User login
Radiation Recall Dermatitis Triggered by Prednisone
To the Editor:
A 69-year-old woman presented to the allergy clinic for evaluation of a rash on the left breast. The patient had a history of breast cancer that was treated with a lumpectomy followed by external beam radiation therapy (total dose, 6000 cGy) to the lateral aspect of the left breast approximately 4 years prior. She developed acute breast dermatitis from the radiation, which was self-treated with over-the-counter hydrocortisone cream. The patient subsequently developed a blistering skin eruption over the area where she applied the cream. She did not recall the subtype of hydrocortisone she used (butyrate and acetate are available over-the-counter). She discontinued the hydrocortisone and was started on triamcinolone cream 0.1%, which was well tolerated, and the rash resolved.
The patient had a history of a similar reaction to hydrocortisone butyrate after blepharoplasty approximately 10 years prior to the current presentation, characterized by facial erythema, pruritus, and blistering. A patch test confirmed reactivity to hydrocortisone-17-butyrate and tixocortol pivalate. However, a skin-prick test for hydrocortisone acetate cream 1% was negative.
Subsequently, the patient developed acute-onset dyspepsia, gnawing epigastric pain, regurgitation, and bloating. A diagnosis of eosinophilic gastritis was established via biopsy, which found increased eosinophils in the lamina propria (>50 eosinophils per high-power field). Helicobacter pylori was not identified. She was started on the proton-pump inhibitor dexlansoprazole but symptoms did not improve. Her other medications included benazepril, alprazolam as needed, vitamin D, and magnesium. The patient subsequently was started on a trial of oral prednisone 40 mg/d. Three days after initiation, she developed an erythematous macular rash over the left breast.
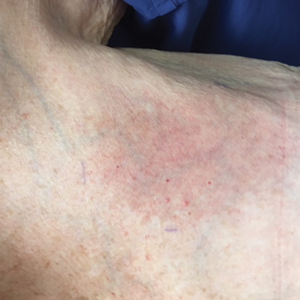
The next day she presented to the allergy clinic. Physical examination of the left breast revealed a 20×10-cm, nipple-sparing patch of well-demarcated erythema without fluctuance or overlying lesions. The area of erythema overlapped with the prior radiation field based on radiation marker tattoos and the lumpectomy scar (Figure). There was no evidence to suggest inflammation of deeper tissue or the pectoral muscles. Vital signs were normal, and the remainder of the examination was unremarkable, including breast, lymph node, and complete skin examinations.
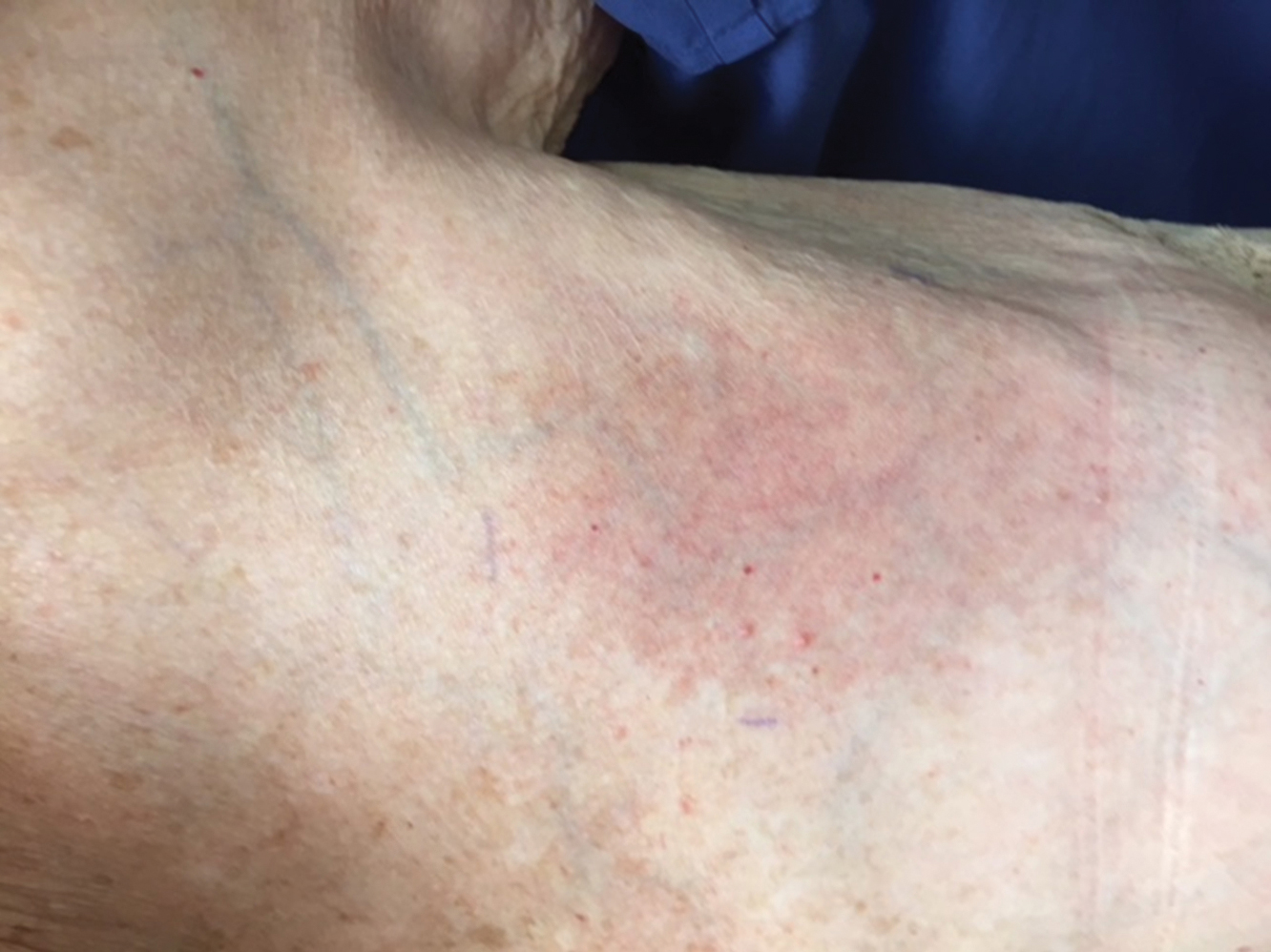
At evaluation, the differential diagnosis included contact dermatitis, fixed drug eruption, infection, tumor recurrence with overlying skin changes, and radiation recall dermatitis. Given that the dermatitis had developed at the site of previously irradiated skin in the absence of fever or an associated mass, the presentation was thought to be most consistent with radiation recall dermatitis.
Oral prednisone was discontinued, and the dermatitis spontaneously improved in a few weeks. Given the patient’s test results and prior tolerance to triamcinolone, eosinophilic gastroenteritis was treated with triamcinolone acetonide 40 mg via intramuscular injection, which was well tolerated.
Radiation recall dermatitis is an acute inflammatory reaction over an area of skin that was previously irradiated. It is most often triggered by chemotherapy agents and occurs in as many as 9% of patients who receive chemotherapy after radiation.1 Commonly implicated chemotherapy agents include anthracyclines, taxanes, antimetabolites, and alkylating agents. Newer targeted cancer treatments also have been reported to trigger radiation recall dermatitis, including epidermal growth factor receptor inhibitors, vascular endothelial growth factor receptor inhibitors, mammalian target of rapamycin inhibitors, and anti–programmed cell death protein 1 monoclonal antibodies.2-5 Radiation recall dermatitis also has been reported to be triggered by intravenous contrast dye.6
The clinical presentation of radiation recall dermatitis ranges from mild rash to skin necrosis and desquamation. Patients often report pruritus or pain in the affected area. The US National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) includes a 5-point scale for grading the severity of radiation recall dermatitis: grade 1, faint erythema or dry desquamation; grade 2, moderate to brisk erythema or patchy moist desquamation, mostly confined to skin folds and creases; grade 3, moist desquamation in areas other than skin folds and creases, with bleeding induced by minor trauma or abrasion; grade 4, skin necrosis or ulceration of full-thickness dermis, with spontaneous bleeding; grade 5, death.7 Based on these criteria, our patient had grade 2 radiation recall dermatitis.
In addition to cutaneous inflammation, additional sites can be inflamed, including the gastrointestinal tract, lungs, and oral mucosa. Cases of myocarditis, sialadenitis, and cystitis also have been reported.⁷
Radiation recall dermatitis can occur even if dermatitis did not occur upon initial treatment. The inflammatory reaction can occur weeks or years after initial irradiation. A study evaluating targeted chemotherapy agents found the median time from initiation of chemotherapy to radiation recall dermatitis was 16.9 weeks (range, 1–86.9 weeks). Inflammation usually lasts approximately 1 to 2 weeks but has been reported to persist as long as 14 weeks.8 Withdrawal of the offending agent in addition to administration of corticosteroids or nonsteroidal anti-inflammatory agents typically results in clinical improvement. Histology on skin biopsy is nonspecific and can reveal mixed infiltrates.7
The pathophysiology of radiation recall dermatitis remains unknown; the condition might be an idiosyncratic drug reaction. It has been hypothesized that prior radiation lowers the threshold for an inflammatory reaction, an example of Ruocco immunocompromised cutaneous districts, in which a prior injury at a cutaneous site increases the likelihood of opportunistic infection, tumor, and immune reactions.9 Because radiation can induce expression of inflammatory cytokines, such as IL-1, IL-6, platelet-derived growth factor β, and tumor necrosis factor α, cells in irradiated areas can continue to secrete low levels of these cytokines after radiation therapy, thus priming an inflammatory reaction in the future.10 An alternative theory is that radiation induces mutations within surviving stem cells, rendering them unable to tolerate or unusually sensitive to subsequent chemotherapy and cytotoxic drugs. However, this premise would not explain how noncytotoxic drugs also can trigger radiation recall dermatitis, as described in our case.11
Prednisone-triggered radiation recall dermatitis is curious, as corticosteroids are used to treat the condition. Corticosteroids are classified by their chemical structure, and patch testing can be used to distinguish allergies across the various classes. Hydrocortisone acetate,
In contrast, triamcinolone is a class B steroid, which has a C16,17-cis-diol or -ketal. Other than budesonide, which can cross-react with D2 steroids, class B steroids do not cross-react with hydrocortisone or prednisone. Triamcinolone does not usually cross-react with D2 corticosteroids, which likely explains why our patient was later able to tolerate triamcinolone to treat eosinophilic gastrointestinal tract disease.
In summary, we present a case of radiation recall dermatitis triggered by prednisone. Radiation can prime an area for a future inflammatory response by upregulating proinflammatory cytokines or triggering stem cell mutation. In our case, clinical reactivity to hydrocortisone-17-butyrate and sensitization to tixocortol pivalate via patch testing could have increased the likelihood of a reaction with prednisone use due to cross-reactivity. This case instructs dermatologists, allergists, and oncologists to be aware of prednisone as a potential trigger of radiation recall dermatitis.
- Kodym E, Kalinska R, Ehringfeld C, et al. Frequency of radiation recall dermatitis in adult cancer patients. Onkologie. 2005;28:18-21.
- Seidel C, Janssen S, Karstens JH, et al. Recall pneumonitis during systemic treatment with sunitinib. Ann Oncol. 2010;21:2119-2120.
- Togashi Y, Masago K, Mishima M, et al. A case of radiation recall pneumonitis induced by erlotinib, which can be related to high plasma concentration. J Thorac Oncol. 2010;5:924-925.
- Bourgier C, Massard C, Moldovan C, et al. Total recall of radiotherapy with mTOR inhibitors: a novel and potentially frequent side-effect? Ann Oncol. 2011;22:485-486.
- Korman AM, Tyler KH, Kaffenberger BH. Radiation recall dermatitis associated with nivolumab for metastatic malignant melanoma. Int J Dermatol. 2017;56:e75-e77.
- Lau SKM, Rahimi A. Radiation recall precipitated by iodinated nonionic contrast. Pract Radiat Oncol. 2015;5:263-266.
- US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic
_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. Published November 27, 2017. Accessed June 10, 2020.] - Levy A, Hollebecque A, Bourgier C, et al. Targeted therapy-induced radiation recall. Eur J Cancer. 2013;49:1662-1668.
- Piccolo V, Baroni A, Russo T, et al. Ruocco’s immunocompromised cutaneous district. Int J Dermatol. 2016;55:135-141.
- Johnson CJ, Piedboeuf P, Rubin P, et al. Early and persistent alterations in the expression of interleukin-1 alpha, interleukin-1 beta and tumour necrosis factor alpha mRNA levels in fibrosis-resistant and sensitive mice after thoracic irradiation. Radiat Res. 1996;145:762-767.
- Azira D, Magné N, Zouhair A, et al. Radiation recall: a well recognized but neglected phenomenon. Cancer Treat Rev. 2005;31:555-570.
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727.
To the Editor:
A 69-year-old woman presented to the allergy clinic for evaluation of a rash on the left breast. The patient had a history of breast cancer that was treated with a lumpectomy followed by external beam radiation therapy (total dose, 6000 cGy) to the lateral aspect of the left breast approximately 4 years prior. She developed acute breast dermatitis from the radiation, which was self-treated with over-the-counter hydrocortisone cream. The patient subsequently developed a blistering skin eruption over the area where she applied the cream. She did not recall the subtype of hydrocortisone she used (butyrate and acetate are available over-the-counter). She discontinued the hydrocortisone and was started on triamcinolone cream 0.1%, which was well tolerated, and the rash resolved.
The patient had a history of a similar reaction to hydrocortisone butyrate after blepharoplasty approximately 10 years prior to the current presentation, characterized by facial erythema, pruritus, and blistering. A patch test confirmed reactivity to hydrocortisone-17-butyrate and tixocortol pivalate. However, a skin-prick test for hydrocortisone acetate cream 1% was negative.
Subsequently, the patient developed acute-onset dyspepsia, gnawing epigastric pain, regurgitation, and bloating. A diagnosis of eosinophilic gastritis was established via biopsy, which found increased eosinophils in the lamina propria (>50 eosinophils per high-power field). Helicobacter pylori was not identified. She was started on the proton-pump inhibitor dexlansoprazole but symptoms did not improve. Her other medications included benazepril, alprazolam as needed, vitamin D, and magnesium. The patient subsequently was started on a trial of oral prednisone 40 mg/d. Three days after initiation, she developed an erythematous macular rash over the left breast.
The next day she presented to the allergy clinic. Physical examination of the left breast revealed a 20×10-cm, nipple-sparing patch of well-demarcated erythema without fluctuance or overlying lesions. The area of erythema overlapped with the prior radiation field based on radiation marker tattoos and the lumpectomy scar (Figure). There was no evidence to suggest inflammation of deeper tissue or the pectoral muscles. Vital signs were normal, and the remainder of the examination was unremarkable, including breast, lymph node, and complete skin examinations.

At evaluation, the differential diagnosis included contact dermatitis, fixed drug eruption, infection, tumor recurrence with overlying skin changes, and radiation recall dermatitis. Given that the dermatitis had developed at the site of previously irradiated skin in the absence of fever or an associated mass, the presentation was thought to be most consistent with radiation recall dermatitis.
Oral prednisone was discontinued, and the dermatitis spontaneously improved in a few weeks. Given the patient’s test results and prior tolerance to triamcinolone, eosinophilic gastroenteritis was treated with triamcinolone acetonide 40 mg via intramuscular injection, which was well tolerated.
Radiation recall dermatitis is an acute inflammatory reaction over an area of skin that was previously irradiated. It is most often triggered by chemotherapy agents and occurs in as many as 9% of patients who receive chemotherapy after radiation.1 Commonly implicated chemotherapy agents include anthracyclines, taxanes, antimetabolites, and alkylating agents. Newer targeted cancer treatments also have been reported to trigger radiation recall dermatitis, including epidermal growth factor receptor inhibitors, vascular endothelial growth factor receptor inhibitors, mammalian target of rapamycin inhibitors, and anti–programmed cell death protein 1 monoclonal antibodies.2-5 Radiation recall dermatitis also has been reported to be triggered by intravenous contrast dye.6
The clinical presentation of radiation recall dermatitis ranges from mild rash to skin necrosis and desquamation. Patients often report pruritus or pain in the affected area. The US National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) includes a 5-point scale for grading the severity of radiation recall dermatitis: grade 1, faint erythema or dry desquamation; grade 2, moderate to brisk erythema or patchy moist desquamation, mostly confined to skin folds and creases; grade 3, moist desquamation in areas other than skin folds and creases, with bleeding induced by minor trauma or abrasion; grade 4, skin necrosis or ulceration of full-thickness dermis, with spontaneous bleeding; grade 5, death.7 Based on these criteria, our patient had grade 2 radiation recall dermatitis.
In addition to cutaneous inflammation, additional sites can be inflamed, including the gastrointestinal tract, lungs, and oral mucosa. Cases of myocarditis, sialadenitis, and cystitis also have been reported.⁷
Radiation recall dermatitis can occur even if dermatitis did not occur upon initial treatment. The inflammatory reaction can occur weeks or years after initial irradiation. A study evaluating targeted chemotherapy agents found the median time from initiation of chemotherapy to radiation recall dermatitis was 16.9 weeks (range, 1–86.9 weeks). Inflammation usually lasts approximately 1 to 2 weeks but has been reported to persist as long as 14 weeks.8 Withdrawal of the offending agent in addition to administration of corticosteroids or nonsteroidal anti-inflammatory agents typically results in clinical improvement. Histology on skin biopsy is nonspecific and can reveal mixed infiltrates.7
The pathophysiology of radiation recall dermatitis remains unknown; the condition might be an idiosyncratic drug reaction. It has been hypothesized that prior radiation lowers the threshold for an inflammatory reaction, an example of Ruocco immunocompromised cutaneous districts, in which a prior injury at a cutaneous site increases the likelihood of opportunistic infection, tumor, and immune reactions.9 Because radiation can induce expression of inflammatory cytokines, such as IL-1, IL-6, platelet-derived growth factor β, and tumor necrosis factor α, cells in irradiated areas can continue to secrete low levels of these cytokines after radiation therapy, thus priming an inflammatory reaction in the future.10 An alternative theory is that radiation induces mutations within surviving stem cells, rendering them unable to tolerate or unusually sensitive to subsequent chemotherapy and cytotoxic drugs. However, this premise would not explain how noncytotoxic drugs also can trigger radiation recall dermatitis, as described in our case.11
Prednisone-triggered radiation recall dermatitis is curious, as corticosteroids are used to treat the condition. Corticosteroids are classified by their chemical structure, and patch testing can be used to distinguish allergies across the various classes. Hydrocortisone acetate,
In contrast, triamcinolone is a class B steroid, which has a C16,17-cis-diol or -ketal. Other than budesonide, which can cross-react with D2 steroids, class B steroids do not cross-react with hydrocortisone or prednisone. Triamcinolone does not usually cross-react with D2 corticosteroids, which likely explains why our patient was later able to tolerate triamcinolone to treat eosinophilic gastrointestinal tract disease.
In summary, we present a case of radiation recall dermatitis triggered by prednisone. Radiation can prime an area for a future inflammatory response by upregulating proinflammatory cytokines or triggering stem cell mutation. In our case, clinical reactivity to hydrocortisone-17-butyrate and sensitization to tixocortol pivalate via patch testing could have increased the likelihood of a reaction with prednisone use due to cross-reactivity. This case instructs dermatologists, allergists, and oncologists to be aware of prednisone as a potential trigger of radiation recall dermatitis.
To the Editor:
A 69-year-old woman presented to the allergy clinic for evaluation of a rash on the left breast. The patient had a history of breast cancer that was treated with a lumpectomy followed by external beam radiation therapy (total dose, 6000 cGy) to the lateral aspect of the left breast approximately 4 years prior. She developed acute breast dermatitis from the radiation, which was self-treated with over-the-counter hydrocortisone cream. The patient subsequently developed a blistering skin eruption over the area where she applied the cream. She did not recall the subtype of hydrocortisone she used (butyrate and acetate are available over-the-counter). She discontinued the hydrocortisone and was started on triamcinolone cream 0.1%, which was well tolerated, and the rash resolved.
The patient had a history of a similar reaction to hydrocortisone butyrate after blepharoplasty approximately 10 years prior to the current presentation, characterized by facial erythema, pruritus, and blistering. A patch test confirmed reactivity to hydrocortisone-17-butyrate and tixocortol pivalate. However, a skin-prick test for hydrocortisone acetate cream 1% was negative.
Subsequently, the patient developed acute-onset dyspepsia, gnawing epigastric pain, regurgitation, and bloating. A diagnosis of eosinophilic gastritis was established via biopsy, which found increased eosinophils in the lamina propria (>50 eosinophils per high-power field). Helicobacter pylori was not identified. She was started on the proton-pump inhibitor dexlansoprazole but symptoms did not improve. Her other medications included benazepril, alprazolam as needed, vitamin D, and magnesium. The patient subsequently was started on a trial of oral prednisone 40 mg/d. Three days after initiation, she developed an erythematous macular rash over the left breast.
The next day she presented to the allergy clinic. Physical examination of the left breast revealed a 20×10-cm, nipple-sparing patch of well-demarcated erythema without fluctuance or overlying lesions. The area of erythema overlapped with the prior radiation field based on radiation marker tattoos and the lumpectomy scar (Figure). There was no evidence to suggest inflammation of deeper tissue or the pectoral muscles. Vital signs were normal, and the remainder of the examination was unremarkable, including breast, lymph node, and complete skin examinations.

At evaluation, the differential diagnosis included contact dermatitis, fixed drug eruption, infection, tumor recurrence with overlying skin changes, and radiation recall dermatitis. Given that the dermatitis had developed at the site of previously irradiated skin in the absence of fever or an associated mass, the presentation was thought to be most consistent with radiation recall dermatitis.
Oral prednisone was discontinued, and the dermatitis spontaneously improved in a few weeks. Given the patient’s test results and prior tolerance to triamcinolone, eosinophilic gastroenteritis was treated with triamcinolone acetonide 40 mg via intramuscular injection, which was well tolerated.
Radiation recall dermatitis is an acute inflammatory reaction over an area of skin that was previously irradiated. It is most often triggered by chemotherapy agents and occurs in as many as 9% of patients who receive chemotherapy after radiation.1 Commonly implicated chemotherapy agents include anthracyclines, taxanes, antimetabolites, and alkylating agents. Newer targeted cancer treatments also have been reported to trigger radiation recall dermatitis, including epidermal growth factor receptor inhibitors, vascular endothelial growth factor receptor inhibitors, mammalian target of rapamycin inhibitors, and anti–programmed cell death protein 1 monoclonal antibodies.2-5 Radiation recall dermatitis also has been reported to be triggered by intravenous contrast dye.6
The clinical presentation of radiation recall dermatitis ranges from mild rash to skin necrosis and desquamation. Patients often report pruritus or pain in the affected area. The US National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) includes a 5-point scale for grading the severity of radiation recall dermatitis: grade 1, faint erythema or dry desquamation; grade 2, moderate to brisk erythema or patchy moist desquamation, mostly confined to skin folds and creases; grade 3, moist desquamation in areas other than skin folds and creases, with bleeding induced by minor trauma or abrasion; grade 4, skin necrosis or ulceration of full-thickness dermis, with spontaneous bleeding; grade 5, death.7 Based on these criteria, our patient had grade 2 radiation recall dermatitis.
In addition to cutaneous inflammation, additional sites can be inflamed, including the gastrointestinal tract, lungs, and oral mucosa. Cases of myocarditis, sialadenitis, and cystitis also have been reported.⁷
Radiation recall dermatitis can occur even if dermatitis did not occur upon initial treatment. The inflammatory reaction can occur weeks or years after initial irradiation. A study evaluating targeted chemotherapy agents found the median time from initiation of chemotherapy to radiation recall dermatitis was 16.9 weeks (range, 1–86.9 weeks). Inflammation usually lasts approximately 1 to 2 weeks but has been reported to persist as long as 14 weeks.8 Withdrawal of the offending agent in addition to administration of corticosteroids or nonsteroidal anti-inflammatory agents typically results in clinical improvement. Histology on skin biopsy is nonspecific and can reveal mixed infiltrates.7
The pathophysiology of radiation recall dermatitis remains unknown; the condition might be an idiosyncratic drug reaction. It has been hypothesized that prior radiation lowers the threshold for an inflammatory reaction, an example of Ruocco immunocompromised cutaneous districts, in which a prior injury at a cutaneous site increases the likelihood of opportunistic infection, tumor, and immune reactions.9 Because radiation can induce expression of inflammatory cytokines, such as IL-1, IL-6, platelet-derived growth factor β, and tumor necrosis factor α, cells in irradiated areas can continue to secrete low levels of these cytokines after radiation therapy, thus priming an inflammatory reaction in the future.10 An alternative theory is that radiation induces mutations within surviving stem cells, rendering them unable to tolerate or unusually sensitive to subsequent chemotherapy and cytotoxic drugs. However, this premise would not explain how noncytotoxic drugs also can trigger radiation recall dermatitis, as described in our case.11
Prednisone-triggered radiation recall dermatitis is curious, as corticosteroids are used to treat the condition. Corticosteroids are classified by their chemical structure, and patch testing can be used to distinguish allergies across the various classes. Hydrocortisone acetate,
In contrast, triamcinolone is a class B steroid, which has a C16,17-cis-diol or -ketal. Other than budesonide, which can cross-react with D2 steroids, class B steroids do not cross-react with hydrocortisone or prednisone. Triamcinolone does not usually cross-react with D2 corticosteroids, which likely explains why our patient was later able to tolerate triamcinolone to treat eosinophilic gastrointestinal tract disease.
In summary, we present a case of radiation recall dermatitis triggered by prednisone. Radiation can prime an area for a future inflammatory response by upregulating proinflammatory cytokines or triggering stem cell mutation. In our case, clinical reactivity to hydrocortisone-17-butyrate and sensitization to tixocortol pivalate via patch testing could have increased the likelihood of a reaction with prednisone use due to cross-reactivity. This case instructs dermatologists, allergists, and oncologists to be aware of prednisone as a potential trigger of radiation recall dermatitis.
- Kodym E, Kalinska R, Ehringfeld C, et al. Frequency of radiation recall dermatitis in adult cancer patients. Onkologie. 2005;28:18-21.
- Seidel C, Janssen S, Karstens JH, et al. Recall pneumonitis during systemic treatment with sunitinib. Ann Oncol. 2010;21:2119-2120.
- Togashi Y, Masago K, Mishima M, et al. A case of radiation recall pneumonitis induced by erlotinib, which can be related to high plasma concentration. J Thorac Oncol. 2010;5:924-925.
- Bourgier C, Massard C, Moldovan C, et al. Total recall of radiotherapy with mTOR inhibitors: a novel and potentially frequent side-effect? Ann Oncol. 2011;22:485-486.
- Korman AM, Tyler KH, Kaffenberger BH. Radiation recall dermatitis associated with nivolumab for metastatic malignant melanoma. Int J Dermatol. 2017;56:e75-e77.
- Lau SKM, Rahimi A. Radiation recall precipitated by iodinated nonionic contrast. Pract Radiat Oncol. 2015;5:263-266.
- US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic
_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. Published November 27, 2017. Accessed June 10, 2020.] - Levy A, Hollebecque A, Bourgier C, et al. Targeted therapy-induced radiation recall. Eur J Cancer. 2013;49:1662-1668.
- Piccolo V, Baroni A, Russo T, et al. Ruocco’s immunocompromised cutaneous district. Int J Dermatol. 2016;55:135-141.
- Johnson CJ, Piedboeuf P, Rubin P, et al. Early and persistent alterations in the expression of interleukin-1 alpha, interleukin-1 beta and tumour necrosis factor alpha mRNA levels in fibrosis-resistant and sensitive mice after thoracic irradiation. Radiat Res. 1996;145:762-767.
