User login
When atopic dermatitis is really contact dermatitis
ATLANTA – When patients present with atopic dermatitis that worsens, changes distribution, fails to improve, or immediately rebounds, think contact dermatitis, Luz Fonacier, MD, advised at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Clinical signs of contact dermatitis include lesions with an atypical distribution/pattern, such as head, eyelid, or cheilitis/perioral predominance, or lesions on the hand or foot. Also elevate your suspicion in patients with therapy-resistant hand eczema, adult- or childhood-onset atopic dermatitis without childhood eczema, as well as in cases of severe or widespread dermatitis prior to initiating a systemic immunosuppressant. The list of potential allergens to consider includes metal (especially nickel, cobalt, and potassium dichromate), fragrances such as formaldehyde and balsam of Peru, preservatives, as well as topical emollients, corticosteroids, antibiotics, and antiseptics.
Dr. Fonacier, professor of medicine at the State University of New York at Stony Brook and section head of allergy at Winthrop University Hospital, Mineola, N.Y., recommends loading acrylates, fragrances, and allergens in an aqueous vehicle immediately before application. She noted that delayed patch test readings are common to metals, topical antibiotics, and topical corticosteroids, and that positive reactions to gold are often not clinically relevant. “The patch test positivity of gold can be as high as 30% in adults and a little bit less in children, but results from two large studies show clinical relevance in only 10%-15% of cases,” she said. A trial of gold avoidance may be warranted in patients with suspected jewelry allergy, facial or eyelid dermatitis, or exposure through gold dental restorations.
She went on to share tips for reading skin patch tests. The first reading should be done after 48 hours, while the second should be done 3, 4, or 7 days after application. “The second reading helps distinguish irritant from allergic responses,” she said. “Thirty percent of negative tests at 48 hours may be positive on delayed readings.” Most true allergic reactions occur between 72 and 96 hours. Allergens that may peak early include thiuram mix, carba mix, and balsam of Peru. Those that disappear after 5 days include balsam of Peru, benzoic acid, disperse blue #124, fragrance mix, mercury, methyldibromo glutaronitrile, phenoxyethanol, and octyl gallate. Delayed patch test reactions after five days include metals (gold potassium dichromate, nickel, and cobalt), topical antibiotics (neomycin and bacitracin) as well as topic corticosteroids.
Resources she recommended to attendees include the American Contact Dermatitis Society and the Contact Dermatitis Institute. Health and safety information about household products can be found here.
Dr. Fonacier disclosed that she has received research and educational grants from Baxter and Genentech. She is also a consultant to Church and Dwight and Regeneron.
ATLANTA – When patients present with atopic dermatitis that worsens, changes distribution, fails to improve, or immediately rebounds, think contact dermatitis, Luz Fonacier, MD, advised at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Clinical signs of contact dermatitis include lesions with an atypical distribution/pattern, such as head, eyelid, or cheilitis/perioral predominance, or lesions on the hand or foot. Also elevate your suspicion in patients with therapy-resistant hand eczema, adult- or childhood-onset atopic dermatitis without childhood eczema, as well as in cases of severe or widespread dermatitis prior to initiating a systemic immunosuppressant. The list of potential allergens to consider includes metal (especially nickel, cobalt, and potassium dichromate), fragrances such as formaldehyde and balsam of Peru, preservatives, as well as topical emollients, corticosteroids, antibiotics, and antiseptics.
Dr. Fonacier, professor of medicine at the State University of New York at Stony Brook and section head of allergy at Winthrop University Hospital, Mineola, N.Y., recommends loading acrylates, fragrances, and allergens in an aqueous vehicle immediately before application. She noted that delayed patch test readings are common to metals, topical antibiotics, and topical corticosteroids, and that positive reactions to gold are often not clinically relevant. “The patch test positivity of gold can be as high as 30% in adults and a little bit less in children, but results from two large studies show clinical relevance in only 10%-15% of cases,” she said. A trial of gold avoidance may be warranted in patients with suspected jewelry allergy, facial or eyelid dermatitis, or exposure through gold dental restorations.
She went on to share tips for reading skin patch tests. The first reading should be done after 48 hours, while the second should be done 3, 4, or 7 days after application. “The second reading helps distinguish irritant from allergic responses,” she said. “Thirty percent of negative tests at 48 hours may be positive on delayed readings.” Most true allergic reactions occur between 72 and 96 hours. Allergens that may peak early include thiuram mix, carba mix, and balsam of Peru. Those that disappear after 5 days include balsam of Peru, benzoic acid, disperse blue #124, fragrance mix, mercury, methyldibromo glutaronitrile, phenoxyethanol, and octyl gallate. Delayed patch test reactions after five days include metals (gold potassium dichromate, nickel, and cobalt), topical antibiotics (neomycin and bacitracin) as well as topic corticosteroids.
Resources she recommended to attendees include the American Contact Dermatitis Society and the Contact Dermatitis Institute. Health and safety information about household products can be found here.
Dr. Fonacier disclosed that she has received research and educational grants from Baxter and Genentech. She is also a consultant to Church and Dwight and Regeneron.
ATLANTA – When patients present with atopic dermatitis that worsens, changes distribution, fails to improve, or immediately rebounds, think contact dermatitis, Luz Fonacier, MD, advised at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Clinical signs of contact dermatitis include lesions with an atypical distribution/pattern, such as head, eyelid, or cheilitis/perioral predominance, or lesions on the hand or foot. Also elevate your suspicion in patients with therapy-resistant hand eczema, adult- or childhood-onset atopic dermatitis without childhood eczema, as well as in cases of severe or widespread dermatitis prior to initiating a systemic immunosuppressant. The list of potential allergens to consider includes metal (especially nickel, cobalt, and potassium dichromate), fragrances such as formaldehyde and balsam of Peru, preservatives, as well as topical emollients, corticosteroids, antibiotics, and antiseptics.
Dr. Fonacier, professor of medicine at the State University of New York at Stony Brook and section head of allergy at Winthrop University Hospital, Mineola, N.Y., recommends loading acrylates, fragrances, and allergens in an aqueous vehicle immediately before application. She noted that delayed patch test readings are common to metals, topical antibiotics, and topical corticosteroids, and that positive reactions to gold are often not clinically relevant. “The patch test positivity of gold can be as high as 30% in adults and a little bit less in children, but results from two large studies show clinical relevance in only 10%-15% of cases,” she said. A trial of gold avoidance may be warranted in patients with suspected jewelry allergy, facial or eyelid dermatitis, or exposure through gold dental restorations.
She went on to share tips for reading skin patch tests. The first reading should be done after 48 hours, while the second should be done 3, 4, or 7 days after application. “The second reading helps distinguish irritant from allergic responses,” she said. “Thirty percent of negative tests at 48 hours may be positive on delayed readings.” Most true allergic reactions occur between 72 and 96 hours. Allergens that may peak early include thiuram mix, carba mix, and balsam of Peru. Those that disappear after 5 days include balsam of Peru, benzoic acid, disperse blue #124, fragrance mix, mercury, methyldibromo glutaronitrile, phenoxyethanol, and octyl gallate. Delayed patch test reactions after five days include metals (gold potassium dichromate, nickel, and cobalt), topical antibiotics (neomycin and bacitracin) as well as topic corticosteroids.
Resources she recommended to attendees include the American Contact Dermatitis Society and the Contact Dermatitis Institute. Health and safety information about household products can be found here.
Dr. Fonacier disclosed that she has received research and educational grants from Baxter and Genentech. She is also a consultant to Church and Dwight and Regeneron.
EXPERT ANALYSIS AT THE 2017 AAAAI ANNUAL MEETING
Long-term peanut sublingual immunotherapy found safe
ATLANTA – Peanut sublingual immunotherapy induces clinically significant desensitization in the majority of subjects and can induce sustained unresponsiveness in a subset of children treated for 36-60 months, results from a small study suggest.
“Sublingual immunotherapy [SLIT] is an easy-to-administer treatment that appears to be safe, and with extended treatment, may provide a clinically significant amount of protection with the potential for a lasting effect,” one of the study authors, Edwin H. Kim, MD, said in an interview in advance of the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
To find out, the researchers treated 37 patients with 2 mg of peanut SLIT for 36-60 months and then assessed a 5,000-mg peanut oral food challenge to further assess desensitization. Those who passed the challenge discontinued SLIT for 2-4 weeks and were then re-challenged with 5,000 mg of peanut protein to assess for sustained unresponsiveness.
“Existing data suggested that about 50% of patients on oral immunotherapy develop sustained unresponsiveness, which was defined by being able to tolerate the same full amount of peanut 1 month after stopping therapy,” Dr. Kim said. “As the assumption was that SLIT would have a more modest effect, it was unclear if any patients at all on SLIT would develop sustained unresponsiveness.”
Of the 37 subjects who completed the study, 32 (86%) safely ingested more than 300 mg of peanut and 12 (32%) passed the oral food challenge at the end of SLIT therapy. The median amount of peanut tolerated was 1,750 mg (compared with 1,710 mg in the original 12-month paper). The 12 subjects who passed the oral food challenge were re-challenged with 5,000 mg of peanut 2-4 weeks after discontinuing SLIT. Of these, 10 (27%) demonstrated sustained unresponsiveness. Dr. Kim characterized the results as “better than we would have expected.”
He acknowledged certain limitations to the study, including the lack of an entry food challenge to determine a baseline reaction threshold and the lack of a placebo arm for the study’s extended maintenance phase.
Dr. Kim reported having no financial disclosures.
[email protected]
ATLANTA – Peanut sublingual immunotherapy induces clinically significant desensitization in the majority of subjects and can induce sustained unresponsiveness in a subset of children treated for 36-60 months, results from a small study suggest.
“Sublingual immunotherapy [SLIT] is an easy-to-administer treatment that appears to be safe, and with extended treatment, may provide a clinically significant amount of protection with the potential for a lasting effect,” one of the study authors, Edwin H. Kim, MD, said in an interview in advance of the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
To find out, the researchers treated 37 patients with 2 mg of peanut SLIT for 36-60 months and then assessed a 5,000-mg peanut oral food challenge to further assess desensitization. Those who passed the challenge discontinued SLIT for 2-4 weeks and were then re-challenged with 5,000 mg of peanut protein to assess for sustained unresponsiveness.
“Existing data suggested that about 50% of patients on oral immunotherapy develop sustained unresponsiveness, which was defined by being able to tolerate the same full amount of peanut 1 month after stopping therapy,” Dr. Kim said. “As the assumption was that SLIT would have a more modest effect, it was unclear if any patients at all on SLIT would develop sustained unresponsiveness.”
Of the 37 subjects who completed the study, 32 (86%) safely ingested more than 300 mg of peanut and 12 (32%) passed the oral food challenge at the end of SLIT therapy. The median amount of peanut tolerated was 1,750 mg (compared with 1,710 mg in the original 12-month paper). The 12 subjects who passed the oral food challenge were re-challenged with 5,000 mg of peanut 2-4 weeks after discontinuing SLIT. Of these, 10 (27%) demonstrated sustained unresponsiveness. Dr. Kim characterized the results as “better than we would have expected.”
He acknowledged certain limitations to the study, including the lack of an entry food challenge to determine a baseline reaction threshold and the lack of a placebo arm for the study’s extended maintenance phase.
Dr. Kim reported having no financial disclosures.
[email protected]
ATLANTA – Peanut sublingual immunotherapy induces clinically significant desensitization in the majority of subjects and can induce sustained unresponsiveness in a subset of children treated for 36-60 months, results from a small study suggest.
“Sublingual immunotherapy [SLIT] is an easy-to-administer treatment that appears to be safe, and with extended treatment, may provide a clinically significant amount of protection with the potential for a lasting effect,” one of the study authors, Edwin H. Kim, MD, said in an interview in advance of the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
To find out, the researchers treated 37 patients with 2 mg of peanut SLIT for 36-60 months and then assessed a 5,000-mg peanut oral food challenge to further assess desensitization. Those who passed the challenge discontinued SLIT for 2-4 weeks and were then re-challenged with 5,000 mg of peanut protein to assess for sustained unresponsiveness.
“Existing data suggested that about 50% of patients on oral immunotherapy develop sustained unresponsiveness, which was defined by being able to tolerate the same full amount of peanut 1 month after stopping therapy,” Dr. Kim said. “As the assumption was that SLIT would have a more modest effect, it was unclear if any patients at all on SLIT would develop sustained unresponsiveness.”
Of the 37 subjects who completed the study, 32 (86%) safely ingested more than 300 mg of peanut and 12 (32%) passed the oral food challenge at the end of SLIT therapy. The median amount of peanut tolerated was 1,750 mg (compared with 1,710 mg in the original 12-month paper). The 12 subjects who passed the oral food challenge were re-challenged with 5,000 mg of peanut 2-4 weeks after discontinuing SLIT. Of these, 10 (27%) demonstrated sustained unresponsiveness. Dr. Kim characterized the results as “better than we would have expected.”
He acknowledged certain limitations to the study, including the lack of an entry food challenge to determine a baseline reaction threshold and the lack of a placebo arm for the study’s extended maintenance phase.
Dr. Kim reported having no financial disclosures.
[email protected]
AT THE 2017 AAAAI ANNUAL MEETING
Key clinical point:
Major finding: Of the children who completed the study, 86% safely ingested more than 300 mg of peanut and 32% passed the oral food challenge at the end of SLIT therapy.
Data source: A study of 37 patients who were treated with 2 mg of peanut SLIT for 36-60 months.
Disclosures: Dr. Kim reported having no financial disclosures.
Teledermatology shows potential for grading patch test results
ORLANDO – Store-and-forward teledermatology may be useful for grading patch test results.
Erin Warshaw, MD, and Sara Hylwa, MD, both of the University of Minnesota, Minneapolis, sought to compare readings of patch test results both in person and via store-and-forward teledermatology. They patch tested patients at the Hennepin County (Minn.) Medical Center with the North American Contact Dermatitis Group screening series; photos were obtained at the 48-hour reading and the final reading (96-160 hours).
Almost all (101 of 107) of patients eligible for the trial were enrolled. Patients were overwhelmingly female (72%) with an average age of 50 years in this single-site study. Most screening panels were applied to the back.
Teledermatology assessment was categorized as successful if it matched the in-person assessment and as a failure if it did not; investigators labeled assessed pairs that did not fully match as indeterminate. Successful matches indicated there was no clinically significant difference between teledermatology and in-person assessment, indeterminate matches indicated that there was possible clinically significant difference, and failure to match indicated definite clinically significant difference.
All readings that were negative both in person and via teledermatology were excluded from the analysis.
At 48 hours, 47.2% of 705 reading pairs were labeled successful and 51.3% were labeled indeterminate. Failure, or complete disagreement, occurred in 1.6%, or 11 individual antigen pairs.
More successes – and failures – were seen at the final reading, with 53.8% of 420 final readings labeled successful, 39.8% labeled indeterminate, and 6.4%, or 27 individual antigen pairs, labeled as failures.
In general, teledermatology was more likely to miss or downplay the severity of reactions in the indeterminate pairs, Dr. Warshaw said. “This makes intuitive sense because when you are with a patient live, often the lighting catches an irritant wrinkle reaction or you can feel the lesion and be much more likely to call it irritant or a mild reaction than you would be from a flat photo.”
In the failure group, teledermatology generally overstated reactions, she added.
Dr. Warshaw said that logistical changes would be needed to make teledermatology more effective for reading patch test reactions in her practice. Their method of marking the patch test grid is to use a surgical marker on the corners, but a highlighter to mark the grid between the antigens. The highlighter simply did not show up well in photographs, she noted.
While not perfect, teledermatology does have promise for reading patch test reactions, she added. “I would love to save patients from having to come for their 48-hour reading... In Minnesota we have these horrible snowstorms. Last week there was a blizzard that was predicted. A third of our patients live 2 hours away from the clinic. If they could have taken photographs instead of trying to come through a blizzard for their final reading, that would be helpful.”
Dr. Warshaw noted that their study assessed only the 70 antigens of the North American Contact Dermatitis Research Group series and that it could have been strengthened by using additional series or the patients’ own products.
[email protected]
On Twitter @denisefulton
ORLANDO – Store-and-forward teledermatology may be useful for grading patch test results.
Erin Warshaw, MD, and Sara Hylwa, MD, both of the University of Minnesota, Minneapolis, sought to compare readings of patch test results both in person and via store-and-forward teledermatology. They patch tested patients at the Hennepin County (Minn.) Medical Center with the North American Contact Dermatitis Group screening series; photos were obtained at the 48-hour reading and the final reading (96-160 hours).
Almost all (101 of 107) of patients eligible for the trial were enrolled. Patients were overwhelmingly female (72%) with an average age of 50 years in this single-site study. Most screening panels were applied to the back.
Teledermatology assessment was categorized as successful if it matched the in-person assessment and as a failure if it did not; investigators labeled assessed pairs that did not fully match as indeterminate. Successful matches indicated there was no clinically significant difference between teledermatology and in-person assessment, indeterminate matches indicated that there was possible clinically significant difference, and failure to match indicated definite clinically significant difference.
All readings that were negative both in person and via teledermatology were excluded from the analysis.
At 48 hours, 47.2% of 705 reading pairs were labeled successful and 51.3% were labeled indeterminate. Failure, or complete disagreement, occurred in 1.6%, or 11 individual antigen pairs.
More successes – and failures – were seen at the final reading, with 53.8% of 420 final readings labeled successful, 39.8% labeled indeterminate, and 6.4%, or 27 individual antigen pairs, labeled as failures.
In general, teledermatology was more likely to miss or downplay the severity of reactions in the indeterminate pairs, Dr. Warshaw said. “This makes intuitive sense because when you are with a patient live, often the lighting catches an irritant wrinkle reaction or you can feel the lesion and be much more likely to call it irritant or a mild reaction than you would be from a flat photo.”
In the failure group, teledermatology generally overstated reactions, she added.
Dr. Warshaw said that logistical changes would be needed to make teledermatology more effective for reading patch test reactions in her practice. Their method of marking the patch test grid is to use a surgical marker on the corners, but a highlighter to mark the grid between the antigens. The highlighter simply did not show up well in photographs, she noted.
While not perfect, teledermatology does have promise for reading patch test reactions, she added. “I would love to save patients from having to come for their 48-hour reading... In Minnesota we have these horrible snowstorms. Last week there was a blizzard that was predicted. A third of our patients live 2 hours away from the clinic. If they could have taken photographs instead of trying to come through a blizzard for their final reading, that would be helpful.”
Dr. Warshaw noted that their study assessed only the 70 antigens of the North American Contact Dermatitis Research Group series and that it could have been strengthened by using additional series or the patients’ own products.
[email protected]
On Twitter @denisefulton
ORLANDO – Store-and-forward teledermatology may be useful for grading patch test results.
Erin Warshaw, MD, and Sara Hylwa, MD, both of the University of Minnesota, Minneapolis, sought to compare readings of patch test results both in person and via store-and-forward teledermatology. They patch tested patients at the Hennepin County (Minn.) Medical Center with the North American Contact Dermatitis Group screening series; photos were obtained at the 48-hour reading and the final reading (96-160 hours).
Almost all (101 of 107) of patients eligible for the trial were enrolled. Patients were overwhelmingly female (72%) with an average age of 50 years in this single-site study. Most screening panels were applied to the back.
Teledermatology assessment was categorized as successful if it matched the in-person assessment and as a failure if it did not; investigators labeled assessed pairs that did not fully match as indeterminate. Successful matches indicated there was no clinically significant difference between teledermatology and in-person assessment, indeterminate matches indicated that there was possible clinically significant difference, and failure to match indicated definite clinically significant difference.
All readings that were negative both in person and via teledermatology were excluded from the analysis.
