User login
VIDEO: When to consider systemic exposure in patients with contact dermatitis
SAN FRANCISCO – When patients with contact dermatitis who have had a patch test positive to an allergen and are not improving despite avoiding cutaneous exposure, it’s important to consider the possibility of systemic exposure, according to Nina Botto, MD, of the department of dermatology, at the University of California, San Francisco.
“Theoretically, any allergen can cause a systemic contact dermatitis. The ones that we think about and encounter more frequently are earth metals like nickel and balsam of Peru, which is a component of many fragrances and flavorings,” she said in a video interview at the annual meeting of the Pacific Dermatologic Association.
In the interview, Dr. Botto, who is codirector of the Occupational and Contact Dermatitis Clinic at UCSF, provides recommendations on how to approach patients with systemic contact dermatitis, including dietary avoidance. But following these diets can be challenging. She recommends starting with avoiding cutaneous exposure to the suspected allergen. For patients not improving after two months of avoidance, “it may be reasonable to consider a diet,”she advised.
Dr. Botto cited the following two publications with tables and guidelines for diets as helpful resources for patients: Dermatitis. 2013 Jul-Aug;24(4):153-60 (for a diet low in balsam of Peru); and Dermatitis. 2013 Jul-Aug; 24(4):190-5 (for a diet low in nickel).
Another useful resource is the American Contact Dermatitis Society website, which produces a customized list of safe products for patients after they enter the allergen into the system.
Dr. Botto had no disclosures.
SAN FRANCISCO – When patients with contact dermatitis who have had a patch test positive to an allergen and are not improving despite avoiding cutaneous exposure, it’s important to consider the possibility of systemic exposure, according to Nina Botto, MD, of the department of dermatology, at the University of California, San Francisco.
“Theoretically, any allergen can cause a systemic contact dermatitis. The ones that we think about and encounter more frequently are earth metals like nickel and balsam of Peru, which is a component of many fragrances and flavorings,” she said in a video interview at the annual meeting of the Pacific Dermatologic Association.
In the interview, Dr. Botto, who is codirector of the Occupational and Contact Dermatitis Clinic at UCSF, provides recommendations on how to approach patients with systemic contact dermatitis, including dietary avoidance. But following these diets can be challenging. She recommends starting with avoiding cutaneous exposure to the suspected allergen. For patients not improving after two months of avoidance, “it may be reasonable to consider a diet,”she advised.
Dr. Botto cited the following two publications with tables and guidelines for diets as helpful resources for patients: Dermatitis. 2013 Jul-Aug;24(4):153-60 (for a diet low in balsam of Peru); and Dermatitis. 2013 Jul-Aug; 24(4):190-5 (for a diet low in nickel).
Another useful resource is the American Contact Dermatitis Society website, which produces a customized list of safe products for patients after they enter the allergen into the system.
Dr. Botto had no disclosures.
SAN FRANCISCO – When patients with contact dermatitis who have had a patch test positive to an allergen and are not improving despite avoiding cutaneous exposure, it’s important to consider the possibility of systemic exposure, according to Nina Botto, MD, of the department of dermatology, at the University of California, San Francisco.
“Theoretically, any allergen can cause a systemic contact dermatitis. The ones that we think about and encounter more frequently are earth metals like nickel and balsam of Peru, which is a component of many fragrances and flavorings,” she said in a video interview at the annual meeting of the Pacific Dermatologic Association.
In the interview, Dr. Botto, who is codirector of the Occupational and Contact Dermatitis Clinic at UCSF, provides recommendations on how to approach patients with systemic contact dermatitis, including dietary avoidance. But following these diets can be challenging. She recommends starting with avoiding cutaneous exposure to the suspected allergen. For patients not improving after two months of avoidance, “it may be reasonable to consider a diet,”she advised.
Dr. Botto cited the following two publications with tables and guidelines for diets as helpful resources for patients: Dermatitis. 2013 Jul-Aug;24(4):153-60 (for a diet low in balsam of Peru); and Dermatitis. 2013 Jul-Aug; 24(4):190-5 (for a diet low in nickel).
Another useful resource is the American Contact Dermatitis Society website, which produces a customized list of safe products for patients after they enter the allergen into the system.
Dr. Botto had no disclosures.
AT THE ANNUAL MEETING OF THE PACIFIC DERMATOLOGIC ASSOCIATION
Bar soaps may be better than body washes for contact dermatitis patients
SAN FRANCISCO – Chronic contact dermatitis often is tied to hidden allergens found in shampoos, soaps, and body washes, according to Cory Dunnick, MD.“A lot of patients who get referred to my patch test clinic will have chronic dermatitis that isn’t responding to treatment or is worsening despite treatment, or they present with a pattern that is suggestive of contact dermatitis,” she said in an interview.
There is also a common perception that liquid body washes are better than bar soaps because they may be more moisturizing, but the results of a recently published study suggest otherwise, Dr. Dunnick of the department of dermatology at the University of Colorado at Denver, Aurora, said at the annual meeting of the Pacific Dermatologic Association.
Dr. Dunnick was one of the investigators in a study that compared ingredients in the top-selling 50 bar soaps and 50 body washes on Amazon.com to determine if there was a difference with respect to allergen content. They obtained the ingredients list for all the products and compared them with the American Contact Dermatitis Society Core Allergen Series. Counter to the common belief, results of the study indicated that liquid soaps were likely the worse choice for sensitive patients: They contained far more preservative and surfactant allergens than bar soaps, and there was no difference in fragrance content between the two classes (Dermatitis. 2017 May 23. doi: 10.1097/DER.0000000000000289).
Of the 50 liquid soaps, 44 had one or more preservative allergens, compared with none of the bar soaps (P less than .001), and 34 had at least one surfactant allergen, compared with seven of the bar soaps (P less than .001). Forty-eight body washes had fragrance, as did 47 of the bar soaps.
The most common allergens in body washes were methylisothiazolinone (19 of 50), quaternium-15 (16), sodium benzoate (15), methylchloroisothiazolinone/methylisothiazolinone (12), DMDM hydantoin (10), and phenoxyethanol (9). None of these allergens appeared in any of the bar soaps.
“If you have a patient who you suspect has a contact allergy to a preservative or surfactant ingredient, then you can recommend perhaps switching to a bar soap, maybe one that is fragrance free,” advised Dr. Dunnick.
The most common allergen they found in body washes, methylisothiazolinone (MI), is becoming an increasing concern, she said. It has been around for many years but became more prevalent when the Food and Drug Administration decided in 2005 to allow higher concentrations of MI to be used in skin care products. “It’s a pretty strong sensitizer. As a result, we’re seeing a lot more allergy,” she noted.
This soap/body-wash allergen study sends a clear message to dermatologists to individualize recommendations, she said. “A lot of dermatologists recommend what they think are mild soaps, but they don’t necessarily think about what contact allergens might be in those soaps, so maybe they need to make more specific recommendations. They might recommend Dove soap,” but there are different Dove soaps, she pointed out.
A bigger challenge is finding a shampoo for sensitive patients. Almost all contain fragrances, and MI is an ingredient in many shampoos as well. Dr. Dunnick has found the DHS brand, which is fragrance free, to be helpful in some cases, and the Nonscents brand, also fragrance free, is sometimes recommended as safe.
But, in the end, recommendations must be individualized for the patient’s specific allergies, and that requires a thorough work-up. “You don’t know what they are unless you do the patch test,” she said.
Dr. Dunnick reported having no relevant financial disclosures.
SAN FRANCISCO – Chronic contact dermatitis often is tied to hidden allergens found in shampoos, soaps, and body washes, according to Cory Dunnick, MD.“A lot of patients who get referred to my patch test clinic will have chronic dermatitis that isn’t responding to treatment or is worsening despite treatment, or they present with a pattern that is suggestive of contact dermatitis,” she said in an interview.
There is also a common perception that liquid body washes are better than bar soaps because they may be more moisturizing, but the results of a recently published study suggest otherwise, Dr. Dunnick of the department of dermatology at the University of Colorado at Denver, Aurora, said at the annual meeting of the Pacific Dermatologic Association.
Dr. Dunnick was one of the investigators in a study that compared ingredients in the top-selling 50 bar soaps and 50 body washes on Amazon.com to determine if there was a difference with respect to allergen content. They obtained the ingredients list for all the products and compared them with the American Contact Dermatitis Society Core Allergen Series. Counter to the common belief, results of the study indicated that liquid soaps were likely the worse choice for sensitive patients: They contained far more preservative and surfactant allergens than bar soaps, and there was no difference in fragrance content between the two classes (Dermatitis. 2017 May 23. doi: 10.1097/DER.0000000000000289).
Of the 50 liquid soaps, 44 had one or more preservative allergens, compared with none of the bar soaps (P less than .001), and 34 had at least one surfactant allergen, compared with seven of the bar soaps (P less than .001). Forty-eight body washes had fragrance, as did 47 of the bar soaps.
The most common allergens in body washes were methylisothiazolinone (19 of 50), quaternium-15 (16), sodium benzoate (15), methylchloroisothiazolinone/methylisothiazolinone (12), DMDM hydantoin (10), and phenoxyethanol (9). None of these allergens appeared in any of the bar soaps.
“If you have a patient who you suspect has a contact allergy to a preservative or surfactant ingredient, then you can recommend perhaps switching to a bar soap, maybe one that is fragrance free,” advised Dr. Dunnick.
The most common allergen they found in body washes, methylisothiazolinone (MI), is becoming an increasing concern, she said. It has been around for many years but became more prevalent when the Food and Drug Administration decided in 2005 to allow higher concentrations of MI to be used in skin care products. “It’s a pretty strong sensitizer. As a result, we’re seeing a lot more allergy,” she noted.
This soap/body-wash allergen study sends a clear message to dermatologists to individualize recommendations, she said. “A lot of dermatologists recommend what they think are mild soaps, but they don’t necessarily think about what contact allergens might be in those soaps, so maybe they need to make more specific recommendations. They might recommend Dove soap,” but there are different Dove soaps, she pointed out.
A bigger challenge is finding a shampoo for sensitive patients. Almost all contain fragrances, and MI is an ingredient in many shampoos as well. Dr. Dunnick has found the DHS brand, which is fragrance free, to be helpful in some cases, and the Nonscents brand, also fragrance free, is sometimes recommended as safe.
But, in the end, recommendations must be individualized for the patient’s specific allergies, and that requires a thorough work-up. “You don’t know what they are unless you do the patch test,” she said.
Dr. Dunnick reported having no relevant financial disclosures.
SAN FRANCISCO – Chronic contact dermatitis often is tied to hidden allergens found in shampoos, soaps, and body washes, according to Cory Dunnick, MD.“A lot of patients who get referred to my patch test clinic will have chronic dermatitis that isn’t responding to treatment or is worsening despite treatment, or they present with a pattern that is suggestive of contact dermatitis,” she said in an interview.
There is also a common perception that liquid body washes are better than bar soaps because they may be more moisturizing, but the results of a recently published study suggest otherwise, Dr. Dunnick of the department of dermatology at the University of Colorado at Denver, Aurora, said at the annual meeting of the Pacific Dermatologic Association.
Dr. Dunnick was one of the investigators in a study that compared ingredients in the top-selling 50 bar soaps and 50 body washes on Amazon.com to determine if there was a difference with respect to allergen content. They obtained the ingredients list for all the products and compared them with the American Contact Dermatitis Society Core Allergen Series. Counter to the common belief, results of the study indicated that liquid soaps were likely the worse choice for sensitive patients: They contained far more preservative and surfactant allergens than bar soaps, and there was no difference in fragrance content between the two classes (Dermatitis. 2017 May 23. doi: 10.1097/DER.0000000000000289).
Of the 50 liquid soaps, 44 had one or more preservative allergens, compared with none of the bar soaps (P less than .001), and 34 had at least one surfactant allergen, compared with seven of the bar soaps (P less than .001). Forty-eight body washes had fragrance, as did 47 of the bar soaps.
The most common allergens in body washes were methylisothiazolinone (19 of 50), quaternium-15 (16), sodium benzoate (15), methylchloroisothiazolinone/methylisothiazolinone (12), DMDM hydantoin (10), and phenoxyethanol (9). None of these allergens appeared in any of the bar soaps.
“If you have a patient who you suspect has a contact allergy to a preservative or surfactant ingredient, then you can recommend perhaps switching to a bar soap, maybe one that is fragrance free,” advised Dr. Dunnick.
The most common allergen they found in body washes, methylisothiazolinone (MI), is becoming an increasing concern, she said. It has been around for many years but became more prevalent when the Food and Drug Administration decided in 2005 to allow higher concentrations of MI to be used in skin care products. “It’s a pretty strong sensitizer. As a result, we’re seeing a lot more allergy,” she noted.
This soap/body-wash allergen study sends a clear message to dermatologists to individualize recommendations, she said. “A lot of dermatologists recommend what they think are mild soaps, but they don’t necessarily think about what contact allergens might be in those soaps, so maybe they need to make more specific recommendations. They might recommend Dove soap,” but there are different Dove soaps, she pointed out.
A bigger challenge is finding a shampoo for sensitive patients. Almost all contain fragrances, and MI is an ingredient in many shampoos as well. Dr. Dunnick has found the DHS brand, which is fragrance free, to be helpful in some cases, and the Nonscents brand, also fragrance free, is sometimes recommended as safe.
But, in the end, recommendations must be individualized for the patient’s specific allergies, and that requires a thorough work-up. “You don’t know what they are unless you do the patch test,” she said.
Dr. Dunnick reported having no relevant financial disclosures.
AT PDA 2017
Expert shares tips for spotting allergic contact dermatitis in children
CHICAGO – If severe eczema persists in a pediatric patient despite your best treatment efforts, think allergic contact dermatitis.
“Or, if your eczema patients tell you that they have a cream that’s making things worse, you should think about a contact allergen,” Catalina Matiz, MD, said at the World Congress of Pediatric Dermatology.
Allergic contact dermatitis (ACD) is a type IV delayed-type hypersensitivity reaction to haptens that come into contact with the skin. Poison ivy is a common plant-based culprit, while nickel is the most common metal allergen in adults and children. “The skin barrier also plays a role,” said Dr. Matiz of the department of dermatology at Rady Children’s Hospital–San Diego, and the University of California, San Diego. “Compared with adults, children have a thinner stratum corneum, and some haptens can penetrate the skin. Some studies suggest that patients with atopic dermatitis may have increased rates of allergic sensitization, and filaggrin mutations have been found in patients with atopic dermatitis and in patients with ACD to nickel. Filaggrin helps to aggregate the cytoskeletal proteins that form the cornified cell envelope. Without filaggrin, the skin barrier is defective.”
The top 10 pediatric allergens found in personal hygiene products across five studies in the medical literature include neomycin, balsam of Peru, fragrance mix, benzalkonium chloride, lanolin, cocamidopropyl betaine, formaldehyde, methylchloroisothiazolinone/methylisothiazolinone (MCI/MI), propylene glycol, and corticosteroids. Dr. Matiz makes it practice to patch test as a last resort. “I always try to get a history, try to improve their symptoms, and have them start avoidance first, following the preemptive avoidance list,” she said (Expert Rev Clin Immunol. 2016;12[5]:551-61).
The T.R.U.E. test includes 35 allergens. “The T.R.U.E test is a good tool, which can capture up to 70% of relevant reactions in children with the inconvenience that some of the allergens in the test are not that relevant in children, and it’s not yet [Food and Drug Administration] approved to use in children,” she noted. The comprehensive chamber test allows you to select from unlimited number of allergens, “but that’s difficult. You have to have specialized staff to help you make the cells.”
A list of the minimum 20 allergens you should test for in children and the recommended supplemental allergens depending on history and locations of their dermatitis can be found in the following article: Curr Allergy Asthma Rep 2014;14[6]:444. “I always tell patients when they come for consultations to bring in everything they’re using: their shampoos, creams, and medications, because we want to see what they’re exposed to, so we can select the right allergens and also test their own products,” Dr. Matiz said. She recommends avoiding testing for strong sensitizers such as paraphenylenediamine, in children younger than 12 years of age who don’t have a history of exposure.
Testing tips for children younger than age 5 include decreasing concentrations to half for nickel, formaldehyde, and rubber accelerators. “Don’t test for paraphenylenediamine unless there is high suspicion,” she said. “Consider removing patches by 24 hours in the very young.”
The best antidote to contact dermatitis is avoidance of the known trigger. “You want to spend a lot of time with patients and parents on this,” she advised. “Give a list of safe products to use from the American Contact Dermatitis Society’s Contact Allergen Management Program [www.contactderm.org], and provide handouts about the location and history of positive allergens [www.truetest.com].” And, she added, “make a plan of treatment and follow-up in 6 weeks.”
Dr. Matiz disclosed that she is a subinvestigator in the Clinical Evaluation of T.R.U.E Test Panel 3.3 in Children and Adolescents study.
CHICAGO – If severe eczema persists in a pediatric patient despite your best treatment efforts, think allergic contact dermatitis.
“Or, if your eczema patients tell you that they have a cream that’s making things worse, you should think about a contact allergen,” Catalina Matiz, MD, said at the World Congress of Pediatric Dermatology.
Allergic contact dermatitis (ACD) is a type IV delayed-type hypersensitivity reaction to haptens that come into contact with the skin. Poison ivy is a common plant-based culprit, while nickel is the most common metal allergen in adults and children. “The skin barrier also plays a role,” said Dr. Matiz of the department of dermatology at Rady Children’s Hospital–San Diego, and the University of California, San Diego. “Compared with adults, children have a thinner stratum corneum, and some haptens can penetrate the skin. Some studies suggest that patients with atopic dermatitis may have increased rates of allergic sensitization, and filaggrin mutations have been found in patients with atopic dermatitis and in patients with ACD to nickel. Filaggrin helps to aggregate the cytoskeletal proteins that form the cornified cell envelope. Without filaggrin, the skin barrier is defective.”
The top 10 pediatric allergens found in personal hygiene products across five studies in the medical literature include neomycin, balsam of Peru, fragrance mix, benzalkonium chloride, lanolin, cocamidopropyl betaine, formaldehyde, methylchloroisothiazolinone/methylisothiazolinone (MCI/MI), propylene glycol, and corticosteroids. Dr. Matiz makes it practice to patch test as a last resort. “I always try to get a history, try to improve their symptoms, and have them start avoidance first, following the preemptive avoidance list,” she said (Expert Rev Clin Immunol. 2016;12[5]:551-61).
The T.R.U.E. test includes 35 allergens. “The T.R.U.E test is a good tool, which can capture up to 70% of relevant reactions in children with the inconvenience that some of the allergens in the test are not that relevant in children, and it’s not yet [Food and Drug Administration] approved to use in children,” she noted. The comprehensive chamber test allows you to select from unlimited number of allergens, “but that’s difficult. You have to have specialized staff to help you make the cells.”
A list of the minimum 20 allergens you should test for in children and the recommended supplemental allergens depending on history and locations of their dermatitis can be found in the following article: Curr Allergy Asthma Rep 2014;14[6]:444. “I always tell patients when they come for consultations to bring in everything they’re using: their shampoos, creams, and medications, because we want to see what they’re exposed to, so we can select the right allergens and also test their own products,” Dr. Matiz said. She recommends avoiding testing for strong sensitizers such as paraphenylenediamine, in children younger than 12 years of age who don’t have a history of exposure.
Testing tips for children younger than age 5 include decreasing concentrations to half for nickel, formaldehyde, and rubber accelerators. “Don’t test for paraphenylenediamine unless there is high suspicion,” she said. “Consider removing patches by 24 hours in the very young.”
The best antidote to contact dermatitis is avoidance of the known trigger. “You want to spend a lot of time with patients and parents on this,” she advised. “Give a list of safe products to use from the American Contact Dermatitis Society’s Contact Allergen Management Program [www.contactderm.org], and provide handouts about the location and history of positive allergens [www.truetest.com].” And, she added, “make a plan of treatment and follow-up in 6 weeks.”
Dr. Matiz disclosed that she is a subinvestigator in the Clinical Evaluation of T.R.U.E Test Panel 3.3 in Children and Adolescents study.
CHICAGO – If severe eczema persists in a pediatric patient despite your best treatment efforts, think allergic contact dermatitis.
“Or, if your eczema patients tell you that they have a cream that’s making things worse, you should think about a contact allergen,” Catalina Matiz, MD, said at the World Congress of Pediatric Dermatology.
Allergic contact dermatitis (ACD) is a type IV delayed-type hypersensitivity reaction to haptens that come into contact with the skin. Poison ivy is a common plant-based culprit, while nickel is the most common metal allergen in adults and children. “The skin barrier also plays a role,” said Dr. Matiz of the department of dermatology at Rady Children’s Hospital–San Diego, and the University of California, San Diego. “Compared with adults, children have a thinner stratum corneum, and some haptens can penetrate the skin. Some studies suggest that patients with atopic dermatitis may have increased rates of allergic sensitization, and filaggrin mutations have been found in patients with atopic dermatitis and in patients with ACD to nickel. Filaggrin helps to aggregate the cytoskeletal proteins that form the cornified cell envelope. Without filaggrin, the skin barrier is defective.”
The top 10 pediatric allergens found in personal hygiene products across five studies in the medical literature include neomycin, balsam of Peru, fragrance mix, benzalkonium chloride, lanolin, cocamidopropyl betaine, formaldehyde, methylchloroisothiazolinone/methylisothiazolinone (MCI/MI), propylene glycol, and corticosteroids. Dr. Matiz makes it practice to patch test as a last resort. “I always try to get a history, try to improve their symptoms, and have them start avoidance first, following the preemptive avoidance list,” she said (Expert Rev Clin Immunol. 2016;12[5]:551-61).
The T.R.U.E. test includes 35 allergens. “The T.R.U.E test is a good tool, which can capture up to 70% of relevant reactions in children with the inconvenience that some of the allergens in the test are not that relevant in children, and it’s not yet [Food and Drug Administration] approved to use in children,” she noted. The comprehensive chamber test allows you to select from unlimited number of allergens, “but that’s difficult. You have to have specialized staff to help you make the cells.”
A list of the minimum 20 allergens you should test for in children and the recommended supplemental allergens depending on history and locations of their dermatitis can be found in the following article: Curr Allergy Asthma Rep 2014;14[6]:444. “I always tell patients when they come for consultations to bring in everything they’re using: their shampoos, creams, and medications, because we want to see what they’re exposed to, so we can select the right allergens and also test their own products,” Dr. Matiz said. She recommends avoiding testing for strong sensitizers such as paraphenylenediamine, in children younger than 12 years of age who don’t have a history of exposure.
Testing tips for children younger than age 5 include decreasing concentrations to half for nickel, formaldehyde, and rubber accelerators. “Don’t test for paraphenylenediamine unless there is high suspicion,” she said. “Consider removing patches by 24 hours in the very young.”
The best antidote to contact dermatitis is avoidance of the known trigger. “You want to spend a lot of time with patients and parents on this,” she advised. “Give a list of safe products to use from the American Contact Dermatitis Society’s Contact Allergen Management Program [www.contactderm.org], and provide handouts about the location and history of positive allergens [www.truetest.com].” And, she added, “make a plan of treatment and follow-up in 6 weeks.”
Dr. Matiz disclosed that she is a subinvestigator in the Clinical Evaluation of T.R.U.E Test Panel 3.3 in Children and Adolescents study.
AT WCPD 2017
Pediatric Pearls From the AAD Annual Meeting
This article exhibits key pediatric dermatology pearls garnered at the 2017 Annual Meeting of the American Academy of Dermatology (AAD) in Orlando, Florida (March 3–7, 2017). Highlights from both the Society for Pediatric Dermatology pre-AAD meeting (March 2, 2017) and the AAD general meeting sessions are included. This discussion is intended to help maximize care of our pediatric patients in dermatology and present high-yield take-home points from the AAD that can be readily transferred to our patient care.
“New Tools for Your Therapeutic Toolbox” by Erin Mathes, MD (University of California, San Francisco)
During this lecture at the Society for Pediatric Dermatology meeting, Dr. Mathes discussed a randomized controlled trial that took place in 2014 in both the United States and the United Kingdom to assess skin barrier enhancement to reduce the incidence of atopic dermatitis (AD) in 124 high-risk infants.1 The high-risk infants had either a parent or sibling with physician-diagnosed AD, asthma, or rhinitis, or a first-degree relative with an aforementioned condition. Full-body emollient therapy was applied at least once daily within 3 weeks of birth for 6 months, while the control arm did not use emollient. Parents were allowed to choose from the following emollients: sunflower seed oil, moisturizing cream, or ointment. The primary outcome was the incidence of AD at 6 months. The authors found a 43% incidence of AD in the control group compared to 22% in the emollient group, amounting to a relative risk reduction of approximately 50%.1
Emollients in AD are hypothesized to help through the enhanced barrier function and decreased penetration of irritant substances and allergens. This study is vital given the ease of use of emollients and the foreseeable substantial impact on reduced health care costs associated with the decreased incidence of AD.
Take-Home Point
Full-body emollient therapy within 3 weeks of birth may reduce the incidence of AD in high-risk infants.
Dr. Mathes also discussed the novel topical phosphodiesterase 4 inhibitor crisaborole and its emerging role in AD. She reviewed the results of a large phase 3 trial of crisaborole therapy for patients aged 2 years or older with mild to moderate AD.2 Crisaborole ointment was applied twice daily for 28 days. The primary outcome measured was an investigator static global assessment score of clear or almost clear, which is a score for AD based on the degree of erythema, presence of oozing and crusting, and presence of induration or papulation. Overall, 32.8% of patients treated with crisaborole achieved success compared to 25.4% of vehicle-treated patients. The control patients were still given a vehicle to apply, which can function as therapy to help repair the barrier of AD and thus theoretically reduced the percentage gap between patients who met success with and without crisaborole therapy. Furthermore, only 4% of patients reported adverse effects such as burning and stinging with application of crisaborole in contrast to topical calcineurin inhibitors, which can elicit symptoms up to 50% of the time.2 In summary, this lecture reviewed the first new topical treatment for AD in 15 years.
Take-Home Point
Crisaborole ointment is a novel topical phosphodiesterase 4 inhibitor approved for mild to moderate AD in patients 2 years of age and older.
“The Truth About Pediatric Contact Dermatitis” by Sharon Jacob, MD (Loma Linda University, California)
In this session, Dr. Jacob discussed how she approaches pediatric patients with suspected contact dermatitis and elaborated on the common allergens unique to this patient population. Furthermore, she explained the substantial role of nickel in pediatric contact dermatitis, citing a study performed in Denmark and the United States, which tested 212 toys for nickel using the dimethylglyoxime test and found that 34.4% of toys did in fact release nickel.3 Additional studies have shown that nickel released from children’s toys is deposited on the skin, even with short contact times such as 30 minutes on one or more occasions within 2 weeks.3,4 She is currently evaluating the presence of nickel in locales frequented by children such as schools, libraries, and supermarkets. Interestingly, she anecdotally found that a pediatric eczematous eruption in a spiralized distribution of the legs can be attributed to the presence of nickel in school chairs, and the morphology is secondary to children wrapping their legs around the chairs. In conclusion, she reiterated that nickel continues to be the top allergen among pediatric patients, and states that additional allergens for patch testing in this population are unique to their adult counterparts.
Take-Home Point
Nickel is an ubiquitous allergen for pediatric contact dermatitis; additionally, the list of allergens for patch testing should be tailored to this patient population.
“When to Image, When to Sedate” by Annette Wagner, MD (Northwestern Medicine, Chicago, Illinois)
This lecture was a 3-part discussion on the safety of general anesthesia in children, when to image children, and when sedation may be worth the risk. Dr. Wagner shared her pearls for when children younger than 3 years may benefit from dermatologic procedures that involve general anesthesia. Large congenital lesions of the scalp or face that require tissue expansion or multiple stages may be best performed at a younger age due to the flexibility of the infant scalp, providing the best outcome. Additional considerations include a questionable malignant diagnosis in which a punch biopsy is not enough, rapidly growing facial lesions, Spitz nevi of the face, congenital lesions with no available therapy, and nonhealing refractory lesions causing severe pain. The general rule proposed was intervention for single procedures lasting less than 1 hour that otherwise would result in a worse outcome if postponed. Finally, she concluded to always advocate for your patient, to wait if the outcome will be the same regardless of timing, and to be frank about not knowing the risks of general anesthesia in this population. The resource, SmartTots (http://smarttots.org) provides current consensus statements and ongoing research on the use and safety of general anesthesia in children.
Take-Home Point
General sedation may be considered for short pediatric procedures that will result in a worse outcome if postponed.
“Highlights From the Pediatric Literature” by Katherine Marks, DO (Geisinger, Danville and Wilkes-Barre, Pennsylvania)
Dr. Marks discussed numerous emerging pediatric dermatology articles. One article looked at 40 infants with proliferating infantile hemangiomas (IHs) who had timolol gel 0.5% applied twice daily.5 The primary outcomes were the urinary excretion and serum levels of timolol as well as the clinical response to therapy measured by a visual analog scale at monthly visits. A urinalysis collected 3 to 4 hours after timolol application was found to be positive in 83% (20/24) of the tested patients; the first 3 positive infants were then sent to have their serum timolol levels drawn and also were found to be positive, though substantially small levels (median, 0.16 ng/mL). The 3 patients tested had small IHs on the face with no ulceration. None of these patients experienced adverse effects and all of the IHs significantly (P<.001) improved with therapy. The authors stated that even though the absorption was minimal, it is wise to be cognizant about the use of timolol in certain patient demographics such as preterm or young infants with large ulcerating IHs.5
Take-Home Point
Systemic absorption with topical timolol occurs, albeit substantially small; be judicious about giving this medication in select patient populations with ulcerated hemangiomas.
Acknowledgment
The author thanks the presenters for their review and contributions to this article.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel phosphodiesterase 4 inhibitor for the topical treatment of AD in children and adults [published online July 11, 2016]. J Am Acad Dermatol. 2016;75:494-503.
- Jensen P, Hamann D, Hamann CR, et al. Nickel and cobalt release from children’s toys purchased in Denmark and the United States. Dermatitis. 2014;25:356-365.
- Overgaard LE, Engebretsen KA, Jensen P, et al. Nickel released from children’s toys is deposited on the skin. Contact Dermatitis. 2016;74:380-381.
- Weibel L, Barysch MJ, Scheer HS, et al. Topical timolol for infantile hemangiomas: evidence for efficacy and degree of systemic absorption [published online February 3, 2016]. Pediatr Dermatol. 2016;33:184-190.
This article exhibits key pediatric dermatology pearls garnered at the 2017 Annual Meeting of the American Academy of Dermatology (AAD) in Orlando, Florida (March 3–7, 2017). Highlights from both the Society for Pediatric Dermatology pre-AAD meeting (March 2, 2017) and the AAD general meeting sessions are included. This discussion is intended to help maximize care of our pediatric patients in dermatology and present high-yield take-home points from the AAD that can be readily transferred to our patient care.
“New Tools for Your Therapeutic Toolbox” by Erin Mathes, MD (University of California, San Francisco)
During this lecture at the Society for Pediatric Dermatology meeting, Dr. Mathes discussed a randomized controlled trial that took place in 2014 in both the United States and the United Kingdom to assess skin barrier enhancement to reduce the incidence of atopic dermatitis (AD) in 124 high-risk infants.1 The high-risk infants had either a parent or sibling with physician-diagnosed AD, asthma, or rhinitis, or a first-degree relative with an aforementioned condition. Full-body emollient therapy was applied at least once daily within 3 weeks of birth for 6 months, while the control arm did not use emollient. Parents were allowed to choose from the following emollients: sunflower seed oil, moisturizing cream, or ointment. The primary outcome was the incidence of AD at 6 months. The authors found a 43% incidence of AD in the control group compared to 22% in the emollient group, amounting to a relative risk reduction of approximately 50%.1
Emollients in AD are hypothesized to help through the enhanced barrier function and decreased penetration of irritant substances and allergens. This study is vital given the ease of use of emollients and the foreseeable substantial impact on reduced health care costs associated with the decreased incidence of AD.
Take-Home Point
Full-body emollient therapy within 3 weeks of birth may reduce the incidence of AD in high-risk infants.
Dr. Mathes also discussed the novel topical phosphodiesterase 4 inhibitor crisaborole and its emerging role in AD. She reviewed the results of a large phase 3 trial of crisaborole therapy for patients aged 2 years or older with mild to moderate AD.2 Crisaborole ointment was applied twice daily for 28 days. The primary outcome measured was an investigator static global assessment score of clear or almost clear, which is a score for AD based on the degree of erythema, presence of oozing and crusting, and presence of induration or papulation. Overall, 32.8% of patients treated with crisaborole achieved success compared to 25.4% of vehicle-treated patients. The control patients were still given a vehicle to apply, which can function as therapy to help repair the barrier of AD and thus theoretically reduced the percentage gap between patients who met success with and without crisaborole therapy. Furthermore, only 4% of patients reported adverse effects such as burning and stinging with application of crisaborole in contrast to topical calcineurin inhibitors, which can elicit symptoms up to 50% of the time.2 In summary, this lecture reviewed the first new topical treatment for AD in 15 years.
Take-Home Point
Crisaborole ointment is a novel topical phosphodiesterase 4 inhibitor approved for mild to moderate AD in patients 2 years of age and older.
“The Truth About Pediatric Contact Dermatitis” by Sharon Jacob, MD (Loma Linda University, California)
In this session, Dr. Jacob discussed how she approaches pediatric patients with suspected contact dermatitis and elaborated on the common allergens unique to this patient population. Furthermore, she explained the substantial role of nickel in pediatric contact dermatitis, citing a study performed in Denmark and the United States, which tested 212 toys for nickel using the dimethylglyoxime test and found that 34.4% of toys did in fact release nickel.3 Additional studies have shown that nickel released from children’s toys is deposited on the skin, even with short contact times such as 30 minutes on one or more occasions within 2 weeks.3,4 She is currently evaluating the presence of nickel in locales frequented by children such as schools, libraries, and supermarkets. Interestingly, she anecdotally found that a pediatric eczematous eruption in a spiralized distribution of the legs can be attributed to the presence of nickel in school chairs, and the morphology is secondary to children wrapping their legs around the chairs. In conclusion, she reiterated that nickel continues to be the top allergen among pediatric patients, and states that additional allergens for patch testing in this population are unique to their adult counterparts.
Take-Home Point
Nickel is an ubiquitous allergen for pediatric contact dermatitis; additionally, the list of allergens for patch testing should be tailored to this patient population.
“When to Image, When to Sedate” by Annette Wagner, MD (Northwestern Medicine, Chicago, Illinois)
This lecture was a 3-part discussion on the safety of general anesthesia in children, when to image children, and when sedation may be worth the risk. Dr. Wagner shared her pearls for when children younger than 3 years may benefit from dermatologic procedures that involve general anesthesia. Large congenital lesions of the scalp or face that require tissue expansion or multiple stages may be best performed at a younger age due to the flexibility of the infant scalp, providing the best outcome. Additional considerations include a questionable malignant diagnosis in which a punch biopsy is not enough, rapidly growing facial lesions, Spitz nevi of the face, congenital lesions with no available therapy, and nonhealing refractory lesions causing severe pain. The general rule proposed was intervention for single procedures lasting less than 1 hour that otherwise would result in a worse outcome if postponed. Finally, she concluded to always advocate for your patient, to wait if the outcome will be the same regardless of timing, and to be frank about not knowing the risks of general anesthesia in this population. The resource, SmartTots (http://smarttots.org) provides current consensus statements and ongoing research on the use and safety of general anesthesia in children.
Take-Home Point
General sedation may be considered for short pediatric procedures that will result in a worse outcome if postponed.
“Highlights From the Pediatric Literature” by Katherine Marks, DO (Geisinger, Danville and Wilkes-Barre, Pennsylvania)
Dr. Marks discussed numerous emerging pediatric dermatology articles. One article looked at 40 infants with proliferating infantile hemangiomas (IHs) who had timolol gel 0.5% applied twice daily.5 The primary outcomes were the urinary excretion and serum levels of timolol as well as the clinical response to therapy measured by a visual analog scale at monthly visits. A urinalysis collected 3 to 4 hours after timolol application was found to be positive in 83% (20/24) of the tested patients; the first 3 positive infants were then sent to have their serum timolol levels drawn and also were found to be positive, though substantially small levels (median, 0.16 ng/mL). The 3 patients tested had small IHs on the face with no ulceration. None of these patients experienced adverse effects and all of the IHs significantly (P<.001) improved with therapy. The authors stated that even though the absorption was minimal, it is wise to be cognizant about the use of timolol in certain patient demographics such as preterm or young infants with large ulcerating IHs.5
Take-Home Point
Systemic absorption with topical timolol occurs, albeit substantially small; be judicious about giving this medication in select patient populations with ulcerated hemangiomas.
Acknowledgment
The author thanks the presenters for their review and contributions to this article.
This article exhibits key pediatric dermatology pearls garnered at the 2017 Annual Meeting of the American Academy of Dermatology (AAD) in Orlando, Florida (March 3–7, 2017). Highlights from both the Society for Pediatric Dermatology pre-AAD meeting (March 2, 2017) and the AAD general meeting sessions are included. This discussion is intended to help maximize care of our pediatric patients in dermatology and present high-yield take-home points from the AAD that can be readily transferred to our patient care.
“New Tools for Your Therapeutic Toolbox” by Erin Mathes, MD (University of California, San Francisco)
During this lecture at the Society for Pediatric Dermatology meeting, Dr. Mathes discussed a randomized controlled trial that took place in 2014 in both the United States and the United Kingdom to assess skin barrier enhancement to reduce the incidence of atopic dermatitis (AD) in 124 high-risk infants.1 The high-risk infants had either a parent or sibling with physician-diagnosed AD, asthma, or rhinitis, or a first-degree relative with an aforementioned condition. Full-body emollient therapy was applied at least once daily within 3 weeks of birth for 6 months, while the control arm did not use emollient. Parents were allowed to choose from the following emollients: sunflower seed oil, moisturizing cream, or ointment. The primary outcome was the incidence of AD at 6 months. The authors found a 43% incidence of AD in the control group compared to 22% in the emollient group, amounting to a relative risk reduction of approximately 50%.1
Emollients in AD are hypothesized to help through the enhanced barrier function and decreased penetration of irritant substances and allergens. This study is vital given the ease of use of emollients and the foreseeable substantial impact on reduced health care costs associated with the decreased incidence of AD.
Take-Home Point
Full-body emollient therapy within 3 weeks of birth may reduce the incidence of AD in high-risk infants.
Dr. Mathes also discussed the novel topical phosphodiesterase 4 inhibitor crisaborole and its emerging role in AD. She reviewed the results of a large phase 3 trial of crisaborole therapy for patients aged 2 years or older with mild to moderate AD.2 Crisaborole ointment was applied twice daily for 28 days. The primary outcome measured was an investigator static global assessment score of clear or almost clear, which is a score for AD based on the degree of erythema, presence of oozing and crusting, and presence of induration or papulation. Overall, 32.8% of patients treated with crisaborole achieved success compared to 25.4% of vehicle-treated patients. The control patients were still given a vehicle to apply, which can function as therapy to help repair the barrier of AD and thus theoretically reduced the percentage gap between patients who met success with and without crisaborole therapy. Furthermore, only 4% of patients reported adverse effects such as burning and stinging with application of crisaborole in contrast to topical calcineurin inhibitors, which can elicit symptoms up to 50% of the time.2 In summary, this lecture reviewed the first new topical treatment for AD in 15 years.
Take-Home Point
Crisaborole ointment is a novel topical phosphodiesterase 4 inhibitor approved for mild to moderate AD in patients 2 years of age and older.
“The Truth About Pediatric Contact Dermatitis” by Sharon Jacob, MD (Loma Linda University, California)
In this session, Dr. Jacob discussed how she approaches pediatric patients with suspected contact dermatitis and elaborated on the common allergens unique to this patient population. Furthermore, she explained the substantial role of nickel in pediatric contact dermatitis, citing a study performed in Denmark and the United States, which tested 212 toys for nickel using the dimethylglyoxime test and found that 34.4% of toys did in fact release nickel.3 Additional studies have shown that nickel released from children’s toys is deposited on the skin, even with short contact times such as 30 minutes on one or more occasions within 2 weeks.3,4 She is currently evaluating the presence of nickel in locales frequented by children such as schools, libraries, and supermarkets. Interestingly, she anecdotally found that a pediatric eczematous eruption in a spiralized distribution of the legs can be attributed to the presence of nickel in school chairs, and the morphology is secondary to children wrapping their legs around the chairs. In conclusion, she reiterated that nickel continues to be the top allergen among pediatric patients, and states that additional allergens for patch testing in this population are unique to their adult counterparts.
Take-Home Point
Nickel is an ubiquitous allergen for pediatric contact dermatitis; additionally, the list of allergens for patch testing should be tailored to this patient population.
“When to Image, When to Sedate” by Annette Wagner, MD (Northwestern Medicine, Chicago, Illinois)
This lecture was a 3-part discussion on the safety of general anesthesia in children, when to image children, and when sedation may be worth the risk. Dr. Wagner shared her pearls for when children younger than 3 years may benefit from dermatologic procedures that involve general anesthesia. Large congenital lesions of the scalp or face that require tissue expansion or multiple stages may be best performed at a younger age due to the flexibility of the infant scalp, providing the best outcome. Additional considerations include a questionable malignant diagnosis in which a punch biopsy is not enough, rapidly growing facial lesions, Spitz nevi of the face, congenital lesions with no available therapy, and nonhealing refractory lesions causing severe pain. The general rule proposed was intervention for single procedures lasting less than 1 hour that otherwise would result in a worse outcome if postponed. Finally, she concluded to always advocate for your patient, to wait if the outcome will be the same regardless of timing, and to be frank about not knowing the risks of general anesthesia in this population. The resource, SmartTots (http://smarttots.org) provides current consensus statements and ongoing research on the use and safety of general anesthesia in children.
Take-Home Point
General sedation may be considered for short pediatric procedures that will result in a worse outcome if postponed.
“Highlights From the Pediatric Literature” by Katherine Marks, DO (Geisinger, Danville and Wilkes-Barre, Pennsylvania)
Dr. Marks discussed numerous emerging pediatric dermatology articles. One article looked at 40 infants with proliferating infantile hemangiomas (IHs) who had timolol gel 0.5% applied twice daily.5 The primary outcomes were the urinary excretion and serum levels of timolol as well as the clinical response to therapy measured by a visual analog scale at monthly visits. A urinalysis collected 3 to 4 hours after timolol application was found to be positive in 83% (20/24) of the tested patients; the first 3 positive infants were then sent to have their serum timolol levels drawn and also were found to be positive, though substantially small levels (median, 0.16 ng/mL). The 3 patients tested had small IHs on the face with no ulceration. None of these patients experienced adverse effects and all of the IHs significantly (P<.001) improved with therapy. The authors stated that even though the absorption was minimal, it is wise to be cognizant about the use of timolol in certain patient demographics such as preterm or young infants with large ulcerating IHs.5
Take-Home Point
Systemic absorption with topical timolol occurs, albeit substantially small; be judicious about giving this medication in select patient populations with ulcerated hemangiomas.
Acknowledgment
The author thanks the presenters for their review and contributions to this article.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel phosphodiesterase 4 inhibitor for the topical treatment of AD in children and adults [published online July 11, 2016]. J Am Acad Dermatol. 2016;75:494-503.
- Jensen P, Hamann D, Hamann CR, et al. Nickel and cobalt release from children’s toys purchased in Denmark and the United States. Dermatitis. 2014;25:356-365.
- Overgaard LE, Engebretsen KA, Jensen P, et al. Nickel released from children’s toys is deposited on the skin. Contact Dermatitis. 2016;74:380-381.
- Weibel L, Barysch MJ, Scheer HS, et al. Topical timolol for infantile hemangiomas: evidence for efficacy and degree of systemic absorption [published online February 3, 2016]. Pediatr Dermatol. 2016;33:184-190.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel phosphodiesterase 4 inhibitor for the topical treatment of AD in children and adults [published online July 11, 2016]. J Am Acad Dermatol. 2016;75:494-503.
- Jensen P, Hamann D, Hamann CR, et al. Nickel and cobalt release from children’s toys purchased in Denmark and the United States. Dermatitis. 2014;25:356-365.
- Overgaard LE, Engebretsen KA, Jensen P, et al. Nickel released from children’s toys is deposited on the skin. Contact Dermatitis. 2016;74:380-381.
- Weibel L, Barysch MJ, Scheer HS, et al. Topical timolol for infantile hemangiomas: evidence for efficacy and degree of systemic absorption [published online February 3, 2016]. Pediatr Dermatol. 2016;33:184-190.
Wearable Health Device Dermatitis: A Case of Acrylate-Related Contact Allergy
Mobile health devices enable patients and clinicians to monitor the type, quantity, and quality of everyday activities and hold the promise of improving patient health and health care practices.1 In 2013, 75% of surveyed consumers in the United States owned a fitness technology product, either a dedicated fitness device, application, or portable blood pressure monitor.2 Ownership of dedicated wearable fitness devices among consumers in the United States increased from 3% in 2012 to 9% in 2013. The immense popularity of wearable fitness devices is evident in the trajectory of their reported sales, which increased from $43 million in 2009 to $854 million in 2013.2 Recognizing that “widespread adoption and use of mobile technologies is opening new and innovative ways to improve health,”3 the US Food and Drug Administration (FDA) ruled that “[technologies] that can pose a greater risk to patients will require FDA review.” One popular class of mobile technologies—activity and sleep sensors—falls outside the FDA’s regulatory guidance. To enable continuous monitoring, these sensors often are embedded into wearable devices.
Reports in the media have documented skin rashes arising in conjunction with use of one type of device,4 which may be related to nickel contact allergy, and the manufacturer has reported that the metal housing consists of surgical stainless steel that is known to contain nickel. We report a complication related to continuous use of an unregulated, commercially available, watchlike wearable sensor that was linked not to nickel but to an acrylate-containing component.
Case Report
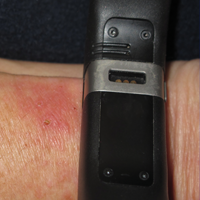
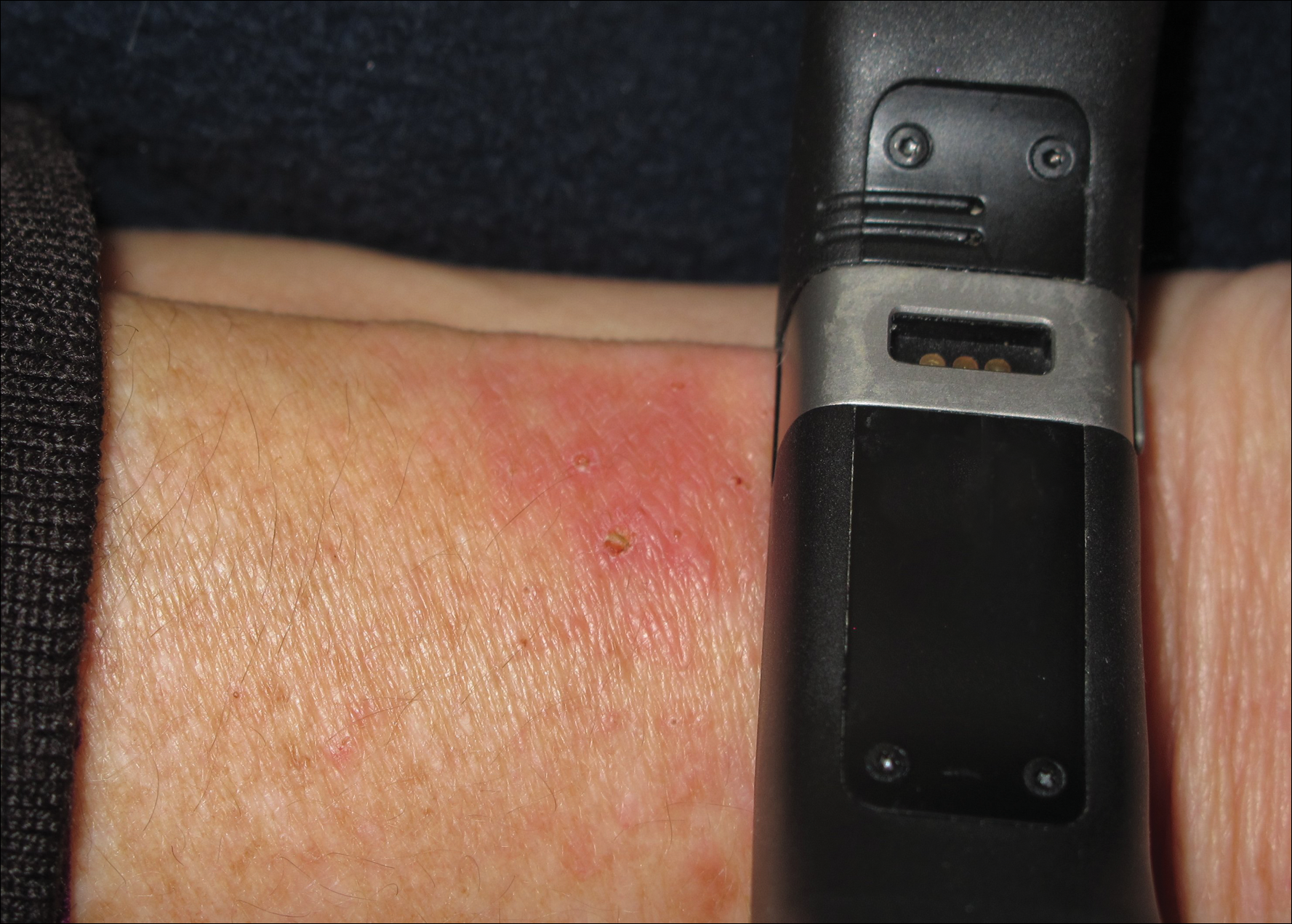
An otherwise healthy 52-year-old woman with no history of contact allergy presented with an intensely itchy eruption involving the left wrist arising 4 days after continuous use of a new watchlike wearable fitness sensor. By day 11, the eruption evolved into a well-demarcated, erythematous, scaly plaque at the location where the device’s rechargeable battery metal housing came into contact with skin (Figure 1).
Dimethylglyoxime testing of the metal housing and clips was negative, but testing of contacts within the housing was positive for nickel (Figure 2). Epicutaneous patch testing of the patient using a modified North American Contact Dermatitis Group patch test series (Table) demonstrated no reaction to nickel, instead showing a strong positive (2+) reaction at 48 and 72 hours to methyl methacrylate 2% and a positive (1+) reaction at 96 hours to ethyl acrylate 0.1% (Figure 3).



