User login
Orbital Granuloma Formation Following Autoinjection of Paraffin Oil: Management Considerations
To the Editor:
Injectable fillers are an increasingly common means of achieving minimally invasive facial rejuvenation. In the hands of well-trained practitioners, these compounds typically are well tolerated, effective, and have a strong safety profile1; however, there have been reports of complications, including vision loss,2 orbital infarction,3 persistent inflammatory nodules,4 and infection.4,5 Paraffin, a derivative of mineral oil, currently is used in cosmetic products and medical ointments.6 In the early 1900s, it often was injected into the body for various medical procedures, such as to create prosthetic testicles, to treat bladder incontinence, and eventually to correct facial contour defects.7,8 Due to adverse effects, injection of paraffin oil was discontinued in the Western medical community around the time of World War I.7 Unfortunately, some patients continue to self-inject paraffin oil for cosmetic purposes today. We present a case of foreign-body granuloma formation mimicking periorbital cellulitis following self-injection of paraffin oil. Our patient developed serious periorbital sequelae that required surgical intervention to restore normal anatomic function.
A 60-year-old woman who was otherwise healthy presented to the emergency department with facial swelling and a rash of 2 weeks’ duration. She reported that she had purchased what she believed was a cosmetic product at a local flea market 2 weeks prior to presentation. Her purchase included needles and a syringe with verbal instructions for injection into the face. She was told the product was used to treat wrinkles and referred to the injectable material as “oil” when providing her history. She reported that she had injected the material into the bilateral lower eyelids, left lateral lip, and left lateral chin. Three days later, she developed tingling and itching with swelling and redness at the injection sites. The patient was evaluated by the emergency department team and was prescribed a 10-day course of clindamycin empirically for suspected facial cellulitis.
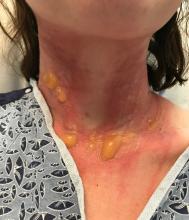
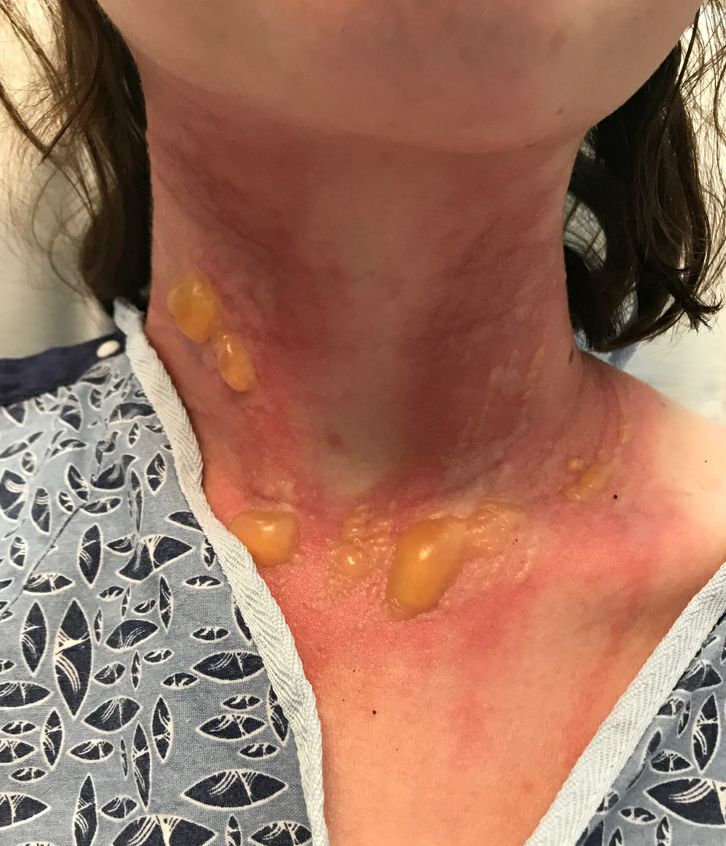
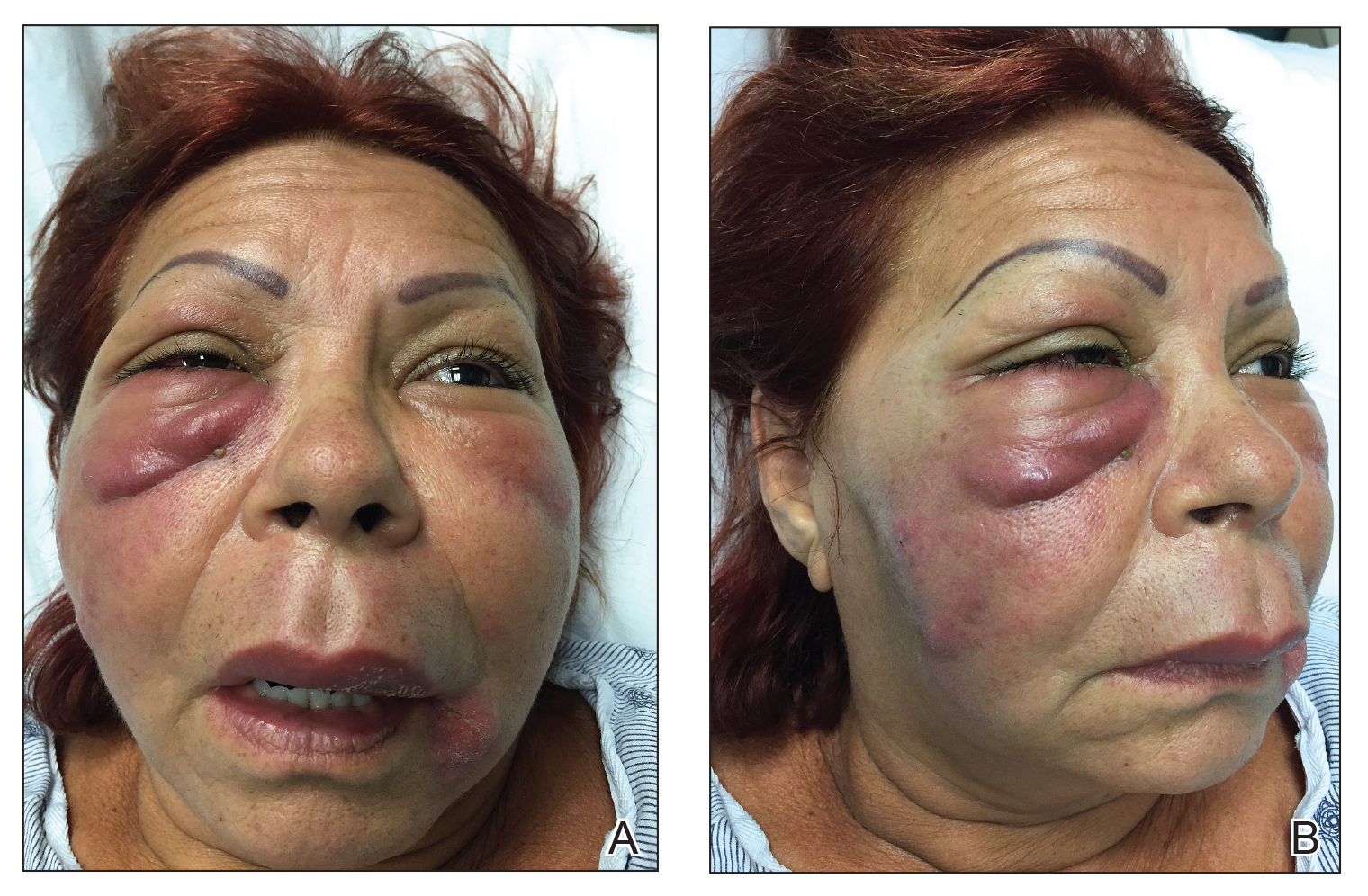
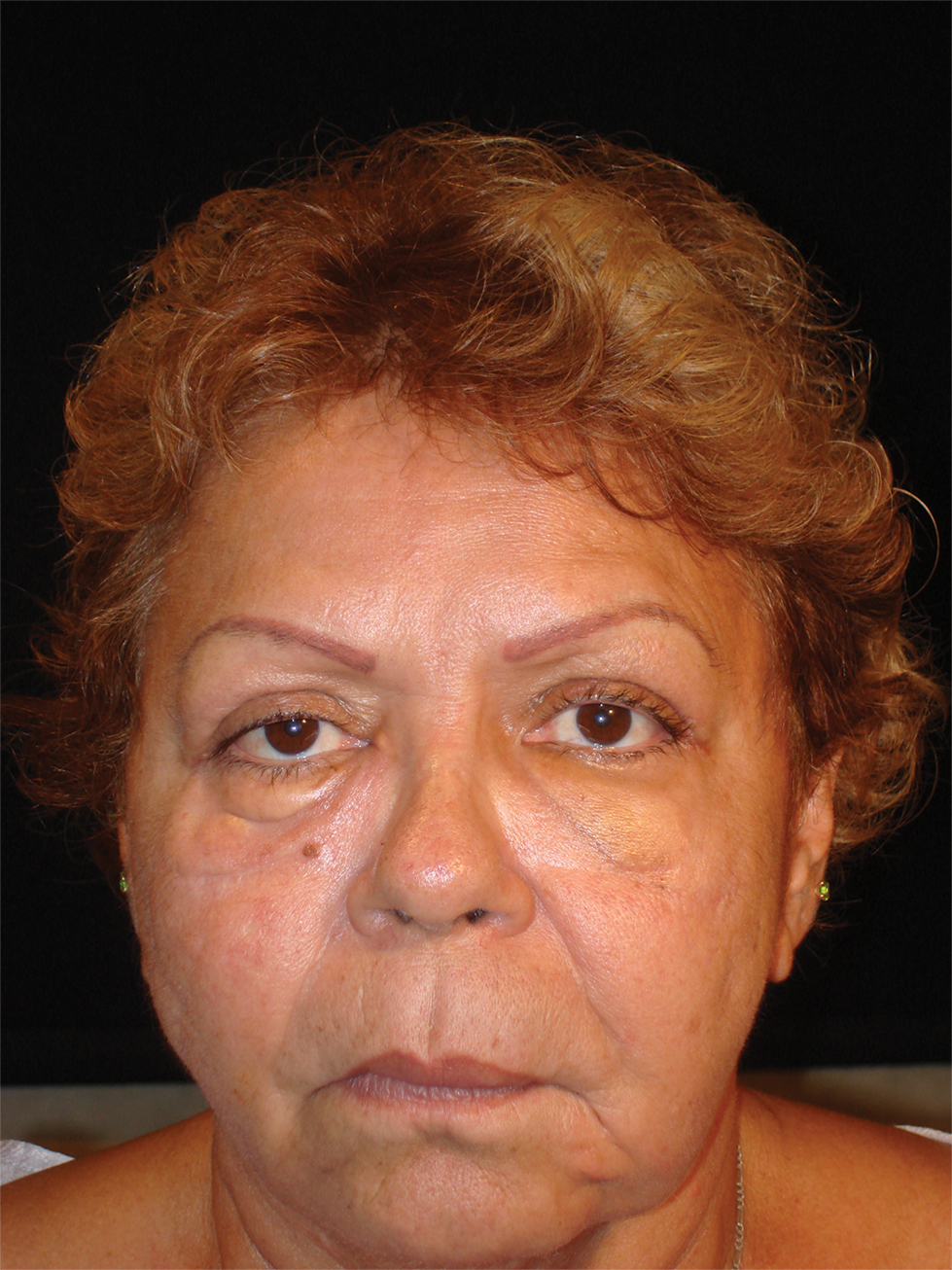
The patient returned to the emergency department 12 days later upon completion of the antibiotic course with worsening edema and erythema. Examination revealed indurated, erythematous, and edematous warm plaques on the face that were concentrated around the prior injection sites with substantial periorbital erythema and edema (Figure 1). A consultation with oculoplastic surgery was obtained. Mechanical ptosis of the right eyelid was noted. Visual acuity was 20/30 in both eyes with habitual correction. Intraocular pressure was soft to palpation, and the pupils were round and reactive with no evidence of a relative afferent pupillary defect. Extraocular motility was intact bilaterally. Examination of the conjunctiva and sclera revealed bilateral conjunctival injection with chemosis of the right eye. The remainder of the anterior and posterior segment examination was within normal limits bilaterally.
Computed tomography of the face showed extensive facial and periorbital swelling without abscess. A dermatology consultation was obtained. Two 4-mm punch biopsies were obtained from the left lower face and were sent for hematoxylin and eosin stain and tissue culture (bacterial, fungal, and acid-fast bacillus). Given the possibility of facial and periorbital cellulitis, empiric intravenous antibiotic therapy was initiated.
The tissue culture revealed normal skin flora. The biopsy results indicated a foreign-body reaction consistent with paraffin granuloma (Figures 2 and 3). Fite-Faraco, Grocott-Gomori methenamine-silver, and periodic acid–Schiff stains were all negative for infection. A diagnosis of foreign-body granuloma was established. Oral minocycline at a dosage of 100 mg twice daily was started, and the patient was discharged.


After 4 weeks of minocycline therapy, the patient showed no improvement and returned to the emergency department with worsening symptoms. She was readmitted and started on intravenous prednisone (1.5 mg/kg/d). Over the ensuing 5 days, the edema, erythema, conjunctival injection, and chemosis demonstrated notable improvement. She was subsequently discharged on an oral prednisone taper. Unfortunately, she did not respond to a trial of intralesional steroid injections to an area of granuloma formation on the left chin performed in the hospital before she was discharged.
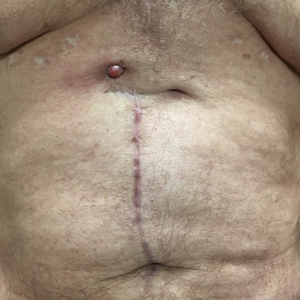
In the ensuing months, she began to develop cicatricial ectropion of the right lower eyelid and mechanical ptosis of the right upper eyelid. Ten months after initial self-injection, staged surgical excision was initiated by an oculoplastic surgeon (I.V.) with the goal of debulking the periorbital region to correct the ectropion and mechanical ptosis. A transconjunctival approach was used to carefully excise the material while still maintaining the architecture of the lower eyelid. The ectropion was surgically corrected concurrently.
One month after excision, serial injections of 5-fluorouracil (5-FU) and triamcinolone acetonide 40 mg/mL were administered to the right lower eyelid and anterior orbit for 3 months. Fifteen weeks after the first surgery, a second surgery was performed to address residual medial right lower eyelid induration, right upper eyelid mechanical ptosis, and left orbital inflammation. During the postoperative period, serial monthly injections of 5-FU and triamcinolone acetonide were again performed beginning at the first postoperative month.
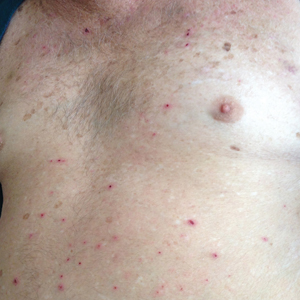
The surgical excisions resulted in notable improvement 3 months following excision (Figure 4). The patient noted improved ocular surface comfort with decreased foreign-body sensation and tearing. She also was pleased with the improved cosmetic outcome.
Crude substances such as paraffin, petroleum jelly, and lanolin were used for aesthetic purposes in the late 19th and early 20th centuries, initially with satisfying results; however, long-term adverse effects such as hardening of the skin, swelling, granuloma formation, ulceration, infections, and abscesses have discouraged its use by medical professionals today.5 Since paraffin is resistant to degradation and absorption, foreign-body reactions may occur upon injection. These reactions are characterized by replacement of normal subcutaneous tissue by cystic spaces of paraffin oil and/or calcification, similar to the appearance of Swiss cheese on histology and surrounded by various inflammatory cells and fibrous tissue.9,10
Clinically, there is an acute inflammatory phase followed by a latent phase of chronic granulomatous inflammation that can last for years.10 Our patient presented during the acute phase, with erythematous and edematous warm plaques around the eye mimicking an orbital infection.
The treatment of choice for paraffin granuloma is complete surgical excision to prevent recurrence.6,9 However, intralesional corticosteroids are preferred in the facial area, especially if complete removal is not possible.10 Intralesional corticosteroid injections inhibit fibroblast and macrophage activity as well as the deposition of collagen, leading to reduced pain and swelling in most cases.11 Additionally, combining antimitotic agents such as 5-FU with a corticosteroid might reduce the risk for cortisone skin atrophy.12 In our case, the patient did not respond to combined 5-FU with intralesional steroids and required oral corticosteroids while awaiting serial excisions.
Our case highlights several important points in the management of paraffin granuloma. First, the clinician must perform a thorough patient history, as surreptitious use of non–medical-grade fillers is more common than one might think.13 Second, the initial presentation of these patients can mimic an infectious process. Careful history, testing, and observation can aid in making the appropriate diagnosis. Finally, treatment of these patients is complex. The mainstays of therapy are systemic anti-inflammatory medications, time, and supportive care. In some cases, surgery may be required. When processes such as paraffin granulomas involve the periorbital region, particular care is required to avoid cicatricial lagophthalmos, ectropion, or retraction. Thoughtful surgical manipulation is required to avoid these complications, which indeed may occur even with the most appropriate interventions.
- Duker D, Erdmann R, Hartmann V, et al. The impact of adverse reactions to injectable filler substances on quality of life: results from the Berlin Injectable Filler Safety (IFS)—study. J Eur Acad Dermatol Venereol. 2016;30:1013-1020.
- Prado G, Rodriguez-Feliz J. Ocular pain and impending blindness during facial cosmetic injections: is your office prepared? [published online December 28, 2016]. Aesthetic Plast Surg. 2017;41:199-203.
- Roberts SA, Arthurs BP. Severe visual loss and orbital infarction following periorbital aesthetic poly-(L)-lactic acid (PLLA) injection. Ophthalmic Plast Reconstr Surg. 2012;28:E68-E70.
- Cassuto D, Pignatti M, Pacchioni L, et al. Management of complications caused by permanent fillers in the face: a treatment algorithm. Plast Reconstr Surg. 2016;138:215E-227E.
- Haneke E. Adverse effects of fillers and their histopathology. Facial Plast Surg. 2014;30:599-614.
- Friedrich RE, Zustin J. Paraffinoma of lips and oral mucosa: case report and brief review of literature. GMS Interdiscip Plast Reconstr Surg DGPW. 2014;3:Doc05.
- Matton G, Anseeuw A, De Keyser F. The history of injectable biomaterials and the biology of collagen. Aesthetic Plast Surg. 1985;9:133-140.
- Glicenstein J. Les premiers fillers, Vaseline et paraffine. du miracle a la catastrope. Ann Chir Plast Esthet. 2007;52:157-161.
- Cohen JL, Keoleian CM, Krull EA. Penile paraffinoma: self-injection with mineral oil. J Am Acad Dermatol 2002;47:S251-S253.
- Legaspi-Vicerra ME, Field LM. Paraffin granulomata, “witch’s chin,” and nasal deformities excision and reconstruction with reduction chinplasty and open rhinotomy resection. J Clin Aesthet Dermatol 2010;3:54-58.
- Carlos-Fabuel L, Marzal-Gamarra C, Marti-Alamo S, et al. Foreign body granulomatous reactions to cosmetic fillers. J Clin Exp Dent. 2012;4:E244-E247.
- Lemperle G, Gauthier-Hazan N. Foreign body granulomas after all injectable dermal fillers: part 2. treatment options. Plast Reconstr Surg. 2009;123:1864-1873.
- Seok J, Hong JY, Park KY, et al. Delayed immunologic complications due to injectable fillers by unlicensed practitioners: our experiences and a review of the literature. Dermatol Ther. 2016;29:41-44.
To the Editor:
Injectable fillers are an increasingly common means of achieving minimally invasive facial rejuvenation. In the hands of well-trained practitioners, these compounds typically are well tolerated, effective, and have a strong safety profile1; however, there have been reports of complications, including vision loss,2 orbital infarction,3 persistent inflammatory nodules,4 and infection.4,5 Paraffin, a derivative of mineral oil, currently is used in cosmetic products and medical ointments.6 In the early 1900s, it often was injected into the body for various medical procedures, such as to create prosthetic testicles, to treat bladder incontinence, and eventually to correct facial contour defects.7,8 Due to adverse effects, injection of paraffin oil was discontinued in the Western medical community around the time of World War I.7 Unfortunately, some patients continue to self-inject paraffin oil for cosmetic purposes today. We present a case of foreign-body granuloma formation mimicking periorbital cellulitis following self-injection of paraffin oil. Our patient developed serious periorbital sequelae that required surgical intervention to restore normal anatomic function.
A 60-year-old woman who was otherwise healthy presented to the emergency department with facial swelling and a rash of 2 weeks’ duration. She reported that she had purchased what she believed was a cosmetic product at a local flea market 2 weeks prior to presentation. Her purchase included needles and a syringe with verbal instructions for injection into the face. She was told the product was used to treat wrinkles and referred to the injectable material as “oil” when providing her history. She reported that she had injected the material into the bilateral lower eyelids, left lateral lip, and left lateral chin. Three days later, she developed tingling and itching with swelling and redness at the injection sites. The patient was evaluated by the emergency department team and was prescribed a 10-day course of clindamycin empirically for suspected facial cellulitis.
The patient returned to the emergency department 12 days later upon completion of the antibiotic course with worsening edema and erythema. Examination revealed indurated, erythematous, and edematous warm plaques on the face that were concentrated around the prior injection sites with substantial periorbital erythema and edema (Figure 1). A consultation with oculoplastic surgery was obtained. Mechanical ptosis of the right eyelid was noted. Visual acuity was 20/30 in both eyes with habitual correction. Intraocular pressure was soft to palpation, and the pupils were round and reactive with no evidence of a relative afferent pupillary defect. Extraocular motility was intact bilaterally. Examination of the conjunctiva and sclera revealed bilateral conjunctival injection with chemosis of the right eye. The remainder of the anterior and posterior segment examination was within normal limits bilaterally.

Computed tomography of the face showed extensive facial and periorbital swelling without abscess. A dermatology consultation was obtained. Two 4-mm punch biopsies were obtained from the left lower face and were sent for hematoxylin and eosin stain and tissue culture (bacterial, fungal, and acid-fast bacillus). Given the possibility of facial and periorbital cellulitis, empiric intravenous antibiotic therapy was initiated.
The tissue culture revealed normal skin flora. The biopsy results indicated a foreign-body reaction consistent with paraffin granuloma (Figures 2 and 3). Fite-Faraco, Grocott-Gomori methenamine-silver, and periodic acid–Schiff stains were all negative for infection. A diagnosis of foreign-body granuloma was established. Oral minocycline at a dosage of 100 mg twice daily was started, and the patient was discharged.


After 4 weeks of minocycline therapy, the patient showed no improvement and returned to the emergency department with worsening symptoms. She was readmitted and started on intravenous prednisone (1.5 mg/kg/d). Over the ensuing 5 days, the edema, erythema, conjunctival injection, and chemosis demonstrated notable improvement. She was subsequently discharged on an oral prednisone taper. Unfortunately, she did not respond to a trial of intralesional steroid injections to an area of granuloma formation on the left chin performed in the hospital before she was discharged.
In the ensuing months, she began to develop cicatricial ectropion of the right lower eyelid and mechanical ptosis of the right upper eyelid. Ten months after initial self-injection, staged surgical excision was initiated by an oculoplastic surgeon (I.V.) with the goal of debulking the periorbital region to correct the ectropion and mechanical ptosis. A transconjunctival approach was used to carefully excise the material while still maintaining the architecture of the lower eyelid. The ectropion was surgically corrected concurrently.
One month after excision, serial injections of 5-fluorouracil (5-FU) and triamcinolone acetonide 40 mg/mL were administered to the right lower eyelid and anterior orbit for 3 months. Fifteen weeks after the first surgery, a second surgery was performed to address residual medial right lower eyelid induration, right upper eyelid mechanical ptosis, and left orbital inflammation. During the postoperative period, serial monthly injections of 5-FU and triamcinolone acetonide were again performed beginning at the first postoperative month.
The surgical excisions resulted in notable improvement 3 months following excision (Figure 4). The patient noted improved ocular surface comfort with decreased foreign-body sensation and tearing. She also was pleased with the improved cosmetic outcome.

Crude substances such as paraffin, petroleum jelly, and lanolin were used for aesthetic purposes in the late 19th and early 20th centuries, initially with satisfying results; however, long-term adverse effects such as hardening of the skin, swelling, granuloma formation, ulceration, infections, and abscesses have discouraged its use by medical professionals today.5 Since paraffin is resistant to degradation and absorption, foreign-body reactions may occur upon injection. These reactions are characterized by replacement of normal subcutaneous tissue by cystic spaces of paraffin oil and/or calcification, similar to the appearance of Swiss cheese on histology and surrounded by various inflammatory cells and fibrous tissue.9,10
Clinically, there is an acute inflammatory phase followed by a latent phase of chronic granulomatous inflammation that can last for years.10 Our patient presented during the acute phase, with erythematous and edematous warm plaques around the eye mimicking an orbital infection.
The treatment of choice for paraffin granuloma is complete surgical excision to prevent recurrence.6,9 However, intralesional corticosteroids are preferred in the facial area, especially if complete removal is not possible.10 Intralesional corticosteroid injections inhibit fibroblast and macrophage activity as well as the deposition of collagen, leading to reduced pain and swelling in most cases.11 Additionally, combining antimitotic agents such as 5-FU with a corticosteroid might reduce the risk for cortisone skin atrophy.12 In our case, the patient did not respond to combined 5-FU with intralesional steroids and required oral corticosteroids while awaiting serial excisions.
