User login
Hypersensitivity Reactions to Orthopedic Implants: What’s All the Hype?
Hypersensitivity to metal implants remains a controversial field in contact dermatitis and patch testing. With positive reactions to nickel hovering around 20% in patch-tested populations,1 the question remains whether metal-allergic patients can safely receive metal implants. Unfortunately, large controlled studies are lacking, in part due to ethical concerns of knowingly placing a metal implant in a metal-allergic patient. Much of the focus of implant hypersensitivity reactions (IHRs) has been on orthopedic joints including hips, knees, and shoulders, as well as fixed orthopedic implanted materials such as screws and plates. However, there have been reports of IHRs to cardiac devices including defibrillators, pacemakers, and intracardiac devices; dental hardware including implants, crowns, dentures, and braces; and neurologic and gynecologic devices. For the purposes of this review, we will focus on IHRs to orthopedic implants.
Making the Case for IHRs
There are multiple case reports and series documenting likely orthopedic IHRs in the literature2-5; however, large prospective studies are lacking. Some of the largest series are from Danish registry studies. In 2009, Thyssen et al6 reviewed356 patients who had undergone both total hip arthroplasty and patch testing. Metal allergy frequencies were similar between patch-tested registry patients and patch test controls, showing no increase in positive patch tests to metals after receiving implants. Additionally, implant revision rates were comparable between registry patients with and without patch testing. The group concluded that the risk for revision after hip implantation in metal-allergic patients and the risk for development of metal allergy after implantation were both low.6 In 2015, Münch et al7 compared 327 patients who had undergone both total knee arthroplasty and patch testing and found that prevalence of allergy to nickel, cobalt, and chromium was similar between patients who had undergone revision surgery and those who had not; however, for patients who had 2 or more knee revisions, there was a higher prevalence of postimplant metal allergy. This study also showed that metal allergy identified before implantation did not increase the risk for postimplantation knee revision surgery or implant failure.7 These larger studies suggest that although individual cases of IHR exist, it is likely quite rare.
Patients have been found to have increased levels of chromium (serum and urine) and titanium (serum) following total hip arthroplasty.8 Additionally, metal wear particles have been identified in postmortem livers and spleens, which was more prevalent in patients with a history of failed hip arthroplasty.9 It is difficult to determine the meaning of this data, as the presence of metal ions does not necessarily indicate allergy or IHR. In 2001, Hallab et al10 pooled data from several implant cohort studies and concluded that in comparison to a baseline metal sensitivity prevalence of approximately 10%, patients with well-functioning implants had a metal sensitivity–weighted average of 25%, and those with poorly functioning implants had a weighted average of 60%. Again, positive patch testing to metals does not necessarily implicate allergy as the cause of implant failure.
Some small studies have shown that patients with evidence of metal hypersensitivity improve with revision. Zondervan et al11 reviewed results of 46 orthopedic revisions following painful total knee arthroplasty. Patients with knee pain and lymphocyte transformation testing (LTT) positive for metals received hypoallergenic revisions, and those with LTT negative for metals received standard revisions. The group who received hypoallergenic revisions had more pain reduction compared to the standard revision group (37.8% reduction in pain vs 27%). However, this study was limited in that the diagnosis of metal allergy was made entirely on results of LTT.11 In 2012, Atanaskova Mesinkovska et al12 described 41 patients who underwent orthopedic patch testing following implantation for symptoms including pain, dermatitis, pruritus, joint loosening, edema, and impaired wound healing. Fifteen (37%) patients had positive patch test reactions to metals, and 10 (67%) of them had reactions to metals that were present in their implants. Six (60%) of these patients had their implants removed and their symptoms resolved; the remaining 4 continued to experience implant symptoms.12 These studies support the existence of rare metal-related orthopedic IHRs and support the concept of proceeding with orthopedic implant revision when indicated, safe, and agreed upon by the surgeon and patient. However, as noted in the series by Zondervan et al,11 not every patient with confirmed metal allergy who undergoes revision improves, so an informed conversation between the patient and surgeon is mandatory.
Types of Orthopedic Implants
Orthopedic implanted materials consist of either dynamic (knees, hips) or static (screws, plates) components. Several generations of hip implants have evolved since the 1960s. First-generation implanted hips were metal-on-metal and had high rates of metal release and sensitization. Metal-on-plastic implants may be less likely to release metal but instead release large polyethylene wear particles. Second-generation metal-on-metal implants reportedly have lower wear rates. With these implants, wear particles are generated but are reportedly smaller than first-generation particles.13
Allergens in IHRs
Metals
Metals are the most commonly implicated allergens in orthopedic IHRs. Potentially relevant metal alloys include 316L stainless steel, cobalt-chromium-molybdenum steel, Vitallium alloy, titanium alloy, titanium-tantalum-niobium alloy, and Oxinium (Smith & Nephew).14,15 Each alloy contains several metals, which can include nickel, chromium, cobalt, manganese, molybdenum, iron, titanium, aluminum, vanadium, niobium, tantalum, and zirconium, among others. For example, 316L stainless steel contains iron, nickel, chromium, manganese, molybdenum, nitrogen, carbon, sulfur, silicon, and phosphorus, whereas Oxinium contains only oxidized zirconium and niobium.
Bone Cement
Bone cement also has been reported in cases of orthopedic IHRs and can contain several chemicals, including methyl methacrylate, N,N-dimethyl-p-toluidine, benzoyl peroxide, hydroquinone, and gentamicin.14 Other potential exposures include adhesives (cyanoacrylates) and topical antibiotics.
Clinical Presentation
Several clinical presentations of orthopedic IHRs have been described. Perhaps the most commonly recognized is a localized cutaneous eczematous eruption, with dermatitis typically overlying the site of the implanted material.1,2,16 Generalized cutaneous eczematous IHRs also have been reported, including diffuse generalized dermatitis from a stainless steel orthopedic screw4 and nummular dermatitis attributed to vanadium in an orthopedic plate.5 Urticaria, vasculitis, and bullous cutaneous reactions, as well as extracutaneous complications, also have been reported.14,15 Pain, edema, joint loosening or failure, and poor wound healing have been reported,12 but it remains unclear whether these symptoms represent IHR.
Patch Testing for IHR
Several groups have published recommended patch test series for IHR.12,14,15 Common components of implant patch testing panels include metals, adhesives (acrylates, epoxy resins) and antibiotics. Importantly, obtaining product information from the manufacturer of the suspected implant can guide which allergens to include in patch testing. Implant and metal panels also are available for commercial purchase.
Other Diagnostic Tests
We rarely (almost never) order LTTs in the workup for potential IHRs. This is an in vitro test that includes lymphocytes, metal ions, and the radioactive marker methyl-3H-thymidine. The goal of the test is to evaluate if patient lymphocytes are reactive or responsive to metal ions. A positive LTT suggests that lymphocytes can respond to the presence of metal ions but does not confirm allergy or the presence of IHR.
Typically, skin or tissue biopsies are not required to make a diagnosis of IHR; however, if performed, histopathology suggestive of IHR can support a suspected diagnosis. Typical findings include but are not limited to spongiotic dermatitis. Eosinophils may or may not be present. Metal disc testing has been utilized for orthopedic IHR but is not currently recommended due to low diagnostic yield. Prick testing rarely is used and also is not a primary method for diagnosis of IHR.17
Preimplantation Patch Testing
Expert opinion guidelines published by the American Contact Dermatitis Society (ACDS) state that routine preimplantation patch testing is not necessary; however, for those patients with a clear history of contact reactions to metal, preimplantation patch testing can be considered.17
Patch test results can influence the orthopedic surgeon’s choice of implant material. In one study, when preimplantation patch testing showed a positive patch test reaction to metals, the results influenced the surgeon’s decision-making in all cases.12
Postimplantation Patch Testing: Diagnostic Criteria for Metal IHR After Implantation
From 2012 to 2013, Schalock and Thyssen18 surveyed expert attendees at meetings of the European Society of Contact Dermatitis and the ACDS for their opinions on proposed diagnostic criteria for metal IHRs. Based on these results (N=119), the authors stratified 4 major and 5 minor diagnostic criteria, which were defined based on overall responses of meeting attendees. Major criteria included (1) chronic dermatitis beginning weeks to months after metallic implantation, (2) complete recovery after removal of the offending implant, (3) eruption overlying the metal implant, and (4) positive patch test reaction to a metal used in the implant. Minor criteria included (1) histology consistent with allergic contact dermatitis, (2) morphology consistent with dermatitis (ie, erythema, induration, papules, vesicles), (3) positive in vitro test to metals (eg, lymphocyte transformation test), (4) systemic allergic dermatitis reaction, and (5) therapy-resistant dermatitis reaction. The authors did not describe a scoring system for evaluation and confirmation of a diagnosis of IHR. Instead, the criteria should be used as general guidelines when evaluating patients for possible IHRs. From a standpoint of available diagnostic tests for metal IHR, 86.1% of experts agreed that a positive patch test reaction to a metal used in the implant was suggestive of a diagnosis, whereas a positive in vitro test to metals (LTT) was suggestive of a diagnosis for only 32.2% of respondents. This study was designed specifically for metal IHRs and therefore is not necessarily generalizable for nonmetal IHRs.18
Final Interpretation
We follow the 2016 ACDS guidelines17 and complete preimplantation patch testing only in the setting of suspected metal allergy and postimplantation patch testing based on the guidelines described by Schalock and Thyssen.18 However, an extended conversation is warranted prior to patch testing to ensure the patient fully understands the limitations of the test. Although we have both ordered the LTT, interpretation remains murky, and until this test is standardized, routine use is unlikely to benefit the patient. Until we are more reliably able to predict who will develop hypersensitivity to implanted metals, the decision to remove or revise an implant is one that should be made by a multidisciplinary team that includes the surgeon and the patient.
- Dekoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Gao X, He RX, Yan SG, et al. Dermatitis associated with chromium following total knee arthroplasty. J Arthroplasty. 2011;26:665.E613-665.E616.
- Treudler R, Simon JC. Benzoyl peroxide: is it a relevant bone cement allergen in patients with orthopaedic implants? Contact Dermatitis. 2007;57:177-180.
- Barranco VP, Soloman H. Eczematous dermatitis from nickel. JAMA. 1972;220:1244.
- Engelhart S, Segal RJ. Allergic reaction to vanadium causes a diffuse eczematous eruption and titanium alloy orthopedic implant failure. Cutis. 2017;99:245-249.
- Thyssen JP, Jakobsen SS, Engkilde K, et al. The association between metal allergy, total hip arthroplasty, and revision. Acta Orthop. 2009;80:646-652.
- Münch HJ, Jacobsen SS, Olesen JT, et al. The association between metal allergy, total knee arthroplasty, and revision: study based on the Danish Knee Arthroplasty Register. Acta Orthop. 2015;86:378-383.
- Jacobs JJ, Skipor AK, Patterson LM, et al. Metal release in patients who have had a primary total hip arthroplasty. a prospective, controlled, longitudinal study. J Bone Joint Surg Am. 1998;80:1447-1458.
- Urban RM, Jacobs JJ, Tomlinson MJ, et al. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J Bone Joint Surg Am. 2000;82:457-476.
- Hallab N, Merritt K, Jacobs JJ. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am. 2001;83:428-436.
- Zondervan RL, Vaux JJ, Blackmer MJ, et al. Improved outcomes in patients with positive metal sensitivity following revision total knee arthroplasty. J Orthop Surg Res. 2019;14:182.
- Atanaskova Mesinkovska N, Tellez A, Molina L, et al. The effect of patch testing on surgical practices and outcomes in orthopedic patients with metal implants. Arch Dermatol. 2012;148:687-693.
- Kovochich M, Fung ES, Donovan E, et al. Characterization of wear debris from metal-on-metal hip implants during normal wear versus edge-loading conditions. J Biomed Mater Res B Appl Biomater. 2018;106:986-996.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Schalock PC, Menné T, Johansen JD, et al. Hypersensitivity reactions to metallic implants—diagnostic algorithm and suggested patch test series for clinical use. Contact Dermatitis. 2012;66:4-19.
- Thomas P, Gollwitzer H, Maier S, et al. Osteosynthesis associated contact dermatitis with unusual perpetuation of hyperreactivity in a nickel allergic patient. Contact Dermatitis. 2006;54:222-225.
- Schalock PC, Crawford G, Nedorost S, et al. Patch testing for evaluation of hypersensitivity to implanted metal devices: a perspective from the American Contact Dermatitis Society. Dermatitis. 2016;27:241-247.
- Schalock PC, Thyssen JP. Patch testers’ opinions regarding diagnostic criteria for metal hypersensitivity reactions to metallic implants. Dermatitis. 2013;24:183-185.
Hypersensitivity to metal implants remains a controversial field in contact dermatitis and patch testing. With positive reactions to nickel hovering around 20% in patch-tested populations,1 the question remains whether metal-allergic patients can safely receive metal implants. Unfortunately, large controlled studies are lacking, in part due to ethical concerns of knowingly placing a metal implant in a metal-allergic patient. Much of the focus of implant hypersensitivity reactions (IHRs) has been on orthopedic joints including hips, knees, and shoulders, as well as fixed orthopedic implanted materials such as screws and plates. However, there have been reports of IHRs to cardiac devices including defibrillators, pacemakers, and intracardiac devices; dental hardware including implants, crowns, dentures, and braces; and neurologic and gynecologic devices. For the purposes of this review, we will focus on IHRs to orthopedic implants.
Making the Case for IHRs
There are multiple case reports and series documenting likely orthopedic IHRs in the literature2-5; however, large prospective studies are lacking. Some of the largest series are from Danish registry studies. In 2009, Thyssen et al6 reviewed356 patients who had undergone both total hip arthroplasty and patch testing. Metal allergy frequencies were similar between patch-tested registry patients and patch test controls, showing no increase in positive patch tests to metals after receiving implants. Additionally, implant revision rates were comparable between registry patients with and without patch testing. The group concluded that the risk for revision after hip implantation in metal-allergic patients and the risk for development of metal allergy after implantation were both low.6 In 2015, Münch et al7 compared 327 patients who had undergone both total knee arthroplasty and patch testing and found that prevalence of allergy to nickel, cobalt, and chromium was similar between patients who had undergone revision surgery and those who had not; however, for patients who had 2 or more knee revisions, there was a higher prevalence of postimplant metal allergy. This study also showed that metal allergy identified before implantation did not increase the risk for postimplantation knee revision surgery or implant failure.7 These larger studies suggest that although individual cases of IHR exist, it is likely quite rare.
Patients have been found to have increased levels of chromium (serum and urine) and titanium (serum) following total hip arthroplasty.8 Additionally, metal wear particles have been identified in postmortem livers and spleens, which was more prevalent in patients with a history of failed hip arthroplasty.9 It is difficult to determine the meaning of this data, as the presence of metal ions does not necessarily indicate allergy or IHR. In 2001, Hallab et al10 pooled data from several implant cohort studies and concluded that in comparison to a baseline metal sensitivity prevalence of approximately 10%, patients with well-functioning implants had a metal sensitivity–weighted average of 25%, and those with poorly functioning implants had a weighted average of 60%. Again, positive patch testing to metals does not necessarily implicate allergy as the cause of implant failure.
Some small studies have shown that patients with evidence of metal hypersensitivity improve with revision. Zondervan et al11 reviewed results of 46 orthopedic revisions following painful total knee arthroplasty. Patients with knee pain and lymphocyte transformation testing (LTT) positive for metals received hypoallergenic revisions, and those with LTT negative for metals received standard revisions. The group who received hypoallergenic revisions had more pain reduction compared to the standard revision group (37.8% reduction in pain vs 27%). However, this study was limited in that the diagnosis of metal allergy was made entirely on results of LTT.11 In 2012, Atanaskova Mesinkovska et al12 described 41 patients who underwent orthopedic patch testing following implantation for symptoms including pain, dermatitis, pruritus, joint loosening, edema, and impaired wound healing. Fifteen (37%) patients had positive patch test reactions to metals, and 10 (67%) of them had reactions to metals that were present in their implants. Six (60%) of these patients had their implants removed and their symptoms resolved; the remaining 4 continued to experience implant symptoms.12 These studies support the existence of rare metal-related orthopedic IHRs and support the concept of proceeding with orthopedic implant revision when indicated, safe, and agreed upon by the surgeon and patient. However, as noted in the series by Zondervan et al,11 not every patient with confirmed metal allergy who undergoes revision improves, so an informed conversation between the patient and surgeon is mandatory.
Types of Orthopedic Implants
Orthopedic implanted materials consist of either dynamic (knees, hips) or static (screws, plates) components. Several generations of hip implants have evolved since the 1960s. First-generation implanted hips were metal-on-metal and had high rates of metal release and sensitization. Metal-on-plastic implants may be less likely to release metal but instead release large polyethylene wear particles. Second-generation metal-on-metal implants reportedly have lower wear rates. With these implants, wear particles are generated but are reportedly smaller than first-generation particles.13
Allergens in IHRs
Metals
Metals are the most commonly implicated allergens in orthopedic IHRs. Potentially relevant metal alloys include 316L stainless steel, cobalt-chromium-molybdenum steel, Vitallium alloy, titanium alloy, titanium-tantalum-niobium alloy, and Oxinium (Smith & Nephew).14,15 Each alloy contains several metals, which can include nickel, chromium, cobalt, manganese, molybdenum, iron, titanium, aluminum, vanadium, niobium, tantalum, and zirconium, among others. For example, 316L stainless steel contains iron, nickel, chromium, manganese, molybdenum, nitrogen, carbon, sulfur, silicon, and phosphorus, whereas Oxinium contains only oxidized zirconium and niobium.
Bone Cement
Bone cement also has been reported in cases of orthopedic IHRs and can contain several chemicals, including methyl methacrylate, N,N-dimethyl-p-toluidine, benzoyl peroxide, hydroquinone, and gentamicin.14 Other potential exposures include adhesives (cyanoacrylates) and topical antibiotics.
Clinical Presentation
Several clinical presentations of orthopedic IHRs have been described. Perhaps the most commonly recognized is a localized cutaneous eczematous eruption, with dermatitis typically overlying the site of the implanted material.1,2,16 Generalized cutaneous eczematous IHRs also have been reported, including diffuse generalized dermatitis from a stainless steel orthopedic screw4 and nummular dermatitis attributed to vanadium in an orthopedic plate.5 Urticaria, vasculitis, and bullous cutaneous reactions, as well as extracutaneous complications, also have been reported.14,15 Pain, edema, joint loosening or failure, and poor wound healing have been reported,12 but it remains unclear whether these symptoms represent IHR.
Patch Testing for IHR
Several groups have published recommended patch test series for IHR.12,14,15 Common components of implant patch testing panels include metals, adhesives (acrylates, epoxy resins) and antibiotics. Importantly, obtaining product information from the manufacturer of the suspected implant can guide which allergens to include in patch testing. Implant and metal panels also are available for commercial purchase.
Other Diagnostic Tests
We rarely (almost never) order LTTs in the workup for potential IHRs. This is an in vitro test that includes lymphocytes, metal ions, and the radioactive marker methyl-3H-thymidine. The goal of the test is to evaluate if patient lymphocytes are reactive or responsive to metal ions. A positive LTT suggests that lymphocytes can respond to the presence of metal ions but does not confirm allergy or the presence of IHR.
Typically, skin or tissue biopsies are not required to make a diagnosis of IHR; however, if performed, histopathology suggestive of IHR can support a suspected diagnosis. Typical findings include but are not limited to spongiotic dermatitis. Eosinophils may or may not be present. Metal disc testing has been utilized for orthopedic IHR but is not currently recommended due to low diagnostic yield. Prick testing rarely is used and also is not a primary method for diagnosis of IHR.17
Preimplantation Patch Testing
Expert opinion guidelines published by the American Contact Dermatitis Society (ACDS) state that routine preimplantation patch testing is not necessary; however, for those patients with a clear history of contact reactions to metal, preimplantation patch testing can be considered.17
Patch test results can influence the orthopedic surgeon’s choice of implant material. In one study, when preimplantation patch testing showed a positive patch test reaction to metals, the results influenced the surgeon’s decision-making in all cases.12
Postimplantation Patch Testing: Diagnostic Criteria for Metal IHR After Implantation
From 2012 to 2013, Schalock and Thyssen18 surveyed expert attendees at meetings of the European Society of Contact Dermatitis and the ACDS for their opinions on proposed diagnostic criteria for metal IHRs. Based on these results (N=119), the authors stratified 4 major and 5 minor diagnostic criteria, which were defined based on overall responses of meeting attendees. Major criteria included (1) chronic dermatitis beginning weeks to months after metallic implantation, (2) complete recovery after removal of the offending implant, (3) eruption overlying the metal implant, and (4) positive patch test reaction to a metal used in the implant. Minor criteria included (1) histology consistent with allergic contact dermatitis, (2) morphology consistent with dermatitis (ie, erythema, induration, papules, vesicles), (3) positive in vitro test to metals (eg, lymphocyte transformation test), (4) systemic allergic dermatitis reaction, and (5) therapy-resistant dermatitis reaction. The authors did not describe a scoring system for evaluation and confirmation of a diagnosis of IHR. Instead, the criteria should be used as general guidelines when evaluating patients for possible IHRs. From a standpoint of available diagnostic tests for metal IHR, 86.1% of experts agreed that a positive patch test reaction to a metal used in the implant was suggestive of a diagnosis, whereas a positive in vitro test to metals (LTT) was suggestive of a diagnosis for only 32.2% of respondents. This study was designed specifically for metal IHRs and therefore is not necessarily generalizable for nonmetal IHRs.18
Final Interpretation
We follow the 2016 ACDS guidelines17 and complete preimplantation patch testing only in the setting of suspected metal allergy and postimplantation patch testing based on the guidelines described by Schalock and Thyssen.18 However, an extended conversation is warranted prior to patch testing to ensure the patient fully understands the limitations of the test. Although we have both ordered the LTT, interpretation remains murky, and until this test is standardized, routine use is unlikely to benefit the patient. Until we are more reliably able to predict who will develop hypersensitivity to implanted metals, the decision to remove or revise an implant is one that should be made by a multidisciplinary team that includes the surgeon and the patient.
Hypersensitivity to metal implants remains a controversial field in contact dermatitis and patch testing. With positive reactions to nickel hovering around 20% in patch-tested populations,1 the question remains whether metal-allergic patients can safely receive metal implants. Unfortunately, large controlled studies are lacking, in part due to ethical concerns of knowingly placing a metal implant in a metal-allergic patient. Much of the focus of implant hypersensitivity reactions (IHRs) has been on orthopedic joints including hips, knees, and shoulders, as well as fixed orthopedic implanted materials such as screws and plates. However, there have been reports of IHRs to cardiac devices including defibrillators, pacemakers, and intracardiac devices; dental hardware including implants, crowns, dentures, and braces; and neurologic and gynecologic devices. For the purposes of this review, we will focus on IHRs to orthopedic implants.
Making the Case for IHRs
There are multiple case reports and series documenting likely orthopedic IHRs in the literature2-5; however, large prospective studies are lacking. Some of the largest series are from Danish registry studies. In 2009, Thyssen et al6 reviewed356 patients who had undergone both total hip arthroplasty and patch testing. Metal allergy frequencies were similar between patch-tested registry patients and patch test controls, showing no increase in positive patch tests to metals after receiving implants. Additionally, implant revision rates were comparable between registry patients with and without patch testing. The group concluded that the risk for revision after hip implantation in metal-allergic patients and the risk for development of metal allergy after implantation were both low.6 In 2015, Münch et al7 compared 327 patients who had undergone both total knee arthroplasty and patch testing and found that prevalence of allergy to nickel, cobalt, and chromium was similar between patients who had undergone revision surgery and those who had not; however, for patients who had 2 or more knee revisions, there was a higher prevalence of postimplant metal allergy. This study also showed that metal allergy identified before implantation did not increase the risk for postimplantation knee revision surgery or implant failure.7 These larger studies suggest that although individual cases of IHR exist, it is likely quite rare.
Patients have been found to have increased levels of chromium (serum and urine) and titanium (serum) following total hip arthroplasty.8 Additionally, metal wear particles have been identified in postmortem livers and spleens, which was more prevalent in patients with a history of failed hip arthroplasty.9 It is difficult to determine the meaning of this data, as the presence of metal ions does not necessarily indicate allergy or IHR. In 2001, Hallab et al10 pooled data from several implant cohort studies and concluded that in comparison to a baseline metal sensitivity prevalence of approximately 10%, patients with well-functioning implants had a metal sensitivity–weighted average of 25%, and those with poorly functioning implants had a weighted average of 60%. Again, positive patch testing to metals does not necessarily implicate allergy as the cause of implant failure.
Some small studies have shown that patients with evidence of metal hypersensitivity improve with revision. Zondervan et al11 reviewed results of 46 orthopedic revisions following painful total knee arthroplasty. Patients with knee pain and lymphocyte transformation testing (LTT) positive for metals received hypoallergenic revisions, and those with LTT negative for metals received standard revisions. The group who received hypoallergenic revisions had more pain reduction compared to the standard revision group (37.8% reduction in pain vs 27%). However, this study was limited in that the diagnosis of metal allergy was made entirely on results of LTT.11 In 2012, Atanaskova Mesinkovska et al12 described 41 patients who underwent orthopedic patch testing following implantation for symptoms including pain, dermatitis, pruritus, joint loosening, edema, and impaired wound healing. Fifteen (37%) patients had positive patch test reactions to metals, and 10 (67%) of them had reactions to metals that were present in their implants. Six (60%) of these patients had their implants removed and their symptoms resolved; the remaining 4 continued to experience implant symptoms.12 These studies support the existence of rare metal-related orthopedic IHRs and support the concept of proceeding with orthopedic implant revision when indicated, safe, and agreed upon by the surgeon and patient. However, as noted in the series by Zondervan et al,11 not every patient with confirmed metal allergy who undergoes revision improves, so an informed conversation between the patient and surgeon is mandatory.
Types of Orthopedic Implants
Orthopedic implanted materials consist of either dynamic (knees, hips) or static (screws, plates) components. Several generations of hip implants have evolved since the 1960s. First-generation implanted hips were metal-on-metal and had high rates of metal release and sensitization. Metal-on-plastic implants may be less likely to release metal but instead release large polyethylene wear particles. Second-generation metal-on-metal implants reportedly have lower wear rates. With these implants, wear particles are generated but are reportedly smaller than first-generation particles.13
Allergens in IHRs
Metals
Metals are the most commonly implicated allergens in orthopedic IHRs. Potentially relevant metal alloys include 316L stainless steel, cobalt-chromium-molybdenum steel, Vitallium alloy, titanium alloy, titanium-tantalum-niobium alloy, and Oxinium (Smith & Nephew).14,15 Each alloy contains several metals, which can include nickel, chromium, cobalt, manganese, molybdenum, iron, titanium, aluminum, vanadium, niobium, tantalum, and zirconium, among others. For example, 316L stainless steel contains iron, nickel, chromium, manganese, molybdenum, nitrogen, carbon, sulfur, silicon, and phosphorus, whereas Oxinium contains only oxidized zirconium and niobium.
Bone Cement
Bone cement also has been reported in cases of orthopedic IHRs and can contain several chemicals, including methyl methacrylate, N,N-dimethyl-p-toluidine, benzoyl peroxide, hydroquinone, and gentamicin.14 Other potential exposures include adhesives (cyanoacrylates) and topical antibiotics.
Clinical Presentation
Several clinical presentations of orthopedic IHRs have been described. Perhaps the most commonly recognized is a localized cutaneous eczematous eruption, with dermatitis typically overlying the site of the implanted material.1,2,16 Generalized cutaneous eczematous IHRs also have been reported, including diffuse generalized dermatitis from a stainless steel orthopedic screw4 and nummular dermatitis attributed to vanadium in an orthopedic plate.5 Urticaria, vasculitis, and bullous cutaneous reactions, as well as extracutaneous complications, also have been reported.14,15 Pain, edema, joint loosening or failure, and poor wound healing have been reported,12 but it remains unclear whether these symptoms represent IHR.
Patch Testing for IHR
Several groups have published recommended patch test series for IHR.12,14,15 Common components of implant patch testing panels include metals, adhesives (acrylates, epoxy resins) and antibiotics. Importantly, obtaining product information from the manufacturer of the suspected implant can guide which allergens to include in patch testing. Implant and metal panels also are available for commercial purchase.
Other Diagnostic Tests
We rarely (almost never) order LTTs in the workup for potential IHRs. This is an in vitro test that includes lymphocytes, metal ions, and the radioactive marker methyl-3H-thymidine. The goal of the test is to evaluate if patient lymphocytes are reactive or responsive to metal ions. A positive LTT suggests that lymphocytes can respond to the presence of metal ions but does not confirm allergy or the presence of IHR.
Typically, skin or tissue biopsies are not required to make a diagnosis of IHR; however, if performed, histopathology suggestive of IHR can support a suspected diagnosis. Typical findings include but are not limited to spongiotic dermatitis. Eosinophils may or may not be present. Metal disc testing has been utilized for orthopedic IHR but is not currently recommended due to low diagnostic yield. Prick testing rarely is used and also is not a primary method for diagnosis of IHR.17
Preimplantation Patch Testing
Expert opinion guidelines published by the American Contact Dermatitis Society (ACDS) state that routine preimplantation patch testing is not necessary; however, for those patients with a clear history of contact reactions to metal, preimplantation patch testing can be considered.17
Patch test results can influence the orthopedic surgeon’s choice of implant material. In one study, when preimplantation patch testing showed a positive patch test reaction to metals, the results influenced the surgeon’s decision-making in all cases.12
Postimplantation Patch Testing: Diagnostic Criteria for Metal IHR After Implantation
From 2012 to 2013, Schalock and Thyssen18 surveyed expert attendees at meetings of the European Society of Contact Dermatitis and the ACDS for their opinions on proposed diagnostic criteria for metal IHRs. Based on these results (N=119), the authors stratified 4 major and 5 minor diagnostic criteria, which were defined based on overall responses of meeting attendees. Major criteria included (1) chronic dermatitis beginning weeks to months after metallic implantation, (2) complete recovery after removal of the offending implant, (3) eruption overlying the metal implant, and (4) positive patch test reaction to a metal used in the implant. Minor criteria included (1) histology consistent with allergic contact dermatitis, (2) morphology consistent with dermatitis (ie, erythema, induration, papules, vesicles), (3) positive in vitro test to metals (eg, lymphocyte transformation test), (4) systemic allergic dermatitis reaction, and (5) therapy-resistant dermatitis reaction. The authors did not describe a scoring system for evaluation and confirmation of a diagnosis of IHR. Instead, the criteria should be used as general guidelines when evaluating patients for possible IHRs. From a standpoint of available diagnostic tests for metal IHR, 86.1% of experts agreed that a positive patch test reaction to a metal used in the implant was suggestive of a diagnosis, whereas a positive in vitro test to metals (LTT) was suggestive of a diagnosis for only 32.2% of respondents. This study was designed specifically for metal IHRs and therefore is not necessarily generalizable for nonmetal IHRs.18
Final Interpretation
We follow the 2016 ACDS guidelines17 and complete preimplantation patch testing only in the setting of suspected metal allergy and postimplantation patch testing based on the guidelines described by Schalock and Thyssen.18 However, an extended conversation is warranted prior to patch testing to ensure the patient fully understands the limitations of the test. Although we have both ordered the LTT, interpretation remains murky, and until this test is standardized, routine use is unlikely to benefit the patient. Until we are more reliably able to predict who will develop hypersensitivity to implanted metals, the decision to remove or revise an implant is one that should be made by a multidisciplinary team that includes the surgeon and the patient.
- Dekoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Gao X, He RX, Yan SG, et al. Dermatitis associated with chromium following total knee arthroplasty. J Arthroplasty. 2011;26:665.E613-665.E616.
- Treudler R, Simon JC. Benzoyl peroxide: is it a relevant bone cement allergen in patients with orthopaedic implants? Contact Dermatitis. 2007;57:177-180.
- Barranco VP, Soloman H. Eczematous dermatitis from nickel. JAMA. 1972;220:1244.
- Engelhart S, Segal RJ. Allergic reaction to vanadium causes a diffuse eczematous eruption and titanium alloy orthopedic implant failure. Cutis. 2017;99:245-249.
- Thyssen JP, Jakobsen SS, Engkilde K, et al. The association between metal allergy, total hip arthroplasty, and revision. Acta Orthop. 2009;80:646-652.
- Münch HJ, Jacobsen SS, Olesen JT, et al. The association between metal allergy, total knee arthroplasty, and revision: study based on the Danish Knee Arthroplasty Register. Acta Orthop. 2015;86:378-383.
- Jacobs JJ, Skipor AK, Patterson LM, et al. Metal release in patients who have had a primary total hip arthroplasty. a prospective, controlled, longitudinal study. J Bone Joint Surg Am. 1998;80:1447-1458.
- Urban RM, Jacobs JJ, Tomlinson MJ, et al. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J Bone Joint Surg Am. 2000;82:457-476.
- Hallab N, Merritt K, Jacobs JJ. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am. 2001;83:428-436.
- Zondervan RL, Vaux JJ, Blackmer MJ, et al. Improved outcomes in patients with positive metal sensitivity following revision total knee arthroplasty. J Orthop Surg Res. 2019;14:182.
- Atanaskova Mesinkovska N, Tellez A, Molina L, et al. The effect of patch testing on surgical practices and outcomes in orthopedic patients with metal implants. Arch Dermatol. 2012;148:687-693.
- Kovochich M, Fung ES, Donovan E, et al. Characterization of wear debris from metal-on-metal hip implants during normal wear versus edge-loading conditions. J Biomed Mater Res B Appl Biomater. 2018;106:986-996.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Schalock PC, Menné T, Johansen JD, et al. Hypersensitivity reactions to metallic implants—diagnostic algorithm and suggested patch test series for clinical use. Contact Dermatitis. 2012;66:4-19.
- Thomas P, Gollwitzer H, Maier S, et al. Osteosynthesis associated contact dermatitis with unusual perpetuation of hyperreactivity in a nickel allergic patient. Contact Dermatitis. 2006;54:222-225.
- Schalock PC, Crawford G, Nedorost S, et al. Patch testing for evaluation of hypersensitivity to implanted metal devices: a perspective from the American Contact Dermatitis Society. Dermatitis. 2016;27:241-247.
- Schalock PC, Thyssen JP. Patch testers’ opinions regarding diagnostic criteria for metal hypersensitivity reactions to metallic implants. Dermatitis. 2013;24:183-185.
- Dekoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Gao X, He RX, Yan SG, et al. Dermatitis associated with chromium following total knee arthroplasty. J Arthroplasty. 2011;26:665.E613-665.E616.
- Treudler R, Simon JC. Benzoyl peroxide: is it a relevant bone cement allergen in patients with orthopaedic implants? Contact Dermatitis. 2007;57:177-180.
- Barranco VP, Soloman H. Eczematous dermatitis from nickel. JAMA. 1972;220:1244.
- Engelhart S, Segal RJ. Allergic reaction to vanadium causes a diffuse eczematous eruption and titanium alloy orthopedic implant failure. Cutis. 2017;99:245-249.
- Thyssen JP, Jakobsen SS, Engkilde K, et al. The association between metal allergy, total hip arthroplasty, and revision. Acta Orthop. 2009;80:646-652.
- Münch HJ, Jacobsen SS, Olesen JT, et al. The association between metal allergy, total knee arthroplasty, and revision: study based on the Danish Knee Arthroplasty Register. Acta Orthop. 2015;86:378-383.
