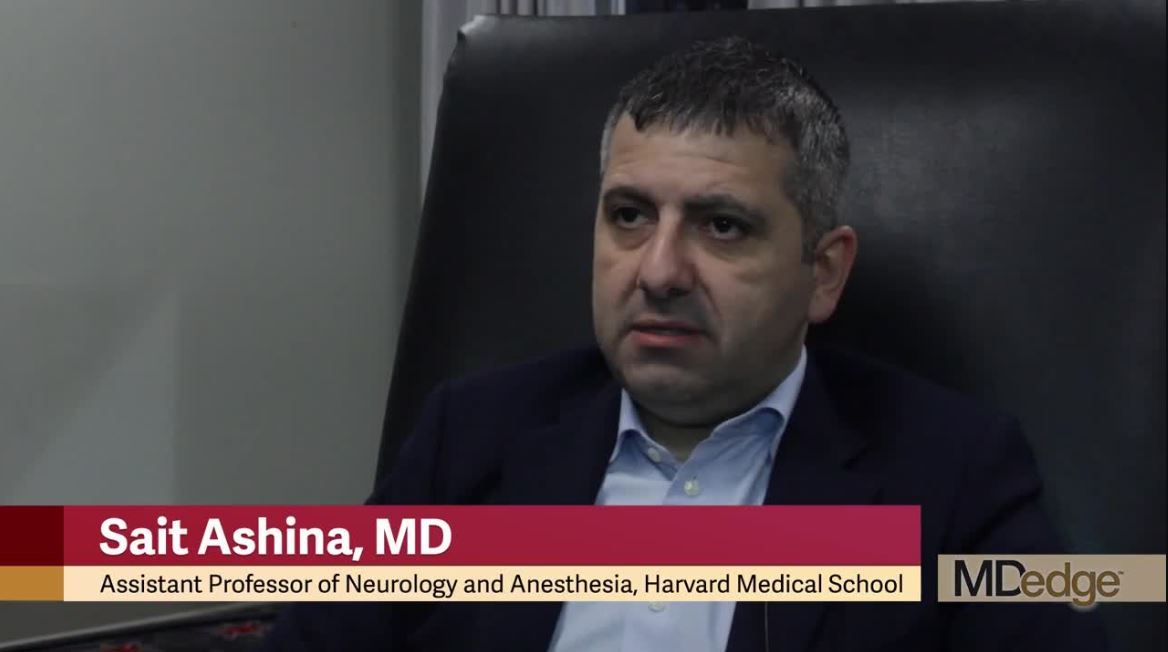
To evaluate opioid use in people with migraine, Sait Ashina, MD, of Harvard Medical School and Beth Israel Deaconess Medical Center in Boston, and the research colleagues analyzed data from 21,143 people with migraine who participated in the OVERCOME (Observational Survey of the Epidemiology, Treatment and Care of Migraine), a Web-based study of a representative U.S. sample. OVERCOME enrolled participants in the fall of 2018.
The researchers classified self-reported opioid use for migraine as current use in the past 12 months, former use, or never. Participants had a mean age of 42 years, and 74% were female. The researchers used a multivariable logistic regression model adjusted for age and sex in their analyses.
“Strikingly, we were able to find 19% of people with migraine were reporting current use of opioids,” Dr. Ashina said.
Among 12,299 patients with 0-3 migraine headache days per month, 59% were never, 26% former, and 15% current users of opioids for migraine. Among 8,844 patients with 4 or more migraine headache days per month, 44.9% were never, 31.2% former, and 23.9% current users of opioids for migraine.
There was an increased likelihood of opioid use for migraine in people with pain comorbidities such as back pain, neck pain, and fibromyalgia and in people with anxiety and depression.
Approximately 30%-40% of those who used opioids for migraine were using strong opioids, as defined by the World Health Organization, Dr. Ashina noted. Preliminary analyses indicate that patients tended to receive opioids in a primary care setting, he said.
Eli Lilly funded the OVERCOME study. Dr. Ashina has consulted for Novartis, Amgen, Promius, Supernus, Satsuma, and Allergan. He is on the Editorial Advisory Board for Neurology Reviews.
PHILADELPHIA – People with 4 or more migraine headache days per month are more likely to use opioids, compared with people with fewer migraine headache days per month, researchers said. Opioid use for migraine “remains alarmingly high,” the investigators said at the annual meeting of the American Headache Society.
Although opioid use for the treatment of migraine typically is discouraged, studies indicate that it is common. Evidence suggests that opioids may increase the risk of progression from episodic to chronic migraine.
To evaluate opioid use in people with migraine, Sait Ashina, MD, of Harvard Medical School and Beth Israel Deaconess Medical Center in Boston, and the research colleagues analyzed data from 21,143 people with migraine who participated in the OVERCOME (Observational Survey of the Epidemiology, Treatment and Care of Migraine), a Web-based study of a representative U.S. sample. OVERCOME enrolled participants in the fall of 2018.
The researchers classified self-reported opioid use for migraine as current use in the past 12 months, former use, or never. Participants had a mean age of 42 years, and 74% were female. The researchers used a multivariable logistic regression model adjusted for age and sex in their analyses.
“Strikingly, we were able to find 19% of people with migraine were reporting current use of opioids,” Dr. Ashina said.
Among 12,299 patients with 0-3 migraine headache days per month, 59% were never, 26% former, and 15% current users of opioids for migraine. Among 8,844 patients with 4 or more migraine headache days per month, 44.9% were never, 31.2% former, and 23.9% current users of opioids for migraine.
There was an increased likelihood of opioid use for migraine in people with pain comorbidities such as back pain, neck pain, and fibromyalgia and in people with anxiety and depression.
Approximately 30%-40% of those who used opioids for migraine were using strong opioids, as defined by the World Health Organization, Dr. Ashina noted. Preliminary analyses indicate that patients tended to receive opioids in a primary care setting, he said.
Eli Lilly funded the OVERCOME study. Dr. Ashina has consulted for Novartis, Amgen, Promius, Supernus, Satsuma, and Allergan. He is on the Editorial Advisory Board for Neurology Reviews.
PHILADELPHIA – People with 4 or more migraine headache days per month are more likely to use opioids, compared with people with fewer migraine headache days per month, researchers said. Opioid use for migraine “remains alarmingly high,” the investigators said at the annual meeting of the American Headache Society.
Although opioid use for the treatment of migraine typically is discouraged, studies indicate that it is common. Evidence suggests that opioids may increase the risk of progression from episodic to chronic migraine.
To evaluate opioid use in people with migraine, Sait Ashina, MD, of Harvard Medical School and Beth Israel Deaconess Medical Center in Boston, and the research colleagues analyzed data from 21,143 people with migraine who participated in the OVERCOME (Observational Survey of the Epidemiology, Treatment and Care of Migraine), a Web-based study of a representative U.S. sample. OVERCOME enrolled participants in the fall of 2018.
The researchers classified self-reported opioid use for migraine as current use in the past 12 months, former use, or never. Participants had a mean age of 42 years, and 74% were female. The researchers used a multivariable logistic regression model adjusted for age and sex in their analyses.
“Strikingly, we were able to find 19% of people with migraine were reporting current use of opioids,” Dr. Ashina said.
Among 12,299 patients with 0-3 migraine headache days per month, 59% were never, 26% former, and 15% current users of opioids for migraine. Among 8,844 patients with 4 or more migraine headache days per month, 44.9% were never, 31.2% former, and 23.9% current users of opioids for migraine.
There was an increased likelihood of opioid use for migraine in people with pain comorbidities such as back pain, neck pain, and fibromyalgia and in people with anxiety and depression.
Approximately 30%-40% of those who used opioids for migraine were using strong opioids, as defined by the World Health Organization, Dr. Ashina noted. Preliminary analyses indicate that patients tended to receive opioids in a primary care setting, he said.
Eli Lilly funded the OVERCOME study. Dr. Ashina has consulted for Novartis, Amgen, Promius, Supernus, Satsuma, and Allergan. He is on the Editorial Advisory Board for Neurology Reviews.
EXPERT ANALYSIS FROM AHS 2019
CARMELINA confirms linagliptin’s renal, CV safety, but it’s still third-line for type 2 diabetes
SAN FRANCISCO – The dipeptidyl peptidase-4 inhibitor linagliptin (Tradjenta) is safe on the kidneys, the cardiovascular system, and in older people with type 2 diabetes, according to findings presented at the annual scientific sessions of the American Diabetes Association.
Investigators in the international Cardiovascular and Renal Microvascular Outcome Study with Linagliptin (CARMELINA) randomized 6,979 patients with type 2 diabetes who also had cardiovascular and/or kidney disease 1:1 to daily oral linagliptin 5 mg or placebo on top of standard of care, and they followed them for a median of 2.2 years. The mean age was 65.9 years, baseline hemoglobin A1c was 8.0%, and disease duration was about 15 years. Almost 63% of the patients were men, and about a quarter had a history of heart failure at baseline (JAMA. 2019;321[1]:69-79).
The study was unusual among other DPP-4 trials in that almost 60% of the patients were older than 65 years and 62.3% had impaired renal function with an estimated glomerular filtration rate (eGFR) of less than 60 ml/min per 1.73 m2.
There was no increased risk with linagliptin, compared with placebo, in the primary composite outcome of cardiovascular death, nonfatal stroke, or nonfatal myocardial infarction (12.4% vs. 12.1%, respectively; hazard ratio, 1.02; P = .74), and there was no difference between the individual components even when broken down by age (younger than 65, 65-75, or older than 75 years) or by renal function (eGFR 60 or more, 45 to less than 60, 30 to less than 45, or less than 30 ml/min per 1.73 m2), according to investigator Mark Cooper, MBBS, PhD, of the department of diabetes at Monash University, Melbourne, who presented the findings.
There was no increase in the number of hospitalizations for heart failure with linagliptin, compared with placebo (6% vs. 6.5%, respectively; HR, 0.90; P = .26) – a concern with some DPP-4 inhibitors – and no increase in hypoglycemia (just over a quarter in both groups), even when broken down by age and renal function.
A decrease in albuminuria with linagliptin held across all renal subgroups. It is not known if that was because of glucose lowering or some other effect, but Dr. Cooper said he believed there was “a modest renal protective effect, [although] not at the level one would expect to translate into hard renal outcomes.”
Robert Eckel, MD, a professor of medicine at the University of Colorado at Denver, Aurora, who moderated the session, said the results were reassuring. “Ultimately, linagliptin seems safe,” even in older people with reduced eGFR. “It does not improve cardiovascular outcomes, but based on many DPP-4 trials, we didn’t expect it to,” he said.
“I don’t think DPP-4s are going to fall into any different place in the [treatment] algorithm” based on these results, he added. The class is currently third-line after metformin or insulin, followed by sodium-glucose cotransporter 2 inhibitors or glucagonlike peptide–1 receptor agonists for cardiovascular protection.
“When we look at [cardiovascular outcomes], ultimately, the SGLT2 inhibitors and the GLP-1 receptor agonists win,” he said. In addition, the blood glucose effects of linagliptin are “pretty modest, so if lowering hemoglobin A1c is the focus, this drug would be lower down on the list.”
Overall, linagliptin “falls into a lesser class, but a safe class for certain circumstances,” said Dr. Eckel, who gave the example of a woman in her late 70s with moderate to severe kidney function, an HbA1c level of 7.9%, and no cardiovascular disease. Her HbA1c might get down to 7.6% or so with linagliptin, he said, “but I’m not sure we have absolute proof of the benefit” of such a modest decline.
Boehringer Ingelheim, the maker of linagliptin, funded the study. The presenter disclosed honoraria, speaking fees, and grants from the company. A number of the investigators were employees of the company.
SAN FRANCISCO – The dipeptidyl peptidase-4 inhibitor linagliptin (Tradjenta) is safe on the kidneys, the cardiovascular system, and in older people with type 2 diabetes, according to findings presented at the annual scientific sessions of the American Diabetes Association.
Investigators in the international Cardiovascular and Renal Microvascular Outcome Study with Linagliptin (CARMELINA) randomized 6,979 patients with type 2 diabetes who also had cardiovascular and/or kidney disease 1:1 to daily oral linagliptin 5 mg or placebo on top of standard of care, and they followed them for a median of 2.2 years. The mean age was 65.9 years, baseline hemoglobin A1c was 8.0%, and disease duration was about 15 years. Almost 63% of the patients were men, and about a quarter had a history of heart failure at baseline (JAMA. 2019;321[1]:69-79).
The study was unusual among other DPP-4 trials in that almost 60% of the patients were older than 65 years and 62.3% had impaired renal function with an estimated glomerular filtration rate (eGFR) of less than 60 ml/min per 1.73 m2.
There was no increased risk with linagliptin, compared with placebo, in the primary composite outcome of cardiovascular death, nonfatal stroke, or nonfatal myocardial infarction (12.4% vs. 12.1%, respectively; hazard ratio, 1.02; P = .74), and there was no difference between the individual components even when broken down by age (younger than 65, 65-75, or older than 75 years) or by renal function (eGFR 60 or more, 45 to less than 60, 30 to less than 45, or less than 30 ml/min per 1.73 m2), according to investigator Mark Cooper, MBBS, PhD, of the department of diabetes at Monash University, Melbourne, who presented the findings.
There was no increase in the number of hospitalizations for heart failure with linagliptin, compared with placebo (6% vs. 6.5%, respectively; HR, 0.90; P = .26) – a concern with some DPP-4 inhibitors – and no increase in hypoglycemia (just over a quarter in both groups), even when broken down by age and renal function.
A decrease in albuminuria with linagliptin held across all renal subgroups. It is not known if that was because of glucose lowering or some other effect, but Dr. Cooper said he believed there was “a modest renal protective effect, [although] not at the level one would expect to translate into hard renal outcomes.”
Robert Eckel, MD, a professor of medicine at the University of Colorado at Denver, Aurora, who moderated the session, said the results were reassuring. “Ultimately, linagliptin seems safe,” even in older people with reduced eGFR. “It does not improve cardiovascular outcomes, but based on many DPP-4 trials, we didn’t expect it to,” he said.
“I don’t think DPP-4s are going to fall into any different place in the [treatment] algorithm” based on these results, he added. The class is currently third-line after metformin or insulin, followed by sodium-glucose cotransporter 2 inhibitors or glucagonlike peptide–1 receptor agonists for cardiovascular protection.
“When we look at [cardiovascular outcomes], ultimately, the SGLT2 inhibitors and the GLP-1 receptor agonists win,” he said. In addition, the blood glucose effects of linagliptin are “pretty modest, so if lowering hemoglobin A1c is the focus, this drug would be lower down on the list.”
Overall, linagliptin “falls into a lesser class, but a safe class for certain circumstances,” said Dr. Eckel, who gave the example of a woman in her late 70s with moderate to severe kidney function, an HbA1c level of 7.9%, and no cardiovascular disease. Her HbA1c might get down to 7.6% or so with linagliptin, he said, “but I’m not sure we have absolute proof of the benefit” of such a modest decline.
Boehringer Ingelheim, the maker of linagliptin, funded the study. The presenter disclosed honoraria, speaking fees, and grants from the company. A number of the investigators were employees of the company.
SAN FRANCISCO – The dipeptidyl peptidase-4 inhibitor linagliptin (Tradjenta) is safe on the kidneys, the cardiovascular system, and in older people with type 2 diabetes, according to findings presented at the annual scientific sessions of the American Diabetes Association.
Investigators in the international Cardiovascular and Renal Microvascular Outcome Study with Linagliptin (CARMELINA) randomized 6,979 patients with type 2 diabetes who also had cardiovascular and/or kidney disease 1:1 to daily oral linagliptin 5 mg or placebo on top of standard of care, and they followed them for a median of 2.2 years. The mean age was 65.9 years, baseline hemoglobin A1c was 8.0%, and disease duration was about 15 years. Almost 63% of the patients were men, and about a quarter had a history of heart failure at baseline (JAMA. 2019;321[1]:69-79).
The study was unusual among other DPP-4 trials in that almost 60% of the patients were older than 65 years and 62.3% had impaired renal function with an estimated glomerular filtration rate (eGFR) of less than 60 ml/min per 1.73 m2.
There was no increased risk with linagliptin, compared with placebo, in the primary composite outcome of cardiovascular death, nonfatal stroke, or nonfatal myocardial infarction (12.4% vs. 12.1%, respectively; hazard ratio, 1.02; P = .74), and there was no difference between the individual components even when broken down by age (younger than 65, 65-75, or older than 75 years) or by renal function (eGFR 60 or more, 45 to less than 60, 30 to less than 45, or less than 30 ml/min per 1.73 m2), according to investigator Mark Cooper, MBBS, PhD, of the department of diabetes at Monash University, Melbourne, who presented the findings.
There was no increase in the number of hospitalizations for heart failure with linagliptin, compared with placebo (6% vs. 6.5%, respectively; HR, 0.90; P = .26) – a concern with some DPP-4 inhibitors – and no increase in hypoglycemia (just over a quarter in both groups), even when broken down by age and renal function.
A decrease in albuminuria with linagliptin held across all renal subgroups. It is not known if that was because of glucose lowering or some other effect, but Dr. Cooper said he believed there was “a modest renal protective effect, [although] not at the level one would expect to translate into hard renal outcomes.”
Robert Eckel, MD, a professor of medicine at the University of Colorado at Denver, Aurora, who moderated the session, said the results were reassuring. “Ultimately, linagliptin seems safe,” even in older people with reduced eGFR. “It does not improve cardiovascular outcomes, but based on many DPP-4 trials, we didn’t expect it to,” he said.
“I don’t think DPP-4s are going to fall into any different place in the [treatment] algorithm” based on these results, he added. The class is currently third-line after metformin or insulin, followed by sodium-glucose cotransporter 2 inhibitors or glucagonlike peptide–1 receptor agonists for cardiovascular protection.
“When we look at [cardiovascular outcomes], ultimately, the SGLT2 inhibitors and the GLP-1 receptor agonists win,” he said. In addition, the blood glucose effects of linagliptin are “pretty modest, so if lowering hemoglobin A1c is the focus, this drug would be lower down on the list.”
Overall, linagliptin “falls into a lesser class, but a safe class for certain circumstances,” said Dr. Eckel, who gave the example of a woman in her late 70s with moderate to severe kidney function, an HbA1c level of 7.9%, and no cardiovascular disease. Her HbA1c might get down to 7.6% or so with linagliptin, he said, “but I’m not sure we have absolute proof of the benefit” of such a modest decline.
Boehringer Ingelheim, the maker of linagliptin, funded the study. The presenter disclosed honoraria, speaking fees, and grants from the company. A number of the investigators were employees of the company.
REPORTING FROM ADA 2019
The costs and benefits of SGLT2 inhibitors & GLP-1 RAs
The options for treating type 2 diabetes without insulin have grown beyond metformin to include a long list of sodium-glucose cotransporter 2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) receptor agonists that can be taken with or without metformin. These new drugs have cardiovascular and kidney benefits and help with weight loss, but they also carry risks and, according to some experts, their costs can be prohibitively expensive.
Given the medical community’s long-term experience with treating patients with metformin, and metformin’s lower cost, most of the physicians interviewed for this article advise using SGLT2 inhibitors and GLP-1 receptor agonists as second-line treatments. Others said that they would prefer to use the newer drugs as first-line therapies in select high-risk patients, but prior authorization hurdles created by insurance companies make that approach too burdensome.
“The economics of U.S. health care is stacked against many of our patients with diabetes in the current era,” Robert H. Hopkins Jr., MD, said in an interview.
Even when their insurance approves the drugs, patients still may not be able to afford the copay, explained Dr. Hopkins, professor of internal medicine and pediatrics and director of the division of general internal medicine at the University of Arkansas for Medical Sciences, Little Rock. “Sometimes patients can purchase drugs at a lower cost than the copay to purchase with the ‘drug coverage’ in their insurance plan – unfortunately, this is not the case with the newer diabetes medications we are discussing here.”
“SGLT2 inhibitors and GLP-1 agonists can cost several hundred dollars a month, and insurers often balk at paying for them. They’ll say, ‘Have you tried metformin?’ ” explained endocrinologist Victor Lawrence Roberts, MD, in a interview. “We have to work with insurance companies the best we can in a stepwise fashion.”
According to Dr. Roberts, 80% of his patients with diabetes struggle with the cost of medicine in general. “They’re either underinsured or not insured or their formulary is limited.
Douglas S. Paauw, MD, agreed in an interview that the newer drugs can be problematic on the insurance front.
“For some patients they aren’t affordable, especially for the uninsured if you can’t get them on an assistance program,” said Dr. Paauw, who is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the university.
Dr. Hopkins, who is on the Internal Medicine News board, noted that “unfortunately, the treatment of type 2 diabetes in patients who cannot achieve control with metformin, diet, weight control, and exercise is a story of the ‘haves’ and the ‘have nots.’ The ‘haves’ are those who have pharmacy benefits which make access to newer agents like SGLT2 inhibitors and GLP-1 agonists a possibility.”
“I have had very few of the ‘have nots’ who have been able to even consider these newer agents, which carry price tags of $600-$1,300 a month even with the availability of discounting coupons in the marketplace,” he added. “Most of these patients end up requiring a sulfonylurea or TZD [thiazolidinedione] as a second agent to achieve glycemic control. This makes it very difficult to achieve sufficient weight and metabolic control to avoid an eventual switch to insulin.”
Fatima Z. Syed, MD, an endocrine-trained general internist at DukeHealth in Durham, N.C., said she prescribes SGLT2 inhibitors and GLP-1 receptor agonists in combination with metformin. “I prescribe them frequently, but they are not first-line treatments,” she explained.
“Nothing replaces diet and exercise” as therapy for patients with type 2 diabetes, she added.
Neil S. Skolnik, MD, said that insurance companies were not preventing patients from using these drugs in his experience. He also provided an optimistic take on the accessibility of these drugs in the near future.
“Most insurance companies are now covering select SGLT2 inhibitors and GLP-1 receptor agonists for appropriate patients and those companies that currently do not will soon have to,” said Dr. Skolnik, who is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
“The outcomes [associated with use of the new drugs] are robust, the benefits are large, and are well worth the cost,” he added.
The side effects
While others praised these drugs for their beneficial effects, they also noted that the side effects of these drugs are serious and must be discussed with patients.
GLP-1 receptor agonists are linked to gastrointestinal symptoms, especially nausea, while SGLT2 inhibitors have been linked to kidney failure, ketoacidosis, and more. The Food and Drug Administration warned in 2018 that the SGLT2 inhibitors can cause a rare serious infection known as Fournier’s gangrene – necrotizing fasciitis of the perineum.
“We have to tell our patients to let us know right away if they get pain or swelling in the genital area,” Dr. Paauw, who is on the Internal Medicine News board, noted. “The chance that an infection could explode quickly is higher in those who take these drugs.”
Amputation risks also are associated with taking the SGLT2 inhibitor canagliflozin (Invokana). The FDA requires the manufacturer of this drug to include a black-box warning about the risk of “lower-limb amputations, most frequently of the toe and midfoot,” but also the leg. In approval trials, the risk doubled versus placebo.
These amputation risks “put a damper on some of the enthusiasm on behalf of physicians and patients ... for taking this drug,” noted Dr. Roberts, who is a professor of internal medicine at the University of Central Florida, Orlando.
While a manufacturer-funded study released last year found no link to amputations, the results weren’t powerful enough to rule out a moderately increased risk.
“[If] you are at high risk for having an amputation, we really have to take this risk very seriously,” said John B. Buse, MD, chief of the division of endocrinology at the University of North Carolina at Chapel Hill, in a presentation about the study at the 2018 annual scientific sessions of the American Diabetes Association.
The benefits
Despite these risks of adverse events, most interviewed agreed that the many benefits observed in those taking SGLT2 inhibitors or GLP-1 receptor agonists make them worth prescribing, at least to those who are able to afford them.
Both SGLT2 inhibitors and GLP-1 receptor agonists appear to have significant cardiovascular benefits. A 2019 meta-analysis and systematic review found that both drugs reduced major adverse cardiac events by about 12% (Circulation. 2019 Apr 23;139[17]:2022-31).
“They don’t cause hypoglycemia, they lower blood pressure, they don’t cause weight gain, and they might promote weight loss,” noted Dr. Paauw.
SGLT2 inhibitors also have shown signs of kidney benefits. The CREDENCE trial linked canagliflozin to a lowering of kidney disorders versus placebo (N Engl J Med. 2019 Jun 13;380[24]:2295-306). “The relative risk of the renal-specific composite of end-stage kidney disease, a doubling of the creatinine level, or death from renal causes was lower by 34% (hazard ratio, 0.66; 95% confidence interval, 0.53-0.81; P less than .001), and the relative risk of end-stage kidney disease was lower by 32% (HR, 0.68; 95% CI, 0.54-0.86; P = .002),” the trial investigators wrote.
“They showed very nicely that the drug improved the kidney function of those patients and reduced the kidney deterioration,” said Yehuda Handelsman, MD, an endocrinologist in Tarzana, Calif., who chaired the 2011 and 2015 American Association of Clinical Endocrinologists’ Comprehensive Diabetes Guidelines. The study was especially impressive, he added, because it included patients with low kidney function.
SGLT2 inhibitors’ “diuretic mechanism explains why there is a substantial reduction in heart failure hospitalizations in patients who take these drugs,” said cardiologist Marc E. Goldschmidt, MD, director of the Heart Success Program at Atlantic Health System’s Morristown (N.J.) Medical Center, in an interview. “Both the EMPA-REG Outcome and the CREDENCE trials demonstrated substantial benefit of this class of medications by showing a lower risk of cardiovascular death as well as death from any cause and a lower risk of hospitalization for heart failure."
Overall, the SGLT2 trial data have been very consistent with a benefit for cardiovascular risk reduction, particularly in regard to heart failure hospitalizations and even in potentially preventing heart failure in diabetics,” he added.
Dr. Skolnik, a columnist for Family Practice News, cited SGLT2 inhibitors and GLP-1 receptor agonists’ ability to slow renal disease progression, promote weight loss, and prevent poor cardiac outcomes.“These drugs should be used, in addition to metformin, in all patients with diabetes and vascular disease. These proven outcomes are far better than we ever were able to achieve previously and the strength of the evidence at this point is very strong,” said Dr. Skolnik. “In addition to the benefits of decreasing the development of cardiovascular disease, serious heart failure, and slowing progression of renal disease, these two classes of medication have additional benefits. Both classes help patients lose weight, which is very different from what was found with either sulfonylureas or insulin, which cause patients to gain weight. Also both the SGLT2 inhibitors and the GLP-1 RAs [receptor agonists] have a low incidence of hypoglycemia. For all these reasons, these have become important medications for us to use in primary care.”
Other recent trials offer “very powerful data” about SGLT2 inhibitors, Dr. Roberts said. That’s good news, since “our approach needs to be toward cardiovascular protection and preservation as well as managing blood sugar.”An Israeli trial, whose results were released in May 2019 at the annual meeting of the American College of Cardiology, found that, compared with other glucose-lowering drugs, taking an SGLT2 inhibitor was associated with lower risks of heart failure hospitalization and all-cause mortality (HR, 0.54; 95% CI, 0.44-0.65; P less than .001). This trial also offered a new detail: The patients gained the benefit regardless of whether their baseline left ventricular ejection fraction was preserved or reduced (J Coll Cardiol. 2019 Mar;73[9]:suppl 1). The SGLT2 inhibitors used in this trial included dapagliflozin (Farxiga) and empagliflozin (Jardiance).
In another study released this year, a subanalysis of the DECLARE-TIMI 58 trial, researchers reported that the SGLT2 inhibitor dapagliflozin reduced risks of both major adverse cardiovascular events and heart failure hospitalization in the subset of patients with type 2 diabetes and prior myocardial infarction versus controls (Circulation. 2019 May 28;139[22]:2516-27). The absolute risk reduction for major adverse cardiovascular events was 1.9% (HR, 0.81; 95% CI, 0.65-1.00; P = .046), while it was 0.6% for heart failure hospitalization (HR, 0.85; 95% CI, 0.72-1.00; P = .055).
These and other studies “speak volumes about the efficacy of managing blood sugar and addressing our biggest nemesis, which is cardiovascular disease,” Dr. Roberts said. “It’s irrefutable. The data [are] very good.”
Dr. Paauw said an SGLT2 inhibitor or GLP-1 receptor agonist is best reserved for use in select patients with cardiovascular risks and type 2 diabetes that need management beyond metformin.
For example, they might fit a 70-year-old with persistent hypertension who’s already taking a couple of blood pressure medications. “If they have another cardiovascular risk factor, the cardiovascular protection piece will be a bigger deal,” he said. Also, “it will probably help lower their blood pressure so they can avoid taking another blood pressure medicine.”