Our case highlights several important points in the management of paraffin granuloma. First, the clinician must perform a thorough patient history, as surreptitious use of non–medical-grade fillers is more common than one might think.13 Second, the initial presentation of these patients can mimic an infectious process. Careful history, testing, and observation can aid in making the appropriate diagnosis. Finally, treatment of these patients is complex. The mainstays of therapy are systemic anti-inflammatory medications, time, and supportive care. In some cases, surgery may be required. When processes such as paraffin granulomas involve the periorbital region, particular care is required to avoid cicatricial lagophthalmos, ectropion, or retraction. Thoughtful surgical manipulation is required to avoid these complications, which indeed may occur even with the most appropriate interventions.
To the Editor:
Injectable fillers are an increasingly common means of achieving minimally invasive facial rejuvenation. In the hands of well-trained practitioners, these compounds typically are well tolerated, effective, and have a strong safety profile1; however, there have been reports of complications, including vision loss,2 orbital infarction,3 persistent inflammatory nodules,4 and infection.4,5 Paraffin, a derivative of mineral oil, currently is used in cosmetic products and medical ointments.6 In the early 1900s, it often was injected into the body for various medical procedures, such as to create prosthetic testicles, to treat bladder incontinence, and eventually to correct facial contour defects.7,8 Due to adverse effects, injection of paraffin oil was discontinued in the Western medical community around the time of World War I.7 Unfortunately, some patients continue to self-inject paraffin oil for cosmetic purposes today. We present a case of foreign-body granuloma formation mimicking periorbital cellulitis following self-injection of paraffin oil. Our patient developed serious periorbital sequelae that required surgical intervention to restore normal anatomic function.
A 60-year-old woman who was otherwise healthy presented to the emergency department with facial swelling and a rash of 2 weeks’ duration. She reported that she had purchased what she believed was a cosmetic product at a local flea market 2 weeks prior to presentation. Her purchase included needles and a syringe with verbal instructions for injection into the face. She was told the product was used to treat wrinkles and referred to the injectable material as “oil” when providing her history. She reported that she had injected the material into the bilateral lower eyelids, left lateral lip, and left lateral chin. Three days later, she developed tingling and itching with swelling and redness at the injection sites. The patient was evaluated by the emergency department team and was prescribed a 10-day course of clindamycin empirically for suspected facial cellulitis.
The patient returned to the emergency department 12 days later upon completion of the antibiotic course with worsening edema and erythema. Examination revealed indurated, erythematous, and edematous warm plaques on the face that were concentrated around the prior injection sites with substantial periorbital erythema and edema (Figure 1). A consultation with oculoplastic surgery was obtained. Mechanical ptosis of the right eyelid was noted. Visual acuity was 20/30 in both eyes with habitual correction. Intraocular pressure was soft to palpation, and the pupils were round and reactive with no evidence of a relative afferent pupillary defect. Extraocular motility was intact bilaterally. Examination of the conjunctiva and sclera revealed bilateral conjunctival injection with chemosis of the right eye. The remainder of the anterior and posterior segment examination was within normal limits bilaterally.

Computed tomography of the face showed extensive facial and periorbital swelling without abscess. A dermatology consultation was obtained. Two 4-mm punch biopsies were obtained from the left lower face and were sent for hematoxylin and eosin stain and tissue culture (bacterial, fungal, and acid-fast bacillus). Given the possibility of facial and periorbital cellulitis, empiric intravenous antibiotic therapy was initiated.
The tissue culture revealed normal skin flora. The biopsy results indicated a foreign-body reaction consistent with paraffin granuloma (Figures 2 and 3). Fite-Faraco, Grocott-Gomori methenamine-silver, and periodic acid–Schiff stains were all negative for infection. A diagnosis of foreign-body granuloma was established. Oral minocycline at a dosage of 100 mg twice daily was started, and the patient was discharged.


After 4 weeks of minocycline therapy, the patient showed no improvement and returned to the emergency department with worsening symptoms. She was readmitted and started on intravenous prednisone (1.5 mg/kg/d). Over the ensuing 5 days, the edema, erythema, conjunctival injection, and chemosis demonstrated notable improvement. She was subsequently discharged on an oral prednisone taper. Unfortunately, she did not respond to a trial of intralesional steroid injections to an area of granuloma formation on the left chin performed in the hospital before she was discharged.
In the ensuing months, she began to develop cicatricial ectropion of the right lower eyelid and mechanical ptosis of the right upper eyelid. Ten months after initial self-injection, staged surgical excision was initiated by an oculoplastic surgeon (I.V.) with the goal of debulking the periorbital region to correct the ectropion and mechanical ptosis. A transconjunctival approach was used to carefully excise the material while still maintaining the architecture of the lower eyelid. The ectropion was surgically corrected concurrently.
One month after excision, serial injections of 5-fluorouracil (5-FU) and triamcinolone acetonide 40 mg/mL were administered to the right lower eyelid and anterior orbit for 3 months. Fifteen weeks after the first surgery, a second surgery was performed to address residual medial right lower eyelid induration, right upper eyelid mechanical ptosis, and left orbital inflammation. During the postoperative period, serial monthly injections of 5-FU and triamcinolone acetonide were again performed beginning at the first postoperative month.
The surgical excisions resulted in notable improvement 3 months following excision (Figure 4). The patient noted improved ocular surface comfort with decreased foreign-body sensation and tearing. She also was pleased with the improved cosmetic outcome.

Crude substances such as paraffin, petroleum jelly, and lanolin were used for aesthetic purposes in the late 19th and early 20th centuries, initially with satisfying results; however, long-term adverse effects such as hardening of the skin, swelling, granuloma formation, ulceration, infections, and abscesses have discouraged its use by medical professionals today.5 Since paraffin is resistant to degradation and absorption, foreign-body reactions may occur upon injection. These reactions are characterized by replacement of normal subcutaneous tissue by cystic spaces of paraffin oil and/or calcification, similar to the appearance of Swiss cheese on histology and surrounded by various inflammatory cells and fibrous tissue.9,10
Clinically, there is an acute inflammatory phase followed by a latent phase of chronic granulomatous inflammation that can last for years.10 Our patient presented during the acute phase, with erythematous and edematous warm plaques around the eye mimicking an orbital infection.
The treatment of choice for paraffin granuloma is complete surgical excision to prevent recurrence.6,9 However, intralesional corticosteroids are preferred in the facial area, especially if complete removal is not possible.10 Intralesional corticosteroid injections inhibit fibroblast and macrophage activity as well as the deposition of collagen, leading to reduced pain and swelling in most cases.11 Additionally, combining antimitotic agents such as 5-FU with a corticosteroid might reduce the risk for cortisone skin atrophy.12 In our case, the patient did not respond to combined 5-FU with intralesional steroids and required oral corticosteroids while awaiting serial excisions.
Our case highlights several important points in the management of paraffin granuloma. First, the clinician must perform a thorough patient history, as surreptitious use of non–medical-grade fillers is more common than one might think.13 Second, the initial presentation of these patients can mimic an infectious process. Careful history, testing, and observation can aid in making the appropriate diagnosis. Finally, treatment of these patients is complex. The mainstays of therapy are systemic anti-inflammatory medications, time, and supportive care. In some cases, surgery may be required. When processes such as paraffin granulomas involve the periorbital region, particular care is required to avoid cicatricial lagophthalmos, ectropion, or retraction. Thoughtful surgical manipulation is required to avoid these complications, which indeed may occur even with the most appropriate interventions.
- Duker D, Erdmann R, Hartmann V, et al. The impact of adverse reactions to injectable filler substances on quality of life: results from the Berlin Injectable Filler Safety (IFS)—study. J Eur Acad Dermatol Venereol. 2016;30:1013-1020.
- Prado G, Rodriguez-Feliz J. Ocular pain and impending blindness during facial cosmetic injections: is your office prepared? [published online December 28, 2016]. Aesthetic Plast Surg. 2017;41:199-203.
- Roberts SA, Arthurs BP. Severe visual loss and orbital infarction following periorbital aesthetic poly-(L)-lactic acid (PLLA) injection. Ophthalmic Plast Reconstr Surg. 2012;28:E68-E70.
- Cassuto D, Pignatti M, Pacchioni L, et al. Management of complications caused by permanent fillers in the face: a treatment algorithm. Plast Reconstr Surg. 2016;138:215E-227E.
- Haneke E. Adverse effects of fillers and their histopathology. Facial Plast Surg. 2014;30:599-614.
- Friedrich RE, Zustin J. Paraffinoma of lips and oral mucosa: case report and brief review of literature. GMS Interdiscip Plast Reconstr Surg DGPW. 2014;3:Doc05.
- Matton G, Anseeuw A, De Keyser F. The history of injectable biomaterials and the biology of collagen. Aesthetic Plast Surg. 1985;9:133-140.
- Glicenstein J. Les premiers fillers, Vaseline et paraffine. du miracle a la catastrope. Ann Chir Plast Esthet. 2007;52:157-161.
- Cohen JL, Keoleian CM, Krull EA. Penile paraffinoma: self-injection with mineral oil. J Am Acad Dermatol 2002;47:S251-S253.
- Legaspi-Vicerra ME, Field LM. Paraffin granulomata, “witch’s chin,” and nasal deformities excision and reconstruction with reduction chinplasty and open rhinotomy resection. J Clin Aesthet Dermatol 2010;3:54-58.
- Carlos-Fabuel L, Marzal-Gamarra C, Marti-Alamo S, et al. Foreign body granulomatous reactions to cosmetic fillers. J Clin Exp Dent. 2012;4:E244-E247.
- Lemperle G, Gauthier-Hazan N. Foreign body granulomas after all injectable dermal fillers: part 2. treatment options. Plast Reconstr Surg. 2009;123:1864-1873.
- Seok J, Hong JY, Park KY, et al. Delayed immunologic complications due to injectable fillers by unlicensed practitioners: our experiences and a review of the literature. Dermatol Ther. 2016;29:41-44.
- Duker D, Erdmann R, Hartmann V, et al. The impact of adverse reactions to injectable filler substances on quality of life: results from the Berlin Injectable Filler Safety (IFS)—study. J Eur Acad Dermatol Venereol. 2016;30:1013-1020.
- Prado G, Rodriguez-Feliz J. Ocular pain and impending blindness during facial cosmetic injections: is your office prepared? [published online December 28, 2016]. Aesthetic Plast Surg. 2017;41:199-203.
- Roberts SA, Arthurs BP. Severe visual loss and orbital infarction following periorbital aesthetic poly-(L)-lactic acid (PLLA) injection. Ophthalmic Plast Reconstr Surg. 2012;28:E68-E70.
- Cassuto D, Pignatti M, Pacchioni L, et al. Management of complications caused by permanent fillers in the face: a treatment algorithm. Plast Reconstr Surg. 2016;138:215E-227E.
- Haneke E. Adverse effects of fillers and their histopathology. Facial Plast Surg. 2014;30:599-614.
- Friedrich RE, Zustin J. Paraffinoma of lips and oral mucosa: case report and brief review of literature. GMS Interdiscip Plast Reconstr Surg DGPW. 2014;3:Doc05.
- Matton G, Anseeuw A, De Keyser F. The history of injectable biomaterials and the biology of collagen. Aesthetic Plast Surg. 1985;9:133-140.
- Glicenstein J. Les premiers fillers, Vaseline et paraffine. du miracle a la catastrope. Ann Chir Plast Esthet. 2007;52:157-161.
- Cohen JL, Keoleian CM, Krull EA. Penile paraffinoma: self-injection with mineral oil. J Am Acad Dermatol 2002;47:S251-S253.
- Legaspi-Vicerra ME, Field LM. Paraffin granulomata, “witch’s chin,” and nasal deformities excision and reconstruction with reduction chinplasty and open rhinotomy resection. J Clin Aesthet Dermatol 2010;3:54-58.
- Carlos-Fabuel L, Marzal-Gamarra C, Marti-Alamo S, et al. Foreign body granulomatous reactions to cosmetic fillers. J Clin Exp Dent. 2012;4:E244-E247.
- Lemperle G, Gauthier-Hazan N. Foreign body granulomas after all injectable dermal fillers: part 2. treatment options. Plast Reconstr Surg. 2009;123:1864-1873.
- Seok J, Hong JY, Park KY, et al. Delayed immunologic complications due to injectable fillers by unlicensed practitioners: our experiences and a review of the literature. Dermatol Ther. 2016;29:41-44.
Practice Points
- The initial presentation of a foreign-body granulomatous process in a patient with surreptitious use of nonmedical filler can mimic infection; thus, careful history and diagnostic measures are paramount.
- Treatment of paraffin oil granuloma can be multifactorial and involves supportive care, systemic anti-inflammatory medications, time, and surgery.
- When a paraffin granuloma involves the orbital region, particular care is required to avoid long-term complications including cicatricial lagophthalmos, ectropion, or retractions, which can be mitigated with the help of oculoplastic surgery.
A teen presents with a severe, tender rash on the extremities
“There’s rue for you, and here’s some for me; we may call it herb of grace o’ Sundays. O, you must wear your rue with a difference.”
— Ophelia in Hamlet by William Shakespeare
The patient was admitted to the hospital for IV fluids, pain control, and observation. The following day she admitted using the leaves of a plant on the trail as a bug repellent, as one time was taught by her grandfather. She rubbed some of the leaves on the brother as well. The grandfather shared some pictures of the bushes, and the plant was identified as Ruta graveolens.
The blisters were deroofed, cleaned with saline, and wrapped with triamcinolone ointment and petrolatum. The patient was also started on a prednisone taper and received analgesics for the severe pain.
Ruta graveolens also known as common rue or herb of grace, is an ornamental plant from the Rutaceae family. This plant is also used as a medicinal herb, condiment, and as an insect repellent. If ingested in large doses, it can cause severe abdominal pain and vomiting. It also can be hepatotoxic.
The herb contains furocumarines, such as 8-methoxypsoralen and 5-methoxypsoralen and furoquinoline alkaloids. These chemicals when exposed to UVA radiation cause cell injury and inflammation of the skin. This is considered a phototoxic reaction of the skin, compared with allergic reactions, such as poison ivy dermatitis, which need a prior sensitization to the allergen for the T cells to be activated and cause injury in the skin. Other common plants and fruits that can cause phytophotodermatitis include citrus fruits, figs, carrots, celery, parsnips, parsley, and other wildflowers like hogweed.
Depending on the degree of injury, the patients can be treated with topical corticosteroids, petrolatum wraps, and pain control. In severe cases like our patient, systemic prednisone may help stop the progression of the lesions and help with the inflammation. Skin hyperpigmentation after the initial injury may take months to clear, and some patient can develop scars.
The differential diagnosis should include severe bullous contact dermatitis like exposure to urushiol in poison ivy; second- and third-degree burns; severe medications reactions such Stevens-Johnson syndrome or toxic epidermal necrolysis, and inmunobullous diseases such as bullous lupus erythematosus, pemphigus vulgaris, or bullous pemphigoid. If there is no history of exposure or there are any other systemic symptoms, consider performing a skin biopsy of one of the lesions.
In this patient’s case, the history of exposure and skin findings helped the dermatologist on call make the right diagnosis.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
J Burn Care Res. 2018 Oct 23;39(6):1064-6.
Dermatitis. 2007 Mar;18(1):52-5.
BMJ Case Rep. 2015 Dec 23;2015:bcr2015213388.
“There’s rue for you, and here’s some for me; we may call it herb of grace o’ Sundays. O, you must wear your rue with a difference.”
— Ophelia in Hamlet by William Shakespeare
The patient was admitted to the hospital for IV fluids, pain control, and observation. The following day she admitted using the leaves of a plant on the trail as a bug repellent, as one time was taught by her grandfather. She rubbed some of the leaves on the brother as well. The grandfather shared some pictures of the bushes, and the plant was identified as Ruta graveolens.
The blisters were deroofed, cleaned with saline, and wrapped with triamcinolone ointment and petrolatum. The patient was also started on a prednisone taper and received analgesics for the severe pain.
Ruta graveolens also known as common rue or herb of grace, is an ornamental plant from the Rutaceae family. This plant is also used as a medicinal herb, condiment, and as an insect repellent. If ingested in large doses, it can cause severe abdominal pain and vomiting. It also can be hepatotoxic.
The herb contains furocumarines, such as 8-methoxypsoralen and 5-methoxypsoralen and furoquinoline alkaloids. These chemicals when exposed to UVA radiation cause cell injury and inflammation of the skin. This is considered a phototoxic reaction of the skin, compared with allergic reactions, such as poison ivy dermatitis, which need a prior sensitization to the allergen for the T cells to be activated and cause injury in the skin. Other common plants and fruits that can cause phytophotodermatitis include citrus fruits, figs, carrots, celery, parsnips, parsley, and other wildflowers like hogweed.
Depending on the degree of injury, the patients can be treated with topical corticosteroids, petrolatum wraps, and pain control. In severe cases like our patient, systemic prednisone may help stop the progression of the lesions and help with the inflammation. Skin hyperpigmentation after the initial injury may take months to clear, and some patient can develop scars.
The differential diagnosis should include severe bullous contact dermatitis like exposure to urushiol in poison ivy; second- and third-degree burns; severe medications reactions such Stevens-Johnson syndrome or toxic epidermal necrolysis, and inmunobullous diseases such as bullous lupus erythematosus, pemphigus vulgaris, or bullous pemphigoid. If there is no history of exposure or there are any other systemic symptoms, consider performing a skin biopsy of one of the lesions.
In this patient’s case, the history of exposure and skin findings helped the dermatologist on call make the right diagnosis.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
J Burn Care Res. 2018 Oct 23;39(6):1064-6.
Dermatitis. 2007 Mar;18(1):52-5.
BMJ Case Rep. 2015 Dec 23;2015:bcr2015213388.
“There’s rue for you, and here’s some for me; we may call it herb of grace o’ Sundays. O, you must wear your rue with a difference.”
— Ophelia in Hamlet by William Shakespeare
The patient was admitted to the hospital for IV fluids, pain control, and observation. The following day she admitted using the leaves of a plant on the trail as a bug repellent, as one time was taught by her grandfather. She rubbed some of the leaves on the brother as well. The grandfather shared some pictures of the bushes, and the plant was identified as Ruta graveolens.
The blisters were deroofed, cleaned with saline, and wrapped with triamcinolone ointment and petrolatum. The patient was also started on a prednisone taper and received analgesics for the severe pain.
Ruta graveolens also known as common rue or herb of grace, is an ornamental plant from the Rutaceae family. This plant is also used as a medicinal herb, condiment, and as an insect repellent. If ingested in large doses, it can cause severe abdominal pain and vomiting. It also can be hepatotoxic.
The herb contains furocumarines, such as 8-methoxypsoralen and 5-methoxypsoralen and furoquinoline alkaloids. These chemicals when exposed to UVA radiation cause cell injury and inflammation of the skin. This is considered a phototoxic reaction of the skin, compared with allergic reactions, such as poison ivy dermatitis, which need a prior sensitization to the allergen for the T cells to be activated and cause injury in the skin. Other common plants and fruits that can cause phytophotodermatitis include citrus fruits, figs, carrots, celery, parsnips, parsley, and other wildflowers like hogweed.
Depending on the degree of injury, the patients can be treated with topical corticosteroids, petrolatum wraps, and pain control. In severe cases like our patient, systemic prednisone may help stop the progression of the lesions and help with the inflammation. Skin hyperpigmentation after the initial injury may take months to clear, and some patient can develop scars.
The differential diagnosis should include severe bullous contact dermatitis like exposure to urushiol in poison ivy; second- and third-degree burns; severe medications reactions such Stevens-Johnson syndrome or toxic epidermal necrolysis, and inmunobullous diseases such as bullous lupus erythematosus, pemphigus vulgaris, or bullous pemphigoid. If there is no history of exposure or there are any other systemic symptoms, consider performing a skin biopsy of one of the lesions.
In this patient’s case, the history of exposure and skin findings helped the dermatologist on call make the right diagnosis.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
J Burn Care Res. 2018 Oct 23;39(6):1064-6.
Dermatitis. 2007 Mar;18(1):52-5.
BMJ Case Rep. 2015 Dec 23;2015:bcr2015213388.
She started taking lithium for depression and anxiety 3 weeks prior to her developing the rash. She denies taking any other medications, supplements, or recreational drugs.
She denied any prior history of photosensitivity, no history of mouth ulcers, joint pain, muscle weakness, hair loss, or any other symptoms.
Besides her brother, there are no other affected family members, and no history of immune bullous disorders or other skin conditions.
On physical exam, the girl appears in a lot of pain and is uncomfortable. The skin is red and hot, and there are tense bullae on the neck, arms, and legs. There are no ocular or mucosal lesions.
Patch Testing 101, Part 1: Performing the Test
Our apologies, dear reader. It seems we have gotten ahead of ourselves. While we were writing about the Allergen of the Year, systemic dermatitis, and patch testing in children, we forgot to start with the basics. Let us remedy that. This is the first of a 2-part series addressing the basics of patch testing. In this article, we examine patch test systems, allergens, patch test readings, testing while on medications, and patch testing pearls and pitfalls. Let us begin!
Patch Test Systems
There are 2 patch test systems in North America: the Thin-layer Rapid Use Epicutaneous (T.R.U.E.) test (SmartPractice), which is approved by the US Food and Drug Administration for those 6 years and older, and the chamber method.
The T.R.U.E. test consists of 3 panels with 35 allergens and 1 negative control. The T.R.U.E. package insert1 describes surgical tape with individual polyester patches, each coated with an allergen film. Benefits of T.R.U.E. include ease of use (ie, easy storage and preparation, quick and straightforward application) and a readily identifiable set of allergens. The main drawback of T.R.U.E. is that only a limited number of allergens are tested, and as a result, it may miss the identification of some contact allergies. In an analysis of the 2015-2016 North American Contact Dermatitis Group (NACDG) patch test screening series, 25% to 40% of positive patch tests would have been missed if patch testing was performed with T.R.U.E. alone.2
Chamber method patch testing describes the process by which allergens are loaded into either metal or plastic chambers and then applied to the patient’s skin. The major benefit of the chamber method is that patches may be truly customized for the patient. The chamber method is time and labor intensive for patch preparation and application. Most comprehensive patch test clinics in North America use the chamber method, including the NACDG.
Patch test chambers largely can be divided into 2 categories: metal (aluminum) or plastic. Aluminum chambers, also known as Finn chambers, traditionally are used in patch testing. There are rare reports of hypersensitivity to aluminum chambers with associated diffuse positive patch test reactions,3,4 which may be more common in the pediatric population and likely is due to the fact that aluminum is present as an adjuvant in many childhood vaccines. As a precaution, some patch text experts recommend using plastic chambers in children younger than 16 to 18 years (M.R. and A.R.A., personal communication). Metal chambers require the additional application of diffusion discs for liquid allergens, and plastic chambers typically already contain the necessary diffusion discs. Finn chambers traditionally are applied with hypoallergenic porous surgical tape, but a waterproof tape also is available. To keep the chambers in place for the necessary 48 hours, additional tape may be applied over the patches.
Allergens
In patch test clinics, many dermatologists use a standard or screening allergen series. An appropriate standard series encompasses allergens that are most likely to be positive and relevant in the tested population. Some patch test experts recommend that allergens with a positive patch test frequency of greater than 0.5% to 1% should be included in a standard series.5 However, geographic differences in positive reactions can influence which allergens are appropriate to include. As a result, there is no universal standard series. Examples of standard or screening series include the American Contact Dermatitis Society (ACDS) allergen series,6 North American Baseline Series or North American 80 Comprehensive Series, European Baseline Series, NACDG series,2 and the Pediatric Baseline Series,7 as well as many other country- or region-specific series. There currently are 2 major commercial allergen distribution companies—Chemotechnique Diagnostics/Dormer Laboratories (series, individual allergens) and SmartPractice/allerGEAZE (series, individual allergens, T.R.U.E.).
In addition to a properly selected standard or screening series, supplemental patch testing with additional allergens can increase the diagnostic yield. Numerous supplemental series exist, including cosmetic, dental, textile, rubber, adhesive, plastics, and glue, among many others. In the NACDG 2015-2016 patch test cycle, it was found that 23% of 5597 patients reacted to an allergen that was not present on the NACDG screening series.2
In some situations, it is appropriate to patch test patient products, or nonstandard allergens. An abundance of caution, understanding of patch testing, and experience is necessary; for example, some chemicals are not recommended for testing, such as cleaning products, certain industrial chemicals, and those that may be carcinogens. We frequently consult De Groot’s Patch Testing8 for recommended allergen test concentrations and vehicles.
Patch Test Readings
The timing of the patch test reading is an important component of the test. Most North American comprehensive patch test clinics perform both first and delayed readings. After application, patches remain in place for 48 hours and then are removed, and a first reading is completed. Results are recorded as +/− (weak/doubtful), + (mild), ++ (strong), +++ (very strong), irritant, and negative.2 Many patch test specialists use side lighting to achieve the best reading and palpate to confirm the presence of induration; panel alignment devices commonly are utilized. There are some scenarios where shorter or longer application times are indicated, but this is beyond the scope of this article. A second, or delayed, reading should be completed 72 to 144 hours after initial application. We usually complete the delayed reading at 96 to 120 hours.
Certain patch test reactions may peak at different times, with fragrances often reacting earlier, and metals, topical antibiotics, and textile dyes reacting later.9 In the scenario of delayed peak reactions, third readings may be indicated.
