User login
More tools for the COVID toolbox
I was recently asked to see a 16-year-old, unvaccinated (against COVID-19) adolescent with hypothyroidism and obesity (body mass index 37 kg/m2) seen in the pediatric emergency department with tachycardia, O2 saturation 96%, urinary tract infection, poor appetite, and nausea. Her chest x-ray had low lung volumes but no infiltrates. She was noted to be dehydrated. Testing for COVID-19 was PCR positive.1
She was observed overnight, tolerated oral rehydration, and was being readied for discharge. Pediatric Infectious Diseases was called about prescribing remdesivir.
Remdesivir was not indicated as its current use is limited to inpatients with oxygen desaturations less than 94%. Infectious Diseases Society of America guidelines do recommend the use of monoclonal antibodies against the SARS-CoV-2 spike protein for prevention of COVID disease progression in high-risk individuals. Specifically, the IDSA guidelines say, “Among ambulatory patients with mild to moderate COVID-19 at high risk for progression to severe disease, bamlanivimab/etesevimab, casirivimab/imdevimab, or sotrovimab rather than no neutralizing antibody treatment.”
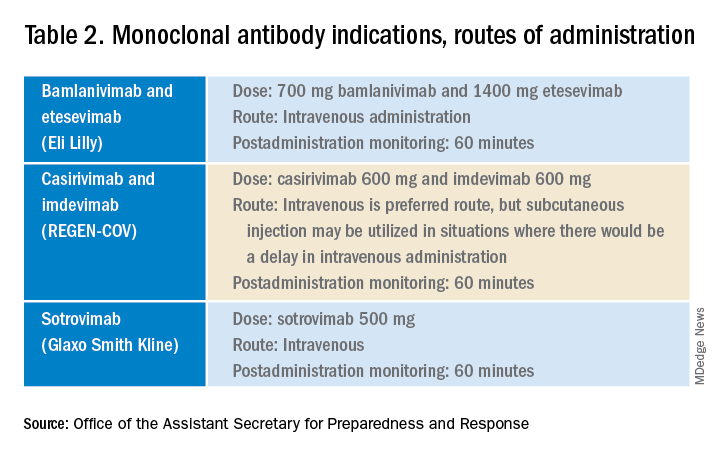
The Food and Drug Administration’s Emergency Use Authorization (EUA) allowed use of specific monoclonal antibodies (casirivimab/imdevimab in combination, bamlanivimab/etesevimab in combination, and sotrovimab alone) for individuals 12 years and above with a minimum weight of 40 kg with high-risk conditions, describing the evidence as moderate certainty.2
Several questions have arisen regarding their use. Which children qualify under the EUA? Are the available monoclonal antibodies effective for SARS-CoV-2 variants? What adverse events were observed? Are there implementation hurdles?
Unlike the EUA for prophylactic use, which targeted unvaccinated individuals and those unlikely to have a good antibody response to vaccine, use of monoclonal antibody for prevention of progression does not have such restrictions. Effectiveness may vary by local variant susceptibility and should be considered in the choice of the most appropriate monoclonal antibody therapy. Reductions in hospitalization and progression to critical disease status were reported from phase 3 studies; reductions were also observed in mortality in some, but not all, studies. Enhanced viral clearance on day 7 was observed with few subjects having persistent high viral load.
Which children qualify under the EUA? Adolescents 12 years and older and over 40 kg are eligible if a high risk condition is present. High-risk conditions include body mass index at the 85th percentile or higher, immunosuppressive disease, or receipt of immunosuppressive therapies, or baseline (pre-COVID infection) medical-related technological dependence such as tracheostomy or positive pressure ventilation. Additional high-risk conditions are neurodevelopmental disorders, sickle cell disease, congenital or acquired heart disease, asthma, or reactive airway or other chronic respiratory disease that requires daily medication for control, diabetes, chronic kidney disease, or pregnancy.3
Are the available monoclonal antibodies effective for SARS-CoV-2 variants? Of course, this is a critical question and relies on knowledge of the dominant variant in a specific geographic location. The CDC data on which variants are susceptible to which monoclonal therapies were updated as of Oct. 21 online (see Table 1). Local departments of public health often will have current data on the dominant variant in the community. Currently, the dominant variant in the United States is Delta and it is anticipated to be susceptible to the three monoclonal treatments authorized under the EUA based on in vitro neutralizing assays.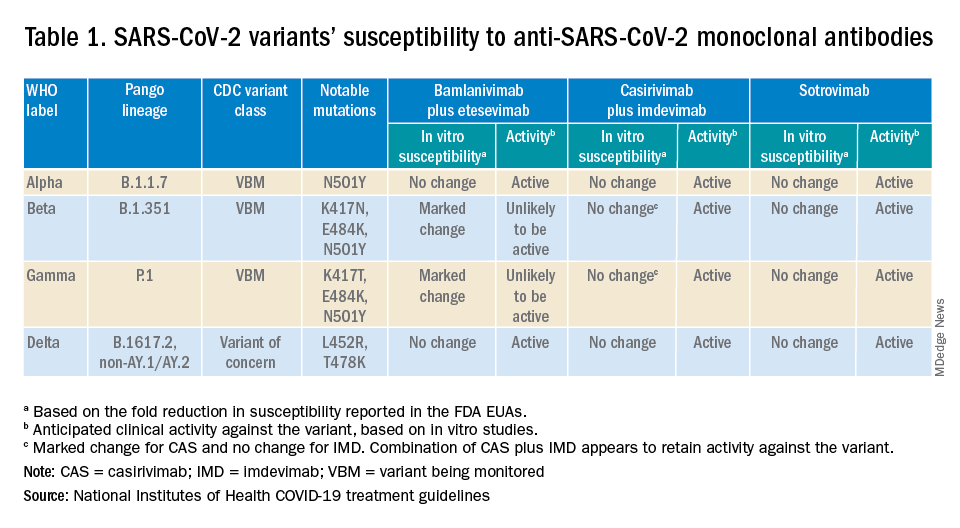
What adverse events were observed? Monoclonal antibody infusions are in general safe but anaphylaxis has been reported. Other infusion-related adverse events include urticaria, pruritis, flushing, pyrexia, shortness of breath, chest tightness, nausea, vomiting, and rash. Nearly all events were grade 1, mild, or grade 2, moderate. For nonsevere infusion-related reactions, consider slowing the infusion; if necessary, the infusion should be stopped.
Implementation challenges
The first challenge is finding a location to infuse the monoclonal antibodies. Although they can be given subcutaneously, the dose is large and little, if any, time is saved as the recommendation is for observation post administration for 1 hour. The challenge we and other centers may face is that the patients are COVID PCR+ and therefore our usual infusion program, which often is occupied by individuals already compromised and at high risk for severe COVID, is an undesirable location. We are planning to use the emergency department to accommodate such patients currently, but even that solution creates challenges for a busy, urban medical center.
Summary
Anti–SARS-CoV-2 monoclonal antibodies are an important part of the therapeutic approach to minimizing disease severity. Clinicians should review high-risk conditions in adolescents who are PCR+ for SARS-CoV-2 and have mild to moderate symptoms. Medical care systems should implement programs to make monoclonal infusions available for such high-risk adolescents.4 Obesity and asthma reactive airways or requiring daily medication for control are the two most common conditions that place adolescents with COVID-19 at risk for progression to hospitalization and severe disease in addition to the more traditional immune-compromising conditions and medical fragility.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and senior attending physician in pediatric infectious diseases, Boston Medical Center. Email him at [email protected].
References
1. Federal Response to COVID-19: Monoclonal Antibody Clinical Implementation Guide. U.S. Department of Health and Human Services. 2021 Sep 2.
2. Bhimraj A et al. IDSA Guidelines on the Treatment and Management of Patients with COVID-19. Last updated 2021 Nov 9.
3. Anti-SARS-CoV-2 Monoclonal Antibodies. National Institutes of Health’s COVID 19 Treatment Guidelines. Last updated 2021 Oct 19.
4. Spreading the Word on the Benefits of Monoclonal Antibodies for COVID-19, by Hannah R. Buchdahl. CDC Foundation, 2021 Jul 2.
I was recently asked to see a 16-year-old, unvaccinated (against COVID-19) adolescent with hypothyroidism and obesity (body mass index 37 kg/m2) seen in the pediatric emergency department with tachycardia, O2 saturation 96%, urinary tract infection, poor appetite, and nausea. Her chest x-ray had low lung volumes but no infiltrates. She was noted to be dehydrated. Testing for COVID-19 was PCR positive.1
She was observed overnight, tolerated oral rehydration, and was being readied for discharge. Pediatric Infectious Diseases was called about prescribing remdesivir.
Remdesivir was not indicated as its current use is limited to inpatients with oxygen desaturations less than 94%. Infectious Diseases Society of America guidelines do recommend the use of monoclonal antibodies against the SARS-CoV-2 spike protein for prevention of COVID disease progression in high-risk individuals. Specifically, the IDSA guidelines say, “Among ambulatory patients with mild to moderate COVID-19 at high risk for progression to severe disease, bamlanivimab/etesevimab, casirivimab/imdevimab, or sotrovimab rather than no neutralizing antibody treatment.”
The Food and Drug Administration’s Emergency Use Authorization (EUA) allowed use of specific monoclonal antibodies (casirivimab/imdevimab in combination, bamlanivimab/etesevimab in combination, and sotrovimab alone) for individuals 12 years and above with a minimum weight of 40 kg with high-risk conditions, describing the evidence as moderate certainty.2
Several questions have arisen regarding their use. Which children qualify under the EUA? Are the available monoclonal antibodies effective for SARS-CoV-2 variants? What adverse events were observed? Are there implementation hurdles?
Unlike the EUA for prophylactic use, which targeted unvaccinated individuals and those unlikely to have a good antibody response to vaccine, use of monoclonal antibody for prevention of progression does not have such restrictions. Effectiveness may vary by local variant susceptibility and should be considered in the choice of the most appropriate monoclonal antibody therapy. Reductions in hospitalization and progression to critical disease status were reported from phase 3 studies; reductions were also observed in mortality in some, but not all, studies. Enhanced viral clearance on day 7 was observed with few subjects having persistent high viral load.
Which children qualify under the EUA? Adolescents 12 years and older and over 40 kg are eligible if a high risk condition is present. High-risk conditions include body mass index at the 85th percentile or higher, immunosuppressive disease, or receipt of immunosuppressive therapies, or baseline (pre-COVID infection) medical-related technological dependence such as tracheostomy or positive pressure ventilation. Additional high-risk conditions are neurodevelopmental disorders, sickle cell disease, congenital or acquired heart disease, asthma, or reactive airway or other chronic respiratory disease that requires daily medication for control, diabetes, chronic kidney disease, or pregnancy.3
Are the available monoclonal antibodies effective for SARS-CoV-2 variants? Of course, this is a critical question and relies on knowledge of the dominant variant in a specific geographic location. The CDC data on which variants are susceptible to which monoclonal therapies were updated as of Oct. 21 online (see Table 1). Local departments of public health often will have current data on the dominant variant in the community. Currently, the dominant variant in the United States is Delta and it is anticipated to be susceptible to the three monoclonal treatments authorized under the EUA based on in vitro neutralizing assays.
What adverse events were observed? Monoclonal antibody infusions are in general safe but anaphylaxis has been reported. Other infusion-related adverse events include urticaria, pruritis, flushing, pyrexia, shortness of breath, chest tightness, nausea, vomiting, and rash. Nearly all events were grade 1, mild, or grade 2, moderate. For nonsevere infusion-related reactions, consider slowing the infusion; if necessary, the infusion should be stopped.
Implementation challenges
The first challenge is finding a location to infuse the monoclonal antibodies. Although they can be given subcutaneously, the dose is large and little, if any, time is saved as the recommendation is for observation post administration for 1 hour. The challenge we and other centers may face is that the patients are COVID PCR+ and therefore our usual infusion program, which often is occupied by individuals already compromised and at high risk for severe COVID, is an undesirable location. We are planning to use the emergency department to accommodate such patients currently, but even that solution creates challenges for a busy, urban medical center.

Summary
Anti–SARS-CoV-2 monoclonal antibodies are an important part of the therapeutic approach to minimizing disease severity. Clinicians should review high-risk conditions in adolescents who are PCR+ for SARS-CoV-2 and have mild to moderate symptoms. Medical care systems should implement programs to make monoclonal infusions available for such high-risk adolescents.4 Obesity and asthma reactive airways or requiring daily medication for control are the two most common conditions that place adolescents with COVID-19 at risk for progression to hospitalization and severe disease in addition to the more traditional immune-compromising conditions and medical fragility.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and senior attending physician in pediatric infectious diseases, Boston Medical Center. Email him at [email protected].
References
1. Federal Response to COVID-19: Monoclonal Antibody Clinical Implementation Guide. U.S. Department of Health and Human Services. 2021 Sep 2.
2. Bhimraj A et al. IDSA Guidelines on the Treatment and Management of Patients with COVID-19. Last updated 2021 Nov 9.
3. Anti-SARS-CoV-2 Monoclonal Antibodies. National Institutes of Health’s COVID 19 Treatment Guidelines. Last updated 2021 Oct 19.
4. Spreading the Word on the Benefits of Monoclonal Antibodies for COVID-19, by Hannah R. Buchdahl. CDC Foundation, 2021 Jul 2.
I was recently asked to see a 16-year-old, unvaccinated (against COVID-19) adolescent with hypothyroidism and obesity (body mass index 37 kg/m2) seen in the pediatric emergency department with tachycardia, O2 saturation 96%, urinary tract infection, poor appetite, and nausea. Her chest x-ray had low lung volumes but no infiltrates. She was noted to be dehydrated. Testing for COVID-19 was PCR positive.1
She was observed overnight, tolerated oral rehydration, and was being readied for discharge. Pediatric Infectious Diseases was called about prescribing remdesivir.
Remdesivir was not indicated as its current use is limited to inpatients with oxygen desaturations less than 94%. Infectious Diseases Society of America guidelines do recommend the use of monoclonal antibodies against the SARS-CoV-2 spike protein for prevention of COVID disease progression in high-risk individuals. Specifically, the IDSA guidelines say, “Among ambulatory patients with mild to moderate COVID-19 at high risk for progression to severe disease, bamlanivimab/etesevimab, casirivimab/imdevimab, or sotrovimab rather than no neutralizing antibody treatment.”
The Food and Drug Administration’s Emergency Use Authorization (EUA) allowed use of specific monoclonal antibodies (casirivimab/imdevimab in combination, bamlanivimab/etesevimab in combination, and sotrovimab alone) for individuals 12 years and above with a minimum weight of 40 kg with high-risk conditions, describing the evidence as moderate certainty.2
Several questions have arisen regarding their use. Which children qualify under the EUA? Are the available monoclonal antibodies effective for SARS-CoV-2 variants? What adverse events were observed? Are there implementation hurdles?
Unlike the EUA for prophylactic use, which targeted unvaccinated individuals and those unlikely to have a good antibody response to vaccine, use of monoclonal antibody for prevention of progression does not have such restrictions. Effectiveness may vary by local variant susceptibility and should be considered in the choice of the most appropriate monoclonal antibody therapy. Reductions in hospitalization and progression to critical disease status were reported from phase 3 studies; reductions were also observed in mortality in some, but not all, studies. Enhanced viral clearance on day 7 was observed with few subjects having persistent high viral load.