Comment
Acrylates are used as adhesives to bond metal to plastic and as part of lithium ion polymer batteries, presumably similar to the one used in this device.5 Our patient had a history of using acrylic nail polish, which may have been a source of prior sensitization. Exposure to sweat or other moisture could theoretically dissolve such a water-soluble polymer,6 allowing for skin contact. Other acrylate polymers have been reported to break down slowly in contact with water, leading to contact sensitization to the monomer.7 The manufacturer of the device was contacted for additional information but declined to provide specific details regarding the device’s composition (personal communication, January 2014).
Although not considered toxic,8 acrylate was named Allergen of the Year in 2012 by the American Contact Dermatitis Society.9-11 Nickel might be a source of allergy for some other patients who wear mobile health devices, but we concluded that this particular patient developed allergic contact dermatitis from prolonged exposure to low levels of methyl methacrylate or another acrylate due to gradual breakdown of the acrylate polymer used in the rechargeable battery housing for this wearable health device.
Given the FDA’s tailored risk approach to regulation, many wearable sensors that may contain potential contact allergens such as nickel and acrylates do not fall under the FDA regulatory framework. This case should alert physicians to the lack of regulatory oversight for many mobile technologies. They should consider a screening history for contact allergens before recommending wearable sensors and broader testing for contact allergens should exposed patients develop reactions. Future wearable sensor materials and designs should minimize exposure to allergens given prolonged contact with continuous use. In the absence of regulation, manufacturers of these devices should consider due care testing prior to commercialization.
Acknowledgment
We are indebted to Alexander S. Rattner, PhD (State College, Pennsylvania), who provided his engineering expertise and insight during conversations with the authors.
- Dobkin BH, Dorsch A. The promise of mHealth: daily activity monitoring and outcome assessments by wearable sensors. Neurorehabil Neural Repair. 2011;25:788-798.
- Consumer interest in purchasing wearable fitness devices in 2014 quadruples, according to CEA Study [press release]. Arlington, VA: Consumer Electronics Association; December 11, 2013.
- US Food and Drug Administration. Mobile medical applications. http://www.fda.gov/medicaldevices/digitalhealth/mobilemedicalapplications/default.htm. Updated September 22, 2015. Accessed July 26, 2017.
- Northrup L. Fitbit Force is an amazing device, except for my contact dermatitis. Consumerist website. http://consumerist.com/2014/01/13/fitbit-force-is-an-amazing-device-except-for-my-contact-dermatitis/. Published January 13, 2014. Accessed January 12, 2017.
- Stern B. Inside Fitbit Force. Adafruit website. http://learn.adafruit.com/fitbit-force-teardown/inside-fitbit-force. Published December 11, 2013. Updated May 4, 2015. Accessed January 12, 2017.
- Pemberton MA, Lohmann BS. Risk assessment of residual monomer migrating from acrylic polymers and causing allergic contact dermatitis during normal handling and use. Regul Toxicol Pharmacol. 2014;69:467-475.
- Guin JD, Baas K, Nelson-Adesokan P. Contact sensitization to cyanoacrylate adhesive as a cause of severe onychodystrophy. Int J Dermatol. 1998;37:31-36.
- Zondlo Fiume M. Final report on the safety assessment of Acrylates Copolymer and 33 related cosmetic ingredients. Int J Toxicol. 2002;21(suppl 3):1-50.
- Sasseville D. Acrylates. Dermatitis. 2012;23:3-5.
- Bowen C, Bidinger J, Hivnor C, et al. Allergic contact dermatitis to 2-octyl cyanoacrylate. Cutis. 2014;94:183-186.
- Spencer A, Gazzani P, Thompson DA. Acrylate and methacrylate contact allergy and allergic contact disease: a 13-year review [published online July 11, 2016]. Contact Dermatitis. 2016;75:157-164.
Mobile health devices enable patients and clinicians to monitor the type, quantity, and quality of everyday activities and hold the promise of improving patient health and health care practices.1 In 2013, 75% of surveyed consumers in the United States owned a fitness technology product, either a dedicated fitness device, application, or portable blood pressure monitor.2 Ownership of dedicated wearable fitness devices among consumers in the United States increased from 3% in 2012 to 9% in 2013. The immense popularity of wearable fitness devices is evident in the trajectory of their reported sales, which increased from $43 million in 2009 to $854 million in 2013.2 Recognizing that “widespread adoption and use of mobile technologies is opening new and innovative ways to improve health,”3 the US Food and Drug Administration (FDA) ruled that “[technologies] that can pose a greater risk to patients will require FDA review.” One popular class of mobile technologies—activity and sleep sensors—falls outside the FDA’s regulatory guidance. To enable continuous monitoring, these sensors often are embedded into wearable devices.
Reports in the media have documented skin rashes arising in conjunction with use of one type of device,4 which may be related to nickel contact allergy, and the manufacturer has reported that the metal housing consists of surgical stainless steel that is known to contain nickel. We report a complication related to continuous use of an unregulated, commercially available, watchlike wearable sensor that was linked not to nickel but to an acrylate-containing component.
Case Report
An otherwise healthy 52-year-old woman with no history of contact allergy presented with an intensely itchy eruption involving the left wrist arising 4 days after continuous use of a new watchlike wearable fitness sensor. By day 11, the eruption evolved into a well-demarcated, erythematous, scaly plaque at the location where the device’s rechargeable battery metal housing came into contact with skin (Figure 1).