Neglecting to complete a delayed reading is a potential pitfall and can increase the risk for both false-positive and false-negative reactions.10,11 In 1996, Uter et al10 published a large study of 9946 patients who were patch tested over a 4-year period. The authors compared patch test reactions at 48 and 72 hours and found that 34.5% of all positive reactions occurred at 72 hours; an additional 15.1% were positive at 96 hours. Importantly, one reading at 48 hours missed approximately one-third of positive patch test reactions, emphasizing the importance of delayed patch test readings.10 Furthermore, another study of 9997 consecutively patch tested patients examined reactions that were either negative or doubtful between days 3 or 4 and followed to see which of those reactions were positive at days 6 or 7. Of the negative reactions, the authors found that 4.4% were positive on days 6 or 7, and of the doubtful reactions, 9.1% were positive on days 6 or 7, meaning that up to 13.5% of positive reactions can be missed when a later reading is not performed.11
Medications During Patch Testing
Topical Medications
Topical medications generally can be continued during patch testing; however, patients should not apply topical medications to the patch test application site. Ideally, there should be no topical medication applied to the patch test application site for 1 to 2 weeks prior to patch test placement.12 Use of topical medications such as corticosteroids, calcineurin inhibitors, and theoretically even phosphodiesterase 4 inhibitors can not only result in suppression of positive patch test reactions but also can make patch adherence difficult.
Phototherapy
Phototherapy can result in local cutaneous immune suppression; therefore, it is recommended that it not be applied to the patch test area either during the patch test process or for 1 to 2 weeks prior to patch test application. In addition, if heat or sweating are generated during phototherapy, they can affect the success of patch testing by poor patch adherence and/or disruption of allergen distribution.
Systemic Medications
Oral antihistamines do not affect patch testing and can be continued during the patch test process.
It is ideal to avoid systemic immunomodulatory agents during the patch test process, but they occasionally are unavoidable, either because they are necessary to manage other medical conditions or because they are needed to achieve clear enough skin to proceed with patch testing. If it is required, prednisone is not recommended to exceed 10 mg daily.12,13 If intramuscular triamcinolone acetonide has been administered, patch testing should occur at least 1 month after the most recent injection.12 Oral methotrexate can probably be continued during patch testing but should be kept at the lowest possible dose and should be held during the week of testing, if possible. Adalimumab, etanercept, infliximab, and ustekinumab can be continued, as they are unlikely to interfere with patch testing.12 There are reports of positive patch test reactions on dupilumab,14,15 and some authors have described the response as variable and potentially allergen dependent.16,17 We believe that it generally is acceptable to continue dupilumab during patch testing. Data on cyclosporine during patch testing are mixed, and caution is advised as higher doses may suppress a positive patch test. Azathioprine and mycophenolate should be avoided, if possible.12
Pearls and Pitfalls
A few tips along the way can help assure your success in patch testing.
- Proper patient counseling determines a successful test. Provide your patient with verbal and written instructions about the patch test process, patch care, and any other necessary information.
- A simple sponge bath is permissible during patch testing provided the back stays dry. One of the authors (A.R.A.) advises patients to sit in a small amount of water in a bathtub to bathe, wash only the front of the body in the shower, and wash hair in the sink.
- No sweating, swimming, heavy exercise, or heavy physical labor. If your patient is planning to run a marathon the week of patch testing, they will be sorely disappointed when you tell them no sweating or showering is allowed! Patients with an occupation that requires physical labor may require a work excuse.
- Tape does not adhere to areas of the skin with excess hair. A scissor trim or electric shave will help the patches stay occluded and in place. We use an electric razor with a disposable replaceable head. A traditional straight razor should not be used, as it can increase the risk for folliculitis, which can make patch readings quite difficult.
- Securing the patches in place with an extra layer of tape provides added security. Large sheets of transparent medical dressings work particularly well for children or if there is difficulty with tape adherence.
Avoid application of patches to areas of the skin with tattoos. In theory, tattooed skin may have a decreased immune response, and tattoo pigment can obscure results.18 However, this is sometimes unavoidable, and Fowler and McTigue18 described a case of successful patch testing on a diffusely tattooed back.
- Avoid skin lesions (eg, scars, seborrheic keratoses, dermatitis) that can affect tape application, patch adherence, or patch readings.
Final Interpretation
The first step to excellent patch testing is understanding the patch test process. Patch test systems include T.R.U.E. and the chamber method. There are several allergen screening series, and the best series for each patient is determined based on geographic region, exposures, and allergen prevalence. The timing and practice of the patch test reading is vital, and physicians should be cognizant of medications and phototherapy use during the patch test process. An understanding of common pearls and pitfalls makes the difference between a good and great patch tester.
Now that you are an expert in performing the test, watch out for part 2 of this series on patch test interpretation, relevance, education, and counseling. Happy testing!
- T.R.U.E TEST [package insert]. Phoenix, AZ: SmartPractice; 1994.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Ward JM, Walsh RK, Bellet JS, et al. Allergic contact dermatitis to aluminum-based chambers during routine patch testing. Paper presented at: American Contact Dermatitis Society Annual Meeting; March 19, 2020; Denver, CO.
- Deleuran MG, Ahlström MG, Zachariae C, et al. Patch test reactivity to aluminum chambers. Contact Dermatitis. 2019;81:318-319.
- Bruze M, Condé-Salazar L, Goossens A, et al. European Society of Contact Dermatitis. thoughts on sensitizers in a standard patch test series. Contact Dermatitis. 1999;41:241-250.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2017 update. Dermatitis. 2017;28:141-143.
- Yu J, Atwater AR, Brod B, et al. Pediatric baseline patch test series: Pediatric Contact Dermatitis Workgroup. Dermatitis. 2018;29:206-212.
- De Groot AC. Patch Testing: Test Concentrations and Vehicles for 4900 Chemicals. 4th ed. Wapserveen, The Netherlands: Acdegroot Publishing; 2018.
- Chaudhry HM, Drage LA, El-Azhary RA, et al. Delayed patch-test reading after 5 days: an update from the Mayo Clinic Contact Dermatitis Group. Dermatitis. 2017;28:253-260.
- Uter WJ, Geier J, Schnuch A. Good clinical practice in patch testing: readings beyond day 2 are necessary: a confirmatory analysis. Members of the Information Network of Departments of Dermatology. Am J Contact Dermat. 1996;7:231-237.
- Madsen JT, Andersen KE. Outcome of a second patch test reading of T.R.U.E. Tests® on D6/7. Contact Dermatitis. 2013;68:94-97.
- Lampel H, Atwater AR. Patch testing tools of the trade: use of immunosuppressants and antihistamines during patch testing. J Dermatol Nurses’ Assoc. 2016;8:209-211.
- Fowler JF, Maibach HI, Zirwas M, et al. Effects of immunomodulatory agents on patch testing: expert opinion 2012. Dermatitis. 2012;23:301-303.
- Puza CJ, Atwater AR. Positive patch test reaction in a patient taking dupilumab. Dermatitis. 2018;29:89.
- Hoot JW, Douglas JD, Falo LD. Patch testing in a patient on dupilumab. Dermatitis. 2018;29:164.
- Stout M, Silverberg JI. Variable impact of dupilumab on patch testing results and allergic contact dermatitis in adults with atopic dermatitis. J Am Acad Dermatol. 2019;81:157-162.
- Raffi J, Botto N. Patch testing and allergen-specific inhibition in a patient taking dupilumab. JAMA Dermatol. 2019;155:120-121.
- Fowler JF, McTigue MK. Patch testing over tattoos. Am J Contact Dermat. 2002;13:19-20.
Our apologies, dear reader. It seems we have gotten ahead of ourselves. While we were writing about the Allergen of the Year, systemic dermatitis, and patch testing in children, we forgot to start with the basics. Let us remedy that. This is the first of a 2-part series addressing the basics of patch testing. In this article, we examine patch test systems, allergens, patch test readings, testing while on medications, and patch testing pearls and pitfalls. Let us begin!
Patch Test Systems
There are 2 patch test systems in North America: the Thin-layer Rapid Use Epicutaneous (T.R.U.E.) test (SmartPractice), which is approved by the US Food and Drug Administration for those 6 years and older, and the chamber method.
The T.R.U.E. test consists of 3 panels with 35 allergens and 1 negative control. The T.R.U.E. package insert1 describes surgical tape with individual polyester patches, each coated with an allergen film. Benefits of T.R.U.E. include ease of use (ie, easy storage and preparation, quick and straightforward application) and a readily identifiable set of allergens. The main drawback of T.R.U.E. is that only a limited number of allergens are tested, and as a result, it may miss the identification of some contact allergies. In an analysis of the 2015-2016 North American Contact Dermatitis Group (NACDG) patch test screening series, 25% to 40% of positive patch tests would have been missed if patch testing was performed with T.R.U.E. alone.2
Chamber method patch testing describes the process by which allergens are loaded into either metal or plastic chambers and then applied to the patient’s skin. The major benefit of the chamber method is that patches may be truly customized for the patient. The chamber method is time and labor intensive for patch preparation and application. Most comprehensive patch test clinics in North America use the chamber method, including the NACDG.
Patch test chambers largely can be divided into 2 categories: metal (aluminum) or plastic. Aluminum chambers, also known as Finn chambers, traditionally are used in patch testing. There are rare reports of hypersensitivity to aluminum chambers with associated diffuse positive patch test reactions,3,4 which may be more common in the pediatric population and likely is due to the fact that aluminum is present as an adjuvant in many childhood vaccines. As a precaution, some patch text experts recommend using plastic chambers in children younger than 16 to 18 years (M.R. and A.R.A., personal communication). Metal chambers require the additional application of diffusion discs for liquid allergens, and plastic chambers typically already contain the necessary diffusion discs. Finn chambers traditionally are applied with hypoallergenic porous surgical tape, but a waterproof tape also is available. To keep the chambers in place for the necessary 48 hours, additional tape may be applied over the patches.
Allergens
In patch test clinics, many dermatologists use a standard or screening allergen series. An appropriate standard series encompasses allergens that are most likely to be positive and relevant in the tested population. Some patch test experts recommend that allergens with a positive patch test frequency of greater than 0.5% to 1% should be included in a standard series.5 However, geographic differences in positive reactions can influence which allergens are appropriate to include. As a result, there is no universal standard series. Examples of standard or screening series include the American Contact Dermatitis Society (ACDS) allergen series,6 North American Baseline Series or North American 80 Comprehensive Series, European Baseline Series, NACDG series,2 and the Pediatric Baseline Series,7 as well as many other country- or region-specific series. There currently are 2 major commercial allergen distribution companies—Chemotechnique Diagnostics/Dormer Laboratories (series, individual allergens) and SmartPractice/allerGEAZE (series, individual allergens, T.R.U.E.).
In addition to a properly selected standard or screening series, supplemental patch testing with additional allergens can increase the diagnostic yield. Numerous supplemental series exist, including cosmetic, dental, textile, rubber, adhesive, plastics, and glue, among many others. In the NACDG 2015-2016 patch test cycle, it was found that 23% of 5597 patients reacted to an allergen that was not present on the NACDG screening series.2
In some situations, it is appropriate to patch test patient products, or nonstandard allergens. An abundance of caution, understanding of patch testing, and experience is necessary; for example, some chemicals are not recommended for testing, such as cleaning products, certain industrial chemicals, and those that may be carcinogens. We frequently consult De Groot’s Patch Testing8 for recommended allergen test concentrations and vehicles.
Patch Test Readings
The timing of the patch test reading is an important component of the test. Most North American comprehensive patch test clinics perform both first and delayed readings. After application, patches remain in place for 48 hours and then are removed, and a first reading is completed. Results are recorded as +/− (weak/doubtful), + (mild), ++ (strong), +++ (very strong), irritant, and negative.2 Many patch test specialists use side lighting to achieve the best reading and palpate to confirm the presence of induration; panel alignment devices commonly are utilized. There are some scenarios where shorter or longer application times are indicated, but this is beyond the scope of this article. A second, or delayed, reading should be completed 72 to 144 hours after initial application. We usually complete the delayed reading at 96 to 120 hours.
Certain patch test reactions may peak at different times, with fragrances often reacting earlier, and metals, topical antibiotics, and textile dyes reacting later.9 In the scenario of delayed peak reactions, third readings may be indicated.
Neglecting to complete a delayed reading is a potential pitfall and can increase the risk for both false-positive and false-negative reactions.10,11 In 1996, Uter et al10 published a large study of 9946 patients who were patch tested over a 4-year period. The authors compared patch test reactions at 48 and 72 hours and found that 34.5% of all positive reactions occurred at 72 hours; an additional 15.1% were positive at 96 hours. Importantly, one reading at 48 hours missed approximately one-third of positive patch test reactions, emphasizing the importance of delayed patch test readings.10 Furthermore, another study of 9997 consecutively patch tested patients examined reactions that were either negative or doubtful between days 3 or 4 and followed to see which of those reactions were positive at days 6 or 7. Of the negative reactions, the authors found that 4.4% were positive on days 6 or 7, and of the doubtful reactions, 9.1% were positive on days 6 or 7, meaning that up to 13.5% of positive reactions can be missed when a later reading is not performed.11
Medications During Patch Testing
Topical Medications
Topical medications generally can be continued during patch testing; however, patients should not apply topical medications to the patch test application site. Ideally, there should be no topical medication applied to the patch test application site for 1 to 2 weeks prior to patch test placement.12 Use of topical medications such as corticosteroids, calcineurin inhibitors, and theoretically even phosphodiesterase 4 inhibitors can not only result in suppression of positive patch test reactions but also can make patch adherence difficult.
Phototherapy
Phototherapy can result in local cutaneous immune suppression; therefore, it is recommended that it not be applied to the patch test area either during the patch test process or for 1 to 2 weeks prior to patch test application. In addition, if heat or sweating are generated during phototherapy, they can affect the success of patch testing by poor patch adherence and/or disruption of allergen distribution.
Systemic Medications
Oral antihistamines do not affect patch testing and can be continued during the patch test process.
It is ideal to avoid systemic immunomodulatory agents during the patch test process, but they occasionally are unavoidable, either because they are necessary to manage other medical conditions or because they are needed to achieve clear enough skin to proceed with patch testing. If it is required, prednisone is not recommended to exceed 10 mg daily.12,13 If intramuscular triamcinolone acetonide has been administered, patch testing should occur at least 1 month after the most recent injection.12 Oral methotrexate can probably be continued during patch testing but should be kept at the lowest possible dose and should be held during the week of testing, if possible. Adalimumab, etanercept, infliximab, and ustekinumab can be continued, as they are unlikely to interfere with patch testing.12 There are reports of positive patch test reactions on dupilumab,14,15 and some authors have described the response as variable and potentially allergen dependent.16,17 We believe that it generally is acceptable to continue dupilumab during patch testing. Data on cyclosporine during patch testing are mixed, and caution is advised as higher doses may suppress a positive patch test. Azathioprine and mycophenolate should be avoided, if possible.12
Pearls and Pitfalls
A few tips along the way can help assure your success in patch testing.
- Proper patient counseling determines a successful test. Provide your patient with verbal and written instructions about the patch test process, patch care, and any other necessary information.
- A simple sponge bath is permissible during patch testing provided the back stays dry. One of the authors (A.R.A.) advises patients to sit in a small amount of water in a bathtub to bathe, wash only the front of the body in the shower, and wash hair in the sink.
- No sweating, swimming, heavy exercise, or heavy physical labor. If your patient is planning to run a marathon the week of patch testing, they will be sorely disappointed when you tell them no sweating or showering is allowed! Patients with an occupation that requires physical labor may require a work excuse.
- Tape does not adhere to areas of the skin with excess hair. A scissor trim or electric shave will help the patches stay occluded and in place. We use an electric razor with a disposable replaceable head. A traditional straight razor should not be used, as it can increase the risk for folliculitis, which can make patch readings quite difficult.
- Securing the patches in place with an extra layer of tape provides added security. Large sheets of transparent medical dressings work particularly well for children or if there is difficulty with tape adherence.
Avoid application of patches to areas of the skin with tattoos. In theory, tattooed skin may have a decreased immune response, and tattoo pigment can obscure results.18 However, this is sometimes unavoidable, and Fowler and McTigue18 described a case of successful patch testing on a diffusely tattooed back.
- Avoid skin lesions (eg, scars, seborrheic keratoses, dermatitis) that can affect tape application, patch adherence, or patch readings.
Final Interpretation
The first step to excellent patch testing is understanding the patch test process. Patch test systems include T.R.U.E. and the chamber method. There are several allergen screening series, and the best series for each patient is determined based on geographic region, exposures, and allergen prevalence. The timing and practice of the patch test reading is vital, and physicians should be cognizant of medications and phototherapy use during the patch test process. An understanding of common pearls and pitfalls makes the difference between a good and great patch tester.
Now that you are an expert in performing the test, watch out for part 2 of this series on patch test interpretation, relevance, education, and counseling. Happy testing!
Our apologies, dear reader. It seems we have gotten ahead of ourselves. While we were writing about the Allergen of the Year, systemic dermatitis, and patch testing in children, we forgot to start with the basics. Let us remedy that. This is the first of a 2-part series addressing the basics of patch testing. In this article, we examine patch test systems, allergens, patch test readings, testing while on medications, and patch testing pearls and pitfalls. Let us begin!
Patch Test Systems
There are 2 patch test systems in North America: the Thin-layer Rapid Use Epicutaneous (T.R.U.E.) test (SmartPractice), which is approved by the US Food and Drug Administration for those 6 years and older, and the chamber method.
The T.R.U.E. test consists of 3 panels with 35 allergens and 1 negative control. The T.R.U.E. package insert1 describes surgical tape with individual polyester patches, each coated with an allergen film. Benefits of T.R.U.E. include ease of use (ie, easy storage and preparation, quick and straightforward application) and a readily identifiable set of allergens. The main drawback of T.R.U.E. is that only a limited number of allergens are tested, and as a result, it may miss the identification of some contact allergies. In an analysis of the 2015-2016 North American Contact Dermatitis Group (NACDG) patch test screening series, 25% to 40% of positive patch tests would have been missed if patch testing was performed with T.R.U.E. alone.2
Chamber method patch testing describes the process by which allergens are loaded into either metal or plastic chambers and then applied to the patient’s skin. The major benefit of the chamber method is that patches may be truly customized for the patient. The chamber method is time and labor intensive for patch preparation and application. Most comprehensive patch test clinics in North America use the chamber method, including the NACDG.
Patch test chambers largely can be divided into 2 categories: metal (aluminum) or plastic. Aluminum chambers, also known as Finn chambers, traditionally are used in patch testing. There are rare reports of hypersensitivity to aluminum chambers with associated diffuse positive patch test reactions,3,4 which may be more common in the pediatric population and likely is due to the fact that aluminum is present as an adjuvant in many childhood vaccines. As a precaution, some patch text experts recommend using plastic chambers in children younger than 16 to 18 years (M.R. and A.R.A., personal communication). Metal chambers require the additional application of diffusion discs for liquid allergens, and plastic chambers typically already contain the necessary diffusion discs. Finn chambers traditionally are applied with hypoallergenic porous surgical tape, but a waterproof tape also is available. To keep the chambers in place for the necessary 48 hours, additional tape may be applied over the patches.
Allergens
In patch test clinics, many dermatologists use a standard or screening allergen series. An appropriate standard series encompasses allergens that are most likely to be positive and relevant in the tested population. Some patch test experts recommend that allergens with a positive patch test frequency of greater than 0.5% to 1% should be included in a standard series.5 However, geographic differences in positive reactions can influence which allergens are appropriate to include. As a result, there is no universal standard series. Examples of standard or screening series include the American Contact Dermatitis Society (ACDS) allergen series,6 North American Baseline Series or North American 80 Comprehensive Series, European Baseline Series, NACDG series,2 and the Pediatric Baseline Series,7 as well as many other country- or region-specific series. There currently are 2 major commercial allergen distribution companies—Chemotechnique Diagnostics/Dormer Laboratories (series, individual allergens) and SmartPractice/allerGEAZE (series, individual allergens, T.R.U.E.).
In addition to a properly selected standard or screening series, supplemental patch testing with additional allergens can increase the diagnostic yield. Numerous supplemental series exist, including cosmetic, dental, textile, rubber, adhesive, plastics, and glue, among many others. In the NACDG 2015-2016 patch test cycle, it was found that 23% of 5597 patients reacted to an allergen that was not present on the NACDG screening series.2
In some situations, it is appropriate to patch test patient products, or nonstandard allergens. An abundance of caution, understanding of patch testing, and experience is necessary; for example, some chemicals are not recommended for testing, such as cleaning products, certain industrial chemicals, and those that may be carcinogens. We frequently consult De Groot’s Patch Testing8 for recommended allergen test concentrations and vehicles.
Patch Test Readings
The timing of the patch test reading is an important component of the test. Most North American comprehensive patch test clinics perform both first and delayed readings. After application, patches remain in place for 48 hours and then are removed, and a first reading is completed. Results are recorded as +/− (weak/doubtful), + (mild), ++ (strong), +++ (very strong), irritant, and negative.2 Many patch test specialists use side lighting to achieve the best reading and palpate to confirm the presence of induration; panel alignment devices commonly are utilized. There are some scenarios where shorter or longer application times are indicated, but this is beyond the scope of this article. A second, or delayed, reading should be completed 72 to 144 hours after initial application. We usually complete the delayed reading at 96 to 120 hours.
Certain patch test reactions may peak at different times, with fragrances often reacting earlier, and metals, topical antibiotics, and textile dyes reacting later.9 In the scenario of delayed peak reactions, third readings may be indicated.
Neglecting to complete a delayed reading is a potential pitfall and can increase the risk for both false-positive and false-negative reactions.10,11 In 1996, Uter et al10 published a large study of 9946 patients who were patch tested over a 4-year period. The authors compared patch test reactions at 48 and 72 hours and found that 34.5% of all positive reactions occurred at 72 hours; an additional 15.1% were positive at 96 hours. Importantly, one reading at 48 hours missed approximately one-third of positive patch test reactions, emphasizing the importance of delayed patch test readings.10 Furthermore, another study of 9997 consecutively patch tested patients examined reactions that were either negative or doubtful between days 3 or 4 and followed to see which of those reactions were positive at days 6 or 7. Of the negative reactions, the authors found that 4.4% were positive on days 6 or 7, and of the doubtful reactions, 9.1% were positive on days 6 or 7, meaning that up to 13.5% of positive reactions can be missed when a later reading is not performed.11
Medications During Patch Testing
Topical Medications
Topical medications generally can be continued during patch testing; however, patients should not apply topical medications to the patch test application site. Ideally, there should be no topical medication applied to the patch test application site for 1 to 2 weeks prior to patch test placement.12 Use of topical medications such as corticosteroids, calcineurin inhibitors, and theoretically even phosphodiesterase 4 inhibitors can not only result in suppression of positive patch test reactions but also can make patch adherence difficult.
Phototherapy
Phototherapy can result in local cutaneous immune suppression; therefore, it is recommended that it not be applied to the patch test area either during the patch test process or for 1 to 2 weeks prior to patch test application. In addition, if heat or sweating are generated during phototherapy, they can affect the success of patch testing by poor patch adherence and/or disruption of allergen distribution.
Systemic Medications
Oral antihistamines do not affect patch testing and can be continued during the patch test process.
It is ideal to avoid systemic immunomodulatory agents during the patch test process, but they occasionally are unavoidable, either because they are necessary to manage other medical conditions or because they are needed to achieve clear enough skin to proceed with patch testing. If it is required, prednisone is not recommended to exceed 10 mg daily.12,13 If intramuscular triamcinolone acetonide has been administered, patch testing should occur at least 1 month after the most recent injection.12 Oral methotrexate can probably be continued during patch testing but should be kept at the lowest possible dose and should be held during the week of testing, if possible. Adalimumab, etanercept, infliximab, and ustekinumab can be continued, as they are unlikely to interfere with patch testing.12 There are reports of positive patch test reactions on dupilumab,14,15 and some authors have described the response as variable and potentially allergen dependent.16,17 We believe that it generally is acceptable to continue dupilumab during patch testing. Data on cyclosporine during patch testing are mixed, and caution is advised as higher doses may suppress a positive patch test. Azathioprine and mycophenolate should be avoided, if possible.12
Pearls and Pitfalls
A few tips along the way can help assure your success in patch testing.
- Proper patient counseling determines a successful test. Provide your patient with verbal and written instructions about the patch test process, patch care, and any other necessary information.
- A simple sponge bath is permissible during patch testing provided the back stays dry. One of the authors (A.R.A.) advises patients to sit in a small amount of water in a bathtub to bathe, wash only the front of the body in the shower, and wash hair in the sink.
- No sweating, swimming, heavy exercise, or heavy physical labor. If your patient is planning to run a marathon the week of patch testing, they will be sorely disappointed when you tell them no sweating or showering is allowed! Patients with an occupation that requires physical labor may require a work excuse.
- Tape does not adhere to areas of the skin with excess hair. A scissor trim or electric shave will help the patches stay occluded and in place. We use an electric razor with a disposable replaceable head. A traditional straight razor should not be used, as it can increase the risk for folliculitis, which can make patch readings quite difficult.
- Securing the patches in place with an extra layer of tape provides added security. Large sheets of transparent medical dressings work particularly well for children or if there is difficulty with tape adherence.
Avoid application of patches to areas of the skin with tattoos. In theory, tattooed skin may have a decreased immune response, and tattoo pigment can obscure results.18 However, this is sometimes unavoidable, and Fowler and McTigue18 described a case of successful patch testing on a diffusely tattooed back.
- Avoid skin lesions (eg, scars, seborrheic keratoses, dermatitis) that can affect tape application, patch adherence, or patch readings.
Final Interpretation
The first step to excellent patch testing is understanding the patch test process. Patch test systems include T.R.U.E. and the chamber method. There are several allergen screening series, and the best series for each patient is determined based on geographic region, exposures, and allergen prevalence. The timing and practice of the patch test reading is vital, and physicians should be cognizant of medications and phototherapy use during the patch test process. An understanding of common pearls and pitfalls makes the difference between a good and great patch tester.
Now that you are an expert in performing the test, watch out for part 2 of this series on patch test interpretation, relevance, education, and counseling. Happy testing!
- T.R.U.E TEST [package insert]. Phoenix, AZ: SmartPractice; 1994.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Ward JM, Walsh RK, Bellet JS, et al. Allergic contact dermatitis to aluminum-based chambers during routine patch testing. Paper presented at: American Contact Dermatitis Society Annual Meeting; March 19, 2020; Denver, CO.
- Deleuran MG, Ahlström MG, Zachariae C, et al. Patch test reactivity to aluminum chambers. Contact Dermatitis. 2019;81:318-319.
- Bruze M, Condé-Salazar L, Goossens A, et al. European Society of Contact Dermatitis. thoughts on sensitizers in a standard patch test series. Contact Dermatitis. 1999;41:241-250.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2017 update. Dermatitis. 2017;28:141-143.
- Yu J, Atwater AR, Brod B, et al. Pediatric baseline patch test series: Pediatric Contact Dermatitis Workgroup. Dermatitis. 2018;29:206-212.
- De Groot AC. Patch Testing: Test Concentrations and Vehicles for 4900 Chemicals. 4th ed. Wapserveen, The Netherlands: Acdegroot Publishing; 2018.
- Chaudhry HM, Drage LA, El-Azhary RA, et al. Delayed patch-test reading after 5 days: an update from the Mayo Clinic Contact Dermatitis Group. Dermatitis. 2017;28:253-260.
- Uter WJ, Geier J, Schnuch A. Good clinical practice in patch testing: readings beyond day 2 are necessary: a confirmatory analysis. Members of the Information Network of Departments of Dermatology. Am J Contact Dermat. 1996;7:231-237.
- Madsen JT, Andersen KE. Outcome of a second patch test reading of T.R.U.E. Tests® on D6/7. Contact Dermatitis. 2013;68:94-97.
- Lampel H, Atwater AR. Patch testing tools of the trade: use of immunosuppressants and antihistamines during patch testing. J Dermatol Nurses’ Assoc. 2016;8:209-211.
- Fowler JF, Maibach HI, Zirwas M, et al. Effects of immunomodulatory agents on patch testing: expert opinion 2012. Dermatitis. 2012;23:301-303.
- Puza CJ, Atwater AR. Positive patch test reaction in a patient taking dupilumab. Dermatitis. 2018;29:89.
- Hoot JW, Douglas JD, Falo LD. Patch testing in a patient on dupilumab. Dermatitis. 2018;29:164.
- Stout M, Silverberg JI. Variable impact of dupilumab on patch testing results and allergic contact dermatitis in adults with atopic dermatitis. J Am Acad Dermatol. 2019;81:157-162.
- Raffi J, Botto N. Patch testing and allergen-specific inhibition in a patient taking dupilumab. JAMA Dermatol. 2019;155:120-121.
- Fowler JF, McTigue MK. Patch testing over tattoos. Am J Contact Dermat. 2002;13:19-20.
- T.R.U.E TEST [package insert]. Phoenix, AZ: SmartPractice; 1994.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Ward JM, Walsh RK, Bellet JS, et al. Allergic contact dermatitis to aluminum-based chambers during routine patch testing. Paper presented at: American Contact Dermatitis Society Annual Meeting; March 19, 2020; Denver, CO.
- Deleuran MG, Ahlström MG, Zachariae C, et al. Patch test reactivity to aluminum chambers. Contact Dermatitis. 2019;81:318-319.
- Bruze M, Condé-Salazar L, Goossens A, et al. European Society of Contact Dermatitis. thoughts on sensitizers in a standard patch test series. Contact Dermatitis. 1999;41:241-250.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2017 update. Dermatitis. 2017;28:141-143.
- Yu J, Atwater AR, Brod B, et al. Pediatric baseline patch test series: Pediatric Contact Dermatitis Workgroup. Dermatitis. 2018;29:206-212.
- De Groot AC. Patch Testing: Test Concentrations and Vehicles for 4900 Chemicals. 4th ed. Wapserveen, The Netherlands: Acdegroot Publishing; 2018.
- Chaudhry HM, Drage LA, El-Azhary RA, et al. Delayed patch-test reading after 5 days: an update from the Mayo Clinic Contact Dermatitis Group. Dermatitis. 2017;28:253-260.
- Uter WJ, Geier J, Schnuch A. Good clinical practice in patch testing: readings beyond day 2 are necessary: a confirmatory analysis. Members of the Information Network of Departments of Dermatology. Am J Contact Dermat. 1996;7:231-237.
- Madsen JT, Andersen KE. Outcome of a second patch test reading of T.R.U.E. Tests® on D6/7. Contact Dermatitis. 2013;68:94-97.
- Lampel H, Atwater AR. Patch testing tools of the trade: use of immunosuppressants and antihistamines during patch testing. J Dermatol Nurses’ Assoc. 2016;8:209-211.
- Fowler JF, Maibach HI, Zirwas M, et al. Effects of immunomodulatory agents on patch testing: expert opinion 2012. Dermatitis. 2012;23:301-303.
- Puza CJ, Atwater AR. Positive patch test reaction in a patient taking dupilumab. Dermatitis. 2018;29:89.
- Hoot JW, Douglas JD, Falo LD. Patch testing in a patient on dupilumab. Dermatitis. 2018;29:164.
- Stout M, Silverberg JI. Variable impact of dupilumab on patch testing results and allergic contact dermatitis in adults with atopic dermatitis. J Am Acad Dermatol. 2019;81:157-162.
- Raffi J, Botto N. Patch testing and allergen-specific inhibition in a patient taking dupilumab. JAMA Dermatol. 2019;155:120-121.
- Fowler JF, McTigue MK. Patch testing over tattoos. Am J Contact Dermat. 2002;13:19-20.
Practice Points
- There are 2 basic patch testing systems: the T.R.U.E. test and the chamber method.
- Patches should be applied to the upper back and should be removed after 48 hours. A delayed reading is necessary and should be performed 72 to 144 hours after placement.
- Certain oral and topical medications and phototherapy may interfere with patch test results.
Erythematous Abdominal Nodule
The Diagnosis: Foreign Body Reaction With Sinus Tract
A delayed foreign body reaction is a rare complication of retained temporary pacing wires following cardiovascular surgery. These epicardial pacing wires are important for the management of postoperative arrhythmia and normally are removed by external traction after normal rhythm has been re-established. However, it is not uncommon for these wires to be cut at the skin surface in the setting of difficult removal, as retained pacing wires generally are viewed as benign.1 These reactions often take years after placement of the pacing wire to present themselves and most often resolve with either complete removal of the wire or resection of the distal end.2
Our patient was referred to dermatology and underwent a shave biopsy. Results were consistent with chronic inflammatory granulation tissue. Bacterial tissue culture grew Staphylococcus epidermidis. Cultures for acid-fast bacilli and fungi were negative. The patient was referred to cardiothoracic surgery. Computed tomography identified a retained temporary pacing wire extending to the base of the lesion. The lesion was excised and the distal aspect of the pacing wire was removed, which resulted in resolution of the nodule.
The differential diagnosis includes pyoderma gangrenosum, nodular basal cell carcinoma, Sister Mary Joseph nodule, and pyogenic granuloma. Pyoderma gangrenosum is a neutrophilic dermatosis that presents as a rapidly progressing, painful, necrotic ulcer. It is classically associated with inflammatory bowel disease and other systemic diseases but also can occur in isolation.3
Nodular basal cell carcinomas often develop in chronically sun-exposed areas of the body. Morphologically, they present as pink, pearl appearing papules with rolled borders and overlying arborizing telangiectasia. Nodular basal cell carcinomas may present with recurrent bleeding but typically do not have continuous drainage.4
Sister Mary Joseph nodule represents a periumbilical lymphatic metastasis from an underlying (usually intra-abdominal) malignancy. It typically presents as an umbilical or periumbilical nodule measuring 0.5 to 15 cm in diameter. The nodules often are painful and discharge a serous fluid. It is estimated that they are present in 1% to 3% of cases of abdominopelvic malignancy, but Sister Mary Joseph nodules also have been reported in several other types of solid organ tumors.5
Pyogenic granuloma is a benign vascular lesion that classically develops rapidly over the course of a few weeks. It often presents as a single red, moist, friable papule with a collarette of scale and frequently is associated with pain, bleeding, and ulceration. Keratinized skin or mucosa can be affected, and pyogenic granuloma is most common in children and young adults.6
- Chung DA, Smith EE. Delayed presentation of foreign body reaction secondary to retained pacing wires. Ann Thorac Surg. 1998;66:550-551.
- Gentry WH, Hassan AA. Complications of retained epicardial pacing wires (an unusual bronchial foreign body. Ann Thorac Surg. 1993;56:1391-1393.
- Ahn C, Negus D, Huang W. Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol. 2018;14:225-233.
- Tanese K. Diagnosis and treatment of basal cell carcinoma. Curr Treat Options Oncol. 2019;20:13
- Tso S, Brockley J, Recica H, et al. Sister Mary Joseph's nodule: an unusual but important physical finding characteristic of widespread internal malignancy. Br J Gen Pract. 2013;63:551-552.
- Mashiah J, Hadj-Rabia S, Slodownik D, et al. Effectiveness of topical propranolol 4% gel in the treatment of pyogenic granuloma in children. J Dermatol. 2019;46:245-248.
The Diagnosis: Foreign Body Reaction With Sinus Tract
A delayed foreign body reaction is a rare complication of retained temporary pacing wires following cardiovascular surgery. These epicardial pacing wires are important for the management of postoperative arrhythmia and normally are removed by external traction after normal rhythm has been re-established. However, it is not uncommon for these wires to be cut at the skin surface in the setting of difficult removal, as retained pacing wires generally are viewed as benign.1 These reactions often take years after placement of the pacing wire to present themselves and most often resolve with either complete removal of the wire or resection of the distal end.2
Our patient was referred to dermatology and underwent a shave biopsy. Results were consistent with chronic inflammatory granulation tissue. Bacterial tissue culture grew Staphylococcus epidermidis. Cultures for acid-fast bacilli and fungi were negative. The patient was referred to cardiothoracic surgery. Computed tomography identified a retained temporary pacing wire extending to the base of the lesion. The lesion was excised and the distal aspect of the pacing wire was removed, which resulted in resolution of the nodule.
The differential diagnosis includes pyoderma gangrenosum, nodular basal cell carcinoma, Sister Mary Joseph nodule, and pyogenic granuloma. Pyoderma gangrenosum is a neutrophilic dermatosis that presents as a rapidly progressing, painful, necrotic ulcer. It is classically associated with inflammatory bowel disease and other systemic diseases but also can occur in isolation.3
Nodular basal cell carcinomas often develop in chronically sun-exposed areas of the body. Morphologically, they present as pink, pearl appearing papules with rolled borders and overlying arborizing telangiectasia. Nodular basal cell carcinomas may present with recurrent bleeding but typically do not have continuous drainage.4
Sister Mary Joseph nodule represents a periumbilical lymphatic metastasis from an underlying (usually intra-abdominal) malignancy. It typically presents as an umbilical or periumbilical nodule measuring 0.5 to 15 cm in diameter. The nodules often are painful and discharge a serous fluid. It is estimated that they are present in 1% to 3% of cases of abdominopelvic malignancy, but Sister Mary Joseph nodules also have been reported in several other types of solid organ tumors.5
Pyogenic granuloma is a benign vascular lesion that classically develops rapidly over the course of a few weeks. It often presents as a single red, moist, friable papule with a collarette of scale and frequently is associated with pain, bleeding, and ulceration. Keratinized skin or mucosa can be affected, and pyogenic granuloma is most common in children and young adults.6
The Diagnosis: Foreign Body Reaction With Sinus Tract
A delayed foreign body reaction is a rare complication of retained temporary pacing wires following cardiovascular surgery. These epicardial pacing wires are important for the management of postoperative arrhythmia and normally are removed by external traction after normal rhythm has been re-established. However, it is not uncommon for these wires to be cut at the skin surface in the setting of difficult removal, as retained pacing wires generally are viewed as benign.1 These reactions often take years after placement of the pacing wire to present themselves and most often resolve with either complete removal of the wire or resection of the distal end.2
Our patient was referred to dermatology and underwent a shave biopsy. Results were consistent with chronic inflammatory granulation tissue. Bacterial tissue culture grew Staphylococcus epidermidis. Cultures for acid-fast bacilli and fungi were negative. The patient was referred to cardiothoracic surgery. Computed tomography identified a retained temporary pacing wire extending to the base of the lesion. The lesion was excised and the distal aspect of the pacing wire was removed, which resulted in resolution of the nodule.
The differential diagnosis includes pyoderma gangrenosum, nodular basal cell carcinoma, Sister Mary Joseph nodule, and pyogenic granuloma. Pyoderma gangrenosum is a neutrophilic dermatosis that presents as a rapidly progressing, painful, necrotic ulcer. It is classically associated with inflammatory bowel disease and other systemic diseases but also can occur in isolation.3
Nodular basal cell carcinomas often develop in chronically sun-exposed areas of the body. Morphologically, they present as pink, pearl appearing papules with rolled borders and overlying arborizing telangiectasia. Nodular basal cell carcinomas may present with recurrent bleeding but typically do not have continuous drainage.4
Sister Mary Joseph nodule represents a periumbilical lymphatic metastasis from an underlying (usually intra-abdominal) malignancy. It typically presents as an umbilical or periumbilical nodule measuring 0.5 to 15 cm in diameter. The nodules often are painful and discharge a serous fluid. It is estimated that they are present in 1% to 3% of cases of abdominopelvic malignancy, but Sister Mary Joseph nodules also have been reported in several other types of solid organ tumors.5
Pyogenic granuloma is a benign vascular lesion that classically develops rapidly over the course of a few weeks. It often presents as a single red, moist, friable papule with a collarette of scale and frequently is associated with pain, bleeding, and ulceration. Keratinized skin or mucosa can be affected, and pyogenic granuloma is most common in children and young adults.6
- Chung DA, Smith EE. Delayed presentation of foreign body reaction secondary to retained pacing wires. Ann Thorac Surg. 1998;66:550-551.
- Gentry WH, Hassan AA. Complications of retained epicardial pacing wires (an unusual bronchial foreign body. Ann Thorac Surg. 1993;56:1391-1393.
- Ahn C, Negus D, Huang W. Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol. 2018;14:225-233.
- Tanese K. Diagnosis and treatment of basal cell carcinoma. Curr Treat Options Oncol. 2019;20:13
- Tso S, Brockley J, Recica H, et al. Sister Mary Joseph's nodule: an unusual but important physical finding characteristic of widespread internal malignancy. Br J Gen Pract. 2013;63:551-552.
- Mashiah J, Hadj-Rabia S, Slodownik D, et al. Effectiveness of topical propranolol 4% gel in the treatment of pyogenic granuloma in children. J Dermatol. 2019;46:245-248.
- Chung DA, Smith EE. Delayed presentation of foreign body reaction secondary to retained pacing wires. Ann Thorac Surg. 1998;66:550-551.
- Gentry WH, Hassan AA. Complications of retained epicardial pacing wires (an unusual bronchial foreign body. Ann Thorac Surg. 1993;56:1391-1393.
- Ahn C, Negus D, Huang W. Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol. 2018;14:225-233.
- Tanese K. Diagnosis and treatment of basal cell carcinoma. Curr Treat Options Oncol. 2019;20:13
- Tso S, Brockley J, Recica H, et al. Sister Mary Joseph's nodule: an unusual but important physical finding characteristic of widespread internal malignancy. Br J Gen Pract. 2013;63:551-552.
- Mashiah J, Hadj-Rabia S, Slodownik D, et al. Effectiveness of topical propranolol 4% gel in the treatment of pyogenic granuloma in children. J Dermatol. 2019;46:245-248.
A 71-year-old man presented with an inflamed erythematous papule on the right subcostal region of 12 months’ duration. It began as a small pimplelike bump that slowly enlarged. The patient did not report any pain or pruritus, but the lesion intermittently drained purulent fluid. The patient had a pacemaker and a history of severe aortic stenosis for which he underwent bioprosthetic aortic valve repair approximately 3 years prior to presentation. His postoperative course was complicated by sternal wound infection and sepsis, prompting surgical replacement of the graft and the pacemaker. He then developed aortitis secondary to bacterial endocarditis with multiple associated septic emboli and is now on lifelong levofloxacin and minocycline therapy. Physical examination revealed a 1.5-cm, erythematous, soft, protuberant nodule with surrounding skin dimpling on the right subcostal region adjacent to a well-healed surgical scar. Approximately 1 to 2 mL of purulent fluid was expressed.
Approximation of Alcohol-Based Hand Sanitizer Volume Using a Toothpaste Cap
Practice Gap
The Centers for Disease Control and Prevention recommends handwashing with soap and water or using alcohol-based hand sanitizers to prevent transmission of coronavirus disease 2019. Five steps are delineated for effective handwashing: wetting, lathering, scrubbing, rinsing, and drying. Although alcohol-based sanitizers may be perceived as more damaging to the skin, they are less likely to cause dermatitis than handwashing with soap and water.1 Instructions are precise for handwashing, while there are no recommendations for effective use of alcohol-based hand sanitizers. A common inquiry regarding alcohol-based hand sanitizers is the volume needed for efficacy without causing skin irritation.
The Technique
Approximately 1 mL of alcohol-based hand sanitizer is recommended by some manufacturers. However, abundant evidence refutes this recommendation, including a study that tested the microbial efficacy of alcohol-based sanitizers by volume. A volume of 2 mL was necessary to achieve the 2.0 log reduction of contaminants as required by the US Food and Drug Administration for antimicrobial efficacy.2 The precise measurement of hand sanitizer using a calibrated syringe before each use is impractical. Thus, we recommend using a screw-top toothpaste cap to assist in approximating the necessary volume (Figure). The cap holds approximately 1 mL of liquid as measured using a syringe; therefore, 2 caps filled with sanitizer should be used.

Practice Implications
The general public may be underutilizing hand sanitizer due to fear of excessive skin irritation or supply shortages, which will reduce efficacy. Patients and physicians can use this simple visual approximation to ensure adequate use of hand sanitizer volume.
- Stutz N, Becker D, Jappe U, et al. Nurses’ perceptions of the benefits and adverse effects of hand disinfection: alcohol-based hand rubs vs. hygienic handwashing: a multicentre questionnaire study with additional patch testing by the German Contact Dermatitis Research Group. Br J Dermatol. 2009;160:565-572.
- Kampf G, Ruselack S, Eggerstedt S, et al. Less and less-influence of volume on hand coverage and bactericidal efficacy in hand disinfection. BMC Infect Dis. 2013;13:472.
Practice Gap
The Centers for Disease Control and Prevention recommends handwashing with soap and water or using alcohol-based hand sanitizers to prevent transmission of coronavirus disease 2019. Five steps are delineated for effective handwashing: wetting, lathering, scrubbing, rinsing, and drying. Although alcohol-based sanitizers may be perceived as more damaging to the skin, they are less likely to cause dermatitis than handwashing with soap and water.1 Instructions are precise for handwashing, while there are no recommendations for effective use of alcohol-based hand sanitizers. A common inquiry regarding alcohol-based hand sanitizers is the volume needed for efficacy without causing skin irritation.
The Technique
Approximately 1 mL of alcohol-based hand sanitizer is recommended by some manufacturers. However, abundant evidence refutes this recommendation, including a study that tested the microbial efficacy of alcohol-based sanitizers by volume. A volume of 2 mL was necessary to achieve the 2.0 log reduction of contaminants as required by the US Food and Drug Administration for antimicrobial efficacy.2 The precise measurement of hand sanitizer using a calibrated syringe before each use is impractical. Thus, we recommend using a screw-top toothpaste cap to assist in approximating the necessary volume (Figure). The cap holds approximately 1 mL of liquid as measured using a syringe; therefore, 2 caps filled with sanitizer should be used.

Practice Implications
The general public may be underutilizing hand sanitizer due to fear of excessive skin irritation or supply shortages, which will reduce efficacy. Patients and physicians can use this simple visual approximation to ensure adequate use of hand sanitizer volume.
Practice Gap
The Centers for Disease Control and Prevention recommends handwashing with soap and water or using alcohol-based hand sanitizers to prevent transmission of coronavirus disease 2019. Five steps are delineated for effective handwashing: wetting, lathering, scrubbing, rinsing, and drying. Although alcohol-based sanitizers may be perceived as more damaging to the skin, they are less likely to cause dermatitis than handwashing with soap and water.1 Instructions are precise for handwashing, while there are no recommendations for effective use of alcohol-based hand sanitizers. A common inquiry regarding alcohol-based hand sanitizers is the volume needed for efficacy without causing skin irritation.
The Technique
Approximately 1 mL of alcohol-based hand sanitizer is recommended by some manufacturers. However, abundant evidence refutes this recommendation, including a study that tested the microbial efficacy of alcohol-based sanitizers by volume. A volume of 2 mL was necessary to achieve the 2.0 log reduction of contaminants as required by the US Food and Drug Administration for antimicrobial efficacy.2 The precise measurement of hand sanitizer using a calibrated syringe before each use is impractical. Thus, we recommend using a screw-top toothpaste cap to assist in approximating the necessary volume (Figure). The cap holds approximately 1 mL of liquid as measured using a syringe; therefore, 2 caps filled with sanitizer should be used.

Practice Implications
The general public may be underutilizing hand sanitizer due to fear of excessive skin irritation or supply shortages, which will reduce efficacy. Patients and physicians can use this simple visual approximation to ensure adequate use of hand sanitizer volume.
- Stutz N, Becker D, Jappe U, et al. Nurses’ perceptions of the benefits and adverse effects of hand disinfection: alcohol-based hand rubs vs. hygienic handwashing: a multicentre questionnaire study with additional patch testing by the German Contact Dermatitis Research Group. Br J Dermatol. 2009;160:565-572.
- Kampf G, Ruselack S, Eggerstedt S, et al. Less and less-influence of volume on hand coverage and bactericidal efficacy in hand disinfection. BMC Infect Dis. 2013;13:472.
- Stutz N, Becker D, Jappe U, et al. Nurses’ perceptions of the benefits and adverse effects of hand disinfection: alcohol-based hand rubs vs. hygienic handwashing: a multicentre questionnaire study with additional patch testing by the German Contact Dermatitis Research Group. Br J Dermatol. 2009;160:565-572.
- Kampf G, Ruselack S, Eggerstedt S, et al. Less and less-influence of volume on hand coverage and bactericidal efficacy in hand disinfection. BMC Infect Dis. 2013;13:472.
Biologic responses to metal implants: Dermatologic implications
Hypersensitivity to implantable devices, albeit rare, is a growing problem. according to a report on biological responses to metal implants released by the Food and Drug Administration in September 2019. Large controlled studies are lacking, and the FDA has initiated extensive postmarketing reviews of certain metal implants in response to safety concerns. Further research is needed on the composition of these implants, the diverse spectrum of metals used, the physical environment in which they are implanted, and the immune response associated with implants.
Local and systemic type IV hypersensitivity reactions can result from exposure to metal ions, which are thought to act as haptens and bind to proteins. The hapten-protein complex acts as the antigen for the T cell. Additionally, both acute and chronic inflammatory responses secondary to wound healing and foreign body reactions can occur. Neutrophils and macrophages elicit a tissue response, which can cause aseptic infection, loosening of joints, and tissue damage. Furthermore, corrosion of metal implants can lead to release of metal ions, which can have genotoxic and carcinogenic effects.
Clinical and subclinical effects of implantable devices depend on the device itself, the composition of the device, the tissue type, and an individual’s immune characteristics. Metal debris released from implants can activate innate and adaptive immune responses through a variety of different mechanisms, depending on the implant type and in what tissues the implant is placed. In the case of orthopedic implants, the most common implants, osteoclasts can sense metal and induce proinflammatory cytokines, which can result in corrosion and uptake of metal particles. Metal devices used in the central nervous system, such as intracerebral electrodes, can cause inflammatory responses leading to tissue encapsulation of electrodes. Corrosion of electrodes and release of metal ions can also impede ion channels in the CNS, blocking critical neuron-signaling pathways. Inflammatory reactions surrounding cardiac and vascular implants containing metal activate coagulation cascades, resulting in endothelial injury and activation of thrombi.
Despite the commonly used term “metal allergy” that delineates a type IV hypersensitivity reaction, reports in the literature supports the existence of both innate and adaptive immune responses to metal implanted in tissues. The recommended terminology is “adverse reactions to metal debris.” The clinical presentation may not be straightforward or easily attributed to the implant. Diagnostic tools are limited and may not detect a causal relationship.
Clinical symptoms can range from local rashes and pruritus to cardiac damage, depression, vertigo, and neurologic symptoms; autoimmune/autoinflammatory reactions including chronic fatigue and autoimmune-like systemic symptoms, such as joint pain, headaches, and hair loss, have also been reported in association with implants containing metal. In addition to pruritus, dermatologic manifestations can include erythema, edema, papules, vesicles, as well as systemic hypersensitivity reactions. Typically, cutaneous reactions usually present within 2 days to 24 months of implantation and may be considered surgical-site infections. Although these reactions can be treated with topical or oral corticosteroids, removal of the device is frequently needed for complete clearance.
In clinical practice, it has been frustrating that potential adverse reactions to metal implants are often overlooked because they are thought to be so rare. There are case series documenting metal implant hypersensitivity, but the actual prevalence of hypersensitivity or autoinflammatory reactions is not known. Testing methods are often inaccurate; therefore, identification of at-risk individuals and management of symptomatic patients with implants is important.
The 2016 American Contact Dermatitis Society guidelines do not recommend preimplantation patch testing unless there is a suspected metal allergy. However, patch testing cannot identify the extent of corrosion, autoinflammatory reactions, and foreign body reactions that can occur.
We must keep an open mind in patients who have implanted devices and have unusual or otherwise undefined symptoms. Often, the symptoms do not directly correspond to the site of implantation and the only way to discern whether the implant is the cause and to treat symptoms is removal of the implanted device.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
Food and Drug Administration. Biological Responses to Metal Implants. 2019 Sep. https://www.fda.gov/media/131150/download.
Atwater AR, Reeder M. Cutis. 2020 Feb;105(2):68-70.
Schalock PC et al. Dermatitis. Sep-Oct 2016;27(5):241-7.
Hypersensitivity to implantable devices, albeit rare, is a growing problem. according to a report on biological responses to metal implants released by the Food and Drug Administration in September 2019. Large controlled studies are lacking, and the FDA has initiated extensive postmarketing reviews of certain metal implants in response to safety concerns. Further research is needed on the composition of these implants, the diverse spectrum of metals used, the physical environment in which they are implanted, and the immune response associated with implants.
Local and systemic type IV hypersensitivity reactions can result from exposure to metal ions, which are thought to act as haptens and bind to proteins. The hapten-protein complex acts as the antigen for the T cell. Additionally, both acute and chronic inflammatory responses secondary to wound healing and foreign body reactions can occur. Neutrophils and macrophages elicit a tissue response, which can cause aseptic infection, loosening of joints, and tissue damage. Furthermore, corrosion of metal implants can lead to release of metal ions, which can have genotoxic and carcinogenic effects.
Clinical and subclinical effects of implantable devices depend on the device itself, the composition of the device, the tissue type, and an individual’s immune characteristics. Metal debris released from implants can activate innate and adaptive immune responses through a variety of different mechanisms, depending on the implant type and in what tissues the implant is placed. In the case of orthopedic implants, the most common implants, osteoclasts can sense metal and induce proinflammatory cytokines, which can result in corrosion and uptake of metal particles. Metal devices used in the central nervous system, such as intracerebral electrodes, can cause inflammatory responses leading to tissue encapsulation of electrodes. Corrosion of electrodes and release of metal ions can also impede ion channels in the CNS, blocking critical neuron-signaling pathways. Inflammatory reactions surrounding cardiac and vascular implants containing metal activate coagulation cascades, resulting in endothelial injury and activation of thrombi.
Despite the commonly used term “metal allergy” that delineates a type IV hypersensitivity reaction, reports in the literature supports the existence of both innate and adaptive immune responses to metal implanted in tissues. The recommended terminology is “adverse reactions to metal debris.” The clinical presentation may not be straightforward or easily attributed to the implant. Diagnostic tools are limited and may not detect a causal relationship.
Clinical symptoms can range from local rashes and pruritus to cardiac damage, depression, vertigo, and neurologic symptoms; autoimmune/autoinflammatory reactions including chronic fatigue and autoimmune-like systemic symptoms, such as joint pain, headaches, and hair loss, have also been reported in association with implants containing metal. In addition to pruritus, dermatologic manifestations can include erythema, edema, papules, vesicles, as well as systemic hypersensitivity reactions. Typically, cutaneous reactions usually present within 2 days to 24 months of implantation and may be considered surgical-site infections. Although these reactions can be treated with topical or oral corticosteroids, removal of the device is frequently needed for complete clearance.
In clinical practice, it has been frustrating that potential adverse reactions to metal implants are often overlooked because they are thought to be so rare. There are case series documenting metal implant hypersensitivity, but the actual prevalence of hypersensitivity or autoinflammatory reactions is not known. Testing methods are often inaccurate; therefore, identification of at-risk individuals and management of symptomatic patients with implants is important.
The 2016 American Contact Dermatitis Society guidelines do not recommend preimplantation patch testing unless there is a suspected metal allergy. However, patch testing cannot identify the extent of corrosion, autoinflammatory reactions, and foreign body reactions that can occur.
We must keep an open mind in patients who have implanted devices and have unusual or otherwise undefined symptoms. Often, the symptoms do not directly correspond to the site of implantation and the only way to discern whether the implant is the cause and to treat symptoms is removal of the implanted device.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
Food and Drug Administration. Biological Responses to Metal Implants. 2019 Sep. https://www.fda.gov/media/131150/download.
Atwater AR, Reeder M. Cutis. 2020 Feb;105(2):68-70.
Schalock PC et al. Dermatitis. Sep-Oct 2016;27(5):241-7.
Hypersensitivity to implantable devices, albeit rare, is a growing problem. according to a report on biological responses to metal implants released by the Food and Drug Administration in September 2019. Large controlled studies are lacking, and the FDA has initiated extensive postmarketing reviews of certain metal implants in response to safety concerns. Further research is needed on the composition of these implants, the diverse spectrum of metals used, the physical environment in which they are implanted, and the immune response associated with implants.
Local and systemic type IV hypersensitivity reactions can result from exposure to metal ions, which are thought to act as haptens and bind to proteins. The hapten-protein complex acts as the antigen for the T cell. Additionally, both acute and chronic inflammatory responses secondary to wound healing and foreign body reactions can occur. Neutrophils and macrophages elicit a tissue response, which can cause aseptic infection, loosening of joints, and tissue damage. Furthermore, corrosion of metal implants can lead to release of metal ions, which can have genotoxic and carcinogenic effects.
Clinical and subclinical effects of implantable devices depend on the device itself, the composition of the device, the tissue type, and an individual’s immune characteristics. Metal debris released from implants can activate innate and adaptive immune responses through a variety of different mechanisms, depending on the implant type and in what tissues the implant is placed. In the case of orthopedic implants, the most common implants, osteoclasts can sense metal and induce proinflammatory cytokines, which can result in corrosion and uptake of metal particles. Metal devices used in the central nervous system, such as intracerebral electrodes, can cause inflammatory responses leading to tissue encapsulation of electrodes. Corrosion of electrodes and release of metal ions can also impede ion channels in the CNS, blocking critical neuron-signaling pathways. Inflammatory reactions surrounding cardiac and vascular implants containing metal activate coagulation cascades, resulting in endothelial injury and activation of thrombi.
Despite the commonly used term “metal allergy” that delineates a type IV hypersensitivity reaction, reports in the literature supports the existence of both innate and adaptive immune responses to metal implanted in tissues. The recommended terminology is “adverse reactions to metal debris.” The clinical presentation may not be straightforward or easily attributed to the implant. Diagnostic tools are limited and may not detect a causal relationship.
Clinical symptoms can range from local rashes and pruritus to cardiac damage, depression, vertigo, and neurologic symptoms; autoimmune/autoinflammatory reactions including chronic fatigue and autoimmune-like systemic symptoms, such as joint pain, headaches, and hair loss, have also been reported in association with implants containing metal. In addition to pruritus, dermatologic manifestations can include erythema, edema, papules, vesicles, as well as systemic hypersensitivity reactions. Typically, cutaneous reactions usually present within 2 days to 24 months of implantation and may be considered surgical-site infections. Although these reactions can be treated with topical or oral corticosteroids, removal of the device is frequently needed for complete clearance.
In clinical practice, it has been frustrating that potential adverse reactions to metal implants are often overlooked because they are thought to be so rare. There are case series documenting metal implant hypersensitivity, but the actual prevalence of hypersensitivity or autoinflammatory reactions is not known. Testing methods are often inaccurate; therefore, identification of at-risk individuals and management of symptomatic patients with implants is important.
The 2016 American Contact Dermatitis Society guidelines do not recommend preimplantation patch testing unless there is a suspected metal allergy. However, patch testing cannot identify the extent of corrosion, autoinflammatory reactions, and foreign body reactions that can occur.
We must keep an open mind in patients who have implanted devices and have unusual or otherwise undefined symptoms. Often, the symptoms do not directly correspond to the site of implantation and the only way to discern whether the implant is the cause and to treat symptoms is removal of the implanted device.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
Food and Drug Administration. Biological Responses to Metal Implants. 2019 Sep. https://www.fda.gov/media/131150/download.
Atwater AR, Reeder M. Cutis. 2020 Feb;105(2):68-70.
Schalock PC et al. Dermatitis. Sep-Oct 2016;27(5):241-7.
Tattoo Hypersensitivity Reactions: Inky Business
Sometimes regrettable yet increasingly common, tattoos are an ancient art form used in modern times as a mark of artistic and cultural expression. Allergic contact dermatitis (ACD) to tattoo ink is rare, but the popularity of tattoos makes ACD an increasingly recognized occurrence. In a retrospective study of 38,543 patch-tested patients, only 29 (0.08%) had tattoo-related ACD, with the majority of patients being female and young adults. The most common contact allergy was to paraphenylenediamine (PPD), which occurred in 22 (76%) patients.1 In this article, we will walk you through the rainbow of tattoo ACD, covering hypersensitivity reactions to both temporary and permanent tattoo inks.
Temporary Tattoo Inks
Henna is the most common temporary tattoo ink. Derived from the plant Lawsonia inermis, henna is an orange dye that has been used in many parts of the world, particularly in Islamic and Hindu cultures, to dye skin, hair, and fabrics. Application of henna tattoos is common for weddings and other celebrations, and brides may wear elaborate henna patterns. To create these tattoos, henna powder is mixed with water and sometimes essential oils and is then applied to the skin for several hours. After application, the henna pigment lawsone (2-hydroxy-1,4-naphthoquinone) interacts with keratin and leaves a red-orange stain on the skin2; longer application time leads to a deeper color. Most traditional cutaneous henna designs fade in 2 to 6 weeks, but some last longer. Red henna generally is considered safe with low incidence of contact allergy. What is referred to as black henna usually is red henna mixed with PPD, a black dye, which is added to deepen the color. Paraphenylenediamine is highly sensitizing; patients can become sensitized to the PPD in the tattoo itself.2 One study confirmed the presence of PPD in black henna tattoos, with chemical analysis of common preparations revealing concentrations ranging from less than 1% to 30%.2 Patients who undergo patch testing for tattoo reactions often are strongly positive to PPD and have concomitant reactions to azo dyes, black rubber, and anesthetics. Other aromatic amines including aminophenols have been identified in black henna tattoo ink, and these chemicals also may contribute to ACD.3 Less common sources of contact allergy from temporary black henna tattoos include resorcinol,4 para-tertiary butylphenol formaldehyde resin,5 and fragrance.6
Clinically, ACD to PPD in temporary tattoos presents 1 to 3 days after application if the patient is already sensitized or 4 to 14 days if the patient is sensitized by the tattoo ink.2 Most patients notice erythema, edema, vesicles, papules, and/or bullae, but other less common reactions including generalized dermatitis, systemic symptoms, urticaria, and pustules have been described.2 Postinflammatory hypopigmentation or hyperpigmentation also can occur.
Because of the sensitizing nature of black henna tattoos, consumers are turning to natural temporary tattoos. Jagua temporary tattoos, with pigment derived from the sap of fruit from the Genipa americana tree, have been associated with ACD.7 This black dye is applied and washed off in a similar fashion to henna tattoos. Importantly, a recent analysis of jagua dye identified no PPD. In one case, a patient who developed ACD to a jagua tattoo was patch tested to components of the dye and had a positive reaction to genipin, a component of the fruit extract.7 Thus, jagua tattoos often are marketed as safe but are an emerging source of contact dermatitis to temporary tattoos.
Permanent Tattoo Inks
Permanent tattoos are created by injecting small amounts of ink into the dermis. As the name suggests, these tattoos are permanent. Tattoos are common; nearly one-third of Americans have at least 1 tattoo.1 Historically, tattoos were created using black pigment composed of amorphous carbon or black iron oxides.8,9 Metallic pigments (eg, mercury, chromium, cobalt, cadmium) were once used to add color to tattoos, but these metals are now only rarely used; in fact, a 2019 study of tattoo ink components identified 44 distinct pigments in 1416 permanent inks, with an average of 3 pigments per ink.8 Of the 44 pigments, 10 had metallic components including iron, barium, zinc, copper, molybdenum, and titanium. The remaining 34 pigments contained carbon, azo, diketopyrrolopyrrole, quinacridone, anthraquinone, dioxazine (purple), or quinophthalone (yellow) dyes. The authors noted that nearly one-quarter of the tattoo pigments identified in their study had been reported as contact allergens.8
Typically, reactions to permanent tattoo inks manifest as an eczematous dermatitis occurring weeks to years after tattoo application.9,10 The dermatitis usually is locally confined to the tattoo and may be limited to particular colors; occasionally, a new tattoo reaction may trigger concurrent inflammation in older tattoos. Many tattoo reactions occur as a response to red pigment but also have occurred with other tattoo ink components.9 Many researchers have speculated as to whether the reaction is related to the ink component itself or from the photochemical breakdown of the ink by exposure to UV radiation and/or laser therapy.9
Red Pigment
Red ink is the most common color reported to cause tattoo hypersensitivity reactions. Historically, red tattoo pigments include mercuric sulfide (vermilion, cinnabar), scarlet lake, cadmium red, carmine, and cochineal,11 but today’s tattoo inks primarily are composed of other pigments, such as quinacridone and azo dyes.12 Several cases of red tattoo ink hypersensitivity reactions exist in the literature, many without completion of patch tests or without positive patch tests to relevant red pigments.11-15
Black Pigment
In general, reactions to permanent black tattoo ink are rare; however, a few case reports exist. Black pigment can be created with India ink (carbon), logwood (chrome), iron oxide, and titanium.16,17 Shellac can be used as a binding agent in tattoo ink; there is at least one report of a reaction to black tattoo ink with a positive patch test to shellac and the original black ink.18
Metals
When utilized in tattoos, metals can create a variety of colors; several have been reported to cause ACD. There has been at least one reported case of a tattoo hypersensitivity reaction to a gold tattoo, with positive patch testing for gold sodium thiosulfate.19 Green tattoo inks also have been confirmed to contain metal. One case of nickel allergy from a green tattoo has been reported, with a positive patch test for nickel sulfate and tissue confirmation of the presence of nickel with micro X-ray fluorescence and laser ablation inductively coupled plasma mass spectrometry.20 Another case series described 3 patients with pruritus and chronic dermatitis associated with green tattoos who had positive patch tests to potassium dichromate, and the green tattoo pigment flared during patch testing. Chromium oxide was presumed to be present in the green tattoo pigment, and potassium dichromate avoidance in products and food improved both the pruritus and dermatitis.21
Azo Pigments
Azo pigments frequently are used in modern tattoos due to their vibrant colors. One case of hypersensitivity to azo pigment involved an eczematous ulcerated plaque overlying yellow, red, and green ink in a recently applied tattoo. Patch testing with the inks originally used in the tattoo was negative. The authors noted that the 3 problematic ink colors all contained pigment yellow 65—an azo pigment—and attributed the reaction to this dye.22 In another azo reaction, a patient had erythema and pruritus overlying a tattoo applied 1 month prior. Patch testing was positive for aminoazobenzene, an azo pigment that was present in the orange ink of the tattoo.23
Management of Tattoo Hypersensitivity Reactions
Hypersensitivity reactions to temporary tattoos are just that—temporary. Topical steroids and time generally will allow these reactions to resolve. In the setting of vigorous reactions, patients may develop postinflammatory hypopigmentation or hyperpigmentation that may last for months. Unfortunately, bullous tattoo reactions can lead to scarring and keloid formation, requiring more aggressive therapy.
Management of reactions to permanent tattoos is more challenging. High-potency topical steroids under occlusion or intralesional corticosteroid injections may aid in treating pruritus or discomfort. For severe reactions, oral corticosteroids may be required. Patients also may consider laser tattoo removal; however, providers should be aware that there have been rare reports of systemic urticarial reactions from this procedure.24,25 Obviously limited by location and size, excision also may be offered.
Patch Testing for Tattoo Ink Contact Allergy
When patients present for evaluation and management of tattoo ACD, it is important to also consider other causes, including granulomatous tattoo reaction, pseudolymphoma, and lichenoid tattoo reaction. A biopsy can be helpful if the diagnosis is in question.
Patch testing for contact allergy to temporary tattoo inks should include PPD, fragrance, aminophenols, resorcinol, para-tertiary butylphenol formaldehyde, and essential oils. Jagua currently is not available for commercial purchase but also should be considered if the patient has the original product or in research settings. If the individual tattoo ingredients can be identified, they also should be tested. In this scenario, recall reactions may occur; testing with the tattoo paste should be avoided if the prior reaction was severe. Importantly, patients with a PPD allergy should be counseled to avoid hair dyes that contain PPD. Many patients who are sensitized to PPD have strong reactions on patch testing and are at risk for severe reactions if PPD or PPD-related compounds are encountered in hair dye.
Patch testing for ACD to permanent tattoos is complex. In most cases, patch testing is of limited utility because many of the chemicals that have been reported to cause ACD are not commercially available. Additionally, a 2014 study of 90 patients with chronic tattoo reactions found that the majority had negative patch testing to the European baseline series (66%), disperse dyes (87%), and tattoo inks (87%–92%). The investigators theorized that the allergens causing tattoo reactions are formed by haptenization of “parent” chemicals in the dermis, meaning application of chemicals present in the original tattoo ink may not identify the relevant allergen.26 If patch testing is performed, it is most ideal if individual pigment ingredients can be identified. Allergens to be considered for testing include azo dyes, aromatic amines, iron oxide, barium, zinc, copper, molybdenum, titanium, gold sodium thiosulfate, nickel sulfate, carbon, shellac, diketopyrrolopyrrole, quinacridone, anthraquinone, dioxazine (purple), quinophthalone (yellow) dyes, mercuric sulfide (vermilion, cinnabar), scarlet lake, cadmium red, carmine, and cochineal, many of which are not commercially available for purchase.
Final Interpretation
As tattoos become increasingly trendy, tattoo ACD should be recognized by the astute dermatologist. The most common allergen associated with tattoo ACD is PPD, but other potential allergens include azo dyes and newer pigments. Unlike tattoos of the past, today’s inks are unlikely to contain toxic metals. Diagnosing ACD caused by permanent tattoo inks requires a high degree of suspicion, as patch testing may be of limited utility.
- Warshaw EM, Schlarbaum JP, Taylor JS, et al. Allergic reactions to tattoos: retrospective analysis of North American Contact Dermatitis Group data, 2001-2016. J Am Acad Dermatol. 2020;82:E61-E62.
- de Groot AC. Side-effects of henna and semi-permanent ‘black henna’ tattoos: a full review. Contact Dermatitis. 2013;69:1-25.
- Romita P, Foti C, Mascia P, et al. Eyebrow allergic contact dermatitis caused by m-aminophenol and toluene-2,5-diamine secondary to a temporary black henna tattoo. Contact Dermatitis. 2018;79:51-52.
- Ormerod E, Hughes TM, Stone N. Allergic contact dermatitis caused by resorcinol following a temporary black henna tattoo. Contact Dermatitis. 2017;77:187-188.
- Rodrigo-Nicolás B, de la Cuadra J, Sierra C, et al. Contact dermatitis from a temporary tattoo in a boy with contact allergy to p-tert butyl phenol formaldehyde resin. Dermatitis. 2014;25:37-38.
- Temesvári E, Podányi B, Pónyai G, et al. Fragrance sensitization caused by temporary henna tattoo. Contact Dermatitis. 2002;47:240.
- Bircher AJ, Scherer Hofmeier K, Schlegel U, et al. Genipin in temporary jagua tattoos—black dye causing severe allergic dermatitis. Dermatitis. 2019;30:375-376.
- Liszewski W, Warshaw EM. Pigments in American tattoo inks and their propensity to elicit allergic contact dermatitis. J Am Acad Dermatol. 2019;81:379-385.
- Serup J, Hutton Carlsen K, Dommershausen N, et al. Identification of pigments related to allergic tattoo reactions in 104 human skin biopsies. Contact Dermatitis. 2020;82:73-82.
- Bjerre RD, Ulrich NH, Linneberg A, et al. Adverse reactions to tattoos in the general population of Denmark. J Am Acad Dermatol. 2018;79:770-772.
- Bhardwaj SS, Brodell RT, Taylor JS. Red tattoo reactions. Contact Dermatitis. 2003;48:236-237.
- Gaudron S, Ferrier-Le Bouëdec MC, Franck F, et al. Azo pigments and quinacridones induce delayed hypersensitivity in red tattoos. Contact Dermatitis. 2015;72:97-105.
- de Winter RW, van der Bent SAS, van Esch M, et al. Allergic reaction to red cosmetic lip tattoo treated with hydroxychloroquine. Dermatitis. 2019;30:82-83.
- Greve B, Chytry R, Raulin C. Contact dermatitis from red tattoo pigment (quinacridone) with secondary spread. Contact Dermatitis. 2003;49:265-266.
- Ruiz-Villaverde R, Fernandez-Crehuet P, Aguayo-Carreras P, et al. Inflammatory reactions to red tattoo inks: three cases highlighting an emerging problem. Sultan Qaboos Univ Med J. 2018;18:E215-E218.
- Gallo R, Parodi A, Cozzani E, et al. Allergic reaction to India ink in a black tattoo. Contact Dermatitis. 1998;38:346-347.
- de Cuyper C, Lodewick E, Schreiver I, et al. Are metals involved in tattoo-related hypersensitivity reactions? a case report. Contact Dermatitis. 2017;77:397-405.
- González-Villanueva I, Hispán Ocete P, Silvestre Salvador JF. Allergic contact dermatitis caused by a black tattoo ink in a patient allergic to shellac. Contact Dermatitis. 2016;75:247-248.
- Tammaro A, Tuchinda P, Persechino S, et al. Contact allergic dermatitis to gold in a tattoo: a case report. Int J Immunopathol Pharmacol. 2011;24:1111-1113.
- van der Bent SAS, Berg T, Karst U, et al. Allergic reaction to a green tattoo with nickel as a possible allergen. Contact Dermatitis. 2019;81:64-66.
- Jacob SE, Castanedo-Tardan MP, Blyumin ML. Inflammation in green (chromium) tattoos during patch testing. Dermatitis. 2008;19:E33-E34.
- González-Villanueva I, Álvarez-Chinchilla P, Silvestre JF. Allergic reaction to 3 tattoo inks containing pigment yellow 65. Contact Dermatitis. 2018;79:107-108.
- Tammaro A, De Marco G, D’Arino A, et al. Aminoazobenzene in tattoo: another case of allergic contact dermatitis. Int J Dermatol. 2017;56:E79-E81.
- Willardson HB, Kobayashi TT, Arnold JG, et al. Diffuse urticarial reaction associated with titanium dioxide following laser tattoo removal treatments. Photomed Laser Surg. 2017;35:176‐180.
- England RW, Vogel P, Hagan L. Immediate cutaneous hypersensitivity after treatment of tattoo with Nd:YAG laser: a case report and review of the literature. Ann Allergy Asthma Immunol. 2002;89:215‐217.
- Serup J, Carlsen KH. Patch test study of 90 patients with tattoo reactions: negative outcome of allergy patch test to baseline batteries and culprit inks suggests allergen(s) are generated in the skin through haptenization. Contact Dermatitis. 2014;71:255-263.
Sometimes regrettable yet increasingly common, tattoos are an ancient art form used in modern times as a mark of artistic and cultural expression. Allergic contact dermatitis (ACD) to tattoo ink is rare, but the popularity of tattoos makes ACD an increasingly recognized occurrence. In a retrospective study of 38,543 patch-tested patients, only 29 (0.08%) had tattoo-related ACD, with the majority of patients being female and young adults. The most common contact allergy was to paraphenylenediamine (PPD), which occurred in 22 (76%) patients.1 In this article, we will walk you through the rainbow of tattoo ACD, covering hypersensitivity reactions to both temporary and permanent tattoo inks.
Temporary Tattoo Inks
Henna is the most common temporary tattoo ink. Derived from the plant Lawsonia inermis, henna is an orange dye that has been used in many parts of the world, particularly in Islamic and Hindu cultures, to dye skin, hair, and fabrics. Application of henna tattoos is common for weddings and other celebrations, and brides may wear elaborate henna patterns. To create these tattoos, henna powder is mixed with water and sometimes essential oils and is then applied to the skin for several hours. After application, the henna pigment lawsone (2-hydroxy-1,4-naphthoquinone) interacts with keratin and leaves a red-orange stain on the skin2; longer application time leads to a deeper color. Most traditional cutaneous henna designs fade in 2 to 6 weeks, but some last longer. Red henna generally is considered safe with low incidence of contact allergy. What is referred to as black henna usually is red henna mixed with PPD, a black dye, which is added to deepen the color. Paraphenylenediamine is highly sensitizing; patients can become sensitized to the PPD in the tattoo itself.2 One study confirmed the presence of PPD in black henna tattoos, with chemical analysis of common preparations revealing concentrations ranging from less than 1% to 30%.2 Patients who undergo patch testing for tattoo reactions often are strongly positive to PPD and have concomitant reactions to azo dyes, black rubber, and anesthetics. Other aromatic amines including aminophenols have been identified in black henna tattoo ink, and these chemicals also may contribute to ACD.3 Less common sources of contact allergy from temporary black henna tattoos include resorcinol,4 para-tertiary butylphenol formaldehyde resin,5 and fragrance.6
Clinically, ACD to PPD in temporary tattoos presents 1 to 3 days after application if the patient is already sensitized or 4 to 14 days if the patient is sensitized by the tattoo ink.2 Most patients notice erythema, edema, vesicles, papules, and/or bullae, but other less common reactions including generalized dermatitis, systemic symptoms, urticaria, and pustules have been described.2 Postinflammatory hypopigmentation or hyperpigmentation also can occur.
Because of the sensitizing nature of black henna tattoos, consumers are turning to natural temporary tattoos. Jagua temporary tattoos, with pigment derived from the sap of fruit from the Genipa americana tree, have been associated with ACD.7 This black dye is applied and washed off in a similar fashion to henna tattoos. Importantly, a recent analysis of jagua dye identified no PPD. In one case, a patient who developed ACD to a jagua tattoo was patch tested to components of the dye and had a positive reaction to genipin, a component of the fruit extract.7 Thus, jagua tattoos often are marketed as safe but are an emerging source of contact dermatitis to temporary tattoos.
Permanent Tattoo Inks
Permanent tattoos are created by injecting small amounts of ink into the dermis. As the name suggests, these tattoos are permanent. Tattoos are common; nearly one-third of Americans have at least 1 tattoo.1 Historically, tattoos were created using black pigment composed of amorphous carbon or black iron oxides.8,9 Metallic pigments (eg, mercury, chromium, cobalt, cadmium) were once used to add color to tattoos, but these metals are now only rarely used; in fact, a 2019 study of tattoo ink components identified 44 distinct pigments in 1416 permanent inks, with an average of 3 pigments per ink.8 Of the 44 pigments, 10 had metallic components including iron, barium, zinc, copper, molybdenum, and titanium. The remaining 34 pigments contained carbon, azo, diketopyrrolopyrrole, quinacridone, anthraquinone, dioxazine (purple), or quinophthalone (yellow) dyes. The authors noted that nearly one-quarter of the tattoo pigments identified in their study had been reported as contact allergens.8
Typically, reactions to permanent tattoo inks manifest as an eczematous dermatitis occurring weeks to years after tattoo application.9,10 The dermatitis usually is locally confined to the tattoo and may be limited to particular colors; occasionally, a new tattoo reaction may trigger concurrent inflammation in older tattoos. Many tattoo reactions occur as a response to red pigment but also have occurred with other tattoo ink components.9 Many researchers have speculated as to whether the reaction is related to the ink component itself or from the photochemical breakdown of the ink by exposure to UV radiation and/or laser therapy.9
Red Pigment
Red ink is the most common color reported to cause tattoo hypersensitivity reactions. Historically, red tattoo pigments include mercuric sulfide (vermilion, cinnabar), scarlet lake, cadmium red, carmine, and cochineal,11 but today’s tattoo inks primarily are composed of other pigments, such as quinacridone and azo dyes.12 Several cases of red tattoo ink hypersensitivity reactions exist in the literature, many without completion of patch tests or without positive patch tests to relevant red pigments.11-15
Black Pigment
In general, reactions to permanent black tattoo ink are rare; however, a few case reports exist. Black pigment can be created with India ink (carbon), logwood (chrome), iron oxide, and titanium.16,17 Shellac can be used as a binding agent in tattoo ink; there is at least one report of a reaction to black tattoo ink with a positive patch test to shellac and the original black ink.18
Metals
When utilized in tattoos, metals can create a variety of colors; several have been reported to cause ACD. There has been at least one reported case of a tattoo hypersensitivity reaction to a gold tattoo, with positive patch testing for gold sodium thiosulfate.19 Green tattoo inks also have been confirmed to contain metal. One case of nickel allergy from a green tattoo has been reported, with a positive patch test for nickel sulfate and tissue confirmation of the presence of nickel with micro X-ray fluorescence and laser ablation inductively coupled plasma mass spectrometry.20 Another case series described 3 patients with pruritus and chronic dermatitis associated with green tattoos who had positive patch tests to potassium dichromate, and the green tattoo pigment flared during patch testing. Chromium oxide was presumed to be present in the green tattoo pigment, and potassium dichromate avoidance in products and food improved both the pruritus and dermatitis.21
Azo Pigments
Azo pigments frequently are used in modern tattoos due to their vibrant colors. One case of hypersensitivity to azo pigment involved an eczematous ulcerated plaque overlying yellow, red, and green ink in a recently applied tattoo. Patch testing with the inks originally used in the tattoo was negative. The authors noted that the 3 problematic ink colors all contained pigment yellow 65—an azo pigment—and attributed the reaction to this dye.22 In another azo reaction, a patient had erythema and pruritus overlying a tattoo applied 1 month prior. Patch testing was positive for aminoazobenzene, an azo pigment that was present in the orange ink of the tattoo.23
Management of Tattoo Hypersensitivity Reactions
Hypersensitivity reactions to temporary tattoos are just that—temporary. Topical steroids and time generally will allow these reactions to resolve. In the setting of vigorous reactions, patients may develop postinflammatory hypopigmentation or hyperpigmentation that may last for months. Unfortunately, bullous tattoo reactions can lead to scarring and keloid formation, requiring more aggressive therapy.
Management of reactions to permanent tattoos is more challenging. High-potency topical steroids under occlusion or intralesional corticosteroid injections may aid in treating pruritus or discomfort. For severe reactions, oral corticosteroids may be required. Patients also may consider laser tattoo removal; however, providers should be aware that there have been rare reports of systemic urticarial reactions from this procedure.24,25 Obviously limited by location and size, excision also may be offered.
Patch Testing for Tattoo Ink Contact Allergy
When patients present for evaluation and management of tattoo ACD, it is important to also consider other causes, including granulomatous tattoo reaction, pseudolymphoma, and lichenoid tattoo reaction. A biopsy can be helpful if the diagnosis is in question.
Patch testing for contact allergy to temporary tattoo inks should include PPD, fragrance, aminophenols, resorcinol, para-tertiary butylphenol formaldehyde, and essential oils. Jagua currently is not available for commercial purchase but also should be considered if the patient has the original product or in research settings. If the individual tattoo ingredients can be identified, they also should be tested. In this scenario, recall reactions may occur; testing with the tattoo paste should be avoided if the prior reaction was severe. Importantly, patients with a PPD allergy should be counseled to avoid hair dyes that contain PPD. Many patients who are sensitized to PPD have strong reactions on patch testing and are at risk for severe reactions if PPD or PPD-related compounds are encountered in hair dye.
Patch testing for ACD to permanent tattoos is complex. In most cases, patch testing is of limited utility because many of the chemicals that have been reported to cause ACD are not commercially available. Additionally, a 2014 study of 90 patients with chronic tattoo reactions found that the majority had negative patch testing to the European baseline series (66%), disperse dyes (87%), and tattoo inks (87%–92%). The investigators theorized that the allergens causing tattoo reactions are formed by haptenization of “parent” chemicals in the dermis, meaning application of chemicals present in the original tattoo ink may not identify the relevant allergen.26 If patch testing is performed, it is most ideal if individual pigment ingredients can be identified. Allergens to be considered for testing include azo dyes, aromatic amines, iron oxide, barium, zinc, copper, molybdenum, titanium, gold sodium thiosulfate, nickel sulfate, carbon, shellac, diketopyrrolopyrrole, quinacridone, anthraquinone, dioxazine (purple), quinophthalone (yellow) dyes, mercuric sulfide (vermilion, cinnabar), scarlet lake, cadmium red, carmine, and cochineal, many of which are not commercially available for purchase.
Final Interpretation
As tattoos become increasingly trendy, tattoo ACD should be recognized by the astute dermatologist. The most common allergen associated with tattoo ACD is PPD, but other potential allergens include azo dyes and newer pigments. Unlike tattoos of the past, today’s inks are unlikely to contain toxic metals. Diagnosing ACD caused by permanent tattoo inks requires a high degree of suspicion, as patch testing may be of limited utility.
Sometimes regrettable yet increasingly common, tattoos are an ancient art form used in modern times as a mark of artistic and cultural expression. Allergic contact dermatitis (ACD) to tattoo ink is rare, but the popularity of tattoos makes ACD an increasingly recognized occurrence. In a retrospective study of 38,543 patch-tested patients, only 29 (0.08%) had tattoo-related ACD, with the majority of patients being female and young adults. The most common contact allergy was to paraphenylenediamine (PPD), which occurred in 22 (76%) patients.1 In this article, we will walk you through the rainbow of tattoo ACD, covering hypersensitivity reactions to both temporary and permanent tattoo inks.
Temporary Tattoo Inks
Henna is the most common temporary tattoo ink. Derived from the plant Lawsonia inermis, henna is an orange dye that has been used in many parts of the world, particularly in Islamic and Hindu cultures, to dye skin, hair, and fabrics. Application of henna tattoos is common for weddings and other celebrations, and brides may wear elaborate henna patterns. To create these tattoos, henna powder is mixed with water and sometimes essential oils and is then applied to the skin for several hours. After application, the henna pigment lawsone (2-hydroxy-1,4-naphthoquinone) interacts with keratin and leaves a red-orange stain on the skin2; longer application time leads to a deeper color. Most traditional cutaneous henna designs fade in 2 to 6 weeks, but some last longer. Red henna generally is considered safe with low incidence of contact allergy. What is referred to as black henna usually is red henna mixed with PPD, a black dye, which is added to deepen the color. Paraphenylenediamine is highly sensitizing; patients can become sensitized to the PPD in the tattoo itself.2 One study confirmed the presence of PPD in black henna tattoos, with chemical analysis of common preparations revealing concentrations ranging from less than 1% to 30%.2 Patients who undergo patch testing for tattoo reactions often are strongly positive to PPD and have concomitant reactions to azo dyes, black rubber, and anesthetics. Other aromatic amines including aminophenols have been identified in black henna tattoo ink, and these chemicals also may contribute to ACD.3 Less common sources of contact allergy from temporary black henna tattoos include resorcinol,4 para-tertiary butylphenol formaldehyde resin,5 and fragrance.6
Clinically, ACD to PPD in temporary tattoos presents 1 to 3 days after application if the patient is already sensitized or 4 to 14 days if the patient is sensitized by the tattoo ink.2 Most patients notice erythema, edema, vesicles, papules, and/or bullae, but other less common reactions including generalized dermatitis, systemic symptoms, urticaria, and pustules have been described.2 Postinflammatory hypopigmentation or hyperpigmentation also can occur.
Because of the sensitizing nature of black henna tattoos, consumers are turning to natural temporary tattoos. Jagua temporary tattoos, with pigment derived from the sap of fruit from the Genipa americana tree, have been associated with ACD.7 This black dye is applied and washed off in a similar fashion to henna tattoos. Importantly, a recent analysis of jagua dye identified no PPD. In one case, a patient who developed ACD to a jagua tattoo was patch tested to components of the dye and had a positive reaction to genipin, a component of the fruit extract.7 Thus, jagua tattoos often are marketed as safe but are an emerging source of contact dermatitis to temporary tattoos.
Permanent Tattoo Inks
Permanent tattoos are created by injecting small amounts of ink into the dermis. As the name suggests, these tattoos are permanent. Tattoos are common; nearly one-third of Americans have at least 1 tattoo.1 Historically, tattoos were created using black pigment composed of amorphous carbon or black iron oxides.8,9 Metallic pigments (eg, mercury, chromium, cobalt, cadmium) were once used to add color to tattoos, but these metals are now only rarely used; in fact, a 2019 study of tattoo ink components identified 44 distinct pigments in 1416 permanent inks, with an average of 3 pigments per ink.8 Of the 44 pigments, 10 had metallic components including iron, barium, zinc, copper, molybdenum, and titanium. The remaining 34 pigments contained carbon, azo, diketopyrrolopyrrole, quinacridone, anthraquinone, dioxazine (purple), or quinophthalone (yellow) dyes. The authors noted that nearly one-quarter of the tattoo pigments identified in their study had been reported as contact allergens.8
Typically, reactions to permanent tattoo inks manifest as an eczematous dermatitis occurring weeks to years after tattoo application.9,10 The dermatitis usually is locally confined to the tattoo and may be limited to particular colors; occasionally, a new tattoo reaction may trigger concurrent inflammation in older tattoos. Many tattoo reactions occur as a response to red pigment but also have occurred with other tattoo ink components.9 Many researchers have speculated as to whether the reaction is related to the ink component itself or from the photochemical breakdown of the ink by exposure to UV radiation and/or laser therapy.9
Red Pigment
Red ink is the most common color reported to cause tattoo hypersensitivity reactions. Historically, red tattoo pigments include mercuric sulfide (vermilion, cinnabar), scarlet lake, cadmium red, carmine, and cochineal,11 but today’s tattoo inks primarily are composed of other pigments, such as quinacridone and azo dyes.12 Several cases of red tattoo ink hypersensitivity reactions exist in the literature, many without completion of patch tests or without positive patch tests to relevant red pigments.11-15
Black Pigment
In general, reactions to permanent black tattoo ink are rare; however, a few case reports exist. Black pigment can be created with India ink (carbon), logwood (chrome), iron oxide, and titanium.16,17 Shellac can be used as a binding agent in tattoo ink; there is at least one report of a reaction to black tattoo ink with a positive patch test to shellac and the original black ink.18
Metals
When utilized in tattoos, metals can create a variety of colors; several have been reported to cause ACD. There has been at least one reported case of a tattoo hypersensitivity reaction to a gold tattoo, with positive patch testing for gold sodium thiosulfate.19 Green tattoo inks also have been confirmed to contain metal. One case of nickel allergy from a green tattoo has been reported, with a positive patch test for nickel sulfate and tissue confirmation of the presence of nickel with micro X-ray fluorescence and laser ablation inductively coupled plasma mass spectrometry.20 Another case series described 3 patients with pruritus and chronic dermatitis associated with green tattoos who had positive patch tests to potassium dichromate, and the green tattoo pigment flared during patch testing. Chromium oxide was presumed to be present in the green tattoo pigment, and potassium dichromate avoidance in products and food improved both the pruritus and dermatitis.21
Azo Pigments
Azo pigments frequently are used in modern tattoos due to their vibrant colors. One case of hypersensitivity to azo pigment involved an eczematous ulcerated plaque overlying yellow, red, and green ink in a recently applied tattoo. Patch testing with the inks originally used in the tattoo was negative. The authors noted that the 3 problematic ink colors all contained pigment yellow 65—an azo pigment—and attributed the reaction to this dye.22 In another azo reaction, a patient had erythema and pruritus overlying a tattoo applied 1 month prior. Patch testing was positive for aminoazobenzene, an azo pigment that was present in the orange ink of the tattoo.23
Management of Tattoo Hypersensitivity Reactions
Hypersensitivity reactions to temporary tattoos are just that—temporary. Topical steroids and time generally will allow these reactions to resolve. In the setting of vigorous reactions, patients may develop postinflammatory hypopigmentation or hyperpigmentation that may last for months. Unfortunately, bullous tattoo reactions can lead to scarring and keloid formation, requiring more aggressive therapy.
Management of reactions to permanent tattoos is more challenging. High-potency topical steroids under occlusion or intralesional corticosteroid injections may aid in treating pruritus or discomfort. For severe reactions, oral corticosteroids may be required. Patients also may consider laser tattoo removal; however, providers should be aware that there have been rare reports of systemic urticarial reactions from this procedure.24,25 Obviously limited by location and size, excision also may be offered.
Patch Testing for Tattoo Ink Contact Allergy
When patients present for evaluation and management of tattoo ACD, it is important to also consider other causes, including granulomatous tattoo reaction, pseudolymphoma, and lichenoid tattoo reaction. A biopsy can be helpful if the diagnosis is in question.
Patch testing for contact allergy to temporary tattoo inks should include PPD, fragrance, aminophenols, resorcinol, para-tertiary butylphenol formaldehyde, and essential oils. Jagua currently is not available for commercial purchase but also should be considered if the patient has the original product or in research settings. If the individual tattoo ingredients can be identified, they also should be tested. In this scenario, recall reactions may occur; testing with the tattoo paste should be avoided if the prior reaction was severe. Importantly, patients with a PPD allergy should be counseled to avoid hair dyes that contain PPD. Many patients who are sensitized to PPD have strong reactions on patch testing and are at risk for severe reactions if PPD or PPD-related compounds are encountered in hair dye.
Patch testing for ACD to permanent tattoos is complex. In most cases, patch testing is of limited utility because many of the chemicals that have been reported to cause ACD are not commercially available. Additionally, a 2014 study of 90 patients with chronic tattoo reactions found that the majority had negative patch testing to the European baseline series (66%), disperse dyes (87%), and tattoo inks (87%–92%). The investigators theorized that the allergens causing tattoo reactions are formed by haptenization of “parent” chemicals in the dermis, meaning application of chemicals present in the original tattoo ink may not identify the relevant allergen.26 If patch testing is performed, it is most ideal if individual pigment ingredients can be identified. Allergens to be considered for testing include azo dyes, aromatic amines, iron oxide, barium, zinc, copper, molybdenum, titanium, gold sodium thiosulfate, nickel sulfate, carbon, shellac, diketopyrrolopyrrole, quinacridone, anthraquinone, dioxazine (purple), quinophthalone (yellow) dyes, mercuric sulfide (vermilion, cinnabar), scarlet lake, cadmium red, carmine, and cochineal, many of which are not commercially available for purchase.
Final Interpretation
As tattoos become increasingly trendy, tattoo ACD should be recognized by the astute dermatologist. The most common allergen associated with tattoo ACD is PPD, but other potential allergens include azo dyes and newer pigments. Unlike tattoos of the past, today’s inks are unlikely to contain toxic metals. Diagnosing ACD caused by permanent tattoo inks requires a high degree of suspicion, as patch testing may be of limited utility.
- Warshaw EM, Schlarbaum JP, Taylor JS, et al. Allergic reactions to tattoos: retrospective analysis of North American Contact Dermatitis Group data, 2001-2016. J Am Acad Dermatol. 2020;82:E61-E62.
- de Groot AC. Side-effects of henna and semi-permanent ‘black henna’ tattoos: a full review. Contact Dermatitis. 2013;69:1-25.
- Romita P, Foti C, Mascia P, et al. Eyebrow allergic contact dermatitis caused by m-aminophenol and toluene-2,5-diamine secondary to a temporary black henna tattoo. Contact Dermatitis. 2018;79:51-52.
- Ormerod E, Hughes TM, Stone N. Allergic contact dermatitis caused by resorcinol following a temporary black henna tattoo. Contact Dermatitis. 2017;77:187-188.
- Rodrigo-Nicolás B, de la Cuadra J, Sierra C, et al. Contact dermatitis from a temporary tattoo in a boy with contact allergy to p-tert butyl phenol formaldehyde resin. Dermatitis. 2014;25:37-38.
- Temesvári E, Podányi B, Pónyai G, et al. Fragrance sensitization caused by temporary henna tattoo. Contact Dermatitis. 2002;47:240.
- Bircher AJ, Scherer Hofmeier K, Schlegel U, et al. Genipin in temporary jagua tattoos—black dye causing severe allergic dermatitis. Dermatitis. 2019;30:375-376.
- Liszewski W, Warshaw EM. Pigments in American tattoo inks and their propensity to elicit allergic contact dermatitis. J Am Acad Dermatol. 2019;81:379-385.
- Serup J, Hutton Carlsen K, Dommershausen N, et al. Identification of pigments related to allergic tattoo reactions in 104 human skin biopsies. Contact Dermatitis. 2020;82:73-82.
- Bjerre RD, Ulrich NH, Linneberg A, et al. Adverse reactions to tattoos in the general population of Denmark. J Am Acad Dermatol. 2018;79:770-772.
- Bhardwaj SS, Brodell RT, Taylor JS. Red tattoo reactions. Contact Dermatitis. 2003;48:236-237.
- Gaudron S, Ferrier-Le Bouëdec MC, Franck F, et al. Azo pigments and quinacridones induce delayed hypersensitivity in red tattoos. Contact Dermatitis. 2015;72:97-105.
- de Winter RW, van der Bent SAS, van Esch M, et al. Allergic reaction to red cosmetic lip tattoo treated with hydroxychloroquine. Dermatitis. 2019;30:82-83.
- Greve B, Chytry R, Raulin C. Contact dermatitis from red tattoo pigment (quinacridone) with secondary spread. Contact Dermatitis. 2003;49:265-266.
- Ruiz-Villaverde R, Fernandez-Crehuet P, Aguayo-Carreras P, et al. Inflammatory reactions to red tattoo inks: three cases highlighting an emerging problem. Sultan Qaboos Univ Med J. 2018;18:E215-E218.
- Gallo R, Parodi A, Cozzani E, et al. Allergic reaction to India ink in a black tattoo. Contact Dermatitis. 1998;38:346-347.
- de Cuyper C, Lodewick E, Schreiver I, et al. Are metals involved in tattoo-related hypersensitivity reactions? a case report. Contact Dermatitis. 2017;77:397-405.
- González-Villanueva I, Hispán Ocete P, Silvestre Salvador JF. Allergic contact dermatitis caused by a black tattoo ink in a patient allergic to shellac. Contact Dermatitis. 2016;75:247-248.
- Tammaro A, Tuchinda P, Persechino S, et al. Contact allergic dermatitis to gold in a tattoo: a case report. Int J Immunopathol Pharmacol. 2011;24:1111-1113.
- van der Bent SAS, Berg T, Karst U, et al. Allergic reaction to a green tattoo with nickel as a possible allergen. Contact Dermatitis. 2019;81:64-66.
- Jacob SE, Castanedo-Tardan MP, Blyumin ML. Inflammation in green (chromium) tattoos during patch testing. Dermatitis. 2008;19:E33-E34.
- González-Villanueva I, Álvarez-Chinchilla P, Silvestre JF. Allergic reaction to 3 tattoo inks containing pigment yellow 65. Contact Dermatitis. 2018;79:107-108.
- Tammaro A, De Marco G, D’Arino A, et al. Aminoazobenzene in tattoo: another case of allergic contact dermatitis. Int J Dermatol. 2017;56:E79-E81.
- Willardson HB, Kobayashi TT, Arnold JG, et al. Diffuse urticarial reaction associated with titanium dioxide following laser tattoo removal treatments. Photomed Laser Surg. 2017;35:176‐180.
- England RW, Vogel P, Hagan L. Immediate cutaneous hypersensitivity after treatment of tattoo with Nd:YAG laser: a case report and review of the literature. Ann Allergy Asthma Immunol. 2002;89:215‐217.
- Serup J, Carlsen KH. Patch test study of 90 patients with tattoo reactions: negative outcome of allergy patch test to baseline batteries and culprit inks suggests allergen(s) are generated in the skin through haptenization. Contact Dermatitis. 2014;71:255-263.
- Warshaw EM, Schlarbaum JP, Taylor JS, et al. Allergic reactions to tattoos: retrospective analysis of North American Contact Dermatitis Group data, 2001-2016. J Am Acad Dermatol. 2020;82:E61-E62.
- de Groot AC. Side-effects of henna and semi-permanent ‘black henna’ tattoos: a full review. Contact Dermatitis. 2013;69:1-25.
- Romita P, Foti C, Mascia P, et al. Eyebrow allergic contact dermatitis caused by m-aminophenol and toluene-2,5-diamine secondary to a temporary black henna tattoo. Contact Dermatitis. 2018;79:51-52.
- Ormerod E, Hughes TM, Stone N. Allergic contact dermatitis caused by resorcinol following a temporary black henna tattoo. Contact Dermatitis. 2017;77:187-188.
- Rodrigo-Nicolás B, de la Cuadra J, Sierra C, et al. Contact dermatitis from a temporary tattoo in a boy with contact allergy to p-tert butyl phenol formaldehyde resin. Dermatitis. 2014;25:37-38.
- Temesvári E, Podányi B, Pónyai G, et al. Fragrance sensitization caused by temporary henna tattoo. Contact Dermatitis. 2002;47:240.
- Bircher AJ, Scherer Hofmeier K, Schlegel U, et al. Genipin in temporary jagua tattoos—black dye causing severe allergic dermatitis. Dermatitis. 2019;30:375-376.
- Liszewski W, Warshaw EM. Pigments in American tattoo inks and their propensity to elicit allergic contact dermatitis. J Am Acad Dermatol. 2019;81:379-385.
- Serup J, Hutton Carlsen K, Dommershausen N, et al. Identification of pigments related to allergic tattoo reactions in 104 human skin biopsies. Contact Dermatitis. 2020;82:73-82.
- Bjerre RD, Ulrich NH, Linneberg A, et al. Adverse reactions to tattoos in the general population of Denmark. J Am Acad Dermatol. 2018;79:770-772.
- Bhardwaj SS, Brodell RT, Taylor JS. Red tattoo reactions. Contact Dermatitis. 2003;48:236-237.
- Gaudron S, Ferrier-Le Bouëdec MC, Franck F, et al. Azo pigments and quinacridones induce delayed hypersensitivity in red tattoos. Contact Dermatitis. 2015;72:97-105.
- de Winter RW, van der Bent SAS, van Esch M, et al. Allergic reaction to red cosmetic lip tattoo treated with hydroxychloroquine. Dermatitis. 2019;30:82-83.
- Greve B, Chytry R, Raulin C. Contact dermatitis from red tattoo pigment (quinacridone) with secondary spread. Contact Dermatitis. 2003;49:265-266.
- Ruiz-Villaverde R, Fernandez-Crehuet P, Aguayo-Carreras P, et al. Inflammatory reactions to red tattoo inks: three cases highlighting an emerging problem. Sultan Qaboos Univ Med J. 2018;18:E215-E218.
- Gallo R, Parodi A, Cozzani E, et al. Allergic reaction to India ink in a black tattoo. Contact Dermatitis. 1998;38:346-347.
- de Cuyper C, Lodewick E, Schreiver I, et al. Are metals involved in tattoo-related hypersensitivity reactions? a case report. Contact Dermatitis. 2017;77:397-405.
- González-Villanueva I, Hispán Ocete P, Silvestre Salvador JF. Allergic contact dermatitis caused by a black tattoo ink in a patient allergic to shellac. Contact Dermatitis. 2016;75:247-248.
- Tammaro A, Tuchinda P, Persechino S, et al. Contact allergic dermatitis to gold in a tattoo: a case report. Int J Immunopathol Pharmacol. 2011;24:1111-1113.
- van der Bent SAS, Berg T, Karst U, et al. Allergic reaction to a green tattoo with nickel as a possible allergen. Contact Dermatitis. 2019;81:64-66.