At 48 hours, 47.2% of 705 reading pairs were labeled successful and 51.3% were labeled indeterminate. Failure, or complete disagreement, occurred in 1.6%, or 11 individual antigen pairs.
More successes – and failures – were seen at the final reading, with 53.8% of 420 final readings labeled successful, 39.8% labeled indeterminate, and 6.4%, or 27 individual antigen pairs, labeled as failures.
In general, teledermatology was more likely to miss or downplay the severity of reactions in the indeterminate pairs, Dr. Warshaw said. “This makes intuitive sense because when you are with a patient live, often the lighting catches an irritant wrinkle reaction or you can feel the lesion and be much more likely to call it irritant or a mild reaction than you would be from a flat photo.”
In the failure group, teledermatology generally overstated reactions, she added.
Dr. Warshaw said that logistical changes would be needed to make teledermatology more effective for reading patch test reactions in her practice. Their method of marking the patch test grid is to use a surgical marker on the corners, but a highlighter to mark the grid between the antigens. The highlighter simply did not show up well in photographs, she noted.
While not perfect, teledermatology does have promise for reading patch test reactions, she added. “I would love to save patients from having to come for their 48-hour reading... In Minnesota we have these horrible snowstorms. Last week there was a blizzard that was predicted. A third of our patients live 2 hours away from the clinic. If they could have taken photographs instead of trying to come through a blizzard for their final reading, that would be helpful.”
Dr. Warshaw noted that their study assessed only the 70 antigens of the North American Contact Dermatitis Research Group series and that it could have been strengthened by using additional series or the patients’ own products.
[email protected]
On Twitter @denisefulton
Key clinical point:
Major finding: Teledermatology readings failed to match in-person final readings 6% of the time.
Data source: Single-site study of 101 patients patch tested with the North American Contact Dermatitis Group series.
Disclosures: Dr. Warshaw declared no relevant conflicts of interest.
Ecofriendly surfactant is allergen of the year
ORLANDO – Alkyl glucosides, mild surfactants derived from natural, sustainable sources, have been named allergen of the year by the American Contact Dermatitis Society.
The ecofriendly nature of these compounds has led to their inclusion in more personal care products in the last decade and a half. Alkyl glucosides are derived from coconut, palm, or rapeseed oil with glucose supplied by corn, wheat starch, or potatoes. They can be found in rinse-off products such as shampoos, shower gels, and liquid cleansers but also in leave-on products such as deodorants, sunscreens, and moisturizers, investigators said at the annual meeting of the American Contact Dermatitis Society, held just prior to the start of the American Academy of Dermatology’s annual meeting.
Camille Loranger, MD, of the department of dermatology, McGill University Health Center, Montreal, presented her institution’s experience with allergic contact dermatitis caused by alkyl glucosides. A total of 3,095 patients were patch tested at the clinic between January 2009 and June 2016. Researchers used the North American Contact Dermatitis Group 65-allergen series, which includes decyl glucoside (5% in petrolatum). Slightly more than half of patients (1,628) also were tested for reactions to lauryl glucoside (3% in petrolatum) as part of an additional cosmetic series. Twenty patients in the larger series reacted to decyl glucoside, while 15 of those who tested for lauryl glucoside reacted. Of those 15 patients, 6 were found to be allergic to decyl glucoside as well (Dermatitis. 2017 Jan/Feb;28[1]:5-13).
Allergy to alkyl glucosides became more common over time in the McGill series. The rate of positivity was low in the early years of the series, but increased from 1.37% of 437 patients in 2014 to 2.2% of 227 patients tested in the first half of 2016, Dr. Loranger said.
“Most of our patients were women with an average age of 48 years,” she added. “Body sites most commonly affected were the head and the hands. Only one case could be attributed to occupational exposure.”
Most patients – 86% – also were atopic (asthma, eczema, and rhinitis).
Products identified as most commonly causing a positive reaction were leave-on moisturizers and hand creams.
Donald V. Belsito, MD, professor of dermatology at Columbia University, N.Y., introduced the allergen of the year, pointing out that the compounds selected are not necessarily “bad actors.”
“The allergen of the year is really chosen to educate dermatologists about allergens that may be of low prevalence but a high relevance,” Dr. Belsito said. The allergens selected “are difficult to test for because they are tested for at irritant concentrations. It doesn’t mean they are these horrible substances that are damaging the world necessarily.”
The ACDS has been naming an allergen of the year since 2004.
[email protected]
On Twitter @denisefulton
ORLANDO – Alkyl glucosides, mild surfactants derived from natural, sustainable sources, have been named allergen of the year by the American Contact Dermatitis Society.
The ecofriendly nature of these compounds has led to their inclusion in more personal care products in the last decade and a half. Alkyl glucosides are derived from coconut, palm, or rapeseed oil with glucose supplied by corn, wheat starch, or potatoes. They can be found in rinse-off products such as shampoos, shower gels, and liquid cleansers but also in leave-on products such as deodorants, sunscreens, and moisturizers, investigators said at the annual meeting of the American Contact Dermatitis Society, held just prior to the start of the American Academy of Dermatology’s annual meeting.
Camille Loranger, MD, of the department of dermatology, McGill University Health Center, Montreal, presented her institution’s experience with allergic contact dermatitis caused by alkyl glucosides. A total of 3,095 patients were patch tested at the clinic between January 2009 and June 2016. Researchers used the North American Contact Dermatitis Group 65-allergen series, which includes decyl glucoside (5% in petrolatum). Slightly more than half of patients (1,628) also were tested for reactions to lauryl glucoside (3% in petrolatum) as part of an additional cosmetic series. Twenty patients in the larger series reacted to decyl glucoside, while 15 of those who tested for lauryl glucoside reacted. Of those 15 patients, 6 were found to be allergic to decyl glucoside as well (Dermatitis. 2017 Jan/Feb;28[1]:5-13).
Allergy to alkyl glucosides became more common over time in the McGill series. The rate of positivity was low in the early years of the series, but increased from 1.37% of 437 patients in 2014 to 2.2% of 227 patients tested in the first half of 2016, Dr. Loranger said.
“Most of our patients were women with an average age of 48 years,” she added. “Body sites most commonly affected were the head and the hands. Only one case could be attributed to occupational exposure.”
Most patients – 86% – also were atopic (asthma, eczema, and rhinitis).
Products identified as most commonly causing a positive reaction were leave-on moisturizers and hand creams.
Donald V. Belsito, MD, professor of dermatology at Columbia University, N.Y., introduced the allergen of the year, pointing out that the compounds selected are not necessarily “bad actors.”
“The allergen of the year is really chosen to educate dermatologists about allergens that may be of low prevalence but a high relevance,” Dr. Belsito said. The allergens selected “are difficult to test for because they are tested for at irritant concentrations. It doesn’t mean they are these horrible substances that are damaging the world necessarily.”
The ACDS has been naming an allergen of the year since 2004.
[email protected]
On Twitter @denisefulton
ORLANDO – Alkyl glucosides, mild surfactants derived from natural, sustainable sources, have been named allergen of the year by the American Contact Dermatitis Society.
The ecofriendly nature of these compounds has led to their inclusion in more personal care products in the last decade and a half. Alkyl glucosides are derived from coconut, palm, or rapeseed oil with glucose supplied by corn, wheat starch, or potatoes. They can be found in rinse-off products such as shampoos, shower gels, and liquid cleansers but also in leave-on products such as deodorants, sunscreens, and moisturizers, investigators said at the annual meeting of the American Contact Dermatitis Society, held just prior to the start of the American Academy of Dermatology’s annual meeting.
Camille Loranger, MD, of the department of dermatology, McGill University Health Center, Montreal, presented her institution’s experience with allergic contact dermatitis caused by alkyl glucosides. A total of 3,095 patients were patch tested at the clinic between January 2009 and June 2016. Researchers used the North American Contact Dermatitis Group 65-allergen series, which includes decyl glucoside (5% in petrolatum). Slightly more than half of patients (1,628) also were tested for reactions to lauryl glucoside (3% in petrolatum) as part of an additional cosmetic series. Twenty patients in the larger series reacted to decyl glucoside, while 15 of those who tested for lauryl glucoside reacted. Of those 15 patients, 6 were found to be allergic to decyl glucoside as well (Dermatitis. 2017 Jan/Feb;28[1]:5-13).
Allergy to alkyl glucosides became more common over time in the McGill series. The rate of positivity was low in the early years of the series, but increased from 1.37% of 437 patients in 2014 to 2.2% of 227 patients tested in the first half of 2016, Dr. Loranger said.
“Most of our patients were women with an average age of 48 years,” she added. “Body sites most commonly affected were the head and the hands. Only one case could be attributed to occupational exposure.”
Most patients – 86% – also were atopic (asthma, eczema, and rhinitis).
Products identified as most commonly causing a positive reaction were leave-on moisturizers and hand creams.
Donald V. Belsito, MD, professor of dermatology at Columbia University, N.Y., introduced the allergen of the year, pointing out that the compounds selected are not necessarily “bad actors.”
“The allergen of the year is really chosen to educate dermatologists about allergens that may be of low prevalence but a high relevance,” Dr. Belsito said. The allergens selected “are difficult to test for because they are tested for at irritant concentrations. It doesn’t mean they are these horrible substances that are damaging the world necessarily.”
The ACDS has been naming an allergen of the year since 2004.
[email protected]
On Twitter @denisefulton
Using Patch Testing to Identify Culprit Agents in Suspected Drug Eruptions
VIDEO: Consider PPIs as a cause of cutaneous reactions
WAILEA, HAWAII – Any proton pump inhibitor (PPI) has the potential to cause skin reactions, so it is important to ask patients about their use, according to J. Mark Jackson, MD, of the University of Louisville (Ky.).
If patients are going to react to a PPI, they usually will do so within 3 or 4 months of starting treatment, rather than in the first week or so of treatment, Dr. Jackson said in a video interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Skin reactions to PPIs are often maculopapular, with a flat and a raised component that can be nonspecific, Dr. Jackson noted.
Interestingly, he added, many times patients can switch to a different PPI and not get a skin reaction. However, there are some patients who develop a lupuslike reaction on the skin, and in these cases, there tends to be cross reactivity, “so they couldn’t switch to a different PPI and be risk-free” of the same reaction, he noted.
Dr. Jackson disclosed financial relationships with companies including AbbVie, Amgen, Celgene, Dermira, Galderma, Genentech, Janssen, Lilly, Medimetriks, Merck, Novartis, Pfizer, Promius, and Top MD.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, HAWAII – Any proton pump inhibitor (PPI) has the potential to cause skin reactions, so it is important to ask patients about their use, according to J. Mark Jackson, MD, of the University of Louisville (Ky.).
If patients are going to react to a PPI, they usually will do so within 3 or 4 months of starting treatment, rather than in the first week or so of treatment, Dr. Jackson said in a video interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Skin reactions to PPIs are often maculopapular, with a flat and a raised component that can be nonspecific, Dr. Jackson noted.
Interestingly, he added, many times patients can switch to a different PPI and not get a skin reaction. However, there are some patients who develop a lupuslike reaction on the skin, and in these cases, there tends to be cross reactivity, “so they couldn’t switch to a different PPI and be risk-free” of the same reaction, he noted.
Dr. Jackson disclosed financial relationships with companies including AbbVie, Amgen, Celgene, Dermira, Galderma, Genentech, Janssen, Lilly, Medimetriks, Merck, Novartis, Pfizer, Promius, and Top MD.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, HAWAII – Any proton pump inhibitor (PPI) has the potential to cause skin reactions, so it is important to ask patients about their use, according to J. Mark Jackson, MD, of the University of Louisville (Ky.).
If patients are going to react to a PPI, they usually will do so within 3 or 4 months of starting treatment, rather than in the first week or so of treatment, Dr. Jackson said in a video interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Skin reactions to PPIs are often maculopapular, with a flat and a raised component that can be nonspecific, Dr. Jackson noted.
Interestingly, he added, many times patients can switch to a different PPI and not get a skin reaction. However, there are some patients who develop a lupuslike reaction on the skin, and in these cases, there tends to be cross reactivity, “so they couldn’t switch to a different PPI and be risk-free” of the same reaction, he noted.
Dr. Jackson disclosed financial relationships with companies including AbbVie, Amgen, Celgene, Dermira, Galderma, Genentech, Janssen, Lilly, Medimetriks, Merck, Novartis, Pfizer, Promius, and Top MD.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF HAWAII DERMATOLOGY SEMINAR
Contact dermatitis gets personal
Parabens have been eliminated from many personal care products because of health concerns, but from a contact dermatitis standpoint, they have “very low rates of irritancy and allergenicity” and are considered safe and well tolerated, according to Jonathan I. Silverberg, MD, of Northwestern University, Chicago.
“I almost never see a positive reaction to parabens,” Dr. Silverberg said in a presentation on contact dermatitis at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
However, the confirmation of estrogenic activity associated with parabens has led to their replacement in many products – especially personal care products – with other
“MCI/MI is now a common cause of contact dermatitis and can cause severe reactions,” said Dr. Silverberg, director of the Northwestern Medicine Multidisciplinary Eczema Center in Chicago.
An alternative to MCI/MI – methyldibromoglutaronitrile/phenoxyethanol (Euxyl K 400) – also appears in personal care products, such as soaps and shampoos, as well as industrial products such as paints, glues, wood preservatives, and metal-working fluids, Dr. Silverberg noted. This preservative is relatively uncommon in the United States, and in Europe it has been banned from leave-on products since 2005 and from rinse-off products since 2007, he said.
Another paraben alternative, iodopropynyl butylcarbamate, is a relatively uncommon preservative, but it frequently occurs as a positive patch test reaction, Dr. Silverberg said.
Lanolin, a natural ingredient used in topical skin emollients and cosmetics, also has been associated with skin reactions, he added. In addition to personal care products, the increasing range of personal technology products can be sources of contact dermatitis, Dr. Silverberg pointed out. Consider nickel exposure not only from jewelry, but from items such as iPads, iPhones, laptops, and Xbox controllers, when evaluating contact dermatitis in adults and children, he said.
Dr. Silverberg disclosed relationships with companies including AbbVie, Anacor, Celgene, Chugai, Galderma, GlaxoSmithKline, Lilly, Puricore, Medimmune-AstraZeneca, Pfizer, Proctor & Gamble, Puricore, Hoffmann-La Roche, and Regeneron-Sanofi.
SDEF and this news organization are owned by the same parent company.
Parabens have been eliminated from many personal care products because of health concerns, but from a contact dermatitis standpoint, they have “very low rates of irritancy and allergenicity” and are considered safe and well tolerated, according to Jonathan I. Silverberg, MD, of Northwestern University, Chicago.
“I almost never see a positive reaction to parabens,” Dr. Silverberg said in a presentation on contact dermatitis at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
However, the confirmation of estrogenic activity associated with parabens has led to their replacement in many products – especially personal care products – with other
“MCI/MI is now a common cause of contact dermatitis and can cause severe reactions,” said Dr. Silverberg, director of the Northwestern Medicine Multidisciplinary Eczema Center in Chicago.
An alternative to MCI/MI – methyldibromoglutaronitrile/phenoxyethanol (Euxyl K 400) – also appears in personal care products, such as soaps and shampoos, as well as industrial products such as paints, glues, wood preservatives, and metal-working fluids, Dr. Silverberg noted. This preservative is relatively uncommon in the United States, and in Europe it has been banned from leave-on products since 2005 and from rinse-off products since 2007, he said.
Another paraben alternative, iodopropynyl butylcarbamate, is a relatively uncommon preservative, but it frequently occurs as a positive patch test reaction, Dr. Silverberg said.
Lanolin, a natural ingredient used in topical skin emollients and cosmetics, also has been associated with skin reactions, he added. In addition to personal care products, the increasing range of personal technology products can be sources of contact dermatitis, Dr. Silverberg pointed out. Consider nickel exposure not only from jewelry, but from items such as iPads, iPhones, laptops, and Xbox controllers, when evaluating contact dermatitis in adults and children, he said.
Dr. Silverberg disclosed relationships with companies including AbbVie, Anacor, Celgene, Chugai, Galderma, GlaxoSmithKline, Lilly, Puricore, Medimmune-AstraZeneca, Pfizer, Proctor & Gamble, Puricore, Hoffmann-La Roche, and Regeneron-Sanofi.
SDEF and this news organization are owned by the same parent company.
Parabens have been eliminated from many personal care products because of health concerns, but from a contact dermatitis standpoint, they have “very low rates of irritancy and allergenicity” and are considered safe and well tolerated, according to Jonathan I. Silverberg, MD, of Northwestern University, Chicago.
“I almost never see a positive reaction to parabens,” Dr. Silverberg said in a presentation on contact dermatitis at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
However, the confirmation of estrogenic activity associated with parabens has led to their replacement in many products – especially personal care products – with other
“MCI/MI is now a common cause of contact dermatitis and can cause severe reactions,” said Dr. Silverberg, director of the Northwestern Medicine Multidisciplinary Eczema Center in Chicago.
An alternative to MCI/MI – methyldibromoglutaronitrile/phenoxyethanol (Euxyl K 400) – also appears in personal care products, such as soaps and shampoos, as well as industrial products such as paints, glues, wood preservatives, and metal-working fluids, Dr. Silverberg noted. This preservative is relatively uncommon in the United States, and in Europe it has been banned from leave-on products since 2005 and from rinse-off products since 2007, he said.
Another paraben alternative, iodopropynyl butylcarbamate, is a relatively uncommon preservative, but it frequently occurs as a positive patch test reaction, Dr. Silverberg said.
Lanolin, a natural ingredient used in topical skin emollients and cosmetics, also has been associated with skin reactions, he added. In addition to personal care products, the increasing range of personal technology products can be sources of contact dermatitis, Dr. Silverberg pointed out. Consider nickel exposure not only from jewelry, but from items such as iPads, iPhones, laptops, and Xbox controllers, when evaluating contact dermatitis in adults and children, he said.
Dr. Silverberg disclosed relationships with companies including AbbVie, Anacor, Celgene, Chugai, Galderma, GlaxoSmithKline, Lilly, Puricore, Medimmune-AstraZeneca, Pfizer, Proctor & Gamble, Puricore, Hoffmann-La Roche, and Regeneron-Sanofi.
SDEF and this news organization are owned by the same parent company.
FROM SDEF HAWAII DERMATOLOGY SEMINAR
Questioning the Specificity and Sensitivity of ELISA for Bullous Pemphigoid Diagnosis
Bullous pemphigoid (BP) is the most common autoimmune blistering disease. The classic presentation of BP is a generalized, pruritic, bullous eruption in elderly patients, which is occasionally preceded by an urticarial prodrome. Immunopathologically, BP is characterized by IgG and sometimes IgE autoantibodies that target basement membrane zone proteins BP180 and BP230 of the epidermis.1
The diagnosis of BP should be suspected when an elderly patient presents with tense blisters and can be confirmed via diagnostic testing, including tissue histology and direct immunofluorescence (DIF) as the gold standard, as well as indirect immunofluorescence (IIF), enzyme-linked immunosorbent assay (ELISA), and most recently biochip technology as supportive tests.2 Since its advent, ELISA has gained popularity as a trustworthy diagnostic test for BP. The specificity of ELISA for BP diagnosis is reported to be 98% to 100%, which leads clinicians to believe that a positive ELISA equals certain diagnosis of BP; however, misdiagnosis of BP based on a positive ELISA result can occur.3-13 The treatment of BP often involves lifelong immunosuppressive therapy. Complications of immunosuppressive therapy contribute to morbidity and mortality in these patients, thus an accurate diagnosis is paramount before introducing therapy.14
We present the case of a 74-year-old man with a history of a pruritic nonbullous eruption who was diagnosed with BP and treated for 3 years based on positive ELISA results in the absence of confirmatory histology or DIF.