Dimethylglyoxime testing of the metal housing and clips was negative, but testing of contacts within the housing was positive for nickel (Figure 2). Epicutaneous patch testing of the patient using a modified North American Contact Dermatitis Group patch test series (Table) demonstrated no reaction to nickel, instead showing a strong positive (2+) reaction at 48 and 72 hours to methyl methacrylate 2% and a positive (1+) reaction at 96 hours to ethyl acrylate 0.1% (Figure 3).



Comment
Acrylates are used as adhesives to bond metal to plastic and as part of lithium ion polymer batteries, presumably similar to the one used in this device.5 Our patient had a history of using acrylic nail polish, which may have been a source of prior sensitization. Exposure to sweat or other moisture could theoretically dissolve such a water-soluble polymer,6 allowing for skin contact. Other acrylate polymers have been reported to break down slowly in contact with water, leading to contact sensitization to the monomer.7 The manufacturer of the device was contacted for additional information but declined to provide specific details regarding the device’s composition (personal communication, January 2014).
Although not considered toxic,8 acrylate was named Allergen of the Year in 2012 by the American Contact Dermatitis Society.9-11 Nickel might be a source of allergy for some other patients who wear mobile health devices, but we concluded that this particular patient developed allergic contact dermatitis from prolonged exposure to low levels of methyl methacrylate or another acrylate due to gradual breakdown of the acrylate polymer used in the rechargeable battery housing for this wearable health device.
Given the FDA’s tailored risk approach to regulation, many wearable sensors that may contain potential contact allergens such as nickel and acrylates do not fall under the FDA regulatory framework. This case should alert physicians to the lack of regulatory oversight for many mobile technologies. They should consider a screening history for contact allergens before recommending wearable sensors and broader testing for contact allergens should exposed patients develop reactions. Future wearable sensor materials and designs should minimize exposure to allergens given prolonged contact with continuous use. In the absence of regulation, manufacturers of these devices should consider due care testing prior to commercialization.
Acknowledgment
We are indebted to Alexander S. Rattner, PhD (State College, Pennsylvania), who provided his engineering expertise and insight during conversations with the authors.
Mobile health devices enable patients and clinicians to monitor the type, quantity, and quality of everyday activities and hold the promise of improving patient health and health care practices.1 In 2013, 75% of surveyed consumers in the United States owned a fitness technology product, either a dedicated fitness device, application, or portable blood pressure monitor.2 Ownership of dedicated wearable fitness devices among consumers in the United States increased from 3% in 2012 to 9% in 2013. The immense popularity of wearable fitness devices is evident in the trajectory of their reported sales, which increased from $43 million in 2009 to $854 million in 2013.2 Recognizing that “widespread adoption and use of mobile technologies is opening new and innovative ways to improve health,”3 the US Food and Drug Administration (FDA) ruled that “[technologies] that can pose a greater risk to patients will require FDA review.” One popular class of mobile technologies—activity and sleep sensors—falls outside the FDA’s regulatory guidance. To enable continuous monitoring, these sensors often are embedded into wearable devices.
Reports in the media have documented skin rashes arising in conjunction with use of one type of device,4 which may be related to nickel contact allergy, and the manufacturer has reported that the metal housing consists of surgical stainless steel that is known to contain nickel. We report a complication related to continuous use of an unregulated, commercially available, watchlike wearable sensor that was linked not to nickel but to an acrylate-containing component.
Case Report
An otherwise healthy 52-year-old woman with no history of contact allergy presented with an intensely itchy eruption involving the left wrist arising 4 days after continuous use of a new watchlike wearable fitness sensor. By day 11, the eruption evolved into a well-demarcated, erythematous, scaly plaque at the location where the device’s rechargeable battery metal housing came into contact with skin (Figure 1).

Dimethylglyoxime testing of the metal housing and clips was negative, but testing of contacts within the housing was positive for nickel (Figure 2). Epicutaneous patch testing of the patient using a modified North American Contact Dermatitis Group patch test series (Table) demonstrated no reaction to nickel, instead showing a strong positive (2+) reaction at 48 and 72 hours to methyl methacrylate 2% and a positive (1+) reaction at 96 hours to ethyl acrylate 0.1% (Figure 3).