- Jacobs JJ, Skipor AK, Patterson LM, et al. Metal release in patients who have had a primary total hip arthroplasty. a prospective, controlled, longitudinal study. J Bone Joint Surg Am. 1998;80:1447-1458.
- Urban RM, Jacobs JJ, Tomlinson MJ, et al. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J Bone Joint Surg Am. 2000;82:457-476.
- Hallab N, Merritt K, Jacobs JJ. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am. 2001;83:428-436.
- Zondervan RL, Vaux JJ, Blackmer MJ, et al. Improved outcomes in patients with positive metal sensitivity following revision total knee arthroplasty. J Orthop Surg Res. 2019;14:182.
- Atanaskova Mesinkovska N, Tellez A, Molina L, et al. The effect of patch testing on surgical practices and outcomes in orthopedic patients with metal implants. Arch Dermatol. 2012;148:687-693.
- Kovochich M, Fung ES, Donovan E, et al. Characterization of wear debris from metal-on-metal hip implants during normal wear versus edge-loading conditions. J Biomed Mater Res B Appl Biomater. 2018;106:986-996.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Schalock PC, Menné T, Johansen JD, et al. Hypersensitivity reactions to metallic implants—diagnostic algorithm and suggested patch test series for clinical use. Contact Dermatitis. 2012;66:4-19.
- Thomas P, Gollwitzer H, Maier S, et al. Osteosynthesis associated contact dermatitis with unusual perpetuation of hyperreactivity in a nickel allergic patient. Contact Dermatitis. 2006;54:222-225.
- Schalock PC, Crawford G, Nedorost S, et al. Patch testing for evaluation of hypersensitivity to implanted metal devices: a perspective from the American Contact Dermatitis Society. Dermatitis. 2016;27:241-247.
- Schalock PC, Thyssen JP. Patch testers’ opinions regarding diagnostic criteria for metal hypersensitivity reactions to metallic implants. Dermatitis. 2013;24:183-185.
Practice Points
- Common clinical presentations of orthopedic implant hypersensitivity reactions include localized cutaneous eruptions, generalized cutaneous eruptions, and noncutaneous reactions.
- Allergens implicated in orthopedic implant hypersensitivity reactions include metals and bone cement components.
- Routine preimplant patch testing for orthopedic hypersensitivity reactions is not recommended but can be performed when there is strong concern for metal allergy.
- Postimplant patch testing should be performed when symptoms are consistent with potential orthopedic implant hypersensitivity reactions.
Makeup is contaminated with pathogenic bacteria
Recalcitrant acne is a common, unwavering problem in dermatology practices nationwide. However, both gram positive and gram negative infections of the skin can go undiagnosed in patients with acne resistant to the armamentarium of oral and topical therapeutics. Although I often use isotretinoin in patients with cystic or recalcitrant acne, I almost always do a culture prior to initiating therapy, and more often than not, have discovered patients have gram negative and gram positive skin infections resistant to antibiotics commonly used to treat acne.
In a study by Bashir and Lambert published in the Journal of Applied Microbiology, 70%-90% of makeup products tested – including lipstick, lip gloss, beauty blenders, eyeliners, and mascara – were found to be contaminated with bacteria. Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli were the most common culprits, and the product with the highest contamination rates were beauty blenders (the small sponges used to apply makeup), which also had high rates of fungal contamination.
Expiration dates on cosmetic products are used to indicate the length of time a preservative in a product can control bacterial contamination. They are printed on packaging as an open jar symbol with the 3M, 6M, 9M, and 12M label for the number of months the product can be opened and used. Unfortunately and unknowingly, most consumers use products beyond the expiration date, and the most common offender is mascara.
Gram positive and gram negative skin infections should be ruled out in all cases of recalcitrant acne. A reminder to note on all culture requisitions to grow gram negatives because not all labs will grow gram negatives on a skin swab. Counseling should also be given to those patients who wear makeup, which should include techniques to clean and sanitize makeup applicators including brushes, tools, and towels. Blenders are known to be used “wet” and are not dried when washed.
It is my recommendation that blenders be a one-time-use-only tool and disposed of after EVERY application. Instructions provided in my clinic are to wash all devices and brushes once a week with hot soapy water, and blow dry with a hair dryer immediately afterward. Lipsticks, mascara wands, and lip glosses should be sanitized with alcohol once a month. Finally, all products need to be disposed of after their expiry.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Resource
Basher A, Lambert P. J Appl Microbiol. 2019. doi: 10.1111/jam.14479.
Recalcitrant acne is a common, unwavering problem in dermatology practices nationwide. However, both gram positive and gram negative infections of the skin can go undiagnosed in patients with acne resistant to the armamentarium of oral and topical therapeutics. Although I often use isotretinoin in patients with cystic or recalcitrant acne, I almost always do a culture prior to initiating therapy, and more often than not, have discovered patients have gram negative and gram positive skin infections resistant to antibiotics commonly used to treat acne.
In a study by Bashir and Lambert published in the Journal of Applied Microbiology, 70%-90% of makeup products tested – including lipstick, lip gloss, beauty blenders, eyeliners, and mascara – were found to be contaminated with bacteria. Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli were the most common culprits, and the product with the highest contamination rates were beauty blenders (the small sponges used to apply makeup), which also had high rates of fungal contamination.
Expiration dates on cosmetic products are used to indicate the length of time a preservative in a product can control bacterial contamination. They are printed on packaging as an open jar symbol with the 3M, 6M, 9M, and 12M label for the number of months the product can be opened and used. Unfortunately and unknowingly, most consumers use products beyond the expiration date, and the most common offender is mascara.
Gram positive and gram negative skin infections should be ruled out in all cases of recalcitrant acne. A reminder to note on all culture requisitions to grow gram negatives because not all labs will grow gram negatives on a skin swab. Counseling should also be given to those patients who wear makeup, which should include techniques to clean and sanitize makeup applicators including brushes, tools, and towels. Blenders are known to be used “wet” and are not dried when washed.
It is my recommendation that blenders be a one-time-use-only tool and disposed of after EVERY application. Instructions provided in my clinic are to wash all devices and brushes once a week with hot soapy water, and blow dry with a hair dryer immediately afterward. Lipsticks, mascara wands, and lip glosses should be sanitized with alcohol once a month. Finally, all products need to be disposed of after their expiry.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Resource
Basher A, Lambert P. J Appl Microbiol. 2019. doi: 10.1111/jam.14479.
Recalcitrant acne is a common, unwavering problem in dermatology practices nationwide. However, both gram positive and gram negative infections of the skin can go undiagnosed in patients with acne resistant to the armamentarium of oral and topical therapeutics. Although I often use isotretinoin in patients with cystic or recalcitrant acne, I almost always do a culture prior to initiating therapy, and more often than not, have discovered patients have gram negative and gram positive skin infections resistant to antibiotics commonly used to treat acne.
In a study by Bashir and Lambert published in the Journal of Applied Microbiology, 70%-90% of makeup products tested – including lipstick, lip gloss, beauty blenders, eyeliners, and mascara – were found to be contaminated with bacteria. Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli were the most common culprits, and the product with the highest contamination rates were beauty blenders (the small sponges used to apply makeup), which also had high rates of fungal contamination.
Expiration dates on cosmetic products are used to indicate the length of time a preservative in a product can control bacterial contamination. They are printed on packaging as an open jar symbol with the 3M, 6M, 9M, and 12M label for the number of months the product can be opened and used. Unfortunately and unknowingly, most consumers use products beyond the expiration date, and the most common offender is mascara.
Gram positive and gram negative skin infections should be ruled out in all cases of recalcitrant acne. A reminder to note on all culture requisitions to grow gram negatives because not all labs will grow gram negatives on a skin swab. Counseling should also be given to those patients who wear makeup, which should include techniques to clean and sanitize makeup applicators including brushes, tools, and towels. Blenders are known to be used “wet” and are not dried when washed.
It is my recommendation that blenders be a one-time-use-only tool and disposed of after EVERY application. Instructions provided in my clinic are to wash all devices and brushes once a week with hot soapy water, and blow dry with a hair dryer immediately afterward. Lipsticks, mascara wands, and lip glosses should be sanitized with alcohol once a month. Finally, all products need to be disposed of after their expiry.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Resource
Basher A, Lambert P. J Appl Microbiol. 2019. doi: 10.1111/jam.14479.
Pruritic and Erythematous Rash Resembling Marks Caused by a Lashing
Shiitake Mushroom Dermatitis
Given the scattered and erythematous 1- to 2-mm macules and patches in a flagellate pattern on the shoulders, back, and neck, the differential diagnosis included shiitake mushroom dermatitis, bleomycin-induced flagellate dermatitis, dermatomyositis flagellate erythema, excoriation disorder, dermatographism, and keratosis lichenoides chronica. On further questioning, the patient indicated that he had consumed raw shiitake mushrooms 3 days before the onset of the rash. Although the clinical variability for all the conditions on the differential is notable, our patient had a history of consuming raw shiitake mushrooms, denied taking any medications, reported no repeated trauma, and had no muscular involvement or systemic symptoms, making shiitake mushroom dermatitis the most likely diagnosis.1 Skin biopsy and blood tests were deemed unnecessary. Instead, the patient was prescribed triamcinolone cream 0.1%, counseled to avoid raw or undercooked shiitake mushrooms in the future, and told to follow-up if symptoms did not resolve. The patient's symptoms resolved, as expected.
Nakamura2 first described shiitake mushroom dermatitis in 1977. The condition also is known as flagellate mushroom dermatitis or shiitake toxicoderma. It classically manifests in susceptible patients as a pruritic, linear, flagellated dermatitis within 24 to 48 hours after the consumption of raw or undercooked shiitake mushrooms (Lentinula edodes).3 Although the complete pathogenesis remains unclear, research suggests that either a toxic reaction or a type IV hypersensitivity reaction to lentinan causes the eruption. Lentinan is a thermolabile polysaccharide within the mushroom that is believed to increase the production of IL-1 and cause vasodilatation.4
Shiitake mushroom dermatitis is a clinical diagnosis based on the most common findings of a flagellate-pattern dermatitis consisting of pruritic and erythematous papular or urticarial lesions, usually found on the trunk. Laboratory tests, skin biopsies, and allergy tests have been shown to be nonspecific and inconsistent.5
Shiitake mushroom dermatitis is a self-limiting condition, with the majority of symptoms resolving after one to several weeks.5 The mainstay of treatment is aimed at symptomatic management and usually consists of topical corticosteroids and antihistamines.3 More rapid resolution of symptoms has been reported with the use of short-term balneo-psoralen plus UVA therapy.6
Although a 2017 review of the literature described only 9 published cases of shiitake mushroom dermatitis within the United States as of July 2015, this number may be misleading given the possibility of more variable and subtle presentations misdiagnosed as a nonspecific dermatitis.5 Given this information and the fact that shiitake mushrooms continue to be a popular choice in American cuisine, this case reminds health care providers of the clinical manifestations, differential diagnosis, and management of flagellate dermatitis caused by consuming shiitake mushrooms.
- Adler MJ, Larsen WG. Clinical variability of shiitake dermatitis. J Am Acad Dermatol. 2012;67:e140-e141.
- Nakamura T. Toxicoderma caused by Shiitake (Lentinus edodes). Japan J Clin Dermatol. 1977;31:65-68.
- Nakamura T. Shiitake (Lentinus edodes) dermatitis. Contact Dermatitis. 1992;27:65-70.
- Corazza M, Zauli S, Ricci M, et al. Shiitake dermatitis: toxic or allergic reaction? J Eur Acad Dermatol Venereol. 2015;29:1449-1451.
- Nguyen AH, Gonzaga MI, Lim VM, et al. Clinical features of shiitake dermatitis: a systematic review. Int J Dermatol. 2017;56:610-616.
- Scheiba N, Andrulis M, Helmbold P. Treatment of shiitake dermatitis by balneo PUVA therapy. J Am Acad Dermatol. 2011;65:453-455.
Shiitake Mushroom Dermatitis
Given the scattered and erythematous 1- to 2-mm macules and patches in a flagellate pattern on the shoulders, back, and neck, the differential diagnosis included shiitake mushroom dermatitis, bleomycin-induced flagellate dermatitis, dermatomyositis flagellate erythema, excoriation disorder, dermatographism, and keratosis lichenoides chronica. On further questioning, the patient indicated that he had consumed raw shiitake mushrooms 3 days before the onset of the rash. Although the clinical variability for all the conditions on the differential is notable, our patient had a history of consuming raw shiitake mushrooms, denied taking any medications, reported no repeated trauma, and had no muscular involvement or systemic symptoms, making shiitake mushroom dermatitis the most likely diagnosis.1 Skin biopsy and blood tests were deemed unnecessary. Instead, the patient was prescribed triamcinolone cream 0.1%, counseled to avoid raw or undercooked shiitake mushrooms in the future, and told to follow-up if symptoms did not resolve. The patient's symptoms resolved, as expected.
Nakamura2 first described shiitake mushroom dermatitis in 1977. The condition also is known as flagellate mushroom dermatitis or shiitake toxicoderma. It classically manifests in susceptible patients as a pruritic, linear, flagellated dermatitis within 24 to 48 hours after the consumption of raw or undercooked shiitake mushrooms (Lentinula edodes).3 Although the complete pathogenesis remains unclear, research suggests that either a toxic reaction or a type IV hypersensitivity reaction to lentinan causes the eruption. Lentinan is a thermolabile polysaccharide within the mushroom that is believed to increase the production of IL-1 and cause vasodilatation.4
Shiitake mushroom dermatitis is a clinical diagnosis based on the most common findings of a flagellate-pattern dermatitis consisting of pruritic and erythematous papular or urticarial lesions, usually found on the trunk. Laboratory tests, skin biopsies, and allergy tests have been shown to be nonspecific and inconsistent.5
Shiitake mushroom dermatitis is a self-limiting condition, with the majority of symptoms resolving after one to several weeks.5 The mainstay of treatment is aimed at symptomatic management and usually consists of topical corticosteroids and antihistamines.3 More rapid resolution of symptoms has been reported with the use of short-term balneo-psoralen plus UVA therapy.6
Although a 2017 review of the literature described only 9 published cases of shiitake mushroom dermatitis within the United States as of July 2015, this number may be misleading given the possibility of more variable and subtle presentations misdiagnosed as a nonspecific dermatitis.5 Given this information and the fact that shiitake mushrooms continue to be a popular choice in American cuisine, this case reminds health care providers of the clinical manifestations, differential diagnosis, and management of flagellate dermatitis caused by consuming shiitake mushrooms.
Shiitake Mushroom Dermatitis
Given the scattered and erythematous 1- to 2-mm macules and patches in a flagellate pattern on the shoulders, back, and neck, the differential diagnosis included shiitake mushroom dermatitis, bleomycin-induced flagellate dermatitis, dermatomyositis flagellate erythema, excoriation disorder, dermatographism, and keratosis lichenoides chronica. On further questioning, the patient indicated that he had consumed raw shiitake mushrooms 3 days before the onset of the rash. Although the clinical variability for all the conditions on the differential is notable, our patient had a history of consuming raw shiitake mushrooms, denied taking any medications, reported no repeated trauma, and had no muscular involvement or systemic symptoms, making shiitake mushroom dermatitis the most likely diagnosis.1 Skin biopsy and blood tests were deemed unnecessary. Instead, the patient was prescribed triamcinolone cream 0.1%, counseled to avoid raw or undercooked shiitake mushrooms in the future, and told to follow-up if symptoms did not resolve. The patient's symptoms resolved, as expected.
Nakamura2 first described shiitake mushroom dermatitis in 1977. The condition also is known as flagellate mushroom dermatitis or shiitake toxicoderma. It classically manifests in susceptible patients as a pruritic, linear, flagellated dermatitis within 24 to 48 hours after the consumption of raw or undercooked shiitake mushrooms (Lentinula edodes).3 Although the complete pathogenesis remains unclear, research suggests that either a toxic reaction or a type IV hypersensitivity reaction to lentinan causes the eruption. Lentinan is a thermolabile polysaccharide within the mushroom that is believed to increase the production of IL-1 and cause vasodilatation.4
Shiitake mushroom dermatitis is a clinical diagnosis based on the most common findings of a flagellate-pattern dermatitis consisting of pruritic and erythematous papular or urticarial lesions, usually found on the trunk. Laboratory tests, skin biopsies, and allergy tests have been shown to be nonspecific and inconsistent.5
Shiitake mushroom dermatitis is a self-limiting condition, with the majority of symptoms resolving after one to several weeks.5 The mainstay of treatment is aimed at symptomatic management and usually consists of topical corticosteroids and antihistamines.3 More rapid resolution of symptoms has been reported with the use of short-term balneo-psoralen plus UVA therapy.6
Although a 2017 review of the literature described only 9 published cases of shiitake mushroom dermatitis within the United States as of July 2015, this number may be misleading given the possibility of more variable and subtle presentations misdiagnosed as a nonspecific dermatitis.5 Given this information and the fact that shiitake mushrooms continue to be a popular choice in American cuisine, this case reminds health care providers of the clinical manifestations, differential diagnosis, and management of flagellate dermatitis caused by consuming shiitake mushrooms.
- Adler MJ, Larsen WG. Clinical variability of shiitake dermatitis. J Am Acad Dermatol. 2012;67:e140-e141.
- Nakamura T. Toxicoderma caused by Shiitake (Lentinus edodes). Japan J Clin Dermatol. 1977;31:65-68.
- Nakamura T. Shiitake (Lentinus edodes) dermatitis. Contact Dermatitis. 1992;27:65-70.
- Corazza M, Zauli S, Ricci M, et al. Shiitake dermatitis: toxic or allergic reaction? J Eur Acad Dermatol Venereol. 2015;29:1449-1451.
- Nguyen AH, Gonzaga MI, Lim VM, et al. Clinical features of shiitake dermatitis: a systematic review. Int J Dermatol. 2017;56:610-616.
- Scheiba N, Andrulis M, Helmbold P. Treatment of shiitake dermatitis by balneo PUVA therapy. J Am Acad Dermatol. 2011;65:453-455.
- Adler MJ, Larsen WG. Clinical variability of shiitake dermatitis. J Am Acad Dermatol. 2012;67:e140-e141.
- Nakamura T. Toxicoderma caused by Shiitake (Lentinus edodes). Japan J Clin Dermatol. 1977;31:65-68.
- Nakamura T. Shiitake (Lentinus edodes) dermatitis. Contact Dermatitis. 1992;27:65-70.
- Corazza M, Zauli S, Ricci M, et al. Shiitake dermatitis: toxic or allergic reaction? J Eur Acad Dermatol Venereol. 2015;29:1449-1451.
- Nguyen AH, Gonzaga MI, Lim VM, et al. Clinical features of shiitake dermatitis: a systematic review. Int J Dermatol. 2017;56:610-616.
- Scheiba N, Andrulis M, Helmbold P. Treatment of shiitake dermatitis by balneo PUVA therapy. J Am Acad Dermatol. 2011;65:453-455.
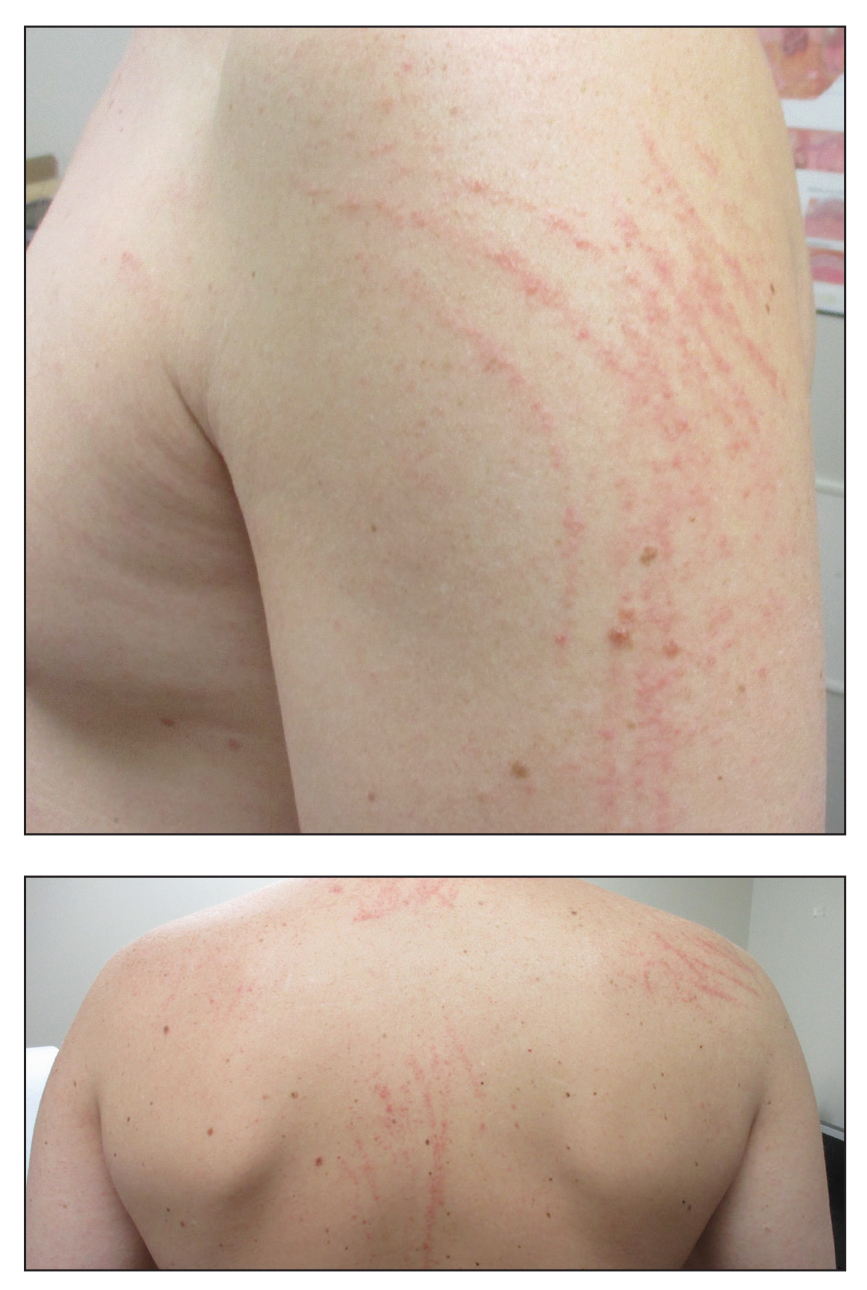
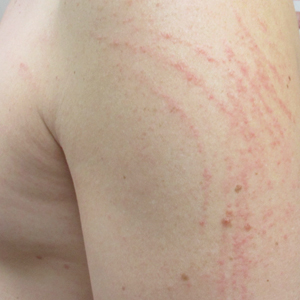
A 40-year-old man presented to the clinic with a sudden-onset pruritic rash of 4 days' duration. He denied any trauma and had no notable medical history. Furthermore, he denied taking any prescription or over-the-counter medications and had no known food or drug allergies. A review of systems was negative. He appeared well on physical examination with normal vital signs. A full-body skin examination displayed no mucosal lesions, but he had multiple areas of scattered and erythematous 1- to 2-mm macules and patches in a curvilinear parallel array on the shoulders (top), back (bottom), and neck.
Systemic Contact Dermatitis: Sometimes It Is the Food
One of the perils of patch testing is fielding questions about which type of allergens will be used. Patients often ask if the patch test includes milk, foods, dander, mold, pets, and grass. Most patch testers spend a substantial amount of time explaining that the purpose of patch testing is to detect applied chemical allergens: It’s not what you eat; rather, it’s what touches your skin. However, the big caveat is that some oral, parenteral, inhaled, and even cutaneous allergens can produce systemic contact dermatitis (SCD), which represents a unique clinical scenario that we will review in this month’s Final Interpretation column.
There are many patterns of SCD. Familiarity with potential clinical presentations can aid in diagnosis and counseling. Systemic contact dermatitis tends to be symmetrical. Dyshidrotic hand dermatitis is a reported pattern for systemic metal allergy, most commonly nickel. Refractory eyelid or genital dermatitis can reflect a systemic exposure, particularly if the dermatitis is in areas not caused by direct skin contact with the allergen. Systemic drug-related intertriginous and flexural exanthema is, as the name describes, an eruption involving axillae, genital skin, and flexural sites. It usually is a type of drug reaction, but the culprit can be an ingested allergen. So-called baboon syndrome SCD can cause persistent genital and intertriginous dermatitis. Other clues to SCD include dermatitis flare at the patch test site and erythema multiforme. Some patients also describe systemic symptoms, including headache, fatigue, and malaise.
Rhus Dermatitis
Poison ivy is the most common cause of acute contact dermatitis but also can be a cause of SCD. From the family Anacardiaceae, this sneaky plant is common in many parts of the United States; most allergic patients are familiar with their allergy from prior exposure.
In 1982, 54 Little League baseball attendees developed diffuse vesicular dermatitis involving the flexures after ingesting packaged cashews contaminated with cashew shells.1 In the same family as poison ivy, the cashew nut tree (Anacardium occidentale) produces a cashew apple containing the cashew nut. The cashew shell is the site that contains the allergenic oils. Typically, cashews are processed to remove the shell and oil prior to consumption. Ingestion of raw cashews is more likely to lead to SCD than roasted cashews because the heat in the roasting process can break down any allergenic oil.2
Metals
Systemic exposures to nickel usually are dietary. Clinically, SCD from nickel most commonly presents as refractory dyshidrotic hand eczema or papular elbow dermatitis.3 Nickel is commonly found in vitamins and supplements as well as certain whole grains, vegetables, beans, coffee, chocolate, and tea.4 Sometimes, cookware also can be a source of nickel exposure, particularly with steel cookware, from which nickel can leach into food.
In general, a diet lower than 150 μg/d is needed to prevent flares.5 A point-based diet is available for nickel-allergic patients.5 Patients should ingest a restricted amount of nickel (15 points daily); those who are extremely allergic might need to limit nickel ingestion to less than 5 points daily. Because of the challenges associated with maintaining a low-nickel diet, chelation therapy has been recommended to prevent nickel absorption. Disulfiram3 and ascorbic acid5 have been recommended, but larger studies are lacking.
Cobalt and chromium are other metals that, when ingested, can lead to SCD; both can be found in multivitamins. Other sources of dietary chromium include vegetables, coffee, beans, certain meats, and seafood.4 For cobalt, the dietary exposures are similar with the addition of nuts, apricots, and whole-grain flour. A point-based cobalt avoidance diet has been published. This diet recommends less than 12 μg of cobalt daily; patients can ingest up to 12 cobalt points daily.6
Likewise, gold has been reported to cause SCD, with one case attributed to gold in a homeopathic cardiac medication.7 Gold SCD also should be considered in the setting of ingested gold-containing alcoholic beverages and historically has been associated with intramuscular gold sodium thiomalate for the treatment of rheumatoid arthritis.8
Metal implants, including prosthetic joints, stents, and other devices, have been implicated in SCD. (More to come on this topic soon; yes, dear reader, that is a teaser!)
Fragrances
Balsam of Peru
Secreted by the tree Myroxylon balsamum var pereirae, balsam of Peru (BOP) contains several potential allergens, including cinnamon oils (eg, eugenol, vanillin, cinnamates), coniferin derivatives, and benzoic acid derivatives.9 Foods and beverages associated with BOP include citrus, pickled vegetables, chocolate, ice cream, chili, pizza, tomatoes, wine, beer, gin, vermouth, flavored tea, and soft drinks.10 Flavoring agents, spices (eg, cloves, curry, vanilla, cinnamon, allspice, ginger, anise), and condiments (eg, ketchup, barbeque sauce) are potential sources, as are cough medicines, lozenges, and flavored tobacco.
Salam and Fowler10 described BOP-allergic patients whose condition improved with dietary restriction of BOP. Avoidance of tomatoes, citrus, spices, and cola most commonly contributed to improvement.10 Scheman et al9 proposed BOP subgroups, including the eugenol, vanillin, cinnamate, benzoate, ferulic acid, and coniferin groups. Targeted patch testing can identify relevant subgroups, and patients can focus dietary restrictions by subgroup.
Plants
Systemic contact dermatitis has been reported in association with a number of plants and herbals, including chamomile in tea,11 goldenrod in a medicated extract,12Hosta plantaginea roots,13 and garlic extract for hyperlipidemia.14 Many more have been described.
Propolis
Also known as bee glue, propolis comprises a mixture of balsams, resins, waxes, essential oils, pollen, cinnamic alcohol, and vitamins. It can be found in many cosmetic products, foods, and chewing gum.15 Propolis has been reported to be the source of SCD from ingestion of propolis capsules, which have been used to promote immune stimulation,15 and propolis solution as a natural tonic.16
Propylene Glycol
Propylene glycol (PG) can be found in (believe it or not) foods and medications. In foods, it typically is used for its softening, humectant, and preservative properties.17 Common food sources of PG include sauces, desserts, snack foods, and salad dressings.
Many topical prescription medications, including corticosteroids and newer nonsteroidal anti-inflammatory topicals, might contain PG; providers must specifically request PG-free products for PG-allergic patients. A detailed PG-avoidance diet lists products to avoid and products that are PG free.18
Preservatives
Sulfites
These compounds are preservatives found in cosmetics, hair dyes, and certain foods. Systemic contact dermatitis caused by sulfites in food has been described in numerous patients. One unfortunate vacationer developed axillary and groin dermatitis after ingesting large amounts of grapes, wine, shrimp, and french fries while vacationing in Italy.19 Among dietary sources, beer and wine contain higher levels of sulfites. Sulfites also can be found in some pickled foods; bottled citrus juice; dried fruits; and commercial prepared foods, such as powdered potatoes and gravy mixes. Other reports of SCD from sulfites include an enema preparation20 and anesthetics21 as the source of the allergen.
Formaldehyde
Formaldehyde can cause SCD after ingestion of aspartame, which is hydrolyzed to phenylalanine, aspartic acid, and aspartic acid methyl ester in the intestine.22 The methyl ester is converted to methyl alcohol, which is transported to the liver and oxidized to formaldehyde, which is then converted to formic acid. Hill and Belsito22 reported a case of SCD presenting as eyelid dermatitis after ingestion of an aspartame-based artificial sweetener. A similar case of eyelid, neck, and leg dermatitis was reported after ingestion of drinks and candy sweetened with aspartame.23
Parabens
Although parabens are rare contact sensitizers, there are a few reports of paraben SCD. Cases include a predominantly flexural pattern from ingestion of a mucolytic-containing methylparaben,24 a generalized eczematous eruption after intramuscular injection of ampicillin preserved with methylparaben and propylparaben,25 and diffuse dermatitis from methylparaben in a local anesthetic.26
Sorbic Acid
Sorbic acid is utilized as a preservative in foods and occurs naturally in red fruit, such as strawberries and cranberries.27 It is a rare allergen, but several cases of sorbic acid SCD have been reported, including perianal and buttock dermatitis,27 hand dermatitis in an infant,28 and hand-and-foot dermatitis in a storekeeper.29
Carmine
Carmine, or cochineal extract, is a red dye derived from dried pulverized scale insects of the family Coccidae. This chemical can be used in a multitude of foods and medications, including candies, yogurt, red velvet items, popsicles, food coloring, frozen meat and fish, ice cream, syrups, ketchup, sausage, donuts, cake pops, applesauce, canned fruits, soups, and drinks.30 Machler and Jacob31 described a child with recurrent episodes of erythroderma and periorbital edema in whom patch testing revealed a reaction to carmine. The patient’s mother connected the flares with ingestion of red velvet cupcakes.31 Ferris et al32 reported a likely case of SCD attributed to carmine in a multivitamin.
Steroids
Ingested and injected corticosteroids have been associated with SCD, which is illustrated by a case of a generalized cutaneous eruption several days after joint injection with triamcinolone acetonide.33 In another report, a patient developed an eruption in the body folds, later generalized, after topical application of a corticosteroid, first in ear drops and later in nasal spray.34 Traditional corticosteroid classification systems might be less reliable in predicting relevant allergens in corticosteroid SCD; comprehensive testing, including oral challenge, might be necessary to identify alternatives.33
Ethylenediamine
Ethylenediamine is an uncommon allergen in patch test populations. It is present in aminophylline35 and is utilized in the production of hydroxyzine36 and other piperazine-derived medications, such as cetirizine, levocetirizine, meclizine, and olanzapine. Several cases of SCD caused by aminophylline,35 cetirizine,36 and hydroxyzine37 have been reported, all in the setting of a positive patch test reaction to ethylenediamine.
When to Counsel About Systemic Exposures
In general, we usually do not counsel on systemic exposures to allergens at the final patch test reading unless the pattern of dermatitis or clinical history strongly suggests systemic exposure. In most cases, we find that counseling on topical allergen avoidance alone is sufficient to treat allergic contact dermatitis. Because of the restrictive nature of allergen-avoidance diets, counseling all patients on the potential for SCD might cause undue stress without much benefit. However, if a patient experiences persistent dermatitis on follow-up with topical avoidance alone, we often will delve into systemic exposures and counsel on further avoidance strategies, including medication and diet.
Final Interpretation
A multitude of chemicals have been reported as the source of SCD; these exposures can occur through ingestion, injection, and inhaled and cutaneous routes. Chemicals present in foods, medications, and beverages have been implicated. Systemic contact dermatitis is rare and should be considered when traditional avoidance of contact allergens is unsuccessful and the clinical pattern is consistent with SCD.
- Marks JG, DeMelfi T, McCarthy MA, et al. Dermatitis from cashew nuts. J Am Acad Dermatol. 1984;10:627-631.
- Hamilton TK, Zug KA. Systemic contact dermatitis to raw cashew nuts in a pesto sauce. Am J Contact Dermat. 1998;9:51-54.
- Fabbro SK, Zirwas MJ. Systemic contact dermatitis to foods: nickel, BOP, and more. Curr Allergy Asthma Rep. 2014;14:463.
- American Contact Dermatitis Society. Contact Allergy Management Program (CAMP). https://www.contactderm.org/resources/acds-camp. Accessed October 23, 2019.
- Mislankar M, Zirwas MJ. Low-nickel diet scoring system for systemic nickel allergy. Dermatitis. 2013;24:190-195.
- Stuckert J, Nedorost S. Low-cobalt diet for dyshidrotic eczema patients. Contact Dermatitis. 2008;59:361-365.
- Malinauskiene L, Isaksson M, Bruze M. Systemic contact dermatitis in a gold-allergic patient after treatment with an oral homeopathic drug. J Am Acad Dermatol. 2013;68:e58.
- Wicks IP, Wong D, McCullagh RB, et al. Contact allergy to gold after systemic administration of gold for rheumatoid arthritis. Ann Rheum Dis. 1988;47:421-422.
- Scheman A, Rakowski EM, Chou V, et al. Balsam of Peru: past and future. Dermatitis. 2013;24:153-160.
- Salam TN, Fowler JF Jr. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Rodríguez-Serna M, Sánchez-Motilla JM, Ramón R, et al. Allergic and systemic contact dermatitis from Matricaria chamomilla tea. Contact Dermatitis. 1998;39:192-193.
- Schätzle M, Agathos M, Breit R. Allergic contact dermatitis from goldenrod (Herba solidaginis) after systemic administration. Contact Dermatitis. 1998;39:271-272.
- Yun SJ, Lee JY, Kim GH, et al. Systemic contact dermatitis induced by roots of Hosta plantaginea. J Eur Acad Dermatol Venereol. 2018;32:e28-e29.