- Jacob SE, Castanedo-Tardan MP, Blyumin ML. Inflammation in green (chromium) tattoos during patch testing. Dermatitis. 2008;19:E33-E34.
- González-Villanueva I, Álvarez-Chinchilla P, Silvestre JF. Allergic reaction to 3 tattoo inks containing pigment yellow 65. Contact Dermatitis. 2018;79:107-108.
- Tammaro A, De Marco G, D’Arino A, et al. Aminoazobenzene in tattoo: another case of allergic contact dermatitis. Int J Dermatol. 2017;56:E79-E81.
- Willardson HB, Kobayashi TT, Arnold JG, et al. Diffuse urticarial reaction associated with titanium dioxide following laser tattoo removal treatments. Photomed Laser Surg. 2017;35:176‐180.
- England RW, Vogel P, Hagan L. Immediate cutaneous hypersensitivity after treatment of tattoo with Nd:YAG laser: a case report and review of the literature. Ann Allergy Asthma Immunol. 2002;89:215‐217.
- Serup J, Carlsen KH. Patch test study of 90 patients with tattoo reactions: negative outcome of allergy patch test to baseline batteries and culprit inks suggests allergen(s) are generated in the skin through haptenization. Contact Dermatitis. 2014;71:255-263.
Practice Points
- Temporary tattoo pigments include red henna, black henna, and jagua.
- Black henna tattoos contain paraphenylenediamine, the most common allergen in temporary tattoos.
- Modern permanent tattoo ink components include metals, carbon, azo, diketopyrrolopyrrole, quinacridone, anthraquinone, dioxazine (purple), or quinophthalone (yellow) dyes.
- Patch testing for tattoo contact allergy is complex and challenging.
Database offers snapshot of common causes of pediatric allergic contact dermatitis
, according to an analysis of data from the Pediatric Allergic Contact Dermatitis Registry.
The registry is the first multicenter prospective database in the United States with a focus on pediatric allergic contact dermatitis. JiaDe (Jeff) Yu, MD, a dermatologist at Massachusetts General Hospital, Boston, was awarded a Dermatology Foundation Career Development Grant and formed the registry in 2018 “in an effort to gain a better understanding of allergic contact dermatitis in children,” Idy Tam, MS, said during the virtual annual meeting of the Society for Pediatric Dermatology. “There is currently limited data regarding the pediatric allergic contact dermatitis in the U.S., despite as many as 20% of children having allergic contact dermatitis.”
To date, the Pediatric Allergic Contact Dermatitis Registry consists of 10 academic medical centers with high volume pediatric patch testing across the United States: Massachusetts General Hospital, Boston; Brigham and Women’s Hospital, Boston; the University of Missouri–Columbia; Stanford (Calif.) University; the Medical University of South Carolina, Charleston; Texas Children’s Hospital, Houston; Northwestern University, Chicago; Emory University, Atlanta; Washington University, St. Louis; and the University of California, San Diego.
For the current analysis, Ms. Tam, a research fellow in the department of dermatology at Massachusetts General Hospital, and colleagues collected data on 218 patients under age 18 who were referred for an evaluation of allergic contact dermatitis at one of the 10 participating sites between January 2016 and June 2020.
The mean age of children at the time of their patch testing was 10 years, 62% were girls, and 66% had a history of atopic dermatitis (AD). Most (75%) were White, 14% were Black, 6% were Asian, the rest were from other racial backgrounds. The distribution of dermatitis varied; the top five most commonly affected sites were the face (62%), arms (35%), legs (29%), hands (27%), and neck (20%).
Ms. Tam reported that the mean number of allergens patch tested per child was 78. In all, 81% of children had one or more positive patch test reactions, with a similar rate among those with and without a history of AD (80% vs. 82%, respectively; P = .21). The five most common allergens were hydroperoxides of linalool (22%), nickel sulfate (19%), methylisothiazolinone (17%), cobalt chloride (13%), and fragrance mix I (12%).
The top two treatments at the time of patch testing were a topical corticosteroid (78%) and a topical calcineurin inhibitor (26%).
“This study has allowed for the increased collaboration among dermatologists with expertise in pediatric dermatology and allergic contact dermatitis,” concluded Ms. Tam, a fourth-year medical student at Tufts University, Boston. “We continue to actively seek further collaboration with a goal of creating the most comprehensive pediatric allergic contact dermatitis registry, which can improve our understanding of this condition in children and hopefully guide future research in this field.”
The work was recognized as one of the top poster abstracts at the meeting. The researchers reported having no relevant disclosures.
, according to an analysis of data from the Pediatric Allergic Contact Dermatitis Registry.
The registry is the first multicenter prospective database in the United States with a focus on pediatric allergic contact dermatitis. JiaDe (Jeff) Yu, MD, a dermatologist at Massachusetts General Hospital, Boston, was awarded a Dermatology Foundation Career Development Grant and formed the registry in 2018 “in an effort to gain a better understanding of allergic contact dermatitis in children,” Idy Tam, MS, said during the virtual annual meeting of the Society for Pediatric Dermatology. “There is currently limited data regarding the pediatric allergic contact dermatitis in the U.S., despite as many as 20% of children having allergic contact dermatitis.”
To date, the Pediatric Allergic Contact Dermatitis Registry consists of 10 academic medical centers with high volume pediatric patch testing across the United States: Massachusetts General Hospital, Boston; Brigham and Women’s Hospital, Boston; the University of Missouri–Columbia; Stanford (Calif.) University; the Medical University of South Carolina, Charleston; Texas Children’s Hospital, Houston; Northwestern University, Chicago; Emory University, Atlanta; Washington University, St. Louis; and the University of California, San Diego.
For the current analysis, Ms. Tam, a research fellow in the department of dermatology at Massachusetts General Hospital, and colleagues collected data on 218 patients under age 18 who were referred for an evaluation of allergic contact dermatitis at one of the 10 participating sites between January 2016 and June 2020.
The mean age of children at the time of their patch testing was 10 years, 62% were girls, and 66% had a history of atopic dermatitis (AD). Most (75%) were White, 14% were Black, 6% were Asian, the rest were from other racial backgrounds. The distribution of dermatitis varied; the top five most commonly affected sites were the face (62%), arms (35%), legs (29%), hands (27%), and neck (20%).
Ms. Tam reported that the mean number of allergens patch tested per child was 78. In all, 81% of children had one or more positive patch test reactions, with a similar rate among those with and without a history of AD (80% vs. 82%, respectively; P = .21). The five most common allergens were hydroperoxides of linalool (22%), nickel sulfate (19%), methylisothiazolinone (17%), cobalt chloride (13%), and fragrance mix I (12%).
The top two treatments at the time of patch testing were a topical corticosteroid (78%) and a topical calcineurin inhibitor (26%).
“This study has allowed for the increased collaboration among dermatologists with expertise in pediatric dermatology and allergic contact dermatitis,” concluded Ms. Tam, a fourth-year medical student at Tufts University, Boston. “We continue to actively seek further collaboration with a goal of creating the most comprehensive pediatric allergic contact dermatitis registry, which can improve our understanding of this condition in children and hopefully guide future research in this field.”
The work was recognized as one of the top poster abstracts at the meeting. The researchers reported having no relevant disclosures.
, according to an analysis of data from the Pediatric Allergic Contact Dermatitis Registry.
The registry is the first multicenter prospective database in the United States with a focus on pediatric allergic contact dermatitis. JiaDe (Jeff) Yu, MD, a dermatologist at Massachusetts General Hospital, Boston, was awarded a Dermatology Foundation Career Development Grant and formed the registry in 2018 “in an effort to gain a better understanding of allergic contact dermatitis in children,” Idy Tam, MS, said during the virtual annual meeting of the Society for Pediatric Dermatology. “There is currently limited data regarding the pediatric allergic contact dermatitis in the U.S., despite as many as 20% of children having allergic contact dermatitis.”
To date, the Pediatric Allergic Contact Dermatitis Registry consists of 10 academic medical centers with high volume pediatric patch testing across the United States: Massachusetts General Hospital, Boston; Brigham and Women’s Hospital, Boston; the University of Missouri–Columbia; Stanford (Calif.) University; the Medical University of South Carolina, Charleston; Texas Children’s Hospital, Houston; Northwestern University, Chicago; Emory University, Atlanta; Washington University, St. Louis; and the University of California, San Diego.
For the current analysis, Ms. Tam, a research fellow in the department of dermatology at Massachusetts General Hospital, and colleagues collected data on 218 patients under age 18 who were referred for an evaluation of allergic contact dermatitis at one of the 10 participating sites between January 2016 and June 2020.
The mean age of children at the time of their patch testing was 10 years, 62% were girls, and 66% had a history of atopic dermatitis (AD). Most (75%) were White, 14% were Black, 6% were Asian, the rest were from other racial backgrounds. The distribution of dermatitis varied; the top five most commonly affected sites were the face (62%), arms (35%), legs (29%), hands (27%), and neck (20%).
Ms. Tam reported that the mean number of allergens patch tested per child was 78. In all, 81% of children had one or more positive patch test reactions, with a similar rate among those with and without a history of AD (80% vs. 82%, respectively; P = .21). The five most common allergens were hydroperoxides of linalool (22%), nickel sulfate (19%), methylisothiazolinone (17%), cobalt chloride (13%), and fragrance mix I (12%).
The top two treatments at the time of patch testing were a topical corticosteroid (78%) and a topical calcineurin inhibitor (26%).
“This study has allowed for the increased collaboration among dermatologists with expertise in pediatric dermatology and allergic contact dermatitis,” concluded Ms. Tam, a fourth-year medical student at Tufts University, Boston. “We continue to actively seek further collaboration with a goal of creating the most comprehensive pediatric allergic contact dermatitis registry, which can improve our understanding of this condition in children and hopefully guide future research in this field.”
The work was recognized as one of the top poster abstracts at the meeting. The researchers reported having no relevant disclosures.
FROM SPD 2020
Patch testing in children: An evolving science
“Time needs to be allocated for a patch test consultation, placement, removal, and reading,” she said at the virtual annual meeting of the Society for Pediatric Dermatology. “You will need more time in the day that you’re reading the patch test for patient education. However, your staff will need more time on the front end of the patch test process for application. Also, if they are customizing patch tests, they’ll need time to make the patch tests along with access to a refrigerator and plenty of counter space.”
Other factors to consider are the site of service, your payer mix, and if you need to complete prior authorizations for patch testing.
Dr. Martin, associate professor of dermatology and child health at the University of Missouri–Columbia, said that the diagnosis of allergic contact dermatitis (ACD) crosses her mind when she sees a patient with new dermatitis, especially in an older child; if the dermatitis is patterned or regional; if there’s exacerbation of an underlying, previously stable skin disease; or if it’s a pattern known to be associated with systemic contact dermatitis. “In fact, 13%-25% of healthy, asymptomatic kids have allergen sensitization,” she said. “If you take that a step further and look at kids who are suspected of having allergic contact dermatitis, 25%-96% have allergen sensitization. Still, that doesn’t mean that those tests are relevant to the dermatitis that’s going on. If you take kids who are referred to tertiary centers for patch testing, about half will have relevant patch test results.”
Pediatric ACD differs from adult ACD in three ways, Dr. Martin said. First, children have a different clinical morphology and distribution on presentation, compared with adults. “In adults, the most common clinical presentation is hand dermatitis, while kids more often present with a scattered generalized morphology of dermatitis,” she said. “This occurs in about one-third of children with ACD. Their patterns of allergen exposure are also different. For the most part, adults are in control of their own environments and what is placed on their skin, whereas kids are not. When thinking about what you might need to patch test a child to if you’re considering ACD, it’s important to think about not only what the parent or caregiver puts directly on the child’s skin but also any connubial or consort allergen exposure – the most common ones coming from the caregivers themselves, such as fragrance or hair dyes that are transferred to a young child.”
The third factor that differs between pediatric and adult ACD is the allergen source. Dr. Martin noted that children and adults use different personal care products, wear different types of clothing, and spend different amounts of time in play versus work. “Children have many more hobbies in general that are unfortunately lost as many of us age,” she said. That means “thinking through the child’s entire day and how the seasons differ for them, such as what sports they’re in and what protective equipment may be involved with where their dermatitis is, or what musical instruments they play.”
Applying the T.R.U.E. patch test panel or a customized patch test panel to young children poses certain challenges, considering their limited body surface area and propensity to squirm. Dr. Martin often employs distraction techniques when placing patches on young patients, including the use of bubbles, music, movies, and games. “The goal is always to get as much of the patches on the back or the flanks as possible,” she said. “If you need additional space you can use the upper outer arms, the abdomen, or the anterior lateral thighs. Another thing to consider is how to set up your week for pediatric patch testing. There’s a standardized process for adults where we place the patches on day 0, read them on day 2, with removal of the patches at that time, and then perform a delayed read between day 4-7.”
The process is similar for postpubescent children, despite the lack of clear guidelines in the medical literature. “There is much controversy and different practices between different pediatric patch test centers,” Dr. Martin said. “There is more consensus between the older kids and the prepubescent group ages 6-12. Most clinicians will still do a similar placement on day 0 with removal and initial read on day 2, with a delayed read on day 4-7. However, some groups will remove patches at 24 hours, especially in those with atopic dermatitis (AD) or a generalized dermatitis, to reduce irritant reactions. Others will also use half-strength concentrations of allergens.”
The most controversy lies with children younger than 6 years, she said. For those aged 3-6 years, who do not have AD, most practices use a standardized pediatric tray with a 24- to 48-hour contact time. However, patch testing can be “very challenging” for children who are under 3 years of age, and children with AD who are under 6 years, “so there needs to be a very high degree of suspicion for ACD and very careful selection of the allergens and contact time that is used in those particular cases,” she noted.
The most common allergens in children are nickel, fragrance mix I, cobalt, balsam of Peru, neomycin, and bacitracin, which largely match the common allergens seen in adults. However, allergens more common in children, compared with adults, include gold, propylene glycol, 2-Bromo-2-nitropropane-1,3-diol, and cocamidopropyl betaine. “If the child presents with a regional dermatitis or a patterned dermatitis, sometimes you can hone in on your suspected allergens and only test for a few,” Dr. Martin said. “In a child with eyelid dermatitis, you’re going to worry more about cocamidopropyl betaine in their shampoos and cleansers. Also, a metal allergen could be transferred from their hands from toys or coins, specifically nickel and cobalt. They also may have different sports gear such as goggles that may be affecting their eyelid dermatitis, which you would not necessarily see in an adult.”
Periorificial contact dermatitis can also differ in presentation between children and adults. “In kids, think about musical instruments, flavored lip balms, gum, and pacifiers,” she said. “For ACD on the buttocks and posterior thighs, think about toilet seat allergens, especially those in the potty training ages, and the nickel bolts on school chairs.”
In 2018, Dr. Martin and her colleagues on the Pediatric Contact Dermatitis Workgroup published a pediatric baseline patch test series as a way to expand on the T.R.U.E. test (Dermatitis. 2018;29[4]:206-12). “It’s nice to have this panel available as a baseline screening tool when you’re unsure of possible triggers of the dermatitis but you still have high suspicion of allergic dermatitis,” Dr. Martin said. “This also is helpful for patients who present with generalized dermatitis. It’s still not perfect. We are collecting prospective data to fine-tune this baseline series.”
She reported having no financial disclosures.
“Time needs to be allocated for a patch test consultation, placement, removal, and reading,” she said at the virtual annual meeting of the Society for Pediatric Dermatology. “You will need more time in the day that you’re reading the patch test for patient education. However, your staff will need more time on the front end of the patch test process for application. Also, if they are customizing patch tests, they’ll need time to make the patch tests along with access to a refrigerator and plenty of counter space.”
Other factors to consider are the site of service, your payer mix, and if you need to complete prior authorizations for patch testing.
Dr. Martin, associate professor of dermatology and child health at the University of Missouri–Columbia, said that the diagnosis of allergic contact dermatitis (ACD) crosses her mind when she sees a patient with new dermatitis, especially in an older child; if the dermatitis is patterned or regional; if there’s exacerbation of an underlying, previously stable skin disease; or if it’s a pattern known to be associated with systemic contact dermatitis. “In fact, 13%-25% of healthy, asymptomatic kids have allergen sensitization,” she said. “If you take that a step further and look at kids who are suspected of having allergic contact dermatitis, 25%-96% have allergen sensitization. Still, that doesn’t mean that those tests are relevant to the dermatitis that’s going on. If you take kids who are referred to tertiary centers for patch testing, about half will have relevant patch test results.”
Pediatric ACD differs from adult ACD in three ways, Dr. Martin said. First, children have a different clinical morphology and distribution on presentation, compared with adults. “In adults, the most common clinical presentation is hand dermatitis, while kids more often present with a scattered generalized morphology of dermatitis,” she said. “This occurs in about one-third of children with ACD. Their patterns of allergen exposure are also different. For the most part, adults are in control of their own environments and what is placed on their skin, whereas kids are not. When thinking about what you might need to patch test a child to if you’re considering ACD, it’s important to think about not only what the parent or caregiver puts directly on the child’s skin but also any connubial or consort allergen exposure – the most common ones coming from the caregivers themselves, such as fragrance or hair dyes that are transferred to a young child.”
The third factor that differs between pediatric and adult ACD is the allergen source. Dr. Martin noted that children and adults use different personal care products, wear different types of clothing, and spend different amounts of time in play versus work. “Children have many more hobbies in general that are unfortunately lost as many of us age,” she said. That means “thinking through the child’s entire day and how the seasons differ for them, such as what sports they’re in and what protective equipment may be involved with where their dermatitis is, or what musical instruments they play.”
Applying the T.R.U.E. patch test panel or a customized patch test panel to young children poses certain challenges, considering their limited body surface area and propensity to squirm. Dr. Martin often employs distraction techniques when placing patches on young patients, including the use of bubbles, music, movies, and games. “The goal is always to get as much of the patches on the back or the flanks as possible,” she said. “If you need additional space you can use the upper outer arms, the abdomen, or the anterior lateral thighs. Another thing to consider is how to set up your week for pediatric patch testing. There’s a standardized process for adults where we place the patches on day 0, read them on day 2, with removal of the patches at that time, and then perform a delayed read between day 4-7.”
The process is similar for postpubescent children, despite the lack of clear guidelines in the medical literature. “There is much controversy and different practices between different pediatric patch test centers,” Dr. Martin said. “There is more consensus between the older kids and the prepubescent group ages 6-12. Most clinicians will still do a similar placement on day 0 with removal and initial read on day 2, with a delayed read on day 4-7. However, some groups will remove patches at 24 hours, especially in those with atopic dermatitis (AD) or a generalized dermatitis, to reduce irritant reactions. Others will also use half-strength concentrations of allergens.”
The most controversy lies with children younger than 6 years, she said. For those aged 3-6 years, who do not have AD, most practices use a standardized pediatric tray with a 24- to 48-hour contact time. However, patch testing can be “very challenging” for children who are under 3 years of age, and children with AD who are under 6 years, “so there needs to be a very high degree of suspicion for ACD and very careful selection of the allergens and contact time that is used in those particular cases,” she noted.
The most common allergens in children are nickel, fragrance mix I, cobalt, balsam of Peru, neomycin, and bacitracin, which largely match the common allergens seen in adults. However, allergens more common in children, compared with adults, include gold, propylene glycol, 2-Bromo-2-nitropropane-1,3-diol, and cocamidopropyl betaine. “If the child presents with a regional dermatitis or a patterned dermatitis, sometimes you can hone in on your suspected allergens and only test for a few,” Dr. Martin said. “In a child with eyelid dermatitis, you’re going to worry more about cocamidopropyl betaine in their shampoos and cleansers. Also, a metal allergen could be transferred from their hands from toys or coins, specifically nickel and cobalt. They also may have different sports gear such as goggles that may be affecting their eyelid dermatitis, which you would not necessarily see in an adult.”
Periorificial contact dermatitis can also differ in presentation between children and adults. “In kids, think about musical instruments, flavored lip balms, gum, and pacifiers,” she said. “For ACD on the buttocks and posterior thighs, think about toilet seat allergens, especially those in the potty training ages, and the nickel bolts on school chairs.”
In 2018, Dr. Martin and her colleagues on the Pediatric Contact Dermatitis Workgroup published a pediatric baseline patch test series as a way to expand on the T.R.U.E. test (Dermatitis. 2018;29[4]:206-12). “It’s nice to have this panel available as a baseline screening tool when you’re unsure of possible triggers of the dermatitis but you still have high suspicion of allergic dermatitis,” Dr. Martin said. “This also is helpful for patients who present with generalized dermatitis. It’s still not perfect. We are collecting prospective data to fine-tune this baseline series.”
She reported having no financial disclosures.
“Time needs to be allocated for a patch test consultation, placement, removal, and reading,” she said at the virtual annual meeting of the Society for Pediatric Dermatology. “You will need more time in the day that you’re reading the patch test for patient education. However, your staff will need more time on the front end of the patch test process for application. Also, if they are customizing patch tests, they’ll need time to make the patch tests along with access to a refrigerator and plenty of counter space.”
Other factors to consider are the site of service, your payer mix, and if you need to complete prior authorizations for patch testing.
Dr. Martin, associate professor of dermatology and child health at the University of Missouri–Columbia, said that the diagnosis of allergic contact dermatitis (ACD) crosses her mind when she sees a patient with new dermatitis, especially in an older child; if the dermatitis is patterned or regional; if there’s exacerbation of an underlying, previously stable skin disease; or if it’s a pattern known to be associated with systemic contact dermatitis. “In fact, 13%-25% of healthy, asymptomatic kids have allergen sensitization,” she said. “If you take that a step further and look at kids who are suspected of having allergic contact dermatitis, 25%-96% have allergen sensitization. Still, that doesn’t mean that those tests are relevant to the dermatitis that’s going on. If you take kids who are referred to tertiary centers for patch testing, about half will have relevant patch test results.”
Pediatric ACD differs from adult ACD in three ways, Dr. Martin said. First, children have a different clinical morphology and distribution on presentation, compared with adults. “In adults, the most common clinical presentation is hand dermatitis, while kids more often present with a scattered generalized morphology of dermatitis,” she said. “This occurs in about one-third of children with ACD. Their patterns of allergen exposure are also different. For the most part, adults are in control of their own environments and what is placed on their skin, whereas kids are not. When thinking about what you might need to patch test a child to if you’re considering ACD, it’s important to think about not only what the parent or caregiver puts directly on the child’s skin but also any connubial or consort allergen exposure – the most common ones coming from the caregivers themselves, such as fragrance or hair dyes that are transferred to a young child.”
The third factor that differs between pediatric and adult ACD is the allergen source. Dr. Martin noted that children and adults use different personal care products, wear different types of clothing, and spend different amounts of time in play versus work. “Children have many more hobbies in general that are unfortunately lost as many of us age,” she said. That means “thinking through the child’s entire day and how the seasons differ for them, such as what sports they’re in and what protective equipment may be involved with where their dermatitis is, or what musical instruments they play.”
Applying the T.R.U.E. patch test panel or a customized patch test panel to young children poses certain challenges, considering their limited body surface area and propensity to squirm. Dr. Martin often employs distraction techniques when placing patches on young patients, including the use of bubbles, music, movies, and games. “The goal is always to get as much of the patches on the back or the flanks as possible,” she said. “If you need additional space you can use the upper outer arms, the abdomen, or the anterior lateral thighs. Another thing to consider is how to set up your week for pediatric patch testing. There’s a standardized process for adults where we place the patches on day 0, read them on day 2, with removal of the patches at that time, and then perform a delayed read between day 4-7.”
The process is similar for postpubescent children, despite the lack of clear guidelines in the medical literature. “There is much controversy and different practices between different pediatric patch test centers,” Dr. Martin said. “There is more consensus between the older kids and the prepubescent group ages 6-12. Most clinicians will still do a similar placement on day 0 with removal and initial read on day 2, with a delayed read on day 4-7. However, some groups will remove patches at 24 hours, especially in those with atopic dermatitis (AD) or a generalized dermatitis, to reduce irritant reactions. Others will also use half-strength concentrations of allergens.”
The most controversy lies with children younger than 6 years, she said. For those aged 3-6 years, who do not have AD, most practices use a standardized pediatric tray with a 24- to 48-hour contact time. However, patch testing can be “very challenging” for children who are under 3 years of age, and children with AD who are under 6 years, “so there needs to be a very high degree of suspicion for ACD and very careful selection of the allergens and contact time that is used in those particular cases,” she noted.
The most common allergens in children are nickel, fragrance mix I, cobalt, balsam of Peru, neomycin, and bacitracin, which largely match the common allergens seen in adults. However, allergens more common in children, compared with adults, include gold, propylene glycol, 2-Bromo-2-nitropropane-1,3-diol, and cocamidopropyl betaine. “If the child presents with a regional dermatitis or a patterned dermatitis, sometimes you can hone in on your suspected allergens and only test for a few,” Dr. Martin said. “In a child with eyelid dermatitis, you’re going to worry more about cocamidopropyl betaine in their shampoos and cleansers. Also, a metal allergen could be transferred from their hands from toys or coins, specifically nickel and cobalt. They also may have different sports gear such as goggles that may be affecting their eyelid dermatitis, which you would not necessarily see in an adult.”
Periorificial contact dermatitis can also differ in presentation between children and adults. “In kids, think about musical instruments, flavored lip balms, gum, and pacifiers,” she said. “For ACD on the buttocks and posterior thighs, think about toilet seat allergens, especially those in the potty training ages, and the nickel bolts on school chairs.”
In 2018, Dr. Martin and her colleagues on the Pediatric Contact Dermatitis Workgroup published a pediatric baseline patch test series as a way to expand on the T.R.U.E. test (Dermatitis. 2018;29[4]:206-12). “It’s nice to have this panel available as a baseline screening tool when you’re unsure of possible triggers of the dermatitis but you still have high suspicion of allergic dermatitis,” Dr. Martin said. “This also is helpful for patients who present with generalized dermatitis. It’s still not perfect. We are collecting prospective data to fine-tune this baseline series.”
