User login
A Learning Health System Approach to Long COVID Care
The Veterans Health Administration (VHA)—along with systems across the world—has spent the past 2 years continuously adapting to meet the emerging needs of persons infected with COVID-19. With the development of effective vaccines and global efforts to mitigate transmission, attention has now shifted to long COVID care as the need for further outpatient health care becomes increasingly apparent.1,2
Background
Multiple terms describe the lingering, multisystem sequelae of COVID-19 that last longer than 4 weeks: long COVID, postacute COVID-19 syndrome, post-COVID condition, postacute sequalae of COVID-19, and COVID long hauler.1,3 Common symptoms include fatigue, shortness of breath, cough, sleep disorders, brain fog or cognitive dysfunction, depression, anxiety, pain, and changes in taste or smell that impact a person’s functioning.4,5 The multisystem nature of the postacute course of COVID-19 necessitates an interdisciplinary approach to devise comprehensive and individualized care plans.6-9 Research is needed to better understand this postacute state (eg, prevalence, underlying effects, characteristics of those who experience long COVID) to establish and evaluate cost-effective treatment approaches.
Many patients who are experiencing symptoms beyond the acute course of COVID-19 have been referred to general outpatient clinics or home health, which may lack the capacity and knowledge of this novel disease to effectively manage complex long COVID cases.2,3 To address this growing need, clinicians and leadership across a variety of disciplines and settings in the VHA created a community of practice (CoP) to create a mechanism for cross-facility communication, identify gaps in long COVID care and research, and cocreate knowledge on best practices for care delivery.
In this spirit, we are embracing a learning health system (LHS) approach that uses rapid-cycle methods to integrate data and real-world experience to iteratively evaluate and adapt models of long COVID care.10 Our clinically identified and data-driven objective is to provide high value health care to patients with long COVID sequalae by creating a framework to learn about this novel condition and develop innovative care models. This article provides an overview of our emerging LHS approach to the study of long COVID care that is fostering innovation and adaptability within the VHA. We describe 3 aspects of our engagement approach central to LHS: the ongoing development of a long COVID CoP dedicated to iteratively informing the bidirectional cycle of data from practice to research, results of a broad environmental scan of VHA long COVID care, and results of a survey administered to CoP members to inform ongoing needs of the community and identify early successful outcomes from participation.
Learning Health System Approach
The VHA is one of the largest integrated health care systems in the United States serving more than 9 million veterans.11 Since 2017, the VHA has articulated a vision to become an LHS that informs and improves patient-centered care through practice-based and data-driven research (eAppendix).12 During the early COVID-19 pandemic, an LHS approach in the VHA was critical to rapidly establishing a data infrastructure for disease surveillance, coordinating data-driven solutions, leveraging use of technology, collaborating across the globe to identify best practices, and implementing systematic responses (eg, policies, workforce adjustments).
Our long COVID CoP was developed as clinical observations and ongoing conversations with stakeholders (eg, veterans, health care practitioners [HCPs], leadership) identified a need to effectively identify and treat the growing number of veterans with long COVID. This clinical issue is compounded by the limited but emerging evidence on the clinical presentation of prolonged COVID-19 symptoms, treatment, and subsequent care pathways. The VHA’s efforts and lessons learned within the lens of an LHS are applicable to other systems confronting the complex identification and management of patients with persistent and encumbering long COVID symptoms. The VHA is building upon the LHS approach to proactively prepare for and address future clinical or public health challenges that require cross-system and sector collaborations, expediency, inclusivity, and patient/family centeredness.11
Community of Practice
As of January 25, 2022, our workgroup consisted of 128 VHA employees representing 29 VHA medical centers. Members of the multidisciplinary workgroup have diverse backgrounds with HCPs from primary care (eg, physicians, nurse practitioners), rehabilitation (eg, physical therapists), specialty care (eg, pulmonologists, physiatrists), mental health (eg, psychologists), and complementary and integrated health/Whole Health services (eg, practitoners of services such as yoga, tai chi, mindfulness, acupuncture). Members also include clinical, operations, and research leadership at local, regional, and national VHA levels. Our first objective as a large, diverse group was to establish shared goals, which included: (1) determining efficient communication pathways; (2) identifying gaps in care or research; and (3) cocreating knowledge to provide solutions to identified gaps.
Communication Mechanisms
Our first goal was to create an efficient mechanism for cross-facility communication. The initial CoP was formed in April 2021 and the first virtual meeting focused on reaching a consensus regarding the best way to communicate and proceed. We agreed to convene weekly at a consistent time, created a standard agenda template, and elected a lead facilitator of meeting proceedings. In addition, a member of the CoP recorded and took extensive meeting notes, which were later distributed to the entire CoP to accommodate varying schedules and ability to attend live meetings. Approximately 20 to 30 participants attend the meetings in real-time.
To consolidate working documents, information, and resources in one location, we created a platform to communicate via a Microsoft Teams channel. All CoP members are given access to the folders and allowed to add to the growing library of resources. Resources include clinical assessment and note templates for electronic documentation of care, site-specific process maps, relevant literature on screening and interventions identified by practice members, and meeting notes along with the recordings. A chat feature alerts CoP members to questions posed by other members. Any resources or information shared on the chat discussion are curated by CoP leaders to disseminate to all members. Importantly, this platform allowed us to communicate efficiently within the VHA organization by creating a centralized space for documents and the ability to correspond with all or select members of the CoP. Additional VHA employees can easily be referred and request access.
To increase awareness of the CoP, expand reach, and diversify perspectives, every participant was encouraged to invite colleagues and stakeholders with interest or experience in long COVID care to join. While patients are not included in this CoP, we are working closely with the VHA user experience workgroup (many members overlap) that is gathering patient and caregiver perspectives on their COVID-19 experience and long COVID care. Concurrently, CoP members and leadership facilitate communication and set up formal collaborations with other non-VHA health care systems to create an intersystem network of collaboration for long COVID care. This approach further enhances the speed at which we can work together to share lessons learned and stay up-to-date on emerging evidence surrounding long COVID care.
Identifying Gaps in Care and Research
Our second goal was to identify gaps in care or knowledge to inform future research and quality improvement initiatives, while also creating a foundation to cocreate knowledge about safe, effective care management of the novel long COVID sequelae. To translate knowledge, we must first identify and understand the gaps between the current, best available evidence and current care practices or policies impacting that delivery.13 As such, the structured meeting agenda and facilitated meeting discussions focused on understanding current clinical decision making and the evidence base. We shared VHA evidence synthesis reports and living rapid reviews on complications following COVID-19 illness (ie, major organ damage and posthospitalization health care use) that provided an objective evidence base on common long COVID complications.14,15
Since long COVID is a novel condition, we drew from literature in similar patient populations and translated that information in the context of our current knowledge of this unique syndrome. For example, we discussed the predominant and persistent symptom of fatigue post-COVID.5 In particular, the CoP discussed challenges in identifying and treating post-COVID fatigue, which is often a vague symptom with multiple or interacting etiologies that require a comprehensive, interdisciplinary approach. As such, we reviewed, adapted, and translated identification and treatment strategies from the literature on chronic fatigue syndrome to patients with post-COVID syndrome.16,17 We continue to work collaboratively and engage the appropriate stakeholders to provide input on the gaps to prioritize targeting.
Cocreate Knowledge
Our third goal was to cocreate knowledge regarding the care of patients with long COVID. To accomplish this, our structured meetings and communication pathways invited members to share experiences on the who (delivers and receives care), what (type of care or HCPs), when (identification of post-COVID and access), and how (eg, telehealth) of care to patients post-COVID. As part of the workgroup, we identified and shared resources on standardized, facility-level practices to reduce variability across the VHA system. These resources included intake/assessment forms, care processes, and batteries of tests/measures used for screening and assessment. The knowledge obtained from outside the CoP and cocreated within is being used to inform data-driven tools to support and evaluate care for patients with long COVID. As such, members of the workgroup are in the formative stages of participating in quality improvement innovation pilots to test technologies and processes designed to improve and validate long COVID care pathways. These technologies include screening tools, clinical decision support tools, and population health management technologies. In addition, we are developing a formal collaboration with the VHA Office of Research and Development to create standardized intake forms across VHA long COVID clinics to facilitate both clinical monitoring and research.
Surveys
The US Department of Veterans Affairs Central Office collaborated with our workgroup to draft an initial set of survey questions designed to understand how each VHA facility defines, identifies, and provides care to veterans experiencing post-COVID sequalae. The 41-question survey was distributed through regional directors and chief medical officers at 139 VHA facilities in August 2021. One hundred nineteen responses (86%) were received. Sixteen facilities indicated they had established programs and 26 facilities were considering a program. Our CoP had representation from the 16 facilities with established programs indicating the deep and well-connected nature of our grassroots efforts to bring together stakeholders to learn as part of a CoP.
A separate, follow-up survey generated responses from 18 facilities and identified the need to capture evolving innovations and to develop smaller workstreams (eg, best practices, electronic documentation templates, pathway for referrals, veteran engagement, outcome measures). The survey not only exposed ongoing challenges to providing long COVID care, but importantly, outlined the ways in which CoP members were leveraging community knowledge and resources to inform innovations and processes of care changes at their specific sites. Fourteen of 18 facilities with long COVID programs in place explicitly identified the CoP as a resource they have found most beneficial when employing such innovations. Specific innovations reported included changes in care delivery, engagement in active outreach with veterans and local facility, and infrastructure development to sustain local long COVID clinics (Table).
Future Directions
Our CoP strives to contribute to an evidence base for long COVID care. At the system level, the CoP has the potential to impact access and continuity of care by identifying appropriate processes and ensuring that VHA patients receive outreach and an opportunity for post-COVID care. Comprehensive care requires input from HCP, clinical leadership, and operations levels. In this sense, our CoP provides an opportunity for diverse stakeholders to come together, discuss barriers to screening and delivering post-COVID care, and create an action plan to remove or lessen such barriers.18 Part of the process to remove barriers is to identify and support efficient resource allocation. Our CoP has worked to address issues in resource allocation (eg, space, personnel) for post-COVID care. For example, one facility is currently implementing interdisciplinary virtual post-COVID care. Another facility identified and restructured working assignments for psychologists who served in different capacities throughout the system to fill the need within the long COVID team.
At the HCP level, the CoP is currently developing workshops, media campaigns, written clinical resources, skills training, publications, and webinars/seminars with continuing medical education credits.19 The CoP may also provide learning and growth opportunities, such as clinical or VHA operational fellowships and research grants.
We are still in the formative stages of post-COVID care and future efforts will explore patient-centered outcomes. We are drawing on the Centers for Disease Control and Prevention’s guidance for evaluating patients with long COVID symptoms and examining the feasibility within VHA, as well as patient perspectives on post-COVID sequalae, to ensure we are selecting assessments that measure patient-centered constructs.18
Conclusions
A VHA-wide LHS approach is identifying issues related to the identification, delivery, and evaluation of long COVID care. This long COVID CoP has developed an infrastructure for communication, identified gaps in care, and cocreated knowledge related to best current practices for post-COVID care. This work is contributing to systemwide LHS efforts dedicated to creating a culture of quality care and innovation and is a process that is transferrable to other areas of care in the VHA, as well as other health care systems. The LHS approach continues to be highly relevant as we persist through the COVID-19 pandemic and reimagine a postpandemic world.
Acknowledg
We thank all the members of the Veterans Health Administration long COVID Community of Practice who participate in the meetings and contribute to the sharing and spread of knowledge.
1. Sivan M, Halpin S, Hollingworth L, Snook N, Hickman K, Clifton I. Development of an integrated rehabilitation pathway for individuals recovering from COVID-19 in the community. J Rehabil Med. 2020;52(8):jrm00089. doi:10.2340/16501977-2727
2. Understanding the long-term health effects of COVID-19. EClinicalMedicine. 2020;26:100586. doi:10.1016/j.eclinm.2020.100586
3. Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. Published online August 11, 2020:m3026. doi:10.1136/bmj.m3026
4. Iwua CJ, Iwu CD, Wiysonge CS. The occurrence of long COVID: a rapid review. Pan Afr Med J. 2021;38. doi:10.11604/pamj.2021.38.65.27366
5. Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-605. doi:10.1001/jama.2020.12603
6. Gemelli Against COVID-19 Post-Acute Care Study Group. Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. 2020;32(8):1613-1620. doi:10.1007/s40520-020-01616-x
7. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi:10.1038/s41591-022-01689-3
8. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi:10.1038/s41586-021-03553-9
9. Ayoubkhani D, Bermingham C, Pouwels KB, et al. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ. 2022;377:e069676. doi:10.1136/bmj-2021-069676
10. Institute of Medicine (US) Roundtable on Evidence-Based Medicine, Olsen L, Aisner D, McGinnis JM, eds. The Learning Healthcare System: Workshop Summary. Washington (DC): National Academies Press (US); 2007. doi:10.17226/11903
11. Romanelli RJ, Azar KMJ, Sudat S, Hung D, Frosch DL, Pressman AR. Learning health system in crisis: lessons from the COVID-19 pandemic. Mayo Clin Proc Innov Qual Outcomes. 2021;5(1):171-176. doi:10.1016/j.mayocpiqo.2020.10.004
12. Atkins D, Kilbourne AM, Shulkin D. Moving from discovery to system-wide change: the role of research in a learning health care system: experience from three decades of health systems research in the Veterans Health Administration. Annu Rev Public Health. 2017;38:467-487. doi:10.1146/annurev-publhealth-031816-044255
13. Kitson A, Straus SE. The knowledge-to-action cycle: identifying the gaps. CMAJ. 2010;182(2):E73-77. doi:10.1503/cmaj.081231
14. Greer N, Bart B, Billington C, et al. COVID-19 post-acute care major organ damage: a living rapid review. Updated September 2021. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid-organ-damage.pdf
15. Sharpe JA, Burke C, Gordon AM, et al. COVID-19 post-hospitalization health care utilization: a living review. Updated February 2022. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid19-post-hosp.pdf
16. Bested AC, Marshall LM. Review of Myalgic Encephalomyelitis/chronic fatigue syndrome: an evidence-based approach to diagnosis and management by clinicians. Rev Environ Health. 2015;30(4):223-249. doi:10.1515/reveh-2015-0026
17. Yancey JR, Thomas SM. Chronic fatigue syndrome: diagnosis and treatment. Am Fam Physician. 2012;86(8):741-746.
18. Kotter JP, Cohen DS. Change Leadership The Kotter Collection. Harvard Business Review Press; 2014.
19. Brownson RC, Eyler AA, Harris JK, Moore JB, Tabak RG. Getting the word out: new approaches for disseminating public health science. J Public Health Manag Pract. 2018;24(2):102-111. doi:10.1097/PHH.0000000000000673
The Veterans Health Administration (VHA)—along with systems across the world—has spent the past 2 years continuously adapting to meet the emerging needs of persons infected with COVID-19. With the development of effective vaccines and global efforts to mitigate transmission, attention has now shifted to long COVID care as the need for further outpatient health care becomes increasingly apparent.1,2
Background
Multiple terms describe the lingering, multisystem sequelae of COVID-19 that last longer than 4 weeks: long COVID, postacute COVID-19 syndrome, post-COVID condition, postacute sequalae of COVID-19, and COVID long hauler.1,3 Common symptoms include fatigue, shortness of breath, cough, sleep disorders, brain fog or cognitive dysfunction, depression, anxiety, pain, and changes in taste or smell that impact a person’s functioning.4,5 The multisystem nature of the postacute course of COVID-19 necessitates an interdisciplinary approach to devise comprehensive and individualized care plans.6-9 Research is needed to better understand this postacute state (eg, prevalence, underlying effects, characteristics of those who experience long COVID) to establish and evaluate cost-effective treatment approaches.
Many patients who are experiencing symptoms beyond the acute course of COVID-19 have been referred to general outpatient clinics or home health, which may lack the capacity and knowledge of this novel disease to effectively manage complex long COVID cases.2,3 To address this growing need, clinicians and leadership across a variety of disciplines and settings in the VHA created a community of practice (CoP) to create a mechanism for cross-facility communication, identify gaps in long COVID care and research, and cocreate knowledge on best practices for care delivery.
In this spirit, we are embracing a learning health system (LHS) approach that uses rapid-cycle methods to integrate data and real-world experience to iteratively evaluate and adapt models of long COVID care.10 Our clinically identified and data-driven objective is to provide high value health care to patients with long COVID sequalae by creating a framework to learn about this novel condition and develop innovative care models. This article provides an overview of our emerging LHS approach to the study of long COVID care that is fostering innovation and adaptability within the VHA. We describe 3 aspects of our engagement approach central to LHS: the ongoing development of a long COVID CoP dedicated to iteratively informing the bidirectional cycle of data from practice to research, results of a broad environmental scan of VHA long COVID care, and results of a survey administered to CoP members to inform ongoing needs of the community and identify early successful outcomes from participation.
Learning Health System Approach
The VHA is one of the largest integrated health care systems in the United States serving more than 9 million veterans.11 Since 2017, the VHA has articulated a vision to become an LHS that informs and improves patient-centered care through practice-based and data-driven research (eAppendix).12 During the early COVID-19 pandemic, an LHS approach in the VHA was critical to rapidly establishing a data infrastructure for disease surveillance, coordinating data-driven solutions, leveraging use of technology, collaborating across the globe to identify best practices, and implementing systematic responses (eg, policies, workforce adjustments).