- Azira D, Magné N, Zouhair A, et al. Radiation recall: a well recognized but neglected phenomenon. Cancer Treat Rev. 2005;31:555-570.
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727.
- Kodym E, Kalinska R, Ehringfeld C, et al. Frequency of radiation recall dermatitis in adult cancer patients. Onkologie. 2005;28:18-21.
- Seidel C, Janssen S, Karstens JH, et al. Recall pneumonitis during systemic treatment with sunitinib. Ann Oncol. 2010;21:2119-2120.
- Togashi Y, Masago K, Mishima M, et al. A case of radiation recall pneumonitis induced by erlotinib, which can be related to high plasma concentration. J Thorac Oncol. 2010;5:924-925.
- Bourgier C, Massard C, Moldovan C, et al. Total recall of radiotherapy with mTOR inhibitors: a novel and potentially frequent side-effect? Ann Oncol. 2011;22:485-486.
- Korman AM, Tyler KH, Kaffenberger BH. Radiation recall dermatitis associated with nivolumab for metastatic malignant melanoma. Int J Dermatol. 2017;56:e75-e77.
- Lau SKM, Rahimi A. Radiation recall precipitated by iodinated nonionic contrast. Pract Radiat Oncol. 2015;5:263-266.
- US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic
_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. Published November 27, 2017. Accessed June 10, 2020.] - Levy A, Hollebecque A, Bourgier C, et al. Targeted therapy-induced radiation recall. Eur J Cancer. 2013;49:1662-1668.
- Piccolo V, Baroni A, Russo T, et al. Ruocco’s immunocompromised cutaneous district. Int J Dermatol. 2016;55:135-141.
- Johnson CJ, Piedboeuf P, Rubin P, et al. Early and persistent alterations in the expression of interleukin-1 alpha, interleukin-1 beta and tumour necrosis factor alpha mRNA levels in fibrosis-resistant and sensitive mice after thoracic irradiation. Radiat Res. 1996;145:762-767.
- Azira D, Magné N, Zouhair A, et al. Radiation recall: a well recognized but neglected phenomenon. Cancer Treat Rev. 2005;31:555-570.
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727.
Practice Points
- Consider the diagnosis of radiation recall dermatitis for a skin eruption that occurs in the same location as prior radiation exposure.
- Prednisone may be a trigger for radiation recall dermatitis in patients with sensitization to cross-reactive topical steroids such as tixocortol pivalate.
- Radiation therapy may prime the skin for a future inflammatory response by upregulating proinflammatory cytokines that persist after the conclusion of treatment.
Isobornyl Acrylate and Diabetic Devices Steal the Show for the 2020 American Contact Dermatitis Society Allergen of the Year
Each year, the American Contact Dermatitis Society names an Allergen of the Year with the purpose of promoting greater awareness of a key allergen and its impact on patients. Often, the Allergen of the Year is an emerging allergen that may represent an underrecognized or novel cause of allergic contact dermatitis (ACD).In 2020, the American Contact Dermatitis Society chose isobornyl acrylate as the Allergen of the Year.1 Not only has isobornyl acrylate been implicated in an epidemic of contact allergy to diabetic devices, but it also illustrates the challenges of investigating contact allergy to medical devices in general.
What Is Isobornyl Acrylate?
Isobornyl acrylate, also known as the isobornyl ester of acrylic acid, is a chemical used in glues, adhesives, coatings, sealants, inks, and paints. Similar to other acrylates, such as those involved in gel nail treatments, it is photopolymerizable; that is, when exposed to UV light, it can transform from a liquid monomer into a hard polymer, contributing to its utility as an adhesive. Prior to its recent implication in diabetic device contact allergy, isobornyl acrylate was not thought to be a common skin sensitizer. In a 2013 Dutch study of patients with acrylate allergy, only 1 of 14 patients with a contact allergy to other acrylates had a positive patch test reaction to isobornyl acrylate, which led the authors to conclude that adding it to their acrylate patch test series was not indicated.2
Isobornyl Acrylate in Diabetic Devices
Devices such as glucose monitoring systems and insulin pumps are used by millions of patients with diabetes worldwide. Not only are continuous glucose monitoring devices more convenient than self-monitoring of blood glucose, but they also are associated with a reduction in hemoglobin A1c levels and lower risk for hypoglycemia.3 However, these devices have been increasingly recognized as a source of irritant contact dermatitis and ACD.
Early cases of contact allergy to isobornyl acrylate in diabetic devices were reported in 1995 when 2 Belgian patients using insulin pumps developed ACD.4 The patients had positive patch test reactions to isobornyl acrylate 0.1% in petrolatum and other allergens including acrylates. In addition, patch testing with plastic scrapings from their insulin pumps also was positive, and it was determined that the glue affixing the needle to the plastic had diffused into the plastic. The patients were switched to insulin pumps produced by heat staking instead of glue, and their symptoms resolved. In retrospect, this case series may seem prescient, as it was written 2 decades before isobornyl acrylate became recognized as a widespread cause of ACD in users of diabetic devices. Admittedly, other acrylate components of the glue also were positive on patch testing in these patients, so it was not until much later that the focus turned more exclusively to isobornyl acrylate.4
Similar to the insulin pumps in the 1995 Belgian series, diffusion of glue to other parts of modern glucose sensors also appears to cause isobornyl acrylate contact allergy. This theory was supported by a 2017 study from Belgian and Swedish investigators in which gas chromatography–mass spectrometry was used to identify concentrations of isobornyl acrylate in various components of a popular continuous glucose monitoring sensor.5 The concentration of isobornyl acrylate was approximately 100-fold higher at the site where the top and bottom plastic components of the sensor were joined as compared to the adhesive patch in contact with the patient’s skin. Therefore, the adhesive patch itself was not the source of the isobornyl acrylate exposure; rather, the isobornyl acrylate diffused into the adhesive patch from the glue used to join the components of the sensor together.5 One ramification is that patients with diabetic device contact allergy can have a false-negative patch test result if the adhesive patch is tested by itself, whereas they may react to patch testing with the whole sensor or an acetonic extract thereof.
Frequency of Sensitization to Isobornyl Acrylate
It is difficult to estimate the frequency of sensitization to isobornyl acrylate among users of diabetic devices, in part because those with mild allergy may not seek medical treatment. Nevertheless, there are studies that demonstrate a high prevalence of sensitization among users with suspected allergy. In a 2019 Finnish study of 6567 patients using an isobornyl acrylate–containing glucose sensor, 63 were patch tested for suspected ACD.6 Of these 63 patients, 51 (81%) had positive patch test reactions to isobornyl acrylate 0.1% in petrolatum. These findings were consistent with the original 2017 study from Belgium and Sweden, in which 10 of 11 (91%) patients who used an isobornyl acrylate–containing glucose sensor and had suspected contact allergy had positive patch test reactions to isobornyl acrylate 0.1% in petrolatum compared to no positive reactions in the 14 control patients.5 Given that there are more than 1.5 million users of this isobornyl acrylate–containing glucose sensor across 46 countries,7 it requires no stretch of the imagination to understand why investigators refer to isobornyl acrylate allergy as an epidemic, even if only a small percentage of users are sensitized to the device.
The Journey to Discover Isobornyl Acrylate as a Culprit Allergen
Similar to the discoveries of radiography and penicillin, the discovery of isobornyl acrylate as a culprit allergen in a modern glucose sensor was purely accidental. In 2016, a 9-year-old boy with diabetes presented to a Belgian dermatology department with ACD to a glucose sensor.1 A patch test nurse serendipitously applied isobornyl acrylate—0.01%, 0.05%, and 0.1% in petrolatum—which was not intended to be applied as part of the typical acrylate series. The only positive patch test reactions in this patient were to isobornyl acrylate at all 3 concentrations. This lucky error inspired isobornyl acrylate to be tested at multiple other dermatology departments in Europe in patients with ACD to their glucose sensors, leading to its discovery as a culprit allergen.1
One challenge facing investigators was obtaining information and materials from the diabetic device industry. Medical device manufacturers are not required to disclose chemicals present in a device on its label.8 Therefore, for patients or investigators to determine whether a potential allergen is present in a given device, they must request that information from the manufacturer, which can be a time-consuming and frustrating effort. Luckily, investigators collaborated with one another, and Belgian investigators suggested that Swedish investigators performing chemical analyses on a glucose monitoring device should focus on isobornyl acrylate, which enabled its detection in an extract from the device.5
Testing for Isobornyl Acrylate Allergy in Your Clinic
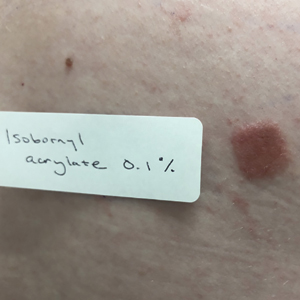
Patients with suspected ACD to a diabetic device—insulin pump or glucose sensor—should be patch tested with isobornyl acrylate, in addition to other previously reported allergens. The vehicle typically is petrolatum, and the commonly tested concentration is 0.1%. Testing with lower concentrations such as 0.01% can result in false-negative reactions,9 and testing at higher concentrations such as 0.3% can result in irritant skin reactions.2 Isobornyl acrylate 0.1% in petrolatum currently is available from one commercial allergen supplier (Chemotechnique Diagnostics). A positive patch test reaction to isobornyl acrylate 0.1% in petrolatum is shown in the Figure.
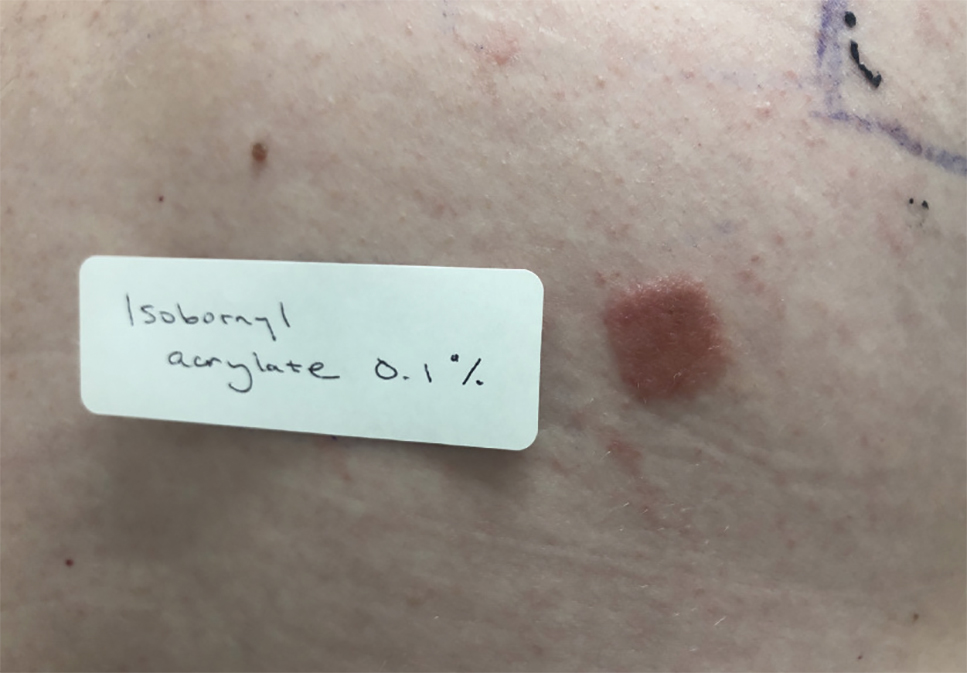
Management of Diabetic Device ACD
For patients with diabetic device ACD, there are several strategies that can reduce direct contact between the device and the patient’s skin. Methods that have been tried with varying success to allow patients to continue using their glucose sensors include barrier sprays (eg, Cavilon [3M], Silesse Skin Barrier [ConvaTec]); barrier pads (eg, Compeed [HRA Pharma], Surround skin protectors [Eakin], DuoDERM dressings [ConvaTec], Tegaderm dressings [3M]); and topical corticosteroids, calcineurin inhibitors, and phosphodiesterase 4 inhibitors. Nevertheless, a 2019 Finnish study showed that only 14 of 63 (22%) patients with ACD to their isobornyl acrylate–containing glucose sensor were able to continue using the device, with all 14 requiring use of a barrier agent. Despite using the barrier agent, 13 (93%) of these patients had residual dermatitis.6 There also is concern that use of barrier methods might hamper the proper functioning of glucose sensors and related devices.
Patients with known isobornyl acrylate contact allergy also may switch to a different diabetic device. A 2019 German study showed that in 5 patients with isobornyl acrylate ACD, none had reactions to the one particular system that has been shown by gas chromatography–mass spectrometry to not contain isobornyl acrylate.10 However, as a word of caution, the same device also has been associated with ACD11,12 but has been resolved by using heat staking during the production process.13 As manufacturers update device components, identification of other isobornyl acrylate–free devices may require a degree of trial and error, as neither isobornyl acrylate nor any other potential allergen is listed on device labels.
Final Interpretation
Isobornyl acrylate is not a common sensitizer in general patch test populations but is a recently identified major culprit in ACD to diabetic devices. Patch testing with isobornyl acrylate 0.1% in petrolatum is not necessary in standard screening panels but should be considered in patients with suspected ACD to glucose sensors or insulin pumps. If a patient with ACD wants to continue to experience the convenience provided by a diabetic device, options include using topical steroids or barrier agents and/or changing the brand of the diabetic device, though none of these methods are foolproof. Hopefully, the identification of isobornyl acrylate as a culprit allergen will help to improve the lives of patients who use diabetic devices worldwide.
- Aerts O, Herman A, Mowitz M, et al. Isobornyl acrylate. Dermatitis. 2020;31:4-12.
- Christoffers WA, Coenraads PJ, Schuttelaar ML. Two decades of occupational (meth)acrylate patch test results and focus on isobornyl acrylate. Contact Dermatitis. 2013;69:86-92.
- Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805.
- Busschots AM, Meuleman V, Poesen N, et al. Contact allergy to components of glue in insulin pump infusion sets. Contact Dermatitis. 1995;33:205-206.
- Herman A, Aerts O, Baeck M, et al. Allergic contact dermatitis caused by isobornyl acrylate in Freestyle® Libre, a newly introduced glucose sensor. Contact Dermatitis. 2017;77:367-373.
- Hyry HSI, Liippo JP, Virtanen HM. Allergic contact dermatitis caused by glucose sensors in type 1 diabetes patients. Contact Dermatitis. 2019;81:161-166.
- Abbott’s Revolutionary FreeStyle® Libre system now reimbursed in the two largest provinces in Canada [press release]. Abbott Park, IL: Abbott; September 13, 2019. https://abbott.mediaroom.com/2019-09-13-Abbotts-Revolutionary-FreeStyle-R-Libre-System-Now-Reimbursed-in-the-Two-Largest-Provinces-in-Canada. Accessed May 14, 2020.
- Herman A, Goossens A. The need to disclose the composition of medical devices at the European level. Contact Dermatitis. 2019;81:159-160.
- Raison-Peyron N, Mowitz M, Bonardel N, et al. Allergic contact dermatitis caused by isobornyl acrylate in OmniPod, an innovative tubeless insulin pump. Contact Dermatitis. 2018;79:76-80.
- Oppel E, Kamann S, Reichl FX, et al. The Dexcom glucose monitoring system—an isobornyl acrylate-free alternative for diabetic patients. Contact Dermatitis. 2019;81:32-36.
- Peeters C, Herman A, Goossens A, et al. Allergic contact dermatitis caused by 2-ethyl cyanoacrylate contained in glucose sensor sets in two diabetic adults. Contact Dermatitis. 2017;77:426-429.
- Aschenbeck KA, Hylwa SA. A diabetic’s allergy: ethyl cyanoacrylate in glucose sensor adhesive. Dermatitis. 2017;28:289-291.
- Gisin V, Chan A, Welsh B. Manufacturing process changes and reduced skin irritations of an adhesive patch used for continuous glucose monitoring devices. J Diabetes Sci Technol. 2018;12:725-726.
Each year, the American Contact Dermatitis Society names an Allergen of the Year with the purpose of promoting greater awareness of a key allergen and its impact on patients. Often, the Allergen of the Year is an emerging allergen that may represent an underrecognized or novel cause of allergic contact dermatitis (ACD).In 2020, the American Contact Dermatitis Society chose isobornyl acrylate as the Allergen of the Year.1 Not only has isobornyl acrylate been implicated in an epidemic of contact allergy to diabetic devices, but it also illustrates the challenges of investigating contact allergy to medical devices in general.
What Is Isobornyl Acrylate?
Isobornyl acrylate, also known as the isobornyl ester of acrylic acid, is a chemical used in glues, adhesives, coatings, sealants, inks, and paints. Similar to other acrylates, such as those involved in gel nail treatments, it is photopolymerizable; that is, when exposed to UV light, it can transform from a liquid monomer into a hard polymer, contributing to its utility as an adhesive. Prior to its recent implication in diabetic device contact allergy, isobornyl acrylate was not thought to be a common skin sensitizer. In a 2013 Dutch study of patients with acrylate allergy, only 1 of 14 patients with a contact allergy to other acrylates had a positive patch test reaction to isobornyl acrylate, which led the authors to conclude that adding it to their acrylate patch test series was not indicated.2
Isobornyl Acrylate in Diabetic Devices
Devices such as glucose monitoring systems and insulin pumps are used by millions of patients with diabetes worldwide. Not only are continuous glucose monitoring devices more convenient than self-monitoring of blood glucose, but they also are associated with a reduction in hemoglobin A1c levels and lower risk for hypoglycemia.3 However, these devices have been increasingly recognized as a source of irritant contact dermatitis and ACD.
Early cases of contact allergy to isobornyl acrylate in diabetic devices were reported in 1995 when 2 Belgian patients using insulin pumps developed ACD.4 The patients had positive patch test reactions to isobornyl acrylate 0.1% in petrolatum and other allergens including acrylates. In addition, patch testing with plastic scrapings from their insulin pumps also was positive, and it was determined that the glue affixing the needle to the plastic had diffused into the plastic. The patients were switched to insulin pumps produced by heat staking instead of glue, and their symptoms resolved. In retrospect, this case series may seem prescient, as it was written 2 decades before isobornyl acrylate became recognized as a widespread cause of ACD in users of diabetic devices. Admittedly, other acrylate components of the glue also were positive on patch testing in these patients, so it was not until much later that the focus turned more exclusively to isobornyl acrylate.4
Similar to the insulin pumps in the 1995 Belgian series, diffusion of glue to other parts of modern glucose sensors also appears to cause isobornyl acrylate contact allergy. This theory was supported by a 2017 study from Belgian and Swedish investigators in which gas chromatography–mass spectrometry was used to identify concentrations of isobornyl acrylate in various components of a popular continuous glucose monitoring sensor.5 The concentration of isobornyl acrylate was approximately 100-fold higher at the site where the top and bottom plastic components of the sensor were joined as compared to the adhesive patch in contact with the patient’s skin. Therefore, the adhesive patch itself was not the source of the isobornyl acrylate exposure; rather, the isobornyl acrylate diffused into the adhesive patch from the glue used to join the components of the sensor together.5 One ramification is that patients with diabetic device contact allergy can have a false-negative patch test result if the adhesive patch is tested by itself, whereas they may react to patch testing with the whole sensor or an acetonic extract thereof.
Frequency of Sensitization to Isobornyl Acrylate
It is difficult to estimate the frequency of sensitization to isobornyl acrylate among users of diabetic devices, in part because those with mild allergy may not seek medical treatment. Nevertheless, there are studies that demonstrate a high prevalence of sensitization among users with suspected allergy. In a 2019 Finnish study of 6567 patients using an isobornyl acrylate–containing glucose sensor, 63 were patch tested for suspected ACD.6 Of these 63 patients, 51 (81%) had positive patch test reactions to isobornyl acrylate 0.1% in petrolatum. These findings were consistent with the original 2017 study from Belgium and Sweden, in which 10 of 11 (91%) patients who used an isobornyl acrylate–containing glucose sensor and had suspected contact allergy had positive patch test reactions to isobornyl acrylate 0.1% in petrolatum compared to no positive reactions in the 14 control patients.5 Given that there are more than 1.5 million users of this isobornyl acrylate–containing glucose sensor across 46 countries,7 it requires no stretch of the imagination to understand why investigators refer to isobornyl acrylate allergy as an epidemic, even if only a small percentage of users are sensitized to the device.
The Journey to Discover Isobornyl Acrylate as a Culprit Allergen
Similar to the discoveries of radiography and penicillin, the discovery of isobornyl acrylate as a culprit allergen in a modern glucose sensor was purely accidental. In 2016, a 9-year-old boy with diabetes presented to a Belgian dermatology department with ACD to a glucose sensor.1 A patch test nurse serendipitously applied isobornyl acrylate—0.01%, 0.05%, and 0.1% in petrolatum—which was not intended to be applied as part of the typical acrylate series. The only positive patch test reactions in this patient were to isobornyl acrylate at all 3 concentrations. This lucky error inspired isobornyl acrylate to be tested at multiple other dermatology departments in Europe in patients with ACD to their glucose sensors, leading to its discovery as a culprit allergen.1
One challenge facing investigators was obtaining information and materials from the diabetic device industry. Medical device manufacturers are not required to disclose chemicals present in a device on its label.8 Therefore, for patients or investigators to determine whether a potential allergen is present in a given device, they must request that information from the manufacturer, which can be a time-consuming and frustrating effort. Luckily, investigators collaborated with one another, and Belgian investigators suggested that Swedish investigators performing chemical analyses on a glucose monitoring device should focus on isobornyl acrylate, which enabled its detection in an extract from the device.5
Testing for Isobornyl Acrylate Allergy in Your Clinic
Patients with suspected ACD to a diabetic device—insulin pump or glucose sensor—should be patch tested with isobornyl acrylate, in addition to other previously reported allergens. The vehicle typically is petrolatum, and the commonly tested concentration is 0.1%. Testing with lower concentrations such as 0.01% can result in false-negative reactions,9 and testing at higher concentrations such as 0.3% can result in irritant skin reactions.2 Isobornyl acrylate 0.1% in petrolatum currently is available from one commercial allergen supplier (Chemotechnique Diagnostics). A positive patch test reaction to isobornyl acrylate 0.1% in petrolatum is shown in the Figure.

Management of Diabetic Device ACD
For patients with diabetic device ACD, there are several strategies that can reduce direct contact between the device and the patient’s skin. Methods that have been tried with varying success to allow patients to continue using their glucose sensors include barrier sprays (eg, Cavilon [3M], Silesse Skin Barrier [ConvaTec]); barrier pads (eg, Compeed [HRA Pharma], Surround skin protectors [Eakin], DuoDERM dressings [ConvaTec], Tegaderm dressings [3M]); and topical corticosteroids, calcineurin inhibitors, and phosphodiesterase 4 inhibitors. Nevertheless, a 2019 Finnish study showed that only 14 of 63 (22%) patients with ACD to their isobornyl acrylate–containing glucose sensor were able to continue using the device, with all 14 requiring use of a barrier agent. Despite using the barrier agent, 13 (93%) of these patients had residual dermatitis.6 There also is concern that use of barrier methods might hamper the proper functioning of glucose sensors and related devices.
Patients with known isobornyl acrylate contact allergy also may switch to a different diabetic device. A 2019 German study showed that in 5 patients with isobornyl acrylate ACD, none had reactions to the one particular system that has been shown by gas chromatography–mass spectrometry to not contain isobornyl acrylate.10 However, as a word of caution, the same device also has been associated with ACD11,12 but has been resolved by using heat staking during the production process.13 As manufacturers update device components, identification of other isobornyl acrylate–free devices may require a degree of trial and error, as neither isobornyl acrylate nor any other potential allergen is listed on device labels.
Final Interpretation
Isobornyl acrylate is not a common sensitizer in general patch test populations but is a recently identified major culprit in ACD to diabetic devices. Patch testing with isobornyl acrylate 0.1% in petrolatum is not necessary in standard screening panels but should be considered in patients with suspected ACD to glucose sensors or insulin pumps. If a patient with ACD wants to continue to experience the convenience provided by a diabetic device, options include using topical steroids or barrier agents and/or changing the brand of the diabetic device, though none of these methods are foolproof. Hopefully, the identification of isobornyl acrylate as a culprit allergen will help to improve the lives of patients who use diabetic devices worldwide.
Each year, the American Contact Dermatitis Society names an Allergen of the Year with the purpose of promoting greater awareness of a key allergen and its impact on patients. Often, the Allergen of the Year is an emerging allergen that may represent an underrecognized or novel cause of allergic contact dermatitis (ACD).In 2020, the American Contact Dermatitis Society chose isobornyl acrylate as the Allergen of the Year.1 Not only has isobornyl acrylate been implicated in an epidemic of contact allergy to diabetic devices, but it also illustrates the challenges of investigating contact allergy to medical devices in general.
What Is Isobornyl Acrylate?
Isobornyl acrylate, also known as the isobornyl ester of acrylic acid, is a chemical used in glues, adhesives, coatings, sealants, inks, and paints. Similar to other acrylates, such as those involved in gel nail treatments, it is photopolymerizable; that is, when exposed to UV light, it can transform from a liquid monomer into a hard polymer, contributing to its utility as an adhesive. Prior to its recent implication in diabetic device contact allergy, isobornyl acrylate was not thought to be a common skin sensitizer. In a 2013 Dutch study of patients with acrylate allergy, only 1 of 14 patients with a contact allergy to other acrylates had a positive patch test reaction to isobornyl acrylate, which led the authors to conclude that adding it to their acrylate patch test series was not indicated.2
Isobornyl Acrylate in Diabetic Devices
Devices such as glucose monitoring systems and insulin pumps are used by millions of patients with diabetes worldwide. Not only are continuous glucose monitoring devices more convenient than self-monitoring of blood glucose, but they also are associated with a reduction in hemoglobin A1c levels and lower risk for hypoglycemia.3 However, these devices have been increasingly recognized as a source of irritant contact dermatitis and ACD.
Early cases of contact allergy to isobornyl acrylate in diabetic devices were reported in 1995 when 2 Belgian patients using insulin pumps developed ACD.4 The patients had positive patch test reactions to isobornyl acrylate 0.1% in petrolatum and other allergens including acrylates. In addition, patch testing with plastic scrapings from their insulin pumps also was positive, and it was determined that the glue affixing the needle to the plastic had diffused into the plastic. The patients were switched to insulin pumps produced by heat staking instead of glue, and their symptoms resolved. In retrospect, this case series may seem prescient, as it was written 2 decades before isobornyl acrylate became recognized as a widespread cause of ACD in users of diabetic devices. Admittedly, other acrylate components of the glue also were positive on patch testing in these patients, so it was not until much later that the focus turned more exclusively to isobornyl acrylate.4
Similar to the insulin pumps in the 1995 Belgian series, diffusion of glue to other parts of modern glucose sensors also appears to cause isobornyl acrylate contact allergy. This theory was supported by a 2017 study from Belgian and Swedish investigators in which gas chromatography–mass spectrometry was used to identify concentrations of isobornyl acrylate in various components of a popular continuous glucose monitoring sensor.5 The concentration of isobornyl acrylate was approximately 100-fold higher at the site where the top and bottom plastic components of the sensor were joined as compared to the adhesive patch in contact with the patient’s skin. Therefore, the adhesive patch itself was not the source of the isobornyl acrylate exposure; rather, the isobornyl acrylate diffused into the adhesive patch from the glue used to join the components of the sensor together.5 One ramification is that patients with diabetic device contact allergy can have a false-negative patch test result if the adhesive patch is tested by itself, whereas they may react to patch testing with the whole sensor or an acetonic extract thereof.