Case Report
A 74-year-old man with diabetes mellitus, hypertension, hyperlipidemia, benign prostatic hypertrophy, and obstructive sleep apnea presented for further evaluation and confirmation of a prior diagnosis of BP by an outside dermatologist. He reported a pruritic rash on the trunk, back, and extremities of 3 years’ duration. He denied occurrence of blisters at any time.
On presentation to an outside dermatologist 3 years ago, a biopsy was performed along with serologic studies due to the patient’s age and the possibility of an urticarial prodrome in BP. The biopsy revealed epidermal acanthosis, subepidermal separation, and a perivascular and interstitial infiltrate of lymphocytes and eosinophils in the papillary dermis. Direct immunofluorescence was nondiagnostic with a weak discontinuous pattern of IgG and IgA linearly along the basement membrane zone as well as few scattered and clumped cytoid bodies of IgM and IgA. Indirect immunofluoresence revealed a positive IgG titer of 1:40 on monkey esophagus substrate and a positive epidermal pattern on human split-skin substrate with a titer of 1:80. An ELISA for IgG autoantibodies against BP180 and BP230 yielded 15 U and 6 U, respectively (cut off value, 9 U). Based on the positive ELISA for IgG against BP180, a diagnosis of BP was made.
Over the following 3 years, the treatment included prednisone, tetracycline, nicotinamide, doxycycline, and dapsone. Therapy was suboptimal due to the patient’s comorbidities and socioeconomic status. Poorly controlled diabetes mellitus precluded consistent use of prednisone as recommended for BP. Tetracycline and nicotinamide were transiently effective in controlling the patient’s symptoms but were discontinued due to changes in his health insurance. Doxycycline and dapsone were ineffective. Throughout this 3-year period, the patient remained blister free, but the pruritic eruption was persistent.
The patient presented to our clinic due to his frustration with the lack of improvement and doubts about the BP diagnosis given the persistent absence of bullous lesions. Physical examination revealed numerous eroded, scaly, crusted papules on erythematous edematous plaques on all extremities, trunk, and back (Figure 1). The head, neck, face, and oral mucosa were spared. His history and clinical findings were atypical for BP and skin biopsies were performed. Histology revealed epidermal erosion with parakeratosis, spongiosis, and superficial perivascular lymphocytic inflammation with rare eosinophils without subepidermal split (Figure 2). Direct immunofluorescence was negative for IgG, IgA, IgM, C3, and C1q. Additionally, further review of the initial histology by another dermatopathologist revealed that the subepidermal separation reported was more likely artifactual clefts. These findings were not consistent with BP.
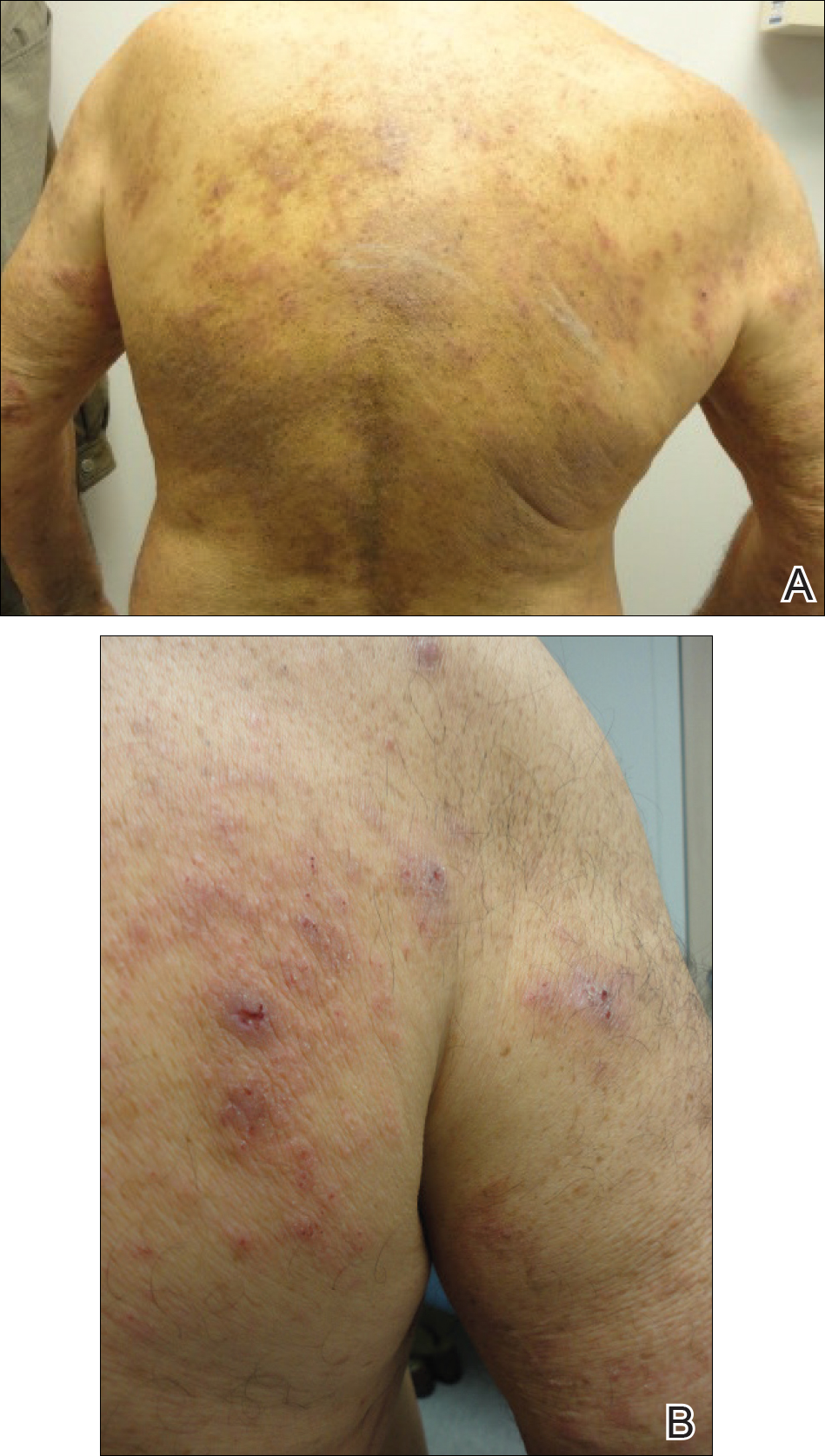

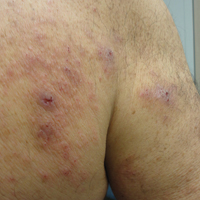
Given the patient’s clinical history, lack of bullae, and twice-negative DIF, the diagnosis was determined to be more consistent with eczematous spongiotic dermatitis. He refused a referral for phototherapy due to scheduling inconvenience. The patient was started on cyclosporine 0.5 mg/kg twice daily. After 10 days of treatment, he returned for follow-up and reported notable improvement in the pruritus. On physical examination, his dermatitis was improved with decreased erythema and inflammation.
The patient is being continued on extensive dry skin care with thick moisturizers and additional topical corticosteroid application on an as-needed basis.
Comment
Chronic immunosuppression contributes to morbidity and mortality in patients with BP; therefore, accurate diagnosis of BP is of utmost importance.14 A meta-analysis described ELISA as a test with high sensitivity and specificity (87% and 98%–100%, respectively) for diagnosis of BP.3 Nevertheless, there are opportunities for misdiagnosis using ELISA, as demonstrated in our case. To determine if the reported sensitivity and specificity of ELISA is accurate and reliable for clinical use, individual studies from the meta-analysis were reviewed.4,5,7-10,13,15 Issues identified in our review included dissimilar diagnostic procedures and patient populations among individual studies, several reports of positive ELISA in patients without BP, and a lack of explanation for these false-positive results.
There are notable differences in diagnostic procedures and patient populations among reports that establish the sensitivity and specificity of ELISA for BP diagnosis.3-13 Studies have detected IgG that targets the NC16A domain of the BP180 kD antigen, the C-terminal of the BP180 kD antigen, or the entire ectodomain of the BP180 kD antigen. Study patient populations varied in disease activity, stage, and treatment. Control patients included healthy patients as well as those with many dermatoses, including pemphigus vulgaris, systemic scleroderma, systemic lupus erythematosus, rheumatoid arthritis, lichen planus, and discoid lupus erythematosus.3-13 Due to these differences between individual studies, we believe the results that determine the overall sensitivity and specificity of ELISA for BP diagnosis must be interpreted with caution. For ELISA statistics to be clinically applicable to a specific patient, he/she should be similar to the patients studied. Therefore, we believe each study must be evaluated individually for applicability, given the differences that exist between them.
Furthermore, there have been several reports of false-positive ELISA results in patients with other dermatologic disorders, specifically in elderly patients with pruritus who do not fulfill clinical criteria for diagnosis with BP.16-18 In a population of elderly patients with pruritus for which no specific dermatological or systemic cause was identified, Hofmann et al18 found that 12% (3/25) of patients showed IgG reactivity to BP180 despite having negative DIF results. In another study of elderly patients with pruritic dermatoses, Feliciani et al17 found that 33% (5/15) of patients had IgG reactivity against BP230 or BP180, though they did not fulfill BP criteria based on clinical presentation and showed negative DIF and IIF results. These findings suggest that IgG reactivity against BP autoantibodies as determined by ELISA is not uncommon in pruritic diseases of the elderly.
Explanations for false-positive ELISA results were rare. A few authors suggested that false-positives could be attributed to an excessively low cutoff value,7-9 which was consistent with reports that the titer of autoantibodies to BP180 correlates with disease severity, suggesting that the higher titer of antibodies correlates with more severe disease and likely more accurate diagnosis.10,19,20 It is important to consider that patients who have low titers of BP180 autoantibodies with inconsistent clinical characteristics and DIF results may not truly have BP. Furthermore, to determine the clinical value of ELISA in identifying patients in the initial phase of BP, sera of BP patients should be compared with sera of elderly patients with pruritic skin disorders because they comprise the patient population that often requires diagnosis.18
Given the issues identified in our review of the literature, the published sensitivity and specificity of ELISA for BP diagnosis are likely overstated. In conclusion, ELISA should not be relied on as a single criterion adequate for diagnosis of BP.12,21 Rather, the diagnosis of BP can be obtained with a positive predictive value of 95% when a patient meets 3 of 4 clinical criteria (ie, absence of atrophic scars, absence of head and neck involvement, absence of mucosal involvement, and older than 70 years) and demonstrates linear deposits of predominantly IgG and/or C3 along the basement membrane zone of a perilesional biopsy on DIF.15 The gold standard for diagnosis of BP remains clinical presentation along with DIF, which can be supported by histology, IIF, and ELISA.22
- Delaporte E, Dubost-Brama A, Ghohestani R, et al. IgE autoantibodies directed against the major bullous pemphigoid antigen in patients with a severe form of pemphigoid. J Immunol. 1996;157:3642-3647.
- Schmidt E, Zillikens D. Diagnosis and clinical severity markers of bullous pemphigoid. F1000 Med Rep. 2009;1:15.
- Tampoia M, Giavarina D, Di Giorgio C, et al. Diagnostic accuracy of enzyme-linked immunosorbent assays (ELISA) to detect anti-skin autoantibodies in autoimmune blistering diseases: a systematic review and meta-analysis. Autoimmun Rev. 2012;12:121-126.
- Zillikens D, Mascaro JM, Rose PA, et al. A highly sensitive enzyme-linked immunosorbent assay for the detection of circulating anti-BP180 autoantibodies in patients with bullous pemphigoid. J Invest Dermatol. 1997;109:679-683.
- Sitaru C, Dahnrich C, Probst C, et al. Enzyme-linked immunosorbent assay using multimers of the 16th non-collagenous domain of the BP180 antigen for sensitive and specific detection of pemphigoid autoantibodies. Exp Dermatol. 2007;16:770-777.
- Yang B, Wang C, Chen S, et al. Evaluation of the combination of BP180-NC16a enzyme-linked immunosorbent assay and BP230 enzyme-linked immunosorbent assay in the diagnosis of bullous pemphigoid. Indian J Dermatol Venereol Leprol. 2012;78:722-727.
- Sakuma-Oyama Y, Powell AM, Oyama N, et al. Evaluation of a BP180-NC16a enzyme-linked immunosorbent assay in the initial diagnosis of bullous pemphigoid. Br J Dermatol. 2004;151:126-131.
- Tampoia M, Lattanzi V, Zucano A, et al. Evaluation of a new ELISA assay for detection of BP230 autoantibodies in bullous pemphigoid. Ann N Y Acad Sci. 2009;1173:15-20.
- Feng S, Lin L, Jin P, et al. Role of BP180NC16a-enzyme-linked immunosorbent assay (ELISA) in the diagnosis of bullous pemphigoid in China. Int J Dermatol. 2008;47:24-28.
- Kobayashi M, Amagai M, Kuroda-Kinoshita K, et al. BP180 ELISA using bacterial recombinant NC16a protein as a diagnostic and monitoring tool for bullous pemphigoid. J Dermatol Sci. 2002;30:224-232.
- Roussel A, Benichou J, Arivelo Randriamanantany Z, et al. Enzyme-linked immunosorbent assay for the combination of bullous pemphigoid antigens 1 and 2 in the diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147:293-298.
- Chan, Lawrence S. ELISA instead of indirect IF in patients with BP. Arch Dermatol. 2011;147:291-292.
- Barnadas MA, Rubiales V, González J, et al. Enzyme-linked immunosorbent assay (ELISA) and indirect immunofluorescence testing in a bullous pemphigoid and pemphigoid gestationis. Int J Dermatol. 2008;47:1245-1249.
- Borradori L, Bernard P. Pemphigoid group. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. New York, NY: Mosby; 2003:469.
- Vaillant L, Bernard P, Joly P, et al. Evaluation of clinical criteria for diagnosis of bullous pemphigoid. Arch Dermatol. 1998;134:1075-1080.
- Fania L, Caldarola G, Muller R, et al. IgE recognition of bullous pemphigoid (BP)180 and BP230 in BP patients and elderly individuals with pruritic dermatoses. Clin Immunol. 2012;143:236-245.
- Feliciani C, Caldarola G, Kneisel A, et al. IgG autoantibody reactivity against bullous pemphigoid (BP) 180 and BP230 in elderly patients with pruritic dermatoses. Br J Dermatol. 2009;61:306-312.
- Hofmann SC, Tamm K, Hertl M, et al. Diagnostic value of an enzyme-linked immunosorbent assay using BP180 recombinant proteins in elderly patients with pruritic skin disorders. Br J Dermatol. 2003;149:910-911.
- Schmidt E, Obe K, Brocker EB, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Arch Dermatol. 2000;136:174-178.
- Feng S, Wu Q, Jin P, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Int J Dermatol. 2008;47:225-228.
- Di Zenzo G, Joly P, Zambruno G, et al. Sensitivity of immunofluorescence studies vs enzyme-linked immunosorbent assay for diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147:1454-1456.
- Schmidt E, Zillikens D. Modern diagnosis of autoimmune blistering skin diseases. Autoimmun Rev. 2010;10:84-89.
Bullous pemphigoid (BP) is the most common autoimmune blistering disease. The classic presentation of BP is a generalized, pruritic, bullous eruption in elderly patients, which is occasionally preceded by an urticarial prodrome. Immunopathologically, BP is characterized by IgG and sometimes IgE autoantibodies that target basement membrane zone proteins BP180 and BP230 of the epidermis.1
The diagnosis of BP should be suspected when an elderly patient presents with tense blisters and can be confirmed via diagnostic testing, including tissue histology and direct immunofluorescence (DIF) as the gold standard, as well as indirect immunofluorescence (IIF), enzyme-linked immunosorbent assay (ELISA), and most recently biochip technology as supportive tests.2 Since its advent, ELISA has gained popularity as a trustworthy diagnostic test for BP. The specificity of ELISA for BP diagnosis is reported to be 98% to 100%, which leads clinicians to believe that a positive ELISA equals certain diagnosis of BP; however, misdiagnosis of BP based on a positive ELISA result can occur.3-13 The treatment of BP often involves lifelong immunosuppressive therapy. Complications of immunosuppressive therapy contribute to morbidity and mortality in these patients, thus an accurate diagnosis is paramount before introducing therapy.14
We present the case of a 74-year-old man with a history of a pruritic nonbullous eruption who was diagnosed with BP and treated for 3 years based on positive ELISA results in the absence of confirmatory histology or DIF.
Case Report
A 74-year-old man with diabetes mellitus, hypertension, hyperlipidemia, benign prostatic hypertrophy, and obstructive sleep apnea presented for further evaluation and confirmation of a prior diagnosis of BP by an outside dermatologist. He reported a pruritic rash on the trunk, back, and extremities of 3 years’ duration. He denied occurrence of blisters at any time.
On presentation to an outside dermatologist 3 years ago, a biopsy was performed along with serologic studies due to the patient’s age and the possibility of an urticarial prodrome in BP. The biopsy revealed epidermal acanthosis, subepidermal separation, and a perivascular and interstitial infiltrate of lymphocytes and eosinophils in the papillary dermis. Direct immunofluorescence was nondiagnostic with a weak discontinuous pattern of IgG and IgA linearly along the basement membrane zone as well as few scattered and clumped cytoid bodies of IgM and IgA. Indirect immunofluoresence revealed a positive IgG titer of 1:40 on monkey esophagus substrate and a positive epidermal pattern on human split-skin substrate with a titer of 1:80. An ELISA for IgG autoantibodies against BP180 and BP230 yielded 15 U and 6 U, respectively (cut off value, 9 U). Based on the positive ELISA for IgG against BP180, a diagnosis of BP was made.
Over the following 3 years, the treatment included prednisone, tetracycline, nicotinamide, doxycycline, and dapsone. Therapy was suboptimal due to the patient’s comorbidities and socioeconomic status. Poorly controlled diabetes mellitus precluded consistent use of prednisone as recommended for BP. Tetracycline and nicotinamide were transiently effective in controlling the patient’s symptoms but were discontinued due to changes in his health insurance. Doxycycline and dapsone were ineffective. Throughout this 3-year period, the patient remained blister free, but the pruritic eruption was persistent.
The patient presented to our clinic due to his frustration with the lack of improvement and doubts about the BP diagnosis given the persistent absence of bullous lesions. Physical examination revealed numerous eroded, scaly, crusted papules on erythematous edematous plaques on all extremities, trunk, and back (Figure 1). The head, neck, face, and oral mucosa were spared. His history and clinical findings were atypical for BP and skin biopsies were performed. Histology revealed epidermal erosion with parakeratosis, spongiosis, and superficial perivascular lymphocytic inflammation with rare eosinophils without subepidermal split (Figure 2). Direct immunofluorescence was negative for IgG, IgA, IgM, C3, and C1q. Additionally, further review of the initial histology by another dermatopathologist revealed that the subepidermal separation reported was more likely artifactual clefts. These findings were not consistent with BP.


Given the patient’s clinical history, lack of bullae, and twice-negative DIF, the diagnosis was determined to be more consistent with eczematous spongiotic dermatitis. He refused a referral for phototherapy due to scheduling inconvenience. The patient was started on cyclosporine 0.5 mg/kg twice daily. After 10 days of treatment, he returned for follow-up and reported notable improvement in the pruritus. On physical examination, his dermatitis was improved with decreased erythema and inflammation.
The patient is being continued on extensive dry skin care with thick moisturizers and additional topical corticosteroid application on an as-needed basis.