Our long COVID CoP was developed as clinical observations and ongoing conversations with stakeholders (eg, veterans, health care practitioners [HCPs], leadership) identified a need to effectively identify and treat the growing number of veterans with long COVID. This clinical issue is compounded by the limited but emerging evidence on the clinical presentation of prolonged COVID-19 symptoms, treatment, and subsequent care pathways. The VHA’s efforts and lessons learned within the lens of an LHS are applicable to other systems confronting the complex identification and management of patients with persistent and encumbering long COVID symptoms. The VHA is building upon the LHS approach to proactively prepare for and address future clinical or public health challenges that require cross-system and sector collaborations, expediency, inclusivity, and patient/family centeredness.11
Community of Practice
As of January 25, 2022, our workgroup consisted of 128 VHA employees representing 29 VHA medical centers. Members of the multidisciplinary workgroup have diverse backgrounds with HCPs from primary care (eg, physicians, nurse practitioners), rehabilitation (eg, physical therapists), specialty care (eg, pulmonologists, physiatrists), mental health (eg, psychologists), and complementary and integrated health/Whole Health services (eg, practitoners of services such as yoga, tai chi, mindfulness, acupuncture). Members also include clinical, operations, and research leadership at local, regional, and national VHA levels. Our first objective as a large, diverse group was to establish shared goals, which included: (1) determining efficient communication pathways; (2) identifying gaps in care or research; and (3) cocreating knowledge to provide solutions to identified gaps.
Communication Mechanisms
Our first goal was to create an efficient mechanism for cross-facility communication. The initial CoP was formed in April 2021 and the first virtual meeting focused on reaching a consensus regarding the best way to communicate and proceed. We agreed to convene weekly at a consistent time, created a standard agenda template, and elected a lead facilitator of meeting proceedings. In addition, a member of the CoP recorded and took extensive meeting notes, which were later distributed to the entire CoP to accommodate varying schedules and ability to attend live meetings. Approximately 20 to 30 participants attend the meetings in real-time.
To consolidate working documents, information, and resources in one location, we created a platform to communicate via a Microsoft Teams channel. All CoP members are given access to the folders and allowed to add to the growing library of resources. Resources include clinical assessment and note templates for electronic documentation of care, site-specific process maps, relevant literature on screening and interventions identified by practice members, and meeting notes along with the recordings. A chat feature alerts CoP members to questions posed by other members. Any resources or information shared on the chat discussion are curated by CoP leaders to disseminate to all members. Importantly, this platform allowed us to communicate efficiently within the VHA organization by creating a centralized space for documents and the ability to correspond with all or select members of the CoP. Additional VHA employees can easily be referred and request access.
To increase awareness of the CoP, expand reach, and diversify perspectives, every participant was encouraged to invite colleagues and stakeholders with interest or experience in long COVID care to join. While patients are not included in this CoP, we are working closely with the VHA user experience workgroup (many members overlap) that is gathering patient and caregiver perspectives on their COVID-19 experience and long COVID care. Concurrently, CoP members and leadership facilitate communication and set up formal collaborations with other non-VHA health care systems to create an intersystem network of collaboration for long COVID care. This approach further enhances the speed at which we can work together to share lessons learned and stay up-to-date on emerging evidence surrounding long COVID care.
Identifying Gaps in Care and Research
Our second goal was to identify gaps in care or knowledge to inform future research and quality improvement initiatives, while also creating a foundation to cocreate knowledge about safe, effective care management of the novel long COVID sequelae. To translate knowledge, we must first identify and understand the gaps between the current, best available evidence and current care practices or policies impacting that delivery.13 As such, the structured meeting agenda and facilitated meeting discussions focused on understanding current clinical decision making and the evidence base. We shared VHA evidence synthesis reports and living rapid reviews on complications following COVID-19 illness (ie, major organ damage and posthospitalization health care use) that provided an objective evidence base on common long COVID complications.14,15
Since long COVID is a novel condition, we drew from literature in similar patient populations and translated that information in the context of our current knowledge of this unique syndrome. For example, we discussed the predominant and persistent symptom of fatigue post-COVID.5 In particular, the CoP discussed challenges in identifying and treating post-COVID fatigue, which is often a vague symptom with multiple or interacting etiologies that require a comprehensive, interdisciplinary approach. As such, we reviewed, adapted, and translated identification and treatment strategies from the literature on chronic fatigue syndrome to patients with post-COVID syndrome.16,17 We continue to work collaboratively and engage the appropriate stakeholders to provide input on the gaps to prioritize targeting.
Cocreate Knowledge
Our third goal was to cocreate knowledge regarding the care of patients with long COVID. To accomplish this, our structured meetings and communication pathways invited members to share experiences on the who (delivers and receives care), what (type of care or HCPs), when (identification of post-COVID and access), and how (eg, telehealth) of care to patients post-COVID. As part of the workgroup, we identified and shared resources on standardized, facility-level practices to reduce variability across the VHA system. These resources included intake/assessment forms, care processes, and batteries of tests/measures used for screening and assessment. The knowledge obtained from outside the CoP and cocreated within is being used to inform data-driven tools to support and evaluate care for patients with long COVID. As such, members of the workgroup are in the formative stages of participating in quality improvement innovation pilots to test technologies and processes designed to improve and validate long COVID care pathways. These technologies include screening tools, clinical decision support tools, and population health management technologies. In addition, we are developing a formal collaboration with the VHA Office of Research and Development to create standardized intake forms across VHA long COVID clinics to facilitate both clinical monitoring and research.
Surveys
The US Department of Veterans Affairs Central Office collaborated with our workgroup to draft an initial set of survey questions designed to understand how each VHA facility defines, identifies, and provides care to veterans experiencing post-COVID sequalae. The 41-question survey was distributed through regional directors and chief medical officers at 139 VHA facilities in August 2021. One hundred nineteen responses (86%) were received. Sixteen facilities indicated they had established programs and 26 facilities were considering a program. Our CoP had representation from the 16 facilities with established programs indicating the deep and well-connected nature of our grassroots efforts to bring together stakeholders to learn as part of a CoP.
A separate, follow-up survey generated responses from 18 facilities and identified the need to capture evolving innovations and to develop smaller workstreams (eg, best practices, electronic documentation templates, pathway for referrals, veteran engagement, outcome measures). The survey not only exposed ongoing challenges to providing long COVID care, but importantly, outlined the ways in which CoP members were leveraging community knowledge and resources to inform innovations and processes of care changes at their specific sites. Fourteen of 18 facilities with long COVID programs in place explicitly identified the CoP as a resource they have found most beneficial when employing such innovations. Specific innovations reported included changes in care delivery, engagement in active outreach with veterans and local facility, and infrastructure development to sustain local long COVID clinics (Table).
Future Directions
Our CoP strives to contribute to an evidence base for long COVID care. At the system level, the CoP has the potential to impact access and continuity of care by identifying appropriate processes and ensuring that VHA patients receive outreach and an opportunity for post-COVID care. Comprehensive care requires input from HCP, clinical leadership, and operations levels. In this sense, our CoP provides an opportunity for diverse stakeholders to come together, discuss barriers to screening and delivering post-COVID care, and create an action plan to remove or lessen such barriers.18 Part of the process to remove barriers is to identify and support efficient resource allocation. Our CoP has worked to address issues in resource allocation (eg, space, personnel) for post-COVID care. For example, one facility is currently implementing interdisciplinary virtual post-COVID care. Another facility identified and restructured working assignments for psychologists who served in different capacities throughout the system to fill the need within the long COVID team.
At the HCP level, the CoP is currently developing workshops, media campaigns, written clinical resources, skills training, publications, and webinars/seminars with continuing medical education credits.19 The CoP may also provide learning and growth opportunities, such as clinical or VHA operational fellowships and research grants.
We are still in the formative stages of post-COVID care and future efforts will explore patient-centered outcomes. We are drawing on the Centers for Disease Control and Prevention’s guidance for evaluating patients with long COVID symptoms and examining the feasibility within VHA, as well as patient perspectives on post-COVID sequalae, to ensure we are selecting assessments that measure patient-centered constructs.18
Conclusions
A VHA-wide LHS approach is identifying issues related to the identification, delivery, and evaluation of long COVID care. This long COVID CoP has developed an infrastructure for communication, identified gaps in care, and cocreated knowledge related to best current practices for post-COVID care. This work is contributing to systemwide LHS efforts dedicated to creating a culture of quality care and innovation and is a process that is transferrable to other areas of care in the VHA, as well as other health care systems. The LHS approach continues to be highly relevant as we persist through the COVID-19 pandemic and reimagine a postpandemic world.
Acknowledg
We thank all the members of the Veterans Health Administration long COVID Community of Practice who participate in the meetings and contribute to the sharing and spread of knowledge.
The Veterans Health Administration (VHA)—along with systems across the world—has spent the past 2 years continuously adapting to meet the emerging needs of persons infected with COVID-19. With the development of effective vaccines and global efforts to mitigate transmission, attention has now shifted to long COVID care as the need for further outpatient health care becomes increasingly apparent.1,2
Background
Multiple terms describe the lingering, multisystem sequelae of COVID-19 that last longer than 4 weeks: long COVID, postacute COVID-19 syndrome, post-COVID condition, postacute sequalae of COVID-19, and COVID long hauler.1,3 Common symptoms include fatigue, shortness of breath, cough, sleep disorders, brain fog or cognitive dysfunction, depression, anxiety, pain, and changes in taste or smell that impact a person’s functioning.4,5 The multisystem nature of the postacute course of COVID-19 necessitates an interdisciplinary approach to devise comprehensive and individualized care plans.6-9 Research is needed to better understand this postacute state (eg, prevalence, underlying effects, characteristics of those who experience long COVID) to establish and evaluate cost-effective treatment approaches.
Many patients who are experiencing symptoms beyond the acute course of COVID-19 have been referred to general outpatient clinics or home health, which may lack the capacity and knowledge of this novel disease to effectively manage complex long COVID cases.2,3 To address this growing need, clinicians and leadership across a variety of disciplines and settings in the VHA created a community of practice (CoP) to create a mechanism for cross-facility communication, identify gaps in long COVID care and research, and cocreate knowledge on best practices for care delivery.
In this spirit, we are embracing a learning health system (LHS) approach that uses rapid-cycle methods to integrate data and real-world experience to iteratively evaluate and adapt models of long COVID care.10 Our clinically identified and data-driven objective is to provide high value health care to patients with long COVID sequalae by creating a framework to learn about this novel condition and develop innovative care models. This article provides an overview of our emerging LHS approach to the study of long COVID care that is fostering innovation and adaptability within the VHA. We describe 3 aspects of our engagement approach central to LHS: the ongoing development of a long COVID CoP dedicated to iteratively informing the bidirectional cycle of data from practice to research, results of a broad environmental scan of VHA long COVID care, and results of a survey administered to CoP members to inform ongoing needs of the community and identify early successful outcomes from participation.
Learning Health System Approach
The VHA is one of the largest integrated health care systems in the United States serving more than 9 million veterans.11 Since 2017, the VHA has articulated a vision to become an LHS that informs and improves patient-centered care through practice-based and data-driven research (eAppendix).12 During the early COVID-19 pandemic, an LHS approach in the VHA was critical to rapidly establishing a data infrastructure for disease surveillance, coordinating data-driven solutions, leveraging use of technology, collaborating across the globe to identify best practices, and implementing systematic responses (eg, policies, workforce adjustments).
Our long COVID CoP was developed as clinical observations and ongoing conversations with stakeholders (eg, veterans, health care practitioners [HCPs], leadership) identified a need to effectively identify and treat the growing number of veterans with long COVID. This clinical issue is compounded by the limited but emerging evidence on the clinical presentation of prolonged COVID-19 symptoms, treatment, and subsequent care pathways. The VHA’s efforts and lessons learned within the lens of an LHS are applicable to other systems confronting the complex identification and management of patients with persistent and encumbering long COVID symptoms. The VHA is building upon the LHS approach to proactively prepare for and address future clinical or public health challenges that require cross-system and sector collaborations, expediency, inclusivity, and patient/family centeredness.11
Community of Practice
As of January 25, 2022, our workgroup consisted of 128 VHA employees representing 29 VHA medical centers. Members of the multidisciplinary workgroup have diverse backgrounds with HCPs from primary care (eg, physicians, nurse practitioners), rehabilitation (eg, physical therapists), specialty care (eg, pulmonologists, physiatrists), mental health (eg, psychologists), and complementary and integrated health/Whole Health services (eg, practitoners of services such as yoga, tai chi, mindfulness, acupuncture). Members also include clinical, operations, and research leadership at local, regional, and national VHA levels. Our first objective as a large, diverse group was to establish shared goals, which included: (1) determining efficient communication pathways; (2) identifying gaps in care or research; and (3) cocreating knowledge to provide solutions to identified gaps.
Communication Mechanisms
Our first goal was to create an efficient mechanism for cross-facility communication. The initial CoP was formed in April 2021 and the first virtual meeting focused on reaching a consensus regarding the best way to communicate and proceed. We agreed to convene weekly at a consistent time, created a standard agenda template, and elected a lead facilitator of meeting proceedings. In addition, a member of the CoP recorded and took extensive meeting notes, which were later distributed to the entire CoP to accommodate varying schedules and ability to attend live meetings. Approximately 20 to 30 participants attend the meetings in real-time.
To consolidate working documents, information, and resources in one location, we created a platform to communicate via a Microsoft Teams channel. All CoP members are given access to the folders and allowed to add to the growing library of resources. Resources include clinical assessment and note templates for electronic documentation of care, site-specific process maps, relevant literature on screening and interventions identified by practice members, and meeting notes along with the recordings. A chat feature alerts CoP members to questions posed by other members. Any resources or information shared on the chat discussion are curated by CoP leaders to disseminate to all members. Importantly, this platform allowed us to communicate efficiently within the VHA organization by creating a centralized space for documents and the ability to correspond with all or select members of the CoP. Additional VHA employees can easily be referred and request access.
To increase awareness of the CoP, expand reach, and diversify perspectives, every participant was encouraged to invite colleagues and stakeholders with interest or experience in long COVID care to join. While patients are not included in this CoP, we are working closely with the VHA user experience workgroup (many members overlap) that is gathering patient and caregiver perspectives on their COVID-19 experience and long COVID care. Concurrently, CoP members and leadership facilitate communication and set up formal collaborations with other non-VHA health care systems to create an intersystem network of collaboration for long COVID care. This approach further enhances the speed at which we can work together to share lessons learned and stay up-to-date on emerging evidence surrounding long COVID care.
Identifying Gaps in Care and Research
Our second goal was to identify gaps in care or knowledge to inform future research and quality improvement initiatives, while also creating a foundation to cocreate knowledge about safe, effective care management of the novel long COVID sequelae. To translate knowledge, we must first identify and understand the gaps between the current, best available evidence and current care practices or policies impacting that delivery.13 As such, the structured meeting agenda and facilitated meeting discussions focused on understanding current clinical decision making and the evidence base. We shared VHA evidence synthesis reports and living rapid reviews on complications following COVID-19 illness (ie, major organ damage and posthospitalization health care use) that provided an objective evidence base on common long COVID complications.14,15
Since long COVID is a novel condition, we drew from literature in similar patient populations and translated that information in the context of our current knowledge of this unique syndrome. For example, we discussed the predominant and persistent symptom of fatigue post-COVID.5 In particular, the CoP discussed challenges in identifying and treating post-COVID fatigue, which is often a vague symptom with multiple or interacting etiologies that require a comprehensive, interdisciplinary approach. As such, we reviewed, adapted, and translated identification and treatment strategies from the literature on chronic fatigue syndrome to patients with post-COVID syndrome.16,17 We continue to work collaboratively and engage the appropriate stakeholders to provide input on the gaps to prioritize targeting.
Cocreate Knowledge
Our third goal was to cocreate knowledge regarding the care of patients with long COVID. To accomplish this, our structured meetings and communication pathways invited members to share experiences on the who (delivers and receives care), what (type of care or HCPs), when (identification of post-COVID and access), and how (eg, telehealth) of care to patients post-COVID. As part of the workgroup, we identified and shared resources on standardized, facility-level practices to reduce variability across the VHA system. These resources included intake/assessment forms, care processes, and batteries of tests/measures used for screening and assessment. The knowledge obtained from outside the CoP and cocreated within is being used to inform data-driven tools to support and evaluate care for patients with long COVID. As such, members of the workgroup are in the formative stages of participating in quality improvement innovation pilots to test technologies and processes designed to improve and validate long COVID care pathways. These technologies include screening tools, clinical decision support tools, and population health management technologies. In addition, we are developing a formal collaboration with the VHA Office of Research and Development to create standardized intake forms across VHA long COVID clinics to facilitate both clinical monitoring and research.
Surveys
The US Department of Veterans Affairs Central Office collaborated with our workgroup to draft an initial set of survey questions designed to understand how each VHA facility defines, identifies, and provides care to veterans experiencing post-COVID sequalae. The 41-question survey was distributed through regional directors and chief medical officers at 139 VHA facilities in August 2021. One hundred nineteen responses (86%) were received. Sixteen facilities indicated they had established programs and 26 facilities were considering a program. Our CoP had representation from the 16 facilities with established programs indicating the deep and well-connected nature of our grassroots efforts to bring together stakeholders to learn as part of a CoP.
A separate, follow-up survey generated responses from 18 facilities and identified the need to capture evolving innovations and to develop smaller workstreams (eg, best practices, electronic documentation templates, pathway for referrals, veteran engagement, outcome measures). The survey not only exposed ongoing challenges to providing long COVID care, but importantly, outlined the ways in which CoP members were leveraging community knowledge and resources to inform innovations and processes of care changes at their specific sites. Fourteen of 18 facilities with long COVID programs in place explicitly identified the CoP as a resource they have found most beneficial when employing such innovations. Specific innovations reported included changes in care delivery, engagement in active outreach with veterans and local facility, and infrastructure development to sustain local long COVID clinics (Table).
Future Directions
Our CoP strives to contribute to an evidence base for long COVID care. At the system level, the CoP has the potential to impact access and continuity of care by identifying appropriate processes and ensuring that VHA patients receive outreach and an opportunity for post-COVID care. Comprehensive care requires input from HCP, clinical leadership, and operations levels. In this sense, our CoP provides an opportunity for diverse stakeholders to come together, discuss barriers to screening and delivering post-COVID care, and create an action plan to remove or lessen such barriers.18 Part of the process to remove barriers is to identify and support efficient resource allocation. Our CoP has worked to address issues in resource allocation (eg, space, personnel) for post-COVID care. For example, one facility is currently implementing interdisciplinary virtual post-COVID care. Another facility identified and restructured working assignments for psychologists who served in different capacities throughout the system to fill the need within the long COVID team.
