User login
Racial disparities in colon cancer survival mainly driven by tumor stage at presentation
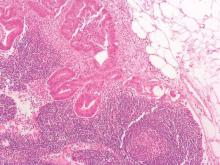
Although black patients with colon cancer received significantly less treatment than white patients, particularly for late stage disease, much of the overall survival disparity between black and white patients was explained by tumor presentation at diagnosis rather than treatment differences, according to an analysis of SEER data.
Among demographically matched black and white patients, the 5-year survival difference was 8.3% (P less than .0001). Presentation match reduced the difference to 5.0% (P less than .0001), which accounted for 39.8% of the overall disparity. Additional matching by treatment reduced the difference only slightly to 4.9% (P less than .0001), which accounted for 1.2% of the overall disparity. Black patients had lower rates for most treatments, including surgery, than presentation-matched white patients (88.5% vs. 91.4%), and these differences were most pronounced at advanced stages. For example, significant differences between black and white patients in the use of chemotherapy was observed for stage III (53.1% vs. 64.2%; P less than .0001) and stage IV (56.1% vs. 63.3%; P = .001).
“Our results indicate that tumor presentation, including tumor stage, is indeed one of the most important factors contributing to the racial disparity in colon cancer survival. We observed that, after controlling for demographic factors, black patients in comparison with white patients had a significantly higher proportion of stage IV and lower proportions of stages I and II disease. Adequately matching on tumor presentation variables (e.g., stage, grade, size, and comorbidity) significantly reduced survival disparities,” wrote Dr. Yinzhi Lai of the Department of Medical Oncology at Sidney Kimmel Cancer Center, Philadelphia, and colleagues (Gastroenterology. 2016 Apr 4. doi: 10.1053/j.gastro.2016.01.030).
Treatment differences in advanced-stage patients, compared with early-stage patients, explained a higher proportion of the demographic-matched survival disparity. For example, in stage II patients, treatment match resulted in modest reductions in 2-, 3-, and 5-year survival rate disparities (2.7%-2.8%, 4.1%-3.6%, and 4.6%-4.0%, respectively); by contrast, in stage III patients, treatment match resulted in more substantial reductions in 2-, 3-, and 5-year survival rate disparities (4.5%-2.2%, 3.1%-2.0%, and 4.3%-2.8%, respectively). A similar effect was observed in patients with stage IV disease. The results suggest that, “to control survival disparity, more efforts may need to be tailored to minimize treatment disparities (especially chemotherapy use) in patients with advanced-stage disease,” the investigators wrote.
The retrospective data analysis used patient information from 68,141 patients (6,190 black, 61,951 white) aged 66 years and older with colon cancer identified from the National Cancer Institute SEER-Medicare database. Using a novel minimum distance matching strategy, investigators drew from the pool of white patients to match three distinct comparison cohorts to the same 6,190 black patients. Close matches between black and white patients bypassed the need for model-based analysis.
The primary matching analysis was limited by the inability to control for substantial differences in socioeconomic status, marital status, and urban/rural residence. A subcohort analysis of 2,000 matched black and white patients showed that when socioeconomic status was added to the demographic match, survival differences were reduced, indicating the important role of socioeconomic status on racial survival disparities.
Significantly better survival was observed in all patients who were diagnosed in 2004 or later, the year the Food and Drug Administration approved the important chemotherapy medicines oxaliplatin and bevacizumab. Separating the cohorts into those who were diagnosed before and after 2004 revealed that the racial survival disparity was lower in the more recent group, indicating a favorable impact of oxaliplatin and/or bevacizumab in reducing the survival disparity.
Prior studies have documented racial disparities in the incidence and outcomes of colon cancer in the United States. Black men and women have a higher overall incidence and more advanced stage of disease at diagnosis than white men and women, while being less likely to receive guideline-concordant treatment.

|
| Dr. Jennifer Lund |
To extend this work, the authors evaluated treatment disparities between black and white colon cancer patients aged 66 years and older and examined the impact of a variety of patient characteristics on racial disparities in overall survival using a novel, sequential matching algorithm that minimized the overall distance between black and white patients based on demographic-, tumor specific–, and treatment-related variables. The authors found that differences in overall survival were mainly driven by tumor presentation; however, advanced-stage black colon cancer patients received less guideline concordant-treatment than white patients. While this minimum-distance algorithm provided close black-white matches on prespecified factors, it could not accommodate other factors (for example, socioeconomic, marital, and urban/rural status); therefore, methodologic improvements to this method and comparisons to other commonly used approaches (that is, propensity score matching and weighting) are warranted.
Finally, these results apply to older black and white colon cancer patients with Medicare fee-for-service coverage only. Additional research using similar methods in older Medicare Advantage populations or younger adults may uncover unique drivers of overall survival disparities by race, which may require tailored interventions.
Jennifer L. Lund, Ph.D., is an assistant professor, department of epidemiology, University of North Carolina at Chapel Hill. She receives research support from the UNC Oncology Clinical Translational Research Training Program (K12 CA120780), as well as through a Research Starter Award from the PhRMA Foundation to the UNC Department of Epidemiology.
Prior studies have documented racial disparities in the incidence and outcomes of colon cancer in the United States. Black men and women have a higher overall incidence and more advanced stage of disease at diagnosis than white men and women, while being less likely to receive guideline-concordant treatment.

|
| Dr. Jennifer Lund |
To extend this work, the authors evaluated treatment disparities between black and white colon cancer patients aged 66 years and older and examined the impact of a variety of patient characteristics on racial disparities in overall survival using a novel, sequential matching algorithm that minimized the overall distance between black and white patients based on demographic-, tumor specific–, and treatment-related variables. The authors found that differences in overall survival were mainly driven by tumor presentation; however, advanced-stage black colon cancer patients received less guideline concordant-treatment than white patients. While this minimum-distance algorithm provided close black-white matches on prespecified factors, it could not accommodate other factors (for example, socioeconomic, marital, and urban/rural status); therefore, methodologic improvements to this method and comparisons to other commonly used approaches (that is, propensity score matching and weighting) are warranted.
Finally, these results apply to older black and white colon cancer patients with Medicare fee-for-service coverage only. Additional research using similar methods in older Medicare Advantage populations or younger adults may uncover unique drivers of overall survival disparities by race, which may require tailored interventions.
Jennifer L. Lund, Ph.D., is an assistant professor, department of epidemiology, University of North Carolina at Chapel Hill. She receives research support from the UNC Oncology Clinical Translational Research Training Program (K12 CA120780), as well as through a Research Starter Award from the PhRMA Foundation to the UNC Department of Epidemiology.
Prior studies have documented racial disparities in the incidence and outcomes of colon cancer in the United States. Black men and women have a higher overall incidence and more advanced stage of disease at diagnosis than white men and women, while being less likely to receive guideline-concordant treatment.

|
| Dr. Jennifer Lund |
To extend this work, the authors evaluated treatment disparities between black and white colon cancer patients aged 66 years and older and examined the impact of a variety of patient characteristics on racial disparities in overall survival using a novel, sequential matching algorithm that minimized the overall distance between black and white patients based on demographic-, tumor specific–, and treatment-related variables. The authors found that differences in overall survival were mainly driven by tumor presentation; however, advanced-stage black colon cancer patients received less guideline concordant-treatment than white patients. While this minimum-distance algorithm provided close black-white matches on prespecified factors, it could not accommodate other factors (for example, socioeconomic, marital, and urban/rural status); therefore, methodologic improvements to this method and comparisons to other commonly used approaches (that is, propensity score matching and weighting) are warranted.
Finally, these results apply to older black and white colon cancer patients with Medicare fee-for-service coverage only. Additional research using similar methods in older Medicare Advantage populations or younger adults may uncover unique drivers of overall survival disparities by race, which may require tailored interventions.
Jennifer L. Lund, Ph.D., is an assistant professor, department of epidemiology, University of North Carolina at Chapel Hill. She receives research support from the UNC Oncology Clinical Translational Research Training Program (K12 CA120780), as well as through a Research Starter Award from the PhRMA Foundation to the UNC Department of Epidemiology.
Although black patients with colon cancer received significantly less treatment than white patients, particularly for late stage disease, much of the overall survival disparity between black and white patients was explained by tumor presentation at diagnosis rather than treatment differences, according to an analysis of SEER data.
Among demographically matched black and white patients, the 5-year survival difference was 8.3% (P less than .0001). Presentation match reduced the difference to 5.0% (P less than .0001), which accounted for 39.8% of the overall disparity. Additional matching by treatment reduced the difference only slightly to 4.9% (P less than .0001), which accounted for 1.2% of the overall disparity. Black patients had lower rates for most treatments, including surgery, than presentation-matched white patients (88.5% vs. 91.4%), and these differences were most pronounced at advanced stages. For example, significant differences between black and white patients in the use of chemotherapy was observed for stage III (53.1% vs. 64.2%; P less than .0001) and stage IV (56.1% vs. 63.3%; P = .001).
“Our results indicate that tumor presentation, including tumor stage, is indeed one of the most important factors contributing to the racial disparity in colon cancer survival. We observed that, after controlling for demographic factors, black patients in comparison with white patients had a significantly higher proportion of stage IV and lower proportions of stages I and II disease. Adequately matching on tumor presentation variables (e.g., stage, grade, size, and comorbidity) significantly reduced survival disparities,” wrote Dr. Yinzhi Lai of the Department of Medical Oncology at Sidney Kimmel Cancer Center, Philadelphia, and colleagues (Gastroenterology. 2016 Apr 4. doi: 10.1053/j.gastro.2016.01.030).
Treatment differences in advanced-stage patients, compared with early-stage patients, explained a higher proportion of the demographic-matched survival disparity. For example, in stage II patients, treatment match resulted in modest reductions in 2-, 3-, and 5-year survival rate disparities (2.7%-2.8%, 4.1%-3.6%, and 4.6%-4.0%, respectively); by contrast, in stage III patients, treatment match resulted in more substantial reductions in 2-, 3-, and 5-year survival rate disparities (4.5%-2.2%, 3.1%-2.0%, and 4.3%-2.8%, respectively). A similar effect was observed in patients with stage IV disease. The results suggest that, “to control survival disparity, more efforts may need to be tailored to minimize treatment disparities (especially chemotherapy use) in patients with advanced-stage disease,” the investigators wrote.
The retrospective data analysis used patient information from 68,141 patients (6,190 black, 61,951 white) aged 66 years and older with colon cancer identified from the National Cancer Institute SEER-Medicare database. Using a novel minimum distance matching strategy, investigators drew from the pool of white patients to match three distinct comparison cohorts to the same 6,190 black patients. Close matches between black and white patients bypassed the need for model-based analysis.
The primary matching analysis was limited by the inability to control for substantial differences in socioeconomic status, marital status, and urban/rural residence. A subcohort analysis of 2,000 matched black and white patients showed that when socioeconomic status was added to the demographic match, survival differences were reduced, indicating the important role of socioeconomic status on racial survival disparities.
Significantly better survival was observed in all patients who were diagnosed in 2004 or later, the year the Food and Drug Administration approved the important chemotherapy medicines oxaliplatin and bevacizumab. Separating the cohorts into those who were diagnosed before and after 2004 revealed that the racial survival disparity was lower in the more recent group, indicating a favorable impact of oxaliplatin and/or bevacizumab in reducing the survival disparity.
Although black patients with colon cancer received significantly less treatment than white patients, particularly for late stage disease, much of the overall survival disparity between black and white patients was explained by tumor presentation at diagnosis rather than treatment differences, according to an analysis of SEER data.
Among demographically matched black and white patients, the 5-year survival difference was 8.3% (P less than .0001). Presentation match reduced the difference to 5.0% (P less than .0001), which accounted for 39.8% of the overall disparity. Additional matching by treatment reduced the difference only slightly to 4.9% (P less than .0001), which accounted for 1.2% of the overall disparity. Black patients had lower rates for most treatments, including surgery, than presentation-matched white patients (88.5% vs. 91.4%), and these differences were most pronounced at advanced stages. For example, significant differences between black and white patients in the use of chemotherapy was observed for stage III (53.1% vs. 64.2%; P less than .0001) and stage IV (56.1% vs. 63.3%; P = .001).
“Our results indicate that tumor presentation, including tumor stage, is indeed one of the most important factors contributing to the racial disparity in colon cancer survival. We observed that, after controlling for demographic factors, black patients in comparison with white patients had a significantly higher proportion of stage IV and lower proportions of stages I and II disease. Adequately matching on tumor presentation variables (e.g., stage, grade, size, and comorbidity) significantly reduced survival disparities,” wrote Dr. Yinzhi Lai of the Department of Medical Oncology at Sidney Kimmel Cancer Center, Philadelphia, and colleagues (Gastroenterology. 2016 Apr 4. doi: 10.1053/j.gastro.2016.01.030).
Treatment differences in advanced-stage patients, compared with early-stage patients, explained a higher proportion of the demographic-matched survival disparity. For example, in stage II patients, treatment match resulted in modest reductions in 2-, 3-, and 5-year survival rate disparities (2.7%-2.8%, 4.1%-3.6%, and 4.6%-4.0%, respectively); by contrast, in stage III patients, treatment match resulted in more substantial reductions in 2-, 3-, and 5-year survival rate disparities (4.5%-2.2%, 3.1%-2.0%, and 4.3%-2.8%, respectively). A similar effect was observed in patients with stage IV disease. The results suggest that, “to control survival disparity, more efforts may need to be tailored to minimize treatment disparities (especially chemotherapy use) in patients with advanced-stage disease,” the investigators wrote.
The retrospective data analysis used patient information from 68,141 patients (6,190 black, 61,951 white) aged 66 years and older with colon cancer identified from the National Cancer Institute SEER-Medicare database. Using a novel minimum distance matching strategy, investigators drew from the pool of white patients to match three distinct comparison cohorts to the same 6,190 black patients. Close matches between black and white patients bypassed the need for model-based analysis.