Trials of both GLP-1 receptor agonists and SGLT2 inhibitors have shown benefits “in improving [major adverse cardiac events], with the SGLT2 class showing substantial benefit in improving both heart failure and renal outcomes as well,” noted Dr. Skolnik. “It is in this context that one must address the question of whether the price of the medications are worthwhile. With such substantial benefit, there is no question in my mind that – for patients who have underlying cardiovascular illness, which includes patients with existent coronary disease, history of stroke, transient ischemic attack, or peripheral vascular disease – it is far and away worth it to prescribe these classes of medications.”
Indeed, the American Diabetes Association and the European Association for the Study of Diabetes’ most recent guidelines now call for a GLP-1 receptor agonist – instead of insulin – to be the first injectable used to treat type 2 diabetes (Diabetes Care 2018 Dec; 41[12]:2669-701).
“For the relatively small number of my patients who have been able to access and use these medications for months or longer, more have tolerated the GLP-1 agonists than SGLT2 inhibitors primarily due to urinary issues,” noted Dr. Hopkins.
Dipeptidyl peptidase–4 inhibitors are another option in patients with type 2 diabetes, but research suggests they may not be a top option for patients with cardiovascular risk. A 2018 review noted that cardiovascular outcome trials for alogliptin (Nesina), saxagliptin (Onglyza), and sitagliptin (Januvia) showed noninferiority but failed to demonstrate any superiority, compared with placebo in patients with type 2 diabetes mellitus and high cardiovascular risk (Circ Res. 2018 May 11;122[10]:1439-59).
The combination therapies
Many of the newer drugs are available as combinations with other types of diabetes drugs. In some cases, physicians create their own form of combination therapy by separately prescribing two or more diabetes drugs. Earlier this year, a study suggested the benefits of this kind of add-on therapy: Diabetes outcomes improved in patients who took the GLP-1 receptor agonist semaglutide and an SGLT2 inhibitor (Lancet Diabetes Endocrinol. 2019 Mar 1. doi: 10.1016/S2213-8587[19]30066-X).
Dr. Roberts suggested caution, however, when prescribing combination therapies. “My recommendation is always to begin with the individual medications to see if the patient tolerates the drugs and then decide which component needs to be titrated. It’s hard to titrate a combination drug, and it doesn’t leave a lot of flexibility. You never know which drug is doing what.
Dr. Handelsman said some patients may need to take three medications such as metformin, an SGLT2 inhibitor, and a GLP-1 receptor agonist.
“I don’t recommend using the combinations if you’re not familiar with the drugs ... These are relatively new pharmaceuticals, and most of us are on a learning curve as to how they fit into the armamentarium. If a drug is tolerated with a good response, you can certainly consider going to the combination tablets,” he added.
There is at least one drug that combines these three classes: The newly FDA-approved Qternmet XR, which combines dapagliflozin (an SGLT2 inhibitor), saxagliptin (a GLP-1 receptor agonist), and metformin. As of mid-June 2019, it was not yet available in the United States. Its sister drug Qtern, which combines dapagliflozin and saxagliptin, costs more than $500 a month with a free coupon, according to goodrx.com. In contrast, metformin is extremely inexpensive, costing just a few dollars a month for a common starting dose.
What about adding insulin?
“Both [SGLT2 inhibitors and GLP-1 receptor agonists] work very well with insulin,” Dr. Handelsman said. “There is a nice additive effect on the reduction of [hemoglobin] A1c. The only caution is that, although neither SGLT2 inhibitors nor GLP-1 receptor agonists cause hypoglycemia, in combination with insulin they do increase the risk of hypoglycemia. You may have to adjust the dose of insulin.”
Dr. Hopkins warned that cost becomes an even bigger issue when you add insulin into the mix.
“When insulin comes into the discussion, we are again stuck with astronomical costs which many struggle to afford,” he explained.
Indeed, the price tag on these drugs seems to be the biggest problem physicians have with them.
“The challenges in managing patients with diabetes aren’t the risks associated with the drugs. It’s dealing with their insurers,” noted Dr. Roberts.
Dr. Hopkins, Dr. Paauw, Dr. Roberts, and Dr. Syed reported no disclosures. Dr. Buse is an investigator for Johnson and Johnson. Dr. Goldschmidt is paid to speak by Novartis. Dr. Handelsman reported research grants, consulting work, and speaker honoraria from Amgen, Gilead, Lilly, Merck, Novo Nordisk, and others. Dr Skolnik reported nonfinancial support from AstraZeneca, Boehringer Ingelheim, Sanofi, and GlaxoSmithKline and personal fees from AstraZeneca, Boehringer Ingelheim, and Eli Lilly. He also serves on the advisory boards of AstraZeneca, Boehringer Ingelheim, Teva Pharmaceutical, Eli Lilly, Sanofi, Janssen Pharmaceuticals, Intarcia, Mylan, and GlaxoSmithKline.
Dr. Paauw and Dr. Skolnik are columnists for Family Practice News and Internal Medicine News.
M. Alexander Otto contributed to this report.
The options for treating type 2 diabetes without insulin have grown beyond metformin to include a long list of sodium-glucose cotransporter 2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) receptor agonists that can be taken with or without metformin. These new drugs have cardiovascular and kidney benefits and help with weight loss, but they also carry risks and, according to some experts, their costs can be prohibitively expensive.
Given the medical community’s long-term experience with treating patients with metformin, and metformin’s lower cost, most of the physicians interviewed for this article advise using SGLT2 inhibitors and GLP-1 receptor agonists as second-line treatments. Others said that they would prefer to use the newer drugs as first-line therapies in select high-risk patients, but prior authorization hurdles created by insurance companies make that approach too burdensome.
“The economics of U.S. health care is stacked against many of our patients with diabetes in the current era,” Robert H. Hopkins Jr., MD, said in an interview.
Even when their insurance approves the drugs, patients still may not be able to afford the copay, explained Dr. Hopkins, professor of internal medicine and pediatrics and director of the division of general internal medicine at the University of Arkansas for Medical Sciences, Little Rock. “Sometimes patients can purchase drugs at a lower cost than the copay to purchase with the ‘drug coverage’ in their insurance plan – unfortunately, this is not the case with the newer diabetes medications we are discussing here.”
“SGLT2 inhibitors and GLP-1 agonists can cost several hundred dollars a month, and insurers often balk at paying for them. They’ll say, ‘Have you tried metformin?’ ” explained endocrinologist Victor Lawrence Roberts, MD, in a interview. “We have to work with insurance companies the best we can in a stepwise fashion.”
According to Dr. Roberts, 80% of his patients with diabetes struggle with the cost of medicine in general. “They’re either underinsured or not insured or their formulary is limited.
Douglas S. Paauw, MD, agreed in an interview that the newer drugs can be problematic on the insurance front.
“For some patients they aren’t affordable, especially for the uninsured if you can’t get them on an assistance program,” said Dr. Paauw, who is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the university.
Dr. Hopkins, who is on the Internal Medicine News board, noted that “unfortunately, the treatment of type 2 diabetes in patients who cannot achieve control with metformin, diet, weight control, and exercise is a story of the ‘haves’ and the ‘have nots.’ The ‘haves’ are those who have pharmacy benefits which make access to newer agents like SGLT2 inhibitors and GLP-1 agonists a possibility.”
“I have had very few of the ‘have nots’ who have been able to even consider these newer agents, which carry price tags of $600-$1,300 a month even with the availability of discounting coupons in the marketplace,” he added. “Most of these patients end up requiring a sulfonylurea or TZD [thiazolidinedione] as a second agent to achieve glycemic control. This makes it very difficult to achieve sufficient weight and metabolic control to avoid an eventual switch to insulin.”
Fatima Z. Syed, MD, an endocrine-trained general internist at DukeHealth in Durham, N.C., said she prescribes SGLT2 inhibitors and GLP-1 receptor agonists in combination with metformin. “I prescribe them frequently, but they are not first-line treatments,” she explained.
“Nothing replaces diet and exercise” as therapy for patients with type 2 diabetes, she added.
Neil S. Skolnik, MD, said that insurance companies were not preventing patients from using these drugs in his experience. He also provided an optimistic take on the accessibility of these drugs in the near future.
“Most insurance companies are now covering select SGLT2 inhibitors and GLP-1 receptor agonists for appropriate patients and those companies that currently do not will soon have to,” said Dr. Skolnik, who is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
“The outcomes [associated with use of the new drugs] are robust, the benefits are large, and are well worth the cost,” he added.
The side effects
While others praised these drugs for their beneficial effects, they also noted that the side effects of these drugs are serious and must be discussed with patients.
GLP-1 receptor agonists are linked to gastrointestinal symptoms, especially nausea, while SGLT2 inhibitors have been linked to kidney failure, ketoacidosis, and more. The Food and Drug Administration warned in 2018 that the SGLT2 inhibitors can cause a rare serious infection known as Fournier’s gangrene – necrotizing fasciitis of the perineum.
“We have to tell our patients to let us know right away if they get pain or swelling in the genital area,” Dr. Paauw, who is on the Internal Medicine News board, noted. “The chance that an infection could explode quickly is higher in those who take these drugs.”
Amputation risks also are associated with taking the SGLT2 inhibitor canagliflozin (Invokana). The FDA requires the manufacturer of this drug to include a black-box warning about the risk of “lower-limb amputations, most frequently of the toe and midfoot,” but also the leg. In approval trials, the risk doubled versus placebo.
These amputation risks “put a damper on some of the enthusiasm on behalf of physicians and patients ... for taking this drug,” noted Dr. Roberts, who is a professor of internal medicine at the University of Central Florida, Orlando.
While a manufacturer-funded study released last year found no link to amputations, the results weren’t powerful enough to rule out a moderately increased risk.
“[If] you are at high risk for having an amputation, we really have to take this risk very seriously,” said John B. Buse, MD, chief of the division of endocrinology at the University of North Carolina at Chapel Hill, in a presentation about the study at the 2018 annual scientific sessions of the American Diabetes Association.
The benefits
Despite these risks of adverse events, most interviewed agreed that the many benefits observed in those taking SGLT2 inhibitors or GLP-1 receptor agonists make them worth prescribing, at least to those who are able to afford them.
Both SGLT2 inhibitors and GLP-1 receptor agonists appear to have significant cardiovascular benefits. A 2019 meta-analysis and systematic review found that both drugs reduced major adverse cardiac events by about 12% (Circulation. 2019 Apr 23;139[17]:2022-31).
“They don’t cause hypoglycemia, they lower blood pressure, they don’t cause weight gain, and they might promote weight loss,” noted Dr. Paauw.
SGLT2 inhibitors also have shown signs of kidney benefits. The CREDENCE trial linked canagliflozin to a lowering of kidney disorders versus placebo (N Engl J Med. 2019 Jun 13;380[24]:2295-306). “The relative risk of the renal-specific composite of end-stage kidney disease, a doubling of the creatinine level, or death from renal causes was lower by 34% (hazard ratio, 0.66; 95% confidence interval, 0.53-0.81; P less than .001), and the relative risk of end-stage kidney disease was lower by 32% (HR, 0.68; 95% CI, 0.54-0.86; P = .002),” the trial investigators wrote.
“They showed very nicely that the drug improved the kidney function of those patients and reduced the kidney deterioration,” said Yehuda Handelsman, MD, an endocrinologist in Tarzana, Calif., who chaired the 2011 and 2015 American Association of Clinical Endocrinologists’ Comprehensive Diabetes Guidelines. The study was especially impressive, he added, because it included patients with low kidney function.
SGLT2 inhibitors’ “diuretic mechanism explains why there is a substantial reduction in heart failure hospitalizations in patients who take these drugs,” said cardiologist Marc E. Goldschmidt, MD, director of the Heart Success Program at Atlantic Health System’s Morristown (N.J.) Medical Center, in an interview. “Both the EMPA-REG Outcome and the CREDENCE trials demonstrated substantial benefit of this class of medications by showing a lower risk of cardiovascular death as well as death from any cause and a lower risk of hospitalization for heart failure."
Overall, the SGLT2 trial data have been very consistent with a benefit for cardiovascular risk reduction, particularly in regard to heart failure hospitalizations and even in potentially preventing heart failure in diabetics,” he added.
Dr. Skolnik, a columnist for Family Practice News, cited SGLT2 inhibitors and GLP-1 receptor agonists’ ability to slow renal disease progression, promote weight loss, and prevent poor cardiac outcomes.“These drugs should be used, in addition to metformin, in all patients with diabetes and vascular disease. These proven outcomes are far better than we ever were able to achieve previously and the strength of the evidence at this point is very strong,” said Dr. Skolnik. “In addition to the benefits of decreasing the development of cardiovascular disease, serious heart failure, and slowing progression of renal disease, these two classes of medication have additional benefits. Both classes help patients lose weight, which is very different from what was found with either sulfonylureas or insulin, which cause patients to gain weight. Also both the SGLT2 inhibitors and the GLP-1 RAs [receptor agonists] have a low incidence of hypoglycemia. For all these reasons, these have become important medications for us to use in primary care.”
Other recent trials offer “very powerful data” about SGLT2 inhibitors, Dr. Roberts said. That’s good news, since “our approach needs to be toward cardiovascular protection and preservation as well as managing blood sugar.”An Israeli trial, whose results were released in May 2019 at the annual meeting of the American College of Cardiology, found that, compared with other glucose-lowering drugs, taking an SGLT2 inhibitor was associated with lower risks of heart failure hospitalization and all-cause mortality (HR, 0.54; 95% CI, 0.44-0.65; P less than .001). This trial also offered a new detail: The patients gained the benefit regardless of whether their baseline left ventricular ejection fraction was preserved or reduced (J Coll Cardiol. 2019 Mar;73[9]:suppl 1). The SGLT2 inhibitors used in this trial included dapagliflozin (Farxiga) and empagliflozin (Jardiance).
In another study released this year, a subanalysis of the DECLARE-TIMI 58 trial, researchers reported that the SGLT2 inhibitor dapagliflozin reduced risks of both major adverse cardiovascular events and heart failure hospitalization in the subset of patients with type 2 diabetes and prior myocardial infarction versus controls (Circulation. 2019 May 28;139[22]:2516-27). The absolute risk reduction for major adverse cardiovascular events was 1.9% (HR, 0.81; 95% CI, 0.65-1.00; P = .046), while it was 0.6% for heart failure hospitalization (HR, 0.85; 95% CI, 0.72-1.00; P = .055).
These and other studies “speak volumes about the efficacy of managing blood sugar and addressing our biggest nemesis, which is cardiovascular disease,” Dr. Roberts said. “It’s irrefutable. The data [are] very good.”
Dr. Paauw said an SGLT2 inhibitor or GLP-1 receptor agonist is best reserved for use in select patients with cardiovascular risks and type 2 diabetes that need management beyond metformin.
For example, they might fit a 70-year-old with persistent hypertension who’s already taking a couple of blood pressure medications. “If they have another cardiovascular risk factor, the cardiovascular protection piece will be a bigger deal,” he said. Also, “it will probably help lower their blood pressure so they can avoid taking another blood pressure medicine.”
Trials of both GLP-1 receptor agonists and SGLT2 inhibitors have shown benefits “in improving [major adverse cardiac events], with the SGLT2 class showing substantial benefit in improving both heart failure and renal outcomes as well,” noted Dr. Skolnik. “It is in this context that one must address the question of whether the price of the medications are worthwhile. With such substantial benefit, there is no question in my mind that – for patients who have underlying cardiovascular illness, which includes patients with existent coronary disease, history of stroke, transient ischemic attack, or peripheral vascular disease – it is far and away worth it to prescribe these classes of medications.”
Indeed, the American Diabetes Association and the European Association for the Study of Diabetes’ most recent guidelines now call for a GLP-1 receptor agonist – instead of insulin – to be the first injectable used to treat type 2 diabetes (Diabetes Care 2018 Dec; 41[12]:2669-701).
“For the relatively small number of my patients who have been able to access and use these medications for months or longer, more have tolerated the GLP-1 agonists than SGLT2 inhibitors primarily due to urinary issues,” noted Dr. Hopkins.
Dipeptidyl peptidase–4 inhibitors are another option in patients with type 2 diabetes, but research suggests they may not be a top option for patients with cardiovascular risk. A 2018 review noted that cardiovascular outcome trials for alogliptin (Nesina), saxagliptin (Onglyza), and sitagliptin (Januvia) showed noninferiority but failed to demonstrate any superiority, compared with placebo in patients with type 2 diabetes mellitus and high cardiovascular risk (Circ Res. 2018 May 11;122[10]:1439-59).
The combination therapies
Many of the newer drugs are available as combinations with other types of diabetes drugs. In some cases, physicians create their own form of combination therapy by separately prescribing two or more diabetes drugs. Earlier this year, a study suggested the benefits of this kind of add-on therapy: Diabetes outcomes improved in patients who took the GLP-1 receptor agonist semaglutide and an SGLT2 inhibitor (Lancet Diabetes Endocrinol. 2019 Mar 1. doi: 10.1016/S2213-8587[19]30066-X).
Dr. Roberts suggested caution, however, when prescribing combination therapies. “My recommendation is always to begin with the individual medications to see if the patient tolerates the drugs and then decide which component needs to be titrated. It’s hard to titrate a combination drug, and it doesn’t leave a lot of flexibility. You never know which drug is doing what.
Dr. Handelsman said some patients may need to take three medications such as metformin, an SGLT2 inhibitor, and a GLP-1 receptor agonist.
“I don’t recommend using the combinations if you’re not familiar with the drugs ... These are relatively new pharmaceuticals, and most of us are on a learning curve as to how they fit into the armamentarium. If a drug is tolerated with a good response, you can certainly consider going to the combination tablets,” he added.
There is at least one drug that combines these three classes: The newly FDA-approved Qternmet XR, which combines dapagliflozin (an SGLT2 inhibitor), saxagliptin (a GLP-1 receptor agonist), and metformin. As of mid-June 2019, it was not yet available in the United States. Its sister drug Qtern, which combines dapagliflozin and saxagliptin, costs more than $500 a month with a free coupon, according to goodrx.com. In contrast, metformin is extremely inexpensive, costing just a few dollars a month for a common starting dose.
What about adding insulin?
“Both [SGLT2 inhibitors and GLP-1 receptor agonists] work very well with insulin,” Dr. Handelsman said. “There is a nice additive effect on the reduction of [hemoglobin] A1c. The only caution is that, although neither SGLT2 inhibitors nor GLP-1 receptor agonists cause hypoglycemia, in combination with insulin they do increase the risk of hypoglycemia. You may have to adjust the dose of insulin.”
Dr. Hopkins warned that cost becomes an even bigger issue when you add insulin into the mix.
“When insulin comes into the discussion, we are again stuck with astronomical costs which many struggle to afford,” he explained.
Indeed, the price tag on these drugs seems to be the biggest problem physicians have with them.
“The challenges in managing patients with diabetes aren’t the risks associated with the drugs. It’s dealing with their insurers,” noted Dr. Roberts.
Dr. Hopkins, Dr. Paauw, Dr. Roberts, and Dr. Syed reported no disclosures. Dr. Buse is an investigator for Johnson and Johnson. Dr. Goldschmidt is paid to speak by Novartis. Dr. Handelsman reported research grants, consulting work, and speaker honoraria from Amgen, Gilead, Lilly, Merck, Novo Nordisk, and others. Dr Skolnik reported nonfinancial support from AstraZeneca, Boehringer Ingelheim, Sanofi, and GlaxoSmithKline and personal fees from AstraZeneca, Boehringer Ingelheim, and Eli Lilly. He also serves on the advisory boards of AstraZeneca, Boehringer Ingelheim, Teva Pharmaceutical, Eli Lilly, Sanofi, Janssen Pharmaceuticals, Intarcia, Mylan, and GlaxoSmithKline.
Dr. Paauw and Dr. Skolnik are columnists for Family Practice News and Internal Medicine News.
M. Alexander Otto contributed to this report.

The options for treating type 2 diabetes without insulin have grown beyond metformin to include a long list of sodium-glucose cotransporter 2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) receptor agonists that can be taken with or without metformin. These new drugs have cardiovascular and kidney benefits and help with weight loss, but they also carry risks and, according to some experts, their costs can be prohibitively expensive.
Given the medical community’s long-term experience with treating patients with metformin, and metformin’s lower cost, most of the physicians interviewed for this article advise using SGLT2 inhibitors and GLP-1 receptor agonists as second-line treatments. Others said that they would prefer to use the newer drugs as first-line therapies in select high-risk patients, but prior authorization hurdles created by insurance companies make that approach too burdensome.
“The economics of U.S. health care is stacked against many of our patients with diabetes in the current era,” Robert H. Hopkins Jr., MD, said in an interview.
Even when their insurance approves the drugs, patients still may not be able to afford the copay, explained Dr. Hopkins, professor of internal medicine and pediatrics and director of the division of general internal medicine at the University of Arkansas for Medical Sciences, Little Rock. “Sometimes patients can purchase drugs at a lower cost than the copay to purchase with the ‘drug coverage’ in their insurance plan – unfortunately, this is not the case with the newer diabetes medications we are discussing here.”
“SGLT2 inhibitors and GLP-1 agonists can cost several hundred dollars a month, and insurers often balk at paying for them. They’ll say, ‘Have you tried metformin?’ ” explained endocrinologist Victor Lawrence Roberts, MD, in a interview. “We have to work with insurance companies the best we can in a stepwise fashion.”
According to Dr. Roberts, 80% of his patients with diabetes struggle with the cost of medicine in general. “They’re either underinsured or not insured or their formulary is limited.
Douglas S. Paauw, MD, agreed in an interview that the newer drugs can be problematic on the insurance front.
“For some patients they aren’t affordable, especially for the uninsured if you can’t get them on an assistance program,” said Dr. Paauw, who is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the university.
Dr. Hopkins, who is on the Internal Medicine News board, noted that “unfortunately, the treatment of type 2 diabetes in patients who cannot achieve control with metformin, diet, weight control, and exercise is a story of the ‘haves’ and the ‘have nots.’ The ‘haves’ are those who have pharmacy benefits which make access to newer agents like SGLT2 inhibitors and GLP-1 agonists a possibility.”
“I have had very few of the ‘have nots’ who have been able to even consider these newer agents, which carry price tags of $600-$1,300 a month even with the availability of discounting coupons in the marketplace,” he added. “Most of these patients end up requiring a sulfonylurea or TZD [thiazolidinedione] as a second agent to achieve glycemic control. This makes it very difficult to achieve sufficient weight and metabolic control to avoid an eventual switch to insulin.”
Fatima Z. Syed, MD, an endocrine-trained general internist at DukeHealth in Durham, N.C., said she prescribes SGLT2 inhibitors and GLP-1 receptor agonists in combination with metformin. “I prescribe them frequently, but they are not first-line treatments,” she explained.
“Nothing replaces diet and exercise” as therapy for patients with type 2 diabetes, she added.
Neil S. Skolnik, MD, said that insurance companies were not preventing patients from using these drugs in his experience. He also provided an optimistic take on the accessibility of these drugs in the near future.
“Most insurance companies are now covering select SGLT2 inhibitors and GLP-1 receptor agonists for appropriate patients and those companies that currently do not will soon have to,” said Dr. Skolnik, who is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
“The outcomes [associated with use of the new drugs] are robust, the benefits are large, and are well worth the cost,” he added.
The side effects
While others praised these drugs for their beneficial effects, they also noted that the side effects of these drugs are serious and must be discussed with patients.
GLP-1 receptor agonists are linked to gastrointestinal symptoms, especially nausea, while SGLT2 inhibitors have been linked to kidney failure, ketoacidosis, and more. The Food and Drug Administration warned in 2018 that the SGLT2 inhibitors can cause a rare serious infection known as Fournier’s gangrene – necrotizing fasciitis of the perineum.
“We have to tell our patients to let us know right away if they get pain or swelling in the genital area,” Dr. Paauw, who is on the Internal Medicine News board, noted. “The chance that an infection could explode quickly is higher in those who take these drugs.”
Amputation risks also are associated with taking the SGLT2 inhibitor canagliflozin (Invokana). The FDA requires the manufacturer of this drug to include a black-box warning about the risk of “lower-limb amputations, most frequently of the toe and midfoot,” but also the leg. In approval trials, the risk doubled versus placebo.
These amputation risks “put a damper on some of the enthusiasm on behalf of physicians and patients ... for taking this drug,” noted Dr. Roberts, who is a professor of internal medicine at the University of Central Florida, Orlando.
While a manufacturer-funded study released last year found no link to amputations, the results weren’t powerful enough to rule out a moderately increased risk.
“[If] you are at high risk for having an amputation, we really have to take this risk very seriously,” said John B. Buse, MD, chief of the division of endocrinology at the University of North Carolina at Chapel Hill, in a presentation about the study at the 2018 annual scientific sessions of the American Diabetes Association.
The benefits
Despite these risks of adverse events, most interviewed agreed that the many benefits observed in those taking SGLT2 inhibitors or GLP-1 receptor agonists make them worth prescribing, at least to those who are able to afford them.
Both SGLT2 inhibitors and GLP-1 receptor agonists appear to have significant cardiovascular benefits. A 2019 meta-analysis and systematic review found that both drugs reduced major adverse cardiac events by about 12% (Circulation. 2019 Apr 23;139[17]:2022-31).
“They don’t cause hypoglycemia, they lower blood pressure, they don’t cause weight gain, and they might promote weight loss,” noted Dr. Paauw.
SGLT2 inhibitors also have shown signs of kidney benefits. The CREDENCE trial linked canagliflozin to a lowering of kidney disorders versus placebo (N Engl J Med. 2019 Jun 13;380[24]:2295-306). “The relative risk of the renal-specific composite of end-stage kidney disease, a doubling of the creatinine level, or death from renal causes was lower by 34% (hazard ratio, 0.66; 95% confidence interval, 0.53-0.81; P less than .001), and the relative risk of end-stage kidney disease was lower by 32% (HR, 0.68; 95% CI, 0.54-0.86; P = .002),” the trial investigators wrote.
“They showed very nicely that the drug improved the kidney function of those patients and reduced the kidney deterioration,” said Yehuda Handelsman, MD, an endocrinologist in Tarzana, Calif., who chaired the 2011 and 2015 American Association of Clinical Endocrinologists’ Comprehensive Diabetes Guidelines. The study was especially impressive, he added, because it included patients with low kidney function.
SGLT2 inhibitors’ “diuretic mechanism explains why there is a substantial reduction in heart failure hospitalizations in patients who take these drugs,” said cardiologist Marc E. Goldschmidt, MD, director of the Heart Success Program at Atlantic Health System’s Morristown (N.J.) Medical Center, in an interview. “Both the EMPA-REG Outcome and the CREDENCE trials demonstrated substantial benefit of this class of medications by showing a lower risk of cardiovascular death as well as death from any cause and a lower risk of hospitalization for heart failure."
Overall, the SGLT2 trial data have been very consistent with a benefit for cardiovascular risk reduction, particularly in regard to heart failure hospitalizations and even in potentially preventing heart failure in diabetics,” he added.
Dr. Skolnik, a columnist for Family Practice News, cited SGLT2 inhibitors and GLP-1 receptor agonists’ ability to slow renal disease progression, promote weight loss, and prevent poor cardiac outcomes.“These drugs should be used, in addition to metformin, in all patients with diabetes and vascular disease. These proven outcomes are far better than we ever were able to achieve previously and the strength of the evidence at this point is very strong,” said Dr. Skolnik. “In addition to the benefits of decreasing the development of cardiovascular disease, serious heart failure, and slowing progression of renal disease, these two classes of medication have additional benefits. Both classes help patients lose weight, which is very different from what was found with either sulfonylureas or insulin, which cause patients to gain weight. Also both the SGLT2 inhibitors and the GLP-1 RAs [receptor agonists] have a low incidence of hypoglycemia. For all these reasons, these have become important medications for us to use in primary care.”
Other recent trials offer “very powerful data” about SGLT2 inhibitors, Dr. Roberts said. That’s good news, since “our approach needs to be toward cardiovascular protection and preservation as well as managing blood sugar.”An Israeli trial, whose results were released in May 2019 at the annual meeting of the American College of Cardiology, found that, compared with other glucose-lowering drugs, taking an SGLT2 inhibitor was associated with lower risks of heart failure hospitalization and all-cause mortality (HR, 0.54; 95% CI, 0.44-0.65; P less than .001). This trial also offered a new detail: The patients gained the benefit regardless of whether their baseline left ventricular ejection fraction was preserved or reduced (J Coll Cardiol. 2019 Mar;73[9]:suppl 1). The SGLT2 inhibitors used in this trial included dapagliflozin (Farxiga) and empagliflozin (Jardiance).
In another study released this year, a subanalysis of the DECLARE-TIMI 58 trial, researchers reported that the SGLT2 inhibitor dapagliflozin reduced risks of both major adverse cardiovascular events and heart failure hospitalization in the subset of patients with type 2 diabetes and prior myocardial infarction versus controls (Circulation. 2019 May 28;139[22]:2516-27). The absolute risk reduction for major adverse cardiovascular events was 1.9% (HR, 0.81; 95% CI, 0.65-1.00; P = .046), while it was 0.6% for heart failure hospitalization (HR, 0.85; 95% CI, 0.72-1.00; P = .055).