- Burden AD, Wilkinson SM, Beck MH, et al. Garlic-induced systemic contact dermatitis. Contact Dermatitis. 1994;30:299-300.
- Komericki P, Kränke B. Maculopapular exanthem from propolis: case report and review of systemic cutaneous and non-cutaneous reactions. Contact Dermatitis. 2009;61:353-355.
- Cho E, Lee JD, Cho SH. Systemic contact dermatitis from propolis ingestion. Ann Dermatol. 2011;23:85-88.
- Lowther A, McCormick T, Nedorost S. Systemic contact dermatitis from propylene glycol. Dermatitis. 2008;19:105-108.
- Scheman A, Cha C, Jacob SE, et al. Food avoidance diets for systemic, lip, and oral contact allergy: an American Contact Alternatives Group article. Dermatitis. 2012;23:248-257.
- Cussans A, McFadden J, Ostlere L. Systemic sodium metabisulfite allergy. Contact Dermatitis. 2015;73:316-317.
- Borges AS, Valejo Coelho MM, Fernandes C, et al. Systemic allergic dermatitis caused by sodium metabisulfite in rectal enemas. Contact Dermatitis. 2018;78:429-430.
- Guha-Niyogi B, Sabroe R, Holden C. An unusual case of a systemic delayed hypersensitivity reaction to sodium metabisulfite. Contact Dermatitis. 2018;79:246-247.
- Hill AM, Belsito DV. Systemic contact dermatitis of the eyelids caused by formaldehyde derived from aspartame? Contact Dermatitis. 2003;49:258-259.
- Veien NK, Lomholt HB. Systemic allergic dermatitis presumably caused by formaldehyde derived from aspartame. Contact Dermatitis. 2012;67:315-316.
- Sánchez-Pérez J, Diez MB, Pérez AA, et al. Allergic and systemic contact dermatitis to methylparaben. Contact Dermatitis. 2006;54:117-118.
- Carradori S, Peluso AM, Faccioli M. Systemic contact dermatitis due to parabens. Contact Dermatitis. 1990;22:238-239.
- Aeling JL, Nuss DD. Systemic eczematous “contact-type” dermatitis medicamentosa caused by parabens. Arch Dermatol. 1974;110:640.
- Giordano-Labadie F, Pech-Ormieres C, Bazex J. Systemic contact dermatitis from sorbic acid. Contact Dermatitis. 1996;34:61-62.
- Raison-Peyron N, Meynadier JM, Meynadier J. Sorbic acid: an unusual cause of systemic contact dermatitis in an infant. Contact Dermatitis. 2000;43:247-248.
- Dejobert Y, Delaporte E, Piette F, et al. Vesicular eczema and systemic contact dermatitis from sorbic acid. Contact Dermatitis. 2001;45:291.
- Rundle CW, Jacob SE, Machler BC. Contact dermatitis to carmine. Dermatitis. 2018;29:244-249.
- Machler BC, Jacob SE. Carmine red: a potentially overlooked allergen in children. Dermatitis. 2018;29:92-93.
- Ferris GJ, Wat M, Nedorost S. Multifactorial dermatitis with probable systemic contact dermatitis to carmine. Dermatitis. 2017;28:293-294.
- Santos-Alarcón S, Benavente-Villegas FC, Farzanegan-Miñano R, et al. Delayed hypersensitivity to topical and systemic corticosteroids. Contact Dermatitis. 2018;78:86-88.
- Faber MA, Sabato V, Ebo DG, et al. Systemic allergic dermatitis caused by prednisone derivatives in nose and ear drops. Contact Dermatitis. 2015;73:317-320.
- Isaksson M, Ljunggren B. Systemic contact dermatitis from ethylenediamine in an aminophylline preparation presenting as the baboon syndrome. Acta Derm Venereol. 2003;83:69-70.
- Cusano F, Ferrara G, Crisman G, et al. Clinicopathologic features of systemic contact dermatitis from ethylenediamine in cetirizine and levocetirizine. Dermatology. 2006;213:353-355.
- Ash S, Scheman AJ. Systemic contact dermatitis to hydroxyzine. Am J Contact Dermat. 1997;8:2-5.
One of the perils of patch testing is fielding questions about which type of allergens will be used. Patients often ask if the patch test includes milk, foods, dander, mold, pets, and grass. Most patch testers spend a substantial amount of time explaining that the purpose of patch testing is to detect applied chemical allergens: It’s not what you eat; rather, it’s what touches your skin. However, the big caveat is that some oral, parenteral, inhaled, and even cutaneous allergens can produce systemic contact dermatitis (SCD), which represents a unique clinical scenario that we will review in this month’s Final Interpretation column.
There are many patterns of SCD. Familiarity with potential clinical presentations can aid in diagnosis and counseling. Systemic contact dermatitis tends to be symmetrical. Dyshidrotic hand dermatitis is a reported pattern for systemic metal allergy, most commonly nickel. Refractory eyelid or genital dermatitis can reflect a systemic exposure, particularly if the dermatitis is in areas not caused by direct skin contact with the allergen. Systemic drug-related intertriginous and flexural exanthema is, as the name describes, an eruption involving axillae, genital skin, and flexural sites. It usually is a type of drug reaction, but the culprit can be an ingested allergen. So-called baboon syndrome SCD can cause persistent genital and intertriginous dermatitis. Other clues to SCD include dermatitis flare at the patch test site and erythema multiforme. Some patients also describe systemic symptoms, including headache, fatigue, and malaise.
Rhus Dermatitis
Poison ivy is the most common cause of acute contact dermatitis but also can be a cause of SCD. From the family Anacardiaceae, this sneaky plant is common in many parts of the United States; most allergic patients are familiar with their allergy from prior exposure.
In 1982, 54 Little League baseball attendees developed diffuse vesicular dermatitis involving the flexures after ingesting packaged cashews contaminated with cashew shells.1 In the same family as poison ivy, the cashew nut tree (Anacardium occidentale) produces a cashew apple containing the cashew nut. The cashew shell is the site that contains the allergenic oils. Typically, cashews are processed to remove the shell and oil prior to consumption. Ingestion of raw cashews is more likely to lead to SCD than roasted cashews because the heat in the roasting process can break down any allergenic oil.2
Metals
Systemic exposures to nickel usually are dietary. Clinically, SCD from nickel most commonly presents as refractory dyshidrotic hand eczema or papular elbow dermatitis.3 Nickel is commonly found in vitamins and supplements as well as certain whole grains, vegetables, beans, coffee, chocolate, and tea.4 Sometimes, cookware also can be a source of nickel exposure, particularly with steel cookware, from which nickel can leach into food.
In general, a diet lower than 150 μg/d is needed to prevent flares.5 A point-based diet is available for nickel-allergic patients.5 Patients should ingest a restricted amount of nickel (15 points daily); those who are extremely allergic might need to limit nickel ingestion to less than 5 points daily. Because of the challenges associated with maintaining a low-nickel diet, chelation therapy has been recommended to prevent nickel absorption. Disulfiram3 and ascorbic acid5 have been recommended, but larger studies are lacking.
Cobalt and chromium are other metals that, when ingested, can lead to SCD; both can be found in multivitamins. Other sources of dietary chromium include vegetables, coffee, beans, certain meats, and seafood.4 For cobalt, the dietary exposures are similar with the addition of nuts, apricots, and whole-grain flour. A point-based cobalt avoidance diet has been published. This diet recommends less than 12 μg of cobalt daily; patients can ingest up to 12 cobalt points daily.6
Likewise, gold has been reported to cause SCD, with one case attributed to gold in a homeopathic cardiac medication.7 Gold SCD also should be considered in the setting of ingested gold-containing alcoholic beverages and historically has been associated with intramuscular gold sodium thiomalate for the treatment of rheumatoid arthritis.8
Metal implants, including prosthetic joints, stents, and other devices, have been implicated in SCD. (More to come on this topic soon; yes, dear reader, that is a teaser!)
Fragrances
Balsam of Peru
Secreted by the tree Myroxylon balsamum var pereirae, balsam of Peru (BOP) contains several potential allergens, including cinnamon oils (eg, eugenol, vanillin, cinnamates), coniferin derivatives, and benzoic acid derivatives.9 Foods and beverages associated with BOP include citrus, pickled vegetables, chocolate, ice cream, chili, pizza, tomatoes, wine, beer, gin, vermouth, flavored tea, and soft drinks.10 Flavoring agents, spices (eg, cloves, curry, vanilla, cinnamon, allspice, ginger, anise), and condiments (eg, ketchup, barbeque sauce) are potential sources, as are cough medicines, lozenges, and flavored tobacco.
Salam and Fowler10 described BOP-allergic patients whose condition improved with dietary restriction of BOP. Avoidance of tomatoes, citrus, spices, and cola most commonly contributed to improvement.10 Scheman et al9 proposed BOP subgroups, including the eugenol, vanillin, cinnamate, benzoate, ferulic acid, and coniferin groups. Targeted patch testing can identify relevant subgroups, and patients can focus dietary restrictions by subgroup.
Plants
Systemic contact dermatitis has been reported in association with a number of plants and herbals, including chamomile in tea,11 goldenrod in a medicated extract,12Hosta plantaginea roots,13 and garlic extract for hyperlipidemia.14 Many more have been described.
Propolis
Also known as bee glue, propolis comprises a mixture of balsams, resins, waxes, essential oils, pollen, cinnamic alcohol, and vitamins. It can be found in many cosmetic products, foods, and chewing gum.15 Propolis has been reported to be the source of SCD from ingestion of propolis capsules, which have been used to promote immune stimulation,15 and propolis solution as a natural tonic.16
Propylene Glycol
Propylene glycol (PG) can be found in (believe it or not) foods and medications. In foods, it typically is used for its softening, humectant, and preservative properties.17 Common food sources of PG include sauces, desserts, snack foods, and salad dressings.
Many topical prescription medications, including corticosteroids and newer nonsteroidal anti-inflammatory topicals, might contain PG; providers must specifically request PG-free products for PG-allergic patients. A detailed PG-avoidance diet lists products to avoid and products that are PG free.18
Preservatives
Sulfites
These compounds are preservatives found in cosmetics, hair dyes, and certain foods. Systemic contact dermatitis caused by sulfites in food has been described in numerous patients. One unfortunate vacationer developed axillary and groin dermatitis after ingesting large amounts of grapes, wine, shrimp, and french fries while vacationing in Italy.19 Among dietary sources, beer and wine contain higher levels of sulfites. Sulfites also can be found in some pickled foods; bottled citrus juice; dried fruits; and commercial prepared foods, such as powdered potatoes and gravy mixes. Other reports of SCD from sulfites include an enema preparation20 and anesthetics21 as the source of the allergen.
Formaldehyde
Formaldehyde can cause SCD after ingestion of aspartame, which is hydrolyzed to phenylalanine, aspartic acid, and aspartic acid methyl ester in the intestine.22 The methyl ester is converted to methyl alcohol, which is transported to the liver and oxidized to formaldehyde, which is then converted to formic acid. Hill and Belsito22 reported a case of SCD presenting as eyelid dermatitis after ingestion of an aspartame-based artificial sweetener. A similar case of eyelid, neck, and leg dermatitis was reported after ingestion of drinks and candy sweetened with aspartame.23
Parabens
Although parabens are rare contact sensitizers, there are a few reports of paraben SCD. Cases include a predominantly flexural pattern from ingestion of a mucolytic-containing methylparaben,24 a generalized eczematous eruption after intramuscular injection of ampicillin preserved with methylparaben and propylparaben,25 and diffuse dermatitis from methylparaben in a local anesthetic.26
Sorbic Acid
Sorbic acid is utilized as a preservative in foods and occurs naturally in red fruit, such as strawberries and cranberries.27 It is a rare allergen, but several cases of sorbic acid SCD have been reported, including perianal and buttock dermatitis,27 hand dermatitis in an infant,28 and hand-and-foot dermatitis in a storekeeper.29
Carmine
Carmine, or cochineal extract, is a red dye derived from dried pulverized scale insects of the family Coccidae. This chemical can be used in a multitude of foods and medications, including candies, yogurt, red velvet items, popsicles, food coloring, frozen meat and fish, ice cream, syrups, ketchup, sausage, donuts, cake pops, applesauce, canned fruits, soups, and drinks.30 Machler and Jacob31 described a child with recurrent episodes of erythroderma and periorbital edema in whom patch testing revealed a reaction to carmine. The patient’s mother connected the flares with ingestion of red velvet cupcakes.31 Ferris et al32 reported a likely case of SCD attributed to carmine in a multivitamin.
Steroids
Ingested and injected corticosteroids have been associated with SCD, which is illustrated by a case of a generalized cutaneous eruption several days after joint injection with triamcinolone acetonide.33 In another report, a patient developed an eruption in the body folds, later generalized, after topical application of a corticosteroid, first in ear drops and later in nasal spray.34 Traditional corticosteroid classification systems might be less reliable in predicting relevant allergens in corticosteroid SCD; comprehensive testing, including oral challenge, might be necessary to identify alternatives.33
Ethylenediamine
Ethylenediamine is an uncommon allergen in patch test populations. It is present in aminophylline35 and is utilized in the production of hydroxyzine36 and other piperazine-derived medications, such as cetirizine, levocetirizine, meclizine, and olanzapine. Several cases of SCD caused by aminophylline,35 cetirizine,36 and hydroxyzine37 have been reported, all in the setting of a positive patch test reaction to ethylenediamine.
When to Counsel About Systemic Exposures
In general, we usually do not counsel on systemic exposures to allergens at the final patch test reading unless the pattern of dermatitis or clinical history strongly suggests systemic exposure. In most cases, we find that counseling on topical allergen avoidance alone is sufficient to treat allergic contact dermatitis. Because of the restrictive nature of allergen-avoidance diets, counseling all patients on the potential for SCD might cause undue stress without much benefit. However, if a patient experiences persistent dermatitis on follow-up with topical avoidance alone, we often will delve into systemic exposures and counsel on further avoidance strategies, including medication and diet.
Final Interpretation
A multitude of chemicals have been reported as the source of SCD; these exposures can occur through ingestion, injection, and inhaled and cutaneous routes. Chemicals present in foods, medications, and beverages have been implicated. Systemic contact dermatitis is rare and should be considered when traditional avoidance of contact allergens is unsuccessful and the clinical pattern is consistent with SCD.
One of the perils of patch testing is fielding questions about which type of allergens will be used. Patients often ask if the patch test includes milk, foods, dander, mold, pets, and grass. Most patch testers spend a substantial amount of time explaining that the purpose of patch testing is to detect applied chemical allergens: It’s not what you eat; rather, it’s what touches your skin. However, the big caveat is that some oral, parenteral, inhaled, and even cutaneous allergens can produce systemic contact dermatitis (SCD), which represents a unique clinical scenario that we will review in this month’s Final Interpretation column.
There are many patterns of SCD. Familiarity with potential clinical presentations can aid in diagnosis and counseling. Systemic contact dermatitis tends to be symmetrical. Dyshidrotic hand dermatitis is a reported pattern for systemic metal allergy, most commonly nickel. Refractory eyelid or genital dermatitis can reflect a systemic exposure, particularly if the dermatitis is in areas not caused by direct skin contact with the allergen. Systemic drug-related intertriginous and flexural exanthema is, as the name describes, an eruption involving axillae, genital skin, and flexural sites. It usually is a type of drug reaction, but the culprit can be an ingested allergen. So-called baboon syndrome SCD can cause persistent genital and intertriginous dermatitis. Other clues to SCD include dermatitis flare at the patch test site and erythema multiforme. Some patients also describe systemic symptoms, including headache, fatigue, and malaise.
Rhus Dermatitis
Poison ivy is the most common cause of acute contact dermatitis but also can be a cause of SCD. From the family Anacardiaceae, this sneaky plant is common in many parts of the United States; most allergic patients are familiar with their allergy from prior exposure.
In 1982, 54 Little League baseball attendees developed diffuse vesicular dermatitis involving the flexures after ingesting packaged cashews contaminated with cashew shells.1 In the same family as poison ivy, the cashew nut tree (Anacardium occidentale) produces a cashew apple containing the cashew nut. The cashew shell is the site that contains the allergenic oils. Typically, cashews are processed to remove the shell and oil prior to consumption. Ingestion of raw cashews is more likely to lead to SCD than roasted cashews because the heat in the roasting process can break down any allergenic oil.2
Metals
Systemic exposures to nickel usually are dietary. Clinically, SCD from nickel most commonly presents as refractory dyshidrotic hand eczema or papular elbow dermatitis.3 Nickel is commonly found in vitamins and supplements as well as certain whole grains, vegetables, beans, coffee, chocolate, and tea.4 Sometimes, cookware also can be a source of nickel exposure, particularly with steel cookware, from which nickel can leach into food.
In general, a diet lower than 150 μg/d is needed to prevent flares.5 A point-based diet is available for nickel-allergic patients.5 Patients should ingest a restricted amount of nickel (15 points daily); those who are extremely allergic might need to limit nickel ingestion to less than 5 points daily. Because of the challenges associated with maintaining a low-nickel diet, chelation therapy has been recommended to prevent nickel absorption. Disulfiram3 and ascorbic acid5 have been recommended, but larger studies are lacking.
Cobalt and chromium are other metals that, when ingested, can lead to SCD; both can be found in multivitamins. Other sources of dietary chromium include vegetables, coffee, beans, certain meats, and seafood.4 For cobalt, the dietary exposures are similar with the addition of nuts, apricots, and whole-grain flour. A point-based cobalt avoidance diet has been published. This diet recommends less than 12 μg of cobalt daily; patients can ingest up to 12 cobalt points daily.6
Likewise, gold has been reported to cause SCD, with one case attributed to gold in a homeopathic cardiac medication.7 Gold SCD also should be considered in the setting of ingested gold-containing alcoholic beverages and historically has been associated with intramuscular gold sodium thiomalate for the treatment of rheumatoid arthritis.8
Metal implants, including prosthetic joints, stents, and other devices, have been implicated in SCD. (More to come on this topic soon; yes, dear reader, that is a teaser!)
Fragrances
Balsam of Peru
Secreted by the tree Myroxylon balsamum var pereirae, balsam of Peru (BOP) contains several potential allergens, including cinnamon oils (eg, eugenol, vanillin, cinnamates), coniferin derivatives, and benzoic acid derivatives.9 Foods and beverages associated with BOP include citrus, pickled vegetables, chocolate, ice cream, chili, pizza, tomatoes, wine, beer, gin, vermouth, flavored tea, and soft drinks.10 Flavoring agents, spices (eg, cloves, curry, vanilla, cinnamon, allspice, ginger, anise), and condiments (eg, ketchup, barbeque sauce) are potential sources, as are cough medicines, lozenges, and flavored tobacco.
Salam and Fowler10 described BOP-allergic patients whose condition improved with dietary restriction of BOP. Avoidance of tomatoes, citrus, spices, and cola most commonly contributed to improvement.10 Scheman et al9 proposed BOP subgroups, including the eugenol, vanillin, cinnamate, benzoate, ferulic acid, and coniferin groups. Targeted patch testing can identify relevant subgroups, and patients can focus dietary restrictions by subgroup.
Plants
Systemic contact dermatitis has been reported in association with a number of plants and herbals, including chamomile in tea,11 goldenrod in a medicated extract,12Hosta plantaginea roots,13 and garlic extract for hyperlipidemia.14 Many more have been described.
Propolis
Also known as bee glue, propolis comprises a mixture of balsams, resins, waxes, essential oils, pollen, cinnamic alcohol, and vitamins. It can be found in many cosmetic products, foods, and chewing gum.15 Propolis has been reported to be the source of SCD from ingestion of propolis capsules, which have been used to promote immune stimulation,15 and propolis solution as a natural tonic.16
Propylene Glycol
Propylene glycol (PG) can be found in (believe it or not) foods and medications. In foods, it typically is used for its softening, humectant, and preservative properties.17 Common food sources of PG include sauces, desserts, snack foods, and salad dressings.
Many topical prescription medications, including corticosteroids and newer nonsteroidal anti-inflammatory topicals, might contain PG; providers must specifically request PG-free products for PG-allergic patients. A detailed PG-avoidance diet lists products to avoid and products that are PG free.18
Preservatives
Sulfites
These compounds are preservatives found in cosmetics, hair dyes, and certain foods. Systemic contact dermatitis caused by sulfites in food has been described in numerous patients. One unfortunate vacationer developed axillary and groin dermatitis after ingesting large amounts of grapes, wine, shrimp, and french fries while vacationing in Italy.19 Among dietary sources, beer and wine contain higher levels of sulfites. Sulfites also can be found in some pickled foods; bottled citrus juice; dried fruits; and commercial prepared foods, such as powdered potatoes and gravy mixes. Other reports of SCD from sulfites include an enema preparation20 and anesthetics21 as the source of the allergen.
Formaldehyde
Formaldehyde can cause SCD after ingestion of aspartame, which is hydrolyzed to phenylalanine, aspartic acid, and aspartic acid methyl ester in the intestine.22 The methyl ester is converted to methyl alcohol, which is transported to the liver and oxidized to formaldehyde, which is then converted to formic acid. Hill and Belsito22 reported a case of SCD presenting as eyelid dermatitis after ingestion of an aspartame-based artificial sweetener. A similar case of eyelid, neck, and leg dermatitis was reported after ingestion of drinks and candy sweetened with aspartame.23
Parabens
Although parabens are rare contact sensitizers, there are a few reports of paraben SCD. Cases include a predominantly flexural pattern from ingestion of a mucolytic-containing methylparaben,24 a generalized eczematous eruption after intramuscular injection of ampicillin preserved with methylparaben and propylparaben,25 and diffuse dermatitis from methylparaben in a local anesthetic.26
Sorbic Acid
Sorbic acid is utilized as a preservative in foods and occurs naturally in red fruit, such as strawberries and cranberries.27 It is a rare allergen, but several cases of sorbic acid SCD have been reported, including perianal and buttock dermatitis,27 hand dermatitis in an infant,28 and hand-and-foot dermatitis in a storekeeper.29
Carmine
Carmine, or cochineal extract, is a red dye derived from dried pulverized scale insects of the family Coccidae. This chemical can be used in a multitude of foods and medications, including candies, yogurt, red velvet items, popsicles, food coloring, frozen meat and fish, ice cream, syrups, ketchup, sausage, donuts, cake pops, applesauce, canned fruits, soups, and drinks.30 Machler and Jacob31 described a child with recurrent episodes of erythroderma and periorbital edema in whom patch testing revealed a reaction to carmine. The patient’s mother connected the flares with ingestion of red velvet cupcakes.31 Ferris et al32 reported a likely case of SCD attributed to carmine in a multivitamin.
Steroids
Ingested and injected corticosteroids have been associated with SCD, which is illustrated by a case of a generalized cutaneous eruption several days after joint injection with triamcinolone acetonide.33 In another report, a patient developed an eruption in the body folds, later generalized, after topical application of a corticosteroid, first in ear drops and later in nasal spray.34 Traditional corticosteroid classification systems might be less reliable in predicting relevant allergens in corticosteroid SCD; comprehensive testing, including oral challenge, might be necessary to identify alternatives.33
Ethylenediamine
Ethylenediamine is an uncommon allergen in patch test populations. It is present in aminophylline35 and is utilized in the production of hydroxyzine36 and other piperazine-derived medications, such as cetirizine, levocetirizine, meclizine, and olanzapine. Several cases of SCD caused by aminophylline,35 cetirizine,36 and hydroxyzine37 have been reported, all in the setting of a positive patch test reaction to ethylenediamine.
When to Counsel About Systemic Exposures
In general, we usually do not counsel on systemic exposures to allergens at the final patch test reading unless the pattern of dermatitis or clinical history strongly suggests systemic exposure. In most cases, we find that counseling on topical allergen avoidance alone is sufficient to treat allergic contact dermatitis. Because of the restrictive nature of allergen-avoidance diets, counseling all patients on the potential for SCD might cause undue stress without much benefit. However, if a patient experiences persistent dermatitis on follow-up with topical avoidance alone, we often will delve into systemic exposures and counsel on further avoidance strategies, including medication and diet.
Final Interpretation
A multitude of chemicals have been reported as the source of SCD; these exposures can occur through ingestion, injection, and inhaled and cutaneous routes. Chemicals present in foods, medications, and beverages have been implicated. Systemic contact dermatitis is rare and should be considered when traditional avoidance of contact allergens is unsuccessful and the clinical pattern is consistent with SCD.
- Marks JG, DeMelfi T, McCarthy MA, et al. Dermatitis from cashew nuts. J Am Acad Dermatol. 1984;10:627-631.
- Hamilton TK, Zug KA. Systemic contact dermatitis to raw cashew nuts in a pesto sauce. Am J Contact Dermat. 1998;9:51-54.
- Fabbro SK, Zirwas MJ. Systemic contact dermatitis to foods: nickel, BOP, and more. Curr Allergy Asthma Rep. 2014;14:463.
- American Contact Dermatitis Society. Contact Allergy Management Program (CAMP). https://www.contactderm.org/resources/acds-camp. Accessed October 23, 2019.
- Mislankar M, Zirwas MJ. Low-nickel diet scoring system for systemic nickel allergy. Dermatitis. 2013;24:190-195.
- Stuckert J, Nedorost S. Low-cobalt diet for dyshidrotic eczema patients. Contact Dermatitis. 2008;59:361-365.
- Malinauskiene L, Isaksson M, Bruze M. Systemic contact dermatitis in a gold-allergic patient after treatment with an oral homeopathic drug. J Am Acad Dermatol. 2013;68:e58.
- Wicks IP, Wong D, McCullagh RB, et al. Contact allergy to gold after systemic administration of gold for rheumatoid arthritis. Ann Rheum Dis. 1988;47:421-422.
- Scheman A, Rakowski EM, Chou V, et al. Balsam of Peru: past and future. Dermatitis. 2013;24:153-160.
- Salam TN, Fowler JF Jr. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Rodríguez-Serna M, Sánchez-Motilla JM, Ramón R, et al. Allergic and systemic contact dermatitis from Matricaria chamomilla tea. Contact Dermatitis. 1998;39:192-193.
- Schätzle M, Agathos M, Breit R. Allergic contact dermatitis from goldenrod (Herba solidaginis) after systemic administration. Contact Dermatitis. 1998;39:271-272.
- Yun SJ, Lee JY, Kim GH, et al. Systemic contact dermatitis induced by roots of Hosta plantaginea. J Eur Acad Dermatol Venereol. 2018;32:e28-e29.
- Burden AD, Wilkinson SM, Beck MH, et al. Garlic-induced systemic contact dermatitis. Contact Dermatitis. 1994;30:299-300.
- Komericki P, Kränke B. Maculopapular exanthem from propolis: case report and review of systemic cutaneous and non-cutaneous reactions. Contact Dermatitis. 2009;61:353-355.
- Cho E, Lee JD, Cho SH. Systemic contact dermatitis from propolis ingestion. Ann Dermatol. 2011;23:85-88.
- Lowther A, McCormick T, Nedorost S. Systemic contact dermatitis from propylene glycol. Dermatitis. 2008;19:105-108.
- Scheman A, Cha C, Jacob SE, et al. Food avoidance diets for systemic, lip, and oral contact allergy: an American Contact Alternatives Group article. Dermatitis. 2012;23:248-257.
- Cussans A, McFadden J, Ostlere L. Systemic sodium metabisulfite allergy. Contact Dermatitis. 2015;73:316-317.
- Borges AS, Valejo Coelho MM, Fernandes C, et al. Systemic allergic dermatitis caused by sodium metabisulfite in rectal enemas. Contact Dermatitis. 2018;78:429-430.
- Guha-Niyogi B, Sabroe R, Holden C. An unusual case of a systemic delayed hypersensitivity reaction to sodium metabisulfite. Contact Dermatitis. 2018;79:246-247.
- Hill AM, Belsito DV. Systemic contact dermatitis of the eyelids caused by formaldehyde derived from aspartame? Contact Dermatitis. 2003;49:258-259.
- Veien NK, Lomholt HB. Systemic allergic dermatitis presumably caused by formaldehyde derived from aspartame. Contact Dermatitis. 2012;67:315-316.
- Sánchez-Pérez J, Diez MB, Pérez AA, et al. Allergic and systemic contact dermatitis to methylparaben. Contact Dermatitis. 2006;54:117-118.
- Carradori S, Peluso AM, Faccioli M. Systemic contact dermatitis due to parabens. Contact Dermatitis. 1990;22:238-239.
- Aeling JL, Nuss DD. Systemic eczematous “contact-type” dermatitis medicamentosa caused by parabens. Arch Dermatol. 1974;110:640.
- Giordano-Labadie F, Pech-Ormieres C, Bazex J. Systemic contact dermatitis from sorbic acid. Contact Dermatitis. 1996;34:61-62.
- Raison-Peyron N, Meynadier JM, Meynadier J. Sorbic acid: an unusual cause of systemic contact dermatitis in an infant. Contact Dermatitis. 2000;43:247-248.
- Dejobert Y, Delaporte E, Piette F, et al. Vesicular eczema and systemic contact dermatitis from sorbic acid. Contact Dermatitis. 2001;45:291.
- Rundle CW, Jacob SE, Machler BC. Contact dermatitis to carmine. Dermatitis. 2018;29:244-249.
- Machler BC, Jacob SE. Carmine red: a potentially overlooked allergen in children. Dermatitis. 2018;29:92-93.
- Ferris GJ, Wat M, Nedorost S. Multifactorial dermatitis with probable systemic contact dermatitis to carmine. Dermatitis. 2017;28:293-294.
- Santos-Alarcón S, Benavente-Villegas FC, Farzanegan-Miñano R, et al. Delayed hypersensitivity to topical and systemic corticosteroids. Contact Dermatitis. 2018;78:86-88.
- Faber MA, Sabato V, Ebo DG, et al. Systemic allergic dermatitis caused by prednisone derivatives in nose and ear drops. Contact Dermatitis. 2015;73:317-320.
- Isaksson M, Ljunggren B. Systemic contact dermatitis from ethylenediamine in an aminophylline preparation presenting as the baboon syndrome. Acta Derm Venereol. 2003;83:69-70.
- Cusano F, Ferrara G, Crisman G, et al. Clinicopathologic features of systemic contact dermatitis from ethylenediamine in cetirizine and levocetirizine. Dermatology. 2006;213:353-355.
- Ash S, Scheman AJ. Systemic contact dermatitis to hydroxyzine. Am J Contact Dermat. 1997;8:2-5.
- Marks JG, DeMelfi T, McCarthy MA, et al. Dermatitis from cashew nuts. J Am Acad Dermatol. 1984;10:627-631.
- Hamilton TK, Zug KA. Systemic contact dermatitis to raw cashew nuts in a pesto sauce. Am J Contact Dermat. 1998;9:51-54.
- Fabbro SK, Zirwas MJ. Systemic contact dermatitis to foods: nickel, BOP, and more. Curr Allergy Asthma Rep. 2014;14:463.
- American Contact Dermatitis Society. Contact Allergy Management Program (CAMP). https://www.contactderm.org/resources/acds-camp. Accessed October 23, 2019.
- Mislankar M, Zirwas MJ. Low-nickel diet scoring system for systemic nickel allergy. Dermatitis. 2013;24:190-195.
- Stuckert J, Nedorost S. Low-cobalt diet for dyshidrotic eczema patients. Contact Dermatitis. 2008;59:361-365.
- Malinauskiene L, Isaksson M, Bruze M. Systemic contact dermatitis in a gold-allergic patient after treatment with an oral homeopathic drug. J Am Acad Dermatol. 2013;68:e58.
- Wicks IP, Wong D, McCullagh RB, et al. Contact allergy to gold after systemic administration of gold for rheumatoid arthritis. Ann Rheum Dis. 1988;47:421-422.
- Scheman A, Rakowski EM, Chou V, et al. Balsam of Peru: past and future. Dermatitis. 2013;24:153-160.
- Salam TN, Fowler JF Jr. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Rodríguez-Serna M, Sánchez-Motilla JM, Ramón R, et al. Allergic and systemic contact dermatitis from Matricaria chamomilla tea. Contact Dermatitis. 1998;39:192-193.
- Schätzle M, Agathos M, Breit R. Allergic contact dermatitis from goldenrod (Herba solidaginis) after systemic administration. Contact Dermatitis. 1998;39:271-272.
- Yun SJ, Lee JY, Kim GH, et al. Systemic contact dermatitis induced by roots of Hosta plantaginea. J Eur Acad Dermatol Venereol. 2018;32:e28-e29.
- Burden AD, Wilkinson SM, Beck MH, et al. Garlic-induced systemic contact dermatitis. Contact Dermatitis. 1994;30:299-300.
- Komericki P, Kränke B. Maculopapular exanthem from propolis: case report and review of systemic cutaneous and non-cutaneous reactions. Contact Dermatitis. 2009;61:353-355.
- Cho E, Lee JD, Cho SH. Systemic contact dermatitis from propolis ingestion. Ann Dermatol. 2011;23:85-88.
- Lowther A, McCormick T, Nedorost S. Systemic contact dermatitis from propylene glycol. Dermatitis. 2008;19:105-108.
- Scheman A, Cha C, Jacob SE, et al. Food avoidance diets for systemic, lip, and oral contact allergy: an American Contact Alternatives Group article. Dermatitis. 2012;23:248-257.
- Cussans A, McFadden J, Ostlere L. Systemic sodium metabisulfite allergy. Contact Dermatitis. 2015;73:316-317.
- Borges AS, Valejo Coelho MM, Fernandes C, et al. Systemic allergic dermatitis caused by sodium metabisulfite in rectal enemas. Contact Dermatitis. 2018;78:429-430.
- Guha-Niyogi B, Sabroe R, Holden C. An unusual case of a systemic delayed hypersensitivity reaction to sodium metabisulfite. Contact Dermatitis. 2018;79:246-247.
- Hill AM, Belsito DV. Systemic contact dermatitis of the eyelids caused by formaldehyde derived from aspartame? Contact Dermatitis. 2003;49:258-259.
- Veien NK, Lomholt HB. Systemic allergic dermatitis presumably caused by formaldehyde derived from aspartame. Contact Dermatitis. 2012;67:315-316.
- Sánchez-Pérez J, Diez MB, Pérez AA, et al. Allergic and systemic contact dermatitis to methylparaben. Contact Dermatitis. 2006;54:117-118.
- Carradori S, Peluso AM, Faccioli M. Systemic contact dermatitis due to parabens. Contact Dermatitis. 1990;22:238-239.
- Aeling JL, Nuss DD. Systemic eczematous “contact-type” dermatitis medicamentosa caused by parabens. Arch Dermatol. 1974;110:640.
- Giordano-Labadie F, Pech-Ormieres C, Bazex J. Systemic contact dermatitis from sorbic acid. Contact Dermatitis. 1996;34:61-62.
- Raison-Peyron N, Meynadier JM, Meynadier J. Sorbic acid: an unusual cause of systemic contact dermatitis in an infant. Contact Dermatitis. 2000;43:247-248.
- Dejobert Y, Delaporte E, Piette F, et al. Vesicular eczema and systemic contact dermatitis from sorbic acid. Contact Dermatitis. 2001;45:291.
- Rundle CW, Jacob SE, Machler BC. Contact dermatitis to carmine. Dermatitis. 2018;29:244-249.
- Machler BC, Jacob SE. Carmine red: a potentially overlooked allergen in children. Dermatitis. 2018;29:92-93.
