User login
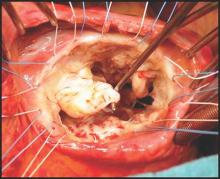
Aortic valve replacement: Transcatheter soars past surgical
In Germany, almost all of the marked increase in the use of transcatheter aortic valve replacement (TAVR) since the procedure’s introduction in 2007 occurred in patients unsuited to a surgical approach because of their advanced age or elevated risk, according to an analysis published online Dec. 17 in the New England Journal of Medicine.
The surgical aortic valve replacement (SAVR) is still the standard of care, but many have questioned how the relatively new transcatheter approach has affected clinical practice overall, said Dr. Jochen Reinöhl of the Heart Center, University of Freiburg (Germany) and his associates.
To assess the evolution of treatment since TAVR was introduced, the investigators analyzed data from the Institute for the Hospital Remuneration System, which tracks all patient data regarding diagnoses, comorbidities, and procedures throughout the country. They focused on all 88,573 admissions for isolated surgical aortic valve replacements (55,992 procedures) and for isolated TAVR (32,581 procedures) performed in Germany during 2007-2013.
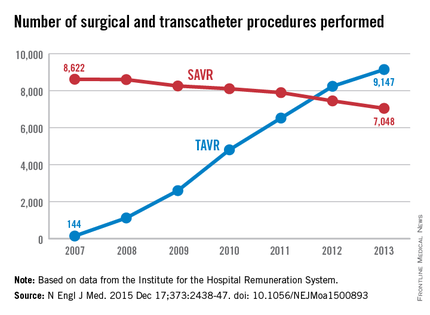
The number of TAVR procedures increased markedly over time, from 144 to 9,147 per year, while the number of SAVRs declined only slightly, from 8,622 to 7,048 per year. Patients aged 80 years and older accounted for almost all of the dramatic increase in transcatheter procedures, the investigators said (N Engl J Med. 2015 Dec 17;373:2438-47 [doi:10.1056/NEJMoa1500893]).
Overall in-hospital mortality was significantly higher with TAVR (6.5%) than with SAVR (2.8%), for an odds ratio of 2.41. This likely reflects the significantly greater risk of patients selected for TAVR, compared with those undergoing surgery, they said.
Mortality decreased over time in both patient groups, from 3.8% to 2.2% with surgery and from 13.2% to 5.4% with TAVR. In the case of TAVR, this decline is likely from a “learning curve” effect among clinicians, improvements in patient care, and advances in treatment devices. In the case of surgery, the mortality decline is probably due in part to the shift of high-risk patients from SAVR to the transcatheter approach, Dr. Reinöhl and his associates said.
Similarly, complications were significantly more common with TAVR. The need for permanent pacemaker implantation was the most frequently reported complication of TAVR, occurring in 17.7% of the transcatheter group but only 4.0% of the surgical group. Stroke rates (2.5% vs. 1.8%) and rates of acute kidney injury (5.5% vs. 3.0%) followed a similar pattern. In contrast, bleeding complications were more frequent with surgery (14.0% vs. 8.2%).
In Germany, almost all of the marked increase in the use of transcatheter aortic valve replacement (TAVR) since the procedure’s introduction in 2007 occurred in patients unsuited to a surgical approach because of their advanced age or elevated risk, according to an analysis published online Dec. 17 in the New England Journal of Medicine.
The surgical aortic valve replacement (SAVR) is still the standard of care, but many have questioned how the relatively new transcatheter approach has affected clinical practice overall, said Dr. Jochen Reinöhl of the Heart Center, University of Freiburg (Germany) and his associates.

To assess the evolution of treatment since TAVR was introduced, the investigators analyzed data from the Institute for the Hospital Remuneration System, which tracks all patient data regarding diagnoses, comorbidities, and procedures throughout the country. They focused on all 88,573 admissions for isolated surgical aortic valve replacements (55,992 procedures) and for isolated TAVR (32,581 procedures) performed in Germany during 2007-2013.
The number of TAVR procedures increased markedly over time, from 144 to 9,147 per year, while the number of SAVRs declined only slightly, from 8,622 to 7,048 per year. Patients aged 80 years and older accounted for almost all of the dramatic increase in transcatheter procedures, the investigators said (N Engl J Med. 2015 Dec 17;373:2438-47 [doi:10.1056/NEJMoa1500893]).
Overall in-hospital mortality was significantly higher with TAVR (6.5%) than with SAVR (2.8%), for an odds ratio of 2.41. This likely reflects the significantly greater risk of patients selected for TAVR, compared with those undergoing surgery, they said.
Mortality decreased over time in both patient groups, from 3.8% to 2.2% with surgery and from 13.2% to 5.4% with TAVR. In the case of TAVR, this decline is likely from a “learning curve” effect among clinicians, improvements in patient care, and advances in treatment devices. In the case of surgery, the mortality decline is probably due in part to the shift of high-risk patients from SAVR to the transcatheter approach, Dr. Reinöhl and his associates said.
Similarly, complications were significantly more common with TAVR. The need for permanent pacemaker implantation was the most frequently reported complication of TAVR, occurring in 17.7% of the transcatheter group but only 4.0% of the surgical group. Stroke rates (2.5% vs. 1.8%) and rates of acute kidney injury (5.5% vs. 3.0%) followed a similar pattern. In contrast, bleeding complications were more frequent with surgery (14.0% vs. 8.2%).
In Germany, almost all of the marked increase in the use of transcatheter aortic valve replacement (TAVR) since the procedure’s introduction in 2007 occurred in patients unsuited to a surgical approach because of their advanced age or elevated risk, according to an analysis published online Dec. 17 in the New England Journal of Medicine.
The surgical aortic valve replacement (SAVR) is still the standard of care, but many have questioned how the relatively new transcatheter approach has affected clinical practice overall, said Dr. Jochen Reinöhl of the Heart Center, University of Freiburg (Germany) and his associates.

To assess the evolution of treatment since TAVR was introduced, the investigators analyzed data from the Institute for the Hospital Remuneration System, which tracks all patient data regarding diagnoses, comorbidities, and procedures throughout the country. They focused on all 88,573 admissions for isolated surgical aortic valve replacements (55,992 procedures) and for isolated TAVR (32,581 procedures) performed in Germany during 2007-2013.
The number of TAVR procedures increased markedly over time, from 144 to 9,147 per year, while the number of SAVRs declined only slightly, from 8,622 to 7,048 per year. Patients aged 80 years and older accounted for almost all of the dramatic increase in transcatheter procedures, the investigators said (N Engl J Med. 2015 Dec 17;373:2438-47 [doi:10.1056/NEJMoa1500893]).
Overall in-hospital mortality was significantly higher with TAVR (6.5%) than with SAVR (2.8%), for an odds ratio of 2.41. This likely reflects the significantly greater risk of patients selected for TAVR, compared with those undergoing surgery, they said.
Mortality decreased over time in both patient groups, from 3.8% to 2.2% with surgery and from 13.2% to 5.4% with TAVR. In the case of TAVR, this decline is likely from a “learning curve” effect among clinicians, improvements in patient care, and advances in treatment devices. In the case of surgery, the mortality decline is probably due in part to the shift of high-risk patients from SAVR to the transcatheter approach, Dr. Reinöhl and his associates said.
Similarly, complications were significantly more common with TAVR. The need for permanent pacemaker implantation was the most frequently reported complication of TAVR, occurring in 17.7% of the transcatheter group but only 4.0% of the surgical group. Stroke rates (2.5% vs. 1.8%) and rates of acute kidney injury (5.5% vs. 3.0%) followed a similar pattern. In contrast, bleeding complications were more frequent with surgery (14.0% vs. 8.2%).
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: In Germany, almost all of the marked increase in the use of transcatheter aortic valve replacement occurred among patients unsuited for a surgical approach.
Major finding: The number of TAVR procedures increased markedly over time, from 144 to 9,147 per year, while the number of surgical procedures declined only slightly, from 8,622 to 7,048 per year.
Data source: A retrospective analysis of all 88,573 surgical and TAVR performed in Germany in 2007-2013.
Disclosures: This study was supported by the Heart Center at Freiburg University. Dr. Reinöhl and one of his associates reported receiving personal fees from Edwards Lifesciences and Direct Flow Medical.
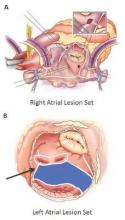
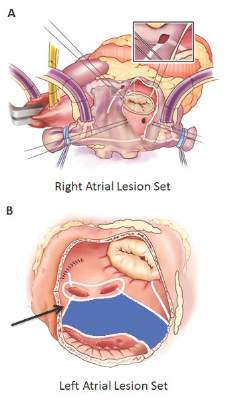
Should annular dilation be a marker for concomitant tricuspid valve repair?
The idea of performing a tricuspid valve repair during a mitral valve procedure has fueled considerable debate among cardiovascular surgeons largely because the grading of tricuspid regurgitation (TR) has been an unreliable marker, so now may be the time to use a new parameter, Dr. Robert Dion of Genk, Belgium, argues in an expert opinion in the Journal of Thoracic and Cardiovascular Surgery (2015 Nov 20;150:1040-3).
“Therefore, we need a parameter hardly depending on preload,” Dr. Dion said. He noted that many authors have validated the use of annual dilatation of 40 mm or 21mm/m2.
Further, preoperative functional New York Heart Association (NYHA) class plays a major role, Dr. Dion said. TR is progressive in nature and the existence of concomitant mitral valve disease can aggravate annular dilation. The earlier surgeons operate on the mitral valve, the less frequently patients will require tricuspid valve repair at the same time, he said.
The controversy was aired in a report at the 2015 American Association for Thoracic Surgery meeting when Dr. Joanna Chikwe of Mount Sinai Hospital in New York discussed an approach that led to tricuspid valve repair at the time of mitral valve surgery in almost two-thirds of patients. Dr. Chikwe and her coauthors opted for concomitant surgery when patients had moderate TR or tricuspid annular dilatation of 40 mm or greater – a strategy that Dr. Dion said was validated because they reported comparable outcomes in terms of death, morbidity, or pacemaker need. The concomitant repair cured TR and prevented progression at seven years of follow-up; and it induced right ventricle recovery and reduced pulmonary hypertension.
In this opinion piece, Dr. Dion took issue with comments made by Dr. Tirone E. David of the University of Toronto during Dr. Chikwe’s AATS presentation. Dr. David, for whom the David reimplantation technique for aortic root replacement is named, called the technique “overkill,” according to Dr. Dion.
Specifically, Dr. Dion questioned Dr. David’s assertions that the use of rigid rings in mitral valve repair causes TR and that no evidence validates the 40-mm diameter minimum in patients with degenerative mitral valve disease. On the first point, Dr. Dion said no evidence has linked the rigidity of the mitral valve ring and progression of tricuspid regurgitation. On the second, Dr. Dion cited eight studies of progressive tricuspid annular dilation with mitral regurgitation, most of which proposed the 40-mm threshold for concomitant tricuspid valve repair. Since then, the 40-mm threshold has been adopted for both European and American guidelines and validated by five reports from 2011 to 2014.
Dr. Dion said the rationale for using annular dilation rather than TR grade rests on “three poles”: annular dilation does not depend on preload whereas the right ventricle does; documented discrepancies between clinical and hemodynamic data (J Cardiol Surg. 1994 Mar;9(2 suppl):237-41; J Am Coll Cardiol. 2004 Feb. 4;43:405-9); and the idea that TR is “bad” for the patient. He also cited disparities in the number of concomitant repairs performed at leading centers: 7%-10% at the Mayo Clinic and Dr. David’s Toronto center, 25% in Leipzig, 40%-45% in his own clinic and two others, and 65% in Dr. Chikwe’s facility.
But early intervention for mitral valve dysfunction is a key indicator of the need for concomitant tricuspid valve repair, Dr. Dion said. “The earlier we operate on the MV, the less frequently patients will require tricuspid valve repair,” he said. In his own approach, Dr. Dion uses a transseptal approach of the mitral valve. For TR greater than grade 2, he performs tricuspid repair using a semirigid ring sized on the area of the anterior leaflet tissue; and if the tenting distance is 8 mm or greater, he includes anterior leaflet augmentation. When TR grade is 2 or less, he also performs concomitant tricuspid repair when the tricuspid annulus is 40 mm or greater or when the tricuspid annulus is 3.5-4 mm in the setting of a host of other cardiac problems, from atrial fibrillation to left valve dysfunction. “Otherwise: abstention,” he said.
“The major issue here is to do everything possible to avoid the risk and outcomes of reoperative tricuspid valve surgery,” Dr. Dion said, citing in-hospital death rates of 13.2% that Dr. David reported (Ann Thorac Surg. 2013 Jan;95:119-24.) and 14.6% the Leipzig group reported (J Thorac Cardiovasc Surg. 2013 Oct; 146:841-7).While he acknowledged calls for a prospective, randomized clinical trial, Dr. Dion said the contraindications for concomitant tricuspid valve repair with mitral valve repair have already been well documented.
Dr. Dion reports consulting fees from Sorin, Edwards, Johnson & Johnson, and St. Jude Medical.
In their invited commentary, Dr. Tirone E. David of the University of Toronto and his colleagues said that performing a tricuspid valve annuloplasty (TVA) in the setting of tricuspid regurgitation (TR) or a tricuspid annulus diameter greater than 40 mm does not completely prevent the onset of new TR (J Thorac Cardiovasc Surg. 2015 Nov;150:1043-4). “Other factors play a role in its development,” they said. “Longstanding atrial fibrillation is one of them.”
“Are patients who have mitral valve (MV) repair for degenerative disease of the MV likely to develop functional TR if there is only trivial or mild TR before surgery?” Dr. David and his coauthors asked. “We are certain that some patients do, but it does not appear to be as common as patients who had MV replacement for rheumatic disease. Is it solely because the incidence of atrial fibrillation is higher in rheumatic patients?”
In a second invited commentary, Dr. Richard J. Shemin of the University of California, Los Angeles, said the discrepancies between the rates of concomitant tricuspid repair among the various centers that Dr. Robert A. Dion cited beg for resolution (J Thorac Cardiovasc Surg. 2015 Nov;150:1045-6). “The wide discrepancy can perhaps be partially resolved with a re-review of the Toronto experience and follow-up,” Dr. Shemin said. “The subset of patients with TVA greater than 40 who were not repaired and the late follow-up would be very helpful.”
The cardiothoracic surgeon faces conflicting principles when considering concomitant tricuspid valve repair, Dr. Shemin said: avoiding an unnecessary surgery when functional TR exists, or leaving a residual lesion that could lead to a risky reoperation. Hence, accurate measurements of the tricuspid valve annulus and TR are essential, Dr. Shemin said.
“The tricuspid valve has been rediscovered and further investigation will resolve the questions,” Dr. Shemin said. Likewise, Dr. David and his colleagues said the “time has come” for a multicentered clinical trial to put the issue to rest for both mitral valve replacement and repair.
In their invited commentary, Dr. Tirone E. David of the University of Toronto and his colleagues said that performing a tricuspid valve annuloplasty (TVA) in the setting of tricuspid regurgitation (TR) or a tricuspid annulus diameter greater than 40 mm does not completely prevent the onset of new TR (J Thorac Cardiovasc Surg. 2015 Nov;150:1043-4). “Other factors play a role in its development,” they said. “Longstanding atrial fibrillation is one of them.”
“Are patients who have mitral valve (MV) repair for degenerative disease of the MV likely to develop functional TR if there is only trivial or mild TR before surgery?” Dr. David and his coauthors asked. “We are certain that some patients do, but it does not appear to be as common as patients who had MV replacement for rheumatic disease. Is it solely because the incidence of atrial fibrillation is higher in rheumatic patients?”
In a second invited commentary, Dr. Richard J. Shemin of the University of California, Los Angeles, said the discrepancies between the rates of concomitant tricuspid repair among the various centers that Dr. Robert A. Dion cited beg for resolution (J Thorac Cardiovasc Surg. 2015 Nov;150:1045-6). “The wide discrepancy can perhaps be partially resolved with a re-review of the Toronto experience and follow-up,” Dr. Shemin said. “The subset of patients with TVA greater than 40 who were not repaired and the late follow-up would be very helpful.”
The cardiothoracic surgeon faces conflicting principles when considering concomitant tricuspid valve repair, Dr. Shemin said: avoiding an unnecessary surgery when functional TR exists, or leaving a residual lesion that could lead to a risky reoperation. Hence, accurate measurements of the tricuspid valve annulus and TR are essential, Dr. Shemin said.
“The tricuspid valve has been rediscovered and further investigation will resolve the questions,” Dr. Shemin said. Likewise, Dr. David and his colleagues said the “time has come” for a multicentered clinical trial to put the issue to rest for both mitral valve replacement and repair.
In their invited commentary, Dr. Tirone E. David of the University of Toronto and his colleagues said that performing a tricuspid valve annuloplasty (TVA) in the setting of tricuspid regurgitation (TR) or a tricuspid annulus diameter greater than 40 mm does not completely prevent the onset of new TR (J Thorac Cardiovasc Surg. 2015 Nov;150:1043-4). “Other factors play a role in its development,” they said. “Longstanding atrial fibrillation is one of them.”
“Are patients who have mitral valve (MV) repair for degenerative disease of the MV likely to develop functional TR if there is only trivial or mild TR before surgery?” Dr. David and his coauthors asked. “We are certain that some patients do, but it does not appear to be as common as patients who had MV replacement for rheumatic disease. Is it solely because the incidence of atrial fibrillation is higher in rheumatic patients?”
In a second invited commentary, Dr. Richard J. Shemin of the University of California, Los Angeles, said the discrepancies between the rates of concomitant tricuspid repair among the various centers that Dr. Robert A. Dion cited beg for resolution (J Thorac Cardiovasc Surg. 2015 Nov;150:1045-6). “The wide discrepancy can perhaps be partially resolved with a re-review of the Toronto experience and follow-up,” Dr. Shemin said. “The subset of patients with TVA greater than 40 who were not repaired and the late follow-up would be very helpful.”
The cardiothoracic surgeon faces conflicting principles when considering concomitant tricuspid valve repair, Dr. Shemin said: avoiding an unnecessary surgery when functional TR exists, or leaving a residual lesion that could lead to a risky reoperation. Hence, accurate measurements of the tricuspid valve annulus and TR are essential, Dr. Shemin said.
“The tricuspid valve has been rediscovered and further investigation will resolve the questions,” Dr. Shemin said. Likewise, Dr. David and his colleagues said the “time has come” for a multicentered clinical trial to put the issue to rest for both mitral valve replacement and repair.
The idea of performing a tricuspid valve repair during a mitral valve procedure has fueled considerable debate among cardiovascular surgeons largely because the grading of tricuspid regurgitation (TR) has been an unreliable marker, so now may be the time to use a new parameter, Dr. Robert Dion of Genk, Belgium, argues in an expert opinion in the Journal of Thoracic and Cardiovascular Surgery (2015 Nov 20;150:1040-3).