Which children qualify under the EUA? Adolescents 12 years and older and over 40 kg are eligible if a high risk condition is present. High-risk conditions include body mass index at the 85th percentile or higher, immunosuppressive disease, or receipt of immunosuppressive therapies, or baseline (pre-COVID infection) medical-related technological dependence such as tracheostomy or positive pressure ventilation. Additional high-risk conditions are neurodevelopmental disorders, sickle cell disease, congenital or acquired heart disease, asthma, or reactive airway or other chronic respiratory disease that requires daily medication for control, diabetes, chronic kidney disease, or pregnancy.3
Are the available monoclonal antibodies effective for SARS-CoV-2 variants? Of course, this is a critical question and relies on knowledge of the dominant variant in a specific geographic location. The CDC data on which variants are susceptible to which monoclonal therapies were updated as of Oct. 21 online (see Table 1). Local departments of public health often will have current data on the dominant variant in the community. Currently, the dominant variant in the United States is Delta and it is anticipated to be susceptible to the three monoclonal treatments authorized under the EUA based on in vitro neutralizing assays.
What adverse events were observed? Monoclonal antibody infusions are in general safe but anaphylaxis has been reported. Other infusion-related adverse events include urticaria, pruritis, flushing, pyrexia, shortness of breath, chest tightness, nausea, vomiting, and rash. Nearly all events were grade 1, mild, or grade 2, moderate. For nonsevere infusion-related reactions, consider slowing the infusion; if necessary, the infusion should be stopped.
Implementation challenges
The first challenge is finding a location to infuse the monoclonal antibodies. Although they can be given subcutaneously, the dose is large and little, if any, time is saved as the recommendation is for observation post administration for 1 hour. The challenge we and other centers may face is that the patients are COVID PCR+ and therefore our usual infusion program, which often is occupied by individuals already compromised and at high risk for severe COVID, is an undesirable location. We are planning to use the emergency department to accommodate such patients currently, but even that solution creates challenges for a busy, urban medical center.

Summary
Anti–SARS-CoV-2 monoclonal antibodies are an important part of the therapeutic approach to minimizing disease severity. Clinicians should review high-risk conditions in adolescents who are PCR+ for SARS-CoV-2 and have mild to moderate symptoms. Medical care systems should implement programs to make monoclonal infusions available for such high-risk adolescents.4 Obesity and asthma reactive airways or requiring daily medication for control are the two most common conditions that place adolescents with COVID-19 at risk for progression to hospitalization and severe disease in addition to the more traditional immune-compromising conditions and medical fragility.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and senior attending physician in pediatric infectious diseases, Boston Medical Center. Email him at [email protected].
References
1. Federal Response to COVID-19: Monoclonal Antibody Clinical Implementation Guide. U.S. Department of Health and Human Services. 2021 Sep 2.
2. Bhimraj A et al. IDSA Guidelines on the Treatment and Management of Patients with COVID-19. Last updated 2021 Nov 9.
3. Anti-SARS-CoV-2 Monoclonal Antibodies. National Institutes of Health’s COVID 19 Treatment Guidelines. Last updated 2021 Oct 19.
4. Spreading the Word on the Benefits of Monoclonal Antibodies for COVID-19, by Hannah R. Buchdahl. CDC Foundation, 2021 Jul 2.
With COVID-19 prevalent in deer, experts urge precautions
With deer hunting season underway or starting in states across the United States, people should wear a mask and gloves when handling deer to prevent coronavirus transmission, experts say.
A recent study by researchers at Penn State University found that more than 80% of the deer sampled during last year’s hunting season in counties across Iowa tested positive for COVID-19. Overall, a third of the deer sampled between September 2020 and January 2021 tested positive.
“The findings suggest that white-tailed deer may be a reservoir for the virus to continually circulate and raise concerns about the emergence of new strains that may prove a threat to wildlife and, possibly, to humans,” the researchers said in a statement.
The deer were likely infected due to “multiple human-to-deer spillover events and deer-to-deer transmission,” they noted.
Studies haven’t shown whether deer have infected humans, but public health experts are recommending that hunters and deer handlers consider possible transmission avenues, The Plain Dealer, a Cleveland newspaper, reported.
To limit deer-to-deer transmission, homeowners and hunters should avoid concentrating deer at backyard feeders or in hunting situations, according to the Ohio Department of Natural Resources Division of Wildlife. The department also urged people to not allow contact between wildlife and domestic animals, including pets and hunting dogs.
Eating venison shouldn’t be a concern if people cook the meat thoroughly, the newspaper reported. Until more is known, people should wear a mask and gloves when handling deer.
In the study, Penn State researchers examined 283 deer between December 2020 and January 2021. They took samples from lymph nodes in the head and neck as part of Iowa’s chronic wasting disease surveillance program.
“This is the first direct evidence of SARS-CoV-2 virus in any free-living species, and our findings have important implications for the ecology and long-term persistence of the virus,” Suresh Kuchipudi, PhD, associate director of the Animal Diagnostics Laboratory at Penn State, said in the statement.
“These include spillover to other free-living or captive animals and potential spillback to human hosts,” he said. “Of course, this highlights that many urgent steps are needed to monitor the spread of the virus in deer and prevent spillback to humans.”
The research team sequenced genomes from all the positive samples and identified 12 coronavirus lineages. The prominent ones corresponded to the same lineages found in humans at the time.
The U.S. Department of Agriculture has also inspected 480 samples this year from white-tailed deer in Illinois, Michigan, New York, and Pennsylvania. Researchers detected virus antibodies in 33% of samples, according to a statement from the department. The department has confirmed the virus in deer in Ohio as well.
Health officials have recommended that hunters also take precautions while around other people by getting vaccinated and wearing a mask, according to WMTV in Wisconsin.
“If someone comes to deer camp and they have COVID and other folks aren’t vaccinated, in that enclosed space with the laughing and good times that are had, the likelihood that those other hunters would be infected is pretty high,” Jeff Pothof, MD, an emergency medicine doctor at UW Health, told the news outlet.
“I think the biggest risk to deer hunters is going to be other hunters, not so much the deer,” he said.
A version of this article first appeared on Medscape.com.
With deer hunting season underway or starting in states across the United States, people should wear a mask and gloves when handling deer to prevent coronavirus transmission, experts say.
A recent study by researchers at Penn State University found that more than 80% of the deer sampled during last year’s hunting season in counties across Iowa tested positive for COVID-19. Overall, a third of the deer sampled between September 2020 and January 2021 tested positive.
“The findings suggest that white-tailed deer may be a reservoir for the virus to continually circulate and raise concerns about the emergence of new strains that may prove a threat to wildlife and, possibly, to humans,” the researchers said in a statement.
The deer were likely infected due to “multiple human-to-deer spillover events and deer-to-deer transmission,” they noted.
Studies haven’t shown whether deer have infected humans, but public health experts are recommending that hunters and deer handlers consider possible transmission avenues, The Plain Dealer, a Cleveland newspaper, reported.
To limit deer-to-deer transmission, homeowners and hunters should avoid concentrating deer at backyard feeders or in hunting situations, according to the Ohio Department of Natural Resources Division of Wildlife. The department also urged people to not allow contact between wildlife and domestic animals, including pets and hunting dogs.
Eating venison shouldn’t be a concern if people cook the meat thoroughly, the newspaper reported. Until more is known, people should wear a mask and gloves when handling deer.
In the study, Penn State researchers examined 283 deer between December 2020 and January 2021. They took samples from lymph nodes in the head and neck as part of Iowa’s chronic wasting disease surveillance program.
“This is the first direct evidence of SARS-CoV-2 virus in any free-living species, and our findings have important implications for the ecology and long-term persistence of the virus,” Suresh Kuchipudi, PhD, associate director of the Animal Diagnostics Laboratory at Penn State, said in the statement.
“These include spillover to other free-living or captive animals and potential spillback to human hosts,” he said. “Of course, this highlights that many urgent steps are needed to monitor the spread of the virus in deer and prevent spillback to humans.”
The research team sequenced genomes from all the positive samples and identified 12 coronavirus lineages. The prominent ones corresponded to the same lineages found in humans at the time.
The U.S. Department of Agriculture has also inspected 480 samples this year from white-tailed deer in Illinois, Michigan, New York, and Pennsylvania. Researchers detected virus antibodies in 33% of samples, according to a statement from the department. The department has confirmed the virus in deer in Ohio as well.
Health officials have recommended that hunters also take precautions while around other people by getting vaccinated and wearing a mask, according to WMTV in Wisconsin.
“If someone comes to deer camp and they have COVID and other folks aren’t vaccinated, in that enclosed space with the laughing and good times that are had, the likelihood that those other hunters would be infected is pretty high,” Jeff Pothof, MD, an emergency medicine doctor at UW Health, told the news outlet.
“I think the biggest risk to deer hunters is going to be other hunters, not so much the deer,” he said.
A version of this article first appeared on Medscape.com.
With deer hunting season underway or starting in states across the United States, people should wear a mask and gloves when handling deer to prevent coronavirus transmission, experts say.
A recent study by researchers at Penn State University found that more than 80% of the deer sampled during last year’s hunting season in counties across Iowa tested positive for COVID-19. Overall, a third of the deer sampled between September 2020 and January 2021 tested positive.
“The findings suggest that white-tailed deer may be a reservoir for the virus to continually circulate and raise concerns about the emergence of new strains that may prove a threat to wildlife and, possibly, to humans,” the researchers said in a statement.
The deer were likely infected due to “multiple human-to-deer spillover events and deer-to-deer transmission,” they noted.
Studies haven’t shown whether deer have infected humans, but public health experts are recommending that hunters and deer handlers consider possible transmission avenues, The Plain Dealer, a Cleveland newspaper, reported.
To limit deer-to-deer transmission, homeowners and hunters should avoid concentrating deer at backyard feeders or in hunting situations, according to the Ohio Department of Natural Resources Division of Wildlife. The department also urged people to not allow contact between wildlife and domestic animals, including pets and hunting dogs.
Eating venison shouldn’t be a concern if people cook the meat thoroughly, the newspaper reported. Until more is known, people should wear a mask and gloves when handling deer.
In the study, Penn State researchers examined 283 deer between December 2020 and January 2021. They took samples from lymph nodes in the head and neck as part of Iowa’s chronic wasting disease surveillance program.
“This is the first direct evidence of SARS-CoV-2 virus in any free-living species, and our findings have important implications for the ecology and long-term persistence of the virus,” Suresh Kuchipudi, PhD, associate director of the Animal Diagnostics Laboratory at Penn State, said in the statement.
“These include spillover to other free-living or captive animals and potential spillback to human hosts,” he said. “Of course, this highlights that many urgent steps are needed to monitor the spread of the virus in deer and prevent spillback to humans.”
The research team sequenced genomes from all the positive samples and identified 12 coronavirus lineages. The prominent ones corresponded to the same lineages found in humans at the time.
The U.S. Department of Agriculture has also inspected 480 samples this year from white-tailed deer in Illinois, Michigan, New York, and Pennsylvania. Researchers detected virus antibodies in 33% of samples, according to a statement from the department. The department has confirmed the virus in deer in Ohio as well.
Health officials have recommended that hunters also take precautions while around other people by getting vaccinated and wearing a mask, according to WMTV in Wisconsin.
“If someone comes to deer camp and they have COVID and other folks aren’t vaccinated, in that enclosed space with the laughing and good times that are had, the likelihood that those other hunters would be infected is pretty high,” Jeff Pothof, MD, an emergency medicine doctor at UW Health, told the news outlet.
“I think the biggest risk to deer hunters is going to be other hunters, not so much the deer,” he said.
A version of this article first appeared on Medscape.com.
Should you worry about picking up COVID or other infections from public bathrooms?
but some experts disagree with the study’s conclusions. The study was published in Science of the Total Environment.
Sotiris Vardoulakis, PhD, of the Australian National University, Canberra, and colleagues reviewed studies of infections associated with public washrooms.
The researchers used keywords to identify potential articles. After screening study abstracts to ensure that only publicly available washrooms with toilets, sinks, and hand dryers were included, 65 studies remained. The investigators excluded washrooms on public transportation (ships, planes, trains, and buses).
“What most of the studies concluded was that what’s really important is to have good hand hygiene and proper maintenance and ventilation of washrooms,” Dr. Vardoulakis said in an interview. “So if the hand washing and drying is effective in the first place, it’s unlikely that the bathroom air or surfaces will pose an infectious disease transmission risk.”
There has been ongoing debate on whether electric hand dryers or paper towels are better. Some studies focused on hygiene. Others focused on the environmental cost of paper towels. One concern is that air dryers might spread germs further.
One study focused on the idea that the air recirculation from electric dryers may spread infective aerosols. Another study determined that the Airblade filters in some electric dryers clean more than 99% of the bacteria. The first study, published in Mayo Clinic Proceedings by Cunrui Huang, MMed, MSPH, and colleagues, concluded that “drying hands thoroughly with single-use, disposable paper towels is the preferred method of hand drying in terms of hand hygiene.” Many people prefer to use paper towels because they can be used as a barrier when opening the washroom door.
Dr. Vardoulakis dismissed the air-versus-paper debate, saying, “If the hand washing and drying is effective in the first place, it’s unlikely that the bathroom air or surfaces will pose an infectious disease transmission risk.”
Although Dr. Vardoulakis’ review did not find that public washrooms pose a risk for infection, other researchers have shown that some settings do pose problems. For example, toilet plumes are thought to have contributed to the 2003 outbreak of severe acute respiratory syndrome at the Amoy Gardens housing complex in Hong Kong and nearby buildings by aerosolization of fecal waste. Also, norovirus has long been shown to be transmitted by aerosolized particles in vomitus or stool.
Rodney E. Rohde, PhD, professor and chair, clinical lab science program, Texas State University, San Marcos, expressed concern about this systematic review in an interview with this news organization. “I believe one of the major limitations is that studies which involved restrooms on planes, hotels, camping (those camp kids are nasty), and other similar public-access restrooms MUST be included in this type of review. I also believe they excluded restrooms from low-income/rural areas. WHAT? Their ultimate conclusions seem to be in line with the most current understanding about hand hygiene (including drying without devices that create strong air currents, which may create widespread emission of microbes).”
In an interview, Emanuel Goldman, PhD, professor of microbiology, biochemistry, and molecular genetics, New Jersey Medical School, Newark, focused on the COVID-specific aspects of the review. “The chances are less than 1 in 10,000 of getting COVID from a fomite, and that’s very conservative,” he said. “I think it’s a lot lower than that. The virus is fragile. It dies very quickly outside of a human host.” He emphasized, “virtually no infectious virus has been found on fomites over the last 2 years. ... A big mistake in a lot of papers is they confuse viral RNA with the virus. It’s not the same. Viral RNA is the genetic material of the virus, but it also is the ghost of the virus after the virus is dead, and that’s what people are finding. They’re finding the ghost of the virus.”
Because “studies show that the transfer from a surface to fingers is in the neighborhood of 10% efficiency” and one’s fingers also kill the virus, “transmission through your fingers is not easy,” Dr. Goldman said. “You’ve got to really work at it to deliberately infect yourself” with COVID from a fomite.
Dr. Rohde’s conclusion about Dr. Vardoulakis’s review? “So, the question may be, have there been enough studies, in general, of these other areas to include in a review? Otherwise, can we really generalize from this study? I don’t think so.”
Dr. Goldman is not worried about COVID transmission in public bathrooms. His summation: “I think indoor dining is more risky than anything else right now.”
The study was funded by Dyson Technology. Dr. Vardoulakis is a member of the Dyson scientific advisory board.
A version of this article first appeared on Medscape.com.
but some experts disagree with the study’s conclusions. The study was published in Science of the Total Environment.
Sotiris Vardoulakis, PhD, of the Australian National University, Canberra, and colleagues reviewed studies of infections associated with public washrooms.
The researchers used keywords to identify potential articles. After screening study abstracts to ensure that only publicly available washrooms with toilets, sinks, and hand dryers were included, 65 studies remained. The investigators excluded washrooms on public transportation (ships, planes, trains, and buses).
“What most of the studies concluded was that what’s really important is to have good hand hygiene and proper maintenance and ventilation of washrooms,” Dr. Vardoulakis said in an interview. “So if the hand washing and drying is effective in the first place, it’s unlikely that the bathroom air or surfaces will pose an infectious disease transmission risk.”