- Ferris GJ, Wat M, Nedorost S. Multifactorial dermatitis with probable systemic contact dermatitis to carmine. Dermatitis. 2017;28:293-294.
- Santos-Alarcón S, Benavente-Villegas FC, Farzanegan-Miñano R, et al. Delayed hypersensitivity to topical and systemic corticosteroids. Contact Dermatitis. 2018;78:86-88.
- Faber MA, Sabato V, Ebo DG, et al. Systemic allergic dermatitis caused by prednisone derivatives in nose and ear drops. Contact Dermatitis. 2015;73:317-320.
- Isaksson M, Ljunggren B. Systemic contact dermatitis from ethylenediamine in an aminophylline preparation presenting as the baboon syndrome. Acta Derm Venereol. 2003;83:69-70.
- Cusano F, Ferrara G, Crisman G, et al. Clinicopathologic features of systemic contact dermatitis from ethylenediamine in cetirizine and levocetirizine. Dermatology. 2006;213:353-355.
- Ash S, Scheman AJ. Systemic contact dermatitis to hydroxyzine. Am J Contact Dermat. 1997;8:2-5.
Practice Points
- Although most cases of allergic contact dermatitis are from direct skin contact, systemic contact dermatitis (SCD) can occur from ingesting certain allergens.
- Systemic contact dermatitis tends to present as a symmetric pruritic eruption, which may involve the flexural or intertriginous surfaces, eyelids, hands, or genital skin.
- Allergens known to cause SCD include certain plants, fragrances, metals, preservatives, and medications.
Patch Testing in Children: Not Just Little Adults
The pediatric population has a unique product exposure profile due to the many care products specifically marketed for use in children. In fact, the prevalence of allergic contact dermatitis (ACD) in children may be as high as 24.5% in the United States.1 In patch tested children, relevant positive reaction rates of 56.7% and 48% have been reported by the North American Contact Dermatitis Group and the Pediatric Contact Dermatitis Registry, respectively.2,3 In this article, we provide an overview of current trends in pediatric patch testing as well as specific considerations in this patient population.
Patch Test Reactions in Children
Several publications have documented pediatric patch test reactions. The North American Contact Dermatitis Group reported patch test results in 883 children from the United States and Canada (2005-2012).2 The most common reactions were nickel (28.1%), cobalt (12.3%), neomycin (7.1%), balsam of Peru (5.7%), lanolin (5.5%), and fragrance mix I (5.2%). When compared to adults, children were more likely to have relevant positive patch tests to nickel, cobalt, and compositae mix.2 In comparison, data from the Pediatric Contact Dermatitis Registry showed that the most common reactions in 1142 children in the United States (2015-2016) were nickel (22%), fragrance mix I (11%), cobalt (9.1%), balsam of Peru (8.4%), neomycin (7.2%), and propylene glycol (6.8%).3
Allergen sensitivities may vary based on geographic region. In Spain, children showed the highest sensitivities to thiomersal (10.2%), cobalt (9.1%), colophony (9.1%), paraphenylenediamine (8.3%), mercury (7.9%), potassium dichromate (7.9%), and nickel (6.4%).4
Pediatric Patch Testing Pearls
History of Product Use
From diapers to drama club, pediatric exposures and sources of ACD are not the same as those seen in adults. Because obtaining a medical history from a toddler can be exasperating, the patient’s caregivers should be asked about potential exposures, ranging from personal care products and diapers to school activities, hobbies, and sports.5,6 It is important to keep in mind that the patient’s primary caregiver may not be the only individual who applies products to the child.7
Application of Allergens
Children are not merely small adults, but they usually do have smaller backs than adult patients. This reduced surface area means that the patch tester must carefully select the allergens to be patch tested. For reference, the back of a typical 6-year-old child can fit 40 to 60 allergens during patch testing.8
Patch Test Chambers
In children, the use of plastic patch test chambers may be preferred over aluminum chambers. Children with persistent pruritic subcutaneous nodules induced by aluminum-based vaccines also may have delayed-type sensitivity reactions to aluminum.9 These patients could react to the aluminum present in some patch test chambers, making interpretation of the results difficult. The authors (A.R.A. and M.R.) typically use plastic chambers in the pediatric population.
Managing Expectations
As with other procedures in the pediatric population, patch testing can elicit emotions of fear, anxiety, and distrust. Video distraction and/or role-playing games may help capture the attention of children and can be particularly helpful during patch application. Children may be apprehensive about the term allergy testing if they are familiar with the term needle testing from previous allergies.5
Securing Patches
Young children can be quite active, posing another challenge for keeping patches in place. We recommend using extra tape to secure the patches in place on a child’s back. In addition, a large transparent film dressing (ie, 12×8 in) can be used if quick application is needed. For extra precaution, the use of a tight T-shirt or favorite onesie during the patch test process may be helpful, making it more difficult for little fingers to remove tape edges.
Duration of Patch Testing
Some authors have proposed application of patch tests for 24 hours in pediatric patients, as compared to 48 hours in adults.10 This recommendation is based on a theory that the reduced application period will decrease the risk for irritant reactions in pediatric patients.
Pediatric Patch Test Screening Series
A summary of the published screening series for patch testing in the pediatric population is provided (Table).
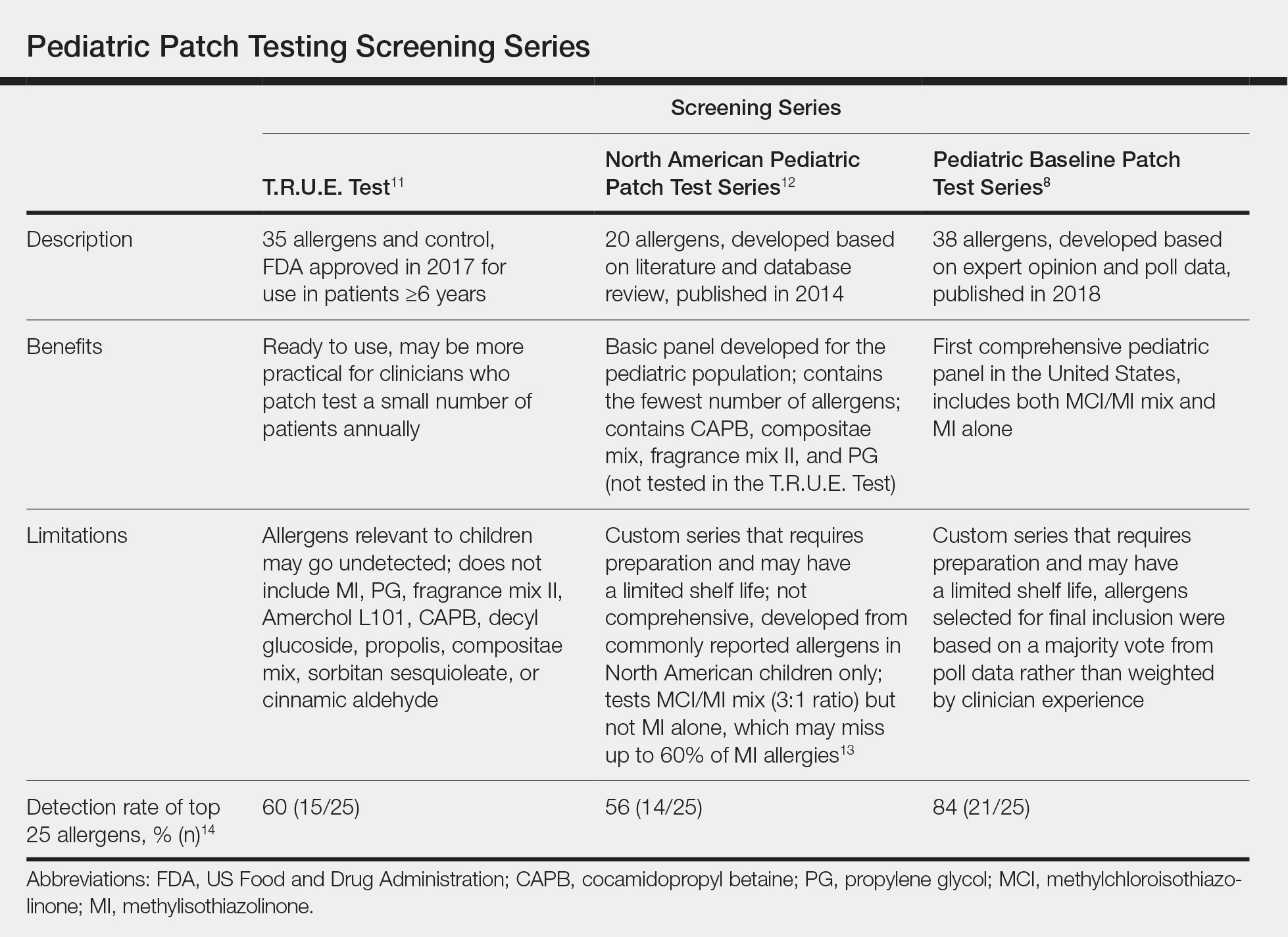
The T.R.U.E. Test (SmartPractice) is approved by the US Food and Drug Administration for use in patients 6 years and older11; however, it may not adequately represent allergen exposures in the pediatric population. Brankov and Jacob14 found that 10 (40%) of their proposed top 25 pediatric allergens were not detected using the T.R.U.E. Test.
In 2014, the North American Pediatric Patch Test Series was proposed as a basic screening panel for children aged 6 to 12 years.12 This series of 20 allergens was developed based on a literature review of pediatric patch test results and case reports as well as a database review. The authors proposed additional allergens to be considered based on patient history.12
More recently, a 2017 American Contact Dermatitis Society physician work group proposed the Pediatric Baseline Patch Test Series. This series of 38 allergens for children aged 6 to 18 years was developed based on expert consensus.8 Studies to determine the efficacy of this series have yet to be conducted, but it may have high sensitivity in detecting relevant allergens in children as demonstrated by a theoretical detection rate of 84%.14
There are 2 recommended patch test series for allergic diaper dermatitis.15 The first series focuses on 23 potential allergens found in wet wipes and topical diaper preparations. The second series contains 10 potential allergens found in diapers. These series contain common topical medications for children including corticosteroids, antimicrobials, and sensitizers specific to diapers such as rubbers and adhesives.15
Similar to adults, it may be difficult to designate one screening panel that can identify all relevant allergens in children; thus, it is always important to obtain a thorough exposure history and customize testing to suspected allergens and/or patient products based on history and clinical relevance.
Unique Pediatric Allergens
Hobbies
Sports gear such as shin guards and splints often contain allergens such as formaldehyde resin, thiuram mix, and dialkyl thioureas.16 Perioral dermatitis may be caused by musical instrument mouthpieces containing nickel.6
Preservatives
Commonly reported causes of ACD in children include methylisothiazolinone (MI) and methylchloroisothiazolinone (MCI) found in wet wipes. A 2016 analysis of diaper wipes showed a low prevalence of MI (6.3%) and MCI (1.6%) in these products, which may reflect the industry’s awareness of these potential allergens and a subsequent change in the preservatives they utilize.17 However, the prevalence of MCI/MI contact allergy may be on the rise due to the popularity of homemade slime, which is made from common household products such as laundry detergent, dishwashing soap, and liquid glue. The Pediatric Baseline Patch Test Series captures most of the potential allergens in these homemade slime recipes and is recommended for use in pediatric patients suspected of having dermatitis secondary to playing with slime.8,18
Toilet Seat Dermatitis
Toilet seat dermatitis presents as a pruritic dermatitis on the posterior upper thighs and buttocks. Although most cases of toilet seat dermatitis are irritant rather than allergic, potential allergens include plastics, fragrances, and components of cleaning products. Thus, physicians should maintain a high index of suspicion for ACD to toilet seats.19
Fragrance and Natural Ingredients
A 2018 study evaluating personal care products marketed specifically for infants and children found that 55% of products (294/533) contained at least 1 common allergen, with fragrance being the most common (48% [255/533]). Other common allergens include betaines (18%), propylene glycol (9%), lanolin (6%), and MCI/MI (3%).20 Caregivers should be advised against the myth that natural products are safer and less allergenic and should be provided with resources such as the Contact Allergen Management Program (CAMP) database (https://www.contactderm.org/resources/acds-camp) for safe alternative personal care products.
Metal Allergens
Nickel, the American Contact Dermatitis Society 2008 Allergen of the Year, is another common allergen that affects children. Nickel allergy, commonly thought to affect the ears due to jewelry and ear piercing, may actually be found in a wide range of daily items such as braces, eyeglasses, keys, zippers, school chairs, electronics, toys, and even food.3,6,21,22 With increased use of electronics in children of all ages, nickel found in mobile phones and other devices may be of particular concern. Caregivers can use a case or cover for metallic-appearing electronics.
Final Interpretation
Pediatric ACD is common. With limited surface area for patch testing in children, we recommend customized panels based on patient history and exposure. It is important for clinicians to recognize the unique causes of ACD in children and develop age-appropriate management plans.
- Bruckner AL, Weston WL, Morelli JG. Does sensitization to contact allergens begin in infancy? Pediatrics. 2000;105:e3.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355.
- Goldenberg A, Mousdicas N, Silverberg N, et al. Pediatric Contact Dermatitis Registry inaugural case data. Dermatitis. 2016;27:293-302.
- Ortiz Salvador JM, Esteve Martinez A, Subiabre Ferrer D, et al. Pediatric allergic contact dermatitis: clinical and epidemiological study in a tertiary hospital. Actas Dermosifiliogr. 2017;108:571-578.
- Jacob SE, Steele T, Brod B, et al. Dispelling the myths behind pediatric patch testing—experience from our tertiary care patch testing centers. Pediatr Dermatol. 2008;25:296-300.
- Brod BA, Treat JR, Rothe MJ, et al. Allergic contact dermatitis: kids are not just little people. Clin Dermatol. 2015;33:605-612.
- Elliott JF, Ramzy A, Nilsson U, et al. Severe intractable eyelid dermatitis probably caused by exposure to hydroperoxides of linalool in a heavily fragranced shampoo. Contact Dermatitis. 2017;76:114-115.
- Yu J, Atwater AR, Brod B, et al. Pediatric Baseline Patch Test Series: Pediatric Contact Dermatitis Workgroup. Dermatitis. 2018;29:206-212.
- Bergfors E, Inerot A, Falk L, et al. Patch testing children with aluminium chloride hexahydrate in petrolatum: a review and a recommendation. Contact Dermatitis. 2019;81:81-88.
- Worm M, Aberer W, Agathos M, et al. Patch testing in children—recommendations of the German Contact Dermatitis Research Group (DKG). J Dtsch Dermatol Ges. 2007;5:107-109.
- T.R.U.E. Test (Thin-Layer Rapid Use Epicutaneous Patch Test) [package insert]. Hillerød, Denmark: SmartPractice Denmark ApS; 2017.
- Jacob SE, Admani S, Herro EM. Invited commentary: recommendation for a North American pediatric patch test series. Curr Allergy Asthma Rep. 2014;14:444.
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013;24:2-6.
- Brankov N, Jacob SE. Pre-emptive avoidance strategy 2016: update on pediatric contact dermatitis allergens. Expert Rev Clin Immunol. 2017;13:93-95.
- Yu J, Treat J, Brod B. Patch test series for allergic perineal dermatitis in the diapered infant. Dermatitis. 2017;28:70-75.
- Sung CT, McGowan MA, Jacob SE. Allergic contact dermatitis evaluation: strategies for the preschooler. Curr Allergy Asthma Rep. 2018;18:49.
- Yu J, Treat J, Chaney K, et al. Potential allergens in disposable diaper wipes, topical diaper preparations, and disposable diapers: under-recognized etiology of pediatric perineal dermatitis. Dermatitis. 2016;27:110-118.
- Anderson LE, Treat JR, Brod BA, et al. “Slime” contact dermatitis: case report and review of relevant allergens. Pediatr Dermatol. 2019;36:335-337.
- Dorfman CO, Barros MA, Zaenglein AL. Contact dermatitis to training toilet seat (potty seat dermatitis). Pediatr Dermatol. 2018;35:e251-e252.
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018;29:81-84.
- Chen JK, Jacob SE, Nedorost ST, et al. A pragmatic approach to patch testing atopic dermatitis patients: clinical recommendations based on expert consensus opinion. Dermatitis. 2016;27:186-192.
- Goldenberg A, Silverberg N, Silverberg JI, et al. Pediatric allergic contact dermatitis: lessons for better care. J Allergy Clin Immunol Pract. 2015;3:661-667; quiz 668.
The pediatric population has a unique product exposure profile due to the many care products specifically marketed for use in children. In fact, the prevalence of allergic contact dermatitis (ACD) in children may be as high as 24.5% in the United States.1 In patch tested children, relevant positive reaction rates of 56.7% and 48% have been reported by the North American Contact Dermatitis Group and the Pediatric Contact Dermatitis Registry, respectively.2,3 In this article, we provide an overview of current trends in pediatric patch testing as well as specific considerations in this patient population.
Patch Test Reactions in Children
Several publications have documented pediatric patch test reactions. The North American Contact Dermatitis Group reported patch test results in 883 children from the United States and Canada (2005-2012).2 The most common reactions were nickel (28.1%), cobalt (12.3%), neomycin (7.1%), balsam of Peru (5.7%), lanolin (5.5%), and fragrance mix I (5.2%). When compared to adults, children were more likely to have relevant positive patch tests to nickel, cobalt, and compositae mix.2 In comparison, data from the Pediatric Contact Dermatitis Registry showed that the most common reactions in 1142 children in the United States (2015-2016) were nickel (22%), fragrance mix I (11%), cobalt (9.1%), balsam of Peru (8.4%), neomycin (7.2%), and propylene glycol (6.8%).3
Allergen sensitivities may vary based on geographic region. In Spain, children showed the highest sensitivities to thiomersal (10.2%), cobalt (9.1%), colophony (9.1%), paraphenylenediamine (8.3%), mercury (7.9%), potassium dichromate (7.9%), and nickel (6.4%).4
Pediatric Patch Testing Pearls
History of Product Use
From diapers to drama club, pediatric exposures and sources of ACD are not the same as those seen in adults. Because obtaining a medical history from a toddler can be exasperating, the patient’s caregivers should be asked about potential exposures, ranging from personal care products and diapers to school activities, hobbies, and sports.5,6 It is important to keep in mind that the patient’s primary caregiver may not be the only individual who applies products to the child.7
Application of Allergens
Children are not merely small adults, but they usually do have smaller backs than adult patients. This reduced surface area means that the patch tester must carefully select the allergens to be patch tested. For reference, the back of a typical 6-year-old child can fit 40 to 60 allergens during patch testing.8
Patch Test Chambers
In children, the use of plastic patch test chambers may be preferred over aluminum chambers. Children with persistent pruritic subcutaneous nodules induced by aluminum-based vaccines also may have delayed-type sensitivity reactions to aluminum.9 These patients could react to the aluminum present in some patch test chambers, making interpretation of the results difficult. The authors (A.R.A. and M.R.) typically use plastic chambers in the pediatric population.
Managing Expectations
As with other procedures in the pediatric population, patch testing can elicit emotions of fear, anxiety, and distrust. Video distraction and/or role-playing games may help capture the attention of children and can be particularly helpful during patch application. Children may be apprehensive about the term allergy testing if they are familiar with the term needle testing from previous allergies.5
Securing Patches
Young children can be quite active, posing another challenge for keeping patches in place. We recommend using extra tape to secure the patches in place on a child’s back. In addition, a large transparent film dressing (ie, 12×8 in) can be used if quick application is needed. For extra precaution, the use of a tight T-shirt or favorite onesie during the patch test process may be helpful, making it more difficult for little fingers to remove tape edges.
Duration of Patch Testing
Some authors have proposed application of patch tests for 24 hours in pediatric patients, as compared to 48 hours in adults.10 This recommendation is based on a theory that the reduced application period will decrease the risk for irritant reactions in pediatric patients.
Pediatric Patch Test Screening Series
A summary of the published screening series for patch testing in the pediatric population is provided (Table).

The T.R.U.E. Test (SmartPractice) is approved by the US Food and Drug Administration for use in patients 6 years and older11; however, it may not adequately represent allergen exposures in the pediatric population. Brankov and Jacob14 found that 10 (40%) of their proposed top 25 pediatric allergens were not detected using the T.R.U.E. Test.
In 2014, the North American Pediatric Patch Test Series was proposed as a basic screening panel for children aged 6 to 12 years.12 This series of 20 allergens was developed based on a literature review of pediatric patch test results and case reports as well as a database review. The authors proposed additional allergens to be considered based on patient history.12
More recently, a 2017 American Contact Dermatitis Society physician work group proposed the Pediatric Baseline Patch Test Series. This series of 38 allergens for children aged 6 to 18 years was developed based on expert consensus.8 Studies to determine the efficacy of this series have yet to be conducted, but it may have high sensitivity in detecting relevant allergens in children as demonstrated by a theoretical detection rate of 84%.14
There are 2 recommended patch test series for allergic diaper dermatitis.15 The first series focuses on 23 potential allergens found in wet wipes and topical diaper preparations. The second series contains 10 potential allergens found in diapers. These series contain common topical medications for children including corticosteroids, antimicrobials, and sensitizers specific to diapers such as rubbers and adhesives.15
Similar to adults, it may be difficult to designate one screening panel that can identify all relevant allergens in children; thus, it is always important to obtain a thorough exposure history and customize testing to suspected allergens and/or patient products based on history and clinical relevance.
Unique Pediatric Allergens
Hobbies
Sports gear such as shin guards and splints often contain allergens such as formaldehyde resin, thiuram mix, and dialkyl thioureas.16 Perioral dermatitis may be caused by musical instrument mouthpieces containing nickel.6
Preservatives
Commonly reported causes of ACD in children include methylisothiazolinone (MI) and methylchloroisothiazolinone (MCI) found in wet wipes. A 2016 analysis of diaper wipes showed a low prevalence of MI (6.3%) and MCI (1.6%) in these products, which may reflect the industry’s awareness of these potential allergens and a subsequent change in the preservatives they utilize.17 However, the prevalence of MCI/MI contact allergy may be on the rise due to the popularity of homemade slime, which is made from common household products such as laundry detergent, dishwashing soap, and liquid glue. The Pediatric Baseline Patch Test Series captures most of the potential allergens in these homemade slime recipes and is recommended for use in pediatric patients suspected of having dermatitis secondary to playing with slime.8,18
Toilet Seat Dermatitis
Toilet seat dermatitis presents as a pruritic dermatitis on the posterior upper thighs and buttocks. Although most cases of toilet seat dermatitis are irritant rather than allergic, potential allergens include plastics, fragrances, and components of cleaning products. Thus, physicians should maintain a high index of suspicion for ACD to toilet seats.19
Fragrance and Natural Ingredients
A 2018 study evaluating personal care products marketed specifically for infants and children found that 55% of products (294/533) contained at least 1 common allergen, with fragrance being the most common (48% [255/533]). Other common allergens include betaines (18%), propylene glycol (9%), lanolin (6%), and MCI/MI (3%).20 Caregivers should be advised against the myth that natural products are safer and less allergenic and should be provided with resources such as the Contact Allergen Management Program (CAMP) database (https://www.contactderm.org/resources/acds-camp) for safe alternative personal care products.
Metal Allergens
Nickel, the American Contact Dermatitis Society 2008 Allergen of the Year, is another common allergen that affects children. Nickel allergy, commonly thought to affect the ears due to jewelry and ear piercing, may actually be found in a wide range of daily items such as braces, eyeglasses, keys, zippers, school chairs, electronics, toys, and even food.3,6,21,22 With increased use of electronics in children of all ages, nickel found in mobile phones and other devices may be of particular concern. Caregivers can use a case or cover for metallic-appearing electronics.
Final Interpretation
Pediatric ACD is common. With limited surface area for patch testing in children, we recommend customized panels based on patient history and exposure. It is important for clinicians to recognize the unique causes of ACD in children and develop age-appropriate management plans.
The pediatric population has a unique product exposure profile due to the many care products specifically marketed for use in children. In fact, the prevalence of allergic contact dermatitis (ACD) in children may be as high as 24.5% in the United States.1 In patch tested children, relevant positive reaction rates of 56.7% and 48% have been reported by the North American Contact Dermatitis Group and the Pediatric Contact Dermatitis Registry, respectively.2,3 In this article, we provide an overview of current trends in pediatric patch testing as well as specific considerations in this patient population.
Patch Test Reactions in Children
Several publications have documented pediatric patch test reactions. The North American Contact Dermatitis Group reported patch test results in 883 children from the United States and Canada (2005-2012).2 The most common reactions were nickel (28.1%), cobalt (12.3%), neomycin (7.1%), balsam of Peru (5.7%), lanolin (5.5%), and fragrance mix I (5.2%). When compared to adults, children were more likely to have relevant positive patch tests to nickel, cobalt, and compositae mix.2 In comparison, data from the Pediatric Contact Dermatitis Registry showed that the most common reactions in 1142 children in the United States (2015-2016) were nickel (22%), fragrance mix I (11%), cobalt (9.1%), balsam of Peru (8.4%), neomycin (7.2%), and propylene glycol (6.8%).3
Allergen sensitivities may vary based on geographic region. In Spain, children showed the highest sensitivities to thiomersal (10.2%), cobalt (9.1%), colophony (9.1%), paraphenylenediamine (8.3%), mercury (7.9%), potassium dichromate (7.9%), and nickel (6.4%).4
Pediatric Patch Testing Pearls
History of Product Use
From diapers to drama club, pediatric exposures and sources of ACD are not the same as those seen in adults. Because obtaining a medical history from a toddler can be exasperating, the patient’s caregivers should be asked about potential exposures, ranging from personal care products and diapers to school activities, hobbies, and sports.5,6 It is important to keep in mind that the patient’s primary caregiver may not be the only individual who applies products to the child.7
Application of Allergens
Children are not merely small adults, but they usually do have smaller backs than adult patients. This reduced surface area means that the patch tester must carefully select the allergens to be patch tested. For reference, the back of a typical 6-year-old child can fit 40 to 60 allergens during patch testing.8
Patch Test Chambers
In children, the use of plastic patch test chambers may be preferred over aluminum chambers. Children with persistent pruritic subcutaneous nodules induced by aluminum-based vaccines also may have delayed-type sensitivity reactions to aluminum.9 These patients could react to the aluminum present in some patch test chambers, making interpretation of the results difficult. The authors (A.R.A. and M.R.) typically use plastic chambers in the pediatric population.
Managing Expectations
As with other procedures in the pediatric population, patch testing can elicit emotions of fear, anxiety, and distrust. Video distraction and/or role-playing games may help capture the attention of children and can be particularly helpful during patch application. Children may be apprehensive about the term allergy testing if they are familiar with the term needle testing from previous allergies.5
Securing Patches
Young children can be quite active, posing another challenge for keeping patches in place. We recommend using extra tape to secure the patches in place on a child’s back. In addition, a large transparent film dressing (ie, 12×8 in) can be used if quick application is needed. For extra precaution, the use of a tight T-shirt or favorite onesie during the patch test process may be helpful, making it more difficult for little fingers to remove tape edges.
Duration of Patch Testing
Some authors have proposed application of patch tests for 24 hours in pediatric patients, as compared to 48 hours in adults.10 This recommendation is based on a theory that the reduced application period will decrease the risk for irritant reactions in pediatric patients.
Pediatric Patch Test Screening Series
A summary of the published screening series for patch testing in the pediatric population is provided (Table).

The T.R.U.E. Test (SmartPractice) is approved by the US Food and Drug Administration for use in patients 6 years and older11; however, it may not adequately represent allergen exposures in the pediatric population. Brankov and Jacob14 found that 10 (40%) of their proposed top 25 pediatric allergens were not detected using the T.R.U.E. Test.
In 2014, the North American Pediatric Patch Test Series was proposed as a basic screening panel for children aged 6 to 12 years.12 This series of 20 allergens was developed based on a literature review of pediatric patch test results and case reports as well as a database review. The authors proposed additional allergens to be considered based on patient history.12
More recently, a 2017 American Contact Dermatitis Society physician work group proposed the Pediatric Baseline Patch Test Series. This series of 38 allergens for children aged 6 to 18 years was developed based on expert consensus.8 Studies to determine the efficacy of this series have yet to be conducted, but it may have high sensitivity in detecting relevant allergens in children as demonstrated by a theoretical detection rate of 84%.14
There are 2 recommended patch test series for allergic diaper dermatitis.15 The first series focuses on 23 potential allergens found in wet wipes and topical diaper preparations. The second series contains 10 potential allergens found in diapers. These series contain common topical medications for children including corticosteroids, antimicrobials, and sensitizers specific to diapers such as rubbers and adhesives.15
Similar to adults, it may be difficult to designate one screening panel that can identify all relevant allergens in children; thus, it is always important to obtain a thorough exposure history and customize testing to suspected allergens and/or patient products based on history and clinical relevance.
Unique Pediatric Allergens
Hobbies
Sports gear such as shin guards and splints often contain allergens such as formaldehyde resin, thiuram mix, and dialkyl thioureas.16 Perioral dermatitis may be caused by musical instrument mouthpieces containing nickel.6
Preservatives
Commonly reported causes of ACD in children include methylisothiazolinone (MI) and methylchloroisothiazolinone (MCI) found in wet wipes. A 2016 analysis of diaper wipes showed a low prevalence of MI (6.3%) and MCI (1.6%) in these products, which may reflect the industry’s awareness of these potential allergens and a subsequent change in the preservatives they utilize.17 However, the prevalence of MCI/MI contact allergy may be on the rise due to the popularity of homemade slime, which is made from common household products such as laundry detergent, dishwashing soap, and liquid glue. The Pediatric Baseline Patch Test Series captures most of the potential allergens in these homemade slime recipes and is recommended for use in pediatric patients suspected of having dermatitis secondary to playing with slime.8,18
Toilet Seat Dermatitis
Toilet seat dermatitis presents as a pruritic dermatitis on the posterior upper thighs and buttocks. Although most cases of toilet seat dermatitis are irritant rather than allergic, potential allergens include plastics, fragrances, and components of cleaning products. Thus, physicians should maintain a high index of suspicion for ACD to toilet seats.19
Fragrance and Natural Ingredients
A 2018 study evaluating personal care products marketed specifically for infants and children found that 55% of products (294/533) contained at least 1 common allergen, with fragrance being the most common (48% [255/533]). Other common allergens include betaines (18%), propylene glycol (9%), lanolin (6%), and MCI/MI (3%).20 Caregivers should be advised against the myth that natural products are safer and less allergenic and should be provided with resources such as the Contact Allergen Management Program (CAMP) database (https://www.contactderm.org/resources/acds-camp) for safe alternative personal care products.
Metal Allergens
Nickel, the American Contact Dermatitis Society 2008 Allergen of the Year, is another common allergen that affects children. Nickel allergy, commonly thought to affect the ears due to jewelry and ear piercing, may actually be found in a wide range of daily items such as braces, eyeglasses, keys, zippers, school chairs, electronics, toys, and even food.3,6,21,22 With increased use of electronics in children of all ages, nickel found in mobile phones and other devices may be of particular concern. Caregivers can use a case or cover for metallic-appearing electronics.
Final Interpretation
Pediatric ACD is common. With limited surface area for patch testing in children, we recommend customized panels based on patient history and exposure. It is important for clinicians to recognize the unique causes of ACD in children and develop age-appropriate management plans.
- Bruckner AL, Weston WL, Morelli JG. Does sensitization to contact allergens begin in infancy? Pediatrics. 2000;105:e3.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355.
- Goldenberg A, Mousdicas N, Silverberg N, et al. Pediatric Contact Dermatitis Registry inaugural case data. Dermatitis. 2016;27:293-302.
- Ortiz Salvador JM, Esteve Martinez A, Subiabre Ferrer D, et al. Pediatric allergic contact dermatitis: clinical and epidemiological study in a tertiary hospital. Actas Dermosifiliogr. 2017;108:571-578.
- Jacob SE, Steele T, Brod B, et al. Dispelling the myths behind pediatric patch testing—experience from our tertiary care patch testing centers. Pediatr Dermatol. 2008;25:296-300.
- Brod BA, Treat JR, Rothe MJ, et al. Allergic contact dermatitis: kids are not just little people. Clin Dermatol. 2015;33:605-612.
- Elliott JF, Ramzy A, Nilsson U, et al. Severe intractable eyelid dermatitis probably caused by exposure to hydroperoxides of linalool in a heavily fragranced shampoo. Contact Dermatitis. 2017;76:114-115.
- Yu J, Atwater AR, Brod B, et al. Pediatric Baseline Patch Test Series: Pediatric Contact Dermatitis Workgroup. Dermatitis. 2018;29:206-212.
- Bergfors E, Inerot A, Falk L, et al. Patch testing children with aluminium chloride hexahydrate in petrolatum: a review and a recommendation. Contact Dermatitis. 2019;81:81-88.
- Worm M, Aberer W, Agathos M, et al. Patch testing in children—recommendations of the German Contact Dermatitis Research Group (DKG). J Dtsch Dermatol Ges. 2007;5:107-109.
- T.R.U.E. Test (Thin-Layer Rapid Use Epicutaneous Patch Test) [package insert]. Hillerød, Denmark: SmartPractice Denmark ApS; 2017.
- Jacob SE, Admani S, Herro EM. Invited commentary: recommendation for a North American pediatric patch test series. Curr Allergy Asthma Rep. 2014;14:444.
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013;24:2-6.
- Brankov N, Jacob SE. Pre-emptive avoidance strategy 2016: update on pediatric contact dermatitis allergens. Expert Rev Clin Immunol. 2017;13:93-95.
- Yu J, Treat J, Brod B. Patch test series for allergic perineal dermatitis in the diapered infant. Dermatitis. 2017;28:70-75.
- Sung CT, McGowan MA, Jacob SE. Allergic contact dermatitis evaluation: strategies for the preschooler. Curr Allergy Asthma Rep. 2018;18:49.
- Yu J, Treat J, Chaney K, et al. Potential allergens in disposable diaper wipes, topical diaper preparations, and disposable diapers: under-recognized etiology of pediatric perineal dermatitis. Dermatitis. 2016;27:110-118.
- Anderson LE, Treat JR, Brod BA, et al. “Slime” contact dermatitis: case report and review of relevant allergens. Pediatr Dermatol. 2019;36:335-337.
- Dorfman CO, Barros MA, Zaenglein AL. Contact dermatitis to training toilet seat (potty seat dermatitis). Pediatr Dermatol. 2018;35:e251-e252.
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018;29:81-84.
- Chen JK, Jacob SE, Nedorost ST, et al. A pragmatic approach to patch testing atopic dermatitis patients: clinical recommendations based on expert consensus opinion. Dermatitis. 2016;27:186-192.
- Goldenberg A, Silverberg N, Silverberg JI, et al. Pediatric allergic contact dermatitis: lessons for better care. J Allergy Clin Immunol Pract. 2015;3:661-667; quiz 668.
- Bruckner AL, Weston WL, Morelli JG. Does sensitization to contact allergens begin in infancy? Pediatrics. 2000;105:e3.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355.
- Goldenberg A, Mousdicas N, Silverberg N, et al. Pediatric Contact Dermatitis Registry inaugural case data. Dermatitis. 2016;27:293-302.
- Ortiz Salvador JM, Esteve Martinez A, Subiabre Ferrer D, et al. Pediatric allergic contact dermatitis: clinical and epidemiological study in a tertiary hospital. Actas Dermosifiliogr. 2017;108:571-578.