At the HCP level, the CoP is currently developing workshops, media campaigns, written clinical resources, skills training, publications, and webinars/seminars with continuing medical education credits.19 The CoP may also provide learning and growth opportunities, such as clinical or VHA operational fellowships and research grants.
We are still in the formative stages of post-COVID care and future efforts will explore patient-centered outcomes. We are drawing on the Centers for Disease Control and Prevention’s guidance for evaluating patients with long COVID symptoms and examining the feasibility within VHA, as well as patient perspectives on post-COVID sequalae, to ensure we are selecting assessments that measure patient-centered constructs.18
Conclusions
A VHA-wide LHS approach is identifying issues related to the identification, delivery, and evaluation of long COVID care. This long COVID CoP has developed an infrastructure for communication, identified gaps in care, and cocreated knowledge related to best current practices for post-COVID care. This work is contributing to systemwide LHS efforts dedicated to creating a culture of quality care and innovation and is a process that is transferrable to other areas of care in the VHA, as well as other health care systems. The LHS approach continues to be highly relevant as we persist through the COVID-19 pandemic and reimagine a postpandemic world.
Acknowledg
We thank all the members of the Veterans Health Administration long COVID Community of Practice who participate in the meetings and contribute to the sharing and spread of knowledge.
1. Sivan M, Halpin S, Hollingworth L, Snook N, Hickman K, Clifton I. Development of an integrated rehabilitation pathway for individuals recovering from COVID-19 in the community. J Rehabil Med. 2020;52(8):jrm00089. doi:10.2340/16501977-2727
2. Understanding the long-term health effects of COVID-19. EClinicalMedicine. 2020;26:100586. doi:10.1016/j.eclinm.2020.100586
3. Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. Published online August 11, 2020:m3026. doi:10.1136/bmj.m3026
4. Iwua CJ, Iwu CD, Wiysonge CS. The occurrence of long COVID: a rapid review. Pan Afr Med J. 2021;38. doi:10.11604/pamj.2021.38.65.27366
5. Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-605. doi:10.1001/jama.2020.12603
6. Gemelli Against COVID-19 Post-Acute Care Study Group. Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. 2020;32(8):1613-1620. doi:10.1007/s40520-020-01616-x
7. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi:10.1038/s41591-022-01689-3
8. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi:10.1038/s41586-021-03553-9
9. Ayoubkhani D, Bermingham C, Pouwels KB, et al. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ. 2022;377:e069676. doi:10.1136/bmj-2021-069676
10. Institute of Medicine (US) Roundtable on Evidence-Based Medicine, Olsen L, Aisner D, McGinnis JM, eds. The Learning Healthcare System: Workshop Summary. Washington (DC): National Academies Press (US); 2007. doi:10.17226/11903
11. Romanelli RJ, Azar KMJ, Sudat S, Hung D, Frosch DL, Pressman AR. Learning health system in crisis: lessons from the COVID-19 pandemic. Mayo Clin Proc Innov Qual Outcomes. 2021;5(1):171-176. doi:10.1016/j.mayocpiqo.2020.10.004
12. Atkins D, Kilbourne AM, Shulkin D. Moving from discovery to system-wide change: the role of research in a learning health care system: experience from three decades of health systems research in the Veterans Health Administration. Annu Rev Public Health. 2017;38:467-487. doi:10.1146/annurev-publhealth-031816-044255
13. Kitson A, Straus SE. The knowledge-to-action cycle: identifying the gaps. CMAJ. 2010;182(2):E73-77. doi:10.1503/cmaj.081231
14. Greer N, Bart B, Billington C, et al. COVID-19 post-acute care major organ damage: a living rapid review. Updated September 2021. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid-organ-damage.pdf
15. Sharpe JA, Burke C, Gordon AM, et al. COVID-19 post-hospitalization health care utilization: a living review. Updated February 2022. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid19-post-hosp.pdf
16. Bested AC, Marshall LM. Review of Myalgic Encephalomyelitis/chronic fatigue syndrome: an evidence-based approach to diagnosis and management by clinicians. Rev Environ Health. 2015;30(4):223-249. doi:10.1515/reveh-2015-0026
17. Yancey JR, Thomas SM. Chronic fatigue syndrome: diagnosis and treatment. Am Fam Physician. 2012;86(8):741-746.
18. Kotter JP, Cohen DS. Change Leadership The Kotter Collection. Harvard Business Review Press; 2014.
19. Brownson RC, Eyler AA, Harris JK, Moore JB, Tabak RG. Getting the word out: new approaches for disseminating public health science. J Public Health Manag Pract. 2018;24(2):102-111. doi:10.1097/PHH.0000000000000673
1. Sivan M, Halpin S, Hollingworth L, Snook N, Hickman K, Clifton I. Development of an integrated rehabilitation pathway for individuals recovering from COVID-19 in the community. J Rehabil Med. 2020;52(8):jrm00089. doi:10.2340/16501977-2727
2. Understanding the long-term health effects of COVID-19. EClinicalMedicine. 2020;26:100586. doi:10.1016/j.eclinm.2020.100586
3. Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. Published online August 11, 2020:m3026. doi:10.1136/bmj.m3026
4. Iwua CJ, Iwu CD, Wiysonge CS. The occurrence of long COVID: a rapid review. Pan Afr Med J. 2021;38. doi:10.11604/pamj.2021.38.65.27366
5. Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-605. doi:10.1001/jama.2020.12603
6. Gemelli Against COVID-19 Post-Acute Care Study Group. Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. 2020;32(8):1613-1620. doi:10.1007/s40520-020-01616-x
7. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi:10.1038/s41591-022-01689-3
8. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi:10.1038/s41586-021-03553-9
9. Ayoubkhani D, Bermingham C, Pouwels KB, et al. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ. 2022;377:e069676. doi:10.1136/bmj-2021-069676
10. Institute of Medicine (US) Roundtable on Evidence-Based Medicine, Olsen L, Aisner D, McGinnis JM, eds. The Learning Healthcare System: Workshop Summary. Washington (DC): National Academies Press (US); 2007. doi:10.17226/11903
11. Romanelli RJ, Azar KMJ, Sudat S, Hung D, Frosch DL, Pressman AR. Learning health system in crisis: lessons from the COVID-19 pandemic. Mayo Clin Proc Innov Qual Outcomes. 2021;5(1):171-176. doi:10.1016/j.mayocpiqo.2020.10.004
12. Atkins D, Kilbourne AM, Shulkin D. Moving from discovery to system-wide change: the role of research in a learning health care system: experience from three decades of health systems research in the Veterans Health Administration. Annu Rev Public Health. 2017;38:467-487. doi:10.1146/annurev-publhealth-031816-044255
13. Kitson A, Straus SE. The knowledge-to-action cycle: identifying the gaps. CMAJ. 2010;182(2):E73-77. doi:10.1503/cmaj.081231
14. Greer N, Bart B, Billington C, et al. COVID-19 post-acute care major organ damage: a living rapid review. Updated September 2021. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid-organ-damage.pdf
15. Sharpe JA, Burke C, Gordon AM, et al. COVID-19 post-hospitalization health care utilization: a living review. Updated February 2022. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid19-post-hosp.pdf
16. Bested AC, Marshall LM. Review of Myalgic Encephalomyelitis/chronic fatigue syndrome: an evidence-based approach to diagnosis and management by clinicians. Rev Environ Health. 2015;30(4):223-249. doi:10.1515/reveh-2015-0026
17. Yancey JR, Thomas SM. Chronic fatigue syndrome: diagnosis and treatment. Am Fam Physician. 2012;86(8):741-746.
18. Kotter JP, Cohen DS. Change Leadership The Kotter Collection. Harvard Business Review Press; 2014.
19. Brownson RC, Eyler AA, Harris JK, Moore JB, Tabak RG. Getting the word out: new approaches for disseminating public health science. J Public Health Manag Pract. 2018;24(2):102-111. doi:10.1097/PHH.0000000000000673
Pfizer plans a vaccine to target all coronaviruses
Ask the sibling of any celebrity and they’ll tell you they don’t get anywhere near the same attention. The same is true for coronaviruses – the one that causes COVID-19 has been in the spotlight for more than 2 years now, while the others at the moment circulate in relative obscurity.
With the knowledge that any of the other coronaviruses could pose a serious future threat, Pfizer and its partner BioNTech announced plans on June 29 to develop a vaccine that will work against SARS-CoV-2 (the virus that causes COVID-19) and the entire class, or family, of related coronaviruses.
Trials in people of this “pan-coronavirus” vaccine are scheduled to start this fall, Reuters reported.
“I applaud the sentiment that is long overdue,” said Eric Topol, MD, when asked to comment. “It is crucial that we get ahead of the virus, and the best way is to develop pan-betacoronavirus vaccines that are variant-proof.”
“We had potential to get them into clinical trials many months ago, but this is the first sign it may happen,” said Dr. Topol, executive vice president of Scripps Research and editor-in-chief for Medscape, WebMD’s sister site for health care professionals.
SARS-CoV-2 is not the first troublemaker in the coronavirus family. SARS, a coronavirus that causes acute respiratory syndrome, emerged in late 2002. A decade later, officials sounded the alarm about the coronavirus behind Middle East respiratory syndrome (MERS).
The coronavirus family is large, but only seven coronavirus types can infect humans, the CDC reports. Most cause mild to moderate upper respiratory tract infections, although some people can get pneumonia or bronchiolitis.
Unless you’re a virologist, immunologist, or public health official, you may be unaware that coronaviruses are one of the causes of the common cold, for example.
A version of this article first appeared on WebMD.com.
Ask the sibling of any celebrity and they’ll tell you they don’t get anywhere near the same attention. The same is true for coronaviruses – the one that causes COVID-19 has been in the spotlight for more than 2 years now, while the others at the moment circulate in relative obscurity.
With the knowledge that any of the other coronaviruses could pose a serious future threat, Pfizer and its partner BioNTech announced plans on June 29 to develop a vaccine that will work against SARS-CoV-2 (the virus that causes COVID-19) and the entire class, or family, of related coronaviruses.
Trials in people of this “pan-coronavirus” vaccine are scheduled to start this fall, Reuters reported.
“I applaud the sentiment that is long overdue,” said Eric Topol, MD, when asked to comment. “It is crucial that we get ahead of the virus, and the best way is to develop pan-betacoronavirus vaccines that are variant-proof.”
“We had potential to get them into clinical trials many months ago, but this is the first sign it may happen,” said Dr. Topol, executive vice president of Scripps Research and editor-in-chief for Medscape, WebMD’s sister site for health care professionals.
SARS-CoV-2 is not the first troublemaker in the coronavirus family. SARS, a coronavirus that causes acute respiratory syndrome, emerged in late 2002. A decade later, officials sounded the alarm about the coronavirus behind Middle East respiratory syndrome (MERS).
The coronavirus family is large, but only seven coronavirus types can infect humans, the CDC reports. Most cause mild to moderate upper respiratory tract infections, although some people can get pneumonia or bronchiolitis.
Unless you’re a virologist, immunologist, or public health official, you may be unaware that coronaviruses are one of the causes of the common cold, for example.
A version of this article first appeared on WebMD.com.
Ask the sibling of any celebrity and they’ll tell you they don’t get anywhere near the same attention. The same is true for coronaviruses – the one that causes COVID-19 has been in the spotlight for more than 2 years now, while the others at the moment circulate in relative obscurity.
With the knowledge that any of the other coronaviruses could pose a serious future threat, Pfizer and its partner BioNTech announced plans on June 29 to develop a vaccine that will work against SARS-CoV-2 (the virus that causes COVID-19) and the entire class, or family, of related coronaviruses.
Trials in people of this “pan-coronavirus” vaccine are scheduled to start this fall, Reuters reported.
“I applaud the sentiment that is long overdue,” said Eric Topol, MD, when asked to comment. “It is crucial that we get ahead of the virus, and the best way is to develop pan-betacoronavirus vaccines that are variant-proof.”
“We had potential to get them into clinical trials many months ago, but this is the first sign it may happen,” said Dr. Topol, executive vice president of Scripps Research and editor-in-chief for Medscape, WebMD’s sister site for health care professionals.
SARS-CoV-2 is not the first troublemaker in the coronavirus family. SARS, a coronavirus that causes acute respiratory syndrome, emerged in late 2002. A decade later, officials sounded the alarm about the coronavirus behind Middle East respiratory syndrome (MERS).
The coronavirus family is large, but only seven coronavirus types can infect humans, the CDC reports. Most cause mild to moderate upper respiratory tract infections, although some people can get pneumonia or bronchiolitis.
Unless you’re a virologist, immunologist, or public health official, you may be unaware that coronaviruses are one of the causes of the common cold, for example.
A version of this article first appeared on WebMD.com.
ACC/AHA issue clinical lexicon for complications of COVID-19
The American College of Cardiology and the American Heart Association have jointly issued a comprehensive set of data standards to help clarify definitions of the cardiovascular (CV) and non-CV complications of COVID-19.
It’s the work of the ACC/AHA Task Force on Clinical Data Standards and has been endorsed by the Heart Failure Society of America and Society for Cardiac Angiography and Interventions.
There is increased importance to understanding the acute and long-term impact of COVID-19 on CV health, the writing group notes. Until now, however, there has not been “clarity or consensus” on definitions of CV conditions related to COVID-19, with different diagnostic terminologies being used for overlapping conditions, such as “myocardial injury,” “myocarditis,” “type Il myocardial infarction,” “stress cardiomyopathy,” and “inflammatory cardiomyopathy,” they point out.
“We, as a research community, did some things right and some things wrong surrounding the COVID pandemic,” Sandeep Das, MD, MPH, vice chair of the writing group, noted in an interview with this news organization.
“The things that we really did right is that everybody responded with enthusiasm, kind of all hands on deck with a massive crisis response, and that was fantastic,” Dr. Das said.
“However, because of the need to hurry, we didn’t structure and organize in the way that we typically would for something that was sort of a slow burn kind of problem rather than an emergency. One of the consequences of that was fragmentation of how things are collected, reported, et cetera, and that leads to confusion,” he added.
The report was published simultaneously June 23 in the Journal of the American College of Cardiology and Circulation: Cardiovascular Quality and Outcomes.
A necessary but not glamorous project
The new data standards for COVID-19 will help standardize definitions and set the framework to capture and better understand how COVID-19 affects CV health.
“It wasn’t exactly a glamorous-type project but, at the same time, it’s super necessary to kind of get everybody on the same page and working together,” Dr. Das said.
Broad agreement on common vocabulary and definitions will help with efforts to pool or compare data from electronic health records, clinical registries, administrative datasets, and other databases, and determine whether these data apply to clinical practice and research endeavors, the writing group says.
They considered data elements relevant to the full range of care provided to COVID-19 patients in all care settings. Among the key items included in the document are:
- Case definitions for confirmed, probable, and suspected acute COVID-19, as well as postacute sequelae of COVID-19.
- Definitions for acute CV complications related to COVID-19, including acute myocardial injury, heart failure, shock, arrhythmia, thromboembolic complications, and .
- Data elements related to COVID-19 vaccination status, comorbidities, and preexisting CV conditions.
- Definitions for postacute CV sequelae of SARS-CoV-2 infection and long-term CV complications of COVID-19.
- Data elements for CV mortality during acute COVID-19.
- Data elements for non-CV complications to help document severity of illness and other competing diagnoses and complications that might affect CV outcomes.
- A list of symptoms and signs related to COVID-19 and CV complications.
- Data elements for diagnostic and therapeutic strategies for COVID-19 and CV conditions.
- A discussion of advanced therapies, including , extracorporeal membrane oxygenation, and end-of-life management strategies.
These data standards will be useful for researchers, registry developers, and clinicians, and they are proposed as a framework for ICD-10 code development of COVID-19–related CV conditions, the writing group says.
The standards are also of “great importance” to patients, clinicians, investigators, scientists, administrators, public health officials, policymakers, and payers, the group says.
Dr. Das said that, although there is no formal plan in place to update the document, he could see sections that might be refined.
“For example, there’s a nice long list of all the various variants, and unfortunately, I suspect that that is going to change and evolve over time,” Dr. Das told this news organization.
“We tried very hard not to include things like specifying specific treatments so we didn’t get proscriptive. We wanted to make it descriptive, so hopefully it will stand the test of time pretty well,” he added.
This research had no commercial funding. The writing group has no relevant disclosures.
A version of this article first appeared on Medscape.com.
The American College of Cardiology and the American Heart Association have jointly issued a comprehensive set of data standards to help clarify definitions of the cardiovascular (CV) and non-CV complications of COVID-19.
It’s the work of the ACC/AHA Task Force on Clinical Data Standards and has been endorsed by the Heart Failure Society of America and Society for Cardiac Angiography and Interventions.
There is increased importance to understanding the acute and long-term impact of COVID-19 on CV health, the writing group notes. Until now, however, there has not been “clarity or consensus” on definitions of CV conditions related to COVID-19, with different diagnostic terminologies being used for overlapping conditions, such as “myocardial injury,” “myocarditis,” “type Il myocardial infarction,” “stress cardiomyopathy,” and “inflammatory cardiomyopathy,” they point out.
“We, as a research community, did some things right and some things wrong surrounding the COVID pandemic,” Sandeep Das, MD, MPH, vice chair of the writing group, noted in an interview with this news organization.
“The things that we really did right is that everybody responded with enthusiasm, kind of all hands on deck with a massive crisis response, and that was fantastic,” Dr. Das said.
“However, because of the need to hurry, we didn’t structure and organize in the way that we typically would for something that was sort of a slow burn kind of problem rather than an emergency. One of the consequences of that was fragmentation of how things are collected, reported, et cetera, and that leads to confusion,” he added.
The report was published simultaneously June 23 in the Journal of the American College of Cardiology and Circulation: Cardiovascular Quality and Outcomes.
A necessary but not glamorous project
The new data standards for COVID-19 will help standardize definitions and set the framework to capture and better understand how COVID-19 affects CV health.
“It wasn’t exactly a glamorous-type project but, at the same time, it’s super necessary to kind of get everybody on the same page and working together,” Dr. Das said.