There has been ongoing debate on whether electric hand dryers or paper towels are better. Some studies focused on hygiene. Others focused on the environmental cost of paper towels. One concern is that air dryers might spread germs further.
One study focused on the idea that the air recirculation from electric dryers may spread infective aerosols. Another study determined that the Airblade filters in some electric dryers clean more than 99% of the bacteria. The first study, published in Mayo Clinic Proceedings by Cunrui Huang, MMed, MSPH, and colleagues, concluded that “drying hands thoroughly with single-use, disposable paper towels is the preferred method of hand drying in terms of hand hygiene.” Many people prefer to use paper towels because they can be used as a barrier when opening the washroom door.
Dr. Vardoulakis dismissed the air-versus-paper debate, saying, “If the hand washing and drying is effective in the first place, it’s unlikely that the bathroom air or surfaces will pose an infectious disease transmission risk.”
Although Dr. Vardoulakis’ review did not find that public washrooms pose a risk for infection, other researchers have shown that some settings do pose problems. For example, toilet plumes are thought to have contributed to the 2003 outbreak of severe acute respiratory syndrome at the Amoy Gardens housing complex in Hong Kong and nearby buildings by aerosolization of fecal waste. Also, norovirus has long been shown to be transmitted by aerosolized particles in vomitus or stool.
Rodney E. Rohde, PhD, professor and chair, clinical lab science program, Texas State University, San Marcos, expressed concern about this systematic review in an interview with this news organization. “I believe one of the major limitations is that studies which involved restrooms on planes, hotels, camping (those camp kids are nasty), and other similar public-access restrooms MUST be included in this type of review. I also believe they excluded restrooms from low-income/rural areas. WHAT? Their ultimate conclusions seem to be in line with the most current understanding about hand hygiene (including drying without devices that create strong air currents, which may create widespread emission of microbes).”
In an interview, Emanuel Goldman, PhD, professor of microbiology, biochemistry, and molecular genetics, New Jersey Medical School, Newark, focused on the COVID-specific aspects of the review. “The chances are less than 1 in 10,000 of getting COVID from a fomite, and that’s very conservative,” he said. “I think it’s a lot lower than that. The virus is fragile. It dies very quickly outside of a human host.” He emphasized, “virtually no infectious virus has been found on fomites over the last 2 years. ... A big mistake in a lot of papers is they confuse viral RNA with the virus. It’s not the same. Viral RNA is the genetic material of the virus, but it also is the ghost of the virus after the virus is dead, and that’s what people are finding. They’re finding the ghost of the virus.”
Because “studies show that the transfer from a surface to fingers is in the neighborhood of 10% efficiency” and one’s fingers also kill the virus, “transmission through your fingers is not easy,” Dr. Goldman said. “You’ve got to really work at it to deliberately infect yourself” with COVID from a fomite.
Dr. Rohde’s conclusion about Dr. Vardoulakis’s review? “So, the question may be, have there been enough studies, in general, of these other areas to include in a review? Otherwise, can we really generalize from this study? I don’t think so.”
Dr. Goldman is not worried about COVID transmission in public bathrooms. His summation: “I think indoor dining is more risky than anything else right now.”
The study was funded by Dyson Technology. Dr. Vardoulakis is a member of the Dyson scientific advisory board.
A version of this article first appeared on Medscape.com.
but some experts disagree with the study’s conclusions. The study was published in Science of the Total Environment.
Sotiris Vardoulakis, PhD, of the Australian National University, Canberra, and colleagues reviewed studies of infections associated with public washrooms.
The researchers used keywords to identify potential articles. After screening study abstracts to ensure that only publicly available washrooms with toilets, sinks, and hand dryers were included, 65 studies remained. The investigators excluded washrooms on public transportation (ships, planes, trains, and buses).
“What most of the studies concluded was that what’s really important is to have good hand hygiene and proper maintenance and ventilation of washrooms,” Dr. Vardoulakis said in an interview. “So if the hand washing and drying is effective in the first place, it’s unlikely that the bathroom air or surfaces will pose an infectious disease transmission risk.”
There has been ongoing debate on whether electric hand dryers or paper towels are better. Some studies focused on hygiene. Others focused on the environmental cost of paper towels. One concern is that air dryers might spread germs further.
One study focused on the idea that the air recirculation from electric dryers may spread infective aerosols. Another study determined that the Airblade filters in some electric dryers clean more than 99% of the bacteria. The first study, published in Mayo Clinic Proceedings by Cunrui Huang, MMed, MSPH, and colleagues, concluded that “drying hands thoroughly with single-use, disposable paper towels is the preferred method of hand drying in terms of hand hygiene.” Many people prefer to use paper towels because they can be used as a barrier when opening the washroom door.
Dr. Vardoulakis dismissed the air-versus-paper debate, saying, “If the hand washing and drying is effective in the first place, it’s unlikely that the bathroom air or surfaces will pose an infectious disease transmission risk.”
Although Dr. Vardoulakis’ review did not find that public washrooms pose a risk for infection, other researchers have shown that some settings do pose problems. For example, toilet plumes are thought to have contributed to the 2003 outbreak of severe acute respiratory syndrome at the Amoy Gardens housing complex in Hong Kong and nearby buildings by aerosolization of fecal waste. Also, norovirus has long been shown to be transmitted by aerosolized particles in vomitus or stool.
Rodney E. Rohde, PhD, professor and chair, clinical lab science program, Texas State University, San Marcos, expressed concern about this systematic review in an interview with this news organization. “I believe one of the major limitations is that studies which involved restrooms on planes, hotels, camping (those camp kids are nasty), and other similar public-access restrooms MUST be included in this type of review. I also believe they excluded restrooms from low-income/rural areas. WHAT? Their ultimate conclusions seem to be in line with the most current understanding about hand hygiene (including drying without devices that create strong air currents, which may create widespread emission of microbes).”
In an interview, Emanuel Goldman, PhD, professor of microbiology, biochemistry, and molecular genetics, New Jersey Medical School, Newark, focused on the COVID-specific aspects of the review. “The chances are less than 1 in 10,000 of getting COVID from a fomite, and that’s very conservative,” he said. “I think it’s a lot lower than that. The virus is fragile. It dies very quickly outside of a human host.” He emphasized, “virtually no infectious virus has been found on fomites over the last 2 years. ... A big mistake in a lot of papers is they confuse viral RNA with the virus. It’s not the same. Viral RNA is the genetic material of the virus, but it also is the ghost of the virus after the virus is dead, and that’s what people are finding. They’re finding the ghost of the virus.”
Because “studies show that the transfer from a surface to fingers is in the neighborhood of 10% efficiency” and one’s fingers also kill the virus, “transmission through your fingers is not easy,” Dr. Goldman said. “You’ve got to really work at it to deliberately infect yourself” with COVID from a fomite.
Dr. Rohde’s conclusion about Dr. Vardoulakis’s review? “So, the question may be, have there been enough studies, in general, of these other areas to include in a review? Otherwise, can we really generalize from this study? I don’t think so.”
Dr. Goldman is not worried about COVID transmission in public bathrooms. His summation: “I think indoor dining is more risky than anything else right now.”
The study was funded by Dyson Technology. Dr. Vardoulakis is a member of the Dyson scientific advisory board.
A version of this article first appeared on Medscape.com.
FROM SCIENCE OF THE TOTAL ENVIRONMENT
Retiform Purpura on the Buttocks in 6 Critically Ill COVID-19 Patients
To the Editor:
There is emerging evidence of skin findings in patients with COVID-19, including perniolike changes of the toes as well as urticarial and vesicular eruptions.1 Magro et al2 reported 3 cases of livedoid and purpuric skin eruptions in critically ill COVID-19 patients with evidence of thrombotic vasculopathy on skin biopsy, including a 32-year-old man with striking buttocks retiform purpura. Histopathologic analysis revealed thrombotic vasculopathy and pressure-induced ischemic necrosis. Since that patient was first evaluated (March 2020), we identified 6 more cases of critically ill COVID-19 patients from a single academic hospital in New York City with essentially identical clinical findings. Herein, we report those 6 cases of critically ill and intubated patients with COVID-19 who developed retiform purpura on the buttocks only, approximately 11 to 21 days after onset of COVID-19 symptoms.
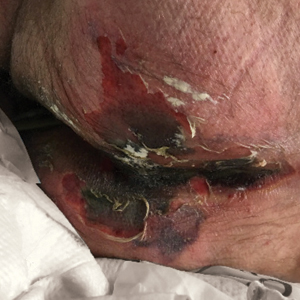
We provided consultation for 5 men and 1 woman (age range, 42–78 years) who were critically ill with COVID-19 and developed retiform purpura on the buttocks (Figures 1 and 2). All had an elevated D-dimer concentration: 2 patients, >700 ng/mL; 2 patients, >2000 ng/mL; 2 patients, >6000 ng/mL (reference, 229 ng/mL). Three patients experienced a peak D-dimer concentration on the day retiform purpura was reported.
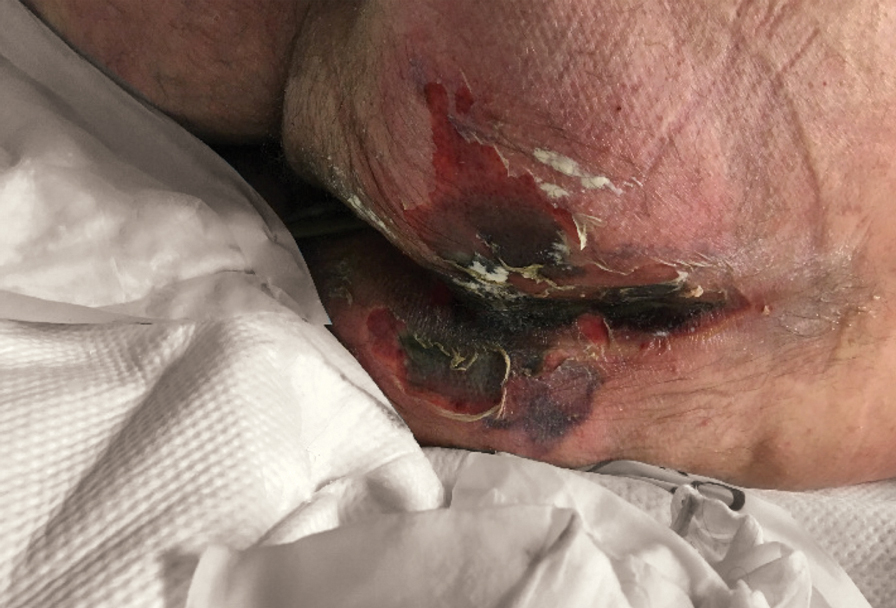
Further evidence of coagulopathy in these patients included 1 patient with a newly diagnosed left popliteal deep vein thrombosis and 1 patient with a known history of protein C deficiency and deep vein thromboses. Five patients were receiving anticoagulation on the day the skin changes were documented; anticoagulation was contraindicated in the sixth patient because of oropharyngeal bleeding. Anticoagulation was continued at the treatment dosage (enoxaparin 80 mg twice daily) in 3 patients, and in 2 patients receiving a prophylactic dose (enoxaparin 40 mg daily), anticoagulation was escalated to treatment dose due to rising D-dimer levels and newly diagnosed retiform purpura. Skin biopsy was deferred for all patients due to positional and ventilatory restrictions. At that point in their care, 3 patients remained admitted on medicine floors, 2 were in the intensive care unit, and 1 had died.
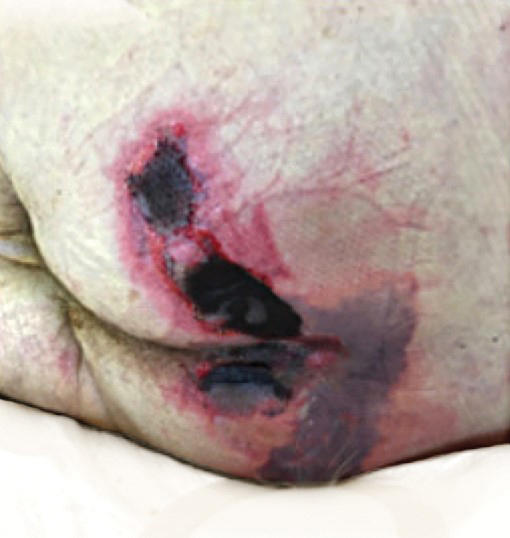
Although the differential diagnosis for retiform purpura is broad and should be fully considered in any patient with this finding, based on the elevated D-dimer concentration, critical illness secondary to COVID-19, and striking similarity to earlier reported case of buttocks retiform purpura with thrombotic vasculopathy and pressure injury noted histopathologically,2 we suspect the buttocks retiform purpura in our 6 cases also represent a combination of cutaneous thrombosis and pressure injury. In addition to acral livedoid eruptions (also reported by Magro and colleagues2), we suspect that this cutaneous manifestation might be associated with a hypercoagulable state in some patients, especially in the setting of a rising D-dimer concentration. One study found that 31% of 184 patients with severe COVID-19 had thrombotic complications,3 a clinical picture that portends a poor prognosis.4
COVID-19 patients presenting with retiform purpura should be fully evaluated based on the broad differential for this morphology. We present 6 cases of buttocks retiform purpura in critically ill COVID-19 patients—all with strikingly similar morphologic findings, an elevated D-dimer concentration, and critical illness due to COVID-19—to alert clinicians to this constellation of findings and propose that this cutaneous manifestation could indicate an associated hypercoaguable state and should prompt a hematology consultation. Additionally, biopsy of this skin finding should be considered, especially if biopsy results might serve to guide management; however, obtaining a biopsy specimen can be technically difficult because of ventilatory requirements.
Given the magnitude of the COVID-19 pandemic and the propensity of these patients to experience thrombotic events, recognition of this skin finding in COVID-19 is important and might allow timely intervention.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212-e213. doi:10.1111/jdv.16387
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013
- Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. doi:10.1111/jth.14768
To the Editor:
There is emerging evidence of skin findings in patients with COVID-19, including perniolike changes of the toes as well as urticarial and vesicular eruptions.1 Magro et al2 reported 3 cases of livedoid and purpuric skin eruptions in critically ill COVID-19 patients with evidence of thrombotic vasculopathy on skin biopsy, including a 32-year-old man with striking buttocks retiform purpura. Histopathologic analysis revealed thrombotic vasculopathy and pressure-induced ischemic necrosis. Since that patient was first evaluated (March 2020), we identified 6 more cases of critically ill COVID-19 patients from a single academic hospital in New York City with essentially identical clinical findings. Herein, we report those 6 cases of critically ill and intubated patients with COVID-19 who developed retiform purpura on the buttocks only, approximately 11 to 21 days after onset of COVID-19 symptoms.
We provided consultation for 5 men and 1 woman (age range, 42–78 years) who were critically ill with COVID-19 and developed retiform purpura on the buttocks (Figures 1 and 2). All had an elevated D-dimer concentration: 2 patients, >700 ng/mL; 2 patients, >2000 ng/mL; 2 patients, >6000 ng/mL (reference, 229 ng/mL). Three patients experienced a peak D-dimer concentration on the day retiform purpura was reported.

Further evidence of coagulopathy in these patients included 1 patient with a newly diagnosed left popliteal deep vein thrombosis and 1 patient with a known history of protein C deficiency and deep vein thromboses. Five patients were receiving anticoagulation on the day the skin changes were documented; anticoagulation was contraindicated in the sixth patient because of oropharyngeal bleeding. Anticoagulation was continued at the treatment dosage (enoxaparin 80 mg twice daily) in 3 patients, and in 2 patients receiving a prophylactic dose (enoxaparin 40 mg daily), anticoagulation was escalated to treatment dose due to rising D-dimer levels and newly diagnosed retiform purpura. Skin biopsy was deferred for all patients due to positional and ventilatory restrictions. At that point in their care, 3 patients remained admitted on medicine floors, 2 were in the intensive care unit, and 1 had died.