- Jacob SE, Steele T, Brod B, et al. Dispelling the myths behind pediatric patch testing—experience from our tertiary care patch testing centers. Pediatr Dermatol. 2008;25:296-300.
- Brod BA, Treat JR, Rothe MJ, et al. Allergic contact dermatitis: kids are not just little people. Clin Dermatol. 2015;33:605-612.
- Elliott JF, Ramzy A, Nilsson U, et al. Severe intractable eyelid dermatitis probably caused by exposure to hydroperoxides of linalool in a heavily fragranced shampoo. Contact Dermatitis. 2017;76:114-115.
- Yu J, Atwater AR, Brod B, et al. Pediatric Baseline Patch Test Series: Pediatric Contact Dermatitis Workgroup. Dermatitis. 2018;29:206-212.
- Bergfors E, Inerot A, Falk L, et al. Patch testing children with aluminium chloride hexahydrate in petrolatum: a review and a recommendation. Contact Dermatitis. 2019;81:81-88.
- Worm M, Aberer W, Agathos M, et al. Patch testing in children—recommendations of the German Contact Dermatitis Research Group (DKG). J Dtsch Dermatol Ges. 2007;5:107-109.
- T.R.U.E. Test (Thin-Layer Rapid Use Epicutaneous Patch Test) [package insert]. Hillerød, Denmark: SmartPractice Denmark ApS; 2017.
- Jacob SE, Admani S, Herro EM. Invited commentary: recommendation for a North American pediatric patch test series. Curr Allergy Asthma Rep. 2014;14:444.
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013;24:2-6.
- Brankov N, Jacob SE. Pre-emptive avoidance strategy 2016: update on pediatric contact dermatitis allergens. Expert Rev Clin Immunol. 2017;13:93-95.
- Yu J, Treat J, Brod B. Patch test series for allergic perineal dermatitis in the diapered infant. Dermatitis. 2017;28:70-75.
- Sung CT, McGowan MA, Jacob SE. Allergic contact dermatitis evaluation: strategies for the preschooler. Curr Allergy Asthma Rep. 2018;18:49.
- Yu J, Treat J, Chaney K, et al. Potential allergens in disposable diaper wipes, topical diaper preparations, and disposable diapers: under-recognized etiology of pediatric perineal dermatitis. Dermatitis. 2016;27:110-118.
- Anderson LE, Treat JR, Brod BA, et al. “Slime” contact dermatitis: case report and review of relevant allergens. Pediatr Dermatol. 2019;36:335-337.
- Dorfman CO, Barros MA, Zaenglein AL. Contact dermatitis to training toilet seat (potty seat dermatitis). Pediatr Dermatol. 2018;35:e251-e252.
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018;29:81-84.
- Chen JK, Jacob SE, Nedorost ST, et al. A pragmatic approach to patch testing atopic dermatitis patients: clinical recommendations based on expert consensus opinion. Dermatitis. 2016;27:186-192.
- Goldenberg A, Silverberg N, Silverberg JI, et al. Pediatric allergic contact dermatitis: lessons for better care. J Allergy Clin Immunol Pract. 2015;3:661-667; quiz 668.
Practice Points
- Pediatric allergic contact dermatitis (ACD) is common with children having unique product exposures.
- Children suspected to have ACD should be patch tested with customized panels based on history and exposure.
- Common pediatric allergens have been identified in personal care products, household products, and recreational gear and toys.
Discoloration and Bullous Lesions on the Hands
The Diagnosis: Irritant Contact Dermatitis and Hyperpigmentation Due to Juglone
Clinical suspicion, resemblance to similar cases, and questioning the patient about his behavior prior to the onset of symptoms led to the diagnosis of irritant contact dermatitis and hyperpigmentation due to juglone in this case. Walnuts belong to the botanical family of Juglandaceae and are the seed of the trees of the genus Juglans, which encompass 24 different species. The nuts from all species included in this genus are edible.1 The most well-known species of walnut is the common walnut (Juglans regia), which is native to the Balkans region in southeast Europe, southwest and central Asia extending to the Himalayas, and southwest China.1
Walnut fruits are rich in phenolic compounds. Thirteen phenolic compounds have been identified in walnut husks including chlorogenic acid, caffeic acid, ferulic acid, sinapic acid, gallic acid, ellagic acid, protocatechuic acid, syringic acid, vanillic acid, catechin, epicatechin, myricetin, and juglone.2 Juglone, also called 5-hydroxy-1,4-napthoquinone, is a yellow naphthoquinone pigment that occurs naturally in the leaves, roots, husks, and bark of plants in the Juglandaceae family, particularly the black walnut (Juglans nigra).3,4
Juglans regia, also known as English or Persian walnut, contains potent chemical constituents and has been used to treat diverse ailments such as diarrhea, hyperglycemia, cancer, infectious diseases, anorexia, asthma, helminthiasis, arthritis, sinusitis, stomachache, and skin disorders (eg, eczema; acne; alopecia; scalp itching, peeling, and dandruff), and as an adjunctive emollient and itch-relieving treatment.5,6
The juice of walnut shells from the J regia tree have been used for centuries to color the skin and hair.7 Irritation and skin hyperpigmentation have been associated with topical walnut use.5 As a naphthoquinone, juglone also is reported to exert some toxic effects on normal tissues including acute irritant contact dermatitis.4 As the active ingredient from the green husk of walnuts, it has been considered a strong sensitizer in guinea pigs,1 but contact sensitivity in humans rarely has been reported.7
Juglone is known to react with the keratin proteins present in the skin to form sclerojuglonic compounds, which have UV protection properties and a red-brown color.8 The resulting reaction gives rise to chromophore groups with a strong pigmenting action that absorbs visible colors (especially violet) and reflects yellow and red, resulting in the coloration ranging from red to deep brown.7 The mechanism of skin pigmentation does not involve the melanocytes. Hyperchromia involving the hands--particularly the palms, fingers, and nails--lasts 1 to 4 weeks depending on the intensity of the pigmentation. Housewives and agricultural workers are the at-risk population.7 Acute irritant contact dermatitis and hyperpigmentation due to juglone mainly has been observed during the early autumn in agricultural workers and housewives who remove the green husk of walnuts.9
Addison disease can present with pigmentary changes in the skin and mucous membranes; it also is accompanied by fatigue, anorexia, weakness, and weight loss, none of which were noted in our patient. A fixed drug eruption tends to have an annular or oval form and is related to the intake of medication (mostly antibiotics) up to 2 weeks prior to the onset of the dermatosis. Our patient did not have any chronic disease or take any medication prior to the dermatosis and lacked the classic clinical morphology of this entity. Hemochromatosis affects not only the skin but also the liver, myocardial fibers, and other internal organs. Our patient did not have any clinical manifestations of liver or heart failure or diabetes mellitus.
Our patient was treated with drainage of the blisters. Due to the extent of the dermatosis, prednisone 25 mg/d also was initiated. The patient was instructed to avoid direct contact with the husk of walnuts. At 1-month follow-up, the hyperpigmentation had resolved with no relapse (Figure).
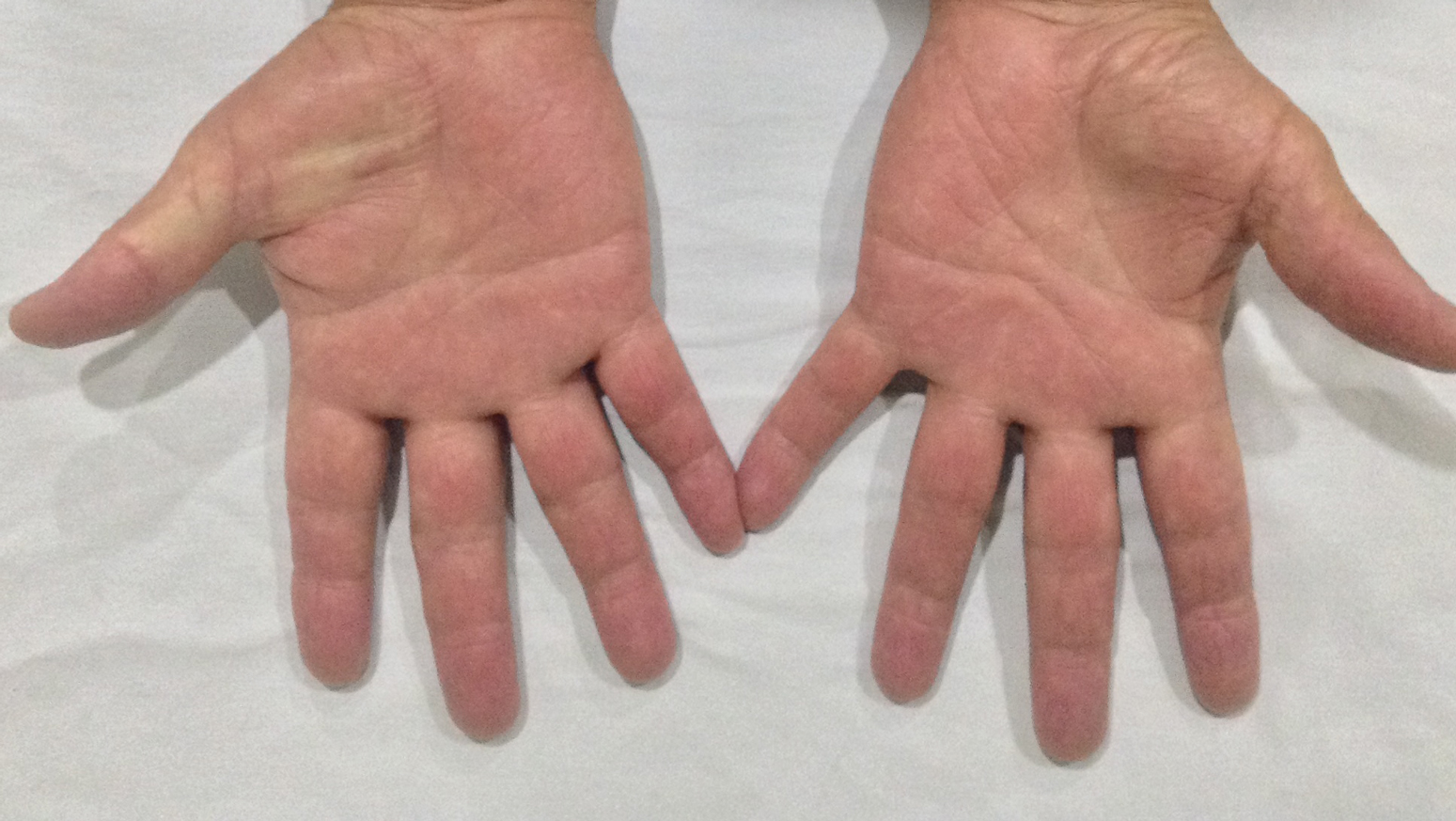
- Costa J, Carrapatoso I, Oliveira MB, et al. Walnut allergens: molecular characterization, detection and clinical relevance. Clin Exp Allergy. 2013;44:319-341.
- Cosmulescu S, Trandafir I, Achim G, et al. Phenolics of green husk in mature walnut fruits. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2010;38:53-56.
- Cosmulescu S, Trandafir I, Achim G, et al. Juglone content in leaf and green husk of five walnut (Juglans regia L.) cultivars. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2011;39:237-240.
- Aithal BK, Sunil Kumar MR, Rao BN, et al. Evaluation of pharmacokinetic, biodistribution, pharmacodynamic, and toxicity profile of free juglone and its sterically stabilized liposomes. J Pharm Sci. 2011;100:3517-3528.
- Panth N, Paudel KR, Karki R. Phytochemical profile and biological activity of Juglans regia. J Integr Med. 2016;14:359-373.
- Aburjai T, Natsheh FM. Plants used in cosmetics. Phytother Res. 2003;17:987-1000.
- Bonamonte D, Foti C, Angelini G. Hyperpigmentation and contact dermatitis due to Juglans regia. Contact Dermatitis. 2001;44:101-102.
- Dweck AC. Natural ingredients for colouring and styling. Int J Cosmet Sci. 2002;24:287-302.
- Neri I, Bianchi F, Giacomini F, et al. Acute irritant contact dermatitis due to Juglans regia. Contact Dermatitis. 2006;55:62-63.
The Diagnosis: Irritant Contact Dermatitis and Hyperpigmentation Due to Juglone
Clinical suspicion, resemblance to similar cases, and questioning the patient about his behavior prior to the onset of symptoms led to the diagnosis of irritant contact dermatitis and hyperpigmentation due to juglone in this case. Walnuts belong to the botanical family of Juglandaceae and are the seed of the trees of the genus Juglans, which encompass 24 different species. The nuts from all species included in this genus are edible.1 The most well-known species of walnut is the common walnut (Juglans regia), which is native to the Balkans region in southeast Europe, southwest and central Asia extending to the Himalayas, and southwest China.1
Walnut fruits are rich in phenolic compounds. Thirteen phenolic compounds have been identified in walnut husks including chlorogenic acid, caffeic acid, ferulic acid, sinapic acid, gallic acid, ellagic acid, protocatechuic acid, syringic acid, vanillic acid, catechin, epicatechin, myricetin, and juglone.2 Juglone, also called 5-hydroxy-1,4-napthoquinone, is a yellow naphthoquinone pigment that occurs naturally in the leaves, roots, husks, and bark of plants in the Juglandaceae family, particularly the black walnut (Juglans nigra).3,4
Juglans regia, also known as English or Persian walnut, contains potent chemical constituents and has been used to treat diverse ailments such as diarrhea, hyperglycemia, cancer, infectious diseases, anorexia, asthma, helminthiasis, arthritis, sinusitis, stomachache, and skin disorders (eg, eczema; acne; alopecia; scalp itching, peeling, and dandruff), and as an adjunctive emollient and itch-relieving treatment.5,6
The juice of walnut shells from the J regia tree have been used for centuries to color the skin and hair.7 Irritation and skin hyperpigmentation have been associated with topical walnut use.5 As a naphthoquinone, juglone also is reported to exert some toxic effects on normal tissues including acute irritant contact dermatitis.4 As the active ingredient from the green husk of walnuts, it has been considered a strong sensitizer in guinea pigs,1 but contact sensitivity in humans rarely has been reported.7
Juglone is known to react with the keratin proteins present in the skin to form sclerojuglonic compounds, which have UV protection properties and a red-brown color.8 The resulting reaction gives rise to chromophore groups with a strong pigmenting action that absorbs visible colors (especially violet) and reflects yellow and red, resulting in the coloration ranging from red to deep brown.7 The mechanism of skin pigmentation does not involve the melanocytes. Hyperchromia involving the hands--particularly the palms, fingers, and nails--lasts 1 to 4 weeks depending on the intensity of the pigmentation. Housewives and agricultural workers are the at-risk population.7 Acute irritant contact dermatitis and hyperpigmentation due to juglone mainly has been observed during the early autumn in agricultural workers and housewives who remove the green husk of walnuts.9
Addison disease can present with pigmentary changes in the skin and mucous membranes; it also is accompanied by fatigue, anorexia, weakness, and weight loss, none of which were noted in our patient. A fixed drug eruption tends to have an annular or oval form and is related to the intake of medication (mostly antibiotics) up to 2 weeks prior to the onset of the dermatosis. Our patient did not have any chronic disease or take any medication prior to the dermatosis and lacked the classic clinical morphology of this entity. Hemochromatosis affects not only the skin but also the liver, myocardial fibers, and other internal organs. Our patient did not have any clinical manifestations of liver or heart failure or diabetes mellitus.
Our patient was treated with drainage of the blisters. Due to the extent of the dermatosis, prednisone 25 mg/d also was initiated. The patient was instructed to avoid direct contact with the husk of walnuts. At 1-month follow-up, the hyperpigmentation had resolved with no relapse (Figure).

The Diagnosis: Irritant Contact Dermatitis and Hyperpigmentation Due to Juglone
Clinical suspicion, resemblance to similar cases, and questioning the patient about his behavior prior to the onset of symptoms led to the diagnosis of irritant contact dermatitis and hyperpigmentation due to juglone in this case. Walnuts belong to the botanical family of Juglandaceae and are the seed of the trees of the genus Juglans, which encompass 24 different species. The nuts from all species included in this genus are edible.1 The most well-known species of walnut is the common walnut (Juglans regia), which is native to the Balkans region in southeast Europe, southwest and central Asia extending to the Himalayas, and southwest China.1
Walnut fruits are rich in phenolic compounds. Thirteen phenolic compounds have been identified in walnut husks including chlorogenic acid, caffeic acid, ferulic acid, sinapic acid, gallic acid, ellagic acid, protocatechuic acid, syringic acid, vanillic acid, catechin, epicatechin, myricetin, and juglone.2 Juglone, also called 5-hydroxy-1,4-napthoquinone, is a yellow naphthoquinone pigment that occurs naturally in the leaves, roots, husks, and bark of plants in the Juglandaceae family, particularly the black walnut (Juglans nigra).3,4
Juglans regia, also known as English or Persian walnut, contains potent chemical constituents and has been used to treat diverse ailments such as diarrhea, hyperglycemia, cancer, infectious diseases, anorexia, asthma, helminthiasis, arthritis, sinusitis, stomachache, and skin disorders (eg, eczema; acne; alopecia; scalp itching, peeling, and dandruff), and as an adjunctive emollient and itch-relieving treatment.5,6
The juice of walnut shells from the J regia tree have been used for centuries to color the skin and hair.7 Irritation and skin hyperpigmentation have been associated with topical walnut use.5 As a naphthoquinone, juglone also is reported to exert some toxic effects on normal tissues including acute irritant contact dermatitis.4 As the active ingredient from the green husk of walnuts, it has been considered a strong sensitizer in guinea pigs,1 but contact sensitivity in humans rarely has been reported.7
Juglone is known to react with the keratin proteins present in the skin to form sclerojuglonic compounds, which have UV protection properties and a red-brown color.8 The resulting reaction gives rise to chromophore groups with a strong pigmenting action that absorbs visible colors (especially violet) and reflects yellow and red, resulting in the coloration ranging from red to deep brown.7 The mechanism of skin pigmentation does not involve the melanocytes. Hyperchromia involving the hands--particularly the palms, fingers, and nails--lasts 1 to 4 weeks depending on the intensity of the pigmentation. Housewives and agricultural workers are the at-risk population.7 Acute irritant contact dermatitis and hyperpigmentation due to juglone mainly has been observed during the early autumn in agricultural workers and housewives who remove the green husk of walnuts.9
Addison disease can present with pigmentary changes in the skin and mucous membranes; it also is accompanied by fatigue, anorexia, weakness, and weight loss, none of which were noted in our patient. A fixed drug eruption tends to have an annular or oval form and is related to the intake of medication (mostly antibiotics) up to 2 weeks prior to the onset of the dermatosis. Our patient did not have any chronic disease or take any medication prior to the dermatosis and lacked the classic clinical morphology of this entity. Hemochromatosis affects not only the skin but also the liver, myocardial fibers, and other internal organs. Our patient did not have any clinical manifestations of liver or heart failure or diabetes mellitus.
Our patient was treated with drainage of the blisters. Due to the extent of the dermatosis, prednisone 25 mg/d also was initiated. The patient was instructed to avoid direct contact with the husk of walnuts. At 1-month follow-up, the hyperpigmentation had resolved with no relapse (Figure).

- Costa J, Carrapatoso I, Oliveira MB, et al. Walnut allergens: molecular characterization, detection and clinical relevance. Clin Exp Allergy. 2013;44:319-341.
- Cosmulescu S, Trandafir I, Achim G, et al. Phenolics of green husk in mature walnut fruits. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2010;38:53-56.
- Cosmulescu S, Trandafir I, Achim G, et al. Juglone content in leaf and green husk of five walnut (Juglans regia L.) cultivars. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2011;39:237-240.
- Aithal BK, Sunil Kumar MR, Rao BN, et al. Evaluation of pharmacokinetic, biodistribution, pharmacodynamic, and toxicity profile of free juglone and its sterically stabilized liposomes. J Pharm Sci. 2011;100:3517-3528.
- Panth N, Paudel KR, Karki R. Phytochemical profile and biological activity of Juglans regia. J Integr Med. 2016;14:359-373.
- Aburjai T, Natsheh FM. Plants used in cosmetics. Phytother Res. 2003;17:987-1000.
- Bonamonte D, Foti C, Angelini G. Hyperpigmentation and contact dermatitis due to Juglans regia. Contact Dermatitis. 2001;44:101-102.
- Dweck AC. Natural ingredients for colouring and styling. Int J Cosmet Sci. 2002;24:287-302.
- Neri I, Bianchi F, Giacomini F, et al. Acute irritant contact dermatitis due to Juglans regia. Contact Dermatitis. 2006;55:62-63.
- Costa J, Carrapatoso I, Oliveira MB, et al. Walnut allergens: molecular characterization, detection and clinical relevance. Clin Exp Allergy. 2013;44:319-341.
- Cosmulescu S, Trandafir I, Achim G, et al. Phenolics of green husk in mature walnut fruits. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2010;38:53-56.
- Cosmulescu S, Trandafir I, Achim G, et al. Juglone content in leaf and green husk of five walnut (Juglans regia L.) cultivars. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2011;39:237-240.
- Aithal BK, Sunil Kumar MR, Rao BN, et al. Evaluation of pharmacokinetic, biodistribution, pharmacodynamic, and toxicity profile of free juglone and its sterically stabilized liposomes. J Pharm Sci. 2011;100:3517-3528.
- Panth N, Paudel KR, Karki R. Phytochemical profile and biological activity of Juglans regia. J Integr Med. 2016;14:359-373.
- Aburjai T, Natsheh FM. Plants used in cosmetics. Phytother Res. 2003;17:987-1000.
- Bonamonte D, Foti C, Angelini G. Hyperpigmentation and contact dermatitis due to Juglans regia. Contact Dermatitis. 2001;44:101-102.
- Dweck AC. Natural ingredients for colouring and styling. Int J Cosmet Sci. 2002;24:287-302.
- Neri I, Bianchi F, Giacomini F, et al. Acute irritant contact dermatitis due to Juglans regia. Contact Dermatitis. 2006;55:62-63.
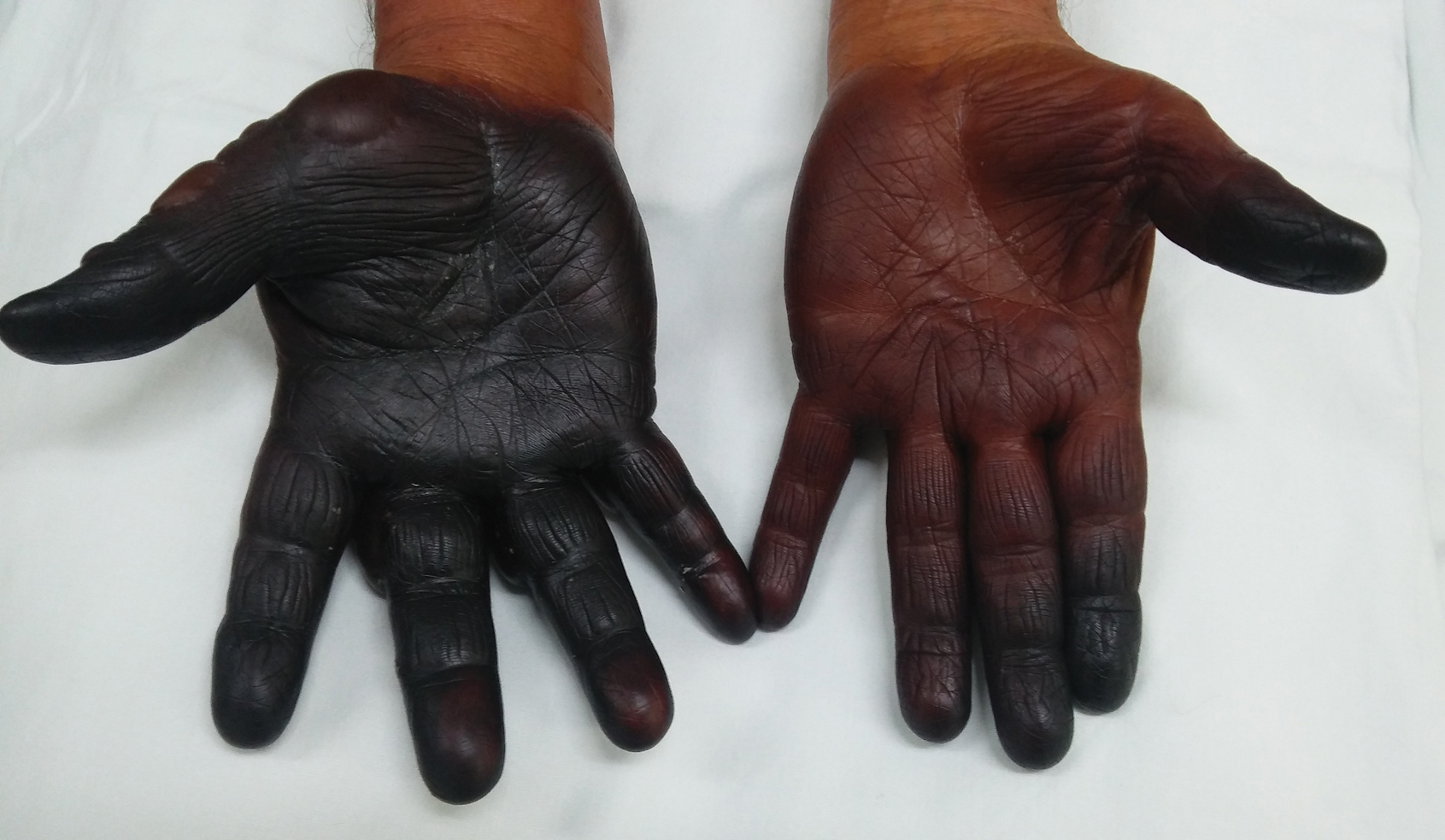
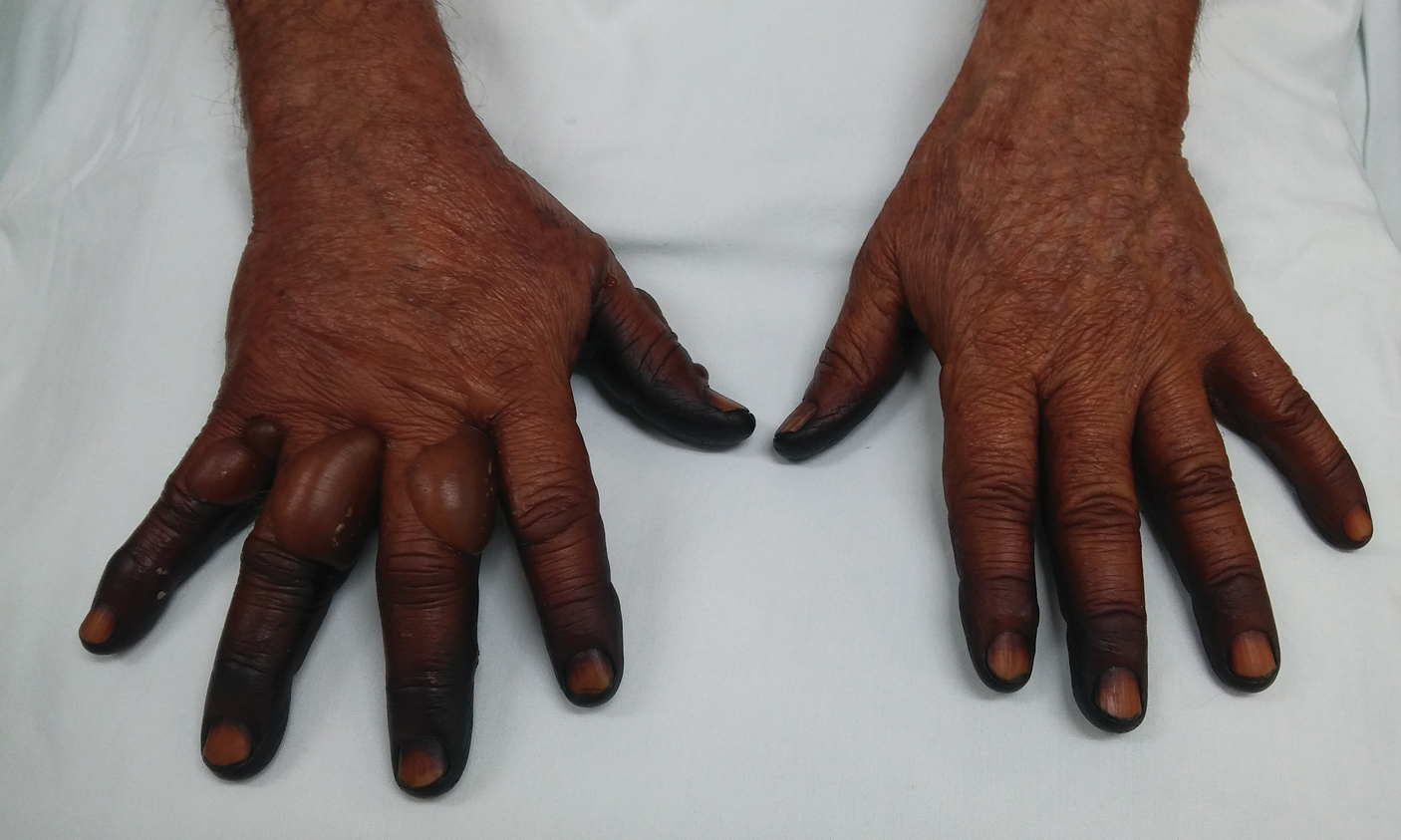
A 71-year-old man presented for evaluation of discoloration and blisters of 1 day's duration on both hands that were more severe on the right hand. The lesions were preceded by a sensation of stinging pain. One hour prior to the onset of symptoms, he had peeled approximately 100 walnuts. He had no relevant medical history. Physical examination revealed dark brown to black discoloration involving both hands (top) extending to the fingernails. Blisters filled with clear fluid also were present on the fingers (bottom).
Symmetrical Pruriginous Nasal Rash
The Diagnosis: Irritant Contact Dermatitis
A slang term for volatile alkyl nitrites, poppers are inhaled for recreational purposes. They produce rapid-onset euphoria and sexual arousal, as well as relax anal and vaginal sphincters, facilitating sexual intercourse. Alkyl nitrites initially were developed to treat coronary disease and angina but were replaced by more potent drugs.1 Because of their psychoactive effects and smooth muscle relaxation properties, they are widely used by homosexual and bisexual men.1-3 The term poppers was originated by the sound generated when the glass vials are crushed; currently, they also may be found in other formats.1
Nausea, hypotension, and headache are mild common adverse effects of volatile alkyl nitrites1; cardiac arrhythmia, oxidative hemolysis,4 and poppers maculopathy5,6 with permanent eye damage also have been reported.7 On the skin, volatile alkyl nitrites induce irritant contact dermatitis that heals without scarring, characteristically involving the face and upper thoracic region, as they are volatile vapors.2 However, the reaction can occur elsewhere. There have been reports of contact dermatitis on other locations, such as the thigh or the ankle, due to vials broken while stored in pockets or on the cuff of the socks.1 There also is a report of irritant contact dermatitis manifesting as a penile ulcer.3 Albeit rare, allergic contact dermatitis to volatile alkyl nitrites and other nitrites also can occur.8
The abuse of alkyl nitrites may increase the risk for sexually transmitted infections (STIs), as they may decrease safer sexual practices and increase the propensity to engage in risky sexual behavior. It has been suggested to screen for STIs in patients with history of volatile alkyl nitrite use. In the past, volatile alkyl nitrites were believed to be a potential vector of human immunodeficiency virus.9 Other popular drugs used in social context or "club drugs," such as 3,4-methylenedioxymethamphetamine, gamma hydroxybutyrate, methamphetamine, and ketamine, do not produce irritant dermatitis as an adverse cutaneous reaction.10 The differential diagnosis in our patient included herpes simplex virus and contagious impetigo1 as well as bullous lupus erythematosus and periorificial dermatitis; however, the clinical picture, acute onset of the reaction, and the patient's medical history were critical in making the correct diagnosis.
The patient was treated with topical hydrocortisone and fusidic acid cream twice daily for 7 days with complete response. Sexually transmitted infection screening was unremarkable. We suggest performing an STI workup on patients with history of volatile alkyl nitrite use.
- Schauber J, Herzinger T. 'Poppers' dermatitis. Clin Exp Dermatol. 2012;37:587-588.
- Foroozan M, Studer M, Splingard B, et al. Facial dermatitis due to inhalation of poppers [in French]. Ann Dermatol Venereol. 2009;136:298-299.
- Latini A, Lora V, Zaccarelli M, et al. Unusual presentation of poppers dermatitis. JAMA Dermatol. 2017;153:233-234.
- Shortt J, Polizzotto MN, Opat SS, et al. Oxidative haemolysis due to 'poppers'. Br J Haematol. 2008;142:328.
- Davies AJ, Kelly SP, Naylor SG, et al. Adverse ophthalmic reaction in poppers users: case series of 'poppers maculopathy'. Eye (Lond). 2012;26:1479-1486.
- Davies AJ, Kelly SP, Bhatt PR. 'Poppers maculopathy'--an emerging ophthalmic reaction to recreational substance abuse. Eye (Lond). 2012;26:888.
- Vignal-Clermont C, Audo I, Sahel JA, et al. Poppers-associated retinal toxicity. N Engl J Med. 2010;363:1583-1585.
- Bos JD, Jansen FC, Timmer JG. Allergic contact dermatitis to amyl nitrite ('poppers'). Contact Dermatitis. 1985;12:109.
- Stratford M, Wilson PD. Agitation effects on microbial cell-cell interactions. Lett Appl Microbiol. 1990;11:1-6.
- Romanelli F, Smith KM, Thornton AC, et al. Poppers: epidemiology and clinical management of inhaled nitrite abuse. Pharmacotherapy. 2004;24:69-78.
The Diagnosis: Irritant Contact Dermatitis
A slang term for volatile alkyl nitrites, poppers are inhaled for recreational purposes. They produce rapid-onset euphoria and sexual arousal, as well as relax anal and vaginal sphincters, facilitating sexual intercourse. Alkyl nitrites initially were developed to treat coronary disease and angina but were replaced by more potent drugs.1 Because of their psychoactive effects and smooth muscle relaxation properties, they are widely used by homosexual and bisexual men.1-3 The term poppers was originated by the sound generated when the glass vials are crushed; currently, they also may be found in other formats.1
Nausea, hypotension, and headache are mild common adverse effects of volatile alkyl nitrites1; cardiac arrhythmia, oxidative hemolysis,4 and poppers maculopathy5,6 with permanent eye damage also have been reported.7 On the skin, volatile alkyl nitrites induce irritant contact dermatitis that heals without scarring, characteristically involving the face and upper thoracic region, as they are volatile vapors.2 However, the reaction can occur elsewhere. There have been reports of contact dermatitis on other locations, such as the thigh or the ankle, due to vials broken while stored in pockets or on the cuff of the socks.1 There also is a report of irritant contact dermatitis manifesting as a penile ulcer.3 Albeit rare, allergic contact dermatitis to volatile alkyl nitrites and other nitrites also can occur.8
The abuse of alkyl nitrites may increase the risk for sexually transmitted infections (STIs), as they may decrease safer sexual practices and increase the propensity to engage in risky sexual behavior. It has been suggested to screen for STIs in patients with history of volatile alkyl nitrite use. In the past, volatile alkyl nitrites were believed to be a potential vector of human immunodeficiency virus.9 Other popular drugs used in social context or "club drugs," such as 3,4-methylenedioxymethamphetamine, gamma hydroxybutyrate, methamphetamine, and ketamine, do not produce irritant dermatitis as an adverse cutaneous reaction.10 The differential diagnosis in our patient included herpes simplex virus and contagious impetigo1 as well as bullous lupus erythematosus and periorificial dermatitis; however, the clinical picture, acute onset of the reaction, and the patient's medical history were critical in making the correct diagnosis.