“Therefore, we need a parameter hardly depending on preload,” Dr. Dion said. He noted that many authors have validated the use of annual dilatation of 40 mm or 21mm/m2.
Further, preoperative functional New York Heart Association (NYHA) class plays a major role, Dr. Dion said. TR is progressive in nature and the existence of concomitant mitral valve disease can aggravate annular dilation. The earlier surgeons operate on the mitral valve, the less frequently patients will require tricuspid valve repair at the same time, he said.
The controversy was aired in a report at the 2015 American Association for Thoracic Surgery meeting when Dr. Joanna Chikwe of Mount Sinai Hospital in New York discussed an approach that led to tricuspid valve repair at the time of mitral valve surgery in almost two-thirds of patients. Dr. Chikwe and her coauthors opted for concomitant surgery when patients had moderate TR or tricuspid annular dilatation of 40 mm or greater – a strategy that Dr. Dion said was validated because they reported comparable outcomes in terms of death, morbidity, or pacemaker need. The concomitant repair cured TR and prevented progression at seven years of follow-up; and it induced right ventricle recovery and reduced pulmonary hypertension.
In this opinion piece, Dr. Dion took issue with comments made by Dr. Tirone E. David of the University of Toronto during Dr. Chikwe’s AATS presentation. Dr. David, for whom the David reimplantation technique for aortic root replacement is named, called the technique “overkill,” according to Dr. Dion.
Specifically, Dr. Dion questioned Dr. David’s assertions that the use of rigid rings in mitral valve repair causes TR and that no evidence validates the 40-mm diameter minimum in patients with degenerative mitral valve disease. On the first point, Dr. Dion said no evidence has linked the rigidity of the mitral valve ring and progression of tricuspid regurgitation. On the second, Dr. Dion cited eight studies of progressive tricuspid annular dilation with mitral regurgitation, most of which proposed the 40-mm threshold for concomitant tricuspid valve repair. Since then, the 40-mm threshold has been adopted for both European and American guidelines and validated by five reports from 2011 to 2014.
Dr. Dion said the rationale for using annular dilation rather than TR grade rests on “three poles”: annular dilation does not depend on preload whereas the right ventricle does; documented discrepancies between clinical and hemodynamic data (J Cardiol Surg. 1994 Mar;9(2 suppl):237-41; J Am Coll Cardiol. 2004 Feb. 4;43:405-9); and the idea that TR is “bad” for the patient. He also cited disparities in the number of concomitant repairs performed at leading centers: 7%-10% at the Mayo Clinic and Dr. David’s Toronto center, 25% in Leipzig, 40%-45% in his own clinic and two others, and 65% in Dr. Chikwe’s facility.
But early intervention for mitral valve dysfunction is a key indicator of the need for concomitant tricuspid valve repair, Dr. Dion said. “The earlier we operate on the MV, the less frequently patients will require tricuspid valve repair,” he said. In his own approach, Dr. Dion uses a transseptal approach of the mitral valve. For TR greater than grade 2, he performs tricuspid repair using a semirigid ring sized on the area of the anterior leaflet tissue; and if the tenting distance is 8 mm or greater, he includes anterior leaflet augmentation. When TR grade is 2 or less, he also performs concomitant tricuspid repair when the tricuspid annulus is 40 mm or greater or when the tricuspid annulus is 3.5-4 mm in the setting of a host of other cardiac problems, from atrial fibrillation to left valve dysfunction. “Otherwise: abstention,” he said.
“The major issue here is to do everything possible to avoid the risk and outcomes of reoperative tricuspid valve surgery,” Dr. Dion said, citing in-hospital death rates of 13.2% that Dr. David reported (Ann Thorac Surg. 2013 Jan;95:119-24.) and 14.6% the Leipzig group reported (J Thorac Cardiovasc Surg. 2013 Oct; 146:841-7).While he acknowledged calls for a prospective, randomized clinical trial, Dr. Dion said the contraindications for concomitant tricuspid valve repair with mitral valve repair have already been well documented.
Dr. Dion reports consulting fees from Sorin, Edwards, Johnson & Johnson, and St. Jude Medical.
The idea of performing a tricuspid valve repair during a mitral valve procedure has fueled considerable debate among cardiovascular surgeons largely because the grading of tricuspid regurgitation (TR) has been an unreliable marker, so now may be the time to use a new parameter, Dr. Robert Dion of Genk, Belgium, argues in an expert opinion in the Journal of Thoracic and Cardiovascular Surgery (2015 Nov 20;150:1040-3).
“Therefore, we need a parameter hardly depending on preload,” Dr. Dion said. He noted that many authors have validated the use of annual dilatation of 40 mm or 21mm/m2.
Further, preoperative functional New York Heart Association (NYHA) class plays a major role, Dr. Dion said. TR is progressive in nature and the existence of concomitant mitral valve disease can aggravate annular dilation. The earlier surgeons operate on the mitral valve, the less frequently patients will require tricuspid valve repair at the same time, he said.
The controversy was aired in a report at the 2015 American Association for Thoracic Surgery meeting when Dr. Joanna Chikwe of Mount Sinai Hospital in New York discussed an approach that led to tricuspid valve repair at the time of mitral valve surgery in almost two-thirds of patients. Dr. Chikwe and her coauthors opted for concomitant surgery when patients had moderate TR or tricuspid annular dilatation of 40 mm or greater – a strategy that Dr. Dion said was validated because they reported comparable outcomes in terms of death, morbidity, or pacemaker need. The concomitant repair cured TR and prevented progression at seven years of follow-up; and it induced right ventricle recovery and reduced pulmonary hypertension.
In this opinion piece, Dr. Dion took issue with comments made by Dr. Tirone E. David of the University of Toronto during Dr. Chikwe’s AATS presentation. Dr. David, for whom the David reimplantation technique for aortic root replacement is named, called the technique “overkill,” according to Dr. Dion.
Specifically, Dr. Dion questioned Dr. David’s assertions that the use of rigid rings in mitral valve repair causes TR and that no evidence validates the 40-mm diameter minimum in patients with degenerative mitral valve disease. On the first point, Dr. Dion said no evidence has linked the rigidity of the mitral valve ring and progression of tricuspid regurgitation. On the second, Dr. Dion cited eight studies of progressive tricuspid annular dilation with mitral regurgitation, most of which proposed the 40-mm threshold for concomitant tricuspid valve repair. Since then, the 40-mm threshold has been adopted for both European and American guidelines and validated by five reports from 2011 to 2014.
Dr. Dion said the rationale for using annular dilation rather than TR grade rests on “three poles”: annular dilation does not depend on preload whereas the right ventricle does; documented discrepancies between clinical and hemodynamic data (J Cardiol Surg. 1994 Mar;9(2 suppl):237-41; J Am Coll Cardiol. 2004 Feb. 4;43:405-9); and the idea that TR is “bad” for the patient. He also cited disparities in the number of concomitant repairs performed at leading centers: 7%-10% at the Mayo Clinic and Dr. David’s Toronto center, 25% in Leipzig, 40%-45% in his own clinic and two others, and 65% in Dr. Chikwe’s facility.
But early intervention for mitral valve dysfunction is a key indicator of the need for concomitant tricuspid valve repair, Dr. Dion said. “The earlier we operate on the MV, the less frequently patients will require tricuspid valve repair,” he said. In his own approach, Dr. Dion uses a transseptal approach of the mitral valve. For TR greater than grade 2, he performs tricuspid repair using a semirigid ring sized on the area of the anterior leaflet tissue; and if the tenting distance is 8 mm or greater, he includes anterior leaflet augmentation. When TR grade is 2 or less, he also performs concomitant tricuspid repair when the tricuspid annulus is 40 mm or greater or when the tricuspid annulus is 3.5-4 mm in the setting of a host of other cardiac problems, from atrial fibrillation to left valve dysfunction. “Otherwise: abstention,” he said.
“The major issue here is to do everything possible to avoid the risk and outcomes of reoperative tricuspid valve surgery,” Dr. Dion said, citing in-hospital death rates of 13.2% that Dr. David reported (Ann Thorac Surg. 2013 Jan;95:119-24.) and 14.6% the Leipzig group reported (J Thorac Cardiovasc Surg. 2013 Oct; 146:841-7).While he acknowledged calls for a prospective, randomized clinical trial, Dr. Dion said the contraindications for concomitant tricuspid valve repair with mitral valve repair have already been well documented.
Dr. Dion reports consulting fees from Sorin, Edwards, Johnson & Johnson, and St. Jude Medical.
Key clinical point: Controversy surrounds the need for concomitant tricuspid valve repair with a mitral valve procedure and what parameters the decision should be based on.
Major finding: Increasing reports have supported the use of annular dilation of 40 mm or greater as a threshold for performing concomitant tricuspid valve repair rather than grading of tricuspid regurgitation.
Data source: This Expert Opinion piece cites studies along with American and European clinical guidelines that support the 40-mm threshold.
Disclosures: Dr. Dion reports consulting fees from Sorin, Edwards, Johnson & Johnson, and St. Jude Medical.
Perioperative statins for cardiac surgery didn’t reduce kidney injury
SAN DIEGO – High-dose perioperative atorvastatin treatment did not reduce acute kidney injury following elective cardiac surgery, and it may increase risk in patients with chronic kidney disease (CKD) who are naive to statin treatment, results from a large, randomized trial showed.
“Despite advances in patient management that have reduced mortality during cardiac surgery, acute kidney injury continues to complicate the postoperative course in 20%-30% of patients,” Dr. Frederic Tremaine Billings said during a press briefing at a meeting sponsored by the American Society of Nephrology.
“Its diagnosis is independently associated with a fivefold increase in mortality following the surgery,” Dr. Tremaine added. “Statins affect several mechanisms underlying postoperative acute kidney injury. Widely prescribed to reduce cholesterol synthesis, these drugs also reduce lipid modification of intracellular signaling molecules, which have been shown to improve perfusion and reduce oxidative stress – both mechanisms important in acute kidney injury following cardiac surgery.”
Dr. Billings of the department of anesthesiology and critical care medicine at Vanderbilt University Medical Center, Nashville, Tenn., and his associates tested the hypothesis that short-term, high-dose perioperative (preoperative, intraoperative, and postoperative) atorvastatin reduces acute kidney injury (AKI) following elective cardiac surgery.
The researchers randomly assigned preoperative statin-naive patients to 80 mg of atorvastatin on the morning before surgery, 40 mg on the morning of surgery, and 40 mg daily throughout hospitalization, or to a matching placebo regimen. In addition, they randomly assigned patients who were already using statins prior to surgery to 80 mg of atorvastatin the morning of surgery, and 40 mg on the morning after surgery, or to a matching placebo regimen.
“We felt it was important to not withhold statin treatment in patients already using statins prior to surgery, beyond what is typically done in clinical practice,” Dr. Billings explained. “For this reason, preoperative statin–using subjects continued their statin up until the day before surgery, and then resumed their statin use on postoperative day 2.”
The primary endpoint of the study was the incidence of AKI as determined by Acute Kidney Injury Network criteria (a 0.3 mg/dL increase in serum creatinine concentrations within 48 hours of surgery). Secondary endpoints included the maximum creatinine increase from baseline to 48 hours after surgery, ICU delirium diagnosed by the Confusion Assessment Method for the ICU, myocardial injury, and the incidence of atrial fibrillation, pneumonia, and stroke. Safety endpoints included liver toxicity, muscle toxicity, and adverse events.
The study was limited to adults having elective cardiac surgery and excluded those with statin intolerance, acute coronary syndrome, liver dysfunction, use of CYP3A4 inhibitors, kidney transplant recipients, those currently on dialysis, and those who were pregnant.
From November 2009 to October 2014, the researchers recruited 653 patients. But the trial was halted on recommendation of Vanderbilt’s data and safety monitoring board because of futility and an increased incidence of AKI among statin-naive patients with CKD randomized to atorvastatin.
Among all patients, AKI occurred in 20.8% of those randomized to atorvastatin, compared with 19.5% of those randomized to placebo, a difference that was neither clinically nor statistically significant (P = .75), Dr. Billings reported.
However, among the 199 patients who were statin naive, AKI occurred in 21.6% of those randomized to atorvastatin, compared with 13.4% of those randomized to placebo (P = .14). “An 8% difference in the incidence of AKI is of clinical importance, if true,” he said.
Among the 36 statin-naive patients with CKD, AKI occurred in 52.9% of those randomized to atorvastatin, compared with 15.8% of those randomized to placebo (P = .03). “While the number of patients in this subgroup is small, the magnitude of effect is striking,” Dr. Billings said.
Among the 416 patients who were using statins prior to surgery, AKI occurred in 20.4% of those randomized to atorvastatin, compared with 22.4% of those randomized to placebo (P = .63). Results were similar among the subset of those patients who had CKD (31.3% vs. 36.3%; P = .59).
Safety endpoints were similar between the two groups.
Strengths of the study, Dr. Billings said, include the fact that it’s the largest randomized, controlled trial to date to test this hypothesis, the pragmatic design of the protocol, and rigorous methodology.
Limitations include the “small number of patients in the statin-naive CKD subgroup,” he noted. “And the short duration of treatment among prestudy statin-using patients could limit the observation that short-term withdrawal is not harmful – although we felt it appropriate not to limit statins beyond what’s typical in clinical practice, based on prior reports that even short-term statin withdrawal may be harmful.”
The National Institutes of Health and the department of anesthesiology at Vanderbilt University supported the study. Dr. Billings reported having no financial disclosures.
SAN DIEGO – High-dose perioperative atorvastatin treatment did not reduce acute kidney injury following elective cardiac surgery, and it may increase risk in patients with chronic kidney disease (CKD) who are naive to statin treatment, results from a large, randomized trial showed.
“Despite advances in patient management that have reduced mortality during cardiac surgery, acute kidney injury continues to complicate the postoperative course in 20%-30% of patients,” Dr. Frederic Tremaine Billings said during a press briefing at a meeting sponsored by the American Society of Nephrology.
“Its diagnosis is independently associated with a fivefold increase in mortality following the surgery,” Dr. Tremaine added. “Statins affect several mechanisms underlying postoperative acute kidney injury. Widely prescribed to reduce cholesterol synthesis, these drugs also reduce lipid modification of intracellular signaling molecules, which have been shown to improve perfusion and reduce oxidative stress – both mechanisms important in acute kidney injury following cardiac surgery.”
Dr. Billings of the department of anesthesiology and critical care medicine at Vanderbilt University Medical Center, Nashville, Tenn., and his associates tested the hypothesis that short-term, high-dose perioperative (preoperative, intraoperative, and postoperative) atorvastatin reduces acute kidney injury (AKI) following elective cardiac surgery.
The researchers randomly assigned preoperative statin-naive patients to 80 mg of atorvastatin on the morning before surgery, 40 mg on the morning of surgery, and 40 mg daily throughout hospitalization, or to a matching placebo regimen. In addition, they randomly assigned patients who were already using statins prior to surgery to 80 mg of atorvastatin the morning of surgery, and 40 mg on the morning after surgery, or to a matching placebo regimen.
“We felt it was important to not withhold statin treatment in patients already using statins prior to surgery, beyond what is typically done in clinical practice,” Dr. Billings explained. “For this reason, preoperative statin–using subjects continued their statin up until the day before surgery, and then resumed their statin use on postoperative day 2.”
The primary endpoint of the study was the incidence of AKI as determined by Acute Kidney Injury Network criteria (a 0.3 mg/dL increase in serum creatinine concentrations within 48 hours of surgery). Secondary endpoints included the maximum creatinine increase from baseline to 48 hours after surgery, ICU delirium diagnosed by the Confusion Assessment Method for the ICU, myocardial injury, and the incidence of atrial fibrillation, pneumonia, and stroke. Safety endpoints included liver toxicity, muscle toxicity, and adverse events.
The study was limited to adults having elective cardiac surgery and excluded those with statin intolerance, acute coronary syndrome, liver dysfunction, use of CYP3A4 inhibitors, kidney transplant recipients, those currently on dialysis, and those who were pregnant.
From November 2009 to October 2014, the researchers recruited 653 patients. But the trial was halted on recommendation of Vanderbilt’s data and safety monitoring board because of futility and an increased incidence of AKI among statin-naive patients with CKD randomized to atorvastatin.
Among all patients, AKI occurred in 20.8% of those randomized to atorvastatin, compared with 19.5% of those randomized to placebo, a difference that was neither clinically nor statistically significant (P = .75), Dr. Billings reported.
However, among the 199 patients who were statin naive, AKI occurred in 21.6% of those randomized to atorvastatin, compared with 13.4% of those randomized to placebo (P = .14). “An 8% difference in the incidence of AKI is of clinical importance, if true,” he said.
Among the 36 statin-naive patients with CKD, AKI occurred in 52.9% of those randomized to atorvastatin, compared with 15.8% of those randomized to placebo (P = .03). “While the number of patients in this subgroup is small, the magnitude of effect is striking,” Dr. Billings said.
Among the 416 patients who were using statins prior to surgery, AKI occurred in 20.4% of those randomized to atorvastatin, compared with 22.4% of those randomized to placebo (P = .63). Results were similar among the subset of those patients who had CKD (31.3% vs. 36.3%; P = .59).
Safety endpoints were similar between the two groups.
Strengths of the study, Dr. Billings said, include the fact that it’s the largest randomized, controlled trial to date to test this hypothesis, the pragmatic design of the protocol, and rigorous methodology.
Limitations include the “small number of patients in the statin-naive CKD subgroup,” he noted. “And the short duration of treatment among prestudy statin-using patients could limit the observation that short-term withdrawal is not harmful – although we felt it appropriate not to limit statins beyond what’s typical in clinical practice, based on prior reports that even short-term statin withdrawal may be harmful.”
The National Institutes of Health and the department of anesthesiology at Vanderbilt University supported the study. Dr. Billings reported having no financial disclosures.
SAN DIEGO – High-dose perioperative atorvastatin treatment did not reduce acute kidney injury following elective cardiac surgery, and it may increase risk in patients with chronic kidney disease (CKD) who are naive to statin treatment, results from a large, randomized trial showed.
“Despite advances in patient management that have reduced mortality during cardiac surgery, acute kidney injury continues to complicate the postoperative course in 20%-30% of patients,” Dr. Frederic Tremaine Billings said during a press briefing at a meeting sponsored by the American Society of Nephrology.
“Its diagnosis is independently associated with a fivefold increase in mortality following the surgery,” Dr. Tremaine added. “Statins affect several mechanisms underlying postoperative acute kidney injury. Widely prescribed to reduce cholesterol synthesis, these drugs also reduce lipid modification of intracellular signaling molecules, which have been shown to improve perfusion and reduce oxidative stress – both mechanisms important in acute kidney injury following cardiac surgery.”