The primary matching analysis was limited by the inability to control for substantial differences in socioeconomic status, marital status, and urban/rural residence. A subcohort analysis of 2,000 matched black and white patients showed that when socioeconomic status was added to the demographic match, survival differences were reduced, indicating the important role of socioeconomic status on racial survival disparities.
Significantly better survival was observed in all patients who were diagnosed in 2004 or later, the year the Food and Drug Administration approved the important chemotherapy medicines oxaliplatin and bevacizumab. Separating the cohorts into those who were diagnosed before and after 2004 revealed that the racial survival disparity was lower in the more recent group, indicating a favorable impact of oxaliplatin and/or bevacizumab in reducing the survival disparity.
FROM GASTROENTEROLOGY
Key clinical point: Tumor stage at diagnosis had a greater effect on survival disparities between black and white patients with colon cancer than treatment differences.
Major finding: Among demographically matched black and white patients, the 5-year survival difference was 8.3% (P less than .0001); matching by presentation reduced the difference to 5.0% (P less than .0001), and additional matching by treatment reduced the difference only slightly to 4.9% (P less than .0001).
Data sources: In total, 68,141 patients (6,190 black, 61,951 white) aged 66 years and older with colon cancer were identified from the National Cancer Institute SEER-Medicare database. Three white comparison cohorts were assembled and matched to the same 6,190 black patients.
Disclosures: Dr. Lai and coauthors reported having no disclosures.
Thromboprophylaxis efficacy similar before and after colorectal surgery
CHICAGO – Lower extremity duplex scans should be performed prior to colorectal surgery, and anticoagulation should be tailored to the result, findings from a randomized clinical trial suggest.
The findings also raise questions about the fairness of financial penalties imposed by the Centers for Medicare & Medicaid Services for perioperative venous thromboembolism, Dr. Karen Zaghiyan of Cedars Sinai Medical Center, Los Angeles said at the annual meeting of the American Surgical Association.
In 376 consecutive adult patients undergoing laparoscopic or open major colorectal surgery who had no occult preoperative deep vein thrombosis (DVT) on lower extremity venous duplex scan and who were randomized to preoperative or postoperative chemical thromboprophylaxis (CTP) with 5,000 U of subcutaneous heparin, no differences were seen with respect to the primary outcome of venous thromboembolism within 48 hours of surgery, Dr. Zaghiyan said.
“There was no significant difference in our primary outcome – early postoperative VTE [venous thromboembolism] – in patients managed with postoperative or preoperative prophylaxis,” she said, noting that three patients in each group developed asymptomatic intraoperative DVT, and two additional patients in the postoperative treatment group developed asymptomatic DVT between postoperative day 0 and 2.
Two additional patients in the postoperative treatment group developed clinically significant DVT between postoperative day 2 and 30.
“Both patients had a complicated prolonged hospital course, and developed DVT while still hospitalized. This difference still did not reach statistical significance, and there were no post-discharge DVT or PEs [pulmonary embolisms] in the entire cohort,” she said.
Bleeding complications, including estimated blood loss and number receiving transfusion, were similar in the two groups, she said, noting that no patients developed heparin-induced thrombocytopenia, and that hospital stay, readmissions, and overall complications were similar between the two groups.
Study subjects had a mean age of 53 years, and 52% were women. The preoperative- and postoperative treatment groups were similar with respect to demographics and preoperative characteristics. They underwent lower extremity venous duplex just prior to surgery, immediately after surgery in the recovery room, on day 2 after surgery, and subsequently as clinically indicated.
Thromboprophylaxis in the preoperative treatment group was given in the “pre-op holding area” then 8 hours after surgery and every 8 hours thereafter until discharge. Thromboprophylaxis in the postoperative treatment group was given within 24 hours after surgery, and then every 8 hours until discharge.
Preoperative and postoperative CTP were equally safe and effective, and since occult preoperative DVT is twice as common as postoperative DVT, occurring in a surprising 4% of patients in this study, the findings support preoperative scans and anticoagulation based on the results – especially in older patients and those with comorbid disease, Dr. Zaghiyan said.
The findings could help improve patients care; although VTE prevention and chemical prophylaxis in colorectal surgery have been extensively studied, current guidelines are vague, with both the American College of Chest Physicians and the Surgical Care Improvement Project recommending that prophylaxis be initiated 24 hours prior to or after major colorectal surgery, she said.
The findings could also help avoid CMS penalties for postoperatively identified VTE,” she added.
Further, those penalties may not be supported by the clinical data; in this study, the majority of early postoperative DVTs were unpreventable, with no additional protection provided with preoperative prophylaxis, she explained.
“CMS should reevaluate the financial penalties, taking preventability into account,” she said.
Dr. Zaghiyan reported having no disclosures.
The complete manuscript of this presentation is anticipated to be published in the Annals of Surgery pending editorial review.
CHICAGO – Lower extremity duplex scans should be performed prior to colorectal surgery, and anticoagulation should be tailored to the result, findings from a randomized clinical trial suggest.
The findings also raise questions about the fairness of financial penalties imposed by the Centers for Medicare & Medicaid Services for perioperative venous thromboembolism, Dr. Karen Zaghiyan of Cedars Sinai Medical Center, Los Angeles said at the annual meeting of the American Surgical Association.
In 376 consecutive adult patients undergoing laparoscopic or open major colorectal surgery who had no occult preoperative deep vein thrombosis (DVT) on lower extremity venous duplex scan and who were randomized to preoperative or postoperative chemical thromboprophylaxis (CTP) with 5,000 U of subcutaneous heparin, no differences were seen with respect to the primary outcome of venous thromboembolism within 48 hours of surgery, Dr. Zaghiyan said.
“There was no significant difference in our primary outcome – early postoperative VTE [venous thromboembolism] – in patients managed with postoperative or preoperative prophylaxis,” she said, noting that three patients in each group developed asymptomatic intraoperative DVT, and two additional patients in the postoperative treatment group developed asymptomatic DVT between postoperative day 0 and 2.
Two additional patients in the postoperative treatment group developed clinically significant DVT between postoperative day 2 and 30.
“Both patients had a complicated prolonged hospital course, and developed DVT while still hospitalized. This difference still did not reach statistical significance, and there were no post-discharge DVT or PEs [pulmonary embolisms] in the entire cohort,” she said.
Bleeding complications, including estimated blood loss and number receiving transfusion, were similar in the two groups, she said, noting that no patients developed heparin-induced thrombocytopenia, and that hospital stay, readmissions, and overall complications were similar between the two groups.
Study subjects had a mean age of 53 years, and 52% were women. The preoperative- and postoperative treatment groups were similar with respect to demographics and preoperative characteristics. They underwent lower extremity venous duplex just prior to surgery, immediately after surgery in the recovery room, on day 2 after surgery, and subsequently as clinically indicated.
Thromboprophylaxis in the preoperative treatment group was given in the “pre-op holding area” then 8 hours after surgery and every 8 hours thereafter until discharge. Thromboprophylaxis in the postoperative treatment group was given within 24 hours after surgery, and then every 8 hours until discharge.
Preoperative and postoperative CTP were equally safe and effective, and since occult preoperative DVT is twice as common as postoperative DVT, occurring in a surprising 4% of patients in this study, the findings support preoperative scans and anticoagulation based on the results – especially in older patients and those with comorbid disease, Dr. Zaghiyan said.
The findings could help improve patients care; although VTE prevention and chemical prophylaxis in colorectal surgery have been extensively studied, current guidelines are vague, with both the American College of Chest Physicians and the Surgical Care Improvement Project recommending that prophylaxis be initiated 24 hours prior to or after major colorectal surgery, she said.
The findings could also help avoid CMS penalties for postoperatively identified VTE,” she added.
Further, those penalties may not be supported by the clinical data; in this study, the majority of early postoperative DVTs were unpreventable, with no additional protection provided with preoperative prophylaxis, she explained.
“CMS should reevaluate the financial penalties, taking preventability into account,” she said.
Dr. Zaghiyan reported having no disclosures.
The complete manuscript of this presentation is anticipated to be published in the Annals of Surgery pending editorial review.
CHICAGO – Lower extremity duplex scans should be performed prior to colorectal surgery, and anticoagulation should be tailored to the result, findings from a randomized clinical trial suggest.
The findings also raise questions about the fairness of financial penalties imposed by the Centers for Medicare & Medicaid Services for perioperative venous thromboembolism, Dr. Karen Zaghiyan of Cedars Sinai Medical Center, Los Angeles said at the annual meeting of the American Surgical Association.
In 376 consecutive adult patients undergoing laparoscopic or open major colorectal surgery who had no occult preoperative deep vein thrombosis (DVT) on lower extremity venous duplex scan and who were randomized to preoperative or postoperative chemical thromboprophylaxis (CTP) with 5,000 U of subcutaneous heparin, no differences were seen with respect to the primary outcome of venous thromboembolism within 48 hours of surgery, Dr. Zaghiyan said.
“There was no significant difference in our primary outcome – early postoperative VTE [venous thromboembolism] – in patients managed with postoperative or preoperative prophylaxis,” she said, noting that three patients in each group developed asymptomatic intraoperative DVT, and two additional patients in the postoperative treatment group developed asymptomatic DVT between postoperative day 0 and 2.
Two additional patients in the postoperative treatment group developed clinically significant DVT between postoperative day 2 and 30.
“Both patients had a complicated prolonged hospital course, and developed DVT while still hospitalized. This difference still did not reach statistical significance, and there were no post-discharge DVT or PEs [pulmonary embolisms] in the entire cohort,” she said.
Bleeding complications, including estimated blood loss and number receiving transfusion, were similar in the two groups, she said, noting that no patients developed heparin-induced thrombocytopenia, and that hospital stay, readmissions, and overall complications were similar between the two groups.
Study subjects had a mean age of 53 years, and 52% were women. The preoperative- and postoperative treatment groups were similar with respect to demographics and preoperative characteristics. They underwent lower extremity venous duplex just prior to surgery, immediately after surgery in the recovery room, on day 2 after surgery, and subsequently as clinically indicated.
Thromboprophylaxis in the preoperative treatment group was given in the “pre-op holding area” then 8 hours after surgery and every 8 hours thereafter until discharge. Thromboprophylaxis in the postoperative treatment group was given within 24 hours after surgery, and then every 8 hours until discharge.
Preoperative and postoperative CTP were equally safe and effective, and since occult preoperative DVT is twice as common as postoperative DVT, occurring in a surprising 4% of patients in this study, the findings support preoperative scans and anticoagulation based on the results – especially in older patients and those with comorbid disease, Dr. Zaghiyan said.
The findings could help improve patients care; although VTE prevention and chemical prophylaxis in colorectal surgery have been extensively studied, current guidelines are vague, with both the American College of Chest Physicians and the Surgical Care Improvement Project recommending that prophylaxis be initiated 24 hours prior to or after major colorectal surgery, she said.
The findings could also help avoid CMS penalties for postoperatively identified VTE,” she added.
Further, those penalties may not be supported by the clinical data; in this study, the majority of early postoperative DVTs were unpreventable, with no additional protection provided with preoperative prophylaxis, she explained.
“CMS should reevaluate the financial penalties, taking preventability into account,” she said.
Dr. Zaghiyan reported having no disclosures.
The complete manuscript of this presentation is anticipated to be published in the Annals of Surgery pending editorial review.
AT THE ASA ANNUAL MEETING
Key clinical point: Lower extremity duplex scans should be performed prior to colorectal surgery, and anticoagulation should be tailored to the result, findings from a randomized clinical trial suggest.
Major finding: No differences were seen with respect to the primary outcome of venous thromboembolism within 48 hours of surgery in patients treated with pre- or post-operative chemical thromboprophylaxis.
Data source: A randomized clinical trial of 376 patients.
Disclosures: Dr. Zaghiyan reported having no disclosures.
VIDEO: Anesthesia services during colonoscopy increase risk of near-term complications
Receiving anesthesia services while undergoing a colonoscopy may not be in your patients’ best interest, as doing so could significantly increase the likelihood of patients experiencing serious complications within 30 days of the procedure.
This is according to a new study published in the April issue of Gastroenterology, in which Dr. Karen J. Wernli and her coinvestigators analyzed claims data, collected from the Truven Health MarketScan Research Database, related to 3,168,228 colonoscopy procedures that took place between 2008 and 2011, to determine whether patients who received anesthesia were at a higher risk of developing complications after the procedure (doi: 10.1053/j.gastro.2015.12.018).
Source: American Gastroenterological Association
“The involvement of anesthesia services for colonoscopy sedation, mainly to administer propofol, has increased accordingly, from 11.0% of colonoscopies in 2001 to 23.4% in 2006, with projections of more than 50% in 2015,” wrote Dr. Wernli of the Group Health Research Institute in Seattle, and her coauthors. “Whether the use of propofol is associated with higher rates of short-term complications compared with standard sedation is not well understood.”
Men and women whose data was included in the study were between 40 and 64 years of age; men accounted for 46.8% of those receiving standard sedation (53.2% women) and 46.5% of those receiving anesthesia services (53.5% women). A total of 4,939,993 individuals were initially screened for enrollment, with 39,784 excluded because of a previous colorectal cancer diagnosis, 240,038 for “noncancer exclusions,” and 1,491,943 for being enrolled in the study less than 1 year.
Standard sedation was done in 2,079,784 (65.6%) of the procedures included in the study, while the other 1,088,444 (34.4%) colonoscopies involved anesthesia services. Use of anesthesia services resulted in a 13% increase in likelihood for patients to experience some kind of complication within 30 days of colonoscopy (95% confidence interval, 1.12-1.14). The most common complications were perforation (odds ratio, 1.07; 95% CI, 1.00-1.15), hemorrhage (OR, 1.28; 95% CI, 1.27-1.30), abdominal pain (OR, 1.07; 95% CI, 1.05-1.08), complications secondary to anesthesia (OR, 1.15; 95% CI, 1.05-1.28), and “stroke and other central nervous system events” (OR, 1.04; 95% CI, 1.00-1.08).