Comment
Acrylates are used as adhesives to bond metal to plastic and as part of lithium ion polymer batteries, presumably similar to the one used in this device.5 Our patient had a history of using acrylic nail polish, which may have been a source of prior sensitization. Exposure to sweat or other moisture could theoretically dissolve such a water-soluble polymer,6 allowing for skin contact. Other acrylate polymers have been reported to break down slowly in contact with water, leading to contact sensitization to the monomer.7 The manufacturer of the device was contacted for additional information but declined to provide specific details regarding the device’s composition (personal communication, January 2014).
Although not considered toxic,8 acrylate was named Allergen of the Year in 2012 by the American Contact Dermatitis Society.9-11 Nickel might be a source of allergy for some other patients who wear mobile health devices, but we concluded that this particular patient developed allergic contact dermatitis from prolonged exposure to low levels of methyl methacrylate or another acrylate due to gradual breakdown of the acrylate polymer used in the rechargeable battery housing for this wearable health device.
Given the FDA’s tailored risk approach to regulation, many wearable sensors that may contain potential contact allergens such as nickel and acrylates do not fall under the FDA regulatory framework. This case should alert physicians to the lack of regulatory oversight for many mobile technologies. They should consider a screening history for contact allergens before recommending wearable sensors and broader testing for contact allergens should exposed patients develop reactions. Future wearable sensor materials and designs should minimize exposure to allergens given prolonged contact with continuous use. In the absence of regulation, manufacturers of these devices should consider due care testing prior to commercialization.
Acknowledgment
We are indebted to Alexander S. Rattner, PhD (State College, Pennsylvania), who provided his engineering expertise and insight during conversations with the authors.
- Dobkin BH, Dorsch A. The promise of mHealth: daily activity monitoring and outcome assessments by wearable sensors. Neurorehabil Neural Repair. 2011;25:788-798.
- Consumer interest in purchasing wearable fitness devices in 2014 quadruples, according to CEA Study [press release]. Arlington, VA: Consumer Electronics Association; December 11, 2013.
- US Food and Drug Administration. Mobile medical applications. http://www.fda.gov/medicaldevices/digitalhealth/mobilemedicalapplications/default.htm. Updated September 22, 2015. Accessed July 26, 2017.
- Northrup L. Fitbit Force is an amazing device, except for my contact dermatitis. Consumerist website. http://consumerist.com/2014/01/13/fitbit-force-is-an-amazing-device-except-for-my-contact-dermatitis/. Published January 13, 2014. Accessed January 12, 2017.
- Stern B. Inside Fitbit Force. Adafruit website. http://learn.adafruit.com/fitbit-force-teardown/inside-fitbit-force. Published December 11, 2013. Updated May 4, 2015. Accessed January 12, 2017.
- Pemberton MA, Lohmann BS. Risk assessment of residual monomer migrating from acrylic polymers and causing allergic contact dermatitis during normal handling and use. Regul Toxicol Pharmacol. 2014;69:467-475.
- Guin JD, Baas K, Nelson-Adesokan P. Contact sensitization to cyanoacrylate adhesive as a cause of severe onychodystrophy. Int J Dermatol. 1998;37:31-36.
- Zondlo Fiume M. Final report on the safety assessment of Acrylates Copolymer and 33 related cosmetic ingredients. Int J Toxicol. 2002;21(suppl 3):1-50.
- Sasseville D. Acrylates. Dermatitis. 2012;23:3-5.
- Bowen C, Bidinger J, Hivnor C, et al. Allergic contact dermatitis to 2-octyl cyanoacrylate. Cutis. 2014;94:183-186.
- Spencer A, Gazzani P, Thompson DA. Acrylate and methacrylate contact allergy and allergic contact disease: a 13-year review [published online July 11, 2016]. Contact Dermatitis. 2016;75:157-164.
- Dobkin BH, Dorsch A. The promise of mHealth: daily activity monitoring and outcome assessments by wearable sensors. Neurorehabil Neural Repair. 2011;25:788-798.
- Consumer interest in purchasing wearable fitness devices in 2014 quadruples, according to CEA Study [press release]. Arlington, VA: Consumer Electronics Association; December 11, 2013.
- US Food and Drug Administration. Mobile medical applications. http://www.fda.gov/medicaldevices/digitalhealth/mobilemedicalapplications/default.htm. Updated September 22, 2015. Accessed July 26, 2017.
- Northrup L. Fitbit Force is an amazing device, except for my contact dermatitis. Consumerist website. http://consumerist.com/2014/01/13/fitbit-force-is-an-amazing-device-except-for-my-contact-dermatitis/. Published January 13, 2014. Accessed January 12, 2017.
- Stern B. Inside Fitbit Force. Adafruit website. http://learn.adafruit.com/fitbit-force-teardown/inside-fitbit-force. Published December 11, 2013. Updated May 4, 2015. Accessed January 12, 2017.
- Pemberton MA, Lohmann BS. Risk assessment of residual monomer migrating from acrylic polymers and causing allergic contact dermatitis during normal handling and use. Regul Toxicol Pharmacol. 2014;69:467-475.
- Guin JD, Baas K, Nelson-Adesokan P. Contact sensitization to cyanoacrylate adhesive as a cause of severe onychodystrophy. Int J Dermatol. 1998;37:31-36.
- Zondlo Fiume M. Final report on the safety assessment of Acrylates Copolymer and 33 related cosmetic ingredients. Int J Toxicol. 2002;21(suppl 3):1-50.
- Sasseville D. Acrylates. Dermatitis. 2012;23:3-5.
- Bowen C, Bidinger J, Hivnor C, et al. Allergic contact dermatitis to 2-octyl cyanoacrylate. Cutis. 2014;94:183-186.
- Spencer A, Gazzani P, Thompson DA. Acrylate and methacrylate contact allergy and allergic contact disease: a 13-year review [published online July 11, 2016]. Contact Dermatitis. 2016;75:157-164.
Practice Points
- Mobile wearable health devices are likely to become an important potential source of contact sensitization as their use increases given their often prolonged contact time with the skin.
- Mobile wearable health devices may pose a risk for allergic contact dermatitis as a result of a variety of components that come into contact with the skin, including but not limited to metals, rubber components, adhesives, and dyes.
Evaluation of Patch Test Reactivities in Patients With Chronic Idiopathic Urticaria
Chronic urticaria (CU) is clinically defined as the daily or almost daily presence of wheals on the skin for at least 6 weeks.1 Chronic urticaria severely affects patients’ quality of life and can cause emotional disability and distress.2 In clinical practice, CU is one of the most common and challenging conditions for general practitioners, dermatologists, and allergists. It can be provoked by a wide variety of different causes or may be the clinical presentation of certain systemic diseases3,4; thus, CU often requires a detailed and time-consuming diagnostic procedure that includes screening for allergies, autoimmune diseases, parasites, malignancies, infections, and metabolic disorders.5,6 In many patients (up to 50% in some case series), the cause or pathogenic mechanism cannot be identified, and the disease is then classified as chronic idiopathic urticaria (CIU).7
It has previously been shown that contact sensitization could have some relation with CIU,8 which was further explored in this study. This study sought to evaluate if contact allergy may play a role in disease development in CIU patients in Saudi Arabia and if patch testing should be routinely performed for CIU patients to determine if any allergens can be avoided.
Methods
This prospective study was conducted at the King Khalid University Hospital Allergy Clinic (Riyadh, Saudi Arabia) in patients aged 18 to 60 years who had CU for more than 6 weeks. It was a clinic-based study conducted over a period of 2 years (March 2010 to February 2012). The study protocol was approved by the local ethics committee at King Khalid University Hospital. Valid written consent was obtained from each patient.
Patients were excluded if they had CU caused by physical factors (eg, hot or cold temperature, water, physical contact) or drug reactions that were possible causative factors or if they had taken oral prednisolone or other oral immunosuppressive drugs (eg, azathioprine, cyclosporine) in the last month. However, patients taking antihistamines were not excluded because it was impossible for the patients to discontinue their urticaria treatment. Other exclusion criteria included CU associated with any systemic disease, thyroid disease, diabetes mellitus, autoimmune disorder, or atopic dermatitis. Pregnant and lactating women were not included in this study.
All new adult CU patients (ie, disease duration >6 weeks) were worked up using the routine diagnostic tests that are typically performed for any new CU patient, including complete blood cell count with differential, erythrocyte sedimentation rate, liver function tests, urine analysis, and hepatitis B and C screenings. Further diagnostic tests also were carried out when appropriate according to the patient’s history and physical examination, including levels of urea, electrolytes, thyrotropin, thyroid antibodies (antithyroglobulin and antimicrosomal), and antinuclear antibodies, as well as a Helicobacter pylori test.
All of the patients enrolled in the study were evaluated by skin prick testing to establish the link between CU and its cause. Patch testing was performed in patients who were negative on skin prick testing.
Skin Prick Testing
All patients were advised to temporarily discontinue the use of antihistamines and corticosteroids 5 to 6 days prior to testing.
Patch Testing
Patch tests were carried out using a ready-to-use epicutaneous patch test system for the diagnosis of allergic contact dermatitis (ACD).10 A European standard series was used with the addition of 4 allergens of local relevance: black seed oil, local perfume mix, henna, and myrrh (a topical herbal medicine used to promote healing).
Assessment of Improvement
Assessment of urticaria severity using the Chronic Urticaria Severity Score (CUSS), a simple semiquantitative assessment of disease activity, was calculated as the sum of the number of wheals and the degree of itch severity graded from 0 (none) to 3 (severe), according to the guidelines established by the Dermatology Section of the European Academy of Allergology and Clinical Immunology, the Global Allergy and Asthma European Network, the European Dermatology Forum, and the World Allergy Organization.11 The avoidance group of patients was assessed at baseline and after 1 month to evaluate changes in their CUSS after allergen avoidance for 8 weeks.
Statistical Analysis
All of the statistical analyses were carried out using SPSS software version 16. Results were presented as the median with the range or the mean (SD).
Results
During the study period, a total of 120 CU patients were seen at the clinic. Ninety-three patients with CU met our selection criteria (77.5%) and were enrolled in the study. The mean age (SD) of the patients was 34.7 (12.4) years. Women comprised 68.8% (64/93) of the study population (Table 1).
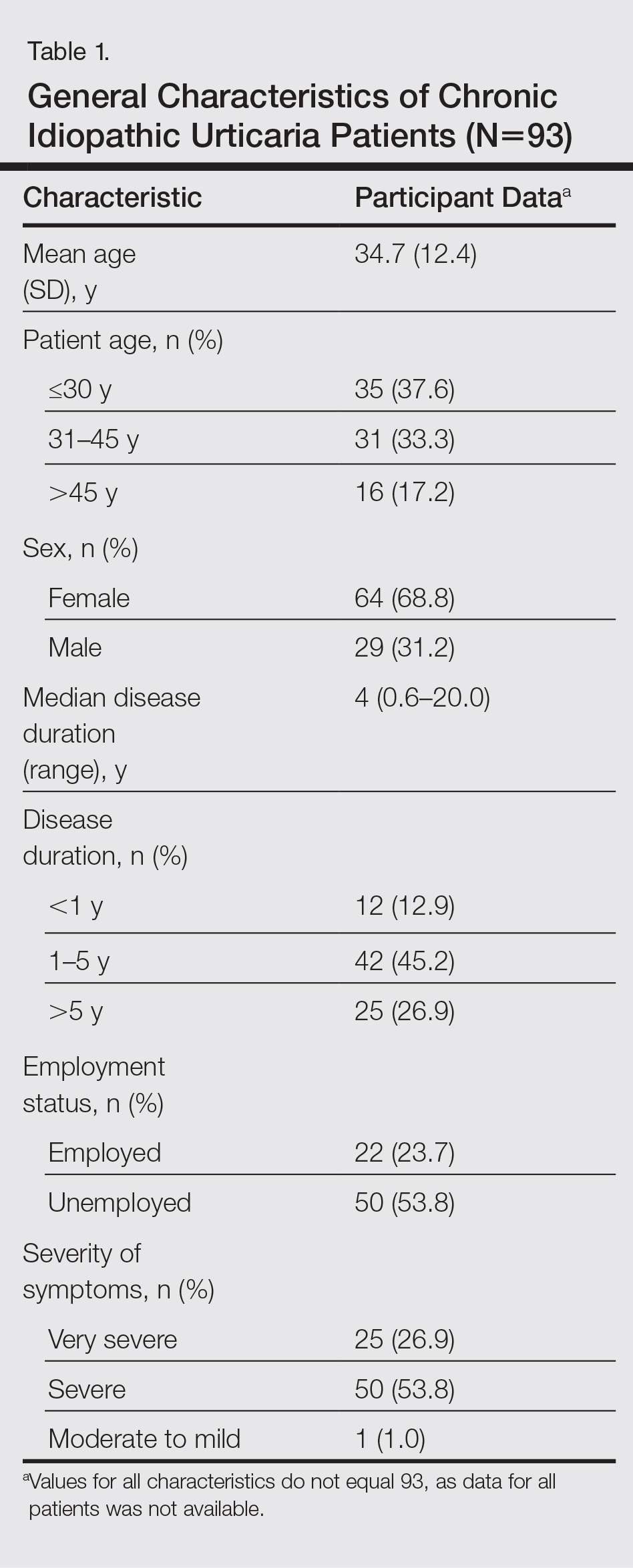
The duration of urticaria ranged from 0.6 to 20 years, with a median duration of 4 years. Approximately half of the patients (50/93) experienced severe symptoms of urticaria, but only 26.9% (25/93) had graded their urticaria as very severe.
Negative results from the skin prick test were reported in 62.4% (58/93) of patients and were subsequently patch tested. These patients also had no other etiologic factors (eg, infection; thyroid, autoimmune, or metabolic disease). Patients who had positive skin prick test results (35/93 [37.6%]) were not considered to be cases of CIU, according to diagnostic recommendations.12 Of the 58 CIU patients who were patch tested, 31 (53.4%) had positive results and 27 (46.5%) had negative results to both skin prick and patch tests (Figure).
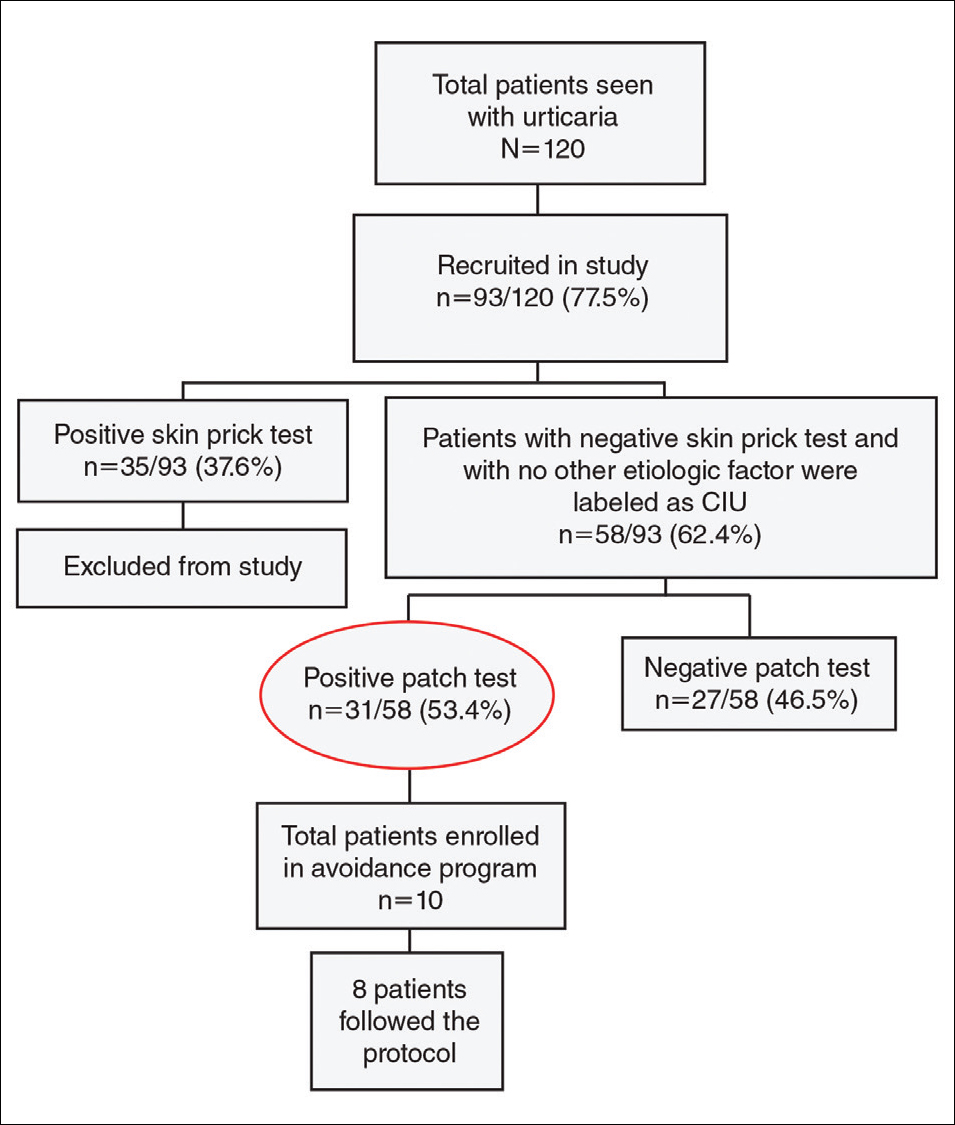
Univariate analysis revealed significant associations between age, gender, and duration of urticaria and patch test positivity (χ2 test, P<.05). T
Of the 31 patients with positive patch tests, there were 20 positive reactions to nickel, 6 to formaldehyde, 4 to phenylenediamine, 3 to cobalt, and 3 to a fragrance mix (Table 2). Some patients showed patch test reactivity to more than 1 allergen concomitantly. Overall, these 31 patients had positive reactions to 16 allergens. None of the patients showed actual signs of contact dermatitis (Table 2).
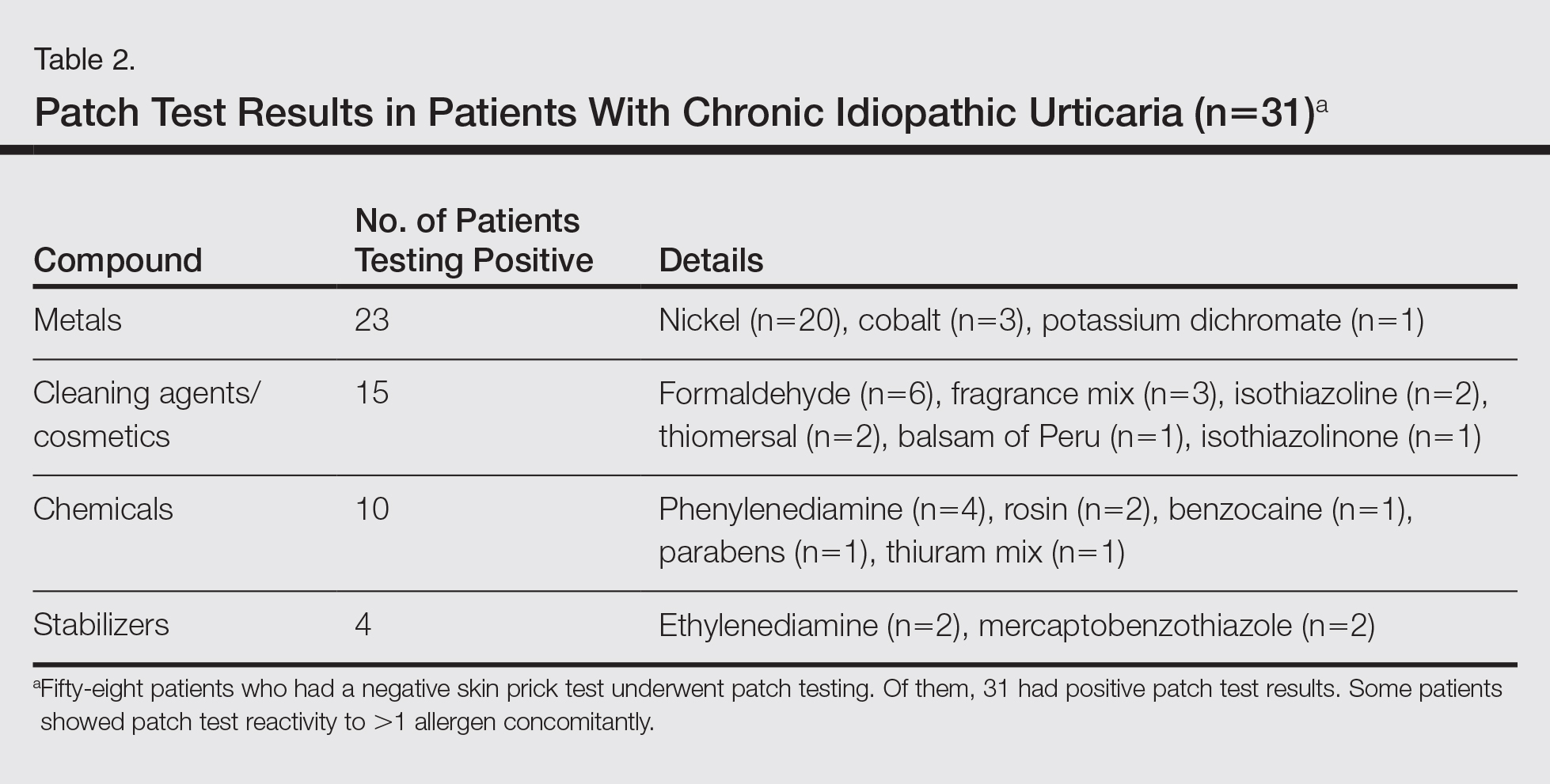
Of
Comment
Chronic idiopathic urticaria is the diagnosis given when urticarial vasculitis, physical urticaria, and all other possible etiologic factors have been excluded in patients with CU. Our study was designed to assess patch test reactivity in patients with CU without any identifiable systemic etiologic factor after detailed laboratory testing and negative skin prick tests.
Chronic idiopathic urticaria can be an extremely disabling and difficult-to-treat condition. Because the cause is unknown, the management of CIU often is frustrating. The
Patch testing is commonly performed to diagnose ACD, and if contact allergens are found via patch testing, patients can often be cured of their dermatitis by avoiding these agents. However, patch testing is not routinely performed in the evaluation of patients with CIU. It is a relatively inexpensive and safe procedure to determine a causal link between sensitization to a specific agent and ACD. In patch test clinics, agents often are tested in standard and screening series. Sensitization that is not suspected from the patient’s history and/or clinical examination can be detected in this manner. Requirements for the inclusion of a chemical in a standard series have been formulated by Bruze et al.13 In addition, ready-to-use materials relevant to the specific leisure activities and working conditions also can be selected for patch testing.
A study conducted in Saudi Arabia showed that the European standard series is suitable for patch testing patients in this community14; however, 3 allergens of local relevance were added in our study: black seed oil, local perfume mix, and henna. Moreover, in our study we added a local allergen known as myrrh, which is a topical herbal medicine used to promote healing that has been reported to cause ACD in some cases.15 We sought to determine if contact allergens can be identified with patch testing in patients with CU and if avoiding these contact allergens would resolve the CU.
Urticaria was once considered an IgE-mediated hypersensitivity reaction, but recent studies have demonstrated the existence of different subgroupsof urticaria, some with an autoimmune mechanism.1-4,11 In CU, skin prick tests are recommended for etiologic workup, while patch testing generally is not recommended.16
It has been observed in clinical practice that a substantial number of patients with CU are positive to patch tests, even without a clear clinical history or signs of contact dermatitis.17 In 2007, Guerra et al17 reported that of 121 patients with CU, 50 (41.3%) tested positive for contact allergens. In all of the patch test–positive patients, avoidance measures led to complete remission within 10 days to 1 month. Therefore, this result suggested that testing for contact sensitization could be helpful in the management of CU. Patients with nickel sensitivity were subsequently allowed to ingest small amounts of nickel-containing foods after 8 weeks of a completely nickel-free diet, and remission persisted.17
Contact dermatitis affects approximately 20% of the general population18; however, there has been little investigation (limited to nickel) into the relationship between contact allergens and CU,19,20 and the underlying mechanisms of the disease are unknown. It has been hypothesized that small amounts of the substances are absorbed through the skin or the digestive tract into the bloodstream over the long-term and are delivered to antigen-presenting cells in the skin, which provide the necessary signals for mast cell activation. Nonetheless, the reasons for a selectively cutaneous localization of the reaction remain largely unclear.
Management of CU is debated among physicians, and several diagnostic flowcharts have been proposed.1,2 In general, patch tests for contact dermatitis are not recommended as a fundamental part of the diagnostic procedure, but Guerra et al17 suggested that contact allergy often plays a role in CU.
There have been inadequate reports of CU found to be caused by common contact sensitizers.21-24 Interestingly, no signs of contact allergy were demonstrated in CU patients before urticarial attack.
Our findings supported our patient selection criteria and also confirmed that contact sensitization may be one of the many possible mechanisms involved in the etiology of CU. Urticaria may have a delayed-type hypersensitivity reaction element, and patients with CU without an obvious causal factor can have positive patch test results.
The
The main findings of our study were that 53.4% of patients with CIU had positive patch test results and that avoidance of the sensitizing substance was effective in 5 of 8 patients who completed an avoidance program. Almost all of the patients showed notable remission of symptoms after limiting their exposure to the offending allergens. This study clearly showed that a cause or pathogenesis for CIU could be identified, thus showing that CIU occurs less frequently than is usually assumed.
Our study had limitations. The first is our lack of a controlled challenge test, which is important to confirm an allergen as a cause of CIU.26 Nonetheless, avoidance of the revealed contact allergen was associated with comparable improvement of CIU severity after 1 month in 5 of 8 patients, though such measures were not tested in all 31 of 58 CIU patients who had positive patch test results.
Conclusion
We propose that patch tests should be performed while investigating CU because they give effective diagnostic and therapeutic results in a substantial number of patients. Urticaria, or at least a subgroup of the disease, may have a delayed-type reaction element, which may explain the disease etiology for many CIU patients. Patients with CU without a detectable underlying etiologic factor can have positive patch test results.
- Zuberbier T, Bindslev-Jensen C, Canonica W, et al. Guidelines, definition, classification and diagnosis of urticaria. Allergy. 2006;61:316-331.
- Kaplan AP. Chronic urticaria: pathogenesis and treatment. J Allergy Clin Immunol. 2004;114:465-474.
- Champion RH. Urticaria: then and now. Br J Dermatol. 1988;119:427-436.
- Green GA, Koelsche GA, Kierland R. Etiology and pathogenesis of chronic urticaria. Ann Allergy. 1965;23:30-36.
- Kaplan AP. Chronic urticaria and angioedema. N Engl J Med. 2002;346:175-179.
- Dreskin SC, Andrews KY. The thyroid and urticaria. Curr Opin Allergy Clin Immunol. 2005;5:408-412.
- Greaves M. Chronic urticaria. J Allergy Clin Immunol. 2000;105:664-672.
- Sharma AD. Use of patch testing for identifying allergen causing chronic urticaria. Indian J Dermatol Venereol Leprol. 2008;74:114-117.
- Li JT, Andrist D, Bamlet WR, et al. Accuracy of patient prediction of allergy skin test results. Ann Allergy Asthma Immunol. 2000;85:382-384.
- Nelson JL, Mowad CM. Allergic contact dermatitis: patch testing beyond the TRUE test. J Clin Aesthet Dermatol. 2010;3:36-41.
- Zuberbier T, Asero R, Bindslev-Jensen C, et al; Dermatology Section of the European Academy of Allergology and Clinical Immunology; Global Allergy and Asthma European Network; European Dermatology Forum; World Allergy Organization. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009;64:1417-1426.
- Bindslev-Jensen C, Finzi A, Greaves M, et al. Chronic urticaria: diagnostic recommendations. Eur Acad Dermatol Venereol. 2000;14:175-180.
- Bruze M, Conde-Slazar L, Goossens A, et al. Thoughts on sensitizers in a standard patch test series. Contact Dermatitis. 1999;41:241-250.
- Al-Sheikh OA, Gad El-Rab MO. Allergic contact dermatitis: clinical features and profile of sensitizing allergens in Riyadh, Saudi Arabia. Int J Dermatol. 1996;35:493-497.
- Al-Suwaidan SN, Gad El Rab MO, Al-Fakhiry S, et al. Allergic contact dermatitis from myrrh, a topical herbal medicine used to promote healing. Contact Dermatitis. 1998;39:137.
- Henz BM, Zuberbier T. Causes of urticaria. In: Henz B, Zuberbier T, Grabbe J, et al, eds. Urticaria: Clinical Diagnostic and Therapeutic Aspects. Berlin, Germany: Springer; 1998:19.
- Guerra L, Rogkakou A, Massacane P, et al. Role of contact sensitization in chronic urticaria. J Am Acad Dermatol. 2007;56:88-90.
- Thyssen JP, Linneberg A, Menné T, et al. The epidemiology of contact allergy in the general population—prevalence and main findings. Contact Dermatitis. 2007;57:287-299.
- Smart GA, Sherlock JC. Nickel in foods and the diet. Food Addit Contam. 1987;4:61-71.
- Abeck D, Traenckner I, Steinkraus V, et al. Chronic urticaria due to nickel intake. Acta Derm Venereol. 1993;73:438-439.
- Moneret-Vautrin DA. Allergic and pseudo-allergic reactions to foods in chronic urticaria [in French]. Ann Dermatol Venereol. 2003;130(Spec No 1):1S35-1S42.
- Wedi B, Raap U, Kapp A. Chronic urticaria and infections. Curr Opin Allergy Clin Immunol. 2004;4:387-396.
- Foti C, Nettis E, Cassano N, et al. Acute allergic reactions to Anisakis simplex after ingestion of anchovies. Acta Derm Venerol. 2002;82:121-123.
- Uter W, Hegewald J, Aberer W, et al. The European standard series in 9 European countries, 2002/2003: first results of the European Surveillance System on Contact Allergies. Contact Dermatitis. 2005;53:136-145.
- Magen E, Mishal J, Menachem S. Impact of contact sensitization in chronic spontaneous urticaria. Am J Med Sci. 2011;341:202-206.
- Antico A, Soana R. Chronic allergic-like dermatopathies in nickel sensitive patients: results of dietary restrictions and challenge with nickel salts. Allergy Asthma Proc. 1999;20:235-242.
Chronic urticaria (CU) is clinically defined as the daily or almost daily presence of wheals on the skin for at least 6 weeks.1 Chronic urticaria severely affects patients’ quality of life and can cause emotional disability and distress.2 In clinical practice, CU is one of the most common and challenging conditions for general practitioners, dermatologists, and allergists. It can be provoked by a wide variety of different causes or may be the clinical presentation of certain systemic diseases3,4; thus, CU often requires a detailed and time-consuming diagnostic procedure that includes screening for allergies, autoimmune diseases, parasites, malignancies, infections, and metabolic disorders.5,6 In many patients (up to 50% in some case series), the cause or pathogenic mechanism cannot be identified, and the disease is then classified as chronic idiopathic urticaria (CIU).7
It has previously been shown that contact sensitization could have some relation with CIU,8 which was further explored in this study. This study sought to evaluate if contact allergy may play a role in disease development in CIU patients in Saudi Arabia and if patch testing should be routinely performed for CIU patients to determine if any allergens can be avoided.
Methods
This prospective study was conducted at the King Khalid University Hospital Allergy Clinic (Riyadh, Saudi Arabia) in patients aged 18 to 60 years who had CU for more than 6 weeks. It was a clinic-based study conducted over a period of 2 years (March 2010 to February 2012). The study protocol was approved by the local ethics committee at King Khalid University Hospital. Valid written consent was obtained from each patient.
Patients were excluded if they had CU caused by physical factors (eg, hot or cold temperature, water, physical contact) or drug reactions that were possible causative factors or if they had taken oral prednisolone or other oral immunosuppressive drugs (eg, azathioprine, cyclosporine) in the last month. However, patients taking antihistamines were not excluded because it was impossible for the patients to discontinue their urticaria treatment. Other exclusion criteria included CU associated with any systemic disease, thyroid disease, diabetes mellitus, autoimmune disorder, or atopic dermatitis. Pregnant and lactating women were not included in this study.
All new adult CU patients (ie, disease duration >6 weeks) were worked up using the routine diagnostic tests that are typically performed for any new CU patient, including complete blood cell count with differential, erythrocyte sedimentation rate, liver function tests, urine analysis, and hepatitis B and C screenings. Further diagnostic tests also were carried out when appropriate according to the patient’s history and physical examination, including levels of urea, electrolytes, thyrotropin, thyroid antibodies (antithyroglobulin and antimicrosomal), and antinuclear antibodies, as well as a Helicobacter pylori test.
All of the patients enrolled in the study were evaluated by skin prick testing to establish the link between CU and its cause. Patch testing was performed in patients who were negative on skin prick testing.
Skin Prick Testing
All patients were advised to temporarily discontinue the use of antihistamines and corticosteroids 5 to 6 days prior to testing.
Patch Testing
Patch tests were carried out using a ready-to-use epicutaneous patch test system for the diagnosis of allergic contact dermatitis (ACD).10 A European standard series was used with the addition of 4 allergens of local relevance: black seed oil, local perfume mix, henna, and myrrh (a topical herbal medicine used to promote healing).
Assessment of Improvement
Assessment of urticaria severity using the Chronic Urticaria Severity Score (CUSS), a simple semiquantitative assessment of disease activity, was calculated as the sum of the number of wheals and the degree of itch severity graded from 0 (none) to 3 (severe), according to the guidelines established by the Dermatology Section of the European Academy of Allergology and Clinical Immunology, the Global Allergy and Asthma European Network, the European Dermatology Forum, and the World Allergy Organization.11 The avoidance group of patients was assessed at baseline and after 1 month to evaluate changes in their CUSS after allergen avoidance for 8 weeks.
Statistical Analysis
All of the statistical analyses were carried out using SPSS software version 16. Results were presented as the median with the range or the mean (SD).
Results
During the study period, a total of 120 CU patients were seen at the clinic. Ninety-three patients with CU met our selection criteria (77.5%) and were enrolled in the study. The mean age (SD) of the patients was 34.7 (12.4) years. Women comprised 68.8% (64/93) of the study population (Table 1).