Dr. Billings of the department of anesthesiology and critical care medicine at Vanderbilt University Medical Center, Nashville, Tenn., and his associates tested the hypothesis that short-term, high-dose perioperative (preoperative, intraoperative, and postoperative) atorvastatin reduces acute kidney injury (AKI) following elective cardiac surgery.
The researchers randomly assigned preoperative statin-naive patients to 80 mg of atorvastatin on the morning before surgery, 40 mg on the morning of surgery, and 40 mg daily throughout hospitalization, or to a matching placebo regimen. In addition, they randomly assigned patients who were already using statins prior to surgery to 80 mg of atorvastatin the morning of surgery, and 40 mg on the morning after surgery, or to a matching placebo regimen.
“We felt it was important to not withhold statin treatment in patients already using statins prior to surgery, beyond what is typically done in clinical practice,” Dr. Billings explained. “For this reason, preoperative statin–using subjects continued their statin up until the day before surgery, and then resumed their statin use on postoperative day 2.”
The primary endpoint of the study was the incidence of AKI as determined by Acute Kidney Injury Network criteria (a 0.3 mg/dL increase in serum creatinine concentrations within 48 hours of surgery). Secondary endpoints included the maximum creatinine increase from baseline to 48 hours after surgery, ICU delirium diagnosed by the Confusion Assessment Method for the ICU, myocardial injury, and the incidence of atrial fibrillation, pneumonia, and stroke. Safety endpoints included liver toxicity, muscle toxicity, and adverse events.
The study was limited to adults having elective cardiac surgery and excluded those with statin intolerance, acute coronary syndrome, liver dysfunction, use of CYP3A4 inhibitors, kidney transplant recipients, those currently on dialysis, and those who were pregnant.
From November 2009 to October 2014, the researchers recruited 653 patients. But the trial was halted on recommendation of Vanderbilt’s data and safety monitoring board because of futility and an increased incidence of AKI among statin-naive patients with CKD randomized to atorvastatin.
Among all patients, AKI occurred in 20.8% of those randomized to atorvastatin, compared with 19.5% of those randomized to placebo, a difference that was neither clinically nor statistically significant (P = .75), Dr. Billings reported.
However, among the 199 patients who were statin naive, AKI occurred in 21.6% of those randomized to atorvastatin, compared with 13.4% of those randomized to placebo (P = .14). “An 8% difference in the incidence of AKI is of clinical importance, if true,” he said.
Among the 36 statin-naive patients with CKD, AKI occurred in 52.9% of those randomized to atorvastatin, compared with 15.8% of those randomized to placebo (P = .03). “While the number of patients in this subgroup is small, the magnitude of effect is striking,” Dr. Billings said.
Among the 416 patients who were using statins prior to surgery, AKI occurred in 20.4% of those randomized to atorvastatin, compared with 22.4% of those randomized to placebo (P = .63). Results were similar among the subset of those patients who had CKD (31.3% vs. 36.3%; P = .59).
Safety endpoints were similar between the two groups.
Strengths of the study, Dr. Billings said, include the fact that it’s the largest randomized, controlled trial to date to test this hypothesis, the pragmatic design of the protocol, and rigorous methodology.
Limitations include the “small number of patients in the statin-naive CKD subgroup,” he noted. “And the short duration of treatment among prestudy statin-using patients could limit the observation that short-term withdrawal is not harmful – although we felt it appropriate not to limit statins beyond what’s typical in clinical practice, based on prior reports that even short-term statin withdrawal may be harmful.”
The National Institutes of Health and the department of anesthesiology at Vanderbilt University supported the study. Dr. Billings reported having no financial disclosures.
AT KIDNEY WEEK 2015
Key clinical point: The use of high-dose perioperative atorvastatin did not reduce acute kidney injury in patients undergoing elective cardiac surgery.
Major finding: Among all patients, acute kidney injury occurred in 20.8% of those randomized to atorvastatin, compared with 19.5% of those randomized to placebo, a difference that is neither clinically nor statistically significant (P = .75).
Data source: A randomized, controlled trial of 653 patients to test the hypothesis that short-term, high-dose perioperative atorvastatin reduces acute kidney injury following elective cardiac surgery.
Disclosures: The National Institutes of Health and the department of anesthesiology at Vanderbilt University supported the study. Dr. Billings reported having no financial disclosures.
Endobronchial valves improve pulmonary function in emphysema
Endobronchial valves improved pulmonary function, exercise capacity, and quality of life in a prospective randomized controlled trial involving 68 adults with severe emphysema, according to a report published online Dec. 10 in the New England Journal of Medicine.
“The improvements we found were of greater magnitude than those noted with pharmacologic treatment in comparable patients and were similar to improvements with surgical lung-volume reduction, but with significantly less morbidity,” said Karin Klooster of the department of pulmonary diseases, University Medical Center Groningen (the Netherlands) and her associates.
Previous research suggested that bronchoscopic lung-volume reduction using one-way endobronchial valves to block inspiratory but not expiratory air flow would be most effective in patients who had a complete rather than an incomplete fissure between the targeted lobe and the adjacent lobe on high-resolution CT. “A complete fissure on HRCT [high-resolution computed tomography] is a surrogate finding for the absence of interlobar collateral ventilation; if there is collateral ventilation, an occluded lobe can be reinflated through its collaterals,” defeating the purpose of the procedure, the researchers wrote.
During a 3-year period, Ms. Klooster and her associates studied emphysema patients who were older than 35 years (mean age, 58-59) and had a postbronchodilator forced expiratory volume in 1 second (FEV1) less than 60% of predicted volume, a total lung capacity more than 100% of the predicted value, and residual volume more than 150% of predicted volume. On HRCT, all the study participants showed a complete or nearly complete fissure between the targeted lobe and the adjacent lobe. They were randomly assigned to receive endobronchial valves (34 patients) or usual care (34 control subjects) and followed for 6 months. At that time, control subjects were allowed to crossover and receive endobronchial valves as well.
The median procedure time was 18 minutes (range, 6-51 minutes), and the median number of valves placed in each patient was 4 (range, 2-7 valves). The median hospital stay was 1 day (range, 1-13 days).
Compared with the control subjects, patients who received endobronchial valves showed a reduction in target lobar volume of 1,366 mL. This was accompanied by improvements in FEV1 by 191 mL, in forced vital capacity by 442 mL, in residual lung volume, in longer 6-minute walk distance by 106 meters, in scores on the Clinical COPD Questionnaire measuring daily functioning, and in scores on the St. George’s Respiratory Questionnaire measuring quality of life. The results for the control subjects who crossed over to the active-treatment group were very similar, the investigators said (N Engl J Med. 2015 Dec 10;373:2325-35. doi:10.1056/NEJMoa1507807).
However, several adverse effects occurred, and close monitoring of this patient population is crucial. The most common complication was pneumothorax, which developed in 6 of the 34 patients (18%), usually within 1 day of undergoing the procedure. Pneumothorax resolved spontaneously in one patient but required chest-tube drainage in the other five; it resolved in one patient after temporary removal of the valves to promote healing, and in another after permanent removal of all valves.
Other adverse effects, some of which required repeat bronchoscopy, included torsion of the lower-lobe bronchus after upper-lobe treatment (two patients), pneumonia distal to the valves (one patient), increased dyspnea and sputum production (two patients), valve migration (two patients), valve dislocation because of granulation-tissue formation (one patient), and persistent cough (one patient). Despite these setbacks, “the overall outcome of treatment was positive,” Ms. Klooster and her associates said.
All patients who underwent valve removal recovered without any further adverse effects, indicating that this treatment “is fully reversible and doesn’t preclude further therapeutic options,” they added.
The study was supported by the Netherlands Organization for Health Research and Development and the University Medical Center Groningen. Ms. Klooster reported receiving fees, devices, travel support, and grant support from Pulmonx and PneumRx/BTG; her associates reported ties to numerous industry sponsors. Pulmonx commercially supplied the endobronchial valves for the study.
Endobronchial valves improved pulmonary function, exercise capacity, and quality of life in a prospective randomized controlled trial involving 68 adults with severe emphysema, according to a report published online Dec. 10 in the New England Journal of Medicine.
“The improvements we found were of greater magnitude than those noted with pharmacologic treatment in comparable patients and were similar to improvements with surgical lung-volume reduction, but with significantly less morbidity,” said Karin Klooster of the department of pulmonary diseases, University Medical Center Groningen (the Netherlands) and her associates.
Previous research suggested that bronchoscopic lung-volume reduction using one-way endobronchial valves to block inspiratory but not expiratory air flow would be most effective in patients who had a complete rather than an incomplete fissure between the targeted lobe and the adjacent lobe on high-resolution CT. “A complete fissure on HRCT [high-resolution computed tomography] is a surrogate finding for the absence of interlobar collateral ventilation; if there is collateral ventilation, an occluded lobe can be reinflated through its collaterals,” defeating the purpose of the procedure, the researchers wrote.
During a 3-year period, Ms. Klooster and her associates studied emphysema patients who were older than 35 years (mean age, 58-59) and had a postbronchodilator forced expiratory volume in 1 second (FEV1) less than 60% of predicted volume, a total lung capacity more than 100% of the predicted value, and residual volume more than 150% of predicted volume. On HRCT, all the study participants showed a complete or nearly complete fissure between the targeted lobe and the adjacent lobe. They were randomly assigned to receive endobronchial valves (34 patients) or usual care (34 control subjects) and followed for 6 months. At that time, control subjects were allowed to crossover and receive endobronchial valves as well.
The median procedure time was 18 minutes (range, 6-51 minutes), and the median number of valves placed in each patient was 4 (range, 2-7 valves). The median hospital stay was 1 day (range, 1-13 days).
Compared with the control subjects, patients who received endobronchial valves showed a reduction in target lobar volume of 1,366 mL. This was accompanied by improvements in FEV1 by 191 mL, in forced vital capacity by 442 mL, in residual lung volume, in longer 6-minute walk distance by 106 meters, in scores on the Clinical COPD Questionnaire measuring daily functioning, and in scores on the St. George’s Respiratory Questionnaire measuring quality of life. The results for the control subjects who crossed over to the active-treatment group were very similar, the investigators said (N Engl J Med. 2015 Dec 10;373:2325-35. doi:10.1056/NEJMoa1507807).
However, several adverse effects occurred, and close monitoring of this patient population is crucial. The most common complication was pneumothorax, which developed in 6 of the 34 patients (18%), usually within 1 day of undergoing the procedure. Pneumothorax resolved spontaneously in one patient but required chest-tube drainage in the other five; it resolved in one patient after temporary removal of the valves to promote healing, and in another after permanent removal of all valves.
Other adverse effects, some of which required repeat bronchoscopy, included torsion of the lower-lobe bronchus after upper-lobe treatment (two patients), pneumonia distal to the valves (one patient), increased dyspnea and sputum production (two patients), valve migration (two patients), valve dislocation because of granulation-tissue formation (one patient), and persistent cough (one patient). Despite these setbacks, “the overall outcome of treatment was positive,” Ms. Klooster and her associates said.
All patients who underwent valve removal recovered without any further adverse effects, indicating that this treatment “is fully reversible and doesn’t preclude further therapeutic options,” they added.
The study was supported by the Netherlands Organization for Health Research and Development and the University Medical Center Groningen. Ms. Klooster reported receiving fees, devices, travel support, and grant support from Pulmonx and PneumRx/BTG; her associates reported ties to numerous industry sponsors. Pulmonx commercially supplied the endobronchial valves for the study.
Endobronchial valves improved pulmonary function, exercise capacity, and quality of life in a prospective randomized controlled trial involving 68 adults with severe emphysema, according to a report published online Dec. 10 in the New England Journal of Medicine.
“The improvements we found were of greater magnitude than those noted with pharmacologic treatment in comparable patients and were similar to improvements with surgical lung-volume reduction, but with significantly less morbidity,” said Karin Klooster of the department of pulmonary diseases, University Medical Center Groningen (the Netherlands) and her associates.
Previous research suggested that bronchoscopic lung-volume reduction using one-way endobronchial valves to block inspiratory but not expiratory air flow would be most effective in patients who had a complete rather than an incomplete fissure between the targeted lobe and the adjacent lobe on high-resolution CT. “A complete fissure on HRCT [high-resolution computed tomography] is a surrogate finding for the absence of interlobar collateral ventilation; if there is collateral ventilation, an occluded lobe can be reinflated through its collaterals,” defeating the purpose of the procedure, the researchers wrote.
During a 3-year period, Ms. Klooster and her associates studied emphysema patients who were older than 35 years (mean age, 58-59) and had a postbronchodilator forced expiratory volume in 1 second (FEV1) less than 60% of predicted volume, a total lung capacity more than 100% of the predicted value, and residual volume more than 150% of predicted volume. On HRCT, all the study participants showed a complete or nearly complete fissure between the targeted lobe and the adjacent lobe. They were randomly assigned to receive endobronchial valves (34 patients) or usual care (34 control subjects) and followed for 6 months. At that time, control subjects were allowed to crossover and receive endobronchial valves as well.
The median procedure time was 18 minutes (range, 6-51 minutes), and the median number of valves placed in each patient was 4 (range, 2-7 valves). The median hospital stay was 1 day (range, 1-13 days).
Compared with the control subjects, patients who received endobronchial valves showed a reduction in target lobar volume of 1,366 mL. This was accompanied by improvements in FEV1 by 191 mL, in forced vital capacity by 442 mL, in residual lung volume, in longer 6-minute walk distance by 106 meters, in scores on the Clinical COPD Questionnaire measuring daily functioning, and in scores on the St. George’s Respiratory Questionnaire measuring quality of life. The results for the control subjects who crossed over to the active-treatment group were very similar, the investigators said (N Engl J Med. 2015 Dec 10;373:2325-35. doi:10.1056/NEJMoa1507807).
However, several adverse effects occurred, and close monitoring of this patient population is crucial. The most common complication was pneumothorax, which developed in 6 of the 34 patients (18%), usually within 1 day of undergoing the procedure. Pneumothorax resolved spontaneously in one patient but required chest-tube drainage in the other five; it resolved in one patient after temporary removal of the valves to promote healing, and in another after permanent removal of all valves.
Other adverse effects, some of which required repeat bronchoscopy, included torsion of the lower-lobe bronchus after upper-lobe treatment (two patients), pneumonia distal to the valves (one patient), increased dyspnea and sputum production (two patients), valve migration (two patients), valve dislocation because of granulation-tissue formation (one patient), and persistent cough (one patient). Despite these setbacks, “the overall outcome of treatment was positive,” Ms. Klooster and her associates said.
All patients who underwent valve removal recovered without any further adverse effects, indicating that this treatment “is fully reversible and doesn’t preclude further therapeutic options,” they added.
The study was supported by the Netherlands Organization for Health Research and Development and the University Medical Center Groningen. Ms. Klooster reported receiving fees, devices, travel support, and grant support from Pulmonx and PneumRx/BTG; her associates reported ties to numerous industry sponsors. Pulmonx commercially supplied the endobronchial valves for the study.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Endobronchial valves improved pulmonary function, exercise capacity, and QOL in severe emphysema.
Major finding: Patients who received endobronchial valves showed improved FEV1 by 191 mL, forced vital capacity by 442 mL, residual lung volume, 6-minute walk distance by 106 meters, and QOL scores.
Data source: A prospective randomized controlled trial involving 68 patients treated during a 3-year period at a single medical center.
Disclosures: The Netherlands Organization for Health Research and Development and the University Medical Center Groningen funded the study. Ms. Klooster reported receiving fees, devices, travel support, and grant support from Pulmonx and PneumRx/BTG; her associates reported ties to numerous industry sponsors. Pulmonx commercially supplied the endobronchial valves for this study.
Does position matter in ViV implantation?
With transcatheter valve-in-valve implantation emerging as a novel treatment for high-risk patients whose existing bioprostheses have deteriorated, a team of investigators at University Heart Center in Hamburg, Germany, has found that the procedure can be done successfully in four different anatomic positions with a variety of bioprostheses.
The findings from the single-center study were published in the December issue of the Journal of Thoracic and Cardiovascular Surgery. (J Thorac Cardiovasc Surg. 2015;150:1557-67). They retrospectively analyzed results of 75 patients who had transcatheter valve-in-valve (ViV) replacement at their institution from 2008 to 2014.
“ViV can be performed in all anatomic positions with acceptable hemodynamic and clinical outcome in high-risk patients,” wrote Dr. Lenard Conradi and coauthors. “Increasing importance of ViV can be anticipated considering growing use of surgical bioprostheses.”
Replacement of biological valves is becoming more common. For surgical aortic valve replacement (SAVR), biological procedures have largely replaced mechanical valve implantation, comprising 87% of all such procedures by 2014, according to data from the German Society for Thoracic and Cardiovascular Surgery (Thorac Cardiovasc Surg. 2014;62:380-92). “Therefore, increasing caseload of patients with deteriorated bioprostheses can be expected,” wrote Dr. Conradi and coauthors.
The four anatomic positions in which the investigators performed the procedures and their share of cases are: aortic (54 patients/72%), mitral (17/22.7%), and tricuspid and pulmonary positions (2/2.7% each). The average interval between the index procedure and ViV was 9 years, with a deviation of nearly 5 years among all procedures. Dr. Conradi and coinvestigators said their study focused on technical aspects of ViV procedures from each position to provide guidance for surgeons.
Overall, the study authors performed ViV successfully in 97.3% of patients, with two patients requiring sequential transcatheter heart valve implantation for initial malpositioning. Thirty-day mortality was 8%, which “ranged lower” than expected when compared to standard preoperative risk stratification, they wrote. Mortality was at 5.6% in the aortic group and 17.6% in the mitral group.
That none of the currently available surgical bioprostheses or transcatheter heart valves (THV) were designed for later ViV procedures in deteriorated bioprostheses – although the CoreValve and Sapein THV have approvals for the indication – “may explain some of the apparent shortcomings of ViV therapy,” the researchers wrote.
The most significant challenge of ViV therapy is dealing with elevated residual gradients, which positioning can influence, according to the study findings. “This is not so much an issue for mitral, tricuspid, or pulmonary positions since surgical bioprostheses implanted in these positions are usually of sufficient size to accommodate the THV,” the researchers noted. “However, in the aortic position, more severe spatial restrictions may apply.”
They cited other reports that described a reverse relationship between size of the bioprosthetic and resulting transvalvular gradient after ViV (JACC Cardiovasc Interv. 2011;4:1218-27; JAMA 2014;312:162-70).
To reduce gradients, the investigators used post-ballooning after aortic ViV with a self-expandable THV in 16 cases, succeeding in 12. “Likely, further THV expansion with active compression of soft leaflet and/or pannus tissue and tighter apposition of THV against the frames of surgical bioprostheses contributed to this desired effect,” wrote the researchers. Patient-prosthesis mismatch probably explained the four cases in which gradients could not be further reduced, they noted.
They issued one “word of caution” regarding aortic ViV in small-sized surgical bioprostheses: “Elevated postprocedural gradients have to be expected and must be weighed against expected benefits and against risk of repeat open heart surgery.”