Comment
Chronic immunosuppression contributes to morbidity and mortality in patients with BP; therefore, accurate diagnosis of BP is of utmost importance.14 A meta-analysis described ELISA as a test with high sensitivity and specificity (87% and 98%–100%, respectively) for diagnosis of BP.3 Nevertheless, there are opportunities for misdiagnosis using ELISA, as demonstrated in our case. To determine if the reported sensitivity and specificity of ELISA is accurate and reliable for clinical use, individual studies from the meta-analysis were reviewed.4,5,7-10,13,15 Issues identified in our review included dissimilar diagnostic procedures and patient populations among individual studies, several reports of positive ELISA in patients without BP, and a lack of explanation for these false-positive results.
There are notable differences in diagnostic procedures and patient populations among reports that establish the sensitivity and specificity of ELISA for BP diagnosis.3-13 Studies have detected IgG that targets the NC16A domain of the BP180 kD antigen, the C-terminal of the BP180 kD antigen, or the entire ectodomain of the BP180 kD antigen. Study patient populations varied in disease activity, stage, and treatment. Control patients included healthy patients as well as those with many dermatoses, including pemphigus vulgaris, systemic scleroderma, systemic lupus erythematosus, rheumatoid arthritis, lichen planus, and discoid lupus erythematosus.3-13 Due to these differences between individual studies, we believe the results that determine the overall sensitivity and specificity of ELISA for BP diagnosis must be interpreted with caution. For ELISA statistics to be clinically applicable to a specific patient, he/she should be similar to the patients studied. Therefore, we believe each study must be evaluated individually for applicability, given the differences that exist between them.
Furthermore, there have been several reports of false-positive ELISA results in patients with other dermatologic disorders, specifically in elderly patients with pruritus who do not fulfill clinical criteria for diagnosis with BP.16-18 In a population of elderly patients with pruritus for which no specific dermatological or systemic cause was identified, Hofmann et al18 found that 12% (3/25) of patients showed IgG reactivity to BP180 despite having negative DIF results. In another study of elderly patients with pruritic dermatoses, Feliciani et al17 found that 33% (5/15) of patients had IgG reactivity against BP230 or BP180, though they did not fulfill BP criteria based on clinical presentation and showed negative DIF and IIF results. These findings suggest that IgG reactivity against BP autoantibodies as determined by ELISA is not uncommon in pruritic diseases of the elderly.
Explanations for false-positive ELISA results were rare. A few authors suggested that false-positives could be attributed to an excessively low cutoff value,7-9 which was consistent with reports that the titer of autoantibodies to BP180 correlates with disease severity, suggesting that the higher titer of antibodies correlates with more severe disease and likely more accurate diagnosis.10,19,20 It is important to consider that patients who have low titers of BP180 autoantibodies with inconsistent clinical characteristics and DIF results may not truly have BP. Furthermore, to determine the clinical value of ELISA in identifying patients in the initial phase of BP, sera of BP patients should be compared with sera of elderly patients with pruritic skin disorders because they comprise the patient population that often requires diagnosis.18
Given the issues identified in our review of the literature, the published sensitivity and specificity of ELISA for BP diagnosis are likely overstated. In conclusion, ELISA should not be relied on as a single criterion adequate for diagnosis of BP.12,21 Rather, the diagnosis of BP can be obtained with a positive predictive value of 95% when a patient meets 3 of 4 clinical criteria (ie, absence of atrophic scars, absence of head and neck involvement, absence of mucosal involvement, and older than 70 years) and demonstrates linear deposits of predominantly IgG and/or C3 along the basement membrane zone of a perilesional biopsy on DIF.15 The gold standard for diagnosis of BP remains clinical presentation along with DIF, which can be supported by histology, IIF, and ELISA.22
Bullous pemphigoid (BP) is the most common autoimmune blistering disease. The classic presentation of BP is a generalized, pruritic, bullous eruption in elderly patients, which is occasionally preceded by an urticarial prodrome. Immunopathologically, BP is characterized by IgG and sometimes IgE autoantibodies that target basement membrane zone proteins BP180 and BP230 of the epidermis.1
The diagnosis of BP should be suspected when an elderly patient presents with tense blisters and can be confirmed via diagnostic testing, including tissue histology and direct immunofluorescence (DIF) as the gold standard, as well as indirect immunofluorescence (IIF), enzyme-linked immunosorbent assay (ELISA), and most recently biochip technology as supportive tests.2 Since its advent, ELISA has gained popularity as a trustworthy diagnostic test for BP. The specificity of ELISA for BP diagnosis is reported to be 98% to 100%, which leads clinicians to believe that a positive ELISA equals certain diagnosis of BP; however, misdiagnosis of BP based on a positive ELISA result can occur.3-13 The treatment of BP often involves lifelong immunosuppressive therapy. Complications of immunosuppressive therapy contribute to morbidity and mortality in these patients, thus an accurate diagnosis is paramount before introducing therapy.14
We present the case of a 74-year-old man with a history of a pruritic nonbullous eruption who was diagnosed with BP and treated for 3 years based on positive ELISA results in the absence of confirmatory histology or DIF.
Case Report
A 74-year-old man with diabetes mellitus, hypertension, hyperlipidemia, benign prostatic hypertrophy, and obstructive sleep apnea presented for further evaluation and confirmation of a prior diagnosis of BP by an outside dermatologist. He reported a pruritic rash on the trunk, back, and extremities of 3 years’ duration. He denied occurrence of blisters at any time.
On presentation to an outside dermatologist 3 years ago, a biopsy was performed along with serologic studies due to the patient’s age and the possibility of an urticarial prodrome in BP. The biopsy revealed epidermal acanthosis, subepidermal separation, and a perivascular and interstitial infiltrate of lymphocytes and eosinophils in the papillary dermis. Direct immunofluorescence was nondiagnostic with a weak discontinuous pattern of IgG and IgA linearly along the basement membrane zone as well as few scattered and clumped cytoid bodies of IgM and IgA. Indirect immunofluoresence revealed a positive IgG titer of 1:40 on monkey esophagus substrate and a positive epidermal pattern on human split-skin substrate with a titer of 1:80. An ELISA for IgG autoantibodies against BP180 and BP230 yielded 15 U and 6 U, respectively (cut off value, 9 U). Based on the positive ELISA for IgG against BP180, a diagnosis of BP was made.
Over the following 3 years, the treatment included prednisone, tetracycline, nicotinamide, doxycycline, and dapsone. Therapy was suboptimal due to the patient’s comorbidities and socioeconomic status. Poorly controlled diabetes mellitus precluded consistent use of prednisone as recommended for BP. Tetracycline and nicotinamide were transiently effective in controlling the patient’s symptoms but were discontinued due to changes in his health insurance. Doxycycline and dapsone were ineffective. Throughout this 3-year period, the patient remained blister free, but the pruritic eruption was persistent.
The patient presented to our clinic due to his frustration with the lack of improvement and doubts about the BP diagnosis given the persistent absence of bullous lesions. Physical examination revealed numerous eroded, scaly, crusted papules on erythematous edematous plaques on all extremities, trunk, and back (Figure 1). The head, neck, face, and oral mucosa were spared. His history and clinical findings were atypical for BP and skin biopsies were performed. Histology revealed epidermal erosion with parakeratosis, spongiosis, and superficial perivascular lymphocytic inflammation with rare eosinophils without subepidermal split (Figure 2). Direct immunofluorescence was negative for IgG, IgA, IgM, C3, and C1q. Additionally, further review of the initial histology by another dermatopathologist revealed that the subepidermal separation reported was more likely artifactual clefts. These findings were not consistent with BP.


Given the patient’s clinical history, lack of bullae, and twice-negative DIF, the diagnosis was determined to be more consistent with eczematous spongiotic dermatitis. He refused a referral for phototherapy due to scheduling inconvenience. The patient was started on cyclosporine 0.5 mg/kg twice daily. After 10 days of treatment, he returned for follow-up and reported notable improvement in the pruritus. On physical examination, his dermatitis was improved with decreased erythema and inflammation.
The patient is being continued on extensive dry skin care with thick moisturizers and additional topical corticosteroid application on an as-needed basis.
Comment
Chronic immunosuppression contributes to morbidity and mortality in patients with BP; therefore, accurate diagnosis of BP is of utmost importance.14 A meta-analysis described ELISA as a test with high sensitivity and specificity (87% and 98%–100%, respectively) for diagnosis of BP.3 Nevertheless, there are opportunities for misdiagnosis using ELISA, as demonstrated in our case. To determine if the reported sensitivity and specificity of ELISA is accurate and reliable for clinical use, individual studies from the meta-analysis were reviewed.4,5,7-10,13,15 Issues identified in our review included dissimilar diagnostic procedures and patient populations among individual studies, several reports of positive ELISA in patients without BP, and a lack of explanation for these false-positive results.
There are notable differences in diagnostic procedures and patient populations among reports that establish the sensitivity and specificity of ELISA for BP diagnosis.3-13 Studies have detected IgG that targets the NC16A domain of the BP180 kD antigen, the C-terminal of the BP180 kD antigen, or the entire ectodomain of the BP180 kD antigen. Study patient populations varied in disease activity, stage, and treatment. Control patients included healthy patients as well as those with many dermatoses, including pemphigus vulgaris, systemic scleroderma, systemic lupus erythematosus, rheumatoid arthritis, lichen planus, and discoid lupus erythematosus.3-13 Due to these differences between individual studies, we believe the results that determine the overall sensitivity and specificity of ELISA for BP diagnosis must be interpreted with caution. For ELISA statistics to be clinically applicable to a specific patient, he/she should be similar to the patients studied. Therefore, we believe each study must be evaluated individually for applicability, given the differences that exist between them.
Furthermore, there have been several reports of false-positive ELISA results in patients with other dermatologic disorders, specifically in elderly patients with pruritus who do not fulfill clinical criteria for diagnosis with BP.16-18 In a population of elderly patients with pruritus for which no specific dermatological or systemic cause was identified, Hofmann et al18 found that 12% (3/25) of patients showed IgG reactivity to BP180 despite having negative DIF results. In another study of elderly patients with pruritic dermatoses, Feliciani et al17 found that 33% (5/15) of patients had IgG reactivity against BP230 or BP180, though they did not fulfill BP criteria based on clinical presentation and showed negative DIF and IIF results. These findings suggest that IgG reactivity against BP autoantibodies as determined by ELISA is not uncommon in pruritic diseases of the elderly.
Explanations for false-positive ELISA results were rare. A few authors suggested that false-positives could be attributed to an excessively low cutoff value,7-9 which was consistent with reports that the titer of autoantibodies to BP180 correlates with disease severity, suggesting that the higher titer of antibodies correlates with more severe disease and likely more accurate diagnosis.10,19,20 It is important to consider that patients who have low titers of BP180 autoantibodies with inconsistent clinical characteristics and DIF results may not truly have BP. Furthermore, to determine the clinical value of ELISA in identifying patients in the initial phase of BP, sera of BP patients should be compared with sera of elderly patients with pruritic skin disorders because they comprise the patient population that often requires diagnosis.18
Given the issues identified in our review of the literature, the published sensitivity and specificity of ELISA for BP diagnosis are likely overstated. In conclusion, ELISA should not be relied on as a single criterion adequate for diagnosis of BP.12,21 Rather, the diagnosis of BP can be obtained with a positive predictive value of 95% when a patient meets 3 of 4 clinical criteria (ie, absence of atrophic scars, absence of head and neck involvement, absence of mucosal involvement, and older than 70 years) and demonstrates linear deposits of predominantly IgG and/or C3 along the basement membrane zone of a perilesional biopsy on DIF.15 The gold standard for diagnosis of BP remains clinical presentation along with DIF, which can be supported by histology, IIF, and ELISA.22
- Delaporte E, Dubost-Brama A, Ghohestani R, et al. IgE autoantibodies directed against the major bullous pemphigoid antigen in patients with a severe form of pemphigoid. J Immunol. 1996;157:3642-3647.
- Schmidt E, Zillikens D. Diagnosis and clinical severity markers of bullous pemphigoid. F1000 Med Rep. 2009;1:15.
- Tampoia M, Giavarina D, Di Giorgio C, et al. Diagnostic accuracy of enzyme-linked immunosorbent assays (ELISA) to detect anti-skin autoantibodies in autoimmune blistering diseases: a systematic review and meta-analysis. Autoimmun Rev. 2012;12:121-126.
- Zillikens D, Mascaro JM, Rose PA, et al. A highly sensitive enzyme-linked immunosorbent assay for the detection of circulating anti-BP180 autoantibodies in patients with bullous pemphigoid. J Invest Dermatol. 1997;109:679-683.
- Sitaru C, Dahnrich C, Probst C, et al. Enzyme-linked immunosorbent assay using multimers of the 16th non-collagenous domain of the BP180 antigen for sensitive and specific detection of pemphigoid autoantibodies. Exp Dermatol. 2007;16:770-777.
- Yang B, Wang C, Chen S, et al. Evaluation of the combination of BP180-NC16a enzyme-linked immunosorbent assay and BP230 enzyme-linked immunosorbent assay in the diagnosis of bullous pemphigoid. Indian J Dermatol Venereol Leprol. 2012;78:722-727.
- Sakuma-Oyama Y, Powell AM, Oyama N, et al. Evaluation of a BP180-NC16a enzyme-linked immunosorbent assay in the initial diagnosis of bullous pemphigoid. Br J Dermatol. 2004;151:126-131.
- Tampoia M, Lattanzi V, Zucano A, et al. Evaluation of a new ELISA assay for detection of BP230 autoantibodies in bullous pemphigoid. Ann N Y Acad Sci. 2009;1173:15-20.
- Feng S, Lin L, Jin P, et al. Role of BP180NC16a-enzyme-linked immunosorbent assay (ELISA) in the diagnosis of bullous pemphigoid in China. Int J Dermatol. 2008;47:24-28.
- Kobayashi M, Amagai M, Kuroda-Kinoshita K, et al. BP180 ELISA using bacterial recombinant NC16a protein as a diagnostic and monitoring tool for bullous pemphigoid. J Dermatol Sci. 2002;30:224-232.
- Roussel A, Benichou J, Arivelo Randriamanantany Z, et al. Enzyme-linked immunosorbent assay for the combination of bullous pemphigoid antigens 1 and 2 in the diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147:293-298.
- Chan, Lawrence S. ELISA instead of indirect IF in patients with BP. Arch Dermatol. 2011;147:291-292.
- Barnadas MA, Rubiales V, González J, et al. Enzyme-linked immunosorbent assay (ELISA) and indirect immunofluorescence testing in a bullous pemphigoid and pemphigoid gestationis. Int J Dermatol. 2008;47:1245-1249.
- Borradori L, Bernard P. Pemphigoid group. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. New York, NY: Mosby; 2003:469.
- Vaillant L, Bernard P, Joly P, et al. Evaluation of clinical criteria for diagnosis of bullous pemphigoid. Arch Dermatol. 1998;134:1075-1080.
- Fania L, Caldarola G, Muller R, et al. IgE recognition of bullous pemphigoid (BP)180 and BP230 in BP patients and elderly individuals with pruritic dermatoses. Clin Immunol. 2012;143:236-245.
- Feliciani C, Caldarola G, Kneisel A, et al. IgG autoantibody reactivity against bullous pemphigoid (BP) 180 and BP230 in elderly patients with pruritic dermatoses. Br J Dermatol. 2009;61:306-312.
- Hofmann SC, Tamm K, Hertl M, et al. Diagnostic value of an enzyme-linked immunosorbent assay using BP180 recombinant proteins in elderly patients with pruritic skin disorders. Br J Dermatol. 2003;149:910-911.
- Schmidt E, Obe K, Brocker EB, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Arch Dermatol. 2000;136:174-178.
- Feng S, Wu Q, Jin P, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Int J Dermatol. 2008;47:225-228.
- Di Zenzo G, Joly P, Zambruno G, et al. Sensitivity of immunofluorescence studies vs enzyme-linked immunosorbent assay for diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147:1454-1456.
- Schmidt E, Zillikens D. Modern diagnosis of autoimmune blistering skin diseases. Autoimmun Rev. 2010;10:84-89.
- Delaporte E, Dubost-Brama A, Ghohestani R, et al. IgE autoantibodies directed against the major bullous pemphigoid antigen in patients with a severe form of pemphigoid. J Immunol. 1996;157:3642-3647.
- Schmidt E, Zillikens D. Diagnosis and clinical severity markers of bullous pemphigoid. F1000 Med Rep. 2009;1:15.
- Tampoia M, Giavarina D, Di Giorgio C, et al. Diagnostic accuracy of enzyme-linked immunosorbent assays (ELISA) to detect anti-skin autoantibodies in autoimmune blistering diseases: a systematic review and meta-analysis. Autoimmun Rev. 2012;12:121-126.
- Zillikens D, Mascaro JM, Rose PA, et al. A highly sensitive enzyme-linked immunosorbent assay for the detection of circulating anti-BP180 autoantibodies in patients with bullous pemphigoid. J Invest Dermatol. 1997;109:679-683.
- Sitaru C, Dahnrich C, Probst C, et al. Enzyme-linked immunosorbent assay using multimers of the 16th non-collagenous domain of the BP180 antigen for sensitive and specific detection of pemphigoid autoantibodies. Exp Dermatol. 2007;16:770-777.
- Yang B, Wang C, Chen S, et al. Evaluation of the combination of BP180-NC16a enzyme-linked immunosorbent assay and BP230 enzyme-linked immunosorbent assay in the diagnosis of bullous pemphigoid. Indian J Dermatol Venereol Leprol. 2012;78:722-727.
- Sakuma-Oyama Y, Powell AM, Oyama N, et al. Evaluation of a BP180-NC16a enzyme-linked immunosorbent assay in the initial diagnosis of bullous pemphigoid. Br J Dermatol. 2004;151:126-131.
- Tampoia M, Lattanzi V, Zucano A, et al. Evaluation of a new ELISA assay for detection of BP230 autoantibodies in bullous pemphigoid. Ann N Y Acad Sci. 2009;1173:15-20.
- Feng S, Lin L, Jin P, et al. Role of BP180NC16a-enzyme-linked immunosorbent assay (ELISA) in the diagnosis of bullous pemphigoid in China. Int J Dermatol. 2008;47:24-28.
- Kobayashi M, Amagai M, Kuroda-Kinoshita K, et al. BP180 ELISA using bacterial recombinant NC16a protein as a diagnostic and monitoring tool for bullous pemphigoid. J Dermatol Sci. 2002;30:224-232.
- Roussel A, Benichou J, Arivelo Randriamanantany Z, et al. Enzyme-linked immunosorbent assay for the combination of bullous pemphigoid antigens 1 and 2 in the diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147:293-298.
- Chan, Lawrence S. ELISA instead of indirect IF in patients with BP. Arch Dermatol. 2011;147:291-292.
- Barnadas MA, Rubiales V, González J, et al. Enzyme-linked immunosorbent assay (ELISA) and indirect immunofluorescence testing in a bullous pemphigoid and pemphigoid gestationis. Int J Dermatol. 2008;47:1245-1249.
- Borradori L, Bernard P. Pemphigoid group. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. New York, NY: Mosby; 2003:469.
- Vaillant L, Bernard P, Joly P, et al. Evaluation of clinical criteria for diagnosis of bullous pemphigoid. Arch Dermatol. 1998;134:1075-1080.
- Fania L, Caldarola G, Muller R, et al. IgE recognition of bullous pemphigoid (BP)180 and BP230 in BP patients and elderly individuals with pruritic dermatoses. Clin Immunol. 2012;143:236-245.
- Feliciani C, Caldarola G, Kneisel A, et al. IgG autoantibody reactivity against bullous pemphigoid (BP) 180 and BP230 in elderly patients with pruritic dermatoses. Br J Dermatol. 2009;61:306-312.