The duration of urticaria ranged from 0.6 to 20 years, with a median duration of 4 years. Approximately half of the patients (50/93) experienced severe symptoms of urticaria, but only 26.9% (25/93) had graded their urticaria as very severe.
Negative results from the skin prick test were reported in 62.4% (58/93) of patients and were subsequently patch tested. These patients also had no other etiologic factors (eg, infection; thyroid, autoimmune, or metabolic disease). Patients who had positive skin prick test results (35/93 [37.6%]) were not considered to be cases of CIU, according to diagnostic recommendations.12 Of the 58 CIU patients who were patch tested, 31 (53.4%) had positive results and 27 (46.5%) had negative results to both skin prick and patch tests (Figure).

Univariate analysis revealed significant associations between age, gender, and duration of urticaria and patch test positivity (χ2 test, P<.05). T
Of the 31 patients with positive patch tests, there were 20 positive reactions to nickel, 6 to formaldehyde, 4 to phenylenediamine, 3 to cobalt, and 3 to a fragrance mix (Table 2). Some patients showed patch test reactivity to more than 1 allergen concomitantly. Overall, these 31 patients had positive reactions to 16 allergens. None of the patients showed actual signs of contact dermatitis (Table 2).

Of
Comment
Chronic idiopathic urticaria is the diagnosis given when urticarial vasculitis, physical urticaria, and all other possible etiologic factors have been excluded in patients with CU. Our study was designed to assess patch test reactivity in patients with CU without any identifiable systemic etiologic factor after detailed laboratory testing and negative skin prick tests.
Chronic idiopathic urticaria can be an extremely disabling and difficult-to-treat condition. Because the cause is unknown, the management of CIU often is frustrating. The
Patch testing is commonly performed to diagnose ACD, and if contact allergens are found via patch testing, patients can often be cured of their dermatitis by avoiding these agents. However, patch testing is not routinely performed in the evaluation of patients with CIU. It is a relatively inexpensive and safe procedure to determine a causal link between sensitization to a specific agent and ACD. In patch test clinics, agents often are tested in standard and screening series. Sensitization that is not suspected from the patient’s history and/or clinical examination can be detected in this manner. Requirements for the inclusion of a chemical in a standard series have been formulated by Bruze et al.13 In addition, ready-to-use materials relevant to the specific leisure activities and working conditions also can be selected for patch testing.
A study conducted in Saudi Arabia showed that the European standard series is suitable for patch testing patients in this community14; however, 3 allergens of local relevance were added in our study: black seed oil, local perfume mix, and henna. Moreover, in our study we added a local allergen known as myrrh, which is a topical herbal medicine used to promote healing that has been reported to cause ACD in some cases.15 We sought to determine if contact allergens can be identified with patch testing in patients with CU and if avoiding these contact allergens would resolve the CU.
Urticaria was once considered an IgE-mediated hypersensitivity reaction, but recent studies have demonstrated the existence of different subgroupsof urticaria, some with an autoimmune mechanism.1-4,11 In CU, skin prick tests are recommended for etiologic workup, while patch testing generally is not recommended.16
It has been observed in clinical practice that a substantial number of patients with CU are positive to patch tests, even without a clear clinical history or signs of contact dermatitis.17 In 2007, Guerra et al17 reported that of 121 patients with CU, 50 (41.3%) tested positive for contact allergens. In all of the patch test–positive patients, avoidance measures led to complete remission within 10 days to 1 month. Therefore, this result suggested that testing for contact sensitization could be helpful in the management of CU. Patients with nickel sensitivity were subsequently allowed to ingest small amounts of nickel-containing foods after 8 weeks of a completely nickel-free diet, and remission persisted.17
Contact dermatitis affects approximately 20% of the general population18; however, there has been little investigation (limited to nickel) into the relationship between contact allergens and CU,19,20 and the underlying mechanisms of the disease are unknown. It has been hypothesized that small amounts of the substances are absorbed through the skin or the digestive tract into the bloodstream over the long-term and are delivered to antigen-presenting cells in the skin, which provide the necessary signals for mast cell activation. Nonetheless, the reasons for a selectively cutaneous localization of the reaction remain largely unclear.
Management of CU is debated among physicians, and several diagnostic flowcharts have been proposed.1,2 In general, patch tests for contact dermatitis are not recommended as a fundamental part of the diagnostic procedure, but Guerra et al17 suggested that contact allergy often plays a role in CU.
There have been inadequate reports of CU found to be caused by common contact sensitizers.21-24 Interestingly, no signs of contact allergy were demonstrated in CU patients before urticarial attack.
Our findings supported our patient selection criteria and also confirmed that contact sensitization may be one of the many possible mechanisms involved in the etiology of CU. Urticaria may have a delayed-type hypersensitivity reaction element, and patients with CU without an obvious causal factor can have positive patch test results.
The
The main findings of our study were that 53.4% of patients with CIU had positive patch test results and that avoidance of the sensitizing substance was effective in 5 of 8 patients who completed an avoidance program. Almost all of the patients showed notable remission of symptoms after limiting their exposure to the offending allergens. This study clearly showed that a cause or pathogenesis for CIU could be identified, thus showing that CIU occurs less frequently than is usually assumed.
Our study had limitations. The first is our lack of a controlled challenge test, which is important to confirm an allergen as a cause of CIU.26 Nonetheless, avoidance of the revealed contact allergen was associated with comparable improvement of CIU severity after 1 month in 5 of 8 patients, though such measures were not tested in all 31 of 58 CIU patients who had positive patch test results.
Conclusion
We propose that patch tests should be performed while investigating CU because they give effective diagnostic and therapeutic results in a substantial number of patients. Urticaria, or at least a subgroup of the disease, may have a delayed-type reaction element, which may explain the disease etiology for many CIU patients. Patients with CU without a detectable underlying etiologic factor can have positive patch test results.
Chronic urticaria (CU) is clinically defined as the daily or almost daily presence of wheals on the skin for at least 6 weeks.1 Chronic urticaria severely affects patients’ quality of life and can cause emotional disability and distress.2 In clinical practice, CU is one of the most common and challenging conditions for general practitioners, dermatologists, and allergists. It can be provoked by a wide variety of different causes or may be the clinical presentation of certain systemic diseases3,4; thus, CU often requires a detailed and time-consuming diagnostic procedure that includes screening for allergies, autoimmune diseases, parasites, malignancies, infections, and metabolic disorders.5,6 In many patients (up to 50% in some case series), the cause or pathogenic mechanism cannot be identified, and the disease is then classified as chronic idiopathic urticaria (CIU).7
It has previously been shown that contact sensitization could have some relation with CIU,8 which was further explored in this study. This study sought to evaluate if contact allergy may play a role in disease development in CIU patients in Saudi Arabia and if patch testing should be routinely performed for CIU patients to determine if any allergens can be avoided.
Methods
This prospective study was conducted at the King Khalid University Hospital Allergy Clinic (Riyadh, Saudi Arabia) in patients aged 18 to 60 years who had CU for more than 6 weeks. It was a clinic-based study conducted over a period of 2 years (March 2010 to February 2012). The study protocol was approved by the local ethics committee at King Khalid University Hospital. Valid written consent was obtained from each patient.
Patients were excluded if they had CU caused by physical factors (eg, hot or cold temperature, water, physical contact) or drug reactions that were possible causative factors or if they had taken oral prednisolone or other oral immunosuppressive drugs (eg, azathioprine, cyclosporine) in the last month. However, patients taking antihistamines were not excluded because it was impossible for the patients to discontinue their urticaria treatment. Other exclusion criteria included CU associated with any systemic disease, thyroid disease, diabetes mellitus, autoimmune disorder, or atopic dermatitis. Pregnant and lactating women were not included in this study.
All new adult CU patients (ie, disease duration >6 weeks) were worked up using the routine diagnostic tests that are typically performed for any new CU patient, including complete blood cell count with differential, erythrocyte sedimentation rate, liver function tests, urine analysis, and hepatitis B and C screenings. Further diagnostic tests also were carried out when appropriate according to the patient’s history and physical examination, including levels of urea, electrolytes, thyrotropin, thyroid antibodies (antithyroglobulin and antimicrosomal), and antinuclear antibodies, as well as a Helicobacter pylori test.
All of the patients enrolled in the study were evaluated by skin prick testing to establish the link between CU and its cause. Patch testing was performed in patients who were negative on skin prick testing.
Skin Prick Testing
All patients were advised to temporarily discontinue the use of antihistamines and corticosteroids 5 to 6 days prior to testing.
Patch Testing
Patch tests were carried out using a ready-to-use epicutaneous patch test system for the diagnosis of allergic contact dermatitis (ACD).10 A European standard series was used with the addition of 4 allergens of local relevance: black seed oil, local perfume mix, henna, and myrrh (a topical herbal medicine used to promote healing).
Assessment of Improvement
Assessment of urticaria severity using the Chronic Urticaria Severity Score (CUSS), a simple semiquantitative assessment of disease activity, was calculated as the sum of the number of wheals and the degree of itch severity graded from 0 (none) to 3 (severe), according to the guidelines established by the Dermatology Section of the European Academy of Allergology and Clinical Immunology, the Global Allergy and Asthma European Network, the European Dermatology Forum, and the World Allergy Organization.11 The avoidance group of patients was assessed at baseline and after 1 month to evaluate changes in their CUSS after allergen avoidance for 8 weeks.
Statistical Analysis
All of the statistical analyses were carried out using SPSS software version 16. Results were presented as the median with the range or the mean (SD).
Results
During the study period, a total of 120 CU patients were seen at the clinic. Ninety-three patients with CU met our selection criteria (77.5%) and were enrolled in the study. The mean age (SD) of the patients was 34.7 (12.4) years. Women comprised 68.8% (64/93) of the study population (Table 1).

The duration of urticaria ranged from 0.6 to 20 years, with a median duration of 4 years. Approximately half of the patients (50/93) experienced severe symptoms of urticaria, but only 26.9% (25/93) had graded their urticaria as very severe.
Negative results from the skin prick test were reported in 62.4% (58/93) of patients and were subsequently patch tested. These patients also had no other etiologic factors (eg, infection; thyroid, autoimmune, or metabolic disease). Patients who had positive skin prick test results (35/93 [37.6%]) were not considered to be cases of CIU, according to diagnostic recommendations.12 Of the 58 CIU patients who were patch tested, 31 (53.4%) had positive results and 27 (46.5%) had negative results to both skin prick and patch tests (Figure).

Univariate analysis revealed significant associations between age, gender, and duration of urticaria and patch test positivity (χ2 test, P<.05). T
Of the 31 patients with positive patch tests, there were 20 positive reactions to nickel, 6 to formaldehyde, 4 to phenylenediamine, 3 to cobalt, and 3 to a fragrance mix (Table 2). Some patients showed patch test reactivity to more than 1 allergen concomitantly. Overall, these 31 patients had positive reactions to 16 allergens. None of the patients showed actual signs of contact dermatitis (Table 2).

Of
Comment
Chronic idiopathic urticaria is the diagnosis given when urticarial vasculitis, physical urticaria, and all other possible etiologic factors have been excluded in patients with CU. Our study was designed to assess patch test reactivity in patients with CU without any identifiable systemic etiologic factor after detailed laboratory testing and negative skin prick tests.
Chronic idiopathic urticaria can be an extremely disabling and difficult-to-treat condition. Because the cause is unknown, the management of CIU often is frustrating. The
Patch testing is commonly performed to diagnose ACD, and if contact allergens are found via patch testing, patients can often be cured of their dermatitis by avoiding these agents. However, patch testing is not routinely performed in the evaluation of patients with CIU. It is a relatively inexpensive and safe procedure to determine a causal link between sensitization to a specific agent and ACD. In patch test clinics, agents often are tested in standard and screening series. Sensitization that is not suspected from the patient’s history and/or clinical examination can be detected in this manner. Requirements for the inclusion of a chemical in a standard series have been formulated by Bruze et al.13 In addition, ready-to-use materials relevant to the specific leisure activities and working conditions also can be selected for patch testing.
A study conducted in Saudi Arabia showed that the European standard series is suitable for patch testing patients in this community14; however, 3 allergens of local relevance were added in our study: black seed oil, local perfume mix, and henna. Moreover, in our study we added a local allergen known as myrrh, which is a topical herbal medicine used to promote healing that has been reported to cause ACD in some cases.15 We sought to determine if contact allergens can be identified with patch testing in patients with CU and if avoiding these contact allergens would resolve the CU.
Urticaria was once considered an IgE-mediated hypersensitivity reaction, but recent studies have demonstrated the existence of different subgroupsof urticaria, some with an autoimmune mechanism.1-4,11 In CU, skin prick tests are recommended for etiologic workup, while patch testing generally is not recommended.16
It has been observed in clinical practice that a substantial number of patients with CU are positive to patch tests, even without a clear clinical history or signs of contact dermatitis.17 In 2007, Guerra et al17 reported that of 121 patients with CU, 50 (41.3%) tested positive for contact allergens. In all of the patch test–positive patients, avoidance measures led to complete remission within 10 days to 1 month. Therefore, this result suggested that testing for contact sensitization could be helpful in the management of CU. Patients with nickel sensitivity were subsequently allowed to ingest small amounts of nickel-containing foods after 8 weeks of a completely nickel-free diet, and remission persisted.17
Contact dermatitis affects approximately 20% of the general population18; however, there has been little investigation (limited to nickel) into the relationship between contact allergens and CU,19,20 and the underlying mechanisms of the disease are unknown. It has been hypothesized that small amounts of the substances are absorbed through the skin or the digestive tract into the bloodstream over the long-term and are delivered to antigen-presenting cells in the skin, which provide the necessary signals for mast cell activation. Nonetheless, the reasons for a selectively cutaneous localization of the reaction remain largely unclear.
Management of CU is debated among physicians, and several diagnostic flowcharts have been proposed.1,2 In general, patch tests for contact dermatitis are not recommended as a fundamental part of the diagnostic procedure, but Guerra et al17 suggested that contact allergy often plays a role in CU.
There have been inadequate reports of CU found to be caused by common contact sensitizers.21-24 Interestingly, no signs of contact allergy were demonstrated in CU patients before urticarial attack.
Our findings supported our patient selection criteria and also confirmed that contact sensitization may be one of the many possible mechanisms involved in the etiology of CU. Urticaria may have a delayed-type hypersensitivity reaction element, and patients with CU without an obvious causal factor can have positive patch test results.
The
The main findings of our study were that 53.4% of patients with CIU had positive patch test results and that avoidance of the sensitizing substance was effective in 5 of 8 patients who completed an avoidance program. Almost all of the patients showed notable remission of symptoms after limiting their exposure to the offending allergens. This study clearly showed that a cause or pathogenesis for CIU could be identified, thus showing that CIU occurs less frequently than is usually assumed.
Our study had limitations. The first is our lack of a controlled challenge test, which is important to confirm an allergen as a cause of CIU.26 Nonetheless, avoidance of the revealed contact allergen was associated with comparable improvement of CIU severity after 1 month in 5 of 8 patients, though such measures were not tested in all 31 of 58 CIU patients who had positive patch test results.
Conclusion
We propose that patch tests should be performed while investigating CU because they give effective diagnostic and therapeutic results in a substantial number of patients. Urticaria, or at least a subgroup of the disease, may have a delayed-type reaction element, which may explain the disease etiology for many CIU patients. Patients with CU without a detectable underlying etiologic factor can have positive patch test results.
- Zuberbier T, Bindslev-Jensen C, Canonica W, et al. Guidelines, definition, classification and diagnosis of urticaria. Allergy. 2006;61:316-331.
- Kaplan AP. Chronic urticaria: pathogenesis and treatment. J Allergy Clin Immunol. 2004;114:465-474.
- Champion RH. Urticaria: then and now. Br J Dermatol. 1988;119:427-436.
- Green GA, Koelsche GA, Kierland R. Etiology and pathogenesis of chronic urticaria. Ann Allergy. 1965;23:30-36.
- Kaplan AP. Chronic urticaria and angioedema. N Engl J Med. 2002;346:175-179.
- Dreskin SC, Andrews KY. The thyroid and urticaria. Curr Opin Allergy Clin Immunol. 2005;5:408-412.
- Greaves M. Chronic urticaria. J Allergy Clin Immunol. 2000;105:664-672.
- Sharma AD. Use of patch testing for identifying allergen causing chronic urticaria. Indian J Dermatol Venereol Leprol. 2008;74:114-117.
- Li JT, Andrist D, Bamlet WR, et al. Accuracy of patient prediction of allergy skin test results. Ann Allergy Asthma Immunol. 2000;85:382-384.
- Nelson JL, Mowad CM. Allergic contact dermatitis: patch testing beyond the TRUE test. J Clin Aesthet Dermatol. 2010;3:36-41.
- Zuberbier T, Asero R, Bindslev-Jensen C, et al; Dermatology Section of the European Academy of Allergology and Clinical Immunology; Global Allergy and Asthma European Network; European Dermatology Forum; World Allergy Organization. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009;64:1417-1426.
- Bindslev-Jensen C, Finzi A, Greaves M, et al. Chronic urticaria: diagnostic recommendations. Eur Acad Dermatol Venereol. 2000;14:175-180.
- Bruze M, Conde-Slazar L, Goossens A, et al. Thoughts on sensitizers in a standard patch test series. Contact Dermatitis. 1999;41:241-250.
- Al-Sheikh OA, Gad El-Rab MO. Allergic contact dermatitis: clinical features and profile of sensitizing allergens in Riyadh, Saudi Arabia. Int J Dermatol. 1996;35:493-497.
- Al-Suwaidan SN, Gad El Rab MO, Al-Fakhiry S, et al. Allergic contact dermatitis from myrrh, a topical herbal medicine used to promote healing. Contact Dermatitis. 1998;39:137.
- Henz BM, Zuberbier T. Causes of urticaria. In: Henz B, Zuberbier T, Grabbe J, et al, eds. Urticaria: Clinical Diagnostic and Therapeutic Aspects. Berlin, Germany: Springer; 1998:19.
- Guerra L, Rogkakou A, Massacane P, et al. Role of contact sensitization in chronic urticaria. J Am Acad Dermatol. 2007;56:88-90.
- Thyssen JP, Linneberg A, Menné T, et al. The epidemiology of contact allergy in the general population—prevalence and main findings. Contact Dermatitis. 2007;57:287-299.
- Smart GA, Sherlock JC. Nickel in foods and the diet. Food Addit Contam. 1987;4:61-71.
- Abeck D, Traenckner I, Steinkraus V, et al. Chronic urticaria due to nickel intake. Acta Derm Venereol. 1993;73:438-439.
- Moneret-Vautrin DA. Allergic and pseudo-allergic reactions to foods in chronic urticaria [in French]. Ann Dermatol Venereol. 2003;130(Spec No 1):1S35-1S42.
- Wedi B, Raap U, Kapp A. Chronic urticaria and infections. Curr Opin Allergy Clin Immunol. 2004;4:387-396.
- Foti C, Nettis E, Cassano N, et al. Acute allergic reactions to Anisakis simplex after ingestion of anchovies. Acta Derm Venerol. 2002;82:121-123.
- Uter W, Hegewald J, Aberer W, et al. The European standard series in 9 European countries, 2002/2003: first results of the European Surveillance System on Contact Allergies. Contact Dermatitis. 2005;53:136-145.
- Magen E, Mishal J, Menachem S. Impact of contact sensitization in chronic spontaneous urticaria. Am J Med Sci. 2011;341:202-206.
- Antico A, Soana R. Chronic allergic-like dermatopathies in nickel sensitive patients: results of dietary restrictions and challenge with nickel salts. Allergy Asthma Proc. 1999;20:235-242.
- Zuberbier T, Bindslev-Jensen C, Canonica W, et al. Guidelines, definition, classification and diagnosis of urticaria. Allergy. 2006;61:316-331.
- Kaplan AP. Chronic urticaria: pathogenesis and treatment. J Allergy Clin Immunol. 2004;114:465-474.
- Champion RH. Urticaria: then and now. Br J Dermatol. 1988;119:427-436.
- Green GA, Koelsche GA, Kierland R. Etiology and pathogenesis of chronic urticaria. Ann Allergy. 1965;23:30-36.
- Kaplan AP. Chronic urticaria and angioedema. N Engl J Med. 2002;346:175-179.
- Dreskin SC, Andrews KY. The thyroid and urticaria. Curr Opin Allergy Clin Immunol. 2005;5:408-412.
- Greaves M. Chronic urticaria. J Allergy Clin Immunol. 2000;105:664-672.
- Sharma AD. Use of patch testing for identifying allergen causing chronic urticaria. Indian J Dermatol Venereol Leprol. 2008;74:114-117.
- Li JT, Andrist D, Bamlet WR, et al. Accuracy of patient prediction of allergy skin test results. Ann Allergy Asthma Immunol. 2000;85:382-384.
- Nelson JL, Mowad CM. Allergic contact dermatitis: patch testing beyond the TRUE test. J Clin Aesthet Dermatol. 2010;3:36-41.
- Zuberbier T, Asero R, Bindslev-Jensen C, et al; Dermatology Section of the European Academy of Allergology and Clinical Immunology; Global Allergy and Asthma European Network; European Dermatology Forum; World Allergy Organization. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009;64:1417-1426.
- Bindslev-Jensen C, Finzi A, Greaves M, et al. Chronic urticaria: diagnostic recommendations. Eur Acad Dermatol Venereol. 2000;14:175-180.
- Bruze M, Conde-Slazar L, Goossens A, et al. Thoughts on sensitizers in a standard patch test series. Contact Dermatitis. 1999;41:241-250.
- Al-Sheikh OA, Gad El-Rab MO. Allergic contact dermatitis: clinical features and profile of sensitizing allergens in Riyadh, Saudi Arabia. Int J Dermatol. 1996;35:493-497.
- Al-Suwaidan SN, Gad El Rab MO, Al-Fakhiry S, et al. Allergic contact dermatitis from myrrh, a topical herbal medicine used to promote healing. Contact Dermatitis. 1998;39:137.
- Henz BM, Zuberbier T. Causes of urticaria. In: Henz B, Zuberbier T, Grabbe J, et al, eds. Urticaria: Clinical Diagnostic and Therapeutic Aspects. Berlin, Germany: Springer; 1998:19.
- Guerra L, Rogkakou A, Massacane P, et al. Role of contact sensitization in chronic urticaria. J Am Acad Dermatol. 2007;56:88-90.
- Thyssen JP, Linneberg A, Menné T, et al. The epidemiology of contact allergy in the general population—prevalence and main findings. Contact Dermatitis. 2007;57:287-299.
- Smart GA, Sherlock JC. Nickel in foods and the diet. Food Addit Contam. 1987;4:61-71.
- Abeck D, Traenckner I, Steinkraus V, et al. Chronic urticaria due to nickel intake. Acta Derm Venereol. 1993;73:438-439.
- Moneret-Vautrin DA. Allergic and pseudo-allergic reactions to foods in chronic urticaria [in French]. Ann Dermatol Venereol. 2003;130(Spec No 1):1S35-1S42.
- Wedi B, Raap U, Kapp A. Chronic urticaria and infections. Curr Opin Allergy Clin Immunol. 2004;4:387-396.
- Foti C, Nettis E, Cassano N, et al. Acute allergic reactions to Anisakis simplex after ingestion of anchovies. Acta Derm Venerol. 2002;82:121-123.
- Uter W, Hegewald J, Aberer W, et al. The European standard series in 9 European countries, 2002/2003: first results of the European Surveillance System on Contact Allergies. Contact Dermatitis. 2005;53:136-145.
- Magen E, Mishal J, Menachem S. Impact of contact sensitization in chronic spontaneous urticaria. Am J Med Sci. 2011;341:202-206.
- Antico A, Soana R. Chronic allergic-like dermatopathies in nickel sensitive patients: results of dietary restrictions and challenge with nickel salts. Allergy Asthma Proc. 1999;20:235-242.
Practice Points
- Patients with chronic urticaria (CU) without a detectable underlying etiologic factor can have positive patch test results.
- Avoidance of the sensitizing substance can be effective in CU patients and remission of symptoms can be possible after limiting their exposure to the offending allergens.
Contact dermatitis in children: The top 10 allergens
Contact dermatitis should be suspected in patients with eczematous dermatitis atypical in location, geometric or symmetric in distribution, or unresponsive to or worsened by common therapies, Dr. Catalina Matiz emphasized.
Allergic contact dermatitis is a complex disorder characterized by a type IV delayed-type hypersensitivity reaction that occurs when a patient’s skin is exposed to a substance easily penetrates the skin barrier.
Increasingly recognized in the United States, many pediatric patients are becoming sensitized to contact allergens found in personal care products, including skin care products, topical medications, and clothing.
A study published by Hill et al. in 2016 reviewed pediatric patch test studies to determine the top contact allergens in children (Expert Rev Clin Immunol. 2016;12[5]:551-61).
Dr. Matiz presented the top 10 allergens discovered by this group, and offered practical advice for allergen avoidance.
Topping the list in descending order are the following:
- Tixocortol pivalate (a corticosteroid).
- Propylene glycol.
- Methylchloroisothiazolinone (MCI)/methylisothiazolinone (MI).
- Formaldehyde.
- Cocamidopropyl betaine.
- Lanolin.
- Benzalkonium chloride.
- Fragrance and balsam of peru.
- Neomycin.
- Nickel.
Dr. Matiz educated meeting attendees on allergen-containing products and clinical correlations suggestive of allergic sensitization to common allergens. Tixocortol pivilate is a corticosteroid present in Class A corticosteroids including hydrocortisone acetate. She highlighted the cross reactivity between class A and class D2 corticosteroids including hydrocortisone butyrate and valerate.
“Usually topical corticosteroid–contact sensitivity manifests as a failure to improve or worsening of existing dermatitis,” emphasized Dr. Matiz of departments of dermatology and pediatrics at the university.
Propylene glycol, benzalkonium chloride, and neomycin also represent top contact allergens frequently found in topical medications. Similarly, lanolin contact sensitivity often presents as refractory or worsening atopic dermatitis. “Lanolin, also known as wool alcohol, is commonly found in emollients, medications, and personal care products used by atopic dermatitis patients,” Dr. Matiz stressed.
Methylchloroisothiazolinone/methylisothiazolinone represent preservatives commonly found in wet wipes, hypoallergenic, and sensitive skin products. This was the allergen of the year in 2013 and was subsequently removed from many wet wipe formulations and products in response.
In addition to acute management of allergic contact dermatitis with corticosteroids, Dr. Matiz further emphasized the importance of preemptive avoidance of the top ten allergens.
She recommends patch testing if no clinical improvement is evident after 8 weeks of common allergen avoidance, for definitive allergen identification.
Dr. Matiz said she had no relevant financial disclosures.
Contact dermatitis should be suspected in patients with eczematous dermatitis atypical in location, geometric or symmetric in distribution, or unresponsive to or worsened by common therapies, Dr. Catalina Matiz emphasized.
Allergic contact dermatitis is a complex disorder characterized by a type IV delayed-type hypersensitivity reaction that occurs when a patient’s skin is exposed to a substance easily penetrates the skin barrier.
Increasingly recognized in the United States, many pediatric patients are becoming sensitized to contact allergens found in personal care products, including skin care products, topical medications, and clothing.
A study published by Hill et al. in 2016 reviewed pediatric patch test studies to determine the top contact allergens in children (Expert Rev Clin Immunol. 2016;12[5]:551-61).
Dr. Matiz presented the top 10 allergens discovered by this group, and offered practical advice for allergen avoidance.
Topping the list in descending order are the following:
- Tixocortol pivalate (a corticosteroid).
- Propylene glycol.
- Methylchloroisothiazolinone (MCI)/methylisothiazolinone (MI).
- Formaldehyde.
- Cocamidopropyl betaine.
- Lanolin.
- Benzalkonium chloride.
- Fragrance and balsam of peru.
- Neomycin.
- Nickel.
Dr. Matiz educated meeting attendees on allergen-containing products and clinical correlations suggestive of allergic sensitization to common allergens. Tixocortol pivilate is a corticosteroid present in Class A corticosteroids including hydrocortisone acetate. She highlighted the cross reactivity between class A and class D2 corticosteroids including hydrocortisone butyrate and valerate.
“Usually topical corticosteroid–contact sensitivity manifests as a failure to improve or worsening of existing dermatitis,” emphasized Dr. Matiz of departments of dermatology and pediatrics at the university.
Propylene glycol, benzalkonium chloride, and neomycin also represent top contact allergens frequently found in topical medications. Similarly, lanolin contact sensitivity often presents as refractory or worsening atopic dermatitis. “Lanolin, also known as wool alcohol, is commonly found in emollients, medications, and personal care products used by atopic dermatitis patients,” Dr. Matiz stressed.
Methylchloroisothiazolinone/methylisothiazolinone represent preservatives commonly found in wet wipes, hypoallergenic, and sensitive skin products. This was the allergen of the year in 2013 and was subsequently removed from many wet wipe formulations and products in response.
In addition to acute management of allergic contact dermatitis with corticosteroids, Dr. Matiz further emphasized the importance of preemptive avoidance of the top ten allergens.
She recommends patch testing if no clinical improvement is evident after 8 weeks of common allergen avoidance, for definitive allergen identification.
Dr. Matiz said she had no relevant financial disclosures.
Contact dermatitis should be suspected in patients with eczematous dermatitis atypical in location, geometric or symmetric in distribution, or unresponsive to or worsened by common therapies, Dr. Catalina Matiz emphasized.
Allergic contact dermatitis is a complex disorder characterized by a type IV delayed-type hypersensitivity reaction that occurs when a patient’s skin is exposed to a substance easily penetrates the skin barrier.
Increasingly recognized in the United States, many pediatric patients are becoming sensitized to contact allergens found in personal care products, including skin care products, topical medications, and clothing.
A study published by Hill et al. in 2016 reviewed pediatric patch test studies to determine the top contact allergens in children (Expert Rev Clin Immunol. 2016;12[5]:551-61).
Dr. Matiz presented the top 10 allergens discovered by this group, and offered practical advice for allergen avoidance.
Topping the list in descending order are the following:
- Tixocortol pivalate (a corticosteroid).
- Propylene glycol.
- Methylchloroisothiazolinone (MCI)/methylisothiazolinone (MI).
- Formaldehyde.
- Cocamidopropyl betaine.
- Lanolin.
- Benzalkonium chloride.
- Fragrance and balsam of peru.
- Neomycin.
- Nickel.
Dr. Matiz educated meeting attendees on allergen-containing products and clinical correlations suggestive of allergic sensitization to common allergens. Tixocortol pivilate is a corticosteroid present in Class A corticosteroids including hydrocortisone acetate. She highlighted the cross reactivity between class A and class D2 corticosteroids including hydrocortisone butyrate and valerate.
“Usually topical corticosteroid–contact sensitivity manifests as a failure to improve or worsening of existing dermatitis,” emphasized Dr. Matiz of departments of dermatology and pediatrics at the university.
Propylene glycol, benzalkonium chloride, and neomycin also represent top contact allergens frequently found in topical medications. Similarly, lanolin contact sensitivity often presents as refractory or worsening atopic dermatitis. “Lanolin, also known as wool alcohol, is commonly found in emollients, medications, and personal care products used by atopic dermatitis patients,” Dr. Matiz stressed.
Methylchloroisothiazolinone/methylisothiazolinone represent preservatives commonly found in wet wipes, hypoallergenic, and sensitive skin products. This was the allergen of the year in 2013 and was subsequently removed from many wet wipe formulations and products in response.
In addition to acute management of allergic contact dermatitis with corticosteroids, Dr. Matiz further emphasized the importance of preemptive avoidance of the top ten allergens.
She recommends patch testing if no clinical improvement is evident after 8 weeks of common allergen avoidance, for definitive allergen identification.
Dr. Matiz said she had no relevant financial disclosures.
Isothiazolinone allergy frequent and underdiagnosed in children
Sensitization to the isothiazolinones MCI (methylchloroisothiazolinone) and MI (methylisothiazolinone), which are used as preservatives in a wide variety of personal and household products, is both frequent and underdiagnosed in U.S. children, according to a report published in Pediatric Dermatology.
These agents are compatible with surfactants and emulsifiers, and because they maintain biocidal activity across a broad range of pH levels they are frequently used as preservatives in products such as wet wipes; shampoos and hair conditioners; soaps, cleansers, and disinfectants; and laundry products. However, they are known to cause contact dermatitis very frequently, and are among the top five contact allergens identified in infants’ patch tests.
A recent survey showed that among 152 pediatric skin care products available at major retail stores, 20% contained MI. These were specifically targeted to infants and children, advertised as being “hypoallergenic,” “natural,” good for “sensitive” skin, and containing “gentle ingredients,” said Alina Goldenberg, MD, of the department of dermatology at the University of California, San Diego, and her associates.
During the past 10 years, only 35 U.S. cases of a positive patch-test reaction to MCI and/or MI have been reported in the literature. To get a more accurate estimate of the true prevalence of pediatric sensitization to MCI and MI, the investigators analyzed information in a database of patch-test results, the Provider Contact Dermatitis Registry. They focused on 1,056 patch tests performed during a 1-year period.
They found 37 positive reactions to combined MCI/MI and another 39 reactions that were negative to combined MCI/MI but positive to MI alone. This shows how important it is to test for sensitization to both formulations separately, Dr. Goldenberg and her associates noted (Pediatr Dermatol. 2017 Mar;34[2]:138-43).
In stark contrast to the reported 35 cases across the entire country during a 10-year period, the investigators found 76 cases (1%) in 1,056 patch tests during a 1-year period.
When test results for MCI/MI and MI alone were compared with those for all other allergens, children sensitized to the isothiazolinones showed marked differences: They were significantly younger, and the location of their dermatitis was more likely to involve the groin and buttocks. This probably is due to the increased use of wet wipes containing MCI and MI being used to clean up urinary and fecal accidents in young children, the researchers said.
The Society for Pediatric Dermatology supported the work. Dr. Goldenberg reported having no relevant financial disclosures; an associate reported serving as a consultant for Johnson & Johnson.
Sensitization to the isothiazolinones MCI (methylchloroisothiazolinone) and MI (methylisothiazolinone), which are used as preservatives in a wide variety of personal and household products, is both frequent and underdiagnosed in U.S. children, according to a report published in Pediatric Dermatology.
These agents are compatible with surfactants and emulsifiers, and because they maintain biocidal activity across a broad range of pH levels they are frequently used as preservatives in products such as wet wipes; shampoos and hair conditioners; soaps, cleansers, and disinfectants; and laundry products. However, they are known to cause contact dermatitis very frequently, and are among the top five contact allergens identified in infants’ patch tests.
A recent survey showed that among 152 pediatric skin care products available at major retail stores, 20% contained MI. These were specifically targeted to infants and children, advertised as being “hypoallergenic,” “natural,” good for “sensitive” skin, and containing “gentle ingredients,” said Alina Goldenberg, MD, of the department of dermatology at the University of California, San Diego, and her associates.
During the past 10 years, only 35 U.S. cases of a positive patch-test reaction to MCI and/or MI have been reported in the literature. To get a more accurate estimate of the true prevalence of pediatric sensitization to MCI and MI, the investigators analyzed information in a database of patch-test results, the Provider Contact Dermatitis Registry. They focused on 1,056 patch tests performed during a 1-year period.
They found 37 positive reactions to combined MCI/MI and another 39 reactions that were negative to combined MCI/MI but positive to MI alone. This shows how important it is to test for sensitization to both formulations separately, Dr. Goldenberg and her associates noted (Pediatr Dermatol. 2017 Mar;34[2]:138-43).
In stark contrast to the reported 35 cases across the entire country during a 10-year period, the investigators found 76 cases (1%) in 1,056 patch tests during a 1-year period.
When test results for MCI/MI and MI alone were compared with those for all other allergens, children sensitized to the isothiazolinones showed marked differences: They were significantly younger, and the location of their dermatitis was more likely to involve the groin and buttocks. This probably is due to the increased use of wet wipes containing MCI and MI being used to clean up urinary and fecal accidents in young children, the researchers said.
The Society for Pediatric Dermatology supported the work. Dr. Goldenberg reported having no relevant financial disclosures; an associate reported serving as a consultant for Johnson & Johnson.
Sensitization to the isothiazolinones MCI (methylchloroisothiazolinone) and MI (methylisothiazolinone), which are used as preservatives in a wide variety of personal and household products, is both frequent and underdiagnosed in U.S. children, according to a report published in Pediatric Dermatology.
These agents are compatible with surfactants and emulsifiers, and because they maintain biocidal activity across a broad range of pH levels they are frequently used as preservatives in products such as wet wipes; shampoos and hair conditioners; soaps, cleansers, and disinfectants; and laundry products. However, they are known to cause contact dermatitis very frequently, and are among the top five contact allergens identified in infants’ patch tests.
A recent survey showed that among 152 pediatric skin care products available at major retail stores, 20% contained MI. These were specifically targeted to infants and children, advertised as being “hypoallergenic,” “natural,” good for “sensitive” skin, and containing “gentle ingredients,” said Alina Goldenberg, MD, of the department of dermatology at the University of California, San Diego, and her associates.
During the past 10 years, only 35 U.S. cases of a positive patch-test reaction to MCI and/or MI have been reported in the literature. To get a more accurate estimate of the true prevalence of pediatric sensitization to MCI and MI, the investigators analyzed information in a database of patch-test results, the Provider Contact Dermatitis Registry. They focused on 1,056 patch tests performed during a 1-year period.
They found 37 positive reactions to combined MCI/MI and another 39 reactions that were negative to combined MCI/MI but positive to MI alone. This shows how important it is to test for sensitization to both formulations separately, Dr. Goldenberg and her associates noted (Pediatr Dermatol. 2017 Mar;34[2]:138-43).
In stark contrast to the reported 35 cases across the entire country during a 10-year period, the investigators found 76 cases (1%) in 1,056 patch tests during a 1-year period.
When test results for MCI/MI and MI alone were compared with those for all other allergens, children sensitized to the isothiazolinones showed marked differences: They were significantly younger, and the location of their dermatitis was more likely to involve the groin and buttocks. This probably is due to the increased use of wet wipes containing MCI and MI being used to clean up urinary and fecal accidents in young children, the researchers said.
The Society for Pediatric Dermatology supported the work. Dr. Goldenberg reported having no relevant financial disclosures; an associate reported serving as a consultant for Johnson & Johnson.
FROM PEDIATRIC DERMATOLOGY
Key clinical point:
Major finding: There were 37 positive patch-test reactions to combined MCI/MI and another 39 reactions that were negative to combined MCI/MI but positive to MI alone.
Data source: An analysis of 1,056 patch-test results recorded in a database by clinicians during a 1-year period.
Disclosures: The Society for Pediatric Dermatology supported the work. Dr. Goldenberg reported having no relevant financial disclosures; an associate reported serving as a consultant for Johnson & Johnson.
Black Linear Streaks on the Face With Pruritic Plaques on the Trunk and Arms
The Diagnosis: Toxicodendron Dermatitis
Toxicodendron dermatitis is an allergic contact dermatitis that can occur after exposure to a plant from the Toxicodendron genus including poison ivy (Toxicodendron radicans), poison oak (Toxicodendron diversilobum), and poison sumac (Toxicodendron vernix). These plants produce urushiol in their oleoresinous sap, which causes intense pruritus, streaks of erythema, and edematous papules followed by vesicles and bullae. Previously sensitized individuals develop symptoms as quickly as 24 to 48 hours after exposure, with a range of 5 hours to 15 days.1-3 Rarely, black spots also can be found on the skin, most prominently after 72 hours of exposure.4
The color change of urushiol-containing sap from pale to black was first documented by Peter Kalm, a Swedish botanist who traveled to North America in the 1700s.5 The black-spot test can be used to identify Toxicodendron species because the sap will turn black when expressed on white paper after a few minutes.6 Manifestation of black lacquer streaks on the skin is rare because concentrated sap is necessary, which typically requires an unusually prolonged exposure with Toxicodendron plants.7
Without treatment, typical Toxicodendron dermatitis resolves in approximately 3 weeks, though it may take up to 6 weeks to clear.2 Early intervention is critical, as urushiol will fully absorb after 30 minutes.2 After contact, complete removal of the oleoresin by washing with mild soap and water within 10 minutes can prevent dermatitis. Early topical corticosteroid application can reduce erythema and pruritus. Extensive or severe involvement, which includes Toxicodendron dermatitis with black spots, is treated with systemic corticosteroids such as prednisone that is tapered over 2 to 3 weeks.1
Our patient had classic findings of Toxicodendron dermatitis; however, initially there was concern for levamisole toxicity by the emergency department, as well-demarcated purpuric or dark skin lesions can be due to morbid conditions such as leukocytoclastic vasculitis or skin necrosis from drug toxicities or infectious etiologies. Dermatology was consulted and these concerns were alleviated on closer skin examination and further questioning. The patient reported that he spent several hours cutting brush that was known to be T vernix (poison sumac) 3 days prior to presentation. Interestingly, the patient did not have similar black streaks on the left side of the face, as he held the weed-trimming saw in his right hand and in effect protected the left side of the face from debris. Furthermore, he had pruritic erythematous plaques on both forearms. The facial black lacquer-like streaks were the result of urushiol oxidation in the setting of prolonged exposure to the poison sumac oleoresin sap during weed trimming. After dermatologic evaluation, the patient was discharged from the emergency department on a 15-day taper of oral prednisone, and he was instructed to wash involved areas and exposed clothing with soap and water, which led to complete resolution.
- Lee NP, Arriola ER. Poison ivy, oak, and sumac dermatitis. West J Med. 1999;171:354-355.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128.
- Gross M, Baer H, Fales H. Urushiols of poisonous anacardiaceae. Phytochemistry. 1975;14:2263-2266.
- Mallory SB, Miller OF 3dU, Tyler WB. Toxicodendron radicans dermatitis with black lacquer deposit on the skin. J Am Acad Dermatol. 1982;6:363-368.
- Benson AB. Peter Kalm's Travels in North America: The English Version of 1770. New York, NY: Dover Publications; 1937.
- Guin JD. The black spot test for recognizing poison ivy and related species. J Am Acad Dermatol. 1980;2:332-333.
- Kurlan JG, Lucky AW. Black spot poison ivy: a report of 5 cases and a review of the literature. J Am Acad Dermatol. 2001;45:246-249.
The Diagnosis: Toxicodendron Dermatitis
Toxicodendron dermatitis is an allergic contact dermatitis that can occur after exposure to a plant from the Toxicodendron genus including poison ivy (Toxicodendron radicans), poison oak (Toxicodendron diversilobum), and poison sumac (Toxicodendron vernix). These plants produce urushiol in their oleoresinous sap, which causes intense pruritus, streaks of erythema, and edematous papules followed by vesicles and bullae. Previously sensitized individuals develop symptoms as quickly as 24 to 48 hours after exposure, with a range of 5 hours to 15 days.1-3 Rarely, black spots also can be found on the skin, most prominently after 72 hours of exposure.4
The color change of urushiol-containing sap from pale to black was first documented by Peter Kalm, a Swedish botanist who traveled to North America in the 1700s.5 The black-spot test can be used to identify Toxicodendron species because the sap will turn black when expressed on white paper after a few minutes.6 Manifestation of black lacquer streaks on the skin is rare because concentrated sap is necessary, which typically requires an unusually prolonged exposure with Toxicodendron plants.7
Without treatment, typical Toxicodendron dermatitis resolves in approximately 3 weeks, though it may take up to 6 weeks to clear.2 Early intervention is critical, as urushiol will fully absorb after 30 minutes.2 After contact, complete removal of the oleoresin by washing with mild soap and water within 10 minutes can prevent dermatitis. Early topical corticosteroid application can reduce erythema and pruritus. Extensive or severe involvement, which includes Toxicodendron dermatitis with black spots, is treated with systemic corticosteroids such as prednisone that is tapered over 2 to 3 weeks.1
Our patient had classic findings of Toxicodendron dermatitis; however, initially there was concern for levamisole toxicity by the emergency department, as well-demarcated purpuric or dark skin lesions can be due to morbid conditions such as leukocytoclastic vasculitis or skin necrosis from drug toxicities or infectious etiologies. Dermatology was consulted and these concerns were alleviated on closer skin examination and further questioning. The patient reported that he spent several hours cutting brush that was known to be T vernix (poison sumac) 3 days prior to presentation. Interestingly, the patient did not have similar black streaks on the left side of the face, as he held the weed-trimming saw in his right hand and in effect protected the left side of the face from debris. Furthermore, he had pruritic erythematous plaques on both forearms. The facial black lacquer-like streaks were the result of urushiol oxidation in the setting of prolonged exposure to the poison sumac oleoresin sap during weed trimming. After dermatologic evaluation, the patient was discharged from the emergency department on a 15-day taper of oral prednisone, and he was instructed to wash involved areas and exposed clothing with soap and water, which led to complete resolution.
The Diagnosis: Toxicodendron Dermatitis
Toxicodendron dermatitis is an allergic contact dermatitis that can occur after exposure to a plant from the Toxicodendron genus including poison ivy (Toxicodendron radicans), poison oak (Toxicodendron diversilobum), and poison sumac (Toxicodendron vernix). These plants produce urushiol in their oleoresinous sap, which causes intense pruritus, streaks of erythema, and edematous papules followed by vesicles and bullae. Previously sensitized individuals develop symptoms as quickly as 24 to 48 hours after exposure, with a range of 5 hours to 15 days.1-3 Rarely, black spots also can be found on the skin, most prominently after 72 hours of exposure.4
The color change of urushiol-containing sap from pale to black was first documented by Peter Kalm, a Swedish botanist who traveled to North America in the 1700s.5 The black-spot test can be used to identify Toxicodendron species because the sap will turn black when expressed on white paper after a few minutes.6 Manifestation of black lacquer streaks on the skin is rare because concentrated sap is necessary, which typically requires an unusually prolonged exposure with Toxicodendron plants.7
Without treatment, typical Toxicodendron dermatitis resolves in approximately 3 weeks, though it may take up to 6 weeks to clear.2 Early intervention is critical, as urushiol will fully absorb after 30 minutes.2 After contact, complete removal of the oleoresin by washing with mild soap and water within 10 minutes can prevent dermatitis. Early topical corticosteroid application can reduce erythema and pruritus. Extensive or severe involvement, which includes Toxicodendron dermatitis with black spots, is treated with systemic corticosteroids such as prednisone that is tapered over 2 to 3 weeks.1
Our patient had classic findings of Toxicodendron dermatitis; however, initially there was concern for levamisole toxicity by the emergency department, as well-demarcated purpuric or dark skin lesions can be due to morbid conditions such as leukocytoclastic vasculitis or skin necrosis from drug toxicities or infectious etiologies. Dermatology was consulted and these concerns were alleviated on closer skin examination and further questioning. The patient reported that he spent several hours cutting brush that was known to be T vernix (poison sumac) 3 days prior to presentation. Interestingly, the patient did not have similar black streaks on the left side of the face, as he held the weed-trimming saw in his right hand and in effect protected the left side of the face from debris. Furthermore, he had pruritic erythematous plaques on both forearms. The facial black lacquer-like streaks were the result of urushiol oxidation in the setting of prolonged exposure to the poison sumac oleoresin sap during weed trimming. After dermatologic evaluation, the patient was discharged from the emergency department on a 15-day taper of oral prednisone, and he was instructed to wash involved areas and exposed clothing with soap and water, which led to complete resolution.
- Lee NP, Arriola ER. Poison ivy, oak, and sumac dermatitis. West J Med. 1999;171:354-355.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128.
- Gross M, Baer H, Fales H. Urushiols of poisonous anacardiaceae. Phytochemistry. 1975;14:2263-2266.
- Mallory SB, Miller OF 3dU, Tyler WB. Toxicodendron radicans dermatitis with black lacquer deposit on the skin. J Am Acad Dermatol. 1982;6:363-368.
- Benson AB. Peter Kalm's Travels in North America: The English Version of 1770. New York, NY: Dover Publications; 1937.
- Guin JD. The black spot test for recognizing poison ivy and related species. J Am Acad Dermatol. 1980;2:332-333.
- Kurlan JG, Lucky AW. Black spot poison ivy: a report of 5 cases and a review of the literature. J Am Acad Dermatol. 2001;45:246-249.
- Lee NP, Arriola ER. Poison ivy, oak, and sumac dermatitis. West J Med. 1999;171:354-355.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128.
- Gross M, Baer H, Fales H. Urushiols of poisonous anacardiaceae. Phytochemistry. 1975;14:2263-2266.
- Mallory SB, Miller OF 3dU, Tyler WB. Toxicodendron radicans dermatitis with black lacquer deposit on the skin. J Am Acad Dermatol. 1982;6:363-368.
- Benson AB. Peter Kalm's Travels in North America: The English Version of 1770. New York, NY: Dover Publications; 1937.
- Guin JD. The black spot test for recognizing poison ivy and related species. J Am Acad Dermatol. 1980;2:332-333.
- Kurlan JG, Lucky AW. Black spot poison ivy: a report of 5 cases and a review of the literature. J Am Acad Dermatol. 2001;45:246-249.
A 68-year-old man presented to the emergency department with pruritic, edematous, pink plaques on the trunk and arms, as well as black linear streaks on the face, prompting dermatology consultation for possible tissue necrosis. The patient reported working outdoors in his garden 3 days prior to presentation.
Allergic Reaction to Vanadium Causes a Diffuse Eczematous Eruption and Titanium Alloy Orthopedic Implant Failure
Metal allergy in patients with orthopedic implants can cause serious problems including dermatitis and implant failure.1 As life expectancy increases, the general population ages, and more metallic orthopedic implants are placed,2 allergy to these implants is expected to be a problem of greater significance. Uncertainty remains regarding best practice for patients with suspected metal implant allergy.1 The major questions are: Who should be tested? When should they be tested? What are the optimal tests to diagnose metal allergy?3-8
We report the case of a patient with vanadium allergy who developed a diffuse eczematous dermatitis and implant failure after receiving a vanadium-containing titanium alloy orthopedic implant in the left foot. This case is remarkable because hypersensitivity reactions to titanium-based hardware are rare, as they traditionally have not been thought to provoke allergic reactions.9
Case Report
A 62-year-old woman who was otherwise healthy presented with an eruption of more than 80 pruritic, nummular, eczematous plaques on the arms, legs, back, and buttocks of 3 weeks’ duration (Figure 1). She had a history of allergy to metal used in costume jewelry. Six weeks prior, the patient underwent implantation of a titanium alloy plate in the left foot for surgical repair of painful deforming osteoarthritis. A radiograph of the foot showed appropriate placement. According to the manufacturer, the plate was composed of the compound Ti6Al4V, which contained 90% titanium, 6% aluminum, and 4% vanadium. The lesions developed on the skin close to but not directly over the surgical site.
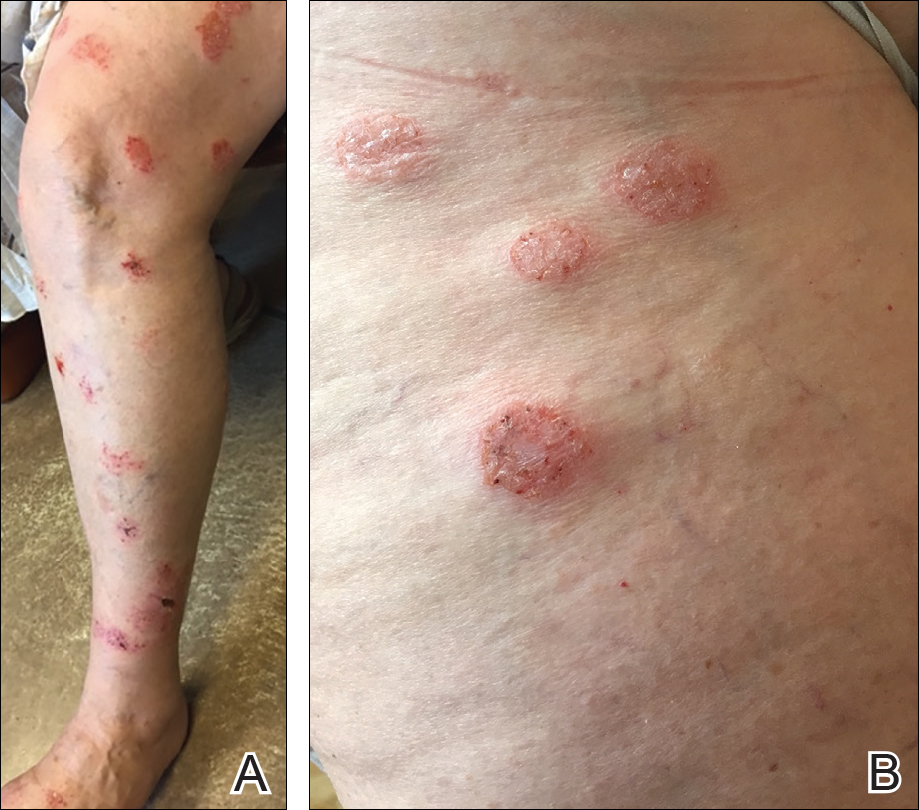
A punch biopsy of one of the lesions on the shoulder showed lymphoeosinophilic spongiosis consistent with a delayed hypersensitivity reaction (Figure 2). There was mild clinical improvement of the eruption with topical steroids. A course of prednisone for systemic effect resulted in clearing of the eruption, but it promptly recurred on cessation of the steroids. The patient was then patch tested using the North American 80 Comprehensive Series, with an additional 59 common textile, shampoo, fragrance, and several metal allergens, all of which were negative.
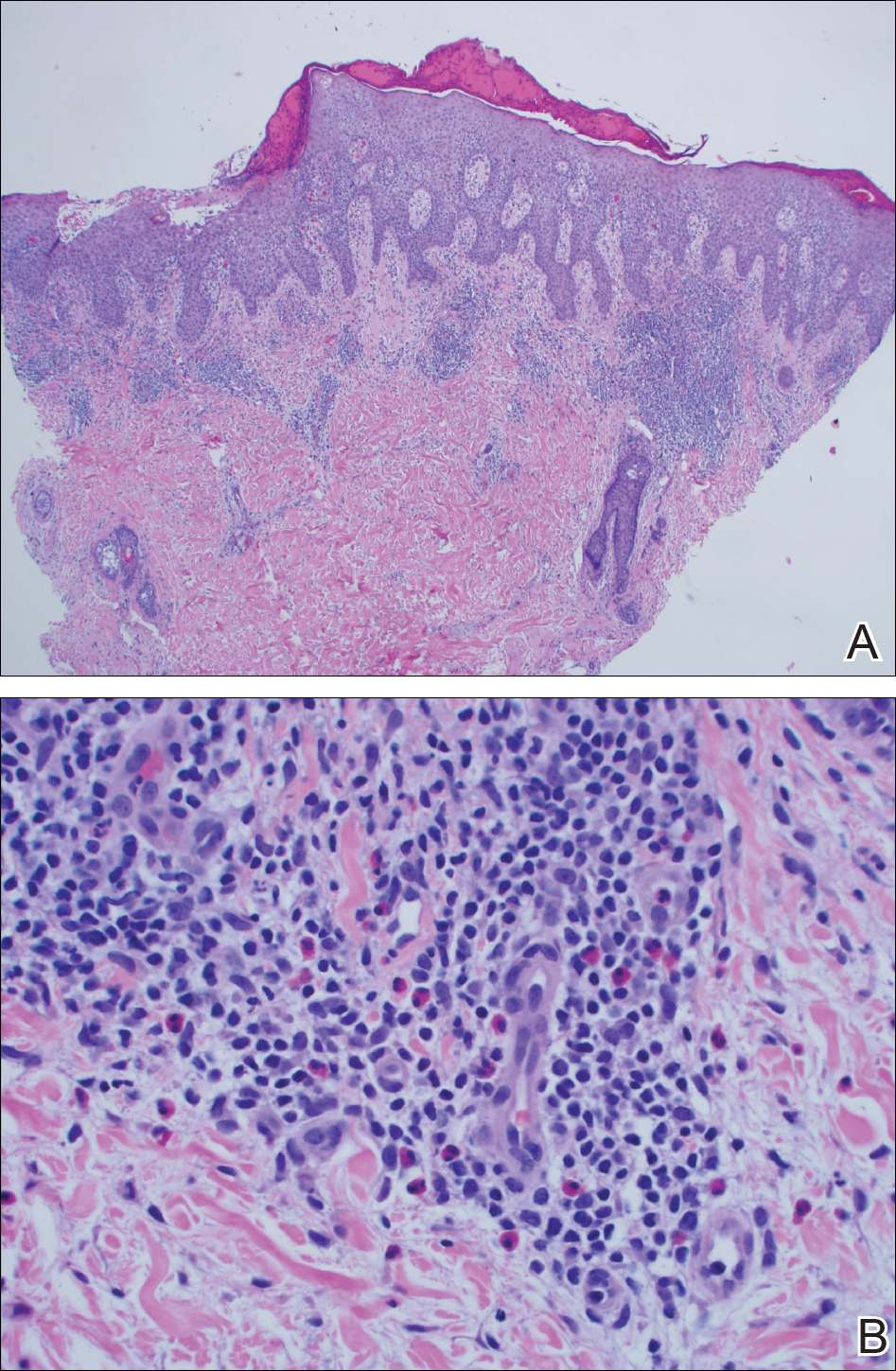
The patient had persistent pain and swelling at the surgical site, and radiographs taken postoperatively at 6 months showed implant failure (Figure 3). The hardware was surgically removed 8 months after implantation (Figure 4) and the plate and screws were submitted to the Institute for Mineral Resources Geosciences LA-ICP-MS Facility and the Lunar and Planetary Laboratory at the University of Arizona (Tucson, Arizona) for analysis. The skin lesions began to improve days after the hardware was removed and the eruption cleared over the following 3 weeks with no additional treatment.