The six transcatheter heart valves the investigators used were Edwards Sapien (XT)/Sapien3 (52%, 39/75); Medtronic CoreValve/CoreValveEvolut (34.7%, 26); St. Jude Portico and Boston Scientific Lotus (4%, three each); and JenaValve and Medtronic Engager (2.7%, two each). The study also looked at different access routes: transapical in 53.3% (40), transfemoral (transarterial or transvenous) in 42.7% (32), transaortic in 2.7% (2), and transjugular in 1.3% (1).
Dr. Conradi and coauthors Dr. Moritz Seiffert, Dr. Ulrich Schaefer, and Dr. Hendrik Treede disclosed ties with Edwards Lifesciences, JenaValve Technology, Medtronic, Symetis, and St. Jude Medical. Four other coauthors reported no disclosures.
As the population ages and younger patients choose bioprosthetic valves to avoid lifelong warfarin, surgeons are going to face more situations where they will have to decide whether to perform surgical or transcatheter reoperative valve surgery, Dr. Jessica Forcillo of Emory University, Atlanta, and coauthors wrote in an invited commentary (J Thorac Cardiovasc Surg. 2015;150:1568-9).
They called the 8% 30-day mortality rate in the Hamburg study “high” even though the average age of the study population was a “relatively young” 74 years. The Hamburg authors may have learned more had they evaluated fewer prostheses. “With a small number of patients and at the beginning of an experience, focusing on one or two available prostheses may have resulted in more accurate and reliable results,” noted Dr. Forcillo and her colleagues. That 53% of the procedures were done via the transapical approach may also explain the mortality rate, they said.
The overall 30-day mortality rate along with a 17.6% mortality in the mitral ViV group are causes for “some caution against overzealous performance of this procedure and continued monitoring of outcomes in other series,” wrote Dr. Forcillo and her colleagues.
But ViV implantation is a “transformative” technology, they said. “For the elderly, high-risk patients with [structural valve degeneration], transcatheter options may provide improved short-term outcomes,” they added. “The valve community eagerly awaits larger series with adjudicated outcomes of the transcatheter valve-in-valve procedure.”
Dr. Forcillo and coauthor Lillian Tsai had no disclosures. Dr. Vinod Thourani disclosed ties with St. Jude Medical, Edwards Lifesciences, Boston Scientific, Abbott Medical, Medtronic, Directflow, and Sorin Medical.
As the population ages and younger patients choose bioprosthetic valves to avoid lifelong warfarin, surgeons are going to face more situations where they will have to decide whether to perform surgical or transcatheter reoperative valve surgery, Dr. Jessica Forcillo of Emory University, Atlanta, and coauthors wrote in an invited commentary (J Thorac Cardiovasc Surg. 2015;150:1568-9).
They called the 8% 30-day mortality rate in the Hamburg study “high” even though the average age of the study population was a “relatively young” 74 years. The Hamburg authors may have learned more had they evaluated fewer prostheses. “With a small number of patients and at the beginning of an experience, focusing on one or two available prostheses may have resulted in more accurate and reliable results,” noted Dr. Forcillo and her colleagues. That 53% of the procedures were done via the transapical approach may also explain the mortality rate, they said.
The overall 30-day mortality rate along with a 17.6% mortality in the mitral ViV group are causes for “some caution against overzealous performance of this procedure and continued monitoring of outcomes in other series,” wrote Dr. Forcillo and her colleagues.
But ViV implantation is a “transformative” technology, they said. “For the elderly, high-risk patients with [structural valve degeneration], transcatheter options may provide improved short-term outcomes,” they added. “The valve community eagerly awaits larger series with adjudicated outcomes of the transcatheter valve-in-valve procedure.”
Dr. Forcillo and coauthor Lillian Tsai had no disclosures. Dr. Vinod Thourani disclosed ties with St. Jude Medical, Edwards Lifesciences, Boston Scientific, Abbott Medical, Medtronic, Directflow, and Sorin Medical.
As the population ages and younger patients choose bioprosthetic valves to avoid lifelong warfarin, surgeons are going to face more situations where they will have to decide whether to perform surgical or transcatheter reoperative valve surgery, Dr. Jessica Forcillo of Emory University, Atlanta, and coauthors wrote in an invited commentary (J Thorac Cardiovasc Surg. 2015;150:1568-9).
They called the 8% 30-day mortality rate in the Hamburg study “high” even though the average age of the study population was a “relatively young” 74 years. The Hamburg authors may have learned more had they evaluated fewer prostheses. “With a small number of patients and at the beginning of an experience, focusing on one or two available prostheses may have resulted in more accurate and reliable results,” noted Dr. Forcillo and her colleagues. That 53% of the procedures were done via the transapical approach may also explain the mortality rate, they said.
The overall 30-day mortality rate along with a 17.6% mortality in the mitral ViV group are causes for “some caution against overzealous performance of this procedure and continued monitoring of outcomes in other series,” wrote Dr. Forcillo and her colleagues.
But ViV implantation is a “transformative” technology, they said. “For the elderly, high-risk patients with [structural valve degeneration], transcatheter options may provide improved short-term outcomes,” they added. “The valve community eagerly awaits larger series with adjudicated outcomes of the transcatheter valve-in-valve procedure.”
Dr. Forcillo and coauthor Lillian Tsai had no disclosures. Dr. Vinod Thourani disclosed ties with St. Jude Medical, Edwards Lifesciences, Boston Scientific, Abbott Medical, Medtronic, Directflow, and Sorin Medical.
With transcatheter valve-in-valve implantation emerging as a novel treatment for high-risk patients whose existing bioprostheses have deteriorated, a team of investigators at University Heart Center in Hamburg, Germany, has found that the procedure can be done successfully in four different anatomic positions with a variety of bioprostheses.
The findings from the single-center study were published in the December issue of the Journal of Thoracic and Cardiovascular Surgery. (J Thorac Cardiovasc Surg. 2015;150:1557-67). They retrospectively analyzed results of 75 patients who had transcatheter valve-in-valve (ViV) replacement at their institution from 2008 to 2014.
“ViV can be performed in all anatomic positions with acceptable hemodynamic and clinical outcome in high-risk patients,” wrote Dr. Lenard Conradi and coauthors. “Increasing importance of ViV can be anticipated considering growing use of surgical bioprostheses.”
Replacement of biological valves is becoming more common. For surgical aortic valve replacement (SAVR), biological procedures have largely replaced mechanical valve implantation, comprising 87% of all such procedures by 2014, according to data from the German Society for Thoracic and Cardiovascular Surgery (Thorac Cardiovasc Surg. 2014;62:380-92). “Therefore, increasing caseload of patients with deteriorated bioprostheses can be expected,” wrote Dr. Conradi and coauthors.
The four anatomic positions in which the investigators performed the procedures and their share of cases are: aortic (54 patients/72%), mitral (17/22.7%), and tricuspid and pulmonary positions (2/2.7% each). The average interval between the index procedure and ViV was 9 years, with a deviation of nearly 5 years among all procedures. Dr. Conradi and coinvestigators said their study focused on technical aspects of ViV procedures from each position to provide guidance for surgeons.
Overall, the study authors performed ViV successfully in 97.3% of patients, with two patients requiring sequential transcatheter heart valve implantation for initial malpositioning. Thirty-day mortality was 8%, which “ranged lower” than expected when compared to standard preoperative risk stratification, they wrote. Mortality was at 5.6% in the aortic group and 17.6% in the mitral group.
That none of the currently available surgical bioprostheses or transcatheter heart valves (THV) were designed for later ViV procedures in deteriorated bioprostheses – although the CoreValve and Sapein THV have approvals for the indication – “may explain some of the apparent shortcomings of ViV therapy,” the researchers wrote.
The most significant challenge of ViV therapy is dealing with elevated residual gradients, which positioning can influence, according to the study findings. “This is not so much an issue for mitral, tricuspid, or pulmonary positions since surgical bioprostheses implanted in these positions are usually of sufficient size to accommodate the THV,” the researchers noted. “However, in the aortic position, more severe spatial restrictions may apply.”
They cited other reports that described a reverse relationship between size of the bioprosthetic and resulting transvalvular gradient after ViV (JACC Cardiovasc Interv. 2011;4:1218-27; JAMA 2014;312:162-70).
To reduce gradients, the investigators used post-ballooning after aortic ViV with a self-expandable THV in 16 cases, succeeding in 12. “Likely, further THV expansion with active compression of soft leaflet and/or pannus tissue and tighter apposition of THV against the frames of surgical bioprostheses contributed to this desired effect,” wrote the researchers. Patient-prosthesis mismatch probably explained the four cases in which gradients could not be further reduced, they noted.
They issued one “word of caution” regarding aortic ViV in small-sized surgical bioprostheses: “Elevated postprocedural gradients have to be expected and must be weighed against expected benefits and against risk of repeat open heart surgery.”
The six transcatheter heart valves the investigators used were Edwards Sapien (XT)/Sapien3 (52%, 39/75); Medtronic CoreValve/CoreValveEvolut (34.7%, 26); St. Jude Portico and Boston Scientific Lotus (4%, three each); and JenaValve and Medtronic Engager (2.7%, two each). The study also looked at different access routes: transapical in 53.3% (40), transfemoral (transarterial or transvenous) in 42.7% (32), transaortic in 2.7% (2), and transjugular in 1.3% (1).
Dr. Conradi and coauthors Dr. Moritz Seiffert, Dr. Ulrich Schaefer, and Dr. Hendrik Treede disclosed ties with Edwards Lifesciences, JenaValve Technology, Medtronic, Symetis, and St. Jude Medical. Four other coauthors reported no disclosures.
With transcatheter valve-in-valve implantation emerging as a novel treatment for high-risk patients whose existing bioprostheses have deteriorated, a team of investigators at University Heart Center in Hamburg, Germany, has found that the procedure can be done successfully in four different anatomic positions with a variety of bioprostheses.
The findings from the single-center study were published in the December issue of the Journal of Thoracic and Cardiovascular Surgery. (J Thorac Cardiovasc Surg. 2015;150:1557-67). They retrospectively analyzed results of 75 patients who had transcatheter valve-in-valve (ViV) replacement at their institution from 2008 to 2014.
“ViV can be performed in all anatomic positions with acceptable hemodynamic and clinical outcome in high-risk patients,” wrote Dr. Lenard Conradi and coauthors. “Increasing importance of ViV can be anticipated considering growing use of surgical bioprostheses.”
Replacement of biological valves is becoming more common. For surgical aortic valve replacement (SAVR), biological procedures have largely replaced mechanical valve implantation, comprising 87% of all such procedures by 2014, according to data from the German Society for Thoracic and Cardiovascular Surgery (Thorac Cardiovasc Surg. 2014;62:380-92). “Therefore, increasing caseload of patients with deteriorated bioprostheses can be expected,” wrote Dr. Conradi and coauthors.
The four anatomic positions in which the investigators performed the procedures and their share of cases are: aortic (54 patients/72%), mitral (17/22.7%), and tricuspid and pulmonary positions (2/2.7% each). The average interval between the index procedure and ViV was 9 years, with a deviation of nearly 5 years among all procedures. Dr. Conradi and coinvestigators said their study focused on technical aspects of ViV procedures from each position to provide guidance for surgeons.
Overall, the study authors performed ViV successfully in 97.3% of patients, with two patients requiring sequential transcatheter heart valve implantation for initial malpositioning. Thirty-day mortality was 8%, which “ranged lower” than expected when compared to standard preoperative risk stratification, they wrote. Mortality was at 5.6% in the aortic group and 17.6% in the mitral group.
That none of the currently available surgical bioprostheses or transcatheter heart valves (THV) were designed for later ViV procedures in deteriorated bioprostheses – although the CoreValve and Sapein THV have approvals for the indication – “may explain some of the apparent shortcomings of ViV therapy,” the researchers wrote.
The most significant challenge of ViV therapy is dealing with elevated residual gradients, which positioning can influence, according to the study findings. “This is not so much an issue for mitral, tricuspid, or pulmonary positions since surgical bioprostheses implanted in these positions are usually of sufficient size to accommodate the THV,” the researchers noted. “However, in the aortic position, more severe spatial restrictions may apply.”
They cited other reports that described a reverse relationship between size of the bioprosthetic and resulting transvalvular gradient after ViV (JACC Cardiovasc Interv. 2011;4:1218-27; JAMA 2014;312:162-70).
To reduce gradients, the investigators used post-ballooning after aortic ViV with a self-expandable THV in 16 cases, succeeding in 12. “Likely, further THV expansion with active compression of soft leaflet and/or pannus tissue and tighter apposition of THV against the frames of surgical bioprostheses contributed to this desired effect,” wrote the researchers. Patient-prosthesis mismatch probably explained the four cases in which gradients could not be further reduced, they noted.
They issued one “word of caution” regarding aortic ViV in small-sized surgical bioprostheses: “Elevated postprocedural gradients have to be expected and must be weighed against expected benefits and against risk of repeat open heart surgery.”
The six transcatheter heart valves the investigators used were Edwards Sapien (XT)/Sapien3 (52%, 39/75); Medtronic CoreValve/CoreValveEvolut (34.7%, 26); St. Jude Portico and Boston Scientific Lotus (4%, three each); and JenaValve and Medtronic Engager (2.7%, two each). The study also looked at different access routes: transapical in 53.3% (40), transfemoral (transarterial or transvenous) in 42.7% (32), transaortic in 2.7% (2), and transjugular in 1.3% (1).
Dr. Conradi and coauthors Dr. Moritz Seiffert, Dr. Ulrich Schaefer, and Dr. Hendrik Treede disclosed ties with Edwards Lifesciences, JenaValve Technology, Medtronic, Symetis, and St. Jude Medical. Four other coauthors reported no disclosures.
Key clinical point: Transcatheter valve-in-valve (ViV) implantation is a relatively safe treatment for patients with a deteriorated bioprostheses.
Major finding: A ViV implantation when performed in four different positions with six different transcatheter heart valves had a 30-day mortality of 8%.
Data source: Retrospective analysis of 75 consecutive patients receiving ViV procedures from 2008 to 2014 at a single institution.
Disclosures: Dr. Conradi and coauthors Dr. Moritz Seiffert, Dr. Ulrich Schaefer, and Dr. Hendrik Treede disclosed relationships with Edwards Lifesciences, JenaValve Technology, Medtronic, Symetis, and St. Jude Medical. Four other coauthors reported no disclosures.
Surgical ablation endures at 5 years
The Cox-Maze IV procedure (CMPIV) has become the standard for surgical ablation for atrial fibrillation (AF), yet little information has been available on how late outcomes compare with catheter-based ablation. A recent analysis of 576 procedures found that after 5 years, most people who had the procedure remained free of atrial tachyarrhythmias and anticoagulation.
The study, by investigators from Washington University, Barnes-Jewish Hospital in St. Louis, was published in the Journal of Thoracic and Cardiovascular Surgery (J Thorac Cardiovasc Surg. 2015;150:1168-78). The researchers first presented the study in April at the American Association for Thoracic Surgery meeting in Seattle.
“The results of the CMPIV remain superior to those reported for catheter ablation and other forms of surgical AF ablation, especially for patients with persistent or long-standing AF,” wrote Dr. Matthew C. Henn and his colleagues.
They set out to evaluate late outcomes after CMPIV using current consensus definitions of treatment failure, noting that such outcomes had yet to be reported. They followed 576 patients with atrial fibrillation who had a CMPIV from 2002 to 2014 and compared long-term freedom from atrial fibrillation on and off antiarrhythmic drugs (AADs) across various subgroups. They included the left-sided CMPIV lesion in the analysis because, they said, it had success rates similar to those of biatrial CMPIV.
The Cox-Maze procedure was first introduced by Dr. James Cox in 1987 and updated from the original “cut-and-sew” technique in 2002 to combine bipolar radiofrequency and cryothermal ablation lines in place of most surgical incisions. This iteration was called the Cox-Maze IV procedure. In 2005, CMPIV was modified to include a superior connecting lesion, which formed a “box lesion” by completely isolating the entire posterior left atrium. The study included 512 people who underwent the “box lesion” set procedure.
“The modifications of the CMPIV have allowed it to be performed through a right minithoracotomy (RMT) approach, which has further reduced major morbidity, mortality, and hospital stay compared to those who underwent sternotomy while enjoying equivalent outcomes with regards to freedom from AF,” wrote Dr. Henn and his coauthors.
In the entire cohort, the overall freedom from atrial tachyarrhythmias (ATAs) and anticoagulation were 92% at 1 year, 88% at 2 years, 87% at 3 years, 81% at 4 years, and 73% at 5 years. Overall freedom from ATAs off antiarrhythmic drugs for the entire cohort ranged from 81% at 1 year to 61% at 5 years, and freedom from anticoagulation ranged from 65% at 1 year to 55% at 5 years.
“Freedoms from ATAs on or off AADs were significantly higher in those who underwent box lesion sets when compared to those who did not at 5 years,” noted Dr. Henn and his coauthors. Among the box lesion set group, 78% of those on AADs remained free of ATAs vs. 45% in the non–box lesion set group, and for those off AADs, 66% had no ATAs at 5 years while 33% of the non–box lesion set group did.
Of the overall study population, 41% had paroxysmal AF and 58% had nonparoxysmal AF. Among the latter group, 20% had persistent and 80% had long-standing persistent AF. The nonparoxysmal AF group had a longer duration of preoperative AF, larger left atria and more failed catheter ablations, Dr. Henn and coauthors reported. But, the study showed no differences in freedom from atrial fibrillation on or off AADs at 5 years between patients with paroxysmal AF or persistent/long-standing persistent AF, or between those who underwent stand-alone procedure and those who received a concomitant Cox-Maze procedure. Among those who had a concomitant procedure, 50% had a concomitant mitral valve procedure and 23% had coronary artery bypass grafting.
“The CMPIV results in our series were better than what has been achieved with catheter ablation,” the researchers wrote. They cited studies that showed arrhythmia-free survival after a single ablation procedure ranging from 17% to 29% and “equally poor results.” (Circ Arrhythm Electrophysiol. 2015;8:18-24; J Am Coll Cardiol. 2011;57:160-166; J Am Heart Assoc. 2013;2:e004549.)
“The CMPIV remains the most successful surgical treatment for AF, even in patients with non-paroxysmal AF and regardless of the complexity of the concomitant procedures,” Dr. Henn and his coauthors concluded.
Inconsistencies in this study of the Cox-Maze IV procedure include differing types of atrial fibrillation, heterogeneous concomitant operations, multiple lesion sets and energy sources and inconsistent postablation monitoring, all of which make direct comparisons of surgical ablation strategies or even catheter ablation difficult, Dr. Robert Hawkins and Dr. Gorav Ailawadi of the University of Virginia noted in their invited commentary (J Thorac Cardiovasc Surg. 2015;150:1179-80). “Moreover, without controls or selection criteria, it is difficult to account for selection bias,” they wrote.