- Hofmann SC, Tamm K, Hertl M, et al. Diagnostic value of an enzyme-linked immunosorbent assay using BP180 recombinant proteins in elderly patients with pruritic skin disorders. Br J Dermatol. 2003;149:910-911.
- Schmidt E, Obe K, Brocker EB, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Arch Dermatol. 2000;136:174-178.
- Feng S, Wu Q, Jin P, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Int J Dermatol. 2008;47:225-228.
- Di Zenzo G, Joly P, Zambruno G, et al. Sensitivity of immunofluorescence studies vs enzyme-linked immunosorbent assay for diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147:1454-1456.
- Schmidt E, Zillikens D. Modern diagnosis of autoimmune blistering skin diseases. Autoimmun Rev. 2010;10:84-89.
Practice Points
- A low serum level of autoantibodies to BP180 should be interpreted with caution because it is more likely to represent a false-positive than a high serum level.
- Rely on the gold standard for diagnosis of bullous pemphigoid: clinical presentation along with direct immunofluorescence, which can be supported by histology, indirect immunofluorescence, and enzyme-linked immunosorbent assay (ELISA) rather than ELISA alone.
Patch Testing for Adverse Drug Reactions
Adverse drug reactions account for 3% to 6% of hospital admissions in the United States and occur in 10% to 15% of hospitalized patients.1,2 The most common culprits are antibiotics and nonsteroidal anti-inflammatory drugs (NSAIDs).3-12 In most cases, diagnoses are made clinically without diagnostic testing. To identify drug allergies associated with diagnostic testing, one center selected patients with suspected cutaneous drug reactions (2006-2010) for further evaluation.13 Of 612 patients who were evaluated, 141 had a high suspicion of drug allergy and were included in the analysis. The excluded patients had pseudoallergic reactions, reactive exanthemas due to infection, histopathologic exclusion of drug allergy, angioedema, or other dermatological conditions such as contact dermatitis and eczema. Of the included patients, 107 were diagnosed with drug reactions, while the remainder had non–drug-related exanthemas or unknown etiology after testing. Identified culprit drugs were predominantly antibiotics (39.8%) and NSAIDs (21.2%); contrast media, anticoagulants, anticonvulsants, antimalarials, antifungals, glucocorticoids, antihypertensives, and proton pump inhibitors also were implicated. They were identified with skin prick, intradermal, and patch tests (62.6%); lymphocyte transformation test (17.7%); oral rechallenge (5.6%); or without skin testing (6.5%). One quarter of patients with a high suspicion for drug allergy did not have a confirmed drug eruption in this study. Another study found that 10% to 20% of patients with reported penicillin allergy had confirmation via skin prick testing.14 These findings suggest that confirmation of suspected drug allergy may require more than one diagnostic test.
Tests for Adverse Drug Reactions
The following tests have been shown to aid in the identification of cutaneous drug eruptions: (1) patch tests15-21; (2) intradermal tests14,15,19,20; (3) drug provocation tests15,20; and (4) lymphocyte transformation tests.20 Intradermal or skin prick tests are most useful in urticarial eruptions but can be considered in nonurticarial eruptions with delayed inspection of test sites up to 1 week after testing. Drug provocation tests are considered the gold standard but involve patient risk. Lymphocyte transformation tests use the principle that T lymphocytes proliferate in the presence of drugs to which the patient is sensitized. Patch tests will be discussed in greater detail below. Immunohistochemistry can determine immunologic mechanisms of eruptions but cannot identify causative agents.16,17,22
A retrospective study of patients referred for evaluation of adverse drug reactions between 1996 and 2006 found the collective negative predictive value (NPV)—the percentage of truly negative skin tests based on provocation or substitution testing—of cutaneous drug tests including patch, prick, and intradermal tests to be 89.6% (95% confidence interval, 85.9%-93.3%).23 The NPVs of each test were not reported. Patients with negative cutaneous tests had subsequent oral rechallenge or substitution testing with medication from the same drug class.23 Another study16 found the NPV of patch testing to be at least 79% after review of data from other studies using patch and provocation testing.16,24 These studies suggest that cutaneous testing can be useful, albeit imperfect, in the evaluation and diagnosis of drug allergy.
Review of the Patch Test
Patch tests can be helpful in diagnosis of delayed hypersensitivities.18 Patch testing is most commonly and effectively used to diagnose allergic contact dermatitis, but its utility in other applications, such as diagnosis of cutaneous drug eruptions, has not been extensively studied.
The development of patch tests to diagnose systemic drug allergies is inhibited by the uncertainty of percutaneous drug penetration, a dearth of studies to determine the best test concentrations of active drug in the patch test, and the potential for nonimmunologic contact urticaria upon skin exposure. Furthermore, cutaneous metabolism of many antigens is well documented, but correlation to systemic metabolism often is unknown, which can confound patch test results and lead to false-negative results when the skin’s metabolic capacity does not match the body’s capacity to generate antigens capable of eliciting immunogenic responses.21 Additionally, the method used to suspend and disperse drugs in patch test vehicles is unfamiliar to most pharmacists, and standardized concentrations and vehicles are available only for some medications.25 Studies sufficient to obtain US Food and Drug Administration approval of patch tests for systemic drug eruptions would be costly and therefore prohibitive to investigators. The majority of the literature consists of case reports and data extrapolated from reviews. Patch test results of many drugs have been reported in the literature, with the highest frequencies of positive results associated with anticonvulsants,26 antibiotics, corticosteroids, calcium channel blockers, and benzodiazepines.21
Patch test placement affects the diagnostic value of the test. Placing patch tests on previously involved sites of fixed drug eruptions improves yield over placement on uninvolved skin.27 Placing patch tests on previously involved sites of other drug eruptions such as toxic epidermal necrolysis also may aid in diagnosis, though the literature is sparse.25,26,28
Patch Testing in Drug Eruptions
Morbilliform eruptions account for 48% to 91% of patients with adverse drug reactions.4-6 Other drug eruptions include urticarial eruptions, acute generalized exanthematous pustulosis (AGEP), drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, toxic epidermal necrolysis, Stevens-Johnson syndrome, lichenoid drug eruption, symmetric drug-related intertriginous and flexural exanthema (SDRIFE), erythema multiforme (EM), and systemic contact dermatitis. The Table summarizes reports of positive patch tests with various medications for these drug eruptions.
In general, antimicrobials and NSAIDs were the most implicated drugs with positive patch test results in AGEP, DRESS syndrome, EM, fixed drug eruptions, and morbilliform eruptions. In AGEP, positive results also were reported for other drugs, including terbinafine and morphine.29-38 In fixed drug eruptions, patch testing on involved skin showed positive results to NSAIDs, analgesics, platelet inhibitors, and antimicrobials.27,52-55 Patch testing in DRESS syndrome has shown many positive reactions to antiepileptics and antipsychotics.39-43 One study used patch tests in SDRIFE, reporting positive results with antimicrobials, antineoplastics, decongestants, and glucocorticoids.61 Nonsteroidal anti-inflammatory drugs, antimicrobials, calcium channel blockers, and histamine antagonists were implicated in EM.47-51 Positive patch tests were seen in morbilliform eruptions with selective serotonin reuptake inhibitors, antiepileptics/benzodiazepines, NSAIDs, and antimicrobials.28,57-60 In toxic epidermal necrolysis, diagnosis with patch testing was made using patches placed on previously involved skin with sulfamethoxazole.62
Systemic Contact Dermatitis
Drugs historically recognized as causing allergic contact dermatitis (eg, topical gentamycin) can cause systemic contact dermatitis, which can be patch tested. In these situations, systemic contact dermatitis may be due to either the active drug or excipients in the medication formulation. Excipients are inactive ingredients in medications that provide a suitable consistency, appearance, or form. Often overlooked as culprits of drug hypersensitivity because they are theoretically inert, excipients are increasingly implicated in drug allergy. Swerlick and Campbell63 described 11 cases in which chronic unexplained pruritus responded to medication changes to avoid coloring agents. The most common culprits were FD&C Blue No. 1 and FD&C Blue No. 2. Patch testing for allergies to dyes can be clinically useful, though a lack of commercially available patch tests makes diagnosis difficult.64
Other excipients can cause cutaneous reactions. Propylene glycol, commonly implicated in allergic contact dermatitis, also can cause cutaneous eruptions upon systemic exposure.65 Corticosteroid-induced systemic contact dermatitis has been reported, though it is less prevalent than allergic contact dermatitis.66 These reactions usually are due to nonmethylated and nonhalogenated corticosteroids including budesonide, cortisone, hydrocortisone, prednisolone, and methylprednisolone.67,68 Patch testing in these situations is complicated by the possibility of false-negative results due to the anti-inflammatory effects of the corticosteroids. Therefore, patch testing should be performed using standardized and not treatment concentrations.
In our clinic, we have anecdotally observed several patients with chronic dermatitis and suspected NSAID allergies have positive patch test results with propylene glycol and not the suspected drug. Excipients encountered in multiple drugs and foods are more likely to present as chronic dermatitis, while active drug ingredients started in hospital settings more often present as acute dermatitis.
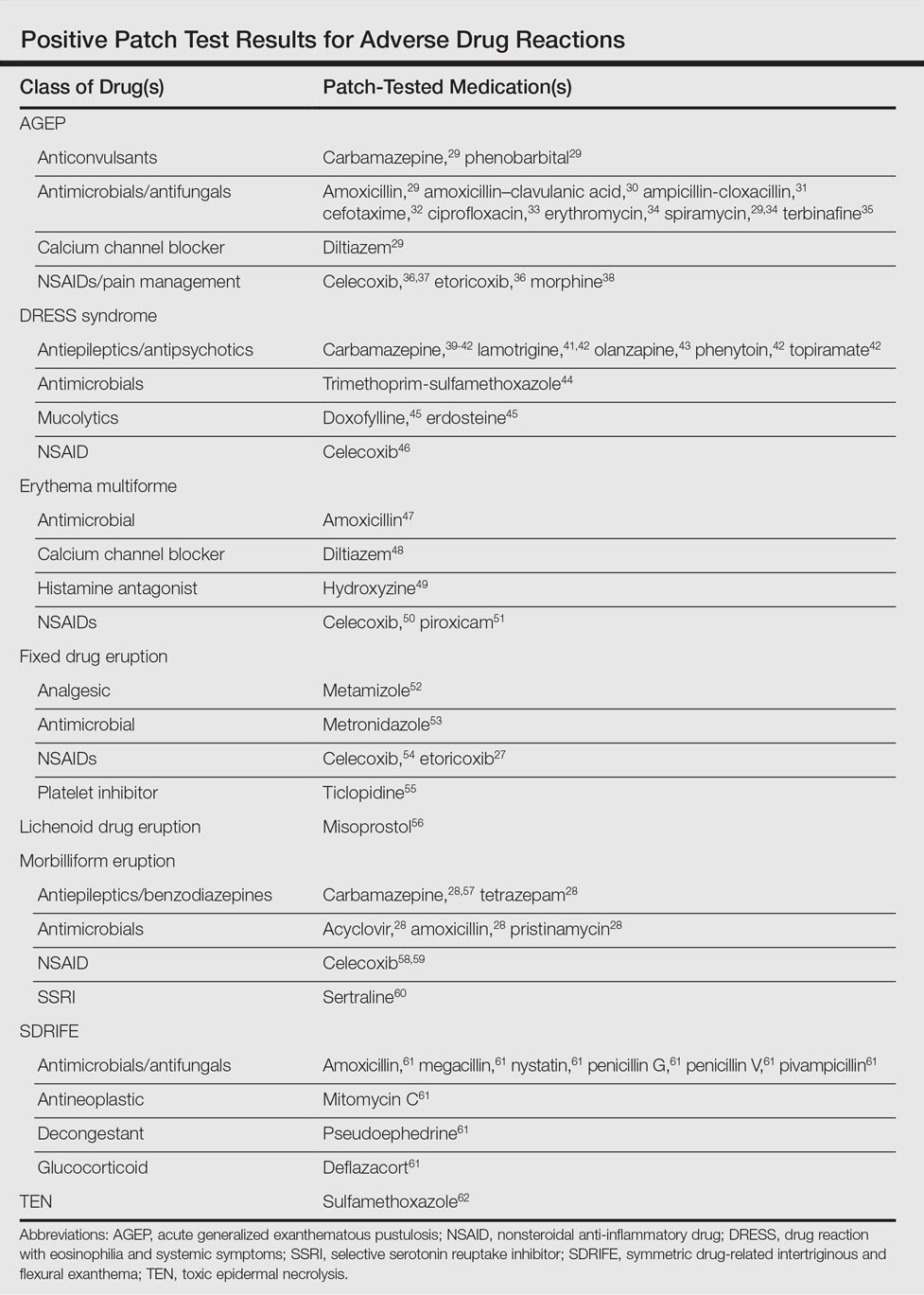
Our Experience
We have patch tested a handful of patients with suspected drug eruptions (University Hospitals Cleveland Medical Center institutional review board #07-12-27). Medications, excipients, and their concentrations (in % weight per weight) and vehicles that were tested include ibuprofen (10% petrolatum), aspirin (10% petrolatum), hydrochlorothiazide (10% petrolatum), captopril (5% petrolatum), and propylene glycol (30% water or 5% petrolatum). Patch tests were read at 48 and 72 hours and scored according to the International Contact Dermatitis Research Group patch test scoring guidelines.69 Two patients tested for ibuprofen reacted positively only to propylene glycol; the 3 other patients did not react to aspirin, hydrochlorothiazide, and captopril. Overall, we observed no positive patch tests to medications and 2 positive tests to propylene glycol in 5 patients tested (unpublished data).
Areas of Uncertainty
Although tests for immediate-type hypersensitivity reactions to drugs exist as skin prick tests, diagnostic testing for the majority of drug reactions does not exist. Drug allergy diagnosis is made with history and temporality, potentially resulting in unnecessary avoidance of helpful medications. Ideal patch test concentrations and vehicles as well as the sensitivity and specificity of these tests are unknown.
Guidelines From Professional Societies
Drug allergy testing guidelines are available from the British Society for Allergy and Clinical Immunology70 and American Academy of Allergy, Asthma and Immunology.71 The guidelines recommend diagnosis by history and temporality, and it is stated that patch testing is potentially useful in maculopapular rashes, AGEP, fixed drug eruptions, and DRESS syndrome.
Conclusion
Case reports in the literature suggest the utility of patch testing in some drug allergies. We suggest testing excipients such as propylene glycol and benzoic acid to rule out systemic contact dermatitis when patch testing with active drugs to confirm cause of suspected adverse cutaneous reactions to medications.
- Arndt KA, Jick H. Rates of cutaneous reactions to drugs. a report from the Boston Collaborative Drug Surveillance Program. JAMA. 1976;235:918-922.
- Bigby M, Jick S, Jick H, et al. Drug-induced cutaneous reactions. a report from the Boston Collaborative Drug Surveillance Program on 15,483 consecutive inpatients, 1975 to 1982. JAMA. 1986;256:3358-3363.
- Fiszenson-Albala F, Auzerie V, Mahe E, et al. A 6-month prospective survey of cutaneous drug reactions in a hospital setting. Br J Dermatol. 2003;149:1018-1022.
- Thong BY, Leong KP, Tang CY, et al. Drug allergy in a general hospital: results of a novel prospective inpatient reporting system. Ann Allergy Asthma Immunol. 2003;90:342-347.
- Hunziker T, Kunzi UP, Braunschweig S, et al. Comprehensive hospital drug monitoring (CHDM): adverse skin reactions, a 20-year survey. Allergy. 1997;52:388-393.
- Swanbeck G, Dahlberg E. Cutaneous drug reactions. an attempt to quantitative estimation. Arch Dermatol Res. 1992;284:215-218.
- Naldi L, Conforti A, Venegoni M, et al. Cutaneous reactions to drugs. an analysis of spontaneous reports in four Italian regions. Br J Clin Pharmacol. 1999;48:839-846.
- French LE, Prins C. Erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis. In: Bolognia, JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:319-333.
- Vasconcelos C, Magina S, Quirino P, et al. Cutaneous drug reactions to piroxicam. Contact Dermatitis. 1998;39:145.
- Gerber D. Adverse reactions of piroxicam. Drug Intell Clin Pharm. 1987;21:707-710.
- Revuz J, Valeyrie-Allanore L. Drug reactions. In: Bolognia, JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:335-356.
- Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: part II. management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1-709.e9; quiz 718-720.
- Heinzerling LM, Tomsitz D, Anliker MD. Is drug allergy less prevalent than previously assumed? a 5-year analysis. Br J Dermatol. 2012;166:107-114.
- Salkind AR, Cuddy PG. Is this patient allergic to penicillin?: an evidence-based analysis of the likelihood of penicillin allergy. JAMA. 2001;285:2498-2505.
- Torres MJ, Gomez F, Doña I, et al. Diagnostic evaluation of patients with nonimmediate cutaneous hypersensitivity reactions to iodinated contrast media. Allergy. 2012;67:929-935.
- Cham PM, Warshaw EM. Patch testing for evaluating drug reactions due to systemic antibiotics. Dermatitis. 2007;18:63-77.
- Andrade P, Brinca A, Gonçalo M. Patch testing in fixed drug eruptions—a 20-year review. Contact Dermatitis. 2011;65:195-201.
- Romano A, Viola M, Gaeta F, et al. Patch testing in non-immediate drug eruptions. Allergy Asthma Clin Immunol. 2008;4:66-74.
- Rosso R, Mattiacci G, Bernardi ML, et al. Very delayed reactions to beta-lactam antibiotics. Contact Dermatitis. 2000;42:293-295.
- Romano A, Torres MJ, Castells M, et al. Diagnosis and management of drug hypersensitivity reactions. J Allergy Clin Immunol. 2011;127(3 suppl):S67-S73.
- Friedmann PS, Ardern-Jones M. Patch testing in drug allergy. Curr Opin Allergy Clin Immunol. 2010;10:291-296.
- Torres MJ, Mayorga C, Blanca M. Nonimmediate allergic reactions induced by drugs: pathogenesis and diagnostic tests. J Investig Allergol Clin Immunol. 2009;19:80-90.
- Waton J, Tréchot P, Loss-Ayay C, et al. Negative predictive value of drug skin tests in investigating cutaneous adverse drug reactions. Br J Dermatol. 2009;160:786-794.
- Romano A, Viola M, Mondino C, et al. Diagnosing nonimmediate reactions to penicillins by in vivo tests. Int Arch Allergy Immunol. 2002;129:169-174.
- De Groot AC. Patch Testing. Test Concentrations and Vehicles for 4350 Chemicals. 3rd ed. Wapserveen, Netherlands: acdegroot publishing; 2008.
- Elzagallaai AA, Knowles SR, Rieder MJ, et al. Patch testing for the diagnosis of anticonvulsant hypersensitivity syndrome: a systematic review. Drug Saf. 2009;32:391-408.
- Andrade P, Gonçalo M. Fixed drug eruption caused by etoricoxib—2 cases confirmed by patch testing. Contact Dermatitis. 2011;64:118-120.
- Barbaud A, Reichert-Penetrat S, Tréchot P, et al. The use of skin testing in the investigation of cutaneous adverse drug reactions. Br J Dermatol. 1998;139:49-58.
- Wolkenstein P, Chosidow O, Fléchet ML, et al. Patch testing in severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Dermatitis. 1996;35:234-236.
- Harries MJ, McIntyre SJ, Kingston TP. Co-amoxiclav-induced acute generalized exanthematous pustulosis confirmed by patch testing. Contact Dermatitis. 2006;55:372.