Analysis of geographic distribution of colonoscopies performed with and without anesthesia services showed that all areas of the United States had a higher likelihood of postcolonoscopy complications associated with anesthesia except in the Southeast, where there was no association between the two. Additionally, in the western U.S., use of anesthesia services was less common than in any other geographic area, but was associated with a staggering 60% higher chance of complication within 30 days for patients who did opt for it.
“Although the use of anesthesia agents can directly impact colonoscopy outcomes, it is not solely the anesthesia agent that could lead to additional complications,” the study authors wrote. “In the absence of patient feedback, increased colonic-wall tension from colonoscopy pressure may not be identified by the endoscopist, and, consistent with our results, could lead to increased risks of colonic complications, such as perforation and abdominal pain.”
Dr. Wernli and her coauthors did not report any relevant financial disclosures.
We are approaching a time when half of all colonoscopies are performed with anesthesia assistance, most using propofol. Undeniably, some patients require anesthesia support for medical reasons, or because they do not sedate adequately with opiate-benzodiazepine combinations endoscopists can administer. The popularity of propofol-based anesthesia for routine colonoscopy, however, is based on several perceived benefits: patient demand for a discomfort-free procedure, rapid sedation followed by quick recovery, and good reimbursement for the anesthesia service itself, added to the benefits of faster overall procedure turnaround time. And presently, there is no disincentive — financial or otherwise — to continuing or expanding this practice. Colonoscopy with anesthesia looks like a win-win for both patient and endoscopist, as long as the added cost of anesthesia can be justified.
However, while anesthesia-assisted colonoscopy appears to possess several advantages, growing evidence suggests that a lower risk of complications is not one of them.
A smaller study (165,000 colonoscopies) using NCI SEER registry data suggested that adding anesthesia to colonoscopy may increase some adverse events. Cooper et al. (JAMA Intern Med. 2013;173:551-6) showed an increase in overall complications and, specifically, aspiration, although not in technical complications of colonoscopy, including perforation and splenic rupture. However, this study did not include patients who underwent polypectomy. Wernli, et al. now show evidence derived from over 3 million patients demonstrating that adding anesthesia to colonoscopy increases complications significantly — not only aspiration, but also technical aspects of colonoscopy, including perforation, bleeding, and abdominal pain.
Colonoscopy is extremely safe, so complications are infrequent. Thus, data sets of colonoscopy complications large enough to be statistically meaningful for studies of this type require an extraordinarily large patient pool. For this prospective, observational cohort study, the authors obtained the large sample size by mining administrative claims data for 3 years, not through examining clinical data. As a result, several assumptions were made. These 3 million colonoscopies represented all indications — not just colorectal cancer screening. Billing claims for anesthesia represented surrogate markers for administration of propofol-based anesthesia. While anesthesia assistance was associated with increased risk of perforation, hemorrhage, abdominal pain, anesthesia complications, and stroke; risk of perforation associated with anesthesia was increased only in patients who underwent polypectomy.
Study methodology and confounding variables aside, it is hard to ignore the core message here: a large body of data analyzed rigorously demonstrate that anesthesia support for colonoscopy increases risk of procedure-related complications.
Patients who are ill, have certain cardiopulmonary issues, or do not sedate adequately with moderate sedation benefit from anesthesia assistance for colonoscopy. But for patients undergoing routine colonoscopy, without such issues, who could safely undergo colonoscopy under moderate sedation without unreasonable discomfort, we must now ask ourselves and discuss with our patients honestly, not only whether the added cost of anesthesia is reasonable — but also whether the apparent added risk of anesthesia justifies perceived benefits.
Dr. John A. Martin is senior associate consultant and associate professor, associate chair for endoscopy, Mayo Clinic, Rochester, Minn. He has no conflicts of interest to disclose.
We are approaching a time when half of all colonoscopies are performed with anesthesia assistance, most using propofol. Undeniably, some patients require anesthesia support for medical reasons, or because they do not sedate adequately with opiate-benzodiazepine combinations endoscopists can administer. The popularity of propofol-based anesthesia for routine colonoscopy, however, is based on several perceived benefits: patient demand for a discomfort-free procedure, rapid sedation followed by quick recovery, and good reimbursement for the anesthesia service itself, added to the benefits of faster overall procedure turnaround time. And presently, there is no disincentive — financial or otherwise — to continuing or expanding this practice. Colonoscopy with anesthesia looks like a win-win for both patient and endoscopist, as long as the added cost of anesthesia can be justified.
However, while anesthesia-assisted colonoscopy appears to possess several advantages, growing evidence suggests that a lower risk of complications is not one of them.
A smaller study (165,000 colonoscopies) using NCI SEER registry data suggested that adding anesthesia to colonoscopy may increase some adverse events. Cooper et al. (JAMA Intern Med. 2013;173:551-6) showed an increase in overall complications and, specifically, aspiration, although not in technical complications of colonoscopy, including perforation and splenic rupture. However, this study did not include patients who underwent polypectomy. Wernli, et al. now show evidence derived from over 3 million patients demonstrating that adding anesthesia to colonoscopy increases complications significantly — not only aspiration, but also technical aspects of colonoscopy, including perforation, bleeding, and abdominal pain.
Colonoscopy is extremely safe, so complications are infrequent. Thus, data sets of colonoscopy complications large enough to be statistically meaningful for studies of this type require an extraordinarily large patient pool. For this prospective, observational cohort study, the authors obtained the large sample size by mining administrative claims data for 3 years, not through examining clinical data. As a result, several assumptions were made. These 3 million colonoscopies represented all indications — not just colorectal cancer screening. Billing claims for anesthesia represented surrogate markers for administration of propofol-based anesthesia. While anesthesia assistance was associated with increased risk of perforation, hemorrhage, abdominal pain, anesthesia complications, and stroke; risk of perforation associated with anesthesia was increased only in patients who underwent polypectomy.
Study methodology and confounding variables aside, it is hard to ignore the core message here: a large body of data analyzed rigorously demonstrate that anesthesia support for colonoscopy increases risk of procedure-related complications.
Patients who are ill, have certain cardiopulmonary issues, or do not sedate adequately with moderate sedation benefit from anesthesia assistance for colonoscopy. But for patients undergoing routine colonoscopy, without such issues, who could safely undergo colonoscopy under moderate sedation without unreasonable discomfort, we must now ask ourselves and discuss with our patients honestly, not only whether the added cost of anesthesia is reasonable — but also whether the apparent added risk of anesthesia justifies perceived benefits.
Dr. John A. Martin is senior associate consultant and associate professor, associate chair for endoscopy, Mayo Clinic, Rochester, Minn. He has no conflicts of interest to disclose.
We are approaching a time when half of all colonoscopies are performed with anesthesia assistance, most using propofol. Undeniably, some patients require anesthesia support for medical reasons, or because they do not sedate adequately with opiate-benzodiazepine combinations endoscopists can administer. The popularity of propofol-based anesthesia for routine colonoscopy, however, is based on several perceived benefits: patient demand for a discomfort-free procedure, rapid sedation followed by quick recovery, and good reimbursement for the anesthesia service itself, added to the benefits of faster overall procedure turnaround time. And presently, there is no disincentive — financial or otherwise — to continuing or expanding this practice. Colonoscopy with anesthesia looks like a win-win for both patient and endoscopist, as long as the added cost of anesthesia can be justified.
However, while anesthesia-assisted colonoscopy appears to possess several advantages, growing evidence suggests that a lower risk of complications is not one of them.
A smaller study (165,000 colonoscopies) using NCI SEER registry data suggested that adding anesthesia to colonoscopy may increase some adverse events. Cooper et al. (JAMA Intern Med. 2013;173:551-6) showed an increase in overall complications and, specifically, aspiration, although not in technical complications of colonoscopy, including perforation and splenic rupture. However, this study did not include patients who underwent polypectomy. Wernli, et al. now show evidence derived from over 3 million patients demonstrating that adding anesthesia to colonoscopy increases complications significantly — not only aspiration, but also technical aspects of colonoscopy, including perforation, bleeding, and abdominal pain.
Colonoscopy is extremely safe, so complications are infrequent. Thus, data sets of colonoscopy complications large enough to be statistically meaningful for studies of this type require an extraordinarily large patient pool. For this prospective, observational cohort study, the authors obtained the large sample size by mining administrative claims data for 3 years, not through examining clinical data. As a result, several assumptions were made. These 3 million colonoscopies represented all indications — not just colorectal cancer screening. Billing claims for anesthesia represented surrogate markers for administration of propofol-based anesthesia. While anesthesia assistance was associated with increased risk of perforation, hemorrhage, abdominal pain, anesthesia complications, and stroke; risk of perforation associated with anesthesia was increased only in patients who underwent polypectomy.
Study methodology and confounding variables aside, it is hard to ignore the core message here: a large body of data analyzed rigorously demonstrate that anesthesia support for colonoscopy increases risk of procedure-related complications.
Patients who are ill, have certain cardiopulmonary issues, or do not sedate adequately with moderate sedation benefit from anesthesia assistance for colonoscopy. But for patients undergoing routine colonoscopy, without such issues, who could safely undergo colonoscopy under moderate sedation without unreasonable discomfort, we must now ask ourselves and discuss with our patients honestly, not only whether the added cost of anesthesia is reasonable — but also whether the apparent added risk of anesthesia justifies perceived benefits.
Dr. John A. Martin is senior associate consultant and associate professor, associate chair for endoscopy, Mayo Clinic, Rochester, Minn. He has no conflicts of interest to disclose.
Receiving anesthesia services while undergoing a colonoscopy may not be in your patients’ best interest, as doing so could significantly increase the likelihood of patients experiencing serious complications within 30 days of the procedure.
This is according to a new study published in the April issue of Gastroenterology, in which Dr. Karen J. Wernli and her coinvestigators analyzed claims data, collected from the Truven Health MarketScan Research Database, related to 3,168,228 colonoscopy procedures that took place between 2008 and 2011, to determine whether patients who received anesthesia were at a higher risk of developing complications after the procedure (doi: 10.1053/j.gastro.2015.12.018).
Source: American Gastroenterological Association
“The involvement of anesthesia services for colonoscopy sedation, mainly to administer propofol, has increased accordingly, from 11.0% of colonoscopies in 2001 to 23.4% in 2006, with projections of more than 50% in 2015,” wrote Dr. Wernli of the Group Health Research Institute in Seattle, and her coauthors. “Whether the use of propofol is associated with higher rates of short-term complications compared with standard sedation is not well understood.”
Men and women whose data was included in the study were between 40 and 64 years of age; men accounted for 46.8% of those receiving standard sedation (53.2% women) and 46.5% of those receiving anesthesia services (53.5% women). A total of 4,939,993 individuals were initially screened for enrollment, with 39,784 excluded because of a previous colorectal cancer diagnosis, 240,038 for “noncancer exclusions,” and 1,491,943 for being enrolled in the study less than 1 year.
Standard sedation was done in 2,079,784 (65.6%) of the procedures included in the study, while the other 1,088,444 (34.4%) colonoscopies involved anesthesia services. Use of anesthesia services resulted in a 13% increase in likelihood for patients to experience some kind of complication within 30 days of colonoscopy (95% confidence interval, 1.12-1.14). The most common complications were perforation (odds ratio, 1.07; 95% CI, 1.00-1.15), hemorrhage (OR, 1.28; 95% CI, 1.27-1.30), abdominal pain (OR, 1.07; 95% CI, 1.05-1.08), complications secondary to anesthesia (OR, 1.15; 95% CI, 1.05-1.28), and “stroke and other central nervous system events” (OR, 1.04; 95% CI, 1.00-1.08).
Analysis of geographic distribution of colonoscopies performed with and without anesthesia services showed that all areas of the United States had a higher likelihood of postcolonoscopy complications associated with anesthesia except in the Southeast, where there was no association between the two. Additionally, in the western U.S., use of anesthesia services was less common than in any other geographic area, but was associated with a staggering 60% higher chance of complication within 30 days for patients who did opt for it.
“Although the use of anesthesia agents can directly impact colonoscopy outcomes, it is not solely the anesthesia agent that could lead to additional complications,” the study authors wrote. “In the absence of patient feedback, increased colonic-wall tension from colonoscopy pressure may not be identified by the endoscopist, and, consistent with our results, could lead to increased risks of colonic complications, such as perforation and abdominal pain.”
Dr. Wernli and her coauthors did not report any relevant financial disclosures.
Receiving anesthesia services while undergoing a colonoscopy may not be in your patients’ best interest, as doing so could significantly increase the likelihood of patients experiencing serious complications within 30 days of the procedure.
This is according to a new study published in the April issue of Gastroenterology, in which Dr. Karen J. Wernli and her coinvestigators analyzed claims data, collected from the Truven Health MarketScan Research Database, related to 3,168,228 colonoscopy procedures that took place between 2008 and 2011, to determine whether patients who received anesthesia were at a higher risk of developing complications after the procedure (doi: 10.1053/j.gastro.2015.12.018).
Source: American Gastroenterological Association
“The involvement of anesthesia services for colonoscopy sedation, mainly to administer propofol, has increased accordingly, from 11.0% of colonoscopies in 2001 to 23.4% in 2006, with projections of more than 50% in 2015,” wrote Dr. Wernli of the Group Health Research Institute in Seattle, and her coauthors. “Whether the use of propofol is associated with higher rates of short-term complications compared with standard sedation is not well understood.”
Men and women whose data was included in the study were between 40 and 64 years of age; men accounted for 46.8% of those receiving standard sedation (53.2% women) and 46.5% of those receiving anesthesia services (53.5% women). A total of 4,939,993 individuals were initially screened for enrollment, with 39,784 excluded because of a previous colorectal cancer diagnosis, 240,038 for “noncancer exclusions,” and 1,491,943 for being enrolled in the study less than 1 year.