Yet, this study has “some important findings” despite its shortcomings, namely the “respectable” rates of atrial tachyarrhythmias off antiarrhythmic drugs. These results are superior to other clinical trials, “in part due to the expertise at Washington University,” noted Dr. Hawkins and Dr. Ailawadi.
Adding patients who had the box lesion set approach also improved 5-year outcomes in the study substantially, and left atrium (LA) ablation alone has good results in patients with paroxysmal AF, left atria less than 5.0 cm, and no right atrial enlargement. “Yet, a direct comparison between biatrial and LA lesion sets cannot be made due to the above listed limitations,” they wrote.
The study makes a case for surgical ablation when the preoperative duration of AF is less than 5-10 years and left atrium size is not a problem, and the lesion-set requires further investigation, they said. “Finally, this study highlights the continued need for rigorous monitoring and comparisons of homogeneous patient populations to make stronger conclusions.”
Dr. Ailawadi disclosed relationships with Abbot Vascular, Mitralign, Edwards Lifesciences and St. Jude Medical. Dr. Hawkins had no relationships to disclose.
Inconsistencies in this study of the Cox-Maze IV procedure include differing types of atrial fibrillation, heterogeneous concomitant operations, multiple lesion sets and energy sources and inconsistent postablation monitoring, all of which make direct comparisons of surgical ablation strategies or even catheter ablation difficult, Dr. Robert Hawkins and Dr. Gorav Ailawadi of the University of Virginia noted in their invited commentary (J Thorac Cardiovasc Surg. 2015;150:1179-80). “Moreover, without controls or selection criteria, it is difficult to account for selection bias,” they wrote.
Yet, this study has “some important findings” despite its shortcomings, namely the “respectable” rates of atrial tachyarrhythmias off antiarrhythmic drugs. These results are superior to other clinical trials, “in part due to the expertise at Washington University,” noted Dr. Hawkins and Dr. Ailawadi.
Adding patients who had the box lesion set approach also improved 5-year outcomes in the study substantially, and left atrium (LA) ablation alone has good results in patients with paroxysmal AF, left atria less than 5.0 cm, and no right atrial enlargement. “Yet, a direct comparison between biatrial and LA lesion sets cannot be made due to the above listed limitations,” they wrote.
The study makes a case for surgical ablation when the preoperative duration of AF is less than 5-10 years and left atrium size is not a problem, and the lesion-set requires further investigation, they said. “Finally, this study highlights the continued need for rigorous monitoring and comparisons of homogeneous patient populations to make stronger conclusions.”
Dr. Ailawadi disclosed relationships with Abbot Vascular, Mitralign, Edwards Lifesciences and St. Jude Medical. Dr. Hawkins had no relationships to disclose.
Inconsistencies in this study of the Cox-Maze IV procedure include differing types of atrial fibrillation, heterogeneous concomitant operations, multiple lesion sets and energy sources and inconsistent postablation monitoring, all of which make direct comparisons of surgical ablation strategies or even catheter ablation difficult, Dr. Robert Hawkins and Dr. Gorav Ailawadi of the University of Virginia noted in their invited commentary (J Thorac Cardiovasc Surg. 2015;150:1179-80). “Moreover, without controls or selection criteria, it is difficult to account for selection bias,” they wrote.
Yet, this study has “some important findings” despite its shortcomings, namely the “respectable” rates of atrial tachyarrhythmias off antiarrhythmic drugs. These results are superior to other clinical trials, “in part due to the expertise at Washington University,” noted Dr. Hawkins and Dr. Ailawadi.
Adding patients who had the box lesion set approach also improved 5-year outcomes in the study substantially, and left atrium (LA) ablation alone has good results in patients with paroxysmal AF, left atria less than 5.0 cm, and no right atrial enlargement. “Yet, a direct comparison between biatrial and LA lesion sets cannot be made due to the above listed limitations,” they wrote.
The study makes a case for surgical ablation when the preoperative duration of AF is less than 5-10 years and left atrium size is not a problem, and the lesion-set requires further investigation, they said. “Finally, this study highlights the continued need for rigorous monitoring and comparisons of homogeneous patient populations to make stronger conclusions.”
Dr. Ailawadi disclosed relationships with Abbot Vascular, Mitralign, Edwards Lifesciences and St. Jude Medical. Dr. Hawkins had no relationships to disclose.
The Cox-Maze IV procedure (CMPIV) has become the standard for surgical ablation for atrial fibrillation (AF), yet little information has been available on how late outcomes compare with catheter-based ablation. A recent analysis of 576 procedures found that after 5 years, most people who had the procedure remained free of atrial tachyarrhythmias and anticoagulation.
The study, by investigators from Washington University, Barnes-Jewish Hospital in St. Louis, was published in the Journal of Thoracic and Cardiovascular Surgery (J Thorac Cardiovasc Surg. 2015;150:1168-78). The researchers first presented the study in April at the American Association for Thoracic Surgery meeting in Seattle.
“The results of the CMPIV remain superior to those reported for catheter ablation and other forms of surgical AF ablation, especially for patients with persistent or long-standing AF,” wrote Dr. Matthew C. Henn and his colleagues.
They set out to evaluate late outcomes after CMPIV using current consensus definitions of treatment failure, noting that such outcomes had yet to be reported. They followed 576 patients with atrial fibrillation who had a CMPIV from 2002 to 2014 and compared long-term freedom from atrial fibrillation on and off antiarrhythmic drugs (AADs) across various subgroups. They included the left-sided CMPIV lesion in the analysis because, they said, it had success rates similar to those of biatrial CMPIV.
The Cox-Maze procedure was first introduced by Dr. James Cox in 1987 and updated from the original “cut-and-sew” technique in 2002 to combine bipolar radiofrequency and cryothermal ablation lines in place of most surgical incisions. This iteration was called the Cox-Maze IV procedure. In 2005, CMPIV was modified to include a superior connecting lesion, which formed a “box lesion” by completely isolating the entire posterior left atrium. The study included 512 people who underwent the “box lesion” set procedure.
“The modifications of the CMPIV have allowed it to be performed through a right minithoracotomy (RMT) approach, which has further reduced major morbidity, mortality, and hospital stay compared to those who underwent sternotomy while enjoying equivalent outcomes with regards to freedom from AF,” wrote Dr. Henn and his coauthors.
In the entire cohort, the overall freedom from atrial tachyarrhythmias (ATAs) and anticoagulation were 92% at 1 year, 88% at 2 years, 87% at 3 years, 81% at 4 years, and 73% at 5 years. Overall freedom from ATAs off antiarrhythmic drugs for the entire cohort ranged from 81% at 1 year to 61% at 5 years, and freedom from anticoagulation ranged from 65% at 1 year to 55% at 5 years.
“Freedoms from ATAs on or off AADs were significantly higher in those who underwent box lesion sets when compared to those who did not at 5 years,” noted Dr. Henn and his coauthors. Among the box lesion set group, 78% of those on AADs remained free of ATAs vs. 45% in the non–box lesion set group, and for those off AADs, 66% had no ATAs at 5 years while 33% of the non–box lesion set group did.
Of the overall study population, 41% had paroxysmal AF and 58% had nonparoxysmal AF. Among the latter group, 20% had persistent and 80% had long-standing persistent AF. The nonparoxysmal AF group had a longer duration of preoperative AF, larger left atria and more failed catheter ablations, Dr. Henn and coauthors reported. But, the study showed no differences in freedom from atrial fibrillation on or off AADs at 5 years between patients with paroxysmal AF or persistent/long-standing persistent AF, or between those who underwent stand-alone procedure and those who received a concomitant Cox-Maze procedure. Among those who had a concomitant procedure, 50% had a concomitant mitral valve procedure and 23% had coronary artery bypass grafting.
“The CMPIV results in our series were better than what has been achieved with catheter ablation,” the researchers wrote. They cited studies that showed arrhythmia-free survival after a single ablation procedure ranging from 17% to 29% and “equally poor results.” (Circ Arrhythm Electrophysiol. 2015;8:18-24; J Am Coll Cardiol. 2011;57:160-166; J Am Heart Assoc. 2013;2:e004549.)
“The CMPIV remains the most successful surgical treatment for AF, even in patients with non-paroxysmal AF and regardless of the complexity of the concomitant procedures,” Dr. Henn and his coauthors concluded.
The Cox-Maze IV procedure (CMPIV) has become the standard for surgical ablation for atrial fibrillation (AF), yet little information has been available on how late outcomes compare with catheter-based ablation. A recent analysis of 576 procedures found that after 5 years, most people who had the procedure remained free of atrial tachyarrhythmias and anticoagulation.
The study, by investigators from Washington University, Barnes-Jewish Hospital in St. Louis, was published in the Journal of Thoracic and Cardiovascular Surgery (J Thorac Cardiovasc Surg. 2015;150:1168-78). The researchers first presented the study in April at the American Association for Thoracic Surgery meeting in Seattle.
“The results of the CMPIV remain superior to those reported for catheter ablation and other forms of surgical AF ablation, especially for patients with persistent or long-standing AF,” wrote Dr. Matthew C. Henn and his colleagues.
They set out to evaluate late outcomes after CMPIV using current consensus definitions of treatment failure, noting that such outcomes had yet to be reported. They followed 576 patients with atrial fibrillation who had a CMPIV from 2002 to 2014 and compared long-term freedom from atrial fibrillation on and off antiarrhythmic drugs (AADs) across various subgroups. They included the left-sided CMPIV lesion in the analysis because, they said, it had success rates similar to those of biatrial CMPIV.
The Cox-Maze procedure was first introduced by Dr. James Cox in 1987 and updated from the original “cut-and-sew” technique in 2002 to combine bipolar radiofrequency and cryothermal ablation lines in place of most surgical incisions. This iteration was called the Cox-Maze IV procedure. In 2005, CMPIV was modified to include a superior connecting lesion, which formed a “box lesion” by completely isolating the entire posterior left atrium. The study included 512 people who underwent the “box lesion” set procedure.
“The modifications of the CMPIV have allowed it to be performed through a right minithoracotomy (RMT) approach, which has further reduced major morbidity, mortality, and hospital stay compared to those who underwent sternotomy while enjoying equivalent outcomes with regards to freedom from AF,” wrote Dr. Henn and his coauthors.
In the entire cohort, the overall freedom from atrial tachyarrhythmias (ATAs) and anticoagulation were 92% at 1 year, 88% at 2 years, 87% at 3 years, 81% at 4 years, and 73% at 5 years. Overall freedom from ATAs off antiarrhythmic drugs for the entire cohort ranged from 81% at 1 year to 61% at 5 years, and freedom from anticoagulation ranged from 65% at 1 year to 55% at 5 years.
“Freedoms from ATAs on or off AADs were significantly higher in those who underwent box lesion sets when compared to those who did not at 5 years,” noted Dr. Henn and his coauthors. Among the box lesion set group, 78% of those on AADs remained free of ATAs vs. 45% in the non–box lesion set group, and for those off AADs, 66% had no ATAs at 5 years while 33% of the non–box lesion set group did.
Of the overall study population, 41% had paroxysmal AF and 58% had nonparoxysmal AF. Among the latter group, 20% had persistent and 80% had long-standing persistent AF. The nonparoxysmal AF group had a longer duration of preoperative AF, larger left atria and more failed catheter ablations, Dr. Henn and coauthors reported. But, the study showed no differences in freedom from atrial fibrillation on or off AADs at 5 years between patients with paroxysmal AF or persistent/long-standing persistent AF, or between those who underwent stand-alone procedure and those who received a concomitant Cox-Maze procedure. Among those who had a concomitant procedure, 50% had a concomitant mitral valve procedure and 23% had coronary artery bypass grafting.
“The CMPIV results in our series were better than what has been achieved with catheter ablation,” the researchers wrote. They cited studies that showed arrhythmia-free survival after a single ablation procedure ranging from 17% to 29% and “equally poor results.” (Circ Arrhythm Electrophysiol. 2015;8:18-24; J Am Coll Cardiol. 2011;57:160-166; J Am Heart Assoc. 2013;2:e004549.)
“The CMPIV remains the most successful surgical treatment for AF, even in patients with non-paroxysmal AF and regardless of the complexity of the concomitant procedures,” Dr. Henn and his coauthors concluded.
Key clinical point: Outcomes with the Cox-Maze IV procedure for surgical ablation are superior to catheter ablation and other forms of surgical ablation for atrial fibrillation for up to 5 years duration.
Major finding: Seventy-three percent of the study population was free from atrial tachyarrhythmias and 55% were free from anticoagulation at 5 years.
Data source: Prospective analysis of 576 consecutive patients with atrial fibrillation who had Cox-Maze IV procedure or a left-sized Cox-Maze IV procedure from 2002 to 2014 at a single institution
Disclosures: The National Institutes of Health provided grants for the study. Coauthor Dr. Ralph J. Damiano Jr. disclosed research grants and educational funding from AtriCure and Edwards LifeSciences. The other authors had no disclosures.
Pediatric heart transplant results not improving
A 25-year study of heart transplants in children with congenital heart disease (CHD) at one institution has found that results haven’t improved over time despite advances in technology and techniques. To improve outcomes, transplant surgeons may need to do a better job of selecting patients and matching patients and donors, according to study in the December issue of the Journal of Thoracic and Cardiovascular Surgery (J Thorac Cardiovasc Surg. 2015;150:1455-62).
“Strategies to improve outcomes in CHD patients might need to address selection criteria, transplantation timing, pretransplant and posttransplant care,” noted Dr. Bahaaldin Alsoufi, of the division of cardiothoracic surgery, Children’s Healthcare of Atlanta, Emory University. “The effect of donor/recipient race mismatch warrants further investigation and might impact organ allocation algorithms or immunosuppression management,” wrote Dr. Alsoufi and his colleagues.
The researchers analyzed results of 124 children with CHD who had heart transplants from 1988 to 2013 at Emory University and Children’s Healthcare of Atlanta. Median age was 3.8 years; 61% were boys. Ten years after heart transplantation, 44% (54) of patients were alive without a second transplant, 13% (17) had a second transplant and 43% (53) died without a second transplant. After the second transplant, 9 of the 17 patients were alive, but 3 of them had gone onto a third transplant. Overall 15-year survival following the first transplant was 41% (51).
The study cited data from the Registry of the International Society for Heart and Lung Transplantation that reported more than 11,000 pediatric heart transplants worldwide in 2013, and CHD represents about 54% of all heart transplants in infants.
A multivariate analysis identified the following risk factors for early mortality after transplant: age younger than 12 months (hazard ration [HR] 7.2) and prolonged cardiopulmonary bypass (HR 5). Late-phase mortality risk factors were age younger than 12 months (HR 3) and donor/recipient race mismatch (HR 2.2).
“Survival was not affected by era, underlying anomaly, prior Fontan, sensitization or pulmonary artery augmentation,” wrote Dr. Alsoufi and his colleagues.
Among the risk factors, longer bypass times may be a surrogate for a more complicated operation, the authors said. But where prior sternotomy is a risk factor following a heart transplant in adults, the study found no such risk in children. Another risk factor previous reports identified is pulmonary artery augmentation, but, again, this study found no risk in the pediatric group.
The researchers looked at days on the waiting list, with a median wait of 39 days in the study group. In all, 175 children were listed for transplants, but 51 did not go through for various reasons. Most of the children with CHD who had a heart transplant had previous surgery; only 13% had a primary heart transplant, mostly in the earlier phase of the study.
Dr. Alsoufi and coauthors also identified African American race as a risk factor for lower survival, which is consistent with other reports. But this study agreed with a previous report that donor/recipient race mismatch was a significant risk factor in white and African American patients (Ann Thorac Surg. 2009;87:204-9). “While our finding might be anecdotal and specific to our geographic population, this warrants some investigation and might have some impact on future organ allocation algorithms and immunosuppression management,” the researchers wrote.
The authors had no relevant disclosures. Emory University School of Medicine, Children’s Healthcare of Atlanta provided study funding.
In his invited commentary, Dr. Robert D.B. Jaquiss of Duke University, Durham, N.C., took issue with the study authors’ “distress” at the lack of improvement in survival over the 25-year term of the study (J Thorac Cardiovasc Surg. 2015;150:1463-4) . Using the year 2000 as a demarcation line for early and late-phase results, Dr. Jaquiss said, “It must be pointed out that in the latter period recipients were much more ill.” He noted that 89% of post-2000 heart transplant patients had UNOS status 1 vs. 49% in the pre-2000 period.

|
Dr. Robert Jaquiss |
“Considering these between-era differences, an alternative, less ‘discouraging’ interpretation is that excellent outcomes were maintained despite the trend toward transplantation in sicker patients, undergoing more complex transplants, with longer ischemic times,” he said.
Dr. Jaquiss also cited “remarkably outstanding outcomes” in Fontan patients, reporting only one operative death in 33 patients. He found the lower survival for African-American patients in the study group “more sobering,” but also controversial because, among other reasons, “a complete mechanistic explanation remains elusive.” How these findings influence pediatric heart transplant practice “requires thoughtful and extensive investigation and discussion,” he said.
Wait-list mortality and mechanical bridge to transplant also deserve mention, he noted. “Though they are only briefly mentioned, the patients who died prior to transplant provide mute testimony to the lack of timely access to suitable donors,” Dr. Jaquiss said. Durable mechanical circulatory support can provide a bridge for these patients, but was not available through the majority of the study period.
“It is striking that no patient in this report was supported by a ventricular assist device (VAD), and only a small number (5%) had been on [extracorporeal membrane oxygenation] support,” Dr. Jaquiss said. “This is an unfortunate and unavoidable weakness of this report, given the recent introduction of VADs for pediatric heart transplant candidates.” The use of VAD in patients with CHD is “increasing rapidly,” he said.
Dr. Jaquiss had no disclosures.
In his invited commentary, Dr. Robert D.B. Jaquiss of Duke University, Durham, N.C., took issue with the study authors’ “distress” at the lack of improvement in survival over the 25-year term of the study (J Thorac Cardiovasc Surg. 2015;150:1463-4) . Using the year 2000 as a demarcation line for early and late-phase results, Dr. Jaquiss said, “It must be pointed out that in the latter period recipients were much more ill.” He noted that 89% of post-2000 heart transplant patients had UNOS status 1 vs. 49% in the pre-2000 period.

|
Dr. Robert Jaquiss |
“Considering these between-era differences, an alternative, less ‘discouraging’ interpretation is that excellent outcomes were maintained despite the trend toward transplantation in sicker patients, undergoing more complex transplants, with longer ischemic times,” he said.
Dr. Jaquiss also cited “remarkably outstanding outcomes” in Fontan patients, reporting only one operative death in 33 patients. He found the lower survival for African-American patients in the study group “more sobering,” but also controversial because, among other reasons, “a complete mechanistic explanation remains elusive.” How these findings influence pediatric heart transplant practice “requires thoughtful and extensive investigation and discussion,” he said.