- Matsumoto Y, Okubo Y, Yamamoto T, et al. Case of acute generalized exanthematous pustulosis caused by ampicillin/cloxacillin sodium in a pregnant woman. J Dermatol. 2008;35:362-364.
- Chaabane A, Aouam K, Gassab L, et al. Acute generalized exanthematous pustulosis (AGEP) induced by cefotaxime. Fundam Clin Pharmacol. 2010;24:429-432.
- Hausermann P, Scherer K, Weber M, et al. Ciprofloxacin-induced acute generalized exanthematous pustulosis mimicking bullous drug eruption confirmed by a positive patch test. Dermatology. 2005;211:277-280.
- Moreau A, Dompmartin A, Castel B, et al. Drug-induced acute generalized exanthematous pustulosis with positive patch tests. Int J Dermatol. 1995;34:263-266.
- Kempinaire A, De Raevea L, Merckx M, et al. Terbinafine-induced acute generalized exanthematous pustulosis confirmed by a positive patch-test result. J Am Acad Dermatol. 1997;37:653-655.
- Mäkelä L, Lammintausta K. Etoricoxib-induced acute generalized exanthematous pustulosis. Acta Derm Venereol. 2008;88:200-201.
- Yang CC, Lee JY, Chen WC. Acute generalized exanthematous pustulosis caused by celecoxib. J Formos Med Assoc. 2004;103:555-557.
- Kardaun SH, de Monchy JG. Acute generalized exanthematous pustulosis caused by morphine, confirmed by positive patch test and lymphocyte transformation test. J Am Acad Dermatol. 2006;55(2 suppl):S21-S23.
- Inadomi T. Drug rash with eosinophilia and systemic symptoms (DRESS): changing carbamazepine to phenobarbital controlled epilepsy without the recurrence of DRESS. Eur J Dermatol. 2010;20:220-222.
- Buyuktiryaki AB, Bezirganoglu H, Sahiner UM, et al. Patch testing is an effective method for the diagnosis of carbamazepine-induced drug reaction, eosinophilia and systemic symptoms (DRESS) syndrome in an 8-year-old girl. Australas J Dermatol. 2012;53:274-277.
- Aouam K, Ben Romdhane F, Loussaief C, et al. Hypersensitivity syndrome induced by anticonvulsants: possible cross-reactivity between carbamazepine and lamotrigine. J Clin Pharmacol. 2009;49:1488-1491.
- Santiago F, Gonçalo M, Vieira R, et al. Epicutaneous patch testing in drug hypersensitivity syndrome (DRESS). Contact Dermatitis. 2010;62:47-53.
- Prevost P, Bédry R, Lacoste D, et al. Hypersensitivity syndrome with olanzapine confirmed by patch tests. Eur J Dermatol. 2012;22:126-127.
- Hubiche T, Milpied B, Cazeau C, et al. Association of immunologically confirmed delayed drug reaction and human herpesvirus 6 viremia in a pediatric case of drug-induced hypersensitivity syndrome. Dermatology. 2011;222:140-141.
- Song WJ, Shim EJ, Kang MG, et al. Severe drug hypersensitivity induced by erdosteine and doxofylline as confirmed by patch and lymphocyte transformation tests: a case report. J Investig Allergol Clin Immunol. 2012;22:230-232.
- Lee JH, Park HK, Heo J, et al. Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome induced by celecoxib and anti-tuberculosis drugs. J Korean Med Sci. 2008;23:521-525.
- González-Delgado P, Blanes M, Soriano V, et al. Erythema multiforme to amoxicillin with concurrent infection by Epstein-Barr virus. Allergol Immunopathol. 2006;34:76-78.
- Gonzalo Garijo MA, Pérez Calderón R, de Argila Fernández-Durán D, et al. Cutaneous reactions due to diltiazem and cross reactivity with other calcium channel blockers. Allergol Immunopathol (Madr). 2005;33:238-240.
- Peña AL, Henriquezsantana A, Gonzalez-Seco E, et al. Exudative erythema multiforme induced by hydroxyzine. Eur J Dermatol. 2008;18:194-195.
- Arakawa Y, Nakai N, Katoh N. Celecoxib-induced erythema multiforme-type drug eruption with a positive patch test. J Dermatol. 2011;38:1185-1188.
- Prieto A, De barrio M, Pérez C, et al. Piroxicam-induced erythema multiforme. Contact Dermatitis. 2004;50:263.
- Dalmau J, Serra-baldrich E, Roé E, et al. Use of patch test in fixed drug eruption due to metamizole (Nolotil). Contact Dermatitis. 2006;54:127-128.
- Gastaminza G, Anda M, Audicana MT, et al. Fixed-drug eruption due to metronidazole with positive topical provocation. Contact Dermatitis. 2001;44:36.
- Bellini V, Stingeni L, Lisi P. Multifocal fixed drug eruption due to celecoxib. Dermatitis. 2009;20:174-176.
- García CM, Carmena R, García R, et al. Fixed drug eruption from ticlopidine, with positive lesional patch test. Contact Dermatitis. 2001;44:40-41.
- Cruz MJ, Duarte AF, Baudrier T, et al. Lichenoid drug eruption induced by misoprostol. Contact Dermatitis. 2009;61:240-242.
- Alanko K. Patch testing in cutaneous reactions caused by carbamazepine. Contact Dermatitis. 1993;29:254-257.
- Grob M, Scheidegger P, Wüthrich B. Allergic skin reaction to celecoxib. Dermatology. 2000;201:383.
- Alonso JC, Ortega JD, Gonzalo MJ. Cutaneous reaction to oral celecoxib with positive patch test. Contact Dermatitis. 2004;50:48-49.
- Fernandes B, Brites M, Gonçalo M, et al. Maculopapular eruption from sertraline with positive patch tests. Contact Dermatitis. 2000;42:287.
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Klein CE, Trautmann A, Zillikens D, et al. Patch testing in an unusual case of toxic epidermal necrolysis. Contact Dermatitis. 1996;35:175-176.
- Swerlick RA, Campbell CF. Medication dyes as a source of drug allergy. J Drugs Dermatol. 2013;12:99-102.
- Guin JD. Patch testing to FD&C and D&C dyes. Contact Dermatitis. 2003;49:217-218.
- Lowther A, McCormick T, Nedorost S. Systemic contact dermatitis from propylene glycol. Dermatitis. 2008;19:105-108.
- Baeck M, Goossens A. Systemic contact dermatitis to corticosteroids. Allergy. 2012;67:1580-1585.
- Baeck M, Goossens A. Immediate and delayed allergic hypersensitivity to corticosteroids: practical guidelines. Contact Dermatitis. 2012;66:38-45.
- Basedow S, Eigelshoven S, Homey B. Immediate and delayed hypersensitivity to corticosteroids. J Dtsch Dermatol Ges. 2011;9:885-888.
- Johansen JD, Aalto-korte K, Agner T, et al. European Society of Contact Dermatitis guideline for diagnostic patch testing—recommendations on best practice. Contact Dermatitis. 2015;73:195-221.
- Mirakian R, Ewan PW, Durham SR, et al. BSACI guidelines for the management of drug allergy. Clin Exp Allergy. 2009;39:43-61.
- Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259-273.
Adverse drug reactions account for 3% to 6% of hospital admissions in the United States and occur in 10% to 15% of hospitalized patients.1,2 The most common culprits are antibiotics and nonsteroidal anti-inflammatory drugs (NSAIDs).3-12 In most cases, diagnoses are made clinically without diagnostic testing. To identify drug allergies associated with diagnostic testing, one center selected patients with suspected cutaneous drug reactions (2006-2010) for further evaluation.13 Of 612 patients who were evaluated, 141 had a high suspicion of drug allergy and were included in the analysis. The excluded patients had pseudoallergic reactions, reactive exanthemas due to infection, histopathologic exclusion of drug allergy, angioedema, or other dermatological conditions such as contact dermatitis and eczema. Of the included patients, 107 were diagnosed with drug reactions, while the remainder had non–drug-related exanthemas or unknown etiology after testing. Identified culprit drugs were predominantly antibiotics (39.8%) and NSAIDs (21.2%); contrast media, anticoagulants, anticonvulsants, antimalarials, antifungals, glucocorticoids, antihypertensives, and proton pump inhibitors also were implicated. They were identified with skin prick, intradermal, and patch tests (62.6%); lymphocyte transformation test (17.7%); oral rechallenge (5.6%); or without skin testing (6.5%). One quarter of patients with a high suspicion for drug allergy did not have a confirmed drug eruption in this study. Another study found that 10% to 20% of patients with reported penicillin allergy had confirmation via skin prick testing.14 These findings suggest that confirmation of suspected drug allergy may require more than one diagnostic test.
Tests for Adverse Drug Reactions
The following tests have been shown to aid in the identification of cutaneous drug eruptions: (1) patch tests15-21; (2) intradermal tests14,15,19,20; (3) drug provocation tests15,20; and (4) lymphocyte transformation tests.20 Intradermal or skin prick tests are most useful in urticarial eruptions but can be considered in nonurticarial eruptions with delayed inspection of test sites up to 1 week after testing. Drug provocation tests are considered the gold standard but involve patient risk. Lymphocyte transformation tests use the principle that T lymphocytes proliferate in the presence of drugs to which the patient is sensitized. Patch tests will be discussed in greater detail below. Immunohistochemistry can determine immunologic mechanisms of eruptions but cannot identify causative agents.16,17,22
A retrospective study of patients referred for evaluation of adverse drug reactions between 1996 and 2006 found the collective negative predictive value (NPV)—the percentage of truly negative skin tests based on provocation or substitution testing—of cutaneous drug tests including patch, prick, and intradermal tests to be 89.6% (95% confidence interval, 85.9%-93.3%).23 The NPVs of each test were not reported. Patients with negative cutaneous tests had subsequent oral rechallenge or substitution testing with medication from the same drug class.23 Another study16 found the NPV of patch testing to be at least 79% after review of data from other studies using patch and provocation testing.16,24 These studies suggest that cutaneous testing can be useful, albeit imperfect, in the evaluation and diagnosis of drug allergy.
Review of the Patch Test
Patch tests can be helpful in diagnosis of delayed hypersensitivities.18 Patch testing is most commonly and effectively used to diagnose allergic contact dermatitis, but its utility in other applications, such as diagnosis of cutaneous drug eruptions, has not been extensively studied.
The development of patch tests to diagnose systemic drug allergies is inhibited by the uncertainty of percutaneous drug penetration, a dearth of studies to determine the best test concentrations of active drug in the patch test, and the potential for nonimmunologic contact urticaria upon skin exposure. Furthermore, cutaneous metabolism of many antigens is well documented, but correlation to systemic metabolism often is unknown, which can confound patch test results and lead to false-negative results when the skin’s metabolic capacity does not match the body’s capacity to generate antigens capable of eliciting immunogenic responses.21 Additionally, the method used to suspend and disperse drugs in patch test vehicles is unfamiliar to most pharmacists, and standardized concentrations and vehicles are available only for some medications.25 Studies sufficient to obtain US Food and Drug Administration approval of patch tests for systemic drug eruptions would be costly and therefore prohibitive to investigators. The majority of the literature consists of case reports and data extrapolated from reviews. Patch test results of many drugs have been reported in the literature, with the highest frequencies of positive results associated with anticonvulsants,26 antibiotics, corticosteroids, calcium channel blockers, and benzodiazepines.21
Patch test placement affects the diagnostic value of the test. Placing patch tests on previously involved sites of fixed drug eruptions improves yield over placement on uninvolved skin.27 Placing patch tests on previously involved sites of other drug eruptions such as toxic epidermal necrolysis also may aid in diagnosis, though the literature is sparse.25,26,28
Patch Testing in Drug Eruptions
Morbilliform eruptions account for 48% to 91% of patients with adverse drug reactions.4-6 Other drug eruptions include urticarial eruptions, acute generalized exanthematous pustulosis (AGEP), drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, toxic epidermal necrolysis, Stevens-Johnson syndrome, lichenoid drug eruption, symmetric drug-related intertriginous and flexural exanthema (SDRIFE), erythema multiforme (EM), and systemic contact dermatitis. The Table summarizes reports of positive patch tests with various medications for these drug eruptions.
In general, antimicrobials and NSAIDs were the most implicated drugs with positive patch test results in AGEP, DRESS syndrome, EM, fixed drug eruptions, and morbilliform eruptions. In AGEP, positive results also were reported for other drugs, including terbinafine and morphine.29-38 In fixed drug eruptions, patch testing on involved skin showed positive results to NSAIDs, analgesics, platelet inhibitors, and antimicrobials.27,52-55 Patch testing in DRESS syndrome has shown many positive reactions to antiepileptics and antipsychotics.39-43 One study used patch tests in SDRIFE, reporting positive results with antimicrobials, antineoplastics, decongestants, and glucocorticoids.61 Nonsteroidal anti-inflammatory drugs, antimicrobials, calcium channel blockers, and histamine antagonists were implicated in EM.47-51 Positive patch tests were seen in morbilliform eruptions with selective serotonin reuptake inhibitors, antiepileptics/benzodiazepines, NSAIDs, and antimicrobials.28,57-60 In toxic epidermal necrolysis, diagnosis with patch testing was made using patches placed on previously involved skin with sulfamethoxazole.62
Systemic Contact Dermatitis
Drugs historically recognized as causing allergic contact dermatitis (eg, topical gentamycin) can cause systemic contact dermatitis, which can be patch tested. In these situations, systemic contact dermatitis may be due to either the active drug or excipients in the medication formulation. Excipients are inactive ingredients in medications that provide a suitable consistency, appearance, or form. Often overlooked as culprits of drug hypersensitivity because they are theoretically inert, excipients are increasingly implicated in drug allergy. Swerlick and Campbell63 described 11 cases in which chronic unexplained pruritus responded to medication changes to avoid coloring agents. The most common culprits were FD&C Blue No. 1 and FD&C Blue No. 2. Patch testing for allergies to dyes can be clinically useful, though a lack of commercially available patch tests makes diagnosis difficult.64
Other excipients can cause cutaneous reactions. Propylene glycol, commonly implicated in allergic contact dermatitis, also can cause cutaneous eruptions upon systemic exposure.65 Corticosteroid-induced systemic contact dermatitis has been reported, though it is less prevalent than allergic contact dermatitis.66 These reactions usually are due to nonmethylated and nonhalogenated corticosteroids including budesonide, cortisone, hydrocortisone, prednisolone, and methylprednisolone.67,68 Patch testing in these situations is complicated by the possibility of false-negative results due to the anti-inflammatory effects of the corticosteroids. Therefore, patch testing should be performed using standardized and not treatment concentrations.
In our clinic, we have anecdotally observed several patients with chronic dermatitis and suspected NSAID allergies have positive patch test results with propylene glycol and not the suspected drug. Excipients encountered in multiple drugs and foods are more likely to present as chronic dermatitis, while active drug ingredients started in hospital settings more often present as acute dermatitis.

Our Experience
We have patch tested a handful of patients with suspected drug eruptions (University Hospitals Cleveland Medical Center institutional review board #07-12-27). Medications, excipients, and their concentrations (in % weight per weight) and vehicles that were tested include ibuprofen (10% petrolatum), aspirin (10% petrolatum), hydrochlorothiazide (10% petrolatum), captopril (5% petrolatum), and propylene glycol (30% water or 5% petrolatum). Patch tests were read at 48 and 72 hours and scored according to the International Contact Dermatitis Research Group patch test scoring guidelines.69 Two patients tested for ibuprofen reacted positively only to propylene glycol; the 3 other patients did not react to aspirin, hydrochlorothiazide, and captopril. Overall, we observed no positive patch tests to medications and 2 positive tests to propylene glycol in 5 patients tested (unpublished data).
Areas of Uncertainty
Although tests for immediate-type hypersensitivity reactions to drugs exist as skin prick tests, diagnostic testing for the majority of drug reactions does not exist. Drug allergy diagnosis is made with history and temporality, potentially resulting in unnecessary avoidance of helpful medications. Ideal patch test concentrations and vehicles as well as the sensitivity and specificity of these tests are unknown.
Guidelines From Professional Societies
Drug allergy testing guidelines are available from the British Society for Allergy and Clinical Immunology70 and American Academy of Allergy, Asthma and Immunology.71 The guidelines recommend diagnosis by history and temporality, and it is stated that patch testing is potentially useful in maculopapular rashes, AGEP, fixed drug eruptions, and DRESS syndrome.
Conclusion
Case reports in the literature suggest the utility of patch testing in some drug allergies. We suggest testing excipients such as propylene glycol and benzoic acid to rule out systemic contact dermatitis when patch testing with active drugs to confirm cause of suspected adverse cutaneous reactions to medications.
Adverse drug reactions account for 3% to 6% of hospital admissions in the United States and occur in 10% to 15% of hospitalized patients.1,2 The most common culprits are antibiotics and nonsteroidal anti-inflammatory drugs (NSAIDs).3-12 In most cases, diagnoses are made clinically without diagnostic testing. To identify drug allergies associated with diagnostic testing, one center selected patients with suspected cutaneous drug reactions (2006-2010) for further evaluation.13 Of 612 patients who were evaluated, 141 had a high suspicion of drug allergy and were included in the analysis. The excluded patients had pseudoallergic reactions, reactive exanthemas due to infection, histopathologic exclusion of drug allergy, angioedema, or other dermatological conditions such as contact dermatitis and eczema. Of the included patients, 107 were diagnosed with drug reactions, while the remainder had non–drug-related exanthemas or unknown etiology after testing. Identified culprit drugs were predominantly antibiotics (39.8%) and NSAIDs (21.2%); contrast media, anticoagulants, anticonvulsants, antimalarials, antifungals, glucocorticoids, antihypertensives, and proton pump inhibitors also were implicated. They were identified with skin prick, intradermal, and patch tests (62.6%); lymphocyte transformation test (17.7%); oral rechallenge (5.6%); or without skin testing (6.5%). One quarter of patients with a high suspicion for drug allergy did not have a confirmed drug eruption in this study. Another study found that 10% to 20% of patients with reported penicillin allergy had confirmation via skin prick testing.14 These findings suggest that confirmation of suspected drug allergy may require more than one diagnostic test.
Tests for Adverse Drug Reactions
The following tests have been shown to aid in the identification of cutaneous drug eruptions: (1) patch tests15-21; (2) intradermal tests14,15,19,20; (3) drug provocation tests15,20; and (4) lymphocyte transformation tests.20 Intradermal or skin prick tests are most useful in urticarial eruptions but can be considered in nonurticarial eruptions with delayed inspection of test sites up to 1 week after testing. Drug provocation tests are considered the gold standard but involve patient risk. Lymphocyte transformation tests use the principle that T lymphocytes proliferate in the presence of drugs to which the patient is sensitized. Patch tests will be discussed in greater detail below. Immunohistochemistry can determine immunologic mechanisms of eruptions but cannot identify causative agents.16,17,22
A retrospective study of patients referred for evaluation of adverse drug reactions between 1996 and 2006 found the collective negative predictive value (NPV)—the percentage of truly negative skin tests based on provocation or substitution testing—of cutaneous drug tests including patch, prick, and intradermal tests to be 89.6% (95% confidence interval, 85.9%-93.3%).23 The NPVs of each test were not reported. Patients with negative cutaneous tests had subsequent oral rechallenge or substitution testing with medication from the same drug class.23 Another study16 found the NPV of patch testing to be at least 79% after review of data from other studies using patch and provocation testing.16,24 These studies suggest that cutaneous testing can be useful, albeit imperfect, in the evaluation and diagnosis of drug allergy.