Broad agreement on common vocabulary and definitions will help with efforts to pool or compare data from electronic health records, clinical registries, administrative datasets, and other databases, and determine whether these data apply to clinical practice and research endeavors, the writing group says.
They considered data elements relevant to the full range of care provided to COVID-19 patients in all care settings. Among the key items included in the document are:
- Case definitions for confirmed, probable, and suspected acute COVID-19, as well as postacute sequelae of COVID-19.
- Definitions for acute CV complications related to COVID-19, including acute myocardial injury, heart failure, shock, arrhythmia, thromboembolic complications, and .
- Data elements related to COVID-19 vaccination status, comorbidities, and preexisting CV conditions.
- Definitions for postacute CV sequelae of SARS-CoV-2 infection and long-term CV complications of COVID-19.
- Data elements for CV mortality during acute COVID-19.
- Data elements for non-CV complications to help document severity of illness and other competing diagnoses and complications that might affect CV outcomes.
- A list of symptoms and signs related to COVID-19 and CV complications.
- Data elements for diagnostic and therapeutic strategies for COVID-19 and CV conditions.
- A discussion of advanced therapies, including , extracorporeal membrane oxygenation, and end-of-life management strategies.
These data standards will be useful for researchers, registry developers, and clinicians, and they are proposed as a framework for ICD-10 code development of COVID-19–related CV conditions, the writing group says.
The standards are also of “great importance” to patients, clinicians, investigators, scientists, administrators, public health officials, policymakers, and payers, the group says.
Dr. Das said that, although there is no formal plan in place to update the document, he could see sections that might be refined.
“For example, there’s a nice long list of all the various variants, and unfortunately, I suspect that that is going to change and evolve over time,” Dr. Das told this news organization.
“We tried very hard not to include things like specifying specific treatments so we didn’t get proscriptive. We wanted to make it descriptive, so hopefully it will stand the test of time pretty well,” he added.
This research had no commercial funding. The writing group has no relevant disclosures.
A version of this article first appeared on Medscape.com.
The American College of Cardiology and the American Heart Association have jointly issued a comprehensive set of data standards to help clarify definitions of the cardiovascular (CV) and non-CV complications of COVID-19.
It’s the work of the ACC/AHA Task Force on Clinical Data Standards and has been endorsed by the Heart Failure Society of America and Society for Cardiac Angiography and Interventions.
There is increased importance to understanding the acute and long-term impact of COVID-19 on CV health, the writing group notes. Until now, however, there has not been “clarity or consensus” on definitions of CV conditions related to COVID-19, with different diagnostic terminologies being used for overlapping conditions, such as “myocardial injury,” “myocarditis,” “type Il myocardial infarction,” “stress cardiomyopathy,” and “inflammatory cardiomyopathy,” they point out.
“We, as a research community, did some things right and some things wrong surrounding the COVID pandemic,” Sandeep Das, MD, MPH, vice chair of the writing group, noted in an interview with this news organization.
“The things that we really did right is that everybody responded with enthusiasm, kind of all hands on deck with a massive crisis response, and that was fantastic,” Dr. Das said.
“However, because of the need to hurry, we didn’t structure and organize in the way that we typically would for something that was sort of a slow burn kind of problem rather than an emergency. One of the consequences of that was fragmentation of how things are collected, reported, et cetera, and that leads to confusion,” he added.
The report was published simultaneously June 23 in the Journal of the American College of Cardiology and Circulation: Cardiovascular Quality and Outcomes.
A necessary but not glamorous project
The new data standards for COVID-19 will help standardize definitions and set the framework to capture and better understand how COVID-19 affects CV health.
“It wasn’t exactly a glamorous-type project but, at the same time, it’s super necessary to kind of get everybody on the same page and working together,” Dr. Das said.
Broad agreement on common vocabulary and definitions will help with efforts to pool or compare data from electronic health records, clinical registries, administrative datasets, and other databases, and determine whether these data apply to clinical practice and research endeavors, the writing group says.
They considered data elements relevant to the full range of care provided to COVID-19 patients in all care settings. Among the key items included in the document are:
- Case definitions for confirmed, probable, and suspected acute COVID-19, as well as postacute sequelae of COVID-19.
- Definitions for acute CV complications related to COVID-19, including acute myocardial injury, heart failure, shock, arrhythmia, thromboembolic complications, and .
- Data elements related to COVID-19 vaccination status, comorbidities, and preexisting CV conditions.
- Definitions for postacute CV sequelae of SARS-CoV-2 infection and long-term CV complications of COVID-19.
- Data elements for CV mortality during acute COVID-19.
- Data elements for non-CV complications to help document severity of illness and other competing diagnoses and complications that might affect CV outcomes.
- A list of symptoms and signs related to COVID-19 and CV complications.
- Data elements for diagnostic and therapeutic strategies for COVID-19 and CV conditions.
- A discussion of advanced therapies, including , extracorporeal membrane oxygenation, and end-of-life management strategies.
These data standards will be useful for researchers, registry developers, and clinicians, and they are proposed as a framework for ICD-10 code development of COVID-19–related CV conditions, the writing group says.
The standards are also of “great importance” to patients, clinicians, investigators, scientists, administrators, public health officials, policymakers, and payers, the group says.
Dr. Das said that, although there is no formal plan in place to update the document, he could see sections that might be refined.
“For example, there’s a nice long list of all the various variants, and unfortunately, I suspect that that is going to change and evolve over time,” Dr. Das told this news organization.
“We tried very hard not to include things like specifying specific treatments so we didn’t get proscriptive. We wanted to make it descriptive, so hopefully it will stand the test of time pretty well,” he added.
This research had no commercial funding. The writing group has no relevant disclosures.
A version of this article first appeared on Medscape.com.
Acute hepatitis cases in children show declining trend; adenovirus, COVID-19 remain key leads
LONDON – Case numbers of acute hepatitis in children show “a declining trajectory,” and COVID-19 and adenovirus remain the most likely, but as yet unproven, causative agents, said experts in an update at the annual International Liver Congress sponsored by the European Association for the Study of the Liver.
Philippa Easterbrook, MD, medical expert at the World Health Organization Global HIV, Hepatitis, and STI Programme, shared the latest case numbers and working hypotheses of possible causative agents in the outbreak of acute hepatitis among children in Europe and beyond.
Global data across the five WHO regions show there were 244 cases in the past month, bringing the total to 894 probable cases reported since October 2021 from 33 countries.
“It’s important to remember that this includes new cases, as well as retrospectively identified cases,” Dr.Easterbrook said. “Over half (52%) are from the European region, while 262 cases (30% of the global total) are from the United Kingdom.”
Data from Europe and the United States show a declining trajectory of reports of new cases. “This is a positive development,” she said.
The second highest reporting region is the Americas, she said, with 368 cases total, 290 cases of which come from the United States, accounting for 35% of the global total.
“Together the United Kingdom and the United States make up 65% of the global total,” she said.
Dr. Easterbrook added that 17 of the 33 reporting countries had more than five cases. Most cases (75%) are in young children under 5 years of age.
Serious cases are relatively few, but 44 (5%) children have required liver transplantation. Data from the European region show that 30% have required intensive care at some point during their hospitalization. There have been 18 (2%) reported deaths.
Possible post-COVID phenomenon, adenovirus most commonly reported
Dr. Easterbrook acknowledged the emerging hypothesis of a post-COVID phenomenon.
“Is this a variant of the rare but recognized multisystem inflammatory syndrome condition in children that’s been reported, often 1-2 months after COVID, causing widespread organ damage?” But she pointed out that the reported COVID cases with hepatitis “don’t seem to fit these features.”
Adenovirus remains the most commonly detected virus in acute hepatitis in children, found in 53% of cases overall, she said. The adenovirus detection rate is higher in the United Kingdom, at 68%.
“There are quite high rates of detection, but they’re not in all cases. There does seem to be a high rate of detection in the younger age groups and in those who are developing severe disease, so perhaps there is some link to severity,” Dr. Easterbrook said.
The working hypotheses continue to favor adenovirus together with past or current SARS-CoV-2 infection, as proposed early in the outbreak, she said. “These either work independently or work together as cofactors in some way to result in hepatitis. And there has been some clear progress on this. WHO is bringing together the data from different countries on some of these working hypotheses.”
Dr. Easterbrook highlighted the importance of procuring global data, especially given that two countries are reporting the majority of cases and in high numbers. “It’s a mixed picture with different rates of adenovirus detection and of COVID,” she said. “We need good-quality data collected in a standardized way.” WHO is requesting that countries provide these data.
She also highlighted the need for good in-depth studies, citing the UK Health Security Agency as an example of this. “There’s only a few countries that have the capacity or the patient numbers to look at this in detail, for example, the U.K. and the UKHSA.”
She noted that the UKHSA had laid out a comprehensive, systematic set of further investigations. For example, a case-control study is trying to establish whether there is a difference in the rate of adenovirus detection in children with hepatitis compared with other hospitalized children at the same time. “This aims to really tease out whether adenovirus is a cause or just a bystander,” she said.
She added that there were also genetic studies investigating whether genes were predisposing some children to develop a more severe form of disease. Other studies are evaluating the immune response of the patients.
Dr. Easterbrook added that the WHO will soon launch a global survey asking whether the reports of acute hepatitis are greater than the expected background rate for cases of hepatitis of unknown etiology.
Acute hepatitis is not new, but high caseload is
Also speaking at the ILC special briefing was Maria Buti, MD, PhD, policy and public health chair for the European Association for the Study of the Liver, and chief of the internal medicine and hepatology department at Hospital General Universitari Valle Hebron in Barcelona.
Dr. Buti drew attention to the fact that severe acute hepatitis of unknown etiology in children is not new.
“We have cases of acute hepatitis that even needed liver transplantation some years ago, and every year in our clinics we see these type of patients,” Dr. Buti remarked. What is really new, she added, is the amount of cases, particularly in the United Kingdom.
Dr. Easterbrook and Dr. Buti have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LONDON – Case numbers of acute hepatitis in children show “a declining trajectory,” and COVID-19 and adenovirus remain the most likely, but as yet unproven, causative agents, said experts in an update at the annual International Liver Congress sponsored by the European Association for the Study of the Liver.
Philippa Easterbrook, MD, medical expert at the World Health Organization Global HIV, Hepatitis, and STI Programme, shared the latest case numbers and working hypotheses of possible causative agents in the outbreak of acute hepatitis among children in Europe and beyond.
Global data across the five WHO regions show there were 244 cases in the past month, bringing the total to 894 probable cases reported since October 2021 from 33 countries.
“It’s important to remember that this includes new cases, as well as retrospectively identified cases,” Dr.Easterbrook said. “Over half (52%) are from the European region, while 262 cases (30% of the global total) are from the United Kingdom.”
Data from Europe and the United States show a declining trajectory of reports of new cases. “This is a positive development,” she said.
The second highest reporting region is the Americas, she said, with 368 cases total, 290 cases of which come from the United States, accounting for 35% of the global total.
“Together the United Kingdom and the United States make up 65% of the global total,” she said.
Dr. Easterbrook added that 17 of the 33 reporting countries had more than five cases. Most cases (75%) are in young children under 5 years of age.
Serious cases are relatively few, but 44 (5%) children have required liver transplantation. Data from the European region show that 30% have required intensive care at some point during their hospitalization. There have been 18 (2%) reported deaths.
Possible post-COVID phenomenon, adenovirus most commonly reported
Dr. Easterbrook acknowledged the emerging hypothesis of a post-COVID phenomenon.
“Is this a variant of the rare but recognized multisystem inflammatory syndrome condition in children that’s been reported, often 1-2 months after COVID, causing widespread organ damage?” But she pointed out that the reported COVID cases with hepatitis “don’t seem to fit these features.”
Adenovirus remains the most commonly detected virus in acute hepatitis in children, found in 53% of cases overall, she said. The adenovirus detection rate is higher in the United Kingdom, at 68%.
“There are quite high rates of detection, but they’re not in all cases. There does seem to be a high rate of detection in the younger age groups and in those who are developing severe disease, so perhaps there is some link to severity,” Dr. Easterbrook said.
The working hypotheses continue to favor adenovirus together with past or current SARS-CoV-2 infection, as proposed early in the outbreak, she said. “These either work independently or work together as cofactors in some way to result in hepatitis. And there has been some clear progress on this. WHO is bringing together the data from different countries on some of these working hypotheses.”
Dr. Easterbrook highlighted the importance of procuring global data, especially given that two countries are reporting the majority of cases and in high numbers. “It’s a mixed picture with different rates of adenovirus detection and of COVID,” she said. “We need good-quality data collected in a standardized way.” WHO is requesting that countries provide these data.
She also highlighted the need for good in-depth studies, citing the UK Health Security Agency as an example of this. “There’s only a few countries that have the capacity or the patient numbers to look at this in detail, for example, the U.K. and the UKHSA.”
She noted that the UKHSA had laid out a comprehensive, systematic set of further investigations. For example, a case-control study is trying to establish whether there is a difference in the rate of adenovirus detection in children with hepatitis compared with other hospitalized children at the same time. “This aims to really tease out whether adenovirus is a cause or just a bystander,” she said.
She added that there were also genetic studies investigating whether genes were predisposing some children to develop a more severe form of disease. Other studies are evaluating the immune response of the patients.
Dr. Easterbrook added that the WHO will soon launch a global survey asking whether the reports of acute hepatitis are greater than the expected background rate for cases of hepatitis of unknown etiology.
Acute hepatitis is not new, but high caseload is
Also speaking at the ILC special briefing was Maria Buti, MD, PhD, policy and public health chair for the European Association for the Study of the Liver, and chief of the internal medicine and hepatology department at Hospital General Universitari Valle Hebron in Barcelona.
Dr. Buti drew attention to the fact that severe acute hepatitis of unknown etiology in children is not new.
“We have cases of acute hepatitis that even needed liver transplantation some years ago, and every year in our clinics we see these type of patients,” Dr. Buti remarked. What is really new, she added, is the amount of cases, particularly in the United Kingdom.
Dr. Easterbrook and Dr. Buti have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LONDON – Case numbers of acute hepatitis in children show “a declining trajectory,” and COVID-19 and adenovirus remain the most likely, but as yet unproven, causative agents, said experts in an update at the annual International Liver Congress sponsored by the European Association for the Study of the Liver.
Philippa Easterbrook, MD, medical expert at the World Health Organization Global HIV, Hepatitis, and STI Programme, shared the latest case numbers and working hypotheses of possible causative agents in the outbreak of acute hepatitis among children in Europe and beyond.
Global data across the five WHO regions show there were 244 cases in the past month, bringing the total to 894 probable cases reported since October 2021 from 33 countries.
“It’s important to remember that this includes new cases, as well as retrospectively identified cases,” Dr.Easterbrook said. “Over half (52%) are from the European region, while 262 cases (30% of the global total) are from the United Kingdom.”
Data from Europe and the United States show a declining trajectory of reports of new cases. “This is a positive development,” she said.
The second highest reporting region is the Americas, she said, with 368 cases total, 290 cases of which come from the United States, accounting for 35% of the global total.
“Together the United Kingdom and the United States make up 65% of the global total,” she said.
Dr. Easterbrook added that 17 of the 33 reporting countries had more than five cases. Most cases (75%) are in young children under 5 years of age.
Serious cases are relatively few, but 44 (5%) children have required liver transplantation. Data from the European region show that 30% have required intensive care at some point during their hospitalization. There have been 18 (2%) reported deaths.
Possible post-COVID phenomenon, adenovirus most commonly reported
Dr. Easterbrook acknowledged the emerging hypothesis of a post-COVID phenomenon.
“Is this a variant of the rare but recognized multisystem inflammatory syndrome condition in children that’s been reported, often 1-2 months after COVID, causing widespread organ damage?” But she pointed out that the reported COVID cases with hepatitis “don’t seem to fit these features.”
Adenovirus remains the most commonly detected virus in acute hepatitis in children, found in 53% of cases overall, she said. The adenovirus detection rate is higher in the United Kingdom, at 68%.
“There are quite high rates of detection, but they’re not in all cases. There does seem to be a high rate of detection in the younger age groups and in those who are developing severe disease, so perhaps there is some link to severity,” Dr. Easterbrook said.
The working hypotheses continue to favor adenovirus together with past or current SARS-CoV-2 infection, as proposed early in the outbreak, she said. “These either work independently or work together as cofactors in some way to result in hepatitis. And there has been some clear progress on this. WHO is bringing together the data from different countries on some of these working hypotheses.”
Dr. Easterbrook highlighted the importance of procuring global data, especially given that two countries are reporting the majority of cases and in high numbers. “It’s a mixed picture with different rates of adenovirus detection and of COVID,” she said. “We need good-quality data collected in a standardized way.” WHO is requesting that countries provide these data.
She also highlighted the need for good in-depth studies, citing the UK Health Security Agency as an example of this. “There’s only a few countries that have the capacity or the patient numbers to look at this in detail, for example, the U.K. and the UKHSA.”
She noted that the UKHSA had laid out a comprehensive, systematic set of further investigations. For example, a case-control study is trying to establish whether there is a difference in the rate of adenovirus detection in children with hepatitis compared with other hospitalized children at the same time. “This aims to really tease out whether adenovirus is a cause or just a bystander,” she said.
She added that there were also genetic studies investigating whether genes were predisposing some children to develop a more severe form of disease. Other studies are evaluating the immune response of the patients.
Dr. Easterbrook added that the WHO will soon launch a global survey asking whether the reports of acute hepatitis are greater than the expected background rate for cases of hepatitis of unknown etiology.
Acute hepatitis is not new, but high caseload is
Also speaking at the ILC special briefing was Maria Buti, MD, PhD, policy and public health chair for the European Association for the Study of the Liver, and chief of the internal medicine and hepatology department at Hospital General Universitari Valle Hebron in Barcelona.
Dr. Buti drew attention to the fact that severe acute hepatitis of unknown etiology in children is not new.
“We have cases of acute hepatitis that even needed liver transplantation some years ago, and every year in our clinics we see these type of patients,” Dr. Buti remarked. What is really new, she added, is the amount of cases, particularly in the United Kingdom.
Dr. Easterbrook and Dr. Buti have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ILC 2022
COVID-19 tied to increased risk for Alzheimer’s disease and Parkinson’s disease
a new study suggests. However, the research also showed there was no excess risk of these neurologic disorders following COVID than other respiratory infections such as influenza or community-acquired bacterial pneumonia.
Considering these results, study investigator Pardis Zarifkar, MD, department of neurology, Rigshospitalet, Copenhagen University Hospital, urged doctors to “keep an eye on” COVID patients and use “a critical mindset” if these patients present with neurologic issues.
“They should consider whether the patient’s condition is something new or if there were already signs and symptoms before they had COVID-19,” she said.
The findings were presented at the 2022 congress of the European Academy of Neurology and published online in Frontiers in Neurology.
‘Surprising’ increased risk
Previous research shows more than 80% of patients hospitalized with COVID-19 have neurologic symptoms including anosmia, dysgeusia, headache, dizziness, memory and concentration difficulties, fatigue, and irritability.
However, it’s unclear whether COVID-19 affects the risk for specific neurologic diseases and if so, whether this association differs from other respiratory infections.
From electronic health records covering about half the Danish population, researchers identified adults who were tested for COVID-19 or diagnosed with community-acquired bacterial pneumonia from February 2020 to November 2021. They also flagged individuals with influenza in the corresponding prepandemic period (February 2018–November 2019).
Dr. Zarifkar noted influenza A or B and community-acquired bacterial pneumonia are two of the most common respiratory tract infections.
The investigators tracked neurologic diseases up to 12 months after a positive test. They looked at two neurodegenerative diseases, Alzheimer’s disease and Parkinson’s disease, as well as cerebrovascular disorders including ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage.
The study included 43,262 individuals with a positive COVID test without a history of influenza A/B in the past year and 876,356 without a positive COVID test. It also included 1,474 individuals with community-acquired pneumonia without a history of COVID and 8,102 with influenza A or B.
“We wanted to investigate whether COVID-19 is really that much worse than all these other common respiratory infections that we have had for ages and see every single year,” said Dr. Zarifkar.
After 12 months, the relative risk for Alzheimer’s disease was 3.4 (95% confidence interval, 2.3-5.1) in the COVID-positive group versus the COVID-negative group. The risks were greater among inpatients versus outpatients.
These results were rather unexpected, said Dr. Zarifkar. “I would have expected a small increase, but the extent of the increase was quite surprising.”
However, there was no difference when comparing the COVID-19 group with the influenza or bacterial pneumonia groups, which Dr. Zarifkar said was “very reassuring.”
The findings were similar for Parkinson’s disease, where there was a 2.2-fold increased risk of a Parkinson’s disease diagnosis within the first 12 months in COVID-positive individuals, compared with COVID-negative people (RR, 2.2; 95% CI, 1.5-3.4). Again, there was no excess risk, compared with influenza or bacterial pneumonia.
Potential mechanisms
Dr. Zarifkar believes a “constellation” of factors may explain higher risks of these diagnoses in COVID patients. Part of it could be a result of neuroinflammation, which can lead to a toxic accumulation of beta amyloid in Alzheimer’s disease and alpha-synuclein in Parkinson’s disease.
“It can accelerate a neurodegenerative disease already in the making,” she said. But perhaps the biggest driver of differences between the groups is the “scientific focus” on COVID patients. “In Denmark, almost everyone who has had COVID-19, especially severe COVID-19, is offered some sort of cognitive testing, and if you hand out MoCAs [Montreal Cognitive Assessments] which is the cognitive test we use, to almost everyone you’re meeting, you’re going to catch these disorders earlier than you might have otherwise.”
As for cerebrovascular disorders, the study showed an increased risk of ischemic stroke in COVID-positive versus COVID-negative subjects at 12 months (RR, 2.87; 95% confidence interval, 2.2-3.2).
The relatively strong inflammatory response associated with COVID-19, which may create a hypercoagulable state, may help explain the increased ischemic stroke risk in COVID patients, said Dr. Zarifkar.
The study did not show an increased risk for subarachnoid hemorrhage in COVID-positive, compared with COVID-negative, subjects but did reveal an increased risk of intracerebral hemorrhage after 12 months (RR, 4.8; 95% CI, 1.8-12.9).
This could be explained by COVID-positive subjects having a higher risk for ischemic stroke and receiving thrombolysis that may increase risk for bleeding in the brain. However, an analysis accounting for medication use found differences in thrombolysis rates didn’t change the result, said Dr. Zarifkar.
It’s also possible that extracorporeal membrane oxygenation and mechanical ventilation – interventions more frequently used in COVID-19 patients – may increase the risk for bleeding in brain, she added.
The researchers did not find an increased risk for multiple sclerosis, myasthenia gravis, Guillain-Barré syndrome, or narcolepsy in COVID patients. However, Dr. Zarifkar noted that it can take years to detect an association with autoimmune disorders.
The investigators did not stratify risk by disease severity, although this would be an important step, she said. “The threshold of being admitted to the hospital with COVID-19 has been much lower than for influenza or bacterial pneumonia where you’re typically quite ill before you’re admitted, so this might actually dilute the findings and underestimate our findings.”
A national, registry-based study that includes the entire Danish population and additional information on vaccination status, virus variants, socioeconomic status, and comorbidities is needed, said Dr. Zarifkar.
The study was supported by Lundbeck Foundation and Novo Nordisk. Dr. Zarifkar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new study suggests. However, the research also showed there was no excess risk of these neurologic disorders following COVID than other respiratory infections such as influenza or community-acquired bacterial pneumonia.
Considering these results, study investigator Pardis Zarifkar, MD, department of neurology, Rigshospitalet, Copenhagen University Hospital, urged doctors to “keep an eye on” COVID patients and use “a critical mindset” if these patients present with neurologic issues.
“They should consider whether the patient’s condition is something new or if there were already signs and symptoms before they had COVID-19,” she said.
The findings were presented at the 2022 congress of the European Academy of Neurology and published online in Frontiers in Neurology.
‘Surprising’ increased risk
Previous research shows more than 80% of patients hospitalized with COVID-19 have neurologic symptoms including anosmia, dysgeusia, headache, dizziness, memory and concentration difficulties, fatigue, and irritability.
However, it’s unclear whether COVID-19 affects the risk for specific neurologic diseases and if so, whether this association differs from other respiratory infections.
From electronic health records covering about half the Danish population, researchers identified adults who were tested for COVID-19 or diagnosed with community-acquired bacterial pneumonia from February 2020 to November 2021. They also flagged individuals with influenza in the corresponding prepandemic period (February 2018–November 2019).
Dr. Zarifkar noted influenza A or B and community-acquired bacterial pneumonia are two of the most common respiratory tract infections.
The investigators tracked neurologic diseases up to 12 months after a positive test. They looked at two neurodegenerative diseases, Alzheimer’s disease and Parkinson’s disease, as well as cerebrovascular disorders including ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage.
The study included 43,262 individuals with a positive COVID test without a history of influenza A/B in the past year and 876,356 without a positive COVID test. It also included 1,474 individuals with community-acquired pneumonia without a history of COVID and 8,102 with influenza A or B.
“We wanted to investigate whether COVID-19 is really that much worse than all these other common respiratory infections that we have had for ages and see every single year,” said Dr. Zarifkar.
After 12 months, the relative risk for Alzheimer’s disease was 3.4 (95% confidence interval, 2.3-5.1) in the COVID-positive group versus the COVID-negative group. The risks were greater among inpatients versus outpatients.
These results were rather unexpected, said Dr. Zarifkar. “I would have expected a small increase, but the extent of the increase was quite surprising.”
However, there was no difference when comparing the COVID-19 group with the influenza or bacterial pneumonia groups, which Dr. Zarifkar said was “very reassuring.”
The findings were similar for Parkinson’s disease, where there was a 2.2-fold increased risk of a Parkinson’s disease diagnosis within the first 12 months in COVID-positive individuals, compared with COVID-negative people (RR, 2.2; 95% CI, 1.5-3.4). Again, there was no excess risk, compared with influenza or bacterial pneumonia.
Potential mechanisms
Dr. Zarifkar believes a “constellation” of factors may explain higher risks of these diagnoses in COVID patients. Part of it could be a result of neuroinflammation, which can lead to a toxic accumulation of beta amyloid in Alzheimer’s disease and alpha-synuclein in Parkinson’s disease.
“It can accelerate a neurodegenerative disease already in the making,” she said. But perhaps the biggest driver of differences between the groups is the “scientific focus” on COVID patients. “In Denmark, almost everyone who has had COVID-19, especially severe COVID-19, is offered some sort of cognitive testing, and if you hand out MoCAs [Montreal Cognitive Assessments] which is the cognitive test we use, to almost everyone you’re meeting, you’re going to catch these disorders earlier than you might have otherwise.”
As for cerebrovascular disorders, the study showed an increased risk of ischemic stroke in COVID-positive versus COVID-negative subjects at 12 months (RR, 2.87; 95% confidence interval, 2.2-3.2).
The relatively strong inflammatory response associated with COVID-19, which may create a hypercoagulable state, may help explain the increased ischemic stroke risk in COVID patients, said Dr. Zarifkar.
The study did not show an increased risk for subarachnoid hemorrhage in COVID-positive, compared with COVID-negative, subjects but did reveal an increased risk of intracerebral hemorrhage after 12 months (RR, 4.8; 95% CI, 1.8-12.9).
This could be explained by COVID-positive subjects having a higher risk for ischemic stroke and receiving thrombolysis that may increase risk for bleeding in the brain. However, an analysis accounting for medication use found differences in thrombolysis rates didn’t change the result, said Dr. Zarifkar.
It’s also possible that extracorporeal membrane oxygenation and mechanical ventilation – interventions more frequently used in COVID-19 patients – may increase the risk for bleeding in brain, she added.
The researchers did not find an increased risk for multiple sclerosis, myasthenia gravis, Guillain-Barré syndrome, or narcolepsy in COVID patients. However, Dr. Zarifkar noted that it can take years to detect an association with autoimmune disorders.
The investigators did not stratify risk by disease severity, although this would be an important step, she said. “The threshold of being admitted to the hospital with COVID-19 has been much lower than for influenza or bacterial pneumonia where you’re typically quite ill before you’re admitted, so this might actually dilute the findings and underestimate our findings.”
A national, registry-based study that includes the entire Danish population and additional information on vaccination status, virus variants, socioeconomic status, and comorbidities is needed, said Dr. Zarifkar.
The study was supported by Lundbeck Foundation and Novo Nordisk. Dr. Zarifkar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new study suggests. However, the research also showed there was no excess risk of these neurologic disorders following COVID than other respiratory infections such as influenza or community-acquired bacterial pneumonia.
Considering these results, study investigator Pardis Zarifkar, MD, department of neurology, Rigshospitalet, Copenhagen University Hospital, urged doctors to “keep an eye on” COVID patients and use “a critical mindset” if these patients present with neurologic issues.
“They should consider whether the patient’s condition is something new or if there were already signs and symptoms before they had COVID-19,” she said.
The findings were presented at the 2022 congress of the European Academy of Neurology and published online in Frontiers in Neurology.
‘Surprising’ increased risk
Previous research shows more than 80% of patients hospitalized with COVID-19 have neurologic symptoms including anosmia, dysgeusia, headache, dizziness, memory and concentration difficulties, fatigue, and irritability.
However, it’s unclear whether COVID-19 affects the risk for specific neurologic diseases and if so, whether this association differs from other respiratory infections.
From electronic health records covering about half the Danish population, researchers identified adults who were tested for COVID-19 or diagnosed with community-acquired bacterial pneumonia from February 2020 to November 2021. They also flagged individuals with influenza in the corresponding prepandemic period (February 2018–November 2019).
Dr. Zarifkar noted influenza A or B and community-acquired bacterial pneumonia are two of the most common respiratory tract infections.
The investigators tracked neurologic diseases up to 12 months after a positive test. They looked at two neurodegenerative diseases, Alzheimer’s disease and Parkinson’s disease, as well as cerebrovascular disorders including ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage.
The study included 43,262 individuals with a positive COVID test without a history of influenza A/B in the past year and 876,356 without a positive COVID test. It also included 1,474 individuals with community-acquired pneumonia without a history of COVID and 8,102 with influenza A or B.
“We wanted to investigate whether COVID-19 is really that much worse than all these other common respiratory infections that we have had for ages and see every single year,” said Dr. Zarifkar.
After 12 months, the relative risk for Alzheimer’s disease was 3.4 (95% confidence interval, 2.3-5.1) in the COVID-positive group versus the COVID-negative group. The risks were greater among inpatients versus outpatients.
These results were rather unexpected, said Dr. Zarifkar. “I would have expected a small increase, but the extent of the increase was quite surprising.”
However, there was no difference when comparing the COVID-19 group with the influenza or bacterial pneumonia groups, which Dr. Zarifkar said was “very reassuring.”
The findings were similar for Parkinson’s disease, where there was a 2.2-fold increased risk of a Parkinson’s disease diagnosis within the first 12 months in COVID-positive individuals, compared with COVID-negative people (RR, 2.2; 95% CI, 1.5-3.4). Again, there was no excess risk, compared with influenza or bacterial pneumonia.
Potential mechanisms
Dr. Zarifkar believes a “constellation” of factors may explain higher risks of these diagnoses in COVID patients. Part of it could be a result of neuroinflammation, which can lead to a toxic accumulation of beta amyloid in Alzheimer’s disease and alpha-synuclein in Parkinson’s disease.
“It can accelerate a neurodegenerative disease already in the making,” she said. But perhaps the biggest driver of differences between the groups is the “scientific focus” on COVID patients. “In Denmark, almost everyone who has had COVID-19, especially severe COVID-19, is offered some sort of cognitive testing, and if you hand out MoCAs [Montreal Cognitive Assessments] which is the cognitive test we use, to almost everyone you’re meeting, you’re going to catch these disorders earlier than you might have otherwise.”
As for cerebrovascular disorders, the study showed an increased risk of ischemic stroke in COVID-positive versus COVID-negative subjects at 12 months (RR, 2.87; 95% confidence interval, 2.2-3.2).
The relatively strong inflammatory response associated with COVID-19, which may create a hypercoagulable state, may help explain the increased ischemic stroke risk in COVID patients, said Dr. Zarifkar.
The study did not show an increased risk for subarachnoid hemorrhage in COVID-positive, compared with COVID-negative, subjects but did reveal an increased risk of intracerebral hemorrhage after 12 months (RR, 4.8; 95% CI, 1.8-12.9).
This could be explained by COVID-positive subjects having a higher risk for ischemic stroke and receiving thrombolysis that may increase risk for bleeding in the brain. However, an analysis accounting for medication use found differences in thrombolysis rates didn’t change the result, said Dr. Zarifkar.
It’s also possible that extracorporeal membrane oxygenation and mechanical ventilation – interventions more frequently used in COVID-19 patients – may increase the risk for bleeding in brain, she added.
The researchers did not find an increased risk for multiple sclerosis, myasthenia gravis, Guillain-Barré syndrome, or narcolepsy in COVID patients. However, Dr. Zarifkar noted that it can take years to detect an association with autoimmune disorders.
The investigators did not stratify risk by disease severity, although this would be an important step, she said. “The threshold of being admitted to the hospital with COVID-19 has been much lower than for influenza or bacterial pneumonia where you’re typically quite ill before you’re admitted, so this might actually dilute the findings and underestimate our findings.”
A national, registry-based study that includes the entire Danish population and additional information on vaccination status, virus variants, socioeconomic status, and comorbidities is needed, said Dr. Zarifkar.
The study was supported by Lundbeck Foundation and Novo Nordisk. Dr. Zarifkar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM FRONTIERS IN NEUROLOGY
Pandemic stress tied to increased headache burden in teens
Contrary to previous research findings, the stress of the COVID-19 pandemic has been linked to an increased headache burden in teens.
Investigators found factors contributing to headache for preteens and teens during the pandemic included increased screen time for online learning, depression, anxiety, female sex, and weight gain.
“The stressors and pressures of the pandemic may have eventually taken their toll,” lead author Ayşe Nur Özdağ Acarli, MD, Ermenek State Hospital, department of neurology, Karaman, Turkey, told this news organization.
“Limiting screen time and providing more psychosocial supports would help lessen the burden of the COVID-19 pandemic on adolescents with headache.”
The findings were presented at the Congress of the European Academy of Neurology (EAN) 2022.
Most common neurological problem in kids
Headache is the most common neurological problem in children and adolescents. Potential factors contributing to headache in this population include lack of sleep and physical activity, mental health problems, and socioeconomic conditions.
The COVID-19 pandemic has had a “striking” impact on every aspect of life for young people, said Dr. Acarli.
Some studies reported an improvement in headache prevalence among adolescents during COVID-19, which was attributed to less school-related stress. However, said Dr. Acarli in her personal clinical experience, young patients suffered more frequent and severe headaches during the pandemic.
She noted previous research examining the impact of the pandemic on headache in youth was conducted only in the early days of the pandemic and examined shorter-term effects. Research examining the long-term effects of the pandemic on headache in this patient population has been “lacking,” she said.
The study included 851 participants aged 10-18 years (mean age 14.9 years and 62% female) who were seen at a neurology or pediatric outpatient clinic from August-December 2021. The study excluded subjects with neurological problems, intellectual deficits, autism spectrum disorder, and epilepsy.
Participants completed detailed questionnaires providing data on demographics, exposure to COVID-19, and electronics, as well as information on depressive symptoms as assessed by the Patient Health Questionnaire-9 and anxiety symptoms using the Generalized Anxiety Disorder-7 and COVID-related anxiety.
“We used two distinct scales for anxiety: one for generalized anxiety and the other for COVID-related anxiety,” said Dr. Acarli.
Of the total study population, 756 (89%) reported headaches. This headache prevalence in children and adolescents is like that found in other studies.
Dr. Acarli noted several differences in the headache group versus the non-headache group. The female/male ratio was 2:1 versus 1:1, the mean age was 15.0 versus 14.4, and depression and generalized anxiety scores were significantly higher. There was no significant difference in COVID-19 history in those with and without headache.
Researchers categorized those with headache into four groups: worsening headaches (27%), improved headaches (3%), new onset headaches (10%), and stable headaches (61%).
Compared with the other groups, the worsened headache group included significantly more females and older individuals with more severe and frequent headaches. This group also had more participants reporting at least 15 headache attacks a month and using painkillers at least once a month.
The study showed headache severity was significantly increased with age, headache duration, depression, generalized anxiety (all P < .001), and COVID-19 anxiety (P < .01). Headache frequency, measured as attacks per month, was significantly increased with age, depression, and generalized anxiety (all P < .001).
Worsening headache outcomes during the pandemic were associated with longer exposure to computer screens (odds ratio, 1.7; 95% confidence interval, 1.2-2.3; P < .01), lack of suitable conditions for online learning (OR, 2.6; 95% CI, 1.8-3.8; P < .001), depression (OR, 2.0; 95% CI, 1.4-2.8; P < .001); and COVID-19 anxiety (OR, 3.2; 95% CI, 1.3-8.0; P < .01). Other contributing factors included school exams, living in a city, female sex, and weight gain.
There may be a link between COVID-related headaches and anxiety or depression, but it’s unclear what’s causing what. “We don’t know which is the chicken and which is the egg,” said Dr. Acarli.
Headache triggers
Commenting for this news organization, Raquel Gil-Gouveia, MD, PhD, head of the neurology department, Hospital da Luz, Lisbon, Portugal, who co-chaired the session where the research was presented, said the information collected for the study was “extensive.”
Some results were expected, including the fact that patients with headaches were more anxious and depressed, said Dr. Gil-Gouveia.
“Anxiety and depression are frequent comorbidities of headache and can act as a triggering factor for headache attacks but can also be a consequence of intense or chronic pain,” she said.
She agreed the new results differ from those of studies carried out during the first pandemic lockdown, which showed an improvement in headache, but noted online learning was not fully implemented at that time, “so it was much like being on vacation.”
In addition to isolation, anxiety, and prolonged screen exposure, the lack of peer contact and fewer sports and leisure activities may also have contributed to worsening headaches during the COVID lockdown, but these were not explored in this study, said Dr. Gil-Gouveia.
The study was supported by the Global Migraine and Pain Society. The investigators and Dr. Gil-Gouveia report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Contrary to previous research findings, the stress of the COVID-19 pandemic has been linked to an increased headache burden in teens.
Investigators found factors contributing to headache for preteens and teens during the pandemic included increased screen time for online learning, depression, anxiety, female sex, and weight gain.
“The stressors and pressures of the pandemic may have eventually taken their toll,” lead author Ayşe Nur Özdağ Acarli, MD, Ermenek State Hospital, department of neurology, Karaman, Turkey, told this news organization.
“Limiting screen time and providing more psychosocial supports would help lessen the burden of the COVID-19 pandemic on adolescents with headache.”
The findings were presented at the Congress of the European Academy of Neurology (EAN) 2022.
Most common neurological problem in kids
Headache is the most common neurological problem in children and adolescents. Potential factors contributing to headache in this population include lack of sleep and physical activity, mental health problems, and socioeconomic conditions.
The COVID-19 pandemic has had a “striking” impact on every aspect of life for young people, said Dr. Acarli.
Some studies reported an improvement in headache prevalence among adolescents during COVID-19, which was attributed to less school-related stress. However, said Dr. Acarli in her personal clinical experience, young patients suffered more frequent and severe headaches during the pandemic.
She noted previous research examining the impact of the pandemic on headache in youth was conducted only in the early days of the pandemic and examined shorter-term effects. Research examining the long-term effects of the pandemic on headache in this patient population has been “lacking,” she said.
The study included 851 participants aged 10-18 years (mean age 14.9 years and 62% female) who were seen at a neurology or pediatric outpatient clinic from August-December 2021. The study excluded subjects with neurological problems, intellectual deficits, autism spectrum disorder, and epilepsy.
Participants completed detailed questionnaires providing data on demographics, exposure to COVID-19, and electronics, as well as information on depressive symptoms as assessed by the Patient Health Questionnaire-9 and anxiety symptoms using the Generalized Anxiety Disorder-7 and COVID-related anxiety.
“We used two distinct scales for anxiety: one for generalized anxiety and the other for COVID-related anxiety,” said Dr. Acarli.
Of the total study population, 756 (89%) reported headaches. This headache prevalence in children and adolescents is like that found in other studies.
Dr. Acarli noted several differences in the headache group versus the non-headache group. The female/male ratio was 2:1 versus 1:1, the mean age was 15.0 versus 14.4, and depression and generalized anxiety scores were significantly higher. There was no significant difference in COVID-19 history in those with and without headache.
Researchers categorized those with headache into four groups: worsening headaches (27%), improved headaches (3%), new onset headaches (10%), and stable headaches (61%).
Compared with the other groups, the worsened headache group included significantly more females and older individuals with more severe and frequent headaches. This group also had more participants reporting at least 15 headache attacks a month and using painkillers at least once a month.
The study showed headache severity was significantly increased with age, headache duration, depression, generalized anxiety (all P < .001), and COVID-19 anxiety (P < .01). Headache frequency, measured as attacks per month, was significantly increased with age, depression, and generalized anxiety (all P < .001).
Worsening headache outcomes during the pandemic were associated with longer exposure to computer screens (odds ratio, 1.7; 95% confidence interval, 1.2-2.3; P < .01), lack of suitable conditions for online learning (OR, 2.6; 95% CI, 1.8-3.8; P < .001), depression (OR, 2.0; 95% CI, 1.4-2.8; P < .001); and COVID-19 anxiety (OR, 3.2; 95% CI, 1.3-8.0; P < .01). Other contributing factors included school exams, living in a city, female sex, and weight gain.
There may be a link between COVID-related headaches and anxiety or depression, but it’s unclear what’s causing what. “We don’t know which is the chicken and which is the egg,” said Dr. Acarli.
Headache triggers
Commenting for this news organization, Raquel Gil-Gouveia, MD, PhD, head of the neurology department, Hospital da Luz, Lisbon, Portugal, who co-chaired the session where the research was presented, said the information collected for the study was “extensive.”
Some results were expected, including the fact that patients with headaches were more anxious and depressed, said Dr. Gil-Gouveia.
“Anxiety and depression are frequent comorbidities of headache and can act as a triggering factor for headache attacks but can also be a consequence of intense or chronic pain,” she said.
She agreed the new results differ from those of studies carried out during the first pandemic lockdown, which showed an improvement in headache, but noted online learning was not fully implemented at that time, “so it was much like being on vacation.”
In addition to isolation, anxiety, and prolonged screen exposure, the lack of peer contact and fewer sports and leisure activities may also have contributed to worsening headaches during the COVID lockdown, but these were not explored in this study, said Dr. Gil-Gouveia.
The study was supported by the Global Migraine and Pain Society. The investigators and Dr. Gil-Gouveia report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Contrary to previous research findings, the stress of the COVID-19 pandemic has been linked to an increased headache burden in teens.
Investigators found factors contributing to headache for preteens and teens during the pandemic included increased screen time for online learning, depression, anxiety, female sex, and weight gain.
“The stressors and pressures of the pandemic may have eventually taken their toll,” lead author Ayşe Nur Özdağ Acarli, MD, Ermenek State Hospital, department of neurology, Karaman, Turkey, told this news organization.
“Limiting screen time and providing more psychosocial supports would help lessen the burden of the COVID-19 pandemic on adolescents with headache.”
The findings were presented at the Congress of the European Academy of Neurology (EAN) 2022.
Most common neurological problem in kids
Headache is the most common neurological problem in children and adolescents. Potential factors contributing to headache in this population include lack of sleep and physical activity, mental health problems, and socioeconomic conditions.
The COVID-19 pandemic has had a “striking” impact on every aspect of life for young people, said Dr. Acarli.
Some studies reported an improvement in headache prevalence among adolescents during COVID-19, which was attributed to less school-related stress. However, said Dr. Acarli in her personal clinical experience, young patients suffered more frequent and severe headaches during the pandemic.
She noted previous research examining the impact of the pandemic on headache in youth was conducted only in the early days of the pandemic and examined shorter-term effects. Research examining the long-term effects of the pandemic on headache in this patient population has been “lacking,” she said.
The study included 851 participants aged 10-18 years (mean age 14.9 years and 62% female) who were seen at a neurology or pediatric outpatient clinic from August-December 2021. The study excluded subjects with neurological problems, intellectual deficits, autism spectrum disorder, and epilepsy.
Participants completed detailed questionnaires providing data on demographics, exposure to COVID-19, and electronics, as well as information on depressive symptoms as assessed by the Patient Health Questionnaire-9 and anxiety symptoms using the Generalized Anxiety Disorder-7 and COVID-related anxiety.
“We used two distinct scales for anxiety: one for generalized anxiety and the other for COVID-related anxiety,” said Dr. Acarli.
Of the total study population, 756 (89%) reported headaches. This headache prevalence in children and adolescents is like that found in other studies.
Dr. Acarli noted several differences in the headache group versus the non-headache group. The female/male ratio was 2:1 versus 1:1, the mean age was 15.0 versus 14.4, and depression and generalized anxiety scores were significantly higher. There was no significant difference in COVID-19 history in those with and without headache.
Researchers categorized those with headache into four groups: worsening headaches (27%), improved headaches (3%), new onset headaches (10%), and stable headaches (61%).
Compared with the other groups, the worsened headache group included significantly more females and older individuals with more severe and frequent headaches. This group also had more participants reporting at least 15 headache attacks a month and using painkillers at least once a month.
The study showed headache severity was significantly increased with age, headache duration, depression, generalized anxiety (all P < .001), and COVID-19 anxiety (P < .01). Headache frequency, measured as attacks per month, was significantly increased with age, depression, and generalized anxiety (all P < .001).
Worsening headache outcomes during the pandemic were associated with longer exposure to computer screens (odds ratio, 1.7; 95% confidence interval, 1.2-2.3; P < .01), lack of suitable conditions for online learning (OR, 2.6; 95% CI, 1.8-3.8; P < .001), depression (OR, 2.0; 95% CI, 1.4-2.8; P < .001); and COVID-19 anxiety (OR, 3.2; 95% CI, 1.3-8.0; P < .01). Other contributing factors included school exams, living in a city, female sex, and weight gain.
There may be a link between COVID-related headaches and anxiety or depression, but it’s unclear what’s causing what. “We don’t know which is the chicken and which is the egg,” said Dr. Acarli.
Headache triggers
Commenting for this news organization, Raquel Gil-Gouveia, MD, PhD, head of the neurology department, Hospital da Luz, Lisbon, Portugal, who co-chaired the session where the research was presented, said the information collected for the study was “extensive.”
Some results were expected, including the fact that patients with headaches were more anxious and depressed, said Dr. Gil-Gouveia.
“Anxiety and depression are frequent comorbidities of headache and can act as a triggering factor for headache attacks but can also be a consequence of intense or chronic pain,” she said.
She agreed the new results differ from those of studies carried out during the first pandemic lockdown, which showed an improvement in headache, but noted online learning was not fully implemented at that time, “so it was much like being on vacation.”
In addition to isolation, anxiety, and prolonged screen exposure, the lack of peer contact and fewer sports and leisure activities may also have contributed to worsening headaches during the COVID lockdown, but these were not explored in this study, said Dr. Gil-Gouveia.
The study was supported by the Global Migraine and Pain Society. The investigators and Dr. Gil-Gouveia report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EAN 2022
FDA panel backs adding Omicron component to COVID boosters
A federal advisory panel on June 28 recommended updating COVID-19 booster vaccines in the United States to include an Omicron component, while urging the need for more information on how well these shots work on emerging strains of the virus.
The Vaccines and Related Biological Products Advisory Committee of the Food and Drug Administration voted 19-2 in favor of a new formulation – although what that formulation will be is yet to be determined. The FDA often incorporates the views of its advisers into its decisions, although it is not bound to do so.
In this case, though, top FDA staff at the meeting seemed inclined to encourage the development of COVID vaccines modified to keep up with an evolving virus. Two Omicron subvariants, BA.4 and BA.5, which first appeared in South Africa in March 2022, have spread to the United States and have begun to increase rapidly in proportion to the virus population, the FDA said in a briefing for the meeting.
New information from the Centers for Disease Control and Prevention shows the two highly infectious subvariants now make up more than half the number of new COVID cases in the US.
Double-duty vaccine
In summarizing the message of the advisory committee, Peter W. Marks, MD, PhD, the director of the FDA’s Center for Biologics Evaluation & Research, said panelists had lent support to modifying vaccines to protect against both the original, or “ancestral” viral strain, and against Omicron, perhaps emphasizing the newly emerging subvariants.
Dr. Marks emphasized that this is a challenging decision, as no one has a “crystal ball” to forecast how SARS-CoV-2 will evolve.
“We are trying to use every last ounce of what we can from predictive modeling and from the data that we have that’s emerging, to try to get ahead of a virus that has been very crafty,” he said.”It’s pretty darn crafty.”
Limited data
Voting “no” were Paul Offit, MD, of Children’s Hospital of Philadelphia and Henry Bernstein, DO, MHCM, of Hofstra/Northwell Health in New Hyde Park, N.Y.
Both Dr. Offit and Dr. Bernstein earlier in the meeting expressed doubts about the evidence gathered to date in favor of a strain change. Dr. Offit had noted that protection seems to persist from the vaccines now available.
“To date, the current prototypical vaccines, the ancestral strain vaccines do protect against serious illness,” he said. “We don’t yet have a variant that is resistant to protection against serious illness.“
Dr. Bernstein said he was “struggling” with the question as well, given the limited data gathered to date about the vaccines and emerging strains of the virus.
Other panelists also expressed reservations, while supporting the concept of altering vaccines to teach the body to fight the emerging strains as well as the original one.
Panelist Wayne Marasco, MD, PhD, of Harvard Medical School, Boston, who voted yes, noted the difficulties of keeping up with the rapidly evolving virus, saying it’s possible that Omicron strains BA.4 and BA.5 could peak within months. That could be before the vaccines are even distributed – if all goes to plan – in the fall.
“This is a step in the right direction, but we have to reevaluate this as we move forward,” Dr. Marasco said, adding that a good strategy would be to elicit antibody response to bridge more than one variant of the virus.
Even panelists like Dr. Marasco who voted yes stressed the need for further data collection about how vaccines may be adapted to a changing virus. But they also acknowledged a need to give vaccine makers a clear indication of what the medical community expects in terms of changes to these shots.
“With the waning vaccine efficacy and the confluence of risk this fall, we need to make a move sooner rather than later and direct our sponsors in the proper direction,” said FDA panelist Michael Nelson, MD, PhD, of the University of Virginia, Charlottesville, said before the vote.
A version of this article first appeared on Medscape.com.
A federal advisory panel on June 28 recommended updating COVID-19 booster vaccines in the United States to include an Omicron component, while urging the need for more information on how well these shots work on emerging strains of the virus.
The Vaccines and Related Biological Products Advisory Committee of the Food and Drug Administration voted 19-2 in favor of a new formulation – although what that formulation will be is yet to be determined. The FDA often incorporates the views of its advisers into its decisions, although it is not bound to do so.
In this case, though, top FDA staff at the meeting seemed inclined to encourage the development of COVID vaccines modified to keep up with an evolving virus. Two Omicron subvariants, BA.4 and BA.5, which first appeared in South Africa in March 2022, have spread to the United States and have begun to increase rapidly in proportion to the virus population, the FDA said in a briefing for the meeting.
New information from the Centers for Disease Control and Prevention shows the two highly infectious subvariants now make up more than half the number of new COVID cases in the US.
Double-duty vaccine
In summarizing the message of the advisory committee, Peter W. Marks, MD, PhD, the director of the FDA’s Center for Biologics Evaluation & Research, said panelists had lent support to modifying vaccines to protect against both the original, or “ancestral” viral strain, and against Omicron, perhaps emphasizing the newly emerging subvariants.
Dr. Marks emphasized that this is a challenging decision, as no one has a “crystal ball” to forecast how SARS-CoV-2 will evolve.
“We are trying to use every last ounce of what we can from predictive modeling and from the data that we have that’s emerging, to try to get ahead of a virus that has been very crafty,” he said.”It’s pretty darn crafty.”
Limited data
Voting “no” were Paul Offit, MD, of Children’s Hospital of Philadelphia and Henry Bernstein, DO, MHCM, of Hofstra/Northwell Health in New Hyde Park, N.Y.
Both Dr. Offit and Dr. Bernstein earlier in the meeting expressed doubts about the evidence gathered to date in favor of a strain change. Dr. Offit had noted that protection seems to persist from the vaccines now available.
“To date, the current prototypical vaccines, the ancestral strain vaccines do protect against serious illness,” he said. “We don’t yet have a variant that is resistant to protection against serious illness.“
Dr. Bernstein said he was “struggling” with the question as well, given the limited data gathered to date about the vaccines and emerging strains of the virus.
Other panelists also expressed reservations, while supporting the concept of altering vaccines to teach the body to fight the emerging strains as well as the original one.
Panelist Wayne Marasco, MD, PhD, of Harvard Medical School, Boston, who voted yes, noted the difficulties of keeping up with the rapidly evolving virus, saying it’s possible that Omicron strains BA.4 and BA.5 could peak within months. That could be before the vaccines are even distributed – if all goes to plan – in the fall.
“This is a step in the right direction, but we have to reevaluate this as we move forward,” Dr. Marasco said, adding that a good strategy would be to elicit antibody response to bridge more than one variant of the virus.
Even panelists like Dr. Marasco who voted yes stressed the need for further data collection about how vaccines may be adapted to a changing virus. But they also acknowledged a need to give vaccine makers a clear indication of what the medical community expects in terms of changes to these shots.
“With the waning vaccine efficacy and the confluence of risk this fall, we need to make a move sooner rather than later and direct our sponsors in the proper direction,” said FDA panelist Michael Nelson, MD, PhD, of the University of Virginia, Charlottesville, said before the vote.
A version of this article first appeared on Medscape.com.
A federal advisory panel on June 28 recommended updating COVID-19 booster vaccines in the United States to include an Omicron component, while urging the need for more information on how well these shots work on emerging strains of the virus.
The Vaccines and Related Biological Products Advisory Committee of the Food and Drug Administration voted 19-2 in favor of a new formulation – although what that formulation will be is yet to be determined. The FDA often incorporates the views of its advisers into its decisions, although it is not bound to do so.
In this case, though, top FDA staff at the meeting seemed inclined to encourage the development of COVID vaccines modified to keep up with an evolving virus. Two Omicron subvariants, BA.4 and BA.5, which first appeared in South Africa in March 2022, have spread to the United States and have begun to increase rapidly in proportion to the virus population, the FDA said in a briefing for the meeting.
New information from the Centers for Disease Control and Prevention shows the two highly infectious subvariants now make up more than half the number of new COVID cases in the US.
Double-duty vaccine
In summarizing the message of the advisory committee, Peter W. Marks, MD, PhD, the director of the FDA’s Center for Biologics Evaluation & Research, said panelists had lent support to modifying vaccines to protect against both the original, or “ancestral” viral strain, and against Omicron, perhaps emphasizing the newly emerging subvariants.
Dr. Marks emphasized that this is a challenging decision, as no one has a “crystal ball” to forecast how SARS-CoV-2 will evolve.
“We are trying to use every last ounce of what we can from predictive modeling and from the data that we have that’s emerging, to try to get ahead of a virus that has been very crafty,” he said.”It’s pretty darn crafty.”
Limited data
Voting “no” were Paul Offit, MD, of Children’s Hospital of Philadelphia and Henry Bernstein, DO, MHCM, of Hofstra/Northwell Health in New Hyde Park, N.Y.
Both Dr. Offit and Dr. Bernstein earlier in the meeting expressed doubts about the evidence gathered to date in favor of a strain change. Dr. Offit had noted that protection seems to persist from the vaccines now available.
“To date, the current prototypical vaccines, the ancestral strain vaccines do protect against serious illness,” he said. “We don’t yet have a variant that is resistant to protection against serious illness.“
Dr. Bernstein said he was “struggling” with the question as well, given the limited data gathered to date about the vaccines and emerging strains of the virus.
Other panelists also expressed reservations, while supporting the concept of altering vaccines to teach the body to fight the emerging strains as well as the original one.
Panelist Wayne Marasco, MD, PhD, of Harvard Medical School, Boston, who voted yes, noted the difficulties of keeping up with the rapidly evolving virus, saying it’s possible that Omicron strains BA.4 and BA.5 could peak within months. That could be before the vaccines are even distributed – if all goes to plan – in the fall.
“This is a step in the right direction, but we have to reevaluate this as we move forward,” Dr. Marasco said, adding that a good strategy would be to elicit antibody response to bridge more than one variant of the virus.
Even panelists like Dr. Marasco who voted yes stressed the need for further data collection about how vaccines may be adapted to a changing virus. But they also acknowledged a need to give vaccine makers a clear indication of what the medical community expects in terms of changes to these shots.
“With the waning vaccine efficacy and the confluence of risk this fall, we need to make a move sooner rather than later and direct our sponsors in the proper direction,” said FDA panelist Michael Nelson, MD, PhD, of the University of Virginia, Charlottesville, said before the vote.
A version of this article first appeared on Medscape.com.
COVID subvariants could cause ‘substantial’ summer cases
As the coronavirus continues to evolve, Omicron subvariants such as BA.4 and BA.5 are expected to lead to many COVID-19 cases in the coming months.
Researchers recently reported that the subvariants have mutated for better “immune escape,” or the ability to avoid antibodies from vaccination or previous infection.
“That has changed our view for what will happen this summer,” Ali Mokdad, PhD, an epidemiologist who has developed COVID-19 forecasts for the University of Washington’s Institute for Health Metrics and Evaluation in Seattle, told The Boston Globe.
Until recently, Dr. Mokdad expected the United States to have a “very good summer” in terms of cases, hospitalizations, and deaths through September. The U.S. is reporting about 100,000 new cases per day, according to the data tracker by The New York Times, which has remained flat throughout June. Cases will likely decrease this summer, Dr. Mokdad said, though the decline will be slower and smaller than first thought.
As of June 18, BA.4 and BA.5 accounted for about 35% of cases in the United States, according to the latest CDC data, with BA.5 making up 23.5% and BA.4 making up 11.4%. The two subvariants will likely take over BA.2.12.1 as top subvariants in coming weeks.
“I expect that BA.5 will likely become the dominant virus in the United States this summer,” Dan Barouch, MD, director of the Center for Virology and Vaccine Research at Beth Israel Deaconess Medical Center in Boston, told the Globe.
Dr. Barouch said the Omicron subvariants will likely create a summer of “substantial infections” but low rates of hospitalization and death. He published a recent study in the New England Journal of Medicine that found BA.4 and BA.5 are better at escaping antibodies than other coronavirus strains – about three times better than the Omicron variants BA.1 and BA.2 and 20 times better than the first coronavirus strain.
“What we’re seeing with each subsequent variant is iteratively higher levels of transmissibility and higher levels of antibody immune escape,” he said. “We’re seeing high levels of infection in populations that are highly vaccinated, as well as populations that have a high level of natural immunity to the prior variants.”
At the same time, current antibodies still appear to protect people against the worst outcomes, Dr. Barouch said.
“If people have vaccine immunity or natural immunity, then they have substantial protection against severe disease,” he said.
So far, researchers have found that Omicron subvariants tend to cause less severe disease than other variants, such as Delta. Dr. Mokdad estimated that 80% of Omicron infections don’t show symptoms.
He said there is a “remote possibility” of another wave during the summer, but he expects cases to rise significantly around the beginning of October, when the seasons change, and most people’s immunity will wane. Other things could play into the predictions this summer, he noted, such as coronavirus mutations and new variants.
“Anybody that models this more than a couple of weeks out is basically just using pixie dust,” Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, told the newspaper.
“There is no pattern whatsoever developing from a seasonality standpoint. It’s all being driven by the variants,” he said. “We just have to be humble and acknowledge that we don’t know.”
A version of this article first appeared on WebMD.com.
As the coronavirus continues to evolve, Omicron subvariants such as BA.4 and BA.5 are expected to lead to many COVID-19 cases in the coming months.
Researchers recently reported that the subvariants have mutated for better “immune escape,” or the ability to avoid antibodies from vaccination or previous infection.
“That has changed our view for what will happen this summer,” Ali Mokdad, PhD, an epidemiologist who has developed COVID-19 forecasts for the University of Washington’s Institute for Health Metrics and Evaluation in Seattle, told The Boston Globe.
Until recently, Dr. Mokdad expected the United States to have a “very good summer” in terms of cases, hospitalizations, and deaths through September. The U.S. is reporting about 100,000 new cases per day, according to the data tracker by The New York Times, which has remained flat throughout June. Cases will likely decrease this summer, Dr. Mokdad said, though the decline will be slower and smaller than first thought.
As of June 18, BA.4 and BA.5 accounted for about 35% of cases in the United States, according to the latest CDC data, with BA.5 making up 23.5% and BA.4 making up 11.4%. The two subvariants will likely take over BA.2.12.1 as top subvariants in coming weeks.
“I expect that BA.5 will likely become the dominant virus in the United States this summer,” Dan Barouch, MD, director of the Center for Virology and Vaccine Research at Beth Israel Deaconess Medical Center in Boston, told the Globe.
Dr. Barouch said the Omicron subvariants will likely create a summer of “substantial infections” but low rates of hospitalization and death. He published a recent study in the New England Journal of Medicine that found BA.4 and BA.5 are better at escaping antibodies than other coronavirus strains – about three times better than the Omicron variants BA.1 and BA.2 and 20 times better than the first coronavirus strain.
“What we’re seeing with each subsequent variant is iteratively higher levels of transmissibility and higher levels of antibody immune escape,” he said. “We’re seeing high levels of infection in populations that are highly vaccinated, as well as populations that have a high level of natural immunity to the prior variants.”
At the same time, current antibodies still appear to protect people against the worst outcomes, Dr. Barouch said.
“If people have vaccine immunity or natural immunity, then they have substantial protection against severe disease,” he said.
So far, researchers have found that Omicron subvariants tend to cause less severe disease than other variants, such as Delta. Dr. Mokdad estimated that 80% of Omicron infections don’t show symptoms.
He said there is a “remote possibility” of another wave during the summer, but he expects cases to rise significantly around the beginning of October, when the seasons change, and most people’s immunity will wane. Other things could play into the predictions this summer, he noted, such as coronavirus mutations and new variants.
“Anybody that models this more than a couple of weeks out is basically just using pixie dust,” Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, told the newspaper.
“There is no pattern whatsoever developing from a seasonality standpoint. It’s all being driven by the variants,” he said. “We just have to be humble and acknowledge that we don’t know.”
A version of this article first appeared on WebMD.com.
As the coronavirus continues to evolve, Omicron subvariants such as BA.4 and BA.5 are expected to lead to many COVID-19 cases in the coming months.
Researchers recently reported that the subvariants have mutated for better “immune escape,” or the ability to avoid antibodies from vaccination or previous infection.
“That has changed our view for what will happen this summer,” Ali Mokdad, PhD, an epidemiologist who has developed COVID-19 forecasts for the University of Washington’s Institute for Health Metrics and Evaluation in Seattle, told The Boston Globe.
Until recently, Dr. Mokdad expected the United States to have a “very good summer” in terms of cases, hospitalizations, and deaths through September. The U.S. is reporting about 100,000 new cases per day, according to the data tracker by The New York Times, which has remained flat throughout June. Cases will likely decrease this summer, Dr. Mokdad said, though the decline will be slower and smaller than first thought.
As of June 18, BA.4 and BA.5 accounted for about 35% of cases in the United States, according to the latest CDC data, with BA.5 making up 23.5% and BA.4 making up 11.4%. The two subvariants will likely take over BA.2.12.1 as top subvariants in coming weeks.
“I expect that BA.5 will likely become the dominant virus in the United States this summer,” Dan Barouch, MD, director of the Center for Virology and Vaccine Research at Beth Israel Deaconess Medical Center in Boston, told the Globe.
Dr. Barouch said the Omicron subvariants will likely create a summer of “substantial infections” but low rates of hospitalization and death. He published a recent study in the New England Journal of Medicine that found BA.4 and BA.5 are better at escaping antibodies than other coronavirus strains – about three times better than the Omicron variants BA.1 and BA.2 and 20 times better than the first coronavirus strain.
“What we’re seeing with each subsequent variant is iteratively higher levels of transmissibility and higher levels of antibody immune escape,” he said. “We’re seeing high levels of infection in populations that are highly vaccinated, as well as populations that have a high level of natural immunity to the prior variants.”
At the same time, current antibodies still appear to protect people against the worst outcomes, Dr. Barouch said.
“If people have vaccine immunity or natural immunity, then they have substantial protection against severe disease,” he said.
So far, researchers have found that Omicron subvariants tend to cause less severe disease than other variants, such as Delta. Dr. Mokdad estimated that 80% of Omicron infections don’t show symptoms.
He said there is a “remote possibility” of another wave during the summer, but he expects cases to rise significantly around the beginning of October, when the seasons change, and most people’s immunity will wane. Other things could play into the predictions this summer, he noted, such as coronavirus mutations and new variants.
“Anybody that models this more than a couple of weeks out is basically just using pixie dust,” Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, told the newspaper.
“There is no pattern whatsoever developing from a seasonality standpoint. It’s all being driven by the variants,” he said. “We just have to be humble and acknowledge that we don’t know.”
A version of this article first appeared on WebMD.com.
Children and COVID: Vaccination off to slow start for the newly eligible
New cases of COVID-19 continue to drop among children, but the vaccination effort in those under age 5 years began with something less than a bang.
In the first 2 days after their respective approvals, almost 99,000 children aged 5-11 years and over 675,000 children aged 12-15 were vaccinated, according to data from the Centers for Disease Control and Prevention. Children aged 0-4 years represent almost 6% of the overall population, compared with 8.7% for the 5- to 11-year-olds and 5.1% for those aged 12-15.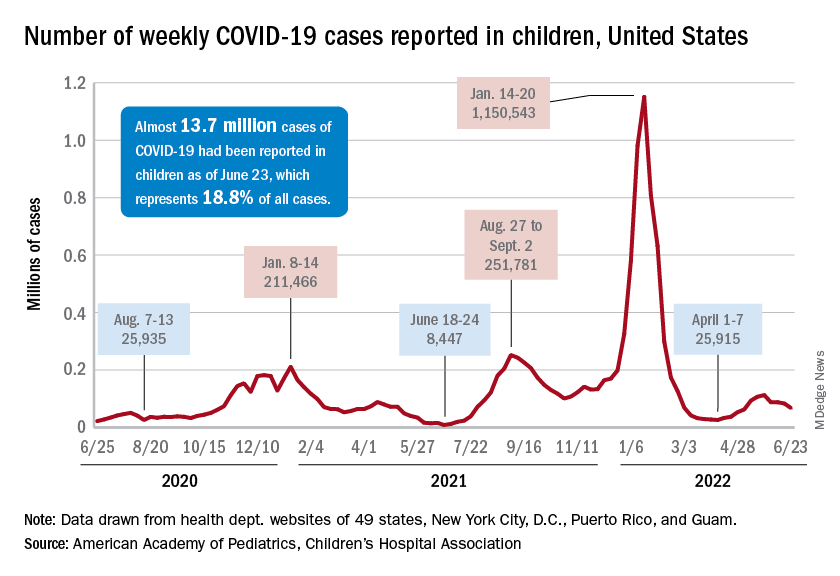
The recent decline in new cases over the past 4 weeks and the substantial decline since the Omicron surge could be a factor in the lack of response, but it is worth noting that the almost 68,000 new child cases reported in the past week, June 17-23, are “far higher than 1 year ago, June 24, 2021, when 8,400 child cases were reported,” the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
That total for June 17-23 was 19% lower than the previous week and down by 40% since new cases hit a spring peak of 112,000 in late May. Regionally, new cases were down in the Midwest, the South, and the West, the AAP/CHA report showed, but the Northeast saw a small increase, which could be a signal of things to come for the summer.
The decline in new cases, however, has not been accompanied by decreases in hospitalizations or emergency department visits. New admissions of children aged 0-17 with confirmed COVID were at 0.31 per 100,000 population on June 24 after reaching that level on June 15, so no drop-off has occurred yet but there are signs of leveling off, based on CDC data.
The ED visit rates have been fairly steady through June, although COVID-related visits were up to 3.4% of all ED visits on June 22 for children aged 0-11 years, after being below 3% for the first 2 weeks of the month. The rate for children aged 12-15 has been between 1.6% and 1.9% for the past 3 weeks and the rate for 16- and 17-year-olds has been hovering between 1.7% and 2.2% for most of June, after going as high as 2.7% in late May, the CDC said on its COVID Data Tracker.
New cases of COVID-19 continue to drop among children, but the vaccination effort in those under age 5 years began with something less than a bang.
In the first 2 days after their respective approvals, almost 99,000 children aged 5-11 years and over 675,000 children aged 12-15 were vaccinated, according to data from the Centers for Disease Control and Prevention. Children aged 0-4 years represent almost 6% of the overall population, compared with 8.7% for the 5- to 11-year-olds and 5.1% for those aged 12-15.
The recent decline in new cases over the past 4 weeks and the substantial decline since the Omicron surge could be a factor in the lack of response, but it is worth noting that the almost 68,000 new child cases reported in the past week, June 17-23, are “far higher than 1 year ago, June 24, 2021, when 8,400 child cases were reported,” the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
That total for June 17-23 was 19% lower than the previous week and down by 40% since new cases hit a spring peak of 112,000 in late May. Regionally, new cases were down in the Midwest, the South, and the West, the AAP/CHA report showed, but the Northeast saw a small increase, which could be a signal of things to come for the summer.
The decline in new cases, however, has not been accompanied by decreases in hospitalizations or emergency department visits. New admissions of children aged 0-17 with confirmed COVID were at 0.31 per 100,000 population on June 24 after reaching that level on June 15, so no drop-off has occurred yet but there are signs of leveling off, based on CDC data.
The ED visit rates have been fairly steady through June, although COVID-related visits were up to 3.4% of all ED visits on June 22 for children aged 0-11 years, after being below 3% for the first 2 weeks of the month. The rate for children aged 12-15 has been between 1.6% and 1.9% for the past 3 weeks and the rate for 16- and 17-year-olds has been hovering between 1.7% and 2.2% for most of June, after going as high as 2.7% in late May, the CDC said on its COVID Data Tracker.
New cases of COVID-19 continue to drop among children, but the vaccination effort in those under age 5 years began with something less than a bang.
In the first 2 days after their respective approvals, almost 99,000 children aged 5-11 years and over 675,000 children aged 12-15 were vaccinated, according to data from the Centers for Disease Control and Prevention. Children aged 0-4 years represent almost 6% of the overall population, compared with 8.7% for the 5- to 11-year-olds and 5.1% for those aged 12-15.
The recent decline in new cases over the past 4 weeks and the substantial decline since the Omicron surge could be a factor in the lack of response, but it is worth noting that the almost 68,000 new child cases reported in the past week, June 17-23, are “far higher than 1 year ago, June 24, 2021, when 8,400 child cases were reported,” the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
That total for June 17-23 was 19% lower than the previous week and down by 40% since new cases hit a spring peak of 112,000 in late May. Regionally, new cases were down in the Midwest, the South, and the West, the AAP/CHA report showed, but the Northeast saw a small increase, which could be a signal of things to come for the summer.
The decline in new cases, however, has not been accompanied by decreases in hospitalizations or emergency department visits. New admissions of children aged 0-17 with confirmed COVID were at 0.31 per 100,000 population on June 24 after reaching that level on June 15, so no drop-off has occurred yet but there are signs of leveling off, based on CDC data.