Wait-list mortality and mechanical bridge to transplant also deserve mention, he noted. “Though they are only briefly mentioned, the patients who died prior to transplant provide mute testimony to the lack of timely access to suitable donors,” Dr. Jaquiss said. Durable mechanical circulatory support can provide a bridge for these patients, but was not available through the majority of the study period.
“It is striking that no patient in this report was supported by a ventricular assist device (VAD), and only a small number (5%) had been on [extracorporeal membrane oxygenation] support,” Dr. Jaquiss said. “This is an unfortunate and unavoidable weakness of this report, given the recent introduction of VADs for pediatric heart transplant candidates.” The use of VAD in patients with CHD is “increasing rapidly,” he said.
Dr. Jaquiss had no disclosures.
In his invited commentary, Dr. Robert D.B. Jaquiss of Duke University, Durham, N.C., took issue with the study authors’ “distress” at the lack of improvement in survival over the 25-year term of the study (J Thorac Cardiovasc Surg. 2015;150:1463-4) . Using the year 2000 as a demarcation line for early and late-phase results, Dr. Jaquiss said, “It must be pointed out that in the latter period recipients were much more ill.” He noted that 89% of post-2000 heart transplant patients had UNOS status 1 vs. 49% in the pre-2000 period.

|
Dr. Robert Jaquiss |
“Considering these between-era differences, an alternative, less ‘discouraging’ interpretation is that excellent outcomes were maintained despite the trend toward transplantation in sicker patients, undergoing more complex transplants, with longer ischemic times,” he said.
Dr. Jaquiss also cited “remarkably outstanding outcomes” in Fontan patients, reporting only one operative death in 33 patients. He found the lower survival for African-American patients in the study group “more sobering,” but also controversial because, among other reasons, “a complete mechanistic explanation remains elusive.” How these findings influence pediatric heart transplant practice “requires thoughtful and extensive investigation and discussion,” he said.
Wait-list mortality and mechanical bridge to transplant also deserve mention, he noted. “Though they are only briefly mentioned, the patients who died prior to transplant provide mute testimony to the lack of timely access to suitable donors,” Dr. Jaquiss said. Durable mechanical circulatory support can provide a bridge for these patients, but was not available through the majority of the study period.
“It is striking that no patient in this report was supported by a ventricular assist device (VAD), and only a small number (5%) had been on [extracorporeal membrane oxygenation] support,” Dr. Jaquiss said. “This is an unfortunate and unavoidable weakness of this report, given the recent introduction of VADs for pediatric heart transplant candidates.” The use of VAD in patients with CHD is “increasing rapidly,” he said.
Dr. Jaquiss had no disclosures.
A 25-year study of heart transplants in children with congenital heart disease (CHD) at one institution has found that results haven’t improved over time despite advances in technology and techniques. To improve outcomes, transplant surgeons may need to do a better job of selecting patients and matching patients and donors, according to study in the December issue of the Journal of Thoracic and Cardiovascular Surgery (J Thorac Cardiovasc Surg. 2015;150:1455-62).
“Strategies to improve outcomes in CHD patients might need to address selection criteria, transplantation timing, pretransplant and posttransplant care,” noted Dr. Bahaaldin Alsoufi, of the division of cardiothoracic surgery, Children’s Healthcare of Atlanta, Emory University. “The effect of donor/recipient race mismatch warrants further investigation and might impact organ allocation algorithms or immunosuppression management,” wrote Dr. Alsoufi and his colleagues.
The researchers analyzed results of 124 children with CHD who had heart transplants from 1988 to 2013 at Emory University and Children’s Healthcare of Atlanta. Median age was 3.8 years; 61% were boys. Ten years after heart transplantation, 44% (54) of patients were alive without a second transplant, 13% (17) had a second transplant and 43% (53) died without a second transplant. After the second transplant, 9 of the 17 patients were alive, but 3 of them had gone onto a third transplant. Overall 15-year survival following the first transplant was 41% (51).
The study cited data from the Registry of the International Society for Heart and Lung Transplantation that reported more than 11,000 pediatric heart transplants worldwide in 2013, and CHD represents about 54% of all heart transplants in infants.
A multivariate analysis identified the following risk factors for early mortality after transplant: age younger than 12 months (hazard ration [HR] 7.2) and prolonged cardiopulmonary bypass (HR 5). Late-phase mortality risk factors were age younger than 12 months (HR 3) and donor/recipient race mismatch (HR 2.2).
“Survival was not affected by era, underlying anomaly, prior Fontan, sensitization or pulmonary artery augmentation,” wrote Dr. Alsoufi and his colleagues.
Among the risk factors, longer bypass times may be a surrogate for a more complicated operation, the authors said. But where prior sternotomy is a risk factor following a heart transplant in adults, the study found no such risk in children. Another risk factor previous reports identified is pulmonary artery augmentation, but, again, this study found no risk in the pediatric group.
The researchers looked at days on the waiting list, with a median wait of 39 days in the study group. In all, 175 children were listed for transplants, but 51 did not go through for various reasons. Most of the children with CHD who had a heart transplant had previous surgery; only 13% had a primary heart transplant, mostly in the earlier phase of the study.
Dr. Alsoufi and coauthors also identified African American race as a risk factor for lower survival, which is consistent with other reports. But this study agreed with a previous report that donor/recipient race mismatch was a significant risk factor in white and African American patients (Ann Thorac Surg. 2009;87:204-9). “While our finding might be anecdotal and specific to our geographic population, this warrants some investigation and might have some impact on future organ allocation algorithms and immunosuppression management,” the researchers wrote.
The authors had no relevant disclosures. Emory University School of Medicine, Children’s Healthcare of Atlanta provided study funding.
A 25-year study of heart transplants in children with congenital heart disease (CHD) at one institution has found that results haven’t improved over time despite advances in technology and techniques. To improve outcomes, transplant surgeons may need to do a better job of selecting patients and matching patients and donors, according to study in the December issue of the Journal of Thoracic and Cardiovascular Surgery (J Thorac Cardiovasc Surg. 2015;150:1455-62).
“Strategies to improve outcomes in CHD patients might need to address selection criteria, transplantation timing, pretransplant and posttransplant care,” noted Dr. Bahaaldin Alsoufi, of the division of cardiothoracic surgery, Children’s Healthcare of Atlanta, Emory University. “The effect of donor/recipient race mismatch warrants further investigation and might impact organ allocation algorithms or immunosuppression management,” wrote Dr. Alsoufi and his colleagues.
The researchers analyzed results of 124 children with CHD who had heart transplants from 1988 to 2013 at Emory University and Children’s Healthcare of Atlanta. Median age was 3.8 years; 61% were boys. Ten years after heart transplantation, 44% (54) of patients were alive without a second transplant, 13% (17) had a second transplant and 43% (53) died without a second transplant. After the second transplant, 9 of the 17 patients were alive, but 3 of them had gone onto a third transplant. Overall 15-year survival following the first transplant was 41% (51).
The study cited data from the Registry of the International Society for Heart and Lung Transplantation that reported more than 11,000 pediatric heart transplants worldwide in 2013, and CHD represents about 54% of all heart transplants in infants.
A multivariate analysis identified the following risk factors for early mortality after transplant: age younger than 12 months (hazard ration [HR] 7.2) and prolonged cardiopulmonary bypass (HR 5). Late-phase mortality risk factors were age younger than 12 months (HR 3) and donor/recipient race mismatch (HR 2.2).
“Survival was not affected by era, underlying anomaly, prior Fontan, sensitization or pulmonary artery augmentation,” wrote Dr. Alsoufi and his colleagues.
Among the risk factors, longer bypass times may be a surrogate for a more complicated operation, the authors said. But where prior sternotomy is a risk factor following a heart transplant in adults, the study found no such risk in children. Another risk factor previous reports identified is pulmonary artery augmentation, but, again, this study found no risk in the pediatric group.
The researchers looked at days on the waiting list, with a median wait of 39 days in the study group. In all, 175 children were listed for transplants, but 51 did not go through for various reasons. Most of the children with CHD who had a heart transplant had previous surgery; only 13% had a primary heart transplant, mostly in the earlier phase of the study.
Dr. Alsoufi and coauthors also identified African American race as a risk factor for lower survival, which is consistent with other reports. But this study agreed with a previous report that donor/recipient race mismatch was a significant risk factor in white and African American patients (Ann Thorac Surg. 2009;87:204-9). “While our finding might be anecdotal and specific to our geographic population, this warrants some investigation and might have some impact on future organ allocation algorithms and immunosuppression management,” the researchers wrote.
The authors had no relevant disclosures. Emory University School of Medicine, Children’s Healthcare of Atlanta provided study funding.
Key clinical point: Pediatric heart transplantation outcomes for congenital heart disease haven’t improved in the current era, indicating ongoing challenges.
Major finding: Ten years following heart transplantation, 13% of patients had undergone retransplantation, 43% had died without retransplantation, and 44% were alive without retransplantation.
Data source: A review of 124 children with congenital heart disease who had heart transplantation at a single center.
Disclosures: The study authors had no relationships to disclose.
AHA: Broadening evidence for CABG over PCI in diabetics
ORLANDO – New evidence further strengthens the case for coronary artery bypass grafting over percutaneous coronary intervention as the preferred revascularization strategy in diabetic patients with multivessel coronary artery disease.
An analysis of 4,819 such patients in a British Columbia province-wide registry who underwent revascularization during 2007-2014 led to the conclusion that the 30-day adjusted risk of the primary composite endpoint of death, MI, or stroke was 39% lower in those who underwent coronary artery bypass grafting (CABG), Dr. Krishnan Ramanathan reported at the American Heart Association scientific sessions.
From day 31 through 5 years of follow-up, the relative risk reduction was 36% in favor of CABG, again after adjustment for age, sex, presentation with stable ischemic heart disease or stabilized acute coronary syndrome (ACS), urgency, renal insufficiency, liver disease, peripheral arterial disease, and left ventricular ejection fraction, added Dr. Ramanathan of the University of British Columbia in Vancouver.
These results in a broad patient population in real-world clinical practice, albeit in an observational, nonrandomized setting, mirror those in the earlier randomized FREEDOM trial sponsored by the National Heart, Lung, and Blood Institute. The 5-year rate of the composite of death, MI, or stroke in FREEDOM was 18.7% in the CABG group and 26.6% with percutaneous coronary intervention (PCI). The number needed to treat with CABG instead of PCI in FREEDOM in order to avoid one case of the composite endpoint was 12.6 (N Engl J Med. 2012 Dec 20;367[25]:2375-84).
An important difference between the British Columbia and the FREEDOM trial is that the registry contained a much higher proportion of diabetic patients with multivessel disease who presented with acute coronary syndrome. Indeed, the ratio of stabilized ACS to stable ischemic heart disease patients in the registry was 62% to 38% as compared with 36% to 64% in FREEDOM. The registry patients underwent a median of 7.8 days of stabilization from the time of initial catheterization to CABG.
The registry data really shore up the advantages of CABG over PCI in diabetics with multivessel disease and ACS, Dr. Ramanathan noted. In the registry, the 30-day composite endpoint occurred in 4.4% of CABG-treated ACS patients, compared with 8.3% with PCI. For 31 days through 5 years, the rates were 21.4% versus 34.7%. The differences favoring CABG were highly significant at both time points.
FREEDOM led to two important revisions in the 2014 guidelines on coronary revascularization, issued by the American College of Cardiology, American Heart Association, American Academy of Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiac Angiography and Interventions, and Society of Thoracic Surgeons. In a focused update issued earlier this year, a new class I recommendation was issued calling for a heart team approach to revascularization in patients with diabetes mellitus and complex multivessel coronary artery disease (CAD). And a recommendation for CABG over PCI for improving survival in diabetic patients with multivessel CAD was upgraded from class IIa to class I.
Even though FREEDOM was a major randomized trial that might be expected to be practice changing, that hasn’t happened. A report from the ACC’s National Cardiovascular Data ACTION Registry presented earlier this year at the ACC annual scientific session documented that the most common revascularization strategy for diabetic patients with multivessel CAD following a non–ST elevation ACS remains PCI, as has been the case since the registry was created. In fact, following the 2012 publication of FREEDOM, the proportion of such patients treated by PCI took a sharp uptick in 2013-2014, according to Dr. Ramanathan.
In the British Columbia registry, 64% of diabetic patients with multivessel disease and an ACS were treated by PCI, 36% by CABG.
“Overall, CABG in stabilized ACS patients is underutilized,” he said.
Discussant Dr. Marc Ruel declared, “The salient finding about the British Columbia study is not so much about the stable ischemic heart disease patients, but about the high proportion of ACS patients in the study. CABG was better than PCI for all major adverse cardiovascular events and each component of the endpoint.”
Dr. Ruel, professor and chair of cardiac surgery at the University of Ottawa Heart Institute, coauthored a meta-analysis of randomized controlled trials comparing CABG to PCI in diabetic patients. Half of the trials used later-generation drug-eluting stents. At 5 years of follow-up, the relative risk of all-cause mortality in diabetic patients allocated to CABG was 0.67 (Lancet Diabetes Endocrinol. 2013 Dec;1[4]:317-28).
“Remember that 0.67 because it’s a recurring number in our literature,” he advised.
For example, in the British Columbia study the relative risk of death, MI, or stroke from 31 days to 5 years was 0.64 in the CABG as compared with the PCI group. And in a patient-level meta-analysis of the FREEDOM, BARI-2D, and COURAGE trials presented by Dr. John Mancini earlier at the AHA scientific sessions in Orlando, CABG plus optimal medical therapy was associated with a 0.65 relative risk of death, MI, or stroke, compared with PCI plus optimal medical therapy, which in turn wasn’t significantly better than optimal medical therapy alone, Dr. Ruel noted.
The 2015 European Society of Cardiology guidelines on management of ACS state that a heart team discussion involving a cardiologist and cardiac surgeon does not need to take place for every patient presenting with multivessel disease and a non–ST elevation ACS. The British Columbia study, coupled with the other evidence, “gives new credence” to a call for revision of that recommendation in diabetic patients, he said.
The British Columbia study was supported by the British Columbia Provincial Health Services Authority. Dr. Ramanathan reported having no financial conflicts.
ORLANDO – New evidence further strengthens the case for coronary artery bypass grafting over percutaneous coronary intervention as the preferred revascularization strategy in diabetic patients with multivessel coronary artery disease.
An analysis of 4,819 such patients in a British Columbia province-wide registry who underwent revascularization during 2007-2014 led to the conclusion that the 30-day adjusted risk of the primary composite endpoint of death, MI, or stroke was 39% lower in those who underwent coronary artery bypass grafting (CABG), Dr. Krishnan Ramanathan reported at the American Heart Association scientific sessions.
From day 31 through 5 years of follow-up, the relative risk reduction was 36% in favor of CABG, again after adjustment for age, sex, presentation with stable ischemic heart disease or stabilized acute coronary syndrome (ACS), urgency, renal insufficiency, liver disease, peripheral arterial disease, and left ventricular ejection fraction, added Dr. Ramanathan of the University of British Columbia in Vancouver.
These results in a broad patient population in real-world clinical practice, albeit in an observational, nonrandomized setting, mirror those in the earlier randomized FREEDOM trial sponsored by the National Heart, Lung, and Blood Institute. The 5-year rate of the composite of death, MI, or stroke in FREEDOM was 18.7% in the CABG group and 26.6% with percutaneous coronary intervention (PCI). The number needed to treat with CABG instead of PCI in FREEDOM in order to avoid one case of the composite endpoint was 12.6 (N Engl J Med. 2012 Dec 20;367[25]:2375-84).
An important difference between the British Columbia and the FREEDOM trial is that the registry contained a much higher proportion of diabetic patients with multivessel disease who presented with acute coronary syndrome. Indeed, the ratio of stabilized ACS to stable ischemic heart disease patients in the registry was 62% to 38% as compared with 36% to 64% in FREEDOM. The registry patients underwent a median of 7.8 days of stabilization from the time of initial catheterization to CABG.
The registry data really shore up the advantages of CABG over PCI in diabetics with multivessel disease and ACS, Dr. Ramanathan noted. In the registry, the 30-day composite endpoint occurred in 4.4% of CABG-treated ACS patients, compared with 8.3% with PCI. For 31 days through 5 years, the rates were 21.4% versus 34.7%. The differences favoring CABG were highly significant at both time points.
FREEDOM led to two important revisions in the 2014 guidelines on coronary revascularization, issued by the American College of Cardiology, American Heart Association, American Academy of Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiac Angiography and Interventions, and Society of Thoracic Surgeons. In a focused update issued earlier this year, a new class I recommendation was issued calling for a heart team approach to revascularization in patients with diabetes mellitus and complex multivessel coronary artery disease (CAD). And a recommendation for CABG over PCI for improving survival in diabetic patients with multivessel CAD was upgraded from class IIa to class I.
Even though FREEDOM was a major randomized trial that might be expected to be practice changing, that hasn’t happened. A report from the ACC’s National Cardiovascular Data ACTION Registry presented earlier this year at the ACC annual scientific session documented that the most common revascularization strategy for diabetic patients with multivessel CAD following a non–ST elevation ACS remains PCI, as has been the case since the registry was created. In fact, following the 2012 publication of FREEDOM, the proportion of such patients treated by PCI took a sharp uptick in 2013-2014, according to Dr. Ramanathan.
In the British Columbia registry, 64% of diabetic patients with multivessel disease and an ACS were treated by PCI, 36% by CABG.
“Overall, CABG in stabilized ACS patients is underutilized,” he said.
Discussant Dr. Marc Ruel declared, “The salient finding about the British Columbia study is not so much about the stable ischemic heart disease patients, but about the high proportion of ACS patients in the study. CABG was better than PCI for all major adverse cardiovascular events and each component of the endpoint.”
Dr. Ruel, professor and chair of cardiac surgery at the University of Ottawa Heart Institute, coauthored a meta-analysis of randomized controlled trials comparing CABG to PCI in diabetic patients. Half of the trials used later-generation drug-eluting stents. At 5 years of follow-up, the relative risk of all-cause mortality in diabetic patients allocated to CABG was 0.67 (Lancet Diabetes Endocrinol. 2013 Dec;1[4]:317-28).
“Remember that 0.67 because it’s a recurring number in our literature,” he advised.
For example, in the British Columbia study the relative risk of death, MI, or stroke from 31 days to 5 years was 0.64 in the CABG as compared with the PCI group. And in a patient-level meta-analysis of the FREEDOM, BARI-2D, and COURAGE trials presented by Dr. John Mancini earlier at the AHA scientific sessions in Orlando, CABG plus optimal medical therapy was associated with a 0.65 relative risk of death, MI, or stroke, compared with PCI plus optimal medical therapy, which in turn wasn’t significantly better than optimal medical therapy alone, Dr. Ruel noted.