She reported having no financial disclosures.
FROM SPD 2020
Don't Let the Bedbugs Bite: An Unusual Presentation of Bedbug Infestation Resulting in Life-Threatening Anemia
To the Editor:
A 61-year-old man presented to the emergency department with a rash on the right leg, generalized pruritus, and chest pain. The patient described intermittent exertional pressure-like chest pain over the last few days but had no known prior cardiac history. He also noted worsening edema of the right leg with erythema. Three months prior he had been hospitalized for a similar presentation and was diagnosed with cellulitis of the right leg. The patient was treated with a course of trimethoprim-sulfamethoxazole and permethrin cream for presumed scabies and followed up with dermatology for the persistent generalized pruritic rash and cellulitis. At that time, he was diagnosed with stasis dermatitis with dermatitis neglecta and excoriations. He was educated on general hygiene and treated with triamcinolone, hydrophilic ointment, and pramoxine lotion for pruritus. He also was empirically treated again for scabies.
At the current presentation, preliminary investigation showed profound anemia with a hemoglobin level of 6.2 g/dL (baseline hemoglobin level 3 months prior, 13.1 g/dL). He was subsequently admitted to the general medicine ward for further investigation of severe symptomatic anemia. A medical history revealed moderate chronic obstructive pulmonary disease, hypertension, gastroesophageal reflux disease, xerosis, and fracture of the right ankle following open reduction internal fixation 6 years prior to admission. There was no history of blood loss, antiplatelet agents, or anticoagulants. He was on disability and lived in a single-room occupancy hotel. He did not report any high-risk sexual behaviors or abuse of alcohol or drugs. He actively smoked 1.5 packs of cigarettes per day for the last 30 years. He denied any allergies.
Physical examination revealed the patient was afebrile, nontoxic, disheveled, and in no acute distress. He had anicteric sclera and pale conjunctiva. The right leg appeared more erythematous and edematous compared to the left leg but without warmth or tenderness to palpation. He had innumerable 4- to 5-mm, erythematous, excoriated papules on the skin (Figure). His bed sheets were noted to have multiple rusty-black specks thought to be related to the crusted lesions. Physical examination was otherwise unremarkable.
Laboratory workup revealed severe iron-deficiency anemia without any evidence of hemolysis, marrow suppression, infection, or immune compromise (Table). He had a vitamin B12 deficiency (197 pg/mL [reference range, 239-931 pg/mL]), but we felt it was very unlikely to be responsible for his profound, sudden-onset microcytic anemia. Further evaluation for occult bleeding revealed an unremarkable upper endoscopy with push enteroscopy and colonoscopy. An alternate etiology of the anemia could not be identified.
Subsequently, he reported multiple pruritic bug bites sustained at the hotel room where he resided and continued to note pruritus while hospitalized. Pest control inspected the hospital room and identified bedbugs, Cimex lectularius, among his belongings. Upon further review, his clothes and walker were found to be completely infested with these organisms in different stages of development. Treatment included blood transfusions, iron supplementation, and environmental control of the infested living space both in the hospital and at his residence, with subsequent resolution of symptoms and anemia. Two weeks following discharge, the patient no longer reported pruritus, and his hemoglobin level had returned to baseline.
Over the last decade there has been an exponential resurgence in C lectularius infestations in developed countries attributed to increasing global travel, growing pesticide resistance, lack of public awareness, and inadequate pest control programs. This re-emergence has resulted in a public health problem. Although bedbugs are not known to transmit infectious diseases, severe infestation can result in notable dermatitis, iron-deficiency anemia from chronic blood loss, superinfection, allergic reactions including anaphylaxis in rare cases, and psychologic distress.
Iron-deficiency anemia caused by excessive bedbug biting in infants and children has been documented as early as the 1960s.1 Our knowledge of severe anemia due to bedbug infestation is limited to only 4 cases in the literature, according to a PubMed search of articles indexed for MEDLINE using the terms bedbugs anemia and cimex anemia.1-4 All cases reported bedbug infestations involving personal clothing, belongings, and/or living spaces. Patient concerns at presentation ranged from lethargy and fatigue with pruritic rash to chest pain and syncope with findings of severe microcytic or normocytic anemia (hemoglobin level, 5-8 g/dL). All cases were treated supportively with blood transfusion and iron supplementation, with hemoglobin recovery after several weeks. Environmental extermination also was required to prevent recurrence.1-4 Given that each bedbug blood meal is on average 7 mm3, one would have to incur a minimum of 143,000 bites to experience a blood loss of 1 L.3
The differential diagnosis for a patient with generalized pruritus should be broad and includes dermatologic conditions (eg, xerosis, atopic dermatitis, contact dermatitis, urticaria, dermatophytosis, lichen simplex chronicus, psoriasis, scabies, pediculosis corporis and pubis, other arthropod bites, bullous pemphigoid), systemic disorders (eg, renal disease, diabetes mellitus, thyroid disease, cholestasis, human immunodeficiency virus), malignancy, connective tissue disease, medication side effects, and psychogenic and neuropathic itch.
The diagnosis of C lectularius infestation is confirmed by finding the wingless, reddish brown, flat and ovular arthropod, with adult lengths of 4 to 7 mm, approximately the size of an apple seed.5-11 Bedbugs typically are active at night and feed for 3 to 10 minutes. After their feed or during the day, bedbugs will return to their nest in furniture, mattresses, beds, walls, and floors. Bedbug bites appear as small clusters or lines of pruritic erythematous papules with a central hemorrhagic puncta. Other cutaneous symptoms include isolated pruritus, papules, nodules, and bullous eruptions.7 Additional signs of bedbug infestation include black fecal stains in areas of inhabitation as well as actual bedbugs feeding during the day due to overcrowding.
Treatment of pruritic localized cutaneous reactions is supportive and includes antipruritic agents, topical steroids, topical anesthetics, antihistamines, or topical or systemic antibiotics for secondary infections.5-11 Systemic reactions, including anaphylaxis, are treated with epinephrine, antihistamines, and/or corticosteroids, while severe anemia is treated supportively with blood transfusions and iron supplementation.5-11 To prevent reoccurrence, environmental control in the form of nonchemical and chemical treatments is crucial in controlling bedbug infestations.5-11
This case highlights the relevance of a rare but notable morbidity associated with bedbug infestation and the adverse effects of bedbugs on public health. This patient's living situation in a single-room occupancy hotel, poor hygiene, and possible cognitive impairment from his multiple medical conditions may have increased his risk for extreme bedbug infestation. With a good history, physical examination, proper inspection of the patient's belongings, and provider awareness of this epidemic, the severity of this patient's anemia may have been circumvented on the prior hospital admission and follow-up office visit. Once such an infestation is confirmed, a multidisciplinary approach including social work assistance, health services, and pest control is needed to appropriately treat the patient and the environment. Methods in preventing and managing this growing public health problem include improving hygiene, avoiding secondhand goods, and increasing awareness in the identification and proper elimination of bedbugs.5-7
- Venkatachalam PS, Belavady B. Loss of haemoglobin iron due to excessive biting by bed bugs. a possible aetiological factor in the iron deficiency anaemia of infants and children. Trans R Soc Trop Med Hyg. 1962;56:218-221.
- Pritchard MJ, Hwang SW. Severe anemia from bedbugs. CMAJ. 2009;181:287-288.
- Paulke-Korinek M, Széll M, Laferl H, et al. Bed bugs can cause severe anaemia in adults. Parasitol Res. 2012;110:2577-2579.
- Sabou M, Imperiale DG, Andrés E, et al. Bed bugs reproductive life cycle in the clothes of a patient suffering from Alzheimer's disease results in iron deficiency anemia. Parasite. 2013;20:16.
- Studdiford JS, Conniff KM, Trayes KP, et al. Bedbug infestation. Am Fam Physician. 2012;86:653-658.
- Goddard J, deShazo R. Bed bugs (Cimex lectularis) and clinical consequences of their bites. JAMA. 2009;301:1358-1366.
- Bernardeschi C, Le Cleach L, Delaunay P, et al. Bed bug infestation. BMJ. 2013;346:f138.
- Silvia Munoz-Price L, Safdar N, Beier JC, et al. Bed bugs inhealthcare settings. Infect Control Hosp Epidemiol. 2012;33:1137-1142.
- Huntington MK. When bed bugs bite. J Fam Pract. 2012;61:384-388.
- Delaunay P, Blanc V, Del Giudice P, et al. Bedbugs and infectious diseases. Clin Infect Dis. 2011;52:200-212.
- Doggett SL, Dwyer DE, Penas PF, et al. Bed bugs: clinical relevance and control options. Clin Microbiol Rev. 2012;25:164-192.
To the Editor:
A 61-year-old man presented to the emergency department with a rash on the right leg, generalized pruritus, and chest pain. The patient described intermittent exertional pressure-like chest pain over the last few days but had no known prior cardiac history. He also noted worsening edema of the right leg with erythema. Three months prior he had been hospitalized for a similar presentation and was diagnosed with cellulitis of the right leg. The patient was treated with a course of trimethoprim-sulfamethoxazole and permethrin cream for presumed scabies and followed up with dermatology for the persistent generalized pruritic rash and cellulitis. At that time, he was diagnosed with stasis dermatitis with dermatitis neglecta and excoriations. He was educated on general hygiene and treated with triamcinolone, hydrophilic ointment, and pramoxine lotion for pruritus. He also was empirically treated again for scabies.
At the current presentation, preliminary investigation showed profound anemia with a hemoglobin level of 6.2 g/dL (baseline hemoglobin level 3 months prior, 13.1 g/dL). He was subsequently admitted to the general medicine ward for further investigation of severe symptomatic anemia. A medical history revealed moderate chronic obstructive pulmonary disease, hypertension, gastroesophageal reflux disease, xerosis, and fracture of the right ankle following open reduction internal fixation 6 years prior to admission. There was no history of blood loss, antiplatelet agents, or anticoagulants. He was on disability and lived in a single-room occupancy hotel. He did not report any high-risk sexual behaviors or abuse of alcohol or drugs. He actively smoked 1.5 packs of cigarettes per day for the last 30 years. He denied any allergies.
Physical examination revealed the patient was afebrile, nontoxic, disheveled, and in no acute distress. He had anicteric sclera and pale conjunctiva. The right leg appeared more erythematous and edematous compared to the left leg but without warmth or tenderness to palpation. He had innumerable 4- to 5-mm, erythematous, excoriated papules on the skin (Figure). His bed sheets were noted to have multiple rusty-black specks thought to be related to the crusted lesions. Physical examination was otherwise unremarkable.

Laboratory workup revealed severe iron-deficiency anemia without any evidence of hemolysis, marrow suppression, infection, or immune compromise (Table). He had a vitamin B12 deficiency (197 pg/mL [reference range, 239-931 pg/mL]), but we felt it was very unlikely to be responsible for his profound, sudden-onset microcytic anemia. Further evaluation for occult bleeding revealed an unremarkable upper endoscopy with push enteroscopy and colonoscopy. An alternate etiology of the anemia could not be identified.

Subsequently, he reported multiple pruritic bug bites sustained at the hotel room where he resided and continued to note pruritus while hospitalized. Pest control inspected the hospital room and identified bedbugs, Cimex lectularius, among his belongings. Upon further review, his clothes and walker were found to be completely infested with these organisms in different stages of development. Treatment included blood transfusions, iron supplementation, and environmental control of the infested living space both in the hospital and at his residence, with subsequent resolution of symptoms and anemia. Two weeks following discharge, the patient no longer reported pruritus, and his hemoglobin level had returned to baseline.
Over the last decade there has been an exponential resurgence in C lectularius infestations in developed countries attributed to increasing global travel, growing pesticide resistance, lack of public awareness, and inadequate pest control programs. This re-emergence has resulted in a public health problem. Although bedbugs are not known to transmit infectious diseases, severe infestation can result in notable dermatitis, iron-deficiency anemia from chronic blood loss, superinfection, allergic reactions including anaphylaxis in rare cases, and psychologic distress.
Iron-deficiency anemia caused by excessive bedbug biting in infants and children has been documented as early as the 1960s.1 Our knowledge of severe anemia due to bedbug infestation is limited to only 4 cases in the literature, according to a PubMed search of articles indexed for MEDLINE using the terms bedbugs anemia and cimex anemia.1-4 All cases reported bedbug infestations involving personal clothing, belongings, and/or living spaces. Patient concerns at presentation ranged from lethargy and fatigue with pruritic rash to chest pain and syncope with findings of severe microcytic or normocytic anemia (hemoglobin level, 5-8 g/dL). All cases were treated supportively with blood transfusion and iron supplementation, with hemoglobin recovery after several weeks. Environmental extermination also was required to prevent recurrence.1-4 Given that each bedbug blood meal is on average 7 mm3, one would have to incur a minimum of 143,000 bites to experience a blood loss of 1 L.3
The differential diagnosis for a patient with generalized pruritus should be broad and includes dermatologic conditions (eg, xerosis, atopic dermatitis, contact dermatitis, urticaria, dermatophytosis, lichen simplex chronicus, psoriasis, scabies, pediculosis corporis and pubis, other arthropod bites, bullous pemphigoid), systemic disorders (eg, renal disease, diabetes mellitus, thyroid disease, cholestasis, human immunodeficiency virus), malignancy, connective tissue disease, medication side effects, and psychogenic and neuropathic itch.
The diagnosis of C lectularius infestation is confirmed by finding the wingless, reddish brown, flat and ovular arthropod, with adult lengths of 4 to 7 mm, approximately the size of an apple seed.5-11 Bedbugs typically are active at night and feed for 3 to 10 minutes. After their feed or during the day, bedbugs will return to their nest in furniture, mattresses, beds, walls, and floors. Bedbug bites appear as small clusters or lines of pruritic erythematous papules with a central hemorrhagic puncta. Other cutaneous symptoms include isolated pruritus, papules, nodules, and bullous eruptions.7 Additional signs of bedbug infestation include black fecal stains in areas of inhabitation as well as actual bedbugs feeding during the day due to overcrowding.
Treatment of pruritic localized cutaneous reactions is supportive and includes antipruritic agents, topical steroids, topical anesthetics, antihistamines, or topical or systemic antibiotics for secondary infections.5-11 Systemic reactions, including anaphylaxis, are treated with epinephrine, antihistamines, and/or corticosteroids, while severe anemia is treated supportively with blood transfusions and iron supplementation.5-11 To prevent reoccurrence, environmental control in the form of nonchemical and chemical treatments is crucial in controlling bedbug infestations.5-11
This case highlights the relevance of a rare but notable morbidity associated with bedbug infestation and the adverse effects of bedbugs on public health. This patient's living situation in a single-room occupancy hotel, poor hygiene, and possible cognitive impairment from his multiple medical conditions may have increased his risk for extreme bedbug infestation. With a good history, physical examination, proper inspection of the patient's belongings, and provider awareness of this epidemic, the severity of this patient's anemia may have been circumvented on the prior hospital admission and follow-up office visit. Once such an infestation is confirmed, a multidisciplinary approach including social work assistance, health services, and pest control is needed to appropriately treat the patient and the environment. Methods in preventing and managing this growing public health problem include improving hygiene, avoiding secondhand goods, and increasing awareness in the identification and proper elimination of bedbugs.5-7
To the Editor:
A 61-year-old man presented to the emergency department with a rash on the right leg, generalized pruritus, and chest pain. The patient described intermittent exertional pressure-like chest pain over the last few days but had no known prior cardiac history. He also noted worsening edema of the right leg with erythema. Three months prior he had been hospitalized for a similar presentation and was diagnosed with cellulitis of the right leg. The patient was treated with a course of trimethoprim-sulfamethoxazole and permethrin cream for presumed scabies and followed up with dermatology for the persistent generalized pruritic rash and cellulitis. At that time, he was diagnosed with stasis dermatitis with dermatitis neglecta and excoriations. He was educated on general hygiene and treated with triamcinolone, hydrophilic ointment, and pramoxine lotion for pruritus. He also was empirically treated again for scabies.
At the current presentation, preliminary investigation showed profound anemia with a hemoglobin level of 6.2 g/dL (baseline hemoglobin level 3 months prior, 13.1 g/dL). He was subsequently admitted to the general medicine ward for further investigation of severe symptomatic anemia. A medical history revealed moderate chronic obstructive pulmonary disease, hypertension, gastroesophageal reflux disease, xerosis, and fracture of the right ankle following open reduction internal fixation 6 years prior to admission. There was no history of blood loss, antiplatelet agents, or anticoagulants. He was on disability and lived in a single-room occupancy hotel. He did not report any high-risk sexual behaviors or abuse of alcohol or drugs. He actively smoked 1.5 packs of cigarettes per day for the last 30 years. He denied any allergies.
Physical examination revealed the patient was afebrile, nontoxic, disheveled, and in no acute distress. He had anicteric sclera and pale conjunctiva. The right leg appeared more erythematous and edematous compared to the left leg but without warmth or tenderness to palpation. He had innumerable 4- to 5-mm, erythematous, excoriated papules on the skin (Figure). His bed sheets were noted to have multiple rusty-black specks thought to be related to the crusted lesions. Physical examination was otherwise unremarkable.

Laboratory workup revealed severe iron-deficiency anemia without any evidence of hemolysis, marrow suppression, infection, or immune compromise (Table). He had a vitamin B12 deficiency (197 pg/mL [reference range, 239-931 pg/mL]), but we felt it was very unlikely to be responsible for his profound, sudden-onset microcytic anemia. Further evaluation for occult bleeding revealed an unremarkable upper endoscopy with push enteroscopy and colonoscopy. An alternate etiology of the anemia could not be identified.

Subsequently, he reported multiple pruritic bug bites sustained at the hotel room where he resided and continued to note pruritus while hospitalized. Pest control inspected the hospital room and identified bedbugs, Cimex lectularius, among his belongings. Upon further review, his clothes and walker were found to be completely infested with these organisms in different stages of development. Treatment included blood transfusions, iron supplementation, and environmental control of the infested living space both in the hospital and at his residence, with subsequent resolution of symptoms and anemia. Two weeks following discharge, the patient no longer reported pruritus, and his hemoglobin level had returned to baseline.
Over the last decade there has been an exponential resurgence in C lectularius infestations in developed countries attributed to increasing global travel, growing pesticide resistance, lack of public awareness, and inadequate pest control programs. This re-emergence has resulted in a public health problem. Although bedbugs are not known to transmit infectious diseases, severe infestation can result in notable dermatitis, iron-deficiency anemia from chronic blood loss, superinfection, allergic reactions including anaphylaxis in rare cases, and psychologic distress.
Iron-deficiency anemia caused by excessive bedbug biting in infants and children has been documented as early as the 1960s.1 Our knowledge of severe anemia due to bedbug infestation is limited to only 4 cases in the literature, according to a PubMed search of articles indexed for MEDLINE using the terms bedbugs anemia and cimex anemia.1-4 All cases reported bedbug infestations involving personal clothing, belongings, and/or living spaces. Patient concerns at presentation ranged from lethargy and fatigue with pruritic rash to chest pain and syncope with findings of severe microcytic or normocytic anemia (hemoglobin level, 5-8 g/dL). All cases were treated supportively with blood transfusion and iron supplementation, with hemoglobin recovery after several weeks. Environmental extermination also was required to prevent recurrence.1-4 Given that each bedbug blood meal is on average 7 mm3, one would have to incur a minimum of 143,000 bites to experience a blood loss of 1 L.3
The differential diagnosis for a patient with generalized pruritus should be broad and includes dermatologic conditions (eg, xerosis, atopic dermatitis, contact dermatitis, urticaria, dermatophytosis, lichen simplex chronicus, psoriasis, scabies, pediculosis corporis and pubis, other arthropod bites, bullous pemphigoid), systemic disorders (eg, renal disease, diabetes mellitus, thyroid disease, cholestasis, human immunodeficiency virus), malignancy, connective tissue disease, medication side effects, and psychogenic and neuropathic itch.
The diagnosis of C lectularius infestation is confirmed by finding the wingless, reddish brown, flat and ovular arthropod, with adult lengths of 4 to 7 mm, approximately the size of an apple seed.5-11 Bedbugs typically are active at night and feed for 3 to 10 minutes. After their feed or during the day, bedbugs will return to their nest in furniture, mattresses, beds, walls, and floors. Bedbug bites appear as small clusters or lines of pruritic erythematous papules with a central hemorrhagic puncta. Other cutaneous symptoms include isolated pruritus, papules, nodules, and bullous eruptions.7 Additional signs of bedbug infestation include black fecal stains in areas of inhabitation as well as actual bedbugs feeding during the day due to overcrowding.
Treatment of pruritic localized cutaneous reactions is supportive and includes antipruritic agents, topical steroids, topical anesthetics, antihistamines, or topical or systemic antibiotics for secondary infections.5-11 Systemic reactions, including anaphylaxis, are treated with epinephrine, antihistamines, and/or corticosteroids, while severe anemia is treated supportively with blood transfusions and iron supplementation.5-11 To prevent reoccurrence, environmental control in the form of nonchemical and chemical treatments is crucial in controlling bedbug infestations.5-11
This case highlights the relevance of a rare but notable morbidity associated with bedbug infestation and the adverse effects of bedbugs on public health. This patient's living situation in a single-room occupancy hotel, poor hygiene, and possible cognitive impairment from his multiple medical conditions may have increased his risk for extreme bedbug infestation. With a good history, physical examination, proper inspection of the patient's belongings, and provider awareness of this epidemic, the severity of this patient's anemia may have been circumvented on the prior hospital admission and follow-up office visit. Once such an infestation is confirmed, a multidisciplinary approach including social work assistance, health services, and pest control is needed to appropriately treat the patient and the environment. Methods in preventing and managing this growing public health problem include improving hygiene, avoiding secondhand goods, and increasing awareness in the identification and proper elimination of bedbugs.5-7
- Venkatachalam PS, Belavady B. Loss of haemoglobin iron due to excessive biting by bed bugs. a possible aetiological factor in the iron deficiency anaemia of infants and children. Trans R Soc Trop Med Hyg. 1962;56:218-221.
- Pritchard MJ, Hwang SW. Severe anemia from bedbugs. CMAJ. 2009;181:287-288.
- Paulke-Korinek M, Széll M, Laferl H, et al. Bed bugs can cause severe anaemia in adults. Parasitol Res. 2012;110:2577-2579.
- Sabou M, Imperiale DG, Andrés E, et al. Bed bugs reproductive life cycle in the clothes of a patient suffering from Alzheimer's disease results in iron deficiency anemia. Parasite. 2013;20:16.
- Studdiford JS, Conniff KM, Trayes KP, et al. Bedbug infestation. Am Fam Physician. 2012;86:653-658.
- Goddard J, deShazo R. Bed bugs (Cimex lectularis) and clinical consequences of their bites. JAMA. 2009;301:1358-1366.
- Bernardeschi C, Le Cleach L, Delaunay P, et al. Bed bug infestation. BMJ. 2013;346:f138.
- Silvia Munoz-Price L, Safdar N, Beier JC, et al. Bed bugs inhealthcare settings. Infect Control Hosp Epidemiol. 2012;33:1137-1142.
- Huntington MK. When bed bugs bite. J Fam Pract. 2012;61:384-388.
- Delaunay P, Blanc V, Del Giudice P, et al. Bedbugs and infectious diseases. Clin Infect Dis. 2011;52:200-212.
- Doggett SL, Dwyer DE, Penas PF, et al. Bed bugs: clinical relevance and control options. Clin Microbiol Rev. 2012;25:164-192.
- Venkatachalam PS, Belavady B. Loss of haemoglobin iron due to excessive biting by bed bugs. a possible aetiological factor in the iron deficiency anaemia of infants and children. Trans R Soc Trop Med Hyg. 1962;56:218-221.
- Pritchard MJ, Hwang SW. Severe anemia from bedbugs. CMAJ. 2009;181:287-288.
- Paulke-Korinek M, Széll M, Laferl H, et al. Bed bugs can cause severe anaemia in adults. Parasitol Res. 2012;110:2577-2579.
- Sabou M, Imperiale DG, Andrés E, et al. Bed bugs reproductive life cycle in the clothes of a patient suffering from Alzheimer's disease results in iron deficiency anemia. Parasite. 2013;20:16.
- Studdiford JS, Conniff KM, Trayes KP, et al. Bedbug infestation. Am Fam Physician. 2012;86:653-658.
- Goddard J, deShazo R. Bed bugs (Cimex lectularis) and clinical consequences of their bites. JAMA. 2009;301:1358-1366.
- Bernardeschi C, Le Cleach L, Delaunay P, et al. Bed bug infestation. BMJ. 2013;346:f138.
- Silvia Munoz-Price L, Safdar N, Beier JC, et al. Bed bugs inhealthcare settings. Infect Control Hosp Epidemiol. 2012;33:1137-1142.
- Huntington MK. When bed bugs bite. J Fam Pract. 2012;61:384-388.
- Delaunay P, Blanc V, Del Giudice P, et al. Bedbugs and infectious diseases. Clin Infect Dis. 2011;52:200-212.
- Doggett SL, Dwyer DE, Penas PF, et al. Bed bugs: clinical relevance and control options. Clin Microbiol Rev. 2012;25:164-192.
Practice Points
- There has been a resurgence in bedbug (Cimex lectularius) infestations in developed countries.
- Although rare, anemia due to bedbug infestation should be considered in patients presenting with anemia and a widespread pruritic papular eruption.
- A thorough history and physical examination are essential to prevent a delay in diagnosis and avoid a costly and unnecessary workup.
- Successful treatment requires a multidisciplinary approach, which includes medical management, social services, and pest control.