Although the differential diagnosis for retiform purpura is broad and should be fully considered in any patient with this finding, based on the elevated D-dimer concentration, critical illness secondary to COVID-19, and striking similarity to earlier reported case of buttocks retiform purpura with thrombotic vasculopathy and pressure injury noted histopathologically,2 we suspect the buttocks retiform purpura in our 6 cases also represent a combination of cutaneous thrombosis and pressure injury. In addition to acral livedoid eruptions (also reported by Magro and colleagues2), we suspect that this cutaneous manifestation might be associated with a hypercoagulable state in some patients, especially in the setting of a rising D-dimer concentration. One study found that 31% of 184 patients with severe COVID-19 had thrombotic complications,3 a clinical picture that portends a poor prognosis.4
COVID-19 patients presenting with retiform purpura should be fully evaluated based on the broad differential for this morphology. We present 6 cases of buttocks retiform purpura in critically ill COVID-19 patients—all with strikingly similar morphologic findings, an elevated D-dimer concentration, and critical illness due to COVID-19—to alert clinicians to this constellation of findings and propose that this cutaneous manifestation could indicate an associated hypercoaguable state and should prompt a hematology consultation. Additionally, biopsy of this skin finding should be considered, especially if biopsy results might serve to guide management; however, obtaining a biopsy specimen can be technically difficult because of ventilatory requirements.
Given the magnitude of the COVID-19 pandemic and the propensity of these patients to experience thrombotic events, recognition of this skin finding in COVID-19 is important and might allow timely intervention.
To the Editor:
There is emerging evidence of skin findings in patients with COVID-19, including perniolike changes of the toes as well as urticarial and vesicular eruptions.1 Magro et al2 reported 3 cases of livedoid and purpuric skin eruptions in critically ill COVID-19 patients with evidence of thrombotic vasculopathy on skin biopsy, including a 32-year-old man with striking buttocks retiform purpura. Histopathologic analysis revealed thrombotic vasculopathy and pressure-induced ischemic necrosis. Since that patient was first evaluated (March 2020), we identified 6 more cases of critically ill COVID-19 patients from a single academic hospital in New York City with essentially identical clinical findings. Herein, we report those 6 cases of critically ill and intubated patients with COVID-19 who developed retiform purpura on the buttocks only, approximately 11 to 21 days after onset of COVID-19 symptoms.
We provided consultation for 5 men and 1 woman (age range, 42–78 years) who were critically ill with COVID-19 and developed retiform purpura on the buttocks (Figures 1 and 2). All had an elevated D-dimer concentration: 2 patients, >700 ng/mL; 2 patients, >2000 ng/mL; 2 patients, >6000 ng/mL (reference, 229 ng/mL). Three patients experienced a peak D-dimer concentration on the day retiform purpura was reported.

Further evidence of coagulopathy in these patients included 1 patient with a newly diagnosed left popliteal deep vein thrombosis and 1 patient with a known history of protein C deficiency and deep vein thromboses. Five patients were receiving anticoagulation on the day the skin changes were documented; anticoagulation was contraindicated in the sixth patient because of oropharyngeal bleeding. Anticoagulation was continued at the treatment dosage (enoxaparin 80 mg twice daily) in 3 patients, and in 2 patients receiving a prophylactic dose (enoxaparin 40 mg daily), anticoagulation was escalated to treatment dose due to rising D-dimer levels and newly diagnosed retiform purpura. Skin biopsy was deferred for all patients due to positional and ventilatory restrictions. At that point in their care, 3 patients remained admitted on medicine floors, 2 were in the intensive care unit, and 1 had died.

Although the differential diagnosis for retiform purpura is broad and should be fully considered in any patient with this finding, based on the elevated D-dimer concentration, critical illness secondary to COVID-19, and striking similarity to earlier reported case of buttocks retiform purpura with thrombotic vasculopathy and pressure injury noted histopathologically,2 we suspect the buttocks retiform purpura in our 6 cases also represent a combination of cutaneous thrombosis and pressure injury. In addition to acral livedoid eruptions (also reported by Magro and colleagues2), we suspect that this cutaneous manifestation might be associated with a hypercoagulable state in some patients, especially in the setting of a rising D-dimer concentration. One study found that 31% of 184 patients with severe COVID-19 had thrombotic complications,3 a clinical picture that portends a poor prognosis.4
COVID-19 patients presenting with retiform purpura should be fully evaluated based on the broad differential for this morphology. We present 6 cases of buttocks retiform purpura in critically ill COVID-19 patients—all with strikingly similar morphologic findings, an elevated D-dimer concentration, and critical illness due to COVID-19—to alert clinicians to this constellation of findings and propose that this cutaneous manifestation could indicate an associated hypercoaguable state and should prompt a hematology consultation. Additionally, biopsy of this skin finding should be considered, especially if biopsy results might serve to guide management; however, obtaining a biopsy specimen can be technically difficult because of ventilatory requirements.
Given the magnitude of the COVID-19 pandemic and the propensity of these patients to experience thrombotic events, recognition of this skin finding in COVID-19 is important and might allow timely intervention.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212-e213. doi:10.1111/jdv.16387
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013
- Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. doi:10.1111/jth.14768
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212-e213. doi:10.1111/jdv.16387
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013
- Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. doi:10.1111/jth.14768
Practice Points
- Retiform purpura in a severely ill patient with COVID-19 and a markedly elevated D-dimer concentration might be a cutaneous sign of systemic coagulopathy.
- This constellation of findings should prompt consideration of skin biopsy and hematology consultation.
COVID-19 vaccines: Lower serologic response among IBD, rheumatic diseases
Patients with immune-mediated inflammatory diseases (IMIDs), such as inflammatory bowel disease and rheumatic conditions, have a reduced serologic response to a 2-dose vaccination regimen with mRNA COVID-19 vaccines, according to the findings of a meta-analysis.
“These results suggest that IMID patients receiving mRNA vaccines should complete the vaccine series without delay and support the strategy of providing a third dose of the vaccine,” wrote study authors Atsushi Sakuraba, MD, of the University of Chicago Medicine, and colleagues in Gastroenterology.
During the COVID-19 pandemic, concerns were raised about the susceptibility of patients with pre-existing conditions to infection with the novel coronavirus, the authors noted. Likewise, ongoing concerns have centered on the risk of worse COVID-19–related outcomes among patients with IMIDs who are treated with immunosuppressive agents.
Since the onset of the pandemic, several registries have been established to gauge the incidence and prognosis of COVID-19 in patients with IMID, including the Surveillance Epidemiology of Coronavirus Under Research Exclusion (SECURE)–Inflammatory Bowel Disease (IBD) registry and the COVID-19 Global Rheumatology Alliance 75 (C19-GRA), which includes patients with rheumatic diseases.
Authorization of COVID-19 mRNA vaccines provided hope that the COVID-19 pandemic could soon come to an end given the overwhelming safety and efficacy data supporting the use of these vaccines for preventing hospitalization and death. Despite these data, little is known regarding the efficacy of mRNA COVID-19 vaccines in patients with IMIDs and/or patients treated with immunosuppressive therapies, as these patients were excluded from the regulatory vaccine studies.
The study by Dr. Sakuraba and colleagues was a meta-analysis of 25 observational studies that reported serologic response rates to COVID-19 vaccination in a pooled cohort of 5,360 patients with IMIDs. Data regarding the reference population, medications, vaccination, and proportion of patients who achieved a serologic response were extracted from the observational studies and included in the meta-analysis.
In the analyzed studies, serologic response was evaluated separately after one or two vaccine doses. The researchers also examined the post-vaccine serologic response rate in patients with IMIDs versus controls without IMIDs.
A total of 23 studies used the BNT162b2 or mRNA-1273 vaccines, while 3 studies reported that 50% to 75.9% of patients received the AZD1222 vaccine. Some studies also included patients who received other COVID-19 vaccines, including CoronaVac, BBV152, and Ad26.COV2.S.
While 6 studies assessed serologic response to COVID-19 after just 1 dose, 20 studies assessed the post-vaccination serologic response following 2 doses. In most cases, researchers evaluated serologic response at 2 to 3 weeks after the first dose. After the second vaccine dose, most studies examined serologic response at 1 to 3 weeks.
The serologic response after 1 dose of the mRNA vaccines was 73.2% (95% CI 65.7-79.5). In a multivariate meta-regression analysis, the researchers found that a significantly greater proportion of patients with IMIDs who took anti-tumor necrosis factor (anti-TNF) therapies had a lower serologic response rate (coefficient, –2.60; 95% CI –4.49 to –0.72; P =.0069). The investigators indicated this “likely contributed to the difference in serologic response rates and overall heterogeneity.”
Studies with patients with IBD reported a lower serologic response rate compared with studies that included patients with rheumatoid arthritis (49.2% vs. 65.0%, respectively), which the investigators explained was likely reflective of the increased use of anti-TNF agents in patients with IBD.
After 2 doses of the mRNA vaccines, the pooled serologic response was 83.4% (95% CI, 76.8%-88.4%). Multivariate meta-regression found that a significantly greater proportion of patients who took anti-CD20 treatments had a lower serologic response (coefficient, -6.08; 95% CI -9.40 to -2.76; P <.001). The investigators found that older age was significantly associated with lower serologic response after 2 doses (coefficient, -0.044; 95% CI -0.083 to -0.0050; P =.027).
For the non-mRNA COVID-19 vaccines, the rates of serologic response after 2 doses were 93.5% with AZD1222, 22.9% with CoronaVac, and 55.6% with BBV152.
Compared with controls without IMIDs, those with IMIDs were significantly less likely to achieve a serologic response following 2 mRNA vaccine doses (odds ratio, 0.086; 95% CI 0.036-0.206; P <.001). The investigators noted that there were not enough studies to examine and compare serologic response rates to adenoviral or inactivated vaccines between patients and controls.
In terms of limitations, the researchers wrote that additional studies examining humoral and cellular immunity to COVID-19 vaccines are needed to determine vaccine efficacy and durability in patients with IMIDs. Additionally, there is a need for studies with larger patient populations to determine serologic response to COVID-19 vaccines in the broader IMID population.
The researchers reported no funding for the study and no relevant conflicts of interest with the pharmaceutical industry.
Patients with immune-mediated inflammatory diseases (IMIDs), such as inflammatory bowel disease and rheumatic conditions, have a reduced serologic response to a 2-dose vaccination regimen with mRNA COVID-19 vaccines, according to the findings of a meta-analysis.
“These results suggest that IMID patients receiving mRNA vaccines should complete the vaccine series without delay and support the strategy of providing a third dose of the vaccine,” wrote study authors Atsushi Sakuraba, MD, of the University of Chicago Medicine, and colleagues in Gastroenterology.
During the COVID-19 pandemic, concerns were raised about the susceptibility of patients with pre-existing conditions to infection with the novel coronavirus, the authors noted. Likewise, ongoing concerns have centered on the risk of worse COVID-19–related outcomes among patients with IMIDs who are treated with immunosuppressive agents.
Since the onset of the pandemic, several registries have been established to gauge the incidence and prognosis of COVID-19 in patients with IMID, including the Surveillance Epidemiology of Coronavirus Under Research Exclusion (SECURE)–Inflammatory Bowel Disease (IBD) registry and the COVID-19 Global Rheumatology Alliance 75 (C19-GRA), which includes patients with rheumatic diseases.
Authorization of COVID-19 mRNA vaccines provided hope that the COVID-19 pandemic could soon come to an end given the overwhelming safety and efficacy data supporting the use of these vaccines for preventing hospitalization and death. Despite these data, little is known regarding the efficacy of mRNA COVID-19 vaccines in patients with IMIDs and/or patients treated with immunosuppressive therapies, as these patients were excluded from the regulatory vaccine studies.
The study by Dr. Sakuraba and colleagues was a meta-analysis of 25 observational studies that reported serologic response rates to COVID-19 vaccination in a pooled cohort of 5,360 patients with IMIDs. Data regarding the reference population, medications, vaccination, and proportion of patients who achieved a serologic response were extracted from the observational studies and included in the meta-analysis.
In the analyzed studies, serologic response was evaluated separately after one or two vaccine doses. The researchers also examined the post-vaccine serologic response rate in patients with IMIDs versus controls without IMIDs.
A total of 23 studies used the BNT162b2 or mRNA-1273 vaccines, while 3 studies reported that 50% to 75.9% of patients received the AZD1222 vaccine. Some studies also included patients who received other COVID-19 vaccines, including CoronaVac, BBV152, and Ad26.COV2.S.
While 6 studies assessed serologic response to COVID-19 after just 1 dose, 20 studies assessed the post-vaccination serologic response following 2 doses. In most cases, researchers evaluated serologic response at 2 to 3 weeks after the first dose. After the second vaccine dose, most studies examined serologic response at 1 to 3 weeks.
The serologic response after 1 dose of the mRNA vaccines was 73.2% (95% CI 65.7-79.5). In a multivariate meta-regression analysis, the researchers found that a significantly greater proportion of patients with IMIDs who took anti-tumor necrosis factor (anti-TNF) therapies had a lower serologic response rate (coefficient, –2.60; 95% CI –4.49 to –0.72; P =.0069). The investigators indicated this “likely contributed to the difference in serologic response rates and overall heterogeneity.”
Studies with patients with IBD reported a lower serologic response rate compared with studies that included patients with rheumatoid arthritis (49.2% vs. 65.0%, respectively), which the investigators explained was likely reflective of the increased use of anti-TNF agents in patients with IBD.
After 2 doses of the mRNA vaccines, the pooled serologic response was 83.4% (95% CI, 76.8%-88.4%). Multivariate meta-regression found that a significantly greater proportion of patients who took anti-CD20 treatments had a lower serologic response (coefficient, -6.08; 95% CI -9.40 to -2.76; P <.001). The investigators found that older age was significantly associated with lower serologic response after 2 doses (coefficient, -0.044; 95% CI -0.083 to -0.0050; P =.027).
For the non-mRNA COVID-19 vaccines, the rates of serologic response after 2 doses were 93.5% with AZD1222, 22.9% with CoronaVac, and 55.6% with BBV152.
Compared with controls without IMIDs, those with IMIDs were significantly less likely to achieve a serologic response following 2 mRNA vaccine doses (odds ratio, 0.086; 95% CI 0.036-0.206; P <.001). The investigators noted that there were not enough studies to examine and compare serologic response rates to adenoviral or inactivated vaccines between patients and controls.
In terms of limitations, the researchers wrote that additional studies examining humoral and cellular immunity to COVID-19 vaccines are needed to determine vaccine efficacy and durability in patients with IMIDs. Additionally, there is a need for studies with larger patient populations to determine serologic response to COVID-19 vaccines in the broader IMID population.
The researchers reported no funding for the study and no relevant conflicts of interest with the pharmaceutical industry.
Patients with immune-mediated inflammatory diseases (IMIDs), such as inflammatory bowel disease and rheumatic conditions, have a reduced serologic response to a 2-dose vaccination regimen with mRNA COVID-19 vaccines, according to the findings of a meta-analysis.
“These results suggest that IMID patients receiving mRNA vaccines should complete the vaccine series without delay and support the strategy of providing a third dose of the vaccine,” wrote study authors Atsushi Sakuraba, MD, of the University of Chicago Medicine, and colleagues in Gastroenterology.
During the COVID-19 pandemic, concerns were raised about the susceptibility of patients with pre-existing conditions to infection with the novel coronavirus, the authors noted. Likewise, ongoing concerns have centered on the risk of worse COVID-19–related outcomes among patients with IMIDs who are treated with immunosuppressive agents.
Since the onset of the pandemic, several registries have been established to gauge the incidence and prognosis of COVID-19 in patients with IMID, including the Surveillance Epidemiology of Coronavirus Under Research Exclusion (SECURE)–Inflammatory Bowel Disease (IBD) registry and the COVID-19 Global Rheumatology Alliance 75 (C19-GRA), which includes patients with rheumatic diseases.
Authorization of COVID-19 mRNA vaccines provided hope that the COVID-19 pandemic could soon come to an end given the overwhelming safety and efficacy data supporting the use of these vaccines for preventing hospitalization and death. Despite these data, little is known regarding the efficacy of mRNA COVID-19 vaccines in patients with IMIDs and/or patients treated with immunosuppressive therapies, as these patients were excluded from the regulatory vaccine studies.
The study by Dr. Sakuraba and colleagues was a meta-analysis of 25 observational studies that reported serologic response rates to COVID-19 vaccination in a pooled cohort of 5,360 patients with IMIDs. Data regarding the reference population, medications, vaccination, and proportion of patients who achieved a serologic response were extracted from the observational studies and included in the meta-analysis.
In the analyzed studies, serologic response was evaluated separately after one or two vaccine doses. The researchers also examined the post-vaccine serologic response rate in patients with IMIDs versus controls without IMIDs.
A total of 23 studies used the BNT162b2 or mRNA-1273 vaccines, while 3 studies reported that 50% to 75.9% of patients received the AZD1222 vaccine. Some studies also included patients who received other COVID-19 vaccines, including CoronaVac, BBV152, and Ad26.COV2.S.
While 6 studies assessed serologic response to COVID-19 after just 1 dose, 20 studies assessed the post-vaccination serologic response following 2 doses. In most cases, researchers evaluated serologic response at 2 to 3 weeks after the first dose. After the second vaccine dose, most studies examined serologic response at 1 to 3 weeks.
The serologic response after 1 dose of the mRNA vaccines was 73.2% (95% CI 65.7-79.5). In a multivariate meta-regression analysis, the researchers found that a significantly greater proportion of patients with IMIDs who took anti-tumor necrosis factor (anti-TNF) therapies had a lower serologic response rate (coefficient, –2.60; 95% CI –4.49 to –0.72; P =.0069). The investigators indicated this “likely contributed to the difference in serologic response rates and overall heterogeneity.”
Studies with patients with IBD reported a lower serologic response rate compared with studies that included patients with rheumatoid arthritis (49.2% vs. 65.0%, respectively), which the investigators explained was likely reflective of the increased use of anti-TNF agents in patients with IBD.
After 2 doses of the mRNA vaccines, the pooled serologic response was 83.4% (95% CI, 76.8%-88.4%). Multivariate meta-regression found that a significantly greater proportion of patients who took anti-CD20 treatments had a lower serologic response (coefficient, -6.08; 95% CI -9.40 to -2.76; P <.001). The investigators found that older age was significantly associated with lower serologic response after 2 doses (coefficient, -0.044; 95% CI -0.083 to -0.0050; P =.027).
For the non-mRNA COVID-19 vaccines, the rates of serologic response after 2 doses were 93.5% with AZD1222, 22.9% with CoronaVac, and 55.6% with BBV152.
Compared with controls without IMIDs, those with IMIDs were significantly less likely to achieve a serologic response following 2 mRNA vaccine doses (odds ratio, 0.086; 95% CI 0.036-0.206; P <.001). The investigators noted that there were not enough studies to examine and compare serologic response rates to adenoviral or inactivated vaccines between patients and controls.
In terms of limitations, the researchers wrote that additional studies examining humoral and cellular immunity to COVID-19 vaccines are needed to determine vaccine efficacy and durability in patients with IMIDs. Additionally, there is a need for studies with larger patient populations to determine serologic response to COVID-19 vaccines in the broader IMID population.
The researchers reported no funding for the study and no relevant conflicts of interest with the pharmaceutical industry.
FROM GASTROENTEROLOGY
The neurological super powers of grandma are real
Deer, COVID, how?
Usually humans cannot get close enough to a deer to really be face-to-face, so it’s easy to question how on Earth deer are contracting COVID-19. Well, stranger things have happened, and honestly, we’ve just stopped questioning most of them.
Exhibit A comes to us from a Penn State University study: Eighty percent of deer sampled in Iowa in December 2020 and January 2021 – as part of the state’s chronic wasting disease surveillance program – were found to be positive for COVID-19.
A statement from the university said that “white-tailed deer may be a reservoir for the virus to continually circulate and raise concerns about the emergence of new strains that may prove a threat to wildlife and, possibly, to humans.” The investigators also suggested that deer probably caught the virus from humans and then transmitted it to other deer.
If you or someone you know is a hunter or a white-tailed deer, it’s best to proceed with caution. There’s no evidence that COVID-19 has jumped from deer to humans, but hunters should wear masks and gloves while working with deer, worrying not just about the deer’s face, but also … you know, the gastrointestinal parts, Robert Salata, MD, of University Hospitals Cleveland Medical Center, told Syracuse.com. It also shouldn’t be too risky to eat venison, he said, just make sure the meat is cooked thoroughly.
The more you know!
The neurological super powers of grandma are real
What is it about grandmothers that makes them seem almost magical at times? They somehow always know how you feel. And they can almost always tell when something is wrong. They also seem to be the biggest ally a child will have against his or her parents.
So what makes these super matriarchs? The answer is in the brain.
Apparently there’s a function in the brains of grandmothers geared toward “emotional empathy.” James Rilling, PhD, of Emory University, lead author of a recent study focused on looking at the brain function of grandmothers, suggested that they’re neurologically tapped into feeling how their grandchildren feel: “If their grandchild is smiling, they’re feeling the child’s joy. And if their grandchild is crying, they’re feeling the child’s pain and distress.”
And then there’s the cute factor. Never underestimate a child’s ability to manipulate his or her grandmother’s brain.
So how do the researchers know this? Functional MRI showed more brain activity in the parts of the brain that deal with emotional empathy and movement in the participating grandmas when shown pictures of their grandchildren. Images of their own adult children lit up areas more associated with cognitive empathy. So less emotional and more mental/logical understanding.
Kids, don’t tell Mom about the secret midnight snacks with grandma. She wouldn’t get it.
Then there’s the grandmother hypothesis, which suggests that women tend to live longer to provide some kind of evolutionary benefit to their children and grandchildren. Evidence also exists that children with positive engagement from their grandmothers tend to have better social and academic outcomes, behavior, and physical health.
A lot of credit on how children turn out, of course, goes to parents, but more can be said about grandmas. Don’t let the age and freshly baked cookies fool you. They have neurologic superpowers within.
Brain cleanup on aisle 5
You’ve got your local grocery store down. You know the ins and outs; you know where everything is. Last week you did your trip in record time. This week, however, you have to stop at a different store. Same chain, but a different location. You stroll in, confidently walk toward the first aisle for your fruits and veggies, and ... it’s all ice cream. Oops.
There’s a lot we don’t understand about the brain, including how it remembers familiar environments to avoid confusion. Or why it fails to do so, as with our grocery store example. However, thanks to a study from the University of Arizona, we may have an answer.
For the experiment, a group of participants watched a video tour of three virtual cities. Those cities were very similar, being laid out in basically identical fashion. Stores could be found in the same places, but the identity of those stores varied. Some stores were in all three cities, some were in two, and some were unique. Participants were asked to memorize the layouts, and those who got things more than 80% correct ran through the test again, only this time their brain activity was monitored through MRI.
In general, brain activity was similar for the participants; after all, they were recalling similar environments. However, when asked about stores that appeared in multiple cities, brain activity varied dramatically. This indicated to the researchers that the brain was recalling shared stores as if they were more dissimilar than two completely disparate and unique stores, a concept often known to brain scientists as “repulsion.” It also indicates that the memories regarding shared environments are stored in the prefrontal cortex, not the hippocampus, which typically handles memory.
The researchers plan to apply this information to questions about diseases such as Alzheimer’s, so the next time you get turned around in a weirdly unfamiliar grocery store, just think: “It’s okay, I’m helping to solve a terrible brain disease.”
The real endgame: Friction is the winner
Spoiler alert! If you haven’t seen “Avengers: Infinity War” yet, we’re about to ruin it for you.
For those still with us, here’s the spoiler: Thanos would not have been able to snap his fingers while wearing the Infinity Gauntlet.
Saad Bhamla, PhD, of Georgia Tech University’s school of chemical and biomolecular engineering, had been studying powerful and ultrafast motions in living organisms along with several colleagues before the movie came out in 2018, and when they saw the finger-snapping scene it got them wondering.
Being scientists of course, they had no choice. They got out their high-speed imaging equipment, automated image processing software, and dynamic force sensors and analyzed finger snaps, paying close attention to friction by covering fingers with “different materials, including metallic thimbles to simulate the effects of trying to snap while wearing a metallic gauntlet, much like Thanos,” according to a statement on Eurekalert.
With finger snaps, it’s all about the rotational velocity. The angular acceleration involved is the fastest ever measured in a human, with a professional baseball pitcher’s throwing arm a distant second.
Dr. Bhamla’s reaction to their work explains why scientists are the ones doing science. “When I first saw the data, I jumped out of my chair,” he said in the written statement.
Rotational velocities dropped dramatically when the friction-reducing thimbles were used, so there was no snap. Which means that billions and billions of fictional lives could have been saved if the filmmakers had just talked to the right scientist.
That scientist, clearly, is Dr. Bhamla, who said that “this is the only scientific project in my lab in which we could snap our fingers and get data.”
Deer, COVID, how?
Usually humans cannot get close enough to a deer to really be face-to-face, so it’s easy to question how on Earth deer are contracting COVID-19. Well, stranger things have happened, and honestly, we’ve just stopped questioning most of them.
Exhibit A comes to us from a Penn State University study: Eighty percent of deer sampled in Iowa in December 2020 and January 2021 – as part of the state’s chronic wasting disease surveillance program – were found to be positive for COVID-19.
A statement from the university said that “white-tailed deer may be a reservoir for the virus to continually circulate and raise concerns about the emergence of new strains that may prove a threat to wildlife and, possibly, to humans.” The investigators also suggested that deer probably caught the virus from humans and then transmitted it to other deer.
If you or someone you know is a hunter or a white-tailed deer, it’s best to proceed with caution. There’s no evidence that COVID-19 has jumped from deer to humans, but hunters should wear masks and gloves while working with deer, worrying not just about the deer’s face, but also … you know, the gastrointestinal parts, Robert Salata, MD, of University Hospitals Cleveland Medical Center, told Syracuse.com. It also shouldn’t be too risky to eat venison, he said, just make sure the meat is cooked thoroughly.
The more you know!
The neurological super powers of grandma are real
What is it about grandmothers that makes them seem almost magical at times? They somehow always know how you feel. And they can almost always tell when something is wrong. They also seem to be the biggest ally a child will have against his or her parents.
So what makes these super matriarchs? The answer is in the brain.
Apparently there’s a function in the brains of grandmothers geared toward “emotional empathy.” James Rilling, PhD, of Emory University, lead author of a recent study focused on looking at the brain function of grandmothers, suggested that they’re neurologically tapped into feeling how their grandchildren feel: “If their grandchild is smiling, they’re feeling the child’s joy. And if their grandchild is crying, they’re feeling the child’s pain and distress.”
And then there’s the cute factor. Never underestimate a child’s ability to manipulate his or her grandmother’s brain.
So how do the researchers know this? Functional MRI showed more brain activity in the parts of the brain that deal with emotional empathy and movement in the participating grandmas when shown pictures of their grandchildren. Images of their own adult children lit up areas more associated with cognitive empathy. So less emotional and more mental/logical understanding.
Kids, don’t tell Mom about the secret midnight snacks with grandma. She wouldn’t get it.
Then there’s the grandmother hypothesis, which suggests that women tend to live longer to provide some kind of evolutionary benefit to their children and grandchildren. Evidence also exists that children with positive engagement from their grandmothers tend to have better social and academic outcomes, behavior, and physical health.
A lot of credit on how children turn out, of course, goes to parents, but more can be said about grandmas. Don’t let the age and freshly baked cookies fool you. They have neurologic superpowers within.
Brain cleanup on aisle 5
You’ve got your local grocery store down. You know the ins and outs; you know where everything is. Last week you did your trip in record time. This week, however, you have to stop at a different store. Same chain, but a different location. You stroll in, confidently walk toward the first aisle for your fruits and veggies, and ... it’s all ice cream. Oops.
There’s a lot we don’t understand about the brain, including how it remembers familiar environments to avoid confusion. Or why it fails to do so, as with our grocery store example. However, thanks to a study from the University of Arizona, we may have an answer.
For the experiment, a group of participants watched a video tour of three virtual cities. Those cities were very similar, being laid out in basically identical fashion. Stores could be found in the same places, but the identity of those stores varied. Some stores were in all three cities, some were in two, and some were unique. Participants were asked to memorize the layouts, and those who got things more than 80% correct ran through the test again, only this time their brain activity was monitored through MRI.
In general, brain activity was similar for the participants; after all, they were recalling similar environments. However, when asked about stores that appeared in multiple cities, brain activity varied dramatically. This indicated to the researchers that the brain was recalling shared stores as if they were more dissimilar than two completely disparate and unique stores, a concept often known to brain scientists as “repulsion.” It also indicates that the memories regarding shared environments are stored in the prefrontal cortex, not the hippocampus, which typically handles memory.
The researchers plan to apply this information to questions about diseases such as Alzheimer’s, so the next time you get turned around in a weirdly unfamiliar grocery store, just think: “It’s okay, I’m helping to solve a terrible brain disease.”
The real endgame: Friction is the winner
Spoiler alert! If you haven’t seen “Avengers: Infinity War” yet, we’re about to ruin it for you.
For those still with us, here’s the spoiler: Thanos would not have been able to snap his fingers while wearing the Infinity Gauntlet.
Saad Bhamla, PhD, of Georgia Tech University’s school of chemical and biomolecular engineering, had been studying powerful and ultrafast motions in living organisms along with several colleagues before the movie came out in 2018, and when they saw the finger-snapping scene it got them wondering.
Being scientists of course, they had no choice. They got out their high-speed imaging equipment, automated image processing software, and dynamic force sensors and analyzed finger snaps, paying close attention to friction by covering fingers with “different materials, including metallic thimbles to simulate the effects of trying to snap while wearing a metallic gauntlet, much like Thanos,” according to a statement on Eurekalert.
With finger snaps, it’s all about the rotational velocity. The angular acceleration involved is the fastest ever measured in a human, with a professional baseball pitcher’s throwing arm a distant second.
Dr. Bhamla’s reaction to their work explains why scientists are the ones doing science. “When I first saw the data, I jumped out of my chair,” he said in the written statement.
Rotational velocities dropped dramatically when the friction-reducing thimbles were used, so there was no snap. Which means that billions and billions of fictional lives could have been saved if the filmmakers had just talked to the right scientist.
That scientist, clearly, is Dr. Bhamla, who said that “this is the only scientific project in my lab in which we could snap our fingers and get data.”
Deer, COVID, how?
Usually humans cannot get close enough to a deer to really be face-to-face, so it’s easy to question how on Earth deer are contracting COVID-19. Well, stranger things have happened, and honestly, we’ve just stopped questioning most of them.
Exhibit A comes to us from a Penn State University study: Eighty percent of deer sampled in Iowa in December 2020 and January 2021 – as part of the state’s chronic wasting disease surveillance program – were found to be positive for COVID-19.
A statement from the university said that “white-tailed deer may be a reservoir for the virus to continually circulate and raise concerns about the emergence of new strains that may prove a threat to wildlife and, possibly, to humans.” The investigators also suggested that deer probably caught the virus from humans and then transmitted it to other deer.