The 2015 European Society of Cardiology guidelines on management of ACS state that a heart team discussion involving a cardiologist and cardiac surgeon does not need to take place for every patient presenting with multivessel disease and a non–ST elevation ACS. The British Columbia study, coupled with the other evidence, “gives new credence” to a call for revision of that recommendation in diabetic patients, he said.
The British Columbia study was supported by the British Columbia Provincial Health Services Authority. Dr. Ramanathan reported having no financial conflicts.
ORLANDO – New evidence further strengthens the case for coronary artery bypass grafting over percutaneous coronary intervention as the preferred revascularization strategy in diabetic patients with multivessel coronary artery disease.
An analysis of 4,819 such patients in a British Columbia province-wide registry who underwent revascularization during 2007-2014 led to the conclusion that the 30-day adjusted risk of the primary composite endpoint of death, MI, or stroke was 39% lower in those who underwent coronary artery bypass grafting (CABG), Dr. Krishnan Ramanathan reported at the American Heart Association scientific sessions.
From day 31 through 5 years of follow-up, the relative risk reduction was 36% in favor of CABG, again after adjustment for age, sex, presentation with stable ischemic heart disease or stabilized acute coronary syndrome (ACS), urgency, renal insufficiency, liver disease, peripheral arterial disease, and left ventricular ejection fraction, added Dr. Ramanathan of the University of British Columbia in Vancouver.
These results in a broad patient population in real-world clinical practice, albeit in an observational, nonrandomized setting, mirror those in the earlier randomized FREEDOM trial sponsored by the National Heart, Lung, and Blood Institute. The 5-year rate of the composite of death, MI, or stroke in FREEDOM was 18.7% in the CABG group and 26.6% with percutaneous coronary intervention (PCI). The number needed to treat with CABG instead of PCI in FREEDOM in order to avoid one case of the composite endpoint was 12.6 (N Engl J Med. 2012 Dec 20;367[25]:2375-84).
An important difference between the British Columbia and the FREEDOM trial is that the registry contained a much higher proportion of diabetic patients with multivessel disease who presented with acute coronary syndrome. Indeed, the ratio of stabilized ACS to stable ischemic heart disease patients in the registry was 62% to 38% as compared with 36% to 64% in FREEDOM. The registry patients underwent a median of 7.8 days of stabilization from the time of initial catheterization to CABG.
The registry data really shore up the advantages of CABG over PCI in diabetics with multivessel disease and ACS, Dr. Ramanathan noted. In the registry, the 30-day composite endpoint occurred in 4.4% of CABG-treated ACS patients, compared with 8.3% with PCI. For 31 days through 5 years, the rates were 21.4% versus 34.7%. The differences favoring CABG were highly significant at both time points.
FREEDOM led to two important revisions in the 2014 guidelines on coronary revascularization, issued by the American College of Cardiology, American Heart Association, American Academy of Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiac Angiography and Interventions, and Society of Thoracic Surgeons. In a focused update issued earlier this year, a new class I recommendation was issued calling for a heart team approach to revascularization in patients with diabetes mellitus and complex multivessel coronary artery disease (CAD). And a recommendation for CABG over PCI for improving survival in diabetic patients with multivessel CAD was upgraded from class IIa to class I.
Even though FREEDOM was a major randomized trial that might be expected to be practice changing, that hasn’t happened. A report from the ACC’s National Cardiovascular Data ACTION Registry presented earlier this year at the ACC annual scientific session documented that the most common revascularization strategy for diabetic patients with multivessel CAD following a non–ST elevation ACS remains PCI, as has been the case since the registry was created. In fact, following the 2012 publication of FREEDOM, the proportion of such patients treated by PCI took a sharp uptick in 2013-2014, according to Dr. Ramanathan.
In the British Columbia registry, 64% of diabetic patients with multivessel disease and an ACS were treated by PCI, 36% by CABG.
“Overall, CABG in stabilized ACS patients is underutilized,” he said.
Discussant Dr. Marc Ruel declared, “The salient finding about the British Columbia study is not so much about the stable ischemic heart disease patients, but about the high proportion of ACS patients in the study. CABG was better than PCI for all major adverse cardiovascular events and each component of the endpoint.”
Dr. Ruel, professor and chair of cardiac surgery at the University of Ottawa Heart Institute, coauthored a meta-analysis of randomized controlled trials comparing CABG to PCI in diabetic patients. Half of the trials used later-generation drug-eluting stents. At 5 years of follow-up, the relative risk of all-cause mortality in diabetic patients allocated to CABG was 0.67 (Lancet Diabetes Endocrinol. 2013 Dec;1[4]:317-28).
“Remember that 0.67 because it’s a recurring number in our literature,” he advised.
For example, in the British Columbia study the relative risk of death, MI, or stroke from 31 days to 5 years was 0.64 in the CABG as compared with the PCI group. And in a patient-level meta-analysis of the FREEDOM, BARI-2D, and COURAGE trials presented by Dr. John Mancini earlier at the AHA scientific sessions in Orlando, CABG plus optimal medical therapy was associated with a 0.65 relative risk of death, MI, or stroke, compared with PCI plus optimal medical therapy, which in turn wasn’t significantly better than optimal medical therapy alone, Dr. Ruel noted.
The 2015 European Society of Cardiology guidelines on management of ACS state that a heart team discussion involving a cardiologist and cardiac surgeon does not need to take place for every patient presenting with multivessel disease and a non–ST elevation ACS. The British Columbia study, coupled with the other evidence, “gives new credence” to a call for revision of that recommendation in diabetic patients, he said.
The British Columbia study was supported by the British Columbia Provincial Health Services Authority. Dr. Ramanathan reported having no financial conflicts.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: New evidence reinforces CABG as preferred over PCI for revascularization in diabetic patients with multivessel CAD.
Major finding: Diabetic patients with multivessel CAD were 36% less likely to experience death, MI, or stroke within the next 5 years if they underwent revascularization with CABG than PCI.
Data source: This observational registry study included 4,819 diabetic patients with multivessel CAD who underwent CABG or isolated PCI for stable ischemic heart disease or stabilized acute coronary syndrome.
Disclosures: The study was supported by the British Columbia Provincial Health Services Authority. The presenter reported having no financial conflicts of interest.
AHA: DAPT score helps decide whether DAPT continues
ORLANDO – The common challenge faced by clinicians in deciding whether or not to continue dual antiplatelet therapy beyond a year in a patient who underwent percutaneous coronary intervention has gotten easier.
Researchers have devised a simple, eight-element scoring system using information already available in a patient’s records to help determine whether an individual patient will be more likely to benefit from continuing or stopping dual antiplatelet therapy (DAPT).
“The DAPT score may help clinicians decide who should and who should not be treated with extended DAPT,” Dr. Robert W. Yeh said at the American Heart Association Scientific Sessions.
“This is a step forward for an issue we deal with daily, balancing an individual patient’s risk from ischemia and bleeding,” commented Dr. Alice Jacobs, professor of medicine at Boston University and director of the cardiac catheterization laboratory and interventional cardiology at Boston Medical Center.
Dr. Yeh and his associates devised the DAPT score from the data collected in the DAPT study, which enrolled more than 25,000 patients and randomized about 10,000 to test whether patients fared better by stopping or continuing DAPT after completing their initial year of DAPT following percutaneous coronary intervention (PCI). The DAPT study results showed that after 18 additional months, continuing DAPT cut the rate of definite or probable stent thrombosis by 1 percentage point and the combined rate of death, MI, or stroke by 1.6 percentage points, both statistically significant differences, compared with patients randomized to treatment with aspirin plus placebo. The results also showed that continued DAPT increased GUSTO moderate or severe bleeding events by 1 percentage point, compared with the control patients (N Engl J Med. 2014 Dec 4;371[23]:2155-66).
The researchers used data collected in the DAPT study to build risk models using patient- and procedure-specific variables that predicted the ischemic and bleeding outcomes, and then combined the two into a single model. That meant abandoning some variables that had significant impact on both outcomes.
The result was a scoring system that includes eight variables that result in a score that ranges from –2 to 9. The analysis showed that a score of 1 or less identified patients for whom the risk for bleeding outweighs their potential gain by avoiding an ischemic event by about 2.5-fold, and hence likely would fare better by stopping DAPT. A score of 2 or higher flagged patients who benefited about eightfold more from avoided ischemic events, compared with their risk for a moderate or severe bleed.
Patient scores showed a classic bell-shaped curve, with roughly a quarter of the DAPT study patients having a score of 1 and about a quarter with a score of 2, about 16% had a score of 0 and about 16% had a score of 3, and about 8% had a score of –1 or –2, while about 9% had a score of 4 or more.
The investigators validated the scoring system using data collected in the PROTECT trial, which included 8,791 patients who underwent PCI during 2007-2008. Dr. Yeh acknowledged that the discrimination strength of the models he and his associated developed was “modest,” but added that its efficacy was greater than what has been shown in validation cohorts for the commonly used CH2ADS2-VASc and HAS-BLED scoring systems.
Dr. Yeh stressed that using the DAPT score “cannot trump clinical judgment.” He suggested that a clinician use the score to help facilitate a conversation with a PCI patient when the time comes to decide whether or not to continue DAPT beyond 1 year.
Other factors that could influence the decision include the length of the stented coronary lesions or prior radiation exposure to the patient’s coronary arteries, said Dr. Laura Mauri, who led the DAPT study and collaborated on developing the DAPT score. “It requires judgment to decide [on whether to continue DAPT] for patients who are on the borderline” for risk and benefit. “This gives patients a way to better understand what they might gain or lose” by continuing treatment. Without the quantification that the DAPT score provides, the balance of risk and benefit “is somewhat nebulous,” said Dr. Mauri, an interventional cardiologist and director of the Center for Clinical Biometrics in the division of cardiovascular medicine at Brigham and Women’s Hospital in Boston.
The investigators who ran the DAPT study realized several years before the study finished that development of the DAPT score was a critical part of applying the findings from the study into clinical practice, she said in an interview.
Dr. Yeh cautioned that the score is only appropriate for patients who match those enrolled into the DAPT study: patients who went through their first year post PCI on DAPT without having any ischemic or bleeding complications. For these patients, “we feel the DAPT score is incredibly valuable,” said Dr. Yeh, an interventional cardiologist and director of the Center for Outcomes Research in Cardiology at Beth Israel Deaconess Medical Center in Boston. He and his associates are now using data from the DAPT study to model bleeding and ischemia risks during the first year following PCI to try to come up with risk models that can address DAPT use during this period.
Dr. Yeh and Dr. Mauri have placed a link to the electronic DAPT score calculator on the website for the DAPT study (www.daptstudy.org), and Dr. Yeh said that an app version will soon become available. Interventionalists in the programs that Dr. Yeh and Mauri are affiliated with have recently begun using the DAPT score calculator in their routine practice, Dr. Yeh said.
The DAPT study received funding from Abbott, Boston Scientific, Cordis, Medtronic, Bristol-Myers Squib-Sanofi, Eli Lilly, and Daiichi Sankyo. Derivation of the DAPT score was funded by the National Institutes of Health. Dr. Yeh has received honoraria from Abbott, Boston Scientific, and Merck. Dr. Jacobs had no disclosures. Dr. Mauri has been a consultant to Biotronik, Medtronic, and St. Jude and has received research funding from several companies.
On Twitter @mitchelzoler
The DAPT score is a clever and innovative idea. It is a major step forward in helping clinicians decide which patients should continue dual antiplatelet therapy after safely completing a year on this therapy following percutaneous coronary intervention. The DAPT score was data driven and provides a tool to help personalize decision making with a simple, practical solution to a common clinical dilemma. It’s a welcome addition to our decision-making process.
The competing risks from bleeding events caused by continued dual antiplatelet therapy (DAPT) beyond 1 year and ischemic events caused by stopping DAPT creates difficulty in determining whether or not to continue or stop DAPT for an individual patient. The DAPT score helps make that decision.

|
| Mitchel L. Zoler/Frontline Medical News Dr. James de Lemos |
The analysis performed by Dr. Yeh and his associates produced a clear and convincing result. The primary caveat is that it is only applicable to patients who entered the randomized phase of the DAPT study, specifically patients who underwent a full first year of DAPT treatment following PCI without an ischemic or major bleeding event. I would like to see replication of the score’s validation in an additional data set, although few data sets exist that are suitable for such replication. Although the discrimination produced by the DAPT score is moderate, it compares favorably with other widely used clinical decision scores such as the CHA2ADS2-VASc.
The added decision-making ability facilitated by this score revises my interpretation of the results from the DAPT study. When the results of the trial appeared in 2014, I considered the outcome null because of the problem it highlighted in balancing the competing risks of ischemic and bleeding events when deciding about continuing DAPT beyond 1 year. The DAPT score helps produce a much clearer risk versus benefit decision for a sizable subset of patients who undergo percutaneous coronary intervention.
Dr. James de Lemos is a professor of medicine at UT Southwestern Medical Center, Dallas, and chief of the cardiology service at Parkland Memorial Hospital in Dallas. He has received honoraria from Novo Nordisk and St. Jude and research funding from Roche Diagnostics and Abbott Diagnostics. He made these comments as the designated discussant for Dr. Yeh’s report.
The DAPT score is a clever and innovative idea. It is a major step forward in helping clinicians decide which patients should continue dual antiplatelet therapy after safely completing a year on this therapy following percutaneous coronary intervention. The DAPT score was data driven and provides a tool to help personalize decision making with a simple, practical solution to a common clinical dilemma. It’s a welcome addition to our decision-making process.
The competing risks from bleeding events caused by continued dual antiplatelet therapy (DAPT) beyond 1 year and ischemic events caused by stopping DAPT creates difficulty in determining whether or not to continue or stop DAPT for an individual patient. The DAPT score helps make that decision.

|
| Mitchel L. Zoler/Frontline Medical News Dr. James de Lemos |
The analysis performed by Dr. Yeh and his associates produced a clear and convincing result. The primary caveat is that it is only applicable to patients who entered the randomized phase of the DAPT study, specifically patients who underwent a full first year of DAPT treatment following PCI without an ischemic or major bleeding event. I would like to see replication of the score’s validation in an additional data set, although few data sets exist that are suitable for such replication. Although the discrimination produced by the DAPT score is moderate, it compares favorably with other widely used clinical decision scores such as the CHA2ADS2-VASc.
The added decision-making ability facilitated by this score revises my interpretation of the results from the DAPT study. When the results of the trial appeared in 2014, I considered the outcome null because of the problem it highlighted in balancing the competing risks of ischemic and bleeding events when deciding about continuing DAPT beyond 1 year. The DAPT score helps produce a much clearer risk versus benefit decision for a sizable subset of patients who undergo percutaneous coronary intervention.
Dr. James de Lemos is a professor of medicine at UT Southwestern Medical Center, Dallas, and chief of the cardiology service at Parkland Memorial Hospital in Dallas. He has received honoraria from Novo Nordisk and St. Jude and research funding from Roche Diagnostics and Abbott Diagnostics. He made these comments as the designated discussant for Dr. Yeh’s report.
The DAPT score is a clever and innovative idea. It is a major step forward in helping clinicians decide which patients should continue dual antiplatelet therapy after safely completing a year on this therapy following percutaneous coronary intervention. The DAPT score was data driven and provides a tool to help personalize decision making with a simple, practical solution to a common clinical dilemma. It’s a welcome addition to our decision-making process.
The competing risks from bleeding events caused by continued dual antiplatelet therapy (DAPT) beyond 1 year and ischemic events caused by stopping DAPT creates difficulty in determining whether or not to continue or stop DAPT for an individual patient. The DAPT score helps make that decision.

|
| Mitchel L. Zoler/Frontline Medical News Dr. James de Lemos |
The analysis performed by Dr. Yeh and his associates produced a clear and convincing result. The primary caveat is that it is only applicable to patients who entered the randomized phase of the DAPT study, specifically patients who underwent a full first year of DAPT treatment following PCI without an ischemic or major bleeding event. I would like to see replication of the score’s validation in an additional data set, although few data sets exist that are suitable for such replication. Although the discrimination produced by the DAPT score is moderate, it compares favorably with other widely used clinical decision scores such as the CHA2ADS2-VASc.
The added decision-making ability facilitated by this score revises my interpretation of the results from the DAPT study. When the results of the trial appeared in 2014, I considered the outcome null because of the problem it highlighted in balancing the competing risks of ischemic and bleeding events when deciding about continuing DAPT beyond 1 year. The DAPT score helps produce a much clearer risk versus benefit decision for a sizable subset of patients who undergo percutaneous coronary intervention.
Dr. James de Lemos is a professor of medicine at UT Southwestern Medical Center, Dallas, and chief of the cardiology service at Parkland Memorial Hospital in Dallas. He has received honoraria from Novo Nordisk and St. Jude and research funding from Roche Diagnostics and Abbott Diagnostics. He made these comments as the designated discussant for Dr. Yeh’s report.
ORLANDO – The common challenge faced by clinicians in deciding whether or not to continue dual antiplatelet therapy beyond a year in a patient who underwent percutaneous coronary intervention has gotten easier.
Researchers have devised a simple, eight-element scoring system using information already available in a patient’s records to help determine whether an individual patient will be more likely to benefit from continuing or stopping dual antiplatelet therapy (DAPT).
“The DAPT score may help clinicians decide who should and who should not be treated with extended DAPT,” Dr. Robert W. Yeh said at the American Heart Association Scientific Sessions.
“This is a step forward for an issue we deal with daily, balancing an individual patient’s risk from ischemia and bleeding,” commented Dr. Alice Jacobs, professor of medicine at Boston University and director of the cardiac catheterization laboratory and interventional cardiology at Boston Medical Center.
Dr. Yeh and his associates devised the DAPT score from the data collected in the DAPT study, which enrolled more than 25,000 patients and randomized about 10,000 to test whether patients fared better by stopping or continuing DAPT after completing their initial year of DAPT following percutaneous coronary intervention (PCI). The DAPT study results showed that after 18 additional months, continuing DAPT cut the rate of definite or probable stent thrombosis by 1 percentage point and the combined rate of death, MI, or stroke by 1.6 percentage points, both statistically significant differences, compared with patients randomized to treatment with aspirin plus placebo. The results also showed that continued DAPT increased GUSTO moderate or severe bleeding events by 1 percentage point, compared with the control patients (N Engl J Med. 2014 Dec 4;371[23]:2155-66).
The researchers used data collected in the DAPT study to build risk models using patient- and procedure-specific variables that predicted the ischemic and bleeding outcomes, and then combined the two into a single model. That meant abandoning some variables that had significant impact on both outcomes.
The result was a scoring system that includes eight variables that result in a score that ranges from –2 to 9. The analysis showed that a score of 1 or less identified patients for whom the risk for bleeding outweighs their potential gain by avoiding an ischemic event by about 2.5-fold, and hence likely would fare better by stopping DAPT. A score of 2 or higher flagged patients who benefited about eightfold more from avoided ischemic events, compared with their risk for a moderate or severe bleed.