The ED visit rates have been fairly steady through June, although COVID-related visits were up to 3.4% of all ED visits on June 22 for children aged 0-11 years, after being below 3% for the first 2 weeks of the month. The rate for children aged 12-15 has been between 1.6% and 1.9% for the past 3 weeks and the rate for 16- and 17-year-olds has been hovering between 1.7% and 2.2% for most of June, after going as high as 2.7% in late May, the CDC said on its COVID Data Tracker.
Racial/ethnic disparities exacerbated maternal death rise during 2020 pandemic.
U.S. maternal deaths – those during pregnancy or within 42 days of pregnancy – increased substantially by 33.3% after March 2020 corresponding to the COVID-19 pandemic onset, according to new research published in JAMA Network Open.
Data from the National Center for Health Statistics (NCHS) revealed this rise in maternal deaths was higher than the 22% overall excess death estimate associated with the pandemic in 2020.
Increases were highest for Hispanic and non-Hispanic Black women, exacerbating already high rates of disparity in comparison with White women, wrote Marie E. Thoma, PhD, an associate professor at the University of Maryland, College Park, and Eugene R. Declercq, PhD, a professor at Boston University.
The authors noted that this spike in maternal deaths might be caused either by conditions directly related to COVID-19, such as respiratory or viral infections, or by conditions worsened by pandemic-associated health care disruptions including those for diabetes or cardiovascular disease.
The precise causes, however, could not be discerned from the data, the authors noted.
The NCHS reported an 18.4% increase in U.S. maternal mortality from 2019 to 2020. The relative increase was 44.4% among Hispanic, 25.7% among non-Hispanic Black, and 6.1% among non-Hispanic White women.
“The rise in maternal mortality among Hispanic women was unprecedented,” Dr. Thoma said in an interview. Given a 16.8% increase in overall U.S. mortality in 2020, largely attributed to the COVID-19 pandemic, the authors examined the pandemic’s role in [the higher] maternal death rates for 2020.
“Prior to this report, the NCHS released an e-report that there had been a rise in maternal mortality in 2020, but questions remained about the role of the pandemic in this rise that their report hadn’t addressed,” Dr. Thoma said in an interview “So we decided to look at the data further to assess whether the rise coincided with the pandemic and how this differed by race/ethnicity, whether there were changes in the causes of maternal death, and how often COVID-19 was listed as a contributory factor in those deaths.”
A total of 1,588 maternal deaths (18.8 per 100,000 live births) occurred before the pandemic versus 684 deaths (25.1 per 100,000 live births) during the 2020 phase of the pandemic, for a relative increase of 33.3%.
Direct obstetrical causes of death included diabetes, hypertensive and liver disorders, pregnancy-related infections, and obstetrical hemorrhage and embolism. Indirect causes comprised, among others, nonobstetrical infections and diseases of the circulatory and respiratory systems as well as mental and nervous disorders.
Relative increases in direct causes (27.7%) were mostly associated with diabetes (95.9%), hypertensive disorders (39.0%), and other specified pregnancy-related conditions (48.0%).
COVID-19 was commonly listed as a lethal condition along with other viral diseases (16 of 16 deaths and diseases of the respiratory system (11 of 19 deaths).
Late maternal mortality – defined as more than 42 days but less than 1 year after pregnancy – increased by 41%. “This was surprising as we might anticipate risk being higher during pregnancy given that pregnant women may be more susceptible, but we see that this rise was also found among people in the later postpartum period,” Dr. Thoma said.
Absolute and relative changes were highest for Hispanic women (8.9 per 100,000 live births and 74.2%, respectively) and non-Hispanic Black women (16.8 per 100,000 live births and 40.2%). In contrast, non-Hispanic White women saw increases of just 2.9 per 100,000 live births and 17.2%.
“Overall, we found the rise in maternal mortality in 2020 was concentrated after the start of pandemic, particularly for non-Hispanic Black and Hispanic women, and we saw a dramatic rise in respiratory-related conditions,” Dr. Thoma said.
In a comment, Steven Woolf, MD, MPH, director emeritus of the Center on Society and Health at Virginia Commonwealth University, Richmond, said the findings are very consistent with his and others research showing dramatic increases in overall death rates from many causes during the pandemic, with these ranging from COVID-19 leading conditions such as diabetes, cardiovascular and Alzheimer’s disease to less-studied causes such as drug overdoses and alcoholism caused by the stresses of the pandemic. Again, deaths were likely caused by both COVID-19 infections and disruptions in diagnosis and care.
“So a rise in maternal mortality would unfortunately also be expected, and these researchers have shown that,” he said in an interview. In addition, they have confirmed “the pattern of stark health disparities in the Hispanic and Black populations relative to the White. Our group has shown marked decreases in the life expectancies of the Black and Hispanic populations relative to the White population.”
While he might take issue with the study’s research methodology, Dr. Woolf said, “The work is useful partly because we need to work out the best research methods to do this kind of analysis because we really need to understand the effects on maternal mortality.”
He said sorting out the best way to do this type of research will be important for looking at excess deaths and maternal mortality following other events, for example, in the wake of the Supreme Court’s recent decision to reverse Roe v. Wade.
The authors acknowledged certain study limitations, including the large percentage of COVID-19 cases with a nonspecific underlying cause. According to Dr. Thoma and Dr. Declercq, that reflects a maternal death coding problem that needs to be addressed, as well as a partitioning of data. The latter resulted in small numbers for some categories, with rates suppressed for fewer than 16 deaths because of reduced reliability.
“We found that more specific information is often available on death certificates but is lost in the process of coding,” said Dr. Thoma. “We were able to reclassify many of these causes to a more specific cause that we attributed to be the primary cause of death.”
The authors said future studies of maternal death should examine the contribution of the pandemic to racial and ethnic disparities and should identify specific causes of maternal deaths overall and associated with COVID-19.
In earlier research, the authors previously warned of possible misclassifications of maternal deaths.
They found evidence of both underreporting and overreporting of deaths, with possible overreporting predominant, whereas accurate data are essential for measuring the effectiveness of maternal mortality reduction programs.
Dr. Thoma’s group will continue to monitor mortality trends with the release of 2021 data. “We hope we will see improvements in 2021 given greater access to vaccines, treatments, and fewer health care disruptions,” Dr. Thoma said. “It will be important to continue to stress the importance of COVID-19 vaccines for pregnant and postpartum people.”
This study had no external funding. The authors disclosed no competing interests. Dr. Woolf declared no conflicts of interest.
U.S. maternal deaths – those during pregnancy or within 42 days of pregnancy – increased substantially by 33.3% after March 2020 corresponding to the COVID-19 pandemic onset, according to new research published in JAMA Network Open.
Data from the National Center for Health Statistics (NCHS) revealed this rise in maternal deaths was higher than the 22% overall excess death estimate associated with the pandemic in 2020.
Increases were highest for Hispanic and non-Hispanic Black women, exacerbating already high rates of disparity in comparison with White women, wrote Marie E. Thoma, PhD, an associate professor at the University of Maryland, College Park, and Eugene R. Declercq, PhD, a professor at Boston University.
The authors noted that this spike in maternal deaths might be caused either by conditions directly related to COVID-19, such as respiratory or viral infections, or by conditions worsened by pandemic-associated health care disruptions including those for diabetes or cardiovascular disease.
The precise causes, however, could not be discerned from the data, the authors noted.
The NCHS reported an 18.4% increase in U.S. maternal mortality from 2019 to 2020. The relative increase was 44.4% among Hispanic, 25.7% among non-Hispanic Black, and 6.1% among non-Hispanic White women.
“The rise in maternal mortality among Hispanic women was unprecedented,” Dr. Thoma said in an interview. Given a 16.8% increase in overall U.S. mortality in 2020, largely attributed to the COVID-19 pandemic, the authors examined the pandemic’s role in [the higher] maternal death rates for 2020.
“Prior to this report, the NCHS released an e-report that there had been a rise in maternal mortality in 2020, but questions remained about the role of the pandemic in this rise that their report hadn’t addressed,” Dr. Thoma said in an interview “So we decided to look at the data further to assess whether the rise coincided with the pandemic and how this differed by race/ethnicity, whether there were changes in the causes of maternal death, and how often COVID-19 was listed as a contributory factor in those deaths.”
A total of 1,588 maternal deaths (18.8 per 100,000 live births) occurred before the pandemic versus 684 deaths (25.1 per 100,000 live births) during the 2020 phase of the pandemic, for a relative increase of 33.3%.
Direct obstetrical causes of death included diabetes, hypertensive and liver disorders, pregnancy-related infections, and obstetrical hemorrhage and embolism. Indirect causes comprised, among others, nonobstetrical infections and diseases of the circulatory and respiratory systems as well as mental and nervous disorders.
Relative increases in direct causes (27.7%) were mostly associated with diabetes (95.9%), hypertensive disorders (39.0%), and other specified pregnancy-related conditions (48.0%).
COVID-19 was commonly listed as a lethal condition along with other viral diseases (16 of 16 deaths and diseases of the respiratory system (11 of 19 deaths).
Late maternal mortality – defined as more than 42 days but less than 1 year after pregnancy – increased by 41%. “This was surprising as we might anticipate risk being higher during pregnancy given that pregnant women may be more susceptible, but we see that this rise was also found among people in the later postpartum period,” Dr. Thoma said.
Absolute and relative changes were highest for Hispanic women (8.9 per 100,000 live births and 74.2%, respectively) and non-Hispanic Black women (16.8 per 100,000 live births and 40.2%). In contrast, non-Hispanic White women saw increases of just 2.9 per 100,000 live births and 17.2%.
“Overall, we found the rise in maternal mortality in 2020 was concentrated after the start of pandemic, particularly for non-Hispanic Black and Hispanic women, and we saw a dramatic rise in respiratory-related conditions,” Dr. Thoma said.
In a comment, Steven Woolf, MD, MPH, director emeritus of the Center on Society and Health at Virginia Commonwealth University, Richmond, said the findings are very consistent with his and others research showing dramatic increases in overall death rates from many causes during the pandemic, with these ranging from COVID-19 leading conditions such as diabetes, cardiovascular and Alzheimer’s disease to less-studied causes such as drug overdoses and alcoholism caused by the stresses of the pandemic. Again, deaths were likely caused by both COVID-19 infections and disruptions in diagnosis and care.
“So a rise in maternal mortality would unfortunately also be expected, and these researchers have shown that,” he said in an interview. In addition, they have confirmed “the pattern of stark health disparities in the Hispanic and Black populations relative to the White. Our group has shown marked decreases in the life expectancies of the Black and Hispanic populations relative to the White population.”
While he might take issue with the study’s research methodology, Dr. Woolf said, “The work is useful partly because we need to work out the best research methods to do this kind of analysis because we really need to understand the effects on maternal mortality.”
He said sorting out the best way to do this type of research will be important for looking at excess deaths and maternal mortality following other events, for example, in the wake of the Supreme Court’s recent decision to reverse Roe v. Wade.
The authors acknowledged certain study limitations, including the large percentage of COVID-19 cases with a nonspecific underlying cause. According to Dr. Thoma and Dr. Declercq, that reflects a maternal death coding problem that needs to be addressed, as well as a partitioning of data. The latter resulted in small numbers for some categories, with rates suppressed for fewer than 16 deaths because of reduced reliability.
“We found that more specific information is often available on death certificates but is lost in the process of coding,” said Dr. Thoma. “We were able to reclassify many of these causes to a more specific cause that we attributed to be the primary cause of death.”
The authors said future studies of maternal death should examine the contribution of the pandemic to racial and ethnic disparities and should identify specific causes of maternal deaths overall and associated with COVID-19.
In earlier research, the authors previously warned of possible misclassifications of maternal deaths.
They found evidence of both underreporting and overreporting of deaths, with possible overreporting predominant, whereas accurate data are essential for measuring the effectiveness of maternal mortality reduction programs.
Dr. Thoma’s group will continue to monitor mortality trends with the release of 2021 data. “We hope we will see improvements in 2021 given greater access to vaccines, treatments, and fewer health care disruptions,” Dr. Thoma said. “It will be important to continue to stress the importance of COVID-19 vaccines for pregnant and postpartum people.”
This study had no external funding. The authors disclosed no competing interests. Dr. Woolf declared no conflicts of interest.
U.S. maternal deaths – those during pregnancy or within 42 days of pregnancy – increased substantially by 33.3% after March 2020 corresponding to the COVID-19 pandemic onset, according to new research published in JAMA Network Open.
Data from the National Center for Health Statistics (NCHS) revealed this rise in maternal deaths was higher than the 22% overall excess death estimate associated with the pandemic in 2020.
Increases were highest for Hispanic and non-Hispanic Black women, exacerbating already high rates of disparity in comparison with White women, wrote Marie E. Thoma, PhD, an associate professor at the University of Maryland, College Park, and Eugene R. Declercq, PhD, a professor at Boston University.
The authors noted that this spike in maternal deaths might be caused either by conditions directly related to COVID-19, such as respiratory or viral infections, or by conditions worsened by pandemic-associated health care disruptions including those for diabetes or cardiovascular disease.
The precise causes, however, could not be discerned from the data, the authors noted.
The NCHS reported an 18.4% increase in U.S. maternal mortality from 2019 to 2020. The relative increase was 44.4% among Hispanic, 25.7% among non-Hispanic Black, and 6.1% among non-Hispanic White women.
“The rise in maternal mortality among Hispanic women was unprecedented,” Dr. Thoma said in an interview. Given a 16.8% increase in overall U.S. mortality in 2020, largely attributed to the COVID-19 pandemic, the authors examined the pandemic’s role in [the higher] maternal death rates for 2020.
“Prior to this report, the NCHS released an e-report that there had been a rise in maternal mortality in 2020, but questions remained about the role of the pandemic in this rise that their report hadn’t addressed,” Dr. Thoma said in an interview “So we decided to look at the data further to assess whether the rise coincided with the pandemic and how this differed by race/ethnicity, whether there were changes in the causes of maternal death, and how often COVID-19 was listed as a contributory factor in those deaths.”
A total of 1,588 maternal deaths (18.8 per 100,000 live births) occurred before the pandemic versus 684 deaths (25.1 per 100,000 live births) during the 2020 phase of the pandemic, for a relative increase of 33.3%.
Direct obstetrical causes of death included diabetes, hypertensive and liver disorders, pregnancy-related infections, and obstetrical hemorrhage and embolism. Indirect causes comprised, among others, nonobstetrical infections and diseases of the circulatory and respiratory systems as well as mental and nervous disorders.
Relative increases in direct causes (27.7%) were mostly associated with diabetes (95.9%), hypertensive disorders (39.0%), and other specified pregnancy-related conditions (48.0%).
COVID-19 was commonly listed as a lethal condition along with other viral diseases (16 of 16 deaths and diseases of the respiratory system (11 of 19 deaths).
Late maternal mortality – defined as more than 42 days but less than 1 year after pregnancy – increased by 41%. “This was surprising as we might anticipate risk being higher during pregnancy given that pregnant women may be more susceptible, but we see that this rise was also found among people in the later postpartum period,” Dr. Thoma said.
Absolute and relative changes were highest for Hispanic women (8.9 per 100,000 live births and 74.2%, respectively) and non-Hispanic Black women (16.8 per 100,000 live births and 40.2%). In contrast, non-Hispanic White women saw increases of just 2.9 per 100,000 live births and 17.2%.
“Overall, we found the rise in maternal mortality in 2020 was concentrated after the start of pandemic, particularly for non-Hispanic Black and Hispanic women, and we saw a dramatic rise in respiratory-related conditions,” Dr. Thoma said.
In a comment, Steven Woolf, MD, MPH, director emeritus of the Center on Society and Health at Virginia Commonwealth University, Richmond, said the findings are very consistent with his and others research showing dramatic increases in overall death rates from many causes during the pandemic, with these ranging from COVID-19 leading conditions such as diabetes, cardiovascular and Alzheimer’s disease to less-studied causes such as drug overdoses and alcoholism caused by the stresses of the pandemic. Again, deaths were likely caused by both COVID-19 infections and disruptions in diagnosis and care.
“So a rise in maternal mortality would unfortunately also be expected, and these researchers have shown that,” he said in an interview. In addition, they have confirmed “the pattern of stark health disparities in the Hispanic and Black populations relative to the White. Our group has shown marked decreases in the life expectancies of the Black and Hispanic populations relative to the White population.”
While he might take issue with the study’s research methodology, Dr. Woolf said, “The work is useful partly because we need to work out the best research methods to do this kind of analysis because we really need to understand the effects on maternal mortality.”
He said sorting out the best way to do this type of research will be important for looking at excess deaths and maternal mortality following other events, for example, in the wake of the Supreme Court’s recent decision to reverse Roe v. Wade.
The authors acknowledged certain study limitations, including the large percentage of COVID-19 cases with a nonspecific underlying cause. According to Dr. Thoma and Dr. Declercq, that reflects a maternal death coding problem that needs to be addressed, as well as a partitioning of data. The latter resulted in small numbers for some categories, with rates suppressed for fewer than 16 deaths because of reduced reliability.
“We found that more specific information is often available on death certificates but is lost in the process of coding,” said Dr. Thoma. “We were able to reclassify many of these causes to a more specific cause that we attributed to be the primary cause of death.”
The authors said future studies of maternal death should examine the contribution of the pandemic to racial and ethnic disparities and should identify specific causes of maternal deaths overall and associated with COVID-19.
In earlier research, the authors previously warned of possible misclassifications of maternal deaths.
They found evidence of both underreporting and overreporting of deaths, with possible overreporting predominant, whereas accurate data are essential for measuring the effectiveness of maternal mortality reduction programs.
Dr. Thoma’s group will continue to monitor mortality trends with the release of 2021 data. “We hope we will see improvements in 2021 given greater access to vaccines, treatments, and fewer health care disruptions,” Dr. Thoma said. “It will be important to continue to stress the importance of COVID-19 vaccines for pregnant and postpartum people.”
This study had no external funding. The authors disclosed no competing interests. Dr. Woolf declared no conflicts of interest.
FROM JAMA NETWORK OPEN