Standard sedation was done in 2,079,784 (65.6%) of the procedures included in the study, while the other 1,088,444 (34.4%) colonoscopies involved anesthesia services. Use of anesthesia services resulted in a 13% increase in likelihood for patients to experience some kind of complication within 30 days of colonoscopy (95% confidence interval, 1.12-1.14). The most common complications were perforation (odds ratio, 1.07; 95% CI, 1.00-1.15), hemorrhage (OR, 1.28; 95% CI, 1.27-1.30), abdominal pain (OR, 1.07; 95% CI, 1.05-1.08), complications secondary to anesthesia (OR, 1.15; 95% CI, 1.05-1.28), and “stroke and other central nervous system events” (OR, 1.04; 95% CI, 1.00-1.08).
Analysis of geographic distribution of colonoscopies performed with and without anesthesia services showed that all areas of the United States had a higher likelihood of postcolonoscopy complications associated with anesthesia except in the Southeast, where there was no association between the two. Additionally, in the western U.S., use of anesthesia services was less common than in any other geographic area, but was associated with a staggering 60% higher chance of complication within 30 days for patients who did opt for it.
“Although the use of anesthesia agents can directly impact colonoscopy outcomes, it is not solely the anesthesia agent that could lead to additional complications,” the study authors wrote. “In the absence of patient feedback, increased colonic-wall tension from colonoscopy pressure may not be identified by the endoscopist, and, consistent with our results, could lead to increased risks of colonic complications, such as perforation and abdominal pain.”
Dr. Wernli and her coauthors did not report any relevant financial disclosures.
FROM GASTROENTEROLOGY
Key clinical point: Using anesthesia services on individuals receiving colonoscopy increases the overall risk of complications associated with the procedure.
Major finding: Colonoscopy patients who received anesthesia had a 13% higher risk of complication within 30 days, including perforation, hemorrhage, abdominal pain, and stroke.
Data source: A prospective cohort study of claims data from 3,168,228 colonoscopy procedures in the Truven Health MarketScan Research Databases from 2008 to 2011.
Disclosures: Funding provided by the Agency for Healthcare Research and Quality and the National Institutes of Health. Dr. Wernli and her coauthors did not report any relevant financial disclosures.
Late-week discharges to home after CRC surgery prone to readmission
BOSTON – The day of the week a patient is discharged from the hospital may have an impact the likelihood of readmission.
Patients discharged home from the hospital on a Thursday after colorectal cancer surgery are more likely to be readmitted within 30 days than those discharged on any other day of the week, investigators found.
In contrast, there were no significant day-dependent differences in readmission rates among patients discharged to a skilled nursing facility or acute rehabilitation program, although patients admitted to clinical facilities had higher overall readmission rates, reported Anna Gustin and coinvestigators at the Levine Cancer Institute at the Carolinas Medical Center in Charlotte, N.C.
“For a patient discharged on a Thursday, if you’re going to get an infection, it’s going to be probably during the weekend, when it’s difficult to contact your primary physician, and when other resources are not as readily available,” said Ms. Gustin, who conducts epidemiologic research at Levine Cancer Center and is also a pre-med student and Japanese major at Wake Forest University in Winston-Salem, N.C.
In a study presented in a poster session at the annual Society of Surgical Oncology Cancer Symposium, Ms. Gustin and her coauthors looked at factors influencing readmission rates among patients undergoing surgery for primary, nonmetastatic colorectal cancer resections.
They drew on the to evaluate outcomes for 93,04 SEER-(Surveillance, Epidemiology, and End Results) Medicare database seven patients aged 66 years and older treated for primary colorectal cancer from 1998 through 2009.
They looked at potential contributing factors such as patient demographics, socioeconomic status, length of stay, days of admission and discharge, and discharge setting (home or clinical facility).
They use multivariate logistic regression models to analyze readmission rates at 14 and 30 days after initial discharge.
Focusing on home discharges, they found that as the week progressed, there was a significant likelihood that a patient discharged home would be readmitted (P less then .001 by chi-square and Cochran-Armitage tests). As noted before, the highest rate of readmission was for patients discharged on Thursday, at 12.4%, compared with 10.1% for patients discharged on Sunday, the discharge day least likely to be associated with rehospitalization.
In multivariate analysis, factors significantly associated with risk for 30-day readmission included male vs. female (hazard ratio, 1.16), black vs. other race (HR, 1.22), length of stay 5, 6-7, or 8-10 vs. 12 or more days (HR, 0.48, 0.59, 0.77, respectively), Charlson comorbidity index score 0, 1 or 3 vs. 3 (HR, 0.59, 0.73, 0.82, respectively), and home discharge vs. other (HR, 0.66; all above comparisons significant as shown by 95% confidence intervals).
The authors concluded that although home discharge itself reduces the likelihood of readmission, “improvements in preparing patients for discharge to home are needed. Additional outpatient interventions could rescue patients from readmission.”
They also suggested reexamining staffing policies and weekend availability of resources for patients, and call for addressing disparities in readmissions based on race, sex, length of stay, and comorbidities.
The study was internally supported. The authors reported having no relevant disclosures.
BOSTON – The day of the week a patient is discharged from the hospital may have an impact the likelihood of readmission.
Patients discharged home from the hospital on a Thursday after colorectal cancer surgery are more likely to be readmitted within 30 days than those discharged on any other day of the week, investigators found.
In contrast, there were no significant day-dependent differences in readmission rates among patients discharged to a skilled nursing facility or acute rehabilitation program, although patients admitted to clinical facilities had higher overall readmission rates, reported Anna Gustin and coinvestigators at the Levine Cancer Institute at the Carolinas Medical Center in Charlotte, N.C.
“For a patient discharged on a Thursday, if you’re going to get an infection, it’s going to be probably during the weekend, when it’s difficult to contact your primary physician, and when other resources are not as readily available,” said Ms. Gustin, who conducts epidemiologic research at Levine Cancer Center and is also a pre-med student and Japanese major at Wake Forest University in Winston-Salem, N.C.
In a study presented in a poster session at the annual Society of Surgical Oncology Cancer Symposium, Ms. Gustin and her coauthors looked at factors influencing readmission rates among patients undergoing surgery for primary, nonmetastatic colorectal cancer resections.
They drew on the to evaluate outcomes for 93,04 SEER-(Surveillance, Epidemiology, and End Results) Medicare database seven patients aged 66 years and older treated for primary colorectal cancer from 1998 through 2009.
They looked at potential contributing factors such as patient demographics, socioeconomic status, length of stay, days of admission and discharge, and discharge setting (home or clinical facility).
They use multivariate logistic regression models to analyze readmission rates at 14 and 30 days after initial discharge.
Focusing on home discharges, they found that as the week progressed, there was a significant likelihood that a patient discharged home would be readmitted (P less then .001 by chi-square and Cochran-Armitage tests). As noted before, the highest rate of readmission was for patients discharged on Thursday, at 12.4%, compared with 10.1% for patients discharged on Sunday, the discharge day least likely to be associated with rehospitalization.
In multivariate analysis, factors significantly associated with risk for 30-day readmission included male vs. female (hazard ratio, 1.16), black vs. other race (HR, 1.22), length of stay 5, 6-7, or 8-10 vs. 12 or more days (HR, 0.48, 0.59, 0.77, respectively), Charlson comorbidity index score 0, 1 or 3 vs. 3 (HR, 0.59, 0.73, 0.82, respectively), and home discharge vs. other (HR, 0.66; all above comparisons significant as shown by 95% confidence intervals).
The authors concluded that although home discharge itself reduces the likelihood of readmission, “improvements in preparing patients for discharge to home are needed. Additional outpatient interventions could rescue patients from readmission.”
They also suggested reexamining staffing policies and weekend availability of resources for patients, and call for addressing disparities in readmissions based on race, sex, length of stay, and comorbidities.
The study was internally supported. The authors reported having no relevant disclosures.
BOSTON – The day of the week a patient is discharged from the hospital may have an impact the likelihood of readmission.
Patients discharged home from the hospital on a Thursday after colorectal cancer surgery are more likely to be readmitted within 30 days than those discharged on any other day of the week, investigators found.
In contrast, there were no significant day-dependent differences in readmission rates among patients discharged to a skilled nursing facility or acute rehabilitation program, although patients admitted to clinical facilities had higher overall readmission rates, reported Anna Gustin and coinvestigators at the Levine Cancer Institute at the Carolinas Medical Center in Charlotte, N.C.
“For a patient discharged on a Thursday, if you’re going to get an infection, it’s going to be probably during the weekend, when it’s difficult to contact your primary physician, and when other resources are not as readily available,” said Ms. Gustin, who conducts epidemiologic research at Levine Cancer Center and is also a pre-med student and Japanese major at Wake Forest University in Winston-Salem, N.C.
In a study presented in a poster session at the annual Society of Surgical Oncology Cancer Symposium, Ms. Gustin and her coauthors looked at factors influencing readmission rates among patients undergoing surgery for primary, nonmetastatic colorectal cancer resections.
They drew on the to evaluate outcomes for 93,04 SEER-(Surveillance, Epidemiology, and End Results) Medicare database seven patients aged 66 years and older treated for primary colorectal cancer from 1998 through 2009.
They looked at potential contributing factors such as patient demographics, socioeconomic status, length of stay, days of admission and discharge, and discharge setting (home or clinical facility).
They use multivariate logistic regression models to analyze readmission rates at 14 and 30 days after initial discharge.
Focusing on home discharges, they found that as the week progressed, there was a significant likelihood that a patient discharged home would be readmitted (P less then .001 by chi-square and Cochran-Armitage tests). As noted before, the highest rate of readmission was for patients discharged on Thursday, at 12.4%, compared with 10.1% for patients discharged on Sunday, the discharge day least likely to be associated with rehospitalization.
In multivariate analysis, factors significantly associated with risk for 30-day readmission included male vs. female (hazard ratio, 1.16), black vs. other race (HR, 1.22), length of stay 5, 6-7, or 8-10 vs. 12 or more days (HR, 0.48, 0.59, 0.77, respectively), Charlson comorbidity index score 0, 1 or 3 vs. 3 (HR, 0.59, 0.73, 0.82, respectively), and home discharge vs. other (HR, 0.66; all above comparisons significant as shown by 95% confidence intervals).
The authors concluded that although home discharge itself reduces the likelihood of readmission, “improvements in preparing patients for discharge to home are needed. Additional outpatient interventions could rescue patients from readmission.”
They also suggested reexamining staffing policies and weekend availability of resources for patients, and call for addressing disparities in readmissions based on race, sex, length of stay, and comorbidities.
The study was internally supported. The authors reported having no relevant disclosures.
Key clinical point: Patients discharged home on a Thursday following surgery for primary colorectal cancer are more likely to be readmitted with 30 days than are patients discharged home on any other day of the week.
Major finding: The highest rate of readmission was for patients discharged on Thursday, at 12.4%, compared with lowest rate of 10.1% for patients discharged on Sunday.
Data source: Retrospective SEER-Medicare database review of records on 93,047 patients treated for colorectal cancer.
Disclosures: The study was internally supported. The authors reported having no relevant disclosures.
Elective CRC resections increase with universal insurance
BOSTON – Expanding access to health insurance for low- and moderate-income families has apparently improved colorectal cancer care in Massachusetts, and may do the same for other states that participate in Medicaid expansion under the Affordable Care Act.
That assertion comes from investigators at Massachusetts General Hospital in Boston. They found that following the introduction in 2006 of a universal health insurance law in the Bay State – the law that would serve as a model for the Affordable Care Act – the rate of elective colorectal resections increased while the rate of emergent resections decreased.
In contrast, in three states used as controls, the opposite occurred.
“This could be due to a variety of different factors, including earlier diagnosis, presenting with disease more amenable to surgical resection. It could also be due to increased referrals from primary care providers or GI doctors,” said Dr. Andrew P. Loehrer from the Massachusetts General Hospital Department of Surgery, at the annual Society of Surgical Oncology Cancer Symposium.
He acknowledged, however, that the administrative dataset he and his colleagues used in the study lacks information about clinical staging or use of neoadjuvant therapy, making it difficult to determine whether insured patients actually present at an earlier, more readily treatable disease stage.
Nonetheless, “from a cancer standpoint, my study provides early, hopeful evidence. In order to definitively say that this improves care, we need to have some more of the cancer-specific variables, but with this study, combined with some other work that we and other groups have done, we see that patients in Massachusetts are presenting with earlier stage disease, whether it’s acute disease or cancer, and they’re getting more appropriate care in a more timely fashion,” he said in an interview.
Role model
Dr. Loehrer noted that disparities in access to health care have been shown in previous studies to be associated with the likelihood of unfavorable outcomes for patients with colorectal cancer. For example, a 2008 study (Lancet Oncol. 2008 Mar;9:222-31) showed that uninsured patients with colorectal cancer had a twofold greater risk for presenting with advanced disease than privately insured patients. Additionally, a 2004 study (Br J Surg. 91:605-9) showed that patients who presented with colorectal cancer requiring emergent resection had significantly lower 5-year overall survival than patients who underwent elective resection.
Massachusetts implemented its pioneering health insurance reform law in 2006. The law increased eligibility for persons with incomes up to 150% of the Federal Poverty Level, created government-subsidized insurance for those with incomes from 150% to 300% of the poverty line, mandated that all Bay State residents have some form of health insurance, and allowed young adults up to the age of 26 to remain on their parents’ plans.
To see whether insurance reform could have a salutary effect on cancer care, the investigators drew on Agency for Health Research and Quality (AHRQ) State Inpatient Databases for Massachusetts and for Florida, New Jersey, and New York as control states. They used ICD-9 diagnosis codes to identify patients with colorectal cancer, including those who underwent resection.
To establish procedure rates, they used U.S. Census Bureau data to establish the population of denominators, which included all adults 18-54 years of age who were insured either through Medicaid, Commonwealth Care (in Massachusetts), or were listed as uninsured or self-pay. Medicare-insured patients were not included, as they were not directly affected by the reform law.
They identified 18,598 patients admitted to Massachusetts hospitals for colorectal cancer from 2001 through 2011, and 147,482 admitted during the same period to hospitals in the control states.
The authors created Poisson difference-in-differences models which compare changes in the selected outcomes in Massachusetts with changes in the control states. The models were adjusted for age, sex, race, hospital type, and secular trends.
