User login
Telemedicine Alopecia Assessment: Highlighting Patients With Skin of Color
Practice Gap
In accordance with World Health Organization guidelines on social distancing to limit transmission of SARS-CoV-2, dermatologists have relied on teledermatology (TD) to develop novel adaptations of traditional workflows, optimize patient care, and limit in-person appointments during the COVID-19 pandemic. Pandemic-induced physical and emotional stress were anticipated to increase the incidence of dermatologic diseases with psychologic triggers.
The connection between hair loss and emotional stress is well documented for telogen effluvium and alopecia areata.1,2 As anticipated, dermatology visits increased during the COVID-19 pandemic for the diagnosis of alopecia1-4; a survey performed during the pandemic found that alopecia was one of the most common diagnoses dermatologists made through telehealth platforms.5
This article provides a practical guide for dermatology practitioners to efficiently and accurately assess alopecia by TD in all patients, with added considerations for skin of color patients.
Diagnostic Tools
The intersection of TD, as an effective mechanism for the diagnosis and treatment of dermatologic disorders, and the increase in alopecia observed during the COVID-19 pandemic prompted us to develop a workflow for conducting virtual scalp examinations. Seven dermatologists (A.M., A.A., O.A., N.E., V.C., C.M.B., S.C.T.) who are experts in hair disorders contributed to developing workflows to optimize the assessment of alopecia through a virtual scalp examination, with an emphasis on patients of color. These experts completed a 7-question survey (Table) detailing their approach to the virtual scalp examination. One author (B.N.W.) served as an independent reviewer and collated responses into the following workflows.

Telemedicine Previsit Workflow
Components of the previsit workflow include:
• Instruct patients to provide all laboratory values and biopsy reports before the appointment.
• Test for a stable Wi-Fi connection using a speed test (available at https://www.speedtest.net/). A speed of 10 megabits/second or more is required for high-quality video via TD.6
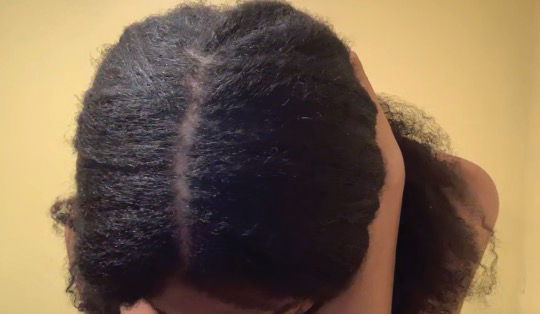
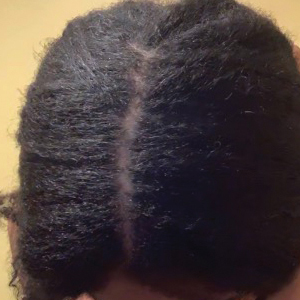
• Provide a handout illustrating the required photographs of the anterior hairline; the mid scalp, including vertex, bilateral parietal, and occipital scalp; and posterior hairline. Photographs should be uploaded 2 hours before the visit. Figures 1 and 2 are examples of photographs that should be requested.
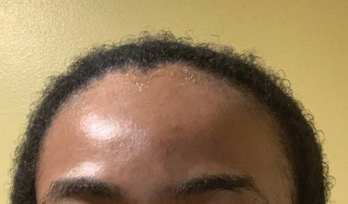
• Request images with 2 or 3 different angles of the area of the scalp with the greatest involvement to help appreciate primary and secondary characteristics.
• Encourage patients to present with clean, recently shampooed, dried, and detangled natural hair, unless they have an itchy or flaky scalp.
• For concerns of scalp, hairline, eyebrow, or facial flaking and scaling, instruct the patient to avoid applying a moisturizer before the visit.
• Instruct the patient to remove false eyelashes, eyelash extensions, eyebrow pencil, hair camouflage, hair accessories, braids, extensions, weaves, twists, and other hairstyles so that the hair can be maneuvered to expose the scalp surface.
• Instruct the patient to have a comb, pic, or brush, or more than one of these implements, available during the visit.
Telemedicine Visit Workflow
Components of the visit workflow include:
• If a stable Wi-Fi connection cannot be established, switch to an audio-only visit to collect a pertinent history. Advise the patient that in-person follow-up must be scheduled.
• Confirm that (1) the patient is in a private setting where the scalp can be viewed and (2) lighting is positioned in front of the patient.
• Ensure that the patient’s hairline, full face, eyebrows, and eyelashes and, upon request, the vertex and posterior scalp, are completely visible.
• Initiate the virtual scalp examination by instructing the patient how to perform a hair pull test. Then, examine the pattern and distribution of hair loss alongside supplemental photographs.
• Instruct the patient to apply pressure with the fingertips throughout the scalp to help localize tenderness, which, in combination with the pattern of hair loss observed, might inform the diagnosis.
• Instruct the patient to scan the scalp with the fingertips for “bumps” to locate papules, pustules, and keloidal scars.
Diagnostic Pearls
Distribution of Alopecia—The experts noted that the pattern, distribution, and location of hair loss determined from the telemedicine alopecia assessment provided important clues to distinguish the type of alopecia.
Diagnostic clues for diffuse or generalized alopecia include:
• Either of these findings might be indicative of telogen effluvium or acquired trichorrhexis nodosa. Results of the hair pull test can help distinguish between these diagnoses.
• Recent stressful life events along with the presence of telogen hairs extracted during a hair pull test support the diagnosis of telogen effluvium.
• A history of external stress on the hair—thermal, traction, or chemical—along with broken hair shafts following the hair pull test support the diagnosis of acquired trichorrhexis nodosa.
Diagnostic clues for focal or patchy alopecia include:
• Alopecia areata generally presents as focal hair loss in an annular distribution; pruritus, erythema, and scale are absent.
• Seborrheic dermatitis can present as pruritic erythematous patches with scale distributed on the scalp and, in some cases, in the eyebrows, nasolabial folds, or paranasal skin.7 Some skin of color patients present with petaloid seborrheic dermatitis—pink or hypopigmented polycyclic coalescing rings with minimal scale.7,8
• Discoid lupus erythematosus, similar to seborrheic dermatitis, might present as pruritic, scaly, hypopigmented patches. However, in the experience of the experts, a more common presentation is tender erythematous patches of hair loss with central hypopigmentation and surrounding hyperpigmentation.
Diagnostic clues for vertex and mid scalp alopecia include:
• Androgenetic alopecia typically presents as a reduction of terminal hair density in the vertex and mid scalp regions (with widening through the midline part) and fine hair along the anterior hairline.9 Signs of concomitant hyperandrogenism, including facial hirsutism, acne, and obesity, might be observed.10
• Central centrifugal cicatricial alopecia typically affects the vertex and mid scalp with a shiny scalp appearance and follicular dropout.
Diagnostic clues for frontotemporal alopecia include:
• Frontal fibrosing alopecia (FFA) often presents with spared single terminal hairs (lonely hair sign).
• Traction alopecia commonly presents with the fringe hair sign.
Scalp Symptoms—The experts noted that the presence of symptoms (eg, pain, tenderness, pruritus) in conjunction with the pattern of hair loss might support the diagnosis of an inflammatory scarring alopecia.
When do symptoms raise suspicion of central centrifugal cicatricial alopecia?
• Suspected in the setting of vertex alopecia associated with tenderness, pain, or itching.
When do symptoms raise suspicion of FFA?
• Suspected when patients experience frontotemporal tenderness, pain, or burning associated with alopecia.
• The skin hue of the affected area might be lighter in color than, and contrast with, the darker hue of the photoaged upper forehead.11
• The lonely hair sign can aid in diagnosing FFA and distinguish it from the fringe sign of traction alopecia.
• Concurrent madarosis, flesh-colored papules on the cheeks, or lichen planus pigmentosus identified by visual inspection of the face confirms the diagnosis.9,12 Madarosis of the eyebrow was frequently cited by the experts as an associated symptom of FFA.
When do symptoms raise suspicion of lichen planopilaris?
• Suspected in the presence of pruritus, burning, tenderness, or pain associated with perifollicular erythema and scale in the setting of vertex and parietal alopecia.13
• Anagen hair release is observed during the hair pull test.11,14• The experts cited flesh-colored papules and lichen planus pigmentosus as frequently associated symptoms of lichen planopilaris.
Practice Implications
There are limitations to a virtual scalp examination—the inability to perform a scalp biopsy or administer certain treatments—but the consensus of the expert panel is that an initial alopecia assessment can be completed successfully utilizing TD. Although TD is not a replacement for an in-person dermatology visit, this technology has allowed for the diagnosis, treatment, and continuing care of many common dermatologic conditions without the patient needing to travel to the office.5
With the increased frequency of hair loss concerns documented over the last year and more patients seeking TD, it is imperative that dermatologists feel confident performing a virtual hair and scalp examination on all patients.1,3,4
- Kutlu Ö, Aktas¸ H, I·mren IG, et al. Short-term stress-related increasing cases of alopecia areata during the COVID-19 pandemic. J Dermatolog Treat. 2020;1. doi:10.1080/09546634.2020.1782820
- Cline A, Kazemi A, Moy J, et al. A surge in the incidence of telogen effluvium in minority predominant communities heavily impacted by COVID-19. J Am Acad Dermatol. 2021;84:773-775. doi:10.1016/j.jaad.2020.11.032
- Kutlu Ö, Metin A. Relative changes in the pattern of diseases presenting in dermatology outpatient clinic in the era of the COVID-19 pandemic. Dermatol Ther. 2020;33:e14096. doi:10.1111/dth.14096
- Tanacan E, Aksoy Sarac G, Emeksiz MAC, et al. Changing trends in dermatology practice during COVID-19 pandemic: a single tertiary center experience. Dermatol Ther. 2020;33:e14136. doi:10.1111/dth.14136
- Sharma A, Jindal V, Singla P, et al. Will teledermatology be the silver lining during and after COVID-19? Dermatol Ther. 2020;33:e13643. doi:10.1111/dth.13643
- Iscrupe L. How to receive virtual medical treatment while under quarantine. Allconnect website. Published March 26, 2020. Accessed December 9, 2021. https://www.allconnect.com/blog/online-doctor-visit-faq
- Elgash M, Dlova N, Ogunleye T, et al. Seborrheic dermatitis in skin of color: clinical considerations. J Drugs Dermatol. 2019;18:24-27.
- McLaurin CI. Annular facial dermatoses in blacks. Cutis. 1983;32:369-370, 384.
- Suchonwanit P, Hector CE, Bin Saif GA, McMichael AJ. Factors affecting the severity of central centrifugal cicatricial alopecia. Int J Dermatol. 2016;55:e338-343. doi:10.1111/ijd.13061
- Gabros S, Masood S. Central centrifugal cicatricial alopecia. StatPearls [Internet]. StatPearls Publishing; 2021. Updated July 20, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559187/
- Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37. doi:10.1016/j.jaad.2004.06.015
- Cobos G, Kim RH, Meehan S, et al. Lichen planus pigmentosus and lichen planopilaris. Dermatol Online J. 2016;22:13030/qt7hp8n6dn.
- Lyakhovitsky A, Amichai B, Sizopoulou C, et al. A case series of 46 patients with lichen planopilaris: demographics, clinical evaluation, and treatment experience. J Dermatolog Treat. 2015;26:275-279. doi:10.3109/09546634.2014.933165
- Tan E, Martinka M, Ball N, et al. Primary cicatricial alopecias: clinicopathology of 112 cases. J Am Acad Dermatol. 2004;50:25-32. doi:10.1016/j.jaad.2003.04.001
Practice Gap
In accordance with World Health Organization guidelines on social distancing to limit transmission of SARS-CoV-2, dermatologists have relied on teledermatology (TD) to develop novel adaptations of traditional workflows, optimize patient care, and limit in-person appointments during the COVID-19 pandemic. Pandemic-induced physical and emotional stress were anticipated to increase the incidence of dermatologic diseases with psychologic triggers.
The connection between hair loss and emotional stress is well documented for telogen effluvium and alopecia areata.1,2 As anticipated, dermatology visits increased during the COVID-19 pandemic for the diagnosis of alopecia1-4; a survey performed during the pandemic found that alopecia was one of the most common diagnoses dermatologists made through telehealth platforms.5
This article provides a practical guide for dermatology practitioners to efficiently and accurately assess alopecia by TD in all patients, with added considerations for skin of color patients.
Diagnostic Tools
The intersection of TD, as an effective mechanism for the diagnosis and treatment of dermatologic disorders, and the increase in alopecia observed during the COVID-19 pandemic prompted us to develop a workflow for conducting virtual scalp examinations. Seven dermatologists (A.M., A.A., O.A., N.E., V.C., C.M.B., S.C.T.) who are experts in hair disorders contributed to developing workflows to optimize the assessment of alopecia through a virtual scalp examination, with an emphasis on patients of color. These experts completed a 7-question survey (Table) detailing their approach to the virtual scalp examination. One author (B.N.W.) served as an independent reviewer and collated responses into the following workflows.

Telemedicine Previsit Workflow
Components of the previsit workflow include:
• Instruct patients to provide all laboratory values and biopsy reports before the appointment.
• Test for a stable Wi-Fi connection using a speed test (available at https://www.speedtest.net/). A speed of 10 megabits/second or more is required for high-quality video via TD.6

• Provide a handout illustrating the required photographs of the anterior hairline; the mid scalp, including vertex, bilateral parietal, and occipital scalp; and posterior hairline. Photographs should be uploaded 2 hours before the visit. Figures 1 and 2 are examples of photographs that should be requested.

• Request images with 2 or 3 different angles of the area of the scalp with the greatest involvement to help appreciate primary and secondary characteristics.
• Encourage patients to present with clean, recently shampooed, dried, and detangled natural hair, unless they have an itchy or flaky scalp.
• For concerns of scalp, hairline, eyebrow, or facial flaking and scaling, instruct the patient to avoid applying a moisturizer before the visit.
• Instruct the patient to remove false eyelashes, eyelash extensions, eyebrow pencil, hair camouflage, hair accessories, braids, extensions, weaves, twists, and other hairstyles so that the hair can be maneuvered to expose the scalp surface.
• Instruct the patient to have a comb, pic, or brush, or more than one of these implements, available during the visit.
Telemedicine Visit Workflow
Components of the visit workflow include:
• If a stable Wi-Fi connection cannot be established, switch to an audio-only visit to collect a pertinent history. Advise the patient that in-person follow-up must be scheduled.
• Confirm that (1) the patient is in a private setting where the scalp can be viewed and (2) lighting is positioned in front of the patient.
• Ensure that the patient’s hairline, full face, eyebrows, and eyelashes and, upon request, the vertex and posterior scalp, are completely visible.
• Initiate the virtual scalp examination by instructing the patient how to perform a hair pull test. Then, examine the pattern and distribution of hair loss alongside supplemental photographs.
• Instruct the patient to apply pressure with the fingertips throughout the scalp to help localize tenderness, which, in combination with the pattern of hair loss observed, might inform the diagnosis.
• Instruct the patient to scan the scalp with the fingertips for “bumps” to locate papules, pustules, and keloidal scars.
Diagnostic Pearls
Distribution of Alopecia—The experts noted that the pattern, distribution, and location of hair loss determined from the telemedicine alopecia assessment provided important clues to distinguish the type of alopecia.
Diagnostic clues for diffuse or generalized alopecia include:
• Either of these findings might be indicative of telogen effluvium or acquired trichorrhexis nodosa. Results of the hair pull test can help distinguish between these diagnoses.
• Recent stressful life events along with the presence of telogen hairs extracted during a hair pull test support the diagnosis of telogen effluvium.
• A history of external stress on the hair—thermal, traction, or chemical—along with broken hair shafts following the hair pull test support the diagnosis of acquired trichorrhexis nodosa.
Diagnostic clues for focal or patchy alopecia include:
• Alopecia areata generally presents as focal hair loss in an annular distribution; pruritus, erythema, and scale are absent.
• Seborrheic dermatitis can present as pruritic erythematous patches with scale distributed on the scalp and, in some cases, in the eyebrows, nasolabial folds, or paranasal skin.7 Some skin of color patients present with petaloid seborrheic dermatitis—pink or hypopigmented polycyclic coalescing rings with minimal scale.7,8
• Discoid lupus erythematosus, similar to seborrheic dermatitis, might present as pruritic, scaly, hypopigmented patches. However, in the experience of the experts, a more common presentation is tender erythematous patches of hair loss with central hypopigmentation and surrounding hyperpigmentation.
Diagnostic clues for vertex and mid scalp alopecia include:
• Androgenetic alopecia typically presents as a reduction of terminal hair density in the vertex and mid scalp regions (with widening through the midline part) and fine hair along the anterior hairline.9 Signs of concomitant hyperandrogenism, including facial hirsutism, acne, and obesity, might be observed.10
• Central centrifugal cicatricial alopecia typically affects the vertex and mid scalp with a shiny scalp appearance and follicular dropout.
Diagnostic clues for frontotemporal alopecia include:
• Frontal fibrosing alopecia (FFA) often presents with spared single terminal hairs (lonely hair sign).
• Traction alopecia commonly presents with the fringe hair sign.
Scalp Symptoms—The experts noted that the presence of symptoms (eg, pain, tenderness, pruritus) in conjunction with the pattern of hair loss might support the diagnosis of an inflammatory scarring alopecia.
When do symptoms raise suspicion of central centrifugal cicatricial alopecia?
• Suspected in the setting of vertex alopecia associated with tenderness, pain, or itching.
When do symptoms raise suspicion of FFA?
• Suspected when patients experience frontotemporal tenderness, pain, or burning associated with alopecia.
• The skin hue of the affected area might be lighter in color than, and contrast with, the darker hue of the photoaged upper forehead.11
• The lonely hair sign can aid in diagnosing FFA and distinguish it from the fringe sign of traction alopecia.
• Concurrent madarosis, flesh-colored papules on the cheeks, or lichen planus pigmentosus identified by visual inspection of the face confirms the diagnosis.9,12 Madarosis of the eyebrow was frequently cited by the experts as an associated symptom of FFA.
When do symptoms raise suspicion of lichen planopilaris?
• Suspected in the presence of pruritus, burning, tenderness, or pain associated with perifollicular erythema and scale in the setting of vertex and parietal alopecia.13
• Anagen hair release is observed during the hair pull test.11,14• The experts cited flesh-colored papules and lichen planus pigmentosus as frequently associated symptoms of lichen planopilaris.
Practice Implications
There are limitations to a virtual scalp examination—the inability to perform a scalp biopsy or administer certain treatments—but the consensus of the expert panel is that an initial alopecia assessment can be completed successfully utilizing TD. Although TD is not a replacement for an in-person dermatology visit, this technology has allowed for the diagnosis, treatment, and continuing care of many common dermatologic conditions without the patient needing to travel to the office.5
With the increased frequency of hair loss concerns documented over the last year and more patients seeking TD, it is imperative that dermatologists feel confident performing a virtual hair and scalp examination on all patients.1,3,4
Practice Gap
In accordance with World Health Organization guidelines on social distancing to limit transmission of SARS-CoV-2, dermatologists have relied on teledermatology (TD) to develop novel adaptations of traditional workflows, optimize patient care, and limit in-person appointments during the COVID-19 pandemic. Pandemic-induced physical and emotional stress were anticipated to increase the incidence of dermatologic diseases with psychologic triggers.
The connection between hair loss and emotional stress is well documented for telogen effluvium and alopecia areata.1,2 As anticipated, dermatology visits increased during the COVID-19 pandemic for the diagnosis of alopecia1-4; a survey performed during the pandemic found that alopecia was one of the most common diagnoses dermatologists made through telehealth platforms.5
This article provides a practical guide for dermatology practitioners to efficiently and accurately assess alopecia by TD in all patients, with added considerations for skin of color patients.
Diagnostic Tools
The intersection of TD, as an effective mechanism for the diagnosis and treatment of dermatologic disorders, and the increase in alopecia observed during the COVID-19 pandemic prompted us to develop a workflow for conducting virtual scalp examinations. Seven dermatologists (A.M., A.A., O.A., N.E., V.C., C.M.B., S.C.T.) who are experts in hair disorders contributed to developing workflows to optimize the assessment of alopecia through a virtual scalp examination, with an emphasis on patients of color. These experts completed a 7-question survey (Table) detailing their approach to the virtual scalp examination. One author (B.N.W.) served as an independent reviewer and collated responses into the following workflows.

Telemedicine Previsit Workflow
Components of the previsit workflow include:
• Instruct patients to provide all laboratory values and biopsy reports before the appointment.
• Test for a stable Wi-Fi connection using a speed test (available at https://www.speedtest.net/). A speed of 10 megabits/second or more is required for high-quality video via TD.6

• Provide a handout illustrating the required photographs of the anterior hairline; the mid scalp, including vertex, bilateral parietal, and occipital scalp; and posterior hairline. Photographs should be uploaded 2 hours before the visit. Figures 1 and 2 are examples of photographs that should be requested.

• Request images with 2 or 3 different angles of the area of the scalp with the greatest involvement to help appreciate primary and secondary characteristics.
• Encourage patients to present with clean, recently shampooed, dried, and detangled natural hair, unless they have an itchy or flaky scalp.
• For concerns of scalp, hairline, eyebrow, or facial flaking and scaling, instruct the patient to avoid applying a moisturizer before the visit.
• Instruct the patient to remove false eyelashes, eyelash extensions, eyebrow pencil, hair camouflage, hair accessories, braids, extensions, weaves, twists, and other hairstyles so that the hair can be maneuvered to expose the scalp surface.
• Instruct the patient to have a comb, pic, or brush, or more than one of these implements, available during the visit.
Telemedicine Visit Workflow
Components of the visit workflow include:
• If a stable Wi-Fi connection cannot be established, switch to an audio-only visit to collect a pertinent history. Advise the patient that in-person follow-up must be scheduled.
• Confirm that (1) the patient is in a private setting where the scalp can be viewed and (2) lighting is positioned in front of the patient.
• Ensure that the patient’s hairline, full face, eyebrows, and eyelashes and, upon request, the vertex and posterior scalp, are completely visible.
• Initiate the virtual scalp examination by instructing the patient how to perform a hair pull test. Then, examine the pattern and distribution of hair loss alongside supplemental photographs.
• Instruct the patient to apply pressure with the fingertips throughout the scalp to help localize tenderness, which, in combination with the pattern of hair loss observed, might inform the diagnosis.
• Instruct the patient to scan the scalp with the fingertips for “bumps” to locate papules, pustules, and keloidal scars.
Diagnostic Pearls
Distribution of Alopecia—The experts noted that the pattern, distribution, and location of hair loss determined from the telemedicine alopecia assessment provided important clues to distinguish the type of alopecia.
Diagnostic clues for diffuse or generalized alopecia include:
• Either of these findings might be indicative of telogen effluvium or acquired trichorrhexis nodosa. Results of the hair pull test can help distinguish between these diagnoses.
• Recent stressful life events along with the presence of telogen hairs extracted during a hair pull test support the diagnosis of telogen effluvium.
• A history of external stress on the hair—thermal, traction, or chemical—along with broken hair shafts following the hair pull test support the diagnosis of acquired trichorrhexis nodosa.
Diagnostic clues for focal or patchy alopecia include:
• Alopecia areata generally presents as focal hair loss in an annular distribution; pruritus, erythema, and scale are absent.
• Seborrheic dermatitis can present as pruritic erythematous patches with scale distributed on the scalp and, in some cases, in the eyebrows, nasolabial folds, or paranasal skin.7 Some skin of color patients present with petaloid seborrheic dermatitis—pink or hypopigmented polycyclic coalescing rings with minimal scale.7,8
• Discoid lupus erythematosus, similar to seborrheic dermatitis, might present as pruritic, scaly, hypopigmented patches. However, in the experience of the experts, a more common presentation is tender erythematous patches of hair loss with central hypopigmentation and surrounding hyperpigmentation.
Diagnostic clues for vertex and mid scalp alopecia include:
• Androgenetic alopecia typically presents as a reduction of terminal hair density in the vertex and mid scalp regions (with widening through the midline part) and fine hair along the anterior hairline.9 Signs of concomitant hyperandrogenism, including facial hirsutism, acne, and obesity, might be observed.10
• Central centrifugal cicatricial alopecia typically affects the vertex and mid scalp with a shiny scalp appearance and follicular dropout.
Diagnostic clues for frontotemporal alopecia include:
• Frontal fibrosing alopecia (FFA) often presents with spared single terminal hairs (lonely hair sign).
• Traction alopecia commonly presents with the fringe hair sign.
Scalp Symptoms—The experts noted that the presence of symptoms (eg, pain, tenderness, pruritus) in conjunction with the pattern of hair loss might support the diagnosis of an inflammatory scarring alopecia.
When do symptoms raise suspicion of central centrifugal cicatricial alopecia?
• Suspected in the setting of vertex alopecia associated with tenderness, pain, or itching.
When do symptoms raise suspicion of FFA?
• Suspected when patients experience frontotemporal tenderness, pain, or burning associated with alopecia.
• The skin hue of the affected area might be lighter in color than, and contrast with, the darker hue of the photoaged upper forehead.11
• The lonely hair sign can aid in diagnosing FFA and distinguish it from the fringe sign of traction alopecia.
• Concurrent madarosis, flesh-colored papules on the cheeks, or lichen planus pigmentosus identified by visual inspection of the face confirms the diagnosis.9,12 Madarosis of the eyebrow was frequently cited by the experts as an associated symptom of FFA.
When do symptoms raise suspicion of lichen planopilaris?
• Suspected in the presence of pruritus, burning, tenderness, or pain associated with perifollicular erythema and scale in the setting of vertex and parietal alopecia.13
• Anagen hair release is observed during the hair pull test.11,14• The experts cited flesh-colored papules and lichen planus pigmentosus as frequently associated symptoms of lichen planopilaris.
Practice Implications
There are limitations to a virtual scalp examination—the inability to perform a scalp biopsy or administer certain treatments—but the consensus of the expert panel is that an initial alopecia assessment can be completed successfully utilizing TD. Although TD is not a replacement for an in-person dermatology visit, this technology has allowed for the diagnosis, treatment, and continuing care of many common dermatologic conditions without the patient needing to travel to the office.5
With the increased frequency of hair loss concerns documented over the last year and more patients seeking TD, it is imperative that dermatologists feel confident performing a virtual hair and scalp examination on all patients.1,3,4
- Kutlu Ö, Aktas¸ H, I·mren IG, et al. Short-term stress-related increasing cases of alopecia areata during the COVID-19 pandemic. J Dermatolog Treat. 2020;1. doi:10.1080/09546634.2020.1782820
- Cline A, Kazemi A, Moy J, et al. A surge in the incidence of telogen effluvium in minority predominant communities heavily impacted by COVID-19. J Am Acad Dermatol. 2021;84:773-775. doi:10.1016/j.jaad.2020.11.032
- Kutlu Ö, Metin A. Relative changes in the pattern of diseases presenting in dermatology outpatient clinic in the era of the COVID-19 pandemic. Dermatol Ther. 2020;33:e14096. doi:10.1111/dth.14096
- Tanacan E, Aksoy Sarac G, Emeksiz MAC, et al. Changing trends in dermatology practice during COVID-19 pandemic: a single tertiary center experience. Dermatol Ther. 2020;33:e14136. doi:10.1111/dth.14136
- Sharma A, Jindal V, Singla P, et al. Will teledermatology be the silver lining during and after COVID-19? Dermatol Ther. 2020;33:e13643. doi:10.1111/dth.13643
- Iscrupe L. How to receive virtual medical treatment while under quarantine. Allconnect website. Published March 26, 2020. Accessed December 9, 2021. https://www.allconnect.com/blog/online-doctor-visit-faq
- Elgash M, Dlova N, Ogunleye T, et al. Seborrheic dermatitis in skin of color: clinical considerations. J Drugs Dermatol. 2019;18:24-27.
- McLaurin CI. Annular facial dermatoses in blacks. Cutis. 1983;32:369-370, 384.
- Suchonwanit P, Hector CE, Bin Saif GA, McMichael AJ. Factors affecting the severity of central centrifugal cicatricial alopecia. Int J Dermatol. 2016;55:e338-343. doi:10.1111/ijd.13061
- Gabros S, Masood S. Central centrifugal cicatricial alopecia. StatPearls [Internet]. StatPearls Publishing; 2021. Updated July 20, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559187/
- Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37. doi:10.1016/j.jaad.2004.06.015
- Cobos G, Kim RH, Meehan S, et al. Lichen planus pigmentosus and lichen planopilaris. Dermatol Online J. 2016;22:13030/qt7hp8n6dn.
- Lyakhovitsky A, Amichai B, Sizopoulou C, et al. A case series of 46 patients with lichen planopilaris: demographics, clinical evaluation, and treatment experience. J Dermatolog Treat. 2015;26:275-279. doi:10.3109/09546634.2014.933165
- Tan E, Martinka M, Ball N, et al. Primary cicatricial alopecias: clinicopathology of 112 cases. J Am Acad Dermatol. 2004;50:25-32. doi:10.1016/j.jaad.2003.04.001
- Kutlu Ö, Aktas¸ H, I·mren IG, et al. Short-term stress-related increasing cases of alopecia areata during the COVID-19 pandemic. J Dermatolog Treat. 2020;1. doi:10.1080/09546634.2020.1782820
- Cline A, Kazemi A, Moy J, et al. A surge in the incidence of telogen effluvium in minority predominant communities heavily impacted by COVID-19. J Am Acad Dermatol. 2021;84:773-775. doi:10.1016/j.jaad.2020.11.032
- Kutlu Ö, Metin A. Relative changes in the pattern of diseases presenting in dermatology outpatient clinic in the era of the COVID-19 pandemic. Dermatol Ther. 2020;33:e14096. doi:10.1111/dth.14096
- Tanacan E, Aksoy Sarac G, Emeksiz MAC, et al. Changing trends in dermatology practice during COVID-19 pandemic: a single tertiary center experience. Dermatol Ther. 2020;33:e14136. doi:10.1111/dth.14136
- Sharma A, Jindal V, Singla P, et al. Will teledermatology be the silver lining during and after COVID-19? Dermatol Ther. 2020;33:e13643. doi:10.1111/dth.13643
- Iscrupe L. How to receive virtual medical treatment while under quarantine. Allconnect website. Published March 26, 2020. Accessed December 9, 2021. https://www.allconnect.com/blog/online-doctor-visit-faq
- Elgash M, Dlova N, Ogunleye T, et al. Seborrheic dermatitis in skin of color: clinical considerations. J Drugs Dermatol. 2019;18:24-27.
- McLaurin CI. Annular facial dermatoses in blacks. Cutis. 1983;32:369-370, 384.
- Suchonwanit P, Hector CE, Bin Saif GA, McMichael AJ. Factors affecting the severity of central centrifugal cicatricial alopecia. Int J Dermatol. 2016;55:e338-343. doi:10.1111/ijd.13061
- Gabros S, Masood S. Central centrifugal cicatricial alopecia. StatPearls [Internet]. StatPearls Publishing; 2021. Updated July 20, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559187/
- Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37. doi:10.1016/j.jaad.2004.06.015
- Cobos G, Kim RH, Meehan S, et al. Lichen planus pigmentosus and lichen planopilaris. Dermatol Online J. 2016;22:13030/qt7hp8n6dn.
- Lyakhovitsky A, Amichai B, Sizopoulou C, et al. A case series of 46 patients with lichen planopilaris: demographics, clinical evaluation, and treatment experience. J Dermatolog Treat. 2015;26:275-279. doi:10.3109/09546634.2014.933165
- Tan E, Martinka M, Ball N, et al. Primary cicatricial alopecias: clinicopathology of 112 cases. J Am Acad Dermatol. 2004;50:25-32. doi:10.1016/j.jaad.2003.04.001
Pursuit of a Research Year or Dual Degree by Dermatology Residency Applicants: A Cross-Sectional Study
To the Editor:
Securing a dermatology residency position is extraordinarily competitive. The match rate for US allopathic seniors for dermatology is 84.7%, among the lowest of all medical specialties. Matched dermatology applicants boast a mean US Medical Licensing Examination (USMLE) Step 1 score of 248, the second highest of all specialties.1 To gain an edge, applicants are faced with decisions regarding pursuit of dedicated research time and additional professional degrees.
We conducted a cross-sectional study to determine how many dermatology residency applicants pursue additional years of training and how this decision relates to USMLE scores and other metrics. This study was approved by the University of Michigan institutional review board. Using Electronic Residency Application Service applicant data, all applicants to the University of Michigan Medical School (Ann Arbor, Michigan) dermatology residency program for the 2018-2019 application cycle were included.
Analysis of variance was performed to determine differences in mean USMLE Step 1 scores, Step 2 Clinical Knowledge scores, and number of research experiences (eg, presentations, publications) between groups. A 2-tailed z test of independent samples was performed for individual pairwise subgroup analyses.
There were 608 (377 female, 231 male; mean age, 27.9 years) applicants from 199 different medical schools; 550 graduated with an MD degree, 40 with a DO degree, and 18 were international medical graduates (IMGs)(eg, MBBS, MBBCh, BAO, MBChB). One hundred eighty-four applicants (30.2%) pursued either a second professional degree or a dedicated research period lasting at least 12 months. Twenty-eight applicants (4.6%) obtained a master’s degree, 21 (3.5%) obtained a doctorate, and 135 (22.2%) pursued dedicated research.
Of the 40 DO applicants, 1 (2.5%) pursued dedicated research time; 0 (zero) completed a dual degree. None (zero) of the 18 IMGs pursued a dual degree or dedicated research time. When the scores of applicants who pursued additional training and the scores of applicants who did not were compared, neither mean USMLE Step 1 scores nor mean USMLE Step 2 Clinical Knowledge scores were statistically different (P=.31 and P=.44, respectively). Applicants who completed medical school in 4 years had fewer research experiences (mean [SD] experiences, 13.9 [13.2]) than students with a master’s degree (18.5 [8.4]), doctorate (24.5 [17.5]), or dedicated research time (23.9 [14.9])(P<.001).
Utilizing US News & World Report rankings (2019 Best Medical Schools: Research), we determined that 146 applicants (24.0%) attended a top 25 medical school in 2019.2 Of those 146 applicants, 77 (52.7%) pursued additional training through dedicated research or a second professional degree. Only 107 of the 462 applicants (23.2%) from medical schools that were not in the top 25 as determined by the US News & World Report pursued additional training (P<.0001)(Figure).
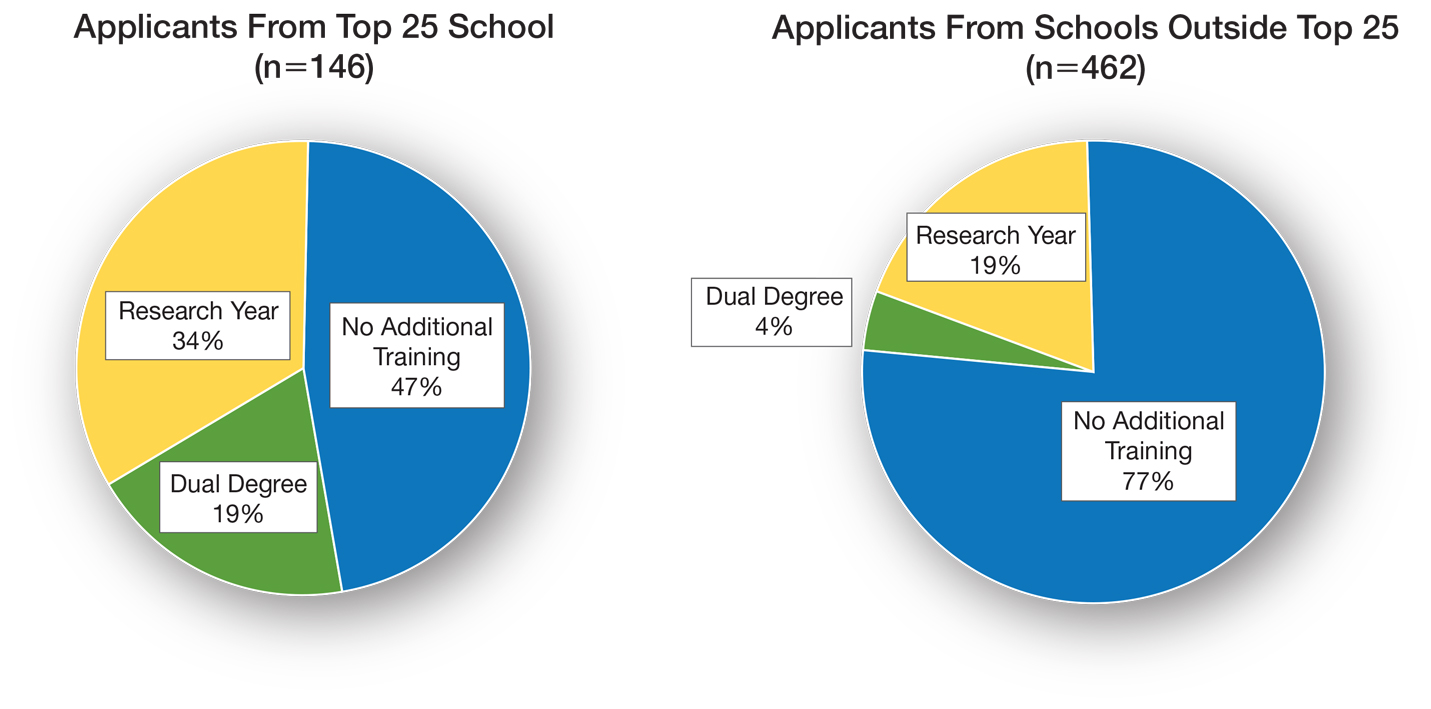
There is sentiment among applicants that a weaker dermatology residency application can be bolstered through a dedicated research year or a second professional degree. Whether this additional training has an impact on an applicant’s chances of matching is unclear and requires further investigation. Our data showed that applicants from the top 25 medical schools were more likely to pursue additional training than graduates at other institutions. These highly ranked academic institutions might encourage students to pursue a dual degree or research fellowship. In addition, year-long research opportunities might be more available through top medical schools; these schools might be more likely to offer dual-degree programs or provide funding to support student research opportunities.
It is important to comment on the potential importance of funding to support research years; the unpaid nature of many research fellowships in dermatology tends to favor applicants from a higher socioeconomic background. In that respect, the pervasive trend of encouraging research years in dermatology might widen already apparent disparities in our field, likely impacting underrepresented minorities disproportionately.3 Importantly, students with an MD degree represent nearly all applicants who completed a dual degree or dedicated research time. This might be due to fewer opportunities available to IMGs and DO students or secondary to incentivization by MD institutions.
Our data also suggest that students who pursue additional training have academic achievement metrics similar to those who do not. Additional training might increase medical students’ debt burden, thus catering to more affluent applicants, which, in turn, might have an impact on the diversity of the dermatology residency applicant pool.
Our data come from a single institution during a single application cycle, comprising 608 applicants. Nationwide, there were 701 dermatology residency applicants for the 2018-2019 application cycle; our pool therefore represents most (86.7%) but not all applicants.
We decided to use the US News & World Report 2019 rankings to identify top medical schools. Although this ranking system is imperfect and inherently subjective, it is widely utilized by prospective applicants and administrative faculty; we deemed it the best ranking that we could utilize to identify top medical schools. Because the University of Michigan Medical School was in the top 25 of Best Medical Schools: Research, according to the US News & World Report 2019 rankings, our applicant pool might be skewed to applicants interested in a more academic, research-focused residency program.
Our study revealed that 30% (n=184) of dermatology residency applicants pursued a second professional degree or dedicated research time. There was no difference in UMLE Step 1 and Step 2 scores for those who pursued additional training compared to those who did not.
- Charting outcomes in the match: U.S. allopathic seniors. 2nd ed. National Residency Matching Program. Published July 2020. Accessed January 3, 2022. https://www.nrmp.org/wp-content/uploads/2021/08/Charting-Outcomes-in-the-Match-2020_MD-Senior_final.pdf
- 2019 Best Medical Schools: Research. US News & World Report; 2019.
- Oussedik E. Important considerations for diversity in the selection of dermatology applicants. JAMA Dermatol. 2017;153:948-949. doi:10.1001/jamadermatol.2017.1814
To the Editor:
Securing a dermatology residency position is extraordinarily competitive. The match rate for US allopathic seniors for dermatology is 84.7%, among the lowest of all medical specialties. Matched dermatology applicants boast a mean US Medical Licensing Examination (USMLE) Step 1 score of 248, the second highest of all specialties.1 To gain an edge, applicants are faced with decisions regarding pursuit of dedicated research time and additional professional degrees.
We conducted a cross-sectional study to determine how many dermatology residency applicants pursue additional years of training and how this decision relates to USMLE scores and other metrics. This study was approved by the University of Michigan institutional review board. Using Electronic Residency Application Service applicant data, all applicants to the University of Michigan Medical School (Ann Arbor, Michigan) dermatology residency program for the 2018-2019 application cycle were included.
Analysis of variance was performed to determine differences in mean USMLE Step 1 scores, Step 2 Clinical Knowledge scores, and number of research experiences (eg, presentations, publications) between groups. A 2-tailed z test of independent samples was performed for individual pairwise subgroup analyses.
There were 608 (377 female, 231 male; mean age, 27.9 years) applicants from 199 different medical schools; 550 graduated with an MD degree, 40 with a DO degree, and 18 were international medical graduates (IMGs)(eg, MBBS, MBBCh, BAO, MBChB). One hundred eighty-four applicants (30.2%) pursued either a second professional degree or a dedicated research period lasting at least 12 months. Twenty-eight applicants (4.6%) obtained a master’s degree, 21 (3.5%) obtained a doctorate, and 135 (22.2%) pursued dedicated research.
Of the 40 DO applicants, 1 (2.5%) pursued dedicated research time; 0 (zero) completed a dual degree. None (zero) of the 18 IMGs pursued a dual degree or dedicated research time. When the scores of applicants who pursued additional training and the scores of applicants who did not were compared, neither mean USMLE Step 1 scores nor mean USMLE Step 2 Clinical Knowledge scores were statistically different (P=.31 and P=.44, respectively). Applicants who completed medical school in 4 years had fewer research experiences (mean [SD] experiences, 13.9 [13.2]) than students with a master’s degree (18.5 [8.4]), doctorate (24.5 [17.5]), or dedicated research time (23.9 [14.9])(P<.001).
Utilizing US News & World Report rankings (2019 Best Medical Schools: Research), we determined that 146 applicants (24.0%) attended a top 25 medical school in 2019.2 Of those 146 applicants, 77 (52.7%) pursued additional training through dedicated research or a second professional degree. Only 107 of the 462 applicants (23.2%) from medical schools that were not in the top 25 as determined by the US News & World Report pursued additional training (P<.0001)(Figure).

There is sentiment among applicants that a weaker dermatology residency application can be bolstered through a dedicated research year or a second professional degree. Whether this additional training has an impact on an applicant’s chances of matching is unclear and requires further investigation. Our data showed that applicants from the top 25 medical schools were more likely to pursue additional training than graduates at other institutions. These highly ranked academic institutions might encourage students to pursue a dual degree or research fellowship. In addition, year-long research opportunities might be more available through top medical schools; these schools might be more likely to offer dual-degree programs or provide funding to support student research opportunities.
It is important to comment on the potential importance of funding to support research years; the unpaid nature of many research fellowships in dermatology tends to favor applicants from a higher socioeconomic background. In that respect, the pervasive trend of encouraging research years in dermatology might widen already apparent disparities in our field, likely impacting underrepresented minorities disproportionately.3 Importantly, students with an MD degree represent nearly all applicants who completed a dual degree or dedicated research time. This might be due to fewer opportunities available to IMGs and DO students or secondary to incentivization by MD institutions.
Our data also suggest that students who pursue additional training have academic achievement metrics similar to those who do not. Additional training might increase medical students’ debt burden, thus catering to more affluent applicants, which, in turn, might have an impact on the diversity of the dermatology residency applicant pool.
Our data come from a single institution during a single application cycle, comprising 608 applicants. Nationwide, there were 701 dermatology residency applicants for the 2018-2019 application cycle; our pool therefore represents most (86.7%) but not all applicants.
We decided to use the US News & World Report 2019 rankings to identify top medical schools. Although this ranking system is imperfect and inherently subjective, it is widely utilized by prospective applicants and administrative faculty; we deemed it the best ranking that we could utilize to identify top medical schools. Because the University of Michigan Medical School was in the top 25 of Best Medical Schools: Research, according to the US News & World Report 2019 rankings, our applicant pool might be skewed to applicants interested in a more academic, research-focused residency program.
Our study revealed that 30% (n=184) of dermatology residency applicants pursued a second professional degree or dedicated research time. There was no difference in UMLE Step 1 and Step 2 scores for those who pursued additional training compared to those who did not.
To the Editor:
Securing a dermatology residency position is extraordinarily competitive. The match rate for US allopathic seniors for dermatology is 84.7%, among the lowest of all medical specialties. Matched dermatology applicants boast a mean US Medical Licensing Examination (USMLE) Step 1 score of 248, the second highest of all specialties.1 To gain an edge, applicants are faced with decisions regarding pursuit of dedicated research time and additional professional degrees.
We conducted a cross-sectional study to determine how many dermatology residency applicants pursue additional years of training and how this decision relates to USMLE scores and other metrics. This study was approved by the University of Michigan institutional review board. Using Electronic Residency Application Service applicant data, all applicants to the University of Michigan Medical School (Ann Arbor, Michigan) dermatology residency program for the 2018-2019 application cycle were included.
Analysis of variance was performed to determine differences in mean USMLE Step 1 scores, Step 2 Clinical Knowledge scores, and number of research experiences (eg, presentations, publications) between groups. A 2-tailed z test of independent samples was performed for individual pairwise subgroup analyses.
There were 608 (377 female, 231 male; mean age, 27.9 years) applicants from 199 different medical schools; 550 graduated with an MD degree, 40 with a DO degree, and 18 were international medical graduates (IMGs)(eg, MBBS, MBBCh, BAO, MBChB). One hundred eighty-four applicants (30.2%) pursued either a second professional degree or a dedicated research period lasting at least 12 months. Twenty-eight applicants (4.6%) obtained a master’s degree, 21 (3.5%) obtained a doctorate, and 135 (22.2%) pursued dedicated research.
Of the 40 DO applicants, 1 (2.5%) pursued dedicated research time; 0 (zero) completed a dual degree. None (zero) of the 18 IMGs pursued a dual degree or dedicated research time. When the scores of applicants who pursued additional training and the scores of applicants who did not were compared, neither mean USMLE Step 1 scores nor mean USMLE Step 2 Clinical Knowledge scores were statistically different (P=.31 and P=.44, respectively). Applicants who completed medical school in 4 years had fewer research experiences (mean [SD] experiences, 13.9 [13.2]) than students with a master’s degree (18.5 [8.4]), doctorate (24.5 [17.5]), or dedicated research time (23.9 [14.9])(P<.001).
Utilizing US News & World Report rankings (2019 Best Medical Schools: Research), we determined that 146 applicants (24.0%) attended a top 25 medical school in 2019.2 Of those 146 applicants, 77 (52.7%) pursued additional training through dedicated research or a second professional degree. Only 107 of the 462 applicants (23.2%) from medical schools that were not in the top 25 as determined by the US News & World Report pursued additional training (P<.0001)(Figure).

There is sentiment among applicants that a weaker dermatology residency application can be bolstered through a dedicated research year or a second professional degree. Whether this additional training has an impact on an applicant’s chances of matching is unclear and requires further investigation. Our data showed that applicants from the top 25 medical schools were more likely to pursue additional training than graduates at other institutions. These highly ranked academic institutions might encourage students to pursue a dual degree or research fellowship. In addition, year-long research opportunities might be more available through top medical schools; these schools might be more likely to offer dual-degree programs or provide funding to support student research opportunities.
It is important to comment on the potential importance of funding to support research years; the unpaid nature of many research fellowships in dermatology tends to favor applicants from a higher socioeconomic background. In that respect, the pervasive trend of encouraging research years in dermatology might widen already apparent disparities in our field, likely impacting underrepresented minorities disproportionately.3 Importantly, students with an MD degree represent nearly all applicants who completed a dual degree or dedicated research time. This might be due to fewer opportunities available to IMGs and DO students or secondary to incentivization by MD institutions.
Our data also suggest that students who pursue additional training have academic achievement metrics similar to those who do not. Additional training might increase medical students’ debt burden, thus catering to more affluent applicants, which, in turn, might have an impact on the diversity of the dermatology residency applicant pool.
Our data come from a single institution during a single application cycle, comprising 608 applicants. Nationwide, there were 701 dermatology residency applicants for the 2018-2019 application cycle; our pool therefore represents most (86.7%) but not all applicants.
We decided to use the US News & World Report 2019 rankings to identify top medical schools. Although this ranking system is imperfect and inherently subjective, it is widely utilized by prospective applicants and administrative faculty; we deemed it the best ranking that we could utilize to identify top medical schools. Because the University of Michigan Medical School was in the top 25 of Best Medical Schools: Research, according to the US News & World Report 2019 rankings, our applicant pool might be skewed to applicants interested in a more academic, research-focused residency program.
Our study revealed that 30% (n=184) of dermatology residency applicants pursued a second professional degree or dedicated research time. There was no difference in UMLE Step 1 and Step 2 scores for those who pursued additional training compared to those who did not.
- Charting outcomes in the match: U.S. allopathic seniors. 2nd ed. National Residency Matching Program. Published July 2020. Accessed January 3, 2022. https://www.nrmp.org/wp-content/uploads/2021/08/Charting-Outcomes-in-the-Match-2020_MD-Senior_final.pdf
- 2019 Best Medical Schools: Research. US News & World Report; 2019.
- Oussedik E. Important considerations for diversity in the selection of dermatology applicants. JAMA Dermatol. 2017;153:948-949. doi:10.1001/jamadermatol.2017.1814
- Charting outcomes in the match: U.S. allopathic seniors. 2nd ed. National Residency Matching Program. Published July 2020. Accessed January 3, 2022. https://www.nrmp.org/wp-content/uploads/2021/08/Charting-Outcomes-in-the-Match-2020_MD-Senior_final.pdf
- 2019 Best Medical Schools: Research. US News & World Report; 2019.
- Oussedik E. Important considerations for diversity in the selection of dermatology applicants. JAMA Dermatol. 2017;153:948-949. doi:10.1001/jamadermatol.2017.1814
PRACTICE POINTS
- In our study of dermatology residency applicants (N11=608), 30% pursued a second professional degree or dedicated research time.