These and other studies “speak volumes about the efficacy of managing blood sugar and addressing our biggest nemesis, which is cardiovascular disease,” Dr. Roberts said. “It’s irrefutable. The data [are] very good.”
Dr. Paauw said an SGLT2 inhibitor or GLP-1 receptor agonist is best reserved for use in select patients with cardiovascular risks and type 2 diabetes that need management beyond metformin.
For example, they might fit a 70-year-old with persistent hypertension who’s already taking a couple of blood pressure medications. “If they have another cardiovascular risk factor, the cardiovascular protection piece will be a bigger deal,” he said. Also, “it will probably help lower their blood pressure so they can avoid taking another blood pressure medicine.”
Trials of both GLP-1 receptor agonists and SGLT2 inhibitors have shown benefits “in improving [major adverse cardiac events], with the SGLT2 class showing substantial benefit in improving both heart failure and renal outcomes as well,” noted Dr. Skolnik. “It is in this context that one must address the question of whether the price of the medications are worthwhile. With such substantial benefit, there is no question in my mind that – for patients who have underlying cardiovascular illness, which includes patients with existent coronary disease, history of stroke, transient ischemic attack, or peripheral vascular disease – it is far and away worth it to prescribe these classes of medications.”
Indeed, the American Diabetes Association and the European Association for the Study of Diabetes’ most recent guidelines now call for a GLP-1 receptor agonist – instead of insulin – to be the first injectable used to treat type 2 diabetes (Diabetes Care 2018 Dec; 41[12]:2669-701).
“For the relatively small number of my patients who have been able to access and use these medications for months or longer, more have tolerated the GLP-1 agonists than SGLT2 inhibitors primarily due to urinary issues,” noted Dr. Hopkins.
Dipeptidyl peptidase–4 inhibitors are another option in patients with type 2 diabetes, but research suggests they may not be a top option for patients with cardiovascular risk. A 2018 review noted that cardiovascular outcome trials for alogliptin (Nesina), saxagliptin (Onglyza), and sitagliptin (Januvia) showed noninferiority but failed to demonstrate any superiority, compared with placebo in patients with type 2 diabetes mellitus and high cardiovascular risk (Circ Res. 2018 May 11;122[10]:1439-59).
The combination therapies
Many of the newer drugs are available as combinations with other types of diabetes drugs. In some cases, physicians create their own form of combination therapy by separately prescribing two or more diabetes drugs. Earlier this year, a study suggested the benefits of this kind of add-on therapy: Diabetes outcomes improved in patients who took the GLP-1 receptor agonist semaglutide and an SGLT2 inhibitor (Lancet Diabetes Endocrinol. 2019 Mar 1. doi: 10.1016/S2213-8587[19]30066-X).
Dr. Roberts suggested caution, however, when prescribing combination therapies. “My recommendation is always to begin with the individual medications to see if the patient tolerates the drugs and then decide which component needs to be titrated. It’s hard to titrate a combination drug, and it doesn’t leave a lot of flexibility. You never know which drug is doing what.
Dr. Handelsman said some patients may need to take three medications such as metformin, an SGLT2 inhibitor, and a GLP-1 receptor agonist.
“I don’t recommend using the combinations if you’re not familiar with the drugs ... These are relatively new pharmaceuticals, and most of us are on a learning curve as to how they fit into the armamentarium. If a drug is tolerated with a good response, you can certainly consider going to the combination tablets,” he added.
There is at least one drug that combines these three classes: The newly FDA-approved Qternmet XR, which combines dapagliflozin (an SGLT2 inhibitor), saxagliptin (a GLP-1 receptor agonist), and metformin. As of mid-June 2019, it was not yet available in the United States. Its sister drug Qtern, which combines dapagliflozin and saxagliptin, costs more than $500 a month with a free coupon, according to goodrx.com. In contrast, metformin is extremely inexpensive, costing just a few dollars a month for a common starting dose.
What about adding insulin?
“Both [SGLT2 inhibitors and GLP-1 receptor agonists] work very well with insulin,” Dr. Handelsman said. “There is a nice additive effect on the reduction of [hemoglobin] A1c. The only caution is that, although neither SGLT2 inhibitors nor GLP-1 receptor agonists cause hypoglycemia, in combination with insulin they do increase the risk of hypoglycemia. You may have to adjust the dose of insulin.”
Dr. Hopkins warned that cost becomes an even bigger issue when you add insulin into the mix.
“When insulin comes into the discussion, we are again stuck with astronomical costs which many struggle to afford,” he explained.
Indeed, the price tag on these drugs seems to be the biggest problem physicians have with them.
“The challenges in managing patients with diabetes aren’t the risks associated with the drugs. It’s dealing with their insurers,” noted Dr. Roberts.
Dr. Hopkins, Dr. Paauw, Dr. Roberts, and Dr. Syed reported no disclosures. Dr. Buse is an investigator for Johnson and Johnson. Dr. Goldschmidt is paid to speak by Novartis. Dr. Handelsman reported research grants, consulting work, and speaker honoraria from Amgen, Gilead, Lilly, Merck, Novo Nordisk, and others. Dr Skolnik reported nonfinancial support from AstraZeneca, Boehringer Ingelheim, Sanofi, and GlaxoSmithKline and personal fees from AstraZeneca, Boehringer Ingelheim, and Eli Lilly. He also serves on the advisory boards of AstraZeneca, Boehringer Ingelheim, Teva Pharmaceutical, Eli Lilly, Sanofi, Janssen Pharmaceuticals, Intarcia, Mylan, and GlaxoSmithKline.
Dr. Paauw and Dr. Skolnik are columnists for Family Practice News and Internal Medicine News.
M. Alexander Otto contributed to this report.

Court halts rule requiring drug list pricing in advertising
The Department of Health & Human Services lacks the authority to require drug manufacturers to disclose the list price of drugs in television advertising, a district court judge has ruled.
“The court finds that HHS lacks the statutory authority under the Social Security Act to adopt the [Wholesale Acquisition Cost] Disclosure Rule,” Judge Amit P. Mehta of the U.S. District Court for the District of Columbia, said in his July 8 ruling.
“Neither the [Social Security] Act’s text, structure, nor context evince an intent by Congress to empower HHS to issue a rule that compels drug manufacturers to disclose list prices. The rule is therefore invalid,” according to the ruling.
The ruling stems from a case brought against HHS by Merck & Co. Inc., Eli Lilly and Company, and Amgen Inc., along with the National Association of Advertisers Inc., in response to the May 8 final rule that required pharmaceutical manufacturers to include the wholesale acquisition cost (WAC) if above $35, in all television advertising. The final rule also required a disclaimer that if a person had health insurance that covers drugs, “your cost may be different.”
In addition to not having authority, the court took exception to the HHS issuing the rule through the Centers for Medicare & Medicaid Services.
“It has adopted a rule that regulates the conduct of market actors that are not direct participants in the Medicare or Medicaid programs,” the ruling states. “Pharmaceutical manufacturers are not health care providers, private plan carriers, or beneficiaries – each of whom plays a direct role in the public health insurance programs. They do not receive payment for their products from CMS. Their pricing decisions, of course, affect the cost of pharmaceutical benefits offered under the Medicare and Medicaid programs. But those decisions impact the program costs in an indirect way.”
The American Medical Association expressed disappointment that the final rule is not moving forward.
“The AMA supported the Trump Administration’s effort to require pricing information in direct-to-consumer television advertising of prescription drugs,” AMA President Patrice Harris, MD, said in a statement. “Last year, the AMA called for regulations requiring the ads to include the manufacturer’s list price of those drugs, and we have supported similar legislative efforts.”
Dr. Harris noted that having the list price would provide a vital piece of information when patients and their physicians are discussing treatment options, as it would “help patients have a more complete picture when faced with prescription drug ads. While current ads outline the potential benefits and side effects, a crucial factor for patients – the drug’s price – is not included. Patients, especially those who pay a drug’s list price or whose cost-sharing is based on the list price, would benefit by having another tool in their toolbox as they work with their physicians to determine their prescription drug regimens.”
Likewise, AARP also expressed disappointment in the decision, calling it “a step backward in the battle against skyrocketing drug prices and providing more information to consumers. Americans should be trusted to evaluate drug price information and discuss any concerns with their health care providers.”
Judge Mehta noted in his ruling that “the court does not question HHS’ motives in adopting the WAC Disclosure Rule. ... That policy very well could be an effective tool in halting the rising cost of prescription drugs. But no matter how vexing the problem of spiraling drug costs may be, HHS cannot do more than what Congress has authorized. The responsibility rests with Congress to act in the first instance.”
The Department of Health & Human Services lacks the authority to require drug manufacturers to disclose the list price of drugs in television advertising, a district court judge has ruled.
“The court finds that HHS lacks the statutory authority under the Social Security Act to adopt the [Wholesale Acquisition Cost] Disclosure Rule,” Judge Amit P. Mehta of the U.S. District Court for the District of Columbia, said in his July 8 ruling.
“Neither the [Social Security] Act’s text, structure, nor context evince an intent by Congress to empower HHS to issue a rule that compels drug manufacturers to disclose list prices. The rule is therefore invalid,” according to the ruling.
The ruling stems from a case brought against HHS by Merck & Co. Inc., Eli Lilly and Company, and Amgen Inc., along with the National Association of Advertisers Inc., in response to the May 8 final rule that required pharmaceutical manufacturers to include the wholesale acquisition cost (WAC) if above $35, in all television advertising. The final rule also required a disclaimer that if a person had health insurance that covers drugs, “your cost may be different.”
In addition to not having authority, the court took exception to the HHS issuing the rule through the Centers for Medicare & Medicaid Services.
“It has adopted a rule that regulates the conduct of market actors that are not direct participants in the Medicare or Medicaid programs,” the ruling states. “Pharmaceutical manufacturers are not health care providers, private plan carriers, or beneficiaries – each of whom plays a direct role in the public health insurance programs. They do not receive payment for their products from CMS. Their pricing decisions, of course, affect the cost of pharmaceutical benefits offered under the Medicare and Medicaid programs. But those decisions impact the program costs in an indirect way.”
The American Medical Association expressed disappointment that the final rule is not moving forward.
“The AMA supported the Trump Administration’s effort to require pricing information in direct-to-consumer television advertising of prescription drugs,” AMA President Patrice Harris, MD, said in a statement. “Last year, the AMA called for regulations requiring the ads to include the manufacturer’s list price of those drugs, and we have supported similar legislative efforts.”
Dr. Harris noted that having the list price would provide a vital piece of information when patients and their physicians are discussing treatment options, as it would “help patients have a more complete picture when faced with prescription drug ads. While current ads outline the potential benefits and side effects, a crucial factor for patients – the drug’s price – is not included. Patients, especially those who pay a drug’s list price or whose cost-sharing is based on the list price, would benefit by having another tool in their toolbox as they work with their physicians to determine their prescription drug regimens.”
Likewise, AARP also expressed disappointment in the decision, calling it “a step backward in the battle against skyrocketing drug prices and providing more information to consumers. Americans should be trusted to evaluate drug price information and discuss any concerns with their health care providers.”
Judge Mehta noted in his ruling that “the court does not question HHS’ motives in adopting the WAC Disclosure Rule. ... That policy very well could be an effective tool in halting the rising cost of prescription drugs. But no matter how vexing the problem of spiraling drug costs may be, HHS cannot do more than what Congress has authorized. The responsibility rests with Congress to act in the first instance.”
The Department of Health & Human Services lacks the authority to require drug manufacturers to disclose the list price of drugs in television advertising, a district court judge has ruled.
“The court finds that HHS lacks the statutory authority under the Social Security Act to adopt the [Wholesale Acquisition Cost] Disclosure Rule,” Judge Amit P. Mehta of the U.S. District Court for the District of Columbia, said in his July 8 ruling.
“Neither the [Social Security] Act’s text, structure, nor context evince an intent by Congress to empower HHS to issue a rule that compels drug manufacturers to disclose list prices. The rule is therefore invalid,” according to the ruling.
The ruling stems from a case brought against HHS by Merck & Co. Inc., Eli Lilly and Company, and Amgen Inc., along with the National Association of Advertisers Inc., in response to the May 8 final rule that required pharmaceutical manufacturers to include the wholesale acquisition cost (WAC) if above $35, in all television advertising. The final rule also required a disclaimer that if a person had health insurance that covers drugs, “your cost may be different.”
In addition to not having authority, the court took exception to the HHS issuing the rule through the Centers for Medicare & Medicaid Services.
“It has adopted a rule that regulates the conduct of market actors that are not direct participants in the Medicare or Medicaid programs,” the ruling states. “Pharmaceutical manufacturers are not health care providers, private plan carriers, or beneficiaries – each of whom plays a direct role in the public health insurance programs. They do not receive payment for their products from CMS. Their pricing decisions, of course, affect the cost of pharmaceutical benefits offered under the Medicare and Medicaid programs. But those decisions impact the program costs in an indirect way.”
The American Medical Association expressed disappointment that the final rule is not moving forward.
“The AMA supported the Trump Administration’s effort to require pricing information in direct-to-consumer television advertising of prescription drugs,” AMA President Patrice Harris, MD, said in a statement. “Last year, the AMA called for regulations requiring the ads to include the manufacturer’s list price of those drugs, and we have supported similar legislative efforts.”
Dr. Harris noted that having the list price would provide a vital piece of information when patients and their physicians are discussing treatment options, as it would “help patients have a more complete picture when faced with prescription drug ads. While current ads outline the potential benefits and side effects, a crucial factor for patients – the drug’s price – is not included. Patients, especially those who pay a drug’s list price or whose cost-sharing is based on the list price, would benefit by having another tool in their toolbox as they work with their physicians to determine their prescription drug regimens.”
Likewise, AARP also expressed disappointment in the decision, calling it “a step backward in the battle against skyrocketing drug prices and providing more information to consumers. Americans should be trusted to evaluate drug price information and discuss any concerns with their health care providers.”
Judge Mehta noted in his ruling that “the court does not question HHS’ motives in adopting the WAC Disclosure Rule. ... That policy very well could be an effective tool in halting the rising cost of prescription drugs. But no matter how vexing the problem of spiraling drug costs may be, HHS cannot do more than what Congress has authorized. The responsibility rests with Congress to act in the first instance.”
Industry payments influence prescription choices
Two studies show a link between industry payments by drug manufacturers to physicians and doctors’ prescribing patterns for certain medications.
In the first study, lead author Taeho Greg Rhee, PhD, of the University of Connecticut, Farmington, and colleagues analyzed Centers for Medicare & Medicaid Services Part D data and Open Payments data for general payments from industry to physicians associated with gabapentinoids.
Specifically, investigators examined data for three brand name products: Gralise (Assertio) and Horizant (Arbor), both of which are extended release formulas approved for the treatment of seizure disorders and postherpetic neuralgia, and Lyrica (Pfizer), which is approved for treatment of seizure disorders, postherpetic neuralgia, neuropathic pain, and fibromyalgia. To evaluate prescribing patterns, researchers estimated physician prescribing as the physician’s proportion of prescription days filled for the three brand-name gabapentinoids in aggregate of all gabapentinoid prescription days filled.
Between 2014 and 2016, manufacturers of the three brand-name gabapentinoids made approximately 510,000 general payments ($11.5 million) to 51,005 physicians, according to Dr. Rhee and colleagues. The doctors represented 14% of physicians who prescribed any gabapentinoid product under Part D during the same time period.
Among physicians who prescribed any gabapentinoid, generic forms of Gralise (gabapentin; 87%) and Lyrica (pregabalin; 12%) were most frequently prescribed. However, physicians receiving payments from industry were more likely to prescribe the three brand-name gabapentinoids than they were gabapentin (JAMA Intern Med. 2019 July 8. doi: 10.1001/jamainternmed.2019.1082).
Generalist physicians received the majority of payments (62%) payments totaling about $4 million, followed by about $7 million for pain medication specialists and $1 million for other physicians.
The majority of payments were for food and beverages, gifts, or educational materials. In addition, industry payments were most commonly paid to physicians in the southern and eastern regions of the United States.
Among physicians who prescribed gabapentinoids, industry payment was associated with a higher likelihood of prescribing brand-name products than generic gabapentin and that such prescribing patterns increase Medicare spending. Data show that brand name gabapentinoids typically cost account for nearly $2,500 in mean Medicare spending per beneficiary in 2016, compared with less than $20 for a 1-month supply of gabapentin, authors noted.
In the second study, Rishad Khan, MD, of the University of Toronto and colleagues examined the association between industry payments to physicians and Medicare spending on adalimumab (Humira; AbbVie) and certolizumab (Cimzia; Union Chimique Belge), both of which are approved for Crohn’s disease and numerous other indications. Investigators analyzed CMS Part D data and Open Payments data linked to the prescribing of adalimumab and certolizumab. Payments were considered relevant if a gastroenterologist received them from a drug manufacturer the year that the medication was prescribed.
From 2014 to 2016, drug makers made more than $10 million in payments to gastroenterologists prescribing adalimumab or certolizumab, the study found. Investigators found that for every $1 in physician payments, there was a $3.16 increase in spending for adalimumab and a $4.72 increase for certolizumab (JAMA Intern Med. 2019 July 8. doi: 10.1001/jamainternmed.2019.0999).
For adalimumab, payments totaled $5.5 million for speaking and consulting, $4.9 million for food, travel,and lodging expenses, and $13,000 for education. For certolizumab, payments totaled $180,000 for speaking and consulting, $117,000 for food, travel,and lodging expenses, and $60,000 for education.
Dr. Khan and associates concluded that the findings suggest a significant association between industry payments by drug manufacturers to physicians and Medicare spending.
The studies by Rhee et. al. and Khan et. al. add to previous research finding that marketing to physicians is associated with increased sales of a company’s product and higher Medicare expenditures.
While the analyses do not account for other influences on prescribing, such as direct-to-consumer advertising, the pattern they illustrate is indisputable.
Drug manufacturers market to physicians because they write the prescriptions; however, that marketing can obscure the fact that generic drugs are just as effective and generally less expensive than brand-name medications. When there are choices, the generics should be prescribed.
The growing research demonstrating a link between industry payments and physicians’ prescribing of brand-name medications raise troubling questions about whether such payments are in the best interest of patients.
Robert Steinbrook, MD, is editor at large for JAMA Internal Medicine. His comments are adapted from an editorial (JAMA Intern Med. 2019 July 8. doi:10.1001/jamainternmed.2019.1081) accompanying the studies by Rhee et al. and Khan et al.
The studies by Rhee et. al. and Khan et. al. add to previous research finding that marketing to physicians is associated with increased sales of a company’s product and higher Medicare expenditures.
While the analyses do not account for other influences on prescribing, such as direct-to-consumer advertising, the pattern they illustrate is indisputable.
Drug manufacturers market to physicians because they write the prescriptions; however, that marketing can obscure the fact that generic drugs are just as effective and generally less expensive than brand-name medications. When there are choices, the generics should be prescribed.
The growing research demonstrating a link between industry payments and physicians’ prescribing of brand-name medications raise troubling questions about whether such payments are in the best interest of patients.
Robert Steinbrook, MD, is editor at large for JAMA Internal Medicine. His comments are adapted from an editorial (JAMA Intern Med. 2019 July 8. doi:10.1001/jamainternmed.2019.1081) accompanying the studies by Rhee et al. and Khan et al.
The studies by Rhee et. al. and Khan et. al. add to previous research finding that marketing to physicians is associated with increased sales of a company’s product and higher Medicare expenditures.
While the analyses do not account for other influences on prescribing, such as direct-to-consumer advertising, the pattern they illustrate is indisputable.
Drug manufacturers market to physicians because they write the prescriptions; however, that marketing can obscure the fact that generic drugs are just as effective and generally less expensive than brand-name medications. When there are choices, the generics should be prescribed.
The growing research demonstrating a link between industry payments and physicians’ prescribing of brand-name medications raise troubling questions about whether such payments are in the best interest of patients.
Robert Steinbrook, MD, is editor at large for JAMA Internal Medicine. His comments are adapted from an editorial (JAMA Intern Med. 2019 July 8. doi:10.1001/jamainternmed.2019.1081) accompanying the studies by Rhee et al. and Khan et al.
Two studies show a link between industry payments by drug manufacturers to physicians and doctors’ prescribing patterns for certain medications.
In the first study, lead author Taeho Greg Rhee, PhD, of the University of Connecticut, Farmington, and colleagues analyzed Centers for Medicare & Medicaid Services Part D data and Open Payments data for general payments from industry to physicians associated with gabapentinoids.
Specifically, investigators examined data for three brand name products: Gralise (Assertio) and Horizant (Arbor), both of which are extended release formulas approved for the treatment of seizure disorders and postherpetic neuralgia, and Lyrica (Pfizer), which is approved for treatment of seizure disorders, postherpetic neuralgia, neuropathic pain, and fibromyalgia. To evaluate prescribing patterns, researchers estimated physician prescribing as the physician’s proportion of prescription days filled for the three brand-name gabapentinoids in aggregate of all gabapentinoid prescription days filled.
Between 2014 and 2016, manufacturers of the three brand-name gabapentinoids made approximately 510,000 general payments ($11.5 million) to 51,005 physicians, according to Dr. Rhee and colleagues. The doctors represented 14% of physicians who prescribed any gabapentinoid product under Part D during the same time period.
Among physicians who prescribed any gabapentinoid, generic forms of Gralise (gabapentin; 87%) and Lyrica (pregabalin; 12%) were most frequently prescribed. However, physicians receiving payments from industry were more likely to prescribe the three brand-name gabapentinoids than they were gabapentin (JAMA Intern Med. 2019 July 8. doi: 10.1001/jamainternmed.2019.1082).
Generalist physicians received the majority of payments (62%) payments totaling about $4 million, followed by about $7 million for pain medication specialists and $1 million for other physicians.
The majority of payments were for food and beverages, gifts, or educational materials. In addition, industry payments were most commonly paid to physicians in the southern and eastern regions of the United States.
Among physicians who prescribed gabapentinoids, industry payment was associated with a higher likelihood of prescribing brand-name products than generic gabapentin and that such prescribing patterns increase Medicare spending. Data show that brand name gabapentinoids typically cost account for nearly $2,500 in mean Medicare spending per beneficiary in 2016, compared with less than $20 for a 1-month supply of gabapentin, authors noted.
In the second study, Rishad Khan, MD, of the University of Toronto and colleagues examined the association between industry payments to physicians and Medicare spending on adalimumab (Humira; AbbVie) and certolizumab (Cimzia; Union Chimique Belge), both of which are approved for Crohn’s disease and numerous other indications. Investigators analyzed CMS Part D data and Open Payments data linked to the prescribing of adalimumab and certolizumab. Payments were considered relevant if a gastroenterologist received them from a drug manufacturer the year that the medication was prescribed.
From 2014 to 2016, drug makers made more than $10 million in payments to gastroenterologists prescribing adalimumab or certolizumab, the study found. Investigators found that for every $1 in physician payments, there was a $3.16 increase in spending for adalimumab and a $4.72 increase for certolizumab (JAMA Intern Med. 2019 July 8. doi: 10.1001/jamainternmed.2019.0999).
For adalimumab, payments totaled $5.5 million for speaking and consulting, $4.9 million for food, travel,and lodging expenses, and $13,000 for education. For certolizumab, payments totaled $180,000 for speaking and consulting, $117,000 for food, travel,and lodging expenses, and $60,000 for education.
Dr. Khan and associates concluded that the findings suggest a significant association between industry payments by drug manufacturers to physicians and Medicare spending.
Two studies show a link between industry payments by drug manufacturers to physicians and doctors’ prescribing patterns for certain medications.
In the first study, lead author Taeho Greg Rhee, PhD, of the University of Connecticut, Farmington, and colleagues analyzed Centers for Medicare & Medicaid Services Part D data and Open Payments data for general payments from industry to physicians associated with gabapentinoids.
Specifically, investigators examined data for three brand name products: Gralise (Assertio) and Horizant (Arbor), both of which are extended release formulas approved for the treatment of seizure disorders and postherpetic neuralgia, and Lyrica (Pfizer), which is approved for treatment of seizure disorders, postherpetic neuralgia, neuropathic pain, and fibromyalgia. To evaluate prescribing patterns, researchers estimated physician prescribing as the physician’s proportion of prescription days filled for the three brand-name gabapentinoids in aggregate of all gabapentinoid prescription days filled.
Between 2014 and 2016, manufacturers of the three brand-name gabapentinoids made approximately 510,000 general payments ($11.5 million) to 51,005 physicians, according to Dr. Rhee and colleagues. The doctors represented 14% of physicians who prescribed any gabapentinoid product under Part D during the same time period.
Among physicians who prescribed any gabapentinoid, generic forms of Gralise (gabapentin; 87%) and Lyrica (pregabalin; 12%) were most frequently prescribed. However, physicians receiving payments from industry were more likely to prescribe the three brand-name gabapentinoids than they were gabapentin (JAMA Intern Med. 2019 July 8. doi: 10.1001/jamainternmed.2019.1082).
Generalist physicians received the majority of payments (62%) payments totaling about $4 million, followed by about $7 million for pain medication specialists and $1 million for other physicians.
The majority of payments were for food and beverages, gifts, or educational materials. In addition, industry payments were most commonly paid to physicians in the southern and eastern regions of the United States.
Among physicians who prescribed gabapentinoids, industry payment was associated with a higher likelihood of prescribing brand-name products than generic gabapentin and that such prescribing patterns increase Medicare spending. Data show that brand name gabapentinoids typically cost account for nearly $2,500 in mean Medicare spending per beneficiary in 2016, compared with less than $20 for a 1-month supply of gabapentin, authors noted.
In the second study, Rishad Khan, MD, of the University of Toronto and colleagues examined the association between industry payments to physicians and Medicare spending on adalimumab (Humira; AbbVie) and certolizumab (Cimzia; Union Chimique Belge), both of which are approved for Crohn’s disease and numerous other indications. Investigators analyzed CMS Part D data and Open Payments data linked to the prescribing of adalimumab and certolizumab. Payments were considered relevant if a gastroenterologist received them from a drug manufacturer the year that the medication was prescribed.
From 2014 to 2016, drug makers made more than $10 million in payments to gastroenterologists prescribing adalimumab or certolizumab, the study found. Investigators found that for every $1 in physician payments, there was a $3.16 increase in spending for adalimumab and a $4.72 increase for certolizumab (JAMA Intern Med. 2019 July 8. doi: 10.1001/jamainternmed.2019.0999).
For adalimumab, payments totaled $5.5 million for speaking and consulting, $4.9 million for food, travel,and lodging expenses, and $13,000 for education. For certolizumab, payments totaled $180,000 for speaking and consulting, $117,000 for food, travel,and lodging expenses, and $60,000 for education.
Dr. Khan and associates concluded that the findings suggest a significant association between industry payments by drug manufacturers to physicians and Medicare spending.
EULAR keeps csDMARDs as top PsA drugs
MADRID – The draft revision of the European League Against Rheumatism’s recommendations for managing psoriatic arthritis sticks with the group’s already-existing conviction that psoriatic arthritis treatment best starts with an NSAID, and if that fails follow with a conventional synthetic antirheumatic drug such as methotrexate, a position in stark contrast with the 2018 recommendation from the American College of Rheumatology to first treat with a tumor necrosis factor (TNF) inhibitor.
For patients with psoriatic arthritis (PsA) manifesting with polyarthritis, conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) “should be first,” and should “start rapidly” if brief, initial treatment with an NSAID proves inadequate, Laure Gossec, MD, PhD, said while presenting a draft version of an update to the PsA management recommendations from EULAR at the European Congress of Rheumatology.
The EULAR recommendations-revision panel had about the same advice for managing PsA patients with oligoarthritis, monoarthritis, or peripheral arthritis. For oligo- and monoarthritis, “consider a csDMARD after failing NSAIDS, but also consider the patient’s prognostic factors,” like structural damage, and dactylitis. For PsA patients with peripheral arthritis, “it still makes sense to keep csDMARDs as the first line treatment,” said Dr. Gossec, professor of rheumatology at Pitie-Salpétriere Hospital and Sorbonne University, Paris. Once published, the revision will replace existing EULAR recommendations from 2015 (Ann Rheum Dis. 2016 Mar;75[3]:499-510).
The list of csDMARDs she cited included not just methotrexate, still the top csDMARD, but also sulfasalazine and leflunomide as alternatives, she noted, with methotrexate also the preferred csDMARD for patients with skin involvement. When a PsA patient fails at least one csDMARD, then switching to a biologic DMARD is recommended. For a patient with skin involvement, a drug that targets interleukin-17 or IL-12 and -23 is preferred. If skin involvement is not a major issue, then treatment with a TNF inhibitor is equally valid, she said.