Frequency of Sensitization to Isobornyl Acrylate
It is difficult to estimate the frequency of sensitization to isobornyl acrylate among users of diabetic devices, in part because those with mild allergy may not seek medical treatment. Nevertheless, there are studies that demonstrate a high prevalence of sensitization among users with suspected allergy. In a 2019 Finnish study of 6567 patients using an isobornyl acrylate–containing glucose sensor, 63 were patch tested for suspected ACD.6 Of these 63 patients, 51 (81%) had positive patch test reactions to isobornyl acrylate 0.1% in petrolatum. These findings were consistent with the original 2017 study from Belgium and Sweden, in which 10 of 11 (91%) patients who used an isobornyl acrylate–containing glucose sensor and had suspected contact allergy had positive patch test reactions to isobornyl acrylate 0.1% in petrolatum compared to no positive reactions in the 14 control patients.5 Given that there are more than 1.5 million users of this isobornyl acrylate–containing glucose sensor across 46 countries,7 it requires no stretch of the imagination to understand why investigators refer to isobornyl acrylate allergy as an epidemic, even if only a small percentage of users are sensitized to the device.
The Journey to Discover Isobornyl Acrylate as a Culprit Allergen
Similar to the discoveries of radiography and penicillin, the discovery of isobornyl acrylate as a culprit allergen in a modern glucose sensor was purely accidental. In 2016, a 9-year-old boy with diabetes presented to a Belgian dermatology department with ACD to a glucose sensor.1 A patch test nurse serendipitously applied isobornyl acrylate—0.01%, 0.05%, and 0.1% in petrolatum—which was not intended to be applied as part of the typical acrylate series. The only positive patch test reactions in this patient were to isobornyl acrylate at all 3 concentrations. This lucky error inspired isobornyl acrylate to be tested at multiple other dermatology departments in Europe in patients with ACD to their glucose sensors, leading to its discovery as a culprit allergen.1
One challenge facing investigators was obtaining information and materials from the diabetic device industry. Medical device manufacturers are not required to disclose chemicals present in a device on its label.8 Therefore, for patients or investigators to determine whether a potential allergen is present in a given device, they must request that information from the manufacturer, which can be a time-consuming and frustrating effort. Luckily, investigators collaborated with one another, and Belgian investigators suggested that Swedish investigators performing chemical analyses on a glucose monitoring device should focus on isobornyl acrylate, which enabled its detection in an extract from the device.5
Testing for Isobornyl Acrylate Allergy in Your Clinic
Patients with suspected ACD to a diabetic device—insulin pump or glucose sensor—should be patch tested with isobornyl acrylate, in addition to other previously reported allergens. The vehicle typically is petrolatum, and the commonly tested concentration is 0.1%. Testing with lower concentrations such as 0.01% can result in false-negative reactions,9 and testing at higher concentrations such as 0.3% can result in irritant skin reactions.2 Isobornyl acrylate 0.1% in petrolatum currently is available from one commercial allergen supplier (Chemotechnique Diagnostics). A positive patch test reaction to isobornyl acrylate 0.1% in petrolatum is shown in the Figure.

Management of Diabetic Device ACD
For patients with diabetic device ACD, there are several strategies that can reduce direct contact between the device and the patient’s skin. Methods that have been tried with varying success to allow patients to continue using their glucose sensors include barrier sprays (eg, Cavilon [3M], Silesse Skin Barrier [ConvaTec]); barrier pads (eg, Compeed [HRA Pharma], Surround skin protectors [Eakin], DuoDERM dressings [ConvaTec], Tegaderm dressings [3M]); and topical corticosteroids, calcineurin inhibitors, and phosphodiesterase 4 inhibitors. Nevertheless, a 2019 Finnish study showed that only 14 of 63 (22%) patients with ACD to their isobornyl acrylate–containing glucose sensor were able to continue using the device, with all 14 requiring use of a barrier agent. Despite using the barrier agent, 13 (93%) of these patients had residual dermatitis.6 There also is concern that use of barrier methods might hamper the proper functioning of glucose sensors and related devices.
Patients with known isobornyl acrylate contact allergy also may switch to a different diabetic device. A 2019 German study showed that in 5 patients with isobornyl acrylate ACD, none had reactions to the one particular system that has been shown by gas chromatography–mass spectrometry to not contain isobornyl acrylate.10 However, as a word of caution, the same device also has been associated with ACD11,12 but has been resolved by using heat staking during the production process.13 As manufacturers update device components, identification of other isobornyl acrylate–free devices may require a degree of trial and error, as neither isobornyl acrylate nor any other potential allergen is listed on device labels.
Final Interpretation
Isobornyl acrylate is not a common sensitizer in general patch test populations but is a recently identified major culprit in ACD to diabetic devices. Patch testing with isobornyl acrylate 0.1% in petrolatum is not necessary in standard screening panels but should be considered in patients with suspected ACD to glucose sensors or insulin pumps. If a patient with ACD wants to continue to experience the convenience provided by a diabetic device, options include using topical steroids or barrier agents and/or changing the brand of the diabetic device, though none of these methods are foolproof. Hopefully, the identification of isobornyl acrylate as a culprit allergen will help to improve the lives of patients who use diabetic devices worldwide.
- Aerts O, Herman A, Mowitz M, et al. Isobornyl acrylate. Dermatitis. 2020;31:4-12.
- Christoffers WA, Coenraads PJ, Schuttelaar ML. Two decades of occupational (meth)acrylate patch test results and focus on isobornyl acrylate. Contact Dermatitis. 2013;69:86-92.
- Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805.
- Busschots AM, Meuleman V, Poesen N, et al. Contact allergy to components of glue in insulin pump infusion sets. Contact Dermatitis. 1995;33:205-206.
- Herman A, Aerts O, Baeck M, et al. Allergic contact dermatitis caused by isobornyl acrylate in Freestyle® Libre, a newly introduced glucose sensor. Contact Dermatitis. 2017;77:367-373.
- Hyry HSI, Liippo JP, Virtanen HM. Allergic contact dermatitis caused by glucose sensors in type 1 diabetes patients. Contact Dermatitis. 2019;81:161-166.
- Abbott’s Revolutionary FreeStyle® Libre system now reimbursed in the two largest provinces in Canada [press release]. Abbott Park, IL: Abbott; September 13, 2019. https://abbott.mediaroom.com/2019-09-13-Abbotts-Revolutionary-FreeStyle-R-Libre-System-Now-Reimbursed-in-the-Two-Largest-Provinces-in-Canada. Accessed May 14, 2020.
- Herman A, Goossens A. The need to disclose the composition of medical devices at the European level. Contact Dermatitis. 2019;81:159-160.
- Raison-Peyron N, Mowitz M, Bonardel N, et al. Allergic contact dermatitis caused by isobornyl acrylate in OmniPod, an innovative tubeless insulin pump. Contact Dermatitis. 2018;79:76-80.
- Oppel E, Kamann S, Reichl FX, et al. The Dexcom glucose monitoring system—an isobornyl acrylate-free alternative for diabetic patients. Contact Dermatitis. 2019;81:32-36.
- Peeters C, Herman A, Goossens A, et al. Allergic contact dermatitis caused by 2-ethyl cyanoacrylate contained in glucose sensor sets in two diabetic adults. Contact Dermatitis. 2017;77:426-429.
- Aschenbeck KA, Hylwa SA. A diabetic’s allergy: ethyl cyanoacrylate in glucose sensor adhesive. Dermatitis. 2017;28:289-291.
- Gisin V, Chan A, Welsh B. Manufacturing process changes and reduced skin irritations of an adhesive patch used for continuous glucose monitoring devices. J Diabetes Sci Technol. 2018;12:725-726.
- Aerts O, Herman A, Mowitz M, et al. Isobornyl acrylate. Dermatitis. 2020;31:4-12.
- Christoffers WA, Coenraads PJ, Schuttelaar ML. Two decades of occupational (meth)acrylate patch test results and focus on isobornyl acrylate. Contact Dermatitis. 2013;69:86-92.
- Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805.
- Busschots AM, Meuleman V, Poesen N, et al. Contact allergy to components of glue in insulin pump infusion sets. Contact Dermatitis. 1995;33:205-206.
- Herman A, Aerts O, Baeck M, et al. Allergic contact dermatitis caused by isobornyl acrylate in Freestyle® Libre, a newly introduced glucose sensor. Contact Dermatitis. 2017;77:367-373.
- Hyry HSI, Liippo JP, Virtanen HM. Allergic contact dermatitis caused by glucose sensors in type 1 diabetes patients. Contact Dermatitis. 2019;81:161-166.
- Abbott’s Revolutionary FreeStyle® Libre system now reimbursed in the two largest provinces in Canada [press release]. Abbott Park, IL: Abbott; September 13, 2019. https://abbott.mediaroom.com/2019-09-13-Abbotts-Revolutionary-FreeStyle-R-Libre-System-Now-Reimbursed-in-the-Two-Largest-Provinces-in-Canada. Accessed May 14, 2020.
- Herman A, Goossens A. The need to disclose the composition of medical devices at the European level. Contact Dermatitis. 2019;81:159-160.
- Raison-Peyron N, Mowitz M, Bonardel N, et al. Allergic contact dermatitis caused by isobornyl acrylate in OmniPod, an innovative tubeless insulin pump. Contact Dermatitis. 2018;79:76-80.
- Oppel E, Kamann S, Reichl FX, et al. The Dexcom glucose monitoring system—an isobornyl acrylate-free alternative for diabetic patients. Contact Dermatitis. 2019;81:32-36.
- Peeters C, Herman A, Goossens A, et al. Allergic contact dermatitis caused by 2-ethyl cyanoacrylate contained in glucose sensor sets in two diabetic adults. Contact Dermatitis. 2017;77:426-429.
- Aschenbeck KA, Hylwa SA. A diabetic’s allergy: ethyl cyanoacrylate in glucose sensor adhesive. Dermatitis. 2017;28:289-291.
- Gisin V, Chan A, Welsh B. Manufacturing process changes and reduced skin irritations of an adhesive patch used for continuous glucose monitoring devices. J Diabetes Sci Technol. 2018;12:725-726.
Practice Points
- In patients with suspected allergic contact dermatitis (ACD) to a diabetic device, patch testing with isobornyl acrylate 0.1% in petrolatum should be considered.
- If patients with ACD to their diabetic device want to continue using the device, options include utilizing topical steroids or barrier agents and/or changing the brand of the diabetic device, though these steps may not be effective for every patient.
Hand Hygiene in Preventing COVID-19 Transmission
Handwashing with antimicrobial soaps or alcohol-based sanitizers is an effective measure in preventing microbial disease transmission. In the context of coronavirus disease 2019 (COVID-19) prevention, the World Health Organization and Centers for Disease Control and Prevention have recommended handwashing with soap and water after coughing/sneezing, visiting a public place, touching surfaces outside the home, and taking care of a sick person(s), as well as before and after eating. When soap and water are not available, alcohol-based sanitizers may be used.1,2
Irritant contact dermatitis (ICD) is most commonly associated with wet work and is frequently seen in health care workers in relation to hand hygiene, with survey-based studies reporting 25% to 55% of nurses affected.3-5 In a prospective study (N=102), health care workers who washed their hands more than 10 times per day were55% more likely to develop hand dermatitis.6 Frequent ICD of the hands has been reported in Chinese health care workers in association with COVID-19.7 Handwashing and/or glove wearing may be newly prioritized by workers who handle frequently touched goods and surfaces, such as flight attendants (Figure). Patients with obsessive-compulsive disorder may be another vulnerable population.8
Alcohol-based sanitizers and detergents or antimicrobials in soaps may cause ICD of the hands by denaturation of stratum corneum proteins, depletion of intercellular lipids, and decreased corneocyte cohesion. These agents alter the skin flora, with increased colonization by staphylococci and gram-negative bacilli.9 Clinical findings include xerosis, scaling, fissuring, and bleeding. Physicians may evaluate severity of ICD of the hands using the
Cleansing the hands with alcohol-based sanitizers has consistently shown equivalent or greater efficacy than antimicrobial soaps for eradication of most microbes, with exception of bacterial spores and protozoan oocysts.11 In an in vivo experiment, 70% ethanol solution was more effective in eradicating rotavirus from the fingerpads of adults than 10% povidone-iodine solution, nonmedicated soaps, and soaps containing chloroxylenol 4.8% or chlorhexidine gluconate 4%.12 Coronavirus disease 2019 is a lipophilic enveloped virus. The lipid-dissolving effects of alcohol-based sanitizers is especially effective against these kinds of viruses. An in vitro experiment showed that alcohol solutions are effective against enveloped viruses including severe acute respiratory syndrome coronavirus, Ebola virus, and Zika virus.13 There are limited data for the virucidal efficacy of non–alcohol-based sanitizers containing quaternary ammonium compounds (most commonly benzalkonium chloride) and therefore they are not recommended for protection against COVID-19. Handwashing is preferred over alcohol-based solutions when hands are visibly dirty.
Alcohol-based sanitizers typically are less likely to cause ICD than handwashing with detergent-based or antimicrobial soaps. Antimicrobial ingredients in soaps such as chlorhexidine, chloroxylenol, and triclosan are frequent culprits.11 Detergents in soap such as sodium laureth sulfate cause more skin irritation and transepidermal water loss than alcohol14; however, among health care workers, alcohol-based sanitizers often are perceived as more damaging to the skin.15 During the 2014 Ebola outbreak, use of alcohol-based sanitizers vs handwashing resulted in lower hand eczema severity index scores (n=108).16
Propensity for ICD is a limiting factor in hand hygiene adherence.17 In a double-blind randomized trial (N=54), scheduled use of an oil-containing lotion was shown to increase compliance with hand hygiene protocols in health care workers by preventing cracks, scaling, and pain.18 Using sanitizers containing humectants (eg, aloe vera gel) or moisturizers with petrolatum, liquid paraffin, glycerin, or mineral oil have all been shown to decrease the incidence of ICD in frequent handwashers.19,20 Thorough hand drying also is important in preventing dermatitis. Drying with disposable paper towels is preferred over automated air dryers to prevent aerosolization of microbes.21 Because latex has been implicated in development of ICD, use of latex-free gloves is recommended.22
Alcohol-based sanitizer is not only an effective virucidal agent but also is less likely to cause ICD, therefore promoting hand hygiene adherence. Handwashing with soap still is necessary when hands are visibly dirty but should be performed less frequently if feasible. Hand hygiene and emollient usage education is important for physicians and patients alike, particularly during the COVID-19 crisis.
- Centers for Disease Control and Prevention. Coronavirus disease 2019. how to protect yourself & others. https://www.cdc.gov/coronavirus/2019-ncov/prepare/prevention.html. Updated April 13, 2020. Accessed April 21, 2020.
- World Health Organization. Coronavirus disease (COVID-19) advice for the public. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Updated March 31, 2020. Accessed April 21, 2020.
- Carøe TK, Ebbehøj NE, Bonde JPE, et al. Hand eczema and wet work: dose-response relationship and effect of leaving the profession. Contact Dermatitis. 2018;78:341-347.
- Larson E, Friedman C, Cohran J, et al. Prevalence and correlates of skin damage on the hands of nurses. Heart Lung. 1997;26:404-412.
- Lampel HP, Patel N, Boyse K, et al. Prevalence of hand dermatitis in inpatient nurses at a United States hospital. Dermatitis. 2007;18:140-142.
- Callahan A, Baron E, Fekedulegn D, et al. Winter season, frequent hand washing, and irritant patch test reactions to detergents are associated with hand dermatitis in health care workers. Dermatitis. 2013;24:170-175.
- Lan J, Song Z, Miao X, et al. Skin damage among healthcare workers managing coronavirus disease-2019 [published online March 18, 2020]. J Am Acad Dermatol. 2020;82:1215-1216.
- Katz RJ, Landau P, DeVeaugh-Geiss J, et al. Pharmacological responsiveness of dermatitis secondary to compulsive washing. Psychiatry Res. 1990;34:223-226.
- Larson EL, Hughes CA, Pyrek JD, et al. Changes in bacterial flora associated with skin damage on hands of health care personnel. Am J Infect Control. 1998;26:513-521.
- Held E, Skoet R, Johansen JD, et al. The hand eczema severity index (HECSI): a scoring system for clinical assessment of hand eczema. a study of inter- and intraobserver reliability. Br J Dermatol. 2005;152:302-307.
- Boyce JM, Pittet D, Healthcare Infection Control Practices Advisory Committee, et al. Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HIPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Am J Infect Control. 2002;30:S1-S46.
- Ansari SA, Sattar SA, Springthorpe VS, et al. Invivo protocol for testing efficacy of hand-washing agents against viruses and bacteria—experiments with rotavirus and Escherichi coli. Appl Environ Microbiol. 1989;55:3113-3118.
- Siddharta A, Pfaender S, Vielle NJ, et al. virucidal activity of world health organization-recommended formulations against enveloped viruses, including Zika, Ebola, and emerging coronaviruses. J Infect Dis. 2017;215:902-906.
- Pedersen LK, Held E, Johansen JD, et al. Less skin irritation from alcohol-based disinfectant than from detergent used for hand disinfection. Br J Dermatol. 2005;153:1142-1146.
- Stutz N, Becker D, Jappe U, et al. Nurses’ perceptions of the benefits and adverse effects of hand disinfection: alcohol-based hand rubs vs. hygienic handwashing: a multicentre questionnaire study with additional patch testing by the German Contact Dermatitis Research Group. Br J Dermatol. 2009;160:565-572.
- Wolfe MK, Wells E, Mitro B, et al. Seeking clearer recommendations for hand hygiene in communities facing Ebola: a randomized trial investigating the impact of six handwashing methods on skin irritation and dermatitis. PLoS One. 2016;11:e0167378.
- Pittet D, Allegranzi B, Storr J. The WHO Clean Care is Safer Care programme: field-testing to enhance sustainability and spread of hand hygiene improvements. J Infect Public Health. 2008;1:4-10.
- McCormick RD, Buchman TL, Maki DG. Double-blind, randomized trial of scheduled use of a novel barrier cream and an oil-containing lotion for protecting the hands of health care workers. Am J Infect Control. 2000;28:302-310.
- Berndt U, Wigger-Alberti W, Gabard B, et al. Efficacy of a barrier cream and its vehicle as protective measures against occupational irritant contact dermatitis. Contact Dermatitis. 2000;42:77-80.
- Kampf G, Ennen J. Regular use of a hand cream can attenuate skin dryness and roughness caused by frequent hand washing. BMC Dermatol. 2006;6:1.
- Gammon J, Hunt J. The neglected element of hand hygiene - significance of hand drying, efficiency of different methods, and clinical implication: a review. J Infect Prev. 2019;20:66-74.
- Elston DM. Letter from the editor: occupational skin disease among healthcare workers during the coronavirus (COVID-19) epidemic [published online March 18, 2020]. J Am Acad Dermatol. 2020;82:1085-1086.
Handwashing with antimicrobial soaps or alcohol-based sanitizers is an effective measure in preventing microbial disease transmission. In the context of coronavirus disease 2019 (COVID-19) prevention, the World Health Organization and Centers for Disease Control and Prevention have recommended handwashing with soap and water after coughing/sneezing, visiting a public place, touching surfaces outside the home, and taking care of a sick person(s), as well as before and after eating. When soap and water are not available, alcohol-based sanitizers may be used.1,2
Irritant contact dermatitis (ICD) is most commonly associated with wet work and is frequently seen in health care workers in relation to hand hygiene, with survey-based studies reporting 25% to 55% of nurses affected.3-5 In a prospective study (N=102), health care workers who washed their hands more than 10 times per day were55% more likely to develop hand dermatitis.6 Frequent ICD of the hands has been reported in Chinese health care workers in association with COVID-19.7 Handwashing and/or glove wearing may be newly prioritized by workers who handle frequently touched goods and surfaces, such as flight attendants (Figure). Patients with obsessive-compulsive disorder may be another vulnerable population.8

Alcohol-based sanitizers and detergents or antimicrobials in soaps may cause ICD of the hands by denaturation of stratum corneum proteins, depletion of intercellular lipids, and decreased corneocyte cohesion. These agents alter the skin flora, with increased colonization by staphylococci and gram-negative bacilli.9 Clinical findings include xerosis, scaling, fissuring, and bleeding. Physicians may evaluate severity of ICD of the hands using the
Cleansing the hands with alcohol-based sanitizers has consistently shown equivalent or greater efficacy than antimicrobial soaps for eradication of most microbes, with exception of bacterial spores and protozoan oocysts.11 In an in vivo experiment, 70% ethanol solution was more effective in eradicating rotavirus from the fingerpads of adults than 10% povidone-iodine solution, nonmedicated soaps, and soaps containing chloroxylenol 4.8% or chlorhexidine gluconate 4%.12 Coronavirus disease 2019 is a lipophilic enveloped virus. The lipid-dissolving effects of alcohol-based sanitizers is especially effective against these kinds of viruses. An in vitro experiment showed that alcohol solutions are effective against enveloped viruses including severe acute respiratory syndrome coronavirus, Ebola virus, and Zika virus.13 There are limited data for the virucidal efficacy of non–alcohol-based sanitizers containing quaternary ammonium compounds (most commonly benzalkonium chloride) and therefore they are not recommended for protection against COVID-19. Handwashing is preferred over alcohol-based solutions when hands are visibly dirty.
Alcohol-based sanitizers typically are less likely to cause ICD than handwashing with detergent-based or antimicrobial soaps. Antimicrobial ingredients in soaps such as chlorhexidine, chloroxylenol, and triclosan are frequent culprits.11 Detergents in soap such as sodium laureth sulfate cause more skin irritation and transepidermal water loss than alcohol14; however, among health care workers, alcohol-based sanitizers often are perceived as more damaging to the skin.15 During the 2014 Ebola outbreak, use of alcohol-based sanitizers vs handwashing resulted in lower hand eczema severity index scores (n=108).16
Propensity for ICD is a limiting factor in hand hygiene adherence.17 In a double-blind randomized trial (N=54), scheduled use of an oil-containing lotion was shown to increase compliance with hand hygiene protocols in health care workers by preventing cracks, scaling, and pain.18 Using sanitizers containing humectants (eg, aloe vera gel) or moisturizers with petrolatum, liquid paraffin, glycerin, or mineral oil have all been shown to decrease the incidence of ICD in frequent handwashers.19,20 Thorough hand drying also is important in preventing dermatitis. Drying with disposable paper towels is preferred over automated air dryers to prevent aerosolization of microbes.21 Because latex has been implicated in development of ICD, use of latex-free gloves is recommended.22
Alcohol-based sanitizer is not only an effective virucidal agent but also is less likely to cause ICD, therefore promoting hand hygiene adherence. Handwashing with soap still is necessary when hands are visibly dirty but should be performed less frequently if feasible. Hand hygiene and emollient usage education is important for physicians and patients alike, particularly during the COVID-19 crisis.
Handwashing with antimicrobial soaps or alcohol-based sanitizers is an effective measure in preventing microbial disease transmission. In the context of coronavirus disease 2019 (COVID-19) prevention, the World Health Organization and Centers for Disease Control and Prevention have recommended handwashing with soap and water after coughing/sneezing, visiting a public place, touching surfaces outside the home, and taking care of a sick person(s), as well as before and after eating. When soap and water are not available, alcohol-based sanitizers may be used.1,2
Irritant contact dermatitis (ICD) is most commonly associated with wet work and is frequently seen in health care workers in relation to hand hygiene, with survey-based studies reporting 25% to 55% of nurses affected.3-5 In a prospective study (N=102), health care workers who washed their hands more than 10 times per day were55% more likely to develop hand dermatitis.6 Frequent ICD of the hands has been reported in Chinese health care workers in association with COVID-19.7 Handwashing and/or glove wearing may be newly prioritized by workers who handle frequently touched goods and surfaces, such as flight attendants (Figure). Patients with obsessive-compulsive disorder may be another vulnerable population.8

Alcohol-based sanitizers and detergents or antimicrobials in soaps may cause ICD of the hands by denaturation of stratum corneum proteins, depletion of intercellular lipids, and decreased corneocyte cohesion. These agents alter the skin flora, with increased colonization by staphylococci and gram-negative bacilli.9 Clinical findings include xerosis, scaling, fissuring, and bleeding. Physicians may evaluate severity of ICD of the hands using the
Cleansing the hands with alcohol-based sanitizers has consistently shown equivalent or greater efficacy than antimicrobial soaps for eradication of most microbes, with exception of bacterial spores and protozoan oocysts.11 In an in vivo experiment, 70% ethanol solution was more effective in eradicating rotavirus from the fingerpads of adults than 10% povidone-iodine solution, nonmedicated soaps, and soaps containing chloroxylenol 4.8% or chlorhexidine gluconate 4%.12 Coronavirus disease 2019 is a lipophilic enveloped virus. The lipid-dissolving effects of alcohol-based sanitizers is especially effective against these kinds of viruses. An in vitro experiment showed that alcohol solutions are effective against enveloped viruses including severe acute respiratory syndrome coronavirus, Ebola virus, and Zika virus.13 There are limited data for the virucidal efficacy of non–alcohol-based sanitizers containing quaternary ammonium compounds (most commonly benzalkonium chloride) and therefore they are not recommended for protection against COVID-19. Handwashing is preferred over alcohol-based solutions when hands are visibly dirty.
Alcohol-based sanitizers typically are less likely to cause ICD than handwashing with detergent-based or antimicrobial soaps. Antimicrobial ingredients in soaps such as chlorhexidine, chloroxylenol, and triclosan are frequent culprits.11 Detergents in soap such as sodium laureth sulfate cause more skin irritation and transepidermal water loss than alcohol14; however, among health care workers, alcohol-based sanitizers often are perceived as more damaging to the skin.15 During the 2014 Ebola outbreak, use of alcohol-based sanitizers vs handwashing resulted in lower hand eczema severity index scores (n=108).16
Propensity for ICD is a limiting factor in hand hygiene adherence.17 In a double-blind randomized trial (N=54), scheduled use of an oil-containing lotion was shown to increase compliance with hand hygiene protocols in health care workers by preventing cracks, scaling, and pain.18 Using sanitizers containing humectants (eg, aloe vera gel) or moisturizers with petrolatum, liquid paraffin, glycerin, or mineral oil have all been shown to decrease the incidence of ICD in frequent handwashers.19,20 Thorough hand drying also is important in preventing dermatitis. Drying with disposable paper towels is preferred over automated air dryers to prevent aerosolization of microbes.21 Because latex has been implicated in development of ICD, use of latex-free gloves is recommended.22
Alcohol-based sanitizer is not only an effective virucidal agent but also is less likely to cause ICD, therefore promoting hand hygiene adherence. Handwashing with soap still is necessary when hands are visibly dirty but should be performed less frequently if feasible. Hand hygiene and emollient usage education is important for physicians and patients alike, particularly during the COVID-19 crisis.
- Centers for Disease Control and Prevention. Coronavirus disease 2019. how to protect yourself & others. https://www.cdc.gov/coronavirus/2019-ncov/prepare/prevention.html. Updated April 13, 2020. Accessed April 21, 2020.
- World Health Organization. Coronavirus disease (COVID-19) advice for the public. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Updated March 31, 2020. Accessed April 21, 2020.
- Carøe TK, Ebbehøj NE, Bonde JPE, et al. Hand eczema and wet work: dose-response relationship and effect of leaving the profession. Contact Dermatitis. 2018;78:341-347.
- Larson E, Friedman C, Cohran J, et al. Prevalence and correlates of skin damage on the hands of nurses. Heart Lung. 1997;26:404-412.
- Lampel HP, Patel N, Boyse K, et al. Prevalence of hand dermatitis in inpatient nurses at a United States hospital. Dermatitis. 2007;18:140-142.