Review of the Patch Test
Patch tests can be helpful in diagnosis of delayed hypersensitivities.18 Patch testing is most commonly and effectively used to diagnose allergic contact dermatitis, but its utility in other applications, such as diagnosis of cutaneous drug eruptions, has not been extensively studied.
The development of patch tests to diagnose systemic drug allergies is inhibited by the uncertainty of percutaneous drug penetration, a dearth of studies to determine the best test concentrations of active drug in the patch test, and the potential for nonimmunologic contact urticaria upon skin exposure. Furthermore, cutaneous metabolism of many antigens is well documented, but correlation to systemic metabolism often is unknown, which can confound patch test results and lead to false-negative results when the skin’s metabolic capacity does not match the body’s capacity to generate antigens capable of eliciting immunogenic responses.21 Additionally, the method used to suspend and disperse drugs in patch test vehicles is unfamiliar to most pharmacists, and standardized concentrations and vehicles are available only for some medications.25 Studies sufficient to obtain US Food and Drug Administration approval of patch tests for systemic drug eruptions would be costly and therefore prohibitive to investigators. The majority of the literature consists of case reports and data extrapolated from reviews. Patch test results of many drugs have been reported in the literature, with the highest frequencies of positive results associated with anticonvulsants,26 antibiotics, corticosteroids, calcium channel blockers, and benzodiazepines.21
Patch test placement affects the diagnostic value of the test. Placing patch tests on previously involved sites of fixed drug eruptions improves yield over placement on uninvolved skin.27 Placing patch tests on previously involved sites of other drug eruptions such as toxic epidermal necrolysis also may aid in diagnosis, though the literature is sparse.25,26,28
Patch Testing in Drug Eruptions
Morbilliform eruptions account for 48% to 91% of patients with adverse drug reactions.4-6 Other drug eruptions include urticarial eruptions, acute generalized exanthematous pustulosis (AGEP), drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, toxic epidermal necrolysis, Stevens-Johnson syndrome, lichenoid drug eruption, symmetric drug-related intertriginous and flexural exanthema (SDRIFE), erythema multiforme (EM), and systemic contact dermatitis. The Table summarizes reports of positive patch tests with various medications for these drug eruptions.
In general, antimicrobials and NSAIDs were the most implicated drugs with positive patch test results in AGEP, DRESS syndrome, EM, fixed drug eruptions, and morbilliform eruptions. In AGEP, positive results also were reported for other drugs, including terbinafine and morphine.29-38 In fixed drug eruptions, patch testing on involved skin showed positive results to NSAIDs, analgesics, platelet inhibitors, and antimicrobials.27,52-55 Patch testing in DRESS syndrome has shown many positive reactions to antiepileptics and antipsychotics.39-43 One study used patch tests in SDRIFE, reporting positive results with antimicrobials, antineoplastics, decongestants, and glucocorticoids.61 Nonsteroidal anti-inflammatory drugs, antimicrobials, calcium channel blockers, and histamine antagonists were implicated in EM.47-51 Positive patch tests were seen in morbilliform eruptions with selective serotonin reuptake inhibitors, antiepileptics/benzodiazepines, NSAIDs, and antimicrobials.28,57-60 In toxic epidermal necrolysis, diagnosis with patch testing was made using patches placed on previously involved skin with sulfamethoxazole.62
Systemic Contact Dermatitis
Drugs historically recognized as causing allergic contact dermatitis (eg, topical gentamycin) can cause systemic contact dermatitis, which can be patch tested. In these situations, systemic contact dermatitis may be due to either the active drug or excipients in the medication formulation. Excipients are inactive ingredients in medications that provide a suitable consistency, appearance, or form. Often overlooked as culprits of drug hypersensitivity because they are theoretically inert, excipients are increasingly implicated in drug allergy. Swerlick and Campbell63 described 11 cases in which chronic unexplained pruritus responded to medication changes to avoid coloring agents. The most common culprits were FD&C Blue No. 1 and FD&C Blue No. 2. Patch testing for allergies to dyes can be clinically useful, though a lack of commercially available patch tests makes diagnosis difficult.64
Other excipients can cause cutaneous reactions. Propylene glycol, commonly implicated in allergic contact dermatitis, also can cause cutaneous eruptions upon systemic exposure.65 Corticosteroid-induced systemic contact dermatitis has been reported, though it is less prevalent than allergic contact dermatitis.66 These reactions usually are due to nonmethylated and nonhalogenated corticosteroids including budesonide, cortisone, hydrocortisone, prednisolone, and methylprednisolone.67,68 Patch testing in these situations is complicated by the possibility of false-negative results due to the anti-inflammatory effects of the corticosteroids. Therefore, patch testing should be performed using standardized and not treatment concentrations.
In our clinic, we have anecdotally observed several patients with chronic dermatitis and suspected NSAID allergies have positive patch test results with propylene glycol and not the suspected drug. Excipients encountered in multiple drugs and foods are more likely to present as chronic dermatitis, while active drug ingredients started in hospital settings more often present as acute dermatitis.

Our Experience
We have patch tested a handful of patients with suspected drug eruptions (University Hospitals Cleveland Medical Center institutional review board #07-12-27). Medications, excipients, and their concentrations (in % weight per weight) and vehicles that were tested include ibuprofen (10% petrolatum), aspirin (10% petrolatum), hydrochlorothiazide (10% petrolatum), captopril (5% petrolatum), and propylene glycol (30% water or 5% petrolatum). Patch tests were read at 48 and 72 hours and scored according to the International Contact Dermatitis Research Group patch test scoring guidelines.69 Two patients tested for ibuprofen reacted positively only to propylene glycol; the 3 other patients did not react to aspirin, hydrochlorothiazide, and captopril. Overall, we observed no positive patch tests to medications and 2 positive tests to propylene glycol in 5 patients tested (unpublished data).
Areas of Uncertainty
Although tests for immediate-type hypersensitivity reactions to drugs exist as skin prick tests, diagnostic testing for the majority of drug reactions does not exist. Drug allergy diagnosis is made with history and temporality, potentially resulting in unnecessary avoidance of helpful medications. Ideal patch test concentrations and vehicles as well as the sensitivity and specificity of these tests are unknown.
Guidelines From Professional Societies
Drug allergy testing guidelines are available from the British Society for Allergy and Clinical Immunology70 and American Academy of Allergy, Asthma and Immunology.71 The guidelines recommend diagnosis by history and temporality, and it is stated that patch testing is potentially useful in maculopapular rashes, AGEP, fixed drug eruptions, and DRESS syndrome.
Conclusion
Case reports in the literature suggest the utility of patch testing in some drug allergies. We suggest testing excipients such as propylene glycol and benzoic acid to rule out systemic contact dermatitis when patch testing with active drugs to confirm cause of suspected adverse cutaneous reactions to medications.
- Arndt KA, Jick H. Rates of cutaneous reactions to drugs. a report from the Boston Collaborative Drug Surveillance Program. JAMA. 1976;235:918-922.
- Bigby M, Jick S, Jick H, et al. Drug-induced cutaneous reactions. a report from the Boston Collaborative Drug Surveillance Program on 15,483 consecutive inpatients, 1975 to 1982. JAMA. 1986;256:3358-3363.
- Fiszenson-Albala F, Auzerie V, Mahe E, et al. A 6-month prospective survey of cutaneous drug reactions in a hospital setting. Br J Dermatol. 2003;149:1018-1022.
- Thong BY, Leong KP, Tang CY, et al. Drug allergy in a general hospital: results of a novel prospective inpatient reporting system. Ann Allergy Asthma Immunol. 2003;90:342-347.
- Hunziker T, Kunzi UP, Braunschweig S, et al. Comprehensive hospital drug monitoring (CHDM): adverse skin reactions, a 20-year survey. Allergy. 1997;52:388-393.
- Swanbeck G, Dahlberg E. Cutaneous drug reactions. an attempt to quantitative estimation. Arch Dermatol Res. 1992;284:215-218.
- Naldi L, Conforti A, Venegoni M, et al. Cutaneous reactions to drugs. an analysis of spontaneous reports in four Italian regions. Br J Clin Pharmacol. 1999;48:839-846.
- French LE, Prins C. Erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis. In: Bolognia, JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:319-333.
- Vasconcelos C, Magina S, Quirino P, et al. Cutaneous drug reactions to piroxicam. Contact Dermatitis. 1998;39:145.
- Gerber D. Adverse reactions of piroxicam. Drug Intell Clin Pharm. 1987;21:707-710.
- Revuz J, Valeyrie-Allanore L. Drug reactions. In: Bolognia, JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:335-356.
- Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: part II. management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1-709.e9; quiz 718-720.
- Heinzerling LM, Tomsitz D, Anliker MD. Is drug allergy less prevalent than previously assumed? a 5-year analysis. Br J Dermatol. 2012;166:107-114.
- Salkind AR, Cuddy PG. Is this patient allergic to penicillin?: an evidence-based analysis of the likelihood of penicillin allergy. JAMA. 2001;285:2498-2505.
- Torres MJ, Gomez F, Doña I, et al. Diagnostic evaluation of patients with nonimmediate cutaneous hypersensitivity reactions to iodinated contrast media. Allergy. 2012;67:929-935.
- Cham PM, Warshaw EM. Patch testing for evaluating drug reactions due to systemic antibiotics. Dermatitis. 2007;18:63-77.
- Andrade P, Brinca A, Gonçalo M. Patch testing in fixed drug eruptions—a 20-year review. Contact Dermatitis. 2011;65:195-201.
- Romano A, Viola M, Gaeta F, et al. Patch testing in non-immediate drug eruptions. Allergy Asthma Clin Immunol. 2008;4:66-74.
- Rosso R, Mattiacci G, Bernardi ML, et al. Very delayed reactions to beta-lactam antibiotics. Contact Dermatitis. 2000;42:293-295.
- Romano A, Torres MJ, Castells M, et al. Diagnosis and management of drug hypersensitivity reactions. J Allergy Clin Immunol. 2011;127(3 suppl):S67-S73.
- Friedmann PS, Ardern-Jones M. Patch testing in drug allergy. Curr Opin Allergy Clin Immunol. 2010;10:291-296.
- Torres MJ, Mayorga C, Blanca M. Nonimmediate allergic reactions induced by drugs: pathogenesis and diagnostic tests. J Investig Allergol Clin Immunol. 2009;19:80-90.
- Waton J, Tréchot P, Loss-Ayay C, et al. Negative predictive value of drug skin tests in investigating cutaneous adverse drug reactions. Br J Dermatol. 2009;160:786-794.
- Romano A, Viola M, Mondino C, et al. Diagnosing nonimmediate reactions to penicillins by in vivo tests. Int Arch Allergy Immunol. 2002;129:169-174.
- De Groot AC. Patch Testing. Test Concentrations and Vehicles for 4350 Chemicals. 3rd ed. Wapserveen, Netherlands: acdegroot publishing; 2008.
- Elzagallaai AA, Knowles SR, Rieder MJ, et al. Patch testing for the diagnosis of anticonvulsant hypersensitivity syndrome: a systematic review. Drug Saf. 2009;32:391-408.
- Andrade P, Gonçalo M. Fixed drug eruption caused by etoricoxib—2 cases confirmed by patch testing. Contact Dermatitis. 2011;64:118-120.
- Barbaud A, Reichert-Penetrat S, Tréchot P, et al. The use of skin testing in the investigation of cutaneous adverse drug reactions. Br J Dermatol. 1998;139:49-58.
- Wolkenstein P, Chosidow O, Fléchet ML, et al. Patch testing in severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Dermatitis. 1996;35:234-236.
- Harries MJ, McIntyre SJ, Kingston TP. Co-amoxiclav-induced acute generalized exanthematous pustulosis confirmed by patch testing. Contact Dermatitis. 2006;55:372.
- Matsumoto Y, Okubo Y, Yamamoto T, et al. Case of acute generalized exanthematous pustulosis caused by ampicillin/cloxacillin sodium in a pregnant woman. J Dermatol. 2008;35:362-364.
- Chaabane A, Aouam K, Gassab L, et al. Acute generalized exanthematous pustulosis (AGEP) induced by cefotaxime. Fundam Clin Pharmacol. 2010;24:429-432.
- Hausermann P, Scherer K, Weber M, et al. Ciprofloxacin-induced acute generalized exanthematous pustulosis mimicking bullous drug eruption confirmed by a positive patch test. Dermatology. 2005;211:277-280.
- Moreau A, Dompmartin A, Castel B, et al. Drug-induced acute generalized exanthematous pustulosis with positive patch tests. Int J Dermatol. 1995;34:263-266.
- Kempinaire A, De Raevea L, Merckx M, et al. Terbinafine-induced acute generalized exanthematous pustulosis confirmed by a positive patch-test result. J Am Acad Dermatol. 1997;37:653-655.
- Mäkelä L, Lammintausta K. Etoricoxib-induced acute generalized exanthematous pustulosis. Acta Derm Venereol. 2008;88:200-201.
- Yang CC, Lee JY, Chen WC. Acute generalized exanthematous pustulosis caused by celecoxib. J Formos Med Assoc. 2004;103:555-557.
- Kardaun SH, de Monchy JG. Acute generalized exanthematous pustulosis caused by morphine, confirmed by positive patch test and lymphocyte transformation test. J Am Acad Dermatol. 2006;55(2 suppl):S21-S23.
- Inadomi T. Drug rash with eosinophilia and systemic symptoms (DRESS): changing carbamazepine to phenobarbital controlled epilepsy without the recurrence of DRESS. Eur J Dermatol. 2010;20:220-222.
- Buyuktiryaki AB, Bezirganoglu H, Sahiner UM, et al. Patch testing is an effective method for the diagnosis of carbamazepine-induced drug reaction, eosinophilia and systemic symptoms (DRESS) syndrome in an 8-year-old girl. Australas J Dermatol. 2012;53:274-277.
- Aouam K, Ben Romdhane F, Loussaief C, et al. Hypersensitivity syndrome induced by anticonvulsants: possible cross-reactivity between carbamazepine and lamotrigine. J Clin Pharmacol. 2009;49:1488-1491.
- Santiago F, Gonçalo M, Vieira R, et al. Epicutaneous patch testing in drug hypersensitivity syndrome (DRESS). Contact Dermatitis. 2010;62:47-53.
- Prevost P, Bédry R, Lacoste D, et al. Hypersensitivity syndrome with olanzapine confirmed by patch tests. Eur J Dermatol. 2012;22:126-127.
- Hubiche T, Milpied B, Cazeau C, et al. Association of immunologically confirmed delayed drug reaction and human herpesvirus 6 viremia in a pediatric case of drug-induced hypersensitivity syndrome. Dermatology. 2011;222:140-141.
- Song WJ, Shim EJ, Kang MG, et al. Severe drug hypersensitivity induced by erdosteine and doxofylline as confirmed by patch and lymphocyte transformation tests: a case report. J Investig Allergol Clin Immunol. 2012;22:230-232.
- Lee JH, Park HK, Heo J, et al. Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome induced by celecoxib and anti-tuberculosis drugs. J Korean Med Sci. 2008;23:521-525.
- González-Delgado P, Blanes M, Soriano V, et al. Erythema multiforme to amoxicillin with concurrent infection by Epstein-Barr virus. Allergol Immunopathol. 2006;34:76-78.
- Gonzalo Garijo MA, Pérez Calderón R, de Argila Fernández-Durán D, et al. Cutaneous reactions due to diltiazem and cross reactivity with other calcium channel blockers. Allergol Immunopathol (Madr). 2005;33:238-240.
- Peña AL, Henriquezsantana A, Gonzalez-Seco E, et al. Exudative erythema multiforme induced by hydroxyzine. Eur J Dermatol. 2008;18:194-195.
- Arakawa Y, Nakai N, Katoh N. Celecoxib-induced erythema multiforme-type drug eruption with a positive patch test. J Dermatol. 2011;38:1185-1188.
- Prieto A, De barrio M, Pérez C, et al. Piroxicam-induced erythema multiforme. Contact Dermatitis. 2004;50:263.
- Dalmau J, Serra-baldrich E, Roé E, et al. Use of patch test in fixed drug eruption due to metamizole (Nolotil). Contact Dermatitis. 2006;54:127-128.
- Gastaminza G, Anda M, Audicana MT, et al. Fixed-drug eruption due to metronidazole with positive topical provocation. Contact Dermatitis. 2001;44:36.
- Bellini V, Stingeni L, Lisi P. Multifocal fixed drug eruption due to celecoxib. Dermatitis. 2009;20:174-176.
- García CM, Carmena R, García R, et al. Fixed drug eruption from ticlopidine, with positive lesional patch test. Contact Dermatitis. 2001;44:40-41.
- Cruz MJ, Duarte AF, Baudrier T, et al. Lichenoid drug eruption induced by misoprostol. Contact Dermatitis. 2009;61:240-242.
- Alanko K. Patch testing in cutaneous reactions caused by carbamazepine. Contact Dermatitis. 1993;29:254-257.
- Grob M, Scheidegger P, Wüthrich B. Allergic skin reaction to celecoxib. Dermatology. 2000;201:383.
- Alonso JC, Ortega JD, Gonzalo MJ. Cutaneous reaction to oral celecoxib with positive patch test. Contact Dermatitis. 2004;50:48-49.
- Fernandes B, Brites M, Gonçalo M, et al. Maculopapular eruption from sertraline with positive patch tests. Contact Dermatitis. 2000;42:287.
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Klein CE, Trautmann A, Zillikens D, et al. Patch testing in an unusual case of toxic epidermal necrolysis. Contact Dermatitis. 1996;35:175-176.
- Swerlick RA, Campbell CF. Medication dyes as a source of drug allergy. J Drugs Dermatol. 2013;12:99-102.
- Guin JD. Patch testing to FD&C and D&C dyes. Contact Dermatitis. 2003;49:217-218.
- Lowther A, McCormick T, Nedorost S. Systemic contact dermatitis from propylene glycol. Dermatitis. 2008;19:105-108.
- Baeck M, Goossens A. Systemic contact dermatitis to corticosteroids. Allergy. 2012;67:1580-1585.
- Baeck M, Goossens A. Immediate and delayed allergic hypersensitivity to corticosteroids: practical guidelines. Contact Dermatitis. 2012;66:38-45.
- Basedow S, Eigelshoven S, Homey B. Immediate and delayed hypersensitivity to corticosteroids. J Dtsch Dermatol Ges. 2011;9:885-888.
- Johansen JD, Aalto-korte K, Agner T, et al. European Society of Contact Dermatitis guideline for diagnostic patch testing—recommendations on best practice. Contact Dermatitis. 2015;73:195-221.
- Mirakian R, Ewan PW, Durham SR, et al. BSACI guidelines for the management of drug allergy. Clin Exp Allergy. 2009;39:43-61.
- Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259-273.
- Arndt KA, Jick H. Rates of cutaneous reactions to drugs. a report from the Boston Collaborative Drug Surveillance Program. JAMA. 1976;235:918-922.
- Bigby M, Jick S, Jick H, et al. Drug-induced cutaneous reactions. a report from the Boston Collaborative Drug Surveillance Program on 15,483 consecutive inpatients, 1975 to 1982. JAMA. 1986;256:3358-3363.
- Fiszenson-Albala F, Auzerie V, Mahe E, et al. A 6-month prospective survey of cutaneous drug reactions in a hospital setting. Br J Dermatol. 2003;149:1018-1022.
- Thong BY, Leong KP, Tang CY, et al. Drug allergy in a general hospital: results of a novel prospective inpatient reporting system. Ann Allergy Asthma Immunol. 2003;90:342-347.
- Hunziker T, Kunzi UP, Braunschweig S, et al. Comprehensive hospital drug monitoring (CHDM): adverse skin reactions, a 20-year survey. Allergy. 1997;52:388-393.