The 2018 PsA management guideline from the American College of Rheumatology (ACR) proposed a strikingly different sequence, endorsing initial treatment with a TNF inhibitor first over all other options, including methotrexate and other “oral small molecules” (the ACR term for csDMARD), and also including NSAIDs (Arthritis Rheumatol. 2019 Jan;71[1]:5-32).
This schism between EULAR and the ACR could be seen as predictable, given the different constraints the two societies have set for themselves.
“EULAR recommendations take into account drug costs; the ACR guideline is supposed to be agnostic to costs,” explained Philip J. Mease, MD, a rheumatologist at Swedish Medical Center in Seattle and a member of the ACR panel that wrote the 2018 PsA guideline.
In fact it was a study Dr. Mease recently led and reported results from that provided the most recent and perhaps best assessment of a TNF inhibitor, compared with methotrexate, as initial treatment for PsA, with findings that suggest that, although the advice from the two societies may sharply differ, the viewpoints of both groups are evidence based.
The SEAM-PsA (Study of Etanercept and Methotrexate in Subjects with Psoriatic Arthritis) trial randomized 851 PsA patients receiving their first treatment to methotrexate only, the TNF inhibitor etanercept (Enbrel) only, or both drugs. The study’s two coprimary outcomes, the ACR 20 and minimal disease activity responses after 24 weeks, showed that etanercept monotherapy produced these responses in 61% and 36% of patients, respectively, while methotrexate monotherapy produced response rates of 51% and 23%, respectively. Both these differences between etanercept monotherapy and methotrexate monotherapy were statistically significant. Combining methotrexate with etanercept did not produce a significant improvement over etanercept alone.
Interpreting the meaning of this finding for clinical practice “depends on the lens you look through,” Dr. Mease said in an interview. “A lot of patients respond to methotrexate, which is good when treatment resources are challenged. But when there is no resource challenge, the data support going straight to a TNF inhibitor.”
Dr. Gossec confirmed the importance of the SEAM-PsA findings in the writing panel’s decision during discussion of the draft, replying to a question about consideration of the study’s findings. “We carefully looked at the SEAM-PsA trial results, which provide some of the only data we have on methotrexate” for PsA. “We felt that the results were in favor of methotrexate’s efficacy, and therefore did not go against our proposal to keep a graduated approach starting with a csDMARD.”
Patients who fail to receive adequate relief from a csDMARD could then try a biologic DMARD – a TNF inhibitor, IL-17 inhibitor, or IL-12/23 inhibitor, Dr. Gossec said. When skin involvement is minimal, any of these options are possible, she said. If skin involvement is significant, the panel recommended preferentially using an IL-17 or IL-12/23 inhibitor based on head-to-head trials in patients with psoriasis, she said.
When a biologic DMARD is not appropriate or fails, another option is to then try a targeted synthetic DMARD, such as a Janus kinase inhibitor. When none of these options are appropriate, or they all fail, another option for patients with mild oligo- or monoarthritis or in patients with limited skin involvement is apremilast (Otezla), a phosphodiesterase-4 inhibitor. The draft recommendations also advise clinicians to be sure to distinguish fibromyalgia pain from enthesitis involvement, and they introduce the possibility of, with “great caution,” tapering down DMARD treatment in PsA patients who show sustained remission.
Dr. Gossec and Dr. Mease have both been consultants to and received honoraria from several companies. SEAM-PsA was sponsored by Amgen, the company that markets Enbrel.
MADRID – The draft revision of the European League Against Rheumatism’s recommendations for managing psoriatic arthritis sticks with the group’s already-existing conviction that psoriatic arthritis treatment best starts with an NSAID, and if that fails follow with a conventional synthetic antirheumatic drug such as methotrexate, a position in stark contrast with the 2018 recommendation from the American College of Rheumatology to first treat with a tumor necrosis factor (TNF) inhibitor.
For patients with psoriatic arthritis (PsA) manifesting with polyarthritis, conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) “should be first,” and should “start rapidly” if brief, initial treatment with an NSAID proves inadequate, Laure Gossec, MD, PhD, said while presenting a draft version of an update to the PsA management recommendations from EULAR at the European Congress of Rheumatology.
The EULAR recommendations-revision panel had about the same advice for managing PsA patients with oligoarthritis, monoarthritis, or peripheral arthritis. For oligo- and monoarthritis, “consider a csDMARD after failing NSAIDS, but also consider the patient’s prognostic factors,” like structural damage, and dactylitis. For PsA patients with peripheral arthritis, “it still makes sense to keep csDMARDs as the first line treatment,” said Dr. Gossec, professor of rheumatology at Pitie-Salpétriere Hospital and Sorbonne University, Paris. Once published, the revision will replace existing EULAR recommendations from 2015 (Ann Rheum Dis. 2016 Mar;75[3]:499-510).
The list of csDMARDs she cited included not just methotrexate, still the top csDMARD, but also sulfasalazine and leflunomide as alternatives, she noted, with methotrexate also the preferred csDMARD for patients with skin involvement. When a PsA patient fails at least one csDMARD, then switching to a biologic DMARD is recommended. For a patient with skin involvement, a drug that targets interleukin-17 or IL-12 and -23 is preferred. If skin involvement is not a major issue, then treatment with a TNF inhibitor is equally valid, she said.
The 2018 PsA management guideline from the American College of Rheumatology (ACR) proposed a strikingly different sequence, endorsing initial treatment with a TNF inhibitor first over all other options, including methotrexate and other “oral small molecules” (the ACR term for csDMARD), and also including NSAIDs (Arthritis Rheumatol. 2019 Jan;71[1]:5-32).
This schism between EULAR and the ACR could be seen as predictable, given the different constraints the two societies have set for themselves.
“EULAR recommendations take into account drug costs; the ACR guideline is supposed to be agnostic to costs,” explained Philip J. Mease, MD, a rheumatologist at Swedish Medical Center in Seattle and a member of the ACR panel that wrote the 2018 PsA guideline.
In fact it was a study Dr. Mease recently led and reported results from that provided the most recent and perhaps best assessment of a TNF inhibitor, compared with methotrexate, as initial treatment for PsA, with findings that suggest that, although the advice from the two societies may sharply differ, the viewpoints of both groups are evidence based.
The SEAM-PsA (Study of Etanercept and Methotrexate in Subjects with Psoriatic Arthritis) trial randomized 851 PsA patients receiving their first treatment to methotrexate only, the TNF inhibitor etanercept (Enbrel) only, or both drugs. The study’s two coprimary outcomes, the ACR 20 and minimal disease activity responses after 24 weeks, showed that etanercept monotherapy produced these responses in 61% and 36% of patients, respectively, while methotrexate monotherapy produced response rates of 51% and 23%, respectively. Both these differences between etanercept monotherapy and methotrexate monotherapy were statistically significant. Combining methotrexate with etanercept did not produce a significant improvement over etanercept alone.
Interpreting the meaning of this finding for clinical practice “depends on the lens you look through,” Dr. Mease said in an interview. “A lot of patients respond to methotrexate, which is good when treatment resources are challenged. But when there is no resource challenge, the data support going straight to a TNF inhibitor.”
Dr. Gossec confirmed the importance of the SEAM-PsA findings in the writing panel’s decision during discussion of the draft, replying to a question about consideration of the study’s findings. “We carefully looked at the SEAM-PsA trial results, which provide some of the only data we have on methotrexate” for PsA. “We felt that the results were in favor of methotrexate’s efficacy, and therefore did not go against our proposal to keep a graduated approach starting with a csDMARD.”
Patients who fail to receive adequate relief from a csDMARD could then try a biologic DMARD – a TNF inhibitor, IL-17 inhibitor, or IL-12/23 inhibitor, Dr. Gossec said. When skin involvement is minimal, any of these options are possible, she said. If skin involvement is significant, the panel recommended preferentially using an IL-17 or IL-12/23 inhibitor based on head-to-head trials in patients with psoriasis, she said.
When a biologic DMARD is not appropriate or fails, another option is to then try a targeted synthetic DMARD, such as a Janus kinase inhibitor. When none of these options are appropriate, or they all fail, another option for patients with mild oligo- or monoarthritis or in patients with limited skin involvement is apremilast (Otezla), a phosphodiesterase-4 inhibitor. The draft recommendations also advise clinicians to be sure to distinguish fibromyalgia pain from enthesitis involvement, and they introduce the possibility of, with “great caution,” tapering down DMARD treatment in PsA patients who show sustained remission.
Dr. Gossec and Dr. Mease have both been consultants to and received honoraria from several companies. SEAM-PsA was sponsored by Amgen, the company that markets Enbrel.
MADRID – The draft revision of the European League Against Rheumatism’s recommendations for managing psoriatic arthritis sticks with the group’s already-existing conviction that psoriatic arthritis treatment best starts with an NSAID, and if that fails follow with a conventional synthetic antirheumatic drug such as methotrexate, a position in stark contrast with the 2018 recommendation from the American College of Rheumatology to first treat with a tumor necrosis factor (TNF) inhibitor.
For patients with psoriatic arthritis (PsA) manifesting with polyarthritis, conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) “should be first,” and should “start rapidly” if brief, initial treatment with an NSAID proves inadequate, Laure Gossec, MD, PhD, said while presenting a draft version of an update to the PsA management recommendations from EULAR at the European Congress of Rheumatology.
The EULAR recommendations-revision panel had about the same advice for managing PsA patients with oligoarthritis, monoarthritis, or peripheral arthritis. For oligo- and monoarthritis, “consider a csDMARD after failing NSAIDS, but also consider the patient’s prognostic factors,” like structural damage, and dactylitis. For PsA patients with peripheral arthritis, “it still makes sense to keep csDMARDs as the first line treatment,” said Dr. Gossec, professor of rheumatology at Pitie-Salpétriere Hospital and Sorbonne University, Paris. Once published, the revision will replace existing EULAR recommendations from 2015 (Ann Rheum Dis. 2016 Mar;75[3]:499-510).
The list of csDMARDs she cited included not just methotrexate, still the top csDMARD, but also sulfasalazine and leflunomide as alternatives, she noted, with methotrexate also the preferred csDMARD for patients with skin involvement. When a PsA patient fails at least one csDMARD, then switching to a biologic DMARD is recommended. For a patient with skin involvement, a drug that targets interleukin-17 or IL-12 and -23 is preferred. If skin involvement is not a major issue, then treatment with a TNF inhibitor is equally valid, she said.
The 2018 PsA management guideline from the American College of Rheumatology (ACR) proposed a strikingly different sequence, endorsing initial treatment with a TNF inhibitor first over all other options, including methotrexate and other “oral small molecules” (the ACR term for csDMARD), and also including NSAIDs (Arthritis Rheumatol. 2019 Jan;71[1]:5-32).
This schism between EULAR and the ACR could be seen as predictable, given the different constraints the two societies have set for themselves.
“EULAR recommendations take into account drug costs; the ACR guideline is supposed to be agnostic to costs,” explained Philip J. Mease, MD, a rheumatologist at Swedish Medical Center in Seattle and a member of the ACR panel that wrote the 2018 PsA guideline.
In fact it was a study Dr. Mease recently led and reported results from that provided the most recent and perhaps best assessment of a TNF inhibitor, compared with methotrexate, as initial treatment for PsA, with findings that suggest that, although the advice from the two societies may sharply differ, the viewpoints of both groups are evidence based.
The SEAM-PsA (Study of Etanercept and Methotrexate in Subjects with Psoriatic Arthritis) trial randomized 851 PsA patients receiving their first treatment to methotrexate only, the TNF inhibitor etanercept (Enbrel) only, or both drugs. The study’s two coprimary outcomes, the ACR 20 and minimal disease activity responses after 24 weeks, showed that etanercept monotherapy produced these responses in 61% and 36% of patients, respectively, while methotrexate monotherapy produced response rates of 51% and 23%, respectively. Both these differences between etanercept monotherapy and methotrexate monotherapy were statistically significant. Combining methotrexate with etanercept did not produce a significant improvement over etanercept alone.
Interpreting the meaning of this finding for clinical practice “depends on the lens you look through,” Dr. Mease said in an interview. “A lot of patients respond to methotrexate, which is good when treatment resources are challenged. But when there is no resource challenge, the data support going straight to a TNF inhibitor.”
Dr. Gossec confirmed the importance of the SEAM-PsA findings in the writing panel’s decision during discussion of the draft, replying to a question about consideration of the study’s findings. “We carefully looked at the SEAM-PsA trial results, which provide some of the only data we have on methotrexate” for PsA. “We felt that the results were in favor of methotrexate’s efficacy, and therefore did not go against our proposal to keep a graduated approach starting with a csDMARD.”
Patients who fail to receive adequate relief from a csDMARD could then try a biologic DMARD – a TNF inhibitor, IL-17 inhibitor, or IL-12/23 inhibitor, Dr. Gossec said. When skin involvement is minimal, any of these options are possible, she said. If skin involvement is significant, the panel recommended preferentially using an IL-17 or IL-12/23 inhibitor based on head-to-head trials in patients with psoriasis, she said.
When a biologic DMARD is not appropriate or fails, another option is to then try a targeted synthetic DMARD, such as a Janus kinase inhibitor. When none of these options are appropriate, or they all fail, another option for patients with mild oligo- or monoarthritis or in patients with limited skin involvement is apremilast (Otezla), a phosphodiesterase-4 inhibitor. The draft recommendations also advise clinicians to be sure to distinguish fibromyalgia pain from enthesitis involvement, and they introduce the possibility of, with “great caution,” tapering down DMARD treatment in PsA patients who show sustained remission.
Dr. Gossec and Dr. Mease have both been consultants to and received honoraria from several companies. SEAM-PsA was sponsored by Amgen, the company that markets Enbrel.
REPORTING FROM EULAR 2019 CONGRESS
To help patients stay on diabetes regimens: Communicate, educate, and use technology
SAN FRANCISCO – Are you having trouble helping patients take their diabetes medications as directed? Try installing 32-inch screens in the examination rooms for a lab result show-and-tell. Keep pharmaceutical marketers out of your hair (and office).
Those are among the suggestions offered by two physicians during a symposium on drug adherence at the annual scientific sessions of the American Diabetes Association.
“Nonadherence is not a case of patients being bad,” said internist and researcher Niteesh K. Choudhry, MD, PhD, of Harvard Medical School and Brigham and Women’s Hospital, Boston. “When half of your patients are nonadherent, I can guarantee you [they] aren’t trying to hurt themselves.”
According to Dr. Choudhry’s own research published in 2011 and based on 2008 data, about 25% of patients do not fill prescriptions after leaving their doctors’ offices. That level for diabetes medications – 42% of patients – is especially high (Am J Med. 2011;124[11]:1081.e9-22).
Other findings, he said, have suggested that half of patients fail to adhere to evidence-based prescribed regimens over the long term. And three groups have especially low levels of adherence: people of color, women, and patients who are caregivers (possibly because they are too busy caring for others to care for themselves).
Various factors affect adherence, including forgetfulness, drug interactions or side effects, and the different colors and shapes of pills. The latter can confuse patients because colors and shapes may be different from prescription to prescription even for the same medication, he said.
Dr. Choudhry added that there’s another factor: multiple prescriptions from multiple physicians that require multiple pharmacy visits. His findings suggest that adherence improves when prescriptions are consolidated to limit the need to visit the drugstore. “The chaos of our health care system leads to nonadherence,” he said (Arch Intern Med. 2011;171[9]:814-22).
Internist Lawrence Garber, MD, of Reliant Medical Group in Worcester, Mass., offered these tips about boosting drug adherence:
- Develop trust with patients. “They need to trust that I’m their advocate, and that they’re my No. 1 reason for prescribing the medication, and not making myself more money,” he said.
- Provide educational resources. “We give them resources online. If their EHR [electronic health record] identifies that they’re diabetic, then they get information about diabetes printed out.”
- Use technology to promote messages about diabetes. Dr. Garber said his clinic has installed screens in the examination rooms so that he can show patients their data. “It [makes it] very clear for them to see why what they’re doing now is not working,’’ and why there is a need to change to a different regimen. In addition, screens in the waiting room can display educational slides about diabetes.
- Set up clinic-wide medication protocols. “We’ve set up protocols and pathways for diabetes, hypertension, and high cholesterol to make it easy to prescribe medications that are lower cost and to make sure we’re following the same path,” Dr. Garber said.
- Stay independent. “I haven’t seen a drug rep in decades. It’s an organizational policy that we don’t see them, so we’re less likely to be biased.”
- Make it easier for patients to take medications. Dr. Garber urged colleagues to talk to their patients about using strategies such as printed pill schedules, weekly pill organizers, auto refills, and smartphone alarm reminders to facilitate adherence.
And, he said, you may wish to make it clear that you will check on whether prescriptions are filled. That way, “the patients know that you’re looking,” and it can actually lead to improved adherence.
Dr. Choudhry reported that his research has been funded by unrestricted grants to his institution from insurers, government funders, nonprofit foundations, pharmaceutical companies (including Merck, Sanofi, and Astra Zeneca), and device makers (including Medisafe). Dr. Garber reported no relevant disclosures.
SAN FRANCISCO – Are you having trouble helping patients take their diabetes medications as directed? Try installing 32-inch screens in the examination rooms for a lab result show-and-tell. Keep pharmaceutical marketers out of your hair (and office).
Those are among the suggestions offered by two physicians during a symposium on drug adherence at the annual scientific sessions of the American Diabetes Association.
“Nonadherence is not a case of patients being bad,” said internist and researcher Niteesh K. Choudhry, MD, PhD, of Harvard Medical School and Brigham and Women’s Hospital, Boston. “When half of your patients are nonadherent, I can guarantee you [they] aren’t trying to hurt themselves.”
According to Dr. Choudhry’s own research published in 2011 and based on 2008 data, about 25% of patients do not fill prescriptions after leaving their doctors’ offices. That level for diabetes medications – 42% of patients – is especially high (Am J Med. 2011;124[11]:1081.e9-22).
Other findings, he said, have suggested that half of patients fail to adhere to evidence-based prescribed regimens over the long term. And three groups have especially low levels of adherence: people of color, women, and patients who are caregivers (possibly because they are too busy caring for others to care for themselves).
Various factors affect adherence, including forgetfulness, drug interactions or side effects, and the different colors and shapes of pills. The latter can confuse patients because colors and shapes may be different from prescription to prescription even for the same medication, he said.
Dr. Choudhry added that there’s another factor: multiple prescriptions from multiple physicians that require multiple pharmacy visits. His findings suggest that adherence improves when prescriptions are consolidated to limit the need to visit the drugstore. “The chaos of our health care system leads to nonadherence,” he said (Arch Intern Med. 2011;171[9]:814-22).
Internist Lawrence Garber, MD, of Reliant Medical Group in Worcester, Mass., offered these tips about boosting drug adherence:
- Develop trust with patients. “They need to trust that I’m their advocate, and that they’re my No. 1 reason for prescribing the medication, and not making myself more money,” he said.
- Provide educational resources. “We give them resources online. If their EHR [electronic health record] identifies that they’re diabetic, then they get information about diabetes printed out.”
- Use technology to promote messages about diabetes. Dr. Garber said his clinic has installed screens in the examination rooms so that he can show patients their data. “It [makes it] very clear for them to see why what they’re doing now is not working,’’ and why there is a need to change to a different regimen. In addition, screens in the waiting room can display educational slides about diabetes.
- Set up clinic-wide medication protocols. “We’ve set up protocols and pathways for diabetes, hypertension, and high cholesterol to make it easy to prescribe medications that are lower cost and to make sure we’re following the same path,” Dr. Garber said.
- Stay independent. “I haven’t seen a drug rep in decades. It’s an organizational policy that we don’t see them, so we’re less likely to be biased.”
- Make it easier for patients to take medications. Dr. Garber urged colleagues to talk to their patients about using strategies such as printed pill schedules, weekly pill organizers, auto refills, and smartphone alarm reminders to facilitate adherence.
And, he said, you may wish to make it clear that you will check on whether prescriptions are filled. That way, “the patients know that you’re looking,” and it can actually lead to improved adherence.
Dr. Choudhry reported that his research has been funded by unrestricted grants to his institution from insurers, government funders, nonprofit foundations, pharmaceutical companies (including Merck, Sanofi, and Astra Zeneca), and device makers (including Medisafe). Dr. Garber reported no relevant disclosures.
SAN FRANCISCO – Are you having trouble helping patients take their diabetes medications as directed? Try installing 32-inch screens in the examination rooms for a lab result show-and-tell. Keep pharmaceutical marketers out of your hair (and office).
Those are among the suggestions offered by two physicians during a symposium on drug adherence at the annual scientific sessions of the American Diabetes Association.
“Nonadherence is not a case of patients being bad,” said internist and researcher Niteesh K. Choudhry, MD, PhD, of Harvard Medical School and Brigham and Women’s Hospital, Boston. “When half of your patients are nonadherent, I can guarantee you [they] aren’t trying to hurt themselves.”
According to Dr. Choudhry’s own research published in 2011 and based on 2008 data, about 25% of patients do not fill prescriptions after leaving their doctors’ offices. That level for diabetes medications – 42% of patients – is especially high (Am J Med. 2011;124[11]:1081.e9-22).
Other findings, he said, have suggested that half of patients fail to adhere to evidence-based prescribed regimens over the long term. And three groups have especially low levels of adherence: people of color, women, and patients who are caregivers (possibly because they are too busy caring for others to care for themselves).
Various factors affect adherence, including forgetfulness, drug interactions or side effects, and the different colors and shapes of pills. The latter can confuse patients because colors and shapes may be different from prescription to prescription even for the same medication, he said.
Dr. Choudhry added that there’s another factor: multiple prescriptions from multiple physicians that require multiple pharmacy visits. His findings suggest that adherence improves when prescriptions are consolidated to limit the need to visit the drugstore. “The chaos of our health care system leads to nonadherence,” he said (Arch Intern Med. 2011;171[9]:814-22).
Internist Lawrence Garber, MD, of Reliant Medical Group in Worcester, Mass., offered these tips about boosting drug adherence:
- Develop trust with patients. “They need to trust that I’m their advocate, and that they’re my No. 1 reason for prescribing the medication, and not making myself more money,” he said.
- Provide educational resources. “We give them resources online. If their EHR [electronic health record] identifies that they’re diabetic, then they get information about diabetes printed out.”
- Use technology to promote messages about diabetes. Dr. Garber said his clinic has installed screens in the examination rooms so that he can show patients their data. “It [makes it] very clear for them to see why what they’re doing now is not working,’’ and why there is a need to change to a different regimen. In addition, screens in the waiting room can display educational slides about diabetes.
- Set up clinic-wide medication protocols. “We’ve set up protocols and pathways for diabetes, hypertension, and high cholesterol to make it easy to prescribe medications that are lower cost and to make sure we’re following the same path,” Dr. Garber said.
- Stay independent. “I haven’t seen a drug rep in decades. It’s an organizational policy that we don’t see them, so we’re less likely to be biased.”
- Make it easier for patients to take medications. Dr. Garber urged colleagues to talk to their patients about using strategies such as printed pill schedules, weekly pill organizers, auto refills, and smartphone alarm reminders to facilitate adherence.
And, he said, you may wish to make it clear that you will check on whether prescriptions are filled. That way, “the patients know that you’re looking,” and it can actually lead to improved adherence.
Dr. Choudhry reported that his research has been funded by unrestricted grants to his institution from insurers, government funders, nonprofit foundations, pharmaceutical companies (including Merck, Sanofi, and Astra Zeneca), and device makers (including Medisafe). Dr. Garber reported no relevant disclosures.
REPORTING FROM ADA 2019
Type 2 diabetes: Evolving concepts and treatment
Insights from basic and clinical research are changing the way we treat diabetes mellitus. In 2016, several key diabetes organizations, ie, the American Diabetes Association (ADA), the Juvenile Diabetes Research Foundation (JDRF), the European Association for the Study of Diabetes (EASD), and the American Association of Clinical Endocrinologists (AACE), called for bringing therapeutic approaches in line with our updated understanding of disease pathophysiology, replacing “one-size-fits-all” management with a tailored approach.1 This message has since been reiterated.2
Here, we review advances in our understanding of diabetes and how these inform a new model of diabetes treatment.
BETA CELLS ARE KEY
High levels of glucose and lipids damage and eventually kill beta cells through mechanisms including that of oxidative stress, so that glucose control deteriorates over time. The same processes are active in the target-organ damage seen in diabetes.3,4 These 2 insights—that the disease arises from combinatorial, nondiscrete pressures and that it proceeds through common processes of cell damage—leads us to a more unified understanding of the mechanism of diabetes, and may eventually replace current classifications of type 1, type 2, or latent autoimmune diabetes in adults, as well as nomenclature such as “microvascular” and “macrovascular” disease.3
FIRST-LINE LIFESTYLE INTERVENTIONS
Lifestyle interventions are the first-line therapy for elevated blood glucose. Achieving and maintaining a healthy body mass index is essential to help correct insulin resistance and minimize beta-cell dysfunction.
Lifestyle modifications for overweight or obese patients with diabetes mellitus include optimal caloric intake, decreased intake of simple carbohydrates, increased physical activity, and a 3% to 5% reduction in body weight.5 Weight-loss drugs may be indicated in obese patients. Normalization of lipids and hypertension should be an early goal.
RIGHT MEDICATIONS, RIGHT PATIENTS
While all of the drugs approved for treating diabetes lower glucose levels, some are more beneficial than others, possessing actions beyond their effect on plasma glucose levels, both good and bad.
The AACE guideline for use of various antidiabetic medications6 grades factors such as risks of hypoglycemia, ketoacidosis, weight gain, cardiovascular events, and renal, gastrointestinal, and bone concerns. This represents a much-needed first step toward guidance on selecting the right medications for the right patients. Risk factors (such as heart failure) and comorbidities (such as nonalcoholic fatty liver disease and nonalcoholic steatohepatitis) are among the considerations for choosing treatment.
Two principles
We propose 2 principles when choosing treatment:
Use “gentle” agents, ie, those that are least likely to exhaust beta cells or damage the organs involved in diabetes-related complications. Since the disease course depends on the health of the beta cells, give preference to agents that appear to best support beta cells—ie, agents that create the least oxidative stress or wear-and-tear—as will be outlined in this article.
Diabetes is associated with risks of cardiovascular disease, cardiac events, heart failure, and accelerated renal decompensation. Thus, it is equally important to prevent damage to the cardiovascular system, kidneys, and other tissues subject to damage through glucolipotoxicity.
Balancing glycemic control and risk
The hemoglobin A1c level is the chief target of care and an important barometer of risk of diabetes-related complications. In 2018, the American College of Physicians (ACP) relaxed its target for hemoglobin A1c from 7% to 8%.8 This move was apparently to give physicians greater “wiggle room” for achieving goals in hypoglycemia-prone patients. This, however, may take a toll.
Hypoglycemia is closely tied to cardiovascular disease. Even mild and asymptomatic hypoglycemia that goes undiagnosed and unnoticed by patients has been found to be associated with higher rates of all-cause mortality, prolonged QT interval, angina, arrhythmias, myocardial dysfunction, disturbances in autonomic balance, and sudden death.9–11
However, the ADA, AACE, American Association of Diabetes Educators (AADE), and the Endocrine Society jointly issued a strong indictment of the ACP recommendation.12 They argue that tight glucose control and its well-documented “legacy effects” on long-term outcomes should not be sacrificed.12,13 Indeed, there is no need to abandon evidence-based best practices in care when at least 8 of the 11 classes of antidiabetes agents do not introduce the same level of risk for hypoglycemia.
Current guidelines argue for tight glucose control but generally stop short of discriminating or stratifying the mechanisms of action of the individual classes of drugs. These guidelines also do not stress targeting the particular pathways of hyperglycemia present in any given patient. However, the 2016 ADA joint statement acknowledges the need to “characterize the many paths to beta-cell dysfunction or demise and identify therapeutic approaches that best target each path.”1
PROFILES OF DIABETES DRUGS
The sections below highlight some of the recent data on the profiles of most of the currently available agents.
Metformin: Still the first-line treatment
Current guidelines from the ACP, ADA, and AACE keep metformin14 as the backbone of treatment, although debate continues as to whether newer agents such as GLP-1 receptor agonists are superior for first-line therapy.
Pathways affected. Metformin improves insulin resistance in the liver, increases endogenous GLP-1 levels via the gut, and appears to modulate gut flora composition, which is increasingly suspected to contribute to dysmetabolism.
Advantages, benefits. Metformin is easy to use and does not cause hypoglycemia. It was found to modestly reduce the number of cardiovascular events and deaths in a number of clinical outcome studies.15–19
Disadvantages, adverse effects. In some patients, tolerability restricts the use of this drug at higher doses. The most common adverse effects of metformin are gastrointestinal symptoms (diarrhea, nausea, vomiting, flatulence); other risks include lactic acidosis in patients with impaired kidney function, heart failure, hypoxemia, alcoholism, cirrhosis, contrast exposure, sepsis, and shock.
GLP-1 receptor agonists
GLP-1 receptor agonists20–25 are injectable medications approved for adults with type 2 diabetes. Exenatide and liraglutide lower hemoglobin A1c by 1 to 1.5 absolute percentage points and reduce body weight; these effects persist over the long term.26 Newer once-weekly GLP-1 receptor agonists (albiglutide,20 dulaglutide,21 and semaglutide25) have similar benefits. In 2019, new drug applications were submitted to the FDA for the first-in-kind oral GLP-1 receptor agonists, which would improve convenience and adherence and make this class even more attractive.
Pathways affected. GLP-1 receptor agonists address multiple pathways of hyperglycemia. They increase insulin production and release, promote weight loss, and reduce insulin resistance, glucagon secretion, and inflammation. They also increase amylin, help overcome GLP-1 resistance, slow gastric emptying, and favorably modify gut flora.27
Advantages, benefits. The cardioprotective actions of GLP-1 receptor agonists include reducing inflammation and dysfunction in endothelial and myocardial cells; slowing atherosclerosis; reducing oxidative stress-induced injury and scavenging of reactive oxygen species in coronary endothelial, smooth muscle, and other cells; and enhancing endogenous antioxidant defenses.27 GLP-1 receptor agonism has also been found to inhibit apoptosis in cardiomyocytes, as well as in beta cells.
Several large-scale studies have shown improved outcomes with GLP-1 receptor agonists. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial26 found that liraglutide reduced major adverse cardiovascular events by 13% and myocardial infarctions by 22% in more than 9,000 adults with type 2 diabetes who were at high risk of major adverse cardiovascular events compared with placebo. Rates of microvascular outcomes were also reduced.
A retrospective database analysis of 39,275 patients with type 2 diabetes who were treated with exenatide reported a lower incidence of cardiovascular events than in patients not treated with exenatide.28
However, no effect on cardiovascular outcomes was found with a third GLP-1 agent, lixisenatide, in a large-scale trial in high-risk patients with diabetes.29
The most recently evaluated GLP-1 receptor agonist is semaglutide. The Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6) demonstrated a reduced risk of major adverse cardiovascular events.30
Disadvantages, adverse effects. The most common adverse effects in this class include nausea, hypoglycemia, diarrhea, constipation, vomiting, headache, decreased appetite, dyspepsia, fatigue, dizziness, abdominal pain, and increased lipase. The nausea can be mitigated by advising patients to stop eating at first sensation of stomach fullness.
DPP-4 inhibitors
Dipeptidyl peptidase 4 (DPP-4) is a ubiquitous enzyme that rapidly degrades GLP-1 and other endogenous peptides.31 Saxagliptin,32 sitagliptin,33 linagliptin,34 and alogliptin35 are approved for use in the United States, and vildagliptin36 is available in Europe.
Pathways affected. These agents modify 3 pathways of hyperglycemia: they increase insulin secretion, decrease glucagon levels, and help overcome GLP-1 resistance.
Advantages, benefits. DPP-4 inhibitors have been used safely and effectively in clinically challenging populations of patients with long-standing type 2 diabetes (> 10 years).
Disadvantages, adverse effects. As this class increases GLP-1 levels only 2- to 4-fold, their efficacy is more modest than that of GLP-1 receptor agonists (hemoglobin A1c reductions of 0.5% to 1%; neutral effects on weight).37
Outcome trials have largely been neutral. Saxagliptin has been associated with an increase in admissions for heart failure. There have been a very small but statistically significant number of drug-related cases of acute pancreatitis.38
The most common adverse effects with this class include headache, nasopharyngitis, urinary tract infection, upper respiratory tract infection, and elevated liver enzymes.
SGLT2 inhibitors
Drugs of this class currently available in the United States are canagliflozin,39 dapagliflozin,40 empagliflozin,41 and ertugliflozin.42
Pathways affected. SGLT2 inhibitors lower the glucose reabsorption threshold in the kidney so that more glucose is excreted in the urine; they also decrease insulin resistance in muscle, liver, and fat cells (via weight loss) and possibly preserve beta-cell function by reducing glucotoxicity. A nonrenal mechanism—delayed gut absorption reducing postprandial glucose excursion—has been proposed to contribute to the glucose-lowering effects of canagliflozin.43
Advantages, benefits. These agents reduce hemoglobin A1c by about 0.5% to 1.0% from a baseline of about 8%. Because their action is independent of insulin, they can be used at any stage of type 2 diabetes, even after insulin secretion has significantly waned. Additional potential advantages include weight loss (up to 3.5% of body mass index) and lowering of systolic blood pressure (2–4 mm Hg) and diastolic blood pressure (1–2 mm Hg).39–42
Canagliflozin was shown in the Canagliflozin Cardiovascular Assessment Study (CANVAS)44 to significantly reduce the overall risk of cardiovascular disease by 14% and risk of heart failure hospitalization by 33% while significantly slowing the progression of renal disease.
In the BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME),45 empagliflozin reduced heart failure hospitalizations by 35%, cardiovascular deaths by 38%, and all-cause mortality by about 32%. These benefits are thought to be due to less arterial stiffness, lower sympathetic tone, and decreased arrhythmias. Notably, these dramatic benefits accrued in only about 3 years with use of add-on therapy, even though the reduction in hemoglobin A1c was modest (0.6%), suggesting that pleiotropic effects are at work.
Disadvantages, adverse effects. The most common adverse effects of this class include urinary tract infections, yeast infections, dehydration, and hypovolemic symptoms; these can often be prevented. A trend toward increased incidence of amputations in earlier studies was not borne out in a 2018 meta-analysis of 4 observational databases.46
Thiazolidinediones
There are currently 2 approved thiazolidinediones in the United States, pioglitazone47 and rosiglitazone.48 Only pioglitazone is in common use, as rosiglitazone is associated with safety issues.49
Pathways affected. Pioglitazone reduces insulin resistance in muscle, liver, and adipose tissue.
Advantages, benefits. Decreased levels of low-density lipoprotein cholesterol and triglycerides and increased high-density lipoprotein cholesterol levels49 could plausibly account for the cardiovascular benefits reported in the Prospective Pioglitazone Clinical Trial in Macrovascular Events.50 Pioglitazone has also been found to improve insulin secretion, endothelial function, and diastolic dysfunction; reduce inflammation; decrease plasminogen activator inhibitor 1; reverse lipotoxicity; and help correct nonalcoholic fatty liver disease and steatohepatitis.
Pioglitazone has also been found to reduce plaque in carotid and coronary arteries51; improve outcomes in patients with heart failure and myocardial infarction compared with insulin-sensitizing drugs52; and reduce stroke and myocardial infarction in patients with insulin resistance (but not diabetes) and a recent history of ischemic stroke or transient ischemic attack (in the Insulin Resistance Intervention After Stroke trial).53 It may also help maintain beta-cell function; the Actos Now for the Prevention of Diabetes Study found that pioglitazone reduced the risk of conversion of impaired glucose tolerance to frank diabetes by 72%.54
Disadvantages, adverse effects. The most common adverse effects seen with this class include weight gain and salt retention, swelling, edema,55 and related cardiovascular consequences in certain patients. While this may be mitigatable with lifestyle changes or use in combination with a GLP-1 receptor agonist or SGLT2 inhibitor,56 pioglitazone is contraindicated in patients with heart failure, hemodynamic instability, or hepatic dysfunction.
Concerns that pioglitazone might increase the risk of bladder cancer seem to have been put to rest when a study in nearly 200,000 patients found no statistically significant association,57 but the warning remains in the US label.
Long-term use of this class of drugs has been associated with an increased risk of bone fractures,58 which warrants a risk-benefit assessment in each patient.
Injected insulin: Less safe than thought
Recent research suggests that injected insulin has a less favorable safety profile than previously thought.15–19,59 Studies of the long-term safety of insulin therapy have had inconsistent results but suggest that injected insulin is associated with poorer cardiovascular and renal outcomes (in some of the same studies that showed metformin or other agents to improve outcomes),17–19 and the association was dose-dependent. Several studies attempted to cancel out the poorer outcomes by adjusting for hemoglobin A1c levels, stage of disease,17–19,26,27 or severe hypoglycemic episodes.60 However, it may be inappropriate to reduce the impact of these variables, as these may themselves be the mediators of any deleterious effects of exogenous insulin.
When exogenous insulin is introduced into the peripheral circulation it causes a state of persistent iatrogenic hyperinsulinemia, which leads to insulin resistance and also appears to compromise the cardiovascular system. In contrast, endogenous insulin is released into the portal system in tightly controlled amounts.5,61 This suggests that the same insulin peptide may not be equivalently beneficial when introduced in an artificial manner.
Before starting insulin therapy, consider its side effects such as weight gain and hypoglycemia. Most (about 85%) episodes of hypoglycemia occur with basal-bolus insulin regimens.62 Moreover, iatrogenic hyperinsulinemia can damage the vascular system.63,64
We recommend. Insulin therapy is used early in the course of the disease as a short-term intervention for glucolipotoxicity. However, this can be accomplished without attendant risks of hypoglycemia and weight gain by using agents such as SGLT2 inhibitors and incretins. When insulin therapy is necessary, using it as add-on therapy might be considered instead of drug-switching. We have found alternate pharmacologic approaches successful in avoiding or delaying bolus insulin therapy. And in some patients taking insulin, we have had success in progressively introducing a noninsulin agent and were ultimately able to eliminate insulin altogether.
Bromocriptine-QR
Bromocriptine-QR (quick release)65 is a short-acting dopamine agonist that mimics the morning dopamine surge in the suprachiasmatic nucleus—the biologic clock.
Pathways affected. Bromocriptine addresses part of the brain contribution to hyperglycemia, with resultant reductions in both peripheral insulin resistance and sympathetic tone. This reduces muscle, liver, and adipose insulin resistance. It is moderately effective in glucose-lowering, especially in patients with significant insulin resistance.66
Advantages, benefits. A 1-year clinical trial reported that bromocriptine reduced cardiovascular adverse outcomes by 39%, and the composite end point of myocardial infarction, stroke, and cardiovascular death by 52% compared with placebo.67
Disadvantages, adverse effects. The most common adverse effects are nausea, rhinitis, headache, asthenia, dizziness, constipation, and sinusitis.
Alpha-glucosidase inhibitors
Alpha-glucosidase inhibitors (acarbose,68 miglitol69) work by decreasing the rate of absorption of glucose from the gastrointestinal tract.
Advantages, benefits. These drugs decrease hemoglobin A1c by 0.5% to 0.8%.70 They are weight-neutral and do not pose a risk of hypoglycemia. Clinical studies suggest that they may delay or prevent diabetes progression. They were also found to reduce cardiovascular events, acute myocardial infarction, and the onset of hypertension.69
Disadvantages, adverse effects. Their use remains limited due to gastrointestinal adverse effects. They may be contraindicated in patients with inflammatory bowel disease, partial bowel obstruction, or severe renal or hepatic disease.
Pramlintide
Pramlintide71 is an injectable amylin analogue. It is used as monotherapy or in combination with a sulfonylurea, metformin, or insulin glargine.
Pathways affected. Pramlintide decreases appetite, reduces glucagon levels, and minimizes absorption of glucose in the gut.
Disadvantages, adverse effects. Common side effects include mild to moderate hypoglycemia and nausea. Nausea may help explain the ability of pramlintide to confer weight loss when used in combination with insulin.
Sulfonylureas and meglitinides
These classes are still widely used in the treatment of type 2 diabetes, although the AACE6 and ADA72 guidelines de-emphasize their use based on associated risks of hypoglycemia, weight gain, morbidity, mortality, and loss of effect over time.
Pathways affected. Sulfonylureas stimulate insulin secretion from beta cells.
Disadvantages, adverse effects. Sulfonylureas and glinides are associated with poorer outcomes than newer agents in clinical trials15–19,59,60 and may be generally less beta-cell friendly.73 Their harmful effects are difficult to measure in vivo, but these drugs sometimes appear to be associated with more rapid beta-cell failure and progression to insulin dependence compared with newer ones. Several large-scale registry studies have found sulfonylureas and glinides to be associated with poorer outcomes (reviewed by Herman et al).74
Adverse effects include asthenia, headache, dizziness, nausea, diarrhea, epigastric fullness, and heartburn. Although they are often selected based on their low cost, other factors may offset their cost-effectiveness, such as need for glucose monitoring and hospital charges due to sulfonylurea-induced hypoglycemia. Their utility is also limited by dependence on beta-cell function.
Colesevelam
Colesevelam75 is a bile acid sequestrant and low-density lipoprotein cholesterol-reducing agent that has been approved for use in diabetes. The mode of action of colesevelam in this capacity is under investigation. Its effect on hemoglobin A1c is modest. It is associated with gastrointestinal adverse effects, particularly constipation.
Ranolazine
Ranolazine76 is an antianginal drug that also lowers glucose by increasing insulin release. It also possesses cardioprotective properties. In patients with diabetes and non-ST-segment elevation acute coronary syndromes, ranolazine reduced hemoglobin A1c by 1.2% and appeared to be weight-neutral.76 Ranolazine is under clinical development for use in diabetes. Adverse effects include dizziness, headache, constipation, and nausea.
Rational combinations of agents
The ideal strategy would use combinations of agents that mechanistically complement one another and address each path of hyperglycemia present in a patient. This approach should supplant the former approaches of adding-on agents only after treatment failure or sequentially trying first-, second-, and third-line treatments.
Examples of synergistic combinations include those that target fasting plasma glucose and postprandial glucose, reduce reliance on insulin with add-on therapies, or manage hyperglycemia in specific patient groups, such as renal-impaired patients.
Large-scale long-term clinical studies are needed to determine the safety, efficacy, and outcomes of various combinations and whether they confer additive benefits. Some studies have begun to explore possible combinations.
Combined metformin, pioglitazone, and exenatide was reported to delay progression of diabetes in early dysglycemia.77,78 Notably, this combination addresses multiple mediating pathways of hyperglycemia (Table 1).
A GLP-1 receptor agonist with an SGLT2 inhibitor would be another intriguing combination, as the mechanisms of action of these 2 classes complement one another. In limited clinical trials—the DURATION-8 study (lasting 26 weeks),79 the Canagliflozin Cardiovascular Assessment Study (18 weeks),80 and a 24-week study in nondiabetic obese patients81—additive benefits were also seen in systolic blood pressure, body weight, and cardiac risk factors by adding an SGLT2 inhibitor to a GLP-1 receptor agonist, compared with either agent alone. In theory, these improvements might slow or reverse cardiorenal compromise. Lower doses of 1 or more may be possible, and the regimen could prove cost-effective and life-sparing should it slow the progression of the disease and the onset of its complications. A clinical study of this combination is under way (Ralph DeFronzo, personal communication, July 2018). Similarly, the combination of metformin, saxagliptin and dapagliflozin has been shown to be effective.82
CONCLUSION
Care for diabetes mellitus can be particularly challenging for the primary care physician. The progressive nature of diabetes, with worsening hyperglycemia over the course of the disease, further complicates disease management.
Best practices for care nonetheless need to evolve with well-evidenced data, and without years of delay for “trickle-down” education from the specialties to primary care. We have arrived at a juncture to leverage therapies that address the 11 mediating pathways of hyperglycemia, optimally protect beta cells, minimize hypoglycemia, manage risk factors associated with diabetes, and improve diabetes-related outcomes.
- Skyler JS, Bakris GL, Bonifacio E, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes 2017; 66(2):241–255. doi:10.2337/db16-0806
- Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 41(12):2669–2701. doi:10.2337/dci18-0033
- Schwartz SS, Epstein S, Corkey BE, Grant SF, Gavin JR 3rd, Aguilar RB. The time is right for a new classification system for diabetes mellitus: rationale and implications of the beta-cell centric classification schema. Diabetes Care 2016; 39(2):179–186. doi:10.2337/dc15-1585
- Shah MS, Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res 2016; 118(11):1808–1829. doi:10.1161/CIRCRESAHA.116.306923
- Schwartz SS, Jellinger PS, Herman ME. Obviating much of the need for insulin therapy in type 2 diabetes mellitus: a re-assessment of insulin therapy’s safety profile. Postgrad Med 2016; 128(6):609–619. doi:10.1080/00325481.2016.1191955
- Garber AJ, Abrahamson MJ, Barzilay JE, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2019 executive summary. Endocr Pract 2019; 25(1):69–100. doi:10.4158/CS-2018-0535
- Sniderman AD, LaChapelle KJ, Rachon NA , Furberg CD. The necessity for clinical reasoning in the era of evidence-based medicine. Mayo Clin Proc 2013; 88(10):1108–1114. doi:10.1016/j.mayocp.2013.07.012
- Qaseem A, Wilt TJ, Kansagara D, Horwitch C, Barry MJ, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med 2018; 168(8):569–576. doi:10.7326/M17-0939
- Frier BM, Schernthaner G, Heller SR. Hypoglycemia and cardiovascular risks. Diabetes Care 2011; 34(suppl 2):S132–S137. doi:10.2337/dc11-s220
- Chico A, Vidal-Ríos P, Subira M, Novials A. The continuous glucose monitoring system is useful for detecting unrecognized hypoglycemias in patients with type 1 and type 2 diabetes but is not better than frequent capillary glucose measurements for improving metabolic control. Diabetes Care 2003; 26(4):1153–1157. pmid:12663589
- Weber KK, Lohmann T, Busch K, Donati-Hirsch I, Riel R. High frequency of unrecognized hypoglycaemias in patients with type 2 diabetes is discovered by continuous glucose monitoring. Exp Clin Endocrinol Diabetes 2007; 115(8):491–494. doi:10.1055/s-2007-984452
- American Diabetes Association (ADA). The American Diabetes Association, the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators and the Endocrine Society strongly disagree with the American College of Physicians’ guidance for higher blood glucose targets for people with type 2 diabetes www.diabetes.org/newsroom/press-releases/2018/joint-acp-guidance-response.html. Accessed June 6, 2019.
- Freed S; Diabetes in Control. American College of Physicians recommending controversial increase in A1c of 7% to 8%. www.diabetesincontrol.com/american-college-of-physicians-recommending-controversial-increase-in-a1c-of-7-to-8. Accessed June 6, 2019.
- Glucophage XR (metformin hydrochloride) extended release tablets prescribing information. Princeton, NJ, Bristol-Myers Squibb Company, 2009.
- Mellbin LG, Malmberg K, Norhammar A, Wedel H, Rydén L; DIGAMI 2 Investigators. The impact of glucose lowering treatment on long-term prognosis in patients with type 2 diabetes and myocardial infarction: a report from the DIGAMI 2 trial. Eur Heart J 2008; 29(2):166–176. doi:10.1093/eurheartj/ehm518
- Anselmino M, Ohrvik J, Malmberg K, Standl E, Rydén L; Euro Heart Survey Investigators. Glucose lowering treatment in patients with coronary artery disease is prognostically important not only in established but also in newly detected diabetes mellitus: a report from the Euro Heart Survey on Diabetes and the Heart. Eur Heart J 2008; 29(2):177–184. doi:10.1093/eurheartj/ehm519
- Smooke S, Horwich TB, Fonarow GC. Insulin-treated diabetes is associated with a marked increase in mortality in patients with advanced heart failure. Am Heart J 2005; 149(1):168–174. doi:10.1016/j.ahj.2004.07.005
- Colayco DC, Niu F, McCombs JS, Cheetham TC. A1C and cardiovascular outcomes in type 2 diabetes: a nested case-control study. Diabetes Care 2011; 34(1):77–83. doi:10.2337/dc10-1318
- Holden SE, Jenkins-Jones S, Morgan CL, Schernthaner G, Currie CJ. Glucose-lowering with exogenous insulin monotherapy in type 2 diabetes: dose association with all-cause mortality, cardiovascular events and cancer. Diabetes Obes Metab 2015; 17(4):350–362. doi:10.1111/dom.12412
- Tanzeum (albiglutide) prescribing information. Wilmington, DE, GlaxoSmithKline LLC, 2014.
- Trulicity (dulaglutide) prescribing information. Indianapolis, IN, Eli Lilly and Company, 2014.
- Byetta (exenatide) prescribing information. Wilmington, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Victoza (liraglutide injection) prescribing information. Plainsboro, NJ, Novo Nordisk Inc, 2013.
- Adlyxin (lixisenatide injection) prescribing information. Bridgewater, NJ, Sanofi, 2016.
- Ozempic (semaglutide) prescribing information. Plainsboro, NJ, Novo Nordisk, 2017.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375(4):311–322. doi:10.1056/NEJMoa1603827
- Chang G, Zhang D, Yu H, et al. Cardioprotective effects of exenatide against oxidative stress-induced injury. Int J Mol Med 2013; 32(5):1011–1020. doi:10.3892/ijmm.2013.1475
- Best JH, Hoogwerf BJ, Herman WH, et al. Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon-like peptide 1 (GLP-1) receptor agonist exenatide twice daily or other glucose-lowering therapies: a retrospective analysis of the LifeLink database. Diabetes Care; 34(1):90–95. doi:10.2337/dc10-1393
- Pfeffer MA, Claggett B, Diaz R, et al; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373(23):2247–2257. doi:10.1056/NEJMoa1509225
- Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375(19):1834–1844. doi:10.1056/NEJMoa1607141
- Mentlein R. Mechanisms underlying the rapid degradation and elimination of the incretin hormones GLP-1 and GIP. Best Pract Res Clin Endocrinol Metab 2009; 23(4):443–452. doi:10.1016/j.beem.2009.03.005
- Onglyza (saxagliptin) tablets prescribing information. Wilmington, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Januvia (sitagliptin) tablets prescribing information. Whitehouse Station, NJ, Merck & Co., Inc, 2014.
- Tradjenta (linagliptin) tablets prescribing information. Ingelheim, Germany, Boehringer Ingelheim International GmbH, 2014.
- Nesina (alogliptin) tablets prescribing information. Deerfield, IL, Takeda Pharmaceuticals America, Inc, 2013.
- Galvus (vildagliptin) prescribing information. North Ryde, Australia, Novartis Pharmaceuticals, 2014.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368(9548):1696–1705. doi:10.1016/S0140-6736(06)69705-5
- Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369(14):1317–1326. doi:10.1056/NEJMoa1307684
- Invokana (canagliflozin) tablets prescribing information. Titusville, NJ: Janssen Pharmaceuticals, Inc, 2013.
- Farxiga (dapagliflozin) prescribing information. Princeton, NJ, Bristol-Myers Squibb, 2014.
- Jardiance (empagliflozin) prescribing information. Ridgefield, CT, Boehringer Ingelheim Pharmaceuticals, Inc, 2014.
- Steglatro (ertugliflozin) prescribing information. Whitehouse Station, NJ, Merck, Sharp & Dohme Corp, 2017.
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159(4):262–274. doi:10.7326/0003-4819-159-4-201308200-00007
- Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377(7):644–657. doi:10.1056/NEJMoa1611925
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373(22):2117–2128. doi:10.1056/NEJMoa1504720
- Ryan PB, Buse JB, Schuemie MJ, et al. Comparative effectiveness of canagliflozin, SGLT2 inhibitors and non-SGLT2 inhibitors on the risk of hospitalization for heart failure and amputation in patients with type 2 diabetes mellitus: a real-world meta-analysis of 4 observational databases (OBSERVE-4D). Diabetes Obes Metab 2018; 20(11):2485–2597. doi:10.1111/dom.13424
- Actos (pioglitazone) tablets for oral use prescribing information. Deerfield, IL, Takeda Pharmaceuticals America, Inc, 2013.
- Avandia (rosiglitazone maleate tablets) prescribing information. Research Triangle Park, NC, GlaxoSmithKline, 1999.
- Goldberg RB, Kendall DK, Deeg MA, et al; GLAI Study Investigators. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care 2005; 28(7):1547–1554. pmid:15983299
- Dormandy JA, Charbonnel B, Eckland DJ, et al; PROactive Investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone clinical trial in macroVascular Events): a randomised controlled trial. Lancet 2005; 366:1279–1289. doi:10.1016/S0140-6736(05)67528-9
- Nissen SE, Nicholls SJ, Wolski K, et al; PERISCOPE Investigators. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes the PERISCOPE randomized controlled trial. JAMA 2008; 299(13):1561–1573. doi:10.1001/jama.299.13.1561
- Masoudi FA, Inzucchi SE, Wang Y, Havranek EP, Foody JM, Krumholz HM. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation 2005; 111(5):583–590. doi:10.1161/01.CIR.0000154542.13412.B1
- Kernan WN, Viscoli CM, Furie KL, et al; IRIS Trial Investigators. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 2016; 374(14):1321–1331. doi:10.1056/NEJMoa1506930
- DeFronzo RA, Tripathy D, Schwenke DC, et al; ACT NOW Study. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011; 364(12):1104–1115. doi:10.1056/NEJMoa1010949
- Nesto RW, Bell D, Bonow RO, et al; American Heart Association; American Diabetes Association. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. October 7, 2003. Circulation 2003; 108(23):2941–2948. doi:10.1161/01.CIR.0000103683.99399.7E
- Kushner RF, Sujak M. Prevention of weight gain in adult patients with type 2 diabetes treated with pioglitazone. Obesity (Silver Spring) 2009; 17(5):1017–1022. doi:10.1038/oby.2008.651
- Lewis JD, Habel LA, Quesenberry CP, et al. Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes. JAMA 2015; 314(3):265–277. doi:10.1001/jama.2015.7996
- Meier C, Kraenzlin ME, Bodmer M, Jick SS, Jick H, Meier CR. Use of thiazolidinediones and fracture risk. Arch Intern Med 2008; 168(8):820–825. doi:10.1001/archinte.168.8.820
- Gamble JM, Chibrikov E, Twells LK, et al. Association of insulin dosage with mortality or major adverse cardiovascular events: a retrospective cohort study. Lancet Diabetes Endocrinol 2017; 5(1):43–52. doi:10.1016/S2213-8587(16)30316-3
- Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010; 340:b4909. doi:10.1136/bmj.b4909
- Wang X, Yu C, Zhang B, Wang Y. The injurious effects of hyperinsulinism on blood vessels. Cell Biochem Biophys 2014; 69(2):213–218. doi:10.1007/s12013-013-9810-6
- Garber AJ, King AB, Del Prato S, et al; NN1250-3582 (BEGIN BB T2D) Trial Investigators. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet 2012; 379(9825):1498–1507. doi:10.1016/S0140-6736(12)60205-0
- Hanefeld M, Monnier L, Schnell O, Owens D. Early treatment with basal insulin glargine in people with type 2 diabetes: lessons from ORIGIN and other cardiovascular trials. Diabetes Ther 2016; 7(2):187–201. doi:10.1007/s13300-016-0153-3
- Nolan CJ, Ruderman NB, Prentki M. Intensive insulin for type 2 diabetes: the risk of causing harm. Lancet Diabetes Endocrinol 2013; 1(1):9–10. doi:10.1016/S2213-8587(13)70027-5
- Cycloset (bromocriptine mesylate) tablets prescribing information. Tiverton, RI, VeroScience LLC, 2019.
- Schwartz S, Zangeneh F. Evidence-based practice use of quick-release bromocriptine across the natural history of type 2 diabetes mellitus. Postgrad Med 2016; 128(8):828–838. doi:10.1080/00325481.2016.1214059
- Gaziano JM, Cincotta AH, Vinik A, Blonde L, Bohannon N, Scranton R. Effect of bromocriptine-QR (a quick-release formulation of bromocriptine mesylate) on major adverse cardiovascular events in type 2 diabetes subjects. J Am Heart Assoc 2012; 1(5):e002279. doi:10.1161/JAHA.112.002279
- Precose (acarbose) tablets prescribing information. Germany, Bayer HealthCare Pharmaceuticals Inc, 2011.
- Glyset (miglitol) tablets prescribing information. Germany, Bayer HealthCare Pharmaceuticals, Inc, 2012.
- Van de Laar FA, Lucassen PL, Akkermans RP, Van de Lisdonk EH, Rutten GE, Van Weel C. Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev 2005; (2):CD003639. doi:10.1002/14651858.CD003639.pub2
- Symlin (pramlintide acetate) injection for subcutaneous use prescribing information. Wilmongton, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2015; 58(3):429–442. doi:10.1007/s00125-014-3460-0
- Wajchenberg BL. Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev 2007; 28(2):187–218. doi:10.1210/10.1210/er.2006-0038
- Herman ME, O’Keefe JH, Bell DSH, Schwartz SS. Insulin therapy increases cardiovascular risk in type 2 diabetes. Prog Cardiovasc Dis 2017; 60(3):422–434. doi:10.1016/j.pcad.2017.09.001
- Welchol (colesevelam hydrochloride) prescribing information. Parsippany, NJ, Daiichi Sankyo Inc, 2014.
- Ranexa (ranolazine) prescribing information. Foster City, CA: Gilead Sciences, Inc, 2016.
- Armato J, DeFronzo R, Abdul-Ghani M, Ruby R. Successful treatment of prediabetes in clinical practice: targeting insulin resistance and beta-cell dysfunction. Endocr Pract 2012; 18(3):342–350. doi:10.4158/EP11194.OR
- Abdul-Ghani MA, Puckett C, Triplitt C, et al. Initial combination therapy with metformin, pioglitazone and exenatide is more effective than sequential add-on therapy in subjects with new-onset diabetes. Results from the efficacy and durability of initial combination therapy for type 2 diabetes (EDICT): a randomized trial. Diabetes Obes Metab 2015; 17(3):268–275. doi:10.1111/dom.12417
- Frías JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol 2016; 4(12):1004–1016. doi:10.1016/S2213-8587(16)30267-4
- Fulcher G, Matthews DR, Perkovic V, et al; CANVAS trial collaborative group. Efficacy and safety of canagliflozin when used in conjunction with incretin-mimetic therapy in patients with type 2 diabetes. Diabetes Obes Metab 2016; 18(1):82–91. doi:10.1111/dom.12589
- Lundkvist P, Sjöström CD, Amini S, Pereira MJ, Johnsson E, Eriksson JW. Dapagliflozin once-daily and exenatide once-weekly dual therapy: a 24-week randomized, placebo-controlled, phase II study examining effects on body weight and prediabetes in obese adults without diabetes. Diabetes Obes Metab 2017; 19(1):49–60. doi:10.1111/dom.12779
- Del Prato S, Rosenstock J, Garcia-Sanchez R, et al. Safety and tolerability of dapagliflozin, saxagliptin and metformin in combination: post-hoc analysis of concomitant add-on versus sequential add-on to metformin and of triple versus dual therapy with metformin. Diabetes Obes Metab 2018; 20(6):1542–1546. doi:10.1111/dom.13258
Insights from basic and clinical research are changing the way we treat diabetes mellitus. In 2016, several key diabetes organizations, ie, the American Diabetes Association (ADA), the Juvenile Diabetes Research Foundation (JDRF), the European Association for the Study of Diabetes (EASD), and the American Association of Clinical Endocrinologists (AACE), called for bringing therapeutic approaches in line with our updated understanding of disease pathophysiology, replacing “one-size-fits-all” management with a tailored approach.1 This message has since been reiterated.2
Here, we review advances in our understanding of diabetes and how these inform a new model of diabetes treatment.
BETA CELLS ARE KEY
High levels of glucose and lipids damage and eventually kill beta cells through mechanisms including that of oxidative stress, so that glucose control deteriorates over time. The same processes are active in the target-organ damage seen in diabetes.3,4 These 2 insights—that the disease arises from combinatorial, nondiscrete pressures and that it proceeds through common processes of cell damage—leads us to a more unified understanding of the mechanism of diabetes, and may eventually replace current classifications of type 1, type 2, or latent autoimmune diabetes in adults, as well as nomenclature such as “microvascular” and “macrovascular” disease.3
FIRST-LINE LIFESTYLE INTERVENTIONS
Lifestyle interventions are the first-line therapy for elevated blood glucose. Achieving and maintaining a healthy body mass index is essential to help correct insulin resistance and minimize beta-cell dysfunction.
Lifestyle modifications for overweight or obese patients with diabetes mellitus include optimal caloric intake, decreased intake of simple carbohydrates, increased physical activity, and a 3% to 5% reduction in body weight.5 Weight-loss drugs may be indicated in obese patients. Normalization of lipids and hypertension should be an early goal.
RIGHT MEDICATIONS, RIGHT PATIENTS
While all of the drugs approved for treating diabetes lower glucose levels, some are more beneficial than others, possessing actions beyond their effect on plasma glucose levels, both good and bad.
The AACE guideline for use of various antidiabetic medications6 grades factors such as risks of hypoglycemia, ketoacidosis, weight gain, cardiovascular events, and renal, gastrointestinal, and bone concerns. This represents a much-needed first step toward guidance on selecting the right medications for the right patients. Risk factors (such as heart failure) and comorbidities (such as nonalcoholic fatty liver disease and nonalcoholic steatohepatitis) are among the considerations for choosing treatment.
Two principles
We propose 2 principles when choosing treatment:
Use “gentle” agents, ie, those that are least likely to exhaust beta cells or damage the organs involved in diabetes-related complications. Since the disease course depends on the health of the beta cells, give preference to agents that appear to best support beta cells—ie, agents that create the least oxidative stress or wear-and-tear—as will be outlined in this article.
Diabetes is associated with risks of cardiovascular disease, cardiac events, heart failure, and accelerated renal decompensation. Thus, it is equally important to prevent damage to the cardiovascular system, kidneys, and other tissues subject to damage through glucolipotoxicity.
Balancing glycemic control and risk
The hemoglobin A1c level is the chief target of care and an important barometer of risk of diabetes-related complications. In 2018, the American College of Physicians (ACP) relaxed its target for hemoglobin A1c from 7% to 8%.8 This move was apparently to give physicians greater “wiggle room” for achieving goals in hypoglycemia-prone patients. This, however, may take a toll.
Hypoglycemia is closely tied to cardiovascular disease. Even mild and asymptomatic hypoglycemia that goes undiagnosed and unnoticed by patients has been found to be associated with higher rates of all-cause mortality, prolonged QT interval, angina, arrhythmias, myocardial dysfunction, disturbances in autonomic balance, and sudden death.9–11
However, the ADA, AACE, American Association of Diabetes Educators (AADE), and the Endocrine Society jointly issued a strong indictment of the ACP recommendation.12 They argue that tight glucose control and its well-documented “legacy effects” on long-term outcomes should not be sacrificed.12,13 Indeed, there is no need to abandon evidence-based best practices in care when at least 8 of the 11 classes of antidiabetes agents do not introduce the same level of risk for hypoglycemia.
Current guidelines argue for tight glucose control but generally stop short of discriminating or stratifying the mechanisms of action of the individual classes of drugs. These guidelines also do not stress targeting the particular pathways of hyperglycemia present in any given patient. However, the 2016 ADA joint statement acknowledges the need to “characterize the many paths to beta-cell dysfunction or demise and identify therapeutic approaches that best target each path.”1
PROFILES OF DIABETES DRUGS
The sections below highlight some of the recent data on the profiles of most of the currently available agents.
Metformin: Still the first-line treatment
Current guidelines from the ACP, ADA, and AACE keep metformin14 as the backbone of treatment, although debate continues as to whether newer agents such as GLP-1 receptor agonists are superior for first-line therapy.
Pathways affected. Metformin improves insulin resistance in the liver, increases endogenous GLP-1 levels via the gut, and appears to modulate gut flora composition, which is increasingly suspected to contribute to dysmetabolism.
Advantages, benefits. Metformin is easy to use and does not cause hypoglycemia. It was found to modestly reduce the number of cardiovascular events and deaths in a number of clinical outcome studies.15–19
Disadvantages, adverse effects. In some patients, tolerability restricts the use of this drug at higher doses. The most common adverse effects of metformin are gastrointestinal symptoms (diarrhea, nausea, vomiting, flatulence); other risks include lactic acidosis in patients with impaired kidney function, heart failure, hypoxemia, alcoholism, cirrhosis, contrast exposure, sepsis, and shock.
GLP-1 receptor agonists
GLP-1 receptor agonists20–25 are injectable medications approved for adults with type 2 diabetes. Exenatide and liraglutide lower hemoglobin A1c by 1 to 1.5 absolute percentage points and reduce body weight; these effects persist over the long term.26 Newer once-weekly GLP-1 receptor agonists (albiglutide,20 dulaglutide,21 and semaglutide25) have similar benefits. In 2019, new drug applications were submitted to the FDA for the first-in-kind oral GLP-1 receptor agonists, which would improve convenience and adherence and make this class even more attractive.
Pathways affected. GLP-1 receptor agonists address multiple pathways of hyperglycemia. They increase insulin production and release, promote weight loss, and reduce insulin resistance, glucagon secretion, and inflammation. They also increase amylin, help overcome GLP-1 resistance, slow gastric emptying, and favorably modify gut flora.27
Advantages, benefits. The cardioprotective actions of GLP-1 receptor agonists include reducing inflammation and dysfunction in endothelial and myocardial cells; slowing atherosclerosis; reducing oxidative stress-induced injury and scavenging of reactive oxygen species in coronary endothelial, smooth muscle, and other cells; and enhancing endogenous antioxidant defenses.27 GLP-1 receptor agonism has also been found to inhibit apoptosis in cardiomyocytes, as well as in beta cells.
Several large-scale studies have shown improved outcomes with GLP-1 receptor agonists. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial26 found that liraglutide reduced major adverse cardiovascular events by 13% and myocardial infarctions by 22% in more than 9,000 adults with type 2 diabetes who were at high risk of major adverse cardiovascular events compared with placebo. Rates of microvascular outcomes were also reduced.
A retrospective database analysis of 39,275 patients with type 2 diabetes who were treated with exenatide reported a lower incidence of cardiovascular events than in patients not treated with exenatide.28
However, no effect on cardiovascular outcomes was found with a third GLP-1 agent, lixisenatide, in a large-scale trial in high-risk patients with diabetes.29
The most recently evaluated GLP-1 receptor agonist is semaglutide. The Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6) demonstrated a reduced risk of major adverse cardiovascular events.30
Disadvantages, adverse effects. The most common adverse effects in this class include nausea, hypoglycemia, diarrhea, constipation, vomiting, headache, decreased appetite, dyspepsia, fatigue, dizziness, abdominal pain, and increased lipase. The nausea can be mitigated by advising patients to stop eating at first sensation of stomach fullness.
DPP-4 inhibitors
Dipeptidyl peptidase 4 (DPP-4) is a ubiquitous enzyme that rapidly degrades GLP-1 and other endogenous peptides.31 Saxagliptin,32 sitagliptin,33 linagliptin,34 and alogliptin35 are approved for use in the United States, and vildagliptin36 is available in Europe.
Pathways affected. These agents modify 3 pathways of hyperglycemia: they increase insulin secretion, decrease glucagon levels, and help overcome GLP-1 resistance.
Advantages, benefits. DPP-4 inhibitors have been used safely and effectively in clinically challenging populations of patients with long-standing type 2 diabetes (> 10 years).
Disadvantages, adverse effects. As this class increases GLP-1 levels only 2- to 4-fold, their efficacy is more modest than that of GLP-1 receptor agonists (hemoglobin A1c reductions of 0.5% to 1%; neutral effects on weight).37
Outcome trials have largely been neutral. Saxagliptin has been associated with an increase in admissions for heart failure. There have been a very small but statistically significant number of drug-related cases of acute pancreatitis.38
The most common adverse effects with this class include headache, nasopharyngitis, urinary tract infection, upper respiratory tract infection, and elevated liver enzymes.
SGLT2 inhibitors
Drugs of this class currently available in the United States are canagliflozin,39 dapagliflozin,40 empagliflozin,41 and ertugliflozin.42
Pathways affected. SGLT2 inhibitors lower the glucose reabsorption threshold in the kidney so that more glucose is excreted in the urine; they also decrease insulin resistance in muscle, liver, and fat cells (via weight loss) and possibly preserve beta-cell function by reducing glucotoxicity. A nonrenal mechanism—delayed gut absorption reducing postprandial glucose excursion—has been proposed to contribute to the glucose-lowering effects of canagliflozin.43
Advantages, benefits. These agents reduce hemoglobin A1c by about 0.5% to 1.0% from a baseline of about 8%. Because their action is independent of insulin, they can be used at any stage of type 2 diabetes, even after insulin secretion has significantly waned. Additional potential advantages include weight loss (up to 3.5% of body mass index) and lowering of systolic blood pressure (2–4 mm Hg) and diastolic blood pressure (1–2 mm Hg).39–42
Canagliflozin was shown in the Canagliflozin Cardiovascular Assessment Study (CANVAS)44 to significantly reduce the overall risk of cardiovascular disease by 14% and risk of heart failure hospitalization by 33% while significantly slowing the progression of renal disease.
In the BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME),45 empagliflozin reduced heart failure hospitalizations by 35%, cardiovascular deaths by 38%, and all-cause mortality by about 32%. These benefits are thought to be due to less arterial stiffness, lower sympathetic tone, and decreased arrhythmias. Notably, these dramatic benefits accrued in only about 3 years with use of add-on therapy, even though the reduction in hemoglobin A1c was modest (0.6%), suggesting that pleiotropic effects are at work.
Disadvantages, adverse effects. The most common adverse effects of this class include urinary tract infections, yeast infections, dehydration, and hypovolemic symptoms; these can often be prevented. A trend toward increased incidence of amputations in earlier studies was not borne out in a 2018 meta-analysis of 4 observational databases.46
Thiazolidinediones
There are currently 2 approved thiazolidinediones in the United States, pioglitazone47 and rosiglitazone.48 Only pioglitazone is in common use, as rosiglitazone is associated with safety issues.49
Pathways affected. Pioglitazone reduces insulin resistance in muscle, liver, and adipose tissue.
Advantages, benefits. Decreased levels of low-density lipoprotein cholesterol and triglycerides and increased high-density lipoprotein cholesterol levels49 could plausibly account for the cardiovascular benefits reported in the Prospective Pioglitazone Clinical Trial in Macrovascular Events.50 Pioglitazone has also been found to improve insulin secretion, endothelial function, and diastolic dysfunction; reduce inflammation; decrease plasminogen activator inhibitor 1; reverse lipotoxicity; and help correct nonalcoholic fatty liver disease and steatohepatitis.
Pioglitazone has also been found to reduce plaque in carotid and coronary arteries51; improve outcomes in patients with heart failure and myocardial infarction compared with insulin-sensitizing drugs52; and reduce stroke and myocardial infarction in patients with insulin resistance (but not diabetes) and a recent history of ischemic stroke or transient ischemic attack (in the Insulin Resistance Intervention After Stroke trial).53 It may also help maintain beta-cell function; the Actos Now for the Prevention of Diabetes Study found that pioglitazone reduced the risk of conversion of impaired glucose tolerance to frank diabetes by 72%.54
Disadvantages, adverse effects. The most common adverse effects seen with this class include weight gain and salt retention, swelling, edema,55 and related cardiovascular consequences in certain patients. While this may be mitigatable with lifestyle changes or use in combination with a GLP-1 receptor agonist or SGLT2 inhibitor,56 pioglitazone is contraindicated in patients with heart failure, hemodynamic instability, or hepatic dysfunction.
Concerns that pioglitazone might increase the risk of bladder cancer seem to have been put to rest when a study in nearly 200,000 patients found no statistically significant association,57 but the warning remains in the US label.
Long-term use of this class of drugs has been associated with an increased risk of bone fractures,58 which warrants a risk-benefit assessment in each patient.
Injected insulin: Less safe than thought
Recent research suggests that injected insulin has a less favorable safety profile than previously thought.15–19,59 Studies of the long-term safety of insulin therapy have had inconsistent results but suggest that injected insulin is associated with poorer cardiovascular and renal outcomes (in some of the same studies that showed metformin or other agents to improve outcomes),17–19 and the association was dose-dependent. Several studies attempted to cancel out the poorer outcomes by adjusting for hemoglobin A1c levels, stage of disease,17–19,26,27 or severe hypoglycemic episodes.60 However, it may be inappropriate to reduce the impact of these variables, as these may themselves be the mediators of any deleterious effects of exogenous insulin.
When exogenous insulin is introduced into the peripheral circulation it causes a state of persistent iatrogenic hyperinsulinemia, which leads to insulin resistance and also appears to compromise the cardiovascular system. In contrast, endogenous insulin is released into the portal system in tightly controlled amounts.5,61 This suggests that the same insulin peptide may not be equivalently beneficial when introduced in an artificial manner.
Before starting insulin therapy, consider its side effects such as weight gain and hypoglycemia. Most (about 85%) episodes of hypoglycemia occur with basal-bolus insulin regimens.62 Moreover, iatrogenic hyperinsulinemia can damage the vascular system.63,64
We recommend. Insulin therapy is used early in the course of the disease as a short-term intervention for glucolipotoxicity. However, this can be accomplished without attendant risks of hypoglycemia and weight gain by using agents such as SGLT2 inhibitors and incretins. When insulin therapy is necessary, using it as add-on therapy might be considered instead of drug-switching. We have found alternate pharmacologic approaches successful in avoiding or delaying bolus insulin therapy. And in some patients taking insulin, we have had success in progressively introducing a noninsulin agent and were ultimately able to eliminate insulin altogether.
Bromocriptine-QR
Bromocriptine-QR (quick release)65 is a short-acting dopamine agonist that mimics the morning dopamine surge in the suprachiasmatic nucleus—the biologic clock.
Pathways affected. Bromocriptine addresses part of the brain contribution to hyperglycemia, with resultant reductions in both peripheral insulin resistance and sympathetic tone. This reduces muscle, liver, and adipose insulin resistance. It is moderately effective in glucose-lowering, especially in patients with significant insulin resistance.66
Advantages, benefits. A 1-year clinical trial reported that bromocriptine reduced cardiovascular adverse outcomes by 39%, and the composite end point of myocardial infarction, stroke, and cardiovascular death by 52% compared with placebo.67
Disadvantages, adverse effects. The most common adverse effects are nausea, rhinitis, headache, asthenia, dizziness, constipation, and sinusitis.
Alpha-glucosidase inhibitors
Alpha-glucosidase inhibitors (acarbose,68 miglitol69) work by decreasing the rate of absorption of glucose from the gastrointestinal tract.
Advantages, benefits. These drugs decrease hemoglobin A1c by 0.5% to 0.8%.70 They are weight-neutral and do not pose a risk of hypoglycemia. Clinical studies suggest that they may delay or prevent diabetes progression. They were also found to reduce cardiovascular events, acute myocardial infarction, and the onset of hypertension.69
Disadvantages, adverse effects. Their use remains limited due to gastrointestinal adverse effects. They may be contraindicated in patients with inflammatory bowel disease, partial bowel obstruction, or severe renal or hepatic disease.
Pramlintide
Pramlintide71 is an injectable amylin analogue. It is used as monotherapy or in combination with a sulfonylurea, metformin, or insulin glargine.
Pathways affected. Pramlintide decreases appetite, reduces glucagon levels, and minimizes absorption of glucose in the gut.
Disadvantages, adverse effects. Common side effects include mild to moderate hypoglycemia and nausea. Nausea may help explain the ability of pramlintide to confer weight loss when used in combination with insulin.
Sulfonylureas and meglitinides
These classes are still widely used in the treatment of type 2 diabetes, although the AACE6 and ADA72 guidelines de-emphasize their use based on associated risks of hypoglycemia, weight gain, morbidity, mortality, and loss of effect over time.
Pathways affected. Sulfonylureas stimulate insulin secretion from beta cells.
Disadvantages, adverse effects. Sulfonylureas and glinides are associated with poorer outcomes than newer agents in clinical trials15–19,59,60 and may be generally less beta-cell friendly.73 Their harmful effects are difficult to measure in vivo, but these drugs sometimes appear to be associated with more rapid beta-cell failure and progression to insulin dependence compared with newer ones. Several large-scale registry studies have found sulfonylureas and glinides to be associated with poorer outcomes (reviewed by Herman et al).74
Adverse effects include asthenia, headache, dizziness, nausea, diarrhea, epigastric fullness, and heartburn. Although they are often selected based on their low cost, other factors may offset their cost-effectiveness, such as need for glucose monitoring and hospital charges due to sulfonylurea-induced hypoglycemia. Their utility is also limited by dependence on beta-cell function.
Colesevelam
Colesevelam75 is a bile acid sequestrant and low-density lipoprotein cholesterol-reducing agent that has been approved for use in diabetes. The mode of action of colesevelam in this capacity is under investigation. Its effect on hemoglobin A1c is modest. It is associated with gastrointestinal adverse effects, particularly constipation.
Ranolazine
Ranolazine76 is an antianginal drug that also lowers glucose by increasing insulin release. It also possesses cardioprotective properties. In patients with diabetes and non-ST-segment elevation acute coronary syndromes, ranolazine reduced hemoglobin A1c by 1.2% and appeared to be weight-neutral.76 Ranolazine is under clinical development for use in diabetes. Adverse effects include dizziness, headache, constipation, and nausea.
Rational combinations of agents
The ideal strategy would use combinations of agents that mechanistically complement one another and address each path of hyperglycemia present in a patient. This approach should supplant the former approaches of adding-on agents only after treatment failure or sequentially trying first-, second-, and third-line treatments.
Examples of synergistic combinations include those that target fasting plasma glucose and postprandial glucose, reduce reliance on insulin with add-on therapies, or manage hyperglycemia in specific patient groups, such as renal-impaired patients.
Large-scale long-term clinical studies are needed to determine the safety, efficacy, and outcomes of various combinations and whether they confer additive benefits. Some studies have begun to explore possible combinations.
Combined metformin, pioglitazone, and exenatide was reported to delay progression of diabetes in early dysglycemia.77,78 Notably, this combination addresses multiple mediating pathways of hyperglycemia (Table 1).
A GLP-1 receptor agonist with an SGLT2 inhibitor would be another intriguing combination, as the mechanisms of action of these 2 classes complement one another. In limited clinical trials—the DURATION-8 study (lasting 26 weeks),79 the Canagliflozin Cardiovascular Assessment Study (18 weeks),80 and a 24-week study in nondiabetic obese patients81—additive benefits were also seen in systolic blood pressure, body weight, and cardiac risk factors by adding an SGLT2 inhibitor to a GLP-1 receptor agonist, compared with either agent alone. In theory, these improvements might slow or reverse cardiorenal compromise. Lower doses of 1 or more may be possible, and the regimen could prove cost-effective and life-sparing should it slow the progression of the disease and the onset of its complications. A clinical study of this combination is under way (Ralph DeFronzo, personal communication, July 2018). Similarly, the combination of metformin, saxagliptin and dapagliflozin has been shown to be effective.82
CONCLUSION
Care for diabetes mellitus can be particularly challenging for the primary care physician. The progressive nature of diabetes, with worsening hyperglycemia over the course of the disease, further complicates disease management.
Best practices for care nonetheless need to evolve with well-evidenced data, and without years of delay for “trickle-down” education from the specialties to primary care. We have arrived at a juncture to leverage therapies that address the 11 mediating pathways of hyperglycemia, optimally protect beta cells, minimize hypoglycemia, manage risk factors associated with diabetes, and improve diabetes-related outcomes.
Insights from basic and clinical research are changing the way we treat diabetes mellitus. In 2016, several key diabetes organizations, ie, the American Diabetes Association (ADA), the Juvenile Diabetes Research Foundation (JDRF), the European Association for the Study of Diabetes (EASD), and the American Association of Clinical Endocrinologists (AACE), called for bringing therapeutic approaches in line with our updated understanding of disease pathophysiology, replacing “one-size-fits-all” management with a tailored approach.1 This message has since been reiterated.2
Here, we review advances in our understanding of diabetes and how these inform a new model of diabetes treatment.
BETA CELLS ARE KEY
High levels of glucose and lipids damage and eventually kill beta cells through mechanisms including that of oxidative stress, so that glucose control deteriorates over time. The same processes are active in the target-organ damage seen in diabetes.3,4 These 2 insights—that the disease arises from combinatorial, nondiscrete pressures and that it proceeds through common processes of cell damage—leads us to a more unified understanding of the mechanism of diabetes, and may eventually replace current classifications of type 1, type 2, or latent autoimmune diabetes in adults, as well as nomenclature such as “microvascular” and “macrovascular” disease.3
FIRST-LINE LIFESTYLE INTERVENTIONS
Lifestyle interventions are the first-line therapy for elevated blood glucose. Achieving and maintaining a healthy body mass index is essential to help correct insulin resistance and minimize beta-cell dysfunction.
Lifestyle modifications for overweight or obese patients with diabetes mellitus include optimal caloric intake, decreased intake of simple carbohydrates, increased physical activity, and a 3% to 5% reduction in body weight.5 Weight-loss drugs may be indicated in obese patients. Normalization of lipids and hypertension should be an early goal.
RIGHT MEDICATIONS, RIGHT PATIENTS
While all of the drugs approved for treating diabetes lower glucose levels, some are more beneficial than others, possessing actions beyond their effect on plasma glucose levels, both good and bad.
The AACE guideline for use of various antidiabetic medications6 grades factors such as risks of hypoglycemia, ketoacidosis, weight gain, cardiovascular events, and renal, gastrointestinal, and bone concerns. This represents a much-needed first step toward guidance on selecting the right medications for the right patients. Risk factors (such as heart failure) and comorbidities (such as nonalcoholic fatty liver disease and nonalcoholic steatohepatitis) are among the considerations for choosing treatment.
Two principles
We propose 2 principles when choosing treatment:
Use “gentle” agents, ie, those that are least likely to exhaust beta cells or damage the organs involved in diabetes-related complications. Since the disease course depends on the health of the beta cells, give preference to agents that appear to best support beta cells—ie, agents that create the least oxidative stress or wear-and-tear—as will be outlined in this article.
Diabetes is associated with risks of cardiovascular disease, cardiac events, heart failure, and accelerated renal decompensation. Thus, it is equally important to prevent damage to the cardiovascular system, kidneys, and other tissues subject to damage through glucolipotoxicity.
Balancing glycemic control and risk
The hemoglobin A1c level is the chief target of care and an important barometer of risk of diabetes-related complications. In 2018, the American College of Physicians (ACP) relaxed its target for hemoglobin A1c from 7% to 8%.8 This move was apparently to give physicians greater “wiggle room” for achieving goals in hypoglycemia-prone patients. This, however, may take a toll.
Hypoglycemia is closely tied to cardiovascular disease. Even mild and asymptomatic hypoglycemia that goes undiagnosed and unnoticed by patients has been found to be associated with higher rates of all-cause mortality, prolonged QT interval, angina, arrhythmias, myocardial dysfunction, disturbances in autonomic balance, and sudden death.9–11
However, the ADA, AACE, American Association of Diabetes Educators (AADE), and the Endocrine Society jointly issued a strong indictment of the ACP recommendation.12 They argue that tight glucose control and its well-documented “legacy effects” on long-term outcomes should not be sacrificed.12,13 Indeed, there is no need to abandon evidence-based best practices in care when at least 8 of the 11 classes of antidiabetes agents do not introduce the same level of risk for hypoglycemia.
Current guidelines argue for tight glucose control but generally stop short of discriminating or stratifying the mechanisms of action of the individual classes of drugs. These guidelines also do not stress targeting the particular pathways of hyperglycemia present in any given patient. However, the 2016 ADA joint statement acknowledges the need to “characterize the many paths to beta-cell dysfunction or demise and identify therapeutic approaches that best target each path.”1
PROFILES OF DIABETES DRUGS
The sections below highlight some of the recent data on the profiles of most of the currently available agents.
Metformin: Still the first-line treatment
Current guidelines from the ACP, ADA, and AACE keep metformin14 as the backbone of treatment, although debate continues as to whether newer agents such as GLP-1 receptor agonists are superior for first-line therapy.
Pathways affected. Metformin improves insulin resistance in the liver, increases endogenous GLP-1 levels via the gut, and appears to modulate gut flora composition, which is increasingly suspected to contribute to dysmetabolism.
Advantages, benefits. Metformin is easy to use and does not cause hypoglycemia. It was found to modestly reduce the number of cardiovascular events and deaths in a number of clinical outcome studies.15–19
Disadvantages, adverse effects. In some patients, tolerability restricts the use of this drug at higher doses. The most common adverse effects of metformin are gastrointestinal symptoms (diarrhea, nausea, vomiting, flatulence); other risks include lactic acidosis in patients with impaired kidney function, heart failure, hypoxemia, alcoholism, cirrhosis, contrast exposure, sepsis, and shock.
GLP-1 receptor agonists
GLP-1 receptor agonists20–25 are injectable medications approved for adults with type 2 diabetes. Exenatide and liraglutide lower hemoglobin A1c by 1 to 1.5 absolute percentage points and reduce body weight; these effects persist over the long term.26 Newer once-weekly GLP-1 receptor agonists (albiglutide,20 dulaglutide,21 and semaglutide25) have similar benefits. In 2019, new drug applications were submitted to the FDA for the first-in-kind oral GLP-1 receptor agonists, which would improve convenience and adherence and make this class even more attractive.
Pathways affected. GLP-1 receptor agonists address multiple pathways of hyperglycemia. They increase insulin production and release, promote weight loss, and reduce insulin resistance, glucagon secretion, and inflammation. They also increase amylin, help overcome GLP-1 resistance, slow gastric emptying, and favorably modify gut flora.27
Advantages, benefits. The cardioprotective actions of GLP-1 receptor agonists include reducing inflammation and dysfunction in endothelial and myocardial cells; slowing atherosclerosis; reducing oxidative stress-induced injury and scavenging of reactive oxygen species in coronary endothelial, smooth muscle, and other cells; and enhancing endogenous antioxidant defenses.27 GLP-1 receptor agonism has also been found to inhibit apoptosis in cardiomyocytes, as well as in beta cells.
Several large-scale studies have shown improved outcomes with GLP-1 receptor agonists. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial26 found that liraglutide reduced major adverse cardiovascular events by 13% and myocardial infarctions by 22% in more than 9,000 adults with type 2 diabetes who were at high risk of major adverse cardiovascular events compared with placebo. Rates of microvascular outcomes were also reduced.
A retrospective database analysis of 39,275 patients with type 2 diabetes who were treated with exenatide reported a lower incidence of cardiovascular events than in patients not treated with exenatide.28
However, no effect on cardiovascular outcomes was found with a third GLP-1 agent, lixisenatide, in a large-scale trial in high-risk patients with diabetes.29
The most recently evaluated GLP-1 receptor agonist is semaglutide. The Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6) demonstrated a reduced risk of major adverse cardiovascular events.30
Disadvantages, adverse effects. The most common adverse effects in this class include nausea, hypoglycemia, diarrhea, constipation, vomiting, headache, decreased appetite, dyspepsia, fatigue, dizziness, abdominal pain, and increased lipase. The nausea can be mitigated by advising patients to stop eating at first sensation of stomach fullness.
DPP-4 inhibitors
Dipeptidyl peptidase 4 (DPP-4) is a ubiquitous enzyme that rapidly degrades GLP-1 and other endogenous peptides.31 Saxagliptin,32 sitagliptin,33 linagliptin,34 and alogliptin35 are approved for use in the United States, and vildagliptin36 is available in Europe.
Pathways affected. These agents modify 3 pathways of hyperglycemia: they increase insulin secretion, decrease glucagon levels, and help overcome GLP-1 resistance.
Advantages, benefits. DPP-4 inhibitors have been used safely and effectively in clinically challenging populations of patients with long-standing type 2 diabetes (> 10 years).
Disadvantages, adverse effects. As this class increases GLP-1 levels only 2- to 4-fold, their efficacy is more modest than that of GLP-1 receptor agonists (hemoglobin A1c reductions of 0.5% to 1%; neutral effects on weight).37
Outcome trials have largely been neutral. Saxagliptin has been associated with an increase in admissions for heart failure. There have been a very small but statistically significant number of drug-related cases of acute pancreatitis.38
The most common adverse effects with this class include headache, nasopharyngitis, urinary tract infection, upper respiratory tract infection, and elevated liver enzymes.
SGLT2 inhibitors
Drugs of this class currently available in the United States are canagliflozin,39 dapagliflozin,40 empagliflozin,41 and ertugliflozin.42
Pathways affected. SGLT2 inhibitors lower the glucose reabsorption threshold in the kidney so that more glucose is excreted in the urine; they also decrease insulin resistance in muscle, liver, and fat cells (via weight loss) and possibly preserve beta-cell function by reducing glucotoxicity. A nonrenal mechanism—delayed gut absorption reducing postprandial glucose excursion—has been proposed to contribute to the glucose-lowering effects of canagliflozin.43
Advantages, benefits. These agents reduce hemoglobin A1c by about 0.5% to 1.0% from a baseline of about 8%. Because their action is independent of insulin, they can be used at any stage of type 2 diabetes, even after insulin secretion has significantly waned. Additional potential advantages include weight loss (up to 3.5% of body mass index) and lowering of systolic blood pressure (2–4 mm Hg) and diastolic blood pressure (1–2 mm Hg).39–42
Canagliflozin was shown in the Canagliflozin Cardiovascular Assessment Study (CANVAS)44 to significantly reduce the overall risk of cardiovascular disease by 14% and risk of heart failure hospitalization by 33% while significantly slowing the progression of renal disease.
In the BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME),45 empagliflozin reduced heart failure hospitalizations by 35%, cardiovascular deaths by 38%, and all-cause mortality by about 32%. These benefits are thought to be due to less arterial stiffness, lower sympathetic tone, and decreased arrhythmias. Notably, these dramatic benefits accrued in only about 3 years with use of add-on therapy, even though the reduction in hemoglobin A1c was modest (0.6%), suggesting that pleiotropic effects are at work.
Disadvantages, adverse effects. The most common adverse effects of this class include urinary tract infections, yeast infections, dehydration, and hypovolemic symptoms; these can often be prevented. A trend toward increased incidence of amputations in earlier studies was not borne out in a 2018 meta-analysis of 4 observational databases.46
Thiazolidinediones
There are currently 2 approved thiazolidinediones in the United States, pioglitazone47 and rosiglitazone.48 Only pioglitazone is in common use, as rosiglitazone is associated with safety issues.49
Pathways affected. Pioglitazone reduces insulin resistance in muscle, liver, and adipose tissue.
Advantages, benefits. Decreased levels of low-density lipoprotein cholesterol and triglycerides and increased high-density lipoprotein cholesterol levels49 could plausibly account for the cardiovascular benefits reported in the Prospective Pioglitazone Clinical Trial in Macrovascular Events.50 Pioglitazone has also been found to improve insulin secretion, endothelial function, and diastolic dysfunction; reduce inflammation; decrease plasminogen activator inhibitor 1; reverse lipotoxicity; and help correct nonalcoholic fatty liver disease and steatohepatitis.
Pioglitazone has also been found to reduce plaque in carotid and coronary arteries51; improve outcomes in patients with heart failure and myocardial infarction compared with insulin-sensitizing drugs52; and reduce stroke and myocardial infarction in patients with insulin resistance (but not diabetes) and a recent history of ischemic stroke or transient ischemic attack (in the Insulin Resistance Intervention After Stroke trial).53 It may also help maintain beta-cell function; the Actos Now for the Prevention of Diabetes Study found that pioglitazone reduced the risk of conversion of impaired glucose tolerance to frank diabetes by 72%.54
Disadvantages, adverse effects. The most common adverse effects seen with this class include weight gain and salt retention, swelling, edema,55 and related cardiovascular consequences in certain patients. While this may be mitigatable with lifestyle changes or use in combination with a GLP-1 receptor agonist or SGLT2 inhibitor,56 pioglitazone is contraindicated in patients with heart failure, hemodynamic instability, or hepatic dysfunction.
Concerns that pioglitazone might increase the risk of bladder cancer seem to have been put to rest when a study in nearly 200,000 patients found no statistically significant association,57 but the warning remains in the US label.
Long-term use of this class of drugs has been associated with an increased risk of bone fractures,58 which warrants a risk-benefit assessment in each patient.
Injected insulin: Less safe than thought
Recent research suggests that injected insulin has a less favorable safety profile than previously thought.15–19,59 Studies of the long-term safety of insulin therapy have had inconsistent results but suggest that injected insulin is associated with poorer cardiovascular and renal outcomes (in some of the same studies that showed metformin or other agents to improve outcomes),17–19 and the association was dose-dependent. Several studies attempted to cancel out the poorer outcomes by adjusting for hemoglobin A1c levels, stage of disease,17–19,26,27 or severe hypoglycemic episodes.60 However, it may be inappropriate to reduce the impact of these variables, as these may themselves be the mediators of any deleterious effects of exogenous insulin.
When exogenous insulin is introduced into the peripheral circulation it causes a state of persistent iatrogenic hyperinsulinemia, which leads to insulin resistance and also appears to compromise the cardiovascular system. In contrast, endogenous insulin is released into the portal system in tightly controlled amounts.5,61 This suggests that the same insulin peptide may not be equivalently beneficial when introduced in an artificial manner.
Before starting insulin therapy, consider its side effects such as weight gain and hypoglycemia. Most (about 85%) episodes of hypoglycemia occur with basal-bolus insulin regimens.62 Moreover, iatrogenic hyperinsulinemia can damage the vascular system.63,64
We recommend. Insulin therapy is used early in the course of the disease as a short-term intervention for glucolipotoxicity. However, this can be accomplished without attendant risks of hypoglycemia and weight gain by using agents such as SGLT2 inhibitors and incretins. When insulin therapy is necessary, using it as add-on therapy might be considered instead of drug-switching. We have found alternate pharmacologic approaches successful in avoiding or delaying bolus insulin therapy. And in some patients taking insulin, we have had success in progressively introducing a noninsulin agent and were ultimately able to eliminate insulin altogether.
Bromocriptine-QR
Bromocriptine-QR (quick release)65 is a short-acting dopamine agonist that mimics the morning dopamine surge in the suprachiasmatic nucleus—the biologic clock.
Pathways affected. Bromocriptine addresses part of the brain contribution to hyperglycemia, with resultant reductions in both peripheral insulin resistance and sympathetic tone. This reduces muscle, liver, and adipose insulin resistance. It is moderately effective in glucose-lowering, especially in patients with significant insulin resistance.66
Advantages, benefits. A 1-year clinical trial reported that bromocriptine reduced cardiovascular adverse outcomes by 39%, and the composite end point of myocardial infarction, stroke, and cardiovascular death by 52% compared with placebo.67
Disadvantages, adverse effects. The most common adverse effects are nausea, rhinitis, headache, asthenia, dizziness, constipation, and sinusitis.
Alpha-glucosidase inhibitors
Alpha-glucosidase inhibitors (acarbose,68 miglitol69) work by decreasing the rate of absorption of glucose from the gastrointestinal tract.
Advantages, benefits. These drugs decrease hemoglobin A1c by 0.5% to 0.8%.70 They are weight-neutral and do not pose a risk of hypoglycemia. Clinical studies suggest that they may delay or prevent diabetes progression. They were also found to reduce cardiovascular events, acute myocardial infarction, and the onset of hypertension.69
Disadvantages, adverse effects. Their use remains limited due to gastrointestinal adverse effects. They may be contraindicated in patients with inflammatory bowel disease, partial bowel obstruction, or severe renal or hepatic disease.
Pramlintide
Pramlintide71 is an injectable amylin analogue. It is used as monotherapy or in combination with a sulfonylurea, metformin, or insulin glargine.
Pathways affected. Pramlintide decreases appetite, reduces glucagon levels, and minimizes absorption of glucose in the gut.
Disadvantages, adverse effects. Common side effects include mild to moderate hypoglycemia and nausea. Nausea may help explain the ability of pramlintide to confer weight loss when used in combination with insulin.
Sulfonylureas and meglitinides
These classes are still widely used in the treatment of type 2 diabetes, although the AACE6 and ADA72 guidelines de-emphasize their use based on associated risks of hypoglycemia, weight gain, morbidity, mortality, and loss of effect over time.
Pathways affected. Sulfonylureas stimulate insulin secretion from beta cells.
Disadvantages, adverse effects. Sulfonylureas and glinides are associated with poorer outcomes than newer agents in clinical trials15–19,59,60 and may be generally less beta-cell friendly.73 Their harmful effects are difficult to measure in vivo, but these drugs sometimes appear to be associated with more rapid beta-cell failure and progression to insulin dependence compared with newer ones. Several large-scale registry studies have found sulfonylureas and glinides to be associated with poorer outcomes (reviewed by Herman et al).74
Adverse effects include asthenia, headache, dizziness, nausea, diarrhea, epigastric fullness, and heartburn. Although they are often selected based on their low cost, other factors may offset their cost-effectiveness, such as need for glucose monitoring and hospital charges due to sulfonylurea-induced hypoglycemia. Their utility is also limited by dependence on beta-cell function.
Colesevelam
Colesevelam75 is a bile acid sequestrant and low-density lipoprotein cholesterol-reducing agent that has been approved for use in diabetes. The mode of action of colesevelam in this capacity is under investigation. Its effect on hemoglobin A1c is modest. It is associated with gastrointestinal adverse effects, particularly constipation.
Ranolazine
Ranolazine76 is an antianginal drug that also lowers glucose by increasing insulin release. It also possesses cardioprotective properties. In patients with diabetes and non-ST-segment elevation acute coronary syndromes, ranolazine reduced hemoglobin A1c by 1.2% and appeared to be weight-neutral.76 Ranolazine is under clinical development for use in diabetes. Adverse effects include dizziness, headache, constipation, and nausea.
Rational combinations of agents
The ideal strategy would use combinations of agents that mechanistically complement one another and address each path of hyperglycemia present in a patient. This approach should supplant the former approaches of adding-on agents only after treatment failure or sequentially trying first-, second-, and third-line treatments.
Examples of synergistic combinations include those that target fasting plasma glucose and postprandial glucose, reduce reliance on insulin with add-on therapies, or manage hyperglycemia in specific patient groups, such as renal-impaired patients.
Large-scale long-term clinical studies are needed to determine the safety, efficacy, and outcomes of various combinations and whether they confer additive benefits. Some studies have begun to explore possible combinations.
Combined metformin, pioglitazone, and exenatide was reported to delay progression of diabetes in early dysglycemia.77,78 Notably, this combination addresses multiple mediating pathways of hyperglycemia (Table 1).
A GLP-1 receptor agonist with an SGLT2 inhibitor would be another intriguing combination, as the mechanisms of action of these 2 classes complement one another. In limited clinical trials—the DURATION-8 study (lasting 26 weeks),79 the Canagliflozin Cardiovascular Assessment Study (18 weeks),80 and a 24-week study in nondiabetic obese patients81—additive benefits were also seen in systolic blood pressure, body weight, and cardiac risk factors by adding an SGLT2 inhibitor to a GLP-1 receptor agonist, compared with either agent alone. In theory, these improvements might slow or reverse cardiorenal compromise. Lower doses of 1 or more may be possible, and the regimen could prove cost-effective and life-sparing should it slow the progression of the disease and the onset of its complications. A clinical study of this combination is under way (Ralph DeFronzo, personal communication, July 2018). Similarly, the combination of metformin, saxagliptin and dapagliflozin has been shown to be effective.82
CONCLUSION
Care for diabetes mellitus can be particularly challenging for the primary care physician. The progressive nature of diabetes, with worsening hyperglycemia over the course of the disease, further complicates disease management.
Best practices for care nonetheless need to evolve with well-evidenced data, and without years of delay for “trickle-down” education from the specialties to primary care. We have arrived at a juncture to leverage therapies that address the 11 mediating pathways of hyperglycemia, optimally protect beta cells, minimize hypoglycemia, manage risk factors associated with diabetes, and improve diabetes-related outcomes.
- Skyler JS, Bakris GL, Bonifacio E, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes 2017; 66(2):241–255. doi:10.2337/db16-0806
- Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 41(12):2669–2701. doi:10.2337/dci18-0033
- Schwartz SS, Epstein S, Corkey BE, Grant SF, Gavin JR 3rd, Aguilar RB. The time is right for a new classification system for diabetes mellitus: rationale and implications of the beta-cell centric classification schema. Diabetes Care 2016; 39(2):179–186. doi:10.2337/dc15-1585
- Shah MS, Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res 2016; 118(11):1808–1829. doi:10.1161/CIRCRESAHA.116.306923
- Schwartz SS, Jellinger PS, Herman ME. Obviating much of the need for insulin therapy in type 2 diabetes mellitus: a re-assessment of insulin therapy’s safety profile. Postgrad Med 2016; 128(6):609–619. doi:10.1080/00325481.2016.1191955
- Garber AJ, Abrahamson MJ, Barzilay JE, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2019 executive summary. Endocr Pract 2019; 25(1):69–100. doi:10.4158/CS-2018-0535
- Sniderman AD, LaChapelle KJ, Rachon NA , Furberg CD. The necessity for clinical reasoning in the era of evidence-based medicine. Mayo Clin Proc 2013; 88(10):1108–1114. doi:10.1016/j.mayocp.2013.07.012
- Qaseem A, Wilt TJ, Kansagara D, Horwitch C, Barry MJ, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med 2018; 168(8):569–576. doi:10.7326/M17-0939
- Frier BM, Schernthaner G, Heller SR. Hypoglycemia and cardiovascular risks. Diabetes Care 2011; 34(suppl 2):S132–S137. doi:10.2337/dc11-s220
- Chico A, Vidal-Ríos P, Subira M, Novials A. The continuous glucose monitoring system is useful for detecting unrecognized hypoglycemias in patients with type 1 and type 2 diabetes but is not better than frequent capillary glucose measurements for improving metabolic control. Diabetes Care 2003; 26(4):1153–1157. pmid:12663589
- Weber KK, Lohmann T, Busch K, Donati-Hirsch I, Riel R. High frequency of unrecognized hypoglycaemias in patients with type 2 diabetes is discovered by continuous glucose monitoring. Exp Clin Endocrinol Diabetes 2007; 115(8):491–494. doi:10.1055/s-2007-984452
- American Diabetes Association (ADA). The American Diabetes Association, the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators and the Endocrine Society strongly disagree with the American College of Physicians’ guidance for higher blood glucose targets for people with type 2 diabetes www.diabetes.org/newsroom/press-releases/2018/joint-acp-guidance-response.html. Accessed June 6, 2019.
- Freed S; Diabetes in Control. American College of Physicians recommending controversial increase in A1c of 7% to 8%. www.diabetesincontrol.com/american-college-of-physicians-recommending-controversial-increase-in-a1c-of-7-to-8. Accessed June 6, 2019.
- Glucophage XR (metformin hydrochloride) extended release tablets prescribing information. Princeton, NJ, Bristol-Myers Squibb Company, 2009.
- Mellbin LG, Malmberg K, Norhammar A, Wedel H, Rydén L; DIGAMI 2 Investigators. The impact of glucose lowering treatment on long-term prognosis in patients with type 2 diabetes and myocardial infarction: a report from the DIGAMI 2 trial. Eur Heart J 2008; 29(2):166–176. doi:10.1093/eurheartj/ehm518
- Anselmino M, Ohrvik J, Malmberg K, Standl E, Rydén L; Euro Heart Survey Investigators. Glucose lowering treatment in patients with coronary artery disease is prognostically important not only in established but also in newly detected diabetes mellitus: a report from the Euro Heart Survey on Diabetes and the Heart. Eur Heart J 2008; 29(2):177–184. doi:10.1093/eurheartj/ehm519
- Smooke S, Horwich TB, Fonarow GC. Insulin-treated diabetes is associated with a marked increase in mortality in patients with advanced heart failure. Am Heart J 2005; 149(1):168–174. doi:10.1016/j.ahj.2004.07.005
- Colayco DC, Niu F, McCombs JS, Cheetham TC. A1C and cardiovascular outcomes in type 2 diabetes: a nested case-control study. Diabetes Care 2011; 34(1):77–83. doi:10.2337/dc10-1318
- Holden SE, Jenkins-Jones S, Morgan CL, Schernthaner G, Currie CJ. Glucose-lowering with exogenous insulin monotherapy in type 2 diabetes: dose association with all-cause mortality, cardiovascular events and cancer. Diabetes Obes Metab 2015; 17(4):350–362. doi:10.1111/dom.12412
- Tanzeum (albiglutide) prescribing information. Wilmington, DE, GlaxoSmithKline LLC, 2014.
- Trulicity (dulaglutide) prescribing information. Indianapolis, IN, Eli Lilly and Company, 2014.
- Byetta (exenatide) prescribing information. Wilmington, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Victoza (liraglutide injection) prescribing information. Plainsboro, NJ, Novo Nordisk Inc, 2013.
- Adlyxin (lixisenatide injection) prescribing information. Bridgewater, NJ, Sanofi, 2016.
- Ozempic (semaglutide) prescribing information. Plainsboro, NJ, Novo Nordisk, 2017.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375(4):311–322. doi:10.1056/NEJMoa1603827
- Chang G, Zhang D, Yu H, et al. Cardioprotective effects of exenatide against oxidative stress-induced injury. Int J Mol Med 2013; 32(5):1011–1020. doi:10.3892/ijmm.2013.1475
- Best JH, Hoogwerf BJ, Herman WH, et al. Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon-like peptide 1 (GLP-1) receptor agonist exenatide twice daily or other glucose-lowering therapies: a retrospective analysis of the LifeLink database. Diabetes Care; 34(1):90–95. doi:10.2337/dc10-1393
- Pfeffer MA, Claggett B, Diaz R, et al; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373(23):2247–2257. doi:10.1056/NEJMoa1509225
- Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375(19):1834–1844. doi:10.1056/NEJMoa1607141
- Mentlein R. Mechanisms underlying the rapid degradation and elimination of the incretin hormones GLP-1 and GIP. Best Pract Res Clin Endocrinol Metab 2009; 23(4):443–452. doi:10.1016/j.beem.2009.03.005
- Onglyza (saxagliptin) tablets prescribing information. Wilmington, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Januvia (sitagliptin) tablets prescribing information. Whitehouse Station, NJ, Merck & Co., Inc, 2014.
- Tradjenta (linagliptin) tablets prescribing information. Ingelheim, Germany, Boehringer Ingelheim International GmbH, 2014.
- Nesina (alogliptin) tablets prescribing information. Deerfield, IL, Takeda Pharmaceuticals America, Inc, 2013.
- Galvus (vildagliptin) prescribing information. North Ryde, Australia, Novartis Pharmaceuticals, 2014.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368(9548):1696–1705. doi:10.1016/S0140-6736(06)69705-5
- Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369(14):1317–1326. doi:10.1056/NEJMoa1307684
- Invokana (canagliflozin) tablets prescribing information. Titusville, NJ: Janssen Pharmaceuticals, Inc, 2013.
- Farxiga (dapagliflozin) prescribing information. Princeton, NJ, Bristol-Myers Squibb, 2014.
- Jardiance (empagliflozin) prescribing information. Ridgefield, CT, Boehringer Ingelheim Pharmaceuticals, Inc, 2014.
- Steglatro (ertugliflozin) prescribing information. Whitehouse Station, NJ, Merck, Sharp & Dohme Corp, 2017.
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159(4):262–274. doi:10.7326/0003-4819-159-4-201308200-00007
- Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377(7):644–657. doi:10.1056/NEJMoa1611925
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373(22):2117–2128. doi:10.1056/NEJMoa1504720
- Ryan PB, Buse JB, Schuemie MJ, et al. Comparative effectiveness of canagliflozin, SGLT2 inhibitors and non-SGLT2 inhibitors on the risk of hospitalization for heart failure and amputation in patients with type 2 diabetes mellitus: a real-world meta-analysis of 4 observational databases (OBSERVE-4D). Diabetes Obes Metab 2018; 20(11):2485–2597. doi:10.1111/dom.13424
- Actos (pioglitazone) tablets for oral use prescribing information. Deerfield, IL, Takeda Pharmaceuticals America, Inc, 2013.
- Avandia (rosiglitazone maleate tablets) prescribing information. Research Triangle Park, NC, GlaxoSmithKline, 1999.
- Goldberg RB, Kendall DK, Deeg MA, et al; GLAI Study Investigators. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care 2005; 28(7):1547–1554. pmid:15983299
- Dormandy JA, Charbonnel B, Eckland DJ, et al; PROactive Investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone clinical trial in macroVascular Events): a randomised controlled trial. Lancet 2005; 366:1279–1289. doi:10.1016/S0140-6736(05)67528-9
- Nissen SE, Nicholls SJ, Wolski K, et al; PERISCOPE Investigators. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes the PERISCOPE randomized controlled trial. JAMA 2008; 299(13):1561–1573. doi:10.1001/jama.299.13.1561
- Masoudi FA, Inzucchi SE, Wang Y, Havranek EP, Foody JM, Krumholz HM. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation 2005; 111(5):583–590. doi:10.1161/01.CIR.0000154542.13412.B1
- Kernan WN, Viscoli CM, Furie KL, et al; IRIS Trial Investigators. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 2016; 374(14):1321–1331. doi:10.1056/NEJMoa1506930
- DeFronzo RA, Tripathy D, Schwenke DC, et al; ACT NOW Study. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011; 364(12):1104–1115. doi:10.1056/NEJMoa1010949
- Nesto RW, Bell D, Bonow RO, et al; American Heart Association; American Diabetes Association. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. October 7, 2003. Circulation 2003; 108(23):2941–2948. doi:10.1161/01.CIR.0000103683.99399.7E
- Kushner RF, Sujak M. Prevention of weight gain in adult patients with type 2 diabetes treated with pioglitazone. Obesity (Silver Spring) 2009; 17(5):1017–1022. doi:10.1038/oby.2008.651
- Lewis JD, Habel LA, Quesenberry CP, et al. Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes. JAMA 2015; 314(3):265–277. doi:10.1001/jama.2015.7996
- Meier C, Kraenzlin ME, Bodmer M, Jick SS, Jick H, Meier CR. Use of thiazolidinediones and fracture risk. Arch Intern Med 2008; 168(8):820–825. doi:10.1001/archinte.168.8.820
- Gamble JM, Chibrikov E, Twells LK, et al. Association of insulin dosage with mortality or major adverse cardiovascular events: a retrospective cohort study. Lancet Diabetes Endocrinol 2017; 5(1):43–52. doi:10.1016/S2213-8587(16)30316-3
- Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010; 340:b4909. doi:10.1136/bmj.b4909
- Wang X, Yu C, Zhang B, Wang Y. The injurious effects of hyperinsulinism on blood vessels. Cell Biochem Biophys 2014; 69(2):213–218. doi:10.1007/s12013-013-9810-6
- Garber AJ, King AB, Del Prato S, et al; NN1250-3582 (BEGIN BB T2D) Trial Investigators. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet 2012; 379(9825):1498–1507. doi:10.1016/S0140-6736(12)60205-0
- Hanefeld M, Monnier L, Schnell O, Owens D. Early treatment with basal insulin glargine in people with type 2 diabetes: lessons from ORIGIN and other cardiovascular trials. Diabetes Ther 2016; 7(2):187–201. doi:10.1007/s13300-016-0153-3
- Nolan CJ, Ruderman NB, Prentki M. Intensive insulin for type 2 diabetes: the risk of causing harm. Lancet Diabetes Endocrinol 2013; 1(1):9–10. doi:10.1016/S2213-8587(13)70027-5
- Cycloset (bromocriptine mesylate) tablets prescribing information. Tiverton, RI, VeroScience LLC, 2019.
- Schwartz S, Zangeneh F. Evidence-based practice use of quick-release bromocriptine across the natural history of type 2 diabetes mellitus. Postgrad Med 2016; 128(8):828–838. doi:10.1080/00325481.2016.1214059
- Gaziano JM, Cincotta AH, Vinik A, Blonde L, Bohannon N, Scranton R. Effect of bromocriptine-QR (a quick-release formulation of bromocriptine mesylate) on major adverse cardiovascular events in type 2 diabetes subjects. J Am Heart Assoc 2012; 1(5):e002279. doi:10.1161/JAHA.112.002279
- Precose (acarbose) tablets prescribing information. Germany, Bayer HealthCare Pharmaceuticals Inc, 2011.
- Glyset (miglitol) tablets prescribing information. Germany, Bayer HealthCare Pharmaceuticals, Inc, 2012.
- Van de Laar FA, Lucassen PL, Akkermans RP, Van de Lisdonk EH, Rutten GE, Van Weel C. Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev 2005; (2):CD003639. doi:10.1002/14651858.CD003639.pub2
- Symlin (pramlintide acetate) injection for subcutaneous use prescribing information. Wilmongton, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2015; 58(3):429–442. doi:10.1007/s00125-014-3460-0
- Wajchenberg BL. Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev 2007; 28(2):187–218. doi:10.1210/10.1210/er.2006-0038
- Herman ME, O’Keefe JH, Bell DSH, Schwartz SS. Insulin therapy increases cardiovascular risk in type 2 diabetes. Prog Cardiovasc Dis 2017; 60(3):422–434. doi:10.1016/j.pcad.2017.09.001
- Welchol (colesevelam hydrochloride) prescribing information. Parsippany, NJ, Daiichi Sankyo Inc, 2014.
- Ranexa (ranolazine) prescribing information. Foster City, CA: Gilead Sciences, Inc, 2016.
- Armato J, DeFronzo R, Abdul-Ghani M, Ruby R. Successful treatment of prediabetes in clinical practice: targeting insulin resistance and beta-cell dysfunction. Endocr Pract 2012; 18(3):342–350. doi:10.4158/EP11194.OR
- Abdul-Ghani MA, Puckett C, Triplitt C, et al. Initial combination therapy with metformin, pioglitazone and exenatide is more effective than sequential add-on therapy in subjects with new-onset diabetes. Results from the efficacy and durability of initial combination therapy for type 2 diabetes (EDICT): a randomized trial. Diabetes Obes Metab 2015; 17(3):268–275. doi:10.1111/dom.12417
- Frías JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol 2016; 4(12):1004–1016. doi:10.1016/S2213-8587(16)30267-4
- Fulcher G, Matthews DR, Perkovic V, et al; CANVAS trial collaborative group. Efficacy and safety of canagliflozin when used in conjunction with incretin-mimetic therapy in patients with type 2 diabetes. Diabetes Obes Metab 2016; 18(1):82–91. doi:10.1111/dom.12589
- Lundkvist P, Sjöström CD, Amini S, Pereira MJ, Johnsson E, Eriksson JW. Dapagliflozin once-daily and exenatide once-weekly dual therapy: a 24-week randomized, placebo-controlled, phase II study examining effects on body weight and prediabetes in obese adults without diabetes. Diabetes Obes Metab 2017; 19(1):49–60. doi:10.1111/dom.12779
- Del Prato S, Rosenstock J, Garcia-Sanchez R, et al. Safety and tolerability of dapagliflozin, saxagliptin and metformin in combination: post-hoc analysis of concomitant add-on versus sequential add-on to metformin and of triple versus dual therapy with metformin. Diabetes Obes Metab 2018; 20(6):1542–1546. doi:10.1111/dom.13258
- Skyler JS, Bakris GL, Bonifacio E, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes 2017; 66(2):241–255. doi:10.2337/db16-0806
- Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 41(12):2669–2701. doi:10.2337/dci18-0033
- Schwartz SS, Epstein S, Corkey BE, Grant SF, Gavin JR 3rd, Aguilar RB. The time is right for a new classification system for diabetes mellitus: rationale and implications of the beta-cell centric classification schema. Diabetes Care 2016; 39(2):179–186. doi:10.2337/dc15-1585
- Shah MS, Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res 2016; 118(11):1808–1829. doi:10.1161/CIRCRESAHA.116.306923
- Schwartz SS, Jellinger PS, Herman ME. Obviating much of the need for insulin therapy in type 2 diabetes mellitus: a re-assessment of insulin therapy’s safety profile. Postgrad Med 2016; 128(6):609–619. doi:10.1080/00325481.2016.1191955
- Garber AJ, Abrahamson MJ, Barzilay JE, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2019 executive summary. Endocr Pract 2019; 25(1):69–100. doi:10.4158/CS-2018-0535
- Sniderman AD, LaChapelle KJ, Rachon NA , Furberg CD. The necessity for clinical reasoning in the era of evidence-based medicine. Mayo Clin Proc 2013; 88(10):1108–1114. doi:10.1016/j.mayocp.2013.07.012
- Qaseem A, Wilt TJ, Kansagara D, Horwitch C, Barry MJ, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med 2018; 168(8):569–576. doi:10.7326/M17-0939
- Frier BM, Schernthaner G, Heller SR. Hypoglycemia and cardiovascular risks. Diabetes Care 2011; 34(suppl 2):S132–S137. doi:10.2337/dc11-s220
- Chico A, Vidal-Ríos P, Subira M, Novials A. The continuous glucose monitoring system is useful for detecting unrecognized hypoglycemias in patients with type 1 and type 2 diabetes but is not better than frequent capillary glucose measurements for improving metabolic control. Diabetes Care 2003; 26(4):1153–1157. pmid:12663589
- Weber KK, Lohmann T, Busch K, Donati-Hirsch I, Riel R. High frequency of unrecognized hypoglycaemias in patients with type 2 diabetes is discovered by continuous glucose monitoring. Exp Clin Endocrinol Diabetes 2007; 115(8):491–494. doi:10.1055/s-2007-984452
- American Diabetes Association (ADA). The American Diabetes Association, the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators and the Endocrine Society strongly disagree with the American College of Physicians’ guidance for higher blood glucose targets for people with type 2 diabetes www.diabetes.org/newsroom/press-releases/2018/joint-acp-guidance-response.html. Accessed June 6, 2019.
- Freed S; Diabetes in Control. American College of Physicians recommending controversial increase in A1c of 7% to 8%. www.diabetesincontrol.com/american-college-of-physicians-recommending-controversial-increase-in-a1c-of-7-to-8. Accessed June 6, 2019.
- Glucophage XR (metformin hydrochloride) extended release tablets prescribing information. Princeton, NJ, Bristol-Myers Squibb Company, 2009.
- Mellbin LG, Malmberg K, Norhammar A, Wedel H, Rydén L; DIGAMI 2 Investigators. The impact of glucose lowering treatment on long-term prognosis in patients with type 2 diabetes and myocardial infarction: a report from the DIGAMI 2 trial. Eur Heart J 2008; 29(2):166–176. doi:10.1093/eurheartj/ehm518
- Anselmino M, Ohrvik J, Malmberg K, Standl E, Rydén L; Euro Heart Survey Investigators. Glucose lowering treatment in patients with coronary artery disease is prognostically important not only in established but also in newly detected diabetes mellitus: a report from the Euro Heart Survey on Diabetes and the Heart. Eur Heart J 2008; 29(2):177–184. doi:10.1093/eurheartj/ehm519
- Smooke S, Horwich TB, Fonarow GC. Insulin-treated diabetes is associated with a marked increase in mortality in patients with advanced heart failure. Am Heart J 2005; 149(1):168–174. doi:10.1016/j.ahj.2004.07.005
- Colayco DC, Niu F, McCombs JS, Cheetham TC. A1C and cardiovascular outcomes in type 2 diabetes: a nested case-control study. Diabetes Care 2011; 34(1):77–83. doi:10.2337/dc10-1318
- Holden SE, Jenkins-Jones S, Morgan CL, Schernthaner G, Currie CJ. Glucose-lowering with exogenous insulin monotherapy in type 2 diabetes: dose association with all-cause mortality, cardiovascular events and cancer. Diabetes Obes Metab 2015; 17(4):350–362. doi:10.1111/dom.12412
- Tanzeum (albiglutide) prescribing information. Wilmington, DE, GlaxoSmithKline LLC, 2014.
- Trulicity (dulaglutide) prescribing information. Indianapolis, IN, Eli Lilly and Company, 2014.
- Byetta (exenatide) prescribing information. Wilmington, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Victoza (liraglutide injection) prescribing information. Plainsboro, NJ, Novo Nordisk Inc, 2013.
- Adlyxin (lixisenatide injection) prescribing information. Bridgewater, NJ, Sanofi, 2016.
- Ozempic (semaglutide) prescribing information. Plainsboro, NJ, Novo Nordisk, 2017.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375(4):311–322. doi:10.1056/NEJMoa1603827
- Chang G, Zhang D, Yu H, et al. Cardioprotective effects of exenatide against oxidative stress-induced injury. Int J Mol Med 2013; 32(5):1011–1020. doi:10.3892/ijmm.2013.1475
- Best JH, Hoogwerf BJ, Herman WH, et al. Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon-like peptide 1 (GLP-1) receptor agonist exenatide twice daily or other glucose-lowering therapies: a retrospective analysis of the LifeLink database. Diabetes Care; 34(1):90–95. doi:10.2337/dc10-1393
- Pfeffer MA, Claggett B, Diaz R, et al; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373(23):2247–2257. doi:10.1056/NEJMoa1509225
- Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375(19):1834–1844. doi:10.1056/NEJMoa1607141
- Mentlein R. Mechanisms underlying the rapid degradation and elimination of the incretin hormones GLP-1 and GIP. Best Pract Res Clin Endocrinol Metab 2009; 23(4):443–452. doi:10.1016/j.beem.2009.03.005
- Onglyza (saxagliptin) tablets prescribing information. Wilmington, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Januvia (sitagliptin) tablets prescribing information. Whitehouse Station, NJ, Merck & Co., Inc, 2014.
- Tradjenta (linagliptin) tablets prescribing information. Ingelheim, Germany, Boehringer Ingelheim International GmbH, 2014.
- Nesina (alogliptin) tablets prescribing information. Deerfield, IL, Takeda Pharmaceuticals America, Inc, 2013.
- Galvus (vildagliptin) prescribing information. North Ryde, Australia, Novartis Pharmaceuticals, 2014.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368(9548):1696–1705. doi:10.1016/S0140-6736(06)69705-5
- Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369(14):1317–1326. doi:10.1056/NEJMoa1307684
- Invokana (canagliflozin) tablets prescribing information. Titusville, NJ: Janssen Pharmaceuticals, Inc, 2013.
- Farxiga (dapagliflozin) prescribing information. Princeton, NJ, Bristol-Myers Squibb, 2014.
- Jardiance (empagliflozin) prescribing information. Ridgefield, CT, Boehringer Ingelheim Pharmaceuticals, Inc, 2014.
- Steglatro (ertugliflozin) prescribing information. Whitehouse Station, NJ, Merck, Sharp & Dohme Corp, 2017.
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159(4):262–274. doi:10.7326/0003-4819-159-4-201308200-00007
- Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377(7):644–657. doi:10.1056/NEJMoa1611925
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373(22):2117–2128. doi:10.1056/NEJMoa1504720
- Ryan PB, Buse JB, Schuemie MJ, et al. Comparative effectiveness of canagliflozin, SGLT2 inhibitors and non-SGLT2 inhibitors on the risk of hospitalization for heart failure and amputation in patients with type 2 diabetes mellitus: a real-world meta-analysis of 4 observational databases (OBSERVE-4D). Diabetes Obes Metab 2018; 20(11):2485–2597. doi:10.1111/dom.13424
- Actos (pioglitazone) tablets for oral use prescribing information. Deerfield, IL, Takeda Pharmaceuticals America, Inc, 2013.
- Avandia (rosiglitazone maleate tablets) prescribing information. Research Triangle Park, NC, GlaxoSmithKline, 1999.
- Goldberg RB, Kendall DK, Deeg MA, et al; GLAI Study Investigators. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care 2005; 28(7):1547–1554. pmid:15983299
- Dormandy JA, Charbonnel B, Eckland DJ, et al; PROactive Investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone clinical trial in macroVascular Events): a randomised controlled trial. Lancet 2005; 366:1279–1289. doi:10.1016/S0140-6736(05)67528-9
- Nissen SE, Nicholls SJ, Wolski K, et al; PERISCOPE Investigators. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes the PERISCOPE randomized controlled trial. JAMA 2008; 299(13):1561–1573. doi:10.1001/jama.299.13.1561
- Masoudi FA, Inzucchi SE, Wang Y, Havranek EP, Foody JM, Krumholz HM. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation 2005; 111(5):583–590. doi:10.1161/01.CIR.0000154542.13412.B1
- Kernan WN, Viscoli CM, Furie KL, et al; IRIS Trial Investigators. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 2016; 374(14):1321–1331. doi:10.1056/NEJMoa1506930
- DeFronzo RA, Tripathy D, Schwenke DC, et al; ACT NOW Study. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011; 364(12):1104–1115. doi:10.1056/NEJMoa1010949
- Nesto RW, Bell D, Bonow RO, et al; American Heart Association; American Diabetes Association. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. October 7, 2003. Circulation 2003; 108(23):2941–2948. doi:10.1161/01.CIR.0000103683.99399.7E
- Kushner RF, Sujak M. Prevention of weight gain in adult patients with type 2 diabetes treated with pioglitazone. Obesity (Silver Spring) 2009; 17(5):1017–1022. doi:10.1038/oby.2008.651
- Lewis JD, Habel LA, Quesenberry CP, et al. Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes. JAMA 2015; 314(3):265–277. doi:10.1001/jama.2015.7996
- Meier C, Kraenzlin ME, Bodmer M, Jick SS, Jick H, Meier CR. Use of thiazolidinediones and fracture risk. Arch Intern Med 2008; 168(8):820–825. doi:10.1001/archinte.168.8.820
- Gamble JM, Chibrikov E, Twells LK, et al. Association of insulin dosage with mortality or major adverse cardiovascular events: a retrospective cohort study. Lancet Diabetes Endocrinol 2017; 5(1):43–52. doi:10.1016/S2213-8587(16)30316-3
- Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010; 340:b4909. doi:10.1136/bmj.b4909
- Wang X, Yu C, Zhang B, Wang Y. The injurious effects of hyperinsulinism on blood vessels. Cell Biochem Biophys 2014; 69(2):213–218. doi:10.1007/s12013-013-9810-6
- Garber AJ, King AB, Del Prato S, et al; NN1250-3582 (BEGIN BB T2D) Trial Investigators. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet 2012; 379(9825):1498–1507. doi:10.1016/S0140-6736(12)60205-0
- Hanefeld M, Monnier L, Schnell O, Owens D. Early treatment with basal insulin glargine in people with type 2 diabetes: lessons from ORIGIN and other cardiovascular trials. Diabetes Ther 2016; 7(2):187–201. doi:10.1007/s13300-016-0153-3
- Nolan CJ, Ruderman NB, Prentki M. Intensive insulin for type 2 diabetes: the risk of causing harm. Lancet Diabetes Endocrinol 2013; 1(1):9–10. doi:10.1016/S2213-8587(13)70027-5
- Cycloset (bromocriptine mesylate) tablets prescribing information. Tiverton, RI, VeroScience LLC, 2019.
- Schwartz S, Zangeneh F. Evidence-based practice use of quick-release bromocriptine across the natural history of type 2 diabetes mellitus. Postgrad Med 2016; 128(8):828–838. doi:10.1080/00325481.2016.1214059
- Gaziano JM, Cincotta AH, Vinik A, Blonde L, Bohannon N, Scranton R. Effect of bromocriptine-QR (a quick-release formulation of bromocriptine mesylate) on major adverse cardiovascular events in type 2 diabetes subjects. J Am Heart Assoc 2012; 1(5):e002279. doi:10.1161/JAHA.112.002279
- Precose (acarbose) tablets prescribing information. Germany, Bayer HealthCare Pharmaceuticals Inc, 2011.
- Glyset (miglitol) tablets prescribing information. Germany, Bayer HealthCare Pharmaceuticals, Inc, 2012.
- Van de Laar FA, Lucassen PL, Akkermans RP, Van de Lisdonk EH, Rutten GE, Van Weel C. Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev 2005; (2):CD003639. doi:10.1002/14651858.CD003639.pub2
- Symlin (pramlintide acetate) injection for subcutaneous use prescribing information. Wilmongton, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2015; 58(3):429–442. doi:10.1007/s00125-014-3460-0
- Wajchenberg BL. Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev 2007; 28(2):187–218. doi:10.1210/10.1210/er.2006-0038
- Herman ME, O’Keefe JH, Bell DSH, Schwartz SS. Insulin therapy increases cardiovascular risk in type 2 diabetes. Prog Cardiovasc Dis 2017; 60(3):422–434. doi:10.1016/j.pcad.2017.09.001
- Welchol (colesevelam hydrochloride) prescribing information. Parsippany, NJ, Daiichi Sankyo Inc, 2014.
- Ranexa (ranolazine) prescribing information. Foster City, CA: Gilead Sciences, Inc, 2016.
- Armato J, DeFronzo R, Abdul-Ghani M, Ruby R. Successful treatment of prediabetes in clinical practice: targeting insulin resistance and beta-cell dysfunction. Endocr Pract 2012; 18(3):342–350. doi:10.4158/EP11194.OR
- Abdul-Ghani MA, Puckett C, Triplitt C, et al. Initial combination therapy with metformin, pioglitazone and exenatide is more effective than sequential add-on therapy in subjects with new-onset diabetes. Results from the efficacy and durability of initial combination therapy for type 2 diabetes (EDICT): a randomized trial. Diabetes Obes Metab 2015; 17(3):268–275. doi:10.1111/dom.12417
- Frías JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol 2016; 4(12):1004–1016. doi:10.1016/S2213-8587(16)30267-4
- Fulcher G, Matthews DR, Perkovic V, et al; CANVAS trial collaborative group. Efficacy and safety of canagliflozin when used in conjunction with incretin-mimetic therapy in patients with type 2 diabetes. Diabetes Obes Metab 2016; 18(1):82–91. doi:10.1111/dom.12589
- Lundkvist P, Sjöström CD, Amini S, Pereira MJ, Johnsson E, Eriksson JW. Dapagliflozin once-daily and exenatide once-weekly dual therapy: a 24-week randomized, placebo-controlled, phase II study examining effects on body weight and prediabetes in obese adults without diabetes. Diabetes Obes Metab 2017; 19(1):49–60. doi:10.1111/dom.12779
- Del Prato S, Rosenstock J, Garcia-Sanchez R, et al. Safety and tolerability of dapagliflozin, saxagliptin and metformin in combination: post-hoc analysis of concomitant add-on versus sequential add-on to metformin and of triple versus dual therapy with metformin. Diabetes Obes Metab 2018; 20(6):1542–1546. doi:10.1111/dom.13258
KEY POINTS
- At least 11 pathways lead to hyperglycemia; of these, beta-cell dysfunction is central.
- As different classes of diabetes drugs act on different pathways, we can target the pathways contributing to hyperglycemia in the individual patient, using fewer agents and lessening the risk of hypoglycemic episodes.
- In selecting treatment, we should favor drugs that are “gentle” on beta cells, do not cause dangerous hypoglycemia, and improve long-term outcomes as shown in randomized clinical trials.
Clinical trials: More to learn than the results
The clinical update of giant cell arteritis (GCA) by Rinden et al in this issue of the Journal reminded me of just how much of our management of this disease has, for decades, been based on retrospective studies (we owe a lot to clinicians from the Mayo Clinic for their compiled observations) tempered by our own recalled experiences, which we may at times twist a bit to fit prevailing paradigms. Several prospective interventional studies, perhaps most importantly the Giant-Cell Arteritis Actemra (GIACTA) trial,1 evaluated the ability of the interleukin 6 (IL-6) antagonist tocilizumab to supplant the protracted use of glucocorticoids in the treatment of GCA. But I learned much more from this trial, in the form of collected clinical tidbits, than just the bottom-line abstract conclusion that IL-6 antagonism is at least a promising approach in many patients with GCA.
As teachers, we tell students to read the entire published clinical trial report, not just the abstract and conclusions. Over the years, I have been impatient with those who violated this dictum, but I now often find myself among the ranks of those who would have been targets of my disapproval. Usually, the articles that I merely skim lie outside my subsubspecialty areas of interest, as time constraints make this abridged reading a necessity for survival, but that excuse does not diminish the self-recognition of my often less-than-complete understanding of the clinical condition being reported. Delving into the nuances of GIACTA truly emphasized that point.
The external validity of any trial rests on understanding the trial’s methods. In the case of GIACTA, there was much more to be learned and affirmed from the trial1 than that 1 year of tocilizumab treatment met the primary end point of increasing the percent of patients achieving sustained remission at week 52 after a rapid 26-week tapering off of prednisone compared with placebo.
One treatment group in the GIACTA trial underwent an aggressive 6-month tapering of prednisone, while another underwent a more protracted tapering over 12 months (more in line with common practice). Patients tapered over 6 months also received either the IL-6 antagonist or placebo for the full year. The concept was that if IL-6 blockade is a correct approach, then it will maintain remission in more patients, and significantly reduce the total amount of steroid needed to control the disease, despite rapid, aggressive steroid tapering. This turned out to be correct, although more than 20% of the drug-treated patients still experienced a flare of GCA (vs 68% of the placebo-treated group).
Somewhat surprising was that almost 20% of the entered patients did not achieve an initial remission despite receiving high-dose prednisone. The traditional teaching is that if a patient diagnosed with GCA does not respond to high-dose steroids, the diagnosis should be questioned.
Another interesting facet of the study relates to the diagnosis. We are becoming more aware of the different GCA phenotypes, which include prominent polymyalgia rheumatica or constitutional features, “classic” GCA with cranial symptoms, and dominant large-vessel vasculitis (aortitis and major aortic branch disease). In GIACTA, even though imaging was not mandated, 37% of participants were enrolled based in part on imaging results (CT, MRI, angiography, or PET-CT), not on the results of temporal artery biopsy. This forces us to think more broadly about diagnosing and staging GCA, and to wonder if we should even modify our approach to other clinical challenges, including unexplained fever and wasting in older patients.
Another tidbit that came out of the study relates to the relationship between the acute-phase response and clinical flares. We already knew that a rise in the erythrocyte sedimentation rate is a nonspecific sign and does not equate with a flare. In this trial one-third of patients in the placebo group who had a flare had a normal sedimentation rate or C-reactive protein during the flare, and about one-third of patients in the placebo group were receiving more than 10 mg of prednisone. In preliminary reports of follow-up after 52 weeks of treatment,2 patients who had achieved complete remission with the IL-6 antagonist and were off of prednisone were still not out of the woods; when the drug was discontinued, many flares continued to occur over the 2-year study extension. We have more to learn about what triggers and drives flares in this disease.
Thus, in addition to informing us of a successful “steroid-sparing” and rescue drug option for our patients with GCA, the details of this well-conducted trial both challenge and reaffirm some of our clinical impressions. Clearly, GCA must be defined for many patients as a very chronic disease, perhaps with occult vascular reservoirs, the biologic basis of which remains to be defined.
- Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017; 377(4):317–328. doi:10.1056/NEJMoa1613849
- Stone JH, Bao M, Han J, et al. Long-term outcome of tocilizumab for patients with giant cell arteritis: results from part 2 of the GIACTA trial (abstract). Ann Rheum Dis 2019; 78:145–146. doi:10.1136/annrheumdis-2019-eular.2099
The clinical update of giant cell arteritis (GCA) by Rinden et al in this issue of the Journal reminded me of just how much of our management of this disease has, for decades, been based on retrospective studies (we owe a lot to clinicians from the Mayo Clinic for their compiled observations) tempered by our own recalled experiences, which we may at times twist a bit to fit prevailing paradigms. Several prospective interventional studies, perhaps most importantly the Giant-Cell Arteritis Actemra (GIACTA) trial,1 evaluated the ability of the interleukin 6 (IL-6) antagonist tocilizumab to supplant the protracted use of glucocorticoids in the treatment of GCA. But I learned much more from this trial, in the form of collected clinical tidbits, than just the bottom-line abstract conclusion that IL-6 antagonism is at least a promising approach in many patients with GCA.
As teachers, we tell students to read the entire published clinical trial report, not just the abstract and conclusions. Over the years, I have been impatient with those who violated this dictum, but I now often find myself among the ranks of those who would have been targets of my disapproval. Usually, the articles that I merely skim lie outside my subsubspecialty areas of interest, as time constraints make this abridged reading a necessity for survival, but that excuse does not diminish the self-recognition of my often less-than-complete understanding of the clinical condition being reported. Delving into the nuances of GIACTA truly emphasized that point.
The external validity of any trial rests on understanding the trial’s methods. In the case of GIACTA, there was much more to be learned and affirmed from the trial1 than that 1 year of tocilizumab treatment met the primary end point of increasing the percent of patients achieving sustained remission at week 52 after a rapid 26-week tapering off of prednisone compared with placebo.
One treatment group in the GIACTA trial underwent an aggressive 6-month tapering of prednisone, while another underwent a more protracted tapering over 12 months (more in line with common practice). Patients tapered over 6 months also received either the IL-6 antagonist or placebo for the full year. The concept was that if IL-6 blockade is a correct approach, then it will maintain remission in more patients, and significantly reduce the total amount of steroid needed to control the disease, despite rapid, aggressive steroid tapering. This turned out to be correct, although more than 20% of the drug-treated patients still experienced a flare of GCA (vs 68% of the placebo-treated group).
Somewhat surprising was that almost 20% of the entered patients did not achieve an initial remission despite receiving high-dose prednisone. The traditional teaching is that if a patient diagnosed with GCA does not respond to high-dose steroids, the diagnosis should be questioned.
Another interesting facet of the study relates to the diagnosis. We are becoming more aware of the different GCA phenotypes, which include prominent polymyalgia rheumatica or constitutional features, “classic” GCA with cranial symptoms, and dominant large-vessel vasculitis (aortitis and major aortic branch disease). In GIACTA, even though imaging was not mandated, 37% of participants were enrolled based in part on imaging results (CT, MRI, angiography, or PET-CT), not on the results of temporal artery biopsy. This forces us to think more broadly about diagnosing and staging GCA, and to wonder if we should even modify our approach to other clinical challenges, including unexplained fever and wasting in older patients.
Another tidbit that came out of the study relates to the relationship between the acute-phase response and clinical flares. We already knew that a rise in the erythrocyte sedimentation rate is a nonspecific sign and does not equate with a flare. In this trial one-third of patients in the placebo group who had a flare had a normal sedimentation rate or C-reactive protein during the flare, and about one-third of patients in the placebo group were receiving more than 10 mg of prednisone. In preliminary reports of follow-up after 52 weeks of treatment,2 patients who had achieved complete remission with the IL-6 antagonist and were off of prednisone were still not out of the woods; when the drug was discontinued, many flares continued to occur over the 2-year study extension. We have more to learn about what triggers and drives flares in this disease.
Thus, in addition to informing us of a successful “steroid-sparing” and rescue drug option for our patients with GCA, the details of this well-conducted trial both challenge and reaffirm some of our clinical impressions. Clearly, GCA must be defined for many patients as a very chronic disease, perhaps with occult vascular reservoirs, the biologic basis of which remains to be defined.
The clinical update of giant cell arteritis (GCA) by Rinden et al in this issue of the Journal reminded me of just how much of our management of this disease has, for decades, been based on retrospective studies (we owe a lot to clinicians from the Mayo Clinic for their compiled observations) tempered by our own recalled experiences, which we may at times twist a bit to fit prevailing paradigms. Several prospective interventional studies, perhaps most importantly the Giant-Cell Arteritis Actemra (GIACTA) trial,1 evaluated the ability of the interleukin 6 (IL-6) antagonist tocilizumab to supplant the protracted use of glucocorticoids in the treatment of GCA. But I learned much more from this trial, in the form of collected clinical tidbits, than just the bottom-line abstract conclusion that IL-6 antagonism is at least a promising approach in many patients with GCA.
As teachers, we tell students to read the entire published clinical trial report, not just the abstract and conclusions. Over the years, I have been impatient with those who violated this dictum, but I now often find myself among the ranks of those who would have been targets of my disapproval. Usually, the articles that I merely skim lie outside my subsubspecialty areas of interest, as time constraints make this abridged reading a necessity for survival, but that excuse does not diminish the self-recognition of my often less-than-complete understanding of the clinical condition being reported. Delving into the nuances of GIACTA truly emphasized that point.
The external validity of any trial rests on understanding the trial’s methods. In the case of GIACTA, there was much more to be learned and affirmed from the trial1 than that 1 year of tocilizumab treatment met the primary end point of increasing the percent of patients achieving sustained remission at week 52 after a rapid 26-week tapering off of prednisone compared with placebo.
One treatment group in the GIACTA trial underwent an aggressive 6-month tapering of prednisone, while another underwent a more protracted tapering over 12 months (more in line with common practice). Patients tapered over 6 months also received either the IL-6 antagonist or placebo for the full year. The concept was that if IL-6 blockade is a correct approach, then it will maintain remission in more patients, and significantly reduce the total amount of steroid needed to control the disease, despite rapid, aggressive steroid tapering. This turned out to be correct, although more than 20% of the drug-treated patients still experienced a flare of GCA (vs 68% of the placebo-treated group).
Somewhat surprising was that almost 20% of the entered patients did not achieve an initial remission despite receiving high-dose prednisone. The traditional teaching is that if a patient diagnosed with GCA does not respond to high-dose steroids, the diagnosis should be questioned.
Another interesting facet of the study relates to the diagnosis. We are becoming more aware of the different GCA phenotypes, which include prominent polymyalgia rheumatica or constitutional features, “classic” GCA with cranial symptoms, and dominant large-vessel vasculitis (aortitis and major aortic branch disease). In GIACTA, even though imaging was not mandated, 37% of participants were enrolled based in part on imaging results (CT, MRI, angiography, or PET-CT), not on the results of temporal artery biopsy. This forces us to think more broadly about diagnosing and staging GCA, and to wonder if we should even modify our approach to other clinical challenges, including unexplained fever and wasting in older patients.
Another tidbit that came out of the study relates to the relationship between the acute-phase response and clinical flares. We already knew that a rise in the erythrocyte sedimentation rate is a nonspecific sign and does not equate with a flare. In this trial one-third of patients in the placebo group who had a flare had a normal sedimentation rate or C-reactive protein during the flare, and about one-third of patients in the placebo group were receiving more than 10 mg of prednisone. In preliminary reports of follow-up after 52 weeks of treatment,2 patients who had achieved complete remission with the IL-6 antagonist and were off of prednisone were still not out of the woods; when the drug was discontinued, many flares continued to occur over the 2-year study extension. We have more to learn about what triggers and drives flares in this disease.
Thus, in addition to informing us of a successful “steroid-sparing” and rescue drug option for our patients with GCA, the details of this well-conducted trial both challenge and reaffirm some of our clinical impressions. Clearly, GCA must be defined for many patients as a very chronic disease, perhaps with occult vascular reservoirs, the biologic basis of which remains to be defined.
- Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017; 377(4):317–328. doi:10.1056/NEJMoa1613849
- Stone JH, Bao M, Han J, et al. Long-term outcome of tocilizumab for patients with giant cell arteritis: results from part 2 of the GIACTA trial (abstract). Ann Rheum Dis 2019; 78:145–146. doi:10.1136/annrheumdis-2019-eular.2099
- Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017; 377(4):317–328. doi:10.1056/NEJMoa1613849
- Stone JH, Bao M, Han J, et al. Long-term outcome of tocilizumab for patients with giant cell arteritis: results from part 2 of the GIACTA trial (abstract). Ann Rheum Dis 2019; 78:145–146. doi:10.1136/annrheumdis-2019-eular.2099