Patient scores showed a classic bell-shaped curve, with roughly a quarter of the DAPT study patients having a score of 1 and about a quarter with a score of 2, about 16% had a score of 0 and about 16% had a score of 3, and about 8% had a score of –1 or –2, while about 9% had a score of 4 or more.
The investigators validated the scoring system using data collected in the PROTECT trial, which included 8,791 patients who underwent PCI during 2007-2008. Dr. Yeh acknowledged that the discrimination strength of the models he and his associated developed was “modest,” but added that its efficacy was greater than what has been shown in validation cohorts for the commonly used CH2ADS2-VASc and HAS-BLED scoring systems.
Dr. Yeh stressed that using the DAPT score “cannot trump clinical judgment.” He suggested that a clinician use the score to help facilitate a conversation with a PCI patient when the time comes to decide whether or not to continue DAPT beyond 1 year.
Other factors that could influence the decision include the length of the stented coronary lesions or prior radiation exposure to the patient’s coronary arteries, said Dr. Laura Mauri, who led the DAPT study and collaborated on developing the DAPT score. “It requires judgment to decide [on whether to continue DAPT] for patients who are on the borderline” for risk and benefit. “This gives patients a way to better understand what they might gain or lose” by continuing treatment. Without the quantification that the DAPT score provides, the balance of risk and benefit “is somewhat nebulous,” said Dr. Mauri, an interventional cardiologist and director of the Center for Clinical Biometrics in the division of cardiovascular medicine at Brigham and Women’s Hospital in Boston.
The investigators who ran the DAPT study realized several years before the study finished that development of the DAPT score was a critical part of applying the findings from the study into clinical practice, she said in an interview.
Dr. Yeh cautioned that the score is only appropriate for patients who match those enrolled into the DAPT study: patients who went through their first year post PCI on DAPT without having any ischemic or bleeding complications. For these patients, “we feel the DAPT score is incredibly valuable,” said Dr. Yeh, an interventional cardiologist and director of the Center for Outcomes Research in Cardiology at Beth Israel Deaconess Medical Center in Boston. He and his associates are now using data from the DAPT study to model bleeding and ischemia risks during the first year following PCI to try to come up with risk models that can address DAPT use during this period.
Dr. Yeh and Dr. Mauri have placed a link to the electronic DAPT score calculator on the website for the DAPT study (www.daptstudy.org), and Dr. Yeh said that an app version will soon become available. Interventionalists in the programs that Dr. Yeh and Mauri are affiliated with have recently begun using the DAPT score calculator in their routine practice, Dr. Yeh said.
The DAPT study received funding from Abbott, Boston Scientific, Cordis, Medtronic, Bristol-Myers Squib-Sanofi, Eli Lilly, and Daiichi Sankyo. Derivation of the DAPT score was funded by the National Institutes of Health. Dr. Yeh has received honoraria from Abbott, Boston Scientific, and Merck. Dr. Jacobs had no disclosures. Dr. Mauri has been a consultant to Biotronik, Medtronic, and St. Jude and has received research funding from several companies.
On Twitter @mitchelzoler
ORLANDO – The common challenge faced by clinicians in deciding whether or not to continue dual antiplatelet therapy beyond a year in a patient who underwent percutaneous coronary intervention has gotten easier.
Researchers have devised a simple, eight-element scoring system using information already available in a patient’s records to help determine whether an individual patient will be more likely to benefit from continuing or stopping dual antiplatelet therapy (DAPT).
“The DAPT score may help clinicians decide who should and who should not be treated with extended DAPT,” Dr. Robert W. Yeh said at the American Heart Association Scientific Sessions.
“This is a step forward for an issue we deal with daily, balancing an individual patient’s risk from ischemia and bleeding,” commented Dr. Alice Jacobs, professor of medicine at Boston University and director of the cardiac catheterization laboratory and interventional cardiology at Boston Medical Center.
Dr. Yeh and his associates devised the DAPT score from the data collected in the DAPT study, which enrolled more than 25,000 patients and randomized about 10,000 to test whether patients fared better by stopping or continuing DAPT after completing their initial year of DAPT following percutaneous coronary intervention (PCI). The DAPT study results showed that after 18 additional months, continuing DAPT cut the rate of definite or probable stent thrombosis by 1 percentage point and the combined rate of death, MI, or stroke by 1.6 percentage points, both statistically significant differences, compared with patients randomized to treatment with aspirin plus placebo. The results also showed that continued DAPT increased GUSTO moderate or severe bleeding events by 1 percentage point, compared with the control patients (N Engl J Med. 2014 Dec 4;371[23]:2155-66).
The researchers used data collected in the DAPT study to build risk models using patient- and procedure-specific variables that predicted the ischemic and bleeding outcomes, and then combined the two into a single model. That meant abandoning some variables that had significant impact on both outcomes.
The result was a scoring system that includes eight variables that result in a score that ranges from –2 to 9. The analysis showed that a score of 1 or less identified patients for whom the risk for bleeding outweighs their potential gain by avoiding an ischemic event by about 2.5-fold, and hence likely would fare better by stopping DAPT. A score of 2 or higher flagged patients who benefited about eightfold more from avoided ischemic events, compared with their risk for a moderate or severe bleed.

Patient scores showed a classic bell-shaped curve, with roughly a quarter of the DAPT study patients having a score of 1 and about a quarter with a score of 2, about 16% had a score of 0 and about 16% had a score of 3, and about 8% had a score of –1 or –2, while about 9% had a score of 4 or more.
The investigators validated the scoring system using data collected in the PROTECT trial, which included 8,791 patients who underwent PCI during 2007-2008. Dr. Yeh acknowledged that the discrimination strength of the models he and his associated developed was “modest,” but added that its efficacy was greater than what has been shown in validation cohorts for the commonly used CH2ADS2-VASc and HAS-BLED scoring systems.
Dr. Yeh stressed that using the DAPT score “cannot trump clinical judgment.” He suggested that a clinician use the score to help facilitate a conversation with a PCI patient when the time comes to decide whether or not to continue DAPT beyond 1 year.
Other factors that could influence the decision include the length of the stented coronary lesions or prior radiation exposure to the patient’s coronary arteries, said Dr. Laura Mauri, who led the DAPT study and collaborated on developing the DAPT score. “It requires judgment to decide [on whether to continue DAPT] for patients who are on the borderline” for risk and benefit. “This gives patients a way to better understand what they might gain or lose” by continuing treatment. Without the quantification that the DAPT score provides, the balance of risk and benefit “is somewhat nebulous,” said Dr. Mauri, an interventional cardiologist and director of the Center for Clinical Biometrics in the division of cardiovascular medicine at Brigham and Women’s Hospital in Boston.
The investigators who ran the DAPT study realized several years before the study finished that development of the DAPT score was a critical part of applying the findings from the study into clinical practice, she said in an interview.
Dr. Yeh cautioned that the score is only appropriate for patients who match those enrolled into the DAPT study: patients who went through their first year post PCI on DAPT without having any ischemic or bleeding complications. For these patients, “we feel the DAPT score is incredibly valuable,” said Dr. Yeh, an interventional cardiologist and director of the Center for Outcomes Research in Cardiology at Beth Israel Deaconess Medical Center in Boston. He and his associates are now using data from the DAPT study to model bleeding and ischemia risks during the first year following PCI to try to come up with risk models that can address DAPT use during this period.
Dr. Yeh and Dr. Mauri have placed a link to the electronic DAPT score calculator on the website for the DAPT study (www.daptstudy.org), and Dr. Yeh said that an app version will soon become available. Interventionalists in the programs that Dr. Yeh and Mauri are affiliated with have recently begun using the DAPT score calculator in their routine practice, Dr. Yeh said.
The DAPT study received funding from Abbott, Boston Scientific, Cordis, Medtronic, Bristol-Myers Squib-Sanofi, Eli Lilly, and Daiichi Sankyo. Derivation of the DAPT score was funded by the National Institutes of Health. Dr. Yeh has received honoraria from Abbott, Boston Scientific, and Merck. Dr. Jacobs had no disclosures. Dr. Mauri has been a consultant to Biotronik, Medtronic, and St. Jude and has received research funding from several companies.
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: A year after percutaneous coronary intervention, the DAPT score helps clinicians decide whether to continue dual antiplatelet therapy.
Major finding: DAPT scores of 1 or less were linked with a 2.5-fold higher risk of bleeding than ischemic events prevented by continued DAPT; scores of 2 or more were linked with an eightfold increased rate of ischemic events prevented, compared with bleeding events triggered.
Data source: The DAPT study, a multicenter, international, randomized trial that enrolled 25,682 patients.
Disclosures: The DAPT study received funding from Abbott, Boston Scientific, Cordis, Medtronic, Bristol-Myers Squibb-Sanofi, Eli Lilly, and Daiichi Sankyo. Derivation of the DAPT score was funded by the National Institutes of Health. Dr. Yeh has received honoraria from Abbott, Boston Scientific, and Merck.
ACC/AHA guidelines upgrade multivessel PCI, downgrade thrombectomy
ORLANDO – Two guideline panels jointly upgraded percutaneous coronary interventions in noninfarct coronary arteries in patients with a recent MI, and downgraded manual aspiration thrombectomy in acute MI patients.
The panels, organized by the American College of Cardiology and the American Heart Association, cover, respectively, percutaneous coronary intervention (PCI) and ST-elevation MI (STEMI).
The revised guidance on percutaneous coronary intervention for multivessel coronary disease affects roughly half the patients with an acute ST-elevation MI, those who have significant stenoses in noninfarct coronaries. The 2011 guidelines from the PCI panel (J Am Coll Cardiol. 2011 Dec;58[24]:e44-122) and the 2013 guidelines from the STEMI committee (J Am Coll Cardiol. 2013 Jan;61[4]:e78-140) only endorsed revascularization of stenotic, noninfarct arteries in patients with cardiogenic shock or severe heart failure. Multivessel PCI for patients without hemodynamic compromise at the time of primary PCI was deemed a class III indication, “thought to confer harm,” based on the results of several observational studies, Dr. Patrick T. O’Gara said at the American Heart Association scientific sessions.
After the 2013 STEMI guidelines came out, results from four studies showed either benefit or no harm from multivessel PCI in patients with appropriate anatomy performed either at the time of primary PCI or as a scheduled, staged procedure soon after primary PCI, said Dr. O’Gara, professor and director of clinical cardiology at Brigham and Women’s Hospital in Boston. Three of the four studies, PRAMI (N Engl J Med. 2013 Sept 19;369[12]:1115-23), CvLPRIT (J Am Coll Cardiol. 2015 March;65[10]:963-72), and DANAMI-3–PRIMULTI (Lancet. 2015 Aug 15;386[9994]:665-71), are published, while results from the fourth, PRAGUE-13, came out in a meeting report this year but as of mid-November had not been published.
Based on these data the two committees reset PCI of noninfarct arteries as a class IIb recommendation that “could be considered” in selected STEMI patients with multivessel disease who are hemodynamically stable.
The guidelines further said that “insufficient data exist to inform a recommendation” about the optimal time for this multivessel PCI, which could be coincident with primary PCI or 2 days, 3 days, a week, or even later after initial PCI, said Dr. O’Gara. “The writing committees do not advocate for routine use of multivessel PCI; clinical judgment is obviously needed,” he cautioned.
The two committees also reassessed manual aspiration thrombectomy during primary PCI for STEMI, which until now has been a class IIa recommendation, “reasonable” for STEMI patients undergoing primary PCI. But based on the neutral results for thrombectomy in the recent findings from the two largest randomized trials of thrombectomy, TOTAL (N Engl J Med. 2015 April 9;372[15]:1389-98) and TASTE (N Engl J Med. 2013 Oct 24;369[17]:1587-97), as well as a recent, 17-trial meta-analysis, the revised guidelines rate routine aspiration thrombectomy as class III, with “no benefit” and “not useful,” and selective and bailout thrombectomy as a class IIb recommendation, with usefulness that is “not well established.”
Selective use of thrombectomy will require good judgment, noted Dr. O’Gara, who added “it will be important to see what effect, if any, these observations, this evidence, and this distillation of the recommendations from the writing committees have on a change in practice” – using thrombectomy to treat STEMI patients.
Dr. O’Gara chairs the STEMI writing panel of the American College of Cardiology and American Heart Association.
On Twitter @mitchelzoler
ORLANDO – Two guideline panels jointly upgraded percutaneous coronary interventions in noninfarct coronary arteries in patients with a recent MI, and downgraded manual aspiration thrombectomy in acute MI patients.
The panels, organized by the American College of Cardiology and the American Heart Association, cover, respectively, percutaneous coronary intervention (PCI) and ST-elevation MI (STEMI).
The revised guidance on percutaneous coronary intervention for multivessel coronary disease affects roughly half the patients with an acute ST-elevation MI, those who have significant stenoses in noninfarct coronaries. The 2011 guidelines from the PCI panel (J Am Coll Cardiol. 2011 Dec;58[24]:e44-122) and the 2013 guidelines from the STEMI committee (J Am Coll Cardiol. 2013 Jan;61[4]:e78-140) only endorsed revascularization of stenotic, noninfarct arteries in patients with cardiogenic shock or severe heart failure. Multivessel PCI for patients without hemodynamic compromise at the time of primary PCI was deemed a class III indication, “thought to confer harm,” based on the results of several observational studies, Dr. Patrick T. O’Gara said at the American Heart Association scientific sessions.
After the 2013 STEMI guidelines came out, results from four studies showed either benefit or no harm from multivessel PCI in patients with appropriate anatomy performed either at the time of primary PCI or as a scheduled, staged procedure soon after primary PCI, said Dr. O’Gara, professor and director of clinical cardiology at Brigham and Women’s Hospital in Boston. Three of the four studies, PRAMI (N Engl J Med. 2013 Sept 19;369[12]:1115-23), CvLPRIT (J Am Coll Cardiol. 2015 March;65[10]:963-72), and DANAMI-3–PRIMULTI (Lancet. 2015 Aug 15;386[9994]:665-71), are published, while results from the fourth, PRAGUE-13, came out in a meeting report this year but as of mid-November had not been published.
Based on these data the two committees reset PCI of noninfarct arteries as a class IIb recommendation that “could be considered” in selected STEMI patients with multivessel disease who are hemodynamically stable.
The guidelines further said that “insufficient data exist to inform a recommendation” about the optimal time for this multivessel PCI, which could be coincident with primary PCI or 2 days, 3 days, a week, or even later after initial PCI, said Dr. O’Gara. “The writing committees do not advocate for routine use of multivessel PCI; clinical judgment is obviously needed,” he cautioned.
The two committees also reassessed manual aspiration thrombectomy during primary PCI for STEMI, which until now has been a class IIa recommendation, “reasonable” for STEMI patients undergoing primary PCI. But based on the neutral results for thrombectomy in the recent findings from the two largest randomized trials of thrombectomy, TOTAL (N Engl J Med. 2015 April 9;372[15]:1389-98) and TASTE (N Engl J Med. 2013 Oct 24;369[17]:1587-97), as well as a recent, 17-trial meta-analysis, the revised guidelines rate routine aspiration thrombectomy as class III, with “no benefit” and “not useful,” and selective and bailout thrombectomy as a class IIb recommendation, with usefulness that is “not well established.”
Selective use of thrombectomy will require good judgment, noted Dr. O’Gara, who added “it will be important to see what effect, if any, these observations, this evidence, and this distillation of the recommendations from the writing committees have on a change in practice” – using thrombectomy to treat STEMI patients.
Dr. O’Gara chairs the STEMI writing panel of the American College of Cardiology and American Heart Association.
On Twitter @mitchelzoler
ORLANDO – Two guideline panels jointly upgraded percutaneous coronary interventions in noninfarct coronary arteries in patients with a recent MI, and downgraded manual aspiration thrombectomy in acute MI patients.
The panels, organized by the American College of Cardiology and the American Heart Association, cover, respectively, percutaneous coronary intervention (PCI) and ST-elevation MI (STEMI).
The revised guidance on percutaneous coronary intervention for multivessel coronary disease affects roughly half the patients with an acute ST-elevation MI, those who have significant stenoses in noninfarct coronaries. The 2011 guidelines from the PCI panel (J Am Coll Cardiol. 2011 Dec;58[24]:e44-122) and the 2013 guidelines from the STEMI committee (J Am Coll Cardiol. 2013 Jan;61[4]:e78-140) only endorsed revascularization of stenotic, noninfarct arteries in patients with cardiogenic shock or severe heart failure. Multivessel PCI for patients without hemodynamic compromise at the time of primary PCI was deemed a class III indication, “thought to confer harm,” based on the results of several observational studies, Dr. Patrick T. O’Gara said at the American Heart Association scientific sessions.
After the 2013 STEMI guidelines came out, results from four studies showed either benefit or no harm from multivessel PCI in patients with appropriate anatomy performed either at the time of primary PCI or as a scheduled, staged procedure soon after primary PCI, said Dr. O’Gara, professor and director of clinical cardiology at Brigham and Women’s Hospital in Boston. Three of the four studies, PRAMI (N Engl J Med. 2013 Sept 19;369[12]:1115-23), CvLPRIT (J Am Coll Cardiol. 2015 March;65[10]:963-72), and DANAMI-3–PRIMULTI (Lancet. 2015 Aug 15;386[9994]:665-71), are published, while results from the fourth, PRAGUE-13, came out in a meeting report this year but as of mid-November had not been published.
Based on these data the two committees reset PCI of noninfarct arteries as a class IIb recommendation that “could be considered” in selected STEMI patients with multivessel disease who are hemodynamically stable.
The guidelines further said that “insufficient data exist to inform a recommendation” about the optimal time for this multivessel PCI, which could be coincident with primary PCI or 2 days, 3 days, a week, or even later after initial PCI, said Dr. O’Gara. “The writing committees do not advocate for routine use of multivessel PCI; clinical judgment is obviously needed,” he cautioned.
The two committees also reassessed manual aspiration thrombectomy during primary PCI for STEMI, which until now has been a class IIa recommendation, “reasonable” for STEMI patients undergoing primary PCI. But based on the neutral results for thrombectomy in the recent findings from the two largest randomized trials of thrombectomy, TOTAL (N Engl J Med. 2015 April 9;372[15]:1389-98) and TASTE (N Engl J Med. 2013 Oct 24;369[17]:1587-97), as well as a recent, 17-trial meta-analysis, the revised guidelines rate routine aspiration thrombectomy as class III, with “no benefit” and “not useful,” and selective and bailout thrombectomy as a class IIb recommendation, with usefulness that is “not well established.”
Selective use of thrombectomy will require good judgment, noted Dr. O’Gara, who added “it will be important to see what effect, if any, these observations, this evidence, and this distillation of the recommendations from the writing committees have on a change in practice” – using thrombectomy to treat STEMI patients.
Dr. O’Gara chairs the STEMI writing panel of the American College of Cardiology and American Heart Association.
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS