User login
Psychiatrists’ income, wealth gain ground despite COVID-19 challenges
Although many physicians endured pandemic-related income struggles in 2020, psychiatrists are doing fairly well with building their nest egg and paying down debt, according to the Medscape Psychiatrist Wealth and Debt Report 2021.
Surprisingly, despite COVID-19, psychiatrists’ income improved somewhat this year – from $268,000 in 2020 to $275,000 in 2021.
However, that still puts psychiatrists among the lower-paid specialists.
The highest-paying specialty is plastic surgery ($526,000), followed by orthopedics and orthopedic surgery ($511,000) and cardiology ($459,000), according to the overall Medscape Physician Wealth and Debt Report 2021. The report is based on responses from nearly 18,000 physicians in 29 specialties. All were surveyed between Oct. 6, 2020, and Feb. 11, 2021.
Psychiatrists’ overall wealth gained some ground over the past year, with 40% reporting a net worth of $1 million to $5 million this year – up from 38% last year. Just 6% of psychiatrists have a net worth north of $5 million, up slightly from 5% last year.
Keeping up with bills
based in St. Louis Park, Minn. He noted that the rise in the stock market also played a role, with the S&P 500 finishing the year up over 18%.
“I’ve seen clients accumulate cash, which has added to their net worth. They cut back on spending because they were worried about big declines in income and also because there was simply less to spend money on,” Dr. Greenwald said.
The percentage of psychiatrists with a net worth under $500,000 decreased from 37% last year to 32% this year. Psychiatry is still among the specialties reporting a high percentage of members with net worth below $500,000.
But gender matters. Earnings overall are higher for male than female psychiatrists, and that is reflected in net worth. Fewer female than male psychiatrists are worth more than $5 million (4% vs. 7%), and more female psychiatrists have a net worth of less than $500,000 (41% vs. 26%).
As in prior years, most psychiatrists are paying down a home mortgage on their primary residence (66%). Psychiatrists’ mortgage payments span a wide range, from less than $100,000 (23%) to more than $500,000 (15%). However, 27% report having no mortgage.
Mortgage aside, other top expenses or debts for psychiatrists are car loan payments (36%), paying off college and medical school debt (26%), credit card debt (25%), and medical expenses for self or loved ones (19%).
Other expenses include college tuition for children (16%), car lease payments (14%), mortgage on a second home (13%), private-school tuition for a child (12%), and child care (12%).
Despite some financially challenging months, the vast majority of psychiatrists (94%) kept up with paying their bills.
That’s better than what much of America experienced. According to a U.S. Census Bureau survey conducted last July, roughly 25% of adults missed a mortgage or rent payment because of COVID-related difficulties.
About half of psychiatrists pool their income to pay for bills. One-quarter do not have joint accounts with a spouse or partner.
Spender or saver?
About three-quarters of psychiatrists continued to spend as usual in 2020. About one-quarter took significant steps to lower their expenses, such as refinancing their home or moving to a less costly home.
In line with prior Medscape surveys, about half of psychiatrists have a general idea of how much they spend and on what, but they do not track or formalize it.
According to a recent survey by Intuit, only 35% of Americans say they know how much they spent last month. Viewed by age, 27% of millennials, 34% of Gen Xers, and 46% of baby boomers knew how much they spent.
Many psychiatrists have a higher-than-average number of credit cards; 42% have at least five. By comparison, the average American has four.
Savings was mixed for psychiatrists this past year; 61% put in the same amount or more each month into their 401(k) plans, but 33% put in less money, compared with last year.
For taxable savings accounts, half of psychiatrists put the same amount or more into after-tax accounts – but 22% put in less money, compared with last year. Another one-quarter did not use these savings accounts at all.
The percentage of psychiatrists who experienced losses because of practice problems rose from 6% to 9% in the past year. Much of that was likely because of COVID. However, about the same percentage reported no financial losses this year (76%), compared with last year (75%).
The vast majority of psychiatrists report living within or below their means; only 5% live above their means.
“There are certainly folks who believe that, as long as they pay off their credit card each month and contribute to their 401(k) enough to get their employer match, they’re doing okay,” Dr. Greenwald said.
However, “living within one’s means is having a 3-6 months’ emergency fund; saving at least 20% of gross income toward retirement; adequately funding 529 college accounts; and, for younger docs, paying down high-interest-rate debt at a good clip,” he added.
A version of this article first appeared on Medscape.com.
Although many physicians endured pandemic-related income struggles in 2020, psychiatrists are doing fairly well with building their nest egg and paying down debt, according to the Medscape Psychiatrist Wealth and Debt Report 2021.
Surprisingly, despite COVID-19, psychiatrists’ income improved somewhat this year – from $268,000 in 2020 to $275,000 in 2021.
However, that still puts psychiatrists among the lower-paid specialists.
The highest-paying specialty is plastic surgery ($526,000), followed by orthopedics and orthopedic surgery ($511,000) and cardiology ($459,000), according to the overall Medscape Physician Wealth and Debt Report 2021. The report is based on responses from nearly 18,000 physicians in 29 specialties. All were surveyed between Oct. 6, 2020, and Feb. 11, 2021.
Psychiatrists’ overall wealth gained some ground over the past year, with 40% reporting a net worth of $1 million to $5 million this year – up from 38% last year. Just 6% of psychiatrists have a net worth north of $5 million, up slightly from 5% last year.
Keeping up with bills
based in St. Louis Park, Minn. He noted that the rise in the stock market also played a role, with the S&P 500 finishing the year up over 18%.
“I’ve seen clients accumulate cash, which has added to their net worth. They cut back on spending because they were worried about big declines in income and also because there was simply less to spend money on,” Dr. Greenwald said.
The percentage of psychiatrists with a net worth under $500,000 decreased from 37% last year to 32% this year. Psychiatry is still among the specialties reporting a high percentage of members with net worth below $500,000.
But gender matters. Earnings overall are higher for male than female psychiatrists, and that is reflected in net worth. Fewer female than male psychiatrists are worth more than $5 million (4% vs. 7%), and more female psychiatrists have a net worth of less than $500,000 (41% vs. 26%).
As in prior years, most psychiatrists are paying down a home mortgage on their primary residence (66%). Psychiatrists’ mortgage payments span a wide range, from less than $100,000 (23%) to more than $500,000 (15%). However, 27% report having no mortgage.
Mortgage aside, other top expenses or debts for psychiatrists are car loan payments (36%), paying off college and medical school debt (26%), credit card debt (25%), and medical expenses for self or loved ones (19%).
Other expenses include college tuition for children (16%), car lease payments (14%), mortgage on a second home (13%), private-school tuition for a child (12%), and child care (12%).
Despite some financially challenging months, the vast majority of psychiatrists (94%) kept up with paying their bills.
That’s better than what much of America experienced. According to a U.S. Census Bureau survey conducted last July, roughly 25% of adults missed a mortgage or rent payment because of COVID-related difficulties.
About half of psychiatrists pool their income to pay for bills. One-quarter do not have joint accounts with a spouse or partner.
Spender or saver?
About three-quarters of psychiatrists continued to spend as usual in 2020. About one-quarter took significant steps to lower their expenses, such as refinancing their home or moving to a less costly home.
In line with prior Medscape surveys, about half of psychiatrists have a general idea of how much they spend and on what, but they do not track or formalize it.
According to a recent survey by Intuit, only 35% of Americans say they know how much they spent last month. Viewed by age, 27% of millennials, 34% of Gen Xers, and 46% of baby boomers knew how much they spent.
Many psychiatrists have a higher-than-average number of credit cards; 42% have at least five. By comparison, the average American has four.
Savings was mixed for psychiatrists this past year; 61% put in the same amount or more each month into their 401(k) plans, but 33% put in less money, compared with last year.
For taxable savings accounts, half of psychiatrists put the same amount or more into after-tax accounts – but 22% put in less money, compared with last year. Another one-quarter did not use these savings accounts at all.
The percentage of psychiatrists who experienced losses because of practice problems rose from 6% to 9% in the past year. Much of that was likely because of COVID. However, about the same percentage reported no financial losses this year (76%), compared with last year (75%).
The vast majority of psychiatrists report living within or below their means; only 5% live above their means.
“There are certainly folks who believe that, as long as they pay off their credit card each month and contribute to their 401(k) enough to get their employer match, they’re doing okay,” Dr. Greenwald said.
However, “living within one’s means is having a 3-6 months’ emergency fund; saving at least 20% of gross income toward retirement; adequately funding 529 college accounts; and, for younger docs, paying down high-interest-rate debt at a good clip,” he added.
A version of this article first appeared on Medscape.com.
Although many physicians endured pandemic-related income struggles in 2020, psychiatrists are doing fairly well with building their nest egg and paying down debt, according to the Medscape Psychiatrist Wealth and Debt Report 2021.
Surprisingly, despite COVID-19, psychiatrists’ income improved somewhat this year – from $268,000 in 2020 to $275,000 in 2021.
However, that still puts psychiatrists among the lower-paid specialists.
The highest-paying specialty is plastic surgery ($526,000), followed by orthopedics and orthopedic surgery ($511,000) and cardiology ($459,000), according to the overall Medscape Physician Wealth and Debt Report 2021. The report is based on responses from nearly 18,000 physicians in 29 specialties. All were surveyed between Oct. 6, 2020, and Feb. 11, 2021.
Psychiatrists’ overall wealth gained some ground over the past year, with 40% reporting a net worth of $1 million to $5 million this year – up from 38% last year. Just 6% of psychiatrists have a net worth north of $5 million, up slightly from 5% last year.
Keeping up with bills
based in St. Louis Park, Minn. He noted that the rise in the stock market also played a role, with the S&P 500 finishing the year up over 18%.
“I’ve seen clients accumulate cash, which has added to their net worth. They cut back on spending because they were worried about big declines in income and also because there was simply less to spend money on,” Dr. Greenwald said.
The percentage of psychiatrists with a net worth under $500,000 decreased from 37% last year to 32% this year. Psychiatry is still among the specialties reporting a high percentage of members with net worth below $500,000.
But gender matters. Earnings overall are higher for male than female psychiatrists, and that is reflected in net worth. Fewer female than male psychiatrists are worth more than $5 million (4% vs. 7%), and more female psychiatrists have a net worth of less than $500,000 (41% vs. 26%).
As in prior years, most psychiatrists are paying down a home mortgage on their primary residence (66%). Psychiatrists’ mortgage payments span a wide range, from less than $100,000 (23%) to more than $500,000 (15%). However, 27% report having no mortgage.
Mortgage aside, other top expenses or debts for psychiatrists are car loan payments (36%), paying off college and medical school debt (26%), credit card debt (25%), and medical expenses for self or loved ones (19%).
Other expenses include college tuition for children (16%), car lease payments (14%), mortgage on a second home (13%), private-school tuition for a child (12%), and child care (12%).
Despite some financially challenging months, the vast majority of psychiatrists (94%) kept up with paying their bills.
That’s better than what much of America experienced. According to a U.S. Census Bureau survey conducted last July, roughly 25% of adults missed a mortgage or rent payment because of COVID-related difficulties.
About half of psychiatrists pool their income to pay for bills. One-quarter do not have joint accounts with a spouse or partner.
Spender or saver?
About three-quarters of psychiatrists continued to spend as usual in 2020. About one-quarter took significant steps to lower their expenses, such as refinancing their home or moving to a less costly home.
In line with prior Medscape surveys, about half of psychiatrists have a general idea of how much they spend and on what, but they do not track or formalize it.
According to a recent survey by Intuit, only 35% of Americans say they know how much they spent last month. Viewed by age, 27% of millennials, 34% of Gen Xers, and 46% of baby boomers knew how much they spent.
Many psychiatrists have a higher-than-average number of credit cards; 42% have at least five. By comparison, the average American has four.
Savings was mixed for psychiatrists this past year; 61% put in the same amount or more each month into their 401(k) plans, but 33% put in less money, compared with last year.
For taxable savings accounts, half of psychiatrists put the same amount or more into after-tax accounts – but 22% put in less money, compared with last year. Another one-quarter did not use these savings accounts at all.
The percentage of psychiatrists who experienced losses because of practice problems rose from 6% to 9% in the past year. Much of that was likely because of COVID. However, about the same percentage reported no financial losses this year (76%), compared with last year (75%).
The vast majority of psychiatrists report living within or below their means; only 5% live above their means.
“There are certainly folks who believe that, as long as they pay off their credit card each month and contribute to their 401(k) enough to get their employer match, they’re doing okay,” Dr. Greenwald said.
However, “living within one’s means is having a 3-6 months’ emergency fund; saving at least 20% of gross income toward retirement; adequately funding 529 college accounts; and, for younger docs, paying down high-interest-rate debt at a good clip,” he added.
A version of this article first appeared on Medscape.com.
Children and COVID: New cases rise to winter levels
Weekly cases of COVID-19 in children topped 100,000 for the first time since early February, according to the American Academy of Pediatrics and the Children’s Hospital Association.
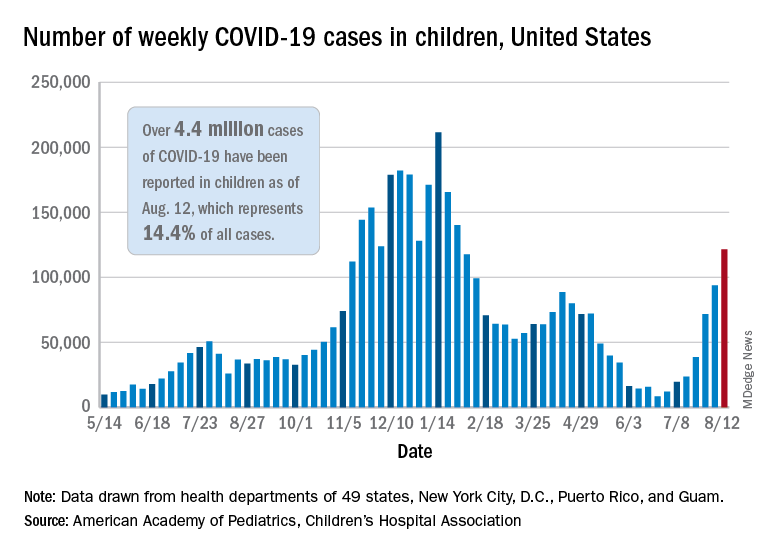
the AAP and CHA said in their weekly COVD-19 report. The recent surge in child COVID has also brought a record high in hospitalizations and shortages of pediatric ICU beds in some areas.
The 121,000 new cases represent an increase of almost 1,400% since June 18-24, when the weekly tally was just 8,447 and at its lowest point in over a year, the AAP/CHA data show.
On the vaccination front in the last week (Aug. 10-16), vaccine initiation for 12- to 17-year-olds was fairly robust but still down slightly, compared with the previous week. Just over 402,000 children aged 12-15 years received a first vaccination, which was down slightly from 411,000 the week before but still higher than any of the 6 weeks from June 22 to Aug. 2, based on data from the Centers for Disease Control and Prevention. Vaccinations were down by a similar margin for 15- to-17-year-olds.
Over 10.9 million children aged 12-17 have had at least one dose of COVID-19 vaccine administered, of whom 8.1 million are fully vaccinated. Among those aged 12-15 years, 44.5% have gotten at least one dose and 31.8% are fully vaccinated, with corresponding figures of 53.9% and 42.5% for 16- and 17-year-olds, according to the CDC’s COVID Data Tracker.
The number of COVID-19 cases reported in children since the start of the pandemic is up to 4.4 million, which makes up 14.4% of all cases in the United States, the AAP and CHA said. Other cumulative figures through Aug. 12 include almost 18,000 hospitalizations – reported by 23 states and New York City – and 378 deaths – reported by 43 states, New York City, Puerto Rico, and Guam.
In the latest edition of their ongoing report, compiled using state data since the summer of 2020, the two groups noted that, “in the summer of 2021, some states have revised cases counts previously reported, begun reporting less frequently, or dropped metrics previously reported.” Among those states are Nebraska, which shut down its online COVID dashboard in late June, and Alabama, which stopped reporting cumulative cases and deaths after July 29.
Weekly cases of COVID-19 in children topped 100,000 for the first time since early February, according to the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and CHA said in their weekly COVD-19 report. The recent surge in child COVID has also brought a record high in hospitalizations and shortages of pediatric ICU beds in some areas.
The 121,000 new cases represent an increase of almost 1,400% since June 18-24, when the weekly tally was just 8,447 and at its lowest point in over a year, the AAP/CHA data show.
On the vaccination front in the last week (Aug. 10-16), vaccine initiation for 12- to 17-year-olds was fairly robust but still down slightly, compared with the previous week. Just over 402,000 children aged 12-15 years received a first vaccination, which was down slightly from 411,000 the week before but still higher than any of the 6 weeks from June 22 to Aug. 2, based on data from the Centers for Disease Control and Prevention. Vaccinations were down by a similar margin for 15- to-17-year-olds.
Over 10.9 million children aged 12-17 have had at least one dose of COVID-19 vaccine administered, of whom 8.1 million are fully vaccinated. Among those aged 12-15 years, 44.5% have gotten at least one dose and 31.8% are fully vaccinated, with corresponding figures of 53.9% and 42.5% for 16- and 17-year-olds, according to the CDC’s COVID Data Tracker.
The number of COVID-19 cases reported in children since the start of the pandemic is up to 4.4 million, which makes up 14.4% of all cases in the United States, the AAP and CHA said. Other cumulative figures through Aug. 12 include almost 18,000 hospitalizations – reported by 23 states and New York City – and 378 deaths – reported by 43 states, New York City, Puerto Rico, and Guam.
In the latest edition of their ongoing report, compiled using state data since the summer of 2020, the two groups noted that, “in the summer of 2021, some states have revised cases counts previously reported, begun reporting less frequently, or dropped metrics previously reported.” Among those states are Nebraska, which shut down its online COVID dashboard in late June, and Alabama, which stopped reporting cumulative cases and deaths after July 29.
Weekly cases of COVID-19 in children topped 100,000 for the first time since early February, according to the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and CHA said in their weekly COVD-19 report. The recent surge in child COVID has also brought a record high in hospitalizations and shortages of pediatric ICU beds in some areas.
The 121,000 new cases represent an increase of almost 1,400% since June 18-24, when the weekly tally was just 8,447 and at its lowest point in over a year, the AAP/CHA data show.
On the vaccination front in the last week (Aug. 10-16), vaccine initiation for 12- to 17-year-olds was fairly robust but still down slightly, compared with the previous week. Just over 402,000 children aged 12-15 years received a first vaccination, which was down slightly from 411,000 the week before but still higher than any of the 6 weeks from June 22 to Aug. 2, based on data from the Centers for Disease Control and Prevention. Vaccinations were down by a similar margin for 15- to-17-year-olds.
Over 10.9 million children aged 12-17 have had at least one dose of COVID-19 vaccine administered, of whom 8.1 million are fully vaccinated. Among those aged 12-15 years, 44.5% have gotten at least one dose and 31.8% are fully vaccinated, with corresponding figures of 53.9% and 42.5% for 16- and 17-year-olds, according to the CDC’s COVID Data Tracker.
The number of COVID-19 cases reported in children since the start of the pandemic is up to 4.4 million, which makes up 14.4% of all cases in the United States, the AAP and CHA said. Other cumulative figures through Aug. 12 include almost 18,000 hospitalizations – reported by 23 states and New York City – and 378 deaths – reported by 43 states, New York City, Puerto Rico, and Guam.
In the latest edition of their ongoing report, compiled using state data since the summer of 2020, the two groups noted that, “in the summer of 2021, some states have revised cases counts previously reported, begun reporting less frequently, or dropped metrics previously reported.” Among those states are Nebraska, which shut down its online COVID dashboard in late June, and Alabama, which stopped reporting cumulative cases and deaths after July 29.
COVID-19 hospitalizations for 30- to 39-year-olds hit record high
Hospitals are reporting record numbers of COVID-19 patients in their 30s, largely because of the contagious Delta variant, according to The Wall Street Journal.
The rate of new hospitalizations for ages 30-39 reached 2.5 per 100,000 people last week, according to the latest CDC data, which is up from the previous peak of 2 per 100,000 people in January.
What’s more, new hospital admissions for patients in their 30s reached an average of 1,113 a day during the last week, which was up from 908 the week before.
“It means Delta is really bad,” James Lawler, MD, an infectious disease doctor and codirector of the Global Center for Health Security at the University of Nebraska Medical Center, told the newspaper.
People in the age group mostly avoided hospitalization throughout the pandemic because of their relatively good health and young age, the newspaper reported. But in recent weeks, those between ages 30 and 39 are contracting the coronavirus because of their active lifestyle – for many in their 30s, these are prime years for working, parenting, and socializing.
Hospitalizations are mostly among unvaccinated adults, according to the Wall Street Journal. Nationally, less than half of those ages 25-39 are fully vaccinated, compared with 61% of all adults, according to CDC data updated Sunday.
“It loves social mobility,” James Fiorica, MD, chief medical officer of Sarasota Memorial Health Care System in Florida, told the newspaper.
“An unvaccinated 30-year-old can be a perfect carrier,” he said.
On top of that, COVID-19 patients in their 30s are arriving at hospitals with more severe disease than in earlier waves, the Journal reported. At the University of Arkansas for Medical Sciences hospital, for instance, doctors are now monitoring younger patients daily with a scoring system for possible organ failure. That wasn’t necessary earlier in the pandemic for people in their 30s.
“This age group pretty much went unscathed,” Nikhil Meena, MD, director of the hospital’s Medical Intensive Care Unit, told the newspaper.
Now, he said, “they’re all out there doing their thing and getting infected and getting sick enough to be in this hospital.”
A version of this article first appeared on WebMD.com.
Hospitals are reporting record numbers of COVID-19 patients in their 30s, largely because of the contagious Delta variant, according to The Wall Street Journal.
The rate of new hospitalizations for ages 30-39 reached 2.5 per 100,000 people last week, according to the latest CDC data, which is up from the previous peak of 2 per 100,000 people in January.
What’s more, new hospital admissions for patients in their 30s reached an average of 1,113 a day during the last week, which was up from 908 the week before.
“It means Delta is really bad,” James Lawler, MD, an infectious disease doctor and codirector of the Global Center for Health Security at the University of Nebraska Medical Center, told the newspaper.
People in the age group mostly avoided hospitalization throughout the pandemic because of their relatively good health and young age, the newspaper reported. But in recent weeks, those between ages 30 and 39 are contracting the coronavirus because of their active lifestyle – for many in their 30s, these are prime years for working, parenting, and socializing.
Hospitalizations are mostly among unvaccinated adults, according to the Wall Street Journal. Nationally, less than half of those ages 25-39 are fully vaccinated, compared with 61% of all adults, according to CDC data updated Sunday.
“It loves social mobility,” James Fiorica, MD, chief medical officer of Sarasota Memorial Health Care System in Florida, told the newspaper.
“An unvaccinated 30-year-old can be a perfect carrier,” he said.
On top of that, COVID-19 patients in their 30s are arriving at hospitals with more severe disease than in earlier waves, the Journal reported. At the University of Arkansas for Medical Sciences hospital, for instance, doctors are now monitoring younger patients daily with a scoring system for possible organ failure. That wasn’t necessary earlier in the pandemic for people in their 30s.
“This age group pretty much went unscathed,” Nikhil Meena, MD, director of the hospital’s Medical Intensive Care Unit, told the newspaper.
Now, he said, “they’re all out there doing their thing and getting infected and getting sick enough to be in this hospital.”
A version of this article first appeared on WebMD.com.
Hospitals are reporting record numbers of COVID-19 patients in their 30s, largely because of the contagious Delta variant, according to The Wall Street Journal.
The rate of new hospitalizations for ages 30-39 reached 2.5 per 100,000 people last week, according to the latest CDC data, which is up from the previous peak of 2 per 100,000 people in January.
What’s more, new hospital admissions for patients in their 30s reached an average of 1,113 a day during the last week, which was up from 908 the week before.
“It means Delta is really bad,” James Lawler, MD, an infectious disease doctor and codirector of the Global Center for Health Security at the University of Nebraska Medical Center, told the newspaper.
People in the age group mostly avoided hospitalization throughout the pandemic because of their relatively good health and young age, the newspaper reported. But in recent weeks, those between ages 30 and 39 are contracting the coronavirus because of their active lifestyle – for many in their 30s, these are prime years for working, parenting, and socializing.
Hospitalizations are mostly among unvaccinated adults, according to the Wall Street Journal. Nationally, less than half of those ages 25-39 are fully vaccinated, compared with 61% of all adults, according to CDC data updated Sunday.
“It loves social mobility,” James Fiorica, MD, chief medical officer of Sarasota Memorial Health Care System in Florida, told the newspaper.
“An unvaccinated 30-year-old can be a perfect carrier,” he said.
On top of that, COVID-19 patients in their 30s are arriving at hospitals with more severe disease than in earlier waves, the Journal reported. At the University of Arkansas for Medical Sciences hospital, for instance, doctors are now monitoring younger patients daily with a scoring system for possible organ failure. That wasn’t necessary earlier in the pandemic for people in their 30s.
“This age group pretty much went unscathed,” Nikhil Meena, MD, director of the hospital’s Medical Intensive Care Unit, told the newspaper.
Now, he said, “they’re all out there doing their thing and getting infected and getting sick enough to be in this hospital.”
A version of this article first appeared on WebMD.com.
U.S. pediatric hospitals in peril as Delta hits children
Over the course of the pandemic, COVID-19 has been a less serious illness for children than it has been for adults, and that continues to be true. But with the arrival of Delta, the risk for kids is rising, and that’s creating a perilous situation for hospitals across the United States that treat them.
Roughly 1,800 kids were hospitalized with COVID-19 in the United States last week, a 500% increase in the rate of COVID-19 hospitalizations for children since early July, according to data from the Centers for Disease Control and Prevention.
Emerging data from a large study in Canada suggest that children who test positive for COVID-19 during the Delta wave may be more than twice as likely to be hospitalized as they were when previous variants were dominating transmission. The new data support what many pediatric infectious disease experts say they’ve been seeing: Younger kids with more serious symptoms.
That may sound concerning, but keep in mind that the overall risk of hospitalization for kids who have COVID-19 is still very low – about one child for every hundred who test positive for the virus will end up needing hospital care for their symptoms, according to current statistics maintained by the American Academy of Pediatrics.
‘This is different’
At Le Bonheur Children’s Hospital in Memphis, they saw Delta coming.
Since last year, every kid that comes to the emergency department at the hospital gets a screening test for COVID-19.
In past waves, doctors usually found kids who were infected by accident – they tested positive after coming in for some other problem, a broken leg or appendicitis, said Nick Hysmith, MD, medical director of infection prevention at the hospital. But within the last few weeks, kids with fevers, sore throats, coughs, and runny noses started testing positive for COVID-19.
“We have seen our positive numbers go from, you know, close to about 8%-10% jump up to 20%, and then in recent weeks, we can get as high as 26% or 30%,” Dr. Hysmith said. “Then we started seeing kids sick enough to be admitted.”
“Over the last week, we’ve really seen an increase,” he said. As of August 16, the hospital had 24 children with COVID-19 admitted. Seven of the children were in the PICU, and two were on ventilators.
Arkansas Children’s Hospital had 23 young COVID-19 patients, 10 in intensive care, and five on ventilators, as of Friday, according to the Washington Post. At Children’s of Mississippi, the only hospital for kids in that state, 22 youth were hospitalized as of Monday, with three in intensive care as of August 16, according to the hospital. The nonprofit relief organization Samaritan’s Purse is setting up a second field hospital in the basement of Children’s to expand the hospital’s capacity.
“This is different,” Dr. Hysmith said. “What we’re seeing now is previously healthy kids coming in with symptomatic infection.”
This increased virulence is happening at a bad time. Schools around the United States are reopening for in-person classes, some for the first time in more than a year. Eight states have blocked districts from requiring masks, while many more have made them optional.
Children under 12 still have no access to a vaccine, so they are facing increased exposure to a germ that’s become more dangerous with little protection, especially in schools that have eschewed masks.
More than just COVID-19
Then there are the latent effects of the virus to contend with.
“We’re not only seeing more children now with acute SARS-CoV-2 in the hospital, we’re starting also to see an uptick of MISC – or Multisystem Inflammatory Syndrome in Children,” said Charlotte Hobbs, MD, a pediatric infectious disease specialist at Mississippi Children’s Hospital. “We are just beginning to [see] those cases, and we anticipate that’s going to get worse.”
Adding to COVID-19’s misery, another virus is also capitalizing on this increased mixing of kids back into the community. Respiratory syncytial virus (RSV) hospitalizes about 58,000 children under age 5 in the United States each year. The typical RSV season starts in the fall and peaks in February, along with influenza. This year, the RSV season is early, and it is ferocious.
The combination of the two infections is hitting children’s hospitals hard, and it’s layered on top of the indirect effects of the pandemic, such as the increased population of kids and teens who need mental health care in the wake of the crisis.
“It’s all these things happening at the same time,” said Mark Wietecha, CEO of the Children’s Hospital Association. “To have our hospitals this crowded in August is unusual.
And children’s hospitals are grappling with the same workforce shortages as hospitals that treat adults, while their pool of potential staff is much smaller.
“We can’t easily recruit physicians and nurses from adult hospitals in any practical way to staff a kids’ hospital,” Mr. Wietecha said.
Although pediatric doctors and nurses were trained to care for adults before they specialized, clinicians who primarily care for adults typically haven’t been taught how to care for kids.
Clinicians have fewer tools to fight COVID-19 infections in children than are available for adults.
“There have been many studies in terms of therapies and treatments for acute SARS-CoV-2 infection in adults. We have less data and information in children, and on top of that, some of these treatments aren’t even available under an EUA [emergency use authorization] to children: For example, the monoclonal antibodies,” Dr. Hobbs said.
Antibody treatments are being widely deployed to ease the pressure on hospitals that treat adults. But these therapies aren’t available for kids.
That means children’s hospitals could quickly become overwhelmed, especially in areas where community transmission is high, vaccination rates are low, and parents are screaming about masks.
“So we really have this constellation of events that really doesn’t favor children under the age of 12,” Dr. Hobbs said.
“Universal masking shouldn’t be a debate, because it’s the one thing, with adult vaccination, that can be done to protect this vulnerable population,” she said. “This isn’t a political issue. It’s a public health issue. Period.”
A version of this article first appeared on Medscape.com.
Over the course of the pandemic, COVID-19 has been a less serious illness for children than it has been for adults, and that continues to be true. But with the arrival of Delta, the risk for kids is rising, and that’s creating a perilous situation for hospitals across the United States that treat them.
Roughly 1,800 kids were hospitalized with COVID-19 in the United States last week, a 500% increase in the rate of COVID-19 hospitalizations for children since early July, according to data from the Centers for Disease Control and Prevention.
Emerging data from a large study in Canada suggest that children who test positive for COVID-19 during the Delta wave may be more than twice as likely to be hospitalized as they were when previous variants were dominating transmission. The new data support what many pediatric infectious disease experts say they’ve been seeing: Younger kids with more serious symptoms.
That may sound concerning, but keep in mind that the overall risk of hospitalization for kids who have COVID-19 is still very low – about one child for every hundred who test positive for the virus will end up needing hospital care for their symptoms, according to current statistics maintained by the American Academy of Pediatrics.
‘This is different’
At Le Bonheur Children’s Hospital in Memphis, they saw Delta coming.
Since last year, every kid that comes to the emergency department at the hospital gets a screening test for COVID-19.
In past waves, doctors usually found kids who were infected by accident – they tested positive after coming in for some other problem, a broken leg or appendicitis, said Nick Hysmith, MD, medical director of infection prevention at the hospital. But within the last few weeks, kids with fevers, sore throats, coughs, and runny noses started testing positive for COVID-19.
“We have seen our positive numbers go from, you know, close to about 8%-10% jump up to 20%, and then in recent weeks, we can get as high as 26% or 30%,” Dr. Hysmith said. “Then we started seeing kids sick enough to be admitted.”
“Over the last week, we’ve really seen an increase,” he said. As of August 16, the hospital had 24 children with COVID-19 admitted. Seven of the children were in the PICU, and two were on ventilators.
Arkansas Children’s Hospital had 23 young COVID-19 patients, 10 in intensive care, and five on ventilators, as of Friday, according to the Washington Post. At Children’s of Mississippi, the only hospital for kids in that state, 22 youth were hospitalized as of Monday, with three in intensive care as of August 16, according to the hospital. The nonprofit relief organization Samaritan’s Purse is setting up a second field hospital in the basement of Children’s to expand the hospital’s capacity.
“This is different,” Dr. Hysmith said. “What we’re seeing now is previously healthy kids coming in with symptomatic infection.”
This increased virulence is happening at a bad time. Schools around the United States are reopening for in-person classes, some for the first time in more than a year. Eight states have blocked districts from requiring masks, while many more have made them optional.
Children under 12 still have no access to a vaccine, so they are facing increased exposure to a germ that’s become more dangerous with little protection, especially in schools that have eschewed masks.
More than just COVID-19
Then there are the latent effects of the virus to contend with.
“We’re not only seeing more children now with acute SARS-CoV-2 in the hospital, we’re starting also to see an uptick of MISC – or Multisystem Inflammatory Syndrome in Children,” said Charlotte Hobbs, MD, a pediatric infectious disease specialist at Mississippi Children’s Hospital. “We are just beginning to [see] those cases, and we anticipate that’s going to get worse.”
Adding to COVID-19’s misery, another virus is also capitalizing on this increased mixing of kids back into the community. Respiratory syncytial virus (RSV) hospitalizes about 58,000 children under age 5 in the United States each year. The typical RSV season starts in the fall and peaks in February, along with influenza. This year, the RSV season is early, and it is ferocious.
The combination of the two infections is hitting children’s hospitals hard, and it’s layered on top of the indirect effects of the pandemic, such as the increased population of kids and teens who need mental health care in the wake of the crisis.
“It’s all these things happening at the same time,” said Mark Wietecha, CEO of the Children’s Hospital Association. “To have our hospitals this crowded in August is unusual.
And children’s hospitals are grappling with the same workforce shortages as hospitals that treat adults, while their pool of potential staff is much smaller.
“We can’t easily recruit physicians and nurses from adult hospitals in any practical way to staff a kids’ hospital,” Mr. Wietecha said.
Although pediatric doctors and nurses were trained to care for adults before they specialized, clinicians who primarily care for adults typically haven’t been taught how to care for kids.
Clinicians have fewer tools to fight COVID-19 infections in children than are available for adults.
“There have been many studies in terms of therapies and treatments for acute SARS-CoV-2 infection in adults. We have less data and information in children, and on top of that, some of these treatments aren’t even available under an EUA [emergency use authorization] to children: For example, the monoclonal antibodies,” Dr. Hobbs said.
Antibody treatments are being widely deployed to ease the pressure on hospitals that treat adults. But these therapies aren’t available for kids.
That means children’s hospitals could quickly become overwhelmed, especially in areas where community transmission is high, vaccination rates are low, and parents are screaming about masks.
“So we really have this constellation of events that really doesn’t favor children under the age of 12,” Dr. Hobbs said.
“Universal masking shouldn’t be a debate, because it’s the one thing, with adult vaccination, that can be done to protect this vulnerable population,” she said. “This isn’t a political issue. It’s a public health issue. Period.”
A version of this article first appeared on Medscape.com.
Over the course of the pandemic, COVID-19 has been a less serious illness for children than it has been for adults, and that continues to be true. But with the arrival of Delta, the risk for kids is rising, and that’s creating a perilous situation for hospitals across the United States that treat them.
Roughly 1,800 kids were hospitalized with COVID-19 in the United States last week, a 500% increase in the rate of COVID-19 hospitalizations for children since early July, according to data from the Centers for Disease Control and Prevention.
Emerging data from a large study in Canada suggest that children who test positive for COVID-19 during the Delta wave may be more than twice as likely to be hospitalized as they were when previous variants were dominating transmission. The new data support what many pediatric infectious disease experts say they’ve been seeing: Younger kids with more serious symptoms.
That may sound concerning, but keep in mind that the overall risk of hospitalization for kids who have COVID-19 is still very low – about one child for every hundred who test positive for the virus will end up needing hospital care for their symptoms, according to current statistics maintained by the American Academy of Pediatrics.
‘This is different’
At Le Bonheur Children’s Hospital in Memphis, they saw Delta coming.
Since last year, every kid that comes to the emergency department at the hospital gets a screening test for COVID-19.
In past waves, doctors usually found kids who were infected by accident – they tested positive after coming in for some other problem, a broken leg or appendicitis, said Nick Hysmith, MD, medical director of infection prevention at the hospital. But within the last few weeks, kids with fevers, sore throats, coughs, and runny noses started testing positive for COVID-19.
“We have seen our positive numbers go from, you know, close to about 8%-10% jump up to 20%, and then in recent weeks, we can get as high as 26% or 30%,” Dr. Hysmith said. “Then we started seeing kids sick enough to be admitted.”
“Over the last week, we’ve really seen an increase,” he said. As of August 16, the hospital had 24 children with COVID-19 admitted. Seven of the children were in the PICU, and two were on ventilators.
Arkansas Children’s Hospital had 23 young COVID-19 patients, 10 in intensive care, and five on ventilators, as of Friday, according to the Washington Post. At Children’s of Mississippi, the only hospital for kids in that state, 22 youth were hospitalized as of Monday, with three in intensive care as of August 16, according to the hospital. The nonprofit relief organization Samaritan’s Purse is setting up a second field hospital in the basement of Children’s to expand the hospital’s capacity.
“This is different,” Dr. Hysmith said. “What we’re seeing now is previously healthy kids coming in with symptomatic infection.”
This increased virulence is happening at a bad time. Schools around the United States are reopening for in-person classes, some for the first time in more than a year. Eight states have blocked districts from requiring masks, while many more have made them optional.
Children under 12 still have no access to a vaccine, so they are facing increased exposure to a germ that’s become more dangerous with little protection, especially in schools that have eschewed masks.
More than just COVID-19
Then there are the latent effects of the virus to contend with.
“We’re not only seeing more children now with acute SARS-CoV-2 in the hospital, we’re starting also to see an uptick of MISC – or Multisystem Inflammatory Syndrome in Children,” said Charlotte Hobbs, MD, a pediatric infectious disease specialist at Mississippi Children’s Hospital. “We are just beginning to [see] those cases, and we anticipate that’s going to get worse.”
Adding to COVID-19’s misery, another virus is also capitalizing on this increased mixing of kids back into the community. Respiratory syncytial virus (RSV) hospitalizes about 58,000 children under age 5 in the United States each year. The typical RSV season starts in the fall and peaks in February, along with influenza. This year, the RSV season is early, and it is ferocious.
The combination of the two infections is hitting children’s hospitals hard, and it’s layered on top of the indirect effects of the pandemic, such as the increased population of kids and teens who need mental health care in the wake of the crisis.
“It’s all these things happening at the same time,” said Mark Wietecha, CEO of the Children’s Hospital Association. “To have our hospitals this crowded in August is unusual.
And children’s hospitals are grappling with the same workforce shortages as hospitals that treat adults, while their pool of potential staff is much smaller.
“We can’t easily recruit physicians and nurses from adult hospitals in any practical way to staff a kids’ hospital,” Mr. Wietecha said.
Although pediatric doctors and nurses were trained to care for adults before they specialized, clinicians who primarily care for adults typically haven’t been taught how to care for kids.
Clinicians have fewer tools to fight COVID-19 infections in children than are available for adults.
“There have been many studies in terms of therapies and treatments for acute SARS-CoV-2 infection in adults. We have less data and information in children, and on top of that, some of these treatments aren’t even available under an EUA [emergency use authorization] to children: For example, the monoclonal antibodies,” Dr. Hobbs said.
Antibody treatments are being widely deployed to ease the pressure on hospitals that treat adults. But these therapies aren’t available for kids.
That means children’s hospitals could quickly become overwhelmed, especially in areas where community transmission is high, vaccination rates are low, and parents are screaming about masks.
“So we really have this constellation of events that really doesn’t favor children under the age of 12,” Dr. Hobbs said.
“Universal masking shouldn’t be a debate, because it’s the one thing, with adult vaccination, that can be done to protect this vulnerable population,” she said. “This isn’t a political issue. It’s a public health issue. Period.”
A version of this article first appeared on Medscape.com.
Youngest children more likely to spread SARS-CoV-2 to family: Study
Young children are more likely than are their older siblings to transmit SARS-CoV-2 in their households, according to an analysis of public health records in Ontario, Canada – a finding that upends the common belief that children play a minimal role in COVID-19 spread.
The study by researchers from Public Health Ontario, published online in JAMA Pediatrics, found that teenagers (14- to 17-year-olds) were more likely than were their younger siblings to bring the virus into the household, while infants and toddlers (up to age 3) were about 43% more likely than were the older teens to spread it to others in the home.
Children or teens were the source of SARS-CoV-2 in about 1 in 13 Ontario households between June and December 2020, the study shows. The researchers analyzed health records from 6,280 households with a pediatric COVID-19 case and a subset of 1,717 households in which a child up to age 17 was the source of transmission in a household.
When analyzing the data, the researchers controlled for gender differences, month of disease onset, testing delay, and mean family size.
The role of young children in transmission seemed logical to some experts who have been tracking the evolution of the pandemic. “I think what was more surprising was how long the narrative persisted that children weren’t transmitting SARS-CoV-2,” said Samuel Scarpino, PhD, managing director of pathogen surveillance at the Rockefeller Foundation.
Meanwhile, less mask-wearing, the return to school and activities, and the onslaught of the Delta variant have changed the dynamics of spread, said Andrew Pavia, MD, chief of the division of pediatric infectious diseases at the University of Utah.
“Adolescents and high-school-aged kids have had much, much higher rates of infection in the past,” he said. “Now when we look at the rates of school-aged kids, they are the same as high-school-aged kids, and we’re seeing more and more in the preschool age groups.”
Cases may be underestimated
If anything, the study may underestimate the role young children play in spreading COVID-19 in families, since it included only symptomatic cases as the initial source and young children are more likely to be asymptomatic, Dr. Pavia said.
The Delta variant heightens the concern; it is more than twice as infectious as previous strains and has spurred a rise in pediatric cases, including some coinfection with other circulating respiratory diseases, such as respiratory syncytial virus (RSV).
The Ontario study covers a period before vaccination and the spread of the Delta variant. “As the number of pediatric cases increases worldwide, the role of children in household transmission will continue to grow,” the authors concluded.
Following recommended respiratory hygiene is clearly more difficult with very young children. For example, parents, caregivers, and older siblings aren’t going to stay 6 feet away from a sick baby or toddler, Susan Coffin, MD, MPH, a pediatric infectious disease physician, and David Rubin, MD, a pediatrician and director of PolicyLab at Children’s Hospital of Philadelphia, noted in an accompanying commentary.
“Cuddling and touching are part and parcel of taking care of a sick young child, and that will obviously come with an increased risk of transmission to parents as well as to older siblings who may be helping to care for their sick brother or sister,” they wrote.
While parents may wash their hands more frequently when caring for a sick child, they aren’t likely to wear a mask, said William Schaffner, MD, an infectious disease specialist at Vanderbilt University, Nashville, Tenn.
“I imagine some moms even take a sick child into bed with them,” he said. “It’s probably just the extensive contact one has with a sick, very small child that augments their capacity to transmit this infection.”
What can be done
What can be done, then, to reduce the household spread of COVID-19? “The obvious solution to protect a household with a sick young infant or toddler is to make sure that all eligible members of the household are vaccinated,” Dr. Coffin and Dr. Rubin stated in their commentary.
The American Academy of Pediatrics recently wrote to Janet Woodcock, MD, acting commissioner of the Food and Drug Administration, asking for the agency to authorize use of SARS-CoV-2 vaccines for children under age 12 “as soon as possible,” noting that “the Delta variant has created a new and pressing risk to children and adolescents across this country, as it has also done for unvaccinated adults.”
The FDA reportedly asked vaccine makers Pfizer and Moderna to expand the clinical trials of children, which may delay authorization for younger age groups. Pfizer has said it plans to submit a request for emergency use authorization of its vaccine for 5- to 11-year-olds in September or October.
As with adult vaccination, hesitancy is likely to be a barrier. Less than half of parents said they are very or somewhat likely to have their children get a COVID-19 vaccine, according to a national survey conducted by researchers at the University of California, Los Angeles.
The Ontario study provides valuable evidence to support taking steps to protect children from transmission in schools, including mask requirements, frequent testing, and improved ventilation, said Dr. Scarpino.
“We’re not going to be able to control COVID without vaccinating younger individuals,” he said.
Dr. Pavia has consulted for GlaxoSmithKline on non–COVID-19–related issues. Sarah Buchan, PhD, study author and scientist at Public Health Ontario, reported grants from the Canadian Institutes of Health Research for research on influenza, RSV, and COVID-19, and grants from the Canadian Immunity Task Force for COVID-19 outside the submitted work. Dr. Coffin reported grants as a Centers for Disease Control and Prevention coinvestigator at a Vaccine and Treatment Evaluation Unit site conducting COVID-19 vaccine trials in children. Dr. Scarpino holds unexercised options in ILiAD Biotechnologies, which is focused on the prevention and treatment of pertussis. Dr. Schaffner is a consultant for VBI Vaccines.
A version of this article first appeared on Medscape.com.
Young children are more likely than are their older siblings to transmit SARS-CoV-2 in their households, according to an analysis of public health records in Ontario, Canada – a finding that upends the common belief that children play a minimal role in COVID-19 spread.
The study by researchers from Public Health Ontario, published online in JAMA Pediatrics, found that teenagers (14- to 17-year-olds) were more likely than were their younger siblings to bring the virus into the household, while infants and toddlers (up to age 3) were about 43% more likely than were the older teens to spread it to others in the home.
Children or teens were the source of SARS-CoV-2 in about 1 in 13 Ontario households between June and December 2020, the study shows. The researchers analyzed health records from 6,280 households with a pediatric COVID-19 case and a subset of 1,717 households in which a child up to age 17 was the source of transmission in a household.
When analyzing the data, the researchers controlled for gender differences, month of disease onset, testing delay, and mean family size.
The role of young children in transmission seemed logical to some experts who have been tracking the evolution of the pandemic. “I think what was more surprising was how long the narrative persisted that children weren’t transmitting SARS-CoV-2,” said Samuel Scarpino, PhD, managing director of pathogen surveillance at the Rockefeller Foundation.
Meanwhile, less mask-wearing, the return to school and activities, and the onslaught of the Delta variant have changed the dynamics of spread, said Andrew Pavia, MD, chief of the division of pediatric infectious diseases at the University of Utah.
“Adolescents and high-school-aged kids have had much, much higher rates of infection in the past,” he said. “Now when we look at the rates of school-aged kids, they are the same as high-school-aged kids, and we’re seeing more and more in the preschool age groups.”
Cases may be underestimated
If anything, the study may underestimate the role young children play in spreading COVID-19 in families, since it included only symptomatic cases as the initial source and young children are more likely to be asymptomatic, Dr. Pavia said.
The Delta variant heightens the concern; it is more than twice as infectious as previous strains and has spurred a rise in pediatric cases, including some coinfection with other circulating respiratory diseases, such as respiratory syncytial virus (RSV).
The Ontario study covers a period before vaccination and the spread of the Delta variant. “As the number of pediatric cases increases worldwide, the role of children in household transmission will continue to grow,” the authors concluded.
Following recommended respiratory hygiene is clearly more difficult with very young children. For example, parents, caregivers, and older siblings aren’t going to stay 6 feet away from a sick baby or toddler, Susan Coffin, MD, MPH, a pediatric infectious disease physician, and David Rubin, MD, a pediatrician and director of PolicyLab at Children’s Hospital of Philadelphia, noted in an accompanying commentary.
“Cuddling and touching are part and parcel of taking care of a sick young child, and that will obviously come with an increased risk of transmission to parents as well as to older siblings who may be helping to care for their sick brother or sister,” they wrote.
While parents may wash their hands more frequently when caring for a sick child, they aren’t likely to wear a mask, said William Schaffner, MD, an infectious disease specialist at Vanderbilt University, Nashville, Tenn.
“I imagine some moms even take a sick child into bed with them,” he said. “It’s probably just the extensive contact one has with a sick, very small child that augments their capacity to transmit this infection.”
What can be done
What can be done, then, to reduce the household spread of COVID-19? “The obvious solution to protect a household with a sick young infant or toddler is to make sure that all eligible members of the household are vaccinated,” Dr. Coffin and Dr. Rubin stated in their commentary.
The American Academy of Pediatrics recently wrote to Janet Woodcock, MD, acting commissioner of the Food and Drug Administration, asking for the agency to authorize use of SARS-CoV-2 vaccines for children under age 12 “as soon as possible,” noting that “the Delta variant has created a new and pressing risk to children and adolescents across this country, as it has also done for unvaccinated adults.”
The FDA reportedly asked vaccine makers Pfizer and Moderna to expand the clinical trials of children, which may delay authorization for younger age groups. Pfizer has said it plans to submit a request for emergency use authorization of its vaccine for 5- to 11-year-olds in September or October.
As with adult vaccination, hesitancy is likely to be a barrier. Less than half of parents said they are very or somewhat likely to have their children get a COVID-19 vaccine, according to a national survey conducted by researchers at the University of California, Los Angeles.
The Ontario study provides valuable evidence to support taking steps to protect children from transmission in schools, including mask requirements, frequent testing, and improved ventilation, said Dr. Scarpino.
“We’re not going to be able to control COVID without vaccinating younger individuals,” he said.
Dr. Pavia has consulted for GlaxoSmithKline on non–COVID-19–related issues. Sarah Buchan, PhD, study author and scientist at Public Health Ontario, reported grants from the Canadian Institutes of Health Research for research on influenza, RSV, and COVID-19, and grants from the Canadian Immunity Task Force for COVID-19 outside the submitted work. Dr. Coffin reported grants as a Centers for Disease Control and Prevention coinvestigator at a Vaccine and Treatment Evaluation Unit site conducting COVID-19 vaccine trials in children. Dr. Scarpino holds unexercised options in ILiAD Biotechnologies, which is focused on the prevention and treatment of pertussis. Dr. Schaffner is a consultant for VBI Vaccines.
A version of this article first appeared on Medscape.com.
Young children are more likely than are their older siblings to transmit SARS-CoV-2 in their households, according to an analysis of public health records in Ontario, Canada – a finding that upends the common belief that children play a minimal role in COVID-19 spread.
The study by researchers from Public Health Ontario, published online in JAMA Pediatrics, found that teenagers (14- to 17-year-olds) were more likely than were their younger siblings to bring the virus into the household, while infants and toddlers (up to age 3) were about 43% more likely than were the older teens to spread it to others in the home.
Children or teens were the source of SARS-CoV-2 in about 1 in 13 Ontario households between June and December 2020, the study shows. The researchers analyzed health records from 6,280 households with a pediatric COVID-19 case and a subset of 1,717 households in which a child up to age 17 was the source of transmission in a household.
When analyzing the data, the researchers controlled for gender differences, month of disease onset, testing delay, and mean family size.
The role of young children in transmission seemed logical to some experts who have been tracking the evolution of the pandemic. “I think what was more surprising was how long the narrative persisted that children weren’t transmitting SARS-CoV-2,” said Samuel Scarpino, PhD, managing director of pathogen surveillance at the Rockefeller Foundation.
Meanwhile, less mask-wearing, the return to school and activities, and the onslaught of the Delta variant have changed the dynamics of spread, said Andrew Pavia, MD, chief of the division of pediatric infectious diseases at the University of Utah.
“Adolescents and high-school-aged kids have had much, much higher rates of infection in the past,” he said. “Now when we look at the rates of school-aged kids, they are the same as high-school-aged kids, and we’re seeing more and more in the preschool age groups.”
Cases may be underestimated
If anything, the study may underestimate the role young children play in spreading COVID-19 in families, since it included only symptomatic cases as the initial source and young children are more likely to be asymptomatic, Dr. Pavia said.
The Delta variant heightens the concern; it is more than twice as infectious as previous strains and has spurred a rise in pediatric cases, including some coinfection with other circulating respiratory diseases, such as respiratory syncytial virus (RSV).
The Ontario study covers a period before vaccination and the spread of the Delta variant. “As the number of pediatric cases increases worldwide, the role of children in household transmission will continue to grow,” the authors concluded.
Following recommended respiratory hygiene is clearly more difficult with very young children. For example, parents, caregivers, and older siblings aren’t going to stay 6 feet away from a sick baby or toddler, Susan Coffin, MD, MPH, a pediatric infectious disease physician, and David Rubin, MD, a pediatrician and director of PolicyLab at Children’s Hospital of Philadelphia, noted in an accompanying commentary.
“Cuddling and touching are part and parcel of taking care of a sick young child, and that will obviously come with an increased risk of transmission to parents as well as to older siblings who may be helping to care for their sick brother or sister,” they wrote.
While parents may wash their hands more frequently when caring for a sick child, they aren’t likely to wear a mask, said William Schaffner, MD, an infectious disease specialist at Vanderbilt University, Nashville, Tenn.
“I imagine some moms even take a sick child into bed with them,” he said. “It’s probably just the extensive contact one has with a sick, very small child that augments their capacity to transmit this infection.”
What can be done
What can be done, then, to reduce the household spread of COVID-19? “The obvious solution to protect a household with a sick young infant or toddler is to make sure that all eligible members of the household are vaccinated,” Dr. Coffin and Dr. Rubin stated in their commentary.
The American Academy of Pediatrics recently wrote to Janet Woodcock, MD, acting commissioner of the Food and Drug Administration, asking for the agency to authorize use of SARS-CoV-2 vaccines for children under age 12 “as soon as possible,” noting that “the Delta variant has created a new and pressing risk to children and adolescents across this country, as it has also done for unvaccinated adults.”
The FDA reportedly asked vaccine makers Pfizer and Moderna to expand the clinical trials of children, which may delay authorization for younger age groups. Pfizer has said it plans to submit a request for emergency use authorization of its vaccine for 5- to 11-year-olds in September or October.
As with adult vaccination, hesitancy is likely to be a barrier. Less than half of parents said they are very or somewhat likely to have their children get a COVID-19 vaccine, according to a national survey conducted by researchers at the University of California, Los Angeles.
The Ontario study provides valuable evidence to support taking steps to protect children from transmission in schools, including mask requirements, frequent testing, and improved ventilation, said Dr. Scarpino.
“We’re not going to be able to control COVID without vaccinating younger individuals,” he said.
Dr. Pavia has consulted for GlaxoSmithKline on non–COVID-19–related issues. Sarah Buchan, PhD, study author and scientist at Public Health Ontario, reported grants from the Canadian Institutes of Health Research for research on influenza, RSV, and COVID-19, and grants from the Canadian Immunity Task Force for COVID-19 outside the submitted work. Dr. Coffin reported grants as a Centers for Disease Control and Prevention coinvestigator at a Vaccine and Treatment Evaluation Unit site conducting COVID-19 vaccine trials in children. Dr. Scarpino holds unexercised options in ILiAD Biotechnologies, which is focused on the prevention and treatment of pertussis. Dr. Schaffner is a consultant for VBI Vaccines.
A version of this article first appeared on Medscape.com.
U.S. reports record COVID-19 hospitalizations of children
The number of children hospitalized with COVID-19 in the U.S. hit a record high on Aug. 14, with more than 1,900 in hospitals.
Hospitals across the South are running out of beds as the contagious Delta variant spreads, mostly among unvaccinated people. Children make up about 2.4% of the country’s COVID-19 hospitalizations, and those under 12 are particularly vulnerable since they’re not eligible to receive a vaccine.
“This is not last year’s COVID,” Sally Goza, MD, former president of the American Academy of Pediatrics, told CNN on Aug. 14.
“This one is worse, and our children are the ones that are going to be affected by it the most,” she said.
The number of newly hospitalized COVID-19 patients for ages 18-49 also hit record highs during the week of Aug. 9. A fifth of the nation’s hospitalizations are in Florida, where the number of COVID-19 patients hit a record high of 16,100 on Aug. 14. More than 90% of the state’s intensive care unit beds are filled.
More than 90% of the ICU beds in Texas are full as well. On Aug. 13, there were no pediatric ICU beds available in Dallas or the 19 surrounding counties, which means that young patients would be transported father away for care – even Oklahoma City.
“That means if your child’s in a car wreck, if your child has a congenital heart defect or something and needs an ICU bed, or more likely, if they have COVID and need an ICU bed, we don’t have one,” Clay Jenkins, a Dallas County judge, said on Aug. 13.
“Your child will wait for another child to die,” he said.
As children return to classes, educators are talking about the possibility of vaccine mandates. The National Education Association announced its support of mandatory vaccination for its members.
“Our students under 12 can’t get vaccinated,” Becky Pringle, president of the association, told CNN.
“It’s our responsibility to keep them safe,” she said. “Keeping them safe means that everyone who can be vaccinated should be vaccinated.”
The U.S. now has an average of about 129,000 new COVID-19 cases per day, Reuters reported, which has doubled in about 2 weeks. The number of hospitalized patients is at a 6-month high, and about 600 people are dying each day.
Arkansas, Florida, Louisiana, Mississippi, and Oregon have reported record numbers of COVID-19 hospitalizations.
In addition, eight states make up half of all the COVID-19 hospitalizations in the U.S. but only 24% of the nation’s population – Alabama, Arkansas, Florida, Georgia, Louisiana, Mississippi, Nevada, and Texas. These states have vaccination rates lower than the national average, and their COVID-19 patients account for at least 15% of their overall hospitalizations.
To address the surge in hospitalizations, Oregon Gov. Kate Brown has ordered the deployment of up to 1,500 Oregon National Guard members to help health care workers.
“I know this is not the summer many of us envisioned,” Gov. Brown said Aug. 13. “The harsh and frustrating reality is that the Delta variant has changed everything. Delta is highly contagious, and we must take action now.”
A version of this article first appeared on WebMD.com.
The number of children hospitalized with COVID-19 in the U.S. hit a record high on Aug. 14, with more than 1,900 in hospitals.
Hospitals across the South are running out of beds as the contagious Delta variant spreads, mostly among unvaccinated people. Children make up about 2.4% of the country’s COVID-19 hospitalizations, and those under 12 are particularly vulnerable since they’re not eligible to receive a vaccine.
“This is not last year’s COVID,” Sally Goza, MD, former president of the American Academy of Pediatrics, told CNN on Aug. 14.
“This one is worse, and our children are the ones that are going to be affected by it the most,” she said.
The number of newly hospitalized COVID-19 patients for ages 18-49 also hit record highs during the week of Aug. 9. A fifth of the nation’s hospitalizations are in Florida, where the number of COVID-19 patients hit a record high of 16,100 on Aug. 14. More than 90% of the state’s intensive care unit beds are filled.
More than 90% of the ICU beds in Texas are full as well. On Aug. 13, there were no pediatric ICU beds available in Dallas or the 19 surrounding counties, which means that young patients would be transported father away for care – even Oklahoma City.
“That means if your child’s in a car wreck, if your child has a congenital heart defect or something and needs an ICU bed, or more likely, if they have COVID and need an ICU bed, we don’t have one,” Clay Jenkins, a Dallas County judge, said on Aug. 13.
“Your child will wait for another child to die,” he said.
As children return to classes, educators are talking about the possibility of vaccine mandates. The National Education Association announced its support of mandatory vaccination for its members.
“Our students under 12 can’t get vaccinated,” Becky Pringle, president of the association, told CNN.
“It’s our responsibility to keep them safe,” she said. “Keeping them safe means that everyone who can be vaccinated should be vaccinated.”
The U.S. now has an average of about 129,000 new COVID-19 cases per day, Reuters reported, which has doubled in about 2 weeks. The number of hospitalized patients is at a 6-month high, and about 600 people are dying each day.
Arkansas, Florida, Louisiana, Mississippi, and Oregon have reported record numbers of COVID-19 hospitalizations.
In addition, eight states make up half of all the COVID-19 hospitalizations in the U.S. but only 24% of the nation’s population – Alabama, Arkansas, Florida, Georgia, Louisiana, Mississippi, Nevada, and Texas. These states have vaccination rates lower than the national average, and their COVID-19 patients account for at least 15% of their overall hospitalizations.
To address the surge in hospitalizations, Oregon Gov. Kate Brown has ordered the deployment of up to 1,500 Oregon National Guard members to help health care workers.
“I know this is not the summer many of us envisioned,” Gov. Brown said Aug. 13. “The harsh and frustrating reality is that the Delta variant has changed everything. Delta is highly contagious, and we must take action now.”
A version of this article first appeared on WebMD.com.
The number of children hospitalized with COVID-19 in the U.S. hit a record high on Aug. 14, with more than 1,900 in hospitals.
Hospitals across the South are running out of beds as the contagious Delta variant spreads, mostly among unvaccinated people. Children make up about 2.4% of the country’s COVID-19 hospitalizations, and those under 12 are particularly vulnerable since they’re not eligible to receive a vaccine.
“This is not last year’s COVID,” Sally Goza, MD, former president of the American Academy of Pediatrics, told CNN on Aug. 14.
“This one is worse, and our children are the ones that are going to be affected by it the most,” she said.
The number of newly hospitalized COVID-19 patients for ages 18-49 also hit record highs during the week of Aug. 9. A fifth of the nation’s hospitalizations are in Florida, where the number of COVID-19 patients hit a record high of 16,100 on Aug. 14. More than 90% of the state’s intensive care unit beds are filled.
More than 90% of the ICU beds in Texas are full as well. On Aug. 13, there were no pediatric ICU beds available in Dallas or the 19 surrounding counties, which means that young patients would be transported father away for care – even Oklahoma City.
“That means if your child’s in a car wreck, if your child has a congenital heart defect or something and needs an ICU bed, or more likely, if they have COVID and need an ICU bed, we don’t have one,” Clay Jenkins, a Dallas County judge, said on Aug. 13.
“Your child will wait for another child to die,” he said.
As children return to classes, educators are talking about the possibility of vaccine mandates. The National Education Association announced its support of mandatory vaccination for its members.
“Our students under 12 can’t get vaccinated,” Becky Pringle, president of the association, told CNN.
“It’s our responsibility to keep them safe,” she said. “Keeping them safe means that everyone who can be vaccinated should be vaccinated.”
The U.S. now has an average of about 129,000 new COVID-19 cases per day, Reuters reported, which has doubled in about 2 weeks. The number of hospitalized patients is at a 6-month high, and about 600 people are dying each day.
Arkansas, Florida, Louisiana, Mississippi, and Oregon have reported record numbers of COVID-19 hospitalizations.
In addition, eight states make up half of all the COVID-19 hospitalizations in the U.S. but only 24% of the nation’s population – Alabama, Arkansas, Florida, Georgia, Louisiana, Mississippi, Nevada, and Texas. These states have vaccination rates lower than the national average, and their COVID-19 patients account for at least 15% of their overall hospitalizations.
To address the surge in hospitalizations, Oregon Gov. Kate Brown has ordered the deployment of up to 1,500 Oregon National Guard members to help health care workers.
“I know this is not the summer many of us envisioned,” Gov. Brown said Aug. 13. “The harsh and frustrating reality is that the Delta variant has changed everything. Delta is highly contagious, and we must take action now.”
A version of this article first appeared on WebMD.com.
Universal masking is the key to safe school attendance
“I want my child to go back to school,” the mother said to me. “I just want you to tell me it will be safe.”
As the summer break winds down for children across the United States, pediatric COVID-19 cases are rising. According to the American Academy of Pediatrics, nearly 94,000 cases were reported for the week ending Aug. 5, more than double the case count from 2 weeks earlier.1
Anecdotally, some children’s hospitals are reporting an increase in pediatric COVID-19 admissions. In the hospital in which I practice, we are seeing numbers similar to those we saw in December and January: a typical daily census of 10 kids admitted with COVID-19, with 4 of them in the intensive care unit. It is a stark contrast to June when, most days, we had no patients with COVID-19 in the hospital. About half of our hospitalized patients are too young to be vaccinated against COVID-19, while the rest are unvaccinated children 12 years and older.
Vaccination of eligible children and teachers is an essential strategy for preventing the spread of COVID-19 in schools, but as children head back to school, immunization rates of educators are largely unknown and are suboptimal among students in most states. As of Aug. 11, 10.7 million U.S. children had received at least one dose of COVID-19 vaccine, representing 43% of 12- to 15-year-olds and 53% of 16- to 17-year-olds.2 Rates vary substantially by state, with more than 70% of kids in Vermont receiving at least one dose of vaccine, compared with less than 25% in Wyoming and Alabama.
Still, in the absence of robust immunization rates, we have data that schools can still reopen successfully. We need to follow the science and implement universal masking, a safe, effective, and practical mitigation strategy.
It worked in Wisconsin. Seventeen K-12 schools in rural Wisconsin opened last fall for in-person instruction.3 Reported compliance with masking was high, ranging from 92.1% to 97.4%, and in-school transmission of COVID-19 was low, with seven cases among 4,876 students.
It worked in Salt Lake City.4 In 20 elementary schools open for in-person instruction Dec. 3, 2020, to Jan. 31, 2021, compliance with mask-wearing was high and in-school transmission was very low, despite a high community incidence of COVID-19. Notably, students’ classroom seats were less than 6 feet apart, suggesting that consistent mask-wearing works even when physical distancing is challenging.
One of the best examples of successful school reopening happened in North Carolina, where pediatricians, pediatric infectious disease specialists, and other experts affiliated with Duke University formed the ABC Science Collaborative to support school districts that requested scientific input to help guide return-to-school policies during the COVID-19 pandemic. From Oct. 26, 2020, to Feb. 28, 2021, the ABC Science Collaborative worked with 13 school districts that were open for in-person instruction using basic mitigation strategies, including universal masking.5 During this time period, there were 4,969 community-acquired SARS-CoV-2 infections in the more than 100,000 students and staff present in schools. Transmission to school contacts was identified in only 209 individuals for a secondary attack rate of less than 1%.
Duke investigator Kanecia Zimmerman, MD, told Duke Today, “We know that, if our goal is to reduce transmission of COVID-19 in schools, there are two effective ways to do that: 1. vaccination, 2. masking. In the setting of schools ... the science suggests masking can be extremely effective, particularly for those who can’t get vaccinated while COVID-19 is still circulating.”
Both the AAP6 and the Pediatric Infectious Diseases Society7 have emphasized the importance of in-person instruction and endorsed universal masking in school. Mask-optional policies or “mask-if-you-are-unvaccinated” policies don’t work, as we have seen in society at large. They are likely to be especially challenging in school settings. Given an option, many, if not most kids, will take off their masks. Kids who leave them on run the risk of stigmatization or bullying.
On Aug. 4, the Centers for Disease Control and Prevention updated its guidance to recommend universal indoor masking for all students, staff, teachers, and visitors to K-12 schools, regardless of vaccination status. Now we’ll have to wait and see if school districts, elected officials, and parents will get on board with masks. ... and we’ll be left to count the number of rising COVID-19 cases that occur until they do.
Case in point: Kids in Greater Clark County, Ind., headed back to school on July 28. Masks were not required on school property, although unvaccinated students and teachers were “strongly encouraged” to wear them.8
Over the first 8 days of in-person instruction, schools in Greater Clark County identified 70 cases of COVID-19 in students and quarantined more than 1,100 of the district’s 10,300 students. Only the unvaccinated were required to quarantine. The district began requiring masks in all school buildings on Aug. 9.9
The worried mother had one last question for me. “What’s the best mask for a child to wear?” For most kids, a simple, well-fitting cloth mask is fine. The best mask is ultimately the mask a child will wear. A toolkit with practical tips for helping children successfully wear a mask is available on the ABC Science Collaborative website.
Dr. Bryant, president of the Pediatric Infectious Diseases Society, is a pediatrician at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. American Academy of Pediatrics. “Children and COVID-19: State-level data report.”
2. American Academy of Pediatrics. “Children and COVID-19 vaccination trends.”
3. Falk A et al. MMWR Morb Mortal Wkly Rep. 2021;70:136-40.
4. Hershow RB et al. MMWR Morb Mortal Wkly Rep 2021;70:442-8.
5. Zimmerman KO et al. Pediatrics. 2021 Jul;e2021052686. doi: 10.1542/peds.2021-052686.
6. American Academy of Pediatrics. “American Academy of Pediatrics updates recommendations for opening schools in fall 2021.”
7. Pediatric Infectious Diseases Society. “PIDS supports universal masking for students, school staff.”
8. Courtney Hayden. WHAS11. “Greater Clark County Schools return to class July 28.”
9. Dustin Vogt. WAVE3 News. “Greater Clark Country Schools to require masks amid 70 positive cases.”
“I want my child to go back to school,” the mother said to me. “I just want you to tell me it will be safe.”
As the summer break winds down for children across the United States, pediatric COVID-19 cases are rising. According to the American Academy of Pediatrics, nearly 94,000 cases were reported for the week ending Aug. 5, more than double the case count from 2 weeks earlier.1
Anecdotally, some children’s hospitals are reporting an increase in pediatric COVID-19 admissions. In the hospital in which I practice, we are seeing numbers similar to those we saw in December and January: a typical daily census of 10 kids admitted with COVID-19, with 4 of them in the intensive care unit. It is a stark contrast to June when, most days, we had no patients with COVID-19 in the hospital. About half of our hospitalized patients are too young to be vaccinated against COVID-19, while the rest are unvaccinated children 12 years and older.
Vaccination of eligible children and teachers is an essential strategy for preventing the spread of COVID-19 in schools, but as children head back to school, immunization rates of educators are largely unknown and are suboptimal among students in most states. As of Aug. 11, 10.7 million U.S. children had received at least one dose of COVID-19 vaccine, representing 43% of 12- to 15-year-olds and 53% of 16- to 17-year-olds.2 Rates vary substantially by state, with more than 70% of kids in Vermont receiving at least one dose of vaccine, compared with less than 25% in Wyoming and Alabama.
Still, in the absence of robust immunization rates, we have data that schools can still reopen successfully. We need to follow the science and implement universal masking, a safe, effective, and practical mitigation strategy.
It worked in Wisconsin. Seventeen K-12 schools in rural Wisconsin opened last fall for in-person instruction.3 Reported compliance with masking was high, ranging from 92.1% to 97.4%, and in-school transmission of COVID-19 was low, with seven cases among 4,876 students.
It worked in Salt Lake City.4 In 20 elementary schools open for in-person instruction Dec. 3, 2020, to Jan. 31, 2021, compliance with mask-wearing was high and in-school transmission was very low, despite a high community incidence of COVID-19. Notably, students’ classroom seats were less than 6 feet apart, suggesting that consistent mask-wearing works even when physical distancing is challenging.
One of the best examples of successful school reopening happened in North Carolina, where pediatricians, pediatric infectious disease specialists, and other experts affiliated with Duke University formed the ABC Science Collaborative to support school districts that requested scientific input to help guide return-to-school policies during the COVID-19 pandemic. From Oct. 26, 2020, to Feb. 28, 2021, the ABC Science Collaborative worked with 13 school districts that were open for in-person instruction using basic mitigation strategies, including universal masking.5 During this time period, there were 4,969 community-acquired SARS-CoV-2 infections in the more than 100,000 students and staff present in schools. Transmission to school contacts was identified in only 209 individuals for a secondary attack rate of less than 1%.
Duke investigator Kanecia Zimmerman, MD, told Duke Today, “We know that, if our goal is to reduce transmission of COVID-19 in schools, there are two effective ways to do that: 1. vaccination, 2. masking. In the setting of schools ... the science suggests masking can be extremely effective, particularly for those who can’t get vaccinated while COVID-19 is still circulating.”
Both the AAP6 and the Pediatric Infectious Diseases Society7 have emphasized the importance of in-person instruction and endorsed universal masking in school. Mask-optional policies or “mask-if-you-are-unvaccinated” policies don’t work, as we have seen in society at large. They are likely to be especially challenging in school settings. Given an option, many, if not most kids, will take off their masks. Kids who leave them on run the risk of stigmatization or bullying.
On Aug. 4, the Centers for Disease Control and Prevention updated its guidance to recommend universal indoor masking for all students, staff, teachers, and visitors to K-12 schools, regardless of vaccination status. Now we’ll have to wait and see if school districts, elected officials, and parents will get on board with masks. ... and we’ll be left to count the number of rising COVID-19 cases that occur until they do.
Case in point: Kids in Greater Clark County, Ind., headed back to school on July 28. Masks were not required on school property, although unvaccinated students and teachers were “strongly encouraged” to wear them.8
Over the first 8 days of in-person instruction, schools in Greater Clark County identified 70 cases of COVID-19 in students and quarantined more than 1,100 of the district’s 10,300 students. Only the unvaccinated were required to quarantine. The district began requiring masks in all school buildings on Aug. 9.9
The worried mother had one last question for me. “What’s the best mask for a child to wear?” For most kids, a simple, well-fitting cloth mask is fine. The best mask is ultimately the mask a child will wear. A toolkit with practical tips for helping children successfully wear a mask is available on the ABC Science Collaborative website.
Dr. Bryant, president of the Pediatric Infectious Diseases Society, is a pediatrician at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. American Academy of Pediatrics. “Children and COVID-19: State-level data report.”
2. American Academy of Pediatrics. “Children and COVID-19 vaccination trends.”
3. Falk A et al. MMWR Morb Mortal Wkly Rep. 2021;70:136-40.
4. Hershow RB et al. MMWR Morb Mortal Wkly Rep 2021;70:442-8.
5. Zimmerman KO et al. Pediatrics. 2021 Jul;e2021052686. doi: 10.1542/peds.2021-052686.
6. American Academy of Pediatrics. “American Academy of Pediatrics updates recommendations for opening schools in fall 2021.”
7. Pediatric Infectious Diseases Society. “PIDS supports universal masking for students, school staff.”
8. Courtney Hayden. WHAS11. “Greater Clark County Schools return to class July 28.”
9. Dustin Vogt. WAVE3 News. “Greater Clark Country Schools to require masks amid 70 positive cases.”
“I want my child to go back to school,” the mother said to me. “I just want you to tell me it will be safe.”
As the summer break winds down for children across the United States, pediatric COVID-19 cases are rising. According to the American Academy of Pediatrics, nearly 94,000 cases were reported for the week ending Aug. 5, more than double the case count from 2 weeks earlier.1
Anecdotally, some children’s hospitals are reporting an increase in pediatric COVID-19 admissions. In the hospital in which I practice, we are seeing numbers similar to those we saw in December and January: a typical daily census of 10 kids admitted with COVID-19, with 4 of them in the intensive care unit. It is a stark contrast to June when, most days, we had no patients with COVID-19 in the hospital. About half of our hospitalized patients are too young to be vaccinated against COVID-19, while the rest are unvaccinated children 12 years and older.
Vaccination of eligible children and teachers is an essential strategy for preventing the spread of COVID-19 in schools, but as children head back to school, immunization rates of educators are largely unknown and are suboptimal among students in most states. As of Aug. 11, 10.7 million U.S. children had received at least one dose of COVID-19 vaccine, representing 43% of 12- to 15-year-olds and 53% of 16- to 17-year-olds.2 Rates vary substantially by state, with more than 70% of kids in Vermont receiving at least one dose of vaccine, compared with less than 25% in Wyoming and Alabama.
Still, in the absence of robust immunization rates, we have data that schools can still reopen successfully. We need to follow the science and implement universal masking, a safe, effective, and practical mitigation strategy.
It worked in Wisconsin. Seventeen K-12 schools in rural Wisconsin opened last fall for in-person instruction.3 Reported compliance with masking was high, ranging from 92.1% to 97.4%, and in-school transmission of COVID-19 was low, with seven cases among 4,876 students.
It worked in Salt Lake City.4 In 20 elementary schools open for in-person instruction Dec. 3, 2020, to Jan. 31, 2021, compliance with mask-wearing was high and in-school transmission was very low, despite a high community incidence of COVID-19. Notably, students’ classroom seats were less than 6 feet apart, suggesting that consistent mask-wearing works even when physical distancing is challenging.
One of the best examples of successful school reopening happened in North Carolina, where pediatricians, pediatric infectious disease specialists, and other experts affiliated with Duke University formed the ABC Science Collaborative to support school districts that requested scientific input to help guide return-to-school policies during the COVID-19 pandemic. From Oct. 26, 2020, to Feb. 28, 2021, the ABC Science Collaborative worked with 13 school districts that were open for in-person instruction using basic mitigation strategies, including universal masking.5 During this time period, there were 4,969 community-acquired SARS-CoV-2 infections in the more than 100,000 students and staff present in schools. Transmission to school contacts was identified in only 209 individuals for a secondary attack rate of less than 1%.
Duke investigator Kanecia Zimmerman, MD, told Duke Today, “We know that, if our goal is to reduce transmission of COVID-19 in schools, there are two effective ways to do that: 1. vaccination, 2. masking. In the setting of schools ... the science suggests masking can be extremely effective, particularly for those who can’t get vaccinated while COVID-19 is still circulating.”
Both the AAP6 and the Pediatric Infectious Diseases Society7 have emphasized the importance of in-person instruction and endorsed universal masking in school. Mask-optional policies or “mask-if-you-are-unvaccinated” policies don’t work, as we have seen in society at large. They are likely to be especially challenging in school settings. Given an option, many, if not most kids, will take off their masks. Kids who leave them on run the risk of stigmatization or bullying.
On Aug. 4, the Centers for Disease Control and Prevention updated its guidance to recommend universal indoor masking for all students, staff, teachers, and visitors to K-12 schools, regardless of vaccination status. Now we’ll have to wait and see if school districts, elected officials, and parents will get on board with masks. ... and we’ll be left to count the number of rising COVID-19 cases that occur until they do.
Case in point: Kids in Greater Clark County, Ind., headed back to school on July 28. Masks were not required on school property, although unvaccinated students and teachers were “strongly encouraged” to wear them.8
Over the first 8 days of in-person instruction, schools in Greater Clark County identified 70 cases of COVID-19 in students and quarantined more than 1,100 of the district’s 10,300 students. Only the unvaccinated were required to quarantine. The district began requiring masks in all school buildings on Aug. 9.9
The worried mother had one last question for me. “What’s the best mask for a child to wear?” For most kids, a simple, well-fitting cloth mask is fine. The best mask is ultimately the mask a child will wear. A toolkit with practical tips for helping children successfully wear a mask is available on the ABC Science Collaborative website.
Dr. Bryant, president of the Pediatric Infectious Diseases Society, is a pediatrician at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. American Academy of Pediatrics. “Children and COVID-19: State-level data report.”
2. American Academy of Pediatrics. “Children and COVID-19 vaccination trends.”
3. Falk A et al. MMWR Morb Mortal Wkly Rep. 2021;70:136-40.
4. Hershow RB et al. MMWR Morb Mortal Wkly Rep 2021;70:442-8.
5. Zimmerman KO et al. Pediatrics. 2021 Jul;e2021052686. doi: 10.1542/peds.2021-052686.
6. American Academy of Pediatrics. “American Academy of Pediatrics updates recommendations for opening schools in fall 2021.”
7. Pediatric Infectious Diseases Society. “PIDS supports universal masking for students, school staff.”
8. Courtney Hayden. WHAS11. “Greater Clark County Schools return to class July 28.”
9. Dustin Vogt. WAVE3 News. “Greater Clark Country Schools to require masks amid 70 positive cases.”
CDC officially endorses third dose of mRNA vaccines for immunocompromised
Centers for Disease Control and Prevention Director Rochelle Walensky, MD, has officially signed off on a recommendation by an independent panel of 11 experts to allow people with weakened immune function to get a third dose of certain COVID-19 vaccines.
The decision follows a unanimous vote by the CDC’s Advisory Committee on Immunization Practices (ACIP), which in turn came hours after the U.S. Food and Drug Administration updated its Emergency Use Authorization (EUA) for the Pfizer and Moderna mRNA vaccines.
About 7 million adults in the United States have moderately to severely impaired immune function because of a medical condition they live with or a medication they take to manage a health condition.
People who fall into this category are at higher risk of being hospitalized or dying if they get COVID-19. They are also more likely to transmit the infection. About 40% of vaccinated patients who are hospitalized with breakthrough cases are immunocompromised.
Recent studies have shown that between one-third and one-half of immunocompromised people who didn’t develop antibodies after two doses of a vaccine do get some level of protection after a third dose.
Even then, however, the protection immunocompromised people get from vaccines is not as robust as someone who has healthy immune function, and some panel members were concerned that a third dose might come with a false sense of security.
“My only concern with adding a third dose for the immunocompromised is the impression that our immunocompromised population [will] then be safe,” said ACIP member Helen Talbot, MD, MPH, an associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn.
“I think the reality is they’ll be safer but still at incredibly high risk for severe disease and death,” she said.
In updating its EUA, the FDA stressed that, even after a third dose, people who are immunocompromised will still need to wear a mask indoors, socially distance, and avoid large crowds. In addition, family members and other close contacts should be fully vaccinated to protect these vulnerable individuals.
Johnson & Johnson not in the mix
The boosters will be available to children as young as 12 years of age who’ve had a Pfizer vaccine or those ages 18 and older who’ve gotten the Moderna vaccine.
For now, people who’ve had the one-dose Johnson & Johnson vaccine have not been cleared to get a second dose of any vaccine.
FDA experts acknowledged the gap but said that people who had received the Johnson & Johnson vaccine represented a small slice of vaccinated Americans, and said they couldn’t act before the FDA had updated its authorization for that vaccine, which the agency is actively exploring.
“We had to do what we’re doing based on the data we have in hand,” said Peter Marks, MD, director of the Center for Biologics Evaluation and Research at the FDA, the division of the agency that regulates vaccines.
“We think at least there is a solution here for the very large majority of immunocompromised individuals, and we believe we will probably have a solution for the remainder in the not-too-distant future,” Dr. Marks said.
In its updated EUA, the FDA said that the third shots were intended for people who had undergone solid organ transplants or have an “equivalent level of immunocompromise.”
The details
Clinical experts on the CDC panel spent a good deal of time trying to suss out exactly what conditions might fall under the FDA’s umbrella for a third dose.
In a presentation to the committee, Neela Goswami, MD, PhD, an assistant professor of infectious diseases at Emory University School of Medicine and of epidemiology at the Emory Rollins School of Public Health, Atlanta, stressed that the shots are intended for patients who are moderately or severely immunocompromised, in close consultation with their doctors, but that people who should qualify would include those:
- Receiving treatment for solid tumors or blood cancers
- Taking immunosuppressing medications after a solid organ transplant
- Within 2 years of receiving CAR-T therapy or a stem cell transplant
- Who have primary immunodeficiencies – rare genetic disorders that prevent the immune system from working properly
- With advanced or untreated
- Taking high-dose corticosteroids (more than 20 milligrams of or its equivalent daily), alkylating agents, antimetabolites, chemotherapy, TNF blockers, or other immunomodulating or immunosuppressing biologics
- With certain chronic medical conditions, such as or asplenia – living without a spleen
- Receiving dialysis
In discussion, CDC experts clarified that these third doses were not intended for people whose immune function had waned with age, such as elderly residents of long-term care facilities or people with chronic diseases like diabetes.
The idea is to try to get a third dose of the vaccine they’ve already had – Moderna or Pfizer – but if that’s not feasible, it’s fine for the third dose to be different from what someone has had before. The third dose should be given at least 28 days after a second dose, and, ideally, before the initiation of immunosuppressive therapy.
Participants in the meeting said that the CDC would post updated materials on its website to help guide physicians on exactly who should receive third doses.
Ultimately, however, the extra doses will be given on an honor system; no prescriptions or other kinds of clinical documentation will be required for people to get a third dose of these shots.
Tests to measure neutralizing antibodies are also not recommended before the shots are given because of differences in the types of tests used to measure these antibodies and the difficulty in interpreting them. It’s unclear right now what level of neutralizing antibodies is needed for protection.
‘Peace of mind’
In public testimony, Heather Braaten, a 44-year-old being treated for ovarian cancer, said she was grateful to have gotten two shots of the Pfizer vaccine last winter, in between rounds of chemotherapy, but she knew she was probably not well protected. She said she’d become obsessive over the past few months reading medical studies and trying to understand her risk.
“I have felt distraught over the situation. My prognosis is poor. I most likely have about two to three years left to live, so everything counts,” Ms. Braaten said.
She said her life ambitions were humble. She wants to visit with friends and family and not have to worry that she’ll be a breakthrough case. She wants to go grocery shopping again and “not panic and leave the store after five minutes.” She’d love to feel free to travel, she said.
“While I understand I still need to be cautious, I am hopeful for the peace of mind and greater freedom a third shot can provide,” Ms. Braaten said.
More boosters on the way?
In the second half of the meeting, the CDC also signaled that it was considering the use of boosters for people whose immunity might have waned in the months since they had completed their vaccine series, particularly seniors. About 75% of people hospitalized with vaccine breakthrough cases are over age 65, according to CDC data.
Those considerations are becoming more urgent as the Delta variant continues to pummel less vaccinated states and counties.
In its presentation to the ACIP, Heather Scobie, PhD, MPH, a member of the CDC’s COVID Response Team, highlighted data from Canada, Israel, Qatar, and the United Kingdom showing that, while the Pfizer vaccine was still highly effective at preventing hospitalizations and death, it’s far less likely when faced with Delta to prevent an infection that causes symptoms.
In Israel, Pfizer’s vaccine prevented symptoms an average of 41% of the time. In Qatar, which is also using the Moderna vaccine, Pfizer’s prevented symptomatic infections with Delta about 54% of the time compared with 85% with Moderna’s.
Dr. Scobie noted that Pfizer’s waning efficacy may have something to do with the fact that it uses a lower dosage than Moderna’s. Pfizer’s recommended dosing interval is also shorter – 3 weeks compared with 4 weeks for Moderna’s. Stretching the time between shots has been shown to boost vaccine effectiveness, she said.
New data from the Mayo clinic, published ahead of peer review, also suggest that Pfizer’s protection may be fading more quickly than Moderna’s.
In February, both shots were nearly 100% effective at preventing the SARS-CoV-2 infection, but by July, against Delta, Pfizer’s efficacy had dropped to somewhere between 13% and 62%, while Moderna’s was still effective at preventing infection between 58% and 87% of the time.
In July, Pfizer’s was between 24% and 94% effective at preventing hospitalization with a COVID-19 infection and Moderna’s was between 33% and 96% effective at preventing hospitalization.
While that may sound like cause for concern, Dr. Scobie noted that, as of August 2, severe COVD-19 outcomes after vaccination are still very rare. Among 164 million fully vaccinated people in the United States there have been about 7,000 hospitalizations and 1,500 deaths; nearly three out of four of these have been in people over the age of 65.
The ACIP will next meet on August 24 to focus solely on the COVID-19 vaccines.
A version of this article first appeared on Medscape.com.
Centers for Disease Control and Prevention Director Rochelle Walensky, MD, has officially signed off on a recommendation by an independent panel of 11 experts to allow people with weakened immune function to get a third dose of certain COVID-19 vaccines.
The decision follows a unanimous vote by the CDC’s Advisory Committee on Immunization Practices (ACIP), which in turn came hours after the U.S. Food and Drug Administration updated its Emergency Use Authorization (EUA) for the Pfizer and Moderna mRNA vaccines.
About 7 million adults in the United States have moderately to severely impaired immune function because of a medical condition they live with or a medication they take to manage a health condition.
People who fall into this category are at higher risk of being hospitalized or dying if they get COVID-19. They are also more likely to transmit the infection. About 40% of vaccinated patients who are hospitalized with breakthrough cases are immunocompromised.
Recent studies have shown that between one-third and one-half of immunocompromised people who didn’t develop antibodies after two doses of a vaccine do get some level of protection after a third dose.
Even then, however, the protection immunocompromised people get from vaccines is not as robust as someone who has healthy immune function, and some panel members were concerned that a third dose might come with a false sense of security.
“My only concern with adding a third dose for the immunocompromised is the impression that our immunocompromised population [will] then be safe,” said ACIP member Helen Talbot, MD, MPH, an associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn.
“I think the reality is they’ll be safer but still at incredibly high risk for severe disease and death,” she said.
In updating its EUA, the FDA stressed that, even after a third dose, people who are immunocompromised will still need to wear a mask indoors, socially distance, and avoid large crowds. In addition, family members and other close contacts should be fully vaccinated to protect these vulnerable individuals.
Johnson & Johnson not in the mix
The boosters will be available to children as young as 12 years of age who’ve had a Pfizer vaccine or those ages 18 and older who’ve gotten the Moderna vaccine.
For now, people who’ve had the one-dose Johnson & Johnson vaccine have not been cleared to get a second dose of any vaccine.
FDA experts acknowledged the gap but said that people who had received the Johnson & Johnson vaccine represented a small slice of vaccinated Americans, and said they couldn’t act before the FDA had updated its authorization for that vaccine, which the agency is actively exploring.
“We had to do what we’re doing based on the data we have in hand,” said Peter Marks, MD, director of the Center for Biologics Evaluation and Research at the FDA, the division of the agency that regulates vaccines.
“We think at least there is a solution here for the very large majority of immunocompromised individuals, and we believe we will probably have a solution for the remainder in the not-too-distant future,” Dr. Marks said.
In its updated EUA, the FDA said that the third shots were intended for people who had undergone solid organ transplants or have an “equivalent level of immunocompromise.”
The details
Clinical experts on the CDC panel spent a good deal of time trying to suss out exactly what conditions might fall under the FDA’s umbrella for a third dose.
In a presentation to the committee, Neela Goswami, MD, PhD, an assistant professor of infectious diseases at Emory University School of Medicine and of epidemiology at the Emory Rollins School of Public Health, Atlanta, stressed that the shots are intended for patients who are moderately or severely immunocompromised, in close consultation with their doctors, but that people who should qualify would include those:
- Receiving treatment for solid tumors or blood cancers
- Taking immunosuppressing medications after a solid organ transplant
- Within 2 years of receiving CAR-T therapy or a stem cell transplant
- Who have primary immunodeficiencies – rare genetic disorders that prevent the immune system from working properly
- With advanced or untreated
- Taking high-dose corticosteroids (more than 20 milligrams of or its equivalent daily), alkylating agents, antimetabolites, chemotherapy, TNF blockers, or other immunomodulating or immunosuppressing biologics
- With certain chronic medical conditions, such as or asplenia – living without a spleen
- Receiving dialysis
In discussion, CDC experts clarified that these third doses were not intended for people whose immune function had waned with age, such as elderly residents of long-term care facilities or people with chronic diseases like diabetes.
The idea is to try to get a third dose of the vaccine they’ve already had – Moderna or Pfizer – but if that’s not feasible, it’s fine for the third dose to be different from what someone has had before. The third dose should be given at least 28 days after a second dose, and, ideally, before the initiation of immunosuppressive therapy.
Participants in the meeting said that the CDC would post updated materials on its website to help guide physicians on exactly who should receive third doses.
Ultimately, however, the extra doses will be given on an honor system; no prescriptions or other kinds of clinical documentation will be required for people to get a third dose of these shots.
Tests to measure neutralizing antibodies are also not recommended before the shots are given because of differences in the types of tests used to measure these antibodies and the difficulty in interpreting them. It’s unclear right now what level of neutralizing antibodies is needed for protection.
‘Peace of mind’
In public testimony, Heather Braaten, a 44-year-old being treated for ovarian cancer, said she was grateful to have gotten two shots of the Pfizer vaccine last winter, in between rounds of chemotherapy, but she knew she was probably not well protected. She said she’d become obsessive over the past few months reading medical studies and trying to understand her risk.
“I have felt distraught over the situation. My prognosis is poor. I most likely have about two to three years left to live, so everything counts,” Ms. Braaten said.
She said her life ambitions were humble. She wants to visit with friends and family and not have to worry that she’ll be a breakthrough case. She wants to go grocery shopping again and “not panic and leave the store after five minutes.” She’d love to feel free to travel, she said.
“While I understand I still need to be cautious, I am hopeful for the peace of mind and greater freedom a third shot can provide,” Ms. Braaten said.
More boosters on the way?
In the second half of the meeting, the CDC also signaled that it was considering the use of boosters for people whose immunity might have waned in the months since they had completed their vaccine series, particularly seniors. About 75% of people hospitalized with vaccine breakthrough cases are over age 65, according to CDC data.
Those considerations are becoming more urgent as the Delta variant continues to pummel less vaccinated states and counties.
In its presentation to the ACIP, Heather Scobie, PhD, MPH, a member of the CDC’s COVID Response Team, highlighted data from Canada, Israel, Qatar, and the United Kingdom showing that, while the Pfizer vaccine was still highly effective at preventing hospitalizations and death, it’s far less likely when faced with Delta to prevent an infection that causes symptoms.
In Israel, Pfizer’s vaccine prevented symptoms an average of 41% of the time. In Qatar, which is also using the Moderna vaccine, Pfizer’s prevented symptomatic infections with Delta about 54% of the time compared with 85% with Moderna’s.
Dr. Scobie noted that Pfizer’s waning efficacy may have something to do with the fact that it uses a lower dosage than Moderna’s. Pfizer’s recommended dosing interval is also shorter – 3 weeks compared with 4 weeks for Moderna’s. Stretching the time between shots has been shown to boost vaccine effectiveness, she said.
New data from the Mayo clinic, published ahead of peer review, also suggest that Pfizer’s protection may be fading more quickly than Moderna’s.
In February, both shots were nearly 100% effective at preventing the SARS-CoV-2 infection, but by July, against Delta, Pfizer’s efficacy had dropped to somewhere between 13% and 62%, while Moderna’s was still effective at preventing infection between 58% and 87% of the time.
In July, Pfizer’s was between 24% and 94% effective at preventing hospitalization with a COVID-19 infection and Moderna’s was between 33% and 96% effective at preventing hospitalization.
While that may sound like cause for concern, Dr. Scobie noted that, as of August 2, severe COVD-19 outcomes after vaccination are still very rare. Among 164 million fully vaccinated people in the United States there have been about 7,000 hospitalizations and 1,500 deaths; nearly three out of four of these have been in people over the age of 65.
The ACIP will next meet on August 24 to focus solely on the COVID-19 vaccines.
A version of this article first appeared on Medscape.com.
Centers for Disease Control and Prevention Director Rochelle Walensky, MD, has officially signed off on a recommendation by an independent panel of 11 experts to allow people with weakened immune function to get a third dose of certain COVID-19 vaccines.
The decision follows a unanimous vote by the CDC’s Advisory Committee on Immunization Practices (ACIP), which in turn came hours after the U.S. Food and Drug Administration updated its Emergency Use Authorization (EUA) for the Pfizer and Moderna mRNA vaccines.
About 7 million adults in the United States have moderately to severely impaired immune function because of a medical condition they live with or a medication they take to manage a health condition.
People who fall into this category are at higher risk of being hospitalized or dying if they get COVID-19. They are also more likely to transmit the infection. About 40% of vaccinated patients who are hospitalized with breakthrough cases are immunocompromised.
Recent studies have shown that between one-third and one-half of immunocompromised people who didn’t develop antibodies after two doses of a vaccine do get some level of protection after a third dose.
Even then, however, the protection immunocompromised people get from vaccines is not as robust as someone who has healthy immune function, and some panel members were concerned that a third dose might come with a false sense of security.
“My only concern with adding a third dose for the immunocompromised is the impression that our immunocompromised population [will] then be safe,” said ACIP member Helen Talbot, MD, MPH, an associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn.
“I think the reality is they’ll be safer but still at incredibly high risk for severe disease and death,” she said.
In updating its EUA, the FDA stressed that, even after a third dose, people who are immunocompromised will still need to wear a mask indoors, socially distance, and avoid large crowds. In addition, family members and other close contacts should be fully vaccinated to protect these vulnerable individuals.
Johnson & Johnson not in the mix
The boosters will be available to children as young as 12 years of age who’ve had a Pfizer vaccine or those ages 18 and older who’ve gotten the Moderna vaccine.
For now, people who’ve had the one-dose Johnson & Johnson vaccine have not been cleared to get a second dose of any vaccine.
FDA experts acknowledged the gap but said that people who had received the Johnson & Johnson vaccine represented a small slice of vaccinated Americans, and said they couldn’t act before the FDA had updated its authorization for that vaccine, which the agency is actively exploring.
“We had to do what we’re doing based on the data we have in hand,” said Peter Marks, MD, director of the Center for Biologics Evaluation and Research at the FDA, the division of the agency that regulates vaccines.
“We think at least there is a solution here for the very large majority of immunocompromised individuals, and we believe we will probably have a solution for the remainder in the not-too-distant future,” Dr. Marks said.
In its updated EUA, the FDA said that the third shots were intended for people who had undergone solid organ transplants or have an “equivalent level of immunocompromise.”
The details
Clinical experts on the CDC panel spent a good deal of time trying to suss out exactly what conditions might fall under the FDA’s umbrella for a third dose.
In a presentation to the committee, Neela Goswami, MD, PhD, an assistant professor of infectious diseases at Emory University School of Medicine and of epidemiology at the Emory Rollins School of Public Health, Atlanta, stressed that the shots are intended for patients who are moderately or severely immunocompromised, in close consultation with their doctors, but that people who should qualify would include those:
- Receiving treatment for solid tumors or blood cancers
- Taking immunosuppressing medications after a solid organ transplant
- Within 2 years of receiving CAR-T therapy or a stem cell transplant
- Who have primary immunodeficiencies – rare genetic disorders that prevent the immune system from working properly
- With advanced or untreated
- Taking high-dose corticosteroids (more than 20 milligrams of or its equivalent daily), alkylating agents, antimetabolites, chemotherapy, TNF blockers, or other immunomodulating or immunosuppressing biologics
- With certain chronic medical conditions, such as or asplenia – living without a spleen
- Receiving dialysis
In discussion, CDC experts clarified that these third doses were not intended for people whose immune function had waned with age, such as elderly residents of long-term care facilities or people with chronic diseases like diabetes.
The idea is to try to get a third dose of the vaccine they’ve already had – Moderna or Pfizer – but if that’s not feasible, it’s fine for the third dose to be different from what someone has had before. The third dose should be given at least 28 days after a second dose, and, ideally, before the initiation of immunosuppressive therapy.
Participants in the meeting said that the CDC would post updated materials on its website to help guide physicians on exactly who should receive third doses.
Ultimately, however, the extra doses will be given on an honor system; no prescriptions or other kinds of clinical documentation will be required for people to get a third dose of these shots.
Tests to measure neutralizing antibodies are also not recommended before the shots are given because of differences in the types of tests used to measure these antibodies and the difficulty in interpreting them. It’s unclear right now what level of neutralizing antibodies is needed for protection.
‘Peace of mind’
In public testimony, Heather Braaten, a 44-year-old being treated for ovarian cancer, said she was grateful to have gotten two shots of the Pfizer vaccine last winter, in between rounds of chemotherapy, but she knew she was probably not well protected. She said she’d become obsessive over the past few months reading medical studies and trying to understand her risk.
“I have felt distraught over the situation. My prognosis is poor. I most likely have about two to three years left to live, so everything counts,” Ms. Braaten said.
She said her life ambitions were humble. She wants to visit with friends and family and not have to worry that she’ll be a breakthrough case. She wants to go grocery shopping again and “not panic and leave the store after five minutes.” She’d love to feel free to travel, she said.
“While I understand I still need to be cautious, I am hopeful for the peace of mind and greater freedom a third shot can provide,” Ms. Braaten said.
More boosters on the way?
In the second half of the meeting, the CDC also signaled that it was considering the use of boosters for people whose immunity might have waned in the months since they had completed their vaccine series, particularly seniors. About 75% of people hospitalized with vaccine breakthrough cases are over age 65, according to CDC data.
Those considerations are becoming more urgent as the Delta variant continues to pummel less vaccinated states and counties.
In its presentation to the ACIP, Heather Scobie, PhD, MPH, a member of the CDC’s COVID Response Team, highlighted data from Canada, Israel, Qatar, and the United Kingdom showing that, while the Pfizer vaccine was still highly effective at preventing hospitalizations and death, it’s far less likely when faced with Delta to prevent an infection that causes symptoms.
In Israel, Pfizer’s vaccine prevented symptoms an average of 41% of the time. In Qatar, which is also using the Moderna vaccine, Pfizer’s prevented symptomatic infections with Delta about 54% of the time compared with 85% with Moderna’s.
Dr. Scobie noted that Pfizer’s waning efficacy may have something to do with the fact that it uses a lower dosage than Moderna’s. Pfizer’s recommended dosing interval is also shorter – 3 weeks compared with 4 weeks for Moderna’s. Stretching the time between shots has been shown to boost vaccine effectiveness, she said.
New data from the Mayo clinic, published ahead of peer review, also suggest that Pfizer’s protection may be fading more quickly than Moderna’s.
In February, both shots were nearly 100% effective at preventing the SARS-CoV-2 infection, but by July, against Delta, Pfizer’s efficacy had dropped to somewhere between 13% and 62%, while Moderna’s was still effective at preventing infection between 58% and 87% of the time.
In July, Pfizer’s was between 24% and 94% effective at preventing hospitalization with a COVID-19 infection and Moderna’s was between 33% and 96% effective at preventing hospitalization.
While that may sound like cause for concern, Dr. Scobie noted that, as of August 2, severe COVD-19 outcomes after vaccination are still very rare. Among 164 million fully vaccinated people in the United States there have been about 7,000 hospitalizations and 1,500 deaths; nearly three out of four of these have been in people over the age of 65.
The ACIP will next meet on August 24 to focus solely on the COVID-19 vaccines.
A version of this article first appeared on Medscape.com.
Heparin’s COVID-19 benefit greatest in moderately ill patients
Critically ill derive no benefit
Therapeutic levels of heparin can have widely varying effects on COVID-19 patients depending on the severity of their disease, according to a multiplatform clinical trial that analyzed patient data from three international trials.
COVID-19 patients in the ICU, or at least receiving ICU-level care, derived no benefit from anticoagulation with heparin, while non–critically ill COVID-19 patients – those who were hospitalized but not receiving ICU-level care – on the same anticoagulation were less likely to progress to need respiratory or cardiovascular organ support despite a slightly heightened risk of bleeding events.
Reporting in two articles published online in the New England Journal of Medicine, authors of three international trials combined their data into one multiplatform trial that makes a strong case for prescribing therapeutic levels of heparin in hospitalized patients not receiving ICU-level care were non–critically ill and critically ill.
“I think this is going to be a game changer,” said Jeffrey S. Berger, MD, ACTIV-4a co–principal investigator and co–first author of the study of non–critically ill patients. “I think that using therapeutic-dose anticoagulation should improve outcomes in the tens of thousands of patients worldwide. I hope our data can have a global impact.”
Outcomes based on disease severity
The multiplatform trial analyzed data from the Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC); A Multicenter, Adaptive, Randomized Controlled Platform Trial of the Safety and Efficacy of Antithrombotic Strategies in Hospitalized Adults with COVID-19 (ACTIV-4a); and Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP).
The trial evaluated 2,219 non–critically ill hospitalized patients, 1,181 of whom were randomized to therapeutic-dose anticoagulation; and 1,098 critically ill patients, 534 of whom were prescribed therapeutic levels of heparin.
In the critically ill patients, those on heparin were no more likely to get discharged or spend fewer days on respiratory or CV organ support – oxygen, mechanical ventilation, life support, vasopressors or inotropes – than were those on usual-care thromboprophylaxis. The investigators stopped the trial in both patient populations: in critically ill patients when it became obvious therapeutic-dose anticoagulation was having no impact; and in moderately ill patients when the trial met the prespecified criteria for the superiority of therapeutic-dose anticoagulation.
ICU patients on therapeutic-level heparin spent an average of 1 day free of organ support vs. 4 for patients on usual-care prophylactic antithrombotic drugs. The percentage of patients who survived to hospital discharge was similar in the therapeutic-level and usual-care critically ill patients: 62.7% and 64.5%, respectively. Major bleeding occurred in 3.8% and 2.8%, respectively. Demographic and clinical characteristics were similar between both patient groups.
However, in non–critically ill patients, therapeutic levels of heparin resulted in a marked improvement in outcomes. The researchers estimated that, for every 1,000 hospitalized patients with what they labeled moderate disease, an initial treatment with therapeutic-dose heparin resulted in 40 additional patients surviving compared to usual-care thromboprophylaxis.
The percentages of patients not needing organ support before hospital discharge was 80.2% on therapeutic-dose heparin and 76.4% on usual-care therapy. In terms of adjusted odds ratio, the anticoagulation group had a 27% improved chance of not needing daily organ support.
Those improvements came with an additional seven major bleeding events per 1,000 patients. That broke down to a rate of 1.9% in the therapeutic-dose and 0.9% in the usual-care patients.
As the Delta variant of COVID-19 spreads, Patrick R. Lawler, MD, MPH, principal investigator of the ATTACC trial, said there’s no reason these findings shouldn’t apply for all variants of the disease.
Dr. Lawler, a physician-scientist at Peter Munk Cardiac Centre at Toronto General Hospital, noted that the multiplatform study did not account for disease variant. “Ongoing clinical trials are tracking the variant patients have or the variants that are most prevalent in an area at that time,” he said. “It may be easier in future trials to look at that question.”
Explaining heparin’s varying effects
The study did not specifically sort out why moderately ill patients fared better on heparin than their critically ill counterparts, but Dr. Lawler speculated on possible reasons. “One might be that the extent of illness severity is too extreme in the ICU-level population for heparin to have a beneficial extent,” he said.
He acknowledged that higher rates of macrovascular thrombosis, such as venous thromboembolism, in ICU patients would suggest that heparin would have a greater beneficial effect, but, he added, “it may also suggest how advanced that process is, and perhaps heparin is not adequate to reverse the course at that point given relatively extensive thrombosis and associate organ failure.”
As clinicians have gained experience dealing with COVID-19, they’ve learned that infected patients carry a high burden of macro- and microthrombosis, Dr. Berger said, which may explain why critically ill patients didn’t respond as well to therapeutic levels of heparin. “I think the cat is out of the bag; patients who are severe are too ill to benefit,” he said. “I would think there’s too much microthrombosis that is already in their bodies.”
However, this doesn’t completely rule out therapeutic levels of heparin in critically ill COVID-19 patients. There are some scenarios where it’s needed, said Dr. Berger, associate professor of medicine and surgery and director of the Center for the Prevention of Cardiovascular Disease at New York University Langone Health. “Anyone who has a known clot already, like a known macrothrombosis in their leg or lung, needs to be on full-dose heparin,” he said.
That rationale can help reconcile the different outcomes in the critically and non–critically ill COVID-19 patients, wrote Hugo ten Cate, MD, PhD, of Maastricht University in the Netherlands, wrote in an accompanying editorial. But differences in the study populations may also explain the divergent outcomes, Dr. ten Cate noted.
The studies suggest that critically ill patients may need hon-heparin antithrombotic approaches “or even profibrinolytic strategies,” Dr. Cate wrote, and that the safety and effectiveness of thromboprophylaxis “remains an important question.” Nonetheless, he added, treating physicians must deal with the bleeding risk when using heparin or low-molecular-weight heparin in moderately ill COVID-19 patients.
Deepak L. Bhatt MD, MPH, of Brigham and Women’s Hospital Heart & Vascular Center, Boston, said in an interview that reconciling the two studies was “a bit challenging,” because effective therapies tend to have a greater impact in sicker patients.
“Of course, with antithrombotic therapies, bleeding side effects can sometimes overwhelm benefits in patients who are at high risk of both bleeding and ischemic complications, though that does not seem to be the explanation here,” Dr. Bhatt said. “I do think we need more data to clarify exactly which COVID patients benefit from various antithrombotic regimens, and fortunately, there are other ongoing studies, some of which will report relatively soon.”
He concurred with Dr. Berger that patients who need anticoagulation should receive it “apart from their COVID status,” Dr. Bhatt said. “Sick, hospitalized patients with or without COVID should receive appropriate prophylactic doses of anticoagulation.” However, he added, “Whether we should routinely go beyond that in COVID-positive inpatients, I think we need more data.”
The ATTACC platform received grants from the Canadian Institutes of Health Research and several other research foundations. The ACTIV-4a platform received funding from the National Heart, Lung, and Blood Institute. REMAP-CAP received funding from the European Union and several international research foundations, as well as Amgen and Eisai.
Dr. Lawler had no relationships to disclose. Dr. Berger disclosed receiving grants from the NHLBI, and financial relationships with AstraZeneca, Janssen, and Amgen outside the submitted work. Dr. ten Cate reported relationships with Alveron, Coagulation Profile, Portola/Alexion, Bayer, Pfizer, Stago, Leo Pharma, Daiichi, and Gilead/Galapagos. Dr. Bhatt is chair of the data safety and monitoring board of the FREEDOM COVID anticoagulation clinical trial.
Critically ill derive no benefit
Critically ill derive no benefit
Therapeutic levels of heparin can have widely varying effects on COVID-19 patients depending on the severity of their disease, according to a multiplatform clinical trial that analyzed patient data from three international trials.
COVID-19 patients in the ICU, or at least receiving ICU-level care, derived no benefit from anticoagulation with heparin, while non–critically ill COVID-19 patients – those who were hospitalized but not receiving ICU-level care – on the same anticoagulation were less likely to progress to need respiratory or cardiovascular organ support despite a slightly heightened risk of bleeding events.
Reporting in two articles published online in the New England Journal of Medicine, authors of three international trials combined their data into one multiplatform trial that makes a strong case for prescribing therapeutic levels of heparin in hospitalized patients not receiving ICU-level care were non–critically ill and critically ill.
“I think this is going to be a game changer,” said Jeffrey S. Berger, MD, ACTIV-4a co–principal investigator and co–first author of the study of non–critically ill patients. “I think that using therapeutic-dose anticoagulation should improve outcomes in the tens of thousands of patients worldwide. I hope our data can have a global impact.”
Outcomes based on disease severity
The multiplatform trial analyzed data from the Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC); A Multicenter, Adaptive, Randomized Controlled Platform Trial of the Safety and Efficacy of Antithrombotic Strategies in Hospitalized Adults with COVID-19 (ACTIV-4a); and Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP).
The trial evaluated 2,219 non–critically ill hospitalized patients, 1,181 of whom were randomized to therapeutic-dose anticoagulation; and 1,098 critically ill patients, 534 of whom were prescribed therapeutic levels of heparin.
In the critically ill patients, those on heparin were no more likely to get discharged or spend fewer days on respiratory or CV organ support – oxygen, mechanical ventilation, life support, vasopressors or inotropes – than were those on usual-care thromboprophylaxis. The investigators stopped the trial in both patient populations: in critically ill patients when it became obvious therapeutic-dose anticoagulation was having no impact; and in moderately ill patients when the trial met the prespecified criteria for the superiority of therapeutic-dose anticoagulation.
ICU patients on therapeutic-level heparin spent an average of 1 day free of organ support vs. 4 for patients on usual-care prophylactic antithrombotic drugs. The percentage of patients who survived to hospital discharge was similar in the therapeutic-level and usual-care critically ill patients: 62.7% and 64.5%, respectively. Major bleeding occurred in 3.8% and 2.8%, respectively. Demographic and clinical characteristics were similar between both patient groups.
However, in non–critically ill patients, therapeutic levels of heparin resulted in a marked improvement in outcomes. The researchers estimated that, for every 1,000 hospitalized patients with what they labeled moderate disease, an initial treatment with therapeutic-dose heparin resulted in 40 additional patients surviving compared to usual-care thromboprophylaxis.
The percentages of patients not needing organ support before hospital discharge was 80.2% on therapeutic-dose heparin and 76.4% on usual-care therapy. In terms of adjusted odds ratio, the anticoagulation group had a 27% improved chance of not needing daily organ support.
Those improvements came with an additional seven major bleeding events per 1,000 patients. That broke down to a rate of 1.9% in the therapeutic-dose and 0.9% in the usual-care patients.
As the Delta variant of COVID-19 spreads, Patrick R. Lawler, MD, MPH, principal investigator of the ATTACC trial, said there’s no reason these findings shouldn’t apply for all variants of the disease.
Dr. Lawler, a physician-scientist at Peter Munk Cardiac Centre at Toronto General Hospital, noted that the multiplatform study did not account for disease variant. “Ongoing clinical trials are tracking the variant patients have or the variants that are most prevalent in an area at that time,” he said. “It may be easier in future trials to look at that question.”
Explaining heparin’s varying effects
The study did not specifically sort out why moderately ill patients fared better on heparin than their critically ill counterparts, but Dr. Lawler speculated on possible reasons. “One might be that the extent of illness severity is too extreme in the ICU-level population for heparin to have a beneficial extent,” he said.
He acknowledged that higher rates of macrovascular thrombosis, such as venous thromboembolism, in ICU patients would suggest that heparin would have a greater beneficial effect, but, he added, “it may also suggest how advanced that process is, and perhaps heparin is not adequate to reverse the course at that point given relatively extensive thrombosis and associate organ failure.”
As clinicians have gained experience dealing with COVID-19, they’ve learned that infected patients carry a high burden of macro- and microthrombosis, Dr. Berger said, which may explain why critically ill patients didn’t respond as well to therapeutic levels of heparin. “I think the cat is out of the bag; patients who are severe are too ill to benefit,” he said. “I would think there’s too much microthrombosis that is already in their bodies.”
However, this doesn’t completely rule out therapeutic levels of heparin in critically ill COVID-19 patients. There are some scenarios where it’s needed, said Dr. Berger, associate professor of medicine and surgery and director of the Center for the Prevention of Cardiovascular Disease at New York University Langone Health. “Anyone who has a known clot already, like a known macrothrombosis in their leg or lung, needs to be on full-dose heparin,” he said.
That rationale can help reconcile the different outcomes in the critically and non–critically ill COVID-19 patients, wrote Hugo ten Cate, MD, PhD, of Maastricht University in the Netherlands, wrote in an accompanying editorial. But differences in the study populations may also explain the divergent outcomes, Dr. ten Cate noted.
The studies suggest that critically ill patients may need hon-heparin antithrombotic approaches “or even profibrinolytic strategies,” Dr. Cate wrote, and that the safety and effectiveness of thromboprophylaxis “remains an important question.” Nonetheless, he added, treating physicians must deal with the bleeding risk when using heparin or low-molecular-weight heparin in moderately ill COVID-19 patients.
Deepak L. Bhatt MD, MPH, of Brigham and Women’s Hospital Heart & Vascular Center, Boston, said in an interview that reconciling the two studies was “a bit challenging,” because effective therapies tend to have a greater impact in sicker patients.
“Of course, with antithrombotic therapies, bleeding side effects can sometimes overwhelm benefits in patients who are at high risk of both bleeding and ischemic complications, though that does not seem to be the explanation here,” Dr. Bhatt said. “I do think we need more data to clarify exactly which COVID patients benefit from various antithrombotic regimens, and fortunately, there are other ongoing studies, some of which will report relatively soon.”
He concurred with Dr. Berger that patients who need anticoagulation should receive it “apart from their COVID status,” Dr. Bhatt said. “Sick, hospitalized patients with or without COVID should receive appropriate prophylactic doses of anticoagulation.” However, he added, “Whether we should routinely go beyond that in COVID-positive inpatients, I think we need more data.”
The ATTACC platform received grants from the Canadian Institutes of Health Research and several other research foundations. The ACTIV-4a platform received funding from the National Heart, Lung, and Blood Institute. REMAP-CAP received funding from the European Union and several international research foundations, as well as Amgen and Eisai.
Dr. Lawler had no relationships to disclose. Dr. Berger disclosed receiving grants from the NHLBI, and financial relationships with AstraZeneca, Janssen, and Amgen outside the submitted work. Dr. ten Cate reported relationships with Alveron, Coagulation Profile, Portola/Alexion, Bayer, Pfizer, Stago, Leo Pharma, Daiichi, and Gilead/Galapagos. Dr. Bhatt is chair of the data safety and monitoring board of the FREEDOM COVID anticoagulation clinical trial.
Therapeutic levels of heparin can have widely varying effects on COVID-19 patients depending on the severity of their disease, according to a multiplatform clinical trial that analyzed patient data from three international trials.
COVID-19 patients in the ICU, or at least receiving ICU-level care, derived no benefit from anticoagulation with heparin, while non–critically ill COVID-19 patients – those who were hospitalized but not receiving ICU-level care – on the same anticoagulation were less likely to progress to need respiratory or cardiovascular organ support despite a slightly heightened risk of bleeding events.
Reporting in two articles published online in the New England Journal of Medicine, authors of three international trials combined their data into one multiplatform trial that makes a strong case for prescribing therapeutic levels of heparin in hospitalized patients not receiving ICU-level care were non–critically ill and critically ill.
“I think this is going to be a game changer,” said Jeffrey S. Berger, MD, ACTIV-4a co–principal investigator and co–first author of the study of non–critically ill patients. “I think that using therapeutic-dose anticoagulation should improve outcomes in the tens of thousands of patients worldwide. I hope our data can have a global impact.”
Outcomes based on disease severity
The multiplatform trial analyzed data from the Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC); A Multicenter, Adaptive, Randomized Controlled Platform Trial of the Safety and Efficacy of Antithrombotic Strategies in Hospitalized Adults with COVID-19 (ACTIV-4a); and Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP).
The trial evaluated 2,219 non–critically ill hospitalized patients, 1,181 of whom were randomized to therapeutic-dose anticoagulation; and 1,098 critically ill patients, 534 of whom were prescribed therapeutic levels of heparin.
In the critically ill patients, those on heparin were no more likely to get discharged or spend fewer days on respiratory or CV organ support – oxygen, mechanical ventilation, life support, vasopressors or inotropes – than were those on usual-care thromboprophylaxis. The investigators stopped the trial in both patient populations: in critically ill patients when it became obvious therapeutic-dose anticoagulation was having no impact; and in moderately ill patients when the trial met the prespecified criteria for the superiority of therapeutic-dose anticoagulation.
ICU patients on therapeutic-level heparin spent an average of 1 day free of organ support vs. 4 for patients on usual-care prophylactic antithrombotic drugs. The percentage of patients who survived to hospital discharge was similar in the therapeutic-level and usual-care critically ill patients: 62.7% and 64.5%, respectively. Major bleeding occurred in 3.8% and 2.8%, respectively. Demographic and clinical characteristics were similar between both patient groups.
However, in non–critically ill patients, therapeutic levels of heparin resulted in a marked improvement in outcomes. The researchers estimated that, for every 1,000 hospitalized patients with what they labeled moderate disease, an initial treatment with therapeutic-dose heparin resulted in 40 additional patients surviving compared to usual-care thromboprophylaxis.
The percentages of patients not needing organ support before hospital discharge was 80.2% on therapeutic-dose heparin and 76.4% on usual-care therapy. In terms of adjusted odds ratio, the anticoagulation group had a 27% improved chance of not needing daily organ support.
Those improvements came with an additional seven major bleeding events per 1,000 patients. That broke down to a rate of 1.9% in the therapeutic-dose and 0.9% in the usual-care patients.
As the Delta variant of COVID-19 spreads, Patrick R. Lawler, MD, MPH, principal investigator of the ATTACC trial, said there’s no reason these findings shouldn’t apply for all variants of the disease.
Dr. Lawler, a physician-scientist at Peter Munk Cardiac Centre at Toronto General Hospital, noted that the multiplatform study did not account for disease variant. “Ongoing clinical trials are tracking the variant patients have or the variants that are most prevalent in an area at that time,” he said. “It may be easier in future trials to look at that question.”
Explaining heparin’s varying effects
The study did not specifically sort out why moderately ill patients fared better on heparin than their critically ill counterparts, but Dr. Lawler speculated on possible reasons. “One might be that the extent of illness severity is too extreme in the ICU-level population for heparin to have a beneficial extent,” he said.
He acknowledged that higher rates of macrovascular thrombosis, such as venous thromboembolism, in ICU patients would suggest that heparin would have a greater beneficial effect, but, he added, “it may also suggest how advanced that process is, and perhaps heparin is not adequate to reverse the course at that point given relatively extensive thrombosis and associate organ failure.”
As clinicians have gained experience dealing with COVID-19, they’ve learned that infected patients carry a high burden of macro- and microthrombosis, Dr. Berger said, which may explain why critically ill patients didn’t respond as well to therapeutic levels of heparin. “I think the cat is out of the bag; patients who are severe are too ill to benefit,” he said. “I would think there’s too much microthrombosis that is already in their bodies.”
However, this doesn’t completely rule out therapeutic levels of heparin in critically ill COVID-19 patients. There are some scenarios where it’s needed, said Dr. Berger, associate professor of medicine and surgery and director of the Center for the Prevention of Cardiovascular Disease at New York University Langone Health. “Anyone who has a known clot already, like a known macrothrombosis in their leg or lung, needs to be on full-dose heparin,” he said.
That rationale can help reconcile the different outcomes in the critically and non–critically ill COVID-19 patients, wrote Hugo ten Cate, MD, PhD, of Maastricht University in the Netherlands, wrote in an accompanying editorial. But differences in the study populations may also explain the divergent outcomes, Dr. ten Cate noted.
The studies suggest that critically ill patients may need hon-heparin antithrombotic approaches “or even profibrinolytic strategies,” Dr. Cate wrote, and that the safety and effectiveness of thromboprophylaxis “remains an important question.” Nonetheless, he added, treating physicians must deal with the bleeding risk when using heparin or low-molecular-weight heparin in moderately ill COVID-19 patients.
Deepak L. Bhatt MD, MPH, of Brigham and Women’s Hospital Heart & Vascular Center, Boston, said in an interview that reconciling the two studies was “a bit challenging,” because effective therapies tend to have a greater impact in sicker patients.
“Of course, with antithrombotic therapies, bleeding side effects can sometimes overwhelm benefits in patients who are at high risk of both bleeding and ischemic complications, though that does not seem to be the explanation here,” Dr. Bhatt said. “I do think we need more data to clarify exactly which COVID patients benefit from various antithrombotic regimens, and fortunately, there are other ongoing studies, some of which will report relatively soon.”
He concurred with Dr. Berger that patients who need anticoagulation should receive it “apart from their COVID status,” Dr. Bhatt said. “Sick, hospitalized patients with or without COVID should receive appropriate prophylactic doses of anticoagulation.” However, he added, “Whether we should routinely go beyond that in COVID-positive inpatients, I think we need more data.”
The ATTACC platform received grants from the Canadian Institutes of Health Research and several other research foundations. The ACTIV-4a platform received funding from the National Heart, Lung, and Blood Institute. REMAP-CAP received funding from the European Union and several international research foundations, as well as Amgen and Eisai.
Dr. Lawler had no relationships to disclose. Dr. Berger disclosed receiving grants from the NHLBI, and financial relationships with AstraZeneca, Janssen, and Amgen outside the submitted work. Dr. ten Cate reported relationships with Alveron, Coagulation Profile, Portola/Alexion, Bayer, Pfizer, Stago, Leo Pharma, Daiichi, and Gilead/Galapagos. Dr. Bhatt is chair of the data safety and monitoring board of the FREEDOM COVID anticoagulation clinical trial.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Tachycardia syndrome may be distinct marker for long COVID
Tachycardia is commonly reported in patients with post-acute COVID-19 syndrome (PACS), also known as long COVID, authors report in a new article. The researchers say tachycardia syndrome should be considered a distinct phenotype.
The study by Marcus Ståhlberg, MD, PhD, of Karolinska University Hospital, Stockholm, and colleagues was published online August 11 in The American Journal of Medicine.
Dr. Ståhlberg told this news organization that although much attention has been paid to cases of clotting and perimyocarditis in patients after COVID, relatively little attention has been paid to tachycardia, despite case reports that show that palpitations are a common complaint.
“We have diagnosed a large number of patients with postural orthostatic tachycardia syndrome [POTS] and other forms of COVID-related tachycardia at our post-COVID outpatient clinic at Karolinska University Hospital and wanted to highlight this phenomenon,” he said.
Between 25% and 50% of patients at the clinic report tachycardia and/or palpitations that last 12 weeks or longer, the authors report.
“Systematic investigations suggest that 9% of Post-acute COVID-19 syndrome patients report palpitations at six months,” the authors write.
The findings also shed light on potential tests and treatments, he said.
“Physicians should be liberal in performing a basic cardiological workup, including an ECG [electrocardiogram], echocardiography, and Holter ECG monitoring in patients complaining of palpitations and/or chest pain,” Dr. Ståhlberg said.
“If orthostatic intolerance is also reported – such as vertigo, nausea, dyspnea – suspicion of POTS should be raised and a head-up tilt test or at least an active standing test should be performed,” he said.
If POTS is confirmed, he said, patients should be offered a heart rate–lowering drug, such as low-dose propranolol or ivabradine. Compression garments, increased fluid intake, and a structured rehabilitation program also help.
“According to our clinical experience, ivabradine can also reduce symptoms in patients with inappropriate sinus tachycardia and post-COVID,” Dr. Ståhlberg said. “Another finding on Holter-ECG to look out for is frequent premature extrasystoles, which could indicate myocarditis and should warrant a cardiac MRI.”
Dr. Ståhlberg said the researchers think the mechanism underlying the tachycardia is autoimmune and that primary SARS-CoV-2 infections trigger an autoimmune response with formation of autoantibodies that can activate receptors regulating blood pressure and heart rate.
Long-lasting symptoms from COVID are prevalent, the authors note, especially in patients who experienced severe forms of the disease.
In the longest follow-up study to date of patients hospitalized with COVID, more than 60% experienced fatigue or muscle weakness 6 months after hospitalization.
PACS should not be considered a single syndrome; the term denotes an array of subsyndromes and phenotypes, the authors write. Typical symptoms include headache, fatigue, dyspnea, and mental fog but can involve multiple organs and systems.
Tachycardia can also be used as a marker to help gauge the severity of long COVID, the authors write.
“[T]achycardia can be considered a universal and easily obtainable quantitative marker of Post-acute COVID-19 syndrome and its severity rather than patient-reported symptoms, blood testing, and thoracic CT-scans,” they write.
An underrecognized complication
Erin D. Michos, MD, MHS, director of women’s cardiovascular health and associate director of preventive cardiology at Johns Hopkins University, Baltimore, said in an interview that she has seen many similar symptoms in the long-COVID patients referred to her practice.
Dr. Michos, who is also an associate professor of medicine and epidemiology, said she’s been receiving a “huge number” of referrals of long-COVID patients with postural tachycardia, inappropriate sinus tachycardia, and POTS.
“I think this is all in the spectrum of autonomic dysfunction that has been recognized a lot since COVID. POTS has been thought to have [a potentially] viral cause that triggers an autoimmune response. Even before COVID, many patients had POTS triggered by a viral infection. The question is whether COVID-related POTS for long COVID is different from other kinds of POTS.”
She says she treats long-COVID patients who complain of elevated heart rates with many of the cardiac workup procedures the authors list and that she treats them in a way similar to the way she treats patients with POTS.
She recommends checking resting oxygen levels and having patients walk the halls and measure their oxygen levels after walking, because their elevated heart rate may be related to ongoing lung injury from COVID.
Eric Adler, MD, a cardiologist with University of San Diego Health, told this news organization that the findings by Dr. Ståhlberg and colleagues are consistent with what he’s seeing in his clinical practice.
Dr. Adler agrees with the authors that tachycardia is an underrecognized complication of long COVID.
He said the article represents further proof that though people may survive COVID, the threat of long-term symptoms, such as heart palpitations, is real and supports the case for vaccinations.
The authors, Dr. Michos, and Dr. Adler have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Tachycardia is commonly reported in patients with post-acute COVID-19 syndrome (PACS), also known as long COVID, authors report in a new article. The researchers say tachycardia syndrome should be considered a distinct phenotype.
The study by Marcus Ståhlberg, MD, PhD, of Karolinska University Hospital, Stockholm, and colleagues was published online August 11 in The American Journal of Medicine.
Dr. Ståhlberg told this news organization that although much attention has been paid to cases of clotting and perimyocarditis in patients after COVID, relatively little attention has been paid to tachycardia, despite case reports that show that palpitations are a common complaint.
“We have diagnosed a large number of patients with postural orthostatic tachycardia syndrome [POTS] and other forms of COVID-related tachycardia at our post-COVID outpatient clinic at Karolinska University Hospital and wanted to highlight this phenomenon,” he said.
Between 25% and 50% of patients at the clinic report tachycardia and/or palpitations that last 12 weeks or longer, the authors report.
“Systematic investigations suggest that 9% of Post-acute COVID-19 syndrome patients report palpitations at six months,” the authors write.
The findings also shed light on potential tests and treatments, he said.
“Physicians should be liberal in performing a basic cardiological workup, including an ECG [electrocardiogram], echocardiography, and Holter ECG monitoring in patients complaining of palpitations and/or chest pain,” Dr. Ståhlberg said.
“If orthostatic intolerance is also reported – such as vertigo, nausea, dyspnea – suspicion of POTS should be raised and a head-up tilt test or at least an active standing test should be performed,” he said.
If POTS is confirmed, he said, patients should be offered a heart rate–lowering drug, such as low-dose propranolol or ivabradine. Compression garments, increased fluid intake, and a structured rehabilitation program also help.
“According to our clinical experience, ivabradine can also reduce symptoms in patients with inappropriate sinus tachycardia and post-COVID,” Dr. Ståhlberg said. “Another finding on Holter-ECG to look out for is frequent premature extrasystoles, which could indicate myocarditis and should warrant a cardiac MRI.”
Dr. Ståhlberg said the researchers think the mechanism underlying the tachycardia is autoimmune and that primary SARS-CoV-2 infections trigger an autoimmune response with formation of autoantibodies that can activate receptors regulating blood pressure and heart rate.
Long-lasting symptoms from COVID are prevalent, the authors note, especially in patients who experienced severe forms of the disease.
In the longest follow-up study to date of patients hospitalized with COVID, more than 60% experienced fatigue or muscle weakness 6 months after hospitalization.
PACS should not be considered a single syndrome; the term denotes an array of subsyndromes and phenotypes, the authors write. Typical symptoms include headache, fatigue, dyspnea, and mental fog but can involve multiple organs and systems.
Tachycardia can also be used as a marker to help gauge the severity of long COVID, the authors write.
“[T]achycardia can be considered a universal and easily obtainable quantitative marker of Post-acute COVID-19 syndrome and its severity rather than patient-reported symptoms, blood testing, and thoracic CT-scans,” they write.
An underrecognized complication
Erin D. Michos, MD, MHS, director of women’s cardiovascular health and associate director of preventive cardiology at Johns Hopkins University, Baltimore, said in an interview that she has seen many similar symptoms in the long-COVID patients referred to her practice.
Dr. Michos, who is also an associate professor of medicine and epidemiology, said she’s been receiving a “huge number” of referrals of long-COVID patients with postural tachycardia, inappropriate sinus tachycardia, and POTS.
“I think this is all in the spectrum of autonomic dysfunction that has been recognized a lot since COVID. POTS has been thought to have [a potentially] viral cause that triggers an autoimmune response. Even before COVID, many patients had POTS triggered by a viral infection. The question is whether COVID-related POTS for long COVID is different from other kinds of POTS.”
She says she treats long-COVID patients who complain of elevated heart rates with many of the cardiac workup procedures the authors list and that she treats them in a way similar to the way she treats patients with POTS.
She recommends checking resting oxygen levels and having patients walk the halls and measure their oxygen levels after walking, because their elevated heart rate may be related to ongoing lung injury from COVID.
Eric Adler, MD, a cardiologist with University of San Diego Health, told this news organization that the findings by Dr. Ståhlberg and colleagues are consistent with what he’s seeing in his clinical practice.
Dr. Adler agrees with the authors that tachycardia is an underrecognized complication of long COVID.
He said the article represents further proof that though people may survive COVID, the threat of long-term symptoms, such as heart palpitations, is real and supports the case for vaccinations.
The authors, Dr. Michos, and Dr. Adler have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Tachycardia is commonly reported in patients with post-acute COVID-19 syndrome (PACS), also known as long COVID, authors report in a new article. The researchers say tachycardia syndrome should be considered a distinct phenotype.
The study by Marcus Ståhlberg, MD, PhD, of Karolinska University Hospital, Stockholm, and colleagues was published online August 11 in The American Journal of Medicine.
Dr. Ståhlberg told this news organization that although much attention has been paid to cases of clotting and perimyocarditis in patients after COVID, relatively little attention has been paid to tachycardia, despite case reports that show that palpitations are a common complaint.
“We have diagnosed a large number of patients with postural orthostatic tachycardia syndrome [POTS] and other forms of COVID-related tachycardia at our post-COVID outpatient clinic at Karolinska University Hospital and wanted to highlight this phenomenon,” he said.
Between 25% and 50% of patients at the clinic report tachycardia and/or palpitations that last 12 weeks or longer, the authors report.
“Systematic investigations suggest that 9% of Post-acute COVID-19 syndrome patients report palpitations at six months,” the authors write.
The findings also shed light on potential tests and treatments, he said.
“Physicians should be liberal in performing a basic cardiological workup, including an ECG [electrocardiogram], echocardiography, and Holter ECG monitoring in patients complaining of palpitations and/or chest pain,” Dr. Ståhlberg said.
“If orthostatic intolerance is also reported – such as vertigo, nausea, dyspnea – suspicion of POTS should be raised and a head-up tilt test or at least an active standing test should be performed,” he said.
If POTS is confirmed, he said, patients should be offered a heart rate–lowering drug, such as low-dose propranolol or ivabradine. Compression garments, increased fluid intake, and a structured rehabilitation program also help.
“According to our clinical experience, ivabradine can also reduce symptoms in patients with inappropriate sinus tachycardia and post-COVID,” Dr. Ståhlberg said. “Another finding on Holter-ECG to look out for is frequent premature extrasystoles, which could indicate myocarditis and should warrant a cardiac MRI.”
Dr. Ståhlberg said the researchers think the mechanism underlying the tachycardia is autoimmune and that primary SARS-CoV-2 infections trigger an autoimmune response with formation of autoantibodies that can activate receptors regulating blood pressure and heart rate.
Long-lasting symptoms from COVID are prevalent, the authors note, especially in patients who experienced severe forms of the disease.
In the longest follow-up study to date of patients hospitalized with COVID, more than 60% experienced fatigue or muscle weakness 6 months after hospitalization.
PACS should not be considered a single syndrome; the term denotes an array of subsyndromes and phenotypes, the authors write. Typical symptoms include headache, fatigue, dyspnea, and mental fog but can involve multiple organs and systems.
Tachycardia can also be used as a marker to help gauge the severity of long COVID, the authors write.
“[T]achycardia can be considered a universal and easily obtainable quantitative marker of Post-acute COVID-19 syndrome and its severity rather than patient-reported symptoms, blood testing, and thoracic CT-scans,” they write.
An underrecognized complication
Erin D. Michos, MD, MHS, director of women’s cardiovascular health and associate director of preventive cardiology at Johns Hopkins University, Baltimore, said in an interview that she has seen many similar symptoms in the long-COVID patients referred to her practice.
Dr. Michos, who is also an associate professor of medicine and epidemiology, said she’s been receiving a “huge number” of referrals of long-COVID patients with postural tachycardia, inappropriate sinus tachycardia, and POTS.
“I think this is all in the spectrum of autonomic dysfunction that has been recognized a lot since COVID. POTS has been thought to have [a potentially] viral cause that triggers an autoimmune response. Even before COVID, many patients had POTS triggered by a viral infection. The question is whether COVID-related POTS for long COVID is different from other kinds of POTS.”
She says she treats long-COVID patients who complain of elevated heart rates with many of the cardiac workup procedures the authors list and that she treats them in a way similar to the way she treats patients with POTS.
She recommends checking resting oxygen levels and having patients walk the halls and measure their oxygen levels after walking, because their elevated heart rate may be related to ongoing lung injury from COVID.
Eric Adler, MD, a cardiologist with University of San Diego Health, told this news organization that the findings by Dr. Ståhlberg and colleagues are consistent with what he’s seeing in his clinical practice.
Dr. Adler agrees with the authors that tachycardia is an underrecognized complication of long COVID.
He said the article represents further proof that though people may survive COVID, the threat of long-term symptoms, such as heart palpitations, is real and supports the case for vaccinations.
The authors, Dr. Michos, and Dr. Adler have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.