After the hardware was removed, it was analyzed to determine the elemental composition of the plate and screws, and the patient was then patch tested with the major metal components of the implant: aluminum chloride hexahydrate 2.0% pet, elemental titanium 10.0% pet, titanium dioxide 10.0% pet, titanium (III) nitride 5.0% pet, titanium (III) oxalate decahydrate 5.0% pet, elemental vanadium 5.0% pet, and vanadium (III) chloride 1.0% pet. She demonstrated a 1+ reaction (erythema and induration) to vanadium trichloride at 72 and 96 hours.
The plate and screws removed from the patient were sterilized and submitted for analysis. Electron microprobe analysis confirmed that the major elemental composition of the plate and screws essentially matched the manufacturer’s listing (Table 1). The trace elements were determined using laser ablative inductively coupled mass spectroscopy, which demonstrated that the screws were of different metal composition from the plate (Table 2). Electron microprobe analysis also was used to determine the microstructure of the plate and screws. The plate had 2 distinct phases consisting of a titanium-aluminum phase and a vanadium phase, whereas the screw was much more homogeneous. Basic electrochemical studies were performed in a salt solution replicating the tissue of the foot. These studies showed that galvanic corrosion could have occurred between the plate and screws due to the differences of composition.
Comment
Titanium is an attractive metal to use in orthopedic implants. It has a high strength-to-weight ratio, a low modulus of elasticity, and good resistance to corrosion. Titanium can be categorized as either commercially pure titanium (cp-Ti) or a titanium alloy. Colloquially, both cp-Ti and titanium alloys are often referred to simply as titanium, but the distinction is important when it comes to medical implants and devices. Commercially pure titanium is more than 99% pure titanium, but up to 1% of its volume can be comprised of impurities.10 In titanium alloys, the alloy elements are intentionally added to create a material with optimal properties. The 2 most common types of titanium that are used for orthopedic implants are cp-Ti and Ti6Al4V, a titanium alloy containing approximately 90% titanium, 6% aluminum, and 4% vanadium. Similar to cp-Ti, titanium alloys also can contain impurities such as aluminum, beryllium, cobalt, chromium, iron, nickel, and palladium, among many others. Although these impurities often are considered negligible from a metallurgy perspective, as they do not change the properties of the material, these trace elements may be present in large enough quantities to cause hypersensitivity reactions.11
Several weeks after implantation of a titanium alloy metal plate in the left foot, a widespread eczematous eruption developed in our patient who had no prior skin disease. The eruption was steroid responsive but did not clear until the plate was removed. Detailed metallurgy analysis confirmed that vanadium was present and was not homogeneously distributed in the plate. The plate also was different in composition from the screws. Additional studies showed that galvanic corrosion between the plate and the chemically different screws might have contributed to the release of vanadium in the tissue.
Vanadium is known to be allergenic, especially in the presence of implant failure.12,13 In our patient, patch testing with more than 100 allergens was negative, except for vanadium trichloride 1%. Our patient’s presentation strongly suggested that she developed a vanadium allergy manifesting as systemic allergic contact dermatitis. She demonstrated no history of skin disease, a widespread eczematous eruption after exposure, histology consistent with systemic contact allergy, a positive patch test to vanadium, and clearance of the eruption on removal of the antigen, which have been proposed as objective criteria that support a diagnosis of metal implant allergy.14 She refused our suggestion to reimplant a portion of the remaining plate under the skin without screws and monitor for recurrence of the eruption. She did not have a lesion overlying the surgical site, but she did develop lesions near the surgical scar. The literature indicates that cutaneous manifestations of allergy to metallic implants can be both localized and generalized.14
Although reports are rare, other researchers have found vanadium allergy in patients with metal orthopedic implants.5,12,13,15 The scarcity of literature on vanadium allergy seems to suggest that it is a rare entity, but we believe that it may be more common. Vanadium allergy may be underdiagnosed because it is not a standard patch test allergen. Furthermore, many of those who do choose to test for it use what we believe to be ineffective formulas of vanadium when patch testing patients. Our patient demonstrated a positive patch test reaction only to vanadium trichloride and not to pure vanadium, which is consistent with the small number of other studies that investigated vanadium allergy.5,12,13,15 We believe that vanadium trichloride is more water soluble than elemental vanadium,16 and thus more likely to identify true vanadium allergy than other test materials.
Although reports of vanadium allergy in patients with metal implants are rare in the medical literature, the material science literature clearly states that vanadium is toxic and that vanadium-containing implants are problematic.17-20 It has been shown that although Ti6Al4V implants are considered highly resistant to corrosion, they will slowly and continuously corrode in a physiologic environment and release titanium, aluminum, and vanadium ions, both systemically and into the peri-implant space.11 To address these problems with vanadium, vanadium-free titanium alloys such as Ti6Al7Nb have specifically been developed for medical use to address the problems caused by vanadium. Ti6Al7Nb contains 7% niobium rather than vanadium and appears to have some improved qualities in surgical implants.17
There is still a great deal of uncertainty around metal implant allergy. Allergy to metal implants can be difficult to diagnose for several reasons. Some metals are not conducive to patch testing because of their low bioavailability. Additionally, we lack validated and standardized patch test formulas for metals that can be diagnosed by patch testing. Furthermore, there is uncertainty about what to do after allergy to a metal implant is diagnosed; in some cases (eg, with more extensive procedures such as total joint replacements), removal or replacement of the implant may be associated with increased risk of further complications.6,21
Conclusion
We suggest that manufacturers consider vanadium-free alloys such as Ti7Al6Nb, which contains niobium instead of vanadium, in their surgical implants,22 and if surgeons have a choice, they should consider using titanium implants with niobium rather than vanadium.10 We suggest that clinicians consider vanadium allergy in patients with Ti6Al4V surgical implants and signs of a hypersensitivity reaction, and include vanadium trichloride 1% when patch testing.
Acknowledgment
The authors would like to thank Nicholas R. Krasnow, PhD (Tucson, Arizona), for his invaluable help coordinating, performing, and interpreting the metal analyses.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785.
- Thyssen JP, Johansen JD, Menné T, et al. Hypersensitivity reactions from metallic implants: a future challenge that needs to be addressed. Br J Dermatol. 2010;162:235-236.
- Aquino M, Mucci T. Systemic contact dermatitis and allergy to biomedical devices. Curr Allergy Asthma Rep. 2013;13:518-527.
- Krecisz B, Kiec-Swierczynska M, Chomiczewska-Skora D. Allergy to orthopedic metal implants—a prospective study. Int J Occup Med Environ Health. 2012;25:463-469.
- Atanaskova Mesinkovska N, Tellez A, Molina L, et al. The effect of patch testing on surgical practices and outcomes in orthopedic patients with metal implants. Arch Dermatol. 2012;148:687-693.
- Frigerio E, Pigatto PD, Guzzi G, et al. Metal sensitivity in patients with orthopaedic implants: a prospective study. Contact Dermatitis. 2011;64:273-279.
- Amini M, Mayes WH, Tzeng TH, et al. Evaluation and management of metal hypersensitivity in total joint arthroplasty: a systematic review. J Long Term Eff Med Implants. 2014;24:25-36.
- Thomas P, Bandl WD, Maier S, et al. Hypersensitivity to titanium osteosynthesis with impaired fracture healing, eczema, and T-cell hyperresponsiveness in vitro: case report and review of the literature. Contact Dermatitis. 2006;55:199-202.
- Wood MM, Warshaw EM. Hypersensitivity reactions to titanium: diagnosis and management. Dermatitis. 2015;26:7-25.
- Cadosch D, Chan E, Gautschi OP, et al. Metal is not inert: role of metal ions released by biocorrosion in aseptic loosening—current concepts. J Biomed Mater Res A. 2009;91:1252-1262.
- Granchi D, Cenni E, Trisolino G, et al. Sensitivity to implant materials in patients undergoing total hip replacement. J Biomed Mater Res B Appl Biomater. 2006;77:257-264.
- Granchi D, Cenni E, Tigani D, et al. Sensitivity to implant materials in patients with total knee arthroplasties. Biomaterials. 2008;29:1494-1500.
- Thyssen JP, Menné T, Schalock PC, et al. Pragmatic approach to the clinical work-up of patients with putative allergic disease to metallic orthopaedic implants before and after surgery. Br J Dermatol. 2011;164:473-478.
- Kręcisz B, Kieć-Świerczyńska M, Bąkowicz-Mitura K. Allergy to metals as a cause of orthopedic implant failure. Int J Occup Med Environ Health. 2006;19:178-180.
- Costigan M, Cary R, Dobson S. Vanadium Pentoxide and Other Inorganic Vanadium Compounds. Geneva, Switzerland: World Health Organization; 2001.
- Challa VS, Mali S, Misra RD. Reduced toxicity and superior cellular response of preosteoblasts to Ti-6Al-7Nb alloy and comparison with Ti-6Al-4V. J Biomed Mater Res A. 2013;101:2083-2089.
- Okazaki Y, Rao S, Ito Y, et al. Corrosion resistance, mechanical properties, corrosion fatigue strength and cytocompatibility of new Ti alloys without Al and V. Biomaterials. 1998;19:1197-1215.
- Paszenda Z, Walke W, Jadacka S. Electrochemical investigations of Ti6Al4V and Ti6Al7Nb alloys used on implants in bone surgery. J Achievements Materials Manufacturing Eng. 2010;38:24-32.
- Wang K. The use of titanium for medical applications in the USA. Materials Sci Eng A. 1996:134-137.
- Haseeb M, Butt MF, Altaf T, et al. Indications of implant removal: a study of 83 cases. Int J Health Sci (Qassim). 2017;11:1-7.
- Geetha M, Singh AK, Asokamani R, et al. Ti based biomaterials, the ultimate choice for orthopaedic implants—a review. Progress Materials Sci. 2009;54:397-425.
Metal allergy in patients with orthopedic implants can cause serious problems including dermatitis and implant failure.1 As life expectancy increases, the general population ages, and more metallic orthopedic implants are placed,2 allergy to these implants is expected to be a problem of greater significance. Uncertainty remains regarding best practice for patients with suspected metal implant allergy.1 The major questions are: Who should be tested? When should they be tested? What are the optimal tests to diagnose metal allergy?3-8
We report the case of a patient with vanadium allergy who developed a diffuse eczematous dermatitis and implant failure after receiving a vanadium-containing titanium alloy orthopedic implant in the left foot. This case is remarkable because hypersensitivity reactions to titanium-based hardware are rare, as they traditionally have not been thought to provoke allergic reactions.9
Case Report
A 62-year-old woman who was otherwise healthy presented with an eruption of more than 80 pruritic, nummular, eczematous plaques on the arms, legs, back, and buttocks of 3 weeks’ duration (Figure 1). She had a history of allergy to metal used in costume jewelry. Six weeks prior, the patient underwent implantation of a titanium alloy plate in the left foot for surgical repair of painful deforming osteoarthritis. A radiograph of the foot showed appropriate placement. According to the manufacturer, the plate was composed of the compound Ti6Al4V, which contained 90% titanium, 6% aluminum, and 4% vanadium. The lesions developed on the skin close to but not directly over the surgical site.