- US Medical Licensing Examination Step 1 and Step 2 scores did not differ among applicants who pursued additional training and those who did not.
- Additional training might increase medical students’ debt burden, thus catering to more affluent applicants and reducing the diversity of applicant and resident pools.
Medicaid implements waivers for some clinical trial coverage
Federal officials will allow some flexibility in meeting new requirements on covering the costs of clinical trials for people enrolled in Medicaid, seeking to accommodate states where legislatures will not meet in time to make needed changes in rules.
Congress in 2020 ordered U.S. states to have their Medicaid programs cover expenses related to participation in certain clinical trials, a move that was hailed by the American Society of Clinical Oncology (ASCO) and other groups as a boost to trials as well as to patients with serious illness who have lower incomes.
The mandate went into effect on Jan. 1, but the Centers for Medicare & Medicaid Services will allow accommodations in terms of implementation time for states that have not yet been able to make needed legislative changes, Daniel Tsai, deputy administrator and director of the Center for Medicaid and CHIP Services, wrote in a Dec. 7 letter. Mr. Tsai’s letter doesn’t mention specific states. The CMS did not immediately respond to a request seeking information on the states expected to apply for waivers.
Medicaid has in recent years been a rare large U.S. insurance program that does not cover the costs of clinical trials. The Affordable Care Act of 2010 mandated this coverage for people in private insurance plans. The federal government in 2000 decided that Medicare would do so.
‘A hidden opportunity’
A perspective article last May in the New England Journal of Medicine referred to the new Medicaid mandate on clinical trials as a “hidden opportunity,” referring to its genesis as an add-on in a massive federal spending package enacted in December 2020.
In the article, Samuel U. Takvorian, MD, MSHP, of the University of Pennsylvania, Philadelphia, and coauthors noted that rates of participation in clinical trials remain low for racial and ethnic minority groups, due in part to the lack of Medicaid coverage.
“For example, non-Hispanic White patients are nearly twice as likely as Black patients and three times as likely as Hispanic patients to enroll in cancer clinical trials – a gap that has widened over time,” Dr. Takvorian and coauthors wrote. “Inequities in enrollment have also manifested during the COVID-19 pandemic, which has disproportionately affected non-White patients, without their commensurate representation in trials of COVID-19 therapeutics.”
In October, researchers from the Arthur G. James Cancer Hospital and Ohio State University, Columbus, published results of a retrospective study of patients with stage I-IV pancreatic cancer that also found inequities in enrollment. Mariam F. Eskander, MD, MPH, and coauthors reported what they found by examining records for 1,127 patients (0.4%) enrolled in clinical trials and 301,340 (99.6%) who did not enroll. They found that enrollment in trials increased over the study period, but not for Black patients or patients on Medicaid.
In an interview, Dr. Eskander said the new Medicaid policy will remove a major obstacle to participation in clinical trials. An oncologist, Dr. Eskander said she is looking forward to being able to help more of her patients get access to experimental medicines and treatments.
But that may not be enough to draw more people with low incomes into these studies, said Dr. Eskander, who is now at Rutgers Cancer Institute of New Jersey in New Brunswick. She urges greater use of patient navigators to help people on Medicaid understand the resources available to them, as well as broad use of Medicaid’s nonemergency medical transportation (NEMT) benefit.
“Some patients will be offered clinical trial enrollment and some will accept, but I really worry about the challenges low-income people face with things like transportation and getting time off work,” she said.
A version of this article first appeared on Medscape.com.
Federal officials will allow some flexibility in meeting new requirements on covering the costs of clinical trials for people enrolled in Medicaid, seeking to accommodate states where legislatures will not meet in time to make needed changes in rules.
Congress in 2020 ordered U.S. states to have their Medicaid programs cover expenses related to participation in certain clinical trials, a move that was hailed by the American Society of Clinical Oncology (ASCO) and other groups as a boost to trials as well as to patients with serious illness who have lower incomes.
The mandate went into effect on Jan. 1, but the Centers for Medicare & Medicaid Services will allow accommodations in terms of implementation time for states that have not yet been able to make needed legislative changes, Daniel Tsai, deputy administrator and director of the Center for Medicaid and CHIP Services, wrote in a Dec. 7 letter. Mr. Tsai’s letter doesn’t mention specific states. The CMS did not immediately respond to a request seeking information on the states expected to apply for waivers.
Medicaid has in recent years been a rare large U.S. insurance program that does not cover the costs of clinical trials. The Affordable Care Act of 2010 mandated this coverage for people in private insurance plans. The federal government in 2000 decided that Medicare would do so.
‘A hidden opportunity’
A perspective article last May in the New England Journal of Medicine referred to the new Medicaid mandate on clinical trials as a “hidden opportunity,” referring to its genesis as an add-on in a massive federal spending package enacted in December 2020.
In the article, Samuel U. Takvorian, MD, MSHP, of the University of Pennsylvania, Philadelphia, and coauthors noted that rates of participation in clinical trials remain low for racial and ethnic minority groups, due in part to the lack of Medicaid coverage.
“For example, non-Hispanic White patients are nearly twice as likely as Black patients and three times as likely as Hispanic patients to enroll in cancer clinical trials – a gap that has widened over time,” Dr. Takvorian and coauthors wrote. “Inequities in enrollment have also manifested during the COVID-19 pandemic, which has disproportionately affected non-White patients, without their commensurate representation in trials of COVID-19 therapeutics.”
In October, researchers from the Arthur G. James Cancer Hospital and Ohio State University, Columbus, published results of a retrospective study of patients with stage I-IV pancreatic cancer that also found inequities in enrollment. Mariam F. Eskander, MD, MPH, and coauthors reported what they found by examining records for 1,127 patients (0.4%) enrolled in clinical trials and 301,340 (99.6%) who did not enroll. They found that enrollment in trials increased over the study period, but not for Black patients or patients on Medicaid.
In an interview, Dr. Eskander said the new Medicaid policy will remove a major obstacle to participation in clinical trials. An oncologist, Dr. Eskander said she is looking forward to being able to help more of her patients get access to experimental medicines and treatments.
But that may not be enough to draw more people with low incomes into these studies, said Dr. Eskander, who is now at Rutgers Cancer Institute of New Jersey in New Brunswick. She urges greater use of patient navigators to help people on Medicaid understand the resources available to them, as well as broad use of Medicaid’s nonemergency medical transportation (NEMT) benefit.
“Some patients will be offered clinical trial enrollment and some will accept, but I really worry about the challenges low-income people face with things like transportation and getting time off work,” she said.
A version of this article first appeared on Medscape.com.
Federal officials will allow some flexibility in meeting new requirements on covering the costs of clinical trials for people enrolled in Medicaid, seeking to accommodate states where legislatures will not meet in time to make needed changes in rules.
Congress in 2020 ordered U.S. states to have their Medicaid programs cover expenses related to participation in certain clinical trials, a move that was hailed by the American Society of Clinical Oncology (ASCO) and other groups as a boost to trials as well as to patients with serious illness who have lower incomes.
The mandate went into effect on Jan. 1, but the Centers for Medicare & Medicaid Services will allow accommodations in terms of implementation time for states that have not yet been able to make needed legislative changes, Daniel Tsai, deputy administrator and director of the Center for Medicaid and CHIP Services, wrote in a Dec. 7 letter. Mr. Tsai’s letter doesn’t mention specific states. The CMS did not immediately respond to a request seeking information on the states expected to apply for waivers.
Medicaid has in recent years been a rare large U.S. insurance program that does not cover the costs of clinical trials. The Affordable Care Act of 2010 mandated this coverage for people in private insurance plans. The federal government in 2000 decided that Medicare would do so.
‘A hidden opportunity’
A perspective article last May in the New England Journal of Medicine referred to the new Medicaid mandate on clinical trials as a “hidden opportunity,” referring to its genesis as an add-on in a massive federal spending package enacted in December 2020.
In the article, Samuel U. Takvorian, MD, MSHP, of the University of Pennsylvania, Philadelphia, and coauthors noted that rates of participation in clinical trials remain low for racial and ethnic minority groups, due in part to the lack of Medicaid coverage.
“For example, non-Hispanic White patients are nearly twice as likely as Black patients and three times as likely as Hispanic patients to enroll in cancer clinical trials – a gap that has widened over time,” Dr. Takvorian and coauthors wrote. “Inequities in enrollment have also manifested during the COVID-19 pandemic, which has disproportionately affected non-White patients, without their commensurate representation in trials of COVID-19 therapeutics.”
In October, researchers from the Arthur G. James Cancer Hospital and Ohio State University, Columbus, published results of a retrospective study of patients with stage I-IV pancreatic cancer that also found inequities in enrollment. Mariam F. Eskander, MD, MPH, and coauthors reported what they found by examining records for 1,127 patients (0.4%) enrolled in clinical trials and 301,340 (99.6%) who did not enroll. They found that enrollment in trials increased over the study period, but not for Black patients or patients on Medicaid.
In an interview, Dr. Eskander said the new Medicaid policy will remove a major obstacle to participation in clinical trials. An oncologist, Dr. Eskander said she is looking forward to being able to help more of her patients get access to experimental medicines and treatments.
But that may not be enough to draw more people with low incomes into these studies, said Dr. Eskander, who is now at Rutgers Cancer Institute of New Jersey in New Brunswick. She urges greater use of patient navigators to help people on Medicaid understand the resources available to them, as well as broad use of Medicaid’s nonemergency medical transportation (NEMT) benefit.
“Some patients will be offered clinical trial enrollment and some will accept, but I really worry about the challenges low-income people face with things like transportation and getting time off work,” she said.
A version of this article first appeared on Medscape.com.
Study finds more adverse maternal outcomes in women with disabilities
Women with physical, intellectual, and sensory disabilities had higher risk for almost all pregnancy complications, obstetric interventions, and adverse outcomes, including severe maternal morbidity (SMM) and mortality compared to women without disabilities, according to an analysis of a large, retrospective cohort.
The findings, published in JAMA Network Open (2021;4[12]:e2138414 doi: 10.1001/jamanetworkopen.2021.38414), “may be a direct reflection of the challenges women with all types of disabilities face when accessing and receiving care, which is likely compounded by poorer preconception health,” suggested lead author Jessica L. Gleason, PhD, MPH, and co-authors, all from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Md.
“Women with disabilities have long been ignored in obstetric research and clinical practice,” added Hilary K. Brown, PhD, from the University of Toronto, in an accompanying editorial. “Inclusion of disability indicators needs to be the norm – not the exception – in health administrative data so that these disparities can be regularly tracked and addressed.”
The investigators used data from the Consortium on Safe Labor (CSL), a retrospective cohort of deliveries from 12 U.S. clinical centers between Jan. 2002 and Jan. 2008, to analyze obstetric interventions and adverse maternal outcomes in women with and without disabilities.
The analysis included a total of 223,385 women, mean age 27.6 years, of whom 2,074 (0.9%) had a disability, and 221,311 did not. Among those with disabilities, 1,733 (83.5%) were physical, 91 (4.4%) were intellectual, and 250 (12.1%) were sensory. While almost half (49.4%) of the women were White, 22.5% were Black, 17.5% were Hispanic, and 4.1% were Asian or Pacific Islander.
Outcomes were analyzed with three composite measures:
- Pregnancy-related complications (pregnancy-related hypertensive diseases, gestational diabetes, placental abruption, placenta previa, premature rupture of membranes, preterm PROM);
- All labor, delivery, and postpartum complications (chorioamnionitis, hemorrhage, blood transfusion, thromboembolism, postpartum fever, infection, cardiovascular events, cardiomyopathy, and maternal death);
- SMM only, including severe pre-eclampsia/eclampsia, hemorrhage, thromboembolism, fever, infection, cardiomyopathy, and cardiovascular events during labor and delivery.
After adjustment for covariates, women with disabilities had higher risk of pregnancy-related complications. This included a 48% higher risk of mild pre-eclampsia and double the risk of severe pre-eclampsia/eclampsia. The composite risk of any pregnancy complication was 27% higher for women with physical disabilities, 49% higher for women with intellectual disabilities, and 53% higher for women with sensory disabilities.
The findings were similar for labor, delivery, and postpartum complications, showing women with disabilities had higher risk for a range of obstetrical interventions, including cesarean delivery – both planned and intrapartum (aRR, 1.34). Additionally, women with disabilities were less likely to have a cesarean delivery that was “solely clinically indicated” (aRR, 0.79), and more likely to have a cesarean delivery for “softer” mixed indication (aRR, 1.16), “supporting a possible overuse of cesarean delivery among women with disability,” they suggested.
Women with disabilities also had a higher risk of postpartum hemorrhage (aRR, 1.27), blood transfusion (aRR, 1.64), and maternal mortality (aRR, 11.19), as well as individual markers of severe maternal morbidity, such as cardiovascular events (aRR, 4.02), infection (aRR, 2.69), and venous thromboembolism (aRR, 6.08).
The authors speculate that the increased risks for women with disabilities “may be the result of a combination of independent risk factors, including the higher rate of obstetric intervention via cesarean delivery, under-recognition of women with disabilities as a population with higher-risk pregnancies, and lack of health care practitioner knowledge or comfort in managing pregnancies among women with disabilities.”
Dr. Brown noted in her commentary that there is a need for better education of health care professionals in this area. “Given that 12% of reproductive-aged women have a disability, that pregnancy rates are similar among women with and without disabilities, and that women with disabilities are at elevated risk of a range of adverse maternal outcomes, including severe maternal morbidity and maternal mortality, disability modules should be a mandatory component of education for obstetricians and midwives as well as other obstetrical health care professionals.”
Calling the study “a serious wake-up call,” Monika Mitra, PhD, told this publication that the findings highlight the need for “urgent attention” on improving obstetric care for people with disabilities “with a focus on accessibility and inclusion, changing clinical practice to better serve disabled people, integrating disability-related training for health care practitioners, and developing evidence-based interventions to support people with disabilities during this time.” The associate professor and director of the Lurie Institute for Disability Policy, in Brandeis University, Waltham, Mass. said the risk factors for poor outcomes are present early in pregnancy or even preconception. “We know that disabled women report barriers in accessing health care and receive lower-quality care compared to nondisabled women and are more likely to experience poverty, housing and food insecurity, educational and employment barriers, abuse, chronic health conditions, and mental illness than women without disabilities.”
She noted that the study’s sample of people with disabilities was small, and the measure of disability used was based on ICD-9 codes, which captures only severe disabilities. “As noted in the commentary by [Dr.] Brown, our standard sources of health administrative data do not give us the full picture on disability, and we need other, more equitable ways of identifying disability based, for example, on self-reports of activity or participation limitations if we are to be able to understand the effects on obstetric outcomes of health and health care disparities and of social determinants of health. Moreover, researchers have generally not yet begun to incorporate knowledge of the experiences of transgender people during pregnancy, which will impact our measures and study of obstetric outcomes among people with disabilities as well as the language we use.”
The study was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The study authors and Dr. Brown reported no conflicts of interest. Dr. Mitra receives funding from the NICHD and the National Institute on Disability, Independent Living for research on pregnancy outcomes among people with disabilities.
Women with physical, intellectual, and sensory disabilities had higher risk for almost all pregnancy complications, obstetric interventions, and adverse outcomes, including severe maternal morbidity (SMM) and mortality compared to women without disabilities, according to an analysis of a large, retrospective cohort.
The findings, published in JAMA Network Open (2021;4[12]:e2138414 doi: 10.1001/jamanetworkopen.2021.38414), “may be a direct reflection of the challenges women with all types of disabilities face when accessing and receiving care, which is likely compounded by poorer preconception health,” suggested lead author Jessica L. Gleason, PhD, MPH, and co-authors, all from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Md.
“Women with disabilities have long been ignored in obstetric research and clinical practice,” added Hilary K. Brown, PhD, from the University of Toronto, in an accompanying editorial. “Inclusion of disability indicators needs to be the norm – not the exception – in health administrative data so that these disparities can be regularly tracked and addressed.”
The investigators used data from the Consortium on Safe Labor (CSL), a retrospective cohort of deliveries from 12 U.S. clinical centers between Jan. 2002 and Jan. 2008, to analyze obstetric interventions and adverse maternal outcomes in women with and without disabilities.
The analysis included a total of 223,385 women, mean age 27.6 years, of whom 2,074 (0.9%) had a disability, and 221,311 did not. Among those with disabilities, 1,733 (83.5%) were physical, 91 (4.4%) were intellectual, and 250 (12.1%) were sensory. While almost half (49.4%) of the women were White, 22.5% were Black, 17.5% were Hispanic, and 4.1% were Asian or Pacific Islander.
Outcomes were analyzed with three composite measures:
- Pregnancy-related complications (pregnancy-related hypertensive diseases, gestational diabetes, placental abruption, placenta previa, premature rupture of membranes, preterm PROM);
- All labor, delivery, and postpartum complications (chorioamnionitis, hemorrhage, blood transfusion, thromboembolism, postpartum fever, infection, cardiovascular events, cardiomyopathy, and maternal death);
- SMM only, including severe pre-eclampsia/eclampsia, hemorrhage, thromboembolism, fever, infection, cardiomyopathy, and cardiovascular events during labor and delivery.
After adjustment for covariates, women with disabilities had higher risk of pregnancy-related complications. This included a 48% higher risk of mild pre-eclampsia and double the risk of severe pre-eclampsia/eclampsia. The composite risk of any pregnancy complication was 27% higher for women with physical disabilities, 49% higher for women with intellectual disabilities, and 53% higher for women with sensory disabilities.
The findings were similar for labor, delivery, and postpartum complications, showing women with disabilities had higher risk for a range of obstetrical interventions, including cesarean delivery – both planned and intrapartum (aRR, 1.34). Additionally, women with disabilities were less likely to have a cesarean delivery that was “solely clinically indicated” (aRR, 0.79), and more likely to have a cesarean delivery for “softer” mixed indication (aRR, 1.16), “supporting a possible overuse of cesarean delivery among women with disability,” they suggested.
Women with disabilities also had a higher risk of postpartum hemorrhage (aRR, 1.27), blood transfusion (aRR, 1.64), and maternal mortality (aRR, 11.19), as well as individual markers of severe maternal morbidity, such as cardiovascular events (aRR, 4.02), infection (aRR, 2.69), and venous thromboembolism (aRR, 6.08).
The authors speculate that the increased risks for women with disabilities “may be the result of a combination of independent risk factors, including the higher rate of obstetric intervention via cesarean delivery, under-recognition of women with disabilities as a population with higher-risk pregnancies, and lack of health care practitioner knowledge or comfort in managing pregnancies among women with disabilities.”
Dr. Brown noted in her commentary that there is a need for better education of health care professionals in this area. “Given that 12% of reproductive-aged women have a disability, that pregnancy rates are similar among women with and without disabilities, and that women with disabilities are at elevated risk of a range of adverse maternal outcomes, including severe maternal morbidity and maternal mortality, disability modules should be a mandatory component of education for obstetricians and midwives as well as other obstetrical health care professionals.”
Calling the study “a serious wake-up call,” Monika Mitra, PhD, told this publication that the findings highlight the need for “urgent attention” on improving obstetric care for people with disabilities “with a focus on accessibility and inclusion, changing clinical practice to better serve disabled people, integrating disability-related training for health care practitioners, and developing evidence-based interventions to support people with disabilities during this time.” The associate professor and director of the Lurie Institute for Disability Policy, in Brandeis University, Waltham, Mass. said the risk factors for poor outcomes are present early in pregnancy or even preconception. “We know that disabled women report barriers in accessing health care and receive lower-quality care compared to nondisabled women and are more likely to experience poverty, housing and food insecurity, educational and employment barriers, abuse, chronic health conditions, and mental illness than women without disabilities.”
She noted that the study’s sample of people with disabilities was small, and the measure of disability used was based on ICD-9 codes, which captures only severe disabilities. “As noted in the commentary by [Dr.] Brown, our standard sources of health administrative data do not give us the full picture on disability, and we need other, more equitable ways of identifying disability based, for example, on self-reports of activity or participation limitations if we are to be able to understand the effects on obstetric outcomes of health and health care disparities and of social determinants of health. Moreover, researchers have generally not yet begun to incorporate knowledge of the experiences of transgender people during pregnancy, which will impact our measures and study of obstetric outcomes among people with disabilities as well as the language we use.”
The study was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The study authors and Dr. Brown reported no conflicts of interest. Dr. Mitra receives funding from the NICHD and the National Institute on Disability, Independent Living for research on pregnancy outcomes among people with disabilities.
Women with physical, intellectual, and sensory disabilities had higher risk for almost all pregnancy complications, obstetric interventions, and adverse outcomes, including severe maternal morbidity (SMM) and mortality compared to women without disabilities, according to an analysis of a large, retrospective cohort.
The findings, published in JAMA Network Open (2021;4[12]:e2138414 doi: 10.1001/jamanetworkopen.2021.38414), “may be a direct reflection of the challenges women with all types of disabilities face when accessing and receiving care, which is likely compounded by poorer preconception health,” suggested lead author Jessica L. Gleason, PhD, MPH, and co-authors, all from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Md.
“Women with disabilities have long been ignored in obstetric research and clinical practice,” added Hilary K. Brown, PhD, from the University of Toronto, in an accompanying editorial. “Inclusion of disability indicators needs to be the norm – not the exception – in health administrative data so that these disparities can be regularly tracked and addressed.”
The investigators used data from the Consortium on Safe Labor (CSL), a retrospective cohort of deliveries from 12 U.S. clinical centers between Jan. 2002 and Jan. 2008, to analyze obstetric interventions and adverse maternal outcomes in women with and without disabilities.
The analysis included a total of 223,385 women, mean age 27.6 years, of whom 2,074 (0.9%) had a disability, and 221,311 did not. Among those with disabilities, 1,733 (83.5%) were physical, 91 (4.4%) were intellectual, and 250 (12.1%) were sensory. While almost half (49.4%) of the women were White, 22.5% were Black, 17.5% were Hispanic, and 4.1% were Asian or Pacific Islander.
Outcomes were analyzed with three composite measures:
- Pregnancy-related complications (pregnancy-related hypertensive diseases, gestational diabetes, placental abruption, placenta previa, premature rupture of membranes, preterm PROM);
- All labor, delivery, and postpartum complications (chorioamnionitis, hemorrhage, blood transfusion, thromboembolism, postpartum fever, infection, cardiovascular events, cardiomyopathy, and maternal death);
- SMM only, including severe pre-eclampsia/eclampsia, hemorrhage, thromboembolism, fever, infection, cardiomyopathy, and cardiovascular events during labor and delivery.
After adjustment for covariates, women with disabilities had higher risk of pregnancy-related complications. This included a 48% higher risk of mild pre-eclampsia and double the risk of severe pre-eclampsia/eclampsia. The composite risk of any pregnancy complication was 27% higher for women with physical disabilities, 49% higher for women with intellectual disabilities, and 53% higher for women with sensory disabilities.
The findings were similar for labor, delivery, and postpartum complications, showing women with disabilities had higher risk for a range of obstetrical interventions, including cesarean delivery – both planned and intrapartum (aRR, 1.34). Additionally, women with disabilities were less likely to have a cesarean delivery that was “solely clinically indicated” (aRR, 0.79), and more likely to have a cesarean delivery for “softer” mixed indication (aRR, 1.16), “supporting a possible overuse of cesarean delivery among women with disability,” they suggested.
Women with disabilities also had a higher risk of postpartum hemorrhage (aRR, 1.27), blood transfusion (aRR, 1.64), and maternal mortality (aRR, 11.19), as well as individual markers of severe maternal morbidity, such as cardiovascular events (aRR, 4.02), infection (aRR, 2.69), and venous thromboembolism (aRR, 6.08).
The authors speculate that the increased risks for women with disabilities “may be the result of a combination of independent risk factors, including the higher rate of obstetric intervention via cesarean delivery, under-recognition of women with disabilities as a population with higher-risk pregnancies, and lack of health care practitioner knowledge or comfort in managing pregnancies among women with disabilities.”
Dr. Brown noted in her commentary that there is a need for better education of health care professionals in this area. “Given that 12% of reproductive-aged women have a disability, that pregnancy rates are similar among women with and without disabilities, and that women with disabilities are at elevated risk of a range of adverse maternal outcomes, including severe maternal morbidity and maternal mortality, disability modules should be a mandatory component of education for obstetricians and midwives as well as other obstetrical health care professionals.”
Calling the study “a serious wake-up call,” Monika Mitra, PhD, told this publication that the findings highlight the need for “urgent attention” on improving obstetric care for people with disabilities “with a focus on accessibility and inclusion, changing clinical practice to better serve disabled people, integrating disability-related training for health care practitioners, and developing evidence-based interventions to support people with disabilities during this time.” The associate professor and director of the Lurie Institute for Disability Policy, in Brandeis University, Waltham, Mass. said the risk factors for poor outcomes are present early in pregnancy or even preconception. “We know that disabled women report barriers in accessing health care and receive lower-quality care compared to nondisabled women and are more likely to experience poverty, housing and food insecurity, educational and employment barriers, abuse, chronic health conditions, and mental illness than women without disabilities.”
She noted that the study’s sample of people with disabilities was small, and the measure of disability used was based on ICD-9 codes, which captures only severe disabilities. “As noted in the commentary by [Dr.] Brown, our standard sources of health administrative data do not give us the full picture on disability, and we need other, more equitable ways of identifying disability based, for example, on self-reports of activity or participation limitations if we are to be able to understand the effects on obstetric outcomes of health and health care disparities and of social determinants of health. Moreover, researchers have generally not yet begun to incorporate knowledge of the experiences of transgender people during pregnancy, which will impact our measures and study of obstetric outcomes among people with disabilities as well as the language we use.”
The study was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The study authors and Dr. Brown reported no conflicts of interest. Dr. Mitra receives funding from the NICHD and the National Institute on Disability, Independent Living for research on pregnancy outcomes among people with disabilities.
How does atopic dermatitis present in skin of color?
“We see very heterogenous and broad clinical presentations across the diverse patient populations that we see,” Andrew F. Alexis, MD, MPH, said at the Revolutionizing Atopic Dermatitis symposium. “Some of these differences might be related to population variations in skin barrier function, immunologic factors, genetic factors, and environmental factors, which all interplay to produce variations in the clinical presentation and overall impact of AD. Many nongenetic factors also contribute to differences that we see, including some socioeconomic and other factors that feed into health disparities.”
Dr. Alexis, professor of clinical dermatology at Weill Cornell Medicine, New York, discussed four main clinical features of AD in skin of color.
Erythema is less visible because it is masked by pigment
“There can be some masking of the redness and alteration of that color such that it doesn’t look bright red as it would in the background of lightly pigmented skin,” Dr. Alexis said. “Instead, the [AD lesions] have shades of grayish-red or grayish-brown or reddish-brown. It’s important to recognize this clinical presentation and look carefully and assess the patient – not just visually but with palpation and take into consideration symptomatology so that you don’t fall into the trap of calling an AD lesion postinflammatory hyperpigmentation. It’s also helpful to isolate the islands of normal or nonlesional skin and contrast that with the areas of lesional skin, to get a sense of how active and inflamed the areas are. Palpation really helps to appreciate the elevation of the lesions that are involved.”
Follicular accentuation
Morphological variants common in skin of color include the follicular variant or micropapular variant of AD. “You might just see a collection of papules that are 1-2 mm in size and pruritic and in typical sites of predilection [for] eczema,” he said. Prurigo nodularis–like lesions or prurigo nodularis in association with AD are also seen more frequently in skin of color.
Lichenification
The lichenoid variant of AD is characterized by a violaceous hue and other features that resemble lichen planus and has been reported to be more common in individuals of African descent. A prospective study of about 1,000 patients with AD seen over 2 years at a dermatology clinic in southeastern Nigeria found that 54% of patients had papular lichenoid lesions. In addition, 51% had elevated blood eosinophil counts, especially those with severe disease.
Dr. Alexis added that psoriasiform features have been reported in studies of East Asian populations with AD. These plaques may be more well demarcated and have clinical and histologic features that resemble psoriasis.
Dyspigmentation
One common feature across the spectrum of patients with skin of color “is the risk of longstanding pigmentary sequelae in the form of hyperpigmentation or hypopigmentation,” said Dr. Alexis, who is also vice chair for diversity and inclusion for the department of dermatology at Weill Cornell Medicine. “In very severe longstanding areas with chronic excoriation to the point of breaking of the skin, eroding of the skin, causing permanent damage to the melanocytes, dyspigmentation that resembles vitiligo can be seen. We can also see hypopigmentation as a consequence of topical corticosteroids, particularly those that are class I or class II and are used for prolonged periods of time.”
Dr. Alexis noted that delays in treatment and undertreatment can contribute to a higher risk of pigmentary and other long-term sequelae. “New therapies show promise in improving outcomes in AD patients with skin of color. When it comes to therapeutic responses, there are some post hoc studies that have investigated potential differences in safety and efficacy of the agents that have been recently approved. We clearly need more data to better understand if there are potential racial or ethnic differences.”
Dr. Alexis reported no relevant financial relationships.
Commentary by Lawrence F. Eichenfield, MD
Atopic dermatitis (AD) is highly heterogenous, with tremendous variations in extent, qualities of eczema, symptom complex, and physical presentation. Prior studies have reported disparities of care delivered to racial and ethnic minorities in the United States, as well as higher susceptibility to AD and odds of persistent disease into adulthood from child-onset AD. Recognizing some differences in presentation of AD in patients with skin of color is important as we select our therapeutic interventions, including assessing new treatments being added to our armamentarium. Erythema may be harder to notice in darker skin, but attempting to blanch the skin with pressure can help to assess the color and inflammation. Appreciating lichenoid changes, including papular and “micropapular” AD, and psoriasiform-like thickening in certain patients (reportedly more common in East Asian populations) are important as well. And dyspigmentation is an important aspect of the disease presentation and patient and parental concern, given both hypopigmentaton and hyperpigmentation commonly seen over the course of AD.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
“We see very heterogenous and broad clinical presentations across the diverse patient populations that we see,” Andrew F. Alexis, MD, MPH, said at the Revolutionizing Atopic Dermatitis symposium. “Some of these differences might be related to population variations in skin barrier function, immunologic factors, genetic factors, and environmental factors, which all interplay to produce variations in the clinical presentation and overall impact of AD. Many nongenetic factors also contribute to differences that we see, including some socioeconomic and other factors that feed into health disparities.”
Dr. Alexis, professor of clinical dermatology at Weill Cornell Medicine, New York, discussed four main clinical features of AD in skin of color.
Erythema is less visible because it is masked by pigment
“There can be some masking of the redness and alteration of that color such that it doesn’t look bright red as it would in the background of lightly pigmented skin,” Dr. Alexis said. “Instead, the [AD lesions] have shades of grayish-red or grayish-brown or reddish-brown. It’s important to recognize this clinical presentation and look carefully and assess the patient – not just visually but with palpation and take into consideration symptomatology so that you don’t fall into the trap of calling an AD lesion postinflammatory hyperpigmentation. It’s also helpful to isolate the islands of normal or nonlesional skin and contrast that with the areas of lesional skin, to get a sense of how active and inflamed the areas are. Palpation really helps to appreciate the elevation of the lesions that are involved.”
Follicular accentuation
Morphological variants common in skin of color include the follicular variant or micropapular variant of AD. “You might just see a collection of papules that are 1-2 mm in size and pruritic and in typical sites of predilection [for] eczema,” he said. Prurigo nodularis–like lesions or prurigo nodularis in association with AD are also seen more frequently in skin of color.
Lichenification
The lichenoid variant of AD is characterized by a violaceous hue and other features that resemble lichen planus and has been reported to be more common in individuals of African descent. A prospective study of about 1,000 patients with AD seen over 2 years at a dermatology clinic in southeastern Nigeria found that 54% of patients had papular lichenoid lesions. In addition, 51% had elevated blood eosinophil counts, especially those with severe disease.
Dr. Alexis added that psoriasiform features have been reported in studies of East Asian populations with AD. These plaques may be more well demarcated and have clinical and histologic features that resemble psoriasis.
Dyspigmentation
One common feature across the spectrum of patients with skin of color “is the risk of longstanding pigmentary sequelae in the form of hyperpigmentation or hypopigmentation,” said Dr. Alexis, who is also vice chair for diversity and inclusion for the department of dermatology at Weill Cornell Medicine. “In very severe longstanding areas with chronic excoriation to the point of breaking of the skin, eroding of the skin, causing permanent damage to the melanocytes, dyspigmentation that resembles vitiligo can be seen. We can also see hypopigmentation as a consequence of topical corticosteroids, particularly those that are class I or class II and are used for prolonged periods of time.”
Dr. Alexis noted that delays in treatment and undertreatment can contribute to a higher risk of pigmentary and other long-term sequelae. “New therapies show promise in improving outcomes in AD patients with skin of color. When it comes to therapeutic responses, there are some post hoc studies that have investigated potential differences in safety and efficacy of the agents that have been recently approved. We clearly need more data to better understand if there are potential racial or ethnic differences.”
Dr. Alexis reported no relevant financial relationships.
Commentary by Lawrence F. Eichenfield, MD
Atopic dermatitis (AD) is highly heterogenous, with tremendous variations in extent, qualities of eczema, symptom complex, and physical presentation. Prior studies have reported disparities of care delivered to racial and ethnic minorities in the United States, as well as higher susceptibility to AD and odds of persistent disease into adulthood from child-onset AD. Recognizing some differences in presentation of AD in patients with skin of color is important as we select our therapeutic interventions, including assessing new treatments being added to our armamentarium. Erythema may be harder to notice in darker skin, but attempting to blanch the skin with pressure can help to assess the color and inflammation. Appreciating lichenoid changes, including papular and “micropapular” AD, and psoriasiform-like thickening in certain patients (reportedly more common in East Asian populations) are important as well. And dyspigmentation is an important aspect of the disease presentation and patient and parental concern, given both hypopigmentaton and hyperpigmentation commonly seen over the course of AD.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
“We see very heterogenous and broad clinical presentations across the diverse patient populations that we see,” Andrew F. Alexis, MD, MPH, said at the Revolutionizing Atopic Dermatitis symposium. “Some of these differences might be related to population variations in skin barrier function, immunologic factors, genetic factors, and environmental factors, which all interplay to produce variations in the clinical presentation and overall impact of AD. Many nongenetic factors also contribute to differences that we see, including some socioeconomic and other factors that feed into health disparities.”
Dr. Alexis, professor of clinical dermatology at Weill Cornell Medicine, New York, discussed four main clinical features of AD in skin of color.
Erythema is less visible because it is masked by pigment
“There can be some masking of the redness and alteration of that color such that it doesn’t look bright red as it would in the background of lightly pigmented skin,” Dr. Alexis said. “Instead, the [AD lesions] have shades of grayish-red or grayish-brown or reddish-brown. It’s important to recognize this clinical presentation and look carefully and assess the patient – not just visually but with palpation and take into consideration symptomatology so that you don’t fall into the trap of calling an AD lesion postinflammatory hyperpigmentation. It’s also helpful to isolate the islands of normal or nonlesional skin and contrast that with the areas of lesional skin, to get a sense of how active and inflamed the areas are. Palpation really helps to appreciate the elevation of the lesions that are involved.”
Follicular accentuation
Morphological variants common in skin of color include the follicular variant or micropapular variant of AD. “You might just see a collection of papules that are 1-2 mm in size and pruritic and in typical sites of predilection [for] eczema,” he said. Prurigo nodularis–like lesions or prurigo nodularis in association with AD are also seen more frequently in skin of color.
Lichenification
The lichenoid variant of AD is characterized by a violaceous hue and other features that resemble lichen planus and has been reported to be more common in individuals of African descent. A prospective study of about 1,000 patients with AD seen over 2 years at a dermatology clinic in southeastern Nigeria found that 54% of patients had papular lichenoid lesions. In addition, 51% had elevated blood eosinophil counts, especially those with severe disease.
Dr. Alexis added that psoriasiform features have been reported in studies of East Asian populations with AD. These plaques may be more well demarcated and have clinical and histologic features that resemble psoriasis.
Dyspigmentation
One common feature across the spectrum of patients with skin of color “is the risk of longstanding pigmentary sequelae in the form of hyperpigmentation or hypopigmentation,” said Dr. Alexis, who is also vice chair for diversity and inclusion for the department of dermatology at Weill Cornell Medicine. “In very severe longstanding areas with chronic excoriation to the point of breaking of the skin, eroding of the skin, causing permanent damage to the melanocytes, dyspigmentation that resembles vitiligo can be seen. We can also see hypopigmentation as a consequence of topical corticosteroids, particularly those that are class I or class II and are used for prolonged periods of time.”
Dr. Alexis noted that delays in treatment and undertreatment can contribute to a higher risk of pigmentary and other long-term sequelae. “New therapies show promise in improving outcomes in AD patients with skin of color. When it comes to therapeutic responses, there are some post hoc studies that have investigated potential differences in safety and efficacy of the agents that have been recently approved. We clearly need more data to better understand if there are potential racial or ethnic differences.”
Dr. Alexis reported no relevant financial relationships.
Commentary by Lawrence F. Eichenfield, MD
Atopic dermatitis (AD) is highly heterogenous, with tremendous variations in extent, qualities of eczema, symptom complex, and physical presentation. Prior studies have reported disparities of care delivered to racial and ethnic minorities in the United States, as well as higher susceptibility to AD and odds of persistent disease into adulthood from child-onset AD. Recognizing some differences in presentation of AD in patients with skin of color is important as we select our therapeutic interventions, including assessing new treatments being added to our armamentarium. Erythema may be harder to notice in darker skin, but attempting to blanch the skin with pressure can help to assess the color and inflammation. Appreciating lichenoid changes, including papular and “micropapular” AD, and psoriasiform-like thickening in certain patients (reportedly more common in East Asian populations) are important as well. And dyspigmentation is an important aspect of the disease presentation and patient and parental concern, given both hypopigmentaton and hyperpigmentation commonly seen over the course of AD.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
FROM REVOLUTIONIZING AD 2021
Fixing the maternal health problem in the U.S.: Signs of hope?
In the United States, nearly 4 million women a year prepare to give birth, looking forward to the joy to come. But for some, the dream turns tragic. And another 60,000 have pregnancy-related or childbirth-related health issues.
Causes of death vary greatly, including hemorrhage during pregnancy or during delivery, heart conditions, and mental health issues such as substance abuse and suicide after the birth.
In 2019, the U.S. maternal death rate was 20.1 per 100,000 women, according to the CDC, significantly higher than the 17.4 per 100,000 recorded in 2018. For Black women, the maternal death rate was more than double the overall – 44 per 100,000 in 2019.
“We have to address our horrendous maternal health care system and also need to address the inequities,” says Laurie Zephyrin, MD, vice president for advancing health equity for the Commonwealth Fund, a foundation supporting independent research on health care issues. “This is an issue that has needed national attention for a long time.”
“If we look overall, our maternal death rate is more than twice that of more than 10 other high-income countries,” she said.
As sobering as the problem is, recent developments have sparked hope that reversing the course is possible. Among them:
U.S. News & World Report, long known for its rankings of hospitals, issued its first ever “Best Hospitals for Maternity” rankings Dec. 7, highlighting facilities that perform well on key quality indicators. It plans to update the report annually.
At the first-ever White House Maternal Health Day of Action on Dec. 7, Vice President Kamala Harris urged a call to action to reduce maternal deaths and pregnancy-related health problems, with extension of postpartum coverage through Medicaid programs, among other actions.
A new hospital designation called ‘’Birthing Friendly” will be established by the Centers for Medicare & Medicaid Services. The label will be given to facilities that take part in a program aimed at improving maternal outcomes and that use patient safety practices.
President Joe Biden’s proposed Build Back Better plan includes maternal health provisions, including $3 billion in new maternal health funding. The money will aim to grow and diversify the workforce caring for pregnant women, coordinate care better, and step up research on maternal health, among other projects.
Ongoing efforts in Congress are aimed at fixing the wide disparities in maternal health affecting Black women. Regardless of income level or education, Black women are at a higher risk of maternal death and other health issues than are White women. A Black woman with a college education is at 60% higher risk of maternal death than a White or Hispanic woman who didn’t graduate high school, according to the Commonwealth Fund.
Best hospitals for maternity
For its rankings, U.S. News and World Report reached out to the 2,700 U.S. hospitals that offer maternity services, said Ben Harder, chief of health analysis and managing editor at U.S. News & World Report.
To be recognized, a hospital had to submit data from 2019 and meet the publication’s maternity care standards. The publication received responses from just 571 hospitals, representing about two of every five births in the country.
Of those, 237 were identified as best for maternity.
As to why the response rate was not higher, Mr. Harder cited the reporting burden and says it is understandable. Some hospitals likely did not have the staff available, especially during the pandemic, to gather the data needed to be evaluated by U.S. News & World Report.
On their other evaluations, the rankings are based on Medicare data, “so hospitals don’t have to lift a finger.” He expects more hospitals will respond for their future evaluations of maternity care.
The evaluators focused on five quality measures, making a score based on the cesarean section delivery rate among first-time mothers, early elective delivery rates, unexpected newborn complication rates, breastfeeding rates, and option for vaginal birth after C-section.
A call to action: Expand coverage
Speaking at the White House Maternal Health Day of Action, Mrs. Harris told participants: “The challenge is urgent, and it is important, and it will take all of us.”
Being pregnant and giving birth, she said, should not carry such great risks. She zeroed in on systemic inequities in the way women are treated and the dramatic impact maternal death and health issues have on the economy.
“A healthy economy requires healthy mothers and healthy babies,” Mrs. Harris said.
“Before, during, and after childbirth, women in our nation are dying at a higher rate than any other developed nation in our world,” she said, noting that research shows that Black women, Native Americans, and women in rural America more likely to suffer.
A major strategy in the call to action, according to Mrs. Harris, is encouraging states to expand postpartum coverage to pregnant women enrolled in Medicaid or the Children’s Health Insurance Program from the existing 60 days to a full year. Together, these two programs cover over 42% of births in the country, so expanding the coverage is expected to have a great impact.
The 60 days of coverage is not enough, as many deaths and complications happen more than 60 days after childbirth, Mrs. Harris said. The logistics for states to extend coverage were established by the American Rescue Plan and will become available by April 2022. Some states have already extended the postpartum coverage.
According to the Centers for Medicare and Medicaid Services, if every state did adopt an extension, as the Build Back Better Act proposes, the number of Americans getting coverage for a full year after childbirth would about double, extending the coverage for about 720,000 each year.
Congressional actions
Congress is working on the issue as well. The Black Maternal Health Momnibus Act of 2021, for instance, proposes several measures, including improving maternal nutrition, expanding affordable housing, and extending the maternal workforce to include more doulas and midwives.
“And for so many women, let’s note doulas are literally a lifeline,” Mrs. Harris said at the White House event.
Doulas are trained to offer women physical, emotional, and informational support before, during, and after childbirth. No reliable statistics are available on their numbers in the United States, but a March of Dimes report estimates that about 9,000 were included in a registration database in 2018.
Explaining and fixing the disparities
No one can explain for sure why Black women, in particular, are at higher risk of dying from pregnancy-related complications. Systemic inequity is one likely reason, Mrs. Harris said, noting there are differences in how people are treated based on who they are.
Inherent and unconscious bias in offering women treatment plays a role, experts say. Training could reverse or reduce that bias. Some women of color also may have less access to care, as do women in some rural areas.
According to Mrs. Harris, more than 20 companies and nonprofits have pledged to invest more than $20 million in maternal health efforts in the United States and more than $150 million globally. Among the proposed programs: remote-care monitors in rural areas, better care models for the postpartum period, and improved education programs for maternal health providers.
When statistics hit home
Many who work to improve maternal health have gone through issues themselves or had loved ones who did.
Jill Arnold, founder of the Maternal Safety Foundation in Bentonville, Ark., became a consumer advocate after giving birth to her two daughters, now teenagers. With the first birth, Ms. Arnold said she was intensely pressured at the last minute to have a C-section. She held out, resisted, and delivered a healthy baby vaginally.
For her second childbirth, she chose an accredited birth center that allowed her to have a doula and a midwife.
“The care I received was night and day,” she said. “The overwhelming pressure to consent to a C-section wasn’t there.”
She welcomes the information provided by the new U.S. News & World Report rankings as well as the upcoming “Birthing Friendly” designations.
“The onus shouldn’t be on patients, on individuals, on pregnant people to do the research,” Ms. Arnold said.
Rather, women and their partners need information at their fingertips so they can make an informed decision about how to give birth and where.
U.S. Rep. Lauren Underwood (D-Ill.), who cofounded the Black Maternal Health Caucus in April 2019, with Rep. Alma Adams (D-N.C.), wrote a touching blog in the journal Health Affairs to explain her passion in improving maternal health.
Her former classmate, Shalon Irving, who went on to become a CDC epidemiologist, died in February 2017 at age 36, just 3 weeks after giving birth, when she developed complications from high blood pressure.
In the blog, Ms. Underwood cited statistics and provides details of the Black Maternal Health Momnibus Act of 2021, then ends the blog, published in 2020, with an update on how Ms. Irving’s then 3-year-old daughter, raised by her grandmother, is doing. While Soleil is “curious, joyful, and brilliant,” the grandmother told Ms. Underwood that she has also walked into a room and found the little girl clutching a framed photograph of her mother.
The child’s question is understandable and heartbreaking: She wants to know where her mommy is.
“Soleil’s question is my motivation,” Ms. Underwood wrote. “To honor Shalon, and all the women like her who we have lost, let us take the serious and urgent action that is required to save our moms.”
A version of this article first appeared on WebMD.com.
In the United States, nearly 4 million women a year prepare to give birth, looking forward to the joy to come. But for some, the dream turns tragic. And another 60,000 have pregnancy-related or childbirth-related health issues.
Causes of death vary greatly, including hemorrhage during pregnancy or during delivery, heart conditions, and mental health issues such as substance abuse and suicide after the birth.
In 2019, the U.S. maternal death rate was 20.1 per 100,000 women, according to the CDC, significantly higher than the 17.4 per 100,000 recorded in 2018. For Black women, the maternal death rate was more than double the overall – 44 per 100,000 in 2019.
“We have to address our horrendous maternal health care system and also need to address the inequities,” says Laurie Zephyrin, MD, vice president for advancing health equity for the Commonwealth Fund, a foundation supporting independent research on health care issues. “This is an issue that has needed national attention for a long time.”
“If we look overall, our maternal death rate is more than twice that of more than 10 other high-income countries,” she said.
As sobering as the problem is, recent developments have sparked hope that reversing the course is possible. Among them:
U.S. News & World Report, long known for its rankings of hospitals, issued its first ever “Best Hospitals for Maternity” rankings Dec. 7, highlighting facilities that perform well on key quality indicators. It plans to update the report annually.
At the first-ever White House Maternal Health Day of Action on Dec. 7, Vice President Kamala Harris urged a call to action to reduce maternal deaths and pregnancy-related health problems, with extension of postpartum coverage through Medicaid programs, among other actions.
A new hospital designation called ‘’Birthing Friendly” will be established by the Centers for Medicare & Medicaid Services. The label will be given to facilities that take part in a program aimed at improving maternal outcomes and that use patient safety practices.
President Joe Biden’s proposed Build Back Better plan includes maternal health provisions, including $3 billion in new maternal health funding. The money will aim to grow and diversify the workforce caring for pregnant women, coordinate care better, and step up research on maternal health, among other projects.
Ongoing efforts in Congress are aimed at fixing the wide disparities in maternal health affecting Black women. Regardless of income level or education, Black women are at a higher risk of maternal death and other health issues than are White women. A Black woman with a college education is at 60% higher risk of maternal death than a White or Hispanic woman who didn’t graduate high school, according to the Commonwealth Fund.
Best hospitals for maternity
For its rankings, U.S. News and World Report reached out to the 2,700 U.S. hospitals that offer maternity services, said Ben Harder, chief of health analysis and managing editor at U.S. News & World Report.
To be recognized, a hospital had to submit data from 2019 and meet the publication’s maternity care standards. The publication received responses from just 571 hospitals, representing about two of every five births in the country.
Of those, 237 were identified as best for maternity.
As to why the response rate was not higher, Mr. Harder cited the reporting burden and says it is understandable. Some hospitals likely did not have the staff available, especially during the pandemic, to gather the data needed to be evaluated by U.S. News & World Report.
On their other evaluations, the rankings are based on Medicare data, “so hospitals don’t have to lift a finger.” He expects more hospitals will respond for their future evaluations of maternity care.
The evaluators focused on five quality measures, making a score based on the cesarean section delivery rate among first-time mothers, early elective delivery rates, unexpected newborn complication rates, breastfeeding rates, and option for vaginal birth after C-section.
A call to action: Expand coverage
Speaking at the White House Maternal Health Day of Action, Mrs. Harris told participants: “The challenge is urgent, and it is important, and it will take all of us.”
Being pregnant and giving birth, she said, should not carry such great risks. She zeroed in on systemic inequities in the way women are treated and the dramatic impact maternal death and health issues have on the economy.
“A healthy economy requires healthy mothers and healthy babies,” Mrs. Harris said.
“Before, during, and after childbirth, women in our nation are dying at a higher rate than any other developed nation in our world,” she said, noting that research shows that Black women, Native Americans, and women in rural America more likely to suffer.
A major strategy in the call to action, according to Mrs. Harris, is encouraging states to expand postpartum coverage to pregnant women enrolled in Medicaid or the Children’s Health Insurance Program from the existing 60 days to a full year. Together, these two programs cover over 42% of births in the country, so expanding the coverage is expected to have a great impact.
The 60 days of coverage is not enough, as many deaths and complications happen more than 60 days after childbirth, Mrs. Harris said. The logistics for states to extend coverage were established by the American Rescue Plan and will become available by April 2022. Some states have already extended the postpartum coverage.
According to the Centers for Medicare and Medicaid Services, if every state did adopt an extension, as the Build Back Better Act proposes, the number of Americans getting coverage for a full year after childbirth would about double, extending the coverage for about 720,000 each year.
Congressional actions
Congress is working on the issue as well. The Black Maternal Health Momnibus Act of 2021, for instance, proposes several measures, including improving maternal nutrition, expanding affordable housing, and extending the maternal workforce to include more doulas and midwives.
“And for so many women, let’s note doulas are literally a lifeline,” Mrs. Harris said at the White House event.
Doulas are trained to offer women physical, emotional, and informational support before, during, and after childbirth. No reliable statistics are available on their numbers in the United States, but a March of Dimes report estimates that about 9,000 were included in a registration database in 2018.
Explaining and fixing the disparities
No one can explain for sure why Black women, in particular, are at higher risk of dying from pregnancy-related complications. Systemic inequity is one likely reason, Mrs. Harris said, noting there are differences in how people are treated based on who they are.
Inherent and unconscious bias in offering women treatment plays a role, experts say. Training could reverse or reduce that bias. Some women of color also may have less access to care, as do women in some rural areas.
According to Mrs. Harris, more than 20 companies and nonprofits have pledged to invest more than $20 million in maternal health efforts in the United States and more than $150 million globally. Among the proposed programs: remote-care monitors in rural areas, better care models for the postpartum period, and improved education programs for maternal health providers.
When statistics hit home
Many who work to improve maternal health have gone through issues themselves or had loved ones who did.
Jill Arnold, founder of the Maternal Safety Foundation in Bentonville, Ark., became a consumer advocate after giving birth to her two daughters, now teenagers. With the first birth, Ms. Arnold said she was intensely pressured at the last minute to have a C-section. She held out, resisted, and delivered a healthy baby vaginally.
For her second childbirth, she chose an accredited birth center that allowed her to have a doula and a midwife.
“The care I received was night and day,” she said. “The overwhelming pressure to consent to a C-section wasn’t there.”
She welcomes the information provided by the new U.S. News & World Report rankings as well as the upcoming “Birthing Friendly” designations.
“The onus shouldn’t be on patients, on individuals, on pregnant people to do the research,” Ms. Arnold said.
Rather, women and their partners need information at their fingertips so they can make an informed decision about how to give birth and where.
U.S. Rep. Lauren Underwood (D-Ill.), who cofounded the Black Maternal Health Caucus in April 2019, with Rep. Alma Adams (D-N.C.), wrote a touching blog in the journal Health Affairs to explain her passion in improving maternal health.
Her former classmate, Shalon Irving, who went on to become a CDC epidemiologist, died in February 2017 at age 36, just 3 weeks after giving birth, when she developed complications from high blood pressure.
In the blog, Ms. Underwood cited statistics and provides details of the Black Maternal Health Momnibus Act of 2021, then ends the blog, published in 2020, with an update on how Ms. Irving’s then 3-year-old daughter, raised by her grandmother, is doing. While Soleil is “curious, joyful, and brilliant,” the grandmother told Ms. Underwood that she has also walked into a room and found the little girl clutching a framed photograph of her mother.
The child’s question is understandable and heartbreaking: She wants to know where her mommy is.
“Soleil’s question is my motivation,” Ms. Underwood wrote. “To honor Shalon, and all the women like her who we have lost, let us take the serious and urgent action that is required to save our moms.”
A version of this article first appeared on WebMD.com.
In the United States, nearly 4 million women a year prepare to give birth, looking forward to the joy to come. But for some, the dream turns tragic. And another 60,000 have pregnancy-related or childbirth-related health issues.
Causes of death vary greatly, including hemorrhage during pregnancy or during delivery, heart conditions, and mental health issues such as substance abuse and suicide after the birth.
In 2019, the U.S. maternal death rate was 20.1 per 100,000 women, according to the CDC, significantly higher than the 17.4 per 100,000 recorded in 2018. For Black women, the maternal death rate was more than double the overall – 44 per 100,000 in 2019.
“We have to address our horrendous maternal health care system and also need to address the inequities,” says Laurie Zephyrin, MD, vice president for advancing health equity for the Commonwealth Fund, a foundation supporting independent research on health care issues. “This is an issue that has needed national attention for a long time.”
“If we look overall, our maternal death rate is more than twice that of more than 10 other high-income countries,” she said.
As sobering as the problem is, recent developments have sparked hope that reversing the course is possible. Among them:
U.S. News & World Report, long known for its rankings of hospitals, issued its first ever “Best Hospitals for Maternity” rankings Dec. 7, highlighting facilities that perform well on key quality indicators. It plans to update the report annually.
At the first-ever White House Maternal Health Day of Action on Dec. 7, Vice President Kamala Harris urged a call to action to reduce maternal deaths and pregnancy-related health problems, with extension of postpartum coverage through Medicaid programs, among other actions.
A new hospital designation called ‘’Birthing Friendly” will be established by the Centers for Medicare & Medicaid Services. The label will be given to facilities that take part in a program aimed at improving maternal outcomes and that use patient safety practices.
President Joe Biden’s proposed Build Back Better plan includes maternal health provisions, including $3 billion in new maternal health funding. The money will aim to grow and diversify the workforce caring for pregnant women, coordinate care better, and step up research on maternal health, among other projects.
Ongoing efforts in Congress are aimed at fixing the wide disparities in maternal health affecting Black women. Regardless of income level or education, Black women are at a higher risk of maternal death and other health issues than are White women. A Black woman with a college education is at 60% higher risk of maternal death than a White or Hispanic woman who didn’t graduate high school, according to the Commonwealth Fund.
Best hospitals for maternity
For its rankings, U.S. News and World Report reached out to the 2,700 U.S. hospitals that offer maternity services, said Ben Harder, chief of health analysis and managing editor at U.S. News & World Report.
To be recognized, a hospital had to submit data from 2019 and meet the publication’s maternity care standards. The publication received responses from just 571 hospitals, representing about two of every five births in the country.
Of those, 237 were identified as best for maternity.
As to why the response rate was not higher, Mr. Harder cited the reporting burden and says it is understandable. Some hospitals likely did not have the staff available, especially during the pandemic, to gather the data needed to be evaluated by U.S. News & World Report.
On their other evaluations, the rankings are based on Medicare data, “so hospitals don’t have to lift a finger.” He expects more hospitals will respond for their future evaluations of maternity care.
The evaluators focused on five quality measures, making a score based on the cesarean section delivery rate among first-time mothers, early elective delivery rates, unexpected newborn complication rates, breastfeeding rates, and option for vaginal birth after C-section.
A call to action: Expand coverage
Speaking at the White House Maternal Health Day of Action, Mrs. Harris told participants: “The challenge is urgent, and it is important, and it will take all of us.”
Being pregnant and giving birth, she said, should not carry such great risks. She zeroed in on systemic inequities in the way women are treated and the dramatic impact maternal death and health issues have on the economy.
“A healthy economy requires healthy mothers and healthy babies,” Mrs. Harris said.
“Before, during, and after childbirth, women in our nation are dying at a higher rate than any other developed nation in our world,” she said, noting that research shows that Black women, Native Americans, and women in rural America more likely to suffer.
A major strategy in the call to action, according to Mrs. Harris, is encouraging states to expand postpartum coverage to pregnant women enrolled in Medicaid or the Children’s Health Insurance Program from the existing 60 days to a full year. Together, these two programs cover over 42% of births in the country, so expanding the coverage is expected to have a great impact.
The 60 days of coverage is not enough, as many deaths and complications happen more than 60 days after childbirth, Mrs. Harris said. The logistics for states to extend coverage were established by the American Rescue Plan and will become available by April 2022. Some states have already extended the postpartum coverage.
According to the Centers for Medicare and Medicaid Services, if every state did adopt an extension, as the Build Back Better Act proposes, the number of Americans getting coverage for a full year after childbirth would about double, extending the coverage for about 720,000 each year.
Congressional actions
Congress is working on the issue as well. The Black Maternal Health Momnibus Act of 2021, for instance, proposes several measures, including improving maternal nutrition, expanding affordable housing, and extending the maternal workforce to include more doulas and midwives.
“And for so many women, let’s note doulas are literally a lifeline,” Mrs. Harris said at the White House event.
Doulas are trained to offer women physical, emotional, and informational support before, during, and after childbirth. No reliable statistics are available on their numbers in the United States, but a March of Dimes report estimates that about 9,000 were included in a registration database in 2018.
Explaining and fixing the disparities
No one can explain for sure why Black women, in particular, are at higher risk of dying from pregnancy-related complications. Systemic inequity is one likely reason, Mrs. Harris said, noting there are differences in how people are treated based on who they are.
Inherent and unconscious bias in offering women treatment plays a role, experts say. Training could reverse or reduce that bias. Some women of color also may have less access to care, as do women in some rural areas.
According to Mrs. Harris, more than 20 companies and nonprofits have pledged to invest more than $20 million in maternal health efforts in the United States and more than $150 million globally. Among the proposed programs: remote-care monitors in rural areas, better care models for the postpartum period, and improved education programs for maternal health providers.
When statistics hit home
Many who work to improve maternal health have gone through issues themselves or had loved ones who did.
Jill Arnold, founder of the Maternal Safety Foundation in Bentonville, Ark., became a consumer advocate after giving birth to her two daughters, now teenagers. With the first birth, Ms. Arnold said she was intensely pressured at the last minute to have a C-section. She held out, resisted, and delivered a healthy baby vaginally.
For her second childbirth, she chose an accredited birth center that allowed her to have a doula and a midwife.
“The care I received was night and day,” she said. “The overwhelming pressure to consent to a C-section wasn’t there.”
She welcomes the information provided by the new U.S. News & World Report rankings as well as the upcoming “Birthing Friendly” designations.
“The onus shouldn’t be on patients, on individuals, on pregnant people to do the research,” Ms. Arnold said.
Rather, women and their partners need information at their fingertips so they can make an informed decision about how to give birth and where.
U.S. Rep. Lauren Underwood (D-Ill.), who cofounded the Black Maternal Health Caucus in April 2019, with Rep. Alma Adams (D-N.C.), wrote a touching blog in the journal Health Affairs to explain her passion in improving maternal health.
Her former classmate, Shalon Irving, who went on to become a CDC epidemiologist, died in February 2017 at age 36, just 3 weeks after giving birth, when she developed complications from high blood pressure.
In the blog, Ms. Underwood cited statistics and provides details of the Black Maternal Health Momnibus Act of 2021, then ends the blog, published in 2020, with an update on how Ms. Irving’s then 3-year-old daughter, raised by her grandmother, is doing. While Soleil is “curious, joyful, and brilliant,” the grandmother told Ms. Underwood that she has also walked into a room and found the little girl clutching a framed photograph of her mother.
The child’s question is understandable and heartbreaking: She wants to know where her mommy is.
“Soleil’s question is my motivation,” Ms. Underwood wrote. “To honor Shalon, and all the women like her who we have lost, let us take the serious and urgent action that is required to save our moms.”
A version of this article first appeared on WebMD.com.
A case-based framework for de-escalating conflict
Hospital medicine can be a demanding and fast-paced environment where resources are stretched thin, with both clinicians and patients stressed. A hospitalist’s role is dynamic, serving as an advocate, leader, or role model while working with interdisciplinary and diverse teams for the welfare of the patient. This constellation of pressures makes a degree of conflict inevitable.
Often, an unexpected scenario can render the hospitalist uncertain and yet the hospitalist’s response can escalate or deescalate conflict. The multiple roles that a hospitalist represents may buckle to the single role of advocating for themselves, a colleague, or a patient in a tense scenario. When this happens, many hospitalists feel disempowered to respond.
De-escalation is a practical skill that involves being calm, respectful, and open minded toward the other person, while also maintaining boundaries. Here we provide case-based tips and skills that highlight the role for de-escalation.
Questions to ask yourself in midst of conflict:
- How did the problematic behavior make you feel?
- What will be your approach in handling this?
- When should you address this?
- What is the outcome you are hoping to achieve?
- What is the outcome the other person is hoping to achieve?
Case 1
There is a female physician rounding with your team. Introductions were made at the start of a patient encounter. The patient repeatedly calls the female physician by her first name and refers to a male colleague as “doctor.”
Commentary: This scenario is commonly encountered by women who are physicians. They may be mistaken for the nurse, a technician, or a housekeeper. This exacerbates inequality and impostor syndrome as women can feel unheard, undervalued, and not recognized for their expertise and achievements. It can be challenging for a woman to reaffirm herself as she worries that the patient will not respect her or will think that she is being aggressive.
Approach: It is vital to interject by firmly reintroducing the female physician by her correct title. If you are the subject of this scenario, you may interject by firmly reintroducing yourself. If the patient or a colleague continues to refer to her by her first name, it is appropriate to say, “Please call her Dr. XYZ.” There is likely another female colleague or trainee nearby that will view this scenario as a model for setting boundaries.
To prevent similar future situations, consistently refer to all peers by their title in front of patients and peers in all professional settings (such as lectures, luncheons, etc.) to establish this as a cultural norm. Also, utilize hospital badges that clearly display roles in large letters.
Case 2
During sign out from a colleague, the colleague repeatedly refers to a patient hospitalized with sickle cell disease as a “frequent flyer” and “drug seeker,” and then remarks, “you know how these patients are.”
Commentary: A situation like this raises concerns about bias and stereotyping. Everyone has implicit bias. Recognizing and acknowledging when implicit bias affects objectivity in patient care is vital to providing appropriate care. It can be intimidating to broach this subject with a colleague as it may cause the colleague to become defensive and uncomfortable as revealing another person’s bias can be difficult. But physicians owe it to a patient’s wellbeing to remain objective and to prevent future colleagues from providing subpar care as a result.
Approach: In this case, saying, “Sometimes my previous experiences can affect my thinking. Will you explain what behaviors the patient has shown this admission that are concerning to you? This will allow me to grasp the complexity of the situation.” Another strategy is to share that there are new recommendations for how to use language about patients with sickle cell disease and patients who require opioids as a part of their treatment plan. Your hospitalist group could have a journal club on how bias affects patients and about the best practices in the care of people with sickle cell disease. A next step could be to build a quality improvement project to review the care of patients hospitalized for sickle cell disease or opioid use.
Case 3
You are conducting bedside rounds with your team. Your intern, a person of color, begins to present. The patient interjects by requesting that the intern leave as he “does not want a foreigner taking care” of him.
Commentary: Requests like this can be shocking. The team leader has a responsibility to immediately act to ensure the psychological safety of the team. Ideally, your response should set firm boundaries and expectations that support the learner as a valued and respected clinician and allow the intern to complete the presentation. In this scenario, regardless of the response the patient takes, it is vital to maintain a safe environment for the trainee. It is crucial to debrief with the team immediately after as an exchange of thoughts and emotions in a safe space can allow for everyone to feel welcome. Additionally, this debrief can provide insights to the team leader of how to address similar situations in the future. The opportunity to allow the intern to no longer follow the patient should be offered, and if the intern opts to no longer follow the patient, accommodations should be made.
Approach: “This physician is a member of the medical team, and we are all working together to provide you with the best care. Everyone on this team is an equal. We value diversity of our team members as it allows us to take care of all our patients. We respect you and expect respect for each member of the team. If you feel that you are unable to respect our team members right now, we will leave for now and return later.” To ensure the patient is provided with appropriate care, be sure to debrief with the patient’s nurse.
Conclusion
These scenarios represent some of the many complex interpersonal challenges hospitalists encounter. These approaches are suggestions that are open to improvement as de-escalation of a conflict is a critical and evolving skill and practice.
For more tips on managing conflict, consider reading “Crucial Conversations” by Kerry Patterson and colleagues. These skills can provide the tools we need to recenter ourselves when we are in the midst of these challenging situations.
Dr. Rawal is clinical assistant professor of medicine at the University of Pittsburgh Medical Center. Dr. Ashford is assistant professor and program director in the department of internal medicine/pediatrics at the University of Nebraska Medical Center, Omaha. Dr. Lee and Dr. Barrett are based in the department of internal medicine, University of New Mexico School of Medicine, Albuquerque. This article is sponsored by the SHM Physicians in Training (PIT) committee, which submits quarterly content to The Hospitalist on topics relevant to trainees and early career hospitalists.
Hospital medicine can be a demanding and fast-paced environment where resources are stretched thin, with both clinicians and patients stressed. A hospitalist’s role is dynamic, serving as an advocate, leader, or role model while working with interdisciplinary and diverse teams for the welfare of the patient. This constellation of pressures makes a degree of conflict inevitable.
Often, an unexpected scenario can render the hospitalist uncertain and yet the hospitalist’s response can escalate or deescalate conflict. The multiple roles that a hospitalist represents may buckle to the single role of advocating for themselves, a colleague, or a patient in a tense scenario. When this happens, many hospitalists feel disempowered to respond.
De-escalation is a practical skill that involves being calm, respectful, and open minded toward the other person, while also maintaining boundaries. Here we provide case-based tips and skills that highlight the role for de-escalation.
Questions to ask yourself in midst of conflict:
- How did the problematic behavior make you feel?
- What will be your approach in handling this?
- When should you address this?
- What is the outcome you are hoping to achieve?
- What is the outcome the other person is hoping to achieve?
Case 1
There is a female physician rounding with your team. Introductions were made at the start of a patient encounter. The patient repeatedly calls the female physician by her first name and refers to a male colleague as “doctor.”
Commentary: This scenario is commonly encountered by women who are physicians. They may be mistaken for the nurse, a technician, or a housekeeper. This exacerbates inequality and impostor syndrome as women can feel unheard, undervalued, and not recognized for their expertise and achievements. It can be challenging for a woman to reaffirm herself as she worries that the patient will not respect her or will think that she is being aggressive.
Approach: It is vital to interject by firmly reintroducing the female physician by her correct title. If you are the subject of this scenario, you may interject by firmly reintroducing yourself. If the patient or a colleague continues to refer to her by her first name, it is appropriate to say, “Please call her Dr. XYZ.” There is likely another female colleague or trainee nearby that will view this scenario as a model for setting boundaries.
To prevent similar future situations, consistently refer to all peers by their title in front of patients and peers in all professional settings (such as lectures, luncheons, etc.) to establish this as a cultural norm. Also, utilize hospital badges that clearly display roles in large letters.
Case 2
During sign out from a colleague, the colleague repeatedly refers to a patient hospitalized with sickle cell disease as a “frequent flyer” and “drug seeker,” and then remarks, “you know how these patients are.”
Commentary: A situation like this raises concerns about bias and stereotyping. Everyone has implicit bias. Recognizing and acknowledging when implicit bias affects objectivity in patient care is vital to providing appropriate care. It can be intimidating to broach this subject with a colleague as it may cause the colleague to become defensive and uncomfortable as revealing another person’s bias can be difficult. But physicians owe it to a patient’s wellbeing to remain objective and to prevent future colleagues from providing subpar care as a result.
Approach: In this case, saying, “Sometimes my previous experiences can affect my thinking. Will you explain what behaviors the patient has shown this admission that are concerning to you? This will allow me to grasp the complexity of the situation.” Another strategy is to share that there are new recommendations for how to use language about patients with sickle cell disease and patients who require opioids as a part of their treatment plan. Your hospitalist group could have a journal club on how bias affects patients and about the best practices in the care of people with sickle cell disease. A next step could be to build a quality improvement project to review the care of patients hospitalized for sickle cell disease or opioid use.
Case 3
You are conducting bedside rounds with your team. Your intern, a person of color, begins to present. The patient interjects by requesting that the intern leave as he “does not want a foreigner taking care” of him.
Commentary: Requests like this can be shocking. The team leader has a responsibility to immediately act to ensure the psychological safety of the team. Ideally, your response should set firm boundaries and expectations that support the learner as a valued and respected clinician and allow the intern to complete the presentation. In this scenario, regardless of the response the patient takes, it is vital to maintain a safe environment for the trainee. It is crucial to debrief with the team immediately after as an exchange of thoughts and emotions in a safe space can allow for everyone to feel welcome. Additionally, this debrief can provide insights to the team leader of how to address similar situations in the future. The opportunity to allow the intern to no longer follow the patient should be offered, and if the intern opts to no longer follow the patient, accommodations should be made.
Approach: “This physician is a member of the medical team, and we are all working together to provide you with the best care. Everyone on this team is an equal. We value diversity of our team members as it allows us to take care of all our patients. We respect you and expect respect for each member of the team. If you feel that you are unable to respect our team members right now, we will leave for now and return later.” To ensure the patient is provided with appropriate care, be sure to debrief with the patient’s nurse.
Conclusion
These scenarios represent some of the many complex interpersonal challenges hospitalists encounter. These approaches are suggestions that are open to improvement as de-escalation of a conflict is a critical and evolving skill and practice.
For more tips on managing conflict, consider reading “Crucial Conversations” by Kerry Patterson and colleagues. These skills can provide the tools we need to recenter ourselves when we are in the midst of these challenging situations.
Dr. Rawal is clinical assistant professor of medicine at the University of Pittsburgh Medical Center. Dr. Ashford is assistant professor and program director in the department of internal medicine/pediatrics at the University of Nebraska Medical Center, Omaha. Dr. Lee and Dr. Barrett are based in the department of internal medicine, University of New Mexico School of Medicine, Albuquerque. This article is sponsored by the SHM Physicians in Training (PIT) committee, which submits quarterly content to The Hospitalist on topics relevant to trainees and early career hospitalists.
Hospital medicine can be a demanding and fast-paced environment where resources are stretched thin, with both clinicians and patients stressed. A hospitalist’s role is dynamic, serving as an advocate, leader, or role model while working with interdisciplinary and diverse teams for the welfare of the patient. This constellation of pressures makes a degree of conflict inevitable.
Often, an unexpected scenario can render the hospitalist uncertain and yet the hospitalist’s response can escalate or deescalate conflict. The multiple roles that a hospitalist represents may buckle to the single role of advocating for themselves, a colleague, or a patient in a tense scenario. When this happens, many hospitalists feel disempowered to respond.
De-escalation is a practical skill that involves being calm, respectful, and open minded toward the other person, while also maintaining boundaries. Here we provide case-based tips and skills that highlight the role for de-escalation.
Questions to ask yourself in midst of conflict:
- How did the problematic behavior make you feel?
- What will be your approach in handling this?
- When should you address this?
- What is the outcome you are hoping to achieve?
- What is the outcome the other person is hoping to achieve?
Case 1
There is a female physician rounding with your team. Introductions were made at the start of a patient encounter. The patient repeatedly calls the female physician by her first name and refers to a male colleague as “doctor.”
Commentary: This scenario is commonly encountered by women who are physicians. They may be mistaken for the nurse, a technician, or a housekeeper. This exacerbates inequality and impostor syndrome as women can feel unheard, undervalued, and not recognized for their expertise and achievements. It can be challenging for a woman to reaffirm herself as she worries that the patient will not respect her or will think that she is being aggressive.
Approach: It is vital to interject by firmly reintroducing the female physician by her correct title. If you are the subject of this scenario, you may interject by firmly reintroducing yourself. If the patient or a colleague continues to refer to her by her first name, it is appropriate to say, “Please call her Dr. XYZ.” There is likely another female colleague or trainee nearby that will view this scenario as a model for setting boundaries.
To prevent similar future situations, consistently refer to all peers by their title in front of patients and peers in all professional settings (such as lectures, luncheons, etc.) to establish this as a cultural norm. Also, utilize hospital badges that clearly display roles in large letters.
Case 2
During sign out from a colleague, the colleague repeatedly refers to a patient hospitalized with sickle cell disease as a “frequent flyer” and “drug seeker,” and then remarks, “you know how these patients are.”
Commentary: A situation like this raises concerns about bias and stereotyping. Everyone has implicit bias. Recognizing and acknowledging when implicit bias affects objectivity in patient care is vital to providing appropriate care. It can be intimidating to broach this subject with a colleague as it may cause the colleague to become defensive and uncomfortable as revealing another person’s bias can be difficult. But physicians owe it to a patient’s wellbeing to remain objective and to prevent future colleagues from providing subpar care as a result.
Approach: In this case, saying, “Sometimes my previous experiences can affect my thinking. Will you explain what behaviors the patient has shown this admission that are concerning to you? This will allow me to grasp the complexity of the situation.” Another strategy is to share that there are new recommendations for how to use language about patients with sickle cell disease and patients who require opioids as a part of their treatment plan. Your hospitalist group could have a journal club on how bias affects patients and about the best practices in the care of people with sickle cell disease. A next step could be to build a quality improvement project to review the care of patients hospitalized for sickle cell disease or opioid use.
Case 3
You are conducting bedside rounds with your team. Your intern, a person of color, begins to present. The patient interjects by requesting that the intern leave as he “does not want a foreigner taking care” of him.
Commentary: Requests like this can be shocking. The team leader has a responsibility to immediately act to ensure the psychological safety of the team. Ideally, your response should set firm boundaries and expectations that support the learner as a valued and respected clinician and allow the intern to complete the presentation. In this scenario, regardless of the response the patient takes, it is vital to maintain a safe environment for the trainee. It is crucial to debrief with the team immediately after as an exchange of thoughts and emotions in a safe space can allow for everyone to feel welcome. Additionally, this debrief can provide insights to the team leader of how to address similar situations in the future. The opportunity to allow the intern to no longer follow the patient should be offered, and if the intern opts to no longer follow the patient, accommodations should be made.
Approach: “This physician is a member of the medical team, and we are all working together to provide you with the best care. Everyone on this team is an equal. We value diversity of our team members as it allows us to take care of all our patients. We respect you and expect respect for each member of the team. If you feel that you are unable to respect our team members right now, we will leave for now and return later.” To ensure the patient is provided with appropriate care, be sure to debrief with the patient’s nurse.
Conclusion
These scenarios represent some of the many complex interpersonal challenges hospitalists encounter. These approaches are suggestions that are open to improvement as de-escalation of a conflict is a critical and evolving skill and practice.