- Swanbeck G, Dahlberg E. Cutaneous drug reactions. an attempt to quantitative estimation. Arch Dermatol Res. 1992;284:215-218.
- Naldi L, Conforti A, Venegoni M, et al. Cutaneous reactions to drugs. an analysis of spontaneous reports in four Italian regions. Br J Clin Pharmacol. 1999;48:839-846.
- French LE, Prins C. Erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis. In: Bolognia, JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:319-333.
- Vasconcelos C, Magina S, Quirino P, et al. Cutaneous drug reactions to piroxicam. Contact Dermatitis. 1998;39:145.
- Gerber D. Adverse reactions of piroxicam. Drug Intell Clin Pharm. 1987;21:707-710.
- Revuz J, Valeyrie-Allanore L. Drug reactions. In: Bolognia, JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:335-356.
- Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: part II. management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1-709.e9; quiz 718-720.
- Heinzerling LM, Tomsitz D, Anliker MD. Is drug allergy less prevalent than previously assumed? a 5-year analysis. Br J Dermatol. 2012;166:107-114.
- Salkind AR, Cuddy PG. Is this patient allergic to penicillin?: an evidence-based analysis of the likelihood of penicillin allergy. JAMA. 2001;285:2498-2505.
- Torres MJ, Gomez F, Doña I, et al. Diagnostic evaluation of patients with nonimmediate cutaneous hypersensitivity reactions to iodinated contrast media. Allergy. 2012;67:929-935.
- Cham PM, Warshaw EM. Patch testing for evaluating drug reactions due to systemic antibiotics. Dermatitis. 2007;18:63-77.
- Andrade P, Brinca A, Gonçalo M. Patch testing in fixed drug eruptions—a 20-year review. Contact Dermatitis. 2011;65:195-201.
- Romano A, Viola M, Gaeta F, et al. Patch testing in non-immediate drug eruptions. Allergy Asthma Clin Immunol. 2008;4:66-74.
- Rosso R, Mattiacci G, Bernardi ML, et al. Very delayed reactions to beta-lactam antibiotics. Contact Dermatitis. 2000;42:293-295.
- Romano A, Torres MJ, Castells M, et al. Diagnosis and management of drug hypersensitivity reactions. J Allergy Clin Immunol. 2011;127(3 suppl):S67-S73.
- Friedmann PS, Ardern-Jones M. Patch testing in drug allergy. Curr Opin Allergy Clin Immunol. 2010;10:291-296.
- Torres MJ, Mayorga C, Blanca M. Nonimmediate allergic reactions induced by drugs: pathogenesis and diagnostic tests. J Investig Allergol Clin Immunol. 2009;19:80-90.
- Waton J, Tréchot P, Loss-Ayay C, et al. Negative predictive value of drug skin tests in investigating cutaneous adverse drug reactions. Br J Dermatol. 2009;160:786-794.
- Romano A, Viola M, Mondino C, et al. Diagnosing nonimmediate reactions to penicillins by in vivo tests. Int Arch Allergy Immunol. 2002;129:169-174.
- De Groot AC. Patch Testing. Test Concentrations and Vehicles for 4350 Chemicals. 3rd ed. Wapserveen, Netherlands: acdegroot publishing; 2008.
- Elzagallaai AA, Knowles SR, Rieder MJ, et al. Patch testing for the diagnosis of anticonvulsant hypersensitivity syndrome: a systematic review. Drug Saf. 2009;32:391-408.
- Andrade P, Gonçalo M. Fixed drug eruption caused by etoricoxib—2 cases confirmed by patch testing. Contact Dermatitis. 2011;64:118-120.
- Barbaud A, Reichert-Penetrat S, Tréchot P, et al. The use of skin testing in the investigation of cutaneous adverse drug reactions. Br J Dermatol. 1998;139:49-58.
- Wolkenstein P, Chosidow O, Fléchet ML, et al. Patch testing in severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Dermatitis. 1996;35:234-236.
- Harries MJ, McIntyre SJ, Kingston TP. Co-amoxiclav-induced acute generalized exanthematous pustulosis confirmed by patch testing. Contact Dermatitis. 2006;55:372.
- Matsumoto Y, Okubo Y, Yamamoto T, et al. Case of acute generalized exanthematous pustulosis caused by ampicillin/cloxacillin sodium in a pregnant woman. J Dermatol. 2008;35:362-364.
- Chaabane A, Aouam K, Gassab L, et al. Acute generalized exanthematous pustulosis (AGEP) induced by cefotaxime. Fundam Clin Pharmacol. 2010;24:429-432.
- Hausermann P, Scherer K, Weber M, et al. Ciprofloxacin-induced acute generalized exanthematous pustulosis mimicking bullous drug eruption confirmed by a positive patch test. Dermatology. 2005;211:277-280.
- Moreau A, Dompmartin A, Castel B, et al. Drug-induced acute generalized exanthematous pustulosis with positive patch tests. Int J Dermatol. 1995;34:263-266.
- Kempinaire A, De Raevea L, Merckx M, et al. Terbinafine-induced acute generalized exanthematous pustulosis confirmed by a positive patch-test result. J Am Acad Dermatol. 1997;37:653-655.
- Mäkelä L, Lammintausta K. Etoricoxib-induced acute generalized exanthematous pustulosis. Acta Derm Venereol. 2008;88:200-201.
- Yang CC, Lee JY, Chen WC. Acute generalized exanthematous pustulosis caused by celecoxib. J Formos Med Assoc. 2004;103:555-557.
- Kardaun SH, de Monchy JG. Acute generalized exanthematous pustulosis caused by morphine, confirmed by positive patch test and lymphocyte transformation test. J Am Acad Dermatol. 2006;55(2 suppl):S21-S23.
- Inadomi T. Drug rash with eosinophilia and systemic symptoms (DRESS): changing carbamazepine to phenobarbital controlled epilepsy without the recurrence of DRESS. Eur J Dermatol. 2010;20:220-222.
- Buyuktiryaki AB, Bezirganoglu H, Sahiner UM, et al. Patch testing is an effective method for the diagnosis of carbamazepine-induced drug reaction, eosinophilia and systemic symptoms (DRESS) syndrome in an 8-year-old girl. Australas J Dermatol. 2012;53:274-277.
- Aouam K, Ben Romdhane F, Loussaief C, et al. Hypersensitivity syndrome induced by anticonvulsants: possible cross-reactivity between carbamazepine and lamotrigine. J Clin Pharmacol. 2009;49:1488-1491.
- Santiago F, Gonçalo M, Vieira R, et al. Epicutaneous patch testing in drug hypersensitivity syndrome (DRESS). Contact Dermatitis. 2010;62:47-53.
- Prevost P, Bédry R, Lacoste D, et al. Hypersensitivity syndrome with olanzapine confirmed by patch tests. Eur J Dermatol. 2012;22:126-127.
- Hubiche T, Milpied B, Cazeau C, et al. Association of immunologically confirmed delayed drug reaction and human herpesvirus 6 viremia in a pediatric case of drug-induced hypersensitivity syndrome. Dermatology. 2011;222:140-141.
- Song WJ, Shim EJ, Kang MG, et al. Severe drug hypersensitivity induced by erdosteine and doxofylline as confirmed by patch and lymphocyte transformation tests: a case report. J Investig Allergol Clin Immunol. 2012;22:230-232.
- Lee JH, Park HK, Heo J, et al. Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome induced by celecoxib and anti-tuberculosis drugs. J Korean Med Sci. 2008;23:521-525.
- González-Delgado P, Blanes M, Soriano V, et al. Erythema multiforme to amoxicillin with concurrent infection by Epstein-Barr virus. Allergol Immunopathol. 2006;34:76-78.
- Gonzalo Garijo MA, Pérez Calderón R, de Argila Fernández-Durán D, et al. Cutaneous reactions due to diltiazem and cross reactivity with other calcium channel blockers. Allergol Immunopathol (Madr). 2005;33:238-240.
- Peña AL, Henriquezsantana A, Gonzalez-Seco E, et al. Exudative erythema multiforme induced by hydroxyzine. Eur J Dermatol. 2008;18:194-195.
- Arakawa Y, Nakai N, Katoh N. Celecoxib-induced erythema multiforme-type drug eruption with a positive patch test. J Dermatol. 2011;38:1185-1188.
- Prieto A, De barrio M, Pérez C, et al. Piroxicam-induced erythema multiforme. Contact Dermatitis. 2004;50:263.
- Dalmau J, Serra-baldrich E, Roé E, et al. Use of patch test in fixed drug eruption due to metamizole (Nolotil). Contact Dermatitis. 2006;54:127-128.
- Gastaminza G, Anda M, Audicana MT, et al. Fixed-drug eruption due to metronidazole with positive topical provocation. Contact Dermatitis. 2001;44:36.
- Bellini V, Stingeni L, Lisi P. Multifocal fixed drug eruption due to celecoxib. Dermatitis. 2009;20:174-176.
- García CM, Carmena R, García R, et al. Fixed drug eruption from ticlopidine, with positive lesional patch test. Contact Dermatitis. 2001;44:40-41.
- Cruz MJ, Duarte AF, Baudrier T, et al. Lichenoid drug eruption induced by misoprostol. Contact Dermatitis. 2009;61:240-242.
- Alanko K. Patch testing in cutaneous reactions caused by carbamazepine. Contact Dermatitis. 1993;29:254-257.
- Grob M, Scheidegger P, Wüthrich B. Allergic skin reaction to celecoxib. Dermatology. 2000;201:383.
- Alonso JC, Ortega JD, Gonzalo MJ. Cutaneous reaction to oral celecoxib with positive patch test. Contact Dermatitis. 2004;50:48-49.
- Fernandes B, Brites M, Gonçalo M, et al. Maculopapular eruption from sertraline with positive patch tests. Contact Dermatitis. 2000;42:287.
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Klein CE, Trautmann A, Zillikens D, et al. Patch testing in an unusual case of toxic epidermal necrolysis. Contact Dermatitis. 1996;35:175-176.
- Swerlick RA, Campbell CF. Medication dyes as a source of drug allergy. J Drugs Dermatol. 2013;12:99-102.
- Guin JD. Patch testing to FD&C and D&C dyes. Contact Dermatitis. 2003;49:217-218.
- Lowther A, McCormick T, Nedorost S. Systemic contact dermatitis from propylene glycol. Dermatitis. 2008;19:105-108.
- Baeck M, Goossens A. Systemic contact dermatitis to corticosteroids. Allergy. 2012;67:1580-1585.
- Baeck M, Goossens A. Immediate and delayed allergic hypersensitivity to corticosteroids: practical guidelines. Contact Dermatitis. 2012;66:38-45.
- Basedow S, Eigelshoven S, Homey B. Immediate and delayed hypersensitivity to corticosteroids. J Dtsch Dermatol Ges. 2011;9:885-888.
- Johansen JD, Aalto-korte K, Agner T, et al. European Society of Contact Dermatitis guideline for diagnostic patch testing—recommendations on best practice. Contact Dermatitis. 2015;73:195-221.
- Mirakian R, Ewan PW, Durham SR, et al. BSACI guidelines for the management of drug allergy. Clin Exp Allergy. 2009;39:43-61.
- Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259-273.
Practice Points
- Consider patch testing in suspected eczematous drug rashes and fixed drug eruption.
- Patch test to inactive excipients as well as active ingredients.
- Caution patients that sensitivity of patch testing for systemic drug reactions is unknown and likely lower than specificity.
Oral Fixed Drug Eruption Due to Tinidazole
To the Editor:
A 50-year-old man presented with a painful ulcer and a burning sensation on the tongue of 2 days’ duration (Figure, A). The ulcer had a yellowish white appearance with erythematous borders. The patient started taking tinidazole 500 mg twice daily 2 days prior, which was prescribed by his primary care physician for an episode of gastroenteritis. He was not taking any other medications and did not smoke or drink. Routine laboratory test results did not reveal any abnormalities. Based on the physical examination as well as the patient’s medical and medication history, a provisional diagnosis of fixed drug eruption (FDE) due to tinidazole was made. Tinidazole was immediately withdrawn and the patient was prescribed beclomethasone dipropionate ointment twice daily to relieve the burning sensation. A punch biopsy of the lesion was recommended; however, the patient opted to wait a week after discontinuing the drug. At follow-up 1 week later, complete healing of the ulcer was observed with no scarring and the burning sensation had resolved (Figure, B). After obtaining informed consent from the patient, an oral challenge test was conducted in the office with 50 mg of tinidazole. Four hours after taking the drug orally, the patient felt a burning sensation and a small ulcerative lesion was observed on the tongue at the same site the next day. The patient was informed of the fixed drug reaction to tinidazole, a drug belonging to the nitroimidazole group, and this information also was conveyed to the patient’s primary care physician.
Tinidazole is a synthetic antiprotozoal and antibacterial agent used primarily in infections such as amebiasis, giardiasis, and trichomoniasis.1 Tinidazole may be a therapeutic alternative to metronidazole. Fixed drug eruption is a distinctive variant of drug eruption with characteristic recurrence at the same site of skin or mucous membranes. It is characterized by onset of round/oval, erythematous, well-defined macules on the skin and/or mucosa associated with itching and burning.1 Fixed drug eruption generally is restricted to the mucous membrane and skin, with the lips, palms, soles, glans penis, and groin area being the most common sites. Intraoral involvement, excluding the lips, of FDE is rare. The tongue is a rare site of an FDE.2 Fixed drug eruption on the tongue has been reported with clarithromycin.3 Dental clinicians have to be aware of the possibility of FDE due to commonly used drugs such tinidazole, which would help in prompt diagnosis of these lesions.
- Prieto A, De Barrio M, Infante S, et al. Recurrent fixed drug eruption due to metronidazole elicited by patch test with tinidazole. Contact Dermatitis. 2005;53:169-170.
- Dhar S, Kanwar AJ. Fixed drug eruption on the tongue of a 4-year-old boy. Pediatr Dermatol. 1995;12:51-52.
- Alonso JC, Melgosa AC, Gonzalo MJ, et al. Fixed drug eruption on the tongue due to clarithromycin. Contact Dermatitis. 2005;53:121-122.
To the Editor:
A 50-year-old man presented with a painful ulcer and a burning sensation on the tongue of 2 days’ duration (Figure, A). The ulcer had a yellowish white appearance with erythematous borders. The patient started taking tinidazole 500 mg twice daily 2 days prior, which was prescribed by his primary care physician for an episode of gastroenteritis. He was not taking any other medications and did not smoke or drink. Routine laboratory test results did not reveal any abnormalities. Based on the physical examination as well as the patient’s medical and medication history, a provisional diagnosis of fixed drug eruption (FDE) due to tinidazole was made. Tinidazole was immediately withdrawn and the patient was prescribed beclomethasone dipropionate ointment twice daily to relieve the burning sensation. A punch biopsy of the lesion was recommended; however, the patient opted to wait a week after discontinuing the drug. At follow-up 1 week later, complete healing of the ulcer was observed with no scarring and the burning sensation had resolved (Figure, B). After obtaining informed consent from the patient, an oral challenge test was conducted in the office with 50 mg of tinidazole. Four hours after taking the drug orally, the patient felt a burning sensation and a small ulcerative lesion was observed on the tongue at the same site the next day. The patient was informed of the fixed drug reaction to tinidazole, a drug belonging to the nitroimidazole group, and this information also was conveyed to the patient’s primary care physician.

Tinidazole is a synthetic antiprotozoal and antibacterial agent used primarily in infections such as amebiasis, giardiasis, and trichomoniasis.1 Tinidazole may be a therapeutic alternative to metronidazole. Fixed drug eruption is a distinctive variant of drug eruption with characteristic recurrence at the same site of skin or mucous membranes. It is characterized by onset of round/oval, erythematous, well-defined macules on the skin and/or mucosa associated with itching and burning.1 Fixed drug eruption generally is restricted to the mucous membrane and skin, with the lips, palms, soles, glans penis, and groin area being the most common sites. Intraoral involvement, excluding the lips, of FDE is rare. The tongue is a rare site of an FDE.2 Fixed drug eruption on the tongue has been reported with clarithromycin.3 Dental clinicians have to be aware of the possibility of FDE due to commonly used drugs such tinidazole, which would help in prompt diagnosis of these lesions.
To the Editor:
A 50-year-old man presented with a painful ulcer and a burning sensation on the tongue of 2 days’ duration (Figure, A). The ulcer had a yellowish white appearance with erythematous borders. The patient started taking tinidazole 500 mg twice daily 2 days prior, which was prescribed by his primary care physician for an episode of gastroenteritis. He was not taking any other medications and did not smoke or drink. Routine laboratory test results did not reveal any abnormalities. Based on the physical examination as well as the patient’s medical and medication history, a provisional diagnosis of fixed drug eruption (FDE) due to tinidazole was made. Tinidazole was immediately withdrawn and the patient was prescribed beclomethasone dipropionate ointment twice daily to relieve the burning sensation. A punch biopsy of the lesion was recommended; however, the patient opted to wait a week after discontinuing the drug. At follow-up 1 week later, complete healing of the ulcer was observed with no scarring and the burning sensation had resolved (Figure, B). After obtaining informed consent from the patient, an oral challenge test was conducted in the office with 50 mg of tinidazole. Four hours after taking the drug orally, the patient felt a burning sensation and a small ulcerative lesion was observed on the tongue at the same site the next day. The patient was informed of the fixed drug reaction to tinidazole, a drug belonging to the nitroimidazole group, and this information also was conveyed to the patient’s primary care physician.

Tinidazole is a synthetic antiprotozoal and antibacterial agent used primarily in infections such as amebiasis, giardiasis, and trichomoniasis.1 Tinidazole may be a therapeutic alternative to metronidazole. Fixed drug eruption is a distinctive variant of drug eruption with characteristic recurrence at the same site of skin or mucous membranes. It is characterized by onset of round/oval, erythematous, well-defined macules on the skin and/or mucosa associated with itching and burning.1 Fixed drug eruption generally is restricted to the mucous membrane and skin, with the lips, palms, soles, glans penis, and groin area being the most common sites. Intraoral involvement, excluding the lips, of FDE is rare. The tongue is a rare site of an FDE.2 Fixed drug eruption on the tongue has been reported with clarithromycin.3 Dental clinicians have to be aware of the possibility of FDE due to commonly used drugs such tinidazole, which would help in prompt diagnosis of these lesions.
- Prieto A, De Barrio M, Infante S, et al. Recurrent fixed drug eruption due to metronidazole elicited by patch test with tinidazole. Contact Dermatitis. 2005;53:169-170.
- Dhar S, Kanwar AJ. Fixed drug eruption on the tongue of a 4-year-old boy. Pediatr Dermatol. 1995;12:51-52.
- Alonso JC, Melgosa AC, Gonzalo MJ, et al. Fixed drug eruption on the tongue due to clarithromycin. Contact Dermatitis. 2005;53:121-122.
- Prieto A, De Barrio M, Infante S, et al. Recurrent fixed drug eruption due to metronidazole elicited by patch test with tinidazole. Contact Dermatitis. 2005;53:169-170.
- Dhar S, Kanwar AJ. Fixed drug eruption on the tongue of a 4-year-old boy. Pediatr Dermatol. 1995;12:51-52.
- Alonso JC, Melgosa AC, Gonzalo MJ, et al. Fixed drug eruption on the tongue due to clarithromycin. Contact Dermatitis. 2005;53:121-122.
Practice Points
- Fixed drug eruption (FDE) is characterized by onset of round/oval, erythematous, well-defined macules on the skin and/or mucosa associated with itching and burning.
- Intraoral involvement of FDE is rare.
- Tinidazole may cause FDE and should be suspected in patients with a spontaneous eruption of macules on mucous membranes.