- Callahan A, Baron E, Fekedulegn D, et al. Winter season, frequent hand washing, and irritant patch test reactions to detergents are associated with hand dermatitis in health care workers. Dermatitis. 2013;24:170-175.
- Lan J, Song Z, Miao X, et al. Skin damage among healthcare workers managing coronavirus disease-2019 [published online March 18, 2020]. J Am Acad Dermatol. 2020;82:1215-1216.
- Katz RJ, Landau P, DeVeaugh-Geiss J, et al. Pharmacological responsiveness of dermatitis secondary to compulsive washing. Psychiatry Res. 1990;34:223-226.
- Larson EL, Hughes CA, Pyrek JD, et al. Changes in bacterial flora associated with skin damage on hands of health care personnel. Am J Infect Control. 1998;26:513-521.
- Held E, Skoet R, Johansen JD, et al. The hand eczema severity index (HECSI): a scoring system for clinical assessment of hand eczema. a study of inter- and intraobserver reliability. Br J Dermatol. 2005;152:302-307.
- Boyce JM, Pittet D, Healthcare Infection Control Practices Advisory Committee, et al. Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HIPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Am J Infect Control. 2002;30:S1-S46.
- Ansari SA, Sattar SA, Springthorpe VS, et al. Invivo protocol for testing efficacy of hand-washing agents against viruses and bacteria—experiments with rotavirus and Escherichi coli. Appl Environ Microbiol. 1989;55:3113-3118.
- Siddharta A, Pfaender S, Vielle NJ, et al. virucidal activity of world health organization-recommended formulations against enveloped viruses, including Zika, Ebola, and emerging coronaviruses. J Infect Dis. 2017;215:902-906.
- Pedersen LK, Held E, Johansen JD, et al. Less skin irritation from alcohol-based disinfectant than from detergent used for hand disinfection. Br J Dermatol. 2005;153:1142-1146.
- Stutz N, Becker D, Jappe U, et al. Nurses’ perceptions of the benefits and adverse effects of hand disinfection: alcohol-based hand rubs vs. hygienic handwashing: a multicentre questionnaire study with additional patch testing by the German Contact Dermatitis Research Group. Br J Dermatol. 2009;160:565-572.
- Wolfe MK, Wells E, Mitro B, et al. Seeking clearer recommendations for hand hygiene in communities facing Ebola: a randomized trial investigating the impact of six handwashing methods on skin irritation and dermatitis. PLoS One. 2016;11:e0167378.
- Pittet D, Allegranzi B, Storr J. The WHO Clean Care is Safer Care programme: field-testing to enhance sustainability and spread of hand hygiene improvements. J Infect Public Health. 2008;1:4-10.
- McCormick RD, Buchman TL, Maki DG. Double-blind, randomized trial of scheduled use of a novel barrier cream and an oil-containing lotion for protecting the hands of health care workers. Am J Infect Control. 2000;28:302-310.
- Berndt U, Wigger-Alberti W, Gabard B, et al. Efficacy of a barrier cream and its vehicle as protective measures against occupational irritant contact dermatitis. Contact Dermatitis. 2000;42:77-80.
- Kampf G, Ennen J. Regular use of a hand cream can attenuate skin dryness and roughness caused by frequent hand washing. BMC Dermatol. 2006;6:1.
- Gammon J, Hunt J. The neglected element of hand hygiene - significance of hand drying, efficiency of different methods, and clinical implication: a review. J Infect Prev. 2019;20:66-74.
- Elston DM. Letter from the editor: occupational skin disease among healthcare workers during the coronavirus (COVID-19) epidemic [published online March 18, 2020]. J Am Acad Dermatol. 2020;82:1085-1086.
- Centers for Disease Control and Prevention. Coronavirus disease 2019. how to protect yourself & others. https://www.cdc.gov/coronavirus/2019-ncov/prepare/prevention.html. Updated April 13, 2020. Accessed April 21, 2020.
- World Health Organization. Coronavirus disease (COVID-19) advice for the public. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Updated March 31, 2020. Accessed April 21, 2020.
- Carøe TK, Ebbehøj NE, Bonde JPE, et al. Hand eczema and wet work: dose-response relationship and effect of leaving the profession. Contact Dermatitis. 2018;78:341-347.
- Larson E, Friedman C, Cohran J, et al. Prevalence and correlates of skin damage on the hands of nurses. Heart Lung. 1997;26:404-412.
- Lampel HP, Patel N, Boyse K, et al. Prevalence of hand dermatitis in inpatient nurses at a United States hospital. Dermatitis. 2007;18:140-142.
- Callahan A, Baron E, Fekedulegn D, et al. Winter season, frequent hand washing, and irritant patch test reactions to detergents are associated with hand dermatitis in health care workers. Dermatitis. 2013;24:170-175.
- Lan J, Song Z, Miao X, et al. Skin damage among healthcare workers managing coronavirus disease-2019 [published online March 18, 2020]. J Am Acad Dermatol. 2020;82:1215-1216.
- Katz RJ, Landau P, DeVeaugh-Geiss J, et al. Pharmacological responsiveness of dermatitis secondary to compulsive washing. Psychiatry Res. 1990;34:223-226.
- Larson EL, Hughes CA, Pyrek JD, et al. Changes in bacterial flora associated with skin damage on hands of health care personnel. Am J Infect Control. 1998;26:513-521.
- Held E, Skoet R, Johansen JD, et al. The hand eczema severity index (HECSI): a scoring system for clinical assessment of hand eczema. a study of inter- and intraobserver reliability. Br J Dermatol. 2005;152:302-307.
- Boyce JM, Pittet D, Healthcare Infection Control Practices Advisory Committee, et al. Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HIPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Am J Infect Control. 2002;30:S1-S46.
- Ansari SA, Sattar SA, Springthorpe VS, et al. Invivo protocol for testing efficacy of hand-washing agents against viruses and bacteria—experiments with rotavirus and Escherichi coli. Appl Environ Microbiol. 1989;55:3113-3118.
- Siddharta A, Pfaender S, Vielle NJ, et al. virucidal activity of world health organization-recommended formulations against enveloped viruses, including Zika, Ebola, and emerging coronaviruses. J Infect Dis. 2017;215:902-906.
- Pedersen LK, Held E, Johansen JD, et al. Less skin irritation from alcohol-based disinfectant than from detergent used for hand disinfection. Br J Dermatol. 2005;153:1142-1146.
- Stutz N, Becker D, Jappe U, et al. Nurses’ perceptions of the benefits and adverse effects of hand disinfection: alcohol-based hand rubs vs. hygienic handwashing: a multicentre questionnaire study with additional patch testing by the German Contact Dermatitis Research Group. Br J Dermatol. 2009;160:565-572.
- Wolfe MK, Wells E, Mitro B, et al. Seeking clearer recommendations for hand hygiene in communities facing Ebola: a randomized trial investigating the impact of six handwashing methods on skin irritation and dermatitis. PLoS One. 2016;11:e0167378.
- Pittet D, Allegranzi B, Storr J. The WHO Clean Care is Safer Care programme: field-testing to enhance sustainability and spread of hand hygiene improvements. J Infect Public Health. 2008;1:4-10.
- McCormick RD, Buchman TL, Maki DG. Double-blind, randomized trial of scheduled use of a novel barrier cream and an oil-containing lotion for protecting the hands of health care workers. Am J Infect Control. 2000;28:302-310.
- Berndt U, Wigger-Alberti W, Gabard B, et al. Efficacy of a barrier cream and its vehicle as protective measures against occupational irritant contact dermatitis. Contact Dermatitis. 2000;42:77-80.
- Kampf G, Ennen J. Regular use of a hand cream can attenuate skin dryness and roughness caused by frequent hand washing. BMC Dermatol. 2006;6:1.
- Gammon J, Hunt J. The neglected element of hand hygiene - significance of hand drying, efficiency of different methods, and clinical implication: a review. J Infect Prev. 2019;20:66-74.
- Elston DM. Letter from the editor: occupational skin disease among healthcare workers during the coronavirus (COVID-19) epidemic [published online March 18, 2020]. J Am Acad Dermatol. 2020;82:1085-1086.
Practice Points
- Alcohol-based sanitizers are as or even more effective as handwashing with soap and water for preventing disease transmission of enveloped viruses such as severe acute respiratory syndrome coronavirus.
- Although perceived as more irritating, alcohol-based sanitizers are less likely to cause irritant contact dermatitis of the hands than handwashing with soap and water.
- Use of humectants, moisturizers, and/or emollients in combination with alcohol-based sanitizers allows for effective hand hygiene without irritating the skin.
Sensitizer prevalent in many hypoallergenic products for children
(AD) and allergic contact dermatitis, according to a research letter in the Journal of the American Academy of Dermatology.
In the letter, the authors, Reid W. Collis, of Washington University in St. Louis, and David M. Sheinbein, MD, of the division of dermatology at the university, referred to a previous study showing an association between contact sensitivity with CAPB and people with a history of AD. This was supported by the results of their own recent study in pediatric patients, they wrote, which found that reactions to CAPB were “exclusively” in patients with AD.
In the survey, they looked at children’s shampoo and soap products available on online databases of six of the biggest retailers, and analyzed the top 20 best-selling products for each retailer in 2018. Of the unique products, CAPB was found to be an ingredient in 52% (39 of 75) of the shampoos and 44% (29 of 66) of the soap products. But each of these products “contained the term ‘hypoallergenic; on the product itself or in the product’s description,” they noted.
“CAPB is a prevalent sensitizer in pediatric patients and should be avoided in patients with AD,” the investigators wrote. That said, it’s not included among the 35 prevalent allergens in the T.R.U.E. test, and they recommended that pediatricians and dermatologists “be aware of common products containing CAPB when counseling patients about their product choices,” considering that CAPB sensitivity is more likely in patients with AD.
The study had no funding source, and the authors had no disclosures.
[email protected]
SOURCE: Cho SI et al. J Am Acad Dermatol. 2020 May. doi: 10.1016/j.jaad.2019.12.036.
(AD) and allergic contact dermatitis, according to a research letter in the Journal of the American Academy of Dermatology.
In the letter, the authors, Reid W. Collis, of Washington University in St. Louis, and David M. Sheinbein, MD, of the division of dermatology at the university, referred to a previous study showing an association between contact sensitivity with CAPB and people with a history of AD. This was supported by the results of their own recent study in pediatric patients, they wrote, which found that reactions to CAPB were “exclusively” in patients with AD.
In the survey, they looked at children’s shampoo and soap products available on online databases of six of the biggest retailers, and analyzed the top 20 best-selling products for each retailer in 2018. Of the unique products, CAPB was found to be an ingredient in 52% (39 of 75) of the shampoos and 44% (29 of 66) of the soap products. But each of these products “contained the term ‘hypoallergenic; on the product itself or in the product’s description,” they noted.
“CAPB is a prevalent sensitizer in pediatric patients and should be avoided in patients with AD,” the investigators wrote. That said, it’s not included among the 35 prevalent allergens in the T.R.U.E. test, and they recommended that pediatricians and dermatologists “be aware of common products containing CAPB when counseling patients about their product choices,” considering that CAPB sensitivity is more likely in patients with AD.
The study had no funding source, and the authors had no disclosures.
[email protected]
SOURCE: Cho SI et al. J Am Acad Dermatol. 2020 May. doi: 10.1016/j.jaad.2019.12.036.
(AD) and allergic contact dermatitis, according to a research letter in the Journal of the American Academy of Dermatology.
In the letter, the authors, Reid W. Collis, of Washington University in St. Louis, and David M. Sheinbein, MD, of the division of dermatology at the university, referred to a previous study showing an association between contact sensitivity with CAPB and people with a history of AD. This was supported by the results of their own recent study in pediatric patients, they wrote, which found that reactions to CAPB were “exclusively” in patients with AD.
In the survey, they looked at children’s shampoo and soap products available on online databases of six of the biggest retailers, and analyzed the top 20 best-selling products for each retailer in 2018. Of the unique products, CAPB was found to be an ingredient in 52% (39 of 75) of the shampoos and 44% (29 of 66) of the soap products. But each of these products “contained the term ‘hypoallergenic; on the product itself or in the product’s description,” they noted.
“CAPB is a prevalent sensitizer in pediatric patients and should be avoided in patients with AD,” the investigators wrote. That said, it’s not included among the 35 prevalent allergens in the T.R.U.E. test, and they recommended that pediatricians and dermatologists “be aware of common products containing CAPB when counseling patients about their product choices,” considering that CAPB sensitivity is more likely in patients with AD.
The study had no funding source, and the authors had no disclosures.
[email protected]
SOURCE: Cho SI et al. J Am Acad Dermatol. 2020 May. doi: 10.1016/j.jaad.2019.12.036.
Minimally Hyperpigmented Plaque With Skin Thickening on the Neck
The Diagnosis: Fiddler's Neck
A thorough patient history revealed that the patient was retired and played violin regularly in the local orchestra. Fiddler's neck, or violin hickey, is an uncommon physical examination finding and often is considered a badge of honor by musicians who develop it. Fiddler's neck is a hobby-related callus seen in highly dedicated violin and viola players, and in some circles, it is known as a mark of greatness. In one instance, members of the public were asked to display a violin hickey before they were allowed to play a $3.5 million violin on public display in London, England.1 Fiddler's neck is a benign submandibular lesion caused by pressure and friction on the skin from extensive time spent playing the instrument. The primary cause is thought to be mechanical, but it is not fully understood why the lesion occurs in some musicians and not others, regardless of playing time.1 This submandibular fiddler's neck is distinct from a similarly named supraclavicular lesion, which represents an allergic contact dermatitis to the nickel bracket of the instrument's chin rest and presents with eczematous scale and/or vesicles.2,3 Submandibular fiddler's neck presents with some combination of erythema, edema, lichenification, and scarring just below the angle of the jaw. Occasionally, papules, pustules, and even cyst formation may be noted. Lesions are sometimes mistaken for malignancy or lymphedema. Therefore, a thorough history and clinical expertise are important, as surgical excision should be avoided.2
Depending on presentation, the differential diagnosis also may include malignant melanoma due to irregular pigmentation, branchial cleft cyst or sialolithiasis due to location and texture, or a tumor of the salivary gland.
Management of fiddler's neck may include topical steroids, neck or instrument padding, or decreased playing time. However, the lesion often is worn with pride, seen as a testament to the musician's dedication, and reassurance generally is most appropriate.1
- Roberts C. How to prevent or even cure a violin hickey. Strings. February 1, 2011. https://stringsmagazine.com/how-to-prevent-or-even-cure-a-violin-hickey/. Accessed January 31, 2020.
- Myint CW, Rutt AL, Sataloff RT. Fiddler's neck: a review. Ear Nose Throat J. 2017;96:76-79.
- Jue MS, Kim YS, Ro YS. Fiddler's neck accompanied by allergic contact dermatitis to nickel in a viola player. Ann Dermatol. 2010;22:88-90.
The Diagnosis: Fiddler's Neck
A thorough patient history revealed that the patient was retired and played violin regularly in the local orchestra. Fiddler's neck, or violin hickey, is an uncommon physical examination finding and often is considered a badge of honor by musicians who develop it. Fiddler's neck is a hobby-related callus seen in highly dedicated violin and viola players, and in some circles, it is known as a mark of greatness. In one instance, members of the public were asked to display a violin hickey before they were allowed to play a $3.5 million violin on public display in London, England.1 Fiddler's neck is a benign submandibular lesion caused by pressure and friction on the skin from extensive time spent playing the instrument. The primary cause is thought to be mechanical, but it is not fully understood why the lesion occurs in some musicians and not others, regardless of playing time.1 This submandibular fiddler's neck is distinct from a similarly named supraclavicular lesion, which represents an allergic contact dermatitis to the nickel bracket of the instrument's chin rest and presents with eczematous scale and/or vesicles.2,3 Submandibular fiddler's neck presents with some combination of erythema, edema, lichenification, and scarring just below the angle of the jaw. Occasionally, papules, pustules, and even cyst formation may be noted. Lesions are sometimes mistaken for malignancy or lymphedema. Therefore, a thorough history and clinical expertise are important, as surgical excision should be avoided.2
Depending on presentation, the differential diagnosis also may include malignant melanoma due to irregular pigmentation, branchial cleft cyst or sialolithiasis due to location and texture, or a tumor of the salivary gland.
Management of fiddler's neck may include topical steroids, neck or instrument padding, or decreased playing time. However, the lesion often is worn with pride, seen as a testament to the musician's dedication, and reassurance generally is most appropriate.1
The Diagnosis: Fiddler's Neck
A thorough patient history revealed that the patient was retired and played violin regularly in the local orchestra. Fiddler's neck, or violin hickey, is an uncommon physical examination finding and often is considered a badge of honor by musicians who develop it. Fiddler's neck is a hobby-related callus seen in highly dedicated violin and viola players, and in some circles, it is known as a mark of greatness. In one instance, members of the public were asked to display a violin hickey before they were allowed to play a $3.5 million violin on public display in London, England.1 Fiddler's neck is a benign submandibular lesion caused by pressure and friction on the skin from extensive time spent playing the instrument. The primary cause is thought to be mechanical, but it is not fully understood why the lesion occurs in some musicians and not others, regardless of playing time.1 This submandibular fiddler's neck is distinct from a similarly named supraclavicular lesion, which represents an allergic contact dermatitis to the nickel bracket of the instrument's chin rest and presents with eczematous scale and/or vesicles.2,3 Submandibular fiddler's neck presents with some combination of erythema, edema, lichenification, and scarring just below the angle of the jaw. Occasionally, papules, pustules, and even cyst formation may be noted. Lesions are sometimes mistaken for malignancy or lymphedema. Therefore, a thorough history and clinical expertise are important, as surgical excision should be avoided.2
Depending on presentation, the differential diagnosis also may include malignant melanoma due to irregular pigmentation, branchial cleft cyst or sialolithiasis due to location and texture, or a tumor of the salivary gland.
Management of fiddler's neck may include topical steroids, neck or instrument padding, or decreased playing time. However, the lesion often is worn with pride, seen as a testament to the musician's dedication, and reassurance generally is most appropriate.1
- Roberts C. How to prevent or even cure a violin hickey. Strings. February 1, 2011. https://stringsmagazine.com/how-to-prevent-or-even-cure-a-violin-hickey/. Accessed January 31, 2020.
- Myint CW, Rutt AL, Sataloff RT. Fiddler's neck: a review. Ear Nose Throat J. 2017;96:76-79.
- Jue MS, Kim YS, Ro YS. Fiddler's neck accompanied by allergic contact dermatitis to nickel in a viola player. Ann Dermatol. 2010;22:88-90.
- Roberts C. How to prevent or even cure a violin hickey. Strings. February 1, 2011. https://stringsmagazine.com/how-to-prevent-or-even-cure-a-violin-hickey/. Accessed January 31, 2020.
- Myint CW, Rutt AL, Sataloff RT. Fiddler's neck: a review. Ear Nose Throat J. 2017;96:76-79.
- Jue MS, Kim YS, Ro YS. Fiddler's neck accompanied by allergic contact dermatitis to nickel in a viola player. Ann Dermatol. 2010;22:88-90.
A 74-year-old man with a history of melanoma and basal cell carcinoma presented for an annual skin examination and displayed asymptomatic stable thickening of skin on the left side of the neck below the jawline of several years' duration. Physical examination revealed a 4×2-cm minimally hyperpigmented plaque with skin thickening and a pebbly appearing surface on the left lateral neck just inferior to the angle of the mandible.
Phytophotodermatitis in a Butterfly Enthusiast Induced by Common Rue
To the Editor:
Phytophotodermatitis is common in dermatology during the summer months, especially in individuals who spend time outdoors; however, identification of the offending plant can be challenging. We report a case of phytophotodermatitis in which the causative plant, common rue, was not identified until it was revealed that the patient was a butterfly enthusiast.
A 60-year-old woman presented to the outpatient dermatology clinic in late summer for a routine skin examination. An eruption was noted over the right thigh and knee that had first appeared approximately 2 weeks prior. The rash started as pruritic blisters but gradually progressed to erythema and then eventually to brown markings, which were observed at the current presentation. Physical examination revealed hyperpigmented, brown, streaky, linear patches and plaques over the right thigh, knee, and lower leg (Figure). When asked about her hobbies, the patient reported an affinity for butterflies and noted that she attracts them with specific species of plants in her garden. She recalled recently planting the herb of grace, or common rue, to attract the giant swallowtail butterfly (Papilio cresphontes). Upon further inquiry, she remembered working in the garden on her knees and digging up roots near the common rue plant while wearing shorts approximately 2 weeks prior to the current presentation. Given the streaky linear pattern of the eruption along with recent sun exposure and exposure to the common rue plant, a diagnosis of phytophotodermatitis was made. No further treatment was sought, as the eruption was not bothersome to her. She was intrigued that the common rue plant had caused the dermatitis and planned on taking proper precautions when working near the plant in the future.
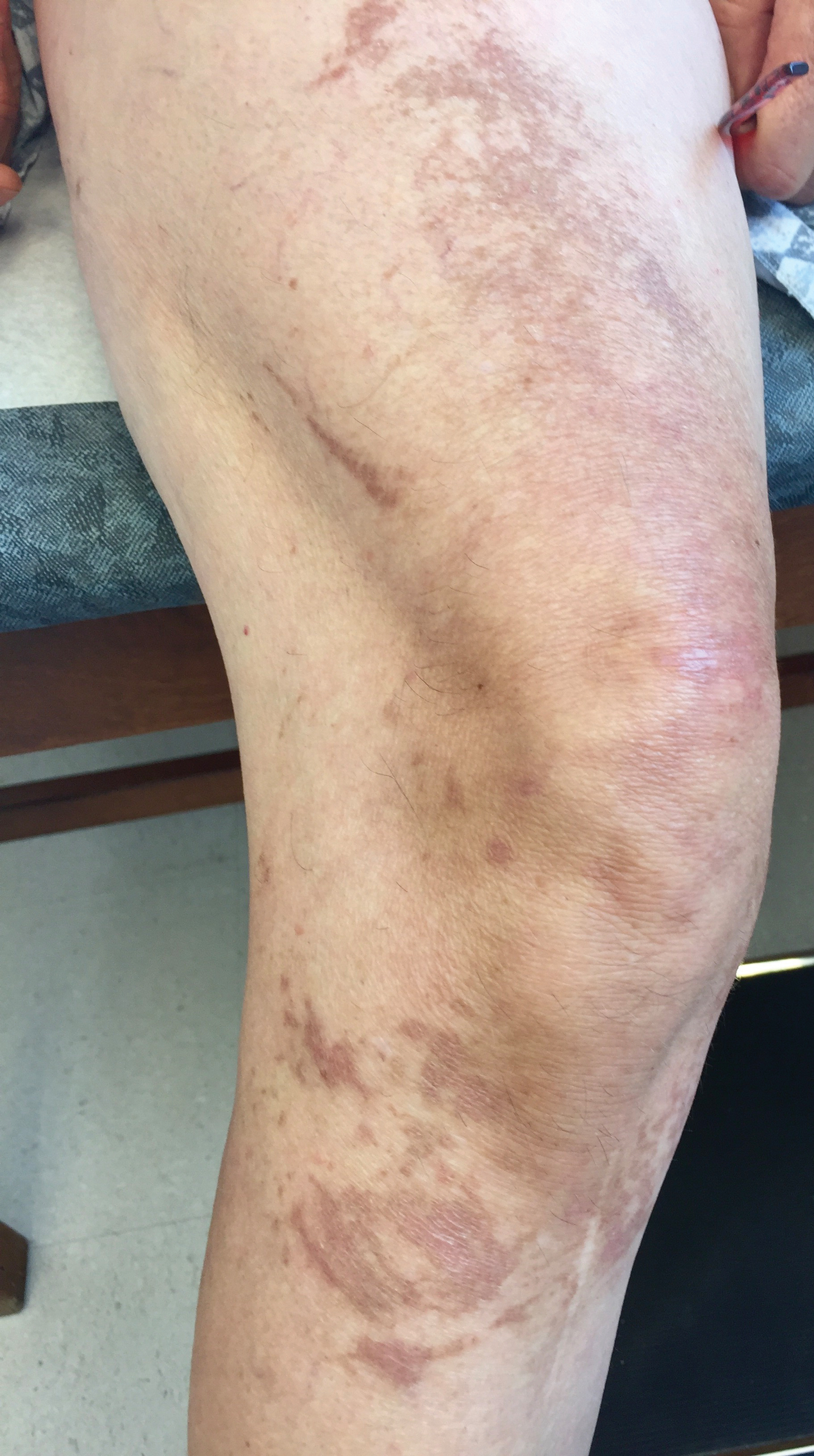
In this case, the observed phototoxic skin findings resulted from exposure to common rue (Ruta graveolens),a pungently scented evergreen shrub native to the Mediterranean region and a member of the Rutaceae family. Extracts have been used in homeopathic practices for bruises, sprains, headache, neck stiffness, rheumatologic pain, neuralgia, stomach problems, and phlebitis, as well as in seasonings, soaps, creams, and perfumes.1 The most commonly encountered plants known to cause phytophotodermatitis belong to the Apiaceae and Rutaceae families.2 Members of Apiaceae include angelica, celery, dill, fennel, hogweed, parsley, and parsnip. Aside from the common rue plant, the Rutaceae family also includes bergamot orange, bitter orange, burning bush (or gas plant), grapefruit, lemon, and lime. Other potential offending agents are fig, mustard, buttercup, St. John’s wort, and scurfpea. The phototoxic properties are due to furocoumarins, which include psoralens and angelicins. They are inert until activated by UVA radiation, which inflicts direct cellular damage, causing vacuolization and apoptosis of keratinocytes, similar to a sunburn.3 Clinical findings typically present 24 hours after sun exposure with erythema, edema, pain, and occasionally vesicles or bullae in severe cases. Unlike sunburn, lesions often present in linear, streaky, or bizarre patterns, reflective of the direct contact with the plant. The lesions eventually transition to hyperpigmentation, which may take months to years to resolve.
Other considerations in cases of suspected phytophotodermatitis include polymorphic light eruption, actinic prurigo, hydroa vacciniforme, chronic actinic dermatitis, solar urticaria, drug reactions, porphyria, Smith-Lemli-Opitz syndrome, lupus erythematosus, and dermatomyositis.4 Clinicians should suspect phytophotodermatitis with phototoxic findings in bartenders, citrus farm workers, gardeners, chefs, and kitchen workers, especially those handling limes and celery. As in our case, phytophotodermatitis also should be considered in butterfly enthusiasts trying to attract the giant swallowtail butterfly. The caterpillars feed on the leaves of the common rue plant, one of a select few plants that giant swallowtail butterflies use as a host due to its bitter leaves that aid in avoiding predators.5
This case illustrates a unique perspective of phytophotodermatitis, as butterfly enthusiasm is not commonly reported in association with the common rue plant with respect to phytophotodermatitis. This case underscores the importance of inquiring about patients’ professions and hobbies, both in dermatology and other specialties.
- Atta AH, Alkofahi A. Anti-nociceptive and anti-inflammatory effects of some Jordanian medicinal plant extracts. J Ethnopharmacol. 1998;60:117-124.
- McGovern TW. Dermatoses due to plants. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. 2nd ed. Edinburgh, Scotland: Mosby; 2007:265-283.
- Hawk JLM, Calonje E. The photosensitivity disorders. In: Elder DE, ed. Lever’s Histopathology of the Skin. 9th ed. Philadelphia, Pennsylvania: Lippincott Williams and Wilkins; 2005:345-353.
- Lim HW. Abnormal responses to ultraviolet radiation: photosensitivity induced by exogenous agents. In: Wolff K, Goldsmith LA, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012:1066-1074.
- McAuslane H. Giant swallowtail. University of Florida Department of Entomology and Nematology Featured Creatures website. http://entnemdept.ufl.edu/creatures/citrus/giantswallowtail.htm. Revised January 2018. Accessed April 10, 2020.
To the Editor:
Phytophotodermatitis is common in dermatology during the summer months, especially in individuals who spend time outdoors; however, identification of the offending plant can be challenging. We report a case of phytophotodermatitis in which the causative plant, common rue, was not identified until it was revealed that the patient was a butterfly enthusiast.
A 60-year-old woman presented to the outpatient dermatology clinic in late summer for a routine skin examination. An eruption was noted over the right thigh and knee that had first appeared approximately 2 weeks prior. The rash started as pruritic blisters but gradually progressed to erythema and then eventually to brown markings, which were observed at the current presentation. Physical examination revealed hyperpigmented, brown, streaky, linear patches and plaques over the right thigh, knee, and lower leg (Figure). When asked about her hobbies, the patient reported an affinity for butterflies and noted that she attracts them with specific species of plants in her garden. She recalled recently planting the herb of grace, or common rue, to attract the giant swallowtail butterfly (Papilio cresphontes). Upon further inquiry, she remembered working in the garden on her knees and digging up roots near the common rue plant while wearing shorts approximately 2 weeks prior to the current presentation. Given the streaky linear pattern of the eruption along with recent sun exposure and exposure to the common rue plant, a diagnosis of phytophotodermatitis was made. No further treatment was sought, as the eruption was not bothersome to her. She was intrigued that the common rue plant had caused the dermatitis and planned on taking proper precautions when working near the plant in the future.

In this case, the observed phototoxic skin findings resulted from exposure to common rue (Ruta graveolens),a pungently scented evergreen shrub native to the Mediterranean region and a member of the Rutaceae family. Extracts have been used in homeopathic practices for bruises, sprains, headache, neck stiffness, rheumatologic pain, neuralgia, stomach problems, and phlebitis, as well as in seasonings, soaps, creams, and perfumes.1 The most commonly encountered plants known to cause phytophotodermatitis belong to the Apiaceae and Rutaceae families.2 Members of Apiaceae include angelica, celery, dill, fennel, hogweed, parsley, and parsnip. Aside from the common rue plant, the Rutaceae family also includes bergamot orange, bitter orange, burning bush (or gas plant), grapefruit, lemon, and lime. Other potential offending agents are fig, mustard, buttercup, St. John’s wort, and scurfpea. The phototoxic properties are due to furocoumarins, which include psoralens and angelicins. They are inert until activated by UVA radiation, which inflicts direct cellular damage, causing vacuolization and apoptosis of keratinocytes, similar to a sunburn.3 Clinical findings typically present 24 hours after sun exposure with erythema, edema, pain, and occasionally vesicles or bullae in severe cases. Unlike sunburn, lesions often present in linear, streaky, or bizarre patterns, reflective of the direct contact with the plant. The lesions eventually transition to hyperpigmentation, which may take months to years to resolve.
Other considerations in cases of suspected phytophotodermatitis include polymorphic light eruption, actinic prurigo, hydroa vacciniforme, chronic actinic dermatitis, solar urticaria, drug reactions, porphyria, Smith-Lemli-Opitz syndrome, lupus erythematosus, and dermatomyositis.4 Clinicians should suspect phytophotodermatitis with phototoxic findings in bartenders, citrus farm workers, gardeners, chefs, and kitchen workers, especially those handling limes and celery. As in our case, phytophotodermatitis also should be considered in butterfly enthusiasts trying to attract the giant swallowtail butterfly. The caterpillars feed on the leaves of the common rue plant, one of a select few plants that giant swallowtail butterflies use as a host due to its bitter leaves that aid in avoiding predators.5
This case illustrates a unique perspective of phytophotodermatitis, as butterfly enthusiasm is not commonly reported in association with the common rue plant with respect to phytophotodermatitis. This case underscores the importance of inquiring about patients’ professions and hobbies, both in dermatology and other specialties.
To the Editor:
Phytophotodermatitis is common in dermatology during the summer months, especially in individuals who spend time outdoors; however, identification of the offending plant can be challenging. We report a case of phytophotodermatitis in which the causative plant, common rue, was not identified until it was revealed that the patient was a butterfly enthusiast.
A 60-year-old woman presented to the outpatient dermatology clinic in late summer for a routine skin examination. An eruption was noted over the right thigh and knee that had first appeared approximately 2 weeks prior. The rash started as pruritic blisters but gradually progressed to erythema and then eventually to brown markings, which were observed at the current presentation. Physical examination revealed hyperpigmented, brown, streaky, linear patches and plaques over the right thigh, knee, and lower leg (Figure). When asked about her hobbies, the patient reported an affinity for butterflies and noted that she attracts them with specific species of plants in her garden. She recalled recently planting the herb of grace, or common rue, to attract the giant swallowtail butterfly (Papilio cresphontes). Upon further inquiry, she remembered working in the garden on her knees and digging up roots near the common rue plant while wearing shorts approximately 2 weeks prior to the current presentation. Given the streaky linear pattern of the eruption along with recent sun exposure and exposure to the common rue plant, a diagnosis of phytophotodermatitis was made. No further treatment was sought, as the eruption was not bothersome to her. She was intrigued that the common rue plant had caused the dermatitis and planned on taking proper precautions when working near the plant in the future.

In this case, the observed phototoxic skin findings resulted from exposure to common rue (Ruta graveolens),a pungently scented evergreen shrub native to the Mediterranean region and a member of the Rutaceae family. Extracts have been used in homeopathic practices for bruises, sprains, headache, neck stiffness, rheumatologic pain, neuralgia, stomach problems, and phlebitis, as well as in seasonings, soaps, creams, and perfumes.1 The most commonly encountered plants known to cause phytophotodermatitis belong to the Apiaceae and Rutaceae families.2 Members of Apiaceae include angelica, celery, dill, fennel, hogweed, parsley, and parsnip. Aside from the common rue plant, the Rutaceae family also includes bergamot orange, bitter orange, burning bush (or gas plant), grapefruit, lemon, and lime. Other potential offending agents are fig, mustard, buttercup, St. John’s wort, and scurfpea. The phototoxic properties are due to furocoumarins, which include psoralens and angelicins. They are inert until activated by UVA radiation, which inflicts direct cellular damage, causing vacuolization and apoptosis of keratinocytes, similar to a sunburn.3 Clinical findings typically present 24 hours after sun exposure with erythema, edema, pain, and occasionally vesicles or bullae in severe cases. Unlike sunburn, lesions often present in linear, streaky, or bizarre patterns, reflective of the direct contact with the plant. The lesions eventually transition to hyperpigmentation, which may take months to years to resolve.
Other considerations in cases of suspected phytophotodermatitis include polymorphic light eruption, actinic prurigo, hydroa vacciniforme, chronic actinic dermatitis, solar urticaria, drug reactions, porphyria, Smith-Lemli-Opitz syndrome, lupus erythematosus, and dermatomyositis.4 Clinicians should suspect phytophotodermatitis with phototoxic findings in bartenders, citrus farm workers, gardeners, chefs, and kitchen workers, especially those handling limes and celery. As in our case, phytophotodermatitis also should be considered in butterfly enthusiasts trying to attract the giant swallowtail butterfly. The caterpillars feed on the leaves of the common rue plant, one of a select few plants that giant swallowtail butterflies use as a host due to its bitter leaves that aid in avoiding predators.5
This case illustrates a unique perspective of phytophotodermatitis, as butterfly enthusiasm is not commonly reported in association with the common rue plant with respect to phytophotodermatitis. This case underscores the importance of inquiring about patients’ professions and hobbies, both in dermatology and other specialties.
- Atta AH, Alkofahi A. Anti-nociceptive and anti-inflammatory effects of some Jordanian medicinal plant extracts. J Ethnopharmacol. 1998;60:117-124.
- McGovern TW. Dermatoses due to plants. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. 2nd ed. Edinburgh, Scotland: Mosby; 2007:265-283.
- Hawk JLM, Calonje E. The photosensitivity disorders. In: Elder DE, ed. Lever’s Histopathology of the Skin. 9th ed. Philadelphia, Pennsylvania: Lippincott Williams and Wilkins; 2005:345-353.
- Lim HW. Abnormal responses to ultraviolet radiation: photosensitivity induced by exogenous agents. In: Wolff K, Goldsmith LA, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012:1066-1074.
- McAuslane H. Giant swallowtail. University of Florida Department of Entomology and Nematology Featured Creatures website. http://entnemdept.ufl.edu/creatures/citrus/giantswallowtail.htm. Revised January 2018. Accessed April 10, 2020.
- Atta AH, Alkofahi A. Anti-nociceptive and anti-inflammatory effects of some Jordanian medicinal plant extracts. J Ethnopharmacol. 1998;60:117-124.
- McGovern TW. Dermatoses due to plants. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. 2nd ed. Edinburgh, Scotland: Mosby; 2007:265-283.
- Hawk JLM, Calonje E. The photosensitivity disorders. In: Elder DE, ed. Lever’s Histopathology of the Skin. 9th ed. Philadelphia, Pennsylvania: Lippincott Williams and Wilkins; 2005:345-353.
- Lim HW. Abnormal responses to ultraviolet radiation: photosensitivity induced by exogenous agents. In: Wolff K, Goldsmith LA, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012:1066-1074.
- McAuslane H. Giant swallowtail. University of Florida Department of Entomology and Nematology Featured Creatures website. http://entnemdept.ufl.edu/creatures/citrus/giantswallowtail.htm. Revised January 2018. Accessed April 10, 2020.
Practice Points
- It is important to inquire about patients’ professions and hobbies, which may lead to the diagnosis, as in this case of a butterfly enthusiast trying to attract the giant swallowtail butterfly with the common rue plant.
- One should suspect phytophotodermatitis with phototoxic findings in bartenders, citrus farm workers, gardeners, chefs, and kitchen workers, especially those handling limes and celery
Essential Oils Debunked: Separating Fact From Myth
What is an essential oil?
An essential oil (EO) is defined by the International Organization for Standardization as a ‘‘product obtained from a natural raw material of plant origin, by steam distillation, by mechanical processes from the epicarp of citrus fruits, or by dry distillation, after separation of the aqueous phase—if any—by physical processes.’’1 Steam distillation is the primary method used for the production of commercial EOs,2 and believe it or not, most EOs contain 100 to 250 individual chemical components.3
The term essential oil often is incorrectly used for a variety of products obtained from plant material by methods other than distillation or cold-pressing, such as extraction. Products that are obtained via the extraction method include absolutes found in fine fragrances; hydrolates such as rose water; concretes such as jasmine or violet leaves; and vegetable oils including olive oil, coconut oil, and sesame oil.2 These products are not true EOs.
Where do EOs come from?
Essential oils are produced in many countries around the world.4 Individual oils may be obtained from species of different plants, from different parts of the same plant, or from various cultivars (plants selectively bred to obtain desirable levels of chemical constituents such as monoterpenes or sesquiterpenes and biochemical properties such as antibacterial or antioxidant activities).3,5 It is estimated that EOs can be obtained from approximately 30,000 plant species, but only 150 EOs are produced commercially.2,6
Why are people using EOs? What is their claim to fame?
Essential oils are employed by the flavor, food (eg, soft drinks, milk, candies, chocolate, meats, sausages, alcoholic beverages, spices, herbs, tea, preservatives, animal foods), fragrance, cosmetic, tobacco, and pharmaceutical industries. They also are used in household products (eg, detergents, fabric softeners, air fresheners, candles, incense) and for medicinal purposes (eg, folk and traditional medicine, phytotherapy, balneotherapy, aromatherapy).2 The oils usually are applied to the skin but also can be administered orally, inhaled, diffused through the air, or used by other means.4 One 2019 survey of Minnesota State Fair attendees (N=282) found the most common reasons for using EOs were a desire for alternative treatments (53.4%), the opinion that EOs are safer than traditional therapies (47.6%), and/or failure of standard medical treatments (10.7%). The survey results also indicated that 46.7% of EO users utilized EOs to treat medical conditions or symptoms.7 Of note, review of the website of an international company that produces EOs confirmed that EOs are marketed not only for adults but also for children to help them concentrate,8 sleep,9 improve the appearance of their skin,10 soothe upset stomachs,11 and decrease sniffles due to colds.12
Why are people selling EOs to family and friends? They must be making major bucks!
In general, the cost of EOs depends on the complexity of cultivated plant species; the mode of harvesting, which is sometimes done by hand; and the yield of oil. Prices range from $4.50 to an incredible $150,000 per kilogram.2 On average, one bottle containing 5 to 15 mL of an EO or oil blend can cost anywhere from $7 to $251.13 In the United States, the consumer EO market is partially composed of multilevel/network marketing companies in which direct consumer sales occur via a hierarchy of individual distributors. Goodier et al7 found that 36.4% of participants who obtained EOs from family and friends purchased them through multilevel/network marketing companies. In 2018, individual distributors of an international EO-producing company made on average anywhere from $4 to as much as $1.54 million annually by selling the company’s EO products and enrolling additional members/individual distributors to purchase or sell the company’s EO products.14
Sometimes EOs are described as natural and pure, but are they really?
Just because a product is labeled as “pure” or “natural” does not ensure that it is a good-quality EO. Organically produced (ie, grown without the use of herbicides or pesticides) plant material can include up to 30% of extraneous herbs and weeds, which can change the composition of the oil.2
Lesser-quality EOs are the result of adulteration, contamination, inadequate oil production, or aging.2 Adulteration (eg, cutting, stretching, bouquetting) occurs when foreign substances are introduced into pure EOs for the benefit of a higher profit; to ensure a sufficient supply of oils; or to meet demands for cheaper oils by “stretching” a more expensive, pure oil by combining with a cheaper, less pure oil. Inadequate oil production leading to lower-quality oils can occur when a biomass is incorrectly distilled, either from too much steam or temperatures that are too high or due to lack of adequate cooling units. Aging occurs when the oils are not stored properly, resulting in a change in the chemical composition due to esterification, reduction, and oxidization of chemicals, which leads to the formation of peroxides and hydroperoxides that can be contact allergens.15
Can patients develop contact allergies to EOs?
The short answer is yes! Contact allergy to almost 80 EOs has been reported,15 including tea tree oil,16,17 ylang-ylang oil,17,18 lavender oil, peppermint oil,18 jasmine absolute,17 geranium oil, rose oil,18 turpentine oil,19,20 and sandalwood oil.18 The recent increased prevalence of allergic reactions to EOs likely is due to increased consumer use as well as increased detection from availability of commercial patch-test preparations.
Essential oils have many common ingredients. De Groot and Schmidt3 documented that 14 of 23 chemicals present in more than 80% of EOs have been reported to cause contact allergy. Interestingly, allergic patients often react to more than one EO, which may be explained by the many shared chemical components in EOs.
Essential oils are “natural” so they must be safe?
In general, most safety profiles are good, but rare toxic reactions from EOs have been observed.4 A recent Australian study reviewed EO exposure calls to the New South Wales Poisons Information Centre.21 The majority of EO poisonings were accidental or the result of therapeutic error such as mistaking EOs for liquid pharmaceuticals. Additionally, this study found that from July 2014 to June 2018, there was a 5% increase in the number of calls per year. More than half of EO poisoning calls involved children, with toddlers being the most frequent cases, suggesting the need for child-resistant top closures. The most frequently involved EOs in poisonings were eucalyptus (46.4% [n=2049]), tea tree (17% [n=749]), lavender (6.1% [n=271]), clove (4.1% [n=179]), and peppermint (3.5% [n=154]).21 Essential oils do not come without potential pitfalls.
What is the clinical presentation and workup?
The workup of EO allergic contact dermatitis begins with obtaining a history to evaluate for use of EO diffusers, perfumes, hygiene products, cosmetics, massage oils, toothpastes, and/or pharmaceutical products. Exploration of potential exposures through occupation, environment, and hobbies also is indicated. Clinical presentation is dependent on the mechanism of exposure. Contact allergy may result from direct application of an allergen to the skin or mucous membranes, contact with a contaminated environmental item (eg, lavender oil on a pillow), contact with EOs used by partners or coworkers (consort dermatitis), airborne exposure (EO diffusers), or systemic exposure (flavorings). Airborne dermatitis from EO diffusers may involve the exposed areas of the face, neck, forearms, arms, behind the earlobes, bilateral eyelids, nasolabial folds, and under the chin. History and clinical presentation can raise suspicion for allergic contact dermatitis, and patch testing is necessary to confirm the diagnosis.
How do we patch test for EO contact allergy?
There are many EOs commercially available for patch testing, and they typically are tested at 2% to 5% concentrations in petrolatum.15 A North American and European study of 62,354 patch-tested patients found that 7.4% of EO-positive individuals did not react to fragrance allergens in a standard screening series including fragrance mix I, fragrance mix II, and balsam of Peru, highlighting the importance of patch testing with specific EOs.22 Currently, only 3 EOs—tea tree oil, peppermint oil, and ylang-ylang oil—are included in the 2019-2020 North American Contact Dermatitis Group screening series, making supplemental testing for other EOs important if contact allergy is suspected; however, testing the patient’s own products is imperative, as there is strong variability in the composition of EOs. Additionally, aged oils may have been exposed to light, oxygen, or varying temperatures, which could result in the formation of additional allergenic chemicals not present in commercially available preparations.15 In addition to commercially available allergens, we test patient-provided EOs either as is in semi-open fashion (ie, EOs are applied to patient’s back with a cotton swab, allowed to dry, covered with adhesive tape, and read at the same interval as other patch tests23) or occasionally dilute them to 1% or 10% (in olive oil or mineral oil).
How should I manage a positive patch-test reaction to EOs?
Patients should avoid relevant EO allergens in their products and environment, which can be easily achieved with the use of the American Contact Dermatitis Society’s Contact Allergen Management Program or similar databases.
Final Interpretation
We are ubiquitously exposed to EOs every day—through the products we use at home, at work, and in our environment. Essential oils make their place in the world by providing sweet-smelling aromas in addition to their alleged therapeutic properties; however, beware, EOs may be the culprit of your next patient’s allergic contact dermatitis.
- International Organization for Standardization. ISO 9235:2013. aromatic natural raw materials—vocabulary. https://www.iso.org/obp/ui/#iso:std:iso:9235:ed-2:v1:en. Accessed March 24, 2020.
- De Groot AC, Schmidt E. Essential oils: part II: general aspects. Dermatitis. 2016;27:43-49.
De Groot AC, Schmidt E. Essential oils: part III: chemical composition. Dermatitis. 2016;27:161-169. - De Groot AC, Schmidt E. Essential oils: part I: introduction. Dermatitis. 2016;27:39-42.
- Insawang S, Pripdeevech P, Tanapichatsakul C, et al. Essential oil compositions and antibacterial and antioxidant activities of five Lavandula stoechas cultivars grown in Thailand. Chem Biodivers. 2019;16:e1900371.
- Lawrence BM. A preliminary report on the world production of some selected essential oils and countries. Perfum Flavor. 2009;34:38-44.
- Goodier MC, Zhang AJ, Nikle AB, et al. Use of essential oils: a general population survey. Contact Dermatitis. 2019;80:391-393.
- KidScents GeneYus. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-geneyus. Accessed March 25, 2020.
- KidScents SleepyIze. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-sleepyize-5ml. Accessed March 25, 2020.
- KidScents® Lotion. Young Living Essential Oils website. www.youngliving.com/en_US/products/kidscents-lotion. Accessed March 25, 2020.
- KidScents TummyGize. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-tummygize-5ml. Accessed March 25, 2020.
- KidScents SniffleEase. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-sniffleease. Accessed March 25, 2020.
- 2019 Product Guide. Young Living Essential Oils website. https://issuu.com/youngliving/docs/yl_productguide. Accessed March 25, 2020.
- 2018 Income Disclosure Statement. Young Living Essential Oils website. https://www.youngliving.com/en_US/opportunity/income-disclosure. Accessed March 25, 2020.
- De Groot AC, Schmidt E. Essential oils, part IV: contact allergy. Dermatitis. 2016;27:170-175.
- Pirker C, Hausen BM, Uter W, et al. Sensitization to tea tree oil in Germany and Austria. a multicenter study of the German Contact Dermatitis Group. J Dtsch Dermatol Ges. 2003;1:629-634.
- Larsen W, Nakayama H, Fischer T, et al. Fragrance contact dermatitis: a worldwide multicenter investigation (part II). Contact Dermatitis. 2001;44:344-346.
- Bleasel N, Tate B, Rademaker M. Allergic contact dermatitis following exposure to essential oils. Australas J Dermatol. 2002;43:211-213.
- Noiles K, Pratt M. Contact dermatitis to Vicks VapoRub. Dermatitis. 2010;21:167-169.
- Barchino-Ortiz L, Cabeza-Martinez R, Leis-Dosil VM, et al. Allergic contact hobby dermatitis from turpentine. Allergol Immunopathol (Madr). 2008;36:117-119.
- Lee KA, Harnett JE, Cairns R. Essential oil exposures in Australia: analysis of cases reported to the NSW Poisons Information Centre. Med J Aust. 2020;212:132-133.
- Warshaw EM, Zug KA, Belsito DV, et al. Positive patch test reactions to essential oils in consecutive patients: results from North America and central Europe. Dermatitis. 2017;28:246-252.
- Lazzarini R, Duarte I, Ferreira AL. Patch tests. An Bras Dermatol. 2013;88:879-888.
What is an essential oil?
An essential oil (EO) is defined by the International Organization for Standardization as a ‘‘product obtained from a natural raw material of plant origin, by steam distillation, by mechanical processes from the epicarp of citrus fruits, or by dry distillation, after separation of the aqueous phase—if any—by physical processes.’’1 Steam distillation is the primary method used for the production of commercial EOs,2 and believe it or not, most EOs contain 100 to 250 individual chemical components.3
The term essential oil often is incorrectly used for a variety of products obtained from plant material by methods other than distillation or cold-pressing, such as extraction. Products that are obtained via the extraction method include absolutes found in fine fragrances; hydrolates such as rose water; concretes such as jasmine or violet leaves; and vegetable oils including olive oil, coconut oil, and sesame oil.2 These products are not true EOs.
Where do EOs come from?
Essential oils are produced in many countries around the world.4 Individual oils may be obtained from species of different plants, from different parts of the same plant, or from various cultivars (plants selectively bred to obtain desirable levels of chemical constituents such as monoterpenes or sesquiterpenes and biochemical properties such as antibacterial or antioxidant activities).3,5 It is estimated that EOs can be obtained from approximately 30,000 plant species, but only 150 EOs are produced commercially.2,6
Why are people using EOs? What is their claim to fame?
Essential oils are employed by the flavor, food (eg, soft drinks, milk, candies, chocolate, meats, sausages, alcoholic beverages, spices, herbs, tea, preservatives, animal foods), fragrance, cosmetic, tobacco, and pharmaceutical industries. They also are used in household products (eg, detergents, fabric softeners, air fresheners, candles, incense) and for medicinal purposes (eg, folk and traditional medicine, phytotherapy, balneotherapy, aromatherapy).2 The oils usually are applied to the skin but also can be administered orally, inhaled, diffused through the air, or used by other means.4 One 2019 survey of Minnesota State Fair attendees (N=282) found the most common reasons for using EOs were a desire for alternative treatments (53.4%), the opinion that EOs are safer than traditional therapies (47.6%), and/or failure of standard medical treatments (10.7%). The survey results also indicated that 46.7% of EO users utilized EOs to treat medical conditions or symptoms.7 Of note, review of the website of an international company that produces EOs confirmed that EOs are marketed not only for adults but also for children to help them concentrate,8 sleep,9 improve the appearance of their skin,10 soothe upset stomachs,11 and decrease sniffles due to colds.12
Why are people selling EOs to family and friends? They must be making major bucks!
In general, the cost of EOs depends on the complexity of cultivated plant species; the mode of harvesting, which is sometimes done by hand; and the yield of oil. Prices range from $4.50 to an incredible $150,000 per kilogram.2 On average, one bottle containing 5 to 15 mL of an EO or oil blend can cost anywhere from $7 to $251.13 In the United States, the consumer EO market is partially composed of multilevel/network marketing companies in which direct consumer sales occur via a hierarchy of individual distributors. Goodier et al7 found that 36.4% of participants who obtained EOs from family and friends purchased them through multilevel/network marketing companies. In 2018, individual distributors of an international EO-producing company made on average anywhere from $4 to as much as $1.54 million annually by selling the company’s EO products and enrolling additional members/individual distributors to purchase or sell the company’s EO products.14
Sometimes EOs are described as natural and pure, but are they really?
Just because a product is labeled as “pure” or “natural” does not ensure that it is a good-quality EO. Organically produced (ie, grown without the use of herbicides or pesticides) plant material can include up to 30% of extraneous herbs and weeds, which can change the composition of the oil.2
Lesser-quality EOs are the result of adulteration, contamination, inadequate oil production, or aging.2 Adulteration (eg, cutting, stretching, bouquetting) occurs when foreign substances are introduced into pure EOs for the benefit of a higher profit; to ensure a sufficient supply of oils; or to meet demands for cheaper oils by “stretching” a more expensive, pure oil by combining with a cheaper, less pure oil. Inadequate oil production leading to lower-quality oils can occur when a biomass is incorrectly distilled, either from too much steam or temperatures that are too high or due to lack of adequate cooling units. Aging occurs when the oils are not stored properly, resulting in a change in the chemical composition due to esterification, reduction, and oxidization of chemicals, which leads to the formation of peroxides and hydroperoxides that can be contact allergens.15
Can patients develop contact allergies to EOs?
The short answer is yes! Contact allergy to almost 80 EOs has been reported,15 including tea tree oil,16,17 ylang-ylang oil,17,18 lavender oil, peppermint oil,18 jasmine absolute,17 geranium oil, rose oil,18 turpentine oil,19,20 and sandalwood oil.18 The recent increased prevalence of allergic reactions to EOs likely is due to increased consumer use as well as increased detection from availability of commercial patch-test preparations.
Essential oils have many common ingredients. De Groot and Schmidt3 documented that 14 of 23 chemicals present in more than 80% of EOs have been reported to cause contact allergy. Interestingly, allergic patients often react to more than one EO, which may be explained by the many shared chemical components in EOs.
Essential oils are “natural” so they must be safe?
In general, most safety profiles are good, but rare toxic reactions from EOs have been observed.4 A recent Australian study reviewed EO exposure calls to the New South Wales Poisons Information Centre.21 The majority of EO poisonings were accidental or the result of therapeutic error such as mistaking EOs for liquid pharmaceuticals. Additionally, this study found that from July 2014 to June 2018, there was a 5% increase in the number of calls per year. More than half of EO poisoning calls involved children, with toddlers being the most frequent cases, suggesting the need for child-resistant top closures. The most frequently involved EOs in poisonings were eucalyptus (46.4% [n=2049]), tea tree (17% [n=749]), lavender (6.1% [n=271]), clove (4.1% [n=179]), and peppermint (3.5% [n=154]).21 Essential oils do not come without potential pitfalls.
What is the clinical presentation and workup?
The workup of EO allergic contact dermatitis begins with obtaining a history to evaluate for use of EO diffusers, perfumes, hygiene products, cosmetics, massage oils, toothpastes, and/or pharmaceutical products. Exploration of potential exposures through occupation, environment, and hobbies also is indicated. Clinical presentation is dependent on the mechanism of exposure. Contact allergy may result from direct application of an allergen to the skin or mucous membranes, contact with a contaminated environmental item (eg, lavender oil on a pillow), contact with EOs used by partners or coworkers (consort dermatitis), airborne exposure (EO diffusers), or systemic exposure (flavorings). Airborne dermatitis from EO diffusers may involve the exposed areas of the face, neck, forearms, arms, behind the earlobes, bilateral eyelids, nasolabial folds, and under the chin. History and clinical presentation can raise suspicion for allergic contact dermatitis, and patch testing is necessary to confirm the diagnosis.
How do we patch test for EO contact allergy?
There are many EOs commercially available for patch testing, and they typically are tested at 2% to 5% concentrations in petrolatum.15 A North American and European study of 62,354 patch-tested patients found that 7.4% of EO-positive individuals did not react to fragrance allergens in a standard screening series including fragrance mix I, fragrance mix II, and balsam of Peru, highlighting the importance of patch testing with specific EOs.22 Currently, only 3 EOs—tea tree oil, peppermint oil, and ylang-ylang oil—are included in the 2019-2020 North American Contact Dermatitis Group screening series, making supplemental testing for other EOs important if contact allergy is suspected; however, testing the patient’s own products is imperative, as there is strong variability in the composition of EOs. Additionally, aged oils may have been exposed to light, oxygen, or varying temperatures, which could result in the formation of additional allergenic chemicals not present in commercially available preparations.15 In addition to commercially available allergens, we test patient-provided EOs either as is in semi-open fashion (ie, EOs are applied to patient’s back with a cotton swab, allowed to dry, covered with adhesive tape, and read at the same interval as other patch tests23) or occasionally dilute them to 1% or 10% (in olive oil or mineral oil).
How should I manage a positive patch-test reaction to EOs?
Patients should avoid relevant EO allergens in their products and environment, which can be easily achieved with the use of the American Contact Dermatitis Society’s Contact Allergen Management Program or similar databases.
Final Interpretation
We are ubiquitously exposed to EOs every day—through the products we use at home, at work, and in our environment. Essential oils make their place in the world by providing sweet-smelling aromas in addition to their alleged therapeutic properties; however, beware, EOs may be the culprit of your next patient’s allergic contact dermatitis.
What is an essential oil?
An essential oil (EO) is defined by the International Organization for Standardization as a ‘‘product obtained from a natural raw material of plant origin, by steam distillation, by mechanical processes from the epicarp of citrus fruits, or by dry distillation, after separation of the aqueous phase—if any—by physical processes.’’1 Steam distillation is the primary method used for the production of commercial EOs,2 and believe it or not, most EOs contain 100 to 250 individual chemical components.3
The term essential oil often is incorrectly used for a variety of products obtained from plant material by methods other than distillation or cold-pressing, such as extraction. Products that are obtained via the extraction method include absolutes found in fine fragrances; hydrolates such as rose water; concretes such as jasmine or violet leaves; and vegetable oils including olive oil, coconut oil, and sesame oil.2 These products are not true EOs.
Where do EOs come from?
Essential oils are produced in many countries around the world.4 Individual oils may be obtained from species of different plants, from different parts of the same plant, or from various cultivars (plants selectively bred to obtain desirable levels of chemical constituents such as monoterpenes or sesquiterpenes and biochemical properties such as antibacterial or antioxidant activities).3,5 It is estimated that EOs can be obtained from approximately 30,000 plant species, but only 150 EOs are produced commercially.2,6
Why are people using EOs? What is their claim to fame?
Essential oils are employed by the flavor, food (eg, soft drinks, milk, candies, chocolate, meats, sausages, alcoholic beverages, spices, herbs, tea, preservatives, animal foods), fragrance, cosmetic, tobacco, and pharmaceutical industries. They also are used in household products (eg, detergents, fabric softeners, air fresheners, candles, incense) and for medicinal purposes (eg, folk and traditional medicine, phytotherapy, balneotherapy, aromatherapy).2 The oils usually are applied to the skin but also can be administered orally, inhaled, diffused through the air, or used by other means.4 One 2019 survey of Minnesota State Fair attendees (N=282) found the most common reasons for using EOs were a desire for alternative treatments (53.4%), the opinion that EOs are safer than traditional therapies (47.6%), and/or failure of standard medical treatments (10.7%). The survey results also indicated that 46.7% of EO users utilized EOs to treat medical conditions or symptoms.7 Of note, review of the website of an international company that produces EOs confirmed that EOs are marketed not only for adults but also for children to help them concentrate,8 sleep,9 improve the appearance of their skin,10 soothe upset stomachs,11 and decrease sniffles due to colds.12
Why are people selling EOs to family and friends? They must be making major bucks!
In general, the cost of EOs depends on the complexity of cultivated plant species; the mode of harvesting, which is sometimes done by hand; and the yield of oil. Prices range from $4.50 to an incredible $150,000 per kilogram.2 On average, one bottle containing 5 to 15 mL of an EO or oil blend can cost anywhere from $7 to $251.13 In the United States, the consumer EO market is partially composed of multilevel/network marketing companies in which direct consumer sales occur via a hierarchy of individual distributors. Goodier et al7 found that 36.4% of participants who obtained EOs from family and friends purchased them through multilevel/network marketing companies. In 2018, individual distributors of an international EO-producing company made on average anywhere from $4 to as much as $1.54 million annually by selling the company’s EO products and enrolling additional members/individual distributors to purchase or sell the company’s EO products.14
Sometimes EOs are described as natural and pure, but are they really?
Just because a product is labeled as “pure” or “natural” does not ensure that it is a good-quality EO. Organically produced (ie, grown without the use of herbicides or pesticides) plant material can include up to 30% of extraneous herbs and weeds, which can change the composition of the oil.2
Lesser-quality EOs are the result of adulteration, contamination, inadequate oil production, or aging.2 Adulteration (eg, cutting, stretching, bouquetting) occurs when foreign substances are introduced into pure EOs for the benefit of a higher profit; to ensure a sufficient supply of oils; or to meet demands for cheaper oils by “stretching” a more expensive, pure oil by combining with a cheaper, less pure oil. Inadequate oil production leading to lower-quality oils can occur when a biomass is incorrectly distilled, either from too much steam or temperatures that are too high or due to lack of adequate cooling units. Aging occurs when the oils are not stored properly, resulting in a change in the chemical composition due to esterification, reduction, and oxidization of chemicals, which leads to the formation of peroxides and hydroperoxides that can be contact allergens.15
Can patients develop contact allergies to EOs?
The short answer is yes! Contact allergy to almost 80 EOs has been reported,15 including tea tree oil,16,17 ylang-ylang oil,17,18 lavender oil, peppermint oil,18 jasmine absolute,17 geranium oil, rose oil,18 turpentine oil,19,20 and sandalwood oil.18 The recent increased prevalence of allergic reactions to EOs likely is due to increased consumer use as well as increased detection from availability of commercial patch-test preparations.
Essential oils have many common ingredients. De Groot and Schmidt3 documented that 14 of 23 chemicals present in more than 80% of EOs have been reported to cause contact allergy. Interestingly, allergic patients often react to more than one EO, which may be explained by the many shared chemical components in EOs.
Essential oils are “natural” so they must be safe?
In general, most safety profiles are good, but rare toxic reactions from EOs have been observed.4 A recent Australian study reviewed EO exposure calls to the New South Wales Poisons Information Centre.21 The majority of EO poisonings were accidental or the result of therapeutic error such as mistaking EOs for liquid pharmaceuticals. Additionally, this study found that from July 2014 to June 2018, there was a 5% increase in the number of calls per year. More than half of EO poisoning calls involved children, with toddlers being the most frequent cases, suggesting the need for child-resistant top closures. The most frequently involved EOs in poisonings were eucalyptus (46.4% [n=2049]), tea tree (17% [n=749]), lavender (6.1% [n=271]), clove (4.1% [n=179]), and peppermint (3.5% [n=154]).21 Essential oils do not come without potential pitfalls.
What is the clinical presentation and workup?
The workup of EO allergic contact dermatitis begins with obtaining a history to evaluate for use of EO diffusers, perfumes, hygiene products, cosmetics, massage oils, toothpastes, and/or pharmaceutical products. Exploration of potential exposures through occupation, environment, and hobbies also is indicated. Clinical presentation is dependent on the mechanism of exposure. Contact allergy may result from direct application of an allergen to the skin or mucous membranes, contact with a contaminated environmental item (eg, lavender oil on a pillow), contact with EOs used by partners or coworkers (consort dermatitis), airborne exposure (EO diffusers), or systemic exposure (flavorings). Airborne dermatitis from EO diffusers may involve the exposed areas of the face, neck, forearms, arms, behind the earlobes, bilateral eyelids, nasolabial folds, and under the chin. History and clinical presentation can raise suspicion for allergic contact dermatitis, and patch testing is necessary to confirm the diagnosis.
How do we patch test for EO contact allergy?
There are many EOs commercially available for patch testing, and they typically are tested at 2% to 5% concentrations in petrolatum.15 A North American and European study of 62,354 patch-tested patients found that 7.4% of EO-positive individuals did not react to fragrance allergens in a standard screening series including fragrance mix I, fragrance mix II, and balsam of Peru, highlighting the importance of patch testing with specific EOs.22 Currently, only 3 EOs—tea tree oil, peppermint oil, and ylang-ylang oil—are included in the 2019-2020 North American Contact Dermatitis Group screening series, making supplemental testing for other EOs important if contact allergy is suspected; however, testing the patient’s own products is imperative, as there is strong variability in the composition of EOs. Additionally, aged oils may have been exposed to light, oxygen, or varying temperatures, which could result in the formation of additional allergenic chemicals not present in commercially available preparations.15 In addition to commercially available allergens, we test patient-provided EOs either as is in semi-open fashion (ie, EOs are applied to patient’s back with a cotton swab, allowed to dry, covered with adhesive tape, and read at the same interval as other patch tests23) or occasionally dilute them to 1% or 10% (in olive oil or mineral oil).
How should I manage a positive patch-test reaction to EOs?
Patients should avoid relevant EO allergens in their products and environment, which can be easily achieved with the use of the American Contact Dermatitis Society’s Contact Allergen Management Program or similar databases.
Final Interpretation
We are ubiquitously exposed to EOs every day—through the products we use at home, at work, and in our environment. Essential oils make their place in the world by providing sweet-smelling aromas in addition to their alleged therapeutic properties; however, beware, EOs may be the culprit of your next patient’s allergic contact dermatitis.
- International Organization for Standardization. ISO 9235:2013. aromatic natural raw materials—vocabulary. https://www.iso.org/obp/ui/#iso:std:iso:9235:ed-2:v1:en. Accessed March 24, 2020.
- De Groot AC, Schmidt E. Essential oils: part II: general aspects. Dermatitis. 2016;27:43-49.
De Groot AC, Schmidt E. Essential oils: part III: chemical composition. Dermatitis. 2016;27:161-169. - De Groot AC, Schmidt E. Essential oils: part I: introduction. Dermatitis. 2016;27:39-42.
- Insawang S, Pripdeevech P, Tanapichatsakul C, et al. Essential oil compositions and antibacterial and antioxidant activities of five Lavandula stoechas cultivars grown in Thailand. Chem Biodivers. 2019;16:e1900371.
- Lawrence BM. A preliminary report on the world production of some selected essential oils and countries. Perfum Flavor. 2009;34:38-44.
- Goodier MC, Zhang AJ, Nikle AB, et al. Use of essential oils: a general population survey. Contact Dermatitis. 2019;80:391-393.
- KidScents GeneYus. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-geneyus. Accessed March 25, 2020.
- KidScents SleepyIze. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-sleepyize-5ml. Accessed March 25, 2020.
- KidScents® Lotion. Young Living Essential Oils website. www.youngliving.com/en_US/products/kidscents-lotion. Accessed March 25, 2020.
- KidScents TummyGize. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-tummygize-5ml. Accessed March 25, 2020.
- KidScents SniffleEase. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-sniffleease. Accessed March 25, 2020.
- 2019 Product Guide. Young Living Essential Oils website. https://issuu.com/youngliving/docs/yl_productguide. Accessed March 25, 2020.
- 2018 Income Disclosure Statement. Young Living Essential Oils website. https://www.youngliving.com/en_US/opportunity/income-disclosure. Accessed March 25, 2020.
- De Groot AC, Schmidt E. Essential oils, part IV: contact allergy. Dermatitis. 2016;27:170-175.
- Pirker C, Hausen BM, Uter W, et al. Sensitization to tea tree oil in Germany and Austria. a multicenter study of the German Contact Dermatitis Group. J Dtsch Dermatol Ges. 2003;1:629-634.
- Larsen W, Nakayama H, Fischer T, et al. Fragrance contact dermatitis: a worldwide multicenter investigation (part II). Contact Dermatitis. 2001;44:344-346.
- Bleasel N, Tate B, Rademaker M. Allergic contact dermatitis following exposure to essential oils. Australas J Dermatol. 2002;43:211-213.
- Noiles K, Pratt M. Contact dermatitis to Vicks VapoRub. Dermatitis. 2010;21:167-169.
- Barchino-Ortiz L, Cabeza-Martinez R, Leis-Dosil VM, et al. Allergic contact hobby dermatitis from turpentine. Allergol Immunopathol (Madr). 2008;36:117-119.
- Lee KA, Harnett JE, Cairns R. Essential oil exposures in Australia: analysis of cases reported to the NSW Poisons Information Centre. Med J Aust. 2020;212:132-133.
- Warshaw EM, Zug KA, Belsito DV, et al. Positive patch test reactions to essential oils in consecutive patients: results from North America and central Europe. Dermatitis. 2017;28:246-252.
- Lazzarini R, Duarte I, Ferreira AL. Patch tests. An Bras Dermatol. 2013;88:879-888.
- International Organization for Standardization. ISO 9235:2013. aromatic natural raw materials—vocabulary. https://www.iso.org/obp/ui/#iso:std:iso:9235:ed-2:v1:en. Accessed March 24, 2020.
- De Groot AC, Schmidt E. Essential oils: part II: general aspects. Dermatitis. 2016;27:43-49.
De Groot AC, Schmidt E. Essential oils: part III: chemical composition. Dermatitis. 2016;27:161-169. - De Groot AC, Schmidt E. Essential oils: part I: introduction. Dermatitis. 2016;27:39-42.
- Insawang S, Pripdeevech P, Tanapichatsakul C, et al. Essential oil compositions and antibacterial and antioxidant activities of five Lavandula stoechas cultivars grown in Thailand. Chem Biodivers. 2019;16:e1900371.
- Lawrence BM. A preliminary report on the world production of some selected essential oils and countries. Perfum Flavor. 2009;34:38-44.
- Goodier MC, Zhang AJ, Nikle AB, et al. Use of essential oils: a general population survey. Contact Dermatitis. 2019;80:391-393.
- KidScents GeneYus. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-geneyus. Accessed March 25, 2020.
- KidScents SleepyIze. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-sleepyize-5ml. Accessed March 25, 2020.
- KidScents® Lotion. Young Living Essential Oils website. www.youngliving.com/en_US/products/kidscents-lotion. Accessed March 25, 2020.
- KidScents TummyGize. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-tummygize-5ml. Accessed March 25, 2020.
- KidScents SniffleEase. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-sniffleease. Accessed March 25, 2020.
- 2019 Product Guide. Young Living Essential Oils website. https://issuu.com/youngliving/docs/yl_productguide. Accessed March 25, 2020.
- 2018 Income Disclosure Statement. Young Living Essential Oils website. https://www.youngliving.com/en_US/opportunity/income-disclosure. Accessed March 25, 2020.
- De Groot AC, Schmidt E. Essential oils, part IV: contact allergy. Dermatitis. 2016;27:170-175.
- Pirker C, Hausen BM, Uter W, et al. Sensitization to tea tree oil in Germany and Austria. a multicenter study of the German Contact Dermatitis Group. J Dtsch Dermatol Ges. 2003;1:629-634.
- Larsen W, Nakayama H, Fischer T, et al. Fragrance contact dermatitis: a worldwide multicenter investigation (part II). Contact Dermatitis. 2001;44:344-346.
- Bleasel N, Tate B, Rademaker M. Allergic contact dermatitis following exposure to essential oils. Australas J Dermatol. 2002;43:211-213.
- Noiles K, Pratt M. Contact dermatitis to Vicks VapoRub. Dermatitis. 2010;21:167-169.
- Barchino-Ortiz L, Cabeza-Martinez R, Leis-Dosil VM, et al. Allergic contact hobby dermatitis from turpentine. Allergol Immunopathol (Madr). 2008;36:117-119.
- Lee KA, Harnett JE, Cairns R. Essential oil exposures in Australia: analysis of cases reported to the NSW Poisons Information Centre. Med J Aust. 2020;212:132-133.
- Warshaw EM, Zug KA, Belsito DV, et al. Positive patch test reactions to essential oils in consecutive patients: results from North America and central Europe. Dermatitis. 2017;28:246-252.
- Lazzarini R, Duarte I, Ferreira AL. Patch tests. An Bras Dermatol. 2013;88:879-888.
Practice Points
- Essential oils (EOs) are present in many consumer products, including foods, cosmetics, pharmaceuticals, and household products; patients can develop contact allergy to EOs.
- Common EO allergens include tea tree oil, ylang-ylang oil, lavender oil, peppermint oil, jasmine absolute, geranium oil, rose oil, turpentine oil, and sandalwood oil.
- In general, EOs have good safety profiles, but caution must be taken when storing them.
- When patch testing for potential EO contact allergy, supplemental testing with both commercially available EOs as well as a patient’s own products is necessary given there is strong variability in the composition of EO products.
Cutaneous Id Reaction After Using Cyanoacrylate for Wound Closure
To the Editor:
In 1998, 2-octyl-cyanoacrylate (2-CA) tissue adhesive gained US Food and Drug Administration approval for topical application to easily hold closed approximated skin edges from surgical excisions and simple trauma-induced lacerations.1 It has since been employed for a number of off-label indications, including sutureless circumcision,2 skin graft fixation,3 pericatheter leakage,4 and intracorporeal use to control air leaks during lung resection.5 Animal investigations additionally have attempted to elucidate potential future uses of 2-CA for procedures such as inguinal hernia repair,6 bowel anastomosis,7 incisional hernia repair with mesh,8 and microvascular anastomosis.9 Compared to sutures, 2-CA offers ease and rapidity of application, a water-resistant barrier, and equivalent cosmetic results, as well as eliminates the need for suture removal.10 As 2-CA is used with increasing frequency across a variety of settings, there arises a greater need to be mindful of the potential complications of its use, such as irritant contact dermatitis (ICD), allergic contact dermatitis (ACD), and cutaneous id reaction.
A 14-year-old adolescent boy with no notable medical history and no known allergies underwent a minimally invasive Nuss procedure11 (performed by P.L.G.) for the repair of severe pectus excavatum. Two 4-cm incisions were made—one in each lateral chest wall at the approximately eighth intercostal space—to facilitate the introduction of the Nuss bar. The surgical wounds were closed with 2 layers of running polyglactin 910 suture before 2-CA was applied topically to the incision sites. The surgery was well tolerated, and the patient’s wounds healed without incident. When the patient was evaluated for Nuss bar removal 3 years later, incision sites were noted to be well healed, and he exhibited no other skin lesions. The original incision sites (bilateral chest walls) were utilized to facilitate surgical Nuss bar removal. The wounds were closed in 4 layers and 2-CA was again applied topically to the incision sites. There were no intraoperative complications; no devices, drains, or tissue implants were left in the patient at the conclusion of the procedure.
One week later, via text message and digital photographs, the patient reported intense pruritus at the bilateral chest wall incision sites, which were now surrounded by symmetric 1-cm erythematous plaques and associated sparse erythematous satellite papules (Figure 1). The patient denied any fevers, pain, swelling, or purulent discharge from the wounds. He was started on hydrocortisone cream 1% twice daily as well as oral diphenhydramine 25 mg at bedtime with initial good effect.
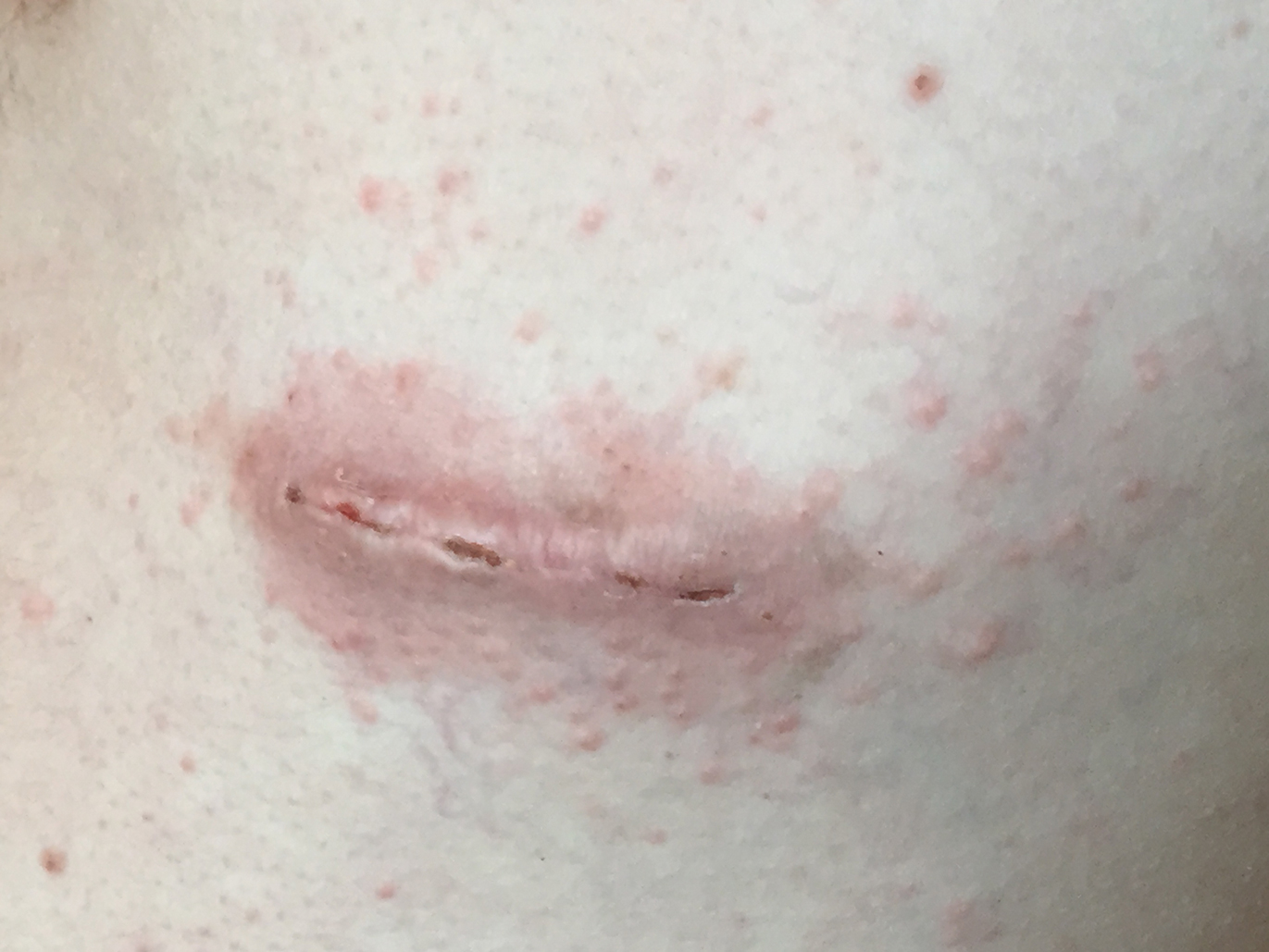
Three days later, the patient sent digital photographs of a morphologically similar–appearing rash that had progressed beyond the lateral chest walls to include the central chest and bilateral upper and lower extremities (Figure 2). He continued to deny any local or systemic signs of infection. Dermatology was consulted, and a diagnosis of ACD with cutaneous id reaction was made. The patient’s medication regimen was modified to include triamcinolone acetonide cream 0.1% applied twice daily to the rash away from the wounds, clobetasol propionate ointment 0.05% applied twice daily to the rash at the wound sites, oral levocetirizine 5 mg once daily, and oral hydroxyzine 25 to 50 mg every 6 hours as needed for pruritus. Additional recommendations included the use of a fragrance-free soap and application of an over-the-counter anti-itch lotion containing menthol and camphor applied as needed. Within 24 hours of starting this modified treatment regimen, the patient began to notice an improvement in symptoms, with full resolution over the course of the ensuing 2 weeks. The patient was counseled to inform his physicians—present and future—of his allergy to 2-CA.

Contact dermatitis associated with the use of 2-CA has been described in the literature.12-15 We report progression to an id reaction, which is characterized by the diffuse symmetric spread of a cutaneous eruption at a site distant from the primary localized dermatitis that develops within a few days of the primary lesion and exhibits the same morphologic and histopathologic findings.16,17 In our patient, pruritic erythematous papules and plaques symmetrically distributed on the arms, legs, and chest appeared 3 days after he first reported a similar eruption at the 2-CA application sites. It is theorized that id reactions develop when the sensitization phase of a type IV hypersensitivity reaction generates a population of T cells that not only recognizes a hapten but also recognizes keratinocyte-derived epitopes.16 A hapten is a small molecule (<500 Da) that is capable of penetrating the stratum corneum and binding skin components. A contact allergen is a hapten that has bound epidermal proteins to create a new antigenic determinant.18 The secondary dermatitis that characterizes id reactions results from an abnormal autoimmune response. Id reactions associated with exposure to adhesive material are rare.19
Allergic contact dermatitis is a type IV hypersensitivity reaction that appears after initial sensitization to an allergen followed by re-exposure. Our patient presented with symmetric erythematous plaques at the surgical incision sites 1 week after 2-CA had been applied. During this interval, sensitization to the inciting allergen occurred. The allergen is taken up by antigen-presenting cells, which then migrate to lymph nodes where they encounter naïve T lymphocytes that subsequently undergo clonal expansion to produce a cohort of T cells that are capable of recognizing the allergen. If subsequent exposure to the specific allergen takes place, an elicitation phase occurs in which primed T cells are incited to release mediators of inflammation that engender the manifestations of ACD within 24 to 72 hours.18,20 Sensitization may be promoted by skin barrier impairments such as dermatitis or a frank wound.12,20 In most cases, the patient is unaware that sensitization has occurred, though a primary ACD within 5 to 15 days after initial exposure to the inciting allergen rarely may be observed.18 Although our patient had 2-CA applied to his surgical wounds at 14 years of age, it was unlikely that sensitization took place at that time, as it was 1 week rather than 1 to 3 days before he experienced the cutaneous eruption associated with his second 2-CA exposure at 17 years of age.
Cyanoacrylate tissue adhesive also may cause ICD resulting from histotoxic degradation products such as formaldehyde and cyanoacetate that are capable of compromising cutaneous barrier function. Keratinocytes that have had their membranes disturbed release proinflammatory cytokines, which recruit cells of the innate immune system as well as T lymphocytes to the site of insult to facilitate the inflammatory response. The manifestations of ICD include erythema, edema, and local necrosis that can compromise wound healing.20 The speed at which a given cyanoacrylate adhesive degrades is proportional to the length of its carbon side chain. Those with shorter side chains—ethyl and methyl cyanoacrylate—degrade more rapidly into formaldehyde and cyanoacetate; 2-CA possesses a longer side chain and therefore degrades more slowly, which should, in theory, lessen its potential to cause ICD.20 Because it may take 7 to 14 days before 2-CA will spontaneously peel from the application site, however, its potential to evoke ICD nevertheless exists.
Treatment of ICD entails removing the irritant while concurrently working to restore the skin’s barrier with emollients. Although topical corticosteroids often are reflexively prescribed to treat rashes, some believe that their use should be avoided in cases of ICD, as their inhibitory effects on epidermal lipid synthesis may further impair the skin’s barrier.21 For cases of ACD, with or without an accompanying id reaction, topical corticosteroids are the mainstay of therapy. It is customary to start with a higher-potency topical steroid such as clobetasol and taper to lower-potency steroids as the patient’s condition improves. Steroid ointments are petroleum based and are capable of causing 2-CA to separate from the skin.10 As a result, they should be used with care when being applied to an area where 2-CA is maintaining dermal closure. Systemic corticosteroids may be warranted in cases with involvement of more than 20% of the body surface area and should start to provide relief within 12 to 24 hours.22 Oral antihistamines and cold water compresses can be added to help address pruritus and discomfort in both ACD and ICD.
Instances of contact dermatitis caused by 2-CA are rare, and progression to an id reaction is rarer still. Physicians should be aware of the possibility of encountering a patient that manifests one or both of these complications whenever 2-CA is employed for skin closure. Physicians who employ 2-CA for skin closure should first ask patients about prior cutaneous reactions to cyanoacrylates including 2-CA and other commonly encountered acrylate-containing products including adhesive wound dressings, dental cements and prostheses, superglue, artificial nails, and adhesives for wigs and false eyelashes. Still, many patients who exhibit acrylate-induced contact dermatitis, with or without an associated id reaction, will not attest to a history of adverse reactions; they simply may not recognize acrylate as the inciting agent. Practitioners across a range of specialties outside of dermatology—surgeons, emergency physicians, and primary care providers—should be prepared to both recognize contact dermatitis and id reaction arising from the use of 2-CA and implement a basic treatment plan that will bring the patient relief without compromising wound closure.
- US Food and Drug Administration. Premarket approval (PMA). https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=p960052. Accessed March 4, 2020.
- Elmore JM, Smith EA, Kirsch AJ. Sutureless circumcision using 2-octyl cyanoacrylate (Dermabond): appraisal after 18-month experience. Urology. 2007;70:803-806.
- Kilic A, Ozdengil E. Skin graft fixation by applying cyanoacrylate without any complication. Plast Reconstr Surg. 2002;110:370-371.
- Gurnaney H, Kraemer FW, Ganesh A. Dermabond decreases pericatheter local anesthetic leakage after continuous perineural infusions. Anesth Analg. 2011;113:206.
- Carr JA. The intracorporeal use of 2-octyl cyanoacrylate resin to control air leaks after lung resection. Eur J Cardiothorac Surg. 2011;39:579-583.
- Miyano G, Yamataka A, Kato Y, et al. Laparoscopic injection of Dermabond tissue adhesive for the repair of inguinal hernia: short- and long-term follow-up. J Pediatr Surg. 2004;39:1867-1870.
- Paral J, Subrt Z, Lochman P, et al. Suture-free anastomosis of the colon. experimental comparison of two cyanoacrylate adhesives. J Gastrointest Surg. 2011;15:451-459.
- Birch DW, Park A. Octylcyanoacrylate tissue adhesive as an alternative to mechanical fixation of expanded polytetrafluoroethylene prosthesis. Am Surg. 2001;67:974-978.
- Ang ES, Tan KC, Tan LH, et al. 2-octylcyanoacrylate-assisted microvascular anastomosis: comparison with a conventional suture technique in rat femoral arteries. J Reconstr Microsurg. 2001;17:193-201.
- Bruns TB, Worthington JM. Using tissue adhesive for wound repair: a practical guide to Dermabond. Am Fam Physician. 2000;61:1383-1388.
- Nuss D, Kelly RE Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg. 1998;33:545-552.
- Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octylcyanoacrylate. Arch Dermatol. 2008;144:814-815.
- Howard BK, Downey SE. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
- Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
- Sachse MM, Junghans T, Rose C, et al. Allergic contact dermatitis caused by topical 2-octyl-cyanoacrylate. Contact Dermatitis. 2013;68:317-319.
- Fehr BS, Takashima A, Bergstresser PR, et al. T cells reactive to keratinocyte antigens are generated during induction of contact hypersensitivity in mice. a model for autoeczematization in humans? Am J Contact Dermat. 2000;11:145-154.
- Gonzalez-Amaro R, Baranda L, Abud-Mendoza C, et al. Autoeczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol. 1993;28:56-60.
- Vocanson M, Hennino A, Rozières A, et al. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy. 2009;64:1699-1714.
- Sommer LL, Hejazi EZ, Heymann WR. An acute linear pruritic eruption following allergic contact dermatitis. J Clin Aesthet Dermatol. 2014;7:42-44.
- Rietschel RL, Fowler JF. Plastics, adhesives, and synthetic resins. In: Rietschek RL, Fowler JF, eds. Fisher’s Contact Dermatitis. Hamilton, BC: Decker Inc; 2008:542-560.
- Kao JS, Fluhr JW, Man M, et al. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J Invest Dermatol. 2003;120:456-464.
- American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology. Contact dermatitis: a practice parameter. Ann Allergy Asthma Immunol. 2006;97(3 suppl 2):S1-S38.
To the Editor:
In 1998, 2-octyl-cyanoacrylate (2-CA) tissue adhesive gained US Food and Drug Administration approval for topical application to easily hold closed approximated skin edges from surgical excisions and simple trauma-induced lacerations.1 It has since been employed for a number of off-label indications, including sutureless circumcision,2 skin graft fixation,3 pericatheter leakage,4 and intracorporeal use to control air leaks during lung resection.5 Animal investigations additionally have attempted to elucidate potential future uses of 2-CA for procedures such as inguinal hernia repair,6 bowel anastomosis,7 incisional hernia repair with mesh,8 and microvascular anastomosis.9 Compared to sutures, 2-CA offers ease and rapidity of application, a water-resistant barrier, and equivalent cosmetic results, as well as eliminates the need for suture removal.10 As 2-CA is used with increasing frequency across a variety of settings, there arises a greater need to be mindful of the potential complications of its use, such as irritant contact dermatitis (ICD), allergic contact dermatitis (ACD), and cutaneous id reaction.
A 14-year-old adolescent boy with no notable medical history and no known allergies underwent a minimally invasive Nuss procedure11 (performed by P.L.G.) for the repair of severe pectus excavatum. Two 4-cm incisions were made—one in each lateral chest wall at the approximately eighth intercostal space—to facilitate the introduction of the Nuss bar. The surgical wounds were closed with 2 layers of running polyglactin 910 suture before 2-CA was applied topically to the incision sites. The surgery was well tolerated, and the patient’s wounds healed without incident. When the patient was evaluated for Nuss bar removal 3 years later, incision sites were noted to be well healed, and he exhibited no other skin lesions. The original incision sites (bilateral chest walls) were utilized to facilitate surgical Nuss bar removal. The wounds were closed in 4 layers and 2-CA was again applied topically to the incision sites. There were no intraoperative complications; no devices, drains, or tissue implants were left in the patient at the conclusion of the procedure.
One week later, via text message and digital photographs, the patient reported intense pruritus at the bilateral chest wall incision sites, which were now surrounded by symmetric 1-cm erythematous plaques and associated sparse erythematous satellite papules (Figure 1). The patient denied any fevers, pain, swelling, or purulent discharge from the wounds. He was started on hydrocortisone cream 1% twice daily as well as oral diphenhydramine 25 mg at bedtime with initial good effect.

Three days later, the patient sent digital photographs of a morphologically similar–appearing rash that had progressed beyond the lateral chest walls to include the central chest and bilateral upper and lower extremities (Figure 2). He continued to deny any local or systemic signs of infection. Dermatology was consulted, and a diagnosis of ACD with cutaneous id reaction was made. The patient’s medication regimen was modified to include triamcinolone acetonide cream 0.1% applied twice daily to the rash away from the wounds, clobetasol propionate ointment 0.05% applied twice daily to the rash at the wound sites, oral levocetirizine 5 mg once daily, and oral hydroxyzine 25 to 50 mg every 6 hours as needed for pruritus. Additional recommendations included the use of a fragrance-free soap and application of an over-the-counter anti-itch lotion containing menthol and camphor applied as needed. Within 24 hours of starting this modified treatment regimen, the patient began to notice an improvement in symptoms, with full resolution over the course of the ensuing 2 weeks. The patient was counseled to inform his physicians—present and future—of his allergy to 2-CA.

Contact dermatitis associated with the use of 2-CA has been described in the literature.12-15 We report progression to an id reaction, which is characterized by the diffuse symmetric spread of a cutaneous eruption at a site distant from the primary localized dermatitis that develops within a few days of the primary lesion and exhibits the same morphologic and histopathologic findings.16,17 In our patient, pruritic erythematous papules and plaques symmetrically distributed on the arms, legs, and chest appeared 3 days after he first reported a similar eruption at the 2-CA application sites. It is theorized that id reactions develop when the sensitization phase of a type IV hypersensitivity reaction generates a population of T cells that not only recognizes a hapten but also recognizes keratinocyte-derived epitopes.16 A hapten is a small molecule (<500 Da) that is capable of penetrating the stratum corneum and binding skin components. A contact allergen is a hapten that has bound epidermal proteins to create a new antigenic determinant.18 The secondary dermatitis that characterizes id reactions results from an abnormal autoimmune response. Id reactions associated with exposure to adhesive material are rare.19
Allergic contact dermatitis is a type IV hypersensitivity reaction that appears after initial sensitization to an allergen followed by re-exposure. Our patient presented with symmetric erythematous plaques at the surgical incision sites 1 week after 2-CA had been applied. During this interval, sensitization to the inciting allergen occurred. The allergen is taken up by antigen-presenting cells, which then migrate to lymph nodes where they encounter naïve T lymphocytes that subsequently undergo clonal expansion to produce a cohort of T cells that are capable of recognizing the allergen. If subsequent exposure to the specific allergen takes place, an elicitation phase occurs in which primed T cells are incited to release mediators of inflammation that engender the manifestations of ACD within 24 to 72 hours.18,20 Sensitization may be promoted by skin barrier impairments such as dermatitis or a frank wound.12,20 In most cases, the patient is unaware that sensitization has occurred, though a primary ACD within 5 to 15 days after initial exposure to the inciting allergen rarely may be observed.18 Although our patient had 2-CA applied to his surgical wounds at 14 years of age, it was unlikely that sensitization took place at that time, as it was 1 week rather than 1 to 3 days before he experienced the cutaneous eruption associated with his second 2-CA exposure at 17 years of age.
Cyanoacrylate tissue adhesive also may cause ICD resulting from histotoxic degradation products such as formaldehyde and cyanoacetate that are capable of compromising cutaneous barrier function. Keratinocytes that have had their membranes disturbed release proinflammatory cytokines, which recruit cells of the innate immune system as well as T lymphocytes to the site of insult to facilitate the inflammatory response. The manifestations of ICD include erythema, edema, and local necrosis that can compromise wound healing.20 The speed at which a given cyanoacrylate adhesive degrades is proportional to the length of its carbon side chain. Those with shorter side chains—ethyl and methyl cyanoacrylate—degrade more rapidly into formaldehyde and cyanoacetate; 2-CA possesses a longer side chain and therefore degrades more slowly, which should, in theory, lessen its potential to cause ICD.20 Because it may take 7 to 14 days before 2-CA will spontaneously peel from the application site, however, its potential to evoke ICD nevertheless exists.
Treatment of ICD entails removing the irritant while concurrently working to restore the skin’s barrier with emollients. Although topical corticosteroids often are reflexively prescribed to treat rashes, some believe that their use should be avoided in cases of ICD, as their inhibitory effects on epidermal lipid synthesis may further impair the skin’s barrier.21 For cases of ACD, with or without an accompanying id reaction, topical corticosteroids are the mainstay of therapy. It is customary to start with a higher-potency topical steroid such as clobetasol and taper to lower-potency steroids as the patient’s condition improves. Steroid ointments are petroleum based and are capable of causing 2-CA to separate from the skin.10 As a result, they should be used with care when being applied to an area where 2-CA is maintaining dermal closure. Systemic corticosteroids may be warranted in cases with involvement of more than 20% of the body surface area and should start to provide relief within 12 to 24 hours.22 Oral antihistamines and cold water compresses can be added to help address pruritus and discomfort in both ACD and ICD.
Instances of contact dermatitis caused by 2-CA are rare, and progression to an id reaction is rarer still. Physicians should be aware of the possibility of encountering a patient that manifests one or both of these complications whenever 2-CA is employed for skin closure. Physicians who employ 2-CA for skin closure should first ask patients about prior cutaneous reactions to cyanoacrylates including 2-CA and other commonly encountered acrylate-containing products including adhesive wound dressings, dental cements and prostheses, superglue, artificial nails, and adhesives for wigs and false eyelashes. Still, many patients who exhibit acrylate-induced contact dermatitis, with or without an associated id reaction, will not attest to a history of adverse reactions; they simply may not recognize acrylate as the inciting agent. Practitioners across a range of specialties outside of dermatology—surgeons, emergency physicians, and primary care providers—should be prepared to both recognize contact dermatitis and id reaction arising from the use of 2-CA and implement a basic treatment plan that will bring the patient relief without compromising wound closure.
To the Editor:
In 1998, 2-octyl-cyanoacrylate (2-CA) tissue adhesive gained US Food and Drug Administration approval for topical application to easily hold closed approximated skin edges from surgical excisions and simple trauma-induced lacerations.1 It has since been employed for a number of off-label indications, including sutureless circumcision,2 skin graft fixation,3 pericatheter leakage,4 and intracorporeal use to control air leaks during lung resection.5 Animal investigations additionally have attempted to elucidate potential future uses of 2-CA for procedures such as inguinal hernia repair,6 bowel anastomosis,7 incisional hernia repair with mesh,8 and microvascular anastomosis.9 Compared to sutures, 2-CA offers ease and rapidity of application, a water-resistant barrier, and equivalent cosmetic results, as well as eliminates the need for suture removal.10 As 2-CA is used with increasing frequency across a variety of settings, there arises a greater need to be mindful of the potential complications of its use, such as irritant contact dermatitis (ICD), allergic contact dermatitis (ACD), and cutaneous id reaction.
A 14-year-old adolescent boy with no notable medical history and no known allergies underwent a minimally invasive Nuss procedure11 (performed by P.L.G.) for the repair of severe pectus excavatum. Two 4-cm incisions were made—one in each lateral chest wall at the approximately eighth intercostal space—to facilitate the introduction of the Nuss bar. The surgical wounds were closed with 2 layers of running polyglactin 910 suture before 2-CA was applied topically to the incision sites. The surgery was well tolerated, and the patient’s wounds healed without incident. When the patient was evaluated for Nuss bar removal 3 years later, incision sites were noted to be well healed, and he exhibited no other skin lesions. The original incision sites (bilateral chest walls) were utilized to facilitate surgical Nuss bar removal. The wounds were closed in 4 layers and 2-CA was again applied topically to the incision sites. There were no intraoperative complications; no devices, drains, or tissue implants were left in the patient at the conclusion of the procedure.
One week later, via text message and digital photographs, the patient reported intense pruritus at the bilateral chest wall incision sites, which were now surrounded by symmetric 1-cm erythematous plaques and associated sparse erythematous satellite papules (Figure 1). The patient denied any fevers, pain, swelling, or purulent discharge from the wounds. He was started on hydrocortisone cream 1% twice daily as well as oral diphenhydramine 25 mg at bedtime with initial good effect.

Three days later, the patient sent digital photographs of a morphologically similar–appearing rash that had progressed beyond the lateral chest walls to include the central chest and bilateral upper and lower extremities (Figure 2). He continued to deny any local or systemic signs of infection. Dermatology was consulted, and a diagnosis of ACD with cutaneous id reaction was made. The patient’s medication regimen was modified to include triamcinolone acetonide cream 0.1% applied twice daily to the rash away from the wounds, clobetasol propionate ointment 0.05% applied twice daily to the rash at the wound sites, oral levocetirizine 5 mg once daily, and oral hydroxyzine 25 to 50 mg every 6 hours as needed for pruritus. Additional recommendations included the use of a fragrance-free soap and application of an over-the-counter anti-itch lotion containing menthol and camphor applied as needed. Within 24 hours of starting this modified treatment regimen, the patient began to notice an improvement in symptoms, with full resolution over the course of the ensuing 2 weeks. The patient was counseled to inform his physicians—present and future—of his allergy to 2-CA.

Contact dermatitis associated with the use of 2-CA has been described in the literature.12-15 We report progression to an id reaction, which is characterized by the diffuse symmetric spread of a cutaneous eruption at a site distant from the primary localized dermatitis that develops within a few days of the primary lesion and exhibits the same morphologic and histopathologic findings.16,17 In our patient, pruritic erythematous papules and plaques symmetrically distributed on the arms, legs, and chest appeared 3 days after he first reported a similar eruption at the 2-CA application sites. It is theorized that id reactions develop when the sensitization phase of a type IV hypersensitivity reaction generates a population of T cells that not only recognizes a hapten but also recognizes keratinocyte-derived epitopes.16 A hapten is a small molecule (<500 Da) that is capable of penetrating the stratum corneum and binding skin components. A contact allergen is a hapten that has bound epidermal proteins to create a new antigenic determinant.18 The secondary dermatitis that characterizes id reactions results from an abnormal autoimmune response. Id reactions associated with exposure to adhesive material are rare.19
Allergic contact dermatitis is a type IV hypersensitivity reaction that appears after initial sensitization to an allergen followed by re-exposure. Our patient presented with symmetric erythematous plaques at the surgical incision sites 1 week after 2-CA had been applied. During this interval, sensitization to the inciting allergen occurred. The allergen is taken up by antigen-presenting cells, which then migrate to lymph nodes where they encounter naïve T lymphocytes that subsequently undergo clonal expansion to produce a cohort of T cells that are capable of recognizing the allergen. If subsequent exposure to the specific allergen takes place, an elicitation phase occurs in which primed T cells are incited to release mediators of inflammation that engender the manifestations of ACD within 24 to 72 hours.18,20 Sensitization may be promoted by skin barrier impairments such as dermatitis or a frank wound.12,20 In most cases, the patient is unaware that sensitization has occurred, though a primary ACD within 5 to 15 days after initial exposure to the inciting allergen rarely may be observed.18 Although our patient had 2-CA applied to his surgical wounds at 14 years of age, it was unlikely that sensitization took place at that time, as it was 1 week rather than 1 to 3 days before he experienced the cutaneous eruption associated with his second 2-CA exposure at 17 years of age.
Cyanoacrylate tissue adhesive also may cause ICD resulting from histotoxic degradation products such as formaldehyde and cyanoacetate that are capable of compromising cutaneous barrier function. Keratinocytes that have had their membranes disturbed release proinflammatory cytokines, which recruit cells of the innate immune system as well as T lymphocytes to the site of insult to facilitate the inflammatory response. The manifestations of ICD include erythema, edema, and local necrosis that can compromise wound healing.20 The speed at which a given cyanoacrylate adhesive degrades is proportional to the length of its carbon side chain. Those with shorter side chains—ethyl and methyl cyanoacrylate—degrade more rapidly into formaldehyde and cyanoacetate; 2-CA possesses a longer side chain and therefore degrades more slowly, which should, in theory, lessen its potential to cause ICD.20 Because it may take 7 to 14 days before 2-CA will spontaneously peel from the application site, however, its potential to evoke ICD nevertheless exists.
Treatment of ICD entails removing the irritant while concurrently working to restore the skin’s barrier with emollients. Although topical corticosteroids often are reflexively prescribed to treat rashes, some believe that their use should be avoided in cases of ICD, as their inhibitory effects on epidermal lipid synthesis may further impair the skin’s barrier.21 For cases of ACD, with or without an accompanying id reaction, topical corticosteroids are the mainstay of therapy. It is customary to start with a higher-potency topical steroid such as clobetasol and taper to lower-potency steroids as the patient’s condition improves. Steroid ointments are petroleum based and are capable of causing 2-CA to separate from the skin.10 As a result, they should be used with care when being applied to an area where 2-CA is maintaining dermal closure. Systemic corticosteroids may be warranted in cases with involvement of more than 20% of the body surface area and should start to provide relief within 12 to 24 hours.22 Oral antihistamines and cold water compresses can be added to help address pruritus and discomfort in both ACD and ICD.
Instances of contact dermatitis caused by 2-CA are rare, and progression to an id reaction is rarer still. Physicians should be aware of the possibility of encountering a patient that manifests one or both of these complications whenever 2-CA is employed for skin closure. Physicians who employ 2-CA for skin closure should first ask patients about prior cutaneous reactions to cyanoacrylates including 2-CA and other commonly encountered acrylate-containing products including adhesive wound dressings, dental cements and prostheses, superglue, artificial nails, and adhesives for wigs and false eyelashes. Still, many patients who exhibit acrylate-induced contact dermatitis, with or without an associated id reaction, will not attest to a history of adverse reactions; they simply may not recognize acrylate as the inciting agent. Practitioners across a range of specialties outside of dermatology—surgeons, emergency physicians, and primary care providers—should be prepared to both recognize contact dermatitis and id reaction arising from the use of 2-CA and implement a basic treatment plan that will bring the patient relief without compromising wound closure.
- US Food and Drug Administration. Premarket approval (PMA). https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=p960052. Accessed March 4, 2020.
- Elmore JM, Smith EA, Kirsch AJ. Sutureless circumcision using 2-octyl cyanoacrylate (Dermabond): appraisal after 18-month experience. Urology. 2007;70:803-806.
- Kilic A, Ozdengil E. Skin graft fixation by applying cyanoacrylate without any complication. Plast Reconstr Surg. 2002;110:370-371.
- Gurnaney H, Kraemer FW, Ganesh A. Dermabond decreases pericatheter local anesthetic leakage after continuous perineural infusions. Anesth Analg. 2011;113:206.
- Carr JA. The intracorporeal use of 2-octyl cyanoacrylate resin to control air leaks after lung resection. Eur J Cardiothorac Surg. 2011;39:579-583.
- Miyano G, Yamataka A, Kato Y, et al. Laparoscopic injection of Dermabond tissue adhesive for the repair of inguinal hernia: short- and long-term follow-up. J Pediatr Surg. 2004;39:1867-1870.
- Paral J, Subrt Z, Lochman P, et al. Suture-free anastomosis of the colon. experimental comparison of two cyanoacrylate adhesives. J Gastrointest Surg. 2011;15:451-459.
- Birch DW, Park A. Octylcyanoacrylate tissue adhesive as an alternative to mechanical fixation of expanded polytetrafluoroethylene prosthesis. Am Surg. 2001;67:974-978.
- Ang ES, Tan KC, Tan LH, et al. 2-octylcyanoacrylate-assisted microvascular anastomosis: comparison with a conventional suture technique in rat femoral arteries. J Reconstr Microsurg. 2001;17:193-201.
- Bruns TB, Worthington JM. Using tissue adhesive for wound repair: a practical guide to Dermabond. Am Fam Physician. 2000;61:1383-1388.
- Nuss D, Kelly RE Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg. 1998;33:545-552.
- Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octylcyanoacrylate. Arch Dermatol. 2008;144:814-815.
- Howard BK, Downey SE. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
- Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
- Sachse MM, Junghans T, Rose C, et al. Allergic contact dermatitis caused by topical 2-octyl-cyanoacrylate. Contact Dermatitis. 2013;68:317-319.
- Fehr BS, Takashima A, Bergstresser PR, et al. T cells reactive to keratinocyte antigens are generated during induction of contact hypersensitivity in mice. a model for autoeczematization in humans? Am J Contact Dermat. 2000;11:145-154.
- Gonzalez-Amaro R, Baranda L, Abud-Mendoza C, et al. Autoeczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol. 1993;28:56-60.
- Vocanson M, Hennino A, Rozières A, et al. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy. 2009;64:1699-1714.
- Sommer LL, Hejazi EZ, Heymann WR. An acute linear pruritic eruption following allergic contact dermatitis. J Clin Aesthet Dermatol. 2014;7:42-44.
- Rietschel RL, Fowler JF. Plastics, adhesives, and synthetic resins. In: Rietschek RL, Fowler JF, eds. Fisher’s Contact Dermatitis. Hamilton, BC: Decker Inc; 2008:542-560.
- Kao JS, Fluhr JW, Man M, et al. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J Invest Dermatol. 2003;120:456-464.
- American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology. Contact dermatitis: a practice parameter. Ann Allergy Asthma Immunol. 2006;97(3 suppl 2):S1-S38.
- US Food and Drug Administration. Premarket approval (PMA). https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=p960052. Accessed March 4, 2020.
- Elmore JM, Smith EA, Kirsch AJ. Sutureless circumcision using 2-octyl cyanoacrylate (Dermabond): appraisal after 18-month experience. Urology. 2007;70:803-806.
- Kilic A, Ozdengil E. Skin graft fixation by applying cyanoacrylate without any complication. Plast Reconstr Surg. 2002;110:370-371.
- Gurnaney H, Kraemer FW, Ganesh A. Dermabond decreases pericatheter local anesthetic leakage after continuous perineural infusions. Anesth Analg. 2011;113:206.
- Carr JA. The intracorporeal use of 2-octyl cyanoacrylate resin to control air leaks after lung resection. Eur J Cardiothorac Surg. 2011;39:579-583.
- Miyano G, Yamataka A, Kato Y, et al. Laparoscopic injection of Dermabond tissue adhesive for the repair of inguinal hernia: short- and long-term follow-up. J Pediatr Surg. 2004;39:1867-1870.
- Paral J, Subrt Z, Lochman P, et al. Suture-free anastomosis of the colon. experimental comparison of two cyanoacrylate adhesives. J Gastrointest Surg. 2011;15:451-459.
- Birch DW, Park A. Octylcyanoacrylate tissue adhesive as an alternative to mechanical fixation of expanded polytetrafluoroethylene prosthesis. Am Surg. 2001;67:974-978.
- Ang ES, Tan KC, Tan LH, et al. 2-octylcyanoacrylate-assisted microvascular anastomosis: comparison with a conventional suture technique in rat femoral arteries. J Reconstr Microsurg. 2001;17:193-201.
- Bruns TB, Worthington JM. Using tissue adhesive for wound repair: a practical guide to Dermabond. Am Fam Physician. 2000;61:1383-1388.
- Nuss D, Kelly RE Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg. 1998;33:545-552.
- Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octylcyanoacrylate. Arch Dermatol. 2008;144:814-815.
- Howard BK, Downey SE. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
- Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
- Sachse MM, Junghans T, Rose C, et al. Allergic contact dermatitis caused by topical 2-octyl-cyanoacrylate. Contact Dermatitis. 2013;68:317-319.
- Fehr BS, Takashima A, Bergstresser PR, et al. T cells reactive to keratinocyte antigens are generated during induction of contact hypersensitivity in mice. a model for autoeczematization in humans? Am J Contact Dermat. 2000;11:145-154.
- Gonzalez-Amaro R, Baranda L, Abud-Mendoza C, et al. Autoeczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol. 1993;28:56-60.
- Vocanson M, Hennino A, Rozières A, et al. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy. 2009;64:1699-1714.
- Sommer LL, Hejazi EZ, Heymann WR. An acute linear pruritic eruption following allergic contact dermatitis. J Clin Aesthet Dermatol. 2014;7:42-44.
- Rietschel RL, Fowler JF. Plastics, adhesives, and synthetic resins. In: Rietschek RL, Fowler JF, eds. Fisher’s Contact Dermatitis. Hamilton, BC: Decker Inc; 2008:542-560.
- Kao JS, Fluhr JW, Man M, et al. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J Invest Dermatol. 2003;120:456-464.
- American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology. Contact dermatitis: a practice parameter. Ann Allergy Asthma Immunol. 2006;97(3 suppl 2):S1-S38.
Practice Points
- 2-Octyl-cyanoacrylate (2-CA) tissue adhesive has been reported to cause contact dermatitis when applied topically for surgical site closure.
- Id reactions resulting from the use of 2-CA tissue adhesive are possible, though less commonly observed.