They found that admission rates for colorectal cancer increased over time in Massachusetts by 13.3 per 100,000 residents per quarter, compared with 8.3/100,000 in the control states, translating into an adjusted rate ratio (ARR) of 1.13. Resection rates for cancer, the primary study outcome, also grew by a significantly larger margin in Massachusetts, by 5.5/100,000, compared with 0.5/100,000 in control states, with an ARR of 1.37 (P less than .001 for both comparisons).
For the secondary outcome of changes in emergent and elective resections after admission, they found that emergent surgeries in Massachusetts declined by 2.7/100,000, but increased by 4.4/100,000 in the states without insurance reform. Similarly, elective resections after admission increased in the Bay State by 7.4/100,000, but decreased by 1.8/100,000 in control states.
Relative to controls, the adjusted probability that a patient with colorectal cancer in Massachusetts would have emergent surgery after admission declined by 6.1% (P = .014) and the probability that he or she would have elective resection increased by 7.8% (P = .005).
An analysis of the odds ratio of resection during admission, adjusted for age, race, presentation with metastatic disease, hospital type, and secular trends, showed that prior to reform uninsured patients in both Massachusetts and control states were significantly less likely than privately insured patients to have resections (odds ratio, 0.42 in Mass.; 0.45 in control states).
However, after the implementation of reform the gap between previously uninsured and privately insured in Massachusetts narrowed (OR, 0.63) but remained the same in control states (OR, 0.44).
Dr. Loehrer acknowledged in an interview that Massachusetts differs from other states in some regards, including in concentrations of health providers and in requirements for insurance coverage that were in place even before the 2006 reforms, but is optimistic that improvements in colorectal cancer care can occur in states that have embraced the Affordable Care Act.
“There are a lot of services that were available and we had high colonoscopy rates prior to all of this, but that said, the mechanism is exactly the same, there are still vulnerable populations, and at this point I think it’s hopeful and promising that we will see similar results in other states,” he said.
BOSTON – Expanding access to health insurance for low- and moderate-income families has apparently improved colorectal cancer care in Massachusetts, and may do the same for other states that participate in Medicaid expansion under the Affordable Care Act.
That assertion comes from investigators at Massachusetts General Hospital in Boston. They found that following the introduction in 2006 of a universal health insurance law in the Bay State – the law that would serve as a model for the Affordable Care Act – the rate of elective colorectal resections increased while the rate of emergent resections decreased.
In contrast, in three states used as controls, the opposite occurred.
“This could be due to a variety of different factors, including earlier diagnosis, presenting with disease more amenable to surgical resection. It could also be due to increased referrals from primary care providers or GI doctors,” said Dr. Andrew P. Loehrer from the Massachusetts General Hospital Department of Surgery, at the annual Society of Surgical Oncology Cancer Symposium.
He acknowledged, however, that the administrative dataset he and his colleagues used in the study lacks information about clinical staging or use of neoadjuvant therapy, making it difficult to determine whether insured patients actually present at an earlier, more readily treatable disease stage.
Nonetheless, “from a cancer standpoint, my study provides early, hopeful evidence. In order to definitively say that this improves care, we need to have some more of the cancer-specific variables, but with this study, combined with some other work that we and other groups have done, we see that patients in Massachusetts are presenting with earlier stage disease, whether it’s acute disease or cancer, and they’re getting more appropriate care in a more timely fashion,” he said in an interview.
Role model
Dr. Loehrer noted that disparities in access to health care have been shown in previous studies to be associated with the likelihood of unfavorable outcomes for patients with colorectal cancer. For example, a 2008 study (Lancet Oncol. 2008 Mar;9:222-31) showed that uninsured patients with colorectal cancer had a twofold greater risk for presenting with advanced disease than privately insured patients. Additionally, a 2004 study (Br J Surg. 91:605-9) showed that patients who presented with colorectal cancer requiring emergent resection had significantly lower 5-year overall survival than patients who underwent elective resection.
Massachusetts implemented its pioneering health insurance reform law in 2006. The law increased eligibility for persons with incomes up to 150% of the Federal Poverty Level, created government-subsidized insurance for those with incomes from 150% to 300% of the poverty line, mandated that all Bay State residents have some form of health insurance, and allowed young adults up to the age of 26 to remain on their parents’ plans.
To see whether insurance reform could have a salutary effect on cancer care, the investigators drew on Agency for Health Research and Quality (AHRQ) State Inpatient Databases for Massachusetts and for Florida, New Jersey, and New York as control states. They used ICD-9 diagnosis codes to identify patients with colorectal cancer, including those who underwent resection.
To establish procedure rates, they used U.S. Census Bureau data to establish the population of denominators, which included all adults 18-54 years of age who were insured either through Medicaid, Commonwealth Care (in Massachusetts), or were listed as uninsured or self-pay. Medicare-insured patients were not included, as they were not directly affected by the reform law.
They identified 18,598 patients admitted to Massachusetts hospitals for colorectal cancer from 2001 through 2011, and 147,482 admitted during the same period to hospitals in the control states.
The authors created Poisson difference-in-differences models which compare changes in the selected outcomes in Massachusetts with changes in the control states. The models were adjusted for age, sex, race, hospital type, and secular trends.
They found that admission rates for colorectal cancer increased over time in Massachusetts by 13.3 per 100,000 residents per quarter, compared with 8.3/100,000 in the control states, translating into an adjusted rate ratio (ARR) of 1.13. Resection rates for cancer, the primary study outcome, also grew by a significantly larger margin in Massachusetts, by 5.5/100,000, compared with 0.5/100,000 in control states, with an ARR of 1.37 (P less than .001 for both comparisons).
For the secondary outcome of changes in emergent and elective resections after admission, they found that emergent surgeries in Massachusetts declined by 2.7/100,000, but increased by 4.4/100,000 in the states without insurance reform. Similarly, elective resections after admission increased in the Bay State by 7.4/100,000, but decreased by 1.8/100,000 in control states.
Relative to controls, the adjusted probability that a patient with colorectal cancer in Massachusetts would have emergent surgery after admission declined by 6.1% (P = .014) and the probability that he or she would have elective resection increased by 7.8% (P = .005).
An analysis of the odds ratio of resection during admission, adjusted for age, race, presentation with metastatic disease, hospital type, and secular trends, showed that prior to reform uninsured patients in both Massachusetts and control states were significantly less likely than privately insured patients to have resections (odds ratio, 0.42 in Mass.; 0.45 in control states).
However, after the implementation of reform the gap between previously uninsured and privately insured in Massachusetts narrowed (OR, 0.63) but remained the same in control states (OR, 0.44).
Dr. Loehrer acknowledged in an interview that Massachusetts differs from other states in some regards, including in concentrations of health providers and in requirements for insurance coverage that were in place even before the 2006 reforms, but is optimistic that improvements in colorectal cancer care can occur in states that have embraced the Affordable Care Act.
“There are a lot of services that were available and we had high colonoscopy rates prior to all of this, but that said, the mechanism is exactly the same, there are still vulnerable populations, and at this point I think it’s hopeful and promising that we will see similar results in other states,” he said.
BOSTON – Expanding access to health insurance for low- and moderate-income families has apparently improved colorectal cancer care in Massachusetts, and may do the same for other states that participate in Medicaid expansion under the Affordable Care Act.
That assertion comes from investigators at Massachusetts General Hospital in Boston. They found that following the introduction in 2006 of a universal health insurance law in the Bay State – the law that would serve as a model for the Affordable Care Act – the rate of elective colorectal resections increased while the rate of emergent resections decreased.
In contrast, in three states used as controls, the opposite occurred.
“This could be due to a variety of different factors, including earlier diagnosis, presenting with disease more amenable to surgical resection. It could also be due to increased referrals from primary care providers or GI doctors,” said Dr. Andrew P. Loehrer from the Massachusetts General Hospital Department of Surgery, at the annual Society of Surgical Oncology Cancer Symposium.
He acknowledged, however, that the administrative dataset he and his colleagues used in the study lacks information about clinical staging or use of neoadjuvant therapy, making it difficult to determine whether insured patients actually present at an earlier, more readily treatable disease stage.
Nonetheless, “from a cancer standpoint, my study provides early, hopeful evidence. In order to definitively say that this improves care, we need to have some more of the cancer-specific variables, but with this study, combined with some other work that we and other groups have done, we see that patients in Massachusetts are presenting with earlier stage disease, whether it’s acute disease or cancer, and they’re getting more appropriate care in a more timely fashion,” he said in an interview.
Role model
Dr. Loehrer noted that disparities in access to health care have been shown in previous studies to be associated with the likelihood of unfavorable outcomes for patients with colorectal cancer. For example, a 2008 study (Lancet Oncol. 2008 Mar;9:222-31) showed that uninsured patients with colorectal cancer had a twofold greater risk for presenting with advanced disease than privately insured patients. Additionally, a 2004 study (Br J Surg. 91:605-9) showed that patients who presented with colorectal cancer requiring emergent resection had significantly lower 5-year overall survival than patients who underwent elective resection.
Massachusetts implemented its pioneering health insurance reform law in 2006. The law increased eligibility for persons with incomes up to 150% of the Federal Poverty Level, created government-subsidized insurance for those with incomes from 150% to 300% of the poverty line, mandated that all Bay State residents have some form of health insurance, and allowed young adults up to the age of 26 to remain on their parents’ plans.
To see whether insurance reform could have a salutary effect on cancer care, the investigators drew on Agency for Health Research and Quality (AHRQ) State Inpatient Databases for Massachusetts and for Florida, New Jersey, and New York as control states. They used ICD-9 diagnosis codes to identify patients with colorectal cancer, including those who underwent resection.
To establish procedure rates, they used U.S. Census Bureau data to establish the population of denominators, which included all adults 18-54 years of age who were insured either through Medicaid, Commonwealth Care (in Massachusetts), or were listed as uninsured or self-pay. Medicare-insured patients were not included, as they were not directly affected by the reform law.
They identified 18,598 patients admitted to Massachusetts hospitals for colorectal cancer from 2001 through 2011, and 147,482 admitted during the same period to hospitals in the control states.
The authors created Poisson difference-in-differences models which compare changes in the selected outcomes in Massachusetts with changes in the control states. The models were adjusted for age, sex, race, hospital type, and secular trends.
They found that admission rates for colorectal cancer increased over time in Massachusetts by 13.3 per 100,000 residents per quarter, compared with 8.3/100,000 in the control states, translating into an adjusted rate ratio (ARR) of 1.13. Resection rates for cancer, the primary study outcome, also grew by a significantly larger margin in Massachusetts, by 5.5/100,000, compared with 0.5/100,000 in control states, with an ARR of 1.37 (P less than .001 for both comparisons).
For the secondary outcome of changes in emergent and elective resections after admission, they found that emergent surgeries in Massachusetts declined by 2.7/100,000, but increased by 4.4/100,000 in the states without insurance reform. Similarly, elective resections after admission increased in the Bay State by 7.4/100,000, but decreased by 1.8/100,000 in control states.
Relative to controls, the adjusted probability that a patient with colorectal cancer in Massachusetts would have emergent surgery after admission declined by 6.1% (P = .014) and the probability that he or she would have elective resection increased by 7.8% (P = .005).
An analysis of the odds ratio of resection during admission, adjusted for age, race, presentation with metastatic disease, hospital type, and secular trends, showed that prior to reform uninsured patients in both Massachusetts and control states were significantly less likely than privately insured patients to have resections (odds ratio, 0.42 in Mass.; 0.45 in control states).
However, after the implementation of reform the gap between previously uninsured and privately insured in Massachusetts narrowed (OR, 0.63) but remained the same in control states (OR, 0.44).
Dr. Loehrer acknowledged in an interview that Massachusetts differs from other states in some regards, including in concentrations of health providers and in requirements for insurance coverage that were in place even before the 2006 reforms, but is optimistic that improvements in colorectal cancer care can occur in states that have embraced the Affordable Care Act.
“There are a lot of services that were available and we had high colonoscopy rates prior to all of this, but that said, the mechanism is exactly the same, there are still vulnerable populations, and at this point I think it’s hopeful and promising that we will see similar results in other states,” he said.
FROM SSO 2016
Key clinical point: Outcomes for patients with colorectal cancer (CRC) who undergo elective resection are better than for those who require emergent resections.
Major finding: Elective CRC resection rates increased and emergent resections decreased after universal insurance was instituted in Massachusetts in 2006.
Data source: Retrospective study comparing differences over time between CRC resection rates in Massachusetts vs. those in Florida, New Jersey, and New York.
Disclosures: The study was supported in part by a grant from the National Institute on Aging. Dr. Loehrer and his coauthors reported no conflicts of interest.
TAMIS for rectal cancer holds its own vs. TEM
JACKSONVILLE, FLA. – Over the past 30 years, transanal endoscopic microsurgery (TEM) has emerged as a technique for localized rectal cancer, but the need for expensive specialized equipment put it beyond the reach of most hospitals.
Now, early results with transanal minimally invasive surgery (TAMIS) may open the door to an option that achieves the benefits of TEM while using commonly available and less expensive equipment, according to a study presented at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress.
Dr. John Costello, general surgery resident at Georgetown University, Washington, presented a poster summarizing the findings of a systematic literature review of TEM and TAMIS studies. The experience with TAMIS is more limited since Dr. Sam Atallah of Sebring, Fla., first introduced it in 2010. The review included the only head-to-head study of the technical aspects of TAMIS and TEM to date.
“Overall the results are very similar between the two approaches,” Dr. Costello said. “In many ways there are, at least anecdotally, some benefits potentially toward the TAMIS technique aside from cost: The perioperative morbidity may be a little lower and, particularly, there seemed to be fewer early problems with continence after surgery.”
The review found similar outcomes between the two approaches: low recurrence rates for small tumors (up to 3 cm) of 4% for TAMIS and 5% for TEM, although the study found that the recurrence rate for TEM increased with larger tumors. Surgery-related deaths with TAMIS ranged from 7.4% to19% and TEM from 6% to 31% across the studies reviewed.