If you or someone you know is a hunter or a white-tailed deer, it’s best to proceed with caution. There’s no evidence that COVID-19 has jumped from deer to humans, but hunters should wear masks and gloves while working with deer, worrying not just about the deer’s face, but also … you know, the gastrointestinal parts, Robert Salata, MD, of University Hospitals Cleveland Medical Center, told Syracuse.com. It also shouldn’t be too risky to eat venison, he said, just make sure the meat is cooked thoroughly.
The more you know!
The neurological super powers of grandma are real
What is it about grandmothers that makes them seem almost magical at times? They somehow always know how you feel. And they can almost always tell when something is wrong. They also seem to be the biggest ally a child will have against his or her parents.
So what makes these super matriarchs? The answer is in the brain.
Apparently there’s a function in the brains of grandmothers geared toward “emotional empathy.” James Rilling, PhD, of Emory University, lead author of a recent study focused on looking at the brain function of grandmothers, suggested that they’re neurologically tapped into feeling how their grandchildren feel: “If their grandchild is smiling, they’re feeling the child’s joy. And if their grandchild is crying, they’re feeling the child’s pain and distress.”
And then there’s the cute factor. Never underestimate a child’s ability to manipulate his or her grandmother’s brain.
So how do the researchers know this? Functional MRI showed more brain activity in the parts of the brain that deal with emotional empathy and movement in the participating grandmas when shown pictures of their grandchildren. Images of their own adult children lit up areas more associated with cognitive empathy. So less emotional and more mental/logical understanding.
Kids, don’t tell Mom about the secret midnight snacks with grandma. She wouldn’t get it.
Then there’s the grandmother hypothesis, which suggests that women tend to live longer to provide some kind of evolutionary benefit to their children and grandchildren. Evidence also exists that children with positive engagement from their grandmothers tend to have better social and academic outcomes, behavior, and physical health.
A lot of credit on how children turn out, of course, goes to parents, but more can be said about grandmas. Don’t let the age and freshly baked cookies fool you. They have neurologic superpowers within.
Brain cleanup on aisle 5
You’ve got your local grocery store down. You know the ins and outs; you know where everything is. Last week you did your trip in record time. This week, however, you have to stop at a different store. Same chain, but a different location. You stroll in, confidently walk toward the first aisle for your fruits and veggies, and ... it’s all ice cream. Oops.
There’s a lot we don’t understand about the brain, including how it remembers familiar environments to avoid confusion. Or why it fails to do so, as with our grocery store example. However, thanks to a study from the University of Arizona, we may have an answer.
For the experiment, a group of participants watched a video tour of three virtual cities. Those cities were very similar, being laid out in basically identical fashion. Stores could be found in the same places, but the identity of those stores varied. Some stores were in all three cities, some were in two, and some were unique. Participants were asked to memorize the layouts, and those who got things more than 80% correct ran through the test again, only this time their brain activity was monitored through MRI.
In general, brain activity was similar for the participants; after all, they were recalling similar environments. However, when asked about stores that appeared in multiple cities, brain activity varied dramatically. This indicated to the researchers that the brain was recalling shared stores as if they were more dissimilar than two completely disparate and unique stores, a concept often known to brain scientists as “repulsion.” It also indicates that the memories regarding shared environments are stored in the prefrontal cortex, not the hippocampus, which typically handles memory.
The researchers plan to apply this information to questions about diseases such as Alzheimer’s, so the next time you get turned around in a weirdly unfamiliar grocery store, just think: “It’s okay, I’m helping to solve a terrible brain disease.”
The real endgame: Friction is the winner
Spoiler alert! If you haven’t seen “Avengers: Infinity War” yet, we’re about to ruin it for you.
For those still with us, here’s the spoiler: Thanos would not have been able to snap his fingers while wearing the Infinity Gauntlet.
Saad Bhamla, PhD, of Georgia Tech University’s school of chemical and biomolecular engineering, had been studying powerful and ultrafast motions in living organisms along with several colleagues before the movie came out in 2018, and when they saw the finger-snapping scene it got them wondering.
Being scientists of course, they had no choice. They got out their high-speed imaging equipment, automated image processing software, and dynamic force sensors and analyzed finger snaps, paying close attention to friction by covering fingers with “different materials, including metallic thimbles to simulate the effects of trying to snap while wearing a metallic gauntlet, much like Thanos,” according to a statement on Eurekalert.
With finger snaps, it’s all about the rotational velocity. The angular acceleration involved is the fastest ever measured in a human, with a professional baseball pitcher’s throwing arm a distant second.
Dr. Bhamla’s reaction to their work explains why scientists are the ones doing science. “When I first saw the data, I jumped out of my chair,” he said in the written statement.
Rotational velocities dropped dramatically when the friction-reducing thimbles were used, so there was no snap. Which means that billions and billions of fictional lives could have been saved if the filmmakers had just talked to the right scientist.
That scientist, clearly, is Dr. Bhamla, who said that “this is the only scientific project in my lab in which we could snap our fingers and get data.”
Text-based COVID monitoring system could reduce deaths, relieve ED in winter surge
COVID Watch, a text message–based remote monitoring program developed by the University of Pennsylvania Health System, was associated with a 68% reduction in the risk of death, compared with those who received usual care. This was the main finding of a paper published in the Annals of Internal Medicine.
The investigators also determined that patients who enrolled in the program were more likely to seek care in the ED and when they did, they came in on average 2 days sooner than those who received usual care.
“When our clinical team designed COVID Watch the goal was to facilitate hospital care for patients who require it, while supporting access to care for patients who can safely remain at home,” study author M. Kit Delgado, MD, MS, an assistant professor of emergency medicine and epidemiology at Penn Presbyterian Medical Center in Philadelphia, said in an interview.
Researchers had initially hoped COVID Watch would relieve pressure on EDs, Dr. Delgado said.
Significantly lower mortality seen among COVID Watch group
For the study, Dr. Delgado and colleagues enrolled 3,488 patients in COVID Watch and 4,377 in the usual care group to compare outcomes at 30 and 60 days.
“We didn’t include patients who were diagnosed with COVID in the ER or hospital, so this is a lower-risk cohort of patients who test positive in outpatient settings,” Dr. Delgado noted. “Outpatients who received usual care and COVID Watch both had relatively low mortality, but it was significantly lower in those who were in COVID Watch.”
The researchers found that 3 patients in the COVID Watch group died within 30 days of their enrollment, compared with 12 in the control group. At 60 days after enrollment, 5 people within COVID Watch died, compared with 16 not using the system. More than one-third of the deaths in the usual care group occurred outside the hospital, compared with zero deaths among those in COVID Watch.
More than half of program participants were Black or Latino
The messaging system also reduced mortality rates among “all major racial and ethnic subgroups,” the researchers said, with more than 50% of the patients enrolled in COVID Watch having been Black or Latino.
“This is important because Black and Hispanic communities have experienced higher exposure and infection rates, decreased access to care, and have had higher mortality rates,” Dr. Delgado said. “Therefore, the results imply that this type of program could play a role in decreasing disparities in COVID outcomes if scaled more broadly.”
Outside expert: COVID Watch bring new approach to digital health monitoring
The study not only highlights the efficacy and sustainment of the COVID Watch program, but it sheds light on the possibility of using text message monitoring systems on other chronic disease conditions, said Jamie Faro, PhD, who was not involved in the study.
“It brings a new approach to health monitoring using digital means, which may lessen the burden on health care providers and be more cost effective than usual care approaches,” said Dr. Faro, who is assistant professor at the department of population and quantitative health sciences at the University of Massachusetts, Worcester. “Text messaging, which is used by over 80% of Americans, can allow us to reach a large percentage of the population for remote health care monitoring.”
Researchers of the current study said the findings “reveal a model for outpatient health system management of patients with COVID-19 and possibly other conditions where the early detection of clinical declines is critical.” Dr. Faro said that COVID Watch can have a measurable impact on an outcome that is truly life or death. However, it would be critical to understand how to reach those who either “were not offered or refused to take part in the program.”
The authors of the paper and Dr. Faro had no disclosures.
COVID Watch, a text message–based remote monitoring program developed by the University of Pennsylvania Health System, was associated with a 68% reduction in the risk of death, compared with those who received usual care. This was the main finding of a paper published in the Annals of Internal Medicine.
The investigators also determined that patients who enrolled in the program were more likely to seek care in the ED and when they did, they came in on average 2 days sooner than those who received usual care.
“When our clinical team designed COVID Watch the goal was to facilitate hospital care for patients who require it, while supporting access to care for patients who can safely remain at home,” study author M. Kit Delgado, MD, MS, an assistant professor of emergency medicine and epidemiology at Penn Presbyterian Medical Center in Philadelphia, said in an interview.
Researchers had initially hoped COVID Watch would relieve pressure on EDs, Dr. Delgado said.
Significantly lower mortality seen among COVID Watch group
For the study, Dr. Delgado and colleagues enrolled 3,488 patients in COVID Watch and 4,377 in the usual care group to compare outcomes at 30 and 60 days.
“We didn’t include patients who were diagnosed with COVID in the ER or hospital, so this is a lower-risk cohort of patients who test positive in outpatient settings,” Dr. Delgado noted. “Outpatients who received usual care and COVID Watch both had relatively low mortality, but it was significantly lower in those who were in COVID Watch.”
The researchers found that 3 patients in the COVID Watch group died within 30 days of their enrollment, compared with 12 in the control group. At 60 days after enrollment, 5 people within COVID Watch died, compared with 16 not using the system. More than one-third of the deaths in the usual care group occurred outside the hospital, compared with zero deaths among those in COVID Watch.
More than half of program participants were Black or Latino
The messaging system also reduced mortality rates among “all major racial and ethnic subgroups,” the researchers said, with more than 50% of the patients enrolled in COVID Watch having been Black or Latino.
“This is important because Black and Hispanic communities have experienced higher exposure and infection rates, decreased access to care, and have had higher mortality rates,” Dr. Delgado said. “Therefore, the results imply that this type of program could play a role in decreasing disparities in COVID outcomes if scaled more broadly.”
Outside expert: COVID Watch bring new approach to digital health monitoring
The study not only highlights the efficacy and sustainment of the COVID Watch program, but it sheds light on the possibility of using text message monitoring systems on other chronic disease conditions, said Jamie Faro, PhD, who was not involved in the study.
“It brings a new approach to health monitoring using digital means, which may lessen the burden on health care providers and be more cost effective than usual care approaches,” said Dr. Faro, who is assistant professor at the department of population and quantitative health sciences at the University of Massachusetts, Worcester. “Text messaging, which is used by over 80% of Americans, can allow us to reach a large percentage of the population for remote health care monitoring.”
Researchers of the current study said the findings “reveal a model for outpatient health system management of patients with COVID-19 and possibly other conditions where the early detection of clinical declines is critical.” Dr. Faro said that COVID Watch can have a measurable impact on an outcome that is truly life or death. However, it would be critical to understand how to reach those who either “were not offered or refused to take part in the program.”
The authors of the paper and Dr. Faro had no disclosures.
COVID Watch, a text message–based remote monitoring program developed by the University of Pennsylvania Health System, was associated with a 68% reduction in the risk of death, compared with those who received usual care. This was the main finding of a paper published in the Annals of Internal Medicine.
The investigators also determined that patients who enrolled in the program were more likely to seek care in the ED and when they did, they came in on average 2 days sooner than those who received usual care.
“When our clinical team designed COVID Watch the goal was to facilitate hospital care for patients who require it, while supporting access to care for patients who can safely remain at home,” study author M. Kit Delgado, MD, MS, an assistant professor of emergency medicine and epidemiology at Penn Presbyterian Medical Center in Philadelphia, said in an interview.
Researchers had initially hoped COVID Watch would relieve pressure on EDs, Dr. Delgado said.
Significantly lower mortality seen among COVID Watch group
For the study, Dr. Delgado and colleagues enrolled 3,488 patients in COVID Watch and 4,377 in the usual care group to compare outcomes at 30 and 60 days.
“We didn’t include patients who were diagnosed with COVID in the ER or hospital, so this is a lower-risk cohort of patients who test positive in outpatient settings,” Dr. Delgado noted. “Outpatients who received usual care and COVID Watch both had relatively low mortality, but it was significantly lower in those who were in COVID Watch.”
The researchers found that 3 patients in the COVID Watch group died within 30 days of their enrollment, compared with 12 in the control group. At 60 days after enrollment, 5 people within COVID Watch died, compared with 16 not using the system. More than one-third of the deaths in the usual care group occurred outside the hospital, compared with zero deaths among those in COVID Watch.
More than half of program participants were Black or Latino
The messaging system also reduced mortality rates among “all major racial and ethnic subgroups,” the researchers said, with more than 50% of the patients enrolled in COVID Watch having been Black or Latino.
“This is important because Black and Hispanic communities have experienced higher exposure and infection rates, decreased access to care, and have had higher mortality rates,” Dr. Delgado said. “Therefore, the results imply that this type of program could play a role in decreasing disparities in COVID outcomes if scaled more broadly.”
Outside expert: COVID Watch bring new approach to digital health monitoring
The study not only highlights the efficacy and sustainment of the COVID Watch program, but it sheds light on the possibility of using text message monitoring systems on other chronic disease conditions, said Jamie Faro, PhD, who was not involved in the study.
“It brings a new approach to health monitoring using digital means, which may lessen the burden on health care providers and be more cost effective than usual care approaches,” said Dr. Faro, who is assistant professor at the department of population and quantitative health sciences at the University of Massachusetts, Worcester. “Text messaging, which is used by over 80% of Americans, can allow us to reach a large percentage of the population for remote health care monitoring.”
Researchers of the current study said the findings “reveal a model for outpatient health system management of patients with COVID-19 and possibly other conditions where the early detection of clinical declines is critical.” Dr. Faro said that COVID Watch can have a measurable impact on an outcome that is truly life or death. However, it would be critical to understand how to reach those who either “were not offered or refused to take part in the program.”
The authors of the paper and Dr. Faro had no disclosures.
FROM ANNALS OF INTERNAL MEDICINE
Children and COVID: Youngest vaccinees off to a slower start

Specific figures for children aged 5-11 years are not yet available, but CDC data show that 1.55 million children under the age of 12 years had received at least one dose of COVID-19 vaccine as of Nov. 15, of whom almost 204,000 already had been vaccinated before Nov. 2. For children aged 12-15, the first 2 weeks after approval on May 12 produced almost 2.1 million vaccine initiations, according to the CDC’s COVID Data Tracker.
That dataset reveals several other noteworthy differences between the two age groups in the 10 days after approval:
- There were over 7,000 vaccine initiations on the first day in the 12-15 group; the younger group had 32.
- The older children reached 100,000 per day in 3 days; the younger children took 8 days.
- The older group topped 200,000 vaccinations per day on six different days; the younger group didn’t get above 175,000.
Children under 12 made up 27.5% of vaccine initiations in all age groups during the 2 weeks from Nov. 2 to Nov. 15, versus 3.4% for 12- to 15-year-olds and 1.2% for 16- and 17-year-olds, the CDC said, while also reporting that 3.6% of children under age 12 had received at least one dose of the COVID vaccine, compared with 57.8% of those aged 12-15 and 64.4% of 16- to 17-year-olds.
Meanwhile, the first full week of November marked the second consecutive increase in the number of weekly child COVID cases, with 122,000 reported for Nov. 5-11. The number of new cases has now surpassed 100,000 for 14 consecutive weeks, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report. That report, which covers state health departments, has not included current information from Alabama, Nebraska, and Texas since the summer.
Regionally, the increases over the past 2 weeks were spread out among the East, the Midwest, and the West, while the decline that had been going on for several weeks in the South has largely come to a halt. The states with the highest percent increases over those 2 weeks are all in New England: Maine, New Hampshire, and Vermont, the AAP and CHA noted. In a separate report, the AAP said that Vermont has the second-highest child vaccination rate (81%) in the country, just behind Massachusetts (82%).