The patient was treated with topical hydrocortisone and fusidic acid cream twice daily for 7 days with complete response. Sexually transmitted infection screening was unremarkable. We suggest performing an STI workup on patients with history of volatile alkyl nitrite use.
The Diagnosis: Irritant Contact Dermatitis
A slang term for volatile alkyl nitrites, poppers are inhaled for recreational purposes. They produce rapid-onset euphoria and sexual arousal, as well as relax anal and vaginal sphincters, facilitating sexual intercourse. Alkyl nitrites initially were developed to treat coronary disease and angina but were replaced by more potent drugs.1 Because of their psychoactive effects and smooth muscle relaxation properties, they are widely used by homosexual and bisexual men.1-3 The term poppers was originated by the sound generated when the glass vials are crushed; currently, they also may be found in other formats.1
Nausea, hypotension, and headache are mild common adverse effects of volatile alkyl nitrites1; cardiac arrhythmia, oxidative hemolysis,4 and poppers maculopathy5,6 with permanent eye damage also have been reported.7 On the skin, volatile alkyl nitrites induce irritant contact dermatitis that heals without scarring, characteristically involving the face and upper thoracic region, as they are volatile vapors.2 However, the reaction can occur elsewhere. There have been reports of contact dermatitis on other locations, such as the thigh or the ankle, due to vials broken while stored in pockets or on the cuff of the socks.1 There also is a report of irritant contact dermatitis manifesting as a penile ulcer.3 Albeit rare, allergic contact dermatitis to volatile alkyl nitrites and other nitrites also can occur.8
The abuse of alkyl nitrites may increase the risk for sexually transmitted infections (STIs), as they may decrease safer sexual practices and increase the propensity to engage in risky sexual behavior. It has been suggested to screen for STIs in patients with history of volatile alkyl nitrite use. In the past, volatile alkyl nitrites were believed to be a potential vector of human immunodeficiency virus.9 Other popular drugs used in social context or "club drugs," such as 3,4-methylenedioxymethamphetamine, gamma hydroxybutyrate, methamphetamine, and ketamine, do not produce irritant dermatitis as an adverse cutaneous reaction.10 The differential diagnosis in our patient included herpes simplex virus and contagious impetigo1 as well as bullous lupus erythematosus and periorificial dermatitis; however, the clinical picture, acute onset of the reaction, and the patient's medical history were critical in making the correct diagnosis.
The patient was treated with topical hydrocortisone and fusidic acid cream twice daily for 7 days with complete response. Sexually transmitted infection screening was unremarkable. We suggest performing an STI workup on patients with history of volatile alkyl nitrite use.
- Schauber J, Herzinger T. 'Poppers' dermatitis. Clin Exp Dermatol. 2012;37:587-588.
- Foroozan M, Studer M, Splingard B, et al. Facial dermatitis due to inhalation of poppers [in French]. Ann Dermatol Venereol. 2009;136:298-299.
- Latini A, Lora V, Zaccarelli M, et al. Unusual presentation of poppers dermatitis. JAMA Dermatol. 2017;153:233-234.
- Shortt J, Polizzotto MN, Opat SS, et al. Oxidative haemolysis due to 'poppers'. Br J Haematol. 2008;142:328.
- Davies AJ, Kelly SP, Naylor SG, et al. Adverse ophthalmic reaction in poppers users: case series of 'poppers maculopathy'. Eye (Lond). 2012;26:1479-1486.
- Davies AJ, Kelly SP, Bhatt PR. 'Poppers maculopathy'--an emerging ophthalmic reaction to recreational substance abuse. Eye (Lond). 2012;26:888.
- Vignal-Clermont C, Audo I, Sahel JA, et al. Poppers-associated retinal toxicity. N Engl J Med. 2010;363:1583-1585.
- Bos JD, Jansen FC, Timmer JG. Allergic contact dermatitis to amyl nitrite ('poppers'). Contact Dermatitis. 1985;12:109.
- Stratford M, Wilson PD. Agitation effects on microbial cell-cell interactions. Lett Appl Microbiol. 1990;11:1-6.
- Romanelli F, Smith KM, Thornton AC, et al. Poppers: epidemiology and clinical management of inhaled nitrite abuse. Pharmacotherapy. 2004;24:69-78.
- Schauber J, Herzinger T. 'Poppers' dermatitis. Clin Exp Dermatol. 2012;37:587-588.
- Foroozan M, Studer M, Splingard B, et al. Facial dermatitis due to inhalation of poppers [in French]. Ann Dermatol Venereol. 2009;136:298-299.
- Latini A, Lora V, Zaccarelli M, et al. Unusual presentation of poppers dermatitis. JAMA Dermatol. 2017;153:233-234.
- Shortt J, Polizzotto MN, Opat SS, et al. Oxidative haemolysis due to 'poppers'. Br J Haematol. 2008;142:328.
- Davies AJ, Kelly SP, Naylor SG, et al. Adverse ophthalmic reaction in poppers users: case series of 'poppers maculopathy'. Eye (Lond). 2012;26:1479-1486.
- Davies AJ, Kelly SP, Bhatt PR. 'Poppers maculopathy'--an emerging ophthalmic reaction to recreational substance abuse. Eye (Lond). 2012;26:888.
- Vignal-Clermont C, Audo I, Sahel JA, et al. Poppers-associated retinal toxicity. N Engl J Med. 2010;363:1583-1585.
- Bos JD, Jansen FC, Timmer JG. Allergic contact dermatitis to amyl nitrite ('poppers'). Contact Dermatitis. 1985;12:109.
- Stratford M, Wilson PD. Agitation effects on microbial cell-cell interactions. Lett Appl Microbiol. 1990;11:1-6.
- Romanelli F, Smith KM, Thornton AC, et al. Poppers: epidemiology and clinical management of inhaled nitrite abuse. Pharmacotherapy. 2004;24:69-78.
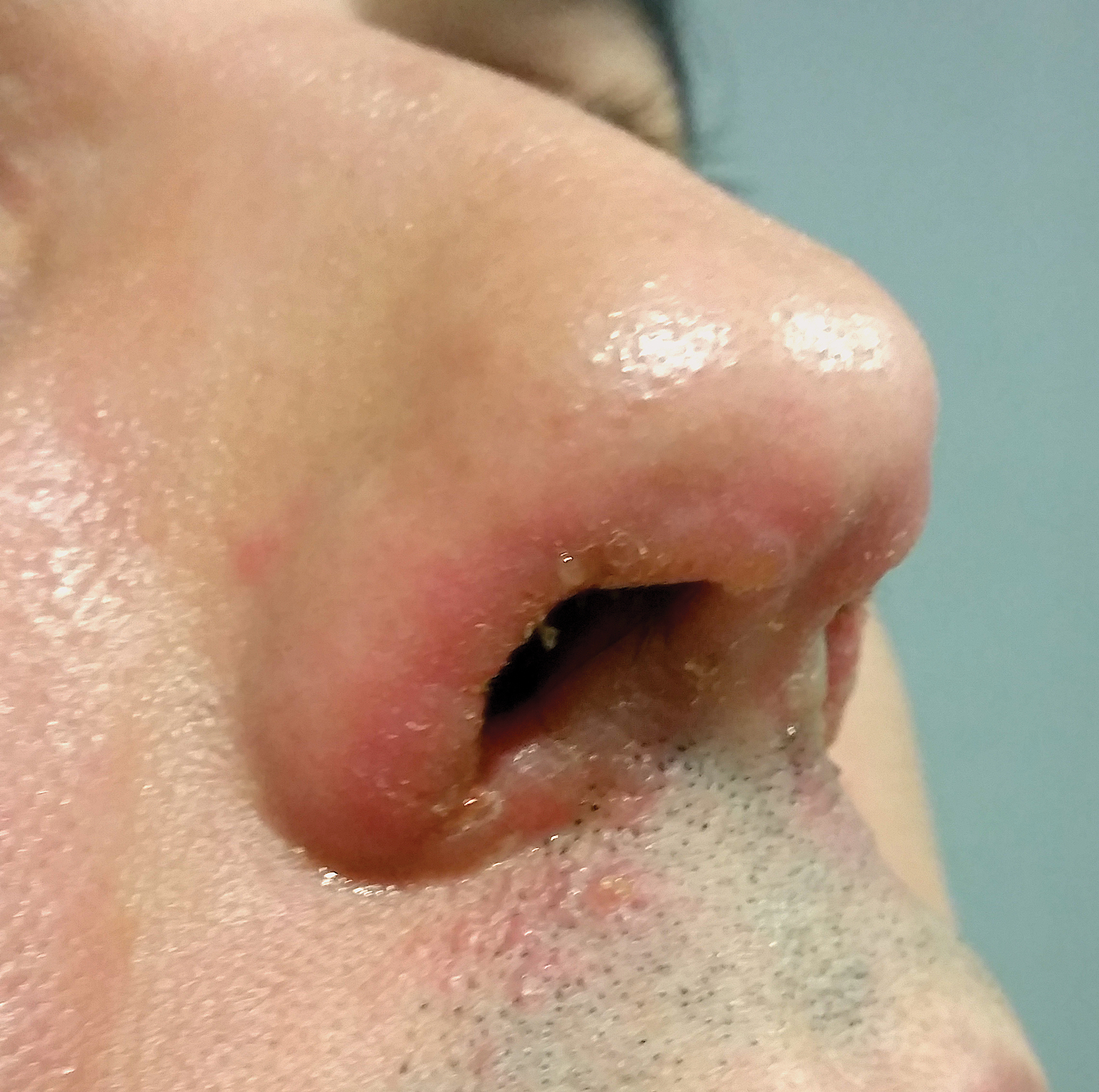
A 44-year-old man was referred to the department of dermatology for a pruriginous nasal rash. Physical examination revealed vesicles with clear content and crusts symmetrically in both nostrils and philtra. The remainder of the examination was otherwise unremarkable. The patient reported inhalation of poppers the prior night during a party. No history of connective tissue diseases was present. The patient was in overall good health with no fever or chills.
Allergic Contact Dermatitis From Sorbitans in Beer and Bread
Sorbitan sesquioleate (SSO), sorbitan monooleate (SMO), and related compounds are increasingly recognized contact allergens. Sorbitan sesquioleate and SMO are nonionic emulsifying agents derived from sorbitol.1
Sorbitan sesquioleate, SMO, and other sorbitol derivatives are used as emulsifiers and dispersing agents in cosmetics, topical medications, topical emollients, produce, and other commercial products. Related compounds also are found in foods such as apples, berries, cherries, and sucrose-free cakes and cookies.1 We present a case of allergic contact dermatitis (ACD) with positive patch testing to sorbitans and clinical correlation with beer and bread exposure.
Case Report
A 62-year-old man presented with a persistent pruritic rash of 6 months’ duration. Erythematous eczematous papules and plaques were observed on the face, neck, chest, abdomen, back, and upper and lower extremities, affecting approximately 60% of the body surface area. His current list of medications was reviewed and included a multivitamin, fish oil, and vitamin C. A punch biopsy revealed spongiotic dermatitis with eosinophils. Patch testing using the North American Contact Dermatitis Group Standard Series with supplemental allergens found in toiletries revealed a positive reaction to SSO and SMO that was persistent at 48 and 96 hours. Notably, patch testing for sodium benzoate, nickel, potassium dichromate, and balsam of Peru were negative. Investigation into the personal care products the patient used identified the presence of sorbitol solution in Vanicream bar soap and Vanicream moisturizing cream (Pharmaceutical Specialties Inc). These products were started after the development of the rash and were discontinued after positive patch testing, but the patient continued to experience the eruption with no improvement.
Retrospectively, the patient was able to correlate exacerbations with drinking beer and eating sandwiches. He habitually ate a sandwich on the same type of bread every single day and enjoyed the same brand of beer 2 to 4 times per week without much variation. To limit allergens, the patient gave up the daily sandwich and avoided bread altogether, noting remarkable clinical improvement over a few weeks. Later, he described even more improvement while on a trip where he did not have access to his usual beer. The eruption recurred when he returned home and excessively indulged in his favorite beer. He also noted recurrence with exposure to certain breads. No new lesions developed with avoidance of beer and bread, and he had less than 1% body surface area involvement at 2-month follow-up and 0% involvement at 1 year. For educational purposes, follow-up patch testing was performed using Vanicream sorbitol solution and the specific beer and bread the patient consumed. The Vanicream solution was obtained from the manufacturer. The beer was placed directly onto a test disc. The bread was moistened with a drop of saline and then placed directly onto a test disc. All were negative at 48 and 96 hours.
Comment
Sorbitol Ingredients
We report a case of systemic ACD with a positive patch test to sorbitans that was exacerbated with consumption of beer and bread and resolved with avoidance of these products. Although it was determined that the patient used personal care products containing a sorbitol solution, discontinuation did not result in clinical improvement. Sorbitol, sorbitans, and sorbitol derivatives are not commonly reported in the ingredient lists of foods such as beer and bread. Both beer and bread are created with the addition of yeast cultures, for fermentation in beer and for leavening in bread. Sorbitol is used as an osmotic stabilizer in the preparation of yeast strains2 and also is a by-product of fermentation by certain bacteria3 found in beer. Additionally, review of commercially available preparations of baker’s and brewer’s yeasts, such as Fleischmann’s and Red Star, list sorbitan monostearate in the ingredients.4-7 We propose that trace amounts are present in the yeast preparations for brewing and baking.
In this case, the offending beer and bread were locally made products (Abita Beer, Covington, Louisiana; Leidenheimer Bread, New Orleans, Louisiana). Both companies were unable to share their yeast sources, limiting our ability to confirm the use of sorbitol in their preparation. We hypothesize that if sorbitol is commonly used in yeast culture preparation and can be a by-product of fermentation, then it is present in trace amounts in many beers and breads and is not specific to these two products.
Contact Allergy
There are few prior reports of ACD due to beer. A case series in 1969 described 4 patients with positive patch testing to ethanol and alcohol by-products and clinical resolution with avoidance of alcohol.8 Another case from 1985 described ACD to beer where patch testing was positive to the beer itself.9 Other published cases of cutaneous reactions to beer demonstrated immediate-type hypersensitivity resulting from both ingestion and skin contact, which is thought to be caused by IgE antibodies to malt and barley proteins.10,11
It is important to distinguish between systemic ACD and oral allergy syndrome (OAS). Although the defining features and criteria for diagnosing OAS have not been officially established, OAS is an IgE-mediated immune reaction commonly described as itching, tingling, or swelling, usually confined to the oral cavity after recent consumption of foods such as raw fruits, vegetables, and nuts.12 Oral allergy syndrome is treated with antihistamines and avoidance of known food allergens. In comparison, ACD is a type IV hypersensitivity, delayed cell-mediated reaction, commonly presenting with widespread rash.
Occupational contact dermatitis is common in bakers and food handlers and is more often irritant than allergic. Several relevant allergens have been identified in these groups13,14 and do not include sorbitans; our patient tested positive to both SSO and SMO. Sorbitan sesquioleate and SMO have been increasingly recognized as contact allergens over the last several years, both as standalone allergens and as potential cross-reactors.1 Sorbitan sesquioleate, SMO, and other sorbitol derivatives are found in cosmetics, topical and oral medications, topical emollients, produce, and other commercial products, including but not limited to topical clindamycin, topical metronidazole, topical ketoconazole, tazarotene cream 0.05% and 0.1%, toothpastes, acetaminophen maximum strength liquid, apples, berries, and sucrose-free cakes and cookies.1,15,16
In 2014, a study evaluated 12 oral antihistamines as potential sources for systemic contact allergens; 55% of these 12 oral antihistamine preparations included at least 1 of 10 allergen groups specifically identified. The sorbitans and sorbitol derivatives group ranked highest among the group of allergens found listed in these oral medications.17
Most patients found to have a contact allergy to the products containing SSO, SMO, or sorbitol derivatives reported notable improvement with discontinuation and change to sorbitol-free product use.1,18 It should be noted that SSO is added as an emulsifier to many of the fragrances used for patch testing. A positive patch test to fragrance mix without concomitant sorbitan testing may incorrectly diagnose the allergen.19
Patients with atopic dermatitis, particularly those with a filaggrin mutation, are at increased risk for ACD to sorbitans due to a compromised skin barrier and frequent use of topical steroids. In one study, 75% of patients (n=12) with a positive patch test to SSO were using a topical steroid emulsified with sorbitol or sorbitan derivatives.19
Conclusion
Sorbitan sesquioleate and SMO are increasingly relevant contact allergens. Sorbitol and related substances have been identified in numerous products and may be present in yeast-fermented and leavened goods. When patch testing is positive to SSO and SMO, the dermatologist should inquire about dietary habits with specific attention to beer and bread, in addition to inventorying other dietary preferences, prescription and over-the-counter medications, and personal care products. We suggest dietary considerations only if topical exposures have been eliminated and the rash has not improved.
- Asarch A, Scheinman PL. Sorbitan sesquioleate: an emerging contact allergen. Dermatitis. 2008;19:339-341.
- Lundblad V, Struhl K. Yeast. In: Adelman K, Ausubel F, Brent R, et al. Current Protocols in Molecular Biology, Supplement 64. New York, NY: John Wiley & Sons, Inc; 2008:13.0.1-13.0.4. https://onlinelibrary.wiley.com. Accessed August 19, 2019.
- Spitaels F, Wieme A, Balzarini T, et al. Gluconobacter cerevisiae sp. nov., isolated from the brewery environment. Int J Sys Evol Microbiol. 2014;64(pt 4):1134-1141.
- Fleischmann’s, n.d. Product Label for Rapid Rise Instant Yeast. Memphis, TN. 2017.
- Fleischmann’s, n.d. Product Label for Active Dry Yeast. Memphis, TN. 2017.
- Red Star, n.d. Product Label for Quick-Rise. Milwaukee, WI. 2017.
- Red Star, n.d. Product Label for Platinum Superior Baking Yeast. Milwaukee, WI. 2017.
- Fregert S, Groth O, Hjorth N, et al. Alcohol dermatitis. Acta Derm Venereol. 1969;49:493-497.
- Clarke P. Contact dermatitis due to beer. Med J Aust. 1985;143:92.
- Koelemij I, Van Zuuren EJ. Contact urticaria from beer. Clin Exp Dermatol. 2014;39:395-407.
- Santucci B, Cristaudo A, Cannistraci C, et al. Urticaria from beer in 3 patients. Contact Dermatitis. 1996;34:368.
- Kohn JB. What is oral allergy syndrome? J Acad Nutr Diet. 2017;117:988.
- Vincenzi C, Stinchi C, Ricci C, et al. Contact dermatitis due to an emulsifying agent in a baker. Contact Dermatitis. 1995;32:57.
- Nethercott JR, Holness DL. Occupational dermatitis in food handlers and bakers. J Am Acad Dermatol. 1989;21:485-490.
- Pereira F, Cunha H, Dias M. Contact dermatitis due to emulsifiers. Contact Dermatitis. 1997;36:114.
- Gao Z, Maurousset L, Lemoine R, et al. Cloning, expression, and characterization of sorbitol transporters from developing sour cherry fruit and leaf sink tissues. Plant Physiol. 2003;131:1566-1575.
- McEnery-Stonelake M, Silvestri DL. Contact allergens in oral antihistamines. Dermatitis. 2014;25:83-88.
- Asarch A, Scheinman PL. Sorbitan sesquioleate, a common emulsifier in topical steroids, is an important contact allergen. Dermatitis. 2008;19:323-327.
- Hald M, Menné T, Johansen JD, et al. Allergic contact dermatitis caused by sorbitan sesquioleate imitating severe glove dermatitis in a patient with filaggrin mutation. Contact Dermatitis. 2013;69:311-322.
Sorbitan sesquioleate (SSO), sorbitan monooleate (SMO), and related compounds are increasingly recognized contact allergens. Sorbitan sesquioleate and SMO are nonionic emulsifying agents derived from sorbitol.1
Sorbitan sesquioleate, SMO, and other sorbitol derivatives are used as emulsifiers and dispersing agents in cosmetics, topical medications, topical emollients, produce, and other commercial products. Related compounds also are found in foods such as apples, berries, cherries, and sucrose-free cakes and cookies.1 We present a case of allergic contact dermatitis (ACD) with positive patch testing to sorbitans and clinical correlation with beer and bread exposure.
Case Report
A 62-year-old man presented with a persistent pruritic rash of 6 months’ duration. Erythematous eczematous papules and plaques were observed on the face, neck, chest, abdomen, back, and upper and lower extremities, affecting approximately 60% of the body surface area. His current list of medications was reviewed and included a multivitamin, fish oil, and vitamin C. A punch biopsy revealed spongiotic dermatitis with eosinophils. Patch testing using the North American Contact Dermatitis Group Standard Series with supplemental allergens found in toiletries revealed a positive reaction to SSO and SMO that was persistent at 48 and 96 hours. Notably, patch testing for sodium benzoate, nickel, potassium dichromate, and balsam of Peru were negative. Investigation into the personal care products the patient used identified the presence of sorbitol solution in Vanicream bar soap and Vanicream moisturizing cream (Pharmaceutical Specialties Inc). These products were started after the development of the rash and were discontinued after positive patch testing, but the patient continued to experience the eruption with no improvement.
Retrospectively, the patient was able to correlate exacerbations with drinking beer and eating sandwiches. He habitually ate a sandwich on the same type of bread every single day and enjoyed the same brand of beer 2 to 4 times per week without much variation. To limit allergens, the patient gave up the daily sandwich and avoided bread altogether, noting remarkable clinical improvement over a few weeks. Later, he described even more improvement while on a trip where he did not have access to his usual beer. The eruption recurred when he returned home and excessively indulged in his favorite beer. He also noted recurrence with exposure to certain breads. No new lesions developed with avoidance of beer and bread, and he had less than 1% body surface area involvement at 2-month follow-up and 0% involvement at 1 year. For educational purposes, follow-up patch testing was performed using Vanicream sorbitol solution and the specific beer and bread the patient consumed. The Vanicream solution was obtained from the manufacturer. The beer was placed directly onto a test disc. The bread was moistened with a drop of saline and then placed directly onto a test disc. All were negative at 48 and 96 hours.
Comment
Sorbitol Ingredients
We report a case of systemic ACD with a positive patch test to sorbitans that was exacerbated with consumption of beer and bread and resolved with avoidance of these products. Although it was determined that the patient used personal care products containing a sorbitol solution, discontinuation did not result in clinical improvement. Sorbitol, sorbitans, and sorbitol derivatives are not commonly reported in the ingredient lists of foods such as beer and bread. Both beer and bread are created with the addition of yeast cultures, for fermentation in beer and for leavening in bread. Sorbitol is used as an osmotic stabilizer in the preparation of yeast strains2 and also is a by-product of fermentation by certain bacteria3 found in beer. Additionally, review of commercially available preparations of baker’s and brewer’s yeasts, such as Fleischmann’s and Red Star, list sorbitan monostearate in the ingredients.4-7 We propose that trace amounts are present in the yeast preparations for brewing and baking.
In this case, the offending beer and bread were locally made products (Abita Beer, Covington, Louisiana; Leidenheimer Bread, New Orleans, Louisiana). Both companies were unable to share their yeast sources, limiting our ability to confirm the use of sorbitol in their preparation. We hypothesize that if sorbitol is commonly used in yeast culture preparation and can be a by-product of fermentation, then it is present in trace amounts in many beers and breads and is not specific to these two products.
Contact Allergy
There are few prior reports of ACD due to beer. A case series in 1969 described 4 patients with positive patch testing to ethanol and alcohol by-products and clinical resolution with avoidance of alcohol.8 Another case from 1985 described ACD to beer where patch testing was positive to the beer itself.9 Other published cases of cutaneous reactions to beer demonstrated immediate-type hypersensitivity resulting from both ingestion and skin contact, which is thought to be caused by IgE antibodies to malt and barley proteins.10,11
It is important to distinguish between systemic ACD and oral allergy syndrome (OAS). Although the defining features and criteria for diagnosing OAS have not been officially established, OAS is an IgE-mediated immune reaction commonly described as itching, tingling, or swelling, usually confined to the oral cavity after recent consumption of foods such as raw fruits, vegetables, and nuts.12 Oral allergy syndrome is treated with antihistamines and avoidance of known food allergens. In comparison, ACD is a type IV hypersensitivity, delayed cell-mediated reaction, commonly presenting with widespread rash.
Occupational contact dermatitis is common in bakers and food handlers and is more often irritant than allergic. Several relevant allergens have been identified in these groups13,14 and do not include sorbitans; our patient tested positive to both SSO and SMO. Sorbitan sesquioleate and SMO have been increasingly recognized as contact allergens over the last several years, both as standalone allergens and as potential cross-reactors.1 Sorbitan sesquioleate, SMO, and other sorbitol derivatives are found in cosmetics, topical and oral medications, topical emollients, produce, and other commercial products, including but not limited to topical clindamycin, topical metronidazole, topical ketoconazole, tazarotene cream 0.05% and 0.1%, toothpastes, acetaminophen maximum strength liquid, apples, berries, and sucrose-free cakes and cookies.1,15,16
In 2014, a study evaluated 12 oral antihistamines as potential sources for systemic contact allergens; 55% of these 12 oral antihistamine preparations included at least 1 of 10 allergen groups specifically identified. The sorbitans and sorbitol derivatives group ranked highest among the group of allergens found listed in these oral medications.17
Most patients found to have a contact allergy to the products containing SSO, SMO, or sorbitol derivatives reported notable improvement with discontinuation and change to sorbitol-free product use.1,18 It should be noted that SSO is added as an emulsifier to many of the fragrances used for patch testing. A positive patch test to fragrance mix without concomitant sorbitan testing may incorrectly diagnose the allergen.19
Patients with atopic dermatitis, particularly those with a filaggrin mutation, are at increased risk for ACD to sorbitans due to a compromised skin barrier and frequent use of topical steroids. In one study, 75% of patients (n=12) with a positive patch test to SSO were using a topical steroid emulsified with sorbitol or sorbitan derivatives.19
Conclusion
Sorbitan sesquioleate and SMO are increasingly relevant contact allergens. Sorbitol and related substances have been identified in numerous products and may be present in yeast-fermented and leavened goods. When patch testing is positive to SSO and SMO, the dermatologist should inquire about dietary habits with specific attention to beer and bread, in addition to inventorying other dietary preferences, prescription and over-the-counter medications, and personal care products. We suggest dietary considerations only if topical exposures have been eliminated and the rash has not improved.
Sorbitan sesquioleate (SSO), sorbitan monooleate (SMO), and related compounds are increasingly recognized contact allergens. Sorbitan sesquioleate and SMO are nonionic emulsifying agents derived from sorbitol.1
Sorbitan sesquioleate, SMO, and other sorbitol derivatives are used as emulsifiers and dispersing agents in cosmetics, topical medications, topical emollients, produce, and other commercial products. Related compounds also are found in foods such as apples, berries, cherries, and sucrose-free cakes and cookies.1 We present a case of allergic contact dermatitis (ACD) with positive patch testing to sorbitans and clinical correlation with beer and bread exposure.
Case Report
A 62-year-old man presented with a persistent pruritic rash of 6 months’ duration. Erythematous eczematous papules and plaques were observed on the face, neck, chest, abdomen, back, and upper and lower extremities, affecting approximately 60% of the body surface area. His current list of medications was reviewed and included a multivitamin, fish oil, and vitamin C. A punch biopsy revealed spongiotic dermatitis with eosinophils. Patch testing using the North American Contact Dermatitis Group Standard Series with supplemental allergens found in toiletries revealed a positive reaction to SSO and SMO that was persistent at 48 and 96 hours. Notably, patch testing for sodium benzoate, nickel, potassium dichromate, and balsam of Peru were negative. Investigation into the personal care products the patient used identified the presence of sorbitol solution in Vanicream bar soap and Vanicream moisturizing cream (Pharmaceutical Specialties Inc). These products were started after the development of the rash and were discontinued after positive patch testing, but the patient continued to experience the eruption with no improvement.
Retrospectively, the patient was able to correlate exacerbations with drinking beer and eating sandwiches. He habitually ate a sandwich on the same type of bread every single day and enjoyed the same brand of beer 2 to 4 times per week without much variation. To limit allergens, the patient gave up the daily sandwich and avoided bread altogether, noting remarkable clinical improvement over a few weeks. Later, he described even more improvement while on a trip where he did not have access to his usual beer. The eruption recurred when he returned home and excessively indulged in his favorite beer. He also noted recurrence with exposure to certain breads. No new lesions developed with avoidance of beer and bread, and he had less than 1% body surface area involvement at 2-month follow-up and 0% involvement at 1 year. For educational purposes, follow-up patch testing was performed using Vanicream sorbitol solution and the specific beer and bread the patient consumed. The Vanicream solution was obtained from the manufacturer. The beer was placed directly onto a test disc. The bread was moistened with a drop of saline and then placed directly onto a test disc. All were negative at 48 and 96 hours.
Comment
Sorbitol Ingredients
We report a case of systemic ACD with a positive patch test to sorbitans that was exacerbated with consumption of beer and bread and resolved with avoidance of these products. Although it was determined that the patient used personal care products containing a sorbitol solution, discontinuation did not result in clinical improvement. Sorbitol, sorbitans, and sorbitol derivatives are not commonly reported in the ingredient lists of foods such as beer and bread. Both beer and bread are created with the addition of yeast cultures, for fermentation in beer and for leavening in bread. Sorbitol is used as an osmotic stabilizer in the preparation of yeast strains2 and also is a by-product of fermentation by certain bacteria3 found in beer. Additionally, review of commercially available preparations of baker’s and brewer’s yeasts, such as Fleischmann’s and Red Star, list sorbitan monostearate in the ingredients.4-7 We propose that trace amounts are present in the yeast preparations for brewing and baking.
In this case, the offending beer and bread were locally made products (Abita Beer, Covington, Louisiana; Leidenheimer Bread, New Orleans, Louisiana). Both companies were unable to share their yeast sources, limiting our ability to confirm the use of sorbitol in their preparation. We hypothesize that if sorbitol is commonly used in yeast culture preparation and can be a by-product of fermentation, then it is present in trace amounts in many beers and breads and is not specific to these two products.
Contact Allergy
There are few prior reports of ACD due to beer. A case series in 1969 described 4 patients with positive patch testing to ethanol and alcohol by-products and clinical resolution with avoidance of alcohol.8 Another case from 1985 described ACD to beer where patch testing was positive to the beer itself.9 Other published cases of cutaneous reactions to beer demonstrated immediate-type hypersensitivity resulting from both ingestion and skin contact, which is thought to be caused by IgE antibodies to malt and barley proteins.10,11
It is important to distinguish between systemic ACD and oral allergy syndrome (OAS). Although the defining features and criteria for diagnosing OAS have not been officially established, OAS is an IgE-mediated immune reaction commonly described as itching, tingling, or swelling, usually confined to the oral cavity after recent consumption of foods such as raw fruits, vegetables, and nuts.12 Oral allergy syndrome is treated with antihistamines and avoidance of known food allergens. In comparison, ACD is a type IV hypersensitivity, delayed cell-mediated reaction, commonly presenting with widespread rash.
Occupational contact dermatitis is common in bakers and food handlers and is more often irritant than allergic. Several relevant allergens have been identified in these groups13,14 and do not include sorbitans; our patient tested positive to both SSO and SMO. Sorbitan sesquioleate and SMO have been increasingly recognized as contact allergens over the last several years, both as standalone allergens and as potential cross-reactors.1 Sorbitan sesquioleate, SMO, and other sorbitol derivatives are found in cosmetics, topical and oral medications, topical emollients, produce, and other commercial products, including but not limited to topical clindamycin, topical metronidazole, topical ketoconazole, tazarotene cream 0.05% and 0.1%, toothpastes, acetaminophen maximum strength liquid, apples, berries, and sucrose-free cakes and cookies.1,15,16
In 2014, a study evaluated 12 oral antihistamines as potential sources for systemic contact allergens; 55% of these 12 oral antihistamine preparations included at least 1 of 10 allergen groups specifically identified. The sorbitans and sorbitol derivatives group ranked highest among the group of allergens found listed in these oral medications.17
Most patients found to have a contact allergy to the products containing SSO, SMO, or sorbitol derivatives reported notable improvement with discontinuation and change to sorbitol-free product use.1,18 It should be noted that SSO is added as an emulsifier to many of the fragrances used for patch testing. A positive patch test to fragrance mix without concomitant sorbitan testing may incorrectly diagnose the allergen.19
Patients with atopic dermatitis, particularly those with a filaggrin mutation, are at increased risk for ACD to sorbitans due to a compromised skin barrier and frequent use of topical steroids. In one study, 75% of patients (n=12) with a positive patch test to SSO were using a topical steroid emulsified with sorbitol or sorbitan derivatives.19
Conclusion
Sorbitan sesquioleate and SMO are increasingly relevant contact allergens. Sorbitol and related substances have been identified in numerous products and may be present in yeast-fermented and leavened goods. When patch testing is positive to SSO and SMO, the dermatologist should inquire about dietary habits with specific attention to beer and bread, in addition to inventorying other dietary preferences, prescription and over-the-counter medications, and personal care products. We suggest dietary considerations only if topical exposures have been eliminated and the rash has not improved.
- Asarch A, Scheinman PL. Sorbitan sesquioleate: an emerging contact allergen. Dermatitis. 2008;19:339-341.
- Lundblad V, Struhl K. Yeast. In: Adelman K, Ausubel F, Brent R, et al. Current Protocols in Molecular Biology, Supplement 64. New York, NY: John Wiley & Sons, Inc; 2008:13.0.1-13.0.4. https://onlinelibrary.wiley.com. Accessed August 19, 2019.
- Spitaels F, Wieme A, Balzarini T, et al. Gluconobacter cerevisiae sp. nov., isolated from the brewery environment. Int J Sys Evol Microbiol. 2014;64(pt 4):1134-1141.
- Fleischmann’s, n.d. Product Label for Rapid Rise Instant Yeast. Memphis, TN. 2017.
- Fleischmann’s, n.d. Product Label for Active Dry Yeast. Memphis, TN. 2017.
- Red Star, n.d. Product Label for Quick-Rise. Milwaukee, WI. 2017.
- Red Star, n.d. Product Label for Platinum Superior Baking Yeast. Milwaukee, WI. 2017.
- Fregert S, Groth O, Hjorth N, et al. Alcohol dermatitis. Acta Derm Venereol. 1969;49:493-497.
- Clarke P. Contact dermatitis due to beer. Med J Aust. 1985;143:92.
- Koelemij I, Van Zuuren EJ. Contact urticaria from beer. Clin Exp Dermatol. 2014;39:395-407.
- Santucci B, Cristaudo A, Cannistraci C, et al. Urticaria from beer in 3 patients. Contact Dermatitis. 1996;34:368.
- Kohn JB. What is oral allergy syndrome? J Acad Nutr Diet. 2017;117:988.