The challenge with the systematic review was that the population of patients who had TAMIS was fewer than 500.
Dr. Costello elucidated the reasons that rectal cancer surgery has proved so challenging to surgeons over the years. The choice of operation was either limited to transabdominal or transanal excision, but the transanal approach had limitations anatomically and was found to be oncologically inferior for early stage cancer. Even with the evolution of the TEM approach, its adoption has been slow.
Either TEM or TAMIS would be a good option for patients too frail for the radical resection that low anterior resection or abdominal perineal resection demand, and would offer an option for palliation for advanced disease, Dr. Costello said. “You could locally resect patients in a way that they go home the same day or at most stay one day in the hospital,” he said.
“The challenge with TEM is that, although the oncologic outcomes are quite good with early-stage disease, the adoption has been very poor over 3 decades mainly because it requires specialized equipment with a very large upfront cost that is limited to use in the rectum,” Dr. Costello said. He estimated the initial capital investment cost for TEM equipment at up to $60,000 on average.
The TAMIS approach, on the other hand, carries a per-procedure equipment cost of about $500 over traditional laparoscopic surgery, he said. It can utilize the single-incision laparoscopic port (SILS) for the transanal approach. TAMIS sacrifices the three-dimensional view of TEM for two-dimensional, but it does provide 360-degree visualization. The surgeon must also be facile with the laparoscopic technique. “In the past that was a big challenge, but now all trainees are very familiar with laparoscopic surgery,” Dr. Costello said.
While the paucity of data on the TAMIS approach makes it difficult to make a strong case for the procedure, the path forward is clear, Dr. Costello said.
“We feel, as do a number of authors of the most papers, that the time truly is now for an actual prospective randomized trial to compare these techniques head-to-head, because colorectal surgeons now have the skill set to be facile at both,” Dr. Costello said.
The investigators had no financial relationships to disclose.
JACKSONVILLE, FLA. – Over the past 30 years, transanal endoscopic microsurgery (TEM) has emerged as a technique for localized rectal cancer, but the need for expensive specialized equipment put it beyond the reach of most hospitals.
Now, early results with transanal minimally invasive surgery (TAMIS) may open the door to an option that achieves the benefits of TEM while using commonly available and less expensive equipment, according to a study presented at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress.
Dr. John Costello, general surgery resident at Georgetown University, Washington, presented a poster summarizing the findings of a systematic literature review of TEM and TAMIS studies. The experience with TAMIS is more limited since Dr. Sam Atallah of Sebring, Fla., first introduced it in 2010. The review included the only head-to-head study of the technical aspects of TAMIS and TEM to date.
“Overall the results are very similar between the two approaches,” Dr. Costello said. “In many ways there are, at least anecdotally, some benefits potentially toward the TAMIS technique aside from cost: The perioperative morbidity may be a little lower and, particularly, there seemed to be fewer early problems with continence after surgery.”
The review found similar outcomes between the two approaches: low recurrence rates for small tumors (up to 3 cm) of 4% for TAMIS and 5% for TEM, although the study found that the recurrence rate for TEM increased with larger tumors. Surgery-related deaths with TAMIS ranged from 7.4% to19% and TEM from 6% to 31% across the studies reviewed.
The challenge with the systematic review was that the population of patients who had TAMIS was fewer than 500.
Dr. Costello elucidated the reasons that rectal cancer surgery has proved so challenging to surgeons over the years. The choice of operation was either limited to transabdominal or transanal excision, but the transanal approach had limitations anatomically and was found to be oncologically inferior for early stage cancer. Even with the evolution of the TEM approach, its adoption has been slow.
Either TEM or TAMIS would be a good option for patients too frail for the radical resection that low anterior resection or abdominal perineal resection demand, and would offer an option for palliation for advanced disease, Dr. Costello said. “You could locally resect patients in a way that they go home the same day or at most stay one day in the hospital,” he said.
“The challenge with TEM is that, although the oncologic outcomes are quite good with early-stage disease, the adoption has been very poor over 3 decades mainly because it requires specialized equipment with a very large upfront cost that is limited to use in the rectum,” Dr. Costello said. He estimated the initial capital investment cost for TEM equipment at up to $60,000 on average.
The TAMIS approach, on the other hand, carries a per-procedure equipment cost of about $500 over traditional laparoscopic surgery, he said. It can utilize the single-incision laparoscopic port (SILS) for the transanal approach. TAMIS sacrifices the three-dimensional view of TEM for two-dimensional, but it does provide 360-degree visualization. The surgeon must also be facile with the laparoscopic technique. “In the past that was a big challenge, but now all trainees are very familiar with laparoscopic surgery,” Dr. Costello said.
While the paucity of data on the TAMIS approach makes it difficult to make a strong case for the procedure, the path forward is clear, Dr. Costello said.
“We feel, as do a number of authors of the most papers, that the time truly is now for an actual prospective randomized trial to compare these techniques head-to-head, because colorectal surgeons now have the skill set to be facile at both,” Dr. Costello said.
The investigators had no financial relationships to disclose.
JACKSONVILLE, FLA. – Over the past 30 years, transanal endoscopic microsurgery (TEM) has emerged as a technique for localized rectal cancer, but the need for expensive specialized equipment put it beyond the reach of most hospitals.
Now, early results with transanal minimally invasive surgery (TAMIS) may open the door to an option that achieves the benefits of TEM while using commonly available and less expensive equipment, according to a study presented at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress.
Dr. John Costello, general surgery resident at Georgetown University, Washington, presented a poster summarizing the findings of a systematic literature review of TEM and TAMIS studies. The experience with TAMIS is more limited since Dr. Sam Atallah of Sebring, Fla., first introduced it in 2010. The review included the only head-to-head study of the technical aspects of TAMIS and TEM to date.
“Overall the results are very similar between the two approaches,” Dr. Costello said. “In many ways there are, at least anecdotally, some benefits potentially toward the TAMIS technique aside from cost: The perioperative morbidity may be a little lower and, particularly, there seemed to be fewer early problems with continence after surgery.”
The review found similar outcomes between the two approaches: low recurrence rates for small tumors (up to 3 cm) of 4% for TAMIS and 5% for TEM, although the study found that the recurrence rate for TEM increased with larger tumors. Surgery-related deaths with TAMIS ranged from 7.4% to19% and TEM from 6% to 31% across the studies reviewed.
The challenge with the systematic review was that the population of patients who had TAMIS was fewer than 500.
Dr. Costello elucidated the reasons that rectal cancer surgery has proved so challenging to surgeons over the years. The choice of operation was either limited to transabdominal or transanal excision, but the transanal approach had limitations anatomically and was found to be oncologically inferior for early stage cancer. Even with the evolution of the TEM approach, its adoption has been slow.
Either TEM or TAMIS would be a good option for patients too frail for the radical resection that low anterior resection or abdominal perineal resection demand, and would offer an option for palliation for advanced disease, Dr. Costello said. “You could locally resect patients in a way that they go home the same day or at most stay one day in the hospital,” he said.
“The challenge with TEM is that, although the oncologic outcomes are quite good with early-stage disease, the adoption has been very poor over 3 decades mainly because it requires specialized equipment with a very large upfront cost that is limited to use in the rectum,” Dr. Costello said. He estimated the initial capital investment cost for TEM equipment at up to $60,000 on average.
The TAMIS approach, on the other hand, carries a per-procedure equipment cost of about $500 over traditional laparoscopic surgery, he said. It can utilize the single-incision laparoscopic port (SILS) for the transanal approach. TAMIS sacrifices the three-dimensional view of TEM for two-dimensional, but it does provide 360-degree visualization. The surgeon must also be facile with the laparoscopic technique. “In the past that was a big challenge, but now all trainees are very familiar with laparoscopic surgery,” Dr. Costello said.
While the paucity of data on the TAMIS approach makes it difficult to make a strong case for the procedure, the path forward is clear, Dr. Costello said.
“We feel, as do a number of authors of the most papers, that the time truly is now for an actual prospective randomized trial to compare these techniques head-to-head, because colorectal surgeons now have the skill set to be facile at both,” Dr. Costello said.
The investigators had no financial relationships to disclose.
AT THE ACADEMIC SURGICAL CONGRESS
Key clinical point: TAMIS for removal of rectal tumors achieved equal outcomes to TEM with measurable cost savings.
Major finding: The review found similar outcomes between the two procedures and low recurrence rates for small tumors (up to 3 cm) of 4% for TAMIS and 5% for TEM.
Data source: Systematic literature review of fewer than 500 cases of TAMIS, compared with results of TEM literature.
Disclosures: The study authors reported having no financial disclosures.
Robotic colectomy takes longer, comparable results
JACKSONVILLE, FLA. – Robotic-assisted colectomy took longer than the laparoscopic operation but didn’t result in better surgical outcomes in a large NSQIP data–based study.
As health care moves away from fee-for-service to a value-based model, the longer operative times and comparative outcomes to laparoscopic colectomy suggest that the use of robotic technologies in straightforward colon resections may not be justified at this time, investigators at Duke University concluded.
“This is the largest analysis to date of robotic-assisted vs. laparoscopic colectomy,” Dr. Brian Ezekian, general surgery resident at Duke, reported at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress. “While the robotic approach is still in its infancy, the technology is associated with increased operative times without improved clinical outcomes, so our study suggests that the routine use of robotic surgery for colectomy may not be financially justifiable at this time.”
The study sampled the American College of Surgeons’ National Surgical Quality Improvement Program (NSQIP) database for patients who had either a robotic or laparoscopic colectomy from 2012 to 2013. Among the 15,976 patients included, 498 of them (3.1%) had robotic colectomy, Dr. Ezekian said.
“The major finding of our study was that robotic-assisted colectomy was associated with roughly 30-minute longer operative times, whereas the short-term clinical outcomes were comparable between the two groups,” Dr. Ezekian said. “This held true for a subset analysis of patients undergoing segmental colectomy only.”
The analysis found no significant difference between the two approaches in rates of wound complications, urinary tract infections, cardiopulmonary or thromboembolic complications, kidney failure or insufficiency, anastomotic leaks, transfusions, unplanned readmissions, or 30-day death.
The key difference was in the operative times associated with each approach. The median time for robotic-assisted colectomy was 196 minutes vs. 166 minutes for the laparoscopic approach. The study found a similar gap for segmental resections only: 190 minutes for the robotic-assisted approach vs. 153 minutes for the laparoscopic approach.
Dr. Ezekian acknowledged that this observation might merely reflect an early experience with this novel technology. “A future direction for this research is to see if operative times for robotic-assisted surgery decrease over time once there are more years in the NSQIP database or in single-institution studies,” he said.
The authors had no financial relationships to disclose.
JACKSONVILLE, FLA. – Robotic-assisted colectomy took longer than the laparoscopic operation but didn’t result in better surgical outcomes in a large NSQIP data–based study.
As health care moves away from fee-for-service to a value-based model, the longer operative times and comparative outcomes to laparoscopic colectomy suggest that the use of robotic technologies in straightforward colon resections may not be justified at this time, investigators at Duke University concluded.
“This is the largest analysis to date of robotic-assisted vs. laparoscopic colectomy,” Dr. Brian Ezekian, general surgery resident at Duke, reported at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress. “While the robotic approach is still in its infancy, the technology is associated with increased operative times without improved clinical outcomes, so our study suggests that the routine use of robotic surgery for colectomy may not be financially justifiable at this time.”
The study sampled the American College of Surgeons’ National Surgical Quality Improvement Program (NSQIP) database for patients who had either a robotic or laparoscopic colectomy from 2012 to 2013. Among the 15,976 patients included, 498 of them (3.1%) had robotic colectomy, Dr. Ezekian said.
“The major finding of our study was that robotic-assisted colectomy was associated with roughly 30-minute longer operative times, whereas the short-term clinical outcomes were comparable between the two groups,” Dr. Ezekian said. “This held true for a subset analysis of patients undergoing segmental colectomy only.”
The analysis found no significant difference between the two approaches in rates of wound complications, urinary tract infections, cardiopulmonary or thromboembolic complications, kidney failure or insufficiency, anastomotic leaks, transfusions, unplanned readmissions, or 30-day death.
The key difference was in the operative times associated with each approach. The median time for robotic-assisted colectomy was 196 minutes vs. 166 minutes for the laparoscopic approach. The study found a similar gap for segmental resections only: 190 minutes for the robotic-assisted approach vs. 153 minutes for the laparoscopic approach.
Dr. Ezekian acknowledged that this observation might merely reflect an early experience with this novel technology. “A future direction for this research is to see if operative times for robotic-assisted surgery decrease over time once there are more years in the NSQIP database or in single-institution studies,” he said.
The authors had no financial relationships to disclose.
JACKSONVILLE, FLA. – Robotic-assisted colectomy took longer than the laparoscopic operation but didn’t result in better surgical outcomes in a large NSQIP data–based study.
As health care moves away from fee-for-service to a value-based model, the longer operative times and comparative outcomes to laparoscopic colectomy suggest that the use of robotic technologies in straightforward colon resections may not be justified at this time, investigators at Duke University concluded.
“This is the largest analysis to date of robotic-assisted vs. laparoscopic colectomy,” Dr. Brian Ezekian, general surgery resident at Duke, reported at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress. “While the robotic approach is still in its infancy, the technology is associated with increased operative times without improved clinical outcomes, so our study suggests that the routine use of robotic surgery for colectomy may not be financially justifiable at this time.”
The study sampled the American College of Surgeons’ National Surgical Quality Improvement Program (NSQIP) database for patients who had either a robotic or laparoscopic colectomy from 2012 to 2013. Among the 15,976 patients included, 498 of them (3.1%) had robotic colectomy, Dr. Ezekian said.
“The major finding of our study was that robotic-assisted colectomy was associated with roughly 30-minute longer operative times, whereas the short-term clinical outcomes were comparable between the two groups,” Dr. Ezekian said. “This held true for a subset analysis of patients undergoing segmental colectomy only.”
The analysis found no significant difference between the two approaches in rates of wound complications, urinary tract infections, cardiopulmonary or thromboembolic complications, kidney failure or insufficiency, anastomotic leaks, transfusions, unplanned readmissions, or 30-day death.
The key difference was in the operative times associated with each approach. The median time for robotic-assisted colectomy was 196 minutes vs. 166 minutes for the laparoscopic approach. The study found a similar gap for segmental resections only: 190 minutes for the robotic-assisted approach vs. 153 minutes for the laparoscopic approach.
Dr. Ezekian acknowledged that this observation might merely reflect an early experience with this novel technology. “A future direction for this research is to see if operative times for robotic-assisted surgery decrease over time once there are more years in the NSQIP database or in single-institution studies,” he said.
The authors had no financial relationships to disclose.
AT THE ACADEMIC SURGICAL CONGRESS
Key clinical point: Robotic-assisted colectomy for straightforward resections involves longer operative times than laparoscopic surgery.
Major finding: Robotic-assisted colectomy was associated with roughly 30-minute longer operative times than laparoscopic surgery with comparable short-term clinical outcomes.
Data source: Analysis of 15,976 cases of colectomy in the American College of Surgeons National Surgical Quality Improvement Program performed from 2012 to 2014.
Disclosures: The study authors reported having no financial disclosures.
Early elective colon resection common in diverticulitis
A significant number of elective colon resections for uncomplicated diverticulitis are done in individuals who have experienced fewer than three episodes.
Researchers analyzed nationwide data from 87,461 immunocompetent patients with at least one claim for diverticulitis, of whom 5,604 (6.4%) underwent a resection.
According to a paper published online Feb. 10 in JAMA Surgery, 94.9% of resections, in a final cohort of 3,054 patients, occurred in individuals with fewer than three episodes of diverticulitis, if only inpatient claims were counted (doi:10.1001/jamasurg.2015.5478).
If both inpatient and outpatient claims for diverticulitis were counted, 80.5% of patients who underwent resection had experienced fewer than three episodes, and if all types of claims (including antibiotic prescription claims for diverticulitis) were counted, that figure dropped to 56.3%.
Individuals who underwent early resection were slightly more likely to be male (risk ratio [RR], 1.07; 95% confidence interval [CI], 1.02-1.13; P = .004) but were of a similar age to those who underwent resection after three or more episodes of diverticulitis.
The mean time between the last two episodes of diverticulitis was longer in individuals who underwent early surgery compared to those who delayed surgery (157 days vs. 96 days; P less than .001).
Patients residing in the South were also significantly more likely to undergo early surgery than were those residing in an other regions, with 60.5% of policy holders there undergoing early surgery compared to 50.7% in the West.
Insurance status also influenced the likelihood of early surgery, as patients with HMO or capitated insurance plans were less likely to undergo early surgery than were patients with other plan types.
In the last decade, professional guidelines have moved toward recommending elective surgery for diverticulitis after three or more episodes, but at the same time, the incidence of elective resection has more than doubled, reported Dr. Vlad V. Simianu of the University of Washington, Seattle, and coauthors.
This study covered a period of data in which guidelines on elective resection have remained in a relatively steady state, offering an opportunity to assess guideline adherence.
“Within this context, the suspected drivers of early elective surgery (younger age, laparoscopy, more frequent episodes, and personal financial risk) were not found to be associated with earlier operations for diverticulitis,” the authors wrote.
The analysis found laparoscopy was not associated with early surgery, which the authors said challenged the hypothesis that the threshold for early surgical resection might be lowered by the availability of laparoscopy.
The lack of an age difference between those undergoing early resection also challenged the notion that younger patients may experience more severe diverticulitis and suffer a greater impact on their quality of life and that this may drive physicians to operate earlier.
The authors noted that patient factors such as quality of life and anxiety about future episodes of diverticulitis, and surgeon-related factors such as training, local practice, and referral patterns were not tested, and that these may account for some of decisions about early surgery.
The National Institute of Diabetes and Digestive and Kidney Diseases and the University of Washington supported the study. No conflicts of interest were declared.
A significant number of elective colon resections for uncomplicated diverticulitis are done in individuals who have experienced fewer than three episodes.
Researchers analyzed nationwide data from 87,461 immunocompetent patients with at least one claim for diverticulitis, of whom 5,604 (6.4%) underwent a resection.
According to a paper published online Feb. 10 in JAMA Surgery, 94.9% of resections, in a final cohort of 3,054 patients, occurred in individuals with fewer than three episodes of diverticulitis, if only inpatient claims were counted (doi:10.1001/jamasurg.2015.5478).
If both inpatient and outpatient claims for diverticulitis were counted, 80.5% of patients who underwent resection had experienced fewer than three episodes, and if all types of claims (including antibiotic prescription claims for diverticulitis) were counted, that figure dropped to 56.3%.
Individuals who underwent early resection were slightly more likely to be male (risk ratio [RR], 1.07; 95% confidence interval [CI], 1.02-1.13; P = .004) but were of a similar age to those who underwent resection after three or more episodes of diverticulitis.
The mean time between the last two episodes of diverticulitis was longer in individuals who underwent early surgery compared to those who delayed surgery (157 days vs. 96 days; P less than .001).
Patients residing in the South were also significantly more likely to undergo early surgery than were those residing in an other regions, with 60.5% of policy holders there undergoing early surgery compared to 50.7% in the West.
Insurance status also influenced the likelihood of early surgery, as patients with HMO or capitated insurance plans were less likely to undergo early surgery than were patients with other plan types.
In the last decade, professional guidelines have moved toward recommending elective surgery for diverticulitis after three or more episodes, but at the same time, the incidence of elective resection has more than doubled, reported Dr. Vlad V. Simianu of the University of Washington, Seattle, and coauthors.
This study covered a period of data in which guidelines on elective resection have remained in a relatively steady state, offering an opportunity to assess guideline adherence.
“Within this context, the suspected drivers of early elective surgery (younger age, laparoscopy, more frequent episodes, and personal financial risk) were not found to be associated with earlier operations for diverticulitis,” the authors wrote.
The analysis found laparoscopy was not associated with early surgery, which the authors said challenged the hypothesis that the threshold for early surgical resection might be lowered by the availability of laparoscopy.
The lack of an age difference between those undergoing early resection also challenged the notion that younger patients may experience more severe diverticulitis and suffer a greater impact on their quality of life and that this may drive physicians to operate earlier.
The authors noted that patient factors such as quality of life and anxiety about future episodes of diverticulitis, and surgeon-related factors such as training, local practice, and referral patterns were not tested, and that these may account for some of decisions about early surgery.
The National Institute of Diabetes and Digestive and Kidney Diseases and the University of Washington supported the study. No conflicts of interest were declared.
A significant number of elective colon resections for uncomplicated diverticulitis are done in individuals who have experienced fewer than three episodes.
Researchers analyzed nationwide data from 87,461 immunocompetent patients with at least one claim for diverticulitis, of whom 5,604 (6.4%) underwent a resection.
According to a paper published online Feb. 10 in JAMA Surgery, 94.9% of resections, in a final cohort of 3,054 patients, occurred in individuals with fewer than three episodes of diverticulitis, if only inpatient claims were counted (doi:10.1001/jamasurg.2015.5478).
If both inpatient and outpatient claims for diverticulitis were counted, 80.5% of patients who underwent resection had experienced fewer than three episodes, and if all types of claims (including antibiotic prescription claims for diverticulitis) were counted, that figure dropped to 56.3%.
Individuals who underwent early resection were slightly more likely to be male (risk ratio [RR], 1.07; 95% confidence interval [CI], 1.02-1.13; P = .004) but were of a similar age to those who underwent resection after three or more episodes of diverticulitis.
The mean time between the last two episodes of diverticulitis was longer in individuals who underwent early surgery compared to those who delayed surgery (157 days vs. 96 days; P less than .001).
Patients residing in the South were also significantly more likely to undergo early surgery than were those residing in an other regions, with 60.5% of policy holders there undergoing early surgery compared to 50.7% in the West.
Insurance status also influenced the likelihood of early surgery, as patients with HMO or capitated insurance plans were less likely to undergo early surgery than were patients with other plan types.
In the last decade, professional guidelines have moved toward recommending elective surgery for diverticulitis after three or more episodes, but at the same time, the incidence of elective resection has more than doubled, reported Dr. Vlad V. Simianu of the University of Washington, Seattle, and coauthors.
This study covered a period of data in which guidelines on elective resection have remained in a relatively steady state, offering an opportunity to assess guideline adherence.
“Within this context, the suspected drivers of early elective surgery (younger age, laparoscopy, more frequent episodes, and personal financial risk) were not found to be associated with earlier operations for diverticulitis,” the authors wrote.
The analysis found laparoscopy was not associated with early surgery, which the authors said challenged the hypothesis that the threshold for early surgical resection might be lowered by the availability of laparoscopy.
The lack of an age difference between those undergoing early resection also challenged the notion that younger patients may experience more severe diverticulitis and suffer a greater impact on their quality of life and that this may drive physicians to operate earlier.
The authors noted that patient factors such as quality of life and anxiety about future episodes of diverticulitis, and surgeon-related factors such as training, local practice, and referral patterns were not tested, and that these may account for some of decisions about early surgery.
The National Institute of Diabetes and Digestive and Kidney Diseases and the University of Washington supported the study. No conflicts of interest were declared.
FROM JAMA SURGERY
Key clinical point: A majority of elective colon resections for uncomplicated diverticulitis are done in individuals who have experienced fewer than three episodes.
Major finding: More than 90% of elective resections occur in patients who have experienced fewer than three inpatient-managed episodes of diverticulitis.
Data source: Retrospective cohort study of 87,461 patients who underwent surgical resection.
Disclosures: The National Institute of Diabetes and Digestive and Kidney Diseases and the University of Washington supported the study. No conflicts of interest were declared.
ERAS eliminated racial disparities in postop hospital stay
JACKSONVILLE, FLA. – An enhanced recovery protocol after colorectal surgery nearly eliminated differences in hospital stays between black and white patients, according to a study based on data from the University of Alabama at Birmingham.
Dr. Tyler S. Wahl, a resident at UAB reported on the institution’s experience with the Enhanced Recovery After Surgery (ERAS) pathway at the Association of Academic Surgery/Society of University Surgeons Academic Surgical Congress. “ERAS has been shown to reduce length of stay, cost, and perioperative complications without compromising readmission or mortality rates,” Dr. Wahl said. Dr. Daniel Chu was senior author.
Surgical literature has increasingly demonstrated disparities among black patients undergoing major surgery: longer lengths of stay, more readmissions, increased postoperative mortality and lower survival rates after colorectal cancer resections, Dr. Wahl said. The UAB investigators set out to determine whether the ERAS pathway would reduce disparities in length of stay among black and white patients when compared to the traditional pathway.
Before UAB started using ERAS for colorectal patients, the average length of stay for patients undergoing colorectal surgery was 6.7 days with significant differences between black and white patients: 8 days vs. 6.1 days, respectively. However, after implementation of the ERAS pathway in January 2015, average length of stay declined to 4.7 days overall. Black patients had dramatic reductions in length of stay, compared with white patients, with stays of 3.9 days vs. 5 days, respectively.
“Not only were patients leaving much earlier, but their length of stay was also shorter than predicted using the American College of Surgeons Risk Calculator,” Dr. Wahl said.
The UAB study was a retrospective, matched cohort analysis of 258 patients – 129 patients from pre-ERAS years were compared with 129 ERAS patients from January to October 2015.
Study subjects were similar in many patient- and procedure-specific factors; however, differences in operative approach, indication, ostomy formation, and operative time did not change the predicted length of stay among races, Dr. Wahl said.
Dr. Wahl said the racial makeup of the study differs from most ERAS literature in colorectal patients. “The overall percentage of the African American population was 30% within our study, as most ERAS literature has 10% or less,” he added.
“Further work needs to be pursued to find what’s driving these dramatic results among the black population,” he said.
Dr. Wahl and coauthors had no disclosures.
JACKSONVILLE, FLA. – An enhanced recovery protocol after colorectal surgery nearly eliminated differences in hospital stays between black and white patients, according to a study based on data from the University of Alabama at Birmingham.
Dr. Tyler S. Wahl, a resident at UAB reported on the institution’s experience with the Enhanced Recovery After Surgery (ERAS) pathway at the Association of Academic Surgery/Society of University Surgeons Academic Surgical Congress. “ERAS has been shown to reduce length of stay, cost, and perioperative complications without compromising readmission or mortality rates,” Dr. Wahl said. Dr. Daniel Chu was senior author.
Surgical literature has increasingly demonstrated disparities among black patients undergoing major surgery: longer lengths of stay, more readmissions, increased postoperative mortality and lower survival rates after colorectal cancer resections, Dr. Wahl said. The UAB investigators set out to determine whether the ERAS pathway would reduce disparities in length of stay among black and white patients when compared to the traditional pathway.
Before UAB started using ERAS for colorectal patients, the average length of stay for patients undergoing colorectal surgery was 6.7 days with significant differences between black and white patients: 8 days vs. 6.1 days, respectively. However, after implementation of the ERAS pathway in January 2015, average length of stay declined to 4.7 days overall. Black patients had dramatic reductions in length of stay, compared with white patients, with stays of 3.9 days vs. 5 days, respectively.
“Not only were patients leaving much earlier, but their length of stay was also shorter than predicted using the American College of Surgeons Risk Calculator,” Dr. Wahl said.
The UAB study was a retrospective, matched cohort analysis of 258 patients – 129 patients from pre-ERAS years were compared with 129 ERAS patients from January to October 2015.
Study subjects were similar in many patient- and procedure-specific factors; however, differences in operative approach, indication, ostomy formation, and operative time did not change the predicted length of stay among races, Dr. Wahl said.
Dr. Wahl said the racial makeup of the study differs from most ERAS literature in colorectal patients. “The overall percentage of the African American population was 30% within our study, as most ERAS literature has 10% or less,” he added.
“Further work needs to be pursued to find what’s driving these dramatic results among the black population,” he said.
Dr. Wahl and coauthors had no disclosures.
JACKSONVILLE, FLA. – An enhanced recovery protocol after colorectal surgery nearly eliminated differences in hospital stays between black and white patients, according to a study based on data from the University of Alabama at Birmingham.
Dr. Tyler S. Wahl, a resident at UAB reported on the institution’s experience with the Enhanced Recovery After Surgery (ERAS) pathway at the Association of Academic Surgery/Society of University Surgeons Academic Surgical Congress. “ERAS has been shown to reduce length of stay, cost, and perioperative complications without compromising readmission or mortality rates,” Dr. Wahl said. Dr. Daniel Chu was senior author.
Surgical literature has increasingly demonstrated disparities among black patients undergoing major surgery: longer lengths of stay, more readmissions, increased postoperative mortality and lower survival rates after colorectal cancer resections, Dr. Wahl said. The UAB investigators set out to determine whether the ERAS pathway would reduce disparities in length of stay among black and white patients when compared to the traditional pathway.
Before UAB started using ERAS for colorectal patients, the average length of stay for patients undergoing colorectal surgery was 6.7 days with significant differences between black and white patients: 8 days vs. 6.1 days, respectively. However, after implementation of the ERAS pathway in January 2015, average length of stay declined to 4.7 days overall. Black patients had dramatic reductions in length of stay, compared with white patients, with stays of 3.9 days vs. 5 days, respectively.
“Not only were patients leaving much earlier, but their length of stay was also shorter than predicted using the American College of Surgeons Risk Calculator,” Dr. Wahl said.
The UAB study was a retrospective, matched cohort analysis of 258 patients – 129 patients from pre-ERAS years were compared with 129 ERAS patients from January to October 2015.
Study subjects were similar in many patient- and procedure-specific factors; however, differences in operative approach, indication, ostomy formation, and operative time did not change the predicted length of stay among races, Dr. Wahl said.
Dr. Wahl said the racial makeup of the study differs from most ERAS literature in colorectal patients. “The overall percentage of the African American population was 30% within our study, as most ERAS literature has 10% or less,” he added.
“Further work needs to be pursued to find what’s driving these dramatic results among the black population,” he said.
Dr. Wahl and coauthors had no disclosures.
FROM THE ACADEMIC SURGICAL CONGRESS
Key clinical point: Use of the ERAS pathway reduced hospital stays for all patients after colorectal surgery, with results most dramatic in black patients.
Major finding: Hospital stays declined from 6.7 days before ERAS to 4.7 days afterward, with stays for blacks declining from 8 days before ERAS to 3.9 days afterward.
Data source: Retrospective, matched cohort analysis of 258 patients – 129 patients from pre-ERAS years were compared to 129 ERAS patients from January to October 2015.
Disclosures: The study authors reported having no financial disclosures.
Pain scores point to hospital quality in colorectal surgery
Post-surgical pain scores may be an overlooked quality indicator among hospitals, according to new research linking patient-reported pain scores with institutional pain management practices and also surgical outcomes.
A retrospective cohort study of patient-reported pain scores after colorectal resections at 52 Michigan hospitals, published in Annals of Surgery (2016 Jan 7; epub ahead of print; doi: 10.1097/SLA.0000000000001541), found that patients treated at the best-performing hospitals for postoperative pain scores were more likely to have received patient-controlled analgesia, compared with those in the worst-performing ones (56.5% vs. 22.8%; P less than .001).
For their research, Dr. Scott E. Regenbogen of the University of Michigan, Ann Arbor, and his colleagues looked at patient-reported pain scores on the first morning post-surgery for 7,221 colorectal operations between 2012 and 2014. The participating hospitals were part of a statewide collaborative that collects data on surgery patients with the aim of improving quality.
Dr. Regenbogen and his colleagues found that patients in the quartile of hospitals with the best pain scores stayed fewer days (6.5 vs. 7.9, P less than .007) and had fewer post-surgical complications (20.3% vs. 26.4%; P less than .001), compared with those in the worst-performing quartile of hospitals.
In addition, Dr. Regenbogen and his colleagues found postoperative emergency department visits, readmissions, and pulmonary complications to be significantly lower in the quartile of hospitals with the best pain scores. The fewer pulmonary complications seen linked with better pain control “could be an indicator of better pulmonary toilet or lesser respiratory depression,” they noted.
The correlation between surgical outcomes and pain scores, the investigators wrote, suggests “consistency in the overall quality performance across both clinical and patient-reported outcomes for colectomy.”
Mean self-rated pain scores, in which patients characterize the intensity of their pain on a scale of 0 to 10, ranged from 4 to 6 across the hospitals in the study, with 5.1 (standard deviation 2.44) reported for the cohort as a whole. The type of surgery also affected pain scores, with minimally invasive procedures associated with lower scores, compared with open or converted procedures. The type of anesthesia used (local or epidural) also significantly affected scores.
Hospitals with better pain scores tended to be somewhat larger than those with poor scores, and performed more colorectal resections per year, the investigators found.
The researchers noted that while a previous meta-analysis showed that patient-controlled analgesia post-surgery provided superior pain control, compared with intermittent treatment (Cochrane Database Syst Rev. 2006 Oct 18;18:CD003348), the hospitals in this study varied widely in their approaches, with 89% of the poorly performing quartile of hospitals using intermittent parenteral narcotics, compared with 66% in the best-performing quartile.
Dr. Regenbogen and his colleagues noted in their analysis that it was possible that the association between pain control and clinical outcomes such as readmissions and complications was driven by case or patient complexity differences among institutions. The 52 hospitals in the study varied in size and type, with community and academic hospitals as well as rural and urban institutions represented.
However, they wrote, it is more likely that “both pain scores and clinical outcomes reflect … global features of the quality of care in hospitals’ surgical performance. Thus, hospitals with the most streamlined, high-quality perioperative care pathways experience the best pain scores, as well as improved clinical outcomes.”
The findings, they concluded, “reveal systematic clinical care variation that could be reduced to improve patients’ experience of pain after colorectal resections.”
The researchers noted as a limitation of the study its reliance on patient-reported pain measures, and that it did not include data on patients’ pain history, opioid use prior to admission, or the administration of pre-emptive analgesia before surgery. The study was funded by the Michigan Surgical Quality Collaborative, which receives support from Blue Cross Blue Shield. None of the study authors declared conflicts of interest.
Post-surgical pain scores may be an overlooked quality indicator among hospitals, according to new research linking patient-reported pain scores with institutional pain management practices and also surgical outcomes.
A retrospective cohort study of patient-reported pain scores after colorectal resections at 52 Michigan hospitals, published in Annals of Surgery (2016 Jan 7; epub ahead of print; doi: 10.1097/SLA.0000000000001541), found that patients treated at the best-performing hospitals for postoperative pain scores were more likely to have received patient-controlled analgesia, compared with those in the worst-performing ones (56.5% vs. 22.8%; P less than .001).
For their research, Dr. Scott E. Regenbogen of the University of Michigan, Ann Arbor, and his colleagues looked at patient-reported pain scores on the first morning post-surgery for 7,221 colorectal operations between 2012 and 2014. The participating hospitals were part of a statewide collaborative that collects data on surgery patients with the aim of improving quality.
Dr. Regenbogen and his colleagues found that patients in the quartile of hospitals with the best pain scores stayed fewer days (6.5 vs. 7.9, P less than .007) and had fewer post-surgical complications (20.3% vs. 26.4%; P less than .001), compared with those in the worst-performing quartile of hospitals.
In addition, Dr. Regenbogen and his colleagues found postoperative emergency department visits, readmissions, and pulmonary complications to be significantly lower in the quartile of hospitals with the best pain scores. The fewer pulmonary complications seen linked with better pain control “could be an indicator of better pulmonary toilet or lesser respiratory depression,” they noted.
The correlation between surgical outcomes and pain scores, the investigators wrote, suggests “consistency in the overall quality performance across both clinical and patient-reported outcomes for colectomy.”
Mean self-rated pain scores, in which patients characterize the intensity of their pain on a scale of 0 to 10, ranged from 4 to 6 across the hospitals in the study, with 5.1 (standard deviation 2.44) reported for the cohort as a whole. The type of surgery also affected pain scores, with minimally invasive procedures associated with lower scores, compared with open or converted procedures. The type of anesthesia used (local or epidural) also significantly affected scores.
Hospitals with better pain scores tended to be somewhat larger than those with poor scores, and performed more colorectal resections per year, the investigators found.
The researchers noted that while a previous meta-analysis showed that patient-controlled analgesia post-surgery provided superior pain control, compared with intermittent treatment (Cochrane Database Syst Rev. 2006 Oct 18;18:CD003348), the hospitals in this study varied widely in their approaches, with 89% of the poorly performing quartile of hospitals using intermittent parenteral narcotics, compared with 66% in the best-performing quartile.
Dr. Regenbogen and his colleagues noted in their analysis that it was possible that the association between pain control and clinical outcomes such as readmissions and complications was driven by case or patient complexity differences among institutions. The 52 hospitals in the study varied in size and type, with community and academic hospitals as well as rural and urban institutions represented.
However, they wrote, it is more likely that “both pain scores and clinical outcomes reflect … global features of the quality of care in hospitals’ surgical performance. Thus, hospitals with the most streamlined, high-quality perioperative care pathways experience the best pain scores, as well as improved clinical outcomes.”
The findings, they concluded, “reveal systematic clinical care variation that could be reduced to improve patients’ experience of pain after colorectal resections.”
The researchers noted as a limitation of the study its reliance on patient-reported pain measures, and that it did not include data on patients’ pain history, opioid use prior to admission, or the administration of pre-emptive analgesia before surgery. The study was funded by the Michigan Surgical Quality Collaborative, which receives support from Blue Cross Blue Shield. None of the study authors declared conflicts of interest.
Post-surgical pain scores may be an overlooked quality indicator among hospitals, according to new research linking patient-reported pain scores with institutional pain management practices and also surgical outcomes.
A retrospective cohort study of patient-reported pain scores after colorectal resections at 52 Michigan hospitals, published in Annals of Surgery (2016 Jan 7; epub ahead of print; doi: 10.1097/SLA.0000000000001541), found that patients treated at the best-performing hospitals for postoperative pain scores were more likely to have received patient-controlled analgesia, compared with those in the worst-performing ones (56.5% vs. 22.8%; P less than .001).
For their research, Dr. Scott E. Regenbogen of the University of Michigan, Ann Arbor, and his colleagues looked at patient-reported pain scores on the first morning post-surgery for 7,221 colorectal operations between 2012 and 2014. The participating hospitals were part of a statewide collaborative that collects data on surgery patients with the aim of improving quality.
Dr. Regenbogen and his colleagues found that patients in the quartile of hospitals with the best pain scores stayed fewer days (6.5 vs. 7.9, P less than .007) and had fewer post-surgical complications (20.3% vs. 26.4%; P less than .001), compared with those in the worst-performing quartile of hospitals.
In addition, Dr. Regenbogen and his colleagues found postoperative emergency department visits, readmissions, and pulmonary complications to be significantly lower in the quartile of hospitals with the best pain scores. The fewer pulmonary complications seen linked with better pain control “could be an indicator of better pulmonary toilet or lesser respiratory depression,” they noted.
The correlation between surgical outcomes and pain scores, the investigators wrote, suggests “consistency in the overall quality performance across both clinical and patient-reported outcomes for colectomy.”
Mean self-rated pain scores, in which patients characterize the intensity of their pain on a scale of 0 to 10, ranged from 4 to 6 across the hospitals in the study, with 5.1 (standard deviation 2.44) reported for the cohort as a whole. The type of surgery also affected pain scores, with minimally invasive procedures associated with lower scores, compared with open or converted procedures. The type of anesthesia used (local or epidural) also significantly affected scores.
Hospitals with better pain scores tended to be somewhat larger than those with poor scores, and performed more colorectal resections per year, the investigators found.
The researchers noted that while a previous meta-analysis showed that patient-controlled analgesia post-surgery provided superior pain control, compared with intermittent treatment (Cochrane Database Syst Rev. 2006 Oct 18;18:CD003348), the hospitals in this study varied widely in their approaches, with 89% of the poorly performing quartile of hospitals using intermittent parenteral narcotics, compared with 66% in the best-performing quartile.
Dr. Regenbogen and his colleagues noted in their analysis that it was possible that the association between pain control and clinical outcomes such as readmissions and complications was driven by case or patient complexity differences among institutions. The 52 hospitals in the study varied in size and type, with community and academic hospitals as well as rural and urban institutions represented.
However, they wrote, it is more likely that “both pain scores and clinical outcomes reflect … global features of the quality of care in hospitals’ surgical performance. Thus, hospitals with the most streamlined, high-quality perioperative care pathways experience the best pain scores, as well as improved clinical outcomes.”
The findings, they concluded, “reveal systematic clinical care variation that could be reduced to improve patients’ experience of pain after colorectal resections.”
The researchers noted as a limitation of the study its reliance on patient-reported pain measures, and that it did not include data on patients’ pain history, opioid use prior to admission, or the administration of pre-emptive analgesia before surgery. The study was funded by the Michigan Surgical Quality Collaborative, which receives support from Blue Cross Blue Shield. None of the study authors declared conflicts of interest.
FROM ANNALS OF SURGERY
Key clinical point: Hospitals delivering better patient-reported pain control after colorectal resection also saw better surgical outcomes.
Major finding: Patients in the quartile of hospitals with the best pain scores stayed fewer days (6.5 vs. 7.9, P less than .007) and had fewer post-surgical complications (20.3% vs. 26.4%; P less than .001), compared with those in the worst-performing quartile of hospitals.
Data source: A retrospective cohort study reviewing more than 7,000 colorectal resections at 52 Michigan hospitals between 2012 and 2014.
Disclosures: The Michigan Surgical Quality Collaborative, funded by Blue Cross Blue Shield, sponsored the study. Investigators declared no conflicts of interest.