Specific figures for children aged 5-11 years are not yet available, but CDC data show that 1.55 million children under the age of 12 years had received at least one dose of COVID-19 vaccine as of Nov. 15, of whom almost 204,000 already had been vaccinated before Nov. 2. For children aged 12-15, the first 2 weeks after approval on May 12 produced almost 2.1 million vaccine initiations, according to the CDC’s COVID Data Tracker.
That dataset reveals several other noteworthy differences between the two age groups in the 10 days after approval:
- There were over 7,000 vaccine initiations on the first day in the 12-15 group; the younger group had 32.
- The older children reached 100,000 per day in 3 days; the younger children took 8 days.
- The older group topped 200,000 vaccinations per day on six different days; the younger group didn’t get above 175,000.
Children under 12 made up 27.5% of vaccine initiations in all age groups during the 2 weeks from Nov. 2 to Nov. 15, versus 3.4% for 12- to 15-year-olds and 1.2% for 16- and 17-year-olds, the CDC said, while also reporting that 3.6% of children under age 12 had received at least one dose of the COVID vaccine, compared with 57.8% of those aged 12-15 and 64.4% of 16- to 17-year-olds.
Meanwhile, the first full week of November marked the second consecutive increase in the number of weekly child COVID cases, with 122,000 reported for Nov. 5-11. The number of new cases has now surpassed 100,000 for 14 consecutive weeks, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report. That report, which covers state health departments, has not included current information from Alabama, Nebraska, and Texas since the summer.
Regionally, the increases over the past 2 weeks were spread out among the East, the Midwest, and the West, while the decline that had been going on for several weeks in the South has largely come to a halt. The states with the highest percent increases over those 2 weeks are all in New England: Maine, New Hampshire, and Vermont, the AAP and CHA noted. In a separate report, the AAP said that Vermont has the second-highest child vaccination rate (81%) in the country, just behind Massachusetts (82%).

Specific figures for children aged 5-11 years are not yet available, but CDC data show that 1.55 million children under the age of 12 years had received at least one dose of COVID-19 vaccine as of Nov. 15, of whom almost 204,000 already had been vaccinated before Nov. 2. For children aged 12-15, the first 2 weeks after approval on May 12 produced almost 2.1 million vaccine initiations, according to the CDC’s COVID Data Tracker.
That dataset reveals several other noteworthy differences between the two age groups in the 10 days after approval:
- There were over 7,000 vaccine initiations on the first day in the 12-15 group; the younger group had 32.
- The older children reached 100,000 per day in 3 days; the younger children took 8 days.
- The older group topped 200,000 vaccinations per day on six different days; the younger group didn’t get above 175,000.
Children under 12 made up 27.5% of vaccine initiations in all age groups during the 2 weeks from Nov. 2 to Nov. 15, versus 3.4% for 12- to 15-year-olds and 1.2% for 16- and 17-year-olds, the CDC said, while also reporting that 3.6% of children under age 12 had received at least one dose of the COVID vaccine, compared with 57.8% of those aged 12-15 and 64.4% of 16- to 17-year-olds.
Meanwhile, the first full week of November marked the second consecutive increase in the number of weekly child COVID cases, with 122,000 reported for Nov. 5-11. The number of new cases has now surpassed 100,000 for 14 consecutive weeks, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report. That report, which covers state health departments, has not included current information from Alabama, Nebraska, and Texas since the summer.
Regionally, the increases over the past 2 weeks were spread out among the East, the Midwest, and the West, while the decline that had been going on for several weeks in the South has largely come to a halt. The states with the highest percent increases over those 2 weeks are all in New England: Maine, New Hampshire, and Vermont, the AAP and CHA noted. In a separate report, the AAP said that Vermont has the second-highest child vaccination rate (81%) in the country, just behind Massachusetts (82%).
New x-ray technique shows COVID-19 lung in unprecedented detail
A recent article published in Nature Methods highlights how hierarchical phase-contrast tomography (HiP-CT), an x-ray phase propagation technique that uses spatial coherence to conduct three-dimensional scans of organs ex vivo, may offer clinicians greater insights into disease processes.
“It is not a clinical technique as such,” said Claire Walsh PhD, a biophysicist and senior research fellow at the Center for Advanced Biomedical Imaging, University College London, and one of the authors of the article. She stressed that HiP-CT is used ex vivo.
“This technology uses x-rays from a fourth-generation x-ray source, the European Synchrotron Radiation Facility’s Extremely Brilliant Source. It is an incredibly bright x-ray source,” said Dr. Walsh in an interview. She said synchrotron x-ray tomography provides a much enhanced view of the lungs of persons who had had COVID-19. “We are looking at a different property of the x-ray waves. We are looking at a phase shift. [HiP-CT] is much, much more sensitive to small changes in the tissue than x-ray or CT. Another massive advantage of HiP-CT is the resolution it offers. The resolution goes down to single cells inside an intact human organ,” she said.
The resolution permits researchers to view blood vessels 5 μm in diameter in an intact lung. In comparison, clinical CT images show blood vessels of around 1 mm in diameter – 200 times larger.
“This technique will help us understand the structure of organs at a more fundamental level,” said Dr. Walsh. She noted that the technology has been valuable in allowing greater understanding of COVID-19 disease process. “This is about building an understanding of what the disease is doing in our bodies. If we don’t understand what the disease is changing structurally, it is very hard to understand how to go about developing treatments,” she said.
There are few synchrotron radiation facilities, so this technology is not widely available. Because of the very high radiation dose, the technique will be used ex vivo for the foreseeable future, Dr. Walsh said.
“The x-ray dose is incredibly high; 2-kg normal CT scans are approximately 100 mG [milligauss]. This is 20,000 times more than a medical CT scan,” explained Dr. Walsh. “We don’t really have plans for this to become an in vivo human technique. We are aiming that we will be able to register clinical scans to HiP-CT in a few cases, and so HiP-CT will become a calibration for analyzing clinical techniques.”
Elsie T. Nguyen, MD, FRCPC, vice-president of the Canadian Society of Thoracic Radiology and associate professor of radiology, University of Toronto, noted that the technology will be valuable in pathology and radiology.
“HiP-CT appears to be an exciting new development that can help physicians, including radiologists, understand pathology that was once beyond the spatial resolution of computed tomography scans,” said Dr. Nguyen in an interview. “The fact that vascular abnormalities particularly relating to severe COVID-19 pneumonia can be visualized to the micron level is very novel and exciting. This will help us understand better from a mechanistic point of view what is happening to the blood vessels that contributes to worse outcomes, like shunting of blood or blood clots, and may have applications for prognostication to predict which patients are likely to survive severe COVID-19 pneumonia.”
Dr. Nguyen noted that HiP-CT could help thoracic radiologists better visualize honeycomb cysts associated with fibrotic interstitial lung disease (ILD). It could help to classify the type of fibrotic ILD and inform patient prognosis.
“Currently, we struggle to differentiate early honeycomb cysts, which are a sign of more advanced lung destruction, from traction bronchiolectasis, that is, dilated airways due to surrounding fibrotic lung, on high-resolution computed tomography of the lungs,” said Dr. Nguyen. She said HiP-CT was very promising and had many applications in addition to visualizing the lungs.
The research was funded by the Chan Zuckerberg Initiative, the ESRF, the UK-MRC, and the Royal Academy of Engineering. Dr. Walsh and Dr. Nguyen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A recent article published in Nature Methods highlights how hierarchical phase-contrast tomography (HiP-CT), an x-ray phase propagation technique that uses spatial coherence to conduct three-dimensional scans of organs ex vivo, may offer clinicians greater insights into disease processes.
“It is not a clinical technique as such,” said Claire Walsh PhD, a biophysicist and senior research fellow at the Center for Advanced Biomedical Imaging, University College London, and one of the authors of the article. She stressed that HiP-CT is used ex vivo.
“This technology uses x-rays from a fourth-generation x-ray source, the European Synchrotron Radiation Facility’s Extremely Brilliant Source. It is an incredibly bright x-ray source,” said Dr. Walsh in an interview. She said synchrotron x-ray tomography provides a much enhanced view of the lungs of persons who had had COVID-19. “We are looking at a different property of the x-ray waves. We are looking at a phase shift. [HiP-CT] is much, much more sensitive to small changes in the tissue than x-ray or CT. Another massive advantage of HiP-CT is the resolution it offers. The resolution goes down to single cells inside an intact human organ,” she said.
The resolution permits researchers to view blood vessels 5 μm in diameter in an intact lung. In comparison, clinical CT images show blood vessels of around 1 mm in diameter – 200 times larger.
“This technique will help us understand the structure of organs at a more fundamental level,” said Dr. Walsh. She noted that the technology has been valuable in allowing greater understanding of COVID-19 disease process. “This is about building an understanding of what the disease is doing in our bodies. If we don’t understand what the disease is changing structurally, it is very hard to understand how to go about developing treatments,” she said.
There are few synchrotron radiation facilities, so this technology is not widely available. Because of the very high radiation dose, the technique will be used ex vivo for the foreseeable future, Dr. Walsh said.
“The x-ray dose is incredibly high; 2-kg normal CT scans are approximately 100 mG [milligauss]. This is 20,000 times more than a medical CT scan,” explained Dr. Walsh. “We don’t really have plans for this to become an in vivo human technique. We are aiming that we will be able to register clinical scans to HiP-CT in a few cases, and so HiP-CT will become a calibration for analyzing clinical techniques.”
Elsie T. Nguyen, MD, FRCPC, vice-president of the Canadian Society of Thoracic Radiology and associate professor of radiology, University of Toronto, noted that the technology will be valuable in pathology and radiology.
“HiP-CT appears to be an exciting new development that can help physicians, including radiologists, understand pathology that was once beyond the spatial resolution of computed tomography scans,” said Dr. Nguyen in an interview. “The fact that vascular abnormalities particularly relating to severe COVID-19 pneumonia can be visualized to the micron level is very novel and exciting. This will help us understand better from a mechanistic point of view what is happening to the blood vessels that contributes to worse outcomes, like shunting of blood or blood clots, and may have applications for prognostication to predict which patients are likely to survive severe COVID-19 pneumonia.”
Dr. Nguyen noted that HiP-CT could help thoracic radiologists better visualize honeycomb cysts associated with fibrotic interstitial lung disease (ILD). It could help to classify the type of fibrotic ILD and inform patient prognosis.
“Currently, we struggle to differentiate early honeycomb cysts, which are a sign of more advanced lung destruction, from traction bronchiolectasis, that is, dilated airways due to surrounding fibrotic lung, on high-resolution computed tomography of the lungs,” said Dr. Nguyen. She said HiP-CT was very promising and had many applications in addition to visualizing the lungs.
The research was funded by the Chan Zuckerberg Initiative, the ESRF, the UK-MRC, and the Royal Academy of Engineering. Dr. Walsh and Dr. Nguyen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A recent article published in Nature Methods highlights how hierarchical phase-contrast tomography (HiP-CT), an x-ray phase propagation technique that uses spatial coherence to conduct three-dimensional scans of organs ex vivo, may offer clinicians greater insights into disease processes.
“It is not a clinical technique as such,” said Claire Walsh PhD, a biophysicist and senior research fellow at the Center for Advanced Biomedical Imaging, University College London, and one of the authors of the article. She stressed that HiP-CT is used ex vivo.
“This technology uses x-rays from a fourth-generation x-ray source, the European Synchrotron Radiation Facility’s Extremely Brilliant Source. It is an incredibly bright x-ray source,” said Dr. Walsh in an interview. She said synchrotron x-ray tomography provides a much enhanced view of the lungs of persons who had had COVID-19. “We are looking at a different property of the x-ray waves. We are looking at a phase shift. [HiP-CT] is much, much more sensitive to small changes in the tissue than x-ray or CT. Another massive advantage of HiP-CT is the resolution it offers. The resolution goes down to single cells inside an intact human organ,” she said.
The resolution permits researchers to view blood vessels 5 μm in diameter in an intact lung. In comparison, clinical CT images show blood vessels of around 1 mm in diameter – 200 times larger.
“This technique will help us understand the structure of organs at a more fundamental level,” said Dr. Walsh. She noted that the technology has been valuable in allowing greater understanding of COVID-19 disease process. “This is about building an understanding of what the disease is doing in our bodies. If we don’t understand what the disease is changing structurally, it is very hard to understand how to go about developing treatments,” she said.
There are few synchrotron radiation facilities, so this technology is not widely available. Because of the very high radiation dose, the technique will be used ex vivo for the foreseeable future, Dr. Walsh said.
“The x-ray dose is incredibly high; 2-kg normal CT scans are approximately 100 mG [milligauss]. This is 20,000 times more than a medical CT scan,” explained Dr. Walsh. “We don’t really have plans for this to become an in vivo human technique. We are aiming that we will be able to register clinical scans to HiP-CT in a few cases, and so HiP-CT will become a calibration for analyzing clinical techniques.”
Elsie T. Nguyen, MD, FRCPC, vice-president of the Canadian Society of Thoracic Radiology and associate professor of radiology, University of Toronto, noted that the technology will be valuable in pathology and radiology.
“HiP-CT appears to be an exciting new development that can help physicians, including radiologists, understand pathology that was once beyond the spatial resolution of computed tomography scans,” said Dr. Nguyen in an interview. “The fact that vascular abnormalities particularly relating to severe COVID-19 pneumonia can be visualized to the micron level is very novel and exciting. This will help us understand better from a mechanistic point of view what is happening to the blood vessels that contributes to worse outcomes, like shunting of blood or blood clots, and may have applications for prognostication to predict which patients are likely to survive severe COVID-19 pneumonia.”
Dr. Nguyen noted that HiP-CT could help thoracic radiologists better visualize honeycomb cysts associated with fibrotic interstitial lung disease (ILD). It could help to classify the type of fibrotic ILD and inform patient prognosis.
“Currently, we struggle to differentiate early honeycomb cysts, which are a sign of more advanced lung destruction, from traction bronchiolectasis, that is, dilated airways due to surrounding fibrotic lung, on high-resolution computed tomography of the lungs,” said Dr. Nguyen. She said HiP-CT was very promising and had many applications in addition to visualizing the lungs.
The research was funded by the Chan Zuckerberg Initiative, the ESRF, the UK-MRC, and the Royal Academy of Engineering. Dr. Walsh and Dr. Nguyen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
High-dose fish oil: ‘Intriguing’ results in COVID-19
A high dose of the purified form of eicosapentaenoic acid, icosapent ethyl (Vascepa, Amarin), failed to significantly reduce hospitalizations or death in patients infected with COVID-19 in the PREPARE-IT 2 study.
The study did, however, show a favorable trend, with a 16% reduction in the primary endpoint of death or an indication for hospitalization. All secondary endpoints were also numerically reduced, but none reached statistical significance.
The product was also well tolerated over the 28 days of the study period, even though a new high-loading dose was used, with no increase in atrial fibrillation or bleeding or other adverse events versus placebo, although there was a slightly higher rate of discontinuation.
The trial was presented at the American Heart Association scientific sessions on Nov. 15 by Rafael Díaz, MD, director of Estudios Clínicos Latinoamérica in Rosario, Argentina.
“Larger, randomized trials powered for a relative risk reduction of around 15% with icosapent ethyl are needed to establish whether or not this product may have a role in the management of COVID-positive outpatients,” Dr. Diaz concluded.
‘Intriguing signals’
Commenting on the study, Manesh Patel, MD, chief of the division of cardiology and codirector of the Heart Center at Duke University, Durham, N.C., and chair of the Scientific Sessions scientific program, said that: “Certainly there are some intriguing signals.”
“I think the trend is valuable, but do we need a larger trial to confirm a benefit? I will leave that to the clinical community to decide,” Dr. Patel added. “But it is hard to power a trial to get that answer, and the world of COVID has changed since this trial started with vaccines now available and new therapeutics coming. So, there’s going to be a competing landscape.”