- Id reactions caused by 2-CA tissue adhesive respond well to treatment with a combination of topical steroids and oral antihistamines. Systemic corticosteroids may be warranted in cases involving greater than 20% body surface area.
Consider toys as culprits in children with contact allergies
A variety of according to the results of a review of 25 published articles.
“In recent years the products have become a reflection of the compounds used frequently in manufacturing, including metals and plastic compounds,” wrote Justine Fenner, MD, and coauthors, from the departments of dermatology and pediatrics at the Icahn School of Medicine at Mount Sinai, New York,
In a study published in Contact Dermatitis, the researchers identified 25 articles describing dermatitis, rash, or eczema associated with a range of toy and play product terms including Nintendo, PlayStation, putty, glue, doll, game, car, bicycle, slime, iPad, and iPhone.
Overall, nickel was the most common allergen. Cases of nickel dermatitis were associated with laptops, videogame controllers, iPads, and cell phones. Cell phones were the most common electronics associated with contact dermatitis, which was observed on the cheek, periauricular area, and hand, as well as the breast in one case of a patient who kept her phone in her bra.
Other sources of metal allergens were identified in toy cars and costume jewelry, the researchers noted.
In addition, temporary tattoos have been associated with contact dermatitis in children, as have homemade “slime” products, which often contain not only borax or other household detergents, but also glue, shaving cream, or coloring.
However, identification of true allergic contact dermatitis from toys “requires both identification of the chemical contents of toys, which are proprietary in nature, and then epicutaneous allergy testing of these ingredients,” the researchers said.
The study findings were limited by several factors including the consideration only of English-language articles and of cases in children, which thus eliminates other potential cases, the researchers noted. However, the results suggest that dermatologists consider toys as a source of contact dermatitis in children, especially if the time to diagnosis is months to years, they said. “Additionally, it may be useful, as it was in several of the above cases, to have the patient bring in his or her favorite toys for the dermatologist to examine and help further understand the etiology of patient’s rash,” they noted. Moreover, “there is an unmet need for corporations to reveal the chemical ingredients of their toys when allergic contact dermatitis is suspected in order to properly evaluate the patient,” they added.
“Contact dermatitis has been underreported in children and constitutes an ongoing concern,” senior author Nanette Silverberg, MD, chief of pediatric dermatology for the Mount Sinai Health System, said in an interview.
“In particular, toy-related allergy is concerning due to the rise in allergen inclusion in common play items,” she commented. The current analysis identified many case reports of allergens that pediatric dermatologists are frequently seeing in their offices, notably metals such as nickel, she pointed out. “The allergen that always stands out ahead of others is nickel,” Dr. Silverberg said. “Nickel allergy affects about 25% of Americans, often starting in early childhood,” she said. “In the European Union, legislation has been passed to reduce nickel release from metals, which has resulted in less sensitization to nickel. We lack such legislation in the United States,” she added.
Other trending allergens include methylchloroisothiazolinone/methylisothiazolinone, which may be components of glue or other ingredients in some “slime” products, Dr. Silverberg said.
She advised clinicians to consider patch testing when addressing localized or persistent dermatitis in children. “Furthermore, consider toys as potential relevant allergens that should be modified in order to achieve skin improvement,” she said.
“Greater reporting of pediatric allergic contact dermatitis is needed,” Dr. Silverberg emphasized. “Additionally, surveillance and monitoring for trends in allergen exposures in toys and personal care items is required to analyze this ongoing concern of childhood,” she said.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Fenner J et al. Contact Dermatitis. 2020 Feb 22. doi: 10.1111/cod.13500.
A variety of according to the results of a review of 25 published articles.
“In recent years the products have become a reflection of the compounds used frequently in manufacturing, including metals and plastic compounds,” wrote Justine Fenner, MD, and coauthors, from the departments of dermatology and pediatrics at the Icahn School of Medicine at Mount Sinai, New York,
In a study published in Contact Dermatitis, the researchers identified 25 articles describing dermatitis, rash, or eczema associated with a range of toy and play product terms including Nintendo, PlayStation, putty, glue, doll, game, car, bicycle, slime, iPad, and iPhone.
Overall, nickel was the most common allergen. Cases of nickel dermatitis were associated with laptops, videogame controllers, iPads, and cell phones. Cell phones were the most common electronics associated with contact dermatitis, which was observed on the cheek, periauricular area, and hand, as well as the breast in one case of a patient who kept her phone in her bra.
Other sources of metal allergens were identified in toy cars and costume jewelry, the researchers noted.
In addition, temporary tattoos have been associated with contact dermatitis in children, as have homemade “slime” products, which often contain not only borax or other household detergents, but also glue, shaving cream, or coloring.
However, identification of true allergic contact dermatitis from toys “requires both identification of the chemical contents of toys, which are proprietary in nature, and then epicutaneous allergy testing of these ingredients,” the researchers said.
The study findings were limited by several factors including the consideration only of English-language articles and of cases in children, which thus eliminates other potential cases, the researchers noted. However, the results suggest that dermatologists consider toys as a source of contact dermatitis in children, especially if the time to diagnosis is months to years, they said. “Additionally, it may be useful, as it was in several of the above cases, to have the patient bring in his or her favorite toys for the dermatologist to examine and help further understand the etiology of patient’s rash,” they noted. Moreover, “there is an unmet need for corporations to reveal the chemical ingredients of their toys when allergic contact dermatitis is suspected in order to properly evaluate the patient,” they added.
“Contact dermatitis has been underreported in children and constitutes an ongoing concern,” senior author Nanette Silverberg, MD, chief of pediatric dermatology for the Mount Sinai Health System, said in an interview.
“In particular, toy-related allergy is concerning due to the rise in allergen inclusion in common play items,” she commented. The current analysis identified many case reports of allergens that pediatric dermatologists are frequently seeing in their offices, notably metals such as nickel, she pointed out. “The allergen that always stands out ahead of others is nickel,” Dr. Silverberg said. “Nickel allergy affects about 25% of Americans, often starting in early childhood,” she said. “In the European Union, legislation has been passed to reduce nickel release from metals, which has resulted in less sensitization to nickel. We lack such legislation in the United States,” she added.
Other trending allergens include methylchloroisothiazolinone/methylisothiazolinone, which may be components of glue or other ingredients in some “slime” products, Dr. Silverberg said.
She advised clinicians to consider patch testing when addressing localized or persistent dermatitis in children. “Furthermore, consider toys as potential relevant allergens that should be modified in order to achieve skin improvement,” she said.
“Greater reporting of pediatric allergic contact dermatitis is needed,” Dr. Silverberg emphasized. “Additionally, surveillance and monitoring for trends in allergen exposures in toys and personal care items is required to analyze this ongoing concern of childhood,” she said.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Fenner J et al. Contact Dermatitis. 2020 Feb 22. doi: 10.1111/cod.13500.
A variety of according to the results of a review of 25 published articles.
“In recent years the products have become a reflection of the compounds used frequently in manufacturing, including metals and plastic compounds,” wrote Justine Fenner, MD, and coauthors, from the departments of dermatology and pediatrics at the Icahn School of Medicine at Mount Sinai, New York,
In a study published in Contact Dermatitis, the researchers identified 25 articles describing dermatitis, rash, or eczema associated with a range of toy and play product terms including Nintendo, PlayStation, putty, glue, doll, game, car, bicycle, slime, iPad, and iPhone.
Overall, nickel was the most common allergen. Cases of nickel dermatitis were associated with laptops, videogame controllers, iPads, and cell phones. Cell phones were the most common electronics associated with contact dermatitis, which was observed on the cheek, periauricular area, and hand, as well as the breast in one case of a patient who kept her phone in her bra.
Other sources of metal allergens were identified in toy cars and costume jewelry, the researchers noted.
In addition, temporary tattoos have been associated with contact dermatitis in children, as have homemade “slime” products, which often contain not only borax or other household detergents, but also glue, shaving cream, or coloring.
However, identification of true allergic contact dermatitis from toys “requires both identification of the chemical contents of toys, which are proprietary in nature, and then epicutaneous allergy testing of these ingredients,” the researchers said.
The study findings were limited by several factors including the consideration only of English-language articles and of cases in children, which thus eliminates other potential cases, the researchers noted. However, the results suggest that dermatologists consider toys as a source of contact dermatitis in children, especially if the time to diagnosis is months to years, they said. “Additionally, it may be useful, as it was in several of the above cases, to have the patient bring in his or her favorite toys for the dermatologist to examine and help further understand the etiology of patient’s rash,” they noted. Moreover, “there is an unmet need for corporations to reveal the chemical ingredients of their toys when allergic contact dermatitis is suspected in order to properly evaluate the patient,” they added.
“Contact dermatitis has been underreported in children and constitutes an ongoing concern,” senior author Nanette Silverberg, MD, chief of pediatric dermatology for the Mount Sinai Health System, said in an interview.
“In particular, toy-related allergy is concerning due to the rise in allergen inclusion in common play items,” she commented. The current analysis identified many case reports of allergens that pediatric dermatologists are frequently seeing in their offices, notably metals such as nickel, she pointed out. “The allergen that always stands out ahead of others is nickel,” Dr. Silverberg said. “Nickel allergy affects about 25% of Americans, often starting in early childhood,” she said. “In the European Union, legislation has been passed to reduce nickel release from metals, which has resulted in less sensitization to nickel. We lack such legislation in the United States,” she added.
Other trending allergens include methylchloroisothiazolinone/methylisothiazolinone, which may be components of glue or other ingredients in some “slime” products, Dr. Silverberg said.
She advised clinicians to consider patch testing when addressing localized or persistent dermatitis in children. “Furthermore, consider toys as potential relevant allergens that should be modified in order to achieve skin improvement,” she said.
“Greater reporting of pediatric allergic contact dermatitis is needed,” Dr. Silverberg emphasized. “Additionally, surveillance and monitoring for trends in allergen exposures in toys and personal care items is required to analyze this ongoing concern of childhood,” she said.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Fenner J et al. Contact Dermatitis. 2020 Feb 22. doi: 10.1111/cod.13500.
FROM CONTACT DERMATITIS
Consider allergic contact dermatitis in children with AD with disease flares, new rash
ORLANDO – Do you have ? Consider patch testing to assess whether they have allergic contact dermatitis.
“Of the patients who are sent to me by local pediatric dermatologists, 50% of them are positive” for allergens, said Jonathan H. Zippin, MD, PhD, director of the contact, occupational, and photodermatitis service at Cornell University, New York.
Speaking at the ODAC Dermatology, Aesthetic, and Surgical Conference, Dr. Zippin noted the prevalence of allergen sensitization is between 13% and 25% among children who are asymptomatic, while the prevalence of sensitization to at least one allergen among children with suspected allergic contact dermatitis (ACD) is between 25% and 96%. In 2014, a study from the National American Contact Dermatitis Group (NACDG) showed that of 883 children who were patch tested, 56.7% had at least one relevant positive patch test (RPPT) result.
“The take-home message here is that pediatric contact dermatitis is common, much more common than a lot of people realize,” Dr. Zippin said.
He described three common scenarios to keep in mind: a worsening rash, a new rash, and failure of a rash to improve after the patient avoids all of his or her positive allergens.
When a rash worsens, patch testing is likely to offer answers. In an analysis of 1,142 patients with suspected ACD aged 18 years or younger (mean age, 10.5 years; 64% female) in the Pediatric Contact Dermatitis Registry study database, 65% had at least one positive patch test, and 48% had at least 1 RPPT (Dermatitis 2016; 27[5] 293-302).
But not all patch testing is the same: The study also found that 24% of the RPPT cases would have been missed if assessed with the T.R.U.E. TEST compared with extended patch testing. If a T.R.U.E. TEST fails to explain generalized atopic dermatitis, the patient should be sent for more comprehensive testing where available, Dr. Zippin advised.
Pediatric patients also have unique allergens clinicians should consider. In the same study, children had a number of allergens similar to those of adults as reported in previous studies, such as nickel, cobalt, and neomycin. However, propylene glycol and cocamidopropyl betaine were allergens identified as unique to the pediatric population.
Another study looking at the same group of patients found that compared with children who did not have AD, children with AD had 7.4 times higher odds of having an RPPT to cocamidopropyl betaine, 7.6 times higher odds of having an RPPT to parthenolide, 5.3 times higher odds of having an RPPT to tixocortol pivalate, 4.2 times higher odds of having an RPPT to wool alcohols, and 4 times higher odds of having an RPPT to lanolin (JAMA Dermatology 2017;153[8]:765-70).
All of these are components of topical medicaments used to treat AD, “either components of emollients that we recommend, or components of steroids that we recommend,” Dr. Zippin pointed out.
One of these allergens could be the culprit when a child develops a new rash but there are no new apparent changes in products, exposures, and activities. Lanolin, also called wool grease, is used in many skin care products, for example. Dr. Zippin described the case of a 6-year-old girl with a history of AD, who presented with a new rash on her scalp and behind her ears, not explained by any obvious changes to products, exposures, or activities. Subsequent patch testing determined that the rash was caused by baby shampoo, which contained cocamidopropyl betaine, which is used in hypoallergenic products. The rash resolved after a different shampoo was used.
“Sometimes, we really have to be thinking when the rash is getting worse, is there something they’re being exposed to that might be an allergen?” Dr. Zippin said.
In patients who have avoided all their positive allergens but a rash has not improved, clinicians should consider systemic contact dermatitis (SCD). Patients can develop SCD through different types of exposures, including transepidermal, transmucosal, oral, intravenous, subcutaneous, intramuscular, inhalation, and implantation routes.
SCD also has a variety of presentations, including pompholyx/dyshidrosis/vesicular dermatitis, maculopapular eruption, chronic pruritus, exfoliative erythroderma/toxiderma, chronic urticaria, erythema multiforme and vasculitis, hyperkeratotic papules of the elbows, acute generalized exanthematous pustulosis, and pruritus ani, according to Dr. Zippin.
SCD should be considered when a patient has a positive patch test to an allergen that is known to cause SCD, and does not clear after avoiding cutaneous exposure to the allergen, Dr. Zippin advised.
Patients will most often develop SCD from plants and herbs, Dr. Zippin noted. Chrysanthemums and chamomile tea are common culprits for compositae allergy and can trigger SCD; other causes are Anacardiaceae, Balsam of Peru, and propolis. Metals (nickel, cobalt, gold, and chromium), medications (aminoglycosides, corticosteroids, and ethylenediamine), and other sources (formaldehyde, propylene glycol in frozen foods, gallates, and methylisothiazolinone) can cause SCD as well.
Methylisothiazolinone in particular is a very common sensitizer, Dr. Zippin said. “If you have a patient who is positive to this, it’s almost always the cause of their problem.”
Balsam of Peru is in a number of different foods, and patients who need to follow a diet free of Balsam of Peru should avoid a long list of foods including citrus; bakery goods; Danish pastry; candy; gum; spices such as cinnamon, cloves, vanilla, curry, allspice, anise, and ginger; spicy condiments such as ketchup, chili sauce, barbecue sauce; chili, pizza, and foods with red sauces; tomatoes; pickles; alcohol (wine, beer, gin, vermouth); tea (perfumed or flavored); tobacco; chocolate and ice cream; and soft drinks (cola or spiced soft drinks).
Patients starting a nickel-free diet should avoid soy, peanuts and other nuts, legumes, chocolate, cocoa, oats, fish, and whole wheat flours. Any elimination diet should last for 3 months but should at least be tried for 3-4 weeks, with gradual reintroduction of foods suspected as triggers once per week. Any type I allergies that are discovered or suspected can be referred to an allergist for allergen challenge and desensitization therapy.
For more information, Dr. Zippin recommended the American Contact Dermatitis Society website for more information.
Dr. Zippin reported that he is the founder and holds stock options at CEP Biotech; is on the medical advisory board and receives stock options from YouV Labs., is a paid consultant and performs industry-sponsored research for Pfizer, receives stock options from Regeneron, and is on the medical advisory board for Hoth Therapeutics Inc. He is on the board of directors for the American Contact Dermatitis Society.
ORLANDO – Do you have ? Consider patch testing to assess whether they have allergic contact dermatitis.
“Of the patients who are sent to me by local pediatric dermatologists, 50% of them are positive” for allergens, said Jonathan H. Zippin, MD, PhD, director of the contact, occupational, and photodermatitis service at Cornell University, New York.
Speaking at the ODAC Dermatology, Aesthetic, and Surgical Conference, Dr. Zippin noted the prevalence of allergen sensitization is between 13% and 25% among children who are asymptomatic, while the prevalence of sensitization to at least one allergen among children with suspected allergic contact dermatitis (ACD) is between 25% and 96%. In 2014, a study from the National American Contact Dermatitis Group (NACDG) showed that of 883 children who were patch tested, 56.7% had at least one relevant positive patch test (RPPT) result.
“The take-home message here is that pediatric contact dermatitis is common, much more common than a lot of people realize,” Dr. Zippin said.
He described three common scenarios to keep in mind: a worsening rash, a new rash, and failure of a rash to improve after the patient avoids all of his or her positive allergens.
When a rash worsens, patch testing is likely to offer answers. In an analysis of 1,142 patients with suspected ACD aged 18 years or younger (mean age, 10.5 years; 64% female) in the Pediatric Contact Dermatitis Registry study database, 65% had at least one positive patch test, and 48% had at least 1 RPPT (Dermatitis 2016; 27[5] 293-302).
But not all patch testing is the same: The study also found that 24% of the RPPT cases would have been missed if assessed with the T.R.U.E. TEST compared with extended patch testing. If a T.R.U.E. TEST fails to explain generalized atopic dermatitis, the patient should be sent for more comprehensive testing where available, Dr. Zippin advised.
Pediatric patients also have unique allergens clinicians should consider. In the same study, children had a number of allergens similar to those of adults as reported in previous studies, such as nickel, cobalt, and neomycin. However, propylene glycol and cocamidopropyl betaine were allergens identified as unique to the pediatric population.
Another study looking at the same group of patients found that compared with children who did not have AD, children with AD had 7.4 times higher odds of having an RPPT to cocamidopropyl betaine, 7.6 times higher odds of having an RPPT to parthenolide, 5.3 times higher odds of having an RPPT to tixocortol pivalate, 4.2 times higher odds of having an RPPT to wool alcohols, and 4 times higher odds of having an RPPT to lanolin (JAMA Dermatology 2017;153[8]:765-70).
All of these are components of topical medicaments used to treat AD, “either components of emollients that we recommend, or components of steroids that we recommend,” Dr. Zippin pointed out.
One of these allergens could be the culprit when a child develops a new rash but there are no new apparent changes in products, exposures, and activities. Lanolin, also called wool grease, is used in many skin care products, for example. Dr. Zippin described the case of a 6-year-old girl with a history of AD, who presented with a new rash on her scalp and behind her ears, not explained by any obvious changes to products, exposures, or activities. Subsequent patch testing determined that the rash was caused by baby shampoo, which contained cocamidopropyl betaine, which is used in hypoallergenic products. The rash resolved after a different shampoo was used.
“Sometimes, we really have to be thinking when the rash is getting worse, is there something they’re being exposed to that might be an allergen?” Dr. Zippin said.
In patients who have avoided all their positive allergens but a rash has not improved, clinicians should consider systemic contact dermatitis (SCD). Patients can develop SCD through different types of exposures, including transepidermal, transmucosal, oral, intravenous, subcutaneous, intramuscular, inhalation, and implantation routes.
SCD also has a variety of presentations, including pompholyx/dyshidrosis/vesicular dermatitis, maculopapular eruption, chronic pruritus, exfoliative erythroderma/toxiderma, chronic urticaria, erythema multiforme and vasculitis, hyperkeratotic papules of the elbows, acute generalized exanthematous pustulosis, and pruritus ani, according to Dr. Zippin.
SCD should be considered when a patient has a positive patch test to an allergen that is known to cause SCD, and does not clear after avoiding cutaneous exposure to the allergen, Dr. Zippin advised.
Patients will most often develop SCD from plants and herbs, Dr. Zippin noted. Chrysanthemums and chamomile tea are common culprits for compositae allergy and can trigger SCD; other causes are Anacardiaceae, Balsam of Peru, and propolis. Metals (nickel, cobalt, gold, and chromium), medications (aminoglycosides, corticosteroids, and ethylenediamine), and other sources (formaldehyde, propylene glycol in frozen foods, gallates, and methylisothiazolinone) can cause SCD as well.
Methylisothiazolinone in particular is a very common sensitizer, Dr. Zippin said. “If you have a patient who is positive to this, it’s almost always the cause of their problem.”
Balsam of Peru is in a number of different foods, and patients who need to follow a diet free of Balsam of Peru should avoid a long list of foods including citrus; bakery goods; Danish pastry; candy; gum; spices such as cinnamon, cloves, vanilla, curry, allspice, anise, and ginger; spicy condiments such as ketchup, chili sauce, barbecue sauce; chili, pizza, and foods with red sauces; tomatoes; pickles; alcohol (wine, beer, gin, vermouth); tea (perfumed or flavored); tobacco; chocolate and ice cream; and soft drinks (cola or spiced soft drinks).
Patients starting a nickel-free diet should avoid soy, peanuts and other nuts, legumes, chocolate, cocoa, oats, fish, and whole wheat flours. Any elimination diet should last for 3 months but should at least be tried for 3-4 weeks, with gradual reintroduction of foods suspected as triggers once per week. Any type I allergies that are discovered or suspected can be referred to an allergist for allergen challenge and desensitization therapy.
For more information, Dr. Zippin recommended the American Contact Dermatitis Society website for more information.
Dr. Zippin reported that he is the founder and holds stock options at CEP Biotech; is on the medical advisory board and receives stock options from YouV Labs., is a paid consultant and performs industry-sponsored research for Pfizer, receives stock options from Regeneron, and is on the medical advisory board for Hoth Therapeutics Inc. He is on the board of directors for the American Contact Dermatitis Society.
ORLANDO – Do you have ? Consider patch testing to assess whether they have allergic contact dermatitis.
“Of the patients who are sent to me by local pediatric dermatologists, 50% of them are positive” for allergens, said Jonathan H. Zippin, MD, PhD, director of the contact, occupational, and photodermatitis service at Cornell University, New York.
Speaking at the ODAC Dermatology, Aesthetic, and Surgical Conference, Dr. Zippin noted the prevalence of allergen sensitization is between 13% and 25% among children who are asymptomatic, while the prevalence of sensitization to at least one allergen among children with suspected allergic contact dermatitis (ACD) is between 25% and 96%. In 2014, a study from the National American Contact Dermatitis Group (NACDG) showed that of 883 children who were patch tested, 56.7% had at least one relevant positive patch test (RPPT) result.
“The take-home message here is that pediatric contact dermatitis is common, much more common than a lot of people realize,” Dr. Zippin said.
He described three common scenarios to keep in mind: a worsening rash, a new rash, and failure of a rash to improve after the patient avoids all of his or her positive allergens.
When a rash worsens, patch testing is likely to offer answers. In an analysis of 1,142 patients with suspected ACD aged 18 years or younger (mean age, 10.5 years; 64% female) in the Pediatric Contact Dermatitis Registry study database, 65% had at least one positive patch test, and 48% had at least 1 RPPT (Dermatitis 2016; 27[5] 293-302).
But not all patch testing is the same: The study also found that 24% of the RPPT cases would have been missed if assessed with the T.R.U.E. TEST compared with extended patch testing. If a T.R.U.E. TEST fails to explain generalized atopic dermatitis, the patient should be sent for more comprehensive testing where available, Dr. Zippin advised.
Pediatric patients also have unique allergens clinicians should consider. In the same study, children had a number of allergens similar to those of adults as reported in previous studies, such as nickel, cobalt, and neomycin. However, propylene glycol and cocamidopropyl betaine were allergens identified as unique to the pediatric population.
Another study looking at the same group of patients found that compared with children who did not have AD, children with AD had 7.4 times higher odds of having an RPPT to cocamidopropyl betaine, 7.6 times higher odds of having an RPPT to parthenolide, 5.3 times higher odds of having an RPPT to tixocortol pivalate, 4.2 times higher odds of having an RPPT to wool alcohols, and 4 times higher odds of having an RPPT to lanolin (JAMA Dermatology 2017;153[8]:765-70).
All of these are components of topical medicaments used to treat AD, “either components of emollients that we recommend, or components of steroids that we recommend,” Dr. Zippin pointed out.
One of these allergens could be the culprit when a child develops a new rash but there are no new apparent changes in products, exposures, and activities. Lanolin, also called wool grease, is used in many skin care products, for example. Dr. Zippin described the case of a 6-year-old girl with a history of AD, who presented with a new rash on her scalp and behind her ears, not explained by any obvious changes to products, exposures, or activities. Subsequent patch testing determined that the rash was caused by baby shampoo, which contained cocamidopropyl betaine, which is used in hypoallergenic products. The rash resolved after a different shampoo was used.
“Sometimes, we really have to be thinking when the rash is getting worse, is there something they’re being exposed to that might be an allergen?” Dr. Zippin said.
In patients who have avoided all their positive allergens but a rash has not improved, clinicians should consider systemic contact dermatitis (SCD). Patients can develop SCD through different types of exposures, including transepidermal, transmucosal, oral, intravenous, subcutaneous, intramuscular, inhalation, and implantation routes.
SCD also has a variety of presentations, including pompholyx/dyshidrosis/vesicular dermatitis, maculopapular eruption, chronic pruritus, exfoliative erythroderma/toxiderma, chronic urticaria, erythema multiforme and vasculitis, hyperkeratotic papules of the elbows, acute generalized exanthematous pustulosis, and pruritus ani, according to Dr. Zippin.
SCD should be considered when a patient has a positive patch test to an allergen that is known to cause SCD, and does not clear after avoiding cutaneous exposure to the allergen, Dr. Zippin advised.
Patients will most often develop SCD from plants and herbs, Dr. Zippin noted. Chrysanthemums and chamomile tea are common culprits for compositae allergy and can trigger SCD; other causes are Anacardiaceae, Balsam of Peru, and propolis. Metals (nickel, cobalt, gold, and chromium), medications (aminoglycosides, corticosteroids, and ethylenediamine), and other sources (formaldehyde, propylene glycol in frozen foods, gallates, and methylisothiazolinone) can cause SCD as well.
Methylisothiazolinone in particular is a very common sensitizer, Dr. Zippin said. “If you have a patient who is positive to this, it’s almost always the cause of their problem.”
Balsam of Peru is in a number of different foods, and patients who need to follow a diet free of Balsam of Peru should avoid a long list of foods including citrus; bakery goods; Danish pastry; candy; gum; spices such as cinnamon, cloves, vanilla, curry, allspice, anise, and ginger; spicy condiments such as ketchup, chili sauce, barbecue sauce; chili, pizza, and foods with red sauces; tomatoes; pickles; alcohol (wine, beer, gin, vermouth); tea (perfumed or flavored); tobacco; chocolate and ice cream; and soft drinks (cola or spiced soft drinks).
Patients starting a nickel-free diet should avoid soy, peanuts and other nuts, legumes, chocolate, cocoa, oats, fish, and whole wheat flours. Any elimination diet should last for 3 months but should at least be tried for 3-4 weeks, with gradual reintroduction of foods suspected as triggers once per week. Any type I allergies that are discovered or suspected can be referred to an allergist for allergen challenge and desensitization therapy.
For more information, Dr. Zippin recommended the American Contact Dermatitis Society website for more information.
Dr. Zippin reported that he is the founder and holds stock options at CEP Biotech; is on the medical advisory board and receives stock options from YouV Labs., is a paid consultant and performs industry-sponsored research for Pfizer, receives stock options from Regeneron, and is on the medical advisory board for Hoth Therapeutics Inc. He is on the board of directors for the American Contact Dermatitis Society.
EXPERT ANALYSIS FROM ODAC 2020