A punch biopsy of one of the lesions on the shoulder showed lymphoeosinophilic spongiosis consistent with a delayed hypersensitivity reaction (Figure 2). There was mild clinical improvement of the eruption with topical steroids. A course of prednisone for systemic effect resulted in clearing of the eruption, but it promptly recurred on cessation of the steroids. The patient was then patch tested using the North American 80 Comprehensive Series, with an additional 59 common textile, shampoo, fragrance, and several metal allergens, all of which were negative.

The patient had persistent pain and swelling at the surgical site, and radiographs taken postoperatively at 6 months showed implant failure (Figure 3). The hardware was surgically removed 8 months after implantation (Figure 4) and the plate and screws were submitted to the Institute for Mineral Resources Geosciences LA-ICP-MS Facility and the Lunar and Planetary Laboratory at the University of Arizona (Tucson, Arizona) for analysis. The skin lesions began to improve days after the hardware was removed and the eruption cleared over the following 3 weeks with no additional treatment.


After the hardware was removed, it was analyzed to determine the elemental composition of the plate and screws, and the patient was then patch tested with the major metal components of the implant: aluminum chloride hexahydrate 2.0% pet, elemental titanium 10.0% pet, titanium dioxide 10.0% pet, titanium (III) nitride 5.0% pet, titanium (III) oxalate decahydrate 5.0% pet, elemental vanadium 5.0% pet, and vanadium (III) chloride 1.0% pet. She demonstrated a 1+ reaction (erythema and induration) to vanadium trichloride at 72 and 96 hours.
The plate and screws removed from the patient were sterilized and submitted for analysis. Electron microprobe analysis confirmed that the major elemental composition of the plate and screws essentially matched the manufacturer’s listing (Table 1). The trace elements were determined using laser ablative inductively coupled mass spectroscopy, which demonstrated that the screws were of different metal composition from the plate (Table 2). Electron microprobe analysis also was used to determine the microstructure of the plate and screws. The plate had 2 distinct phases consisting of a titanium-aluminum phase and a vanadium phase, whereas the screw was much more homogeneous. Basic electrochemical studies were performed in a salt solution replicating the tissue of the foot. These studies showed that galvanic corrosion could have occurred between the plate and screws due to the differences of composition.


Comment
Titanium is an attractive metal to use in orthopedic implants. It has a high strength-to-weight ratio, a low modulus of elasticity, and good resistance to corrosion. Titanium can be categorized as either commercially pure titanium (cp-Ti) or a titanium alloy. Colloquially, both cp-Ti and titanium alloys are often referred to simply as titanium, but the distinction is important when it comes to medical implants and devices. Commercially pure titanium is more than 99% pure titanium, but up to 1% of its volume can be comprised of impurities.10 In titanium alloys, the alloy elements are intentionally added to create a material with optimal properties. The 2 most common types of titanium that are used for orthopedic implants are cp-Ti and Ti6Al4V, a titanium alloy containing approximately 90% titanium, 6% aluminum, and 4% vanadium. Similar to cp-Ti, titanium alloys also can contain impurities such as aluminum, beryllium, cobalt, chromium, iron, nickel, and palladium, among many others. Although these impurities often are considered negligible from a metallurgy perspective, as they do not change the properties of the material, these trace elements may be present in large enough quantities to cause hypersensitivity reactions.11
Several weeks after implantation of a titanium alloy metal plate in the left foot, a widespread eczematous eruption developed in our patient who had no prior skin disease. The eruption was steroid responsive but did not clear until the plate was removed. Detailed metallurgy analysis confirmed that vanadium was present and was not homogeneously distributed in the plate. The plate also was different in composition from the screws. Additional studies showed that galvanic corrosion between the plate and the chemically different screws might have contributed to the release of vanadium in the tissue.
Vanadium is known to be allergenic, especially in the presence of implant failure.12,13 In our patient, patch testing with more than 100 allergens was negative, except for vanadium trichloride 1%. Our patient’s presentation strongly suggested that she developed a vanadium allergy manifesting as systemic allergic contact dermatitis. She demonstrated no history of skin disease, a widespread eczematous eruption after exposure, histology consistent with systemic contact allergy, a positive patch test to vanadium, and clearance of the eruption on removal of the antigen, which have been proposed as objective criteria that support a diagnosis of metal implant allergy.14 She refused our suggestion to reimplant a portion of the remaining plate under the skin without screws and monitor for recurrence of the eruption. She did not have a lesion overlying the surgical site, but she did develop lesions near the surgical scar. The literature indicates that cutaneous manifestations of allergy to metallic implants can be both localized and generalized.14
Although reports are rare, other researchers have found vanadium allergy in patients with metal orthopedic implants.5,12,13,15 The scarcity of literature on vanadium allergy seems to suggest that it is a rare entity, but we believe that it may be more common. Vanadium allergy may be underdiagnosed because it is not a standard patch test allergen. Furthermore, many of those who do choose to test for it use what we believe to be ineffective formulas of vanadium when patch testing patients. Our patient demonstrated a positive patch test reaction only to vanadium trichloride and not to pure vanadium, which is consistent with the small number of other studies that investigated vanadium allergy.5,12,13,15 We believe that vanadium trichloride is more water soluble than elemental vanadium,16 and thus more likely to identify true vanadium allergy than other test materials.
Although reports of vanadium allergy in patients with metal implants are rare in the medical literature, the material science literature clearly states that vanadium is toxic and that vanadium-containing implants are problematic.17-20 It has been shown that although Ti6Al4V implants are considered highly resistant to corrosion, they will slowly and continuously corrode in a physiologic environment and release titanium, aluminum, and vanadium ions, both systemically and into the peri-implant space.11 To address these problems with vanadium, vanadium-free titanium alloys such as Ti6Al7Nb have specifically been developed for medical use to address the problems caused by vanadium. Ti6Al7Nb contains 7% niobium rather than vanadium and appears to have some improved qualities in surgical implants.17
There is still a great deal of uncertainty around metal implant allergy. Allergy to metal implants can be difficult to diagnose for several reasons. Some metals are not conducive to patch testing because of their low bioavailability. Additionally, we lack validated and standardized patch test formulas for metals that can be diagnosed by patch testing. Furthermore, there is uncertainty about what to do after allergy to a metal implant is diagnosed; in some cases (eg, with more extensive procedures such as total joint replacements), removal or replacement of the implant may be associated with increased risk of further complications.6,21
Conclusion
We suggest that manufacturers consider vanadium-free alloys such as Ti7Al6Nb, which contains niobium instead of vanadium, in their surgical implants,22 and if surgeons have a choice, they should consider using titanium implants with niobium rather than vanadium.10 We suggest that clinicians consider vanadium allergy in patients with Ti6Al4V surgical implants and signs of a hypersensitivity reaction, and include vanadium trichloride 1% when patch testing.
Acknowledgment
The authors would like to thank Nicholas R. Krasnow, PhD (Tucson, Arizona), for his invaluable help coordinating, performing, and interpreting the metal analyses.
Metal allergy in patients with orthopedic implants can cause serious problems including dermatitis and implant failure.1 As life expectancy increases, the general population ages, and more metallic orthopedic implants are placed,2 allergy to these implants is expected to be a problem of greater significance. Uncertainty remains regarding best practice for patients with suspected metal implant allergy.1 The major questions are: Who should be tested? When should they be tested? What are the optimal tests to diagnose metal allergy?3-8
We report the case of a patient with vanadium allergy who developed a diffuse eczematous dermatitis and implant failure after receiving a vanadium-containing titanium alloy orthopedic implant in the left foot. This case is remarkable because hypersensitivity reactions to titanium-based hardware are rare, as they traditionally have not been thought to provoke allergic reactions.9
Case Report
A 62-year-old woman who was otherwise healthy presented with an eruption of more than 80 pruritic, nummular, eczematous plaques on the arms, legs, back, and buttocks of 3 weeks’ duration (Figure 1). She had a history of allergy to metal used in costume jewelry. Six weeks prior, the patient underwent implantation of a titanium alloy plate in the left foot for surgical repair of painful deforming osteoarthritis. A radiograph of the foot showed appropriate placement. According to the manufacturer, the plate was composed of the compound Ti6Al4V, which contained 90% titanium, 6% aluminum, and 4% vanadium. The lesions developed on the skin close to but not directly over the surgical site.

A punch biopsy of one of the lesions on the shoulder showed lymphoeosinophilic spongiosis consistent with a delayed hypersensitivity reaction (Figure 2). There was mild clinical improvement of the eruption with topical steroids. A course of prednisone for systemic effect resulted in clearing of the eruption, but it promptly recurred on cessation of the steroids. The patient was then patch tested using the North American 80 Comprehensive Series, with an additional 59 common textile, shampoo, fragrance, and several metal allergens, all of which were negative.

The patient had persistent pain and swelling at the surgical site, and radiographs taken postoperatively at 6 months showed implant failure (Figure 3). The hardware was surgically removed 8 months after implantation (Figure 4) and the plate and screws were submitted to the Institute for Mineral Resources Geosciences LA-ICP-MS Facility and the Lunar and Planetary Laboratory at the University of Arizona (Tucson, Arizona) for analysis. The skin lesions began to improve days after the hardware was removed and the eruption cleared over the following 3 weeks with no additional treatment.


After the hardware was removed, it was analyzed to determine the elemental composition of the plate and screws, and the patient was then patch tested with the major metal components of the implant: aluminum chloride hexahydrate 2.0% pet, elemental titanium 10.0% pet, titanium dioxide 10.0% pet, titanium (III) nitride 5.0% pet, titanium (III) oxalate decahydrate 5.0% pet, elemental vanadium 5.0% pet, and vanadium (III) chloride 1.0% pet. She demonstrated a 1+ reaction (erythema and induration) to vanadium trichloride at 72 and 96 hours.
The plate and screws removed from the patient were sterilized and submitted for analysis. Electron microprobe analysis confirmed that the major elemental composition of the plate and screws essentially matched the manufacturer’s listing (Table 1). The trace elements were determined using laser ablative inductively coupled mass spectroscopy, which demonstrated that the screws were of different metal composition from the plate (Table 2). Electron microprobe analysis also was used to determine the microstructure of the plate and screws. The plate had 2 distinct phases consisting of a titanium-aluminum phase and a vanadium phase, whereas the screw was much more homogeneous. Basic electrochemical studies were performed in a salt solution replicating the tissue of the foot. These studies showed that galvanic corrosion could have occurred between the plate and screws due to the differences of composition.


Comment
Titanium is an attractive metal to use in orthopedic implants. It has a high strength-to-weight ratio, a low modulus of elasticity, and good resistance to corrosion. Titanium can be categorized as either commercially pure titanium (cp-Ti) or a titanium alloy. Colloquially, both cp-Ti and titanium alloys are often referred to simply as titanium, but the distinction is important when it comes to medical implants and devices. Commercially pure titanium is more than 99% pure titanium, but up to 1% of its volume can be comprised of impurities.10 In titanium alloys, the alloy elements are intentionally added to create a material with optimal properties. The 2 most common types of titanium that are used for orthopedic implants are cp-Ti and Ti6Al4V, a titanium alloy containing approximately 90% titanium, 6% aluminum, and 4% vanadium. Similar to cp-Ti, titanium alloys also can contain impurities such as aluminum, beryllium, cobalt, chromium, iron, nickel, and palladium, among many others. Although these impurities often are considered negligible from a metallurgy perspective, as they do not change the properties of the material, these trace elements may be present in large enough quantities to cause hypersensitivity reactions.11
Several weeks after implantation of a titanium alloy metal plate in the left foot, a widespread eczematous eruption developed in our patient who had no prior skin disease. The eruption was steroid responsive but did not clear until the plate was removed. Detailed metallurgy analysis confirmed that vanadium was present and was not homogeneously distributed in the plate. The plate also was different in composition from the screws. Additional studies showed that galvanic corrosion between the plate and the chemically different screws might have contributed to the release of vanadium in the tissue.
Vanadium is known to be allergenic, especially in the presence of implant failure.12,13 In our patient, patch testing with more than 100 allergens was negative, except for vanadium trichloride 1%. Our patient’s presentation strongly suggested that she developed a vanadium allergy manifesting as systemic allergic contact dermatitis. She demonstrated no history of skin disease, a widespread eczematous eruption after exposure, histology consistent with systemic contact allergy, a positive patch test to vanadium, and clearance of the eruption on removal of the antigen, which have been proposed as objective criteria that support a diagnosis of metal implant allergy.14 She refused our suggestion to reimplant a portion of the remaining plate under the skin without screws and monitor for recurrence of the eruption. She did not have a lesion overlying the surgical site, but she did develop lesions near the surgical scar. The literature indicates that cutaneous manifestations of allergy to metallic implants can be both localized and generalized.14
Although reports are rare, other researchers have found vanadium allergy in patients with metal orthopedic implants.5,12,13,15 The scarcity of literature on vanadium allergy seems to suggest that it is a rare entity, but we believe that it may be more common. Vanadium allergy may be underdiagnosed because it is not a standard patch test allergen. Furthermore, many of those who do choose to test for it use what we believe to be ineffective formulas of vanadium when patch testing patients. Our patient demonstrated a positive patch test reaction only to vanadium trichloride and not to pure vanadium, which is consistent with the small number of other studies that investigated vanadium allergy.5,12,13,15 We believe that vanadium trichloride is more water soluble than elemental vanadium,16 and thus more likely to identify true vanadium allergy than other test materials.
Although reports of vanadium allergy in patients with metal implants are rare in the medical literature, the material science literature clearly states that vanadium is toxic and that vanadium-containing implants are problematic.17-20 It has been shown that although Ti6Al4V implants are considered highly resistant to corrosion, they will slowly and continuously corrode in a physiologic environment and release titanium, aluminum, and vanadium ions, both systemically and into the peri-implant space.11 To address these problems with vanadium, vanadium-free titanium alloys such as Ti6Al7Nb have specifically been developed for medical use to address the problems caused by vanadium. Ti6Al7Nb contains 7% niobium rather than vanadium and appears to have some improved qualities in surgical implants.17
There is still a great deal of uncertainty around metal implant allergy. Allergy to metal implants can be difficult to diagnose for several reasons. Some metals are not conducive to patch testing because of their low bioavailability. Additionally, we lack validated and standardized patch test formulas for metals that can be diagnosed by patch testing. Furthermore, there is uncertainty about what to do after allergy to a metal implant is diagnosed; in some cases (eg, with more extensive procedures such as total joint replacements), removal or replacement of the implant may be associated with increased risk of further complications.6,21
Conclusion
We suggest that manufacturers consider vanadium-free alloys such as Ti7Al6Nb, which contains niobium instead of vanadium, in their surgical implants,22 and if surgeons have a choice, they should consider using titanium implants with niobium rather than vanadium.10 We suggest that clinicians consider vanadium allergy in patients with Ti6Al4V surgical implants and signs of a hypersensitivity reaction, and include vanadium trichloride 1% when patch testing.
Acknowledgment
The authors would like to thank Nicholas R. Krasnow, PhD (Tucson, Arizona), for his invaluable help coordinating, performing, and interpreting the metal analyses.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785.
- Thyssen JP, Johansen JD, Menné T, et al. Hypersensitivity reactions from metallic implants: a future challenge that needs to be addressed. Br J Dermatol. 2010;162:235-236.
- Aquino M, Mucci T. Systemic contact dermatitis and allergy to biomedical devices. Curr Allergy Asthma Rep. 2013;13:518-527.
- Krecisz B, Kiec-Swierczynska M, Chomiczewska-Skora D. Allergy to orthopedic metal implants—a prospective study. Int J Occup Med Environ Health. 2012;25:463-469.
- Atanaskova Mesinkovska N, Tellez A, Molina L, et al. The effect of patch testing on surgical practices and outcomes in orthopedic patients with metal implants. Arch Dermatol. 2012;148:687-693.
- Frigerio E, Pigatto PD, Guzzi G, et al. Metal sensitivity in patients with orthopaedic implants: a prospective study. Contact Dermatitis. 2011;64:273-279.
- Amini M, Mayes WH, Tzeng TH, et al. Evaluation and management of metal hypersensitivity in total joint arthroplasty: a systematic review. J Long Term Eff Med Implants. 2014;24:25-36.
- Thomas P, Bandl WD, Maier S, et al. Hypersensitivity to titanium osteosynthesis with impaired fracture healing, eczema, and T-cell hyperresponsiveness in vitro: case report and review of the literature. Contact Dermatitis. 2006;55:199-202.
- Wood MM, Warshaw EM. Hypersensitivity reactions to titanium: diagnosis and management. Dermatitis. 2015;26:7-25.
- Cadosch D, Chan E, Gautschi OP, et al. Metal is not inert: role of metal ions released by biocorrosion in aseptic loosening—current concepts. J Biomed Mater Res A. 2009;91:1252-1262.
- Granchi D, Cenni E, Trisolino G, et al. Sensitivity to implant materials in patients undergoing total hip replacement. J Biomed Mater Res B Appl Biomater. 2006;77:257-264.
- Granchi D, Cenni E, Tigani D, et al. Sensitivity to implant materials in patients with total knee arthroplasties. Biomaterials. 2008;29:1494-1500.
- Thyssen JP, Menné T, Schalock PC, et al. Pragmatic approach to the clinical work-up of patients with putative allergic disease to metallic orthopaedic implants before and after surgery. Br J Dermatol. 2011;164:473-478.
- Kręcisz B, Kieć-Świerczyńska M, Bąkowicz-Mitura K. Allergy to metals as a cause of orthopedic implant failure. Int J Occup Med Environ Health. 2006;19:178-180.
- Costigan M, Cary R, Dobson S. Vanadium Pentoxide and Other Inorganic Vanadium Compounds. Geneva, Switzerland: World Health Organization; 2001.
- Challa VS, Mali S, Misra RD. Reduced toxicity and superior cellular response of preosteoblasts to Ti-6Al-7Nb alloy and comparison with Ti-6Al-4V. J Biomed Mater Res A. 2013;101:2083-2089.
- Okazaki Y, Rao S, Ito Y, et al. Corrosion resistance, mechanical properties, corrosion fatigue strength and cytocompatibility of new Ti alloys without Al and V. Biomaterials. 1998;19:1197-1215.
- Paszenda Z, Walke W, Jadacka S. Electrochemical investigations of Ti6Al4V and Ti6Al7Nb alloys used on implants in bone surgery. J Achievements Materials Manufacturing Eng. 2010;38:24-32.
- Wang K. The use of titanium for medical applications in the USA. Materials Sci Eng A. 1996:134-137.
- Haseeb M, Butt MF, Altaf T, et al. Indications of implant removal: a study of 83 cases. Int J Health Sci (Qassim). 2017;11:1-7.
- Geetha M, Singh AK, Asokamani R, et al. Ti based biomaterials, the ultimate choice for orthopaedic implants—a review. Progress Materials Sci. 2009;54:397-425.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785.
- Thyssen JP, Johansen JD, Menné T, et al. Hypersensitivity reactions from metallic implants: a future challenge that needs to be addressed. Br J Dermatol. 2010;162:235-236.
- Aquino M, Mucci T. Systemic contact dermatitis and allergy to biomedical devices. Curr Allergy Asthma Rep. 2013;13:518-527.
- Krecisz B, Kiec-Swierczynska M, Chomiczewska-Skora D. Allergy to orthopedic metal implants—a prospective study. Int J Occup Med Environ Health. 2012;25:463-469.
- Atanaskova Mesinkovska N, Tellez A, Molina L, et al. The effect of patch testing on surgical practices and outcomes in orthopedic patients with metal implants. Arch Dermatol. 2012;148:687-693.
- Frigerio E, Pigatto PD, Guzzi G, et al. Metal sensitivity in patients with orthopaedic implants: a prospective study. Contact Dermatitis. 2011;64:273-279.
- Amini M, Mayes WH, Tzeng TH, et al. Evaluation and management of metal hypersensitivity in total joint arthroplasty: a systematic review. J Long Term Eff Med Implants. 2014;24:25-36.
- Thomas P, Bandl WD, Maier S, et al. Hypersensitivity to titanium osteosynthesis with impaired fracture healing, eczema, and T-cell hyperresponsiveness in vitro: case report and review of the literature. Contact Dermatitis. 2006;55:199-202.
- Wood MM, Warshaw EM. Hypersensitivity reactions to titanium: diagnosis and management. Dermatitis. 2015;26:7-25.
- Cadosch D, Chan E, Gautschi OP, et al. Metal is not inert: role of metal ions released by biocorrosion in aseptic loosening—current concepts. J Biomed Mater Res A. 2009;91:1252-1262.
- Granchi D, Cenni E, Trisolino G, et al. Sensitivity to implant materials in patients undergoing total hip replacement. J Biomed Mater Res B Appl Biomater. 2006;77:257-264.
- Granchi D, Cenni E, Tigani D, et al. Sensitivity to implant materials in patients with total knee arthroplasties. Biomaterials. 2008;29:1494-1500.
- Thyssen JP, Menné T, Schalock PC, et al. Pragmatic approach to the clinical work-up of patients with putative allergic disease to metallic orthopaedic implants before and after surgery. Br J Dermatol. 2011;164:473-478.
- Kręcisz B, Kieć-Świerczyńska M, Bąkowicz-Mitura K. Allergy to metals as a cause of orthopedic implant failure. Int J Occup Med Environ Health. 2006;19:178-180.
- Costigan M, Cary R, Dobson S. Vanadium Pentoxide and Other Inorganic Vanadium Compounds. Geneva, Switzerland: World Health Organization; 2001.
- Challa VS, Mali S, Misra RD. Reduced toxicity and superior cellular response of preosteoblasts to Ti-6Al-7Nb alloy and comparison with Ti-6Al-4V. J Biomed Mater Res A. 2013;101:2083-2089.
- Okazaki Y, Rao S, Ito Y, et al. Corrosion resistance, mechanical properties, corrosion fatigue strength and cytocompatibility of new Ti alloys without Al and V. Biomaterials. 1998;19:1197-1215.
- Paszenda Z, Walke W, Jadacka S. Electrochemical investigations of Ti6Al4V and Ti6Al7Nb alloys used on implants in bone surgery. J Achievements Materials Manufacturing Eng. 2010;38:24-32.
- Wang K. The use of titanium for medical applications in the USA. Materials Sci Eng A. 1996:134-137.
- Haseeb M, Butt MF, Altaf T, et al. Indications of implant removal: a study of 83 cases. Int J Health Sci (Qassim). 2017;11:1-7.
- Geetha M, Singh AK, Asokamani R, et al. Ti based biomaterials, the ultimate choice for orthopaedic implants—a review. Progress Materials Sci. 2009;54:397-425.
Practice Points
- Vanadium may be an underrecognized allergen in patients with metal implants.
- Consider vanadium allergy in those with surgical implants and signs of hypersensitivity reaction.
- Test for allergy with vanadium trichloride.
- Niobium is an alternative for implants in vanadium-allergic patients.