Discussing the trial at an AHA news briefing, Erin Michos, MD, associate professor of medicine within the division of cardiology at Johns Hopkins University, Baltimore, said: “Results showed that everything trended in the right direction, but did not reach statistical significance largely because there were fewer events than anticipated. COVID hospitalizations are going down because of the broad adoption of vaccines, which meant that this study didn’t quite meet its endpoint.”
But, she added: “Reassuringly, even with the higher loading dose, there was no increased risk of [atrial fibrillation] when used for just 28 days, and no increased risk in bleeding, so there was very good safety.”
“We need a larger trial to really definitely show whether icosapent ethyl can or cannot help COVID-positive outpatients, but I think a better prevention strategy would be the broad adoption of vaccinations globally,” Dr. Michos concluded.
‘A pretty big ask’
Donald Lloyd-Jones, MD, AHA president and designated discussant at the late-breaking science session, congratulated the investigators on conducting “a very nice pragmatic trial in the midst of the COVID pandemic.”
Dr. Lloyd-Jones concluded that the broad range of potentially beneficial actions of icosapent ethyl – including antitriglyceride, anti-inflammatory, antioxidant, and antithrombotic effects – leads to the possibility of it helping in COVID, but he added that “this is a pretty big ask for a fish oil supplement given short term.”
Presenting the study, Dr. Diaz noted that there are limited options for the outpatient treatment of patients with COVID-19 infection, and it is believed that inflammation plays a major role in worsening the severity of the infection.
He pointed out that previous data support a potential role of omega-3 fatty acids in reducing inflammation and infection, and that icosapent ethyl has shown a reduction in major cardiovascular events in the REDUCE-IT trial, with the mechanism thought to involve anti-inflammatory effects.
In the first trial to investigate the role of icosapent ethyl in COVID-19, PREPARE-IT, the product did not prevent uninfected individuals at risk from COVID from becoming infected with the virus, but there was no increase in side effects versus placebo with use over a 60-day period.
A small study last year in 100 COVID-positive patients showed icosapent ethyl reduced C-reactive protein, an inflammatory marker, and also improved symptoms.
PREPARE-IT 2, a pragmatic web-based trial, was conducted to investigate whether icosapent ethyl in nonhospitalized patients with a positive diagnosis of COVID-19 could reduce hospitalization rates and complications.
The trial enrolled 2,052 patients (mean age, 50 years), of whom 1,010 were allocated to the active group and 1,042 to the placebo group. Inclusion criteria included individuals aged 40 years or older with a confirmed COVID-19 diagnosis and no more than 7 days from the onset of symptoms and without a clear indication for hospitalization.
Patients who were allocated to the active arm received icosapent ethyl at a dose of 8 g (four capsules every 12 hours, morning and evening) for the first 3 days, followed by 4 g (two capsules every 12 hours) thereafter (days 4-28).
The primary outcome, COVID-19–related hospitalization (indication for hospitalization or hospitalization) or death at 28 days, occurred in 11.16% of the active group and 13.69% of the placebo group, giving a hazard ratio of 0.84 (95% confidence interval, 0.65-1.08; P = .166)
Secondary outcomes showed similar positive trends, but none were significant. These included: death or still hospitalized at 28 days (HR, 0.74), major events (MI, stroke, death; HR, 0.38), and total mortality (HR, 0.52).
In terms of safety, there was no significant difference in total adverse events between the two groups (16.5% in the active group vs. 14.8% in the placebo group). The most common adverse effects were constipation (2.7%), diarrhea (7.2%), and nausea (4%), but these were not significantly different from placebo. There were, however, more discontinuations in the active group (7% vs. 4%).
Dr. Diaz pointed out that the PREPARE-IT 2 trial was started in May 2020, when there wasn’t much known about the COVID-19 condition, and there were no vaccines or treatments, so hospitalization rates were high.
“We were hoping to see a 25%-30% reduction in hospitalizations with icosapent ethyl, and the trial was powered for that sort of reduction, but today we know we can expect a more modest reduction of about 15%,” Dr. Diaz concluded. “But to show that, we need a much larger trial with 8,000 or 9,000 patients, and that will be much more difficult to conduct.”
The PREPARE-IT 2 study was funded by Amarin. Dr. Diaz has received grants from Dalcor, Amarin, PHRI, and Lepetit.
A version of this article first appeared on Medscape.com.
A high dose of the purified form of eicosapentaenoic acid, icosapent ethyl (Vascepa, Amarin), failed to significantly reduce hospitalizations or death in patients infected with COVID-19 in the PREPARE-IT 2 study.
The study did, however, show a favorable trend, with a 16% reduction in the primary endpoint of death or an indication for hospitalization. All secondary endpoints were also numerically reduced, but none reached statistical significance.
The product was also well tolerated over the 28 days of the study period, even though a new high-loading dose was used, with no increase in atrial fibrillation or bleeding or other adverse events versus placebo, although there was a slightly higher rate of discontinuation.
The trial was presented at the American Heart Association scientific sessions on Nov. 15 by Rafael Díaz, MD, director of Estudios Clínicos Latinoamérica in Rosario, Argentina.
“Larger, randomized trials powered for a relative risk reduction of around 15% with icosapent ethyl are needed to establish whether or not this product may have a role in the management of COVID-positive outpatients,” Dr. Diaz concluded.
‘Intriguing signals’
Commenting on the study, Manesh Patel, MD, chief of the division of cardiology and codirector of the Heart Center at Duke University, Durham, N.C., and chair of the Scientific Sessions scientific program, said that: “Certainly there are some intriguing signals.”
“I think the trend is valuable, but do we need a larger trial to confirm a benefit? I will leave that to the clinical community to decide,” Dr. Patel added. “But it is hard to power a trial to get that answer, and the world of COVID has changed since this trial started with vaccines now available and new therapeutics coming. So, there’s going to be a competing landscape.”
Discussing the trial at an AHA news briefing, Erin Michos, MD, associate professor of medicine within the division of cardiology at Johns Hopkins University, Baltimore, said: “Results showed that everything trended in the right direction, but did not reach statistical significance largely because there were fewer events than anticipated. COVID hospitalizations are going down because of the broad adoption of vaccines, which meant that this study didn’t quite meet its endpoint.”
But, she added: “Reassuringly, even with the higher loading dose, there was no increased risk of [atrial fibrillation] when used for just 28 days, and no increased risk in bleeding, so there was very good safety.”
“We need a larger trial to really definitely show whether icosapent ethyl can or cannot help COVID-positive outpatients, but I think a better prevention strategy would be the broad adoption of vaccinations globally,” Dr. Michos concluded.
‘A pretty big ask’
Donald Lloyd-Jones, MD, AHA president and designated discussant at the late-breaking science session, congratulated the investigators on conducting “a very nice pragmatic trial in the midst of the COVID pandemic.”
Dr. Lloyd-Jones concluded that the broad range of potentially beneficial actions of icosapent ethyl – including antitriglyceride, anti-inflammatory, antioxidant, and antithrombotic effects – leads to the possibility of it helping in COVID, but he added that “this is a pretty big ask for a fish oil supplement given short term.”
Presenting the study, Dr. Diaz noted that there are limited options for the outpatient treatment of patients with COVID-19 infection, and it is believed that inflammation plays a major role in worsening the severity of the infection.
He pointed out that previous data support a potential role of omega-3 fatty acids in reducing inflammation and infection, and that icosapent ethyl has shown a reduction in major cardiovascular events in the REDUCE-IT trial, with the mechanism thought to involve anti-inflammatory effects.
In the first trial to investigate the role of icosapent ethyl in COVID-19, PREPARE-IT, the product did not prevent uninfected individuals at risk from COVID from becoming infected with the virus, but there was no increase in side effects versus placebo with use over a 60-day period.
A small study last year in 100 COVID-positive patients showed icosapent ethyl reduced C-reactive protein, an inflammatory marker, and also improved symptoms.
PREPARE-IT 2, a pragmatic web-based trial, was conducted to investigate whether icosapent ethyl in nonhospitalized patients with a positive diagnosis of COVID-19 could reduce hospitalization rates and complications.
The trial enrolled 2,052 patients (mean age, 50 years), of whom 1,010 were allocated to the active group and 1,042 to the placebo group. Inclusion criteria included individuals aged 40 years or older with a confirmed COVID-19 diagnosis and no more than 7 days from the onset of symptoms and without a clear indication for hospitalization.
Patients who were allocated to the active arm received icosapent ethyl at a dose of 8 g (four capsules every 12 hours, morning and evening) for the first 3 days, followed by 4 g (two capsules every 12 hours) thereafter (days 4-28).
The primary outcome, COVID-19–related hospitalization (indication for hospitalization or hospitalization) or death at 28 days, occurred in 11.16% of the active group and 13.69% of the placebo group, giving a hazard ratio of 0.84 (95% confidence interval, 0.65-1.08; P = .166)
Secondary outcomes showed similar positive trends, but none were significant. These included: death or still hospitalized at 28 days (HR, 0.74), major events (MI, stroke, death; HR, 0.38), and total mortality (HR, 0.52).
In terms of safety, there was no significant difference in total adverse events between the two groups (16.5% in the active group vs. 14.8% in the placebo group). The most common adverse effects were constipation (2.7%), diarrhea (7.2%), and nausea (4%), but these were not significantly different from placebo. There were, however, more discontinuations in the active group (7% vs. 4%).
Dr. Diaz pointed out that the PREPARE-IT 2 trial was started in May 2020, when there wasn’t much known about the COVID-19 condition, and there were no vaccines or treatments, so hospitalization rates were high.
“We were hoping to see a 25%-30% reduction in hospitalizations with icosapent ethyl, and the trial was powered for that sort of reduction, but today we know we can expect a more modest reduction of about 15%,” Dr. Diaz concluded. “But to show that, we need a much larger trial with 8,000 or 9,000 patients, and that will be much more difficult to conduct.”
The PREPARE-IT 2 study was funded by Amarin. Dr. Diaz has received grants from Dalcor, Amarin, PHRI, and Lepetit.
A version of this article first appeared on Medscape.com.
A high dose of the purified form of eicosapentaenoic acid, icosapent ethyl (Vascepa, Amarin), failed to significantly reduce hospitalizations or death in patients infected with COVID-19 in the PREPARE-IT 2 study.
The study did, however, show a favorable trend, with a 16% reduction in the primary endpoint of death or an indication for hospitalization. All secondary endpoints were also numerically reduced, but none reached statistical significance.
The product was also well tolerated over the 28 days of the study period, even though a new high-loading dose was used, with no increase in atrial fibrillation or bleeding or other adverse events versus placebo, although there was a slightly higher rate of discontinuation.
The trial was presented at the American Heart Association scientific sessions on Nov. 15 by Rafael Díaz, MD, director of Estudios Clínicos Latinoamérica in Rosario, Argentina.
“Larger, randomized trials powered for a relative risk reduction of around 15% with icosapent ethyl are needed to establish whether or not this product may have a role in the management of COVID-positive outpatients,” Dr. Diaz concluded.
‘Intriguing signals’
Commenting on the study, Manesh Patel, MD, chief of the division of cardiology and codirector of the Heart Center at Duke University, Durham, N.C., and chair of the Scientific Sessions scientific program, said that: “Certainly there are some intriguing signals.”
“I think the trend is valuable, but do we need a larger trial to confirm a benefit? I will leave that to the clinical community to decide,” Dr. Patel added. “But it is hard to power a trial to get that answer, and the world of COVID has changed since this trial started with vaccines now available and new therapeutics coming. So, there’s going to be a competing landscape.”
Discussing the trial at an AHA news briefing, Erin Michos, MD, associate professor of medicine within the division of cardiology at Johns Hopkins University, Baltimore, said: “Results showed that everything trended in the right direction, but did not reach statistical significance largely because there were fewer events than anticipated. COVID hospitalizations are going down because of the broad adoption of vaccines, which meant that this study didn’t quite meet its endpoint.”
But, she added: “Reassuringly, even with the higher loading dose, there was no increased risk of [atrial fibrillation] when used for just 28 days, and no increased risk in bleeding, so there was very good safety.”
“We need a larger trial to really definitely show whether icosapent ethyl can or cannot help COVID-positive outpatients, but I think a better prevention strategy would be the broad adoption of vaccinations globally,” Dr. Michos concluded.
‘A pretty big ask’
Donald Lloyd-Jones, MD, AHA president and designated discussant at the late-breaking science session, congratulated the investigators on conducting “a very nice pragmatic trial in the midst of the COVID pandemic.”
Dr. Lloyd-Jones concluded that the broad range of potentially beneficial actions of icosapent ethyl – including antitriglyceride, anti-inflammatory, antioxidant, and antithrombotic effects – leads to the possibility of it helping in COVID, but he added that “this is a pretty big ask for a fish oil supplement given short term.”
Presenting the study, Dr. Diaz noted that there are limited options for the outpatient treatment of patients with COVID-19 infection, and it is believed that inflammation plays a major role in worsening the severity of the infection.
He pointed out that previous data support a potential role of omega-3 fatty acids in reducing inflammation and infection, and that icosapent ethyl has shown a reduction in major cardiovascular events in the REDUCE-IT trial, with the mechanism thought to involve anti-inflammatory effects.
In the first trial to investigate the role of icosapent ethyl in COVID-19, PREPARE-IT, the product did not prevent uninfected individuals at risk from COVID from becoming infected with the virus, but there was no increase in side effects versus placebo with use over a 60-day period.
A small study last year in 100 COVID-positive patients showed icosapent ethyl reduced C-reactive protein, an inflammatory marker, and also improved symptoms.
PREPARE-IT 2, a pragmatic web-based trial, was conducted to investigate whether icosapent ethyl in nonhospitalized patients with a positive diagnosis of COVID-19 could reduce hospitalization rates and complications.
The trial enrolled 2,052 patients (mean age, 50 years), of whom 1,010 were allocated to the active group and 1,042 to the placebo group. Inclusion criteria included individuals aged 40 years or older with a confirmed COVID-19 diagnosis and no more than 7 days from the onset of symptoms and without a clear indication for hospitalization.
Patients who were allocated to the active arm received icosapent ethyl at a dose of 8 g (four capsules every 12 hours, morning and evening) for the first 3 days, followed by 4 g (two capsules every 12 hours) thereafter (days 4-28).
The primary outcome, COVID-19–related hospitalization (indication for hospitalization or hospitalization) or death at 28 days, occurred in 11.16% of the active group and 13.69% of the placebo group, giving a hazard ratio of 0.84 (95% confidence interval, 0.65-1.08; P = .166)
Secondary outcomes showed similar positive trends, but none were significant. These included: death or still hospitalized at 28 days (HR, 0.74), major events (MI, stroke, death; HR, 0.38), and total mortality (HR, 0.52).
In terms of safety, there was no significant difference in total adverse events between the two groups (16.5% in the active group vs. 14.8% in the placebo group). The most common adverse effects were constipation (2.7%), diarrhea (7.2%), and nausea (4%), but these were not significantly different from placebo. There were, however, more discontinuations in the active group (7% vs. 4%).
Dr. Diaz pointed out that the PREPARE-IT 2 trial was started in May 2020, when there wasn’t much known about the COVID-19 condition, and there were no vaccines or treatments, so hospitalization rates were high.
“We were hoping to see a 25%-30% reduction in hospitalizations with icosapent ethyl, and the trial was powered for that sort of reduction, but today we know we can expect a more modest reduction of about 15%,” Dr. Diaz concluded. “But to show that, we need a much larger trial with 8,000 or 9,000 patients, and that will be much more difficult to conduct.”
The PREPARE-IT 2 study was funded by Amarin. Dr. Diaz has received grants from Dalcor, Amarin, PHRI, and Lepetit.
A version of this article first appeared on Medscape.com.
FROM AHA 2021