- Vincenzi C, Stinchi C, Ricci C, et al. Contact dermatitis due to an emulsifying agent in a baker. Contact Dermatitis. 1995;32:57.
- Nethercott JR, Holness DL. Occupational dermatitis in food handlers and bakers. J Am Acad Dermatol. 1989;21:485-490.
- Pereira F, Cunha H, Dias M. Contact dermatitis due to emulsifiers. Contact Dermatitis. 1997;36:114.
- Gao Z, Maurousset L, Lemoine R, et al. Cloning, expression, and characterization of sorbitol transporters from developing sour cherry fruit and leaf sink tissues. Plant Physiol. 2003;131:1566-1575.
- McEnery-Stonelake M, Silvestri DL. Contact allergens in oral antihistamines. Dermatitis. 2014;25:83-88.
- Asarch A, Scheinman PL. Sorbitan sesquioleate, a common emulsifier in topical steroids, is an important contact allergen. Dermatitis. 2008;19:323-327.
- Hald M, Menné T, Johansen JD, et al. Allergic contact dermatitis caused by sorbitan sesquioleate imitating severe glove dermatitis in a patient with filaggrin mutation. Contact Dermatitis. 2013;69:311-322.
- Asarch A, Scheinman PL. Sorbitan sesquioleate: an emerging contact allergen. Dermatitis. 2008;19:339-341.
- Lundblad V, Struhl K. Yeast. In: Adelman K, Ausubel F, Brent R, et al. Current Protocols in Molecular Biology, Supplement 64. New York, NY: John Wiley & Sons, Inc; 2008:13.0.1-13.0.4. https://onlinelibrary.wiley.com. Accessed August 19, 2019.
- Spitaels F, Wieme A, Balzarini T, et al. Gluconobacter cerevisiae sp. nov., isolated from the brewery environment. Int J Sys Evol Microbiol. 2014;64(pt 4):1134-1141.
- Fleischmann’s, n.d. Product Label for Rapid Rise Instant Yeast. Memphis, TN. 2017.
- Fleischmann’s, n.d. Product Label for Active Dry Yeast. Memphis, TN. 2017.
- Red Star, n.d. Product Label for Quick-Rise. Milwaukee, WI. 2017.
- Red Star, n.d. Product Label for Platinum Superior Baking Yeast. Milwaukee, WI. 2017.
- Fregert S, Groth O, Hjorth N, et al. Alcohol dermatitis. Acta Derm Venereol. 1969;49:493-497.
- Clarke P. Contact dermatitis due to beer. Med J Aust. 1985;143:92.
- Koelemij I, Van Zuuren EJ. Contact urticaria from beer. Clin Exp Dermatol. 2014;39:395-407.
- Santucci B, Cristaudo A, Cannistraci C, et al. Urticaria from beer in 3 patients. Contact Dermatitis. 1996;34:368.
- Kohn JB. What is oral allergy syndrome? J Acad Nutr Diet. 2017;117:988.
- Vincenzi C, Stinchi C, Ricci C, et al. Contact dermatitis due to an emulsifying agent in a baker. Contact Dermatitis. 1995;32:57.
- Nethercott JR, Holness DL. Occupational dermatitis in food handlers and bakers. J Am Acad Dermatol. 1989;21:485-490.
- Pereira F, Cunha H, Dias M. Contact dermatitis due to emulsifiers. Contact Dermatitis. 1997;36:114.
- Gao Z, Maurousset L, Lemoine R, et al. Cloning, expression, and characterization of sorbitol transporters from developing sour cherry fruit and leaf sink tissues. Plant Physiol. 2003;131:1566-1575.
- McEnery-Stonelake M, Silvestri DL. Contact allergens in oral antihistamines. Dermatitis. 2014;25:83-88.
- Asarch A, Scheinman PL. Sorbitan sesquioleate, a common emulsifier in topical steroids, is an important contact allergen. Dermatitis. 2008;19:323-327.
- Hald M, Menné T, Johansen JD, et al. Allergic contact dermatitis caused by sorbitan sesquioleate imitating severe glove dermatitis in a patient with filaggrin mutation. Contact Dermatitis. 2013;69:311-322.
Practice Points
- Sorbitan sesquioleate (SSO) and sorbitan monooleate (SMO) are increasingly relevant contact allergens that may be present in yeast-fermented and leavened products.
- When patch testing is positive to SSO and SMO, the dermatologist should inquire about dietary habits with specific attention to beer and bread.
- Consider elimination of beer, bread, and other leavened products when rash persists after avoidance of topical exposures.
Beware of natural fruit and nut ingredients in latex-allergic patients
It has been 40 years since the first reported case of IgE-mediated natural rubber latex allergy, which was soon followed by a global epidemic of allergic and anaphylactic reactions.1,2 Resolution came through insightful work in the 1990s that led to the removal of cornstarch powder and a switch to nonpowdered latex and synthetic examination gloves.2 Also discovered during this period was the cross-reactivity of many patients to latex and various fruits.
Research substantiates reports
Blanco et al. conducted a prospective study in their outpatient clinic in 25 patients diagnosed with latex allergy, published in 1994.They used a clinical questionnaire, skin-prick tests, skin test with a latex extract, and identification of total and specific IgE to help ascertain clinical characteristics and cross-reactivity. Of the 23 women and 2 men in the study (mean age 33, plus or minus 9 years), 9 (36%) experienced latex-induced reactions characterized by systemic anaphylaxis. In 13 patients (52%), 42 food allergies were identified, and 23 included systemic anaphylaxis. Avocado (9), chestnut (9), banana (7), kiwi (5), and papaya (3) were the most common foods to cause hypersensitivities. The researchers concluded that their small study supported the reality of a “latex-fruit syndrome.”3
Another study aimed to characterize the cross-reactivity of latex and foods and evaluate clinical significance. Beezhold et al. examined 47 patients allergic to latex and 46 nonallergic controls. The investigators found immunologic reactivity to foods to be prevalent (33 latex-allergic patients and seven controls), with 27% of food skin-prick tests positive in the latex-allergic group. In addition, clinical symptoms were linked to 27% of positive skin-prick tests. Among the 17 patients who displayed clinical allergies to at least one food, 14 showed local sensitivity reactions, with anaphylaxis noted in 11. Avocado (53%), potato (40%), banana (38%), tomato (28%), chestnut (28%), and kiwi (17%) were the foods most frequently cited for provoking a skin test reaction. The authors observed extensive cross-reactivity between latex sensitivity and particular foods, with potatoes and tomatoes reported for the first time.4
In 1997, Brehler et al. studied serum samples from 136 patients whose immediate hypersensitivity to latex proteins was clinically observable and documented. The samples were assessed for IgE antibodies against several fruits, with fruit-specific IgE antibodies recorded in 69.1%. Radioallergosorbent (RAST) -inhibition tests yielded the recognition of cross-reacting IgE antibodies in latex and multiple fruit allergens: avocado, banana, chestnut, fig, kiwi, mango, melon, papaya, passion fruit, peach, pineapple, and tomato. The investigators recorded 112 intolerance reactions and noted that 42.5% of their patients reported allergic symptoms after consuming these fruits. Fruit-specific IgE antibodies were detected in only 32.1% of these patients, suggesting to the researchers that serologic tests were suboptimal in forecasting food hypersensitivities in patients who are allergic to latex.5
Cross-reactivity with banana
Mäkinen-Kiljunen studied 47 patients to investigate banana allergy in patients with latex allergy in 1994, measuring latex-, banana-, and pollen-specific (birch, timothy, and mugwort) IgE. Thirty-one patients were also given skin-prick tests with banana and were queried about reactions after consuming bananas. Of the 47 sera samples, latex RAST results were positive in 31 and banana RAST results in 26. RAST results from latex and banana were correlated (25 of the 31 latex RAST-positive samples were also banana RAST-positive), but not with pollen. Sixteen of the 31 patients who ate banana reported symptoms, and 11 of the 31 patients given the banana skin-prick test showed positive results. The author confirmed the cross-reactivity of IgE antibodies for latex and banana, identifying for the first time a structurally similar antigen/allergen as at least one antigen from banana fused with an antigen from latex in crossed-line immunoelectrophoresis.6
In 1998, Mikkola et al. investigated whether proteins similar to hevein, a major natural rubber latex allergen, are present in banana and account for cross-reactivity between these botanicals. Immunoblotting revealed that 9 of 15 sera from latex-allergic patients with IgE to hevein also bound to 32- and 33-kd banana proteins. Studies using ELISA [enzyme-linked immunosorbent assay] showed that the common presentation of hypersensitivity to banana among patients allergic to latex could be attributed to cross-reacting IgE antibodies binding to epitopes in hevein and in the then-newly identified hevein-like endochitinase found in banana.7
Cross-reactivity with avocado
In response to reports of an association between allergy to natural rubber latex and avocado, Ahlroth et al. investigated cross-reactive proteins between natural rubber latex and avocado in 1995 by using skin-prick tests with fresh avocado on 11 patients and the sera of 18 patients with known latex allergy for IgE antibodies. Fourteen of the 18 sera were found to have IgE antibodies binding to 17 distinct avocado proteins, with multiple immunoblot experiments and skin-prick test results (positive in 7 of 11 patients) revealing marked immunologic cross-reactivity between latex and avocado.8
In 1998, Chen et al. set out to identify the cross-sensitizing allergen between latex and avocado, with hevein suspected. The researchers looked at sera samples from 118 health care workers allergic to latex and 78 patients with spina bifida who were allergic to latex. They noted a robust correlation between the prevalence of seropositive IgE antibodies to avocado in the presence of hevein-specific IgE antibodies in both groups. All members in the spina bifida group and 91 (73%) of the health care workers had positive IgE antibodies to hevein and high IgE values to avocado. Additional results supported the conclusion that sensitization to avocado in the majority of people allergic to latex is engendered by IgE-binding epitopes found in hevein.9
A year later, Diaz-Perales et al. considered the potential relevance of chitinases and complex glycans as factors in the then newly described latex/food syndrome, particularly in avocado, banana, and chestnuts. The investigators culled extracts from 20 various plant foods as well as latex. In immunoblot inhibition assays, the primary allergen and class I chitinase in avocado, Prs a 1, and the latex extract potently or completely blocked IgE binding by these constituents. Polyclonal antibodies to chitinases and sera from patients with latex/fruit allergy responded to reactive proteins of about 30-45 kd (putative class I chitinases) in chestnut, cherimoya, kiwi, mango, papaya, passion fruit, tomato, and wheat flour extracts. The glycans complex was deemed to be irrelevant in latex/fruit cross-reactivity, but the researchers found the putative class I chitinases to be notable players in the latex/fruit syndrome.10
According to Wagner and Breitender, anywhere from 30%-50% of people with known latex allergy also evince a related hypersensitivity or allergy to various plant-derived foods, with avocado, banana, chestnut, kiwi, peach, tomato, potato, and bell pepper among the foods most frequently linked to latex/fruit syndrome. They summarize that several plant defense proteins have been shown to be involved in the syndrome, with the most prominent, class I chitinases with an N-terminal hevein-like domain, having been found to cross-react with hevein (Hev b 6.02), a major IgE-binding allergen for individuals allergic to latex. A beta-1,3-glucanase, a key latex allergen, has also shown cross-reactivity with proteins of bell pepper, and another significant latex allergen, Hev b 7, a patatin-like protein, cross-reacts with its analogous protein in potato.11
Conclusion
It is unknown whether latex allergy precedes or follows food allergy.11 The latex/food syndrome itself merits attention as a significant source of hypersensitivity to natural cosmeceutical ingredients. Dermatologists should be aware of the lengthy list of cross-reacting plant-derived products, particularly when it comes to reviewing topical product ingredients with susceptible or allergic patients. Latex-allergic patients may react to these natural ingredients in food or when topically applied to the skin.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Nutter AF. Br J Dermatol 1979 Nov;101(5):597-8.
2. Kelly KJ et al. J Allergy Clin Immunol Pract. 2017 Sep-Oct;5(5):1212-16.
3. Blanco C et al. Ann Allergy. 1994 Oct;73(4):309-14.
4. Beezhold DH et al. Clin Exp Allergy. 1996 Apr;26(4):416-22.
5. Brehler R et al. Allergy. 1997 Apr;52(4):404-10.
6. Mäkinen-Kiljunen S. J Allergy Clin Immunol. 1994 Jun;93(6):990-6.
7. Mikkola JH et al. J Allergy Clin Immunol. 1998 Dec;102(6 Pt 1):1005-12.
8. Ahlroth M et al. J Allergy Clin Immunol. 1995 Aug;96(2):167-73.
9. Chen Z et al. J Allergy Clin Immunol. 1998 Sep;102(3):476-81.
10. Diaz-Perales A et al. J Allergy Clin Immunol. 1999 Sep;104(3 Pt 1):681-7.
11. Wagner S et al. Biochem Soc Trans. 2002 Nov;30(Pt 6):935-40.
It has been 40 years since the first reported case of IgE-mediated natural rubber latex allergy, which was soon followed by a global epidemic of allergic and anaphylactic reactions.1,2 Resolution came through insightful work in the 1990s that led to the removal of cornstarch powder and a switch to nonpowdered latex and synthetic examination gloves.2 Also discovered during this period was the cross-reactivity of many patients to latex and various fruits.
Research substantiates reports
Blanco et al. conducted a prospective study in their outpatient clinic in 25 patients diagnosed with latex allergy, published in 1994.They used a clinical questionnaire, skin-prick tests, skin test with a latex extract, and identification of total and specific IgE to help ascertain clinical characteristics and cross-reactivity. Of the 23 women and 2 men in the study (mean age 33, plus or minus 9 years), 9 (36%) experienced latex-induced reactions characterized by systemic anaphylaxis. In 13 patients (52%), 42 food allergies were identified, and 23 included systemic anaphylaxis. Avocado (9), chestnut (9), banana (7), kiwi (5), and papaya (3) were the most common foods to cause hypersensitivities. The researchers concluded that their small study supported the reality of a “latex-fruit syndrome.”3
Another study aimed to characterize the cross-reactivity of latex and foods and evaluate clinical significance. Beezhold et al. examined 47 patients allergic to latex and 46 nonallergic controls. The investigators found immunologic reactivity to foods to be prevalent (33 latex-allergic patients and seven controls), with 27% of food skin-prick tests positive in the latex-allergic group. In addition, clinical symptoms were linked to 27% of positive skin-prick tests. Among the 17 patients who displayed clinical allergies to at least one food, 14 showed local sensitivity reactions, with anaphylaxis noted in 11. Avocado (53%), potato (40%), banana (38%), tomato (28%), chestnut (28%), and kiwi (17%) were the foods most frequently cited for provoking a skin test reaction. The authors observed extensive cross-reactivity between latex sensitivity and particular foods, with potatoes and tomatoes reported for the first time.4
In 1997, Brehler et al. studied serum samples from 136 patients whose immediate hypersensitivity to latex proteins was clinically observable and documented. The samples were assessed for IgE antibodies against several fruits, with fruit-specific IgE antibodies recorded in 69.1%. Radioallergosorbent (RAST) -inhibition tests yielded the recognition of cross-reacting IgE antibodies in latex and multiple fruit allergens: avocado, banana, chestnut, fig, kiwi, mango, melon, papaya, passion fruit, peach, pineapple, and tomato. The investigators recorded 112 intolerance reactions and noted that 42.5% of their patients reported allergic symptoms after consuming these fruits. Fruit-specific IgE antibodies were detected in only 32.1% of these patients, suggesting to the researchers that serologic tests were suboptimal in forecasting food hypersensitivities in patients who are allergic to latex.5
Cross-reactivity with banana
Mäkinen-Kiljunen studied 47 patients to investigate banana allergy in patients with latex allergy in 1994, measuring latex-, banana-, and pollen-specific (birch, timothy, and mugwort) IgE. Thirty-one patients were also given skin-prick tests with banana and were queried about reactions after consuming bananas. Of the 47 sera samples, latex RAST results were positive in 31 and banana RAST results in 26. RAST results from latex and banana were correlated (25 of the 31 latex RAST-positive samples were also banana RAST-positive), but not with pollen. Sixteen of the 31 patients who ate banana reported symptoms, and 11 of the 31 patients given the banana skin-prick test showed positive results. The author confirmed the cross-reactivity of IgE antibodies for latex and banana, identifying for the first time a structurally similar antigen/allergen as at least one antigen from banana fused with an antigen from latex in crossed-line immunoelectrophoresis.6
In 1998, Mikkola et al. investigated whether proteins similar to hevein, a major natural rubber latex allergen, are present in banana and account for cross-reactivity between these botanicals. Immunoblotting revealed that 9 of 15 sera from latex-allergic patients with IgE to hevein also bound to 32- and 33-kd banana proteins. Studies using ELISA [enzyme-linked immunosorbent assay] showed that the common presentation of hypersensitivity to banana among patients allergic to latex could be attributed to cross-reacting IgE antibodies binding to epitopes in hevein and in the then-newly identified hevein-like endochitinase found in banana.7
Cross-reactivity with avocado
In response to reports of an association between allergy to natural rubber latex and avocado, Ahlroth et al. investigated cross-reactive proteins between natural rubber latex and avocado in 1995 by using skin-prick tests with fresh avocado on 11 patients and the sera of 18 patients with known latex allergy for IgE antibodies. Fourteen of the 18 sera were found to have IgE antibodies binding to 17 distinct avocado proteins, with multiple immunoblot experiments and skin-prick test results (positive in 7 of 11 patients) revealing marked immunologic cross-reactivity between latex and avocado.8
In 1998, Chen et al. set out to identify the cross-sensitizing allergen between latex and avocado, with hevein suspected. The researchers looked at sera samples from 118 health care workers allergic to latex and 78 patients with spina bifida who were allergic to latex. They noted a robust correlation between the prevalence of seropositive IgE antibodies to avocado in the presence of hevein-specific IgE antibodies in both groups. All members in the spina bifida group and 91 (73%) of the health care workers had positive IgE antibodies to hevein and high IgE values to avocado. Additional results supported the conclusion that sensitization to avocado in the majority of people allergic to latex is engendered by IgE-binding epitopes found in hevein.9
A year later, Diaz-Perales et al. considered the potential relevance of chitinases and complex glycans as factors in the then newly described latex/food syndrome, particularly in avocado, banana, and chestnuts. The investigators culled extracts from 20 various plant foods as well as latex. In immunoblot inhibition assays, the primary allergen and class I chitinase in avocado, Prs a 1, and the latex extract potently or completely blocked IgE binding by these constituents. Polyclonal antibodies to chitinases and sera from patients with latex/fruit allergy responded to reactive proteins of about 30-45 kd (putative class I chitinases) in chestnut, cherimoya, kiwi, mango, papaya, passion fruit, tomato, and wheat flour extracts. The glycans complex was deemed to be irrelevant in latex/fruit cross-reactivity, but the researchers found the putative class I chitinases to be notable players in the latex/fruit syndrome.10
According to Wagner and Breitender, anywhere from 30%-50% of people with known latex allergy also evince a related hypersensitivity or allergy to various plant-derived foods, with avocado, banana, chestnut, kiwi, peach, tomato, potato, and bell pepper among the foods most frequently linked to latex/fruit syndrome. They summarize that several plant defense proteins have been shown to be involved in the syndrome, with the most prominent, class I chitinases with an N-terminal hevein-like domain, having been found to cross-react with hevein (Hev b 6.02), a major IgE-binding allergen for individuals allergic to latex. A beta-1,3-glucanase, a key latex allergen, has also shown cross-reactivity with proteins of bell pepper, and another significant latex allergen, Hev b 7, a patatin-like protein, cross-reacts with its analogous protein in potato.11
Conclusion
It is unknown whether latex allergy precedes or follows food allergy.11 The latex/food syndrome itself merits attention as a significant source of hypersensitivity to natural cosmeceutical ingredients. Dermatologists should be aware of the lengthy list of cross-reacting plant-derived products, particularly when it comes to reviewing topical product ingredients with susceptible or allergic patients. Latex-allergic patients may react to these natural ingredients in food or when topically applied to the skin.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Nutter AF. Br J Dermatol 1979 Nov;101(5):597-8.
2. Kelly KJ et al. J Allergy Clin Immunol Pract. 2017 Sep-Oct;5(5):1212-16.
3. Blanco C et al. Ann Allergy. 1994 Oct;73(4):309-14.
4. Beezhold DH et al. Clin Exp Allergy. 1996 Apr;26(4):416-22.
5. Brehler R et al. Allergy. 1997 Apr;52(4):404-10.
6. Mäkinen-Kiljunen S. J Allergy Clin Immunol. 1994 Jun;93(6):990-6.
7. Mikkola JH et al. J Allergy Clin Immunol. 1998 Dec;102(6 Pt 1):1005-12.
8. Ahlroth M et al. J Allergy Clin Immunol. 1995 Aug;96(2):167-73.
9. Chen Z et al. J Allergy Clin Immunol. 1998 Sep;102(3):476-81.
10. Diaz-Perales A et al. J Allergy Clin Immunol. 1999 Sep;104(3 Pt 1):681-7.
11. Wagner S et al. Biochem Soc Trans. 2002 Nov;30(Pt 6):935-40.
It has been 40 years since the first reported case of IgE-mediated natural rubber latex allergy, which was soon followed by a global epidemic of allergic and anaphylactic reactions.1,2 Resolution came through insightful work in the 1990s that led to the removal of cornstarch powder and a switch to nonpowdered latex and synthetic examination gloves.2 Also discovered during this period was the cross-reactivity of many patients to latex and various fruits.
Research substantiates reports
Blanco et al. conducted a prospective study in their outpatient clinic in 25 patients diagnosed with latex allergy, published in 1994.They used a clinical questionnaire, skin-prick tests, skin test with a latex extract, and identification of total and specific IgE to help ascertain clinical characteristics and cross-reactivity. Of the 23 women and 2 men in the study (mean age 33, plus or minus 9 years), 9 (36%) experienced latex-induced reactions characterized by systemic anaphylaxis. In 13 patients (52%), 42 food allergies were identified, and 23 included systemic anaphylaxis. Avocado (9), chestnut (9), banana (7), kiwi (5), and papaya (3) were the most common foods to cause hypersensitivities. The researchers concluded that their small study supported the reality of a “latex-fruit syndrome.”3
Another study aimed to characterize the cross-reactivity of latex and foods and evaluate clinical significance. Beezhold et al. examined 47 patients allergic to latex and 46 nonallergic controls. The investigators found immunologic reactivity to foods to be prevalent (33 latex-allergic patients and seven controls), with 27% of food skin-prick tests positive in the latex-allergic group. In addition, clinical symptoms were linked to 27% of positive skin-prick tests. Among the 17 patients who displayed clinical allergies to at least one food, 14 showed local sensitivity reactions, with anaphylaxis noted in 11. Avocado (53%), potato (40%), banana (38%), tomato (28%), chestnut (28%), and kiwi (17%) were the foods most frequently cited for provoking a skin test reaction. The authors observed extensive cross-reactivity between latex sensitivity and particular foods, with potatoes and tomatoes reported for the first time.4
In 1997, Brehler et al. studied serum samples from 136 patients whose immediate hypersensitivity to latex proteins was clinically observable and documented. The samples were assessed for IgE antibodies against several fruits, with fruit-specific IgE antibodies recorded in 69.1%. Radioallergosorbent (RAST) -inhibition tests yielded the recognition of cross-reacting IgE antibodies in latex and multiple fruit allergens: avocado, banana, chestnut, fig, kiwi, mango, melon, papaya, passion fruit, peach, pineapple, and tomato. The investigators recorded 112 intolerance reactions and noted that 42.5% of their patients reported allergic symptoms after consuming these fruits. Fruit-specific IgE antibodies were detected in only 32.1% of these patients, suggesting to the researchers that serologic tests were suboptimal in forecasting food hypersensitivities in patients who are allergic to latex.5
Cross-reactivity with banana
Mäkinen-Kiljunen studied 47 patients to investigate banana allergy in patients with latex allergy in 1994, measuring latex-, banana-, and pollen-specific (birch, timothy, and mugwort) IgE. Thirty-one patients were also given skin-prick tests with banana and were queried about reactions after consuming bananas. Of the 47 sera samples, latex RAST results were positive in 31 and banana RAST results in 26. RAST results from latex and banana were correlated (25 of the 31 latex RAST-positive samples were also banana RAST-positive), but not with pollen. Sixteen of the 31 patients who ate banana reported symptoms, and 11 of the 31 patients given the banana skin-prick test showed positive results. The author confirmed the cross-reactivity of IgE antibodies for latex and banana, identifying for the first time a structurally similar antigen/allergen as at least one antigen from banana fused with an antigen from latex in crossed-line immunoelectrophoresis.6
In 1998, Mikkola et al. investigated whether proteins similar to hevein, a major natural rubber latex allergen, are present in banana and account for cross-reactivity between these botanicals. Immunoblotting revealed that 9 of 15 sera from latex-allergic patients with IgE to hevein also bound to 32- and 33-kd banana proteins. Studies using ELISA [enzyme-linked immunosorbent assay] showed that the common presentation of hypersensitivity to banana among patients allergic to latex could be attributed to cross-reacting IgE antibodies binding to epitopes in hevein and in the then-newly identified hevein-like endochitinase found in banana.7
Cross-reactivity with avocado
In response to reports of an association between allergy to natural rubber latex and avocado, Ahlroth et al. investigated cross-reactive proteins between natural rubber latex and avocado in 1995 by using skin-prick tests with fresh avocado on 11 patients and the sera of 18 patients with known latex allergy for IgE antibodies. Fourteen of the 18 sera were found to have IgE antibodies binding to 17 distinct avocado proteins, with multiple immunoblot experiments and skin-prick test results (positive in 7 of 11 patients) revealing marked immunologic cross-reactivity between latex and avocado.8
In 1998, Chen et al. set out to identify the cross-sensitizing allergen between latex and avocado, with hevein suspected. The researchers looked at sera samples from 118 health care workers allergic to latex and 78 patients with spina bifida who were allergic to latex. They noted a robust correlation between the prevalence of seropositive IgE antibodies to avocado in the presence of hevein-specific IgE antibodies in both groups. All members in the spina bifida group and 91 (73%) of the health care workers had positive IgE antibodies to hevein and high IgE values to avocado. Additional results supported the conclusion that sensitization to avocado in the majority of people allergic to latex is engendered by IgE-binding epitopes found in hevein.9
A year later, Diaz-Perales et al. considered the potential relevance of chitinases and complex glycans as factors in the then newly described latex/food syndrome, particularly in avocado, banana, and chestnuts. The investigators culled extracts from 20 various plant foods as well as latex. In immunoblot inhibition assays, the primary allergen and class I chitinase in avocado, Prs a 1, and the latex extract potently or completely blocked IgE binding by these constituents. Polyclonal antibodies to chitinases and sera from patients with latex/fruit allergy responded to reactive proteins of about 30-45 kd (putative class I chitinases) in chestnut, cherimoya, kiwi, mango, papaya, passion fruit, tomato, and wheat flour extracts. The glycans complex was deemed to be irrelevant in latex/fruit cross-reactivity, but the researchers found the putative class I chitinases to be notable players in the latex/fruit syndrome.10
According to Wagner and Breitender, anywhere from 30%-50% of people with known latex allergy also evince a related hypersensitivity or allergy to various plant-derived foods, with avocado, banana, chestnut, kiwi, peach, tomato, potato, and bell pepper among the foods most frequently linked to latex/fruit syndrome. They summarize that several plant defense proteins have been shown to be involved in the syndrome, with the most prominent, class I chitinases with an N-terminal hevein-like domain, having been found to cross-react with hevein (Hev b 6.02), a major IgE-binding allergen for individuals allergic to latex. A beta-1,3-glucanase, a key latex allergen, has also shown cross-reactivity with proteins of bell pepper, and another significant latex allergen, Hev b 7, a patatin-like protein, cross-reacts with its analogous protein in potato.11
Conclusion
It is unknown whether latex allergy precedes or follows food allergy.11 The latex/food syndrome itself merits attention as a significant source of hypersensitivity to natural cosmeceutical ingredients. Dermatologists should be aware of the lengthy list of cross-reacting plant-derived products, particularly when it comes to reviewing topical product ingredients with susceptible or allergic patients. Latex-allergic patients may react to these natural ingredients in food or when topically applied to the skin.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Nutter AF. Br J Dermatol 1979 Nov;101(5):597-8.
2. Kelly KJ et al. J Allergy Clin Immunol Pract. 2017 Sep-Oct;5(5):1212-16.
3. Blanco C et al. Ann Allergy. 1994 Oct;73(4):309-14.
4. Beezhold DH et al. Clin Exp Allergy. 1996 Apr;26(4):416-22.
5. Brehler R et al. Allergy. 1997 Apr;52(4):404-10.
6. Mäkinen-Kiljunen S. J Allergy Clin Immunol. 1994 Jun;93(6):990-6.
7. Mikkola JH et al. J Allergy Clin Immunol. 1998 Dec;102(6 Pt 1):1005-12.
8. Ahlroth M et al. J Allergy Clin Immunol. 1995 Aug;96(2):167-73.
9. Chen Z et al. J Allergy Clin Immunol. 1998 Sep;102(3):476-81.
10. Diaz-Perales A et al. J Allergy Clin Immunol. 1999 Sep;104(3 Pt 1):681-7.
11. Wagner S et al. Biochem Soc Trans. 2002 Nov;30(Pt 6):935-40.
Methylisothiazolinone and Isothiazolinone Allergy
Unless you have been living under a rock, you probably already know that the preservative methylisothiazolinone (MI) has caused an epidemic of allergic contact dermatitis (ACD) and was named the 2013 American Contact Dermatitis Society Allergen of the Year.1 Methylisothiazolinone is not new on the market, but its solo use as a preservative is relatively new. In this article, we review the emergence of MI as a common allergen, discuss North American MI patch test results, and describe common and uncommon sources of MI exposure. We also explore the related isothiazolinones, benzisothiazolinone (BIT) and octylisothiazolinone (OIT).
Background
Methylchloroisothiazolinone (MCI) and MI have been utilized as a preservative in a 3:1 ratio since the 1980s. In 2005, MI was first used alone as a preservative in personal care products in concentrations of up to 100 ppm, which represented a 25-fold increase in exposure to MI in personal care products and thus unleashed an epidemic of ACD.1 In the 2015 to 2016 cycle of the North American Contact Dermatitis Group (NACDG) patch testing results, MI was found to be positive in 13.4% of patch tested patients (N=5597) and also had the highest significance-prevalence index number, a calculation that represents the relevance of positive reactions in relationship to prevalence.2 In Europe, MI is banned in leave-on products and is allowed in rinse-off products in concentrations of up to 15 ppm. In the United States, the Cosmetic Ingredient Review panel concluded that MI is safe at a maximum concentration up to 100 ppm in rinse-off products and safe in leave-on products when formulated to be nonsensitizing, which may be determined based on a quantitative risk assessment.3
It is recommended that MI be patch tested at a concentration of 2000 ppm (0.2% aqueous).4 Testing at lower concentrations may result in missed positives. In addition, it should be noted that MCI/MI is present in the T.R.U.E. Test (SmartPractice), but MI alone is not.
Sources of MI Exposure
The first few case reports of MI contact allergy were associated with occupational exposures. In 2004, Isaksson et al5 reported 2 cases of MI allergy following exposure to wallpaper glue and a chemical burn from a biocide, respectively. Soon after, Thyssen et al6 reported 4 occupational cases of MI allergy at a paint manufacturing plant.
An early case series of MI contact allergy associated with personal care products was published in 2010 in which the authors described adults with ACD from wet wipes and a makeup remover that contained MI.7 A more recent report indicated that MI is now an infrequent ingredient in wet wipes but is still found in a wide variety of household and personal care products.8 A 2017 query of the American Contact Dermatitis Society’s Contact Allergy Management Program (CAMP) database revealed that 12.9% of all products contained MI. Furthermore, CAMP data revealed that MI was the most commonly found preservative in both hair care and household products.9 An additional CAMP database study revealed that 53% of shampoos and 45% of conditioners contained MI, and it also was commonly found in hair dyes, soaps and cleansers, hand cleaners and sanitizers, vaginal hygiene products, sunscreens, and moisturizers.10
Household products represent an important source of MI exposure. A chemical analysis of water-based paints identified the presence of isothiazolinones. Contact allergy from isothiazolinones in paint can present as either direct or airborne-pattern contact dermatitis.11 Sodium bisulfite has been used to inactivate MCI/MI in wall paint and could be utilized in severe cases of airborne contact dermatitis.12 Off-gassing may take up to 5.5 weeks before the paint cures and the isothiazolinone level decreases.13 A 2016 analysis of household products in the CAMP database revealed that MI commonly was found in dishwashing soap (64%), followed by household cleaners (47%), laundry softeners/additives (30%), surface disinfectants (27%), and laundry detergents (13%).10 Because certain chemical ingredients are not always listed on household product labels, patients with MI contact allergy may be at higher risk for unanticipated exposure to this allergen.
Dear reader, we know that you know all of this. We know that you have been watching the MI epidemic and have followed its every turn. But something that may be new to you are the unique MI exposures identified over the last several years.
In 2017, MI was identified in the glue used to affix 3 layers of the upper portion of a shoe.14 In addition, a recent chemical analysis of US consumer adhesives confirmed the presence of isothiazolinones in 50% (19/38) of products; 44.7% (17/38) specifically contained MI.15 Slime, the sticky play substance that children concoct out of household materials, has caused ACD, and not surprisingly, MI has been identified as a culprit allergen. In one case report, contact allergy was caused by MI present in a slime mixture made up of laundry detergent, dish soap, shampoo, and hand cream.16 In another case series, 3 children with MI contact allergy had played with slime that included dishwashing liquid, which contained MI, along with polyvinyl acetate glue and liquid soap components.17 Another case report documented slime made from MI-containing school glue as the source of ACD.18 Isothiazolinones also have been identified as causative allergens in “noise putty,” another homemade play item.19
Additionally, there has been a report of contact allergy to MI in a designer eyeglass frame.20 There also have been several documented cases of ACD to MCI/MI aerosolized from water used during ironing.21,22
There also have been several reports of photoaggravated ACD and possible photoallergic contact dermatitis from MI.23,24 In such cases, patients also may have transient photosensitivity even when MI exposure is discontinued; therefore, MI should be considered for inclusion in photopatch test panels when relevant.
Methylisothiazolinone contact allergy also should be considered for products that do not list MI on the label, which presents another potential exposure. In products that do not list MI as an ingredient on the label, its presence may be due to inclusion of the preservative in raw materials used in production. For example, a patient who reacted to a facial mask gel had a positive patch test reaction to MI, the facial mask gel, and sodium hyaluronate, the raw ingredient in the gel. Further analysis revealed that MI was unexpectedly present in the sodium hyaluronate.25 Similar scenarios have been reported in association with facial wet wipes,26 an exfoliating facial sponge,27 and a polyurethane sponge from a wound vacuum pump,28 among others.
Other Isothiazolinones
Other isothiazolinones also are known to cause ACD, albeit less commonly than MI. Benzisothiazolinone has been identified in glues, cleaning agents, paints, and industrial chemicals; unlike MI, the presence of BIT is infrequent in personal care products.15,29 This chemical is not commonly included in patch test screening series in the United States but is currently present in the NACDG screening series as BIT 0.1% in petrolatum.
Octylisothiazolinone (OIT) has been reported in leather furniture, belts, shoes, and watchbands, as well as industrial chemicals.30,31 Similar to BIT, OIT is not commonly tested in screening series in the United States; the NACDG tests this chemical as OIT 0.025% in petrolatum.