For more tips on managing conflict, consider reading “Crucial Conversations” by Kerry Patterson and colleagues. These skills can provide the tools we need to recenter ourselves when we are in the midst of these challenging situations.
Dr. Rawal is clinical assistant professor of medicine at the University of Pittsburgh Medical Center. Dr. Ashford is assistant professor and program director in the department of internal medicine/pediatrics at the University of Nebraska Medical Center, Omaha. Dr. Lee and Dr. Barrett are based in the department of internal medicine, University of New Mexico School of Medicine, Albuquerque. This article is sponsored by the SHM Physicians in Training (PIT) committee, which submits quarterly content to The Hospitalist on topics relevant to trainees and early career hospitalists.
Racial, other disparities in blood cancer treatment
As compared with White individuals, minorities often face higher barriers to cancer care. Racial and ethnic disparities in patients with solid tumors, particularly those of the prostate and breast, have been well documented. Hematologic malignancies are less common, but an increasing number of studies have documented disparities within this subgroup of cancer, particularly among Black and non-White Hispanics. An increasing armamentarium of therapeutics, including novel chemotherapy agents and targeted molecular, cellular, and immunologic therapies, has highlighted the need for understanding and exploring the differences in care as well as biology, which may lead to disparate outcomes.
Overall, an estimated 186,400 people living in the United States are expected to be diagnosed with leukemia, lymphoma, or myeloma in 2021, and new cases of hematologic malignancies are expected to account for 9.8% of the estimated 1,898,160 new cancer cases diagnosed this year.1
The underlying reasons for disparities are highly complex and multifactorial, and clinicians must consider how the biologic, clinical, demographic, and socioeconomic characteristics of their patients interact. All of these factors can play a role in prognosis and/or access to care.
Disparities in leukemia
Leukemia is a heterogeneous group of diseases affecting both children and adults, but during the past few decades survival rates have steadily improved, particularly among children. Response to therapy and prognosis do vary among leukemia types, but one large analysis reported that there were overall improvements in survival seen across racial/ethnic groups, most age groups, and genders during a 40-year period.2
From 1973 through 2014, survival trends were assessed across four leukemia types: acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), chronic myeloid leukemia (CML), and chronic lymphoid leukemia (CLL). After stratifying survival for each leukemia type by race/ethnicity, improvement rates were not uniform among all groups.
For example, there were substantial improvements of leukemia-specific survival in 2010-2014 among Black (81.0%) and Asian (80.0%) patients with CML, as well as younger patients (20-49 years) with CLL (96.0%). But in contrast, Black patients, those with AML, and individuals over the age of 75 years experienced the lowest improvement in survival.
Studies have found that Hispanics have increased rates of ALL and acute promyelocytic leukemia (APL), but lower rates of AML, as compared to Whites. They also tend to be diagnosed at a younger age and have poorer overall survival.3
Demographics may also play a role, as Hispanics born outside the United States had a higher incidence rate of APL versus U.S.-born Hispanics (incidence rate ratio, 1.79; 1.11-2.94). Thus, the higher incidence rates of increased B-cell ALL may be due to heritable genetic factors, while APL may also be attributable to environmental exposures.4
Hispanics living on the Texas-Mexico border were also found to have a higher incidence of chronic myeloid leukemia (RR, 1.28; 95% CI, 1.07-1.51; P = .02) and acute myeloid leukemia (RR, 1.17; 95% CI, 1.04-1.33; P = .0009) as compared with Hispanics living elsewhere in Texas5 AML and CML were more likely to be observed in patients who resided in this border region, and those with ALL, AML, and CML had worse outcomes compared with Hispanics living elsewhere in Texas. In addition, both Hispanic and non-Hispanic patients along the border have a worse prognosis for ALL than patients in other areas of Texas.
“We don’t yet understand if the differences are due to nonbiologic factors, or if biology plays a role because of the more aggressive disease that we’re seeing,” said study author Anna Eiring, PhD, an assistant professor at Texas Tech University, El Paso. “This is a medically underserved region, and even though we are a safety net hospital, many of the Hispanic patients don’t have health insurance.”
They also tend to have worse socioeconomic status compared with non-Hispanic populations, and there may also be lifestyle and environmental factors. “Exposure to environmental toxins may also play a role, as many work in jobs that could put them at risk,” she said. “Lifestyle factors may also play a role.”
AML is a hematopoietic disorder that is characterized by numerous cytogenetic and molecular aberrations, with poor overall survival. Researchers found that Black patients had shorter survival than White patients, based on an analysis of Surveillance Epidemiology and End Results (SEER) Program data, and performing and performed mutational profiling of 1,339 patients with AML treated on frontline Alliance for Clinical Trials in Oncology (Alliance) protocols.6 The disparity was especially pronounced in Black patients under 60 years old, after adjustment for socioeconomic (SEER) and molecular (Alliance) factors. Black race was an independent prognosticator of poor survival.
“Based on our analyses in Black and White AML patients under the age of 60 years, we believe that a differential impact of molecular aberrations, specifically AML-associated gene mutations, contribute to the observed survival disparities,” said study author Ann-Kathrin Eisfeld, MD, an assistant professor in the division of hematology at the Ohio State University, Columbus, and a member of the leukemia research program at the university’s comprehensive cancer center, the James. “For example, NPM1 mutations seem to lack the known positive prognostic impact we are used to seeing in previous studies with White AML patients.”
She noted that when looking at molecular prognosticators just within Black AML patients, researchers found that FLT3-ITD and also IDH2 mutations were associated with poor overall survival. “While FLT3-ITD is a known adverse prognosticator, the significant impact of IDH2 mutations was surprising to us and is currently being further explored,” said Dr. Eisfeld.
“In general, however, it can’t be highlighted enough that while this study suggests an impact of somatic tumor genomics that needs a lot more attention and investigation and ideally, also prospective studies, structural racism and its impact is still the problem,” she emphasized. “It’s the ‘elephant in the room’ and the major factor that needs to be addressed in order to improve and overcome these survival disparities.”
Disparities in lymphoma
Similar to leukemia, lymphomas are a heterogenous and diverse group of malignancies that range from indolent to highly aggressive. The two main types are listed below:
Non-Hodgkin lymphoma (NHL), the most common subtype, with about 80,000 new cases a year in the United States. There are more than 90 types of NHL, the most common being B-cell lymphomas, which include diffuse large B cell, primary mediastinal B cell, follicular, small lymphocytic lymphoma, and chronic lymphocytic leukemia; marginal zone, mantle zone, and Burkitt lymphomas; and Waldenström macroglobulinemia.
Hodgkin lymphoma (HL), less common than NHL, with about 9,000 people diagnosed every year. There are five types of HL, and it is primarily seen in children and young adults.
Disparities in incidence, age at diagnosis, and overall survival have been observed in lymphoma, which, aside from marginal zone and follicular lymphoma, are more common among men. The incidence of most lymphoma subtypes is generally lower in racial and ethnic minority groups, although Black and Hispanic patients tend to be diagnosed at a younger age, and in Black patients, at a more advanced stage and the lymphomas have higher risk features at initial presentation.7
One study that looked at racial disparities in Hodgkin lymphoma found that HL was significantly more common in Hispanics versus Whites under the age of 65 years. The 5-, 10-, and 15-year overall survival rates were also inferior for Blacks and Hispanics compared with Whites (P less than 0.005 and P less than 0.001, respectively).8
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma in the United States, comprising approximately one-third of lymphomas diagnosed in adults (Lee et al. 2020). In one study that examined ancestry and tumor genomics, recurrent somatic mutations in established driver genes, such as ATM, MGA, SETD2, TET2, DNMT3A, and MLL3, were observed more frequently in patients with African ancestry versus those of European ancestry.9 Other data suggest a variety of disparities in receipt of treatment. For example, patients with localized disease who were Black, uninsured/Medicaid insured, or of lower socioeconomic status were less likely to receive any form of chemotherapy (all P less than 0.0001), and Black race was also associated with being less likely to receive chemoimmunotherapy.
Leveling the field of disparities is complex and requires a multifaceted approach. But one facility found that they could help minority patients overcome some of the hurdles related to nonbiologic factors by the support of a nurse navigator in addition to therapy.10 Their study included 204 patients with DLBCL (47 minority patients and 157 White patients) and following the initiation of the nurse navigator program, virtually all patients received frontline chemotherapy (98% versus 96%). The incidence of relapsed/refractory disease was similar (40% versus 38%) and in the relapsed/refractory population, similar proportions of patients underwent hematopoietic stem cell transplantation (32% versus 29%) or received chimeric antigen receptor T-cell therapy (16% versus 19%). The 2-year overall survival rates were 81% and 76% for minorities and Whites, respectively, and 2-year progression-free survival rates were 62% and 65%, respectively.
“We found that the minority patients often needed more help to get care, and they utilized the nurse navigator more intensively,” said study author Bei Hu, MD, who is with the department of hematologic oncology and blood disorders, Levine Cancer Institute/Atrium Health, Charlotte, N.C. “The nurse navigator was able to help them with things like finances, transportation, and insurance.”
Minorities tended to face more barriers than White patients. “Even something as simple as needing money for gas to get to the clinic can be a barrier to care,” said Dr. Hu. “And many of the patients are often uncomfortable discussing these things with their physician – plus a lot is covered in our appointments and we focus on the cancer. So, they may be more comfortable discussing these issues with the nurse.”
Disparities in multiple myeloma
Multiple myeloma is the malignant clonal proliferation of plasma B cells in the bone marrow and, despite the advent of new therapies, remains incurable and generally fatal. It progresses from the more common but often subclinical precursor states of monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM) to overt and symptomatic multiple myeloma. Racial disparities have been observed in all stages of the disease, and as compared with Whites, individuals who are Black have a higher risk of MGUS and myeloma and a higher mortality rate.11 They have not experienced the same survival gains seen in White patients.
Some research suggests that these disparities may be more related to socioeconomic status as opposed to race. One analysis of 562 patients found that those with higher socioeconomic status had a median overall survival of 62.8 months compared with 53.7 and 48.6 months for middle and low socioeconomic status (P = 0.015).12
After controlling for confounders including race, patients with low socioeconomic status had a 54% increase in mortality rate relative to those with high status. The authors then performed a similar analysis of 45,505 patients with multiple myeloma from the S
“In some homogeneous health systems, such as the VA, we are seeing that Black patients do as well or better than White patients,” said Catherine Marinac, PhD, an assistant professor of medicine, Harvard Medical School, Boston. “Survival is equal or better, as long as treatment is not delayed and they receive the standard of care.”
Black patients generally have a more indolent disease subtype and may experience less aggressive disease, but they have not experienced the same magnitude in survival as White patients following the introduction of new therapeutics. This disparity lends support to the influence of socioeconomic factors, such as unequal access to novel therapies and/or differences in treatment response, and lower rates of autologous stem cell transplantation.13
However, there are racial/ethnic differences in risk for both myeloma and its premalignant conditions, as well as incidence. Blacks have a twofold increased risk of myeloma compared with White individuals and are diagnosed at younger ages. Differences in myeloma incidence is less marked in other racial/ethnic groups, such as Hispanics, where it is only slightly higher than in Whites at 6.7 per 100,00.11 In contrast, the incidence of myeloma is markedly lower in Asians as compared with non-Hispanic Whites (incidence rate of 3.8 versus 6.2 per 100,000). Black persons also have a markedly higher prevalence of MGUS, and these differences suggest that biology, and clinical characteristics, differ by race or ancestry.
“Mortality among Black patients is also higher,” said Dr. Marinac, who is also on the faculty in the division of population sciences at the Dana Farber Cancer Institute, also in Boston. “The higher mortality rate is driven by the higher incidence.”
There are also differences in the prevalence of immunoglobulin isotypes observed across racial/ethnic groups of MGUS patients, Dr. Marinac explained, which is consistent with the hypothesis that there is a biological basis for disparities arising in precursor lesions.
“What we are looking at now is cancer prevention and early intervention,” she said. “There are well-defined precursors to myeloma, and Blacks are three times more likely to have a precursor condition.”
Early detection of precursors followed by preventing progression to full-blown multiple myeloma is one way of addressing disparities, but right now, there are no real screening guidelines. “Most patients now are diagnosed incidentally, and then the only intervention is to monitor them,” Dr. Marinac said. “At Dana Farber, we are now looking to see if we can refine screening, and then see who may need additional monitoring.”
The Promise study, being conducted at Dana Farber, is recruiting participants to examine the molecular changes that occur when precursor conditions develop into full-blown multiple myeloma and is open to individuals considered to be at high risk: Black race and/or have a first-degree relative with multiple myeloma or one of its precursor conditions.
Dr. Marinac also pointed out that there are ongoing clinical trials that are looking at low-risk early interventions in patients with precursor conditions. “We are now looking at lifestyle and metformin,” she said. “The thought is that if we treat them now, we can prevent myeloma from developing.”
Lessening barriers to care
When trying to tease out the strongest/most prominent reasons for the disparities that have been observed in the care of patients with blood cancers, Stephanie Lee, M.D., M.P.H, professor and associate director of the clinical research division at Fred Hutchinson Cancer Research Center, Seattle, thinks that the problem is truly multifactorial.
“Access has been cited many times because some studies show that if access is equitable, sometimes racial/ethnic minorities do the same as non-Hispanic Whites,” she said. “Same thing with quality of care – if all people are treated on clinical trials, sometimes the outcomes are the same.”
That said, many things have to go right to get the best outcomes, and if one factor isn’t optimal, then treatment may never achieve the success that is possible, she noted.
Considering how complex the issue of disparities is, addressing it can seem daunting. Dr. Lee points out that the place to begin is with clinical trials. “I would like to see more studies that test interventions to correct disparities,” said Dr. Lee. “But I have actually seen in my own work that racial and ethnic minorities are less likely to participate in studies, even survey and observational studies where physical risks are low or nonexistent.”
People are studying how to increase minority participation in clinical trials, but thus far, there isn’t one solution. “As with routine care, there are probably a lot of logistical barriers to trial participation that disproportionately affect minority populations,” she noted. “There is also greater distrust of studies.”
But for now, there are some steps that clinicians can take to start to improve these disparities. “I think we can start inquiring about and documenting barriers to care and clinical trial participation, just like we document other aspects of the social history,” Dr. Lee explained. “Truly understanding the problem is the first step toward trying to solve it.”
References
1. Leukemia & Lymphoma Society. 2021. www.lls.org/facts-and-statistics/facts-and-statistics-overview.
2. Utuama O et al. PLoS One. 2019 Aug 19;14(8):e0220864.
3. Pollyea DA et al. J Cancer Prev Curr Res. 2014;1(1):14-19.
4. Bencomo-Alvarez AE et al. Cancer. 2021 Apr 1;127(7):1068-79.
5. Nabhan C et al. Cancer. 2012 Oct 1;118(19):4842-50.
6. Bhatnagar B et al. Blood. 2020;136(Suppl 1):5-7.
7. Shenoy PJ et al. Cancer. 2011;117:2530-40.
8. Evens AM et al. Ann Oncol. 2012 Aug 1;23(8):2128-37.
9. Lee MJ et al. Cancer. 2020;126:3493-3503.
10. Hu B et al. Cancer. 2021 Jul 21. doi: 10.1002/cncr.33779.
11. Marinac CR et al. Blood Cancer J. 2020 Feb 17;10(2):19.
12. Fiala MA et al. Leuk Lymphoma. 2015;56(9):2643-9.
13. Costa LJ et al. Biol Blood Marrow Transplant. 2015 Apr;21(4):701-6.
As compared with White individuals, minorities often face higher barriers to cancer care. Racial and ethnic disparities in patients with solid tumors, particularly those of the prostate and breast, have been well documented. Hematologic malignancies are less common, but an increasing number of studies have documented disparities within this subgroup of cancer, particularly among Black and non-White Hispanics. An increasing armamentarium of therapeutics, including novel chemotherapy agents and targeted molecular, cellular, and immunologic therapies, has highlighted the need for understanding and exploring the differences in care as well as biology, which may lead to disparate outcomes.
Overall, an estimated 186,400 people living in the United States are expected to be diagnosed with leukemia, lymphoma, or myeloma in 2021, and new cases of hematologic malignancies are expected to account for 9.8% of the estimated 1,898,160 new cancer cases diagnosed this year.1
The underlying reasons for disparities are highly complex and multifactorial, and clinicians must consider how the biologic, clinical, demographic, and socioeconomic characteristics of their patients interact. All of these factors can play a role in prognosis and/or access to care.
Disparities in leukemia
Leukemia is a heterogeneous group of diseases affecting both children and adults, but during the past few decades survival rates have steadily improved, particularly among children. Response to therapy and prognosis do vary among leukemia types, but one large analysis reported that there were overall improvements in survival seen across racial/ethnic groups, most age groups, and genders during a 40-year period.2
From 1973 through 2014, survival trends were assessed across four leukemia types: acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), chronic myeloid leukemia (CML), and chronic lymphoid leukemia (CLL). After stratifying survival for each leukemia type by race/ethnicity, improvement rates were not uniform among all groups.
For example, there were substantial improvements of leukemia-specific survival in 2010-2014 among Black (81.0%) and Asian (80.0%) patients with CML, as well as younger patients (20-49 years) with CLL (96.0%). But in contrast, Black patients, those with AML, and individuals over the age of 75 years experienced the lowest improvement in survival.
Studies have found that Hispanics have increased rates of ALL and acute promyelocytic leukemia (APL), but lower rates of AML, as compared to Whites. They also tend to be diagnosed at a younger age and have poorer overall survival.3
Demographics may also play a role, as Hispanics born outside the United States had a higher incidence rate of APL versus U.S.-born Hispanics (incidence rate ratio, 1.79; 1.11-2.94). Thus, the higher incidence rates of increased B-cell ALL may be due to heritable genetic factors, while APL may also be attributable to environmental exposures.4
Hispanics living on the Texas-Mexico border were also found to have a higher incidence of chronic myeloid leukemia (RR, 1.28; 95% CI, 1.07-1.51; P = .02) and acute myeloid leukemia (RR, 1.17; 95% CI, 1.04-1.33; P = .0009) as compared with Hispanics living elsewhere in Texas5 AML and CML were more likely to be observed in patients who resided in this border region, and those with ALL, AML, and CML had worse outcomes compared with Hispanics living elsewhere in Texas. In addition, both Hispanic and non-Hispanic patients along the border have a worse prognosis for ALL than patients in other areas of Texas.
“We don’t yet understand if the differences are due to nonbiologic factors, or if biology plays a role because of the more aggressive disease that we’re seeing,” said study author Anna Eiring, PhD, an assistant professor at Texas Tech University, El Paso. “This is a medically underserved region, and even though we are a safety net hospital, many of the Hispanic patients don’t have health insurance.”
They also tend to have worse socioeconomic status compared with non-Hispanic populations, and there may also be lifestyle and environmental factors. “Exposure to environmental toxins may also play a role, as many work in jobs that could put them at risk,” she said. “Lifestyle factors may also play a role.”
AML is a hematopoietic disorder that is characterized by numerous cytogenetic and molecular aberrations, with poor overall survival. Researchers found that Black patients had shorter survival than White patients, based on an analysis of Surveillance Epidemiology and End Results (SEER) Program data, and performing and performed mutational profiling of 1,339 patients with AML treated on frontline Alliance for Clinical Trials in Oncology (Alliance) protocols.6 The disparity was especially pronounced in Black patients under 60 years old, after adjustment for socioeconomic (SEER) and molecular (Alliance) factors. Black race was an independent prognosticator of poor survival.
“Based on our analyses in Black and White AML patients under the age of 60 years, we believe that a differential impact of molecular aberrations, specifically AML-associated gene mutations, contribute to the observed survival disparities,” said study author Ann-Kathrin Eisfeld, MD, an assistant professor in the division of hematology at the Ohio State University, Columbus, and a member of the leukemia research program at the university’s comprehensive cancer center, the James. “For example, NPM1 mutations seem to lack the known positive prognostic impact we are used to seeing in previous studies with White AML patients.”
She noted that when looking at molecular prognosticators just within Black AML patients, researchers found that FLT3-ITD and also IDH2 mutations were associated with poor overall survival. “While FLT3-ITD is a known adverse prognosticator, the significant impact of IDH2 mutations was surprising to us and is currently being further explored,” said Dr. Eisfeld.
“In general, however, it can’t be highlighted enough that while this study suggests an impact of somatic tumor genomics that needs a lot more attention and investigation and ideally, also prospective studies, structural racism and its impact is still the problem,” she emphasized. “It’s the ‘elephant in the room’ and the major factor that needs to be addressed in order to improve and overcome these survival disparities.”
Disparities in lymphoma
Similar to leukemia, lymphomas are a heterogenous and diverse group of malignancies that range from indolent to highly aggressive. The two main types are listed below:
Non-Hodgkin lymphoma (NHL), the most common subtype, with about 80,000 new cases a year in the United States. There are more than 90 types of NHL, the most common being B-cell lymphomas, which include diffuse large B cell, primary mediastinal B cell, follicular, small lymphocytic lymphoma, and chronic lymphocytic leukemia; marginal zone, mantle zone, and Burkitt lymphomas; and Waldenström macroglobulinemia.
Hodgkin lymphoma (HL), less common than NHL, with about 9,000 people diagnosed every year. There are five types of HL, and it is primarily seen in children and young adults.
Disparities in incidence, age at diagnosis, and overall survival have been observed in lymphoma, which, aside from marginal zone and follicular lymphoma, are more common among men. The incidence of most lymphoma subtypes is generally lower in racial and ethnic minority groups, although Black and Hispanic patients tend to be diagnosed at a younger age, and in Black patients, at a more advanced stage and the lymphomas have higher risk features at initial presentation.7
One study that looked at racial disparities in Hodgkin lymphoma found that HL was significantly more common in Hispanics versus Whites under the age of 65 years. The 5-, 10-, and 15-year overall survival rates were also inferior for Blacks and Hispanics compared with Whites (P less than 0.005 and P less than 0.001, respectively).8
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma in the United States, comprising approximately one-third of lymphomas diagnosed in adults (Lee et al. 2020). In one study that examined ancestry and tumor genomics, recurrent somatic mutations in established driver genes, such as ATM, MGA, SETD2, TET2, DNMT3A, and MLL3, were observed more frequently in patients with African ancestry versus those of European ancestry.9 Other data suggest a variety of disparities in receipt of treatment. For example, patients with localized disease who were Black, uninsured/Medicaid insured, or of lower socioeconomic status were less likely to receive any form of chemotherapy (all P less than 0.0001), and Black race was also associated with being less likely to receive chemoimmunotherapy.
Leveling the field of disparities is complex and requires a multifaceted approach. But one facility found that they could help minority patients overcome some of the hurdles related to nonbiologic factors by the support of a nurse navigator in addition to therapy.10 Their study included 204 patients with DLBCL (47 minority patients and 157 White patients) and following the initiation of the nurse navigator program, virtually all patients received frontline chemotherapy (98% versus 96%). The incidence of relapsed/refractory disease was similar (40% versus 38%) and in the relapsed/refractory population, similar proportions of patients underwent hematopoietic stem cell transplantation (32% versus 29%) or received chimeric antigen receptor T-cell therapy (16% versus 19%). The 2-year overall survival rates were 81% and 76% for minorities and Whites, respectively, and 2-year progression-free survival rates were 62% and 65%, respectively.
“We found that the minority patients often needed more help to get care, and they utilized the nurse navigator more intensively,” said study author Bei Hu, MD, who is with the department of hematologic oncology and blood disorders, Levine Cancer Institute/Atrium Health, Charlotte, N.C. “The nurse navigator was able to help them with things like finances, transportation, and insurance.”
Minorities tended to face more barriers than White patients. “Even something as simple as needing money for gas to get to the clinic can be a barrier to care,” said Dr. Hu. “And many of the patients are often uncomfortable discussing these things with their physician – plus a lot is covered in our appointments and we focus on the cancer. So, they may be more comfortable discussing these issues with the nurse.”
Disparities in multiple myeloma
Multiple myeloma is the malignant clonal proliferation of plasma B cells in the bone marrow and, despite the advent of new therapies, remains incurable and generally fatal. It progresses from the more common but often subclinical precursor states of monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM) to overt and symptomatic multiple myeloma. Racial disparities have been observed in all stages of the disease, and as compared with Whites, individuals who are Black have a higher risk of MGUS and myeloma and a higher mortality rate.11 They have not experienced the same survival gains seen in White patients.
Some research suggests that these disparities may be more related to socioeconomic status as opposed to race. One analysis of 562 patients found that those with higher socioeconomic status had a median overall survival of 62.8 months compared with 53.7 and 48.6 months for middle and low socioeconomic status (P = 0.015).12
After controlling for confounders including race, patients with low socioeconomic status had a 54% increase in mortality rate relative to those with high status. The authors then performed a similar analysis of 45,505 patients with multiple myeloma from the S
“In some homogeneous health systems, such as the VA, we are seeing that Black patients do as well or better than White patients,” said Catherine Marinac, PhD, an assistant professor of medicine, Harvard Medical School, Boston. “Survival is equal or better, as long as treatment is not delayed and they receive the standard of care.”
Black patients generally have a more indolent disease subtype and may experience less aggressive disease, but they have not experienced the same magnitude in survival as White patients following the introduction of new therapeutics. This disparity lends support to the influence of socioeconomic factors, such as unequal access to novel therapies and/or differences in treatment response, and lower rates of autologous stem cell transplantation.13
However, there are racial/ethnic differences in risk for both myeloma and its premalignant conditions, as well as incidence. Blacks have a twofold increased risk of myeloma compared with White individuals and are diagnosed at younger ages. Differences in myeloma incidence is less marked in other racial/ethnic groups, such as Hispanics, where it is only slightly higher than in Whites at 6.7 per 100,00.11 In contrast, the incidence of myeloma is markedly lower in Asians as compared with non-Hispanic Whites (incidence rate of 3.8 versus 6.2 per 100,000). Black persons also have a markedly higher prevalence of MGUS, and these differences suggest that biology, and clinical characteristics, differ by race or ancestry.
“Mortality among Black patients is also higher,” said Dr. Marinac, who is also on the faculty in the division of population sciences at the Dana Farber Cancer Institute, also in Boston. “The higher mortality rate is driven by the higher incidence.”
There are also differences in the prevalence of immunoglobulin isotypes observed across racial/ethnic groups of MGUS patients, Dr. Marinac explained, which is consistent with the hypothesis that there is a biological basis for disparities arising in precursor lesions.
“What we are looking at now is cancer prevention and early intervention,” she said. “There are well-defined precursors to myeloma, and Blacks are three times more likely to have a precursor condition.”
Early detection of precursors followed by preventing progression to full-blown multiple myeloma is one way of addressing disparities, but right now, there are no real screening guidelines. “Most patients now are diagnosed incidentally, and then the only intervention is to monitor them,” Dr. Marinac said. “At Dana Farber, we are now looking to see if we can refine screening, and then see who may need additional monitoring.”
The Promise study, being conducted at Dana Farber, is recruiting participants to examine the molecular changes that occur when precursor conditions develop into full-blown multiple myeloma and is open to individuals considered to be at high risk: Black race and/or have a first-degree relative with multiple myeloma or one of its precursor conditions.
Dr. Marinac also pointed out that there are ongoing clinical trials that are looking at low-risk early interventions in patients with precursor conditions. “We are now looking at lifestyle and metformin,” she said. “The thought is that if we treat them now, we can prevent myeloma from developing.”
Lessening barriers to care
When trying to tease out the strongest/most prominent reasons for the disparities that have been observed in the care of patients with blood cancers, Stephanie Lee, M.D., M.P.H, professor and associate director of the clinical research division at Fred Hutchinson Cancer Research Center, Seattle, thinks that the problem is truly multifactorial.
“Access has been cited many times because some studies show that if access is equitable, sometimes racial/ethnic minorities do the same as non-Hispanic Whites,” she said. “Same thing with quality of care – if all people are treated on clinical trials, sometimes the outcomes are the same.”
That said, many things have to go right to get the best outcomes, and if one factor isn’t optimal, then treatment may never achieve the success that is possible, she noted.
Considering how complex the issue of disparities is, addressing it can seem daunting. Dr. Lee points out that the place to begin is with clinical trials. “I would like to see more studies that test interventions to correct disparities,” said Dr. Lee. “But I have actually seen in my own work that racial and ethnic minorities are less likely to participate in studies, even survey and observational studies where physical risks are low or nonexistent.”
People are studying how to increase minority participation in clinical trials, but thus far, there isn’t one solution. “As with routine care, there are probably a lot of logistical barriers to trial participation that disproportionately affect minority populations,” she noted. “There is also greater distrust of studies.”
But for now, there are some steps that clinicians can take to start to improve these disparities. “I think we can start inquiring about and documenting barriers to care and clinical trial participation, just like we document other aspects of the social history,” Dr. Lee explained. “Truly understanding the problem is the first step toward trying to solve it.”
References
1. Leukemia & Lymphoma Society. 2021. www.lls.org/facts-and-statistics/facts-and-statistics-overview.
2. Utuama O et al. PLoS One. 2019 Aug 19;14(8):e0220864.
3. Pollyea DA et al. J Cancer Prev Curr Res. 2014;1(1):14-19.
4. Bencomo-Alvarez AE et al. Cancer. 2021 Apr 1;127(7):1068-79.
5. Nabhan C et al. Cancer. 2012 Oct 1;118(19):4842-50.
6. Bhatnagar B et al. Blood. 2020;136(Suppl 1):5-7.
7. Shenoy PJ et al. Cancer. 2011;117:2530-40.
8. Evens AM et al. Ann Oncol. 2012 Aug 1;23(8):2128-37.
9. Lee MJ et al. Cancer. 2020;126:3493-3503.
10. Hu B et al. Cancer. 2021 Jul 21. doi: 10.1002/cncr.33779.
11. Marinac CR et al. Blood Cancer J. 2020 Feb 17;10(2):19.
12. Fiala MA et al. Leuk Lymphoma. 2015;56(9):2643-9.
13. Costa LJ et al. Biol Blood Marrow Transplant. 2015 Apr;21(4):701-6.
As compared with White individuals, minorities often face higher barriers to cancer care. Racial and ethnic disparities in patients with solid tumors, particularly those of the prostate and breast, have been well documented. Hematologic malignancies are less common, but an increasing number of studies have documented disparities within this subgroup of cancer, particularly among Black and non-White Hispanics. An increasing armamentarium of therapeutics, including novel chemotherapy agents and targeted molecular, cellular, and immunologic therapies, has highlighted the need for understanding and exploring the differences in care as well as biology, which may lead to disparate outcomes.
Overall, an estimated 186,400 people living in the United States are expected to be diagnosed with leukemia, lymphoma, or myeloma in 2021, and new cases of hematologic malignancies are expected to account for 9.8% of the estimated 1,898,160 new cancer cases diagnosed this year.1
The underlying reasons for disparities are highly complex and multifactorial, and clinicians must consider how the biologic, clinical, demographic, and socioeconomic characteristics of their patients interact. All of these factors can play a role in prognosis and/or access to care.
Disparities in leukemia
Leukemia is a heterogeneous group of diseases affecting both children and adults, but during the past few decades survival rates have steadily improved, particularly among children. Response to therapy and prognosis do vary among leukemia types, but one large analysis reported that there were overall improvements in survival seen across racial/ethnic groups, most age groups, and genders during a 40-year period.2
From 1973 through 2014, survival trends were assessed across four leukemia types: acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), chronic myeloid leukemia (CML), and chronic lymphoid leukemia (CLL). After stratifying survival for each leukemia type by race/ethnicity, improvement rates were not uniform among all groups.
For example, there were substantial improvements of leukemia-specific survival in 2010-2014 among Black (81.0%) and Asian (80.0%) patients with CML, as well as younger patients (20-49 years) with CLL (96.0%). But in contrast, Black patients, those with AML, and individuals over the age of 75 years experienced the lowest improvement in survival.
Studies have found that Hispanics have increased rates of ALL and acute promyelocytic leukemia (APL), but lower rates of AML, as compared to Whites. They also tend to be diagnosed at a younger age and have poorer overall survival.3
Demographics may also play a role, as Hispanics born outside the United States had a higher incidence rate of APL versus U.S.-born Hispanics (incidence rate ratio, 1.79; 1.11-2.94). Thus, the higher incidence rates of increased B-cell ALL may be due to heritable genetic factors, while APL may also be attributable to environmental exposures.4
Hispanics living on the Texas-Mexico border were also found to have a higher incidence of chronic myeloid leukemia (RR, 1.28; 95% CI, 1.07-1.51; P = .02) and acute myeloid leukemia (RR, 1.17; 95% CI, 1.04-1.33; P = .0009) as compared with Hispanics living elsewhere in Texas5 AML and CML were more likely to be observed in patients who resided in this border region, and those with ALL, AML, and CML had worse outcomes compared with Hispanics living elsewhere in Texas. In addition, both Hispanic and non-Hispanic patients along the border have a worse prognosis for ALL than patients in other areas of Texas.
“We don’t yet understand if the differences are due to nonbiologic factors, or if biology plays a role because of the more aggressive disease that we’re seeing,” said study author Anna Eiring, PhD, an assistant professor at Texas Tech University, El Paso. “This is a medically underserved region, and even though we are a safety net hospital, many of the Hispanic patients don’t have health insurance.”
They also tend to have worse socioeconomic status compared with non-Hispanic populations, and there may also be lifestyle and environmental factors. “Exposure to environmental toxins may also play a role, as many work in jobs that could put them at risk,” she said. “Lifestyle factors may also play a role.”
AML is a hematopoietic disorder that is characterized by numerous cytogenetic and molecular aberrations, with poor overall survival. Researchers found that Black patients had shorter survival than White patients, based on an analysis of Surveillance Epidemiology and End Results (SEER) Program data, and performing and performed mutational profiling of 1,339 patients with AML treated on frontline Alliance for Clinical Trials in Oncology (Alliance) protocols.6 The disparity was especially pronounced in Black patients under 60 years old, after adjustment for socioeconomic (SEER) and molecular (Alliance) factors. Black race was an independent prognosticator of poor survival.
“Based on our analyses in Black and White AML patients under the age of 60 years, we believe that a differential impact of molecular aberrations, specifically AML-associated gene mutations, contribute to the observed survival disparities,” said study author Ann-Kathrin Eisfeld, MD, an assistant professor in the division of hematology at the Ohio State University, Columbus, and a member of the leukemia research program at the university’s comprehensive cancer center, the James. “For example, NPM1 mutations seem to lack the known positive prognostic impact we are used to seeing in previous studies with White AML patients.”
She noted that when looking at molecular prognosticators just within Black AML patients, researchers found that FLT3-ITD and also IDH2 mutations were associated with poor overall survival. “While FLT3-ITD is a known adverse prognosticator, the significant impact of IDH2 mutations was surprising to us and is currently being further explored,” said Dr. Eisfeld.
“In general, however, it can’t be highlighted enough that while this study suggests an impact of somatic tumor genomics that needs a lot more attention and investigation and ideally, also prospective studies, structural racism and its impact is still the problem,” she emphasized. “It’s the ‘elephant in the room’ and the major factor that needs to be addressed in order to improve and overcome these survival disparities.”
Disparities in lymphoma
Similar to leukemia, lymphomas are a heterogenous and diverse group of malignancies that range from indolent to highly aggressive. The two main types are listed below:
Non-Hodgkin lymphoma (NHL), the most common subtype, with about 80,000 new cases a year in the United States. There are more than 90 types of NHL, the most common being B-cell lymphomas, which include diffuse large B cell, primary mediastinal B cell, follicular, small lymphocytic lymphoma, and chronic lymphocytic leukemia; marginal zone, mantle zone, and Burkitt lymphomas; and Waldenström macroglobulinemia.
Hodgkin lymphoma (HL), less common than NHL, with about 9,000 people diagnosed every year. There are five types of HL, and it is primarily seen in children and young adults.
Disparities in incidence, age at diagnosis, and overall survival have been observed in lymphoma, which, aside from marginal zone and follicular lymphoma, are more common among men. The incidence of most lymphoma subtypes is generally lower in racial and ethnic minority groups, although Black and Hispanic patients tend to be diagnosed at a younger age, and in Black patients, at a more advanced stage and the lymphomas have higher risk features at initial presentation.7
One study that looked at racial disparities in Hodgkin lymphoma found that HL was significantly more common in Hispanics versus Whites under the age of 65 years. The 5-, 10-, and 15-year overall survival rates were also inferior for Blacks and Hispanics compared with Whites (P less than 0.005 and P less than 0.001, respectively).8
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma in the United States, comprising approximately one-third of lymphomas diagnosed in adults (Lee et al. 2020). In one study that examined ancestry and tumor genomics, recurrent somatic mutations in established driver genes, such as ATM, MGA, SETD2, TET2, DNMT3A, and MLL3, were observed more frequently in patients with African ancestry versus those of European ancestry.9 Other data suggest a variety of disparities in receipt of treatment. For example, patients with localized disease who were Black, uninsured/Medicaid insured, or of lower socioeconomic status were less likely to receive any form of chemotherapy (all P less than 0.0001), and Black race was also associated with being less likely to receive chemoimmunotherapy.
Leveling the field of disparities is complex and requires a multifaceted approach. But one facility found that they could help minority patients overcome some of the hurdles related to nonbiologic factors by the support of a nurse navigator in addition to therapy.10 Their study included 204 patients with DLBCL (47 minority patients and 157 White patients) and following the initiation of the nurse navigator program, virtually all patients received frontline chemotherapy (98% versus 96%). The incidence of relapsed/refractory disease was similar (40% versus 38%) and in the relapsed/refractory population, similar proportions of patients underwent hematopoietic stem cell transplantation (32% versus 29%) or received chimeric antigen receptor T-cell therapy (16% versus 19%). The 2-year overall survival rates were 81% and 76% for minorities and Whites, respectively, and 2-year progression-free survival rates were 62% and 65%, respectively.
“We found that the minority patients often needed more help to get care, and they utilized the nurse navigator more intensively,” said study author Bei Hu, MD, who is with the department of hematologic oncology and blood disorders, Levine Cancer Institute/Atrium Health, Charlotte, N.C. “The nurse navigator was able to help them with things like finances, transportation, and insurance.”
Minorities tended to face more barriers than White patients. “Even something as simple as needing money for gas to get to the clinic can be a barrier to care,” said Dr. Hu. “And many of the patients are often uncomfortable discussing these things with their physician – plus a lot is covered in our appointments and we focus on the cancer. So, they may be more comfortable discussing these issues with the nurse.”
Disparities in multiple myeloma
Multiple myeloma is the malignant clonal proliferation of plasma B cells in the bone marrow and, despite the advent of new therapies, remains incurable and generally fatal. It progresses from the more common but often subclinical precursor states of monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM) to overt and symptomatic multiple myeloma. Racial disparities have been observed in all stages of the disease, and as compared with Whites, individuals who are Black have a higher risk of MGUS and myeloma and a higher mortality rate.11 They have not experienced the same survival gains seen in White patients.
Some research suggests that these disparities may be more related to socioeconomic status as opposed to race. One analysis of 562 patients found that those with higher socioeconomic status had a median overall survival of 62.8 months compared with 53.7 and 48.6 months for middle and low socioeconomic status (P = 0.015).12
After controlling for confounders including race, patients with low socioeconomic status had a 54% increase in mortality rate relative to those with high status. The authors then performed a similar analysis of 45,505 patients with multiple myeloma from the S
“In some homogeneous health systems, such as the VA, we are seeing that Black patients do as well or better than White patients,” said Catherine Marinac, PhD, an assistant professor of medicine, Harvard Medical School, Boston. “Survival is equal or better, as long as treatment is not delayed and they receive the standard of care.”
Black patients generally have a more indolent disease subtype and may experience less aggressive disease, but they have not experienced the same magnitude in survival as White patients following the introduction of new therapeutics. This disparity lends support to the influence of socioeconomic factors, such as unequal access to novel therapies and/or differences in treatment response, and lower rates of autologous stem cell transplantation.13
However, there are racial/ethnic differences in risk for both myeloma and its premalignant conditions, as well as incidence. Blacks have a twofold increased risk of myeloma compared with White individuals and are diagnosed at younger ages. Differences in myeloma incidence is less marked in other racial/ethnic groups, such as Hispanics, where it is only slightly higher than in Whites at 6.7 per 100,00.11 In contrast, the incidence of myeloma is markedly lower in Asians as compared with non-Hispanic Whites (incidence rate of 3.8 versus 6.2 per 100,000). Black persons also have a markedly higher prevalence of MGUS, and these differences suggest that biology, and clinical characteristics, differ by race or ancestry.
“Mortality among Black patients is also higher,” said Dr. Marinac, who is also on the faculty in the division of population sciences at the Dana Farber Cancer Institute, also in Boston. “The higher mortality rate is driven by the higher incidence.”
There are also differences in the prevalence of immunoglobulin isotypes observed across racial/ethnic groups of MGUS patients, Dr. Marinac explained, which is consistent with the hypothesis that there is a biological basis for disparities arising in precursor lesions.
“What we are looking at now is cancer prevention and early intervention,” she said. “There are well-defined precursors to myeloma, and Blacks are three times more likely to have a precursor condition.”
Early detection of precursors followed by preventing progression to full-blown multiple myeloma is one way of addressing disparities, but right now, there are no real screening guidelines. “Most patients now are diagnosed incidentally, and then the only intervention is to monitor them,” Dr. Marinac said. “At Dana Farber, we are now looking to see if we can refine screening, and then see who may need additional monitoring.”
The Promise study, being conducted at Dana Farber, is recruiting participants to examine the molecular changes that occur when precursor conditions develop into full-blown multiple myeloma and is open to individuals considered to be at high risk: Black race and/or have a first-degree relative with multiple myeloma or one of its precursor conditions.
Dr. Marinac also pointed out that there are ongoing clinical trials that are looking at low-risk early interventions in patients with precursor conditions. “We are now looking at lifestyle and metformin,” she said. “The thought is that if we treat them now, we can prevent myeloma from developing.”
Lessening barriers to care
When trying to tease out the strongest/most prominent reasons for the disparities that have been observed in the care of patients with blood cancers, Stephanie Lee, M.D., M.P.H, professor and associate director of the clinical research division at Fred Hutchinson Cancer Research Center, Seattle, thinks that the problem is truly multifactorial.
“Access has been cited many times because some studies show that if access is equitable, sometimes racial/ethnic minorities do the same as non-Hispanic Whites,” she said. “Same thing with quality of care – if all people are treated on clinical trials, sometimes the outcomes are the same.”
That said, many things have to go right to get the best outcomes, and if one factor isn’t optimal, then treatment may never achieve the success that is possible, she noted.
Considering how complex the issue of disparities is, addressing it can seem daunting. Dr. Lee points out that the place to begin is with clinical trials. “I would like to see more studies that test interventions to correct disparities,” said Dr. Lee. “But I have actually seen in my own work that racial and ethnic minorities are less likely to participate in studies, even survey and observational studies where physical risks are low or nonexistent.”
People are studying how to increase minority participation in clinical trials, but thus far, there isn’t one solution. “As with routine care, there are probably a lot of logistical barriers to trial participation that disproportionately affect minority populations,” she noted. “There is also greater distrust of studies.”
But for now, there are some steps that clinicians can take to start to improve these disparities. “I think we can start inquiring about and documenting barriers to care and clinical trial participation, just like we document other aspects of the social history,” Dr. Lee explained. “Truly understanding the problem is the first step toward trying to solve it.”
References
1. Leukemia & Lymphoma Society. 2021. www.lls.org/facts-and-statistics/facts-and-statistics-overview.
2. Utuama O et al. PLoS One. 2019 Aug 19;14(8):e0220864.
3. Pollyea DA et al. J Cancer Prev Curr Res. 2014;1(1):14-19.
4. Bencomo-Alvarez AE et al. Cancer. 2021 Apr 1;127(7):1068-79.
5. Nabhan C et al. Cancer. 2012 Oct 1;118(19):4842-50.
6. Bhatnagar B et al. Blood. 2020;136(Suppl 1):5-7.
7. Shenoy PJ et al. Cancer. 2011;117:2530-40.
8. Evens AM et al. Ann Oncol. 2012 Aug 1;23(8):2128-37.
9. Lee MJ et al. Cancer. 2020;126:3493-3503.
10. Hu B et al. Cancer. 2021 Jul 21. doi: 10.1002/cncr.33779.
11. Marinac CR et al. Blood Cancer J. 2020 Feb 17;10(2):19.
12. Fiala MA et al. Leuk Lymphoma. 2015;56(9):2643-9.
13. Costa LJ et al. Biol Blood Marrow Transplant. 2015 Apr;21(4):701-6.
NORD: Approaching rare cancers through a diversity lens
The National Organization for Rare Disorders (NORD® ) advocates for all rare disease patients, no matter their race, ethnicity, religion, color, national origin, age, disability, sexual orientation, gender identity, etc. Since its inception in 1983, NORD has advocated for marginalized individuals – people living with rare diseases, diagnosed and undiagnosed – who were excluded from conventional clinical care, research, and drug development.
People living with rare diseases often experience a long and arduous journey to diagnosis, due to a dearth of information in medical textbooks, lack of knowledge in the clinical setting, lack of research, and lack of FDA-approved treatments. Furthermore, a substantial amount of research has shown inequity in access to and quality of health care for marginalized groups, especially Black, Brown, indigenous and people of color (BIPOC). The barriers to be accurately diagnosed and provided quality care by specialists already poses threats to quality and length of life of the community at large, but the additional barriers faced by BIPOC communities have deadly consequences due to the lack of access to culturally proficient health care and access to rare disease specialists, in addition to socioeconomic considerations (i.e., insurance access and medical literacy).
In addition to further delayed diagnosis and inequities in care, people of color are consistently underrepresented in clinical trials and registries, resulting in a lack of diversity in clinical studies and some mystery in how effective therapies will be across diverse populations of patients. Because many rare diseases are genetic and certain genetic conditions disproportionately affect communities of color, having a vast majority of white participants creates significant knowledge gaps that can affect patient care and drug development and effectiveness.
Unfortunately, when looking at the rare cancer population within the rare disease community, the same problems persist. Of the approximately 7,000 known rare diseases, 1 more than 500 are rare cancers, 2 and combined, all rare cancers account for slightly more than one out of ever four cancer diagnoses each year and one out of every four cancer-related deaths. 3 Black people have the highest death rate and shortest survival of any racial/ethnic group in the U.S. for most cancers, and Black men have the highest cancer incidence rate. 4 NORD and the NORD Rare Cancer Coalition™, including 27 rare cancer member organizations, are committed to shining a light on the causes of these inequities for rare cancer patients, including but not limited to systemic racism, economic disparities, cultural differences, and issues concerning access to quality health care and inclusive research.
To raise awareness of rare cancers and the issues rare cancer patients face throughout the diagnostic odyssey, in seeking and receiving specialized care and in advocating for awareness, and increased research and drug development, NORD and the NORD Rare Cancer Coalition spearheaded Rare Cancer Day, observed annually on September 30. Through the universal hashtag campaign #RareCancerDay and our social media toolkit of infographics and messaging, NORD brings together the global community of advocates to promote awareness of rare cancers and provide opportunities to educate patients, caregivers, clinicians, and researchers. NORD hosted a free webinar for the rare disease community, Rare Cancers: Breaking Down Barriers to Diagnosis, Treatment and Research, to explore rare cancer challenges and offer insights to assist those who are impacted. Throughout August and September, NORD highlighted the powerful, important, and inspiring stories of the rare cancer community on the NORD blog.
In addition, the 2021 NORD Rare Diseases + Orphan Products Breakthrough Summit, held October 18 and 19, featured a breakout session and a follow-up discussion group on Advancing Rare Cancer Awareness & Education Among Healthcare Professionals. These sessions explored educational gaps and approaches for increasing awareness and delivering quality education for healthcare professionals in optimizing care for rare cancer patients, genomic testing, personalized medicine, and collaboration with researchers and patient advocacy groups.
This issue of Rare Diseases Report: Cancers helps us further our mission to foster the identification, treatment, and cure of rare disorders through programs of education, advocacy, research, and patient services, as well as the work of NORD’s Rare Cancer Coalition™ which aims to unite NORD member organizations working in rare cancers to collaborate on issues facing the greater rare cancer community.
NORD remains steadfastly committed to advocating for all rare disease patients, no matter their race, ethnicity, religion, color, national origin, age, disability, sexual orientation, gender identity, parental status, marital status, political affiliation, gender expression, mental illness, socioeconomic status or background, neuro(a)typicality, or physical appearance. NORD’s work includes advocating for rare cancer patients, raising awareness of rare cancer patients, sharing the stories of people living with rare cancers, and educating patients, caregivers and healthcare professionals about accurate diagnosis, quality care, advancements in research, and available treatment options. Learn more at rarediseases.org.
Rebecca Aune
Director of Education Programs
Debbie Drell
Director of Membership
References
1. Genetic and Rare Diseases Information Center; National Center for Advancing Translational Sciences; FAQs About Rare Diseases; 11/30/2017. https://rarediseases.info.nih.gov/diseases/pages/31/faqs-about-rare-diseases
2. Genetic and Rare Diseases Information Center; National Center for Advancing Translational Sciences; Rare Cancers; 1/25/2019. https://www.youtube.com/watch?v=ES5KylRT1qY, https://rarediseases.info.nih.gov/diseases/diseases-by-category/1/rare-cancers, or https://rarediseases.info.nih.gov/diseases/diseases-by-category/1/rare-cancers
3. NIH National Cancer Institute. Rare Cancer Statistics | Did You Know? [Video]. Youtube. https://www.youtube.com/watch?v=ES5KylRT1qY&t=155s. Published April 5, 2018. Accessed Oct. 20, 2021.
4. American Cancer Society. Cancer Facts; Figures for African Americans 2019-2021. Atlanta: American Cancer Society, 2019. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-facts-and-figures-for-african-americans/cancer-facts-and-figures-for-african-americans-2019-2021.pdf1.
The National Organization for Rare Disorders (NORD® ) advocates for all rare disease patients, no matter their race, ethnicity, religion, color, national origin, age, disability, sexual orientation, gender identity, etc. Since its inception in 1983, NORD has advocated for marginalized individuals – people living with rare diseases, diagnosed and undiagnosed – who were excluded from conventional clinical care, research, and drug development.
People living with rare diseases often experience a long and arduous journey to diagnosis, due to a dearth of information in medical textbooks, lack of knowledge in the clinical setting, lack of research, and lack of FDA-approved treatments. Furthermore, a substantial amount of research has shown inequity in access to and quality of health care for marginalized groups, especially Black, Brown, indigenous and people of color (BIPOC). The barriers to be accurately diagnosed and provided quality care by specialists already poses threats to quality and length of life of the community at large, but the additional barriers faced by BIPOC communities have deadly consequences due to the lack of access to culturally proficient health care and access to rare disease specialists, in addition to socioeconomic considerations (i.e., insurance access and medical literacy).
In addition to further delayed diagnosis and inequities in care, people of color are consistently underrepresented in clinical trials and registries, resulting in a lack of diversity in clinical studies and some mystery in how effective therapies will be across diverse populations of patients. Because many rare diseases are genetic and certain genetic conditions disproportionately affect communities of color, having a vast majority of white participants creates significant knowledge gaps that can affect patient care and drug development and effectiveness.
Unfortunately, when looking at the rare cancer population within the rare disease community, the same problems persist. Of the approximately 7,000 known rare diseases, 1 more than 500 are rare cancers, 2 and combined, all rare cancers account for slightly more than one out of ever four cancer diagnoses each year and one out of every four cancer-related deaths. 3 Black people have the highest death rate and shortest survival of any racial/ethnic group in the U.S. for most cancers, and Black men have the highest cancer incidence rate. 4 NORD and the NORD Rare Cancer Coalition™, including 27 rare cancer member organizations, are committed to shining a light on the causes of these inequities for rare cancer patients, including but not limited to systemic racism, economic disparities, cultural differences, and issues concerning access to quality health care and inclusive research.
To raise awareness of rare cancers and the issues rare cancer patients face throughout the diagnostic odyssey, in seeking and receiving specialized care and in advocating for awareness, and increased research and drug development, NORD and the NORD Rare Cancer Coalition spearheaded Rare Cancer Day, observed annually on September 30. Through the universal hashtag campaign #RareCancerDay and our social media toolkit of infographics and messaging, NORD brings together the global community of advocates to promote awareness of rare cancers and provide opportunities to educate patients, caregivers, clinicians, and researchers. NORD hosted a free webinar for the rare disease community, Rare Cancers: Breaking Down Barriers to Diagnosis, Treatment and Research, to explore rare cancer challenges and offer insights to assist those who are impacted. Throughout August and September, NORD highlighted the powerful, important, and inspiring stories of the rare cancer community on the NORD blog.
In addition, the 2021 NORD Rare Diseases + Orphan Products Breakthrough Summit, held October 18 and 19, featured a breakout session and a follow-up discussion group on Advancing Rare Cancer Awareness & Education Among Healthcare Professionals. These sessions explored educational gaps and approaches for increasing awareness and delivering quality education for healthcare professionals in optimizing care for rare cancer patients, genomic testing, personalized medicine, and collaboration with researchers and patient advocacy groups.
This issue of Rare Diseases Report: Cancers helps us further our mission to foster the identification, treatment, and cure of rare disorders through programs of education, advocacy, research, and patient services, as well as the work of NORD’s Rare Cancer Coalition™ which aims to unite NORD member organizations working in rare cancers to collaborate on issues facing the greater rare cancer community.
NORD remains steadfastly committed to advocating for all rare disease patients, no matter their race, ethnicity, religion, color, national origin, age, disability, sexual orientation, gender identity, parental status, marital status, political affiliation, gender expression, mental illness, socioeconomic status or background, neuro(a)typicality, or physical appearance. NORD’s work includes advocating for rare cancer patients, raising awareness of rare cancer patients, sharing the stories of people living with rare cancers, and educating patients, caregivers and healthcare professionals about accurate diagnosis, quality care, advancements in research, and available treatment options. Learn more at rarediseases.org.
Rebecca Aune
Director of Education Programs
Debbie Drell
Director of Membership
References
1. Genetic and Rare Diseases Information Center; National Center for Advancing Translational Sciences; FAQs About Rare Diseases; 11/30/2017. https://rarediseases.info.nih.gov/diseases/pages/31/faqs-about-rare-diseases
2. Genetic and Rare Diseases Information Center; National Center for Advancing Translational Sciences; Rare Cancers; 1/25/2019. https://www.youtube.com/watch?v=ES5KylRT1qY, https://rarediseases.info.nih.gov/diseases/diseases-by-category/1/rare-cancers, or https://rarediseases.info.nih.gov/diseases/diseases-by-category/1/rare-cancers
3. NIH National Cancer Institute. Rare Cancer Statistics | Did You Know? [Video]. Youtube. https://www.youtube.com/watch?v=ES5KylRT1qY&t=155s. Published April 5, 2018. Accessed Oct. 20, 2021.
4. American Cancer Society. Cancer Facts; Figures for African Americans 2019-2021. Atlanta: American Cancer Society, 2019. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-facts-and-figures-for-african-americans/cancer-facts-and-figures-for-african-americans-2019-2021.pdf1.
The National Organization for Rare Disorders (NORD® ) advocates for all rare disease patients, no matter their race, ethnicity, religion, color, national origin, age, disability, sexual orientation, gender identity, etc. Since its inception in 1983, NORD has advocated for marginalized individuals – people living with rare diseases, diagnosed and undiagnosed – who were excluded from conventional clinical care, research, and drug development.
People living with rare diseases often experience a long and arduous journey to diagnosis, due to a dearth of information in medical textbooks, lack of knowledge in the clinical setting, lack of research, and lack of FDA-approved treatments. Furthermore, a substantial amount of research has shown inequity in access to and quality of health care for marginalized groups, especially Black, Brown, indigenous and people of color (BIPOC). The barriers to be accurately diagnosed and provided quality care by specialists already poses threats to quality and length of life of the community at large, but the additional barriers faced by BIPOC communities have deadly consequences due to the lack of access to culturally proficient health care and access to rare disease specialists, in addition to socioeconomic considerations (i.e., insurance access and medical literacy).
In addition to further delayed diagnosis and inequities in care, people of color are consistently underrepresented in clinical trials and registries, resulting in a lack of diversity in clinical studies and some mystery in how effective therapies will be across diverse populations of patients. Because many rare diseases are genetic and certain genetic conditions disproportionately affect communities of color, having a vast majority of white participants creates significant knowledge gaps that can affect patient care and drug development and effectiveness.
Unfortunately, when looking at the rare cancer population within the rare disease community, the same problems persist. Of the approximately 7,000 known rare diseases, 1 more than 500 are rare cancers, 2 and combined, all rare cancers account for slightly more than one out of ever four cancer diagnoses each year and one out of every four cancer-related deaths. 3 Black people have the highest death rate and shortest survival of any racial/ethnic group in the U.S. for most cancers, and Black men have the highest cancer incidence rate. 4 NORD and the NORD Rare Cancer Coalition™, including 27 rare cancer member organizations, are committed to shining a light on the causes of these inequities for rare cancer patients, including but not limited to systemic racism, economic disparities, cultural differences, and issues concerning access to quality health care and inclusive research.
To raise awareness of rare cancers and the issues rare cancer patients face throughout the diagnostic odyssey, in seeking and receiving specialized care and in advocating for awareness, and increased research and drug development, NORD and the NORD Rare Cancer Coalition spearheaded Rare Cancer Day, observed annually on September 30. Through the universal hashtag campaign #RareCancerDay and our social media toolkit of infographics and messaging, NORD brings together the global community of advocates to promote awareness of rare cancers and provide opportunities to educate patients, caregivers, clinicians, and researchers. NORD hosted a free webinar for the rare disease community, Rare Cancers: Breaking Down Barriers to Diagnosis, Treatment and Research, to explore rare cancer challenges and offer insights to assist those who are impacted. Throughout August and September, NORD highlighted the powerful, important, and inspiring stories of the rare cancer community on the NORD blog.
In addition, the 2021 NORD Rare Diseases + Orphan Products Breakthrough Summit, held October 18 and 19, featured a breakout session and a follow-up discussion group on Advancing Rare Cancer Awareness & Education Among Healthcare Professionals. These sessions explored educational gaps and approaches for increasing awareness and delivering quality education for healthcare professionals in optimizing care for rare cancer patients, genomic testing, personalized medicine, and collaboration with researchers and patient advocacy groups.
This issue of Rare Diseases Report: Cancers helps us further our mission to foster the identification, treatment, and cure of rare disorders through programs of education, advocacy, research, and patient services, as well as the work of NORD’s Rare Cancer Coalition™ which aims to unite NORD member organizations working in rare cancers to collaborate on issues facing the greater rare cancer community.
NORD remains steadfastly committed to advocating for all rare disease patients, no matter their race, ethnicity, religion, color, national origin, age, disability, sexual orientation, gender identity, parental status, marital status, political affiliation, gender expression, mental illness, socioeconomic status or background, neuro(a)typicality, or physical appearance. NORD’s work includes advocating for rare cancer patients, raising awareness of rare cancer patients, sharing the stories of people living with rare cancers, and educating patients, caregivers and healthcare professionals about accurate diagnosis, quality care, advancements in research, and available treatment options. Learn more at rarediseases.org.
Rebecca Aune
Director of Education Programs
Debbie Drell
Director of Membership
References
1. Genetic and Rare Diseases Information Center; National Center for Advancing Translational Sciences; FAQs About Rare Diseases; 11/30/2017. https://rarediseases.info.nih.gov/diseases/pages/31/faqs-about-rare-diseases
2. Genetic and Rare Diseases Information Center; National Center for Advancing Translational Sciences; Rare Cancers; 1/25/2019. https://www.youtube.com/watch?v=ES5KylRT1qY, https://rarediseases.info.nih.gov/diseases/diseases-by-category/1/rare-cancers, or https://rarediseases.info.nih.gov/diseases/diseases-by-category/1/rare-cancers
3. NIH National Cancer Institute. Rare Cancer Statistics | Did You Know? [Video]. Youtube. https://www.youtube.com/watch?v=ES5KylRT1qY&t=155s. Published April 5, 2018. Accessed Oct. 20, 2021.
4. American Cancer Society. Cancer Facts; Figures for African Americans 2019-2021. Atlanta: American Cancer Society, 2019. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-facts-and-figures-for-african-americans/cancer-facts-and-figures-for-african-americans-2019-2021.pdf1.
LGBTQ health care: There is reason to be hopeful
I write a lot about watershed moments in my career, things that proved to be moments of tremendous growth, as a person and as a doctor.
One of these occurred early in my career when I met a new patient with ovarian cancer. When I walked into the exam room, I made eye contact with the woman who was accompanied by a man. I assumed they were married, so I went to her first. I introduced myself, stating that I was here to talk about how best to treat her cancer. She stopped me quickly. “Doctor, I am not the patient,” she said. “He is.”
It was the first time I had cared for a transgender man with ovarian cancer. I recall how awkward the following moments were – for all of us. It was the first time I realized that cancer does not have a gender. Men can get breast cancer. Trans women can get prostate cancer. Trans men can get ovarian cancer.
But even many years later, we are not much further along in how prepared we are as a medical community to care for LGBTQ persons. Lesbian, gay, bisexual, transgender, and queer people are not part of the normal medical school curriculum. For most medical students, LGBTQ health is still approached as an aside – perhaps during an infectious disease clerkship, while learning about STDs and HIV. Students do not learn how to approach the male couple seeking to become parents, STD risk reduction for gays and lesbians, or the trans man with ovarian cancer.
But they should, particularly in light of a 2015 study evaluating bias among U.S. medical students. The analysis found that about 45% of medical students exhibited explicit bias against LGBTQ individuals and 8 in 10 held an implicit bias. Fewer than 20% showed no evidence of bias. This lack of preparedness to treat LGBTQ individuals against a backdrop of bias in the medical community often leads patients to mistrust medicine.
To gain perspective outside of oncology, I spoke to Michelle Forcier (she/they), MD, MPH, assistant dean of admissions and professor of pediatrics at Brown University, Providence, R.I. Dr. Forcier agreed that “LGBTQ/rainbow health has been harmfully treated by the system, by both intention and by ignorance.”
“I have had patients who report that EMTs have tried to look under their clothes to determine their gender and transgender patients who have asked point blank to show a provider the results of gender reassignment surgery, not because it was relevant to the issue at hand, but purely out of curiosity,” Dr. Forcier continued. “Then there are the patients who are addressed by the name on their legal record rather than the name that reflects their actual lived experience and identity. These experiences foster this anticipation that is pervasive in this community, that something will be said or done that doesn’t fit who they are, and that ultimately will out them as ‘other.’ ”
I have also felt this sense of being “other” – something I thought I would be immune to as a physician. I have been asked on multiple occasions what my wife does for a living. Moments like this are always awkward. I’m either forced to come out of the closet yet again, or answer vaguely, as if I should be ashamed of my sexuality.
So, how can we move toward equity? Dr. Forcier explained how she lays the groundwork early. “I love pediatrics because kids know when you are being authentic,” she said. “I say who I am, I use she/they pronouns. I also teach by example. If there are more than just my patient in a room, I say, ‘Let’s go around the room and introduce ourselves’ so all have a chance to tell me who they are and how they have come together. If it’s not clear to me, sometimes I prod: ‘How are you here to support [the patient]?’ ”
The point, according to Dr. Forcier: Don’t make assumptions about relationships when you walk into a room with more than one person. Don’t even make assumptions about who the patient is.
But bringing up gender and sexuality can be awkward. Even I sometimes have a hard time. In oncology, patients are there to talk about their cancer and what can be done about it.
“I think it’s really about how it’s framed,” Dr. Forcier said. “In pediatrics, I might start by prefacing it with ‘I am going to ask you some personal questions, and it might seem invasive, but it’s important for your health care. How do you see yourself in the world? What gender identity fits you the best? Who are you attracted to?’ And then I shut up. Doctors need to learn how to stop and wait, provide the space to answer.”
I can see why understanding our patients more deeply is important. We treat people with cancer, not cancer people. As such, understanding someone more fully includes being cognizant of how they identify.
“I am continuously inspired by my LGBTQ patients who have fought to realize who they are and become their truer selves,” Dr. Forcier said. “They know who they are, and they know what they need. They have learned to demand it, to demand that their rights be respected – both civil and human rights.”
As we look toward a future in medicine where diversity, equity, and inclusion have gained prominence and urgency, I think there is reason to be hopeful. In oncology, one institutional study published in 2017 found that, although only about a third of practicing clinicians surveyed were comfortable treating LGBTQ patients, 92% of them acknowledged our unique needs, 78% wanted more education on how to appropriately care for our community, and 64% wanted to be listed as an LGBTQ-friendly provider.
“As an optimist, I believe that those struggling with homophobia/transphobia are open to doing things better,” Dr. Forcier said. “After all, we all strive to be better doctors. Whether explicit or implicit bias is at play, turning moments where colleagues are being inappropriate and showing them an alternative, more inclusive way to handle things is one mechanism to educate, rather than to shame. The bottom line is simple: You don’t have to be perfect. You just have to try.”
Dr. Dizon is the director of women’s cancers at Lifespan Cancer Institute and director of medical oncology at Rhode Island Hospital, both in Providence. He is also a professor of medicine at Brown University. His research interests are in novel treatments of women’s cancers and issues related to survivorship, particularly as they relate to sexual health after cancer for both men and women.
A version of this article first appeared on Medscape.com.
I write a lot about watershed moments in my career, things that proved to be moments of tremendous growth, as a person and as a doctor.
One of these occurred early in my career when I met a new patient with ovarian cancer. When I walked into the exam room, I made eye contact with the woman who was accompanied by a man. I assumed they were married, so I went to her first. I introduced myself, stating that I was here to talk about how best to treat her cancer. She stopped me quickly. “Doctor, I am not the patient,” she said. “He is.”
It was the first time I had cared for a transgender man with ovarian cancer. I recall how awkward the following moments were – for all of us. It was the first time I realized that cancer does not have a gender. Men can get breast cancer. Trans women can get prostate cancer. Trans men can get ovarian cancer.
But even many years later, we are not much further along in how prepared we are as a medical community to care for LGBTQ persons. Lesbian, gay, bisexual, transgender, and queer people are not part of the normal medical school curriculum. For most medical students, LGBTQ health is still approached as an aside – perhaps during an infectious disease clerkship, while learning about STDs and HIV. Students do not learn how to approach the male couple seeking to become parents, STD risk reduction for gays and lesbians, or the trans man with ovarian cancer.
But they should, particularly in light of a 2015 study evaluating bias among U.S. medical students. The analysis found that about 45% of medical students exhibited explicit bias against LGBTQ individuals and 8 in 10 held an implicit bias. Fewer than 20% showed no evidence of bias. This lack of preparedness to treat LGBTQ individuals against a backdrop of bias in the medical community often leads patients to mistrust medicine.
To gain perspective outside of oncology, I spoke to Michelle Forcier (she/they), MD, MPH, assistant dean of admissions and professor of pediatrics at Brown University, Providence, R.I. Dr. Forcier agreed that “LGBTQ/rainbow health has been harmfully treated by the system, by both intention and by ignorance.”
“I have had patients who report that EMTs have tried to look under their clothes to determine their gender and transgender patients who have asked point blank to show a provider the results of gender reassignment surgery, not because it was relevant to the issue at hand, but purely out of curiosity,” Dr. Forcier continued. “Then there are the patients who are addressed by the name on their legal record rather than the name that reflects their actual lived experience and identity. These experiences foster this anticipation that is pervasive in this community, that something will be said or done that doesn’t fit who they are, and that ultimately will out them as ‘other.’ ”
I have also felt this sense of being “other” – something I thought I would be immune to as a physician. I have been asked on multiple occasions what my wife does for a living. Moments like this are always awkward. I’m either forced to come out of the closet yet again, or answer vaguely, as if I should be ashamed of my sexuality.
So, how can we move toward equity? Dr. Forcier explained how she lays the groundwork early. “I love pediatrics because kids know when you are being authentic,” she said. “I say who I am, I use she/they pronouns. I also teach by example. If there are more than just my patient in a room, I say, ‘Let’s go around the room and introduce ourselves’ so all have a chance to tell me who they are and how they have come together. If it’s not clear to me, sometimes I prod: ‘How are you here to support [the patient]?’ ”
The point, according to Dr. Forcier: Don’t make assumptions about relationships when you walk into a room with more than one person. Don’t even make assumptions about who the patient is.
But bringing up gender and sexuality can be awkward. Even I sometimes have a hard time. In oncology, patients are there to talk about their cancer and what can be done about it.
“I think it’s really about how it’s framed,” Dr. Forcier said. “In pediatrics, I might start by prefacing it with ‘I am going to ask you some personal questions, and it might seem invasive, but it’s important for your health care. How do you see yourself in the world? What gender identity fits you the best? Who are you attracted to?’ And then I shut up. Doctors need to learn how to stop and wait, provide the space to answer.”
I can see why understanding our patients more deeply is important. We treat people with cancer, not cancer people. As such, understanding someone more fully includes being cognizant of how they identify.
“I am continuously inspired by my LGBTQ patients who have fought to realize who they are and become their truer selves,” Dr. Forcier said. “They know who they are, and they know what they need. They have learned to demand it, to demand that their rights be respected – both civil and human rights.”
As we look toward a future in medicine where diversity, equity, and inclusion have gained prominence and urgency, I think there is reason to be hopeful. In oncology, one institutional study published in 2017 found that, although only about a third of practicing clinicians surveyed were comfortable treating LGBTQ patients, 92% of them acknowledged our unique needs, 78% wanted more education on how to appropriately care for our community, and 64% wanted to be listed as an LGBTQ-friendly provider.
“As an optimist, I believe that those struggling with homophobia/transphobia are open to doing things better,” Dr. Forcier said. “After all, we all strive to be better doctors. Whether explicit or implicit bias is at play, turning moments where colleagues are being inappropriate and showing them an alternative, more inclusive way to handle things is one mechanism to educate, rather than to shame. The bottom line is simple: You don’t have to be perfect. You just have to try.”
Dr. Dizon is the director of women’s cancers at Lifespan Cancer Institute and director of medical oncology at Rhode Island Hospital, both in Providence. He is also a professor of medicine at Brown University. His research interests are in novel treatments of women’s cancers and issues related to survivorship, particularly as they relate to sexual health after cancer for both men and women.
A version of this article first appeared on Medscape.com.
I write a lot about watershed moments in my career, things that proved to be moments of tremendous growth, as a person and as a doctor.
One of these occurred early in my career when I met a new patient with ovarian cancer. When I walked into the exam room, I made eye contact with the woman who was accompanied by a man. I assumed they were married, so I went to her first. I introduced myself, stating that I was here to talk about how best to treat her cancer. She stopped me quickly. “Doctor, I am not the patient,” she said. “He is.”
It was the first time I had cared for a transgender man with ovarian cancer. I recall how awkward the following moments were – for all of us. It was the first time I realized that cancer does not have a gender. Men can get breast cancer. Trans women can get prostate cancer. Trans men can get ovarian cancer.
But even many years later, we are not much further along in how prepared we are as a medical community to care for LGBTQ persons. Lesbian, gay, bisexual, transgender, and queer people are not part of the normal medical school curriculum. For most medical students, LGBTQ health is still approached as an aside – perhaps during an infectious disease clerkship, while learning about STDs and HIV. Students do not learn how to approach the male couple seeking to become parents, STD risk reduction for gays and lesbians, or the trans man with ovarian cancer.
But they should, particularly in light of a 2015 study evaluating bias among U.S. medical students. The analysis found that about 45% of medical students exhibited explicit bias against LGBTQ individuals and 8 in 10 held an implicit bias. Fewer than 20% showed no evidence of bias. This lack of preparedness to treat LGBTQ individuals against a backdrop of bias in the medical community often leads patients to mistrust medicine.
To gain perspective outside of oncology, I spoke to Michelle Forcier (she/they), MD, MPH, assistant dean of admissions and professor of pediatrics at Brown University, Providence, R.I. Dr. Forcier agreed that “LGBTQ/rainbow health has been harmfully treated by the system, by both intention and by ignorance.”
“I have had patients who report that EMTs have tried to look under their clothes to determine their gender and transgender patients who have asked point blank to show a provider the results of gender reassignment surgery, not because it was relevant to the issue at hand, but purely out of curiosity,” Dr. Forcier continued. “Then there are the patients who are addressed by the name on their legal record rather than the name that reflects their actual lived experience and identity. These experiences foster this anticipation that is pervasive in this community, that something will be said or done that doesn’t fit who they are, and that ultimately will out them as ‘other.’ ”
I have also felt this sense of being “other” – something I thought I would be immune to as a physician. I have been asked on multiple occasions what my wife does for a living. Moments like this are always awkward. I’m either forced to come out of the closet yet again, or answer vaguely, as if I should be ashamed of my sexuality.
So, how can we move toward equity? Dr. Forcier explained how she lays the groundwork early. “I love pediatrics because kids know when you are being authentic,” she said. “I say who I am, I use she/they pronouns. I also teach by example. If there are more than just my patient in a room, I say, ‘Let’s go around the room and introduce ourselves’ so all have a chance to tell me who they are and how they have come together. If it’s not clear to me, sometimes I prod: ‘How are you here to support [the patient]?’ ”
The point, according to Dr. Forcier: Don’t make assumptions about relationships when you walk into a room with more than one person. Don’t even make assumptions about who the patient is.
But bringing up gender and sexuality can be awkward. Even I sometimes have a hard time. In oncology, patients are there to talk about their cancer and what can be done about it.
“I think it’s really about how it’s framed,” Dr. Forcier said. “In pediatrics, I might start by prefacing it with ‘I am going to ask you some personal questions, and it might seem invasive, but it’s important for your health care. How do you see yourself in the world? What gender identity fits you the best? Who are you attracted to?’ And then I shut up. Doctors need to learn how to stop and wait, provide the space to answer.”
I can see why understanding our patients more deeply is important. We treat people with cancer, not cancer people. As such, understanding someone more fully includes being cognizant of how they identify.
“I am continuously inspired by my LGBTQ patients who have fought to realize who they are and become their truer selves,” Dr. Forcier said. “They know who they are, and they know what they need. They have learned to demand it, to demand that their rights be respected – both civil and human rights.”
As we look toward a future in medicine where diversity, equity, and inclusion have gained prominence and urgency, I think there is reason to be hopeful. In oncology, one institutional study published in 2017 found that, although only about a third of practicing clinicians surveyed were comfortable treating LGBTQ patients, 92% of them acknowledged our unique needs, 78% wanted more education on how to appropriately care for our community, and 64% wanted to be listed as an LGBTQ-friendly provider.
“As an optimist, I believe that those struggling with homophobia/transphobia are open to doing things better,” Dr. Forcier said. “After all, we all strive to be better doctors. Whether explicit or implicit bias is at play, turning moments where colleagues are being inappropriate and showing them an alternative, more inclusive way to handle things is one mechanism to educate, rather than to shame. The bottom line is simple: You don’t have to be perfect. You just have to try.”
Dr. Dizon is the director of women’s cancers at Lifespan Cancer Institute and director of medical oncology at Rhode Island Hospital, both in Providence. He is also a professor of medicine at Brown University. His research interests are in novel treatments of women’s cancers and issues related to survivorship, particularly as they relate to sexual health after cancer for both men and women.
A version of this article first appeared on Medscape.com.