The cross-reaction patterns between the isothiazolinones remain uncertain. A study in mice supported cross-reactivity between MI, OIT, and BIT32; however, several clinical epidemiologic studies suggested that although there is evidence that there may be cross-reactivity between OIT and MI, concomitant positive BIT and MI reactions more likely represent cosensitization.33-35
Final Interpretation
Methylisothiazolinone continues to have high positive patch test rates in North American patch test populations and should be tested at a concentration of 2000 ppm (0.2% aqueous). Methylisothiazolinone may now be rare in wet wipes, but it is still present in numerous personal care products including hair care products, liquid soaps, and cleaning products. Novel exposures to MI include paint, slime, and glues. It also is important to remember that MI can cause photoaggravated or photoallergic contact dermatitis and might be a worthy addition to photopatch test trays. Finally, keep a look out for BIT and OIT, which may be present in industrial chemicals, glues, paints, cleaning products, and leather items
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013;24:2-6. 2. DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Cosmetic Ingredient Review. Amended safety assessment of methylisothiazolinone as used in cosmetics. https://www.cir-safety.org/sites/default/files/mthiaz092014FR_final.pdf. Released October 8, 2014. Accessed July 9, 2019.
- Isaksson M, Ale I, Andersen KE, et al. Multicenter patch testing with methylisothiazolinone and methylchloroisothiazolinone/methylisothiazolinone within the International Contact Dermatitis Research Group. Dermatitis. 2017;28:210-214.
- Isaksson M, Gruvberger B, Bruze M. Occupational contact allergy and dermatitis from methylisothiazolinone after contact with wallcovering glue and after a chemical burn from a biocide. Dermatitis. 2004;15:201-205.
- Thyssen JP, Sederberg-Olsen N, Thomsen JF, et al. Contact dermatitis from methylisothiazolinone in a paint factory. Contact Dermatitis. 2006;54:322-324.
- García-Gavín J, Vansina S, Kerre S, et al. Methylisothiazolinone, an emerging allergen in cosmetics? Contact Dermatitis. 2010;63:96-101.
- Hamann CR, Sahni S, Zug KA. Methylisothiazolinone: still on leave-on products, but no longer on baby wipes. Dermatitis. 2019;30:173-174.
- Beene KM, Scheman A, Severson D, et al. Prevalence of preservatives across all product types in the Contact Allergen Management Program. Dermatitis. 2017;28:81-87.
- Scheman A, Severson D. American Contact Dermatitis Society Contact Allergy Management Program: an epidemiologic tool to quantify ingredient usage. Dermatitis. 2016;27:11-13.
- Goodier MC, Siegel PD, Zang LY, et al. Isothiazolinone in residential interior wall paint: a high-performance liquid chromatographic-mass spectrometry analysis. Dermatitis. 2018;29:332-338.
- Bohn S, Niederer M, Brehm K, et al. Airborne contact dermatitis from methylchloroisothiazolinone in wall paint. abolition of symptoms by chemical allergen inactivation. Contact Dermatitis. 2000;42:196-201.
- Amsler E, Aerts O, Raison-Peyron N, et al; Dermatology Allergy Group (DAG) of the French Society of Dermatology. Airborne allergic contact dermatitis caused by isothiazolinones in water-based paints: a retrospective study of 44 cases. Contact Dermatitis. 2017;77:163-170.
- Silva CA, El-Houri RB, Christensen LP, et al. Contact allergy caused by methylisothiazolinone in shoe glue. Contact Dermatitis. 2017;77:175-176.
- Goodier MC, Zang LY, Siegel PD, et al. Isothiazolinone content of US consumer adhesives: ultrahigh-performance liquid chromatographic mass spectrometry analysis. Dermatitis. 2019;30:129-134.
- Anderson LE, Treat JR, Brod BA, et al. “Slime” contact dermatitis: case report and review of relevant allergens. Pediatr Dermatol. 2019;36:335-337.
- Salman A, Demir G, Apti O. “Slime”: a trending cause of isothiazolinone contact allergy in children. Contact Dermatitis. 2019;80:409-411.
- Zhang AJ, Boyd AH, Asch S, et al. Allergic contact dermatitis to slime: the epidemic of isothiazolinone allergy encompasses school glue. Pediatr Dermatol. 2019;36:e37-e38.
- Ducharme O, Labadie M, Briand SM, et al. Allergic contact dermatitis in a child caused by isothiazolinones in a “noise putty.” Contact Dermatitis. 2018;79:393-394.
- El-Houri RB, Christensen LP, Persson C, et al. Methylisothiazolinone in a designer spectacle frame—a surprising finding. Contact Dermatitis. 2016;75:310-312.
- Atkar R, Todd P. Four cases of allergic contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in ironing water. Contact Dermatitis. 2016;75:316-317.
- Hunter KJ, Shelley JC, Haworth AE. Airborne allergic contact dermatitis to methylchloroisothiazolinone/methylisothiazolinone in ironing water. Contact Dermatitis. 2008;58:183-184.
- Aerts O, Goossens A, Marguery MC, et al. Photoaggravated allergic contact dermatitis and transient photosensitivity caused by methylisothiazolinone. Contact Dermatitis. 2018;78:241-245.
- Trokoudes D, Banerjee P, Fityan A, et al. Photoaggravated contact dermatitis caused by methylisothiazolinone. Contact Dermatitis. 2017;76:303-304.
- Kerre S, Naessens T, Theunis M, et al. Facial dermatitis caused by undeclared methylisothiazolinone in a gel mask: is the preservation of raw materials in cosmetics a cause of concern? Contact Dermatitis. 2018;78:421-424.
- Isaksson M, Persson L. ‘Mislabelled’ make-up remover wet wipes as a cause of severe, recalcitrant facial eczema [published online March 27, 2015]. Contact Dermatitis. 2015;73:56-59.
- Madsen JT, Andersen KE, Nielsen DT, et al. Undisclosed presence of methylisothiazolinone in ‘100% natural’ Konjac® sponge. Contact Dermatitis. 2016;75:308-309.
- Schliemann S, Isaksson M, Persson C, et al. Allergic contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in a medical device. Contact Dermatitis. 2016;75:312-314.
- Kaur-Knudsen D, Menné T, Christina Carlsen B. Systemic allergic dermatitis following airborne exposure to 1,2-benzisothiazolin-3-one. Contact Dermatitis. 2012;67:310-312.
- Aerts O, Meert H, Romaen E, et al. Octylisothiazolinone, an additional cause of allergic contact dermatitis caused by leather: case series and potential implications for the study of cross-reactivity with methylisothiazolinone. Contact Dermatitis. 2016;75:276-284.
- Alipour Tehrany Y, Quenan S, Bugey A, et al. Allergic contact dermatitis caused by octylisothiazolinone in a leather sofa. Contact Dermatitis. 2018;79:188-189.
- Schwensen JF, Menné Bonefeld C, Zachariae C, et al. Cross-reactivity between methylisothiazolinone, octylisothiazolinone and benzisothiazolinone using a modified local lymph node assay. Br J Dermatol. 2017;176:176-183.
- Aalto-Korte K, Suuronen K. Patterns of concomitant allergic reactions in patients suggest cross-sensitization between octylisothiazolinone and methylisothiazolinone. Contact Dermatitis. 2017;77:385-389.
- Craig S, Urwin R, Latheef F, et al. Patch test clinic experience of potential cross-reactivity of isothiazolinones. Contact Dermatitis. 2017;76:299-300.
- Geier J, Lessmann H, Schnuch A, et al. Concomitant reactivity to methylisothiazolinone, benzisothiazolinone, and octylisothiazolinone. International Network of Departments of Dermatology data, 2009-2013. Contact Dermatitis. 2015;72:337-339.
Unless you have been living under a rock, you probably already know that the preservative methylisothiazolinone (MI) has caused an epidemic of allergic contact dermatitis (ACD) and was named the 2013 American Contact Dermatitis Society Allergen of the Year.1 Methylisothiazolinone is not new on the market, but its solo use as a preservative is relatively new. In this article, we review the emergence of MI as a common allergen, discuss North American MI patch test results, and describe common and uncommon sources of MI exposure. We also explore the related isothiazolinones, benzisothiazolinone (BIT) and octylisothiazolinone (OIT).
Background
Methylchloroisothiazolinone (MCI) and MI have been utilized as a preservative in a 3:1 ratio since the 1980s. In 2005, MI was first used alone as a preservative in personal care products in concentrations of up to 100 ppm, which represented a 25-fold increase in exposure to MI in personal care products and thus unleashed an epidemic of ACD.1 In the 2015 to 2016 cycle of the North American Contact Dermatitis Group (NACDG) patch testing results, MI was found to be positive in 13.4% of patch tested patients (N=5597) and also had the highest significance-prevalence index number, a calculation that represents the relevance of positive reactions in relationship to prevalence.2 In Europe, MI is banned in leave-on products and is allowed in rinse-off products in concentrations of up to 15 ppm. In the United States, the Cosmetic Ingredient Review panel concluded that MI is safe at a maximum concentration up to 100 ppm in rinse-off products and safe in leave-on products when formulated to be nonsensitizing, which may be determined based on a quantitative risk assessment.3
It is recommended that MI be patch tested at a concentration of 2000 ppm (0.2% aqueous).4 Testing at lower concentrations may result in missed positives. In addition, it should be noted that MCI/MI is present in the T.R.U.E. Test (SmartPractice), but MI alone is not.
Sources of MI Exposure
The first few case reports of MI contact allergy were associated with occupational exposures. In 2004, Isaksson et al5 reported 2 cases of MI allergy following exposure to wallpaper glue and a chemical burn from a biocide, respectively. Soon after, Thyssen et al6 reported 4 occupational cases of MI allergy at a paint manufacturing plant.
An early case series of MI contact allergy associated with personal care products was published in 2010 in which the authors described adults with ACD from wet wipes and a makeup remover that contained MI.7 A more recent report indicated that MI is now an infrequent ingredient in wet wipes but is still found in a wide variety of household and personal care products.8 A 2017 query of the American Contact Dermatitis Society’s Contact Allergy Management Program (CAMP) database revealed that 12.9% of all products contained MI. Furthermore, CAMP data revealed that MI was the most commonly found preservative in both hair care and household products.9 An additional CAMP database study revealed that 53% of shampoos and 45% of conditioners contained MI, and it also was commonly found in hair dyes, soaps and cleansers, hand cleaners and sanitizers, vaginal hygiene products, sunscreens, and moisturizers.10
Household products represent an important source of MI exposure. A chemical analysis of water-based paints identified the presence of isothiazolinones. Contact allergy from isothiazolinones in paint can present as either direct or airborne-pattern contact dermatitis.11 Sodium bisulfite has been used to inactivate MCI/MI in wall paint and could be utilized in severe cases of airborne contact dermatitis.12 Off-gassing may take up to 5.5 weeks before the paint cures and the isothiazolinone level decreases.13 A 2016 analysis of household products in the CAMP database revealed that MI commonly was found in dishwashing soap (64%), followed by household cleaners (47%), laundry softeners/additives (30%), surface disinfectants (27%), and laundry detergents (13%).10 Because certain chemical ingredients are not always listed on household product labels, patients with MI contact allergy may be at higher risk for unanticipated exposure to this allergen.
Dear reader, we know that you know all of this. We know that you have been watching the MI epidemic and have followed its every turn. But something that may be new to you are the unique MI exposures identified over the last several years.
In 2017, MI was identified in the glue used to affix 3 layers of the upper portion of a shoe.14 In addition, a recent chemical analysis of US consumer adhesives confirmed the presence of isothiazolinones in 50% (19/38) of products; 44.7% (17/38) specifically contained MI.15 Slime, the sticky play substance that children concoct out of household materials, has caused ACD, and not surprisingly, MI has been identified as a culprit allergen. In one case report, contact allergy was caused by MI present in a slime mixture made up of laundry detergent, dish soap, shampoo, and hand cream.16 In another case series, 3 children with MI contact allergy had played with slime that included dishwashing liquid, which contained MI, along with polyvinyl acetate glue and liquid soap components.17 Another case report documented slime made from MI-containing school glue as the source of ACD.18 Isothiazolinones also have been identified as causative allergens in “noise putty,” another homemade play item.19
Additionally, there has been a report of contact allergy to MI in a designer eyeglass frame.20 There also have been several documented cases of ACD to MCI/MI aerosolized from water used during ironing.21,22
There also have been several reports of photoaggravated ACD and possible photoallergic contact dermatitis from MI.23,24 In such cases, patients also may have transient photosensitivity even when MI exposure is discontinued; therefore, MI should be considered for inclusion in photopatch test panels when relevant.
Methylisothiazolinone contact allergy also should be considered for products that do not list MI on the label, which presents another potential exposure. In products that do not list MI as an ingredient on the label, its presence may be due to inclusion of the preservative in raw materials used in production. For example, a patient who reacted to a facial mask gel had a positive patch test reaction to MI, the facial mask gel, and sodium hyaluronate, the raw ingredient in the gel. Further analysis revealed that MI was unexpectedly present in the sodium hyaluronate.25 Similar scenarios have been reported in association with facial wet wipes,26 an exfoliating facial sponge,27 and a polyurethane sponge from a wound vacuum pump,28 among others.
Other Isothiazolinones
Other isothiazolinones also are known to cause ACD, albeit less commonly than MI. Benzisothiazolinone has been identified in glues, cleaning agents, paints, and industrial chemicals; unlike MI, the presence of BIT is infrequent in personal care products.15,29 This chemical is not commonly included in patch test screening series in the United States but is currently present in the NACDG screening series as BIT 0.1% in petrolatum.
Octylisothiazolinone (OIT) has been reported in leather furniture, belts, shoes, and watchbands, as well as industrial chemicals.30,31 Similar to BIT, OIT is not commonly tested in screening series in the United States; the NACDG tests this chemical as OIT 0.025% in petrolatum.
The cross-reaction patterns between the isothiazolinones remain uncertain. A study in mice supported cross-reactivity between MI, OIT, and BIT32; however, several clinical epidemiologic studies suggested that although there is evidence that there may be cross-reactivity between OIT and MI, concomitant positive BIT and MI reactions more likely represent cosensitization.33-35
Final Interpretation
Methylisothiazolinone continues to have high positive patch test rates in North American patch test populations and should be tested at a concentration of 2000 ppm (0.2% aqueous). Methylisothiazolinone may now be rare in wet wipes, but it is still present in numerous personal care products including hair care products, liquid soaps, and cleaning products. Novel exposures to MI include paint, slime, and glues. It also is important to remember that MI can cause photoaggravated or photoallergic contact dermatitis and might be a worthy addition to photopatch test trays. Finally, keep a look out for BIT and OIT, which may be present in industrial chemicals, glues, paints, cleaning products, and leather items
Unless you have been living under a rock, you probably already know that the preservative methylisothiazolinone (MI) has caused an epidemic of allergic contact dermatitis (ACD) and was named the 2013 American Contact Dermatitis Society Allergen of the Year.1 Methylisothiazolinone is not new on the market, but its solo use as a preservative is relatively new. In this article, we review the emergence of MI as a common allergen, discuss North American MI patch test results, and describe common and uncommon sources of MI exposure. We also explore the related isothiazolinones, benzisothiazolinone (BIT) and octylisothiazolinone (OIT).
Background
Methylchloroisothiazolinone (MCI) and MI have been utilized as a preservative in a 3:1 ratio since the 1980s. In 2005, MI was first used alone as a preservative in personal care products in concentrations of up to 100 ppm, which represented a 25-fold increase in exposure to MI in personal care products and thus unleashed an epidemic of ACD.1 In the 2015 to 2016 cycle of the North American Contact Dermatitis Group (NACDG) patch testing results, MI was found to be positive in 13.4% of patch tested patients (N=5597) and also had the highest significance-prevalence index number, a calculation that represents the relevance of positive reactions in relationship to prevalence.2 In Europe, MI is banned in leave-on products and is allowed in rinse-off products in concentrations of up to 15 ppm. In the United States, the Cosmetic Ingredient Review panel concluded that MI is safe at a maximum concentration up to 100 ppm in rinse-off products and safe in leave-on products when formulated to be nonsensitizing, which may be determined based on a quantitative risk assessment.3
It is recommended that MI be patch tested at a concentration of 2000 ppm (0.2% aqueous).4 Testing at lower concentrations may result in missed positives. In addition, it should be noted that MCI/MI is present in the T.R.U.E. Test (SmartPractice), but MI alone is not.
Sources of MI Exposure
The first few case reports of MI contact allergy were associated with occupational exposures. In 2004, Isaksson et al5 reported 2 cases of MI allergy following exposure to wallpaper glue and a chemical burn from a biocide, respectively. Soon after, Thyssen et al6 reported 4 occupational cases of MI allergy at a paint manufacturing plant.
An early case series of MI contact allergy associated with personal care products was published in 2010 in which the authors described adults with ACD from wet wipes and a makeup remover that contained MI.7 A more recent report indicated that MI is now an infrequent ingredient in wet wipes but is still found in a wide variety of household and personal care products.8 A 2017 query of the American Contact Dermatitis Society’s Contact Allergy Management Program (CAMP) database revealed that 12.9% of all products contained MI. Furthermore, CAMP data revealed that MI was the most commonly found preservative in both hair care and household products.9 An additional CAMP database study revealed that 53% of shampoos and 45% of conditioners contained MI, and it also was commonly found in hair dyes, soaps and cleansers, hand cleaners and sanitizers, vaginal hygiene products, sunscreens, and moisturizers.10
Household products represent an important source of MI exposure. A chemical analysis of water-based paints identified the presence of isothiazolinones. Contact allergy from isothiazolinones in paint can present as either direct or airborne-pattern contact dermatitis.11 Sodium bisulfite has been used to inactivate MCI/MI in wall paint and could be utilized in severe cases of airborne contact dermatitis.12 Off-gassing may take up to 5.5 weeks before the paint cures and the isothiazolinone level decreases.13 A 2016 analysis of household products in the CAMP database revealed that MI commonly was found in dishwashing soap (64%), followed by household cleaners (47%), laundry softeners/additives (30%), surface disinfectants (27%), and laundry detergents (13%).10 Because certain chemical ingredients are not always listed on household product labels, patients with MI contact allergy may be at higher risk for unanticipated exposure to this allergen.
Dear reader, we know that you know all of this. We know that you have been watching the MI epidemic and have followed its every turn. But something that may be new to you are the unique MI exposures identified over the last several years.
In 2017, MI was identified in the glue used to affix 3 layers of the upper portion of a shoe.14 In addition, a recent chemical analysis of US consumer adhesives confirmed the presence of isothiazolinones in 50% (19/38) of products; 44.7% (17/38) specifically contained MI.15 Slime, the sticky play substance that children concoct out of household materials, has caused ACD, and not surprisingly, MI has been identified as a culprit allergen. In one case report, contact allergy was caused by MI present in a slime mixture made up of laundry detergent, dish soap, shampoo, and hand cream.16 In another case series, 3 children with MI contact allergy had played with slime that included dishwashing liquid, which contained MI, along with polyvinyl acetate glue and liquid soap components.17 Another case report documented slime made from MI-containing school glue as the source of ACD.18 Isothiazolinones also have been identified as causative allergens in “noise putty,” another homemade play item.19
Additionally, there has been a report of contact allergy to MI in a designer eyeglass frame.20 There also have been several documented cases of ACD to MCI/MI aerosolized from water used during ironing.21,22
There also have been several reports of photoaggravated ACD and possible photoallergic contact dermatitis from MI.23,24 In such cases, patients also may have transient photosensitivity even when MI exposure is discontinued; therefore, MI should be considered for inclusion in photopatch test panels when relevant.
Methylisothiazolinone contact allergy also should be considered for products that do not list MI on the label, which presents another potential exposure. In products that do not list MI as an ingredient on the label, its presence may be due to inclusion of the preservative in raw materials used in production. For example, a patient who reacted to a facial mask gel had a positive patch test reaction to MI, the facial mask gel, and sodium hyaluronate, the raw ingredient in the gel. Further analysis revealed that MI was unexpectedly present in the sodium hyaluronate.25 Similar scenarios have been reported in association with facial wet wipes,26 an exfoliating facial sponge,27 and a polyurethane sponge from a wound vacuum pump,28 among others.
Other Isothiazolinones
Other isothiazolinones also are known to cause ACD, albeit less commonly than MI. Benzisothiazolinone has been identified in glues, cleaning agents, paints, and industrial chemicals; unlike MI, the presence of BIT is infrequent in personal care products.15,29 This chemical is not commonly included in patch test screening series in the United States but is currently present in the NACDG screening series as BIT 0.1% in petrolatum.
Octylisothiazolinone (OIT) has been reported in leather furniture, belts, shoes, and watchbands, as well as industrial chemicals.30,31 Similar to BIT, OIT is not commonly tested in screening series in the United States; the NACDG tests this chemical as OIT 0.025% in petrolatum.
The cross-reaction patterns between the isothiazolinones remain uncertain. A study in mice supported cross-reactivity between MI, OIT, and BIT32; however, several clinical epidemiologic studies suggested that although there is evidence that there may be cross-reactivity between OIT and MI, concomitant positive BIT and MI reactions more likely represent cosensitization.33-35
Final Interpretation
Methylisothiazolinone continues to have high positive patch test rates in North American patch test populations and should be tested at a concentration of 2000 ppm (0.2% aqueous). Methylisothiazolinone may now be rare in wet wipes, but it is still present in numerous personal care products including hair care products, liquid soaps, and cleaning products. Novel exposures to MI include paint, slime, and glues. It also is important to remember that MI can cause photoaggravated or photoallergic contact dermatitis and might be a worthy addition to photopatch test trays. Finally, keep a look out for BIT and OIT, which may be present in industrial chemicals, glues, paints, cleaning products, and leather items
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013;24:2-6. 2. DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Cosmetic Ingredient Review. Amended safety assessment of methylisothiazolinone as used in cosmetics. https://www.cir-safety.org/sites/default/files/mthiaz092014FR_final.pdf. Released October 8, 2014. Accessed July 9, 2019.
- Isaksson M, Ale I, Andersen KE, et al. Multicenter patch testing with methylisothiazolinone and methylchloroisothiazolinone/methylisothiazolinone within the International Contact Dermatitis Research Group. Dermatitis. 2017;28:210-214.
- Isaksson M, Gruvberger B, Bruze M. Occupational contact allergy and dermatitis from methylisothiazolinone after contact with wallcovering glue and after a chemical burn from a biocide. Dermatitis. 2004;15:201-205.
- Thyssen JP, Sederberg-Olsen N, Thomsen JF, et al. Contact dermatitis from methylisothiazolinone in a paint factory. Contact Dermatitis. 2006;54:322-324.
- García-Gavín J, Vansina S, Kerre S, et al. Methylisothiazolinone, an emerging allergen in cosmetics? Contact Dermatitis. 2010;63:96-101.
- Hamann CR, Sahni S, Zug KA. Methylisothiazolinone: still on leave-on products, but no longer on baby wipes. Dermatitis. 2019;30:173-174.
- Beene KM, Scheman A, Severson D, et al. Prevalence of preservatives across all product types in the Contact Allergen Management Program. Dermatitis. 2017;28:81-87.
- Scheman A, Severson D. American Contact Dermatitis Society Contact Allergy Management Program: an epidemiologic tool to quantify ingredient usage. Dermatitis. 2016;27:11-13.
- Goodier MC, Siegel PD, Zang LY, et al. Isothiazolinone in residential interior wall paint: a high-performance liquid chromatographic-mass spectrometry analysis. Dermatitis. 2018;29:332-338.
- Bohn S, Niederer M, Brehm K, et al. Airborne contact dermatitis from methylchloroisothiazolinone in wall paint. abolition of symptoms by chemical allergen inactivation. Contact Dermatitis. 2000;42:196-201.
- Amsler E, Aerts O, Raison-Peyron N, et al; Dermatology Allergy Group (DAG) of the French Society of Dermatology. Airborne allergic contact dermatitis caused by isothiazolinones in water-based paints: a retrospective study of 44 cases. Contact Dermatitis. 2017;77:163-170.
- Silva CA, El-Houri RB, Christensen LP, et al. Contact allergy caused by methylisothiazolinone in shoe glue. Contact Dermatitis. 2017;77:175-176.
- Goodier MC, Zang LY, Siegel PD, et al. Isothiazolinone content of US consumer adhesives: ultrahigh-performance liquid chromatographic mass spectrometry analysis. Dermatitis. 2019;30:129-134.
- Anderson LE, Treat JR, Brod BA, et al. “Slime” contact dermatitis: case report and review of relevant allergens. Pediatr Dermatol. 2019;36:335-337.
- Salman A, Demir G, Apti O. “Slime”: a trending cause of isothiazolinone contact allergy in children. Contact Dermatitis. 2019;80:409-411.
- Zhang AJ, Boyd AH, Asch S, et al. Allergic contact dermatitis to slime: the epidemic of isothiazolinone allergy encompasses school glue. Pediatr Dermatol. 2019;36:e37-e38.
- Ducharme O, Labadie M, Briand SM, et al. Allergic contact dermatitis in a child caused by isothiazolinones in a “noise putty.” Contact Dermatitis. 2018;79:393-394.
- El-Houri RB, Christensen LP, Persson C, et al. Methylisothiazolinone in a designer spectacle frame—a surprising finding. Contact Dermatitis. 2016;75:310-312.
- Atkar R, Todd P. Four cases of allergic contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in ironing water. Contact Dermatitis. 2016;75:316-317.
- Hunter KJ, Shelley JC, Haworth AE. Airborne allergic contact dermatitis to methylchloroisothiazolinone/methylisothiazolinone in ironing water. Contact Dermatitis. 2008;58:183-184.
- Aerts O, Goossens A, Marguery MC, et al. Photoaggravated allergic contact dermatitis and transient photosensitivity caused by methylisothiazolinone. Contact Dermatitis. 2018;78:241-245.
- Trokoudes D, Banerjee P, Fityan A, et al. Photoaggravated contact dermatitis caused by methylisothiazolinone. Contact Dermatitis. 2017;76:303-304.
- Kerre S, Naessens T, Theunis M, et al. Facial dermatitis caused by undeclared methylisothiazolinone in a gel mask: is the preservation of raw materials in cosmetics a cause of concern? Contact Dermatitis. 2018;78:421-424.
- Isaksson M, Persson L. ‘Mislabelled’ make-up remover wet wipes as a cause of severe, recalcitrant facial eczema [published online March 27, 2015]. Contact Dermatitis. 2015;73:56-59.
- Madsen JT, Andersen KE, Nielsen DT, et al. Undisclosed presence of methylisothiazolinone in ‘100% natural’ Konjac® sponge. Contact Dermatitis. 2016;75:308-309.
- Schliemann S, Isaksson M, Persson C, et al. Allergic contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in a medical device. Contact Dermatitis. 2016;75:312-314.
- Kaur-Knudsen D, Menné T, Christina Carlsen B. Systemic allergic dermatitis following airborne exposure to 1,2-benzisothiazolin-3-one. Contact Dermatitis. 2012;67:310-312.
- Aerts O, Meert H, Romaen E, et al. Octylisothiazolinone, an additional cause of allergic contact dermatitis caused by leather: case series and potential implications for the study of cross-reactivity with methylisothiazolinone. Contact Dermatitis. 2016;75:276-284.
- Alipour Tehrany Y, Quenan S, Bugey A, et al. Allergic contact dermatitis caused by octylisothiazolinone in a leather sofa. Contact Dermatitis. 2018;79:188-189.
- Schwensen JF, Menné Bonefeld C, Zachariae C, et al. Cross-reactivity between methylisothiazolinone, octylisothiazolinone and benzisothiazolinone using a modified local lymph node assay. Br J Dermatol. 2017;176:176-183.
- Aalto-Korte K, Suuronen K. Patterns of concomitant allergic reactions in patients suggest cross-sensitization between octylisothiazolinone and methylisothiazolinone. Contact Dermatitis. 2017;77:385-389.
- Craig S, Urwin R, Latheef F, et al. Patch test clinic experience of potential cross-reactivity of isothiazolinones. Contact Dermatitis. 2017;76:299-300.
- Geier J, Lessmann H, Schnuch A, et al. Concomitant reactivity to methylisothiazolinone, benzisothiazolinone, and octylisothiazolinone. International Network of Departments of Dermatology data, 2009-2013. Contact Dermatitis. 2015;72:337-339.
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013;24:2-6. 2. DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Cosmetic Ingredient Review. Amended safety assessment of methylisothiazolinone as used in cosmetics. https://www.cir-safety.org/sites/default/files/mthiaz092014FR_final.pdf. Released October 8, 2014. Accessed July 9, 2019.
- Isaksson M, Ale I, Andersen KE, et al. Multicenter patch testing with methylisothiazolinone and methylchloroisothiazolinone/methylisothiazolinone within the International Contact Dermatitis Research Group. Dermatitis. 2017;28:210-214.
- Isaksson M, Gruvberger B, Bruze M. Occupational contact allergy and dermatitis from methylisothiazolinone after contact with wallcovering glue and after a chemical burn from a biocide. Dermatitis. 2004;15:201-205.
- Thyssen JP, Sederberg-Olsen N, Thomsen JF, et al. Contact dermatitis from methylisothiazolinone in a paint factory. Contact Dermatitis. 2006;54:322-324.
- García-Gavín J, Vansina S, Kerre S, et al. Methylisothiazolinone, an emerging allergen in cosmetics? Contact Dermatitis. 2010;63:96-101.
- Hamann CR, Sahni S, Zug KA. Methylisothiazolinone: still on leave-on products, but no longer on baby wipes. Dermatitis. 2019;30:173-174.
- Beene KM, Scheman A, Severson D, et al. Prevalence of preservatives across all product types in the Contact Allergen Management Program. Dermatitis. 2017;28:81-87.
- Scheman A, Severson D. American Contact Dermatitis Society Contact Allergy Management Program: an epidemiologic tool to quantify ingredient usage. Dermatitis. 2016;27:11-13.
- Goodier MC, Siegel PD, Zang LY, et al. Isothiazolinone in residential interior wall paint: a high-performance liquid chromatographic-mass spectrometry analysis. Dermatitis. 2018;29:332-338.
- Bohn S, Niederer M, Brehm K, et al. Airborne contact dermatitis from methylchloroisothiazolinone in wall paint. abolition of symptoms by chemical allergen inactivation. Contact Dermatitis. 2000;42:196-201.
- Amsler E, Aerts O, Raison-Peyron N, et al; Dermatology Allergy Group (DAG) of the French Society of Dermatology. Airborne allergic contact dermatitis caused by isothiazolinones in water-based paints: a retrospective study of 44 cases. Contact Dermatitis. 2017;77:163-170.
- Silva CA, El-Houri RB, Christensen LP, et al. Contact allergy caused by methylisothiazolinone in shoe glue. Contact Dermatitis. 2017;77:175-176.
- Goodier MC, Zang LY, Siegel PD, et al. Isothiazolinone content of US consumer adhesives: ultrahigh-performance liquid chromatographic mass spectrometry analysis. Dermatitis. 2019;30:129-134.
- Anderson LE, Treat JR, Brod BA, et al. “Slime” contact dermatitis: case report and review of relevant allergens. Pediatr Dermatol. 2019;36:335-337.
- Salman A, Demir G, Apti O. “Slime”: a trending cause of isothiazolinone contact allergy in children. Contact Dermatitis. 2019;80:409-411.
- Zhang AJ, Boyd AH, Asch S, et al. Allergic contact dermatitis to slime: the epidemic of isothiazolinone allergy encompasses school glue. Pediatr Dermatol. 2019;36:e37-e38.
- Ducharme O, Labadie M, Briand SM, et al. Allergic contact dermatitis in a child caused by isothiazolinones in a “noise putty.” Contact Dermatitis. 2018;79:393-394.
- El-Houri RB, Christensen LP, Persson C, et al. Methylisothiazolinone in a designer spectacle frame—a surprising finding. Contact Dermatitis. 2016;75:310-312.
- Atkar R, Todd P. Four cases of allergic contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in ironing water. Contact Dermatitis. 2016;75:316-317.
- Hunter KJ, Shelley JC, Haworth AE. Airborne allergic contact dermatitis to methylchloroisothiazolinone/methylisothiazolinone in ironing water. Contact Dermatitis. 2008;58:183-184.
- Aerts O, Goossens A, Marguery MC, et al. Photoaggravated allergic contact dermatitis and transient photosensitivity caused by methylisothiazolinone. Contact Dermatitis. 2018;78:241-245.
- Trokoudes D, Banerjee P, Fityan A, et al. Photoaggravated contact dermatitis caused by methylisothiazolinone. Contact Dermatitis. 2017;76:303-304.
- Kerre S, Naessens T, Theunis M, et al. Facial dermatitis caused by undeclared methylisothiazolinone in a gel mask: is the preservation of raw materials in cosmetics a cause of concern? Contact Dermatitis. 2018;78:421-424.
- Isaksson M, Persson L. ‘Mislabelled’ make-up remover wet wipes as a cause of severe, recalcitrant facial eczema [published online March 27, 2015]. Contact Dermatitis. 2015;73:56-59.
- Madsen JT, Andersen KE, Nielsen DT, et al. Undisclosed presence of methylisothiazolinone in ‘100% natural’ Konjac® sponge. Contact Dermatitis. 2016;75:308-309.
- Schliemann S, Isaksson M, Persson C, et al. Allergic contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in a medical device. Contact Dermatitis. 2016;75:312-314.
- Kaur-Knudsen D, Menné T, Christina Carlsen B. Systemic allergic dermatitis following airborne exposure to 1,2-benzisothiazolin-3-one. Contact Dermatitis. 2012;67:310-312.
- Aerts O, Meert H, Romaen E, et al. Octylisothiazolinone, an additional cause of allergic contact dermatitis caused by leather: case series and potential implications for the study of cross-reactivity with methylisothiazolinone. Contact Dermatitis. 2016;75:276-284.
- Alipour Tehrany Y, Quenan S, Bugey A, et al. Allergic contact dermatitis caused by octylisothiazolinone in a leather sofa. Contact Dermatitis. 2018;79:188-189.
- Schwensen JF, Menné Bonefeld C, Zachariae C, et al. Cross-reactivity between methylisothiazolinone, octylisothiazolinone and benzisothiazolinone using a modified local lymph node assay. Br J Dermatol. 2017;176:176-183.
- Aalto-Korte K, Suuronen K. Patterns of concomitant allergic reactions in patients suggest cross-sensitization between octylisothiazolinone and methylisothiazolinone. Contact Dermatitis. 2017;77:385-389.
- Craig S, Urwin R, Latheef F, et al. Patch test clinic experience of potential cross-reactivity of isothiazolinones. Contact Dermatitis. 2017;76:299-300.
- Geier J, Lessmann H, Schnuch A, et al. Concomitant reactivity to methylisothiazolinone, benzisothiazolinone, and octylisothiazolinone. International Network of Departments of Dermatology data, 2009-2013. Contact Dermatitis. 2015;72:337-339.
Practice Points
- Methylisothiazolinone (MI) is a preservative found in water-based personal care products and is a common allergen in patch-tested populations.
- Methylisothiazolinone also has been identified in household products, industrial chemicals, paint, adhesives, and other unique sources.
- Benzisothiazolinone and octylisothiazolinone are structurally similar to MI, and a subset of MI-allergic patients may need to avoid them.